Abstract
Background
In well treated human immunodeficiency virus infection (HIV), there is a residual immune activation and immune exhaustion that may contribute to increased risk of comorbidities. T-cell immunoglobulin mucin domain-3 (Tim-3) is an inhibitory molecule involved in HIV-associated T-cell dysfunction. The Tim-3 can be cleaved to soluble Tim-3 (sTim-3) that may serve as a soluble marker of immune exhaustion.
Methods
We measured sTim-3 with enzyme-linked immunosorbent assay DuoSets in a cross-sectional cohort of 1010 people with HIV (PWH) on antiretroviral therapy (ART), and 76 controls from the Copenhagen Co-Morbidity in HIV Infection (COCOMO) study, and in a longitudinal cohort of 60 PWH before and during ART.
Results
In the cross-sectional cohort, levels of sTim-3 were elevated in PWH on ART compared with controls, especially in hepatitis C virus (HCV)-coinfected individuals, and were associated with HCV viremia and inflammation. In the longitudinal cohort, pretreatment sTim-3 correlated with HIV viral load and decreased after ART initiation. Pretreatment sTim-3 correlated inversely with CD4 counts, but it did not predict immunological response in multivariable analyses.
Conclusions
Levels of sTim-3 decreased after ART initiation. In a cross-sectional cohort, levels of sTIM-3 were higher in PWH than in controls and were independently associated with HCV coinfection and high-sensitivity C-reactive protein, representing a potential link between immune exhaustion, inflammation, and risk of comorbidities.
Keywords: hepatitis C, HIV, immune exhaustion, soluble, Tim-3
In our study, levels of soluble Tim-3 decrease after ART initiation, are elevated in treated PWH compared with controls, and are associated with hepatitis C coinfection and low-grade inflammation but not immune reconstitution.
Despite the success of antiretroviral therapy (ART), people with human immunodeficiency virus (PWH) have excess morbidity [1, 2]. Even when viral replication is undetectable in plasma due to ART, the body is unable to completely clear the virus, and the latent reservoir with low-grade viral replication could contribute to a state of persistent immune activation [3]. This nonresolving low-grade immune activation may induce T-cell exhaustion and immunosenescence, which could contribute to the increased burden of comorbidities such as non-acquired immune deficiency syndrome (AIDS)-defining cancer and metabolic and cardiovascular diseases (CVD) in PWH [4–6].
T cells contribute to clearance of viral infections, but they may turn into exhausted T cells when they are unable to clear viral reservoirs, like in chronic human immunodeficiency virus (HIV) infection, with harmful effects on the host due to persistent low-grade antigen exposure [7]. In this process, several inhibitory molecules and receptors (checkpoint inhibitors) are upregulated, such as T-cell immunoglobulin and mucin domain-3 (Tim-3) and programmed cell death protein-1 (PD-1), probably to prevent persistent (too long) and overshooting (too high) T-cell activation, which could harm the host [4, 7].
Human Tim-3 is a transmembrane protein that is expressed on T cells and monocytes [8]. The Tim-3 is shown to play a modulating role in HIV-associated T-cell dysfunction [9], and the expression of Tim-3 seems to be a reliable marker of T-cell exhaustion in disorders characterized by persistent T-cell activation including chronic viral infection [10]. The Tim-3 can be cleaved from the cell surface by certain matrix metalloproteinases (ie, A disintegrin and metalloprotease [ADAM]-10 and ADAM-17) to a soluble form [11]. Although the function of soluble Tim-3 (sTim-3) is not clarified, it seems to reflect the degree of membrane expression of this checkpoint inhibitor. Therefore, sTim-3 could be an attractive soluble marker of persistent T-cell activation and exhaustion in various disorders including HIV [12].
To this end, data on sTim-3 in HIV infection are scarce and restricted to relatively small cohorts (n < 100) [13], with only 1 reporting regulation of sTim-3 during ART [12]. We aimed to investigate sTim-3 in relation to clinical, immunological, and virologic characteristics in 2 well defined cohorts: (1) a large cross-sectional cohort of 1010 PWH and (2) a longitudinal study of 60 PWH initiating ART.
