Abstract
Following a request from the European Commission, the EFSA Panel on Plant Health performed a pest categorisation of Cronartium harknessii, Cronartium kurilense and Cronartium sahoanum, which are well‐defined and distinguishable tree fungal pathogens of the family Cronartiaceae. In 2018, these species were moved from the genus Endocronartium to the genus Cronartium. These pathogens are not known to be present in the EU and are regulated in Council Directive 2000/29/EC (Annex IAI) (as non‐European Endocronartium spp.) as harmful organisms whose introduction into the EU is banned. These three fungi are autoecious rusts completing their life cycle on Pinus spp. C. harknessii is known as the western gall rust or pine‐pine gall rust in North America (Canada, the USA and Mexico). C. kurilense and C. sahoanum are reported from Russia (North Kuril Islands) and Japan. The pathogens could enter the EU via host plants for planting and cut branches. The pathogens could establish in the EU, as climatic conditions are favourable and Pinus spp. are common. The pathogens would be able to spread following establishment by movement of host plants for planting and cut branches, as well as natural spread. Should these pathogens be introduced in the EU, impacts can be expected on pine forests, plantations, ornamental trees and nurseries. The pathogens cause formation of stem galls, which kill young trees and result in stem defect in older trees. The main knowledge gap concerns the limited available information on C. kurilense and C. sahoanum compared to C. harknessii. The criteria assessed by the Panel for consideration of C. harknessii, C. kurilense and C. sahoanum as potential quarantine pests are met, whilst, for regulated non‐quarantine pests, the criterion on the pest presence in the EU is not met.
Keywords: European Union, forest pathology, Peridermium spp., pest risk, plant pest, quarantine, tree health
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorizations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/ pest categorisation is not available.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/20023, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pests categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases are the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocanthus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Endocronartium spp. (non‐EU) is one of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest (RNQP) for the area of the EU.
The term ‘non‐EU’ species is interpreted to imply that the EU is not (or is not known to be) part of the native range of that species. At the time the mandate was received, two species were described within the genus according to Index Fungorum (http://www.indexfungorum.org) (Endocronartium harknessii and Endocronartium sahoanum, of which two different variants were distinguished (var. sahoanum and var. hokkaidoense)). Both species were considered to be non‐EU as the EU is not or is not known to be part of their native range. The two species were moved to the genus Cronartium by Aime et al. (2018), who also split the E. sahoanum species into two species: Cronartium kurilense and Cronartium sahoanum. As this taxonomic revision has been accepted by Index Fungorum, this pest categorisation will thus deal with Cronartium harknessii, Cronartium kurilense and Cronartium sahoanum.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on Endocronartium spp. and the non‐EU species of the genus, i.e. Endocronartium harknessii and Endocronartium sahoanum, was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pests as search term. Relevant papers were reviewed and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plan Protection Organization (EPPO) Global Database (EPPO, 2018) and relevant publications.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTE) of the European Commission, and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States (MS) and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for C. harknessii, C. kurilense and C. sahoanum following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018) and as defined in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
In accordance with the guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018), this work was started following an evaluation of the EU plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union RNQP in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required in accordance with the specific terms of reference received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a RNQP. If one of the criteria is not met, the pest will not qualify. A pest that does not qualify as a quarantine pest may still qualify as a RNQP that needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone; thus, the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism. | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pest must be present in the risk assessment area) |
| Regulatory status (Section 3.3) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future |
The protected zone system aligns with the pest free area system under the International Plant Protection Convention (IPPC). The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone) |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, whereas addressing social impacts is outside the remit of the Panel, in agreement with the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
Yes, the identity of the pest is established.
Endocronartium is a fungal genus of the family Cronartiaceae. At the time the request to conduct a pest categorisation of non‐EU Endocronartium species was received by EFSA, there were two species described within the genus (http://www.indexfungorum.org). Both species were considered to be non‐EU as the EU is not or is not known to be part of their native range:
Endocronartium harknessii – synonyms: Peridermium harknessii, Peridermium cerebroides, Cronartium harknessii (http://www.indexfungorum.org; Farr and Rossman, 2018).
Endocronartium sahoanum, which occurred in two different variants (var. sahoanum and var. hokkaidoense) (http://www.indexfungorum.org).
There were two other species not assigned to the Endocronartium genus that have Endocronartium as synonym and are thus not considered in this pest categorisation:
Peridermium yamabense (synonym Endocronartium yamabense (http://www.indexfungorum.org; Farr and Rossman, 2018)).
Cronartium pini (synonyms: Endocronartium pini and Peridermium pini). In addition, this species is native to the EU (Pretzsch, 1986).
The genus Endocronartium appeared not to be monophyletic (Hiratsuka, 1969), which implied that there could be further taxonomic revision of the genus. Indeed, Endocronartium species were moved to the genus Cronartium, i.e. Endocronartium harknessii (renamed as Cronartium harknessii), Endocronartium sahoanum var. hokkaidoense (renamed as Cronartium kurilense) and E. sahoanum var. sahoanum (renamed as Cronartium sahoanum) (Aime et al., 2018). As these proposals have been accepted by Index Fungorum, the Panel followed the proposals of Aime et al. (2018) and performed a pest categorisation of C. harknessii, C. kurilense and C. sahoanum (see Section 1.2).
3.1.2. Biology of the pest
C. harknessii, C. kurilense and C. sahoanum are autoecious rusts able to complete their life cycle on Pinus species (Hiratsuka, 1969).
C. harknessii, which is the best described of the three species, is known as the western gall rust or pine‐pine gall rust in North America (Sinclair and Lyon, 2005). The hosts are two‐ and three‐needle Pinus spp. (EPPO, 1997). Spores are produced in the spring during the shoot elongation period, normally on branch galls, rarely on stem cankers, 2–4 years after infection (EPPO, 1997; Sinclair and Lyon, 2005). The spores have the morphological characteristics of aeciospores but germinate and function like teliospores (EPPO, 1997).
