Motivational impairments are common in depression and have been linked to deficits in reward processing. Whitton et al. report that markers of reward-related ventrostriatal dopamine function may be useful for identifying depressed individuals likely to respond favourably to a dopaminergic antidepressant.
Keywords: reward learning, depression, dopamine, striatum, pramipexole
Abstract
The efficacy of dopamine agonists in treating major depressive disorder has been hypothesized to stem from effects on ventrostriatal dopamine and reward function. However, an important question is whether dopamine agonists are most beneficial for patients with reward-based deficits. This study evaluated whether measures of reward processing and ventrostriatal dopamine function predicted response to the dopamine agonist, pramipexole (ClinicalTrials.gov Identifier: NCT02033369). Individuals with major depressive disorder (n = 26) and healthy controls (n = 26) (mean ± SD age = 26.5 ± 5.9; 50% female) first underwent assessments of reward learning behaviour and ventrostriatal prediction error signalling (measured using functional MRI). 11C-(+)-PHNO PET before and after oral amphetamine was used to assess ventrostriatal dopamine release. The depressed group then received open-label pramipexole treatment for 6 weeks (0.5 mg/day titrated to a maximum daily dose of 2.5 mg). Symptoms were assessed weekly, and reward learning was reassessed post-treatment. At baseline, relative to controls, the depressed group showed lower reward learning (P = 0.02), a trend towards blunted reward-related prediction error signals (P = 0.07), and a trend towards increased amphetamine-induced dopamine release (P = 0.07). Despite symptom improvements following pramipexole (Cohen’s d ranging from 0.51 to 2.16 across symptom subscales), reward learning did not change after treatment. At a group level, baseline reward learning (P = 0.001) and prediction error signalling (P = 0.004) were both associated with symptom improvement, albeit in a direction opposite to initial predictions: patients with stronger pretreatment reward learning and reward-related prediction error signalling improved most. Baseline D2/3 receptor availability (P = 0.02) and dopamine release (P = 0.05) also predicted improvements in clinical functioning, with lower D2/3 receptor availability and lower dopamine release predicting greater improvements. Although these findings await replication, they suggest that measures of reward-related mesolimbic dopamine function may hold promise for identifying depressed individuals likely to respond favourably to dopaminergic pharmacotherapy.
Introduction
Although several treatments are available for major depressive disorder, response rates are modest and highly varied. Half of patients fail to respond to first-line antidepressants (Levkovitz et al., 2011), and there are no consistently replicated, clinically meaningful predictors of response to specific classes of antidepressant medications. Finding ways to tailor treatment to a given individual is therefore an important step towards reducing the global burden of depression.
One approach is to subtype patients based on symptoms associated with specific underlying neurobiological features, to which personalized treatments can be directed. A promising target for subtyping depression is motivational disturbance, particularly anhedonia, which has been linked to poorer response to selective serotonin reuptake inhibitor treatment (McMakin et al., 2012), psychotherapy (McMakin et al., 2012), and transcranial magnetic stimulation (Downar et al., 2014), suggesting that anhedonic individuals may require alternative treatment approaches. Translational research has linked the reward and motivation-related deficits that characterize anhedonia to mesolimbic dopamine system dysfunction (Berridge and Kringelbach, 2015). For example, manipulating phasic dopamine neuron firing in the ventral tegmental area, which projects to the ventral striatum, alters anhedonic behaviour in rodents (Chaudhury et al., 2013). In psychiatrically healthy humans, PET imaging has shown that blunted ventrostriatal dopamine release is associated with decreased motivation to work for rewards (Treadway et al., 2012). Furthermore, ventrostriatal deep brain stimulation has been found to reduce anhedonia severity (Bewernick et al., 2010). Collectively, these findings suggest that for a subset of depressed individuals with prominent anhedonia, a treatment that specifically targets ventrostriatal dopamine may be warranted. However, to achieve this level of treatment precision, valid indicators of anhedonia-related ventrostriatal dopamine dysfunction are required.
Reward learning is a measure that correlates with mesolimbic dopamine function (Steinberg et al., 2013) and may be useful for identifying individuals likely to benefit from dopaminergic pharmacotherapy. It is the process by which behaviour is updated based on prior reinforcement, and is guided by phasic dopamine neuron firing that encodes differences between anticipated and actual rewards, known as reward prediction errors (Glimcher, 2011). Reward learning is impaired in major depressive disorder, particularly among anhedonic individuals (Pizzagalli et al., 2008b; Fletcher et al., 2015). Similarly, individuals with depression display blunted prediction error signals to reward in the ventral striatum (Kumar et al., 2008, 2018; but see Rutledge et al., 2017) and the extent of this blunting correlates with anhedonia (Greenberg et al., 2015). Further support for the importance of phasic dopamine firing in reward learning comes from studies showing that pharmacological challenges assumed to reduce phasic dopamine signalling disrupt reward learning (Pizzagalli et al., 2008a), whereas administering drugs that enhance striatal dopamine signalling improves reward learning (Der-Avakian et al., 2013; Pergadia et al., 2014). Together, these findings suggest that reward learning and prediction error signalling are both closely linked to ventrostriatal dopamine function, and may be useful for identifying depressed individuals who would benefit from a dopamine-targeting medication.
