Abstract
The Panel on Plant Health performed a pest categorisation of the soil‐borne fungus Fusarium oxysporum f. sp. albedinis, the causal agent of Fusarium wilt of date palm, for the EU. The identity of the pest is well established and reliable methods exist for its detection/identification. The pest is listed in Annex IIAI of Directive 2000/29/EC and is not known to occur in the EU. Fusarium oxysporum f. sp. albedinis is present in Morocco, Algeria and Mauritania. Its major host is Phoenix dactylifera, which is the only Phoenix species known to be affected by the pest. Uncertainty exists about the host status of Lawsonia inermis, Medicago sativa and Trifolium spp. cultivated as intercrops in the infested areas and reported as being symptomless carriers of the pest. The pest could potentially enter the EU on host plants and soil/growing media originating in infested Third countries. The current pest distribution and climate matching suggest that the pest could establish and spread in the EU wherever the host is present. In the infested areas, the pest causes vascular wilt resulting in yield/quality losses and plant death. It is expected that pest introduction and spread in the EU could impact date production. The pest is expected to have high environmental consequences in the Elche area (Spain), which is a UNESCO World Heritage Site, as well as other EU areas where P. dactylifera is grown as an amenity tree. Current EU phytosanitary measures are not fully effective at mitigating the risk of introduction and spread of the pest in the EU. Fusarium oxysporum f. sp. albedinis meets all the criteria assessed by EFSA for consideration as potential Union quarantine pest. As the pest is not known to occur in the EU, this criterion to consider it as Union regulated non‐quarantine pest is not met.
Keywords: Climate, European Union, Fusarium wilt, impacts, pest distribution, Phoenix dactylifera, quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above‐mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorizations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/20023, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pest categorisations should be delivered by end 2020.
For the above‐mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L.,Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Fusarium oxysporum f. sp. albedinis is one of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States (MSs) referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on Fusarium oxysporum f. sp. albedinis was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database. The search focussed on Fusarium oxysporum f. sp. albedinis and its geographic distribution, life cycle, host plants and the damage it causes. The following search terms (TS) and combinations were used: TS = (“Fusarium oxysporum f. sp. albedinis ” OR “Fusarium wilt of date palm” OR “Bayoud*”) AND TS = (geograph* OR distribution OR “life cycle” OR lifecycle OR host OR hosts OR plant* OR damag*). Relevant papers were reviewed and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plan Protection Organization (EPPO) Global Database (EPPO, online) and relevant publications.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network launched by the Directorate General for Health and Consumers (DG SANCO), and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the MSs and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for Fusarium oxysporum f. sp. albedinis, following guiding principles and steps presented in the EFSA guidance on the harmonised framework for pest risk assessment (EFSA PLH Panel, 2010) and as defined in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
In accordance with the guidance on a harmonised framework for pest risk assessment in the EU (EFSA PLH Panel, 2010), this work was initiated following an evaluation of the EU plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union regulated non‐quarantine pest in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required in accordance with the specific terms of reference received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a regulated non‐quarantine pest. If one of the criteria is not met, the pest will not qualify. A pest that does not qualify as a quarantine pest may still qualify as a regulated non‐quarantine pest that needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone; thus, the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism. | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pest must be present in the risk assessment area). |
| Regulatory status (Section 3.3 ) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future. |
The protected zone system aligns with the pest free area system under the International Plant Protection Convention (IPPC). The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone). |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6 ) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, whereas addressing social impacts is outside the remit of the Panel, in agreement with EFSA guidance on a harmonised framework for pest risk assessment (EFSA PLH Panel, 2010).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
Yes, the identity of Fusarium oxysporum f. sp. albedinis is well‐established.
