Abstract
In accordance with Article 6 of Regulation (EC) No 396/2005, the Imazalil Task Force submitted a request to the competent national authority in the Netherlands to change the existing maximum residue levels (MRLs) for the active substance imazalil in products of plant and animal origin. The evaluating Member State (EMS) proposed to modify the existing MRLs only in citrus fruits and bananas and, due to transfer of residues, in bovine and equine livers. EFSA assessed the intended uses in citrus fruits, but not the use in bananas, since compared with the previously performed MRL review no new information was provided in the context of the MRL application. For the intended uses in citrus fruits and for animal products, EFSA did not derive MRL proposals. A decision on the amendment of MRL for the intended uses needs to be postponed, until the risk assessment for the plant metabolite R014821 and for the metabolites identified in livestock (i.e. FK‐772 and FK‐284) is completed with regard to genotoxicity and general toxicity; data need to be provided to derive a conclusion whether the toxicological reference values derived for imazalil are also applicable for the metabolites. Until a conclusion on the toxicological properties of the metabolites is reached, a decision on the residue definition for risk assessment cannot be made which is a prerequisite to perform a reliable dietary risk assessment.
Keywords: imazalil, citrus fruits, pesticide, MRL, R014821, consumer risk assessment
Summary
In accordance with Article 6 of Regulation (EC) No 396/2005, the Imazalil Task Force submitted an application to the competent national authority in the Netherlands (evaluating Member State (EMS)) to modify the existing maximum residue levels (MRLs) for the active substance imazalil in citrus fruits, apples, pears, bananas and potatoes and in products of animal origin. The Netherlands drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA). Since the intended uses on apples, pears and potatoes resulted in a short‐term consumer exposure which exceeded the toxicological reference value set for imazalil, the EMS did not propose MRLs for these crops and consequently, EFSA did not assess these uses in this reasoned opinion. The intended use in bananas is identical with a good agricultural practice (GAP) which was already assessed in the framework of the MRL review; since no new information was provided, the use in bananas was also not assessed in this reasoned opinion. The current reasoned opinion is therefore only dealing with the requested modification of the existing MRLs in citrus fruits related to post‐harvest uses of imazalil and residues in bovine and equine livers.
Based on the conclusions derived by EFSA in the framework of Directive 91/414/EEC, the data evaluated in a previous MRL assessment under Article 12 of Regulation (EC) No 396/2005 and the additional data assessed by the EMS in the framework of this application, the following conclusions were derived.
The toxicological profile of imazalil was assessed in the framework of the EU pesticides peer review and the data were sufficient to set an acceptable daily intake (ADI) of 0.025 mg/kg body weight (bw) per day and an acute reference dose (ARfD) of 0.05 mg/kg bw. The data and information provided in this MRL application were not sufficient to conclude on the toxicological properties of the metabolites formed in plants (R014821) and observed in animal metabolism (FK‐772 and FK‐284).
Metabolism studies representative for post‐harvest use of imazalil in fruit crops have been assessed in the framework of the peer review. Studies investigating the effect of processing on the nature of imazalil showed the active substance to be hydrolytically stable. Investigations of residues in rotational crops were not available and are not required for the post‐harvest treatments.
The residue definition for enforcement proposed during the EU pesticides peer review is applicable to all plant commodities, including citrus fruits, and to processed products. As regards the residue definition for risk assessment, a tentative residue definition was derived, i.e. ‘sum of imazalil and R014821, expressed as imazalil’, pending the full assessment of the toxicological information requested for metabolite R014821 in the framework of the peer review and the MRL review. Taking into account the additional data on the toxicology of plant metabolite R014821 submitted in the context of the MRL application, a final conclusion on the residue definition for risk assessment still cannot be derived. The data were insufficient to clearly rule out a genotoxic potential of R014821 and to conclude whether the toxicological reference values derived for parent imazalil would be appropriate for the metabolite. Therefore, EFSA proposes not to use the previously proposed tentative residue definition for risk assessment for the post‐harvest uses assessed under this application.
The residue trials submitted were found to be sufficient to calculate potential MRL proposals for the three different GAPs in citrus fruits, i.e. 6 mg/kg for dipping/drenching, 4 mg/kg for the low‐volume post‐harvest spraying and 2 mg/kg for waxing. However, reliable risk assessment values cannot be derived as long as no final decision on the residue definition for risk assessment is taken.
Since citrus fruits by‐product such as dried pulp may be used as a feed item, the transfer of residues in commodities of animal origin would have to be assessed. However, for a reliable calculation of the livestock dietary burden, agreement on the residue definition for risk assessment in primary and processed products is required. Thus, the assessment of the need to modify the existing MRLs has to be postponed. In addition, data to assess the possible impact of the livestock metabolism on the isomer ratio are not available. Furthermore, data on two metabolites found in animal metabolism studies (i.e. FK‐772 and FK‐284) are missing to conclude on their toxicological properties, including genotoxicity, and to conclude whether specific reference value need to be derived or if the toxicological reference values derived for the parent compound would be applicable. Additional data to address the metabolic pathway of R014821 in livestock and of imazalil in poultry may need to be provided, if triggered by the revised livestock dietary burden. Because of these data gaps, the previously proposed tentative residue definition for risk assessment for animal products is withdrawn.
Lacking a decision on the appropriate residue definitions for risk assessment which is a consequence of the data gaps identified for the metabolites R014821, FK‐772 and FK‐284, the risk assessment cannot be finalised. In conclusion, EFSA did not derive MRL proposals for citrus fruits and for animal products.
When a decision on the tentative MRL proposals derived for imazalil under Article 12 of Regulation (EC) No 396/2005 is taken, risk managers should take into account the information presented in the current reasoned opinion.
The data gaps identified in the framework of the MRL review on storage stability of R014821 in high water content commodities and on storage condition of the samples of the livestock feedings study in ruminants identified following the MRL review were addressed in this opinion.
Assessment
In accordance with Article 6 of Regulation (EC) No 396/2005, the Imazalil Task Force (Makhteshim Chemical Works, Certis Europe BV and Janssen Pharmaceutica NV) submitted an application to the competent national authority in the Netherlands (evaluating Member State (EMS)) to modify the existing maximum residue levels (MRLs) for the active substance imazalil in citrus fruits, apples, pears, bananas and potatoes. Since potatoes and by‐products of apples and citrus are used as animal feed, the applicant also requested a modification of the existing MRLs in certain products of animal origin. The detailed description of the intended uses of imazalil in citrus fruits, apples, pears, bananas and potatoes which are the basis for the current MRL application is reported in Appendix A.1
The Netherlands drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 8 May 2015. In the framework of the detailed evaluation, EFSA identified data missing which were required to perform complete assessment. On 16 March 2018, the EMS submitted a revised evaluation report (Netherlands, 2015), which contained the assessment of additional data provided by the applicant in response to the request of EFSA. This version of the evaluation report replaced the previously submitted versions. Although the new information provided in the revised evaluation report did not address all data gaps identified (see Section 1), EFSA was asked by the European Commission to resume the assessment based on the available information; the assessment should highlight the existing data gaps and the related uncertainties.