METHODS
Study Design and Populations
Cross-Sectional Cohort
A total of 1099 PWH and 78 controls from the general population were included from the Copenhagen Co-Morbidity in HIV Infection (COCOMO) study, which is a prospective study evaluating the burden of comorbidities in PWH in Copenhagen, Denmark. Inclusion criteria for the COCOMO study were a positive HIV test and age >18 years. Data collection was performed between March 2015 and November 2016. The procedures for recruitment and data collection have been described elsewhere [14]. Of 1099 participants and 78 controls from the general population included in the COCOMO study, 1010 participants received ART and had available plasma samples and 76 controls where plasma samples were available were included in the present study. Sixty-six immunological nonresponders ([INRs] CD4 <350 × 106/L) and 133 immunological responders ([IRs] CD4 >500 × 106/L) matched on sex, age, time with HIV, CD4, and nadir CD4 cell counts were identified from the COCOMO study for subanalyses.
Longitudinal Study
Sixty PWH were included in a prospective study, and plasma samples were obtained before initiation of ART, and at 6 and 12 months thereafter. All individuals were included from the Department of Infectious Diseases, Rigshospitalet, Copenhagen University Hospital, and Department of Infectious Diseases, Hvidovre Hospital as described previously [15].
Biochemistry
Nonfasting venous blood for plasma samples (ethylenediaminetetraacetic acid-anticoagulated) were immediately stored on ice until centrifugation at 4°C, before storing at −80°C. Soluble Tim-3 was analyzed by enzyme immunoassay using DuoSets (catalog no. DY2365) from R&D Systems (Stillwater, MN) in a 384 format using a combination of a SELMA (Jena, Germany) pipetting robot and a BioTek (Winooski, VT) dispenser/washer. In brief, high binding 384-well microtiter plates (UltraCruz; Santa Cruz Biotechnology, Heidelberg, Germany) were coated with 20 μL 2 μg/mL primary antibody (part no. 843115) in phosphate-buffered saline (PBS). Plates were washed 3 times (wash buffer 0.05% tween in PBS, wash similar for all washing steps) and blocked with 80 μL 1% bovine serum albumin (BSA) in PBS for >1 hour. Plates were washed again, and samples (diluted 50 times in 0.5% BSA in wash buffer) and standards were added in parallel and incubated overnight at 4oC. The following morning, plates were washed and 20 μL secondary antibody (20 μg/mL, part no. 843116) was added and incubated at room temperature for 2 hours. Plates were washed again, and 20 μL streptavidin-horseradish peroxidase (part no. 890803) was added and incubated for 20 minutes at room temperature. A final wash was performed, and 20 μL stable TMB solution was added (catalog no. SB02; Life Technologies) and incubated for 10 minutes before 50 μL 1N H2SO4 was added, and absorption was read at 450 nm with wavelength correction set to 540 nm using a Synergy H1 plate reader (BioTek). Intra- and interassay coefficients of variation were <10%. Sensitivity, defined as 3×SD of a low sample, was 36 pg/mL. Average parallelity of 3 samples serially diluted 5 times was 113%, average recovery of 2 samples spiked with low and high sTim-3 was 111%, whereas average level of 4 samples exposed to 2–10 freeze-thaw cycles was 104%. Plasma high-sensitivity C-reactive protein (hsCRP) was analyzed at Biochemical Department, Herlev University Hospital, Copenhagen as previously described [14].
Statistical Analysis
Differences in continuous variables with normal distribution were compared using Students t test, whereas skewed variables were compared using Mann-Whitney U test. Due to differences in age and sex between groups, sTim-3 levels were compared using multivariable regression, using group as a fixed factor and age and sex as covariate. These data are expressed as estimated marginal means and 95% confidence intervals. In the paired situation, levels were first compared with the Friedman test and, if significant, Wilcoxon paired test was used to compare changes in sTim-3 levels between different time points. Categorical variables were compared with χ 2 test. Correlations were investigated using Spearman rank-order test, and multivariable analyses were performed by linear regression, predicting sTim-3 levels after adjustment for relevant covariates (current CD8 count, nadir CD4 count, age, hsCRP, estimated glomerular filtration rate (eGFR), smoking, previous AIDS-defining diagnosis, and hepatitis C coinfection).