Fusarium oxysporum f. sp. albedinis is a well‐identified fungus of the family Nectriaceae. According to Index Fungorum database (www.indexfungorum.org), the pathogen has the following taxonomical identification:
-
Preferred scientific name: Fusarium oxysporum f. sp. albedinis (Kill. & Maire) W.L. Gordon
Family – Nectriaceae
Genus – Fusarium
Species – oxysporum
Forma specialis – albedinis
Synonyms: Cylindrophora albedinis Killian & Maire
Fusarium albedinis (Killian & Maire) Malençon
Fusarium oxysporum var. albedinis (Kill. & Maire) Malençon
Teleomorph: Not known
Preferred common name: Fusarium wilt of date palm
Other common names: Bayoudh (or bayoud) disease of date palm
3.1.2. Biology of the pest
Fusarium oxysporum f. sp. albedinis is a soil‐borne fungal pathogen causing vascular wilt on Phoenix dactylifera (date palm). The pathogen survives as chlamydospores in the soil and in dead host plant tissues, particularly in the roots (Benzohra et al., 2015). Following the disintegration of the host tissues, the chlamydospores are released into the soil, where they survive for more than 8 years. The fungus has been detected at soil depths up to 30 cm, and occasionally at depths of more than 1 m (Tantaoui, 1989). Even small numbers of chlamydospores are sufficient to initiate the disease and infection of only a few roots can result in plant death (EPPO, Online). In the presence of host roots, chlamydospores germinate and enter the vascular system of the roots. Once the pathogen is inside the vascular system, it grows rapidly with the mycelium advancing up to the stem (Ghaemi et al., 2011; Laurence et al., 2012). Subsequently, the mycelium produces conidia (micro‐ and macroconidia) in the vessels, which are carried upwards by the water stream (Benzohra et al., 2015). As the conidia flow up, the vessel is impeded by a cross wall, the conidia germinate, the germ tubes penetrate the wall and then conidial formation is resumed on the other side of the wall. These new conidia are carried along the next transverse wall. This process continues upward to the terminal bud resulting in wilting and subsequent death of the plant. Following the death of the palm, the mycelium continues to grow in the parenchyma forming numerous chlamydospores (Louvet, 1977; Sutherland et al., 2013).
In general, conditions conducive to the growth of the host also favour disease development (EPPO, Online). The optimum temperatures for fungal growth range between 21 and 27.5°C; mycelial growth is slow at 18 and 32°C and stops at 7 and 37°C (Shabani and Kumar, 2013; Shabani et al., 2014). Bounaga (1975) found that the optimal temperature for mycelial growth is 28°C.
3.1.3. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, Fusarium oxysporum f. sp. albedinis can be detected and identified based on isolation of the fungus from affected plant tissues or soil, and the application of molecular methods on the culture.
F. oxysporum f. sp. albedinis is specific to P. dactylifera and causes symptoms (external and internal) similar to those caused by other vascular wilt pathogens. Cross‐pathogenicity with other formae speciales of F. oxysporum has never been observed in nature. Nevertheless, identification of the pathogen based solely on symptomatology is not reliable.
Identifying the pathogen using only cultural/morphological characters is not reliable either because formae speciales of F. oxysporum are identical, and they cannot be differentiated with these techniques from non‐pathogenic or saprophytic strains, of which there is a huge diversity, especially in soil (Summerell et al., 2003).
Vegetative compatibility of nitrate mutants (Djerbi, 1990) and Restriction Fragment Length Polymorphism (RFLP) analysis (Tantaoui et al., 1996) have been used in the past and have demonstrated the homogeneity of F. oxysporum f. sp. albedinis. However, those methods are no longer used for the detection and identification of the pathogen (EPPO, 2002).
Identification should be based on isolation of the fungus in culture media combined with molecular methods. A primer pair (TL3–FOA28) was developed by Fernandéz et al. (1998) for unambiguous diagnosis of pure cultures of Fusarium oxysporum f. sp. albedinis by polymerase chain reaction (PCR). It has been demonstrated that this test differentiates F. oxysporum f. sp. albedinis from F. oxysporum f. sp. canariensis and F. oxysporum f. sp. elaeidis, and also from saprophytic strains of F. oxysporum that can coexist in soil.
All the above methods for the detection and identification of F. oxysporum f. sp. albedinis are described in the EPPO Standard PM 7/16(1) (EPPO, 2002).
