Abstract
The Panel on Food Additives and Flavourings of the European Food Safety Authority was requested to consider in this revision 2 of Flavouring Group Evaluation 201, the additional data on genotoxicity submitted by the Industry on two substances, 2‐methylpent‐2‐enal [FL‐no: 05.090] and 2 methylcrotonaldehyde [FL‐no: 05.095], from subgroup 1.1.2 of FGE.19. In FGE.201Rev1, the Panel concluded that further data were required in order to clarify the genotoxic potential of this subgroup and considered the testing of 2‐methylcrotonaldehyde [FL‐no: 05.095] in a comet assay in liver and duodenum, the first site of contact, as a preferred option to further investigate the genotoxicity in vivo. New genotoxicity studies have been submitted for both 2‐methylpent‐2‐enal [FL‐no: 05.090] and 2‐methylcrotonaldehyde [FL‐no: 05.095]. 2‐Methylpent‐2‐enal [FL‐no: 05.090] tested in a combined micronucleus/comet assay did not induce DNA damage, overruling the weak gene mutation effect observed in bacteria and confirming the negative results observed in the in vitro micronucleus assay. 2‐Methylcrotonaldehyde [FL‐no: 05.095] did not induce gene mutations in liver and glandular stomach of transgenic rats. In addition, 2‐methylcrotonaldehyde [FL‐no: 05.095] tested in an in vivo comet assay in liver and duodenum, it did not induce DNA damage. Overall, the Panel concluded that the genotoxic evidence observed in vitro, was not confirmed in vivo for the representative substances 2‐methylcrotonaldehyde [FL‐no: 05.095] and 2‐methylpent‐2‐enal [FL‐no: 05.090], therefore all the 10 substances in this subgroup [FL‐no: 02.174, 05.033, 05.090, 05.095, 05.105, 05.107, 05.126, 07.261, 12.065 and 12.079] can be evaluated through the Procedure for the evaluation of flavouring substances.
Keywords: α,β‐unsaturated aldehydes; 2‐alkylated substances; FGE.201; FGE.19; subgroup 1.1.2
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The use of flavouring is regulated under Regulation (EC) No 1334/20081 of the European Parliament and Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation, an evaluation and approval are required for flavouring substances.
The Union List of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/20122. The list includes a number of flavouring substances for which the safety evaluation should be completed in accordance with Commission Regulation (EC) No 1565/20003.
The substances in this group were included in the Union list with a footnote 1 (under evaluation by EFSA).
In its opinion about this subgroup of 2012, the EFSA Panel considered that the mutagenicity hazard could not be cleared by the endpoints evaluated in the in vivo micronucleus assay submitted. The Panel therefore conclude that further data were required in order to clarify the genotoxic potential of this subgroup. The Panel considered the Comet assay with [FL‐no: 05.095] as test material and performed on liver, blood and first site of contact, as a preferred option to further investigate the genotoxicity in vivo.
The additional data submitted by the applicant consist essentially of:
a transgenic mutation assay in combination with an in vivo micronucleus assay for the substance 2‐methylcrotonaldehyde [FL‐no: 05.095];
a combination of a Comet/micronucleus assay for the substance 2‐methylpent‐2‐enal [FL‐no: 05.090].
The Panel also considered in this opinion on FGE.201 rev.1 that the additional data on 2‐methylcrotonaldehyde [FL‐no: 05.095] could also be considered representative for the following substances: 2,8‐dithianon‐4‐en‐4‐carboxaldehyde [FL‐no: 12.065] and 2‐(methylthiomethyl)but‐2‐enal [FL‐no: 12.079].
1.1.1. Terms of Reference
The European Commission requests the European Food Safety Authority (EFSA) to evaluate the new information submitted on 2‐methylpent‐2‐enal [FL‐no: 05.090] and 2‐methylcrotonaldehyde [FL‐no: 05.095] including also 2,8‐dithianon‐4‐en‐4‐carboxaldehyde [FL‐no: 12.065] and 2‐(methylthiomethyl)but‐2‐enal [FL‐no: 12.079] and, depending on the outcome, proceed to the full evaluation of the substances of this group listed in the table below, in accordance with Commission Regulation (EC) No 1565/2000, within 9 months.
In case the genotoxic potential cannot be ruled out or the procedure cannot be applied, EFSA is asked to characterise the hazards and also quantify the exposure.
As regards the substance 2,6‐Dimethyl‐2,5,7‐octatriene‐1‐ol acetate ([FL‐no: 09.931] CAS no 999999‐91‐4) the applicant indicate that it is included in this subgroup 1.1.2 of FGE19 (FGE.201). However, this substance has been already evaluated by EFSA in FGE 207 and FGE 72 Rev.1 of 2013.
As regards substance 4‐methyl‐3‐hepten‐5‐one ([FL‐no: 07.261] CAS no 22319‐31‐9) EFSA indicated in its opinion FGE.204 that ‘the 2‐methyl substituted alpha, beta‐unsaturated aldehydes in FGE.201Rev1 can be considered as structurally related to it [FL‐no: 07.261]. Thus the final conclusion on [FL‐no: 07.261] will be drawn based on the outcome of the evaluation of FGE.201Rev1’.
| FL‐no | Chemical name | CAS no |
|---|---|---|
| [05.090] | 2‐Methylpent‐2‐enal | 623‐36‐9 |
| [05.095] | 2‐Methylcrotonaldehyde | 497‐03‐0 |
| [02.174] | 2‐Methylbut‐2‐en‐1‐ol | 4675‐87‐0 |
| [05.033] | 2‐Ethylhept‐2‐enal | 10031‐88‐6 |
| [05.105] | 2‐Butylbut‐2‐enal | 25409‐08‐9 |
| [05.107] | 2‐Isopropyl‐5‐methylhex‐2‐enal | 35158‐25‐9 |
| [05.126] | 2‐Methyloct‐2‐enal | 49576‐57‐0 |
| [07.261] | 4‐methyl‐3‐hepten‐5‐one | 22319‐31‐9 |
| [12.065] | 2,8‐dithianon‐4‐en‐4‐carboxaldehyde | 59902‐01‐1 |
| [12.079] | 2‐(methylthiomethyl)but‐2‐enal | 40878‐72‐6 |
2. Data and methodologies
2.1. History of the evaluation of FGE.19 substances
Flavouring Group Evaluation 19 (FGE.19) contains 360 flavouring substances from the EU Register being α,β‐unsaturated aldehydes or ketones and precursors which could give rise to such carbonyl substances via hydrolysis and/or oxidation (EFSA, 2008a).
The α,β‐unsaturated aldehyde and ketone structures are structural alerts for genotoxicity (EFSA, 2008a). The Panel noted that there were limited genotoxicity data on these flavouring substances but that positive genotoxicity studies were identified for some substances in the group.
The α,β‐unsaturated carbonyls were subdivided into subgroups on the basis of structural similarity (EFSA, 2008a). In an attempt to decide which of the substances could go through the Procedure, a (quantitative) structure‐activity relationship ((Q)SAR) prediction of the genotoxicity of these substances was undertaken considering a number of models that were available at that time (DEREKfW, TOPKAT, DTU‐NFI‐MultiCASE Models and ISS‐Local Models (Gry et al., 2007)).
The Panel noted that for most of these models internal and external validation has been performed, but considered that the outcome of these validations was not always extensive enough to appreciate the validity of the predictions of these models for these α,β‐unsaturated carbonyls. Therefore, the Panel considered it inappropriate to totally rely on (Q)SAR predictions at this point in time and decided not to take substances through the procedure based on negative (Q)SAR predictions only.
The Panel took note of the (Q)SAR predictions by using two ISS Local Models (Benigni and Netzeva, 2007a,b) and four DTU‐NFI MultiCASE Models (Gry et al., 2007; Nikolov et al., 2007) and the fact that there are available data on genotoxicity, in vitro and in vivo, as well as data on carcinogenicity for several substances. Based on these data the Panel decided that 15 subgroups (1.1.1, 1.2.1, 1.2.2, 1.2.3, 2.1, 2.2, 2.3, 2.5, 3.2, 4.3, 4.5, 4.6, 5.1, 5.2 and 5.3) (EFSA, 2008b) could not be evaluated through the Procedure due to concern with respect to genotoxicity. Corresponding to these subgroups, 15 Flavouring Group Evaluations (FGEs) were established: FGE.200, 204, 205, 206, 207, 208, 209, 211, 215, 219, 221, 222, 223, 224 and 225.
For 11 subgroups, the Panel decided, based on the available genotoxicity data and (Q)SAR predictions, that a further scrutiny of the data should take place before requesting additional data from the Flavouring Industry on genotoxicity. These subgroups were evaluated in FGE.201, 202, 203, 210, 212, 213, 214, 216, 217, 218 and 220. For the substances in FGE.202, 214 and 218, it was concluded that a genotoxic potential could be ruled out and accordingly these substances were evaluated using the Procedure. For all or some of the substances in the remaining FGEs, FGE.201, 203, 210, 212, 213, 216, 217 and 220 the genotoxic potential could not be ruled out.
To ease the data retrieval of the large number of structurally related α,β‐unsaturated substances in the different subgroups for which additional data are requested, EFSA worked out a list of representative substances for each subgroup (EFSA, 2008c). In selecting the representative substances, expert judgement was applied. In each subgroup, the representative substances were selected taken into account chain length, branched chain, lipophilicity and possible additional functional groups. Likewise, an EFSA genotoxicity expert group has worked out a test strategy to be followed in the data retrieval for these substances (EFSA, 2008b).
The Flavouring Industry has been requested to submit additional genotoxicity data according to the list of representative substances and test strategy for each subgroup.
The Flavouring Industry has now submitted additional data and the present FGE concerns the evaluation of these data requested on genotoxicity.
2.2. Presentation of the substances in flavouring group evaluation 201Rev2
The FGE.201, originally, concerned 11 substances, which are presented in Appendix A, Table A.1. The 11 substances correspond to subgroup 1.1.2 of FGE.19 (EFSA, 2008b). Eight of these substances are aliphatic acyclic 2‐alkylated α,β‐unsaturated aldehydes with or without additional double bonds [FL‐no: 05.033, 05.090, 05.095, 05.105, 05.107, 05.126, 05.130 and 05.178] and three are precursors for such aldehydes [FL‐no: 02.174, 09.177 and 09.931].
Table A.1.