To accommodate for the intended uses of imazalil, the EMS proposed to raise the existing MRL for citrus fruits from 5 to 8 mg/kg, for bananas from 2 to 8 mg/kg and to set a MRL of 0.04 mg/kg in bovine and equine liver, while the current MRL for bovine and equine liver is set at the limit of quantification of 0.05 mg/kg. Since the intended uses on apples, pears and potatoes resulted in a short‐term consumer exposure which exceeded the toxicological reference value set for imazalil, the EMS did not a propose MRLs for these crops.
In this reasoned opinion, EFSA assessed the post‐harvest uses in citrus2 as described in Appendix A (good agricultural practice (GAP) no 1, 2 and 3) and the impact on MRLs for products of animal origin, based on the information provided in the evaluation report prepared by the EMS. The remaining commodities are not subject of this reasoned opinion for the following reason:
Pears and potatoes (GAP no 5 and 6): the same GAPs were already assessed in the framework of the MRL review (EFSA, 2017a); no new information was provided. In 2017, EFSA identified an acute intake concern.
Bananas (GAP no 7): the same GAPs were already assessed in the framework of the MRL review (EFSA, 2017a); no new information was provided. EFSA identified an acute intake concern3 and data gaps.
Apples and pears (GAP no 4): EFSA has identified an acute consumer intake concern for the GAP with a waiting period of 60 days after the treatment; thus, the more critical GAP with a waiting period of 0 days was not further considered by the EMS in the final version of the evaluation report (Netherlands, 2015).
On 4 May 2018, EFSA consulted Member States on the draft Reasoned Opinion via written procedure. The Member State consultation closed on 18 May 2018.
Imazalil is the ISO common name for (RS)‐1‐(β‐allyloxy‐2,4‐dichlorophenethyl)imidazole or allyl (RS)‐1‐(2,4‐dichlorophenyl)‐2‐imidazol‐1‐ylethyl ether (IUPAC). Imazalil is composed of a racemic mixture of isomers. The chemical structures of the active substance and its main metabolites are reported in Appendix E.
Imazalil was evaluated in the framework of Directive 91/414/EEC4 with Luxembourg designated as rapporteur Member State (RMS).5 Following the first peer review in which EFSA was not yet involved, imazalil was approved as fungicide on 1 January 1999. EFSA carried out the peer review of the pesticide risk assessment for imazalil for its renewal in the framework of Commission Regulation (EC) No 737/20076, with the Netherlands designated as RMS; the representative uses assessed were on citrus fruits (post‐harvest, dipping/drenching or spray waxing), protected tomatoes grown on artificial substrate (foliar use) and on barley and wheat (seed treatment). The draft assessment report (DAR) prepared by the RMS has been peer reviewed by EFSA (2010). Imazalil has been approved under Regulation (EC) No 1107/20097 on 1 January 2012 for the use as a fungicide; however, the manufacturer was requested to provide further residues and environmental studies as confirmatory information by 31 December 2013. The confirmatory data were assessed in a Technical Report of EFSA (2014) and were found sufficient (European Commission, 2015).
The MRL review has been performed (EFSA, 2017a); data gaps were identified which have relevance for the crops assessed in the reasoned opinion. The proposed modifications have not yet been implemented in the European Union (EU) MRL legislation. Currently, the EU MRLs for imazalil are established in Annex II of Regulation (EC) No 396/20058.
EFSA based its assessment on the revised evaluation report submitted by the EMS (Netherlands, 2015), the comments received during the Member State consultation on the draft Reasoned Opinion on imazalil (EFSA, 2018), the DAR and its addendum (Netherlands, 2009a,b) prepared under Commission Regulation (EC) No 737/2007, the Commission review report on imazalil (European Commission, 2015), the conclusions on the EU pesticides peer review, the confirmatory data and the MRL review of the active substance imazalil (EFSA, 2010, 2014, 2017a,b).
For this application, the data requirements established in Regulation (EU) No 544/20119 and the guidance documents applicable at the date of submission of the application to the EMS are applicable (European Commission, 1997a,b,c,d,e,f,g, 2000, 2010a, b, 2017; OECD, 2011). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/201110.
A selected list of end points of the studies assessed by EFSA in the framework of this MRL application including the end points of relevant studies assessed previously, submitted in support of the current MRL application, are presented in Appendix B.
The revised evaluation report submitted by the EMS (Netherlands, 2015) and the comments received during the Member State consultation on the draft Reasoned Opinion on imazalil (EFSA, 2018) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available as background documents to this reasoned opinion.
1. Mammalian toxicology
The toxicological profile of imazalil was assessed during the peer review (EFSA, 2010) where an acceptable daily intake (ADI) of 0.025 mg/kg body weight (bw) per day and an ARfD of 0.05 mg/kg bw were derived.
Following evaluation of the new information as detailed in the post‐approval addendum, which was peer‐reviewed by Member States and EFSA, it was agreed to retain the existing ARfD of 0.05 mg/kg bw (European Commission, 2015).
The toxicological profile of metabolite R014821, a metabolite which was found in significant amounts in metabolism studies in fruit and root crops following post‐harvest treatment, was discussed during the peer review. Metabolite R014821 corresponded to the rat metabolite M11. The RMS confirmed that it was present at 1% in faeces in the metabolism studies. No toxicological information was available for this metabolite. The metabolite is formed by O‐dealkylation of the side chain in the molecule. Lacking further toxicological studies, the experts could not conclude whether the toxicological profile of the metabolite was comparable with the parent compound (EFSA, 2010).
In the framework of the MRL review under Article 12 of Regulation (EC) No 396/2005, EFSA confirmed the data gaps related to the toxicological profile of metabolite R014821, i.e. a full toxicological assessment of this metabolite was requested; in addition, the need for a full toxicological assessment was noted for FK‐722 and FK‐284, two metabolites that were identified in livestock metabolism studies (EFSA, 2017a).
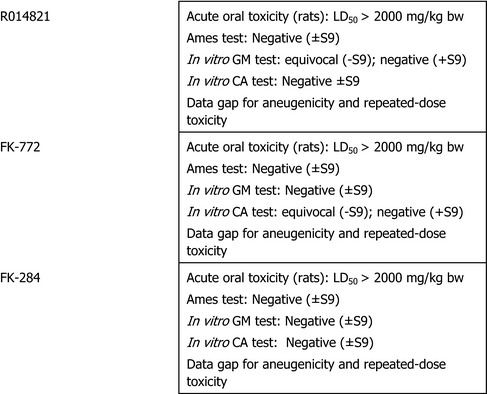
During the detailed assessment of this application, the need to provide additional toxicological data on the metabolites R014821, FK‐722 and FK‐284 was reiterated. In response to the request, the applicant submitted additional data for the three metabolites, including QSAR evaluations, in vitro genotoxicity test battery and acute oral toxicity testing of metabolites R014821, FK‐772 and FK‐284.