Recently, a new forma specialis, F. oxysporum f. sp. palmarum, which affects Phoenix canariensis, Syagrus romanzoffiana, Washingtonia robusta and × Butyagrus nabonnandii (a sterile hybrid of Syagrus romanzoffiana and Butia odorata) and can also be present in soil, has been described (Elliott et al., 2010, 2017; Elliot, 2011). There is no information whether the molecular method developed by Fernandéz et al. (1998) could be used to distinguish F. oxysporum f. sp. albedinis from F. oxysporum f. sp. palmarum.
Symptoms
The pathogen affects young and mature date palms, as well as the offshoots that emerge from their base (Saaidi, 1979). The first symptoms appear on one or more fronds of the middle crown. The affected fronds become ash‐grey in colour and wither from bottom to top in a characteristic way: pinnae or spines situated on one side of the frond wither progressively from the base upward to the apex (Benzohra et al., 2015). Subsequently, the other side of the frond becomes ash‐grey in colour with the withering progressing this time from the top of the frond to the base, until the whole frond dies. A brown stain appears lengthwise on the dorsal side of the rachis and advances from the base to the tip of the frond, corresponding to the passage of the mycelium in the vascular bundles of the rachis. Afterwards, the frond exhibits a characteristic arch, resembling a wet feather and hangs down along the trunk. The same symptoms then start to appear on adjacent fronds. The plant dies when the fungus and its toxins reach the terminal bud. The time between the appearance of the first symptoms on fronds and the death of the plant varies between 6 months to 2 years after the appearance of the first symptoms, depending on the planting conditions and the susceptibility of the cultivar (Bulit et al., 1967; Louvet et al., 1970; Djerbi, 1982). Sometimes, external symptoms develop differently. The brown stain appears in the middle of the rachis on its dorsal side, not unilaterally, and progresses upwards until the rachis becomes so narrow that all tissues are affected, leading to the death of the tip. Thereafter, the whitening and dying of pinnae progress downwards until the frond is killed. Other variations may occur in the early symptoms; a general yellowing may be detected before the appearance of typical symptoms, mainly during autumn and winter. A diseased plant shows relatively few affected (reddish) roots. When cut, palm fronds showing external symptoms exhibit a reddish‐brown discoloration with distinctly coloured vascular bundles. Finally, the pathogen infects the offshoots emerging at the base of the affected palm tree (Saaidi, 1979; Benzohra et al., 2015). There are no reports of the pathogen affecting peduncles, flowers or fruits of P. dactylifera (Koulla and Saaidi, 1985).
The incubation period (time between inoculation and appearance of the first symptoms) on 3‐week‐old P. dactylifera seedlings (at the two‐leaf stage) is 3 weeks (EPPO, 2002).
Morphology
Cultures on potato dextrose agar (PDA) and Czapek dox agar (CDA) are pink in colour (seen from the reverse side of the plate, especially on PDA), slimy in appearance due to the abundant production of conidia, and slow‐growing (6.0–8.5 cm diameter, after 8 days at 25°C on PDA) (EPPO, 2002).
Microconidia on synthetic nutrient poor agar (SNA) medium are produced abundantly on short, simple monophialides (8–14 μm long) arising laterally on the hyphae or from short sparsely branched conidiophores. They are hyaline, variable in form (globose in young cultures and more elongated in older cultures) and dimensions (5–12 × 2.2–3.5 μm), and are produced in mucilagenous slime.
Macroconidia (on SNA), when present (they are sparse in some strains), are borne on more elaborately branched conidiophores or on the surface of Tubercularia‐like sporodochia. They are thin‐walled, generally 3–5‐septate (3‐septate are the most commonly produced in cultures), fusoid‐subulate and pointed at both ends, occasionally fusoid‐falcate, some with a somewhat hooked apex and a pedicellate base. They range in size from 27–46 × 3–5 μm (3‐septate) to 50–66 × 3.5–5 μm (6–7‐septate).
Chlamydospores (on SNA) are abundant, 7–11 μm in diameter, smooth to rough walled, terminal or intercalary, generally solitary but occasionally in pairs or chains.
Morphology of conidia (micro‐ and macroconidia) produced on the host or on culture media other than SNA is usually very variable and thus, it is not a reliable character for the identification of the pathogen.
