Abstract
The EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) provides a scientific opinion re‐evaluating the safety of propane‐1,2‐diol (E 1520) when used as a food additive. In 1996, the Scientific Committee on Food (SCF) established an acceptable daily intake (ADI) of 25 mg/kg body weight (bw) per day for propane‐1,2‐diol. Propane‐1,2‐diol is readily absorbed from the gastrointestinal and is expected to be widely distributed to organs and tissues. The major route of metabolism is oxidation to lactic acid and pyruvic acid. At high concentrations, free propane‐1,2‐diol is excreted in the urine. No treatment‐related effects were observed in subchronic toxicity studies. The available data did not raise concern with respect to genotoxicity. Haematological changes suggestive of an increased red blood cell destruction with a compensatory increased rate of haematopoiesis were observed at the highest dose level (5,000 mg/kg bw per day) in a 2‐year study in dogs. No adverse effects were reported in a 2‐year chronic study in rats with propane‐1,2‐diol (up to 2,500 mg/kg bw per day). The SCF used this study to derive the ADI. No adverse effects were observed in the available reproductive and developmental toxicity studies. Propane‐1,2‐diol (E 1520) is authorised according to Annex III in some food additives, food flavourings, enzymes and nutrients and it is then carried over to the final food. Dietary exposure to E 1520 was assessed based on the use levels and analytical data. The Panel considered that for the food categories for which information was available, the exposure was likely to be overestimated. Considering the toxicity database, the Panel concluded that there was no reason to revise the current ADI of 25 mg/kg bw per day. The Panel also concluded that the mean and the high exposure levels (P95) of the brand‐loyal refined exposure scenario did not exceed the ADI in any of the population groups from the use of propane‐1,2‐diol (E 1520) at the reported use levels and analytical results.
Keywords: propane‐1,2‐diol; propylene glycol; E 1520; CAS No 57‐55‐6; food additive
Summary
Propane‐1,2‐diol (E 1520) is authorised as a food additive in the European Union (EU) according to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/2012.
In 1996, the Scientific Committee on Food (SCF) established an acceptable daily intake (ADI) of 25 mg/kg body weight (bw) per day for propane‐1,2‐diol.
In 1973, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) allocated an ADI of 0–25 mg/kg bw for propane‐1,2‐diol and in 2001 evaluated propane‐1,2‐diol as a food flavouring; however, the Committee did not finalise the evaluation as it wanted confirmation that the substance is actually used as food flavouring. The Panel noted that propane‐1,2‐diol is not authorised as a flavouring substance in the EU according to Regulation 1334/2008.
Propane‐1,2‐diol is readily absorbed from the gastrointestinal tract in experimental animals and in humans and is expected to be widely distributed to organs and tissues. The major route of metabolism is oxidation to lactic acid and pyruvic acid. An alternative route of metabolism of propane‐1,2‐diol to lactic acid is via phosphorylated glycol. At high concentrations, free propane‐1,2‐diol is excreted in the urine as the elimination of propane‐1,2‐diol from the body is saturated at dose levels higher than 20,000 mg/day in humans. The renal clearance in young infants up to the age of approximately 3 months appeared to be much lower than in adults.
The acute oral toxicity of propane‐1,2‐diol was low.
No treatment‐related effects were observed in subchronic toxicity studies in which 1,000 mg propane‐1,2‐diol/kg bw per day was administered by gavage to mice, rats, dogs and monkeys for 92–97 days.
The Panel considered that the available data did not raise concern with respect to genotoxicity of propane‐1,2‐diol.
Except for haematological changes suggestive of an increased red blood cell destruction with a compensatory increased rate of haematopoiesis at the highest dose level (5,000 mg/kg bw per day), no adverse effects were observed in a 2‐year study in dogs administered propane‐1,2‐diol in the diet (2,000 or 5,000 mg/kg bw per day). No adverse effects, including neoplastic findings, were reported in a chronic study in rats administered propane‐1,2‐diol in the diet (up to 2,500 mg/kg bw per day) for 2 years. The SCF and JECFA used the study in rats to derive an ADI of 25 mg/kg bw per day using an uncertainty factor of 100.
No adverse effects were observed in the available reproductive and developmental toxicity studies.
Propane‐1,2‐diol (E 1520) is authorised according to Annex III in some food additives, food flavourings, enzymes and nutrients and it is then carried over to the final food. Dietary exposure to the food additive was assessed based on data received by both industry (use levels) and Member States (analytical data). In the refined exposure assessment scenario, 20 food categories were taken into account. Because propane‐1,2‐diol (E 1520) is authorised only according to Annex III, its labelling is not mandatory, therefore, the Panel considered that the list of uses of this food additive from the Mintel's Global New Products Database (GNPD) was likely not exhaustive.
The exposure assessment was hampered by several uncertainties; overall the Panel considered that for the food categories for which information was available, the exposure was likely to be overestimated. The Panel considered that the brand‐loyal scenario covering the general population was the most appropriate scenario for risk characterisation of E 1520 assuming that the population is likely to be exposed long‐term to the food additive present at the maximum reported use in foods belonging to the food category ‘Flavoured drinks’ and at the mean reported use/analytical data in foods belonging to the other food categories. The highest high level of exposure (P95) to propane‐1,2‐diol (E 1520) according to this scenario was estimated at 23.3 mg/kg bw per day in children.
The Panel also noted that propane‐1,2‐diol could be formed from metabolism of other food additives (e.g. E 405, E 477). In this opinion, a combined exposure assessment was not performed.
Considering the overall toxicity database, the Panel concluded that there was no reason to revise the current ADI of 25 mg/kg bw per day for propane‐1,2‐diol (E 1520).
The Panel also concluded that the mean and the high exposure levels (P95) of the brand‐loyal refined exposure scenario did not exceed the ADI in any of the population groups from the use of propane‐1,2‐diol (E 1520) as a food additive at the reported use levels and analytical results.
The Panel recommended that:
the European Commission considers lowering the current maximum limits for lead in the EU specification for propane‐1,2‐diol (E 1520) in order to ensure that propane‐1,2‐diol (E 1520) as food additives will not be a significant source of exposure to this toxic element in food.
the European Commission considers inclusion of maximum limits for propylene oxide, mono‐ and di‐ethylene glycol and propylene carbonate in the EU specifications for propane‐1,2‐diol (E 1520).
the need for a combined exposure assessment to propane‐1,2‐diol from E 1520 and from other food additives from which propane‐1,2‐diol is formed through metabolism should be considered.
1. Introduction
The present opinion document deals with the re‐evaluation of propane‐1,2‐diol (E 1520) when used as a food additive.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
Regulation (EC) No 1333/20081 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union. In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the European Union before 20 January 2009 has been set up under the Regulation (EU) No 257/20102. This Regulation also foresees that food additives are re‐evaluated whenever necessary in light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU3 of 2001. The report ‘Food additives in Europe 20004’ submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010 the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference
The Commission asks EFSA to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 march 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.2. Information on existing authorisations and evaluations
Propane‐1,2‐diol (E 1520) is an authorised food additive in the EU according to Annex III of Regulation (EC) No 1333/2008 and specific purity criteria have been defined in the Commission Regulation (EU) No 231/20125.
The SCF evaluated several times propane‐1,2‐diol as a food additive (SCF, 1978, 1996, 1997), as a solvent in food (SCF, 1981, 1996, 1997), as monomers and additives for food contact plastics (SCF, 1999). The Committee in its evaluation in 1981 concluded that there were sufficient data available, including long‐term studies in rats and dogs, to establish an acceptable daily intake (ADI), and they agreed with the ADI of 0–25 mg/kg body weight (bw) established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA, 1974). In its evaluation in 1993 of propane‐1,2‐diol as a food additive, the SCF noted an uncertainty with regard to potential mutagenic effects at the germ cell level, which led the Committee to make the ADI temporary (but keeping the figure of 25 mg/kg bw per day) (SCF, 1996). In 1996, the SCF evaluated new genotoxicity studies and although they did not entirely correspond to the SCF's request the Committee concluded nevertheless that the ADI of 25 mg/kg bw per day could be reassigned to propane‐1,2‐diol (SCF, 1997).
In 1973, JECFA allocated an ADI of 0–25 mg/kg bw based on the level in rat and dog causing no toxicological effect of 2,500 mg/kg bw and using an uncertainty factor of 100 (JECFA, 1974). In 2001, JECFA evaluated propane‐1,2‐diol as a food flavouring; however, the Committee did not finalise the evaluation as it wanted confirmation that the substance is actually used as food flavouring (JECFA, 2002a,b). The Panel noted that propane‐1,2‐diol is not authorised as a flavouring substance in the EU according to Regulation 1334/2008.
Propane‐1,2‐diol (Ref. No 23740 and 81840) is included in the Union list of authorised substances that may be intentionally used in the manufacture of plastic layers in plastic materials and articles (Annex I to Commission Regulation (EU) No 10/20116).
The Scientific Committee for Animal Nutrition (SCAN) expressed a provisional opinion on the use of propane‐1,2‐diol as a preservative in animal feed on the health of cats because of claims that when the additive is fed to cats over a prolonged period of time, erythrocytes may develop oxidative damage to the haemoglobin, Heinz body formation and, possibly, reduced resistance to oxidative stress. The Committee concluded provisionally that there was no immediate need to suspend the use of the substance in feed for cats (SCAN, 1991).
The EFSA Panel on Contaminants in the Food Chain (CONTAM) evaluated a list of substances, including propylene glycol, in the Annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and concluded that those would not be of health concern as previous cargoes (EFSA CONTAM Panel, 2011).
The EFSA Panel on Biological Hazards (BIOHAZ) and the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) evaluated the safety and efficacy of a product, containing propane‐1,2‐diol as a carrier, used for the removal of microbial surface contamination of raw poultry products. Both Panels concluded that the potential exposure to propane‐1,2‐diol was several orders of magnitude below the ADI and thus not of toxicological concern (EFSA BIOHAZ Panel and CEF Panel, 2012).
A working group established by the Nordic Council of Ministers concluded that there was no need to re‐evaluate propane‐1,2‐diol (E 1520), but a survey to estimate the likely exposure to the substance was recommended. This recommendation was based on the fact that Danish control examinations revealed levels of up to 28 g/kg in cakes (TemaNord, 2002).
The European Agency for the Evaluation of Medicinal Products (EMA) has evaluated the use of propane‐1,2‐diol as a pharmacological active substance for animal uses and found it acceptable for ‘all food producing species with no special conditions of use’ (EMA, 1996).
The Committee for Medicinal Products for Human Use (CHMP) considered the procedure under Article 5(3) of Regulation (EC) No 726/2004 on the excipient, propylene glycol in medicines for children as per questions posed (EMA, 2014a). In the final document, EMA concluded ‘Nevertheless, clinical data showed that in children from the age of 5 years and adult patients, up to 500 mg/kg/day of propylene glycol could generally be considered safe. In the absence of compelling data this safety threshold is decreased to 50 mg/kg/day in children less than 5 years old, and even to 1 mg/kg/day in pre‐term and term neonates due to known immaturity of both metabolic and renal clearances of propylene glycol in these populations’ (EMA, 2014b).
Propane‐1,2‐diol is permitted in cosmetic products (European Commission database – CosIng7).
2. Data and methodologies
2.1. Data
The Panel was not provided with a newly submitted dossier. EFSA launched public calls for data,8 , 9 to collect relevant information from interested parties.
The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up to February 2018. Attempts were made at retrieving relevant original study reports on which previous evaluations or reviews were based, however not always these were available to the Panel.
Food consumption data from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database10) was used to estimate the dietary exposure to propane‐1,2‐diol (E 1520).
The Mintel's Global New Products Database (GNPD) was used to verify the use of propane‐1,2‐diol (E 1520) in food products. Mintel's GNPD is an online database that contains the compulsory ingredient information present on the label of food products.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing Guidances from the EFSA Scientific Committee.
The ANS Panel assessed the safety of propane‐1,2‐diol (E 1520) as a food additive in line with the principles laid down in Regulation (EU) 257/2010 and the relevant guidance documents: Guidance on submission for food additive evaluations by the SCF (2001).
When the test substance was administered in the feed or in the drinking water, but doses were not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012) for studies in rodents or, in the case of other animal species, by JECFA (2000). In these cases, the daily intake is expressed as ‘equivalent’.
For this evaluation, the Panel used 1.036 g/cm3 (INRS, 2010) as a density for propane‐1,2‐diol.
Dietary exposure to propane‐1,2‐diol (E 1520) from its use as a food additive was estimated combining food consumption data available within the EFSA Comprehensive Database with the reported use levels and analytical data submitted to EFSA following a call for data. Different scenarios were used to calculate exposure (see Section 3.3.1). Uncertainties on the exposure assessment were identified and discussed.
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
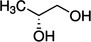
Propane‐1,2‐diol (E 1520) has as chemical formula C3H8O2 and a molecular weight 76.10 g/mol. The substance exists under different enantiomeric forms. Table 1 shows the structural formulae and chemical registry numbers of these different forms.
Table 1.
Structural formulae of enantiomers and registry numbers of propane‐1,2‐diol
| Name | Structural formula | CAS registry numbera | EC (EINECS) numbera |
|---|---|---|---|
| (R)‐(−)‐1,2‐Propanediol |
|
4254‐14‐2 | 610‐038‐5 |
| (S)‐(+)‐1,2‐Propanediol |
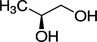
|
4254‐15‐3 | 610‐030‐0 |
| (R,S)‐1,2‐propanediol |
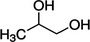
|
57‐55‐6 | 200‐338‐0 |
CAS: Chemical Abstracts Service; EINECS: European Inventory of Existing Chemical Substances.
EC inventory available online: https://echa.europa.eu/information-on-chemicals/ec-inventory
According to Commission Regulation (EU) No 231/2012, the propane‐1,2‐diol (E 1520) has the EINECS Number 200‐338‐0. According to the EC inventory, the EINECS number indicated in the EU specifications for E 1520 refers to CAS 57‐55‐6 that corresponds to (R,S)‐1,2‐propanediol (Scifinder,11 online). The Panel noted that the authorisation of the racemic ((R,S)‐1,2‐propanediol) is not clearly stated in the EU specifications.
Synonyms: propylene glycol; α‐propylene glycol; 1,2‐dihydroxypropane; methyl ethylene glycol.
According to Commission Regulation (EU) No 231/2012, propane‐1,2‐diol (E 1520) is described as a clear, colourless, hygroscopic, viscous liquid. It is soluble in water, ethanol and acetone.
Propane‐1,2‐diol has a melting point of −60°C, a boiling point of 186–190°C and the partition coefficient (octanol/water) (log Pow) is −0.912 (CEFIC, 2012 (Documentation provided to EFSA n. 2)).
3.1.2. Specifications
The specifications for propane‐1,2‐diol (E 1520) as defined in the Commission Regulation (EU) No 231/2012 and by JECFA (2006) are listed in Table 2.
Table 2.
Specifications for propane‐1,2‐diol (E 1520) according to Commission Regulation (EU) No 231/2012 and JECFA (2006)
| Commission Regulation (EU) No 231/2012 | JECFA (2006) | |
|---|---|---|
| Assay | Content not less than 99.5% on the anhydrous basis | Not less than 99.5% on the anhydrous basis |
| Description | Clear, colourless, hygroscopic, viscous liquid | Clear, colourless, hygroscopic, viscous liquid |
| Identification | ||
| Solubility | Soluble in water, ethanol and acetone | Soluble in water, ethanol and acetone |
| Specific gravity (20°C/20°C) | 1.035–1.040 | 1.035–1.040 |
| Refractive index | [n]D 20: 1.431–1.433 | – |
| Infrared absorption | – | The infrared spectrum of a potassium bromide dispersion of the sample corresponds with the infrared spectrum below [in the specifications] |
| Purity | ||
| Distillation | 99.5% of the product distils between 185°C and 189°C. The remaining 0.5% consists mainly of dimers and traces of trimers from propane‐1,2‐diol | 99% v/v distils between 185‐ and 189°C |
| Water | Not more than 1.0% (Karl Fischer method) | Not more than 1.0% (Karl Fischer) |
| Sulphated ash | Not more than 0.07% | Not more than 0.07% |
| Free acid | – | Add 3–6 drops of phenol red TS to 50 mL water, then add 0.1N sodium hydroxide until solution remains red for 30 s. To this solution, add about 50 g of the sample accurately weighed. Titrate with 0.01N sodium hydroxide until the original red colour returns and remains for 15 s. Not more than 1.67 mL of 0.01N sodium hydroxide are consumed by a sample of 50.0 g |
| Lead | Not more than 2 mg/kg | Not more than 2 mg/kg |
The Panel noted that, according to the EU specifications for propane‐1,2‐diol (E 1520), lead, as an impurity, is accepted up to a concentration of 2 mg/kg. Contamination at this level could have a significant impact on the exposure already close to the health‐based guidance value or benchmark doses (lower confidence limits) established by EFSA (EFSA CONTAM Panel, 2010, 2012).
According to the literature (Fowles et al., 2013) and to the information on the manufacturing process (see Section 3.1.3), some by products (mono‐ and di‐ethylene glycol) might be formed next to residual propylene oxide used as a starting material. The Panel noted that there are not maximum limits for propylene oxide and mono‐ and di‐ethylene glycol in the EU specifications for propane‐1,2‐diol (E 1520).
According to information from the literature (Holtbruegge et al. (2015), see Section 3.1.3), propane‐1,2‐diol, which is intended to be used as a food additive, can be manufactured by using as a raw material propylene carbonate, which may not be completely removed from the final product, propane‐1,2‐diol. The Panel noted that no maximum limits for propylene carbonate are set in EU specifications for E 1520.
3.1.3. Manufacturing process
According to industry, propane‐1,2‐diol is made from propylene oxide by reaction with water. The resulting mixture consisting of mono‐, di‐ and tri‐glycols is distilled to obtain purified propane‐1,2‐diol. The product is in accordance with the requirements for the European Pharmacopoeia for use as a pharmaceutical excipient, and for use in food, feed and personal care applications. The production process does not utilise catalysts or solvents and the final product does not contain any additive (e.g. stabilisers, antioxidants) (CEFIC, 2012 (Documentation provided to EFSA n. 2)).
