Abstract
The present opinion deals with the re‐evaluation of glycerol esters of wood rosin (GEWR, E 445) when used as a food additive. Regarding GEWR originating from Pinus palustris (longleaf pine) and Pinus elliottii (slash pine), based on the overall toxicity database, and given the absence of reproductive and developmental toxicity data, the Panel concluded that the current acceptable daily intake (ADI) of 12.5 mg/kg body weight (bw) per day for GEWR (E 445) as established by the Scientific Committee on Food (SCF) in 1994 should be temporary pending the provision of such data. This assessment is restricted to GEWR derived from P. palustris (longleaf pine) and P. elliottii (slash pine) and with a chemical composition in compliance with GEWR used in the toxicological testing. The Panel concluded that the mean and the high exposure levels (P95) of the brand‐loyal refined exposure scenario did not exceed the temporary ADI in any of the population groups from the use of GEWR (E 445) as a food additive at the reported use levels. For GEWR originating from Pinus halepensis and Pinus brutia, the Panel noted that concentrations of the fractions of ‘glycerol monoesters’, ‘free resin acids’ and ‘neutrals’, which are considered to be of particular toxicological relevance, are not known; therefore, the evaluation of chemical equivalence with GEWR originating from P. palustris (longleaf pine) and P. elliottii (slash pine) is not possible; no data on stability were available; no toxicological data were available. Therefore, the Panel concluded that a safety assessment of GEWR originating from P. halepensis and P. brutia could not be performed. The Panel recommended the European Commission to consider an update of the definition of GEWR (E 445) in the EU specifications. It should be indicated that GEWR (E 445) (i) contain, besides the mentioned glycerol di‐ and triesters, a residual fraction of glycerol monoesters, and (ii) contain residual free resin acids and neutrals (non‐acidic other saponifiable and unsaponifiable substances).
Keywords: Glycerol esters of wood rosin, GEWR, E 445, CAS No. 8050‐31‐5, food additive
Summary
The present opinion deals with the re‐evaluation of glycerol esters of wood rosin (GEWR, E 445) when used as a food additive.
GEWR (E 445) are authorised as a food additive in the European Union (EU) in accordance to Annex II of Regulation (EC) No 1333/20081 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/2012.2
According to the Commission Regulation (EU) No 231/1012 and the JECFA specifications (2006), GEWR (E 445) are a complex mixture of ‘tri‐ and diglycerol esters of resin acids’ from wood rosin, obtained by the solvent extraction of aged pine stumps followed by a liquid–liquid solvent refining process. Excluded from the additive's definition are substances derived from gum rosin, an exudate of living pine trees, and substances derived from tall oil rosin, a by‐product of kraft (paper) pulp processing. The source material,3 wood rosin, is composed of approximately 90% resin acids and 10% neutrals (non‐acidic compounds). Its resin acid fraction is a complex mixture of isomeric diterpenoid monocarboxylic acids having the empirical molecular formula of C20H30O2, chiefly abietic acid. The esterified product is purified by steam stripping or by counter current steam distillation.
The recent JECFA definition of GEWR (E 455) (JECFA, 2013b) is in agreement with the EU definition but further specifies, that GEWR (i) contain, besides the mentioned glycerol di‐ and triesters, a residual fraction of glycerol monoesters, (ii) contain, also neutrals (non‐acidic saponifiable and unsaponifiable substances) and residual free resin acids, (iii) are originating exclusively from two species: Pinus palustris (longleaf pine) and Pinus elliottii (slash pine).
According to information from interested parties, the wood rosin is either obtained from P. palustris (longleaf pine) and P. elliottii (slash pine) stumps (Documentation provided to EFSA N. 2) or from Pinus halepensis and Pinus brutia stumps (Documentation provided to EFSA N. 17).
The Panel noted that the EC specifications do not define the source material by its exclusive botanical origin and that the food additive E 455 obtained from other botanical origins, if complying with the current specifications, may also be on the market in the EU.
The Panel noted that the current evaluation is based on the results of toxicological studies performed with GEWR originating from P. palustris (longleaf pine) and P. elliottii (slash pine).
GEWR originating from P. halepensis and P. brutia yielded almost identical infrared (IR) spectra and similar retention times in gas chromatography as GEWR derived from P. palustris (longleaf pine) and P. elliottii (slash pine). However, this information only refers to the resin alcohols obtained after hydrolysis and reduction. In addition, the Panel noted that no information was available, in which concentrations the toxicologically relevant fractions of ‘glycerol monoesters’, ‘free resin acids’ and ‘neutrals’ are present in the GEWR originating from P. halepensis and P. brutia. Therefore, the evaluation of its equivalence with GEWR originating from P. palustris (longleaf pine) and P. elliottii (slash pine) is not possible. Altogether, the Panel concluded that the available data on the chemical composition of GEWR originating from P. halepensis and P. brutia do not allow for read across of toxicological data from GEWR originating from P. palustris (longleaf pine) and P. elliottii (slash pine).
Absorption, distribution, metabolism and excretion (ADME) studies have shown that ester bonds in GEWR are largely resistant to hydrolysis in the gut, the majority of orally applied GEWR being excreted unchanged in the faeces (Documentation provided to EFSA N. 5 and 6; Documentation provided to EFSA N. 15). Only a small fraction, most likely the glycerol monoesters of wood rosin, seems to undergo hydrolysis. Studies with 14C‐labelled GEWR in rats (Documentation provided to EFSA N. 15) on excretion of radioactivity via faeces, bile, urine and exhaled air and disposition in the carcass gave evidence for a low absorption rate of ≤ 5% of the applied dose. Most of the absorbed radioactivity was eliminated in the bile and excreted via faeces. Results of high‐performance liquid chromatography (HPLC) analysis of faeces and bile suggested that absorbed components were hydrolysis products from glycerol monoesters of wood rosin present in GEWR.
A few ADME data were available for free resin acids which might be released in the gastrointestinal tract after hydrolysis of glycerol monoesters or of esters with other alcohols in GEWR. Furthermore, GEWR contain a fraction of residual free resin acids. The experiments in rats with radiolabelled dehydroabietic acid (Documentation provided to EFSA N. 16) revealed an absorption rate of approximately 40% after oral exposure. Most of the radioactivity was excreted via the bile, minor amounts via the urine and only traces were exhaled. Tetrahydroabietic acid and isopimaric acid exhibited an excretion pattern similar to dehydroabietic acid (Documentation provided to EFSA N. 16).
No studies on acute oral toxicity of GEWR were available.
No treatment‐related effects were detected in a 13‐week feeding study (Documentation provided to EFSA N. 3 and 4) conducted with GEWR originated from P. Palustris (longleaf pine) and P. elliottii (slash pine), representative of the food additive on the market, according to current standards after oral exposure of male and female F344 rats to 0, 625, 1,250 or 2,500 mg GEWR/kg body weight (bw) per day. From this study, the Panel identified a no observed adverse effect level (NOAEL) of 2,500 mg GEWR/kg bw per day, the highest dose tested.
In summary, the Panel considered that GEWR, when tested as a mixture, did not show genotoxic potential in the core battery of adequately performed in vitro tests (i.e. bacterial reversion and in vitro micronucleus assays) recommended in the EFSA guideline on genotoxicity testing strategy (EFSA Scientific Committee, 2011). Negative results were also obtained in limited in vitro chromosomal aberration and unscheduled DNA synthesis (UDS) assays. Positive results, of unclear biological relevance and plausibility, were only reported in a limited in vivo study. Based on the overall weight evidence, the Panel considered GEWR as non‐genotoxic.
Limited information is available on individual compounds from the residual free resin acids fraction, which accounts only for 2.3–2.8% of the mixture. A positive mutagenic outcome was only observed for neoabietic acid in limited bacterial and yeast mutation assays (Nestmann et al., 1979; Nestmann and Lee, 1983). The Panel noted that the positive results with neoabietic acid were not corroborated by results with structurally related compounds and that no structural alerts for genotoxicity were identified in neoabietic acid. On this basis, the Panel considered that the mutagenic response of neoabietic acid could possibly be attributed to the presence of impurities in the lot tested, as also suggested by the study authors, as well as to cytotoxic effects through the formation of microcolonies of auxotrophic bacteria (i.e. not true his+ revertants), since no data on the clearance of the background lawn were reported. Therefore, the Panel concluded that the positive findings reported with neoabietic acid are of questionable relevance for the genotoxicity assessment of GEWR. The Panel also noted that, according to the information provided by one interested party (Documentation provided to EFSA N. 24 and 25), the levels of free neoabietic acid in GEWR are below the limit of quantitation of 0.05 wt. %.
Altogether the Panel concluded that GEWR (E 445) containing free neoabietic acid below the limit of quantitation (0.05 weight percent) when used as a food additive are of no concern for genotoxicity.
There were no studies available concerning chronic toxicity or carcinogenicity of GEWR.
There were no studies available on the endpoint reproductive and developmental toxicity of GEWR.
In 1994, the Scientific Committee on Food (SCF) established an acceptable daily intake (ADI) of 12.5 mg/kg bw per day for GEWR from the NOAEL of 2,500 mg/kg bw per day from a 13‐week study in rats by applying an uncertainty factor (UF) of 200 to take into account the 90‐day duration (SCF, 1994). JECFA concluded in 1996 that the documented data on subchronic oral toxicity studies and the studies confirming the non‐bioavailability of GEWR were adequate to establish an ADI of 0–25 mg/kg bw per day for GEWR by applying an UF of 100 to the NOAEL of 2,500 mg/kg bw per day, although there were no studies available on chronic or reproductive toxicity (JECFA, 1996b). This ADI was confirmed in 2013 (JECFA, 2013a).
Based on the overall toxicity database, and given the absence of reproductive and developmental toxicity data, the Panel considered that the current ADI of 12.5 mg/kg bw per day for GEWR (E 445) as established by the SCF in 1994 should be temporary pending the provision of such data.
The Panel considered that the brand‐loyal consumer scenario resulted in more realistic long‐term exposure estimates compared to the regulatory maximum level exposure assessment scenario. Since GEWR (E 445) are authorised and used in a certain type of flavoured drinks, to which consumers may be brand loyal, the Panel selected the refined brand‐loyal scenario as the most relevant exposure scenario for the safety evaluation of this food additive. In this scenario, the highest mean and P95 exposure estimates occurred in the population group of toddlers with 1.07 and 4.06 mg/kg bw per day, respectively. The Panel noted that the mean and the high exposure levels (P95) of the brand‐loyal refined exposure scenario did not exceed the ADI in any of the population groups from the use of GEWR (E 445) as a food additive at the reported use levels.
The Panel considered that the exposure estimates in all exposure scenarios resulted in overestimates of the exposure to GEWR (E 445) from its use as a food additive according to Annex II to Regulation (EC) No 1333/2008 (Section 3.4).
Regarding the GEWR originating from P. palustris (longleaf pine) and P. elliottii (slash pine), based on the overall toxicity database, and given the absence of reproductive and developmental toxicity data, the Panel concluded that the current ADI of 12.5 mg/kg bw per day for GEWR (E 445) as established by the SCF in 1994 should be temporary pending the provision of such data.
This assessment is restricted to GEWR derived from P. palustris (longleaf pine) and P. elliottii (slash pine) and with a chemical composition in compliance with GEWR used in the toxicological testing.
The Panel concluded that the mean and the high exposure levels (P95) of the brand‐loyal refined exposure scenario did not exceed the temporary ADI in any of the population groups from the use of GEWR (E 445) as a food additive at the reported use levels.
For GEWR originating from P. halepensis and P. brutia, the Panel noted that
concentrations of the fractions of ‘glycerol monoesters’, ‘free resin acids’ and ‘neutrals’, which are considered to be of particular toxicological relevance, are not known,
therefore, the evaluation of chemical equivalence with GEWR originating from P. palustris (longleaf pine) and P. elliottii (slash pine) is not possible,
no data on stability were available,
no toxicological data were available.
Therefore, the Panel concluded that a safety assessment of GEWR originating from P. halepensis and P. brutia could not be performed.
The Panel recommended the European Commission to consider:
an update of the definition of GEWR (E 445) in the EU specifications. It should be indicated that GEWR (E 445) (i) contain, besides the mentioned glycerol di‐ and triesters, a residual fraction of glycerol monoesters, and (ii) contain residual free resin acids and neutrals (non‐acidic other saponifiable and unsaponifiable substances); technical terms should be corrected as indicated in Section 3.1.2,
setting limits for glycerol monoesters of resin acids and neutrals in the EU specifications (see Section 3.1.1), in accordance with the analytical data provided for GEWR (E 445) from P. palustris (longleaf pine) and P. elliottii (slash pine), being in compliance with the test material used in the toxicological tests,
setting a maximum level for free neoabietic acid of 0.05 wt. % (limit of quantitation) in the EU specifications, while the concentration of the total of free resin acids in GEWR being limited already by the acid value of the existing specifications,
revising the current limits for the toxic elements lead mercury, cadmium and arsenic in the EC specification to ensure that GEWR (E 445) as a food additive will not be a significant source of exposure to these toxic elements in food. Currently, detected levels of these toxic elements were two orders of magnitude below those defined in the EU specifications,
setting maximum levels for impurities, such as butanetriols, acrolein, chlorinated compounds and 3‐monochloropropane‐1,2‐diol, in the EU specifications, for which limits are defined in the food additive glycerol (E 422),
requesting the provision of a reproductive and developmental toxicity study, in accordance with the applicable OECD test guidelines, using a test material which is representative of the food additive present on the market and taking into account the above recommendations for the update of the specifications.
1. Introduction
The present opinion deals with the re‐evaluation of glycerol esters of wood rosin (GEWR, E 445) when used as a food additive.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background as provided by the European Commission
Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union. In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the European Union before 20 January 2009 has been set up under the Regulation (EU) No 257/20104. This Regulation also foresees that food additives are re‐evaluated whenever necessary in light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU of 2001. The report “Food additives in Europe 2000” submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010 the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference as provided by the European Commission
The Commission asks the European Food Safety Authority to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.2. Information on existing evaluations and authorisations
GEWR (E 445) are authorised as a food additive in the EU in accordance to Annex II of Regulation (EC) No 1333/20081 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/2012.2
In the European Union, GEWR (E 445) has been evaluated by the SCF in 1990 and 1992 (SCF, 1992, 1994). In the latest evaluation, the SCF allocated an acceptable daily intake (ADI) of 12.5 mg/kg body weight (bw) per day. This ADI was derived from the no‐observed‐adverse‐effect level (NOAEL) of 2,500 mg/kg bw per day from a 13‐week study in rats. The Committee applied an uncertainty factor (UF) of 200 to take into account the 90‐day duration (SCF, 1994). However, no toxicological data were specified in the document.
GEWR has also been evaluated several times by JEFCA. In 1996, the Committee considered that the data on repeated dose toxicity studies and the new studies confirming the non‐bioavailability of GEWR were adequate to establish an ADI (JECFA, 1996a,b, 1997), although there were no long‐term toxicity studies or reproductive toxicity available. On the basis of the NOAEL of 2,500 mg/kg bw per day in a 13‐week study in rats with food‐grade GEWR, the Committee allocated an ADI of 0–25 mg/kg bw per day applying an UF of 100. In its 71st meeting in 2009, JECFA decided to include glycerol esters of gum rosin (GEGR) in the ADI for GEWR of 0–25 mg/kg bw per day, thereby establishing a group ADI of 0–25 mg/kg bw per day for GEWR and GEGR (JECFA, 2010a,b). In 2011, the Committee withdrew the group ADI for GEGR and GEWR and established a temporary group ADI for GEGR and GEWR of 0–12.5 mg/kg bw per day applying an additional UF of 2, because new information raised questions about the identity and composition of GEWR as the product in commerce (JECFA, 2011). As requested data on GEGR were not submitted, the Committee withdrew the temporary group ADI of 0–12.5 mg/kg bw per day for GEGR and GEWR in 2013 and re‐established the ADI of 0–25 mg/kg bw per day for GEWR (JECFA, 2013a).
JECFA established in its 71st meeting new specifications for GEWR, which were made tentative pending the submission of infrared spectra that corresponded to the commercially available products, data on the resin acid composition obtained with updated chromatographic techniques, and additional information on methods that enabled the identification of the individual glycerol esters of rosins and their differentiation (JECFA, 2010b). To complete the evaluation of GEWR, additional data were required by the Committee in its 74th meeting to characterise the GEWR in commerce in relation to the composition of (i) the refined wood rosin used as the source rosin for the production of GEWR, (ii) the glycerol ester of wood rosin, (iii) the total glycerol esters of resin acids and (iv) the neutrals. Validated methods for the determination of the substances considered in the specifications were also required (JECFA, 2011). In the 77th meeting of the Committee new information, including compositional data on GEWR were evaluated, the existing tentative specifications were revised and the tentative status was removed (JECFA, 2013a).
In 2010 and 2011, the EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) provided scientific opinions evaluating the safety of GEGR and glycerol esters of tall oil rosin (GETOR) for the proposed uses as food additives. The Panel did not have sufficient information to evaluate the chemical equivalence of GEGR and GEWR or of GETOR and GEWR, respectively. Therefore, the toxicological data obtained with GEWR could not be used for read across. The Panel concluded that the available data were too limited to conclude on the safety of GEGR or GETOR as food additives at the proposed uses and use levels (EFSA ANS Panel, 2010, 2011).
GEWR has also been reviewed by the Nordic Council of Ministers (TemaNord, 2002). The Committee stated that no reproduction/teratogenicity studies or long‐term studies are available. However, the data base, including metabolism data, and the low level of GEWR exposure give no reason for concern. The Committee recommended the implementation of a reproduction/teratogenicity study if the permitted levels are to be raised.
2. Data and methodologies
Data
The Panel was not provided with a newly submitted dossier. EFSA launched public calls for data5 , 6 to collect relevant information from interested parties.
The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up to the last Working Group meeting before the adoption of the opinion.7 Attempts were made at retrieving relevant original study reports on which previous evaluations or reviews were based, however not always these were available to the Panel.
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database8) was used to estimate the dietary exposure.
The Mintel's Global New Products Database (GNPD) is an online database which was used for checking the labelling of products containing glycerol ester of wood rosins (E 445) within the EU's food products as the GNPD shows the compulsory ingredient information presented in the labelling of products.
Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing Guidances from the EFSA Scientific Committee.
The ANS Panel assessed the safety of GEWR (E 445) as a food additive in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the Scientific Committee on Food (SCF, 2001) and taking into consideration the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
When the test substance was administered in the feed or in the drinking water, but doses were not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012) for studies in rodents or, in the case of other animal species, by JECFA (2000). In these cases, the daily intake is expressed as equivalent. When in human studies in adults (aged above 18 years), the dose of the test substance administered was reported in mg/person per day, the dose in mg/kg bw per day was calculated by the Panel using a body weight of 70 kg as default for the adult population as described in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012).
Dietary exposure to GEWR (E 445) from its use as a food additive was estimated combining food consumption data available within the EFSA Comprehensive European Food Consumption Database with the maximum permitted levels (MPLs) and/or reported use levels submitted to EFSA following a call for data. Different exposure scenarios were calculated (see Section 3.4). Uncertainties on the exposure assessment were identified and discussed.
In the context of this re‐evaluation, the Panel followed the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EC) No 257/2010 (EFSA ANS Panel, 2014).
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
According to the Commission Regulation (EU) No 231/1012 and the JECFA (2006), GEWR (E 445)9 are a complex mixture of tri‐ and diglycerol esters of resin acids from wood rosin,10 obtained by the solvent extraction of aged pine stumps followed by a liquid–liquid solvent refining process. Excluded from the additive's definition are substances derived from gum rosin, an exudate of living pine trees, and substances derived from tall oil rosin, a by‐product of kraft (paper) pulp processing. The source material,11 wood rosin, is composed of approximately 90% resin acids and 10% neutrals (non‐acidic compounds). Its resin acid fraction is a complex mixture of isomeric diterpenoid monocarboxylic acids having the empirical molecular formula of C20H30O2, chiefly abietic acid. The esterified product is purified by steam stripping or by counter current steam distillation.
The recent JECFA definition of GEWR (JECFA, 2013b) is in agreement with the EU definition of GEWR (E 455) but further specifies, that GEWR (i) contain a residual fraction of glycerol monoesters, (ii) contain, besides the mentioned glycerol mono‐, di‐ and triesters, also neutrals (non‐acidic saponifiable and unsaponifiable substances) and residual free resin acids, (iii) are originating exclusively from two species: Pinus palustris (longleaf pine) and Pinus elliottii (slash pine).