The airborne spores infect wet, soft current year stems (Sinclair and Lyon, 2005) and/or the cone flowers (Hoffman and Hagle, 2011). The fungus penetrates the cuticle and epidermal cells, grows within the cambial tissue and colonises living host tissues (Adams, 1997; Hoffman and Hagle, 2011).
Stem swelling occurs later in the same growing season or during the spring of the following year (Sinclair and Lyon, 2005). Galls are globose, pear‐shaped or elongated, woody, and typically grow until a diameter of 1–10 cm (Sinclair and Lyon, 2005). Infections can live for many years but eventually lose the ability to produce spores (Adams, 1997). The fungus does not spread beyond the gall (Hoffman and Hagle, 2011). Aecia and aeciospores are produced every spring typically for less than ten years, occasionally for longer (rarely >100 years; Sinclair and Lyon, 2005).
The optimum temperature for germination and infection ranges between 15 and 28°C, but this optimal range may be as wide as 10–30°C (Sinclair and Lyon, 2005). Weather conducive to disease is described as prolonged periods of cool and wet weather during shoot elongation (Adams, 1997). Infection is more common near waterbodies and wet areas (Hoffman and Hagle, 2011). Pine gall rust infection does not occur uniformly year after year: there are occasional years with heavy infection promoted by optimal conditions for both pathogen sporulation and host infection (Adams, 1997).
Infections are mostly found within 3–6 m from the ground in some pine species (e.g. Pinus sylvestris) while others may be infected as tall mature trees (e.g. Pinus ponderosa; Adams, 1997). There are also reports that infection is most common in the lower third part of the crown (Hoffman and Hagle, 2011).
The infection rate of Pinus contorta in British Columbia was not found to be significantly affected by stand density (Van der Kamp and Spence, 1987).
3.1.3. Intraspecific diversity
Spore source provenance of C. harknessii was found to account for 14% of the variation in the susceptibility of lodgepole pine provenances to western gall rust (Van der Kamp, 1988).
3.1.4. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, detection and identification methods are available.
The disease caused by C. harknessii can be identified based on the symptoms and morphological characteristics described (EPPO, 1997 and references therein).
C. harknessii can be identified using a molecular‐based protocol, but without being able to distinguish it from Cronartium quercuum f. sp. fusiforme (Ramsfield and Vogler, 2010). Isozyme and protein pattern analysis was found to be able to differentiate C. harknessii from other Cronartium spp. including C. quercuum (Powers et al., 1989).
A morphological description of C. sahoanum is available in Imazu et al. (1989). Features of C. harknessii in axenic culture have been described by Lundquist et al. (1994). Aeciospores of C. harknessii could be distinguished from those of Cronartium coleosporioides, Cronartium comandrae, Cronartium comptoniae and Cronartium ribicola under scanning electron microscope (Hiratsuka, 1971).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
C. harknessii is reported only from North America (Figure 1; EPPO, 2018). In Canada, the pathogen is reported as widespread (EPPO, 2018). In the USA, the pathogen is found in a large part of the country except in the south‐eastern states (EPPO, 2018). C. harknessii is also reported from Mexico (EPPO, 2018).
Figure 1.
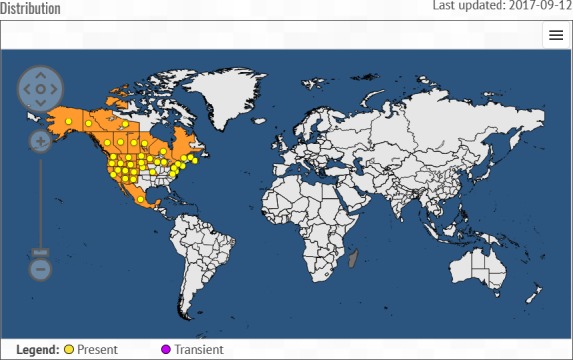
Global distribution map for C. harknessii (extracted from the EPPO Global Database, accessed May 2018). There are no reports of transient populations for this species. Note that the distribution of Cronartium kurilense and Cronartium sahoanum is not included in the map
C. kurilense and C. sahoanum are reported from Russia (North Kuril Islands, Kamchatka region) and Japan (Imazu et al., 2000).
In Japan, C. sahoanum is only known to occur in the mountainous areas of Tohoku and eastern⁄central Hokkaido (Kim et al., 2010).
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
No, the pests are not reported to be present in the EU.
None of these pathogens has been reported to be present in the EU. C. harknessii is reported as absent in Slovenia (no pest record, 2017) (EPPO, 2018) and in the UK (UK Plant Health Register, accessed May 2018, based on surveillance, https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=11787). With this exception, there are no reports of absence available to the Panel that have been confirmed by survey.
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
These pathogens are listed in Council Directive 2000/29/EC as non‐European Endocronartium spp. Details are presented in Tables 2 and 3.
Table 2.
Endocronartium spp. (non‐EU) in Council Directive 2000/29/EC
| Annex I, Part A | Harmful organisms whose introduction into, and spread within, all Member States shall be banned |
| Section I | Harmful organisms not known to occur in any part of the Community and relevant for the entire Community |
| (c) | Fungi |
| Species | |
| 4. | Endocronartium spp. (non‐European) |
3.3.2. Legislation addressing the hosts of Endocronartium spp. (non‐EU)
Table 3.
Regulated hosts and commodities that may involve Endocronartium spp. (non‐EU) in Annexes III, IV and V of Council Directive 2000/29/EC
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all Member States | |
| Description | Country of origin | |
| 1. | Plants of Abies Mill., Cedrus Trew, Chamaecyparis Spach, Juniperus L., Larix Mill., Picea A. Dietr., Pinus L., Pseudotsuga Carr. and Tsuga Carr., other than fruit and seeds | Non‐European countries |
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the Community, before being moved within the Community—in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community | |
| Part A | Plants, plant products and other objects originating in the Community | |
| Section II | Plants, plant products and other objects produced by producers whose production and sale is authorised to persons professionally engaged in plant production, other than those plants, plant products and other objects which are prepared and ready for sale to the final consumer, and for which it is ensured by the responsible official bodies of the Member States, that the production thereof is clearly separate from that of other products | |
| 1.1. | Plants of Abies Mill., Larix Mill., Picea A. Dietr., Pinus L. and Pseudotsuga Carr. | |
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
The hosts of C. harknessii, C. kurilense and C. sahoanum belong to the genus Pinus.