SPSS software and GraphPad Prism were used for the statistical analysis. A 2-sided P < .05 was considered statistically significant.
Ethical Aspects
All patients gave written informed consent before storage of blood samples. The study was approved by the Scientific Ethics Committee of the Capital Region of Denmark (protocol H-15017350 and H-2-2011-089), the Danish Data Protection Agency (jr.nr 30-1454), and the Regional Committees for Medical and Health Research Ethics in Norway (2018/2565-1). All processing of personal data followed national guidelines and regulations.
RESULTS
Clinical Characteristics
The characteristics of the 2 cohorts are shown in Tables 1 and 2. The cross-sectional study consisted of 1010 PWH on stable ART with available sTIM-3 measurements and 76 controls from the general population. The median age was 50 years for PWH (85% male) and 60 years for controls (69% male). Median nadir CD4 count was 235 cells/µL, current median CD4 count was 690 cells/µL, and 18% had a previous AIDS-defining diagnosis. In addition, 11% were anti-hepatitis C virus (HCV) positive, 6% were coinfected with active HCV infection (detectable plasma HCV ribonucleic acid [RNA]), whereas 4% were hepatitis B surface antigen positive (Table 1).
Table 1.
Baseline Characteristics of Cross-Sectional Cohort
| Cross-Sectional Cohort | Correlation With sTim-3 | Regression With sTim-3 as Dependent | |||
|---|---|---|---|---|---|
| PWH (n = 1010) | rho | P | β | P | |
| Age, years, median (IQR) | 50 (43–58) | 0.26 | 0.000 | 0.15 | 0.004 |
| Sex, male, % | 85 | −0.06 | 0.068 | ||
| Mode of transmission, % (n) | |||||
| MSM | 71.0 (712) | ||||
| Heterosexual | 22.0 (216) | ||||
| PWID | 1.4 (14) | ||||
| Other | 5.6 (58) | ||||
| Current viral load <50, % (n) | 95.2 (952) | ||||
| Nadir CD4, cells/µL, median (IQR) | 235 (120–350) | −0.08 | 0.016 | 0.01 | 0.882 |
| CD4, cells/µL, median (IQR) | 690 (520–890) | −0.00 | 0.977 | ||
| CD8, cells/µL, median (IQR) | 840 (632–1188) | 0.07 | 0.029 | 0.01 | 0.867 |
| Viral load, copies/mL, median (IQR) | 19 (19–20) | −0.01 | 0.653 | ||
| hsCRP, mg/L, median (IQR) | 1.2 (0.6–2.5) | 0.24 | 0.000 | 0.18 | 0.000 |
| eGFR, mL/min/1.73m2, mean (SD) | 89 (15.4) | −0.25 | 0.000 | −0.16 | 0.001 |
| Anti-HCV positive % (n) | 10.5 (103) | 0.10 | 0.002 | ||
| HCV RNA positive % (n) | 6 (55) | 0.11 | 0.001 | 0.14 | 0.000 |
| HBsAg positive % (n) | 3.7 (37) | 0.04 | 0.197 | ||
| Anti-HBc positive % (n) | 5.7 (50) | 0.02 | 0.483 | ||
| Anti-HBs positive % (n) | 70.2 (702) | 0.01 | 0.657 | ||
| CD4/CD8 median (IQR) | 0.81 (0.57–1.13) | −0.06 | 0.065 | ||
| CDC AIDS (past) | 18 (184) | 0.08 | 0.008 | 0.02 | 0.654 |
| sTim-3 ng/mL, median (IQR) | 7.0 (5.8–8.8) | ||||
| Time since diagnosis, years, median (IQR) | 13.7 (6.9–21.3) | 0.15 | 0.000 | ||
| Time on ART, years, median (IQR) | 10.5 (5.2–17.0) | 0.14 | 0.000 | ||
| Smoking, packyears in current and previous smokers, median (IQR) | 18.8 (7.0–32.0) | 0.19 | 0.000 | 0.07 | 0.103 |
Abbreviations: ART, antiretroviral therapy; CDC AIDS, Center for Disease Control and Prevention AIDS-defining conditions; eGFR, estimated glomerular filtration rate; HBc, hepatitis B core antibody; Hbs, hepatitis B surface antibody; HbsAg, hepatitis B surface antigen; HCV, hepatitis C virus; hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; MSM, men whom have sex with men; PWH, people with human immunodeficiency virus; PWID, people who inject drugs; RNA, ribonucleic acid; SD, standard deviation; sTim-3, soluble T-cell immunoglobulin mucin domain-3.
