Abstract
The EFSA Panel on Food Additives and Flavourings (FAF) provides a scientific opinion re‐evaluating the safety of thermally oxidised soya bean oil interacted with mono‐ and diglycerides of fatty acids (TOSOM) (E 479b) when used as a food additive. The Scientific Committee on Food (SCF) and the Joint FAO/WHO Expert Committee on Food Additives (JECFA) derived an acceptable daily intake (ADI) of 25 and 30 mg/kg body weight (bw) per day, respectively. There was no reliable information regarding the absorption, distribution, metabolism, excretion (ADME) for TOSOM. No adverse effects have been detected in a limited subchronic toxicity study in pigs. The Panel identified a no observed adverse effect level (NOAEL) of 5,400, the highest dose tested, from a chronic and carcinogenicity study in rats. No genotoxicity data were available. No reliable studies for reproductive or developmental toxicity were available. From the chronic and carcinogenicity study, no lesions in reproductive organs were described and the lack of carcinogenic effect alleviated the concern for genotoxicity at the first site of contact. The Panel concluded that the available toxicological data were insufficient to support the current ADI, in particular, due to the lack of ADME data and absence of developmental toxicity studies TOSOM (E 479b) is only authorised in one food category and only one reported use level that equals the maximum permitted level was submitted. The estimated high (P95) exposure reached an upper value of 10.1 mg/kg bw per day for toddlers. When comparing the highest estimated exposure of 10 mg/kg bw per day in toddlers with the NOAEL of 5,400 mg/kg bw per day (the highest dose tested), the margin of safety (MoS) would be 540. Therefore, the Panel considered the use of TOSOM (E 479b) to be of no safety concern, in particular when considering the limited current use of this food additive. The Panel also recommended some modifications of the EU specifications for E 479b.
Keywords: thermally oxidised soya bean oil interacted with mono‐ and diglycerides of fatty acids, TOSOM, E 479b, food additive
Summary
Thermally oxidised soya bean oil interacted with mono‐ and diglycerides of fatty acids (TOSOM) (E 479b) is defined as a complex mixture of esters of glycerol and fatty acids found in edible fat and fatty acids from thermally oxidised soy bean oil. The Panel noted that the soya bean oil used to produce E 479b (in line with the specifications in Regulation (EU) No 231/2012) is only made from naturally available soya bean varieties or authorised varieties of genetically modified soya bean (according to Regulation (EC) No 1829/2003.
The Panel noted that the Scientific Committee on Food (SCF) and the Joint FAO/WHO Expert Committee on Food Additives (JECFA) derived from the same study an acceptable daily intake (ADI) of 25 and 30 mg/kg body weight (bw) per day, respectively, but starting with different no observed adverse effect levels (NOAELs) and applying different uncertainty factors. Not all of the unpublished reports cited in the evaluations by SCF and the JECFA were available to the Panel.
There was no reliable information regarding the absorption, distribution, metabolism, excretion (ADME) for TOSOM. No adverse effects have been detected in a limited subchronic toxicity study in pigs. No genotoxicity data on TOSOM (E 479b) were available.
A chronic and carcinogenicity study performed in rats did not show any carcinogenic potential. The Panel noted that histopathological examination was conducted in a range of organs including reproductive organs and the incidence of non‐neoplastic lesions was evenly distributed among all groups. In addition, no changes were observed in mortality, clinical, haematological or biochemical parameters. In this study, there was no urinalysis. The Panel identified a NOAEL of 5,400 and 7,400 mg/kg bw per day in males and females, respectively, the highest dose tested.
The Panel considered that some of the potential by‐products (e.g. epoxides), which may result from the manufacturing process of TOSOM, could be reactive to DNA. However, the Panel noted that the maximum permitted level for epoxides in the E479b according to its specifications is very low and that even fully epoxidised soya bean oil did not show genotoxic potential. Moreover, the lack of carcinogenic effect in the chronic and carcinogenicity study alleviated the concern for genotoxicity at the first site of contact.
No reliable studies for reproductive toxicity were available; however, no lesions in reproductive organs were described in the chronic and carcinogenicity study in rats. No developmental toxicity studies were available.
The toxicological data that were available to the Panel were insufficient to support the current ADI, in particular, due to the lack of ADME data and the absence of developmental toxicity studies.
TOSOM (E 479b) is only authorised in FC 02.2.2 ‘Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions’ according to Annex II to Regulation (EC) No 1333/2008. The Panel could only estimate the exposure to TOSOM (E 479b) according to the regulatory maximum level exposure assessment scenario, as the only reported use level equalled the maximum permitted level. According to this scenario, the high (P95) exposure reached an upper value of 10.1 mg/kg bw per day for toddlers. The Panel also noted that according to the Mintel's Global New Products Database (GNPD), only a few products were labelled with TOSOM (E 479b) over the last 5 years, and that since 2015 no new product launches contained this food additive according to the product label. Overall, the Panel considered that the exposure to TOSOM (E 479b) was overestimated.
The Panel noted that when comparing the highest estimated exposure of 10 mg/kg bw per day in toddlers with the NOAEL of 5,400 mg/kg bw per day (the highest dose tested) identified from the chronic and carcinogenicity study, the margin of safety (MoS) would be 540. Based on this MoS, the Panel considered that the use of TOSOM (E 479b) is of no safety concern, in particular when considering the limited current use of this food additive.
The Panel recommended that:
the European Commission considers lowering the current limits for toxic elements (arsenic, lead, mercury and cadmium) in the European Union (EU) specifications for TOSOM (E 479b) in order to ensure that the food additive will not be a significant source of exposure to these toxic elements in food.
the European Commission considers revising the EU specifications for TOSOM (E 479b) including maximum limits for impurities currently included in the EU specifications for the food additive mono‐ and diglycerides of fatty acids (E 471) and recommended by the Panel in the re‐evaluation of E 471 (EFSA ANS Panel, 2017).
the European Commission considers revising the description provided in the EU specifications for ‘Soya bean oil is exclusively made from strains of soya beans’ indicating that the soya bean oil used to produce E 479b is exclusively made from naturally available soya bean varieties or authorised varieties of genetically modified soya bean (Regulation (EC) No 1829/2003).