The EMS assessed the data and concluded that the data on metabolites R014821, FK‐772 and FK‐284 are not sufficient to conclude on the genotoxicity of the three metabolites and considering that no repeated dose toxicity studies are available no specific reference values can be derived nor can it be concluded if the metabolites are covered by the parent (Netherlands, 2015). The summary of the genotoxicity studies are reported in the Appendix B, B.1.
EFSA shares the views of the EMS that the risk assessment for the three metabolites cannot be finalised, pending submission of the following information:
The aneugenicity potential has to be investigated for the three metabolites R014821, FK‐772 and FK‐284.
Equivocal results were obtained regarding the gene mutation potential of R014821 in mammalian cells (without metabolic activation); this result has to be clarified to exclude a mutagenic potential of the metabolite.
Equivocal results were obtained in chromosome aberration assay without metabolic activation with the metabolite FK‐772, this result has to be clarified to exclude a clastogenic potential of the metabolite FK‐772.
Repeated‐dose toxicity studies with the three metabolites R014821, FK‐772 and FK‐284 are needed – unless it can be shown that they are major rat metabolites (i.e. at least 10% of the dose administered is systemically available in toxicokinetics/metabolism studies) to assess the toxicological profile of the metabolites in comparison with the one of the parent compound.
It was noted that the classification of imazalil has been re‐evaluated by ECHA after the peer review and the current harmonised classification and labelling in the EU of the active substance now includes Carcinogenicity Cat. 2 (H351, Suspected of causing cancer11), this information has therefore been included in the Appendix B, B.1 (amending the list of endpoints agreed during the peer review).
2. Residues in plants
2.1. Nature of residues and methods of analysis in plants
2.1.1. Nature of residues in primary crops
The metabolism of imazalil was investigated in different crop groups in the framework of the EU pesticides peer review and the MRL review (EFSA, 2010, 2017a). The studies were conducted after foliar applications on fruit crops, post‐harvest use on fruit and root crops and seed treatment on root crops and cereals/grasses.
Imazalil was the major component of total radioactive residues (TRR) following foliar and post‐harvest uses (69–99% TRR) and after seed treatment in cereal forage and straw (17–24% TRR). Following the post‐harvest application, the compound R014821 was observed in significant amount (11% TRR in apples and 9% TRR in potatoes after a storage period of 6–7 months, respectively); residues of R014821 were shown to increase with the length of the storage. R014821 was also observed in metabolism studies following foliar treatment, but below the trigger value of 10% of the TRR.
Chiral analyses performed on potato tubers after post‐harvest application indicated that the S/R ratio of imazalil isomers remained unchanged during the storage period (EFSA, 2017a). Details of the metabolism studies are presented in Appendix B.
2.1.2. Nature of residues in rotational crops
Since imazalil was proposed for use post‐harvest, investigations on the nature of residues in rotational crops are not required in the framework of this MRL request. Thus, the data gap identified in the framework of the MRL review (EFSA, 2017a) is not relevant for this application.
2.1.3. Nature of residues in processed commodities
Although the effect of processing on the nature of imazalil residues was not investigated with radiolabelled material, the deviation was considered acceptable. Imazalil was concluded to be stable because 94–99% of initial concentration was recovered after the hydrolysis under standard conditions (EFSA, 2014). Details of the study are presented in Appendix B.
2.1.4. Methods of analysis in plants
The MRL review concluded that analytical methods using liquid chromatography with tandem mass spectrometry (LC–MS/MS) were fully validated for the determination of imazalil residues in plant (high water, high acid, high oil and dry) commodities with a limit of quantification (LOQ) of 0.01 mg/kg (EFSA, 2017a).
2.1.5. Stability of residues in plants
The storage stability of imazalil was demonstrated in high water, high acid and high starch content commodities and of R014821 in high water content commodities (EFSA, 2010, 2017a). Additional storage stability data were assessed in the framework of this MRL application. Imazalil and R014821 were shown to be stable under frozen conditions (−20°C) in orange samples for 8 months (Netherlands, 2015). The new data addressed the data gap identified during the MRL review for R014821 in high acid content commodities.
2.1.6. Proposed residue definitions
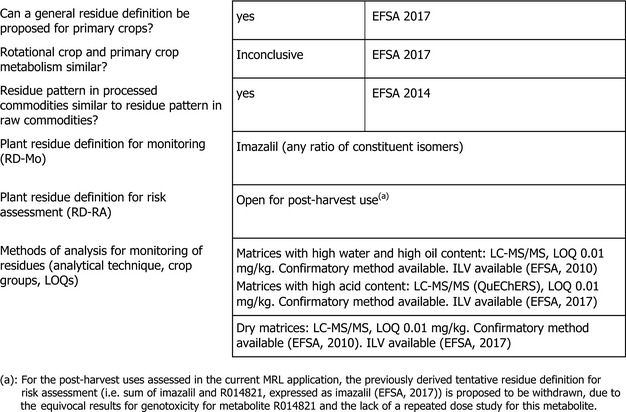
Residue definition for enforcement: The residue definition for enforcement proposed during the EU pesticides peer review for fruit crops and cereals/grasses was imazalil (parent compound (EFSA, 2010).
Based on the metabolic pattern identified in metabolism studies and the results of the hydrolysis study, the MRL review extended the enforcement residue definition parent imazalil to all plant commodities and processed products. Taking into account that the compound is a racemic mixture of isomers and that the information regarding the isomer ratio of the residues is very limited, the residue definition for enforcement was specified as imazalil (any ratio of constituent isomers) (EFSA, 2017a). This residue definition is appropriate for the uses assessed in this opinion.
Residue definition for risk assessment: The peer review and the MRL review had tentatively defined the residue definition for risk assessment as ‘sum of imazalil and R014821, expressed as imazalil’, pending the full assessment of the toxicological information requested for metabolite R014821 (EFSA, 2010, 2017a). It was noted that the metabolite is only relevant when commodities are subject to post‐harvest uses with longer withholding periods since R014821 was found in significant levels only after post‐harvest treatments (EFSA, 2017a,b); in metabolism studies with foliar treatment, R014821 was considered as a minor metabolite.
Taking into account the additional data on the toxicology of plant metabolite R014821 submitted in the context of the MRL application, a final conclusion on the residue definition for risk assessment still cannot be derived. The data were insufficient to clearly rule out a genotoxic potential of R014821 (see Section 1) and to conclude whether the toxicological reference values derived for parent imazalil would be appropriate for the metabolite. Therefore, EFSA proposes not to apply the previously proposed tentative residue definition for risk assessment in plants for post‐harvest uses.