Table 2.
Characteristics of Longitudinal Cohort
| Characteristics | Longitudinal Cohort (n = 60) | ||
|---|---|---|---|
| Baseline | 6 Months After ART | 12 Months After ART | |
| Age, years, median (IQR) | 40 (33–47) | ||
| Sex | 92% male | ||
| Nadir CD4, cells/µL, median (IQR) | 370 (239–520) | ||
| CD4, cells/µL, median (IQR) | 380 (239–537) | 600 (430–750) | 610 (450–770) |
| CD8, cells/µL, median (IQR) | 1000 (525–1500) | 920 (680–1400) | 970 (640–1300) |
| Viral load, copies/mL, median (IQR) | 80 919 (30 059–328 245) | 20 (19–30) | 20 (19–20) |
| CD4/CD8 median (IQR) | 0.34 (0.23–0.52) | 0.60 (0.32–0.92) | 0.63 (0.31–0.92) |
| sTim-3 ng/mL, median (IQR) | 9.3 (6.0–12.4) | 5.8 (4.4–7.2) | 5.8 (4.4–7.4) |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; sTim-3, soluble T-cell immunoglobulin mucin domain-3.
In the longitudinal study with 60 PWH, the median age was 40 years (92% male), and the median CD4 count and viral load before ART initiation were 380 cells/µL and 80 919 RNA copies/mL, respectively (Table 2).
Levels of Soluble T-Cell Immunoglobulin Mucin Domain-3 During Stable Antiretroviral Therapy: The Cross-Sectional Study
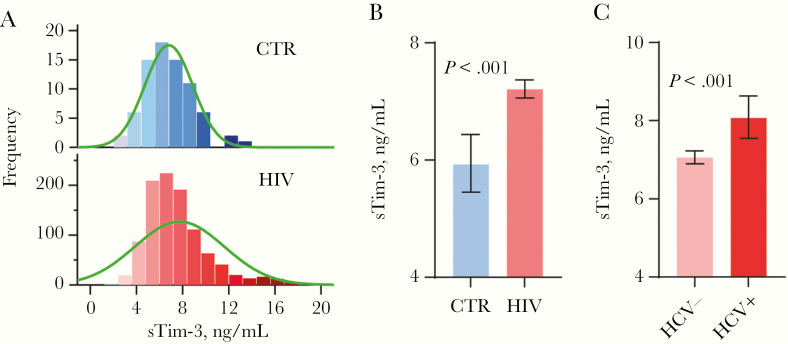
Figure 1A shows the distribution of sTim-3 in PWH and controls. The PWH cohort was younger and with a higher percentage of males, but in multivariable regression analysis adjusting for age and sex significantly higher sTim-3 levels was found in PWH compared with controls (P < .001) (Figure 1B). The difference remained significant after excluding the 55 individuals with HCV coinfection (P < .001).
Figure 1.
Levels of soluble T-cell immunoglobulin mucin domain-3 (sTim-3) in cross-sectional cohort. (A) Distribution of sTim-3 in people with human immunodeficiency virus (PWH) and controls (CTR). (B) Levels of sTim-3 in PWH and noninfected CTR. (C) Levels of sTim-3 in PWH with and without hepatitis C virus (HCV). Data in B and C are presented as estimated marginal means and 95% confidence intervals, adjusted for age and sex.
In PWH, sTim-3 correlated positively with age, hsCRP, time since HIV diagnosis, time on ART, previous AIDS diagnosis, past and present smoking, and HCV serostatus and negatively with nadir CD4 T-cell counts and eGFR (Table 1). In contrast, age did not correlate with sTim-3 in the control group (rho = −0.05, P = .97).
