Abstract
The present scientific opinion deals with the evaluation of the safety of di‐magnesium malate (DMM) proposed as a novel food ingredient and as a source of magnesium for use in foods for the general population, food supplements, total diet replacement for weight control and food for special medical purposes (FSMP), and with the bioavailability of magnesium from this source. Additional information was sought from the applicant during the assessment process. However, despite several requests, the applicant did not provide the additional data. Consequently, the Panel performed this assessment on the basis of the available data and concluded that there was insufficient scientific evidence of a difference between the proposed novel food ingredient named as DMM and magnesium malate already authorised as a source of magnesium included in Annex II to Directive 2002/46/EC. Accordingly, the Panel was unable to assess the safety of DMM as a novel food ingredient. The Panel concluded that based on the data provided it was not possible to assess the dissociation of DMM into magnesium and malic acid. The Panel further concluded that if DMM dissociates, magnesium would be available following ingestion of DMM and the availability would appear similar to values reported for other sources of magnesium already permitted. Finally, the Panel noted that the proposed use levels could result in exposures to magnesium greater than its upper level (UL) (250 mg/day) for food supplements and for food for special medical purposes.
Keywords: DMM, di‐magnesium malate, magnesium, nutrient source, food supplements, food for special medical purposes
Summary
The present scientific opinion deals with the evaluation of the safety of di‐magnesium malate (DMM) proposed as a novel food ingredient and as a source of magnesium for use in foods for the general population, food supplements, total diet replacement for weight control and food for special medical purposes (FSMP), and with the bioavailability of magnesium from this source. The safety of magnesium itself, in terms of amounts that may be consumed, and the consideration of magnesium as a nutrient are outside the remit of this Panel.
Additional information was sought from the applicant during the assessment process. A final request sent by the European Food Safety Authority (EFSA) in June 2017 did not generate a reply. Consequently, the Panel concluded this assessment on the basis of the available data.
The Panel noted that the structural formula of the proposed complex, as indicated in the dossier, is based on expert judgement and not supported by any analytical evidence (e.g. X‐ray crystallography). Following a request for additional information, the applicant did not provide any further data in support to the proposed structural formula of the proposed complex.
The Panel considered that the scientific evidence submitted by the applicant does not demonstrate the difference between ‘dimagnesium malate (DMM)’, which is subject of the application under evaluation and ‘magnesium malate’ which is already included in Annex II to Directive 2002/46/EC as one of the mineral substances which may be added to food supplements, following a scientific opinion by EFSA AFC Panel (2006).
The applicant provided results from eight lots of DMM that were analysed by high‐performance liquid chromatography (HPLC) for maleic and fumaric acids. No maleic acid was detected in DMM. The mean concentration of the fumaric acid present in the samples was 0.29%, with a maximum of 0.4% and a minimum of 0.24%. DMM may contain contamination of lead, arsenic, mercury and cadmium as a result of inherent contamination in the starting materials. However, the Panel noted that the certificates of analysis were not provided by the applicant.
The applicant provided analytical data on particle size of five non‐consecutive lots of DMM. However, the Panel considered that the described vibratory sieve testing method with the smallest sieve opening of 45 μm is not appropriate to determine nano‐size particles. The Panel also noted that an average of 10.03% of the tested material passed through the smallest 45 μm sieve.
The Panel noted that the applicant did not submit any information on proposed uses and use levels for DMM, in the application dossier. Further to a request for additional information, the applicant has submitted data on the proposed use and use levels of DMM as source of supplemental magnesium in foods. However, the Panel noted that the values provided by the applicant were expressed in mg/day instead of mg/kg of food as requested by the EFSA ‘Guidance for submission for food additive evaluations’ (EFSA ANS Panel, 2012). Further to a request for additional information, the applicant has submitted data on the proposed use and use levels of DMM as source of supplemental magnesium in foods. The Panel noted that the food classification system used was level 3 of the FoodEx classification system and as such the Panel was unable to complete an exposure estimate for the proposed use of DMM in foods. The Panel considered converting the data on the proposed use and use levels of DMM made on the basis of the level 3 of the FoodEx classification system into the food categories as expressed in the FAIM tools (version 1). However, the Panel considered the uncertainties associated with this would be too great to provide a reliable estimate. Therefore, the Panel did not use this approach.
The proposed use levels in food supplements indicated by the applicant are intended to provide from 75 to 375 mg/day of supplemental magnesium, corresponding to an intended intake of 375–1,875 mg/day of DMM from the use in food supplements. With respect to the use in FSMP and total diet replacement for weight control, at the proposed use levels for DMM the corresponding intake of magnesium would be 25 mg/day and 150–375 mg/day, respectively. The Panel noted that at the proposed use levels of DMM, the existing upper level (UL) of 250 mg/day for supplemental magnesium may be exceeded.
The Panel further noted that, besides a bioavailability study conducted in human volunteers and an acute toxicity study performed in rats, no further biological and toxicological data on DMM were submitted by the applicant as part of the dossier. The applicant provided a justification for not complying with the Tier 1 requirements of the 2012 ANS Panel ‘Guidance for submission for food additive evaluations’ by stating that, following oral ingestion, DMM readily dissociated into magnesium and malic acid.
The dissociation of DMM into its constituents at low pH was investigated by gel filtration by liquid chromatography (LC), using an acidic solution (pH 2.3), mimicking the pH conditions in the stomach, as a mobile phase. However, the Panel considered based on the data provided, that it is not clear whether the substance under evaluation dissociate into its components (malic acid and magnesium) at pH 2.3. Despite a request for additional information, the applicant did not provide any additional data in support of the non‐dissociation of DMM into magnesium and malic acid at other pHs. The Panel considered that the information provided by the applicant was not sufficient to evaluate the dissociation of DMM into magnesium and malic acid.
On the basis of the available data, the Panel concluded that there was insufficient scientific evidence of a difference between the proposed novel food ingredient named as DMM and magnesium malate already authorised as a source of magnesium included in Annex II to Directive 2002/46/EC. Accordingly, the Panel was unable to assess the safety of DMM as a novel food ingredient.
The Panel concluded that based on the data provided it was not possible to assess the dissociation of DMM into magnesium and malic acid. The Panel further concluded that if DMM dissociates, magnesium would be available following ingestion of DMM and the availability would appear similar to values reported for other sources of magnesium already permitted.
The Panel noted that the proposed use levels could result in exposures to magnesium greater than its UL for food supplements and for FSMP.
1. Introduction
The present scientific opinion deals with the evaluation of the safety of di‐magnesium malate (DMM) proposed as a novel food ingredient and as a source of magnesium for use in foods for the general population, food supplements, total diet replacement for weight control and food for special medical purposes (FSMP), and with the bioavailability of magnesium from this source. The safety of magnesium itself, in terms of amounts that may be consumed, and the consideration of magnesium as a nutrient are outside the remit of this Panel.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
The European Union legislation lists nutritional substances that may be used for nutritional purposes in certain categories of foods as sources of certain nutrients.
The relevant Union legislative measures are:
Regulation (EC) No 258/97 of the European Parliament and the Council concerning novel foods and novel food ingredients1;
Directive 2002/46/EC of the European Parliament and of the Council on the approximation of the laws of the Member States relating to food supplements2;
Regulation (EC) No 1925/2006 on the addition of vitamins and mineral and of certain other substances to foods3;
Regulation (EU) No 609/2013 of the European Parliament and of the Council on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control.4
The dossier relating to di‐magnesium malate (DMM) as a source of magnesium has been submitted to the Food Safety Authority of Ireland (FSAI), the competent authority for novel food in Ireland, for an initial assessment under Article 6(2) of Regulation (EC) No 258/97 concerning novel foods and novel food ingredients. The applicant has asked the authorisation for di‐magnesium malate in many food categories, including food supplements as a source of magnesium.