2.2. Magnitude of residues in plants
2.2.1. Magnitude of residues in primary crops
Citrus fruit, drenching/dipping post‐harvest use (GAP No 1): In total, 34 residue trials in oranges and mandarins with drenching/dipping application were submitted and assessed previously (EFSA, 2010, 2017a). Among these data, 10 trials in mandarins and 12 in oranges were identified by the EMS as valid trials. EFSA disregarded two additional trials (one in oranges and one in mandarins) as they were considered not independent.12
In eight of the valid trials, samples of fruit and pulp were analysed separately for imazalil and R014821. Four of these trials were designed as decline studies with samples taken up to 85–86 days after the treatment which allowed getting a better understanding of the kinetic of residue behaviour for the parent compound and the metabolite. These decline studies provided evidence that following post‐harvest treatment, the concentrations of R014821 increased with time (< 0.01–0.03 mg/kg the day after treatment; < 0.01–0.13 mg/kg after 85 days). A similar observation was made in the metabolism studies in apples and potatoes and in citrus samples collected from the market (unknown storage time), where R014821 was measured at up to 0.88 mg/kg (EFSA, 2017b). A migration of residues from peel to pulp was also observed over the time for both the parent compound and the metabolite R014821 (up to 0.15 mg/kg for imazalil and 0.02 mg/kg for R014821, in the pulp at 85 days).
For the GAP assessed, a MRL proposal of 6 mg/kg13 is calculated which is in line with the previously derived MRL proposal (EFSA, 2017a); the data were considered sufficient for extrapolation to the whole group of citrus fruits. However, reliable risk assessment values cannot be derived as long as no final decision on the residue definition for risk assessment is taken.
Citrus fruit, low‐volume spraying post‐harvest use, (GAP No 2): Among the trials submitted by the applicant, eight GAP‐compliant residue trials (four trials in oranges and four trials in mandarins were identified by the EMS. All samples were analysed for parent imazalil and R014821; separate results for pulp are also available.
The calculated MRL proposal for this GAP is 4 mg/kg. However, reliable risk assessment values cannot be derived as long as no final decision on the residue definition for risk assessment is taken.
Citrus fruit, waxing post‐harvest use (GAP No 3): Residue trials representative for this GAP were previously assessed by EFSA in the framework of the peer review (EFSA; 2010). In addition, six new residue trials (three trials in oranges and three trials in mandarins) were provided. The residue trials already assessed by EFSA as representative use in the framework of the EU pesticides peer review (EFSA, 2010) were analysed only for the parent compound, while the new trials provide information on the parent compound and the metabolite R014821. In the new trials, samples of fruit and pulp were analysed separately for imazalil and R014821.
Based on this data, a MRL proposal of 2 mg/kg was derived. However, reliable risk assessment values cannot be derived as long as no final decision on the residue definition for risk assessment is taken.
A more critical GAP for waxing of citrus fruits was assessed in the framework of the MRL review (EFSA, 2017a) and the data submitted in the MRL request under assessment do not lead to a higher calculated MRL.
Details of the residue trials are presented in Appendix B.
2.2.2. Magnitude of residues in rotational crops
Not required (post‐harvest use).
2.2.3. Magnitude of residues in processed commodities
Specific studies assessing the magnitude of imazalil residues in processed products and the distribution of residues in peel and pulp of fruits with inedible peel were previously assessed by EFSA (EFSA, 2017a). The processing factors (PF) and conversion factors (CF) for fruit crops and the peeling factors for citrus fruits derived may need to be reconsidered upon finalisation of the residue definition for risk assessment.
2.2.4. Proposed MRLs
Based on the available data, EFSA calculated MRL proposals that would be required to cover the three different GAPs in citrus (i.e. 6 mg/kg for the drenching/dipping post‐harvest uses, 4 mg/kg for the low‐volume spray post‐harvest use and 2 mg/kg for the waxing post‐harvest use). However, considering the data gaps identified regarding the toxicological profile of R014821, reliable risk assessment values could not be derived. Therefore, a realistic consumer risk assessment cannot be performed at the moment (see Section 4).
3. Residues in livestock
By‐products of citrus fruits (dried pulp) may be used as a feed item. Therefore, the possible transfer of residues in commodities of animal origin should be assessed.
In the framework of the MRL review, the dietary burden was calculated, taking into account the critical existing uses. Citrus fruits were included in the dietary burden calculation using the supervised trials median residue (STMR) of 1.5 mg/kg derived from a GAP for a post‐harvest treatment (waxing, 300 g a.s./hL) and a default processing factor of 4.2. Based on a feeding study in ruminants,14 tentative MRL proposals were derived for food of animal origin in the MRL review (EFSA, 2017a).
The dietary burden calculation should be updated, once a decision on the residue definition for risk assessment for plant products is derived. However, as long as the toxicological profile of metabolite R014821 is not fully elucidated and a final conclusion on the residue definition for risk assessment is not derived, the calculation of the dietary burden for livestock and a sound assessment of the residues in food of animal products are not possible.
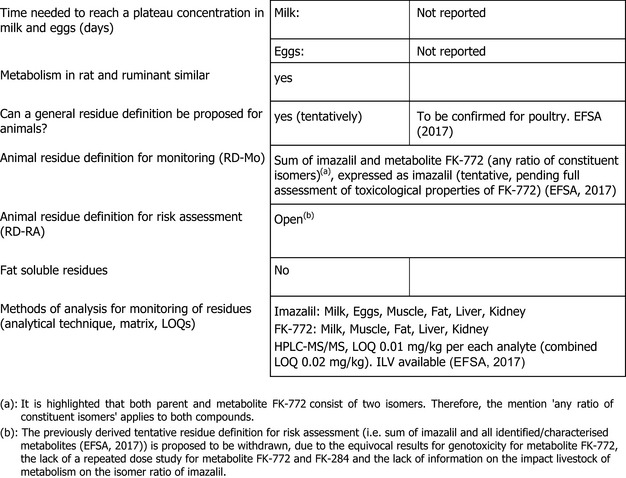
Furthermore, it needs to be highlighted that data gaps were also identified for metabolites identified in metabolism studies in livestock, i.e. metabolites FK‐772 and FK‐284 (see Section 1, data need to be provided to derive a conclusion whether the toxicological reference values derived for imazalil are also applicable for the metabolites). Therefore, EFSA proposes to withdraw the previously proposed tentative residue definition for risk assessment applicable for animal products. In addition, it is noted that the available metabolism studies in livestock did not investigate the impact on the isomer ratio for imazalil and its metabolites.
Additional data to address the metabolic pathway of R014821 in livestock and of imazalil in poultry may need to be provided, if triggered by the revised livestock dietary burden.
Considering the substantial data gaps, the possible revision of the existing MRLs for bovine and equine livers could not be assessed by EFSA at the moment.
4. Consumer risk assessment
In the framework of the MRL review, a comprehensive chronic and acute risk assessment taking into account the existing uses was performed. The risk assessment was based on the tentative residue definitions for risk assessment (i.e. sum of imazalil and R014821, expressed as imazalil for food of plant origin and the sum of imazalil and all identified/characterised metabolites for food of animal origin) assuming that the metabolites would be of similar toxicity as the parent compound (EFSA, 2017a).
