Abstract
Poppy seeds are obtained from the opium poppy (Papaver somniferum L.). They are used as food and to produce edible oil. The opium poppy plant contains narcotic alkaloids such as morphine and codeine. Poppy seeds do not contain the opium alkaloids, but can become contaminated with alkaloids as a result of pest damage and during harvesting. The European Commission asked EFSA to provide an update of the Scientific Opinion on opium alkaloids in poppy seeds. The assessment is based on data on morphine, codeine, thebaine, oripavine, noscapine and papaverine in poppy seed samples. The CONTAM Panel confirms the acute reference dose (ARfD) of 10 μg morphine/kg body weight (bw) and concluded that the concentration of codeine in the poppy seed samples should be taken into account by converting codeine to morphine equivalents, using a factor of 0.2. The ARfD is therefore a group ARfD for morphine and codeine, expressed in morphine equivalents. Mean and high levels of dietary exposure to morphine equivalents from poppy seeds considered to have high levels of opium alkaloids (i.e. poppy seeds from varieties primarily grown for pharmaceutical use) exceed the ARfD in most age groups. For poppy seeds considered to have relatively low concentrations of opium alkaloids (i.e. primarily varieties for food use), some exceedance of the ARfD is also seen at high levels of dietary exposure in most surveys. For noscapine and papaverine, the available data do not allow making a hazard characterisation. However, comparison of the dietary exposure to the recommended therapeutical doses does not suggest a health concern for these alkaloids. For thebaine and oripavine, no risk characterisation was done due to insufficient data. However, for thebaine, limited evidence indicates a higher acute lethality than for morphine and the estimated exposure could present a health risk.
Keywords: poppy seeds, opium alkaloids, morphine, codeine, thebaine, acute reference dose (ARfD)
Summary
In October 2011, the EFSA Panel on Contaminants in the Food Chain (CONTAM) adopted the Scientific Opinion on the risks for public health related to the presence of opium alkaloids in poppy seeds. For that opinion, the European Food Safety Authority (EFSA) received the results from analyses of opium alkaloids, primarily morphine, codeine, thebaine, papaverine and noscapine, in samples of poppy seeds, bakery products and baking ingredients. Based on the relative prevalence of the alkaloids present in poppy seed and food samples analysed, and on their pharmacological potency, the CONTAM Panel concluded that the risk assessment could be based on dietary exposure to morphine alone. The CONTAM Panel established an acute reference dose (ARfD) of 10 μg morphine/kg body weight (bw) based on the lowest known single oral therapeutic dose of 30 μg morphine/kg bw and applying an uncertainty factor of 3 for extrapolation from the lowest‐observed‐effect‐level (LOEL) to a no‐observed‐effect level (NOEL) considering that the LOEL was derived from patients and not from the general population.
The European Commission asked EFSA for an update of the Scientific Opinion on the human health risks related to the presence of opium alkaloids in poppy seeds taking into account the alkaloid profile of the poppy seed samples submitted to EFSA since 2011. Recent occurrence data on the presence of opium alkaloids in poppy seeds available on the European market indicate that the alkaloids profile of the poppy seed samples submitted to EFSA after the 2011 opinion may be different compared to the data used in the EFSA opinion, and this issue is particularly important for thebaine.
The CONTAM Panel concluded that the update of this opinion should comprise: (i) a re‐evaluation of the toxicity of opium alkaloids present in poppy seed samples, for humans considering all relevant toxicological endpoints; (ii) an evaluation of the alkaloid profile (i.e. composition of the alkaloids and their concentration) of the poppy seed samples submitted to EFSA since 2011 and whether the profile is similar to that of the poppy seed samples used in the 2011 opinion; (iii) an estimation of the dietary exposure of the EU population to opium alkaloids from poppy seeds, including the consumption patterns of specific groups of the population if appropriate; (iv) an assessment of the human health risks for the EU population, including specific groups of the population if appropriate, as the consequence of the estimated dietary exposure.
Poppy seeds are obtained from the opium poppy (Papaver somniferum L.). The latex (milky sap) of the opium poppy contains up to 80 different alkaloids, including morphine and codeine, which have been used by man for the treatment of severe pain for generations but are also subject to misuse. The seeds are used as food and to produce edible oil. Alkaloid accumulation in the poppy plant depends on both genetic factors and environmental/cultivation conditions. Breeding is focusing on the development of either cultivars with a high and specific alkaloid content to fulfil the requirements of the pharmaceutical industry or cultivars with a low alkaloid content for seed/oil production. However, poppy seeds from P. somniferum varieties with high alkaloid content especially bred for pharmaceutical purposes are used as a by‐product for food purposes. Mature poppy seeds do not contain the latex, but can become contaminated with opium alkaloids as a result of pest damage and during harvest. Opium alkaloids detected in samples of poppy seeds and poppy seed‐containing foods include the phenanthrenes: principally morphine, codeine, thebaine and oripavine, and the benzylisoquinolines: principally papaverine and noscapine. In this Scientific Opinion, the term ‘opium alkaloids’ refers to these compounds.
Morphine is extensively absorbed from the gastrointestinal (GI) tract and is distributed throughout the body. The oral bioavailability of morphine is reduced by both Phase I and II presystemic metabolism in the GI tract and liver. Though the brain is its primary site of action, morphine does not cross the blood‐brain barrier easily. Morphine diffuses across the placenta, and transfers into milk. Morphine is metabolised via N‐demethylation and O‐glucuronidation in the gut and the liver. Metabolites are normorphine, morphine‐3‐glucuronide (M3G) and morphine‐6‐glucuronide (M6G). M6G has similar or higher activity than morphine. The rate and pathways of opioid metabolism may be influenced by genetic factors and medical conditions.
Codeine is readily and extensively absorbed from the GI tract following oral administration. Codeine is distributed throughout the body, penetrates the placental barrier and enters fetal circulation. Codeine and its metabolites are almost fully excreted via the kidneys mainly as glucuronides. The principal pathways for metabolism of codeine occur in the liver, although some metabolism occurs in the intestine and brain. Codeine is converted to codeine‐6‐glucuronide (C6G), norcodeine and morphine. The formed morphine is further metabolised into normorphine, M3G and M6G. Codeine metabolism to morphine is dependent of the CYP2D6 activity. Individuals can be classified into poor metaboliser, intermediate metaboliser, extensive metaboliser or ultra‐rapid metaboliser. The distribution of poor, extensive and ultra‐rapid metabolisers differs significantly between ethnic groups; the extensive metabolisers represent the majority of the Caucasian population. Significant differences were detected between the groups in areas under the plasma concentration vs time curves (AUCs) and the maximum plasma concentrations (Cmax) of morphine. No new data were identified that provide a basis to change the previous conclusion from 2011 that the maximal metabolic conversion of codeine into morphine does not exceed 20%.
Based on limited information, oral bioavailability of thebaine, oripavine, noscapine and papaverine appears to be reduced due to presystemic metabolism in the GI tract and liver, primarily involving demethylation reactions but also glucuronidation. There are indications that thebaine is metabolised into several metabolites including oripavine and morphine.
In experimental animals, morphine acts on the nervous system and its development. Chronic toxicity, including carcinogenicity, of morphine has not been systematically evaluated. However, based on the lack of carcinogenicity of codeine which is metabolised to morphine, it is unlikely that morphine is carcinogenic. Morphine is genotoxic only in vivo but most likely by a non‐DNA reactive mode of action. Recent studies in rats and mice add to the weight of evidence that morphine has reproductive and developmental effects. Depressed sexual activity, reduced testicular function and spermatogenesis, disruption of ovarian cyclicity and decreased pregnancy rate have been observed in rats exposed to morphine. Oral morphine exposure of pregnant rats or mice affects the normal development of the placenta and the brain which could lead to postnatal neurological and behavioural deficits in the animals including memory loss. It has also been shown that morphine causes immunosuppressive actions. These data do not allow assessment of dose‐response relationships in support of the risk assessment for opioids in food.
Long‐term feeding studies in rats and mice showed no evidence of carcinogenic activity of codeine. Codeine is not genotoxic. Based on limited data, it is concluded that oral administration of codeine did not result in teratogenicity. There are no data to make conclusions on the neurotoxic effects of codeine.
Based on acute toxicity data, thebaine and oripavine are more toxic than morphine. Lower LD50 values have been reported for thebaine compared to morphine, both by oral and parenteral routes. However no other data on the oral toxicity of thebaine were identified and therefore its critical effects are unknown. No oral LD50 values have been reported for oripavine, but lower intraperitoneal (i.p.) and subcutaneous (s.c.) LD50 values have been reported for oripavine than for morphine. Apart from one study showing convulsions following i.p. and s.c. administration, no other data on the toxicity of oripavine were identified. Therefore the toxicological profile of oripavine is unclear.
High LD50 values for noscapine, following both oral and parenteral administration, indicate a lower acute toxicity compared to the other opioid alkaloids. Repeated exposure of rats and dogs to noscapine did not result in adverse effects. Noscapine is an aneugen in vitro most likely by a non‐DNA reactive mechanism. A risk of genotoxic damage caused by noscapine in humans at therapeutic dosages is very unlikely. LD50 values of papaverine by oral and parenteral administration are similar to morphine. However, no evidence of toxicity was seen following oral administration of papaverine to rats and dogs. Therefore the critical effects of papaverine are unclear.
Based on human data, the CONTAM Panel confirms its previous conclusion that the critical effects of morphine are on the central nervous system (CNS) mediated by its high affinity to the μ‐opioid receptor as an agonistic ligand. These adverse effects include nausea, vomiting, sedation, drowsiness, euphoria, miosis, respiratory depression and obstipation. Therapeutic doses of morphine may reduce attentiveness and reactive skills, with potential impact on driving and operating machinery. From data on humans and experimental animals, it is clear that morphine can interfere with brain development of the fetus resulting in behavioural effects at later life‐stages; however, data are too limited to derive a dose–response relationship. The lowest known single oral therapeutic dose reported is 1.9 mg morphine, corresponding to 27 μg/kg bw for an adult weighing 70 kg.
The pharmacology of codeine is strongly related to that of morphine, as it is a precursor of morphine itself. Codeine has some direct activity at the μ‐opioid receptor, but with a much lower potency than morphine, and therefore, a direct effect of codeine is negligible compared to the effect of its metabolite morphine. More recent literature focused on cases of life‐threatening toxicity in paediatric patients given therapeutic doses of codeine. This has been associated with the genotype predisposing to ultra‐rapid metabolism of codeine into morphine by the isoenzyme CYP2D6. The adverse reactions to codeine are similar to those of morphine but seen to a lesser extent at clinical doses. The most frequent side effects of codeine are constipation and nausea. Morphine and codeine have dependency potential. Longer term use can result in tolerance.
For oripavine and thebaine, no data after oral or parenteral exposure of humans are available.
Noscapine is used as a centrally acting antitussive compound and has neither an analgesic, respiratory depressive nor obstipating effect. Although its toxicological properties have not been characterised, less severe adverse effects (e.g. headache, dizziness) are known from the therapeutic uses in humans.
Papaverine does not show opiate‐like pharmacology. It acts as a smooth muscle relaxant that is most pronounced on blood vessels. The side effects of papaverine, that have been reported after oral administration, are dizziness, drowsiness, headache, tiredness, GI disturbance, tachycardia, skin rash, sweating and hypotonia. After long‐term administration eosinophilia, icterus and liver enzyme changes (reversible) have been reported. Overdosage may lead to seizures.
The pharmacological effects of opioid drugs derive from their ability to interact with the μ‐, δ‐ and κ‐opioid receptors. The μ‐opioid receptor plays a crucial role not only in opioid‐induced analgesia but also in the unwanted actions of opioids. Morphine behaves as a potent full agonist at the μ‐receptor and it has 200‐fold greater affinity to the μ‐opioid receptor than codeine. M6G has a similar or higher affinity as morphine. Oripavine displays moderate potency (30‐ to 70‐fold less than morphine) at the μ‐receptor. Thebaine is able to stimulate opioid receptors only at very high concentrations (micromolar range). No indication has been found that the effects of noscapine and papaverine are mediated via opioid receptors.
For morphine, the CONTAM Panel again concluded that the dose–response analysis should be based on the lowest oral therapeutic dose of morphine (1.9 mg morphine/person). Applying the default body weight of 70 kg, this dose corresponds to 27 μg/kg bw per day or 30 μg/kg bw per day when rounded to one significant figure.
The CONTAM Panel previously concluded that the establishment of an ARfD was required for morphine because of the short‐term nature of its effects, and that ensuring exposure is below the ARfD would also protect against possible effects of repeated exposure. An ARfD of 10 μg/kg bw was derived from the lowest known single oral therapeutic dose of morphine, which was regarded as a LOEL, with application of an uncertainty factor of 3. The more recently available data do not provide a basis for revising the 2011 conclusions and therefore the CONTAM Panel confirms the ARfD of 10 μg/kg bw.
The CONTAM Panel considered whether combined exposure to morphine and codeine from poppy seed containing‐foods, expressed as morphine‐equivalents, should be estimated. The currently available occurrence data demonstrate that the concentration of codeine can be much higher than that of morphine in some poppy seed samples on the European market. The CONTAM Panel therefore now concludes that the concentration of codeine in the poppy seed samples should be taken into account in the exposure assessment and risk characterisation, by converting it to morphine equivalents, using a factor of 0.2, based on the maximal metabolic conversion of codeine into morphine. The ARfD is therefore a group ARfD for morphine and codeine, expressed in morphine equivalents.
Oripavine also acts as an agonist to the μ‐opioid receptor, with a lower activity than morphine and a higher activity than codeine. Non‐oral LD50 values in rodents indicate that oripavine is more acutely toxic than morphine. However, the available data are insufficient to characterise the hazard or to identify a factor for conversion to morphine equivalents.
The toxicological properties of thebaine have not been well characterised. A contribution to morphine‐like toxicity due to its possible metabolism into morphine and oripavine may occur, but is expected to be small. Oral LD50 values in rodents indicate that thebaine is more acutely toxic than morphine. The available data are insufficient to identify a factor for conversion to morphine equivalents, to propose a health‐based guidance value (HBGV) or to identify a reference point for calculating margins of exposure.
Noscapine and papaverine do not exhibit morphine‐like properties, and therefore, they are not included in the group ARfD. Their toxicological properties have not been well characterised and it is not possible to establish HBGVs. Based on the limited data available, it appears that noscapine and papaverine are less toxic than the other alkaloids discussed in this opinion.
The CONTAM Panel considered that only acute dietary exposure to opium alkaloids had to be assessed as for the previous opinion. Two batches of analytical results on the occurrence of opium alkaloids in food and poppy seeds were available. A first batch of 2,678 analytical results were collected by EFSA until 2011 and presented in the previous CONTAM opinion on opium alkaloids. This data set contained analytical results from 55 Australian poppy seed samples that were not used for dietary exposure assessment since their opium alkaloid profile was considered different from poppy seed samples available on the EU market at that time. A second batch of data contained 4,991 analytical results and was collected by EFSA between 2012 and 2017.
Considering the limited amount of data on food categories other than poppy seeds, the CONTAM Panel decided to base the assessment on poppy seed samples only. The occurrence data on opium alkaloids show the presence of poppy seed samples with high concentrations of morphine, codeine and/or thebaine. The opium alkaloid profile seems to be related to the country of origin of the poppy seed samples and ultimately to the poppy variety. Based on the available data per country of origin and batch, the CONTAM Panel concluded that the profiles in the two batches are comparable. In addition, the profile in the Australian poppy seeds is compatible with the profile in the European poppy seeds. Therefore, the Australian data were included in the data set and the two batches of data submitted to EFSA were merged.
Based on evidence from the currently available data set and background information, the CONTAM Panel divided the poppy seed samples with known country of origin into two groups. The ‘high‐morphine’ group, which is assumed to represent primarily varieties grown for the pharmaceutical sector and the ‘low‐morphine’ group, which is assumed to represent primarily varieties grown for the food sector. It emerged that both types are available, although in unknown proportion, on the European market, therefore, the CONTAM Panel has elaborated two separate acute exposure scenarios.
In poppy seed samples, highest mean middle bound (MB) concentrations were reported for morphine (147 mg/kg in the ‘high‐morphine’ group and 16.4 mg/kg in the ‘low‐morphine’ group) and thebaine (92.5 and 3.92 mg/kg, respectively) compared to codeine (22.7 and 2.88 mg/kg, respectively) and oripavine (20.0 and 2.14 mg/kg, respectively). For noscapine and papaverine, the mean MB concentrations were smaller and not exceeding 2 mg/kg. In the ‘high‐morphine’ group, thebaine was present at a concentration higher than morphine in more than 25% of the samples and codeine was higher than morphine in about 5% of the samples. In the ‘low‐morphine’ group, morphine was the opium alkaloid present at the highest concentration in almost all samples.
Among consumers of poppy seeds, the average estimated poppy seed consumption from the Comprehensive Food Consumption Database ranged from 0.5 (Other children, Sweden) to 25 g poppy seeds/day (Adults, Hungary) in 44 population groups (age class within a country). Only in 7 population groups, it was possible to calculate the 95th percentile (P95) ranging from 1 (Other children, Sweden) to 55 g poppy seeds/day (Adults, Germany).
The CONTAM Panel calculated the acute dietary exposure for morphine, codeine, thebaine, morphine equivalents, papaverine and noscapine for consumers of poppy seeds only. However, for oripavine, the exposure was not assessed due to the low amount of occurrence data. Considering the low percentage of left‐censored data, and consequently the small difference between lower bound (LB), MB and upper bound (UB) concentrations for morphine, codeine and thebaine, only the MB acute dietary exposure is presented. Due to the higher percentage of left‐censored data for noscapine and papaverine, the LB and UB acute dietary exposure is presented.
For morphine, the mean MB acute dietary exposure for consumers only (based on the mean occurrence and the mean consumption) ranged from 2.1 to 100 μg/kg bw per day across dietary surveys and age groups when considering the poppy seed samples from the ‘high‐morphine’ group. The P95 acute dietary exposure (based on the P95 occurrence and the P95 consumption) ranged from 21 up to 330 μg/kg bw day. For the ‘low‐morphine’ group, the acute mean MB acute dietary exposure ranged from 0.2 to 11 μg/kg bw per day across dietary surveys and age groups and the P95 from 2.8 to 46 μg/kg bw per day. The mean acute dietary exposure was the highest for other children. Maximum exposure at the P95 was similar for all age groups for which data were available.
For codeine, acute dietary exposure was lower compared to morphine, with the P95 dietary exposure similar to the mean dietary exposure to morphine. Acute dietary exposure estimates for morphine and codeine, expressed as morphine equivalents are quite similar compared to morphine alone and codeine makes a minor contribution to the morphine equivalents.
For thebaine, the mean MB acute dietary exposure for consumers only, ranged from 1.3 to 63 μg/kg bw per day across dietary surveys and age groups when considering the poppy seed samples from the ‘high‐morphine’ group. For the ‘low‐morphine’ group, the mean MB acute dietary exposure ranged from 0.06 to 2.7 μg/kg bw per day across dietary surveys and age groups. The P95 acute dietary exposure ranged from 18 to 288 μg/kg bw per day for the ‘high‐morphine’ group and from 0.7 to 11 μg/kg bw per day for the ‘low‐morphine’ group. The mean acute dietary exposure was the highest for other children. Maximum exposure at the P95 was similar for all age groups for which data were available.
For noscapine, the mean dietary exposure for consumers only, ranged from 0.02 to 1.5 μg/kg bw per day (min LB–max UB) across dietary surveys and age groups and was the highest for other children. The P95 acute dietary exposure ranged from 0.3 to 4.4 μg/kg bw per day (min LB–max UB) across dietary surveys and age groups. Among the opium alkaloids considered in this Scientific Opinion, acute dietary exposure for consumers only, was the lowest for papaverine, not exceeding 1 μg/kg bw per day.
It should be noted that exposure estimates are based on reported concentrations of alkaloids in poppy seed samples due to the low amount of occurrence data on food products containing poppy seeds. However, food processing steps (i.e. washing, soaking, heat treatment, grinding) may reduce the alkaloid content in raw poppy seeds by 25–100% in the final product. Therefore, the group ARfD is most likely to be exceeded when large portions are consumed, or if foods containing unprocessed poppy seeds are consumed.
Considering data related to poppy seeds containing relatively high levels of opium alkaloids, such as those from varieties primarily grown for pharmaceutical use (‘high‐morphine’ group), mean and high levels of dietary exposure to morphine from poppy seeds result in the group ARfD of 10 μg morphine equivalents/kg bw being exceeded in most age groups by up to 33‐fold. For poppy seeds considered to have relatively lower concentrations of opium alkaloids (primarily varieties for food use; ‘low‐morphine’ group), exceedance of the group ARfD is also seen at high levels of dietary exposure in most surveys, but to a lesser extent at up to 4‐ to 5‐fold. Based on the mean morphine occurrence, 34 out of 44 population groups are estimated to be exposed for at least 50% of the consumption days to a level of morphine higher than the group ARfD of 10 μg/kg bw for the ‘high‐morphine’ scenario and only one population group for the ‘low‐morphine’ scenario.
Codeine makes a minor contribution to exceedance of the group ARfD. However when considering individual poppy seed samples containing high concentrations of codeine, their consumption might result in a codeine exposure exceeding the group ARfD.
There are few reports of adverse reactions arising from traditional consumption of poppy seeds in foods, but in the absence of formal reporting systems, it cannot be assumed that such reactions do not occur from time to time, particularly in sensitive individuals. Pregnant women, infants, people above 75 years of age and those suffering from health conditions with impaired respiratory function are subgroups that are more sensitive to adverse effects of morphine.
In principle, oripavine could contribute to morphine‐like effects, but the available toxicological and occurrence data were insufficient for risk characterisation. For thebaine, no quantitative risk characterisation could be carried out. The estimated dietary exposures are slightly lower than those for morphine. On the other hand, although the lack of data did not allow the establishment of a HBGV, the CONTAM Panel noted that LD50 values for thebaine are 3‐ to 10‐fold lower than for morphine, suggesting that the estimated exposure levels might pose a health risk. For noscapine, the dietary exposure is at least 80‐fold lower than the recommended therapeutical dose, which does not suggest a health concern. For papaverine, the dietary exposure is about 2,000‐fold lower than the recommended therapeutical dose and it is highly unlikely that this would be a health concern.
The CONTAM Panel concluded that there is a need for toxicological data on thebaine and oripavine, including toxicokinetic data particularly on the formation of active metabolites. More occurrence data for foods containing poppy seeds are required for the opium alkaloids considered in this opinion. There is also a need for clarification of the occurrence of oripavine in poppy seed samples available on the EU market. Certified reference materials of food products that contain opium alkaloids at relevant concentrations should become available, as well as international proficiency tests. Finally, more information on consumption of poppy seeds and poppy seed‐based food in the EU is needed.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
On 5 October 2011, the EFSA Panel on Contaminants in the Food Chain (CONTAM) adopted the Scientific Opinion on the risks for public health related to the presence of opium alkaloids in poppy seeds (EFSA CONTAM Panel, 2011).
For that opinion, EFSA received the results from analyses of opium alkaloids, primarily morphine, codeine, thebaine, papaverine and noscapine, in samples of poppy seeds, bakery products and baking ingredients. Based on the relative prevalence of the alkaloids present in poppy seed and food samples analysed, and on their pharmacological potency, the CONTAM Panel concluded that the risk assessment could be based on dietary exposure to morphine alone. The CONTAM Panel established an acute reference dose (ARfD) of 10 μg morphine/kg body weight (bw). Estimates of dietary exposure to morphine from foods containing poppy seed demonstrate that the ARfD can be exceeded during a single serving by some consumers, particularly children, across the EU. Furthermore, the CONTAM Panel stressed that this risk assessment relates to poppy seed samples with an alkaloid profile comparable to that of the submitted data and should not be extrapolated to poppy seed samples with a qualitatively different alkaloid profile.
In the opinion it is concluded that morphine has a high affinity for the μ‐opioid receptor as an agonistic ligand. The pharmacology of codeine is strongly related to that of morphine, as it is a precursor of morphine itself. Up to 20% of codeine can be converted to morphine. Oripavine and thebaine show only partial agonistic activity at the μ‐receptor and thebaine has been shown to act as an antagonist at higher dosages based on the limited data available. Papaverine and noscapine do not show opiate‐like pharmacology since papaverine acts as a smooth muscle relaxant that is most pronounced on blood vessels, and noscapine is an antitussive agent.
Recent occurrence data on the presence of opium alkaloids in poppy seeds of European origin indicate that the alkaloids profile of the poppy seed samples submitted to EFSA after the 2011 opinion may be different compared to the data used in the EFSA opinion, and this issue is particularly important for thebaine.
Therefore, it seems appropriate to consider an update of the Scientific Opinion taking into account the alkaloid profile of the poppy seed samples submitted to EFSA since 2011.
1.1.2. Terms of Reference
In accordance with Art. 29 (1) of Regulation (EC) No 178/2002, the European Commission asks the European Food Safety Authority for an update of the Scientific Opinion on the human health risks related to the presence of opium alkaloids in poppy seeds taking into account the alkaloid profile of the poppy seeds samples submitted to EFSA since 2011.
1.2. Interpretation of the Terms of Reference
The CONTAM Panel concluded this opinion should comprise the:
re‐evaluation of the toxicity of opium alkaloids present in poppy seed samples, for humans considering all relevant toxicological endpoints;
evaluation of the alkaloid profile (i.e. composition of the alkaloids and their concentrations) of the poppy seed samples submitted to EFSA since 2011 and whether the profile is similar to that of the poppy seed samples used in the 2011 opinion;
estimation of the dietary exposure of the EU population to opium alkaloids from poppy seeds, including the consumption patterns of specific groups of the population if appropriate;
assessment of the human health risks for the EU population, including specific groups of the population if appropriate, as the consequence of the estimated dietary exposure.
1.3. Supporting information for the assessment
1.3.1. Chemical and physical properties
The chemical properties of opium alkaloids have been reviewed in the previous EFSA opinion on opium alkaloids (EFSA CONTAM Panel, 2011). The following chapter is an adapted version of this review.
Opium alkaloids can be divided into two distinct chemical classes, phenanthrenes and benzylisoquinolines. Among the more than 80 alkaloids of poppy, the principal phenanthrenes are morphine, codeine and thebaine whereas the principal benzylisoquinolines are noscapine and papaverine. In this Scientific Opinion, the term ‘opium alkaloids’ refers to these compounds. Figure 1 shows the chemical structures of the predominant alkaloids reported to occur in poppy seed samples. The opium alkaloids with a phenanthrene structure are also called morphinans, which are typified by an aromatic A ring and (partly) saturated B and C ring and with an additional nitrogen‐containing D ring, spanning carbons 9 and 13 of the phenanthrene structure. In the biosynthetic pathway, thebaine is the precursor of oripavine and of codeine, which are both precursors of morphine (Appendix A). The benzylisoquinolines noscapine and papaverine are not closely related in the biosynthetic pathway.
Figure 1.
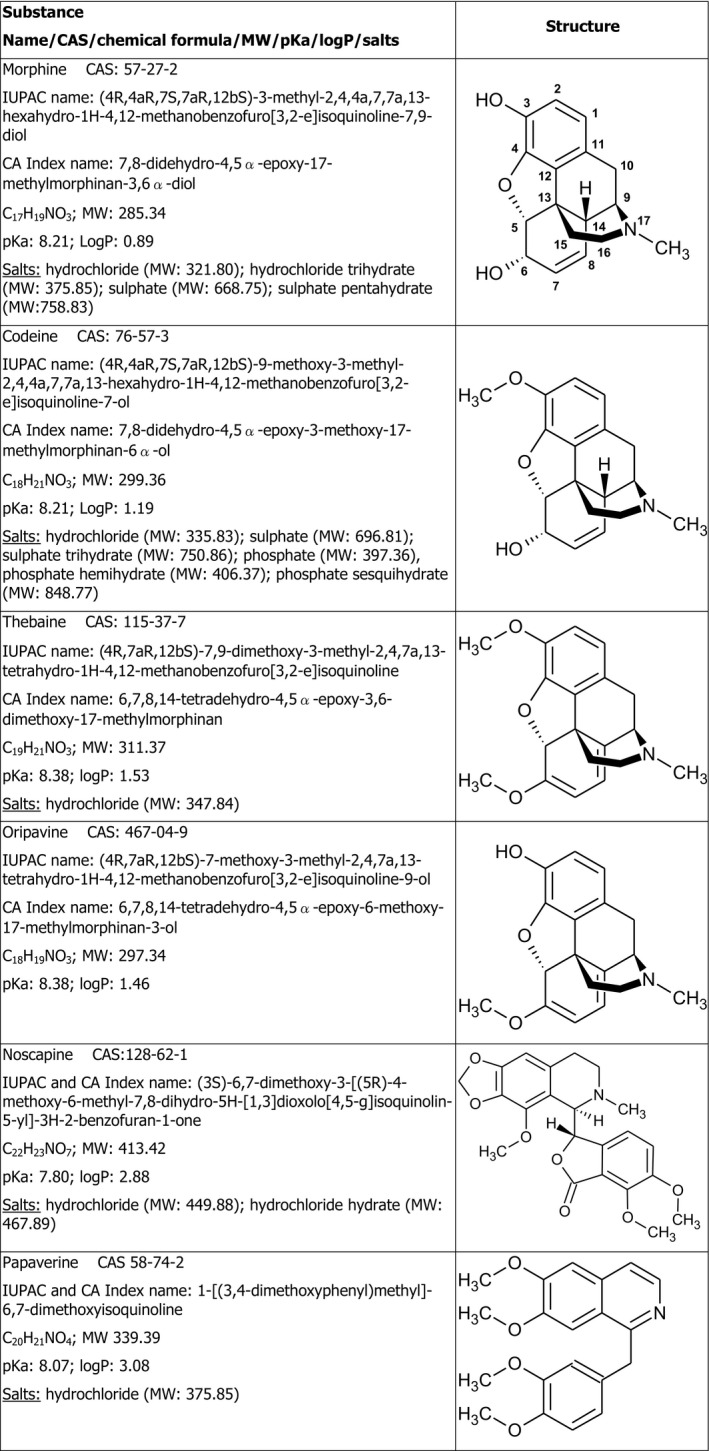
Structures of opium alkaloids considered in this Scientific Opinion
- MW: molecular weight; CAS: chemical abstracts service number; pKa: log of acid dissociation constant; logP: log of octanol: water partition coefficient. Different sources were used for retrieving the chemical structures above.1
1.3.2. Analytical methods
The analytical methods for the detection of opium alkaloids in poppy seeds and food have been reviewed in the previous EFSA opinion on opium alkaloids (EFSA CONTAM Panel, 2011). Only a short update is presented here.
Extraction of opium alkaloids from the biological matrix is often performed with the use of (mixtures) of aqueous or organic solvents under acidic conditions. Depending on the matrix or the limit of detection (LOD) that is required, additional clean‐up by solid‐phase extraction (SPE) or liquid/liquid extraction (LLE) may be necessary. Detection methods most often used are high‐performance liquid chromatography‐ultraviolet (HPLC‐UV), Gas chromatography–mass spectrometry (GC–MS) and liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS). GC–MS and LC–MS/MS have the advantage of higher selectivity and sensitivity than HPLC–UV. GC–MS has as drawback that derivatisation may be required to obtain sufficient sensitivity. Nowadays, LC–MS/MS, equipped with an electrospray ionisation source, has become the most popular technique for targeted analysis of opium alkaloids. Due to the high sensitivity of LC–MS/MS, sample preparation can often be minimal (dilute‐and‐shoot) resulting in a higher sample through‐put (López et al., 2018). For analysis of opium alkaloids in more complex matrices, or at low levels, additional clean‐up steps may still be required in order to reduce matrix interferences and suppression/enhancement effects that may otherwise hamper quantification. GC–MS, LC–MS/MS or liquid chromatography coupled to high‐resolution mass spectrometry (LC–HRMS) techniques are also increasingly used for comprehensive metabolite profiling of poppy cultivars or opium latex (Choe et al., 2011; Desgagné‐Penix et al., 2012; Liu et al., 2016a).
Isotope‐labelled standards are commercially available for the major opium alkaloids and for many of their synthetic derivatives. In most cases, these are D3 or D6 analogues of the unlabelled congener. 13C‐isotope‐labelled standards are much less common, but a 13C4 analogue of morphine and codeine is commercially available. The isotopically labelled standards can be used as internal standards in LC–MS/MS and GC–MS methods to improve the accuracy of the determination of the corresponding unlabelled congeners as the internal standard will correct for matrix effects and recovery.
No progress has been made with respect to the availability of certified reference materials of food products that contain opium alkaloids at relevant concentrations. No international proficiency tests for their determination were identified.
LC–MS‐ and GC–MS‐based analytical methodologies are also widely applied for the determination of opium alkaloids and their metabolites in human biological samples such as urine, plasma, serum, hair, saliva, cerebrospinal fluids, often in relation to forensic cases, drug abuse or palliative care (see for reviews: Bosch et al., 2007; Barroso et al., 2011). As these methods are not directly used for food, they are beyond the scope of this opinion and thus not further described.
1.3.3. Previous assessments
In 2011, the EFSA CONTAM Panel issued an opinion on the risks for public health related to the presence of opium alkaloids in poppy seeds intended for human consumption (EFSA CONTAM Panel, 2011). EFSA received results from analyses of opium alkaloids, primarily morphine, codeine, thebaine, papaverine and noscapine in samples of poppy seeds, bakery products and baking ingredients. Based on the relative prevalence of the alkaloids present in poppy seed and food samples analysed, and on their pharmacological potency, the Panel concluded that the risk assessment could be based on dietary exposure to morphine alone. This assessment included the estimation of morphine‐equivalents based on morphine and codeine concentrations, but the CONTAM Panel concluded that codeine has a minor impact.
The CONTAM Panel established an ARfD of 10 μg morphine/kg bw based on the lowest known single oral therapeutic dose of 30 μg morphine/kg bw and applying an uncertainty factor of 3 for extrapolation from the lowest‐observed‐effect‐level (LOEL) to a no‐observed‐effect level (NOEL) considering that the LOEL was derived from patients and not from the general population.
The CONTAM Panel concluded that a considerable proportion of consumers of foods that contain large amounts of poppy seeds, such as are common in central‐eastern European countries, are likely to exceed the ARfD for morphine on at least some eating occasions. Taking the possible reduction during food processing into account, the ARfD was most likely to be exceeded when single large portions were consumed or if foods containing raw, unground poppy seeds were consumed. The CONTAM Panel stressed that this risk assessment related to poppy seed samples with an alkaloid profile comparable to that of the submitted data and should not be extrapolated to poppy seed samples with a qualitatively different alkaloid profile.
Several institutions, including the European Medicines Agency (EMA), recently re‐evaluated the pharmaceutical use of codeine and developed recommendations restricting the use in infants, children, breast‐feeding mothers and ultra‐rapid metabolisers (see Section 3.1.3.2 Codeine).
1.3.4. Legislation
1.3.4.1. Food
In order to protect public health, Article 2 of the Council Regulation (EEC) No 315/932 stipulates that, where necessary, maximum tolerances for specific contaminants shall be established. Thus, a number of maximum tolerances for contaminants as well as natural plant toxicants are currently laid down in Commission Regulation (EC) No 1881/20063. However, no maximum levels for opium alkaloids in poppy seeds have been established neither under this nor under another Regulation.
In 2014, the European Commission published a recommendation on good practices to prevent and to reduce the presence of opium alkaloids in poppy seeds and poppy seed products.4 These include good agricultural practices to prevent the presence of opium alkaloids during growing, harvesting and storage, and good practices to prevent the presence of opium alkaloids during processing. These recommendations include:
adequate control of pests and diseases,
prevention of bad harvesting conditions caused by lodging of plants,
application of appropriate harvesting conditions (i.e. moisture content and appropriate harvesters),
post‐harvest cleaning including the removal of dust particles and other impurities,
application of ventilated storage,
labelling in case the poppy seeds need to undergo an additional treatment to reduce the opium alkaloid content before human consumption,
application of pretreatments and processing methods reducing the concentration of opium alkaloids.
In addition, several countries have national legislation on the presence of opium alkaloids in poppy seeds.
1.3.4.2. Narcotics
Among the three main international drug control conventions of the United Nations (UN), the Single Convention on Narcotic Drugs of 1961, as amended by the 1972 Protocol, includes the regulation on poppy cultivation (beside coca bush and cannabis plant).5 It was established to achieve more effective, co‐ordinated and universal measures against drug abuse. This convention defines the methods and rules of estimation of drug requirement (each for medical and scientific purposes as well as for manufacture of other drugs) and providing statistics on production (both as quantity and area) and stocks of these drugs, their consumption, export and import. The list of substances (among them concentrate of poppy straw,6 opium, morphine, thebaine and derivatives of morphine and codeine) underlying to this convention, is included in an addendum ‘ST/CND/1/Add.1’. The countries permitting the cultivation of the opium poppy for the production of opium (alkaloids) shall maintain government agencies to carry out the controlling functions defined in the convention. Article 25 especially about control of poppy straw stipulates that the country that permits the cultivation of the opium poppy for purposes other than the production of opium shall take all measures necessary to ensure that opium is not produced from such opium poppies; and that the manufacture of drugs from poppy straw is adequately controlled.
1.3.5. Poppy seeds and opium poppy – botanical origin, ingredients, uses, varieties and cultivation
The botanical origin, uses, varieties and cultivation of poppy seeds and opium poppy has been extensively reviewed in the previous EFSA opinion on opium alkaloids (EFSA CONTAM Panel, 2011). The following chapter is an updated excerpt of this review.
Poppy seed is an oilseed obtained from the opium poppy (Papaver somniferum L.) that is used as food and to produce edible oil. P. somniferum L. is cultivated for production of alkaloids and opium for pharmaceutical purposes. The alkaloids are synthesised, stored and metabolised in the latex (milky sap). The latex permeates all parts of the plant other than the seeds and is to be found in particular in the pericarp of the capsule. The ripened seeds do not contain milky sap and do not naturally contain opium alkaloids. However, opium alkaloids can be present on the surface of the poppy seeds as a result of contamination from dust of capsule walls during harvest and/or improper processing (Fairbairn and El‐Masry, 1968; Rochholz et al., 2004). Contamination may also result from insect damage (weevil; Ceutorhynchus macula‐alba) due to chewing of larvae on the unripe capsule wall resulting in droplets of latex on the seed surface (Bernáth and Németh, 2010). On the other hand, the morphine content on the surface of the seeds can drastically be reduced by professional cleaning machines and especially through washing (e.g. Lo and Chua, 1992; Andresen and Schmoldt, 2004; Commission Recommendation 2014/662/EU7 – see Section 3.1.2.3).
There is a wide range of cultivars and ecotypes of P. somniferum grown all over the world that are differing in morphological (e.g. growth, branching, flower colour, capsule shape, etc.) and chemical characteristics (level and spectrum of alkaloids) and furthermore in the length of vegetation cycle and yield. Eighteen countries are authorised by the UN to produce poppy for pharmaceutical use (Austria, Australia, China, France, Germany, Great Britain, Hungary, India, Japan, Macedonia, the Netherlands, New Zealand, Poland, Romania, Slovakia, Spain, Turkey and Ukraine). To a large extent, this list coincides with the countries that are reported by the Food and Agriculture Organization (FAO)8 as the producers of poppy seed (Austria, Bulgaria, Croatia, the Czech Republic, France, Germany, Hungary, Macedonia, the Netherlands, Occupied Palestinian Territory, Romania, Serbia, Slovakia, Spain, and Turkey) (Lohr, 2017). In the European Union (EU), the main poppy seed producing country is the Czech Republic (Figure 2). Other important producers are Spain, France and Hungary. Own production of the Netherlands is relatively small but it is considered the largest centre of the world regarding poppy seed trade. Globally, the biggest producer of poppy alkaloids is Australia followed by Turkey. Recently, China has also emerged on the market. These three countries are also the main exporters to the EU (Figure 3).
Figure 2.
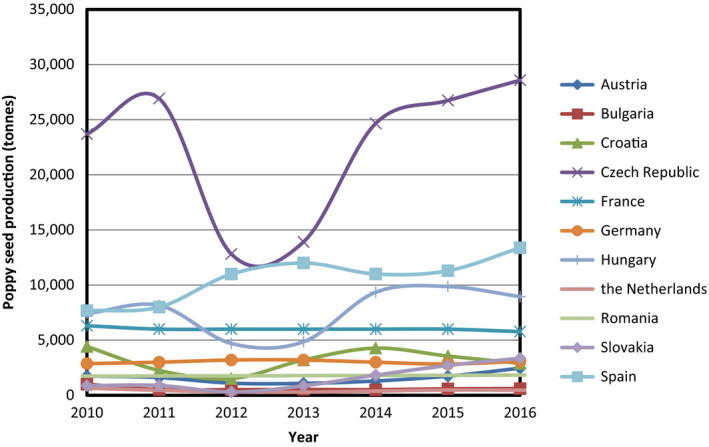
Poppy seed production in EU countries in 2010–2016 (based on data extracted from FAOSTAT)
Figure 3.
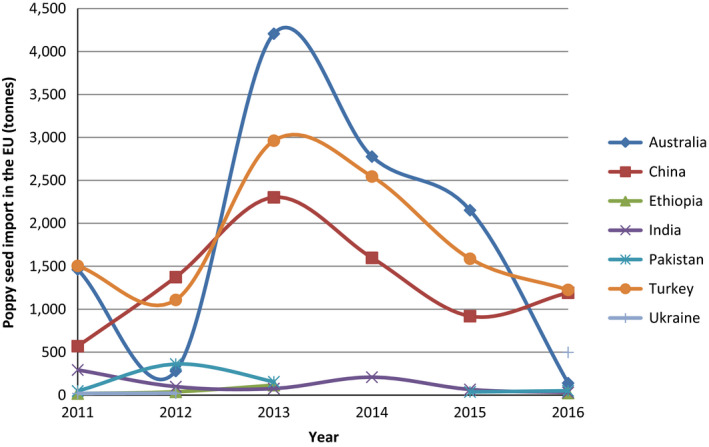
Poppy seed import from third countries into the EU in 2011–2016 (based on data extracted from FAOSTAT)
- Data only shown for third countries from which at least in one year 100 tonnes were imported.
Poppy seeds are used in the food sector. They are used in bakery products, on top of dishes, in fillings of cakes and in desserts and to produce edible oil. The oil content in poppy seeds varies between 32% and 57%. The oil is obtained by solvent or mechanical extraction and the remaining poppy seed cake is crushed and used for cattle feed. Besides the seeds originating from varieties particularly grown for food production, also seeds from varieties grown for pharmaceutical purposes enter the food market (Fist, 2001).
The latex of the immature capsules is released by incisions and when dried is called opium. The opium contains approximately 20–25% alkaloids of which around 50 alkaloids have been isolated in pure form up to now. Poppy straw (i.e. capsules with the seeds removed and with 5–10 cm stem part) contain the same active substances as opium but at a lower concentration (up to 3–4%; Németh‐Zámbori et al., 2011; Desgagné‐Penix et al., 2012). Both opium and poppy straw are used as raw material by the pharmaceutical industry. Stems, leaves and roots accumulate much lower amounts of alkaloids than the capsules, usually well below 1% and sometimes only in traces (Gessner and Orzechowski, 1974; Gurkok et al., 2016).
Alkaloid accumulation in the poppy plant depends on both genetic factors and environmental/cultivation conditions. On the one hand, breeding is focusing on the development of cultivars with high and specific alkaloid content to fulfil the requirements of the pharmaceutical industry (Desgagné‐Penix et al., 2012; Chaturvedi et al., 2014) and on the other hand on cultivars with low alkaloid content for seed/oil production (Bernáth and Németh, 2009). In some European countries (e.g. Germany, the Czech Republic), only ‘low‐morphine’ varieties of P. somniferum are authorised for cultivation. They have been selected for the production of seeds for food use or to produce oil. In some other countries, only varieties for pharmaceutical use of the capsules are grown (e.g. Australia, France, Spain) while in certain countries (e.g. Hungary, Slovakia) both culinary and pharmaceutical varieties are cultivated (Lohr, 2017). A generally defined borderline between the alkaloid contents of these two types of varieties does not exist. However, in some countries (e.g. the Czech Republic9 and Hungary10) national legislation defines the alkaloid content of varieties for food use). Currently, 54 varieties are listed on the EU Plant variety Catalogue.11 The OECD list contains 45 varieties, including 40 varieties in common with the EU list, but also 5 Turkish genotypes.12 Unfortunately, these open databases do not offer information on the alkaloid contents of the varieties. At the same time, several other, non‐registered strains/genotypes/accessions exist which have not been the target of official variety legislation as they seem to be cultivated in closed areas for pharmaceutical industry only (Millgate et al., 2004; Desgagné‐Penix et al., 2012; Chaturvedi et al., 2014).
In course of time, the need of the pharmaceutical industry for specific opium alkaloid profiles has changed with current demands for cultivars not only rich in morphine, but also in codeine, oripavine thebaine or noscapine (Dang and Facchini, 2014).
Besides the genetics, environmental and cultivation conditions may also influence the alkaloid content of the plant. Warm and sunny weather during capsule development and ripening contribute to higher accumulation levels of morphine (Bernáth, 1998), while the opposite effect has been reported for noscapine (Kálmán‐Pál et al., 1987). Nitrogen fertilisation may enhance the accumulation of alkaloids (Kadar et al., 2001); however, only under excessive light conditions (Bernáth, 1986). Severe drought stress may decrease the accumulation of morphinans (Bernáth, 1986). Experimental data showed that accumulation of heavy metals (Cd, Zn) – due to their higher concentrations in the soil – correlates with higher noscapine levels in the seeds (Lachman et al., 2006). Heavy rain may cause a wash out of alkaloids from mature capsules resulting in a marked drop in content. Morphine and codeine are more easily washed out than thebaine or noscapine (Hofman and Menary, 1984a). Pathogens, like viruses, may enhance the concentration of alkaloids in the plant (Zaim et al., 2014) while on the other hand certain fungi can cause a considerable reduction of the morphine yield (Hofman and Menary, 1984b).
The total alkaloid content changes during ontogenesis: it increases until the flowering period and then drops again (Kadar et al., 2001; Frohne and Pfänder, 2004; Blaschek et al., 2006).
2. Data and methodologies
The CONTAM Panel applied the general principles for the assessment of chemicals in food as described by WHO/IPCS (2009) and any EFSA guidance documents pertaining to risk assessment and relevant for the present assessment (see Appendix C, Section C.4).
2.1. Supporting information for the assessment, and previously reported occurrence data and dietary exposure
The CONTAM Panel issued a risk assessment on opium alkaloids in poppy seeds in 2011 and this opinion was used as a starting point for drafting the supporting information. The data was summarised in a narrative way based on expert knowledge/judgement and updated when new information had become available (further details see Appendix C, Section C.1).
2.2. Hazard identification and characterisation
2.2.1. Collection and selection of evidence
A comprehensive search for literature was conducted for peer‐reviewed original research pertaining to adverse health effects in experimental animals and humans. The search strategy was designed to identify scientific literature dealing with toxicity, mode of action, toxicokinetics and human data on morphine, codeine, thebaine, oripavine, papaverine and noscapine. Since this Scientific Opinion is an update of the Scientific Opinion on the human health risks related to the presence of opium alkaloids in poppy seeds published in 2011, the literature search was restricted to papers published in 2011 and after. However, given the limited information available on thebaine and oripavine in the Scientific Opinion published in 2011, no time restrictions were included in the literature search for these 2 substances. An overview of the search terms is given in Appendix C, Section C.2.
The literature search was not restricted to publications in English. A first literature search was performed in February 2017 and has been updated until December 2017. Web of Science,13 PubMed14 and Embase were identified as databases appropriate for retrieving literature for the present evaluation. The references obtained from the literature search were imported and saved using a software package (EndNote15). The references obtained were screened using title and abstract to identify the relevant literature and exclusion criteria are shown in Appendix C, Section C.3. For morphine, Distiller SR was used to screen the title and abstract due to the high number of identified papers.
Additionally, reviews, relevant scientific evaluations by national or international bodies were considered for the current risk assessment.
2.2.2. Appraisal of evidence
The information retrieved has been screened and evaluated by relevant domain experts from the CONTAM Working Group (WG) on opium alkaloids and has been used for the present assessment. Any limitations in the information used are documented in this Scientific Opinion.
Selection of the scientific papers for inclusion or exclusion was based on consideration of the extent to which the study was relevant to the assessment or on general study quality considerations (e.g. sufficient details on the methodology, performance and outcome of the study, on dosing, substance studied and route of administration and on statistical description of the results), irrespective of the results.
2.3. Occurrence data submitted to EFSA
2.3.1. Data collection and validation
At the moment of receiving the request for the update of the scientific opinion from the European Commission, some occurrence data on opium alkaloids in food were already available in the EFSA Chemical Occurrence database; part of those analytical data (2,678) were presented in the previous opinion on opium alkaloids in poppy seeds (EFSA CONTAM Panel, 2011). In order to increase the number of available data for the exposure assessment and to update the data set with more recent data points, in 2017, the EFSA Evidence Management Unit (DATA Unit) alerted the data provider network, requesting data on opium alkaloids in poppy seeds and food commodities. Data providers were specifically invited to submit data on opium alkaloids as single chemicals rather than as sum of opium alkaloids, with a specific focus on the following substances: morphine, codeine, papaverine, noscapine, thebaine, oripavine and narceine. European national food authorities and similar bodies, research institutions, academia and food business operators were invited to submit data through the Call for continuous collection of chemical contaminants occurrence data in food and feed.16 Only data submitted until October 2017 were considered for this Scientific Opinion.
The data submission to EFSA followed the requirements of the EFSA Guidance on Standard Sample Description for Food and Feed (EFSA, 2010a); occurrence data were managed following the EFSA standard operating procedures (SOPs) on ‘Data collection and validation’ and on ‘Data analysis of food consumption and occurrence data’.
2.3.2. Data analysis
Data were thoroughly evaluated, this including cleaning and validation steps. Special attention was paid to the identification of duplicates and to the accuracy of different parameters such as ‘Analytical methods’, ‘Reporting unit’, ‘Sampling strategy’ and the codification of the different samples under FoodEx classification. Upon identification of potential inconsistencies, data providers were contacted to provide further clarification. Among others, particular attention was also devoted to the ‘Country of Origin’ and when this was not reported specific clarification request was sent to the data providers. Details of data cleaning are reported in Annex A (Table A.1) while the outcome of the data analysis is shown in Section 3.2.1.
The left‐censored data (analytical data reported below the LOD/limit of quantification (LOQ)) were treated by the substitution method as recommended in the ‘Principles and Methods for the Risk Assessment of Chemicals in Food’ (WHO/IPCS, 2009). This method is also indicated in the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010b), as an option for the treatment of left‐censored data. According to this guidance the lower bound (LB) and upper bound (UB) approach should be used for chemicals likely to be present in the food (e.g. naturally occurring contaminants, nutrients and mycotoxins). At the LB, results below the LOQ or LOD were replaced by zero; at the UB, the results below the LOD were replaced by the LOD and those below the LOQ were replaced by the value reported as LOQ. Additionally, a middle bound (MB) approach was used by assigning a value of LOD/2 or LOQ/2 to the left‐censored data.
2.4. Food consumption data
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database) provides a compilation of existing national information on food consumption at individual level. It was first built in 2010 (EFSA, 2011a; Huybrechts et al., 2011; Merten et al., 2011). Details on how the Comprehensive Database is used are published in the Guidance of EFSA (2011a). The latest version of the Comprehensive Database contains results from a total of 51 different dietary surveys carried out in 23 different Member States covering 94,532 individuals.
The age classes considered in the EFSA Comprehensive European Food Consumption Database are the following:
Infants: < 12 months old
Toddlers: ≥ 12 months to < 36 months old
Other children: ≥ 36 months to < 10 years old
Adolescents: ≥ 10 years to < 18 years old
Adults: ≥ 18 years to < 65 years old
Elderly: ≥ 65 years to < 75 years old
Very elderly: ≥ 75 years old.
Overall, the food consumption data in the Comprehensive Database are the most complete and detailed data currently available at EU level. Consumption data were collected using single or repeated 24‐ or 48‐h dietary recalls, and dietary records covering from 3 to 7 days per subject. Owing to the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading. Detailed information on the different dietary surveys available in the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) can be found on the dedicated page of the EFSA website.17
2.5. Food classification
Consumption data were classified according to the FoodEx classification system (EFSA, 2011b). FoodEx is a food classification system developed by EFSA in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing the exposure to hazardous substances. It contains 20 main food groups (first level), which are further divided into subgroups having 140 items at the second level, 1,261 items at the third level and reaching about 1,800 endpoints (food names or generic food names) at the fourth level.
In 2011, a new version of FoodEx, named FoodEx2 has been developed and is described in the scientific document ‘Report on the development of a Food Classification and Description System for exposure assessment and guidance on its implementation and use’ (EFSA, 2011c). The last release of FoodEx2 complements the previous hierarchical classification system of basic codes with more detailed food levels and gives the possibility of reporting additional information through the use of facets and facet descriptors (EFSA, 2015).
2.6. Exposure assessment
The CONTAM Panel considered that only acute dietary exposure to opium alkaloids had to be assessed as for the previous opinion (EFSA CONTAM Panel, 2011). Thus, for acute exposure assessment, food consumption data were used from 41 different and most recent dietary surveys carried out in 23 different European countries present in the latest version of the Comprehensive Database (Annex B, Table B.1). Not for all countries consumption information is available for all age groups.
In addition to the reporting of poppy seed consumption, reports on consumption of foods containing poppy seeds were considered. Therefore, the original food descriptors for each of the foods reported in the Comprehensive Database were searched for the term ‘poppy’. In this way, a larger amount of eating occasions of food products containing poppy seeds as ingredient were identified. In order to estimate the amount of poppy seeds in each of these food products, disaggregation factors were worked out from the portfolio of recipes compiled in the 2011 opinion (EFSA CONTAM Panel, 2011). When it was possible to associate more than one recipe to a food product, the average content of poppy seeds from the different recipes was used. Annex B, Table B.2 reports the average disaggregation factors for each food product. Based on this approach, poppy seed consumption was reported in 18 dietary surveys from 13 countries (Annex B, Table B.3).
Exposure estimates were calculated for each dietary survey and age class. Acute dietary exposures were calculated by combining opium alkaloids mean, median, P75, P90, P95 and P99 concentrations in poppy seed samples with the mean and P95 consumption of poppy seeds (expressed on a body weight basis) per day.
All analyses were run using the SAS Statistical Software (SAS enterprise guide 5.1).
2.7. Risk characterisation
The CONTAM Panel applied the general principles of the risk characterisation process for chemicals in food as described by WHO/IPCS (2009) and the relevant EFSA guidance documents (see Appendix C, Section C.4).
3. Assessment
3.1. Hazard identification and characterisation
3.1.1. Toxicokinetics
3.1.1.1. ADME
The information on absorption, distribution, metabolism and excretion (ADME) of opium alkaloids presented below, relates to humans unless specified differently.
Morphine
Morphine is extensively absorbed from the GI tract of humans and rats and is distributed throughout the body. The oral bioavailability of morphine is reduced by both Phase I and II presystemic metabolism in the GI tract and liver. Though the brain is its primary site of action, morphine does not cross the blood–brain barrier easily. Morphine diffuses across the placenta, and transfers into milk. Morphine is metabolised via N‐demethylation and O‐glucuronidation in the gut and the liver. Glucuronidation is the predominant route of metabolism, producing approximately 60% of morphine‐3‐glucuronide (M3G) and 5–10% of morphine‐6‐glucuronide (M6G). These reactions are principally catalysed by uridine diphosphate‐glucuronosyltransferase UGT2B7 and to a lesser extent UGT1A8. Approximately 5% of morphine is N‐demethylated to normorphine via the cytochromes P450 (CYP) enzymes CYP3A4 and CYP2C8. In addition, other minor metabolites are formed: morphine‐3,6‐diglucuronide, codeine and morphine‐3‐sulfate. About 90% of morphine is excreted in the urine within 24 h mainly as M3G, M6G, and only 2–12% is excreted as morphine unchanged. The elimination half‐life of morphine is approximately 2 h in humans. Both morphine and M6G have a high affinity to the μ‐opioid receptor, whereas M3G has a very weak affinity and is therefore considered as an inactive metabolite (see Section 3.1.4) (EFSA CONTAM Panel, 2011).
Morphine has a low plasma protein binding of 35%, whereas the binding for M3G and M6G is even lower: 10% and 15%, respectively (van Dongen et al., 1994). Higher plasma concentrations of M3G and M6G have been measured in pharmacokinetic studies (Sawe et al., 1983). Penetration rate of M3G and M6G across the blood–brain barrier is lower than for morphine itself (Yoshimura et al., 1973).
The rate and pathways of opioid metabolism may be influenced by genetic factors and medical conditions (liver and kidney disease).
More information on the ‘pharmacogenetics’ of transporters has become available since the previous opinion. The distribution of morphine is under the control of several polymorphic genes, which can account for part of the observed interindividual variation in morphine effects. For instance, pain relief variability of morphine was associated with single‐nucleotide polymorphism of the ABCB1 gene (coding for a P‐glycoprotein transporter) (Campa et al., 2008) that may modify the ability of the alkaloid to cross the blood–brain barrier. Similar results were obtained in a separate study when fentanyl was used as analgesic (Dzambazovska‐Trajkovska et al., 2016). The importance of the ABCB1 polymorphism was also confirmed by investigating the risk of respiratory depression in response to morphine administration (Sadhasivam et al., 2015). A similar role in influencing the risk of morphine induced respiratory depression has been recently established for the gene ABCC3 (coding for a transporter that facilitates hepatic morphine metabolite efflux) (Venkatasubramanian et al., 2014; Sadhasivam et al., 2015; Chidambaran et al., 2017). OCT1, a member of the organic cation transporters (OCTs) family, mediates cellular uptake of morphine into hepatocytes. Children with OCT1 homozygous genotypes have lower morphine clearance and have significantly lower M3G and M6G formation. Relatively high allelic frequencies of defective OCT1 variants among the Caucasian population may explain their lower morphine clearance and higher frequencies of adverse effects compared with African American children (Fukuda et al., 2013; Venkatasubramanian et al., 2014). Genetic polymorphisms can lead to substantially low OCT1 activity in up to 9% of the Europeans (Tzvetkov, 2017). Breastfed infants with mothers who have a low OCT1 transporter activity are also at increased risk for toxicity (Tzvetkov, 2017).