On 9 July 2015, FSAI forwarded to the Commission the initial assessment report, concluding that an additional assessment by the European Food Safety Authority is required in line with Article 6(3) of that Regulation.
1.1.2. Terms of reference
In accordance with Article 29(1)(a) of Regulation (EC) No 178/20025, the European Commission asks the European Food Safety Authority to provide a scientific opinion:
By carrying out the additional assessment for di‐magnesium malate (DMM) as a novel food ingredient in the context of Regulation (EC) No 258/97, and
Following the outcome of the novel food assessment by evaluating the safety of di‐magnesium malate (DMM) when added for nutritional purposes as a source of magnesium to food for the general population, food supplements, total diet replacement for weight control and food for special medical purposes, and the bioavailability of magnesium from this source, in the context of Regulation (EC) No 1925/2006, Directive 2002/46/EC and Regulation (EU) No 609/2013.
1.2. Information on existing evaluations and authorisations
The applicant provided information on the regulatory status of magnesium oxide and malic acid. The Panel considered that information on regulatory status of magnesium oxide is not directly relevant for the assessment of DMM.
1.2.1. Magnesium
The setting of a tolerable upper level (UL) for magnesium was considered by the European Union (EU) Scientific Committee on Food (SCF) in 2001. The SCF concluded that osmotic diarrhoea was the critical effect for establishing an UL for magnesium and identified a no‐observed‐adverse‐effect‐level (NOAEL) of 250 mg/day of magnesium and an uncertainty factor of 1 for deriving an UL of 250 mg/day of magnesium for readily dissociable magnesium salts (e.g. chloride, sulfate, aspartate, lactate) and compounds, such as magnesium oxide, in nutritional supplements, water, or added to food and beverages. The UL does not include magnesium normally present in foods and beverages and it only applies to adults, including pregnant and lactating women, and children from 4 years onwards. No UL could be set for children aged 1–3 years due to lack of data (SCF, 2001).
In 2015, the EFSA NDA Panel issued a scientific opinion on dietary reference values for magnesium (EFSA NDA Panel, 2015). In that opinion, data on dietary intake of magnesium in the general population were reported.
1.2.2. Magnesium malate
In 2006, the former EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) issued a scientific opinion on magnesium malate added for nutritional purposes to food supplements as a source of magnesium. The AFC based its opinion on the previous safety evaluations of the individual substances, malic acid and magnesium and concluded that the use of magnesium malate in food supplements as a source of magnesium was of no safety concern (EFSA AFC Panel, 2006). Magnesium malate is therefore included in Annex II of Directive 2002/46/EC on vitamin and mineral substances which may be used in the manufacture of food supplements.
1.2.3. Malic acid and malates
According to the applicant, malic acid is an intermediate in the tricarboxylic acid cycle, also known as the citric acid or Krebs cycle, which is essential for the oxidative metabolism of carbohydrates and the production of adenosine triphosphate (ATP). The l‐isoform of malic acid occurs naturally in apples and many other fruits and plants (Burdock, 2001; PDRNS, 2001).
Both the Joint FAO/WHO Expert Committee on Food Additives (JECFA, 1980) and the SCF (1991) concluded that there was clear evidence that both enantiomers of malic acid are readily metabolised by laboratory animals and humans and that there was no reason to distinguish between l‐malic acid and dl‐malic acid when considering their safe use in food.
Calcium malate and calcium citrate malate are included in Annex II of Directive 2002/46/EC on vitamin and mineral substances which may be used in the manufacture of food supplements, in Annex II of Regulation (EC) No 1925/2006 on vitamin formulations and mineral substances which may be added to foods. Calcium malate and calcium citrate malate are also included in the Union list of substances that may be added to FSMP and total diet replacement for weight control as referred to in Annex of Regulation (EU) 609/2013.
Zinc and potassium malate are included in Annex II of Directive 2002/46/EC on vitamin and mineral substances which may be used in the manufacture of food supplements.
Malic acid, and sodium‐, sodium hydrogen‐, potassium‐, calcium‐ and calcium hydrogen malate (E 296, E 350–352) are authorised food additives in the EU according to Annex II and Annex III to Regulation (EC) No 1333/20086 on food additives. Currently, their re‐evaluation as food additives is still ongoing as foreseen in Regulation (EC) No 257/20107.The acceptable daily intake (ADI) ‘not specified’ was established by JECFA (1980).
2. Data and methodologies
2.1. Data
The present evaluation is based on the data on DMM provided by the applicant in a dossier submitted in support of its application (Documentation provided to EFSA n. 1), an initial assessment performed by FSAI (Documentation provided to EFSA n. 2), the comments raised by Member States during the assessment of DMM as a novel food ingredient (Documentation provided to EFSA n. 3) and subsequent response by the applicant (Documentation provided to EFSA n. 4).
Additional information was sought from the applicant during the assessment process (Documentation provided to EFSA n. 6, 7).
However, despite several requests, the applicant did not provide the additional data. A final request sent by EFSA in June 2017 did also not generate a reply. Consequently, the Panel concluded this assessment on the basis of the available data.
2.2. Methodologies
The assessment was conducted in line with the principles described in the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA, 2009) and following the relevant existing Guidances from the EFSA Scientific Committee.
The ANS Panel assessed the safety of DMM as a novel food ingredient in line with the principles laid down in Commission Recommendation 97/618/EC.8 In particular, where it is stated that ‘Most of the defined chemical substances can probably be tested for their safety similarly to food additives by utilising conventional methods of safety evaluation as described in the SCF Report No 10.’, the Panel considered that to reflect state of the art scientific knowledge and welfare considerations the reference to SCF Report No 10 should be replaced by the latest existing guidance on the safety evaluation of food additives, namely the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
With respect to the evaluation of bioavailability of the nutrient (magnesium) from the source DMM, the principles contained in the ‘Guidance on submissions for safety evaluation of nutrients or of other ingredients proposed for use in the manufacture of foods’ (SCF, 2001) were followed.
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
According to the applicant (‘Documentation provided to EFSA’ n. 1), DMM is a compound containing a magnesium malate complex and is described as follows:
| Chemical name: | Di‐magnesium malate |
| CAS number: | 671197‐50‐5 |
| Synonyms: | DMM; magnesium malate complex; magnesium (II) malate complex; magnesium malate; dimagnesium malate complex; dimagnesium dihydroxy malate; tetra magnesium dimalate; hydroxybutanedioic magnesium; dimagnesium hydroxybutanedioate; tetramagnesium dihydroxybutanedioate |
| Trade name | Dimagnesium malate (DMM) |
| Chemical formula | Mg2(OH)2C4H4O5 |
| Molecular weight | 171.44 g/mol |
| Description of physical state | Free‐flowing and hygroscopic white powder |
| Solubility | Soluble to freely soluble in water and very slightly soluble in ethanol and acetone in accordance with the solubility definition given by United States Pharmacopeia (USP)9 |
The Panel noted that the molecular weight of DMM as provided by the applicant in the dossier (171.44 g/mol) does not match the molecular weight calculated on the basis of the chemical formula provided (214.60 g/mol).
The structural formula of DMM complex as proposed by the applicant is given in Figure 1. The Panel noted that the structural formula of the proposed complex, as indicated in the dossier, is based on expert judgement and not supported by any analytical evidence (e.g. X‐ray crystallography).
Figure 1.