Holtbruegge et al. (2015) proposed an optimisation of the production method of high purity propane‐1,2‐diol, patented by Buchanan et al. (2002), which is intended for various uses, including the use as a food additive. After a transesterification of propylene carbonate with methanol, where sodium methoxide is used as a catalyst, a membrane‐assisted reactive separation process is proposed. According to this method, propane‐1,2‐diol is collected together with non‐reacted propylene carbonate and subsequently the two solvents are separated via distillation.
In the literature other methods for manufacturing of propane‐1,2‐diol are reported or proposed, based on
a liquid‐phase high pressure reaction (600 atmospheres) of synthetic gas in the presence of a rhodium cluster complex (ASTDR, 1997),
an hydrogenolysis of glycerol by using various catalysts like: copper, nickel or noble metals (Bozga et al., 2011), highly active copper/magnesia (magnesium oxide) catalysts (Pudi et al., 2015) or a copper/zinc/magnesia catalyst (Mondal et al., 2017), chromium oxide, or ruthenium or platinum/tungsten catalysts (Kutz et al., 2017),
via fermentation of deoxy sugars (i.e. rhamnose) or other substrates by using Clostridia, Enterobacteriaceae or yeasts (Tanaka et al., 2017; Kutz et al. (2017)).
The Panel noted that none of these methods is suitable for the production of the food additive E 1520, without further purification steps, as they yield propane‐1,2‐diol having a lower purity than required in the EU specifications for E 1520.
3.1.4. Methods of analysis in food
An analytical enzymatic method for the determination of propane‐1,2‐diol in commercial food is available (Hamano et al., 1984). The method is based on the extraction of propane‐1,2‐diol from the food with deionised water followed by its oxidation by propylene glycol dehydrogenase from Microcyclus eburneus. During this step, oxidised nicotinamide adenine dinucleotide (NAD+) is reduced to NADH and the absorbance of the solution is measured at 340 nm. Recoveries ranged from 85% up to 99%. The limit of detection (LOD) was equal to 2 mg/kg.
A gas chromatographic method with flame ionisation detection method is also available for the determination of propane‐1,2‐diol in beer, after extraction with ethyl acetate. The recovery rate of the method was reported to be 88% and the LOD 0.73 mg/L (Williamson and Iverson, 1993).
Tandem mass spectrometry method has been used for identification of propane‐1,2‐diol in fish. The sample was homogenised with methanol. No performance criteria of the method were given (Matusik et al., 1993).
A titrimetric analytical method for the determination of propane‐1,2‐diol in vanilla extract was also described (AOAC, 2000). This method is only applicable when the vanilla extract contains high amounts of the food additive.
Sagadykova et al. (2017) described a method for the determination of propane‐1,2‐diol in wines using gas chromatography–mass spectrometry (GC/MS). According to this method, a solid‐phase microextraction of the semi‐volatile constituents of wine prior to the final injection to the GC–MS apparatus is applied. A standard addition is used to skip the estimation of recovery rates and the LOD is 2 mg/L. A similar approach for the determination of propane‐1,2‐diol in cheese was proposed by Badertscher et al. (2017) where an esterification with phenyl boronic acid preceded the injection into GC–MS. The LOD in this case was reported to be below 1 mg/kg.
A high‐performance liquid chromatography (HPLC) method for the determination of propane‐1,2‐diol in spirits was proposed by Tanaka et al. (2017). In this case, the Cu(II) ions of the mobile phase enable the determination of the substance via an UV‐detector (at 250 nm) with a LOD of 0.1 mL/L.
3.1.5. Stability of the substance, and reaction and fate in food
According to the information provided by industry, propane‐1,2‐diol is hygroscopic and sensitive to sunlight, air, oxidising agents, acids, bases and high temperatures. Partial oxidation in the presence of oxygen may lead to the formation of aldehydes, ketones, acids and dioxolanes. When stored outside in transparent plastic containers, the rate of degradation increases with temperature, the presence of metals and/or exposure to sun (UV) light. It has been shown that no aldehydes, ketones, acids and dioxolanes were formed from propane‐1,2‐diol in a stability test over 2 years when kept out of sunlight and kept at a temperature not exceeding 40°C (CEFIC, 2012 (Documentation provided to EFSA n. 2)). Hence, according to industry, it is generally recommended to store the product in approved, closed containers at temperatures not exceeding 40°C.
According to industry (CEFIC, 2012 (Documentation provided to EFSA n. 2)), the stability of propane‐1,2‐diol as such is representative for the stability of the substance in food because there is no interaction of the substance with food components.
The Panel noted that, as propane‐1,2‐diol may be used in a variety of food, which can also be heat‐treated and/or stored in daylight, it is expected that the substance partially degrades.
3.2. Authorised uses and use levels
Propane‐1,2‐diol (E 1520) is not authorised to be added directly to food according to Annex II to Regulation (EC) No 1333/2008 on food additives. Maximum levels of propane‐1,2‐diol (E 1520) in final foods (via carry‐over from food improving agents or nutrients) have been defined only in Annex III to Regulation (EC) No 1333/2008 on food additives, as amended.
According to Annex III, Part 1 of Regulation (EC) No 1333/2008, propane‐1,2‐diol (E 1520) is authorised as a carrier in food additives (colours, emulsifiers and anti‐oxidants), with a maximum level of 1,000 mg/kg in final food. It is also authorised from all sources in foodstuffs up to 3,000 mg/kg (individually or in combination with triethyl citrate (E 1505), glyceryl diacetate (E 1517) and glyceryl triacetate (E 1518)); in the case of beverages, with the exception of cream liqueurs, the maximum level of E 1520 is 1,000 mg/L from all sources.
According to Annex III, Part 3, propane‐1,2‐diol (E 1520) is authorised as a food additive, only as a carrier, in food enzymes, at the maximum level of 500 g/kg in enzyme preparation and with a maximum level from all sources in foodstuffs of 3,000 mg/kg (individually or in combination with E 1505, E 1517 and E 1518). In the case of beverages, with the exception of cream liqueurs, the maximum level of E 1520 is 1,000 mg/L from all sources.
In addition, according to Annex III, Part 4, propane‐1,2‐diol (E 1520) is authorised in all flavourings individually or in combination with E 1505, E 1517 and E 1518 at the maximum level of 3,000 mg/kg from all sources in foodstuffs as consumed, or as reconstituted according to the instructions of the manufacturers. In the case of beverages, with the exception of cream liqueurs, the maximum level of E 1520 is 1,000 mg/L from all sources.
In addition, according to Annex III, Part 5, Section A of Regulation (EC) No 1333/2008, propane‐1,2‐diol (E 1520) is authorised as a carrier only, in all nutrients (except nutrients intended to be used in foodstuffs for infants and young children listed in point 13.1 of Part E of Annex II) at maximum level of 1,000 mg/kg in final food and with a maximum level for E 1518 and E 1520 from all sources in foodstuffs of 3,000 mg/kg (individually or in combination with E 1505 and E 1517). In the case of beverages, with the exception of cream liqueurs, the maximum level of E 1520 is 1,000 mg/L from all sources.
3.3. Exposure data
3.3.1. Reported use levels or data on analytical levels of propane‐1,2‐diol
Most food additives in the EU are authorised at a specific maximum permitted level (MPL). However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued a public call12 for occurrence data (usage level and/or concentration data) on propane‐1,2‐diol (E 1520). In response to this call, occurrence data on propane‐1,2‐diol (E 1520) were submitted to EFSA by both industry and Member States.
Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with data on use levels (n = 548) of propane‐1,2‐diol (E 1520) in foods for 22 food categories. The provided use levels were submitted by FoodDrinkEurope (FDE), the International Chewing Gum Association (ICGA), Specialised Nutrition Europe (SNE), KRÜGER GmbH & Co and Spanish Association of Postharvest Services and Processes (AGRUPOST). The majority of the submitted data (n = 521) were for the food category ‘Edible ices’ (FC 03).
The Panel noted that two use levels referred to niche products. These products belonged to the food categories ‘Decorations, coatings and fillings’ (FC 05.4) and ‘Dietary foods for special medical purposes’ (FC 13.2). These use level were not considered in the exposure assessment. For FC 05.4, a use level referring to a widely used product was available, whereas FC 13.2 was not included in the exposure assessment (see Section 3.3.3).
The Panel considered that the reported use levels refer to the level of propane‐1,2‐diol (E 1520) in final food from all sources in foodstuffs (as a carrier from food additives, enzymes, flavouring and all nutrients).
Appendix A provides data on the use levels of propane‐1,2‐diol (E 1520) in foods as reported by industry.
Summarised data on analytical results in food submitted by Member States
In total, 162 analytical results were reported to EFSA by four countries: the Czech Republic (n = 7), Germany (n = 72), Denmark (n = 73) and Ireland (n = 10). These data were mainly for flavourings or essence and pastries and cakes covering four food categories. Foods were sampled between 2000 and 2016, and the majority of them (98%) were analysed the year that they were collected.
Around half of the analytical results on propane‐1,2‐diol (E 1520) were left‐censored: either not quantified (< limit of quantification (LOQ)) in 62 samples, or not detected (< LOD) in 25 samples.
Complete information on the methods of analysis (e.g. validation) was not made available to EFSA, but all samples were derived from accredited laboratories.
The Panel noted that eight analytical results were obtained via suspect sampling and were therefore excluded from the exposure assessment.
In order to include recent analytical data in the exposure assessment, only samples sampled in the last 10 years were included. Samples collected before 2008 (n = 39) were therefore also excluded from further analysis.
Overall, 115 analytical results were reported for propane‐1,2‐diol (E 1520) in four food categories and in flavourings.
Appendix B shows the analytical results of propane‐1,2‐diol (E 1520) in foods as reported by Member States.
The Panel noted that the analytical data reported by Member States showed the presence of propane‐1,2‐diol in three food categories for which no uses have been reported by the food manufacturers out of the four food categories for which data were reported. This may relate to the fact that E 1520 is not directly added to the food by the manufacturers, but carried over via uses of food ingredients, such as some food additives, food flavourings, enzymes and nutrients.
3.3.2. Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database that monitors new introductions of packaged goods in the market worldwide. It contains information of over 2.5 million food and beverage products of which more than 900,000 are or have been available on the European food market. The Mintel's GNPD started covering EU's food markets in 1996; currently, it contains data from 20 out of its 28 Member‐States and Norway.13
For the purpose of this Scientific Opinion, the Mintel's GNPD14 was used for checking the labelling of food and beverage products and food supplements for propane‐1,2‐diol (E 1520) within the EU's food market as the database contains the compulsory ingredient information on the label.
As mentioned before, propane‐1,2‐diol (E 1520) is authorised according to Annex III in some food additives, food flavourings, enzymes and nutrients and it is then carried over to the final food. According to legislation, the labelling of food additives used as such is not mandatory. Despite this, propane‐1,2‐diol (E 1520) was labelled on 278 products between January 2013 and January 2018 (456 products on any date) according to the Mintel's GNPD.
The products labelled with the food additive were mainly cakes, pastries and sweet goods, and baking ingredients and mixes.
Appendix C lists the percentage of the food products labelled with propane‐1,2‐diol (E 1520) out of the total number of food products per food sub‐category according to the Mintel's GNPD food classification. The percentages ranged from less than 0.1% in many food sub‐categories to 1.2% in the Mintel's GNPD food subcategory ‘Frozen desserts’. The average percentage of foods labelled to contain propane‐1,2‐diol (E 1520) was 0.1%. However, since the labelling of propane‐1,2‐diol (E 1520) is not mandatory, the information about the use of this food additive from the Mintel's GNPD is very likely not exhaustive. However, the data received from industry seem to be in agreement with the foods labelled with propane‐1,2‐diol (E 1520) according to the Mintel's GNPD.
3.3.3. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). Consumption surveys added in the Comprehensive database in 2015 were also taken into account in this assessment.15
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting, and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database includes the currently best available food consumption data across Europe.
Food consumption data from the following population groups were used for the exposure assessment: infants, toddlers, children, adolescents, adults, and the elderly. For the present assessment, food consumption data were available from 33 dietary surveys carried out in 19 European countries (Table 3).
Table 3.
Population groups considered for the exposure estimates of propane‐1,2‐diol (E 1520)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlersa | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrenb | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlyb | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Netherlands, Sweden, UK |
The term ‘toddlers’ in the EFSA Comprehensive Database corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013.
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D to perform exposure estimates. In practice, the FoodEx food codes were matched to the FCS food categories.
Food categories considered for the exposure assessment of propane‐1,2‐diol (E 1520)
As described in Section 3.2, propane‐1,2‐diol (E 1520) is not authorised to be added directly into foods (no authorisation according to Annex II to Regulation No 1333/2008). Propane‐1,2‐diol (E 1520) is only authorised in some food additives (colours, emulsifiers and antioxidants) or enzymes, flavourings and nutrients. As a result of this authorisation, the majority of foods and beverages, within a diet could contain propane‐1,2‐diol (E 1520) via carry over. However, the available data received suggested that only a few products out of the whole number of products on the European market contained propane‐1,2‐diol (E 1520).
A regulatory maximum level exposure assessment scenario could be calculated by attributing to all foods and cream liqueurs the MPL of 3,000 mg/kg and to beverages, except cream liqueurs, the MPL of 1,000 mg/kg. However, this scenario would be very uncertain and would result in an unrealistically high estimate of the exposure. Thus, the Panel considered that a regulatory maximum level exposure assessment scenario would not provide useful information about the exposure to propane‐1,2‐diol (E 1520) and decided to perform only a refined exposure assessment scenario.
In the refined exposure assessment scenario, only food categories for which data were made available to EFSA (either from industry or from Member States), and for which consumption data were available were included in the exposure assessment. The food categories, which were taken into account, are described below (in ascending order of the FCS codes):
01.7.1 Unripened cheese excluding products falling in category 16,
01.8 Dairy analogues, including beverages whiteners,
03 Edible ices,
04.1 Unprocessed fruits and vegetables,16
04.2.6 Processed potato products,
05.1 Cocoa and chocolate products as covered by Directive 2000/36/EC,
05.2 Other confectionary including breath freshening microsweets,
05.3 Chewing gum,
05.4 Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4,
07.2 Fine bakery wares,
12.2.2 Seasonings and condiments,
12.5 Soups and broths,
12.6 Sauces,
14.1.4 Flavoured drinks,
14.1.5.2 Coffee, coffee and chicory extracts, tea, herbal‐ and fruit‐infusions; coffee substitutes, coffee mixes and mixes for ‘hot beverages’, Other than coffee, coffee extracts
14.2.2 Wine and other products defined by Reg (EC) No 1234/2007, and alcohol‐free counterparts,
14.2.6 Spirits drinks as defined in Reg (EC) No 110/2008, except cream liqueurs,
14.2.6 Spirits drinks as defined in Reg (EC) No 110/2008, only cream liqueurs,
15.1 Potato‐,cereal‐, flour‐ or starch‐based snacks,
16 Desserts,
17.1 Food supplements supplied in a solid form including capsules and tablets and similar forms, excluding chewable forms,
17.3 Food supplements supplied in a syrup‐type or chewable form.
For three food categories, use levels were provided by industry but could not be taken into account in the exposure assessment: FC 01.7.6 Cheese products, FC 13.2 Dietary foods for special medical purposes, and FC 18 processed foods not covered by categories 1 to 17. For FC 01.7.6, no consumption data were available in the EFSA Comprehensive database. The eating occasions belonging to FCs 13.2 (and 13.3) and 18 have been reclassified under food categories in accordance to their main ingredient, as food items belonging to these food categories may be very diverse and, in addition, for FCs 13.2 and 13.3, there is very limited information on their consumption. The reported use levels of E 1520 for FCs 13.2 and 18 (Appendix A) were not considered in the exposure assessment.
Additionally, industry provided use levels for FCs 17.1 and 17.3: food supplements in solid, syrup‐type or chewable form. In the EFSA Comprehensive database, no information is provided on the form of food supplements ingested. In the exposure assessment, all food supplements (including FC 17.2, food supplements in liquid form) were therefore considered together.
The Panel noted that analytical data were provided on both artificial and natural flavourings (Appendix B). To take these data into account in an exposure assessment, information on the levels of these flavourings in each flavoured food as well as a list of flavoured foods would be needed. As this information was not available, conservative assumptions would be needed to include these flavourings in the exposure assessment, which would result in unrealistically high estimates of exposure. The Panel decided therefore not to use these data in the current exposure assessment of propane‐1,2‐diol (E 1520). However, the Panel considered that the reported use levels and the available analytical data on propane‐1,2‐diol (E 1520), included its use in flavouring preparations.
For the refined scenario, 20 food categories were taken into account. The exposure to propane‐1,2‐diol (E 1520) via the ingestion of food supplements was assessed via a separate exposure scenario and included the same food categories as the refined scenario as well as the three food supplement food categories (Appendix B).
3.4. Exposure estimates
3.4.1. Exposure to propane‐1,2‐diol (E 1520) from its use as a food additive
The Panel estimated the chronic dietary exposure to propane‐1,2‐diol (E 1520) for the following population groups: infants, toddlers, children, adolescents, adults and the elderly. Dietary exposure to propane‐1,2‐diol (E 1520) was calculated by multiplying concentrations of propane‐1,2‐diol (E 1520) per food category (Appendix D) with their respective consumption amount per kilogram body weight for each individual in the Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only one day per subject were excluded as they were considered as not adequate to assess repeated exposure.
This was carried out for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 3). Based on these distributions, the mean and the 95th percentile of exposure were calculated per survey and per population group. The 95th percentile of exposure was only calculated for those population groups with a sufficiently large sample size (EFSA, 2011a). Therefore, in the present assessment, the 95th percentile of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain was not estimated.
As described above, a regulatory exposure scenario was not estimated in the current opinion. Thus, solely a refined exposure assessment scenario to propane‐1,2‐diol (E 1520) was carried out by the Panel based on reported use levels and analytical data. This scenario does not consider the consumption of food supplements. This source of exposure was covered in an additional scenario (food supplements consumers only scenario).
Refined exposure assessment scenario
The refined exposure assessment scenario of propane‐1,2‐diol (E 1520) was based on use levels reported by food industry and analytical results reported by Member States. This exposure scenario can consider only food categories for which these data were available to the Panel.