Pramipexole is a high-affinity D2/3 receptor agonist that may be suitable for treatment of anhedonia, as several randomized controlled trials have found it to be efficacious in treating major depressive disorder (Goldberg et al., 2004; Fawcett et al., 2016) as well as motivational symptoms in Parkinson’s disease (Drijgers et al., 2012). Building on our prior report focusing on cross-sectional abnormalities in ventrostriatal dopamine function in medication-naïve individuals with major depressive disorder (Schneier et al., 2018), we tested whether reward learning and ventrostriatal prediction error signalling prospectively predicted response to pramipexole. To directly assess the relationship between ventrostriatal dopamine function and response to pramipexole, we also examined whether baseline ventrostriatal dopamine release, measured using 11C-(+)-PHNO [11C-(+)-propyl-hexahydro-naphtho-oxazin, a D2/3 agonist] PET imaging in conjunction with oral amphetamine, predicted response to pramipexole. Given pramipexole’s known effects on striatal dopamine (Mierau and Schingnitz, 1992), we hypothesized that individuals showing impaired reward learning and blunted ventrostriatal prediction errors to reward would disproportionally benefit from pramipexole treatment (i.e. show greater depressive and anhedonic symptom improvement). Consistent with links between reward learning, ventrostriatal prediction error signalling and ventrostriatal dopamine function, we also expected that lower ventrostriatal dopamine release would predict greater response to pramipexole.
Materials and methods
Participants
Individuals with major depressive disorder (n = 26) and healthy controls (n = 26) were recruited from clinics at the New York State Psychiatric Institute and Icahn School of Medicine at Mount Sinai. Inclusion and exclusion criteria are outlined in the Supplementary material. Procedures were approved by both institutional review boards, and participants provided written informed consent prior to participating, in accordance with the Declaration of Helsinki. The Clinical trials registration can be found at https://clinicaltrials.gov/ct2/show/NCT02033369.
Clinical measures
Three outcome measures assessing depressive symptoms, anhedonia and clinical global improvement were administered weekly across 6 weeks of treatment: the 17-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960), the Snaith-Hamilton Pleasure Scale (SHAPS) (Snaith et al., 1995; Ameli et al., 2014), and the Clinical Global Impression-Change Scale (CGI) (Guy, 1976). Additional assessments are described in the Supplementary material.
Behavioural probabilistic reward task
Reward learning was assessed pre- and post-treatment using the Probabilistic Reward Task (PRT), which has been described in detail (Pizzagalli et al., 2008b). This task uses a differential reinforcement schedule to induce a response bias towards a more frequently rewarded (‘rich’) stimulus (see Supplementary material). Each trial began with a fixation cross (500 ms), followed by a schematic face without a mouth (500 ms). Next, a short (10 mm) or a long (11 mm) mouth was displayed (100 ms). Participants indicated whether the short or long mouth was presented. There were three blocks of 100 trials, and 40 correct trials in each block were followed by monetary reward (‘Correct! You won 20 cents’). Long and short mouths were presented with equal frequency; however, one of the lengths (the ‘rich stimulus’) was rewarded three times more frequently than the other (the ‘lean stimulus’). Participants were not informed of this contingency. Two versions were administered in a counterbalanced order from pre- to post-treatment: one where the mouth length varied and another where the nose length varied.
After quality control, signal detection analysis (Macmillan and Creelman, 1991) was used to calculate response bias (the tendency to bias responding to the rich stimulus). Reward learning (defined as block 3 − block 1 response bias) was evaluated as a predictor of treatment response.
Computational model
To unravel the mechanisms driving any observed association between reward learning and treatment response, we used a reinforcement learning model to compute two parameters for each individual: reward sensitivity and learning rate (see Supplementary material) (Huys et al., 2013). Higher reward sensitivity indicates greater subjective value of a reward, whereas greater learning rate indicates greater weight of immediate prior rewards on future decisions.
Imaging acquisition and analysis
Functional MRI reinforcement learning paradigm
Full details of the functional MRI acquisition, learning paradigm and analysis can be found elsewhere (Schneier et al., 2018) and in the Supplementary material. Scanning was conducted on a GE SIGNA 3 T scanner (GE Healthcare) with a 32-channel head coil. T1-weighted structural images (1 mm isotropic, 200 slices, field of view = 256 mm) and functional echo-planar images (repetition time = 2000 ms, echo time = 28 ms, flip angle = 77°, field of view = 19.2, 3 mm isotropic voxels, 40 slices) were acquired in six runs of 20 trials.
During functional MRI, participants performed a separate two-phase reinforcement learning task (Reinen et al., 2014) consisting of counterbalanced gain (winning money) and loss conditions (avoiding losing money from an endowment). On each trial, participants had to choose one of two shapes. After making a choice they received anticipatory feedback (‘correct’ or ‘incorrect’; 70/30 probability based on choice), followed by a monetary outcome. The trial staging allowed us to model prediction errors separately for anticipatory feedback and monetary outcomes. In the gain condition, ‘correct’ feedback triggered a $1 or $0.50 monetary gain (50/50 probability), whereas ‘incorrect’ feedback triggered a $0.50 or $0 monetary gain (50/50 probability). In the loss condition, correct feedback triggered a loss of $0 or $0.50 (50/50 probability), whereas incorrect feedback triggered a loss of $0.50 or $1 (50/50 probability). This design was used to equate the magnitude of both gain and loss prediction errors, while allowing for differences in motivational context.