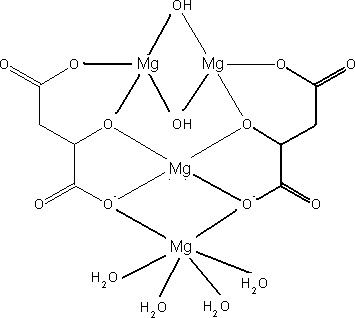
Structural formula of the di‐magnesium malate (DMM) complex, as proposed by the applicant (‘Documentation provided to EFSA’ n. 1)
Further to a request for additional information, the applicant did not provide any additional data in support to the proposed structural formula of the proposed complex.
The Panel noted that according to the information available in the SciFinder database, the formula associated with the CAS number provided by the applicant (CAS 671197‐50‐5) is not specific and the CAS number cannot be unambiguously associated with the material, which is the subject of this application. Further to a request for additional information, the applicant did not provide any additional data on the availability of a CAS number for the proposed complex.
The identity of the product was determined through the analysis of total composition as well as with Fourier‐transforming infrared (FTIR) and mass spectroscopies. The Panel noted that the mass spectrum was not provided in the application dossier. Based on the analysis of infrared and mass spectra, the applicant concluded that DMM was a unique magnesium malate compound rather than a mixture of magnesium and malic acid.
The shifts in the FTIR spectrum of DMM shows coordination between carboxylate and the metal but that do not permit to reach any conclusion on the structure of DMM. The applicant compared the FTIR spectrum of DMM with the one from disodium malate and proposed a molecule with the structure presented in Figure 2. The Panel noted that this structure is different from the one proposed in the same application dossier and presented in Figure 1.
Figure 2.
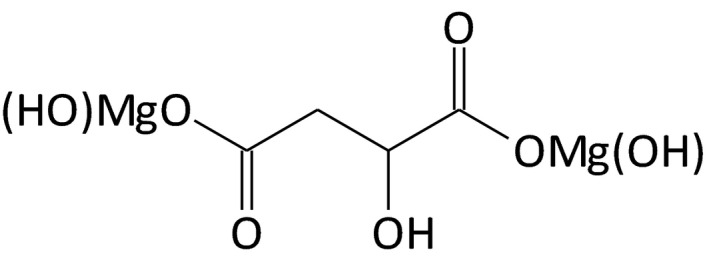
Structural formula for DMM as proposed by the applicant in the report on ‘dimagnesium malate molecular structure data’ Appendix B (‘Documentation provided to EFSA’ n. 1)
However, the Panel considered that these results alone would not be sufficient to characterise the proposed DMM as a complex and that additional analytical evidence (e.g. X‐ray crystallography) has been requested but never provided by the applicant.
Results on the composition of eight lots of DMM were provided by the applicant indicating the concentration of each component (magnesium and malic acid). The results are reported in Table 1.
Table 1.
Concentration of magnesium and malic acid based on results on eight lots of DMM
| Constituent | Average (%) | Maximum (%) | Minimum (%) |
|---|---|---|---|
| Magnesium | 22.05 | 22.53 | 21.46 |
| Malic acid | 54.35 | 56.40 | 53.10 |
| Moisture | 9.83 | 11.14 | 8.26 |
DMM: di‐magnesium malate.
The content of magnesium and malate was analysed using inductively coupled plasma (ICP) spectrometry and high‐performance liquid chromatography (HPLC), respectively. Moisture was calculated theoretically on the basis of the percentage of magnesium and malate.
According to the applicant the purity of DMM can be determined through the analysis of magnesium content using inductively coupled plasmaatomic emission spectrometry (ICP‐AES). According to the applicant, the two geometric isomers fumaric and maleic acid are possible impurities that could occur in malic acid based on the proposed manufacturing process.
Industrially, racemic malic acid is produced by the hydration of the double bond of maleic acid. It is possible that there could be residual maleic or fumaric acids in the malic acid raw material. Neither fumaric nor maleic acids would be formed during the manufacturing of DMM.
The applicant provided results from eight lots of DMM that were analysed by HPLC for maleic and fumaric acids. No maleic acid was detected in DMM. The mean concentration of the fumaric acid present in the samples was 0.29%, with a maximum of 0.40% and a minimum of 0.24%.
According to the applicant, DMM may contain contamination of lead, arsenic, mercury and cadmium as a result of inherent contamination in the starting materials. However, the Panel noted that the certificates of analysis of these toxic elements in DMM were not provided by the applicant.
The Panel noted that, according to the applicant, the DMM is soluble to freely soluble in water.
Upon request from EFSA, the applicant provided analytical data on particle size of five non‐consecutive lots of DMM (Documentation provided to EFSA n. 6) tested using a vibratory sieve testing apparatus. Each lot was analysed without replication. The Panel noted that the applicant provided a description of the method of analysis used. Information on the percentage of the total mass retained above or on top of each sieve, on the cumulative percentage of the total mass retained above or on top of each sieve as a progression through the sieve nest and on the percentage of the total mass that passed through each sieve was provided. The data are summarised in Table 2.
Table 2.
Results of particle size distribution analysis of 5 batches of di‐magnesium malate (DMM)
| US sieve number | Opening (μm) | Percentage above (%) | Cumulative above (%) | Percentage below (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ave | SD | RSD | Ave | SD | RSD | Ave | SD | RSD | ||
| 35 | 500 | 0.02 | 0.03 | 171 | 0.02 | 0.03 | 171 | 99.98 | 0.03 | 0 |
| 45 | 355 | 0.27 | 0.14 | 53 | 0.29 | 0.12 | 43 | 99.71 | 0.12 | 0 |
| 60 | 250 | 0.64 | 0.19 | 30 | 0.92 | 0.28 | 30 | 99.08 | 0.28 | 0 |
| 80 | 180 | 1.45 | 0.32 | 22 | 2.37 | 0.54 | 23 | 97.63 | 0.54 | 1 |
| 120 | 125 | 2.70 | 0.83 | 31 | 5.08 | 1.06 | 21 | 94.92 | 1.06 | 1 |
| 170 | 90 | 17.25 | 6.04 | 35 | 22.33 | 5.90 | 26 | 77.67 | 5.90 | 8 |
| 230 | 63 | 40.46 | 4.94 | 12 | 62.79 | 8.00 | 13 | 37.21 | 8.00 | 21 |
| 325 | 45 | 27.18 | 7.95 | 29 | 89.97 | 3.85 | 4 | 10.03 | 3.85 | 38 |
| Pan | N/A | 10.03 | 3.85 | 38 | 100.00 | 0.00 | 0 | 0.00 | 0.00 | N/A |
Ave: average; SD: standard deviation; RSD: residual standard deviation; N/A: not applicable.
On the basis of the data provided by the applicant, the Panel considered that the described vibratory sieve testing method with the smallest sieve opening of 45 μm is not appropriate to determine nano‐size particles. The Panel also noted that an average of 10% of the tested material passed through the smallest 45 μm sieve.
The Panel noted that there is an inconsistency in the documentation provided by the applicant with regards to the structure of the substance under evaluation: the structural formula presented in the application dossier refers to a complex (Figure 1) and it is different from the structural formula presented in one of the appendixes of the application dossier (Figure 2) (‘Documentation provided to EFSA’ n. 1). Additionally, the Panel considered that the scientific evidence submitted by the applicant (initial application dossier and additional data provided) does not demonstrate the difference between ‘dimagnesium malate (DMM)’, which is subject of the application under evaluation and ‘magnesium malate’ which is already included in the Annex II to Directive 2002/46/EC as one of the mineral substances which may be added to food supplements, following a scientific opinion by EFSA AFC Panel (2006).
3.1.2. Specifications
The specifications for DMM as proposed by the applicant are listed in Table 3.
Table 3.