Appendix D summarises the concentration levels of propane‐1,2‐diol (E 1520) used in the refined exposure assessment scenario. Based on the available data set, the Panel calculated two refined exposure estimates based on two model populations:
-
The brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to propane‐1,2‐diol (E 1520) present at the maximum reported use/P95 of analytical level for one food category. This exposure estimate is calculated as follows:
-
1
– Combining food consumption with the maximum of the reported use levels or the 95th percentile of the analytical results, whichever was highest or available, for the main contributing food category at the individual level.
-
2
– Using the mean of the typical reported use levels or the mean of analytical results, whichever was highest or available, for the remaining food categories.
-
1
The non‐brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to propane‐1,2‐diol (E 1520) present at the mean reported use/analytical levels in food. This exposure estimate is calculated using the mean of the typical reported use levels or the mean of analytical results for all food categories.
In the two refined exposure assessment scenarios, the concentration levels considered by the Panel were extracted from the whole dataset (i.e. reported use levels and analytical results). To consider left‐censored analytical data (i.e. analytical results < LOD or < LOQ), the substitution method as recommended in the ‘Principles and Methods for the Risk Assessment of Chemicals in Food’ (WHO, 2009) and the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010) was used. In the present opinion, analytical data below LOD or LOQ were assigned half of LOD or LOQ, respectively (middle bound (MB)). Subsequently, per food category, the mean or median, whichever is highest, MB concentration was calculated.
Appendix D summarised the concentration levels of propane‐1,2‐diol (E 1520) used in the refined exposure scenarios.
Food supplement consumers only exposure assessment scenario
Use levels for propane‐1,2‐diol (E 1520) were reported by food industry for FC 17: Food supplements as defined in Directive 2002/46/EC excluding food supplements for infants and young children. As exposure via food supplements may deviate largely from that via food, and the number of food supplement consumers may be low depending on populations and surveys, an extra exposure scenario was calculated in order to reflect additional exposure to propane‐1,2‐diol (E 1520) from food supplements compared to exposure to propane‐1,2‐diol (E 1520) excluding these sources. This scenario was estimated assuming that consumers of food supplements were exposed to propane‐1,2‐diol (E 1520) present at the maximum reported use levels in food supplements on a daily basis. For the remaining food categories (20 categories), the mean/median of the typical reported use levels or analytical results was used.
As FC 17 does not consider food supplements for infants and toddlers as defined in the legislation, exposure to propane‐1,2‐diol (E 1520) from food supplements were not estimated for these two population groups.
This scenario included 23 food categories (Appendix D).
Dietary exposure to propane‐1,2‐diol (E 1520)
Table 4 summarises the estimated exposure to propane‐1,2‐diol (E 1520) from its use as a food additive in six population groups (Table 3), according to the different exposure scenarios. Detailed results per population group and survey are presented in Appendix E.
Table 4.
Summary of dietary exposure to propane‐1,2‐diol (E 1520) from its use as a food additive in the refined exposure scenario in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Refined estimated exposure assessment scenario | ||||||
| Brand‐loyal scenario | ||||||
| Mean | 0.2–3.1 | 1.0–11.6 | 3.4–10.5 | 1.8–7.0 | 1.0–3.6 | 0.8–3.0 |
| 95th percentile | 0.4–12.4 | 3.3–21.8 | 7.7–23.3 | 4.4–15.2 | 2.9–9.5 | 2.0–7.3 |
| Non‐brand‐loyal scenario | ||||||
| Mean | 0.1–1.5 | 0.4–8.4 | 1.8–7.8 | 1.3–5.3 | 0.7–2.4 | 0.5–1.2 |
| 95th percentile | 0.3–5.7 | 1.2–16.4 | 5.4–15.3 | 3.8–10.7 | 1.8–6.6 | 1.3–2.9 |
In the brand‐loyal scenario of the refined estimated exposure scenario, the mean exposure to propane‐1,2‐diol (E 1520) from its use as a food additive ranged from 0.2 mg/kg bw per day in infants to 11.6 mg/kg bw per day in toddlers. The high exposure to propane‐1,2‐diol (E 1520) ranged from 0.4 mg/kg bw per day in infants to 23.3 mg/kg bw per day in children.
In the non‐brand‐loyal scenario, mean exposure to propane‐1,2‐diol (E 1520) from its use as a food additive ranged from 0.1 mg/kg bw per day in infants to 8.4 mg/kg bw per day in toddlers. The 95th percentile of exposure to propane‐1,2‐diol (E 1520) ranged from 0.3 mg/kg bw per day in infants to 16.4 mg/kg bw per day in toddlers.
The main contributing food categories from the refined estimated exposure scenarios, both brand‐loyal and non‐brand‐loyal scenario were unprocessed fruit and vegetables and fine bakery wares for infants; for all the other population groups, main contributing food categories were fine bakery wares and flavoured drinks (Appendix F).
In the food supplements consumers only scenario, mean exposure to propane‐1,2‐diol (E 1520) from its use as a food additive ranged from 0.7 mg/kg bw per day for the elderly to 10.9 mg/kg bw per day for children. The 95th percentile of exposure to propane‐1,2‐diol (E 1520) ranged between 1.7 mg/kg bw per day for the elderly to 14.2 mg/kg bw per day for children.
Uncertainty analysis
Uncertainties in the exposure assessment of propane‐1,2‐diol (E 1520) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 5.
Table 5.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/– |
| Use of data from food consumption surveys covering only a few days to estimate high percentiles (95th) long‐term (chronic) exposure | + |
| Correspondence of reported use levels and analytical data to the food items in the EFSA Comprehensive Database: uncertainties to which types of food the levels refer | +/– |
| Uncertainty in possible national differences in use levels of food categories | +/– |
Concentration data:
|
+ +/– – |
Food categories selected for the exposure assessment:
|
– +/– |
Refined exposure assessment scenario:
|
+/– +/– |
MS: Member State; FC: food category; LOD: limit of detection; LOQ: limit of quantification.
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
Propane‐1,2‐diol (E 1520) is only authorised as carrier according to Annex III to Regulation No 1333/2008, and therefore has the potential to be present in the majority of the food and beverage products.
Use levels of the food additive were made available by industry for 22 food categories. Member States provided analytical data on four food categories and flavourings.
The Panel noted that according to the Mintel's GNPD, propane‐1,2‐diol (E 1520) was labelled in less than 300 food products in the last 5 years. For five food subcategories that included foods that were labelled with propane‐1,2‐diol (E 1520) according to the Mintel's classification (Appendix C), no data (reported use levels or analytical data) were provided to EFSA and these food categories were therefore not taken into account in the exposure assessment (Cider, Bread & Bread Products, Poultry Products, Spoonable Yogurt and Fish Products). However, the number of foods labelled with the food additive within these food sub‐categories was very small: maximally 5 (Appendix C). On the other hand, some foods for which data were provided were not found to be labelled in the Mintel's GNPD (cheese products). Furthermore, the percentage of foods per food sub‐category labelled to contain propane‐1,2‐diol (E 1520) was maximally 1.2% (Appendix C), as opposed to the assumption of 100% labelled of the foods belonging to an authorised food category in the exposure assessment. Given that propane‐1,2‐diol (E 1520) is authorised only according to Annex III and industry is not obliged to label the additive, information from the Mintel's GNPD is uncertain.
It should also be noted that the use of MB LOD/LOQ values (half of LOD or LOQ) in the exposure assessment (Section 3.3.1) may have resulted in either an overestimation, where propane‐1,2‐diol (E 1520) was not present, or underestimation, where the concentration was between the MB and LOQ/LOD value, but the analytical method was not able to detect or quantify it. The Panel however estimated that the possible under/overestimation would have been minor as only for three food categories, analytical data were used in the refined exposure assessment scenario.
The Panel considered that based on the data that could be used in the refined exposure assessment scenario (food categories for which information was available) the uncertainties identified would result in an overestimation of the exposure to propane‐1,2‐diol (E 1520) from its use as a food additive, according to Annex III, in European countries considered in the EFSA European database.
3.4.2. Exposure via other sources
Propane‐1,2‐diol is also used in cosmetic products. Propane‐1,2‐diol is used as humectant, solvent and preservative in a wide range of medicinal products (EMA, 2014a).
Lactobacillus parabuchneri, one of the most frequent representatives of lactobacilli present in cheese, and the closely related Lactobacillus buchneri were found to be produced propane‐1,2‐diol in cheese (Badertscher et al., 2017).
Quantification of exposure via these sources was not precisely known and therefore could not be taken into account in this opinion.
The Panel noted that propane‐1,2‐diol could be formed from metabolism of other food additives (e.g. E 405, E 477). In this opinion, a combined exposure assessment was not performed.
3.5. Biological and Toxicological data
Even though it was not specifically indicated, the Panel considered that the propane‐1,2‐diol tested in the biological and toxicological studies considered for this opinion would appear to be in the racemic form.
3.5.1. Absorption, distribution, metabolism and excretion
Animals
Rats
The absorption of propane‐1,2‐diol was investigated by injection of 10 mL/kg bw of a 10% propane‐1,2‐diol solution (1,031 mg/kg bw) into intestinal loops (15 cm long), the stomach and the colon, in several animal species (van Winkle, 1941a). A total number of 72 rats, 16 rabbits and 48 cats were used for the experiments. Absorption of propane‐1,2‐diol was reported to be identical in rabbits and cats showing a very rapid absorption within 30 min from jejunum followed by duodenum, colon, and stomach. Propane‐1,2‐diol was reported to be absorbed more rapidly in rats where a complete absorption from the jejunum occurred within 20–30 min, whereas in rabbits and cats the absorption from jejunum was 91% after 1 h. In cats, absorption of propane‐1,2‐diol from the jejunum was found to be dose‐dependent (after injection of a 5%, 10%, 50% or 100% solution). At the lowest dose (5%), 66.4% propane‐1,2‐diol was absorbed, whereas 49.4% was absorbed at the highest dose (100%). After 1 h, similar absorption levels for propane‐1,2‐diol (88.2–95.2%) were noted at all doses.
In the same publication, the renal excretion of propane‐1,2‐diol was described in rats. For this investigation, groups of five rats were given 5%, 10%, 15% or 20% propane‐1,2‐diol in the drinking water for 1 week (equivalent to 6,216, 12,432, 18,648 or 24,864 mg propane‐1,2‐diol/kg bw per day); thereafter, the propane‐1,2‐diol solutions were replaced by water (van Winkle, 1941a). After a normal fluid balance was obtained, the propane‐1,2‐diol treatment was repeated. Measurement of the propylene concentration in the urine showed that, regardless of the concentration of propane‐1,2‐diol in the drinking water, about one‐third of the amount ingested was excreted unchanged in the urine; the remaining two‐thirds were, according to the authors, metabolised in the body.
The kinetics of propane‐1,2‐diol elimination and metabolism in vivo have been investigated in rats (Morshed et al., 1988). Male Wistar rats (6 animals/group) were given a single oral dose of propane‐1,2‐diol administered as an aqueous solution at concentrations of 4.83, 9.66, 19.32, 38.64 and 77.28 mmol/kg bw. The time required to reach the maximum concentration of propane‐1,2‐diol in blood was linearly related to the dose (10, 20, 30, 60 and 90 min, respectively). The rate of elimination of propane‐1,2‐diol from the blood was found to be a first‐order process. The plot of the observed elimination velocity vs the propane‐1,2‐diol doses indicated that the elimination of propane‐1,2‐diol from the body was saturated at the higher dose levels. Likewise, a linear increase in the urinary excretion of propane‐1,2‐diol was observed for increasing doses (24‐h urine samples) with only 1/5 of the administered dose of propane‐1,2‐diol being excreted within the 24‐h period. According to the authors, the maximal metabolising capacity in rats of 0.63 g/kg bw per hour was equivalent to 1.06 kg/day for an average 70‐kg human.
Rabbits
Rabbits (2 animals) showed increased excretion of glucuronic acid in the urine (22% and 100%, respectively, at 48 h after dosing) following administration of a single dose of 2.3 mL propane‐1,2‐diol/kg bw (2.383 mg/kg bw) by oral gavage, indicating, according to the authors, that propane‐1,2‐diol was conjugated to glucuronic acid and that the conjugate was excreted in the urine (Fellows et al., 1947).
The blood concentration of propane‐1,2‐diol in New Zealand White rabbits (four animals) reached its maximum level 1 h after administration by gavage of 28.4% propane‐1,2‐diol (38.66 mmol/kg bw, equal to 2,942 mg) as an aqueous solution (Morshed et al., 1991a).
Cats
Plasma levels of propane‐1,2‐diol were determined in two cats fed a 12% propane‐1,2‐diol diet (equal to 1,600 mg/kg bw per day) (Christopher et al., 1989a). Plasma levels of propane‐1,2‐diol at 19.1 and 8.4 mM/L were reported at day 24 after introduction of the propane‐1,2‐diol diet.
Dogs
A dose of 2, 8 or 12 mL/kg bw (2,072, 8,288 and 12,432 mg/kg bw) of propane‐1,2‐diol was administered per dog (Lehman and Newman, 1937). For investigating absorption, the dose was given by stomach tube as a 50% solution (v/v) in warm tap water. The dog dosed with 2,072 mg/kg bw, received the same dose intravenously in addition to the oral dose. The dog dosed with 8,288 mg/kg bw was a second time treated with the same dose, now under pentobarbital anaesthesia. A dose of 6 mL (6,216 mg/kg bw) was given into the in situ isolated stomach from an untreated dog. The maximum blood propane‐1,2‐diol level was observed 30 min after administration of 2 mL/kg bw and 2–4 h after administration of 8 or 12 mL/kg bw. Forty‐eight hours after administration, 12% of the total dose was excreted in the urine at 2 mL/kg bw and 39% at 8 mL/kg bw; after administration of 12 mL/kg bw, 36% of the total dose was excreted in the urine after 24 h. As the blood concentration time profile was nearly identical in the dog dosed with 2,072 mg/kg bw orally and intravenously, the author concluded that the absorption was rapid and nearly complete. As the maximum blood concentration in the dog in which a dose of 6,216 mg/kg bw was given into the isolated stomach was at the same level as in the blood of the dog having received 2,072 mg/kg bw, the authors concluded that the absorption by the stomach was low. The Panel agreed with the interpretation of the results.
Plasma levels of propane‐1,2‐diol were determined in female dogs as a function of time after a single dose of 5,000 mg/kg bw (Weil et al., 1971). Peak values were reached at 6–8 h after dosing and levels at 12 h after dosing were approximately half those at 8 h.
Humans
Absorption of propane‐1,2‐diol in human was found to be slower than in dog, as illustrated by an experiment in which three human subjects were given a single oral dose of 1 mL propane‐1,2‐diol/kg bw (1,036 mg/kg bw) (68, −75 mL/subject) (Hanzlik et al., 1939a). The highest concentration of propane‐1,2‐diol measured in blood was reached after 30 min and persisted for approximately 4 h before they began to decline, indicating, according to the authors, ‘a delayed absorption keeping pace with the elimination of propane‐1,2‐diol from the body’. Blood concentrations varied considerably between individuals. About 20–25% was eliminated in the urine within 10 h.
The pharmacokinetic profile of the substance was examined in a multiple oral‐dosing study by Yu et al. (1985). Propane‐1,2‐diol was the solvent for the anti‐epileptic drug phenytoin given to outpatients of a neurological clinic. With the phenytoin, the patients received 20.7 g propane‐1,2‐diol three times daily for a minimum of 3 days (n = 16) or 41.4 g propane‐1,2‐diol twice daily for a period of 3 days (n = 6). Propane‐1,2‐diol was rapidly absorbed from the gastrointestinal tract with maximum plasma concentrations obtained within 1 h of dosing. The average serum half‐life of propane‐1,2‐diol was calculated to be 3.8 h (20.7 g, 3 times daily) and 4.1 h (41.4 g, 2 times daily), respectively. The average apparent total body clearance was approximately 0.1 L/kg per hour. However, a significant variability in clearance rate among individuals was observed. The apparent volume of distribution was approximately 0.5 L/kg.
In a study with intravenous administration of propane‐1,2‐diol (doses between 5.1 and 21 g/day) to 6 patients, the mean clearance was 0.41 L/h per kg bw, and the volume of distribution 0.8 L/kg bw (Speth et al., 1987). The difference in apparent clearance and apparent volume of distribution after oral dosing as compared to intravenous dosing might be explained by the bioavailability but also by a saturated excretion.
A population pharmacokinetic analysis of propane‐1‐2‐diol was done on 372 plasma samples taken from infants (1–11 samples per patient) between 20 min and 20.5 h after intravenous administration of a drug: paracetamol (34), phenobarbital (25) and phenobarbital plus paracetamol (3), which was dissolved in propane‐1‐2‐diol (De Cock et al., 2013). Sixty‐two preterm and term infants were involved in the study, with a gestational age between 24 and 41 weeks at birth and an age between 1 and 82 days, weighing 630–3,980 g. The population value for the clearance was 0.0849 L/h, which is extremely lower than the value for adults (8.64–23.4 L/h per 1.73 m2; Speth et al., 1987). Variability in clearance was explained by body weight at birth. Volume of distribution was dependent on the current body weight. For example, the volume of distribution of a neonate of 1 kg (0.23–0.40 L) was very different compared with a neonate of 4 kg (1.69–3 L).
In a further analysis of their data, De Cock et al. (2014) found out that renal elimination increased from 15% of total clearance after the first dose to 25% of the total clearance, one day later by an unknown mechanism. Compared to the renal clearance in adults which is 45% of the total clearance renal clearance in newborn infants was low, indicating the not yet fully maturated renal function at this age.
The metabolism of propane‐1,2‐diol has been reviewed by different authors (Ruddick, 1972; LaKind et al., 1999) (Figure 1).
Figure 1.