Functional MRI analysis
A Q-learning model generated trial-by-trial prediction error values that were used as regressors for functional MRI analyses. Prediction error beta values generated from the general linear model were extracted from regions of interest in the left and right ventral striatum, defined by automated meta-analysis (neurosynth.org). A higher value for the gain prediction error beta indicates increased ventrostriatal activation for unexpected receipt of reward or better-than-expected feedback, and decreased activation for unexpected omission of reward or worse-than-expected feedback, in the gain condition. Conversely, a higher value for the loss prediction error beta indicates increased ventrostriatal activation for unexpected omission of loss or better-than-expected feedback, and decreased activation for unexpected receipt of loss or worse-than-expected feedback, in the loss condition. Eight prediction error variables were extracted: gain and loss prediction errors, under feedback and outcome conditions, in left and right ventral striatum. The four gain and four loss prediction errors were averaged to create a gain and a loss prediction error that were evaluated as predictors of treatment response.
PET imaging
The PET imaging methods are described in our prior report (Schneier et al., 2018). Subjects completed two 120-min 11C-(+)-PHNO PET scans (5-h apart), before and after 0.5 mg/kg of oral amphetamine. In contrast to functional MRI, which measures task-evoked changes in blood oxygen level-dependent activation, PET imaging calculates regional dopamine release as the difference in binding potential between two scans. Therefore, we chose an anatomical (rather than a functional) ventral striatum region of interest for PET analyses, which was drawn on each individual’s T1 image using criteria for ventral striatum boundary definitions defined in prior PET studies (Mawlawi et al., 2001; Martinez et al., 2003). Time-activity curves were calculated as the mean activity within the region of interest in each time frame. Reference tissue-based kinetic modelling yielded binding potential relative to non-displaceable compartment (BPND) (Innis et al., 2007). Percentage change from baseline BPND following amphetamine (ΔBPND) was used as the measure of dopamine release (Martinez et al., 2003).
Pramipexole treatment
One day after behavioural testing and imaging, participants began 6 weeks of open-label pramipexole monotherapy. Doses (ranging from 0.5 to 2.5 mg/day) were adjusted weekly based on clinical response, and participant’s symptoms were assessed at each weekly visit via clinical interview.
Statistical analysis
Baseline group differences were assessed using the following: response bias: Group (control, depressed) × Block (1, 2, 3) ANOVA; functional MRI analyses: separate Group × Hemisphere (left, right) × Condition (feedback, outcome) ANOVAs for gain and loss prediction errors; PET analyses: paired samples t-tests for dopamine D2/3 receptor availability (BPND) and dopamine release (ΔBPND).
Predictors were then assessed for their ability to predict end-point symptom severity as well as rate of change in symptom improvement across the 6 weeks of treatment. This approach allowed us to examine potential biomarkers of overall versus rapid antidepressant effects (Supplementary material). First, multiple regression assessed whether measures of reward processing (reward learning, prediction error signals) and dopamine function (BPND and ΔBPND) predicted post-treatment symptom severity on the HDRS, SHAPS and CGI, controlling for baseline scores. Next, we used linear mixed effects models (implemented in STATA 13.1) to evaluate whether these measures of reward processing and dopamine function predicted the slope of symptom improvement across 6 weeks of treatment. Models included random intercepts and slopes. The predictors in the model were Baseline symptom scores, Predictor, Week, and a Predictor × Week interaction term. A significant Predictor × Week interaction indicated that the variable predicted the slope of symptom improvement across treatment.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Results
Sample characteristics
Twenty-four controls and 25 patients were considered because they had either valid behavioural, functional MRI or PET data (see CONSORT diagram, Supplementary Fig. 1). Sample characteristics are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of sample
| HC baseline (n = 24) | MDD baseline (n = 25) | P-value (HC versus MDD baseline) | MDD Week 6 (n = 21) | P-value (MDD baseline versus Week 6) | |
|---|---|---|---|---|---|
| Age, mean (SD) | 26.9 (5.5) | 26.5 (6.3) | 0.84 | ||
| Female, n (%) | 12 (50) | 13 (52) | 0.89 | ||
| Caucasian, n (%) | 10 (42) | 10 (40) | 0.93 | ||
| Years of education, mean (SD) | 15.4 (1.5) | 14.6 (1.4) | 0.06 | ||
| Income below $60 000 p.a., n (%) | 14 (74)a | 18 (86)a | 0.34 | ||
| MDD age at onset, mean (SD) | 17.4 (6.4) | ||||
| Number lifetime MDEs, mean (SD) | 2.4 (3.9) | ||||
| HDRS 17-item total, mean (SD) | 0.2 (0.4) | 20.2 (2.7) | <0.001 | 8.1 (5.4) | <0.001 |
| SHAPS, mean (SD) | 18.8 (4.9) | 32.0 (6.7) | <0.001 | 25.3 (6.9) | 0.003 |
| CGI, mean (SD) | 3.0 (0.9)b | 1.8 (0.8) | <0.001 | ||
| MASQ Anhedonic Depression subscale, mean (SD) | 37.7 (9.7) | 82.6 (10.5) | <0.001 | 59.6 (18.5) | <0.001 |
| Apathy Evaluation Scale, mean (SD) | 23.8 (5.0) | 40.9 (8.8) | <0.001 | 31.7 (9.5) | 0.001 |
| TEPS subscale | |||||
| Anticipatory, mean (SD) | 49.0 (5.3) | 36.2 (8.1) | <0.001 | 43.2 (7.9) | 0.02 |
| Consummatory, mean (SD) | 38.4 (7.3) | 30.2 (7.7) | <0.001 | 35.6 (6.5) | 0.007 |
Some participants chose not to report their income, therefore income totals are out of 19 healthy controls and 21 patients.
As the CGI change scale captures change in clinical impairment from one time point to the next, the ‘baseline’ mean and SD for this measure reflects ratings given at Week 1 (which capture changes in clinical impairment from baseline to Week 1).
