Abstract
In June 2016, EFSA received a mandate from the national food competent authorities of five European countries (Denmark, Finland, Iceland, Norway and Sweden) to provide a dietary reference value (DRV) for sugars, with particular attention to added sugars. A draft protocol was developed with the aim of defining as much as possible beforehand the strategy that will be applied for collecting data, appraising the relevant evidence, and analysing and integrating the evidence in order to draw conclusions that will form the basis for the Scientific Opinion on sugars. As EFSA wished to seek advice from stakeholders on this draft protocol, the NDA Panel endorsed it for public consultation on 12 December 2017. The consultation was open from 9 January to 4 March 2018. A technical meeting with stakeholders was held in Brussels on 13 February 2018, during the consultation period. After consultation with stakeholders and the mandate requestors, EFSA interprets this mandate as a request to provide scientific advice on an Tolerable Upper Intake Level (UL) for (total/added/free) sugars, i.e. the maximum level of total chronic daily intake of sugars (from all sources) judged to be unlikely to pose a risk of adverse health effects to humans. The assessment concerns the main types of sugars (mono‐ and disaccharides) found in mixed diets (i.e. glucose, fructose, galactose, sucrose, lactose, maltose and trehalose) taken through the oral route. The health outcomes of interest relate to the development of metabolic diseases and dental caries. The final version of the protocol was endorsed by the EFSA Panel on Dietetic Products, Nutrition and Allergies on 28 June 2018.
Keywords: sugars, tolerable upper intake level, adverse effects, metabolic diseases, dental caries, protocol
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2018.EN-1455/full
1. Introduction and scope of the protocol
This document outlines the protocol for the Scientific Opinion on the Tolerable Upper Intake Level of dietary sugars of the EFSA Panel on Nutrition, Dietetic Products and Allergies (NDA Panel), supported by the ad‐hoc Working Group (WG) on sugars. This draft protocol has been developed with the aim of defining as much as possible beforehand the strategy that will be applied for collecting data (i.e. which data to use for the assessment and how to identify and select them), appraising the relevant evidence, and analysing and integrating the evidence in order to draw conclusions that will form the basis for the Scientific Opinion.
The protocol has been developed following the principles and process illustrated in the EFSA PROMETHEUS project (PROmoting METHods for Evidence Use in Scientific assessments) (EFSA, 2015a).
A draft of this protocol was open for public consultation from 9 January to 4 March 2018. The public consultation included a technical meeting with stakeholders held in Brussels on 13 February 2018. The draft protocol has been amended in view of the comments received. All comments received have been addressed and published in a technical report (EFSA, 2018).
2. Background and rationale of the mandate
In June 2016, the national food competent authorities of five European countries (Denmark, Finland, Iceland, Norway and Sweden) sent a request to the European Food Safety Authority (EFSA) in order to provide a dietary reference value (DRV) for sugars, with particular attention to added sugars, on the basis of most recent scientific evidence. After discussing the mandate at its plenary meeting on 22–23 September 2016, the NDA Panel asked for some clarifications to the requestors, particularly regarding the type of DRV to be established, the exposure of interest, the target population and the health outcomes to be considered. In February 2017, the requestors clarified that they were interested in a science‐based cut‐off value for a daily exposure to added sugars from all sources (i.e. sucrose, fructose, glucose, starch hydrolysates such as glucose syrup, high‐fructose syrup and other isolated sugar preparations used as such or added during food preparation and manufacturing) which is not associated with adverse health effects. The target population for the assessment was defined as the general healthy population, including children, adolescents, adults and the elderly. The requestors also clarified that the request relates to an update of the EFSA's Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre (EFSA NDA Panel, 2010a) in relation to the effects of added sugars on nutrient density, glucose tolerance and insulin sensitivity, serum lipids, other cardiovascular risk factors (blood pressure), body weight, type 2 diabetes and dental caries in adults and children.
In the EFSA's 2010 opinion, the term ‘added sugars’ referred to sucrose, fructose, glucose, starch hydrolysates (glucose syrup, high‐fructose syrup) and other isolated sugar preparations used as such or added during food preparation and manufacturing.
With regard to the effects of added sugar intake, the NDA Panel reached the following conclusions on the outcomes assessed:
-
–
Micronutrient density of the diet: observed negative associations between added sugars intake and micronutrient density of the diet are mainly related to patterns of intake of the foods from which added sugars in the diet are derived rather than to the intake of added sugars per se. The available data are not sufficient to set an upper limit for (added) sugars intake.
-
–
Glucose and insulin response: there are limited, and mainly short‐term, data on the effects of high intakes of sugars on glucose and insulin response. Most studies do not find any adverse effects at intakes of predominantly added sugars up to 20–25% of total energy (E%), provided that body weight is maintained.
-
–
Serum lipids: although there is some evidence that high intakes (> 20 E%) of sugars may increase serum triglycerides and cholesterol concentrations, the available data are not sufficient to set an upper limit for (added) sugar intake.
-
–
Body weight: the evidence relating high intake of sugars (mainly as added sugars), compared to high intakes of starch, to weight gain is inconsistent for solid foods. However, there is some evidence that high intakes of sugars in the form of sugar‐sweetened beverages (SSBs) might contribute to weight gain. The available evidence is insufficient to set an upper limit for sugars based on their effects on body weight.
-
–
Type 2 diabetes: controversial findings on the association between total sugars and/or specific types of sugars and diabetes risk were reported in large prospective cohort studies. However positive associations were found between SSBs and increased type 2 diabetes risk. The available evidence was found insufficient to set a Tolerable Upper Level of Intake (UL) for sugars based on their effects on type 2 diabetes risk.
-
–
Dental caries: available data do not allow the setting of a UL for (added) sugars on the basis of a risk reduction for dental caries, as caries development related to consumption of sucrose and other cariogenic carbohydrates does not depend only on the amount of sugar consumed, but it is also influenced by oral hygiene, exposure to fluoride, frequency of consumption and various other factors.
The NDA Panel concluded that the available data did not allow the setting of a UL for total or added sugars, neither an Adequate Intake (AI) nor a Reference Intake range (RI). However evidence on the relationship between patterns of consumption of sugar‐containing foods and dental caries, weight gain and micronutrient intake should be considered when establishing nutrient goals for populations and recommendations for individuals and when developing food‐based dietary guidelines (FBDG).
3. Terms of reference as provided by the mandate requestor
The request is for scientific assistance in line with Regulation (EC) No 178/2002 in assessing a DRV for added sugars, which would benefit risk managers and substantially support their work with dietary guidelines and nutrient recommendations if they could base their advices on an up‐to‐date assessment by EFSA.
To this end, EFSA has been requested to update its Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre published in 2010 (EFSA NDA Panel, 2010a), on the basis of the most recent scientific evidence, in order to derive a science‐based cut‐off value for a daily exposure to added sugars which is not associated with adverse health effects.
The mandate requestor clarified that the intake of interest is added sugars from all sources, i.e. sucrose, fructose, glucose, starch hydrolysates such as glucose syrup, high‐fructose syrup and other isolated sugar preparations used as such or added during food preparation and manufacturing. The health outcomes of interest are those already addressed in the EFSA 2010 opinion, i.e. micronutrient density of the diet, glucose tolerance and insulin sensitivity, serum lipids, other cardiovascular risk factors (blood pressure), body weight, type 2 diabetes and dental caries in adults and children.
To address this mandate, EFSA is requested to consider published reports from national and international bodies/authorities addressing the health effects of added sugars, as well as systematic reviews and meta‐analysis published since 2010 on this topic.
4. Background information
To address this mandate EFSA is requested to consider, as background information and sources of data, published reports from national and international authorities/bodies addressing the health effects of sugars, as well as systematic reviews and meta‐analysis published since 2010 on this topic.
An overview of the most recent existing DRVs and recommendations issued by other national and international authorities/bodies can be found in Appendix A. These publications will be used as sources of individual studies meeting the inclusion criteria for the present assessment through the scrutiny of their reference list.
A scoping literature search for systematic reviews and meta‐analysis addressing the health effects of sugars or any of its dietary sources published in English since 2009 has also been performed. The list of the references identified and their main characteristics (e.g. exposure and endpoints of interest) can be found in Appendix B. These systematic reviews and meta‐analysis will be used in two ways:
As sources of individual studies meeting the inclusion criteria for the present assessment through the scrutiny of their reference list;
As starting point for the literature searches to be carried out in the context of this assessment, whenever appropriate (see Section 9.2).
Data on the most recent national recommendations on sugars consumption in European countries will also be gathered through a questionnaire to National Competent Authorities (Appendix C).
5. Interpretation of the Terms of Reference
5.1. Definition of the exposure
Different terms and definitions have been used by researches and risk managers for dietary sugars. Among these:
Sugars (total), which are glycaemic carbohydrates composed of 1–2 monomers found in food. The main types of sugars found in mixed diets are the monosaccharides glucose, fructose and galactose, and the disaccharides sucrose, lactose, maltose and trehalose (FAO/WHO, 1998; EFSA NDA Panel, 2010a).
Added sugars, which include sugars (mono‐ and disaccharides) used as ingredients in processed and prepared foods and sugars eaten separately or added to foods at the table. This definition was first applied in the 2000 US Dietary Guidelines for Americans (USDA/HHS, 2000), and then by the IoM (2005), EFSA (EFSA NDA Panel, 2010a) and some European countries (Nordic Council of Ministers, 2014).
Free sugars, which include monosaccharides (glucose, fructose and galactose) and disaccharides (sucrose, lactose, maltose and trehalose) added to foods by the manufacturer, cook, or consumer plus sugars naturally present in honey, syrups, fruit juices and fruit juice concentrates. This term has been used by the World Health Organization (WHO, 2003, 2015).
Non‐milk extrinsic (NME) sugars, defined as sugars not located within the cellular structure of a food, such as those found in fruit juice, honey and syrups, and those added to processed foods, excluding lactose in milk. The term originated from the UK Department of Health (1989) as opposed to intrinsic sugars, which are those located within the cellular structure of a food (e.g. naturally found in fruits and vegetables). On its assessment of 2015, the UK Scientific Advisory Committee on Nutrition (SACN) (2015) adopted the WHO definition of free sugars to provide recommendations on sugar intakes.
This assessment concerns the main types of sugars (mono‐ and disaccharides) found in mixed diets (i.e. glucose, fructose, galactose, sucrose, lactose, maltose and trehalose) taken through the oral route only. Sugar alcohols (polyols), other substances used as sugar replacers and other mono‐ or disaccharides present in the diet in marginal amounts, are not included in the term ‘sugars’ for the purpose of this assessment.
To allow comparability with previous assessments done by EFSA and by other bodies, total sugars, added sugars and free sugars as defined above will be addressed in this protocol.
5.2. Objectives of the risk assessment
This mandate is a request for EFSA to update its Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre published in 2010 (EFSA NDA Panel, 2010a) with respect to (added) sugars, and therefore interpreted as a request in the context of establishing ULs for nutrients for the general European population. The principles for the application of risk assessment to nutrients in general, and for deriving ULs in particular, have been described elsewhere (SCF/EFSA NDA Panel, 2006; EFSA NDA Panel, 2010b).
EFSA interprets this mandate as a request to provide scientific advice on an UL for (total/added/free) sugars, i.e. the maximum level of total chronic daily intake of sugars (from all sources) judged to be unlikely to pose a risk of adverse health effects to humans. ‘Tolerable intake’ in this context connotes what is physiologically tolerable and is a scientific judgement as determined by assessment of risk, i.e. the probability of an adverse effect occurring at some specified level of exposure.
ULs may be derived for various life stage groups in the population. The UL is not a recommended level of intake. If there are no, or insufficient, data on which to base the establishment of a UL, as it was the case in 2010 for total or added sugars (EFSA NDA Panel, 2010a), an indication should be given on the highest level of chronic daily intake (from all sources) where there is reasonable confidence in data on the absence of adverse effects (i.e. a science‐based cut‐off value for a daily exposure which is not associated with adverse health effects). If there are no, or insufficient, data on which to base the establishment of a UL or a cut‐off value for (total/added/free) sugars from all sources because the evidence available relates to one or few sources only, or to a particular type of sugar (e.g. fructose, glucose, sucrose), and the extrapolation of the results to (total/added/free) sugars from all sources is found to be unjustified, scientific advice could be provided on quantitative intakes in relation to one or few sugar sources only, and/or in relation to one type of sugar only (e.g. fructose, glucose, sucrose) (Figure 1).
Figure 1.
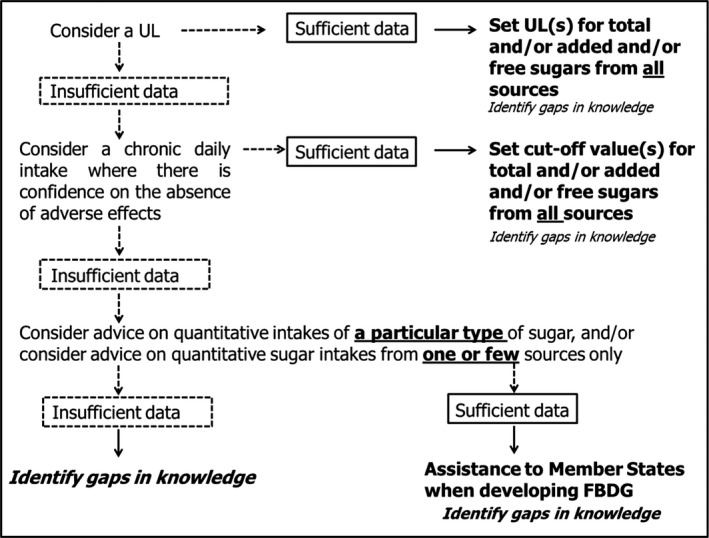
Step‐wise process to provide scientific advice on total/added/free sugars
The characterisation of the risk (SCF/EFSA NDA Panel, 2006) includes a description of the scientific uncertainties associated with the UL estimates in order to indicate the degree of scientific confidence that can be placed in these estimates. It will also include an estimate of intake for population groups as well as an indication of the margin between actual intakes and the UL, and an indication of circumstances, if any, in which risk is likely to arise. If a UL for (total/added/free) sugars cannot be established, other values could be used instead to characterise the risk (Figure 1).
It is out of the scope of this assessment to address possible beneficial health effects of sugars or of particular dietary sources of sugars.
The outcome of the assessment is expected to assist Member States and health professionals in establishing nutrient goals for populations and recommendations for individuals, and when developing FBDG.
5.3. Target population
Adverse effects of nutrients are influenced by physiological changes and common conditions associated with growth and maturation that occur during an individual's lifespan. Therefore, where necessary, and to the extent possible, ULs are derived for each separate life‐stage group, e.g. infants, children, adults, the elderly and women during pregnancy or lactation. Even within relatively homogenous life‐stage groups, there is a range of sensitivities to adverse effects, e.g. sensitivity is influenced by body weight and lean body mass.