Coinfection With Hepatitis C Is Associated With Elevated Soluble T-Cell Immunoglobulin Mucin Domain-3 Levels: Cross-Sectional Study
Because sTim-3 correlated with HCV serostatus, we analyzed a subset of 55 PWH who were coinfected with HCV (detectable HCV RNA in plasma). As shown in Figure 1C, sTim-3 levels were significantly higher in PWH with HCV coinfection (median, 8.1 ng/mL; interquartile range [IQR], 6.3–10.2 ng/mL) than in PWH without HCV coinfection (median, 7.0 ng/mL; IQR, 5.7–8.7 ng/mL) (P = .001).
Predictors of Soluble T-Cell Immunoglobulin Mucin Domain-3 in People With Human Immunodeficiency Virus: Cross-Sectional Study
Linear regression was used to assess predictors of sTim-3 in PWH. Nadir CD4 counts, CD8 counts, age, hsCRP, eGFR, previous AIDS-defining diagnosis, cumulative tobacco pack-years, and presence of HCV RNA were included as variables in a multivariable analysis with sTim-3 as the dependent variable. Hepatitis C viremia (β = 0.14, P < .001), hsCRP (β = 0.20, P < .001), low eGFR (β = −0.17, P < .001), and age (β = 0.17, P < .001) were associated with higher sTim-3 (Table 1). Of the 55 coinfected with HCV, 4 were people who inject drugs (PWID). The PWID status (n = 14) was correlated with sTIM-3 (rho = 0.088, P = .006), but it did not predict sTim-3 in the multivariable regression analysis in the total cohort.
Levels of Soluble T-Cell Immunoglobulin Mucin Domain-3 in Relation to Viral Load Before and During Antiretroviral Therapy: Longitudinal Study
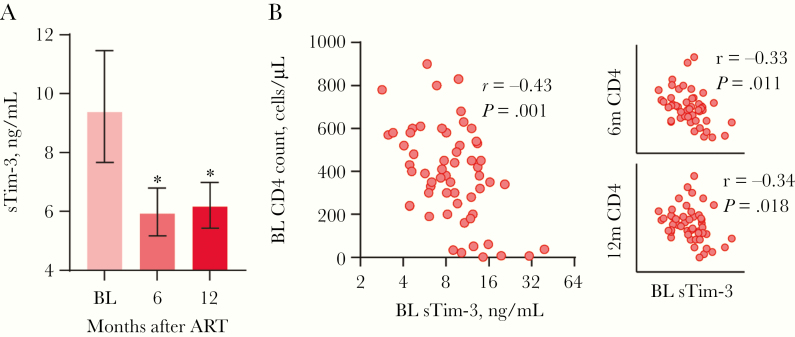
In the longitudinal cohort, there was a significant decrease of 38% in sTim-3 after initiating ART (median, 9.2 ng/mL; IQR, 6.0–12.4 ng/mL) with stabilized levels at 6 (median, 5.8 ng/mL; IQR, 4.4–7.2 ng/mL) and 12 (median, 5.8 ng/mL; IQR, 4.4–7.4 ng/mL) months of follow up (Figure 2A). Furthermore, baseline sTim-3 correlated with baseline viral load (median load, 80 919 copies/mL; rho = 0.51; P < .001) and sTim-3 after 6 months correlated with viral load after 6 months (median load, 20 copies/mL; rho = 0.29; P = .048).
Figure 2.
Levels of sTim-3 in longitudinal cohort. (A) Soluble T-cell immunoglobulin mucin domain-3 (sTim-3) before initiation of antiretroviral therapy ([ART] baseline [BL]) and 6 months and 12 months after initiation of ART (median plasma levels with 95% confidential intervals [CI]). Error bars represent 95% CI: * represent significant differences from baseline, *P < .001. Wilcoxon paired test. (B) Correlations between sTim-3 at baseline and CD4 T-cell counts at BL, 6 months, and 12 months after initiation of ART. Spearman correlation test.
