Abstract
4,15‐Diacetoxyscirpenol (DAS) is a mycotoxin primarily produced by Fusarium fungi and occurring predominantly in cereal grains. As requested by the European Commission, the EFSA Panel on Contaminants in the Food Chain (CONTAM) assessed the risk of DAS to human and animal health related to its presence in food and feed. Very limited information was available on toxicity and on toxicokinetics in experimental and farm animals. Due to the limitations in the available data set, human acute and chronic health‐based guidance values (HBGV) were established based on data obtained in clinical trials of DAS as an anticancer agent (anguidine) after intravenous administration to cancer patients. The CONTAM Panel considered these data as informative for the hazard characterisation of DAS after oral exposure. The main adverse effects after acute and repeated exposure were emesis, with a no‐observed‐adverse‐effect level (NOAEL) of 32 μg DAS/kg body weight (bw), and haematotoxicity, with a NOAEL of 65 μg DAS/kg bw, respectively. An acute reference dose (ARfD) of 3.2 μg DAS/kg bw and a tolerable daily intake (TDI) of 0.65 μg DAS/kg bw were established. Based on over 15,000 occurrence data, the highest acute and chronic dietary exposures were estimated to be 0.8 and 0.49 μg DAS/kg bw per day, respectively, and were not of health concern for humans. The limited information for poultry, pigs and dogs indicated a low risk for these animals at the estimated DAS exposure levels under current feeding practices, with the possible exception of fattening chicken. Assuming similar or lower sensitivity than for poultry, the risk was considered overall low for other farm and companion animal species for which no toxicity data were available. In consideration of the similarities of several trichothecenes and the likelihood of co‐exposure via food and feed, it could be appropriate to perform a cumulative risk assessment for this group of substances.
Keywords: 4,15 ‐ diacetoxyscirpenol; DAS; anguidine; MAS; exposure; toxicity; human and animal risk assessment
Summary
In a request from the European Commission, the EFSA Panel on Contaminants in the Food Chain (CONTAM Panel) was asked to assess whether 4,15‐diacetoxyscirpenol (DAS) in food and feed is a potential risk for public and animal health, taking into account the toxicity of DAS and the occurrence in food and feed. Possible interactions with other Fusarium toxins, in particular group A trichothecenes, as regards toxicity and occurrence should be included in the evaluation. For the assessment of the risks, the impact on different farm animal species and the specific (vulnerable) groups of the human population should be considered.
DAS is a type A trichothecene mycotoxin with low molecular weight, produced by several Fusarium species (mainly F. langsethiae, F. poae, F. sporotrichioides and F. sambucinum). It has mainly been detected in cereal grains, cereal‐based products and coffee but its presence in other foods and feeds cannot be excluded. A naturally occurring modified form of DAS has been identified as DAS‐glucoside.
Liquid chromatography with tandem mass spectrometry (LC–MS/MS) is currently the most widely used and preferred technique for analysis of DAS in foods, feed and biological samples, while high‐performance liquid chromatography‐flame ionisation detection (HPLC‐FLD), gas chromatography‐flame ionisation detection (GC‐FID) or gas chromatography‐electron capture detector (GC‐ECD) and gas chromatography–mass spectrometry (/tandem mass spectrometry (GC–MS(/MS)) have also been applied. None of the applied methods for DAS have been formally validated in interlaboratory studies and certified reference materials are not available. Calibrants are commercially available.
In the published literature, DAS has mainly been reported in various cereal grains (principally wheat, sorghum, maize, barley and oats) and cereal products, but also in potato products, soybeans and coffee. The highest levels have been reported for wheat, sorghum and coffee. DAS has been found to co‐occur with many other mycotoxins in grains and grain‐based products, in particular Fusarium toxins including type A and B trichothecenes, and zearalenone.
Based on the analytical results of food and feed in the EFSA database up to the end of 2017 and the literature, a total of 15,786 and 2,098 analytical results, respectively, fulfilled the quality criteria applied and have been used in the assessment. These concern the data reported to EFSA by Member States (97%) and literature data reported for Europe (3%). The proportion of left‐censored data (results below the limit of quantification (LOQ)) was 98.6% in food and 93.9% in feed. For food, the highest mean concentrations of DAS were recorded in the category of ‘cereal‐based dishes’, and for feed in the categories ‘maize grains and maize silage’ and ‘rice, broken’.
No studies on the effect of cleaning, sorting and milling of grains could be identified, but these practices are expected to result in a redistribution and/or reduction of DAS in the final products, as is the case with other trichothecenes.
In humans, the highest mean acute exposure estimates ranged from 17 ng/kg body weight (bw) to 186 ng/kg bw across different surveys and population groups when applying the probabilistic approach. When considering the 95th percentile, the values ranged from 54 to 799 ng/kg bw. The highest values were recorded for ‘infants’ and ‘toddlers’
The estimates of mean chronic exposure to DAS across different dietary surveys and all age groups ranged from 0.2 ng/kg bw per day (lowest minimum lower bound (LB), observed in ‘infants’ and at ages ≥ 18 years) to 185 ng/kg bw per day (highest maximum upper bound (UB) observed in ‘toddlers’) when applying the deterministic approach. Highest values are generally recorded for young people because of high food consumption when expressed per kg bw. The estimates of 95th percentile chronic exposure ranged from 0.4 ng/kg bw per day (lowest minimum LB, observed in ‘elderly’ and ‘very elderly’) to 491 ng/kg bw per day (highest maximum UB observed in ‘other children’). The most important contributors to the chronic dietary exposure to DAS were ‘grains and grain‐based products’, especially ‘cereal‐based dishes’.
Exposure of farm and companion animals to DAS is primarily from consuming cereal grains and cereal by‐products. Due to the lack of data on compound feeds, exposures for all livestock other than poultry were estimated using data for individual feed materials and their assumed inclusion rates in the diets. The highest estimated exposures were for ruminants (dairy cows and beef cattle) fed with maize silage‐based diets. For dairy cows on a maize silage‐based diet, the estimated mean diet concentration ranged from 1.3 (LB) to 43 (UB) μg/kg dry matter (DM), the latter corresponding to a maximum of 1.8 μg/kg bw per day. Estimated exposures for beef cattle on maize silage‐based diets were marginally lower. For horses, the maximum estimated exposure was 0.58 μg/kg bw per day.
Exposures for starter pigs, growing/fattening pigs, finisher pigs and lactating sows were broadly similar, however, not all mean and P95 LB values could be determined. The mean and P95 UB estimates ranged from 11 to 18 and 20 to 32 μg/kg DM, respectively (corresponding to 0.35–0.92 and 0.61–1.6 μg/kg bw per day). For poultry, the highest diet levels were estimated for laying hens. Mean and P95 exposures ranged from non‐determined (LB) to 27 (UB–mean) or 48 (UB–P95) μg/kg DM (corresponding to 1.61 and 2.9 μg/kg bw per day, respectively). For farmed rabbits and mink, the mean and P95 LB estimates were < 1.0 μg/kg DM. The UB values were highest for mink (2.4 and 4.2 μg/kg DM, respectively, for mean and P95 exposures, corresponding to 0.09 and 0.15 μg/kg bw per day). Similarly, the estimated LB mean and P95 for fish (salmonids and carp) were < 1.0 μg/kg DM. Due to the higher levels of cereals in carp diets, UB estimates were highest for carp (25 and 45 μg/kg DM, for mean and P95 estimates, respectively, corresponding to 0.55 and 1 μg/kg bw per day). Finally, for companion animals (cats and dogs), mean and P95 LB estimates were < 1 μg/kg DM, while the UB mean and P95 exposure estimates were higher for cats (7.3 and 13 μg/kg DM, respectively; corresponding to a maximum of 0.20 μg/kg bw per day) than for dogs (5.1 and 9.1 μg/kg DM, respectively; corresponding to a maximum of 0.13 μg/kg bw per day).
No pharmacokinetic data in humans were available from clinical studies after intravenous (i.v.) administration of DAS and no data after oral administration were identified. After oral administration in rats and mice, the absorption of DAS has not been quantified but the excretion ratio between urine and faeces indicated high absorption. DAS was rapidly distributed to most organs. Tissue concentrations decreased rapidly with no apparent accumulation in any tissue and more than 90% of radiolabelled DAS was excreted within 24 h.
In vitro, DAS is metabolised to a large number of metabolites. The main metabolic processes are deacetylation, hydrolysis, deepoxidations and glucuronide conjugation. Deepoxidation reactions were primarily found after incubation with gastrointestinal and ruminal contents, rumen fluids or faeces. Also, in vivo DAS was rapidly metabolised to a large number of metabolites. Although in vivo studies on the toxicokinetics of DAS in farm animals were rare and not all relevant parameters were determined, a rapid absorption, distribution, metabolism and excretion was generally shown. The main systemic metabolites were determined as 15‐monoacetoxyscirpenol (15‐MAS) and scirpentriol (SCT). In pigs, a large portion of orally administered DAS could be found in faeces, mainly as deepoxidised SCT. In chickens, DAS was rapidly absorbed from the gastrointestinal tract, extensively metabolised and excreted as 15‐MAS and 7‐OH‐DAS in faeces and in urine. No data were available for ruminants, horses, dogs and cats, and farmed rabbits, mink and fish.
There was insufficient evidence to conclude on the transfer of DAS from feed to food of animal origin.
When administered orally to rodents, the LD50 values ranged from 2 to 16 mg/kg bw. Haematological effects such as anaemia, leucopenia and thrombocytopenia were observed. When administered i.v., the LD50 ranged from 1 to 12 mg/kg bw, while the LD50 ranged from 0.8 to 23 mg DAS/kg bw after intraperitoneal (i.p.) administration. Repeated dose studies in rodents were scarce and not of sufficient quality for hazard identification and characterisation.
After a single i.v. administration to dogs, DAS induced emesis, diarrhoea, haematological changes and effects in the bone marrow. Repeated dosing by i.v. in dogs and monkeys induced emesis, diarrhoea, body weight loss, erythema, increased haematocrit, anaemia, leucocytosis and/or leucopenia, neutrophilia, lymphopenia, elevated aspartate aminotransferase, alanine transaminase and blood urea nitrogen, and nucleated erythrocytes. The no‐observed‐adverse‐effect level (NOAEL) for emesis and haematological effects after i.v. administration was 31 and 16 μg/kg bw per day for dogs, respectively, and 125 μg/kg bw per day for monkeys.
Developmental and reproductive toxicity was reported after i.p. administration of DAS in experimental animals, and adverse effects were seen in tissues with high proliferation rate. No NOAEL could be identified; however, the CONTAM identified a lowest observed adverse effect level (LOAEL) of 1 mg/kg bw in mice after a single injection based on increase in resorption, reduction in fetal body weight and gross and skeletal malformations. Furthermore, a LOAEL of 1.7 mg/kg bw in rats was identified based on reduced testicular weight and sperm production and an increased frequency of hypocellular seminiferous tubules.
There was no evidence that DAS induces bacterial reverse mutation in vitro. The only in vivo i.p. genotoxicity study in mice reported chromosomal abnormalities in somatic cells (bone marrow) and in germ cells (spermatocytes). Protein synthesis inhibition is likely to be a mechanism underlying the observed in vivo chromosomal abnormalities. The Panel considered that there are currently insufficient data on the genotoxicity of DAS.
No chronic toxicity or carcinogenicity studies were identified.
The limited available toxicological data for the main metabolites of DAS (4‐ or 15‐MAS, SCT) indicate that their toxicity is equal to or less than the toxicity of DAS in vitro and in vivo after oral exposure.
The CONTAM Panel identified adverse health effects in humans exposed to DAS when it was tested as a cytostatic anticancer drug (named anguidine) in phase I and phase II clinical trials on cancer patients by i.v. administration. Based on the data of the phase I studies, the CONTAM Panel identified nausea and vomiting as the most relevant acute adverse health effects of DAS with a NOAEL at 1.2 mg DAS/m2 (equivalent to 32 μg DAS/kg bw). Haematotoxicity and myelosuppression were the most frequently observed and persistent adverse effects observed in the phase I studies when DAS was given repeatedly (5‐day regimen) in treatment cycles of 3–4 weeks. A NOAEL of 2.4 mg DAS/m2 (equivalent to 65 μg DAS/kg bw per day) was identified. The reported adverse health effects at doses from 3 to 5 mg DAS/m2 (equivalent to 81–135 μg DAS/kg bw per day) of the phase II clinical trials, performed at the proximity of the maximum tolerable doses, supported these findings.
DAS is binding to ribosomes, inducing a ‘ribotoxic stress response’ with activation of ribosome‐associated MAPKs and inhibition of protein synthesis. DAS also possesses cytotoxic properties, while no clear indications for reactive oxygen species (ROS) production are available. An increase in gut satiety hormones (e.g. cholecystokinin (CCK)) levels is considered the mechanism of DAS (and trichothecenes) induced anorexia. In vitro assays indicated cytotoxic properties on haematopoietic progenitors which could be due to stimulation of apoptosis or inhibition of protein synthesis.
Data on combined effects of DAS and other mycotoxins in vivo were available mainly in poultry, and only one experiment was performed in pigs. Although limited, the effects observed for DAS in combination with T‐2 toxin, aflatoxin B1, ochratoxin A and fusaric acid were generally more marked than when DAS was administered alone. The CONTAM Panel noted that because of the lack of dose–response data, it was difficult to draw definitive conclusions concerning the nature of the combined effects and interaction with other Fusarium toxins, including type A trichothecenes.
Based on the conclusions on the genotoxicity and the mode of action (MoA) of DAS, the CONTAM Panel decided to establish health‐based guidance values (HBGV) for both the acute and chronic exposure of DAS to humans. The Panel concluded that the database from the oral studies on rats and guinea pigs and the i.v. studies in dogs and monkey was insufficient for the identification of a reference point (RP) for the human hazard characterisation. For both acute and chronic hazard characterisation, data from clinical studies in patients treated by i.v. administration of DAS (anguidine) for cancer were used. Since the data from experimental and farm animals suggest almost complete absorption following oral administration, the Panel under a conservative approach considered an equivalent bioavailability after oral exposure and i.v. dosing. In addition, the Panel noted that following oral exposure DAS is subject to extensive presystemic degradation in the gastrointestinal tract and hepatic metabolisation. Based on limited information showing that DAS metabolites would have similar or lower toxicity than DAS, oral exposure would not lead to higher systemic toxicity than i.v. administration.
The CONTAM Panel identified 32 μg DAS/kg bw as a RP for acute health effects, based on nausea and emesis observed in clinical studies in cancer patients. The Panel then applied the default uncertainty factor of 10 accounting for interindividual toxicokinetic and toxicodynamic variability and derived an acute reference dose (ARfD) of 3,200 ng DAS/kg bw.
The haematotoxic and myelotoxic effects observed in clinical studies were identified as the critical endpoint for human chronic hazard characterisation. The CONTAM Panel concluded that these effects would not be expected in humans at a dose equal or below 65 μg/kg bw per day which was therefore selected as a RP for chronic effects. The CONTAM Panel considered the default uncertainty factor of 10 as adequate to cover for interindividual toxicokinetic and toxicodynamic variability. In addition, the Panel applied an uncertainty factor of 10 to account for the limited duration and the intermittent dosing regimen of the human clinical studies used for the chronic RP selection. Taking the overall uncertainty factor into account, the CONTAM Panel established a tolerable daily intake (TDI) of 650 ng DAS/kg bw per day.
The CONTAM Panel concluded that data were too scarce to identify a RP for adverse health effects of DAS in ruminants. A few studies on adverse effects of DAS in pigs were available. Oral lesions in the gastrointestinal tract were observed following exposure to ≥ 2.0 mg DAS/kg feed, corresponding to 0.08 mg/kg bw per day. Reduced body weight gain was seen at feed concentrations resulting in doses ≥ 0.12 mg/kg bw per day. No NOAEL could be identified from the available data. For poultry, oral lesions were observed at the lowest level of exposure in the following species: chickens, laying hens, turkeys and ducks with LOAELs of 0.010, 0.054, 0.012 and 0.022 mg DAS/kg bw per day, respectively. At equal or higher doses, reduction of body weight gain, decreased eggs production and decreased fertility were other adverse effects observed in various studies in different poultry species.
No data were available on the oral exposure of dogs. Emesis and haematotoxicity were observed following i.v. administration. Assuming equivalent bioavailability and systemic toxicity of DAS following i.v. and oral administration, the CONTAM Panel identified NOAELs of 0.031 and 0.016 mg/kg bw per day for acute and chronic toxicity, respectively.
In addition to ruminants, no toxicity data suitable for hazard characterisation of DAS were identified for farmed rabbits, farmed fish, farmed mink, horses and cats. In order to obtain an indication on the risk of DAS in these species, the CONTAM Panel considered the lowest LOAEL of 0.01 mg DAS/kg bw per day identified for fattening chicken as indicative for potential adverse health effects.
In humans, all mean and 95th percentile exposure estimates were below the established HBGV values (ARfD and TDI), and therefore not of health concern. The impact of the uncertainties in the human risk assessment of DAS is large and the risk is more likely to be over than underestimated.
Because of the limited data for farm and companion animals, health risk characterisation was carried out only for pigs, poultry and dogs. For pigs, the CONTAM Panel noted that the exposure levels in starter and growing/fattening pigs were 1.2% and 2.0%, respectively, of the identified critical LOAEL of 80 μg/kg bw per day. Although the hazard characterisation was based on very limited data on adverse effects, the Panel concluded that the risk for adverse health effects from feed containing DAS is low for pigs at the estimated exposure levels under current feeding practices. For poultry, the CONTAM Panel noted that the higher exposure estimates for fattening chicken was about up to 25% of the lowest LOAEL identified for oral lesions (10 μg/kg bw per day), indicating a possible risk for adverse effects. The risk was considered low for laying hens, fattening turkeys and ducks at the estimated exposure levels under current feeding practices. In the dog, the estimated exposure levels were less than 1% of the identified NOAELs of 31 and 16 μg/kg bw per day for both acute and chronic effects, indicating a low risk for adverse health effects.
The CONTAM Panel noted that the largest available exposure estimate for species where a RP could not be identified (ruminants, horses, cats, farmed rabbit, fish and mink) was in the majority of cases a small fraction of the LOAEL of 10 μg/kg bw per day for oral lesions in poultry (maximum 18% in dairy cows). Therefore, the adverse health effects from feed containing DAS would be unlikely to occur for these farm and companion animals at the levels of exposure estimated for current feeding practices, with the exception of dairy cows fed on maize silage‐based diets where a possible risk may exist. Conclusions for these animal species are affected by a high degree of uncertainty.
In order to decrease the level of uncertainty in the risk assessments, the CONTAM Panel recommends that a well‐designed 90‐day oral toxicity study be undertaken with rats using purified DAS, in accordance with the relevant OECD guidelines and with special focus on the assessment of haematotoxicity, myelotoxicity and reproductive performance. In addition, studies of the toxicokinetics of DAS after oral and i.v. exposure in experimental animals are required, in addition to in vivo studies on the genotoxicity of DAS. More data would also be needed on the cellular and molecular MoA, in particular for a better understanding of cytotoxicity, DNA and protein synthesis inhibition, stimulation of apoptosis and effects on haematopoietic progenitors and bone marrow. Well‐designed dietary studies on adverse effects (including the investigations for oral and gastrointestinal lesions) of DAS in farm animals other than poultry are also recommended.
More occurrence data on DAS in food and feed obtained with state‐of‐the‐art validated analytical methods with adequate sensitivity, such as LC–MS/MS, are needed to also reduce the uncertainty in the exposure assessment for humans and farm and companion animals.
Finally, in consideration of the similar toxicity profiles and structural similarities of several trichothecenes, together with their likely co‐exposure via food and feed, it would be appropriate to perform a cumulative risk assessment for this group of substances.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
4,15‐Diacetoxyscirpenol (DAS) is one of the trichothecene mycotoxins produced by certain species of Fusarium. DAS is considered to be one of the most toxic trichothecenes and it belongs to the group A trichothecenes. DAS is mainly produced by Fusarium langsethiae, F. poae, F. sporotrichioides. and Fusarium sambucinum. DAS has been found to occur in cereals and cereal‐based products and in coffee beans.
1.1.1.1. Available information (not exhaustive)
In accordance with Article 36 of Regulation (EC) No 178/2002, a report “Scientific information on mycotoxins and natural plant toxicants” has been produced following a grant agreement between the European Food Safety Authority (EFSA) and the author(s) of the report (CFP/EFSA/CONTAM/2008/01). The report presents information, inter alia, regarding diacetoxyscirpenol in feed and food and is available on the EFSA website (http://www.efsa.europa.eu/en/scdocs/doc/24e.pdf).
1.1.1.2. Issue
There might be possible risk for animal and public health, related to the presence of 4,5‐diacetoxyscirpenol in feed and food. The European Commission asks EFSA to assess on the basis of the available information the risk for farm animals and public health in order to enable the European Commission and the competent authorities in the Member States to consider the need for a possible follow up including to fill the knowledge gaps.
1.1.2. Terms of Reference
In accordance with Art. 29 (1) of Regulation (EC) No 178/2002, the European Commission asks the European Food Safety Authority to provide a scientific opinion on the risks for public health related to the presence of diacetoxyscirpenol in feed and food.
The assessment should, based upon the available information, assess if the presence of 4, 15‐diacetoxyscirpenol in food and feed is a potential risk for public and animal health taking into account the toxicity of diacetoxyscirpenol and the occurrence in feed and food and to assess possible interactions with other Fusarium toxins, in particular group A trichothecenes, as regards toxicity and occurrence. For the assessment of the risks, the situation for the different farm animal species and the specific (vulnerable) groups of the human population (e.g. high consumers, children, people following specific diets, etc.) should be considered.
1.2. Interpretation of the Terms of Reference
The CONTAM Panel concluded that the terms of reference provided by the Commission were clear.
1.3. Supporting information for the assessment
1.3.1. Chemistry
4,15‐Diacetoxyscirpenol (3α,4β)‐3‐hydroxy‐12,13‐epoxy‐trichothec‐9‐ene‐4,15‐diyl diacetate; Chemical Abstracts Service (CAS) No. 2270‐40‐8; C19H2607; molecular weight 336.405 Da) or anguidine is a type A trichothecene mycotoxin produced by several Fusarium species (Thrane et al., 2004; Lysoe et al., 2016), see Figure 1 and Table 1.
Figure 1.
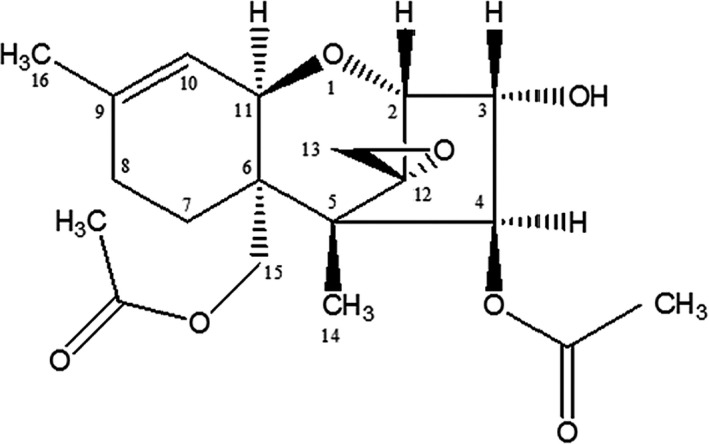
Chemical structure of 4, 15‐DAS
Table 1.
Type A trichothecenes of known relevance for human and animal health
| Compound | Element formula | Molecular weight | logPoct/water a |
|---|---|---|---|
| 4,15‐Diacetoxyscirpenol (DAS) | C19H26O7 | 366 | 0.2 |
| T‐2 Toxin | C24H34O9 | 466 | 0.9 |
| HT‐2 Toxin | C22H32O8 | 424 | 0.4 |
| Neosolaniol | C19H26O8 | 382 | −0.9 |
Data obtained from PubChem repository.
4,15‐Diacetoxyscirpenol (from now onwards indicated as DAS) is naturally present in various crops, in particular cereals. It is a white crystalline compound with a melting point ranging between 162°C and 164°C (Lewis, 1999). The chemical structure of trichothecenes is characterised by a tetracyclic sesquiterpenoid 12,13‐epoxytrichothec‐9‐ene ring with different patterns of substitution around this core. Type A trichothecenes (Table 1) do not have a carbonyl function at C‐8 in contrast to type B trichothecenes with deoxynivalenol as the best‐known example. DAS differs from the type A trichothecene T‐2 toxin only by missing the isovaleryl group at C‐8 (Dellafiora et al., 2017).
Due to the lack of a keto group at C‐8 and less free hydroxyl functions, DAS is less polar compared to the type B trichothecenes (Rodríguez‐Carrasco et al., 2017). DAS is highly soluble in ethyl acetate, acetone, chloroform, methylene chloride and diethyl ether (Battilani et al., 2009) and stable at neutral and acidic pH (Rodriguez et al., 2014). When treated with alkali or dilute solutions of potassium carbonate, sodium hydroxide or ammonium hydroxide DAS is hydrolysed to scirpentriol (SCT)1 (Battilani et al., 2009).
Within the Type A trichothecene group, it is possible to distinguish the scirpentriol subgroup; this comprises SCT, three monoacetoxyscirpenols (MAS), three diacetoxyscirpenols and the completely acetylated triacetoxyscirpenol (Table 2) (Schollenberger et al., 2007, 2011). DAS has been the most studied member of the scirpentriol subgroup.
Table 2.
DAS‐related type A trichothecenes of the scirpentriol subgroup
| Compound | Element formula | Molecular weight | logPoct/water a |
|---|---|---|---|
| Scirpentriol (SCT) | C15H22O5 | 282 | −1 |
| 3‐Monoacetoxyscirpenol (3‐MAS) | C17H24O6 | 324 | −0.4 |
| 4‐Monoacetoxyscirpenol (4‐MAS) | C17H24O6 | 324 | −0.4 |
| 15‐Monoacetoxyscirpenol (15‐MAS) | C17H24O6 | 324 | −0.4 |
| 3,4‐Diacetoxyscirpenol (DAS) | C19H26O7 | 366 | 0.2 |
| 3,15‐Diacetoxyscirpenol (DAS) | C19H26O7 | 366 | 0.2 |
| 4,15‐Diacetoxyscirpenol (DAS) | C19H26O7 | 366 | 0.2 |
| 3,4,15‐Triacetoxyscirpenol (TAS) | C21H28O8 | 408 | 0.8 |
Data obtained from PubChem repository.
Biosynthesis of trichothecenes in the fungi starts with a series of reactions that involve the cyclisation of the isoprenoid‐pathway‐intermediate farnesyl pyrophosphate to trichodiene. This is followed by modification of trichodiene through a series of oxygenation steps to form calonectrin (CAL) among others (Hohn et al., 1993).
The biosynthesis of DAS then proceeds from CAL (Figure 2) to 3,15‐diacetoxyscirpenol (3,15‐DAS) which is acetylated to form 3,4,15‐triacetoxyscirpenol (3,4,15‐TAS). In a subsequent step, 3,4,15‐TAS is deacetylated to DAS (Desjardins et al., 1993; Kimura et al., 2007).
Figure 2.
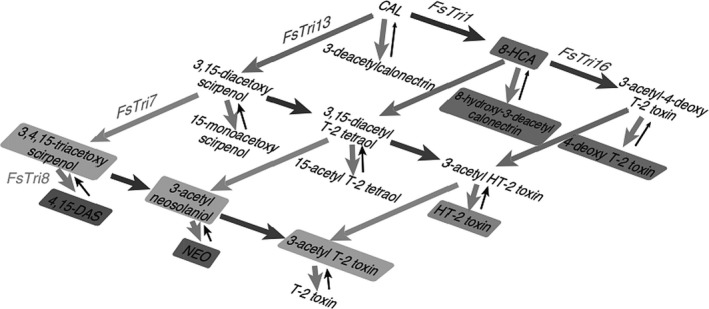
Proposed biosynthetic pathway for DAS and related trichothecenes in Fusarium according to Kimura et al. (2007)2
By deacetylation at C‐4, DAS is transformed into 15‐MAS, which is later deacetylated at C‐15 to form SCT (Perkowski et al., 2003; Schollenberger et al., 2011).
According to Richardson et al. (1989), all eight members of the SCT subgroup are synthesised by F. sambucinum. However, Schollenberger et al. (2011) reported more recently a sequential accumulation pattern of DAS, 15‐MAS and SCT in F. poae and F. sporotrichioides. These observations indicate that at least in in vitro Fusarium culture, the spectrum of DAS related type A trichothecenes of the scirpentriol subgroup may change over time.
Nakagawa et al. (2013a,b) identified DAS‐glucoside and two 15‐MAS‐glucosides as modified forms of DAS and 15‐MAS, respectively, in a contaminated maize powder and using high‐resolution mass spectrometric analysis (Table 3). Although the absolute structure of DAS‐glucoside was not clarified, glucosylation at the 3‐OH position appeared to be the most probable. The two 15‐MAS‐glucosides are isomers.
Table 3.
Modified forms of DAS
| Compound | Element formula | Molecular weight |
|---|---|---|
| Plant modified | ||
| DAS‐glucoside | C25H36O12 | 528 |
| 15‐MAS‐3‐glucoside | C23H34O11 | 486 |
| 15‐MAS‐4‐glucoside | C23H34O11 | 486 |
| Modified through thermal degradation | ||
| DAS‐M1 | C19H28O8 | 384 |
MAS: monoacetoxyscirpenol; DAS: diacetoxyscirpenol.
It was reported that DAS can also be modified through thermal degradation with partly conversion to DAS‐M1, however under conditions that are not representative of household or commercial processing circumstances (Shams et al., 2011). The structure of DAS‐M1 was elucidated with nuclear magnetic resonance (NMR) (Table 3) Shams et al., (2011). While the elucidated structure of DAS‐M1 is new, analogue reaction products were previously reported by Grove and Mortimer (1969).
Both, in vivo and in vitro studies have shown that DAS can be biotransformed in humans and in animals (see Sections 3.1.1 and 3.1.2 on toxicokinetics) to a variety of metabolites (see Yang et al., 2015 and also Table 4). The major metabolic pathways of DAS are hydrolysis, hydroxylation, conjugation and deepoxidation, where deepoxidation takes place in the intestinal tract by microorganisms (Swanson et al., 1987; Fuchs et al., 2002; Yang et al., 2015).
Table 4.
Animal and human phase I and phase II metabolites of 4,15‐diacetoxyscirpenol (DAS)
| Compound | Element formula | Molecular weight |
|---|---|---|
| Phase I metabolites | ||
| 15‐Monoacetoxyscirpenol (15‐MAS), 4‐deacetyl‐DAS | C17H24O6 | 324 |
| 4‐Monoacetoxyscirpenol (4‐MAS), 15‐deacetyl‐DAS | C17H24O6 | 324 |
| Scirpentriol (SCT), bis‐deacetyl‐DAS | C15H22O5 | 282 |
| 8β‐Hydroxy‐DAS (8β‐OH‐DAS) | C19H26O8 | 382 |
| Neosolaniol | C19H26O8 | 382 |
| 7‐Hydroxy‐DAS (7‐OH‐DAS) + isomer | C19H26O8 | 382 |
| Deepoxy‐15‐MAS | C17H24O5 | 308 |
| Deepoxy‐SCT | C15H22O4 | 266 |
| Phase II metabolites | ||
| DAS‐3‐glucuronide | C25H34013 | 542 |
| 15‐MAS‐3‐glucuronide | C23H32O12 | 500 |
| 15‐MAS‐4 glucuronide | C23H32O12 | 500 |
1.3.2. Methods of analysis
1.3.2.1. Sampling and storage
In Commission Regulation (EC) No 519/20143, methods of sampling for the official control of the levels of mycotoxins are laid down. This Regulation No 519/2014 amends the earlier Commission Regulation (EC) No 401/20064. There are no specific requirements or recommendations which should be followed concerning the sampling and the storage of the samples intended for the determination of DAS. However, the same criteria prescribed for other Fusarium toxins should be applied to ensure the reliability of the generated analytical data. Prior to the determination of DAS, a representative sample must be provided. Due to the possible non‐homogeneous distribution of DAS in lots (of grains), sampling may contribute to a significant extent to the variability in analytical results. After sampling, the samples should be stored under appropriate conditions (dry, preferably frozen) until analysis to prevent further Fusarium fungal growth and toxin production.
1.3.2.2. Methods of analysis of DAS
Analytical methods for type A trichothecenes in food, feed and biological samples, including DAS, have been reviewed by Sforza et al. (2006), Zöllner and Mayer‐Helm (2006), Schollenberger et al. (2007), Battilani et al. (2009), Lattanzio et al. (2009) and Berthiller et al. (2017). A limited number of the studies reported in the review papers described analysis of DAS. Other compounds of the scirpentriol subgroup, such as 15‐MAS and SCT were rarely addressed.
The analysis of DAS is mainly part of multicomponent analytical methods (Berger et al., 1999; Asam and Rychlik, 2007; Cavaliere et al., 2007; Malachová et al., 2014; Berthiller et al., 2017). DAS reference standard as well as 13C isotope‐labelled internal standard are commercially available.
Extraction of DAS from different matrices is mostly done with acetonitrile and water (Berthiller et al., 2005; Asam and Rychlik, 2007), or with methanol and water (Cohen and Lapointe, 1984), or with ethylacetate and formic acid (Diana Di Mavungu et al., 2009; García‐Moraleja et al., 2015a,b). In addition, QuEChERS‐based extraction has been described, which is easy to handle (Desmarchelier et al., 2010; Sospedra et al., 2010; Rodríguez‐Carrasco et al., 2013a,b; Tamura et al., 2015). Clean‐up is mainly based on solid‐phase extraction, including MycoSep® and MultiSep® columns (Berger et al., 1999; Klötzel et al., 2005; Asam and Rychlik, 2007; Schollenberger et al., 2007).
Different chromatographic methods have been described in the literature.
Thin‐layer chromatography (TLC) was mainly used in the past but lacks sufficient sensitivity and specificity (Gimeno, 1979; Nowotny et al., 1983; Anaya et al., 2004).
Gas chromatography (GC) followed by flame ionisation detection (FID) (Bata et al., 1983; Furlong and Valente Soares, 1995) and by electron capture detection (ECD) (Cohen and Lapointe, 1984) have been reported. GC coupled to mass spectrometric (MS and MS/MS) detection is still popular (Thrane et al., 2004; Schollenberger et al., 2011; Rodríguez‐Carrasco et al., 2013a,b; Escrivá et al., 2015). However, when using GC, a derivatisation step of the hydroxyl groups is needed to increase volatility and sensitivity. Nielsen and Thrane (2001) described a GC–MS/MS method to detect several trichothecenes simultaneously in Fusarium cultures, including neosolaniol, SCT, DAS, 15‐MAS and 4‐MAS. A two‐dimensional GC–time‐of‐flight‐MS method has also been described for the simultaneous analysis of trichothecenes including SCT, 15‐MAS DAS, and TAS in wheat grain without sample clean‐up (Jelen and Wasowicz, 2008).
High‐performance liquid chromatography coupled to UV detection (HPLC‐UV) is not suitable for DAS because of its low intensity of UV absorption and the need to use a very low wavelength (below 205 nm), unless derivatisation of the hydroxyl groups with a strongly UV absorbing compound is performed (Maycock and Utley, 1985). Also HPLC methods with fluorescence detection of fluorescent derivatives have been developed (Jimenez et al., 2000; Dall'Asta et al., 2004). However, HPLC–MS/MS and ultra‐performance liquid chromatography (UPLC)‐MS/MS have the greatest potential because they can be used for simultaneous detection of several mycotoxins and their metabolites/derivatives and are commonly reported in recent literature for application in all kinds of food, feed and biological matrices (Berger et al., 1999; Schollenberger et al., 2007; Gentili et al., 2007; Njumbe Ediage et al., 2011; Vanheule et al., 2014, 2017). Moreover, high‐resolution mass spectrometry has been used for structural elucidation and quantification of DAS metabolites and modified forms (Rubert et al., 2011; Nakagawa et al., 2013a; Tamura et al., 2015; Yang et al., 2015; Lysoe et al., 2016).
The development of sensitive enzyme‐linked immunoassays (ELISA) for DAS has been reported. Klaffer et al. (1988) obtained a detection limit (LOD) for DAS of about 10 pg/mL using a polyclonal antibody. Monoclonal antibodies (mAb) against DAS were developed by Chu et al. (1984), Pauly et al. (1988) and Hack et al. (1989) with different cross‐reactivities against neosolaniol, 4‐MAS, 15‐MAS, SCT and TAS. Clare Mills et al. (1988) developed an ELISA method for DAS determination in wheat. Mayer et al. (2000) developed a multi‐mycotoxin rapid flow‐through immunoassay for simultaneous detection of seven mycotoxins, including DAS, in wheat and maize.
A comparison of analytical methods and their limits of quantification (LOQ) is shown in Table 5.
Table 5.
Typical examples of the method characteristics and limits of quantification (LOQ) of analytical methods used for the determination of DAS in food and feed
| Analytical technique | Method characteristics | LOQ (μg/kg) | References |
|---|---|---|---|
| TLC | Screening (qualitative – semi‐quantitative) | 2,400–4,000 | Gimeno (1979), Nowotny et al. (1983) |
| ELISA | Screening (qualitative – semi‐quantitative ‐ quantitative) | 5–300 | Clare Mills et al. (1988), Mayer et al. (2000) |
| HPLC‐UV/DAD HPLC‐FLD |
Confirmation (semi‐quantitative – quantitative) Possible multi‐analyte detection |
0.2–800 (= LOD) | Jimenez et al. (2000), Dall'Asta et al. (2004) Omurtag et al. (2007) |
| GC‐FID GC‐ECD |
Confirmation (semi‐quantitative – quantitative) Possible multi‐analyte detection |
25–200 | Cohen and Lapointe (1984), Furlong and Valente Soares (1995), Jimenez and Mateo (1997), Radova et al. (1998), Kotal et al. (1999), Schothorst and Jekel (2001) Schothorst and Jekel, (2003) |
| GC–MS(/MS) |
Confirmation (semi‐quantitative – quantitative) Possible multi‐analyte detection |
4–550 | Schollenberger et al. (1998), Milanez and Valente‐Soares (2006), Rodríguez‐Carrasco et al. (2013a,b), Escrivá et al. (2016) |
| LC–MS(/MS) |
Confirmation (semi‐quantitative – quantitative) Possible multi‐analyte detection |
0.05–125 | Berger et al. (1999), Berthiller et al. (2005), Biselli and Hummert (2005) Sørensen and Elbæk (2005), Diana Di Mavungu et al. (2009), Santini et al. (2009), Desmarchelier et al. (2010), Njumbe Ediage et al. (2011), Yang et al. (2015), Bryła et al. (2014), Capriotti et al. (2014), Malachová et al. (2014), Flores‐Flores and González‐Penas (2015), Dong et al. (2016) |
| LC–HRMS |
Confirmation (semi‐quantitative – quantitative) Possible multi‐analyte detection Identification of unknown compounds |
5–80 | Vaclavik et al. (2010), Rubert et al. (2011), Tamura et al. (2015) |
TLC: thin‐layer chromatography; HPLC: high‐performance liquid chromatography; UV: ultraviolet; DAD: diode array detection; FLD: fluorescence detection; GC: gas chromatography; FID: flame ionisation detection; ECD: electron capture detection; LC: liquid chromatography; MS: mass spectrometry; MS/MS: tandem mass spectrometry; HRMS: high‐resolution mass spectrometry; ELISA: enzyme‐linked immunosorbent assay.
1.3.2.3. Analytical quality assurance: performance criteria, reference materials and proficiency testing for analysis of food
In Annex II of the Regulation (EU) No 401/2006 of 23 February 2006, as amended by the Regulation (EU) No 519/2014 of 16 May 2014, criteria for methods of analysis for the official control of the levels of various mycotoxins are laid down. Performance criteria for methods of analysis of DAS have not (yet) been established. The quality of analytical results regarding accuracy, precision and comparability is essentially linked to the use of reference materials (RMs) and certified reference materials (CRMs). Currently, CRMs are not available for DAS, but non‐certified calibrant solutions of DAS are commercially available. Proficiency tests for the determination of DAS are not organised.
1.3.3. Previous risk and exposure assessments on DAS
Only one scientific risk assessment on DAS in food and/or feed performed by national agencies or national and international independent expert advisory committees was identified by the CONTAM Panel; the recent assessment of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in November 2016 (FAO/WHO technical report 1002, 2017).
For exposure assessment, a total of 11,842 available records in the GEMS/Food contaminants database were analysed by the JECFA. In Europe, the prevalence of DAS in cereals and cereal based food was found to be 1.5%. Low prevalence and low concentration of DAS in European countries were also identified in published literature.
JECFA estimated dietary exposure of DAS using the concentration data from the GEMS/Food contaminants database and the consumption data from the GEMS/Food cluster diets. Using the substitution approach (because of the high proportion of left‐censored data), lower and upper bounds (LB–UB values) were calculated. The highest exposure level in the five worldwide regions for which exposure estimates were available were found in Europe with LB–UB mean and high exposure estimates for adults of 2.8–41 and 5.6–82 ng/kg body weight (bw) per day, respectively. However, the JECFA recognised the huge uncertainty in the exposure estimates due to the high degree of left censorship of these data.
JECFA evaluated previously published toxicological data on DAS and concluded that neither a dose‐effect relationship nor a point of departure could be derived from the limited available toxicological data. The Committee noted (1) the existence of structural similarity between DAS and T‐2/HT‐2 toxin, (2) the similarity of toxic effects of DAS and T‐2/HT‐2 toxin at biochemical and cellular levels, which was consistent with results from in vivo toxicity studies, and (3) the indication for an additive effect of the combined exposure to DAS and T‐2/HT‐2 toxins. JECFA concluded that there was sufficient evidence to support the establishment of a group provisional maximum tolerable daily intake (PMTDI) for T‐2/HT‐2 toxins and DAS. The group PMTDI of 60 ng/kg bw per day established for T‐2/HT‐2 toxins at the fifty‐sixth meeting of JECFA (2001), was considered to be sufficiently conservative to include DAS because of the observation that T‐2 toxin was consistently more potent than DAS when comparing similar in vitro and in vivo endpoints. However, it was recommended to update the JECFA evaluation from 2001 since new toxicity data on T‐2/HT‐2 toxin became available.
In the 2001 JECFA evaluation, the total LB mean dietary exposure of T‐2/HT‐2 toxins from the European population was estimated as 16.3 ng/kg bw per day, but no UB estimate was available at that time. From the total LB dietary exposure estimates of 16 ng/kg bw per day for T‐2/HT‐2 toxin from 2001 and the LB dietary exposure estimates of up to 2.8 ng/kg bw per day for DAS, the Committee estimated a LB mean and high dietary exposure of 19 and 38 ng/kg bw per day (twice the mean), respectively, for the sum of DAS and T‐2/HT‐2 toxin. The Committee concluded that the LB estimates for Europe do not exceed the group PMTDI for T‐2, HT‐2 and DAS. The group toxins exposure was therefore considered of low public health risk concern in the Europe.
1.3.4. Legislation
Worldwide, legal maximum levels (MLs) for DAS in food or feed products are scarce. Canada has a maximum level for DAS in feed for swine at 2,000 μg/kg and in feed for poultry at 1,000 μg/kg. Israel has a maximum level for DAS in all grains for feed at 200 μg/kg (FAO, 2004; Leatherhead Food Research, 2010). In the EU, MLs in food for specific contaminants shall be established if this is necessary to protect public health (Article 2 of Council Regulation (EEC) No 315/93 of February 1993 laying down Community procedures for contaminants in food5). Once adopted, they are laid down in the Annex of Commission Regulation (EC) No 1881/2006. DAS is not included in this Annex. MLs for undesirable substances in feed are laid down in EU Directive 2002/32/EC6. Annex I of this Directive contains MLs of a number of inorganic and organic contaminants in feed. DAS is not regulated under this Directive. While some Fusarium toxins are regulated within Recommendation 2006/576/EC7, in which guidance levels are given for these mycotoxins in certain products intended for animal feed, DAS is not included in the Recommendation.
1.3.5. Other supporting information
The CONTAM Panel noted that several reviews on trichothecenes and Fusarium toxins identified the possible effects of DAS to humans and to several animal species (Ueno, 1983; D'Mello et al. 1999; Conková et al., 2003; Meissonier et al., 2008).
2. Data and methodologies
2.1. Methodology of data collection for supporting information for the assessment
2.1.1. Collection and selection of evidence (search strategy, eligibility criteria) for supporting information
A literature search was carried out for scientific evidence for the Sections 1.3 (Supporting information for the assessment), 3.3.1 (Occurrence data on food and feed reported in the available literature) and 3.4 (Food and feed processing). The collected scientific evidence in these sections, used as background information for the assessment and for contributing data for the exposure assessment (Section 3.3.1), was limited to the most relevant information identified by the experts of the CONTAM Panel Working Group (WG) on Fusarium toxins.
A search for recent reviews was conducted to identify scientific publications dealing with methods of analysis, chemistry, formation in food, exposure and occurrence in food and feed. The literature search was performed in February 2017 and October 2017 for updated information. Web of Science,8 PubMed9 and Embase10 were identified as databases appropriate for retrieving literature for the present evaluation using the word ‘diacetoxyscirpenol’ as the key term word (‘DAS’ was not used as a keyword since it is the acronym of many other unrelated words). The references resulting from the literature search were imported and saved using a software package (EndNote11), which allows effective management of references and citations. Additionally, reviews and relevant scientific evaluations by national or international bodies were considered for the current risk assessment, i.e. JECFA (2016). When relevant papers were identified during the risk assessment process (e.g. from other studies or reviews), they were also considered. The references obtained were screened using title and abstract to identify relevant literature.
2.1.2. Appraisal of evidence for supporting information
The inclusion of studies for the Sections 1.3 (Supporting information for the assessment), and 3.4 (Food and feed processing) was based on consideration by the expert judgement of the CONTAM WG on Fusarium toxins on the extent to which the study was informative and relevant for the assessment and taking account of study quality considerations. With regard to the Section 3.3.1 (Occurrence data on food and feed reported in the available literature), the appraisal and reporting of selected data used for the exposure assessment were in compliance with the quality requirements of EFSA for the occurrence data (see Section 2.3).
2.2. Methodology of data collection for hazard identification and characterisation
2.2.1. Collection and selection of evidence (search strategy, eligibility criteria) for hazard identification and characterisation
A literature search was conducted in scientific databases aimed at identifying relevant studies published in the open scientific literature and in scientific peer‐reviewed journals until 6 February 2017. The collection of scientific studies available in the public domain was done by searching scientific literature databases (Web of Science, PubMed and Embase) using the word ‘diacetoxyscirpenol’ as the key term word (‘DAS’ was not used as a keyword since it is the acronym of many other unrelated words). The search aimed to retrieve as many studies as possible that might be relevant for hazard identification and hazard characterisation of DAS. The search was not limited to the evidence published in English language. No filters were applied regarding language and date of publication. The references resulting from the literature search were imported and managed using a software package (EndNote).
An update of this literature search was conducted on 12 October 2017, in order to retrieve any additional papers published from January 2017.
In addition, on 12 October 2017, a literature search was performed using the diacetoxyscirpenol synonym: ‘anguidine’. In order to avoid duplicates, ‘Diacetoxyscirpenol’ was excluded using the Boolean operator ‘NOT’.
All the references retrieved in February and October 2017 were uploaded in Endnote.
The literature search details are given in Appendix B.
2.2.2. Appraisal of evidence for hazard identification and characterisation
The retrieved evidence was reviewed by the CONTAM WG on Fusarium toxins and has been used for this assessment as considered relevant by expert judgement. Any limitations noted by the WG in the evidence used for the risk assessment of DAS in food and feed are described in this scientific opinion. Selection of the scientific papers considered study quality and the extent to which the study was relevant (e.g. sufficient details on the methodology, performance and outcome of the study, information on dosing and route of administration and details of reporting).
The amount of available data on DAS for different sections of the assessment varied greatly. In a first step, only those data from which it could clearly be concluded that the adverse effects in experimental and farm and companion animals were associated with an oral exposure to DAS alone were included in the sections on hazard characterisation of humans and farm and companion animals. Second, papers reporting oral co‐exposure to DAS and other mycotoxins were included when it was clear from the study description and content that the co‐exposure did not have a substantial impact on toxicity of DAS: for example when the other identified mycotoxins had concentrations that were not considered to induce or notably contribute to the observed adverse effects, or when the other identified mycotoxins were known to have specific adverse effects which could not be attributed to DAS, or when the other identified mycotoxins were not expected to interact with the effects of DAS.