According to an interested party (Documentation provided to EFSA N. 23), the major fractions of the GEWR derived from wood rosin from P. palustris (longleaf pine) and P. elliottii (slash pine) were measured by solid‐phase extraction (SPE) and high‐temperature/high‐resolution gas chromatography (HT/HR GC). Upon analysis of 5 production samples of GEWR, total glycerol esters averaged 84.3% (ranging from 79.7% to 86.3%), while neutrals averaged 13.2% (ranging from 11.4% to 17.6%) and free resin acids averaged 2.6% (ranging from 2.3% to 2.8%). The sum of glycerol di‐ and triesters was determined to be 82.0% (ranging from 78.3% to 83.9%). Glycerol monoesters were measured by size exclusion chromatography (SEC) and averaged 2.2% (ranging from 1.5 to 3.2%). One interested party submitted results of an analysis of five GEWR samples for unreacted (free) neoabietic acid, showing that the results for all five samples tested were below the quantitation limit, which for this analysis was calculated to be 0.05 wt. % (Documentation provided to EFSA N .23). No other information has been provided on the percentages of the various rosin acids (in bound and unbound form). According to the interested party, the GEWR, that was used in the biological and toxicological studies,12 is also derived from P. palustris (longleaf pine) and P. elliottii (slash pine), shares the same manufacturing process and is assumed to be compositionally equivalent to the food additive product in commerce as beforehand described (Documentation provided to EFSA N. 23).
According to another interested party (Documentation provided to EFSA N. 17), GEWR can also be produced from wood rosin derived from Pinus halepensis and Pinus brutia stumps. However, for this product, no indication of the percentage of the different fractions, e.g. the fraction of glycerol monoesters, free resin acids and neutrals are available. For this product, only limited analytical data have been provided to enable the evaluation of equivalence with other GEWR preparations, e.g. the substances tested in the toxicological studies. The similarity of the GEWR derived from P. halepensis and P. brutia stumps was compared to GEWR derived from P. palustris (longleaf pine) and P. elliottii (slash pine) (four lots for each of the two products). The identification tests were performed by gas chromatography (GC) and infrared (IR) spectroscopy, after hydrolysis of the esters and reduction of the resulting resin acids to the corresponding resin alcohols, according to the JECFA GEWR specifications monograph (JECFA, 2013b).
The Panel noted that both GEWRs have almost identical IR spectra, exhibiting relative maxima at the same wavelengths complying with the reference GEWR spectra in the JECFA monograph (JECFA, 2013b) after the correction for the baseline.
The Panel also noted that according to the GC data presented, both GEWRs had similar glycerol retention times and yielded contained detectable levels of pimaric, isopimaric, palustric, dehydroabietic, abietic and neoabietic alcohol after hydrolysis of glycerol ester and reduction.
One interested party is of the opinion that although the relative peak areas of dehydroabietic and abietic alcohol in the GC method differed between the two substances, it is not possible to conclude that these differences represent quantitative differences in the amount of these resin acids in the finished products because the method specified in the JECFA monograph does not require the use of quantitative standards for determining the amount of the resin alcohols (Documentation provided to EFSA N. 17).
Gaefvert et al. (1994) identified five compounds after esterification of abietic acid with glycerol under experimental conditions: abietic acid (unreacted), glycerol triabietate, 1,2‐glycerol diabietate, 1,3‐glycerol diabietate and 1‐glycerol monoabietate (Figure 1).
Figure 1.
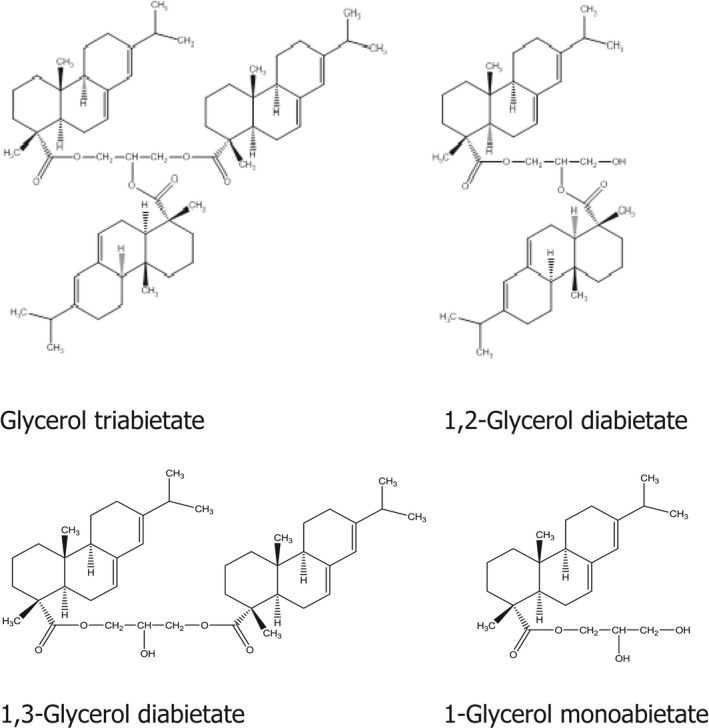
Structural formulae of products formed from esterification of abietic acid with glycerol (according to Gaefvert et al., 1994)
Analytical results on the contents of individual resin acids in GEWR measured after saponification are presented in the EFSA opinion on the safety of GEGR for the proposed use as a food additive for reasons of comparison (EFSA ANS Panel, 2010). In samples of saponificated GEWR (botanical origin not specified), the following resin acid concentrations were measured after saponification (given percentage refers to the total of each resin acid present in GEWR in bound and unbound form): abietic (19.5–26.0%), dehydroabietic (22.7–34.6%), palustric (4.29–5.90%), neoabietic (0.546–2.77%), pimaric (5.31–6.45%), isopimaric (9.35–15.0%), sandaracopimaric (5.76–6.41%) and communic (9.69–10.7%) acids (EFSA ANS panel, 2010). The given concentrations of the individual resin acids measured in GEWR samples after saponification covers the fraction of free resin acids and resin acids as hydrolysis products from esters. The structural formulae of resin acids found in GEWR of unspecified botanical origin are presented in Figure 2.
Figure 2.
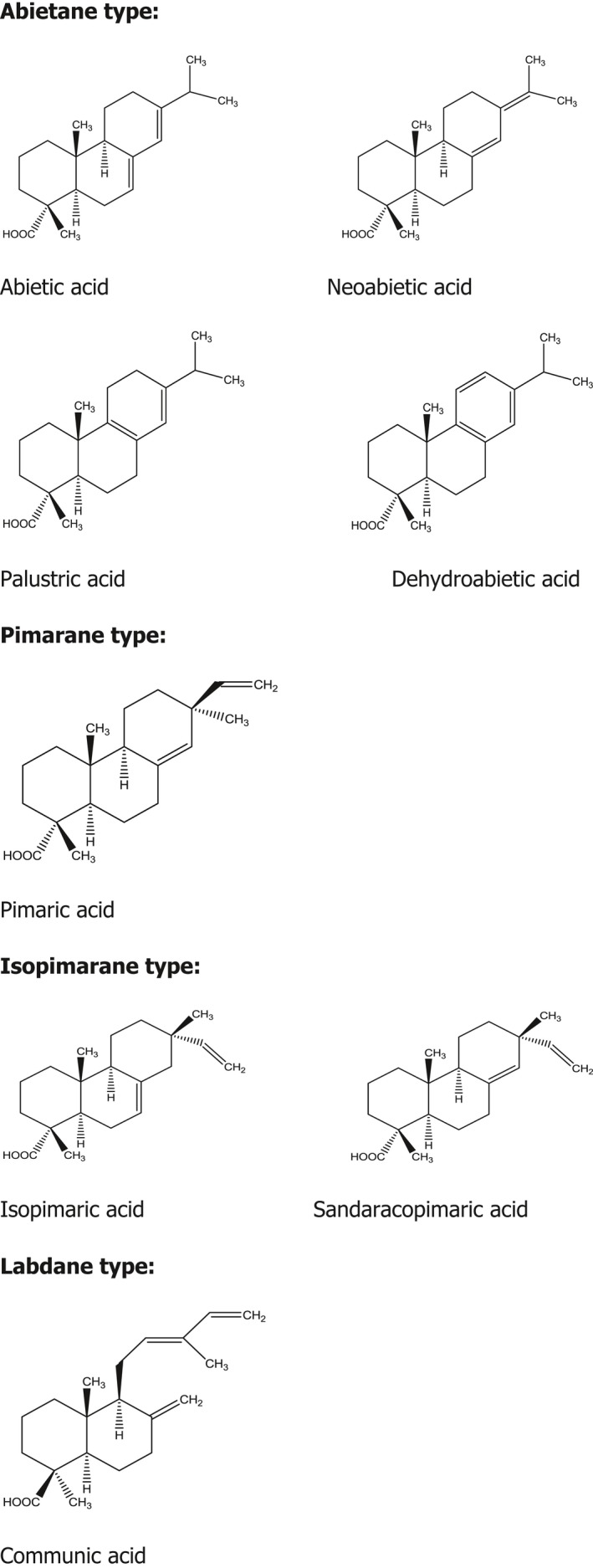
Structural formulae of resin acids found after saponification of GEWR of unspecified botanical origin (EFSA ANS panel, 2010)
Concerning the content and composition of the total glycerol esters of resin acids in GEWR, with regard to glycerol monoesters, 1,2‐glycerol diesters, 1,3‐glycerol diesters and glycerol triesters, an interested party (Documentation provided to EFSA N. 23) is of the opinion that distinguishing between them is not practical or feasible considering the multitude of different isomers and adducts that are formed with identical molecular weights. There are over 20 different possible monoesters based on the various resin acid isomers, for a diester, there are over 400 combinations possible, and for a triester, at least 8,000 combinations are possible.
The Chemical Abstracts Services (CAS) Registry Number 8050‐31‐5 is assigned to the glycerol esters of resin acids and is not only unique to GEWR but also used for GEGR and GETOR.
GEWR are hard, yellow to pale amber‐coloured solids. They are insoluble in water, but soluble in acetone (JECFA, 2013b; Documentation provided to EFSA N. 2).
According to one interested party (Documentation provided to EFSA N. 2), the following names are used as ‘synonyms’: ester gum; abietic acid glycerol ester; glycerol ester of rosin; rosin, glycerol ester; glycerol, rosin polymer; glyceryl abietate; glyceryl rosinate; rosin ester with glycerol; rosin, glycerine ester; rosin, glycerol resin.
Characterisation of the source material wood rosin
According to one interested party (Documentation provided to EFSA N. 23) throughout the history of the manufacture of GEWR, the wood rosin feedstock has always been based on a mixture originating from P. palustris and P. elliottii, which occur naturally in overlapping regions of the south‐eastern USA. The Panel noted that EC specifications do not define the source material by its exclusive botanical origin and that the food additive E 455 obtained from other botanical origins, if complying with the current specifications, may also be on the market in the EU.
Possible neutral components of wood rosin can be organised into 3 classes: (1) monoterpene neutrals, (2) diterpene neutrals and (3) high molecular weight neutrals. Each class consists of several fractions differentiated by structural type. The results of SPE and HT/HR GC analyses provided data on the component types within these fractions. Reversed‐phase ultra‐performance liquid chromatography coupled with ultraviolet photodiode array detection and multimode electrospray‐atmospheric‐pressure‐chemical‐ionization time‐offlight mass spectrometry (UPLC/UV‐EScI(+)‐TOFMS) tentatively identified individual neutral constituents representing six different structural types: (1) methyl esters of rosin, (2) diterpene aldehydes, (3) diterpene nor‐alcohols, (4) diterpene alcohols, (5) diterpene di‐alcohols, and (6) diterpene alcohol esters of rosin. Quantitation has not been achieved. A listing of neutral constituents of wood rosin which could also be components in GEWR has been provided by one interested party (Documentation provided to EFSA N. 23) and is presented in Appendix F.
According to one interested party, refined wood rosin used as the source rosin for the production of GEWR consists of the sum of the resin acids and the neutrals. The content of resin acids in the refined wood rosin originating from P. palustris (longleaf pine) and P. elliottii (slash pine) stumps was quantified using a potentiometric titration method specified in ASTM D1240‐02 (Documentation provided to EFSA N. 23). The results of the analysis for 5 retain samples of Pexite® wood rosin (the trade name for the refined wood rosin used as the source rosin for the GEWR) indicated that the resins acids content averaged at 88% (ranging from 86.9% to 89.3%). Based on the normal variation in acid number, the expected range for resin acids is 83–91%, and the results of the analysis would fall within the expected range. The neutrals content of refined (unesterified) wood rosin obtained by difference using the resin acids content data ranged from 10.7% to 13.1%, which would fall within the expected range of 9% to 17%, based on the normal variation in the refined wood rosin acid number (Documentation provided to EFSA N. 23).
Overall the Panel noted that GEWR (E 445) contain the following three fractions:
the fraction of ‘glycerol esters of resin acids (mono‐, di‐ and tri‐esters)’,
the fraction of ‘free resin acids’ and
the fraction of ‘neutrals (non‐acidic other saponifiables and unsaponifiables)’.
The percentage of the ‘glycerol monoesters of resin acids’, of the fraction of ‘free resin acids’ and of the fraction of ‘neutrals’ (including small amounts of esters of resin acids with alcohols different from glycerol) in GEWR is of particular interest, since these compounds may be absorbed or be hydrolysed to compounds which may be absorbed (see Section 3.5.1).
Evaluation of similarities of GEWR derived from Pinus halepensis and Pinus brutia stumps compared to GEWR derived from Pinus palustris (longleaf pine) and Pinus elliottii (slash pine)
The Panel noted that the current evaluation is based on the results of toxicological studies performed with GEWR originating from P. palustris (longleaf pine) and P. elliottii (slash pine) which are considered to be in compliance with the definition, description, identification and purity criteria of the specifications for GEWR (E 445) as defined in the Commission Regulation (EU) No 231/2012. GEWR originating from P. halepensis and P. brutia have been characterised by one interested party by IR spectroscopy and GC. The Panel noted that both GEWRs yielded almost identical IR spectra and similar retention times by the GC method of resin alcohols obtained after hydrolysis and reduction. However, the Panel also noted that no information was available, in which concentrations the fractions of ‘glycerol monoesters’, ‘free resin acids’ and ‘neutrals’ are present in the GEWR originating from P. halepensis and P. brutia. The concentrations of these fractions are known for GEWR derived from P. palustris (longleaf pine) and P. elliottii (slash pine), which have toxicologically been tested, averaging to 2.2%, 2.6% and 13.2%, respectively. Furthermore, it has been specified that possible concentrations of free neoabietic acid in the GEWR derived from P. palustris (longleaf pine) and P. elliottii (slash pine), are below the limit of quantitation. The concentrations of these fractions and of free neoabietic acid are considered to be of particular relevance for the outcome of the toxicological testing. Since the product derived from P. halepensis and P. brutia stumps is not characterised for the percentages of the fractions of ‘monoglycerol esters’, ‘free resin acids’ (including neoabietic acid) and ‘neutrals’, evaluation of equivalence with GEWR originating from P. palustris (longleaf pine) and P. elliottii (slash pine), tested in the toxicological studies is not possible. Altogether, the Panel concluded that the available data on the chemical composition of GEWR originating from P. halepensis and P. brutia do not allow for read across of toxicological data from GEWR originating from P. palustris (longleaf pine) and P. elliottii (slash pine).
3.1.2. Specifications
The specifications for GEWR (E 445) as defined in the Commission Regulation (EU) No 231/2012 and by the JECFA (2013b) are listed in Table 1.
Table 1.
Specifications for GEWR (E 445) according to Commission Regulation (EU) No 231/2012 and the JECFA (2013b)
| Commission Regulation (EU) No 231/2012 | JECFA (2013b) | |
|---|---|---|
| E 445 | INS No. 445(iii) | |
| Definition | A complex mixture of tri‐ and diglycerol esters of resin acids from wood rosin. The rosin is obtained by the solvent extraction of aged pine stumps followed by a liquid‐liquid solvent refining process. Excluded from these specifications are substances derived from gum rosin, an exudate of living pine trees, and substances derived from tall oil rosin, a by‐product of kraft (paper) pulp processing. The final product is composed of approximately 90% resin acids and 10% neutrals (non‐acidic compounds). The resin acid fraction is a complex mixture of isomeric diterpenoid mono‐carboxylic acids having the empirical molecular formula of C20H30O2, chiefly abietic acid. The substance is purified by steam stripping or by countercurrent steam distillation |
Glycerol ester of wood rosin is a complex mixture of tri‐ and diglycerol esters of resin acids from wood rosin, with a residual fraction of glycerol mono esters. Besides these esters, neutrals (non‐acidic saponifiable and unsaponifiable substances) and residual free resin acids are present. It is obtained by the solvent extraction of aged pine stumps (Pinus palustris (longleaf) and Pinus elliottii (slash) species) followed by a liquid–liquid solvent refining process. The refined wood rosin composed of 90% resin acids and 10% neutrals (non‐acidic saponifiable and unsaponifiable substances). The resin–acid fraction is a complex mixture of isomeric diterpenoid monocarboxylic acids having the typical empirical formula C20H30O2, of which the main component is abietic acid. The substance is purified by steam stripping or by countercurrent steam distillation. These specifications do not cover substances derived from gum rosin, an exudate of living pine trees, and substances derived from tall oil rosin, a by‐product of kraft (paper) pulp processing |
| Description | Hard, yellow to pale amber‐coloured solid | Hard, yellow to pale amber‐coloured solid |
| Functional uses | ‘ |
Emulsifier, density adjustment agent (flavouring oils in beverages), stabiliser, chewing gum base component |
| Identification | ||
| Solubility | Insoluble in water, soluble in acetone | Insoluble in water, soluble in acetone |
| Infrared absorption spectrum | Characteristic of the compound | The infrared spectrum of a thin film of the sample (potassium bromide disc) corresponds with the typical infrared spectrum below |
| Sulfur test | – |
Negative Weigh 40–50 mg of sample into a test tube and add 1–2 drops of a 20% (w/v) solution of sodium formate. Place a strip of lead acetate test paper over the mouth of the test tube. Heat the tube until fumes are formed that contact the test paper. Continue heating for 2–5 min. The formation of a black spot of lead sulfide indicates the presence of sulfur‐containing compounds. (Detection Limit: 50 mg/kg sulfur) |
| Gas chromatography of resin alcohols and glycerol | – |
Passes test See description under TESTS |
| Purity | ||
| Specific gravity of solution | [d]20 25 not less than 0.935 when determined in a 50% solution in d‐limonene (97%, boiling point 175.5–176 °C, d20 4: 0.84) | d20 25: Not less than 0.935 (50% solution in d‐limonene) |
| Ring and ball softening range | Between 82 and 90°C | Not less than 82° (see ‘Specific Methods, Glycerol Esters of Rosins’) |
| Acid value | Not less than 3 and not more than 9 |
Between 3 and 9 (see ‘Specific Methods, Fats, Oils, and Hydrocarbons’) |
| Hydroxyl value | Not less than 15 and not more than 45 | – |
| Arsenic | Not more than 3 mg/kg | – |
| Lead | Not more than 2 mg/kg |
Not more than 1 mg/kg Determine using an AAS/ICP‐AES technique appropriate to the specified level. The selection of sample size and method of sample preparation may be based on the principles of the method described in Volume 4 (under ‘General Methods, Metallic Impurities’) |
| Mercury | Not more than 1 mg/kg | – |
| Cadmium | Not more than 1 mg/kg | – |
| Test for absence of tall oil rosin (sulfur test) | When sulfur‐containing organic compounds are heated in the presence of sodium formate, the sulfur is converted to hydrogen sulfide which can readily be detected by the use of lead acetate paper. A positive test indicates the use of tall oil rosin instead of wood rosin | – |
AAS: atomic absorption spectroscopy; ICP‐AES: inductively coupled plasma atomic emission spectroscopy.
The Panel noted that the terms ‘tri‐ and diglycerol esters of resin acids’ in the EU specifications are not chemically correct and should be replaced by the terms ‘glycerol di‐ and triesters of resin acids’.
As indicated in Section 3.1.1, the JECFA definition of GEWR (E 455) (JECFA, 2013b) gives more precise details for the identity of the food additive and its source material compared to the definition in the EU specifications:
for the food additive GEWR (E 455), in addressing the occurrence of a residual fraction of glycerol monoesters, of a fraction of neutrals and of a fraction of residual free resin acids in GEWR and
for the source material wood rosin, in defining its exclusive origin from the two species P. palustris (longleaf pine) and P. elliottii (slash pine) and being composed of 90% resin acids (main component: abietic acid) and 10% neutrals (non‐acidic saponifiable and unsaponifiable substances).