The derivation of ULs for the general population, divided into various life‐stage groups, accounts for normally expected variability in sensitivity due to e.g. ethnicity, dietary habits, nutritional status, physical activity level (PAL) and metabolic risk profile. This includes individuals within the general population at risk of chronic disease (e.g. with excess body weight, elevated blood pressure, blood glucose and/or blood lipids but not on pharmacological treatment for the condition). It excludes, however, subpopulations with distinct vulnerabilities due to genetic predisposition or other considerations (e.g. individuals with inborn errors of carbohydrate metabolism) because including these would result in ULs which are significantly lower than are needed to protect most people against adverse effects of high intakes. Subpopulations needing special protection (e.g. individuals on pharmacological treatment for obesity, diabetes, hypertension, dyslipidaemia, chronic liver or renal diseases) are better served through the use of public health screening, health care providers, or other individualised strategies.
The following groups will be considered a priori:
Infants ≥ 4 to < 12 months
Toddlers (young children) ≥ 1 to < 3 years
Other children ≥ 3 to < 10 years
Adolescents ≥ 10 to < 18 years
Adults ≥ 18 to < 65 years
Elderly adults ≥ 65 years
Pregnant women
The age ranges may be modified by the NDA Panel depending on the available data, e.g. children may be further categorised according to the type of dentition (primary – milk or secondary – permanent) in relation to dental caries.
For this assessment, it is anticipated that lactating women will be considered together with other women in child‐bearing age.
Infants < 4 months of age will be excluded from the assessment on the assumption that they are exclusively fed with breast milk or breast milk substitutes (EFSA NDA Panel, 2009).
5.4. Adverse effects and endpoints
The assessment will focus on possible adverse effects of sugars intake on endpoints selected based on the scope of the mandate, on previous assessments done by other bodies (Appendix A), on the systematic reviews available (Appendix B) and on the comments received through the public consultation (EFSA, 2018).
Both disease endpoints and other endpoints will be addressed. Disease endpoints are considered to be the most direct, or applicable, to the assessment. Other endpoints are relevant but less direct, and can include upstream indicators, risk factors, intermediate endpoints or measures related to disease endpoints.
The disease endpoints of interest are:
Incidence/severity of dental caries
-
Incidence of metabolic diseases:
Obesity,
Type 2 diabetes mellitus (T2DM) and gestational diabetes mellitus (GDM),
Hypertension,
Dyslipidaemia,
Cardiovascular diseases (CVDs),
Non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis (NAFLD and NASH),
Gout.
Other endpoints of interest are:
Measures of body fatness and body fat distribution; birth weight‐related endpoints,
Ectopic fat deposition (e.g. muscle, liver),
Measures of glucose homoeostasis,
Blood pressure,
Blood lipids,
Uric acid,
The outcome variables that will be investigated in relation to the above‐mentioned endpoints are depicted in Section 9.1.
6. Identification of the assessment subquestions
The identification of the assessment subquestions follows the principles of risk assessment for nutrients (SCF/EFSA NDA Panel, 2006). The assessment subquestions are illustrated in Table 1.
Table 1.
Assessment subquestions to be answered
| Number | Subquestion |
|---|---|
| 1 | What are the levels of (total/added/free) sugars in foods and beverages in Europe? |
| 2 | What is the distribution of intakes of (total/added/free) sugars from all dietary sources (and by food source) by population group? |
| 3 | What are the digestion, absorption and metabolism of different types of sugars from different sources in humans? |
| 4 | What is the relationship between the intake of (total/added/free) sugars and metabolic diseases (disease endpoints and other endpoints) in the target population? |
| 5 | What is the relationship between the intake of (total/added/free sugars) and dental caries in the target population? |
| 6 | Which could be the potential mode(s) of action underlying the adverse effects (if any) of (total/added/free) sugars intake? |
For hazard identification, human studies provide the most relevant data and, when they are of sufficient quality and extent, are given the greatest weight. Owing to the wealth of human studies available on sugars intake and that extrapolation from animal models to humans is subject to considerable uncertainties, the Panel considers that evidence for adverse effects of sugars on humans should be obtained from human studies (subquestions 4 and 5).
Decisions on whether structural or functional alterations observed in human studies represent adverse effects or changes of little or self‐limiting biological importance will be based on scientific judgement and will be taken on a case‐by‐case basis. In this context, it will be important to determine:
the causal significance of an exposure–effect association indicated by observational studies, primarily for disease endpoints. Criteria for judging this include demonstration of a temporal relationship, consistency, strength of association, a dose–response relationship (a biological gradient), specificity, biological plausibility and coherence; and
the clinical significance of an intake–effect relationship indicated by intervention studies primarily on other (than disease) endpoints. For energy‐containing macronutrients, such as sugars, it will be important to determine how such relationships relate to energy‐wise comparable intakes of other energy‐containing macronutrients which are reasonably expected to replace the macronutrient under assessment in the diet. For sugars, the Panel considers that other glycaemic carbohydrates (e.g. starch) are the most appropriate comparison (EFSA NDA Panel, 2010a). Isocaloric comparisons with other macronutrients, however, may provide additional information which could be considered in the assessment.
Relevant experimental data (animal models, in vitro data) and knowledge of the molecular or cellular events underlying the adverse effect (mode of action) can assist in dealing with problems of data interpretation (subquestion 6).
Hazard characterisation includes a dose–response assessment which addresses the relationship between (total/added/free) sugars intake (dose) and adverse effect (in terms of intake and severity). Only human studies will be considered for that purpose (subquestions 4 and 5). The selection of the most appropriate or critical data set(s) for deriving the UL will be made on the basis of data quality, taking into account the related uncertainties and the possibility to derive quantitative estimates. Data on bioavailability (digestion, absorption and metabolism) will be considered to explain any apparent differences in dose response between different types, forms and sources of sugars (subquestion 3). Critical data set(s) should document the route of exposure and magnitude and duration of intake, and the intake that does not produce adverse effects as well as the intake which produces adverse effects. If more than one data set is found to be suitable and the UL that can be derived from each of them differs (e.g. for different disease endpoints), scientific advice will be provided for each of them separately. If there are no, or insufficient, data on which to base the establishment of a UL, an indication will be given on the highest level of intake of (total/added/free) sugars where there is reasonable confidence in data on the absence of adverse effects (Figure 1).
Risk characterisation includes a description of the scientific uncertainties associated with the UL estimates (or alternative estimates as the case may be; Figure 1) to indicate the degree of scientific confidence that can be placed in these estimates. Where data are available, it also includes an estimate of intake (from occurrence data and food consumption data; subquestions 1 and 2) for population groups as well as an indication of the margin between actual intakes and the UL, and an indication of circumstances, if any, in which risk is likely to arise.
7. Methods to answer subquestions 1 and 2
7.1. Levels of sugars in foods and beverages in Europe
7.1.1. Development of a food composition database for total sugars
Since only total sugars are subject to mandatory labelling in Europe and there are no analytical methods to distinguish between added/free sugars and other sugars present in foods, available data on total sugars will be used as starting point to estimate the levels of added and free sugars in foods and beverages. To that end, a food composition database for total sugars will be developed first.
Data on total sugars will be extracted from the EFSA's food composition database, which was compiled as a deliverable of the procurement project ‘Updated food composition database for nutrient intake’ (Roe et al., 2013). The aim of the project was to provide EFSA with an updated food composition database covering approximately 1,750 food entries in the EFSA FoodEx2 classification system1 with additional FoodEx2 facet descriptors, and to expand the data set to include harmonised information on the most common composite recipes of European countries and harmonised information on food supplements. In case no country‐specific data were available for certain food codes, data compilers borrowed compatible data from other countries and/or from similar foods.
The EFSA's food composition database contains raw data for energy, macro‐ and micronutrients from national food composition databases up to 2012 provided by fourteen national food database compiler organisations. Within the EFSA's food composition database, 12 countries provided data on total sugars covering about 1290 FoodEx2 codes. The number of values for total sugars available for a given FoodEx2 code is variable.
For the purpose of this Scientific Opinion, a single European food composition database for total sugars will be developed from the information available in the national food composition databases. To that end, an outlier analysis will be performed to identify any value which deviates from the others for a given food code, which could be either wrongly attributed values (data cleaning) or values describing real variability in total sugars content. For food codes for which no outliers can be identified, the mean will be taken as a unique value.
The outlier assessment will prioritise foods with a high content of total sugars and foods largely consumed by one or more population subgroups. The expected variability in the total sugars content will decrease as the FoodEx2 level increases. Such variability will inform decisions regarding the level of FoodEx2 at which values for total sugars need to be attributed, together with data on the frequency of consumption gathered from the EFSA food consumption database. In this context, the Mintel Global New Products Database (GNPD),2 an online database which monitors product introductions in consumer markets of packaged goods worldwide, will be used to check if the variability detected in the EFSA's food composition database regarding the total sugars content of manufactured foods reflects real variability of products in the market, and also to decide whether more than one scenario needs to be considered for risk characterisation regarding one or more food groups, and at which FoodEx2 level (i.e. whether two extreme values (lowest and highest), rather than a mean value, will need to be assigned to a food code to evaluate the impact of this variability on total sugars intake) (Figure 2).
Figure 2.
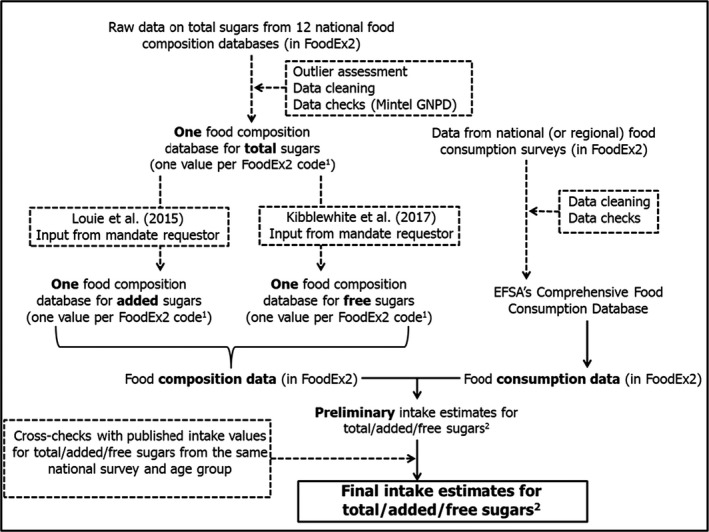
Steps that will be followed to estimate intakes of total, added and free sugars
- Legend to Figure 2: 1) Two values for a given FoodEx2 code may be assigned when both the observed variability in sugars content and the frequency of consumption are high, so that different intake scenarios could be considered; 2) In grams per day and as E% per country and population group.
A similar process has been undertaken to build the EFSA's food composition database for a number of vitamins and minerals in order to support EFSA's scientific opinions on DRVs for nutrients.
7.1.2. Development of food composition databases for added and free sugars
A European food composition database for added sugars in foods and beverages will be developed taking into account the 10‐step methodology described by Louie et al. (2015) for added sugars and adapted by FSANZ3 to determine the amount of added sugars in foods in the AUSNUT 2010–2013 food nutrient database. Although the definition of added sugars (a component of free sugars) in the Australian code includes maltodextrin and similar products, a decision was made by FSANZ not to capture these ingredients in the data set of added sugars (and therefore neither in the data set of free sugars) to maintain consistency with the definition of sugars used in nutrition labelling and with international food composition database practice where total sugars have been defined as being only mono‐ and di‐saccharides. The same approach will be followed by EFSA for the same reasons.
The food composition database for added sugars will be developed for all FoodEx2 codes for which a consumption has been reported in the EFSA Comprehensive Food Consumption Database (see Section 7.2.1) in combination with the relevant FoodEx2 facet descriptors included in the EFSA FoodEx2 classification system (e.g. sugar free facet), using as starting point the food composition database for total sugars (Section 7.1.1). The step‐wise methodology described by Kibblewhite et al. (2017) will be adapted to develop the food composition database for free sugars by applying the definition given in Section 5.1 of this protocol as strictly as possible. Databases for added and free sugars will be developed in close consultation with the mandate requestor (Figure 2). All the steps of the process will be thoroughly documented.
7.2. Estimates of intake of total, added and free sugars from all dietary sources
Estimates of intake of total, added and free sugars from all dietary sources will be obtained using data from the EFSA Comprehensive Food Consumption Database in combination with the food composition databases for total, added and free sugars (Sections 7.1.1 and 7.1.2).
7.2.1. The EFSA Comprehensive Food Consumption Database
Food consumption data from the EFSA Comprehensive Food Consumption Database (hereinafter referred as Comprehensive Database) will be used in order to assess the intake of free sugars. The Comprehensive Database provides a compilation of existing national information on food consumption at individual level. It was first established in 2010 (EFSA, 2011; Huybrechts et al., 2011; Merten et al., 2011). The latest version of the Comprehensive Database, updated on 26 April 2018, contains results from 61 different dietary surveys carried out in 25 different Member States.
Within the dietary surveys, subjects are classified in different age groups as follows:
Infants: 1–11 months old
Toddlers: ≥ 1 year to < 3 years old
Other children: ≥ 3 years to < 10 years old
Adolescents: ≥ 10 years to < 18 years old
Adults: ≥ 18 years to < 65 years old
Elderly: ≥ 65 years to < 75 years old
Very elderly: ≥ 75 years old
Data from infants 4 to 11 months old only will be considered in this assessment.
Overall, the Comprehensive Database is the most complete and detailed collection of food consumption data currently available in the EU. Consumption data were collected using single or repeated 24‐ or 48‐h dietary recalls or dietary records covering from three to 7 days per subject. Surveys with only one observation day per subject, or which used food frequency questionnaires (FFQ) for data collection, were excluded. Owing to the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading. Detailed information on the different dietary surveys included in the Comprehensive Database is shown on the EFSA website,4 including the number of subjects and days available for each age group. If new food consumption surveys become available during the assessment and up to December 2018, the most recent survey for a given country and age group will be used.
The linking between the foods consumed and the food composition databases for total, added and free sugars (Sections 7.1.1 and 7.1.2) will be done through the FoodEx2 system (EFSA, 2015b).
7.2.2. Sugars intake calculation
The intake of total/added/free sugars (in grams per day) will be calculated at individual level by multiplying the average daily consumption for each food or food group with the corresponding concentration of total/added/free sugars, summing up the respective intakes throughout the diet. The intake of total/added/free sugars will also be expressed as percentage of total energy intake (%E) and as percentage of non‐alcohol energy intake (non‐alcohol E%). In line with the Guidance of EFSA for the Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment (EFSA, 2011), chronic total/added/free sugars intake calculations will be performed only for subjects with at least two reporting days. In this context, chronic total/added/free sugars intake refers to the arithmetic mean of all reporting days available for the same subject. The intake will be modelled using the SAS software (SAS Enterprise Guide 5.1, 2013). The mean as well as the 5th, 50th and 95th percentiles of intake will be derived for each survey and age group (and sex group, if appropriate), respectively. Data on the contribution of different food groups to (total/added/free) sugars intake by survey and age group will also be presented.