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
Fusarium oxysporum f. sp. albedinis has a very restricted distribution worldwide. According to EPPO Global Database (last updated 12/9/2017; last accessed 4/12/2017), the pathogen is currently present in Algeria, Morocco and Mauritania (Figure 1 and Table 2).
Figure 1.
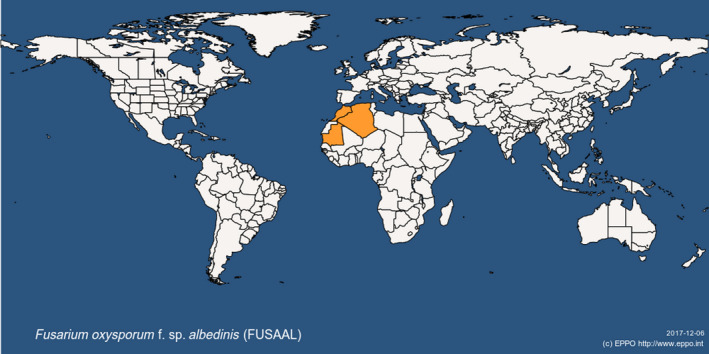
Global distribution map for Fusarium oxysporum f. sp. albedinis (extracted from the EPPO Global Database accessed on 6/12/2017)
Table 2.
Global distribution of Fusarium oxysporum f. sp. albedinis based on information extracted from the EPPO Global Database (last updated: 12/9/2017; last accessed: 6/12/2017)
| Continent | Country | Status | Sources |
|---|---|---|---|
| Africa | Algeria | Present, restricted distribution | EPPO |
| Mauritania | Present, restricted distribution | EPPO | |
| Morocco | Present, restricted distribution | EPPO |
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory?
No, Fusarium oxysporum f. sp. albedinis is not known to be present in the risk assessment area
The pathogen is not known to occur in the EU (EPPO Global Database, last updated 12/9/2017; last accessed: 4/12/2017).
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
Fusarium oxysporum f. sp. albedinis is listed in Council Directive 2000/29/EC. Details are presented in Tables 3 and 4.
Table 3.
Fusarium oxysporum f. sp. albedinis in Council Directive 2000/29/EC
| Annex II, Part A | Harmful organisms whose introduction into, and spread within, all member states shall be banned if they are present on certain plants or plant products | |
|---|---|---|
| Section I | Harmful organisms not known to occur in the community and relevant for the entire community | |
| (c) | Fungi | |
| Species | Subject of contamination | |
| 10. | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon) | Plants of Phoenix spp., other than fruit and seeds |
Table 4.
Regulated hosts and commodities that may involve Fusarium oxysporum f. sp. albedinis in Annexes III, IV and V of Council Directive 2000/29/EC
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all Member States | |
| Description | Country of origin | |
| 17. | Plants of Phoenix spp. other than fruit and seeds | Algeria, Morocco |
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the Community, before being moved within the Community—in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community | |
| Part A | Plants, plant products and other objects originating in the Community | |
| Section I | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community and which must be accompanied by a plant passport | |
| 2.3.1. | Plants of Palmae, intended for planting, having a diameter of the stem at the base of over 5 cm and belonging to the following genera: Brahea Mart., Butia Becc., Chamaerops L., Jubaea Kunth, Livistona R. Br., Phoenix L., Sabal Adans., Syagrus Mart., Trachycarpus H. Wendl., Trithrinax Mart., Washingtonia Raf. | |
| Part B | Plants, plant products and other objects originating in territories, other than those referred to in Part A |
| Section I | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community |
| 2. | Parts of plants, other than fruits and seeds, of:
|
3.3.2. Legislation addressing the hosts of Fusarium oxysporum f. sp. albedinis
Phytosanitary measures are also in place (Annex III, Part A, points 14 & 34 and Annex V, Part B, point 7 of Council Directive 2000/29/EC) for the import into the Member States of soil and growing media from Third countries (see section 3.6.1).