Specification for DMM as proposed by the applicant
| Specification parameter | Specification value | Method of analysis |
|---|---|---|
| Technical characteristics | ||
| Description | White to off‐white powder | Visual inspection |
| Identification | Passes test | FTIR analysis |
| Assay | ||
| Magnesium content | Not less than 20% | ICP‐AES |
| Malic acid content | Not less than 52% | HPLC |
| pH value | 10–11 | pH meter |
| Moisture/Loss on drying | Not more than 12% | Moisture balance |
| Fumaric acid content | Not more than 1.0% | HPLC |
| Maleic acid | Not more than 0.05% | HPLC |
| Heavy metals | ||
| Arsenic | Not more than 3.0 ppm | ICP‐MS |
| Lead | Not more than 1.5 ppm | ICP‐MS |
| Cadmium | Not more than 0.5 ppm | ICP‐MS |
| Mercury | Not more than 0.1 ppm | ICP‐MS |
| Microbiological specifications | ||
| Total plate count | Not more than 1,000 | FDA BAM 8th edition |
| Yeast and moulds | Not more than 100 | AOAC 995.21 |
| Coliform count | Negative | FDA BAM 8th edition |
| Escherichia coli | Negative | FDA BAM 8th edition |
| Staphylococcus aureus | Negative | FDA BAM 8th edition |
| Salmonella | Negative | AOAC 991.12 |
FTIR: Fourier‐transforming infrared; HPLC: high‐performance liquid chromatography; ICP‐MS: inductively coupled plasma mass spectrometry; BAM: bacteriological analytical manual; nd: not detectable information available.
The Panel noted that the description in the proposed specifications (white to white off powder) was more limited than that given in the identity of the substance (free flowing and hygroscopic white powder). The Panel noted that no flowability data were provided by the applicant.
Furthermore, the Panel noted that the currently proposed specifications indicate a minimum limit for magnesium and malic acid whilst no maximum limits are provided.
The Panel also noted that the currently proposed specifications indicate a range of solubility in water for the complex (from soluble to freely soluble). The Panel noted that the results of the solubility test in support of the proposed classification range were not provided in the application dossier (‘Documentation provided to EFSA’ n. 1).
Further to a request for additional information, the applicant did not provide any additional data in support to the currently proposed specifications of DMM.
According to the applicant, the impurities resulting from the manufacturing process include arsenic (≤ 0.003%), lead (≤ 0.0015%), cadmium (≤ 0.0005%) and mercury (≤ 0.0001%) that correspond to ≤ 30 ppm (arsenic), ≤ 15 ppm (lead), ≤ 5 ppm (cadmium) and ≤ 1 ppm (mercury). Specifications for DMM include parameters for all impurities, and each batch manufactured is tested to ensure that the purity criteria are met. However, the Panel noted that analytical data provided by the applicant are not in agreement with those reported in the specifications.
The Panel noted that the applicant submitted results on eight non‐consecutive batch analyses on DMM. However, the certificates of analysis were provided for a single batch that was not included in the analysis. Consequently, on the basis of the information provided the Panel was unable to evaluate whether DMM is produced in compliance with the proposed specifications (‘Documentation provided to EFSA’ n. 1).
3.1.3. Manufacturing process
The Panel noted that the information on the manufacturing process as provided by the applicant in the application dossier (‘Documentation provided to EFSA’ n. 1) was claimed to be confidential.
According to information provided by the applicant, DMM is manufactured from magnesium oxide powder (purity: 96.0–100.5%) and dl‐malic acid (purity: 99–100.5%) as starting materials, under aqueous conditions.
In the process, dl‐malic acid and magnesium oxide are dissolved in water and mixed under controlled temperature. The resulting solution is then spray‐dried and packed.
It is stated that the dl‐malic acid used as raw material meets USP grade specifications and is equivalent to food‐grade material; the magnesium oxide powder also meets Food Chemicals Codex specifications (FCC, 2003a,b).
The applicant provided information indicating that DMM is manufactured under good manufacturing practices (GMP) and in accordance with the Hazard Analysis Critical Control Point system (HACCP).
The Panel considered that the method outlined by the applicant sufficiently described the manufacturing process for a magnesium malate; however, it does not demonstrate the specific production of DMM.
3.1.4. Methods of analysis in food
According to the applicant, the level of magnesium as DMM added to foodstuff may be determined by the inductively coupled plasma/atomic emission spectroscopy (ICP‐AES) method. According to the applicant each milligram of magnesium assayed is equivalent to 5 mg of DMM.
The Panel noted that the applicant did not provide any information on direct method for analysis of DMM in foodstuff. Further to a request for additional information, the applicant did not submit any additional data in support of the availability of direct method for analysis of DMM in food.
3.1.5. Stability of the substance, and reaction and fate in food
The mineral content (magnesium) and pH stability of five non‐consecutive batches of DMM has been evaluated by the applicant over a period of 3 years. According to the applicant, the products were tested at the time of release for all specifications in effect at the time of release. The samples were stored in a polystyrene container with a screw‐top lid at ambient temperature and humidity in a controlled access room. At the end of 3 years, the samples were tested again for all specifications that were initially tested (Documentation provided to EFSA n. 1).
The Panel noted that the stability data submitted from a 3‐year test provided indication only on the variations in the total amount of Mg and pH during that period (Documentation provided to EFSA n. 1).
However, the Panel noted that the data submitted did not provide indications on the stability of the proposed complex of DMM during storage time. Furthermore, the Panel noted that no information on effect of temperature on the stability of DMM in the food matrix was provided by the applicant.
According to the applicant, in the event of degradation of DMM, the expected degradation products would be magnesium ions, hydroxide ions (OH−) and malic acid., It is not expected that magnesium ions or malic acid would cause lipid peroxidation or other organoleptic change (Documentation provided to EFSA n. 1).
Further to a request for additional information, the applicant did not provide any additional data in support to the stability of DMM.
On the basis of the data available, the Panel was unable to conclude on the stability of the complex DMM (Documentation provided to EFSA n. 1).
3.2. Proposed uses and use levels
3.2.1. Use in foods for the general population according to Regulation (EC) No 1925/2006
The Panel noted that the applicant did not submit any information on proposed uses and use levels for DMM, in the application dossier (‘Documentation provided to EFSA’ n. 1).
Further to a request for additional information, the applicant has submitted data on the proposed use and use levels of DMM as a source of supplemental magnesium in foods. However the Panel noted that the values provided by the applicant were expressed in mg/day instead of mg/kg of food as requested by the EFSA ‘Guidance for submission for food additive evaluations’ (EFSA ANS Panel, 2012). The Panel noted that the typical and the maximum use levels, as provided by the applicant, were 300 and 1,875 mg DMM/kg of food, respectively, for all the food categories proposed, corresponding to 60 and 375 mg magnesium/kg of food for the typical and the maximum levels (‘Documentation provided to EFSA’ n. 6).
Moreover, the Panel noted that, the food classification system used the level 3 of the FoodEx classification system and as such the Panel was unable to complete an exposure estimate for the proposed use of DMM in foods (Documentation provided to EFSA n. 7). The additional information submitted is presented in Annex A.
3.2.2. Use in food supplements according to Directive 2002/46/EC
In addition to use in food, the applicant requested the inclusion of DMM as a source of magnesium in food supplements among the authorised substances included in Annex II of Directive 2002/46/EC.
According to the data provided by the applicant, the use of DMM in food supplements would provide from 375 to 1,875 mg/day of DMM. This would correspond to 75–375 mg/day of magnesium (Documentation provided to EFSA n. 6).
3.2.3. Use in food for special medical purposes and total diet replacement for weight control according to Regulation (EU) No 609/2013
The applicant is also proposing the use of DMM in FSMP at levels providing 25 mg/day of magnesium corresponding to 125 mg/day of DMM in individuals consuming FSMP.