1. Introduction
The present opinion deals with the re‐evaluation of thermally oxidised soya bean oil interacted with mono‐ and diglycerides of fatty acids (TOSOM) (E 479b) when used as a food additive.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
Regulation (EC) No 1333/20081 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union. In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the European Union before 20 January 2009 has been set up under the Regulation (EU) No 257/20102. This Regulation also foresees that food additives are re‐evaluated whenever necessary in light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU3 of 2001. The report ‘Food additives in Europe 20004’ submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010 the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference
The Commission asks the European Food Safety Authority to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.2. Information on existing authorisations and evaluations
TOSOM (E 479b) is authorised as a food additive in the European Union (EU) in accordance with Annex II to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/20125.
The SCF identified a no observed adverse effect level (NOAEL) of 2,500 mg/kg body weight (bw) per day in a 2.5‐year chronic and carcinogenicity study in rats, and through application of a 100‐fold uncertainty factor derived an ADI of 25 mg/kg bw per day (SCF, 1989).
The JECFA identified a NOAEL of 6,000 mg/kg bw per day from the same 2.5‐year chronic and carcinogenicity study in rats and through application of a 200‐fold uncertainty factor derived an ADI of 30 mg/kg bw per day (JECFA, 1992).
TOSOM was also evaluated by the Nordic Council of Ministers (TemaNord, 2002). It was concluded that ‘the toxicological data available for SCF include what generally would be required for an ADI to be set for a food additive. Exposure is low. There has been found no information that could necessitate a re‐evaluation of TOSOM.
2. Data and methodologies
2.1. Data
The Panel on Food Additives and Flavourings (FAF) was not provided with a newly submitted dossier. EFSA launched a public call for data6 , 7 , 8 to collect information from interested parties.
The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up June 2018. Attempts were made at retrieving relevant original study reports on which previous evaluations or reviews were based, however not always these were available to the Panel.
Food consumption data used to estimate the dietary exposure to TOSOM (E 479b) were derived from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database9).
The Mintel's Global New Products Database (GNPD) was checked to identify the uses of TOSOM (E 479b) in food and beverage products and food supplements. The Mintel's GNPD is an online database that contains the compulsory ingredient information present on the label of numerous products.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
The FAF Panel assessed the safety of TOSOM (E 479b) as a food additive in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the SCF (2001) and taking into consideration the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
When the test substance was administered in the feed or in the drinking water, but doses were not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012) for studies in rodents or, in the case of other animal species, by JECFA (2000). In these cases, the daily intake was expressed as equivalent.
Dietary exposure to TOSOM (E 479b) from its use as a food additive was estimated by combining the food consumption data available within the EFSA Comprehensive Database with the maximum permitted levels according to Annex II to Regulation (EC) No 1333/2008 and reported use levels submitted to EFSA following a call for data. The exposure was estimated according to different scenarios (see Section 3.4.1). Uncertainties in the exposure assessment were identified and discussed.
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
According to Commission Regulation (EU) No 231/2012, TOSOM (E 479b) is defined as a complex mixture of esters of glycerol and fatty acids found in edible fat and fatty acids from thermally oxidised soya bean oil. The CAS Registry Number is 884318‐13‐2(Scifinder,10 online); an EINECS number has not been assigned.
The general chemical structure of TOSOM (E 479b) is shown in Figure 1. The distribution of the principal components within the ester depends on the proportion of glycerol and fatty acids and the reaction conditions used (EFEMA, 2009 (Documentation provided to EFSA n. 4)).
Figure 1.
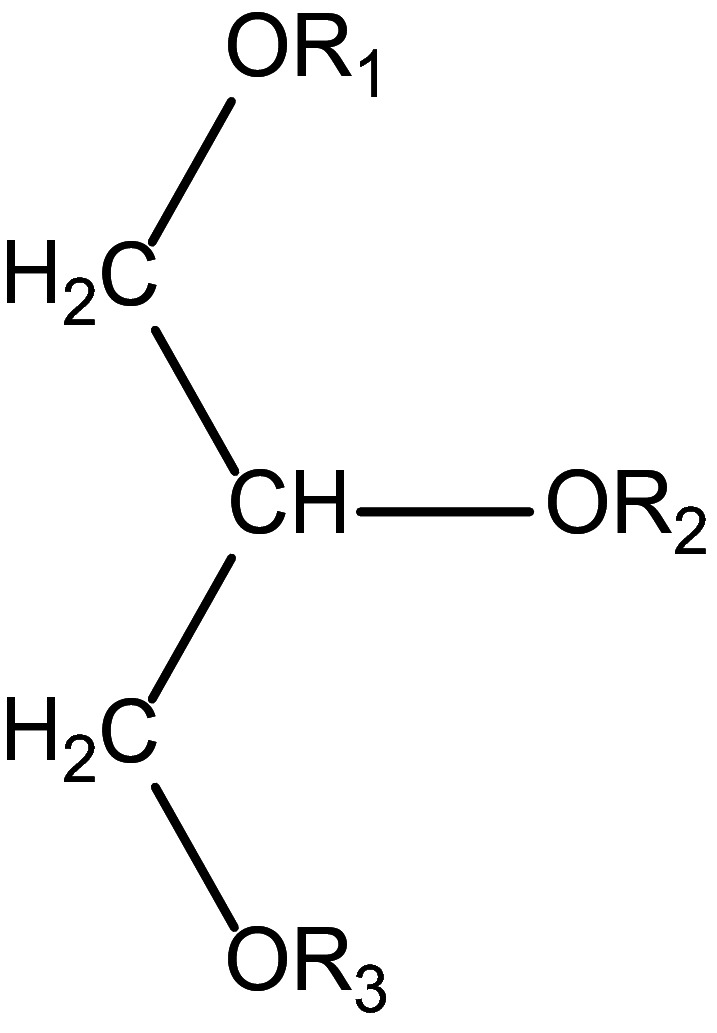
General chemical structure of TOSOM (E 479b) where R1, R2 or R3 may be a normal fatty acid, oxidised fatty acid (e.g. hydroxyl and/or carbonyl compound of fatty acid), hydrogen, short‐chain fatty acid or di‐ and polymer of oxidised fatty acids (JECFA, 2006)
The Panel noted that in addition to hydroxyl and/or carbonyl functions also epoxides moieties may be present in TOSOM (E 479b).