HC = healthy control; MASQ = Mood and Anxiety Symptom Questionnaire; MDD = major depressive disorder; MDE = major depressive episode; TEPS = Temporal Experience of Pleasure Scale (greater scores on TEPS indicate less anhedonia).
Baseline group differences in reward learning, prediction errors and ventrostriatal dopamine function
Reward learning on the Probabilistic Reward Task
A main effect of Block emerged [F(2,80) = 5.62, P = 0.005, ηp2 = 0.12], due to overall higher response bias in block 3 than in block 1 (P = 0.03), indicating that the task effectively induced a response bias. Furthermore, a main effect of Group emerged [F(1,40) = 5.65, P = 0.02, ηp2 = 0.12] due to overall lower response bias in the depressed [mean ± standard deviation (SD) = 0.11 ± 0.15] than control (0.20 ± 0.09) group (Cohen’s d = 0.73; Fig. 1A). The main effect was not qualified by a Group × Block interaction (P = 0.92). Groups did not differ in computationally-defined reward sensitivity [t(40) = 0.40, P = 0.69] or learning rate [t(40) = 0.50, P = 0.62] parameters.
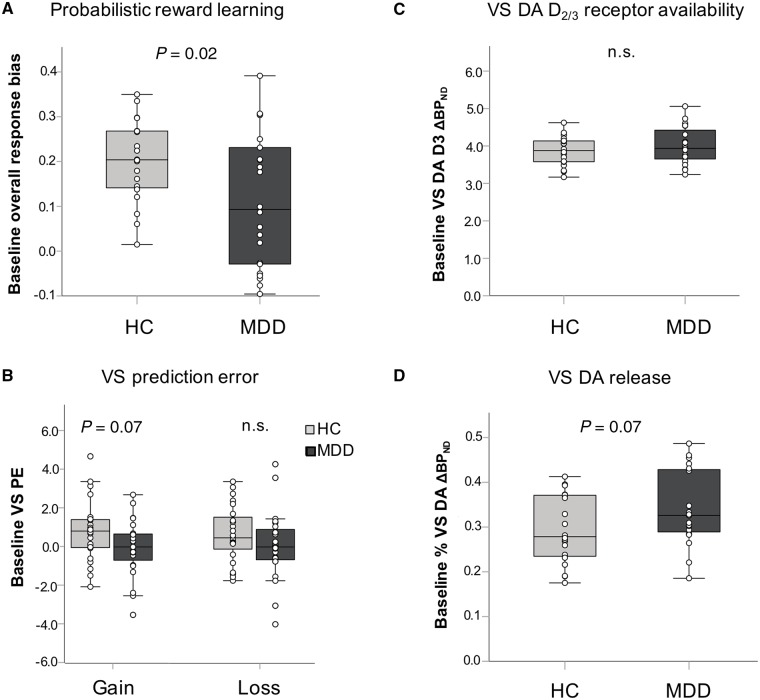
Figure 1.
Group differences in reward learning and measures of ventrostriatal dopamine function. Middle line shows the median and the top and bottom box lines show the first and third quartiles. Individual data points are overlaid onto each box-and-whisker plot. At baseline, relative to the healthy control group, the major depressive disorder group had blunted overall response bias in the Probabilistic Reward Task (A) (Cohen’s d = 0.73), a trend towards blunted ventral striatal gain prediction error signal (d = 0.54) but equivalent loss prediction error (B) (d = 0.61), equivalent dopamine (DA) D2/3 receptor availability (C) (d = 0.03) and a trend towards greater ventral striatal dopamine release (D) (d = 0.58). Note that dopamine release (ΔBPND) is expressed as a percentage change from baseline BPND with the sign reversed for ease of interpretation; higher values indicate more DA release. n.s. = not statistically significant (P < 0.05) or trend (P < 0.1). HC = healthy control; MDD = major depressive disorder; PE = prediction error; VS = ventral striatal.
Ventrostriatal prediction error signals
A trend-level main effect of Group emerged for the gain prediction error signal [F(1,45) = 3.59, P = 0.07, ηp2 = 0.07, d = 0.54]. Averaged across conditions and hemispheres, the depressed group had blunted ventrostriatal prediction error responses when learning to gain rewards compared to controls (Fig. 1B). No group effects emerged for the loss prediction error signal (all P’s > 0.10).
Dopamine function
As previously reported (Schneier et al., 2018), there were no group differences in ventrostriatal dopamine D2/3 receptor availability (BPND) [t(38) = −0.11, P = 0.92, d = 0.03] (Fig. 1C). In contrast, there was a trend for greater ventrostriatal dopamine release (ΔBPND) in the depressed relative to the control group [t(38) = 1.85, P = 0.07, d = 0.58] (Fig. 1D).
Associations between reward learning, prediction error signals, ventrostriatal dopamine function, and symptom severity are reported in the Supplementary material.
Changes in reward learning and symptoms following pramipexole
Among 22 depressed patients who started pramipexole, 21 completed 6 weeks of treatment. The average maximum dose of pramipexole was 1.6 ± 0.7 mg/day. There were significant improvements across all measures from pre- to post-treatment (Supplementary Table 1; see Supplementary Table 2 for treatment-emergent adverse events). Of those who completed treatment, 17 had valid PRT data at baseline and post-treatment. Despite significant improvements in symptoms, there were no changes in response bias, reward sensitivity or learning rate from pre- to post-treatment (all P’s > 0.10).