Specification summary of the substances in the flavouring group evaluation 201 revision 2 (JECFA, 2003; EFFA, 2018)
| FL‐no JECFA‐no | EU register name | Structural formula | FEMA no CoE no CAS no | Phys. form Mol. formula Mol. weight | Solubilitya Solubility in ethanolb | Boiling point, °Cc Melting point, °C ID test Assay minimum | Refrac. indexd Spec. gravitye | Comments |
|---|---|---|---|---|---|---|---|---|
|
02.174 1617 |
2‐Methylbut‐2‐en‐1‐ol |
|
– 10258 4675‐87‐0 |
Liquid C5H10O 86.13 |
– Freely soluble |
137 – – 95% |
1.439–1.445 0.863–0.869 |
|
|
05.033 1216 |
2‐Ethylhept‐2‐enal |

|
2438 120 10031‐88‐6 |
Liquid C9H16O 140.23 |
Insoluble Soluble |
55–60 (5 hPa) – NMR 97% |
1.460–1.466 0.891–0.898 |
|
|
05.090 1209 |
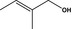
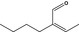
2‐Methylpent‐2‐enal |
|
3194 2129 623‐36‐9 |
Liquid C6H10O 98.15 |
Insoluble Soluble |
137 – IR MS 95% |
1.445–1.453 0.855–0.865 |
Mainly E‐isomer (> 90%). Secondary components (E) and (Z)‐2‐methyl‐2‐pentenoic acid (< 0.5% in fresh samples, up to 1% in samples after storage) |
|
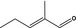
05.095 1201 |
2‐Methylcrotonaldehyde |
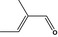
|
3407 2281 497‐03‐0 |
Liquid C5H8O 84.12 |
Slightly soluble Soluble |
117–118 – IR NMR 95% |
1.445–1.450 0.868–0.873 (20°) |
Mainly E‐isomer (> 95%). Secondary components: acetic acid (up to 0.1%), tiglic acid (up to 0.5%) and paraldehyde (up to 1%) (i.e. 2,4,6‐trimethyl‐1,3,5‐trioxane, which is a condensation product – cyclic ether) |
|
05.105 1214 |
2‐Butylbut‐2‐enal |
|
3392 10324 25409‐08‐9 |
Liquid C8H14O 126.20 |
Insoluble Soluble |
50 (18 hPa) – NMR 97% |
1.447–1.453 1.449–1.459 (20°) |
|
|
05.107 1215 |
2‐Isopropyl‐5‐methylhex‐2‐enal |
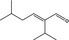
|
3406 10361 35158‐25‐9 |
Liquid C10H18O 154.25 |
Insoluble Soluble |
189 – NMR 95% |
1.448–1.454 0.840–0.846 |
|
|
05.126 1217 |
2‐Methyloct‐2‐enal |
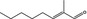
|
3711 10363 49576‐57‐0 |
Liquid C9H16O 140.23 |
Insoluble Soluble |
70–75 (10 hPa) – IR 96% |
1.449–1.459 0.872–0.882 |
|
| 05.130 | alpha‐Sinensal |

|
3141 10380 17909‐77‐2 |
– – 218.34 |
Substance not in the Register | |||
|
05.178 1227 |
beta‐Sinensal |

|
3141 10381 60066‐88‐8 |
Liquid C15H22O 218.34 |
Insoluble Soluble |
180 (1 hPa) – NMR 99% |
1.504–1.513 0.917–0.923 |
Substance not supported by industry |
| 07.261 | 4‐Methyl‐3‐hepten‐5‐one |
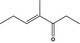
|
22319‐31‐9 |
Liquid C8H14O 126.20 |
Insoluble Freely soluble |
179 – MS 96.12% |
1.442–1.462 0.851–0.871 |
Substance from FGE.204 |
|
09.177 1207 |
2‐Methylallyl butyrate |
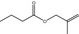
|
2678 572 7149‐29‐3 |
Liquid C8H14O2 142.20 |
Insoluble Soluble |
168 – NMR97% |
1.422–1.428 0.873–0.883 |
Substance not supported by industry |
|
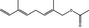
09.931 1226 |
2,6‐Dimethyl‐2,5,7‐octatriene‐1‐ol acetate |
|
3886 – 999999‐91‐4 |
Liquid C12H18O2 194.28 |
Insoluble Soluble |
70 (3 hPa) – IR NMR MS 96% |
1.490–1.500 0.937–0.947 |
Substance already evaluated in FGE.207 |
|
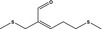
12.065 471 |
2,8‐Dithianon‐4‐en‐4‐carboxaldehyde |
|
3483 11904 59902‐01‐1 |
Liquid C8H14OS2 190.32 |
Slightly soluble |
104–105 (13 hPa) – IR NMR 98% |
1.557–1.567 1.105–1.107 |
Substance from FGE.225 |
|
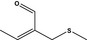
12.079 470 |
2‐(Methylthiomethyl)but‐2‐enal |
|
3601 11549 40878‐72‐6 |
Liquid C6H10OS 130.21 |
Insoluble |
77 (7 hPa) – 99% |
1.5228–1.5328 0.982–0.987 |
Substance from FGE.225 |
FL‐no: FLAVIS number; JECFA: Joint FAO/WHO Expert Committee on Food Additives; FEMA: Flavor and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service; ID: Identity; NMR: nuclear magnetic resonance; IR: infrared; MS: mass spectrometry.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1,013.25 hPa, if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
A summary of their current evaluation status by the JECFA is given in Appendix B, Table B.1 (JECFA, 2004, 2007).
Table B.1.
| FL‐no JECFA‐no | EU Register name | Structural formula | EU MSDIa US MSDI (μg/capita per day) | Classb Evaluation procedure pathc | Outcome on the named compound (d or e) | EFSA conclusion on the named compound (genotoxicity) |
|---|---|---|---|---|---|---|
|
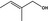
02.174 1617 |
2‐Methylbut‐2‐en‐1‐ol |
|
0.037 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.201Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.033 1216 |
2‐Ethylhept‐2‐enal |

|
0.012 0.1 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.201Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.090 1209 |
2‐Methylpent‐2‐enal |
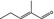
|
3.4 0.2 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.201Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.095 1201 |
2‐Methylcrotonaldehyde |
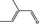
|
0.61 0.2 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.201Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.105 1214 |
2‐Butylbut‐2‐enal |
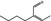
|
0.0 0.01 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.201Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.107 1215 |
2‐Isopropyl‐5‐methylhex‐2‐enal |
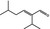
|
0.24 0.01 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.201Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.126 1217 |
2‐Methyloct‐2‐enal |

|
0.0 7.9 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.201Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 05.130 | alpha‐Sinensal |

|
Not evaluated by JECFA | Substance not in the Register | ||
|
05.178 1227 |
beta‐Sinensal |

|
0.91 0.5 |
Class I A3: Intake below threshold |
d | Substance not supported by industry |
| 07.261 | 4‐Methyl‐3‐hepten‐5‐one |
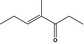
|
– | Not evaluated by JECFA | Evaluated in FGE.201Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure | |
|
09.177 1207 |
2‐Methylallyl butyrate |
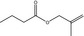
|
ND 0.2 |
Class I A3: Intake below threshold |
d | Substance not supported by industry |
|
09.931 1226 |
2,6‐Dimethyl‐2,5,7‐octariene‐1‐ol acetate |
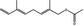
|
1.2 7.7 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.207, as of no genotoxicity concern and evaluated through the Procedure in FGE.72Rev1 |
|
12.065 471 |
2,8‐Dithianon‐4‐en‐4‐carboxaldehyde |
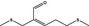
|
0.012 0.1 |
Class I JECFA evaluated at step B5: intake below 1.5 μg/person per day |
d | Evaluated in FGE.201Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
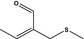
|
12.079 470 |
2‐(Methylthiomethyl)but‐2‐enal |
|
0.024 0.1 |
Class I JECFA evaluated at step B5: intake below 1.5 μg/person per day |
d | Evaluated in FGE.201Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
ND: not determined; JECFA: The Joint FAO/WHO Expert Committee on Food Additives; FL‐no: FLAVIS number; MSDI: maximised survey‐derived daily intake; FGE: Flavouring Group Evaluation.
EU MSDI: Amount added to food as flavour in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita per day.
Thresholds of concern: Class I = 1,800 μg/person/day, Class II = 540 μg/person/day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
No safety concern based on intake calculated by the MSDI approach of the named compound.
Data must be available on the substance or closely related substances to perform a safety evaluation.
The α,β‐unsaturated aldehyde and ketone structures are structural alerts for genotoxicity (EFSA, 2008b). Accordingly, the available data on genotoxic or carcinogenic activity for the eight aldehydes in FGE.201 [FL‐no: 05.033, 05.090, 05.095, 05.105, 05.107, 05.126, 05.130 and 05.178] and the two aldehydes [non‐Register substances, 2,6‐dimethyl‐2,5,7‐octatrienal and 2‐methyl‐2‐propenal, see Table A.1] anticipated to be metabolism products formed from two of the three precursors in FGE.201 [FL‐no: 02.174, 09.177 and 09.931], have been considered in FGE.201. The anticipated metabolism product formed from the third precursor [FL‐no: 02.174] is one of the eight Register aldehydes in this FGE [FL‐no: 05.095].
The Panel has also taken into consideration the outcome of the predictions from five selected (Q)SAR models (Benigni and Netzeva, 2007a; Gry et al., 2007; Nikolov et al., 2007) on the 10 aldehydes [FL‐no: 05.033, 05.090, 05.095, 05.105, 05.107, 05.126, 05.130, 05.178, 2‐methyl‐2‐propenal and 2,6‐dimethyl‐2,5,7‐octatrienal]. The 10 aldehydes and their (Q)SAR predictions are shown in Appendix C Table C.1.
Table C.1.
(Q)SAR predictions on mutagenicity for aldehydes representing the substances in subgroup 1.1.2
| FL‐no JECFA‐no | EU Register name | Structural formulaa | FEMA no CoE no CAS no | ISS local model Ames Test TA100b | MultiCASE Ames testc | MultiCASE Mouse lymphoma testd | MultiCASE Chromosmal aberration test in CHOe | MultiCASE Chromosmal aberration test in CHLf |
|---|---|---|---|---|---|---|---|---|
|
05.095 1201 |
2‐Methylcrotonaldehyde |
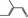
|
3407 2281 497‐03‐0 |
POS | NEG | OD | OD | NEG |
|
05.090 1209 |
2‐Methylpent‐2‐enal |

|
3194 2129 623‐36‐9 |
POS | NEG | OD | NEG | NEG |
|
05.105 1214 |
2‐Butylbut‐2‐enal |
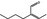
|
3392 10324 25409‐08‐9 |
POS | NEG | OD | OD | NEG |
|
05.107 1215 |
2‐Isopropyl‐5‐methylhex‐2‐enal |
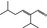
|
3406 10361 35158‐25‐9 |
NEG | NEG | OD | OD | OD |
|
05.033 1216 |
2‐Ethylhept‐2‐enal |
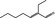
|
2438 120 10031‐88‐6 |
NEG | NEG | OD | OD | NEG |
|
05.126 1217 |
2‐Methyloct‐2‐enal |

|
3711 10363 49576‐57‐0 |
NEG | NEG | NEG | NEG | NEG |
| 05.130 | alpha‐Sinensal |

|
3141 10380 17909‐77‐2 |
NEG | NEG | OD | NEG | NEG |
|
05.178 1227 |
beta‐Sinensal |

|
3141 10381 60066‐88‐8 |
NEG | NEG | OD | NEG | NEG |
| Not in Register | 2‐methyl‐2‐propenal |
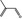
|
– – 78‐85‐3 |
NYA | POS | OD | OD | OD |
| Not in Register | 2,6‐dimethyl‐2,5,7‐octatrienal |

|
– – – |
NYA | NEG | OD | NEG | NEG |
FEMA: Flavor and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service;
Structure group 1.1.2: Aliphatic acyclic α,β‐unsaturated 2‐alkylated aldehydes.
Local model on aldehydes and ketones, Ames TA100. (NEG: Negative; POS: Positive; OD: out of domain; NYA: not yet assessed).
MultiCase Ames test (OD: Out of domain; POS: Positive; NEG: Negative; EQU: Equivocal).
MultiCase Mouse Lymphoma test (OD: Out of domain; POS: Positive; NEG: Negative; EQU: Equivocal).
MultiCase Chromosomal aberration in CHO (OD: Out of domain; POS: Positive; NEG: Negative; EQU: Equivocal).
MultiCase Chromosomal aberration in CHL (OD: Out of domain; POS: Positive; NEG: Negative; EQU: Equivocal).
OD, out of applicability domain: not matching the range of conditions where a reliable prediction can be obtained in this model. These conditions may be physicochemical, structural, biological, etc.
After the publication of FGE.201, Industry withdrew their wish of maintaining three of the substances for use as flavours in EU, [FL‐no: 05.130, 05.178 and 09.177]. One substance, [FL‐no: 09.931], was evaluated in FGE.207 (EFSA CEF Panel, 2013) where the genotoxicity concern was ruled out. In FGE.204 (EFSA CEF Panel, 2012a), the Panel noted that 2‐methyl substituted α,β‐unsaturated aldehydes in FGE.201 can be considered as structurally related to 4‐methyl‐3‐hepten‐5‐one [FL‐no: 07.261]. Thus, the conclusion on [FL‐no: 07.261] will be drawn based on the outcome of the evaluation of FGE.201Rev2.
In FGE.201Rev1 (EFSA CEF Panel, 2012b), the Panel considered that additional data required for 2‐methylcrotonaldehyde [FL‐no: 05.095] could also be considered representative for the substances in FGE.225, 2,8‐dithianon‐4‐en‐4‐carboxaldehyde [FL‐no: 12.065] and 2‐(methylthiomethyl)but‐2‐enal [FL‐no: 12.079].