The Panel noted that the term ‘final product’ in the definition of the EU specifications is misleading and should be replaced by ‘source material wood rosin’.
The Panel also noted that different from the JECFA specifications the EU specifications require the determination of a hydroxyl value but no gas chromatographic test for resin alcohols and glycerol.
According to the information from an interested party, due to extremely high temperatures used in the manufacturing process employed to produce GEWR (E 445) (Section 3.1.3), it is unlikely for the finished product to contain microorganisms (Documentation provided to EFSA N. 2).
An interested party provided data showing compliance with the EU specifications. Six sample lots of GEWR (E 445) were analysed and revealed acid values in the range from 5.6 to 6.3, hydroxyl values in the range from 15 to 26 and a hydroxyl content in the range from 0.45% to 0.79%. Sample levels for arsenic, lead, mercury and cadmium were for each of the elements < 0.02 mg/kg and of heavy metals (measured as lead) were < 0.02 mg/kg (2 samples) or < 0.03 mg/kg (4 samples), respectively (Documentation provided to EFSA N. 2).
The Panel noted that the levels of the toxic elements in the six batches analysed, were about two orders of magnitude below the levels as defined in the Commission Regulation (EU) No 231/2012. According to the EC specifications for GEWR (E 445), impurities of the toxic elements arsenic, cadmium, lead and mercury are accepted up concentrations of 3, 1, 2 and 1 mg/kg, respectively. Contamination at those levels could have a significant impact on the exposure to these toxic elements, for which exposures are already close to the health‐based guidance values or benchmark doses (lower confidence limits) established by EFSA (EFSA CONTAM Panel, 2009, 2010, 2012a,b,c, 2014).
The Panel further noted that the EU specifications for glycerol (E 422) contain the limits for various other impurities (e.g. butanetriols, acrolein, chlorinated compounds, 3‐monochloropropane‐1,2‐diol) and that these contaminants may also be present in the food additive E 455.
Altogether, the Panel recommended an update of the definition and of the purity criteria of glycerol esters of wood rosin (E 445) in the Commission Regulation (EU) No 231/1012, taking the above mentioned parameters and the recent JECFA specifications (JECFA, 2013b) into account. Furthermore, GEWR may be more specifically characterised by indicating the maximum levels of the fraction of glycerol monoesters of resin acids and of the fraction of neutrals (non‐acidic other saponifiables and unsaponifiables) in the definition. Setting limits for glycerol monoesters of resin acids might be relevant for the specifications, since only the glycerol monoesters of GEWR are supposed to undergo hydrolysis in the gastro‐intestinal tract (see Section 3.5.1).
The Panel noted that the concentration of the total of free resin acids in GEWR is limited by the acid value which should not exceed 9. In addition, specifications might require, that levels of free neoabietic acid are below the limit of quantitation of 0.05 wt. %.
3.1.3. Manufacturing process
GEWR (E 445) is manufactured in a two‐phase process. At first, solvent extraction and refining of wood rosin from aged pine stumps is performed. In a second step, the refined wood rosin is esterified and the final product is purified. According to information from interested parties, the wood rosin is either obtained from P. palustris (longleaf pine) and P. elliottii (slash pine) stumps (Documentation provided to EFSA N. 2) or from P. halepensis and P. brutia stumps (Documentation provided to EFSA N. 17).
In detail, solvent extraction of pine wood chips is followed by counter current steam stripping and liquid‐liquid solvent refining of the crude rosin extract. This process removes volatile terpene fractions, as well as less volatile impurities such as metals and chromophoric, polar and oxidised species from the wood rosin prior to the esterification of the wood rosin, the refined wood rosin is subjected to counter current steam stripping once more. Subsequently, glycerol is added to the refined wood rosin in order to produce the glycerol esters of wood rosin. The wood rosin and glycerol are added in a fixed ratio, yielding a product consisting predominantly of glycerol triesters. The esterification reaction is carried out under extreme conditions (temperatures of greater than 250°C) in order to overcome steric hindrance and help the reaction progress to completion. However, once the reaction is complete, the final product will contain small amounts (approximately 5%) of unreacted wood rosin (Zinkel and Russell, 1989 as cited in Documentation provided to EFSA N. 2). The resultant product is a complex mixture comprising not only of primarily tri‐, but also di‐esters of resin acids from wood rosin and glycerol, with residual levels of glycerol monoesters and free resin acids. Following the esterification reaction, the glycerol esters of wood rosin are subjected to purification, which varies depending on the end‐use of the rosin product. Specifically, when intended to be used in chewing gum base, the wood rosin esters are purified by steam stripping, whereas when intended, e.g. to be used in adjusting the density of citrus oils for beverages, the rosin is purified by counter current steam distillation process (Documentation provided to EFSA N. 2).
The carboxylic group of the resin acids is attached to a tertiary carbon which is sterically hindered. In order to esterify this type of hindered carboxylic groups, generally higher temperatures and more drastic conditions have to be used than for other carboxylic acids. These steric effects are also responsible for the resistance of the resin acid ester linkage to cleavage by water, acid, and alkali (Hausen et al., 1982; Documentation provided to EFSA N. 5).
Under temperatures exceeding 200°C dehydroabietic acid, dihydroabietic acid and tetrahydroabietic acid may be formed from abietic acid and other resin acids present in wood rosin (Hausen et al., 1982).
The Panel noted that differences in temperature during esterification may result in differences in degrees of esterification, in stability of formed esters, and in residue concentrations of free acids, which may be relevant for compliance with specifications, as well as for toxicokinetic behaviour and induction of toxicological effects by GEWR.
3.1.4. Methods of analysis in food
Nasirullah Krishnamurthy and Nagaraja (1995) published a paper chromatographic method for the determination of GEWR in ready‐to‐serve beverages and their concentrates. A dried and concentrated chloroform extract is developed on a paper strip using methanol as mobile phase. After derivatisation with bromine, GEWR is detected and quantified by UV spectrometry at 245 nm. A detection limit was not determined.
According to information from interested parties, gas chromatography–mass spectrometry (GC–MS) can be used to detect GEWR on the surface of confectionary products. However, a detailed description of the method is not available (Documentation provided to EFSA N. 2).
Furthermore, methods for the detection of single GEWR components are published. Nilsson et al. (2008) analysed resin acids like abietic acid and dehydroabietic acid in different consumer products like cosmetics, using SPE and HPLC. Jones et al. (2001) analysed dehydroabietic acid in urine using GC‐MS. However, no application of these methods on food was described. Furthermore, similar products like GEGR and glycerol ester of tall oil rosin cannot be distinguished from GEWR.
3.1.5. Stability of the substance, and reaction and fate in food
GEWR is described by the JECFA to be essentially chemically and biologically inert and no reaction in foods and no effect on other nutrients are expected. The steric effects are responsible for the resistance of the resin‐acid ester linkage to cleavage by water, acid, and alkali and explain the stability of the glycerol ester in the gastro‐intestinal tract with only a minor fraction undergoing partial hydrolysis (JECFA, 2013a,b).
A confectionary which was treated with an edible ink containing 20% GEWR was stored at 30°C for 72 h, simulating ageing under accelerated conditions, and compared to freshly printed samples. The samples were analysed by GC/MS and by olfactometry. The GC/MS revealed the presence of free and esterified (methyl‐ and ethyl esters) fatty acids in extraction samples of the aged, printed candy, which were not identified in the control samples. For the ‘aged’ samples, the presence of relative small amounts of some specific free fatty acids and methyl‐ and ethyl esters of fatty acids could be detected, which were not found for the freshly printed samples (Documentation provided to EFSA N. 12). Overall, one interested party concluded from the results that glycerol ester of wood rosin remains stable when used as part of edible inks for application onto confectionary candy (Documentation provided to EFSA N. 2).
3.2. Authorised uses and use levels
Maximum levels of GEWR (E 445) have been defined in Annex II to Regulation (EC) No 1333/200813 on food additives, as amended. In this document, these levels are named maximum permitted levels (MPLs). Table 2 summarises food categories (FCs) that are permitted to contain GEWR (E 445) and the corresponding MPLs as set by Annex II to Regulation (EC) No 1333/2008.
Table 2.
MPLs of GEWR (E 445) in foods according to the Annex II to Regulation (EC) No 1333/2008
| Food category number | Food category name | Restrictions/exception | Maximum level (mg/L or mg/kg as appropriate) |
|---|---|---|---|
| 04.1.1 | Entire fresh fruit and vegetables | Only surface treatment of citrus fruit | 50 |
| 05.2 | Other confectionery including breath freshening microsweets | Only for printing on personalised and/or promotional hard‐coated confectionery products | 320 |
| 14.1.4 | Flavoured drinks | Only cloudy drinks | 100 |
| 14.2.6 | Spirit drinks as defined in Regulation (EC) No 110/2008 | Only cloudy spirit drinks | 100 |
| 14.2.8 | Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol | Only flavoured cloudy alcoholic drinks containing less than 15% of alcohol | 100 |
MPL: maximum permitted level.
GEWR (E 445) does not have other authorisation according to Annex III.
3.3. Exposure data
3.3.1. Reported use levels of GEWR (E 445)
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued a public call14 for occurrence data (usage level and/or concentration data) on GEWR (E 445). In response to this public call, information on the actual use levels of GEWR (E 445) in foods was made available to EFSA by industry. No analytical data on the concentration of GEWR (E 445) in foods were made available by the Member States.
Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with data on use levels (n = 25) in foods for 2 out of the 5 food categories in which GEWR (E 445) are authorised.
Updated information on the actual use levels of GEWR (E 445) in foods was made available to EFSA by Spanish Association of Postharvest Services and Processes (AGRUPOST) (Documentation provided to EFSA N. 26), Specialised Nutrition Europe (SNE) (Documentation provided to EFSA N. 27), FoodDrinkEurope (FDE) (Documentation provided to EFSA N. 28).
The Panel noted that seven usage levels on FC 14.1.4 Flavoured drinks referred to niche products. Out of these, six usage levels were excluded from further analysis in the refined scenarios since other usage levels were available for these food category, and only one record of use levels on niche products referring to flavoured drinks with sweetener was retained.
Appendix A provides data on the use levels of GEWR (E 445) in foods as reported by industry.
3.3.2. Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information of over 2.5 million food and beverage products of which more than 900,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 20 out of its 28 member countries and Norway presented in the Mintel GNPD.15
For the purpose of this Scientific Opinion, the Mintel's GNPD16 was used for checking the labelling of food and beverages products for GEWR (E 445) within the EU's food market as the database contains the compulsory ingredient information on the label.
According to the Mintel's GNPD, GEWR (E 445) was labelled between January 2013 and January 2018 on 1,563 products, mainly in subcategories Carbonated Soft Drinks, Beverage Concentrates, Fruit/Flavoured Still Drinks, Sports Drinks, Energy Drinks and some Flavoured Alcoholic Beverages and Beers.
Appendix B lists the number and percentage of the food products labelled with GEWR (E 445) out of the total number of food products per food subcategories according to the Mintel's GNPD food classification. The percentages ranged from less than 0.1% in many food sub‐categories to 24.4% in the Mintel's GNPD food subcategory ‘Sports drink’, while most of the products (n = 504) were labelled on ‘Carbonated Soft Drinks’. The average percentage of foods labelled to contain GEWR (E 445) was 1.2%.
The Panel noted that GEWR (E 445) was listed as ingredient in 13 food products of the Mintel's GNPD subcategories ‘Cakes, Pastries & Sweet Goods’, ‘Other Sauces & Seasonings’, ‘Sweet Biscuits/Cookies’, ‘Bread & Bread Products’, ‘Prepared Meals’, ‘Poultry Products’, ‘Dairy Based Ice Cream & Frozen Yogurt’, ‘Seasonal Chocolate’, ‘Table Sauces’, ‘Non‐Individually Wrapped Chocolate Pieces’, ‘Confiture & Fruit Spreads’, ‘Dressings & Vinegar’ in which they are not authorised.
3.3.3. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). Consumption surveys added in the Comprehensive database in 2015 were also taken into account in this assessment.8
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database includes the currently best available food consumption data across Europe.
Food consumption data from the following population groups: infants, toddlers, children, adolescents, adults and the elderly were used for the exposure assessment. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 3).
Table 3.
Population groups considered for the exposure estimates of GEWR (E 445)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlersa | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrenb | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlyb | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Netherlands, Sweden, UK |
The term ‘toddlers’ in the EFSA Comprehensive Database corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013.
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure estimates. In practice, the FoodEx food codes were matched to the FCS food categories.
Food categories considered for the exposure assessment of GEWR (E 445)
The food categories in which the use of GEWR (E 445) are authorised were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
For the following food categories, the restrictions/exceptions which apply to the use of GEWR (E 445) could not be taken into account or only can be partially considered. This applies to three food categories (Appendix C) and may have resulted in an overestimation of the exposure:
5.2 Other confectionery including breath freshening microsweets, only for printing on personalised and/or promotional hard‐coated confectionery products. Only hard coated confectionary products were taken into account in order to narrow the food category and approximate the restriction as specifically as possible. Category considered only in the MPL scenario, as use level data was not available.
14.1.4 Flavoured drinks, only cloudy drinks. Information on the turbidity (cloudiness) of the product is not indicated in the FoodEx system in the Comprehensive Database. Based on Mintel information, all soft drinks were taken into account in the assessment. Other types of flavoured drinks such as flavoured milk based drinks and flavoured plant‐based milk substitute drinks were not taken into account, as the additive is considered as not used in them.
14.2.6 Spirit drinks as defined in Regulation (EC) No 110/2008, only cloudy spirit drinks. Information on the turbidity (cloudiness) of the product is not indicated in the FoodEx system in the Comprehensive Database. Based on Mintel information, liquors and rum were included in the assessment, only in the MPL scenario, as use level data was not available.
14.2.8 Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol only flavoured cloudy alcoholic drinks containing less than 15% of alcohol. Information on the turbidity (cloudiness) of the product is not indicated in the FoodEx system in the Comprehensive Database. It was not possible to distinguish these types of products based on their turbidity. As vast majority of them could be considered cloudy, the whole category was considered in the assessment, only in the MPL scenario, as use level data was not available.
For the refined scenario, three food categories were not taken into account because no concentration data were provided for these food categories to EFSA (Appendix C). For the remaining food category FC 04.1.1 Entire fresh fruit and vegetables, only surface treatment of citrus fruits, all citrus fruits in the Comprehensive Database were taken into account.,
Overall, for the regulatory maximum level exposure scenario, five food categories were included, while for the refined scenarios 2 food categories were included in the present exposure assessment to GEWR (E 445) (Appendix C).
3.4. Exposure to GEWR (E 445) from its use as a food additive
The Panel estimated chronic exposure for the following population groups: infants; toddlers, children, adolescents, adults and the elderly. Dietary exposure to GEWR (E 445) was calculated by multiplying GEWR (E 445) concentrations for each food category (Appendix C) with their respective consumption amount per kilogram of body weight for each individual in the Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only one day per subject were excluded as they are considered as not adequate to assess repeated exposure.
This was carried out for all individuals per survey and per population group, resulting in distributions of individual average exposure per survey and population group (Table 4). Based on these distributions, the mean and 95th percentile of exposure was calculated per survey for the total population and per population group. The high percentile exposure was only calculated for those population groups where the sample size was sufficiently large to allow calculation of the 95th percentile of exposure (EFSA, 2011a). Therefore, in the present assessment, high levels of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain were not included.
Table 4.
Summary of dietary exposure to GEWR (E 445) from its use as a food additive in the regulatory maximum level exposure assessment scenario and in the refined exposure scenarios, in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants | Toddlers | Children | Adolescents | Adults | The elderly | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (12 weeks–11 months) | (12–35 months) | (3–9 years) | (10–17 years) | (18–64 years) | (≥ 65 years) | |||||||
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | |
| Regulatory maximum level exposure assessment scenario | ||||||||||||
| Mean | 0 | 0.22 | 0.02 | 1.17 | 0.10 | 1.40 | 0.15 | 1.08 | 0.07 | 0.47 | 0.04 | 0.16 |
| 95th percentile | 0 | 1.40 | 0.13 | 4.31 | 0.50 | 3.60 | 0.61 | 2.61 | 0.32 | 1.70 | 0.15 | 0.64 |
| Refined estimated exposure assessment scenario | ||||||||||||
| Brand‐loyal scenario | ||||||||||||
| Mean | 0 | 0.2 | 0.02 | 1.07 | 0.09 | 1.01 | 0.14 | 0.77 | 0.06 | 0.36 | 0.03 | 0.10 |
| 95th percentile | 0 | 1.32 | 0.10 | 4.06 | 0.46 | 3.24 | 0.58 | 2.04 | 0.3 | 1.39 | 0.13 | 0.42 |
| Non‐brand‐loyal scenario | ||||||||||||
| Mean | 0 | 0.08 | 0.01 | 0.44 | 0.05 | 0.48 | 0.07 | 0.37 | 0.03 | 0.16 | 0.02 | 0.06 |
| 95th percentile | 0 | 0.51 | 0.09 | 1.59 | 0.25 | 1.28 | 0.27 | 0.89 | 0.14 | 0.59 | 0.09 | 0.23 |
Two exposure scenarios were defined and carried out by the ANS Panel regarding the concentration data of GEWR (E 445) used: (1) MPLs as set down in the EU legislation (defined as the regulatory maximum level exposure assessment scenario); and (2) the reported use levels (defined as the refined exposure assessment scenario). These two scenarios are discussed in detail below.
3.4.1.
Regulatory maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario is based on the MPLs as set in Annex II to Regulation (EC) No 1333/2008 and listed in Table 3.
The Panel considers the exposure estimates derived following this scenario as the most conservative since it is assumed that the consumer will be continuously (over a lifetime) exposed to GEWR (E 445) in food at MPL.
Refined exposure assessment scenario
The refined exposure assessment scenario is based on use levels reported by industry. This exposure scenario can consider only food categories for which the above data were available to the Panel.
Appendix C summarises the concentration levels of GEWR (E 445) used in the refined exposure assessment scenario. Based on the available data set, the Panel calculated two refined exposure estimates based on different model populations:
-
The brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to GEWR (E 445) present at the maximum reported use level for one food category. This exposure estimate is calculated as follows:
-
—
Combining food consumption with the maximum of the reported use levels for the main contributing food category at the individual level.
-
—
Using the mean of the typical reported use levels for the remaining food categories.
-
—
The non‐brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to GEWR (E 445) present at the mean reported use levels in food. This exposure estimate is calculated using the mean of the typical reported use levels for all food categories.
Dietary exposure to GEWR (E 445)
Table 4 summarises the estimated exposure to GEWR (E 445) from its use as a food additive in six population groups (Table 3) according to the different exposure scenarios. Detailed results per population group and survey are presented in Appendix D.
From the regulatory maximum level exposure assessment scenario, the mean exposure to GEWR (E 445) from its use as a food additive ranged from 0 mg/kg bw per day in infants to 1.40 mg/kg bw per day in children. The 95th percentile of exposure to GEWR (E 445) ranged from 0 mg/kg bw per day in infants to 4.31 mg/kg bw per day in toddlers.
From the refined estimated exposure scenario, in the brand‐loyal scenario, the mean exposure to GEWR (E 445) from its use as a food additive ranged from 0 mg/kg bw per day in infants to 1.07 mg/kg bw per day in toddlers. The high exposure to GEWR (E 445) ranged from 0 mg/kg bw per day in infants to 4.06 mg/kg bw per day in toddlers.
In the non‐brand‐loyal scenario, the mean exposure to GEWR (E 445) from its use as a food additive ranged from 0 mg/kg bw per day in infants to 0.48 mg/kg bw per day in children. The 95th percentile of exposure to GEWR (E 445) ranged from 0 mg/kg bw per day in infants to 1.59 mg/kg bw per day in toddlers.
Main food categories contributing to exposure to GEWR (E 445) using the maximum level exposure assessment scenario
From the regulatory maximum level exposure assessment scenario, the main contributing food categories to the total mean exposure estimates in all population group were ‘unprocessed fruit and vegetables’ and ‘flavoured drinks’. In addition to them, for children and adolescents confectionary products were also contributing significantly, while for adults and the elderly ‘alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol’ also appeared as a contributor in some surveys (see Appendix E for more details).
Main food categories contributing to exposure to GEWR (E 445) using the refined exposure assessment scenario
As use level data was available for only two food categories, these two, namely the ‘unprocessed fruit and vegetables’ and ‘flavoured drinks’ are appearing as main contributor in all population groups in both brand loyal and non‐brand loyal scenarios.