Pinus banksiana, P. contorta and P. ponderosa are reported as major hosts of C. harknessii in North America (EPPO, 1997, 2018). Other North American Pinus species such as Pinus attenuata, Pinus muricata and Pinus radiata are also recorded as hosts (Old, 1981; EPPO, 1997).
The European species P. sylvestris is reported as susceptible to C. harknessii (Adams, 1997; EPPO, 2018) and Pinus halepensis, Pinus mugo and Pinus nigra have all been found to be infected in North America (Ziller, 1970; EPPO, 1997).
C. kurilense and C. sahoanum have been reported on Pinus pumila, Pinus strobiformis and Pinus strobus (Farr and Rossman, 2018).
Due to the autoecious nature of these fungi, there are no alternate hosts (EPPO, 1997).
In Council Directive 2000/29/EC, these pests are not regulated on a particular host or commodity; their introduction into the EU is banned (Annex IAI).
3.4.2. Entry
Is the pest able to enter into the EU territory? If yes, identify and list the pathways!
Yes, these fungi could enter the EU via host plants for planting and cut branches.
Host commodities on which the pathogens could enter the EU territory are (EPPO, 1997, 2018):
plants for planting of Pinus spp.
cut branches of Pinus spp.
The pathogens may be transported with plants for planting, as observed in the USA (EPPO, 2018). C. harknessii has a long incubation period and latent infection – it may therefore remain undetected (EPPO, 1997). The aeciospores have a relatively good ability to survive in an airborne state (and thus would have considerable potential for long‐distance spread), although spore longevity decreases with increasing temperature and humidity (Chang and Blenis, 1989).
Non‐squared wood is listed as pathway of entry in EPPO (2018) and isolated bark is mentioned in EPPO (1997). However, no reference was provided to support this. Since these fungi are biotrophs and require live host tissue, they would presumably not survive long in wood and bark after harvest. Hoffman and Hagle (2011) state that since the pathogen requires live host tissue to survive, it is not necessary to dispose of infected trees or branches after cutting.
The pathways plants for planting and cut branches of Pinus spp. are regulated due to the ban on importing plants of Pinus spp., other than fruit and seeds, from non‐European countries (see Section 3.3.2).
There is no reported risk associated with movement of seeds or pollen (EPPO, 1997; Ramsfield et al., 2007).
As of May 2018, there were no records of interception of Endocronartium spp. in the Europhyt database.
3.4.3. Establishment
Is the pest able to become established in the EU territory?
Yes, the pathogens could establish in the EU, as hosts are common and climatic conditions are favourable. Moreover, these species do not require the presence of alternate hosts to complete their life cycle.
3.4.3.1. EU distribution of main host plants
C. harknessii, C. kurilense and C. sahoanum can infect a wide range of Pinus spp. (Section 3.4.1). Pine species are widely distributed in the EU (Figure 2).
Figure 2.
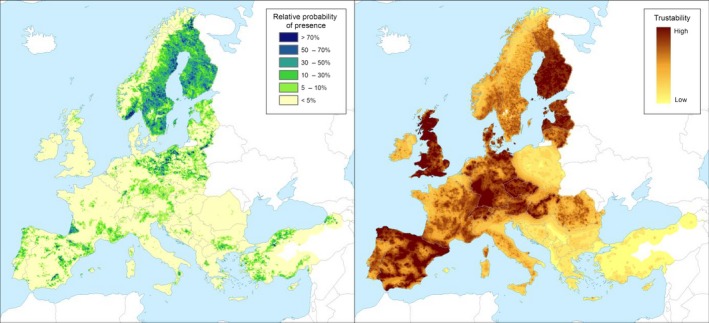
Left‐hand panel: Relative probability of presence (RPP) of the genus Pinus (based on data from the species: P. sylvestris, P. pinaster, P. halepensis, P. nigra, P. pinea, P. contorta, P. cembra, P. mugo, P. radiata, P. canariensis, P. strobus, P. brutia, P. banksiana, P. ponderosa, P. heldreichii, P. leucodermis and P. wallichiana) in Europe, mapped at 100 km2 pixel resolution. The underlying data are from European‐wide forest monitoring data sets and from national forestry inventories based on standard observation plots measuring in the order of hundreds m2. RPP represents the probability of finding at least one individual of the taxon in a standard plot placed randomly within the grid cell. For details, see Appendix A (courtesy of JRC, 2017). Right‐hand panel: Trustability of RPP. This metric expresses the strength of the underlying information in each grid cell and varies according to the spatial variability in forestry inventories. The colour scale of the trustability map is obtained by plotting the cumulative probabilities (0–1) of the underlying index (for details see Appendix A)
The European species P. sylvestris, P. halepensis, P. mugo and P. nigra have all been found to be infected by C. harknessii in North America (Ziller, 1969; EPPO, 1997) and some of the major hosts in the native range are grown in Europe, e.g. P. contorta and P. ponderosa (EPPO, 2018).
P. strobus (host of C. kurilense and C. sahoanum) is also commonly found in Europe, both as ornamental and in forest plantations.
3.4.3.2. Climatic conditions affecting establishment
The distribution of C. harknessii in North America, from Mexico to the northern parts of Canada (Figure 1; Section 3.2.1), covers areas with a wide range of climate types. These climate types overlap to a large extent with the climates found in the EU. Therefore, climate is not assumed to limit the establishment of the pathogen in the EU.