Therefore, substances evaluated in FGE.201Rev2 are [FL‐no: 02.174, 05.033, 05.090, 05.095, 05.105, 05.107, 05.126, 07.261, 12.065 and 12.079]. New genotoxicity data on [FL‐no: 05.095] are considered to cover the genotoxicity concern for the other nine substances [FL‐no: 02.174, 05.033, 05.090, 05.105, 05.107, 05.126, 07.261, 12.065 and 12.079].
2.3. History of the evaluation of the substances belonging to FGE.201
In FGE.201 (EFSA, 2009), the Panel concluded that additional genotoxicity data were required for all 11 α,β‐unsaturated aldehydes and alcohols and related esters considered in the FGE.
In the EFSA Opinion ‘List of α,β‐unsaturated aldehydes and ketones representative of FGE.19 substances for genotoxicity testing’ (EFSA, 2008c), representative flavouring substances have been selected for subgroup 1.1.2, corresponding to FGE.201, for which additional data on genotoxicity were requested, according to the Opinion of the Panel on the ‘Genotoxicity Test Strategy for Substances Belonging to Subgroups of FGE.19’ (EFSA, 2008b) (see Table 1).
Table 1.
Representative substances for subgroup 1.1.2 of FGE.19 (EFSA, 2008c)
| FL‐no JECFA‐no | EU register name | Structural formula | Comments |
|---|---|---|---|
|
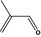
05.090 1209 |
2‐Methylpent‐2‐enal |
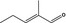
|
Originally selected as representative substance |
|
05.095 1201 |
2‐Methylcrotonaldehyde |
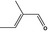
|
Representative substance for all the substances in FGE.201Rev2 |
|
05.178 1227 |
Beta‐Sinesal |

|
Originally selected as representative substance Not supported by industry |
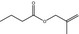
| Not in the EU Register | 2‐Methyl‐2‐propenal or its precursor [09.177] |
|
Originally selected as representative substance |
|
09.177 1207 |
2‐Methylallyl butyrate |
|
Originally selected as representative substance Not supported by industry |
In reply to a data request presented in FGE.201, additional data were provided by Industry (EFFA, 2011) for the representative substance 2‐methylpent‐2‐enal [FL‐no: 05.090] and a non‐representative substance, also from subgroup 1.1.2, 2‐methylcrotonaldehyde [FL‐no: 05.095]. No data were submitted for the other representatives identified by EFSA (EFSA, 2008c), beta‐sinensal [FL‐no: 05.178], 2‐methyl‐2‐propenal (not a Register substance but a precursor for such) or its precursor 2‐methylallyl butyrate [FL‐no: 09.177]. According to Industry, [FL‐no: 05.178 and 09.177] are not any longer supported by the Industry and accordingly no data were submitted for these substances due to the lack of test material for the required genotoxicity testing. Also, alpha‐sinensal [FL‐no: 05.130] is not any longer supported by the Industry. Data submitted were evaluated in FGE.201 Revision 1 (FGE.201Rev1).
In FGE.201Rev1 (EFSA CEF Panel, 2012b), the Panel concluded that there is some evidence for [FL‐no: 05.095] and an indication for [FL‐no: 05.090] to show a potency for the induction of gene mutations in vitro. Furthermore, the Panel considered that the mutagenicity hazard could not be cleared by the endpoints evaluated in the in vivo micronucleus assay and that further data are required in order to clarify the genotoxic potential of this subgroup. A comet assay performed with [FL‐no: 05.095] in liver, blood and first site of contact was considered as a preferred option to further investigate the genotoxicity in vivo. Finally, the Panel concluded that the genotoxicity data for [FL‐no: 05.095] could be representative for [FL‐no: 02.174, 05.033, 05.090, 05.105, 05.107, 05.126, 07.261, 12.065 and 12.079] but not for the remaining substances of this subgroup [FL‐no: 05.130, 05.178, 09.177 and 09.931], for which however, an evaluation by the Panel is not needed any longer.
In response to the requested genotoxicity data in FGE.201Rev1 on representative substances for subgroup 1.1.2, new data on 2‐methylpent‐2‐enal [FL‐no: 05.090] and 2‐methylcrotonaldehyde [FL‐no: 05.095] have been submitted by Industry that are evaluated in the present revision of FGE.201 (FGE.201Rev2).
The new data submitted for [FL‐no: 05.090 and 05.095] are described and evaluated in Section 3 of the present revision FGE.201Rev2. Sections 2.4 and 2.5 report the same information that was presented in FGE.201 (EFSA, 2009) and FGE.201Rev1 (EFSA CEF Panel, 2012b), respectively.
| FGE | Adopted by EFSA | Link | No. of Substances |
|---|---|---|---|
| FGE.201 | 25 September 2008 | http://www.efsa.europa.eu/en/efsajournal/pub/1080 | 11 |
| FGE.201Rev1 | 24 May 2012 | http://www.efsa.europa.eu/en/efsajournal/pub/2749 | 11 |
| FGE.201Rev2 | 13 September 2018 | https://www.efsa.europa.eu/en/efsajournal/pub/5423 | 10 |
2.4. Data evaluated by the Panel in FGE.2014
2.4.1. (Q)SAR Predictions
In Appendix C Table C.1, the outcomes of the (Q)SAR predictions for possible genotoxicity activity in five in vitro (Q)SAR models (ISS‐Local Model‐Ames Test, DTU‐NFI‐MultiCASE‐Ames test, ‐Chromosomal aberration test in Chinese hamster ovary cells (CHO), ‐Chromosomal aberration test in Chinese hamster lung cells (CHL) and ‐Mouse Lymphoma Test) are presented. For the three short‐chain aldehydes [FL‐no: 05.095, 05.090 and 05.105], the predictions in the ISS Local Ames test (TA100) were positive. For 2‐methyl‐2‐propenal, the DTU‐NFI‐MultiCASE Ames test was positive. All other predictions were either negative or out of domain (See Table C.1).
2.4.2. Carcinogenicity studies
No carcinogenicity studies are available for the eight α,β‐unsaturated aldehydes and the α,β‐unsaturated aldehydes anticipated to be formed from the three precursors [FL‐no: 02.174, 09.177 and 09.931] in subgroup 1.1.2.
2.4.3. Genotoxicity studies
Only one study on 2‐methylpent‐2‐enal [FL‐no: 05.090] and one study on 2‐methyl‐2‐propenal [not in Register] is available for the eight aldehydes and the α,β‐unsaturated aldehydes anticipated to be formed from the precursors in subgroup 1.1.2. The study on 2‐methylpent‐2‐enal is a spot test (Florin et al., 1980), which is not in accordance with the OECD guideline. Furthermore, the methods and results are insufficiently reported and the study is considered to be of insufficient validity. The study on 2‐methyl‐2‐propenal (and the structurally related 2‐propyl and 2‐butyl substituted 2‐propenals), in Ames test in Salmonella Typhimurium strain TA100, showed mutagenic effects of all the 2‐alkylated 2‐propenals (Eder and Deininger, 2001). The study was found valid. See Appendix D Table D.1.
Table D.1.
Genotoxicity studies in vitro
| Chemical Name [FL‐no] | Test system | Test object | Concentration | Reported Result | Reference | Commentsb |
|---|---|---|---|---|---|---|
| 2‐Methylpent‐2‐enal [05.090] | Reverse mutation | Salmonella Typhimurium TA98, TA100 | 0.03–3 mmol/plate (2.94–294 mg/plate) | Negativea | Florin et al. (1980) | Insufficient validity (spot test, not according to OECD guideline, methods and results insufficiently reported) |
| S. Typhimurium TA98, TA100, TA102, TA1535 and TA1537 | 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate | Negativea , c | Bowen (2011) | dValid. The study was performed in compliance with GLP and according to OECD TG 471 | ||
| 78.13, 156.13, 312.5, 625, 1,250, 2,500 and 5,000 μg/plate | Negativea , h | f | ||||
| S. Typhimurium TA100 | 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate | Negative (−S9, Plate)a , c Weakly positive (+S9, Plate) | Ballantyne (2011) | gValid. The study was performed in compliance with GLP and according to OECD TG 471 except that only one bacterial strain was used | ||
| 51.2, 128, 320, 800, 2,000 and 5,000 μg/plate | Weakly positive (−S9, Plate)c , e | |||||
| 8.192, 20.48, 51.2, 128, 320, 800, 2,000 and 5,000 μg/plate | Weakly positive (+S9, Plate) Negative (+S9, Pre‐inc)c , h , i | |||||
| 51.2, 128, 320, 800, 2,000 and 5,000 μg/plate | Weakly positive (−S9, Plate)c , e | |||||
| Micronucleus induction | Human peripheral blood lymphocytes | 100, 200 and 300 μg/ml | Negativee , j | Whitwell (2011) | kValid. The study was performed in compliance with GLP and according to OECD TG 487 | |
| 200, 275 and 350 μg/ml | Negativeh , i | |||||
| 20, 50, 70 and 80 μg/ml 100, 175, 260 and 300 | Negativee , l Negativec , j | |||||
| 2‐methylacrolein 2‐Methyl‐2‐propenal | Ames test | S. Typhimurium TA100 | 0−2 μmol/plate (−S9)0−9 μmol/plate (+S9) | Positivea | Eder and Deininger (2001) | Valid. Positive both with and without S9‐mix. Toxic at 1 μmol/plate and above (−S9) and 6 μmol/plate (+S9) evident as a reduction in revertants |
| 2‐Methylcrotonaldehyde [05.095] | Reverse mutation | S. Typhimurium TA98, TA100, TA1535, TA1537, and Escherichia coli WP2uvrA | 8.19, 20.5, 51.2, 128, 320, 800, 2,000 and 5,000 μg/plate | Positivea , c (TA100) | Nakajima (2006a) | mValid. According to the study report, the study was performed in compliance with Japanese GLP standards. The study report contained a certificate of reliability but no details of inspection. The study is in accordance with OECD except that only two plates were used per concentration. No statistics performed. |
| 156, 313, 625, 1,250, 2,500 and 5000 μg/plate | Positivea , c (TA100)Weakly positive (WP2uvrA, −S9) | |||||
| E. coli WP2uvrA | 156, 313, 625, 1,250, 2,500 and 5,000 μg/plate | Negativea | ||||
| S. Typhimurium TA100 | 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate | Weakly positive (+/−S9, Plate)a , c | Ballantyne (2011) | nValid. The study was performed in compliance with GLP and according to OECD TG 471 except that only one bacterial strain was used | ||
| 51.2, 128, 320, 800, 2,000 and 5,000 μg/plate | Weakly positive (+/−S9, Plate)a , c | |||||
| 8.192, 20.48, 51.2, 128, 320, 800, 2,000 and 5,000 μg/plate | Weakly positive (+S9, Pre)h , i | |||||
| 0.32, 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate | Weakly positive (+S9, Plate)c , i | |||||
| Chromosomal aberration | Chinese hamster Pulmonic fibroblasts | 105, 210 and 421 μg/mL without S‐9 treatment and 105, 210, 421 and 841 μg/mL with S‐9 treatment | Positive | Nakajima (2006b) | oValid. According to the study report, the study was performed in compliance with Japanese GLP standards. The study report contained a certificate of reliability but no details of inspection. Mainly in accordance with OECD TG 473 |
With and without metabolic activation.
Validity of genotoxicity studies: Valid. Limited validity (e.g. if certain aspects are not in accordance with OECD guidelines or current standards and/or limited documentation). Insufficient validity (e.g. if main aspects are not in accordance with any recognised guidelines (e.g. OECD) or current standards inappropriate/not validated test system). Validity cannot be evaluated (e.g. insufficient documentation, short abstract only, too little experimental details provided, text not in a Community language).
Plate incorporation method.
Toxicity was observed in TA1537 at 5,000 μg/plate in the presence of S‐9 and in TA102 at 1,000 μg/plate and above in the presence of S‐9.
Without S9 metabolic activation.
Toxicity was observed in all strains in the presence of S‐9 above 2,500 μg/plate and 1,250 μg/plate in TA1537. Study design complied with current recommendations. Acceptable top concentrations were achieved.