TOSOM is typically dispersible in hot water, non‐dispersible in cold water and soluble in edible oils and fats (EFEMA, 2009 (Documentation provided to EFSA n. 4)).
3.1.2. Specifications
The specifications for TOSOM (E 479b) as defined in the Commission Regulation (EU) No 231/2012 and by JECFA (2006) are listed in Table 1.
Table 1.
Specifications for TOSOM (E 479b) according to Commission Regulation (EU) No 231/2012 and JECFA (2006)
| Commission Regulation (EU) No 231/2012 | JECFA (2006) | |
|---|---|---|
| Definition | Thermally oxidised soya bean oil interacted with mono‐ and diglycerides of fatty acids is a complex mixture of esters of glycerol and fatty acids found in edible fat and fatty acids from thermally oxidised soya bean oil. It is produced by interaction and deodorisation under vacuum at 130°C of 10% of thermally oxidised soya bean oil and 90% mono‐ and diglycerides of food fatty acids. Soya bean oil is exclusively made from strains of soya beans | A complex mixture of esters of glycerol and fatty acids found in edible fat and fatty acids from thermally oxidised soya bean oil; produced by interaction and deodorisation under vacuum at 130° of 10% w/w of thermally oxidised soya bean oil (thermally oxidised soya bean oil is obtained by oxidation of refined soya bean oil with air at 190–200°) and 90% w/w of mono‐ and diglycerides of food fatty acids |
| Description | Pale yellow to light brown a waxy or solid consistency | Pale yellow to light brown with a waxy or solid consistency |
| Identification | ||
| Solubility | Insoluble in water; soluble in hot oil or fat | Insoluble in water; soluble in hot fats and oils |
| Purity | ||
| Melting range | 55–65°C | 55–65° |
| Free fatty acids | Not more than 1.5% estimated as oleic acid |
Not more than 1.5% w/w calculated as oleic acid Proceed as directed under Free Fatty Acids using the equivalence factor e = 28.2 |
| Free glycerol | Not more than 2% | Not more than 2% w/w |
| Total fatty acids | 83–90% | 83–90% w/w |
| Total glycerol | 16–22% | 16–22% w/w |
| Fatty acid methyl esters, not forming adduct with urea | Not more than 9% of total fatty acid methyl esters | Not more than 9.0% w/w of total fatty acid methyl esters |
| Fatty acids, insoluble in petroleum ether | Not more than 2% of total fatty acids | Not more than 2% w/w of total fatty acids |
| Peroxide value | Not more than 3 | Not more than 3 |
| Epoxides | Not more than 0.03% oxirane oxygen | Not more than 0.03% w/w oxiran oxygen |
| Arsenic | Not more than 3 mg/kg | – |
| Lead | Not more than 2 mg/kg | Not more than 2 mg/kg |
| Mercury | Not more than 1 mg/kg | – |
| Cadmium | Not more than 1 mg/kg | – |
The Panel noted that the soya bean oil used to produce E 479b (in line with the specifications in Commission Regulation (EU) No 231/2012) is only made from naturally available soya bean varieties or authorised varieties of genetically modified soya bean (Regulation (EC) No 1829/200311). This is not clearly reflected in the description provided in the EU specifications that “Soya bean oil is exclusively made from strains of soya beans”.
The Panel noted that, according to the EU specifications for TOSOM (E 479b), impurities of the toxic elements arsenic, lead, cadmium and mercury are accepted up to concentrations of 3, 2, 1 and 1 mg/kg, respectively. Contamination at those levels could have a significant impact on the exposure already are close to the health based guidance values or benchmark doses (lower confidence limits) established by EFSA (EFSA CONTAM Panel, 2009a,b, 2010, 2012a,b,c, 2014).
The food additive, mono‐ and diglycerides of fatty acids (E 471) is used in the manufacturing processes for TOSOM (E 479b) as a starting material (90% according to EU specifications). Therefore, the Panel considered the need to include maximum limits for impurities currently included in the EU specifications for the food additive mono‐ and diglycerides of fatty acids (E 471) and recommended by the EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) in its re‐evaluation (EFSA ANS Panel, 2017) in the EU specifications for TOSOM (E 479b).
3.1.3. Manufacturing process
According to industry (EFEMA, 2016 (Documentation provided to EFSA n. X)) for manufacturing TOSOM, refined soya bean oil is charged into a reactor vessel and heated. When the temperature reaches 200°C, the oil is oxidised by pumping air through the oil via a sparging ring located at the bottom of the reactor. The oxidation process continues until the refractive index (nD 40) reaches a value of 1.475–1.485. The airflow is then stopped and the reactor temperature is lowered to 140°C.
In a second step, mono‐ and diglycerides of fatty acids (E 471) of vegetable origin are charged into the reactor to interact with the thermally oxidised soya bean oil. The reaction product is steam‐deodorised under partial vacuum at a temperature of 120–130°C. The reaction product is cooled to 100°C and filtered. At room temperature, the product forms a yellowish solid.
3.1.4. Methods of analysis in food
No specific method of analysis for TOSOM in food was identified.
3.1.5. Stability of the substance, and reaction and fate in food
According to industry (Danisco, 2008 [Doc. provided to EFSA n. 1]), the total shelf life is at least 24 months when the product is stored in unbroken packaging preferably not exceeding 10°C and 80% relative humidity. The product should be kept away from sunlight and odorous products.
No information on the reaction and fate in food for TOSOM was available.
3.2. Authorised use and use level
Currently, TOSOM (E 479b) is an authorised food additive in the EU at 5,000 mg/kg in one single food category as indicated in Table 2. In this document, the maximum level of TOSOM (E 479b) as defined in Annex II to Regulation (EC) No 1333/2008 is named maximum permitted level (MPL).
Table 2.
MPL of TOSOM (E 479b) in one food category according to the Annex II to Regulation (EC) No 1333/2008
| Food category number | Food category name | E‐number/group | Restrictions/exception | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|---|
| 02.2.2 | Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions | E 479b | Only fat emulsions for frying purposes | 5,000 |
MPL: maximum permitted level.
TOSOM (E 479b) is not authorised according to Annex III of Regulation (EC) No 1333/2008.