In some studies, UGT2B7 polymorphism has been associated with interindividual variability in the pharmacokinetics and/or adverse effects of morphine, while other studies have dismissed this association (Kwara et al., 2009; Madadi et al., 2009; Fujita et al., 2010). Eissing et al. (2012) have shown that increased UGT2B7 activity is associated with a decrease in active opioid exposure (lower morphine and M6G plasma exposure); however, CYP3A4 (which catalyses the formation of norcodeine and normorphine) inhibitors have only minor influences on active internal opioid exposure (increases of approximately 10% in morphine and 15% M6G exposure).
Reduced renal function has been reported to increase the risk of adverse opioid effects. This may be the consequence of the increase of M6G in case of renal impairment (Gasche et al., 2004; Klimas and Mikus, 2014). However, according to other researchers, elimination of morphine is not significantly impaired in patients with renal dysfunction (Aitkenhead et al., 1984; Sawe et al., 1985; Woolner et al., 1986; Eissing et al., 2012). Based on AUC data, there is a major contribution of M6G to the analgesic effect of morphine (96.6% after oral administration of morphine). In patients with renal insufficiency, 97.6% of the analgesic effect is caused by M6G after oral administration of morphine (Klimas and Mikus, 2014).
In newborns and young infants, morphine elimination is much slower because of immature liver and kidney functions (Lynn and Slattery, 1987; Bhat et al., 1990; Osborne et al., 1993). The ontogeny of UGT2B7 is comparatively slow, and adult activity levels are attained between 2 months and 3 years of age (Strassburg et al., 2002; Edginton et al., 2006).
Codeine
Codeine (as codeine phosphate) is readily and extensively absorbed from the GI tract following oral administration. The maximum plasma concentration is reached after about 1 h. Interindividual variations in bioavailability (40–70%) have been shown. Codeine is distributed throughout the body. It penetrates the placental barrier and enters fetal circulation. Oral bioavailability of codeine in humans is approximately fivefold greater than in rats; however, the amount of codeine converted to morphine is approximately 30‐fold greater in the rat. As a result, internal exposures to morphine from an oral dose of codeine would be predicted to be approximately sevenfold lower in humans relative to rats. Codeine and its metabolites are mainly excreted as glucuronides via the kidney. In healthy adults, the elimination half‐life is 3–5 h and between 9 and 18 h in the case of renal insufficiency. The elimination of codeine is also slower in older people. In lactating women, it is excreted in breast milk and concentrations are approximately 2.5‐fold higher compared to maternal plasma concentrations (EFSA CONTAM Panel, 2011).
The principal pathways for metabolism of codeine occur in the liver, although some metabolism occurs in the intestine and brain. Approximately 50–70% of codeine is converted to codeine‐6‐glucuronide (C6G) by UGT2B7, 10–15% of codeine is N‐demethylated to norcodeine by CYP3A4 and about 0–15% of codeine is O‐demethylated to morphine, the most active metabolite, via CYP2D6 (Yue et al., 1991; Caraco et al., 1996b; Coffman et al., 1997) (Figure 4). Norcodeine is then glucuronidated to norcodeine‐6‐glucuronide (N6G) and a minor part is O‐demethylated to normorphine (Yue et al., 1989; Yue and Sawe, 1997). The formed morphine is metabolised as described above. Codeine, C6G, norcodeine and N6G have weak affinity for the μ‐opioid receptor (Chen et al., 1991; Mignat et al., 1995; Volpe et al., 2011 – see Section 3.1.4). O‐demethylation is regarded to be the bioactivation reaction essential for the analgesic activity of codeine.
Figure 4.
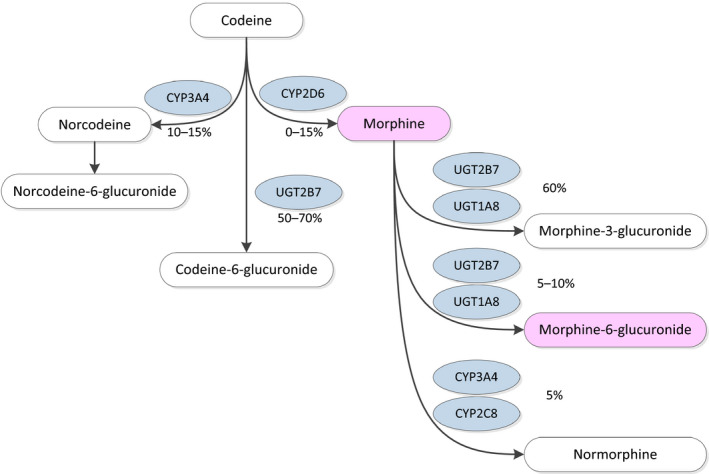
Codeine metabolism pathway in an individual with CYP2D6 extensive metabolism based on Crews et al. (2014)
- Metabolites in pink colour indicate main active metabolites.
Functional CYP2D6 activity is only weakly expressed in fetal liver but increases rapidly after birth. There is considerable interindividual variability in CYP2D6 activity within the first 2–4 weeks of life (Crews et al., 2012).
Codeine metabolism to morphine is dependent of the CYP2D6 activity which relates to the phenotype of the gene. In some cases, individuals have more than two copies of the CYP2D6 gene. CYP2D6 alleles are characterised as wild type (normal function), reduced‐function or non‐functional alleles based on the expected activity of the corresponding enzyme. Each allele is assigned an activity value between 0 and 1.0. If multiple copies of the gene are detected, the activity score is multiplied by the number of copies of each allele present. The total CYP2D6 activity score typically ranges from 0 to 3.0 (sum of the values assigned to each allele) (Crews et al., 2012). Individuals can be classified into poor metaboliser, intermediate metaboliser, extensive metaboliser or ultra‐rapid metaboliser as described in Table 1. As reported, the extensive metabolisers represent the majority of the Caucasian population.
Table 1.
Assignment of likely codeine metabolism phenotypes based on cytochrome P450 2D6 diplotypes – data of Crews et al. (2012, 2014)
| Likely phenotypea | Activity score | Genotype |
|---|---|---|
| Ultra‐rapid metabolisers (~ 1–2% of population) | > 2.0 | An individual carrying more than two copies of functional alleles |
| Extensive metaboliser (~ 77–92% of population) | 1.0–2.0 | An individual carrying two alleles coding for a full or reduced function or one full function allele together with either one non‐functional or one reduced‐function allele |
| Intermediate metaboliser (~ 2–11% of population) | 0.5 | An individual carrying one reduced and one non‐functional alleles |
| Poor metaboliser (~ 5–10% of population) | 0 | An individual carrying no functional alleles |
The frequency estimates are based on data from Caucasians and may differ substantially for other ethnicities.
The distribution of poor, extensive and ultra‐rapid metabolisers differ significantly between ethnic groups (Ingelman‐Sundberg et al., 2007). The highest frequency of ultra‐rapid metabolisers in Europe is in southern Europe (Greece, Italy, Portugal and Spain, about 5–10%) (Ingelman‐Sundberg, 2005; Kirchheiner et al., 2007). The highest frequency of ultra‐rapid metabolisers (16–28%) occurs in the North African, Ethiopian and Arab populations (Wong et al., 2012).
Kirchheiner et al. (2007) studied the pharmacokinetics of codeine and its metabolite morphine in poor, extensive and ultra‐rapid metabolisers after administration of a single oral dose of 30 mg codeine. Significant differences were detected between the groups in areas under the plasma concentration vs time curves (AUCs) and the maximum plasma concentrations (Cmax) of morphine (Table 2). So there is a very sharp increase in both morphine AUC and Cmax following codeine in extensive/ultra‐rapid metabolisers due to CYP2D6 duplications. The genotype‐caused differences in metabolism are also reflected in the metabolic ratio in urine.
Table 2.
Morphine area under the curve (AUC) and maximum plasma concentrations (Cmax) in poor, extensive and ultra‐rapid metabolisers following a single oral dose of 30 mg codeine (Kirchheiner et al., 2007)
| Metaboliser type | AUC median (range) | Cmax median (range) |
|---|---|---|
| Poor | 0.5 (0.5–2.8) | 0.05 (0.03–0.07) |
| Extensive | 11 (5–17) | 2.1 (0.6–4.3) |
| Ultra‐rapid | 16 (10–24) | 2.6 (1.5–4.6) |
Based on the available data, the CONTAM Panel concluded in 2011 that the maximal metabolic conversion of codeine to morphine in CYP2D6 ultra‐rapid metabolisers does not exceed 20%. Since then, no new pharmacokinetic studies were identified that change this conclusion.
In addition to the subjects that are genetically deficient in CYP2D6 (poor metabolisers), subjects that ingest CYP2D6 inhibitors, such as quinidine (an anti‐arrhythmic medication), form only negligible amounts of the codeine metabolites, which results in a loss of pharmacological effect (Sindrup et al., 1990; Desmeules et al., 1991; Otton et al., 1993a,b; Caraco et al., 1996a).
Overall, the documented abilities of active transport proteins, including the P‐glycoprotein, and of CYP2D6 phenotype to modify the pharmacological effects of morphine and/or codeine show that genetic polymorphisms affect the sensitivity of humans to adverse effects of these opium alkaloids or to develop addiction. The risk for opioid intoxication might be increased if other additional factors such as reduction in renal function or further inhibition of other enzymes occur.
Thebaine, oripavine, noscapine and papaverine
Based on limited information, oral bioavailability of thebaine, oripavine, noscapine and papaverine appears to be reduced due to presystemic metabolism in the GI tract and liver primarily involving demethylation reactions but also glucuronidation (EFSA CONTAM Panel, 2011).
Tissue distribution studies on male Albino Wistar rats have shown that the uptake of 131I‐thebaine in the stomach, large intestine, spinal cord and prostate was higher than in the other tissues. The clearance was via the urinary and hepatobiliary systems. The uptake of radiolabelled thebaine in the brain was greater in the midbrain and hypothalamus (Enginar et al., 2013).
Kodaira and Spector (1988) have shown in vitro, that thebaine is transformed to oripavine, codeine, and morphine by rat liver, kidney, and brain rat microsomes in the presence of an NADPH generating system. Morphine biosynthesis from thebaine in mammalian tissues can occur by at least two routes: (i) via codeine: thebaine → neopinone → codeinone → codeine → morphine; and (ii) via oripavine: thebaine → oripavine → 3‐O‐demethylated neopinone → morphinone → morphine. The presence of morphine and codeine in mammalian tissue after dosing with thebaine can be explained by the fact that these compounds are enzymatically synthesised there.
After subcutaneous (s.c.) injection, thebaine is extensively metabolised in the rat and eliminated in urine as free and glucuronide metabolites. Thebaine was metabolised in the brain, intestine, liver, kidney and blood. The metabolic pathways of thebaine in the rat appear to be O‐ and N‐demethylation as well as hydroxylation and glucuronide conjugation. Metabolites are oripavine, 14‐hydroxycodeinone, norcodeine, normorphine, codeine and morphine (Misra et al., [Link]; Donnerer et al., 1986). Oripavine appears to be the major metabolite in Sprague–Dawley rats. The conversion of thebaine to oripavine is catalysed by CYP2D1, the rat orthologue to human CYP2D6 (Mikus et al., 1991).
After oral administration (150 mg dose of either rapidly dissolving tablets or a tablet containing ion exchange resin‐bound noscapine) to five human volunteers, noscapine was rapidly absorbed and gave a maximum plasma concentration after 1 h. From then on, the noscapine levels declined with a half‐life of 124 min (range 95–143 min). The absolute oral bioavailability was 30% (range 16–58%) (Dahlström et al., 1982). After gavage administration of noscapine hydrochloride salt (75, 150 and 300 mg/kg) to mice, noscapine was easily and quickly absorbed at all dose levels (t max < 2 h) and was distributed rapidly and widely. The percent bioavailability was comparable at 75 and 300 mg/kg (between 22% and 27%); it was higher at 150 mg/kg (42–48%) for both males and females. Half‐lives were 2.91/3.02 h, 1.47/0.99 h and 2.52/2.65 h in males and females, respectively (Aneja et al., 2007). Noscapine undergoes extensive ‘first pass’ metabolism mainly by C–C cleavage, O‐demethylation and cleavage of methylenedioxy group in rats, rabbits and humans (Tsunoda and Yoshimura, 1981). Noscapine's metabolism in mice has been shown to result in the formation of at least 20 or more metabolites including a reactive intermediate that undergoes conjugation with glutathione (Fang et al., 2012). Phase I metabolism of noscapine has been shown to result in more than 10 metabolites and Phase II metabolism primarily produces at least 9 glucuronide conjugates (Fang et al., 2012). Several enzymes including CYP and flavin monooxygenases have been shown to catalyse the biotransformation of noscapine.
Several novel phase I metabolites of noscapine were detected after oral gavage of mice, including an N‐demethylated metabolite, two hydroxylated metabolites, one metabolite undergoing both demethylation and cleavage of the methylenedioxy group and a bis‐demethylated metabolite. Additionally, several novel glucuronides were detected (Fang et al., 2012).
Iwase et al. (2017) have shown that papaverine, a GI drug, has an inhibitory effect on CYP3A4 activity in human liver microsomes. CYP3A4 is expressed in the liver and also in the small intestine. It is considered that papaverine causes drug interactions mediated by CYP3A4 inhibition in the small intestine rather than the liver.
3.1.1.2. Kinetic modelling
Various models that describe the link between pharmacokinetics (PK) and pharmacodynamics (PD) of opioids and their metabolites have been developed and have been reviewed for adult (e.g. Lotsch, 2005a,b; Martini et al., 2011; Yassen et al., 2013) and paediatric data (Mahmood, 2011; Holford et al., 2012; Krekels et al., 2012; Emoto et al., 2017). In adults, an obvious sex difference in morphine PD but not PK is visible with greater opioid potency in women (Sarton et al., 2000; Niesters et al., 2010). Interest in models that describe the PK and PD in infants and neonates is growing although the model performance in most studies is limited (see review Krekels et al., 2012). Willmann et al. (2009) described a model that relates morphine plasma concentration in mother and infant from maternal codeine consumption in mothers who breastfed. The most critical factors for morphine accumulation in the neonate are the mother's codeine and morphine clearances and the neonate's morphine clearance. Considering the added effect of low neonatal elimination capacity for morphine, the authors reported that potentially toxic morphine plasma concentrations can be reached within 4 days in the neonate after repeated codeine dosing to the mother. CYP2D6 activity in neonates reaches ~ 3–5% of the adult level activity during the first week of life and increases significantly thereafter. Neonates of extensive and ultra‐rapid metabolising mothers have comparable risks of opioid poisoning (Willmann et al., 2009).
Studies linking PK and PD of morphine action and the role of transporter systems are available in experimental (rodent) and human models (Caraco et al., 1999; Zelcer et al., 2005; Borst et al., 2007; Kalvass et al., 2007; van de Wetering et al., 2007; Emoto et al., 2017). In paediatric patients, changes in cardiac output and age‐dependent developmental changes need to be considered as well as key determinants of variability in morphine clearance (Anderson and Holford, 2009; Vinks et al., 2015; Emoto et al., 2017).
Linares et al. (2015) developed a PK pathway model for codeine and its metabolites that incorporate the effects of genetic polymorphisms of CYP2D6, using the data reported by Kirchheiner et al. (2007) as the starting point for the modelling. According to their model, about 10% of a codeine dose was converted to morphine in poor metabolisers, 40% in extensive metabolisers and 51% in ultra‐rapid metabolisers. The model indicated also that about 4% of morphine formed from codeine was converted to M6G in poor metabolisers, 39% in extensive metabolisers and 58% in ultra‐rapid metabolisers; this is important since M6G has a similar or higher potency than morphine in terms of efficacy and opioid‐related toxicities (overall effects on the central nervous system (CNS) and respiratory depression; see Section 3.1.4). The CONTAM Panel noted substantial inconsistencies in morphine and its glucuronides AUC in this paper compared to the experimental data presented by Kirchheiner et al. (2007). The CONTAM Panel concluded that the paper by Linares et al. does not provide a basis to change its previous conclusion from 2011 that the maximal metabolic conversion of codeine into morphine does not exceed 20%.
3.1.2. Toxicity in experimental animals
3.1.2.1. Acute toxicity (single exposure)
No new data since the previous opinion were identified. Table 3 summarises the oral lethal doses (LD50) reported in the literature for the different compounds (EFSA CONTAM Panel, 2011). Since not for all compounds oral LD50 values were available, the CONTAM Panel also considered other routes of administration for comparison of acute toxicity of the compounds (Table 4).
Table 3.
Summary of oral LD50 values reported in scientific literature for the opium alkaloids addressed in this Scientific Opinion
| Substance | Species | LD50 (mg/kg bw)a |
|---|---|---|
| Morphine | Rat | 335 |
| Mouse | 524 | |
| Codeine | Rat | 427 |
| Mouse | 250b | |
| Codeine phosphate | Rat | 266 |
| Thebaine | Rat | 114 |
| Mouse | 54 | |
| Oripavine | No data | |
| Noscapine | Rat | 1,520b |
| Mouse | 853b–3,500 | |
| Papaverine | Mouse | 162b |
bw: body weight; LD50: lethal dose for 50% of the subjects.
LD50 values available from EFSA CONTAM Panel (2011) unless specified differently.
LD50 values available from https://chem.nlm.nih.gov/chemidplus/
Table 4.
Summary of subcutaneous and intraperitoneal LD50 values reported in scientific literature for the opium alkaloids addressed in this Scientific Opinion
| Substance | Route of administration | Species | LD50 (mg/kg bw)a |
|---|---|---|---|
| Morphine | i.p. | Rat | 100 |
| s.c. | Rat | 109 | |
| s.c. | Rat | 630c | |
| i.p. | Mouse | 140/500b | |
| s.c. | Mouse | 220 | |
| s.c. | Mouse | 430c | |
| Codeine | i.p. | Rat | 100 |
| s.c. | Rat | 229 | |
| i.p. | Mouse | 60 | |
| s.c. | Mouse | 84.1 | |
| Thebaine | i.p. | Mouse | 20 |
| s.c. | Mouse | 31 | |
| Oripavine | s.c. | Rat | 13 |
| i.p. | Mouse | 26.1 | |
| s.c. | Mouse | 11 | |
| Noscapine | i.p. | Mouse | 960 |
| s.c. | Mouse | 725 | |
| Papaverine | i.p. | Rat | 59.6 |
| s.c. | Rat | 151 | |
| i.p. | Mouse | 91 | |
| s.c. | Mouse | 170 |
bw: body weight; i.p.: intraperitoneal; LD50: lethal dose for 50% of the subjects; s.c.: subcutaneous.
s.c. and i.p. LD50 values available from https://chem.nlm.nih.gov/chemidplus/ unless specified differently.
Gómez‐Serranillos et al. (1998).
Yeh (1981).
Morphine and codeine
Morphine and codeine are potent pharmacologically active substances with their specific actions usually mediated via the specific μ‐opioid receptor. The pattern of acute toxicity might be different between animal species due to the specific distribution of several opioid receptors (μ, κ and δ) (EFSA CONTAM Panel, 2011).
Thebaine
Balint (1971) investigated the effects of thebaine administered intraperitoneal (i.p.) in mice. On narcosis induced by hexobarbital sodium, thebaine produced a stronger potentiating effect than morphine. Its analgesic effect is considerably inferior to that of morphine. In contrast to morphine, it does not cause urine‐retention. It was also shown that thebaine inhibits ‘orientation hypermobility’ and spontaneous motility but it had no effect on hypermotility induced by cocaine and benzpropamine.
The dependence potential of thebaine is at least partially attributed to oripavine, which is one of the principal metabolites of thebaine (WHO, 1980).
Oripavine
Oripavine possesses an analgesic potency comparable to morphine and much higher than that of thebaine; however, it is not clinically useful due to severe toxicity and low therapeutic index (WHO Advisory group, 1981). In both mice and rats, toxic doses caused tonic‐clonic seizures of the limbs and mouth followed by death, similar to thebaine (Gómez‐Serranillos et al., 1998). Oripavine has a potential for dependence, which is significantly greater than that of thebaine but slightly less than that of morphine (Toxnet18; Yeh, 1981).
The analgesic dose 50 (AD50) of oripavine and morphine after s.c. administration in the mouse were 4.1 and 4.0 mg/kg bw, respectively. At doses above 7 mg oripavine/kg bw, convulsions were observed in some mice. The analgesic potency of oripavine in the rat is less than that of morphine. The AD50 of oripavine and morphine after s.c. administration in the rat were 7.7 and 4.5 mg/kg bw, respectively. After administration of 10 mg oripavine/kg bw, convulsions were observed in 9/10 rats and 1 animal died. The analgesic doses of oripavine in mice and rats are close to the lethal doses (LD50 of 11 and 13 mg/kg bw for s.c. administration, respectively). This is not the case for morphine where the LD50 were 430 and 630 mg/kg bw for s.c. administration, respectively (Yeh, 1981). In rats, the physical dependence potential of oripavine at a dose of 4 mg/kg is almost comparable to that of morphine at 0.5 mg/kg. Studies carried out on monkeys show that oripavine possesses weak morphine‐antagonist properties (WHO Advisory Group, 1981).
Clonic‐tonic convulsions followed by death were observed in rats or mice after s.c. or i.p. administration of oripavine (Yeh, 1981; Gómez‐Serranillos et al., 1998). In mice, i.p. injection of oripavine (6.5, 8.7 and 13 mg/kg bw) produced a decrease of the alert status, spontaneous activity and reactivity. It showed also CNS excitation as a sacrococcygeal dorsal muscle contraction and a decrease in the respiratory rhythm.
Toxicity does not appear to be mediated at the opioid receptor. Oripavine showed some cross tolerance with morphine, but did not suppress morphine abstinence in the mouse and rat (Yeh, 1981).
From the LD50 values by s.c. and i.p. administration, it can be concluded that thebaine and oripavine are more toxic than morphine and codeine.
Noscapine
It is reported that noscapine is the least toxic of the opium alkaloids (Krueger et al., 1943 cited by Winter and Flataker (1961)). Noscapine administered by gastric gavage of 800, 1,600 or 3,200 mg/kg bw to fasting rats result in 0/5, 3/5 or 5/5 deaths, respectively. Death appeared to be due to asphyxia, preceded by dyspnea and tonic convulsions. At autopsy the lungs were distended and filled with mucus (Winter and Flataker, 1961).
Papaverine
Besides LD50 values, no data on the acute toxicity of papaverine were identified.
3.1.2.2. Short‐term toxicity
No new data since the previous opinion were identified. The text below summarises the previous information.
Morphine
Dietary studies in which rats were exposed to 100–200 mg/kg bw morphine for 4–6 weeks showed clear opiate dependence, but no effects on body weight and feed intake. However, a clear decrease in feed intake and body weight was observed when morphine was withdrawn as well as changes in spontaneous locomotor activity (EFSA CONTAM Panel, 2011).
The data in rats and mice clearly show the dose dependency with marginal effects in rats at a serum concentration of 200 ng/mL, resulting from an oral dose of 60 mg/kg bw (Van der Laan et al., 1988).
Codeine
Decreased body weights and body weight gains were observed in rats exposed to codeine in feed (≥ 125 mg/kg bw per day after 14 days and ≥ 100 mg/kg bw per day after 13 weeks) and mice (3,000 mg/kg bw per day after 14 days). No treatment‐related gross or histopathological lesions were observed (EFSA CONTAM Panel, 2011).
Thebaine
No data were identified.
Oripavine
No data were identified.
Noscapine
No toxic effects were observed in dogs given orally 30 mg noscapine/kg bw per day for 13 weeks or in rats receiving up to 700 mg noscapine/kg bw per day (EFSA CONTAM Panel, 2011).
Papaverine
Oral administration of papaverine (50 and 100 mg/kg bw per day) to rats during 45 days was well tolerated as no changes were reported. In a 6‐month study in dogs, oral administration of 10 mg papaverine/kg bw per day was tolerated without any adverse effects in clinical, biochemical, and histopathological examinations (EFSA CONTAM Panel, 2011).
3.1.2.3. Long‐term toxicity (including carcinogenicity)
Only limited new data were identified since the previous opinion. The text below summarises the previous and new information.
Morphine
No carcinogenicity study is available on morphine. However, based on the lack of carcinogenicity in mice or rats of codeine, which is metabolised to morphine, the CONTAM Panel concluded that morphine is unlikely to be carcinogenic (EFSA CONTAM Panel, 2011).
Tumour promotion effects were reported with s.c. administration of morphine in a transgenic mice model with breast cancer (female transgenic mice carrying a rat C3(1) simian virus 40 large tumour antigen fusion gene (called C3TAG mice) which causes highly invasive breast tumours) (Gupta et al., 2002; Farooqui et al., 2007; Nguyen et al., 2014). Gach et al. (2011) observed both growth‐promoting and growth‐inhibiting effects. The CONTAM Panel considered the data to be too limited to be further considered in this Scientific Opinion.
Codeine
Long‐term feeding toxicity studies in rats and mice showed no evidence of carcinogenic activity. Exposure‐related decreased body weight was noted in treated animals. In rats, at 2 years, there was an exposure‐related decrease in the incidences of adrenal medulla hyperplasia in both sexes, of benign pheochromocytomas in males and of mammary gland fibroadenomas and fibroadenomas or adenocarcinomas (combined). In mice, at 2 years, there was a significant increased incidence of thyroid gland follicular cell hyperplasia. The incidence of hepatocellular adenomas and adenomas or carcinomas (combined) were significantly lower at 400 mg/kg bw per day than in controls. The lower incidence of tumours in high‐dose animals of both species compared with control was considered to be related to the suppression of body weight gain at this level. The no‐observed‐adverse‐effect level (NOAEL) for chronic toxicity was 15 mg/kg bw per day in rats and less than 100 mg/kg bw per day in mice (EFSA CONTAM Panel, 2011).
Thebaine
No data were identified.
Oripavine
No data were identified.
Noscapine
No data were identified in the literature. The use of noscapine as an antitussive drug is intended to be short‐term, therefore not warranting life‐time carcinogenicity studies in accordance with regulatory guidelines (EFSA CONTAM Panel, 2011).
Papaverine
No data were identified.
3.1.2.4. Genotoxicity
Morphine
The CONTAM Panel concluded in 2011 that morphine is genotoxic only in vivo, most likely by a non‐DNA reactive and opioid receptor‐mediated mechanism, which might be caused by species‐specific systems (EFSA CONTAM Panel, 2011). This conclusion was based on the following observations.
In vitro, morphine is not mutagenic in Salmonella spp. or yeast test systems or after short‐term exposure in hprt gene mutation assays. However, a highly significant increase in the mutation frequency at the hprt locus was observed after treatment of human T‐cells for 3–4 days (Shafer et al., 1994). It did not induce chromosomal aberrations in cultured human lymphocytes (Falek et al., 1972) or was equivocal in one chromosomal aberration test (Snyder, 2009) and negative in an in vitro micronucleus test in mouse splenocytes (without S9, 21 h exposure) (Sawant and Couch, 1995). It induced a significant, dose‐dependent increase in DNA damage (single strand breaks, detected in a Comet assay) in cultured human T cells (Shafer et al., 1994). In mice, i.p. injection of morphine induced an increase in the frequency of micronuclei in splenocytes (Couch and Sawant, 1995; Sawant and Couch, 1995). Acute, but not chronic, morphine administration has been shown to increase the frequency of chromosome aberrations and micronuclei in mouse bone marrow cells (Swain et al., 1980; Das and Swain, 1982). Studies show also that morphine induced DNA damage in germ cells of male mice might occur secondary to hyperthermia (EFSA CONTAM Panel, 2011; Kirkland et al., 2011). More generally, it has been shown that chemically and environmentally induced changes in body temperature may be associated with increased micronuclei in rodents (Asanami and Shimono, 1997, 2000; Shuey et al., 2007).
Codeine
In vitro, codeine was not mutagenic in Salmonella Typhimurium or Escherichia coli and it did not induce chromosomal aberrations in Chinese hamster ovary (CHO) cells. It induces significant increases in sister chromatid exchanges (SCE) in CHO cells in the presence and in the absence of S9 mix. In one study, increased frequencies of micronuclei were reported in cultured rat kidney fibroblasts exposed to a combination of codeine phosphate and paracetamol. However, this was probably due to the treatment with paracetamol only. In vivo, negative results were reported in bone marrow micronucleus test in mice after i.p. injection or gavage (EFSA CONTAM Panel, 2011).
Based on these observations, the CONTAM Panel concluded in 2011 that codeine is not genotoxic. No new data were identified.
Thebaine
No data were identified.
Oripavine
No data were identified.
Noscapine
Since the previous opinion, several new studies have become available and the text below gives an overview of previous and new data.
Several studies have shown that noscapine induces polyploidy and is an aneugen in vitro. To clarify if noscapine would have an effect on germ cells in vivo, female mice were exposed by the oral route to noscapine and oocytes were collected. Noscapine did not induce delay of the meiotic progression or induction of chromosome malsegregation. No increases in polyploidy or hyperploid oocytes were found (Tiveron et al., 1993; EFSA CONTAM Panel, 2011). An in vivo micronucleus test in mouse bone marrow cells by i.p. injection was negative (Furukawa et al., 1989 – reported in an abstract only).
The difference between in vitro and in vivo results might be explained by the relatively long treatment (24 h) necessary to induce polyploidy in vitro (Gatehouse et al., 1991; Mitchell et al., 1991). Such a long exposure period at this level will never be reached in vivo because of the pharmacokinetics of noscapine. It has been shown that after oral administration in healthy volunteers, noscapine has a relatively low bioavailability because its systemic availability is greatly reduced by a substantial first‐pass metabolism (Dahlström et al., 1982). A risk of genotoxic damage caused by noscapine in humans at therapeutic dosages is therefore very unlikely. Even after oral administration to relatively high concentrations of noscapine, the GI tract is unlikely to be at risk because of the short period that the cells are exposed to these concentrations (Rauws et al., 1997).
One possible therapeutic application of noscapine, i.e. as an oral effective anticancer drug, results from its capacity to bind to tubulin, alter its conformation and assembly properties and interfere with microtubule dynamics. In this way, noscapine arrests a variety of mammalian cells including drug‐resistant variants in mitosis targeting them for apoptosis. Noscapine inhibits progression of murine melanoma, lymphoma, leukaemia, glioblastoma, colon cancer, ovarian carcinoma, non‐small cell lung cancer and prostate cancer cell lines and of human breast and bladder tumours implanted in nude mice (Ye et al., 1998; Ke et al., 2000; Zhou et al., 2002; Landen et al., 2002, 2004; Jackson et al., 2008; Mahmoudian and Rahimi‐Moghaddam, 2009; Chougule et al., 2011a,b; Afzali et al., 2015). In the in vivo studies, the toxicity to the kidney, heart, liver, bone marrow, spleen and small intestine was low (Aneja et al., 2006; Afzali et al., 2015). These effects were seen in mice receiving 3 mg of noscapine dissolved in 0.2 mL saline (120 mg/kg bw) by i.p. for 3 weeks (Ye et al., 1998) or orally fed 300 mg/kg bw for 80 days (Aneja et al., 2006). Harati et al. (2017) assessed the antiproliferative effects of noscapine on human soft tissue sarcomas (STS) cells. Noscapine exhibited cytostatic effects on STS cells, as assessed using the bromodeoxyuridine (BrdU) and 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) cell proliferation assays at 24 h; however, these effects could not be validated by real‐time cell analysis over a longer time period.
Fellows et al. (2011) tested the ability of the mouse lymphoma L5178Y TK+/– assay to detect aneugens among which noscapine. Noscapine was negative in this test, but this test is not a standard test to detect aneugens.
The aneugenic potential of noscapine was evaluated in an in vitro micronucleus assay in TK6 cells. In the first experiment, cells were exposed continuously during 27 h to 0, 32.8, 41.0 and 64.0 μg/mL in the absence of metabolic activation. A dose‐related and statistically significant increase in micronuclei was observed. In a second experiment, cells were exposed during 4 h to 0, 98.4, 115.8, 160.3, 188.6 and 221.85 μg/mL in the absence of metabolic activation and harvested 40 h later. Noscapine produced an equivocal response as it induced a statistically significant increase in micronuclei only at 188.6 μg/mL, the highest concentration considered acceptable according to the study criteria regarding toxicity (Sobol et al., 2012).
Noscapine has been evaluated for its aneugenic properties in TK6 cells by flow cytometry for micronucleus induction. Cells were exposed continuously for 4 or 24 h to the solvent control (dimethyl sulfoxide (DMSO), 1%) or noscapine (3.1–200 μM) without metabolic activation. Noscapine produced dose‐related increases in micronuclei at the 24‐h time point (from 10 μM) but not at the 4‐h time point. In addition, noscapine treatment at 24 h induced a dose dependent increase in polyploidy between 40 and 200 μM compared to DMSO. It was also shown that noscapine induced a dose‐related increase in the frequency of cells in mitosis while the percentage of cells in G0/G1 declined with dose (Cheung et al., 2015).
Afzali et al. (2015) investigated the selective cytotoxic, genotoxic and apoptosis induction effects of noscapine and papaverine on three human cancer cell lines: HT29 colorectal carcinoma, T47D breast cancer cells and HT‐1080 fibrosarcoma cells. A non‐cancerous mouse NIH‐3T3 cell line was used to compare the effects of the substances between cancerous and non‐cancerous cells. Doxorubicin was used as a positive control. Cells were treated with different concentrations of noscapine, papaverine and doxorubicin and were incubated for 48 h. Cell viability was determined by the MTT assay. The genotoxicity was tested with the comet assay (measure of the tail length). Noscapine and papaverine had a dose‐related cytotoxic effect on HT‐29, T47D and HT‐1080 cell lines, whereas no cytotoxic effect was observed on the non‐cancerous NIH‐3T3 cell line. Doxorubicin induced cytotoxicity in all cell lines. Noscapine and papaverine selectively enhanced DNA damage in cancerous cells compared to non‐cancerous cells, whereas, doxorubicin induced DNA damage in all cell lines.
In summary, noscapine is an aneugen in vitro most likely by a non‐DNA reactive mechanism. No in vivo genotoxicity test conducted on somatic cells, as recommended by the EFSA guidance (EFSA Scientific Committee, 2011) has been identified, with the exception of one negative in vivo micronucleus test in mouse bone marrow by i.p. reported in an abstract. Taking into account also its low systemic availability, a risk of genetic damage caused by noscapine in vivo is very unlikely. The possible therapeutic application of noscapine as an oral effective anticancer drug, results from its capacity to bind to tubulin and interferes with microtubule dynamics. Noscapine arrests a variety of mammalian cells, including drug‐resistant variants, in mitosis targeting them for apoptosis. In this way, noscapine inhibits progression of various tumours implanted in nude mice. It was also shown that, in vitro, noscapine selectively enhanced DNA damage in cancerous cells compared to non‐cancerous cells.
Papaverine
The selective cytotoxic, genotoxic and apoptosis induction effects of papaverine in human cancer cell lines were reported in the section on noscapine above (Afzali et al., 2015). However, the data are too limited to conclude on the genotoxic potential of papaverine.
3.1.2.5. Developmental and reproductive toxicity
Since the identified studies do not provide information on the oral dose‐response relationship, they cannot be used for the identification of a reference point and therefore only few details are reported.
Morphine
Depressed sexual activity was observed in male rats treated with morphine. This effect is reversible and results from lowering serum luteinising hormone and testosterone. Reduced testicular function and spermatogenesis has been reported after prenatal administration of morphine. It was also shown that morphine disrupted ovarian cyclicity, decrease pregnancy rate and increase still births (EFSA CONTAM Panel, 2011).
More recently, Ghosian Moghaddam et al. (2013) reported that oral morphine exposure for 21 days of adult male rats results in significantly lower luteinising hormone level, testosterone level, oestrogen level and progesterone level than the control group and a higher follicle‐stimulating hormone level.
Developmental toxicity studies in experimental animal species are not conclusive. Subcutaneous injection of high doses of morphine to hamster dams has been reported to cause CNS defects in the fetuses. Teratogenic effects, such as exencephaly and cryptorchidism were observed in mice but were probably related to maternal toxicity. These effects could be blocked by administration of naloxone, the opioid antagonist. The primary effect of morphine in rats and rabbits might be on food intake and body weight rather than on organogenesis, causing miscarriages, low birth weight and decreases in crown‐to‐rump length in the newborns. Morphine given late during pregnancy has been associated with placental vasoconstriction with a potential for general fetal effects. Studies have also shown that prenatal exposure of rats to morphine induces long‐term alterations in the adult brain, changes in morphology or effects on neural tube development (EFSA CONTAM Panel, 2011).
Recent publications have confirmed that oral morphine exposure of pregnant rats affects the normal development of the placenta: increased the placenta concentration of corticosterone, increased the thickness of the maternal part of the placenta, and significantly decreased the thickness of the placenta and the lacuna number in the embryonic part (Kazemi et al., 2011, 2012; Dehghani et al., 2013). Time‐dependent effects of oral morphine exposure can inhibit the development and natural functioning of cytotrophoblast and syncytiotrophoblast cells of the placental layers; morphine acting as a mitogenic stimulus for cytotrophoblast cells. The defect in the normal development of placenta leads to either abnormal fetal development or miscarriage (Dehghani et al., 2013).
The immature brain is particularly vulnerable to the actions of morphine. Morphine appears to selectively accumulate in the nervous tissues of offspring because of increased permeability of the blood‐brain barrier. It may affect the development of the CNS and cause a variety of delays in ontogeny. The delay effects of morphine on embryonic cells might be due to the stimulation of opioid receptors in embryonic tissues (Nasiraei‐Moghadam et al., 2005; Sadraie et al., 2008). Oral exposure of pregnant rats to high doses of morphine induces suppression of cell proliferation in the cortical plate and affects the migration and survival of neurons in the embryo which could lead to postnatal neurological and behavioural deficits in the animals (Sadraie et al., 2008). In mice, effects were observed on cell cycle progression of both radial glia and progenitor cells in the developing cerebral cortex (Sargeant et al., 2008). Several studies in rats indicated that oral administration of morphine during pregnancy can delay the development of the neural tube and affect neural plate evolution, can result in defects in the development of the cerebellum and reduce the development of frontal cortex in the embryo (EFSA CONTAM Panel, 2011). Morphine can also affect growth and completion of the spinal cord and decrease the embryos fronto‐occipital length and the number of grey cells (Niknam et al., 2013). Another study showed that the exposure to morphine disrupt the natural organisation of the choroid plexus in the brain of the exposed rat embryos preventing them from functioning properly in the future (Kazemi et al., 2009).
Haydari et al. (2014) showed that male pups from pregnant rats exposed to morphine during pregnancy exhibited anxiogenic behaviour on post‐natal day (PND) 35.
Nasiraei‐Moghadam et al. (2013) studied the effects of prenatal morphine exposure (through drinking water) on inhibitory avoidance memory performance in rat offsprings and whether these deficits are reversible during the postnatal development. Animals were dosed from gestational day 1 to 13 (group 1) or from gestational day 1 to 21 (group 2). Behavioural, molecular and histological studies were conducted on PND 28 and 70, respectively, before and after puberty. Adolescent and adult females failed in passive avoidance retention task in both groups. Adolescent and adult male offspring were similar to control rats in group 1, however, in group 2, retention task was impaired in pre‐pubertal male offspring. This memory loss was repaired in post‐pubertal stage.
Maternal morphine exposure prior to pregnancy can influence the development of future offspring (Byrnes, 2005). Adolescent morphine exposure of female rats delays sexual maturation and long‐lasting alterations in adult gene expression are induced within the endogenous opioid system (Byrnes, 2008). Potential changes in maternal care, communication and/or attachment were observed in the offsprings of rats from mothers exposed to morphine during adolescence. Studies have shown that endogenous opioids are implicated in the display of separation‐induced distress calls and that these effects appear to be opioid receptor specific. Daily s.c. injections of morphine in adolescent rats for 10 days resulted in a significant decrease in body weight and reduced number of distress calls elicited by separation from the dam when compared to control offsprings. The same effects have been observed after saline injection. However, only adolescent morphine exposure results in increased maternal potentiation (i.e. difference between the number of ultrasonic vocalisations during first and second separation) on PND 9 and PND 12. Significant modifications in the functional activation and transcription of the μ‐opioid receptor (OPRM1) and the dopamine D2 receptor are involved (Bodi et al., 2016).
In summary, depressed sexual activity, reduced testicular function and spermatogenesis, disruption of ovarian cyclicity and decreased pregnancy rate have been observed in rats exposed to morphine. Oral morphine exposure of pregnant rats or mice affects the normal development of the placenta and the brain. The effects on brain development of the embryo could lead to postnatal neurological and behavioural deficits in the animals including memory loss.
Codeine
Based on limited data it is concluded that oral administration of codeine did not result in teratogenicity, even at doses inducing embryotoxicity. Embryotoxicity was observed in the presence of maternal toxicity (EFSA CONTAM Panel, 2011).
Thebaine
Geber and Schramm (1975) have administered a single s.c. injection of thebaine (0, 110, 140 or 193 mg/kg bw) to hamster (20, 22 and 80 animals, respectively) on day 8 of gestation. A high maternal mortality rate was produced at the two highest doses (10% and 75%, respectively). No effect was noted on fetuses viability, however, a dose‐related increase in the % of fetuses with congenital malformations was observed (0%, 0%, 2.0% and 4.2%, respectively). Cranioschisis was the most common anomaly observed, mainly at the highest dose (100% of the malformed fetuses). At 140 mg/kg bw, 80% of the malformed fetuses exhibited ‘other lesions’ (micro‐ or anophthalmia, mylocoele, micrognathia, external liver and spina bifida).
Based on one study, it is concluded that s.c. injection of thebaine produces a teratogenic response, but the results are limited by the marked maternal mortality.
Oripavine
No data were identified.
Noscapine
No data were identified.
Papaverine
No data were identified.
3.1.2.6. Neurotoxicity
Morphine
Morphine exerts many different effects, both in the CNS and peripheral nervous system by acting on the opioid receptors. Most pharmacological effects are well characterised with regard not only to their dose–response character but also to their development of tolerance induced by repeated administration. Adverse effects include sedation, drowsiness, nausea and vomiting as well as respiratory depression. Studies focused on neurobehavioural outcome showed that prenatal morphine exposure induces long‐term alterations in the adult brain, not restricted to a single brain area or a single neurotransmitter. Altogether, there is evidence that persistent alterations in neurobehaviour can be attributable to opioid effects on the developing nervous system (EFSA CONTAM Panel, 2011).
The effects of morphine on the nervous system have been largely investigated in experimental animal models, mostly because of the concerns for side effects induced by the high doses used for severe pain treatment (see Section 3.1.3 Observations in humans). Most of the studies are performed using high doses administered s.c. or i.p., thus not relevant for the purpose of this opinion. No neurotoxicity studies in adult animals based on oral administration of low doses were identified. Studies on the effects of oral administration of morphine on the developing nervous system are reported in the ‘Developmental and reproductive toxicity’ section (see Section 3.1.2.5).
Codeine
No data were identified.
Thebaine
The predominant effect of thebaine is CNS stimulation. Hyperirritability and increase in motor activity as well as reflex excitability were observed in mice, rabbits, cats and dogs at doses around 2–10 mg/kg s.c. or intramuscular. In rabbits, thebaine antagonised the effects of phenobarbital and potentiated those of caffeine. Convulsions were observed in mice, guinea pigs, rabbits, cats and dogs after s.c. administration (WHO, 1980).
Oripavine
No data were identified.
Noscapine
No data were identified.
Papaverine
Shehab‐Eldeen et al. (2015) reported in an abstract that papaverine decreases viability of rat neural progenitors’ neurospheres.
3.1.2.7. Immunotoxicity
Morphine
The CONTAM Panel concluded in 2011 that morphine causes immunosuppressive actions: effects on macrophages, neutrophils, mast cells, depression of lymphocytes, decreased expression of the T‐cell E‐receptors, suppression of antibody formation, decrease in spleen and thymus weight associated with several changes in immune cells, decrease of B cells and T cells, lower natural killer cell activity, alteration of cytokine expression, changes in mesenterial lymph node at dosages that induce also physical dependence. These effects may be relevant for the sensitivity of opiate addicts to infectious agents. The involvement of the μ‐opioid receptor was supported by the fact that the effects could be antagonised by naloxone. The changes in immune function are also associated with increases of the levels of corticosterone which is the final effector hormone (Bryant et al., 1987, 1988; Sei et al., 1991; Freier and Fuchs, 1993; Breslow et al., 2011; EFSA CONTAM Panel, 2011; Roy et al., 2011; Zhang et al., 2011; Brown et al., 2012; Sacerdote et al., 2012; Plein and Rittner, 2017).
Since the previous opinion, only studies with s.c. pellet administration have been identified which were considered to be of limited relevance for this opinion and are not described.
Codeine
Codeine may affect immunity responses both locally in the respiratory tract and systemically as shown by immunotoxicological findings observed in immunised guinea pigs (EFSA CONTAM Panel, 2011).
Thebaine
No data were identified.
Oripavine
No data were identified.
Noscapine
No data were identified.
Papaverine
No data were identified.
3.1.3. Observations in humans
This section summarises and updates the human data presented in the ‘Scientific Opinion on the risks for public health related to the presence of opium alkaloids in poppy seeds’ published in 2011 (EFSA CONTAM Panel, 2011).
In humans, opioids are able to bind to three types of receptors, the μ‐, δ‐ and κ‐opioid receptors. Morphine acts as an agonist and has high affinity to the μ‐opioid receptor. Codeine is also able to bind to the μ‐opioid receptor but with a much lower affinity. More information on the interaction of opium alkaloids with the opioid receptors is provided in Section 3.1.4. In addition, morphine and codeine are endogenously formed but their physiological relevance is unknown (reviewed in Stefano et al., 2008). Activation of the μ‐opioid receptor leads to analgesia, predominantly at the supraspinal level, euphoria, dependence, cough calming, respiratory depression, obstipation and miosis,. Besides the supraspinal level, also effects at the spinal level have been reported (Poeaknapo et al., 2004; BfR, 2005; Trescot et al., 2008; Aktories et al., 2009).
3.1.3.1. Morphine
In general, morphine is used for the treatment of severe pain and dyspnoea as morphine sulfate (in the form of morphine sulfate pentahydrate) or as morphine hydrochloride (in the form of morphine hydrochloride trihydrate) (Ph. Eur. 8, 2016; Martindale, 2017). Morphine primarily affects the CNS and the GI system. Tolerance development as well as strong physical and psychological dependence occur in conjunction with the chronic oral intake of morphine. The Panel concluded in 2011 that ‘with sustained release forms in the oral dose range of 7.5–15 mg morphine per day (corresponding to single doses of about 1.3–2.5 mg morphine taken every 4 h) side effects such as sedation, dizziness or light‐headedness occurred in the onset of therapy of opioid‐naïve individuals. [….] This dose range is also associated with nausea, vomiting and obstipation’.
In 2011, the CONTAM Panel concluded that ‘Morphine has high affinity for the μ‐opiate receptor as an agonistic ligand. Activation of μ‐opiate receptors leads to analgesia, euphoria, dependence, miosis, respiratory depression, cough calming and obstipation. Patients with pain tolerate, without severe side effects, larger doses of morphine than pain‐free patients. Therapeutic doses of morphine may also impair the ability to drive or to operate machinery due to changes in attentiveness and reactive skills. The lowest known single oral therapeutic dose reported is 1.9 mg morphine, corresponding to 31.7 μg/kg bw for an adult weighing 60 kg’
From the complex pharmaco‐ and toxicodynamic profile of morphine described in detail in the 2011 opinion (EFSA CONTAM Panel, 2011), several aspects have been reviewed and updated in the newer literature. Relevant information focussing on adverse effects associated with low doses of orally administered morphine (up to 30 mg/day in adults) are summarised in the following (e.g. Caraceni et al., 2012; Katz, 2013; Barton, 2016).
A. Pharmacodynamics, therapeutic applications and dosage
Treatment of severe pain
Newer reviews are reporting on studies on ‘pharmacogenetics’, the genetic variations affecting receptors, metabolising enzymes and transporters, and thus the individual response to morphine by their influence on pain perception, PK and PD, including development of adverse effects, such as respiratory depression (e.g. Branford et al., 2012; Chidambaran and Sadhasivam, 2012; Sadhasivam et al., 2015; Tzvetkov, 2012; Droney et al., 2013; Tzvetkov et al., 2013; Hajj et al., 2013; Rhodin et al., 2013; Biesiada et al., 2014; Chidambaran et al., 2015; Ting and Schug, 2016; Aka et al., 2017; Balyan et al., 2017; Obeng et al., 2017). However, recent knowledge does not yet allow personalised therapies. Therapeutic morphine doses for pain therapy in children of different age and adults, referring to oral immediate‐release forms (aqueous solutions or tablets) as indicated in the 2011 opinion (EFSA CONTAM Panel, 2011) are still valid according to recent recommendations (Merck, 2016) (Table 5).
Table 5.
Examples of recommended doses for orally administered morphine for pain therapy in children and adults (immediate release forms)
| Age group (body weight (kg)) | Single dose Morphine hydrochloride trihydrate dosage (equivalent morphine dosage) | Total daily dose Morphine hydrochloride trihydrate dosage (equivalent morphine dosage) | ||
|---|---|---|---|---|
| Dose (mg) (Merck, 2016) | Dose (mg/kg bw)a | Dose (mg) (Merck, 2016) | Dose (mg/kg bw per day)a | |
| Children up to 2 years (up to 12.5 kg) | Up to 2.5 (Up to 1.9) | Up to 0.20 (Up to 0.15) | Up to 22.5 (Up to 17.1) | Up to 1.80 (Up to 1.37) |
| Children 2–6 years (12.5–20 kg) | 2.5–5 (1.9–3.8) | 0.20–0.40 (0.15–0.30) | 15–30 (11.4–22.8) | 1.20–2.40 (0.91–1.82) |
| Children 6–12 years (20–40 kg) | 5–10 (3.8–7.6) | 0.25–0.50 (0.19–0.38) | 30–60 (22.8–45.6) | 1.50–3.00 (1.14–2.28) |
| Adolescents 12–16 years (40–50 kg) | 10–20 (7.6–15.2) | 0.25–0.50 (0.19–0.38) | 60–120 (45.6–91.1) | 1.50–3.00 (1.14–2.28) |
| Adolescents > 16 years and adults (70 kg) | 10–60 (7.6–45.6) | 0.14–0.86 (0.11–0.65) | Up to 360 (Up to 273.3) | Up to 5.14 (Up to 3.90) |
bw: body weight.
Where dose ranges are shown, the dosage in mg/kg bw or mg/kg bw per day has been calculated using the lower body weight shown in the first column.
According to new information, the British National Formulary for Children (BNF, 2017) indicates lower dosage per kg bw for younger infants than for older infants. The following initial oral doses of morphine are recommended according to children's age, whereby doses should thereafter be adjusted according to response:
1–2 months: 0.05–0.1 mg/kg bw every 4 h;
3–5 months: 0.1–0.15 mg/kg bw every 4 h;
6–11 months: 0.2 mg/kg bw every 4 h;
1 year: 0.2–0.3 mg/kg bw every 4 h;
2–11 years: 0.2–0.3 mg/kg bw every 4 h (maximum single dose per person: 10 mg);
12–17 years: 5–10 mg/person every 4 h.
It is assumed that the pharmacokinetics of morphine in children are similar to those of adults (Martindale, 2017). Neonates, however, demonstrate significantly altered pharmacokinetics and have a longer half‐life for morphine. They have lower plasma clearance, higher volume of distribution, decreased protein binding, decreased hepatic clearance because of their immature CYP system and decreased renal clearance compared to adults. Furthermore, they exhibit an immature blood‐brain barrier that can lead to elevated morphine concentrations in the brain and to increases of the respiratory side effects of morphine (Kesavan, 2015). By 1 year of age, elimination of morphine is almost at adult levels (Lyon and Njoku, 2015).
In general, in view of the risks of severe adverse effects, the paediatric indications for chronic (> 3 month) daily administration of morphine for chronic pain treatment are primarily life‐limiting conditions (Berde et al., 2012).
Management of neonatal abstinence syndrome
Morphine is also used in the management of neonatal abstinence syndrome (NAS) under specialist supervision (e.g. Dahan et al., 2011; Kraft and van den Anker, 2012; Kelly, 2014; Hall and Anand, 2014; Martindale, 2017). The British National Formulary for Children (BNF, 2017) recommends an initial oral dose of 0.040 mg/kg bw every 4 h until symptoms are controlled and an increase of the dose if necessary. The dosage frequency should be reduced gradually over 6–10 days until a dose of 0.040 mg/kg bw once daily is achieved after which the drug should be stopped (British National Formulary for Children (BNF, 2017; Martindale, 2017). From the USA, a standard treatment consisting of an initial dose of 0.4 mg/kg bw per day in six divided doses is reported (Kraft et al., 2011). A survey of neonatal units in the United Kingdom revealed typical maximum doses up to 1.3 mg/kg bw per day in the treatment of NAS (Kraft and van den Anker, 2012).
Treatment of dyspnoea
Morphine is used in the treatment for dyspnoea as a main symptom of several advanced lung or heart diseases, because it is known to reduce ventilatory drive in response to carbon dioxide, hypoxia and exercise (EFSA CONTAM Panel, 2011).
In the treatment of dyspnoea, doses of morphine tend to be smaller than those used for pain relief. Morphine sulfate may be given as an oral solution in carefully titrated doses, starting with as little as 2.5 mg morphine sulfate (equivalent to 1.9 mg morphine corresponding to 27.1 μg/kg bw for an adult weighing 70 kg) every 4 h. For opioid‐naive adult patients, 2.5 mg of morphine sulfate (equivalent to 1.9 mg morphine corresponding to 27.1 μg/kg bw for an adult weighing 70 kg) is recommended as the lowest oral therapeutic single dose which could be applied every 4 h resulting in a daily dose of 11.4 mg morphine per day (Martindale, 2017). In the study of Rocker et al. (2013), therapy was even started by increasing dosages from an initial dosage of 0.5 mg morphine sulfate twice daily, to minimise the likelihood of adverse effects and taking a delay of any therapeutic effect into account. However, since the authors did not report from which dosage onwards therapeutic and/or adverse effects were observed, this study was not considered to be relevant for this risk assessment.
In the preceding opinion (EFSA CONTAM Panel, 2011), it was concluded that results obtained with sustained release forms providing a mean of 12.4 mg morphine/day would be expected to be similar to those obtained following the administration of an immediate release form with 1.9 mg morphine applied every 4 h resulting in a daily dose of 11.4 mg morphine/day. Patients treated with sustained release forms received doses as low as 10 mg morphine sulfate/day (corresponding to 7.52 mg morphine/day). Dosage regimens, as presented in the opinion from the CONTAM Panel from 2011, are confirmed in recent reviews and monographs (e.g. Currow et al., 2014; Kohberg et al., 2016; Cabezon‐Gutierrez et al., 2016; Martindale, 2017). However, in newer literature, further research needs are suggested to investigate upwards titration of dosages under aspects of therapeutical benefits and reduction of adverse effects (Currow et al., 2014; Verberkt et al., 2016).
B. Tolerance and dependence
Chronic oral intake of morphine may induce tolerance as well as strong physical and psychological dependence. A short‐term intake of morphine is not linked to any risk of dependence (EFSA CONTAM Panel, 2011; Blaschek et al., 2015).
C. Adverse reactions
Adverse effects associated with the treatment of severe pain with morphine
Newer literature is in agreement with the reported side effects in the EFSA CONTAM Panel opinion (2011).
Common adverse effects of morphine in the treatment of severe pain are bowel dysfunction and spastic constipation, nausea and vomiting, pruritus, somnolence and cognitive impairment, dry mouth and bladder dysfunction. A severe side effect of morphine in larger doses is respiratory depression. Additional recognised adverse effects are endocrinopathies and sleep disorders. Furthermore myoclonus and morphine induced hyperalgesia are described (Caraceni et al., 2012; Coluzzi et al., 2012; Labianca et al., 2012; Jitpakdee and Mandee, 2014; Gyawali et al., 2015; Nelson and Camilleri, 2015; Riley et al., 2015; Harned and Sloan, 2016; Zecca et al., 2016; Hussain et al., 2016; Martindale, 2017).
Recent reviews describe opioid‐induced respiratory depression as a potentially life‐threatening adverse effect in the treatment of severe acute and chronic pain with estimates of respiratory events in the perioperative setting ranging from 0.5% to 2% (Boom et al., 2012; Dahan, 2013; Overdyk et al., 2014). In the past, respiratory depression had been considered a transient morphine side effect in the treatment of chronic pain to which tolerance quickly developed (e.g. Labianca et al., 2012). However, the influence of morphine and other opioids on sleep‐disordered breathing, including central sleep apnoea, has recently been investigated more carefully (Zutler and Holty, 2011; Jungquist et al., 2012; Javaheri et al., 2014; Rose et al., 2014; Harned and Sloan, 2016). A recent systematic review found the overall prevalence of central sleep apnoea among patients taking chronic opioids to be 24% (Correa et al., 2015). The risk appeared to be linearly related to escalating opioid doses, with the morphine equivalent daily dose and risk of sleep disordered breathing being strongly associated. The authors concluded that an morphine equivalent daily dose of 200 mg or greater was a risk factor for severity of central sleep apnoea (Correa et al., 2015; Harned and Sloan, 2016).
Opioid‐related CNS side effects can be separated into symptoms and signs associated with a lowering level of consciousness (e.g. sedation, drowsiness), cognitive and psychomotor impairment (e.g. impairment of memory, attention, reaction time, motor coordination), and hyperexcitability reactions (hallucinations, myoclonus and hyperalgesia) (Caraceni et al., 2012; Højsted et al., 2012; Matzo and Dawson, 2013). In a recent review on cognitive effects and sedation in chronic non‐malignant pain populations receiving long‐term opioid therapy, memory deficits (73–81%), sleep disturbance (35–57%) and fatigue (10%) are described as the most prevalent observations (Dhingra et al., 2015).