C. kurilense and C. sahoanum are reported from Hokkaido and northern Honshu in Japan, with cool temperate climate, overlapping with the climate found in European mountain ranges (Imazu et al., 2000).
3.4.4. Spread
Is the pest able to spread within the EU territory following establishment? How?
Yes, by natural dispersal and movement of infected plants for planting and cut branches.
RNQPs: Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects?
No, plants for planting are not the main pathway of spread, as wind‐blown spores can travel over considerable distances.
C. harknessii has wind‐borne aeciospores that can travel considerable distances (Chang and Blenis, 1989; EPPO, 1997). A distance of several hundred km is mentioned (Adams, 1997).
The pathogen may also be transported across large distances with plants for planting (EPPO, 1997).
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes, the pest introduction could have an impact on pine forests, plantations, ornamental trees and nurseries.
RNQPs: Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? 4
Yes, the pest introduction could have an impact on the intended use of plants for planting.
C. harknessii may damage the form, lumber content and growth rates of infected Pinus spp. (EPPO, 1997; Hoffman and Hagle, 2011) (Figure 3). Infection of the main bole and branches may lead to girdling or breakage at the gall and individual trees may be killed (Van der Kamp, 1989; Hoffman and Hagle, 2011). Damage by the fungus also predisposes the tree to secondary pests (Hoffman and Hagle, 2011). Trees of all ages are infected but damage is reported as most severe in seedlings and saplings in nurseries, Christmas tree plantations and progeny test plantations (Merrill and Kistler, 1976; Hoffman and Hagle, 2011). In north‐west Canada, severe outbreaks on seedlings of P. ponderosa and P. contorta have been recorded. In Quebec, young natural stands of P. banksiana and P. sylvestris plantations have been observed to suffer serious damage (EPPO, 1997).
Figure 3.

Stem canker caused by Cronartium harknessii on lodgepole pine (Pinus contorta). Photo by Rocky Mountain Research Station, USDA, Forest Service, Bugwood.org. Available online: https://www.forestryimages.org/browse/detail.cfm?imgnum=1473029
In British Columbia, an average mortality of 5% for P. contorta was estimated with a stem infection rate in individual stands found to range between 0% and 50% (references in Van der Kamp, 1989). Heineman et al. (2010) quantified the presence of C. harknessii in 66 P. contorta stands in British Columbia and found the pathogen in all (100%) of the sites with a mean occurrence ranging between 3% and 74% on individual sites. Across all surveyed trees, 21% were affected and 55% of these were rejected as productive trees (Heineman et al., 2010).
Studies on inoculated seedlings showed that seedlings of P. sylvestris were generally less susceptible than P. contorta, but that infection was very variable, with both heavily infected and disease‐free seedlings observed (Van der Kamp, 1989). The author concluded that damage to P. sylvestris would be less serious while damage to P. contorta may be similar to that observed in North America should the fungus be introduced to Europe (Van der Kamp, 1989). However, after 7 years, whilst infection by C. harknessii was much higher on P. contorta than on P. sylvestris, the average number of galls per infected tree was higher on P. sylvestris than on P. contorta (Karlman et al., 1997). Western gall rust caused about 25% growth reductions on both lodgepole and Scots pine trees after 25 years at five test sites in western Canada (Fries, 2017).
C. harknessii is considered to be a serious threat to P. radiata cultivation in exotic forest plantations in Australia and New Zealand (Old, 1981; Ramsfield et al., 2007; Ramsfield and Vogler, 2010).
Compared to what is reported about C. harknessii, there is less available evidence on the observed and potential impacts of C. kurilense and C. sahoanum. Nevertheless, should C. harknessii, C. kurilense and C. sahoanum be introduced to the EU, impacts can be expected in pine forests, plantations, ornamental trees and nurseries.
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
Yes, please see Sections 3.3 and 3.6.1
RNQPs: Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated?
Yes, production of plants for planting in pest free areas can prevent pest presence on plants for planting.
3.6.1. Identification of additional measures
Phytosanitary measures are currently applied to the host species of C. harknessii, C. kurilense and C. sahoanum (see Section 3.3.2).
3.6.1.1. Additional control measures
Potential additional control measures are listed in Table 4.
Table 4.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry/establishment/spread/impact in relation to currently unregulated hosts and pathways. Control measures are measures that have a direct effect on pest abundance
| Information sheet title (with hyperlink to information sheet if available) | Control measure summary | Risk component (entry/establishment/spread/impact) |
|---|---|---|
| http://doi.org/10.5281/zenodo.1175887 |
Plant nurseries should be located far away from infected forest stands (EPPO, 1997) Removal of infected trees surrounding nurseries can reduce the risk of infection in nurseries (Hoffman and Hagle, 2011) |
Entry/spread |
| Chemical treatments on crops including reproductive material | Chemical control at the beginning of spore release can reduce impacts (Merrill and Kistler, 1976; Adams, 1997) | Impact |
| Use of resistant and tolerant plant species/varieties | Selection of resistant trees as seed source can reduce impacts (Van der Kamp, 1989; Hoffman and Hagle, 2011) | Impact |
| http://doi.org/10.5281/zenodo.1181436 |
Removal of infected trees by thinning may reduce the inoculum and be economical (Adams, 1997; EPPO, 1997). The effect may, however, depend on the disease presence in the surrounding landscape (Adams, 1997) Removal of inoculum by pruning branches with galls of ornamental trees can reduce impacts (Hoffman and Hagle, 2011) |
Entry/ spread/ impact |
| http://doi.org/10.5281/zenodo.1181717 | Promotion of tree species diversity may reduce impacts (Hoffman and Hagle, 2011) | Impact |
3.6.1.2. Biological or technical factors limiting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
3.6.1.3. Biological or technical factors limiting the ability to prevent the presence of the pest on plants for planting
3.7. Uncertainty
Compared to C. harknessii, there is limited information regarding C. kurilense and C. sahoanum.
4. Conclusions
C. harknessii, C. kurilense and C. sahoanum meet the criteria assessed by EFSA for consideration as potential quarantine pests (Table 5).