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
The major host of F. oxysporum f. sp. albedinis is P. dactylifera (date palm) (EPPO, Online). EPPO Global Database indicates Lawsonia inermis (henna), Medicago sativa (lucerne) and Trifolium spp. (clovers) as incidental hosts of the pathogen. These plants are often cultivated among date palms in North African and Near East countries and are reported as being symptomless carriers of the pathogen (Djerbi et al., 1985, 1986; Bengyella et al., 2012). However, in their review article, Benzohra et al. (2015) reported that to date, F. oxysporum f. sp. albedinis has only been isolated from asymptomatic L. inermis plants, but without citing any literature.
Based on the above, there is uncertainty about the host status of the so called ‘symptomless carriers of the pathogen’ as the supported literature is very old and no molecular methods existed at that time for a reliable identification of the pathogen.
Of the above‐mentioned hosts (major and incidental), only P. dactylifera is regulated in the EU.
The EU legislation indicates Phoenix spp. as hosts of F. oxysporum f. sp. albedinis. In the past, F. oxysporum f. sp. albedinis was suspected to cause wilt of Canary Island palm (P. canariensis) in Morocco, but subsequent studies on pathological properties, morphology and vegetative compatibility groupings have shown that the causal agent was F. oxysporum f. sp. canariensis and not F. oxysporum f. sp. albedinis (Djerbi et al., 1986, 1990; Sedra and Djerbi, 1986). There is no evidence of Phoenix species other than P. dactylifera being affected by F. oxysporum f. sp. albedinis.
3.4.2. Entry
Is the pest able to enter into the EU territory? If yes, identify and list the pathways!
Yes, under the current EU legislation, Fusarium oxysporum f. sp. albedinis could potentially enter the risk assessment area on (i) plants of Phoenix dactylifera, other than fruit and seeds, originating in Mauritania, (ii) soil and growing media not associated with plants originating in Morocco, and (iii) soil and growing media attached to plants (not necessarily host plants) originating in Algeria and Morocco
The PLH Panel identified the following pathways for the entry of F. oxysporum f. sp. albedinis into the EU territory:
Host plants (plants for planting, offshoots, detached leaves), excluding fruit and seeds, and
-
Soil and growing media
originating in infested Third countries.
No evidence exists for the pathogen to be seed borne or to affect fruit of P. dactylifera (dates).
The current EU legislation prohibits the import into the risk assessment area of (i) Phoenix spp. plants, other than fruit and seeds, from Algeria and Morocco, (ii) soil and growing media not associated with plants and originating in Algeria and Mauritania, and (iii) soil and growing media attached to plants and originating in Mauritania. Therefore, under the EU legislation, the relevant pathways for the entry of the pathogen into the risk assessment area are the following:
host plants, other than fruit and seeds, originating in Mauritania,
soil and growing media not associated with plants originating in Morocco, and
soil and growing media attached to plants (not necessarily host plants) originating in Algeria and Morocco.
There is uncertainty whether plants for planting of L. inermis, M. sativa and Trifolium spp. constitute a pathway of entry, as their role as asymptomatic carriers of the pathogen has not been fully established (see section 3.4.1).
No data exists in EUROSTAT (online) on imports of P. dactylifera plants for planting and soil/growing media into the EU.
There is no record of interception of F. oxysporum f. sp. albedinis in the Europhyt database (online) (search performed on 8 December 2017).
3.4.3. Establishment
Is the pest able to become established in the EU territory? (Yes or No)
Yes, both the biotic (host availability) and abiotic (climate suitability) factors suggest that Fusarium oxysporum f. sp. albedinis could potentially establish in the risk assessment area.
3.4.3.1. EU distribution of main host plants
Phoenix dactylifera is present in the EU territory in nurseries and as an amenity tree in public and private gardens, parks, avenues, etc. The region of Elche (Spain) is the biggest area in Europe where P. dactylifera is grown commercially as a compact forest (Palmeral de Elche) of 180,000 adult palms that produced 4,000 tonnes of fresh dates in 2015 (FAOSTAT, 2015; Rivera et al., 2015). This forest is unique in Europe and was declared by UNESCO as a World Heritage Site in 2000 intended to be maintained as a unique European example of an introduced oasis system, with the date palm as its key agricultural species. Other date production areas in Spain are Abanilla and Huerta de Murcia in Murcia and Albatera, Alicante, Callosa, Crevillent, and Orihuela, Comunidad Valenciana, which contribute to the overall date production of Spain (Rivera et al., 2015). Significant ornamental palm production exists also in nurseries in the Marche region of Italy (Nardi et al., 2009).