Different intake scenarios could be considered in the intake calculation process, especially if more than one value for total/added/free sugars is assigned to one or more FoodEx2 codes in the food composition database (Figure 2).
To evaluate the accuracy of the results obtained, these will be compared with published intake values for total/added/free sugars from the same survey data set and age group, whenever available. These data will be retrieved through a questionnaire to the National Competent Authorities of European countries (Appendix C).
8. Method to answer subquestion 3
In order to address the digestion, absorption and metabolism of different types of free sugars from different food matrices in humans, background information will be gathered by the WG experts and EFSA staff through a narrative review. Recent textbooks, authoritative reviews and research papers retrieved through searches in bibliographic databases, and selected on the basis of their relevance, will be used as sources of information.
9. Methods to answer subquestions 4 and 5
Subquestions 4 and 5 will be answered by performing systematic reviews and, possibly, dose–response meta‐analyses if the available data allow doing so. The conceptual framework for the systematic reviews on sugars intake in relation to disease and other endpoints is shown in Figure 3.
Figure 3.
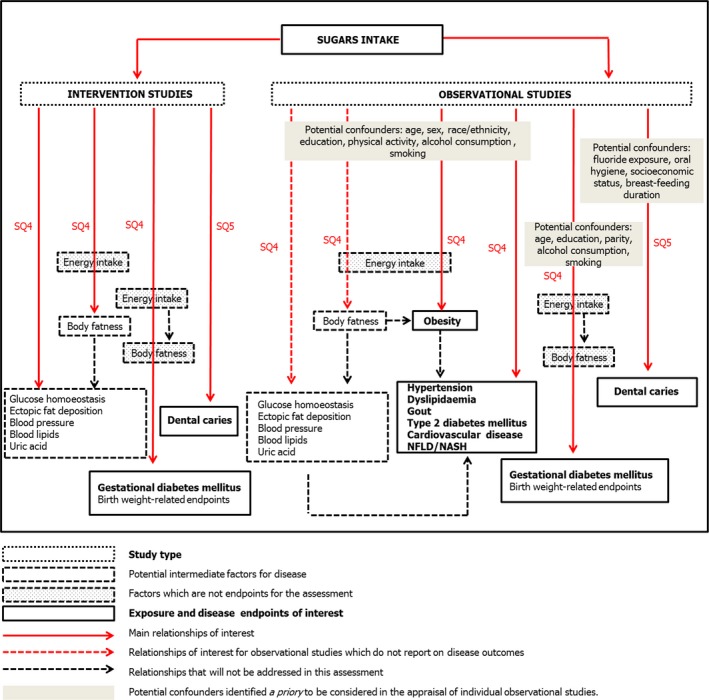
Conceptual framework for the systematic reviews on sugars intake in relation to disease endpoints and other endpoints
9.1. Eligibility criteria for study selection
The selection of human studies relevant to subquestions 4 and 5 will be performed using the eligibility criteria described in Table 2.
Table 2.
Eligibility criteria for human studies to address subquestions 4 and 5
| INTERVENTION STUDIES | ||
| Study design | In |
Randomised controlled trials Non‐randomised, comparative studies of interventionsa |
| Out | Single‐arm intervention studies with no control group | |
| Study duration | In |
Depending on the endpoints addressed, as follows: Measures of body fatness ≥ 6 weeks Ectopic fat deposition ≥ 2 weeks Measures of glucose homoeostasis: ≥ 1 week to ≥ 12 weeksb Blood pressure, blood lipids and uric acid ≥ 4 weeks Dental caries: ≥ 1 year for primary dentition and ≥ 18 months for permanent dentition Pregnancy endpoints: any duration |
| Out | Studies of shorter duration | |
| Study location | In | Any location |
| Population | In |
Adults (≥ 18 years) and children (4 months to < 18 years) from the general population, including overweight or obese subjects, subjects at risk of disease (e.g. with impaired glucose tolerance, impaired fasting glucose, NAFLD), and subjects with one or more features of the metabolic syndrome which are not on pharmacological treatment during the intervention |
| Out |
Studies targeting individuals with a disease (except for obesity), either untreated or under pharmacological/surgical treatment for the disease, or individuals on a therapeutic diet, including weight‐loss diets Studies in individuals under physical training programs (e.g. athletes, military), except for dental caries |
|
| Intervention/control | In |
Studies aiming at:
|
| Out | Studies aiming at:
|
|
| Endpoints of interest | In |
Body fatness and ectopic fat deposition
Dental caries
|
| Out |
|
|
| Language | In | Full‐text document in English |
| Out | Articles with the full text in another language | |
| Publication year | In | Up to March 2018 |
| Publication type | In |
Primary research studies (i.e. studies generating new data) reported in full‐text articles Primary research studies reported in letters to editors if the information provided is sufficient to allow a scientific evaluation of the results Systematic reviews and meta‐analysesc |
| Out |
Narrative reviews, expert opinions, editorials and letters to editors not reporting on primary data Meetings’ abstracts and posters Conference proceedings PhD theses Grey literature |
|
| OBSERVATIONAL STUDIES | ||
| Study design | In | Prospective, longitudinal, observational (prospective cohort and nested case‐control) studies |
| Out |
Retrospective case–control studies Cross‐sectional studies Ecological studies Case studies/case series |
|
| Study duration | In |
≥ 18 months for dental caries for permanent dentition ≥ 1 year follow‐up for all other disease endpoints, including dental caries for primary dentition Pregnancy endpoints: any duration |
| Out | Studies with a shorter follow‐up | |
| Study location | In | Any location |
| Population | In |
Adults (≥ 18 years) and children (4 months to < 18 years) from the general population Studies targeting individuals at risk of disease (e.g. with impaired glucose tolerance, impaired fasting glucose, the metabolic syndrome, overweight, NAFLD) not on pharmacological treatment for the condition Studies in which prevalent cases of the disease endpoint of interest at baseline were excluded for data analysis |
| Out |
Studies targeting individuals with a disease (except for obesity), either untreated or under dietary or pharmacological/surgical treatment for the disease Studies in which prevalent cases of the disease endpoint of interest at baseline were not excluded for data analysis |
|
| Exposure | In |
Method to quantify sugars intake (one or more of the following):
|
| Out |
– Studies not providing sufficient information to allow quantitative estimates of sugars intake, whether total or from one or more dietary sources (e.g. studies reporting only on the frequency of consumption of one or more dietary sources of sugars with unknown sugar content) Eating conditions: under dietary controlled conditions prior to the dietary intake assessment Method to assess intake of sugars: – Any other method |
|
| Endpoints of interest | In |
Disease endpoints:
|
| Out |
|
|
| Language | In | Full‐text document in English |
| Out | Articles with the full text in another language | |
| Publication year | In | Up to March 2018 |
| Publication type | In |
Primary research studies (i.e. studies generating new data) reported in full‐text articles Primary research studies reported in letters to editors if the information provided is sufficient to allow a scientific evaluation of the results Systematic reviews and meta‐analysesc |
| Out |
Narrative reviews, expert opinions, editorials and letters to editors not reporting on primary data Meetings’ abstracts and posters Conference proceedings PhD theses Grey literature |
|
NAFLD: non‐alcoholic fatty liver diseases; DXA: dual‐energy X‐ray absorptiometry; MRI: magnetic resonance imaging; CT: computed tomography; VAT: visceral adipose tissue; HOMA: homeostasis model assessment; QUICKI: Quantitative insulin sensitivity check index; SBP: systolic blood pressure; DBP: diastolic blood pressure; LDL‐c: low‐density lipoprotein cholesterol; HDL‐c: high‐density lipoprotein cholesterol; VLDL‐c: very low‐density lipoprotein cholesterol; GDM: gestational diabetes mellitus; BMI: body mass index; BIA: Bioelectrical impedance analysis; FFQ: Food frequency questionnaire; NASH: non‐alcoholic steatohepatitis.
Prospective studies that compare the effects of two or more interventions which did not use randomisation to allocate individuals or clusters to the comparison groups.
For dynamic indices of insulin sensitivity and/or beta‐cell function ≥ 1 week; for static indices and fructosamine ≥ 4 weeks; for HbA1c ≥ 12 weeks.
Systematic reviews, including meta‐analyses, on this topic that will be identified during the process of literature screening will be collected for the purpose of reviewing the reference list but will not be considered to contribute to the final number of studies considered eligible unless they also contain original data.
For subquestion 4, the minimum study duration for the inclusion of intervention studies has been selected by considering the time generally required for the stabilisation of the endpoints assessed following a nutritional intervention. The minimum study duration for the inclusion of observational studies for subquestion 4, and for the inclusion of intervention and observational studies for subquestion 5, is based on the minimum time estimated to be needed for the disease to develop in individuals free of the disease at baseline (expert judgement).
Regarding the study location, no limits are applied. It is acknowledged, however, that the background diet may affect the relationship between the intake of sugars and the endpoints being addressed, and that major differences in the background diet may limit the extrapolation of the results obtained outside Europe to the European population. This aspect will be considered when synthesising the evidence (Section 9.6).
9.2. Literature searches for studies meeting the eligibility criteria
For subquestions 4 and 5, an extensive literature search will be performed in bibliographic databases. Sources of grey literature and databases of theses/dissertations will not be searched.
The bibliographic databases listed in Table 3 will be searched in order to identify relevant studies.
Table 3.
Bibliographic databases to be searched for relevant studies
| Database | Platform | Types of studies |
|---|---|---|
| Cochrane Library. Cochrane Central Register of Controlled Trials (CENTRAL) | Wiley | Intervention studies |
| Cochrane Library. Cochrane Database of Systematic Reviews (CDSR) | Wiley | Systematic reviews |
| Cochrane Library. Database of Abstracts of Reviews of Effects | Wiley | Systematic reviews |
| Embase | Elsevier | Systematic reviews, intervention studies, observational studies |
| PubMed | NLM | Systematic reviews, intervention studies, observational studies |
| Scopus | Elsevier | Systematic reviews, intervention studies, observational studies |
For subquestion 4, literature searches will be performed by type of endpoint. Previous systematic reviews with similar review questions, similar or broader inclusion criteria and appropriate search strategies were identified during the scoping searches (see Appendix B). Therefore, date limits will be applied to the searches for subquestion 4 (by endpoint) and subquestion 5 using these systematic reviews as starting point whenever possible (Table 4). Studies published before these dates will be retrieved by hand‐searching the reference lists of the systematic reviews (Appendix B) and from existing reports by other authorities/bodies (Appendix A). No retrospective date limits will be applied for endpoints for which no existing systematic review can be taken as starting point.
Table 4.
Date limits applied to the searches and systematic reviews used as sources of relevant studies
| Subquestion | Endpoints | Date limit | Systematic review |
|---|---|---|---|
| 4 | Adipose tissue | Intervention and observational studies: December 2011 | Te Morenga et al. (2013) |
| 4 | Blood pressure | Interventions: August 2013 | Te Morenga et al. (2014) |
| Observational studies: no date limit | |||
| 4 | Blood lipids | Interventions: August 2013 | Te Morenga et al. (2014) |
| Observational studies: no date limit | |||
| 4 | All other endpoints | Intervention and observational studies: no retrospective date limit | |
| 5 | Dental caries | Intervention and observational studies: November 2011 | Moynihan and Kelly (2014) |
Date limits might be changed should new systematic reviews on the topic be identified which are considered to adequately cover the relevant literature. Existing systematic reviews (Appendix B) with narrower inclusion criteria regarding either the exposure (e.g. limited to SSBs) or the study duration (e.g. (Sonestedt et al., 2012; SACN, 2015)) will be hand searched.
The search terms that will be used for the exposure and the various endpoints of interest are depicted in Appendix D. The specific search strategies for each database will be developed at a later stage. The performance of the search strings will be tested against the reference lists of the systematic reviews shown in Appendix B.
The output from the searched databases, including all indexed fields per hit (e.g. title, authors, abstract), will be exported into separate Endnote® files, allowing a count of the individual hits per database. All the studies included in the above‐mentioned systematic reviews will be added to specific Endnote® libraries. Files will then be combined and duplicate records will be removed.
The files obtained will be transferred into DistillerSR® Web‐Based Systematic Review Software (Evidence Partners, Ottawa, Canada) for the selection procedure (see Section 9.3).
9.3. Study selection process
The whole selection process will be performed with DistillerSR®. Studies to be included in the review will be selected using a two‐step selection procedure:
-
1
Screening of title and abstract to identify potentially relevant studies that will be included for full‐text screening, applying the selection criteria described in Section 9.1. If the information contained in the title or abstract is not relevant to the research objectives, the article will not be selected for full‐text assessment. During the screening process, studies will be categorised into two groups corresponding to the two subquestions that are the objectives of these systematic reviews.
This step will be conducted by WG experts and/or EFSA staff in duplicate, and by using machine learning techniques where/when appropriate. The results of the use of machine learning will be evaluated by a reviewer and documented. If the title and/or abstract do not mention the endpoints of interest for the assessment, but the words ‘adverse effects’ or ‘side effects’ are mentioned, the paper will be included to check if these effects have any relation to the endpoints of interest. If there are doubts or divergences which cannot be resolved between the two reviewers, the full article will be screened.
-
2
Screening of full text to assess if the article is relevant to the risk assessment.
This step will be conducted by WG experts and/or EFSA staff, in duplicate, for the references retrieved. Possible divergences will be discussed by the whole WG on sugars, in case these would highlight the need for amendments to the inclusion/exclusion criteria. The content of the full text will be checked against the inclusion and exclusion criteria established in the protocol.
Possible divergences or doubt for inclusion of domain‐specific articles will be discussed together with the relevant expert from the WG, also in case these would highlight the need for amendments to the inclusion/exclusion criteria.
Articles reporting solely on digestion, absorption or metabolism (i.e. without reporting on the endpoints of interest), or reporting only on endpoints other than those listed in Section 9.1, will not be included in this research subquestions but will be flagged for subquestions 3 and 6, where appropriate.
Screeners will be trained using written documentation on study eligibility. Eligibility criteria will be pilot tested on a subset of records, and refined if prone to misinterpretation. The results of the different phases of the study selection process will be reported in a flowchart as recommended in the PRISMA statement on preferred reporting items for systematic reviews and meta‐analyses (Moher et al., 2009).
9.4. Data extraction from included studies
Data will be extracted from the studies using predefined forms that comprise data on the characteristics of the studies (e.g. study design), their key‐elements (e.g. population, intervention/exposure, comparator, outcomes (endpoints), setting and duration), results, aspects related to the internal validity of the studies (e.g. confounders, randomisation) and funding source.