Usually, with new MRL requests, the previously performed risk assessment should be updated, including the risk assessment values for the new MRL proposals and any other information that would be relevant for the risk assessment. In the context of this application the applicant provided data, which in his view would allow to replace the default variability factors (VF) used for citrus fruit in the acute risk assessment risk (i.e. VF of 5 for grapefruit and 7 for other citrus fruit) with the empirical VF of 1.9 reported in public literature (Hamilton et al., 2004). Since the empirical unit‐to‐unit variability of imazalil residues was obtained analysing market samples for which application method, rate and storage were unknown, EFSA was of the opinion that this VF cannot be used. Experimental data on the unit‐to unit variability from citrus fruits treated according to the reported post‐harvest uses and analysed for the full residue definition for risk assessment would be required to derive a specific VF to replace the default VF.
Considering that due to the lack of toxicological data for the metabolites R014821, FK‐772 and FK‐284 (see Section 1), the risk assessment for these metabolites cannot be finalised, EFSA did not derive a decision on appropriate residue definitions for risk assessment. As a consequence, the update of the risk assessment taking into account the intended uses in citrus could not be performed.
5. Conclusion and Recommendations
In conclusion, EFSA did not derive MRL proposals for citrus fruits for the intended post‐harvest uses of imazalil. A decision on the setting of MRL for the intended uses needs to be postponed, until the risk assessment for metabolite R014821 is completed and it can be ruled out that the metabolite is genotoxic; data need to be provided to derive a conclusion whether the toxicological reference values derived for imazalil are also applicable for the metabolite. Until a conclusion on the toxicological properties of the metabolites is reached, a decision on the residue definition for risk assessment cannot be made which is a prerequisite to perform a reliable dietary risk assessment.
As regards the request to amend the existing MRLs for bovine and equine liver, no MRL proposal could be derived, lacking information/data that would allow finalising the risk assessment for metabolites FK‐772 and FK‐284.
When a decision on the tentative MRL proposals derived for imazalil under Article 12 of Regulation (EC) No 396/2005 (EFSA, 2017a,b) is taken, risk managers should take into account the information presented in the current reasoned opinion. In particular, risk managers should be made aware that metabolite R014821 is expected to occur following post‐harvest uses of imazalil. Thus, the tentative MRL proposals for grapefruit, oranges, lemons, limes, mandarins, bananas and melons should be reconsidered with view to the possible occurrence of metabolite R014821. If the proposed tentative MRLs for the before mentioned crops are not accepted by risk managers, the MRL proposals for animal products should be revised, considering a scenario of the dietary burden calculation which would not include the feed items derived from citrus fruit.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CF
conversion factor for enforcement to risk assessment residue definition
- cGAP
critical GAP
- CXL
Codex maximum residue limit
- DALA
days after last application
- DAR
draft assessment report
- DAT
days after treatment
- EC
emulsifiable concentrate
- EMS
evaluating Member State
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- HPLC–MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- ILV
independent laboratory validation
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- LC
liquid chromatography
- LD50
lethal dose, median
- LOQ
limit of quantification
- MRL
maximum residue level
- NEU
northern Europe
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant‐back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- QSAR
quantitative structure–activity relationship
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged, and Safe (analytical method)
- Rber
statistical calculation of the MRL by using a non‐parametric method
- Rmax
statistical calculation of the MRL by using a parametric method
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SC
suspension concentrate
- SEU
southern Europe
- SG
water‐soluble granule
- SL
soluble concentrate
- SMILES
simplified molecular‐input line‐entry system
- SP
water‐soluble powder
- STMR
supervised trials median residue
- TAR
total applied radioactivity
- TRR
total radioactive residue
- UV
ultraviolet (detector)
- VF
variability factors
- WHO
World Health Organization
Appendix A – Summary of intended GAP triggering the amendment of existing EU MRLs
1.
| Crop and/or situation | NEU, SEU, MS or country | F G or Ia | Pests or group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days)d | Remarks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb |
Conc. a.s. |
Method kind | Range of growth stages & seasonc |
number min–max |
Interval between application (min) |
kg a.s./hL min–max |
Water L/ha min–max |
Rate | Unit | ||||||
| 1. Citrus fruits | EU | I | Penicillium digitatum, P. italicum, Diaporthe citri, Diplodia spp., Alternaria citri, Botrytis spp., Phomopsis spp., Diplodia natalensis | EC | 500 g/L | Post‐harvest, drenching/dipping | N/A | 1 | – | 0.05 | – | – | – | Waiting period 0 days | GAP assessed in the MRL review. Safety concern identified for grapefruits and oranges, but new data received to attempt refinement of the risk assessment |
| 2. Citrus fruits | EU | I | EC | 500 g/L | Post‐harvest, spraying (low volume) | N/A | 1 | – | 0.15 | – | – | – | Waiting period 0 days | GAP was reported in the MRL review, but not assessed as it was not the most critical GAP | |
| 3. Citrus fruits | EU | I | EC | 500 g/L | Post‐harvest, waxing | N/A | 1 | – | 0.2 | – | – | – | Waiting period 0 days | A more cGAP was assessed in the MRL review (rate of 0.3 kg/hL) for grapefruits and oranges | |
| 4. Apples/Pears | EU | I | Post‐harvest fungi | SL | 75 g/L | Post‐harvest, drenching/dipping | N/A | 1 | – | 0.0375 | – | – | Waiting period 0 days | A less cGAP assessed in the MRL review (with waiting period of 60 days) and in the original version of the evaluation report with safety concern identified | |
| 5. Pears | EU | I | Penicillium expansum, Botrytis cinerea, Gloeosporium album | SL | 75 g/L | Post‐harvest, drenching/dipping | Within 16 h after harvest | 1 | – | 0.025 | – | – | Waiting period 0 days | GAP assessed in the MRL review. Safety concern identified | |
| 6. Potatoes | EU | I | Silver scurf, Gangrene, Skin spot, Dry rot (Fusarium) | SL | 100 g/L | Post‐harvest, spraying | N/A | 1 | – | – | – | 0.015 | g/t | Waiting period 0 days | According to the evaluation report, the intended GAP was 0.105 kg a.s./ha, which was considered equivalent to 0.015 kg a.s./t; the same GAP was assessed in the MRL review for which a consumer safety concern was identified |
| 7. Bananas | EU | I | Post‐harvest fungi | SG | 750 g/kg | Post‐harvest, dipping/drenching/spraying | 1 | – | 0.06 | – | – | – | Waiting period 35 days | GAP assessed in the MRL review. Safety concerns and data gap identified | |
GAP: good agricultural practice; MRL: maximum residue level; NEU: northern European Union; SEU: southern European Union; MS: Member State; a.s.: active substance; EC: emulsifiable concentrate; SL: soluble concentrate; cGAP: critical GAP; SG: water‐soluble granule.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI: minimum preharvest interval.
GAPs highlighted in grey: GAPs already assessed by EFSA in the framework of the MRL review and not subject to reassessment in this reasoned opinion.
Appendix B – List of end points
B.1. Mammalian toxicology
B.1..1.
B.1..1.1.