The information retrieved has been screened and evaluated by relevant domain experts from the CONTAM WG on fusarium toxins in food and feed and has been used for the present assessment. Any limitations in the information used are documented in this scientific opinion. Selection of the scientific papers for inclusion or exclusion was based on consideration of the extent to which the study was relevant to the assessment or on general study quality considerations (e.g. sufficient details on the methodology, performance and outcome of the study, on dosing, substance studied and route of administration and on statistical description of the results), irrespective of the results.
List of papers assessed with reason(s) for exclusion by the relevant domain expert is stored in the EFSA document management system.
2.3. Occurrence data on DAS used for the assessment
2.3.1. Data collection and validation
Following an European Commission mandate to EFSA, a call for annual collection of chemical contaminant occurrence data in food and feed, including DAS, was issued by the former EFSA Dietary and Chemical Monitoring Unit (now DATA Unit) in December 2010 with a closing date of 1 October of each year. European national authorities and similar bodies, research institutions, academia, food business operators and other stakeholders were invited to submit analytical data on DAS in food and feed.
The data submission to EFSA followed the requirements of the EFSA Guidance on Standard Sample Description for Food and Feed (EFSA, 2010a); occurrence data were managed following the EFSA standard operational procedures on ‘Data collection and validation’ and on ‘Data analysis of food consumption and occurrence data’.
In the data validation phase, data identified as suspect samples were excluded from the present analysis. Suspect samples are usually samples taken from the same site as a consequence of evidence or suspicion of contamination, and are often taken as a follow‐up of demonstrated non‐compliance with legislation. Some of the remaining samples may also have been collected in a more targeted way (i.e. selective sampling, convenient sampling) (see also Section 2.3.2).
Data on DAS in food and feed available in the EFSA database from 2000 onwards to the end of December 2017 were used for the present assessment. Data received after this date were not included in the data set used for further evaluation for this opinion.
In addition to the occurrence data collected from the Member States within the call for data, the CONTAM Panel also searched the published literature for occurrence data of DAS in food and feed for possible inclusion as additional data in the occurrence data sets submitted to EFSA within the call for data and to be used for the exposure assessment. The literature data were included when they conformed to the most important EFSA requirements on data collection and validation, and the details on country of origin, product, analytical method, LODs/LOQs and occurrence levels (e.g. mean, median) were adequately reported.
2.3.2. Data analysis
Following the EFSA Standard Operating Procedure (SOP) on ‘Data analysis of food consumption and occurrence data’ to guarantee an appropriate quality of the data used in the exposure assessment, the initial data set was carefully evaluated applying several data cleaning and validation steps. Special attention was paid to different parameters such as ‘Sampling strategy’, ‘Sampling method’, ‘Sampling year’, ‘Sampling country’, ‘Analytical methods’, ‘Reporting unit’, ‘LOD/LOQ’, and the codification of the different samples under FoodEx classification.
In the analysis of DAS occurrence data, the left‐censored data (results below LOD or below LOQ) were treated by the substitution method as recommended in the ‘Principles and Methods for the Risk Assessment of Chemicals in Food’ (WHO, 2009). The same method is indicated in the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010b) as an option in the treatment of left‐censored data. The guidance suggests that the LB and UB approach should be used for chemicals likely to be present in the food (e.g. naturally occurring contaminants, nutrients and mycotoxins). The LB is obtained by assigning a value of zero (minimum possible value) to all samples reported as lower than the LOD (< LOD) or LOQ (< LOQ). The UB is obtained by assigning the numerical value of LOD to values reported as < LOD and LOQ to values reported as < LOQ (maximum possible value), depending on whether LOD or LOQ is reported by the laboratory.
2.4. Food consumption
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database) provides a compilation of existing national information on food consumption at individual level. It was first built in 2010 (EFSA, 2011a; Huybrechts et al., 2011; Merten et al., 2011). Details on how the Comprehensive Database is used are published in the Guidance of EFSA (EFSA, 2011a). The latest version of the Comprehensive Database updated in 2018 contains results from a total of 60 different dietary surveys carried out in 25 different Member States covering 119,458 individuals.
Within the dietary studies, subjects are classified in different age classes as follows:
Infants: < 12 months old
Toddlers: ≥ 12 months to < 36 months old
Other children: ≥ 36 months to < 10 years old
Adolescents: ≥ 10 years to < 18 years old
Adults: ≥ 18 years to < 65 years old
Elderly: ≥ 65 years to < 75 years old
Very elderly: ≥ 75 years old
Four additional surveys provided information on specific population groups: ‘Pregnant women’ (≥ 15 years to ≤ 45 years old, Latvia; 17 years old to 46 years old, Portugal) and ‘Lactating women’ (≥ 28 years to ≤ 39 years old, Greece; 18 years old to 45 years old, Estonia).
For chronic exposure assessment, food consumption data were available from 53 different dietary surveys carried out in 22 different European countries. When for one particular country and age class two different dietary surveys were available, only the most recent one was used. This resulted in a total of 38 dietary surveys selected to estimate chronic dietary exposure.
For the acute assessment, recent food consumption data was available for 43 surveys of 25 countries.
In a separate Excel document, Annex A_Table 3, these dietary surveys and the number of subjects available for the acute and chronic exposure assessment are described.
The food consumption data gathered by EFSA in the Comprehensive Database are the most complete and detailed data currently available in the EU. Consumption data were collected using single or repeated 24‐ or 48‐h dietary recalls or dietary records covering from 3 to 7 days per subject. Because of the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading.
2.5. Food classification
Consumption data were classified according to the FoodEx classification system (EFSA, 2011c). FoodEx is a food classification system developed by EFSA in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing the exposure to hazardous substances. The system consists of a large number of individual food items aggregated into food groups and broader food categories in a hierarchical parent–child relationship. It contains 20 main food categories (first level), which are further divided into subgroups having 140 items at the second level, 1,261 items at the third level and reaching about 1,800 end‐points (food names or generic food names) at the fourth level.
2.6. Feed consumption
DAS is predominantly found in cereal crops, cereal grains and in by‐products of cereal processing, all of which are widely used as feed for farm animals in Europe. They may be included as ingredients of manufactured complete feedingstuffs, or fed directly as individual feeds to livestock. In 2015, more than 90 million tonnes of cereals and cereal by‐products were used in the manufacture of compound feeds, accounting for 60% of all feed materials used, almost all of which (> 95%) are grown or produced in the EU. In addition, a further 51 million tonnes of cereal grains and by‐products were fed in on‐farm mixes or as single ingredients. However, there are no industry data on the partition of these cereal grains between livestock species (cattle, pigs, poultry, etc.).
There is considerable variation in both the feeds used and the feeding systems adopted for farm livestock, companion animals and fish throughout Europe. This variation is largely due to the availability of feeds and market demands for specific animal products, the quality of the feeds available and nutritional needs of the animals concerned. For many livestock, part or all of the daily ration is provided in the form of manufactured compound feeds and, where data on levels of DAS in species‐specific compound feeds are available, these have been used to estimate exposure. However, for most of the livestock categories information on levels in compound feeds has not been given, or insufficient data have been provided to allow reliable estimates of exposure to be made. Therefore, data on individual feed materials (see Appendix E) and estimates of intake have been used to estimate exposure. It should be stressed that these do not represent ‘average’ diets, nor are the feeding systems ‘typical’ for all of Europe. Instead, they are used to estimate levels of exposure to DAS that might be indicative. They are based on published guidelines on nutrition and feeding (AFRC, 1993; Carabano and Piquer, 1998; NRC, 2007a,b; Leeson and Summers, 2008; OECD, 2009; McDonald et al., 2011; EFSA FEEDAP Panel, 2012) data on EU manufacture of compound feeds (FEFAC, 2009) and expert knowledge of production systems in Europe. For companion animals (cats and dogs), information on typical diet formulations have been provided by The European Pet Food Industry.
Details of feed consumption of farm and companion animals and the rations used are given in Appendix E.
2.7. Feed classification
Feeds were classified based on the catalogue of feed materials specified in the Commission Regulation (EU) No 68/2013 as amended by 2017/201712 creating the Catalogue of feed materials. Where information was available, compound feedingstuffs were classified in groups based on the species/production categories for which the feed is intended.
2.8. Methodology for exposure assessment for DAS
2.8.1. Methodology for DAS exposure assessment in humans
The CONTAM Panel estimated acute and chronic exposure to DAS for all age groups (see Section 3.4). The food categories represented by either very low number of samples (≤ 6 samples) or by all data left‐censored on FoodEx Level 2 were considered not being suitable and were not used in exposure calculation.
For matching the occurrence and the consumption data, standard dilution factors commonly used in EFSA opinions were applied in case of coffee and cereal‐based foods which are to be reconstituted.
The proportion of left‐censored data after excluding the categories containing 100% left‐censored data was generally very high (97%). The chronic dietary exposure cannot be performed accurately if a large proportion of left‐censored data is included (WHO, 2009; EFSA, 2011b). Therefore, the large proportion of left‐censored data and the limited available data add uncertainty to the chronic dietary exposure assessment. Since this was the case for most of the food categories, the results of the present assessment should be interpreted with caution. It should be noted that with a high proportion of left‐censored data, the exposure is likely to be underestimated with the LB approach, whereas it may be highly overestimated with the UB approach (see also Section 4).
2.8.1.1. Acute dietary exposure
Acute dietary exposure to DAS was estimated using a probabilistic approach. For calculating acute dietary exposure to DAS, food consumption and body weight data at the individual level were accessed in the Comprehensive Database. The acute dietary exposure to DAS was calculated for each reporting day, since individual meals are recorded for only a few countries in the consumption database. The preferred option is, therefore, to use individual days of consumption. Days of consumption offer a conservative estimate of the exposure, since it will sum the contribution of all meals during the same day.
Acute exposure was assessed for each reporting day by multiplying the total consumption amount for each food category by an occurrence level randomly drawn among individual results available for that food category. Respective intakes of the foods consumed that day were summed and finally divided by the individual's body weight. This process was iterated 500 times for each day of consumption reported by each participant. For the calculations, occurrence data estimated using the UB approach was used. The UB approach is a conservative approach, which better reflects the purpose of an acute exposure compared to the LB approach. For each of these endpoints, the 95% confidence interval was defined as the 2.5th and 97.5th percentiles obtained from the 1,000 iterations. All analyses were run using the SAS Statistical Software (SAS enterprise guide 5.1), including the modelling of the probabilistic acute exposure.
2.8.1.2. Chronic dietary exposure
As suggested by the EFSA Working Group on Food Consumption and Exposure (EFSA, 2011a), dietary surveys with only 1 day per subject were not considered for chronic exposure as they are not adequate to assess repeated exposure. Similarly, subjects who participated only 1 day in the dietary studies, when the protocol prescribed more reporting days per individual, were also excluded for the chronic exposure assessment. Not all countries provided consumption information for all age groups, and in some cases the same country provided more than one consumption survey.
For calculating chronic dietary exposure to DAS, food consumption and body weight data at the individual level were accessed in the Comprehensive Database. Occurrence data and consumption data were linked at the relevant FoodEx level.
The mean and the high (95th percentile) chronic dietary exposures were calculated by combining DAS mean occurrence values for food samples collected in different countries (pooled European occurrence data) with the average daily consumption for each food at individual level in each dietary survey and age class. Consequently, individual average exposures per day and body weight were obtained for all individuals. On the basis of distributions of individual exposures, the mean and 95th percentile exposure were calculated per survey and per age class. Dietary exposure was assessed using overall European LB and UB mean occurrence of DAS. The contribution (%) of each food category to overall mean chronic exposure of DAS was calculated for each age group and dietary survey. Estimations of chronic exposure using the LB approach, which is considered to be less influenced by results below LOD/LOQ, were used to explain the contribution of the different food categories.
All analyses were run using the SAS Statistical Software (SAS enterprise guide 5.1).
2.8.2. Methodology for DAS exposure assessment in farm and companion animals
Commercially manufactured compound feeds (as complete or complementary feedingstuffs) are important – and frequently the sole – feeds for livestock and companion animals. Ideally, levels of DAS in these feeds should be used, together with estimates of intake (given in Appendix E) to estimate exposure. However, in this Opinion data were only available for poultry starter feeds.
For all other species, the mean and 95th percentile (high) exposures have been made using the levels of DAS in individual feed materials, estimates of their inclusion in the diets of the animals feed consumed and levels of intake. Details on the diet compositions and feed intakes for each livestock category are given in Appendix E. It should be stressed that these do not represent either ‘average’ or ‘extreme’ diets, nor are the feeding systems ‘typical’ for all of Europe. Instead, the diets are used to estimate levels of exposure to DAS that might be indicative. They are based on published guidelines on nutrition and feeding (AFRC, 1993; Carabano and Piquer, 1998; NRC, 2007a,b; Leeson and Summers, 2008; McDonald et al., 2011; EFSA FEEDAP Panel, 2012; OECD, 2013), and expert knowledge of production systems in Europe. Details of the rations used feed intakes and live weights assumed are given in Appendix E.
DAS generally occurs in cereals crops, cereal grains and by‐products of cereal processing, both for human food and biofuel production, and these may account for 60% or more of the diet of farm and companion animals. However, diets also include a wide range of other feed materials, particularly vegetable proteins and by‐products of food manufacture, but since no data are available on levels of DAS in these feeds it has not been possible to estimate the contribution they make to exposure to DAS.
For ruminant livestock and horses, forages represent essential ingredients in their diets. With the exception of maize silage, no data on the presence of DAS in forages were available and therefore it is assumed that, with the exception of maize silage‐based diets forages make no contribution to the exposure to DAS.
According to EFSA (2011a,b,c), caution is needed when calculating acute exposure (95th percentile) where data on less than 60 samples are available, since the results may not be statistically robust. Therefore, in this Opinion estimates of 95th percentile have not been made where data on < 60 samples are available.
2.9. Methodology for risk characterisation
The CONTAM Panel applied the general principles of the risk assessment process for chemicals in food as described by WHO (2009), i.e. hazard identification and characterisation, exposure assessment and risk characterisation. Several EFSA guidance documents were applied in the assessment of DAS in food and feed listed in Appendix A.
3. Assessment
3.1. Hazard identification
3.1.1. Toxicokinetics in experimental animals and humans
3.1.1.1. Absorption
Absorption of DAS after oral administration has not been quantified in experimental animals or humans. However, the excretion ratio in urine vs faeces was 4.5:1 in mice and in rats indicating that a high proportion of DAS is absorbed, see the subsection on excretion below.
3.1.1.2. Distribution
Wang et al. (1989) administered a single oral dose of 0.55 mg 3H‐labelled DAS/kg bw intragastrically to rats and a slightly higher dose of 0.66 mg DAS/kg bw to mice. Four animals of each species were euthanised after 90 min, 24 h and 7 days. No visible signs of toxicity were observed. The distribution patterns, expressed as percentage of the dose, were very similar in mice and rats, with rapid excretion (from 75% to 95%) in the first 24 h. After 7 days, only 1–3% was found in carcass and organs. At 24 h, distribution mainly in intestine, spleen, thymus, femur and testis (mouse) was recorded. The Panel noted that the radioactivity levels were higher and the decrease slower in known target organs compared to other organs.
The radioactivity in the brain was low, but decreased relatively slowly compared to other tissues. Differences in tissue concentrations in rats and mice were small. The mice generally tended to have higher concentrations in kidneys and liver as well as in heart and lymphoid tissues and rats tended to have higher concentrations in the small intestine.
3.1.1.3. Metabolism
In vitro
DAS was incubated with faecal microbiota from rats in a study which also considered cattle, pigs, chickens, horses and dogs (see Section 3.1.2) (Swanson et al., 1988). In rats, DAS was completely transformed to deepoxy MAS (66.5%) and deepoxy SCT (33.5%). In incubations with rat caecal content, DAS was completely transformed to deepoxy MAS (81.9%), deepoxy SCT (17.8%) and SCT (0.3%).
DAS was deacetylated in the position 4 to form 15‐MAS in microsomal incubations with rat or rabbit liver microsomes (Ohta et al., 1978). The in vitro velocity was about six times lower after incubation with liver microsomes from rats than from rabbits. No peaks other than of DAS and MAS were seen in the chromatograms and the sum of DAS and MAS was almost equal to the initial amount of DAS. Carboxylesterases isolated from CD‐1 mouse liver microsomes deacetylated DAS in the position 4 to form 15‐MAS (Wu and Marletta, 1988).
Isolated rat liver was perfused with 2 mg of DAS and the bile was collected and analysed for metabolites with and without enzymatic treatment with glucuronidase (Gareis et al., 1986). In the bile, 340 μg MAS‐glucuronide and 10 μg SCT were found. This approach did, however, not allow a determination of the positions for hydroxylation and glucuronidation reactions to occur.
Yang et al. (2015) performed a comparative study of the in vitro phase I and phase II metabolism of DAS using liver microsomes from rats, chickens, pigs, goats, cows and humans. DAS was incubated with liver microsomes and incubates were later analysed by UHPLC‐QTOF. DAS was extensively metabolised in microsomal incubates from all these species. The main phase I metabolic transformations were hydrolysis (deacetylation) in C‐4 and/or C‐15 positions and hydroxylation in C‐8 or C‐7 positions (Figure 3). The main phase II metabolites found were DAS‐3 glucuronide, 15‐MAS 3 glucuronide and 15‐MAS‐4 glucuronide. The metabolite patterns varied between species. Human liver microsomes had the highest ability to metabolise DAS. 15‐MAS was the main metabolite in the six species studied. It was particularly high in human microsomes. Incubations with rat liver microsomes produced seven phase I metabolites while incubations with human liver microsomes produced five. 4‐MAS was only detected in incubations with rat liver microsomes and only in trace amounts. Three phase II metabolites were described in this in vitro study, namely, DAS‐3 glucuronide, 15‐MAS‐3‐glucuronide and 15‐MAS‐4‐glucuronide All three were detected in incubations with both rat and human liver microsomes. DAS‐3‐glucuronide was the main conjugate in incubations with human liver microsomes (also in the case of pigs, goats and cows), while 15‐MAS‐3‐glucuronide was the main metabolite in incubations with rat liver microsomes.
Figure 3.
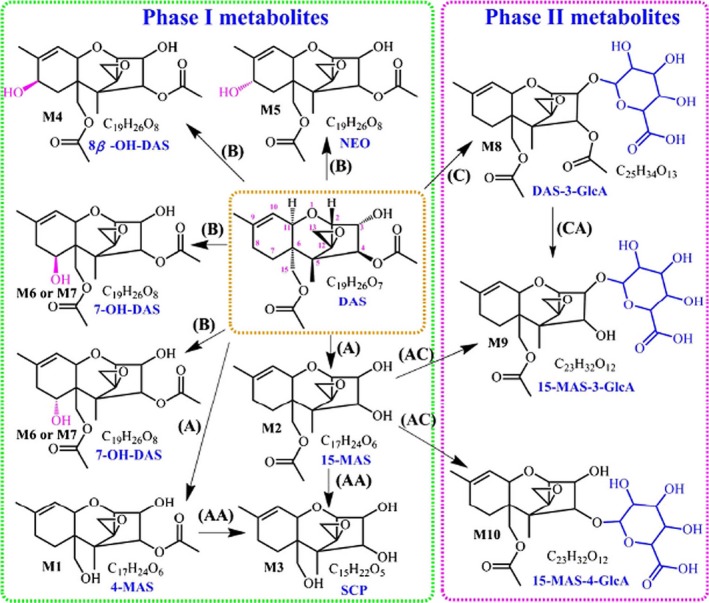
Proposed phase I and II metabolic pathways of 4, 15 – DAS: (A) hydrolysis, (B) hydroxylation, (C) glucuronidation ‐ from Yang et al. (2015)13
In vivo
Male Wistar rats were given three oral doses of 2.8 mg DAS/kg bw on days 0, 7 and 14 (Sakamoto et al., 1986). Urine and faeces were collected daily for 21 days. MAS, SCT, ‐deepoxy MAS and deepoxy SCT were detected in the urine, while only the deepoxide metabolites were detected in the faeces.
Yang et al. (2015) gave a single oral dose of 3 mg DAS (purity > 98%)/kg bw to fasting rats (n = 3/sex) with the aim to verify that the metabolites found in vitro also are formed in vivo. Urine and faeces were collected 0–12 h and 12–24 h post‐dosing. DAS was found together with four metabolites, 4‐MAS, 15‐MAS, neosolaniol and 7‐OH‐DAS in the urine samples collected in the time period 0–12 h post‐dosing, while only 15‐MAS and 7‐OH‐DAS were detected in the faeces. Consistent with the in vitro results, 15‐MAS and 7‐OH‐DAS were the main metabolites found. No metabolites could be detected in the samples collected 12–24 h post‐dosing. In contrast to in vitro studies, no glucuronide conjugates were detected neither in faeces nor in urine. No deepoxide metabolites were detected in this study.
3.1.1.4. Excretion
Urine and faeces samples were collected in rats and mice up to 7 days after a single oral dose of respectively 0.55 or 0.66 mg 3H‐labelled DAS/kg bw (Wang et al., 1989, see also the subsection on distribution above). DAS was rapidly excreted and 93.7% and 90.3% of the dose was excreted in rats and mice, respectively, during the first 24 h. The urinary to faecal excretion ratio was 4.5 in both species. The excretion was accompanied by a corresponding decrease in radiolabelled DAS in gastrointestinal (GI) content, organs and carcass. The radioactivity in the animals levelled out during the following 6 days, indicating that a small fraction of DAS remained in the tissue for a prolonged time period.
3.1.1.5. Summary
In vitro, DAS is metabolised to a large number of metabolites. The main metabolic processes are deacylations, hydroxylations, deepoxidations and glucuronide conjugations. Deepoxidation reactions have primarily been found after incubation with GI content or faeces.
After oral administration in rats and mice, the absorption of DAS has not been quantified but the excretion ratio in urine and faeces indicated high absorption. After absorption, DAS was rapidly distributed to most organs. Tissue concentrations decreased rapidly with no apparent accumulation in any tissue and more than 90% of radiolabelled DAS was excreted within 24 h, with an approximate 80% via urinary excretion. Only 2–3% of orally administered DAS was estimated to remain in the body after a few days. DAS was rapidly metabolised to a large number of metabolites in vivo.
3.1.2. Toxicokinetics in farm and companion animals
A few studies conducted in farm animals either in vitro or in vivo have been identified, and as a result only limited data in a few farm animal species were available. For companion animals, few in vitro data were available for horses and dogs.
3.1.2.1. In vitro studies
Few studies on the in vitro biotransformation of DAS in farm animals have been identified. DAS was incubated for 12, 24 and 48 h under anaerobic conditions in rumen fluids (Swanson et al., 1987). MAS, SCT and their deepoxide metabolites were detected in the incubates. Deepoxy‐DAS was not detected.
In another experiment (see also Section 3.1.1.3), these authors incubated DAS with faecal microbiota from cattle, pigs, chickens, horses and dogs (Swanson et al., 1988). One mg DAS was incubated with 2 mg of faeces or intestinal suspensions for 4 days at 37°C. DAS was completely transformed in the incubations from pigs and cattle, primarily to deepoxy MAS (62% and 32%) and deepoxy SCT (24% and 40%), while smaller amounts of MAS (13% and 2%) and SCT (2% and 4%) were also detected. In addition, 8% of unmetabolised DAS was found in the incubations from cattle. In contrast to this, no deepoxide metabolites of DAS could be detected in faecal incubations from horses, dogs or chickens. However, de‐acetylated metabolites MAS and SCT were found in the faecal incubations from these species, together with unmetabolised DAS.
The rapid conversion of DAS by anaerobic rumen microorganisms to metabolites has been shown in several studies.
The in vitro biotransformation by bovine rumen fluid resulted in the recovery of 24.1% of DAS (as administered), 21.4% of deepoxy‐15‐MAS, 25.7% of SCT and 15.1% of deepoxy‐SCT. DAS was not detected after 48 h of incubation (Swanson et al., 1987). Isolated bacteria cultured from ovine rumen fluid (Matsushima et al., 1996) as well as isolated protozoa from ovine rumen fluid (Kiessling et al., 1984) were capable to deacetylate DAS to 15‐MAS. The protozoa were more active than the bacteria (Kiessling et al., 1984).
Gut microbiota from catfish (Ameriurus nebulosus) which are known to transform DON to its deepoxy metabolite, was also incubated with 50 μg DAS/mL for 96 h (Guan et al., 2009). After the incubation, 54% of the added toxin was present as MAS deepoxide (form not specified), 42% as DAS and 4% as MA (form not specified).
3.1.2.2. In vivo studies
Ruminants
The only available in vivo toxicokinetic study on DAS in ruminants showed a rapid distribution and metabolism after i.v. administration of 0.5 mg DAS/kg bw to four heifers (body weight 74.5–139.1 kg) (Coppock et al., 1987). The volume of distribution was calculated as 1.9–3.5 L/kg, the total body clearance (TBC) as 162.6–502.8 mL/min per kg and the t1/2 (half‐life) in blood as 3.8–10.5 min. DAS was rapidly metabolised to 15‐MAS and SCT, 15 min after i.v. administration only 0.14 ng/mL of DAS being detected in urine, while 15‐MAS amounted to 2.10 ng/mL and SCT to 1.17 ng/mL.
Pigs
Three studies on toxicokinetics of DAS in pigs were available. Bauer et al. (1985) observed a rapid absorption of DAS after a single oral dose of 2 mg/kg bw in female pigs (n = 4, mean bw 20 kg) The highest amounts of DAS (9.6–21.9 ng/mL) were detected within 0.5–1 h in blood serum. The peak concentrations of the metabolite 15‐MAS (5.9–13.2 ng/mL) were measured after 0.5–4 h, while the highest values of SCT (1.6–14.8 ng/mL) appeared after 0.5–6 h. In a follow‐up report, these authors noted that following oral administration of 2 mg DAS per kg bw, the amount of DAS and its metabolites excreted in the urine was low, in contrast to faeces, where much higher values were found (Bauer et al., 1989; Bauer, 1989). Within 72 h, deepoxidised MAS and SCT amounted to 30 and 1,975 μg, respectively, while 42 μg of MAS and 583 μg of SCT were found. The highest proportion was detected in the first 6 h after administration. Regarding the resulting toxicokinetic parameters it should be taken into account, that up to 26% of the administered dose was eliminated, partly presystemic hydrolysed to 15‐MAS (2.3% on average), by vomiting (Bauer et al., 1989). Furthermore, the quantification of the deepoxide metabolites in the faeces was performed by re‐measuring the samples of only one pig (personal communication).
Coppock et al. (1987) surveyed the pharmacokinetics of DAS following i.v. administration. Two groups of pigs (25–49.2 kg) received 0.5 (n = 4) or 1 (n = 3) mg DAS/kg bw over 48 h, respectively, after halothane anaesthesia. The t1/2 (half‐life) in blood varied in the group dosed 0.5 mg/kg between 7.5 and 150.7 min and the TBC between 27.25 and 191.67 mL/min per kg. DAS was extensively metabolised to MAS and SCT, the highest urinary concentrations 15 min after i.v. administration being 0.51 (DAS), 0.80 (MAS) and 10.4 ng/mL (SCT). In plasma, no DAS or MAS could be found 8 h after i.v. administration, while 240 μg/kg of SCT was detected.
Chickens
Only one study was found on the in vivo metabolism in chickens. Yang et al. (2015) administered a single dose of DAS (> 98% pure) of 3 mg/kg bw to three male and three female Avian chickens (1.0–1.2 kg) by gavage. During the 0‐ to 12‐h period after administration, 15‐MAS and 7‐OH‐DAS were detected in the faeces and urine using a LC–MS method. In the following period of 12–24 h post‐administration, no metabolites were detected.
Regarding phase II metabolites, no glucuronide conjugates were detected. The authors concluded that DAS is rapidly absorbed from the GI tract and extensively metabolised and excreted in chickens (Yang et al., 2015).
3.1.2.3. Summary
Although in vivo studies on the toxicokinetics of DAS in farm animals are rare and not all relevant parameters have been determined, a rapid absorption, distribution, metabolism and excretion (ADME) of this mycotoxin was generally shown. In pigs, the majority of DAS and its metabolites were excreted in faeces within one day and plasma peaks of DAS and its metabolites were quickly reached. Following i.v. administration, the half‐life in blood was very short (less than 30 min) and total body clearance was fast. Highest urinary concentrations were seen 15 min after i.v. administration. Neither DAS nor MAS were found in plasma 8 h after i.v. administration. The main systemic metabolites were determined as 15‐MAS and SCT. In pigs, a large portion of orally administered DAS could be found in faeces, mainly as deepoxidised SCT.
The data of only one study in chickens receiving a single oral dose at 3 mg DAS/kg bw, indicated that DAS is rapidly absorbed from the GI tract, extensively metabolised and excreted as 15‐MAS and 7‐OH‐DAS in faeces and 7‐OH‐DAS in urine. No glucuronide conjugates were detected.
3.1.3. Transfer
Only one study was found on the transfer of DAS. Yang et al. (2013) developed a LC–MS method for the detection and determination of DAS and T‐2 and HT‐2 toxins in chicken tissues (heart, liver, spleen, lung, kidney, glandular stomach, muscular stomach, small intestine, muscle, bone and brain). The method was applied following a 6‐week period feeding of two 42‐day‐old chickens with a diet prepared from corn samples inoculated by F. poae producing T‐2 toxin, HT‐2 toxin and DAS. The toxin contents in feed were mentioned as low but the levels were not reported by the authors. The concentrations of DAS in the various tissues were not detected except in the heart and the brain. However, because of the unknown DAS levels in feed and daily consumption by the two birds, no percentages of transfer could be calculated.
No data were available for possible transfer to milk.
The CONTAM Panel considered that there is insufficient evidence to conclude on the transfer of DAS from feed.
3.1.4. Toxicity in experimental animals
3.1.4.1. Acute toxicity
The CONTAM Panel identified several studies which characterise the oral acute toxicity of DAS in rodents as well as acute toxicity after i.v. exposure in other experimental animal species which were tested in preclinical studies for the development of DAS (named anguidine) as a cytostatic anticancer drug. The oral, intraperitoneal (i.p.) and the i.v. routes were considered relevant to describe the acute toxicity (LD50 values summarised in Table 6).
Table 6.
Acute toxicity with DAS LD50 values associated with oral, intravenous and intraperitoneal exposure in rodents and experimental animals
| Species (gender) | Origin and purity of DAS | Doses tested (mg/kg bw) | LD50 (mg/kg bw) | Reference |
|---|---|---|---|---|
| Oral treatment | ||||
| Male CD‐I mouse | ND | 2–22 |
15.5 12 |
Conner et al. (1986), Ueno (1983) |
| Rat | ND | ND | 7.3 | Ueno (1983)a |
| Guinea pig | Crystalline (no more information) | 0, 1, 2, 4, 8 | 2.14 | Kriegleder (1981) |
| Intravenous treatment | ||||
| Mouse | ND | ND | 12 | Ueno (1983)a |
| Rat | ND | ND | 1.3 | Ueno (1983)a |
| Rabbit | ND | ND | 1.0 | Ueno (1983)a |
| Dog | ND | ND | 1.1 (approximate) | Ueno (1983)a |
| Intraperitoneal treatment | ||||
| Male CD‐I mouse | ND | 2–22 | 20 | Conner et al. (1986) |
| Mouse | ND | ND | 23 | Ueno (1983) |
| Rat | ND | ND | 0.75 | Ueno (1983) |
The CONTAM Panel noted that the data from Ueno (1983) are not precisely described in the publication and references were incomplete, it was noted that some LD50 values were extracted from Stähelin et al. (1968) who reported LD50 values after i.v. exposure in mouse, rat, rabbit and dogs in which death occurred after 48 h (except in rabbits, after 4 days). Apathy, reduced breathing, cyanosis, diarrhoea, bloody stool, vomiting, tremor and tachycardia were observed.
Oral administration
Mice
Conner et al. (1986) performed an acute toxicity study in male CD‐I mice. DAS was administered to groups of four to six mice by gastric gavage with doses from 2 to 22 mg/kg bw, doubling the starting dose and later increasing it by a factor of 1.5. The LD50 at 96 h was calculated as 15.5 mg/kg bw. Lethal doses induced intestinal necrosis and extensive necrosis of lympho‐haematopoietic organs. Sublethal doses of DAS induced cell depletion and necrosis in lympho‐haematopoietic organs, multifocal necrosis of intestinal epithelium, and diffuse necrosis of germinal epithelium followed by progressive tubule degeneration in the testes. Leucocytosis, due to both lymphocytosis and neutrophilia in the first few hours after exposure to DAS, followed by lymphopenia, neutropenia and anaemia by 3 days were noted. There was rapid recovery of all sensitive organs after exposure to sublethal doses of DAS except for testis where decreased weights and abnormal spermatogenesis persisted for the 2‐week observation period.
Rats
In order to investigate adverse effects in oesophagus and stomach, female Porton Wistar rats were fed DAS via oral route (gastric gavage) with single doses of 0.0125, 0.06, 0.125, 0.5 and 2.0 mg DAS/kg bw and the vehicle dimethyl sulfoxide (DMSO) alone as control (Craddock et al., 1988) with n = 8 animals per group and sacrificing twp rats per day on days 1–4, respectively, starving overnight before. An increase in cell replication 1 day after treatment in the oesophagus and in the squamous and glandular stomach was observed at the highest dose when compared with the control group. This effect increased further by day 2, and returned to normality by day 4. At the lowest dose, no increase in replication was observed in the oesophagus, but still in the squamous and glandular stomach at the doses from 0.06 to 0.5 mg/kg bw, with maximum increase after 1 day of treatment. At 0.06 mg/kg bw, the response was doubtful and at 0.0125 mg/kg bw staining of cells gave results similar to those of control animals.
Guinea pigs
Kriegleder studied five groups of 5–6 guinea pigs that received vehicle (control group) or DAS at 1, 2, 4 and 8 mg/kg bw by gastric gavage (Kriegleder, 1980, 1981). Lethality was observed at 24 h following the treatment at all doses with exception of the lowest dose where all six animals survived until day 4. Mortality and time to death were clearly dose dependent: at maximum 16 and 22 h at the highest doses of 8 and 4 mg/kg bw and between 24 and 96 h at 2 mg/kg bw. At the lowest dose, all six animals survived by 96 h and no necrosis was observed. The LD50 was identified at 2.14 mg/kg bw. Signs of GI toxicity were noted. On day 4, all surviving animals underwent haematological investigations followed by euthanasia with collection and histological examination of selected organs. The erythrocyte and leucocyte counts and the haemoglobin levels of a total of 12 animals were analysed in the four dose groups (n = 4, 5, 2 and 1 at 1, 2, 4 and 8 mg/kg bw). The authors’ summarised the findings as follows: in animals from each of the different dosage groups, an increased leukocyte count was observed compared to the starting value. An increase in the number of neutrophils and a decrease in the small and large lymphocytes was recorded. Erythrocyte count and haemoglobin concentration appeared to be affected following a single dose of DAS. There were no other significant changes in blood values in the remaining animals.
Intravenous administration
Dogs
A preclinical study in support of anticancer drug development was performed in a total of 18 beagle dogs. One male and one female dog per group were exposed to a single injection of 0, 0.031, 0.063, 0.125, 0.25, 0.5, 1, 2 mg DAS/kg bw by i.v. (IRDC, 1973) with the exception of the group exposed to 1 mg DAS/kg bw where two males and two females were used. Complete haematological and biochemical investigations were performed every second day after treatment in the first week and weekly thereafter. One dog from each dosage level was sacrificed on day 8 (except at the 1 mg/kg dosage level, where two dogs were sacrificed) and the remaining dog(s) at each dosage level were sacrificed on day 45. At the highest dose, emesis, diarrhoea, slight tremors, licking of chops, injection of sclera and ataxia were noted for both dogs. At the lowest dose, no adverse effects compared to control were observed. Emesis, diarrhoea and haematological changes (e.g. neutropenia and lymphopenia) appeared from the dose of 0.063 mg/kg bw onwards and the frequency and severity of the toxicity was dose dependent. At the highest dose, the female dog was found dead 21 h after treatment. Moderate to marked increases in liver enzymes (e.g. alanine transaminase (ALT) and aspartate aminotransferase (AST)), haematocrit and nucleated erythrocyte count, leucocytosis, neutrophilia with increase in non‐segmented neutrophils, blood urea nitrogen (BUN) were recorded. Lymphopenia and thrombocytopenia were noted in the male dog. All values had essentially returned to normal by day 8 of study for this dog. According to the authors, the dose without adverse effect (no‐observed‐adverse‐effect level (NOAEL)) was 0.031 mg/kg bw.
Coppock et al. (1989) performed a study on dogs. A total of eight dogs (four animals per dose group) were exposed to the vehicle or 0.5 mg/kg bw of DAS (purity > 98%) i.v. The animals were euthanatised at 8 h after treatment. Blood samples were taken at half‐hour intervals. Sequential clinical signs of intoxication observed in the treatment dogs included ptyalism (hypersalivation), emesis, diarrhoea, ataxia, muscular weakness and depression. Histopathological investigation revealed lesions in the bone marrow of treatment animals consisting of cellular necrosis in the haematopoietic districts. A marked increase in the number of immature neutrophils and replacement of lymphocytes with immature cells was observed.
Mice, rats and rabbits
Stähelin et al. (1968) reported LD50 values after i.v. exposure of mice, rats, rabbits and dogs (Table 4) with death after 10–48 h except rabbits where some died after 4 days (also reported in Ueno, 1983). Apathy, reduced breathing, cyanosis, diarrhoea, bloody stool, vomiting, tremor and tachycardia was observed.
Intraperitoneal administration
Ueno (1983) reported about LD50 identified after i.p. administration in the mouse and the rat and Conner et al. (1986) in the mouse only (Table 4). Values were ranging from 20 to 23 in mice or 0.75 mg DAS/kg bw in rats.
In summary, DAS showed a high acute toxicity in rodents with oral LD50 levels ranging from 2.14 to 15.5 mg/kg bw, while i.v. and i.p. LD50 levels ranged between 1.3 and 12 or 0.75 and 23 mg/kg bw, respectively. In dogs, a NOAEL of 0.031 mg DAS/kg bw was identified from a single i.v. dose study.
3.1.4.2. Repeated dose toxicity studies
Oral administration
Rats
Moré et al. (1990), exposed male Sprague–Dawley rats orally by oesophageal intubation to 0.1 mg/kg bw per day for 2 days. Treatment with DAS induced changes in the reactivity of gastric glycoproteins compared to controls. Histochemical analysis of the glycoproteins produced by mucus‐secreting cells in the rat stomach showed that they were slightly modified by short‐term treatment. No microscopic lesions were noted in any treated animal.
Van Rensburg et al. (1987) studied male Wistar rat (30 animals/group) exposed to 1 mg/kg bw by gastric intubation three times weekly over a period of 5 weeks. The erythrocyte counts and related parameters (i.e. haematocrit and haemoglobin) appeared to be the most sensitive ones to the toxic action of DAS. A statistically significant increase in the proportion of larger platelets was observed in the treated animals. The leucocyte counts and related parameters were unaffected by the treatment. Lymphocyte karyorrhexis and increasing numbers of granulocytes in the thymic cortex and moderate or mild atrophy in the spleen were observed. Atrophy was also observed in the bone marrow and lymph nodes while the bone marrow showed a predominance of myeloid cells and depletion of megakaryocytes. Signs of lymphoid‐tissue atrophy in the small intestine were also recorded.
In studies from Craddock et al. (1986, 1987, 1988), the effect of DAS on oesophagus and stomach mucosa at different concentrations and with different durations was assessed. However, the studies present several limitations such as low number of animals per group, single dose levels per experiment, high doses tested, high mortality, and the absence of control and combinations of these across the studies. Consequently, the Panel considered that these studies could not be taken into account for hazard identification.
Guinea pigs
Guinea pigs (4 animals per group) received DAS by drinking water, over 30 days in daily doses of 0, 0.6, 1, 1.3 and 1.6 mg/kg bw (Kriegleder, 1980, 1981). With exception of the highest dose, feed intake and body weight gain were not significantly different from controls, although reduction in body weight gain was observed (up to 20%). All animals died in the highest dose group within 10 days and one animal per group died at the other dose levels (accidental death). Weekly haematological investigations were performed and bone marrow smears taken at the end of observation period. The authors reported emesis, loss of appetite and reduced movement after the second day of treatment at the highest dose. An episode of lips necrosis was noted. In the animals that died from day 5 onwards histological signs of necrosis in GI tract, lymph nodes and bone marrow were noted. No significant changes in haematological parameters (n = 8 animals evaluable in the three dose groups) were noted.
Intravenous administration
DAS has been considered as a possible anticancer drug and it has been tested in preclinical studies by i.v. administration in dog and monkeys according to the supposed clinical schedule (5 consecutive days or weekly administrations – IRDC report, 1973) to support the clinical development. In addition, DAS has been also tested by i.v. earlier on in rats, dogs and monkeys (Stähelin et al., 1968).
Rats
Rats (10 males and 10 females per group) were exposed via i.v. for 4 weeks to 0.06, 0.18 and 0.54 mg DAS/kg bw per day (Stähelin et al., 1968). Mortality was observed at low dose (single death) and up to 75% of rats in the high‐dose groups. Adverse effects observed with dose‐relationship included weight reduction, diarrhoea, haematuria, reduced testis and liver weight. Histopathological examination showed findings in the liver (necrotic foci, bile duct and reticuloendothelial cells proliferation), in spleen (follicle hypoplasia) and testis (tubule atrophy and blocked spermatogenesis). Decrease in leucocytes count and minor reduction in erythrocytes count have been observed after 4 weeks starting from animals exposed to the middle dose and with dose dependency. Effects in bone marrow have been noted only at high dose level (e.g. decrease of granulopoiesis).
Six rats were additionally treated by i.v. for 6 weeks with doses increasing during the study from 0.15 up to 0.5 mg DAS/kg bw with treatment‐free periods – no further details available (Stähelin et al., 1968). No mortality was recorded and only haematological effects (e.g. variable degrees of leucopenia and lymphopenia) were observed.
Dogs
In a study performed by Stähelin et al. (1968), four dogs per group were treated via i.v. daily for 4 weeks with 0, 0.02, 0.06 and 0.18 mg DAS/kg bw per day. At the highest dose, three dogs died during the treatment period and vomiting after each administration, bloody stool and weight loss was recorded. No adverse effects were observed at the lowest dose. A decrease in body weight was noted from 0.06 mg/kg bw day. According to the authors, no haematological changes were observed at the lowest dose, while lymphopenia and erythroblastosis in bone marrow appeared from the middle dose. In the survived dog at the highest dose, leucopenia, follicular hyperplasia in spleen, reduction of number of haematopoietic cells in bone marrow were noted. At low doses, it was histologically observed reduction of lymphatic tissue in spleen, proliferation of reticuloendothelial cells and degenerated in follicle germ cells, atrophy and reduced maturation in testis and pyknotic cells in intestinal epithelium. Overall, the CONTAM Panel concluded that in this study the lowest dose of 0.02 mg DAS/kg bw can be considered as a NOAEL.
Daily treatment by i.v was carried out in two beagles (1 male and 1 female/group) during 5 days at dose equal to 0, 0.016, 0.031, 0.063, 0.125 and 0.25 mg/kg bw per day in a preclinical study (IRDC report, 1973) in support to anticancer drug development. Haematological investigations and liver function tests were periodically performed. One dog from each dosage level was sacrificed on day 12 and the remaining dog at each dosage level was sacrificed around day 50 of the observation period of the study. At the lowest dose, no adverse effects compared to control were observed. From 0.031 mg/kg bw per day, the presence of nucleated erythrocytes at haematology together with neutrophilia, lymphopenia (11% and 37% lymphocyte in male and female respectively compared to normal values 20–52%) and signs of slight anaemia, were noted for both dogs. Erythema and emesis after dosing were sporadically noted in dogs exposed to 0.063 mg/kg bw per day. Haematological troubles increased with the increase in the doses. According to the authors, the dose without toxic effect was the lowest dose tested, 0.016 mg/kg bw per day. Overall, the CONTAM Panel concluded that in this study the lowest dose of 0.016 and 0.031 mg DAS/kg bw can be considered as a NOAEL for haematotoxicity and emesis, respectively.
Daily treatment by i.v. was performed in beagle dogs (1 male and 1 female/group) during 5 days followed by 9 days of rest, the treatment was repeated three times (IRDC report, 1973). The doses tested were 0.031 and 0.125 mg/kg bw per day. Complete haematological and biochemical investigations were periodically performed. One dog from each dose level was sacrificed on day 40 of the study and the remaining dog was sacrificed on day 78 at the end of observation period. Emesis was noted at the high dose. At laboratory examinations slight increases in leucocytes, neutrophils, eosinophils and hepatic enzymes (ALT, AST) together with decreased haematocrit were recorded. At the highest dose instead, haematological effects (namely leucopenia, neutropenia, relative lymphocytosis, thrombocytopenia, polychromasia of erythrocytes and slight anaemia) were noted with different severity in both dogs. Slight elevation in hepatic enzymes was also recorded during treatment periods. Overall, the CONTAM Panel concluded that in this study the lowest dose of 0.031 mg DAS/kg bw can be considered as a NOAEL for emesis.
Weekly treatment by i.v. was also performed in beagle dogs (2 animals per group) during 6 weeks at doses of 0.031, 0.063, 0.125 and 0.25 mg/kg bw (IRDC report, 1973). Haematological and biochemical investigations were regularly performed during the treatment period. One dog from each dosage level was sacrificed on day 43 of study and the remaining dog was sacrificed on day 81 of study, at the end of the observation period. At the lowest dose, the only effects noted were slight and sporadic and consisted in anaemia, leuco‐ and thrombocytopenia, reticulocytosis and elevation of liver enzymes. From 0.063 mg/kg bw per week, emesis and slight erythema post‐dosing were frequently noted for the male dog and occasionally for the female dog. More marked anaemia, neutropenia and lymphopenia were noted at laboratory investigations with some dose. Overall, the CONTAM Panel concluded that also in this study the lowest dose of 0.031 mg DAS/kg bw can be considered as a NOAEL for emesis.
Monkeys
Two rhesus monkeys were treated with DAS by i.v. daily administration for 4 weeks with DAS in a preliminary study. The dose administrated increased after each week by the factor of 3, starting with 0.01 mg DAS/kg bw per day up to 0.27 mg DAS/kg bw per day in week 4 (Stähelin et al., 1968). A third monkey served as a control animal. No deaths were observed. Histological changes were comparable with those observed in rats and dogs. It is reported by the author that no unexpected toxic effects were noted. Taking into account the peculiarity of the experimental design (with dose escalation), it is not possible to identify a NOAEL in this study.
DAS has been injected daily intravenously to rhesus monkeys (1 male and 1 female/group) during five consecutive days at doses equal to 0, 0.125, 0.25, 0.50 and 1.0 mg/kg bw per day in a preclinical study (IRDC report, 1973) for anticancer drug development. Complete haematological and biochemical investigations were carried out at regular intervals. One monkey from each dosage level was sacrificed on day 12 and the remaining monkey at each dosage level was sacrificed on day 50 of study. At the lowest dose (0.125 mg/kg bw per day), no adverse effects compared to control were identified. At the dose of 0.25 mg/kg bw per day, during the treatment period, emesis, anorexia, soft stool or diarrhoea and hypoactivity were noted. During and immediately after the 5‐day of treatment, a decrease in the absolute number of leucocytes (up to 70% vs basal values) with lymphopenia and neutropenia was recorded. A change in leucocyte formula was also noted with neutrophils reaching the highest value of 85% (standard values ranging from 7% to 47%) and lymphocytes the lowest value of 14% (with a standard value ranging from 52% to 92%) with similar trend in both animals. At the dose of 0.5 mg/kg bw per day, both monkeys died on day 4. Death was preceded by hypothermia, anorexia, emesis and hypoactivity. At laboratory examinations leucocytosis (twice the basal values) with marked neutrophilia (> 90% of the total leucocytes) was observed in both monkeys. At the highest dose (1 mg/kg bw per day), both monkeys died on day 3. At laboratory, examinations similar but more marked changes in comparison with the dose of 0.5 mg/kg bw per day were recorded for both monkeys (with leucocytes showing values three times the basal values and marked neutrophilia, > 90% of the total leucocytes). Slight increases in some biochemical parameters (namely ALT, AST and BUN) before the death of animals were recorded.
The CONTAM Panel noted that under the experimental conditions applied in this study the lowest dose tested, 0.125 mg/kg bw per day, can be considered as a NOAEL.
Table 7.