According to the data provided by applicant, the use of DMM in food for total diet replacement for weight control would provide from 750 to 1,875 mg/day of DMM. This would correspond to 150–375 mg/day of magnesium.
3.3. Exposure data
The Panel noted that the applicant did not provide any overall estimate for the daily intake of DMM and the corresponding intake of magnesium, resulting from each proposed use of DMM in foods.
The Panel considered converting the data on the proposed use and use levels of DMM made on the basis of the level 3 of the FoodEx classification system into the food categories as expressed in the FAIM tools (version 1). However, the Panel considered the uncertainties associated with this would be too great to provide a reliable estimate. Therefore, the Panel did not use this approach.
The proposed used levels in food supplements indicated by the applicant are intended to provide from 75 to 375 mg/day of supplemental magnesium, corresponding to an intended intake of 375–1,875 mg/day of DMM.
With respect to the use in FSMP and total diet replacement for weight control, at the proposed use levels for DMM the corresponding intake of magnesium would be 25 mg and 150–375 mg/day, respectively.
The Panel noted that at the proposed use levels of DMM, the existing UL of 250 mg/day for supplemental magnesium may be exceeded.
3.4. Biological and toxicological data
The Panel noted that, besides a bioavailability study conducted in human volunteers and an acute toxicity study performed in rats, no further biological and toxicological data on DMM were submitted by the applicant as part of the dossier.
The applicant provided a justification for not complying with the Tier 1 requirements in the ‘Guidance for submission for food additive evaluations’ (EFSA ANS Panel, 2012) by stating that, following oral ingestion, DMM readily dissociated into magnesium and malic acid.
3.4.1. Bioavailability of magnesium from DMM
Data on dissociation of DMM
The Panel noted that the applicant has provided results from an experimental study in support of the dissociation of DMM at low pH into magnesium and malic acid (Documentation provided to EFSA n. 1).
The dissociation of DMM into its constituents at low pH was investigated by gel filtration by liquid chromatography (LC), using an acidic solution (pH 2.3), mimicking the pH conditions in the stomach, as a mobile phase. The study was designed to provide some evidence to support that DMM, when placed into acidic solution like that found in the stomach, dissociated into its individual components (magnesium and malic acid).
Different fractions were collected, evaporated and re‐dissolved. The detection of the eluted organic acids (malic, maleic and fumaric) in each fraction was made by HPLC (UV–vis spectrometer) and magnesium concentrations were determined by titration with EDTA, using Eriochrome black as indicator (Documentation provided to EFSA n. 1).
If the DMM complex was intact, the methodology used (separation based on molecular weight) would result in this being eluted before the organic acids. The liquid chromatogram obtained showed two major peaks (corresponding to the collected fractions 3 and 5, respectively). The analysis of the fractions confirmed that malic acid was the main component in the fraction 3, but this fraction did not contain the magnesium amount that would be expected in a DMM complex, and that fumaric acid was the main component in the fraction 5 but likewise the corresponding fraction showed no magnesium that would indicate complexation. Magnesium was present at the highest concentration in the final fraction (fraction 6) and no other compounds were found in significant amount in this fraction, indicating that magnesium was not complexed to any other constituents in the solution. Additionally, the Panel noted the presence of maleic acid in fractions 2, 4 and 6, even though the applicant has stated that maleic acid is not present in DMM (Section 3.1.1).
However, the Panel considered based on the description of the method as above, that it is not clear whether the substance under evaluation dissociate into its components (malic acid and magnesium) at pH 2.3.
Despite a request for additional information, the applicant did not provide any additional data in support of the non‐dissociation of DMM into magnesium and malic acid at other pHs.
On the basis of the data available, the Panel concluded that the information provided by the applicant is not sufficient to evaluate the dissociation of DMM into magnesium and malic acid; consequently, Tier 1 toxicity studies would be needed to evaluate the safety of DMM.
Human data
The bioavailability and tolerability of magnesium from four magnesium supplements (magnesium oxide, magnesium bisglycinate chelate, magnesium bisglycinate chelate buffered and DMM) was investigated in an unpublished randomised, double‐blind, four‐arm cross‐over study in 14 healthy volunteers, performed in accordance with Good Clinical Practice (GCP) in 2007 (Documentation provided to EFSA n. 1). Each participant received 150 mg as bolus dose of one of the four magnesium supplements per visit, with a minimum of a 2‐ week wash out period between visits for a total of four visits. Bioavailability was determined by serum magnesium levels and tolerability was determined by adverse event reporting.
None of the subjects was discontinued from the study due to adverse events.
Serum samples were taken at 0, 1, 2, 3, 4, 8, 12 and 24 h post‐treatment. The areas under the curve (AUC) of magnesium for magnesium bisglycinate, magnesium bisglycinate buffered, DMM and magnesium oxide were 0.164, 0.225, 0.196 and 0.109, respectively. The results were statistically lower for magnesium oxide in comparison to the other magnesium forms. According to the authors, magnesium from magnesium bisglycinate, magnesium bisglycinate buffered and DMM showed a higher bioavailability compare to that from magnesium oxide. According to the applicant, systemic availability of magnesium from DMM was comparable to that of other sources already permitted.
3.4.2. Acute toxicity
DMM from a single lot has been evaluated in an unpublished single‐dose oral toxicity study conducted in accordance with Good Laboratory Practice (GLP) in three healthy female Wistar albino rats (Documentation provided to EFSA n. 1). No mortality was recorded at 5,000 mg/kg body weight (bw).
3.5. Discussion
The current assessment by the Panel is based on the information submitted in the application dossier (Documentation provided to EFSA n. 1) and the additional information provided by the applicant in response to EFSA's requests (Documentation provided to EFSA n. 6, 7). However, despite several requests, the applicant did not provide the additional data. A final request sent by EFSA in June 2017 also did not generate a reply. Consequently, the Panel concluded this assessment on the basis of the available data.
According to the applicant, DMM is a compound containing a magnesium malate complex.
However, the Panel considered that the identity of DMM as a complex has not been demonstrated.
The Panel noted that the manufacturing process described for DMM does not demonstrate that the synthesis of DMM is different from that of magnesium malate.
In the absence of scientific evidence, the Panel considered that it is not possible to distinguish between the proposed substance and a salt of magnesium and malic acid. The Panel has requested scientific data to demonstrate that the substance under evaluation is different from ‘magnesium malate’, which is already included in the Annex II to Directive 2002/46/EC, and the applicant was not able to provide any scientific evidence. In the absence of these data, the Panel is unable to conclude that the substance presented as DMM would be different from magnesium malate already authorised as a nutrient source.
The applicant has proposed chemical and microbiological specifications for DMM and provided results from eight non‐consecutive batches. However, the certificates of analysis were provided for a single batch that was not included in the analysis. Consequently, on the basis of the information provided the Panel was unable to evaluate whether DMM is produced in compliance with the proposed specifications.
According to the applicant, DMM is expected to remain stable for up to 3 years under normal storage condition. However, the Panel noted that the results on stability tests as provided by the applicant refer to the magnesium content and not to DMM as a complex. Moreover, the Panel noted that no information on the effect of temperature on the stability of DMM in a food matrix was provided by the applicant. On the basis of the data available, the Panel was unable to conclude on the stability of the complex DMM.
The Panel considered that the data provided by the applicant on the proposed uses and use levels were not adequate to allow the reliable calculation of the exposure to DMM.
On the basis of the information provided by the applicant, the Panel noted that the intake of magnesium from food fortified with DMM in addition to the proposed use in food supplements, FSMP and food for total diet replacement for weight control, would lead to an exceedance of the UL for supplemental magnesium (250 mg/day).