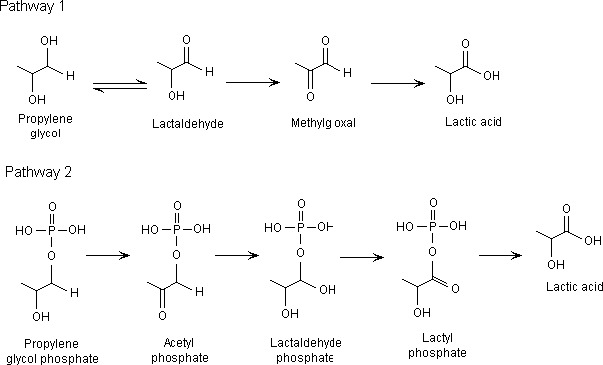
Metabolism of propane‐1,2‐diol in mammals (re‐produced from Ruddick (1972))
Overall, propane‐1,2‐diol is readily absorbed from the gastrointestinal tract in experimental animals and in humans and is expected to be widely distributed to organs and tissues. The major route of metabolism is oxidation to lactic acid and pyruvic acid. An alternative route of metabolism of propane‐1,2‐diol to lactic acid is via phosphorylated glycol. Lactate may be metabolised via the citric acid cycle and excreted as carbon dioxide via exhalation. At high concentrations, free propane‐1,2‐diol is excreted in the urine as the metabolism of propane‐1,2‐diol is saturated at dose levels higher than 20,000 mg/day in humans (Yu et al., 1985). The Panel noted that in premature infants and infants in the first weeks of life the clearance of propane‐1,2‐diol was extremely low (De Cock et al., 2014).
3.5.2. Acute toxicity
3.5.2.1.
Mice
Groups of 10–20 mice were fasted overnight (18 h before treatment) before administration of a single dose of 20.0, 22.5, 25.0 or 30.0 mL propane‐1,2‐diol/kg bw by stomach tube (Laug et al., 1939). The LD50 value was calculated to be 23.9 mL propane‐1,2‐diol/kg bw (24,760 mg/kg bw).
Groups of at least four mice received a single oral dose of propane‐1,2‐diol (six dose levels, not specified) (Latven and Molitor, 1939). The LD50 value was calculated to be 22.0 mL propane‐1,2‐diol/kg bw (22,792 mg/kg bw).
Rats
A single dosage of 26,000, 31,200 or 36,400 mg propane‐1,2‐diol/kg bw was administered orally to groups of rats (5 animals/group) (Weatherby and Haag, 1938). The LD50 value was calculated to be 33,500 mg/kg bw.
Groups of 9–10 rats were fasted overnight (18 h before treatment) before administration of a single dose of 15.0, 17.5, 17.6, 18.6, 20.0, 21.4, 22.5, 22.6 or 25.0 mL propane‐1,2‐diol/kg bw by stomach tube (Laug et al., 1939). The LD50 value was calculated to be 21.0 mL propane‐1,2‐diol/kg bw (21,756 mg/kg bw).
Rats received a single dose of propane‐1,2‐diol (diluted in water) by stomach tube. In most cases, 10 rats/group were used and the maximal dosage was 50,000 mg propane‐1,2‐diol/kg bw (Smyth et al., 1941). The LD50 value was calculated to be 26,380 mg propane‐1,2‐diol/kg bw.
Groups of five female rats received a single dose by gavage of 18.2, 27.7, 31.6, 50.4 or 57.2 mL propane‐1,2‐diol/kg bw (Thomas et al., 1949). The LD50 value was reported to be 28 mL propane‐1,2‐diol/kg bw (29,008 mg/kg bw).
Groups of female F344 rats (5/groups) received a single oral dose of propane‐1,2‐diol by gavage. The LD50 value was reported to be 22,800 mg/kg bw (Clark et al., 1979). Acute haemorrhagic enteritis together with adrenocortical haemorrhage and widespread lymphocyte depletion were observed at 23,500 mg propane‐1,2‐diol/kg bw.
Guinea pigs
Groups of 10 guinea pigs were fasted overnight (18 h before treatment) before administration of a single dose of 15.0, 17.5, 20.0 or 22.5 mL propane‐1,2‐diol/kg bw by stomach tube (Laug et al., 1939). The LD50 value was calculated to be 18.9 mL propane‐1,2‐diol/kg bw (19,580 mg/kg bw).
A single dose of propane‐1,2‐diol diluted in water was administered to guinea pigs by stomach tube. In most cases, 10 animals/group were used and the maximal dosage was 50,000 mg propane‐1,2‐diol/kg bw (Smyth et al., 1941). The LD50 value was calculated to be 18,350 mg/kg bw).
Rabbits
Groups of rabbits (2–9/group) were dosed with 15,750, 18,900, 19,950 or 21,000 mg propane‐1,2‐diol/kg bw (Braun and Cartland, 1936). Deaths occurred within 18–36 h post‐treatment and the minimum lethal dose (MLD) of propane‐1,2‐diol was estimated to 20,000 mg propane‐1,2‐diol/kg bw.
Overall, the acute toxicity for propanediol‐1,2‐diol was low.
3.5.3. Short‐term and subchronic toxicity
3.5.3.1.
Mice
A group of CD1 mice (10 animals/sex) was given 1,000 mg propane‐1,2‐diol/kg bw per day dissolved in distilled water by gavage for 92–93 days (Thackaberry et al., 2010). The study was performed in accordance with the OECD guideline No 408 (limit test) and in compliance with good laboratory practice (GLP). The control group was administered by gavage 20 mg/kg bw per day in distilled water of hydroxypropyl methylcellulose. No differences in clinical observations, body weights, food consumption, ophthalmology, electrocardiograms, clinical pathology (blood and urine) or absolute and relative organ weights were found between the propane‐1,2‐diol group and the control group. No macroscopic or histopathologic changes in any organs (full section) were observed compared to controls.
Rats
The effect of continuous administration of propane‐1,2‐diol in the drinking water was studied by Seidenfeld and Hanzlik (1932). Groups of 5 rats (sex not specified) were given 1%, 2%, 5%, 10%, 25% or 50% solutions of propane‐1,2‐diol in water for 140 days (equal to 1,600, 3,680, 7,700 or 13,200 mg/kg bw per day for the 1%, 2%, 5% or 10% group, respectively). The majority of rats receiving 25 or 50% propane‐1,2‐diol died by the end of day 6 or day 9, respectively; the animals showed an average weight loss of 10–20 g/rat and the fluid intake was less than 0.5 mL/rat. No significant changes in growth and body weight were found in animals given 1%, 2%, 5% or 10% propane‐1,2‐diol solution, and no significant pathological changes in the kidneys, heart, spleen and liver were observed.
In a subchronic toxicity study, a group of six female rats was given a concentration of 3.58% propane‐1,2‐diol (equivalent to 2,890 mg/kg bw per day) via the drinking water for 11 weeks (Holck, 1937). During the experiment, one rat died (week 10). According to the authors, by the end of the study, a significant weight loss was observed for one rat and the remaining rats were beginning to lose weight.
The toxicity of propane‐1,2‐diol was studies in white growing rats by administration of the substance in the drinking water (Weatherby and Haag, 1938). Groups of five male rats at weaning age received concentrations of 0%, 0.1%, 0.3%, 1%, 3% or 10% propane‐1,2‐diol in their drinking water for 100 days. Food and drink were allowed ad libitum. According to the authors, rats receiving 10% propane‐1,2‐diol in their drinking water consumed from 9,600 to 15,800 mg propane‐1,2‐diol/kg bw per day. During the first 10 days of the experiment, a decrease in weight gain together with poor physical conditions was observed for rats receiving 10% propane‐1,2‐diol compared to the other groups. After this period, the animals seemed to recover and increase in weight in the same way as the control group. Two animals from the 10% propane‐1,2‐diol group and one animal from the 1% propane‐1,2‐diol group died before study termination. No different microscopic changes were found in organs for any of the groups receiving propane‐1,2‐diol when compared to the control group.
In rats (5 animals, sex not specified) given daily 10 mL propane‐1,2‐diol/kg bw (10,360 mg/kg bw per day) by gavage for 15 weeks, no effect on the growth rate was observed compared to control rats (van Winkle, 1941b).
Hanzlik et al. (1939a) investigated the toxicity of propane‐1,2‐diol in young growing rats in a continued consumption study and in a paired feeding experiment. In the continued consumption study, rats (5 rats/group) were fed for 24 weeks on a diet where 25%, 50%, 75% or 100% of the carbohydrate in the diet was replaced with propane‐1,2‐diol. The group fed a carbohydrate‐free, 100% propane‐1,2‐diol‐substituted diet showed no weight gain and all rats died within about a month from study initiation. Rats receiving a 75% propane‐1,2‐diol diet showed significant reduced food intake and impaired growth rate; all rats died within 14 weeks after study initiation. A reduced growth rate and decreased food consumption was observed for groups given diets containing 25% or 50% propane‐1,2‐diol (equal to 8,288 or 31,200 mg propane‐1,2‐diol/kg bw per day) though it was most pronounced at the beginning of the study; no mortality occurred within 20 weeks. According to the authors, the reduced initial growth rate observed in groups fed propane‐1,2‐diol diets may be caused by a distaste of the diet due to the propane‐1,2‐diol content. To test this hypothesis, a paired feeding experiment was performed where groups of 6 rats were fed a 25% carbohydrate reduced diet for 5 months. During this period, rats were dosed daily by gavage with propane‐1,2‐diol (equal to 12.8% of total food intake the previous day, or 25% carbohydrate substitution) or water (control). Rats administered propane‐1,2‐diol increased significantly in body weight compared to controls, which, according to the authors is due to a higher calorie intake from propane‐1,2‐diol and showed the lack of any toxic effect of propane‐1,2‐diol.
In a study by van Winkle and Newman (1941), paired‐fed rats (5 rats/group) received propane‐1,2‐diol or water (control) daily by stomach tube for 127–163 days. Rats dosed with propane‐1,2‐diol were kept on a 25% reduced carbohydrate diet and dosed with a caloric equivalent amount of propane‐1,2‐diol (total propane‐1,2‐diol intake ranged from 92.4 to 135.4 mg). Propane‐1,2‐diol dosed rats were found to grow more rapidly and showed an increased weight gain compared to the controls. In addition, propane‐1,2‐diol was found to increase the concentration of liver glycogen with 2–7 times (van Winkle and Newman, 1941).
Administration of 5% or 10% propane‐1,2‐diol (equivalent to 6,250 and 12,500 mg/kg bw per day) in the drinking water to 10 male rats for 5 weeks resulted in significantly decreased red blood cell count (8–11%) (Vaille et al., 1971). A dose‐related increase in the liver weight (relative/absolute 20/11% or 37/27%) and in blood glucose (39% or 78%) was reported in the 5% or 10% group respectively. No histopathological (liver, spleen, kidneys, heart, pancreas, adrenal glands) changes were observed. The Panel noted that the administered doses were high, the highest one being half of the LD50 value.
When CD rats (15/sex per group) were fed a diet containing 50,000 mg propane‐1,2‐diol/kg diet (equivalent to 4,500 mg/kg bw per day) for 15 weeks, no effects on serum and urine parameters, haematology or organ weights (brain, heart, liver, spleen, kidneys, adrenals, gonads, and pituitary) were noted (Gaunt et al., 1972). No abnormalities were seen at necropsy.
Groups of male Wistar rats (6 animals/group) were orally dosed with 38.66 mM/kg bw of propane‐1,2‐diol (294.23 mg/kg bw per day as 1 mL 28.4%/100 g aqueous solution) for 10, 20 or 30 days (Morshed et al., 1991b). A control group received an equal volume of saline. No deaths were observed. The animals were necropsied after the required dosing schedule was finished. The brush border membrane (BBM) from the jejuno‐ileum part of the intestine of the animals was prepared. Various enzymes were measured and sucrose, maltase, alkaline phosphatase, gamma‐glutamyl transpeptidase (GGT) and leucine amino peptidase activity was increased in the BBM of propane‐1,2‐diol treated animals when compared to controls. The absorption of nutrient was measured using the tissue accumulation method (intestinal rings of the jejunum were prepared). Intestinal absorption of dextrose, glycine, l‐aspartic acid, l‐lysine, and calcium was increased for treated groups dosed for 20 or 30 days.
In another study by Morshed and Nagpaul (1995) using the same dose levels as above, propane‐1,2‐diol‐dosed groups showed significantly increased plasma concentrations of lactate (approx. 1.7‐ to 2‐fold) and glucose (approx. 1.3‐ to 1.7‐fold) for all treatment periods. No difference in pyruvate concentrations between treated and control rats were found for any of the treatment periods. The lactate/pyruvate ratio was significantly increased compared to controls after 10 days (approx. 2‐fold) and 20 days (approx. 1.3‐fold) of treatment. A significant decrease in plasma glutathione concentration was reported after 10 days of dosing (approx. 1.6‐fold), but was not significantly different from the control group after 20 or 30 days of dosing. In addition, propane‐1,2‐diol treatment influenced the concentrations of several plasma enzymes; treated rats had significantly decreased glutamic‐oxaloacetic transaminase (GOT) at day 10 (approx. 1.2 fold), significantly increased GGT at day 10–30 (approx. 1.7‐ to 1.8‐fold) and significantly decreased acid phosphatase at day 10–30 (approx. 1.3‐ to 1.4‐fold) (ethanol/propane‐1,2‐diol as a substrate). According to the authors, the changes in plasma glucose, glutathione, lactate/pyruvate ratio and plasma enzyme activities such GOT, GGT and acid suggested a toxic potential of propane‐1,2‐diol. The Panel considered these changes to be most likely adaptive and related to the metabolism of propane‐1,2‐diol to lactate and for a proportion of this, to be metabolised via gluconeogenesis to glucose, and therefore not adverse. A significantly increased activity of intestinal alcohol dehydrogenase was reported after propane‐1,2‐diol treatment for 10 (approx. 1.7/1.8‐fold), 20 (approx. 2.2/2.6 fold) and 30 (approx. 2.7/3.1‐fold) days.
Male Sprague–Dawley rats (n = 10) were fed a basal diet with propane‐1,2‐diol (250 mg/kg diet) for 42 days (equivalent to 30 mg/kg bw per day) (El‐Wahab and Moram, 2013). The control group was fed the basal diet only. By the end of the study period, the animals were sacrificed, blood samples were drawn for examination of haematological and blood biochemistry parameters, and the livers, hearts and kidneys were weighed in order to calculate the relative organ weights. The group fed propane‐1,2‐diol had a significant higher total feed intake, whereas the body weight gain was significantly decreased (p < 0.05) compared to the control group. The mean growth rate for rats fed propane‐1,2‐diol was –22.3% compared to +91% for control rats. No significant difference in the relative weights of the liver, kidneys or heart was found between the propane‐1,2‐diol group and the control group. Significant increases in alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase activities, as well as in bilirubin, urea, creatinine, total protein and albumin were observed in comparison to the control group. Compared to the control group, the haemoglobin concentration and total number of RBC were decreased by 21% and 41%, respectively, in the propane‐1,2‐diol group. Finally, propane‐1,2‐diol treatment was reported to significantly decrease the activity of reduced glutathione and superoxide dismutase activities in the blood and liver, as well as to decrease the glutathione‐S‐transferase activity in liver. The loss of body weight (–22.3%) of the animals of the only dose tested is very difficult to explain; animals should gain weight as was reported for the control group (+91%). The Panel considered this study as not reliable for this assessment because weight loss and liver toxicity was not observed in any of the other studies, in particular when considering the low dose (30 mg/kg bw per day) administered to the animals.
Sprague–Dawley rats (10 animals/sex per group) were given 1,000 mg propane‐1,2‐diol/kg bw per day dissolved in distilled water by gavage for 92–93 days (Thackaberry et al., 2010). The control group was administered 20 mg hydroxypropyl methylcellulose/kg bw per day dissolved in distilled water by daily gavage. The study was performed in accordance with the OECD guideline No. 408 (limit study) and in compliance with GLP. No differences in clinical observations, body weights, food consumption, ophthalmology, electrocardiograms, clinical pathology (blood and urine) or absolute and relative organ weights were found between the propane‐1,2‐diol group and the control group. No macroscopic or histopathological changes in any organs were observed compared to controls.
Rabbits
No effect on growth rate in young rabbits (sex not specified) was found when propane‐1,2‐diol was administered daily by gavage (as a 20% aqueous solution) for 50 days in concentrations of 1,050 (n = 1), 2,100 (n = 2), 3,150 (n = 1), 4,200 (n = 4) and 8,400 (n = 3) mg/kg bw per day (Braun and Cartland, 1936).
Rabbits (seven male animals, fauve de Bourgogne, body weight approximately 3.2 kg) were daily administered with 0% or 5% propane‐1,2‐diol in their drinking water for 8 weeks (equal to 14.8 mL/day per rabbit; calculated by the Panel to be 5,000 mg propane‐1,2‐diol/kg bw per day) (Vaille et al., 1971). In contrary to what was reported in rats in the same publication, induction of hyperglycaemia was not detected.
Dogs
Hanzlik et al. (1939a) studied the toxicity of propane‐1,2‐diol in individual dogs (sex not specified) administered by repeated gastric dosing (intervals of dosing not further specified). The authors concluded that six repeated doses of 1.5 mL/kg bw (1,554 mg/kg bw per day) (1 dog), four repeated doses of 2.0 mL/kg bw per day (2,072 mg/kg bw per day) (1 dog) or two repeated doses of 10.0 mL/kg bw (10,360 mg/kg bw per day) (1 dog) showed no evidence of toxicity.
In a study carried out by Van Winkle and Newman (1941), propane‐1,2‐diol at a concentration of 5% (equal to 5,284 mg propane‐1,2‐diol/kg bw per day) was administered in the drinking water to a group of four female dogs for 5–9 months. The animals received no other fluid. They had access to the propane‐1,2‐diol containing water for 1 h twice daily, thus insuring a higher blood glycol level than if the dogs drank small quantities of the propane‐1,2‐diol containing water at frequent intervals. The dogs had a normal growth rate and no histopathological changes or alteration in the liver and kidney function (galactose excretion, uric acid excretion, rose bengal test, phenolsulfonphthalein test) were found in propane‐1,2‐diol‐treated dogs. An attempt was made to keep a second group of dogs on 10% propane‐1,2‐diol in the drinking water, but this concentration was shown to cause a threefold increase of drinking water intake (hypertonic drinking water) and to result in coma. Dogs died of marked central depression or from a subsequent pneumonia.