Repeated dose intravenous toxicological studies in dogs and monkeys
| Species (number of animals per group) | Dosage (mg/kg bw per day) | Observation (days) | Adverse effects | NOAEL emesis (mg/kg bw per day) | NOAEL haemato‐ toxicity (mg/kg bw per day) | Reference |
|---|---|---|---|---|---|---|
| Dog (1 male, 1 female) | 4 weeks 0.02, 0.06 and 0.18 | 28 | Emesis, bloody stool and weight loss, blood disorders lymphopenia and erythroblastosis | 0.02 | 0.02 | Stähelin (1968) |
| Beagle Dog (1 male, 1 female) | 5 daily injections 0, 0.016, 0.031, 0.063, 0.125, 0.25 | 52 | Leucopenia, neutrophilia and lymphopenia were described as emesis, erythema and polydipsia | 0.031 | 0.016 | IRDC report (1973) |
| Beagle Dog (1 male, 1 female) | 5 daily injections three times with 9 days of rest0.031, 0.125 | 75 | Leucopenia, neutropenia, relative lymphocytosis, thrombocytopenia, polychromasia of erythrocytes | 0.031 | [LOAEL 0.031] | |
| Beagle Dog (1 male, 1 female) | Weekly injection during 6 weeks 0.031, 0.063, 0.125, 0.25 | 43, 81 | Emesis and slight erythema post‐dosing, neutropenia, anaemia, lymphopenia | 0.031 | [LOAEL 0.031] | |
| Rhesus Monkey 1 male and 1 female/group | 5 daily injections 0,0.125,0.25, 0.50, 1.0 | 12, 50 | Emesis, polydipsia, diarrhoea, erythema, increased haematocrit, anaemia, leucocytosis and/or leucopenia, neutrophilia, lymphopenia, elevated ALT and AST activity, elevated BUN and nucleated erythrocytes | 0.125 | 0.125 | IRDC report (1973) |
ALT: alanine transaminase; AST: aspartate aminotransferase; NOAEL: no‐observed‐adverse‐effect level; BUN: blood urea nitrogen; LOAEL: lowest observed adverse effect level.
3.1.4.3. Chronic toxicity
No chronic toxicity studies have been identified in the literature.
Summary
DAS showed acute toxicity in rodents with oral LD50 ranging from 2.1 to 15.5 mg/kg bw. Haematological effects such as anaemia, leucopenia and thrombocytopenia have been observed. When administered i.v., the LD50 resulted to be slightly lower, ranging from 1 to 12 mg/kg bw.
The oral repeated dose toxicity studies performed in rodents are very few and not sufficiently described to be used for identifying a reference point (RP) for the hazard characterisation. However, in guinea pigs, organs with a high rate of cell proliferation such as oesophagus mucosa, small intestine, haematopoietic bone marrow have been identified as a target of adverse effects of DAS; emesis was also noted.
In the well‐conducted and documented repeated dose preclinical toxicity studies performed by i.v. route in dogs and monkeys, the signs of toxicity of DAS (named anguidine) were fairly consistent for both species on the various dosing schedules and included emesis, diarrhoea, erythema, increased haematocrit, anaemia, leucocytosis and/or leucopenia, neutrophilia, lymphopenia, elevated AST and ALT, elevated BUN and nucleated erythrocytes.
The lowest dose without toxic effect (NOAEL) regarding emesis and haematological adverse effects was equal to 0.016 mg/kg bw per day for dogs and to 0.125 mg/kg bw per day for monkeys.
The CONTAM Panel noted that adverse effects observed in preclinical studies performed by i.v. with DAS were similar to those described in acute toxicity studies after oral administration. DAS as other trichothecenes are known to induce leucopenia, agranulocytosis, anaemia, aplastic anaemia due to cytotoxicity on circulating blood cells and on haematopoietic progenitors cells, the source of blood cells renewing in bone marrow (Parent‐Massin, 2004; EFSA CONTAM Panel, 2011, 2017; JECFA, 2016).
3.1.4.4. Developmental and reproductive toxicity
The studies that have been reported on the developmental and reproductive toxicology of DAS all used i.p. administration of the compound.
DAS was injected i.p. to male mice at doses of 1, 5, 10 or 15 mg/kg bw and animals were killed 1 h to 14 days later. Testicular weights were decreased 3 days after DAS exposure (15 mg/kg bw) and this effect persisted throughout the observation period of 14 days. There was progressive depletion of germinal epithelium which was followed by tubule degeneration (Conner et al., 1986).
Conner et al. (1990) investigated the testicular function in male Lewis rats that had been exposed to DAS at 1.7 mg/kg bw by i.p. injection. This dose is 75% of the i.p. LD50 as measured in the authors’ laboratory. The animals were studied up to 90 days after exposure. DAS induced a decrease of the testicular weight and sperm production and an increased frequency of hypocellular seminiferous tubules. These effects became more marked with increasing time after administration of DAS.
Pregnant mice were administered a single i.p. dose of DAS at 1.0, 1.5, 2.0, 3.0 and 6.0 mg/kg bw on one of gestation days 7–11 (Mayura et al., 1987). The two highest doses resulted in maternal toxicity. There was no significant effect on total number of implants at all dose levels tested during the various gestation periods. The incidence of resorption was dependent on dose, reaching 100% at the higher doses, and increases were seen at the lower doses. The exposure caused a reduction in fetal body weight even at the dose of 1.0 mg/kg bw, on all the days tested. Significant incidences of gross and skeletal malformations in mouse foetuses were observed at all doses of DAS tested (1–3 mg/kg bw). The authors commented that DAS is a potent inhibitor of protein synthesis and that this may be responsible for the effects induced by it.
Summary
Developmental and reproductive toxicity has been reported for studies where DAS was administered by the i.p. route to experimental animals. The lowest dose where effects were seen in mice was 1 mg/kg bw (females, incidence of resorption, reduction in fetal body weight and gross and skeletal malformations), and in rats it was 1.7 mg/kg bw (males, decrease of the testicular weight and sperm production and an increased frequency of hypocellular seminiferous tubules). No lower doses were used and thus no NOAELs were observed. The CONTAM Panel noted that tissues with high proliferation rate such as testicular are targets of adverse effect of DAS.
3.1.4.5. Genotoxicity
In vitro assays
Wehner et al. (1978) showed that DAS did not induce mutation in the Salmonella Typhimurium bacterial mutation assay (Ames test) using strains TA98, TA100, TA1535 and TA1537 (0.25–250 μg DAS/plate) with and without metabolic activation with an induced rat liver S9 fraction.
Kuczuk et al. (1978) obtained negative data in the S. Typhimurium bacterial mutation assay (Ames test) using strains TA1535 and TA1537 and TA1538 (0.1–100 μg DAS/plate) with and without metabolic activation with induced rat liver S9 fraction. DAS was also screened for mutagenic activity in Saccharomyces cerevisiae D‐3 and none was detected: DAS was tested in this study at the concentration 100 μg/mL without metabolic activation, and at 50 μg/mL with metabolic activation.
Sinsheimer et al. (1989) found that DAS lacked alkylating activity as measured by its reaction with 4‐(4‐nitrobenzyl)pyridine, and also lacked mutagenicity using S. Typhimurium strain TA 100 (0.01–15 μmol DAS/plate).
The genotoxicity of DAS in Escherichia coli was investigated by Krivobok et al. (1987) using the SOS chromotest, which is an assay to detect the SOS response. No SOS inducing activity was detected, either with or without metabolic activation. The maximal concentration of DAS that was tested was 60 μg/ml.
Cooray (1984) studied the effect of DAS on DNA synthesis, by measurement of [3H]‐thymidine incorporation, and sister chromatid exchange (SCE) frequency in phytohaemagglutinin‐stimulated human peripheral lymphocytes. Addition of DAS to the cell cultures resulted in a dose‐related inhibition of [3H]‐thymidine incorporation, with complete inhibition at a concentration of 8 ng DAS/mL. Toxicity was slightly reduced by addition of rat liver cells to the incubation. No increase in SCE frequency was observed either with or without the presence of rat liver cells (1.5–12.0 ng DAS/mL).
In vivo studies
The genotoxicity of DAS following i.p. injection was investigated in male Swiss albino mice by Hassanane et al. (2000). For both single and repeat dose studies, groups of mice (n = 5) received 0, 0.5, 0.75 and 1 mg DAS/kg bw. In the repeat dose study, the dose was administered on days 1, 10 and 20. The study was carried out on both somatic and germ cells. After single doses, DAS significantly reduced the mitotic activity of the bone marrow cells at all doses tested. Increased structural chromosome abnormalities (chromatid gaps, breaks, centromeric attenuation, endomitosis) were observed after single doses. The increases of centromeric attenuation, endomitosis and total abnormalities were highly significant (p < 0.01) for the two highest doses of DAS. After repeat doses, increased chromatid gaps, breaks and endomitosis were also observed. Finally, there was evidence of chromosome damage in spermatocytes, and sperm abnormality after DAS treatment.
DAS gave negative results in the wing somatic mutation and recombination test (SMART) in Drosophila melanogaster, at concentrations ranging from 5 to 40 μM (Gürbüzel et al., 2015).
Summary
In summary, there was no evidence that DAS induces bacterial reverse mutation in vitro. One in vivo genotoxicity study in mice has been reported where DAS was administered by the i.p. route. Chromosomal abnormalities were observed in somatic cells (bone marrow) and in germ cells (spermatocytes). It is probable that the impairment of DNA synthesis is caused as a secondary event of inhibition of protein synthesis. Protein synthesis inhibition is likely to be the mechanism underlying the observed in vivo chromosomal abnormalities (see Section 3.1.8).
The Panel considered that there are currently insufficient data on the genotoxicity of DAS.
3.1.4.6. Carcinogenicity
Lindenfelser et al. (1974) carried out a study on the initiating and promoting activity of mycotoxins in a 22‐week skin tumour test according to Boutwell (1964) with a focus on the interactions of aflatoxin B1 with T‐2 toxin and DAS. Groups of Charles River female mice (n = 8) were treated, by skin application, with the initiating compound and then after 4 days promoter compounds were administered twice weekly for 22 weeks. The compounds that were tested for either initiation or promotions were aflatoxin B1, T‐2 toxin and DAS, and positive controls were 7,12‐dimethylbenz[a]anthracene (DMBA) (initiation) and croton oil (promotion). A variety of compound combinations were studied, and three of these involved DAS. These were: DMBA initiation (50 μg) with DAS promotion (10 and 25 μg), aflatoxin B1 initiation (25, 50 or 100 μg) with DAS promotion (10 and 25 μg) and DAS initiation (25 μg) with DAS promotion (10 μg). In addition T‐2 ability to act as initiator (25 μg) or promoter (10 and 25 μg) was also investigated. After DMBA initiation and DAS promotion there was a minimal tumour response (1 papilloma in a single mouse). Similarly, after DMBA initiation, one of the eight mice treated with T‐2 developed one papilloma. No papillomas were seen in mice after aflatoxin B1 initiation and DAS or T‐2 promotion. The group administered DAS (or T‐2) over both the initiation and promotion stages developed no papillomas. After DMBA initiation and promotion with the positive control croton oil (1,000 μg) all eight mice had papillomas. After aflatoxin B1 initiation (25, 50 and 100 μg) and croton oil promotion, papillomas were observed in 5, 5 and 6 out of 8 mice, respectively. The authors concluded that there is an indication of weak promoting activity of DAS.
Summary
In summary, no long‐term studies on carcinogenicity have been identified to assess carcinogenicity of DAS. No initiating activity was identified for DAS.
3.1.4.7. Toxicity of metabolites
The toxicity of metabolites of DAS was reviewed by JECFA (2016). The only few data available indicate that the toxicity of the main metabolites of DAS (4 or 15 MAS, SCT) is equal to or less than the toxicity of DAS in vitro (JECFA, 2016; Thompson and Wannemacher, 1986) and in vivo after oral exposure (JECFA, 2016; Thompson and Wannemacher, 1986; Richardson and Hamilton, 1990; Ademoyero and Hamilton, 1991a,b). The observed clinical symptoms after exposure to the metabolites are also similar to the symptoms observed after exposure to DAS.
3.1.5. Adverse effects in farm and companion animals
3.1.5.1. Ruminants
Following single i.v. administration of DAS at 0.5 mg/kg bw (according to the authors equivalent to 25 mg/m2 body surface) to four heifers (body weight 74.5–139.1 kg) animals showed a variety of clinical signs of acute intoxication like ptyalism, fragmentary defecation, abdominal pain, ruminal stasis, diarrhoea, muscular weakness, congestion of mucous membranes, and coma (Coppock et al., 1989). Anuria was not observed and death did not occur. In the same study, haematological changes were monitored. The numbers of metarubricyte, myelocytes, metamyelocytes, and band‐neutrophiles VII were significantly elevated at that dose compare with controls (n = 4). Changes in other parameters like the number of segmented neutrophils and morphologically abnormal blood cells in blood from principal heifers, vacuolated and prominent chromatin strands in the nuclei of myelocytes and metamyelocytes were occasionally observed. Moreover, lesions were found in bone marrow sections from principal calves.
A diet of 5.0 mg DAS/kg feed (corresponding to 0.16 mg DAS/kg bw per day considering the reported intake and initial body weight) for 35 days resulted in a statistically significant reduced feed intake and weight loss in ewe lambs compared with controls (n = 6 per group, 3 lambs in 3 pens, initial mean bw 38–39 kg) (Harvey et al., 1995). Furthermore, the treatment with DAS decreased of about 23% or 32% urea nitrogen and serum cholinesterase, respectively.
Summary
Among ruminants only two small studies on heifers and lambs were identified. Both indicated adverse health effects: in the heifers at the only single tested dose of 0.5 mg DAS/kg bw per day i.v., while in the lambs at the oral repeated dose corresponding to 0.16 mg DAS/kg bw per day. The CONTAM Panel concluded that the data were too scarce to determine a RP for adverse health effects of DAS in ruminants.
3.1.5.2. Pigs
The acute toxicity of DAS in pigs was studied by Weaver et al. (1978). Twelve crossbred fattening pigs weighing from 8 to 48 kg and a 30‐months‐old sow (115 kg) were administered DAS (95% pure in ethanol) i.v. in doses varying from 0.30 to 0.50 mg/kg bw. Seven animals from the test group died within 24 h, the other ones were killed after 2–14 days and underwent haematological and biochemical analysis and bone marrow examination. In this study, the mycotoxin occasionally induced haemorrhagic bowel lesions (two feeder pigs) and mucosal congestion of the jejunum and ileum in a sow. Furthermore, a variety of clinical signs consisting of emesis, lethargy, signs of extreme hunger, frequent defecation of normal stools and posterior paresis have been observed. The i.v. LD50 of DAS in swine was calculated as 0.376 ± 0.043 mg DAS/kg bw.
To investigate the subchronic toxicity in pigs, Weaver et al. (1981) fed purified DAS (94–96% pure) in two separate experiments to male, weanling crossbred pigs (7.1–9.1 kg bw). In the first experiment, the animals were given rations containing 0 (n = 1), 2, 4, or 8 (n = 2 each), or 10 mg/kg (n = 1) of DAS. After 4 weeks, three pigs (one each at 2, 4 and 8 mg DAS/kg feed) were euthanised, the other pigs were euthanised after 9 weeks. In the second experiment, five pigs were fed for four weeks with 4 mg DAS/kg feed, one pig was used as control. Exposure to DAS caused multifocal, proliferative, gingival, buccal and lingual lesions at all dose levels. The small intestine showed glandular and mucosal cell hyperplasia. Furthermore, feed intake and weight gain were decreased at all DAS concentrations used. At 10 mg/kg feed, a total feed refusal was observed. For the observed lesions, the feed intake and the reduced weight gain, a lowest observed adverse effect level (LOAEL) of 2 mg/kg feed could be identified. Assuming a feed efficiency of 2 kg/kg resulting in 37.6 and 14.8 kg final weights of the two pigs, respectively, an intake of about 0.08 mg DAS/kg bw was considered plausible for this experiment.
The toxicity of DAS in pigs was also surveyed by Harvey et al., 1991. The mycotoxin (> 98% pure) was incorporated in the feed of 9‐week‐old crossbred barrows (mean weight 25.0 kg, n = 9) for 28 days at a concentration of 2.0 mg DAS/kg feed. The diets of the control group (n = 9) did not contain detectable (< 10 μg/kg) concentrations of aflatoxin (AF), DAS, T‐2 toxin, zearalenone, deoxynivalenol, patulin, penicillic acid, ochratoxin, or cyclopiazonic acid. Body weight gain and serum iron binding capacity were significantly decreased in the DAS group and such 2.0 mg/kg feed (corresponding to 0.12 mg/kg bw) was identified as LOAEL for these effects.
Coppock et al. (1985) administered DAS (96% purity, solved in ethanol) i.v to 12 pigs (9 female and 3 male, n = 4 per group) at single doses of 0.0 (ethanol only), 0.5, and 1.0 mg/kg bw (according to the authors equivalent to 0, 16.5 and 32.9 mg/m2 body surface, respectively), with mean bw per group of 32.6, 36.6 and 35.5 kg respectively. The authors used i.v. administration to what they called ‘ensure uniform dosing’. One male died after 4 h at the low dose and another at 7 h at the high dose. All surviving animals were euthanatised after 8 h. Clinical signs of intoxication observed in the DAS administered pigs included ptyalism, emesis, diarrhoea, ataxia, mahogany flushing of the skin, muscular weakness, and depression. The haematologic changes included necrosis of bone marrow haematopoietic elements, a sequential increase in the type and number of abnormal cells, a marked left shift in the neutrophil population. Furthermore, metarubricytes and large platelets were found and lymphocytes were replaced with immature cells.
Summary
Only few studies on adverse effects of DAS in pigs were available. An i.v. LD50 was identified at 0.38 mg DAS/kg bw. An oral LOAEL of 2.0 mg DAS/kg feed could be identified for oral lesions (corresponding to about 0.08 mg/kg bw) and reduced body weight gain (corresponding to 0.12 mg DAS/kg bw) as most sensitive effects.
3.1.5.3. Poultry
All studies from the literature in which DAS toxicity was investigated in poultry were performed via oral administration. Description of acute adverse effects including the determination of an LD50, were based on results from either a single dose or multiple daily doses of DAS. Other studies including chronic toxicological experiments were based on the use of doses below the identified LD50 values administered over several consecutive days.
Chickens
Acute Toxicity
The first study on LD50 determination identified in the literature was performed by Chi et al. (1978). Pure DAS (authenticity determined by combined GC–MS) in solution was administered by gastric intubation to one day old chickens as single dose. The oral 7‐day LD50 was 3.82 mg DAS/kg bw. Clinical signs started with asthenia, feed refusal and diarrhoea 4–10 h after dosing followed by coma before death.
Hoerr et al. (1981a) performed two trials on acute toxicity. In the first trial, pure commercially purchased DAS was dissolved at appropriate concentrations, added to the feed (presence of other mycotoxins not reported) and administered by gavage to 7‐day‐old male broiler chickens (Hubbard x Hubbard) at doses of 1, 2, 3, 4, 5 and 6 mg DAS/kg bw in single dose. The 72‐h single oral LD50 was 5.0 mg/kg bw. In the second trial, doses of 2.5, 3.0 and 3.5 mg DAS/kg bw were given daily over 14 consecutive days by gavage to 7‐day old male broiler chickens (Hubbard x Hubbard) and a LD50 of 4.15 mg/kg bw was estimated by extrapolation. Hoerr et al. (1981b), observed as first clinical symptom, after a 2.7 mg/kg single dose given by gavage reduced spontaneous activity and slightly fluid faecal droppings within the first 24 h. The small intestine, skeletal muscles, liver (with disseminated foci) and gall bladder were affected. Necrosis and hyperplasia occurred in various intestinal tissues, lymphoid tissue and bone marrow. Most lesions recovered within 168 h after treatment.
Hoerr et al. (1982a) observed some mortality in comparison with a control group of 10 birds receiving a diet with solvent only: two birds died in each of the two groups fed at 2.5 and 3.5 mg DAS/kg bw and one bird in the group fed at 3.0 mg DAS/kg bw. Reduced body weight gain was observed in surviving birds; the feathers were malformed, and the beak and legs were pale yellow. At necropsy, the lymphoid organs were atrophic, bone marrow was pale red or yellow, the liver was discoloured yellow, and the crop mucosa was ulcerated. Microscopic lesions included necrosis and cell depletion in lymphocytic and haematopoietic tissues and necrosis of hepatocytes, bile duct epithelium, enteric mucosa, and germinal regions of feather barbs. Other hepatic lesions were fatty change of hepatocytes and hyperplasia of bile ductile. Thyroid follicles were small, contained pale colloid and had tall epithelial cells.
In an experiment performed by Richardson and Hamilton (1990) purified DAS (purity tested by NMR and MS) was administered by gavage (to the crop) to one day old chickens. The 24‐h‐LD50 was 2.0 mg/kg bw accompanied with reduced activity, loose faecal droppings and decreased feed consumption. At necropsy, the authors found haemorrhages in the vascular beds of the beak and toe nails, in the hock joint, in the internal and external surfaces of the proventriculus and the ventriculus. In the intestine, they found pinpoint haemorrhages on the liver and the heart.
Subchronic and chronic toxicity
Other studies of different duration including subchronic and chronic toxicological experiments were based on the daily use of doses below the identified LD50 values administered over several consecutive days. All adverse effects including zootechnical parameters such as body weight gain which were observed by the authors are described below. Because of the relevance of these parameters, when possible NOAELs and LOAELs were identified for each of these adverse effects.
In an experiment performed by Hoerr et al. (1982b), 7‐day‐old male broiler (Hubbard x Hubbard) chickens (5–20 birds per group) were fed for 21 days. Commercial broiler starter mash was used as control and added with commercially purchased DAS (purity at least 95% determined by mass spectrometry) to provide diets at levels of 4.0 and 16.0 mg DAS/kg feed, corresponding to 0.7 and 2.6 mg DAS/kg bw per day. No mortality was noticed but reductions of feed consumption and body weight gain were observed in treated birds compared with controls. Oral lesions (focal yellow plaques on the palate, tongue and buccal floor) were observed at day 2, progressing to raised yellowish‐crust which covered ulcers. From this study, the CONTAM Panel identified for 7‐day‐old chickens a LOAEL for body weight gain reduction and oral lesions of 4 mg DAS/kg feed, corresponding to 0.7 mg DAS/kg bw per day.
Burditt et al. (1983a,b)reported a reduction of 77% feed consumption compared to controls in 6 h in 7‐day‐old Selkab chickens (n = 30) when the diet given boilers as starter mash contained 87 mg DAS/kg feed from a culture filtrate of Fusarium roseum. From this study, the CONTAM Panel noticed the high level of the contamination, which was not useful for identification of either a LOAEL or a NOAEL.
Ademoyero and Hamilton (1991a) fed graded dietary levels at 0 (as control), 1.0, 2.0 and 4.0 mg DAS/kg feed to groups of 10 one‐day‐old male broiler chickens (Arbor Acres x Arbor Acres) for 21 days. Crystalline DAS was added to the starter mash containing mainly yellow corn and protein soybean meal shown to be free of T‐2 toxin and SCT, TAS, MAS and DAS (chromatographic determination). Oral lesions were observed already at 1.0 mg DAS/kg feed. Lesions were obvious after one week toxin and the total number of lesions tripled by the end of the second week. The rank orders from greatest to least affected oral sites were upper beak, lower beak, tongue, and angles. Reduction of body weight gain was observed in chickens fed 2.0 and 4.0 mg DAS/kg feed levels. In a follow up study, Ademoyero and Hamilton (1991b) fed groups of 10 one‐day‐old male broiler chickens with several diets at 0, 1.0, 2.0, 4.0 and 8.0 mg DAS/kg feed for 21 days and at the two dietary fat proportions of 6% and 12%. Crystalline DAS was produced from broth cultures of F. sambucinum and its purity was ascertained by TLC in comparison with DAS (standard authenticated by mass spectrometry and nuclear magnetic resonance). There was a highly significant interaction between dietary fat and DAS, whereby increased dietary fat increased the reduction of body weight gain by DAS. The authors concluded that their data were consistent with the high‐fat diet promoting lipid micellar absorption of DAS. DAS, once absorbed, would inhibit protein synthesis at the ribosomal level, a well‐established mechanism of action for trichothecenes. From these two studies on 1‐day‐old chickens, the CONTAM Panel identified the level of 1.0 DAS/kg feed as LOAEL generating mouth lesions and the levels of 2.0 and 1.0 mg DAS/kg feed as LOAEL and NOAEL for body weight gain reduction, respectively.
One‐day‐old male broiler chickens (Peterson x Hubbard; six replicates of 10 broilers per dietary treatment until 3 weeks of age) were fed two dietary treatments (Kubena et al., 1993). A basal diet with 0 mg DAS/kg feed (occurrence of other mycotoxins not reported) as control and a basal diet of 5 mg of pure DAS/kg feed (purity of DAS at least 99%) were examined. Beginning at the eighth day of feeding the diet, body weight gain was reduced without alteration of relative weights of all organs examined (liver, kidney, heart, proventriculus, gizzard, spleen, and pancreas). Oral lesions were observed in all treated birds at the end of the experiment. Among the haematological and biochemical values, only the triglycerides (significant reduction) and the corpuscular volume (decrease) were affected. In a similar experiment, Kubena et al. (1994) treated one day‐old male broiler chicks (Peterson x Hubbard, 8 replicates of 7 broilers per dietary treatment during 19 days) with 6 mg DAS/kg feed. At the end of the experiment, reduction of body weight gain was observed in all treated birds and oral lesions in at least 90% of treated birds. Only the relative weights of proventriculus and gizzard were increased. The mean corpuscular volume and corpuscular haemoglobin were significantly decreased but only creatine kinase activities were significantly reduced among the blood serum parameters. From these two studies despite of discrepancies noticed for biochemical values and enzyme activities, the CONTAM Panel considered the level of 5–6 mg DAS/kg feed as LOAEL for generating oral lesions, reduction of body weight gain.
Two trials were conducted by Brake et al. (1999) on fertility and hatchability of broiler breeders. In a first one, daily allocation of feed containing 0.0 (basal diet as control), 1.25, 2.5, or 5.0 mg DAS/kg diet was provided to 4 groups of 10 broiler breeder males and 4 groups of 25 broiler breeder females from 67 to 69 week of age. Crystalline DAS was produced from broth cultures of F. sambucinum and its purity was ascertained by TLC and GC in comparison with pure DAS authenticated by mass spectrometry and nuclear magnetic resonance and was added to the control diet which was free of aflatoxin and DAS (data on possible contents of other mycotoxins were not reported). A restricted feeding regimen of 154 g feed/bird per day was applied. Fertility was consistently improved at 5.0 mg DAS/kg diet and intermittently at 1.25 and 2.50 mg DAS/kg diet. The effect disappeared upon removal of DAS. Percentage hatchability of fertile eggs was increased at 1.25 and 2.50 mg DAS/kg diet with the 5.0 mg DAS/kg intermediate. The authors concluded that there was an apparent beneficial spermatozoal storage effect caused by DAS in females. In a second trial, broiler breeder males (4 groups of 10) and females (4 groups of 25) were fed a basal diet containing 0 (as control), 5.0, 10.0, or 20.0 mg DAS/kg diet from 25 to 27 week of age. Pure DAS was added to the basal diet as described for the first trial. A restricted feeding regimen of 119–125 g feed/bird per day was applied. Semen was pooled from males within each treatment and used to inseminate females from each treatment. Female‐related fertility was increased at 5.0 and 10.0 mg DAS/kg feed and male‐related fertility was decreased by the 10.0 and 20.0 mg DAS/kg feed. Upon necropsy, mall, fluid‐filled cysts were observed on the testes of many DAS‐treated males. Conversely, dietary levels of DAS at 10 mg DAS/kg and above decreased male related fertility, presumably by direct toxic effects on the testes. Although results appeared inconsistent, the authors concluded that diet levels of DAS at 10 mg/kg feed or less improved female‐related fertility, presumably because of enhanced spermatozoal storage within the oviduct, but only in the presence of sufficient spermatozoa. Dietary levels below 5 mg DAS/kg feed were not detrimental to fertility and hatchability (Brake et al., 1999).
Under a similar study design, Brake et al. (2000) conducted experiments where DAS was prepared and added as described in Brake et al. (1999). In the first experiment, groups of 20 female broiler breeders of 24 weeks of age were fed diets containing DAS levels at 0 (as control), 5.0, 10.0 and 20.0 mg DAS/kg feed during 2 weeks. The feeding of DAS coincided with the initiation of lay. After the week 25, the control diet was fed for the following 7 weeks through 32 weeks of age. Feed consumption and body weight were decreased and mouth lesions were observed in all treated birds compared to control. After cessation of the DAS feeding, feed consumption increased rapidly and returned to levels similar to those of the controls. However, at the end of the experiment, 7 weeks after DAS exposure, the body weight of females fed 20 mg DAS/kg feed remained insignificantly lower compared to controls. In a second trial, 4 groups of 10 broiler breeder males and 4 groups of 20 broiler breeders females were fed diets from 24 weeks to 27 weeks of age containing DAS levels at 0 (as control), 5.0, 10.0 and 20.0 mg DAS/kg feed restricted to 150 g feed per bird and per day using individual feeders. Feed consumption was decreased in males and females but body weight gain was not affected in males whereas a reduction of body weight was observed in females. Mouth lesions in palatine and sublingual area were observed in all DAS levels in all treated birds. In a third experiment reported by Brake et al. (2000), 2 groups of 10 broiler breeder males from 23 weeks to 26 weeks of age were fed diets containing DAS levels at 0 (as control) and 10.0 mg DAS/kg feed restricted to 119 g feed per bird and per day by individual feeders. Feed consumption decreased after 1 week of treatment but not further on and body weight gain was not affected. No information on mouth effects were reported.
From these two papers, the CONTAM Panel considered 5 mg DAS/kg feed as NOAEL for detrimental fertility and hatchability, and 10 mg DAS/kg feed as LOAEL for decrease of fertility in 25‐week‐old male broiler breeders. The CONTAM Panel considered 5 mg DAS/kg feed as LOAEL for the generation of mouth lesions in 24‐week‐old broiler breeder males and females. Furthermore, the CONTAM Panel considered 5 and 10 mg DAS/kg feed as NOAEL and LOAEL, respectively, for decrease of body weight in 24‐week‐old female broiler breeder. Finally, the CONTAM Panel considered 20 mg DAS/kg feed as NOAEL for reduced of body weight gain in 24‐week‐old male broiler breeders.
Curtui (2000) conducted a trial on 1‐day‐old chickens using F. poae extract mixed into a basal diet to prepare a diet containing nivalenol, T‐2 toxin and DAS at levels 6.4, 1.3 and 2.6 mg/kg feed, respectively. No mortality was recorded and the feed refusal was the major syndrome observed in the treated group compared to the control group receiving the basal diet only. The CONTAM Panel noted the occurrence of several mycotoxins and moreover that DAS was not the dominant toxin present in the contaminated diet. Because of the possibility of these toxins to interfere, the CONTAM Panel did not consider this study further for risk assessment.
Sklan et al. (2001) studied 4 groups of 10 one‐day‐old male Ross chickens fed mash diets in which commercial pure DAS added to a basal feed (analysed as free of aflatoxins, T‐2 toxin, ochratoxin and DON) at 0.0 (as control), 0.2, 0.5, 1.0 mg DAS/kg feed for 35 days. After 10 days, oral lesions were observed at the low doses of 0.2 and 0.5 mg DAS/kg feed in 58% and 92% of the chicks, respectively. The severity of oral lesions was maximal between 15 and 20 days and then decreased slightly with time. No difference in body weight gain was observed at day 35 between control and treated birds. At doses of 0.5 and 1.0 mg DAS/kg feed some slight diarrhoea was observed and birds at the highest dose exhibited mild lymphoid depletion in the spleen and occasional small hepatic lymphocytic perivascular aggregates. From this study, the CONTAM Panel identified the LOAEL at 0.2 mg DAS/kg feed for generating mouth lesions in one day old chickens and the level of 1.0 mg DAS/kg feed as NOAEL for reduced body weight gain.
Diaz (2002) conducted an experiment on 1‐day‐old chickens (Ross) for 3 weeks. Groups of seven birds were amended with diets that consisted of a commercial starter ration mixed with the following inclusion rates of DAS: 0.0 (as control), 1.0, 2.0 mg DAS/kg feed. Purified DAS was prepared from broth cultures of Fusarium sambucinum. Toxin purity greater than 95% was confirmed by TLC. The basal commercial diet was free of aflatoxin, ochratoxin A, and four type‐A trichothecene mycotoxins (T‐2 toxin, DAS, HT‐2 toxin, and neosolaniol). Both levels of dietary DAS significantly decreased body weight and feed intake and caused oral lesions, with the effect of 2 mg DAS/kg feed being more severe. Based on these data, the CONTAM Panel considered the level of 1.0 mg DAS/kg feed as LOAEL for oral lesions.
In a case report Konjević et al. (2004) described the death of two out of 10 Brahma chickens approximately 2 months old preceded by signs of depression and loss of appetite. Histopathological analysis revealed vacuolar dystrophy of the liver, necrosis and depletion of lymphocyte in the bursa of Fabricius as well as multiple necrosis in the glandular stomach and gut. A subsequent mycotoxicological analysis revealed the presence of Fusarium spp. in the feed with a T‐2 toxin level of 0.7 mg/kg, DAS at the level of 0.5 mg/kg and DON levels not reported. Because of the presence of several toxins which could interfere for effect interpretation, the CONTAM Panel did not consider this study for further risk assessment.
Table 8.
Summarised data on adverse effects in broiler chickens exposed to DAS in feed from studies considered for further risk assessment
| Species (age) | No. per treatment | Concentration (mg/kg feed) | Toxin source in feed | Exposure time | Adverse effects | LOAEL (mg/kg feed) | NOAEL (mg/kg feed) | Reference |
|---|---|---|---|---|---|---|---|---|
| Hubbard × Hubbard (day 1) | 10 | 15, 18, 21 | Spiked with purified DAS | 10 days | Mortalities, reduction bw gain, hepatic lesions | 15 | Hoerr et al. (1982a) | |
| Hubbard × Hubbard (day 1) | 10 | 4, 16 | Spiked with purified DAS | 21 days | Reduction bw gain oral lesions |
4 4 |
Hoerr et al. (1982b) | |
| Arbor Acres × Arbor Acres (day 1) | 30 | 0.5, 1, 2, 4 | Spiked with pure DAS | 21 days | Reduction bw gain oral lesions |
2 1 |
1 0.5 |
Ademoyero and Hamilton (1991a) |
| Peterson × Hubbard (day 1) | 10 | 5 | Spiked with purified DAS | 8 days | Reduction bw gain | 5 | Kubena et al. (1993) | |
| Petersons × Hubbard (day 1) | 10 | 6 | Spiked with purified DAS | 19 days | Reduction bw gain oral lesions |
6 6 |
Kubena et al. (1994) | |
| Broiler breeders Male female (day 25) |
10 25 |
5, 10, 20 | Purified | 2 weeks |
Decreased male fertility Decreased female bw Decreased male bw oral lesions in males and females |
10 10 5 |
5 5 10 |
Brake et al. (1999, 2000) |
| Male Ross (day 1) | 10 | 0.2, 0.5, 1 | Pure | 35 days |
Oral lesions for half of the birds Reduction of bw gain |
0.2 | 1 | Sklan et al. (2001) |
| Male Ross (day 1) | 7 | 1, 2 | Purified | 3 weeks | Oral lesions and reduction of bw gain | 1 | Diaz (2002) |
DAS: diacetoxyscirpenol; LOAEL: lowest‐observed‐adverse‐effect level; NOAEL: no‐observed‐adverse‐effect level; bw: body weight.
Laying hens
Allen et al. (1982) reported two trials on DAS in White Leghorn hens. In the first, 3 groups (n = 15 each) of 36‐week‐old hens were fed 8 weeks a culture of Fusarium roseum containing 15 μg DAS/g and 30 μg of other unidentified mycotoxins per g at diet levels of 0% (as control), 1% (with an equivalent to 0.15 mg DAS/kg feed), and 2% (0.30 mg DAS/kg feed). Afterwards, all hens were placed on 0% diet for the following 6 weeks. Birds were inseminated weekly with 0.05 mL of pooled semen from males given control diets. During the initial 8 weeks, the 1% and 2% level groups showed no significant effect on body weight change or egg weights but egg production was significantly depressed. Feed consumption, fertility and hatchability of fertile eggs were reduced at the 2% level, the majority of embryo mortality occurring the first week of incubation. Egg production levels returned to normal during the final 6 weeks without exposure. In the second trial, three groups (n = 15 each) of 15 White Leghorn hens (50 weeks old) were fed control (0%), 0.5 mg/kg purified DAS, and 3% F. roseum culture (i.e. 0.45 mg DAS/kg feed) for 4 weeks and followed by 2 weeks with the control diet. In the first 4 weeks, egg weight, body weight, feed consumption, egg production and fertility were not affected, whereas the hatchability of fertile eggs was 58% under DAS treatment and 3% under culture of F. roseum treatment. Percentages of fertility and hatchability of fertile eggs returned to 60–65% after the toxins were removed for both treatments. Oral lesions were not observed. Following authors’ interpretation, the CONTAM Panel could not consider this study for hazard characterisation of laying hens because of the co‐exposure of DAS with other unidentified mycotoxins.
Engster et al. (1982) conducted a study on White Leghorn hens 63‐week‐old. The birds were fed diets at levels of 0.0 (as control), 0.5, 1.0, 2.0, 4.0 and 8.0 mg DAS/kg feed based on F. roseum extract. The basal diet contained no toxins. There were no significant effects of DAS in any dietary levels on egg production or egg weight. The authors indicated that body weight decreased with increased levels of DAS in the ration and mouth lesions were observed not only in the treated birds but also in the control group of birds. The CONTAM Panel noted that only limited experimental details were available to allow a toxicological interpretation. For this reason, the CONTAM Panel did not consider this study suitable for risk assessment.
In an experiment conducted by Diaz et al. (1994), one group of ten 33‐week‐old commercial Single Comb White Leghorn hens were fed a basal diet which was free of aflatoxin, ochratoxin, sterigmatocystin, citrinin, ergot alkaloids, penicillic acid, T‐2 toxin, DAS and zearalenone, and in which only traces of DON (0.3 mg/kg) were detected. A second group of 10 hens was given the basal diet containing 2 mg DAS/kg feed. Hens were kept on these treatments for 24 days. After 48 h of exposure, 4 of the 10 treated hens had oral lesions, and half of them were affected at the end of the experiment. The treated hens consumed significantly less feed compared to the controls. Egg production in the treated hens decreased. However, no statistically significant change in body weight was observed over the experimental period. Plasma activities of aspartate aminotransferase, alanine, aminotransferase, glutamate dehydrogenase, lactate dehydrogenase (LDH) and creatine kinase were not affected. The CONTAM Panel considered the level of 2 mg DAS/kg feed generating oral lesions and reduction of egg production as LOAEL and as NOAEL for reduction of body weight for hens.
In a study conducted by Brake et al. (2000) on broiler breeder females, DAS was prepared as described in Brake et al. (1999). Groups of 20 hens at 24 weeks of age were fed diets containing DAS levels at 0 (as control), 5.0, 10.0 and 20.0 mg DAS/kg feed. Feed consumption and body weight gain decreased at 10 and 20 mg DAS/kg feed. Oral lesions in palatine, tongue and sublingual area were observed from second week on in all three treatment groups with dose‐related severity. A follow up study was conducted by Brake et al. (2002) involving three trials and was focused on egg production. In a first trial, four groups of hens were fed basal breeder diet as control and diets containing 1.25, 2.5, or 5.0 mg DAS/kg feed from 67 to 69 weeks of age followed by a 3 weeks recovery period. In a second trial, four groups of hens were fed diets containing 0.0 (as control), 5, 10, or 20 mg DAS/kg feed from 25 to 27 weeks of age followed by a 4 weeks recovery period. In a third trial, four groups of hens were fed diets containing 0.0 (as control), 5, 10, or 20 mg DAS/kg feed for 2 weeks beginning at week 24, followed by the control diet for 7 weeks. Egg production was not affected by levels of up to 5 mg DAS/kg in 67‐ to 69‐week‐old hens of the first trial. When fed from 25 to 27 weeks of age in the second trial, DAS treatment caused a decrease of egg production but at the 20 mg/kg level only. When fed from 24 to 25 weeks of age in the third trial, DAS treatment had no significant effect on egg production or egg quality. From these studies on laying hens, the CONTAM Panel identified the level of 5 mg DAS/kg feed as LOAEL for oral lesions. The CONTAM Panel considered the levels of 5 and 10 mg DAS/kg feed as NOAEL and LOAEL for reduction of body weight gain, respectively. The CONTAM Panel considered the levels of 10 and 20 mg DAS/kg as NOAEL and LOAEL for decreased egg production, respectively.
Turkeys
Kubena et al. (1997) fed 1‐day‐old female Nicholas Large White turkey poults (n = 24, 4 replicates of 6 poults per dietary treatment during 3 weeks) a basal diet spiked with pure DAS (purity of DAS at least 99%) at 4 mg DAS/kg feed and compared those with a control group fed the basal diet only. Both diets were shown to be free of aflatoxins DON, Cyclopiazonic acid and Zearalenone. By end of week 1, body weight gains, feed consumption and feed efficiency were significantly reduced at 4 mg DAS/kg feed and after 3 weeks oral lesions were observed in most of the exposed birds. The relative weight of the spleen was decreased in the treated group but the relative weights of the kidney, bursa of Fabricius, and proventriculus were not significantly altered. Triglycerides and cholesterol were significantly reduced and increased, respectively. From these study results, the CONTAM Panel considered the level of 4 mg DAS/kg feed as LOAEL generating oral lesions, body weight gain reduction and changes in some biochemical values in turkey poults.
Sklan et al. (2003) studied four groups of 12 one‐day‐old male poults fed mash diets at 0.0 (as control), 0.22, 0.43, 0.86 mg DAS/kg feed for 33 days. Feed intake and feed efficiency were not affected by DAS but oral lesions were apparent at day 7. The severity of the lesions plateaued after 7–15 days. Slight diarrhoea was observed at 0.43, 0.86 mg DAS/kg feed and villus width, length and area in the jejunum was decreased at the highest dose. The proportion of proliferating cells along the villi was not changed in the duodenum but was increased in the jejunum. There were no other gross pathological lesions and no histological abnormalities. The authors concluded that except changes in small intestinal morphology, feeding turkeys of up to 0.86 mg DAS/kg feed for 33 days did not depress but enhanced growth, and did not influence antibody production. From these study results, the CONTAM Panel considered the level of 0.22 mg DAS/kg feed as LOAEL for causing oral lesions and the level of 0.86 mg DAS/kg feed as NOAEL since no adverse effects on body weight gain in turkey poults were noted.
In Fairchild et al. (2005) study, day‐of‐hatch Large White Hybrid turkey poults were fed basal starter diet which was found to be below detection limits for aflatoxin, fumonisin, deoxynivalenol, zearalenone, and T‐2 toxin as control and a diet composed with the control feed plus 5.0 mg DAS/kg feed. DAS was prepared from cultures of Fusarium sambucinum and the identity was confirmed by mass spectroscopy. There were 10 poults per pen with 6 replicate pens per treatment. Poults fed DAS had mouth lesions, lower body weight, significantly lower relative intestine weight, higher relative bursa Fabricius weight and an alteration of the intestinal architecture compared to the control poults. The CONTAM Panel identified that the level at 5 mg DAS/kg feed generated oral lesions and decreased body weight gain in turkey poults.
Ducks
Schlosberg et al. (1986) studied four groups of 10 day‐old domesticated Muscovy ducklings (Cairina moschata) at 0.0 (as control), 0.25, 0.5, or 1 mg DAS/kg of feed (commercially purchased DAS added to commercial chicken starter concentrate) for 7 days. Oral lesions were recorded in all treated groups: incidence, first appearance and severity of the lesions were dose related. In addition, reduced feed intake was observed but no differences in body weight gain were reported. From these data a LOAEL at 0.25 mg DAS/kg feed was identified for the occurrence of oral lesions in ducks.
Summary for poultry
For acute toxicity, available studies were performed only on chickens. Oral LD50 were reported ranging from 3.82 to 5.0 mg DAS/kg bw for 7 day old chickens. An oral 24‐h LD50 was reported at 2.0 mg DAS/kg bw for one day old chickens.
Main acute toxicities were reduced spontaneous activity and slightly fluid faecal droppings within the first 24 h. The small intestine, skeletal muscles, the liver (with disseminated foci) and the gall bladder were affected. Haemorrhages in the vascular beds of the beak and toe nails, in the hock joint were observed. At necropsy, the lymphoid organs were atrophic, bone marrow was pale red or yellow, the liver was discoloured yellow, and the crop mucosa was ulcerated. Microscopic lesions included necrosis and cell depletion in lymphocytic and haematopoietic tissues and necrosis of hepatocytes, bile ductile epithelium, enteric mucosa, and germinal regions of feather barbs. Other hepatic lesions were fatty change of hepatocytes and hyperplasia of bile ductile. Thyroid follicles were small, contained pale colloid and had tall epithelial cells.
For subchronic and chronic toxicity, NOAELs and/or LOAELS were identified specifically to some types of adverse effects for the following poultry species: chickens, broiler breeders, laying hens, turkeys and ducks. For 1‐ to 10‐day‐old chickens, in several studies oral lesions were observed after 7–10 days of DAS exposure. The LOAEL was identified at 0.2 mg DAS/kg feed, corresponding to the dose of 0.010 mg DAS/kg bw per day for 1‐day‐old chickens. Regarding body weight reduction, several studies allowed to identify the levels of 1 and 2 mg DAS/kg feed as NOAEL and LOAEL (corresponding to 0.050–0.065 mg DAS/kg bw per day and 0.100–0.130 mg DAS/kg bw per day, respectively). For 25‐day‐old male broiler breeders, investigated in two studies at the levels of 5 and 10 mg DAS/kg feed, a NOAEL and a LOAEL for decreased fertility (corresponding to the dose of 0.200 and 0.400 mg DAS/kg bw per day, respectively) were identified. Based on three experiments on laying hens, the CONTAM Panel identified 2 mg DAS/kg feed as LOAEL for generating oral lesions (corresponding to 0.054 mg DAS/kg bw per day) and 5 and 10 mg DAS/kg feed as NOAEL and LOAEL causing reduction of body weight gain, corresponding to the dose of 0.333 and 0.598 mg DAS/kg bw per day, respectively. The CONTAM Panel considered the levels at 10 and 20 mg DAS/kg as NOAEL and LOAEL corresponding to 0.598 and 1.012 mg DAS/kg bw per day for decreased egg production, respectively. For turkeys, based on the three studies available, the CONTAM Panel considered 0.22 mg DAS/kg feed (corresponding to 0.012 mg DAS/kg bw per day) as LOAEL for oral lesions, and 0.86 and 4.0 as NOAEL and LOAEL for bw reduction corresponding to 0.047 and 0.286 mg DAS/kg bw per day, respectively.
Based on the data from only one study (Schlosberg et al., 1986) on ducks, the CONTAM Panel considered the level at 0.25 mg DAS/kg feed corresponding to 0.022 mg DAS/kg bw per day as LOAEL for oral lesions.
Oral lesions were the first observed adverse effect at the lowest level of DAS exposure in the poultry species of chickens, turkeys, ducks and laying hens at the doses of 0.010, 0.012, 0.022 and 0.054 mg DAS/kg bw per day, respectively. The Panel noted that oral lesions had been also observed for the type A trichothecenes T‐2 and HT‐2 (EFSA CONTAM Panel, 2011).
3.1.5.4. Horses
No studies have been identified.
3.1.5.5. Farmed rabbits
No data on chronic intoxication by oral ingestion were available from the literature.
The CONTAM Panel could only identify a review of Mézes and Balogh (2009) who reported a LD50 after intravenous injection of 1.0 mg DAS/kg bw (also included in Table 4).
Since no other data were identified, a hazard characterisation of DAS was not possible for farmed rabbits.
3.1.5.6. Farmed fish
No studies have been identified.
3.1.5.7. Farmed mink
No studies have been identified.
3.1.5.8. Cats and dogs
Data on adverse health effects were only available for dogs but not for cats. Data from the preclinical i.v. studies on the acute and repeated dose toxicity of DAS (anguidine) in beagle dogs identified a NOAEL of 0.031 mg DAS/kg bw for acute adverse health effects (emesis, Section 3.1.4.1) and a NOAEL of 0.016 mg DAS/kg bw for chronic adverse health effects (haematotoxicity, Section 3.1.4.2).