Uncertainty analysis
Uncertainties in the exposure assessment of GEWR (E 445) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 5.
Table 5.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
|
Consumption data: Different methodologies/representativeness/underreporting/misreporting/no portion size standard |
+/– |
| Use of data from food consumption surveys covering only a few days to estimate high percentiles (95th) long‐term (chronic) exposure | + |
| Correspondence of reported use levels to the food items in the EFSA Comprehensive Database: uncertainties to which types of food the levels refer | +/– |
| Uncertainty in possible national differences in use levels within food categories | +/– |
|
Concentration data: – Levels considered applicable to all foods within the entire food category – Levels not fully representative of foods on the EU market |
+ +/– |
| Food categories included in the exposure assessment: no data for certain food categories which were therefore not considered in the exposure estimates (n = 3 for the refined scenarios out of the 5 authorised food categories) | – |
| Food categories selected for the exposure assessment: inclusion of food categories without considering the restriction/exception (n = 3 max scenario/n = 1 refined scenarios out of 5 authorised food categories) | + |
|
Regulatory maximum level exposure assessment scenario: – exposure calculations based on the MPLs according to Annex II to Regulation (EC) No 1333/2008 |
+ |
|
Refined exposure assessment scenarios: – exposure calculations based on the maximum or mean levels (reported use from food industries) |
+/– |
MPL: maximum permitted level.
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
GEWR (E 445) are authorised in five food categories from which for two categories use levels were reported. The Panel calculated that out of the foods authorised to contain food additive GEWR (E 445) according to Annex II to Regulation (EC) No 1333/2008, 0.1–82% of the amount of food consumed (by weight) per population group was reported to potentially contain food additive GEWR (E 445) as a food additive.
Furthermore, the Panel noted that information from the Mintel GNPD (Appendix B) taking into account the period between January 2013 and January 2018 indicated that 14 out of 29 food subcategories, categorised according to the Mintel GNPD nomenclature, in which GEWR (E 445) was labelled were included in the current refined exposure assessment. These 14 food subcategories represented approximately 85% of the food products labelled with GEWR (E 445) in the database. In the remaining 15 food subcategories from Mintel, in which GEWR (E 445) was labelled but which were not included in the exposure assessment, GEWR (E 445) was authorised in 4 food subcategories. On the other hand, some foods not authorised according to the EU legislation were found to be labelled in the Mintel's GNPD to contain the additive (sauces, cakes, pastries & sweet goods, etc.). Furthermore, the percentage of foods per subcategory labelled to contain GEWR (E 445) was maximally about 24% (Appendix B), while in the assessment it was assumed that 100% of the foods belonging to an authorised food category contained the additive.
Given these observations, the Panel considered overall that the uncertainties identified would, in general, result in an overestimation of the exposure to GEWR (E 445) from its use as a food additive according to Annex II in both the regulatory maximum level and refined exposure scenario. Based on the assumption that the food additive is not used in those food categories in which it is permitted but for which no usage data were provided by the stakeholders, also the refined scenario would in general result in an overestimation of exposure.
3.5. Biological and Toxicological data
Biological and toxicological studies conducted with GEWR have been evaluated several times by JEFCA and also by the SCF (JECFA, 1975, 1996a,b; SCF, 1992, 1994). Structurally related substances are GEGR and GETOR which are not authorised as food additives in the EU. In recent evaluations by EFSA, the Panel concluded that the data base is not sufficient for evaluation of the chemical equivalence of GEWR and GEGR (EFSA ANS panel, 2010) or GEWR and GETOR (EFSA ANS Panel, 2011). Thus, the toxicological data obtained with GEGR and GETOR cannot be used for a read across to GEWR.
Also unpublished studies conducted with GEWR and the parent compound wood rosin in the early 1960s by Industrial Bio‐Test Laboratories (Documentation provided to EFSA N. 9 and 10; Documentation provided to EFSA N. 19) were available from the EFSA public call for data. The reliability of the studies is questionable since several reports conducted by Industrial Bio‐Test Laboratories have been disqualified by the FDA as a result of an investigation that revealed improper practices in the conducting laboratory (Marshall, 1983; EFSA ANS Panel, 2010). Furthermore, the composition of resin acids in GEWR is expected to differ from that of the parent compound wood rosin. Due to the drastic manufacturing conditions of the glycerol esters of rosins, including heating at high temperatures, isomerisation, oxidation and dehydrogenation/hydrogenation reactions of the resin acids (whether as individual free acids or bound in esters) may occur. Decarboxylation of resin acids may also take place. In consequence, the composition of resin acids in the glycerol esters of rosins is expected to differ from that of the original rosin (Soltes and Zinkel, 1989; Downs and Sansom, 1999). Even though the Panel concluded, that the studies conducted in the early 1960's by Industrial Bio‐Test Laboratories and especially studies on wood rosin are only of limited value for the re‐evaluation of GEWR, they are summarised below for completeness.
Most data referred to in the following sections were identified in the JECFA evaluations on GEWR (JECFA, 1996a,b). In the JECFA evaluations, the Committee referenced to several unpublished studies. The cited original reports were available for the present pre‐evaluation from the EFSA public call for data. No references on the toxicological evaluation were given in the SCF Opinion (SCF, 1994); however, it is presumed that a similar data base was used as in the JECFA evaluations (JECFA, 1996a,b).
The biological and toxicological studies with relevance for the re‐evaluation of GEWR (E 445) (e.g. Documentation provided to EFSA N. 3, 4, 5 and 6; Documentation provided to EFSA N. 15; Documentation provided to EFSA N. 8; Documentation provided to EFSA N. 7; Documentation provided to EFSA N. 14; Mukherjee et al., 1992), were conducted with GEWR described as ‘Ester Gum 8BG’, a commercial preparation of GEWR; beverage grade. The botanical origin of the test material is not described in the protocols. However, according to an interested party ‘the GEWR used for each of the biological and toxicity studies, similar to the food additive product in commerce, is based on a mixture of the 2 species (i.e., P. palustris and P. elliottii). Considering that the GEWR that was used in the toxicological and biological studies shares the same botanical origin as the food additive with the same manufacturing process, it can be deduced that the GEWR used in the toxicity studies is compositionally equivalent to the food additive product in commerce…’ (Documentation provided to EFSA N. 23). The Panel noted that JECFA recognised that the GEWR used for the toxicity studies considered in the JECFA evaluations (JECFA 1996a and b), was based, just as the product now in commerce, on a mixture originating from the two species P. palustris and P. elliottii.
In vivo studies with radiolabelled GEWR indicated the hydrolysis of small amounts of GEWR (see Section 3.5.1). Resin acids as products of hydrolysis of glycerol monoesters or esters with other alcohols might be released in the gastro‐intestinal tract and absorbed. Furthermore, GEWR contain a fraction of residual free resin acids. Therefore, studies on the metabolic fate and for toxicological endpoints of resin acids after oral application are also presented. The hydrolysis product glycerol is considered to only contribute to overall glycerol exposure in minor amounts and is therefore not addressed in this evaluation.
3.5.1. Absorption, distribution, metabolism and excretion
Ester bonds in GEWR are described to be resistant to hydrolysis by water, acids and alkali except under extreme conditions. The stability of the esters is due to the fact that the carboxylic group of the resin acids is attached to a tertiary carbon which causes steric hindrance. (Hausen et al., 1982; Documentation provided to EFSA N. 5). Several in vitro and in vivo studies have been conducted to clarify to which extent GEWR also resists enzymatic hydrolysis in the gastro‐intestinal tract.
3.5.1.1. Studies with GEWR cited in the previous JECFA evaluations
All studies available have been cited in the previous JECFA evaluations (JECFA, 1996a,b).
In vitro studies
In vitro studies were conducted to investigate the stability of GEWR in the gastro‐intestinal tract Documentation provided to EFSA N. 13). Radiolabelled GEWR ([1,3‐14C] glycerol ester of wood rosin; equivalent to Ester Gum 8BG, a commercial preparation of GEWR; beverage grade, used also in in vivo studies) were incubated at a concentration of 4.4 or 0.5 mg/mL with human faecal extract or simulated gastric fluid for 24 h, the negative control with sterile water. Samples were collected after an incubation period of 0, 6, or 24 h. Analysis in a HPLC‐radiodetector system revealed the stability of the test item in working emulsions.
The elution patterns on the radiochromatogram were similar for the different incubation periods at each experimental design. Further analysis was based on changes in radioactivity in seven regions related to the peaks of radioactivity on the radiochromatogram. No significant changes occurred at either concentration in the negative control. With human faecal extracts, only minor differences (authors comment: ‘not substantial’) between time points were occasionally seen in regions of the radiochromatogram containing small peaks. A concentration of 0.5 mg radiolabelled GEWR per mL simulated gastric fluid induced no significant change in the peak regions after 24 h. However, the high concentration of 4.4 mg/mL gastric fluid resulted in a slight decrease after 24 h of incubation (related to 6 h of incubation but not to 0 h) in one specific peak region which is associated with the triglycerides of rosin. The author of the study stated that the emptying time of the stomach is normally about 4 h and changes occurring after that time will have no effect on the stability of GEWR in the stomach in vivo. The authors concluded that no ‘substantial change’ occurred with the longest incubation time in sterile water, gastric fluid or faecal extracts, indicating the stability of GEWR in the human gastrointestinal tract (Documentation provided to EFSA N. 13).
The Panel noted that with regard to use levels, the concentration at the high‐dose level might not realistically simulate the situation in the gastro‐intestinal tract, and that no changes were reported at the low concentration.
In vivo studies
In a feeding study in F344 rats the absorption of unlabelled GEWR from the gastrointestinal tract was studied (Documentation provided to EFSA N. 5 and 6). Groups of six males (initial body weight 223–246 g) and six females (initial body weight 144–161 g) received in the first experiment a diet containing 0.7 or 2.8% GEWR for 24 h (Ester Gum 8BG); no further details. Doses correspond to 83 mg/rat or 274 mg/rat (equivalent to 354 or 1,168 mg/kg bw, respectively; males and females combined for calculation) or in the second experiment groups of 6 male rats a diet supplemented with 1.4% or 2.8% of the test item for 10 days (corresponding to 127 mg/rat per day or 332 mg/rat per day or to 541 or 1,416 mg/kg bw, respectively). The analytical control revealed concentrations within 10% of nominal concentrations as well as homogeneity and stability of the test item in the diet. Feed consumption was measured during the treatment period and data used for calculation of GEWR intake for each experimental group. Faeces were collected in 24‐h intervals during the treatment and for 5 (first experiment) or 4 days (second experiment) in the post‐treatment period. Analysis of faeces was performed via HPLC of solvent extract. Analytical validation showed that the limit of detection was about 25 mg GEWR in 7 g of faecal material. Studies of the recovery of the test material added to rat faecal samples resulted in recoveries of 94–118% of the nominal concentration; the overall relative standard deviation was < 10%. In the first experiment (intake for 24 h), rats excreted the ingested GEWR via the faeces within 48 (low dose) or 72 h (high dose) after the treatment period; no GEWR was detected in faeces thereafter. At the 0.7% level, 75% (mean total recovery 73% in males and 78% in females) of the ingested GEWR was accounted for in the faeces but 95% (97% in males and 9% in females) at the 2.8% level. The author discussed the lower recovery from the 0.7% group as a lack of sensitivity of the analytical method: the concentrations in faeces were near the detection limit. In the second experiment with male rats, the ingestion of the test material via the diet resulted in a mean total recovery of about 102% of the amount at a dietary level of 1.4% and about 91% at the 2.8% level. The excretion of GEWR via faeces increased at days 1 and 2 of the exposure period and remained high through day 12. Approximately 72 h following the completion of dosing, no test article was detected in the faeces. The author concluded that the total faecal recoveries in the 1‐day study at a dose of 2.8% GEWR and in the 10‐day study at doses of 1.4 or 2.8% GEWR were essentially equal to the amounts ingested indicating no absorption from the gastro‐intestinal tract.
In a subsequent study (Documentation provided to EFSA N. 15), radiolabelled GEWR was used. The 14C‐label was positioned at the glycerol moiety ([1,3‐14C] glycerol esters of wood rosin). The author used the same test material (Ester Gum 8BG) for labelling than in the preceding study with unlabelled GEWR performed in Documentation provided to EFSA N. 5 and 6. Groups of Fischer 344 rats received via gavage a single dose of about 200 mg/kg bw 14C‐GEWR after one (5 males and 5 females) or 10 days (5 males) of dietary administration of unlabelled compound (1.4% in the diet). Analytical studies revealed the stability and homogeneity of the labelled test item in the dosing emulsion. The radioactivity eliminated in expired carbon dioxide, urine, and faeces during the 120‐hour interval after gavage and the residual radioactivity in the carcass (including intestinal content) 120 hours after treatment was measured. The extent of hydrolysis of GEWR was determined by reverse‐phase HPLC of extracts of faeces. Male and female rats fed unlabelled GEWR for 1 day excreted ≤ 1% of the administered radiolabel either as expired carbon dioxide or in urine within 120 h. Most of the applied radioactivity (> 95%) was recovered in faeces and cage rinses within 120 h. The majority of the dose was eliminated within 48 h after dosing. Similar results were obtained for male rats given the radiolabel after a 10‐day dietary administration of unlabelled GEWR: < 1.1% of the applied radioactivity was detected in expired carbon dioxide or in urine. In both experiments, only traces (< 0.2%) were found in eight of 15 carcasses obtained 120 h after treatment. According to the author of the study, the traces may have been unabsorbed radiolabel remaining in the intestine. The author concluded that less than 2% of the administered dose was absorbed in male and female rats after a single oral application of 200 mg/kg bw 14C‐GEWR. However, HPLC analysis of the faeces of male rats (no data about females) collected 0–48 h after gavage of 14C‐GEWR showed a higher percentage of a radioactive peak that eluted at the approximate void volume of the column than did a standard solution of 14C‐GEWR, with 0.8% of the administered radioactivity in samples collected at 0–12 h, 2.2% at 12–24 h and 0.8% at 24–48 h after application. Furthermore, two small peaks of radioactivity that were present in the standard solution of 14C‐GEWR and eluted at the approximate retention time of glycerol monoesters of GEWR were not detectable in faeces. According to the authors, these data indicate that only a small percentage of the administered 14C‐GEWR, probably glycerol monoesters, was hydrolysed, however the ester bonds were essentially stable under conditions anticipated in the human gastro‐intestinal tract.
In a separate experiment (Documentation provided to EFSA N. 15), five male F344 rats with jugular vein and biliary duct cannulas received 200 mg/kg bw of 14C‐GEWR by gavage. The radiolabel was measured in bile and blood at 4‐ and 12‐h intervals for 24 h after administration. The rats excreted 1.6–2.9% of the applied radioactivity into bile during a 24‐h interval after gavage. In HPLC analyses of two bile samples obtained 0–4 h after application, total radioactivity eluted at the approximate void volume of the column. According to the author, again, this result suggested the presence of hydrolysis products, which may be the same as those excreted via faeces. Analysis of the labelled GEWR in blood demonstrated only traces of applied radioactivity (< 0.1%) in blood at 4, 8, 12 or 24 h after administration. Also, in liver samples collected from the same rats 24 h after treatment, radiolabel represented only 0.1–0.2% of the applied dose.
The Panel noted that the results of the experiments performed by Documentation provided to EFSA N. 15 indicated that after gavage of 14C‐GEWR in rats, only low levels of the applied radioactivity (5% by combining excretion via bile/faeces, urine and exhaled air) were absorbed and this absorption is correlated with a low hydrolysis or degradation in the gastrointestinal tract. However, due to the particular radiolabelling of 14C‐GEWR, the results of this study are only referring to the metabolic fate of the glycerol moiety in GEWR but not to that of the unradiolabelled resin acids moieties.
3.5.1.2. Studies (in vivo) with free resin acids cited in the previous JECFA evaluations
Data on the ADME of free resin acids were evaluated since these resin acids might be released in the gastro‐intestinal tract after minor hydrolysis of GEWR (Documentation provided to EFSA N. 15). An unpublished study (Documentation provided to EFSA N. 16) cited by the JECFA (1996a) and available from the EFSA public call for data7 reports on a series of ADME experiments with dehydroabietic acid, isopimaric acid and tetrahydroabietic acid. In the studies by Documentation provided to EFSA N. 16, radioactivity in excreta and tissues was quantified by liquid scintillation spectroscopy after oral application of the tritium‐labelled (site of label not reported) and purified resin acids. Analysis of metabolites included paper, thin layer and gas chromatographic methods. All experiments were conducted in rats (strain not reported).
Dehydroabietic acid
The excretion of dehydroabietic acid was investigated in four male rats exposed orally (details not reported) to 100 mg of 3H‐dehydroabietic acid per rat (no data about body weight) (Documentation provided to EFSA N. 16). The author measured radioactivity in faeces and urine over a 15‐day post‐treatment period (no data about exhalation or disposition in the body). Rats excreted an average of 80.0% of the applied radioactivity in faeces and 7.2% in urine (total recovery 87.2%). In a preliminary trial with two male rats (also 100 mg/rat corresponding to about 300 mg/kg bw), the excretion of the radioactivity in faeces peaked 24–36 h after application and declined slowly through the fourth day. Most radioactivity in the urine was detected 0–12 h after treatment followed by rapid decline. In experiments with lower dose levels of labelled dehydroabietic acid (only 2 mg/kg bw), essentially the same results were obtained: male rats (n = 4) excreted an average of 87.8% of applied radioactivity in faeces and 5.3% in urine. Further analysis of urine revealed that most of radioactivity in urine is related to the excreted dehydroabietic acid and/or metabolites and not tritium‐labelled water.
In further experiments on total recovery after oral application (details not reported), rats (n = 4; no data about sex) received 1 mg 3H‐dehydroabietic acid (5.5 μCi) per animal (Documentation provided to EFSA N. 16). Sampling of excreta in metabolism cages was carried out at various time intervals ranging from 28 to 51 h after treatment. The amount of applied radioactivity recovered averaged 70% in faeces and 17% in the gastro‐intestinal tract (combined: 87%), 8% in urine, 1.1 % in the carcass, and only 0.5% in breath. The average total recovery was 97%.
To test the assumption that the test item was absorbed and radioactivity excreted via the bile, the author used bile cannulated rats (sex not reported) (Documentation provided to EFSA N. 16). The animals received orally either 10 (n = 1) or 20 μCi (n = 3) of 3H‐dehydroabietic acid and 50 mg of unlabelled dehydroabietic acid (no further details about dose and administration). The bile was collected for up to 96 h. Approximately 20.4% to 40.8% of the administered dose was recovered in the bile of rats exposed to 20 μCi and 7.3% in the rat exposed to 10 μCi. Excretion via the bile was highest 6–24 h after application. These data indicated that dehydroabietic acid is absorbed and excreted in the bile after oral administration. Combining all available data on excretion of radioactivity via exhaled air, urine and bile, the author concluded that about 40% of the orally applied dose is absorbed.
The distribution of 3H‐dehydroabietic acid and/or metabolites following oral absorption was studied in 4 male and 4 female rats treated with 50 mg (5.5 μCi) of labelled dehydroabietic acid (oral route, no details reported) (Documentation provided to EFSA N. 16). One male and one female were sacrificed at 1, 2, 4 or 8 h after application and distribution of radioactivity measured in heart, liver, spleen, muscle, kidney, brain, lung, fat, and testes. The results showed no accumulation in any organ. Analysis revealed the highest concentration of radioactivity in the liver. Peak levels occurred 2–4 h after treatment.
The author (Documentation provided to EFSA N. 16) investigated also the metabolism of 3H‐dehydroabietic acid using qualitative and quantitative chromatography. Four male rats received orally (no details reported) 2 mg/kg bw of labelled dehydroabietic acid. Metabolites were determined in faeces, bile and urine. Analysis of the faecal samples (samples of highest activity, no further details) revealed unmetabolised dehydroabietic acid as well as a more polar metabolite designated as metabolite C (no quantification available). In the urine of two rats, dehydroabietic acid (13% and 14% of the radioactivity found in the urine) and a metabolite B (60% and 67%) were detected. The bile of two rats revealed mainly metabolite B (61% and 69% of the radioactivity found in the bile) and minor amounts of metabolite C (9% and 10%) and metabolite D (11% and 14%). The author stated that metabolite B in the bile might be converted to metabolite C by the intestinal microflora and further identified in faeces.