3.3. Exposure data
3.3.1. Reported use levels or data on analytical levels of TOSOM (E 479b)
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued a public call8 for occurrence data (usage level and/or concentration data) on TOSOM (E 479b). In response to this public call, limited information on the use levels of TOSOM (E 479b) in foods was made available to EFSA by industry. No analytical data on the concentration of TOSOM (E 479b) in foods were made available by the Member States.
Summarised data on reported use levels in foods provided by industry
Industry (EFEMA, 2017) provided EFSA with one use level equal to the MPL (i.e. 5,000 mg/kg). The Panel noted that EFEMA is a food additive producer/chemical supplier, which does not directly use TOSOM (E 479b) as a food additive in foods. EFEMA indicated that the level provided is a recommended level based on the MPL. Thus, only one exposure scenario, the regulatory maximum level exposure assessment scenario, could be performed (Section 3.4).
3.3.2. Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information of over 2.5 million food and beverage products of which more than 1,000,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 20 out of its 28 member countries and Norway presented in the Mintel's GNPD.12
For the purpose of this Scientific Opinion, the Mintel's GNPD13 was used for checking the labelling of food and beverages products, and food supplements for TOSOM (E 479b) within the EU's food market as the database contains the compulsory ingredient information on the label.
According to the Mintel's GNPD, TOSOM (E 479b) was labelled on three foods belonging to the food categories Snacks, Desserts and Cakes between July 2013 and July 2015. The percentages of foods labelled with TOSOM (E 479b) within these three food categories ranged from less than 0.01% to maximally 0.04%. According to the Mintel's GNPD, no new products placed on the market since 2015 have been labelled with TOSOM (E 479b).
3.3.3. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). Consumption surveys added in the Comprehensive database in 2015 were also taken into account in this assessment.9
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database includes the currently best available food consumption data across Europe.
Food consumption data from the following population groups were used for the exposure assessment: infants, toddlers, children, adolescents, adults and the elderly. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 3).
Table 3.
Population groups considered for the exposure estimates of TOSOM (E 479b)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlersa | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrenb | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlyb | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Netherlands, Sweden, UK |
The term ‘toddlers’ in the EFSA Comprehensive Database corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013.
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure estimates. In practice, the FoodEx food codes were matched to the FCS food categories.
Food categories considered for the exposure assessment of TOSOM (E 479b)
The food category in which the use of TOSOM (E 479b) is authorised was selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system) at FoodEx Level 3 (EFSA, 2011b).
The FC 02.2.2 was included in the assessment taking into account the consumption of all kinds of margarines and other fats from mixed origin as recorded in the Comprehensive Database. The restriction for the use of TOSOM (E 479b) within this food category (only fat emulsions for frying purposes) was not considered, as it was not possible to make a distinction between fats used for frying or other purposes. This may have resulted in an overestimation of the exposure to TOSOM (E 479b).
3.4. Exposure to TOSOM (E 479b) from its use as a food additive
The Panel estimated the chronic dietary exposure to TOSOM (E 479b) for the following population groups: infants, toddlers, children, adolescents, adults and the elderly. Dietary exposure to TOSOM (E 479b) was calculated by multiplying MPL of TOSOM (E 479b) for FC 02.2.2 with their respective consumption amount per kilogram body weight for each individual in the Comprehensive Database. The exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only 1 day per subject were excluded as they are considered as not adequate to assess repeated exposure.
This was carried out for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 3). On the basis of these distributions, the mean and 95th percentile of exposure were calculated per survey and per population group. The 95th percentile of exposure was only calculated for those population groups with a sufficiently large sample size (EFSA, 2011a). Therefore, in the present assessment, the 95th percentile of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain were not estimated.
Exposure assessment to TOSOM (E 479b) was carried out by the FAF Panel based on only the MPL as set down in the EU legislation (defined as the regulatory maximum level exposure assessment scenario). A refined exposure assessment scenario was not performed as the only use level provided equalled the MPL.
The regulatory maximum level exposure assessment scenario for TOSOM (E 479b) was based on the MPL as set in Annex II to Regulation (EC) No 1333/2008 and listed in Table 2. The Panel considers the exposure estimates derived following this scenario as the most conservative since it is assumed that the population will be exposed to the food additive present in food at the MPL over a longer period of time.
3.4.1. Dietary exposure to TOSOM (E 479b)
Table 4 summarises the estimated exposure to TOSOM (E 479b) from its use as a food additive in six population groups (Table 3). Detailed results per population group and survey are presented in Appendix A.
Table 4.
Summary of dietary exposure to TOSOM (E 479b) from its use as a food additive in the regulatory maximum level exposure assessment scenario in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| • Mean | < 0.1–3.1 | < 0.1–4.6 | < 0.1–4.4 | < 0.1–2.1 | < 0.1–1.5 | < 0.1–1.9 |
| • 95th percentile | < 0.1–8.6 | 2.1–10.1 | < 0.1–9.8 | < 0.1–4.8 | < 0.1–4.8 | < 0.1–6.6 |
The mean exposure to TOSOM (E 479b) from its use as a food additive ranged from < 0.1 mg/kg bw per day in all populations to 4.6 mg/kg bw per day in toddlers. The 95th percentile of exposure to TOSOM (E 479b) ranged from < 0.1 mg/kg bw per day to 10.1 mg/kg bw per day in toddlers.
As TOSOM (E 479b) is only allowed in FC 02.2.2 ‘Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions’ albeit only in fat emulsions for frying purposes (Table 2), this food category was responsible for 100% of the exposure to this food additive in all population groups.
3.4.2. Uncertainty analysis
Uncertainties in the exposure assessment of TOSOM (E 479b) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 5.
Table 5.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/− |
| Use of data from food consumption surveys covering only a few days to estimate high percentiles (95th) long‐term (chronic) exposure | + |
Concentration data:
|
+ + |
| Food categories selected for the exposure assessment: inclusion of foods without knowing the final use of the fat (frying purposes or not) | + |
| Possible carry‐over from industrial food processing | − |
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
Given the uncertainties listed in Table 5, the Panel considered that the exposure to TOSOM (E 479b) from its use as a food additive according to Annex II was overestimated, mainly due to the use of the MPL and the assumption that all foods belonging to FC 02.2.2 contained TOSOM (E 479b).