Greater baseline reward learning and reward sensitivity predict lower post-treatment anhedonia
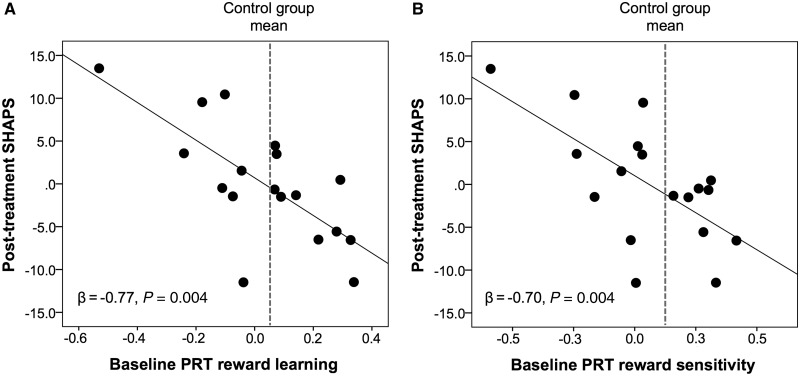
Baseline PRT performance did not predict post-treatment HDRS or CGI scores, after controlling for baseline HDRS or CGI scores, respectively (all P’s > 0.10). However, after controlling for baseline SHAPS scores, baseline reward learning predicted post-treatment SHAPS scores (β = −0.77, P = 0.001); unexpectedly, better—rather than worse—baseline reward learning predicted lower post-treatment anhedonia (Fig. 2A). When the same analysis was run for the reward sensitivity and learning rate parameters (using a separate regression model for each parameter), only reward sensitivity emerged as a significant predictor of post-treatment SHAPS scores, β = −0.70, P = 0.004 (Fig. 2B). The reward learning and reward sensitivity predictors survived correction for multiple comparisons [corrected alpha = 0.05/(three PRT indices × three outcome measures) = 0.0056].
Figure 2.
Baseline reward learning and reward sensitivity predict post-treatment anhedonia. Partial regression plots showing that (A) better baseline reward learning and (B) greater baseline reward sensitivity (as assessed using computational modelling) on the Probabilistic Reward Task (PRT) predicted lower post-treatment anhedonia (as assessed by the SHAPS) after controlling for baseline SHAPS scores. For visualization purposes, the grey dashed line shows the healthy control group mean and indicates that patients with scores equal to or greater than the control group mean (i.e. those with relatively more normative scores) showed the lowest post-treatment anhedonia.
Linear mixed effects models examining predictors of change in HDRS, CGI and SHAPS scores across the 6 weeks of treatment failed to show a Reward learning × Week interaction or a Reward sensitivity × Week interaction (all P’s > 0.10).
Stronger ventrostriatal gain and weaker ventrostriatal loss prediction errors predict symptom improvement
Ventrostriatal gain prediction error signals
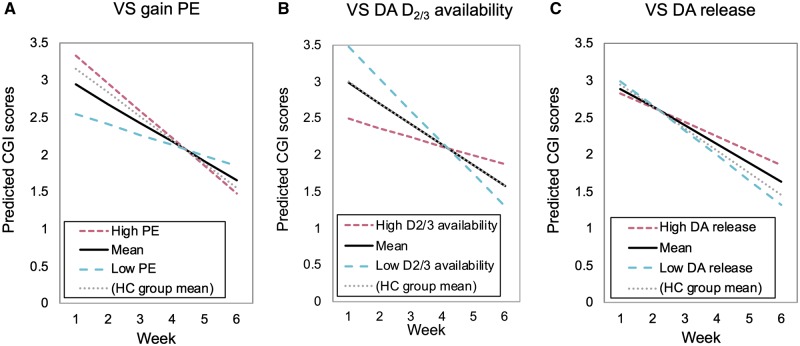
Gain prediction error signals did not predict post-treatment symptom scores. However, there was a significant Gain prediction error × Week interaction [B = −0.08, 95% confidence interval (CI) = −0.13 to −0.02, P = 0.004] for the model predicting CGI scores. Specifically, stronger gain prediction error signals predicted greater improvements in global illness severity across the 6 weeks of treatment (Fig. 3A). In addition, a trend-level Gain prediction error × Week interaction emerged for the model predicting HDRS scores (B = −0.32, 95% CI = −0.68 to 0.04, P = 0.08) where again, stronger gain prediction error signals predicted greater reductions in depressive symptoms across treatment. Contrary to initial predictions, gain prediction error did not predict improvement in SHAPS scores (P > 0.10).
Figure 3.
Predictors of change in global illness severity across the 6 weeks of treatment. Figures show the moderating effect of baseline ventral striatal gain prediction error (A), ventral striatal dopamine D2/3 receptor availability (B) and the trend-level moderating effect of ventral striatal dopamine release (C) on the rate of global clinical improvement on the CGI across the 6 weeks of treatment. For visualization purposes, scores for values at the mean, 1 SD above the mean (‘High’), and 1 SD below the mean (‘Low’) are plotted. Scores for values equal to the healthy control group mean are also shown. Higher baseline gain prediction error signals, lower dopamine D2/3 receptor availability and lower dopamine release, predicted greater global clinical improvement. For the models involving the gain prediction error signal (A) and dopamine release (C) as predictors, patients with scores more similar to the healthy control group mean (i.e. those with relatively more normative scores), were those showing the greatest clinical improvement over the course of treatment. For the model involving ventral striatal dopamine D2/3 receptor availability as the predictor (B), the MDD group mean was equal to and overlapped with the healthy control group mean. DA = dopamine; HC = healthy control; PE = prediction error; VS = ventral striatal.