Studies for which the information provided in the publication(s) does not allow a full scientific evaluation by the WG experts (e.g. studies with missing or ambiguous information) will be excluded at this step. The reasons for exclusion will be clearly indicated.
The data will be extracted in the original units of measurement, which will be subsequently harmonised to allow data analysis. The authors may be contacted to retrieve additional data if needed.
Regarding the intervention/exposure, data on the food source(s) of sugars, on the type of sugars consumed (e.g. fructose, glucose, sucrose) and on the frequency of sugars consumption will be extracted from the included studies whenever available. For observational studies, data on disease endpoints (dichotomous variables, i.e. incidence of hypertension, incidence of type 2 diabetes) will be extracted first whenever available. Data on other endpoints (continuous variables, e.g. blood pressure, fasting glucose) will only be extracted if data on corresponding disease endpoints are not reported (Figure 3).
If a full‐text document reports on more than one study, the individual studies will be identified at this step to allow for data extraction at individual study level. If a single study is reported in more than one publication, different publications reporting on the same study (e.g. on different outcomes (endpoints), at different time points) will be identified at this step. Data will be extracted for the last observation available for each endpoint (corresponding to the longest intervention or follow‐up) for data analysis. Exceptions to this rule will be duly justified (e.g. on the basis of attrition and sample size, compliance with the intervention, other factors).
Clear instructions for extracting data will be developed. The data extraction forms will be created in DistillerSR® (Evidence Partners, Ottawa, Canada) and/or Excel, and pilot tested on a subset of studies. The piloting will also be used to identify sources of contextual (i.e. related to the key elements of the studies) heterogeneity. The forms and instructions will be refined if needed.
Data will be extracted from each individual study by one EFSA staff or one WG expert. In the piloting phase, extracted data will be validated by another EFSA staff or WG expert, in order to identify sources of possible errors. The data extraction will be then conducted by one EFSA staff/WG expert. Data quality checks will be performed for each study (Section 9.6).
9.5. Appraisal of the internal validity of the included studies
The risk of bias (RoB) of a given study in relation to a specific outcome (endpoint) refers to the risk of systematic errors in the design, recruitment, data collection or analysis that results in a mistaken estimation of the true effect of the exposure on the outcome.
The internal validity or RoB of each individual study included in the assessment will be appraised using a customised version of the OHAT/NTP RoB tool, which is suitable for both intervention and observational studies.5 This tool was developed based on guidance from the Agency for Healthcare Research and Quality (Viswanathan et al., 2012), the Cochrane risk‐of‐bias tool for non‐randomised studies of interventions (Sterne et al., 2014), the Cochrane Handbook (Higgins and Green, 2011), CLARITY Group at McMaster University (CLARITY, 2013) and other sources. The OHAT/NTP RoB tool was developed to provide a parallel approach to the evaluation of the RoB in the context of hazard identification for human risk assessment of chemicals, and to facilitate consideration of RoB across evidence streams (i.e. human, animal and mechanistic studies) with common terms and categories for RoB rating. For this assessment, the use of the tool will be limited to the aspects relevant to intervention and prospective observational studies in humans.
For each study, the appraisal will be done at outcome level, because for the same study the design and conduct may affect the RoB differently depending on the endpoints measured. Each study will be appraised by two independent experts from the WG (‘the reviewers’). Possible discrepancies will be discussed by the whole WG. If upon further discussion the WG cannot reach an agreement on a RoB rating for a particular domain, the more conservative judgment (the highest RoB) will be selected.
The OHAT/NTP RoB tool outlines 10 RoB questions, grouped in 6 bias domains (selection, confounding, performance, attrition/exclusion, detection and selective reporting) ‐ plus ‘other sources of bias’ ‐, which help identify the practices that may introduce bias (Table 5). Each RoB question addresses aspects relevant to specific study designs, i.e. 8 questions apply to intervention studies and 7 questions apply to prospective observational (cohort and nested case–control) studies (Table 5). Reviewers are required to answer RoB questions by applying a 4‐level rating scale (Figure 4).
Table 5.
Extracted from OHAT/NTP RoB tool (source: OHAT Handbook – 9 January 2015)b
| Bias Domains and Questions | Controlled interventiona | Observational |
|---|---|---|
| Selection Bias | ||
| 1. Was administered dose or exposure level adequately randomized? | X | |
| 2. Was allocation to study groups adequately concealed? | X | |
| 3. Did selection of study participants result in appropriate comparison groups? | X | |
| Confounding Bias | ||
| 4. Did the study design or analysis account for important confounding and modifying variables? | X | |
| Performance Bias | ||
| 5. Were the research personnel and human subjects blinded to the study group during the study? | X | |
| Attrition/Exclusion Bias | ||
| 6. Were outcome data complete without attrition or exclusion from analysis? | X | X |
| Detection Bias | ||
| 7. Can we be confident in the exposure characterization? | X | X |
| 8. Can we be confident in the outcome assessment? | X | X |
| Selective Reporting Bias | ||
| 9. Were all measured endpoints reported? | X | X |
| Other Sources of Bias | ||
| 10. Were there no other potential threats to internal validity (e.g., statistical methods were appropriate and researchers adhered to the study protocol)? | X | X |
Includes studies in humans with a controlled exposure including randomised controlled trials and non‐randomised intervention studies.
Figure 4.
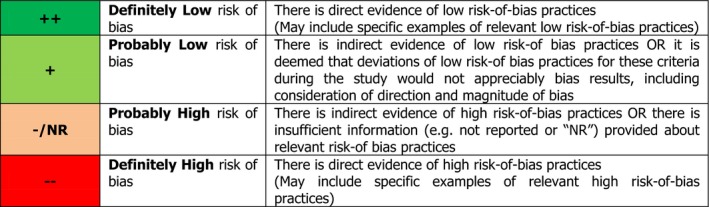
Answer format for the RoB questions (source: OHAT/NTP RoB tool)5
The RoB questions and rating instructions provided in the tool will be tailored to the specific subquestions illustrated in this protocol.
The OHAT/NTP RoB tool encourages judging the direction of bias, when possible. Empirical evidence about the direction of bias is discussed for each of the RoB questions. If there is no clear rationale for judging the likely direction of bias, reviewers are invited to simply outline the evidence and not to attempt a guess. This approach will be followed.
Once customised, the tool will be created in the review management software DistillerSR® to allow web‐based appraisal of the studies.
Specific elements identified a priori and that will be considered in the assessment of confounding and biases related to the exposure and outcome characterisation are discussed below.
9.5.1. Consideration of potential confounders
When assessing the RoB of studies concerned with causality (e.g. which investigate the effect of an exposure on disease), confounding should be addressed regardless of the study design. When present, confounding may lead to a biased estimate of the effect of exposure on disease, either closer to the null (resulting in underestimation of the exposure effect) or departing from the null, and can even reverse the apparent direction of the effect.
There are several requirements for a factor to actually act as a confounder, as described by McNamee (McNamee, 2003) and illustrated below. The factor must:
be a cause of the disease, or a surrogate measure of the cause, in unexposed people; factors satisfying this condition are called ‘risk factors’; and
be correlated, positively or negatively, with exposure in the study populations. If the study population is classified into exposed and unexposed groups, this means that the factor has a different distribution (prevalence) in the two groups; and
not be affected by the exposure. When a factor is an intermediate factor in the relationship between the exposure and the disease, it is affected by both the exposure and the disease, or shares a common cause with the disease which is in turn affected by the exposure, such factor cannot be considered a confounder.
Whereas criteria 1 and 3 can be judged based on current (expert) knowledge of external empirical evidence (e.g. prior research), criteria 2 can only be judged from internal evidence (i.e. evidence from the study under evaluation with respect to the RoB). Therefore, whether potential confounders identified a priori on the basis on current knowledge can actually confound the association between exposure and disease in a particular study by making the study groups not comparable in terms of disease risk is to be addressed on a case‐by‐case basis, in the context of each particular study.
The OHAT/NTP RoB tool does not include a separate question for confounding in human intervention studies because randomisation and allocation concealment should adequately address the issue of confounding. It recognises, however, that in some cases appropriate procedures for randomisation and allocation concealment may fail in accounting for confounding. For example, in the context of this assessment, confounding could be a concern if there are important differences among study groups in baseline characteristics which are risk factors for the outcome (endpoint) but not affected by the exposure (e.g. age, sex, PAL). In accordance with the OHAT/NTP guidance, for intervention studies where confounding is strongly suspected despite the fact that randomisation and allocation concealment are rated at ‘probably low’ or ‘definitely low risk of bias’, confounding will be addressed under ‘other potential threats to internal validity’ (OHAT/NTP, 2015).
In observational studies, potential confounding can be reduced by good design and appropriate statistical analysis (control for measured confounders), although it is acknowledged that the problem of confounding cannot be resolved fully in non‐randomised designs. The aim of controlling for confounders in observational studies is to allow comparability of the study groups for the risk of disease which is not affected by the exposure, so that any observed difference in risk among groups can be attributed to differences in the exposure of interest.
Being sugars an energy‐containing nutrient, an increase in energy intake and/or an increase in body fatness could be considered intermediate factors in the causal pathway between high sugars intake and adverse effects related to glucose homoeostasis, ectopic fat deposition, blood pressure, blood lipids and uric acid, which in turn could mediate the effect of high sugars intake on the incidence of hypertension, dyslipidaemia, gout, T2DM mellitus, CVD and NFLD/NASH (Figure 3). An increase in energy intake and/or an increase in body fatness could also be considered intermediate factors in the causal pathway between high sugars intake and adverse pregnancy outcomes (endpoints). All these intermediate factors could potentially be affected by the exposure and therefore do not meet the criteria to be considered as confounders (McNamee, 2003), but rather as a potential source of over‐adjustment bias. The extent to which an effect of sugars intake on the endpoints of interest (except obesity and dental caries) may be mediated by changes in energy intake and/or body fatness will be explored in the synthesis of the evidence (Section 9.6). In addition, there are dietary factors known to be risk factors for one or more of the above‐mentioned diseases, such the intake of sodium, potassium, saturated and trans fatty acids, or dietary fibre. However, these may or may not be associated with both the exposure and the endpoint within a given study, and therefore, they will be not considered as potential confounders a priori, but rather in the context of individual studies.
Based on recent publications, the Panel identified a priori an indicative list of potential factors that could confound the relationship between the intake of sugars and measures of body fatness, ectopic fat deposition, glucose homoeostasis, blood lipids, blood pressure and uric acid, and the relationship between the intake of sugars and incidence of overweight/obesity, hypertension, dyslipidaemia, gout, T2DM, cardiovascular disease and liver disease: age, sex, race/ethnicity, education (or education of the parents for studies in children), smoking habits, physical activity, alcohol consumption.
The Panel also identified a priori an indicative list of potential factors that could confound the relationship between the intake of sugars and dental caries: fluoride exposure (e.g. water fluoride, use of fluoride toothpaste, supplements), oral hygiene practices, socioeconomic status and breast feeding duration for studies on young children. Frequency of sugars consumption has been highly correlated to the amount of sugars consumed in observational studies and it is likely to be affected by the exposure, so that it will not be considered as a potential confounder in observational studies in relation to dental caries.
As for pregnancy outcomes, the Panel identified a priori the following factors that could potentially confound the relationship between the intake of sugars and birthweight‐related endpoints or the incidence of GDM: parity, maternal age, education, alcohol consumption and smoking.
When assessing RoB in observational studies, the reviewers will consider, for each study, whether these factors can confound the association on a case‐by‐case basis. Additional confounders may be identified by the reviewers. The reviewers will consider whether the confounding variables were measured reliably and consistently within each study and whether the design and/or the data analysis adequately accounted for potential confounding (e.g. multivariable analysis, stratification).
9.5.2. Confidence in the exposure characterisation
The exposure of interest for the assessment is the daily intake of total/added/free sugars from all dietary sources. It is acknowledged however, that few of the available individual studies investigating the health effects of dietary sugars may have used the definition of added or free sugars as described in this protocol (Section 5.1) to characterise the intervention/exposure. In this context, the confidence in the exposure characterisation that will be assessed in relation to the RoB of individual studies refers to the confidence on the quantitative estimates for the sugar fraction that is being investigated in the study, and not the extent to which the exposure investigated on each study reflects the intake of total/added/free sugars from all dietary sources as defined in this protocol. The latter aspect will be discussed when integrating and weighing the evidence in light of the identified uncertainties to provide scientific advice on total/added/free sugars (see Figure 1 and Section 12).
In assessing RoB, reviewers will consider the risk of errors in the estimate of sugar intake for individuals and related risks of misclassification of individuals according to their exposure. The accuracy of sugar intake estimates may be affected by (i) the method (or combination of methods) used to assess the sugar fraction of interest for the study (e.g. 24‐h urinary excretion of fructose and sucrose vs dietary records vs diet recalls vs FFQs; specificity of FFQs for the exposure of interest; validation; number of days recorded); (ii) the accuracy of 24‐h urine collections and the accuracy of reporting dietary intakes (e.g. self vs dietitian assisted compilation of FFQs); (iii) systematic changes in habitual diet prior to the intake assessment. The reviewers will consider the resulting risk of misclassification in appraising the studies.
9.5.3. Confidence in the outcome assessment
Confidence in the outcome (endpoint) requires valid, reliable and sensitive methods to assess the outcome applied consistently across groups (OHAT/NTP, 2015). Outcome misclassification or measurement error may be unrelated to the exposure (non‐differential) or related to the exposure (differential).
Factors that will be considered by the reviewers while assessing bias in relation to the outcome assessment include: (1) the objectivity of the outcome assessment, (2) the consistency in measurement of endpoints, and (3) the blinding of the outcome assessors (for knowledge of the exposure) (OHAT/NTP, 2015).
9.5.4. Summarising the internal validity of each individual study
Each study will be reported using a tabular summary form which will include the key elements of the study and a summary of the results of the critical appraisal.
When all the studies have been summarised in this way, the WG will consider whether and how to combine the scores from the RoB questions at the level of individual studies. The WG may consider using an algorithm to combine the scores in a weighted or unweighted manner: if so, the rationale for the chosen algorithm will be documented. Alternatively, the RoB scores may be kept separate for each RoB question and taken into account in the synthesis of evidence. The results of the RoB assessment will be taken into account in the weight of evidence assessment and uncertainty analysis (Sections 11 and 12).
9.6. Synthesis of the evidence
Data from included individual studies will be considered separately for each of study design and for each endpoint to derive single lines of evidence (Figure 3).