In order to obtain further data on the metabolism of dehydroabietic acid, an additional study was conducted at a higher dose level and using methodological alteration in processing of samples (Documentation provided to EFSA N. 16). Three male rats were administered (no details reported; presumably oral application) approximately 300 mg/kg bw per day 3H‐dehydroabietic acid on 2 successive days. Urine and faeces were collected over a period of 4 days (no further details about sampling period) and excreta of the four rats pooled for analyses. The pooled faeces disclosed 89% of the applied radioactivity and the pooled urine 8.4%. Quantitative chromatography identified 3 metabolites (metabolite A, B and C) beside the unchanged parent compound in the faeces. The percentage of the applied radioactivity recovered as metabolite A in faeces was 33%, metabolite B 8%, metabolite C 14% and unchanged dehydroabietic acid 14%; 20% of the applied radioactivity were not identified. Two metabolites in urine accounted for 7.1% of applied radioactivity as metabolite B, 0.55% metabolite C and 0.13% dehydroabietic acid. The identity of the metabolites was not finally clarified but according to the author there is evidence that metabolite A is more non‐polar than the parent compound and supposed to be a reduction product. Metabolites B and C were more polar than dehydroabietic acid and are believed to be hydroxylated metabolites. None of the metabolites was conjugated.
Tetrahydroabietic acid
Two rats received orally 45.7 μCi of 3H‐tetrahydroabietic acid (sex, dose and application not reported) (Documentation provided to EFSA N. 16). In metabolism cages, faeces were collected for 16 days in 24‐h sampling intervals for the first 4 days after treatment (thereafter in 4‐day intervals), urine for 8 days in 24‐h intervals for the first 4 days (pooled samples for day 5–8) and exhaled air for 5 days also in 24‐h sampling interval. Rats excreted an average of 68% of the applied radioactivity in the faeces, 3.6% in the urine and 2.2% in exhaled air. An amount of 8.7% of the applied radioactivity was reported as ‘incidental’ (no further data). The total recovery was 82% of the administered radioactivity. Excretion via faeces peaked 1 or 2 days after treatment and declined the fourth day to minor amounts. Concerning urinary excretion, most of the radioactivity was detected in samples of day 1, traces later than day 3. The decline in exhaled radioactivity was slowly during the observation period; but rats exhaled only minor amounts of radioactivity (presumably tritium in exhaled water) via this route. Data on excretion of absorbed tetrahydroabietic acid in the bile were not available. Thus, no statement can be given on the absorption rate. However, thin layer chromatography of the faeces revealed that radioactivity was mainly related to the unchanged tetrahydroabietic acid and minor amounts to an unidentified metabolite. Also, in urine, the bulk of radioactivity represented tetrahydroabietic acid itself. According to the authors, the excretion pattern would be similar to that of dehydroabietic acid.
Isopimaric acid
Two rats were orally exposed to 9.19 μCi of 3H‐isopimaric acid (sex, dose and application not reported) and excretion studied in metabolism cages Documentation provided to EFSA N. 16). Faeces, urine and exhaled air were collected for 6–7 days in 12‐h sampling intervals for the first 2 days and thereafter in 24‐h intervals. The rats excreted an average of 76% of the applied radioactivity in the faeces, 13% in the urine and 1.8% was exhaled. In this experiment, the total recovery was 95% of the administered radioactivity. The excretion pattern was similar to dehydroabietic acid and tetrahydroabietic acid. Elimination of radioactivity via faeces peaked one or two days after treatment and declined the fourth or fifth day to minor amounts. Concerning urinary excretion, most radioactivity was detected in samples of day 1, only small amounts at day 4 (rat 1) or 6 (rat 2). Rats excreted minor quantities of radioactivity via exhalation (presumably tritium in exhaled water) during the observation period with a peak at day 2. No data were reported on excretion in the bile. The thin layer chromatography of the faeces indicated that the majority of radioactivity represented isopimaric acid itself and small amounts an unidentified metabolite. The urine was not analysed. According to the authors, the excretion pattern is similar to dehydroabietic acid and tetrahydroabietic acid.
3.5.1.3. Additional studies with free resin acids not cited in the previous JECFA evaluations
Dehydroabietic acid
Asakawa et al. (1986), analysed the metabolites of (+)‐dehydroabietic acid in the urine of six rabbits (strain and sex not reported). The urine samples were collected for 3 days after oral administration of 2 g of the sodium salt of dehydroabietic acid as a suspension in 20 mL of 0.02% aqueous Tween 80. The metabolites were extracted from urine. The analysis by thin layer chromatography revealed the presence of nine different metabolites. The structures of five metabolites were presented in detail: (+)‐methyl 15‐hydroxyabieta‐8,11,13‐trien‐18‐oate, methyl abieta‐8,11,13,15‐tetraen‐18‐oate, (+)‐methyl 16‐hydroxyabieta‐8,11,13‐trien‐18‐oate, (+)‐methyl 16‐acetoxyabieta‐8,11,13‐trien‐18‐oate and methyl 7‐oxo‐abieta‐8,11,13‐trien‐18‐oate.
Matsumoto et al. (1990) identified several metabolites of (+)‐dehydroabietic acid from urine of rabbits orally administered with the sodium salt of dehydroabietic acid (no further details on experimental design were available). On the basis of chemical and spectral data, the authors identified seven different metabolites: (15S)‐8,11.13‐abietatrien‐16,18‐dioic acid, 2 alpha‐hydroxy‐8,11,13,15‐abietatetraen‐18‐oic acid, (15R)‐15,16‐dihydroxy‐8,11,13‐abietatrien‐18‐oic acid, 2 beta,15‐dihydroxy‐8,11,13‐abietatrien‐18‐oic acid, (15S)‐2 beta,16‐dihydroxy‐8,11,13‐abietatrien‐18‐oic acid, 2 alpha,15‐dihydroxy‐8,11,13‐abietatrien‐18‐oic acid and (15S)‐2 alpha,16‐dihydroxy‐8,11,13‐abietatrien‐18‐oic acid.
Abietic acid
Asakawa et al. (1986) identified two metabolites detected in the 72‐h urine of rabbits (n = 6, sex and strain not reported) after oral application (presumably gavage) of 2 g of the sodium salt of (‐)‐abietic acid. Thin‐layer chromatography identified one of these two metabolites: (‐)‐dimethyl abieta‐7,13‐diene‐16,18‐dioate.
Overall, in vitro and in vivo studies demonstrated that ester bonds in GEWR are largely resistant to hydrolysis in the gastrointestinal tract. In rats receiving GEWR radiolabelled on the glycerol moiety, the low levels of radioactivity in bile, urine, carcasses and exhaled air would give evidence for a low absorption (2–5%) of the applied dose. The major fraction of oral dose was mainly excreted unchanged in the faeces. Results of HPLC analysis of faeces and bile suggested that the absorbed components were hydrolysed products from glycerol monoesters of wood rosin present in GEWR. A few metabolic data are available for free resin acids which might be released in the gastrointestinal tract after hydrolysis of glycerol monoesters or of esters with other alcohols in GEWR. Experiments in rats orally administered with radiolabelled dehydroabietic acid revealed absorption of approximately 40% of the dose. Most of the radioactivity was excreted in bile and faeces, minor amounts were found in urine and only traces were exhaled. Tetrahydroabietic acid and isopimaric acid exhibited an excretion pattern similar to dehydroabietic acid.
3.5.2. Acute toxicity
3.5.2.1. Studies with GEWR
No studies on acute oral toxicity of GEWR were available.
3.5.2.2. Studies with free resin acids
Dehydroabietic acid
In an acute toxicity study, dehydroabietic acid (78.3% pure) dissolved in corn oil was administered by oral intubation to male and female Sprague–Dawley rats. The LD50 values obtained for males and females were in the range of 3,000‐4,000 mg/kg bw with no delayed toxicity up to 14 days after treatment (Villeneuve et al., 1977).
3.5.2.3. Studies with wood rosin cited in the previous JECFA evaluations
Oral LD50 values for wood rosin (Pinus species and composition not indicated) determined in Swiss White mice, guinea pigs and Albino rats by intubation of a solution in corn oil ranged from 4,100 to 8,400 mg/kg bw (Documentation provided to EFSA N. 11).
The Panel noted the questionable reliability of the study conducted by Industrial Bio‐Test Laboratories (see Introduction of Section 3.5). However, the Panel considered that available data suggest a low acute oral toxicity of GEWR.
3.5.3. Short‐term and subchronic toxicity
3.5.3.1. Studies with GEWR cited in the previous JECFA evaluations
All studies available have been cited in the previous JECFA evaluations (JECFA, 1996b).
In a subchronic feeding study (Documentation provided to EFSA N. 9), groups of 10 male and 10 female Sprague–Dawley rats received for 90 days a diet containing GEWR (Ester Gum 8D; suspension in corn oil) at levels of 0%, 0.01%, 0.05%, 0.2%, 1.0% or 5.0 % (equivalent to 0, 9, 45, 180, 900 or 4,500 mg/kg bw per day). The diets of treatment and control groups contained 2.3% corn oil except the high‐dose group, which contained 11.7% corn oil. The tested parameters included daily clinical observations, weekly body weight and food consumption measurements, monthly haematology and urinalysis (n = 5 per sex), and at termination organ weights and gross and microscopic pathology. Histopathology was restricted to five rats per sex per dose. During the course of this study, no clinical signs occurred. No significant effects were noted on body weight. At the 5% level, the food intake was reduced and food utilisation increased due to the high amount of corn oil in the diet. No treatment‐related effects were detected in haematology, urinalysis, gross and microscopic pathology. Relative (but not absolute) organ weights of liver, gonads and heart were sporadically decreased in mid‐dose groups; these effects were not considered by the author of the study to be of toxicological relevance due to the lack of a dose response. The author stated that there were no significant histological alterations in any of the test groups which could be attributed to the subacute feeding of the test material. The Panel noted the questionable reliability of the study conducted by Industrial Bio‐Test Laboratories (see Introduction of Section 3.5. However, the NOAEL would be 5% GEWR in the diet, the highest dose tested, (equivalent to 4,500 mg/kg bw per day). The Panel noted that the study results are in agreement with those of the studies by Documentation provided to EFSA N. 3 and 4 described below.
In a 13‐week feeding study according to current standard, groups of 20 male and 20 female F344 rats (6 weeks of age at initiation) were fed a diet containing GEWR (Ester gum 8BG, beverage grade) at concentrations yielding nominal dose levels of 0, 625, 1,250 or 2,500 mg/kg bw per day (Documentation provided to EFSA N. 3 and 4). The concentrations in the diet were adjusted weekly reflecting the corresponding body weight and food consumption. The analytical concentrations of the test item in the diet ranged between 89% and 108% of the desired target concentrations. Further analytical studies revealed the homogeneity and stability of the test item in the diet. The treatment did not induce any clinical signs in any dose group. Slight (< 5%) but significant decreases in body weight occurred occasional in males and females as well as significant effects on food consumption and food efficiency. The author considered these effects to be of no toxicological relevance. Ophthalmology, haematology, clinical chemistry and necropsy showed no treatment‐related effects. Significant increases in treated groups were noted in the relative caecum (full but not the empty caecum) weights of males at 2,500 mg/kg bw per day and relative liver (difference < 10%) and adrenal (left but not the right adrenal) weights of high‐dose females. The increase in relative kidney weight (left but not the right organ) reached significance in females of the low‐ and mid‐dose group (but not at 2,500 mg/kg bw per day). The absolute but not the relative thymus weights were significantly decreased in females at 1,250 and 2,500 mg/kg bw per day. However, all of these organ weight differences were considered by the author to indicate normal biological variation. Furthermore, no treatment‐related histopathological changes were observed in any of these or other examined organs. The author noted a slight increase in the incidence of chronic myocarditis in the high‐dose males (incidence 16/20 vs 10/20 in controls) and females (6/20 vs 3/20 in controls). Incidences comparable to controls were found in low‐ and mid dose. However, chronic myocarditis occurs spontaneously in this strain of rat. Therefore, the author considered these effects not to be treatment related. Overall, the author of the study allocated a NOAEL of 2,500 mg/kg bw per day, the highest dose tested, for male and female rats in this study (Documentation provided to EFSA N. 3 and 4). The Panel agreed with the conclusion of the author. This NOAEL formed the basis for the ADI of 0–25 mg/kg bw per day as allocated for GEWR by the JECFA (JECFA 1996b, 2013a), while the SCF derived an ADI of 12.5 mg/kg bw per day from the NOAEL of 2,500 mg/kg bw per day (no specification of the toxicological data base) by applying an UF of 200 (SCF, 1994).
3.5.3.2. Studies with free resin acids
Dehydroabietic acid
In a subacute toxicity study, male Sprague–Dawley rats weighing 150–225 g were fed diets containing 0, 50, 500 and 5,000 mg dehydroabietic acid/kg diet ad libitum (equivalent to 0, 5, 50, 500 mg/kg bw per day) (Villeneuve et al., 1977). The purity of the test material was 78.3%. Ten animals from each group were killed after 14 and 28 days. Haematological investigations were made at each sacrifice. Food and water intake was measured weekly and body weight was measured daily. All animals underwent a complete autopsy. According to the authors, there were no gross or histopathological changes associated with the exposure up to the highest dose in the diet for 28 days.
3.5.3.3. Study with wood rosin cited in the previous JECFA evaluations
In a 90‐day study in Sprague–Dawley rats (10/sex per group), wood rosin (Pinus species and composition not indicated) was added to the diet at concentrations of 0%, 0.01%, 0.05%, 0.2%, 1.0% or 5.0% (equivalent to 0, 9, 45, 180, 900 or 4,500 mg/kg bw per day) (Documentation provided to EFSA N. 10). Feeding at a 5.0% level was attempted, but discontinued at an early stage of the study, since all the animals died during the first 8 days of the treatment period. The author attributed these deaths to starvation through refusal of the diet. The parameters examined included clinical observations, food consumption, body weights, haematology, urinalysis, organ weights, and gross and microscopic pathology. No mortality in controls or in animals receiving lower doses of wood rosin and no differences between treated groups from controls were observed for haemoglobin, haematocrit, total leucocyte count, differential leucocyte count or urine analysis parameters. Body weights were reduced throughout the study period in male and female rats fed 1.0% wood rosin. Statistically significant changes in absolute and relative weights (to body weight and/or brain weight) of the liver, kidney and spleen were observed in male and female rats at the 1.0% concentration. No macroscopic or microscopic pathological lesions related to wood rosin administration were reported in examined test animal organs. The Panel noted the questionable reliability of the study conducted by Industrial Bio‐Test Laboratories (see Introduction of Section 3.5).
Overall, no treatment‐related effects were detected in a 13‐week feeding study (Documentation provided to EFSA N. 3 and 4) according to current standards after oral exposure of male and female F344 rats to 0, 625, 1,250 or 2,500 mg/kg bw per day. The author of the study allocated a NOAEL of 2,500 mg/kg bw per day, the highest dose tested. Results of an earlier subchronic feeding study in Documentation provided to EFSA N. 9 with limited reliability are in agreement with the study results of Documentation provided to EFSA N. 3 and 4. The NOAEL of 2,500 mg/kg bw per day formed the basis for the ADI of 0–25 mg/kg bw per day as allocated for GEWR by the JECFA (2013a) and for the ADI of 12.5 mg/kg bw per day as derived for GEWR by the SCF (1994).
3.5.4. Genotoxicity
Studies with GEWR
In vitro
In the study by Ishidate et al. (1984) GEWR (ester gum, with no further details) was tested in a bacterial reverse mutation assay with the Salmonella Typhimurium strains TA92, TA1535, TA100, TA1537, TA94 and TA98 using the plate incorporation method both with and without metabolic activation up to 10 mg/plate. The results of this study gave no indications for a mutagenic activity at any dose level both with and without metabolic activation. The study complies with the current OECD guideline no. 471 with the exception that the tester strains S. Typhimurium TA102 or Escherichia coli WP2uvrA, bearing AT mutations were not used.
In the study reported in Documentation provided to EFSA N. 8, GEWR (Ester Gum 8BG) was examined for mutagenic activity in a bacterial reverse mutation test using S. Typhimurium strains TA98, TA100, TA1535, TA1537 and TA1538. Two independent trials, without any modification, were performed using the plate incorporation method both with and without metabolic activation. Triplicate plates per test point were exposed to the test item up to concentration of 500 μg/plate, since precipitation occurred at dose levels of ≥ 156 μg/plate. Two independent experiments were performed. No mutagenic effects were reported both in the absence and presence of metabolic activation. The study complies with the current OECD guideline no. 471 with the exception that the tester strains S. Typhimurium TA102 or E. coli WP2uvrA, bearing AT mutations were not used.
One interested party provided the Panel with a newly performed bacterial reverse mutation test (Documentation provided to EFSA N. 24, 25). A sample of GEWR, (Ester Gum 8BG) spiked with 0.0534 wt. % neoabietic acid near its limit of quantitation (0.05 wt. %), was assayed in form of a solution with S. Typhimurium strains TA98, TA100, TA1535 and TA1537 and E. coli strain WP2 uvrA (pKM101) at a maximum dose level of 5,000 μg/plate (containing 2.7 μg neoabietic acid), both in the absence and presence of S9 metabolic activation, using the plate incorporation method. No mutagenic effect was observed at any concentration tested. In a chromosome aberration assay on 242 food additives, GEWR MSG (ester gums, composition undefined) was assayed for its clastogenic properties in a Chinese hamster lung (CHL) cell line using three concentrations in the absence of S9 metabolic activation only (Ishidate et al., 1984). The highest concentration used was 8.0 mg/mL, selected from a cytotoxicity test based on estimation of the 50% growth inhibition. Negative results were obtained. However, the Panel noted some shortcomings in the experimental protocol which include the treatment of cells only in the absence of S9 metabolic activation, the inclusion of short (3–6 h) treatment and a limited number of metaphases scored. On this basis the results are considered to be of limited relevance.
In a further chromosome aberration assay, GEWR (Ester Gum 8BG OSR) was evaluated for its potential to induce chromosomal aberrations in a Chinese hamster ovary (CHO) cell line both in the absence and presence of S9 metabolic activation (Documentation provided to EFSA N. 14). The cells were exposed to concentrations of 0, 127, 253, 380 or 507 μg/mL for 2 h in the presence of metabolic activation followed by incubation in medium for 8 hours and for 7.3 h in its absence and harvested after a total exposure period of 10 h. Other incubation periods were not tested. The highest dose‐level selected was based on the induced cell cycle delay evaluating the frequencies of the first, second and third metaphases analysed by the fluorochrome plus Giemsa (FPG) method. Duplicate cultures were used and 100 metaphases per culture were analysed. The results obtained indicated that GEWR (Ester Gum 8BG OSR) did not induce any statistically significant increase for both structural chromosomal aberrations and polyploid cells. The Panel noted significant deviation from the current OECD Guideline no. 473 regarding the treatment schedule and harvesting times; however, under these experimental conditions the positive control substances produced a clear positive response both with and without metabolic activation, and the results were considered reliable.
In the study reported in Documentation provided to EFSA N. 7, GEWR (Ester Gum 8BG OSR) was assayed for induction of DNA repair using the in vitro unscheduled DNA synthesis (UDS) assay in rat hepatocytes at dose levels of 0, 5, 13, 25, 51, 76 or 102 μg/mL for 18–19 h. Triplicate cultures were used for each concentration; 150 cells per dose were evaluated. Untreated control, solvent control (acetone) and positive control ran concurrently. Concentrations > 25 μg/mL resulted in precipitates but no cytotoxicity (cell viability measured). The results obtained indicated that GEWR (Ester Gum 8BG OSR) did not produce any evidence of UDS in either the hepatocyte or intestinal mucosal cells. The Panel noted that this assay detects only compounds, which specifically trigger DNA excision repair following DNA adduction.
In the newly performed study submitted by one interested party (Documentation provided to EFSA N. 24), GEWR was evaluated for its potential to induce chromosome aberration in an in vitro micronucleus test in human lymphocytes. The study was in compliance with OECD TG 487 and good laboratory practice (GLP). The substance was assayed at the maximum concentration of 62.5 μg/mL as limited by solubility in the final treatment medium and at two lower dose levels spaced by a factor of 2. Three treatment series were performed: a 3 h treatment in the absence and presence of S9 metabolic activation and a continuous treatment up to 20 h in the absence of S9 metabolic activation. Neither cytotoxicity nor increases in the incidences of micronucleated cells were observed at any concentration tested, in any treatment series.