Soluble T-Cell Immunoglobulin Mucin Domain-3 Is Associated With Pre-Antiretroviral Therapy (ART) CD4 Counts, but Not With Immune Reconstitution During ART: Cross-Sectional and Longitudinal Study
Next, we aimed to investigate whether sTim-3 could predict immune reconstitution during ART. In the longitudinal cohort, baseline sTim-3 correlated inversely with nadir and baseline CD4 counts and with CD4 counts 6 and 12 months after ART initiation (Figure 2B). However, in a multivariable linear regression with CD4 counts 12 months after ART initiation as dependent variable, only nadir CD4 count (β = 0.82, P < .001) but not baseline sTim-3 (β = 0.06, P = .526) predicted CD4 counts after 12 months.
Finally, in a subanalysis of the cross-sectional cohort with 66 INRs (CD4 <350 × 106/L) and 133 matched IRs (CD4 >500 × 106/L), we found no significant difference in sTim-3 between INR (median, 7.6 ng/mL; IQR, 6.1–9.5 ng/mL) and IR (median, 6.9 ng/mL; IQR, 5.6–8.8 ng/mL) (P = .074).
Discussion
Dysregulation of coinhibitory molecules plays a role in T-cell dysfunction and persistent immune activation in HIV infection. In the present study, we aimed to explore the levels of sTim-3 in relation to HIV-specific parameters, coinfections, and demographic parameters as well as its response to ART. Our main findings can be summarized as follows: (1) in a large cross-sectional study, levels of sTim-3 were elevated in PWH on ART compared with controls; (2) in a longitudinal study, levels of sTim-3 correlated with HIV viral load before treatment and decreased after initiation of ART; (3) in the cross-sectional study, coinfection with HCV and systemic inflammation as assessed by hsCRP predicted sTim-3 in linear regression analysis; and (4) in the longitudinal study, baseline sTim-3 correlated inversely with nadir and baseline CD4 counts, and with CD4 counts 6 and 12 months after ART initiation, but in multivariable analyses, sTim-3 did not predict immunological response after ART initiation.
Previous studies have reported elevated levels of Tim-3 expression on T cells in untreated HIV infection, increasing levels with progressive disease, and reduced Tim-3 levels in patients receiving ART [8, 16, 17], but data on sTIM-3 before and after initiation of ART are scarce. A decrease of sTim-3 after initiating ART has been shown during primary HIV infection [12]. In our longitudinal cohort of patients with chronic HIV infection, we found that sTim-3 levels correlated with viral load, nadir, and current CD4 counts after 6 months of ART. In addition, we found that sTim-3 decreased after ART initiation. Moreover, whereas similar sTim-3 levels in PWH on ART and healthy controls have been reported in a small group of patients (n = 20) [13], we show in a large cross-sectional study that PWH (n = 1010) had higher levels of sTim-3 compared with HIV-noninfected controls even after several years on suppressive ART. Thus, even in patients on stable ART, sTim-3 is elevated compared with controls, suggesting a degree of immune exhaustion even in these patients, but the overlap between PWH on ART and controls suggests that it is difficult to use sTim-3 as a specific marker of exhaustion.
People with HIV coinfected with HCV are at higher risk of comorbidities such as diabetes and liver, kidney, and CVD, possibly linked to immune activation [18, 19]. Data on Tim-3 expression on CD8 T cells in PWH that are coinfected with HCV have previously been somewhat contradictory [20, 21]. We show that PWH on ART that were coinfected with HCV had even higher sTim-3 levels than PWH without HCV infection, and notably HCV viremia was one of the strongest predictors of high sTim-3 levels. This may give additional explanation to the increased risk of comorbidities in coinfected patients. Our data suggest that these coinfected individuals have an ever higher degree of T-cell exhaustion and persistent immune activation and underscore the need for treating both infections in PWH that are coinfected with HCV. With the new direct-acting antivirals and cure rates comparable to HCV monoinfected individuals, the main challenge is to identify patients and to prevent reinfection in high-risk groups [22].