Beagle dogs (4 animals/sex) were given 1,000 mg propane‐1,2‐diol/kg bw per day dissolved in distilled water by gavage for 95–97 days (Thackaberry et al., 2010). The control group was administered 20 mg/kg bw per day of hydroxypropyl methylcellulose dissolved in distilled water by daily gavage. The study was performed in accordance with the OECD guidelines No. 409 (limit test) and in compliance with GLP. No differences in clinical observations, body weights, food consumption, ophthalmology, electrocardiograms, clinical pathology (blood and urine) or absolute and relative organ weights were found between the propane‐1,2‐diol treated group and the control group. No macroscopical or histopathological changes in any organs were observed compared to controls.
Monkeys
Cynomolgus monkeys (4 animals/sex) were given 1,000 mg propane‐1,2‐diol/kg bw per day dissolved in distilled water by gavage for 95–97 days (Thackaberry et al., 2010). The control group was administered 20 mg/kg bw per day of hydroxypropyl methylcellulose dissolved in distilled water by daily gavage. The study was performed in accordance with the OECD guidelines No. 409 (limit test) and in compliance with GLP. No differences in clinical observations, body weights, food consumption, ophthalmology, electrocardiograms, clinical pathology (blood and urine) or absolute and relative organ weights were found between the propane‐1,2‐diol group and the control group. No macroscopical or histopathological changes in any organs were observed compared to controls.
Cats
The Panel noted that several studies have been performed in cats ((Dow Chemical, 1979 (Documentation provided to EFSA n. 3); Christopher et al. (1989a,b, 1990); Hickman et al., 1990; Bauer et al. (1992a,b)). The Panel decided not to use these studies because that as described by LaKind et al. (1999) ‘cats do not possess the ability to generate the glucuronide metabolite of PG, a detoxification capacity that acts via enhancement of urinary excretion of this product. The PG glucuronide conjugate represents a substantial fraction of the PG recovered from urine of several species other than cats. This lack of conjugation potential in cats may serve to enhance effective concentrations of PG in the systemic circulation, and thereby yield hematotoxicity in this species. For this reason, while cats were the most sensitive species tested, they are not viewed as being the most predictive of human responses to PG’.
Overall, in the preguideline rat studies propane‐1,2‐diol administered in the diet or drinking water resulted in reduced growth rate at very high dose levels only; no other effects were reported. No differences in clinical observations, body weights, food consumption, ophthalmology, electrocardiograms, clinical pathology (blood and urine), absolute and relative organ weights, or macroscopic or histopathologic changes in any organs were observed in a recent guideline study (limit study) in which propane‐1,2‐diol was administered by gavage (1,000 mg/kg bw per day by gavage) to mice, rats, dogs and monkeys for 92–97 days (Thackaberry et al., 2010).
3.5.4. Genotoxicity
3.5.4.1.
In vitro
Propane‐1,2‐diol was reported to be negative when tested in Salmonella/microsome test (McCann and Ames, 1976). In a reverse mutation assay using Salmonella Typhimurium strains TA98, TA100, TA1535, TA1537 and TA1538, propane‐1,2‐diol (1–10,000 μg/plate) was not mutagenic with or without metabolic activation (Clark et al., 1979). In S. Typhimurium strain TA100, no mutagenic potential was observed for propane‐1,2‐diol when tested in concentrations up to 1,000 μmol/plate with or without metabolic activation (Stolzenberg and Hine, 1979). In another reverse mutation assay using S. Typhimurium strains TA98, TA100, TA1535 and TA1537, propane‐1,2‐diol (230 μg/plate) was also negative with or without metabolic activation (Florin et al., 1980). Similarly, propane‐1,2‐diol showed no mutagenic potential when tested in S. Typhimurium strains TA98 and TA100 with or without metabolic activation, or in Bacillus subtilis rec without metabolic activation (Kawachi et al., 1980). No mutagenic potential of propane‐1,2‐diol was found when tested in S. Typhimurium strains TA98, TA100, TA1535 and TA1537 without metabolic activation at concentrations from 5‐ to 300 μmol/plate (Pfeiffer and Dunkelberg, 1980). Similar results were obtained when propane‐1,2‐diol was tested in S. Typhimurium strains TA98, TA100, TA1535 and TA1537 with or without metabolic activation at concentrations ranging from 100 to 10,000 μg/plate (Haworth et al., 1983). In addition, propane‐1,2‐diol was reported to be negative in the Salmonella/microsome test using S. Typhimurium strains TA92, TA94, TA98, TA100, TA1535 and TA1537 (with and without metabolic activation) (Ishidate et al., 1984).
The Panel noted that propane‐1,2‐diol was not tested in TA102 or Escherichia coli WP2 tester strains. However, since oxidising or cross‐linking activities are not expected to occur, overall, the Panel, considered the negative results observed as reliable.
Propane‐1,2‐diol was assessed for its capability to induce chromosomal aberrations in anaphase in human embryonic lung cells (WI38) at concentrations of 0.001, 0.01 and 0.1 μg/mL only in the absence of S9 metabolic activation and no cytogenetic effects were reported (Litton Bionetics, 1974 (Documentation provided to EFSA n. 10)). However, the Panel noted that the test on induction of chromosomal aberrations in anaphase did not receive further validation and it is currently not employed in genetic toxicology testing. In addition, the assay was only performed in the absence of S9 metabolic activation. On this basis the results obtained are of limited relevance.
In the study by Kawachi et al. (1980), propane‐1,2‐diol was reported to be positive when tested for chromosomal aberrations in hamster lung fibroblasts without and with metabolic activation, but negative for the induction of sister chromatid exchanges (SCEs). However, the Panel noted that the study is poorly reported with no indication of concentrations employed, treatment conditions and number of cells scored. On this basis, the results reported as a single table (with plus or minus signs) were judged to be not reliable.
Propane‐1,2‐diol was reported to be positive in another chromosomal aberration test using a Chinese hamster fibroblast cell line following treatments only in the absence of metabolic activation (Ishidate et al., 1984). The incidence of aberrations was 38% at the highest concentration tested (32 mg/mL) which is above the range of physiological osmotic pressure values, indicating, according to the authors, that the cells may have been affected by the osmotic pressure changes in the culture medium. The Panel agreed with this conclusion and noted that the recommended concentration limits indicated in the current OECD Guideline No 473 correspond to 2 mg/mL. On this basis, the results were judged unreliable.
In the study by Sasaki et al. (1980), propane‐1,2‐diol was tested for the induction of micronuclei and SCE's in the absence of S9 metabolic activation in a human fibroblastic cell line (HE2144) and in a Chinese hamster ovary Don‐6 cell line. Concentrations used were 3.8, 7.6 and 23 mg/mL for both micronuclei and SCEs in the Don‐6 cell line and 3.8 and 7.6 mg/mL for SCE's in the human fibroblast HE2144 cell line. Negative results were observed for induction of micronuclei in both cell lines, whereas slight increases for induction of SCEs up to 1.8‐ and 1.5‐folds over the concurrent control were only observed in the Chinese hamster ovary Don‐6 cells at concentrations of 23 and 7.6 mg/mL, respectively. This result was judged by the Panel to be of no biological relevance since obtained at concentrations exceeding the limit of 2 mg/mL recommended in the current OECD Guideline No 473. Overall, the Panel considered the outcome of this study as negative for both micronuclei and SCEs but with limitations since the assays were only performed in the absence of S9 metabolic activation.
A chromosome aberration assay in peripheral blood human lymphocytes was conducted in compliance with GLP principles (Huntingdon‐Research‐Centre, 1990 (Documentation provided to EFSA n. 7)). Replicate cultures were treated with propane‐1,2‐diol in both the absence and presence of S9 metabolic activation at concentrations of 476, 1,910 and 3,910 μg/mL corresponding to 6, 25 and 51 mM. Cultures were sampled at 24 and 42 h after initiation of treatment. No biologically relevant induction of chromosomal aberrations were observed at any treatment series, though the two higher concentrations assessed far exceeded the limit of 10 mM recommended in the current OECD Guideline No 473. The Panel noted that the study was in compliance with the OECD Guideline No 473 applicable at the time when the study was performed. However, compared to the current version of the OECD Guideline No 473 (2014) there are some limitations (e.g. the length of treatment time in the presence of S9 (2 h vs 3–6 h) and the absence of a short treatment (3 h) in the absence of S9 metabolic activation, selection of the high concentration, number of cells scored). On this basis, the results of the study were considered of limited reliability by the Panel.
Propane‐1,2‐diol was tested in a Chinese hamster ovary cell line (CHO‐K1) for induction of DNA strand‐breaks in the alkaline comet assay and for chromosomal damages in the micronucleus assay both in the absence and presence of S9 metabolic activation at concentrations of 0, 25, 50, 75 and 100 mg/mL, corresponding to 316, 632, 948 and 1,300 mM (Aye et al., 2010). Concentration‐related and statistically significant increases for both the induction of micronuclei and DNA strand breaks were observed at all concentrations employed, with the exception of 25 mg/mL in the presence of metabolic activation. However, the Panel noted that the concentrations used far exceeded the limit of 10 mM recommended in the current OECD Guideline No. 473 (2014). Therefore, the results obtained were considered by the Panel not reliable.
The genotoxicity of propane‐1,2‐diol has recently been assessed in mouse oocytes by an in vitro comet assay. Meiosis II oocytes obtained from prepubescent (< 4 weeks old) female CD‐1 mice and exposed for 1–2 h to 5%, 7% or 15% propane‐1,2‐diol solutions corresponding to 50, 75 and 150 mg/mL (Berthelot‐Ricou et al., 2011). A significant DNA damage was noted at the two higher concentrations (7.5% or 15%) at both exposure durations, whereas no DNA damage was reported at 5% propane‐1,2‐diol. However, the Panel noted that the concentrations used far exceeded the limit of 2 mg/mL recommended in the current OECD Guideline for the in vitro genotoxicity assays (e.g. OECD Guideline No. 473 (2014), in vitro mammalian chromosome aberration assay, No. 476 in vitro mammalian cell gene mutation tests using the Hprt and Xprt genes, No. 487 in vitro mammalian cell micronucleus test, No. 490 in vitro mammalian cell gene mutation tests using the thymidine kinase gene). On this basis, the results obtained were considered by the Panel not reliable.
In vivo
Propane‐1,2‐diol has been investigated in a host‐mediated assay in mice. ICR random‐bred male mice (10 animals, 25–30 g each, at each dose level) were administered propane‐1,2‐diol by oral gavage in both acute (single dose) and subacute studies (once a day for 5 days) (Litton Bionetics, 1974 (Documentation provided to EFSA n. 10)). The dose levels were 30, 2,500 or 5,000 mg/kg bw. The indicator organisms (S. Typhimurium strain TA1530 and G46 for detection of induction of reverse mutation, and Saccharomyces cerevisiae strain D‐3 for detection of mitotic recombination) were given by intraperitoneal injection. The negative control was the vehicle (saline) and the positive control was dimethyl nitrosamine (100 mg/kg) for Salmonella, and ethyl methane sulfonate (350 mg/kg) for Saccharomyces. Propane‐1,2‐diol caused no significantly increased mutant frequencies in Salmonella strain TA1530 or in strain G46 except for the acute high dose level at which, according to the authors of the study report, may have been a weak or a questionable positive result. Saccharomyces strain D3 produced increased recombinant frequencies in the acute treatment at the low and intermediate dose‐levels and at all dose levels in the subacute treatment. However, according to the author, the recoveries of the indicator organism decreased with increasing dose‐levels and in the subacute treatment, they were severely depressed possibly due to cell lethality. In conclusion, the author stated for the strain D3 that ‘data are difficult to interpret and at the dose‐levels used it appears that compound may be recombinogenic’. The Panel agreed with this conclusion but noted that this assay did not receive further validation and it does not belong to the assays recommended for regulatory purposes (EFSA Scientific Committee, 2011). On this basis, the positive outcome obtained was considered to be of low relevance.
Propane‐1,2‐diol has also been investigated in a cytogenetic assay in rats. Male Sprague–Dawley rats (10–12 weeks old, 280–350 g) were administered propane‐1,2‐diol by oral gavage in both acute (single dose, in total 59 animals) and subacute studies (once a day for 5 days, in total 18 animals) (Litton Bionetics, 1974 (Documentation provided to EFSA n. 10)). The dose levels were 30, 2,500 or 5,000 mg/kg bw. The negative control was the vehicle (saline) and the positive control was triethylene melamine (0.3 mg/kg bw). In the acute studies, animals were sacrificed 6, 24 and 48 h after dosing and in the subacute treatment 6 h after the last dose. Bone marrow cells were used to prepare cytogenetic slides following administration of colcemid at 4 mg/kg by intraperitoneal injection to accumulate cells in metaphase before sacrifice of animals. Fifty metaphase spreads for animal were scored for chromosomal aberration analyses. The mitotic indices were also evaluated by scoring 1,000 cells (mitotic and interphase cells) per animal to assess cytotoxicity. The result obtained showed no relevant increases in chromosomal aberration at any dose level and sampling time employed. Slight reduction of mitotic indices in the test substance treatment groups were also observed indicating that the test compound reached the target organ. The Panel noted that although 50 metaphases per animal were scored instead of 100–200 as recommended by latter OECD Guidelines TG 473 (1997, 2014), in consideration of the high dose applied (higher than the maximum recommended) and of the evidence of target cells exposure, the results of this study can be considered as relevant for genotoxicity assessment in a weight of evidence (WoE) approach.
In addition, propane‐1,2‐diol has been investigated in a dominant lethal assay in rats. Male random‐bred rats (10 animals per group, 10–12 week old) were administered propane‐1,2‐diol by oral gavage in both acute (single dose) and subacute studies (once a day for 5 days) (Litton Bionetics, 1974 (Documentation provided to EFSA n. 10)). The dose levels were 30, 2,500 or 5,000 mg/kg bw. The negative control was the vehicle (saline) and the positive control was triethylene melamine (0.3 mg/kg). Following treatment, the males were sequentially mated to 2 females per week for 8 weeks (7 weeks in the subacute study). Females were sacrificed at 14 days after separation from the male and the uterus was examined for corpora lutea, early fetal deaths, late fetal deaths and total implantations. According to the authors of the study report, propane‐1,2‐diol was considered to be non‐mutagenic in rats in the dominant lethal assay when using the dosages employed in this study. The Panel agreed with this conclusion.
In a micronucleus test, propane‐1,2‐diol did not induce micronuclei in femoral bone marrow cells from male ddY mice (6 animals/group) after a single i.p. injection of 2,500, 5,000, 10,000, 15,000 or 20,000 mg/kg bw propane‐1,2‐diol (Hayashi et al., 1988). At 15,000 mg/kg bw, 3/6 mice died, and at a dose of 20,000 mg/kg bw all six mice died. The Panel noted that although 1,000 polychromatic erythrocytes (PCEs) per animal were scored instead of 2,000–4,000 as recommended by latter OECD Guidelines TG 474 (1997, 2014), in consideration of the high doses applied (higher than the maximum recommended) and of the evidence of systemic toxicity in treated animals, the results of this study can be considered as relevant for genotoxicity assessment in a WoE approach.
Overall, the Panel considered that the available data did not raise concern with respect to genotoxicity of propane‐1,2‐diol when used as a food additive.
3.5.5. Chronic toxicity and carcinogenicity
3.5.5.1.
Rats
Morris et al. (1942) examined the chronic oral toxicity of propane‐1,2‐diol in rats. Groups of 10 albino rats (both sexes) were fed 2.45% or 4.9% propane‐1,2‐diol (equivalent to 1,230 or 2,450 mg/kg bw per day) in the diet for two years. No difference in food intake, growth and survival rate (after the first year) was observed between propane‐1,2‐diol groups and the control group. Histopathological examination of the lungs, heart, liver, spleen, kidneys, adrenals, testis, pancreas, stomach, intestines and lymph nodes showed that propane‐1,2‐diol did not induce adverse effects, except for slight chronic liver damage characterised by diffuse or centrilobular atrophy, bile duct proliferation and fatty degeneration.
In a study by Gaunt et al. (1972), groups of CD rats (30/sex per group) were fed on diets containing 0, 6,250, 12,500, 25,000 or 50,000 mg propane‐1,2‐diol/kg diet (equivalent to 0, 312, 625, 1,250, or 2,500 mg/kg bw per day) for 2 years. Propane‐1,2‐diol was not found to influence mortality, body weight gain, food consumption or cumulative death rate. Overall, feeding of propane‐1,2‐diol had no effect on haematology, urinary cell excretion, the urine‐concentrating ability of the kidneys, organ weights, pathological findings or tumour incidence (brain, heart, liver, spleen, kidneys, adrenals, gonads, stomach, small intestine, caecum, salivary gland, trachea, aorta, thymus, lymph nodes, pituitary, urinary bladder, colon, rectum, pancreas, uterus, muscle). No carcinogenic potential was observed up 2,500 mg/kg bw per day, the highest dose tested.