Baldo and Pham (2012) rediscuss the histamine‐releasing and allergenic properties of opioid drugs including morphine. Hypersensitivity reactions such as urticaria are frequently reported after oral intake of morphine (Merck, 2016). The reported incidence of pruritus is 2–20% after oral administration of opioids (Golembiewski, 2013). The pathogenesis of morphine induced pruritus is still not fully known. It is primarily mediated by the binding of morphine to central μ‐opioid receptors in the brain and spinal cord (Jitpakdee and Mandee, 2014). The incidence of other morphine induced dermatological side effects such as exanthema is rare (Merck, 2016). However, a case of erythroderma associated with administration of morphine sulfate to control severe pain has been published some years ago (Arai and Mukai, 2011). Anaphylactoid reactions after administration of morphine are known but are uncommon (AHFS, 2016; Merck, 2016).
In a study of Wiese et al. (2016) to investigate the immunosuppressive potential of opioids, their use in patients with rheumatoid arthritis was associated with an increased risk of hospitalisation for serious infections. Also, according to epidemiological studies, high doses and the initiation of opioid therapy for non‐malignant pain appear to correlate with a higher risk of infectious diseases such as pneumonia. However, the clinical relevance of this observation has not yet been confirmed in controlled clinical studies (Plein and Rittner, 2017).
Disruption of physiological hormonal activity by chronic therapy with morphine and other opioids has been well established and comprises all routes of opioid administration including oral application. In addition to reduced testosterone and oestrogen levels, individuals taking opioid medications have reduced levels of adrenal androgens, suggesting impairment of the hypothalamic–pituitary–adrenal axis in addition to disruption of the hypothalamic–pituitary–gonadal axis. Recent reviews concluded that especially hypogonadism is associated with chronic opioid use and that patients taking opioids equivalent to ≥ 100 mg of morphine daily should be monitored regularly for symptoms of hypogonadism (De Maddalena et al., 2012; Elliott et al., 2012; Brennan, 2013; Elliott and Fibuch, 2013; Inder et al., 2013; Valverde‐Filho et al., 2015; Harned and Sloan, 2016; Girish and Rao, 2017).
A double‐blind, placebo‐controlled study was conducted in 21 participants with chronic low back pain to investigate the potential of morphine to induce neuroplastic changes. In the treatment group (n = 11) receiving a sustained‐release oral formulation of morphine up to a maximum dosage of 120 mg/day for 1 month, decreases of grey matter volume in the right amygdala were observed, while no grey matter decreases were observed in the placebo group (n = 10) (Lin et al., 2016). Reduced grey matter in the right amygdala had also been observed in an earlier study following a month of oral morphine administration via a sustained‐release formulation with a ceiling of the dosage at 120 mg/day (Younger et al., 2011).
Adverse effects associated with the treatment of dyspnoea or moderate cancer pain with morphine (low‐dose range)
In the EFSA CONTAM Panel opinion (2011), it was concluded from studies on the treatment of dyspnoea, that with sustained release forms in the oral dose range of 7.5–15 mg morphine/day (corresponding to single doses of about 1.3–2.5 mg morphine taken every 4 h) side effects such as sedation, dizziness or light‐headedness occurred in the onset of therapy of opioid‐naïve individuals. This dose range was also associated with nausea, vomiting and obstipation. Furthermore, a single low sustained release morphine bedtime dose of 11.55 mg had been shown to significantly affect sleep architecture in healthy adults and lead to reductions in slow‐wave sleep. A threshold for the onset of these side effects was not described and it was not known if these side effects occur below the lowest known single oral therapeutic dose of 1.9 mg morphine (EFSA CONTAM Panel, 2011).
According to newer literature, these conclusions are still valid. Raffa and Pergolizzi (2012) reviewed the effects of morphine on sleep architecture without discussing dose–effect relationships: decrease in the number and duration of rapid eye movement (REM) periods, delay in the onset of the first REM period and increase in the waking state and reduction in slow‐wave sleep in healthy adults. According to Kohberg et al. (2016), no severe adverse effects such as respiratory depression were reported from studies on the treatment of dyspnoea, only constipation was reported as a common side effect. Also Rocker et al. (2013) report that GI symptoms, primarily constipation, were the most frequent and troubling adverse effects observed in the therapy of dyspnoea, besides of nausea, vomiting, bloating, cramping in a few patients. Other adverse effects included sleepiness, itching, dry mouth, sweating, dizziness and skin changes (Rocker et al., 2013). In a 28‐day study by Bandieri et al. (2016) in 118 adults with moderate cancer pain, median doses of 30 mg morphine per day were well tolerated according to the authors. Tiredness, nausea, depression, anxiety, drowsiness, all in mild forms were reported side effects, no shortness of breath being observed (Bandieri et al., 2016). In a study by Navigante et al. (2010) 2 patients out of 30 required dose reduction because of excessive somnolence to a lower dose of 3 mg morphine every 8 h for both patients (the dose causing the somnolence is not reported). A case of angioedema with tongue and lip swelling has been reported in a 85‐year‐old woman, receiving low dose sublingual morphine (2.5 mg every 4 h) (Kohli et al., 2011).
E. Interactions
The side effects of morphine, particularly respiratory depression, sedation, hypotonia or coma, may be amplified by parallel administration of other central depressant drugs including other opioids and alcohol (EFSA CONTAM Panel, 2011; Merck, 2016).
F. Specific groups (including contraindications)
Pregnant women, preterm infants
Morphine crosses the placental barrier. Adequate data are not available that would permit an assessment of the possible teratogenic risk for humans (e.g. Brennan and Rayburn, 2012). However, it has been reported on a possible association of morphine administration with an increased incidence of hernia inguinalis (Merck, 2016).
Newborns of women addicted to morphine or related opioids, having prolonged in utero exposure to the opioid, may develop the NAS exhibiting withdrawal symptoms during the first days of life. It is mainly characterised by signs of CNS and GI dysfunction (Bio et al., 2011; EFSA CONTAM Panel, 2011; Kraft et al., 2011; Kraft and van den Anker, 2012; Brucknerova et al., 2015). Use of prescription opioids including morphine during pregnancy is also associated with a risk of NAS, especially when used long term or late in pregnancy (Desai et al., 2015; Dooley et al., 2015).
Prenatal exposure to morphine increases the number of opioid receptors in the substantia nigra, subthalamic nucleus and the hippocampus, the structures involved in seizures. This corresponds to the increased seizure activity, electroencephalogram (EEG) changes, and increased irritability observed in children of opioid‐abusing mothers (Kesavan, 2015).
Morphine analgesia in preterm neonates was associated with impaired cellular growth in the neonatal period and smaller head circumference/cerebellar volume and poorer neurodevelopmental outcome in early childhood (Ferguson et al., 2012; Steinhorn et al., 2015; Kocek et al., 2016; Zwicker et al., 2016; McPherson, 2016). Opioid receptors are expressed early in the developing brain. Morphine acts on the μ‐, δ‐ and κ‐opioid receptors and activates multiple intracellular signalling pathways, which are implicated in modulation of proliferation, survival and differentiation of neural stem cells, neurons, and glia that express opioid receptors. Therefore, exposure to morphine during a critical period of neuronal maturation may lead via complex mechanisms to permanent changes in the formation and function of the CNS (Kesavan, 2015).
On the basis of the knowledge currently available, it is not possible to identify a threshold dose for developmental and reproductive toxic effects of morphine in humans.
Breast feeding
Morphine is distributed into milk where it may reach higher levels than in the plasma of the mother (AHFS, 2016; Merck, 2016). Hendrickson and McKeown (2012) analysed available data and concluded that they did not identify any reports of suspected infant toxicity from maternal morphine use while breast feeding. Recommendations in monographs are not consistent: while the American Academy of Pediatrics has stated that the administration of morphine is usually compatible with lactation (Martindale, 2017), others advise against breast feeding during oral treatment of the mother with morphine (Merck, 2016).
Children
Morphine can cause significant apnoea in newborn infants and is associated with seizure activity in newborns. Respiratory depression due to morphine treatment is considered to be a main risk in all children; however, neonates and infants under the age of 1 year have an enhanced risk to respiratory depression. Morphine‐associated respiratory depression is especially greater in premature born infants due to the immaturity of their respiratory centre responses to hypoxia and hypercarbia (Kesavan, 2015; Merck, 2016; Martindale, 2017).
Children with obstructive sleep apnoea and significant previous recurrent hypoxemia during sleep have been observed to have a higher sensitivity for morphine. This has been concluded from a study in 22 children aged between 19 and 79 months treated postoperatively after adenotonsillectomy with morphine intravenously (i.v.) (Brown et al., 2006).
In children episodes of recurrent hypoxia from sleep‐disordered breathing, obstructive sleep apnoea and/or tonsillitis are considered to increase sensitivity to morphine for respiratory depression and analgesic effects (Brown et al., 2006; EFSA CONTAM Panel, 2011; Niesters et al., 2013).
Children between 2 and 12 years of age (n = 93), who underwent (adeno)tonsillectomy, were discharged from the hospital on regular paracetamol and ibuprofen for 7 days and a single oral dose of 0.2 mg morphine sulfate/kg bw on the mornings of days 3, 4 and 5 after tonsillectomy. Adverse effects associated with morphine were only seen in 11 patients. There were no major adverse side effects like respiratory depression recorded (Syed et al., 2016).
The elderly
Elder patients (over 75 years) may be more sensitive to morphine (Merck, 2016).
Individuals at higher risks due to certain health conditions
As described in the EFSA CONTAM Panel opinion (2011), individuals under special health conditions may be at higher risks for development of side effects of morphine. For example, morphine has to be avoided in patients at risk of paralytic ileus and has to be used only with caution in obstructive or inflammatory bowel disorders and in patients with renal or hepatic impairment. Morphine is generally contra‐indicated in acute respiratory depression and obstructive airways disease, and must be used with caution in patients with compromised respiratory function (e.g. emphysema, apnoea, severe obesity) (e.g. AHFS, 2016). Also, in recent literature, it is reported that morphine is recognised to worsen obstructive sleep apnoea (Rowsell et al., 2015, 2016) and that morphine treatment may be associated with toxicity in renal insufficiency (Mallappallil et al., 2017).
G. Intoxications
Miosis, respiratory depression and unconsciousness (coma) are the three typical symptoms of acute morphine intoxication. For non‐opiate dependent adults, doses in the range from 200 to 1,100 mg morphine may be acutely lethal. Toddlers and infants are far more sensitive (e.g. EFSA CONTAM Panel, 2011; Bracher et al., 2016; Merck, 2016).
Rivard et al. (2016) report on a case of intoxication of a 3‐year‐old child (bw: 15 kg), who underwent adenoidectomy and tonsillectomy. At the day of surgery, he received three times a single oral dose of 3 mg morphine and 225 mg paracetamol (corresponding to single doses of 0.2 mg morphine and 15 mg paracetamol/kg bw). The day after he was given within 15 h two single oral doses of 3 mg morphine and 225 mg paracetamol (corresponding to single doses of 0.2 mg morphine and 15 mg paracetamol/kg bw) and by mistake two single oral doses of 15 mg morphine (corresponding to single doses of 1 mg morphine/kg bw). Two hours after the last dose, the child developed fever, somnolence, tachycardia, miosis, respiratory depression and convulsion. The child survived after emergency treatment.
3.1.3.2. Codeine
Based on new data on the therapeutic use of codeine in humans, the CONTAM Panel has updated the information from the previous opinion. Codeine is used for medicinal purposes as a monohydrate or as a salt, e.g. codeine hydrochloride (in the form of a dihydrate) or codeine phosphate (in the form of a hemihydrate or a sesquihydrate) (Ph. Eur. 8, 2016). From Swedish national register data, it was concluded that codeine belongs to the most frequently used opioids (Söderberg et al., 2013).
A. Pharmacodynamics, therapeutic applications and dosage
Codeine is used to treat mild to moderately severe pain (often in combination with a non‐opioid analgesic) and also to treat acute diarrhoea but its main use is for the symptomatic treatment of dry coughs. The antitussive effect results from suppression of the cough reflex by acting on the medulla oblongata in the cough centre. Additionally, codeine has slight sedating properties (Bracher et al., 2016; Martindale, 2017).
Codeine is approximately one‐twelfth less potent than morphine as an analgesic and has a correspondingly lower dependence‐liability than morphine (Vree et al., 2000). Codeine may be considered a prodrug of the active compounds morphine and C6G (Srinivasan et al., 1997; Vree et al., 2000). Codeine has little analgesic effect in patients who are CYP2D6 poor metabolisers, whereas the risk of morphine toxicity is higher in ultra‐rapid metabolisers (see Section 3.1.1.1).
For the treatment of cough or pain, the following dose ranges shown in Table 6 are recommended for adolescents over the age of 12 years and adults.
Table 6.
Recommended single and total daily doses for orally administered codeine salts presented as doses of codeine to treat dry cough (Prescribing Information, 2015; Bracher et al., 2016)
| Age group | Single dose mg codeinea | Total daily dose mg codeine |
|---|---|---|
| Adolescents over the age of 12 years and adults | 15–44 mg, can be repeated every 6–8 h; in individual cases up to 100 mg | Up to 200 mg |
Anhydrous codeine base, CAS No 76‐57‐3, MW: 299.36 g/mol.
Observed life‐threatening toxicity in paediatric patients given moderate doses of codeine has been associated with the genotype predisposing to ultra‐rapid metabolism of codeine into morphine by the isoenzyme CYP2D6. Higher plasma morphine concentrations in the blood of ultra‐rapid metabolisers lead to a higher risk of side effects due to morphine's action on the brain and respiratory centre (e.g. Gasche et al., 2004; Ciszkowski et al., 2009; Kelly et al., 2012; Martindale, 2017). Therefore, medicinal uses of codeine especially in children under 12 years have been restricted by responsible authorities (EMA, 2013a,b, 2015a,b; FDA, 2013; Health Canada, 2013; MHRA/CHM, 2013) (see Section 3.1.3.2.E). According to Heintze and Fuchs (2015), in children, who are rapid or ultra‐rapid metabolisers, therapeutically used daily dose of codeine of 1–3 mg/kg bw may result in toxic accumulation of morphine which may lead to respiratory depression and death (for details see Section 3.1.3.2.E Specific groups).
B. Tolerance and dependence
Codeine has dependence potential, with longer term use and high doses resulting in tolerance and physical/psychological dependence. Codeine is subject to abuse, but produces less euphoria and sedation than morphine. There is cross‐tolerance with other opiates (Prescribing Information, 2015; Martindale, 2017).
C. Adverse reactions
The adverse reactions to codeine are similar to those with morphine but seen to a lesser extent at clinical doses. In the case of chronic administration in usual doses, the most frequent side effects are nausea sometimes linked with vomiting (particularly at the beginning of treatment) and constipation (Prescribing Information, 2015; Martindale, 2017).
Confusion is described as an unusual adverse effect, however has been observed recently in a 14‐year‐old girl after treatment with a cough suppressant over 15 days with daily doses of 30–45 mg codeine/person (O'Reilly et al., 2015). The authors noted that the daily doses were within the recommended dose range but the maximum recommended duration of dosage of 3 days was exceeded (no genotyping for CYP2D6).
Codeine phosphate (50 mg/person) alone and with alcohol had a deleterious effect on driving skills in a simulated driving test (Linnoila and Hakkinen, 1974; Martindale, 2017). Medications with codeine have been associated with a significant increase of risk for motor vehicle collisions (Rudisill et al., 2016).
Allergic reactions to codeine are known, as also shown by newly published case reports, but rarely in a severe form (Fathallah et al., 2011; Deverriere et al., 2012; Ariosto, 2014; Yoo et al., 2014; Erfan et al., 2015; Prescribing Information, 2015).
D. Interactions
Combined intake of codeine and other central depressants (e.g. sedatives, hypnotics, psychotropics, antihistamines, antihypertensives) can amplify the sedating or respiratory depressive effect of codeine. In addition, the combination of alcohol with codeine may reduce the psychomotor abilities (supra‐additive effect of individual components) (Prescribing Information, 2015; Bracher et al., 2016; Martindale, 2017).
E. Specific groups (including contraindications)
Pregnant women
As summarised by the CONTAM Panel in 2011, codeine crosses the placental barrier. While codeine administration during the first trimester was statistically significantly associated with respiratory and heart malformations, data on other malformations were inconclusive. Furthermore, studies on the use of codeine to treat pain and ‘high temperature’ during pregnancy did not reveal any elevated risk of malformation. This was confirmed by the outcome of ‘The Norwegian Mother and Child Cohort Study’ (Nezvalova‐Henriksen et al., 2011, 2012; Nordeng et al., 2014). In this study, pregnancy outcomes of 2,666 women who used codeine during pregnancy were compared with 65,316 women without opioids use during pregnancy by logistic regression analyses. Between codeine exposed and unexposed infants no significant differences were found in the survival rate (adjusted odds ratio 0.9, 95% confidence interval (CI) 0.6–1.5) or the congenital malformation rate (adjusted odds ratio 0.9, 95% CI 0.8–1.1). However, third‐trimester maternal codeine intake was associated for the mothers with acute Caesarean delivery (adjusted odds ratio 1.5, 95% CI 1.3–1.8; p < 0.0001) and postpartum haemorrhage (adjusted odds ratio 1.3, 95% CI 1.1–1.5; p < 0.0001).
In a neonate whose mother had taken about 90 mg of codeine daily during the last 2 months of pregnancy, symptoms characteristic of the NAS associated with narcotic withdrawal were seen (Khan and Chang, 1997). Thomas and Wolff (2016) recently reported another case, in which symptoms such as hypoglycaemia, poor feeding, GI disturbance and respiratory distress have been associated with NAS due to oral therapy during pregnancy with codeine in daily doses of 180 mg combined with the analgesic tramadol, which is a synthetic opioid.
The identification of threshold doses for developmental and reproductive toxic effects is not possible on the basis of the knowledge currently available.
CYP2D6 ultra‐rapid metabolisers, breast feeding, children
Several cases of life‐threatening toxicity in patients (and breastfed infants of mothers) given orally moderate doses of codeine were associated with an increased metabolism of codeine resulting in excessive blood levels of morphine in ultra‐rapid metabolisers. The prevalence of ultra‐rapid metabolisers varies by ethnic origin and ranges from 1% to 2% for Northern Europeans up to 29% for Africans (e.g. Crankshaw, 2011; MHRA/CHM, 2013; Tremlett, 2013; Crews et al., 2014; Prescribing Information, 2015; Martindale, 2017). Niesters et al. (2013) noted in their review on opioid induced respiratory depression in paediatrics, that below the age of 6 months the CYP2D6‐dependent conversion of codeine into morphine is predominant since alternative pathways of codeine metabolism are not fully developed. An additional risk factor to develop respiratory depression is seen in recurrent hypoxic episodes from sleep‐disordered breathing, obstructive sleep apnoea and/or tonsillitis. Episodes of recurrent hypoxia are considered to increase sensitivity to opioids (Brown et al., 2006; EFSA CONTAM Panel, 2011; Niesters et al., 2013).
Lactating mothers and breastfed infants
Codeine and its metabolite morphine are distributed into breast milk. If the mother is an ultra‐rapid metaboliser of codeine, breastfed infants are at high risks of severe adverse effects from its metabolite, morphine. CNS depressions including a case of respiratory arrest resulting in death have been reported for breastfed infants of mothers receiving codeine e.g. in the treatment of post‐labour pain (Koren et al., 2006; Madadi et al., 2007, 2009; van den Anker, 2012; Juurlink et al., 2012; Lam et al., 2012, 2013; Berlin and van den Anker, 2013; Prescribing Information, 2015; Anderson et al., 2016; Martindale, 2017).
A newborn male infant was found cyanotic and without vital signs on day 13 after birth. Resuscitation was unsuccessful. Full post‐mortem analysis failed to identify an anatomic cause of death. The mother had been taking orally single dosages of 30 or 60 mg codeine twice daily (initially 60 mg every 12 h, reduced to half that dose from postpartum day 2 because of somnolence and constipation) as part of a combination preparation with paracetamol for about 2 weeks. Assayed morphine concentrations in the mother's breast milk were found to be 87 ng/mL. Post‐mortem toxicological testing of the infants’ blood revealed a blood concentration of morphine at 70 ng/mL. Neonates receiving morphine for analgesia have been reported to have serum concentrations of morphine at 10–12 ng/mL. Later investigations found that the mother's genotype for CYP2D6 classified her as an ultra‐rapid metaboliser of codeine (Koren et al., 2006; Madadi et al., 2007, 2009).
Madadi et al. (2009) reported an additional case of severe neonatal toxicity in a breastfed infant whose mother was a CYP2D6 ultra‐rapid metaboliser. After childbirth, the mother took 120 mg codeine/day in form of a combination product with paracetamol to treat severe muscle pain. During the period, she was taking codeine, she reported feeling sedated, nauseous, dizzy, and weak. Her breastfed infant was described extremely drowsy and feeding poorly. Due to feeding difficulties, breast milk was supplemented with formula. After completely replacing breast milk by formula feeding at 7 days after delivery, the mother noted complete reversal of the infant's symptoms in the following days. Furthermore, the authors examined characteristics of mothers and infants with or without signs of CNS depression following codeine exposure while breastfeeding in a case–control study. They showed that mothers of symptomatic infants (n = 17) consumed a mean 59% higher codeine dose than mothers of asymptomatic infants (n = 55) (1.62 ± 0.79 mg/kg bw per day vs 1.02 ± 0.54 mg/kg bw per day; p = 0.004). Comparison of neonatal and maternal CNS depression exhibited a concordance of 71%. Ultra‐rapid metabolisers were identified in 2 (11.8%) cases. According to the authors the lowest maternal dose of codeine associated with symptoms in the infant was 0.63 mg/kg bw per day corresponding to 44 mg codeine for a 70‐kg woman (Madadi et al., 2009).
Administration to children
Serious adverse effects due to central nervous depression have been repeatedly reported after oral administration of codeine to infants or children after hospital discharge in the management of pain due to surgery or in the therapy of cough (EMA, 2013a, 2015a, Niesters et al., 2013; Subramanyam et al., 2013; Wong et al., 2012). Especially after oral codeine treatment following tonsillectomy, apnoeas partly with fatal outcome have been observed in children who were diagnosed (or suspected of being) extensive or ultra‐rapid metabolisers of codeine (e.g. Voronov et al., 2007; Ciszkowski et al., 2009; Kelly et al., 2011, 2012).
Voronov et al. (2007) described a case of a 29‐month‐old boy (bw: 13.7 kg) who experienced apnoea resulting in brain injury following oral treatment with paracetamol combined with codeine 2 days after adenotonsillectomy. After the surgery, the patient spent 5 h in the hospital and received two oral doses of codeine (1.75 mg/kg bw per dose). At home during postoperative day 1, four doses of codeine and one extra codeine dose during the night were administered to the boy (1.75 mg/kg bw per dose). Four hours later, his mother found him unresponsive and apnoeic. The emergency medical service noted upon arrival that the child was spontaneously breathing, unresponsive and with pinpoint pupils. A quantity of 1.4 mg naloxone was administered leading to some improvement. He came to the intensive care unit with stable vital signs. Magnetic resonance imaging of the head showed bilateral parasagittal watershed infarcts involving the white matter and cerebral cortex. He was discharged from the hospital on post‐operative day 13 with stable conditions. Genotyping was performed and the results interpreted by the authors as indication of extensive metabolism.19
Ciszkowski et al. (2009) report on the case of a healthy 2‐year‐old boy (bw: 13 kg), who underwent adenotonsillectomy. After surgery, instructions were to administer at home orally 10–12.5 mg of codeine and 120 mg paracetamol every 4–6 h as needed (corresponding to single doses of 0.77–0.96 mg codeine/kg bw and about 3–6 mg/kg bw per day). On the second evening after surgery, the child developed fever and wheezing and on the next morning, the boy's vital signs were absent. Post‐mortem examination showed evidence of bronchopneumonia. Analysis of femoral blood resulted in 700 ng codeine/mL and 32 ng morphine/mL without evidence of other drugs or metabolites being present. CYP2D6 genotyping revealed ultra‐rapid metaboliser phenotype. According to the authors’ explanation, the given dose of codeine was within the recommended range of 1–3 mg/kg bw per day. However, respiratory depression has been shown in other young children with serum morphine concentrations exceeding 20 ng/mL. The authors also noted that the existing bronchopneumonia may have enhanced the risk of hypoxemia and recurrent episodes of hypoxemia may lead to alterations in the μ‐opioid receptor and increased sensitivity to morphine.
Two fatalities and a case of severe respiratory depression following the oral use of codeine in three young overweight children, aged 3–5 years, for pain following adenotonsillectomy have been published by the same group of authors (Kelly et al., 2012). The three children were receiving doses within the recommended weight‐adjusted dose of 0.5–1 mg codeine/kg bw every 4–6 h (maximum 6 mg codeine/kg bw per day). Two of the children had CYP2D6 polymorphisms for extensive or ultra‐rapid metabolism phenotypes (which can overlap), and this was suspected in the third child in view of the high morphine blood concentrations found relative to those of codeine. As morphine sparsely distributes to fatty tissue, the authors considered that dosing in the three overweight patients based on total body weight instead of lean mass might have partially contributed to morphine accumulation.
Friedrichsdorf et al. (2013) describe the deaths of three children aged 4–10 years after oral administration of codeine at home in the recommended dose ranges. All three children were overweight or obese and were given codeine after tonsillectomy, orthopaedic surgery or as cough suppressant. They received codeine every 4 h as needed in single oral doses of 0.29 mg/kg bw (tonsillectomy; co‐administration: valproate; death occurred after 4 single doses of codeine); 0.22–0.44 mg/kg bw (respiratory infection; co‐administration: guaifenesin, azithromycin; death occurred after 3 single doses of codeine); or 0.45–0.9 mg/kg bw (orthopaedic surgery; reactive airway disease; co‐administration: diazepam; death occurred after 3 single doses of codeine). In the last case, co‐administration of benzodiazepine and the reactive airway disease were regarded as possible contributing factors by the authors. Testing for CYP2D6 ultra‐rapid metabolism was undertaken in only one of the three cases presented with negative result (no indication which case).
Hermanns‐Clausen et al. (2009) report on the intoxication of 3‐year‐old male twins after the oral use of codeine as an antitussive agent. Further medication consisted of acetaminophen, ibuprofen, an ivy extract. One of the twins with a body weight of 13.4 kg was found lying in vomit and apnoeic at night. He was re‐animated and survived after transfer to an intensive care unit. Two and a half hours later, his twin brother (body weight: 14 kg) was found dead in his bed at home and autopsy showed massive aspiration of gastric content. The monozygotic twins had been given both a codeine solution at a dose of ‘10 drops per day’ for 6 days for the treatment of an upper respiratory tract infection lasting for several days. The weight of ‘10 drops’ was determined experimentally and was found to range from 494 to 940 mg. Thus, the highest possible dose administered was 23.5 mg codeine/day (corresponding to 1.75 mg codeine/kg bw per day based on a body weight of 13.4 kg) instead of the recommended 10 mg codeine/day. The twins had identical CYP2D6 gene polymorphisms of the ‘extensive metaboliser’ type.
An episode of an apparent life‐threatening event with recurrent apnoea was observed in a 17‐day‐old Chinese female neonate born at full‐term, who was treated for mild cough and nasal blockage with chlorpheniramine and a cough suppressant syrup containing 6 mg codeine/2.5 mL (single dose). According to the authors, the patient was given a dose of 6.6 mg codeine/kg bw per day, which exceeded the published dosage recommendation at that time for codeine in children of 1 mg/kg bw per day. At the second day of treatment, the girl experienced a first attack of cyanosis with a decrease in responsiveness and breathing effort 2 h after the last administration of the dose which took place at noon. The attack lasted for 1 min and the patient gradually recovered. The second attack of apnoea occurred 1 h later while the patient was waiting at the emergency department. The patient recovered after treatment in hospital. No CYP2D6 genotyping was performed (Tong and Ng, 2001).
Magnani and Evans (1999) report on a case of codeine intoxication in an infant at 29 days of age, in which codeine was prescribed for cough control in the form of a medicinal preparation. Each 5‐mL of the preparation contained 30 mg of pseudoephedrine hydrochloride, 10 mg of codeine phosphate, 2 mg of chlorpheniramine maleate, and 5% alcohol. The infant received twice 1 mL of the preparation within 6 h, corresponding to a total of 4 mg of codeine phosphate or 1.26 mg of codeine phosphate/kg bw within a 6‐h period. Two hours after the administration of the last dose the mother noted that the baby was not breathing. Despite lifesaving measures, the infant died. No CYP2D6 genotyping was performed.
Near‐fatal consequences were described after oral administration of a medicinal preparation, containing triprolidine, pseudoephedrine hydrochloride and codeine phosphate to a 3‐month‐old boy with a body weight of 3.0 kg for the treatment of an upper respiratory tract infection. Within 24 h after administering the single dose of 5 mL containing 10 mg codeine phosphate twice (total intake: 6.6 mg codeine phosphate/kg bw), his mother noted he was sleepy, breathing heavily, and had small pupils. On arrival in the hospital the baby was collapsed, cold, and semi‐comatose with pin‐point pupils and Cheyne–Stokes breathing. He recovered after i.v. treatment with naloxone. No CYP2D6 genotyping was performed. The authors noted that the total intake of codeine was well above the at that time recommended dose of 1–2 mg codeine/kg bw per day (Wilkes et al., 1981).
The CONTAM Panel noted that therapeutical oral administration of single doses of codeine as low as 0.22–0.44 mg/kg bw, repeated every 4 h as needed, has led to death in children. Case reports and pharmacovigilance data for codeine show that individuals who have CYP2D6 polymorphisms for extensive or ultra‐rapid metabolism phenotypes exhibit higher sensitivity to codeine toxicity compared to their poor or intermediate metabolising counterparts. Especially affected are breastfed infants from ultra‐rapid metabolising mothers and ultra‐rapid metabolising infants and children. In addition breathing problems such as recurrent hypoxic episodes from sleep‐disordered breathing, obstructive sleep apnoea and/or tonsillitis may increase the risk for codeine toxicity.
Current recommendations
Although morphine‐induced side effects after administration of oral codeine may occur at all ages, current evidence suggests that children below 12 years are at special risk of life‐threatening respiratory depression with codeine. EMA considered that the way codeine is converted into morphine in children below 12 years is more variable and unpredictable, making this population at special risk of such side effects (EMA, 2015b). A particular risk is seen in those paediatric patients who might already have compromised airways and who require pain relief following tonsillectomy and/or adenoidectomy (EMA, 2013a,b, 2015a,b; FDA, 2013; Health Canada, 2013; MHRA/CHM, 2013; Prescribing Information, 2015; Martindale, 2017).
Following reviews by the EMA's Pharmacovigilance Risk Assessment Committee (PRAC), which investigated reports of serious and fatal respiratory depression in children after taking codeine, some of the children being ultra‐rapid metabolisers of codeine, EMA (EMA, 2013a,b, 2015a,b) recommended that:
“Codeine‐containing medicines should only be used to treat acute (short‐lived) moderate pain in children above 12 years of age, and only if it cannot be relieved by other painkillers such as paracetamol or ibuprofen, because of the risk of respiratory depression associated with codeine use.”
“Codeine should not be used at all in children (aged below 18 years) who undergo surgery for the removal of the tonsils or adenoids to treat obstructive sleep apnoea, as these patients are more susceptible to respiratory problems.”
“Use of codeine for cough and cold is contraindicated in children below 12 years.”
“Use of codeine for cough and cold is not recommended in children and adolescents between 12 and 18 years who have breathing problems.”
Codeine should not be used in breastfeeding mothers.
Codeine should not be used in people of any age who are known to be ultra‐rapid metabolisers of codeine.
Similar recommendations were given by other authorities (FDA, 2013; Health Canada, 2013; MHRA/CHM, 2013; Stingl and Rotthauwe, 2015).
The elderly
In elder patients, elimination of codeine may be prolonged (Prescribing Information, 2015).
Individuals at higher risks due to certain health conditions
It is known that patients with certain diseases and individuals under special health conditions are at higher risks for development of side effects of codeine, consequently precautionary measures or contraindications are recommended in the medical field due to corresponding known side effects of codeine.
Codeine is generally contraindicated in acute respiratory depression. Codeine must be used with caution in patients with obstructive airways disease, asthma and impaired renal function (Prescribing Information, 2015; Martindale, 2017).
F. Intoxications
Codeine intoxication leads to similar symptoms as morphine intoxication; reflecting the metabolism of codeine to morphine. The most frequently reported symptoms are extreme respiratory depression and pulmonary oedema. Other symptoms are extreme sleepiness, stupor, coma, miosis, vomiting, headaches, urine and faeces retention, cyanosis, hypoxia, cold skin, skeletal muscle tone loss and areflexia and, in some cases, with bradycardia, syncopes and a drop in blood pressure. It is to be noted that in children only cramps occur (BfR, 2005; Dillon et al., 2013; Prescribing Information, 2015; Bracher et al., 2016).
3.1.3.3. Thebaine, oripavine, noscapine and papaverine
Thebaine
Since thebaine is not used pharmaceutically, but only as a starting substance for pharmaceutic synthesis, no information is available about its effects in humans after oral or parental exposure as already noted in the previous opinion in 2011 (EFSA CONTAM Panel, 2011).
Oripavine
Since oripavine is not used pharmaceutically, but only as a starting substance for pharmaceutic synthesis, no information is available about its effects in humans after oral exposure.
Noscapine
As summarised by the CONTAM Panel in 2011, noscapine which is used as centrally acting antitussive compound, does not bind to opioid receptors and has neither an analgesic, respiratory depressive nor obstipating effect. For adults, the oral therapeutic dose of noscapine is 25–50 mg (corresponding to 0.36–0.71 mg/kg bw for an adult weighing 70 kg) three times daily, for children aged between 3 and 12 years 12–25 mg (corresponding to 0.52–1.09 mg/kg bw assuming a body weight of 23 kg20) three times daily and for children aged between 6 months and 3 years 6–12 mg (corresponding to 0.67–1.33 mg/kg bw assuming a body weight of 9 kg or 0.5–1.0 mg/kg bw assuming a body weight of 12 kg21) two to three times daily. Slight sleepiness, light‐headedness, dizziness, headaches, nausea and erythema are indicated as adverse reactions. Very rarely anaphylactic reactions and Steven‐Johnson syndrome may occur.
As already referred to by the CONTAM Panel in 2011, investigations regarding the use of noscapine and its derivatives as anti‐mitotic and tubulin‐binding anti‐cancer agents are still a subject of reviewing articles (e.g. DeBono et al., 2015; Rida et al., 2015; Tomar et al., 2017). From new literature, it is known that the Netherlands Pharmacovigilance Centre received 10 reports of angioedema associated with the use of noscapine between 1987 and 2013.22 Furthermore, two cases of erythema occurring in Japan after noscapine administration have been published (Ishiguro et al., 2011).
Papaverine
Papaverine is normally used pharmaceutically as a smooth muscle relaxant in the form of papaverine hydrochloride (Ph. Eur. 8, 2016) in the treatment of GI disorders and coughs. Orally, 150–300 mg papaverine hydrochloride (equivalent to 135–270 mg papaverine) is administered two to three times daily. The maximum daily dose is indicated as 600 mg papaverine hydrochloride (equivalent to 540 mg papaverine) (Bracher et al., 2016; Martindale, 2017).
In 2011, the CONTAM Panel concluded that papaverine does not show opiate‐like pharmacology since it acts as a smooth muscle relaxant that is most pronounced on blood vessels. The side effects of papaverine, reported after oral administration, are dizziness, drowsiness, headache, tiredness, GI disturbance, tachycardia, skin rash, sweating and hypotonia. After long‐term administration, eosinophilia, icterus and liver enzyme changes (reversible) have been reported (BfR, 2005). Overdosage may lead to seizures.
3.1.3.4. Opium
Opium is the dried latex obtained from the unripe capsules of P. somniferum L. The toxicity of opium is mainly attributed to the toxic effect of morphine and can be increased by codeine. The paralysing effect of opium on the CNS, particularly on the respiratory centre, is described to be lower compared to morphine due to the antagonistic effect of thebaine (Blaschek et al., 2013). Noscapine also has a minor stimulatory effect on respiration (Blaschek et al., 2013).
Mainly the peripheral GI effects of opium are used therapeutically. According to the European Pharmacopoeia (Ph. Eur. 8, 2016), ‘raw opium’ should contain not less than 10% of morphine (anhydrous), not less than 2% of codeine (anhydrous), and not more than 3% of thebaine (anhydrous). ‘Raw opium’ is intended only as the starting material for the manufacture of ‘standardised opium dry extract’, ‘prepared opium’ or ‘standardised opium tincture’, which are orally used in the treatment of severe diarrhoea associated with chronic inflammatory GI diseases. The relaxing action of papaverine and noscapine on intestinal muscle makes the opium preparations more constipating than morphine. They decrease in the number and frequency of bowel movements and slow down intestinal movements (Blaschek et al., 2013; Bracher et al., 2016; Martindale, 2017).
‘Standardised opium tincture’ contains 0.95–1.05% morphine (anhydrous), not less than 0.1% of codeine (anhydrous) and not more than 0.3% of thebaine (anhydrous). In the treatment of pain for adults, single dosages range from 0.5 g to a maximum 1.5 g of tincture, the maximum daily dosage being 5 g of tincture. In the treatment of diarrhoea, the tincture is administered in single dosage of 0.15–0.33 g, given three times daily, the daily dosage being 0.45–1 g/day. This corresponds to single dosages of about 1.5–3.3 mg morphine, not less than 0.15–0.33 mg codeine and not more than 0.45–1 mg thebaine and daily dosages of 4.5–10 mg morphine, not less than mg 0.45–1 mg codeine and not more than 1.35–3 mg thebaine for adults. This calculation informs about the maximum doses of thebaine, which are tolerated by humans in the therapeutic uses of mixtures of opium alkaloids in the treatment of diarrhoea associated with chronic inflammatory GI diseases.
Thus, as noted by the CONTAM Panel in 2011, since the high alkaloid content in poppy seeds is considered to be caused by contamination with the latex, opium alkaloids adhering to poppy seeds may have a distribution pattern similar to that found in opium. In consequence, combined effects reported for the alkaloids in opium may be relevant for alkaloid‐contaminated poppy seeds originating from cultivars traditionally used in opium production. However, the available analytical results show that the alkaloid profile in poppy seed samples may largely deviate from the profile of opium as defined in the European Pharmacopoeia.
3.1.3.5. Poppy seeds
The CONTAM Panel summarised in 2011 one case of intoxication after food use of a consumer reporting an ‘uneasy feeling’ in her head, vomiting and she felt like she had a hangover the next day. Symptoms of intoxications following misuse of poppy seeds included respiratory depression in a 6‐week‐old baby and tonic‐clonic seizures and hallucinations in a 26‐year‐old adult. From studies with healthy subjects, nausea, vomiting, constipation and some system on the CNS like dizziness, headache, and difficulties to concentrate were reported after intake of contaminated poppy seeds.
Since 2011, some cases of intoxications after consumption of homemade tea from poppy seeds, store‐bought or purchased on the Internet, for ‘relaxation’ or ‘treatment of chronic anxiety’ or ‘natural pain control’ have been reported (Castelli and Hendrickson, 2017; Rinner et al., 2017; Spyres and Van Wijk, 2017). Authors associate the intoxications with intake of opioid overdoses via the poppy seed tea without specifying the exposure doses of the opioids.
Schuppener and Corliss (2018) describe a case of a 54‐year‐old woman who had consumed ‘large quantities’ of raw poppy seeds prior to the development of intractable vomiting and death. A cast‐like large bowel obstruction composed of brown‐black poppy seeds (approximately 900 g) was identified at autopsy. The authors report that the post‐mortem blood morphine level was < 10 ng/mL and considered the level to be well below that needed to cause acute opioid toxicity or even intoxication. The codeine level in the blood was below the LOD. Complications of a bowel obstruction, secondary to poppy seed ingestion, were determined to be the cause of death which was not associated with opioid toxicity.
As outlined in the 2011 opinion, immediate‐type allergy (Type I, immunoglobulin E (IgE)‐mediated) and anaphylaxis caused by poppy seeds are well known (EFSA CONTAM Panel, 2011). This is also reflected in the newer literature which includes case reports, studies on cross‐reactivity and reviews (e. g. Armentia et al., 2014; Lavine and Ben‐Shoshan, 2015; van Gasse et al., 2015; Patel and Bahna, 2016).
3.1.4. Mode of action
3.1.4.1. Interaction with opioid receptors: affinity, agonistic/antagonistic effects
The pharmacological effects of opioid drugs derive from their ability to interact with three different G protein coupled receptors namely the μ‐, δ‐ and κ‐opioid receptors. Opioid receptors are present in both the CNS as well as peripheral tissues throughout the body. All the opioid drugs which are currently used in the clinic display high affinity for the μ‐opioid receptor. The crucial role played by this receptor in opioid induced analgesia but also in the unwanted actions of opioids has been clearly documented in receptor knockout studies (Kieffer, 1999). Opioid receptors are coupled with Gi/o proteins, thus their activation reduces cyclic adenosine monophosphate (cAMP) levels, increases potassium conductance and reduces calcium currents. These cellular actions promote a reduction in neuronal firing when the receptors are located post‐synaptically or a reduction in neurotransmitter release when the receptors are located presynaptically. These mechanisms associated with the expression of opioid receptors along the nociceptive neuronal pathways (nociceptors, dorsal horn of the spinal cord, periaqueductal gray, thalamus and limbic system) account for the profound analgesic actions obtained in response to opioid drugs. Opioids drugs used in the clinic act as opioid receptor agonists (eventually with different efficacy). Pure antagonists such as naloxone are used to treat opioid overdose.
The gene coding for the μ‐opioid receptor, OPRM1, is highly polymorphic and OPRM1 polymorphism may alter the activity of the receptor and explain to some extent the interindividual variation in efficacy of opioids in the treatment of pain and their implication in dependence. It has been hypothesised that polymorphism of catechol‐amino transferase (COMT), the enzyme that modulates the neurotransmission of dopamine and norepinephrine, may have a similar effect on the efficacy of opioids. However, there is a lack of evidence linking COMT polymorphism to interindividual variation in opioid dose requirement (Lloyd et al., 2017; Obeng et al., 2017).
A large amount of data from the recent years demonstrated that G protein‐coupled receptor ligands can couple with different efficacies to the multiple pathways activated by a given receptor, a phenomenon most commonly known as functional selectivity or biased agonism (Kenakin, 2015). As far as opioid receptors are concerned, previous studies have demonstrated that the analgesic response to opioid drugs entirely depends on G protein signalling while the interaction between the μ‐opioid receptor and beta‐arrestin‐2 promotes the unwanted side effects of opioid drugs (Siuda et al., 2017). For example, beta‐arrestin‐2 knockout mice display enhanced and prolonged morphine analgesia while being protected from morphine induced respiratory suppression (Bohn et al., 1999).
In receptor binding experiments, morphine displayed the following values of affinity (Ki) 14 nM, 538 nM and > 1,000 nM at the μ‐, δ‐ and κ‐opioid receptor, respectively (Raynor et al., 1994). Nikolaev et al. (2007) reported the following values of affinity at the human μ‐opioid receptor for natural alkaloids: morphine 4 nM, codeine 6,300 nM, oripavine 286 nM and thebaine 7,400 nM. A large difference between morphine and codeine affinity was also reported (1.8 nM for morphine vs 350 nM for codeine) in guinea pig brain membranes by Mignat et al. (1995). No indication has been found that the effects of noscapine and papaverine are mediated via opioid receptors.
The pharmacological effects of codeine, oripavine and thebaine in comparison with morphine were investigated by Zhang et al. (2012) in cells coexpressing the human opioid receptors and chimeric G proteins that force the receptors to signal via the calcium pathway and by Nikolaev et al. (2007) by measuring μ‐mediated activation of Gi with a fluorescence resonance energy transfer assay. The results obtained in the two studies were highly consistent and can be summarised as follows:
Morphine behaved as a potent full agonist at the μ‐opioid receptor, as a potent partial agonist at the κ‐opioid receptor, while at the δ‐opioid receptor it displayed low efficacy associated to very low potency.
Codeine displayed very low potency (200‐ to 1,600‐fold less than morphine) associated with low efficacy both at the μ‐ and κ‐opioid receptors, while being inactive at the δ‐opioid receptor.
Oripavine displayed moderate potency (30‐ to 70‐fold less than morphine) at the μ‐opioid receptor. At κ‐ and δ‐opioid receptors, oripavine produced similar effects as morphine.
Thebaine was able to stimulate opioid receptors only at very high concentrations (micromolar range).
The morphine metabolites M3G and M6G are able to bind the μ‐opioid receptor. M6G displays values of affinity similar to morphine or higher, while M3G is more than 100‐fold less potent (Frances et al., 1992; Mignat et al., 1995; Klimas and Mikus, 2014). Interestingly, morphine and M6G displayed similar analgesic potency after s.c. injection in mice, although M6G exhibited a much higher antinociceptive potency (longer lasting effect) after intracerebroventricular injection (Frances et al., 1992). More information on M6G is available in the review by Dahan et al. (2008). Knockout studies demonstrated that both the desired (antinociceptive) and undesired (respiratory depression) effects of M6G and morphine are due to the μ‐opioid receptor (Romberg et al., 2003). The codeine metabolites C6G, norcodeine and N6G have weak affinity for the μ‐opioid receptor (Chen et al., 1991; Mignat et al., 1995; Volpe et al., 2011; Frost et al., 2012).
3.1.4.2. Apoptosis
Morphine
Apoptotic cell death can be induced by morphine in both adult and developing nervous system. Several in vivo and in vitro studies have shown occurrence of apoptotic cell death in neuronal cells after morphine administration. Apoptotic cells have been observed in several areas including parietal, frontal, temporal, occipital, pyriform and enthorinal cortex, hippocampus and spinal cord. Changes in the levels of Bax and Bcl‐2 as well as activation of caspase 3 have been reported after exposure in adulthood as well as after prenatal exposure. The involvement of Bcl‐2 and Bax, with subsequent activation of caspase 3, suggests that the mitochondrial‐mediated pathway may be a major player in morphine‐induced apoptosis. The studies are presented in more detail in Appendix D.
Codeine
No data were identified.
Thebaine
No data were identified.
Oripavine
No data were identified.
Noscapine
The selective cytotoxic and apoptosis induction effects of noscapine and papaverine were studied in three human cancer cells lines: HT29 colorectal carcinoma, T47D breast cancer cells and HT‐1080 fibrosarcoma cells. Non‐cancerous mouse NIH‐3T3 cell line was used to compare the effects of the substances between cancerous and non‐cancerous cells. Noscapine and papaverine had a dose‐related cytotoxic effect on HT‐29, T47D and HT‐1080 cell lines, whereas no cytotoxic effect was observed on the non‐cancerous NIH‐3T3 cell line. Noscapine and papaverine induced apoptosis in HT‐29 and T47D without any significant effect on NIH‐3T3 cell line. Noscapine increase caspase activity, which was not the case for papaverine. The authors concluded that their results may indicate that caspase‐independent apoptosis pathways are involved in the programmed cell death related to papaverine (Afzali et al., 2015).
Papaverine
See previous paragraph on noscapine.
Opium
Asiabanha et al. (2011) examined the influence of opium exposure by i.p. on brain and liver cells apoptosis in male and female diabetic and non‐diabetic rats. Apoptosis was evaluated by TUNEL (terminal deoxynucleotidyl transferase (TdT)‐mediated dUTP‐X nick end labelling) and DNA fragmentation assays. Brain and liver cells from normal opium‐exposed and diabetic opium‐exposed rats had a significantly higher incidence of apoptosis as compared to cells from unexposed animals. Apoptosis in brain cells was significantly higher in opium‐exposed and diabetic opium‐exposed males than in females, whereas apoptosis in liver cells was significantly higher in opium‐exposed and diabetic opium‐exposed females than in males. The authors concluded that opium probably plays an important role in brain and liver cell apoptosis, leading to neurotoxicity and hepatotoxicity and that there is a difference in susceptibility between males and females for induction of apoptosis by opium in brain or liver cells.
3.1.4.3. Other effects
Administration of morphine (s.c. or i.p) has been shown to change the expression of genes related to mitochondria, cytoskeleton, secretory vesicle and cell adhesion. These modifications are considered to be linked to intracellular adaptation processes. In certain brain areas, such as nucleus accumbens, locus coeruleus or caudate putamen, alterations in gene expression occur and they may have a role in adaptive response to chronic morphine and dependence.
Altogether, the reported changes in gene expression in the brain are consistent with the observations that morphine affects a wide range of cellular and molecular targets including neurotransmitters, neuromodulators, signalling pathways involved in neuronal plasticity, proliferation and apoptosis. The studies are presented in more detail in Appendix D.
3.1.5. Considerations of critical effects and dose–response analysis
3.1.5.1. Considerations of critical effects
The CONTAM Panel previously concluded that the chronic and developmental toxicity of morphine have not been systematically evaluated, but based on the lack of carcinogenicity of codeine, which is metabolised to morphine, it is unlikely that morphine is carcinogenic (EFSA CONTAM Panel, 2011). Furthermore, morphine is genotoxic in vivo but not in vitro, most likely by a non‐DNA reactive mode of action. Recent studies in rats and mice add to the weight of evidence that morphine has reproductive and developmental effects. These effects include depressed sexual activity, reduced testicular function and spermatogenesis, disruption of ovarian cyclicity and decreased pregnancy rate. Oral morphine exposure to pregnant rats or mice affects the normal development of the placenta and the brain, which could lead to postnatal neurological and behavioural deficits in the animals including memory loss. Some evidence from newborns of mothers who were opioid‐addicted or used prescription opioids during pregnancy demonstrates that the CNS of the human fetus can also be affected by morphine. There is also evidence of immunosuppressive effects in humans. These data do not allow assessment of dose–response relationships in support of the risk assessment for opioids in food.
The CONTAM Panel confirms its previous conclusion that the critical effects of morphine are on the CNS, mediated by its high affinity to the μ‐opioid receptor as an agonistic ligand. These effects included analgesia, euphoria, dependence, miosis, respiratory depression, cough calming and obstipation. Therapeutic doses of morphine may also reduce attentiveness and reactive skills, with potential impact on driving and operating machinery.
From data on humans and experimental animals, it is clear that morphine can interfere with brain development of the fetus resulting in behavioural effects at later life‐stages; however, data are too limited to derive a dose–response relationship.
The critical effects of codeine are the same as those of morphine, because morphine is the active metabolite of codeine. Codeine has some direct activity at the μ‐opioid receptor, but with a much lower potency than morphine, and therefore a direct effect of codeine is negligible compared to the effect of its metabolite morphine.
The toxicological properties of thebaine have not been well characterised. It demonstrates little or no activity with opioid receptors. Limited information is available regarding the metabolism of thebaine. However, there are indications that thebaine is metabolised into several metabolites including oripavine and morphine, which act as agonists to the μ‐opioid receptor. Therefore, a contribution to morphine‐like toxicity may occur, but is expected to be small. Lower LD50 values have been reported for thebaine compared to morphine, both by oral and parenteral routes indicating that thebaine is more acutely toxic than morphine. However, no other data on the oral toxicity of thebaine were identified and therefore its critical effects are unknown.
Oripavine also acts as an agonist to the μ‐opioid receptor, with a lower activity than morphine and a higher activity than codeine. No oral LD50 values have been reported, but lower i.p. and s.c. LD50 values have been reported for oripavine than for morphine, indicating that oripavine is more acutely toxic than morphine. Apart from one study showing convulsions following i.p. and s.c. administration, no other data on the toxicity of oripavine were identified. Therefore, the toxicological profile of oripavine is unclear.
High LD50 values for noscapine, following both oral and parenteral administration, indicate a lower acute toxicity compared to the other opioid alkaloids. EFSA's CONTAM Panel (2011) noted that noscapine is used as a centrally acting antitussive compound, does not bind to opioid receptors and has neither an analgesic, respiratory depressive nor obstipating effect. Although its toxicological properties have not been characterised, less severe adverse effects (e.g. headache, dizziness; see Section 3.1.3) are known from the therapeutic uses in humans.
For papaverine, the LD50 values, by oral and parenteral administration are similar to those for morphine. However, evidence of toxicity was not seen following oral administration of papaverine (50 and 100 mg/kg bw per day) to rats for 45 days and to dogs at 10 mg papaverine/kg bw per day (EFSA CONTAM Panel, 2011). Therefore, the critical effects of papaverine are unclear. No data on receptor binding were identified; however, EFSA's CONTAM Panel (2011) concluded that papaverine does not show opiate‐like pharmacology since it acts as a smooth muscle relaxant that is most pronounced on blood vessels. The side effects of papaverine, reported after oral administration, are dizziness, drowsiness, headache, tiredness, GI disturbance, tachycardia, skin rash, sweating and hypotonia. After long‐term administration eosinophilia, icterus and liver enzyme changes (reversible) have been reported (BfR, 2005). Overdosage may lead to seizures.
3.1.5.2. Dose–response analysis
For morphine, the CONTAM Panel selected again the lowest oral therapeutic dose of morphine (1.9 mg morphine/person) in adults to derive a reference point. Applying the default body weight of 70 kg (EFSA Scientific Committee, 2012), this dose corresponds to 27 μg/kg bw per day or 30 μg/kg bw per day when rounded to one significant figure. This conclusion also relates to codeine because morphine is the active metabolite of codeine.
For thebaine, oripavine, noscapine and papaverine, critical effects have not been established and the available data from studies in experimental animals are insufficient for dose‐response analysis. However, based on the very limited available data in experimental animals, it appears that noscapine is less toxic than the other alkaloids discussed in this opinion.
Noscapine is used as a centrally acting antitussive compound. For adults, the oral dosage of noscapine is 25–50 mg (corresponding to 0.36–0.71 mg/kg bw for an adult weighing 70 kg) three times daily, for children aged between 3 and 12 years 12–25 mg (corresponding to 0.52–1.09 mg/kg bw assuming a body weight of 23 kg20) three times daily and for children aged between 6 months and 3 years 6–12 mg (corresponding to 0.67–1.33 mg/kg bw assuming a body weight of 9 kg or 0.5–1.0 mg/kg bw assuming a body weight of 12 kg21) two to three times daily.
Pharmaceutically, papaverine hydrochloride is used orally as a myotropic spasmolytic agent in single dosages of 150–300 mg (equivalent to 135–270 mg papaverine) (corresponding to 1.93–3.85 mg/kg bw for an adult weighing 70 kg) two to three times daily. The maximum daily dose is indicated as 600 mg papaverine hydrochloride (equivalent to 540 mg papaverine; corresponding to 7.71 mg/kg bw per day for an adult weighing 70 kg).
The therapeutic uses of noscapine and papaverine may be accompanied by adverse effects (e.g. dizziness, headache) which are considered less severe compared to those known from the pharmaceutical uses of morphine.
3.1.6. Derivation of a health‐based guidance value
The CONTAM Panel previously concluded that the establishment of an ARfD was required for morphine because of the short‐term nature of its effects, and that ensuring exposure is below the ARfD would also protect against possible effects of repeated exposure. An ARfD of 10 μg/kg bw was derived from the lowest known single oral therapeutic dose of morphine, which was regarded as a LOEL, with application of an uncertainty factor of 3. The more recently available data do not provide a basis for revising the 2011 conclusions and therefore the CONTAM Panel confirms the ARfD of 10 μg/kg bw. This is the dose of morphine from foods for which a person would not be expected to experience adverse effects following consumption of a single meal, or from total consumption over the course of a day.
The CONTAM Panel previously considered whether morphine‐equivalents should be estimated based on combined exposure to morphine and codeine from poppy seed‐containing foods. However, at that time, it was concluded that codeine has a minor impact based on an analysis of the relative concentrations of morphine and codeine in poppy seed samples, using an equivalence factor of 0.2 for codeine (see Table 8 of EFSA CONTAM Panel, 2011). The factor of 0.2 assumes that ≤ 20% of codeine is converted to morphine in ultra‐rapid metabolisers which was primarily based on the data of Kirchheiner et al. (2007). Since 2011, no new data have become available regarding the conversion of codeine to morphine. The CONTAM Panel noted that Linares et al. (2015) had performed pharmacokinetic modelling of the data of Kirchheiner et al. (2007), which suggested that about 51% of codeine is converted to morphine in ultra‐rapid metabolisers. However, there are inconsistencies in the outcome of the modelling, relating to the much larger AUCs for morphine and its glucuronides. The CONTAM Panel therefore concluded that the results of the pharmacokinetic modelling did not provide a robust basis for revising its previous conclusion that the equivalence factor of 0.2 is appropriate.
Table 8.
Descriptive statistics of reported limits of quantification for poppy seed samples (mg/kg; whole weight) and proportion of left‐censored (LC) data (%) for analytical results submitted from 2012 until 2017 (2nd batch)
| Substance | N | % LC | Minimum | P25 | Mean | P75 | Maximum |
|---|---|---|---|---|---|---|---|
| Morphine | 1,164 | 2 | 0.010 | 0.10 | 2.7 | 6.9 | 7.5 |
| Codeine | 962 | 25 | 0.010 | 0.050 | 0.60 | 1.0 | 1.8 |
| Thebaine | 869 | 31 | 0.040 | 0.050 | 2.35 | 1.0 | 27 |
| Oripavine | 41 | 63 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Noscapine | 782 | 60 | 0.050 | 0.10 | 0.65 | 1.0 | 1.0 |
| Papaverine | 304 | 71 | 0.040 | 0.050 | 0.17 | 0.10 | 1.0 |
| Narceine | 41 | 54 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Acetylcodeine | 43 | 100 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
N: number of samples; P: percentile.