Throughout experiments some small but statistically significant increases were seen but these were attributed to normal biological variability, and were generally less than 2‐fold over concurrent vehicle controls.
Pre‐incubation method.
With S9 metabolic activation.
3‐hours incubation with 21‐hours recovery period.
Complies with draft OECD guideline 487. Acceptable levels of cytotoxicity were achieved at the top concentrations used in all parts of the study.
24‐hours incubation with no recovery period.
Study design complied with current recommendations. Acceptable top concentrations were achieved.
Throughout experiments some small but statistically significant increases were generally less than 2‐fold over concurrent vehicle controls.
Dose‐dependent increase in induction of structural chromosomal aberrations with and without S‐9 treatment. No changes in numerical chromosomal aberrations were observed.
2.4.4. Conclusion on genotoxicity
The genotoxicity concern with respect to this group of substances due to the presence of an α,β‐unsaturated aldehyde group (or precursor for this) cannot be ruled out based on the genotoxicity data and (Q)SAR predictions available.
2.4.5. Conclusion
The Panel concluded that a genotoxic potential of the 11 α,β‐unsaturated aldehydes and alcohols and related esters in the present FGE.201 could not be ruled out. Accordingly the 11 substances cannot be evaluated through the Procedure. Additional data on genotoxicity on substances representative for this subgroup should be provided according to the Genotoxicity Test Strategy for Substances Belonging to Subgroups of FGE.19 (EFSA, 2008b).
2.5. Additional genotoxicity data evaluated by the Panel in FGE.201Rev15
2.5.1. In Vitro data
2.5.1.1. Ames tests
2‐Methylpent‐2‐enal [FL‐no: 05.090] was tested in S. Typhimurium strains TA98, TA100, TA102, TA1535 and TA1537 in the presence and absence of metabolic activation by S‐9 (Bowen, 2011). In the first experiment performed using plate incorporation methodology, concentrations of 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate were assessed for all tester strains. Evidence of toxicity was only observed in strains TA1537 at 5,000 μg/plate in the presence of S‐9 and TA102 at 1,000 μg/plate and above in the presence of S‐9. The concentration range was therefore narrowed for a second experiment. In addition, the second experiment included a supplementary S‐9 pre‐incubation step for the S‐9 treatment group to increase the range of assay detection. Following these treatments, evidence of toxicity was observed in the presence of S‐9 in S. Typhimurium strains TA98, TA100, TA1535 and TA102 at 2,500 μg/plate and above and in strain TA1537 at 1,250 μg/plate and above. Toxicity was not observed in the absence of S‐9. In the second experiment, at a single intermediate dose of 2,500 μg/plate without S‐9 treatment in strain TA1537, there was a small (2.1‐fold), statistically significant increase in revertants, but this was not dose‐related and was within the range of historical controls. The Panel concluded that 2‐methylpent‐2‐enal was not mutagenic in this study (Tables D.1 and D.4).
Table D.4.
Ames test with 2‐methylpent‐2‐enal [05.090] (Bowen, 2011) Statistically significant increases, non‐toxic effects
| S9 | Assay | TA98 | TA100 | TA1535 | TA1537 | TA102 | Conclusion | |
|---|---|---|---|---|---|---|---|---|
| Range‐finding experiment | − | Plate |
1.2‐fold < HC d‐r |
Negative | ||||
| + | Plate |
1.2‐fold < HC not d‐r |
Negative | |||||
| Experiment 1 | − | Plate | NS | NS | NS | NS | NS | Negative |
| + | Plate | NS |
1.2‐fold < HC not d‐r |
NS | NS | NS | Negative | |
| Experiment 2 | − | Pre | NS |
1.2‐fold < HC not d‐r |
NS |
2.1‐fold < HC not d‐r |
NS | Negative |
| + | Pre | NS | NS | NS |
1.6‐fold < HC not d‐r |
NS | Negative |
> HC: above historical control; < HC: within historical control.
NS: statistically not significant.
d‐r: dose‐related but only the highest dose statistically significant; D‐R, dose‐related and at least two doses statistically significant.
A bacterial reverse mutation assay was also conducted in S. Typhimurium strains TA98, TA100, TA1535, TA1537, and Escherichia coli strain WP2uvrA with 2‐methylcrotonaldehyde [FL‐no: 05.095] (Nakajima, 2006a), which does not belong to the substances selected as representative by the Panel for this subgroup. In an initial experiment, the concentrations used were 8.19, 20.5, 51.2, 128, 320, 800, 2,000 and 5,000 μg/plate. In strain TA100, 2‐methylcrotonaldehyde increased the number of colonies showing reverse mutations, both with and without metabolic activation by S‐9, in a dose‐dependent manner. In the absence of S‐9, the increases were threefold from 320 to 5,000 μg/plate, and with S‐9 metabolic activation, the increases were up to 3.9‐fold from 320 to 2,000 μg/plate; under these conditions, growth inhibition was observed at 5,000 μg/plate. In all other strains, in the presence and absence of metabolic activation treatment with 2‐methylcrotonaldehyde did not result in an increase of reverse mutant colony (Tables D.1 and D.3).
Table D.3.
Ames test with 2‐methyl‐2‐butenal [05.095] (Nakajima, 2006a) Non‐toxic effects
| S9 | Assay | TA98 | TA100 | TA1535 | TA1537 | WP2uvrA | Comment | Conclusion | |
|---|---|---|---|---|---|---|---|---|---|
| Range‐finding experiment | − | Pre |
1.4‐fold < HC d‐r |
3‐fold > HC d‐r |
2.0‐fold < HC not d‐r |
1.6‐fold < HC not d‐r |
1.2‐fold < HC (d‐r) |
Positive in TA 100 | |
| + | Pre |
1.2‐fold < HC (d‐r) |
3.9‐fold > HC d‐r |
1.4‐fold < HC not d‐r |
1.0‐fold < HC |
1.3‐fold < HC (d‐r) |
Positive in TA 100 | ||
| Experiment 1 | − | Pre |
2.0‐fold > HC not d‐r |
7.2‐fold > HC d‐r |
1.8‐fold < HC not d‐r |
1.9‐fold < HC (d‐r) |
2.1‐fold > HC (d‐r) |
WP2uvrA‐result not clearly reproducible | Positive in TA 100, equivocal in WP2uvrA |
| + | Pre |
1.5‐fold < HC (d‐r) |
4.6‐fold > HC d‐r |
1.5‐fold < HC not d‐r |
1.0‐fold < HC |
1.2‐fold < HC not d‐r |
Positive in TA 100 | ||
| Experiment 2 | − | Pre |
1.4‐fold > HC (d‐r) |
Equivocal | |||||
| + | Pre |
Two plates were used per concentration. No examinations using statistical procedures were conducted.
> HC: above historical control; < HC: within historical control.
d‐r: dose‐related; (d‐r), not clearly dose‐related but the highest dose resulted in the largest increase, not d‐r, not dose‐related.
Pre: Pre‐incubation assay.
In a second experiment using 2‐methylcrotonaldehyde [FL‐no: 05.095] concentrations of 156, 313, 625, 1,250, 2,500 and 5,000 μg/plate, treatment of strain TA100 in the absence of S‐9 activation induced a dose‐dependent increase in reverse mutant colonies (1.5‐ to 7.2‐fold), with growth inhibition observed at 5,000 μg/plate in the absence of S‐9. In the presence of S‐9, treatment of strain TA100 also gave a dose‐dependent (313–2,500 μg/plate) increase in reverse mutant colonies (1.2‐ to 4.6‐fold), with growth inhibition again observed at 5,000 μg/plate. In the absence of S‐9, strain TA1535 gave a 1.8‐fold increase in reverse mutants at 2,500 μg/plate, but this was not dose‐dependent. Strain TA98 had a nearly twofold increase in reverse mutant colonies in the absence of S‐9 at both 2,500 and 5,000 μg/plate. These increases were above the historical control range but they were not clearly dose‐related. In the presence of S‐9, the same strain showed a 1.5‐fold increase at 5,000 μg/plate. E. coli strain WP2uvrA showed a twofold increase in reverse mutant colonies at 5,000 μg/plate treatment without S‐9 which was above the historical control range, but otherwise there was no increase at any other dose or with S‐9 treatment (Tables D.1 and D.3).
Strain WP2uvrA did not show evidence of mutagenicity in the first experiment but produced weakly mutagenic results in the second experiment at the highest concentration tested. Since the repeatability of the result was not confirmed, an additional experiment with this strain was performed from 156 to 5,000 μg/plate and this resulted in a 1.4‐fold increase in revertant colonies at 2,500 and 5,000 μg/plate. The Panel considered these small increases as indication for a weak mutagenic potential because the effects were reproducible in two out of three experiments and were above the historical control range. However, with the exception of strain TA100, there were no increases in reverse mutation colonies exceeding that of the negative control by twofold. The Panel considered that 2‐methylcrotonaldehyde [FL‐no: 05.095] was mutagenic in the TA100 strain in the absence and presence of S‐9 under the specified conditions of the assay.
In order to clarify the ability of 2‐methylcrotonaldehyde [FL‐no: 05.095] to induce reverse mutations and provide cross‐laboratory comparison of results, a third Ames assay was conducted (Ballantyne, 2011) in the same laboratory in which the Bowen 2011 study was performed. In order to directly compare 2‐methylcrotonaldehyde with 2‐methylpent‐2‐enal, the latter was tested additionally in this assay. Both substances were tested in the single S. Typhimurium strain TA100, in the absence and the presence of metabolic activation, in three separate experiments.
The first experiment was conducted using final concentrations of 2‐methylcrotonaldehyde [FL‐no: 05.095] and 2‐methylpent‐2‐enal [FL‐no: 05.090] at 1.6, 8, 40, 200, 1,000 and 5,000 μg/plate in strain TA100 with or without S‐9. Following these treatments, no evidence of toxicity was observed. Statistically significant (p < 0.01) increases in revertants were observed for 2‐methylcrotonaldehyde at 5,000 μg/plate in the absence of S‐9 metabolic activation (1.3‐fold) and at all concentrations tested in the presence of S‐9. In all cases, the increases were small versus concurrent controls (1.2‐ to 1.7‐fold vs concurrent controls) but were above the range of historical controls. Similarly, for 2‐methylpent‐2‐enal, small statistically significant (p < 0.05) increases in revertants (1.2‐fold) were observed at 5,000 μg/plate in the absence of S‐9 metabolic activation and at 1.6, 200 and 5,000 μg/plate (p < 0.01) in the presence of S‐9. In all cases, the increases were small versus concurrent controls (1.2‐ to 1.4‐fold vs concurrent controls) but the results obtained in the presence of S‐9 were above the range of historical controls and dose‐related (Tables D.1 and D.5).
Table D.5.
Ames test with TA100 (Ballantyne, 2011) Statistically significant increases not accompanied by toxicity
| Register name [FL‐no] | S9 | Assay | Exp 1 | Exp 2 | Exp 3 | Comment | Conclusion |
|---|---|---|---|---|---|---|---|
|
2‐Methylcrotonaldehyde [05.095] |
− | Plate |
1.3‐fold > HC Not d‐r |
1.4‐fold > HC d‐r |
Reproducible | Indication for a weak mutagenic activity | |
| + | Plate |
1.7‐fold > HC d‐r |
1.4‐fold > HC d‐r |
1.8‐fold > HC d‐r |
Reproducible | Indication for a weak mutagenic activity | |
| − | Pre | ||||||
| + | Pre |
1.7‐fold > HC d‐r |
Reproducible when compared with the plate‐incorporation experiment | Indication for a weak mutagenic activity | |||
|
2‐Methylpent‐2‐enal [05.090] |
− | Plate |
1.2‐fold < HC d‐r |
2.5‐fold > HC d‐r |
1.3‐fold > HC d‐r |
Reproducible | Indication for a weak mutagenic activity |
| + | Plate |
1.4‐fold > HC d‐r |
1.4‐fold > HC d‐r |
Reproducible | Indication for a weak mutagenic activity | ||
| − | Pre | ||||||
| + | Pre |
NS < HC |
> HC: above historical control; < HC: within historical control.