3.4.3.2. Climatic conditions affecting establishment
The pest is present in North‐East African countries characterised by BWh (Arid Desert hot), BWk (Arid Desert cold), BSh (Desert Steppe, hot), BSk (Arid Steppe cold) and Csa (Temperate dry summer, hot Summer) climate types (Peel et al., 2007). BSk and Csa climates are also present in part of the risk assessment area, mainly in Greece, Italy, Portugal, and Spain (Figure 2).
Figure 2.
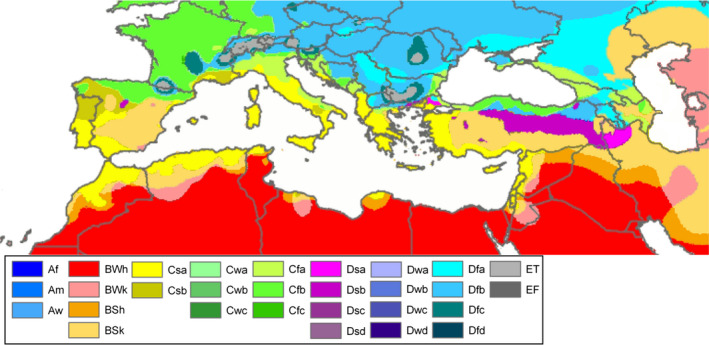
Köppen–Geiger climate type map of the Mediterranean Basin, from Peel et al. (2007)
Based on the above and on the biology of the pest, F. oxysporum f.sp. albedinis could establish in the EU where the host is present.
3.4.4. Spread
3.4.4.1. Vectors and their distribution in the EU (if applicable)
Is the pest able to spread within the EU territory following establishment? Yes
How? By natural and human‐assisted means
Once established, F. oxysporum f. sp. albedinis can spread in the risk assessment area by both natural and human‐assisted means.
Spread by natural means. According to Benzohra et al. (2017), F. oxysporum f. sp. albedinis can spread within a plantation or between plantations of date palm via:
surface water (irrigation, rain or river water). In the infested areas, the spread of the pathogen between plantations located in the same oasis occurs mainly through irrigation water. It is also considered that the pathogen spread from Draa valley in Morocco to Saoura valley in Algeria via the river that connects these two valleys.
wind, which can transport infested soil particles and spores.
root contact between diseased and healthy date palms.
Spread by human‐assisted means. The pathogen can also spread via the movement of infected host plants (plants for planting, including offshoots, detached leaves or other plant parts), infested soil and contaminated tools. It is confirmed that, in the infested areas, the spread of the disease from one oasis to another is mostly correlated to caravan‐routes of nomads who take infected palm plants or offshoots with them (Benzohra et al., 2017).
Little research has been done on the speed of disease spread within a plantation. Based on data obtained from an experimental palm grove containing 125 palm trees of the susceptible variety Bou Feggous in Zagora, Morocco (Toutain, 1970), the whole plantation was destroyed by the disease over a period of 14 years at an average rate of 6% per year.
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes, the introduction of the pest could potentially cause losses to P. dactylifera grown in the risk assessment area.
The date palm (P. dactylifera) is a cultivated plant representing an economic value for several populations in the arid regions (Loth et al., 2015). It is one of the oldest fruit‐producing plants in the world being exploited by humans. Furthermore, the Middle East and North Africa are the major date palm‐producing areas in the world. Dates have been both a food crop for local people and a source of export earnings. Date palms are essential integral components of farming systems in dry and semi‐arid regions and can be produced equally well in small farm units or as larger scale commercial plantation units. The date palm also makes a significant contribution towards the creation of equable microclimates within oasis ecosystems.