In vivo
In the chromosome aberration assay (Mukherjee et al., 1992), four male Swiss mice per group (single sex not justified by the authors but similar toxic effects for males and females in subchronic studies, were administered GEWR, orally in corn oil at dose levels of 0, 50, 100 or 150 mg/kg body weight. Mice were sacrificed 6, 12 or 24 h after administration (i.p. 4 mg colchicine/kg bw 90 min prior to sacrificed). In all mice, bone marrow cells were processed for cytogenetic analysis of 100 metaphases per animal; gaps were counted but excluded from evaluation. Significant and dose‐related increases in the frequency of cell with structural chromosomal aberrations, compared to the concurrent vehicle control, were observed at the two higher dose levels (100 and 150 mg/kg bw) 6 hours after oral treatment. No increase in chromosomal damage compared to the concurrent vehicle controls was observed at the 12‐ and 24‐h sampling times at any dose‐level tested.
The authors of the study classified GEWR as a weak clastogen. The Panel noted that the observed increases were low, and statistical analyses were performed using pooled data from all animals and not for individual animals, which represent the actual statistical units according to OECD guidelines on in vivo genotoxicity testing. This exercise excluded, de facto, the evaluation of inter animal variability, which is an important parameter for a correct statistical analysis. The Panel also noted that significant increases in cells with structural chromosomal aberrations were only observed at the early sampling time (6 h after sacrifice). This pattern indicates a mechanism of clastogenicity S‐independent, typical of ionising radiation and radiomimetic agents, which is rarely observed for chemicals.
Overall, the Panel considered that the positive findings of this study should be considered with caution.
In the same study, Mukherjee et al. (1992) also measured the induction of sister chromatid exchanges (SCE) in the bone marrow cells of mice by GEWR (Ester Gum 8BG), following a single oral administration at the same dose‐levels used for the chromosomal aberration assay (0, 50, 100 or 150 mg/kg) and no significant increases in the frequencies of SCE's were observed at any dose‐level assayed compared with the concurrent vehicle control. However, the Panel noted that this test is not included in the current test batteries for detection of genotoxic compounds.
Studies with free resin acids
In vitro
Data on genotoxicity of resin acids (Nestmann et al., 1979; Nestmann and Lee, 1983) have already been reported in the JECFA evaluation (JECFA, 1996a). No information is available from the EFSA public call for data, but by literature search further references were identified (Douglas et al., 1983; Ozaki et al., 2005; Seifried et al., 2006).
In the study by Nestmann et al. (1979), primaric acid (College point NY, purity 85%), abietic acid (Sigma Chemical Co., purity ≥ 65%) and the following eight acid resins synthesised according to methods outlined in the literature which included neoabietic acid (purity 95%), levopimaric acid (purity 95%), dehydroabietic acid (purity 95%), monochlorodehydroabietic acid (purity 95%), dichlorodehydroabietic acid (purity 95%), sandaracopimaric acid (purity 90%) and isopimaric acid (purity 98%) were evaluated for their mutagenic activity in the bacterial reverse mutation test using S. typhimurium strains TA98, TA100, TA1535, TA1537 and TA1538 both in the absence and presence of S9 metabolic activation. Tested concentrations reached the limit of solubility and/or cytotoxic threshold. Negative and positive controls ran concurrently. The results obtained indicated for neoabietic acid a marked mutagenic response at doses ≥ 400 μg/plate in the absence of S9 with TA1535, TA1538, TA98 and TA100 tester strains of S. typhimurium. Such a marked mutagenic response (> 1,500 revertants/plate for strain TA100 at the top dose of 1,000 μg/plate) was possibly attributed by the authors to the presence of impurities in the lot of neoabietic acid used, since the remaining nine compounds evaluated in the same study which are structurally related, did not produce any mutagenic response. Further arguments from the authors, in support of this hypothesis, comes from the observation that no mutagenicity (data not reported) was observed using a crude sample of material containing 10% neoabietic acid, tested up to 10 mg/plate (i.e. the same final dose of synthetised neoabietic acid). The Panel could not exclude this possibility, which is further supported by the absence of structural alerts for genotoxicity for this compound, as evaluated by the OECD QSAR Toolbox, as well as the possibility that the mutagenic response was mimicked by the formation of microcolonies of auxothropic bacteria (i.e. not true his+ revertants) growing on trace of histidine under conditions of extreme cytotoxicity. This last aspect cannot be excluded since no data on the clearance of the background lawn were reported.
Neoabietic acid (purity undefined) was also tested in a gene conversion assay in Saccharomyces cerevisiae D7 without metabolic activation. No genotoxic activity was detected at concentrations up to the cytotoxic limit (1.5 mg/mL, solvent not reported). However, in parallel gene mutation assays in S. cerevisiae strain XV185‐14C (tested only without metabolic activation) a threefold increase in TRP+ revertants was reported at non‐cytotoxic concentrations (1 mg/mL). Other resin acids like dehydroabietic acid, isopimaric acid, levopimaric acid, pimaric acid and sandaracopimaric acid again revealed a negative response in both assays (Douglas et al., 1983; Nestmann and Lee, 1983). The Panel noted that the test system employed has not received further validation, and it is currently considered obsolete.
Abietic acid was not mutagenic in the bacterial reverse mutation test in S. typhimurium strains TA98, TA100, TA1535, TA1537 and TA1538 at concentrations up to the threshold of cytotoxicity (3.3 mg/plate, solvent not reported) in the presence and absence of a metabolic activation system (Seifried et al., 2006).
The rec‐assay was used as an indicator of DNA damage in bacteria (Ozaki et al., 2005). Dehydroabietic acid and abietic acid produced enlarged killing zones in the Bacillus subtilis strains M45 rec‐, unable to repair DNA damage, compared to the DNA repair‐proficient strain. The Panel noted that this assay did not receive sufficient validation and it is not included in the accepted test batteries for mutagenicity testing.
In the report by Seifried et al. (2006), abietic acid (purity undefined) was indicated to be mutagenic in the mouse lymphoma assay in the absence but not in the presence of metabolic activation. The reported lowest effective dose was 46 μg/mL. However, the Panel noted that the criteria for positive results (a doubling of mutant frequency over the concurrent negative control) are not aligned with those indicated in the current OECD Guideline no. 490. Since no data on cytotoxicity and mutant frequencies were reported, no firm conclusion could be drawn.
In summary, the Panel considered that GEWR, when tested as a mixture, did not show genotoxic potential in the core battery of adequately performed in vitro tests (i.e. bacterial reversion and in vitro micronucleus assays) recommended in the EFSA guideline on genotoxicity testing strategy (EFSA Scientific Committee, 2011a). Negative results were also obtained in limited in vitro chromosomal aberration and UDS assays. Positive results, of unclear biological relevance and plausibility, were only reported in a limited in vivo study. Based on the overall weight evidence, the Panel considered GEWR as non‐genotoxic.
Limited information is available on individual compounds from the residual free resin acids fraction, which accounts only for 2.3–2.8% of the mixture. A positive mutagenic outcome was only observed for neoabietic acid in limited bacterial and yeast mutation assays (Nestmann et al., 1979; Nestmann and Lee, 1983). The Panel noted that the positive results with neoabietic acid were not corroborated by results with structurally related compounds and that no structural alerts for genotoxicity were identified in neoabietic acid. On this basis, the Panel considered that the mutagenic response of neoabietic acid could possibly be attributed to the presence of impurities in the lot tested, as also suggested by the study authors, as well as to cytotoxic effects through the formation of microcolonies of auxotrophic bacteria (i.e. not true his+ revertants), since no data on the clearance of the background lawn were reported. Therefore, the Panel concluded that the positive findings reported with neoabietic acid are of questionable relevance for the genotoxicity assessment of GEWR. The Panel also noted that, according to the information provided by one interested party (Documentation provided to EFSA N. 24 and 25), the levels of free neoabietic acid in GEWR are below the limit of quantitation of 0.05 weight percent.
Altogether the Panel concluded that GEWR (E 445) containing free neoabietic acid below the limit of quantitation (0.05 wt. %) when used as a food additive are of no concern for genotoxicity.
3.5.5. Chronic toxicity and carcinogenicity
Studies with GEWR
No studies on chronic toxicity and carcinogenicity of GEWR and resin acids mentioned in Figure 2 are available.
There are no studies available concerning chronic toxicity or carcinogenicity of GEWR. The literature search in the databases Toxline, Medline, and SciFinder did not identify any additional information and there is also no information available from the EFSA public call for data.
The only report identified was that on an in vitro study of abietic acid and dehydroabietic acid in the Bhas 42 cell‐transformation assay for initiation and promotion (Ohmori and Kawamura, 2009). Tested in the initiation stage, abietic acid and dehydroabietic acid did not increase transformation frequencies. At the promotion stage, however, both chemicals induced transformed foci dose dependently at concentrations of 20–60 nmol/mL (6–18 μg/mL). The authors of the study concluded that abietic acid and dehydroabietic acid may have tumour‐promoting potential (Ohmori and Kawamura, 2009). The Panel noted that the cell‐transformation assay is not currently accepted as a reliable test by the OECD.
Studies with wood rosin cited in the previous JECFA evaluations
Two 2‐year studies conducted by Industrial Bio‐Test Laboratories, in which the source material wood rosin was administered with the diet to rats and dogs (Documentation provided to EFSA n. 19 and 20)) are described below.
Rats
Groups of weanling Sprague–Dawley rats (30/sex per group) were fed wood rosin (Pinus species and composition not indicated) at dietary levels of 0%, 0.05%, 0.2% or 1%, equivalent to approximately 0, 25, 100 and 500 mg/kg bw per day respectively, for a period of 24 months (Kohn,1962a). At the end of the 12 months, five animals of each sex were killed for gross and histopathological examinations. At the end of the 24‐month period all remaining surviving animals were killed. All major organs were collected and weighed and subjected to histopathological examination. No statistical analysis was performed on any of the parameters evaluated, with the exception of body weight gain and organ weight measurements. Body weights of high‐dose animals were reported to be lower than body weights of control animals throughout the study period (not examined statistically). At 12 months, body weight gain was significantly lower in high‐dose male and female rats compared to control animals; however, after 24 months, only high‐dose females continued to exhibit reduced body weight gain. According to the author, the decrease in body weight gain and body weights may have resulted from the reduced food consumption that was also noted in the high‐dose group (not examined statistically), which was attributed to the non‐palatability of the diet mixed with wood rosin. Mortality, haematology, results of urinalysis, gross and histopathology were comparable among the wood rosin treated groups and control animals. Sporadic differences that were statistically significant in the relative (to body weight) liver, kidney, brain, or spleen weights in females were observed at all dose levels tested compared to the control group; however, these findings were considered not to be compound‐related by the authors due to the lack of a dose‐response and the occurrence in one sex only. The Panel noted the questionable reliability of the study conducted by Industrial Bio‐Test Laboratories (see Introduction of Section 3.5).
Dogs
In a 24‐month dietary study, wood rosin (botanical species and composition not indicated) was administered to beagle dogs (3/sex per group) at dietary concentrations of 0%, 0.05% (low‐dose) or 1.0% (high‐dose), equal to 14 and 260 mg/kg bw per day, respectively (Kohn, 1962b; JECFA, 1996b). The parameters examined included body weight, food consumption, mortality and behavioural changes, haematology and urine analysis, liver and kidney function tests, gross and histopathological examinations, and relative organ weight ratio (no statistical analysis was conducted on any of the parameters evaluated). An increase in body weight and food consumption was observed in low‐dose male dogs compared to control animals; however, a decrease in body weight and in food consumption was observed in high‐dose male dogs compared to control animals. No other compound‐related effects were observed in dogs fed wood rosin compared to control animals. The Panel noted the questionable reliability of the study conducted by Industrial Bio‐Test Laboratories (see Introduction of Section 3.5).
Studies with other rosins
Two 2‐year studies conducted by Industrial Bio‐Test Laboratories, in which gum rosin (Pinus species and composition not indicated) was administered with the diet up to 1% to rats and dogs (Documentation provided to EFSA N. 21 and 22), were submitted by an interested party, addressing the similarity in composition of gum rosin and wood rosin.17 Rats of the 1% group displayed a significant growth depression, while survival and incidence of tumour formation in the rats were not influenced by the ingestion of the test material. No adverse effects were reported from the dogs fed the gum rosin.
The Panel noted the questionable reliability of the studies conducted by Industrial Bio‐Test Laboratories (see Introduction of Section 3.5) and noted furthermore that the results of both studies did not add any relevant information for the re‐evaluation of GEWR to the results already described from the two 2‐year studies conducted by Industrial Bio‐Test Laboratories, in which the source material wood rosin was administered with the diet to rats and dogs (Kohn, 1962a,b).
Overall, the Panel noted that no adequate data were available to evaluate the chronic toxicity and carcinogenicity of GEWR.
3.5.6. Reproductive and developmental toxicity
No studies on reproductive and developmental toxicity of GEWR and resin acids mentioned in Figure 2 are available.
3.5.7. Hypersensitivity, allergenicity and food intolerance
Since 1980, the number of reported cases of contact allergy to rosin (colophony), the resin obtained from species of the genus Pinus has increased and rosin has become one of the most important dermal sensitisers (e.g. Oppel and Schnuch, 2006). All types of rosins, including wood rosin, have been classified as skin sensitiser (Karlberg et al., 1999). Positive patch test results have been seen with different types of rosins, modified rosins and their components, including wood rosin and GEWR (e.g. Koch et al., 1971, 1973; Lysell, 1976; Hausen et al., 1982; de Groot et al., 1988; Young et al., 1988; Hausen and Mohnert, 1989; Ogino et al., 1989; Satyawan et al., 1990; Shao et al., 1993; Gaefvert et al., 1994, 1996; Färm, 1997; Inoue et al., 1998; Downs and Sansom, 1999; Gupta and Forsyth, 1999; Wohrl et al., 2001; Geier and Hausen, 2006; Goon and Goh, 2006; Illing et al., 2009; Tsuruta et al., 2011).
Animal studies
Even though it was not possible to sensitise guinea pigs against purified abietic acid (Karlberg et al., 1985), Hausen and Mohnert (1989) found that of 44 dermatologic patients, 17 (39%) reacted positive to purified abietic acid as well as to standard rosin in a patch test. As reviewed by Karlberg et al. (1999) and Downs and Sansom (1999) abietic type acids are easily oxidised when in contact with air, and the oxidation products are thought to be contact sensitisers. Apart from resin acids and esters of resin acids, the non‐acidic part of a rosin has provoked positive reactions in human patch testing (Hausen and Mohnert, 1989).
The allergenicity of glycerol esters of rosins and their components has been investigated in guinea pigs and in human patch tests. In studies in guinea pigs, 1‐glycerol monoabietate was found to be a sensitiser, while glycerol triabietate was not. No significant cross‐reactivity to these compounds was found in the animals sensitive to unmodified rosin. No reactions were seen in rosin‐sensitive patients tested with 1,2‐glycerol diabietate, 1,3‐glycerol diabietate or glycerol triabietate. According to the authors, the difference in contact sensitisation potential of 1‐glycerol monoabietate and glycerol triabietate may be due to their difference in size. 1‐Glycerol monoabietate having only about one‐third of the molecular weight of glycerol triabietate would probably have a higher bioavailability across the skin than the latter. Thus, the esterification of rosins with glycerol might reduce their skin allergenic activity, possibly because of the formation of much larger molecules with reduced bioavailability (Shao et al., 1993; Gaefvert et al., 1994).
Abietic acid has been reported to have anti‐inflammatory properties using in vitro (Takahashi et al., 2003; Kim et al., 2010) and in vivo models (Fernandez et al., 2001; Gao et al., 2016).
Human studies
A case of an 8‐year‐old boy who suffered from recurring perioral dermatitis for 18 months was reported. He frequently chewed gum before each episode of dermatitis. Patch testing was positive for cobalt, rosin, fragrance‐mix, oakmoss and isoeugenol as well as to the previously used chewing gum and bubble gum. The perioral dermatitis improved but did not completely disappear after the child stopped chewing gum. The authors assumed that the perioral dermatitis was due to rosin in chewing gum, but possible sensitivity to allergens other than rosin could not be ruled out (Satyawan et al., 1990).
Two additional cases of adverse reactions in the mouth following chewing gum exposure were reported, in which patients showed positive results of patch testing with rosin (Gupta and Forsyth, 1999).
The Panel noted that there is no evidence of a causal relationship in these three cases between the adverse effects observed and exposure to chewing gum which possibly might have contained rosin or rosin‐derived products.
Literature searches did not reveal reports of allergic responses from ingestion of GEWR in food.
Overall, the Panel noted that, wood rosin has been classified as skin sensitiser (Karlberg et al., 1999). However, there is evidence from experimental data that the esterification of rosins with glycerol reduces their skin allergenic activity (Shao et al., 1993; Gaefvert et al., 1994). Abietic acid was reported to have anti‐inflammatory effects in vitro. Furthermore, no allergic reactions to food containing the food additive GEWR (E 445) have been identified.
3.6. Discussion
According to the Commission Regulation (EU) No 231/1012 and the JECFA (2006), GEWR (E 445) are a complex mixture of ‘tri‐ and diglycerol esters of resin acids’ from wood rosin, obtained by the solvent extraction of aged pine stumps followed by a liquid‐liquid solvent refining process. Excluded from the additive's definition are substances derived from gum rosin, an exudate of living pine trees, and substances derived from tall oil rosin, a by‐product of kraft (paper) pulp processing. The source material,18 wood rosin, is composed of approximately 90% resin acids and 10% neutrals (non‐acidic compounds). Its resin acid fraction is a complex mixture of isomeric diterpenoid monocarboxylic acids having the empirical molecular formula of C20H30O2, chiefly abietic acid. The esterified product is purified by steam stripping or by counter current steam distillation.
The recent JECFA definition of GEWR (E 455) (JECFA, 2013b) is in agreement with the EU definition but further specifies, that GEWR (i) contain, besides the mentioned glycerol di‐ and triesters, a residual fraction of glycerol monoesters, (ii) contain, also neutrals (non‐acidic saponifiable and unsaponifiable substances) and residual free resin acids, (iii) are originating exclusively from two species: P. palustris (longleaf pine) and P. elliottii (slash pine).
According to information from interested parties, the wood rosin is either obtained from P. palustris (longleaf) and P. elliottii (slash pine) stumps (Documentation provided to EFSA N. 2) or from P. halepensis and P. brutia stumps (Documentation provided to EFSA N. 17).
The Panel noted that the EC specifications do not define the source material by its exclusive botanical origin and that the food additive E 455 obtained from other botanical origins, if complying with the current specifications, may also be on the market in the EU.
In samples of saponificated GEWR (botanical origin not specified) the following resin acid concentrations were measured after saponification (given percentage refers to the total of each resin acid present in GEWR in bound and unbound form): abietic (19.5–26.0%), dehydroabietic (22.7–34.6%), palustric (4.29–5.90%), neoabietic (0.546–2.77%), pimaric (5.31–6.45%), isopimaric (9.35–15.0%), sandaracopimaric (5.76–6.41%) and communic (9.69–10.7%) acids (EFSA ANS Panel, 2010). Additional resin acids, such as dihydroabietic acid and tetrahydroabietic acid may occur in GEWR due to formation from abietic acid under high manufacturing temperatures exceeding 200°C (Hausen et al., 1982). According to an interested party (Documentation provided to EFSA N. 23), in GEWR derived from wood rosin from P. palustris (longleaf pine) and P. elliottii (slash pine) total glycerol esters averaged 84.3%, while neutrals averaged 13.2% and free resin acids averaged 2.6%, possible concentrations of free neoabietic acid in the GEWR derived from P. palustris (longleaf pine) and P. elliottii (slash pine), being below the limit of quantitation (0.05 weight percent). The sum of glycerol di‐ and triesters was determined to be in average 82.0%. Glycerol monoesters averaged 2.2%. According to an interested party, the GEWR, that was used in the biological and toxicological studies,12 is also derived from P. palustris (longleaf pine) and P. elliottii (slash pine), shares the same manufacturing process and is assumed to be compositionally equivalent to the food additive product in commerce as beforehand described (Documentation provided to EFSA N. 23). The Panel noted that the current evaluation is based on the results of toxicological studies performed with GEWR originating from P. palustris (longleaf pine) and P. elliottii (slash pine).
GEWR originating from P. halepensis and P. brutia yielded almost identical IR spectra and similar retention times in GC as GEWR derived from P. palustris (longleaf pine) and P. elliottii (slash pine). However, this information only refers to the resin alcohols obtained after hydrolysis and reduction. In addition, the Panel noted that no information was available, in which concentrations the toxicologically relevant fractions of ‘glycerol monoesters’, ‘free resin acids’ and ‘neutrals’ are present in the GEWR originating from P. halepensis and P. brutia. Therefore, the evaluation of its equivalence with GEWR originating from P. palustris (longleaf pine) and P. elliottii (slash pine) is not possible. Altogether, the Panel concluded that the available data on the chemical composition of GEWR originating from P. halepensis and P. brutia do not allow for read across of toxicological data from GEWR originating from P. palustris (longleaf pine) and P. elliottii (slash pine).