NS: statistically not significant.
d‐r: dose‐related but only the highest dose statistically significant; D‐R, dose‐related and at least two doses statistically significant.
In the second experiment, treatment of strain TA100 with 2‐methylcrotonaldehyde [FL‐no: 05.095] and 2‐methylpent‐2‐enal [FL‐no: 05.090] was performed in the absence and in the presence of S‐9 at narrowed concentration intervals. 2‐Methylcrotonaldehyde and 2‐methylpent‐2‐enal were assayed at 51.2, 128, 320, 800, 2,000 and 5,000 μg/plate in the absence of S‐9 using plate incorporation methodology and 8.192, 20.48, 51.2, 128, 320, 800, 2,000 and 5,000 μg/plate in the presence of S‐9 using both plate incorporation and pre‐incubation methodology. Clear evidence of toxicity was only observed following pre‐incubation methodology treatments with 2‐methylcrotonaldehyde in the presence of S‐9 at 5,000 μg/plate and pre‐incubation methodology treatments with 2‐methylpent‐2‐enal in the presence of S‐9 at 2,000 and 5,000 μg/plate. In the absence of S‐9 activation, small but statistically significant increases in revertant colonies showing a dose‐dependent relationship were observed for 2‐methylcrotonaldehyde at 800, 2,000 and 5,000 μg/plate. These were only 1.3‐ to 1.4‐fold over concurrent vehicle controls but above the range of historical controls. In the presence of S‐9 activation, 2‐methylcrotonaldehyde showed a small but statistically significant increase in revertant colonies only at 5,000 μg/plate using plate incorporation (1.4‐fold) and at 800 and 2,000 μg/plate using pre‐incubation methodology (1.7‐fold). These increases were above the historical control range. Similarly, in the presence of S‐9 activation 2‐methylpent‐2‐enal showed a small (1.4‐fold) but statistically significant increase in revertants only at 5,000 μg/plate using plate incorporation methodology, but in contrast gave no increases in revertant colonies using pre‐incubation. In the absence of S‐9 activation, a statistically significant increase in revertant colonies was observed at 5,000 μg/plate that was 2.5‐fold over concurrent vehicle controls. These increases were above the historical control range, likewise (Tables D.1 and D.5).
In order to further investigate the reproducibility and dose relationship of some increases in revertant numbers seen in the first two experiments, a third experiment was performed with 2‐methylcrotonaldehyde [FL‐no: 05.095] in the presence of S‐9 (plate incorporation methodology only) and with 2‐methylpent‐2‐enal in the absence of S‐9. In order to investigate the dose range over which the increases in revertant numbers were previously observed, treatment concentration ranges of 0.32–5,000 μg/plate and 51.2–5,000 μg/plate were employed for 2‐methylcrotonaldehyde and 2‐methylpent‐2‐enal, respectively. Following these treatments, no evidence of toxicity was observed with both substances. For 2‐methylcrotonaldehyde, small (1.3‐ to 1.8‐fold) but statistically significant and dose‐related increases in revertants were observed at 1,000 and 5,000 μg/plate. These increases were above the historical control range. For 2‐methylpent‐2‐enal, a small but statistically significant increase in revertants was observed at 5,000 μg/plate. This increase was only 1.3‐fold vs. concurrent vehicle controls but above the historical control range.
Overall, statistically significant increases in revertant numbers (when the data were analysed at the 1% level using the Dunnett's test) were observed in the absence and presence of S‐9 in each experiment where the TA100 strain was treated with 2‐methylcrotonaldehyde [FL‐no: 05.095]. These increases provided evidence of a dose relationship, with the exception of the first experiment in the absence of S‐9, where the largest increase was observed at the lowest treatment concentration (1.6 μg/plate) and was attributed to an aberrant occurrence that was not reproduced in subsequent experiments. While the magnitude of these increases were small (1.3‐ to 1.8‐fold above the concurrent control levels), these data were considered by the authors of the study report as evidence of 2‐methylcrotonaldehyde mutagenic activity in strain TA100 in the absence and presence of metabolic activation with S‐9. The Panel noted that these increases, although being small, were above the range of historical controls and that these effects were generally reproducible in the different experiments of this study. Thus, the Panel concluded that this study provided an indication for a weak mutagenic activity of 2‐methylcrotonaldehyde [FL‐no: 05.095] in the presence and absence of metabolic activation.
2‐Methylpent‐2‐enal [FL‐no: 05.090] treatments of strain TA100 in the absence and presence of S‐9, assayed simultaneously for comparison to 2‐methylcrotonaldehyde, resulted in statistically significant increases in revertant numbers in the absence and presence of S‐9 in each experiment, with the exception of experiment 2 using pre‐incubation methodology in the presence of S‐9. The maximum treatment concentration in the experiment 2 in the absence of S‐9 resulted in a statistically significant increase of 2.5‐fold over the concurrent control level, though this was not reproduced in experiments 1 or 3, nor previously observed (Bowen, 2011). However, the small increases observed in experiments 1 and 3 were statistically significant and above the range of historical controls. Thus, there is at least some consistency in these three experiments. The authors of the study report (Ballantyne, 2011) noted that all of the observed increases provided at least some evidence of dose dependence (in most cases the only responding concentration being the highest treatment concentration), and were reproducible over most of the treatment occasions. Accordingly, the authors considered that these increases are indicative of weak 2‐methylpent‐2‐enal mutagenic activity in strain TA100 in the absence and in the presence of S‐9 in this assay system. The Panel agreed with the authors.
The details and conclusions for the Ames tests described above are summarised in Tables D.1 and D.3–D.6.
Table D.6.
Ames test results for 2‐methylcrotonaldehyde [FL‐no: 05.095] and 2‐methylpent‐2‐enal [FL‐no: 05.090]
| Register name [FL‐no] | S9 | Nakajima, 2006 | Bowen (2011) | Ballantyne (2011) | Comment | Conclusion |
|---|---|---|---|---|---|---|
| 2‐Methylcrotonaldehyde [05.095] | − | Positive | Indication for a weak mutagenic activity | At least the indication is reproducible | Indication for a mutagenic activity based on two studies | |
| + | Positive | Indication for a weak mutagenic activity | At least the indication is reproducible | Indication for a mutagenic activity based on two studies | ||
| 2‐Methylpent‐2‐enal [05.090] | − | Negative | Indication for a weak mutagenic activity | The indication was not reproducible in different studies. Inconsistent results | Indication for a weak mutagenic activity based on the results of one study and structural similarity with 2‐methylcrotonaldehyde [FL‐no: 05.095] | |
| + | Negative | Indication for a weak mutagenic activity | The indication was not reproducible in different studies. Inconsistent results | Indication for a weak mutagenic activity based on the results of one study and structural similarity with 2‐methylcrotonaldehyde [FL‐no: 05.095] |
2.5.1.2. In vitro Micronucleus assay
2‐Methylpent‐2‐enal [FL‐no: 05.090] was evaluated in an in vitro micronucleus assay in human peripheral blood lymphocytes for its ability to induce chromosomal damage or aneuploidy in the presence and absence of rat liver metabolic activation system (S‐9) as an in vitro metabolising system (Whitwell, 2011). Cells were stimulated for 48 h with phytohaemagglutinin to produce exponential cell growth followed by treatment for 3 h (with a 21 h recovery period) in the presence or absence of S‐9 or 24 h treatment with no recovery in the absence of S‐9. In the first experiment, doses of 0, 100, 200 and 300 μg/mL of 2‐methylpent‐2‐enal were tested. Frequencies of micronucleated binucleate (MNBN) cells were not significantly different (p ≤ 0.05) from that of the negative control in all conditions with the exception of the highest concentration analysed post 3 + 21 h without S‐9 treatment (300.0 μg/mL, inducing 49% cytotoxicity, mean MNBN cells 1.05%). This increase, although statistically significant relative to the concurrent vehicle control, was relatively small and did not exceed the range of historical controls (0.1–1.2% MNBN cells). Furthermore, both replicate cultures at this concentration and all other 2‐methylpent‐2‐enal concentrations analysed were within a normal range of MNBN cell values.
To clarify these data, a 3 + 21 h treatment in the absence of S‐9 was performed in a second experiment with 100.0, 175.0, 260.0 and 300.0 μg/mL concentrations of 2‐methylpent‐2‐enal. Statistically significant increases in micronucleated cell frequencies were not observed. Consistent with current regulatory guideline recommendations for this assay, the maximum concentrations that were analysed induced between 49% and 57% cytotoxicity. The Panel agreed with the authors of the study report and concluded that 2‐methylpent‐2‐enal did not induce micronuclei in cultured human peripheral blood lymphocytes following treatment in the absence and presence of metabolic activation with S‐9 (Table D.1).
2.5.1.3. In vitro chromosomal aberration assay
The ability of 2‐methylcrotonaldehyde [FL‐no: 05.095] to induce chromosomal aberrations was evaluated in an in vitro assay using Chinese hamster pulmonic fibroblasts (Nakajima, 2006b). Microscopic observations were conducted during the chromosome aberration tests using short‐term treatment with three or four different doses respectively, 105, 210 and 421 μg/mL in the absence of S‐9 and 105, 210, 421 and 841 μg/mL in the presence of S‐9. The frequencies of chromosome structural aberrations as a result of short‐term treatment of 2‐methylcrotonaldehyde without metabolic activation by S‐9 were 0.5% in the negative control, 3.5% in the 105 μg/mL treatment, 12.0% in the 210 μg/mL treatment, 55% in the 421 μg/mL treatment and 50.0% in the mitomycin positive control. The appearance of polyploidy cells was not observed at any dose and there were no significant reductions in relative cell growth rate under these test conditions. In the presence of S‐9, the frequencies of chromosome structural aberration as a result of treatment using 2‐methylcrotonaldehyde were 0.05% in the negative control, 1.5% in the 105 μg/mL dose, 1.5% in the 210 μg/mL dose, 33.0% in the 421 μg/mL dose, 96.5% in the 841 μg/mL dose and 39% in the cyclophosphamide positive control treatment group. The frequencies of appearance of polyploidy cells were equivalent to those of the negative control group at all doses and there was no significant reduction in relative cell growth rate. Therefore, a dose‐dependent induction of structural chromosome aberrations was associated with 2‐methylcrotonaldehyde treatment in both short‐term treatments with and without S‐9 under testing conditions. An increase in numerical chromosomal aberrations was not observed. The Panel agreed with the authors of the study report and concluded that 2‐methylcrotonaldehyde induced chromosomal aberrations in cultured mammalian cells in the presence and absence of metabolic activation (Table D.1).
2.5.2. In vivo genotoxicity tests
2.5.2.1. Micronucleus assay
2‐Methylcrotonaldehyde [FL‐no: 05.095] was tested in an in vivo micronucleus assay using BDF1 male mice (Nakajima, 2007). Five mice per group were administered by oral gavage with a dose of 2‐methylcrotonaldehyde once per day for two consecutive days at either 250, 500 or 1,000 mg/kg bw (1,000 mg/kg bw was the maximum tolerable dose based on an initial dose‐finding study in which two out of three animals died after administration of 2,000 mg/kg bw). The bone marrow cells were sampled 24 h after the second dosing. Administration of 2‐methylcrotonaldehyde resulted in one case of piloerection 25 h after the first administration of 1,000 mg/kg. No other treatment groups showed any sign of toxicity by means of visual examination or by body weight loss. There was no statistically significant increase in the frequency of micronucleated erythrocytes in treated groups compared to the negative control group. The ratio of polychromatic erythrocytes (PCE) among total erythrocytes was not changed. Thus, the exposure of the bone marrow to the test substance could not be demonstrated based on that parameter. However, since mortality was observed at a dose of 2,000 mg/kg bw in the dose‐finding study, the Panel considered that systemic availability of the test substance could be assumed at the highest dose of 1,000 mg/kg bw in the micronucleus assay. Although, it may be assumed that the bone marrow was exposed to the test substance since the bone marrow is a well‐perfused tissue, no convincing evidence was provided. The Panel considered the study to be compliant with OECD guideline 474 except that no justification for the use of a single sex was given in the report, i.e. no data demonstrating that there are no substantial differences between sexes in toxicity. It was concluded that 2‐methylcrotonaldehyde did not induce micronuclei in mice bone marrow cells (Table D.2).