Table 5.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1) | The identity of C. harknessii, C. kurilense and C. sahoanum is clear | The identity of C. harknessii, C. kurilense and C. sahoanum is clear | None |
| Absence/presence of the pest in the EU territory (Section 3.2) | The pathogens are not reported to be present in the EU | The pathogens are not reported to be present in the EU | None |
| Regulatory status (Section 3.3) | These pathogens are regulated by Council Directive 2000/29/EC (Annex IAI) (as Endocronartium spp. non‐EU) as harmful organisms whose introduction into, and spread within, all Member States shall be banned | These pathogens are regulated by Council Directive 2000/29/EC (Annex IAI) (as Endocronartium spp. non‐EU) as harmful organisms whose introduction into, and spread within, all Member States shall be banned | None |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) |
Entry: the pathogens could enter the EU via host plants for planting and cut branches Establishment: hosts are common and climatic conditions are favourable in the risk assessment area Spread: the pathogens could spread following establishment by movement of host plants for planting and cut branches, as well as natural spread |
Plants for planting are not the main pathway of spread, given the potential contribution of cut branches and natural spread | None |
| Potential for consequences in the EU territory (Section 3.5) | The introduction of the pathogens would have economic and environmental impacts in pine forests, plantations, ornamental trees and nurseries | The introduction of the pathogens could have an impact on the intended use of plants for planting | None |
| Available measures (Section 3.6) | Import prohibition of host plants, locating nurseries far away from infected forests stands, removing infected trees surrounding nurseries, selecting resistant trees as seed source and promoting tree species diversity are available measures | Production of plants for planting in pest free areas can prevent pest presence on plants for planting | None |
| Conclusion on pest categorisation (Section 4) | The criteria assessed by the Panel for consideration of C. harknessii, C. kurilense and C. sahoanum as potential quarantine pests are met | The criterion on the pest presence in the EU is not met | |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | The main knowledge gap is the limited available information on C. kurilense and C. sahoanum compared to C. harknessii | ||
Glossary
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 1995, 2017)
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 1995, 2017)
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2017)
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2017)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2017)
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2017)
- Measures
Control (of a pest) is defined in ISPM 5 (FAO 2017) as ‘Suppression, containment or eradication of a pest population’ (FAO, 1995) Control measures are measures that have a direct effect on pest abundance Supporting measures are organisational measures or procedures supporting the choice of appropriate Risk Reduction Options that do not directly affect pest abundance
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2017)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2017)
- Protected zones (PZ)
A protected zone is an area recognised at EU level to be free from a harmful organism, which is established in one or more other parts of the Union
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2017)
- Regulated non‐quarantine pest
A non‐quarantine pest whose presence in plants for planting affects the intended use of those plants with an economically unacceptable impact and which is therefore regulated within the territory of the importing contracting party (FAO, 2017)
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2017)
Abbreviations
- C‐SMFA
constrained spatial multi‐scale frequency analysis
- CLC
Corine Land Cover
- DG SANTE
Directorate General for Health and Food Safety
- EPPO
European and Mediterranean Plant Protection Organization
- EUFGIS
European Information System on Forest Genetic Resources
- FAO
Food and Agriculture Organization
- GD2
Georeferenced Data on Genetic Diversity
- IPPC
International Plant Protection Convention
- MS
Member State
- PLH
EFSA Panel on Plant Health
- PZ
Protected Zone
- RPP
relative probability of presence
- ToR
Terms of Reference
Appendix A – Methodological notes on Figure 2
1.
The relative probability of presence (RPP) reported here for Pinus spp. in Figure 2 and in the European Atlas of Forest Tree Species (de Rigo et al., 2016; San‐Miguel‐Ayanz et al., 2016) is the probability of that genus to occur in a given spatial unit (de Rigo et al., 2017). In forestry, such a probability for a single taxon is called ‘relative’. The maps of RPP are produced by means of the constrained spatial multi‐scale frequency analysis (C‐SMFA) (de Rigo et al., 2014, 2017) of species presence data reported in geolocated plots by different forest inventories.
A.1. Geolocated plot databases
The RPP models rely on five geodatabases that provide presence/absence data for tree species and genera: four European‐wide forest monitoring data sets and a harmonised collection of records from national forest inventories (de Rigo et al., 2014, 2016, 2017). The databases report observations made inside geolocalised sample plots positioned in a forested area, but do not provide information about the plot size or consistent quantitative information about the recorded species beyond presence/absence.
The harmonisation of these data sets was performed within the research project at the origin of the European Atlas of Forest Tree Species (de Rigo et al., 2016; San‐Miguel‐Ayanz, 2016; San‐Miguel‐Ayanz et al., 2016). Given the heterogeneity of strategies of field sampling design and establishment of sampling plots in the various national forest inventories (Chirici et al., 2011a,b), and also given legal constraints, the information from the original data sources was harmonised to refer to an INSPIRE compliant geospatial grid, with a spatial resolution of 1 km2 pixel size, using the ETRS89 Lambert Azimuthal Equal‐Area as geospatial projection (EPSG: 3035, http://spatialreference.org/ref/epsg/etrs89-etrs-laea/).
A.1.1. European National Forestry Inventories database
This data set was derived from National Forest Inventory data and provides information on the presence/absence of forest tree species in approximately 375,000 sample points with a spatial resolution of 1 km2/pixel, covering 21 European countries (de Rigo et al., 2014, 2016).
A.1.2. Forest Focus/Monitoring data set
This project is a Community scheme for harmonised long‐term monitoring of air pollution effects in European forest ecosystems, normed by EC Regulation No. 2152/20035. Under this scheme, the monitoring is carried out by participating countries on the basis of a systematic network of observation points (Level I) and a network of observation plots for intensive and continuous monitoring (Level II). For managing the data, the JRC implemented a Forest Focus Monitoring Database System, from which the data used in this project were taken (Hiederer et al., 2007; Houston Durrant and Hiederer, 2009). The complete Forest Focus data set covers 30 European Countries with more than 8600 sample points.