3.1.6. Combined effects of DAS with other mycotoxins
Data were identified for combined effects and interactions of DAS with T‐2 and HT‐2, AF, OTA and fumonisins.
In the study on LD50 determination of DAS performed by Hoerr et al. (1981a) (see Section 3.1.5.3), the determination of LD50 of combined DAS and T‐2 toxin was included. In a first trial, commercially purchased DAS and purified T‐2 toxin (purity percentage non mentioned) were dissolved at appropriate concentrations and then added to the feed and administered alone and in combination to 7 day‐old male broiler chickens (see details in Section 3.1.5.3). The 72‐h single oral dose LD50 for DAS alone and T‐2 toxin alone was 5.0 and 4.0 mg/kg bw, respectively. LD50 of combined DAS and T‐2 toxin (DAS: T‐2 toxin) were determined as 3.05:1.03, 2.26:2.26 and 1.14:3.25 mg/kg bw. In a second trial, multiple oral dose test of DAS alone and T‐2 toxin alone administered as 14 consecutive daily oral doses determined a LD50 of 4.15 and 2.9 mg/kg bw, respectively. Multiple oral dose tests of combined DAS and T‐2 toxin (DAS:T‐2 toxin) at several doses lead to a LD50 of combined DAS and T‐2 toxin as of 2.38:0.88, 1.55:1.55 and 0.88:2.38 mg/kg bw. The authors concluded that combinations of these toxins caused additive lethal effects in the both single and multiple dose tests (Hoerr et al., 1981a).
Diaz et al. (1994) fed four groups of ten 33‐week‐old commercial Single Comb White Leghorn hens during a 24 day experiment. One group was fed a basal diet which was free of aflatoxin, ochratoxin, sterigmatocystin, citrinin, ergot alkaloids, penicillic acid, T‐2 toxin, DAS and zearalenone, and in which only traces of DON (0–3 mg/kg) were detected. Three other groups of 10 hens were given the basal diet containing 2 mg T‐2 toxin, 2 mg DAS and a combination of 2 mg T‐2 toxin and 2 mg DAS per kg feed, respectively. After 48 h of exposure, half of the hens fed diet containing both T‐2 toxin and DAS had oral lesions, while 3 and 4 out of 10 hens were affected in the T‐2 toxin and DAS groups, respectively. The number of affected hens increased gradually during the trial and almost all hens in group fed the combined toxin diet developed oral lesions, while half of the birds in the T‐2 and DAS groups were affected at the end of the experiment. Significantly, less feed was consumed by the treated hens compared to the control. However, hens receiving the combination of T‐2 toxin and DAS showed the lower feed intake than the groups given a single dietary toxin or the control birds. Egg production was affected by the treated diets and the lowest production was observed in the group fed DAS alone or in combination with T‐2 toxin. Plasma activities, of aspartate aminotransferase, alanine, aminotransferase, glutamate dehydrogenase, LDH and creatine kinase were not affected in groups fed DAS alone. But a significant reduction of glutamate dehydrogenase activity was observed in hens receiving T‐2 toxin and the LDH values of hens receiving T‐2 toxin singly or with DAS were significantly higher than those of control hens.
Sklan et al. (2001) evaluates the effects of the combination of DAS and T‐2 toxin and the combination of DAS, T‐2 toxin and aflatoxin B1 (AFB1) (commercially purchased pure DAS, T‐2 toxin and AFB1). Four groups of 10 one day old male Ross chickens were fed basal diet into which pure DAS was added at the levels of 0.0 (as control), 0.2, 0.5, 1.0 mg/kg feed (see details in Section 3.1.5.3). Two additional groups of 10 birds were fed the basal diet into which pure DAS and pure T‐2 toxin was added at levels of 0.5 mg DAS plus 0.5 mg T‐2 toxin/kg feed and DAS, pure T‐2 toxin and pure AFB1 at levels of 0.5 mg DAS plus 0.1 mg T‐2 toxin and plus 0.2 AFB1 mg/kg feed for 35 days. Chicks fed 0.2 mg DAS/kg feed or more developed oral lesions. Similar severities of oral lesions were found in the two groups fed mixtures of toxins. Neither DAS nor the mixture of DAS plus T‐2 toxin diets affected body weight gain and feed efficiency whereas the mixture of DAS plus T‐2 toxin plus AFB1 diet reduced growth and feed efficiency. In chickens fed 0.5 and 1.0 mg DAS/kg feed and the mixture of DAS plus T‐2 toxin some slight diarrhoea was observed. Concentrations of antibodies against Newcastle disease virus were not influenced by the intake of the toxins. The authors concluded that at the tested levels despite of oral lesions, DAS and the mixture of DAS plus T‐2 toxin did not affect body weight gain whereas the mixture of DAS plus T‐2 toxin and plus AFB1 depressed growth and feed efficiency, and neither DAS nor DAS plus T‐2 toxin nor DAS plus T‐2 toxin and plus AFB1 impaired antibody production (Sklan et al., 2001).
Harvey et al. (1991) conducted a study on combined effects of aflatoxins (AFs) and DAS in pigs. Four groups of nine 10‐ to 14‐week‐old growing barrows (Hampshire x Yorkshire x Landrace) were fed basal diets for 28 days as follows: 0 mg AFs and 0 mg DAS/kg feed (as control), 2.5 mg AFs/kg feed, 2.0 mg DAS/kg feed, and 2.5 mg AFs + 2.0 mg DAS/kg feed. The basal diet did not have detectable (below 10 μg/kg of diet) concentrations of AFs, DAS, T‐2 toxin, zearalenone, deoxynivalenol, patulin, penicillic acid, ochratoxin or cyclopiazonic acid. Aflatoxins was produced by fermentation of rice by Aspergillus parasiticus and the AFs content was measured by spectrophotometric analysis and consisted of 79.0% AFB1, 16.0% AFG1, 4.0% AFB2 and 1.0% AFG2. The DAS was at least 98% pure, as determined by nuclear magnetic resonance and mass spectrometry. Body weight gain was significantly decreased by each toxin but more by the combination treatment. The AFs or AFs + DAS treatments induced diffuse hepatocellular vacuolisation, early portal fibrosis, and early bile duct hyperplasia. Aflatoxins increased serum values of creatinine and gamma‐glutamyltransferase (GGT), cholinesterase, and alkaline phosphatase (ALP) activities; increased packed cell volume, haemoglobin and decreased urea nitrogen and total iron binding capacity. DAS alone reduced serum iron binding capacity. The AFs + DAS treatment increased serum GGT and ALP activities, increased haemoglobin, and decreased serum iron binding capacity. The authors concluded that the combination treatment could be described as additive or as less than additive, with most of the effects attributable to AFs.
In the experiment performed by Kubena et al. (1993), see Section 3.1.5.3, the effects of the combination of AFs and DAS were also studied. In addition to control diet and a diet with 5 mg DAS/kg feed, day‐old male broiler chicks were fed 3.5 mg AFs/kg feed and 3.5 mg AFs/kg plus 5 mg DAS/kg feed until 3 weeks of age. AFs were produced through the fermentation of rice by a toxigenic strain Aspergillus parasiticus. The AFs contaminated rice consisted of 79% AFB1, 16% AFG1, 4% AFB2, and 1% AFG2 (by HPLC determination) and then was autoclaved, and ground to a powder incorporated into the feed. When compared with the controls, beginning the 8th day of diet, body weight gain was reduced continuing throughout the experiment by both the treated diets with AFs alone and DAS alone, whereas body weight gains were reduced during the first time period (1–7 days) for the AFs plus DAS combination treatment. Feeding DAS did not alter relative weights of any of the organs (liver, kidney, heart, proventriculus, gizzard, spleen and pancreas), whereas the relative weights of these organs were increased in the chickens consuming the AFs plus DAS diet. Moreover, alterations in haematological values (decrease in both mean corpuscular volume and mean corpuscular haemoglobin) and serum biochemical values (reduction of concentrations of triglycerides, cholesterol, glucose, total protein, and albumin) were observed for the AFs and the AFs and DAS combination, whereas feeding DAS resulted in a significant reduction only in triglyceride concentration and in a decrease in the mean corpuscular volume. Among the serum enzyme activities, feeding AFs alone caused an increase in creatine kinase activity and a decrease in LDH and aspartate amino transferase activities. Feeding the DAS diet resulted in no changes in enzyme activities. Feeding AFs plus DAS resulted in an increase in 7 GGT activity. The authors concluded that apparently a synergistic interaction occurred between AFs and DAS for reduced body weight gain (Kubena et al., 1993).
In the same experiment performed by Kubena et al. (1994), see also 3.1.5.3 with a design similar as in Kubena et al. (1993), the effects of the combination of ochratoxin A (OTA) and DAS were studied. In addition to control diet and a diet with 6 mg DAS/kg feed, day‐old male broiler chicks were fed 2 mg OTA/kg feed and 2 mg OTA/kg plus 6 mg DAS/kg feed during 19 days. OTA was at least 95% pure (HPLC determination). When compared with the controls, OTA and DAS significantly reduced body weight gains when fed alone and in combination. The reduction of body weight gains due to the combination of OA and DAS was almost identical to that of DAS alone. 90% of the chicks fed diets containing DAS with or without OTA had oral lesions. When compared with controls, relative weights of the liver and kidney were increased by feeding OTA singly or in combination with DAS, whereas no changes in these organs were observed when DAS was fed alone, and the relative weights of the proventriculus and gizzard were increased by feeding DAS alone but also by feeding the OTA and DAS combination. The relative weights of the others organs (spleen, pancreas and bursa of Fabricius) were not significantly different from those of the control in any of the treatments. When compared with controls, serum total protein and albumin concentrations were reduced by OA when present alone or in combination with DAS. There was a significant increase in serum uric acid concentrations in birds fed the diet containing OTA alone, whereas uric acid concentrations were not increased in the chickens receiving DAS alone or the OTA plus DAS combination. Serum concentrations of triglycerides were significantly increased, whereas concentrations of cholesterol were decreased in birds fed the diet containing OTA alone. The serum concentrations of triglycerides or cholesterol were not altered in the chickens receiving the diets containing DAS alone or in combination with OTA. No effects of the combination OTA plus DAS were observed for the serum enzyme activities and haematological values. The authors concluded that there was significant interactions between OTA and DAS which could best be characterised as less than additive for body weight gains and for decreased serum albumin A, a significant antagonistic interaction occurred between OTA and DAS for cholesterol and for the uric acid concentrations (Kubena et al., 1994).
In the another experiment performed by Kubena et al. (1997) with study design similar to the Kubena et al. (1993, 1994), the effects of the combination of fumonisin B1 (FB1) and DAS were studied in turkeys. In addition to control diet and a diet with 4 mg DAS/kg feed, 1 day‐old female Nicholas Large White turkey poults were fed a diet at 300 mg FB1/kg feed and a diet at 300 mg FB1/kg plus 4 mg DAS/kg feed in combination until 3 weeks of age. To obtain FB1 contaminated diet, ground Fusarium moniliforme culture material was substituted for ground corn to prepare the desired level of FB1 (300 mg/kg of diet). The diet also contained approximately 89 mg/kg FB2 and 27 mg/kg FB3. When compared with the controls, by the end of week 1, body weight gains were significantly reduced in poults fed the 3 contaminated diets but the birds fed the diet containing FB1 plus DAS in combination had significantly lower body weights than birds fed the 2 other diets (FB1 alone and DAS alone). Feed consumption and feed efficiency were reduced by all three treatments. Oral lesions were observed in most of the birds fed the both DAS contaminated diets (alone and in combination). Relative weights of the liver and gizzard increased and in the opposite relative weight of the heart decreased in birds fed FB1 diet with or without DAS, whereas relative weight of the spleen decreased in all treated groups. The relative weight of the pancreas was increased in birds fed FB1 alone. The relative weights of the kidney, bursa of Fabricius, and proventriculus were not significantly altered by any of the 3 treatments. Regarding serum enzyme activities, the combined diet increased activities of aspartate aminotransferase and LDH. However, serum concentrations of cholesterol were significantly higher in birds fed the DAS diet but were significantly lower in birds fed FB1 or the combination of FB1 and DAS. The authors concluded that their results indicated an additive or less than additive toxicity (e.g. reduction in body weight gain and relative weight of heart), whereas there was a significant antagonistic interaction between FB1 and DAS for cholesterol concentration when turkeys were fed the combined FB1 plus DAS diet (Kubena et al., 1997).
In the Fairchild et al. (2005), study, day‐of‐hatch Large White Hybrid turkey poults were fed basal starter diet which was found to be below detection limits for aflatoxin, fumonisin, deoxynivalenol, zearalenone, and T‐2 toxin as control and 3 different diets composed with the control feed plus 5.0 mg DAS/kg feed, a second plus 119 mg fusaric acid (FA)/kg feed and a third one plus a combination of 5 mg DAS and 137 mg FA per kg feed. The DAS was prepared from cultures of Fusarium sambucinum and FA was commercially purchased. The identity of both toxins was confirmed by mass spectroscopy. There were 10 poults per pen with 6 replicate pens per treatment. Poults fed DAS or the toxin combination had more severe oral lesions than poults fed FA. FA had no effect on body weight or body weight gain at any period compared to control birds. Poults fed the toxin combination had reduced body weight gain compared to controls, while poults fed DAS diet had lower bw than all treatments at every period. Poults fed FA had significantly lower relative intestine weight than poults fed the other diets. The authors concluded that dietary DAS resulted in decreased poult performance, while dietary FA had little or no effect. Fusaric acid fed in combination with DAS resulted in some protective effect towards DAS.
Summary
Combined effect of DAS and others mycotoxins was investigated in vivo mainly in poultry, one experiment has also been performed in pigs. These experiments investigated the effect of DAS with T‐2 toxin, AFB1, OTA and fusaric acid. Except for fusaric acid that reduced the effect observed with DAS, all other experiments revealed a greater effect when DAS was present in combination with T‐2 toxin, AFB1 and OTA. The CONTAM Panel noted that because of the lack of dose–response data, it is difficult to perform a refined statistical analysis and to draw definitive conclusion concerning the nature of the combined effects.
A few in vitro experiments were performed to investigate the combined effect of DAS and other mycotoxins (Alassane‐Kpembi et al., 2017) but they were not sufficient for establishing the nature of combined effects.
The CONTAM Panel noted that the available database describing possible effects of combined exposure to DAS and other mycotoxins is weak and inconclusive.
3.1.7. Human data
The Panel identified data on adverse health effects in humans exposed to DAS in clinical studies and few epidemiological data which suggested an association with the incidence of a disease that possibly related to DAS.
Information on human food poisoning outbreak that has been identified as being possibly associated with DAS refers to the ‘alimentary toxic aleukia’ (AKA) disease. AKA, occurring in the early 20th Century in the Soviet Union has been associated with the consumption of food contaminated with Fusarium toxins, T‐2 and in particular, with DAS, having been suspected although not confirmed (Joffe, 1974; Joffe and Yagen, 1978; Bennett and Klich, 2003). The symptoms included inflammation of the GI tract in the early disease stages, followed by leucopenia, anaemia and other haematotoxicity symptoms, and lesions and necrosis in the mouth and gut. The CONTAM Panel noted that some of these symptoms have close similarity with symptoms observed as clinical side effects of DAS (see below) but the Panel considered the available data unsuitable for hazard characterisation of DAS in humans.
In the 1970s, DAS (named as ‘anguidine’) was developed as a potential anticancer drug and tested in a series of phase I and phase II trials on patients with different cancer types. Exclusively given by intravenous (i.v.) administration, the established route for chemotherapy at these times, DAS doses ranged between 0.1 and 10 mg/m2 in these studies, with different treatment schedules e.g. i.v. injection (bolus) or infusion (up to several hours). Treatment in phase 1 studies was usually at an initial low dose in small patient cohorts and then escalated in subsequent cohorts to high doses with weekly or five consecutive days schedule, repeated 2 or 3 weeks apart. Some studies escalated the dose also within patients. The dose ranges applied expressed as mg/m2 corresponded to 2.7–270 μg/kg bw, when using a transformation factor of 37 (FDA, 2005)14 to convert mg/m2 into μg/kg bw. The clinical development of DAS was discontinued because the treatment showed lack of efficacy on different types of cancer and severe side effects (Bukowski et al., 1982; DeSimone et al., 1986; Thigpen et al., 1981).
The adverse effects reported in these studies were examined by the CONTAM Panel in order to identify relevant human toxicities of DAS and a possible RP for the hazard characterisation. The CONTAM Panel noted that the aim of phase I clinical trial is to identify a dose with acceptable adverse effects to be used in the phase II trials and not to identify a NOAEL. In addition, treatment schedule varied with a total of more than one hundred patients and a small number (between 3 and 6) of patients were usually treated per dose group in different studies. Although sample size of phase II studies were larger (usually from about 20 to about 60 patients each) these studies only provided confirmatory toxicological information. Because of the relatively high and fixed doses used and looking for clinical efficacy while accepting some adverse effects, their usefulness in identifying reference doses was very limited.
Phase I studies
Goodwin et al. (1978), selected a starting dose of 0.2 mg/m2 (5.4 μg/kg bw) for DAS when given as i.v. bolus over 5 min and repeated for 5 consecutive days, escalating the dose by 50% with three or more patients at each dose level. Nausea and vomiting were seen in 10/24 patients at doses equal to or lower than 2.4 mg/m2 (65 μg/kg bw). At these doses 5 out of 24 patients showed leucopenia and 3 out of 24, thrombocytopenia. The percentage of patients with signs of myelotoxicity increased at higher doses. Central nervous system (CNS) symptoms ranging from mild confusion to short periods of coma and hallucinations started at 3.1 mg/m2 (84 μg/kg bw). CNS toxicity and hypotension were dose limiting at 4.5 mg/m2 (121 μg/kg bw) and myelosuppression was unacceptably high at 6 mg/m2 (162 μg/kg bw) when administered by i.v. bolus over 5‐day period. A dosing regimen of 4–8 h infusion at 4.5 mg/m2 per day provided a more acceptable balance between toxicities. Although the dose of 2.4 mg/m2 (65 μg/kg bw) resulted to be well tolerated with some mild adverse effects (nausea, vomiting and haematotoxicity), no sufficient information was available on the paper to identify a NOAEL or a LOAEL.
Murphy et al. (1978) evaluated the toxicity of DAS in 33 cancer patients with various malignancies. The i.v. treatment was scheduled as daily for 5 days every 2 weeks. The dose was escalated from initially 0.1 mg/m2 (2.7 μg/kg bw) up to the dose of 7.5 mg/m2 (278 μg/kg bw). At doses < 2.4 mg/m2 per day (< 65 μg/kg bw), there was no drug related adverse effects reported and at 2.4 mg/m2 only one patient reported mild nausea and vomiting. Above this dose GI symptoms were more frequently noted. At doses of 3 mg/m2 (81 μg/kg bw) and higher significant toxicity including nausea/vomiting was observed in all patients, moderate myelosuppression (79%), hypotension (20%) and also CNS toxicity including ataxia (20%) and fever (12%). Above 5 mg/m2 per day (135 μg/kg bw) severe toxicity was observed in patients with impaired liver function. While all patients suffered nausea and vomiting at this dose, those with abnormal liver function experienced more severe expression of these symptoms.
The NOAEL for acute toxicity (nausea and vomiting) was identified as 1.2 mg/m2 (32 μg/kg bw). No myelosuppression was reported at doses less than 3 mg/m2 (81 μg/kg bw), therefore a NOAEL for myelotoxicity in the subacute/chronic scenario was identified at 2.4 mg/m2 (65 μg/kg bw).
Belt et al. (1979), treated 20 patients weekly (33 courses ranging between 1 and 19 weeks duration, median 4 weeks) by i.v. infusion mostly over 4‐h and with a few high doses over 8 h. The starting dose of 2 mg/m2 (54 μg/kg bw) was escalated in steps of 0.5 to 1 mg/m2 up to 10 mg/m2 (270 μg/kg bw). GI and CNS toxicity symptoms were dose‐limiting at > 4 or > 6 mg/m2 (> 108 or 162 μg/kg bw, respectively) in patients with or without hepatic dysfunction, respectively, but myelosuppression was infrequent. Thrombocytopenia was reported at the lowest dose (2–3.5 mg/m2, 54–94 μg/kg bw) in 50% patients but only in 1 and 2 patients at the highest dose (6.5–10 mg/m2, 176–270 μg/kg bw) and it was not dose related. No information was given on the times when symptoms developed.
From the available data the LOAEL for acute toxicity (nausea and vomiting) ranged from 2 mg/m2 (corresponding to 54 μg/kg bw) to 3.5 mg/m2 (94 μg/kg bw).
DeSimone et al. (1979) also found no myelosuppression in 23 patients with various solid malignancies given a weekly dose of 1.5–7.5 mg/m2 (40–203 μg/kg bw) for three to six weekly treatments over a 3‐h infusion. They reported no adverse effects at 1.5 mg/m2 (40 μg/kg bw) except for one patient with pancreatic cancer (mild nausea/vomiting). Nausea and vomiting also appeared at 3 mg/m2 (81 μg/kg bw). More severe nausea and vomiting lasting longer, together with low grade fever and mild hypertension were reported for 5 mg/m2 (135 μg/kg bw) and CNS symptoms were observed at 7.5 mg/m2 (203 μg/kg bw). As noted by Murphy et al. (1978), toxicity was more severe in patients with liver dysfunction. The LOAEL for acute toxicity (nausea and vomiting) was identified as 1.5 mg DAS/m2 (40.5 μg DAS/kg bw), although considering sporadic nature (1 out of 6), severity (mild) and the susceptibility of the patient (pancreatic cancer) it can be even considered as a NOAEL.
In summary, DAS caused no toxic effects at i.v. dose of 1.2 mg/m2 (32 μg/kg bw) in phase I trials. Significant toxicity was observed in cancer patients treated with daily doses above 2.4 mg/m2 (65 μg/kg bw). The adverse effects included nausea and vomiting, fever, hypotension, myelosuppression and CNS effects that all increased in frequency and severity with dose (Goodwin et al., 1978; Murphy et al., 1978; Belt et al., 1979) although some trials with weekly schedule did not show myelosuppression (DeSimone et al., 1979; Belt et al., 1979). The treatment was poorly tolerated by patients with a significant proportion suffering severe toxicity at doses of 5 mg/m2 (135 μg/kg bw) including some life threatening episodes of hypotension. Patients with impaired liver function constantly showed worse toxicity.
From these studies, the CONTAM Panel overall identified nausea and vomiting as the most frequently observed acute toxic effects with a NOAEL at 1.2 mg/m2 (32 μg/kg bw). For the subacute toxicity, myelosuppression was the most frequently observed adverse effect; a NOAEL was identified at 2.4 mg/m2 (65 μg/kg bw).
Phase II studies
Numerous phase II trials in cancer patients using DAS were conducted typically at a fixed dose for clinical cancer treatment effect. While these studies may further confirm the toxicities identified above they did not provide specific information for determining RPs (e.g. NOAELs) for the hazard characterisation since a large portion of patients experienced toxicity at the dose chosen for the chemotherapy with DAS.
Yap et al. (1979), tested 30 patients with advanced refractory breast cancer with DAS given i.v. at 5 mg/m2 per day (135 μg/kg bw) for 5 days, repeated every three weeks or 3 mg/m2 per day (81 μg/kg bw) if liver function was impaired. The treatment was poorly tolerated, with nausea/vomiting, fever, hypotension, confusion, lethargy occurring as side effects. In addition to nausea and vomiting, the most significant adverse effect noted was hypotension. Myelosuppression resulted to be mild with a nadir (the lowest number) of cells count between the second and the third week following treatment.
Thigpen et al. (1981), conducted a phase II trial on 25 patients with sarcoma, using 4.5 mg/m2 per day (121 μg DAS/kg bw) for 5 days (only one patient received more than 3 courses i.v.). There was no observed effect on the tumours and the most relevant adverse effect was the myelosuppression observed in 16 out of 25 patients (leucopenia and/or thrombocytopenia). Although no deaths were recorded two patients developed life‐threatening myelosuppression. A total of 16 out of 25 patients suffered of various degrees of nausea and vomiting and 6 out of 25 of mild hypotension.
Bukowski et al. (1982), conducted an i.v. phase II study in 134 patients with GI malignancies using doses of 3–4.5 mg/m2 (81–121 μg/kg bw) daily for 5 consecutive days every 28 days. Non‐haematological toxicities were substantial with approximately 50% of patients suffering nausea and vomiting and 40% hypotension. In addition, approximately 20% of patients suffered of mild myelosuppression (thrombocytopenia and leucopenia with nadir in third and fourth week after treatment).
Adler et al. (1984) conducted a phase II study on 276 patients using 3 or 5 mg/m2 per day (81–135 μg/kg bw) for 4 or 5 days continuous i.v. infusion every 4 weeks (cycle). Substantial haematological toxicity was seen in patients with solid tumours, including severe reduction of granulocyte and platelet in approximately 30% of patients, which was sporadically life threatening. Haematological toxicity was not cumulative after three courses. GI and CNS toxicities (16% and 6%, respectively) were also observed.
DeSimone et al. (1986), reported on the effects of 5 mg/m2 (135 μg/kg bw) 3–6 h daily i.v infusion for 5 days every 3‐week in 33 patients with advanced colorectal adenocarcinoma. Hypotension and fever was observed in approximately 25% of patients while 10% experienced CNS or haematological toxicity.
In the paper from Goodwin et al. (1983), it is reported that mild myelosuppression, CNS and GI (nausea and vomiting) symptoms were observed in some patients when treated at 3.5 or 5 mg/m2 (94–135 μg/kg bw) weekly.
When 19 patients with metastatic carcinoma from colorectal cancer were treated with i.v. doses (2–3 h infusion) from 3.5 to 5 mg/m2 (94–135 μg/kg bw) for 5 days every 3 weeks approximately 85% had fever, 75% experienced nausea and vomiting and 35% hypotension (sometimes severe). Myelotoxicity was only moderate with decrease in white blood cells and platelets, no further details were available (Diggs et al., 1978).
Using a prediction model taking into account in vitro data from clonogenic assays on rodents and human haematopoietic progenitors and in vivo data in rodents, Parent‐Massin and Parchment (1998) calculated that the maximum tolerated dose (MTD) in human could be equal to 7.9 mg/m2 (equivalent to 213 μg/kg bw) while clinical data gave 6 mg/m2. It is important to note that in this case, the MTD is not a NOAEL but the dose inducing acceptable adverse effects in patient. MTD was considered to be the dose inducing 90% of cytotoxicity and keeping 10% of haematopoietic progenitors able to renew blood cells.
In summary, the dosing regimen (5 mg/m2 per day or 3 mg/m2 per day, if liver function was impaired) caused in phase II studies similar toxicity as seen at these doses in the phase I trials. Overall, it is clear that doses of 3 mg DAS/m2 day (81 μg/kg bw) and above are associated with substantial toxicities, in particular nausea and vomiting, myelosuppression, haematological toxicity and hypotension which can be life threatening at doses of 5 mg/m2 per day (135 μg/kg bw) and higher.
While these phase II clinical trials confirmed DAS‐related adverse effects, for the characteristics and objectives of the studies they were considered of limited value to identifying RPs for hazard characterisation.
Table 9.
Summary table for IV clinical studies with DAS (anguidine) as an anticancer agent
| Ref | Phase | Organ cancer | Dose mg/m2 per day | Dosea μg/kg bw per day | Duration/intervals days | Route | No. patients treated (no. patient evaluated for toxicity) | Adverse effects |
|---|---|---|---|---|---|---|---|---|
| Goodwin et al. (1978) | I | Advanced malignancies resistant to conventional therapeutic modalities | From 0.2 up to 6 [dose escalation] | From 5.4 up to 162 | Daily for 5 days (one month interval) |
i.v. From bolus up to 8 h infusion |
36 (24) |
Dose ≤ 2.4 nausea, vomiting Dose ≥ 2.4 neurotoxicity, GI toxicity, cardiotoxicity Myelotoxicity (inacceptable for highest dose) |
| Murphy et al. (1978) | I | Advanced malignancies |
From 0.1 and then 0.4 0.6–1 1.2–2.4 3–7.5 [dose Escalation] |
From 2.7 then 11 16–27 32–64 81–203 |
Daily for 5 days at 2‐week intervals |
i.v. 30–60 min Infusion |
39 (33) | Dose ≥ 3, nausea, vomiting, hypotension, central nervous system symptoms (including somnolence, confusion, and ataxia), diarrhoea, chills and fever, generalised burning erythema, stomatitis, shortness of breath, moderate myelosuppression |
| Belt et al. (1979) | I | Advanced malignancies resistant to conventional therapeutic modalities | 2, 3.5, 4, 6, 6.5, 10 Weekly infusion | 54, 94, 108, 162, 175, 270 | Until 36 weeks (5 patients) |
i.v. 4–8 h Infusion |
20 | Dose ≥ 2 gastrointestinal and neurologic toxic effects. Thrombocytopenia at lowest doses (2–3.5) |
| DeSimone et al. (1979) | I |
From 1.5, then 3, 5, 7.5 Weekly infusion |
From 40 then 81, 135, 203 | Repeated for 3–6 weeks |
i.v. 3 h Infusion |
29 (23) | Dose ≥ 3 Nausea and vomiting, hypotension, CNS symptoms (confusion, hallucinations, and psychomotor seizures), chills, fever, and diarrhoea | |
| Diggs et al. (1978) | II | Metastatic Adenocarcinoma colon rectum | 3.5 – liver injuries – or 5 | 94, 135 |
Daily for 5 days at 3‐week intervals |
i.v. 2–3 h infusion |
19 |
Hypotension (acute) Nausea and vomiting tolerable Myelosuppression moderate Fever and chills |
| Yap et al. (1979) | II | Advanced Breast cancer | 3 or 5 mg/m2 | 81, 135 | Daily for 5 days |
i.v. No details available |
30 (25) | Nausea and vomiting, hypotension, CNS symptoms (confusion, hallucinations, and psychomotor seizures), chills, fever, and diarrhoea |
| Bukowski et al. (1982) | II | Colon carcinoma, pancreatic, gastric cancer, miscellaneous | 3.0, 4.5 mg/m2 daily × 5‐day (dose escalation) | 81,121 | Daily for 5 days every 28 days |
i.v. 4‐h infusion |
134 (93) | Thrombocytopenia (19.8%), leucopenia (18.8%), nausea and vomiting (49%), hypotension (37%) confusion (12%) |
| Thigpen et al. (1981) | II | Sarcoma | 4.5 mg/m2 per day | 121 | Daily for 5 days every 21 days. | i.v. 4‐h infusion | 27 (25) | Myelotoxicity, nausea and vomiting, mild to moderate hypotension |
| Goodwin et al. (1983) | II | Nervous system tumour | 3.5–5 mg/m2 Weekly infusion | 94, 135 | with schedule |
i.v. 4‐h infusion |
17 (16) | myelosuppression, gastrointestinal symptoms, and central nervous system symptoms |
| Adler et al. (1984) | II | 7 different types | 3–5 mg/m2 | 94, 135 | 4‐ or 5‐day continuous infusion every 28 days | i.v. Continuous infusion | 276 (177) | Myelotoxicity |
| DeSimone et al. (1986) | II | Colorectal adenocarcinomas | 5 mg/m2 daily x | 135 | Daily for 5 days every 21 days. |
i.v 3–6 h infusion |
33 (29) | Hypotension (7/29), fever (7/29), CNS(3/29), myelotoxicity (3/29) |
bw: body weight; i.v.: intravenous.
Transformation factor of 37 (FDA, 2005) to convert the dose from m2 to kg bw.
Summary
No human epidemiological data on exposure to DAS were identified. DAS has been evaluated as a candidate anticancer drug, from the 1970s until mid‐1980s, in both phase I and II studies.
DAS administrated by i.v. induced various adverse effects during the course of treatments. The adverse effects included GI effects (nausea and vomiting), myelosuppression and haematotoxicity, hypotension, fever and CNS effects. Patients with impaired liver function showed worse symptoms. The drug ceased for development because of the lack of efficacy in cancer treatment and the exacerbation of side effects.
The CONTAM Panel considered the data on the toxicity of DAS collected in these clinical trials on about 500 patients as informative for the hazard characterisation of DAS. From the phase I trials, nausea and vomiting were the most frequently observed acute toxic effects and derived a NOAEL corresponding to a dose of 32 μg DAS/kg bw (1.2 mg/m2).
Accounting for the duration of the treatment and the repeated observation of adverse effect over several cycles (generally covering from 1 to 3–4 months), the CONTAM Panel considered the same studies as informative for the subacute/subchronic toxicity of DAS in humans and identified haematotoxicity and myelosuppression as relevant adverse effects resulting in the identification of a NOAEL as of 65 μg/kg DAS bw (2.4 mg/m2) when administered i.v..
The CONTAM Panel noted that the aim of phase I clinical trial is to identify a dose of acceptable adverse effects to be used in the phase II trial and not to identify a NOAEL. This was considered to limit the accuracy of the NOAEL as RP for hazard characterisation.
Data from phase II studies performed at high fixed doses for the cancer treatment purpose confirmed these toxicities.
3.1.8. Mode of action
Trichothecene mycotoxins, including DAS, are known to target tissues with high proliferative or turnover rates such as epithelium, bone marrow and lymph nodes (Escrivá et al., 2015).
Inhibition of protein synthesis and ribotoxic stress
It is well known that trichotecenes are potent inhibitors of protein synthesis. This inhibitory effect was demonstrated in whole animals, protozoan, rat liver, primary cells and tumour cell lines (Ueno, 1983). The trichothecenes target the ribosome protein synthesis machinery, binding to the 60S subunit (Mc Cormick et al., 2011; Garreau de Loubresse et al., 2014). The mechanism of protein synthesis inhibition can be of two types; inhibition of the initial step of protein synthesis by interference with the activity of peptidyltransferase or inhibition of the elongation‐termination step. In vitro data indicate that DAS as well as T‐2 and HT‐2 especially inhibit the initial step, but at high doses DAS was also found to block the elongation step in both yeast and mammalian cells (Cundliffe and Davies, 1977; Hernandez and Cannon, 1982). Similar inhibition of proteins synthesis was also observed in Hela cells at concentrations much lower than those causing inhibition of DNA synthesis (Liao et al., 1976). As in vitro, trichotecenes exerted no inhibitory effects on DNA or RNA polymerases as well as on other enzymes involved in DNA/RNA synthesis; it is probable that the impairment of DNA synthesis is caused as a secondary event of inhibition of protein synthesis (Ueno, 1980). Inhibition of protein synthesis associated with DAS exposure is therefore likely to be a mechanism underlying the observed in vivo chromosomal abnormalities (Section 3.1.4.5).
For several trichothecenes, the binding to ribosome has been shown to activate also several ribosome‐associated mitogen‐activated protein kinases (MAPKs), including p38, c‐Jun N‐terminal kinase (JNK), and extracellular signal‐regulated kinase 1 and 2 (ERK1/2), an effect called ‘ribotoxic stress response’ (Pestka, 2010; Payros et al., 2016). A similar response can be anticipated for DAS.
Induction of oxidative stress
Among mycotoxins, trichothecenes are able to trigger the production of reactive oxygen species (ROS) (Arunachalam and Doohan, 2013; Da Silva et al., 2018). However data concerning the specific implication of DAS in the induction of oxidative stress are very limited. In yeast cells, a 18% increase in ROS production was observed after treatment with 150 μM DAS for 6 h, however, when the duration of the treatment was increased to 18 h, a 65% decrease in ROS generation was observed (Bin‐Umer et al., 2011).
Effect on proliferation, cell‐cycle and apoptosis
Trichothecenes possess potent cytotoxicity to eukaryotic cells and several studies have demonstrated that DAS is cytotoxic for human cells such as cervical carcinoma cells (HeLa, IC50 = 2.7 × 10−8M, Ueno, 1983), embryonic kidney cells (HEK, IC50 = 2.7 × 10−8M, Ueno, 1983), Jurkat T lymphocytes (IC50 = 5 × 10−9M, Nasri et al., 2006) and haematopoietic progenitors (see below). DAS has also been shown to be cytotoxic for animal cells such as rabbit reticulocytes (IC50 = 8.2 × 10−8M, Ueno et al., 1977); monkey kidney (Vero) cells (IC50 = 3 × 10−8M, Thompson and Wannemacher, 1986) rat spleen lymphocytes (IC50 = 1 × 10−8M, Thompson and Wannemacher, 1986), chicken macrophages (Qureshi et al., 1998) and porcine kidney epithelial cells (Morrison et al., 2002).
As indicated in data obtained on HeLa cells, the cytotoxic effect of DAS is observed at level within the same order of magnitude than the inhibition of protein synthesis (IC50 of 2.7 × 10−8 M for cytotoxicity vs IC50 of 8.2 × 10−8 M for inhibition of protein synthesis, Ueno, 1983).
In Jurkat T cells, it was demonstrated that DAS‐induced cytotoxicity was due in part to apoptosis initiated by caspase‐8 activation and subsequent mitochondrial‐dependent or independent activation of caspase cascades and in part to the interruption of cell cycle progression initiated by downregulation of cyclin‐dependent kinase (CDK) 4 and cyclin B1 proteins (Jun et al., 2007).
Effects on blood cells and haematopoietic progenitors
Type A trichothecenes are known to induce haematological disturbances. The mode of action (MoA) has been explored using in vitro tests.
Murine peritoneal macrophages were pre‐incubated for 4 h with DAS concentrations ranging from of 0.1 ng/mL to 1 μg/mL. At concentrations that did not affect the cell viability, DAS reduced phagocytosis (2 ng/mL), microbicidal activity (1 ng/mL), superoxide anion production (1 ng/mL) and phagosome‐lysosome fusion (0.1 ng/mL) and LDH 10 mg/mL). According to the authors, these data indicated that the inhibition of microbicidal activity arise from both oxidative and non‐oxidative pathways (Ayral et al., 1992).
Aggregation of bovine platelets was inhibited by DAS tested at concentrations ranging from 0.1 to 16 mM when either collagen or adenosine diphosphate was used as the stimulatory agent for aggregation. The degree of inhibition was dependent on mycotoxin concentration but was not dependent on the time of exposure of the platelets to the toxin. T‐2 toxin and HT‐2 toxin were always more toxic than DAS, and DAS was also less toxic than DON (Chan and Gentry, 1984).
Human and murine haematopoietic progenitors have been incubated in the presence of DAS in order to study its effect on viability, proliferation and differentiation in white blood cells, red blood cells and platelets respectively. The authors demonstrated that DAS is cytotoxic at very low concentration (10−7 M) and without any cytotoxicity from 2.5 × 10−8 M for human white blood cell haematopoietic progenitors to 5 × 10−10 M for human red blood cell haematopoietic progenitors. DAS also inhibited the haemoglobin synthesis at higher concentrations (Parent‐Massin et al., 1994, Parent‐Massin and Thouvenot, 1995, Lautraite et al., 1995; Rio et al., 1997, Froquet et al., 2001). The CONTAM Panel noted that concentrations equal to 10−7 M and 5 × 10−9 M for DAS and T‐2 toxin, respectively, are cytotoxic to white blood haematopoietic progenitor cells and that no effect concentrations were equal to 2.5 × 10−8 M and less than 10−10 M for DAS and T‐2 toxin respectively (Lautraite et al., 1995, Lautraite et al., 1997).
When comparing the myelotoxicity of the four major trichothecenes (DAS, DON, HT‐2 and T‐2 toxin) in in vitro clonogenic assays, Parent‐Massin (2004) concluded that T‐2 and HT‐2 among these four mycotoxins were the most myelotoxic ones to human haematopoietic progenitors. The author concluded that DAS was at least ten times less myelotoxic than T‐2 on white blood cell progenitors. In all cases white blood cells progenitors and platelets progenitors were more sensitive to the myelotoxicity of these trichothecenes than the erythroblastic progenitors. The authors also concluded that haematological toxicity induced in vivo by DAS, T‐2 and HT‐2 toxin, was due to a strong cytotoxicity on haematopoietic progenitors in the bone marrow. As life time of circulating white blood cells and platelets is shorter compared to red blood cells (3–4 days vs 4 months) thrombocytopenia and leucopenia occurred before anaemia.
Effects on gut satiety hormones
Anorexia is a hallmark of the trichothecenes adverse effects, including DAS, but the underlying mechanisms are not yet fully understood. In a mouse model, oral gavage of 1 mg/kg bw DAS or intraperitoneal injection of 1 mg/kg bw DAS, resulted in dramatically decreased food intake (Zhang et al., 2017a,b). Therefore an increased concentration of the gut satiety hormones cholecystokinin (CCK), glucagon‐like peptide‐17–36 amide (GLP‐1), peptide YY3‐36 (PYY3‐36) and glucose‐dependent insulinotropic polypeptide (GIP) were observed in the plasma. More specifically, after oral exposure, CCK, PYY3‐36 and GIP peaked at 2 h, whereas for GLP‐1 plasma concentration still increased after 6 h. These findings suggest an implication of gut satiety hormones in DAS‐induced anorexia but specific receptor antagonists have never been used.
Summary
The available data suggested that the mode of action of DAS is by binding to ribosomes, inducing a ‘ribotoxic stress response’ with activation of ribosome‐associated MAPKs and inhibition of protein synthesis. DAS also possesses cytotoxic properties while no clear indications for ROS (reactive oxygen species) production are available. Increase in gut satiety hormones (e.g. CCK) levels is considered the mechanism of DAS (and trichothecenes) induced anorexia.
The CONTAM Panel concluded that the mode of action clearly indicated cytotoxic properties on haematopoietic progenitors which could be due to stimulation of apoptosis or inhibition of protein synthesis.
3.2. Consideration of critical effects and identification of reference points
3.2.1. Human hazard characterisation and derivation of health‐based guidance values
3.2.1.1. Consideration of critical studies
Based on the conclusions on the genotoxicity and the MoA of DAS (see Sections 3.1.4.5 and 3.1.8) the CONTAM Panel decided to establish health‐based guidance values (HBGV) for both the acute and chronic exposure of DAS for humans.
The CONTAM Panel noted that the human risk of DAS in food had not been assessed by EFSA previously and therefore reviewed all available data from studies conducted in experimental animals and humans as described in Section 3.1.
No oral chronic toxicity studies were identified in experimental animals and only few subacute oral toxicity studies were found in rats and guinea pigs, treated over a period of 5 or 4 weeks at the dose(s) of 1 mg/kg bw per day and 0.6–1.6 mg/kg bw per day, respectively. The Panel considered the database of these oral studies insufficient for the derivation of a RP for the human hazard characterisation.
In a next step, the CONTAM Panel noted that the selection of the starting dose in phase I clinical trials was based on the outcome of preclinical studies in dogs and monkeys. To mimic different clinical scenarios, DAS was administrated in these studies repeatedly via i.v. (weekly administration or for five consecutive days every two weeks, eventually repeated a few times) at doses ranging from 0.016 to 2 mg DAS/kg bw per day in dogs and 0.125 to 1 mg DAS/kg bw per day in monkeys. Dose‐dependent emesis, haematotoxicity and myelotoxicity (see Section 3.1.4.2, Table 5) were observed. The CONTAM Panel considered that this database in dogs and monkeys was too limited to be used for the derivation of a RP for the human hazard characterisation.
By contrast human data from clinical studies (summarised in Section 3.1.7) were considered by the CONTAM Panel as relevant, consistent and sufficiently informative to establish HBGV for both acute and chronic exposure of humans.
No clinical studies after oral administration of DAS and no other data in humans that would inform on the hazard of oral exposure (e.g. from a human food poisoning outbreak clearly associated with DAS) were identified. Therefore, the CONTAM Panel decided to base the human hazard characterisation of DAS on the available clinical studies in patients treated by i.v. administration of DAS (anguidine) for cancer.
Data on the toxicity of DAS was available for almost 500 patients recruited in phase I and II clinical trials conducted between 1978 and 1986. The Panel noted that about 100 patients were treated in clinical dose‐finding studies of phase I starting with i.v. doses as low as about 0.1 mg DAS/m2 (corresponding to 0.0027 mg DAS/kg bw) per day. These doses were chosen low enough to avoid signs of toxicity, however in principle not too far from a dose possibly effective against cancer. The dosed used in the phase I study ranged up to 10 mg DAS/m2 (corresponding to 0.27 mg DAS/kg bw) per day where severe toxicity was reported, considered as dose limiting in the phase I studies. DAS treatment was administered in cycles where one cycle usually consisted of 5‐day i.v. infusion every 3 weeks or once a week. Since the treatment period lasted from a few weeks to a few months the CONTAM Panel considered these clinical trials as short‐term studies of DAS. The reported data on clinical side effects (also denoted as adverse effects or events in the context of clinical drug development) were considered as informative for oral toxicity of DAS in humans.
It was also noted that these patients may be considered as a specific vulnerable subpopulation since patients entering phase I studies usually are at advanced cancer stage and in general already pre‐treated with other anticancer drugs.
3.2.1.2. Relationship between oral and i.v. administration and toxicity of DAS
In order to evaluate a relationship between the i.v. and the oral exposure of DAS, the CONTAM Panel assessed all available information pertaining to ADME characteristics in humans and in animals.
No pharmacokinetic data in humans were available from clinical studies after i.v. administration of DAS and no data after oral administration were identified neither in humans nor in experimental animals. Therefore metabolic or kinetic differences between oral and i.v. administration of DAS could not be assessed for humans. In general, oral administration is expected to lead to delayed and lower peak concentrations in the organism, with longer half‐life than after i.v. administration (Timbrell, 2000; Goodman et al., 2001).
In addition after oral ingestion, DAS might be subject to gastro‐intestinal degradation and a strong first‐pass effect with early production of metabolites may occur. There were also indications from in vitro studies that DAS might be deepoxidated or deactylated to a large extent in the gut (Section 3.1.1.3).
The CONTAM Panel considered that the available data from animals are suggestive for an almost complete absorption and/or quick clearance after oral and i.v. administration (see Sections 3.1.1 and 3.1.2). Accounting for some variability in the bioavailability, the levels of DAS after oral administration may reach a fraction of up to about 80% of the i.v. levels. Therefore, considering exposure after oral dosing as equivalent to i.v. dosing can be regarded as a worst‐case scenario.
The limited available experimental data suggested that the toxicity of DAS when given orally was not higher than the toxicity after i.v. administration, as shown in the comparison of LD50 values over various species in Table 4 (Section 3.1.4.1).
In humans, severity of adverse effects observed in clinical use of DAS was correlated with the duration of the i.v. infusion. Bolus injection (e.g. 5–10 min) caused more severe acute effects (mainly nausea, vomiting, hypotension and CNS toxicity) compared to prolonged infusion (e.g. 4–8 h) with a tendency to an increased severity of haematotoxicity and myelotoxicity, see Section 3.1.7.
Available data on the MoA indicate for DAS cytotoxicity and binding with ribosomes, inducing a ‘ribotoxic stress response’ with activation of ribosome‐associated MAPKs and inhibition of protein and DNA synthesis, but without clear indications for ROS production (Section 3.1.8). Therefore, what is currently known about the MoA is not against the assumption of similar toxicity of DAS when given orally or via i.v.
Taking into account the similar or lower toxicity of DAS metabolites (MAS and SCT, see section 3.1.4.7 and the aforementioned ADME aspects), the Panel assumed that the toxicity of DAS after oral administration would not lead to higher systemic toxicity compared to that after i.v. administration.
The CONTAM Panel noted, however, data gaps that prevent a proper assessment of possible GI toxicity (i.e. mucositis) of DAS when orally ingested. Furthermore the available clinical data of patients treated via i.v. do not permit a full assessment of local oral and GI tract toxicity. Oral administration might lead to exposure by contact in the upper GI tract. The Panel noted that GI effects, and particularly oral mucositis, are mainly ascribed to damage of basal mucosal layers after i.v. administration of anticancer cytotoxic chemotherapy (Duncan and Grant, 2003; van Vliet et al., 2010). In clinical trials with cytotoxic drugs mucositis often occurs together with other adverse effects such as GI symptoms, nausea and vomiting, haematotoxicity and CNS toxicity. It should be noted that the toxicity profile of DAS in phase II trials conducted at 3–5 mg/m2 per day was very similar to the profile seen in phase I studies. The absence of effects in the mucosa in a total of 11 independent i.v. clinical studies of DAS would therefore not support the hypothesis that oral toxicity of DAS would be significantly larger than i.v. toxicity at similar doses.