In HIV, immune exhaustion is linked to comorbidities even in patients on ART and represents a potential obstacle for a cure [4]. In the cross-sectional study, we found a strong correlation between hsCRP and sTim-3, reflecting persistent inflammation and immune exhaustion as 2 faces of the dysregulated immunity in HIV even on stable ART. High-sensitivity CRP is linked to aging, immunoscenescense, morbidity, and mortality in PWH [5, 23–25], and future studies should examine whether the combination of these markers (sTim-3 and hsCRP) could give even more prognostic information in PWH. Lipopolysaccharides may contribute to Tim-3 upregulation and shedding [13], possibly linking immune exhaustion to microbial translocation, which also contributes to systemic immune activation in HIV [26], potentially reflecting a complex interacting network that promotes an accelerated aging in PWH.
Immunological nonresponders are PWH who fail to achieve optimal CD4 T-cell reconstitution after ART, and they have higher immune activation, increased risk of comorbidities, progression to AIDS, and higher mortality [25, 27, 28]. We aimed to explore the potential role of sTim-3 in these patients, but we did not find a difference in sTim-3 levels between IRs and INRs. In addition, although pre-ART sTim-3 was associated with CD4 counts 1 year after ART initiation, it did not predict CD4 T-cell count in multivariate analyses. However, the number of patients in the longitudinal study was relative low, and future studies should examine the potential for checkpoint inhibitors in HIV therapy through enhancement of therapeutic vaccine responses, reversing latency, and through enhancing T-cell function to eliminate infected cells [29].
The strengths of this study are well defined cohorts, one with 1010 participants, including 55 coinfected with HCV. However, we did not have baseline sTim-3 measurements in the cross-sectional cohort, so the predictive value of baseline sTim-3 in this cohort (for example, on INR) is unknown. We did not have available peripheral blood mononuclear cells for further characterization of immune function, which would have strengthened the study. Moreover, the noninfected control group was small (n = 76).
Conclusions
In conclusion, sTim-3 decreased after initiation of ART, but treated PWH had higher levels of sTim-3 than controls, with the highest levels in those coinfected with HCV. The correlation between hsCRP and sTim-3 might represent a link between immune activation and exhaustion in well treated PHW contributing to the increased occurrence of comorbidities and accelerated aging in these patients.
Acknowledgments
Author contributions. P. A., M. T., and S. D. N. were responsible for the study concept. S. D. N., T. B., H. U., H. J. H., M. G., and M. H.-S. were responsible for study design and inclusion of patients. T. U., H. H., and A. E. M. carried out the experiments. T. U., H. H., and M. T. did the statistical analyses. H. H., P. A., and M. T. drafted the manuscript. All coauthors participated in discussions about the interpretation of the findings and critically reviewed the manuscript.
Financial support. This work was funded by South-Eastern Norway Regional Health Authorities (Grant 39819).
Potential conflicts of interest. S. D. N. reports grants from Novo Nordisk Foundation and Rigshospitalet Research Foundation, Advisory Board and traveling grant from Gilead, and Advisory Board for GSK, outside the submitted work. T. B. reports grants from Pfizer, NovoNordisk Foundation, Simonsen Foundation, GSK, and personal fees from GSK, Pfizer, Boehringer Ingelheim, Gilead, and MSD, outside the submitted work. H. U. received an unrestricted research grant from Novartis, outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Guaraldi G, Orlando G, Zona S, et al. . Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–6. [DOI] [PubMed] [Google Scholar]
- 2. Hasse B, Ledergerber B, Furrer H, et al. ; Swiss HIV Cohort Study Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011; 53:1130–9. [DOI] [PubMed] [Google Scholar]
- 3. Doitsh G, Galloway NL, Geng X, et al. . Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 2014; 505:509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khaitan A, Unutmaz D. Revisiting immune exhaustion during HIV infection. Curr HIV/AIDS Rep 2011; 8:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sokoya T, Steel HC, Nieuwoudt M, Rossouw TM. HIV as a cause of immune activation and immunosenescence. Mediators Inflamm 2017; 2017:6825493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci 2014; 69:833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol 2005; 6:873–9. [DOI] [PubMed] [Google Scholar]