Table D.2.
Genotoxicity studies in vivo evaluated in FGE.201Rev1
| Chemical Name[FL‐no] | Test system | Test object | Dose | Reported Result | Reference | Commentsa |
|---|---|---|---|---|---|---|
| Trans‐2‐methyl‐2‐butenal[05.095] | In vivo micronucleus induction | BDF1 male mice | 250, 500 and 1,000 mg/kg bw per day by oral gavage | Negative | Nakajima (2007) | Valid. According to the study report, the study was performed in compliance with Japanese GLP standards. The study report contained a certificate of reliability but no details of inspection. The Panel considered the study to be compliant with OECD guideline 474 except that no justification for the use of a single sex was given in the report, i.e. no data demonstrating that there are no substantial differences between sexes in toxicity |
bw: body weight; GLP: Good Laboratory Practice.
Validity of the genotoxicity studies: Valid. Limited validity (e.g. if certain aspects are not in accordance with OECD guidelines or current standards and/or limited documentation). Insufficient validity (e.g. if main aspects are not in accordance with any recognised guidelines (e.g. OECD) or current standards inappropriate/not validated test system). Validity cannot be evaluated (e.g. insufficient documentation, short abstract only, too little experimental details provided, text not in a Community language).
2.5.3. Discussion of the additional data
2‐Methylpent‐2‐enal [FL‐no: 05.090] and 2‐methylcrotonaldehyde [FL‐no: 05.095] were tested in a series of in vitro tests to explore their genotoxicity potential.
For 2‐methylcrotonaldehyde [FL‐no: 05.095], there is some evidence for a mutagenic activity in the presence and absence of metabolic activation. This is based on two reliable studies on the induction of bacterial gene mutations (Nakajima, 2006a; Ballantyne, 2011) with the first study providing clear evidence for mutagenic activity and the latter study providing indication for a weak mutagenic activity.
For the representative substance 2‐methylpent‐2‐enal [FL‐no: 05.090], there is an indication for a weak mutagenic activity both in the presence and absence of metabolic activation. This indication is based on the results of one reliable study (Ballantyne, 2011) and structural similarity with 2‐methylcrotonaldehyde [FL‐no: 05.095].
In vitro assessment of micronucleus induction by 2‐methylpent‐2‐enal in the presence and absence of S‐9 metabolic activation was negative. In the case of 2‐methylcrotonaldehyde, a dose‐dependent induction of structural, but not numerical chromosomal aberrations in vitro was observed, both with and without S‐9. To further investigate the clastogenic potential an in vivo micronucleus assay using BDF1 male mice was performed and no evidence of clastogenic effects was identified but the respective test was inconclusive. These data support the absence of clastogenicity for the two substances 2‐methylpent‐2‐enal [FL‐no: 05.090] and 2‐methylcrotonaldehyde [FL‐no: 05.095].
However, since there is some evidence for 2‐methylcrotonaldehyde [FL‐no: 05.095] and an indication for 2‐methylpent‐2‐enal [FL‐no: 05.090] to show a potency for the induction of gene mutations in vitro the Panel considered the mutagenicity hazard not cleared by the endpoints evaluated in the in vivo micronucleus assay. The Panel therefore concluded that further data are required in order to clarify the genotoxic potential of this subgroup. The Panel considers the in vivo Comet assay as a preferred option to further investigate the genotoxicity in vivo. The Comet assay will also be accountable to explore any genotoxicity at the first site of contact where higher concentrations of the test substance are expected to occur. In this view, the in vivo Comet assay should be performed on liver, blood and first site of contact (e.g. duodenum or stomach). Alternatively, a transgenic rodent gene mutation assay (OECD TG 488) in tissues including first site of contact would also be acceptable.
Since 2‐methylpent‐2‐enal [FL‐no: 05.090] and 2‐methylcrotonaldehyde [FL‐no: 05.095] are closely related chemical structures, it is expected that they will have a similar reactivity behaviour. Since the evidence for a mutagenic activity in vitro is stronger for 2‐methylcrotonaldehyde [FL‐no: 05.095] than for 2‐methylpent‐2‐enal [FL‐no: 05.090], the Panel considers that additional data for the former substance are required. A negative result of 2‐methylcrotonaldehyde [FL‐no: 05.095] in the in vivo assay would be considered representative for the following substances of this subgroup [FL‐no: 02.174, 05.033, 05.090, 05.105, 05.107 and 05.126]. A positive result of 2‐methylcrotonaldehyde [FL‐no: 05.095] in the in vivo assay will require further testing of these six substances [FL‐no: 02.174, 05.033, 05.090, 05.105, 05.107 and 05.126] in order to finalise their evaluation.
The Panel noted that the Industry has communicated that the following two substances [FL‐no: 05.178 and 09.177] are not supported any more. These two substances were selected as representative for the substances in subgroup 1.1.2 and in addition also for substances in subgroup 2.1 (FGE.207). Since no data will be provided for these substances, they cannot further be used as representatives for the substances [FL‐no: 02.122, 09.034, 09.712, 09.809] in FGE.207.
2‐Methylpent‐2‐enal [FL‐no: 05.090] was originally selected as representative for the subgroup 1.1.2 and also for the substances in subgroup 5.3 (FGE.225). Since the Panel now considers that additional data are required for 2‐methylcrotonaldehyde, these data could also be considered representative for the substances in FGE.225 [FL‐no: 12.065, 12.079].
The genotoxicity studies are summarised in Appendix D Tables from D.2 to E.1.
Table E.1.
Summary of additional genotoxicity data submitted for FGE.201Rev2 in vivo
| FL‐no | Chemical name | Test system in vivo | Test object route | Concentrations of substance mg/kg bw per day | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|
| 05.090 | 2‐Methylpent‐2‐enal | Comet (liver and duodenum) |
Han Wistar rats Oral gavage |
0 (corn oil), 350, 700 and 1,400 | Negative | Keig‐Shevlin (2016) | Study performed in accordance with OECD TG 489 |
| Micronucleus (bone marrow) | Negative | Study performed in accordance with OECD TG 474 | |||||
| 05.095 | 2‐Methylbut‐2‐enal | Micronucleus (peripheral blood erythrocytes) | Fisher gpt delta rats Oral gavage | 0 (olive oil), 125, 250 and 500 | Negative | JBRC (2014) | The study was performed in 2014 and analysed in two different phases (2014 and 2016). Study performed in accordance with OECD TG 488 and OECD TG474 |
| Gene Mutation assay gpt/Spi− (liver) | Negative | ||||||
| Gene Mutation assay gpt/Spi− (stomach) | Negative | JBRC (2014, 2016) | |||||
| Comet (liver and duodenum) |
Sprague‐Dawley rats Oral gavage |
0 (corn oil), 250, 500, 1,000 and 2,000 | Negative | Bruce (2018) | Study performed in accordance with OECD TG 489 |
2.5.4. Conclusion
Since there is some evidence for 2‐methylcrotonaldehyde [FL‐no: 05.095] and an indication for 2‐methylpent‐2‐enal [FL‐no: 05.090] to show a potency for the induction of gene mutations in vitro the Panel considered the mutagenicity hazard not cleared by the endpoints evaluated in the in vivo micronucleus assay on 2‐methylcrotonaldehyde [FL‐no: 05.095]. The Panel therefore concluded that further data are required in order to clarify the genotoxic potential of this subgroup. The Panel considers the in vivo Comet assay as a preferred option to further investigate the genotoxicity in vivo. Since the evidence for a mutagenic activity in vitro is stronger for 2‐methylcrotonaldehyde [FL‐no: 05.095] than for 2‐methylpent‐2‐enal [FL‐no: 05.090], the Panel considers that additional data for 2‐methylcrotonaldehyde are required. The Panel concluded that the genotoxicity data for 2‐methylcrotonaldehyde cannot be considered representative for the remaining substances of this subgroup [FL‐no: 05.130, 05.178, 09.177 and 09.931] for which it was already concluded in the previous version of this FGE that the available data were insufficient to evaluate their genotoxicity.
The Panel noted that this conclusion will also have consequences for the read across for substances in subgroups 2.1 and 5.3 (FGE.207 and FGE.225).
3. Assessment
3.1. Additional data evaluated by the Panel in FGE.201Rev2
New genotoxicity data have been provided for two substances, 2‐methylpent‐2‐enal [FL‐no: 05.090] and 2‐methylbut‐2‐enal (2‐methylcrotonaldehyde) [FL‐no: 05.095]. In Table 2, the newly submitted data are listed and they are summarised in Appendix E, Table E.1.
Table 2.
List of genotoxicity studies evaluated in FGE.201Rev2
| Substance name | FL‐no | In vivo test | Reference |
|---|---|---|---|
| 2‐Methylpent‐2‐enal | 05.090 | Combined MN and Comet assay in liver and duodenum of rats | Keig‐Shevlin (2016) |
| 2‐Methylcrotonaldehyde | 05.095 | Comet in liver and duodenum of rats | Bruce (2018) |
| Combined gene mutation and micronucleus assay in transgenic rats | JBRC (2014, 2016) |
3.1.1. Stability and decomposition products
The Panel noted that in the recently provided in vivo genotoxicity study for 2‐methylpent‐2‐enal [FL‐no: 05.090], this substance was stored under nitrogen; this, however, does not correspond to the conditions of storage of the flavouring substances expected under normal conditions of use (i.e. storage for 12 months at temperatures < 18°C and out of direct light and air) (EFFA, 2018 in response to EFSA letter dated 9 August 2017, requesting information on storage conditions and chemical stability of [FL‐no: 05.090] and requesting to test [FL‐no: 05.095] in an in vivo comet assay).
To decide whether the substances subjected to genotoxicity testing can be considered representative of the materials of commerce, the Panel requested information on the stability of 2‐methylpent‐2‐enal [FL‐no: 05.090] under its intended conditions of use. The applicant provided data on both 2‐methylpent‐2‐enal [FL‐no: 05.090] and 2‐methylcrotonaldehyde [FL‐no: 05.095] from capillary gas chromatographic analyses of freshly prepared flavouring substances and of flavouring substances stored up to the end of their shelf‐life or longer.
For 2‐methylpent‐2‐enal [FL‐no: 05.090], the purity of fresh samples was 95–> 98% (this purity covers mainly the E‐isomer that is > 90%). The main secondary component reported was: 2‐methyl‐2‐pentenoic acid (< 0.5% in fresh samples up to 1% in samples after storage) – mainly the (E)‐2‐methyl‐2‐pentenoic acid was reported. The purity of samples stored up to 4 years (maximum expiration time reported) reported was > 96% (the purity covers mainly the E‐isomer) and the main secondary components reported were (E) and (Z)‐2‐methyl‐2‐pentenoic acid (up to 1%).
For 2‐methylcrotonaldehyde [FL‐no: 05.095], the purity of fresh samples was 95–> 98% (this purity covers mainly the E‐isomer that is > 95%). Secondary components reported were: acetic acid (up to 0.1%), tiglic acid (up to 0.5%) and paraldehyde (up to 1%) (i.e. 2,4,6‐trimethyl‐1,3,5‐trioxane, which is a condensation product – cyclic ether).
The purity of samples stored up to 4 years (maximum expiration time reported) reported was 95–> 96%. The composition of the secondary components was reported as follows: acetic acid (< 0.5%), tiglic acid (up to 2%) and paraldehyde (up to 2%) (EFFA, 2018).
The Panel concluded that the materials tested in the genotoxicity studies are representative of the material of commerce.
3.1.2. 2‐Methylpent‐2‐enal [FL‐no: 05.090] – in vivo combined micronucleus and comet assay
The genotoxic potential of 2‐methylpent‐2‐enal [FL‐no: 05.090] (purity 99.1%, stored protected from light under nitrogen) was assessed in vivo using the bone marrow micronucleus assay combined with the Comet assay in liver and duodenum in the same animals (Keig‐Shevlin, 2016). The study was conducted in accordance with GLP, OECD TG 474 (OECD, 2014a) and OECD TG 489 (OECD, 2014b).