Classification with regard to toxicological data (Regulation (EU) No 283/2013, Annex Part A, Section 10)

B.2. Residues in plants
B.2.1. Nature of residues and methods of analysis in plants
B.2.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Fruit crops | Tomato | Foliar, 3 × 0.3 kg/ha | 1 DALA | EFSA (2010) | |
| Foliar, 3 × 1.5 kg/ha | 1 DALA | ||||
| Orange, Apple | Po‐dipping, 1 × 0.05 kg/hL | 2 h to 7 months | EFSA (2010) | ||
| Root crops | Potato | Po, onto the tuber: 1 × 0.015 kg/tonnes | 0, 14, 29, 91, 188 DAT | 14C‐benzoyl‐ring EFSA (2017a,b) | |
| Seed treatment: 1 × 0.015 kg/tonnes | At maturity (of newly produced potatoes) | Treated potatoes stored 3 months in a cold dry chamber prior to planting EFSA (2017a,b) | |||
| Seed treatment: 1 × 0.075 kg/tonnes | |||||
| Cereals/grass | Spring wheat | Seed treatment: 1 × 0.49 kg/tonnes | Forage: 42 DATGrain: 150 DAT | EFSA (2010) | |
| Test substance: 3H‐ or 14C‐imazalil. Further details on the exact position not available | |||||
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Root/tuber crops | – | – | – |
Studies not available EFSA (2010) Not require for Po use |
|
| Leafy crops | – | – | – | ||
| Cereal (small grain) | – | – | – | ||
| Other | – | – | – |
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source |
|---|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Yes | Test substance not radiolabelled. EFSA (2014) | |
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | ||
| Sterilisation (20 min, 120°C, pH 6) | Yes | ||
| Other processing conditions |
Po: post‐harvest; DAT: days after treatment; DALA: days after last application; PBI: plant‐back interval.
B.2.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Applea | −20 | 12 | Months | Imazalil | EFSA (2010) | |
| 12 | Months | R014821 | EFSA (2017a,b) | ||||
| Tomato | −18 | 6 | Months | Imazalil | EFSA (2010) | ||
| High acid content | Orangeb | −20 | 8 | Months | Imazalil | Netherlands (2015) | |
| 8 | Months | R014821 | |||||
| High starch | Cereal grain | −18 | 6 | Months | Imazalil | EFSA (2010) | |
| Specific matrices | Cereal straw | −18 | 6 | Months | Imazalil | EFSA (2010) | |
B.2.2. Magnitude of residues in plants
B.2.2.1. Summary of residues data from the supervised residue trials
| Commodity | Region/indoora | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HRb (mg/kg) | STMRc (mg/kg) | CFd |
|---|---|---|---|---|---|---|---|
| Citrus fruit | EU | GAP No. 1 (Po, drenching/dipping: 50 g/hL; WP 0 day) |
Combined data set of residue trials on oranges and mandarins compliant with the GAP already assessed (EFSA, 2010, 2017a,b). In bold, trials analysed for imazalil only. Four trials designed as decline (underlined). Higher residue level observed at a longer WP (reported under parentheses). Rmax : 4.97 (unrounded) Rber: 5.74 (unrounded) MRLOECD: 6.59 (mean + 4 SD, unrounded) Extrapolation to whole group of citrus fruits would be possible |
7 or 6e | 4.95 | 2.59 | TBE |
|
Mo orange (whole fruit): 1.36, 1.83, 2.06, 2.09, 2.58, 2.59, 2.69, 2.81, 2.89, 3.12, 4.95 Mo mandarin (whole fruit): 1.14, 1.30, 1.51, 2.19, 2.26, 2.66, 2.72, 3.34, 4.84 | |||||||
| RA (orange, mandarin): – (a final residue definition for risk assessment could not be derived) | |||||||
|
Additional information: Imazalil orange (pulp): 0.15 (WP 85 days), 0.19, 0.21, 0.69 Imazalil mandarin (pulp): 0.18, 0.14 (WP 61 days), 0.43, 0.70 R014821 orange (whole fruit): < 0.01, 0.01,0.06 (WP 57 days), 0,08 (WP 85 days) R014821 mandarin (whole fruit): 0.02, 0.03, 0.08 (WP 61 days), 0.13 (WP 86 days) R014821 orange (pulp): 3 × < 0.01, 0.02 (WP 85 days) R014821 mandarin (pulp): 2 × < 0.01, 0.01 (WP 86 days), 0.02 (WP 61 days) | |||||||
| Citrus fruit | EU | GAP No. 2 (Po, low volume spraying, 150 g/hL, WP 0 day) |
Combined data set of residue trials on oranges and mandarins compliant with the GAP. None of the studies was designed as decline study. Rmax: 3.69 (unrounded) Rber: 3.34 (unrounded) MRLOECD: 3.88 (mean + 4 SD, unrounded) Extrapolation to whole group of citrus fruits possible |
4 | 2.4 | 1.07 | TBE |
|
Mo orange (whole fruit): 0.64; 0.87; 1.08; 2.40 Mo mandarin (whole fruit): 0.55; 1.05; 1.16; 2.07 | |||||||
| RA (orange, mandarin): ‐ (a final residue definition for risk assessment could not be derived) | |||||||
|
Additional information: Imazalil orange (pulp): 0.044; 0.049; < 0.01; 0.044 Imazalil mandarin (pulp): 0.022; 0.016; 0.019; 0.711 R014821 orange (whole fruit): < 0.01; < 0.01; < 0.01; 0.018 R014821 mandarin (whole fruit): 0.022; 0.019; 0.032; 0.032 R014821 orange and mandarin (pulp): 8 ×< 0.01 | |||||||
| Citrus fruit | EU | GAP No. 3 (Po, waxing: 200 g/hL, WP 0 day) |
Combined data set of residue trials on oranges and mandarins compliant with the GAP. None of the studies was designed as decline study. Results from trials already assessed (EFSA, 2010) and analysed for imazalil only are in bold. Rmax: 1.30 (unrounded) Rber: 1.75 (unrounded) MRLOECD: 1.68 (mean + 4 SD, unrounded) Extrapolation to whole group of citrus fruits possible. |
2 | 1.14 | 0.62 | TBE |
|
Mo orange (whole fruit): 0.39, 0.49, 0.52, 0.56, 0.77, 0.91, 0.94, 0.97, 1.14 Mo mandarin (whole fruit): 0.20, 0.30, 0.40, 0.61, 2 × 0.62, 0.76 | |||||||
| RA (orange, mandarin): – (a final residue definition for risk assessment could not be derived) | |||||||
|
Additional information: Imazalil orange (pulp): 3 × < 0.01 Imazalil mandarin (pulp): < 0.01, 0.01, 0.04 R014821 orange and mandarin (whole fruit): 6 × < 0.01 R014821 orange and mandarin (pulp): 6 × < 0.01 |
MRL: maximum residue level; GAP: good agricultural practice; Mo: monitoring; RA: risk assessment; Rmax: statistical calculation of the MRL by using a parametric method; Rber: statistical calculation of the MRL by using a non‐parametric method; OECD: Organisation for Economic Co‐operation and Development.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue derived for the residue definition for monitoring. The highest residue refers to the whole commodity and not to the edible portion.