Dogs
Chronic toxicity of propane‐1,2‐diol was studied in dogs by Weil et al. (1971). Groups of Beagle dogs (5/sex per group, 10–14 month old at study initiation) were fed diets containing 0, 2,000 (low dose) or 5,000 (high dose) mg propane‐1,2‐diol/kg bw per day for 2 years. A significantly higher diet utilisation (% g weight change/g diet consumed) was observed for high‐dose male dogs compared to controls during the first three months of the study. Treated female dogs (low or high dose of propane‐1,2‐diol) had significantly lower water intake than controls only after 1 and 2 months (approx. 2.3‐fold), while no difference in water intake was observed between control and treated male dogs. Propane‐1,2‐diol treatment did not affect differential leucocyte counts, erythrocyte fragility, urinary pH and histopathological changes, alkaline phosphatase, bromsulfthalein retention, liver glycogen, blood glucose, total liver lipids, metabolic rate of liver slices using propane‐1,2‐diol, activities of serum glutamic‐oxalacetic and glutamic‐pyruvic transaminases, percentage of water in the liver, or liver weights (absolute and relative). In the low‐dose group, sporadically lower total leucocyte counts were observed in males, and females had a lower level of liver triglyceride; these changes were not observed in the high‐dose group. No difference in spleen weights was noted between control and treated groups. High‐dose females had a slightly increased absolute kidney weight; however, no significant change in relative kidney weight and kidney histopathology was noted for these animals. After 23.5 months of treatment, the high‐dose group showed decreased mean total erythrocyte count (both sexes: males: 17%, females: 15%) and decreased haemoglobin (both sexes: M: 13%, F: 20%) and haematocrit (females: 11%) values. After 23 months feeding in these groups, an increase in percentages of nucleated erythrocytes (females: 4.5‐fold), mean reticulocyte count (females: 2.5‐fold) and the degree of anisocytosis and poikilocytosis (both sexes: 0/5) was reported pointing to destruction of erythrocytes accompanied by accelerated erythropoiesis in the bone marrow. In the high‐dose group, total bilirubin was statistically significantly increased in males after 6 (1.7‐fold) and 24 months (1.8‐fold) (but not after 8, 12, 13 or 23 months) and in females after 6, 8, 12, 13, 23 and 24 months (1.5‐ to 1.8‐fold) (no changes in van den Bergh direct and indirect bilirubin values were reported at the end of the study). Furthermore, urine output was raised in males at 23 months (2.4‐fold) and in females at 6 (1.4‐fold) and 12 (1.3‐fold) months. Treated groups had higher serum propane‐1,2‐diol levels than controls; values were below 0.1% (v/v) 24 h after the daily dose of propane‐1,2‐diol except for one dog where a concentration of 0.2% (v/v) was reported. At study termination, glucose metabolic rates (when glucose was used as substrate), were significantly lower in animals fed propane‐1,2‐diol although only statistically significant for high‐dose males (4 fold). Gross and histopathological examination showed no treatment‐related changes in propane‐1,2‐diol‐treated animals. A slight increase in bone‐marrow activity in high‐dose females was considered by the authors to be physiological and not pathological (Weil et al., 1971). The Panel considered the 2,000 mg/kg bw per day as the no observed adverse effect level (NOAEL) in the 2‐year study in dogs.
Overall, no adverse effects, including neoplastic findings, were reported in a chronic study in rats administered propane‐1,2‐diol in the diet at 2,500 mg/kg bw per day (the highest dose tested) (Gaunt et al., 1972). No adverse effects were observed in a 2‐year study in dogs administered propane‐1,2‐diol in the diet (2,000 or 5,000 mg/kg bw per day), except for haematological changes suggestive of an increased red blood cell destruction with a compensatory increased rate of haematopoiesis at the highest dose level (5,000 mg/kg bw per day) (Weil et al., 1971). The Panel considered 2,000 mg/kg bw per day in dogs as the NOAEL for this study.
3.5.6. Reproductive and developmental toxicity
Reproductive toxicity
Mice
Propane‐1,2‐diol was studied in a Reproductive Assessment by Continuous Breeding (RACB) in CD‐1 mice (NTP, 1985; Morrissey et al., 1989; Lamb et al., 1997). In the reproductive phase of these assessment, the study contained a control (n = 40/sex per group) and three treatment levels (n = 20/sex per group (0%, 1.0%, 2.5% and 5.0% propane‐1,2‐diol in drinking water equal to 0, 1,819, 4,796, and 10,118 mg propane‐1,2‐diol/kg bw per day). The mice were exposed for a 7‐day premating period, during the cohabitation period of 14 weeks, and 3 weeks after the cohabitation period. No differences in the fertility index, mean numbers of litters per pair, mean numbers of live pups per pair, mean numbers of live males/females per pair, proportion of pups born live, and sex of pups born live were noted when compared to the control group.
Bolon et al. (1997) examined the ovaries from 10 mice of the afore mentioned study that had been exposed to 5% propane‐1,2‐diol as gametes, during prenatal and postnatal development, and as young adults were examined for differential ovarian follicle counts (small, growing and large antral follicles). Propane‐1,2‐diol showed no effect on reproductive function in terms of follicle counts compared to controls.
Rats
Propane‐1,2‐diol was used as a vehicle control in a study in rats by Sjöberg et al. (1986). The control group of six male SD rats (35 days old, weight 130–160 g) was administered daily the vehicle propane‐1,2‐diol by gavage for 5 days. The daily volume given to each animal was 2 mL/kg bw per day (2,072 mg propane‐1,2‐diol/kg bw per day). The animals were sacrificed on day 6 and one testis, the ventral lobes of the prostate and the liver were removed, weighed and examined histologically. The number of degenerated cells in 50 seminiferous tubular cross‐sections per testis was counted and the most advanced germ cell type present was recorded. In the propane‐1,2‐diol group, only occasional degenerated cells were observed with the majority (19%) having 1–3 degenerated cells per tubular cross‐section and only 1% having 4–10 degenerated cells per tubular cross‐section. Most of these cells were either in early meiotic prophase or undergoing meiotic divisions.
The reproductive toxicity in Sprague–Dawley rats by daily gavage (vehicle: water) of 1,000 mg propane‐1,2‐diol/kg bw dissolved in distilled water was examined by Enright et al. (2010). The control group was given 20 mg/kg bw hydroxypropyl methylcellulose dissolved in distilled water. The study was performed in compliance with GLP. In the fertility study, female rats (22 animals/group, 9–11 weeks of age at initiation of dosing, weight 203–264 g) were dosed for 2 weeks before and during mating (up to 3 weeks) and from gestation day (GD) 0–7; male rats (22 animals/group, weight 203–264 g) were dosed before mating (3 weeks), during mating (up to 3 weeks) and after mating (3 weeks). Animals were observed twice daily during the dosing period, body weights and food consumption were recorded at regular. Oestrous cyclicity was assessed in all females for 2 weeks before initiation of dosing through the day of mating. Females were sacrificed on GD 14, the uterus was examined to determine pregnancy status, corpora lutea and uterine implants were counted and implants were classified as a live or dead fetus, or resorption (early or late), and a gross examination of the thoracic and abdominal cavities was performed on all F0 females in all dose groups. Males were sacrificed within 3 weeks after the completion of the mating phase and a gross examination of the thoracic and abdominal cavities and reproductive organs was carried on all F0 males in all dose groups. No treatment‐related effects were observed on paternal or reproductive toxicity. The Panel considered 1,000 mg propane‐1,2 diol/kg bw per day (the only dose tested) as the NOAEL for this study.
Developmental toxicity
Mice
Groups of pregnant mice (25 animals/group) were given by gavage propane‐1,2‐diol in distilled water (10 mL/kg bw) at dose levels of 0, 16, 74.3, 345 or 1,600 mg/kg bw per day from GD 6 to 15 (FDRL, 1973 (Documentation provided to EFSA n. 5)). One dam in the 74.3 mg/kg bw per day dose group died before term. No other maternal effects or differences in the number of implantation sites, resorptions, fetal body weight, viability or external, skeletal or visceral abnormalities were apparent. The NOAEL for this study was 1,600 mg propane‐1,2‐diol/kg bw per day, the highest dose tested.
In a developmental screening assay, timed‐pregnant CD‐1 mice (30 animals, 60 days old) dosed daily with propane‐1,2‐diol (10,000 mg/kg bw per day) by oral gavage on GD 8–12 were examined after sacrifice for maternal toxicity (number of pregnant dams, mortality and number of dams with resorptions) and neonatal effects (number of live pups and pup weight on postnatal day 1 and 3). No treatment‐related maternal or developmental effects were observed (Kavlock et al., 1987).
In a developmental toxicity study, timed‐pregnant female CD‐1 mice (30 animals/group) were dosed daily by gavage with 0.5, 5 or 10 mL propane‐1,2‐diol/kg bw (518, 5,180 and 10,360 mg propane‐1,2‐diol/kg bw per day) on GD 6–15; the control group (30 animals) received water (Union Carbide, 1993 (Documentation provided to EFSA n. 14)). The study was performed in accordance with the OECD guideline No. 414 and in compliance with GLP. The dams of the mid‐ and high‐dose groups had an increase water consumption. No other treatment‐related maternal or developmental effects were reported.
Rats
Pregnant rats (20–24 animals/group) were given propane‐1,2‐diol dissolved in distilled water by gavage at dose levels of concentrations of 0, 16, 74.3, 345 and 1,600 mg/kg bw per day from GD 6 to 15 (dose volume was 6.4, 1, 1, 2 and 6.4 mL/kg bw, respectively) (FDRL, 1973 (Documentation provided to EFSA n. 5)). No treatment‐related maternal effects or differences in the number of implantation sites, resorptions, fetal body weight, viability or external and visceral abnormalities were apparent. The number of fetuses with wavy ribs was increased in all groups treated with propane‐1,2 diol compared to the control group; however, no dose relation was observed. The Panel considered the highest dose tested, 1,600 mg propane‐1,2‐diol/kg bw per day, as the NOAEL.
The prenatal developmental toxicity in Sprague–Dawley rats by daily gavage (vehicle: water) of 1,000 mg propane‐1,2‐diol/kg bw per day was examined by Enright et al. (2010). The control group was given 20 mg hydroxypropyl methylcellulose/kg bw per day. Mated female rats (22 animals/group; 11 weeks of age at initiation of dosing; weight 227–300 g) were dosed from GD 6‐17. Clinical observations were made daily and maternal body weights and food intake were recorded on regular intervals. Dams were sacrificed on GD 21, the uterus was examined to determine pregnancy status, corpora lutea and uterine implants were counted and implants were classified as a live or dead fetus, or resorption (early or late), and a gross examination of the thoracic and abdominal cavities was performed on all F0 females in all dose groups. All fetuses were weighed, sexed and examined externally, and then sacrificed. Approximately half of fetuses in each litter underwent a visceral examination; the remaining half underwent a skeletal examination. No treatment‐related effects were observed on maternal or developmental toxicity. The Panel considered the only dose tested, 1,000 mg propane‐1,2 diol/kg bw per day, as the NOAEL for this study.
Hamsters
Pregnant hamsters (21–25 animals/group) were given propane‐1,2‐diol dissolved in distilled water by gavage at dose levels of 0, 15.5, 72, 334.5 and 1,550 mg/kg bw per day from GD 6 to 10 (dose volume was 6.4, 1, 1, 2 and 6.4 mL/kg bw, respectively) (FDRL, 1973 (Documentation provided to EFSA n. 5)). No treatment‐related differences in maternal toxicity and in the number of implantation sites, resorptions, fetal body weight, and visceral and skeletal abnormalities. The number of dead fetuses was increased in the high‐dose group (1 in the control group vs 18 in the high‐dose group; however 16 dead fetuses were found in one litter) and the number of live fetuses accordingly decreased. The authors concluded only that there were no effects on skeletal and visceral abnormalities. No statistical analysis or conclusion on other study data were presented. As the increased number of dead fetuses was mainly due to one litter, the Panel considered 1,550 mg propane‐diol/kg bw per day as the NOAEL for this study. However, the Panel considered this study as having limited reliability.
Rabbits
Pregnant rabbits (11–13 animals/group) were given propane‐1,2‐diol in distilled water by gavage in concentrations of 0, 12.3, 57.1, 267 or 1,230 mg/kg bw per day from GD 6 to 18 (dose volume was 5, 1, 1, 2 and 5 mL/kg bw, respectively) (FDRL, 1973 (Documentation provided to EFSA n. 5)). No effects attributed to propane‐1,2‐diol were observed for maternal or developmental effects. The Panel considered 1,230 mg propane‐1,2‐diol kg bw per day (the highest dose tested) as the NOAEL for this study.
The prenatal developmental toxicity of propane‐1,2 diol was examined in New Zealand White rabbits by daily gavage of 1,000 mg propane‐1,2‐diol/kg bw per day; the control group was given 20 hydroxypropyl methylcellulose mg/kg bw per day (Enright et al., 2010). Mated female rabbits (20 animals/group, 5–6 months of age at initiation of dosing, weight 2.7–3.8 kg) were dosed on GD 7–19. Clinical observations were made daily, maternal body weights, food consumption (visual inspection) were measured regularly. Dams were sacrificed on GD 29, the uterus was examined to determine pregnancy status, corpora lutea and uterine implants were counted and implants were classified as a live or dead fetus, or resorption (early or late), and a gross examination of the thoracic and abdominal cavities and of the placenta was performed. All fetuses were weighed, sexed and examined externally, and then sacrificed. All fetuses underwent a visceral and a skeletal examination. No differences in the examined parameters between treated and control animals were reported. The Panel considered the only dose tested, 1,000 mg propane‐1,2‐diol/kg bw per day, as the NOAEL for this study.
Overall, no adverse effects on reproductive toxicity parameters of propane‐1,2‐diol up to 5% (equal to 10,118 mg propane‐1,2‐diol/kg bw per day) in drinking water were observed in a continuous breeding reproduction study in mice (Morrissey et al., 1989; Lamb et al., 1997) or in a fertility study in male and female rats given propane‐1,2‐diol (1,000 mg/kg bw per day) daily by gavage (Enright et al., 2010). No adverse maternal or developmental effects were observed in prenatal developmental toxicity studies with mice, rats, hamsters and rabbits given propane‐1,2‐diol by oral gavage at dose levels up to 1,600, 1,600, 1,550 and 1,230 mg/kg bw per day, respectively, on GD 6–15, 6–15, 6–10 and 6–18, respectively (FDRL 1973 (Documentation provided to EFSA n. 5)). In an additional developmental toxicity study in mice, no adverse effect was observed in pregnant females dosed daily by gavage from GD 6 to 15 up to 10,360 mg propane‐1,2‐diol/kg bw per day (Union Carbide, 1993 (Documentation provided to EFSA n. 14)). In other prenatal developmental toxicity studies by gavage of 1,000 mg/kg bw per day (the only dose tested) from GD 6 to 17 in rats or in rabbits from GD 7 to 19 (Enright et al., 2010), no effects were observed.
3.5.7. Hypersensitivity, allergenicity and food intolerance
There is a controversy about the contact allergenic potential of propane‐1,2‐diol in patch tests. However, this seemed to be due to its low irritant potency, which often confounds the interpretation of skin sensitisation reaction (Fowles et al., 2013; Jacob et al., 2017; Mc Gowan et al., 2017). The Cosmetic Ingredient Review (CIR) did not report any sensitising potential or immunotoxicity for propane‐1,2‐diol when used in cosmetics (CIR, 2011), and the EFSA CONTAM Panel did not consider propane‐1‐2‐diol as a possible allergen (EFSA CONTAM Panel, 2011). Given the large body of evidence available, the Panel considered that it is very unlikely that propane 1‐2‐diol could be an allergen for humans when used as a food additive.
3.5.8. Other studies
Young animals
C57BL/6 mice were exposed to a single intraperitoneal dose of propane‐1‐2‐diol (dose range 1–10 mg/kg) to examine whether propane‐1,2‐diol could produce apoptosis in the developing central nervous system (Lau et al., 2012). The investigation was performed in mice of different age (postnatal day (PND) 4, 7, 14, 17, 24 and 30). Activated caspase‐3 (Ac‐3) was measured in the brain as this seemed to be a sensitive measure of apoptosis. At 1 mL propane‐1‐2‐diol (1,036 mg)/kg bw, there was no significant difference in Ac‐3 cell counts between propane‐1,2‐diol treated cells and saline animals. Compared to saline, 2 mL propane‐1‐2‐diol (2,072 mg)/kg bw induced a statistically significantly, but less than 10% increase in the number of apoptotic neurons. The highest number of propane‐1,2‐diol‐induced apoptotic cells was measured when mice were exposed on PND 7. The authors concluded that further research was needed to determine the underlying mechanism.
3.6. Discussion
Propane‐1,2‐diol was previously evaluated by the SCF and JECFA, and both committees established an ADI of 25 mg/kg bw per day.
Some by products (mono‐ and di‐ethylene glycol) might be formed next to residual propylene oxide used as starting material. The Panel noted that there are not maximum limits for propylene oxide and mono‐ and di‐ethylene glycol in the EU specifications for propane‐1,2‐diol (E 1520). Additionally, propane‐1,2‐diol can be manufactured by using as a raw material propylene carbonate, which may not be completely removed from the final product. The Panel noted that no maximum limits for propylene carbonate are set in EU specifications for E 1520.
Propane‐1,2‐diol is readily absorbed from the gastrointestinal tract in experimental animals and in humans and is expected to be widely distributed to organs and tissues. The major route of metabolism is oxidation to lactic acid and pyruvic acid. An alternative route of metabolism of propane‐1,2‐diol to lactic acid is via phosphorylated glycol. Lactate is mainly metabolised via the citric acid cycle and excreted as carbon dioxide via exhalation. At high concentrations, free propane‐1,2‐diol is excreted in the urine as the elimination of propane‐1,2‐diol from the body is saturated at dose levels higher than 20,000 mg/day in humans. The renal clearance in young infants up to the age of approx. 3 months appeared to be much lower than in adults.
The acute oral toxicity of propane‐1,2‐diol was low.
No treatment‐related effects were observed in subchronic toxicity studies in which propane‐1,2‐diol was administered by gavage (1,000 mg/kg bw per day) to mice, rats, dogs and monkeys for 92–97 days (Thackaberry et al., 2010).
The Panel considered that the available data did not raise concern with respect to genotoxicity of propane‐1,2‐diol.
Except for haematological changes suggestive of an increased red blood cell destruction with a compensatory increased rate of haematopoiesis at the highest dose level (5,000 mg/kg bw per day), no adverse effects were observed in a 2‐year study in dogs administered propane‐1,2‐diol in the diet (2,000 or 5,000 mg/kg bw per day) (Weil et al., 1971). No adverse effects, including neoplastic findings, were reported in a chronic study in rats administered propane‐1,2‐diol in the diet (up to 2,500 mg/kg bw per day) for 2 years (Gaunt et al., 1972). The SCF and JECFA used the study by Gaunt et al. (1972) in rats to derive an ADI of 25 mg/kg bw per day using an uncertainty factor of 100.