Ventrostriatal loss prediction error signals
Loss prediction error signals did not predict post-treatment scores on any outcome measure. However, significant Loss prediction error × Week interactions emerged for the model predicting SHAPS scores (B = 0.61, 95% CI = 0.20 to 1.02, P = 0.004) and for the model predicting HDRS scores (B = 0.39, 95% CI = 0.04 to 0.73, P = 0.03). For both models, more blunted loss prediction errors (i.e. a reduced ventrostriatal response to monetary loss) predicted greater symptom improvement across treatment.
The Gain prediction error × Week interaction for the model predicting CGI scores and the Loss prediction error × Week interaction for the model predicting SHAPS scores (Table 2) survived correction for multiple comparisons [corrected alpha = 0.05/(two prediction errors × three outcomes) = 0.0083].
Table 2.
Linear mixed effect models showing the moderating effects of striatal prediction error signals on symptom improvement across the 6 weeks of treatment
| Model term a | Coefficient | SE | Z | P |
|---|---|---|---|---|
| Model 1: Ventral striatal gain prediction error predicts change in global illness severity | ||||
| Week | −0.26 | 0.04 | −6.59 | <0.001 |
| Ventral striatal gain prediction error | 0.34 | 0.12 | 2.96 | 0.003 |
| Week × Ventral striatal gain prediction error | −0.08 | 0.03 | −2.87 | 0.004 |
| Model 2: Ventral striatal loss prediction error predicts change in anhedonia | ||||
| Baseline SHAPS | 0.82 | 0.17 | 4.79 | <0.001 |
| Week | −0.77 | 0.31 | −2.50 | 0.012 |
| Ventral striatal loss prediction error | −1.52 | 0.91 | −1.67 | 0.095 |
| Week × Ventral striatal loss prediction error | 0.61 | 0.21 | 2.91 | 0.004 |
The CGI-Change Scale measures changes in global illness severity and was therefore first administered after 1 week of treatment. Accordingly, models do not include a baseline CGI score term.
Lower D2/3 receptor availability and dopamine release predict greater improvement in global illness severity
Dopamine D2/3 receptor availability (BPND)
Dopamine D2/3 receptor availability did not predict post-treatment symptom scores or slope of symptom improvement on the SHAPS or HDRS (P’s > 0.05). However, it did predict the slope of global illness severity improvement on the CGI. Specifically, a BPND × Week interaction emerged (B = 0.20, 95% CI = 0.04 to 0.37, P = 0.02) where lower dopamine D2/3 receptor availability predicted greater improvements in global illness severity across treatment (Fig. 3B).
Dopamine release (ΔBPND)
Dopamine release did not predict post-treatment symptom scores or slope of symptom improvement on the SHAPS or HDRS (all P’s > 0.10). However, the ΔBPND × Week interaction for the model predicting CGI scores was marginally significant (B = −1.04, 95% CI = −2.08 to 0.01, P = 0.05) indicating that lower ventrostriatal dopamine release predicted greater improvements in global illness severity across treatment (Fig. 3C). Neither of the PET predictors survived correction for multiple comparisons [alpha = 0.05 / (two PET indices × three outcomes) = 0.0083].
Discussion
Building on recent PET analyses on this sample (Schneier et al., 2018), which found no differences between controls and individuals with major depressive disorder on measures of striatal dopamine receptor availability or release, this study examined whether measures of reward-related mesolimbic dopamine system function (reward learning, functional MRI-based ventrostriatal prediction error signalling, PET-based ventrostriatal dopamine release) predicted clinical response to dopamine agonist treatment in major depressive disorder. Replicating prior findings (Kumar et al., 2008, 2018; Pizzagalli et al., 2008b), depression was characterized by significantly reduced reward learning and blunted ventrostriatal gain prediction error signals (trend) at baseline. Following pramipexole treatment, the depressed group showed significant reductions in depression, anhedonia and global illness severity. As hypothesized, baseline reward learning and ventrostriatal prediction error signalling were associated with post-treatment anhedonia severity and change in global illness severity, respectively, following 6 weeks of treatment with pramipexole. However, counter to the direction of predictions, individuals with better reward learning, greater reward sensitivity, and stronger ventrostriatal prediction error signalling to gains showed the greatest improvements in anhedonia (reward learning and sensitivity) or global illness severity (ventrostriatal prediction error signals). Although these findings await replication in a larger placebo-controlled study, the results suggest that depressed individuals with more normative reward learning and striatal prediction error signalling may respond favourably to a dopamine agonist. While unexpected, our results are consistent with an earlier literature suggesting that individuals with atypical depression (a subtype characterized by preserved reward sensitivity) may preferentially improve with dopaminergic pharmacotherapy (Stewart and Thase, 2007).
Direct measures of dopamine function also predicted clinical response to pramipexole. We hypothesized that individuals with more pronounced dopamine deficits (i.e. those with reduced ventrostriatal dopamine release), would show the greatest response to pramipexole. Results fit these predictions, where lower baseline ventrostriatal dopamine release predicted greater improvement in global illness severity. However, contrary to predictions, the depressed group did not show blunted ventrostriatal dopamine release relative to controls, but rather, showed a trend for increased ventrostriatal dopamine release. Accordingly, and consistent with the direction of effects observed for reward learning and ventrostriatal prediction error signalling, depressed patients with ventrostriatal dopamine release more similar to controls were those who responded more favourably to pramipexole.