In summary, toxicity after oral exposure of DAS was considered as similar to or lower than that after i.v. administration and equitoxic effects for the same DAS dose were assumed when DAS was administered orally or intravenously. That might lead to an overestimation of the hazard after oral exposure such that the CONTAM Panel concluded that the use of data generated by i.v. administration might represent a worst‐case scenario for hazard characterisation and therefore be adequately protective for the consumers.
3.2.1.3. Critical effects and derivation of an acute reference dose (ARfD) for DAS
Data on acute adverse health effects in humans were identified in clinical phase I studies when DAS was administered to humans in dose‐finding studies with doses ranging from 0.1 to 2.0 mg DAS/m2 (corresponding to 0.0027 to 0.054 mg DAS/kg bw, using the conversion factor of 3715 to express doses from square meters (m2) on a kg/bw basis). The most frequent acute adverse effects observed were nausea, vomiting, some CNS effects and hypotension followed by haematotoxicity (Murphy et al., 1978; Goodwin et al., 1978, DeSimone et al., 1979). The CONTAM Panel identified nausea and emesis as the most prominent acute adverse health effect of DAS in humans and selected this as critical effect to determine a RP for the acute risk of humans exposed to DAS. The dose of 1.2 mg DAS/m2, equivalent to 0.032 mg DAS/kg bw, was indicated as dose without any emetic effect from clinical studies of phase I.
The CONTAM Panel considered these data sufficiently informative to determine a low level of dietary human intake at which no substantial acute health effects occurred. Nausea and vomiting were identified as the critical acute effects to allocate an ARfD for humans exposed to DAS.
Based on these clinical data and conservatively assuming 100% bioavailability, the CONTAM Panel identified 0.032 mg DAS/kg bw as a RP for acute adverse health effects of DAS.
The CONTAM Panel acknowledged that the cancer patients might represent a vulnerable population more prone to develop adverse acute effect (mainly nausea and vomiting) compared with the general population. Nevertheless in order to ensure a more protective approach, the Panel considered the application of an uncertainty factor (UF) for inter‐human variability (EFSA Scientific Committee, 2012) and used the UF of 10 to account for differences in toxicokinetics and toxicodynamics between humans. This is considered as a conservative approach since human variability and toxicokinetic differences would be limited to metabolism and excretion in the case of i.v. administration.
Therefore, applying the UF of 10 to the RP of 0.032 mg DAS/kg bw identified above, the CONTAM Panel established 0.0032 mg DAS/kg bw (i.e. 3,200 ng DAS/kg bw) as ARfD for the acute exposure of humans to DAS.
3.2.1.4. Critical effects and derivation of a tolerable daily intake (TDI) for DAS
The CONTAM Panel identified haematotoxicity and myelotoxicity as critical adverse health effects after repeated exposure to DAS.
The Panel noted that haematotoxicity and myelotoxicity of DAS are expected to induce leucopenia, agranulocytosis, and anaemia due to its cytotoxicity on circulating blood cells and on haematopoietic progenitor cells in bone marrow (Section 3.1.8). These haematological effects were also observed in repeated dose studies in animals after repeated oral or i.v. exposure to DAS (Section 3.1.4.2).
The observed adverse health effects in clinical phase I studies were identified as pivotal data also for human chronic hazard characterisation and the data from three prospectively defined dose‐escalation schemes were investigated for the identification of a NOAEL for haematotoxicity and myelotoxicity (DeSimone et al., 1979; Murphy et al., 1978; Goodwin et al., 1978 – Section 3.1.7). The CONTAM Panel concluded from these data that haematotoxicity and myelotoxicity would not constitute an adverse health effect in humans when exposed at a dose of 2.4 mg/m2 per day (i.e. 65 μg/kg bw per day).
While the phase II clinical trials confirmed DAS‐related adverse effects, for the characteristics and objectives of the studies they were considered of limited value to identifying a RP for hazard characterisation. However, they might be considered as confirmatory for the choice of the critical adverse effect.
In order to account for inter‐human toxicokinetic (possible accumulation in bone marrow cannot be assessed or ruled out) and toxicodynamic variability of DAS, the CONTAM Panel applied the UF of 10 (EFSA Scientific Committee, 2012) on this RP for chronic adverse health effects.
In addition, the Panel noted that the adverse health effects in phase I clinical studies were observed usually within less than 3–6 months (i.e. about 3–8 treatment cycles using intermittent dosing regimens). Taking into account the limited duration of the exposure and the relatively limited number of patients exposed in the phase I studies used for the determination of the RP for chronic exposure above, the Panel decided to apply an additional UF of 10 to adjust for those limitations. Considering the range of factors (2–10) suggested for the extrapolation from short‐term to chronic studies by different agencies (EFSA Scientific Committee, 2012) and accounting for the type of human clinical phase I data available, the CONTAM Panel noted that the use of a factor of 10 to account for these above‐mentioned limitations should be considered as conservative approach in the hazard characterisation of human chronic exposure.
Therefore, the CONTAM Panel applied an overall UF of 100 to the NOAEL of 65 μg DAS/kg bw per day, and established a TDI of 650 ng/kg bw per day for the chronic dietary exposure of humans to DAS. It is noted that the application of two UFs of 10 is a conservative approach.
3.2.2. Consideration of critical effects and identification of reference points for farm and companion animal risk assessment
Because of the limited available data on toxicity after oral exposure, NOAELs and/or LOAELs for adverse effects of DAS for farm and companion animals were only identified for pigs, poultry (Section 3.1.5) and dogs (Section 3.1.4).
3.2.2.1. Pigs
Reduced feed intake and body weight gain, oral lesions and GI hyperplasia were identified as adverse health effects in two short‐term (28–63 days) oral studies on pigs. The CONTAM Panel identified from these data a LOAEL of 2 mg DAS/kg feed for oral lesions corresponding to 0.08 mg DAS/kg bw per day as RP see Table 10. The LOAEL for reduced bw gain was 0.12 mg DAS/kg bw per day. The Panel noted that this hazard characterisation was based on sparse data.
Table 10.
Summary of NOAELs and LOAELs identified for hazard characterisation of DAS for those farm and companion animal species for which data on relevant adverse health effects could be identified. The NOAEL/LOAEL values are reported both as doses (mg DAS/kg bw) and concentrations (mg DAS/kg feed)
| Animal species | Reference point dose | Reference point concentration | Adverse effect | ||
|---|---|---|---|---|---|
| NOAEL (mg DAS/kg bw per day) | LOAEL (mg DAS/kg bw per day) | NOAEL (mg DAS/ kg feed) | LOAEL (mg DAS/kg feed) | ||
| Pigs | n.d. | 0.08 | n.d | 2.0 | Oral lesions |
| n.d. | 0.12 | n.d. | 2.0 | Reduced bw gain | |
| Chicken | n.d. | 0.01 | n.d. | 0.2 | Oral lesions |
| 0.05 | 0.1 | 1.0 | 2.0 | Reduced bw gain | |
| 0.2 | 0.4 | 5.0 | 10.0 | Reduced fertility | |
| Laying hens | n.d. | 0.054 | n.d | 2.0 | Oral lesions |
| 0.33 | 0.60 | 5.0 | 10 | Reduced bw gain | |
| 0.60 | 1.01 | 10 | 20 | Reduced egg production | |
| Ducks | n.d. | 0.022 | n.d. | 0.3 | Oral lesions |
| Turkeys | n.d. | 0.012 | n.d. | 0.2 | Oral lesions |
| 0.047 | 0.29 | 0.86 | 4.0 | Reduced bw gain | |
| Dogs | 0.031 | n.d. | 0.08 | n.d. | Emesis |
| 0.016 | 0.031 | 0.04 | 0.08 | Haematotoxicity | |
DAS: diacetoxyscirpenol; bw: body weight; NOAEL: no observed adverse effect level; LOAEL: lowest observed adverse effect level; n.d.: not determined,
3.2.2.2. Poultry
Oral lesions were the first observed adverse effect and were also observed at the lowest level of DAS in chickens, laying hens, ducks and turkeys.
For chickens, the CONTAM Panel identified oral lesions as the most sensitive critical effects of DAS with a LOAEL of 0.2 mg DAS/kg feed, corresponding to 0.01 mg DAS/kg bw per day. It was not possible to define a NOAEL for this effect. NOAELs (LOAELs) for reduced bw gain were 1.0 (2.0) mg DAS/kg feed corresponding to 0.05 (0.1) mg DAS/kg bw per day (Table 10).
For Broiler breeders, the CONTAM Panel identified only a LOAEL but no NOAEL for the most critical adverse effect i.e. oral lesions; this LOAEL was 5.0 mg DAS/kg feed corresponding to 0.2 mg DAS/kg bw per day.
For laying hens, the CONTAM Panel identified a LOAEL of 2.0 mg DAS/kg feed for oral lesions as the critical effect, corresponding to 0.054 mg DAS/kg bw per day. It was not possible to define a NOAEL for this effect. NOAELs (LOAELs) for reduced bw gain and for reduced egg production were 5.0 (10.0) and 10.0 (20.0) mg DAS/kg feed, respectively, corresponding to 0.33 (0.60) and 0.60 (1.01) mg DAS/kg bw per day (Table 10).
For ducks, the CONTAM Panel identified a LOAEL of 0.25 mg DAS/kg feed for oral lesion, corresponding to 0.022 mg DAS/kg bw per day. NOAELs (LOAELs) for reduced bw gain or other adverse effects were not identified.
For turkeys, the CONTAM Panel identified a LOAEL of 0.22 mg DAS/kg feed for oral lesions as the most sensitive critical effect of DAS, corresponding to 0.012 mg DAS/kg bw per day whereas no NOAEL was identified, see Table 10. The NOAEL (LOAEL) for reduced bw gain was 0.86 (4.0) mg DAS/kg feed, corresponding to 0.047 (0.29) mg DAS/kg bw per day.
3.2.2.3. Dogs
No data were available on the toxicity after oral exposure of DAS for dogs. However, the CONTAM Panel noted that suitable data on the toxicity of DAS were available from several preclinical studies on DAS (anguidine) administered via i.v. in beagle dogs that inform on the toxicity of DAS (section 3.1.4.2). The Panel considered these data as informative and useful for the hazard characterisation of dogs, similarly as the data on i.v. administration which were used for the hazard characterisation of humans. Therefore, when considering the bioavailability of the oral dose as same as for the i.v. dose and assuming equitoxic effects of both administration routes, the Panel identified a NOAEL of 0.031 and 0.016 mg/kg bw per day for acute and chronic risk for dogs, respectively (related to emesis or haematotoxicity, see Table 10). These doses were calculated as equivalent to 0.08 and 0.04 mg DAS/kg feed when assuming a weight of 10 kg and 0.25 kg feed per day. The Panel noted that the use of these preclinical data generated by i.v. administration is subject to uncertainty and might overestimate the risk.
3.2.2.4. Other farm and companion animals
Only one experiment was identified which compared lambs exposed to 5 mg DAS/kg feed (equivalent to 0.16 mg DAS/kg bw per day) with controls (n = 6 per group) and identified reduced feed intake and weight loss as adverse health effects for these ruminants. No RP could be derived from this study. For the other farm animal such as farmed rabbits, fishes and mink, and also for horses and cats no toxicity data were available or suitable for the hazard characterisation of DAS to identify NOAELs/LOAELs.
In order to obtain an indication on the risk of DAS for these species, the CONTAM Panel considered the lowest LOAEL of 0.2 mg DAS/kg feed (corresponding to 0.01 mg DAS/kg bw per day) for oral lesions in poultry as indicative for potential adverse health effects of DAS.
3.3. Occurrence of DAS in food and feed
3.3.1. Occurrence data on food and feed reported in the available literature
Data on the occurrence of DAS in food and feed have been published in the literature, in particular in the last decade. DAS has been reported to co‐occur with many other mycotoxins in grains and grain products DAS co‐occurred in particular with Fusarium toxins including type A and B trichothecenes and zearalenone. In coffee, DAS co‐occurred with type A and B trichothecenes, zearalenone, aflatoxins, fumonisins, ochratoxin A, sterigmatocystin, enniatins and beauvericin.
3.3.1.1. Food
Twenty‐three publications could be identified, which contained relevant information about the occurrence of DAS in food grains and food products, marketed in Europe and sampled from 2000 onwards (Appendix C). Data from previous years were not considered, due to limitations in the performance of the analytical methods used before 2000 and to ascertain a representative picture, because changes in environmental conditions and agricultural practices might have influenced DAS formation by Fusarium species (this also holds true for Section 3.3.1.2). With few exceptions, LC/MS or GC/MS was used as analytical technique to determine DAS. The publications contained data from 12 studies on DAS in food grains and from 13 studies on DAS in food products. Altogether 5562 analytical data on occurrence of DAS were reported. Due to sometimes, incomplete and inconsistent reporting details in the various papers, interpretation of the results was at times difficult and conclusions should therefore be made with caution. There is also an uncertainty for the classification of grains as ‘food grains’. It cannot be excluded that some of these grains were (also) used as ‘feed grains’.
The majority of the samples (n = 3,781; 68%) originated from the studies in which grains were investigated. Only 183 samples (4.8%) were found positive for DAS. In five of the grain studies DAS was not found at all. Highest concentrations were reported in samples of wheat (130 μg/kg) and sorghum (91 μg/kg). Other types of grains (e.g. maize, barley and oats) were only occasionally found contaminated with DAS, while concentrations were at lower levels.
In the studies in which food products were investigated, 1,781 samples were analysed, of which 122 samples (6.9%) were found positive for DAS. In five of the food product studies DAS was not found at all. Highest concentrations were reported in samples of brewed coffee (402 μg/kg, expressed as μg/kg roasted coffee), potato products (21 μg/kg and roasted soy beans (21 μg/kg). DAS was infrequently detected at lower concentrations in corn germ oil, roasted coffee beans and oat products.
The literature data on occurrence of DAS in brewed coffee (García‐Moraleja et al., 2015b) are remarkable, since the mean DAS concentration reported in brewed coffee is a factor 30 times higher than that in roasted coffee beans (all concentrations standardised to solid coffee and therefore comparable), analysed by the same authors in another study. Because the DAS concentrations reported for brewed coffee were inconsistent with the DAS concentrations reported for commercial roasted coffee, the authors were contacted for explanation and further details. Since this did not yield the required information, the literature data on DAS in brewed coffee were not accepted for inclusion in the EFSA database for exposure assessment.
In summary, literature data about DAS in food grains and food products show that DAS has been reported in the literature to occur at relatively low incidences and concentrations in grains and grain products, while DAS has also been found to occur in coffee.
3.3.1.2. Feed
Relatively few studies (published since 2000) have analysed for the presence of DAS in feeds, and in those that have been reported the frequency of detection was low. For example, in samples of maize kernels (n = 21), maize silage (n = 18) and ‘other feed components (n = 15), DAS was only quantified in one sample of each food type analysed (Schollenberger et al., 2005). It was also quantified in 1/21 samples of maize kernels, but not detected in whole plants (n = 9), or other maize‐derived feeds (n = 11) (Schollenberger et al., 2005). DAS was quantified in maize kernels (2/41 samples analysed) and maize by‐products (1/13 samples) by Schollenberger et al. (2006) and in all of the 17 samples of feed oats examined by Gottschalk et al. (2009). Details of levels reported are given in Appendix D.
In complete feedingstuffs, DAS was quantified in 10 of 50 samples of complete feeds for poultry (Labuda et al., 2005) with a mean concentration of 3.6 μg/kg. Dall'Asta et al. (2004) reported the occurrence of DAS in maize‐based feeds for fattening pigs. Of the seven samples analysed (LC/ESI–MS (LOD = 30 μg/kg), five were < LOD, while levels in the remaining two samples were 376 and 847 μg/kg. Since these values significantly exceeded levels reported in cereal grains that would usually used in the production of these compound feeds (see Table 15), the CONTAM Panel concluded that they were unsuitable to use in the estimate of exposure.
Table 15.
Summary of probabilistic acute dietary exposure assessment to DAS (UB) across European dietary surveys by age group
| Age group | n | Mean UB exposure (ng/kg bw per day) | n | 95th percentile dietary UB exposure (ng/kg bw per day)a | ||
|---|---|---|---|---|---|---|
| Min | Max | Min | Max | |||
| Infants | 11 | 22.6 (16.6–29.4) | 181.1 (165.0–196.1) | 10 | 118.3 (90.9–185.2) | 799.1 (673.1–931.6) |
| Toddlers | 15 | 50.4 (46.8–56.8) | 186.0 (163.6–221.5) | 15 | 173.5 (167.1–180.3) | 736.3 (705.7–771.3) |
| Other children | 21 | 34.8 (32.8–37.1) | 175.1 (160.0–195.9) | 21 | 114.6 (104.0–124.6) | 702.5 (670.6–735.3) |
| Adolescents | 21 | 24.6 (23.4–25.8) | 106.2 (92.5–122.5) | 21 | 89.0 (84.0–93.5) | 354.4 (334.8–372.4) |
| Adults | 23 | 21.7 (21.0–22.4) | 85.4 (77.7–97.0) | 23 | 62.5 (58.2–66.9) | 283.1 (264.2–294.1) |
| Elderly | 20 | 17.9 (17.2–18.6) | 75.2 (72.3–78.6) | 20 | 54.0 (48.6–60.7) | 203.3 (182.3–224.8) |
| Very elderly | 17 | 17.1 (16.1–18.2) | 83.2 (81.5–85.2) | 15 | 62.5 (57.9–68.4) | 182.6 (132.2–214.9) |
bw: body weight; n: number of surveys; Min: minimum; Max: maximum.
The corresponding 95% confidence intervals are presented in the brackets.
The 95th percentile estimates obtained on dietary surveys/age classes with less than 60 observations may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
However, in most of the published studies, DAS was not detected. For cereal grains, DAS was not detected in Austrian maize (n = 23) or oats (n = 18) harvested in 2002 (Fuchs et al., 2002), in 446 samples of barley harvested in the UK (Edward, 2009), or in 115 sample of Finnish wheat, rye, barley and oats (Eskola, 2002). DAS was also not quantified in samples of wheat (n = 41) or oats (n = 17), (Schollenberger et al., 2006), nor in 46 samples of Italian wheat collected in 2009 and 2010 (Alkadri et al., 2014).
For non‐cereal feedingstuffs, DAS was not quantified in soybean meal (n = 13), rapeseed meal (n = 12), field peas (n = 25), lupines (n = 9) or other oilseed by‐products (sunflower meal, linseed meal, palm kernel expeller, n = 8) (Schollenberger et al., 2006).
For forages, DAS was looked for, but not detected, neither in maize silage and grass silages in the Netherlands (Driehuis et al., 2008), nor in maize plants (n = 8), maize silage (n = 5) or grass hay (n = 28) in Germany (Schollenberger et al., 2006).
Those studies in which DAS has been identified in feeds are summarised in Appendix D.
3.3.2. Occurrence data submitted to EFSA
From 2000 onwards to December 2017, 20,061 analytical results of food and feed with analytical data on DAS were available in the EFSA database.
During the data cleaning process, data providers were contacted to clarify a number of possible inconsistencies which were identified during the data check. The following modifications were made to the initial data set based on the feedback received:
The product description of a number of records allowed a more accurate FoodEx classification. In these cases, the samples were reclassified to a more specific, detailed level.
56 samples reported as ‘suspect sampling’ were not taken into account in the current assessment.
Results of samples reported to be analysed by the ELISA method (n = 10) were not considered sufficiently reliable to be included in the assessment.
1,152 analytical results were found to be duplicate submission and were disregarded.
1,409 analytical results were submitted with misreported parameter code and therefore they were excluded from the assessment.
One sample on unspecified feed terms was disregarded from the assessment as it was not possible to identify which feedstuff was analysed.
The cleaned database contained 17,433 samples provided by national authorities from Finland, Germany, Ireland, Luxembourg, the Netherlands, Slovenia and the United Kingdom (UK) for food (n =15,485) and France, Germany, the Netherlands, Slovakia, Slovenia, Sweden and the UK for feed (n = 1,948).
3.3.3. Occurrence data used for the assessment
As an outcome of the literature search, additional occurrence data on DAS in food (i.e. 301 analytical results) were obtained from the scientific literature (see Appendix C). Additional occurrence data on DAS in feed (i.e. 150 analytical results) were obtained from the scientific literature (see Appendix D).
In summary, a total of 17,885 analytical results (i.e. 15,786 on food, 2,098 on feed) were available for the exposure assessment. (Annex A, Table 1A‐B, Annex A, Table 2A‐B).
Occurrence levels used in the assessment from the available data set are presented in Tables 11 and 12. Note that the food categories represented by either very low number of samples (≤ 6 samples) or by all data left‐censored on FoodEx Level 2, were considered not being suitable and were not used in exposure calculation.
Table 11.
Occurrence levels (μg/kg bw) of DAS in food categories considered in the exposure assessment scenarios
| Food category | No samples | LB mean | UB mean | LB P95 | UB P95 |
|---|---|---|---|---|---|
| Breakfast cereals | 1,075 | 0.14 | 15.68 | 0.00 | 50.00 |
| Fine bakery wares | 504 | 0.05 | 9.45 | 0.00 | 10.00 |
| Grain milling products | 2,135 | 0.04 | 13.51 | 0.00 | 50.00 |
| Grain for human consumption | 7,101 | 0.14 | 11.43 | 0.00 | 50.00 |
| Pasta (Raw) | 292 | 0.001 | 22.75 | 0.00 | 50.00 |
| Coffee beans, roasted | 103 | 0.76 | 6.05 | 6.50 | 6.50 |
| Oilseeds | 476 | 0.05 | 35.96 | 0.00 | 50.00 |
| Vegetable oil | 136 | 0.09 | 7.18 | 0.00 | 10.00 |
| Cereal‐based food for infants and young children | 265 | 1.06 | 17.76 | 20.00 | 50.00 |
| Ready‐to‐eat meal for children, cereal‐based | 11 | 0.18 | 12.00 | – | – |
| Cereal‐based dishes | 50 | 12.02 | 56.82 | – | – |
| Snack food | 128 | 2.34 | 14.05 | 8.00 | 50.00 |
DAS: diacetoxyscirpenol; bw: body weight; LB: lower bound; UB: upper bound; –: not determined (< 60 samples available, see Section 2.8.2).
Table 12.
Occurrence levels (μg/kg bw) of DAS in food categories considered in the exposure assessment scenarios
| Feed Group level 1 | Feed category | No samples | LB mean | UB mean | LB P95 | UB P95 |
|---|---|---|---|---|---|---|
| Cereal grains, their products and by‐products | Maize_&_Corn | 233 | 0.51 | 28.04 | 0.00 | 50.00 |
| Cereal grains, their products and by‐products | Oats | 380 | 0.92 | 5.93 | 4.44 | 13.91 |
| Cereal grains, their products and by‐products | Rice, broken | 194 | 0.49 | 49.77 | 0.00 | 50.00 |
| Cereal grains, their products and by‐products | Wheat | 591 | 0.01 | 6.14 | 0.00 | 10.00 |
| Compound feed | Complementary/Complete feed (Poultry, starter diets) | 271 | 0.13 | 19.78 | 0.00 | 50.00 |
| Forages and roughage, and products derived thereof | Maize silage | 35 | 1.83 | 42.07 | – | – |
DAS: diacetoxyscirpenol; bw: body weight; LB: lower bound; UB: upper bound; –: not determined (< 60 samples available, see Section 2.8.2).
3.3.3.1.
Analytical methods
Considering all the available analytical results (i.e. the results submitted to EFSA by Member States and the results extracted from the published literature), where classification of the analytical method used for determination of DAS in food was reported by the Member States, results were obtained by LC–MS(/MS) (40%), GC–MS (31%) and HPLC‐based methods (1%). For the remaining 29% of the data, no information on analytical methods was reported.
For feed 74% of the data were analysed by LC–MS(/MS) and 26% by GC‐MS.
3.3.3.2. Food occurrence data used for the assessment
Altogether, including the results submitted to EFSA by Member States and the results extracted from the published literature 15,786 analytical results were available for food from 10 European countries (Table 13). Detailed list of food categories and analytical results on FoodEx levels 2 and 3 is shown in Annex A, Table 1A and 1B respectively. All analytical results shown in the table were expressed on whole weight basis, thus no conversion had to be applied.
Table 13.
Number of food samples in the final data set by countries
| Sampling country | No | % |
|---|---|---|
| Finland | 43 | 0.3 |
| Germany | 4,785 | 30 |
| Ireland | 129 | 0.8 |
| Italy | 14 | 0.1 |
| Luxembourg | 30 | 0.2 |
| Netherlands | 3,790 | 24 |
| Poland | 98 | 0.6 |
| Slovenia | 7 | 0.04 |
| Spain | 103 | 0.7 |
| United Kingdom | 6,787 | 43 |
The origin of the samples was not always the European country who reported the data, i.e. the data set also contained samples originating from North and South America, Africa, Asia and Australia.
The LODs/LOQs of the DAS data from the EFSA database and the scientific literature data varied between laboratories and food matrices (i.e. LOD minimum–maximum 0.1–25 μg/kg, mean 10 μ/kg; LOQ minimum–maximum 0.1–50 μg/kg, mean 21 μg/kg). The lowest mean LOQs were reported for the FoodEx level 2 categories of ‘Grains for human consumption’, ‘Grain milling products’ and ‘Breakfast cereals’. A high percentage of results below LOD/LOQ in combination with high LODs/LOQs with substantial differences between LB and UB scenarios were observed, increasing the uncertainty associated with the dietary exposure estimations.
An evaluation of LOQs was performed for those DAS data for which the results were considered to be suitable for the dietary exposure assessment, and in case of high LOQs data providers were contacted, but as they were confirmed by them no exclusion was made.
3.3.3.3. Feed occurrence data used for the assessment
Altogether, including the results submitted to EFSA by Member States and the results extracted from the published literature 2,098 analytical results were available for feed from seven European countries (Table 14). The detailed list of feed categories and analytical results on levels 2 and 3 is shown in Annex A, Table 2A and 2B, respectively. Analytical results expressed on whole weight basis were transformed to 88% dry matter (DM) basis, thus in the annex tables all the presented data is expressed in 88% DM basis.
Table 14.
Number of feed samples in the final data set by countries
| Sampling country | No | % |
|---|---|---|
| France | 413 | 20 |
| Germany | 100 | 5 |
| Netherlands | 278 | 13 |
| Slovakia | 50 | 2.4 |
| Slovenia | 167 | 8 |
| Sweden | 228 | 11 |
| United Kingdom | 862 | 41 |
The LODs/LOQs of the DAS data from the EFSA database and the scientific literature data varied between laboratories and feed matrices (i.e. LOD minimum–maximum 0.1–50 μg/kg, mean 10 μ/kg; LOQ minimum–maximum 0.1–100 μg/kg, mean 23 μg/kg). The lowest mean LOQs were reported for the FoodEx level 2 categories of ‘Oats’, ‘Wheat’ and ‘Complementary/Complete feed. A high percentage of analytical results below LOD/LOQ in combination with high LODs/LOQs resulted in substantial differences between LB and UB exposure scenarios. This was considered as an uncertainty associated with the dietary exposure estimations.
An evaluation of LOQs was performed for those DAS data for which the results were considered to be suitable for the dietary exposure assessment, and no exclusion was made.
3.3.4. Food and Feed processing
3.3.4.1. Food processing
The extent to which cereals are processed depends on the cereal type and the final feed/food product. Processing reduces Fusarium toxin concentrations in products for human consumption but may increase levels in food or feed by‐products. Published studies on the effects of processing of cereals and cereal products on the concentrations of DAS in food and feed are very scarce. As common for trichothecenes, sorting of cereals by removing those extensively damaged or infected kernels such as those with visible mould growth or shrivelled kernels, has the effect of lowering the concentrations of most mycotoxins, including several trichothecenes, in products that are subsequently produced (Ryu et al., 2008).
No specific studies could be identified on the fate of DAS during cleaning and sorting. However it is assumed that, as for trichothecenes in general, mechanical cleaning of cereals (dehulling) may lead to by‐products (for the feed industry) in which DAS concentrates significantly. This would be expected to result in higher concentrations of DAS in these materials than in the cereals before cleaning.
Rolling and milling
Although no studies could be identified on the specific effects of rolling and milling on DAS in grains, it would be expected that DAS behaves similarly as other trichothecenes. In general, milling has the effect of reduction and redistribution of trichothecenes, probably also for DAS. Higher concentrations of DAS in particular fractions such as the bran and shorts would be expected. During dry milling, DAS is expected to concentrate in the fractions containing the outer parts of the grain rather than in those fractions containing the inner parts of the grain, as also is the case for DON (Abbas et al., 1985; Lešnik et al., 2008). Therefore, the highest levels of DAS would be expected in the germ, screenings, dust and bran while the flour and grits would contain lower levels than those found in the grain before dry milling (Hazel and Patel, 2004).
Cooking and baking
Only one study was identified on the effect of thermal food processing of DAS. Kanimura et al. (1979) investigated the fate of DAS in bread and Japanese and Chinese noodles prepared from wheat flour under various conditions of manufacturing and cooking. When adding chloroform solutions containing DAS, reductions of initial levels of DAS after heating were reported. Since initial and final concentrations of DAS were not given, these data were not used for assessing the role of cooking and baking.
3.3.4.2. Feed processing
No information on impact of feed processing on DAS concentrations, specifically in feed materials, has been identified. However, cereal grains intended for use as animal feed are usually subject to some of the processes used for processing grains for human consumption (cleaning, sorting, drying, rolling/grinding and/or extrusion) before being fed to livestock, and therefore the effects of these processes reported above for food (Section 3.4.1) apply equally to cereal grains for animal feed.
For long‐term storage of cereal grains, a maximum moisture content of approximately 12% is generally recommended. In order to achieve this, air temperatures of up to 125–130°C may be used, resulting in grain temperatures of up to 45°C. Like most trichothecenes, DAS is stable at these temperatures (Schwake‐Anduschus et al., 2010) and therefore drying is unlikely to affect DAS concentrations in grains dried in this way.
Cereal by‐products resulting from the processing of grain for human consumption are widely used in livestock diets. The EC Catalogue of Feed Materials16 lists over 80 cereal by‐products used as animal feeds. In common with other mycotoxins, DAS is found predominantly on the outer layer of the grain. Dry milling generally results in a redistribution of DAS into separate milling fractions, and an increase of mycotoxins in particular fractions such as the bran and grits might be expected, although no data specifically for animal feeds have been identified.
Compound feeds consist of mixtures of feed materials and additives formulated to meet the specific nutritional requirements of the livestock to which they are fed. One of the final stages in the compound feed manufacturing process is the production of feed pellets, which results in an increase in temperature, with temperature increasing up to 95°C (Thomas et al., 1997). Like most trichothecenes, DAS is stable at these temperatures (Schwake‐Anduschus et al., 2010) and therefore compound fed manufacture is unlikely to affect concentrations in the finished product.
3.3.4.3. Summary
Published studies on the effects of food and feed processing on DAS were quite scarce and no firm conclusions could be drawn. However, the CONTAM Panel assumed that these practices are expected to result in a redistribution and/or reduction of DAS in the final products, as is the case with other trichothecenes.
3.4. Exposure assessment
3.4.1. Human exposure assessment
3.4.1.1. Mean and 95th percentile acute dietary exposure
The mean and 95th percentile of acute exposure estimates at the UB level are shown for the different age groups of humans in Table 15 as minimum (Min) and maximum (Max) over from different surveys and age groups. The numbers in brackets indicate the 95% confidence intervals obtained from the probabilistic approach (for more details, see Section 2.8.1), see also Annex A, Table 4.
Infants (< 12 months)
The mean acute dietary exposure ranged from 22.6 to 181.1 ng/kg bw per day, and the 95th percentile from 118.3 to 799.1 ng/kg bw per day.
Toddlers (≥ 12 months to< 36 months old)
The mean acute dietary exposure ranged from 50.4 to 186.0 ng/kg bw per day, and the 95th percentile from 173.5 to 736.3 ng/kg bw per day.
Other children (≥ 36 months to < 10 years old)
The mean acute dietary exposure ranged from 34.8 to 175.1 ng/kg bw per day, and the 95th percentile from 114.6 to 702.5 ng/kg bw per day.
Adolescents (≥ 10 years to < 18 years old)
The mean acute dietary exposure ranged from 24.6 to 106.2 ng/kg bw per day, and the 95th percentile ranged from 89.0 to 354.4 ng/kg bw per day.
Adults (≥ 18 years to < 65 years)
The mean acute dietary exposure ranged from 21.7 to 85.4 ng/kg bw per day, and the 95th percentile ranged from 62.5 to 283.1 ng/kg bw per day.
Acute dietary exposure in the two surveys of ‘Pregnant women’ from Portugal and Latvia, respectively, were within the range of exposure estimates in the adult population with mean exposures of 25.2 (23.3–29.7) and 53.0 (50.9–55.7) ng/kg bw per day and 95th percentile exposure of 78.7 (71.2–87.1) and 179.4 (170.7–187.4) ng/kg bw per day.
In the two surveys of ‘Lactating women’ from Estonia and Greece, respectively, the mean exposures were 44.6 (42.6–46.7) and 102.5 (86.2–128.2) ng/kg bw per day while 95th percentile exposures were 117.5 (106.6–132.6) and 346.8(322.5–371.2) ng/kg bw per day which are exceeding the average exposure of the adult population.
Elderly and very elderly (≥ 65 years old)
The mean dietary exposure ranged from 17.1 to 83.2 ng/kg bw per day for the elderly and very elderly, and the 95th percentiles ranged from 54.0 to 203.3 ng/kg bw per day.
3.4.1.2. Mean and 95th percentile chronic dietary exposure
The mean and 95th percentile of chronic exposure estimates at the UB to DAS obtained for different age groups are shown in Table 16. Detailed mean and 95th percentile dietary exposure estimates calculated for each dietary survey are presented in Annex A, Table 5.
Table 16.
Summary statistics of the chronic dietary exposure to DAS (ng/kg bw per day) across European countries
| Age group | N | Mean dietary exposure (ng/kg bw per day) | |||||
|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||||
| LB | UB | LB | UB | LB | UB | ||
| Infants | 11 | 0.2 | 14.9 | 1.4 | 54.4 | 8.0 | 92.3 |
| Toddlers | 14 | 0.5 | 49.0 | 1.4 | 81.2 | 23.5 | 184.8 |
| Other children | 19 | 0.4 | 34.4 | 1.2 | 70.1 | 27.9 | 174.3 |
| Adolescents | 18 | 0.2 | 23.5 | 1.2 | 44.3 | 18.4 | 105.9 |
| Adults | 19 | 0.2 | 16.3 | 0.9 | 24.5 | 8.9 | 66.2 |
| Elderly | 18 | 0.2 | 12.6 | 0.8 | 19.8 | 5.9 | 46.2 |
| Very elderly | 15 | 0.2 | 12.2 | 0.7 | 23.3 | 4.5 | 39.5 |
| Age classa | N | 95th percentile dietary exposure (ng/kg bw per day) | |||||
|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||||
| LB | UB | LB | UB | LB | UB | ||
| Infants | 10 | 0.5 | 67.3 | 3.9 | 107.6 | 41.2 | 315.9 |
| Toddlers | 12 | 1.0 | 97.6 | 3.8 | 150.3 | 88.0 | 488.1 |
| Other children | 19 | 1.0 | 71.8 | 4.4 | 155.8 | 97.0 | 490.5 |
| Adolescents | 17 | 0.5 | 49.5 | 3.6 | 104.3 | 52.4 | 264.7 |
| Adults | 19 | 0.6 | 35.2 | 2.1 | 55.5 | 35.9 | 194.4 |
| Elderly | 18 | 0.4 | 29.1 | 1.4 | 45.7 | 28.8 | 156.9 |
| Very elderly | 10 | 0.4 | 30.9 | 1.1 | 51.2 | 21.8 | 110.8 |
bw: body weight; LB: lower bound; UB: upper bound; N: number of surveys.
The 95th percentile estimates obtained on dietary surveys/age classes with less than 60 observations may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
Infants (< 12 months)
The mean chronic dietary exposure ranged from 0.2 to 92.3 ng/kg bw per day (minimum LB and maximum UB) and the 95th percentile dietary exposure from 0.5 to 315.9 ng/kg bw per day (minimum LB to maximum UB).
Toddlers (≥ 12 months to < 36 months old)
The mean chronic dietary exposure ranged from 0.5 to 184.8 ng/kg bw per day (minimum LB to maximum UB) and the 95th percentile dietary exposure from 1.0 to 488.1 ng/kg bw per day (minimum LB to maximum UB).
Other children (≥ 36 months to < 10 years old)
The mean chronic dietary exposure ranged from 0.4 to 174.3 ng/kg bw per day (minimum LB to maximum UB) and the 95th percentile dietary exposure from 1.0 to 490.5 ng/kg bw per day (minimum LB to maximum UB).
Adolescents (≥ 10 years to < 18 years old)
The mean chronic dietary exposure ranged from 0.2 to 105.9 ng/kg bw per day (minimum LB to maximum UB) and the 95th percentile dietary exposure from 0.5 to 264.7 ng/kg bw per day (minimum LB to maximum UB).
Adults (≥ 18 years to < 65 years)
The mean chronic dietary exposure to DAS ranged from 0.2 to 66.2 ng/kg bw per day (minimum LB to maximum UB) and the 95th percentile dietary exposure estimate from 0.6 to 194.4 ng/kg bw per day (minimum LB to maximum UB).
Chronic dietary exposure in the two surveys of ‘Pregnant women’ from Portugal and Latvia were within the range of exposure estimates in the adult population.
In the two surveys of ‘Lactating women’ from Estonia and Greece, the mean exposure ranged from 0.4 to 97.0 (minimum LB to maximum UB) and the 95th percentile dietary exposure estimate from 0.9 to 228.5 ng/kg bw per day (minimum LB to maximum UB).
Elderly and very elderly (≥ 65 years old)
The mean dietary exposure to DAS ranged from 0.2 to 46.2 ng/kg bw per day (minimum LB to maximum UB), the 95th percentiles dietary exposure estimate from ranged from 0.4 to 156.9 ng/kg bw per day (minimum LB to maximum UB).
3.4.1.3. Contribution of different food groups to the exposure
Contribution of the different food groups across dietary surveys is summarised by age class in Annex A, Table 6. These contributions were calculated on the basis of the LB exposure estimates. The results are reported as a number of surveys for the following contribution ranges: 0–5, 5–10, 10–25, 25–50, 50–75, > 75%.
In all population groups, ‘Cereal‐based dishes’ was the main contributor.
In the age class ‘Infants’ and ‘Toddlers’, additional important contributors were ‘Cereal‐based food for infants and young children’, and ‘Breakfast cereals’.
‘Snack food’ was contributing mostly in age classes ‘Toddlers’, ‘Other children’ and ‘Adolescents’.
In the adult population groups, ‘Coffee’ also occurs as an additional important contributing category.
3.4.2. Exposure assessment for farm and companion animals
Exposure estimates at the 95th percentile and mean concentrations for farm and companion animals are reported below. These were calculated by using the occurrence data set for feed (Section 3.3.3.2) and feed consumption as reported in Appendix E. For all species, the P95 and mean exposures have been estimated based on the 95th percentile and the mean LB and UB concentrations, respectively. According to EFSA (2010), caution is needed when calculating chronic exposure (95th percentile) where data on less than 60 samples are available, since the results may not be statistically robust. Therefore, in this Opinion, there are no acute exposure estimations where data on < 60 samples are available. Furthermore, EFSA (2010b) has indicated that estimates of chronic exposure based on data for < 10 samples are unreliable, and therefore where data on less than 10 samples have been provided these have not been used to estimate the mean LB and UB exposures.
Sufficient data on levels of DAS in species‐specific compound feeds have been reported for poultry starters diet: there were sufficient data (n = 118) with which to calculate mean and acute (P95) exposures. For all other livestock and companion animals, exposures to DAS have been based on concentrations in individual feed materials – mainly cereal grains – and their levels of inclusion in the diets. Data were provided on levels of DAS in complementary feed for dairy cows (n = 15) and in compound feeds for laying hens (n = 13); however, since all values were left censored they were not used to estimate exposure, and estimates were made using levels of DAS in individual feedingstuffs and their estimated intakes in diets for the individual animal species.
3.4.2.1. Ruminants and horses
Forages, either fresh or conserved, are essential ingredients in the diets of ruminants and horses, and in some cases may be the sole ingredient. However, only data for maize silage (n = 35) have been available for this Opinion and these, together with levels of DAS in non‐forage feeds, have been used to estimate exposure for dairy cows and beef cattle on maize silage‐based rations. For other diets for ruminants and horses, exposures have been made based on levels of DAS in cereal grains and assumed inclusion rates in their diets (for details, see Appendix E). In the absence of data on forages (other than maize silage) it is assumed that they make no contribution to exposure to DAS.
Estimates of exposures for ruminants and horses are given in Table 17.
Table 17.
Estimated 95th percentile (P95) and mean exposure to DAS by ruminants and horses, derived from concentrations in individual feed materials and their relative proportions in diets(a)
| Animal species (diet) | Diet concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw per day | ||||
|---|---|---|---|---|---|---|---|
| Mean | P95 | Mean | P95 | Mean | P95 | ||
| Ruminants on maize‐silage‐based diets | |||||||
| Dairy Cows (maize silage‐based diet) a | LB | 1.3 | nd | 36 | nd | 0.06 | nd |
| UB | 43 | nd | 1,193 | nd | 1.8 | nd | |
| Beef cattle (maize silage‐based diet) a | LB | 1.5 | nd | 10 | nd | 0.03 | nd |
| UB | 35 | nd | 234 | nd | 0.78 | nd | |
| Ruminants and horses on other forage‐based diets | |||||||
| Dairy cows (grass‐based diet) | LB | nd | nd | 0.01 | nd | nd | nd |
| UB | 2.3 | 3.9 | 48 | 80 | 0.07 | 0.12 | |
| Beef cattle (grass‐based diet) | LB | nd | nd | Nd | nd | nd | nd |
| UB | 0.51 | 0.68 | 4.87 | 6.55 | 0.01 | 0.02 | |
| Beef cattle (cereal‐based diet) | LB | nd | nd | Nd | nd | nd | nd |
| UB | 4.3 | 5.8 | 43 | 57 | 0.11 | 0.14 | |
| Lactating sheep | LB | nd | nd | Nd | nd | nd | nd |
| UB | 2.8 | 4.6 | 7.8 | 13 | 0.10 | 0.16 | |
| Lactating goats | LB | 0.17 | nd | 0.59 | nd | 0.01 | nd |
| UB | 7.5 | 15 | 25 | 49 | 0.43 | 0.83 | |
| Horses | LB | 0.11 | nd | 0.16 | nd | nd | nd |
| UB | 3.9 | 15 | 5.8 | 23 | 0.15 | 0.58 | |
bw: body weight; nd: not determined; LB: lower bound; UB: upper bound.
Rounded to the first or second decimal place or to a whole number.
Insufficient samples of maize silage were available to reliably undertake 95th percentile exposure estimates.
3.4.2.2. Pigs and Poultry
Estimates of 95th percentile and mean exposure by pigs and poultry to DAS were derived from data on levels of DAS in cereals and assumed inclusion rates of cereal grains in their diets and are given in Table 18. Details of assumed inclusion rates of cereals are given in Appendix E.
Table 18.
Estimates of 95th percentile (P95) and mean exposure to DAS for pigs and poultry derived from assumed inclusion rates of cereal grains in their diets(a)
| Animal species | Diet concentration μg/kg dry matter | Exposure μg/day | Exposure μg/kg bw per day | ||||
|---|---|---|---|---|---|---|---|
| Mean | P95 | Mean | P95 | Mean | P95 | ||
| Estimates derived from LB and UB concentrations in species‐specific compound feeds | |||||||
| Poultry starters | LB | nd | 0.02 | 0.03 | 0.31 | nd | 0.002 |
| UB | 0.06 | 0.32 | 0.84 | 4.3 | 0.005 | 0.024 | |
| Estimates derived from LB and UB concentrations in feed materials and their relative proportions in diets | |||||||
| Pig starter | LB | nd | nd | nd | nd | nd | nd |
| UB | 18 | 32 | 18 | 32 | 0.92 | 1.6 | |
| Growing/fattening pigs | LB | nd | nd | 0.01 | nd | nd | nd |
| UB | 11 | 20 | 35 | 61 | 0.35 | 0.61 | |
| Pig finisher | LB | nd | nd | 0.01 | nd | nd | nd |
| UB | 11 | 20 | 35 | 61 | 0.35 | 0.61 | |
| Lactating sow | LB | nd | nd | 0.02 | nd | nd | nd |
| UB | 14 | 25 | 86 | 151 | 0.43 | 0.75 | |
| Fattening chickens | LB | 0.22 | nd | 0.03 | nd | 0.01 | nd |
| UB | 24 | 43 | 2.9 | 5.2 | 1.44 | 2.6 | |
| Laying hens | LB | 0.21 | nd | 0.02 | nd | 0.01 | nd |
| UB | 27 | 48 | 3.2 | 5.8 | 1.61 | 2.9 | |
| Fattening turkeys | LB | nd | nd | nd | nd | nd | nd |
| UB | 14 | 24 | 5.7 | 9.8 | 0.48 | 0.81 | |
| Fattening ducks | LB | nd | nd | nd | nd | nd | nd |
| UB | 22 | 38 | 3.0 | 5.4 | 1.0 | 1.8 | |
bw: body weight; nd: not determined; LB: lower bound; UB: upper bound.
Rounded to the first or second decimal place or to a whole number.
3.4.2.3. Farmed fish
In the absence of any data on concentrations of DAS in species‐specific compound feeds for fish, estimates of exposure were made by using example rations and concentrations in individual feed materials (see Appendix E for details of rations used) and are reported in Table 19.
Table 19.
Estimated 95th percentile (P95) and mean exposure to DAS for farmed fish derived from concentrations in cereal grains and their relative proportions in diets(a)
| Animal species | Diet concentration μg/kg dry feed matter | Exposure μg/day | Exposure μg/kg bw per day | ||||
|---|---|---|---|---|---|---|---|
| Mean | P95 | Mean | P95 | Mean | P95 | ||
| Salmonids | LB | nd | nd | nd | nd | nd | nd |
| UB | 8.57 | 15.48 | 0.34 | 0.62 | 0.17 | 0.31 | |
| Carp | LB | 0.06 | nd | nd | nd | nd | nd |
| UB | 25 | 45 | 0.55 | 1.00 | 0.55 | 1.00 | |
bw: body weight; nd: not determined; LB: lower bound; UB: upper bound.
Rounded to the first or second decimal place or to a whole number.
3.4.2.4. Farmed rabbits and mink
In the absence of any data on concentrations of DAS in species‐specific compound feeds for fish, estimates of exposure were made by using example rations and concentrations in individual feed materials (see Appendix E for details of rations used) and are reported in Table 20.
Table 20.
Estimated 95th percentile (P95) and mean exposure to DAS for farmed rabbits and farmed mink derived from concentrations in cereal grains and their relative proportions in diets(a)
| Animal species | Diet concentration μg/kg dry feed matter | Exposure μg/day | Exposure μg/kg bw per day | ||||
|---|---|---|---|---|---|---|---|
| Mean | P95 | Mean | P95 | Mean | P95 | ||
| Farmed rabbits | LB | nd | nd | nd | nd | nd | nd |
| UB | 1.52 | 2.0 | 0.23 | 0.31 | 0.11 | 0.15 | |
| Farmed mink | LB | 0.04 | nd | nd | nd | nd | nd |
| UB | 2.39 | 4.2 | 0.18 | 0.32 | 0.09 | 0.15 | |
bw: body weight; nd: not determined; LB: lower bound; UB: upper bound.
Rounded to the first or second decimal place or to a whole number.
3.4.2.5. Dogs and cats
No data on levels of DAS in proprietary feeds for dogs and cats were available, and therefore exposure was estimated using example rations (see Appendix E for details) and concentrations DAS in cereal grains used. The estimated exposures are reported in Table 21.
Table 21.
Estimated 95th percentile (P95) and mean exposure to DAS by companion animals (dogs and cats) derived from concentrations in cereal grains and their relative proportions in diets(a)
| Animal species | Diet concentration μg/kg dry feed matter | Exposure μg/day | Exposure μg/kg bw per day | ||||
|---|---|---|---|---|---|---|---|
| Mean | P95 | Mean | P95 | Mean | P95 | ||
| Cats | LB | 0.03 | nd | nd | nd | nd | nd |
| UB | 7.3 | 13 | 0.44 | 0.78 | 0.11 | 0.20 | |
| Dogs | LB | 0.04 | 0.02 | 0.01 | 0.01 | nd | nd |
| UB | 5.1 | 9.1 | 1.8 | 3.3 | 0.07 | 0.13 | |
bw: body weight; nd: not determined; LB: lower bound; UB: upper bound.