3.5. Biological and Toxicological data
The Panel noted that the test material named Homodan MO which has been used in some of the toxicological studies, consists of a mixture of mono‐, di‐ and triglycerides of hydrogenated, higher fatty acids from edible oils, partly inter‐esterified with 10% refined soya‐bean oil, which has been thermally oxidised and deodorised. Although detailed information on the conditions of the oxidation and final reaction steps were missing, the Panel considered Homodan MO likely sufficiently similar to TOSOM (E 479b) and included studies with Homodan MO in the safety assessment of E 479b.
No information on the identity of the test materials, named Homodan MO54 and Homodan M54 used in some toxicological studies (Dam, 1952, Asaes‐Jorgensen, 1954 (Documentation provided to EFSA n. X and X)) were available. Based on available information (Colliopoulos and Yanick, 1976), the Panel considered Homodan PT006 to be different from E 479b. Therefore, the Panel did not use studies with PT006 in the safety assessment of E 479b.
3.5.1. Absorption, distribution, metabolism and excretion
The only study with TOSOM (Homodan MO) Phillips et al. (1978b) was briefly described in the JECFA report (JECFA, 1993); however, this study was not available to the Panel. The conclusion from the JECFA evaluation was that ‘TOSOM is absorbed slightly better than unesterified TOSO [a mixture of two part of soya bean oil and one part of oxidised soya bean oil]’. No information on a possible hydrolysis of TOSOM in the gastrointestinal tract was reported.
3.5.2. Acute toxicity
No data were available.
3.5.3. Short‐term and subchronic toxicity
Pigs
Danish Landrace pigs (n = 4 females per group) were exposed via diet to Homodan MO. The dose levels were 0, 0.4, 2 and 10% (equivalent to 0, 160, 800 or 4,000 mg/kg bw per day) with a duration over 98 days (Gyrd‐Hansen and Rasmussen, 1968). Bodyweights were recorded weekly, blood samples were taken 1 week prior to the start, at the start of the test, and at intervals of 2 weeks. The treatment did not induce any modification in haematological, biochemical and urinary parameters, as well as in body weight and body weight gain. Histopathological examination of heart, aortic arch, liver, kidneys, spleen, duodenum, jejunum, pancreas, thyroid gland, adrenals and hypophysis did not demonstrate any adverse effects.
3.5.4. Genotoxicity
No data were available.
3.5.5. Chronic toxicity and carcinogenicity
Rats
The Panel noted that two studies (Harmsen, 1959, 1960 as referred to by JECFA, 1972) were described in the JECFA monograph (1972). These studies were not available to the Panel and very limited details were provided in the JECFA monograph. Therefore, the Panel could not use these studies for hazard characterisation of E 479b. Another study by Harmsen (1961, 1954 (Documentation provided to EFSA n. X)) was also described in JECFA monograph; however from the description of the study available to the Panel, this study was not relevant for this endpoint.
In a study performed with a protocol essentially compliant with the OECD Guideline for the study of carcinogenicity, groups of 60 Wistar rats of each sex were fed diets containing 0, 3, 6 or 12% of the margarine emulsifier TOSOM14 for 2.5 years (Gry et al., 1987 (Documentation provided to EFSA n. X); Meyer et al., 1993). The calculated daily intake was 0, 1,300, 2,700 or 5,400 mg/kg bw per day in males and 1,800, 3,600 or 7,400 mg/kg bw per day in females. Groups of 120 rats of each sex fed a diet containing mono‐ and diglycerides served as controls. The diets given to all groups were isocaloric. Clinical appearance, food consumption, body weight and weight gain, survival, haematology and clinical chemistry parameters were examined. No urinalysis was conducted. Gross and histopathological examinations, including neoplastic and non‐neoplastic lesions, were performed on all groups. Time to occurrence of tumours was recorded. Among females dosed with TOSOM (all dose levels), a tendency to a slightly prolonged survival compared with the controls was observed; however, this finding was not dose dependent. The survival of animals in all the groups at 24 months was more than 50% and more than 25% at termination of the study (31 months). The haematological and clinical chemical results did not indicate any changes that could be attributed to treatment. The non‐neoplastic lesions seen were changes normally seen in ageing rats and they were evenly distributed among all groups. Histopathological examination revealed various tumours in both the control and the dosed groups. The authors concluded that TOSOM did not induce chronic or carcinogenic effects. The Panel agreed with this conclusion and identified a NOAEL of 5,400 and 7,400 mg/kg bw per day, in males and females respectively, the highest dose tested.
3.5.6. Reproductive and developmental toxicity
Reproductive toxicity studies
In a two‐generation reproductive toxicity study in Wistar rats, the animals were dosed via diet with Homodan MO at an average level of 10% (equivalent to 5,000 mg/kg bw per day) (Harmsen, 1961 (Documentation provided to EFSA n. X)). The information in the summary report is poor; there was no information on the number of animals per dose group and no information on reproductive data. Therefore, the Panel was unable to use this study for hazard characterisation.
Developmental studies
No data available.
3.5.7. Hypersensitivity, allergenicity and food intolerance
No data were available.
3.5.8. Studies with other emulsifiers
TOSOM (E 479b) is included in the list of EFEMA index of food emulsifiers (EFEMA, 2015).
In several recent studies, some other emulsifiers have been reported to alter the gut microbiota, to promote gut inflammation, obesity and to impair glycaemic control (Swidsinski et al., 2009a,b; Renz et al., 2012; Merga et al., 2014; Cani and Everard, 2015; Chassaing et al., 2015; Romano‐Keeler and Weitkamp, 2015; Lecomte et al., 2016; Chassaing et al., 2017; Nejrup et al., 2017; Shah et al., 2017; Jiang et al., 2018; Holder and Chassaing, 2018; Viennois and Chassaing, 2018). The Panel noted that, even though some of these effects are not systematically studied in toxicity studies performed according to toxicity testing guidelines, they would be investigated on a case‐by‐case basis if indicated by the results of the general toxicity testing as recommended in the Guidance for submission of food additives (EFSA ANS Panel, 2012). The Panel considered that additional studies would be needed to show the relevance of the effects seen in mice for human health and if salts of fatty acids can induce such effects.