Lower ventrostriatal dopamine D2/3 receptor availability also predicted greater improvement in global illness severity across treatment. Studies examining striatal dopamine receptor availability in depression have produced mixed findings, with nine PET studies reporting no difference, four reporting increases and one reporting decreases in receptor availability (for a review see Schneier et al., 2018). One explanation for higher receptor availability in depression is that depression-related dopamine deficits may cause a compensatory up-regulation of D2/3 receptors (Dunlop and Nemeroff, 2007). Accordingly, depressed individuals with lower ventrostriatal BPND (who showed the greatest global clinical improvement following pramipexole) might be those with more normative D2/3 receptor availability. However, PHNO binding is sensitive to competition with endogenous dopamine, with 42% of ventrostriatal BPND variance estimated to be attributable to endogenous dopamine (Caravaggio et al., 2016). Thus, lower ventrostriatal BPND at baseline could alternatively represent higher levels of endogenous dopamine.
Taken together, these findings suggest that measures of reward processing and striatal dopamine function are associated with lower post-treatment anhedonia severity and greater improvements in global illness severity, respectively, following treatment with a dopamine agonist in individuals with major depressive disorder. However, contrary to conventional assumptions, individuals with more normative rather than more disrupted reward and dopamine function, responded most favourably. These findings are consistent with a recent study showing that greater baseline ventrostriatal prediction error signalling predicted greater reductions in anhedonia in a naturalistic longitudinal study (Eckstrand et al., 2019). Furthermore, they align with studies showing links between better baseline reward processing and superior response to Behavioral Activation Therapy (Carl et al., 2016; Walsh et al., 2017), a therapy thought to specifically target anhedonia (Hopko et al., 2003). A critical next step is to determine whether baseline reward processing predicts superior response to treatments specifically targeting reward processing, or whether it predicts greater treatment responsiveness more generally.
Some limitations must be considered when interpreting the current findings (these are discussed further in the Supplementary material). First, a placebo group could not be included given the costs of intensive multimodal neuroimaging in this study (Schneier et al., 2018). Hence, the findings only point to a relationship between baseline measures of ventrostriatal reward function and pramipexole response at the group, rather individual patient, level. Future studies should test the specificity of our findings using placebo and/or a non-dopaminergic antidepressant control. Second, larger sample sizes are needed to test whether the differential predictive effects observed for reward learning (on anhedonia) and striatal prediction error signalling and ventrostriatal dopamine function (on global illness severity) are robust. Finally, functional MRI and PET imaging were only performed at baseline; therefore, we could not evaluate whether pramipexole altered ventrostriatal prediction error signalling or dopamine function. This is an important area for future research, as it remains unclear whether longer-term treatment with pramipexole may alter brain reward function via allostatic processes (Supplementary material).
Identifying ways to improve treatment precision for individuals with depression represents a major challenge. Using a multimodal approach, our findings suggest that measures of reward processing and ventrostriatal dopamine function may identify individuals with depression likely to respond favourably to a dopaminergic antidepressant. These findings pave the way for larger studies focused on improved antidepressant treatment precision, which is a critical step towards reducing the global burden of depression.
Supplementary Material
Acknowledgements
We thank Roberto Valdovinos and Danielle Moskow for assistance with data collection, and Page van Meter for assistance with data management.
Funding
This study was supported by National Institute of Mental Health Grant No. R01MH099322 (to F.R.S.). D.A.P. was partially supported by Grant No. R37 MH068376 and R01 MH101521. A.E.W. was partially supported by the National Health and Medical Research Council, Grant No. APP1110773.
Competing interests
F.R.S. has received research support from Forest Laboratories/Allergan and Feelmore Labs. M.S. has received research support from Forest Laboratories, Pierre-Fabre, CHDI, and Otsuka; and has provided consultation for Amgen. D.V.I. has received consulting fees from Alkermes, Axsome, Centers of Psychiatric Excellence, Jazz, Lundbeck, MyndAnalytics (CNS Response), Otsuka, Precision Neuroscience, and Sundovion; and has received research support (through his academic institutions) from Alkermes, Astra Zeneca, Brainsway, LiteCure, Neosync, Roche, and Shire. A.A-D. has received research support from Takeda and Forest Pharmaceuticals and has served on advisory boards for Roche, Forum, and Otsuka. D.A.P. has received consulting fees from Blackthorn Therapeutics, Boehringer Ingelheim, Compass, Takeda and an honorarium from Alkermes for activities unrelated to the current research. D.A.P. has a financial interest in BlackThorn Therapeutics, which has licensed the copyright to the Probabilistic Reward Task through Harvard University. D.A.P. interests were reviewed and are managed by McLean Hospital and Partners HealthCare in accordance with their conflict of interest policies. All other authors report no biomedical financial interests or potential conflicts of interest.