Rounded to the first or second decimal place or to a whole number.
Summary
Ideally, estimates of exposures should be made using levels of DAS in species‐specific complete or complementary feeds, but in this opinion, this was only possible for poultry starter diets. Based on these data, the estimated LB and UB exposures, for both the mean and 95th percentile exposures, were all < 1.0 μg/kg diet. For dairy cows, growing/fattening pigs and laying hens, data were also provided, but all were left‐censored and therefore considered unreliable to estimate exposure. Therefore, exposures for all livestock (with the exception of young poultry) were made using the levels of DAS in individual feeds and their inclusion rates in the diets.
With the exception of maize silage, there were only levels of DAS in forages. Consequently, the highest estimated exposures for ruminants were for those fed on maize silage‐based diets. For dairy cows, the estimated mean exposures ranged from 1.3 (LB) to 43 (UB) μg/kg diet DM; while the P95 exposures were not determinable. Estimated exposures for beef cattle on maize silage‐based diets were marginally lower.
For most other livestock and farmed animals, the LB estimates for both the mean and P95 exposures were not determinable or < 1 μg/kg diet DM.
For pigs, the average for the UB mean and P95 estimates were 15 and 26 μg/kg diet DM, respectively. For poultry, the highest UB exposures were estimated for laying hens (27 and 48 μg/kg diet DM, for mean and P95 estimates, respectively), while for farmed rabbits and mink, the UB (rounded) values were highest for mink (2.4 and 4.2 μg/kg diet DM, for mean and P95 exposures, respectively).
For fish, due to the higher inclusion rates of cereals in their diets, UB estimates were highest for carp (25 and 45 μg/kg diet DM, for mean and P95 estimates, respectively).
For companion animals, the UB mean and P95 and exposures were highest for cats (7.3 and 13 μg/kg diet DM, respectively).
3.5. Risk characterisation
3.5.1. Human health risk characterisation
3.5.1.1. Acute human health risk from the dietary exposure to DAS
The CONTAM Panel characterised the human health risk associated with acute dietary exposure to DAS by comparing the mean and 95th percentile acute dietary minimum and maximum exposure estimates (summarised in Table 13) with the derived ARfD of 3,200 ng/kg bw.
The estimated mean acute dietary exposure to DAS in food ranged from 17.1 ng/kg bw (minimum, calculated for the ‘very elderly’) to 186 ng/kg bw (maximum, calculated for the ‘toddlers’) across the dietary surveys and age groups.
The estimated 95th percentile acute dietary exposure DAS in food ranged from 54 ng/kg bw (minimum, calculated for the ‘elderly’) to 799 ng/kg bw (maximum, calculated for the ‘infants’) across the dietary surveys and age groups.
Since even the largest 95th percentile acute dietary exposure estimates were below the ARfD of 3,200 ng/kg bw, the CONTAM Panel concluded that the current acute exposure to DAS in food raised no health concern for humans.
3.5.1.2. Chronic human health risk from the dietary exposure to DAS
The CONTAM Panel characterised the human health risk associated with chronic dietary exposure to DAS by comparing the mean and 95th percentile chronic dietary LB and UB exposure estimates across the European dietary surveys and the age groups (summarised in Table 16) with the TDI of 650 ng/kg bw per day.
The lowest LB to the highest UB chronic mean exposure across age groups and surveys ranged from 0.2 ng/kg bw per day (‘Infants’) to 185 ng/kg bw per day (‘toddlers’). The lowest LB to the highest UB chronic 95th percentile exposure ranged from 0.4 ng/kg bw per day (‘elderly’ and ‘very elderly’) to 491 ng/kg bw per day (‘other children’).
Therefore, the CONTAM Panel concluded that there was no health concern associated with chronic dietary exposure to DAS.
3.5.2. Animal health risk characterisation
3.5.2.1. Animal health risk characterisation
Because of the limited knowledge on the effects of DAS on farm and companion animals and on the absence of a comprehensive database on feed consumption by livestock in the EU, it has not been possible to properly assess the risk of DAS for animal health.
However the exposure estimates at the LB and UB concentrations for DAS in diets have been estimated for the most relevant farm livestock and companion animal categories, based on expected feed intakes and example diets. Those have been compared with the species specific overall LOAELs/NOAELs for pigs, poultry and dogs (see Table 20). With this aim the estimates of dietary exposure to DAS were expressed as concentration in diet (μg/kg DM), exposure per day and exposure per kg bw per day, see Tables 16–19.
Pigs
For pigs, the CONTAM Panel characterised the health risk associated with dietary exposure to DAS by comparing the estimated exposures at the UB mean and UB 95th percentile dietary exposure for DAS with the identified LOAEL of 80 μg/kg bw per day for oral lesions in fattening pigs (see Tables 16 and 20).
The dietary exposure was highest for starter pigs at the estimated UB mean and 95th percentile dietary exposures of 0.92 and 1.61 μg/kg bw per day, i.e. 1.2% and 2.0% of the LOAEL for these pigs, respectively.
For growing/fattening and finisher pigs and lactating sows, the estimated UB mean and 95th percentile dietary exposures ranged between 0.35 and 0.43 μg/kg bw per day and 0.61 and 0.75 μg/kg bw per day, respectively, corresponding to 0.4–0.5% and 0.8–0.9 of the LOAEL (see Tables 10 and 22).
Table 22.
Comparison of reference points (NOAELs/LOAELs) for DAS as availbale for pigs, poultry and dogs only with estimated exposure levels these animal species
| Animal species | Reference point (μg DAS/kg bw) | Estimated exposure (μg DAS/kg bw per day) | Estimated exposure in % of NOAEL or LOAEL | |||
|---|---|---|---|---|---|---|
| NOAEL | LOAEL | mean (UB) | P95 (UB) | Mean (UB) | P95 (UB) | |
|
Pigs growing/fattening starter finisher lactating sow |
n.d. | 80 |
0.35 0.92 0.35 0.43 |
0.61 1.61 0.61 0.75 |
0.4 1.2 0.4 0.5 |
0.8 2.0 0.8 0.9 |
| Poultry Fattening chicken | n.d. | 10 | 1.44 | 2.58 | 14.4 | 25.8 |
|
Laying hens Fattening turkeys |
n.d. n.d. |
54 12 |
1.61 0.48 |
2.90 0.81 |
3.0 4.0 |
5.4 6.8 |
| Fattening Ducks | n.d. | 22 | 1.02 | 1.80 | 5.5 | 8.2 |
| Dogs | 16 | n.d. | 0.07 | 0.13 | 0.4 | 0.8 |
| Other farm and companion animals | n.d. | 10 | 0.01–1.8 | n.d. | 0.1–18 | n.d. |
bw: body weight; NOAEL: no observed adverse effect level; LOAEL: lowest observed adverse effect level; UB: upper bound; n.d.: not determined.
Exposures have been calculated from dietary concentrations expressed on a fresh weight (88% dry matter) basis to make them comparable with the data from which NOAELS/LOAELS have been derived.
Therefore, the Panel concluded that the risk for adverse health effects from feed containing DAS is low for pigs at the estimated exposure levels under current feeding practices. However, the CONTAM Panel noted that the hazard characterisation was based on limited data on adverse effects and as such the risk characterisation of pigs carries substantial uncertainty, in particular, for growing/fattening pigs where the use of specific compound‐feed could not be assessed.
Poultry
For poultry, the CONTAM Panel characterised the health risk associated with dietary exposure to DAS by comparing the estimated exposures at the estimated UB mean and UB 95th percentile dietary exposures with the identified LOAELs of 10, 54, 12 and 22 μg/kg bw per day determined for chicken, laying hens, turkeys and ducks, respectively, (oral lesions being identified as the critical adverse health effects, see Tables 18 and 22).
Among the poultry species, the highest estimated dietary exposure was for fattening chicken, for which the exposure at the estimated UB mean and 95th percentile was 1.44 and 2.58 μg/kg bw per day, corresponding to approximately 14 and 26% of the LOAEL.
For laying hens, fattening turkeys and ducks, the dietary exposure at the estimated UB mean and 95th percentile ranged between 0.48 and 1.61 μg/kg bw per day and 0.81 and 2.90 μg/kg bw per day, respectively, corresponding to 3.0–5.5% and 5.4–8.2% of the LOAEL.
From these results, the CONTAM Panel concluded that for fattening chicken the margins between the LOAEL for oral lesions and the estimated UB dietary exposures to DAS were small and a possible risk for adverse health effects may exist under current feeding practices. The risk for adverse health effects was considered low for laying hens, fattening turkeys and ducks at the estimated exposure levels under current feeding practices.
Dogs
For dogs, the CONTAM Panel characterised the health risk associated with dietary exposure to DAS by comparing the NOAELs of 31 μg/kg bw and 16 μg/kg bw per day for the acute and chronic hazard, respectively, with the estimated dietary exposures at the UB mean and 95th percentile.
The estimated dietary acute and chronic exposure of dogs at the UB were 0.07 and 0.13 μg/kg bw per day, corresponding to 0.4 and 0.8% of the of the identified NOAELs, respectively (see Tables 21 and 22).
Based on this comparison, the CONTAM Panel concluded that the risk for adverse health effects from feed containing DAS was for both acute and chronic exposure negligible for dogs at the estimated exposure levels under current feeding practices.
Ruminants, horses, cats, farmed rabbits, fishes and mink
For all other farm and companion animal species, the CONTAM Panel applied a conservative approach and characterised the health risk associated with dietary exposure to DAS by comparing the estimated exposures at the UB mean and 95th percentile dietary concentrations for DAS with the lowest LOAEL. The LOAEL of 10 μg/kg bw per day for oral lesions in fattening chicken was identified among all farm animals for which a RP was identified (see Tables 10 and 22).
The estimated mean UB exposures of all these other farm and companion animals together ranged between 0.01 μg/kg bw per day (beef cattle with grass‐based diet) and 1.8 μg/kg bw per day (dairy cows fed with maize silage‐based diet), see Tables 17–21. The few available UB 95th percentile exposure estimates ranged between 0.02 μg/kg bw per day (beef cattle with grass‐based diet) and 1.0 μg/kg bw per day (carps). Because of scarcity of exposure data, the UB 95th percentile estimates could not be calculated for all categories of ruminants, e.g. for dairy cows with maize silage‐based diet.
The CONTAM Panel noted that the highest exposure estimates for ruminants, horses, cats, farmed rabbits, fishes and mink was 1.8 μg/kg bw per day corresponding to 18% of the LOAEL of 10 μg/kg bw per day identified for oral lesions in chicken used in the absence of sufficient data for the hazard characterisation.
Therefore, it was concluded that overall adverse health effects from feed containing DAS are unlikely for these farm and companion animals at the levels of exposure estimated for current feeding practices, with the exception of dairy cows fed with maize silage‐based diet where a possible risk may exist. It is also noted that for these animal species conclusions would be affected by a high degree of uncertainty.
4. Uncertainty analysis
Evaluation of the inherent uncertainties in the assessment of exposure to DAS has been performed following the guidance given in the Opinion of the Scientific Committee related to Uncertainties in Dietary Exposure Assessment (EFSA, 2006). In addition, the report on ‘Characterizing and Communicating Uncertainty in Exposure Assessment’ has been considered (WHO, 2008). According to the guidance provided by EFSA (2006) the following sources of uncertainties have been considered: assessment objectives, exposure scenario, exposure model and model input (parameters). In addition to the EFSA opinion (2006), the CONTAM Panel also considered other uncertainties.
4.1. Assessment objectives
The objectives of the assessment were defined in the terms of reference and no uncertainty was associated in the objectives.
4.2. Exposure scenario and model
The analytical results of the samples collected between 2000 and 2016 on the occurrence of DAS in food and feed reported to EFSA, identified as suitable for the evaluation (see Section 3.3), were very limited. These data were augmented by the analytical results collected and extracted from the publications reporting occurrence data in food and feed marketed in Europe and sampled since 2000. Those data were combined with the data reported to EFSA to a final data set when they were considered suitable for evaluation, sufficiently detailed to extract quantitative information on the concentrations in food or feed and compliant with standards established by EFSA. This included two reports with plausible results where the analytical method was not completely specified. For the final data set, analytical results for food were available from 10 European countries and for feed from seven European countries. However, the samples of food and feed did not always originate from the European country which reported the data.
Both the data reported to EFSA as well as those extracted from the published literature showed a high percentage of left censored results (98.6% of the results for human exposure and 93.9% of those for farm and companion animals). From the results submitted to EFSA, only analytical results obtained by non‐targeted sampling were included in the evaluation. In the absence on information on sampling non‐targeted sampling was assumed also for the data from the published literature. Therefore, the final data set may not be fully representative at the European level, which adds uncertainty for the exposure assessments for both humans and farm and companion animals. The use of LB values tends to underestimate, while the use of UB values tends to overestimate dietary exposure when using the substitution method to account for left censored data. The large portion of LC data in the final data sets for food and feed contributed substantially to the overall uncertainty of the exposure assessment.
The lack of analytical methods formally validated through interlaboratory studies contributed to the uncertainty of the currently available results on DAS. The lack of certified reference materials for DAS (as calibrants and in particular matrix materials) is an additional limitation. Both issues contribute to the overall uncertainty of occurrence data and are equally valid for the chemical analysis used in toxicokinetic and toxicity studies. Available information on correction/non‐correction for recovery was not complete and contributes further to that uncertainty.
There were considerable differences in the number of analytical results across the food categories with high prevalence of grains and grain‐based foods and only few or no samples for other food categories. No suitable data were available for several food categories (e.g. ‘bread and rolls’) where the results were all left censored. For other food categories, the estimation of the highest reliable percentile was not possible due to the small numbers of samples. This adds to the overall uncertainty of the human exposure estimates.
The CONTAM Panel noted a large difference between the LB and UB values of the chronic dietary exposure which introduces considerable uncertainty into exposure estimates for humans.
Regarding the exposure of farm and companion animals through feed consumption, only data on the major cereal grains (wheat, barley, oats, and maize), and limited data for maize silage, were available to assess exposure. As cereal by‐products are widely used for animal feeding, the absence of data on DAS concentrations in these products led to a likely underestimation of exposure. A large variety of feed materials and forages are used to formulate diets for farm and companion animals in Europe, and the lack of information on levels of DAS in these feeds added to the overall uncertainty of the estimates of animal exposure. The Panel also noted that the database of DAS was for some animals too scarce to estimate the high exposure at the 95th percentile, in particular for dairy cows and beef cattle and growing/fattening pigs.
In addition, the Panel noted that for almost all animal species no LB values could be estimated, both for the mean and high chronic dietary exposure, which introduces considerable uncertainty for animals.
4.3. Other uncertainties
At least in in vitro Fusarium culture the spectrum of DAS related type A trichothecenes of the scirpentriol subgroup may change over time such that data from previous exposure to one trichothecene may no more reflect human or animal exposure to that mycotoxin at present or in the near future.
Data on toxicokinetics were missing for humans and were very limited for experimental animals. No in vivo data were available for the quantification of absorption after oral exposure in humans and data on distribution, metabolism and excretion were scarce and no studies investigated repeated dosing. For farm and companion animals, data on in vivo toxicokinetics were available only from one study in heifers and one in chicken.
Therefore, the characterisation of the toxicokinetic of DAS in vivo was incomplete and the uncertainty of the toxicokinetics of DAS is large.
No studies were available to assess the differences in toxicokinetics between oral and i.v. administration of DAS, neither for humans nor for experimental animals. This adds to the uncertainty of the risk assessment.
There is a lack of long‐term oral toxicity studies and reproduction toxicity studies in experimental animals. In addition, available in vitro and in vivo data on the mode of action of DAS are currently too limited to conclude firmly on the toxicological evidence of several hypothetical mechanisms of action, which hampered the use of this information for the identification of critical effects for the dose–response assessment of DAS.
There is a lack of genotoxicity data and the mechanism of induction of in vivo chromosomal abnormalities is not fully understood.
In the absence of long‐term toxicity data on the selected critical endpoint a relative large UFs of 10 was used to characterise chronic adverse health effects in humans. The use of this adjustment factor might lead to an overestimation of the risk.
The CONTAM Panel noted that using data from clinical trials on DAS (anguidine) in cancer patients who were pre‐treated with cytostatic/cytotoxic drugs and had only limited life expectancy, might be leading to an overestimation of the risk because of their greater sensitivity to adverse effects of DAS.
No data were available to identify NOAELs in farm animals, therefore LOAELs were considered instead. This is adding additional uncertainties to the assessment.
There is also uncertainty around co‐exposure and potential combined effects of DAS and other trichothecenes; this could lead to underestimation of the risk.
Overall, the CONTAM Panel considered that the uncertainty of this current risk assessment for humans and almost all farm and companion animals as large, in particular due to the lack of toxicokinetic and toxicity data on DAS.
4.4. Summary of uncertainties
In Table 23, a summary of the uncertainty evaluation is presented, highlighting the main sources of uncertainty and indicating an estimate of whether the respective source of uncertainty might have led to an over‐ or underestimation of the exposure or the resulting risk.
Table 23.
Summary of qualitative evaluation of the impact of uncertainties on the risk assessment of the human and animal dietary exposure to DAS
| Sources of uncertainty | Directiona |
|---|---|
| Extrapolation of the occurrence data to the whole of Europe | +/− |
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/− |
| Assuming non‐targeted sampling for literature data used for exposure | + |
| Uncertainty of the analytical measurements | +/− |
| No data for most grain based products and other foods and no data on other feed than grains | − |
| High variability of the composition of feedstuffs used and feeding systems for farm animals in Europe | +/− |
| High percentage of left censored data resulting in large difference between LB and UB exposure estimates | +/− |
| Highest reliable percentile limited by the number of samples | − |
| Lack of data on general toxicity and reproductive and developmental toxicity in experimental and most farm and companion animals | +/− |
| Limited data on the mode of action | +/− |
| Lack of data to asses genotoxicity and carcinogenicity | − |
| Assumption of equivalent exposure and toxicity effects after i.v. and oral administration of the same dose | + |
| Use of large adjustment factors for risk assessment (humans) | + |
| Co‐exposure and potential combined effects of DAS and other trichothecenes | − |
+ = uncertainty with potential to cause over‐estimation of exposure/risk; − = uncertainty with potential to cause under‐estimation of exposure/risk.
The CONTAM Panel concluded that the impact of the uncertainties in the human risk assessment of DAS is large and that the risk is more likely to be over than underestimated.
The impact of the uncertainties in the risk assessment of farm and companion animals is large.
5. Conclusions
4,15‐DAS is a type A trichothecene mycotoxin with low molecular weight, produced by several Fusarium species. It has mainly been detected in cereal grains, cereal‐based products and coffee but presence in other foods and feeds cannot be excluded. A naturally occurring modified form of DAS has been identified as DAS‐glucoside.
LC–MS/MS is currently the most widely used and preferred technique for analysis of DAS in foods, feed and biological samples, while HPLC‐FLD, GC‐FID or GC‐ECD and GC–MS(/MS) have also been applied. None of the applied methods for DAS have been formally validated in interlaboratory studies and certified reference materials are not available. Calibrants are commercially available.
5.1.
5.1.1.
Occurrence
In the open literature, DAS has mainly been reported in various grain cereals (wheat, sorghum, maize, barley and oats) and cereal products, but also in potato products, soybeans and coffee. The highest levels have been reported for wheat, sorghum and coffee.
DAS co‐occurs with many other mycotoxins in grains and grain based products, in particular Fusarium toxins including type A and B trichothecenes, and zearalenone.
For food and feed, a total of 15,786 and 2,098 analytical results, respectively, fulfilled the quality criteria applied and have been used in the assessment. These include the data reported to EFSA by Member States (97%) and literature data reported for Europe (3%).
The proportion of left‐censored data (results below the LOQ) were 98.6% in food and 93.9% in feed.
The highest mean concentrations of DAS were recorded for food in the category of ‘Cereal‐based dishes’, for feed in the categories ‘maize grains and maize silage and ‘Rice, broken’.
Effects of processing
No studies on the effect of cleaning, sorting and milling of grains could be identified, but these practices are expected to result in a redistribution and/or reduction of DAS in the final products, as is the case with other trichothecenes.
Human exposure
Acute exposure estimates at the upper bound ranged for the mean from a minimum of 17 ng/kg bw to a maximum of 186 ng/kg bw across different surveys and population groups when applying the probabilistic approach. When considering the 95th percentile the values were ranging from 54 to 799 ng/kg bw. The highest values being recorded for ‘Infants’ and ‘toddlers’
The estimates of mean chronic exposure to DAS across different dietary surveys and all age groups ranged from 0.2 ng/kg bw per day (lowest minimum LB, observed in ‘infants’ and at ages ≥ 18 years) to 185 ng/kg bw per day (highest maximum UB observed in ‘toddlers’) when applying the deterministic approach. The highest values are generally recorded for young people because of their high food consumption when expressed per kg bw.
The estimates of 95th percentile chronic exposure ranged from 0.4 ng/kg bw per day (lowest minimum LB, observed in ‘elderly’ and ‘very elderly’) to 491 ng/kg bw per day (highest maximum UB observed in ‘other children’).
The most important contributors to the chronic dietary exposure to DAS were ‘Grains and grain‐based products’, especially ‘cereal‐based dishes’.
Farm and companion animal exposure
Animal exposure to DAS is primarily from consuming cereal grains and cereal by‐products.
Due to the lack of data on compound feeds, exposures for all other livestock than poultry were estimated using data for individual feed materials and their assumed inclusion rates in the diets.
The highest estimated exposures were for ruminants (dairy cows and beef cattle) fed on maize silage‐based diets. For dairy cows on a maize silage‐based diet, the estimated mean diet concentration ranged from 1.3 (LB) to 43 (UB) μg/kg DM; corresponding to a maximum of 1.8 μg/kg bw per day. Estimated exposures for beef cattle on maize silage‐based diets were marginally lower. For horses the maximum estimated exposure was 0.58 μg/kg bw per day.
Exposures for starter pigs, growing/fattening pigs, finisher pigs and lactating sows were broadly similar, however not all mean and P95 LB values could be determined. The mean and P95 UB estimates instead were ranging from 11‐18 and 20‐32 μg/kg DM, respectively (corresponding to 0.35–0.92 and 0.61–1.6 μg/kg bw per day).
For poultry, the highest diet levels were estimated for laying hens. The mean and P95 exposures ranged from non‐determined (LB) to 27 (UB) or 48 (UB) μg/kg DM, respectively (corresponding to 1.61 (mean) and 2.9 (P95) μg/kg bw per day).
For farmed rabbits and mink, the mean and P95 LB estimates were < 1.0 μg/kg DM. The UB values were highest for the mink (2.4 and 4.2 μg/kg DM, respectively, for the mean and P95 exposures that are corresponding to 0.09–0.15 μg/kg bw per day).
Similarly, the estimated LB mean and P95 for fish (salmonids and carp) were < 1.0 μg/kg DM. Due to the higher levels of cereals in carp diets, the UB estimates were highest for carp (25 and 45 μg/kg DM, for the mean and P95 estimates, respectively, corresponding to 0.55 and 1 μg/kg bw per day).
For companion animals (cats and dogs), the mean and P95 LB estimates were < 1 μg/kg DM, while the UB mean and P95 exposure estimates were higher for cats (7.3 and 13 μg/kg DM, respectively; corresponding to a maximum of 0.20 μg/kg bw per day) than for dogs (5.1 and 9.1 μg/kg DM, respectively; corresponding to a maximum of 0.13 μg/kg bw per day).
Toxicokinetics and transfer
No pharmacokinetic data in humans were available from clinical studies after i.v. administration of DAS and no data on any oral administration were identified.
After oral administration in rats and mice, the absorption of DAS has not been quantified but the excretion ratio between urine and faeces indicated high absorption. DAS was rapidly distributed to most organs. Tissue concentrations decreased rapidly with no apparent accumulation in any tissue and more than 90% of radiolabelled DAS was excreted within 24 h.
In vitro, DAS is metabolised to a large number of metabolites. The main metabolic processes are deacylations, hydroxylations, deepoxidations and glucuronide conjugations. Deepoxidation reactions have primarily been found after incubation with GI content, rumen fluid or faeces, DAS was rapidly metabolised to a large number of metabolites also in vivo.
Although in vivo studies on the toxicokinetics of DAS in farm animals were rare and not all relevant parameters were determined, a rapid ADME was generally shown. The main systemic metabolites were determined as 15‐MAS and SCT. In pigs, a large portion of orally administered DAS could be found in faeces, mainly as deepoxidised SCT.
In chickens, DAS was rapidly absorbed from the GI tract, extensively metabolised and excreted as 15‐MAS and 7‐OH‐DAS in faeces and in urine
No data were available for ruminants, horses, dogs and cats, and farmed rabbits, mink and fish.
There was insufficient evidence to conclude on the transfer of DAS from feed to food of animal origin.
Toxicity of DAS in experimental animals
The oral LD50 values in rodents ranged from 2 to 16 mg/kg bw. Haematological effects such as anaemia, leucopenia and thrombocytopenia were observed. When administered i.v., the LD50 ranged from 1 to 12 mg/kg bw while the LD50 ranged from 0.8 to 23 mg DAS/kg bw after i.p. administration.
Repeated dose studies in rodents were scarce and not of sufficient quality for hazard identification and characterisation.
After single i.v. administration to dogs, DAS induced emesis, diarrhoea, haematological changes and effects in bone marrow. Repeated dosing by i.v. in dogs and monkeys induced emesis, diarrhoea, body weight loss, erythema, increased haematocrit, anaemia, leucocytosis and/or leucopenia, neutrophilia, lymphopenia, elevated AST, ALT and BUN, and nucleated erythrocytes. The NOAEL for emesis and haematological effects after i.v. administration was 31 and 16 μg/kg bw per day for dog, and 125 μg/kg bw per day for monkeys.
-
Developmental and reproductive toxicity was reported after i.p. administration of DAS in experimental animals, and adverse effects were seen in tissues with high proliferation rate. No NOAEL could be identified, however the CONTAM identified:
-
–
a LOAEL of 1 mg/kg bw in mice after a single injection of DAS, based on increases in resorption, reduction in fetal body weight and observation of gross and skeletal malformations.
-
–
a LOAEL of 1.7 mg/kg bw in rats based on reduced testicular weight and sperm production and an increased frequency of hypocellular seminiferous tubules.
-
–
There was no evidence that DAS induces bacterial reverse mutation in vitro.
The only in vivo genotoxicity study in mice (i.p. exposure) reported chromosomal abnormalities in somatic cells (bone marrow) and in germ cells (spermatocytes). Protein synthesis inhibition is likely to be a mechanism underlying the observed in vivo chromosomal abnormalities. The Panel considered that there are currently insufficient data on the genotoxicity of DAS.
No chronic toxicity or carcinogenicity studies were identified
The limited available toxicological data for the main metabolites of DAS (4 or 15 MAS, SCT) indicate that their toxicity is equal to or less than the toxicity of DAS in vitro and in vivo after oral exposure.
Toxicity of DAS in humans
The CONTAM Panel identified adverse health effects in humans exposed to DAS when it was tested as a cytostatic anticancer drug (named anguidine) in phase I and phase II clinical trials on cancer patients by i.v. administration.
Based on the data of the phase I studies, the CONTAM Panel identified nausea and vomiting as the most relevant acute adverse health effects of DAS when administered i.v. with a NOAEL at 1.2 mg DAS/m2 (equivalent to 32 μg DAS/kg)
Haematotoxicity and myelosuppression were the most frequently observed and persistent adverse effects observed in the phase I studies when DAS was given repeatedly (5‐day regimen) in treatment cycles of 3–4 weeks. A NOAEL of 2.4 mg DAS/m2 (equivalent to 65 μg DAS/kg bw per day) was identified from the same phase I studies.
The reported adverse health effects at doses from 3 to 5 mg DAS/m2 (equivalent to 81‐135 μg DAS/kg bw per day) of the phase II clinical trials, performed at the proximity of the maximum tolerable doses, supported these findings.
Mode of action of DAS
DAS is binding to ribosomes, inducing a ‘ribotoxic stress response’ with activation of ribosome‐associated MAPKs and inhibition of protein synthesis.
DAS also possesses cytotoxic properties, while no clear indications for ROS production are available. Increase in gut satiety hormones (e.g. CCK) levels is considered the mechanism of DAS (and trichothecenes) induced anorexia.
In vitro assays indicated cytotoxic properties on haematopoietic progenitors which could be due to stimulation of apoptosis or inhibition of protein synthesis.
Combined effects of DAS with other mycotoxins
Data on combined effects of DAS and others mycotoxins in vivo were available mainly in poultry and only one experiment was performed in pigs.
Although limited, the effects observed for DAS in combination with T‐2 toxin, AFB1, OTA and fusaric acid were generally more marked than when DAS was administered alone. The CONTAM Panel noted that because of the lack of dose–response data, it was difficult to draw a definitive conclusion concerning the nature of the combined effects and interaction with other Fusarium toxins, including type A trichothecenes.
Adverse health effects and derivation of human health based guidance values
Based on the conclusions on the genotoxicity and the MoA of DAS, the CONTAM Panel decided to establish HBGV for both the acute and chronic exposure of DAS to humans.
The Panel concluded that the database of the oral studies on rats and guinea pigs and the i.v. studies in dogs and monkey was insufficient for the identification of a RP for the human hazard characterisation. For both acute and chronic hazard characterisation, data from clinical studies in patients treated by i.v. administration of DAS (anguidine) for cancer were used.
The data from experimental and farm animals suggest almost complete absorption following oral administration. The Panel therefore under a conservative approach considered an equivalent bioavailability after oral exposure and i.v. dosing.
The CONTAM Panel noted that following oral exposure DAS is subject to extensive pre‐systemic degradation in the GI tract and hepatic metabolisation. Based on limited information, the Panel noted that DAS metabolites would have similar or lower toxicity than DAS and consequently that oral exposure would not lead to higher systemic toxicity than i.v. administration.
The CONTAM Panel identified 32 μg DAS/kg bw as a RP for acute health effects, based on nausea and emesis observed in clinical studies on cancer patients.
The Panel applied the default UF of 10 accounting for interindividual toxicokinetic and toxicodynamic variability and derived an ARfD of 3,200 ng DAS/kg bw.
The haematotoxic and myelotoxic effects observed in clinical studies were identified as the critical endpoint for human chronic hazard characterisation. The CONTAM Panel concluded that these effects would not be expected in humans at a dose equal to or below 65 μg/kg bw per day, and this was therefore selected as a RP for chronic effects.
The CONTAM Panel considered the default UF of 10 as adequate to cover for interindividual toxicokinetic and toxicodynamic variability. In addition the Panel applied an UF of 10 to account for the limited duration and the intermittent dosing regimen of the human clinical studies used for the chronic RP selection. Taking the overall UF into account the CONTAM Panel established a TDI of 650 ng DAS/kg bw per day.
Adverse effects and identification of reference points in farm and companion animals
Among Tolerable Daily Intake ruminants studies indicated adverse health effects in heifers at the only tested dose of 0.5 mg DAS/kg bw per day i.v., and in lambs at an oral dose corresponding to 0.16 mg DAS/kg bw per day. The CONTAM Panel concluded that these data were too scarce to identify a reference point for adverse health effects of DAS in ruminants.
Only few studies on adverse effects of DAS in pigs were available. Oral lesions in the GI tract were observed following exposure to ≥ 2.0 mg DAS/kg feed, corresponding to 0.08 mg/kg bw per day. Reduced body weight gain was seen at feed concentrations resulting in doses ≥ 0.12 mg/kg bw per day. No NOAEL could be identified from the available data.
For poultry, oral lesions were observed at the lowest level of exposure in the following species: chickens, laying hens, turkeys and ducks with LOAELs of 0.010, 0.054, 0.012 and 0.022 mg DAS/kg bw per day, respectively. At equal or higher doses, reduction of body weight gain, decreased eggs production and decreased fertility were other adverse effects observed in various studies in different poultry species.
No data were available on the oral exposure of dogs. Emesis and haematotoxicity were observed following i.v. administration. Assuming equivalent bioavailability and systemic toxicity of DAS following i.v. and oral administration, the CONTAM Panel identified NOAELs of 0.031 and 0.016 mg/kg bw per day for acute and chronic toxicity, respectively.
No toxicity data suitable for hazard characterisation of DAS were identified for ruminants, farmed rabbits, farmed fish, farmed mink, horses and cats. In order to obtain an indication on the risk of DAS in these species, the CONTAM Panel considered the LOAEL of 0.01 mg DAS/kg bw per day identified for fattening chicken.
Human health risk characterisation
All the estimated mean and 95th percentile acute and chronic exposure levels were below the established ARfD and TDI, respectively, and therefore not of health concern.
The impact of the uncertainties in the human risk assessment of DAS is large and the risk is more likely to be over than underestimated.
Farm and companion animal health risk characterisation
Because of the limited data on the effects of DAS on farm and companion animals only the health risk to pigs, poultry and of dogs could be characterised.
For pigs, the CONTAM Panel noted that the exposure levels in starter and growing/fattening pigs were 1.2% and 2.0%, respectively, of the identified critical LOAEL of 80 μg/kg bw per day. Although the hazard characterisation was based on limited data on adverse effects, the Panel concluded that the risk for adverse health effects from feed containing DAS is low for pigs at the estimated exposure levels under current feeding practices
For poultry, the CONTAM Panel noted that the higher exposure estimates for fattening chicken was up to 25% of the lowest LOAEL identified for oral lesions (10 μg/kg bw per day), indicating a possible risk for adverse effects. The risk was considered low for laying hens, fattening turkeys and ducks at the estimated exposure levels under current feeding practices.
In the dog, the estimated exposure levels were lower than 1% of the identified NOAELs of 31 and 16 μg/kg bw per day for both acute and chronic effects, respectively, suggesting a low risk for adverse health effects.
The CONTAM Panel noted that the largest available exposure estimate for species where a RP could not be identified (ruminants, horses, cats and farmed rabbits, fishes and mink) were in the majority of cases a small fraction of the LOAEL of 10 μg/kg bw per day for oral lesions in poultry (maximum 18% in dairy cows). Therefore, the overall adverse health effects from feed containing DAS would be unlikely for these farm and companion animals at the levels of exposure estimated for current feeding practices, with the exception of dairy cows fed with maize silage‐based diet where a possible risk may exist. Conclusions for these animal species are affected by a high degree of uncertainty.
6. Recommendations
A well‐designed 90‐day oral toxicity study in rats using purified DAS performed according to relevant OECD guidelines with special focus on the assessment of haematotoxicity, myelotoxicity and reproductive performance is needed.
Well‐designed studies of the toxicokinetics of DAS after oral and i.v. exposure in experimental animals are required.
Studies on the in vivo genotoxicity of DAS are needed and more data are required on the cellular and molecular MoA of DAS, in particular, for a better understanding of cytotoxicity, DNA and protein synthesis inhibition, stimulation of apoptosis and effects on haematopoietic progenitors and bone marrow.
Well‐designed dietary studies on adverse effects (including the investigations for oral and GI lesions) of DAS are needed in farm animals other than poultry.
More occurrence data on DAS in food and feed obtained with state‐of‐the‐art validated analytical methods with adequate sensitivity, such as LC–MS/MS are needed to reduce the uncertainty in the exposure assessment for humans and farm and companion animals.
In consideration of the similar toxicity profiles and structural similarities of several trichothecenes, together with their likely co‐exposure via food and feed, it could be appropriate to perform a cumulative risk assessment for this group of substances.
Abbreviations
- ADME
absorption, distribution, metabolism, excretion
- AFB1
aflatoxin B1
- AKA
alimentary toxic aleukia
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- ARfD
Acute reference dose
- AST
aspartate aminotransferase
- BUN
blood urea nitrogen
- bw
body weight
- CAL
calonectrin
- CAS
Chemical Abstract Service
- CCK
cholecystokinin
- CDK
cyclin‐dependent kinase
- CI
confidence interval
- CNS
central nervous system
- CONTAM Panel
EFSA Panel on Contaminants in the Food Chain
- CRM
Certified Reference Material
- DAD
diode array detection
- DAS
4,15‐diacetoxyscirpenol
- DMBA
dimethylbenzanthracene
- DM
dry matter
- DMSO
dimethyl sulfoxide
- ECD
electron capture detection
- ERK1/2
extracellular signal‐regulated kinase 1 and 2
- FAO
Food and Agriculture Organization of the United Nations
- FEDIAF
European Pet Food Industry Federation
- FEEDAP Panel
EFSA Panel on Additives and Products or Substances used in Animal Feed
- FID
flame ionisation detection
- FLD
fluorescence detection
- GC
gas chromatography
- GGT
gamma‐glutamyltransferase
- GI
gastrointestinal
- GIP
glucose‐dependent insulinotropic polypeptide
- GLP‐1
glucagon‐like peptide‐17–36 amide
- HBGV
health‐based guidance value
- HPLC
high‐performance liquid chromatography
- i.p.
intraperitoneal
- i.v.
intravenous
- IC50
half maximal inhibitory concentration
- IPCS
International Programme on Chemical Safety (WHO)
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- JNK
c‐Jun N‐terminal kinase
- LB
lower bound
- LC–MS
liquid chromatography–mass spectrometry
- LC‐UV
liquid chromatography‐ultraviolet
- LD50
lethal dose (median)
- LDH
lactate dehydrogenase
- LOAEL
lowest observed adverse effect level
- LOD
limit of detection
- LOQ
limit of quantification
- mAb
monoclonal antibodies
- MAPK
mitogen‐activated protein kinase
- MAS
monoacetoxyscirpenol
- Max
maximum
- Min
minimum
- ML
maximum level
- MoA
mode of action
- MTD
maximum tolerated dose
- NMR
nuclear magnetic resonance
- NOAEL
no‐observed‐adverse‐effect level
- NRC
National Research Council
- OECD
Organisation for Economic Co‐operation and Development
- OTA
ochratoxin A
- PMTDI
provisional maximum tolerable daily intake
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged, and Safe
- ROS
reactive oxygen species
- RP
Reference Point
- SCE
sister chromatid exchange
- SCT
scirpentriol
- SMART
somatic mutation and recombination test
- SOP
Standard Operating Procedure
- TAS
Triacetoxyscirpenol
- TBC
total body clearance
- TDI
tolerable daily intake
- TLC
thin‐layer chromatography
- TOF
time‐of‐flight
- UB
upper bound
- UHPLC
ultra‐high‐performance liquid chromatography
- UPLC
ultra‐performance liquid chromatography
- UV
ultraviolet
- WG
Working group
- WHO
World Health Organization
Appendix A – EFSA guidance documents applied in the assessment of DAS in food and feed
1.
The CONTAM Panel applied the general principles of the risk assessment process for chemicals in food as described by WHO (2009), i.e. hazard identification and characterisation, exposure assessment and risk characterisation. The following EFSA guidance documents were applied in the assessment of DAS in food and feed:
EFSA (European Food Safety Authority), 2006. Guidance of the Scientific Committee on a request from EFSA related to uncertainties in Dietary Exposure Assessment. EFSA Journal 2007;4(12):438, 54 pp. https://doi.org/10.2903/j.efsa.2007.438
EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(5):1051, 22 pp. https://doi.org/10.2903/j.efsa.2009.1051
EFSA (European Food Safety Authority), 2011. Overview of the procedures currently used at EFSA for the assessment of dietary exposure to different chemical substances. EFSA Journal 2011;9(12):2490, 33 pp. https://doi.org/10.2903/j.efsa.2011.2490
EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534, 26 pp. https://doi.org/10.2903/j.efsa.2012.2534
EFSA Scientific Committee, 2011. Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. https://doi.org/10.2903/j.efsa.2011.2379
EFSA Scientific Committee, 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 33 pp. https://doi.org/10.2903/j.efsa.2012.2579
EFSA Scientific Committee, 2012. Scientific Opinion on Risk Assessment Terminology. EFSA Journal 2012;10(5):2664, 43 pp. https://doi.org/10.2903/j.efsa.2012.2664
EFSA Scientific Committee, Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Bresson J‐L, Griffin J, Hougaard Benekou S, van Loveren H, Luttik R, Messean A, Penninks A, Ru G, Stegeman JA, van der Werf W, Westendorf J, Woutersen RA, Barizzone F, Bottex B, Lanzoni A, Georgiadis N and Alexander J, 2017. Guidance on the assessment of the biological relevance of data in scientific assessments. EFSA Journal 2017;15(8):4970, 73 pp. https://doi.org/10.2903/j.efsa.2017.4970
EFSA Scientific Committee, Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry QM, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne J‐L, Fernandez Dumont A, Hempen M, Valtueña Martínez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M, 2017. Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. https://doi.org/10.2903/j.efsa.2017.4971
EFSA Scientific Committee, Hardy A, Benford D, Halldorsson T, Jeger M, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Younes M, Aquilina G, Crebelli R, Gürtler R, Hirsch‐Ernst KI, Mosesso P, Nielsen E, van Benthem J, Carfì M, Georgiadis N, Maurici D, Parra Morte J and Schlatter J, 2017. Scientific Opinion on the clarification of some aspects related to genotoxicity assessment. EFSA Journal 2017;15(12):5113, 25 pp. https://doi.org/10.2903/j.efsa.2017.5113
EFSA Scientific Committee, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. https://doi.org/10.2903/j.efsa.2018.5123
Appendix B – Literature search for supporting information and hazard identification and characterisation
1.
The sources of literature were: ISI Web of Science, Embase and Pubmed
Key word used was “diacetoxyscirpenol”
Search details:
1. Web of Science
“Topic” was selected for all key words for Web of Science
TOPIC: (diacetoxyscirpenol)
Indexes: SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC.
Time span: All years.
2. Pubmed
A free‐text search was performed using diacetoxyscirpenol as a key word:
“diacetoxyscirpenol”[Supplementary Concept] OR “diacetoxyscirpenol”[All Fields]
Database: PubMed
Time span: all years
3. Embase
Advanced search was performed, using the Embase mapping option: “Search as broadly as possible”
‘diacetoxyscirpenol’/exp OR ‘diacetoxyscirpenol’
SourcesEmbase, Embase Classic, MEDLINE
Time span: all years
Conducted on 6 February 2017
In total, 2,281 references were found, after removing duplicates the amount was 796 references.
Literature search for “Anguidine” performed on 12 October 2017
Three databases were used: ISI Web of Science, Embase and Pubmed
A literature search was performed using the diacetoxyscirpenol synonym: “anguidine”. “Diacetoxyscirpenol” was excluded using the Boolean operator NOT.
1. Embase
Advanced search was performed selecting “Search as broadly as possible” option, and using the following search string:
(anguidin* OR anguidin*/exp) NOT (‘diacetoxyscirpenol’/exp OR ‘diacetoxyscirpenol’)
Time span: All years
Results: 5
2. Pubmed
The following search string was used:
anguidin*[All Fields] NOT (“diacetoxyscirpenol”[Supplementary Concept] OR “diacetoxyscirpenol”[All Fields])
Time span: All years
Results: 15
3. Web of Science
A basic search was performed selecting “all databases”, and using the following search string:
TOPIC: (anguidin*) NOT TOPIC: (diacetoxyscirpenol)
Time span: All years
Results: 78
All the references were uploaded in Endnote. The final number of references after removal of duplicates is 79.
An update of the literature search on DAS was performed on 12 October 2017.
Three databases were used: ISI Web of Science, Embase and Pubmed. As in the previous search (February 2017), “DAS” was not used as a keyword since it's the acronym of many other unrelated words.
Search details:
1. Embase
Advanced search was performed selecting “Search as broadly as possible” option, and using the following search string:
(‘diacetoxyscirpenol’/exp OR ‘diacetoxyscirpenol’)
Time span: from 2017
Results: 11
2. Pubmed
The following search string was used:
“diacetoxyscirpenol”[Supplementary Concept] OR “diacetoxyscirpenol”[All Fields]
Time span: from 2017
Results: 6
3. Web of Science
A basic search was performed selecting “all databases”, and using the following search string:
TOPIC: (diacetoxyscirpenol)
Time span: from 2017
Results: 7
All the references were uploaded in Endnote. The final number of references after removal of duplicates is 16.