3.6. Discussion
TOSOM (E 479b) is defined as a complex mixture of esters of glycerol and fatty acids found in edible fat and fatty acids from thermally oxidised soy bean oil. The Panel noted that the soya bean oil used to produce E 479b (in line with the specifications in Regulation (EU) No 231/2012) is only be made from naturally available soya bean varieties or authorised varieties of genetically modified soya bean (Regulation (EC) No 1829/2003).
The food additive, mono‐ and diglycerides of fatty acids (E 471), is used in the manufacturing processes for TOSOM (E 479b) as a starting material (90% according to EU specifications). Therefore, there is a need to include maximum limits for impurities currently included in the EU specifications for the food additive mono‐ and diglycerides of fatty acids (E 471) and recommended by the ANS Panel in its re‐evaluation (EFSA ANS Panel, 2017) in the EU specifications for TOSOM (E 479b).
The Panel noted that the SCF and the JECFA derived from the same study an ADI of 25 and 30 mg/kg bw per day, respectively, but starting with different NOAELs and applying different uncertainty factors. Not all of the unpublished reports cited in the evaluations by SCF and the JECFA were available to the Panel.
The Panel noted that there was no reliable information regarding the ADME for TOSOM.
No adverse effects have been detected in a limited subchronic toxicity study in pigs up to the higher dose of 4,000 mg/kg bw per day.
No genotoxicity data on TOSOM (E 479b) were available.
A chronic and carcinogenicity study performed in rats did not show any carcinogenic potential. The Panel noted that histopathological examination was conducted in a range of organs including reproductive organs and the incidence of non‐neoplastic lesions was evenly distributed among all groups. In addition, no changes were observed in mortality, clinical, haematological or biochemical parameters. In this study, there was no urinalysis. The Panel identified a NOAEL of 5,400 and 7,400 mg/kg bw per day in males and females, respectively, the highest dose tested.
The Panel considered that some of the potential by‐products, which may result from the manufacturing process of TOSOM, could be reactive to DNA. Such by‐products (e.g. epoxides) being highly reactive are not expected to be systemically available; however, they could have local effects at the first site of contact in the gastrointestinal tract. However, the Panel noted that the maximum permitted level for epoxides in the food additive E479b according to its specifications is very low and that even fully epoxidised soya bean oil did not show genotoxic potential (EFSA, 2004). Moreover, the lack of carcinogenic effect in the chronic and carcinogenicity study with a test material complying with the EU specifications for the food additive E479b alleviated the concern for genotoxicity at the first site of contact.
The Panel noted that no reliable studies for reproductive toxicity were available; however, no lesions in reproductive organs were described in the 2.5‐year chronic and carcinogenicity study in rats. Furthermore, no developmental toxicity studies were available.
The toxicological data that were available to the Panel were insufficient to support the current ADI, in particular, due to the lack of ADME data and the absence of developmental toxicity studies.
TOSOM (E 479b) is only authorised in FC 02.2.2 ‘Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions’ according to Annex II to Regulation (EC) No 1333/2008 (Table 2).
The Panel could only estimate the exposure to TOSOM (E 479b) according to the regulatory maximum level exposure assessment scenario, as the only reported use level equalled the MPL (Section 3.3.1). According to this scenario, the high (P95) exposure reached an upper value of 10.1 mg/kg bw per day for toddlers. The Panel also noted that according to Mintel's GNPD, only a few products were labelled with TOSOM (E 479b) over the last 5 years, and that since 2015 no new product launches contained this food additive according to the product label. Overall, the Panel considered that the exposure to TOSOM (E 479b) was overestimated (Section 3.4).
The Panel noted that when comparing the highest estimated exposure of 10 mg/kg bw per day in toddlers with the NOAEL of 5,400 mg/kg bw per day (the highest dose tested) identified from the chronic toxicity and carcinogenicity study in rats, the margin of safety (MoS) would be 540. Based on this MoS, the Panel considered that it is very likely that the use of TOSOM (E 479b) is of no safety concern, in particular when considering the limited current use of this food additive.
4. Conclusions
The Panel concluded that the toxicological data available were insufficient to support the current ADI for which ADME and developmental toxicological studies would be required.
Taking into account the single authorised use of this food additive, the overestimation of the exposure estimate and the absence of adverse effects reported in the highest dose tested in the combined chronic carcinogenicity study, the Panel also concluded that the calculated MoS of 540 indicated that the use of TOSOM (E 479b) is of no safety concern.
5. Recommendations
The Panel recommended that:
the European Commission considers lowering the current limits for toxic elements (arsenic, lead, mercury and cadmium) in the EU specifications for TOSOM (E 479b) in order to ensure that the food additive will not be a significant source of exposure to these toxic elements in food.
the European Commission considers revising the EU specifications for TOSOM (E 479b) including maximum limits for impurities currently included in the EU specifications for the food additive mono‐ and diglycerides of fatty acids (E 471) and recommended by the Panel in the re‐evaluation of E 471 (EFSA ANS Panel, 2017).
the European Commission considers revising the description provided in the EU specifications for ‘Soya bean oil is exclusively made from strains of soya beans’ indicating that the soya bean oil used to produce E 479b is exclusively made from naturally available soya bean varieties or authorised varieties of genetically modified soya bean (Regulation (EC) No 1829/2003).
Documentation provided to EFSA
Aaes‐Jorgensen E, Funch JP, Engel PF and Dam H, 1954. Growth experiments on rats fed on marganite. Submitted by Dupont, March 2018.
Dam H, 1952. Report from Dam H and Engelbreth‐Holm S to Herlow A (Grindstedvaerket). Submitted by Dupont, March 2018
Danisco, 2008. Product Description PD 147‐12.5EN. Material No. 101420. 3p. Submitted by EFEMA on February 2011.
EFEMA (European Food Emulsifier Manufacturers’ Association), 2009. EFEMA index of food emulsifiers. September 2009, 5th Edition. Submitted by EFEMA, January 2011.
EFEMA (European Food Emulsifier Manufacturers’ Association), 2016. Document on thermally oxidized soya bean oil interacted with mono‐ and diglycerides of fatty acids E 479b. Manufacturing process for the food additive including information on the sources used. Submitted by EFEMA on August 2016.
EFEMA (European Food Emulsifier Manufacturers’ Association), 2017. Data on use levels of thermally oxidised soya bean oil interacted with mono‐ and diglycerides of fatty acids E 479b in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 23 October 2017.