F. oxysporum f.sp. albedinis is the most important fungal pathogen of date palm (Djerbi et al., 1991; Azouaou‐Ait Kettout et al., 2010) in North Africa and particularly in Morocco. The disease was first reported in 1870 in Zagora, Morocco. By 1940, the disease had already affected several date plantations in Morocco and one century later, it had practically destroyed more than two‐thirds of the palms (12 million trees) (Fernandéz et al., 1995) as well as those in western and central Algerian Sahara (Killian and Maire, 1930; Toutain, 1965, 1972; Toutain and Louvet, 1972). Data indicate that half of the date palm plantations in Algeria (more than 3 million trees) have been killed by the pathogen (Macheix, 1992; Brac de la Perriére et al., 1995; Benzohra et al., 2015). In Morocco, the disease continues to cause the death of 4.5–12% of date palms per year (Djerbi, 1983). Many high‐quality, high‐yielding cultivars proved highly susceptible with the remaining plants in many affected regions to be of low quality and yield (Benzohra et al., 2017). Oases that formerly had 300–400 palms per hectare have been reduced to 40–50 palms per hectare (Saaidi, 1979; Djerbi et al., 1986). In Mauritania, this disease has contributed to a steady deterioration of oases and loss of some cultivars of interest, in particular good spontaneous seedling date palms, contributing to the depletion of the date palm gene pool in different regions (Sedra, 2015).
In the infested countries, date palms also provide shade for the cultivation of annual crops, so their loss has affected other aspects of agricultural production and has accelerated the processes of desertification as farmers have abandoned their land and moved to urban centres (Toutain and Louvet, 1974).
The impact of introducing F. oxysporum f. sp. albedinis in the EU areas where P. dactylifera is grown for fruit (dates) production is expected to be very high. However, the production of P. dactylifera fruit (dates) in the risk assessment area is restricted to 700 ha (data from 2015) located in the south of Spain and producing 4,000 tonnes (FAOSTAT, 2015). Because of the classification of the palm groves in Elche area as UNESCO World Heritage Site, the environmental impact as a result of the introduction of the pest would be very high. Moreover, the potential introduction of F. oxysporum f. sp. albedinis in the EU areas where P. dactylifera is grown as an amenity tree is expected to have consequences on the aesthetic and environmental value of parks and roads.
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
Yes, the likelihood of pest entry can be mitigated if host plants (plants for planting, offshoots and detached leaves) as well as soil/growing media are sourced from pest‐free areas or pest‐free places of production and are inspected and lab tested for the detection of F. oxysporum f. sp. albedinis, both at the place of origin and the EU entry point. In the infested areas, sanitation measures and agricultural practices, including resistant cultivars, are available for disease management.
Measures for preventing the entry of the pest into the risk assessment area include:
sourcing host plant material and soil/growing media from pest‐free areas or pest‐free places of production;
phytosanitary certificate for the export of host plants (plants for planting, offshoots and detached leaves) and soil/growing media from infested countries;
inspection and testing of host plants and soil/growing media prior to export to the EU and at the EU entry point.
Measures for preventing the establishment and spread of the pest in the risk assessment area include:
use of resistant P. dactylifera varieties;
use of sanitary measures (e.g. removal of infected plants or plant parts and pruning residues, disinfection of pruning/cutting tools, etc.);
crop residue management;
prevent the movement of infected plant material.
3.6.1. Phytosanitary measures
Pest‐free area, pest‐free place of production, pest‐free production site
Plant health inspection
Phytosanitary certification.
3.6.2. Biological or technical factors limiting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
The following biological and technical factors could potentially limit the feasibility and effectiveness of measures to prevent the entry into and spread within the risk assessment area of F. oxysporum f. sp. albedinis (see Section 3.1.3):
The difficulty in identifying the pathogen using only cultural/morphological characters and/or pathogenicity tests.
The difficulty in soil sampling and testing for the detection of the pest, and the uncertainty related to the differentiation of F. oxysporum f. sp. albedinis from F. oxysporum f. sp. palmarum by molecular tools.
The long incubation period (3 weeks) on infected host plant material.
3.6.3. Pest control methods
Disease control in the infested countries (e.g. Algeria, Morocco) relies mainly on internal phytosanitary measures that prevent the movement of plant material from contaminated areas (Benzohra et al., 2017). Disinfestation of soil with chemicals is expensive and difficult, except perhaps when there is an outbreak in a previously disease‐free area (Frederix and Den Brader, 1989).