The levels of toxic elements in the six batches of GEWR (E 455) analysed, were below 0.02 mg/kg for lead, cadmium, mercury and arsenic and were thus about two orders of magnitude below the levels as defined in the Commission Regulation (EU) No 231/2012 (Documentation provided to EFSA N. 2). The Panel noted that, according to the EC specifications for GEWR (E 445), impurities of the toxic elements arsenic, cadmium, lead and mercury are accepted up to concentrations of 3, 1, 2 and 1 mg/kg, respectively. Contamination at those levels could have a significant impact on the exposure to these toxic elements, for which exposures are already close to the health‐based guidance values or benchmark doses (lower Confidence Limits) established by EFSA (EFSA CONTAM Panel, 2009, 2010, 2012a,b,c, 2014).
The Panel recommended an update of the definition and of the purity criteria of GEWR (E 445) in the Commission Regulation (EU) No 231/1012, taking also additional parameters indicated in the recent JECFA specifications (JECFA, 2013b) into account. Furthermore, GEWR may be more specifically characterised by indicating the maximum levels of the fraction of neutrals (non‐acidic other saponifiables and unsaponifiables) in the definition. GEWR components relevant for possible systemic effects are free resin acids and glycerol monoesters (and other esters) of resin esters, from which resin acids may get available after possible hydrolysis in the gastro‐intestinal tract (see Section 3.5.1). Thus, in the view of the Panel, setting limits for glycerol monoesters of resin acids might be relevant for the specifications. In addition, EU specifications should require that levels of free neoabietic acid are below the limit of quantitation of 0.05 weight percent.
GEWR (E 445) is manufactured in a two‐phase process that involves at first solvent extraction and refining of wood rosin from aged pine stumps. In a second step, the refined wood rosin is esterified and the final product is purified. The carboxylic group of the resin acids is attached to a tertiary carbon which causes steric hindrance. In order to esterify this type of sterically hindered carboxylic groups, generally more drastic conditions and higher temperatures, namely of greater than 250°C, have to be used than for other carboxylic acids (Hausen et al., 1982; Documentation provided to EFSA N. 5; Documentation provided to EFSA N. 2).
As a consequence of this steric hindrance ester bonds in GEWR are described to be resistant to hydrolysis by water, acids and alkali except under extreme conditions (Hausen et al., 1982; Documentation provided to EFSA N. 5). To clarify to which extent GEWR also resists enzymatic hydrolysis in the gastro‐intestinal tract, several in vitro and in vivo studies have been conducted (Documentation provided to EFSA N. 13 and 15). From a series of experiments by Documentation provided to EFSA N. 16, data are also available on the absorption and excretion of dehydroabietic acid, isopimaric acid and tetrahydroabietic acid, as possible hydrolysis products of GEWR.
ADME studies have shown that ester bonds in GEWR are largely resistant to hydrolysis in the gut, the majority of orally applied GEWR being excreted unchanged in the faeces (Documentation provided to EFSA N. 5 and 6; Documentation provided to EFSA N. 15). Only a small fraction, most likely the glycerol monoesters of wood rosin, seems to undergo hydrolysis. Studies with 14C‐labelled GEWR in rats (Documentation provided to EFSA N. 15) on excretion of radioactivity via faeces, bile, urine and exhaled air and disposition in the carcass gave evidence for a low absorption rate of ≤ 5% of the applied dose. Most of the absorbed radioactivity was eliminated in the bile and excreted via faeces. Results of HPLC analysis of faeces and bile suggested that absorbed components were hydrolysis products from glycerol monoesters of wood rosin present in GEWR.
A few ADME data were available for free resin acids which might be released in the gastro‐intestinal tract after hydrolysis of glycerol monoesters or of esters with other alcohols in GEWR. Furthermore, GEWR contain a fraction of residual free resin acids. The experiments in rats with radiolabelled dehydroabietic acid (Documentation provided to EFSA N. 16) revealed an absorption rate of approximately 40% after oral exposure. Most of the radioactivity was excreted via the bile, minor amounts via the urine and only traces were exhaled. Tetrahydroabietic acid and isopimaric acid exhibited an excretion pattern similar to dehydroabietic acid (Documentation provided to EFSA N. 16).
No studies on acute oral toxicity of GEWR were available.
No treatment‐related effects were detected in a 13‐week feeding study (Documentation provided to EFSA N. 3 and 4) conducted with GEWR originated from P. Palustris (longleaf pine) and P. elliottii (slash pine), representative of the food additive on the market, according to current standards after oral exposure of male and female F344 rats to 0, 625, 1,250 or 2,500 mg GEWR/kg bw per day. From this study, the Panel identified a NOAEL of 2,500 mg GEWR/kg bw per day, the highest dose tested.
GEWR, when tested as a mixture, did not show genotoxic potential in the core battery of adequately performed in vitro tests (i.e. bacterial reversion and in vitro micronucleus assays) recommended in the EFSA guideline on genotoxicity testing strategy (EFSA Scientific Committee, 2011a). Negative results were also obtained in limited in vitro chromosomal aberration and UDS assays. Positive results, of unclear biological significance, were only reported in a limited in vivo study. Based on the overall weight evidence, the Panel considered GEWR as non‐genotoxic.
When individual compounds from the residual free resin acids fraction, which accounts only for 2.3–2.8% of GEWR, were evaluated, an equivocal mutagenic outcome was only observed for neoabietic acid in limited bacterial and yeast mutation assays (Nestmann et al., 1979; Nestmann and Lee, 1983). The Panel also noted that according to the information provided by one interested party (documentation xxx) levels of free neoabietic acid in GEWR are below the limit of quantitation of 0.05 wt. %. Overall, the Panel concluded that GEWR (E445) containing free neoabietic acid below the limit of quantitation (0.05 wt. %) when used as a food additive are of no concern for genotoxicity.
There were no studies available concerning chronic toxicity or carcinogenicity of GEWR.
There were no studies available on the endpoint reproductive and developmental toxicity of GEWR.
The Panel noted that, wood rosin has been classified as skin sensitiser (Karlberg et al., 1999). However, there is evidence from experimental data that the esterification of rosins with glycerol reduces their skin allergenic activity (Shao et al., 1993; Gaefvert et al., 1994). Furthermore, data on allergic reactions on food containing GEWR have not been identified.
In 1994, the SCF established an ADI of 12.5 mg/kg bw per day for GEWR from the NOAEL of 2,500 mg/kg bw per day from a 13‐week study in rats by applying an UF of 200 to take into account the 90‐day duration (SCF, 1994). JECFA concluded in 1996 that the documented data on subchronic oral toxicity studies and the studies confirming the non‐bioavailability of GEWR were adequate to establish an ADI of 0–25 mg/kg bw per day for GEWR by applying an UF of 100 to the NOAEL of 2,500 mg/kg bw per day, although there were no studies available on chronic or reproductive toxicity (JECFA, 1996b). This ADI was confirmed in 2013 (JECFA, 2013a).
Based on the overall toxicity database, and given the absence of reproductive and developmental toxicity data, the Panel considered that the current ADI of 12.5 mg/kg bw per day for GEWR (E 445) as established by the SCF in 1994 should be temporary pending the provision of such data.
To assess the dietary exposure to GEWR (E 445) from its use as a food additive, the exposure was calculated based on (1) MPLs set out in the EU legislation (defined as the regulatory maximum level exposure assessment scenario) and (2) the reported use levels (defined as the refined exposure assessment scenario).
Based on the available data set, the Panel calculated two refined exposure estimates based on different assumptions as described in Section 3.4: a brand‐loyal consumer scenario and a non‐brand‐loyal scenario. The Panel considered that the brand‐loyal consumer scenario resulted in more realistic long‐term exposure estimates compared to the regulatory maximum level exposure assessment scenario. Since GEWR (E 445) are authorised and used in a certain type of flavoured drinks, to which consumers may be brand loyal, the Panel selected the refined brand‐loyal scenario as the most relevant exposure scenario for the safety evaluation of this food additive. In this scenario, the highest mean and P95 exposure estimates occurred in the population group of toddlers with 1.07 and 4.06 mg/kg bw per day, respectively. The Panel noted that the mean and the high exposure levels (P95) of the brand‐loyal refined exposure scenario did not exceed the ADI in any of the population groups from the use of GEWR (E 445) as a food additive at the reported use levels.
For GEWR (E 445), reported use levels were available. In total, two out of five food categories were taken into account in the present refined exposure estimate. For category 14.1.4 Flavoured drinks, with the restriction ‘only cloudy drinks’, information on the turbidity (cloudiness) of the product was not indicated in the FoodEx system in the Comprehensive Database. Based on Mintel information, all soft drinks were taken into account in the assessment, while other types of flavoured drinks such as flavoured milk based drinks and flavoured plant‐based milk substitute drinks were not taken into account, as the additive is considered as not used in them. This may cause an overestimation of the exposure.
Added to that, approximately 85% of the food products labelled with GEWR (E 445) in the Mintel's GNPD belonged to food subcategories that were considered in the exposure assessment (Appendix C). The Panel considered that the exposure estimates in all exposure scenarios resulted in overestimates of the exposure to GEWR (E 445) from its use as a food additive according to Annex II to Regulation (EC) No 1333/2008 (Section 3.4).
The Panel also noted that the refined exposure estimates are based on information provided on the reported level of use of food additive GEWR (E 445). If actual practice changes these refined estimates may no longer be representative and should be updated. In addition, the Panel noted that GEWR (E 445) was listed as ingredient in Mintel GNPD subcategories where the uses are not authorised (see Section 3.3.2).
4. Conclusions
GEWR originating from Pinus palustris (longleaf pine) and Pinus elliottii (slash pine)
Based on the overall toxicity database, and given the absence of reproductive and developmental toxicity data, the Panel concluded that the current ADI of 12.5 mg/kg bw per day for GEWR (E 445) as established by the SCF in 1994 should be temporary pending the provision of such data.
This assessment is restricted to GEWR derived from P. palustris (longleaf pine) and P. elliottii (slash pine) and with a chemical composition in compliance with GEWR used in the toxicological testing.
The Panel concluded that the mean and the high exposure levels (P95) of the brand‐loyal refined exposure scenario did not exceed the temporary ADI in any of the population groups from the use of GEWR (E 445) as a food additive at the reported use levels.
GEWR originating from Pinus halepensis and Pinus brutia
For GEWR originating from P. halepensis and P. brutia, the Panel noted that:
concentrations of the fractions of ‘glycerol monoesters’, ‘free resin acids’ and ‘neutrals’, which are considered to be of particular toxicological relevance, are not known,
therefore, the evaluation of chemical equivalence with GEWR originating from P. palustris (longleaf pine) and P. elliottii (slash pine) is not possible,
no data on stability were available,
no toxicological data were available.
Therefore, the Panel concluded that a safety assessment of GEWR originating from P. halepensis and P. brutia could not be performed.
5. Recommendations
The Panel recommended the European Commission to consider:
an update of the definition of GEWR (E 445) in the EU specifications. It should be indicated that GEWR (E 445) (i) contain, besides the mentioned glycerol di‐ and triesters, a residual fraction of glycerol monoesters, and (ii) contain residual free resin acids and neutrals (non‐acidic other saponifiable and unsaponifiable substances); technical terms should be corrected as indicated in Section 3.1.2,
setting limits for glycerol monoesters of resin acids and neutrals in the EU specifications (see section 3.1.1), in accordance with the analytical data provided for GEWR (E 445) from P. palustris (longleaf pine) and P. elliottii (slash pine), being in compliance with the test material used in the toxicological tests,
setting a maximum level for free neoabietic acid of 0.05 wt. % (limit of quantitation) in the EU specifications, while the concentration of the total of free resin acids in GEWR being limited already by the acid value of the existing specifications,
revising the current limits for the toxic elements lead mercury, cadmium and arsenic in the EC specification to ensure that GEWR (E 445) as a food additive will not be a significant source of exposure to these toxic elements in food. Currently, detected levels of these toxic elements were two orders of magnitude below those defined in the EU specifications,
setting maximum levels for impurities, such as butanetriols, acrolein, chlorinated compounds and 3‐monochloropropane‐1,2‐diol, in the EU specifications, for which limits are defined in the food additive glycerol (E 422),
requesting the provision of a reproductive and developmental toxicity study, in accordance with the applicable OECD test guidelines, using a test material which is representative of the food additive present on the market and taking into account the above recommendations for the update of the specifications.
Documentation provided to EFSA
Pre‐evaluation document on glycerol esters of wood rosin (E 445). Frauenhofer ITEM. Submitted in March‐April 2013.
Pinova‐Mars Chocolate UK, 2010. Reply to EFSA call for data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents. Application for the approval of Glycerol Esters of Wood Rosin (E 445) for use as an Emulsifier in Edible Inks. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010 and June 2014.
International Research Development Corporation, 1991. 13‐week dietary toxicity study in rats [Ester gum 8BG]. Report No. 548‐007. International Research Development Corporation. Mattawan, Michigan, Dec. 30, 1991. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
International Research Development Corporation, 1992. 13‐week dietary toxicity study in rats [Ester gum 8BG]. Amendment to the final report. Report No. 548‐007. International Research Development Corporation, Mattawan, Michigan, Dec. 30, 1991. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
International Life Sciences Institute, 1994. A dietary excretion study with Ester Gum 8BG in Fischer 344 Rats. Unpublished study No. 3352.1 from Springborn Laboratories, Inc., Spencerville, OH, USA. International Life Sciences Institute. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
International Life Sciences Institute, 1995. A dietary excretion study with Ester Gum 8BG in Fischer 344 Rats. Amended final report. Unpublished study No. 3352.1 from Springborn Laboratories, Inc., Spencerville, OH, USA. International Life Sciences Institute. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
Hazleton Laboratories America, 1988. Mutagenicity test on Ester Gum 8BG in the rat primary hepatocyte unscheduled DNA synthesis assay. Sponsor: Hercules Inc., Wilmington. HLA study no. 10349‐0‐447. Hazleton Laboratories America, Inc., Kensington, Maryland. Oct. 27, 1988. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
Hazleton Laboratories America, 1988. Mutagenicity test on Ester Gum 8BG in the Ames salmonella/microsome reverse mutation assay. HLA study no. 10349‐0‐401. Hazleton Labotarories America, Inc., Wilmington, Delaware; November 3, 1988. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
Industrial BIO‐TEST Laboratories, 1960a. Ninety‐day subacute oral toxicity of Ester Gum 8D. Industrial BIO‐TEST Laboratories, Inc., Northbrook, Illinois, Sep. 8, 1960. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
Industrial BIO‐TEST Laboratories, 1960b. Ninety‐day subacute oral toxicity of N‐wood rosin. Industrial BIO‐TEST Laboratories, Inc., Northbrook, Illinois, June 16, 1960. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
Industrial BIO‐TEST Laboratories, 1961. Summary of acute oral toxicity studies on rosins. Industrial Bio‐Test Laboratories, Inc., Northbrook, Illinois; March 14, 1961. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
TNO, 2010. Investigation of four samples of M&M's. TNO Project number 031.20907/01.01. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
International Life Sciences Institute, 1996. Metabolism study of Ester Gum 8BG in human faecal extracts and simulated human gastric juice. Laboratory Project No. 8871. Report from Southern Research Institute, Birmingham, AL 35205, USA. International Life Sciences Institute. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
Hazleton Laboratories America, 1988. Mutagenicity test on Ester Gum 8BG OSR in an in vitro cytogenetic assay measuring chromosomal aberration frequencies in Chinese hamster ovary (CHO) cells. HLA study no. 10349‐0‐437. Hazleton Laboratories America, Inc., Kensington, Maryland; Oct. 26, 1988. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
International Life Sciences Institute, 1996. Pharmacokinetic study of Ester Gum 8BG in rats. Report project No. 8801 from Southern Research Institute, Birmingham, AL 35205, USA. International Life Sciences Institute. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
Hercules Powder Company, 1965. The absorption, fate and excretion of dehydroabietic acid, isopimaric acid and tetrahydroabietic acid in rats. Hercules Powder Company. University of Miami, School of Medicine, Department of Pharmacology, Coral Gables, Florida, Mar. 1, 1961 ‐ Jul. 31, 1965. Submitted by Cantox on behalf of Pinova and Mars Chocolate UK in October 2010.
T&R Chemicals, 2017. Personal communication to EFSA. Data on the chemistry of Glycerol ester of wood rosin. Submitted on February 2017.
T&R Chemicals, 2017. Personal communication to EFSA. Summary of genotoxicity and carcinogenicity testing of GEGR, GEWR and other pine products. Submitted on April 2017.
Industrial BIO‐TEST Laboratories, 1962a. Two‐year chronic oral toxicity of gum rosin in albino rats. Industrial BIO‐TEST Laboratories, Inc., Northbrook, Illinois, Aug. 24, 1962. Submitted by T&R Chemicals in August 2017.
Industrial BIO‐TEST Laboratories, 1962b. Two‐year chronic oral toxicity of gum rosin in dogs. Industrial BIO‐TEST Laboratories, Inc., Northbrook, Illinois, July 31, 1962. Submitted by T&R Chemicals in August 2017.
Industrial BIO‐TEST Laboratories, 1962c. Two‐year chronic oral toxicity of N‐wood rosin in albino rats. Industrial BIO‐TEST Laboratories, Inc., Northbrook, Illinois, Aug. 24, 1962. Submitted by T&R Chemicals in August 2017.
Industrial BIO‐TEST Laboratories, 1962d. Two‐year chronic oral toxicity of N‐wood rosin in dogs. Industrial BIO‐TEST Laboratories, Inc., Northbrook, Illinois, July 31, 1962. Submitted by T&R Chemicals in August 2017.
Intertek, 2017. Data on the chemistry of glycerol ester of wood rosin. RESPONSE TO REQUEST FOR ADDITIONAL INFORMATION BY EFSA LETTER DATED 09 FEBRUARY 2017. Submitted on April and November 2017.
Intertek, 2018. RE‐EVALUATION OF GLYCEROL ESTERS OF WOOD ROSIN (E 445) – RESPONSE TO REQUEST FOR ADDITIONAL INFORMATION BY EFSA LETTER DATED 09 FEBRUARY 2017 (EFSA‐Q‐2011‐00531). Genotoxicity tests on glycerol ester of wood rosin. Submitted on April 2018.
Intertek, 2018. Pinova® Ester Gum 8BG glycerol ester of wood rosin and Pinova® Ester Gum 8BG glycerol ester of wood rosin spiked with Neoabietic Acid: Bacterial Reverse Mutation Test. Submitted in May‐June 2018.
Spanish Association of Postharvest Services and Processes (AGRUPOST), 2017. Data on usage levels on glycerol ester of wood rosin (E 445) in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 5). Submitted to EFSA by 30 January 2017.
Specialised Nutrition Europe (SNE), 2017. Data on usage levels on glycerol ester of wood rosin (E 445) in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 5). Submitted to EFSA by 31 January 2017.
FoodDrinkEurope (FDE), 2017. Data on usage levels on glycerol ester of wood rosin (E 445) in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 5). Submitted to EFSA by 01 February 2017.
Glossary and Abbreviations
- ADI
acceptable daily intake
- ADME
Absorption, distribution, metabolism and excretion
- AGRUPOST
Spanish Association of Postharvest Services and Processes
- ANS
EFSA Scientific Panel on Food Additives and Nutrient Sources added to Food
- bw
body weight
- CAS
Chemical Abstracts Service
- CHL
Chinese hamster lung
- CHO
Chinese hamster ovary
- CONTAM
EFSA Panel on Contaminants in Food Chain
- FAO
Food and Agriculture Organization of the United Nations
- FCs
food categories
- FCS
food categorisation system
- FDE
FoodDrinkEurope
- FPG
fluorochrome plus Giemsa
- GC
gas chromatography
- GEGR
glycerol esters of gum rosin
- GETOR
glycerol esters of tall oil rosin
- GEWR
glycerol esters of wood rosin
- GLP
good laboratory practice
- GNPD
Global New Products Database
- HPLC
high‐performance liquid chromatography
- HT/HR GC
high‐temperature/high‐resolution gas chromatography
- IR
infrared
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LD50
lethal dose, median
- MPL
maximum permitted level
- MS
mass spectrometry
- NDA
EFSA Panel on Dietetic Products, Nutrition and Allergies
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- SCE
sister chromatid exchange
- SCF
Scientific Committee on Food
- SEC
size exclusion chromatography
- SNE
Specialised Nutrition Europe
- SPE
solid‐phase extraction
- TemaNord
is a publishing series for results of the often research‐based work that working groups or projects under Nordic Council of Ministers have put in motion
- TLC
thin‐layer chromatography
- TOF
time‐of‐flight
- UDS
unscheduled DNA synthesis
- UF
uncertainty factor
- UPLC
ultra‐performance liquid chromatography
- UV
ultraviolet
- WHO
World Health Organization
Appendix A – Summary of the reported use levels (mg/kg) of glycerol esters of wood rosin E445 provided by industry
Appendix B – Number and percentage of food products labelled with glycerol esters of wood rosin (E445) out of the total number of food products present in THE Mintel GNPD per food subcategory between January 2013 and January 2018
Appendix C – Concentration levels of glycerol esters of wood rosin (E445) used in the refined exposure scenarios (mg/kg or mL/kg as appropriate)
Appendix D – Summary of total estimated exposure of glycerol esters of wood rosin (E445) from their use as a food additive for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Appendix E – Main food categories contributing to exposure to glycerol esters of wood rosin (E445) using the maximum level exposure scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Appendix A–E can be found in the online version of this output (‘Supporting information’ section).