Information on inclusion criteria, RoB assessment and endpoints as extracted from the individual studies will be summarised in evidence tables. Data quality checks will be performed for each study. For each variable, the proportion of missing observations will be assessed; range checks will be carried out for all included variables to ensure that all values are reasonable; categorical variables will be tabulated and key variables will be cross‐tabulated to check for internal consistency. For intervention studies, results from intention‐to‐treat analyses will be preferred over per‐protocol analyses if both are reported. In the case of missing data, flexible and transparent strategies will be pursued, such as requesting missing data from the authors, re‐doing the analysis or placing the original results in adequate context according to the feasibility and adequacy of these approaches on a per‐study basis. Effect estimates such as relative risks and odds ratios for dichotomous variables for disease endpoints, and differences in means for continuous variables for other endpoints along with measures of their statistical precision (usually 95% confidence intervals) will be extracted from the studies and reported in the assessment.
Whenever data allow for a meaningful quantitative synthesis of the evidence, effect estimates from intervention studies (for all endpoints; by study design) and observational studies (for disease endpoints) will be pooled separately and assessed through meta‐analysis and dose–response meta‐analysis, using fixed‐ and random‐effects models as appropriate (Orsini et al., 2012; Crippa and Orsini, 2016; Discacciati et al., 2017). Dose–response meta‐analysis is a statistical technique that aims to characterise the smooth and gradual change in non‐linear responses along the range of a quantitative exposure using aggregated data from several studies. Within intervention studies, effect estimates will be pooled separately by study design (e.g. isocaloric exchange of sugars with other macronutrients, changes in sugars intake under free‐living conditions). For observational studies, the suitability of unadjusted and adjusted models will be considered case‐by‐case for data analysis depending on the level of control for potential confounders and the risk of over‐adjustment bias.
In addition to the meta‐analyses stratified by study design, subgroup/stratified analyses will be performed according to age, gender, changes in body fatness (except for obesity and dental caries), type of dentition (for dental caries), type of sugar/food source/frequency of consumption, country or continent, and other factors suspected or known to modify the association between sugars intake and the endpoints assessed. Subgroup and sensitivity analyses will also be performed according to the RoB of the included studies, the degree of control for confounding, the methodology and quality of the exposure assessment, duration of follow‐up for both the intervention and the observational studies, and possibly by the source of study funding. If needed, sensitivity analyses will be performed to evaluate the robustness of the findings and the possible influence of different biases on the summary pooled effect estimates (Arah et al., 2008; Rothman et al., 2012; Corbin et al., 2017). Influence analysis will be carried out by examining whether removal of single studies influences the results of the meta‐analyses, and the reasons underlying such an influence on the effect estimates, if any (Rothman et al., 2012).
Statistical heterogeneity across study‐specific findings will be taken into account in the statistical model and evaluated by visual inspection of forest plots and the I2 statistic (Higgins and Thompson, 2002; Higgins et al., 2003), and an attempt will be made to identify its sources.
The possibility of publication bias will be investigated using one or more of the following approaches: (a) visual inspection of funnel plots to investigate the association between study size and effect size (Altman, 1986); (b) Egger's regression test (Egger et al., 1997; Sterne and Egger, 2005); and (c) trim‐and‐fill analysis (Duval and Tweedie, 2004; Rothstein et al., 2005) following the approach of Peters Jaime et al. (2007).
If there are studies that are relevant but cannot be included in meta‐analyses (e.g. due to differences in study design), their contribution to the assessment will be integrated with the results of the meta‐analysis by a weight of evidence approach (Section 11). If none of the relevant studies for an effect are suitable for meta‐analysis, evidence synthesis for that effect will be performed by a weight of evidence approach (Section 11).
9.7. Plans for updating the literature searches and dealing with newly available evidence
The literature searches performed as detailed above (Section 9.2) will be repeated approximately 3 months before the planned date of endorsement of the draft opinion by the Panel. Databases and keywords will be those of the original searches. Date limits will be defined based on the cut‐off date of the preceding searches. The papers retrieved by these additional searches will be screened for relevance applying the same criteria.
Relevant studies will be reviewed by the WG experts and their contribution to the assessment will be integrated (with the results of a meta‐analysis or otherwise) by a weight of evidence approach (Section 11), but will not be considered for inclusion in any meta‐analysis.
10. Methods to answer subquestion 6
In order to address the mode(s) of action for possible adverse health effects of (total/added/free) sugars identified in subquestions 4 and 5, background information will be gathered by the WG experts and EFSA staff through a narrative review. Recent textbooks, authoritative reviews and research papers retrieved through searches in bibliographic databases, and selected on the basis of their relevance, will be used as sources of information. Articles reporting solely on digestion, absorption or metabolism that are identified/retrieved during the screening of the full text in the context of the systematic reviews described in Section 9 will also be considered, where appropriate. The mode of action by which sugars can contribute to the development of dental caries (subquestion 5), however, is considered to be well known.
11. Methods for integrating and weighing the evidence to set a UL for sugars
Integration of evidence will be performed at a number of levels and by different methods, according to what is appropriate given the available evidence (Figure 5). For subquestions 4 and 5, the following integration steps may be needed:
Where appropriate, different studies (by study design) for the same endpoint will be combined by meta‐analysis.
Results from meta‐analysis will be integrated with evidence from other relevant studies on the same endpoint (if there are any that could not be included in the meta‐analysis) by a weight of evidence approach.
For endpoints where meta‐analysis is not feasible, relevant studies will be integrated by a weight of evidence approach.
Where appropriate, results for different endpoints of the same type (e.g. disease and other endpoints relating to the same chronic disease) may be integrated by a weight of evidence approach.
Figure 5.
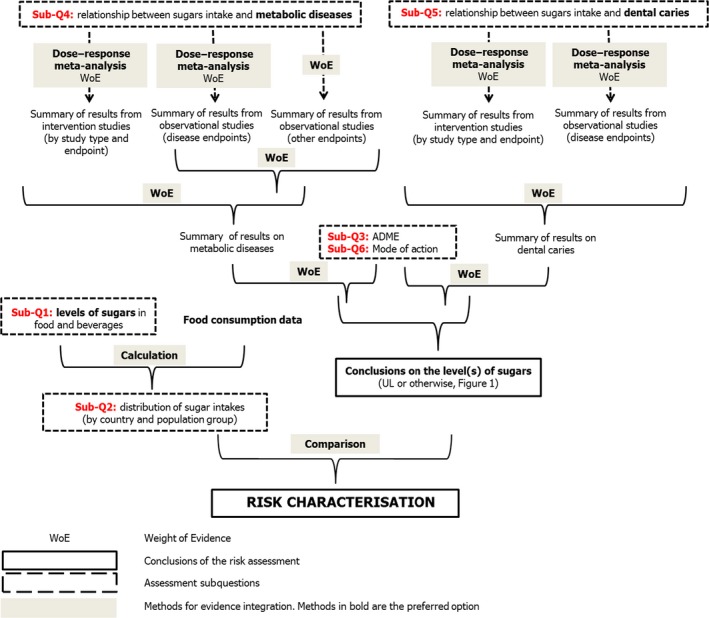
Conceptual framework for evidence integration
The outcome of subquestions 4 and 5 will be integrated with the endpoints of subquestions 3 and 6 by a weight of evidence approach. The results of this will form the Panel's conclusions on the levels of intake for total/added/free sugars (UL or otherwise; Figure 1): this may result in more than one level of intake, depending on whether there is material variation between sugar fraction, effects and/or population groups.
The results of subquestion 1 (levels of total/added/free sugars in foods and beverages) will be integrated by calculation with food consumption data to address subquestion 2, resulting in intake assessments for the European population.
Risk characterisation will be performed by comparing results of the intake assessment (from subquestions 1 and 2) with the levels of intake (UL or otherwise; Figure 1) for total/added/free sugars (from subquestions 3, 4, 5 and 6) (Figure 5).
In several of the steps described above, integration will be performed by a weight of evidence approach using expert judgement. The methods will vary, depending on the evidence to be integrated and the specific considerations involved. In each case, the principles of EFSA's guidance on weight of evidence will be applied (EFSA Scientific Committee, 2017). Evidence will be organised into lines of evidence, where helpful. Relevance, reliability (including the RoB evaluations described in earlier sections) and consistency will be taken into account when weighing the evidence. Formal (EFSA, 2014) or semi‐formal (EFSA Scientific Committee, 2018) methods for expert knowledge elicitation (EKE) will be used where appropriate. Detailed protocols will be established for each stage of the weight of evidence process before it is performed. Each stage of the process will be documented, including the reasons for any deviations from the protocol.
12. Evaluating the uncertainty in the body of evidence
Uncertainties in the estimates of total/added/free sugar intake in European countries may arise from inaccuracies in mapping food consumption data according to the FoodEx2 classification, from analytical errors or from errors in estimating the levels of total sugars in the national food composition tables, from errors in attributing levels of added/free sugars to foods from their content of total sugars, and from replacing missing values by values of similar food groups in the added/free sugars intake estimation process. These uncertainties may, in principle, result in both too high and too low estimates of total/added/free sugars intake.
For disease and other endpoints, once the individual studies are appraised for internal validity and after synthesising the evidence for each endpoint, line of evidence (i.e. intervention studies separately from observational studies; intervention studies by design) and subquestion, the uncertainties in the body of evidence will be identified, including factors such as the consistency of results, the precision of effect/association estimates and/or dose–response models, the internal and external validity (directness, generalisability, applicability) of the included studies, and gaps in knowledge.
Uncertainty analysis will be performed following approaches recommended by EFSA (EFSA Scientific Committee et al., 2018) for case‐specific assessments. Uncertainty affecting each subquestion will be identified, and taken into account when evaluating the overall uncertainty for the main endpoints of the assessment: UL for the intake of total/added/free sugars (or otherwise; Figure 1) and risk characterisation. The overall uncertainty will be evaluated by expert judgement using either formal or semi‐formal EKE methods (EFSA, 2014; EFSA Scientific Committee, 2018). For the UL of total/added/free sugars (or otherwise; Figure 1), the weight of evidence (Section 11) and uncertainty analysis may be addressed together in a single EKE procedure. Detailed protocols cannot be specified in advance, but will be established for each stage of the uncertainty analysis before it is performed. Each stage of the process will be documented, including the reasons for any deviations from the protocol.
Abbreviations
- AHA
American Heart Association
- AI
adequate intake
- ANSES
French Agency for Food, Environmental and Occupational Health & Safety
- AUSNUT
Australian food and nutrient database
- BIA
bioelectrical impedance analysis
- BMI
body mass index
- BP
blood pressure
- CHD
coronary heart disease
- CIGMA
Continuous infusion of glucose with model assessment
- CT
computed tomography
- CVD
cardiovascular disease
- DRV
dietary reference value
- DBP
diastolic blood pressure
- DXA
dual‐energy X‐ray absorptiometry
- EKE
expert knowledge elicitation
- ESPGHAN
European Society for Paediatric Gastroenterology, Hepatology and Nutrition
- FBDG
food‐based dietary guidelines
- FFQ
food frequency questionnaire
- FoodEx2
Standardised food classification and description system developed by EFSA
- FSANZ
Food Standards Australia New Zealand
- FSIGT
Frequently sampled intravenous glucose tolerance tests
- GNPD
Global New Products Database
- GNS
German Nutrition Society
- HDL‐c
high‐density lipoprotein cholesterol
- HFCS
high‐fructose corn syrups
- HOMA
homeostasis model assessment
- IoM
Institute of Medicine
- LDL‐c
low‐density lipoprotein cholesterol
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- NAFLD
non‐alcoholic fatty liver diseases
- NAA
neutron activation analysis
- NASH
non‐alcoholic steatohepatitis
- NDA
EFSA Panel on Nutrition, Dietetic Products and Allergies
- NME
non‐milk extrinsic
- NTP
National toxicology program
- OGTT
oral glucose tolerance test
- OHAT
Office of health assessment and translation
- PAL
physical activity level
- PC
prospective cohort studies
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- PROMETHEUS
PROmoting METHods for Evidence Use in Scientific assessments
- QUICKI
Quantitative insulin sensitivity check index
- RCT
randomised controlled trial
- RI
Reference Intake range
- RoB
risk of bias
- SBP
systolic blood pressure
- SACN
UK Scientific Advisory Committee on Nutrition
- SSB
sugar‐sweetened beverage
- SSD
sugar‐sweetened soft drinks
- T2DM
type 2 diabetes mellitus
- TG
triglycerides
- Total‐c
total cholesterol
- UL
Tolerable upper level of intake
- VAT
visceral adipose tissue
- VLDL‐c
very low‐density lipoprotein cholesterol
- WG
Working Group
- WHO
World Health Organization
Appendix A – Overview of dietary reference values and recommendations
1.
In 2010, in the context of setting dietary reference values for carbohydrates and dietary fibre, the European Food safety Authority (EFSA NDA Panel, 2010a) concluded that the available data did not allow the setting of a UL for total or added sugars, neither an Adequate Intake (AI) nor a Reference Intake range (RI). However, evidence on the relationship between patterns of consumption of sugar‐containing foods and dental caries, weight gain and micronutrient intake should be considered when establishing nutrient goals for populations and recommendations for individuals and when developing food‐based dietary guidelines (FBDG).
The evidence‐based Guideline of the German Nutrition Society (GNS) on carbohydrate intake and prevention of nutrition‐related diseases (Hauner et al., 2012) recommended reducing the consumption of SSBs, but did not provide a quantitative limit for sugar intake or any components of this. The basis for this recommendation was probable evidence that high consumption of SSBs increases the risk of obesity and type 2 diabetes in adults, and the high consumption of SSBs particularly among adolescents and young adults in Germany.
The Nordic Nutrition Recommendations (Nordic Council of Ministers, 2014) limited the intake of added sugars (sucrose, fructose and starch hydrolysates) to < 10% of the total energy intake for the general population to ensure adequate intakes of micronutrients and dietary fibre (micronutrient density of the diet), which was found particularly important for children and persons with a low energy intake. It was also recommended to limit the consumption of SSBs because associated with an increased risk of type 2 diabetes and excess weight gain, and to avoid frequent consumption of sugar‐containing foods to reduce the risk of dental caries.
The UK Scientific Advisory Committee on Nutrition (SACN, 2015) recommended that the average population intake of free sugars (all monosaccharides and disaccharides added to foods by the manufacturer, cook or consumer, plus sugars naturally present in honey, syrups and unsweetened fruit juices) should not exceed 5% of total energy intake for age groups from 2 years upwards. Evidence from intervention studies showing that increasing sugars intake increases energy intake in individuals consuming an ad libitum diet and that SSBs are linked to weight gain in children and adolescents, and evidence from prospective cohort studies showing that the consumption of sugars is associated with increased risk of dental caries and intake of SSBs are associated with an increased risk of type 2 diabetes mellitus were at the basis of this recommendation.