The Panel further noted that the toxicological data set was limited to an acute oral toxicity study and a randomised, double‐blind, four‐arm crossover study performed in healthy human volunteers with limited safety parameters.
According to the applicant, DMM at low pH dissociates into its main components (magnesium and malic acid). Despite a request for additional information, the applicant did not provide any additional data in support of the non‐dissociation of DMM into magnesium and malic acid at other pHs.
On the basis of the data available, the Panel concluded that the information provided by the applicant is not sufficient to evaluate the dissociation of DMM into magnesium and malic acid; consequently, Tier 1 toxicity studies would be needed to evaluate the safety of DMM.
On the basis of the data submitted by the applicant, the Panel considered that magnesium is bioavailable from the source.
4. Conclusions
On the basis of the available data, the Panel concluded that there was insufficient scientific evidence of a difference between the proposed novel food ingredient named as DMM and magnesium malate already authorised as a source of magnesium included in Annex II to Directive 2002/46/EC. Accordingly, the Panel was unable to assess the safety of DMM as a novel food ingredient.
The Panel concluded that based on the data provided it was not possible to assess the dissociation of DMM into magnesium and malic acid. The Panel concluded that if DMM dissociates, magnesium would be available following ingestion of DMM and the availability would appear similar to values reported for other sources of magnesium already permitted.
The Panel noted that the proposed use levels could result in exposures to magnesium greater than its UL for food supplements and for FSMP.
Documentation provided to EFSA
Dossier ‘Application for the approval of di‐magnesium malate as a source of magnesium for use in the manufacture of PARNUTS products, food supplements and fortified foods’. May 2015. Additional data provided on 08 April 2016. Submitted by Albion Laboratories, Inc.
Initial assessment of di‐magnesium malate (DMM) as a novel food ingredient. Food Safety Authority Ireland (FSAI). July 2015.
Member States comments and objections. September 2015.
Response by the applicant to the initial assessment report and the Member States' comments and objections. Submitted by Albion Laboratories, Inc. 20 January 2016.
Letter from the European Commission to the European Food Safety Authority with a request for a scientific opinion on di‐magnesium malate. SANTE/E6/SS/ks D (2015) 368907; Ref. Ares(2016)444515, dated 27 January 2016.
Additional information. February 2017. Submitted by Albion Laboratories, Inc. in response to a request from EFSA.
Additional information. March 2017. Submitted by Albion Laboratories, Inc. in response to a request from EFSA.
Abbreviations
- ADI
acceptable daily intake
- ANS Panel
EFSA Panel on Food additives and Nutrient Sources added to Food
- ATP
adenosine triphosphate
- AUC
area under the curve
- BAM
bacteriological analytical manual
- bw
body weight
- CAS
Chemical Abstracts Service
- DMM
di‐magnesium malate
- FAIM
Food additives Intake Model
- FCC
Food Chemical Codex
- FSMP
Food for special medical purposes
- FTIR
Fourier‐transforming infrared
- GI
gastrointestinal
- GCP
Good Clinical Practice
- GLP
Good Laboratory Practice
- GMP
Good Manufacturing Practices
- HACCP
Hazard Analysis Critical Control Point system
- HPLC
high‐performance liquid chromatography
- ICP/AES
inductively coupled plasma/atomic emission spectrometry
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LC
liquid chromatography
- MS
mass spectroscopy
- NDA Panel
EFSA Panel on Dietetic Products, Nutrition and Allergies
- NOAEL
no‐observed‐adverse‐effect‐level
- SCF
Scientific Committee on Food
- UL
upper level
- USP
United States Pharmacopeia
Annex A – Proposed use and use levels as provided by the applicant (Documentation provided to EFSA n. 7)
1.
| Code | IntendedTree | Corex | Amount DMM/kg food | Maximum DMM/kg food |
|---|---|---|---|---|
| A000J | Grain and grain‐based products | H | ||
| A000K | Cereals and cereal primary derivatives | H | ||
| A000L | Cereal grains (and cereal‐like grains) | M | ||
| A001X | Mixtures of grains | C | 320 mg | 1,925 mg |
| A0D9Y | Barley and similar | C | 320 mg | 1,925 mg |
| A04KH | Buckwheat and other pseudo‐cereals and similar | C | 320 mg | 1,925 mg |
| A000S | Maize and similar | C | 320 mg | 1,925 mg |
| A000Y | Common millet and similar | C | 320 mg | 1,925 mg |
| A000F | Oat and similar | C | 320 mg | 1,925 mg |
| A001C | Rice and similar | C | 320 mg | 1,925 mg |
| A0D9R | Rye and similar | C | 320 mg | 1,925 mg |
| A0D9Q | Sorghum and similar | C | 320 mg | 1,925 mg |
| A04KR | Cereals and cereal‐like grains not separately listed | H | ||
| A001M | Wheat and similar | C | 320 mg | 1,925 mg |
| A04KS | Cereal and cereal‐like flours | M | ||
| A004S | Flour mix (like wheat/rye/barley/oats and other) | C | 320 mg | 1,925 mg |
| A002E | Amaranth flour | C | 320 mg | 1,925 mg |
| A002L | Barley flour | C | 320 mg | 1,925 mg |
| A002G | Buckwheat flour | C | 320 mg | 1,925 mg |
| A0C0Z | Maize, milled | C | 320 mg | 1,925 mg |
| A002T | Millet flour | C | 320 mg | 1,925 mg |
| A002Y | Oat flour | C | 320 mg | 1,925 mg |
| A003F | Rice flour | C | 320 mg | 1,925 mg |
| A003J | Rye flour | C | 320 mg | 1,925 mg |
| A003T | Sorghum flour | C | 320 mg | 1,925 mg |
| A004H | Spelt flour | C | 320 mg | 1,925 mg |
| A003X | Wheat flour | C | 320 mg | 1,925 mg |
| A0BY1 | Groats | M | ||
| A065N | Barley groats | C | 320 mg | 1,925 mg |
| A002H | Buckwheat groats | C | 320 mg | 1,925 mg |
| A002V | Millet groats | C | 320 mg | 1,925 mg |
| A002Z | Oat groats | C | 320 mg | 1,925 mg |
| A003P | Rye groats | C | 320 mg | 1,925 mg |
| A004E | Wheat groat | C | 320 mg | 1,925 mg |
| A004G | Bulgur | C | 320 mg | 1,925 mg |
| A0ETL | Semolina | M | ||
| A002N | Maize semolina | C | 320 mg | 1,925 mg |
| A004F | Wheat semolina | C | 320 mg | 1,925 mg |
| A0F6Q | Rice semolina | C | 320 mg | 1,925 mg |
| A002C | Cereal bran | M | 320 mg | 1,925 mg |
| A003B | Oat bran | C | 320 mg | 1,925 mg |
| A003Q | Rye bran | C | 320 mg | 1,925 mg |
| A004P | Wheat bran | C | 320 mg | 1,925 mg |
| A0F6P | Rice bran | C | 320 mg | 1,925 mg |
| A00CV | Breakfast cereals | H | ||
| A04LH | Breakfast cereals, plain | H | ||
| A04LJ | Cereal rolled grains | M | ||
| A00EH | Mixed cereal rolled grains | C | 320 mg | 1,925 mg |
| A00CY | Barley rolled grains | C | 320 mg | 1,925 mg |