No adverse effects on reproductive toxicity parameters of propane‐1,2‐diol up to 5% in drinking water (equal to 10,118 mg propane‐1,2‐diol/kg bw per day) were observed in a continuous breeding reproduction study in mice (Morrissey et al., 1989; Lamb et al., 1997) or in a fertility study in male and female rats given propane‐1,2‐diol (1,000 mg/kg bw per day) daily by gavage (Enright et al., 2010). No adverse maternal or developmental effects were observed in prenatal developmental toxicity studies with mice, rats, hamsters and rabbits given propane‐1,2‐diol by oral gavage at dose levels up to 1,600, 1,600, 1,550 and 1,230 mg/kg bw per day, respectively, on GD 6–15, 6–15, 6–10 and 6–18, respectively (FDRL 1973 (Documentation provided to EFSA n. 5)). In an additional developmental toxicity study in mice, no adverse effect was observed in pregnant females dosed daily by gavage from GD 6‐15 up to 10,360 mg propane‐1,2‐diol/kg bw per day (Union Carbide, 1993 (Documentation provided to EFSA n. 14)). In other prenatal developmental toxicity studies by gavage of 1,000 mg/kg bw per day (the only dose tested) from GD 6 to 17 in rats or in rabbits from GD 7 to 19 no effects were observed (Enright et al., 2010).
Based on the overall toxicity database, the Panel considered that there was no reason to revise the current ADI of 25 mg/kg bw per day for propane‐1,2‐diol (E 1520).
Propane‐1,2‐diol (E 1520) is authorised according to Annex III in some food additives, food flavourings, enzymes and nutrients and it is then carried over to the final food. Dietary exposure to the food additive was assessed based on data received by both industry (use levels) and Member States (analytical data). In the refined exposure assessment scenario, 20 food categories were taken into account. The data submitted to EFSA were consistent with information from the Mintel's GNPD. However, since the labelling of propane‐1,2‐diol (E 1520) is not mandatory, the information about the use of this food additive from the Mintel's GNPD is very likely not exhaustive.
The exposure assessment was hampered by several uncertainties (Table 5), overall the Panel considered that for the food categories for which information was available, the exposure was likely to be overestimated. For an elaborate discussion of the uncertainties, see Section 3.4.1. The Panel considered that the brand‐loyal scenario covering the general population was the most appropriate scenario for risk characterisation of E 1520 as possible brand‐loyalty to food belonging to the food category ‘Flavoured drinks’ was identified. This scenario assumed that the population is likely to be exposed long‐term to the food additive present at the maximum reported use in foods belonging to the food category ‘Flavoured drinks’ and at the mean reported use/analytical data in foods belonging to the other food categories. The highest high level of exposure (P95) to propane‐1,2‐diol (E 1520) according to this scenario was estimated at 23.3 mg/kg bw per day in children.
The Panel noted that the refined exposure estimates are based on information provided on the reported level of use of food additive (E 1520) and analytical data. If actual practice changes this refined estimates may no longer be representative and should be updated.
The Panel also noted that propane‐1,2‐diol could be formed during the metabolism of other food additives (e.g. E 405, E 477). In this opinion, a combined exposure assessment was not performed.
4. Conclusions
Considering the overall toxicity database, the Panel concluded that there was no reason to revise the current ADI of 25 mg/kg bw per day for propane‐1,2‐diol (E 1520).
The Panel concluded that the mean and the high exposure levels (P95) of the brand‐loyal refined exposure scenario did not exceed the ADI in any of the population groups from the use of propane‐1,2‐diol (E 1520) as a food additive at the reported use levels and analytical results.
5. Recommendations
The Panel recommended that:
the European Commission considers lowering the current maximum limits for lead in the EU specification for propane‐1,2‐diol (E 1520) in order to ensure that propane‐1,2‐diol (E 1520) as food additives will not be a significant source of exposure to this toxic element in food.
the European Commission considers inclusion of maximum limits for propylene oxide, mono‐ and di‐ethylene glycol, and propylene carbonate in the EU specifications for propane‐1,2‐diol (E 1520).
the need for a combined exposure assessment to propane‐1,2‐diol from E 1520 and from other food additives from which propane‐1,2‐diol is formed through metabolism should be considered.
Documentation provided to EFSA
AGRUPOST (Spanish Association of Postharvest Services and Processes), 2016. Data on usage levels of propane‐1,2‐diol (E 1520) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption. Submitted to EFSA on 31 May 2016.
CEFIC (European Chemical Industry Council), 2012. Dossier for the re‐evaluation of propylene glycol as food additive. Submitted by CEFIC, 9 December 2012.
Dow Chemical, 1979. Results of a toxicological study in cats fed diets containing proplyene glycol for up to three months. Quast JF, Humiston CG, Wade CE, Beyer JE, Albee RR, Schuetz DJ and Morden DC. Reviewed by/kociba RJ. Toxicological Research Laboratory Health and Enviroment Sciences, USA. Dow Chemical ISA. Midland, Michigan, USA. Submitted by CEFIC, 11 January 2016.
Dow‐Chemical‐Company, 1995. Metabolism of tripropylene glycol in rats. Authors: Mendrala AL and McNett DA, The Toxicology Research Laboratory, Health and Environmental Sciences, The Dow Chemical Company, Midland, Michigan 48674, United States, 1–36. Submitted by CEFIC, 9 December 2012.
FDRL (Food and Drug Research Laboratories, Inc.), 1973. Teratologic evaluation of FDA 71‐56 (propylene glycol) in mice, rats, hamsters and rabbits. Waverely (NY): Food and Drug Research Laboratories, Inc. Submitted by the FDA, 27 October 2017.
FDE (FoodDrinkEurope), 2016. Data on usage levels of propane‐1,2‐diol (E 1520) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption. Submitted to EFSA on 31st May 2016.
Huntingdon‐Research‐Centre, 1990. Propylene glycol. Metaphase chromosome analysis of human lymphocytes cultured in vitro. Authors: Brooker PC, Akhurst LC and Gray VM, Huntingdon Research Centre Ltd., 1‐26. Submitted by CEFIC, 9 December 2012.
ICGA (International Chewing Gum Association), 2016. Data on usage levels of propane‐1,2‐diol (E 1520) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption. Submitted to EFSA on 31st May 2016.
KRÜGER (KRÜGER GmbH & Co. KG), 2016. Data on usage levels of propane‐1,2‐diol (E 1520) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption. Submitted to EFSA on 25 May 2016.
LittonBionetics Inc., 1974. Mutagenic evaluation of compound FDA 71‐56. Propylene glycol. Contract FDA 71–268, 1–88. Submitted by CEFIC, 9 December 2012.
NTP (National Toxicological Program), 2004. NTP Thechnical report on the toxicology and carcinogenisis studies of dipropylene glycol (CAS No. 25265‐71‐8) in F344/N rats and B6C3F1 mice (drinking water studies). PO Box 12233. Research Triangle Park, NC 27709. NTP TR 511. Submitted by CEFIC, 11 January 2016.
Pre‐evaluation document prepared by DTU, March 2014.
SNE (Specialised Nutrition Europe), 2016. Data on usage levels of propane‐1,2‐diol (E 1520) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption. Submitted to EFSA on 30 May 2016.
Union Carbide, 1993. Propylene glycol: developmental toxicity gavage study III in CD‐1 mice. Authors: Driscoll CD, Kubena MF and Neeper‐Bradley TL, Bushy Run Research Center (BBRC), Union Carbide Chemicals and Plastics Company Inc. (UCC&P), 1‐466. Submitted by CEFIC, 9 December 2012.
Abbreviations
- Ac‐3
Activated caspase‐3
- ADI
Acceptable daily intake
- AGRUPOST
Spanish Association of Postharvest Services and Processes
- ANS
EFSA Scientific Panel on Food Additives and Nutrient Sources added to Food
- AOAC
Association of Official Agricultural Chemists
- AV
Acid value
- BBM
Brush border membrane
- BIOHAZ
EFSA Panel on Biological Hazards
- bw
Body weight
- CAS
Chemical Abstracts Service
- CEF
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CEFIC
EFSA Panel on Food Contact Material, Enzymes, Flavourings and Processing Aids
- CHMP
Committee for Medicinal Products for Human Use
- CIR
Cosmetic Ingredient Review
- CONTAM
EFSA Panel on Contaminants in Food Chain
- EINECS
European Inventory of Existing Chemical Substances
- EMA
European Agency for the Evaluation of Medicinal Products
- FAO
Food and Agriculture Organization of the United Nations
- FCS
Food categorisation system
- FDE
Food Drink Europe
- GC–MS
Gas chromatography–mass spectrometry
- GD
Gestation day
- GGT
Gamma‐glutamyl transpeptidase
- GLP
Good laboratory practice
- GOT
Glutamic‐oxaloacetic transaminase
- GNPD
Global New Products Database
- HPLC
High‐performance liquid chromatography
- ICGA
International Chewing Gum Association
- INRS
Institut National fe Recherche et de Sécurité
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LD50
Lethal dose, median
- LOD
Limit of detection
- LOQ
Limit of quantification
- MB
Middle‐bound
- MLD
Minimum lethal dose
- MPL
Maximum permitted level
- NAD+
Nicotinamide adenine dinucleotide
- NOAEL
No observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- PCE
Polychromatic erythrocyte
- PND
Postnatal day
- RACB
Reproductive Assessment by Continuous Breeding
- SCAN
Scientific Committee for Animal Nutrition
- SCE
Sister chromatid exchange
- SCF
Scientific Committee on Food
- SNE
Specialised Nutrition Europe
- TemaNord
Is a publishing series for results of the often research‐based work that working groups or projects under Nordic Council of Ministers have put in motion
- WHO
World Health Organization
- WoE
weight of evidence
Appendix A – Summary of reported use levels of propane‐1,2‐diol (E 1520) provided by industry (mg/kg)
Appendix B – Summary of reported use levels of propane‐1,2‐diol (E 1520) provided by Member States (mg/kg)
Appendix C – Number and percentage of food products labelled with propane‐1,2‐diol (E 1520) out of the total number of food products present in the Mintel GNPD per food sub‐category between 2013 and 2018
Appendix D – Concentration levels of propane‐1,2‐diol (E 1520) used in the refined exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Appendix E – Summary of total estimated exposure of propane‐1,2‐diol (E 1520) from its use as a food additive for the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Appendix F – Main food categories contributing to exposure to propane‐1,2‐diol (E 1520) at the refined exposure assessment scenarios (> 5% to the total mean exposure)
1.
Appendix Appendix A – Summary of reported use levels of propane‐1,2‐diol (E 1520) provided by industry (mg/kg), Appendix B – Summary of reported use levels of propane‐1,2‐diol (E 1520) provided by Member States (mg/kg), Appendix C – Number and percentage of food products labelled with propane‐1,2‐diol (E 1520) out of the total number of food products present in the Mintel GNPD per food sub‐category between 2013 and 2018, Appendix D – Concentration levels of propane‐1,2‐diol (E 1520) used in the refined exposure assessment scenarios (mg/kg or mL/kg as appropriate), Appendix E – Summary of total estimated exposure of propane‐1,2‐diol (E 1520) from its use as a food additive for the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day), Appendix F – Main food categories contributing to exposure to propane‐1,2‐diol (E 1520) at the refined exposure assessment scenarios (> 5% to the total mean exposure) can be found in the online version of this output (‘Supporting information’ section).
Supporting information
Summary of reported use levels of propane‐1,2‐diol (E 1520) provided by industry (mg/kg)
Summary of reported use levels of propane‐1,2‐diol (E 1520) provided by Member States (mg/kg)
Number and percentage of food products labelled with propane‐1,2‐diol (E 1520) out of the total number of food products present in the Mintel GNPD per food sub‐category between 2013 and 2018
Concentration levels of propane‐1,2‐diol (E 1520) used in the refined exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of propane‐1,2‐diol (E 1520) from its use as a food additive for the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to propane‐1,2‐diol (E 1520) at the refined exposure assessment scenarios (> 5% to the total mean exposure)
Suggested citation: EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) , Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Boon P, Chrysafidis D, Gürtler R, Mosesso P, Parent‐Massin D, Tobback P, Rincon AM, Tard A and Lambré C, 2018. Scientific Opinion on the re‐evaluation of propane‐1,2‐diol (E 1520) as a food additive. EFSA Journal 2018;16(4):5235, 40 pp. 10.2903/j.efsa.2018.5235
Requestor: European Commission
Question number: EFSA‐Q‐2011‐00591
Panel members: Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipič, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Gunter Georg Kuhnle, Claude Lambré, Jean‐Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The ANS Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 13 March 2018
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © Elsevier
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Commission Regulation (EU) No 257/2010 of 25 march 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
COM(2001) 542 final.
Food Additives in Europe 2000, Status of safety assessments of food additives presently permitted in the EU, Nordic Council of Ministers, TemaNord, 2002, 560.
Commission Regulation (EU) No 231/2012 of 9 march 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1–295.
Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. OJ L 12, 15.1.2011, p. 1.
Available online: http://ec.europa.eu/consumers/cosmetics/cosing/index.cfm?fuseaction=search.simple
Call for scientific data on miscellaneous food additives permitted in EU and belonging to several functional classes. Published: 6 July 2012. Available online: http://www.efsa.europa.eu/sites/default/files/consultation/120706a.pdf
Call for food additives usage level and/or concentration data in food and beverages intended for human consumption. Published: 12 October 2015. Available online: http://www.efsa.europa.eu/en/data/call/151012
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
SciFinder® the choice for chemistry researchTM.
Missing Bulgaria, Cyprus, Estonia, Latvia, Lithuania, Luxembourg, Malta and Slovenia.
http://www.gnpd.com/sinatra/home/ accessed on 10/01/2018.
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
Information provided by industry: It is part of a food coating formulation. Fresh fruits and vegetables taken into account are: citrus (all citrus fruits were included), apple, pears, melon, peach, pineapple, pomegranate, mango, avocado, berries, papaya, potato.
References
- AOAC (Association of Official Agricultural Chemists), 2000. AOAC Official method 947.09 Propylene glycol in vanilla extract. Titrimetric method.
- ASTDR (Agency for Toxic Substances and Disease Registry), 1997. Toxicological profile for propylene glycol. [PubMed]
- Aye M, Di Giorgio C, De Mo M, Botta A, Perrin J and Courbiere B, 2010. Assessment of the genotoxicity of three cryoprotectants used for human oocyte vitrification: dimethyl sulfoxide, ethylene glycol and propylene glycol. Food and Chemical Toxicology, 48, 1905–1912. [DOI] [PubMed] [Google Scholar]
- Badertscher R, Freiburghaus C, Wechsler D, Irmler S, 2017. Validated method for the determination of propane‐1,2‐diol, butane‐2,3‐diol and propane‐1,3‐diol in cheese and bacterial cultures using phenylboronic esterification and GC‐MS. Food Chemistry, 230, 372–377. [DOI] [PubMed] [Google Scholar]
- Bauer MC, Weiss DJ and Perman V, 1992a. Hematologic alterations in adult cats fed 6 or 12% propylene glycol. American Journal of Veterinary Research, 53, 69–72. [PubMed] [Google Scholar]
- Bauer MC, Weiss DJ and Perman V, 1992b. Hematological alterations in kittens induced by 6 and 12% dietary propylene glycol. Veterinary and Human Toxicology, 34, 127–131. [PubMed] [Google Scholar]
- Berthelot‐Ricou A, Perrin J, di Giorgio C, de Meo M, Botta A and Courbiere B, 2011. Assessment of 1,2‐propanediol (PrOH) genotoxicity on mouse oocytes by comet assay. Fertility and Sterility, 96, 1002–1007. [DOI] [PubMed] [Google Scholar]
- Bolon B, Bucci TJ, Warbritton AR, Chen JJ, Mattison DR and Heindel JJ, 1997. Differential follicle counts as a screen for chemically induced ovarian toxicity in mice: results from continuous breeding bioassays. Fundamental and Applied Toxicology, 39, 1–10. [DOI] [PubMed] [Google Scholar]
- Bozga ER, Plesu V, Bozga G, Bildea CS and Zaharia E, 2011. Conversion of glycerol to propanediol and acrolein by heterogeneous catalysis. Chemical Reviews, 62, 646–654. [Google Scholar]
- Braun HA and Cartland GF, 1936. The toxicity of propylene glycol. Journal of the American Pharmaceutical Association, 1912–1977, 746–749. [Google Scholar]
- Buchanan JS, Jiang Z, Kowalski JA and Santiesteban JG, 2002. Integrated process for preparing dialkyl carbonates and diols. US Patent 6,407,279.
- Christopher MM, Perman V, White JG and Eaton JW, 1989a. Propylene glycol‐induced Heinz body formation and D‐lactic acidosis in cats. Progress in Clinical and Biological Research, 319, 69–92. [PubMed] [Google Scholar]
- Christopher MM, Perman V and Eaton JW, 1989b. Contribution of propylene glycol‐induced Heinz body formation to anemia in cats. Journal of the American Veterinary Medical Association, 194, 1045–1056. [PubMed] [Google Scholar]
- Christopher MM, White JG and Eaton JW, 1990. Erythrocyte pathology and mechanisms of Heinz body‐mediated hemolysis in cats. Veterinary Pathology, 27, 299–310. [DOI] [PubMed] [Google Scholar]
- CIR (Cosmetic Ingredient Review), 2011. Final Report of the Cosmetic Ingredient Review Expert Panel. On the Safety Assessment of 1,2‐Glycols as Used in Cosmetics. June 2011.