The French Agency for Food, Environmental and Occupational Health & Safety (ANSES) issued an opinion on the establishment of recommendations on sugar intake (ANSES, 2016). The opinion focused on the metabolic effects of sugars in food, whether naturally present or added, and their involvement in the development of chronic diseases (metabolic diseases, cancer and cardiovascular diseases). ANSES set an upper intake limit for total sugars of 100 g/day for the adult healthy population, which excludes lactose and galactose naturally present in milk and dairy products. The upper intake limit was calculated from the minimum daily consumption of fructose (50 g) for which a significant increase in blood concentrations of triglycerides was observed in intervention studies, and considering that an intake of 50 g of fructose corresponds to an intake of 100 g of sucrose.
The Institute of Medicine of the US National Academy of Sciences (IoM, 2005) concluded that there was insufficient evidence to set a UL for added sugars (sugars and syrups that are added to foods during processing or preparation). However, a maximal intake level of ≤ 25% of total energy intake was suggested to prevent the displacement of foods that are major sources of essential micronutrients (micronutrient density of the diet).
The 2015–2020 Dietary Guidelines for Americans (HHS/USDA, 2015) recommended that individuals aged 2 years and older should derive < 10% of total energy intake from added sugars in order to achieve healthy eating patterns within calorie limits (micronutrient density of the diet). This recommendation was based on food pattern modelling and national data on intakes of calories from added sugars.
The World Health Organization (WHO) appraised the evidence available on the effects of free sugars on the risk of non‐communicable diseases in adults and children, with a particular focus on weight gain and dental caries (WHO, 2015). The WHO recommended reducing the intake of free sugars to < 10% of total energy intake in both adults and children, with a conditional recommendation to reduce it further to < 5% of total energy intake.
Finally, two professional associations have issued recommendations on sugar intake for children only (up to 18 years of age).
The American Heart Association (AHA) reviewed the scientific evidence on the cardiovascular health effects of added sugars in children (Vos et al., 2016). Strong evidence was found to support an association between the intake of added sugars and increased cardiovascular disease risk in children through increased energy intake, increased adiposity and dyslipidaemia. AHA provided recommendations that added sugars (all sugars used as ingredients in processed and prepared foods and sugars eaten separately or added to foods at the table) should be consumed up to a maximum amount of 25 g per day by children > 2 years of age and avoided by children < 2 years of age.
In 2017, the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition (Fidler et al., 2017), reviewed the scientific evidence on the relationship between sugars intake and: (a) the development of sweet taste or flavour preference, and (b) health outcomes. The Committee concluded that a preference for sweet taste is driven by interplay of many factors and that the association between high consumption of SSBs in early and late childhood could not be demonstrated to be causal. Building on the conclusions reached by WHO (2015), SACN (2015) and the AHA (Vos et al., 2016) regarding the effect of sugars intake and different health outcomes in paediatric populations, the Committee recommended that intakes of free sugars should be reduced and minimised with a desirable upper limit of < 5% energy intake in children and adolescents aged 2–18 years. This represents 15–28 g of free sugars for girls and 16–37 g for boys. Intakes should be even lower in infants and toddlers < 2 years.
A tabulated overview of these recommendations is given in Table A.1.
Table A.1.
Summary of existing recommendations on sugar intake
| Guideline | Target population | Sugar fraction | Recommendation | Basis (endpoint) | Other endpoints assessed | Review method |
|---|---|---|---|---|---|---|
| EFSA NDA Panel ( 2010a ) | General population | Added sugars | Consider when setting FBDGs |
Dental caries Body weight Micronutrient density |
Glucose homoeostasis, risk of T2DM, blood lipids, blood pressure, CVD risk | Narrative |
| German Nutrition Society (2012) | General population | SSBs | Limit consumption |
Obesity Risk of T2DM |
BP/hypertension, metabolic syndrome, CHD risk, cancer | Systematic |
| Nordic Council of Ministers ( 2014 ) | General population | Added sugars | < 10E% | Micronutrient density |
Dental caries (frequency of intake), weight gain and risk of T2DM (SSBs), glucose homoeostasis, blood lipids, blood pressure, CVD risk, uric acid |
Systematic |
| Health Council of the Netherlands ( 2015 ) | General population | SSBs | Limit consumption |
Obesity Risk of T2DM |
– | Systematic |
| SACN ( 2015 ) | General population (>2 years) | Free sugars | ≤ 5E% | Energy intake |
Dental caries (frequency of intake), weight gain and risk of T2DM (SSBs), blood lipids, blood pressure, CHD, glucose homoeostasis |
Systematic |
| ANSES ( 2016 ) | Adults | Total sugars | 100 g/day | Fasting triglycerides | Weight gain, glucose homoeostasis, blood lipids, intrahepatic lipids and risk of NAFLD, uric acid, blood pressure | Systematic |
| IoM ( 2005 ) | General population | Added sugars | < 25E% | Micronutrient density | CHD risk, energy intake, body weight, blood lipids, cancer | Narrative |
| HHS/USDA ( 2015 ) | General population | Added sugars | < 10E% | Micronutrient density | – | Food pattern modelling and national data on added sugars intake |
| WHO ( 2015 ) | General population | Free sugars |
< 10E% < 5E% conditional |
Body weight Dental caries |
– | Systematic |
| American Heart Association (2016) | Children | Added sugars |
25 g/day ≥ 2 years Avoided < 2 years |
Energy intake Adiposity Dyslipidaemia CVD risk |
Micronutrient density, blood pressure, risk of NAFLD, glucose homoeostasis, risk of T2DM | Narrative |
| ESPGHAN (2017) | Children | Free sugars | ≤ 5E% ≥ 2 years (lower for < 2 years) |
Dental caries Weight gain (SSBs) CVD and T2DM (fructose) |
Preference for sweet taste | Narrative/systematic |
FBDG: food‐based dietary guidelines; T2DM: type 2 diabetes mellitus; CVD: cardiovascular disease; SSB: sugar‐sweetened beverage; CHD: coronary heart disease; NAFLD: non‐alcoholic fatty liver diseases.
Appendix B – Systematic reviews and meta‐analysis on the relationship between added/free sugars and their sources and surrogate/disease endpoints
1.
A scoping literature search was performed to identify systematic reviews and meta‐analysis published in English since 2009 addressing the health effects of added sugars/non‐milk extrinsic sugars/free sugars or any of its dietary sources.
The full list of references identified is reported in Table B.1 together with the specific exposure and outcome(s) of interest. All reviews for which the exposure of interest was added, free or total sugars from all dietary sources are presented in Table B.2. Based on the inclusion criteria identified for subquestions 5 and 6 (see Section 9.1), reviews having the same or wider inclusion criteria were used as a basis to update or build new literature searches (see Section 9.2).
Table B.1.
Overview of systematic reviews and meta‐analysis published since 2009 on the relationship between sugars and their sources and endpoints of interest
| Reference | Endpoint (population subgroup) | Exposure |
|---|---|---|
| Anderson et al. ( 2009 ) | Caries (adults and children) | Sucrose including sucrose‐based carbonated soft drinks, baked goods, sweets and table sugar, as added to other foods and drinks |
| Auerbach et al. ( 2017 ) | Obesity (children) | Fruit juice |
| Avery et al. ( 2015 ) | Obesity (children) | SSBs |
| Bucher Della Torre et al. ( 2016 ) | Obesity (children) | SSBs |
| Chiavaroli et al. ( 2015 ) | Blood lipids (adults and children) | Fructose |
| Chiu et al. ( 2014 ) | NAFLD (adults) | Fructose |
| Chung et al. ( 2014 ) | Liver health (adults) | Fructose |
| Crowe‐White et al. ( 2016 ) | Obesity (children) | Fruit juice |
| Fattore et al. ( 2017 ) | Blood lipids, blood pressure (adults) | Free sugars (fructose, sucrose and glucose) |
| Gibson ( 2008 ) | Obesity (adults and children) | Sugar‐sweetened soft drinks (SSD) |
| Gibson et al. ( 2013 ) | Blood lipids, blood pressure, glucose metabolism (adults) | Sucrose |
| Greenwood et al. ( 2014 ) | Risk of T2DM | SSD |
| Huang et al. ( 2014 ) | Risk of CVD | SSBs |
| Imamura et al. ( 2015 ) | Risk of T2DM | SSB, fruit juice |
| Jayalath et al. ( 2014 ) | Blood pressure | Fructose‐containing sugar (high‐fructose corn syrup, sucrose and fructose) |
| Jayalath et al. ( 2015 ) | Blood pressure | SSBs containing free or bound fructose |
| Kaiser et al. ( 2013 ) | Obesity (adults and children) | SSBs |
| Kelishadi et al. ( 2014 ) | Blood lipids, blood pressure, glucose metabolism | Fructose |
| Keller and Bucher Della Torre ( 2015 ) | Blood lipids, Blood pressure, glucose metabolism, risk of CVD | SSBs |
| Kim and Je ( 2016 ) | Blood pressure | SSBs and artificially sweetened beverages |
| Ma et al. ( 2016 ) | Obesity (adults and children) | Fructose, glucose, SSB, HFCS |
| Malik et al. ( 2010 ) | Risk of T2DM | SSBs |
| Malik et al. ( 2013 ) | Obesity (adults and children) | SSBs |
| Malik et al. ( 2014 ) | Blood pressure | SSBs |
| Mattes et al. ( 2011 ) | Obesity (adults and children) | SSBs |
| Moynihan and Kelly ( 2014 ) | Dental caries (adults and children) | Total sugars, free sugars, added sugars, sucrose, NME sugars |
| Perez‐Morales et al. ( 2013 ) | Obesity (children) | SSBs |
| SACN ( 2015 ) | Blood lipids, blood pressure, dental caries, glucose metabolism, obesity, risk of CVD, risk of T2DM | Total carbohydrate, sugars reported as a nutrient (fructose, sucrose, lactose, glucose), table sugar and other extrinsic sugars (syrups), food format (solid vs. liquid, which includes SSBs) |
| Singh et al. ( 2011 ) | Risk of gout (adults) | SSBs, fructose |
| Sonestedt et al. ( 2012 ) | Blood lipids, blood pressure, glucose metabolism, risk of CVD, risk of T2DM | SSBs, sugars, sucrose and fructose |
| Te Morenga et al. ( 2013 ) | Obesity (adults and children) | Free sugars |
| Te Morenga et al. ( 2014 ) | Blood lipids, blood pressure | Sugar (sucrose) or free sugars |
| David Wang et al. ( 2014 ) | Blood lipids | Fructose |
| Wang et al. ( 2014 ) | Glucose metabolism (adults) | Fruit juice |
| Xi et al. ( 2014 ) | Risk of T2DM | Fruit juice |
| Xi et al. ( 2015 ) | Blood pressure, risk of CVD | SSBs |
| Zheng et al. ( 2015 ) | Obesity (adults and children) | SSBs |
SSB: sugar‐sweetened beverage; NAFLD: non‐alcoholic fatty liver diseases; T2D: type 2 diabetes mellitus; CVD: cardiovascular disease; HFCS: high‐fructose corn syrup.
Table B.2.
Selected systematic reviews with research question partially or completely overlapping with the present work
| Subquestion 4 | Subquestion 5 | |||||
|---|---|---|---|---|---|---|
| Systematic review | Fattore et al. (2017)a | SACN (2015) | Sonestedt et al. (2012) | Te Morenga et al. (2013) | Te Morenga et al. (2014) | Moynihan and Kelly (2014) |
| Endpoints | BP, blood lipids and body weight | All | Glucose metabolism, BP, blood lipids, risk of T2D, risk of CVD | Obesity | BP, blood lipids | Caries |
| Databases |
PubMed/MEDLINE, EMBASE, Cochrane Library Hand search |
Medline MEDLINE In‐Process & Other Non‐Indexed Citations Embase CAB Abstracts ISI Web of Science BIOSIS The Cochrane Library Hand search |
PubMed SveMed+ |
OVID Medline, Embase PubMed, CiNAHL, Scopus, Web of Science |
OVID Medline, Embase PubMed, CiNAHL, Scopus, Web of Science Grey literature Hand search |
MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, LILACS, CNKI, Wanfang South African Department of Health databases |
| Search dates | Up to 22 October 2015 | 1990–December 2010 | January 2000–October 2010Update November 2010–December 2011 | Up to December 2011 | 1960–August 2013 | 1950–November 2011 |
| Language limit | English | English | English or a Nordic language | English | English | No limit |
| Study type | Intervention studies |
RCT PC |
RCT PC |
RCT PC |
RCT | Intervention, cohort, population or cross‐sectional |
| Study duration | ≥ 2 weeks |
RCT > 6 weeks (≥ 3 days for energy intake/satiety) PC > 3 years |
RCT ≥ 4 weeks (drop out ≤ 50%) PC ≥ 4 years |
RT ≥ 2 weeks PC ≥ 1 year |
RCT≥ 2 weeks | |
| Population | Adults | Adults/children | Adults/children | Adults/children | Adults/children | Adults/children |
BP: blood pressure; T2D: type 2 diabetes; CVD: cardiovascular disease; RCT: randomised controlled trial; PC: prospective cohort studies.
Not used as primary source of information as the endpoints were assessed under isocaloric conditions.
Appendix C – Questionnaire to National Competent Authorities of European countries
1.
NAME: 
COUNTRY: 
ALIGFFILIGLIATION: 
E MAIL: 
DATE: 
-
EFSA has been requested to provide a scientific opinion on the Tolerable Upper Intake Level of dietary sugars. The assessment concerns the main types of sugars (mono‐ and disaccharides) found in mixed diets (i.e. glucose, fructose, galactose, sucrose, lactose, maltose and trehalose). Sugar alcohols (polyols), other substances used as sugar replacers, and other mono‐ or disaccharides present in the diet in marginal amounts, are not included in the term “sugars” for the purpose of this assessment. In this context, EFSA will address:
-
–
Total sugars: i.e. the monosaccharides glucose, fructose, and galactose, and the disaccharides sucrose, lactose, maltose and trehalose present in foods;
-
–
Added sugars: sugars used as ingredients in processed and prepared foods and sugars eaten separately or added to foods at the table; and
-
–
Free sugars: added sugars plus sugars naturally present in honey, syrups, fruit juices and fruit juice concentrates.
-
–
In the context, EFSA would like, through this questionnaire, to gather information on the following:
-
–
Data available from national food consumption surveys (or regional food consumption surveys e.g. targeting specific population groups such as infants or pregnant women) on the intake of total/added/free sugars;
-
–
National food composition data on total, added and free sugars if available, together with a description of the methods used to estimate added and free sugars in foods;
-
–
National dietary recommendations on the amount of sugars (whether total, added or free) to be consumed in the diet, if available.