| A00DF | Millet rolled grains | C | 320 mg | 1,925 mg |
| A00DH | Oat rolled grains | C | 320 mg | 1,925 mg |
| A00DQ | Rice rolled grains | C | 320 mg | 1,925 mg |
| A00DV | Rye rolled grains | C | 320 mg | 1,925 mg |
| A00EA | Spelt rolled grains | C | 320 mg | 1,925 mg |
| A00EB | Wheat rolled grains | C | 320 mg | 1,925 mg |
| A00EC | Wheat germs rolled flakes | C | 320 mg | 1,925 mg |
| A00ED | Wheat bran rolled flakes | C | 320 mg | 1,925 mg |
| A00EN | Porridge (in dry form, to be diluted) | M | ||
| A00EX | Barley porridge | C | 320 mg | 1,925 mg |
| A00ET | Cornmeal porridge | C | 320 mg | 1,925 mg |
| A00EQ | Oat porridge | C | 320 mg | 1,925 mg |
| A00ER | Rice porridge | C | 320 mg | 1,925 mg |
| A00ES | Rye porridge | C | 320 mg | 1,925 mg |
| A00EV | Wheat semolina porridge | C | 320 mg | 1,925 mg |
| A00EY | Cereal bars | C | 320 mg | 1,925 mg |
| A010R | Sugar plants | H | ||
| A0ETE | Roots used as sugar source | M | ||
| A0CXQ | Sugar beet roots and similar | C | 320 mg | 1,925 mg |
| A0CXM | Chicory roots and similar | C | 320 mg | 1,925 mg |
| A0ESQ | Stalks/canes/trunk sap or similar for sugar | M | ||
| A0CXP | Sugar canes and similar | C | 320 mg | 1,925 mg |
| A04HB | Other sugar plants | C | 320 mg | 1,925 mg |
| A02LR | Milk and dairy products | H | ||
| A04NN | Milk, whey and cream | H | ||
| A02LT | Milk | M | ||
| A02LV | Cow milk | C | 192 mg/L | 1,925 mg/L |
| A02MB | Goat milk | C | 192 mg/L | 1,925 mg/L |
| A02MP | Flavoured milks | C | 192 mg/L | 1,925 mg/L |
| A02MZ | Fermented milk or cream | H | ||
| A02NQ | Yoghurt drinks, sweetened and/or flavoured | C | 192 mg/L | 1,925 mg/L |
| A02PD | Milk and dairy powders and concentrates | M | ||
| A04NR | Other dairy concentrate | H | ||
| A02PJ | Milk powder | C | 192 mg/L | 1,925 mg/L |
| A02PM | Cream powder | C | 192 mg/L | 1,925 mg/L |
| A02PN | Whey powder | C | 192 mg/L | 1,925 mg/L |
| A032F | Sugar and similar, confectionery and water‐based sweet desserts | H | ||
| A04PA | Sugar and other sweetening ingredients (excluding intensive sweeteners) | H | ||
| A032G | Sugars (mono‐ and di‐saccharides) | H | ||
| A032H | Sucrose (common sugar) | C | 320 mg | 1,925 mg |
| A033R | Syrup (molasses and other syrups) | M | ||
| A033S | Molasses | C | 320 mg | 1,925 mg |
| A033Z | Syrups | C | 320 mg | 1,925 mg |
| A033J | Honey | C | 320 mg | 1,925 mg |
| A04PB | Other sweetening ingredients | H | ||
| A032Z | Polyols | C | 320 mg | 1,925 mg |
| A0F7R | Table‐top sweeteners formulations | M | ||
| A0F7T | Table‐top sweeteners in liquid form | C | 320 mg | 1,925 mg |
| A0F7V | Table‐top sweeteners in powder form | C | 320 mg | 1,925 mg |
| A0F7X | Table‐top sweeteners in tables | C | 320 mg | 1,925 mg |
| A039K | Fruits and vegetable juices and nectars (including concentrates) | H | ||
| A0BX9 | Fruits/vegetable juices and nectars | M | ||
| A03AN | Mixed fruit juice | C | 192 mg/L | 1,925 mg |
| A039M | Juice, apple | C | 192 mg/L | 1,925 mg |
| A03AM | Juice, orange | C | 192 mg/L | 1,925 mg |
| A039T | Juice, cranberry | C | 192 mg/L | 1,925 mg |
| A039N | Juice, apricot | C | 192 mg/L | 1,925 mg |
| A03AL | Juice, grapefruit | C | 192 mg/L | 1,925 mg |
| A03AF | Juice, pineapple | C | 192 mg/L | 1,925 mg |
| A04PN | Other fruit juices | H | ||
| A039P | Juice, black currant | C | 192 mg/L | 1,925 mg |
| A039S | Juice, blackberry | C | 192 mg/L | 1,925 mg |
| A03AG | Juice, citrus | C | 192 mg/L | 1,925 mg |
| A039V | Juice, elderberry | C | 192 mg/L | 1,925 mg |
| A03AK | Juice, grape | C | 192 mg/L | 1,925 mg |
| A039X | Juice, guava | C | 192 mg/L | 1,925 mg |
| A03AH | Juice, lemon | C | 192 mg/L | 1,925 mg |
| A03AJ | Juice, lime | C | 192 mg/L | 1,925 mg |
| A039Y | Juice, mango | C | 192 mg/L | 1,925 mg |
| A039Z | Juice, nectarine | C | 192 mg/L | 1,925 mg |
| A03AA | Juice, passion fruit | C | 192 mg/L | 1,925 mg |
| A03AB | Juice, peach | C | 192 mg/L | 1,925 mg |
| A03AC | Juice, pear | C | 192 mg/L | 1,925 mg |
| A03AD | Juice, pomegranate | C | 192 mg/L | 1,925 mg |
| A03AE | Juice, prune | C | 192 mg/L | 1,925 mg |
| A039R | Juice, red currant | C | 192 mg/L | 1,925 mg |
| A04PS | Other (mixed) fruit and vegetable juices or nectars | H | ||
| A03DC | Juice, apple–carrot | C | 192 mg/L | 1,925 mg |
| A03DD | Juice, multifruit–carrot | C | 192 mg/L | 1,925 mg |
| A04PT | Other mixed fruit and vegetable juices | H | ||
| A03DF | Fruit smoothies | C | 192 mg/L | 1,925 mg |
| A03DH | Multivitamin juices | C | 192 mg/L | 1,925 mg |
| A03BM | Concentrated or dehydrated fruit/vegetables juices | H | ||
| A0ETV | Fruit/vegetable juice concentrate | M | ||
| A03BP | Juice concentrate, apricot | C | 192 mg/L | 1,925 mg |
| A03BQ | Juice concentrate, blackberry | C | 192 mg/L | 1,925 mg |
| A03BR | Juice concentrate, blueberry | C | 192 mg/L | 1,925 mg |
| A03BS | Juice concentrate, black currant | C | 192 mg/L | 1,925 mg |
| A03BT | Juice concentrate, red currant | C | 192 mg/L | 1,925 mg |
| A03BV | Juice concentrate, cranberry | C | 192 mg/L | 1,925 mg |
| A03BX | Juice concentrate, gooseberry | C | 192 mg/L | 1,925 mg |
| A03BY | Juice concentrate, grape | C | 192 mg/L | 1,925 mg |
| A03BZ | Juice concentrate, mandarin | C | 192 mg/L | 1,925 mg |
| A03CA | Juice concentrate, orange | C | 192 mg/L | 1,925 mg |
| A03CB | Juice concentrate, peach | C | 192 mg/L | 1,925 mg |
| A03CC | Juice concentrate, plum | C | 192 mg/L | 1,925 mg |
| A03CD | Juice concentrate, raspberry | C | 192 mg/L | 1,925 mg |
| A03CE | Juice concentrate, strawberry | C | 192 mg/L | 1,925 mg |
| A03CF | Juice concentrate, sweet cherry | C | 192 mg/L | 1,925 mg |
| A03CZ | Vegetable juice concentrate | C | 192 mg/L | 1,925 mg |
| A03DJ | Water and water‐based beverages | H | ||
| A03DK | Drinking water | M | ||
| A03DQ | Natural mineral water | C | 19 mg/L | 384 mg/L |
| A03DT | Bottled drinking water | C | 19 mg/L | 384 mg/L |
| A03DY | Flavoured bottled water | C | 19 mg/L | 384 mg/L |
| A03GC | Fortified bottled water | C | 19 mg/L | 384 mg/L |
| A04PY | Water based beverages | H | ||
| A03FZ | Functional drinks | M | ||
| A03GB | Isotonic and sport drinks | C | 192 mg/L | 1,925 mg/L |
| A04PZ | Beverages concentrates | H | ||
| A03GD | Drink mixes | M | ||
| A03GE | Liquid drink bases (including concentrates and home‐made preparations) | C | 192 mg | 1,925 mg |
| A03GF | Powdered drink bases | C | 192 mg | 1,925 mg |
| A03RQ | Products for non‐standard diets, food imitates and food supplements | H | ||
| A03RR | Food for particular diets | H | ||
| A03RS | Food for weight reduction | M | ||