A.1.3. BioSoil data set
This data set was produced by one of a number of demonstration studies performed in response to the ‘Forest Focus’ Regulation (EC) No. 2152/2003 mentioned above. The aim of the BioSoil project was to provide harmonised soil and forest biodiversity data. It comprised two modules: a Soil Module (Hiederer et al., 2011) and a Biodiversity Module (Houston Durrant et al., 2011). The data set used in the C‐SMFA RPP model came from the Biodiversity module, in which plant species from both the tree layer and the ground vegetation layer were recorded for more than 3,300 sample points in 19 European Countries.
A.1.4. European Information System on Forest Genetic Resources (EUFGIS)
EUFGIS (http://portal.eufgis.org) is a smaller geodatabase providing information on tree species composition in over 3,200 forest plots in 34 European countries. The plots are part of a network of forest stands managed for the genetic conservation of one or more target tree species. Hence, the plots represent the natural environment to which the target tree species are adapted.
A.1.5. Georeferenced Data on Genetic Diversity (GD2)
GD2 (http://gd2.pierroton.inra.fr) provides information about 63 species of interest for genetic conservation. The database covers 6,254 forest plots located in stands of natural populations that are traditionally analysed in genetic surveys. While this database covers fewer species than the others, it covers 66 countries in Europe, North Africa and the Middle East, making it the data set with the largest geographic extent.
A.2. Modelling methodology
For modelling, the data were harmonised in order to have the same spatial resolution (1 km2) and filtered to a study area comprising 36 countries in the European continent. The density of field observations varies greatly throughout the study area and large areas are poorly covered by the plot databases. A low density of field plots is particularly problematic in heterogeneous landscapes, such as mountainous regions and areas with many different land use and cover types, where a plot in one location is not representative of many nearby locations (de Rigo et al., 2014). To account for the spatial variation in plot density, the model used here (C‐SMFA) considers multiple spatial scales when estimating RPP. Furthermore, statistical resampling is systematically applied to mitigate the cumulated data‐driven uncertainty.
The presence or absence of a given forest tree species then refers to an idealised standard field sample of negligible size compared with the 1 km2 pixel size of the harmonised grid. The modelling methodology considered these presence/absence measures as if they were random samples of a binary quantity (the punctual presence/absence, not the pixel one). This binary quantity is a random variable having its own probability distribution which is a function of the unknown average probability of finding the given tree species within a plot of negligible area belonging to the considered 1 km2 pixel (de Rigo et al., 2014). This unknown statistic is denoted hereinafter with the name of ‘probability of presence’.
C‐SMFA performs spatial frequency analysis of the geo‐located plot data to create preliminary RPP maps (de Rigo et al., 2014). For each 1 km2 grid cell, the model estimates kernel densities over a range of kernel sizes to estimate the probability that a given species is present in that cell. The entire array of multi‐scale spatial kernels is aggregated with adaptive weights based on the local pattern of data density. Thus, in areas where plot data are scarce or inconsistent, the method tends to put weight on larger kernels. Wherever denser local data are available, they are privileged ensuring a more detailed local RPP estimation. Therefore, a smooth multi‐scale aggregation of the entire arrays of kernels and data sets is applied instead of selecting a local ‘best performing’ one and discarding the remaining information. This array‐based processing, and the entire data harmonisation procedure, are made possible thanks to the semantic modularisation which defines the Semantic Array Programming modelling paradigm (de Rigo, 2012).
The probability to find a single species (e.g. a particular coniferous tree species) in a 1 km2 grid cell cannot be higher than the probability of presence of all the coniferous species combined. The same logical constraints applied to the case of single broadleaved species with respect to the probability of presence of all the broadleaved species combined. Thus, to improve the accuracy of the maps, the preliminary RPP values were constrained so as not to exceed the local forest‐type cover fraction with an iterative refinement (de Rigo et al., 2014). The forest‐type cover fraction was estimated from the classes of the Corine Land Cover (CLC) maps which contain a component of forest trees (Bossard et al., 2000; Büttner et al., 2012).
The resulting probability of presence is relative to the specific tree taxon, irrespective of the potential co‐occurrence of other tree taxa with the measured plots, and should not be confused with the absolute abundance or proportion of each taxon in the plots. RPP represents the probability of finding at least one individual of the taxon in a plot placed randomly within the grid cell, assuming that the plot has negligible area compared with the cell. As a consequence, the sum of the RPP associated with different taxa in the same area is not constrained to be 100%. For example, in a forest with two codominant tree species which are homogeneously mixed, the RPP of both may be 100% (see e.g. the Glossary in San‐Miguel‐Ayanz et al. (2016), http://forest.jrc.ec.europa.eu/media/atlas/Glossary.pdf).
The robustness of RPP maps depends strongly on sample plot density, as areas with few field observations are mapped with greater uncertainty. This uncertainty is shown qualitatively in maps of ‘RPP trustability’. RPP trustability is computed on the basis of the aggregated equivalent number of sample plots in each grid cell (equivalent local density of plot data). The trustability map scale is relative, ranging from 0 to 1, as it is based on the quantiles of the local plot density map obtained using all field observations for the species. Thus, trustability maps may vary among species based on the number of databases that report a particular species (de Rigo et al., 2014, 2016).
The RPP and relative trustability range from 0 to 1 and are mapped at a 1 km spatial resolution. To improve visualisation, these maps can be aggregated to coarser scales (i.e. 10 × 10 pixels or 25 × 25 pixels, respectively summarising the information for aggregated spatial cells of 100 km2 and 625 km2) by averaging the values in larger grid cells.