In a dose range‐finder assay, groups of three male and three female Han Wistar rats were given three administrations (at 0, 24 and 45 h) of 2‐methylpent‐2‐enal, at 1,000, 1,400 or 2,000 mg/kg bw per day. At 1,000 and 1,400 mg/kg bw per day, no clinical signs of toxicity were observed. At 2,000 mg/kg bw per day, clinical signs of toxicity were observed only after the third administration. These included decreased activity, hunched posture, piloerection, lethargy, ataxia and ptosis. One female animal was killed in extremis. All other animals were back to a normal condition by 8 hours post‐dose. Minor losses in bodyweight were observed in all three males, but no losses in bodyweight were observed in the two remaining females. Based on this study, a maximum tolerated dose (MTD) of 1,400 mg/kg bw per day was established. As no gender specific effects were seen, only male rats were used in the main study. Groups of six male Han Wistar rats per dose group were administered doses by oral gavage of 0 (corn oil), 350, 700 or 1,400 mg/kg bw of 2‐methylpent‐2‐enal on three consecutive days (0, 24 and 45 h). A satellite group of three male rats were given the same dosing regimen as the 1,400 mg/kg bw per day group rats. A positive control group of three male rats were given doses of 150 mg ethyl methanesulfonate/kg bw at the same time intervals as the dosed groups.
Test animals were examined daily for signs of overt toxicity and body weights were recorded. No changes in body weight, clinical chemistry parameters or histopathology were observed.
Rats were euthanised 3 h after the last dose and 48 h after the initial dose. Blood samples were taken at necropsy for clinical chemistry analysis.
Comet assay
Liver and duodenum cells were prepared for Comet scoring. For both tissue types, the tail moment and tail intensity (%) of a total of 150 cells per animal, split over two to three slides, were recorded.
Both in liver and in duodenum, there was no dose‐related increase in %hedgehogs following treatment with 2‐methylpent‐2‐enal demonstrating that treatment did not cause excessive DNA damage that could have interfered with Comet analysis.
In liver, no statistically significant increase in group mean tail intensity and tail moment values was seen in any test substance treatment group compared to the vehicle control treatment group. However, a weak but statistically significant linear trend (p < 0.05) was observed. Although the data did not meet the criteria for a clearly negative result, the increases in tail intensity were minor, not statistically significant and fell at the lower end of the historical vehicle control range for tail intensity (0.08–5.08). Therefore, the data were considered to be within the usual variation seen in this assay and of no biological relevance. The positive control provided statistically significant increases in both tail intensity and tail moment.
In duodenum, there were no dose‐related increases in the tail intensity or tail moment and no statistically significant results compared to the control group. The positive control provided statistically significant increases in both tail intensity and tail moment.
Micronucleus assay
Bone marrow from the femurs was prepared for micronucleus scoring. A total of at least 500 PCE and normochromatic erythrocytes (NCE) was scored to calculate the degree of bone marrow toxicity by the relative decrease in PCE. For micronuclei (MN) analysis, 4,000 PCE per animal were scored for the presence of MN. In order to improve data interpretation, additional 4,000 PCE were analysed to a total of 8,000 PCE per animal.
2‐Methylpent‐2‐enal [FL‐no: 05.090] did not induce micronuclei in bone marrow of rats tested up to 1,400 mg/kg bw per day (MTD). No indication of bone marrow toxicity was observed, because the treatment with 2‐methylpent‐2‐enal [FL‐no: 05.090] did not reduce the percentage of PCE. However, in the range‐finding test, at 2,000 mg/kg bw per day (above the MTD), clinical signs of toxicity were observed (including decreased activity, hunched posture, lethargy, ataxia) that could be considered as a line of evidence of systemic exposure.
In the same animals, 2‐methylpent‐2‐enal [FL‐no: 05.090] showed negative results in the comet assay both in duodenum and liver. The Panel concluded that 2‐methylpent‐2‐enal [FL‐no: 05.090] did not induce DNA damage under the conditions of this study.
3.1.3. 2‐Methylcrotonaldehyde [FL‐no: 05.095] – in vivo combined gene mutation and micronucleus assay in transgenic rats
2‐Methylcrotonaldehyde [FL‐no: 05.095] (purity 99.5%) was tested in a combined gene mutation and micronucleus assay in transgenic Fisher gpt delta rats. Liver and glandular stomach were analysed for gene mutation and peripheral blood erythrocytes for micronuclei (JBRC, 2014 and additional assay 2016). The study was performed in accordance with OECD TG 488 (OECD, 2013) and OECD TG 474 (OECD, 1997).
In a dose range‐finding 14‐day test, a MTD of 500 mg/kg bw per day was established, based on the observation that one out of three animals died in the 1,000 mg/kg bw per day dose group. In the main study, groups of six transgenic rats were administered 2‐methylcrotonaldehyde, by gavage, daily doses of 0, 125, 250 and 500 mg/kg bw per day for 28 days. The solvent, olive oil, was the negative control. As a positive control, one group of six rats was given a single i.p. dose of 125 mg/kg bw of benzo[a]pyrene (B[a]P).
Besides clinical observations of lacrimation and salivation found in some animals in the 250 and 500 mg/kg bw per day groups, no other clear toxicity effects from the test substance were found for body weight, food consumption, gross findings, liver weight, and histopathological lesions of the liver, bone marrow, spleen, stomach, small intestine and large intestine.
Livers from the positive control group were removed 7 days after the treatment. In animals of the other groups, liver and glandular stomach were removed 3 days after the treatment. While liver was analysed immediately for gene mutations (JBRC, 2014), glandular stomach was analysed for gene mutations at a later stage (JBRC, 2016).
For glandular stomach, no positive control was included, but the authors used the results of positive control (B[a]P)in liver. The OECD TG 488 does not provide any suggestion for a positive control to be used in the mutation assay of rat's stomach.
Twenty‐four hours after the final dose had been given, blood samples from the tail vein of treated rats were collected for scoring of micronuclei in the immature erythrocytes, and after a manifestation period of 3 days, rats were euthanised.
No historical control data for the performing laboratory were included in the study report but reference was made to published papers.
3.1.3.1.
Gene mutation assay
Genomic DNA was extracted from liver and glandular stomach of treated rats. The isolated gpt transgene sequences were packaged into a lambda phage shuttle vector and mutations subsequently detected in E. coli YG6020 (6TG selection) and in E. coli XL‐1Blue MRA (Spi− selection).
There were no increases in gpt and Spi− mutant frequencies in the liver of the transgenic rats following treatment with 2‐methylcrotonaldheyde compared to the negative control whereas significant increases were seen in the positive control, B[a]P (JBRC, 2014).
There were no increases in gpt and Spi− mutant frequencies in the glandular stomach of transgenic rats following treatment with 2‐methylcrotonaldehyde compared to the negative control. No positive control was included in the study for the effect in the glandular stomach (JBRC, 2016).
Micronucleus assay
For each slide, 2,000 PCE were scored for MN.
No increases in micronucleated immature erythrocytes in the peripheral blood were observed in the 2‐methylcrotonaldehyde treated groups compared to the negative control, while a statistically significant increase was seen in the positive control group.
Based on these results, the authors of the study concluded that 2‐methylcrotonaldehyde is not mutagenic in the liver nor in the glandular stomach of gpt delta transgenic rats and does not induce micronuclei in the peripheral blood of the same rats.
The Panel noted that there are no indications of bone marrow toxicity because the percentage of PCE is not affected by the treatment with 2‐methylcrotonalheyde. In addition, there are no indications of bone marrow exposure based on the clinical signs of toxicity observed, therefore the micronucleus assay is inconclusive. Since the potential clastogenicity of [FL‐no: 05.095] is not clarified in this study, it was requested to test [FL‐no: 05.095] in an in vivo comet assay (EFSA letter dated 9 August 2017). The Panel concluded that 2‐methylcrotonaldheyde did not induce gene mutations in the liver and the glandular stomach under the conditions applied in this study.
3.1.4. 2‐Methylcrotonaldehyde [FL‐no: 05.095] ‐ in vivo comet assay
2‐Methylcrotonaldehyde [FL‐no: 05.095] (purity ≥ 99%) was tested in a comet assay in liver and duodenum of male Sprague–Dawley rats (Bruce, 2018), in accordance with OECD TG 489 (OECD, 2016) and GLP. Animals were dosed via oral gavage on two consecutive days, the second dose was administered 21 hours after the first dose. The vehicle control used was corn oil and the positive control was ethyl methanesulfonate (EMS). Animals were dosed once with EMS, 3–4 h before euthanasia on day 2.
No dose range‐finding experiment was performed. 2‐Methylcrotonaldehyde was tested at 250, 500, 1,000 and 2,000 mg/kg bw per day (six animals per group). At the highest dose tested, three animals were found dead. In the surviving animals, reductions in mean group body weights were observed in the 2,000 mg/kg bw per day group.
At 250 and 500 mg/kg bw per day, no clinical signs of toxicity were observed except piloerection at 500 mg/kg bw per day. At 1,000 and 2,000 mg/kg bw per day, clinical signs of toxicity, including piloerection, ataxia, prostration, irregular breathing, hunched position, were observed.
Due to the elevated mortality (3 animals out of six) occurred in the highest treatment group (2,000 mg/kg bw per day) the remaining animals were excluded from the Comet analysis.
For both liver and duodenum, the comet assay resulted in no dose‐dependent or statistically significant increases in the % tail DNA in animals dosed with 2‐methylcrotonaldehyde up to and including a dose of 1,000 mg/kg bw per day, compared to the concurrent vehicle control group.
In liver and duodenum cells, the positive control, EMS, induced a statistically significant increase in the % tail DNA as compared to the vehicle control groups (p ≤ 0.05). In the vehicle control group, % tail DNA was within the historical vehicle control range respectively for liver and duodenum.
The authors of the study concluded that under the conditions of this study, 2‐methylcrotonaldehyde did not induce DNA damage in liver and duodenum of rats. The Panel agreed with this conclusion.
3.2. Discussion
In FGE.201Rev1, the Panel considered that for 2‐methylpent‐2‐enal [FL‐no: 05.090] there was an indication for a weak mutagenic activity in bacteria both in the presence and absence of metabolic activation. In vitro assessment of micronucleus induction by 2‐methylpent‐2‐enal [FL‐no: 05.090] in the presence and absence of S9 metabolic activation was negative.
In FGE.201Rev1, the Panel considered that for 2‐methylcrotonaldehyde [FL‐no: 05.095] there is some evidence for a mutagenic activity in bacteria in the presence and absence of metabolic activation. In addition, a dose‐dependent induction of structural chromosomal aberrations in vitro was observed, both with and without S9‐mix. The in vivo micronucleus assay on 2‐methylcrotonaldehyde [FL‐no: 05.095] was not sufficient to overrule the genotoxicity concern; therefore, additional genotoxicity data have been provided that are evaluated in the present revision, FGE.201Rev2.
2‐Methylpent‐2‐enal [FL‐no: 05.090] tested in a combined micronucleus and comet assay did not induce DNA damage, overruling the weak gene mutation effect observed in bacteria and confirming the negative results observed in the in vitro micronucleus assay.
2‐Methylcrotonaldehyde [FL‐no: 05.095] did not induce gene mutations in vivo in liver and glandular stomach of transgenic rats (gpt and Spi‐ assay). In addition, 2‐methylcrotonaldehyde [FL‐no: 05.095] tested in an in vivo comet assay in liver and duodenum, it did not induce DNA damage.
The extrapolation from two representative substances to eight candidate substances is intrinsically associated with the introduction of uncertainty. In this opinion, only one toxicological endpoint is addressed (genotoxicity) related to one well defined structural alert, therefore the uncertainty is considered to be limited. Other toxicological endpoints will be addressed in separate opinions.
3.3. Conclusion
Overall, based on the data obtained the Panel concluded that the genotoxic evidence observed in vitro, was not confirmed in vivo. Since the genotoxicity concern is cleared for the representative substances 2‐methylcrotonaldehyde [FL‐no: 05.095] and 2‐methylpent‐2‐enal [FL‐no: 05.090], all the ten substances in this subgroup [FL‐no: 02.174, 05.033, 05.090, 05.095, 05.105, 05.107, 05.126, 07.261, 12.065 and 12.079] can be evaluated through the Procedure for the evaluation of flavouring substances.