| A03RT | Total daily diet replacement for weight reduction | C | 963 mg | 1,925 mg |
| A03RV | Single meal replacement for weight reduction | C | 320 mg | 1,925 mg |
| A03RX | Food for sporting people | M | ||
| A03RY | Carbohydrate‐rich energy food products for sports people | C | 320 mg | 1,925 mg |
| A03RZ | Carbohydrate‐electrolyte solutions for sports people | C | 192 mg/L | 385 mg/L |
| A03SB | Micronutrients supplement for sports people | C | 963 mg | 1,925 mg |
| A03SD | Dietary foods for special medical purposes | M | ||
| A03SE | Nutritionally complete formulae | C | 963 mg | 1,925 mg |
| A03SF | Nutritionally incomplete formulae | C | 320 mg | 1,925 mg |
| A03SH | Oral rehydration products | C | 192 mg/L | 385 mg/L |
| A03SJ | Food supplements and similar preparations | M | ||
| A03TC | Mixed supplements/formulations | C | 100 g | 900 g |
| A03SM | Mineral only supplements | C | 200 g | 1,000 g |
| A03SN | Combination of vitamin and mineral only supplements | C | 100 g | 1,000 g |
| A046L | Major isolated ingredients, additives, flavours, baking and processing aids | H | ||
| A0EVD | Isolated proteins and other protein products | M | ||
| A02PR | Milk protein | C | 320 mg | 1,925 mg |
| A02PS | Whey protein | C | 320 mg | 1,925 mg |
| A01BG | Soya proteins | C | 320 mg | 1,925 mg |
| A0F5E | Gelatine | C | 320 mg | 1,925 mg |
| A0ETM | Starches | M | ||
| A002R | Maize starch | C | 320 mg | 1,925 mg |
| A003A | Oat starch | C | 320 mg | 1,925 mg |
| A003G | Rice starch | C | 320 mg | 1,925 mg |
| A003R | Rye starch | C | 320 mg | 1,925 mg |
| A004M | Wheat starch | C | 320 mg | 1,925 mg |
| A011F | Potato starch | C | 320 mg | 1,925 mg |
| A011J | Tapioca starch | C | 320 mg | 1,925 mg |
| A0DPT | Maltodextrins and similar | M | ||
| A0BSL | Maltodextrin | C | 320 mg | 1,925 mg |
| A0BSK | Dextrin | C | 320 mg | 1,925 mg |
| A04QR | Other regulated additives | H | ||
| A048C | Acidity regulator | C | 320 mg | 1,925 mg |
| A0F0S | Other ingredients | H | ||
| A0EVF | Chemical elements | M | ||
| A0EXF | Magnesium | C | 100 g | 1 kg |
DMM: di‐magnesium malate.
Suggested citation: EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) , Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gundert‐Remy U, Kuhnle GG, Lambré C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, McArdle H, Tobback P, Pizzo F, Rincon A, Smeraldi C and Gott D, 2018. Scientific Opinion on the evaluation of di‐magnesium malate, used as a novel food ingredient and as a source of magnesium in foods for the general population, food supplements, total diet replacement for weight control and food for special medical purposes.. EFSA Journal 2018;16(6):5292, 24 pp. 10.2903/j.efsa.2018.5292
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00115
Panel members: Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipič, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Gunter Georg Kuhnle, Claude Lambré, Jean‐Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The Panel wishes to thank the member of the Working Group on Applications including: Lieve Herman for the preparatory work on this scientific output, and EFSA staff member: Alexandra Tard for the support provided to this scientific output. The ANS Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 15 May 2018
Notes
Regulation (EC) No 258/97 of the European Parliament and of the Council of 27 January 1997 concerning novel foods and novel food ingredients. OJ L 43, 14.2.1997, p. 1–6.
Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. OJ L 183, 12.7.2002, p. 51–57.
Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. OJ L 404, 30.12.2006, p. 26.
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009. OJ L 181, 29.6.2013, p. 35.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives OJ L 354, 31.12.2008, p. 1–16.
Commission Recommendation No 97/618 (EC) concerning the scientific aspects and the presentation of information necessary to support applications for the placing on the market of novel foods and novel food ingredients and the preparation of initial assessment reports under Regulation (EC) No 258/97 of the European Parliament and of the Council (Text with EEA relevance) OJ L 253, 16.9.1997, p. 1–36.
Solubility definition given by USP. Parts of solvent required for 1 part of solute: less than 1 (very soluble); from 1 to 10 (freely soluble); from 10 to 30 (soluble); from 30 to 100 (sparingly soluble); from 100 to 1,000 (slightly soluble); from 1,000 to 10,000 (very slightly soluble); greater than or equal to 10,000 (practically insoluble).
References
- Burdock GA (ed.), 2001. L‐Malic acid. In: Fenaroli's Handbook of Flavor Ingredients 4th Edition, CRC Press Inc., Boca Raton, Florida. pp. 992–993. [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA AFC Panel (EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food), 2006. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to calcium, magnesium and zinc malate added for nutritional purposes to food supplements as sources for calcium, magnesium and zinc and to calcium malate added for nutritional purposes to foods for particular nutritional uses and foods intended for the general population as source for Calcium. EFSA Journal 2006;4(11):391a, 6 pp. 10.2903/j.efsa.2006.391a http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2006.391a/pdf [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015. Scientific Opinion on Dietary Reference Values for magnesium. EFSA Journal 2015;13(7):4186, 63 pp. 10.2903/j.efsa.2015.4186 [DOI] [Google Scholar]
- FCC (Food Chemicals Codex), 2003a. Magnesium oxide. In: Food Chemicals Codex, 5th Edition. National Academy Press (NAP), Washington, DC. 261 pp. [Google Scholar]
- FCC (Food Chemicals Codex), 2003b. Malic acid. In: Food Chemicals Codex, 5th Edition. National Academy Press (NAP), Washington, DC. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1980. Evaluation of certain food additives. Technical Report Series 648, ISBN 92 4 120648 9.
- PDRNS . 2001. Malic acid. In: PDR For Nutritional Supplements 1st Edition. Physicians' Desk Reference (PDR); Des Moines, Iowa/Medical Economics Data Production Company, Montvale, New Jersey. [Google Scholar]
- SCF (Scientific Committee on Food), 1991. Report on the Scientific Committee for Food (Twenty‐fifth series), ISBN 92‐826‐2483‐8. Available at: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_reports_25.pdf
- SCF (Scientific Committee on Food), 2001. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Magnesium (expressed on 26 September 2001). Available online: http://ec.europa.eu/food/fs/sc/scf/out105_en.pdf