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C., Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Fejer Justesen AM, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent A, Yuen J, Zappalà L, Boberg J, Jeger M, Pautasso M and Dehnen‐Schmutz K, 2018. Scientific Opinion on the pest categorisation of Cronartium harknessii, Cronartium kurilense and Cronartium sahoanum . EFSA Journal 2018;16(10):5443, 26 pp. 10.2903/j.efsa.2018.5443
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00038, EFSA‐Q‐2018‐00750, EFSA‐Q‐2018‐00751, EFSA‐Q‐2018‐00752
Panel members: Claude Bragard, Katharina Dehnen‐Schmutz, Francesco Di Serio, Paolo Gonthier, Marie‐Agnès Jacques, Josep Anton Jaques Miret, Anne Marie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A. Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L. Reignault, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent, Jonathan Yuen and Lucia Zappalà.
Adopted: 27 September 2018
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © EPPO; Figure 2: © European Union; Figure 3: © Bugwood.org
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
See Section 2.1 on what falls outside EFSA's remit.
Council of the European Union, 2003. Regulation (EC) No 2152/2003 of the European Parliament and of the Council of 17 November 2003 concerning monitoring of forests and environmental interactions in the Community (Forest Focus). Official Journal of the European Union 46 (L 324), 1–8.
References
- Adams D, 1997. Western Pines & Western Gall Rust. Tree Notes Nr. 22. California Department of Forestry and Fire Protection, 4 pp. Available online: http://santacruzcountyfire.com/resource_mgmt/treenote22.pdf
- Aime MC, Castlebury LA, Abbasi M, Begerow D, Berndt R, Kirschner R, Marvanová L, Yoshitaka O, Padamsee M, Scholler M, Thines M and Rossman AY, 2018. Competing sexual and asexual generic names in Pucciniomycotina and Ustilaginomycotina (Basidiomycota) and recommendations for use. IMA Fungus, 9, 75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossard M, Feranec J and Otahel J, 2000. CORINE land cover technical guide ‐ Addendum 2000. Tech. Rep. 40, European Environment Agency. Available online: https://www.eea.europa.eu/ds_resolveuid/032TFUPGVR
- Büttner G, Kosztra B, Maucha G and Pataki R, 2012. Implementation and achievements of CLC2006. Tech. rep., European Environment Agency. Available online: http://www.eea.europa.eu/ds_resolveuid/GQ4JECM8TB
- Chang KF and Blenis PV, 1989. Survival of Endocronartium harknessii teliospores in a simulated airborne state. Canadian Journal of Botany, 67, 928–932. [Google Scholar]
- Chirici G, Bertini R, Travaglini D, Puletti N and Chiavetta U, 2011a. The common NFI database In: Chirici G, Winter S. and McRoberts RE. (eds.). National forest inventories: contributions to forest biodiversity assessments. Springer, Berlin: pp. 99–119. [Google Scholar]
- Chirici G, McRoberts RE, Winter S, Barbati A, Brändli U‐B, Abegg M, Beranova J, Rondeux J, Bertini R, Alberdi Asensio I and Condés S, 2011b. Harmonization tests In: Chirici G, Winter S. and McRoberts RE. (eds.). National forest inventories: contributions to forest biodiversity assessments. Springer, Berlin: pp. 121–190. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Hart A, Schans J, Schrader G, Suffert M, Kertesz V, Kozelska S, Mannino MR, Mosbach‐Schulz O, Pautasso M, Stancanelli G, Tramontini S, Vos S and Gilioli G, 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 1997. Data sheets on quarantine pests: Endocronartium harknessii In: Smith IM, McNamara DG, Scott PR. and Holderness M. (eds). Quarantine Pests for Europe. 2nd Edition CABI/EPPO, Wallingford: 1425 pp. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 2018. EPPO Global Database. Available online: https://gd.eppo.int [Accessed May 2018].
- FAO (Food and Agriculture Organization of the United Nations), 1995. ISPM (International standards for phytosanitary measures) No 4. Requirements for the establishment of pest free areas. Available online: https://www.ippc.int/en/publications/614/
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 2017. ISPM (International standards for phytosanitary measures) No 5. Glossary of phytosanitary terms. Available online: https://www.ippc.int/en/publications/622/
- Farr DF and Rossman AY, 2018. Fungal Databases, US National Fungus Collections, Agricultural Research Service, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/ [Accessed May 2018].
- Fries A, 2017. Damage by pathogens and insects to Scots pine and lodgepole pine 25 years after reciprocal plantings in Canada and Sweden. Scandinavian Journal of Forest Research, 32, 459–472. [Google Scholar]
- Heineman JL, Sachs DL, Mather WJ and Simard SW, 2010. Investigating the influence of climate, site, location, and treatment factors on damage to young lodgepole pine in southern British Columbia. Canadian Journal of Forest Research, 40, 1109–1127. [Google Scholar]
- Hiederer R, Houston Durrant T, Granke O, Lambotte M, Lorenz M, Mignon B and Mues V, 2007. Forest focus monitoring database system ‐ validation methodology. Vol. EUR 23020 EN of EUR – Scientific and Technical Research. Office for Official Publications of the European Communities. 10.2788/51364 [DOI]
- Hiederer R, Houston Durrant T and Micheli E, 2011. Evaluation of BioSoil demonstration project ‐ Soil data analysis. Vol. 24729 of EUR ‐ Scientific and Technical Research. Publications Office of the European Union. 10.2788/56105 [DOI]
- Hiratsuka Y, 1969. Endocronartium, a new genus for autoecious pine stem rusts. Canadian Journal of Botany, 47, 1493–1495. [Google Scholar]
- Hiratsuka Y, 1971. Spore surface morphology of pine stem rusts of Canada as observed under a scanning electron microscope. Canadian Journal of Botany, 49, 371–372. [Google Scholar]