Glossary
Abbreviations
- BPND =
non-displaceable binding potential;
- CGI =
Clinical Global Impressions–Change Scale;
- HDRS =
Hamilton Depression Rating Scale;
- SHAPS =
Snaith-Hamilton Pleasure Scale
References
- Ameli R, Luckenbaugh DA, Gould NF, Holmes MK, Lally N, Ballard ED, et al. SHAPS-C: the Snaith-Hamilton pleasure scale modified for clinician administration. PeerJ 2014; 2: e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron 2015; 86: 646–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry 2010; 67: 110–6. [DOI] [PubMed] [Google Scholar]
- Caravaggio F, Kegeles LS, Wilson AA, Remington G, Borlido C, Mamo DC, et al. Estimating the effect of endogenous dopamine on baseline [11C]‐(+)‐PHNO binding in the human brain. Synapse 2016; 70: 453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl H, Walsh E, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, et al. Sustained anterior cingulate cortex activation during reward processing predicts response to psychotherapy in major depressive disorder. J Affect Disord 2016; 203: 204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013; 493: 532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, D'souza M, Pizzagalli D, Markou A. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiatry 2013; 3: e297.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, et al. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry 2014; 76: 176–85. [DOI] [PubMed] [Google Scholar]
- Drijgers RL, Verhey FR, Tissingh G, van Domburg PH, Aalten P, Leentjens AF. The role of the dopaminergic system in mood, motivation and cognition in Parkinson's disease: a double blind randomized placebo-controlled experimental challenge with pramipexole and methylphenidate. J Neurol Sci 2012; 320: 121–6. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 2007; 64: 327–37. [DOI] [PubMed] [Google Scholar]
- Eckstrand KL, Forbes EE, Bertocci MA, Chase HW, Greenberg T, Lockovich J, et al. Anhedonia reduction and the association between left ventral striatal reward response and 6-month improvement in life satisfaction among young adults. JAMA Psychiatry 2019; 76: 958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J, Rush AJ, Vukelich J, Diaz SH, Dunklee L, Romo P, et al. Clinical experience with high-dosage pramipexole in patients with treatment-resistant depressive episodes in unipolar and bipolar depression. Am J Psychiatry 2016; 173: 107–11. [DOI] [PubMed] [Google Scholar]
- Fletcher K, Parker G, Paterson A, Fava M, Iosifescu D, Pizzagalli DA. Anhedonia in melancholic and non-melancholic depressive disorders. J Affect Disord 2015; 184: 81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW. Understanding dopamine and reinforcement learning: the dopamine reward prediction error hypothesis. Proc Natl Acad Sci USA 2011; 108: 15647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JF, Burdick KE, Endick CJ. Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. Am J Psychiatry 2004; 161: 564–6. [DOI] [PubMed] [Google Scholar]
- Greenberg T, Chase HW, Almeida JR, Stiffler R, Zevallos CR, Aslam HA, et al. Moderation of the relationship between reward expectancy and prediction error-related ventral striatal reactivity by anhedonia in unmedicated major depressive disorder: findings from the EMBARC study. Am J Psychiatry 2015; 172: 881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. Assessment manual for psychopharmacology, revised (DHEW publication ABM 76-366). Washington, DC: US Government Printing Office; 1976. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopko DR, Lejuez C, Ruggiero KJ, Eifert GH. Contemporary behavioral activation treatments for depression: procedures, principles, and progress. Clin Psychol Rev 2003; 23: 699–717. [DOI] [PubMed] [Google Scholar]
- Huys QJ, Pizzagalli DA, Bogdan R, Dayan P. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol Mood Anxiety Disord 2013; 3: 12–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007; 27: 1533–9. [DOI] [PubMed] [Google Scholar]
- Kumar P, Goer F, Murray L, Dillon DG, Beltzer ML, Cohen AL, et al. Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology 2018; 43: 1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele J. Abnormal temporal difference reward-learning signals in major depression. Brain 2008; 131: 2084–93. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Tedeschini E, Papakostas GI. Efficacy of antidepressants for dysthymia: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry 2011; 72: 509–14. [DOI] [PubMed] [Google Scholar]
- Macmillan N, Creelman C. Detection theory: a user’s guide. UK: Cambridge University Press; 1991. [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang D-R, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 2003; 23: 285–300. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang D-R, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 2001; 21: 1034–57. [DOI] [PubMed] [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment–resistant depression. J Am Acad Child Adolesc Psychiatry 2012; 51: 404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierau J, Schingnitz G. Biochemical and pharmacological studies on pramipexole, a potent and selective dopamine D2 receptor agonist. Eur J Pharmacol 1992; 215: 161–70. [DOI] [PubMed] [Google Scholar]
- Pergadia ML, Der-Avakian A, D’souza MS, Madden PA, Heath AC, Shiffman S, et al. Association between nicotine withdrawal and reward responsiveness in humans and rats. JAMA Psychiatry 2014; 71: 1238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, et al. Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology 2008a; 196: 221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res 2008b; 43: 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinen J, Smith EE, Insel C, Kribs R, Shohamy D, Wager TD, et al. Patients with schizophrenia are impaired when learning in the context of pursuing rewards. Schizophr Res 2014; 152: 309–10. [DOI] [PubMed] [Google Scholar]
- Rutledge RB, Moutoussis M, Smittenaar P, Zeidman P, Taylor T, Hrynkiewicz L, et al. Association of neural and emotional impacts of reward prediction errors with major depression. JAMA Psychiatry 2017; 74: 790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR, Slifstein M, Whitton AE, Pizzagalli DA, Reinen J, McGrath PJ, et al. Dopamine release in antidepressant-naïve major depressive disorder: a multimodal [11C]-(+)-PHNO positron emission tomography and functional magnetic resonance imaging study. Biol Psychiatry 2018; 84: 563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith R, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith–Hamilton Pleasure Scale. Br J Psychiatry 1995; 167: 99–103. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci 2013; 16: 966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JW, Thase ME. Treating DSM-IV depression with atypical features. J Clin Psychiatry 2007; 68: e10.. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci 2012; 32: 6170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E, Carl H, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, et al. Attenuation of frontostriatal connectivity during reward processing predicts response to psychotherapy in major depressive disorder. Neuropsychopharmacology 2017; 42: 831–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.