Documentation provided to EFSA
Ballantyne M, 2011. Reverse mutation in a single histidine‐requiring strain of Salmonella typhimurium. 2‐Methyl‐2‐butenal. Covance Laboratories Ltd. Study no. 8243635. June 2011. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Benigni R and Netzeva T, 2007a. Report on a QSAR model for prediction of genotoxicity of alpha,beta‐unsaturated aldehydes in S. typhimurium TA 100 and its application for predictions on alpha,beta‐unsaturated aldehydes in Flavouring Group Evaluation 19 (FGE.19). Unpublished report submitted by FLAVIS Secretariat to EFSA.
Benigni R and Netzeva T, 2007b. Report on a QSAR model for prediction of genotoxicity of alpha,beta‐unsaturated ketones in S. typhimurium TA 100 and its application for predictions on alpha,beta‐unsaturated aldehydes in Flavouring Group Evaluation 19 (FGE.19). Unpublished report submitted by FLAVIS Secretariat to EFSA.
Bowen R, 2011. Reverse mutation in five histidine‐requiring strains of Salmonella typhimurium. 2‐Methylpent‐2‐enal. Covance Laboratories Ltd. Study no. 8228189. January 2011. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Bruce S, 2018. 2‐Methylcrotonaldehyde, in vivo alkaline comet assay. BioReliance study number AF08XC.423M.BTL. Unpublished report submitted by EFFA to EFSA.
EFFA (European Flavour Association), 2011. Submission by the European Flavour Association to the European Food Safety Authority (EFSA). Flavouring Group Evaluation 19 Subgroup 1.1.2 (corresponding to FGE.201): Additional genotoxicity data requested in FGE.201 for subgroup 1.1.2 ‐ straight‐ and branched‐chain aliphatic acyclic alpha,beta‐unsaturated aldehydes (2‐alkylated substances with or without additional double‐bonds). 12 August 2011. FLAVIS/8.125.
EFFA (European Flavour Association), 2018. Submission of additional information on substances within FGE.201 (FGE.19 Subgroup 1.1.2). Supplementary data submitted by EFFA to EFSA.
Gry J, Beltoft V, Benigni R, Binderup M‐L, Carere A, Engel K‐H, Gürtler R, Jensen GE, Hulzebos E, Larsen JC, Mennes W, Netzeva T, Niemelä J, Nikolov N, Nørby KK and Wedebye EB, 2007. Description and validation of QSAR genotoxicity models for use in evaluation of flavouring substances in Flavouring Group Evaluation 19 (FGE.19) on 360 alpha,beta‐unsaturated aldehydes and ketones and precursors for these. Unpublished report submitted by FLAVIS Secretariat to EFSA.
Keig‐Shevlin, 2016. 2‐methyl‐2‐pentenal: Rat Micronucleus and Alkaline Comet Assay. Covance Laboratories Ltd. Study no 8312067. Unpublished report.
Nakajima M, 2006a. Bacterial reverse mutation assay using trans‐2‐methyl‐2‐butenal. Biosafety Research Center Foods, Drugs and Pesticides. Assay no. 9683 (079‐348). 6 June, 2006. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Nakajima M, 2006b. Chromosome aberration test of trans‐2‐methyl‐2‐butenal using cultured mammalian cells. Biosafety Research Center Foods, Drugs and Pesticides. Test no. 9684 (079‐349). 6 June, 2006. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Nakajima M, 2007. Micronucleus Assay using trans‐2‐methyl‐2‐butenal‐dosed mice. Biosafety Research Center Foods, Drugs and Pesticides. Test no. A379 (079‐395). 13 August, 2007. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Nikolov N, Jensen GE, Wedebye EB and Niemelä J, 2007. Report on QSAR predictions of 222 alpha,beta‐unsaturated aldehydes and ketones from Flavouring Group Evaluation 19 (FGE.19) on 360 alpha,beta‐unsaturated aldehydes and ketones and precursors for these. Unpublished report submitted by FLAVIS Secretariat to EFSA.
JBRC (Japan bioassay research center), 2014 and 2016. Mutation assay for 2‐methylcrotonaldehyde using gpt delta rats. Study Number 0835. Japan Bioassay Research Center, Japan Industrial Safety and Health Association. Unpublished report.
Whitwell J, 2011. Induction of micronuclei in cultured human peripheral blood lymphocytes. 2‐Methylpent‐2‐enal. Covance Laboratories Ltd. Study no. 8228190. March 2011. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Abbreviations
- bw
body weight
- CAS
Chemical Abstract Service
- CEF
Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CHL
Chinese hamster lung (cells)
- CHO
Chinese hamster ovary (cells)
- CoE
Council of Europe
- DTU‐NFI
Danish Technical University – National Food Institute
- EMS
ethyl methanesulfonate
- FAO
Food and Agriculture Organization of the United Nations
- FAF
Panel on Food Additives and Flavourings
- FEMA
Flavor and Extract Manufacturers Association
- FGE
Flavouring Group Evaluation
- FLAVIS
(FL) Flavour Information System (database)
- GLP
Good Laboratory Practice
- ID
Identity
- i.p.
intraperitoneal
- IR
infrared spectroscopy
- ISS
Istituto Superiore di Sanità
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- MN
micronuclei
- MNBN
micronucleated binucleate cells
- MS
mass spectrometry
- MTD
maximum Tolerated Dose
- MSDI
maximised Survey‐derived Daily Intake
- NCE
normochromatic erythrocytes
- NMR
nuclear magnetic resonance
- No
Number
- OECD
Organisation for Economic Co‐operation and Development
- PCE
polychromatic erythrocytes
- (Q)SAR
(Quantitative) Structure–Activity Relationship
- WHO
World Health Organization
Appendix A – Specification Summary of the Substances in the Flavouring Group Evaluation 201Rev2
1.
Appendix B – Summary of safety evaluation applying the procedure
1.
Appendix C – (Q)SAR predictions
1.
Appendix D – Genotoxicity data evaluated in FGE.201Rev1
1.
In vitro studies available for the group of aldehydes and the aldehydes anticipated to be formed from the precursors in subgroup 1.1.2.
One in vivo study is available for the group of aldehydes and the aldehydes anticipated to be formed from the precursors in subgroup 1.1.2.
Appendix E – Genotoxicity studies evaluated in FGE.201Rev2
1.
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Frutos Fernandez MJ, Fürst P, Gundert‐Remy U, Husøy T, Mennes W, Moldeus P, Oskarsson A, Rainieri S, Shah R, Waalkens‐Berendsen I, Wölfle D, Binderup M‐L, Bolognesi C, Marcon F, Marzin D, Mosesso P, Anastassiadou M, Carfì M, Vianello G and Gürtler R, 2018. Scientific Opinion on Flavouring Group Evaluation 201 Revision 2 (FGE.201Rev2): 2‐alkylated, aliphatic, acyclic alpha,beta‐unsaturated aldehydes and precursors, with or without additional double‐bonds, from chemical subgroup 1.1.2 of FGE.19. EFSA Journal 2018;16(10):5423, 33 pp. 10.2903/j.efsa.2018.5423
Requestor: European Commission
Question numbers: EFSA‐Q‐2017‐00117, EFSA‐Q‐2017‐00118, EFSA‐Q‐2017‐00119, EFSA‐Q‐2017‐00120, EFSA‐Q‐2017‐00121, EFSA‐Q‐2017‐00122, EFSA‐Q‐2017‐00123, EFSA‐Q‐2017‐00124, EFSA‐Q‐2018‐00412, EFSA‐Q‐2018‐00413
Panel members: Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Rainer Gürtler, Ursula Gundert‐Remy, Trine Husøy, Wim Mennes, Peter Moldeus, Agneta Oskarsson, Sandra Rainieri, Romina Shah, Ine Waalkens‐Berendsen, Detlef Wölfle and Maged Younes.
Acknowledgements: The Panel wishes to thank the hearing experts: Vibe Beltoft and Karin Nørby for the support provided to this scientific opinion.
Adopted: 13 September 2018
Notes
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
The data presented in Section 2.4 are cited from the Scientific Opinion FGE.201. These data are the basis for the conclusions in FGE.201 requesting additional genotoxicity data.
The data presented in Section 2.5 are cited from the Scientific Opinion FGE.201Rev1. These data are the basis for the conclusions in FGE.201Rev1 requesting additional genotoxicity data.
References
- Eder E and Deininger C, 2001. Mutagenicity of 2‐alkylpropenals in Salmonella typhimurium strain TA 100: structural influences. Environmental and Molecular Mutagenesis 37, 324–328. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Minutes of the 26th Plenary meeting of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food, Held in Parma on 27 ‐ 29 November 2007. Parma, 7 January 2008.
- EFSA (European Food Safety Authority), 2008b. Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) on Genotoxicity Test Strategy for Substances belonging to Subgroups of FGE.19. EFSA Journal 2008;854, 1–5. 10.2903/j.efsa.2008.854 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008c. Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) on List of alpha, beta‐unsaturated aldehydes and ketones representative of FGE.19 substances for genotoxicity testing. EFSA Journal 2008;6(12):910, 1–5. 10.2903/j.efsa.2008.910 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Flavouring Group Evaluation 201: 2‐Alkylated aliphatic acyclic alpha,beta‐unsaturated aldehydes and precursors with or without additional double bonds from chemical subgroup 1.1.2 of FGE.19. EFSA Journal 2009;7(5):1080, 1–15. 10.2903/j.efsa.2009.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2012a. Scientific Opinion on Flavouring Group Evaluation 204 (FGE.204): consideration of genotoxicity data on representatives for 18 mono‐unsaturated, aliphatic, α,β‐unsaturated ketones and precursors from chemical subgroup 1.2.1 of FGE.19 by EFSA. EFSA Journal 2012;10(12):2992, 25 pp. 10.2903/j.efsa.2012.2992 [DOI] [Google Scholar]
- EFSA CEF Panel (Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2012b. Scientific Opinion on Flavouring Group Evaluation 201Rev1: 2‐Alkylated, aliphatic, acyclic alpha,beta‐unsaturated aldehydes and precursors, with or without additional double‐bonds, from chemical subgroup 1.1.2 of FGE.19. EFSA Journal 2012;10(5):2749, 27 pp. 10.2903/j.efsa.2012.2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2013. Scientific Opinion on Flavouring Group Evaluation 207 (FGE.207). EFSA Journal 2013;11(5):3228, 17 pp. 10.2903/j.efsa.2013.3228 [DOI] [Google Scholar]
- Florin I, Rutberg L, Curvall M and Enzell CR, 1980. Screening of tobacco smoke constituents for mutagenicity using the Ames’ test. Toxicology, 18, 219–232. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Evaluation of certain food additives and contaminants. Fifty‐third meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series no. 896. Geneva, 1‐10 June 1999.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2003. Compendium of food additive specifications. Addendum 11. Joint FAO/WHO Expert Committee of Food Additives 61st session. Rome, 10‐19 June 2003. FAO Food and Nutrition paper 52 Add. 11.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2004. Safety evaluation of certain food additives and contaminants. Sixty‐first meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 52. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2007. Evaluation of certain food additives. Sixty‐eighth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 947. Geneva, 19‐28 June 2007.
- OECD (Organisation for Economic Co‐operation and Development), 1997. Test No. 474: Mammalian erythrocyte micronucleus test. OECD Guideline for testing of chemicals, Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 2013. Test No. 488: Transgenic Rodent Somatic and Germ Cell Gene Mutation Assays. OECD Guidelines for the Testing of Chemicals, Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 2014a. Test No. 474: Mammalian erythrocyte micronucleus test. OECD Guideline for testing of chemicals, Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 2014b. Test No. 489: In Vivo Mammalian Alkaline Comet Assay. OECD Guidelines for the Testing of Chemicals, Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 2016. Test No. 489: In Vivo Mammalian Alkaline Comet Assay. OECD Guidelines for the Testing of Chemicals, Section 4.