However, the occurrence data currently available demonstrate that the concentration of codeine can be much higher than that of morphine in some poppy seed samples on the European market (see Section 3.2.1.5). The CONTAM Panel therefore now concludes that the concentration of codeine in the poppy seed samples should be taken into account in the exposure assessment and risk characterisation by converting to morphine equivalents, using a factor of 0.2, in order to express exposure in morphine equivalents. The ARfD is therefore a group ARfD for morphine and codeine, expressed in morphine equivalents. It relates to the risk assessment of opium alkaloids in poppy seed‐containing foods and not to therapeutic uses of these opioids, which also take into account the benefits of therapeutic use.
Thebaine is exhibiting in rats and mice a higher oral acute toxicity than morphine based on lower LD50 values. Thebaine stimulates the μ‐opioid receptor only at very high concentrations, however due to possible metabolism into morphine and oripavine, a contribution to morphine‐like toxicity may occur, but is expected to be small. However, the available data are insufficient to identify a factor for conversion to morphine equivalents, or to propose a health‐based guidance value (HBGV), or to identify a reference point for calculating margins of exposure. The CONTAM Panel noted that no conclusions could be drawn regarding whether thebaine would enhance narcotic morphine effects as described for hexobarbital in mice.
Oripavine also has the potential to elicit morphine‐like effects, and therefore in principle should be considered for inclusion in the group ARfD. However, the available data are insufficient to characterise the hazard or to identify a factor for conversion to morphine equivalents.
The modes of action of noscapine and papaverine differ from that of morphine, and therefore, these alkaloids cannot be converted to morphine equivalents and are not included in the group ARfD. The available data are insufficient to propose HBGVs. Based on the very limited data available, it appears that noscapine and papaverine are less toxic than the other alkaloids discussed in this opinion.
3.2. Occurrence data
3.2.1. Occurrence data on food submitted to EFSA
3.2.1.1. Overview on analytical results submitted to EFSA before and after 2011
A first batch of 2,678 analytical results on occurrence of opium alkaloids in food and poppy seeds (Table 7) was collected by EFSA until 2011 and these data were already presented in the previous opinion of EFSA's CONTAM Panel on opium alkaloids in poppy seeds (EFSA CONTAM Panel, 2011). The 2011 data set is used in the current document for comparison with occurrence data on opium alkaloids provided to EFSA after the previous opinion was published. The data set available for the 2011 opinion contained 181 analytical results, belonging to 55 Australian poppy seed samples, which were presented in the opinion but not used for dietary exposure assessment. This was due to their opium alkaloid profile that was considered different from poppy seed samples available on the EU market at that time.
Table 7.
Number of analytical results on opium alkaloids in poppy seeds and other food types collected by EFSA
| Reporting country/institution | Substance | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Morphine | Codeine | Thebaine | Oripavine | Noscapine | Papaverine | Narceine | Acetylcodeine | Total | |
| 1st batch a | |||||||||
| Australia | 55 | 43 | 47 | 43 | 0 | 0 | 0 | 0 | 188 |
| Austria | 45 | 45 | 0 | 0 | 45 | 45 | 0 | 0 | 180 |
| Germany | 561 | 374 | 143 | 0 | 87 | 90 | 0 | 0 | 1,255 |
| Hungary | 231 | 231 | 231 | 0 | 231 | 0 | 0 | 0 | 924 |
| Netherlands | 131 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 131 |
| Total | 1,023 | 693 | 421 | 43 | 363 | 135 | 0 | 0 | 2,678 |
| 2nd batch b | |||||||||
| Austria | 9 | 9 | 0 | 0 | 9 | 9 | 0 | 0 | 36 |
| Czech Republic | 146 | 145 | 145 | 0 | 145 | 145 | 0 | 0 | 726 |
| EU food business association | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| France | 63 | 63 | 63 | 0 | 0 | 0 | 0 | 0 | 189 |
| Germany | 173 | 234 | 181 | 90 | 132 | 182 | 0 | 0 | 992 |
| Hungary | 516 | 517 | 518 | 0 | 518 | 0 | 0 | 0 | 2,069 |
| Luxembourg | 17 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Malta | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Spain | 253 | 50 | 50 | 0 | 0 | 0 | 0 | 0 | 353 |
| Netherlands | 44 | 44 | 44 | 44 | 44 | 44 | 44 | 0 | 308 |
| United Kingdom | 81 | 68 | 0 | 0 | 43 | 43 | 0 | 43 | 278 |
| Total | 1,309 | 1,146 | 1,001 | 134 | 891 | 423 | 44 | 43 | 4,991 |
Collected by EFSA until 2011; these results were already presented in the previous opinion from EFSA's CONTAM Panel on opium alkaloids in poppy seeds (EFSA CONTAM Panel, 2011).
Collected by EFSA from 2012 to 2017; presented data show the number of samples after cleaning (see Annex A, Table A.1 for further details regarding data cleaning).
Between November 2012 and October 2017, new occurrence data were submitted to EFSA. This second batch of data contained a total of 5,040 analytical results on opium alkaloids in different foods. The overall data set was carefully evaluated and data cleaning procedures were applied before the estimation of dietary exposure to opium alkaloids. In particular, 49 analytical records were excluded because the reported analyte was indicated as ‘sum of morphine alkaloids’ and could not be included in any of the considered single opium alkaloids used for this opinion; details and criteria for data cleaning and the outcome of clarification requests to data providers are detailed in Annex A, Table A.1. After cleaning, a total of 4,991 analytical results on opium alkaloids in food, submitted by institutions from 10 different EU countries, were available in the second batch (Table 7). Moreover within the second batch, two analytical results on morphine were submitted by an EU food business association. It should be noted that the country of sampling did not necessarily correspond to the country of reporting, since some institutions collected samples abroad. The data set contains analytical results on morphine, codeine, thebaine, oripavine, noscapine and papaverine, as well as a small number of analytical results on narceine (n = 44) and acetylcodeine (n = 43) (Table 7).
3.2.1.2. Distribution of analytical results across different food types
For consistency with the previous opinion on opium alkaloids (first batch) and since some single food categories at the level 4 of the FoodEx classification contained too few analytical results, certain food groups were reclassified. The aggregated food groups are detailed in Annex A, Table A.3, while Annex A, Table A.2 presents for both batches the number of analytical results per substance and reporting country for the aggregated food categories. The three main food categories in the second batch of data were represented by poppy seeds (4,206 analytical results for 1,190 samples), bakery products (599 analytical results for 131 samples) and baking ingredients (106 analytical results for 20 samples). Apart from the three main food categories, some data on other types of food were submitted in the second batch: fish snack product (1 sample), cereal‐based food for infants and young children (2 samples), honey (1 sample), (non‐chocolate) confectionery (3 samples), tap water (40 samples) and well water (11 samples).
In both batches, the vast majority of data was reported on poppy seed samples (Figure 5) and mainly for morphine, followed by codeine, thebaine and noscapine.
Figure 5.
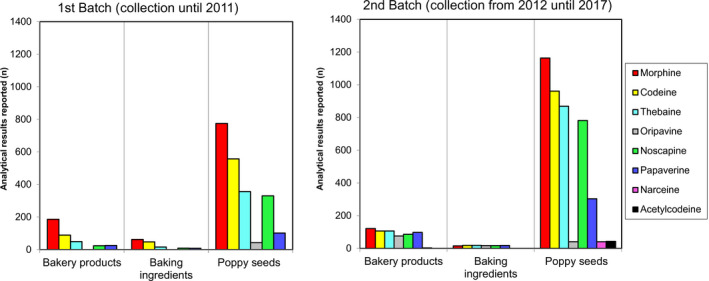
Number of analytical results on opium alkaloids in food available to EFSA according to main food groups and substance
- On the left: analytical results collected until 2011 and presented in the EFSA Scientific Opinion of 2011 (1st batch); on the right: analytical results collected from November 2012 until October 2017 (2nd batch).
3.2.1.3. Analytical methods and limits of quantification
Within the second batch, 3,694 analytical results were generated by LC–MS‐based methods, 309 by HPLC with UV–Vis or fluorescence detection, while for 988 data points no information was provided. This is in accordance with the analytical methods reported for the first batch (see Figure 3 in EFSA CONTAM Panel, 2011).
Table 8 provides an overview of the reported LOQs for poppy seed samples (second batch only) while LODs and LOQs for all matrix types are reported in Annex A, Table A.4. Similar LODs were reported for the first batch (see EFSA CONTAM Panel, 2011 for further details). The LOQs reported for morphine in poppy seed samples in the second batch varied from 0.010 to 7.5 mg/kg and 2% of the data on morphine were left‐censored. The maximum reported LOQ was 27 mg/kg for thebaine; this LOQ was reported for a set of 35 samples measured by HPLC‐UV. However, all samples were quantified and had a thebaine concentration well above the LOQ.
3.2.1.4. Occurrence data in poppy seed samples: a critical comparison between first and second batch
In total, for both batches, 7,669 analytical results on the occurrence of opium alkaloids in various foods were available. Considering the limited amount of data on food categories other than poppy seeds, the CONTAM Panel decided to evaluate the alkaloid profile based on poppy seed samples only. For the latter, a total of 6,369 analytical results related to 1,975 samples, collected throughout the years 1981–2017, were available. A total of 4,206 analytical results (corresponding to 1,190 samples) were submitted to EFSA in the second batch as compared to 2,163 analytical results (corresponding to 785 samples) in the first batch. Annex A, Table A.5 presents an overview of the number of analytical results divided by batch, sampling year and reporting country/organisation.
Table 9 summarises the descriptive statistics for all poppy seed samples and the percentage of left‐censored data. The Australian data collected until 2011 have not been excluded to allow a comparison with the second batch. All data on acetylcodeine were left‐censored and therefore not used in the present opinion. Only one institution provided a limited number of analytical results on narceine (41 samples) and 54% were left‐censored. Therefore, this compound was not included in the risk assessment.
Table 9.
Summary statistics of the concentration (mg/kg; whole weight) of opium alkaloids in poppy seed samples collected until 2011 (1st batch)a and collected after 2011 (2nd batch)
| Substance | Batch | N | LC (%) | Concentration (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | P95b | Maximum | ||||||||
| LB | UB | LB | UB | LB | UB | LB | UB | ||||
| Morphine | 1st | 775 | 11 | 10.0 | 10.0 | 38.2 | 38.6 | 202 | 202 | 630 | 630 |
| 2nd | 1,164 | 2 | 13.6 | 13.6 | 57.8 | 57.8 | 253 | 253 | 596 | 596 | |
| Codeine | 1st | 557 | 32 | 1.40 | 1.40 | 4.95 | 5.23 | 14.9 | 14.9 | 827 | 827 |
| 2nd | 962 | 25 | 1.40 | 1.40 | 5.93 | 6.15 | 28.0 | 28.0 | 298 | 298 | |
| Thebaine | 1st | 356 | 19 | 2.00 | 2.00 | 15.7 | 15.8 | 101 | 101 | 783 | 783 |
| 2nd | 869 | 31 | 1.20 | 1.20 | 12.4 | 12.6 | 64.0 | 64.0 | 455 | 455 | |
| Oripavine | 1st | 43 | 42 | 4.00 | 4.00 | 20.8 | 21.3 | n.a. | n.a. | 233 | 233 |
| 2nd | 41 | 63 | 0 | 0.100 | 1.78 | 1.85 | n.a. | n.a. | 27.5 | 27.5 | |
| Noscapine | 1st | 330 | 69 | 0 | 1.00 | 1.02 | 1.70 | 4.20 | 4.20 | 39.2 | 39.2 |
| 2nd | 782 | 60 | 0 | 1.00 | 1.84 | 2.34 | 6.30 | 6.30 | 86.9 | 86.9 | |
| Papaverine | 1st | 102 | 81 | 0 | 0.750 | 0.090 | 0.590 | 0.400 | 1.00 | 1.79 | 1.79 |
| 2nd | 304 | 71 | 0 | 0.100 | 0.150 | 0.250 | 1.15 | 1.15 | 3.20 | 3.20 | |
| Narceine | 2nd | 41 | 54 | 0 | 0.100 | 0.230 | 0.290 | n.a. | n.a. | 1.61 | 1.61 |
| Acetylcodeine | 2nd | 43 | 100 | 0 | 0.100 | 0 | 0.100 | n.a. | n.a. | 0 | 0.100 |
N: number of samples; LB: lower bound; LC: left‐censored; n.r.: not applicable; P95: 95th percentile; UB: upper bound.
Discrepancies in the descriptive statistics of the 1st batch of data compared to the 2011 opinion are due to the way of handling left‐censored data. In the present report, analytical results showing a concentration below the reported limit of quantification (LOQ) were considered as LC irrespectively of whether or not it was specified by the data provider as below the LOQ.
The 95th percentile with less than 60 observations may not be statistically robust (EFSA, 2011b) and is therefore not calculated.
The comparison between the first and second batch revealed that the latter had a higher mean morphine concentration than the first. However, in both data sets, samples with very high morphine concentrations were found, and the 95th percentile exceeded 200 mg/kg in both batches. This is accordance with the fact that poppy seeds from poppy varieties with high alkaloid content, bred for pharmaceutical purposes, are used as a by‐product for food purposes.
The mean oripavine concentration was considerably lower in the second batch of reported data compared to the data presented for the previous opinion, but in both cases statistics have been calculated on a limited number of observations (about 40).
The CONTAM Panel identified large differences in the concentration of opium alkaloids in relation to the country of origin of the poppy seed samples (Figure 6 and Annex A, Table A.6). The Spanish poppy seed samples collected in the second batch contained a higher median concentration of morphine, codeine and thebaine than those of any other reported country of origin. The median UB morphine concentration in Spanish poppy seeds was 156 mg/kg. Upon a clarification request, the data provider confirmed that ‘analyses were made by the producer on commercial batches for the food industry. It is very important to remark that these poppy seeds are from a variety of plant also cultivated for the pharmaceutical industry’.
Figure 6.
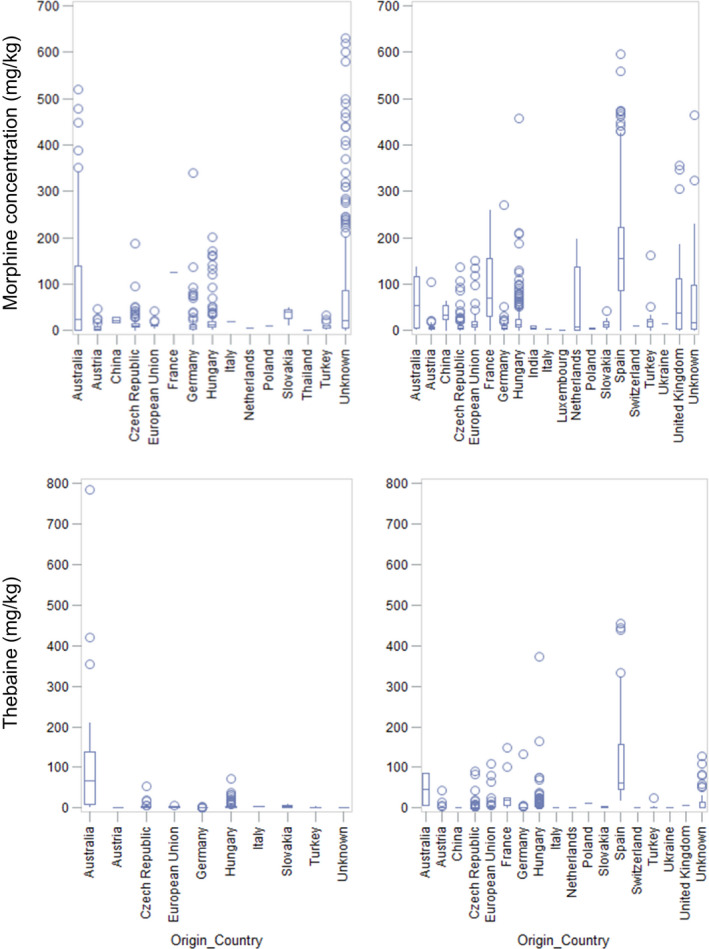
Box plots representing the upper bound concentration (mg/kg whole weight) of morphine (top) and thebaine (down) in poppy seed samples according to country of origin for the 1st (left) and 2nd batch (right) of data
In the second batch, apart from Spanish samples, also poppy seed samples originating from France (69.6 mg/kg), Australia (53.4 mg/kg), the UK (37.6 mg/kg) and China (32.1 mg/kg) had a high median morphine content (at the UB) compared to samples originating from other countries. Moreover French poppy seed samples had a relatively high median codeine (9.6 mg/kg at the UB) and thebaine (17.9 mg/kg at the UB) concentration compared to other countries of origin, although the number of reported samples was limited (n = 15). Likewise, the Australian poppy seed samples showed a higher codeine and thebaine concentration compared to the other samples and this was in line with the data presented in the first batch. However, it should be noted that only a limited number of Australian samples (n = 4) was present in the second data set.
The CONTAM Panel considered the following points:
the high median morphine concentration in poppy seeds originating from Australia in the first batch was confirmed by the samples originating from Australia in the second batch;
high morphine concentrations were reported in the second batch for samples originating from China, France, Spain and the UK, for which no data were available in the first batch (Annex A, Table A.6 and Figure 6);
the concentration of morphine was lower in poppy seeds originating from the other countries (e.g. Austria, the Czech Republic, Germany, Hungary and the Netherlands) with little variation between data from the first and second batch.
Based on these considerations, the CONTAM Panel concluded that the profiles in the two batches are comparable. In addition, the profile in the Australian poppy seeds is compatible with the profile in the European poppy seeds. Therefore, the Australian data were included in the data set and the two batches of data submitted to EFSA were merged. Based on the observed differences in median morphine concentrations between different countries of origin, the CONTAM Panel decided to divide the samples in three groups:
poppy seed samples originating from Australia, China, France, Spain and the UK which are assumed to represent primarily poppy seeds from varieties grown for pharmaceutical use which are used as a by‐product for food purposes; hereafter referred to as ‘high‐morphine’ group;
poppy seed samples originating from other countries which are assumed to represent varieties grown for food use; hereafter referred to as ‘low‐morphine’ group. However, it is known that some of these countries produce also varieties for pharmaceutical purposes and this is also represented by the high maximum morphine concentrations as reported in Annex A, Table A.6;
poppy seed samples with unknown origin.
Besides the fact that countries may produce varieties for both food and pharmaceutical use, the application of cleaning practices might reduce the level of morphine in the poppy seed samples. Furthermore, seeds of different poppy varieties (data providers did not report information on poppy seeds varieties) may be mixed before entering the food market. These factors introduce some uncertainty in the applied division.
Table 10 presents the results for the occurrence data set divided into the above‐mentioned groups. Considering the low percentage of left‐censored data, and consequently the small difference between LB, MB and UB concentrations for morphine, codeine and thebaine, only the MB concentrations are shown in Table 10. LB and UB concentrations are presented in Annex A, Table A.7. Due to the higher percentage of left‐censored data for oripavine, noscapine and papaverine, also the LB and UB concentrations were considered for these opium alkaloids (see discussion below; Annex A, Table A.7).
Table 10.
Descriptive statistics of opium alkaloids MB concentrations (mg/kg whole weight) in poppy seed samples
| Substance | Group | N | Mean | Min | P25 | P50 | P75 | P95a | Max |
|---|---|---|---|---|---|---|---|---|---|
| Morphine | ‘High‐morphine’ | 362 | 147 | 0.05 | 48.3 | 129 | 212 | 383 | 596 |
| ‘Low‐morphine’ | 1,210 | 16.4 | 0.02 | 4.50 | 8.37 | 15.6 | 52.8 | 458 | |
| Unknown | 367 | 64.8 | 0.05 | 5.90 | 16.7 | 71.0 | 309 | 630 | |
| Total | 1,939 | 50.1 | 0.02 | 5.20 | 11.9 | 41.4 | 240 | 630 | |
| Codeine | ‘High‐morphine’ | 144 | 22.7 | 0.02 | 0.50 | 4.77 | 25.3 | 52.0 | 827 |
| ‘Low‐morphine’ | 1,097 | 2.88 | 0.03 | 0.50 | 1.20 | 2.50 | 9.30 | 202 | |
| Unknown | 278 | 7.95 | 0.02 | 0.60 | 1.90 | 3.90 | 26.8 | 262 | |
| Total | 1,519 | 5.82 | 0.02 | 1.00 | 1.40 | 3.14 | 21.0 | 827 | |
| Morphine equivalents | ‘High‐morphine’ | 362 | 152 | 0.05 | 49.7 | 133 | 218 | 390 | 614 |
| ‘Low‐morphine’ | 1,210 | 16.9 | 0.02 | 4.70 | 8.60 | 16.2 | 55.3 | 479 | |
| Unknown | 367 | 67.2 | 0.05 | 6.18 | 17.4 | 73.1 | 318 | 649 | |
| Total | 1,939 | 51.7 | 0.02 | 5.2 | 12.3 | 43.0 | 247 | 649 | |
| Thebaine | ‘High‐morphine’ | 123 | 92.5 | 0.03 | 11.0 | 52.0 | 121 | 334 | 783 |
| ‘Low‐morphine’ | 940 | 3.92 | 0.01 | 0.50 | 1.20 | 3.00 | 12.3 | 373 | |
| Unknown | 162 | 8.56 | 0.01 | 0.29 | 0.86 | 4.20 | 54.9 | 128 | |
| Total | 1,225 | 13.5 | 0.01 | 1.00 | 1.40 | 4.10 | 68.4 | 783 | |
| Oripavine | ‘High‐morphine’ | 46 | 20.0 | 0.05 | 0.50 | 3.50 | 23.0 | n.a. | 233 |
| ‘Low‐morphine’ | 15 | 2.14 | 0.05 | 0.05 | 0.05 | 0.18 | n.a. | 27.5 | |
| Unknown | 23 | 1.19 | 0.05 | 0.05 | 0.05 | 2.52 | n.a. | 7.00 | |
| Total | 84 | 11.8 | 0.10 | 0.10 | 1.00 | 5.00 | 55.0 | 233 | |
| Noscapine | ‘High‐morphine’ | 26 | 0.13 | 0.03 | 0.03 | 0.05 | 0.05 | n.a. | 1.80 |
| ‘Low‐morphine’ | 943 | 2.08 | 0.02 | 0.50 | 0.50 | 1.00 | 6.30 | 86.9 | |
| Unknown | 143 | 0.78 | 0.02 | 0.05 | 0.50 | 0.50 | 2.68 | 27.0 | |
| Total | 1,112 | 2.15 | 0.02 | 1.00 | 1.00 | 1.00 | 5.10 | 86.9 | |
| Papaverine | ‘High‐morphine’ | 26 | 0.09 | 0.03 | 0.03 | 0.05 | 0.05 | n.a. | 1.30 |
| ‘Low‐morphine’ | 273 | 0.27 | 0.01 | 0.03 | 0.09 | 0.50 | 1.15 | 2.60 | |
| Unknown | 107 | 0.19 | 0.01 | 0.03 | 0.05 | 0.07 | 0.92 | 3.20 | |
| Total | 406 | 0.34 | 0.01 | 0.05 | 0.10 | 0.50 | 1.00 | 3.20 |
N: number of samples; n.a.: not applicable; Max: maximum; Min: minimum; P: percentile.
The 95th percentile with less than 60 observations may not be statistically robust (EFSA, 2011b) and is therefore not calculated.
The median morphine concentration in poppy seed samples of the ‘high‐morphine’ group was 15 times higher than in the ‘low‐morphine’ group. Moreover, the median concentration for codeine, thebaine and oripavine in the ‘high‐morphine’ group was 4, 43 and 70 times, respectively, higher compared to the ‘low‐morphine’ group. Similar trends were observed at the P75. The here presented data therefore indicate that seeds from poppy varieties relatively rich in morphine, codeine and thebaine might be used for culinary purpose in Europe. Poppy seed samples with a high morphine, codeine and/or thebaine concentration were also occasionally present in the ‘low‐morphine’ group and in the unknown group. The available information regarding country of origin and country of sampling shows that although poppy seeds grown in one country are mainly sampled in the same country, a part is also sampled and consequently consumed in other EU countries.
As explained in Section 3.1.6, the CONTAM Panel concluded to take the combined exposure to morphine and codeine into account in the risk assessment, by converting the codeine concentration into morphine equivalents, using a factor of 0.2. In 1,485 poppy seed samples, both morphine and codeine were analysed, while in 454 samples only morphine was analysed. In order to use the same data set for the calculation of the exposure to morphine and morphine equivalents, an imputation of the codeine concentration was performed for these samples. After selection of the not left‐censored data for codeine and morphine, the proportion of codeine to morphine was calculated (expressed as percentage). The median proportion was 15% for both the ‘high‐’ and ‘low‐morphine’ group. This proportion was used for the samples in which codeine was not analysed. Morphine equivalents were calculated using the following formula:
Morphine equivalents were very similar to the morphine concentrations (Table 10) since in the majority of the samples codeine is contributing little to the morphine equivalents.
For oripavine, noscapine and papaverine, the percentage of left‐censored data was higher and consequently the difference between LB and UB concentrations was larger. The mean oripavine concentration in poppy seed samples (n = 84) was 11.5–11.8 mg/kg (LB–UB) and the P95 was 55.0 mg/kg (LB = UB). The mean noscapine concentration in poppy seed samples (n = 1,112) was 1.59–2.15 mg/kg (LB–UB) and the P95 was 5.1 mg/kg (LB = UB). The mean papaverine concentration in poppy seed samples (n = 406) was 0.14–0.34 mg/kg (LB–UB) and the P95 was 1.00 mg/kg (LB = UB).
3.2.1.5. Profiles of opium alkaloids in poppy seeds – Ratios to morphine
Variations in the alkaloid profiles in poppy seed samples were explored by calculating the ratio of single opium alkaloids to morphine. Concentrations were expressed as mmol/kg at the UB. It was noted that not all laboratories reported results on the full set of opium alkaloids, and morphine was the most frequently reported substance in poppy seeds (n = 1,939). The overall results (Table 11 – line Total) showed a large variation in the ratios among poppy seed samples.
Table 11.
Opium alkaloids ratios in poppy seed samples compared to morphine
| Substance | Origin | N | Ratio to morphinea | |||||
|---|---|---|---|---|---|---|---|---|
| Min | P25 | Median | P75 | P95b | Max | |||
| Codeine | ‘High‐morphine’ | 142 | < 0.01 | 0.04 | 0.14 | 0.29 | 1.08 | 713 |
| ‘Low‐morphine’ | 1,086 | 0.01 | 0.10 | 0.14 | 0.20 | 0.51 | 0.97 | |
| Unknown | 257 | 0.01 | 0.09 | 0.15 | 0.27 | 0.67 | 2.33 | |
| Total | 1,485 | < 0.01 | 0.10 | 0.14 | 0.21 | 0.75 | 713 | |
| Thebaine | ‘High‐morphine’ | 123 | 0.01 | 0.11 | 0.29 | 14.0 | 135 | 227 |
| ‘Low‐morphine’ | 936 | < 0.01 | 0.08 | 0.14 | 0.22 | 0.48 | 1.86 | |
| Unknown | 143 | < 0.01 | 0.07 | 0.12 | 0.19 | 0.52 | 1.10 | |
| Total | 1,202 | < 0.01 | 0.08 | 0.14 | 0.24 | 0.84 | 227 | |
| Oripavine | ‘High‐morphine’ | 46 | < 0.01 | 0.01 | 0.87 | 4.34 | n.a. | 43.4 |
| ‘Low‐morphine’ | 15 | < 0.01 | 0.01 | 0.01 | 0.02 | n.a. | 0.47 | |
| Unknown | 23 | < 0.01 | 0.01 | 0.02 | 0.05 | n.a. | 0.21 | |
| Total | 84 | < 0.01 | 0.01 | 0.04 | 0.87 | 19.9 | 43.4 | |
| Noscapine | ‘High‐morphine’ | 26 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | n.a. | 0.75 |
| ‘Low‐morphine’ | 939 | < 0.01 | 0.04 | 0.07 | 0.14 | 0.62 | 6.75 | |
| Unknown | 127 | < 0.01 | 0.01 | 0.05 | 0.09 | 0.23 | 0.48 | |
| Total | 1,092 | < 0.01 | 0.03 | 0.07 | 0.13 | 0.57 | 6.75 | |
| Papaverine | ‘High‐morphine’ | 26 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | n.a. | 0.76 |
| ‘Low‐morphine’ | 272 | < 0.01 | 0.01 | 0.02 | 0.11 | 0.76 | 0.76 | |
| Unknown | 87 | < 0.01 | < 0.01 | 0.01 | 0.05 | n.a. | 0.76 | |
| Total | 385 | < 0.01 | 0.01 | 0.02 | 0.09 | 0.76 | 0.76 | |
N: number of samples; n.a.: not applicable; Max: maximum; Min: minimum; P: percentile.
Calculated by dividing the opium alkaloid upper bound concentration by the morphine upper bound concentration. Concentrations were expressed as mmol/kg.
The 95th percentile with less than 60 observations may not be statistically robust (EFSA, 2011b) and is therefore not calculated.
In general, morphine was the predominant alkaloid in poppy seed samples as shown by the median ratios being smaller than 1 for the overall data set (Table 11). However in the ‘high‐morphine’ group, several samples had a thebaine, codeine or oripavine ratio to morphine that was greater than 1, which was not observed for the ‘low‐morphine’ and the unknown group. At the P95, the codeine ratio exceeded 1 in the ‘high‐morphine’ group, while this was not observed for the ‘low‐morphine’ group. In the ‘high‐morphine’ group, a sample (with origin Australia) was reported with a very high concentration of codeine and a ratio to morphine of > 700‐fold. At the P75, the thebaine concentration was 14 times higher than the morphine concentration in the ‘high‐morphine’ group, while in the ‘low‐morphine’ group the ratio was 0.22. At the P75, oripavine was over 4 times higher than morphine in the ‘high‐morphine’ group versus a ratio of 0.02 in the ‘low‐morphine’ group. Ratios according to the country of origin of the poppy seed samples can be found in Annex A, Table A.8.
3.2.1.6. Proportion of alkaloid content in poppy seed samples
For a total of 1,197 poppy seed samples, the concentration of morphine, codeine and thebaine was available. For these samples the proportion of each alkaloid to the total opium alkaloid content was calculated, corrected for molecular weight (Table 12). Annex A, Table A.9 shows the proportion per country of origin.
Table 12.
Percentagea of the opium alkaloid to the sum of morphine, codeine and thebaine per sample
| Substance | Origin | N | Percentagea of the opium alkaloid to the sum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | P25 | Median | P75 | P95 | Max | |||
| Morphine | ‘High‐morphine’ | 119 | 50.6 | 0.13 | 6.6 | 70.0 | 80.0 | 97.6 | 98.5 |
| ‘Low‐morphine’ | 935 | 76.4 | 28.4 | 71.0 | 78.5 | 83.8 | 91.7 | 97.4 | |
| Unknown | 143 | 77.3 | 22.6 | 74.3 | 79.8 | 84.4 | 92.2 | 97.1 | |
| Total | 1,197 | 73.9 | 0.13 | 70.3 | 78.0 | 83.9 | 92.6 | 98.5 | |
| Codeine | ‘High‐morphine’ | 119 | 8.88 | 0.1 | 0.7 | 4.0 | 10.6 | 19.9 | 92.6 |
| ‘Low‐morphine’ | 935 | 11.6 | 0.8 | 8.1 | 10.5 | 13.9 | 22.7 | 35.7 | |
| Unknown | 143 | 11.6 | 1.7 | 6.7 | 9.3 | 12.9 | 32.0 | 52.7 | |
| Total | 1,197 | 11.3 | 0.1 | 7.6 | 10.2 | 13.5 | 22.8 | 92.6 | |
| Thebaine | ‘High‐morphine’ | 119 | 40.5 | 1.26 | 9.7 | 19.2 | 89.4 | 98.6 | 99.4 |
| ‘Low‐morphine’ | 935 | 12.1 | 0.12 | 6.8 | 10.5 | 15.9 | 26.0 | 58.0 | |
| Unknown | 143 | 11.1 | < 0.1 | 6.4 | 10.0 | 14.2 | 24.7 | 32.6 | |
| Total | 1,197 | 14.8 | < 0.1 | 6.8 | 10.8 | 16.6 | 37.2 | 99.4 | |
N: number of samples; Max: maximum; Min: minimum; P: percentile.
Calculated by dividing the opium alkaloid upper bound concentration by the sum of the upper bound concentration of morphine, codeine and thebaine, per sample morphine upper bound concentration. Concentrations were expressed as mmol/kg.
Morphine was overall the main of the three considered opium alkaloids in poppy seeds; for all samples combined the average proportion is 74%, followed by thebaine (15%) and codeine (11%). Morphine was also the largest contributor of the three (51% on average) to the sum of morphine, codeine and thebaine for the ‘high‐morphine’ group. However, thebaine was also an important contributor (41% on average). For the ‘low‐morphine’ group, morphine contributed on average 76% to the sum, with thebaine and codeine both contributing on average 12%.
3.2.1.7. Occurrence data for matrices other than poppy seeds
Descriptive statistics of the concentration of opium alkaloids in food other than poppy seeds are shown in Annex A (Table A.10) according to the food categories described in Section 3.2.1.2, substance and batch of data collection. The following general considerations on data from other food categories were made:
Bakery products: a limited number of analytical results were available to EFSA for this food category. Bakery products represent a heterogeneous category since it consists of different types of products containing different amounts of poppy seeds. In general, the mean opium alkaloids concentrations were in the same order of magnitude in the first and second batch, with a mean UB concentration of 1 mg/kg or below. Oripavine was reported only in the second batch, but with 94% of left‐censored data. The three bakery products analysed for narceine were left‐censored.
Baking ingredients: a limited number of analytical results were available for this category. Under the UB scenario, the average morphine concentration in the first and secnd batch was 3.64 and 1.10 mg/kg, respectively, for codeine 1.19 and 0.17 mg/kg, for thebaine 0.23 and 0.21 mg/kg, and for papaverine 0.18 and 0.11 mg/kg. Noscapine levels were comparable in the first and second batch of data. Oripavine was reported only in the second batch, but 96% of the data were left‐censored.
Other types of food: In the second batch, results on food types other than bakery products and ingredients were reported. Three confectionary products were found to contain morphine (average concentration of 6.60 mg/kg). Tap and well water samples (n = 51) were only analysed for codeine and all samples were left‐censored. Other food categories for which analytical results were reported (honey, cereal‐based food for infants and young children, snack food) had a minor, or negligible, opium alkaloids content. Further details are reported in Annex A, Table A.10.
3.2.2. Previously reported occurrence data in the open literature
In this section, an overview is presented on the reported occurrence data in the open literature. It should be noted that part of the data published may also have been submitted to EFSA and may have been included in the EFSA occurrence data set described in Section 3.2.1.
A summary on the older data on occurrence of opium alkaloids in poppy seeds and baking ingredients (poppy seed fillings) can be found in the report from the Bundesinstitut für Risikobewertung (the German Federal Institute for Risk Assessment; BfR) from 2005 (BfR, 2005). The reported values in (unground) poppy seeds range from 0.1 to 620 mg/kg for morphine, from 0.1 to 57.1 mg/kg for codeine, from 0.3 to 41 mg/kg for thebaine, from 0.84 to 230 mg/kg for noscapine and from < LOQ to 67 mg/kg for papaverine. With respect to the occurrence of morphine in poppy seeds, data were provided for 72 samples, ranging in content from 0.5 to 620 mg/kg. Nineteen samples contained at least 100 mg/kg morphine, while 23 samples contained less than 10 mg/kg morphine. A study conducted by the Bavarian Regional Office for Health and Food Safety on the content of morphine and codeine in 48 poppy seed samples showed for morphine a range of 3.1–330 mg/kg and for codeine a range of < LOQ to 23.7 mg/kg.
BVL (Bundesamt für Verbraucherschutz und Lebensmittelsicherheit) reported on two German surveys held in 2007 on the occurrence of morphine in a total of 446 samples of poppy seeds and poppy seed containing food products collected on the German retail market. Of the 201 samples of unground poppy seeds, 62 (30.8%) contained only low levels (≤ 4 mg/kg) of morphine, 114 (56.7%) contained between 4 and 20 mg/kg, and 25 (12.4%) contained more than 20 mg/kg. The ground poppy seeds (46 samples) tended to have slightly lower concentrations of morphine: 21 (45.7%) contained ≤ 4 mg/kg, 19 (41.3%) between 4 and 20 mg/kg, and 6 samples (13.0%) contained more than 20 mg/kg. The poppy seed containing food products could be divided into baking products (poppy seed cake, rolls, 143 samples) and baking ingredients (ready‐to‐use fillings, 56 samples). The large majority of the pastry samples (140, 97.9%) contained ≤4 mg/kg morphine, while 3 samples contained between 4 and 10 mg/kg. Regarding the ready‐to‐use fillings, 45 (80.3%) contained ≤ 4 mg/kg morphine, 10 (17.9%) contained between 4 and 20 mg/kg while one sample exceeded 20 mg/kg (BVL, 2007).
In the previous opinion, the occurrence data were presented that had been submitted to EFSA up to early 2011 (EFSA CONTAM Panel, 2011). Data had been submitted by five different countries for in total six alkaloids (morphine, codeine, thebaine, oripavine, noscapine and papaverine) in poppy seeds, bakery products and baking ingredients. However, the number of analytical results per individual alkaloid differed significantly, from 43 results for oripavine to 1,023 results for morphine. The majority of submitted data (2,163, results, 80.8%) was on poppy seeds, and smaller contributions were made for bakery products (373 results, 13.9%) and baking ingredients (142 results, 5.3%). The CONTAM Panel noted that only one country (Germany) submitted results for bakery products and ingredients. Regarding the poppy seeds, morphine (775 results) was present in the highest concentrations (based on LB approach): 10.0, 38.2, 202 and 630 mg/kg for the median, mean, P95 and maximum, respectively. For codeine (557 results) the values were: 1.4, 5.0, 14.9 and 827 mg/kg, respectively, and for thebaine (356 results): 2.0, 15.6, 101 and 783 mg/kg, respectively. Data on oripavine were based on a relatively small number of samples (43): resulting in the following values: 4.0, 20.8, 68.0 and 233 mg/kg, respectively. For noscapine (330 results): 0.0, 1.0, 4.2 and 39.2 mg/kg, respectively, and for papaverine (102 results): 0.0, 0.1, 0.4 and 1.8 mg/kg, respectively. The percentage of data below the LOQ (varying between 0.1 and 10 mg/kg) varied from 9% for morphine to 80% for papaverine. In general, morphine was the dominant alkaloid in poppy seed samples, followed by codeine and thebaine. Only in a small number of samples (9 out of 547) codeine was present in a higher concentration than morphine. A notable exception formed the poppy seed data submitted by Australia, in which thebaine was often present in higher concentrations than morphine (29 out of 43). This was presumably due to use of different cultivars selected for use in the pharmaceutical industry. The concentration of opium alkaloids in bakery products and baking ingredients was generally lower: The maximum concentration reported was 8.2 mg/kg morphine in bakery products and 28.8 mg/kg morphine in baking ingredients, while the mean concentrations were 0.5 and 3.2 mg/kg, respectively. The other alkaloids were found at even lower concentrations.
In the framework of an exposure study of consumers in Hungary to opium alkaloids in poppy seeds, Zentai et al. (2012) reported the results obtained for 737 poppy seed samples collected from the Hungarian retail market. Samples were collected during 2001–2010 and were analysed for morphine, codeine, thebaine and noscapine. Morphine was detected above the LOQ (ranging from 1 to 2 mg/kg) in all but one of the samples. Codeine (61.3%) and thebaine (63.0%) were often present, but noscapine was detected only in 6.2% of the samples. With respect to individual alkaloids, morphine was present in the highest concentrations: 11.0, 18.7, 57.6, and 533 mg/kg for the median, mean, P95 and maximum, respectively. For codeine the values were: 2.0, 3.6, 17.2, and 60 mg/kg, respectively and for thebaine: 2.0, 4.0, 15.8, and 120 mg/kg, respectively. Noscapine was the least prominent alkaloid analysed in Hungarian poppy seed samples, with values of 1.2, 1.2, 1.6, and 40 mg/kg, respectively.
Recently, López et al. (2018) reported the results obtained for 35 retail samples of blue and white poppy seeds collected in the Netherlands and for three samples of ground seeds and three ready‐to‐eat products sampled in Germany. Samples were analysed for six alkaloids (morphine, codeine, thebaine, noscapine, papaverine and narceine). Regarding the 32 blue poppy seed samples the following concentrations for the median, mean, P90 and maximum were reported: morphine: 6.7, 39.4, 125 and 241 mg/kg, respectively. For codeine the values were: 1.6, 48.6, 202 and 348 mg/kg, respectively, and for thebaine: 0.8, 20.4, 76.5 and 106 mg/kg, respectively. Levels reported for noscapine, papaverine and narceine were much lower, with maximum concentrations of 6.0, 2.1 and 2.1 mg/kg, respectively. In the poppy seed samples, morphine was generally present in the highest concentration, but in seven samples codeine was dominant and in one sample thebaine was the major alkaloid. Ground poppy seeds, white poppy seeds and the ready‐to‐eat products contained much lower concentrations of alkaloids (maximum for morphine was 19.6 mg/kg in a sample of white poppy seeds.
Within the period covering 2005–2017, the Rapid Alert System for Food and Feed (RASFF) has reported 20 occurrences of high levels of morphine in poppy seeds (Appendix B). Fourteen notifications were based on official control on the market, while six notifications were done by the producing or trading company. The reported values ranged from 40 to 228 mg/kg morphine in (unground) poppy seeds. One sample of ground poppy seeds was reported to contain 59.13 mg/kg morphine. The countries of origin were Hungary (5 reports), the Czech Republic (4), France (3), Australia (2), Slovak Republic (2), Austria (1), the Netherlands (1) and for two consignments the country of origin was not known. In most cases, the products concerned were withdrawn from the market or prevented from entering the market. It should be noted that only three countries have issued reports on the occurrence of high morphine levels in poppy seeds: Hungary and Germany have issued a total of 14 reports in the period 2005–2010, while the Czech Republic has made six notifications in the period 2014–2015.
In conclusion, opium alkaloids are often present in poppy seeds and in bakery products or ingredients containing poppy seeds. In general, morphine is the dominant alkaloid detected, followed by codeine and thebaine. However, in a smaller proportion of samples thebaine or codeine is the dominant alkaloid instead of morphine. Although the majority of raw poppy seed samples contain individual alkaloid levels below 50 mg/kg, a smaller proportion (possibly 5–20%) can contain levels between 100 and 1,000 mg/kg. Bakery products and ingredients contain significantly lower levels of alkaloids, with the large majority of samples well below 20 mg/kg. The CONTAM Panel noted that for these two categories of products only data have been reported for products obtained from the German retail market, which may not be representative for the EU as a whole. Overall, the concentration of opium alkaloids in poppy seeds identified from the open literature and the data submitted to EFSA are in agreement.
3.2.3. Food processing
The reduction of the alkaloid content of poppy seed samples by means of pretreatment and food processing has been reviewed in detail in the previous EFSA opinion on opium alkaloids (EFSA CONTAM Panel, 2011).
Based on this review, the European Commission published in 2014 an overview of recommended pre‐treatments and processing methods reducing the alkaloid content of poppy seeds and poppy seed products as part of a recommendation on good practices to prevent and to reduce the presence of opium alkaloids in poppy seeds and poppy seed products4 (see Section 1.3.4 for further details).
Little new work has been published since then. The following paragraph is an ‘excerpt’ of the information provided in the previous EFSA opinion.
The alkaloid content of poppy seed samples can be reduced by several means of pretreatment and food processing. Washing or soaking of poppy seeds with cold water may reduce morphine levels by 40–75%, and reductions close to 100% can be reached using hot or boiling water (Bjerver et al., 1982; Sproll et al., 2007). Grinding of the seeds results in a moderate reduction (25–34%) in morphine content (Sproll et al., 2007). Baking/heating of poppy seeds gives a reduction in morphine content of around 30% at moderate baking temperatures (135°C) and of 80–90% at high temperatures (220°C) (General et al., 2007). Combination of poppy seed pretreatment and food preparation (baking) has been shown to result in an overall reduction of 80–100% in the final food product (General et al., 2007; Lachenmeier et al., 2010).
It should be noted that the above‐mentioned studies focussed only on the fate of morphine while the stability of other opium alkaloids was not assessed. Leaching from mature capsules due to rainfall was more significant for morphine and codeine than for thebaine, suggesting a lower water solubility of the latter alkaloid (Hofman and Menary, 1984a). This is also reflected in the slightly lower logP values for morphine (0.89) and codeine (1.19) compared to thebaine (1.53) and oripavine (1.46) (Figure 1). So, it could be assumed that reduction by washing or soaking is more effective for morphine and codeine than for thebaine and oripavine. Reactivity to oxygen upon grinding and thermal stability during baking may resemble that of morphine. Nevertheless, experimental data on the fate of other opium alkaloids than morphine during pretreatment and food processing will be required for a better estimate of the reduction that can be obtained during food processing.
3.3. Dietary exposure assessment for humans
Acute dietary exposure to opium alkaloids from poppy seeds was estimated across the European Union following the methodology described in Section 2.6. A total of 41 dietary surveys, carried out in 23 different Member States, were available for this assessment (Annex B, Table B.1). The consumption of poppy seeds (or poppy seed‐containing foods) was reported at least once in 18 dietary surveys (corresponding to 44 population groups23) from 13 different Member States. Poppy seed consumption was reported for up to about 5% of the days covered by the survey. The five population groups most frequently consuming poppy seeds (more than 3% of the days) were other children from the Czech Republic, very elderly from Denmark, adults from Slovakia, adolescents from the Czech Republic and other children from Austria. Among poppy seed consumers, average consumption ranged from 0.5 (Other children, Sweden) to 25 g poppy seeds/day (Adults, Hungary). Only in seven population groups, it was possible to calculate the 95th percentile, which ranged from 1 (Other children, Sweden) to 55 g poppy seeds/day (Adults, Germany). Poppy seeds consumption data are reported in Annex B (Table B.3).
In some dietary surveys, consumption of poppy seed‐containing foods was reported. The disaggregation strategy described in Section 2.6 was used to convert the consumed amount of poppy seed‐containing foods into the corresponding amount of consumed poppy seeds. This disaggregation strategy did not take into consideration any possible reduction of morphine (and other opium alkaloids) due to the processing of poppy seeds, such as heating or milling.
The CONTAM Panel verified the disaggregation strategy by comparing calculated morphine concentrations in bread to reported concentrations in bread. Within the second batch, Germany reported analytical results for morphine both in poppy seeds (58 samples) and in white wheat rolls containing poppy seeds (70 samples). The reported median and maximum levels of morphine in white wheat rolls were 0.19 and 6.3 mg/kg, respectively. These reported values were in line with the morphine concentrations for white wheat rolls calculated from the concentrations in poppy seeds. In fact, the calculated median and maximum concentrations of morphine in bread containing 4% of poppy seeds were 0.20 and 10.9 mg/kg, based on the median and maximum level of morphine in poppy seed samples from Germany (5.1 and 272 mg/kg, respectively). This backward calculation suggests that the used approach is in line with the submitted data on the occurrence in bakery products.
3.3.1. Mean and high dietary exposure
Acute dietary exposure estimates to opium alkaloids were based on two different occurrence data sets: the ‘high‐morphine’ and ‘low‐morphine’ groups. It is assumed that the first represents primarily poppy seeds from varieties grown for pharmaceutical use of which the seeds are used as a by‐product for food purposes, and the second primarily varieties grown for food use (Section 3.2.1.4). In the text and tables below, the mean exposure (based on the mean consumption and mean occurrence) and the 95th exposure (based on 95th percentile consumption and 95th percentile occurrence) are presented. Hereafter, they are just referred to as mean exposure and P95 exposure respectively. Detailed exposure results, including the exposure calculated using other occurrence percentiles are presented in Annex B (Tables B.4 until B.9). Acute dietary exposure estimates are presented for consumers of poppy seeds only, and under the MB (for morphine, codeine, thebaine and morphine equivalents) or LB and UB (papaverine and noscapine) scenario.
A clear difference in the exposure to morphine (Table 13; Annex B, Table B.4) emerges between the ‘high‐’ and the ‘low‐morphine’ scenario: based on the ‘high‐morphine’ data set ‘other children’ displayed the highest mean exposure, while the corresponding exposure for the ‘low‐morphine’ group was about 10 times lower. The mean exposure was also high for ‘toddlers’ for the ‘high‐morphine’ group ranging from 25.7 to 55.3 μg/kg bw per day, however only two surveys were available. The CONTAM Panel noted that the P95 exposure of the ‘low‐morphine’ group was similar to the mean exposure for the ‘high‐morphine’ group; the reason for this is likely due to the already mentioned the presence of poppy seeds with high morphine content in both groups.
Table 13.
Summary statistics of acute dietary exposure to morphine (μg/kg bw per day) estimated at the middle bound across dietary surveys for consumers only
| Groupa | Population class | Mean exposureb | P95 exposurec | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Min | Median | Max | N | Min | Median | Max | ||
| ‘High‐morphine’ | Infants | 2 | 14.9 | n.a. | 37.9 | 0 | n.a. | n.a. | n.a. |
| Toddlers | 2 | 25.7 | n.a. | 55.3 | 1 | 330 | n.a. | 330 | |
| Other children | 9 | 3.81 | 40.9 | 99.5 | 2 | 20.6 | n.a. | 284 | |
| Adolescents | 8 | 2.31 | 27.2 | 56.1 | 0 | n.a. | n.a. | n.a. | |
| Adults | 11 | 4.91 | 28.1 | 53.8 | 4 | 82.3 | 257 | 326 | |
| Elderly | 8 | 2.07 | 25.1 | 41.3 | 0 | n.a. | n.a. | n.a. | |
| Very elderly | 4 | 8.99 | 28.7 | 40.6 | 0 | n.a. | n.a. | n.a. | |
| ‘Low‐morphine’ | Infants | 2 | 1.66 | n.a. | 4.22 | 0 | n.a. | n.a. | n.a. |
| Toddlers | 2 | 2.86 | n.a. | 6.17 | 1 | 45.6 | n.a. | 45.6 | |
| Other children | 9 | 0.42 | 4.56 | 11.1 | 2 | 2.84 | n.a. | 39.2 | |
| Adolescents | 8 | 0.26 | 3.03 | 6.25 | 0 | n.a. | n.a. | n.a. | |
| Adults | 11 | 0.55 | 3.14 | 6.00 | 4 | 11.4 | 35.5 | 45.1 | |
| Elderly | 8 | 0.23 | 2.79 | 4.60 | 0 | n.a. | n.a. | n.a. | |
| Very elderly | 4 | 1.00 | 3.20 | 4.53 | 0 | n.a. | n.a. | n.a. | |
bw: body weight; Max: maximum; Min: minimum; N: number of surveys; n.a. not applicable; P95: 95th percentile.
Group of poppy seed samples. The ‘high‐morphine’ group refers to poppy seed samples originating from Australia, China, France, Spain and the UK which are assumed to represent primarily varieties grown for pharmaceutical use. The ‘low‐morphine’ group refers to poppy seed samples originating from other countries which are assumed to represent varieties grown for food use. See Section 3.2.1.4 for further details.
Mean exposure calculated from the mean consumption of poppy seeds and the mean occurrence of morphine in poppy seed samples.
P95 exposure calculated from the P95 consumption of poppy seeds and the P95 occurrence of morphine in poppy seed samples. The P95 exposure estimates for surveys with less than 60 observations may not be statistically robust and are therefore not calculated.
Acute dietary exposure to codeine (Table 14) is an order of magnitude lower compared to the exposure to morphine. Also in the case of codeine, there is a large difference between the ‘high‐’ and the ‘low‐morphine’ scenario, with the highest mean exposure found for the other children and toddlers in both scenarios. Detailed summary statistics on the exposure estimates calculated for each dietary survey are presented in Annex B, Table B.5.
Table 14.
Summary statistics of acute dietary exposure to codeine (μg/kg bw per day) estimated at the middle bound across dietary surveys for consumers only
| Groupa | Population class | Mean exposureb | P95 exposurec | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Min | Median | Max | N | Min | Median | Max | ||
| ‘High‐morphine’ | Infants | 2 | 2.30 | n.a. | 5.85 | 0 | n.a. | n.a. | n.a. |
| Toddlers | 2 | 3.96 | n.a. | 8.54 | 1 | 44.9 | n.a. | 44.9 | |
| Other children | 9 | 0.59 | 6.31 | 15.4 | 2 | 2.80 | n.a. | 38.6 | |
| Adolescents | 8 | 0.36 | 4.19 | 8.65 | 0 | n.a. | n.a. | n.a. | |
| Adults | 11 | 0.76 | 4.34 | 8.31 | 4 | 11.2 | 35.0 | 44.4 | |
| Elderly | 8 | 0.32 | 3.87 | 6.37 | 0 | n.a. | n.a. | n.a. | |
| Very elderly | 4 | 1.39 | 4.43 | 6.27 | 0 | n.a. | n.a. | n.a. | |
| ‘Low‐morphine’ | Infants | 2 | 0.29 | n.a. | 0.74 | 0 | n.a. | n.a. | n.a. |
| Toddlers | 2 | 0.50 | n.a. | 1.08 | 1 | 8.03 | n.a. | 8.03 | |
| Other children | 9 | 0.07 | 0.80 | 1.95 | 2 | 0.50 | n.a. | 6.90 | |
| Adolescents | 8 | 0.05 | 0.53 | 1.10 | 0 | n.a. | n.a. | n.a. | |
| Adults | 11 | 0.10 | 0.55 | 1.05 | 4 | 2.00 | 6.25 | 7.93 | |
| Elderly | 8 | 0.04 | 0.49 | 0.81 | 0 | n.a. | n.a. | n.a. | |
| Very elderly | 4 | 0.18 | 0.56 | 0.80 | 0 | n.a. | n.a. | n.a. | |
bw: body weight; max: maximum; min: minimum; N: number of surveys; n.a. not applicable; P95: 95th percentile
Group of poppy seed samples. The ‘high‐morphine’ group refers to poppy seed samples originating from Australia, China, France, Spain and the UK which are assumed to represent primarily varieties grown for pharmaceutical use. The ‘low‐morphine’ group refers to poppy seed samples originating from other countries which are assumed to represent varieties grown for food use. See Section 3.2.1.4 for further details.
Mean exposure calculated from the mean consumption of poppy seeds and the mean occurrence of codeine in poppy seed samples.
P95 exposure calculated from the P95 consumption of poppy seeds and the P95 occurrence of morphine in poppy seed samples. The P95 exposure estimates for surveys with less than 60 observations may not be statistically robust and are therefore not calculated.
Table 15 summarises the acute dietary exposure estimates for morphine and codeine, expressed as morphine equivalents. Detailed summary statistics on the exposure estimates calculated for each dietary survey are presented in Annex B, Table B.6. Overall, similar results as for morphine alone were obtained and the data show that codeine makes a minor contribution to the morphine equivalents.
Table 15.
Summary statistics of acute dietary exposure to morphine and codeine, expressed as morphine equivalents (μg/kg bw per day) estimated at the middle bound across dietary surveys for consumers only
| Groupa | Population class | Mean exposureb | P95 exposurec | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Min | Median | Max | N | Min | Median | Max | ||
| ‘High‐morphine’ | Infants | 2 | 15.4 | n.a. | 39.2 | 0 | n.a. | n.a. | n.a. |
| Toddlers | 2 | 26.5 | n.a. | 57.2 | 1 | 336 | n.a. | 336 | |
| Other children | 9 | 3.94 | 42.3 | 103 | 2 | 21.0 | n.a. | 289 | |
| Adolescents | 8 | 5.08 | 29.1 | 55.7 | 0 | n.a. | n.a. | n.a. | |
| Adults | 11 | 2.38 | 28.1 | 57.9 | 4 | 83.8 | 262 | 332 | |
| Elderly | 8 | 2.14 | 25.9 | 42.7 | 0 | n.a. | n.a. | n.a. | |
| Very elderly | 4 | 9.29 | 29.7 | 42.0 | 0 | n.a. | n.a. | n.a. | |
| ‘Low‐morphine’ | Infants | 2 | 1.71 | n.a. | 4.36 | 0 | n.a. | n.a. | n.a. |
| Toddlers | 2 | 2.95 | n.a. | 6.36 | 1 | 47.7 | n.a. | 47.7 | |
| Other children | 9 | 0.44 | 4.70 | 11.4 | 2 | 2.98 | n.a. | 41.0 | |
| Adolescents | 8 | 0.56 | 3.24 | 6.19 | 0 | n.a. | n.a. | n.a. | |
| Adults | 11 | 0.27 | 3.13 | 6.44 | 4 | 11.9 | 37.2 | 47.2 | |
| Elderly | 8 | 0.24 | 2.88 | 4.75 | 0 | n.a. | n.a. | n.a. | |
| Very elderly | 4 | 1.03 | 3.30 | 4.67 | 0 | n.a. | n.a. | n.a. | |
bw: body weight; Max: maximum; Min: minimum; N: number of surveys; n.a. not applicable; P95: 95th percentile
Group of poppy seed samples. The ‘high‐morphine’ group refers to poppy seed samples originating from Australia, China, France, Spain and the UK which are assumed to represent primarily varieties grown for pharmaceutical use. The ‘low‐morphine’ group refers to poppy seed samples originating from other countries which are assumed to represent varieties grown for food use. See Section 3.2.1.4 for further details.
Mean exposure calculated from the mean consumption of poppy seeds and the mean occurrence of morphine and codeine, expressed as morphine equivalents, in poppy seed samples.
P95 exposure calculated from the P95 consumption of poppy seeds and the P95 occurrence of morphine and codeine, expressed as morphine equivalents, in poppy seed samples. The P95 exposure estimates for surveys with less than 60 observations may not be statistically robust and are therefore not calculated.