Supervised trials median residue derived for the residue definition for monitoring. The median residue refers to the whole commodity and not to the edible portion and is derived according to the residue definition for enforcement.
Conversion factor to recalculate residues according to the residue definition for monitoring to the residue definition for risk assessment. To be established (TBE) upon decision on the residue definition for risk assessment after Po‐uses in citrus fruits.
B.2.2.2. Residues in rotational crops

B.2.2.3. Processing factors
| Processed commodity | Number of valid studiesa | Processing Factor (PF) | CFP c | Comment/Source | |
|---|---|---|---|---|---|
| Individual valuesb | Median PF | ||||
|
PFs derived for imazalil in the MRL review CFp to be calculated, once a decision on the residue definition for risk assessment for Po‐use is derived | |||||
| Citrus, peeled | 36d | 0.01; 0.01; 0.01; 0.02; 0.03; 0.04; 0.04; 0.04; 0.04; 0.04; 0.04; 0.04; 0.05; 0.05; 0.05; 0.05; 0.06; 0.07; 0.07; 0.07; 0.08; 0.08; 0.08; 0.08; 0.10; 0.11; 0.12; 0.13; 0.14; 0.15; 0.15; 0.16; 0.20; 0.21; 0.25; 0.28 | 0.07 | TBE | EFSA (2017a,b) |
| Oranges, juice | 10 | 0.01; 0.02; 0.03; 0.05; 0.05; 0.10; 0.11; 0.14; 0.33; 0.35 | 0.08 | TBE | EFSA (2017a,b) |
| Oranges, dry pomace | 10 | 1.0; 1.1; 2.3; 4.03; 4.05; 4.39; 4.48; 4.86; 6.70; 9.56 | 4.20 | TBE | EFSA (2017a,b) |
| Oranges, wet pomace | 7 | 1.74; 1.98; 2.03; 2.04; 2.20; 2.29; 2.72 | 2.00 | TBE | EFSA (2017a,b) |
| Oranges, marmalade | 7 | 0.15; 0.25; 0.25; 0.27; 0.28; 0.56; 0.68 | 0.27 | TBE | EFSA (2017a,b) |
MRL: maximum residue level.
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
In bold, samples analysed also for R014821. Residues were: 2 × 0.02 mg/kg in the RAC (WP 31 days), 2 × < 0.01 mg/kg in juice, 0.18 and 0.28 mg/kg in dry pomace, 0.05 and 0.08 mg/kg in wet pomace, < 0.01 and 0.01 mg/kg in marmalade (Netherlands, 2015).
Conversion factor for risk assessment in the processed commodity. To be established (TBE) upon decision on a residue definition for risk assessment for Po‐use in citrus fruits.
Based on residue trials compliant with the Po GAP (drenching, 50 g/hL; WP 0 days, including replicates) performed on oranges (n=15, ranging from 0.01 to 0.28) and mandarins (n=16, ranging from 0.01 to 0.25) (EFSA, 2017a,b) and considering the PFs derived during the peer review on oranges (0.08), lemons (0.04; 0.04; 0.05) and grapefruits (0.13) (EFSA, 2010).
B.3. Residues in livestock
Livestock dietary burden calculations not performed.
B.3.1. Nature of residues and methods of analysis in livestock
B.3.1.1. Metabolism studies, methods of analysis and residue definitions in livestock
B.4. Consumer risk assessment
Lacking a residue definition for risk assessment for food of plant and animal origin, the consumer risk assessment could not be finalised.
B.5. Recommended MRLs
| Codea | Commodity | Existing EU MRL/MRL proposed in MRL reviewb (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: imazalil (any ratio of constituent isomers) | ||||
|
0110010 0110020 0110030 0110040 0110050 0110990 |
Grapefruits Oranges Lemons Limes Mandarins Other citrus fruits |
5/4c 5/4c 5/6d 5/6d 5/6d 5/– |
No proposals |
The submitted data were sufficient to calculate the MRLs that would be required to cover the three different GAPs for citrus fruits (6 mg/kg for the post‐harvest dipping/drenching use, 4 mg/kg for the post‐harvest low volume spray and 2 mg/kg for the post‐harvest waxing use). However, EFSA did not derived MRL proposals, since the risk assessment for metabolite R014821 needs to be completed first, ruling out that the metabolite is genotoxic; data need to be provided to derive a conclusion whether the toxicological reference values derived for imazalil are also applicable for the metabolite R014821. Lacking this information, a final decision on the residue definition for risk assessment for plant products cannot be derived, and consequently, the risk assessment cannot be completed |
| 1000000 | Products of animal origin | 0.05*/0.03 (bovine and equine liver); 0.02* (other products) | No proposals |
EFSA did not derived MRL proposals for animal products, since the risk assessment for metabolites FK‐772 and FK‐284 needs to be completed first, ruling out that the metabolites are is genotoxic; data need to be provided to derive a conclusion whether the toxicological reference values derived for imazalil are also applicable for the metabolites. Lacking this information, a final decision on the residue definition for risk assessment for animal products cannot be derived and consequently, the risk assessment cannot be completed |
MRL: maximum residue level; GAP: good agricultural practice.
* Indicates that the MRL is set at the limit of quantification.
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
MRL proposals derived in the framework of the MRL review (EFSA, 2017a,b); not yet implemented in EU MRL legislation.
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.
Not applicable.
Appendix D – Input values for the exposure calculations
1.
Not applicable. No input values as no consumer risk assessment can be carried out.
Appendix E – Used compound codes
1.
| Code/trivial name | IUPAC name/SMILES notation/InChiKeya | Structural formulab |
|---|---|---|
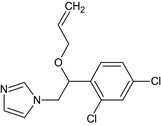
| Imazalil |
(RS)‐1‐(β‐allyloxy‐2,4‐dichlorophenethyl)imidazole or allyl (RS)‐1‐(2,4‐dichlorophenyl)‐2‐imidazol‐1‐ylethyl ether Clc2ccc(C(OCC=C)Cn1ccnc1)c(Cl)c2 PZBPKYOVPCNPJY‐UHFFFAOYSA‐N |
|
|
R014821 (M11) |
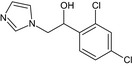
(1RS)‐1‐(2,4‐dichlorophenyl)‐2‐(1H‐imidazol‐1‐yl)ethanol OC(Cn1ccnc1)c2ccc(Cl)cc2Cl UKVLTPAGJIYSGN‐UHFFFAOYSA‐N |
|
| FK‐772 |
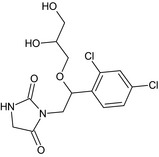
3‐{(2RS)‐2‐(2,4‐dichlorophenyl)‐2‐[(2RS)‐2,3‐dihydroxypropoxy]ethyl}‐2,4‐imidazolidinedione Clc2ccc(C(OCC(O)CO)CN1C(=O)CNC1=O)c(Cl)c2 SPULEQRPBJYQTB‐UHFFFAOYSA‐N |
|
| FK‐284 |
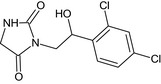
3‐[(2RS)‐2‐(2,4‐dichlorophenyl)‐2‐hydroxyethyl]‐2,4‐imidazolidinedione O=C2NCC(=O)N2CC(O)c1ccc(Cl)cc1Cl FXAPZNQCMLRNEK‐UHFFFAOYSA‐N |
|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system.