To answer this questionnaire, please tick the relevant boxes. If you have doubts or queries in relation to the compilation of this questionnaire, please contact us at: http://nda@efsa.europa.eu
-
1
Please specify the last national food consumption survey(s) carried out in your country, indicating the year (or time frame) in which the survey was conducted, the method used for data collection (food diaries, food records, 24‐h dietary recalls, other), the number of days in which data was collected for each subject, and the age group(s) included in the survey. Regional food consumption surveys should only be indicated if they targeted specially infants (up to 12 months of age) or pregnant women. Food consumption surveys using food–frequency questionnaires for data collection should not be included.
| Name of survey |
Type of survey (National/regional) |
Year (range) | Method for data collection | Number of days/subject | Age groups included |
|---|---|---|---|---|---|
Add as many rows as needed.
-
2
Please indicate whether there are publications available (in any language) on the intake of total sugars, added sugars, and/or free sugars in your country at national level (i.e. from the most recent national food consumption surveys that you have listed in question 1):
| Author/year | Total sugars | Added sugars | Free sugars |
|---|---|---|---|
| □ yes □ no | □ yes □ no | □ yes □ no | |
| □ yes □ no | □ yes □ no | □ yes □ no | |
| □ yes □ no | □ yes □ no | □ yes □ no | |
| □ yes □ no | □ yes □ no | □ yes □ no |
Add as many rows as needed.
Please provide the full list of references, as well as the full text if available to you 
Note: Should you wish to share data not published yet but soon to be (i.e. on a confidential basis/embargo until publication), please specify the foreseen/assumed date of publication, and note that only data published by March 2019 will be accepted. Please also specify if you have anything against sharing unpublished data with the Experts of the Working Group on Sugar, for the purposes of this risk assessment.
If NO publications are available on the intake of total sugars, added sugars, and/or free sugars in your country at national level → please go directly to question 5.
-
3
The national food composition database used to calculate the intake of total sugars, added sugars, and/or free sugars in the afore‐mentioned publications contains data on:

Please provide the link to a website where the database can be downloaded from or a contact address/details of the person(s) responsible for the maintenance/update of the database 
-
4
Your national food composition database contains information on:

If the answer to any of the above is yes, please specify in detail the methodology that has been used to estimate the content of added sugars and/or free sugars in foods 
-
5
Does your country have some type of national recommendations on the amount of sugars to be consumed in the diet?

If the answer is no → please go directly to question 9.
-
6
Please specify the national dietary recommendations to limit the amount of sugars consumed in the diet available in your country, indicating the type of recommendation (e.g. in the context of setting dietary reference values for nutrients, in the context of developing Food–Based Dietary Guidelines (FBDG)) and who was involved in their development (government bodies, scientific societies, industry, non‐profit organisations, other), and the year in which the recommendation was issued. Please provide a link to the full text of the recommendation, if available. If more than one national recommendation is available in your country (e.g. established by different bodies; FBDGs targeting only specific age groups), use as many rows as needed.
National dietary recommendation:
 Body/organisation
Body/organisation  Year
Year 
National dietary recommendation:
 Body/organisation
Body/organisation  Year
Year 
National dietary recommendation:
 Body/organisation
Body/organisation  Year
Year 
-
7
National recommendations on the consumption of dietary sugars (total, added and/or free) are directed to:
Please note that age ranges below are only indicative

-
8
Which diet‐related health problems were considered when developing recommendations for the intake of total, added and/or free sugars consumption in your country? Please tick as many as needed:

-
9
Please add any general or specific comment, you might have:

We kindly ask you to send back the filled survey by e‐mail to: http://nda@efsa.europa.eu
Thank you very much for your participation in this survey
Appendix D – Exposure and endpoints search terms for subquestions 4 and 5
1.
Subject index terms, where available, will be combined with free‐text terms. Free‐text terms will be searched in title and abstract fields in Embase and PubMed; and in title, abstract and keyword fields in the Cochrane Library databases and Scopus.
Exposure
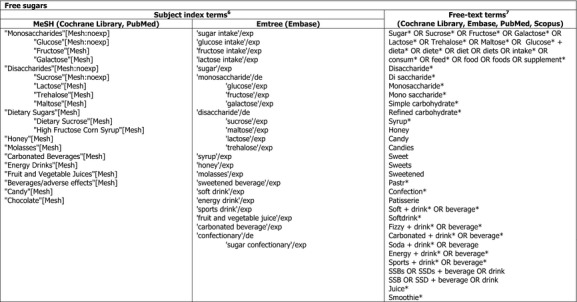
Endpoints
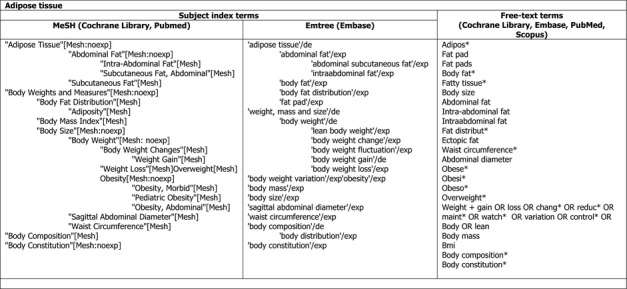
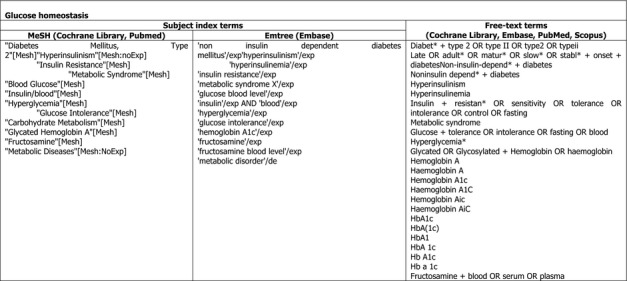
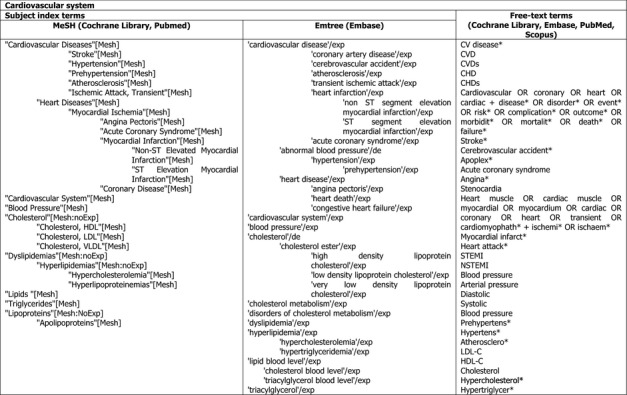
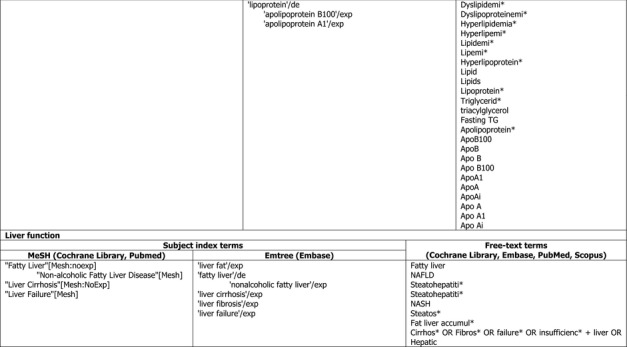
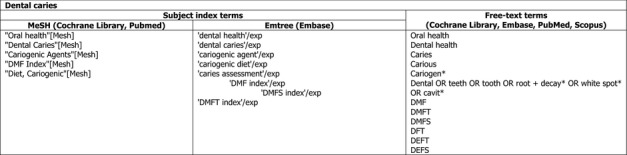
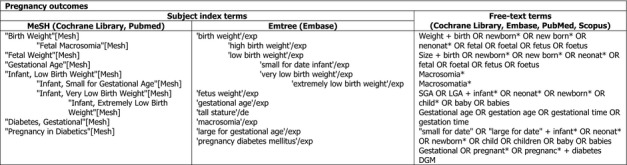
Suggested citation: EFSA (European Food Safety Authority) , 2018. Protocol for the scientific opinion on the Tolerable Upper Intake Level of dietary sugars. EFSA Journal 2018;16(8):5393, 47 pp. 10.2903/j.efsa.2018.5389
Requestors: National Food Agency, Sweden; Finnish Food Safety Authority, EVIRA, Finland;
National Food Institute (DTU), Denmark; Norwegian Scientific Committee for Food Safety, Norway;
Icelandic Food and Veterinary Authority, Iceland
Question number: EFSA‐Q‐2017‐00646
Acknowledgements: EFSA wishes to thank the members of the Panel on Dietetic Products, Nutrition and Allergies (NDA): Jean‐Louis Bresson, Barbara Burlingame, Tara Dean, Susan Fairweather‐Tait, Marina Heinonen, Karen Ildico Hirsch‐Ernst, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Monika Neuhäuser‐Berthold, Grażyna Nowicka, Kristina Pentieva, Yolanda Sanz, Alfonso Siani, Anders Sjödin, Martin Stern, Daniel Tomé, Dominique Turck, Henk Van Loveren, Marco Vinceti and Peter Willatts, the members of the Working Group on Sugars: Roger Adan, Pauline Emmett, Mathilde Kersting, Paula Moynihan, Luc Tappy, and EFSA staff members: Davide Arcella, Lucia Fabiani, Ana García, Andrea Germini, Irene Muñoz Guajardo and Silvia Valtueña Martínez for the support provided to this scientific output.
Approved: 12 July 2018
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2018.EN-1455/full
Notes
The Mintel GNPD contains information on over two million food and beverage products, of which more than 800,000 are or have been available on the European food market. Mintel started covering European Union's food markets in 1996. Twenty out of the 28 EU member countries and Norway are present in the Mintel GNPD. The database provides the compulsory ingredient information presented in the labelling of products and the nutritional facts when available on the labels, which provide information about the use of sugars as ingredients and about the total sugar content of foods. http://www.mintel.com/global-new-products-database
[Mesh] indicates that the MeSH term will be exploded, including in the search the terms below in the MesH hierarchy if available. [Mesh:noexp] indicates that the MesH term will not be exploded, the terms below in the MeSH hierarchy will not be searched. /exp indicates that the Emtree term will be exploded, including in the search terms below in the Emtree hierarchy if available. /de indicates that the Emtree term will not be exploded, the terms below in the Emtree hierarchy will not be searched.
Asterisk symbol '*' indicates truncation. Plus sign '+' indicates the search terms will be linked with the Boolean operator AND.
References
- Altman DG, 1986. Book review. Summing up. The science of reviewing research, Richard J. Light and David B. Pillemer, Harvard University Press, Cambridge, Mass., 1984. Statistics in Medicine, 5, 289.
- Anderson CA, Curzon ME, Van Loveren C, Tatsi C and Duggal MS, 2009. Sucrose and dental caries: a review of the evidence. Obesity Reviews, 10(Suppl 1), 41–54. [DOI] [PubMed] [Google Scholar]
- ANSES (French Agency for Food, Environmental and Occupational Health & Safety), 2016. Opinion of ANSES on the establishment of recommendations on sugar intake.
- Arah OA, Chiba Y and Greenland S, 2008. Bias formulas for external adjustment and sensitivity analysis of unmeasured confounders. Annals of Epidemiology, 18, 637–646. [DOI] [PubMed] [Google Scholar]
- Auerbach BJ, Wolf FM, Hikida A, Vallila‐Buchman P, Littman A, Thompson D, Louden D, Taber DR and Krieger J, 2017. Fruit Juice and Change in BMI: A Meta‐analysis. Pediatrics, 139, e20162454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery A, Bostock L and McCullough F, 2015. A systematic review investigating interventions that can help reduce consumption of sugar‐sweetened beverages in children leading to changes in body fatness. Journal of Human Nutrition and Dietetics, 28(Suppl 1), 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher Della Torre S, Keller A, Laure Depeyre J and Kruseman M, 2016. Sugar‐sweetened beverages and obesity risk in children and adolescents: a systematic analysis on how methodological quality may influence conclusions. Journal of the Academy of Nutrition and Dietetics, 116, 638–659. [DOI] [PubMed] [Google Scholar]
- Chiavaroli L, de Souza RJ, Ha V, Cozma AI, Mirrahimi A, Wang DD, Yu M, Carleton AJ, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Beyene J, Kendall CW, Jenkins DJ and Sievenpiper JL, 2015. Effect of fructose on established lipid targets: a systematic review and meta‐analysis of controlled feeding trials. Journal of the American Heart Association, 4, e001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S, Sievenpiper JL, de Souza RJ, Cozma AI, Mirrahimi A, Carleton AJ, Ha V, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Don‐Wauchope AC, Beyene J, Kendall CW and Jenkins DJ, 2014. Effect of fructose on markers of non‐alcoholic fatty liver disease (NAFLD): a systematic review and meta‐analysis of controlled feeding trials. European Journal of Nutrition, 68, 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M, Ma J, Patel K, Berger S, Lau J and Lichtenstein AH, 2014. Fructose, high‐fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta‐analysis. The American Journal of Clinical Nutrition, 100, 833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARITY (CLARITY Group at McMaster University), 2013. Tools to assess risk of bias in cohort studies, case control studies, randomized controlled trials, and longitudinal symptom research studies aimed at the general population.
- Corbin M, Haslett S, Pearce N, Maule M and Greenland S, 2017. A comparison of sensitivity‐specificity imputation, direct imputation and fully Bayesian analysis to adjust for exposure misclassification when validation data are unavailable. International Journal of Epidemiology, 46, 1063–1072. [DOI] [PubMed] [Google Scholar]
- Crippa A and Orsini N, 2016. Dose‐response meta‐analysis of differences in means. BMC Medical Research Methodology, 16, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe‐White K, O'Neil CE, Parrott JS, Benson‐Davies S, Droke E, Gutschall M, Stote KS, Wolfram T and Ziegler P, 2016. Impact of 100% fruit juice consumption on diet and weight status of children: an evidence‐based review. Critical Reviews in Food Science and Nutrition, 56, 871–884. [DOI] [PubMed] [Google Scholar]
- David Wang D, Sievenpiper JL, de Souza RJ, Cozma AI, Chiavaroli L, Ha V, Mirrahimi A, Carleton AJ, Di Buono M, Jenkins AL, Leiter LA, Wolever TMS, Beyene J, Kendall CWC and Jenkins DJA, 2014. Effect of fructose on postprandial triglycerides: A systematic review and meta‐analysis of controlled feeding trials. Atherosclerosis, 232, 125–133. [DOI] [PubMed] [Google Scholar]
- Discacciati A, Crippa A and Orsini N, 2017. Goodness of fit tools for dose–response meta‐analysis of binary outcomes. Research Synthesis Methods, 8, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S and Tweedie R, 2004. Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics, 56, 455–463. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Guidance on expert knowledge elicitation in food and feed safety risk assessment. EFSA Journal 2014;12(6):3734, 278 pp. 10.2903/j.efsa.2014.3734 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015a. Principles and process for dealing with data and evidence in scientific assessments. EFSA Journal 2015;13(5):4121, 35 pp. 10.2903/j.efsa.2015.4121 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015b. The food classification and description system FoodEx2 (revision 2). EFSA supporting publication 2015;12(5):EN‐804, 90 pp. 10.2903/sp.efsa.2015.en-804 [DOI]
- EFSA (European Food Safety Authority), 2018. Outcome of the public consultation on a draft protocol for the Scientific Opinion on dietary sugars. EFSA supporting publication 2018:EN‐1456, 22 pp. 10.2903/sp.efsa.2018.en-1456, 200 pp. [DOI]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2009. Scientific Opinion on the appropriate age for introduction of complementary feeding of infants. EFSA Journal 2009;7(12):1423, 38 pp. 10.2903/j.efsa.2009.1423 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2010a. Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA Journal 2010;8(3):1462, 77 pp. 10.2903/j.efsa.2010.1462 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2010b. Scientific Opinion on principles for deriving and applying Dietary Reference Values. EFSA Journal 2010;8(3):1458, 30 pp. 10.2903/j.efsa.2010.1458 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger Michael J, Knutsen Helle K, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter Josef R, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry Qasim M, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne JL, Fernandez Dumont A, Hempen M, Valtueña Martínez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M (European Food Safety Authority), 2017. Guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger Michael J, Knutsen Helle K, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter Josef R, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on uncertainty analysis in scientific assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M and Minder C, 1997. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal, 315, 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization and the World Health Organization), 1998. Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation, Rome, Italy, 14‐18 April 1997.