- 8. Jones RB, Ndhlovu LC, Barbour JD, et al. . Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 2008; 205:2763–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sakhdari A, Mujib S, Vali B, et al. . Tim-3 negatively regulates cytotoxicity in exhausted CD8+ T cells in HIV infection. PLoS One 2012; 7:e40146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jin HT, Anderson AC, Tan WG, et al. . Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A 2010; 107:14733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Möller-Hackbarth K, Dewitz C, Schweigert O, et al. . A disintegrin and metalloprotease (ADAM) 10 and ADAM17 are major sheddases of T cell immunoglobulin and mucin domain 3 (Tim-3). J Biol Chem 2013; 288:34529–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zilber E, Martin GE, Willberg CB, et al. ; CHERUB Investigators Soluble plasma programmed death 1 (PD-1) and Tim-3 in primary HIV infection. AIDS 2019; 33:1253–6. [DOI] [PubMed] [Google Scholar]
- 13. Clayton KL, Douglas-Vail MB, Nur-ur Rahman AK, et al. . Soluble T cell immunoglobulin mucin domain 3 is shed from CD8+ T cells by the sheddase ADAM10, is increased in plasma during untreated HIV infection, and correlates with HIV disease progression. J Virol 2015; 89:3723–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ronit A, Haissman J, Kirkegaard-Klitbo DM, et al. . Copenhagen comorbidity in HIV infection (COCOMO) study: a study protocol for a longitudinal, non-interventional assessment of non-AIDS comorbidity in HIV infection in Denmark. BMC Infect Dis 2016; 16:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hartling HJ, Jespersen S, Gaardbo JC, et al. . Reduced IL-7R T cell expression and increased plasma sCD127 in late presenting HIV-infected individuals. J Acquir Immune Defic Syndr 2017; 74:81–90. [DOI] [PubMed] [Google Scholar]
- 16. Kassu A, Marcus RA, D’Souza MB, et al. . Suppression of HIV replication by antiretroviral therapy reduces TIM-3 expression on HIV-specific CD8(+) T cells. AIDS Res Hum Retroviruses 2011; 27:1–3. [DOI] [PubMed] [Google Scholar]
- 17. Rallón N, García M, García-Samaniego J, et al. . Expression of PD-1 and Tim-3 markers of T-cell exhaustion is associated with CD4 dynamics during the course of untreated and treated HIV infection. PLoS One 2018; 13:e0193829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Veenhuis RT, Astemborski J, Chattergoon MA, et al. . Systemic elevation of proinflammatory interleukin 18 in HIV/HCV coinfection versus HIV or HCV monoinfection. Clin Infect Dis 2017; 64:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Operskalski EA, Kovacs A. HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep 2011; 8:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rallón N, García M, García-Samaniego J, et al. . HCV coinfection contributes to HIV pathogenesis by increasing immune exhaustion in CD8 T-cells. PLoS One 2017; 12:e0173943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feuth T, Arends JE, Fransen JH, et al. . Complementary role of HCV and HIV in T-cell activation and exhaustion in HIV/HCV coinfection. PLoS One 2013; 8:e59302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bruno G, Saracino A. Direct-acting antivirals for HIV/HCV co-infected individuals: as good as it gets? Curr HIV Res 2017; 15:422–33. [DOI] [PubMed] [Google Scholar]
- 23. Kuller LH, Tracy R, Belloso W, et al. ; INSIGHT SMART Study Group Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wallet MA, Buford TW, Joseph AM, et al. . Increased inflammation but similar physical composition and function in older-aged, HIV-1 infected subjects. BMC Immunol 2015; 16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vos AG, Idris NS, Barth RE, et al. . Pro-inflammatory markers in relation to cardiovascular disease in HIV infection. a systematic review. PLoS One 2016; 11:e0147484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brenchley JM, Price DA, Schacker TW, et al. . Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 27. Lichtenstein KA, Armon C, Buchacz K, et al. ; HIV Outpatient Study (HOPS) Investigators Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis 2010; 51:435–47. [DOI] [PubMed] [Google Scholar]
- 28. Piconi S, Trabattoni D, Gori A, et al. . Immune activation, apoptosis, and Treg activity are associated with persistently reduced CD4+ T-cell counts during antiretroviral therapy. AIDS 2010; 24:1991–2000. [DOI] [PubMed] [Google Scholar]
- 29. Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol 2018; 18:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]