Acute dietary exposure to thebaine (Table 16) is lower compared to the sum of morphine and codeine, expressed as morphine equivalents, in the ‘low‐morphine’ group and slightly lower in the ‘high‐morphine’ group. Compared to the exposure to codeine, the acute dietary exposure to thebaine is higher in both groups. The mean dietary exposure to thebaine calculated for the ‘high‐morphine’ group ranged from 1.3 to 62.5 μg/kg bw per day across dietary surveys and age groups and was the highest for other children. For the ‘low‐morphine’ group, the acute mean dietary exposure ranged from 0.06 to 2.65 μg/kg bw per day across dietary surveys and age groups. The P95 acute dietary exposure was only calculated for 7 dietary surveys, with exposures ranging from 18.0 to 288 μg/kg bw per day for the ‘high‐morphine’ group and from 0.66 to 10.6 μg/kg bw per day for the ‘low‐morphine’ group. Detailed summary statistics on the exposure estimates calculated for each dietary survey are presented in Annex B, Table B.7.
Table 16.
Summary statistics of acute dietary exposure to thebaine (μg/kg bw per day) estimated at the middle bound across dietary surveys for consumers only
| Groupa | Population class | Mean exposureb | P95 exposurec | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Min | Median | Max | N | Min | Median | Max | ||
| ‘High‐morphine’ | Infants | 2 | 9.34 | n.a. | 23.8 | 0 | n.a. | n.a. | n.a. |
| Toddlers | 2 | 16.1 | n.a. | 34.7 | 1 | 288 | n.a. | 288 | |
| Other children | 9 | 2.39 | 25.7 | 62.5 | 2 | 18.0 | n.a. | 248 | |
| Adolescents | 8 | 1.45 | 17.1 | 35.2 | 0 | n.a. | n.a. | n.a. | |
| Adults | 11 | 3.08 | 17.7 | 33.8 | 4 | 71.9 | 225 | 285 | |
| Elderly | 8 | 1.30 | 15.7 | 25.9 | 0 | n.a. | n.a. | n.a. | |
| Very elderly | 4 | 5.64 | 18.0 | 25.5 | 0 | n.a. | n.a. | n.a. | |
| ‘Low‐morphine’ | Infants | 2 | 0.40 | n.a. | 1.01 | 0 | n.a. | n.a. | n.a. |
| Toddlers | 2 | 0.68 | n.a. | 1.47 | 1 | 10.6 | n.a. | 10.6 | |
| Other children | 9 | 0.10 | 1.09 | 2.65 | 2 | 0.66 | n.a. | 9.09 | |
| Adolescents | 8 | 0.06 | 0.72 | 1.49 | 0 | n.a. | n.a. | n.a. | |
| Adults | 11 | 0.13 | 0.75 | 1.43 | 4 | 2.63 | 8.24 | 10.5 | |
| Elderly | 8 | 0.06 | 0.67 | 1.10 | 0 | n.a. | n.a. | n.a. | |
| Very elderly | 4 | 0.24 | 0.76 | 1.08 | 0 | n.a. | n.a. | n.a. | |
bw: body weight; max: maximum; min: minimum; N: number of surveys; n.a. not applicable; P95: 95th percentile.
Group of poppy seed samples. The ‘high‐morphine’ group refers to poppy seed samples originating from Australia, China, France, Spain and the UK which are assumed to represent primarily varieties grown for pharmaceutical use. The ‘low‐morphine’ group refers to poppy seed samples originating from other countries which are assumed to represent varieties grown for food use. See Section 3.2.1.4 for further details.
Mean exposure calculated from the mean consumption of poppy seeds and the mean occurrence of thebaine in poppy seed samples.
P95 exposure calculated from the P95 consumption of poppy seeds and the P95 occurrence of thebaine in poppy seed samples. The P95 exposure estimates for surveys with less than 60 observations may not be statistically robust and are therefore not calculated.
For oripavine, no calculation of the exposure assessment was performed because of the insufficient amount of occurrence data.
In the case of noscapine and papaverine, dietary exposure was not calculated for the ‘high‐’ and ‘low‐morphine’ group separately, because of the small amount of samples per subgroup. Due to the higher percentage of left‐censored data, acute dietary exposure is presented in Tables 17 and 18 for both the LB and UB. Detailed summary statistics on the exposure estimates calculated for each dietary survey are presented in Annex B, Table B.8 and B.9. For noscapine, the mean dietary exposure ranged from 0.02 to 1.45 μg/kg bw per day (min LB–max UB) across dietary surveys and age groups and was the highest for other children. The P95 acute dietary exposure ranged from 0.27 to 4.40 μg/kg bw per day (min LB–max UB) across dietary surveys and age groups. Among the opium alkaloids considered in this Scientific Opinion, acute dietary exposure was the lowest for papaverine. The mean dietary exposure to papaverine ranged from 0.002 to 0.27 μg/kg bw per day (min LB–max UB) across dietary surveys and age groups and was the highest for other children. The P95 acute dietary exposure ranged from 0.06 to 0.99 μg/kg bw per day (min LB–max UB) across dietary surveys and age groups.
Table 17.
Summary statistics of acute dietary exposure to noscapine (μg/kg bw per day) estimated at the lower and upper bound across dietary surveys for consumers only
| Age group | Mean exposurea | P95 exposureb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Min | Median | Max | N | Min | Median | Max | ||
| Infants | LB | 2 | 0.161 | n.a. | 0.410 | 0 | n.a. | n.a. | n.a. |
| UB | 2 | 0.217 | n.a. | 0.552 | 0 | n.a. | n.a. | n.a. | |
| Toddlers | LB | 2 | 0.278 | n.a. | 0.599 | 1 | 4.40 | n.a. | 4.40 |
| UB | 2 | 0.374 | n.a. | 0.806 | 1 | 4.40 | n.a. | 4.40 | |
| Other children | LB | 9 | 0.041 | 0.442 | 1.076 | 2 | 0.27 | n.a. | 3.78 |
| UB | 9 | 0.056 | 0.596 | 1.449 | 2 | 0.27 | n.a. | 3.78 | |
| Adolescents | LB | 8 | 0.025 | 0.294 | 0.606 | 0 | n.a. | n.a. | n.a. |
| UB | 8 | 0.034 | 0.396 | 0.817 | 0 | n.a. | n.a. | n.a. | |
| Adults | LB | 11 | 0.053 | 0.304 | 0.582 | 4 | 1.10 | 3.43 | 4.35 |
| UB | 11 | 0.072 | 0.410 | 0.784 | 4 | 1.10 | 3.43 | 4.35 | |
| Elderly | LB | 8 | 0.022 | 0.271 | 0.447 | 0 | n.a. | n.a. | n.a. |
| UB | 8 | 0.030 | 0.365 | 0.602 | 0 | n.a. | n.a. | n.a. | |
| Very elderly | LB | 4 | 0.097 | 0.310 | 0.440 | 0 | n.a. | n.a. | n.a. |
| UB | 4 | 0.131 | 0.418 | 0.592 | 0 | n.a. | n.a. | n.a. | |
bw: body weight; max: maximum; min: minimum; N: number of surveys; n.a. not applicable; P95: 95th percentile.
Mean exposure calculated from the mean consumption of poppy seeds and the mean occurrence of noscapine in poppy seed samples.
P95 exposure calculated from the P95 consumption of poppy seeds and the P95 occurrence of noscapine in poppy seed samples. The P95 exposure estimates for surveys with less than 60 observations may not be statistically robust and are therefore not calculated.
Table 18.
Summary statistics of acute dietary exposure to papaverine (μg/kg bw per day) estimated at the lower and upper bound across dietary surveys for consumers only
| Age group | Mean exposurea | P95 exposureb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Min | Median | Max | N | Min | Median | Max | ||
| Infants | LB | 2 | 0.014 | n.a. | 0.035 | 0 | n.a. | n.a. | n.a. |
| UB | 2 | 0.041 | n.a. | 0.104 | 0 | n.a. | n.a. | n.a. | |
| Toddlers | LB | 2 | 0.024 | n.a. | 0.051 | 1 | 0.99 | n.a. | 0.99 |
| UB | 2 | 0.070 | n.a. | 0.151 | 1 | 0.99 | n.a. | 0.99 | |
| Other children | LB | 9 | 0.004 | 0.038 | 0.092 | 2 | 0.06 | n.a. | 0.85 |
| UB | 9 | 0.010 | 0.112 | 0.272 | 2 | 0.06 | n.a. | 0.85 | |
| Adolescents | LB | 8 | 0.002 | 0.025 | 0.052 | 0 | n.a. | n.a. | n.a. |
| UB | 8 | 0.006 | 0.074 | 0.153 | 0 | n.a. | n.a. | n.a. | |
| Adults | LB | 11 | 0.005 | 0.026 | 0.050 | 4 | 0.25 | 0.77 | 0.98 |
| UB | 11 | 0.013 | 0.077 | 0.147 | 4 | 0.25 | 0.77 | 0.98 | |
| Elderly | LB | 8 | 0.002 | 0.023 | 0.038 | 0 | n.a. | n.a. | n.a. |
| UB | 8 | 0.006 | 0.069 | 0.113 | 0 | n.a. | n.a. | n.a. | |
| Very elderly | LB | 4 | 0.008 | 0.027 | 0.038 | 0 | n.a. | n.a. | n.a. |
| UB | 4 | 0.025 | 0.078 | 0.111 | 0 | n.a. | n.a. | n.a. | |
bw: body weight; max: maximum; min: minimum; N: number of surveys; n.a. not applicable; P95: 95th percentile.
Mean exposure calculated from the mean consumption of poppy seeds and the mean occurrence of papaverine in poppy seed samples.
P95 exposure calculated from the P95 consumption of poppy seeds and the P95 occurrence of noscapine in poppy seed samples. The P95 exposure estimates for surveys with less than 60 observations may not be statistically robust and are therefore not calculated.
3.3.2. Previously reported dietary exposure
In the previous opinion, a dietary exposure assessment was performed based on the available occurrence data that had been submitted to EFSA up to early 2011 and the data available on poppy seed consumption in the Comprehensive European Food Consumption Database (EFSA CONTAM Panel, 2011). Only acute dietary exposure was considered relevant and, based on the relative prevalence of the alkaloids present in the poppy seed and food samples combined with the pharmacological potency of the opium alkaloids, it was concluded that the exposure assessment could be focused on morphine alone. It was also considered that the consumption of food products containing substantial amounts of poppy seeds is mainly restricted to countries in Central and Eastern Europe. In the other European countries, poppy seeds are mostly used as decoration or condiment.
Poppy seed consumption data were available from only five different EU Member States (Austria, the Czech Republic, Germany, Hungary and Slovakia). However, of these, only three countries (the Czech Republic, Hungary and Slovakia) had more than 10 consumption days per survey. Based on these limited data the average reported poppy seed consumption for these three countries was used to estimate the dietary exposure to morphine, using lower and upper bound concentrations. For adults, the exposure ranged from 3.1 to 17.4 μg/kg bw per day using the mean morphine concentration in poppy seeds and from 16.4 to 90.9 μg/kg bw per day taking the P95 concentration in poppy seeds. For children in the age range 3–10 years the exposure was lower: 9.2 and 48.3 μg/kg bw per day for the mean and P95 concentration, respectively.
The CONTAM Panel concluded that the lack of available consumption data could lead to a possible underestimation of the true poppy seed intake. To compensate for this, scenarios were developed, using recipes for the preparation of poppy seed containing food products (bread, pastries, biscuits, main dishes, desserts, poppy seed fillings), to estimate the acute exposure for a hypothetical single portion of foods with high poppy seed content. The exposure (UB) to morphine ranged from 37.8 (mean morphine concentration) to 200 μg/kg bw per day for adults (P95 level). For children of the age group of 3–10 years, the exposure ranged from 47.8 to 252 μg/kg bw per day, respectively.
As a worse‐case approach, occurrence data of morphine in poppy seed samples and consumption of bread or fine bakery ware with estimated high or low level poppy seed contents were used, assuming that all bread or fine bakery ware that were consumed on one day contained poppy seeds. Estimated exposure from bread was lower than from fine bakery ware. For adult consumers of foods with high poppy seed content, the estimated dietary exposure to morphine via fine bakery ware ranged from 2.48 to 375 μg morphine/kg bw per day. The estimated dietary exposure was the highest for children of age 3–10 years at 10.2–753 μg morphine/kg bw per day. For adult consumers of low poppy seed content foods, the estimated dietary exposure to morphine via fine bakery ware ranged from 0.05 to 16.9 μg morphine/kg bw per day. The estimated dietary exposure for children age 3–10 years, ranged from 0.16 to 33.6 μg morphine/kg bw per day.
A final exposure assessment was made in which occurrence data was used that had been submitted by Germany on morphine levels measured in fine bakery ware. Using LB and UB concentrations for P95 morphine levels in the occurrence data, the exposure for adults ranged from 0.50 to 14.6 μg/kg bw per day. The exposure was the highest for children in the age of 3–10 years, ranging from 1.88 to 30.7 μg/kg bw per day.
Except for the final approach in which the German occurrence data in fine bakery was used, the effects of processing had not been taken into account in the exposure assessments. The CONTAM Panel noted that food processing steps (washing, grinding, baking) may reduce the alkaloid content in raw poppy seeds by up to 90% in the final product. However, due to the lack of consumption data on poppy seeds and the products as consumed, processing was not taken into account.
From the different exposure scenarios. it could be concluded that children of age 3–10 are the most exposed age group on a body weight basis.
Zentai et al. (2012) conducted a dietary exposure assessment on morphine, focussing on Hungarian consumers. Occurrence data for the period 2001–2010 on morphine, codeine, thebaine and noscapine in poppy seeds sold on the Hungarian market were used and consumption data were available from two 3‐day surveys conducted in Hungary in 2003 and 2009, respectively. The 2003 survey contained consumption data for adults only, while the 2009 survey included also children and adolescents. The overall percentage of poppy seed consumption days was 1.94% in 2003 and 2.18% in 2009. Consumption of poppy seeds was somewhat more frequent in the age group below 18 (2.81%). The 2009 survey provided information on the type of product that was consumed, i.e. 65% of poppy seeds was consumed as ground and 35% was consumed as baked and ground in the form of baked cakes. The effect of processing could therefore be taken into account, including grinding and baking steps while washing was considered not relevant for the Hungarian situation. Processing factors were defined as 0.71 for grinding, 0.31 for baking and 0.22 for the combination of grinding and baking.
Based on the infrequent use of poppy seeds, only the acute exposure was considered relevant. Point estimates for acute exposure were made, taking the 97.5 percentile of daily poppy seed consumption in combination with the 97.5 percentile of the morphine content. Based on the available occurrence data the following P97.5 concentrations in poppy seeds were calculated: 84.8 mg/kg morphine; 25.7 mg/kg codeine; 23.9 mg/kg thebaine and 4.7 mg/kg noscapine. Based on the 2009 survey, the P97.5 for poppy seed consumption (on consumption days) was estimated as 1.26 g/kg bw per day, while the average consumption was 0.44 g/kg bw per day. Ground poppy seeds were consumed about twice as often as baked poppy seeds, resulting in an average consumption of 0.52 g ground poppy seeds/kg bw per day and 0.30 g baked poppy seeds/kg bw per day. The point estimates for the acute exposure at the P97.5, based on raw poppy seeds, for adults and children were 116.7 and 164.7 μg/kg bw per day, respectively. Taking processing into account, the P97.5 values were 78.6 and 116.9 μg/kg bw per day, a decrease of approximately 30%.
The authors also used different probabilistic methods to estimate morphine intake and to model the effect of processing. This resulted in significantly lower estimated values at P97.5: for unprocessed poppy seeds this ranged between 25.6 and 40.9 μg/kg bw per day for adults (6 different options calculated) and between 59.1 and 63.3 μg/kg bw per day for children (2 different options calculated). When the effects of processing were included, the exposure at P97.5 was calculated as 18.3–25.4 μg/kg bw per day for adults (2 options calculated) and 32.9 μg/kg bw per day for children (1 option calculated).
No point estimates or probabilistic calculations were performed for the other opium alkaloids present in the poppy seeds. Overall, the dietary exposure to morphine from poppy seed‐containing foods reported in the open literature is in‐line with the acute dietary exposure estimated in this Scientific Opinion (see Section 3.3.1).
3.3.3. Non‐dietary sources of exposure
Besides the exposure from poppy seed consumption as a food, consumers can be exposed to opium alkaloids from other sources.
As described in Section 3.1.3 (Observations in humans), some opium alkaloids and their derivatives are used as licensed medicines and this can lead to additional exposure.
Morphine has been reported to occur in wastewater, surface water and ground water. Concentrations ranging from 12.3 to 1,346 ng/L have been reported in influents from wastewater treatment plants and concentrations ranging from < 3.95 (LOQ) to 929 ng/L in effluents as reviewed by Pal et al. (2013). In surface water, concentrations ranging from 1.74 to 148 ng/L have been reported and in ground water a concentration of 1.4 ng/L has been reported (Pal et al., 2013; Mendoza et al., 2014). Morphine was not detected in tap water (Mendoza et al., 2014) but it was detected, as well as codeine, in both treated and untreated drinking water (Rodayan et al., 2016). These data indicate a potential but low additional exposure to opium alkaloids via drinking water.
As described by the CONTAM Panel in 2011, poppy seeds are used as an ingredient in warm beverages such as milk to calm infants and to prepare tea which is used as an agent of abuse or to treat opioid dependency. These uses are not considered as food use in this Scientific Opinion.
Also, traditional Chinese medicine herbal preparations, which are used as an antitussive in China, have been reported to contain morphine, codeine, thebaine, noscapine and papaverine. These preparations often contain Pericarpium papaveris (Yin Su Qiao) as one of the ingredients. Liu et al. (2016b) analysed a total of 40 herbal products and the ranges found were: 5.0–1,311 mg/kg morphine, 0.6–14.4 mg/kg codeine, 1.8–113 mg/kg thebaine, 0.7–473 mg/kg noscapine and 1.6–317 mg/kg papaverine.
Pericarpium papaveris is also illicitly used as an additive in hot pot, a popular dish served in Chinese restaurants in China (Guo et al., 2013). A survey was conducted including 29 samples of hot pot broth from local restaurants and supermarkets. Samples were analysed for morphine, codeine, thebaine, noscapine and papaverine with LC–MS/MS (LOQs ranged from 0.01 to 0.1 μg/kg). The survey yielded 3 positive samples, two samples containing 22.5 and 28.9 μg/kg morphine, respectively, while a third sample contained traces of noscapine and papaverine.
3.4. Risk characterisation
The mean and high (P95) acute dietary exposure to morphine alone and to morphine and codeine, expressed as morphine equivalents, calculated from ‘low‐morphine’ poppy seed samples (primarily varieties for food use) and ‘high‐morphine’ poppy seed samples (i.e. those grown primarily for pharmaceutical use), were compared with the ARfD of 10 μg/kg bw (Annex B, Tables B.4 and B.6). It should be noted that the exposure estimates presented in this Scientific Opinion refer to consumers of poppy seeds.
The estimates of high (P95) acute dietary exposure to morphine alone from poppy seed considered to have high levels of opium alkaloids (i.e. those from varieties grown primarily for pharmaceutical use of which the seeds are used as a by‐product for food purposes) demonstrated that the group ARfD of 10 μg morphine equivalents/kg bw was exceeded by all age groups for which data were available. At the highest estimated exposure, which is seen in the toddler age group based on one survey only, the group ARfD is exceeded by up to 33‐fold. The estimates of mean dietary exposure for this type of poppy seeds also exceed the group ARfD in most surveys in all age groups. Based on the mean morphine occurrence in these poppy seed samples, consumers in 34 out of 44 population groups are estimated to be exposed for at least 50% of the consumption days to a level of morphine higher than the group ARfD of 10 μg/kg bw. For poppy seeds, considered to have relatively lower concentrations of opium alkaloids (primarily varieties for food use), exceedance of the group ARfD is also seen at high levels of dietary exposure in most surveys, but to a lesser extent (up to 4‐ to 5‐fold). Based on the mean occurrence of morphine in these poppy seed samples, the estimated exposure to morphine exceeded the group ARfD on less than 50% of the consumption days in all but one population group.
Comparison of the dietary exposure to morphine alone with the combined exposure to morphine and codeine, expressed as morphine equivalents, shows that codeine makes a minor contribution to the sum. Exposures and exceedances of the ARfD are marginally increased by the inclusion of codeine into the sum (Table B.6 of Annex B).
However, when considering individual poppy seed samples containing high concentrations of codeine, their consumption may result in a codeine exposure, expressed in morphine equivalents, that exceeds the group ARfD. Based on the highest P95 consumption of poppy seeds of 55 g/day (see Annex B, Table B.3) and assuming a default body weight of 70 kg for an adult, the group ARfD might be exceeded when consuming poppy seeds contaminated with codeine at a concentration higher than 65 mg/kg.
It should be noted that exposure estimates are based on reported concentrations of alkaloids in poppy seed samples due to the lack of occurrence data on food products containing poppy seeds. However, food processing steps (i.e. washing, soaking, heat treatment, grinding) may reduce the alkaloid content in raw poppy seeds by 25–100% in the final product. Therefore, the group ARfD is most likely to be exceeded when large portions are consumed or if foods containing unprocessed poppy seeds are consumed.
The CONTAM Panel reconfirms that, although there are few reports of adverse reactions arising from traditional consumption of poppy seeds in foods, in the absence of formal reporting systems, it cannot be assumed that such reactions do not occur from time to time. Pregnant women, infants, people above 75 years of age and those suffering from health conditions with impaired respiratory function are subgroups that are more sensitive to adverse effects of morphine.
In principle, oripavine could contribute to morphine‐like effects, but the available toxicological and occurrence data were insufficient for risk characterisation.
For thebaine, the estimated dietary exposures are slightly lower than those for morphine. LD50 values in rodents indicate that thebaine is more acutely toxic than morphine. In view of this evidence, the dietary exposures could be a health risk. However, hazard characterisation is not possible due to the lack of data from which to derive a HBGV and consequently no risk characterisation could be carried out.
The estimated dietary exposures to noscapine and papaverine are lower than for morphine and based on very limited data it appears that they are less toxic than the other alkaloids discussed in this opinion. However, the available data are insufficient to propose HBGVs. Instead, the CONTAM Panel compared estimated dietary exposure to noscapine and papaverine with the limited information regarding the recommended therapeutic doses in humans. Therefore, risk characterisation for these alkaloids is incomplete. For noscapine, dietary exposure is at least 80‐fold lower than the recommended therapeutical dose, which does not suggest a health concern. For papaverine, the dietary exposure is about 2,000‐fold lower than the recommended therapeutical dose and it is highly unlikely that this would be a health concern.
3.5. Uncertainty analysis
The evaluation of the inherent uncertainties in the assessment of exposure to opium alkaloids in poppy seed samples has been performed following the guidance of the Opinion of the Scientific Committee related to Uncertainties in Dietary Exposure Assessment (EFSA, 2006). In addition, the report on ‘Characterizing and Communicating Uncertainty in Exposure Assessment’ has been considered (WHO/IPCS, 2008). According to the guidance provided by the EFSA opinion (2006) the following sources of uncertainties have been considered: assessment objectives, exposure scenario, exposure model, and model input (parameters).
3.5.1. Assessment objectives
The objectives of the assessment were clearly specified in the terms of reference.
3.5.2. Exposure scenario/exposure model
The final data set on opium alkaloids was composed of 7,669 data points (of which 6,369 on poppy seeds). With respect to the occurrence data used in the 2011 EFSA opinion, the number of reporting countries and most importantly the country of origin of the poppy seeds increased. However, a source of uncertainty is the representativity of the data set with respect to the countries of sampling and/or origin of the samples (the largest amount of data were reported by Germany, Hungary and the Czech Republic).
The alkaloid profile strongly differs between P. somniferum cultivars. The CONTAM Panel noted a large variation in the alkaloid profile of the available poppy seed samples and observed a clear trend of association between the country of origin of the poppy seeds and high median morphine concentrations. Therefore, the occurrence data set was divided in three groups based on the country of origin: the poppy seed samples assumed to represent primarily varieties grown for the pharmaceutical sector (‘high‐morphine’ group; samples originating from Australia, China, France, Spain and the UK), poppy seed samples assumed to represent primarily varieties grown for the food sector (‘low‐morphine’ group; samples originating from other countries) and poppy seed samples with unknown origin. However, it should be noted that countries may produce varieties for both food and pharmaceutical use. Consequently, high morphine concentrations were observed in the ‘low‐morphine’ group as well. This may result in an overestimation of P95 dietary exposure for the ‘low‐morphine’ scenario. In addition, the application of cleaning practices after harvest might reduce the level of morphine in the poppy seeds. Furthermore, seeds of different poppy varieties (data providers did not report information on poppy varieties) may be mixed before entering the food market. These factors introduce some uncertainty in the applied division.
Poppy seed samples from unknown origin were not taken into account in the exposure assessment for morphine, codeine, morphine equivalents and thebaine. However, the concentrations reported for the unknown group were similar to the concentrations reported for the ‘low‐’ and ‘high‐morphine’ group and therefore the uncertainty for excluding these data from the exposure assessment is limited.
A further source of uncertainty is related to the assumptions regarding the percentage of poppy seeds in different types of poppy seed‐containing foods. It can be assumed that the presence of poppy seeds in foods was not always captured in the dietary surveys, particularly when small amount of poppy seeds are present in the food. This leads to an underestimation of the frequency of poppy seed consumption and a bias in the consumption statistics for consumers of poppy seeds since particularly low consumption events may be underreported. In addition, the number of consumers of poppy seeds is likely underestimated due to the limited number of reporting days per subject (from 1 to 7 per subject).
Most of the available data in this opinion related to unprocessed poppy seeds and no effect of food processing was taken into account in the assessment of acute dietary exposure reported in this opinion. However, it is expected that the alkaloid content of the poppy seeds is reduced during the pre‐treatment and processing (i.e. washing, soaking, heat treatment, grinding) and reductions of 25–100% have been reported (see Section 3.2.3). A comparison of calculated and reported morphine concentrations in bread, indicate that the uncertainty due to the use of analytical results in poppy seed samples instead of bread may be limited. However, this verification was only done for one food and on a limited data set and therefore there is uncertainty regarding the effect of food processing.
For the calculation of the morphine equivalents, the median percentage of codeine to morphine was imputed for 454 samples for which the codeine concentration was not reported. With few exceptions, the variability of the percentage of codeine to morphine was limited and, considering the low contribution of codeine to the morphine equivalent, this source of uncertainty can be considered as minor.
In relation to the dietary exposure assessment, uncertainties and limitations related to the use of EFSA's Comprehensive Food Consumption Database are described in EFSA's guidance (EFSA, 2011a) and are not further detailed in this opinion. Out of the 41 dietary surveys available for this assessment, consumption of poppy seeds and related foods was reported in only 18 of them. Consumption data were available for Austria, Bulgaria, the Czech Republic, Denmark, Germany, Hungary, Ireland, Poland, Romania, Slovakia, Slovenia, Sweden and the United Kingdom. Moreover, not all age groups were covered for each country. For instance for Slovakia and Slovenia, data on the consumption of poppy seeds were available for adults only. Finally, poppy seed‐containing foods are partially seasonal foods eaten during Christmas or Easter time, and these periods are not always covered by the dietary national surveys. This can represent a source of underestimation of the dietary exposure.
3.5.3. Model input (parameters)
Currently, no official standard methods are available for the analysis of opium alkaloids in foods. Analytical results used for the exposure assessment were therefore obtained using different analytical methods and varying LOQ/LODs. Although for papaverine and noscapine, a large proportion of left‐censored results was found, the difference between LB and UB exposure estimates was limited. For morphine, codeine and thebaine, the proportion of left‐censored results was small and consequently the difference between LB and UB exposure estimates was negligible. This indicates that the uncertainty due to left‐censored data is limited.
3.5.4. Other uncertainties
The uncertainties described in the 2011 opinion remain valid: ‘The ARfD is derived from the lowest known single oral therapeutic dose of morphine. A range of adverse reactions is reported to occur during therapeutic use of morphine, but the extent to which these occur at the lowest known single oral therapeutic dose or even below is not clear. Furthermore, the medical literature refers to contraindications in which morphine treatment should be avoided. There is also uncertainty if the low doses that are efficient in the therapy of dyspnoea via the reduction of ventilatory drive may impose additional risks on persons with different forms of sleep apnoea or sleep disordered breathing’.
Additive effects or interactions with accompanying alkaloids in poppy seeds and/or with concomitant intake of alcohol, opioid drugs or central depressant drugs is not taken into account as is the case for decreased elimination of morphine, codeine or their metabolites, e.g. in consequence of hepatic or renal impairment.
The toxicological properties of noscapine and papaverine are less characterised than for morphine and codeine. However, from the therapeutic uses, information for dose–adverse effect relationships in humans are available for noscapine and papaverine. These adverse effects (e.g. dizziness, headache) are considered less severe compared to those known from the pharmaceutical uses of morphine.
The available data did not allow taking into account the toxicity of thebaine and oripavine, which may be present in poppy seed samples. This could be especially relevant for poppy seeds derived from poppy varieties bred for pharmaceutical uses with specific alkaloid profiles (e.g. high concentrations of thebaine, oripavine).
3.5.5. Summary of uncertainties
In Table 19, a summary of the uncertainty evaluation is presented, highlighting the main sources of uncertainty and indicating an estimate of whether the respective source of uncertainty might have led to an over‐ or underestimation of the exposure or the resulting risk.
Table 19.
Summary of qualitative evaluation of the impact of uncertainties on the risk assessment of opium alkaloids in poppy seeds
| Sources of uncertainty | Directiona |
|---|---|
| Extrapolation of occurrence data to the whole of Europe | +/− |
| Effect of food processing not taken into account in the exposure assessment | + |
| Overestimation of P95 dietary exposure for the ‘low‐morphine’ scenario due to the presence of poppy seed samples from varieties grown for pharmaceutical use | + |
| Underestimation of dietary exposure for the ‘high‐morphine’ scenario due to the possible presence of poppy seed samples from varieties grown for use as food | − |
| Imputation of codeine content in a number of samples for the calculation of morphine equivalents | +/− |
| Use of mean disaggregation factors to convert eating occasions of poppy seed‐containing foods into amounts of consumed poppy seeds | +/− |
| Consumption of foods containing small amounts of poppy seeds not always captured in dietary surveys (e.g. bread roll) | +/− |
| Dietary surveys not capturing possible high consumption of poppy seeds during festivities (e.g. Easter) | − |
| Uncertainty in the LOAEL for establishing the ARfD | − |
| Possible interactions of morphine and codeine with other opium alkaloids contaminating poppy seeds | +/− |
| Additive adverse effects of morphine and codeine with concomitant intake of alcohol, or central depressant drugs are not taken into account | − |
| Intrinsic toxicity of thebaine could not be taken into account in the risk assessment | − |
LOAEL: lowest‐observed‐adverse‐effect level.
+ = uncertainty with potential to cause over‐estimation of exposure/risk; − = uncertainty with potential to cause under‐estimation of exposure/risk.
The CONTAM Panel considered that the impact of the uncertainties on the risk assessment of exposure to opium alkaloids in poppy seed samples is considerable. The exposure is more likely to be overestimated than underestimated. The risk associated with dietary exposure to morphine and codeine is considered to be overestimated. However, risks linked to exposure to thebaine and oripavine could not be characterised.
4. Conclusions
Poppy seeds are obtained from the opium poppy (P. somniferum L.). The latex (milky sap) of the opium poppy contains up to 80 different alkaloids, including morphine and codeine, which have been used by man for the treatment of severe pain for generations but are also subject to misuse. The seeds are used as food and to produce edible oil.
Alkaloid accumulation in the poppy plant depends on both genetic factors and environmental/cultivation conditions. Breeding is focusing on the development of either cultivars with a high and specific alkaloid content to fulfil the requirements of the pharmaceutical industry or cultivars with a low alkaloid content for seed/oil production. However, poppy seeds from P. somniferum varieties with high alkaloid content especially bred for pharmaceutical purposes are used as a by‐product for food purposes. Mature poppy seeds do not contain the latex, but can become contaminated with opium alkaloids as a result of pest damage and during harvest.
Opium alkaloids detected in samples of poppy seeds and poppy seed‐containing foods include the phenanthrenes: principally morphine, codeine, thebaine and oripavine, and the benzylisoquinolines: principally papaverine and noscapine. In this Scientific Opinion, the term ‘opium alkaloids’ refers to these compounds.
4.1. Hazard identification and characterisation
4.1.1. Toxicokinetics
Morphine
Morphine is extensively absorbed from the GI tract and is distributed throughout the body. The oral bioavailability of morphine is reduced by both Phase I and II presystemic metabolism in the GI tract and liver.
Morphine diffuses across the placenta, and transfers into milk.
Morphine is metabolised via N‐demethylation and O‐glucuronidation in the gut and the liver. Metabolites are normorphine, M3G and M6G.
The rate and pathways of opioid metabolism and distribution of morphine may be influenced by genetic factors (active transport proteins, including the P‐glycoprotein) and medical conditions (liver and kidney disease), which can account for part of the observed inter‐individual variation in morphine effects.
Codeine
Codeine is readily and extensively absorbed from the GI tract following oral administration and is distributed throughout the body.
Codeine penetrates the placental barrier and enters fetal circulation. It is also excreted in breast milk.
Codeine is metabolised via O‐demethylation, N‐demethylation and O‐glucuronidation in the liver and gut. Main metabolites are morphine, norcodeine and C6G. The formed morphine is further metabolised into normorphine, M3G and M6G.
Codeine metabolism to morphine is dependent of the CYP2D6 activity. Individuals can be classified into poor metaboliser, intermediate metaboliser, extensive metaboliser or ultra‐rapid metaboliser. The extensive metaboliser represents the majority of the Caucasian population. The phenotypes generated by different CYP2D6 alleles affects the sensitivity of humans to adverse effects of codeine.
There is a sharp increase in both morphine AUC and Cmax following codeine exposure in extensive/ultra‐rapid metabolisers.
No new data were identified that provide a basis to change the previous conclusion from 2011 that the maximal metabolic conversion of codeine into morphine does not exceed 20%.
Thebaine, oripavine, noscapine and papaverine
Based on limited information, oral bioavailability of thebaine, oripavine, noscapine and papaverine appears to be reduced due to pre‐systemic metabolism in the GI tract and liver primarily involving demethylation reactions but also glucuronidation.
There are indications that thebaine is metabolised into several metabolites, including oripavine and morphine.
4.1.2. Toxicity in experimental animals
Morphine
In experimental animals, morphine acts on the nervous system and its development. Most of the available data were generated by using routes of administration and doses that are not relevant for the purpose of the present opinion.
Chronic toxicity, including carcinogenicity, of morphine has not been systematically evaluated. Based on the lack of carcinogenicity of codeine which is metabolised to morphine, it is unlikely that morphine is carcinogenic.
Morphine is genotoxic only in vivo but most likely by a non‐DNA reactive mode of action.
Depressed sexual activity, reduced testicular function and spermatogenesis, disruption of ovarian cyclicity and decreased pregnancy rate have been observed in rats exposed to morphine.
Oral morphine exposure of pregnant rats or mice affects the normal development of the placenta and the brain.
Morphine causes immunosuppressive actions.
Codeine
Long‐term feeding studies in rats and mice showed no evidence of carcinogenic activity.
Codeine is not genotoxic.
Based on limited data it is concluded that oral administration of codeine did not result in teratogenicity.
There are no data to make conclusions on the neurotoxic effects of codeine.
Thebaine and oripavine
Based on LD50 values, thebaine and oripavine are more toxic than morphine.
Noscapine
Noscapine has a lower acute toxicity compared to the other opium alkaloids considered in this Scientific Opinion.
Repeated exposure of rats and dogs to noscapine did not result in adverse effects.
Noscapine is an aneugen in vitro most likely by a non‐DNA reactive mechanism. Taking into account also its low systemic availability, a risk of genotoxic damage in vivo is very unlikely.
Papaverine
The LD50 values, by oral and parenteral administration are similar to those for morphine. However, evidence of toxicity was not seen following oral administration of papaverine to rats at 50 and 100 mg/kg bw per day and to dogs at 10 mg/kg bw per day.
4.1.3. Observations in humans
According to recent literature most conclusions in the 2011 opinion are still valid.
Morphine has a high affinity for the μ‐opioid receptor as an agonistic ligand. Activation of μ‐opioid receptors leads to adverse effects such as nausea, vomiting, sedation, drowsiness, euphoria, miosis, respiratory depression and obstipation. Therapeutic doses of morphine may impair the ability to drive or to operate machinery due to changes in attentiveness and reactive skills.
The lowest known single oral therapeutic dose reported is 1.9 mg morphine, corresponding to 27 μg/kg bw for an adult weighing 70 kg.
The pharmacology of codeine is strongly related to that of morphine, as it is a precursor of morphine itself.
Recent literature focused on cases of life‐threatening toxicity in paediatric patients given therapeutic doses of codeine. This has been associated with the genotype predisposing to ultra‐rapid metabolism of codeine into morphine by the isoenzyme CYP2D6.
Morphine and codeine have dependency potential. Longer term use can result in tolerance.
The adverse reactions to codeine are similar to those of morphine but seen to a lesser extent at clinical doses. The most frequent side effects of codeine are constipation and nausea.
For oripavine and thebaine, no data after oral or parenteral exposure of humans are available.
Noscapine and papaverine do not show opiate‐like pharmacology. Noscapine is used as an antitussive agent. Papaverine acts as a smooth muscle relaxant. Compared to morphine, adverse effects of noscapine and papaverine given orally in therapeutic doses are less severe (e.g. dizziness, headache).
4.1.4. Mode of action
Morphine, codeine, thebaine and oripavine interact with three different receptors namely the μ‐, δ‐ and κ‐opioid receptors. The μ‐opioid receptor plays a crucial role in both the desired (analgesia) and undesired (respiratory depression) effects of morphine and codeine in medicinal use.
The rank order of potency of natural opium alkaloids at the μ‐opioid receptor is morphine ≫ oripavine > codeine > thebaine.
No indication has been found that the effects of noscapine and papaverine are mediated via opioid receptors.
M6G is an active metabolite of morphine with similar or higher activity as morphine, whereas M3G has weak affinity for the μ‐opioid receptor.
The codeine metabolites C6G, norcodeine and N6G have weak affinity for the μ‐opioid receptor.
4.1.5. Considerations of critical effects, dose‐response modelling and possibilities for derivation of a health‐based guidance value
The CONTAM Panel confirms its previous conclusion that the critical effects of morphine are on the CNS mediated by its high affinity to the μ‐opioid receptor as an agonistic ligand.
From data on humans and experimental animals, it is clear that morphine can interfere with brain development of the fetus resulting in behavioural effects at later life‐stages; however, data are too limited to derive a dose‐response relationship.
The critical effects of codeine are the same as those of morphine, because morphine is the active metabolite of codeine.
The data that have become available since the 2011 CONTAM opinion, do not provide a basis for revising the ARfD for morphine of 10 μg/kg bw, which is derived from the lowest therapeutic dose of morphine. In order to characterise the risks of combined exposure to morphine and codeine, the ARfD is a group ARfD for morphine and codeine, expressed in morphine equivalents using an equivalence factor of 0.2 for codeine.
Oripavine also acts as an agonist to the μ‐opioid receptor, with a lower activity than morphine and a higher activity than codeine. Non‐oral LD50 values in rodents indicate that oripavine is more acutely toxic than morphine. However, the available data are insufficient to characterise the hazard or to identify a factor for conversion to morphine equivalents.
The toxicological properties of thebaine have not been well characterised. A contribution to morphine‐like toxicity due to its possible metabolism into morphine and oripavine may occur, but is expected to be small. Oral LD50 values in rodents indicate that thebaine is more acutely toxic than morphine. The available data are insufficient to identify a factor for conversion to morphine equivalents, to propose a HBGV, or to identify a reference point for calculating margins of exposure.
Noscapine and papaverine do not exhibit morphine‐like properties and therefore they are not included in the group ARfD. Their toxicological properties have not been well characterised and it is not possible to establish HBGVs.
Based on the limited data available, it appears that noscapine and papaverine are less toxic than the other alkaloids discussed in this opinion.
4.2. Occurrence/exposure
To estimate acute dietary exposure to opium alkaloids, two batches of analytical results on the occurrence of opium alkaloids in food and poppy seeds were available. A first batch of 2,678 analytical results were collected by EFSA until 2011 and presented in the previous CONTAM opinion on opium alkaloids. This data set contained analytical results from 55 Australian poppy seed samples that were not used for dietary exposure assessment since their opium alkaloid profile was considered different from poppy seed samples available on the EU market at that time. A second batch of data contained 4,991 analytical results and was collected by EFSA between 2012 and 2017.
Considering the limited amount of data on food categories other than poppy seeds, the CONTAM Panel decided to base the assessment on poppy seed samples only.
The occurrence data on opium alkaloids show the presence of poppy seed samples with high concentrations of morphine, codeine and/or thebaine. The opium alkaloid profile seems to be related to the country of origin of the poppy seed samples and ultimately to the poppy variety.
Based on the available data per country of origin and batch, the CONTAM Panel concluded that the profiles in the two batches are comparable. In addition, the profile in the Australian poppy seeds is compatible with the profile in the European poppy seeds. Therefore, the Australian data were included in the data set and the two batches of data submitted to EFSA were merged.
Based on evidence from the currently available data set and background information, the CONTAM Panel divided the poppy seed samples with known country of origin into two groups. The ‘high‐morphine’ group, which is assumed to represent primarily poppy seeds from varieties grown for the pharmaceutical sector and the ‘low‐morphine’ group, which is assumed to represent primarily varieties grown for the food sector. It emerged that both types are available, although in unknown proportion, on the European market, therefore the CONTAM Panel has consequently elaborated two separate acute exposure scenarios.
In poppy seed samples, the highest mean MB concentrations were reported for morphine (147 mg/kg in the ‘high‐morphine’ group and 16.4 mg/kg in the ‘low‐morphine’ group) and thebaine (92.5 and 3.92 mg/kg, respectively) compared to codeine (22.7 and 2.88 mg/kg, respectively) and oripavine (20.0 and 2.14 mg/kg, respectively). For noscapine and papaverine, the mean MB concentrations were smaller and not exceeding 2 mg/kg.
In the ‘high‐morphine’ group, thebaine was present at a concentration higher than morphine in more than 25% of the samples and codeine was higher than morphine in about 5% of the samples. In the ‘low‐morphine’ group, morphine was the opium alkaloid present at the highest concentration in almost all samples.
Among consumers of poppy seeds, the average estimated poppy seed consumption from the Comprehensive Food Consumption Database ranged from 0.5 (Other children, Sweden) to 25 g poppy seeds/day (Adults, Hungary) in 44 population groups. Only in 7 population groups it was possible to calculate the P95 ranging from 1 (Other children, Sweden) to 55 g poppy seeds/day (Adults, Germany).
For morphine, under the MB scenario, the mean acute dietary exposure (based on the mean consumption and mean occurrence) for consumers only, ranged from 2.1 to 100 μg/kg bw per day across dietary surveys and age groups when considering the poppy seed samples from the ‘high‐morphine’ group. The P95 acute dietary exposure (based on the P95 consumption and P95 occurrence) ranged from 21 up to 330 μg/kg bw day. For the ‘low‐morphine’ group, the mean MB acute dietary exposure ranged from 0.2 to 11 μg/kg bw per day across dietary surveys and age groups and the P95 from 2.8 to 46 μg/kg bw per day. The mean acute dietary exposure was the highest for other children. Maximum exposure at the P95 was similar for all age groups for which data were available.
For codeine, acute dietary exposure was lower compared to morphine, with the P95 dietary exposure similar to the mean dietary exposure to morphine.
Acute dietary exposure estimates for morphine and codeine, expressed as morphine equivalents are quite similar compared to morphine alone and codeine makes a minor contribution to the morphine equivalents.
For thebaine, the mean MB acute dietary exposure for consumers only, ranged from 1.3 to 63 μg/kg bw per day across dietary surveys and age groups when considering the poppy seed samples from the ‘high‐morphine’ group. For the ‘low‐morphine’ group, the mean MB acute dietary exposure ranged from 0.06 to 2.7 μg/kg bw per day across dietary surveys and age groups. The P95 acute dietary exposure ranged from 18 to 288 μg/kg bw per day for the ‘high‐morphine’ group and from 0.7 to 11 μg/kg bw per day for the ‘low‐morphine’ group. The mean acute dietary exposure was the highest for other children. Maximum exposure at the P95 was similar for the age groups for which data were available.
No exposure assessment was performed for oripavine due to the very limited amount of occurrence data.
For noscapine, the mean dietary exposure for consumers only, ranged from 0.02 to 1.5 μg/kg bw per day (min LB–max UB) across dietary surveys and age groups and was the highest for other children. The P95 acute dietary exposure ranged from 0.3 to 4.4 μg/kg bw per day (min LB–max UB) across dietary surveys and age groups.
Among the opium alkaloids considered in this Scientific Opinion, acute dietary exposure for consumers only, was the lowest for papaverine, not exceeding 1 μg/kg bw per day.
4.3. Risk characterisation
Exposure estimates are based on reported concentrations of alkaloids in poppy seed samples due to the low amount of occurrence data on food products containing poppy seeds. However, food processing steps (i.e. washing, soaking, heat treatment, grinding) may reduce the alkaloid content in raw poppy seeds by 25–100% in the final product. The group ARfD is most likely to be exceeded when large portions are consumed or if foods containing unprocessed poppy seeds are consumed.
Considering data related to poppy seeds containing relatively high levels of opium alkaloids, such as those from varieties primarily grown for pharmaceutical use, mean and high levels of dietary exposure to morphine from poppy seeds result in the group ARfD of 10 μg morphine equivalents/kg bw being exceeded in most age groups by up to 33‐fold.
For poppy seeds considered to have relatively lower concentrations of opium alkaloids (primarily varieties for food use), exceedance of the group ARfD is also seen at high levels of dietary exposure in most surveys, but to a lesser extent at up to 4 to 5‐fold.
Based on the mean morphine occurrence, 34 out of 44 population groups are estimated to be exposed for at least 50% of the consumption days to a level of morphine higher than the group ARfD of 10 μg/kg bw for the ‘high‐morphine’ scenario and only 1 population group for the ‘low‐morphine’ scenario.
Codeine makes a minor contribution to exceedance of the group ARfD. However when considering individual poppy seed samples containing high concentrations of codeine, their consumption might result in a codeine exposure exceeding the group ARfD.
There are few reports of adverse reactions arising from traditional consumption of poppy seeds in foods, but in the absence of formal reporting systems it cannot be assumed that such reactions do not occur from time to time, particularly in sensitive individuals.
Pregnant women, infants, people above 75 years of age and those suffering from health conditions with impaired respiratory function are subgroups that are more sensitive to adverse effects of morphine.
In principle, oripavine could contribute to morphine‐like effects, but the available toxicological and occurrence data were insufficient for risk characterisation.
For thebaine, no quantitative risk characterisation could be carried out. The estimated dietary exposures are slightly lower than those for morphine. On the other hand, although the lack of data did not allow the establishment of a HBGV, the CONTAM Panel noted that LD50 values for thebaine are 3‐ to 10‐fold lower than for morphine, suggesting that the estimated exposure levels might pose a health risk.
For noscapine, dietary exposure is at least 80‐fold lower than the recommended therapeutical dose, which does not suggest a health concern.
For papaverine, the dietary exposure is about 2000‐fold lower than the recommended therapeutical dose and it is highly unlikely that this would be a health concern.
5. Recommendations
There is a need for toxicological data on thebaine and oripavine, including toxicokinetic data particularly on the formation of active metabolites.
There is a need for more occurrence data of the opium alkaloids considered in this opinion in foods containing poppy seeds.
There is a need for clarification of the occurrence of oripavine in poppy seed samples available on the EU market.
Certified reference materials of food products that contain opium alkaloids at relevant concentrations should become available, as well as international proficiency tests.
More information on consumption of poppy seeds and poppy seed‐based food in the EU is needed.
Abbreviations
- AD
analgesic dose
- ADME
absorption, distribution, metabolism and excretion
- ARfD
acute reference dose
- AUC
Area under the curve
- BfR
Bundesinstitut für Risikobewertung/ the German Federal Institute for Risk Assessment
- BNF
British National Formulary
- BrdU
Bromodeoxyuridine
- BVL
Bundesamt für Verbraucherschutz und Lebensmittelsicherheit
- bw
body weight
- C6G
codeine‐6‐glucuronide
- cAMP
cyclic adenosine monophosphate
- CAS
Chemical Abstracts Service
- CHO
Chinese hamster ovary
- CI
confidence interval
- Cmax
maximum plasma concentrations
- CNS
central nervous system
- COMT
catechol‐amino transferase
- CONTAM Panel
EFSA Panel on Contaminants in the Food Chain
- CYP
cytochrome P450
- DMSO
dimethyl sulfoxide
- EEG
electroencephalogram
- EMA
European Medicines Agency
- FAO
Food and Agriculture Organization of the UN
- GC–MS
gas chromatography–mass spectrometry
- GI
gastrointestinal
- HBGV
health‐based guidance value
- HPLC‐UV
high‐performance liquid chromatography‐ultraviolet
- IgE
immunoglobulin E
- i.p.
intraperitoneal
- i.v.
intravenously
- IPCS
International Programme on Chemical Safety
- IUPAC
International Union of Pure and Applied Chemistry
- LB
lower bound
- LC
left‐censored data (values below the limit of detection or limit of quantification)
- LC–HRMS
liquid chromatography coupled to high‐resolution mass spectrometry
- LC–MS/MS
Liquid chromatography coupled to tandem mass spectrometry
- LD
lethal dose
- LLE
liquid/liquid extraction
- LOAEL
lowest‐observed‐adverse‐effect level
- LOD
limit of detection
- LOEL
lowest‐observed‐effect‐level
- logP
log of octanol:water partition coefficient
- LOQ
limit of quantification
- M3G
morphine‐3‐glucuronide
- M6G
morphine‐6‐glucuronide
- MTT
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide
- MW
molecular weight
- N6G
norcodeine‐6‐glucuronide
- NAS
neonatal abstinence syndrome
- NIH
US National Institute of Health
- NOAEL
no‐observed‐adverse‐effect level
- NOEL
no‐observed‐effect level
- OCT
organic cation transporters
- P5/P95
5th percentile/95th percentile
- PD
pharmacodynamics
- PK
pharmacokinetics
- pKa
log of acid dissociation constant
- PND
post‐natal day
- PRAC
Pharmacovigilance Risk Assessment Committee
- RASFF
Rapid Alert System for Food and Feed
- REM
rapid eye movement
- s.c.
subcutaneous
- SCE
sister chromatid exchanges
- SOP
standard operating procedure
- SPE
solid‐phase extraction
- STS
soft tissue sarcomas
- TUNEL
terminal deoxynucleotidyl transferase (TdT)‐mediated dUTP‐X nick end labelling
- TyDC
tyrosine decarboxylase enzyme
- UB
upper bound
- UGT
uridine diphosphate‐glucuronysyltransferase
- UM
ultra‐rapid metabolisers
- UN
United Nations
- UV
ultraviolet
- WG
Working Group
- WHO
World Health Organization
Appendix A – Biosynthetic pathway opium alkaloids in poppy seeds
1.
Figure A.1.
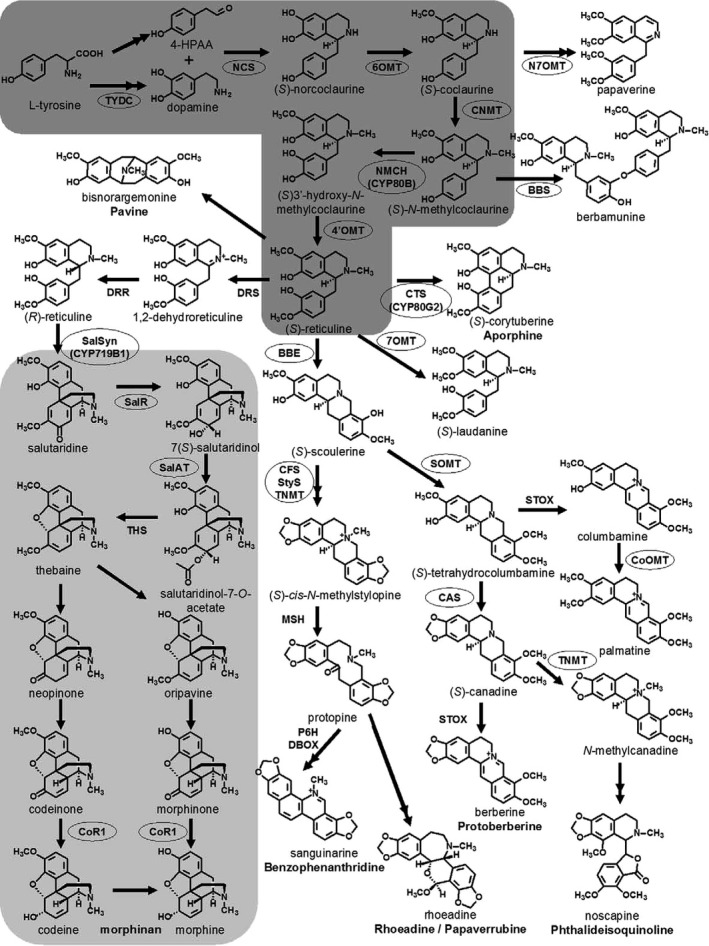
Benzylisoquinoline alkaloid biosynthesis (Ziegler et al., 2009)
- Multiple arrowheads denote more than one enzymatic step. Arrows without labelling reflect conversions that have not been enzymatically characterised. Enzymes that have been characterised are labelled, and those for which cognate cDNAs have been isolated are circled. The basic benzylisoquinoline pathway is shaded in dark grey, whereas the promorphinan and morphinan pathways are shaded in light grey. Abbreviations: 4‐HPAA: p‐hydroxyphenyl acetaldehyde; 4′OMT: (S)3′‐hydroxy‐N‐methylcoclaurine 4′‐O‐methyltransferase; 6OMT: (S)‐norcoclaurine 6‐O‐methyltransferase; 7OMT: (R,S)‐reticuline 7‐O‐methyltransferase; BBE: berberine bridge enzyme; BBS: berbamunine synthase; CAS: (S)‐canadine synthase; CFS: (S)‐cheilanthifoline synthase; CNMT: (S)‐coclaurine N‐methyltransferase; CoOMT: columbamine O‐methyltransferase; CoR1: codeinone reductase 1; CTS: (S)‐corytuberine synthase (CYP80G2); DBOX: dihydrobenzophenanthridine oxidase; DRR: 1,2‐dehydroreticuline reductase; DRS: 1,2‐dehydroreticuline synthase; MSH: methylstylopine 14‐hydroxylase; N7OMT: (S)‐norreticuline7‐O‐methyltransferase; NCS: (S)‐norcoclaurine synthase; NMCH: (S)‐N‐methylcoclaurine 3′‐hydroxylase (CYP80B subfamily); P6H: protopine 6‐hydroxylase; SalAT: 7(S)‐salutaridinol 7‐O‐acetyltransferase; SalR: salutaridine reductase; SalSyn: salutaridine synthase (CYP719B1); SOMT: scoulerine 9‐O‐methyltransferase; STOX: (S)‐tetrahydroprotoberberine oxidase; StyS: (S)‐stylopine synthase; THS: thebaine synthase; TNMT: tetrahydroprotoberberine N‐methyltransferase; TYDC: tyrosine decarboxylase.[Link]
Appendix B – Rapid Alert System for Food and Feed (RASFF) reports on the presence of morphine in poppy seed samples
1.
| Product category | Date | Reference | Product type | Notification type | Notification basis | Notified by | Countries concerned | Subject | Action taken | Distribution status | Risk decision |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuts, nut products and seeds | 22‐6‐2015 | 2015.0798 | Food | Alert | Company's own check | Czech Republic | Czech Republic (D/O), Slovakia (D) | High content of morphine (59.13 mg/kg – ppm) in ground poppy seeds from the Czech Republic | Withdrawal from the market | Distribution restricted to notifying country | Serious |
| Nuts, nut products and seeds | 17‐6‐2015 | 2015.0776 | Food | Alert | Official control on the market | Czech Republic | Czech Republic (D), France (O), Poland, Ukraine (D) | High content of morphine (69.61 mg/kg – ppm) in grey poppy seeds from France, via Poland | No distribution from notifying country | Serious | |
| Nuts, nut products and seeds | 3‐6‐2015 | 2015.0675 | Food | Alert | Official control on the market | Czech Republic | Australia (O), Czech Republic (D), Netherlands | High content of morphine (137 mg/kg – ppm) in blue poppy seeds from Australia, via the Netherlands | Detained by operator | No distribution from notifying country | Serious |
| Nuts, nut products and seeds | 21‐5‐2015 | 2015.0619 | Food | Alert | Official control on the market | Czech Republic | Czech Republic (D), France (O), Poland (D), Ukraine (D) | High content of morphine (96.93 mg/kg – ppm) in poppy seed from France, via Poland | Informing recipients | Distribution to other member countries | Serious |
| Nuts, nut products and seeds | 16‐5‐2014 | 2014.0670 | Food | Alert | Official control on the market | Czech Republic | Commission Services, Czech Republic (D), France (O), Slovakia (O) | High content of morphine (56.2 mg/kg – ppm) in blue poppy seeds from France, packaged in Slovakia | Withdrawal from the market | Distribution to other member countries | Serious |
| Nuts, nut products and seeds | 14‐1‐2014 | 2014.0053 | Food | Alert | Official control on the market | Czech Republic | Czech Republic (D), Hungary (O), Poland (D), Slovakia (D) | High content of morphine (88.1 mg/kg – ppm) in poppy seeds from Hungary | Withdrawal from the market | Distribution to other member countries | Serious |
| Nuts, nut products and seeds | 24‐6‐2010 | 2010.0837 | Food | Alert | Official control on the market | Germany | Germany (D), Hungary (D), Netherlands (O), unknown origin (O) | High content of morphine (72 mg/kg – ppm) in poppy seeds from unknown origin, via the Netherlands | Destruction | Distribution on the market (possible) | Undecided |
| Nuts, nut products and seeds | 26‐10‐2007 | 2007.0774 | Food | Alert | Company's own check | Hungary | Hungary (D/O), Slovakia (D) | High content of morphine (80 mg/kg – ppm) in poppy seeds in bulk from Hungary | Prohibition to trade – sales ban | Distribution on the market (possible) | Undecided |
| Nuts, nut products and seeds | 26‐10‐2007 | 2007.0773 | Food | Alert | Company's own check | Hungary | Hungary (D/O), Netherlands (D), Russia (D), Ukraine (D) | High content of morphine (40 mg/kg – ppm) in poppy seeds in bulk from Hungary | Prohibition to trade – sales ban | Distribution on the market (possible) | Undecided |
| Nuts, nut products and seeds | 7‐9‐2007 | 2007.CCX | Food | Information | Company's own check | Hungary | Hungary (D/O), Russia (D) | High content of morphine (60 mg/kg – ppm) in poppy seed from Hungary | Physical/ chemical treatment | Distribution restricted to notifying country | Undecided |
| Nuts, nut products and seeds | 22‐1‐2007 | 2007.0050 | Food | Alert | Official control on the market | Hungary | Austria (D), Czech Republic (O), Germany (D), Hungary (D), Poland (D), Romania (D), Slovakia (D) | High content of morphine (60.0; 80.0 mg/kg – ppm) in poppy seed from the Czech Republic via the Slovak Republic | Withdrawal from the market | Distribution on the market (possible) | Undecided |
| Nuts, nut products and seeds | 22‐12‐2006 | 2006.0927 | Food | Alert | Official control on the market | Germany | Czech Republic (O), Germany (D), Liechtenstein (O), Netherlands | High content of morphine (93 mg/kg – ppm) in poppy seed from the Czech Republic via the Netherlands and via Liechtenstein | Withdrawal from the market | Distribution on the market (possible) | Undecided |
| Nuts, nut products and seeds | 19‐12‐2006 | 2006.0903 | Food | Alert | Company's own check | Hungary | Hungary (D), Slovakia (O) | High content of morphine (40 mg/kg – ppm) in poppy seed from the Slovak Republic | Official detention | Distribution on the market (possible) | Undecided |
| Nuts, nut products and seeds | 19‐12‐2006 | 2006.0902 | Food | Alert | Company's own check | Hungary | Czech Republic (O), Hungary (D), Slovakia | High content of morphine (53 mg/kg – ppm) in poppy seed from the Czech Republic via the Slovak Republic | Official detention | Distribution on the market (possible) | Undecided |
| Nuts, nut products and seeds | 13‐1‐2006 | 2006.0039 | Food | Alert | Official control on the market | Hungary | Hungary (D), Poland, Slovakia (O) | High content of morphine (44 mg/kg – ppm) in poppy seed from the Slovak Republic | Destination of the product changed | Distribution on the market (possible) | Undecided |
| Nuts, nut products and seeds | 10‐1‐2006 | 2006.0024 | Food | Alert | Official control on the market | Germany | Austria (D), Germany (D), Netherlands (O) | High content of morphine (200 mg/kg – ppm) in poppy seed from the Netherlands | Product recall or withdrawal | Distribution on the market (possible) | Undecided |
| Nuts, nut products and seeds | 28‐12‐2005 | 2005.DHV | Food | Information | Official control on the market | Hungary | Croatia (D), Hungary (D/O), Slovakia (D) | High content of morphine (44.4 mg/kg – ppm) in poppy seed from Hungary | Product recall or withdrawal | Product past use‐by date | Undecided |
| Nuts, nut products and seeds | 21‐12‐2005 | 2005.DFK | Food | Information | Official control on the market | Germany | Germany (D), unknown origin (O) | High content of morphine (200 mg/kg – ppm) in poppy seed from unknown origin | Product recall or withdrawal | Distribution restricted to notifying country | Undecided |
| Nuts, nut products and seeds | 7‐12‐2005 | 2005.892 | Food | Alert | Official control on the market | Hungary | Austria (O), Hungary (D) | High content of morphine (48 mg/kg – ppm) in ground poppy seeds from Austria | Product recall or withdrawal | Distribution on the market (possible) | Undecided |
| Nuts, nut products and seeds | 27‐10‐2005 | 2005.CTA | Food | Information | Official control on the market | Germany | Australia (O), Germany (D) | High content of morphine (228 mg/kg – ppm) in poppy seed from Australia | Product recall or withdrawal | Distribution restricted to notifying country | Undecided |
D: country of distribution; O: country of origin.