Appendix C – Occurrence of DAS in food identified from the open literature
1.
| Country | Food type | Sampling year | Sampling strategy | No of samples | LOD μg/kg | LOQ μg/kg | % pos. | Mean(a) μg/kg | Min. μg/kg | Max. μg/kg | Reference | Considered in the exposure assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgium + Germany | Sorghum | 2014 | Random | 8 + 2 | 1.2 | 2.5 | 70 | 44 | < LOD | 91 | Ediage et al. (2015) | Yes |
| Denmark | Milk | 2004 | Random | 42 | 0.03 | 0.05 | 0 | < LOQ | < LOQ | < LOQ | Sørensen and Elbæk (2005) | No |
| Denmark | Oats | 2006 | Random | 11 | 30 | n.r. | 0 | < LOD | < LOD | < LOD | Rasmussen et al. (2012) | No |
| Triticale | 2007 | 5 | n.r. | 0 | < LOD | < LOD | < LOD | |||||
| Wheat | 2007 | 6 | n.r. | 0 | < LOD | < LOD | < LOD | |||||
| Finland | Wheat | 2001–2002 | Random | 70 | n.r. | 25–50 | 0 | < LOD | < LOD | < LOD | SCOOP (2003) | No |
| Barley | 40 | 0 | < LOD | < LOD | < LOD | |||||||
| Barley malt | 50 | 0 | < LOD | < LOD | < LOD | |||||||
| Oats | 55 | 0 | < LOD | < LOD | < LOD | |||||||
| Rye | 25 | 0 | < LOD | < LOD | < LOD | |||||||
| France | Wheat | 2001 | Random | 52 | n.r. | 20 | 100 | n.r. | n.r. | n.r. | SCOOP (2003) | No |
| Maize | Random | 29 | n.r. | 20 | 100 | n.r. | n.r. | n.r. | ||||
| Barley | Random | 9 | n.r. | 20 | 100 | n.r. | n.r. | n.r. | ||||
| Soft wheat | Random | 31 | n.r. | 20 | 100 | n.r. | n.r. | n.r. | ||||
| Maize | 2001 | Targeted | 25 | 30 | n.r. | 0 | < LOD | < LOD | < LOD | |||
| Soft wheat | 2000–2002 | Targeted | 225 | 30 | 60 | 0 | < LOD | < LOD | < LOD | |||
| Durum wheat | 81 | 30 | 60 | 0 | < LOD | < LOD | < LOD | |||||
| Malting barley | 2001–2002 | Random | 194 | 30 | n.r. | 0 | < LOD | < LOD | < LOD | |||
| Maize | 2000–2001 | Random | 57 | 30 | n.r. | 0 | < LOD | < LOD | < LOD | |||
| Soft wheat | 306 | 30 | n.r. | 3 | < LOD | < LOD | < LOD | |||||
| Germany | Barley | 2009 | Random | 59 | 0.09 | 0.29 | 22 | 0.052 | < LOD | 0.52 | Barthel et al. (2012) | Yes |
| Germany | Semolina | 2000 | Random | 14 | 14 | 28 | 0 | < LOD | < LOD | < LOD | Schollenberger et al. (2005) | No |
| Maize flour | 15 | 0 | < LOD | < LOD | < LOD | |||||||
| Germany | Oat flakes | 2005 | Random | 43 | n.r. | n.r. | 86 | 0.08 | < LOD | 0.38 | Gottschalk et al. (2007) | No |
| Germany | Wheat flour | 2005/2006 | Random | 39 | 0.09 | 0.29 | 3 | 0.01 | < LOD | 0.25 | Gottschalk et al. (2009) | No |
| Whole wheat flour | 11 | 0 | < LOD | < LOD | < LOD | |||||||
| Wheat kernels | 52 | 2 | < LOD | < LOD | 0.25 | |||||||
| Semolina | 13 | 0 | < LOD | < LOD | < LOD | |||||||
| Wheat bran | 10 | 20 | 0.05 | < LOD | 0.25 | |||||||
| Wheat contain. infant food | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Rye flour | 15 | 0 | < LOD | < LOD | < LOD | |||||||
| Whole rye flour | 9 | 0 | < LOD | < LOD | < LOD | |||||||
| Rye kernels | 37 | 0 | < LOD | < LOD | < LOD | |||||||
| Oat flakes | 31 | 71 | 0.08 | < LOD | 0.38 | |||||||
| Fine oat flakes | 23 | 65 | 0.04 | < LOD | 0.17 | |||||||
| Oat kernels | 19 | 47 | 0.04 | < LOD | 0.25 | |||||||
| Oat bran | 12 | 75 | 0.04 | < LOD | 0.12 | |||||||
| Oat contain. infant food | 13 | 31 | 0.03 | < LOD | 0.25 | |||||||
| Germany | Wheat‐based foods | 2000–2001 | Random | 15 | 14 | 28 | 0 | < LOD | < LOD | < LOD | Schollenberger et al. (2005) | No |
| Oat‐based foods | 21 | 0 | < LOD | < LOD | < LOD | |||||||
| Maize‐based foods | 20 | 0 | < LOD | < LOD | < LOD | |||||||
| Pseudocereals | 15 | 0 | < LOD | < LOD | < LOD | |||||||
| Gluten‐free food | 23 | 0 | < LOD | < LOD | < LOD | |||||||
| Potato products | 21 | 5 | n.r. | < LOD | 21 | |||||||
| Red pepper | 10 | 0 | < LOD | < LOD | < LOD | |||||||
| Canned tomatoes | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Legumes | 23 | 0 | < LOD | < LOD | < LOD | |||||||
| Onion powder | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Garlic powder | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Canned asparagus | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Banana chips | 6 | 0 | < LOD | < LOD | < LOD | |||||||
| Tapioca | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Peanuts | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Sunflower seeds | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Pumpkin kernel | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Sesame seeds | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Hazelnut kernel | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Almond kernel | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Desiccated coconut | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Germany | Soybeans | n.r. | Random | 6 | 14 | 28 | 0 | < LOD | < LOD | < LOD | Schollenberger et al. (2007) | No |
| Soybeans roasted | 5 | 20 | n.r. | < LOD | 21 | |||||||
| Soy flour and flakes, not defatted | 10 | 0 | < LOD | < LOD | < LOD | |||||||
| Soy flakes partially defatted and products (crisp) | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Texturised soy protein | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Tofu | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Soy sauce | 4 | 0 | < LOD | < LOD | < LOD | |||||||
| Soy protein isolates | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Germany | Soybean oil | 2004 | Random | 14 | 3 | 6 | 0 | < LOD | < LOD | < LOD | Schollenberger et al. (2008) | No |
| Sunflower seed oil | 16 | 0 | < LOD | < LOD | < LOD | |||||||
| Corn germ oil | 17 | 6 | < LOD | < LOD | < LOD | Yes | ||||||
| Olive oil | 10 | 0 | < LOD | < LOD | < LOD | No | ||||||
| Rapeseed oil | 11 | 0 | < LOD | < LOD | < LOD | |||||||
| Safflower oil | 10 | 0 | < LOD | < LOD | < LOD | |||||||
| Wheat germ oil | 3 | 0 | < LOD | < LOD | < LOD | |||||||
| Pumpkin oil | 4 | 0 | < LOD | < LOD | < LOD | |||||||
| Peanut oil | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Walnut oil | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Grape kernel oil | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Sesame seed oil | 5 | 0 | < LOD | < LOD | < LOD | |||||||
| Linseed oil | 4 | 0 | < LOD | < LOD | < LOD | |||||||
| Palm oil | 1 | 0 | < LOD | < LOD | < LOD | |||||||
| Italy | Durum wheat grain | 2009–2010 | Random | 46 | 2 | 5 | 0 | < LOD | < LOD | < LOD | Alkadri et al. (2014) | No |
| Italy | Wheat | 2003–2004 | Random | 14 | 0.2 | 0.7 | 21 | 100 | < LOD | 130 | Dall'Asta et al. (2004) | Yes |
| Poland | Maize | 2010 | Semi‐targeted | 19 | 0.65 | 1.3 | 26 | 4.9 | < LOD | 5.9 | Aniolowska et al. (2014) | Yes |
| Poland | Wheat | 2014 | Random | 99 | n.r. | 1 | 0 | < LOD | < LOD | < LOD | Bryła et al. (2014) | No |
| Barley | 24 | 0 | < LOD | < LOD | < LOD | |||||||
| Oats | 4 | 0 | < LOD | < LOD | < LOD | |||||||
| Triticale | 20 | 0 | < LOD | < LOD | < LOD | |||||||
| Poland | Oats | 2009–2011 | Semi‐targeted ‐ conventional and organic | 58 | n.r. | n.r. | 43 | 4.1 | < LOD | 20.8 | Twaruzek et al. (2013) | Yes |
| Poland | Oat bran | 2009–2011 | Semi‐targeted ‐ conventional and organic | 11 | 1 | 3 | 0 | n.r. | < LOD | < LOQ | Twaruzek et al. (2013) | No |
| Oat flakes | 21 | 5 | 4.8 | Yes | ||||||||
| Oat flour | 4 | 0 | < LOQ | No | ||||||||
| Oat groats | 1 | 0 | < LOQ | |||||||||
| Oat pasta | 3 | 0 | < LOQ | |||||||||
| Spain | Roasted coffee beans | 2013–2014 | Random | 103 | 3 | 5 | 12 | 6.5 | < LOD | 8.6 | García‐Moraleja et al. (2015a) | Yes |
| Spain | Brewed coffee | 2013–2014 | Random | 169 | 1.38 | 5.99 | 7 | 196 | < LOD | 402 | García‐Moraleja et al. (2015b) | No |
| Spain | Oats | n.r. | Random | 7 | 4 | 10 | 0 | < LOD | < LOD | < LOD | Juan et al. (2013) | No |
| Spelt | 3 | 0 | < LOD | < LOD | < LOD | |||||||
| Wheat | 57 | 0 | < LOD | < LOD | < LOD | |||||||
| Barley | 9 | 0 | < LOD | < LOD | < LOD | |||||||
| Rye | 11 | 0 | < LOD | < LOD | < LOD | |||||||
| Spain | Wheat semolina | 2012 | Random | 49 | 2.5 | 5 | 0 | < LOD | < LOD | < LOD | Rodríguez‐Carrasco et al. (2013a, b) | No |
| Wheat couscous | 14 | 0 | < LOD | < LOD | < LOD | |||||||
| Wheat‐based small pastas | 56 | 0 | < LOD | < LOD | < LOD | |||||||
| Rice crackers | 7 | 5 | 10 | 0 | < LOD | < LOD | < LOD | |||||
| Rice starch | 6 | 0 | < LOD | < LOD | < LOD | |||||||
| Rice dietetic snacks | 10 | 0 | < LOD | < LOD | < LOD | |||||||
| Maize crackers | 4 | 1.25 | 2.5 | 0 | < LOD | < LOD | < LOD | |||||
| Maize starch | 3 | 0 | < LOD | < LOD | < LOD | |||||||
| Maize dietetic snacks | 10 | 0 | < LOD | < LOD | < LOD | |||||||
| Spain | Breadsticks | 2012 | Random | 61 | 0.6‐5 | 1.25‐10 | 0 | < LOD | < LOD | < LOD | Rodríguez‐Carrasco et al. (2014) | No |
| UK | Rice | 2000 | Random | 100 | n.r. | 10 | 0 | < LOD | < LOD | < LOD | SCOOP (2003) | No |
| Cereal fractions | 27 | 0 | < LOD | < LOD | < LOD | |||||||
| Maize products | 2000–2001 | 70 | 0 | < LOD | < LOD | < LOD | ||||||
| Wheat products | 95 | 0 | < LOD | < LOD | < LOD | |||||||
| Wheat flour | 2000 | 29 | 0 | < LOD | < LOD | < LOD | ||||||
| Flour | 8 | 0 | < LOD | < LOD | < LOD | |||||||
| Polenta | 8 | 0 | < LOD | < LOD | < LOD | |||||||
| Biscuits | 54 | 0 | < LOD | < LOD | < LOD | |||||||
| Bread | 56 | 0 | < LOD | < LOD | < LOD | |||||||
| ‘Mixed’ | 2000–2001 | 30 | 0 | < LOD | < LOD | <LOQ | ||||||
| UK | Barley | 2002 | Random | 111 | 10 | n.r. | 0 | < LOD | < LOD | < LOD | Edward (2009) | No |
| Barley | 2003 | 128 | 0 | < LOD | < LOD | < LOD | ||||||
| Barley | 2004 | 110 | 0 | < LOD | < LOD | < LOD | ||||||
| Barley | 2005 | 97 | 0 | < LOD | < LOD | < LOD | ||||||
| Wheat | 2001 | 283 | 0 | < LOD | < LOD | < LOD | Edward (2009) | No | ||||
| Wheat | 2002 | 343 | 0 | < LOD | < LOD | < LOD | ||||||
| Wheat | 2003 | 328 | 0 | < LOD | < LOD | < LOD | ||||||
| Wheat | 2004 | 344 | 0 | < LOD | < LOD | < LOD | ||||||
| Wheat | 2005 | 326 | 0 | < LOD | < LOD | < LOD |
LOD: limit of detection; LOQ: limit of quantification; n.r.: not reported.
In most cases, mean of positives, but this is not always fully clear.
Appendix D – Occurrence of DAS in feed identified from the open literature
1.
| Country | Food type | Sampling year | Sampling strategy | No of samples | LOD μg/kg | LOQ μg/kg | % pos. | Mean μg/kg | Min. μg/kg | Max. μg/kg | Reference | Considered in the exposure assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slovak Republic | Complete feedingstuff (poultry) | 2003–2004 | Random | 50 | 0.3 | nr | 20.0 | 3.6 | nr | nr | Labuda et al. (2005) | Yes |
| Germany | Maize (corn) | 2000–2001 | Random | 41 | 14 | 28 | 4.9 | 49 | nr | nr | Schollenberger et al. (2006) | Yes |
| Maize by‐products | 2000–2001 | Random | 13 | 14 | 28 | 7.7 | 21 | nr | nr | No | ||
| Germany | Maize silage | 2000–2001 | Random | 18 | 14 | 28 | 6 | nr | nr | 64 | Schollenberger et al. (2005) | Yes |
| Maize kernels | 2000–2001 | Random | 24 | 14 | 28 | 4 | nr | nr | 21 | Yes | ||
| Germany | Oat grains | 2009 | Random | 17 | 0.33 | 0.89 | 100 | 1.19 | < LOD | 8.05 | Gottschalk et al. (2009) | Yes |
| Italy | Complete feedingstuff (fattening pigs) | 2003–2004 | Random | 7 | 20 | nr | 29 | 612 | < LOD | nr | Dall'Asta et al. (2004) | No |
LOD: limit of detection; LOQ: limit of quantification; nr: not reported.
Appendix E – Feed intakes and diet composition (livestock)
1.
This Appendix gives details of the feed intakes, live weights and diet compositions for different livestock, fish and companion animals used as the basis to estimate exposures to DAS. These are based on published guidelines on nutrition and feeding (e.g. Carabano and Piquer, 1998; NRC 2000, 2007a,b; Ewing, 2002; Leeson and Summers, 2008; OECD, 2009; McDonald et al., 2011; EBLEX, 2008, 2012; EFSA, 2012) and information provided by European feed manufacturers. They are therefore estimates of the Panel on Contaminants in the Food Chain (CONTAM Panel) but agree with common practice. In Table E.6, the concentrations of DAS and its modified forms in feeds used to estimate exposure are presented.
Table E.6.
Levels of DAS and the sum of its hidden forms (μg/kg DM) in species‐specific compound/complementary feeds and feed materials used to estimate exposure by farmed livestock and companion animals
| Feed type | N | P95 | Mean | ||
|---|---|---|---|---|---|
| LB | UB | LB | UB | ||
| Compound/complementary feeds | |||||
| Pig: growing/fattening | 29 | 48 | 79 | 695 | 695 |
| Poultry: starter diets | 118 | 0.3 | 11 | 4.1 | 57 |
| Feed materials | |||||
| Wheat | 588 | 0.0 | 6.7 | 0.0 | 11 |
| Barley | 336 | 0.0 | 8.5 | 0.0 | 11 |
| Oats | 282 | 0.7 | 4.7 | 3.7 | 11 |
| Maize (corn) | 232 | 0.6 | 32 | 0.0 | 57 |
| Soybean meal | 10 | 0.0 | 62 | 0.0 | 113 |
| Oat feed | 98 | 2.2 | 3.3 | 8.8 | 8.8 |
| Maize silage | 35 | 2.1 | 48 | 0.0 | 148 |
N: Number of samples; DM: dry matter.
E.1. Feed intakes
E.1.1. Ruminants and horses
E.1.1..1.
Dairy cows
The amounts of feed given to lactating dairy cows varies according to the amount and quality of forages and other feeds available, the weight of the cow and its milk yield. In this Opinion, it is assumed that non‐forage (i.e. complementary) feeds are fed at the rate of 0.3 kg/kg of milk produced (Nix, 2010). Exposure to DAS has been estimated for a 650‐kg dairy cow, with a milk yield of 40 kg per day. Assumptions on the amounts of forages and non‐forage feed are given in Table E.1.
Table E.1.
Live weights, growth rate/productivity, dry matter intake for cattle, sheep, goats and horses, and the proportions of the diet as non‐forage
| Animal species | Live weight (kg) | Growth rate or productivity | Dry matter intake (kg/day) | % of diet as non‐forage feed | Reference |
|---|---|---|---|---|---|
| Dairy cows, lactatinga | 650 | 40 kg milk/day | 20.7 | 40 | OECD (2009) |
| Fattening cattle: beefb | 400 | 1 kg/day | 9.6 | 15 | AFRC (1993) |
| Fattening cattle: maize silage‐based ration | 300 | 1.4 kg/day | 6.6 | 25 | Browne et al. (2004) |
| Fattening cattle: cereal straw‐based diet | 300 | 0.9 kg/day | 8.0 | 68 | EBLEX (2008) |
| Fattening cattle: cereal beef | 400 | 1.4 kg/day | 10.0 | 85 | EBLEX (2012) |
| Sheep: lactating | 80 | Feeding twin lambs | 2.8 | 50 | OECD (2009) |
| Goats: milking | 60 | 6 kg milk/day | 3.4 | 65 | NRC (2007a) |
| Goats: fattening | 40 | 0.3 kg/day | 1.5 | 40 | NRC (2007a) |
| Horses | 450 | Moderate activity | 9.0 | 50 | NRC (2007b) |
Months 2–3 of lactation.
Housed castrate cattle, medium maturing breed.
Beef cattle
There are a wide variety of beef production and husbandry systems in Europe. They may be categorised broadly as forage‐based or cereal‐based systems, although combinations of these systems are commonly found. In this opinion, three feeding systems are considered, in which the forages are grass or maize silage with, in each case, appropriate supplementation with non‐forage feed materials. A third system, commonly known as ‘cereal beef’, is also considered. For exposure estimates, live weights of 300 or 400 kg, and feed intakes of between 6.6 and 10 kg dry matter per day have been assumed, depending on the feeding regime, based on guidelines published by EBLEX (2008, 2012), and details are given in Table E.1.
Sheep and goats
Many breeds and systems of management have been developed for sheep and goats to suit the land, climate and husbandry conditions in the EU. As for other ruminants, forages may be the only feeds used after weaning (NRC, 2007a). Common exceptions to this are pregnant and lactating animals, whose feed is usually supplemented with non‐forage feeds or manufactured compound (complementary) feeds (AFRC, 1993; NRC, 2007a). In this Opinion, exposure estimates have been made for lactating sheep and goats. The CONTAM Panel has used a daily dry matter intake of 2.8 kg for an 80‐kg lactating sheep feeding twin lambs to estimate the exposures. For lactating goats, the CONTAM Panel has used a daily dry matter intake of 3.3 kg for a 60‐kg goat for milking (4 kg milk/day); for fattening goats, a body weight of 40 kg and feed intake of 1.5 kg DM/day has been assumed, of which 60% is forage (Table E.1).
Horses
Horses are non‐ruminant herbivores. They generally consume 2–3.5% of their body weight in feed (dry matter) each day, of which a minimum of 50% should be as forage (pasture grass or hay) (NRC, 2007b). Assumed intakes are given in Table E.1.
E.1.2. Non‐ruminant animals
E.1.2..1.
Pigs
Although there is a considerable range of pig production systems in Europe, exposure estimates have been made for piglets (pig starter), finishing pigs and lactating sows (using feed intakes proposed by EFSA FEEDAP Panel (2012). Details are given in Table E.2.
Table E.2.
Live weights and feed intake for pigs, poultry, ducks and fish
| Animal species | Live weight (kg) | Feed intake (kg dry matter/day) | Reference |
|---|---|---|---|
| Pigs: starter | 20 | 1.0 | EFSA (2012) |
| Pigs: finishing | 100 | 3.0 | EFSA (2012) |
| Pigs: lactating sows | 200 | 6.0 | EFSA (2012) |
| Poultry: broiler starters | 0.7 | 0.075 | Leeson and Summers (2008) |
| Poultry: broilersa | 2 | 0.12 | EFSA (2012) |
| Poultry: laying hens | 2 | 0.12 | EFSA (2012) |
| Turkeys: fattening turkeys | 12 | 0.40 | EFSA (2012) |
| Ducks: fattening ducks | 3 | 0.14 | Leeson and Summers (2008) |
| Salmonids | 2 | 0.04 | EFSA (2012) |
| Carp | 1 | 0.02 | Schultz et al. (2012) |
Fattening chickens.
Poultry
The CONTAM Panel applied the live weights and feed intakes reported for fattening chickens (broilers), laying hens and turkeys proposed by EFSA (2009) and for ducks by Leeson and Summers (2008) (Table E.2).
Farmed fish (salmonids and carp)
Commercially reared species include Atlantic salmon, rainbow trout, sea bass, sea bream, cod, halibut, tuna, eel and turbot. In this Scientific Opinion, exposures to DAS and their modified forms have been made for farmed salmon and carp. Details of the body weights and feed intakes used are given in Table E.2.
Rabbits
Feed intakes of 65–80 g/kg bw per day have been reported (Carabano and Piquer, 1998). For the exposure estimates, the CONTAM Panel have assumed a live weight of 2 kg, and a daily feed intake of 75 g/kg bw (derived from Carabano and Piquer, 1998).
Farmed mink
For estimating exposure, the CONTAM Panel have assumed a live weight of 2.07 kg for a male mink at pelting, and with a feed intake of 227 g fresh weight per day (75 g dry matter) (NRC, 1982).
Companion animals: dogs and cats
The amount of food consumed is largely a function of the mature weight of the animal, level of activity, physiological status (e.g. pregnancy or lactation) and the energy content of the diet. In this Scientific Opinion, the CONTAM Panel assumed body weights (kg) and feed intakes (g dry matter/day) for dogs and cats of 25/360 and 4/60, respectively (derived from NRC, 2006).
E.2. Diet compositions and inclusion of diet ingredients to the exposure calculations
Many livestock in the European countries are fed proprietary commercial compound feeds. Where sufficient data have been provided on species‐specific compound feeds, estimates of exposure have been made using these data together with estimated intakes given in Tables E.1 and E.2. Where data on proprietary compound feeds were not available, or were available but in insufficient numbers, estimates of exposure have been made using dietary inclusion rates of feed materials given in this section. Levels of DAS species‐specific compound/complementary feeds or feed materials used to estimate exposure are given in Table E.6.
E.2.1. Cattle, sheep, goats and horses
For most ruminants and horses, forages (either fresh or conserved as silage or hay) are essential ingredients in their diet, but they are normally supplemented with non‐forage feeds such as cereals, cereal by‐products, oilseed meals and by‐products of human food production. Although these non‐forages may be fed as commercially manufactured compound feeds, no data levels of DAS in these feeds were available and therefore example diets and levels of DAS and DAS in individual feeds have been used to estimate exposure.17
With the exception of maize silage, no data on levels of DAS are available. Therefore, two approaches to estimating exposure by ruminants and horses have been taken. The first assumes that the main forages are fresh grass and/or grass silage, and that these make no contribution to exposure. Inclusion rates of feeds, for which levels of DAS are available, are given in Table E.3. Exposures have also been estimated for diets in which maize silage is the main forage. AFSSA (2009) have provided example intakes of dairy cows fed maize silage supplemented with maize grain and soybean meal, while example diets of beef cattle on maize silage are taken from EBLEX (2008, 2012), and these are given in Table E.4.
Table E.3.
Assumed inclusion rates (%) of feeds, for which information on levels of DAS are available, in the diets of ruminants and horses
| Non‐forage feed materials | Dairy cows | Beef cattle | Lactating sheep | Lactating goats | Fattening goats | Horses |
|---|---|---|---|---|---|---|
| Wheat (%) | 15 | ni | 14 | ni | ni | ni |
| Barley (%) | 20 | 40 | 18 | 25 | 20 | ni |
| Oats (%) | ni | ni | ni | 35 | 40 | 40 |
| Soybean meal (%) | 5 | ni | 5 | 10 | 10 | ni |
| % of non‐forage feeds in the diet | 40 | 15 | 50 | 75 | 40 | 50 |
ni: not included in the diet formulations.
Table E.4.
Assumed diet compositions and feed intake of lactating dairy cows and fattening beef cattle fed diets based on maize silage, and beef cattle on cereal‐based dietsa
| Animal species | Forage | Maize grain | Soybean meal | Barley grain | Reference |
|---|---|---|---|---|---|
| Lactating dairy cows: maize silage‐based diet | 15.0 | 9.5 | 2.8 | ni | AFSSA (2009) |
| Fattening beef cattle: maize silage‐based diet | 4.9 | ni | ni | ni | EBLEX (2012) |
| Fattening beef cattle: intensive cereal‐based dietb | 1.5 | ni | ni | 5.5 | EBLEX (2008) |
ni: not included in the diet formulations.
Diets are also supplemented with rapeseed meal, but no data available on levels of DAS in this feed.
Grass silage assumed as the forage.
E.2.2. Pigs and poultry
Data for species‐specific compound feeds for poultry starter diets and fattening pigs were provided (see Table E.6) and these were used to estimate exposure to DAS. For other categories of pigs and poultry, inclusion rates of individual feeds (Table E.6) together with levels of DAS in these feeds (Table E.6) were used to estimate exposure to DAS.18
E.2.3. Rabbits
Rabbits are usually fed a pelleted diet (in the form of complete feedingstuffs) consisting of dried forages, cereals and vegetable proteins supplemented with minerals, vitamins and trace elements. Lebas and Renouf (2009) reviewed diet formulations used in experimental studies: in 58 diets, cereals and cereal by‐products (mostly wheat bran) accounted for up to 40% of all ingredients. In these studies, maize was a major cereal grain and was included in more than one‐third of all diets. In northern Europe, however, maize may be replaced by barley and wheat. In this opinion, the feed ingredients used in a typical French commercial rabbit compound, as provided by T. Gidenne, (personal communication, 2011) have been used, details of which are given in Table E.5.
Table E.5.
Assumed diet composition (%) for farmed fish (salmonids and carp), farmed rabbits, farmed mink and companion animals (cats and dogs)
| Feed materials | Farmed fish | Farmed rabbits | Farmed mink | Companion animals | ||
|---|---|---|---|---|---|---|
| Salmonids | Carp | Cats | Dogs | |||
| Wheat (%) | 15 | 24 | ni | 6 | 10 | 10 |
| Barley (%) | ni | ni | ni | 1 | ni | ni |
| Maize (%) | ni | 10 | 18 | 6 | 5 | 6 |
| Oats (%) | ni | ni | ni | ni | 1 | 0.5 |
| Soybean meal (%) | 12 | 32 | ni | ni | 8 | 4 |
ni: not included in the diet formulations.
E.2.4. Farmed fish (salmonids and carp)
Traditionally, the principal raw materials used for the manufacture of fish feeds in Europe have been fishmeal and fish oils, and although alternative sources of oil and protein (e.g. soybean meals and vegetable oils) are increasingly being used fish‐derived feeds still remain the major ingredients.
For many fish species, digestion of complex carbohydrates and the metabolic utilisation of the absorbed glucose is low, reflecting the scarcity of carbohydrates in the aquatic environment (Guillaume et al., 2001). Instead, fish obtain much of their energy from protein in the diet. Where carbohydrates are used, they generally require some form of pretreatment (e.g. cooking, flaking or toasting).
Berntssen et al. (2010) provided details of the composition of a diet for growing salmonids, and the CONTAM Panel used this feed formulation to estimate the exposures (Table E.5).
In contrast, studies with the common carp (Cyprinus carpio) have demonstrated greater intestinal amylase activity than in carnivorous fish, which accounts for the better utilisation of carbohydrates by these fish. The optimum level of carbohydrates appears to be 30–40% (Food and Agriculture Organization of the United Nations (FAO), Aquaculture Feed and Fertiliser Resources Information System19), which allows for higher levels of cereals than in diets for salmonids. The CONTAM Panel used the ingredients of commercial compound feeds for carp reported by Schultz et al. (2012) to estimate exposure to DAS.
E.2.5. Farmed mink
Mink are carnivorous animals and are fed high protein diets consisting mainly of meat and meat by‐products. Commercially manufactured mink feed consists largely of fish and land animal by‐products, with lesser amounts of cereals and cereal by‐products, and supplemented with mineral/vitamin premixtures. Mink are fed diets high in protein, although their nutritional requirements vary according to the animal's physiological stage (e.g. gestating, lactating and growing) and climatic conditions, particularly temperature. The proportions of cereal grains, their products and by‐products used in estimating the exposure are given in Table E.6.20
E.2.6. Companion animals (dogs and cats)
Most small companion animals derive their nutritional needs from processed food, and in 2010 EU annual sales of pet food products was ~ 8.3 million tonnes.21 Although a wide range of ingredients is used in commercial diets, most dog and cat diets contain at least some animal protein. Other ingredients include cereals (predominantly wheat, rice or maize), cereal by‐products, vegetable proteins and by‐products of human food production. The ingredients will vary depending both on the availability of feed materials and the nutrient requirements of the animals.
The European Pet Food Industry Federation (FEDIAF) has provided information on typical inclusion levels of cereals, cereal by‐products and other feed materials in dry cat and dog food. In the absence of sufficient data on species‐specific manufactured complete feedingstuffs, the CONTAM Panel has used example diets based on information provided by FEDIAF22 (details given in Table E.5).
Annex A – Supporting tables on food and feed occurrence and human exposure
1.
Annex A contains supporting tables on food and feed occurrence and human exposure and is available as excel file under supporting information.
Supporting information
Statistical methods used to estimate the intake–response of serum 25(OH)D concentration on daily supplemental intake of vitamin D and to derive the percentage of infants exceeding a serum 25(OH)D concentration
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Nebbia CS, Oswald IP, Petersen A, Rose M, Roudot A‐C, Schwerdtle T, Vleminckx C, Vollmer G, Wallace H, De Saeger S, Eriksen GS, Farmer P, Fremy J‐M, Gong YY, Meyer K, Parent‐Massin D, van Egmond H, Altieri A, Colombo P, Horváth Z, Levorato S and Edler L, 2018. Scientific Opinion on the risk to human and animal health related to the presence of 4,15‐diacetoxyscirpenol in food and feed. EFSA Journal 2018;16(8):5367, 106 pp. 10.2903/j.efsa.2018.5367
Requestor: European Commission
Question number: EFSA‐Q‐2010‐01010
Panel members: Jan Alexander, Lars Barregård, Margherita Bignami, Beat Brüeschweiler, Sandra Ceccatelli, Bruce Cottrill, Lutz Edler, Michael Dinovi, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Helle Katrine Knutsen, Carlo Stefano Nebbia, Isabelle P. Oswald, Annette Petersen, Martin Rose, Alain‐Claude Roudot, Tanja Schwerdtle, Christiane Vleminckx, Günter Vollmer and Heather Wallace.
Acknowledgments: The CONTAM Panel wishes to thank the hearing expert Chiara Dall'Asta, the WG member Hanspeter Naegeli and the EFSA staff member Mari Eskola for the support provided to this scientific output. The CONTAM Panel acknowledges all European competent institutions and other stakeholders that provided occurrence data on DAS in food and feed, and supported the data collection for the Comprehensive European Food Consumption database.
Adopted: 29 June 2018
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 2: © Taylor & Francis Ltd.; Figure 3: © Nature/Springer/Palgrave
Notes
It is noted that scirpentriol can be abbreviated in different ways (e.g. SCP), the CONTAM Panel decided to use SCT.
Reprinted by permission from Taylor & Francis Ltd, http://www.tandfonline.com; Kimura M et al.; Molecular and genetic studies of Fusarium trichothecene biosynthesis; Bioscence, Biotechnology, and Biochemistry; 71(9), (2007) 2105–2123, (https://doi.org/10.1271/bbb.70183)
Commission Regulation (EU) No 519/2014 of 16 May 2014 amending Regulation (EC) No 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T‐2, HT‐2 toxin and citrinin and screening methods of analysis. OJ L 147, 17.5.2014, p. 29–43.
Regulation (EC) No 401/2006 of the European Parliament and the Council of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. OJ L 70, 9.3.2006, p. 12–34.
Council Regulation (EEC) No 315/93 of February 1993 laying down Community procedures for contaminants in food. OJ L 37, 13.2.1993, p. 1–3.
Directive 2002/32/EC of the European Parliament and the Council of 7 May 2002 on undesirable substances in animal feed. OJ L 140, 30.5.2002, p. 10–21.
Commission Recommendation 2006/576/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T‐2 and HT‐2 and fumonisins in products intended for animal feeding. OJ L 229, 23.8.2006, p. 7–9.
Web of Science (WoS), formerly ISI Web of Knowledge, Thomson Reuters. Available online: http://thomsonreuters.com/thomson-reuters-web-of-science/18
PubMed, Entrez Global Query Cross‐Database Search System, National Center for Biotechnology Information (NCBI), NationalLibrary of Medicine (NLM), Department of the National Institutes of Health (NIH), United States Department of Health andHuman Services. Available online: http://www.ncbi.nlm.nih.gov/pubmed
Embase – Elsevier. Available online: https://www.elsevier.com/solutions/embase-biomedical-research
EndNote X8, Thomson Reuters. Available online: https://endnote.com/
Commission Regulation (EU) No 2017/2017 of 15 June 2017 amending Regulation (EU) No 68/2013 on the Catalogue of feed materials. OJ L 159, p. 48–119.
Reprinted by permission from Nature/Springer/Palgrave; Yang S et al.; Unraveling the in vitro and in vivo metabolism of diacetoxyscirpenol in various animal species and human using ultrahigh‐performance liquid chromatography‐quadrupole/time‐of‐flight hybrid mass spectrometry, Analytical and Bioanalytical Chemistry; (2015) 407: 8571‐8583, (https://doi.org/10.1007/s00216-015-9016-4)
Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers, FDA ‐ Center for drug evaluation and research, July 2005.
US FDA, Guidance for industry; Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers; July 2005.
Commission Regulation (EU) No 2017/2017 of 15 June 2017 amending Regulation (EU) No. 68/2013 on the Catalogue of feed materials OJ L 159, p. 48–119.
Although data were reported on levels of DAS in compound feeds for dairy cows (n = 15), the data were all left censored and therefore considered unreliable for estimating exposure.
Although data were reported on levels of DAS in compound feeds for laying hens (n = 13), the data were all left censored and therefore considered unreliable for estimating exposure.
Diet formulation based on data provided by the Finnish Fur Breeders Association in 2015 and translated from Finnish to English, http://www.profur.fi
Available online: http://www.Fediaf.org
The European Pet Food Industry Federation (FEDIAF), Personal communication by email, May 2016.
References
- Abbas HK, Mirocha CJ, Pawlosky RJ and Pusch DJ, 1985. Effect of cleaning, milling, and baking on deoxynivalenol in wheat. Applied and Environmental Microbiology., 50, 482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ademoyero AA and Hamilton PB, 1991a. High dietary fat increases toxicity of DAS in chickens. Poultry Science, 70, 2271–2274. [DOI] [PubMed] [Google Scholar]
- Ademoyero AA and Hamilton PB, 1991b. Mouth lesions in broiler chickens caused by scirpenol mycotoxins. Poultry Science, 70, 2082–2089. [DOI] [PubMed] [Google Scholar]
- Adler SS, Lowenbraun S, Birch B, Jarrell R and Garrard J, 1984. Anguidine: a broad phase II study of the Southeastern Cancer Study Group. Cancer Treatment Reports, 68, 423–425. [PubMed] [Google Scholar]
- AFRC (Agricultural and Food Research Council), 1993. Energy and protein requirements of ruminants: an advisory manual In: Alderman G, Cottrill BR. (eds.). CAB International, Wallingford, UK, 159 pp. [Google Scholar]
- AFSSA (Agence Française de Securite Sanitaire de Aliments), 2009. Evaluation des risques liés á la présence de mycotoxines dans les chaînes alimentaires humaine et animale. Fremy JM and Thomann C (eds.). AFSSA, Rennes, France, 308 pp.
- Alassane‐Kpembi I, Schatzmayr G, Taranu I, Marin D, Puel O and Oswald IP, 2017. Mycotoxins co‐contamination: methodological aspects and biological relevance of combined toxicity studies. Critical Reviews in Food Science and Nutrition, 57, 3489–3507. [DOI] [PubMed] [Google Scholar]
- Alkadri D, Rubert J, Prodi A, Pisi A, Mañes J and Soler C, 2014. Natural co‐occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chemistry, 157, 111–118. [DOI] [PubMed] [Google Scholar]
- Allen NK, Jevne RL, Mirocha CJ and Lee YW, 1982. The effect of a Fusarium roseum culture and DAS on reproduction of white leghorn females. Poultry Science, 61, 2172–2175. [DOI] [PubMed] [Google Scholar]
- Anaya I, Ventura M, Agut M and Comellas L, 2004. Rapid screening method for deoxynivalenol, T‐2 toxin and diacetoxyscirpenol in maize‐based foods by thin layer chromatography. Affinidad LXI, 510, 124–128. [Google Scholar]
- Aniołowska M and Steininger M, 2014. Determination of trichothecenes and zearalenone in different corn (Zea mays) cultivars for human consumption in Poland. Journal of Food Composition and Analysis, 33, 14–19. [Google Scholar]
- Arunachalam C and Doohan FM, 2013. Trichothecene toxicity in eukaryotes: cellular and molecular mechanisms in plants and animals. Toxicology Letters, 217, 149–158. [DOI] [PubMed] [Google Scholar]
- Asam S and Rychlik M, 2007. Studies on accuracy of trichothecene multitoxin analysis using stable isotope dilution assays. Mycotoxin Research, 23, 191–198. [DOI] [PubMed] [Google Scholar]
- Ayral AM, Dubech N, Le Bars J and Escoula L, 1992. In vitro effect of DAS and DON on microbial activity of murine peritoneal macrophages. Mychopathologia, 120, 121–127. [DOI] [PubMed] [Google Scholar]
- Barthel J, Gottschalk C, Rapp M, Berger M, Bauer J and Meyer K, 2012. Occurrence of type A, B and D trichothecenes in barley and barley products from the Bavarian market. Mycotoxin Research, 28, 97–106. [DOI] [PubMed] [Google Scholar]
- Bata A, Vanyi A and Lasztity R, 1983. Simultaneous detection of some fusariotoxins by gas‐liquid chromatography. Journal Association of Official Analytical Chemists, 66, 577–581. [PubMed] [Google Scholar]
- Battilani P, Costa LG, Dossena A, Gullino ML, Marchelli R, Galaverna G, Pietri A, Dall'Asta C, Giorni P, Spadaro D and Gualla A, 2009. CFP/EFSA/CONTAM/2008/01 Scientific information on mycotoxins and natural plant toxicants.
- Bauer J, Bollwahn W, Gareis M, Gedek B and Heinritzi K, 1985. Kinetic Profiles of Diacetoxyscirpenol and two of its Metabolites in Blood Serum of Pigs. Applied and Environmental Microbiology, 49, 842–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Gareis M and Gedek B, 1989. Metabolism of the trichothecenes T‐2 toxin, diacetoxyscirpenol, and deoxynivalenol by farm animals Chelkowski J. (ed.). Fusarium: Mycotoxins, taxonomy and pathogenicity. Elsevier, Amsterdam: pp. 139–165. [Google Scholar]
- Bauer J, 1995. The metabolism of trichothecenes in swine [Article in German]. Deutsche Tierärztliche Wochenschrift, 102, 50–52. [PubMed] [Google Scholar]
- Belt RJ, Haas CD, Joseph U, Goodwin W, Moore D and Hoogstraten B, 1979. Phase I study of anguidine administered weekly. Cancer Treatment Reports, 63, 1993–1995. [PubMed] [Google Scholar]
- Bennett JW and Klich M, 2003. Mycotoxins. Clinical Microbiology Reviews, 16, 497–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger U, Oehme M and Kuhn F, 1999. Quantitative determination and structure elucidation of type A and B‐trichothecenes by HPLC/ion trap multiple mass spectrometry. Journal of Agricultural and Food Chemistry, 47, 4240–4245. [DOI] [PubMed] [Google Scholar]
- Berntssen MHG, Maage A, Julshamm K, Oeye BE and Lundebye AK, 2010. Carry‐over of dietary organochlorine pesticides, PCDD/Fs, PCBs, and brominated flame retardants to Atlantic salmon (Salmo salar L.) fillets. Chemosphere, 83, 95–103. [DOI] [PubMed] [Google Scholar]
- Berthiller F, Schuhmacher R, Buttinger G and Krska R, 2005. Rapid simultaneous determination of major type A‐ and B‐trichothecenes as well as zearalenone in maize by high performance liquid chromatography–tandem mass spectrometry. Journal of Chromatography A, 1062, 209–216. [DOI] [PubMed] [Google Scholar]
- Berthiller F, Brera C, Iha MH, Krska R, Lattanzio VMT, MacDonald S, Malone RJ, Maragos C, Solfrizzo M, Stranska‐Zachariasova M, Stroka J and Tittlemier SA, 2017. Developments in mycotoxin analysis: an update for 2015–2016. World Mycotoxin Journal, 11, 5–29. [Google Scholar]
- Bin‐Umer MA, McLaughlin JE, Basu D, McCormick S and Tumer NE, 2011. Trichothecene mycotoxins inhibit mitochondrial translation ‐implication for the mechanism of toxicity. Toxins, 3, 1484–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biselli S and Hummert C, 2005. Development of a multicomponent method for Fusarium toxins using LC‐MS/MS and its application during a survey for the content of T‐2 toxin and deoxynivalenol in various feed and food samples. Food Additives and Contaminants Part A, 22, 752–760. [DOI] [PubMed] [Google Scholar]
- Boutwell RK, 1964. Some biological aspects of skin cancerogenesis. Progress in Experimental Tumour Research, 4, 207–250. [DOI] [PubMed] [Google Scholar]
- Brake J, Hamilton PB and Kittrell RS, 1999. Effects of the trichothecene mycotoxin diacetoxyscirpenol on fertility and hatchability of broiler breeders. Poultry Science, 78, 1690–1694. [DOI] [PubMed] [Google Scholar]
- Brake J, Hamilton PB and Kittrell RS, 2000. Effects of the trichothecene mycotoxin diacetoxyscirpenol on feed consumption, body weight and oral lesions of broiler breeders. Poultry Science, 79, 856–863. [DOI] [PubMed] [Google Scholar]
- Brake J, Hamilton PB and Kittrell RS, 2002. Effects of the tricothecene mycotoxin DAS effects of the tricothecene mycotoxin diacetoxyscirpenol on egg production of broiler breeders. Poultry Science, 81, 1807–1810. [DOI] [PubMed] [Google Scholar]
- Browne EM, Juniper DT, Bryant MJ and Fisher AV, 2004. Intake, live‐weight gain and carcass characteristics of beef cattle given diets based on forage maize silage harvested at different stages of maturity. Animal Science, 79, 405–413. 10.1017/S1357729800090275 [DOI] [Google Scholar]
- Bryła M, Jędrzejczak R, Szymczyk K, Roszko M and Obiedziński MW, 2014. An LC‐IT‐MS/MS‐based method to determine trichothecenes in grain products. Food Analytical Methods, 7, 1056–1065. [Google Scholar]
- Bukowski R, Vaughn C, Bottomley R and Chen T, 1982. Phase II study of anguidine in gastrointestinal malignancies: a Southwest Oncology Group study. Cancer Treatment Reports, 66, 381–383. [PubMed] [Google Scholar]
- Burditt SJ, Hagler WM and Hamilton PB, 1983a. Survey of molds and mycotoxins for their ability to cause feed refusal in chickens. Poultry Science, 62, 2187–2191. [DOI] [PubMed] [Google Scholar]
- Burditt SJ, Hagler WM, Hutchins JE and Hamilton PB, 1983b. Models of feed refusal syndrome in poultry. Poultry Science, 62, 2158–2163. [DOI] [PubMed] [Google Scholar]
- Capriotti AL, Cavaliere C, Foglia P, Samperi R, Stampachiacchiere S, Ventura S and Laganà A, 2014. Multiclass analysis of mycotoxins in biscuits by high performance liquid chromatography–tandem mass spectrometry. Comparison of different extraction procedures. Journal of Chromatography A, 1343, 69–78. [DOI] [PubMed] [Google Scholar]
- Carabano R and Piquer J, 1998. The digestive system of the rabbit In: de Blas C. and Wiseman J. (eds.). The Nutrition of the Rabbit. CABI Publishing, Oxfordshire, UK: pp. 1–16. [Google Scholar]
- Cavaliere C, Foglia P, Guarino C, Motto M, Nazzari M, Samperi R, Lagana A and Berardo N, 2007. Mycotoxins produced by Fusarium genus in maize: determination by screening and confirmatory methods based on liquid chromatography tandem mass spectrometry. Food Chemistry, 105, 700–710. [Google Scholar]
- Chan PK‐C and Gentry PA, 1984. Inhibition of bovine platelet function by T‐2 toxin, HT‐2 toxin, diacetoxyscirpenol and deoxynivalenol. Food and Chemical Toxicology, 22, 643–648. [DOI] [PubMed] [Google Scholar]
- Chi SM, Robison TS, Mirocha CJ and Reddy KR, 1978. Acute toxicity of 12,13‐epoxytrichothecenes in one‐day‐old broiler chicks. Applied and Environmental Microbiology, 35, 636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu FS, Chen Liang M and Zhang GS, 1984. Production and characterization of antibody against diacetoxyscirpenol. Applied and Environmental Microbiology, 48, 777–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare Mills EN, Johnston JM, Kemp HA and Morgan MRA, 1988. An enzyme‐linked immunosorbent assay for diacetoxyscirpenol applied to the analysis of wheat. Journal of the Science of Food and Agriculture, 42, 225–233. [Google Scholar]
- Cohen H and Lapointe M, 1984. Capillary gas chromatographic determination of T‐2 toxin, HT‐2 toxin, and diacetoxyscirpenol in cereal grains. Journal Association of Official Analytical Chemists, 67, 1105–1107. [PubMed] [Google Scholar]
- Conková E, Laciaková A, Kovác G and Seidel H, 2003. Fusarial toxins and their role in animal diseases. The Veterinary Journal, 165, 214–220. [DOI] [PubMed] [Google Scholar]
- Conner MW, de Camargo J, Punyarit P, Riengropitak S, Rogers AE and Newberne PM, 1986. Toxicity of anguidine in mice. Fundamental and Applied Toxicology, 7, 153–164. [DOI] [PubMed] [Google Scholar]
- Conner MW, Conner BH, Rogers AE and Newberne PM, 1990. Anguidine‐induced testicular injury in Lewis rats. Reproductive Toxicology, 4, 215–222. [DOI] [PubMed] [Google Scholar]
- Cooray R, 1984. Effects of some mycotoxins on mitogen‐induced blastogenesis and SCE frequency in human lymphocytes. Food and Chemical Toxicology, 22, 529–534. [DOI] [PubMed] [Google Scholar]
- Coppock RW, Gelberg HB, Hoffmann WE and Buck WB, 1985. The acute toxicopathy of intravenous diacetoxyscirpenol (anguidine) administration in Swine. Fundamental and Applied Toxicology, 5, 1034–1049. [DOI] [PubMed] [Google Scholar]
- Coppock RW, Swanson SP, Gelberg HB, Koritz GD, Buck WB and Hoffmann WE, 1987. Pharmacokinetics of DAS in cattle and swine: effects of halothane. American Journal of Veterinary Residues, 48, 691–695. [PubMed] [Google Scholar]
- Coppock RW, Hoffmann WE, Gelberg HB, Bass D and Buck WB, 1989. Hematologic changes induced by intravenous administration of DAS in pigs, dogs, and calves. American Journal of Veterinary Residues, 50, 411–415. [PubMed] [Google Scholar]
- Craddock VM, Sparrow S and Henderson AR, 1986. The effect of the trichothecene mycotoxin DAS on nitrosamine‐induced esophageal cancer in the rat. Cancer Letters, 31, 197–204. [DOI] [PubMed] [Google Scholar]
- Craddock VM, Hill RJ and Henderson AR, 1987. Stimulation of DNA replication in rat esophagus and stomach by the trichothecene mycotoxin DAS. Cancer Letters, 38, 199–208. [DOI] [PubMed] [Google Scholar]
- Craddock VM, Hill RJ and Henderson AR, 1988. Acute and chronic effects of DAS on cell replication in rat esophagus and stomach. Cancer Letters, 41, 287–294. [DOI] [PubMed] [Google Scholar]
- Cundliffe E and Davies JE, 1977. Inhibition of initiation, elongation, and termination of eukaryotic protein synthesis by trichothecene fungal toxins. Antimicrobial Agents Chemotheraphy, 11, 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtui VG, 2000. Effects of feeding a Fusarium poae extract and a natural zeolite to broiler chickens. Mycotoxin Research, 16, 43–52. [DOI] [PubMed] [Google Scholar]
- Da Silva EO, Bracarense APFRL and Oswald IP, 2018. Mycotoxins and oxidative stress: where are we? World Mycotoxin journal, 11, 113–133. [Google Scholar]
- Dall'Asta C, Galaverna G, Biancardi A, Gasparini M, Sforza S, Dossena A and Marchelli R, 2004. Simultaneous liquid chromatography–fluorescence analysis of type A and type B trichothecenes as fluorescent derivatives via reaction with coumarin‐3‐carbonyl chloride. Journal of Chromatography A, 1047, 241–247. [PubMed] [Google Scholar]
- Dellafiora L, Galaverna G and Dall'Asta C, 2017. In silico analysis sheds light on the structural basis underlying the ribotoxicity of trichothecenes – a tool for supporting the hazard identification process. Toxicology Letters, 270, 80–87. [DOI] [PubMed] [Google Scholar]
- D'Mello JPF, Placinta CM and Macdonald AMC, 1999. Fusarium mycotoxins: a review of global implications for animal health, welfare and productivity. Animal Feed Science and Technology, 80, 183–205. [Google Scholar]
- DeSimone PA, Greco FA and Lessner HF, 1979. Phase I evaluation of a weekly schedule of anguidine. Cancer Treatment Reports, 63, 2015–2017. [PubMed] [Google Scholar]
- DeSimone PA, Greco FA, Lessner HF and Bartolucci A, 1986. Phase II evaluation of anguidine (NSC 141537) in 5‐day courses in colorectal adenocarcinoma ‐ A Southeastern Cancer Study Group Trial. American Journal of Clinical Oncology, 9, 187–188. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Hohn TM and McCormick SP, 1993. Trichothecene biosynthesis in Fusarium species: chemistry, genetics, and significance. Microbiological Reviews, 57, 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarchelier A, Oberson J‐M, Tella P, Gremaud E, Seefelder W and Mottier P, 2010. Development and comparison of two multiresidue methods for the analysis of 17 mycotoxins in cereals by liquid chromatography electrospray ionization tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 58, 7510–7519. [DOI] [PubMed] [Google Scholar]
- Diana Di Mavungu J, Monbaliu S, Scippo M‐L, Maghuin‐Rogister G, Schneider Y‐J, Larondelle Y, Callebaut A, Robbens J, Van Peteghem C and De Saeger S, 2009. LC‐MS/MS multi‐analyte method for mycotoxin determination in food supplements. Food Additives and Contaminants, 26, 885–895. [DOI] [PubMed] [Google Scholar]
- Diaz GJ, 2002. Evaluation of the Efficacy of a Feed Additive to Ameliorate the Toxic Effects of 4,15‐Diacetoxiscirpenol in Growing Chicks. Poultry Science, 81, 1492–1495. [DOI] [PubMed] [Google Scholar]
- Diaz GJ, Squires EJ, Julian RJ and Boermans HJ, 1994. Individual and combined effects of T‐2 toxin and DAS in laying hens. British Poultry Science, 35, 393–405. [DOI] [PubMed] [Google Scholar]
- Diggs CH, Scoltock MJ and Wiernik PH, 1978. Phase II evaluation of anguidine NSC‐141537 for adenocarcinoma of the colon or rectum. Cancer Clinical Trials, Winter, 1978, 26–29. [PubMed] [Google Scholar]
- Dong M, Si W, Wang W, Bai B, Nie D, Song W, Zhao Z, Guo Y and Han Z, 2016. Determination of type A trichothecenes in coix seed by magnetic solid‐phase extraction based on magnetic multi‐walled carbon nanotubes coupled with ultra‐high performance liquid chromatography‐tandem mass spectrometry. Analytical and Bioanalytical Chemistry, 408, 6823–6831. [DOI] [PubMed] [Google Scholar]
- Driehuis F, Spanjer MC, Scholten JM and Giffel MC, 2008. Occurrence of mycotoxins in maize, grass and wheat silage for dairy cattle in the Netherlands. Food Additives and Contaminants, 1, 41–50. [DOI] [PubMed] [Google Scholar]
- Duncan M and Grant G, 2003. Oral and intestinal mucositis ‐ causes and possible treatments. Alimentary Pharmacology and Therapeutics, 18, 853–874. [DOI] [PubMed] [Google Scholar]
- EBLEX (English Beef and Lamb Executive), 2008. Feeding growing and finishing cattle for better returns. In: Brown D, Rumans K and Vickers M (eds.). EBLEX Better Return Programme, Huntington, UK, 20 pp.