ACD/Name 2015 ACD/Labs 2015 Release (File version N20E41, Build 75170, 19 December 2014).
ACD/ChemSketch 2015 ACD/Labs 2015 Release (File version C10H41, Build 75059, 17 December 2014).
Suggested citation: EFSA (European Food Safety Authority) , Brancato A, Brocca D, Carrasco Cabrera L, Chiusolo A, Civitella C, Court Marques D, Crivellente F, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Istace F, Jarrah S, Kardassi D, Leuschner R, Lostia A, Lythgo C, Medina P, Mineo D, Miron I, Molnar T, Parra Morte JM, Pedersen R, Reich H, Riemenschneider C, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Terron A, Theobald A, Vagenende B and Villamar‐Bouza L, 2018. Reasoned opinion on the modification of the existing maximum residue levels for imazalil in various commodities. EFSA Journal 2018;16(6):5329, 29 pp. 10.2903/j.efsa.2018.5329
Requestor: European Commission
Question number: EFSA‐Q‐2015‐00283
Approved: 8 June 2018
Amended: 9 October 2018
Amendment: An editorial correction was carried out that does not materially affect the contents or outcome of this scientific output. To avoid confusion, the older version has been removed from the EFSA Journal, but is available on request.
Notes
It is noted that the GAPs submitted under this MRL application exception of the waxing post‐harvest use in citrus have been assessed previously by EFSA in the framework of the MRL review under Article 12 of Regulation (EC) No 396/2005 (MRL review)recently conducted by EFSA (EFSA, 2017a,b).
The same GAP was already assessed in the framework of the MRL review (EFSA, 2017a,b) where an acute consumer health risk was identified. The applicant provided additional information to perform a more refined risk assessment. Therefore the GAP was assessed again under this application.
A corrigendum of the previous reasoned opinion (EFSA, 2017), which will report the acute consumer health risk, is currently under preparation.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32.
Belgium acted as RMS on behalf of Luxembourg.
Commission Regulation (EC) No 737/2007 of 27 June 2007 on laying down the procedure for the renewal of the inclusion of a first group of active substances in Annex I to Council Directive 91/414/EEC and establishing the list of those substances. OJ L 169, 29.6.2007, p. 10–18.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Commission Regulation (EU) No 544/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the data requirements for active substances. OJ L 155, 11.6.2011, p. 1–66.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Commission Regulation (EU) 2015/1221 of 24 July 2015 amending Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures, for the purposes of its adaptation to technical and scientific progress. OJ L 197, 25.7.2015, p. 10–23.
EFSA disregarded the levels of 2.55 mg/kg for mandarins and 2.46 mg/kg for oranges. Each pair of side‐by‐side trial was conducted in the same location, with the same variety of mandarin and orange, with the same experimental conditions and with less than 30 days interval between the application dates.
The MRL of 8 mg/kg proposed by the EMS was derived with the formula ‘CF × 3 mean’ of the OECD MRL calculator. For post‐harvest uses, this formula shall normally be disregarded and the MRL proposed based on the ‘mean + 4 SD’ or the ‘highest residue’ (EFSA, 2015; OECD, 2016).
In the framework of the MRL review, information on the storage conditions of the samples of the feeding studies in ruminants was not available. With the current assessment, the EMS confirmed that the samples from the ruminant and poultry feeding studies were stored under frozen conditions and analysed within 30 days (tissues) or at −4°C and analysed within 3 days (milk, eggs) after collection (Netherlands, 2015). Consequently, investigations on storage stability are not required. This information addressed the data gap on information of the storage conditions of the samples from feeding studies identified during the MRL review (EFSA, 2017a,b).
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, 1–1355.
References
- EFSA (European Food Safety Authority), 2010. Conclusion on the peer review of the pesticide risk assessment of the active substance imazalil. EFSA Journal 2010;8(2):1526, 69 pp. 10.2903/j.efsa.2010.1526 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Outcome of the consultation with Member States, applicant and EFSA on the pesticide risk assessment of confirmatory data submitted for the active substance imazalil. EFSA supporting publication 2014:EN‐674, 13 pp. [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Residues trials and MRL calculations Proposals for a harmonised approach for the selection of the trials and data used for the estimation of MRL, STMR and HR. September 2015.
- EFSA (European Food Safety Authority), 2017a. Review of the existing maximum residue levels for imazalil according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2017;15(9):4977, 66 pp. 10.2903/j.efsa.2017.4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2017b. Member States consultation report on the review of the existing MRLs of imazalil prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005. 31 July 2017.
- EFSA (European Food Safety Authority), 2018. Compilation of comments received during the Member States consultation on the draft reasoned opinion on the modification of the existing maximum residue levels for imazalil in various commodities, prepared by EFSA in the framework of Article 10 of Regulation (EC) No 396/2005. 18 May 2018.
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2015. Review report for the active substance imazalil. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 17 June 2011 in view of the approval of imazalil as active substance in accordance with Regulation (EC) No 1107/2009. SANCO/11021/2011‐Rev 3, 17 June 2011 revised 9 October 2015.
- European Commission , 2017. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev. 10.3, 13 June 2017.
- Hamilton D, Ambrus A, Dieterle R, Felsot A, Harris C, Petersen B, Racke K, Wong SS, Gonzalez R, Tanaka K, Earl M, Roberts G and Bhula R; Advisory Committee on Crop Protection Chemistry, Division of Chemistry and the Environment; of the International Union of Pure and Applied Chemistry , 2004. Pesticide residues in food–acute dietary exposure. Pest Management Science, 60, 311–339. 10.1002/ps.865 [DOI] [PubMed] [Google Scholar]
- Netherlands , 2009a. Assessment Report on the active substance imazalil prepared by the rapporteur Member State Netherlands in consultation with Spain in the framework of Commission Regulation (EC) No 737/2007, May 2009. Available online: www.efsa.europa.eu
- Netherlands , 2009b. Final Addendum to Assessment Report on imazalil, prepared by the rapporteur Member State Netherlands in consultation with Spain in the framework of Commission Regulation (EC) No 737/2007, compiled by EFSA, November 2009. Available online: www.efsa.europa.eu
- Netherlands , 2015. Evaluation report on the modification of MRLs for imazalil in various crops and products of animal origin prepared by the evaluating Member State The Netherlands under Article 8 of Regulation (EC) No 396/2005. March 2015 revised in March 2018, 389 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2016. Guidance document on crop field trials. Series of pesticides No 66. In: Series of Testing and Assessment No 164. ENV/JM/MONO(2011)50/REV1, 7 September 2016.