- Clark CR, Marshall TC, Merickel BS, Sanchez A, Brownstein DG and Hobbs CH, 1979. Toxicological assessment of heat transfer fluids proposed for use in solar energy applications. Toxicology and Applied Pharmacology, 51, 529–535. [DOI] [PubMed] [Google Scholar]
- De Cock RFW, Knibbe CAJ, Kulo A, de Hoon J, Verbesselt R, Danhof M and Allegaert K, 2013. Developmental pharmacokinetics of propylene glycol in preterm and term neonates. British Journal of Clinical Pharmacology, 75, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cock RF, Allegaert K, Vanhaesebrouck S, de Hoon J, Verbesselt R, Danhof M and Knibbe CA, 2014. Low but inducible contribution of renal elimination to clearance of propylene glycol in preterm and term neonates. Therapeutic Drug Monitoring, 36, 278–287. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Scientific opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel and CEF Panel (EFSA Panel on Biological Hazards and EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2012. Scientific Opinion on the evaluation of the safety and efficacy of Cecure® for the removal of microbial surface contamination of raw poultry products. EFSA Journal 2012;10(3):2612, 66 pp. 10.2903/j.efsa.2012.2612 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2011. Scientific Opinion on the evaluation of the substances currently on the list in the Annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part I of III. EFSA Journal 2011;9(12):2482 61 pp. 10.2903/j.efsa.2011.2482 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012. Scientific Opinion on lead dietary exposure in the European population. EFSA Journal 2012;10(7):2831, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- El‐Wahab HMFA and Moram GSE‐D, 2013. Toxic effects of some synthetic food colorants and/or flavor additives on male rats. Toxicology and Industrial Health, 29, 224–232. [DOI] [PubMed] [Google Scholar]
- EMA (European Medicines Agency), 1996. Propylene glycol, Summary report. EMEA/MRL/130/96‐FINAL, 1‐3. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2014/03/WC500163989.pdf
- EMA (European Medicines Agency), 2014a. Assessment report. Propylene glycol in medicinal products for children. EMA/175205/2014. Committee for Medicinal Products for Human Use (CHMP). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2014/03/WC500163989.pdf
- EMA (European Medicines Agency), 2014b. Questions & answers on propylene glycol and esters in the context of the revision of the guideline on ‘Excipients in the label and package leaflet of medicinal products for human use’ (CPMP/463/00 Rev. 1). November 2014.
- Enright BP, McIntyre BS, Thackaberry EA, Treinen KA and Kopytek SJ, 2010. Assessment of hydroxypropyl methylcellulose, propylene glycol, polysorbate 80, and hydroxypropyl‐β‐cyclodextrin for use in developmental and reproductive toxicology studies. Birth Defects Research Part B: Developmental and Reproductive Toxicology, 89, 504–516. [DOI] [PubMed] [Google Scholar]
- Fellows JK, Luduena FP and Hanzlik PJ, 1947. Glucuronic acid excretion after diethylene glycol monoethyl ether (carbitol) and some other glycols. Journal of Pharmacology, 89, 210–213. [PubMed] [Google Scholar]
- Florin I, Rutberg L, Curvall M and Enzell CR, 1980. Screening of tobacco smoke constituents for mutagenicity using the Ames’ test. Toxicology, 15, 219–232. [DOI] [PubMed] [Google Scholar]
- Fowles JR, Banton MI and Pottenger LH, 2013. A toxicological review of propylene glycols. Critical Reviews in Toxicology, 43, 363–390. [DOI] [PubMed] [Google Scholar]
- Gaunt IF, Carpanini FMB, Grasso P and Lansdown ABG, 1972. Long‐term toxicity of propylene glycol in rats. Food and Cosmetics Toxicology, 10, 151–162. [DOI] [PubMed] [Google Scholar]
- Hamano T, Mitsuhashi Y, Tanaka K, Matsuki Y, Nukina M, Oji Y, Okamoto S, 1984. Enzymatic determination of propylene glycol in commercial foods. Agricultural and Biological Chemistry, 48, 2517–2521. [Google Scholar]
- Hanzlik PJ, Newman HW, van Winkle WJ, Lehman AJ and Kennedy NK, 1939a. Toxicity, fate and excretion of propylene glycol and some other glycols. Journal of Pharmacology, 67, 101–113. [Google Scholar]
- Haworth S, Lawlor T, Mortelmans K, Speck W and Zeiger E, 1983. Salmonella mutagenicity test results for 250 chemicals. Environmental Mutagenesis, 5, 3–142. [PubMed] [Google Scholar]
- Hayashi M, Kishi M, Sofuni T and Ishidate M Jr, 1988. Micronucleus tests in mice on 39 food additives and eight miscellaneous chemicals. Food and Chemical Toxicology, 26, 487–500. [DOI] [PubMed] [Google Scholar]
- Hickman MA, Rogers QR and Morris JG, 1990. Effect of diet on Heinz body formation in kittens. American Journal of Veterinary Research, 51, 475–478. [PubMed] [Google Scholar]
- Holck HGO, 1937. Glycerol, ethylene glycol, propylene glycol and diethylene glycol. Report on feeding experiments with rats. Journal of the American Medical Association, 109, 1517–1520. [Google Scholar]
- Holtbruegge J, Kuhlmann H and Lutze P, 2015. Process analysis and economic optimization of intensified process alternatives for simultaneous industrial scale production of dimethyl carbonate and propylene glycol. Chemical, Engineering Research and Design, 93, 411–431. [Google Scholar]
- INRS (Institut National fe Recherche et de Sécurité), 2010. Propylène‐glycol. Fiche toxicologique. Available online: http://www.inrs.fr/publications/bdd/fichetox/fiche.html?refINRS=FICHETOX_226
- Ishidate MJ, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M and Matsuoka A, 1984. Primary mutagenicity screening of food additives currently used in Japan. Food and Chemical Toxicology, 22, 623–636. [DOI] [PubMed] [Google Scholar]
- Jacob SE, Scheman A and McGowan MA, 2017. Allergen of the year: propylene glycol. Dermatitis, 00, 1–3. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO, WHO Expert Committee on Food Additives), 1974. 1,2‐propylene glycol. Toxicological evaluation of some additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. WHO Food Additives Series, 5, 491–495. [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002a. Aliphatic acyclic diols, triols, and related substances. Safety evaluation of certain food additives and contaminants. Prepared by the fifty‐seventh meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additives Series, 48.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002b. Evaluation of certain food additives and contaminants. Fifty‐seventh Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, 1–171.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Monograph 1. Combined compendium of food additive specifications. All specifications monographs from the 1st to the 65th meeting (1956‐2005). Volume 4. Available online: http://www.fao.org/ag/agn/jecfa-additives/search.html
- Kavlock RJ, Short RDJ and Chernoff N, 1987. Further evaluation of an in vivo teratology screen. Teratogenesis, Carcinogenesis, and Mutagenesis, 7, 7–16. [DOI] [PubMed] [Google Scholar]
- Kawachi T, Komatsu T, Kada T, Ishidate M, Sasaki M, Sugiyama T and Tazima Y, 1980. Results of recent studies on the relevance of various short‐term screening tests in Japan In: Kroes R, Waaijers HW, van der Poll KW. and Williams GM. (eds.). The Predictive Value of Short‐term Screening Tests in Carcinogenicity Evaluation. Elsevier/North Holland Biomedical Press; pp. 253–267. [Google Scholar]
- Kutz H, Kuenz A, Prüße U, Willke T and Vorlop KD, 2017. Products components: alcohols. Advances in Biochemical Engineering/Biotechnology, 10.1007/10_2016_74 [DOI] [PubMed] [Google Scholar]
- LaKind JS, McKenna EA, Hubner RP and Tardiff RG, 1999. A review of the comparative mammalian toxicity of ethylene glycol and propylene glycol. Critical Reviews in Toxicology, 29, 331–365. [DOI] [PubMed] [Google Scholar]
- Lamb JC, Gulati DK, Barnes LH and Welch M, 1997. Propylene glycol. Envir Health Perspect, 105(suppl 1), 231–232. [PMC free article] [PubMed] [Google Scholar]
- Latven AR and Molitor H, 1939. Comparison of the toxic, hypnotic and irritating properties of eight organic solvents. Journal of Pharmacology, 65, 89–94. [Google Scholar]
- Lau K, Swiney BS, Rreves N, Noguchi KK and Farber NB, 2012. Propylene glycol produces excessive apoptosis in the developing mouse brain, alone and in combination with phenobarbital. Pediatr Res, 71, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laug EP, Calvery HO, Morris HJ and Woodward G, 1939. The toxicology of some glycols and derivatives. Journal of Industrial Hygiene and Toxicology, 21, 173–201. [Google Scholar]
- Lehman AJ and Newman HW, 1937. Propylene glycol; rate of metabolism, absorption and excretion, with a method for estimation in body fluids. Journal of Pharmacology, 60, 312–322. [Google Scholar]
- Matusik JE, Eilers PP, Waldron EM, Conrad SM and Sphon JA, 1993. Confirmation of identities of propylene and ethylene glycols in anchovies by tandem mass spectroscopy. Journal of AOAC International, 67, 1344–1347. [PubMed] [Google Scholar]
- Mc Gowan MA, Scheman A and Jacob SE, 2017. Propylene glycol in contact dermatitis. A systematic review. Dermatitis, 00, 1–7. [DOI] [PubMed] [Google Scholar]
- McCann J and Ames BN, 1976. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals: discussion. Proceedings of the National Academy of Sciences of the United States of America, 73, 950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S, Arifa AA and Biswas P, 2017. Production of 1,2‐propanediol from renewable glycerol over highly stable and efficient Cu‐Zn(4:1)/MgO catalysts. Catalysis Letters, 147, 2783–2798. 10.1007/s10562-017-2187-1 [DOI] [Google Scholar]
- Morris HJ, Nelson AA and Calvery HO, 1942. Chronic toxicities of propylene glycol, ethylene glycol, diethylene glycol, ethylene glycol monoethyl ether and diethylene glycol monoethyl ether. Journal of Pharmacology, 74, 266–273. [Google Scholar]
- Morrissey RE, Lamb JC IV, Morris RW, Chapin RE, Gulati DK and Heindel JJ, 1989. Results and evaluations of 48 continuous breeding reproduction studies conducted in mice. Fundamental and Applied Toxicology, 13, 747–777. [DOI] [PubMed] [Google Scholar]
- Morshed KM and Nagpaul JP, 1995. Propylene glycol‐induced changes in plasma and mucosal functions in rats: evidence of subchronic toxicity. Toxic Substance Mechanisms, 14, 13–25. [Google Scholar]
- Morshed KM, Nagpaul JP, Majumdar S and Amma MKP, 1988. Kinetics of propylene glycol elimination and metabolism in rat. Biochemical Medicine and Metabolic Biology, 39, 90–97. [DOI] [PubMed] [Google Scholar]
- Morshed KM, L'Helgoualch A, Nagpaul JP, Amma MKP and Desjeux JF, 1991a. The role of propylene glycol metabolism in lactatemia in the rabbit. Biochemical Medicine and Metabolic Biology, 46, 145–151. [DOI] [PubMed] [Google Scholar]
- Morshed KM, Desjeux JF, Nagpaul JP, Majumdar S and Amma MK, 1991b. The effect of propane‐diols on the intestinal uptake of nutrients and brush border membrane enzymes in the rat. Biochemical Medicine and Metabolic Biology, 45, 161–170. [DOI] [PubMed] [Google Scholar]
- NTP (National toxicology program), 1985. Propylene glycol: reproduction and fertility assessment in CD‐1 mice when administered in drinking water. Report by Gulati DK, Barnes LH and Lamb IV JC. Contract no.: NO1‐ES‐2‐5013 NTP‐84FACB‐038 (CAS No. 57‐55‐6).
- OECD (Organisation for Economic Co‐operation and Development), 2014. OECD Guidelines for testing of chemicals, section 4. Test No 474: Mammalian Erythocyte micronucleus test. Adopted 29 July 2016.
- Pfeiffer EH and Dunkelberg H, 1980. Mutagenicity of ethylene oxide and propylene oxide and of the glycols and halohydrins formed from them during the fumigation of foods. Food and Cosmetics Toxicology, 18, 115–118. [DOI] [PubMed] [Google Scholar]
- Pudi SM, Zoeb A, Biswas P and Kumar S, 2015. Liquid phase conversion of glycerol to propanediol over highly active copper/magnesia catalysts. Journal of Chemical Sciences, 127, 833–842. [Google Scholar]
- Ruddick JA, 1972. Toxicology, metabolism, and biochemistry of 1,2‐propanediol. Toxicology and Applied Pharmacology, 21, 102–211. [DOI] [PubMed] [Google Scholar]
- Sagadykova GN, Alimzhanova MB, Nurzhanova YT and Kenessov B, 2017. Determination of semi‐volatile additives in wines using SPME and GC‐MS. Food Chemistry, 220, 162–167. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Sugimura K, Yoshida MA and Abe S, 1980. Cytogenetic effects of 60 chemicals on cultured human and Chinese hamster cells. La Kromosomo II, 20, 574–584. [Google Scholar]
- SCAN (Scientific Committee for Animal Nutrition), 1991. Report of the Scientific Committee for Animal Nutrition on the use of Propane‐1,2‐diol (Propylene glycol; 1,2‐Propanediol), as a feed additive in the feedstuffs for cats. Provisional opinion expressed: 15 January 1991.
- SCF (Scientific Committee for Food), 1978. Report of the Scientific Committee for Food on the provisions relating to additives and processing aids in the draft proposal for a council directive concerning the approximation of the laws of Member States relating to fine bakers’ wares, rusks, pastries and biscuits. (Opinion expressed on 1st May 1978). Reports of the Scientific Committee for Food, Fifth series. 1–25.
- SCF (Scientific Committee for Food), 1981. Reports of the Scientific Committee for Food, Eleventh series. 1–49.
- SCF (Scientific Committee for Food), 1996. Opinion on propylene glycol (opinion expressed on 9 December 1993). Reports of the Scientific Committee for Food, Thirty‐fifth series, 1–3.
- SCF (Scientific Committee for Food), 1997. Opinion on propane‐1,2‐diol (Opinion expressed on 20 September 1996). Reports of the Scientific Committee for Food, Fortieth series, 63–64.
- SCF (Scientific Committee for Food), 1999. Compilation of the evaluations of the Scientific Committee for Food on certain monomers and additives used in the manufacture of plastics materials intended to come into contact with foodstuffs until 21 march 1997. Reports of the Scientific Committee for Food, Forty‐second series 99.
- SCF (Scientific Committee for Food), 2001. Guidance on submissions for food additive evaluations by the Scientific Committee on Food. SCF/CS/ADD/GEN/26 Final. 12 July 2001.
- Seidenfeld MA and Hanzlik PJ, 1932. The general properties, actions and toxicity of propylene glycol. Journal of Pharmacology, 44, 109–121. [Google Scholar]
- Sjöberg P, Bondesson U, Gray TJ and Plöen L, 1986. Effects of di‐(2‐ethylhexyl) phthalate and five of its metabolites on rat testis in vivo and in in vitro. Acta Pharmacology and Toxicology, 58, 225–233. [DOI] [PubMed] [Google Scholar]
- Smyth HFJ, Seaton J and Fischer L, 1941. The single‐dose toxicity of some glycols and derivatives. Journal of Industrial Hygiene and Toxicology, 23, 259–268. [Google Scholar]
- Speth PA, Vree TB, Neilen NF, de Mulder PH, Newell DR, Gore ME and de Pauw BE, 1987. Propylene glycol pharmacokinetics and effects after intravenous infusion in humans. Therapeutic Drug Monitoring, 9, 255–258. [DOI] [PubMed] [Google Scholar]
- Stolzenberg SJ and Hine CH, 1979. Mutagenicity of halogenated and oxygenated three‐carbon compounds. Journal of Toxicology and Environmental Health, 5, 1149–1158. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Dohi T, Aizawa SI, Kemmei T, Terashima H, Taga A, Yamamoto A and Kodama S, 2017. Simultaneous determination of alcohols including diols and triols by HPLC with ultraviolet detection based on the formulation of a copper(II) complex. Journal of Separation Science, 40, 4168–4175. [DOI] [PubMed] [Google Scholar]
- TemaNord (Nordic Council of Ministers), 2002. E 475 Polyglycerol esters of fatty acids. Food Additives in Europe 2000 ‐ Status of safety assessments of food additives presently permitted in the EU, 668‐670.
- Thackaberry EA, Kopytek S, Sherratt P, Trouba K and McIntyre B, 2010. Comprehensive investigation of hydroxypropyl methylcellulose, propylene glycol, polysorbate 80, and hydroxypropyl‐beta‐cyclodextrin for use in general toxicology studies. Toxicological Sciences, 117, 485–492. [DOI] [PubMed] [Google Scholar]
- Thomas JF, Kesel R and Hodge HC, 1949. Range‐finding toxicity tests on propylene glycol in the rat. Journal of Industrial Hygiene and Toxicology, 31, 256–257. [PubMed] [Google Scholar]
- Vaille C, Debray C, Roze C, Souchard M and Martin E, 1971. Hyperglycemic action of propylene glycol. Annales Pharmaceutiques Francaises, 29, 577–582. [PubMed] [Google Scholar]
- Weatherby JH and Haag HB, 1938. Toxicity of propylene glycol. Journal of the American Pharmaceutical Association, 1912–1977, 466–471. [Google Scholar]
- Weil CS, Woodside MD, Smyth HFJ and Carpenter CP, 1971. Results of feeding propylene glycol in the diet to dogs for two years. Food and Cosmetics Toxicology, 9, 479–490. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2009. Principles and Methods for the Risk Assessment of Chemicals in Food. Environment Health Criteria 240. Available online: http://www.who.int/foodsafety/publications/chemical-food/en/
- Williamson SA and Iverson WG, 1993. Determination of short‐chain diols and selected fermentation by‐products in beer. American Society of Brewing Chemist, Inc, 51, 114–118. [Google Scholar]
- van Winkle WJ, 1941a. Quantitative gastrointestinal absorption and renal excretion of propylene glycol. Journal of Pharmacology and Experimental Therapeutics, 72, 344–353. [Google Scholar]
- van Winkle WJ, 1941b. Toxicity and actions of trimethylene glycol. Journal of Pharmacology, 72, 227–232. [Google Scholar]
- van Winkle WJ and Newman HW, 1941. Further results of continued administration of propylene glycol. Food Research, 6, 509–516. [Google Scholar]
- Yu DK, Elmquist WF and Sawchuk RJ, 1985. Pharmacokinetics of propylene glycol in humans during multiple dosing regimens. Journal of Pharmaceutical Sciences, 74, 876–879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of reported use levels of propane‐1,2‐diol (E 1520) provided by industry (mg/kg)
Summary of reported use levels of propane‐1,2‐diol (E 1520) provided by Member States (mg/kg)
Number and percentage of food products labelled with propane‐1,2‐diol (E 1520) out of the total number of food products present in the Mintel GNPD per food sub‐category between 2013 and 2018
Concentration levels of propane‐1,2‐diol (E 1520) used in the refined exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of propane‐1,2‐diol (E 1520) from its use as a food additive for the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to propane‐1,2‐diol (E 1520) at the refined exposure assessment scenarios (> 5% to the total mean exposure)