EFEMA (European Food Emulsifier Manufacturers’ Association), 2018. Communication from EFEMA following a request by EFSA. 14th May 2018.
Gry J, Bille N, Kristiansen E, Madsen C, Meyer O, Olsen P, Roswall K, Thorup I and Wurtzen G, 1987. Thermally oxidized soya‐bean oil interacted with mono‐ and diglycerides of food fatty acids (Esters of glycerol and thermally oxidized soya bean fatty acids). A long‐term study in rats. Report of the Institute of Toxicology of the National Food Agency of Denmark. Submitted by Dupont, March 2018.
Harmsen H, 1961. Report from Dr. Harmsen to Herlow A (Grindstedvaerket). Biological examination of the emulsifier HOMODAN MO with reference to carcinogenous effects, if any. Submitted by Dupont, March 2018.
Kemper F, 1981. Bericht. Über untersuchungen sur subchronichen (91 tage) oralen toxizität von “CARLO”, trenn‐emulsion, an ratten. 31 October 1981. Submitted by Palsgaard, March 2018.
Phillips JC, Topp CE, Cook M and Gangolli SD 1978a. The metabolic disposition of 14C‐labelled soyabean oil and 14C‐labelled HOMODAN O in the rat, guinea‐pig and mouse. Project No. 185/2 from the British Industrial Biological Research Association. Submitted by Dupont, March 2018.
Pre‐evaluation document. Fraunhofer finalised on 31 March 2014.
Abbreviations
- ADME
absorption, distribution, metabolism, excretion
- ADI
acceptable daily intake
- ANS
EFSA Panel on Food Additives and Nutrient Sources added to Food
- bw
body weight
- CAS
Chemical Abstracts Service
- EFEMA
European Food Emulsifiers Manufacturers Association
- EINECS
European Inventory of Existing Commercial Chemical Substances
- FAF
EFSA Panel on Food Additives and Flavourings
- FC
food category
- FCS
food categorisation system
- GNPD
Global New Products Database
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- MPL
maximum permission limit
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- QS
quantum satis
- SCF
Scientific Committee on Food
- TemaNord
is a publishing series for results of the often research‐based work that working groups or projects under Nordic Council of Ministers have put in motion
- TOSOM
thermally oxidised soya bean oil interacted with mono‐ and diglycerides of fatty acids
- WHO
World Health Organization
Appendix A – Summary of total estimated exposure of TOSOM (E 479b) from its use as a food additive for the regulatory maximum level exposure scenario per population group and survey: mean and 95th percentile (mg/kg bw per day)
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section):
Supporting information
Summary of total estimated exposure of TOSOM (E 479b) from its use as a food additive for the regulatory maximum level exposure scenario per population group and survey: mean and 95th percentile (mg/kg bw per day)
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Frutos Fernandez MJ, Fürst P, Gürtler R, Gundert‐Remy U, Husøy T, Mennes W, Moldeus P, Oskarsson A, Rainieri S, Shah R, Waalkens‐Berendsen DH, Wölfle D, Boon P, Parent‐Massin D, Tobback P, Wright M, Chrysafidis D, Rincon AM, Tard A and Lambré C, 2018. Scientific Opinion on the re‐evaluation of oxidised soya bean oil interacted with mono‐ and diglycerides of fatty acids (E 479b) as a food additive. EFSA Journal 2018;16(10):5420, 20 pp. 10.2903/j.efsa.2018.5420
Requestor: European Commission
Question number: EFSA‐Q‐2011‐00567
Panel members: Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Rainer Gürtler, Ursula Gundert‐Remy, Trine Husøy, Wim Mennes, Peter Moldeus, Agneta Oskarsson, Sandra Rainieri, Romina Shah, Dina Hendrika Waalkens‐Berendsen, Detlef Wölfle and Maged Younes.
Acknowledgements: The Panel wishes to thank the Working Group on the re‐evaluation of food additives other than gums and colours of the former EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) for the preparatory work on this scientific output, in particular Pasquale Mosesso and Rudolf Antonius Woutersen. The FAF Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 11 September 2018
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
COM(2001) 542 final.
Food Additives in Europe 2000, Status of safety assessments of food additives presently permitted in the EU, Nordic Council of Ministers, TemaNord 2002, 560.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents. Published: 22 November 2009. Available from: http://www.efsa.europa.eu/en/dataclosed/call/ans091123
Call for technical data on salts of fatty acids (E470a,b) and related food additives authorised in the EU. Published: 19 January 2016. Available online: http://www.efsa.europa.eu/sites/default/files/consultation/160119.pdf
Call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 6). Published 23 February 2017. Available online: http://www.efsa.europa.eu/sites/default/files/consultation/170223.pdf
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
SciFinder® the choice for chemistry researchTM.
Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed. OJ L 261, 18.10.2003.
Missing Bulgaria, Cyprus, Estonia, Latvia, Lithuania, Luxembourg, Malta and Slovenia.
http://www.gnpd.com/sinatra/home accessed on 19/6/2018.
The chemical analysis reported in the study report by Gry et al. (1987 (Documentation provided to EFSA n. X)) showed that the definition of the test material as well as the purity, apart from the maximum levels for some toxic elements, comply with the current EU specifications.
References
- Cani PD and Everard A, 2015. Keeping gut lining at bay: impact of emulsifiers. Trends in Endocrinology and Metabolism, 26, 273–274. [DOI] [PubMed] [Google Scholar]
- Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE and Gewirtz AT, 2015. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature, 519, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, de Wiele TV, De Bodt J, Marzorati M and Gewirtz AT, 2017. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colliopoulos JA and Yanick NS, 1976. Vegetable oil emulsion. United States Patent 3,966,632. June 29, 1976.