- Hoffman J and Hagle S, 2011. Western gall rust management. Insect and Disease Management Series, 14.1. USDA Forest Service, 3 pp. Available online: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5188580.pdf
- Houston Durrant T and Hiederer R, 2009. Applying quality assurance procedures to environmental monitoring data: a case study. Journal of Environmental Monitoring, 11, 774–781. [DOI] [PubMed] [Google Scholar]
- Houston Durrant T, San‐Miguel‐Ayanz J, Schulte E and Suarez Meyer A, 2011. Evaluation of BioSoil demonstration project: forest biodiversity ‐ Analysis of biodiversity module. Vol. 24777 of EUR – Scientific and Technical Research. Publications Office of the European Union. 10.2788/84823 [DOI]
- Imazu M, Kakishima M and Kaneko S, 1989. Endocronartium sahoanum, a new stem rust fungus on Pinus pumila in Japan. Transactions of the Mycological Society of Japan, 30, 301–310. [Google Scholar]
- Imazu M, Azbukina ZM, Kakishima M, Fukushima K, Nishimura K and Miyaji M, 2000. Identification of a rust fungus on Pinus pumila collected in the North Kurils. Russia. Mycoscience, 41, 139–144. [Google Scholar]
- Karlman M, Van Der Kamp B and Witzell J, 1997. Susceptibility of Pinus sylvestris to the stem rusts of Pinus contorta in western Canada. Scandinavian Journal of Forest Research, 12(2), 168–178. [Google Scholar]
- Kim MS, Klopfenstein NB, Ota Y, Lee SK, Woo KS and Kaneko S, 2010. White pine blister rust in Korea, Japan and other Asian regions: comparisons and implications for North America. Forest Pathology, 40, 382–401. [Google Scholar]
- Lundquist JE, Walla JA and Tuskan GA, 1994. Characteristics of Peridermium harknessii in axenic culture. Canadian Journal of Forest Research, 24, 1345–1353. [Google Scholar]
- Merrill W and Kistler BR, 1976. Phenology and control of Endocronartium harknessii in Pennsylvania. Phytopathology, 66, 1246–1248. [Google Scholar]
- Old KM, 1981. Western gall rust, a serious disease of Pinus radiata in California. Australian Forestry, 44, 178–184. [Google Scholar]
- Powers HR, Lin D and Hubbes M, 1989. Interspecific and intraspecific differentiation within the genus Cronartium by isozyme and protein pattern analysis. Plant Disease, 73, 691–694. [Google Scholar]
- Pretzsch H, 1986. Ertragskundliche Merkmale von Kiefernbeständen bei Schädigung durch den Kienzopf (Peridermium pini = Endocronartium pini). Forstarchiv, 57, 137–145. [Google Scholar]
- Ramsfield TD and Vogler DR, 2010. A DNA‐based method for detection of Peridermium harknessii, the causal agent of western gall rust. Australasian Plant Pathology, 39, 247–253. [Google Scholar]
- Ramsfield TD, Kriticos DJ, Vogler DR and Geils BW, 2007. Western gall rust‐a threat to Pinus radiata in New Zealand. New Zealand Journal of Forestry Science, 37, 143–152. [Google Scholar]
- de Rigo D, 2012. Semantic Array Programming for environmental modelling: application of the Mastrave library In: Seppelt R, Voinov AA, Lange S. and Bankamp D. (eds.). International Environmental Modelling and Software Society (iEMSs) 2012 International Congress on Environmental Modelling and Software ‐ Managing Resources of a Limited Planet: Pathways and Visions under Uncertainty, Sixth Biennial Meeting. Leipzig, Germany: pp. 1167–1176. [Google Scholar]
- de Rigo D, Caudullo G, Busetto L and San‐Miguel‐Ayanz J, 2014. Supporting EFSA assessment of the EU environmental suitability for exotic forestry pests: final report. EFSA Supporting Publications, 11, EN‐434. [Google Scholar]
- de Rigo D, Caudullo G, Houston Durrant T and San‐Miguel‐Ayanz J, 2016. The European Atlas of Forest Tree Species: modelling, data and information on forest tree species In: San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T. and Mauri A. (eds.). European Atlas of Forest Tree Species. Publications Office of the European Union, Luxembourg, pp. e01aa69+. [Google Scholar]
- de Rigo D, Caudullo G, San‐Miguel‐Ayanz J and Barredo JI, 2017. Robust modelling of the impacts of climate change on the habitat suitability of forest tree species. Publication Office of the European Union, Publications Office of the European Union, Luxembourg, 58 pp. [Google Scholar]
- San‐Miguel‐Ayanz J, 2016. The European Union Forest Strategy and the Forest Information System for Europe In: San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A. (eds.). European Atlas of Forest Tree Species. Publications Office of the European Union, Luxembourg, pp. e012228+. [Google Scholar]
- San‐Miguel‐Ayanz J, de Rigo D, Caudullo G, Houston Durrant T. and Mauri A. (eds.)., 2016. European Atlas of Forest Tree Species. Publication Office of the European Union, Luxembourg. [Google Scholar]
- Sinclair WA and Lyon HH, 2005. Diseases of Trees and Shrubs. 2nd Edition Cornell University Press, Ithaca, NY, 660 pp. [Google Scholar]
- Van der Kamp BJ, 1988. Susceptibility of lodgepole pine provenances to geographically separate western gall rust spore sources. Canadian Journal of Forest Research, 18, 1203–1205. [Google Scholar]
- Van der Kamp BJ, 1989. The relative susceptibility of Scots and lodgepole pine to western gall rust. European Journal of Forest Pathology, 19, 274–280. [Google Scholar]
- Van der Kamp BJ and Spence M, 1987. Stem diseases of lodgepole pine in the British Columbia interior following juvenile spacing. The Forestry Chronicle, 63, 334–339. [Google Scholar]
- Ziller WG, 1969. The tree rusts of western Canada ‐ annotated lists with keys. Department of Fisheries and Forestry, Forest Research Laboratory, Victoria, BC, Canada. Information Report BC‐X‐034, 24 pp. [Google Scholar]
- Ziller WG, 1970. Studies of western tree rusts. VII. Inoculation experiments with pine stem rusts (Cronartium and Endocronartium). Canadian Journal of Botany, 48, 1313–1319. [Google Scholar]