- EBLEX (English Beef and Lamb Executive), 2012. Experts view: Maize in beef systems. In: Marsh S (ed.). EBLEX Better Returns Programme, Huntington, UK, 3 pp.
- Edward SG, 2009. Fusarium mycotoxin content of UK organic and conventional barley. Food Additives and Contaminants Part A, 26, 1185–1190. 10.1080/02652030902919418 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2006. Guidance of the Scientific Committee on a request from EFSA related to uncertainties in Dietary Exposure Assessment. EFSA Journal 2007;4(12):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Intakes Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011c. Report on the development of a Food Classification and Description System for exposure assessment and guidance on its implementation and use. EFSA Journal 2011;9(12):2489, 84 pp. 10.2903/j.efsa.2011.2489 [DOI] [Google Scholar]
- EFSA CONTAM Panel , 2011. Scientific Opinion on the risks for animal and public health related to the presence of T‐2 and HT‐2 toxin in food and feed. EFSA Journal 2011;9(12):2481, 187 pp. 10.2903/j.efsa.2011.2481 [DOI] [Google Scholar]
- EFSA CONTAM Panel , 2017. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA Journal 2017;15(9):4718, 345 pp. 10.2903/j.efsa.2017.4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534 26 pp. 10.2903/j.efsa.2012.2534 [DOI] [Google Scholar]
- Engster HM, Snetsinger DC, Cover MS and Eldridge LF, 1982. Effects of nutritional fortification on performance and subsequent recovery of laying hens fed diets contaminated with diacetoxyscirpenol. Abstract, 71 annual meeting of Poultry Science Association. Poultry Science, 61, 1403–1572. [Google Scholar]
- Escrivá L, Manyes L, Font G and Berrada H, 2015. Analysis of trichothecenes in laboratory rat feed by gas chromatography‐tandem mass spectrometry. Food Additives and Contaminants: Part A, 66, 329–338. 10.1080/19440049.2015.1124458 [DOI] [PubMed] [Google Scholar]
- Escrivá L, Manyes L, Font G and Berrada H, 2016. Analysis of trichothecenes in laboratory rat feed by gas chromatography‐tandem mass spectrometry. Food Additives and Contaminants: Part A, 33, 329–338. [DOI] [PubMed] [Google Scholar]
- Eskola M, 2002. Study on trichothecenes, zearalenone and ochratoxin A in Finnish cereals: occurrence and analytical techniques. EELA publications 3. National Veterinary and Food Research Innstitute and University of Helsinki [dissertation].
- Ewing WN, 2002. The feeds directory: branded products guide. Context Products Ltd.
- Fairchild AS, Grimes JL, Porter JK, Croom WJ Jr, Daniel LR and Hagler WM Jr, 2005. Effects of diacetoxyscirpenol and fusaric acid on poults: individual and combined effects of dietary diacetoxyscirpenol and fusaric acid on turkey poult performance. International Journal of Poultry Science, 4, 350–355. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 2004. Worldwide regulations for mycotoxins in food and feed in 2003. FAO Food and Nutrition Paper 81. Food and Agriculture Organization of the United Nations, Rome, Italy. Available online: http://www.fao.org/3/a-y5499e.pdf
- FEFAC , 2009. The European Feed Manufacturers Federation – Compound Feed Production 2009.
- Flores‐Flores ME and González‐Penas E, 2015. Development and validation of a high performance liquid chromatographic–mass spectrometry method for the simultaneous quantification of 10 trichothecenes in ultra‐high temperature processed cow milk. Journal of Chromatography A, 1419, 37–44. [DOI] [PubMed] [Google Scholar]
- Froquet R, Sibiril Y and Parent‐Massin D, 2001. Trichothecene toxicity on human megakaryocyte progenitors (CFU‐MK). Human and Experimental Toxicology, 20, 84–89. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Binder EM, Heidler D and Krska R, 2002. Structural characterization of metabolites after the microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Additives & Contaminants, 19, 379–386. [DOI] [PubMed] [Google Scholar]
- Furlong EB and Valente Soares LM, 1995. Gas chromatographic method for quantitation and confirmation of trichothecenes in wheat. Journal of AOAC International, 78, 386–390. [Google Scholar]
- García‐Moraleja A, Font G, Mañes J and Ferrer E, 2015a. Development of a new method for the simultaneous determination of 21 mycotoxins in coffee beverages by liquid chromatography tandem mass spectrometry. Food Research International, 72, 247–255. [Google Scholar]
- García‐Moraleja A, Font G, Mañes J and Ferrer E, 2015b. Simultaneous determination of mycotoxin in commercial coffee. Food Control, 57, 282–292. [Google Scholar]
- Gareis M, Hashem A, Bauer J and Gedek B, 1986. Identification of glucuronide metabolites of T‐2 toxin and diacetoxyscirpenol in the bile of isolated perfused rat liver. Toxicology and Applied Pharmacology, 84, 168–172. [DOI] [PubMed] [Google Scholar]
- Garreau de Loubresse N, Prokhorova I, Holtkamp W, Rodnina MV, Yusupova G and Yusupov M, 2014. Structural basis for the inhibition of the eukaryotic ribosome. Nature. 2014 Sep 25;513(7519):517–522. [DOI] [PubMed] [Google Scholar]
- Gentili A, Caretti F, D'Ascenzo G, Mainero Rocca L, Marchese S, Materazzi S and Perret D, 2007. Simultaneous Determination of Trichothecenes A, B, and D in Maize Food Products by LC–MS–MS. Chromatographia, 66, 669–676. [Google Scholar]
- Gimeno A, 1979. Thin layer chromatographic determination of aflatoxins, ochratoxins, sterigmatocystin, zearalenone, citrinin, t‐2 toxin, diacetoxyscirpenol, penicillic acid, patulin, and penitrem. Journal of Association of Official Analytical Chemistry, 62, 579–585. [PubMed] [Google Scholar]
- Goodman LS, Hardman JG, Limbird LE and Gilman AG, 2001. Goodman & Gilman's the Pharmacological Basis of Therapeutics. 10th Edition Edited by Hardman JG, Limbird LE, Gilman AG. McGraw Hill, New York. [Google Scholar]
- Goodwin W, Haas CD, Fabian C, Heller‐Bettinger I and Hoogstrate B, 1978. Phase I evaluation of anguidine (DAS, NSC ‐141537). American Cancer Society, 42, 23–26. [DOI] [PubMed] [Google Scholar]
- Goodwin W, Bottomley RH, Vaughn CB, Frank J and Pugh RP, 1983. Phase II evaluation of anguidine in central nervous system tumours: a Southwest oncology study group. Cancer Treatment Report, 67, 285. [PubMed] [Google Scholar]
- Gottschalk C, Barthel J, Engelhardt G, Bauer J and Meyer K, 2007. Occurrence of type A trichothecenes in conventionally and organically produced oats and oat products. Molecular Nutrition and Food Research, 51, 1547–1553. [DOI] [PubMed] [Google Scholar]
- Gottschalk C, Barthel J, Engelhardt G, Bauer J and Meyer K, 2009. Simultaneous determination of type A, B and D trichothecenes and their occurrence in cereals and cereal products. Food Additives and Contaminants, 26, 1273–1289. [Google Scholar]
- Grove JF and Mortimer PH, 1969. The cytotoxicity of some transformation products of diacetoxyscirpenol. Biochemical Pharmacology, 18, 1473–1478. [DOI] [PubMed] [Google Scholar]
- Guan S, He J, Young JC, Zhu H, Li X‐Z, Ji C and Zhou T, 2009. Transformation of trichothecene mycotoxins by microorganisms from fish digesta/. Aquaculture, 290, 290–295. [Google Scholar]
- Guillaume J, Kaushik S, Bergot P and Metailler R, 2001. Nutrition and feeding of fish and crustaceans. Praxis Publishing, UK: p. 408. [Google Scholar]
- Gürbüzel M, Uysal H and Kizilet H, 2015. Assessment of genotoxic potential of two mycotoxins in the wing spot test of Drosophila melanogaster. Toxicology and Industrial Health, 31, 261–267. [DOI] [PubMed] [Google Scholar]
- Hack R, Klaffer U and Terplan G, 1989. A monoclonal antibody to the trichothecene mycotoxin diacetoxyscirpenol. Letters in Applied Microbiology, 8, 71–75. [Google Scholar]
- Harvey RB, Kubena LF, Elissalde MH, Corrier DE, Huff WE, Rottinghaus GE and Clement BA, 1991. Cocontamination of swine diets by aflatoxin and diacetoxyscirpenol. Journal of Veterinary Diagnostic Investigations, 3, 155–160. [DOI] [PubMed] [Google Scholar]
- Harvey RB, Edrington TS, Kubena LF, Elissalde MH, Corrier DE and Rottinghaus GE, 1995. Effect of aflatoxin and diacetoxyscirpenol in ewe lambs. Bulletin of Environmental Contamination and Toxicology, 54, 325–330. [DOI] [PubMed] [Google Scholar]
- Hassanane MS, Abdalla ESA, El‐Fiky S, Amer MA and Hamdy A, 2000. Mutagenicity of mycotoxin DAS on somatic and germ cells of mice. Mycotoxin Research, 16, 53–64. [DOI] [PubMed] [Google Scholar]
- Hazel CM and Patel S, 2004. Influence of processing on trichothecene levels. Toxicology Letters, 153, 51–59. [DOI] [PubMed] [Google Scholar]
- Hernandez F and Cannon M, 1982. Inhibition of protein synthesis in Saccharomyces cerevisiae by the 12,13‐epoxytrichothecenes trichodermol, diacetoxyscirpenol and verrucarin A. Reversibility of the effects. The Journal of Antibiotics (Tokyo), 35, 875–881. [DOI] [PubMed] [Google Scholar]
- Hoerr FJ, Carlton WW and Yagen B, 1981a. The toxicity of T‐2 toxin and diacetoxyscirpenol in combination for broiler chickens. Food and Cosmetics Toxicology, 19, 185–188. [DOI] [PubMed] [Google Scholar]
- Hoerr FJ, Carlton WW and Yagen B, 1981b. Mycotoxicosis caused by a single dose of T‐2 toxin or diacetoxyscirpenol in broiler chickens. Veterinary Pathology, 18, 652–664. [DOI] [PubMed] [Google Scholar]
- Hoerr FJ, Carlton WW, Yagen B and Joffe AZ, 1982a. Mycotoxicosis produced in broiler chickens by multiple doses of either T‐2 toxin or DAS. Avian Pathology, 11, 369–383. [DOI] [PubMed] [Google Scholar]
- Hoerr FJ, Carlton WW, Yagen B and Joffe AZ, 1982b. Mycotoxicosis caused by either T‐2 toxin or diacetoxyscirpenol in the diet of broiler chickens. Fundamental and Applied Toxicology, 2, 121–124. [DOI] [PubMed] [Google Scholar]
- Hohn TM, McCormick SP and Desjardins AE, 1993. Evidence for a gene cluster involving trichothecene‐pathway biosynthetic genes in Fusarium sporotrichioides . Current Genetics, 24, 291–295. [DOI] [PubMed] [Google Scholar]
- Huybrechts I, Sioen I, Boon PE, Ruprich J, Lafay L, Turrini A, Amiano P, Hirvonen T, De Neve M, Arcella D, Moschandreas J, Westerlund A, Ribas‐Barba L, Hilbig A, Papoutsou S, Christensen T, Oltarzewski M, Virtanen S, Rehurkova I, Azpiri M, Sette S, Kersting M, Walkiewicz A, Serra‐Majem L, Volatier J‐L, Trolle E, Tornaritis M, Busk L, Kafatos A, Fabiansson S, De Henauw S and Van Klaveren JD, 2011. Dietary exposure assessments for children in Europe (the EXPOCHI project): rationale, methods and design. Archives of Public Health, 69, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRDC report (International Research and Development Corporation), 1973. Anguidine (NSC 141537) preclinical toxicological evaluation in dogs and monkeys. Pages 1‐289
- JECFA (Fifty‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives) , 2001. Evaluation of certain mycotoxins in food: WHO Technical Report series No. 906, 2001: 42–51. [PubMed]
- JECFA (Eighty‐third report of the joint FAO/WHO Expert Committee on Food Additives), 2016. Evaluation of certain contaminants in food. WHO technical report series No. 1002, 2017: 40–54
- Jelen HH and Wasowicz E, 2008. Determination of trichothecenes in wheat grain without sample cleanup using comprehensive two‐dimensional gas chromatography–time‐of‐flight mass spectrometry. Journal of Chromatography A, 1215, 203–207. [DOI] [PubMed] [Google Scholar]
- Jimenez M and Mateo R, 1997. Determination of mycotoxins produced by Fusarium isolates from banana fruits by capillary gas chromatography and high performance liquid chromatography. Journal of Chromatography A, 778, 363–372. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Mateo JJ and Mateo R, 2000. Determination of type A trichothecenes by high‐performance liquid chromatography with coumarin‐3‐carbonyl chloride derivatisation and fluorescence detection. Journal of Chromatography A, 870, 473–481. [DOI] [PubMed] [Google Scholar]
- Joffe AZ, 1974. Toxicity of Fusarium poae and F. sporotrichoides and its relation to alimentary toxic aleukia. Mycotoxins, page 229 [Google Scholar]
- Joffe AZ and Yagen B, 1978. Intoxication produced by toxic fungi Fusarium poae and F.Sporotrichoides on chicks. Toxicon, 16, 263–273. [DOI] [PubMed] [Google Scholar]
- Juan C, Ritieni A and Mañes J, 2013. Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chemistry, 141, 1747–1755. [DOI] [PubMed] [Google Scholar]
- Jun DY, Kim JS, Park HS, Song WS, Bae YS and Kim YH, 2007. Cytotoxicity of diacetoxyscirpenol is associated with apoptosis by activation of caspase‐8 and interruption of cell cycle progression by down‐regulation of cdk4 and cyclin B1 in human Jurkat T cells. Toxicology and Applied Pharmacology, 222, 190–201. [DOI] [PubMed] [Google Scholar]
- Kamimura H, Nishijima M, Saito K, Yasuda K, Ibe A, Nagayama T, Ushiyama H and Naoi Y, 1979The decomposition of trichothecene mycotoxins during food processing (Studies on mycotoxins in foods. XII). Journal Food Hygiene Society of Japan, 20, 352–357. [Google Scholar]
- Kiessling K‐H, Pettersson H, Sandholm K and Olsen M, 1984. Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Applied and Environmental Microbiology, 47, 1070–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Tokai T, Takahashi‐Ando N, Ohsato S and Fujimura M, 2007. Molecular and Genetic Studies of Fusarium Trichothecene Biosynthesis: pathways, Genes, and Evolution. Bioscience, Biotechnology, and Biochemistry, 71(9), 2105–2123. [DOI] [PubMed] [Google Scholar]
- Klaffer U, Märtlbauer E and Terplan G, 1988. Development of a sensitive enzyme‐linked immunosorbent assay for the detection of diacetoxyscirpenol. International Journal of Food Microbiology, 6, 9–17. [DOI] [PubMed] [Google Scholar]
- Klötzel M, Gutsche B, Lauber U and Humpf H‐U, 2005. Determination of 12 Type A and B Trichothecenes in Cereals by Liquid Chromatography‐Electrospray Ionization Tandem Mass Spectrometry. Journal of Agriculture and Food Chemistry, 53, 8904–8910. [DOI] [PubMed] [Google Scholar]
- Konjević D, Srebočan E, Gudan A, Lojkić I, Severin K and Sokolovićet M, 2004. A pathological condition possibly caused by spontaneous trichotecene poisoning in Brahma poultry: first report. Avian pathology, 33(3), 377–380. [DOI] [PubMed] [Google Scholar]
- Kotal F, Holadova K, Hajslova J, Poustka J and Radova Z, 1999. Determination of trichothecenes in cereals. Journal of Chromatography A, 830, 219–225. [DOI] [PubMed] [Google Scholar]
- Kriegleder H, 1980. Morphological findings in guinea‐pigs after administration of Trichothecenes Diacetoxyscirpenol and T‐2 Toxin. [dissertation in German]
- Kriegleder H, 1981. Morphological Findings in Guinea Pigs following Acute and Subacute Intoxication with Diacetoxyscirpenol. [Article in German]. Zentralblatt für Veterinärmedizin, 28, 165–175. [PubMed] [Google Scholar]
- Krivobok S, Olivier P, Marzin DR, Seigle‐Murandi F and Steiman R, 1987. Study of the genotoxic potential of 17 mycotoxins with the SOS chromotest. Mutagenesis, 2(6), 433–439. [DOI] [PubMed] [Google Scholar]
- Kubena LF, Harvey RB, Huff WE, Elissalde MH, Yersin AG, Phillips TD and Rottinghaus GE, 1993. Efficacy of a Hydrated Sodium Calcium Aluminosilicate to Reduce the Toxicity of AF and DAS. Poultry Science, 72, 51–59. [DOI] [PubMed] [Google Scholar]
- Kubena LF, Harvey RB, Edrington TS and Rottinghaus GE, 1994. Influence of ochratoxin A and diacetoxyscirpenol singly and in combination on broiler chickens. Poultry Science, 73, 408–415. [DOI] [PubMed] [Google Scholar]
- Kubena LF, Edrington TS, Harvey RB and Rottinghaus GE, 1997. Individual and Combined Effects of Fumonisin B1 Present in Fusarium moniliforme Culture Material and Diacetoxyscirpenol or Ochratoxin A in Turkey Poults. Poultry Science, 76, 256–264. [DOI] [PubMed] [Google Scholar]
- Kuczuk MH, Benson PM, Heath H and Hayes W, 1978. Evaluation of the mutagenic potential of mycotoxins using Salmonella typhimurium and Saccharomyces cerevisiae . Mutation Research, 53, 11–20. [DOI] [PubMed] [Google Scholar]
- Labuda R, Parich A, Berthiller F and Tancinová D, 2005. Incidence of trichothecenes and zearalenone in poultry feed mixtures from Slovakia. International Journal of Food Microbiology, 105, 19–25. [DOI] [PubMed] [Google Scholar]
- Lattanzio VMT, Pascale M and Visconti A, 2009. Current analytical methods for trichothecene mycotoxins in cereals. Trends in Analytical Chemistry, 28, 758–768. [Google Scholar]
- Lautraite S, Parent‐Massin D, Rio B and Hoellinger H, 1995. Comparison of toxicity induced by T‐2 toxin on human and rat granulo‐monocytic progenitors with an in vitro model. Human and Experimental Toxicology, 14(8), 672–678. [DOI] [PubMed] [Google Scholar]
- Lautraite S, Rio B, Guinard J and Parent‐Massin D, 1997. In vitro effects of DAS on human and rat granulo‐monocytic progenitors. Mycopathologia, 140, 59–64. [DOI] [PubMed] [Google Scholar]
- Leatherhead Food Research Association , 2010. Guide to Contaminants in Foodstuffs. An international overview of maximum limits. Leatherhead Food International Limited, Leatherhead, United Kingdom. ISBN 987‐907895‐09‐8.
- Lebas F and Renouf B, 2009. Raw materials utilization and feeding techniques: new contributions in the 9th World Rabbit Congress. Journée d’étude ASFC, 5 February 2009, Vérone ‐ Ombres & Lumiéres, 30–36.
- Leeson S and Summers JD, 2008. Commercial Poultry Nutrition, 3rd edition Nottingham University Press, Guelph, Ontario, Canada: p. 412. [Google Scholar]
- Lešnik M, Cencič A, Vajs S and Simončič A, 2008. Milling and bread baking techniques significantly affect the mycotoxin (deoxynivalenol and nivalenol) level in bread. Acta Alimentaria, 37, 471–483. [Google Scholar]
- Lewis RJ, 1999. Sax's Dangerous Properties of Industrial Materials. 10th ed. Volumes 1‐3 New York, NY: John Wiley & Sons Inc., p. V2: 26 [Google Scholar]
- Liao LL, Grollman AP and Horwitz SB, 1976. Mechanism of action of the 12,13‐ epoxytrichothecene, anguidine, an inhibitor of protein synthesis. Biochimica et Biophysica Acta, 454, 273–284. [DOI] [PubMed] [Google Scholar]
- Lindenfelser A, Lillehoj EB and Burmeister HR, 1974. Aflatoxin and trichothecene toxins: skin tumor induction and synergistic acute toxicity in white mice. Journal of the National Cancer Institute, 52, 113–116. [DOI] [PubMed] [Google Scholar]
- Lysoe E, Frandsen RJN, Divon HH, Terzi V, Orru L, Lamontanara A, Kolseth A‐K, Nielsen KF and Thrane U, 2016. Draft genome sequence and chemical profiling of Fusarium langsethiae, an emerging producer of type A trichothecenes. International Journal of Food Microbiology, 221, 29–36. [DOI] [PubMed] [Google Scholar]
- Malachová A, Sulyok M, Beltrán E, Berthiller F and Krska R, 2014. Optimization and validation of a quantitative liquid chromatography–tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. Journal of Chromatography A, 1362, 145–156. [DOI] [PubMed] [Google Scholar]
- Matsushima T, Okamoto E, Miyagawa E, Matsui Y, Shimizu H and Asano K, 1996. Deacetylation of diacetoxyscirpenol by rumen bacteria. Journal of general and applied microbiology, 42, 225–234. [Google Scholar]
- Maycock R and Utley D, 1985. Analysis of some trichothecene mycotoxins by liquid chromatography. Journal of Chromatography, 347, 429–433. [DOI] [PubMed] [Google Scholar]
- Mayer B, Schneider E, Usleber E, Dietrich R, Bürk C and Märtlbauer E, 2000. Entwicklung eines immunchemischen Schnelltestverfahrens zum Multimykotoxinnachweis. Mycotoxin Research, 16, 227–230. [DOI] [PubMed] [Google Scholar]
- Mayura K, Smith EE, Clement BA, Harvey RB, Kubena LF and Phillips TD, 1987. Developmental toxicity of diacetoxyscirpenol in the mouse. Toxicology, 45, 245–255. [DOI] [PubMed] [Google Scholar]
- Mc Cormick SP, Stanley AM, Stover NA and Alexander NJ, 2011. Trichothecenes: from simple to complex mycotoxins. Toxins, 2011, 802–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald P, Greenhalgh JFD, Morgan CA, Edwards R, Sinclair L and Wilkinson R, 2011. Animal Nutrition, 7th edition Pearson, London, UK. 712 pp. [Google Scholar]
- Meissonnier GM, Pinton P, Laffitte J, Cossalter AM, Gong YY, Wild CP, Bertin G, Galtier P and Oswald IP, 2008. Immunotoxicity of aflatoxin B1: impairment of the cell‐mediated response to vaccine antigen and modulation of cytokine expression. Toxicology and Applied Pharmacology, 231, 142–149. 10.1016/j.taap.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Merten C, Ferrari P, Bakker M, Boss A, Hearty A, Leclercq C, Lindtner O, Tlustos C, Verger P, Volatier JL and Arcella D, 2011. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food Additives and Contaminants: Part A, 28, 975–995. [DOI] [PubMed] [Google Scholar]
- Mézes M and Balogh K, 2009. Mycotoxins in rabbit feed: a review. World Rabbit Science, 17, 53–62. [Google Scholar]
- Milanez TV and Valente‐Soares LM, 2006. Gas Chromatography ‐ Mass Spectrometry Determination of Trichothecene Mycotoxins in Commercial Corn Harvested in the State of São Paulo. Brazil. J. Braz. Chem. Soc., 17(2), 412–416. [Google Scholar]
- Moré J, Galtier P and Eeckhoutte C, 1990. Effect of low doses of a trichothecene mycotoxin (diacetoxyscirpenol) on rat gastric glycoproteins: a histochemical study. Toxicology Letters, 50, 173–178. [DOI] [PubMed] [Google Scholar]
- Morrison E, Rundberget T, Kosiak B, Aastveit AH and Bernhoft A, 2002. Cytotoxicity of trichothecenes and fusarochromanone produced by Fusarium equiseti strains isolated from Norwegian cereals. Mycopathologia, 153(1), 49–56. [DOI] [PubMed] [Google Scholar]
- Murphy WK, Burgess MA, Valdivieso M, Livingston RB, Bodey GP and Freireich EJ, 1978. Phase I clinical evaluation of anguidine. Cancer Treatment Reports., 62(10), 1497–1502. [PubMed] [Google Scholar]
- Nakagawa H, Sakamoto S, Sago Y, Kushiro M and Nagashima H, 2013a. Detection of masked mycotoxins derived from type A trichothecenes in corn by high‐resolution LC‐Orbitrap mass spectrometer. Food Additives & Contaminants, 30(8), 1407–1414. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Sakamoto S, Sago Y and Nagashima H, 2013b. Detection of Type A Trichothecene Di‐Glucosides Produced in Corn by High‐Resolution Liquid Chromatography‐Orbitrap Mass Spectrometry. Toxins, 5, 590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasri T, Bosch RR, ten Voordea S and Fink‐Gremmels J, 2006. Differential induction of apoptosis by type A and B trichothecenes in Jurkat T‐lymphocytes. Toxicology in vitro, 20, 832–840. [DOI] [PubMed] [Google Scholar]
- Nielsen KF and Thrane U, 2001. Fast methods for screening of trichothecenes in fungal cultures using gas chromatography–tandem mass spectrometry. Journal of Chromatography A, 929, 75–87. [DOI] [PubMed] [Google Scholar]
- Nix JS, 2010. The John Nix Farm Management Pocketbook. 40th Edition. The Anderson Centre
- Njumbe Ediage E, Diana Di Mavungu J, Monbaliu S, Van Peteghem C and De Saeger S, 2011. A Validated Multianalyte LC‐MS/MS Method for Quantification of 25 Mycotoxins in Cassava Flour, Peanut Cake and Maize Samples. Journal of Agriculture and Food Chemistry, 59, 5173–5180. [DOI] [PubMed] [Google Scholar]
- Njumbe Ediage E, Van Poucke C and De Saeger S, 2015. A multi‐analyte LC–MS/MS method for the analysis of 23 mycotoxins in different sorghum varieties: The forgotten sample matrix. Food Chemistry, 177, 397–404. [DOI] [PubMed] [Google Scholar]
- Nowotny P, Baltes W, Krönert W and Weber R, 1983. Dunnschichtchromatographische Methode zur Bestimmung von 22 Mykotoxinen in verschimmelten Lebensmitteln. Chem. Mikrobiol. Technol. Lebensm., 8, 24–28. [Google Scholar]
- NRC (National Research Council), 1982. Nutrient requirements of Mink and Foxes. 2nd Revised edition. The National Academies Press, Washington, USA. 72 pp. 10.17226/1114 [DOI]
- NRC (National Research Council), 2000. Nutrient Requirements of Beef Cattle: Seventh Revised Edition: Update 2000. The National Academies Press, Washington, USA, 248 pp. 10.17226/9791 [DOI]
- NRC (National Research Council), 2006. Nutrient Requirements of Dogs and Cats. The National Academies Press, Washington, DC, 424 pp. 10.17226/10668 [DOI]
- NRC (National Research Council), 2007a. Nutrient requirements of small ruminants: sheep, goats, cervids and new world camelids. The National Academies Press, Washington, DC, 384 pp. 10.17226/11654 [DOI]
- NRC (National Research Council), 2007b. Nutrient requirement of horses, 6th Revised Edition. The National Academies Press, Washington, DC, 360 pp. 10.17226/11653 [DOI]
- OECD (Organisation for Economic Co‐operation and Development), 2009. Guidance Document on Overview of residue chemistry study. Series on Pesticides No. 32, Paris, France, 93 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance Document on Residues in Livestock. Series on Pesticides No. 73. OECD, Paris, France, 77 pp
- Ohta M, Matsumoto H, Ishii K and Ueno Y, 1978. Metabolism of trichothecenes mycotoxin. Journal of biochemistry, 84, 697–706. [DOI] [PubMed] [Google Scholar]
- Omurtag GZ, Tozan A, Sirkecioglu O, Kumbaraci V and Rollas S, 2007. Occurrence of diacetoxyscirpenol (anguidine) in processed cereals and pulses in Turkey by HPLC. Food Control, 18, 970–974. [Google Scholar]
- Parent‐Massin D, 2004. Haematotoxicity of trichothecenes. Toxicology Letters, 153, 75–81. [DOI] [PubMed] [Google Scholar]
- Parent‐Massin D and Parchment RE, 1998. Haematotoxicity of mycotoxins. Revue Médicine Véterinaire, 149, 591–598. [Google Scholar]
- Parent‐Massin D and Thouvenot D, 1995. In vitro toxicity of trichothecenes on rat haematopoietic progenitors. 12, 41–49 [DOI] [PubMed]
- Parent‐Massin D, Fuselier R and Thouvenot D, 1994. In vitro toxicity of trichothecenes on human haematopoietic progenitors. Food additives and contaminants., 11, 441–447. [DOI] [PubMed] [Google Scholar]
- Pauly JU, Bitter‐Suermann D and Dose K, 1988. Production and Characterization of a Monoclonal Antibody to the Trichothecene Mycotoxin Diacetoxyscirpenol. Biol. Chem. Hoppe‐Seyler, 369, 487–492. [DOI] [PubMed] [Google Scholar]
- Payros D, Alassane‐Kpembi I, Pierron A, Loiseau N, Pinton P and Oswald IP, 2016. Toxicology of deoxynivalenol and its acetylated and modified forms. Archive of Toxicology, 90, 2931–2957. [DOI] [PubMed] [Google Scholar]
- Perkowski J, Kiecana I, Stachowiak J and Basinski T, 2003. Natural occurrence of scirpentriol in cereals infected by Fusarium species. Food Additives and Contaminants, 20, 572–578. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, 2010. Deoxynivalenol: mechanisms of action, human exposure and toxicological relevance. Archives of Toxicology, 84, 663–679. [DOI] [PubMed] [Google Scholar]
- Qureshi MA, Brundage MA and Hamilton PB, 1998. beta, 15‐Diacetoxyscirpenol induces cytotoxicity and alterations in phagocytic and Fc‐receptor expression functions in chicken macrophages in vitro. Immunopharmacology and Immunotoxicology., 20(4), 541–553. [DOI] [PubMed] [Google Scholar]
- Radova Z, Holadova K and Hajslova J, 1998. Comparison of two clean‐up principles for determination of trichothecenes in grain extract. Journal of Chromatography A, 829, 259–267. [DOI] [PubMed] [Google Scholar]
- Rasmussen PH, Nielsen KF, Ghorbani F, Spliid NH, Nielsen GC and Jørgensen LN, 2012. Occurrence of different trichothecenes and deoxynivalenol3‐β‐D‐glucoside in naturally and artificially contaminated Danish cereal grains and whole maize plants. Mycotoxins Research, 28, 181–190. [DOI] [PubMed] [Google Scholar]
- Ryu D, Bianchini A and Bullerman LB, 2008. Effects of processing on mycotoxins. Stewart Postharvest Review., 6, 5 10.2212/spr.2008.6 [DOI] [Google Scholar]
- Van Rensburg DFJ, Thiel PG and Jaskiewic K, 1987. Short‐term effects of two fusarium toxins, DAS and NEO monoacetate, in male Wistar rats. Food and Chemical Toxicology, 25, 767–771. [DOI] [PubMed] [Google Scholar]
- Richardson KE and Hamilton PB, 1990. Comparative Toxicity of Scirpentriol and Its Acetylated Derivatives. Poultry Science, 69, 397–402. [DOI] [PubMed] [Google Scholar]
- Richardson KE, Toney GE, Haney CA and Hamilton PB, 1989. Occurrence of Scirpentriol and its Seven Acetylated Derivatives in Culture Extracts of Fusarium sambucinum NRRL 13495. J. Food Protection, 52, 871–876. [DOI] [PubMed] [Google Scholar]
- Rio B, Lautraite S and Parent‐Massin D, 1997. In vitro toxicity of trichothecenes on human erythroblastic progenitors. Human and Experimental Toxicology, 16, 673–679. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Carrasco Y, Font G, Manes J and Berrada H, 2013a. Determination of Mycotoxins in Bee Pollen by Gas Chromatography‐Tandem Mass Spectrometry. Journal of agricultural and food chemistry, 61, 1999–2005. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Carrasco Y, Ruiz MJ, Font G and Berrada H, 2013b. Exposure estimates to Fusarium mycotoxins through cereals intake. Chemosphere, 93, 2297–2303. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Carrasco Y, Font G, Moltó JC and Berrada H, 2014. Quantitative determination of trichothecenesin breadsticks by gas chromatography‐triple quadrupole tandem mass spectrometry. Food Additives & Contaminants: Part A, 31, 1422–1430. 10.1080/19440049.2014.926399 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Rodriguez M, Herrera M, Arino A and Cordoba JJ, 2014. T‐2, HT‐2, and diacetoxyscirpenol toxins from Fusarium In Manual of Security Sensitive Microbes and Toxins. CRC press, Taylor and Francis Group; pp. 545–557. [Google Scholar]
- Rodríguez‐Carrasco Y, Moltó J, Mañes J and Berrada H, 2017. Development of microextraction techniques in combination with GC‐MS/MS for the determination of mycotoxins and metabolites in human urine. Journal of Separation Science, 40, 1572–1582. [DOI] [PubMed] [Google Scholar]
- Rubert J, Manes J, James KJ and Soler C, 2011. Application of hybrid linear ion trap‐high resolution mass spectrometry to the analysis of mycotoxins in beer. Food Additives and Contaminants, 28, 1438–1446. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Swanson SP, Yoshizawa T and Buck WB, 1986. Structures of New Metabolites of diacetoxyscirpenol in the excreta of orally administered rats. Journal of agricultural and food chemistry, 34, 698–701. [Google Scholar]
- Santini A, Ferracane R, Somma MC, Aragon A and Ritieni A, 2009. Multitoxin extraction and detection of trichothecenes in cereals: an improved LC‐MS/MS approach. Journal of the Science of Food and Agriculture, 89, 1145–1153. [Google Scholar]
- Schlosberg AS, Klinger Y and Malkinson MH, 1986. Muscovy ducklings, a particularly sensitive avian bioassay for T‐2 toxin and diacetoxyscirpenol. Avian Diseases, 30, 820–824. [PubMed] [Google Scholar]
- Schollenberger M, Lauber U, Jara HT, Suchy S, Drochner W and Müller H‐M, 1998. Determination of eight trichothecenes by gas chromatography–mass spectrometry after sample clean‐up by a two‐stage solid‐phase extraction. Journal of Chromatography A, 815, 123–132. [DOI] [PubMed] [Google Scholar]
- Schollenberger M, Mueller H‐M and Drochner W, 2005. Fusarium toxin content of maize and maize products purchased in the years 2000 and 2001 in Germany. Mycotoxin Research, 21, 26–28. [DOI] [PubMed] [Google Scholar]
- Schollenberger M, Mueller H‐M, Rüfle M, Suchy S, Plank S and Drochner W, 2006. Natural Occurrence of 16 Fusarium Toxins in Grains and Feedstuffs of Plant Origin from Germany. Mycopathologia, 161, 43–52. [DOI] [PubMed] [Google Scholar]
- Schollenberger M, Drochner W and Mueller H‐M, 2007. Fusarium toxins of the scirpentriol subgroup: a review. Mycopathologia, 164, 101–118. [DOI] [PubMed] [Google Scholar]
- Schollenberger M, Mueller H‐M, Liebscher M, Schlecker C, Berger M and Hermann W, 2011. Accumulation kinetics of three scirpentriol‐based toxins in oats inoculated in vitro with isolates of Fusarium sporotrichioides and Fusarium poae . Toxins, 3, 442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schollenberger M, Muller H‐M, Rufle M and Drochner W, 2008. Natural occurrence of 16 Fusarium toxins in edible oil marketed in Germany. Food Control, 19, 475–482. [Google Scholar]
- Schothorst RC and Jekel AA, 2001. Determination of trichothecenes in wheat by capillary gas chromatography with flame ionization detection. Food Chemistry, 73, 111–117. [Google Scholar]
- Schothorst RC and Jekel AA, 2003. Determination of trichothecenes in beer by capillary gas chromatography with flame ionisation detection. Food Chemistry, 82, 475–479. [Google Scholar]
- Schultz S, Vallant B and Kainz MJ, 2012. Preferential feeding on high quality diets decreases methyl mercury of farm‐raised common carp (Cyprinus carpio L.). Aquaculture, 29, 338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwake‐Anduschus C, Langenkämper G, Unbehend G, Dietrich R, Märtlbauer E and Muenzing K, 2010. Occurrence of Fusarium T‐2 and HT‐2 toxins in oats from cultivar studies in Germany and degradation of the toxins during grain cleaning treatment and food processing. Food Additives and Contaminants ‐ Part A Chemistry, Analysis, Control, Exposure and Risk Assessment, 27, 1253–60. [DOI] [PubMed] [Google Scholar]
- SCOOP (Scientific Co‐Operation), 2003. Collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU Member States. Report of experts participati9ng in Task 3.2.10, April 2003. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_contaminants_catalogue_fusarium_task3210.pdf
- Sforza S, Dall'Asta C and Marchelli R, 2006. Recent advances in mycotoxin determination in food and feed by hyphenated chromatographic techniques/mass spectrometry. Mass Spectrometry Reviews, 25, 54–76. [DOI] [PubMed] [Google Scholar]
- Shams M, Mitterbauer R, Corradini R, Wiesenberger G, Dall'Asta C, Schuhmacher R, Krska R, Adam G and Berthiller F, 2011. Isolation and characterization of a new less‐toxic derivative of the Fusarium mycotoxin diacetoxyscirpenol after thermal treatment. Journal of Agriculture and Food Chemistry, 59, 9709–9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsheimer JE, Chakraborty PK, Messerly EA and Gaddamidi V, 1989. Mutagenicity of oxaspiro compounds with Salmonella. Mutation Research, 224, 171–175. [DOI] [PubMed] [Google Scholar]
- Sklan D, Klipper E, Friedman A, Shelley M and Makovsky B, 2001. The Effect of Chronic Feeding of DAS, T‐2 toxin, AF on Performance, Health, and Antibody Production in Chicks. Journal of Applied Poultry Research, 10, 79–85. [DOI] [PubMed] [Google Scholar]
- Sklan D, Shelly M, Makovsky B, Geyra A, Klipper E and Friedman A, 2003. The effect of chronic feeding of DAS and T‐2 on performance, health, small intestinal physiology and antibody production in turkey poults. British Poultry Science, 44, 46–52. [DOI] [PubMed] [Google Scholar]
- Sørensen LK and Elbæk TH, 2005. Determination of mycotoxins in bovine milk by liquid chromatography tandem mass spectrometry. Journal of Chromatography B, 820, 183–196. [DOI] [PubMed] [Google Scholar]
- Sospedra I, Blesa J, Soriano JM and Manes J, 2010. Use of the modified quick easy cheap effective rugged and safe sample preparation approach for the simultaneous analysis of type A‐ and B‐trichothecenes in wheat flour. Journal of Chromatography A, 1217, 1437–1440. [DOI] [PubMed] [Google Scholar]
- Stähelin H, Kalberer‐Rush ME, Signer E and Lazáry S, 1968. Biological effects of the cytostatic DAS. Arzeneimittelforschung, 18, 989–994. [Google Scholar]
- Swanson SP, Rood HD Jr, Behrens JC and Sanders PE, 1987. Preparation and characterization of the deepoxy trichothecenes: deepoxy HT‐2, deepoxy T‐2 triol, deepoxy T‐2 tetraol, deepoxy 15‐monoacetoxyscirpenol, and deepoxy scirpentriol. App. Environm. Microbiol., 53, 2821–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson SP, Helaszek C, Buck WB, Rood HD Jr and Haschek WM, 1988. The role of intestinal microflora in the metabolism of trichothecenene mycotoxins. Food and Chemical Toxicology, 26, 823–829. [DOI] [PubMed] [Google Scholar]
- Tamura M, Mochizuki N, Nagatomi Y, Harayama K, Toriba A and Hayakawa K, 2015. A Method for Simultaneous Determination of 20 Fusarium Toxins in Cereals by High‐Resolution Liquid Chromatography‐Orbitrap Mass Spectrometry with a Pentafluorophenyl Column. Toxins, 7, 1664–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JT, Vaughn C and Stuckey WJ, 1981. Phase II trial of anguidine in patients with sarcomas unresponsive to prior chemotherapy: a Southwest Oncology Group Study. Cancer Treatment Reports, 65(9‐10), 881–882. [PubMed] [Google Scholar]
- Thomas M, van Zuilichem DJ and van der Poel AFB, 1997. Physical quality of pelleted animal feed. 2. Contribution of processes and its conditions. Animal Feed Science and Technology, 64, 173–192. [Google Scholar]
- Thompson WL and Wannemacher RW, 1986. Structure‐function relationships of 12,13‐epoxytrichothecene mycotoxins in cell culture: comparison to whole animal lethality. Toxicon, 24, 985–994. [DOI] [PubMed] [Google Scholar]
- Thrane U, Adler A, Clasen P‐E, Galvano F, Langseth W, Lew H, Logrieco A, Nielsen KF and Ritieni A, 2004. Diversity in metabolite production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides . International Journal of Food Microbiology, 95, 257–266. [DOI] [PubMed] [Google Scholar]
- Timbrell J, 2000. Principles of biochemical toxicology. Taylor and Francis, third edition [Google Scholar]
- Twaruzek M, Blajet‐Kosicka A, Wenda‐Piesik A, Palubicki J and Grajewski J, 2013. Statistical comparison of Fusarium mycotoxins content in oat grain and related products from two agricultural systems. Food Control, 34, 291–295. [Google Scholar]
- Ueno Y, 1980. Trichothecene mycotoxins: Mycology, chemistry and toxicology. Advances in Food and Nutrition Research, 3, 301–353. [Google Scholar]
- Ueno Y, 1983. General toxicology In: Ueno Y, editor. Developments in Food Science. IV Trichothecenes ‐ Chemical, biological and toxicological aspects. Tokyo/Amsterdam, Kodansha/Elsevier; pp. 135–146. [Google Scholar]
- Ueno Y, Ishii K, Sawano M, Ohtsubo K, Matsuda Y, Tanaka T, Kurata H and Ichinoe M, 1977. Toxicological approach to the metabolites of Fusaria. Japanese Journal of Experimental Medicine., 47, 177–184. [PubMed] [Google Scholar]
- Vaclavik L, Zachariasova M, Hrbek V and Hajslova J, 2010. Analysis of multiple mycotoxins in cereals under ambient conditions using direct analysis in real time (DART) ionization coupled to high resolution mass spectrometry. Talanta, 82, 1950–1957. [DOI] [PubMed] [Google Scholar]
- Vanheule A, Audenaert K, De Boevre M, Landschoot S, Bekaert B, Munaut F, Eeckhout M, Höfte M, De Saeger S and Haesaert G, 2014. The compositional mosaic of Fusarium species and their mycotoxins in unprocessed cereals, food and feed products in Belgium. International Journal of Food Microbiology, 181, 28–36. [DOI] [PubMed] [Google Scholar]
- Vanheule A, De Boevre M, Moretti A, Scauflaire J, Munaut F, De Saeger S, Bekaert B, Haesaert G, Waalwijk C, van der Lee T and Audenaert K, 2017. Genetic diversity and chemotype diversity in the Fusarium head blight pathogen Fusarium poae . Toxins, 9, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet MJ, Harmsen HJ, De Bont ES and Tissing WJ, 2010. The role of intestinal microbiota in the development and severity of chemotherapy‐induced mucositis. PLoS Pathog, 6, e1000879 10.1371/journal.ppat.1000879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J‐S, Busby WF jr and Wogan GN, 1989. Comparative Tissue Distribution and Excretion of Orally Administered [3H]Diacetoxyscirpenol (Anguidine) in Rats and Mice. Toxicology and Applied Pharmacology, 103, 430–440. [DOI] [PubMed] [Google Scholar]
- Weaver GA, Kurtz HJ, Mirocha CJ, Bates FY and Beherens JC, 1978. Acute toxicity of the mycotoxin diacetoxyscirpenol in swine. The Canadian Veterinary Journal, 19, 267–271. [PMC free article] [PubMed] [Google Scholar]
- Weaver GA, Kurtz HJ, Bates FY, Mirocha CJ, Beherens JC and Hagler WM, 1981. Diacetoxyscirpenol toxicity in pigs. Research in Veterinary Sciences, 31, 131–135. [PubMed] [Google Scholar]
- Wehner FC, Marasas WFO and Thiel PG, 1978. Lack of mutagenicity to Salmonella typhimurium of some Fusarium mycotoxins . Applied and Environmental Microbiology, 35, 659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2008. Harmonization Project Document No. 6. Guidance document on characterizing and communicating uncertainty in exposure assessment (Part 1), WHO/IPCS. 152 pp. Available online: http://www.who.int/ipcs/methods/harmonization/areas/uncertainty%20.pdf
- WHO , 2009. International Programme on Chemical Safety. Principles and methods for the risk assessment of chemicals in food. Environmental Health Criteria 240. Available online: http://www.who.int/foodsafety/publications/chemical-food/en/
- Wu S‐E and Marletta MA, 1988. Carboxylesterase isoenzyme specific deacylation of diacetoxyscirpenol (anguidine). Chemical Research and Toxicology, 1, 69–73. [DOI] [PubMed] [Google Scholar]
- Yang S, Li Y, Cao X, Hu D, Wang Z, Wang Y, Shen J and Zhang S, 2013. Metabolic pathways of T‐2 toxin in in vivo and in vitro systems of Wistar rats. Journal of Agricultural and Food Chemistry, 61, 9734–9743. [DOI] [PubMed] [Google Scholar]
- Yang S, De Boevre M, Zhang H, De Ruyck K, Sun F, Wang Z, Cao X, Shen J, De Saeger S and Zhang S, 2015. Unraveling the in vitro and in vivo metabolism of diacetoxyscirpenol in various animal species and human using ultrahigh‐performance liquid chromatography‐quadrupole/time‐of‐flight hybrid mass spectrometry. Analytical and Bioanalytical Chemistry, 407, 8571–8583. [DOI] [PubMed] [Google Scholar]
- Yap H‐Y, Murphy WK, DiStefano A, Blumenschein GR and Bodey GP, 1979. Phase II Study of Anguidine in Advanced Breast Cancer. Cancer Treatment Reports, 63, 789–791. [PubMed] [Google Scholar]
- Zhang J, Liu S, Zhang H, Li Y, Wu W and Zhang H, 2017a. Gut satiety hormones cholecystokinin and glucagon‐like Peptide‐17‐36 amide mediate anorexia induction by trichothecenes T‐2 toxin, HT‐2 toxin, diacetoxyscirpenol and neosolaniol. Toxicology and Applied Pharmacology, 15, 49–55. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jia H, Wang Q, Zhang Y, Wu W and Zhang H, 2017b. Role of Peptide YY3‐36 and Glucose‐Dependent Insulinotropic Polypeptide in Anorexia Induction by Trichothecences T‐2 Toxin, HT‐2 Toxin, Diacetoxyscirpenol, and Neosolaniol. Toxicological Sciences, 159, 203–210. [DOI] [PubMed] [Google Scholar]
- Zöllner P and Mayer‐Helm B, 2006. Trace mycotoxin analysis in complex biological and food matrices by liquid chromatography‐atmospheric pressure ionization mass spectrometry. J. Chromat. A, 1136, 123–169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical methods used to estimate the intake–response of serum 25(OH)D concentration on daily supplemental intake of vitamin D and to derive the percentage of infants exceeding a serum 25(OH)D concentration