Appendix C – Identification and selection of evidence relevant for the risk assessment of opium alkaloids in poppy seeds
C.1. Literature search for supporting information for the assessment, and previously reported occurrence data and exposure
Searches in Google Scholar, Science Direct and Web of Science were conducted to identify scientific papers on up‐to‐date regarding:
the influence of environmental factors and agrotechnical measures on the alkaloid content of poppy capsules;
recent breeding efforts concerning alkaloid spectrum of poppy varieties;
the analysis of opium alkaloids and on the occurrence of and exposure to opium alkaloids in poppy seeds and poppy seed containing products.
Used search terms (without time span): selection and poppy; breeding and poppy; variety and poppy; cultivar and poppy; (inheritance and alkaloid) and Papaver; genetic* and Papaver; (development or ontogenesis) and poppy; poppy and growing; poppy and cultivation; temperature and morphine; temperature and alkaloids; water and alkaloids; (precipitation or irrigation) and poppy; contamination and seed and poppy; Papaver bracteatum; opium alkaloid* and analysis; Papaver somniferum and analysis; poppy seed* and analysis; poppy seed food*; poppy seed product*; survey and poppy seed*; exposure and opium alkaloid*; consumption and poppy seeds*.
The officially registered varieties of Papaver somniferum L. were assessed both from the OECD Variety List24 and the EU Plant Variety Catalogue.11
The Rapid Alert System for Food and Feed (RASFF) of the European Commission was assessed for notifications on the presence of morphine or opium alkaloids in poppy seeds and poppy seed food products.25
C.2. Literature search for hazard identification and characterisation
C.2.1. Search for papers regarding morphine
C.2.1.1. Toxicity
Since the previous assessment was based on a reference point identified in humans, only a limited search in PubMed was conducted to identify papers on toxicity in experimental animals. The following search string was used.
A. PubMed
(“Morphine”[Mesh] OR “morphine”[tiab] OR “morfin”[tiab] OR “morfine”[tiab] OR “morphia”[tiab] OR “morphin”[tiab] OR “morphine”[tiab] OR “morphinium”[tiab] OR “morphium”[tiab] OR 57‐27‐2[rn]) AND (“Reproduction”[Mesh] OR “Prenatal Injuries”[Mesh] OR “Congenital Abnormalities”[Mesh] OR “Maternal Exposure”[Mesh] OR “Embryonic and Fetal Development”[Mesh] OR embryo* development[tiab] OR pregnan*[tiab] OR developmental tox*[tiab] OR “reprotox*”[tiab] OR “embryotox*”[tiab] OR reproductive tox*[tiab] OR “Teratogens”[Mesh] OR “teratogen*”[tiab] OR “Mutagenicity Tests”[Mesh] OR “genotox*”[tiab] OR mutagen*[tiab] OR “Carcinogenicity Tests”[Mesh] OR carcinogen*[tiab] OR “Immune System/drug effects”[Mesh] OR immunotox*[tiab] OR immune effect*[tiab] OR immunosuppress*[tiab] OR “Neurotoxicity Syndromes”[Mesh] OR neurotox*[tiab] OR neurological effect*[tiab] OR “repeated dose”[tiab]) AND (animal*[tiab] OR mouse[tiab] OR mice[tiab] OR rat[tiab] OR rats[tiab] OR rodent*[tiab] OR primate*[tiab] OR monkey*[tiab])
Time span: 2011–2017.
C.2.1.2. Metabolism and kinetics
Since little new information has become available in recent years regarding the metabolism and kinetics of morphine, no comprehensive literature search was conducted but experts in the field identified relevant papers in areas for which new information had become available.
C.2.1.3. Human data
A comprehensive search was carried out in Embase, PubMed and Web of Science to retrieve human data on morphine using the following search strings:
A. Embase
‘morphine’/mj/dd_ae,dd_ct,dd_to OR ((‘morfin’:ti,ab OR ‘morfine’:ti,ab OR ‘morphia’:ti,ab OR ‘morphin’:ti,ab OR ‘morphine’:ti,ab OR ‘morphinium’:ti,ab OR ‘morphium’:ti,ab) AND (‘adverse drug reaction’/exp/mj OR ‘adverse effect*’:ti,ab OR ‘clinical study’/exp/mj OR ‘clinical stud*’:ti,ab OR ‘clinical trial*’:ti,ab OR ‘case report*’:ti,ab OR ‘case stud*’:ti,ab OR ‘human experiment’/exp OR ‘human stud*’:ti,ab OR ‘volunteer’/exp OR ‘human volunteer*’:ti,ab OR ‘drug surveillance program’/exp OR ‘pharmacovigilance’:ti,ab))
Time span: 2011–2017
Database: Embase, Embase classic (w/o Medline)
Quick limit: humans
B. PubMed
((“Morphine/adverse effects”[Mesh] OR “Morphine/contraindications”[Mesh] OR “Morphine/poisoning”[Mesh] OR “Morphine/toxicity” [Mesh]) OR ((“Morphine”[Mesh] OR “morphine”[tiab] OR “morfin”[tiab] OR “morfine”[tiab] OR “morphia”[tiab] OR “morphin”[tiab] OR “morphine”[tiab] OR “morphinium”[tiab] OR “morphium”[tiab] OR 57‐27‐2[rn]) AND (“Drug‐Related Side Effects and Adverse Reactions”[Mesh] OR adverse effect*[tiab] OR “Clinical Study” [Publication Type] OR “Clinical Studies as Topic”[Mesh] OR clinical trial*[tiab] OR clinical stud*[tiab] OR “Case Reports” [Publication Type] OR case report*[tiab] OR “Human Experimentation”[Mesh] OR human stud*[tiab] OR “Volunteers”[Mesh] OR “human volunteer*”[tiab] OR “Pharmacovigilance”[Mesh] OR “pharmacovigilance”[tiab]))) NOT (“Animals”[Mesh] NOT “Humans”[Mesh])
Time span: 2011–2017
C. Web of Science
TOPIC: (morphine OR morfin OR morfine OR morphia OR morphin OR morphine OR morphinium OR morphium) AND TOPIC:(((clinical OR human) NEAR/2 (trial* OR stud*)) OR (case NEAR/2 report*) OR “human volunteer*” OR ((adverse OR toxic*) NEAR/2 effect*) OR pharmacovigilance)
Refined by: Databases: (FSTA OR WOS OR CSCD OR DRCI OR CCC OR SCIELO OR RSCI OR BCI OR KJD OR ZOOREC)
(w/o CABI, Zoological, Medline)
[excluding] RESEARCH AREAS: (VETERINARY SCIENCES OR ZOOLOGY)
Time span: 2011–2017
All the retrieved references were uploaded in Endnote and screened for relevance.
C.2.2. Search for papers regarding codeine
C.2.2.1. Toxicity
Searches in Embase, PubMed and Web of Science were conducted to identify papers on toxicity (in vitro and in vivo) by using the following search strings.
A. Embase
‘codeine’/mj/dd_to OR ((‘codeine’:ti,ab OR ‘codein’:ti,ab OR ‘methyl morphine’:ti,ab OR ‘methyl morfin’:ti,ab OR ‘morphine methyl ether’:ti,ab OR ‘morphine monomethyl ether’:ti,ab) AND (‘toxicity’/exp/mj OR ‘toxicity testing’/exp/mj OR ‘toxicity assay’/exp/mj OR ‘toxic substance’/exp/mj OR carcinogen*:ti,ab OR cardiotox*:ti,ab OR cytotox*:ti,ab OR ‘endocrine toxic*’:ti,ab OR ‘endocrine disruptor*’:ti,ab OR genotox*:ti,ab OR hemotoxic*:ti,ab OR hematotoxic*:ti,ab OR hepatotox*:ti,ab OR immunotox*:ti,ab OR ‘liver toxic*’:ti,ab OR mutagen*:ti,ab OR nephrotox*:ti,ab OR neurotox*:ti,ab OR reprotox*:ti,ab OR terato*:ti,ab OR toxic*:ti,ab OR ‘toxic effect*’:ti,ab OR tumorigen*:ti,ab OR ‘in vivo’:ti,ab OR ‘in vitro’:ti,ab))
Time span: 2011–2017
Database: all
B. PubMed
“Codeine/toxicity”[Mesh] OR ((Codeine[tiab] OR Codein[tiab] OR “N‐Methylmorphine”[tiab] OR “Methyl morphine”[tiab] OR “methyl morfine”[tiab] OR “morphine methyl ether”[tiab] OR “morphine monomethyl ether”[tiab] OR “76‐57‐3”[rn]) AND (“Toxic Actions”[Mesh] OR “Toxicity Tests”[Mesh] OR carcinogen*[tiab] OR cardiotox*[tiab] OR cytotox*[tiab] OR endocrine toxic*[tiab] OR endocrine disruptor*[tiab] OR genotox*[tiab] OR hemotoxic*[tiab] OR hematotoxic*[tiab]OR hepatotox*[tiab]OR immunotox*[tiab] OR liver toxic*[tiab] OR mutagen*[tiab] OR nephrotox*[tiab] OR neurotox*[tiab] OR reprotox*[tiab] OR terato*[tiab] OR toxic*[tiab] OR toxic effect*[tiab] OR tumorigen*[tiab] OR “in vivo”[tiab] OR “in vitro”[tiab]))
Time span: 2011–2017
C. Web of Science
TOPIC: (Codeine OR Codein OR “Methyl morphine” OR “methyl morfine” OR “morphine methyl ether” OR “morphine monomethyl ether”) AND TOPIC: (carcinogen* OR cardiotox* OR cytotox* OR endocrine toxic* OR endocrine disruptor* OR genotox* OR hemotoxic* OR hematotoxic*OR hepatotox*OR immunotox* OR liver toxic* OR mutagen* OR nephrotox* OR neurotox* OR reprotox* OR terato* OR toxic* OR toxic effect* OR tumorigen* OR “in vivo” OR “in vitro”)
Time span: 2011–2017
Database: all
C.2.2.2. Metabolism and kinetics
Searches in Embase, PubMed and Web of Science were conducted to identify papers on metabolism and kinetics by using the following search strings.
A. Embase
‘codeine’/mj/dd_pk,dd_pd OR ((‘codeine’:ti,ab OR ‘codein’:ti,ab OR ‘methyl morphine’:ti,ab OR ‘methyl morfin’:ti,ab OR ‘morphine methyl ether’:ti,ab OR ‘morphine monomethyl ether’:ti,ab) AND (‘biological marker’/exp/mj OR ‘drug receptor’/exp/mj OR ‘metabolism’/exp/mj OR metabolism:ti,ab OR kinetic*:ti,ab OR toxicokinetic*:ti,ab OR pharmacokinetic*:ti,ab OR absorption:ti,ab OR distribution:ti,ab OR excretion:ti,ab OR mechanism*:ti,ab OR mode:ti,ab OR action:ti,ab OR biomarker*:ti,ab OR biotransformation:ti,ab OR pharmacology:ti,ab OR receptor*:ti,ab OR “mu receptor”:ti,ab OR “opioid receptor”:ti,ab))
Time span: 2011–2017
Database: all
B. PubMed
“Codeine/metabolism”[Mesh] OR “Codeine/pharmacokinetics”[Mesh] OR “Codeine/pharmacology”[Mesh] OR ((Codeine[tiab] OR Codein[tiab] OR “N‐Methylmorphine”[tiab] OR “76‐57‐3”[rn]) AND (“Biomarkers”[Mesh] OR “Metabolism”[Mesh] OR “Receptors, Drug”[Mesh] OR “Receptors, Opioid, mu”[Mesh] OR “Pharmacology”[Mesh] OR metabolism[tiab] OR kinetic*[tiab] OR toxicokinetic*[tiab] OR pharmacokinetic*[tiab] OR absorption[tiab] OR distribution[tiab] OR excretion[tiab] OR mechanism*[tiab] OR mode[tiab] OR action[tiab] OR biomarker*[tiab] OR biotransformation[tiab] OR pharmacology[tiab] OR receptor*[tiab] OR “mu receptor”[tiab] OR “opioid receptor”[tiab]))
Time span: 2011–2017
C. Web of Science
(Codeine OR Codein OR “Methyl morphine” OR “methyl morfine” OR “morphine methyl ether” OR “morphine monomethyl ether”) AND TOPIC: (metabolism OR kinetic* OR toxicokinetic* OR pharmacokinetic* OR absorption OR distribution OR excretion OR mechanism* OR mode OR action OR biomarker* OR biotransformation OR pharmacology OR receptor* OR “mu receptor” OR “opioid receptor”)
Time span: 2011–2017
Database: all
C.2.2.3. Human data
Searches in Embase, PubMed and Web of Science were conducted to identify papers on human data by using the following search strings.
A. Embase
‘codeine’/mj/dd_ae OR ((‘codeine’:ti,ab OR ‘codein’:ti,ab OR ‘methyl morphine’:ti,ab OR ‘methyl morfin’:ti,ab OR ‘morphine methyl ether’:ti,ab OR ‘morphine monomethyl ether’:ti,ab) AND (‘epidemiology’/exp/mj OR ‘adverse drug reaction’/exp/mj OR ‘volunteer’/exp OR ‘human experiment’/exp OR ‘epidemiolog*’:ti,ab OR ‘case control’:ti,ab OR ‘case stud*’:ti,ab OR ‘cohort’:ti,ab OR ‘cross sectional’:ti,ab OR ‘adverse effect*’:ti,ab OR ‘biomarker*’:ti,ab OR ‘human volunteers’:ti,ab OR ‘human stud*’:ti,ab))
Time span: 2011–2017
Database: all
‘codeine’/mj/dd_ct OR ((‘codeine’:ti,ab OR ‘codein’:ti,ab OR ‘methyl morphine’:ti,ab OR ‘methyl morfin’:ti,ab OR ‘morphine methyl ether’:ti,ab OR ‘morphine monomethyl ether’:ti,ab) AND (‘clinical study’/exp/mj OR ‘clinical stud*’:ti,ab OR ‘clinical trial*’:ti,ab OR ‘case report*’:ti,ab)) AND [2011‐2017]/py
Time span: 2011–2017
Database: all
B. PubMed
“Codeine/adverse effects”[Mesh] OR ((Codeine[tiab] OR Codein[tiab] OR “N‐Methylmorphine”[tiab] OR “76‐57‐3”[rn]) AND (“Epidemiologic Studies”[Mesh] OR “Drug‐Related Side Effects and Adverse Reactions”[Mesh] OR “Volunteers”[Mesh] OR “Human Experimentation”[Mesh] OR epidemiolog*[tiab] OR “case control”[tiab] OR case stud*[tiab] OR cohort[tiab] OR “cross sectional”[tiab] OR adverse effect*[tiab] OR “human volunteers”[tiab] or human stud*[tiab]))
Time span: 2011–2017
(Codeine[tiab] OR Codein[tiab] OR “N‐Methylmorphine”[tiab] OR “76‐57‐3”[rn]) AND (“Clinical Study” [Publication Type] OR “Clinical Studies as Topic”[Mesh] OR “Case Reports” [Publication Type] OR clinical stud*[tiab] OR clinical trial*[tiab] OR case report*[tiab])
Time span: 2011–2017
C. Web of Science
TOPIC: Codeine OR Codein OR “Methyl morphine” OR “methyl morfine” OR “morphine methyl ether” OR “morphine monomethyl ether”) AND TOPIC: (epidemiolog* OR “case control” OR case stud* OR cohort OR “cross sectional” OR adverse effect* OR “human volunteers” or human stud*)
Time span: 2011–2017
Database: all
TOPIC: (Codeine OR Codein OR “Methyl morphine” OR “methyl morfine” OR “morphine methyl ether”) AND TOPIC: (clinical stud* OR clinical trial* OR case report*)
Time span: 2011–2017
Database: all
All the retrieved references for codeine were uploaded in Endnote and screened for relevance.
C.2.3. Search for papers regarding thebaine
C.2.3.1. Toxicity
Searches in Embase, PubMed and Web of Science were conducted to identify papers on toxicity (in vitro and in vivo) by using the following search strings.
A. Embase
‘thebaine’/mj/dd_to OR ((‘thebaine’:ti,ab OR ‘thebain’:ti,ab OR ‘paramorphine’:ti,ab) AND (‘toxicity’/exp/mj OR ‘toxicity testing’/exp/mj OR ‘toxicity assay’/exp/mj OR ‘toxic substance’/exp/mj OR carcinogen*:ti,ab OR cardiotox*:ti,ab OR cytotox*:ti,ab OR ‘endocrine toxic*’:ti,ab OR ‘endocrine disruptor*’:ti,ab OR genotox*:ti,ab OR hemotoxic*:ti,ab OR hematotoxic*:ti,ab OR hepatotox*:ti,ab OR immunotox*:ti,ab OR ‘liver toxic*’:ti,ab OR mutagen*:ti,ab OR nephrotox*:ti,ab OR neurotox*:ti,ab OR reprotox*:ti,ab OR terato*:ti,ab OR toxic*:ti,ab OR ‘toxic effect*’:ti,ab OR tumorigen*:ti,ab OR ‘in vivo’:ti,ab OR ‘in vitro’:ti,ab))
Time span: all years
Database: all
B. PubMed
“Thebaine/toxicity”[Mesh] OR ((thebaine[tiab] OR thebain[tiab] OR paramorphine[tiab] OR 115‐37‐7[rn]) AND (“Toxic Actions”[Mesh] OR “Toxicity Tests”[Mesh] OR carcinogen*[tiab] OR cardiotox*[tiab] OR cytotox*[tiab] OR endocrine toxic*[tiab] OR endocrine disruptor*[tiab] OR genotox*[tiab] OR hemotoxic*[tiab] OR hematotoxic*[tiab]OR hepatotox*[tiab]OR immunotox*[tiab] OR liver toxic*[tiab] OR mutagen*[tiab] OR nephrotox*[tiab] OR neurotox*[tiab] OR reprotox*[tiab] OR terato*[tiab] OR toxic*[tiab] OR toxic effect*[tiab] OR tumorigen*[tiab] OR “in vivo”[tiab] OR “in vitro”[tiab]))
Time span: all years
C. Web of Science
TOPIC: (thebaine OR thebain OR paramorphine) AND TOPIC: (carcinogen* OR cardiotox* OR cytotox* OR endocrine toxic* OR endocrine disruptor* OR genotox* OR hemotoxic* OR hematotoxic* OR hepatotox*OR immunotox* OR liver toxic* OR mutagen* OR nephrotox* OR neurotox* OR reprotox* OR terato* OR toxic* OR toxic effect* OR tumorigen* OR “in vivo” OR “in vitro”)
Time span: all years
Database: all
C.2.3.2. Metabolism and kinetics
Searches in Embase, PubMed and Web of Science were conducted to identify papers on metabolism and kinetics by using the following search strings.
A. Embase
‘thebaine’/mj/dd_pk,dd_pd OR ((‘thebaine’:ti,ab OR ‘thebain’:ti,ab OR ‘paramorphine’:ti,ab) AND (‘biological marker’/exp/mj OR ‘drug receptor’/exp/mj OR ‘metabolism’/exp/mj OR metabolism:ti,ab OR kinetic*:ti,ab OR toxicokinetic*:ti,ab OR pharmacokinetic*:ti,ab OR absorption:ti,ab OR distribution:ti,ab OR excretion:ti,ab OR mechanism*:ti,ab OR mode:ti,ab OR action:ti,ab OR biomarker*:ti,ab OR biotransformation:ti,ab OR pharmacology:ti,ab OR receptor*:ti,ab OR “mu receptor”:ti,ab OR “opioid receptor”:ti,ab))
Time span: all years
Database: all
B. PubMed
(“Thebaine/metabolism”[Mesh] OR “Thebaine/pharmacokinetics”[Mesh] OR “Thebaine/pharmacology”[Mesh]) OR ((thebaine[tiab] OR thebain[tiab] OR paramorphine[tiab] OR 115‐37‐7[rn]) AND (“Biomarkers”[Mesh] OR “Metabolism”[Mesh] OR “Receptors, Drug”[Mesh] OR “Receptors, Opioid, mu”[Mesh] OR “Pharmacology”[Mesh] OR metabolism[tiab] OR kinetic*[tiab] OR toxicokinetic*[tiab] OR pharmacokinetic*[tiab] OR absorption[tiab] OR distribution[tiab] OR excretion[tiab] OR mechanism*[tiab] OR mode[tiab] OR action[tiab] OR biomarker*[tiab] OR biotransformation[tiab] OR pharmacology[tiab] OR receptor*[tiab] OR “mu receptor”[tiab] OR “opioid receptor”[tiab]))
Time span: all years
C. Web of Science
TOPIC: (thebaine OR thebain OR paramorphine) AND TOPIC: (metabolism OR kinetic* OR toxicokinetic* OR pharmacokinetic* OR absorption OR distribution OR excretion OR mechanism* OR mode OR action OR biomarker* OR biotransformation OR pharmacology OR receptor* OR “mu receptor” OR “opioid receptor”)
Time span: all years
Database: all
C.2.3.3. Human data
Searches in Embase, PubMed and Web of Science were conducted to identify papers on human data by using the following search strings.
A. Embase
‘thebaine’/mj/dd_ae OR ((‘thebaine’:ti,ab OR ‘thebain’:ti,ab OR ‘paramorphine’:ti,ab) AND (‘epidemiology’/exp/mj OR ‘adverse drug reaction’/exp/mj OR ‘volunteer’/exp OR ‘human experiment’/exp OR ‘epidemiolog*’:ti,ab OR ‘case control’:ti,ab OR ‘case stud*’:ti,ab OR ‘cohort’:ti,ab OR ‘cross sectional’:ti,ab OR ‘adverse effect*’:ti,ab OR ‘biomarker*’:ti,ab OR ‘human volunteers’:ti,ab OR ‘human stud*’:ti,ab))
Time span: all years
Database: all
‘thebaine’/mj/dd_ct OR ((‘thebaine’:ti,ab OR ‘thebain’:ti,ab OR ‘paramorphine’:ti,ab) AND (‘clinical study’/exp/mj OR ‘clinical stud*’:ti,ab OR ‘clinical trial*’:ti,ab OR ‘case report*’:ti,ab)) AND [2011‐2017]/py
Time span: all years
Database: all
B. PubMed
“Thebaine/adverse effects”[Mesh] OR “Thebaine/pharmacokinetics”[Mesh] OR “Thebaine/pharmacology”[Mesh] OR ((thebaine[tiab] OR thebain[tiab] OR paramorphine[tiab] OR 115‐37‐7[rn]) AND (“Epidemiologic Studies”[Mesh] OR “Drug‐Related Side Effects and Adverse Reactions”[Mesh] OR “Volunteers”[Mesh] OR “Human Experimentation”[Mesh] OR epidemiolog*[tiab] OR “case control”[tiab] OR case stud*[tiab] OR cohort[tiab] OR “cross sectional”[tiab] OR adverse effect*[tiab] OR “human volunteers”[tiab] or human stud*[tiab]))
Time span: all years
(thebaine[tiab] OR thebain[tiab] OR paramorphine[tiab] OR 115‐37‐7[rn]) AND (“Clinical Study” [Publication Type] OR “Clinical Studies as Topic”[Mesh] OR “Case Reports” [Publication Type] OR clinical stud*[tiab] OR clinical trial*[tiab] OR case report*[tiab])
Time span: all years
C. Web of Science
TOPIC: (thebaine OR thebain OR paramorphine) AND TOPIC: (epidemiolog* OR “case control” OR case stud* OR cohort OR “cross sectional” OR adverse effect* OR “human volunteers” or human stud*)
Time span: all years
Database: all
TOPIC: (thebaine OR thebain OR paramorphine) AND TOPIC: (clinical stud* OR clinical trial* OR case report*)
Time span: all years
Database: all
All the retrieved references were uploaded in Endnote and screened for relevance.
C.2.3.4. Receptor binding
Searches in PubMed and Web of Science were conducted to identify papers on receptor binding by using the following search strings.
A. PubMed
thebaine and receptor
Time span: all years
B. Web of Science
TOPIC: (thebaine and receptor)
Time span: all years
Database: all
All the retrieved references in PubMed (50) and Web of Science (73) were uploaded in Endnote and, after removal of the duplicates, 103 references were screened for relevance.
C.2.4. Search for papers regarding oripavine
C.2.4.1. Toxicity
Searches in Embase, PubMed and Web of Science were conducted to identify papers on toxicity (in vitro and in vivo) by using the following search strings.
A. Embase
‘oripavine’/mj/dd_to OR ((‘oripavine’:ti,ab) AND (‘toxicity’/exp/mj OR ‘toxicity testing’/exp/mj OR ‘toxicity assay’/exp/mj OR ‘toxic substance’/exp/mj OR carcinogen*:ti,ab OR cardiotox*:ti,ab OR cytotox*:ti,ab OR ‘endocrine toxic*’:ti,ab OR ‘endocrine disruptor*’:ti,ab OR genotox*:ti,ab OR hemotoxic*:ti,ab OR hematotoxic*:ti,ab OR hepatotox*:ti,ab OR immunotox*:ti,ab OR ‘liver toxic*’:ti,ab OR mutagen*:ti,ab OR nephrotox*:ti,ab OR neurotox*:ti,ab OR reprotox*:ti,ab OR terato*:ti,ab OR toxic*:ti,ab OR ‘toxic effect*’:ti,ab OR tumorigen*:ti,ab OR ‘in vivo’:ti,ab OR ‘in vitro’:ti,ab))
Time span: all years
Database: all
B. PubMed
(“oripavine”[Supplementary Concept] OR “oripavine”[tiab] OR 467‐04‐9[rn]) AND (“Toxic Actions”[Mesh] OR “Toxicity Tests”[Mesh] OR carcinogen*[tiab] OR cardiotox*[tiab] OR cytotox*[tiab] OR endocrine toxic*[tiab] OR endocrine disruptor*[tiab] OR genotox*[tiab] OR hemotoxic*[tiab] OR hematotoxic*[tiab]OR hepatotox*[tiab]OR immunotox*[tiab] OR liver toxic*[tiab] OR mutagen*[tiab] OR nephrotox*[tiab] OR neurotox*[tiab] OR reprotox*[tiab] OR terato*[tiab] OR toxic*[tiab] OR toxic effect*[tiab] OR tumorigen*[tiab] OR “in vivo”[tiab] OR “in vitro”[tiab])
Time span: all years
C. Web of Science
TOPIC: (oripavine) AND TOPIC: (carcinogen* OR cardiotox* OR cytotox* OR endocrine toxic* OR endocrine disruptor* OR genotox* OR hemotoxic* OR hematotoxic*OR hepatotox*OR immunotox* OR liver toxic* OR mutagen* OR nephrotox* OR neurotox* OR reprotox* OR terato* OR toxic* OR toxic effect* OR tumorigen* OR “in vivo” OR “in vitro”)
Time span: all years
Database: all
C.2.4.2. Metabolism and kinetics
Searches in Embase, PubMed and Web of Science were conducted to identify papers on metabolism and kinetics by using the following search strings.
A. Embase
‘oripavine’/mj/dd_ae,dd_to OR ((‘oripavine’:ti,ab) AND (‘biological marker’/exp/mj OR ‘drug receptor’/exp/mj OR ‘metabolism’/exp/mj OR metabolism:ti,ab OR kinetic*:ti,ab OR toxicokinetic*:ti,ab OR pharmacokinetic*:ti,ab OR absorption:ti,ab OR distribution:ti,ab OR excretion:ti,ab OR mechanism*:ti,ab OR mode:ti,ab OR action:ti,ab OR biomarker*:ti,ab OR biotransformation:ti,ab OR pharmacology:ti,ab OR receptor*:ti,ab OR “mu receptor”:ti,ab OR “opioid receptor”:ti,ab))
Time span: all years
Database: all
B. PubMed
(“oripavine”[Supplementary Concept] OR “oripavine”[tiab] OR 467‐04‐9[rn]) AND (“Biomarkers”[Mesh] OR “Metabolism”[Mesh] OR “Receptors, Drug”[Mesh] OR “Receptors, Opioid, mu”[Mesh] OR “Pharmacology”[Mesh] OR metabolism[tiab] OR kinetic*[tiab] OR toxicokinetic*[tiab] OR pharmacokinetic*[tiab] OR absorption[tiab] OR distribution[tiab] OR excretion[tiab] OR mechanism*[tiab] OR mode[tiab] OR action[tiab] OR biomarker*[tiab] OR biotransformation[tiab] OR pharmacology[tiab] OR receptor*[tiab] OR “mu receptor”[tiab] OR “opioid receptor”[tiab])
Time span: all years
C. Web of Science
TOPIC: (oripavine) AND TOPIC: (metabolism OR kinetic* OR toxicokinetic* OR pharmacokinetic* OR absorption OR distribution OR excretion OR mechanism* OR mode OR action OR biomarker* OR biotransformation OR pharmacology OR receptor* OR “mu receptor” OR “opioid receptor”)
Time span: all years
Database: all
C.2.4.3. Human data
Searches in Embase, PubMed and Web of Science were conducted to identify papers on human data by using the following search strings.
A. Embase
‘oripavine’/mj/dd_ae OR ((‘oripavine’:ti,ab) AND (‘epidemiology’/exp/mj OR ‘adverse drug reaction’/exp/mj OR ‘volunteer’/exp OR ‘human experiment’/exp OR ‘epidemiolog*’:ti,ab OR ‘case control’:ti,ab OR ‘case stud*’:ti,ab OR ‘cohort’:ti,ab OR ‘cross sectional’:ti,ab OR ‘adverse effect*’:ti,ab OR ‘biomarker*’:ti,ab OR ‘human volunteers’:ti,ab OR ‘human stud*’:ti,ab))
Time span: all years
Database: all
‘oripavine’/mj/dd_ct OR (‘oripavine:ti,ab AND (‘clinical study’/exp/mj OR ‘clinical stud*’:ti,ab OR ‘clinical trial*’:ti,ab OR ‘case report*’:ti,ab))
Time span: all years
Database: all
B. PubMed
(“oripavine”[Supplementary Concept] OR “oripavine”[tiab] OR 467‐04‐9[rn]) AND (“Epidemiologic Studies”[Mesh] OR “Drug‐Related Side Effects and Adverse Reactions”[Mesh] OR “Volunteers”[Mesh] OR “Human Experimentation”[Mesh] OR epidemiolog*[tiab] OR “case control”[tiab] OR case stud*[tiab] OR cohort[tiab] OR “cross sectional”[tiab] OR adverse effect*[tiab] OR “human volunteers”[tiab] or human stud*[tiab])
Time span: all years
(“oripavine”[Supplementary Concept] OR “oripavine”[tiab] OR 467‐04‐9[rn]) AND (“Clinical Study” [Publication Type] OR “Clinical Studies as Topic”[Mesh] OR “Case Reports” [Publication Type] OR clinical stud*[tiab] OR clinical trial*[tiab] OR case report*[tiab])
Time span: all years
C. Web of Science
TOPIC: (oripavine) AND TOPIC: (epidemiolog* OR “case control” OR case stud* OR cohort OR “cross sectional” OR adverse effect* OR “human volunteers” or human stud*)
Time span: all years
Database: all
TOPIC: (oripavine) AND TOPIC: (clinical stud* OR clinical trial* OR case report*)
Time span: all years
Database: all
All the retrieved references were uploaded in Endnote and were screened for relevance.
C.2.5. Search for papers regarding noscapine
C.2.5.1. Toxicity
Searches in Embase, PubMed and Web of Science were conducted to identify papers on toxicity (in vitro and in vivo) by using the following search strings.
A. Embase
‘noscapine’/mj/dd_to OR ((‘noscapine’:ti,ab OR ‘noscapin’:ti,ab OR ‘narcotine’:ti,ab) AND (‘toxicity’/exp/mj OR ‘toxicity testing’/exp/mj OR ‘toxicity assay’/exp/mj OR ‘toxic substance’/exp/mj OR carcinogen*:ti,ab OR cardiotox*:ti,ab OR cytotox*:ti,ab OR ‘endocrine toxic*’:ti,ab OR ‘endocrine disruptor*’:ti,ab OR genotox*:ti,ab OR hemotoxic*:ti,ab OR hematotoxic*:ti,ab OR hepatotox*:ti,ab OR immunotox*:ti,ab OR ‘liver toxic*’:ti,ab OR mutagen*:ti,ab OR nephrotox*:ti,ab OR neurotox*:ti,ab OR reprotox*:ti,ab OR terato*:ti,ab OR toxic*:ti,ab OR ‘toxic effect*’:ti,ab OR tumorigen*:ti,ab OR ‘in vivo’:ti,ab OR ‘in vitro’:ti,ab))
Time span: 2011–2017
Database: all
B. PubMed
“Noscapine/toxicity”[Mesh] OR ((“noscapine”[tiab] OR “narcotine”[tiab] OR “noscapin”[tiab] OR 128‐62‐1[rn]) AND (“Toxic Actions”[Mesh] OR “Toxicity Tests”[Mesh] OR carcinogen*[tiab] OR cardiotox*[tiab] OR cytotox*[tiab] OR endocrine toxic*[tiab] OR endocrine disruptor*[tiab] OR genotox*[tiab] OR hemotoxic*[tiab] OR hematotoxic*[tiab]OR hepatotox*[tiab]OR immunotox*[tiab] OR liver toxic*[tiab] OR mutagen*[tiab] OR nephrotox*[tiab] OR neurotox*[tiab] OR reprotox*[tiab] OR terato*[tiab] OR toxic*[tiab] OR toxic effect*[tiab] OR tumorigen*[tiab] OR “in vivo”[tiab] OR “in vitro”[tiab]))
Time span: 2011–2017
C. Web of Science
TOPIC: (Noscapine OR Noscapin OR Narcotine) AND TOPIC: (carcinogen* OR cardiotox* OR cytotox* OR endocrine toxic* OR endocrine disruptor* OR genotox* OR hemotoxic* OR hematotoxic* OR hepatotox*OR immunotox* OR liver toxic* OR mutagen* OR nephrotox* OR neurotox* OR reprotox* OR terato* OR toxic* OR toxic effect* OR tumorigen* OR “in vivo” OR “in vitro”)
Time span: 2011–2017
Database: all
C.2.5.2. Metabolism and kinetics
Searches in Embase, PubMed and Web of Science were conducted to identify papers on metabolism and kinetics by using the following search strings.
A. Embase
‘noscapine’/mj/dd_pk,dd_pd OR ((‘noscapine’:ti,ab OR ‘noscapin’:ti,ab OR ‘narcotine’:ti,ab) AND (‘biological marker’/exp/mj OR ‘drug receptor’/exp/mj OR ‘metabolism’/exp/mj OR metabolism:ti,ab OR kinetic*:ti,ab OR toxicokinetic*:ti,ab OR pharmacokinetic*:ti,ab OR absorption:ti,ab OR distribution:ti,ab OR excretion:ti,ab OR mechanism*:ti,ab OR mode:ti,ab OR action:ti,ab OR biomarker*:ti,ab OR biotransformation:ti,ab OR pharmacology:ti,ab OR receptor*:ti,ab OR “mu receptor”:ti,ab OR “opioid receptor”:ti,ab))
Time span: 2011–2017
Database: all
B. PubMed
“Noscapine/metabolism”[Mesh] OR “Noscapine/pharmacokinetics”[Mesh] OR “Noscapine/pharmacology”[Mesh] OR ((“noscapine”[tiab] OR “narcotine”[tiab] OR “noscapin”[tiab] OR 128‐62‐1[rn]) AND (“Biomarkers”[Mesh] OR “Metabolism”[Mesh] OR “Receptors, Drug”[Mesh] OR “Receptors, Opioid, mu”[Mesh] OR “Pharmacology”[Mesh] OR metabolism[tiab] OR kinetic*[tiab] OR toxicokinetic*[tiab] OR pharmacokinetic*[tiab] OR absorption[tiab] OR distribution[tiab] OR excretion[tiab] OR mechanism*[tiab] OR mode[tiab] OR action[tiab] OR biomarker*[tiab] OR biotransformation[tiab] OR pharmacology[tiab] OR receptor*[tiab] OR “mu receptor”[tiab] OR “opioid receptor”[tiab]))
Time span: 2011–2017
C. Web of Science
TOPIC: (Noscapine OR Noscapin OR Narcotine) AND TOPIC: (metabolism OR kinetic* OR toxicokinetic* OR pharmacokinetic* OR absorption OR distribution OR excretion OR mechanism* OR mode OR action OR biomarker* OR biotransformation OR pharmacology OR receptor* OR “mu receptor” OR “opioid receptor”)
Time span: 2011–2017
Database: all
C.2.5.3. Human data
Searches in Embase, PubMed and Web of Science were conducted to identify papers on human data by using the following search strings.
A. Embase
‘noscapine’/mj/dd_ae OR ((‘noscapine’:ti,ab OR ‘noscapin’:ti,ab OR ‘narcotine’:ti,ab) AND (‘epidemiology’/exp/mj OR ‘adverse drug reaction’/exp/mj OR ‘volunteer’/exp OR ‘human experiment’/exp OR ‘epidemiolog*’:ti,ab OR ‘case control’:ti,ab OR ‘case stud*’:ti,ab OR ‘cohort’:ti,ab OR ‘cross sectional’:ti,ab OR ‘adverse effect*’:ti,ab OR ‘biomarker*’:ti,ab OR ‘human volunteers’:ti,ab OR ‘human stud*’:ti,ab))
Time span: 2011–2017
Database: all
‘noscapine’/mj/dd_ct OR ‘noscapine’:ti,ab OR ‘noscapin’:ti,ab OR ‘narcotine’:ti,ab AND (‘clinical study’/exp/mj OR ‘clinical stud*’:ti,ab OR ‘clinical trial*’:ti,ab OR ‘case report*’:ti,ab) AND [2011‐2017]/py
Time span: 2011–2017
Database: all
B. PubMed
“Noscapine/adverse effects”[Mesh] OR ((“noscapine”[tiab] OR “narcotine”[tiab] OR “noscapin”[tiab] OR 128‐62‐1[rn]) AND (“Epidemiologic Studies”[Mesh] OR “Drug‐Related Side Effects and Adverse Reactions”[Mesh] OR “Volunteers”[Mesh] OR “Human Experimentation”[Mesh] OR epidemiolog*[tiab] OR “case control”[tiab] OR case stud*[tiab] OR cohort[tiab] OR “cross sectional”[tiab] OR adverse effect*[tiab] OR “human volunteers”[tiab] or human stud*[tiab]))
Time span: 2011–2017
(“noscapine”[tiab] OR “narcotine”[tiab] OR “noscapin”[tiab] OR 128‐62‐1[rn]) AND (“Clinical Study” [Publication Type] OR “Clinical Studies as Topic”[Mesh] OR “Case Reports” [Publication Type] OR clinical stud*[tiab] OR clinical trial*[tiab] OR case report*[tiab])
Time span: 2011–2017
C. Web of Science
TOPIC: (Noscapine OR Noscapin OR Narcotine) AND TOPIC: (epidemiolog* OR “case control” OR case stud* OR cohort OR “cross sectional” OR adverse effect* OR “human volunteers” or human stud*)
Time span: 2011–2017
Database: all
TOPIC: (Noscapine OR Noscapin OR Narcotine) AND TOPIC: (clinical stud* OR clinical trial* OR case report*)
Time span: 2011–2017
Database: all
All the retrieved references were uploaded in Endnote and screened for relevance.
C.2.6. Search for papers regarding papaverine
C.2.6.1. Toxicity
Searches in Embase, PubMed and Web of Science were conducted to identify papers on toxicity (in vitro and in vivo) by using the following search strings.
A. Embase
‘papaverine’/mj/dd_to OR ((‘papaverine’:ti,ab OR ‘papaverin’:ti,ab) AND (‘toxicity’/exp/mj OR ‘toxicity testing’/exp/mj OR ‘toxicity assay’/exp/mj OR ‘toxic substance’/exp/mj OR carcinogen*:ti,ab OR cardiotox*:ti,ab OR cytotox*:ti,ab OR ‘endocrine toxic*’:ti,ab OR ‘endocrine disruptor*’:ti,ab OR genotox*:ti,ab OR hemotoxic*:ti,ab OR hematotoxic*:ti,ab OR hepatotox*:ti,ab OR immunotox*:ti,ab OR ‘liver toxic*’:ti,ab OR mutagen*:ti,ab OR nephrotox*:ti,ab OR neurotox*:ti,ab OR reprotox*:ti,ab OR terato*:ti,ab OR toxic*:ti,ab OR ‘toxic effect*’:ti,ab OR tumorigen*:ti,ab OR ‘in vivo’:ti,ab OR ‘in vitro’:ti,ab))
Time span: 2011–2017
Database: all
B. PubMed
“Papaverine/toxicity”[Mesh] OR ((papaverine[tiab] OR papaverin[tiab] OR 58‐74‐2[rn]) AND (“Toxic Actions”[Mesh] OR “Toxicity Tests”[Mesh] OR carcinogen*[tiab] OR cardiotox*[tiab] OR cytotox*[tiab] OR endocrine toxic*[tiab] OR endocrine disruptor*[tiab] OR genotox*[tiab] OR hemotoxic*[tiab] OR hematotoxic*[tiab]OR hepatotox*[tiab]OR immunotox*[tiab] OR liver toxic*[tiab] OR mutagen*[tiab] OR nephrotox*[tiab] OR neurotox*[tiab] OR reprotox*[tiab] OR terato*[tiab] OR toxic*[tiab] OR toxic effect*[tiab] OR tumorigen*[tiab] OR “in vivo”[tiab] OR “in vitro”[tiab]))
Time span: 2011–2017
C. Web of Science
TOPIC: (Papaverine OR Papaverin) AND TOPIC: (carcinogen* OR cardiotox* OR cytotox* OR endocrine toxic* OR endocrine disruptor* OR genotox* OR hemotoxic* OR hematotoxic* OR hepatotox*OR immunotox* OR liver toxic* OR mutagen* OR nephrotox* OR neurotox* OR reprotox* OR terato* OR toxic* OR toxic effect* OR tumorigen* OR “in vivo” OR “in vitro”)
Time span: 2011–2017
Database: all
C.2.6.2. Metabolism and kinetics
Searches in Embase, PubMed and Web of Science were conducted to identify papers on metabolism and kinetics by using the following search strings.
A. Embase
‘papaverine’/mj/dd_pk,dd_pd OR ((‘papaverine’:ti,ab OR ‘papaverin’:ti,ab) AND (‘biological marker’/exp/mj OR ‘drug receptor’/exp/mj OR ‘metabolism’/exp/mj OR metabolism:ti,ab OR kinetic*:ti,ab OR toxicokinetic*:ti,ab OR pharmacokinetic*:ti,ab OR absorption:ti,ab OR distribution:ti,ab OR excretion:ti,ab OR mechanism*:ti,ab OR mode:ti,ab OR action:ti,ab OR biomarker*:ti,ab OR biotransformation:ti,ab OR pharmacology:ti,ab OR receptor*:ti,ab OR “mu receptor”:ti,ab OR “opioid receptor”:ti,ab))
Time span: 2011–2017
Database: all
B. PubMed
“Papaverine/metabolism”[Mesh] OR “Papaverine/pharmacokinetics”[Mesh] OR “Papaverine/pharmacology”[Mesh] OR ((papaverine[tiab] OR papaverin[tiab] OR 58‐74‐2[rn]) AND (“Biomarkers”[Mesh] OR “Metabolism”[Mesh] OR “Receptors, Drug”[Mesh] OR “Receptors, Opioid, mu”[Mesh] OR “Pharmacology”[Mesh] OR metabolism[tiab] OR kinetic*[tiab] OR toxicokinetic*[tiab] OR pharmacokinetic*[tiab] OR absorption[tiab] OR distribution[tiab] OR excretion[tiab] OR mechanism*[tiab] OR mode[tiab] OR action[tiab] OR biomarker*[tiab] OR biotransformation[tiab] OR pharmacology[tiab] OR receptor*[tiab] OR “mu receptor”[tiab] OR “opioid receptor”[tiab]))
Time span: 2011–2017
C. Web of Science
TOPIC: (Papaverine OR Papaverin) AND TOPIC: (metabolism OR kinetic* OR toxicokinetic* OR pharmacokinetic* OR absorption OR distribution OR excretion OR mechanism* OR mode OR action OR biomarker* OR biotransformation OR pharmacology OR receptor* OR “mu receptor” OR “opioid receptor”)
Time span: 2011–2017
Database: all
C.2.6.3. Human data
Searches in Embase, PubMed and Web of Science were conducted to identify papers on human data by using the following search strings.
A. Embase
‘papaverine’/mj/dd_ae OR ((‘papaverine’:ti,ab OR ‘papaverin’:ti,ab) AND (‘epidemiology’/exp/mj OR ‘adverse drug reaction’/exp/mj OR ‘volunteer’/exp OR ‘human experiment’/exp OR ‘epidemiolog*’:ti,ab OR ‘case control’:ti,ab OR ‘case stud*’:ti,ab OR ‘cohort’:ti,ab OR ‘cross sectional’:ti,ab OR ‘adverse effect*’:ti,ab OR ‘biomarker*’:ti,ab OR ‘human volunteers’:ti,ab OR ‘human stud*’:ti,ab))
Time span: 2011–2017
Database: all
‘papaverine’/mj/dd_ct OR ((‘papaverine’:ti,ab OR ‘papaverin’:ti,ab) AND (‘clinical study’/exp/mj OR ‘clinical stud*’:ti,ab OR ‘clinical trial*’:ti,ab OR ‘case report*’:ti,ab)) AND [2011‐2017]/py
Time span: 2011–2017
B. PubMed
“Papaverine/adverse effects”[Mesh] OR ((papaverine[tiab] OR papaverin[tiab] OR 58‐74‐2[rn]) AND (“Epidemiologic Studies”[Mesh] OR “Drug‐Related Side Effects and Adverse Reactions”[Mesh] OR “Volunteers”[Mesh] OR “Human Experimentation”[Mesh] OR epidemiolog*[tiab] OR “case control”[tiab] OR case stud*[tiab] OR cohort[tiab] OR “cross sectional”[tiab] OR adverse effect*[tiab] OR “human volunteers”[tiab] or human stud*[tiab]))
Time span: 2011–2017
(papaverine[tiab] OR papaverin[tiab] OR 58‐74‐2[rn]) AND (“Clinical Study” Publication Type] OR “Clinical Studies as Topic”[Mesh] OR “Case Reports” [Publication Type] OR clinical stud*[tiab] OR clinical trial*[tiab] OR case report*[tiab])
Time span: 2011–2017
C. Web of Science
TOPIC: (Papaverine OR Papaverin) AND TOPIC: (epidemiolog* OR “case control” OR case stud* OR cohort OR “cross sectional” OR adverse effect* OR “human volunteers” or human stud*)
Time span: 2011–2017
Database: all
TOPIC: (Papaverine OR Papaverin) AND TOPIC: (clinical stud* OR clinical trial* OR case report*)
Time span: 2011–2017
Database: all
All the retrieved references were uploaded in Endnote and screened for relevance.
C.2.7. Search for papers regarding poppy seeds
C.2.7.1. Human data
Searches in Embase, PubMed and Web of Science were conducted to identify papers on adverse effects in humans related to the ingestion of poppy seed using the following search strings:
A. Embase
‘poppy seed’/exp OR ‘poppy seed*’:ti,ab AND (‘clinical study’/exp/mj OR ‘clinical stud*’:ti,ab OR ‘clinical trial*’:ti,ab OR ‘case report*’:ti,ab OR ‘case stud*’:ti,ab OR ‘intoxication’/exp/mj OR ‘intoxication*:ti,ab’ OR ‘adverse effect*’:ti,ab OR ‘human experiment’/exp OR ‘human stud*’:ti,ab OR ‘volunteer’/exp OR ‘human volunteer*’:ti,ab) AND [2011‐2017]/py
Time span: 2011–2017
Database: all
B. PubMed
poppy seed*[tiab] AND (“Clinical Study” [Publication Type] OR “Clinical Studies as Topic”[Mesh] OR “Case Reports” [Publication Type] OR clinical stud*[tiab] OR clinical trial*[tiab] OR case report*[tiab] OR case stud*[tiab] OR “Poisoning”[Mesh] OR intoxication*[tiab] OR adverse effect*[tiab] OR “Human Experimentation”[Mesh] OR human stud*[tiab] OR “Volunteers”[Mesh] OR “human volunteer*”[tiab])
Time span: 2011–2017
C. Web of Science
TOPIC: (poppy seed*) AND TOPIC: (clinical stud* OR clinical trial* OR case report* OR case stud* OR adverse effect* OR intoxication* OR poisoning* OR human stud* OR human volunteer*)
Time span: 2011–2017
Database: all
All the retrieved references were uploaded in Endnote and screened for relevance.
C.3. Exclusion criteria for abstracts
The titles and abstracts of the references retrieved from the literature search described in Section C.2 were screened to identify the relevant papers. Papers on the following subjects were excluded:
Papers related to the use of analgesic opioids in the clinical practice and/or in specific category of patients (e.g. cancer patients) not reporting adverse effects (except reviews);
Papers related to opioids sparing effects and rotation in clinical practice (i.e. the strategy applied during opioid therapy for pain treatment that refers to a switch from one opioid to another in an effort to improve clinical outcomes);
Papers related to the pattern of prescription and use of opioids in clinical practice and/or self‐medication;
Papers related to the risk of fall injuries after consumption of analgesic opioids;
Papers related to the development of vaccines against drugs of abuse (including opioids);
Papers related to the detection and/or distribution of opioids in post‐mortem tissue/organs;
Papers describing the analgesic activity and/or adverse effects of opium alkaloids derivatives;
Studies reporting adverse effects in opium smokers (no clear information about the composition of the substances smoked and therefore not useful for identification reference point);
Papers related to repeated use and/or exposure to high doses of opium alkaloids, i.e. misuse, abuse, hyperalgesia and withdrawal syndrome;
Human studies in which morphine was administered post‐operatively for pain control (with the exception of those explicitly reporting opioid‐related adverse effects);
Studies evaluating the effects of morphine on cancer growth/recurrence (except reviews);
Human studies in which opium alkaloids were not administered via oral route;
Toxicity studies in experimental animals in which the route of exposure was not oral (except for acute toxicity, immunotoxicity and genotoxicity studies);
Studies in experimental animals evaluating the molecular mechanism of opioid analgesic effects, tolerance and/or withdrawal syndrome (except studies related to the pharmacology of the opioid receptor).
C.4. EFSA guidance documents applied for the risk assessment
The following EFSA guidance documents pertaining to risk assessment were followed for the development of the risk assessment:
EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA Journal 2005;3(10):282, 31 pp. https://doi.org/10.2903/j.efsa.2005.282
EFSA (European Food Safety Authority), 2006. Guidance of the Scientific Committee on a request from EFSA related to Uncertainties in Dietary Exposure Assessment. EFSA Journal, 4(12):438, 54 pp. https://doi.org/10.2903/j.efsa.2007.438
EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. The EFSA Journal 2009;6(9):1051, 22 pp. https://doi.org/10.2903/j.efsa.2009.1051
EFSA (European Food Safety Authority), 2010. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. https://doi.org/10.2903/j.efsa.2010.1457
EFSA (European Food Safety Authority), 2010. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. https://doi.org/10.2903/j.efsa.2010.1557
EFSA (European Food Safety Authority), 2011. Overview of the procedures currently used at EFSA for the assessment of dietary exposure to different chemical substances. EFSA Journal 2011;9(12):2490, 33 pp. https://doi.org/10.2903/j.efsa.2011.2490
EFSA (European Food Safety Authority), 2011. Use of the EFSA Comprehensive European Food Consumption Database in Intakes Assessment. EFSA Journal 2011;9(3):2097, 34 pp. https://doi.org/10.2903/j.efsa.2011.2097
EFSA Scientific Committee, 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. https://doi.org/10.2903/j.efsa.2011.2379
EFSA Scientific Committee, 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. https://doi.org/10.2903/j.efsa.2012.2579
EFSA Scientific Committee, 2012. Scientific Opinion on Risk Assessment Terminology. EFSA Journal 2012;10(5):2664, 43 pp. https://doi.org/10.2903/j.efsa.2012.2664
EFSA Scientific Committee, Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry QM, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne J‐L, Fernandez Dumont A, Hempen M, Valtueña Martínez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M, 2017. Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. https://doi.org/10.2903/j.efsa.2017.4971
EFSA Scientific Committee, Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Bresson J‐L, Griffin J, Hougaard Benekou S, van Loveren H, Luttik R, Messean A, Penninks A, Ru G, Stegeman JA, van der Werf W, Westendorf J, Woutersen RA, Barizzone F, Bottex B, Lanzoni A, Georgiadis N and Alexander J, 2017. Guidance on the assessment of the biological relevance of data in scientific assessments. EFSA Journal 2017;15(8):4970, 73 pp. https://doi.org/10.2903/j.efsa.2017.4970
Appendix D – Detailed description of studies on mode of action
D.1. Apoptosis
A number of in vivo studies have reported the occurrence of apoptotic cell death in the nervous system upon administration of morphine via different routes.
Intrathecal infusion of 20 μg twice daily for 7 days induces apoptosis in rat spinal cord with increased caspase 3, Bax, decreased Bcl‐2. NMDA receptors were also involved suggesting that glutamate may have a role (Mao et al., 2002). Apoptosis was also induced in rat spinal cord via a caspase‐mediated mechanism following intrathecal administration of 10 μg twice daily for 7 days (Lim et al., 2005).
Morphine administered to rats i.p. (4 mg/kg per day for the first 10 days, 8 mg/kg per day between 11 and 20 days, and 12 mg/kg per day between 21 and 30 days) has been shown to induce apoptosis in the parietal, frontal, temporal, occipital, pyriform, entorhinal cortex as well as in the hippocampal CA1, CA2 and CA3 regions, and in the spinal cord (Atici et al., 2004).
Nasiraei‐Moghadam et al. (2010) have shown that exposure to morphine from the first day of gestation to the time of sampling (embryonic day 9.5 and 13.5) increases neuroblast apoptosis as revealed by changes in the expression of Bax, Bcl‐2 and cleaved caspase 3.