Appendix F – List of neutral constituents in wood rosin which could also be components in GEWR (Documents provided to EFSA n. 23)
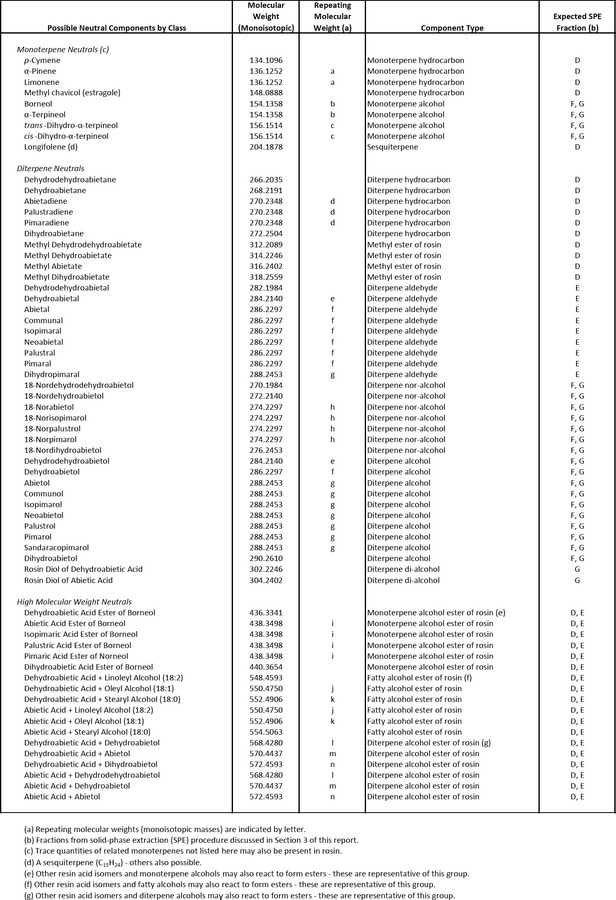
Supporting information
Summary of the reported use levels (mg/kg) of glycerol esters of wood rosin E445 provided by industry
Number and percentage of food products labelled with glycerol esters of wood rosin (E445) out of the total number of food products present in THE Mintel GNPD per food subcategory between January 2013 and January 2018
Concentration levels of glycerol esters of wood rosin (E445) used in the refined exposure scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of glycerol esters of wood rosin (E445) from their use as a food additive for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to glycerol esters of wood rosin (E445) using the maximum level exposure scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Suggested citation: EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Younes M, Aggett P, Aguilar F, Crebelli R, Filipič M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Lambré C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Briemer L, Pasquale M, Christodoulidou A, Horvath Zs, Lodi F, Tard A and Dusemund B, 2018. Scientific Opinion on the re‐evaluation of glycerol esters of wood rosin (E 445) as a food additive. EFSA Journal 2018;16(7):5370, 47 pp. 10.2903/j.efsa.2018.5370
Requestor: European Commission
Question number: EFSA‐Q‐2011‐00531
Panel members: Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipič, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Gunter Georg Kuhnle, Claude Lambré, Jean‐Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Oliver Lindtner, Jacqueline Wiesner and Eleonora Alquati. The ANS Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 27 June 2018
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p 1.
The Commission Regulation (EU) No 231/1012 is using the term “final product” instead of the term “the source material, wood rosin” which may be misinterpreted.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents Deadline: 23 May 2010. Available from: http://www.efsa.europa.eu/en/dataclosed/call/ans091123
Call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 5). Deadline: 31 January 2017. Available from: https://www.efsa.europa.eu/en/data/call/160524
14/06/2018.
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
In this opinion, the term GEWR (E 445) refers to the substance meeting the EU specifications.
The Panel noted that the terms ‘tri‐ and diglycerol esters of resin acids’ in the EU specifications are not chemically correct and should be replaced by the terms ‘glycerol di‐ and triesters of resin acids’.
The Commission Regulation (EU) No 231/1012 is using the term ‘final product’ instead of the term ‘the source material’ which may be misinterpreted.
Test material in biological and toxicological studies is named ‘Ester Gum 8BG’, ‘Ester Gum 8BG OSR’ or ‘Ester Gum 8D’.
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16.
Missing Bulgaria, Cyprus, Estonia, Latvia, Lithuania, Luxembourg, Malta and Slovenia.
http://www.gnpd.com/sinatra/home/ accessed on 23/01/2018.
Wood rosin and gum rosin are both derived from Pinus species and are both described in generally containing approximately 90% resin acids and approximately 10% neutral fraction. However, gum rosin and wood rosin may vary in their composition due to different production processes. While wood rosin is obtained via a multistage purification and refining process involving solvent extraction of ground aged pine stumps, gum rosin is obtained by tapping oleoresin (EFSA ANS Panel, 2010).
The Commission Regulation (EU) No 231/1012 is using the term ‘final product’ instead of the term ‘the source material,, wood rosin’ which may be misinterpreted.
References
- Asakawa Y, Ishida T, Toyota M and Takemoto T, 1986. Terpenoid biotransformation in mammals. IV Biotransformation of (+)‐longifolene, (‐)‐caryophyllene, (‐)‐caryophyllene oxide, (‐)‐cyclocolorenone, (+)‐nootkatone, (‐)‐elemol, (‐)‐abietic acid and (+)‐dehydroabietic acid in rabbits. Xenobiotica, 16, 753–767. [DOI] [PubMed] [Google Scholar]
- Douglas GR, Nestmann ER and McKague AB, 1983. Mutagenicity Of Pulp And Paper Mill Effluent: A Comprehensive Study Of Complex Mixtures. In: Short‐Term Bioassays in the Analysis of Complex Environmental Mixtures III, Environmental Science Research. Vol. 27. Eds. Waters MD, Sandhu SS, Lewtas J, Claxton L, Chernoff N, Nesnow S. Springer Verlag, 431‐459.
- Downs AMR and Sansom JE, 1999. Colophony allergy: a review. Contact Dermatitis, 241, 305–310. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2010. Scientific Opinion on the safety of glycerol esters of gum rosin for the proposed uses as a food additive. EFSA Journal 2010;8(7):1654, 31 pp. 10.2903/j.efsa.2010.1654. Available online: http://www.efsa.europa.eu [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2011. Scientific opinion on the safety of glycerol esters of tall oil rosin for the proposed uses as a food additive. EFSA Journal 2011;9(5):2141, 33 pp. 10.2903/j.efsa.2011.2141 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources Added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2014. Statement on a conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010. EFSA Journal 2014;12(6):3697, 11 pp. 10.2903/j.efsa.2014.3697 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009. Scientific opinion on cadmium in food. EFSA Journal 2009;7(10):980, 139 pp. 10.2903/j.efsa.2009.980 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012a. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 241 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012b. Scientific Opinion on lead dietary exposure in the European population. EFSA Journal 2012;10(7):2831, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012c. Scientific Opinion on cadmium dietary exposure in the European population. EFSA Journal 2012;10(1):2551, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on dietary exposure to inorganic arsenic in the European population. EFSA Journal 2014;12(3):3597, 68 pp. 10.2903/j.efsa.2014.3597, EFSA 2014 (Conceptual framework). [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general Principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69pp. 10.2903/j.efsa.2011.2379. Available online: http://www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579. Available online: http://www.efsa.europa.eu [DOI] [Google Scholar]
- Färm G, 1997. Contact allergy to colophony. Clinical and experimental studies with emphasis on clinical relevance. Acta Dermato‐Venereologica. Supplementum, 201, 1–42. [PubMed] [Google Scholar]
- Fernandez MA, Tornos MP, Garcia MD, De las Heras B, Villar AM and Saenz MT, 2001. Anti‐inflammatory activity of abietic acid, a diterpene isolated from Pimenta racemosa var. grissea. Journal of Pharmacology, 53, 867–872. [DOI] [PubMed] [Google Scholar]
- Gaefvert E, Shao LP, Karlberg A‐T, Nilsson U and Nilsson JLG, 1994. Allergenicity of rosin (colophony) esters (II). Glyceryl monoabietate identified as contact allergen. Contact Dermatitis, 31, 11–17. [DOI] [PubMed] [Google Scholar]
- Gaefvert E, Bordalo O and Karlberg AT, 1996. Patch testing with allergens from modified rosin (colophony) discloses additional cases of contact allergy. Contact Dermatitis, 35, 290–298. [DOI] [PubMed] [Google Scholar]
- Gao Y, et al., 2016. Abietic acid attenuates allergic airway inflammation in a mouse allergic asthma mode. International Immunopharmacology, 38, 261–266. [DOI] [PubMed] [Google Scholar]
- Geier J and Hausen BM, 2006. Epikutantestung mit chemisch modifiziertem Kolophonium. Aktuelle Dermatologie, 32, 239–242. [Google Scholar]
- Goon AT‐J and Goh C‐L, 2006. Patch testing of Singapore children and adolescents: our experience over 18 years. Pediatric Dermatology, 23, 117–120. [DOI] [PubMed] [Google Scholar]
- de Groot AC, Beverdam EG, Ayong CT, Coenraads PJ and Nater JP, 1988. The role of contact allergy in the spectrum of adverse effects caused by cosmetics and toiletries. Contact Dermatitis, 19, 195–201. [DOI] [PubMed] [Google Scholar]
- Gupta G and Forsyth A, 1999. Allergic contact reactions to colophony presenting as oral disease. Contact Dermatitis, 40, 332–333. [DOI] [PubMed] [Google Scholar]
- Hausen BM and Mohnert J, 1989. Contact allergy due to colophony. (V). Patch test results with different types of colophony and modified‐colophony products. Contact Dermatitis, 20, 295–301. [DOI] [PubMed] [Google Scholar]
- Hausen BM, Kuhlwein A and Schulz KH, 1982. Kolophonium‐Allergie. Dermatosen, 30, 107–115. [PubMed] [Google Scholar]
- Illing HPA, Malmfors T and Rodenburg L, 2009. Skin sensitization and possible groupings for “read across” for rosin based substances. Regulatory Toxicology and Pharmacology, 54, 234–241. [DOI] [PubMed] [Google Scholar]
- Inoue A, Shoji A and Aso S, 1998. Allergic lipstick cheilitis due to ester gum and ricinoleic acid. Contact Dermatitis, 39, 39. [DOI] [PubMed] [Google Scholar]
- Ishidate M Jr, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M and Matsuoka A, 1984. Primary mutagenicity screening of food additives currently used in Japan. Food and Chemical Toxicology, 22, 623–636. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1975. 398. Glycerol esters of wood rosins. WHO Food Additives Series No. 6.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1996a. Toxicological Evaluation of Certain Food Additives. Glycerol ester of wood rosin. WHO Food Additive Series No. 37.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1996b. Toxicological Evaluation of Certain Food Additives. Glycerol ester of wood rosin. WHO Food Additive Series No. 35.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1997. Evaluation of certain food additives and contaminants. Forty‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series No. 868. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Safety evaluation of certain food additives and contaminants. 976. Lead. WHO Food Additives Series No. 44, 39p.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Evaluation of certain food additives and contaminants. Sixty‐fourth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series No. 930, 109p.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2010a. Safety Evaluation of Certain Food Additives. Seventy‐first meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additive Series No. 62.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2010b. Evaluation of certain food additives (Seventy‐first report of the Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report Series, No. 956. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2011. Evaluation of certain food additives and contaminants. Seventy‐fourth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series No. 966, 25p.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2013a. Evaluation of certain food additives and contaminants. Seventy‐seventh report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series No. 983, 23p.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2013b. FAO JECFA Monographs 14 (2013) http://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/monograph14/additive-213-m14.pdf
- Jones K, Garfitt SJ, Calverley A, Channa K and Cocker J, 2001. Identification of a possible biomarker for colophony exposure. Occupational Medicine, 51, 507–509. [DOI] [PubMed] [Google Scholar]
- Karlberg AT, Bergstedt E, Boman A, Bohlinder K, Lidén C, Lars J, Nilsson G and Wahlberg JE, 1985. Oct. Is abietic acid the allergenic component of colophony? Contact Dermatitis, 13, 209–215. [DOI] [PubMed] [Google Scholar]
- Karlberg AT, Basketter D, Goossens A and Lepoittevin JP, 1999. Regulatory classification of substances oxidized to skin sensitizers on exposure to air. Contact Dermatitis, 40, 183–188. [DOI] [PubMed] [Google Scholar]
- Kim NH, et al., 2010. Tetrahydroabietic acid, a reduced abietic acid, inhibits the production of inflammatory mediators in RAW 264.7 macrophages activated with lipopolysaccharide. J Clin Biochem Nutr, 46, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Magnusson B and Nyquist G, 1971. Contact allergy to medicaments and materials used in dentistry. II. Sensitivity to eugenol and colophony. Odontologisk Revy, 22, 275–289. [PubMed] [Google Scholar]
- Koch G, Magnusson B, Nobreus N, Nyquist G and Soderholm G, 1973. Contact allergy to medicaments and materials used in dentistry. IV. Sensitizing effect of eugenol‐colophony in surgical dressing. Odontologisk Revy, 24, 109–114. [PubMed] [Google Scholar]
- Lysell L, 1976. Contact allergy to rosin in a periodontal dressing. A case report. Journal of Oral Medicine, 31, 24–25. [PubMed] [Google Scholar]
- Marshall E, 1983. The murky world of toxicity testing. Science, 220, 1130–1132. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Hayashi N, Ishida T and Asakawa Y, 1990. Metabolites of (+)‐dehydroabietic acid in rabbits. Journal of Pharmaceutical Sciences, 79, 540–547. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Agarwal K and Chakrabarti J, 1992. Genotoxicity studies of the food additive ester gum. Food and Chemical Toxicology, 30, 627–630. [DOI] [PubMed] [Google Scholar]
- Nasirullah Krishnamurthy MN and Nagaraja KV, 1995. Detection and determination of ester gum (substitute for brominated vegetable oil) in ready‐to‐serve beverages and their concentrates. Journal of Food Science and Technology, 32, 240–242. [Google Scholar]
- Nestmann ER, Lee EG, Mueller JC and Douglas GR, 1979. Mutagenicity of resin acids identified in pulp and paper mill effluents using the Salmonella/mammalian‐microsome assay. Environmental Mutagenesis, 1, 361–369. [DOI] [PubMed] [Google Scholar]
- Nestmann ER and Lee EG, 1983. Mutagenicity of constituents of pulp and paper mill effluent in growing cells of Saccharomyces cerevisiae. Mutation Research, 119, 273–280. [DOI] [PubMed] [Google Scholar]
- Nilsson U, Berglund N, Lindahl F, Axelsson S, Redeby T, Lassen P and Karlberg A‐T, 2008. SPE and HPLC/UV of resin acids in colophonium‐containing products. Journal of Separation Science, 31, 2784–2790. [DOI] [PubMed] [Google Scholar]
- Ogino Y, Hosokawa K, Suzuki M, Matsunaga K, Hirose O, Arima Y and Hayakawa R, 1989. Allergic contact dermatitis due to ester gum in a lipstick. Skin Research, 31, 180–184. [Google Scholar]
- Ohmori K and Kawamura Y, 2009. Cell transformation activities of abietic acid and dehydroabietic acid: safety assessment of possible contaminants in paper and paperboard for food contact use. Food Additives and Contaminants Part a‐Chemistry Analysis Control Exposure & Risk Assessment, 26, 568–573. [DOI] [PubMed] [Google Scholar]
- Oppel T and Schnuch A, 2006. The most frequent allergens in allergic contact dermatitis. Deutsche Medizinische Wochenschrift, 131, 1584–1589. [DOI] [PubMed] [Google Scholar]
- Ozaki A, Yamaguchi Y, Fujita T, Kuroda K and Endo G, 2005. Safety assessment of paper and board food packaging: chemical analysis and genotoxicity of possible contaminants in packaging. Food Additives and Contaminants, 22, 1053–1060. [DOI] [PubMed] [Google Scholar]
- Satyawan I, Oranje AP and van Joost T, 1990. Perioral dermatitis in a child due to rosin in chewing gum. Contact Dermatitis, 22, 182–183. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food), 1992. Opinion expressed 1990. Report of the Scientific Committee for Food. 26th Series. Food Science and Techniques, 48p.
- SCF (Scientific Committee for Food), 1994. Opinion expressed 1992. Report of the Scientific Committee for Food. 32nd Series. Food Science and Techniques, 37p.
- SCF (Scientific Committee for Food), 2001. Guidance on submissions for food additive evaluations by the Scientific Committee on Food. Opinion expressed on 11 July 2001. Available online: http://ec.europa.eu/food/fs/sc/scf/out98_en.pdf
- Seifried HE, Seifried RM, Clarke JJ, Junghans TB and San RH, 2006. A compilation of two decades of mutagenicity test results with the Ames Salmonella typhimurium and L5178Y mouse lymphoma cell mutation assays. Chemical Research in Toxicology, 19, 627–644. [DOI] [PubMed] [Google Scholar]
- Shao LP, Gafvert E, Karlberg AT, Nilsson U and Nilsson JL, 1993. The allergenicity of glycerol esters and other esters of rosin (colophony). Contact Dermatitis, 28, 229–234. [DOI] [PubMed] [Google Scholar]
- Soltes EJ and Zinkel DF, 1989. Chemistry of rosin In: Zinkel DF. and Russell J. (eds.). Naval Stores. Pulp Chemicals Association Inc, New York: pp. 261–330. [Google Scholar]
- Takahashi N, et al., 2003. Abietic acid activates peroxisome proliferator‐activated receptor‐γ (PPR‐γ) in RAW264.7 macrophages and 3T3‐L1 adipocytes to regulate gene expression involved in inflammation and lipid metabolism. FEBS Letters, 550, 190–194. [DOI] [PubMed] [Google Scholar]
- TemaNord (Nordic Council of Ministers), 2002. E 445: Glycerol esters of wood rosin. Food Additives in Europe 2000 ‐ Status of safety assessments of food additives presently permitted in the EU.
- Tsuruta D, Sowa J, Tsuruta K, Ishii M and Kobayashi H, 2011. Allergic contact dermatitis caused by gum rosin and wood rosin in Tako‐no‐Suidashi ointment. Journal of Dermatology, 38, 993–995. [DOI] [PubMed] [Google Scholar]
- Villeneuve DC, Yagminas AP, Marino IA and Becking GC, 1977. Toxicity studies on dehydroabietic acid. Bulletin of Environmental Contamination and Toxicology, 18, 42–47. [DOI] [PubMed] [Google Scholar]
- Wohrl S, Hemmer W, Focke M, Gotz M and Jarisch R, 2001. The significance of fragrance mix, balsam of Peru, colophony and propolis as screening tools in the detection of fragrance allergy. British Journal of Dermatology, 145, 268–273. [DOI] [PubMed] [Google Scholar]
- Young E, van Weelden H and van Osch L, 1988. Age and sex distribution of the incidence of contact sensitivity to standard allergens. Contact Dermatitis, 19, 307–308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the reported use levels (mg/kg) of glycerol esters of wood rosin E445 provided by industry
Number and percentage of food products labelled with glycerol esters of wood rosin (E445) out of the total number of food products present in THE Mintel GNPD per food subcategory between January 2013 and January 2018
Concentration levels of glycerol esters of wood rosin (E445) used in the refined exposure scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of glycerol esters of wood rosin (E445) from their use as a food additive for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to glycerol esters of wood rosin (E445) using the maximum level exposure scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)