- Fattore E, Botta F, Agostoni C and Bosetti C, 2017. Effects of free sugars on blood pressure and lipids: a systematic review and meta‐analysis of nutritional isoenergetic intervention trials. The American Journal of Clinical Nutrition, 105, 42–56. [DOI] [PubMed] [Google Scholar]
- Fidler Mis N, Braegger C, Bronsky J, Campoy C, Domello M, Embleton N, Hojsak I, Hulst J, Indrio F, Lapillonne A, Mihatsch W, Molgaard C, Vora R and Fewtrell M on Behalf of the ESPGHAN Committee on Nutrition , 2017. Sugar in infants, children and adolescents: a position paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition, 65, 681–696. [DOI] [PubMed] [Google Scholar]
- Gibson S, 2008. Sugar‐sweetened soft drinks and obesity: a systematic review of the evidence from observational studies and interventions. Nutrition Research Reviews, 21, 134–147. [DOI] [PubMed] [Google Scholar]
- Gibson S, Gunn P, Wittekind A and Cottrell R, 2013. The effects of sucrose on metabolic health: a systematic review of human intervention studies in healthy adults. Critical Reviews in Food Science and Nutrition, 53, 591–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DC, Threapleton DE, Evans CEL, Cleghorn CL, Nykjaer C, Woodhead C and Burley VJ, 2014. Association between sugar‐sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose–response meta‐analysis of prospective studies. British Journal of Nutrition, 112, 725–734. [DOI] [PubMed] [Google Scholar]
- Hauner H, Bechthold A, Boeing H, Brönstrup A, Buyken A, Leschik‐Bonnet E, Linseisen J, Schulze M, Strohm D and Wolfram G, 2012. Evidence‐based guideline of the German Nutrition Society: carbohydrate intake and prevention of nutrition‐related diseases. Annals of Nutrition and Metabolism, 60(suppl 1), 1–58. [DOI] [PubMed] [Google Scholar]
- Health Council of the Netherlands , 2015. Dutch dietary guidelines 2015.
- HHS/USDA (U.S. Department of Health and Human Services and U.S. Department of Agriculture), 2015. 2015–2020 Dietary Guidelines for Americans. 8th Edition.
- Higgins J and Green S (The Cochrane Collaboration), 2011. Cochrane Handbook for systematic reviews of interventions version 5.1.0 [updated March 2011].
- Higgins JPT and Thompson SG, 2002. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine, 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ and Altman DG, 2003. Measuring inconsistency in meta‐analyses. British Medical Journal, 327, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Huang JH, Tian Y, Yang X and Gu D, 2014. Sugar sweetened beverages consumption and risk of coronary heart disease: a meta‐analysis of prospective studies. Atherosclerosis, 234, 11–16. [DOI] [PubMed] [Google Scholar]
- Huybrechts I, Sioen I, Boon PE, Ruprich J, Lafay L, Turrini A, Amiano P, Hirvonen T, De Neve M, Arcella D, Moschandreas J, Westerlund A, Ribas‐Barba L, Hilbig A, Papoutsou S, Christensen T, Oltarzewski M, Virtanen S, Rehurkova I, Azpiri M, Sette S, Kersting M, Walkiewicz A, Serra‐Majem L, Volatier J‐L, Trolle E, Tornaritis M, Busk L, Kafatos A, Fabiansson S, De Henauw S and Van Klaveren JD, 2011. Dietary exposure assessments for children in Europe (the EXPOCHI project): rationale, methods and design. Archives of Public Health, 69, 4–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura F, O'Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN and Forouhi NG, 2015. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta‐analysis, and estimation of population attributable fraction. British Medical Journal, 351, h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IoM (Institute of Medicine), 2005. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. [DOI] [PubMed]
- Jayalath VH, Sievenpiper JL, de Souza RJ, Ha V, Mirrahimi A, Santaren ID, Blanco Mejia S, Di Buono M, Jenkins AL, Leiter LA, Wolever TMS, Beyene J, Kendall CWC and Jenkins DJA, 2014. Total fructose intake and risk of hypertension: a systematic review and meta‐analysis of prospective cohorts. Journal of the American College of Nutrition, 33, 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayalath VH, de Souza RJ, Ha V, Mirrahimi A, Blanco‐Mejia S, Di Buono M, Jenkins AL, Leiter LA, Wolever TMS, Beyene J, Kendall CWC, Jenkins DJA and Sievenpiper JL, 2015. Sugar‐sweetened beverage consumption and incident hypertension: a systematic review and meta‐analysis of prospective cohorts. The American Journal of Clinical Nutrition, 102, 914–921. [DOI] [PubMed] [Google Scholar]
- Kaiser KA, Shikany JM, Keating KD and Allison DB, 2013. Will reducing sugar‐sweetened beverage consumption reduce obesity? Evidence supporting conjecture is strong, but evidence when testing effect is weak. Obesity Reviews, 14, 620–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelishadi R, Mansourian M and Heidari‐Beni M, 2014. Association of fructose consumption and components of metabolic syndrome in human studies: A systematic review and meta‐analysis. Nutrition, 30, 503–510. [DOI] [PubMed] [Google Scholar]
- Keller A and Bucher Della Torre S, 2015. Sugar‐sweetened beverages and obesity among children and adolescents: a review of systematic literature reviews. Childhood Obesity, 11, 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibblewhite R, Nettleton A, McLean R, Haszard J, Fleming E, Kruimer D and Te Morenga L, 2017. Estimating free and added sugar intakes in New Zealand. Nutrients, 9, 1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y and Je Y, 2016. Prospective association of sugar‐sweetened and artificially sweetened beverage intake with risk of hypertension. Archives of Cardiovascular Diseases, 109, 242–253. [DOI] [PubMed] [Google Scholar]
- Louie JC, Moshtaghian H, Boylan S, Flood VM, Rangan AM, Barclay AW, Brand‐Miller JC and Gill TP, 2015. A systematic methodology to estimate added sugar content of foods. European Journal of Nutrition, 69, 154–161. [DOI] [PubMed] [Google Scholar]
- Ma J, Karlsen MC, Chung M, Jacques PF, Saltzman E, Smith CE, Fox CS and McKeown NM, 2016. Potential link between excess added sugar intake and ectopic fat: a systematic review of randomized controlled trials. Nutrition Reviews, 74, 18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik VS, Popkin BM, Bray GA, Després J‐P, Willett WC and Hu FB, 2010. Sugar‐Sweetened Beverages and Risk of Metabolic Syndrome and Type 2 Diabetes. Diabetes Care, 33, 2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik VS, Pan A, Willett WC and Hu FB, 2013. Sugar‐sweetened beverages and weight gain in children and adults: a systematic review and meta‐analysis. The American Journal of Clinical Nutrition, 98, 1084–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AH, Akram Y, Shetty S, Malik SS and Yanchou Njike V, 2014. Impact of sugar‐sweetened beverages on blood pressure. The American Journal of Cardiology, 113, 1574–1580. [DOI] [PubMed] [Google Scholar]
- Mattes RD, Shikany JM, Kaiser KA and Allison DB, 2011. Nutritively sweetened beverage consumption and body weight: a systematic review and meta‐analysis of randomized experiments. Obesity Reviews, 12, 346–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee R, 2003. Confounding and confounders. Occupational and Environmental Medicine, 60, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten C, Ferrari P, Bakker M, Boss A, Hearty Á, Leclercq C, Lindtner O, Tlustos C, Verger P, Volatier JL and Arcella D, 2011. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food Additives & Contaminants: Part A, 28, 975–995. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG and The PG, 2009. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. PLoS Medicine, 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan PJ and Kelly SAM, 2014. Effect on caries of restricting sugars intake: systematic review to inform WHO Guidelines. Journal of Dental Research, 93, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordic Council of Ministers , 2014. Nordic Nutrition Recommendations 2012. Integrating nutrition and physical activity. 627 pp.
- OHAT/NTP (Office of Health Assessment and Translation, Division of the National Toxicology Program), 2015. OHAT Risk of Bias Rating Tool for Human and Animal Studies.
- Orsini N, Li R, Wolk A, Khudyakov P and Spiegelman D, 2012. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. American Journal of Epidemiology, 175, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Morales E, Bacardi‐Gascon M and Jimenez‐Cruz A, 2013. Sugar‐sweetened beverage intake before 6 years of age and weight or BMI status among older children; systematic review of prospective studies. Nutricion Hospitalaria, 28, 47–51. [DOI] [PubMed] [Google Scholar]
- Peters Jaime L, Sutton Alex J, Jones David R, Abrams Keith R and Rushton L, 2007. Performance of the trim and fill method in the presence of publication bias and between‐study heterogeneity. Statistics in Medicine, 26, 4544–4562. [DOI] [PubMed] [Google Scholar]
- Roe MA, Bell S, Oseredczuk M, Christensen T, Westenbrink S, Pakkala H, Presser K and Finglas PM, 2013. Updated food composition database for nutrient intake. EFSA supporting publication 2013:EN‐355, 21 pp.
- Rothman KJ, Greenland S and Lash TL, 2012. Modern epidemiology, 3rd edition Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- Rothstein H, Sutton A and Borenstein M, 2005. Publication bias in meta‐analysis: prevention, assessment, and adjustments. Wiley, Chichester, England. [Google Scholar]
- SACN (Scientific Advisory Committee on Nutrition), 2015. Carbohydrates and health.
- SCF/EFSA NDA Panel (Scientific Committee on Food (SCF) and EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA)), 2006. Tolerable upper intake levels for vitamins and minerals.
- Singh JA, Reddy SG and Kundukulam J, 2011. Risk factors for gout and prevention: a systematic review of the literature. Current Opinion in Rheumatology, 23, 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonestedt E, Øverby NC, Laaksonen DE and Birgisdottir BE, 2012. Does high sugar consumption exacerbate cardiometabolic risk factors and increase the risk of type 2 diabetes and cardiovascular disease? Food & Nutrition Research, 56, 10.3402/fnr.v3456i3400.19104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J and Egger M, 2005. Regression methods to detect publication and other bias in meta‐analysis. In: Publication Bias in Meta‐Analysis.
- Sterne J, Higgins J and Reeves B, 2014. A Cochrane risk of bias assessment tool: for non‐randomized studies of interventions (ACROBAT‐NRSI).
- Te Morenga L, Mallard S and Mann J, 2013. Dietary sugars and body weight: systematic review and meta‐analyses of randomised controlled trials and cohort studies. British Medical Journal, 346, e7492. [DOI] [PubMed] [Google Scholar]
- Te Morenga LA, Howatson AJ, Jones RM and Mann J, 2014. Dietary sugars and cardiometabolic risk: systematic review and meta‐analyses of randomized controlled trials of the effects on blood pressure and lipids. The American Journal of Clinical Nutrition, 100, 65–79. [DOI] [PubMed] [Google Scholar]
- UK Department of Health (Department of Health. Committee on Medical Aspects of Food Policy), 1989. Dietary Sugars and Human Disease. Report on Health and Social Subjects no. 37.
- USDA/HHS (U.S. Department of Agriculture and U.S. Department of Health and Human Services), 2000. Dietary Guidelines for Americans. 5th Edition.
- Viswanathan M, Ansari M, Berkman N, Chang S, Hartling L, McPheeters L, Santaguida P, Shamliyan T, Singh K, Tsertsvadze A and Treadwell J (Agency for Healthcare Research and Quality Methods Guide for Comparative Effectiveness Reviews), 2012. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. Agency for Healthcare Research and Quality Publication No. 12‐EHC047‐EF. [PubMed]
- Vos MB, Kaar JL, Welsh JA, Van Horn LV, Feig DI, Anderson CAM, Patel MJ, Cruz Munos J, Krebs NF, Xanthakos SA and Johnson RK, 2016. Added sugars and cardiovascular disease risk in children. Circulation. [DOI] [PMC free article] [PubMed]
- Wang B, Liu K, Mi M and Wang J, 2014. Effect of fruit juice on glucose control and insulin sensitivity in adults: a meta‐analysis of 12 randomized controlled trials. PLoS ONE, 9, e95323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (Word Health Organization), 2003. Diet, nutrition and the prevention of chronic diseases. Report of the joint WHO/FAO expert consultation. WHO Technical Report Series, No. 916 (TRS 916). [PubMed]
- WHO (World Health Organisation), 2015. Guideline: Sugars intake for adults and children. [PubMed]
- Xi B, Li S, Liu Z, Tian H, Yin X, Huai P, Tang W, Zhou D and Steffen LM, 2014. Intake of fruit juice and incidence of type 2 diabetes: a systematic review and meta‐analysis. PLoS ONE, 9, e93471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi B, Huang Y, Reilly KH, Li S, Zheng R, Barrio‐Lopez MT, Martinez‐Gonzalez MA and Zhou D, 2015. Sugar‐sweetened beverages and risk of hypertension and CVD: a dose–response meta‐analysis. British Journal of Nutrition, 113, 709–717. [DOI] [PubMed] [Google Scholar]
- Zheng M, Rangan A, Olsen NJ, Andersen LB, Wedderkopp N, Kristensen P, Grontved A, Ried‐Larsen M, Lempert SM, Allman‐Farinelli M and Heitmann BL, 2015. Substituting sugar‐sweetened beverages with water or milk is inversely associated with body fatness development from childhood to adolescence. Nutrition, 31, 38–44. [DOI] [PubMed] [Google Scholar]