The same group evaluated whether morphine‐induced memory deficit in adolescent and adult rats from dams exposed during pregnancy or 1 week postconception is associated to an increased rate of neuronal apoptosis. Immunostainings with antibodies recognising apoptotic proteins such as Bax, Bcl‐2 and cleaved caspase 3 were performed on sections from hippocampi. The Bax/Bcl‐2 ratio, as well as the level of activated caspase 3 were found significantly increased in the hippocampus of both d1–13 and d1–21 adolescent and adult females, but only in d1–21 adolescent male rats (Nasiraei‐Moghadam et al., 2013).
In vitro studies performed on cultures of neurons and microglia exposed to 10‐6 M morphine also showed the occurrence of apoptosis. In contrast, apoptotic cell death was not observed in astrocytes exposed to morphine at a similar concentration (Hu et al., 2002).
D.2. Other effects
Studies aimed at clarifying the molecular mechanisms behind opiate tolerance and dependence have contributed data that may be relevant for understanding morphine mode of action. As reviewed by Ammon‐Treiber and Höllt (2005), several studies focused on gene expression analysis in the brain after administration of morphine s.c. or i.p.
Acute and chronic morphine was reported to induce a rapid and transient increase in the expression of c‐fos and c‐jun in various areas of the brain such as striatum, cerebral cortex and nucleus accumbens. Chang et al. (1988) showed that in rats acute administration of 10 mg/kg bw s.c increases c‐fos mRNA and protein in the caudate/putamen, suggesting that Fos may be involved in the morphine post‐receptor signal transduction.
Morphine hydrochloride (10 mg/kg s.c. for up to 9 days) differentially affects the expression of beta arrestins 1 and 2 in locus coeruleus, cerebral cortex or hippocampus of rats. Arrestins are involved in the regulation of opioid receptor signaling (Fan et al., 2003).
mRNA of fos related antigen family members and AP‐1 DNA binding activity increase in the nucleus accumbens and striatum in response to chronic morphine (1 pellet s.c. containing 75 mg of morphine base for 5 days). These changes may be part of the adaptive response to chronic morphine and also involved in the modification in dendritic spines observed in the nucleus accumbens after morphine (Nye and Nestler, 1996).
CyclicAMP response element binding protein (CREB) increases in the locus coeruleus in response to chronic morphine (pellets with 75 mg morphine base given s.c. for 5 days) and has been suggested to play a role in development of dependence to opiate (Lane‐Ladd et al., 1997).
Morphine hydrochloride (10–40 mg/kg s.c. up to 5 days) increases dynamin mRNA in the caudate putamen via the μ receptors. The increase may be linked to intracellular adaptation following repeated exposure (Noble et al., 2000).
Several receptors are affected by morphine: after 10 mg/kg i.p for 8 days the mRNAs of dopamine receptors D1, D2 and D3 are differentially regulated in the caudate putamen. While D1R mRNA does not change, D2R mRNA decreases about 25% and D3R mRNA increases 85%. These changes probably occur in the attempt to normalise the abnormal DA transmission thus reducing the effect of morphine (Spangler et al., 2003).
Le Greves et al. (1998) have shown that acute administration of 10 mg/kg s.c. morphine chloride modifies the expression of NMDA NR1, 2A and 2B in the hippocampus and hypothalamus already after 4 h. Since this glutamate receptor subtype is involved in the development of dependency to morphine, the results suggest that this effect starts after the first administration of the drug.
Numan et al. (1998) showed that morphine base 75 mg in s.c. pellet for 5 days, differentially affects nerve growth factors, BDNF and NT3 as well as their receptors. In locus coeruleus, which is implicated in opioid physical dependency and withdrawal, BDNF and NT3 expression increases while tjeir receptors trkB and C are not changed. These modifications may play a critical role in the morphine‐induced changes in plasticity in locus coeruleus.
Other gene expression studies have shown that morphine hydrochloride 10 mg/kg up to 14 days modifies the expression of 159 genes in the nucleus accumbens. Molecules involved in neurotransmission, such as DA, neuropeptides (encephalin, dynorphin, CART, substance P are affected by morphine (Spijker et al., 2004). Acute exposure to 10 mg/kg morphine i.p. alter the expression of genes related to mitochondrial function, cytoskeletal functions, secretory vesicle and cell adhesion, and regulatory proteins (Loguinov et al., 2001).
Several in vitro studies have addressed the effects of very low concentrations of morphine. 10‐14 M morphine has neurotrophic effects and increases the length of the longest process of rat spinal and cortical neurons (24% and 18% respectively) (Brailoiu et al., 2004). At higher concentration (10‐6 M), no significant effects were observed.
Electrophysiological studies on dorsal root ganglion neurons showed that extremely low concentrations of morphine (fM‐nM) induced direct excitatory effects, whereas higher concentrations (micromolar) evoked inhibitory effects (Crain and Shen, 2001).
Bandaru et al. (2011) have shown that transgenic mice expressing gp120 (the HIV virus envelope protein) are highly reactive to morphine. After administration via pellets that delivered 5 mg/day morphine for 5 days s.c., there was a decrease in sphingomyelin and accumulation of ceramide in the brain.
Campbell et al. (2015) have shown that morphine (10 mg/kg bw escalating to 30 mg/kg bw s.c. for 5 days) has protective effects against neurotoxicity induced by intracerebral injection of gp120Bal likely by increasing the level of the chemokine CCL5.
Annex A – Occurrence of opium alkaloids
1.
Tables A.1–A.10 of Annex A can be found in the online version of this output (‘Supporting information’ section): https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2018.5243
Description: Supporting tables on the occurrence of opium alkaloids in poppy seeds and poppy seed‐containing food.
Annex B – Consumption data and dietary exposure for humans
1.
Tables B.1–B.9 of Annex B can be found in the online version of this output (‘Supporting information’ section): https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2018.5243
Description: Supporting tables on poppy seed consumption and human dietary exposure.
Supporting information
Occurrence of opium alkaloids
Consumption data and dietary exposure for humans
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Nebbia CS, Oswald IP, Petersen A, Rose M, Roudot A‐C, Schwerdtle T, Vollmer G, Wallace H, Benford D, Calò G, Dahan A, Dusemund B, Mulder P, Németh‐Zámboriné É, Arcella D, Baert K, Cascio C, Levorato S, Schutte M and Vleminckx C, 2018. Update of the Scientific Opinion on opium alkaloids in poppy seeds. EFSA Journal 2018;16(5):5243, 119 pp. 10.2903/j.efsa.2018.5243
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00812
Panel members: Jan Alexander, Lars Barregård, Margherita Bignami, Beat Brüschweiler, Sandra Ceccatelli, Bruce Cottrill, Michael Dinovi, Lutz Edler, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Helle Katrine Knutsen, Carlo Stefano Nebbia, Isabelle P Oswald, Annette Petersen, Martin Rose, Alain‐Claude Roudot, Tanja Schwerdtle, Christiane Vleminckx, Günter Vollmer and Heather Wallace.
Acknowledgements: The CONTAM Panel wishes to thank the hearing experts: Pavel Cihlar, Daniel Doerge and Vaclav Lohr for the support provided to this scientific output. The CONTAM Panel acknowledges all European competent institutions and other stakeholders that provided occurrence data on opium alkaloids in food, and supported the data collection for the Comprehensive European Food Consumption Database.
Adopted: 22 March 2018
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure A.1 in Appendix A: © Elsevier.
Notes
Sources: http://www.commonchemistry.org; https://www.drugbank.ca; https://pubchem.ncbi.nlm.nih.gov; http://www.chemspider.com; https://www.rsc.org/Merck-Index/monograph
Council Regulation (EEC) No 315/93 of 8 February 1993 laying down Community procedures for contaminants in food. OJ L 37, 13.2.1993, p. 1–3.
Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. OJ L 364, 20.12.2006, p. 5–24.
Commission Recommendation of 10 September 2014 on good practices to prevent and to reduce the presence of opium alkaloids in poppy seeds and poppy seed products (2014/662/EU). OJ L 271, 12.9.2014, p. 96–100.
Single Convention on Narcotic Drugs of 1961 as amended by the 1972 Protocol Convention on Psychotropic Substances of 1971 United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 with final acts and resolutions. https://www.unodc.org/documents/commissions/CND/Int_Drug_Control_Conventions/Ebook/The_International_Drug_Control_Conventions_E.pdf
‘The material arising when poppy straw has entered into a process for the concentration of its alkaloids when such material is made available in trade’. This definition was taken from the addendum ST/CND/1/Add.1/Rev.3 ‘Schedules of the Single Convention on Narcotic Drugs of 1961 as amended by the 1972 Protocol as at April 2017’. Available at https://documents-dds-ny.un.org/doc/UNDOC/GEN/V17/032/70/PDF/V1703270.pdf?OpenElement
Commission Recommendation 2014/662/EU of 10 September 2014 on good practices to prevent and to reduce the presence of opium alkaloids in poppy seeds and poppy seed products. OJ L 271, 12.9.2014, p. 96–100.
Regulation No 399/2013 of the Ministry of the Agriculture amending the Regulation of the Ministry of Agriculture No 329/1997 which implements Section 18 (a), (d), (h), (i), (j) and (k) of Act No 110/1997 on foodstuff and tobacco products and on the change and completion of some related laws, for starch and starch products, legumes and oil seeds, as amended by the Regulation No 418/2000.
19/2015 (II.16.) Government Decree on amendment of the Government Decree 162/2003. (X. 16.) on the regulation of cultivation, trade and utilisation of narcotic plants and of the Government Decree 66/2012 (IV.2.) on the regulation of activities with drugs and psychotropic agents and on the official registration and amendments of list of these compounds.
EU Database of registered plant varieties, http://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases/search/public/index.cfm?event=SearchVariety&ctl_type=A&species_id=246&variety_name=&listed_in=0&show_current=on&show_deleted, Accessed 29.3.2017.
OECD List of Varieties eligible for seed certification http://www.oecd.org/tad/code/oecd-list-of-varieties-eligible-for-certification.htm, Accessed 1.1.2018.
Web of Science (WoS), formally ISI Web of Knowledge, Thomson Reuters. Available at: http://thomsonreuters.com/thomson-reuters-web-of-science/
PubMed, Entrez Global Query Cross‐Database Search System, National Center for Biotechnology Information (NCBI), National Library of Medicine (NLM), Department of the National Institutes of Health (NIH), United States Department of Health and Human Services. Available at: http://www.ncbi.nlm.nih.gov/pubmed/
EndNote X5, Thomson Reuters. Available at: http://endnote.com/
However, it was noted that the authors described it as well as ultra‐rapid metabolism in the title of their report (Voronov et al., 2007). In consequence, the report was repeatedly commented (de Leon, 2008; Lerman, 2008; Stamer and Stuber, 2008; de Wildt and Koren, 2008).
Mean body weight for children from 3 to 10 years old (EFSA Scientific Committee, 2012).
The mean body weight of infants from 6 to 12 months old is 8.8 kg and the default body weight for European toddlers (aged 1–3 years) is 12 kg (EFSA Scientific Committee, 2012).
Age class within a country.
Reprinted from Phytochemistry, volume 70, Ziegler J, Facchini PJ, Geissler R, Schmidt J, Ammer C, Kramell R, Voigtläander S,Gesell A, Pienkny S and Brandt W, Evolution of morphine biosynthesis in opium poppy, 1696–1707, 2009, with permission from Elsevier.
OECD List of Varieties eligible for seed certification, http://www.oecd.org/tad/code/oecd-list-of-varieties-eligible-for-certification.htm, Accessed 1.1.2018.
References
- Afzali M, Ghaeli P, Khanavi M, Parsa M, Montazeri H, Ghahremani MH and Ostad SN, 2015. Non‐addictive opium alkaloids selectively induce apoptosis in cancer cells compared to normal cells. DARU Journal of Pharmaceutical Sciences, 23, 16. 10.1186/s40199-015-0101-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AHFS (American Hospital Formulary Service), 2016. Monograph Morphine Sulfate. American Society of Health‐System Pharmacists, Inc., Maryland, USA. Available online: http://www.ahfsdruginformation.com/ [Google Scholar]
- Aitkenhead AR, Vater M, Achola K, Cooper CM and Smith G, 1984. Pharmacokinetics of single‐dose i.v. morphine in normal volunteers and patients with end‐stage renal failure. British Journal of Anaesthesia, 56, 813–819. [DOI] [PubMed] [Google Scholar]
- Aka I, Bernal CJ, Carroll R, Maxwell‐Horn A, Oshikoya KA and Van Driest SL, 2017. Clinical pharmacogenetics of cytochrome P450‐associated drugs in children. Journal of Personalized Medicine, 7, 10.3390/jpm7040014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktories K, Förstermann U, Hofmann F and Starke K, 2009. Allgemeine und spezielle Pharmakologie und Toxikologie, 10th Edition. Urban & Fischer Verlag/Elsevier GmbH, München, Germany. 1248 pp. [Google Scholar]
- Ammon‐Treiber S and Höllt V, 2005. Morphine‐induced Changes of Gene Expression in the Brain. Addiction Biology, 10, 81–89. [DOI] [PubMed] [Google Scholar]
- Anderson BJ and Holford NH, 2009. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metabolism and Pharmacokinetics, 24, 25–36. [DOI] [PubMed] [Google Scholar]
- Anderson PO, Manoguerra AS and Valdes V, 2016. A review of adverse reactions in infants from medications in breastmilk. Clinical Pediatrics, 55, 236–244. 10.1177/0009922815594586 [DOI] [PubMed] [Google Scholar]
- Andresen H and Schmoldt A, 2004. Führt der Verzehr von Mohnsamen zu positiven Opiat‐Befunden in Urin, Blut und Haaren? [Does the consumption of poppy seeds lead to positive opiate‐test results in urine, blood and hair?]. Blutalkohol, 41, 191–202. [Google Scholar]
- Aneja R, Lopus M, Zhou J, Vangapandu SN, Ghaleb A, Yao J, Nettles JH, Zhou B, Gupta M, Panda D, Chandra R and Joshi HC, 2006. Rational design of the microtubule‐targeting anti‐breast cancer drug EM015. Cancer Research, 66, 3782–3791. 10.1158/0008-5472.can-05-2962 [DOI] [PubMed] [Google Scholar]
- Aneja R, Dhiman N, Idnani J, Awasthi A, Arora SK, Chandra R and Joshi HC, 2007. Preclinical pharmacokinetics and bioavailability of noscapine, a tubulin‐binding anticancer agent. Cancer Chemotherapy and Pharmacology, 60, 831–839. 10.1007/s00280-007-0430-y [DOI] [PubMed] [Google Scholar]
- van den Anker JN, 2012. Is it safe to use opioids for obstetric pain while breastfeeding? Journal of Pediatrics, 160, 4–6. 10.1016/j.jpeds.2011.08.066 [DOI] [PubMed] [Google Scholar]
- Arai S and Mukai H, 2011. Erythroderma induced by morphine sulfate. Journal of Dermatology, 38, 288–289. 10.1111/j.1346-8138.2010.01017.x [DOI] [PubMed] [Google Scholar]
- Ariosto D, 2014. Factors contributing to CPOE opiate allergy alert overrides. AMIA Annual Symposium Proceedings, 2014, 256–265. [PMC free article] [PubMed] [Google Scholar]
- Armentia A, Pineda F, Palacios R, Martin‐Gil FJ, Miguel AS, Arenal JJ, Tejedor J and Tef BM, 2014. Utility of opium seed extract tests in preventing hypersensitivity reactions during surgery. Allergologia et Immunopathologia, 42, 56–63. 10.1016/j.aller.2012.08.010 [DOI] [PubMed] [Google Scholar]
- Asanami S and Shimono K, 1997. High body temperature induces micronuclei in mouse bone marrow. Mutation Research, 390, 79–83. [DOI] [PubMed] [Google Scholar]
- Asanami S and Shimono K, 2000. Effects of chemically‐ and environmentally‐induced hypothermia on micronucleus induction in rats. Mutation Research, 471, 81–86. [DOI] [PubMed] [Google Scholar]
- Asiabanha M, Asadikaram G, Rahnema A, Mahmoodi M, Hasanshahi G, Hashemi M and Khaksari M, 2011. Chronic opium treatment can differentially induce brain and liver cells apoptosis in diabetic and non‐diabetic male and female rats. Korean Journal of Physiology and Pharmacology, 15, 327–332. 10.4196/kjpp.2011.15.6.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atici S, Cinel L, Cinel I, Doruk N, Aktekin M, Akca A, Camdeviren H and Oral U, 2004. Opioid neurotoxicity: comparison of morphine and tramadol in an experimental rat model. International Journal of Neurosciences, 114, 1001–1011. [DOI] [PubMed] [Google Scholar]
- Baldo BA and Pham NH, 2012. Histamine‐releasing and allergenic properties of opioid analgesic drugs: resolving the two. Anaesthesia and Intensive Care, 40, 216–235. [DOI] [PubMed] [Google Scholar]
- Balint G, 1971. Thebaine, as therapeutics? Kitasato Archives of Experimental Medicine, 44, 75–78. [PubMed] [Google Scholar]
- Balyan R, Zhang X, Chidambaran V, Martin LJ, Mizuno T, Fukuda T, Vinks AA and Sadhasivam S, 2017. OCT1 genetic variants are associated with postoperative morphine‐related adverse effects in children. Pharmacogenomics, 18, 621–629. 10.2217/pgs-2017-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, Patel N, Ewaleifoh O and Haughey NJ, 2011. A failure to normalize biochemical and metabolic insults during morphine withdrawal disrupts synaptic repair in mice transgenic for HIV‐gp120. Journal of Neuroimmune Pharmacology, 6, 640–649. 10.1007/s11481-011-9289-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandieri E, Romero M, Ripamonti CI, Artioli F, Sichetti D, Fanizza C, Santini D, Cavanna L, Melotti B, Conte PF, Roila F, Cascinu S, Bruera E, Tognoni G and Luppi M, 2016. Randomized trial of low‐dose morphine versus weak opioids in moderate cancer pain. Journal of Clinical Oncology, 34, 436–442. 10.1200/jco.2015.61.0733 [DOI] [PubMed] [Google Scholar]
- Barroso M, Gallardo E, Vieira DN, Queiroz JA and López‐Rivadulla M, 2011. Bioanalytical procedures and recent developments in the determination of opiates/opioids in human biological samples. Analytical and Bioanalytical Chemistry, 400, 1665–1690. 10.1007/s00216-011-4888-4 [DOI] [PubMed] [Google Scholar]
- Barton MK, 2016. First‐line low‐dose morphine is better for the control of moderate cancer pain than weaker opioids. CA: A Cancer Journal for Clinicians, 66, 177–178. 10.3322/caac.21303 [DOI] [PubMed] [Google Scholar]
- Berde CB, Walco GA, Krane EJ, Anand KJS, Aranda JV, Craig KD, Dampier CD, Finkel JC, Grabois M, Johnston C, Lantos J, Lebel A, Maxwell LG, McGrath P, Oberlander TF, Schanberg LE, Stevens B, Taddio A, von Baeyer CL, Yaster M and Zempsky WT, 2012. Pediatric analgesic clinical trial designs, measures, and extrapolation: report of an FDA scientific workshop. Pediatrics, 129, 354–364. 10.1542/peds.2010-3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin CM Jr and van den Anker JN, 2013. Safety during breastfeeding: drugs, foods, environmental chemicals, and maternal infections. Seminars in Fetal and Neonatal Medicine, 18, 13–18. 10.1016/j.siny.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Bernáth J, 1986. Complex physio‐ecological evaluation of the alkaloid formation of the poppy (Papaver somniferum L.). Herba Hungarica, 25, 43–75. [Google Scholar]
- Bernáth J, 1998. Poppy: the genus Papaver . In: Bernáth J (ed.). Harwood Academic Publishers, Amsterdam, The Netherlands, 357 pp. [Google Scholar]
- Bernáth J and Németh E, 2009. Poppy. In: Vollmann J and Rajcan I (eds.). Handbook of Plant Breeding 4: Oil Crops, Springer Science and Business Media, Dordrecht Heidelberg London New York, pp. 449–468. [Google Scholar]
- Bernáth J and Németh E, 2010. A mák. Termesztés és receptek [Poppy ‐ Cultivation and recipes]. CSER, Kiadó, Budapest, Hungary. 88 pp. [Google Scholar]
- BfR (Bundesinstitut für Risikobewertung ‐ Federal Institute for Risk Assessment), 2005. BfR recommends provisional daily upper intake level and a guidance value for morphine in poppy seeds, BfR Health Assessment No. 012/2006, 27 December 2005. Available online: http://www.bfr.bund.de/cm/245/bfr_recommends_provisional_daily_upper_intake_level_and_a_guidance_value_for_morphine_in_poppy_seeds.pdf
- Bhat R, Chari G, Gulati A, Aldana O, Velamati R and Bhargava H, 1990. Pharmacokinetics of a single dose of morphine in preterm infants during the first week of life. Journal of Pediatrics, 117, 477–481. [DOI] [PubMed] [Google Scholar]
- Biesiada J, Chidambaran V, Wagner M, Zhang X, Martin LJ, Meller J and Sadhasivam S, 2014. Genetic risk signatures of opioid‐induced respiratory depression following pediatric tonsillectomy. Pharmacogenomics, 15, 1749–1762. 10.2217/pgs.14.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bio LL, Siu A and Poon CY, 2011. Update on the pharmacologic management of neonatal abstinence syndrome. Journal of Perinatology, 31, 692–701. 10.1038/jp.2011.116 [DOI] [PubMed] [Google Scholar]
- Bjerver K, Jonsson J, Nilsson A, Schuberth J and Schuberth J, 1982. Morphine intake from poppy seed food. Journal of Pharmacy and Pharmacology, 34, 798–801. 10.1111/j.2042-7158.1982.tb06228.x [DOI] [Google Scholar]
- Blaschek W, Ebel S, Hackenthal E, Holzgrabe U, Keller K, Reichling J and Schulz V, 2006. Hager ROM 2006, Hagers Handbuch der Drogen und Arzneistoffe. Springer Medizin Verlag, Heidelberg. [Google Scholar]
- Blaschek W, Ebel S, Hilgenfeldt U, Holzgrabe U, Reichling J, Schulz V, Barthlott W and Höltje H, 2013. ‘Noscapine’ and ‘Opium’ in Hagers Enzyklopädie der Arzneistoffe und Drogen. Wissenschaftliche Verlagsgesellschaft Stuttgart. DrugBase online, updated (Noscapine): 24.01.2013.
- Blaschek W, Ebel S, Hilgenfeldt U, Holzgrabe U, Reichling J, Schulz V and Barthlott W, 2015. Morphine. In: Blaschek W, Hilgenfeldt U, Holzgrabe U, Reichling J and Hagers PR (eds.). Enzyklopädie der Arzneistoffe und Drogen, Springer‐Verlag, Berlin Heidelberg, pp. 20 000. Available online: https://www.springer.com/de/book/9783662487297 [Google Scholar]
- BNF (British National Formulary), 2017. BNF for children 2017–2018. BMJ Group and Pharmaceutical Press, London, UK. 1050 pp. [Google Scholar]
- Bodi CM, Vassoler FM and Byrnes EM, 2016. Adolescent Experience Affects Postnatal ultrasonic Vocalizations and Gene Expression in Future Offspring. Developmental Psychobiology 58, 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG and Lin FT, 1999. Enhanced morphine analgesia in mice lacking beta‐arrestin 2. Science, 286, 2495–2498. [DOI] [PubMed] [Google Scholar]
- Boom M, Niesters M, Sarton E, Aarts L, Smith TW and Dahan A, 2012. Non‐analgesic effects of opioids: opioid‐induced respiratory depression. Current Pharmaceutical Design, 18, 5994–6004. [DOI] [PubMed] [Google Scholar]
- Borst P, de Wolf C and van de Wetering K, 2007. Multidrug resistance‐associated proteins 3, 4, and 5. Pflugers Archiv: European Journal of Physiology, 453, 661–673. 10.1007/s00424-006-0054-9 [DOI] [PubMed] [Google Scholar]
- Bosch ME, Sanchez AR, Rojas FS and Ojeda CB, 2007. Morphine and its metabolites: analytical methodologies for its determination. Journal of Pharmaceutical and Biomedical Analysis, 43, 799–815. 10.1016/j.jpba.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Bracher F, Heisig P, Langguth P, Mutschler E, Rücker G, Schirmeister T, Scriba G, Stahl‐Biskup E and Troschütz R, 2016. Kommentar zum Europäischen Arzneibuch (Commentary to the Pharmacopoeia Europaea), Wissenschaftliche Verlagsgesellschaft, Stuttgart, Germany. CD‐ROM vol 52 (Deutsch).
- Brailoiu E, Hoard J, Brailoiu GC, Chi M, Godbolde R and Dun NJ, 2004. Ultra low concentrations of morphine increase neurite outgrowth in cultured rat spinal cord and cerebral cortical neurons. Neuroscience Letters, 365, 10–13. 10.1016/j.neulet.2004.03.092 [DOI] [PubMed] [Google Scholar]
- Branford R, Droney J and Ross JR, 2012. Opioid genetics: the key to personalized pain control? Clinical Genetics, 82, 301–310. 10.1111/j.1399-0004.2012.01923.x [DOI] [PubMed] [Google Scholar]
- Brennan MJ, 2013. The effect of opioid therapy on endocrine function. American Journal of Medicine, 126, S12–S18. 10.1016/j.amjmed.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Brennan MC and Rayburn WF, 2012. Counseling about risks of congenital anomalies from prescription opioids. Birth Defects Research Part A‐Clinical and Molecular Teratology, 94, 620–625. 10.1002/bdra.23064 [DOI] [PubMed] [Google Scholar]
- Breslow JM, Monroy MA, Daly JM, Meissler JJ, Gaughan J, Adler MW and Eisenstein TK, 2011. Morphine, but not trauma, sensitizes to systemic Acinetobacter baumannii infection. Journal of Neuroimmune Pharmacology, 6, 551–565. 10.1007/s11481-011-9303-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KA, Laferriere A, Lakheeram I and Moss IR, 2006. Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates. Anesthesiology, 105, 665–669. 10.1097/00000542-200610000-00009 [DOI] [PubMed] [Google Scholar]
- Brown JN, Ortiz GM, Angel TE, Jacobs JM, Gritsenko M, Chan EY, Purdy DE, Murnane RD, Larsen K, Palermo RE, Shukla AK, Clauss TR, Katze MG, McCune JM and Smith RD, 2012. Morphine produces immunosuppressive effects in nonhuman primates at the proteomic and cellular levels. Molecular and Cellular Proteomics, 11, 605–618. 10.1074/mcp.M111.016121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucknerova I, Mach M, Dubovicky M and Ujhazy E, 2015. Neonatal withdrawal syndrome and perinatal asphyxia. How to manage the patient? Case Report. Neuroendocrinology Letters, 36 (Suppl 1), 53–56. [PubMed] [Google Scholar]
- Bryant HU, Bernton EW and Holaday JW, 1987. Immunosuppressive effects of chronic morphine treatment in mice. Life Sciences, 41, 1731–1738. [DOI] [PubMed] [Google Scholar]
- Bryant HU, Bernton EW and Holaday JW, 1988. Morphine pellet‐induced immunomodulation in mice: temporal relationships. Journal of Pharmacology and Experimental Therapeutics, 245, 913–920. [PubMed] [Google Scholar]
- BVL (Bundesamt für Verbraucherschutz und Lebensmittelsicherheit), 2007.Berichte zur Lebensmittelsicherheit 2007: Bundesweiter Überwachungsplan 2007. 26‐28.
- Byrnes EM, 2005. Transgenerational consequences of adolescent morphine exposure in female rats: effects on anxiety‐like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl), 182, 537–544. 10.1007/s00213-005-0122-4 [DOI] [PubMed] [Google Scholar]
- Byrnes EM, 2008. Chronic morphine exposure during puberty induces long‐lasting changes in opioid‐related mRNA expression in the mediobasal hypothalamus. Brain Research, 1190, 186–192. 10.1016/j.brainres.2007.11.018 [DOI] [PubMed] [Google Scholar]
- Cabezon‐Gutierrez L, Khosravi‐Shahi P, Custodio‐Cabello S, Muniz‐Gonzalez F, Cano‐Aguirre Mdel P and Alonso‐Viteri S, 2016. Opioids for management of episodic breathlessness or dyspnea in patients with advanced disease. Supportive Care in Cancer, 24, 4045–4055. 10.1007/s00520-016-3316-x [DOI] [PubMed] [Google Scholar]
- Campa D, Gioia A, Tomei A, Poli P and Barale R, 2008. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clinical Pharmacology and Therapeutics, 83, 559–566. 10.1038/sj.clpt.6100385 [DOI] [PubMed] [Google Scholar]
- Campbell G, Nielsen S, Larance B, Bruno R, Mattick R, Hall W, Lintzeris N, Cohen M, Smith K and Degenhardt L, 2015. Pharmaceutical opioid use and dependence among people living with chronic pain: associations observed within the pain and opioids in treatment (POINT) cohort. Pain Medicine, 16, 1745–1758. 10.1111/pme.12773 [DOI] [PubMed] [Google Scholar]
- Caraceni A, Hanks GR, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, De Conno F, Fallon M, Hanna M, Haugen DF, Juhl G, King S, Klepstad P, Laugsand EA, Maltoni M, Mercadante S, Nabal M, Pigni A, Radbruch L, Reid C, Sjogren P, Stone PC, Tassinari D, Zeppetella G and Epcrc and Eapc , 2012. Use of opioid analgesics in the treatment of cancer pain: evidence‐based recommendations from the EAPC. Lancet Oncology, 13, E58–E68. [DOI] [PubMed] [Google Scholar]
- Caraco Y, Sheller J and Wood AJ, 1996a. Pharmacogenetic determination of the effects of codeine and prediction of drug interactions. Journal of Pharmacology and Experimental Therapeutics, 278, 1165–1174. [PubMed] [Google Scholar]
- Caraco Y, Tateishi T, Guengerich FP and Wood AJ, 1996b. Microsomal codeine N‐demethylation: cosegregation with cytochrome P4503A4 activity. Drug Metabolism and Disposition, 24, 761–764. [PubMed] [Google Scholar]
- Caraco Y, Sheller J and Wood AJ, 1999. Impact of ethnic origin and quinidine coadministration on codeine's disposition and pharmacodynamic effects. Journal of Pharmacology and Experimental Therapeutics, 290, 413–422. [PubMed] [Google Scholar]
- Castelli R and Hendrickson R, 2017. Somniferum somnolence: a case of prolonged opioid toxidrome following poppy seed tea ingestion. Clinical Toxicology, 55, 718. [Google Scholar]
- Chang SL, Squinto SP and Harlan RE, 1988. Morphine activation of c‐fos expression in rat brain. Biochemical and Biophysical Research Communications, 157, 698–704. [DOI] [PubMed] [Google Scholar]
- Chaturvedi N, Singh M, Shukla AK, Shasany AK, Shanker K, Lal RK and Khanuja SP, 2014. Comparative analysis of Papaver somniferum genotypes having contrasting latex and alkaloid profiles. Protoplasma, 251, 857–867. 10.1007/s00709-013-0587-7 [DOI] [PubMed] [Google Scholar]
- Chen ZR, Irvine RJ, Somogyi AA and Bochner F, 1991. Mu receptor binding of some commonly used opioids and their metabolites. Life Sciences, 48, 2165–2171. [DOI] [PubMed] [Google Scholar]
- Cheung JR, Dickinson DA, Moss J, Schuler MJ, Spellman RA and Heard PL, 2015. Histone markers identify the mode of action for compounds positive in the TK6 micronucleus assay. Mutation Research: Genetic Toxicology and Environmental Mutagenesis, 777, 7–16. 10.1016/j.mrgentox.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Chidambaran V and Sadhasivam S, 2012. Pediatric acute and surgical pain management: recent advances and future perspectives. International Anesthesiology Clinics, 50, 66–82. 10.1097/AIA.0b013e31826f3284 [DOI] [PubMed] [Google Scholar]
- Chidambaran V, Mavi J, Esslinger H, Pilipenko V, Martin LJ, Zhang K and Sadhasivam S, 2015. Association of OPRM1 A118G variant with risk of morphine‐induced respiratory depression following spine fusion in adolescents. Pharmacogenomics Journal, 15, 255–262. 10.1038/tpj.2014.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidambaran V, Venkatasubramanian R, Zhang X, Martin LJ, Niu J, Mizuno T, Fukuda T, Meller J, Vinks AA and Sadhasivam S, 2017. ABCC3 genetic variants are associated with postoperative morphine‐induced respiratory depression and morphine pharmacokinetics in children. Pharmacogenomics Journal, 17, 162–169. 10.1038/tpj.2015.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Kim S, Lee C, Yang W, Park Y, Choi H, Chung H, Lee D and Hwang BY, 2011. Species identification of Papaver by metabolite profiling. Forensic Science International, 211, 51–60. 10.1016/j.forsciint.2011.04.015 [DOI] [PubMed] [Google Scholar]
- Chougule M, Patel AR, Sachdeva P, Jackson T and Singh M, 2011a. Anticancer activity of noscapine, an opioid alkaloid in combination with cisplatin in human non‐small cell lung cancer. Lung Cancer, 71, 271–282. 10.1016/j.lungcan.2010.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chougule MB, Patel AR, Jackson T and Singh M, 2011b. Antitumor activity of noscapine in combination with Doxorubicin in triple negative breast cancer. PLoS ONE, 6, e17733. 10.1371/journal.pone.0017733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciszkowski C, Madadi P, Phillips MS, Lauwers AE and Koren G, 2009. Codeine, ultrarapid‐metabolism genotype, and postoperative death. New England Journal of Medicine, 361, 827–828. 10.1056/NEJMc0904266 [DOI] [PubMed] [Google Scholar]
- Coffman BL, Rios GR, King CD and Tephly TR, 1997. Human UGT2B7 catalyzes morphine glucuronidation. Drug Metabolism and Disposition, 25, 1–4. [PubMed] [Google Scholar]
- Coluzzi F, Rocco A, Mandatori I and Mattia C, 2012. Non‐analgesic effects of opioids: opioid‐induced nausea and vomiting: mechanisms and strategies for their limitation. Current Pharmaceutical Design, 18, 6043–6052. [DOI] [PubMed] [Google Scholar]
- Correa D, Farney RJ, Chung F, Prasad A, Lam D and Wong J, 2015. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesthesia and Analgesia, 120, 1273–1285. 10.1213/ane.0000000000000672 [DOI] [PubMed] [Google Scholar]
- Couch DB and Sawant SG, 1995. The clastogenicity of morphine sulfate in vivo . Advances in Experimental Medicine and Biology, 373, 123–129. [DOI] [PubMed] [Google Scholar]
- Crain SM and Shen KF, 2001. Acute thermal hyperalgesia elicited by low‐dose morphine in normal mice is blocked by ultra‐low‐dose naltrexone, unmasking potent opioid analgesia. Brain Research, 888, 75–82. [DOI] [PubMed] [Google Scholar]
- Crankshaw DP, 2011. A case report of codeine intoxication following normal panadeine forte dosage. Pain Medicine (United States), 12, 1695–1696. [Google Scholar]
- Crews KR, Gaedigk A, Dunnenberger HM, Klein TE, Shen DD, Callaghan JT, Kharasch ED and Skaar TC, 2012. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clinical Pharmacology and Therapeutics, 91, 321–326. 10.1038/clpt.2011.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, Haidar CE, Shen DD, Callaghan JT, Sadhasivam S, Prows CA, Kharasch ED and Skaar TC, 2014. Clinical Pharmacogenetics Implementation Consortium Guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clinical Pharmacology and Therapeutics, 95, 376–382. 10.1038/clpt.2013.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currow DC, Ekstrom M and Abernethy AP, 2014. Opioids for chronic refractory breathlessness: right patient, right route? Drugs, 74, 1–6. 10.1007/s40265-013-0162-8 [DOI] [PubMed] [Google Scholar]
- Dahan A, 2013. Reply from the authors. British Journal of Anaesthesia, 110, 844–845. [DOI] [PubMed] [Google Scholar]
- Dahan A, van Dorp E, Smith T and Yassen A, 2008. Morphine‐6‐glucuronide (M6G) for postoperative pain relief. European Journal of Pain, 12, 403–411. 10.1016/j.ejpain.2007.07.009 [DOI] [PubMed] [Google Scholar]
- Dahan S, Elefant E, Girard I, Azcona B, Champion V and Mitanchez D, 2011. Neonatal seizures, buprenorphine abstinence syndrome, and substitutive treatment with morphine. Archives de pédiatrie, 18, 287–290. 10.1016/j.arcped.2010.12.003 [DOI] [PubMed] [Google Scholar]
- Dahlström B, Mellstrand T, Lofdahl CG and Johansson M, 1982. Pharmacokinetic properties of noscapine. European Journal of Clinical Pharmacology, 22, 535–539. [DOI] [PubMed] [Google Scholar]
- Dang TT and Facchini PJ, 2014. Cloning and characterization of canadine synthase involved in noscapine biosynthesis in opium poppy. FEBS Letters, 588, 198–204. 10.1016/j.febslet.2013.11.037 [DOI] [PubMed] [Google Scholar]
- Das RK and Swain N, 1982. Mutagenic evaluation of morphine sulphate and pethidine hydrochloride in mice by the micronucleus test. Indian Journal of Medical Research, 75, 112–117. [PubMed] [Google Scholar]
- De Maddalena C, Bellini M, Berra M, Meriggiola MC and Aloisi AM, 2012. Opioid‐induced hypogonadism: why and how to treat it. Pain Physician, 15, ES111–ES118. [PubMed] [Google Scholar]
- DeBono A, Capuano B and Scammells PJ, 2015. Progress toward the development of noscapine and derivatives as anticancer agents. Journal of Medicinal Chemistry, 58, 5699–5727. 10.1021/jm501180v [DOI] [PubMed] [Google Scholar]
- Dehghani L, Sahraei H, Meamar R and Kazemi M, 2013. Time‐dependent effect of oral morphine consumption on the development of cytotrophoblast and syncytiotrophoblast cells of the placental layers during the three different periods of pregnancy in Wistar rats. Clinical and Developmental Immunology, 2013, 974205. 10.1155/2013/974205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RJ, Huybrechts KF, Hernandez‐Diaz S, Mogun H, Patorno E, Kaltenbach K, Kerzner LS and Bateman BT, 2015. Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. BMJ, 350, h2102. 10.1136/bmj.h2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgagné‐Penix I, Farrow SC, Cram D, Nowak J and Facchini PJ, 2012. Integration of deep transcript and targeted metabolite profiles for eight cultivars of opium poppy. Plant Molecular Biology, 79, 295–313. 10.1007/s11103-012-9913-2 [DOI] [PubMed] [Google Scholar]
- Desmeules J, Gascon MP, Dayer P and Magistris M, 1991. Impact of environmental and genetic factors on codeine analgesia. European Journal of Clinical Pharmacology, 41, 23–26. 10.1007/bf00280101 [DOI] [PubMed] [Google Scholar]
- Deverriere G, Carre D, Nae I, Cailliez D and Boulloche J, 2012. Bullous mastocytosis in infancy: a rare presentation. Archives de pédiatrie, 19, 722–725. 10.1016/j.arcped.2012.04.021 [DOI] [PubMed] [Google Scholar]
- Dhingra L, Ahmed E, Shin J, Scharaga E and Magun M, 2015. Cognitive effects and sedation. Pain Medicine, 16, S37–S43. 10.1111/pme.12912 [DOI] [PubMed] [Google Scholar]
- Dillon R, Johnston PC and Daly G, 2013. Codeine‐induced pulmonary oedema (an unusual cause of dyspnoea). QMJ, 106, 289–290. 10.1093/qjmed/hcs022 [DOI] [PubMed] [Google Scholar]
- van Dongen RT, Crul BJ, Koopman‐Kimenai PM and Vree TB, 1994. Morphine and morphine‐glucuronide concentrations in plasma and CSF during long‐term administration of oral morphine. British Journal of Clinical Pharmacology, 38, 271–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnerer J, Oka K, Brossi A, Rice KC and Spector S, 1986. Presence and formation of codeine and morphine in the rat. Proceedings of the National Academy of Sciences of the United States of America, 83, 4566–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley R, Dooley J, Antone I, Guilfoyle J, Gerber‐Finn L, Kakekagumick K, Cromarty H, Hopman W, Muileboom J, Brunton N and Kelly L, 2015. Narcotic tapering in pregnancy using long‐acting morphine: an 18‐month prospective cohort study in Northwestern Ontario. Canadian Family Physician / Médecin de famille canadien, 61, e88–e95. [PMC free article] [PubMed] [Google Scholar]
- Droney JM, Gretton SK, Sato H, Ross JR, Branford R, Welsh KI, Cookson W and Riley J, 2013. Analgesia and central side‐effects: two separate dimensions of morphine response. British Journal of Clinical Pharmacology, 75, 1340–1350. 10.1111/bcp.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzambazovska‐Trajkovska V, Nojkov J, Kartalov A, Kuzmanovska B, Spiroska T, Seljmani R, Trajkovski G, Matevska‐Geshkovska N and Dimovski A, 2016. Association of Single‐Nucleotide Polymorhism C3435T in the ABCB1 Gene with Opioid Sensitivity in Treatment of Postoperative Pain. Prilozi (Makedonska akademija na naukite i umetnostite. Oddelenie za medicinski nauki), 37, 73–80. 10.1515/prilozi-2016-0019 [DOI] [PubMed] [Google Scholar]
- Edginton AN, Schmitt W and Willmann S, 2006. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clinical Pharmacokinetics, 45, 1013–1034. 10.2165/00003088-200645100-00005 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2006. Guidance of the Scientific Committee on a request from EFSA related to Uncertainties in Dietary Exposure Assessment. EFSA Journal 2006;4(12):438, 454 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Guidance of EFSA on the use of the EFSA Comprehensive European Food Consumption Database in Intakes Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011c. Report on the development of a Food Classification and Description System for exposure assessment and guidance on its implementation and use. EFSA Journal 2011;9(12):2489, 84 pp. 10.2903/j.efsa.2011.2489 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. The food classification and description system FoodEx2 (revision 2). EFSA supporting publication 2015:EN‐804, 90 pp. 10.2903/sp.efsa.2015.EN-804 [DOI]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2011. Scientific opinion on the risks for public health related to the presence of opium alkaloids in poppy seeds. EFSA Journal 2011;9(11):2405, 150 pp. 10.2903/j.efsa.2011.2405 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 2532 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- Eissing T, Lippert J and Willmann S, 2012. Pharmacogenomics of codeine, morphine, and morphine‐6‐glucuronide: model‐based analysis of the influence of CYP2D6 activity, UGT2B7 activity, renal impairment, and CYP3A4 inhibition. Molecular Diagnosis and Therapy, 16, 43–53. 10.2165/11597930-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Elliott JA and Fibuch EE, 2013. Endocrine effects of chronic opioid therapy: implications for clinical management. Pain Management, 3, 237–246. 10.2217/pmt.13.13 [DOI] [PubMed] [Google Scholar]
- Elliott JA, Opper SE, Agarwal S and Fibuch EE, 2012. Non‐analgesic effects of opioids: opioids and the endocrine system. Current Pharmaceutical Design, 18, 6070–6078. [DOI] [PubMed] [Google Scholar]
- EMA (European Medicines Agency), 2013a. Procedure under Article 31 of Directive 2001/83/EC resulting from pharmacovigilance data. Assessment report for codeine‐containing medicinal products indicated in the management of pain in children, EMA Pharmacovigilance Risk Assessment Committee (PRAC), 24 June 2013. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Codeine_containing_medicinal_products/Recommendation_provided_by_Pharmacovigilance_Risk_Assessment_Committee/WC500147065.pdf
- EMA (European Medicines Agency), 2013b. Restrictions on use of codeine for pain relief in children ‐ CMDh endorses PRAC recommendation. 28 June 2013. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2013/06/WC500144851.pdf
- EMA (European Medicines Agency), 2015a. Assessment report, Procedure under Article 31 of Directive 2001/83/EC resulting from pharmacovigilance data, Codeine containing medicinal products for the treatment of cough and/or cold in paediatric patients, EMA Pharmacovigilance Risk Assessment Committee (PRAC), 12 March 2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Codeine_cough_or_cold_in_children/Recommendation_provided_by_Pharmacovigilance_Risk_Assessment_Committee/WC500186522.pdf
- EMA (European Medicines Agency), 2015b. Codeine not to be used in children below 12 years for cough and cold, EMA/249413/2015, 24 April 2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Codeine_cough_or_cold_in_children/Position_provided_by_CMDh/WC500186159.pdf
- Emoto C, Fukuda T, Johnson TN, Neuhoff S, Sadhasivam S and Vinks AA, 2017. Characterization of contributing factors to variability in morphine clearance through PBPK modeling implemented with OCT1 transporter. Cpt‐Pharmacometrics and Systems Pharmacology, 6, 110–119. 10.1002/psp4.12144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enginar H, Ünak P, Lambrecht FY, Biber Müftüler FZ, Seyitoğlu B, Yurt A, Yolcular S, Medine Eİ and Bulduk İ, 2013. Extraction, radiolabeling, and biodistribution of thebaine in rats. Medicinal Chemistry Research, 22, 3459–3465. 10.1007/s00044-012-0360-z [DOI] [Google Scholar]
- Erfan G, Yanik ME, Kaya S, Tasolar K, Oznur M and Kulac M, 2015. Symmetrical drug‐related intertriginous and flexural exanthema due to codeine. Indian Journal of Dermatology, Venereology and Leprology, 81, 405–406. 10.4103/0378-6323.158665 [DOI] [PubMed] [Google Scholar]
- Fairbairn JW and El‐Masry S, 1968. The alkaloids of Papaver somniferum L.—VI. Phytochemistry, 7, 181–187. 10.1016/S0031-9422(00)86313-X [DOI] [Google Scholar]
- Falek A, Jordan RB, King BJ, Arnold PJ and Skelton WD, 1972. Human chromosomes and opiates. Archives of General Psychiatry, 27, 511–515. [DOI] [PubMed] [Google Scholar]
- Fan XL, Zhang JS, Zhang XQ, Yue W and Ma L, 2003. Differential regulation of beta‐arrestin 1 and beta‐arrestin 2 gene expression in rat brain by morphine. Neuroscience, 117, 383–389. [DOI] [PubMed] [Google Scholar]
- Fang ZZ, Krausz KW, Li F, Cheng J, Tanaka N and Gonzalez FJ, 2012. Metabolic map and bioactivation of the anti‐tumour drug noscapine. British Journal of Pharmacology, 167, 1271–1286. 10.1111/j.1476-5381.2012.02067.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui M, Li Y, Rogers T, Poonawala T, Griffin RJ, Song CW and Gupta K, 2007. COX‐2 inhibitor celecoxib prevents chronic morphine‐induced promotion of angiogenesis, tumour growth, metastasis and mortality, without compromising analgesia. British Journal of Cancer, 97, 1523–1531. 10.1038/sj.bjc.6604057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathallah N, Trimech B, Boussofara L, Ghali I, Slim R, Ben Salem C and Bouraoui K, 2011. Codeine‐induced generalized dermatitis. Drug Safety, 34, 946. [Google Scholar]
- FDA (Food and Drug Administration), 2013. Safety review update of codeine use in children; new boxed warning and contraindication on use after tonsillectomy and/or adenoidectomy. Available online: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM339116.pdf
- Fellows MD, Doherty AT, Priestley CC, Howarth V and O'Donovan MR, 2011. The ability of the mouse lymphoma TK assay to detect aneugens. Mutagenesis, 26, 771–781. 10.1093/mutage/ger045 [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Ward WL, Paule MG, Hall RW and Anand KJ, 2012. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicology and Teratology, 34, 47–55. 10.1016/j.ntt.2011.10.008 [DOI] [PubMed] [Google Scholar]
- Fist AJ, 2001. The Tasmanian poppy industry: a case study of the application of science and technology. Proceedings of the 10th Agronomy Conference ‘Science and Technology: Delivering Results for Agriculture?’, January 29–31, 2001, Hobart, Australia. Available online: http://www.regional.org.au/au/asa/2001/plenary/1/fist.htm
- Frances B, Gout R, Monsarrat B, Cros J and Zajac JM, 1992. Further evidence that morphine‐6 beta‐glucuronide is a more potent opioid agonist than morphine. Journal of Pharmacology and Experimental Therapeutics, 262, 25–31. [PubMed] [Google Scholar]
- Freier DO and Fuchs BA, 1993. Morphine‐induced alterations in thymocyte subpopulations of B6C3F1 mice. Journal of Pharmacology and Experimental Therapeutics, 265, 81–88. [PubMed] [Google Scholar]
- Friedrichsdorf SJ, Nugent AP and Strobl AQ, 2013. Codeine‐associated pediatric deaths despite using recommended dosing guidelines: three case reports. Journal of Opioid Management, 9, 151–155. 10.5055/jom.2013.0156 [DOI] [PubMed] [Google Scholar]
- Frohne D and Pfänder HJ, 2004. Giftpflanzen: Ein Handbuch für Apotheker, Ärzte, Toxikologen und Biologen. 5. Auflage. Wissenschaftliche Verlagsgesellschaft mbH Stuttgart. 456 pp. [Google Scholar]
- Frost J, Helland A, Nordrum IS and Slordal L, 2012. Investigation of morphine and morphine glucuronide levels and cytochrome P450 isoenzyme 2D6 genotype in codeine‐related deaths. Forensic Science International, 220, 6–11. 10.1016/j.forsciint.2012.01.019 [DOI] [PubMed] [Google Scholar]
- Fujita K, Ando Y, Yamamoto W, Miya T, Endo H, Sunakawa Y, Araki K, Kodama K, Nagashima F, Ichikawa W, Narabayashi M, Akiyama Y, Kawara K, Shiomi M, Ogata H, Iwasa H, Okazaki Y, Hirose T and Sasaki Y, 2010. Association of UGT2B7 and ABCB1 genotypes with morphine‐induced adverse drug reactions in Japanese patients with cancer. Cancer Chemotherapy and Pharmacology, 65, 251–258. 10.1007/s00280-009-1029-2 [DOI] [PubMed] [Google Scholar]
- Fukuda T, Chidambaran V, Mizuno T, Venkatasubramanian R, Ngamprasertwong P, Olbrecht V, Esslinger HR, Vinks AA and Sadhasivam S, 2013. OCT1 genetic variants influence the pharmacokinetics of morphine in children. Pharmacogenomics, 14, 1141–1151. 10.2217/pgs.13.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa A, Ohuchida A and Wierzba K, 1989. In vivo mutagenicity tests on polyploid inducers. 1989 EMS Abstracts. Environmental and Molecular Mutagenesis, 14, 63–64. 10.1002/em.2850140503 [DOI] [Google Scholar]
- Gach K, Wyrebska A, Fichna J and Janecka A, 2011. The role of morphine in regulation of cancer cell growth. Naunyn‐Schmiedeberg's Archives of Pharmacology, 384, 221–230. 10.1007/s00210-011-0672-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasche Y, Daali Y, Fathi M, Chiappe A, Cottini S, Dayer P and Desmeules J, 2004. Codeine intoxication associated with ultrarapid CYP2D6 metabolism. New England Journal of Medicine, 351, 2827–2831. 10.1056/NEJMoa041888 [DOI] [PubMed] [Google Scholar]
- Gatehouse DG, Stemp G, Pascoe S, Wilcox P, Hawker J and Tweats DJ, 1991. Investigations into the induction of aneuploidy and polyploidy in mammalian cells by the anti‐tussive agent noscapine hydrochloride. Mutagenesis, 6, 279–283. [DOI] [PubMed] [Google Scholar]
- Geber WF and Schramm LC, 1975. Congenital malformations of the central nervous system produced by narcotic analgesics in the hamster. American Journal of Obstetrics and Gynecology, 123, 705–713. [DOI] [PubMed] [Google Scholar]
- General J, Unbehend G, Lindhauer MG, Kniel B and Moser M, 2007. Untersuchungen zur Reduzierung von Morphin in Mohnsamen und Mohngebäcken mit praktikablen technologischen Massnahmen. Getreidetechnologie, 61, 36–42. [Google Scholar]
- Gessner O and Orzechowski G, 1974. Gift‐ und Arzneipflanzen von Mitteleuropa, 3rd Edition. Carl Winter Universitätsverlag, Heidelberg, Deutschland. 582 pp. [Google Scholar]
- Ghosian Moghaddam MH, Khalili M, Maleki M and Ahmad Abadi ME, 2013. The effect of oral feeding of Tribulus terrestris L. on sex hormone and gonadotropin levels in addicted male rats. International Journal of Fertility and Sterility, 7, 57–62. [PMC free article] [PubMed] [Google Scholar]
- Girish S and Rao A, 2017. Opiates use and hypogonadism in a VA endocrine clinic. Endocrine Reviews, 38. [Google Scholar]
- Golembiewski J, 2013. Opioid‐induced pruritus. Journal of Perianesthesia Nursing, 28, 247–249. 10.1016/j.jopan.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Gómez‐Serranillos MP, Palomino OM, Carretero E and Villar A, 1998. Analytical study and analgesic activity of oripavine from Papaver somniferum L. Phytotherapy Research, 12, 346–349. [DOI] [Google Scholar]
- Guo Q, Zhang J, Zhao S and Shao B, 2013. Determination of five alkaloids of Pericarpium papaveris in hot pot broth using ultra‐performance liquid chromatography coupled to triple quadruple mass spectrometry. Food Analytical Methods, 6, 698–704. 10.1007/s12161-012-9479-2 [DOI] [Google Scholar]
- Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D and Hebbel RP, 2002. Morphine stimulates angiogenesis by activating proangiogenic and survival‐promoting signaling and promotes breast tumor growth. Cancer Research, 62, 4491–4498. [PubMed] [Google Scholar]
- Gurkok T, Ozhuner E, Parmaksiz I, Ozcan S, Turktas M, Ipek A, Demirtas I, Okay S and Unver T, 2016. Functional characterization of 4'OMT and 7OMT genes in BIA biosynthesis. Frontiers in Plant Science, 7, 98. 10.3389/fpls.2016.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyawali B, Hayashi N, Tsukuura H, Honda K, Shimokata T and Ando Y, 2015. Opioid‐induced constipation. Scandinavian Journal of Gastroenterology, 50, 1331–1338. 10.3109/00365521.2015.1054423 [DOI] [PubMed] [Google Scholar]
- Hajj A, Khabbaz L, Laplanche JL and Peoc'h K, 2013. Pharmacogenetics of opiates in clinical practice: the visible tip of the iceberg. Pharmacogenomics, 14, 575–585. 10.2217/pgs.13.13 [DOI] [PubMed] [Google Scholar]
- Hall RW and Anand KJS, 2014. Pain management in newborns. Clinics in Perinatology, 41, 895–924. 10.1016/j.clp.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harati K, Behr B, Wallner C, Daigeler A, Hirsch T, Jacobsen F, Renner M, Harati A, Lehnhardt M and Becerikli M, 2017. Anti‐proliferative activity of epigallocatechin‐3‐gallate and silibinin on soft tissue sarcoma cells. Molecular Medicine Reports, 15, 103–110. 10.3892/mmr.2016.5969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harned M and Sloan P, 2016. Safety concerns with long‐term opioid use. Expert Opinion on Drug Safety, 15, 955–962. 10.1080/14740338.2016.1177509 [DOI] [PubMed] [Google Scholar]
- Haydari S, Miladi‐Gorji H, Mokhtari A and Safari M, 2014. Effects of voluntary exercise on anxiety‐like behavior and voluntary morphine consumption in rat pups borne from morphine‐dependent mothers during pregnancy. Neuroscience Letters, 578, 50–54. 10.1016/j.neulet.2014.06.026 [DOI] [PubMed] [Google Scholar]
- Health Canada , 2013. Health Canada's review recommends codeine only be used in patients aged 12 and over (issued 6th June, 2013). Available online: http://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2013/33915a-eng.php
- Heintze K and Fuchs W, 2015. Codeine ultra‐rapid metabolizers: age appears to be a key factor in adverse effects of codeine. Drug Research, 65, 640–644. 10.1055/s-0034-1396885 [DOI] [PubMed] [Google Scholar]
- Hendrickson RG and McKeown NJ, 2012. Is maternal opioid use hazardous to breast‐fed infants? Clinical Toxicology, 50, 1–14. 10.3109/15563650.2011.635147 [DOI] [PubMed] [Google Scholar]
- Hermanns‐Clausen M, Weinmann W, Auwarter V, Ferreiros N, Trittler R, Muller C, Pahl A, Superti‐Furga A and Hentschel R, 2009. Drug dosing error with drops: severe clinical course of codeine intoxication in twins. European Journal of Pediatrics, 168, 819–824. 10.1007/s00431-008-0842-7 [DOI] [PubMed] [Google Scholar]
- Hofman PJ and Menary RC, 1984a. Losses, by leaching, of alkaloids from the capsule of the poppy (Papaver somniferum L.) during maturation. Australian Journal of Agricultural Research, 35, 253–261. 10.1071/ar9840253 [DOI] [Google Scholar]
- Hofman PJ and Menary RC, 1984b. Fungal and enzymic degradation of alkaloids from the capsule of the poppy (Papaver somniferum L.). Australian Journal of Agricultural Research, 35, 263–269. 10.1071/ar9840263 [DOI] [Google Scholar]
- Højsted J, Kurita GP, Kendall S, Lundorff L, de Mattos Pimenta CA and Sjøgren P, 2012. Non‐analgesic effects of opioids: the cognitive effects of opioids in chronic pain of malignant and non‐malignant origin. An update. Current Pharmaceutical Design, 18, 6116–6122. [DOI] [PubMed] [Google Scholar]
- Holford NH, Ma SC and Anderson BJ, 2012. Prediction of morphine dose in humans. Paediatric Anaesthesia, 22, 209–222. 10.1111/j.1460-9592.2011.03782.x [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR and Peterson PK, 2002. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology, 42, 829–836. [DOI] [PubMed] [Google Scholar]
- Hussain Z, Rhee KW, Lee YJ and Park H, 2016. The effect of DA‐9701 in opioid‐induced bowel dysfunction of guinea pig. Journal of Neurogastroenterology and Motility, 22, 529–538. 10.5056/jnm15194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts I, Sioen I, Boon PE, Ruprich J, Lafay L, Turrini A, Amiano P, Hirvonen T, De Neve M, Arcella D, Moschandreas J, Westerlund A, Ribas‐Barba L, Hilbig A, Papoutsou S, Christensen T, Oltarzewski M, Virtanen S, Rehurkova I, Azpiri M, Sette S, Kersting M, Walkiewicz A, Serra‐Majem L, Volatier J‐L, Trolle E, Tornaritis M, Busk L, Kafatos A, Fabiansson S, De Henauw S and Van Klaveren JD, 2011. Dietary exposure assessments for children in Europe (the EXPOCHI project): rationale, methods and design. Archives of Public Health, 69, 4. 10.1186/0778-7367-69-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder WJ, Thomas F, Sorbello J, Ho K, Torpy DJ and Martin J, 2013. Cortisol status in patients with chronic non‐malignant pain treated with opioids. Endocrine Reviews, 34. [Google Scholar]
- Ingelman‐Sundberg M, 2005. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. The Pharmacogenomics Journal, 5, 6–13. 10.1038/sj.tpj.6500285 [DOI] [PubMed] [Google Scholar]
- Ingelman‐Sundberg M, Sim SC, Gomez A and Rodriguez‐Antona C, 2007. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacology and Therapeutics, 116, 496–526. 10.1016/j.pharmthera.2007.09.004 [DOI] [PubMed] [Google Scholar]
- Ishiguro E, Hatamochi A, Hayashi S, Hamasaki Y and Yamazaki S, 2011. Fixed drug eruption caused by noscapine. Journal of Dermatology, 38, 295–297. 10.1111/j.1346-8138.2010.01118.x [DOI] [PubMed] [Google Scholar]
- Iwase M, Nishimura Y, Kurata N, Namba H, Hirai T and Kiuchi Y, 2017. Inhibitory effects of gastrointestinal drugs on CYP activities in human liver microsomes. Biological and Pharmaceutical Bulletin, 40, 1654–1660. [DOI] [PubMed] [Google Scholar]
- Jackson T, Chougule MB, Ichite N, Patlolla RR and Singh M, 2008. Antitumor activity of noscapine in human non‐small cell lung cancer xenograft model. Cancer Chemotherapy and Pharmacology, 63, 117–126. 10.1007/s00280-008-0720-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheri S, Harris N, Howard J and Chung E, 2014. Adaptive servoventilation for treatment of opioid‐associated central sleep apnea. Journal of Clinical Sleep Medicine, 10, 637–643. 10.5664/jcsm.3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitpakdee T and Mandee S, 2014. Strategies for preventing side effects of systemic opioid in postoperative pediatric patients. Pediatric Anesthesia, 24, 561–568. 10.1111/pan.12420 [DOI] [PubMed] [Google Scholar]
- Jungquist CR, Flannery M, Perlis ML and Grace JT, 2012. Relationship of chronic pain and opioid use with respiratory disturbance during sleep. Pain Management Nursing, 13, 70–79. 10.1016/j.pmn.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Juurlink DN, Gomes T, Guttmann A, Hellings C, Sivilotti MLA, Harvey MA and Mamdani MM, 2012. Postpartum maternal codeine therapy and the risk of adverse neonatal outcomes: a retrospective cohort study. Clinical Toxicology, 50, 390–395. [DOI] [PubMed] [Google Scholar]
- Kadar I, Foldesi D, Voros J, Szilagyi J and Lukacs D, 2001. Mineral fertilisation of poppy (Papaver somniferum L.) on calcareous loamy chernozem soil II. Novenytermeles, 50, 467–468. [Google Scholar]
- Kálmán‐Pál Á, Bernáth J and Tétényi P, 1987. Phenotypic variability int he production and alkaloid spectrum of the Papaver somniferum L. hybrid. Herba Hungaricum, 26, 75–82. [Google Scholar]
- Kalvass JC, Olson ER, Cassidy MP, Selley DE and Pollack GM, 2007. Pharmacokinetics and pharmacodynamics of seven opioids in P‐glycoprotein‐competent mice: assessment of unbound brain EC50 and correlation of in vitro, preclinical, and clinical data. Journal of Pharmacology and Experimental Therapeutics, 323, 346–355. 10.1124/jpet.107.119560 [DOI] [PubMed] [Google Scholar]
- Katz MH, 2013. Opioid prescriptions for chronic nonmalignant pain driving on a dangerous road. JAMA Internal Medicine, 173, 178. 10.1001/jamainternmed.2013.1838 [DOI] [PubMed] [Google Scholar]
- Kazemi M, Azarnia M, Sahraei H, Bahadoran H and Saeedabadi S, 2009. The effect of oral morphine consumption on the development of lateral ventricles and choroid plexus in rat embryos. Kowsar Medical Journal, 14, 77–82. [Google Scholar]
- Kazemi M, Sahraei H, Azarnia M, Dehghani L, Bahadoran H and Tekieh E, 2011. The effect of morphine consumption on plasma corticosteron concentration and placenta development in pregnant rats. Iran Journal of Reprodudctive Medicine, 9, 71–76. [PMC free article] [PubMed] [Google Scholar]
- Kazemi M, Sahraei H and Dehghani L, 2012. Identification of site of morphine action in pregnant Wistar rat placenta tissue: a C14‐morphine study. Cell Journal, 14, 122–129. [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Ye K, Grossniklaus HE, Archer DR, Joshi HC and Kapp JA, 2000. Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses. Cancer Immunology, Immunotherapy, 49, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly L, 2014. Oral morphine weaning for neonatal abstinence syndrome at home vs. in hospital. Basic and Clinical Pharmacology and Toxicology, 115, 78. [Google Scholar]
- Kelly L, Rieder M, Veenstra F, Madadi P, Ross C, Sistonen J and Koren G, 2011. Pharmacogenetics of fatal paediatric obstructive sleep apnea cases in response to codeine: additional cases. Journal of Population Therapeutics and Clinical Pharmacology, 18, e345. [Google Scholar]
- Kelly LE, Rieder M, van den Anker J, Malkin B, Ross C, Neely MN, Carleton B, Hayden MR, Madadi P and Koren G, 2012. More codeine fatalities after tonsillectomy in North American children. Pediatrics, 129, e1343–e1347. 10.1542/peds.2011-2538 [DOI] [PubMed] [Google Scholar]
- Kenakin T, 2015. Gaddum Memorial Lecture 2014: receptors as an evolving concept: from switches to biased microprocessors. British Journal of Pharmacology, 172, 4238–4253. 10.1111/bph.13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavan K, 2015. Neurodevelopmental implications of neonatal pain and morphine exposure. Pediatric Annals, 44, e260–e264. 10.3928/00904481-20151112-08 [DOI] [PubMed] [Google Scholar]
- Khan K and Chang J, 1997. Neonatal abstinence syndrome due to codeine. Archives of Disease in Childhood. Fetal and Neonatal Edition, 76, F59–F60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer BL, 1999. Opioids: first lessons from knockout mice. Trends in Pharmacological Sciences, 20, 19–26. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Schmidt H, Tzvetkov M, Keulen JT, Lotsch J, Roots I and Brockmoller J, 2007. Pharmacokinetics of codeine and its metabolite morphine in ultra‐rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics Journal, 7, 257–265. 10.1038/sj.tpj.6500406 [DOI] [PubMed] [Google Scholar]
- Kirkland D, Reeve L, Gatehouse D and Vanparys P, 2011. A core in vitro genotoxicity battery comprising the Ames test plus the in vitro micronucleus test is sufficient to detect rodent carcinogens and in vivo genotoxins. Mutation Research, 721, 27–73. 10.1016/j.mrgentox.2010.12.015 [DOI] [PubMed] [Google Scholar]
- Klimas R and Mikus G, 2014. Morphine‐6‐glucuronide is responsible for the analgesic effect after morphine administration: a quantitative review of morphine, morphine‐6‐glucuronide, and morphine‐3‐glucuronide. British Journal of Anaesthesia, 113, 935–944. 10.1093/bja/aeu186 [DOI] [PubMed] [Google Scholar]
- Kocek M, Wilcox R, Crank C and Patra K, 2016. Evaluation of the relationship between opioid exposure in extremely low birth weight infants in the neonatal intensive care unit and neurodevelopmental outcome at 2 years. Early Human Development, 92, 29–32. 10.1016/j.earlhumdev.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Kodaira H and Spector S, 1988. Transformation of thebaine to oripavine, codeine, and morphine by rat liver, kidney, and brain microsomes. Proceedings of the National Academy of Sciences of the United States of America, 85, 1267–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohberg C, Andersen CU and Bendstrup E, 2016. Opioids: an unexplored option for treatment of dyspnea in IPF. European Clinical Respiratory Journal, 3, 30629. 10.3402/ecrj.v3.30629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli R, Argento V, Skudlarska B and Blagodatny M, 2011. Morphine induced angioedema. Journal of the American Geriatrics Society, 59, S24–S25. 10.1111/j.1532-5415.2011.03416.x [DOI] [PubMed] [Google Scholar]
- Koren G, Cairns J, Chitayat D, Gaedigk A and Leeder SJ, 2006. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine‐prescribed mother. Lancet, 368, 704. 10.1016/s0140-6736(06)69255-6 [DOI] [PubMed] [Google Scholar]
- Kraft WK and van den Anker JN, 2012. Pharmacologic management of the opioid neonatal abstinence syndrome. Pediatric Clinics of North America, 59, 1147–1165. 10.1016/j.pcl.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft WK, Dysart K, Greenspan JS, Gibson E, Kaltenbach K and Ehrlich ME, 2011. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. Addiction, 106, 574–580. 10.1111/j.1360-0443.2010.03170.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krekels EH, Tibboel D, Danhof M and Knibbe CA, 2012. Prediction of morphine clearance in the paediatric population: how accurate are the available pharmacokinetic models? Clinical Pharmacokinetics, 51, 695–709. 10.1007/s40262-012-0006-9 [DOI] [PubMed] [Google Scholar]
- Krueger H, Eddy NB and Sumwalt M, 1943. The pharmacology of the opium alkaloids. Government Printing Office, Washington. 775 pp. [Google Scholar]
- Kwara A, Lartey M, Boamah I, Rezk NL, Oliver‐Commey J, Kenu E, Kashuba AD and Court MH, 2009. Interindividual variability in pharmacokinetics of generic nucleoside reverse transcriptase inhibitors in TB/HIV‐coinfected Ghanaian patients: UGT2B7*1C is associated with faster zidovudine clearance and glucuronidation. Journal of Clinical Pharmacology, 49, 1079–1090. 10.1177/0091270009338482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan JW, Loeber JG, de Groot G and Sekhuis VM, 1988. The concentration of morphine in serum of rats made dependent using a drug‐admixed food method. Pharmacology Biochemistry and Behavior, 31, 123–128. 10.1016/0091-3057(88)90322-x [DOI] [PubMed] [Google Scholar]
- Labianca R, Sarzi‐Puttini P, Zuccaro SM, Cherubino P, Vellucci R and Fornasari D, 2012. Adverse effects associated with non‐opioid and opioid treatment in patients with chronic pain. Clinical Drug Investigation, 32, 53–63. [DOI] [PubMed] [Google Scholar]
- Lachenmeier DW, Sproll C and Musshoff F, 2010. Poppy seed foods and opiate drug testing ‐ where are we today? Therapeutic Drug Monitoring, 32, 11–18. 10.1097/FTD.0b013e3181c0eee0 [DOI] [PubMed] [Google Scholar]
- Lachman J, Hejtmankova A, Miholova D, Kolihova D and Tluka P, 2006. Relations among alkaloids, cadmium and zinc contents in opium poppy (Papaver somniferum L.). Plant Soil and Environment, 52, 282–288. [Google Scholar]
- Lam J, Matlow JN, Ross CJ, Hayden MR, Carleton BC and Madadi P, 2012. Postpartum maternal codeine therapy and the risk of adverse neonatal outcomes: the devil is in the details. Therapeutic Drug Monitoring, 34, 378–380. 10.1097/FTD.0b013e31825da19f [DOI] [PubMed] [Google Scholar]
- Lam J, Madadi P and Koren G, 2013. Opioid toxicity following breastfeeding in infants. In: Zibadi S, Watson RR, Preedy VR (eds.). Handbook of dietary and nutritional aspects of human breast milk. Human Health Handbooks no. 5. Academic Publishers, Wageningen. pp. 691–709. 10.3920/978-90-8686-764-6_41 [DOI] [Google Scholar]
- Landen JW, Lang R, McMahon SJ, Rusan NM, Yvon AM, Adams AW, Sorcinelli MD, Campbell R, Bonaccorsi P, Ansel JC, Archer DR, Wadsworth P, Armstrong CA and Joshi HC, 2002. Noscapine alters microtubule dynamics in living cells and inhibits the progression of melanoma. Cancer Research, 62, 4109–4114. [PubMed] [Google Scholar]
- Landen JW, Hau V, Wang M, Davis T, Ciliax B, Wainer BH, Van Meir EG, Glass JD, Joshi HC and Archer DR, 2004. Noscapine crosses the blood‐brain barrier and inhibits glioblastoma growth. Clinical Cancer Research, 10, 5187–5201. 10.1158/1078-0432.ccr-04-0360 [DOI] [PubMed] [Google Scholar]
- Lane‐Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK and Nestler EJ, 1997. CREB (cAMP response element‐binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. Journal of Neuroscience, 17, 7890–7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine E and Ben‐Shoshan M, 2015. Allergy to sunflower seed and sunflower butter as proposed vehicle for sensitization. Allergy Asthma and Clinical Immunology, 11, 3. 10.1186/s13223-014-0065-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Greves P, Huang W, Zhou Q, Thornwall M and Nyberg F, 1998. Acute effects of morphine on the expression of mRNAs for NMDA receptor subunits in the rat hippocampus, hypothalamus and spinal cord. European Journal of Pharmacology, 341, 161–164. [DOI] [PubMed] [Google Scholar]
- de Leon J, 2008. CYP2D6 genotyping and codeine. Paediatric Anaesthesia, 18, 274–275; author reply 275–276. 10.1111/j.1460-9592.2008.02420.x [DOI] [PubMed] [Google Scholar]
- Lerman J, 2008. Did opioid sensitivity contribute to post‐tonsillectomy arrest? Paediatric Anaesthesia, 18, 691–692. 10.1111/j.1460-9592.2008.02532.x [DOI] [PubMed] [Google Scholar]
- Lim G, Wang S, Lim JA and Mao J, 2005. Activity of adenylyl cyclase and protein kinase A contributes to morphine‐induced spinal apoptosis. Neuroscience Letters, 389, 104–108. 10.1016/j.neulet.2005.07.035 [DOI] [PubMed] [Google Scholar]
- Lin JC, Chu LF, Stringer EA, Baker KS, Sayyid ZN, Sun J, Campbell KA and Younger JW, 2016. One month of oral morphine decreases gray matter volume in the right amygdala of individuals with low back pain: confirmation of previously reported magnetic resonance imaging results. Pain Medicine, 17, 1497–1504. 10.1093/pm/pnv047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares OA, Fudin J, Schiesser WE, Daly Linares AL and Boston RC, 2015. CYP2D6 phenotype‐specific codeine population pharmacokinetics. Journal of Pain and Palliative Care Pharmacotherapy, 29, 4–15. 10.3109/15360288.2014.997854 [DOI] [PubMed] [Google Scholar]
- Linnoila M and Hakkinen S, 1974. Effects of diazepam and codeine, alone and in combination with alcohol, on simulated driving. Clinical Pharmacology and Therapeutics, 15, 368–373. [DOI] [PubMed] [Google Scholar]
- Liu C, Hua Z and Bai Y, 2016a. Classification of opium by UPLC‐Q‐TOF analysis of principal and minor alkaloids. Journal of Forensic Science, 61, 1615–1621. 10.1111/1556-4029.13190 [DOI] [PubMed] [Google Scholar]
- Liu G, Qiao S, Liu T, Yu H, Wang W, Zhou Y, Li Q and Li S, 2016b. Simultaneous determination of 18 chemical constituents in traditional Chinese medicine of antitussive by UPLC‐MS‐MS. Journal of Chromatographic Science, 10.1093/chromsci/bmw099. [DOI] [PubMed] [Google Scholar]
- Lloyd RA, Hotham E, Hall C, Williams M and Suppiah V, 2017. Pharmacogenomics and patient treatment parameters to opioid treatment in chronic pain: a focus on morphine, oxycodone, tramadol, and fentanyl. Pain medicine (Malden, Mass.), 18, 2369–2387. 10.1093/pm/pnw317 [DOI] [PubMed] [Google Scholar]
- Lo DS and Chua TH, 1992. Poppy seeds: implications of consumption. Medicine, Science, and the Law, 32, 296–302. 10.1177/002580249203200403 [DOI] [PubMed] [Google Scholar]
- Loguinov AV, Anderson LM, Crosby GJ and Yukhananov RY, 2001. Gene expression following acute morphine administration. Physiological Genomics, 6, 169–181. 10.1152/physiolgenomics.2001.6.3.169 [DOI] [PubMed] [Google Scholar]
- Lohr V, 2017. Re: Poppy producing countries. Message to Baert K. 29 November 2017. E‐mail.