- EFEMA , 2015. Available online: http://www.emulsifiers.org/files/Index_of_food_emulsifiers_7th%20Edition_FINAL.pdf
- EFSA (European Food Safety Authority), 2004. Scientific opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a request from the Commission related to the use of epoxidised soyabean oil in food contact materials. EFSA Journal 2004;64, 1–17. 10.2903/j.efsa.2004.64 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Scientific opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Boon P, Chrysafidis D, Gürtler R, Mosesso P, Tobback P, Horvath Z, Rincon AM and Lambré C, 2017. Scientific opinion on the re‐evaluation of mono‐ and di‐glycerides of fatty acids (E 471) as a food additive. EFSA Journal 2017;15(11):5045, 27 pp. 10.2903/j.efsa.2017.5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009a. Scientific opinion on cadmium in food. EFSA Journal 2009;7(10):980, 139 pp. 10.2903/j.efsa.2009.98 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009b. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351, 199 pp. 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012a. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 241 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012b. Scientific Opinion on lead dietary exposure in the European population. EFSA Journal 2012;10(7):2831, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012c. Scientific Opinion on cadmium dietary exposure in the European population. EFSA Journal 2012;10(1):2551, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on dietary exposure to inorganic arsenic in the European population. EFSA Journal 2014;12(3):3597, 68 pp. 10.2903/j.efsa.2014.3597 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- Gyrd‐Hansen N and Rasmussen F, 1968. Short‐term feeding study of the emulsifier Homodan MO in pigs. Food and Cosmetics Toxicology, 6, 163–169. [DOI] [PubMed] [Google Scholar]
- Harmsen H, 1959. Unpublished report submitted by Grindstedvaerket Laboratoriet, as referred to by JECFA, 1972.
- Harmsen H, 1960. Unpublished report submitted by Grindstedvaerket Laboratoriet, as referred to by JECFA, 1972.
- Holder MK and Chassaing B, 2018. Impact of food additives on the gut‐brain axis. Physiology and Behavior, 192, 173–176. 10.1016/j.physbeh.2018.02.025 [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1972. Toxicological evaluation of some enzymes, modified starches and certain other substances. Esters of glycerol and thermally oxidized soy bean fatty acids. WHO Food Additives Series, No. 1. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1992. Evaluation of certain food additives and naturally occurring toxicants. Thirty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization Technical Report Series, 828. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1993. Thermally oxidized soya bean oil and thermally oxidized soya bean oil interacted with mono‐ and di‐glycerides of fatty acids. First draft prepared by B Priestly. Food Additives Series 30, JECFA 39/3.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Monograph 1. C. Prepared at the 61st JECFA (2003) and published in FNP 52 Add 11 (2003). Online Edition “Combined compendium of food additive specifications”. Available online: http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/en/
- Jiang Z, Zhao M, Zhang H, Li Y, Liu M and Feng F, 2018. Antimicrobial emulsifier‐ glycerol monolaurate induces metabolic syndrome, gut microbiota dysbiosis and systemic low‐grade inflammation in low‐fat diet fed mice. Molecular Nutrition and Food Research, 1–11. [DOI] [PubMed] [Google Scholar]
- Lecomte M, Couedelo Leslie, Meugnier E, Plaisancie P, Letisse M, Bérengère B, Gabert L, Penhoat A, Durand A, Pineau G, Joffre F, Géloën A, Vaysse C, Laugerette F and Michalski MC, 2016. Dietary emulsifiers from milk and soybean differently impact adiposity and inflammation in association with modulation of colonic goblet in high‐fat fed mice. Molecular Nutrition and Food Research, 60, 606–620. [DOI] [PubMed] [Google Scholar]
- Merga Y, Campbell BJ and Rhodes JM, 2014. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Digestive Diseases, 32, 475–483. [DOI] [PubMed] [Google Scholar]
- Meyer O, Kristianse E, Gry J, Madsen C, Olsen P and Thrup I, 1993. Carcinogenicity study of the emulsifier TOSOM and the release agent TOS in Wistar rats. Food Chemical Toxicology, 31, 825–833. [DOI] [PubMed] [Google Scholar]
- Nejrup RG, Licht TR and Hellgren LI, 2017. Fatty acid composition and phospholipid types used in infant formulas modifies the establishment of human gut bacteria in germ‐free mice. Scientific Reports, 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JC, Topp CE, Cook M and Gangolli SD, 1978b. The metabolic disposition of 14C‐labelled soyabean oil and 14C‐labelled EMULSIFIER MO in the rat, guinea‐pig and mouse. Unpublished Report No. 185/1 from BIBRA. Submitted to WHO through the National Food Agency, Denmark, by Grindstedvaerket A/S, Brabrend, Denmark, as referred to by JECFA, 1993.
- Renz H, Brandtzaeg P and Hornef M, 2012. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nature Reviews‐Immunology, 12, 9–23. [DOI] [PubMed] [Google Scholar]
- Romano‐Keeler J and Weitkamp JH, 2015. Maternal influences on fetal microbial colonization and immune development. Pediatric Research, 77, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food), 1989. Food science and techniques. Reports of the Scientific Committee for Food. Twenty‐first Series. Report of the Scientific Committee for food on emulsifiers, stabilizers, thickeners and gelling agents. Opinion expressed 11 November 1988.
- SCF (Scientific Committee for Food), 2001. Guidance on submissions for food additive evaluations by the Scientific Committee on Food. SCF/CS/ADD/GEN/26 Final. 12 July 2001.
- Shah R, Kolanos R, DiNovi MJ, Mattia A and Kaneko KJ, 2017. Dietary exposures for the safety assessment of seven emulsifiers commonly added to foods in the United States and implications for safety. Food Additives and Contaminants: Part A, 34, 905–917. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Loening‐Baucke V and Herber A, 2009a. Mucosal flora in Crohn's disease and ulcerative colitis ‐ an overview. Journal of Physiology and Pharmacology, 60(Supplement 6), 61–71. [PubMed] [Google Scholar]
- Swidsinski A, Ung V, Sydora BC, Loening‐Baucke V, Doerffel Y, Verstraelen H and Fedorak RN, 2009b. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflammatory Bowel Diseases, 15, 359–364. [DOI] [PubMed] [Google Scholar]
- TemaNord (Nordic Council of Ministers), 2002. E 479b Thermally oxidised soya bean oil interacted with mono‐ and diglycerides of fatty acid (TOSOM). Food Additives in Europe 2000 ‐ Status of safety assessments of food additives presently permitted in the EU.
- Viennois E and Chassaing B, 2018. First victim, later aggressor: how the intestinal microbiota drives the pro‐inflammatory effects of dietary emulsifiers? Gut Microbes, 13: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of total estimated exposure of TOSOM (E 479b) from its use as a food additive for the regulatory maximum level exposure scenario per population group and survey: mean and 95th percentile (mg/kg bw per day)