- López P, Pereboom‐de Fauw DPKH, Mulder PPJ, Spanjer M, de Stoppelaar J, Mol HGJ and de Nijs M, 2018. Straightforward analytical method to determine opium alkaloids in poppy seeds and bakery products. Food Chemistry, 242, 443–450. 10.1016/j.foodchem.2017.08.045 [DOI] [PubMed] [Google Scholar]
- Lotsch J, 2005a. Opioid metabolites. Journal of Pain Symptom Management, 29, S10–S24. 10.1016/j.jpainsymman.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Lotsch J, 2005b. Pharmacokinetic‐pharmacodynamic modeling of opioids. Journal of Pain Symptom Management, 29, S90–S103. 10.1016/j.jpainsymman.2005.01.012 [DOI] [PubMed] [Google Scholar]
- Lynn AM and Slattery JT, 1987. Morphine pharmacokinetics in early infancy. Anesthesiology, 66, 136–139. [DOI] [PubMed] [Google Scholar]
- Lyon C and Njoku DB, 2015. Anesthetic pharmacology: physiologic states, pathophysiologic states, and adverse effects. Essentials of Pediatric Anesthesiology, 27–37. [Google Scholar]
- Madadi P, Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder JS, Teitelbaum R, Karaskov T and Aleksa K, 2007. Safety of codeine during breastfeeding: fatal morphine poisoning in the breastfed neonate of a mother prescribed codeine. Canadian Family Physician, 53, 33–35. [PMC free article] [PubMed] [Google Scholar]
- Madadi P, Ross CJ, Hayden MR, Carleton BC, Gaedigk A, Leeder JS and Koren G, 2009. Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: a case‐control study. Clinical Pharmacology and Therapeutics, 85, 31–35. 10.1038/clpt.2008.157 [DOI] [PubMed] [Google Scholar]
- Magnani B and Evans R, 1999. Codeine intoxication in the neonate. Pediatrics, 104, e75. [DOI] [PubMed] [Google Scholar]
- Mahmood I, 2011. Evaluation of a morphine maturation model for the prediction of morphine clearance in children: how accurate is the predictive performance of the model? British Journal of Clinical Pharmacology, 71, 88–94. 10.1111/j.1365-2125.2010.03802.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudian M and Rahimi‐Moghaddam P, 2009. The anti‐cancer activity of noscapine: a review. Recent Patents on Anti‐Cancer Drug Discovery, 4, 92–97. [DOI] [PubMed] [Google Scholar]
- Mallappallil M, Sabu J, Friedman EA and Salifu M, 2017. What do we know about opioids and the kidney? International Journal of Molecular Sciences, 18, 10.3390/ijms18010223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR and Lim G, 2002. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid‐induced neurotoxic mechanism. Journal of Neuroscience, 22, 7650–7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale , 2017. The complete drug reference. Morphine; online. Pharmaceutical Press, London. Available online: http://www.medicinescomplete.com/mc/martindale/current/
- Martini C, Olofsen E, Yassen A, Aarts L and Dahan A, 2011. Pharmacokinetic‐pharmacodynamic modeling in acute and chronic pain: an overview of the recent literature. Expert Review of Clinical Pharmacology, 4, 719–728. 10.1586/ecp.11.59 [DOI] [PubMed] [Google Scholar]
- Matzo M and Dawson KA, 2013. Opioid‐induced neurotoxicity. American Journal of Nursing, 113, 51–56. 10.1097/01.naj.0000435351.53534.83 [DOI] [PubMed] [Google Scholar]
- McPherson C, 2016. Morphine exposure in preterm infants correlates with impaired cerebellar growth and poorer neurodevelopmental outcome. Evidence‐Based Medicine, 21, 234. 10.1136/ebmed-2016-110511 [DOI] [PubMed] [Google Scholar]
- Mendoza A, Rodríguez‐Gil JL, González‐Alonso S, Mastroianni N, López de Alda M, Barceló D and Valcárcel Y, 2014. Drugs of abuse and benzodiazepines in the Madrid Region (Central Spain): seasonal variation in river waters, occurrence in tap water and potential environmental and human risk. Environment International, 70, 76–87. 10.1016/j.envint.2014.05.009 [DOI] [PubMed] [Google Scholar]
- Merck , 2016. Fachinformation Merck, May 2016. Morphin Merck Tropfen 0.5%/2%. Rote Liste Service GmbH. Berlin, Germany, 1–4. [Google Scholar]
- Merten C, Ferrari P, Bakker M, Boss A, Hearty A, Leclercq C, Lindtner O, Tlustos C, Verger P, Volatier JL and Arcella D, 2011. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food Additives and Contaminants. Part A Chemistry, Analysis, Control, Exposure and Risk Assessment, 28, 975–995. 10.1080/19440049.2011.576440 [DOI] [PubMed] [Google Scholar]
- MHRA/CHM (Medicines and Healthcare products Regulatory Agency/Commission on Human Medicines), 2013. Codeine for analgesia: restricted use in children because of reports of morphine toxicity. Drug Safety Update, 6, July 2013:A1. Available online: https://www.gov.uk/drug-safety-update/codeine-for-analgesia-restricted-use-in-children-because-of-reports-of-morphine-toxicity [Google Scholar]
- Mignat C, Wille U and Ziegler A, 1995. Affinity profiles of morphine, codeine, dihydrocodeine and their glucuronides at opioid receptor subtypes. Life Sciences, 56, 793–799. [DOI] [PubMed] [Google Scholar]
- Mikus G, Somogyi AA, Bochner F and Eichelbaum M, 1991. Thebaine O‐demethylation to oripavine: genetic differences between two rat strains. Xenobiotica, 21, 1501–1509. [DOI] [PubMed] [Google Scholar]
- Millgate AG, Pogson BJ, Wilson IW, Kutchan TM, Zenk MH, Gerlach WL, Fist AJ and Larkin PJ, 2004. Analgesia: morphine‐pathway block in top1 poppies. Nature, 431, 413–414. 10.1038/431413a [DOI] [PubMed] [Google Scholar]
- Misra AL, Pontani RB and Mulé SJ, (1974). Pharmacokinetics and Metabolism of [3H] Thebaine, Xenobiotica, 4, 17–32, 10.3109/00498257409052087 [DOI] [PubMed]
- Mitchell ID, Carlton JB, Chan MY, Robinson A and Sunderland J, 1991. Noscapine‐induced polyploidy in vitro . Mutagenesis, 6, 479–486. [DOI] [PubMed] [Google Scholar]
- Nasiraei‐Moghadam S, Sahraei H, Bahadoran H, Sadooghi M, Salimi SH, Kaka GR, Imani H, Mahdavi‐Nasab H and Dashtnavard H, 2005. Effects of maternal oral morphine consumption on neural tube development in Wistar rats. Brain Research. Developmental Brain Research, 159, 12–17. 10.1016/j.devbrainres.2005.06.012 [DOI] [PubMed] [Google Scholar]
- Nasiraei‐Moghadam S, Kazeminezhad B, Dargahi L and Ahmadiani A, 2010. Maternal oral consumption of morphine increases Bax/Bcl‐2 ratio and caspase 3 activity during early neural system development in rat embryos. Journal of Molecular Neuroscience, 41, 156–164. 10.1007/s12031-009-9312-6 [DOI] [PubMed] [Google Scholar]
- Nasiraei‐Moghadam S, Sherafat MA, Safari MS, Moradi F, Ahmadiani A and Dargahi L, 2013. Reversal of prenatal morphine exposure‐induced memory deficit in male but not female rats. Journal of Molecular Neuroscience, 50, 58–69. 10.1007/s12031-012-9860-z [DOI] [PubMed] [Google Scholar]
- Navigante AH, Castro MA and Cerchietti LC, 2010. Morphine versus midazolam as upfront therapy to control dyspnea perception in cancer patients while its underlying cause is sought or treated. Journal of Pain Symptom Management, 39, 820–830. 10.1016/j.jpainsymman.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Nelson AD and Camilleri M, 2015. Chronic opioid induced constipation in patients with nonmalignant pain: challenges and opportunities. Therapeutic Advances in Gastroenterology, 8, 206–220. 10.1177/1756283x15578608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh‐Zámbori É, Jászberényi C, Rajhárt P and Bernáth J, 2011. Evaluation of alkaloid profiles in hybrid generations of different poppy (Papaver somniferum L.) genotypes. Industrial Crops and Products, 33, 690–696. 10.1016/j.indcrop.2011.01.013 [DOI] [Google Scholar]
- Nezvalova‐Henriksen K, Spigset O and Nordeng H, 2011. Effects of codeine on pregnancy outcome: results from a large population‐based cohort study. European Journal of Clinical Pharmacology, 67, 1253–1261. 10.1007/s00228-011-1069-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezvalová‐Henriksen K, Spigset O and Nordeng H, 2012. Erratum to: Effects of codeine on pregnancy outcome: results from a large population‐based cohort study. European Journal of Clinical Pharmacology, 68, 1689–1690. 10.1007/s00228-012-1434-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen J, Luk K, Vang D, Soto W, Vincent L, Robiner S, Saavedra R, Li Y, Gupta P and Gupta K, 2014. Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. British Journal of Anaesthesia, 113, 4–13. 10.1093/bja/aeu090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesters M, Dahan A, Kest B, Zacny J, Stijnen T, Aarts L and Sarton E, 2010. Do sex differences exist in opioid analgesia? A systematic review and meta‐analysis of human experimental and clinical studies. Pain, 151, 61–68. 10.1016/j.pain.2010.06.012 [DOI] [PubMed] [Google Scholar]
- Niesters M, Overdyk F, Smith T, Aarts L and Dahan A, 2013. Opioid‐induced respiratory depression in paediatrics: a review of case reports. British Journal of Anaesthesia, 110, 175–182. 10.1093/bja/aes447 [DOI] [PubMed] [Google Scholar]
- Niknam NA, Azarnia M, Bahadoran H, Kazemi M, Tekieh E, Ranjbaran M and Sahraei H, 2013. Evaluating the effects of oral morphine on embryonic development of cerebellum in Wistar rats. Basic and Clinical Neuroscience, 4, 130–135. [PMC free article] [PubMed] [Google Scholar]
- Nikolaev VO, Boettcher C, Dees C, Bunemann M, Lohse MJ and Zenk MH, 2007. Live cell monitoring of mu‐opioid receptor‐mediated G‐protein activation reveals strong biological activity of close morphine biosynthetic precursors. Journal of Biological Chemistry, 282, 27126–27132. 10.1074/jbc.M703272200 [DOI] [PubMed] [Google Scholar]
- Noble F, Szücs M, Kieffer B and Roques BP, 2000. Overexpression of dynamin is induced by chronic stimulation of μ‐ but not δ‐opioid receptors: relationships with μ‐related morphine dependence. Molecular Pharmacology, 58, 159–166. [DOI] [PubMed] [Google Scholar]
- Nordeng H, Ystrom E and Eberhard‐Gran M, 2014. Medication safety in pregnancy – results from the MoBa study. Norsk Epidemiologi ‐ Norwegian journal of Epidemology, 24, 161–168. [Google Scholar]
- Numan S, Lane‐Ladd SB, Zhang L, Lundgren KH, Russell DS, Seroogy KB and Nestler EJ, 1998. Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. Journal of Neuroscience, 18, 10700–10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye HE and Nestler EJ, 1996. Induction of chronic Fos‐related antigens in rat brain by chronic morphine administration. Molecular Pharmacology, 49, 636–645. [PubMed] [Google Scholar]
- Obeng AO, Hamadeh I and Smith M, 2017. Review of opioid pharmacogenetics and considerations for pain management. Pharmacotherapy, 37, 1105–1121. 10.1002/phar.1986 [DOI] [PubMed] [Google Scholar]
- O'Reilly D, Thomas M and Moylett E, 2015. Cough, codeine and confusion. BMJ Case Reports, 2015, 10.1136/bcr-2015-212727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne R, Joel S, Grebenik K, Trew D and Slevin M, 1993. The pharmacokinetics of morphine and morphine glucuronides in kidney failure. Clinical Pharmacology and Therapeutics, 54, 158–167. [DOI] [PubMed] [Google Scholar]
- Otton SV, Schadel M, Cheung SW, Kaplan HL, Busto UE and Sellers EM, 1993a. CYP2D6 phenotype determines the metabolic conversion of hydrocodone to hydromorphone. Clinical Pharmacology and Therapeutics, 54, 463–472. [DOI] [PubMed] [Google Scholar]
- Otton SV, Wu D, Joffe RT, Cheung SW and Sellers EM, 1993b. Inhibition by fluoxetine of cytochrome P450 2D6 activity. Clinical Pharmacology and Therapeutics, 53, 401–409. [DOI] [PubMed] [Google Scholar]
- Overdyk F, Dahan A, Roozekrans M, Van Der Schrier R, Aarts L and Niesters M, 2014. Opioid‐induced respiratory depression in the acute care setting: a compendium of case reports. Pain Management, 4, 317–325. 10.2217/pmt.14.19 [DOI] [PubMed] [Google Scholar]
- Pal R, Megharaj M, Kirkbride KP and Naidu R, 2013. Illicit drugs and the environment ‐a review. Science of the Total Environment, 463–464, 1079–1092. 10.1016/j.scitotenv.2012.05.086 [DOI] [PubMed] [Google Scholar]
- Patel A and Bahna SL, 2016. Hypersensitivities to sesame and other common edible seeds. Allergy, 71, 1405–1413. 10.1111/all.12962 [DOI] [PubMed] [Google Scholar]
- Ph. Eur. 8 , 2016. European Pharmacopoeia 8th edition (Ph. Eur.8) including 7 supplements.
- Plein LM and Rittner HL, 2017. Opioids and the immune system ‐ friend or foe. British Journal of Pharmacology, 10.1111/bph.13750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeaknapo C, Schmidt J, Brandsch M, Drager B and Zenk MH, 2004. Endogenous formation of morphine in human cells. Proceedings of the National Academy of Science of the United States of America, 101, 14091–14096. 10.1073/pnas.0405430101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescribing Information , 2015. Fachinformation Codeintropfen‐CT. Rote Liste Service GmbH, Frankfurt, Germany. [Google Scholar]
- Raffa RB and Pergolizzi JV Jr, 2012. Is morphine medicine's biggest misnomer? Annals of Pharmacotherapy, 46, 1122–1123. 10.1345/aph.1R133 [DOI] [PubMed] [Google Scholar]
- Rauws AG, De Waal EJ and Van Der Laan JW, 1997. Sense and non‐sense in toxicity assessment of medicinal products. Advances in Drug Research, 30, 15–72. 10.1016/S0065-2490(97)80004-4 [DOI] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI and Reisine T, 1994. Pharmacological characterization of the cloned κ‐, δ‐, and μ‐opioid receptors. Molecular Pharmacology, 45, 330–334. [PubMed] [Google Scholar]
- Rhodin A, Gronbladh A, Ginya H, Nilsson KW, Rosenblad A, Zhou Q, Enlund M, Hallberg M, Gordh T and Nyberg F, 2013. Combined analysis of circulating beta‐endorphin with gene polymorphisms in OPRM1, CACNAD2 and ABCB1 reveals correlation with pain, opioid sensitivity and opioid‐related side effects. Molecular Brain, 6, 10.1186/1756-6606-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rida PC, LiVecche D, Ogden A, Zhou J and Aneja R, 2015. The noscapine chronicle: a pharmaco‐historic biography of the opiate alkaloid family and its clinical applications. Medicinal Research Review, 35, 1072–1096. 10.1002/med.21357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J, Branford R, Droney J, Gretton S, Sato H, Kennett A, Oyebode C, Thick M, Wells A, Williams J, Welsh K and Ross J, 2015. Morphine or oxycodone for cancer‐related pain? A randomized, open‐label, controlled trial. Journal of Pain and Symptom Management, 49, 161–172. 10.1016/j.jpainsymman.2014.05.021 [DOI] [PubMed] [Google Scholar]
- Rinner G, White CC, Smolinske SC and Warrick BJ, 2017. Case report: a relaxing cup of poppy seed tea goes toxic. Clinical Toxicology, 55, 502. 10.1080/15563650.2017.1309792 [DOI] [Google Scholar]
- Rivard J, Yu L, Tremblay S and Lebel D, 2016. Morphine poisoning in pediatrics: a case report. Canadian Journal of Hospital Pharmacy, 69, 244–248. [PMC free article] [PubMed] [Google Scholar]
- Rochholz G, Westphal F, Wiesbrock UO and Schütz HW, 2004. Opiat‐Nachweis in Urin, Blut und Haaren nach Verzehr mohnsamenhaltiger Backwaren. Blutalkohol, 41, 319–329. [Google Scholar]
- Rocker GM, Simpson AC, Horton R, Sinuff T, Demmons J, Hernandez P and Marciniuk D, 2013. Opioid therapy for refractory dyspnea in patients with advanced chronic obstructive pulmonary disease: patients’ experiences and outcomes. CMAJ Open, 1, E27–E36. 10.9778/cmajo.20120031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodayan A, Afana S, Segura PA, Sultana T, Metcalfe CD and Yargeau V, 2016. Linking drugs of abuse in wastewater to contamination of surface and drinking water. Environmental Toxicology and Chemistry, 35, 843–849. 10.1002/etc.3085 [DOI] [PubMed] [Google Scholar]
- Romberg R, Sarton E, Teppema L, Matthes HW, Kieffer BL and Dahan A, 2003. Comparison of morphine‐6‐glucuronide and morphine on respiratory depressant and antinociceptive responses in wild type and μ‐opioid receptor deficient mice. British Journal of Anaesthesia, 91, 862–870. [DOI] [PubMed] [Google Scholar]
- Rose AR, Catcheside PG, McEvoy RD, Paul D, Kapur D, Peak E, Vakulin A and Antic NA, 2014. Sleep disordered breathing and chronic respiratory failure in patients with chronic pain on long term opioid therapy. Journal of Clinical Sleep Medicine, 10, 847–852. 10.5664/jcsm.3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowsell L, Wong K, Yee B, Eckert D, Grunstein R and Wang D, 2015. The effect of morphine on obstructive sleep apnea – a randomised double‐blind placebo‐controlled crossover trial. Sleep and Biological Rhythms, 13, 55–56. 10.1111/sbr.12132 [DOI] [PubMed] [Google Scholar]
- Rowsell L, Wong K, Yee B, Eckert D, Somogyi A, Duffin J, Grunstein R and Wang D, 2016. Identifying obstructive sleep apnea patients vulnerable to opioid‐induced respiratory depression: Results from a randomized double blind placebo‐controlled crossover trial. Sleep, 39, A110. [Google Scholar]
- Roy S, Ninkovic J, Banerjee S, Charboneau RG, Das S, Dutta R, Kirchner VA, Koodie L, Ma J, Meng J and Barke RA, 2011. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. Journal of Neuroimmune Pharmacology, 6, 442–465. 10.1007/s11481-011-9292-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudisill TM, Zhu M, Kelley GA, Pilkerton C and Rudisill BR, 2016. Medication use and the risk of motor vehicle collisions among licensed drivers: A systematic review. Accident: Analysis and Prevention, 96, 255–270. 10.1016/j.aap.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacerdote P, Franchi S and Panerai AE, 2012. Non‐analgesic effects of opioids: mechanisms and potential clinical relevance of opioid‐induced immunodepression. Current Pharmaceutical Design, 18, 6034–6042. [DOI] [PubMed] [Google Scholar]
- Sadhasivam S, Chidambaran V, Zhang X, Meller J, Esslinger H, Zhang K, Martin LJ and McAuliffe J, 2015. Opioid‐induced respiratory depression: ABCB1 transporter pharmacogenetics. Pharmacogenomics Journal, 15, 119–126. 10.1038/tpj.2014.56 [DOI] [PubMed] [Google Scholar]
- Sadraie SH, Kaka GR, Sahraei H, Dashtnavard H, Bahadoran H, Mofid M, Nasab HM and Jafari F, 2008. Effects of maternal oral administration of morphine sulfate on developing rat fetal cerebrum: a morphometrical evaluation. Brain Research, 1245, 36–40. 10.1016/j.brainres.2008.09.052 [DOI] [PubMed] [Google Scholar]
- Sargeant TJ, Day DJ, Miller JH and Steel RW, 2008. Acute in utero morphine exposure slows G2/M phase transition in radial glial and basal progenitor cells in the dorsal telencephalon of the E15.5 embryonic mouse. European Journal of Neuroscience, 28, 1060–1067. 10.1111/j.1460-9568.2008.06412.x [DOI] [PubMed] [Google Scholar]
- Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, Burm A, Teppema L and Dahan A, 2000. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology, 93, 1245–1254; discussion 1246A. [DOI] [PubMed] [Google Scholar]
- Sawant SG and Couch DB, 1995. Induction of micronuclei in murine lymphocytes by morphine. Environmental and Molecular Mutagenesis, 25, 279–283. [DOI] [PubMed] [Google Scholar]
- Sawe J, Svensson JO and Rane A, 1983. Morphine metabolism in cancer patients on increasing oral doses—no evidence for autoinduction or dose‐dependence. British Journal of Clinical Pharmacology, 16, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawe J, Svensson JO and Odar‐Cederlof I, 1985. Kinetics of morphine in patients with renal failure. Lancet, 2, 211. [DOI] [PubMed] [Google Scholar]
- Schuppener LM and Corliss RF, 2018. Death due to complications of bowel obstruction following raw poppy seed ingestion. Journal of Forensic Sciences, 63, 614–618. 10.1111/1556-4029.13562 [DOI] [PubMed] [Google Scholar]
- Sei Y, Yoshimoto K, McIntyre T, Skolnick P and Arora PK, 1991. Morphine‐induced thymic hypoplasia is glucocorticoid‐dependent. Journal of Immunology, 146, 194–198. [PubMed] [Google Scholar]
- Shafer DA, Xie Y and Falek A, 1994. Detection of opiate‐enhanced increases in DNA damage, HPRT mutants, and the mutation frequency in human HUT‐78 cells. Environmental and Molecular Mutagenesis, 23, 37–44. [DOI] [PubMed] [Google Scholar]
- Shehab‐Eldeen N, Salama M, Lotfy A, Elgamal M, Sheashaa H, Sobh M, Elsherbeeny M and Elmetwally H, 2015. Effect of papaverine on neurospheres. Toxicon, 93, S56. [Google Scholar]
- Shuey DL, Gudi R, Krsmanovic L and Gerson RJ, 2007. Evidence that oxymorphone‐induced increases in micronuclei occur secondary to hyperthermia. Toxicological Sciences, 95, 369–375. 10.1093/toxsci/kfl148 [DOI] [PubMed] [Google Scholar]
- Sindrup SH, Brosen K, Bjerring P, Arendt‐Nielsen L, Larsen U, Angelo HR and Gram LF, 1990. Codeine increases pain thresholds to copper vapor laser stimuli in extensive but not poor metabolizers of sparteine. Clinical Pharmacology and Therapeutics, 48, 686–693. [DOI] [PubMed] [Google Scholar]
- Siuda ER, Carr R 3rd, Rominger DH and Violin JD, 2017. Biased mu‐opioid receptor ligands: a promising new generation of pain therapeutics. Current Opinion in Pharmacology, 32, 77–84. 10.1016/j.coph.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Snyder RD, 2009. An update on the genotoxicity and carcinogenicity of marketed pharmaceuticals with reference to in silico predictivity. Environmental and Molecular Mutagenesis, 50, 435–450. 10.1002/em.20485 [DOI] [PubMed] [Google Scholar]
- Sobol Z, Homiski ML, Dickinson DA, Spellman RA, Li D, Scott A, Cheung JR, Coffing SL, Munzner JB, Sanok KE, Gunther WC, Dobo KL and Schuler M, 2012. Development and validation of an in vitro micronucleus assay platform in TK6 cells. Mutation Research, 746, 29–34. 10.1016/j.mrgentox.2012.02.005 [DOI] [PubMed] [Google Scholar]
- Söderberg KC, Laflamme L and Moller J, 2013. Newly initiated opioid treatment and the risk of fall‐related injuries. A nationwide, register‐based, case‐crossover study in Sweden. CNS Drugs, 27, 155–161. 10.1007/s40263-013-0038-1 [DOI] [PubMed] [Google Scholar]
- Spangler R, Goddard NL, Avena NM, Hoebel BG and Leibowitz SF, 2003. Elevated D3 dopamine receptor mRNA in dopaminergic and dopaminoceptive regions of the rat brain in response to morphine, Brain Research. Molecular Brain Research, 111, 74–83. [DOI] [PubMed] [Google Scholar]
- Spijker S, Houtzager SW, De Gunst MC, De Boer WP, Schoffelmeer AN and Smit AB, 2004. Morphine exposure and abstinence define specific stages of gene expression in the rat nucleus accumbens. FASEB Journal, 18, 848–850. 10.1096/fj.03-0612fje [DOI] [PubMed] [Google Scholar]
- Sproll C, Perz RC, Buschmann R and Lachenmeier DW, 2007. Guidelines for reduction of morphine in poppy seed intended for food purposes. European Food Research and Technology, 226, 307–310. 10.1007/s00217-006-0522-7 [DOI] [Google Scholar]
- Spyres M and Van Wijk X, 2017. Prolonged and severe opioid toxicity after ingestion of poppy seed tea. Journal of Medical Toxicology, 13, 24. 10.1007/s13181-017-0599-3 [DOI] [Google Scholar]
- Srinivasan V, Wielbo D and Tebbett IR, 1997. Analgesic effects of codeine‐6‐glucuronide after intravenous administration. European Journal of Pain, 1, 185–190. [DOI] [PubMed] [Google Scholar]
- Stamer UM and Stuber F, 2008. Codeine induced respiratory depression in a child. Paediatric Anaesthesia, 18, 272–273; author reply 275–276. 10.1111/j.1460-9592.2008.02415.x [DOI] [PubMed] [Google Scholar]
- Stefano GB, Cadet P, Kream RM and Zhu W, 2008. The presence of endogenous morphine signaling in animals. Neurochemical Research, 33, 1933–1939. 10.1007/s11064-008-9674-0 [DOI] [PubMed] [Google Scholar]
- Steinhorn R, McPherson C, Anderson PJ, Neil J, Doyle LW and Inder T, 2015. Neonatal morphine exposure in very preterm infants‐cerebral development and outcomes. Journal of Pediatrics, 166, 1200–1207. 10.1016/j.jpeds.2015.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl JC and Rotthauwe J, 2015. Codeine ‐ restrictions on use for children and teenagers. Deutsche Medizinische Wochenschrif, 140, 1093–1095. 10.1055/s-0041-102948 [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Strassburg A, Kneip S, Barut A, Tukey RH, Rodeck B and Manns MP, 2002. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut, 50, 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanyam R, Varughese A, Willging JP and Sadhasivam S, 2013. Future of pediatric tonsillectomy and perioperative outcomes. International Journal of Pediatric Otorhinolaryngology, 77, 194–199. 10.1016/j.ijporl.2012.10.016 [DOI] [PubMed] [Google Scholar]
- Swain N, Das RK and Paul M, 1980. Cytogenetic assay of potential mutagenicity in vivo of two narcotic analgesics. Mutation Research, 78, 97–100. [DOI] [PubMed] [Google Scholar]
- Syed MI, Magos TA, Singh J and Montague ML, 2016. A new analgesia regimen after (adeno) tonsillectomy in children: a pilot study. Clinical Otolaryngology, 41, 660–665. 10.1111/coa.12579 [DOI] [PubMed] [Google Scholar]
- Thomas J and Wolff K, 2016. Diagnosis of neonatal abstinence syndrome: substance use during pregnancy. Heroin Addiction and Related Clinical Problems, 18, 43–48. [Google Scholar]
- Ting S and Schug S, 2016. The pharmacogenomics of pain management: prospects for personalized medicine. Journal of Pain Research, 9, 49–56. 10.2147/jpr.s55595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiveron C, Hartley‐Asp B, Johansson CJ and Pacchierotti F, 1993. Noscapine does not show aneugenic activity in mouse oocytes. Mutagenesis, 8, 311–315. [DOI] [PubMed] [Google Scholar]
- Tomar V, Kukreti S, Prakash S, Madan J and Chandra R, 2017. Noscapine and its analogs as chemotherapeutic agent: current updates. Current Topics in Medicinal Chemistry, 17, 174–188. [DOI] [PubMed] [Google Scholar]
- Tong TF and Ng KK, 2001. Codeine ingestion and apparent life‐threatening event in a neonate. Pediatrics International, 43, 517–518. [DOI] [PubMed] [Google Scholar]
- Tremlett MR, 2013. Wither codeine? Paediatric Anaesthesia, 23, 677–683. 10.1111/pan.12190 [DOI] [PubMed] [Google Scholar]
- Trescot AM, Datta S, Lee M and Hansen H, 2008. Opioid pharmacology. Pain Physician, 11, S133–S153. [PubMed] [Google Scholar]
- Tsunoda N and Yoshimura H, 1981. Metabolic fate of noscapine. III. Further studies on identification and determination of the metabolites. Xenobiotica, 11, 23–32. [DOI] [PubMed] [Google Scholar]
- Tzvetkov M, 2012. Pharmacogenetics of drug transport: effects of OCT1 polymorphisms on the uptake of weak basic drugs into the liver. Drug Metabolism and Drug Interactions, 27, A33. 10.1515/dmdi-2012-0025 [DOI] [Google Scholar]
- Tzvetkov MV, 2017. OCT1 pharmacogenetics in pain management: is a clinical application within reach? Pharmacogenomics, 18, 1515–1523. 10.2217/pgs-2017-0095 [DOI] [PubMed] [Google Scholar]
- Tzvetkov MV, dos Santos Pereira JN, Meineke I, Saadatmand AR, Stingl JC and Brockmoller J, 2013. Morphine is a substrate of the organic cation transporter OCT1 and polymorphisms in OCT1 gene affect morphine pharmacokinetics after codeine administration. Biochemical Pharmacology, 86, 666–678. 10.1016/j.bcp.2013.06.019 [DOI] [PubMed] [Google Scholar]
- Valverde‐Filho J, da Cunha Carneiro, Neto MB, Fonoff ET, Meirelles EdS and Teixeira MJ, 2015. Chronic spinal and oral morphine‐induced neuroendocrine and metabolic changes in noncancer pain patients. Pain Medicine, 16, 715–725. 10.1111/pme.12661 [DOI] [PubMed] [Google Scholar]
- Van Gasse AL, Hagendorens MM, Sabato V, Bridts CH, De Clerck LS and Ebo DG, 2015. IgE to poppy seed and morphine are not useful tools to diagnose opiate allergy. Journal of allergy and clinical immunology In Practice, 3, 396–399. 10.1016/j.jaip.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian R, Fukuda T, Niu J, Mizuno T, Chidambaran V, Vinks AA and Sadhasivam S, 2014. ABCC3 and OCT1 genotypes influence pharmacokinetics of morphine in children. Pharmacogenomics, 15, 1297–1309. 10.2217/pgs.14.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberkt CA, van den Beuken‐van Everdingen MH, Franssen FM, Dirksen CD, Schols JM, Wouters EF and Janssen DJ, 2016. A randomized controlled trial on the benefits and respiratory adverse effects of morphine for refractory dyspnea in patients with COPD: protocol of the MORDYC study. Contemporary Clinical Trials, 47, 228–234. 10.1016/j.cct.2016.01.007 [DOI] [PubMed] [Google Scholar]
- Vinks AA, Emoto C and Fukuda T, 2015. Modeling and simulation in pediatric drug therapy: application of pharmacometrics to define the right dose for children. Clinical Pharmacology and Therapeutics, 98, 298–308. 10.1002/cpt.169 [DOI] [PubMed] [Google Scholar]
- Volpe DA, McMahon Tobin GA, Mellon RD, Katki AG, Parker RJ, Colatsky T, Kropp TJ and Verbois SL, 2011. Uniform assessment and ranking of opioid mu receptor binding constants for selected opioid drugs. Regulatory Toxicology and Pharmacology, 59, 385–390. 10.1016/j.yrtph.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Voronov P, Przybylo HJ and Jagannathan N, 2007. Apnea in a child after oral codeine: a genetic variant ‐ an ultra‐rapid metabolizer. Paediatric Anaesthesia, 17, 684–687. 10.1111/j.1460-9592.2006.02182.x [DOI] [PubMed] [Google Scholar]
- Vree TB, van Dongen RT and Koopman‐Kimenai PM, 2000. Codeine analgesia is due to codeine‐6‐glucuronide, not morphine. International Journal of Clinical Practice, 54, 395–398. [PubMed] [Google Scholar]
- van de Wetering K, Zelcer N, Kuil A, Feddema W, Hillebrand M, Vlaming ML, Schinkel AH, Beijnen JH and Borst P, 2007. Multidrug resistance proteins 2 and 3 provide alternative routes for hepatic excretion of morphine‐glucuronides. Molecular Pharmacology, 72, 387–394. 10.1124/mol.107.035592 [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 1980. The dependence potential of thebaine. Report of a WHO Advisory Group. Bulletin on Narcotics, 32, 45–54. Available online: http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1980-01-01_1_page006.html [PubMed] [Google Scholar]
- WHO Advisory Group , 1981. Dependence potential of oripavine. Bulletin on Narcotics, 33, 29–35. [PubMed] [Google Scholar]
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2008. Uncertainty and data quality in exposure assessment. Harmonisation project document No. 6. ISBN 978 92 4 156376 5.U.
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety) 2009. Environmental Health Criteria 240. Principles and methods for the assessment of chemicals in food.
- Wiese AD, Griffin MR, Stein CM, Mitchel EF Jr and Grijalva CG, 2016. Opioid analgesics and the risk of serious infections among patients with rheumatoid arthritis: a self‐controlled case series study. Arthritis and Rheumatology, 68, 323–331. 10.1002/art.39462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wildt SN and Koren G, 2008. Re: Apnea in a child after oral codeine: a genetic variant ‐ an ultra‐rapid metabolizer [corrected]. Paediatric Anaesthesia, 18, 273–274; author's reply 275–276. 10.1111/j.1460-9592.2008.02418.x [DOI] [PubMed] [Google Scholar]
- Wilkes TC, Davies DP and Dar MF, 1981. Apnoea in a 3‐month‐old baby prescribed compound linctus containing codeine. Lancet, 1, 1166–1167. [DOI] [PubMed] [Google Scholar]
- Willmann S, Edginton AN, Coboeken K, Ahr G and Lippert J, 2009. Risk to the breast‐fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study. Clinical Pharmacology and Therapeutics, 86, 634–643. 10.1038/clpt.2009.151 [DOI] [PubMed] [Google Scholar]
- Winter C and Flataker L, 1961. Toxicity studies on noscapine. Toxicology and Applied Pharmacology, 3, 96–106. [DOI] [PubMed] [Google Scholar]
- Wong C, Lau E, Palozzi L and Campbell F, 2012. Pain management in children: Part 2 ‐ a transition from codeine to morphine for moderate to severe pain in children. Canadian Pharmacists Journal, 145, 276–279. 10.3821/145.6.cpj276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolner DF, Winter D, Frendin TJ, Begg EJ, Lynn KL and Wright GJ, 1986. Renal failure does not impair the metabolism of morphine. British Journal of Clinical Pharmacology, 22, 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassen A, Passier P, Furuichi Y and Dahan A, 2013. Translational PK‐PD modeling in pain. Journal of Pharmacokinetics and Pharmacodynamics, 40, 401–418. 10.1007/s10928-012-9282-0 [DOI] [PubMed] [Google Scholar]
- Ye K, Ke Y, Keshava N, Shanks J, Kapp JA, Tekmal RR, Petros J and Joshi HC, 1998. Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proceedings of the National Academy of Sciences of the United States of America, 95, 1601–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh SY, 1981. Analgesic activity and toxicity of oripavine and phi‐dihydrothebaine in the mouse and rat. Archives internationales de pharmacodynamie et de thérapie, 254, 227–240. [PubMed] [Google Scholar]
- Yoo HS, Yang EM, Kim MA, Hwang SH, Shin YS, Ye YM, Nahm DH and Park HS, 2014. A case of codeine induced anaphylaxis via oral route. Allergy, Asthma and Immunology Research, 6, 95–97. 10.4168/aair.2014.6.1.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H, Ida S, Oguri K and Tsukamoto H, 1973. Biochemical basis for analgesic activity of morphine‐6‐glucuronide. I. Penetration of morphine‐6‐glucuronide in the brain of rats. Biochemical Pharmacology, 22, 1423–1430. [DOI] [PubMed] [Google Scholar]
- Younger JW, Chu LF, D'Arcy NT, Trott KE, Jastrzab LE and Mackey SC, 2011. Prescription opioid analgesics rapidly change the human brain. Pain, 152, 1803–1810. 10.1016/j.pain.2011.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue QY and Sawe J, 1997. Different effects of inhibitors on the O‐ and N‐demethylation of codeine in human liver microsomes. European Journal of Clinical Pharmacology, 52, 41–47. [DOI] [PubMed] [Google Scholar]
- Yue QY, Svensson JO, Alm C, Sjoqvist F and Sawe J, 1989. Interindividual and interethnic differences in the demethylation and glucuronidation of codeine. British Journal of Clinical Pharmacology, 28, 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue QY, Hasselstrom J, Svensson JO and Sawe J, 1991. Pharmacokinetics of codeine and its metabolites in Caucasian healthy volunteers: comparisons between extensive and poor hydroxylators of debrisoquine. British Journal of Clinical Pharmacology, 31, 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaim M, Lal RK, Verma RK and Pandey R, 2014. Studies on Effect of Poppy Mosaic Virus Infection on Poppy Produce and Some Secondary Metabolites. In: Mathe A (ed). International Symposium on Papaver, Acta Horticulturae, 1036, 151–155. [Google Scholar]
- Zecca E, Brunelli C, Bracchi P, Biancofiore G, De Sangro C, Bortolussi R, Montanari L, Maltoni M, Moro C, Colonna U, Finco G, Roy MT, Ferrari V, Alabiso O, Rosti G, Kaasa S and Caraceni A, 2016. Comparison of the tolerability profile of controlled‐release oral morphine and oxycodone for cancer pain treatment. An open‐label randomized controlled trial. Journal of Pain and Symptom Management, 52, 783–794. 10.1016/j.jpainsymman.2016.05.030 [DOI] [PubMed] [Google Scholar]
- Zelcer N, van de Wetering K, Hillebrand M, Sarton E, Kuil A, Wielinga PR, Tephly T, Dahan A, Beijnen JH and Borst P, 2005. Mice lacking multidrug resistance protein 3 show altered morphine pharmacokinetics and morphine‐6‐glucuronide antinociception. Proceedings of the National Academy of Sciences of the United States of America, 102, 7274–7279. 10.1073/pnas.0502530102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentai A, Sali J, Szeitzne‐Szabo M, Szabo IJ and Ambrus A, 2012. Exposure of consumers to morphine from poppy seeds in Hungary. Food Additives and Contaminants Part A Chemistry, Analysis, Control, Exposure and Risk Assessment, 29, 403–414. 10.1080/19440049.2011.636762 [DOI] [PubMed] [Google Scholar]
- Zhang EY, Xiong J, Parker BL, Chen AY, Fields PE, Ma X, Qiu J and Yankee TM, 2011. Depletion and recovery of lymphoid subsets following morphine administration. British Journal of Pharmacology, 164, 1829–1844. 10.1111/j.1476-5381.2011.01475.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Z, Cox DP and Civelli O, 2012. Study on the activation of the opioid receptors by a set of morphine derivatives in a well‐defined assay system. Neurochemical Research, 37, 410–416. 10.1007/s11064-011-0627-7 [DOI] [PubMed] [Google Scholar]
- Zhou J, Gupta K, Yao J, Ye K, Panda D, Giannakakou P and Joshi HC, 2002. Paclitaxel‐resistant human ovarian cancer cells undergo c‐Jun NH2‐terminal kinase‐mediated apoptosis in response to noscapine. Journal of Biological Chemistry, 277, 39777–39785. 10.1074/jbc.M203927200 [DOI] [PubMed] [Google Scholar]
- Ziegler J, Facchini PJ, Geissler R, Schmidt J, Ammer C, Kramell R, Voigtlander S, Gesell A, Pienkny S and Brandt W, 2009. Evolution of morphine biosynthesis in opium poppy. Phytochemistry, 70, 1696–1707. 10.1016/j.phytochem.2009.07.006 [DOI] [PubMed] [Google Scholar]
- Zutler M and Holty J‐EC, 2011. Opioids, sleep, and sleep‐disordered breathing. Current Pharmaceutical Design, 17, 1443–1449. [DOI] [PubMed] [Google Scholar]
- Zwicker JG, Miller SP, Grunau RE, Chau V, Brant R, Studholme C, Liu M, Synnes A, Poskitt KJ, Stiver ML and Tam EWY, 2016. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. Journal of Pediatrics, 172, 81–87e82. 10.1016/j.jpeds.2015.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Occurrence of opium alkaloids
Consumption data and dietary exposure for humans