It is suggested that certain soils in Morocco and Algeria may be suppressive to the pathogen, which may explain the apparent absence of the disease in some areas (EPPO, Online). Microorganisms, such as non‐pathogenic Fusarium spp. and fluorescent Pseudomonas spp., have been shown to be involved in the suppressive ability of some soils (Sneh, 1998).
The use of resistant date cultivars remains the only effective method for controlling the disease (Benzohra et al., 2017). In 1972, only 6 out of 32 Moroccan varieties showed total resistance to F. oxysporum f. sp. albedinis. Unfortunately, all those varieties produce low quality dates.
The Moroccan breeding programme of date palm is based on directed crossing between resistant and susceptible cultivars of good quality date, to select genotypes combining the two characteristics (El Modafar, 2010). The same programme has been operating since 1981 at Adrar, Algeria (Djerbi, 1982).
3.7. Uncertainty
Entry: there is uncertainty on the host status of L. inermis, M. sativa and Trifolium spp. grown as intercrops in date palm groves in infested Third countries (see Section 3.4.1).
Entry: there is no import data for the following open pathways: (i) host plants from Mauritania, (ii) soil and growing media (associated or not with plants) from Morocco, and (iii) soil and growing media associated with plants from Algeria.
Spread: There is no data about the distribution of P. dactylifera (e.g. in nurseries, parks, etc.) in the EU.
Spread: There is no information on the distance that infested soil particles and conidia of F. oxysporum f. sp. albedinis can travel by wind.
4. Conclusions
Fusarium oxysporum f. sp. albedinis meets all the criteria assessed by EFSA for consideration as potential EU quarantine pest. As the pest is not known to occur in the EU, it does not meet at least one of the criteria assessed by EFSA for consideration as Union regulated non‐quarantine pest (see Table 5).
Table 5.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of the pest (F. oxysporum f. sp. albedinis) is clearly defined and there are reliable methods for its detection and identification | The identity of the pest (F. oxysporum f. sp. albedinis) is clearly defined and there are reliable methods for its detection and identification | None |
| Absence/presence of the pest in the EU territory (Section 3.2 ) | The pest is not known to occur in the EU | The pest is not known to occur in the EU | None |
| Regulatory status (Section 3.3 ) | The pest is currently officially regulated as quarantine pest on Phoenix spp. other than fruit and seeds (Dir 2000/29/EC) | The pest is currently officially regulated as quarantine pest on Phoenix spp. other than fruit and seeds (Dir 2000/29/EC) | None |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) |
The pest could potentially enter, establish and spread in the EU. Pathways of entry:
|
The pest could potentially spread in the EU through movement of host plants for planting, soil and/or growing media, and natural means. Therefore, plants for planting is a main pathway, but not the only one. |
There is uncertainty on the host status of Lawsonia inermis, Medicago sativa and Trifolium spp. (Uncertainty 1). There is no import data on host plants for planting or soil/growing media from infested third countries (Uncertainty 2). There is no data about the distribution of P. dactylifera in the EU (Uncertainty 3). There is no information on the distance that infested soil particles and conidia can travel by wind (Uncertainty 4). |
| Potential for consequences in the EU territory (Section 3.5 ) |
The introduction and spread of the pest in the EU could cause mortality of P. dactylifera trees in nurseries as well as in areas where P. dactylifera is grown for date production (South Eastern of Spain). The introduction and spread of the pest in the EU could have a high environmental impact in the UNESCO World Heritage Site in Elche region (Spain), and consequences on the aesthetic value of parks and roads where P. dactylifera is used as an amenity tree. |
The spread of the pest in the EU could cause losses as regards the intended use of P. dactylifera plants for planting | There is no data about the distribution of P. dactylifera in the EU (Uncertainty 3). |
| Available measures (Section 3.6 ) | There are measures to prevent the entry of the pest into the EU but the currently applied phytosanitary measures are not fully effective:
|
There are no fully effective measures to prevent pest presence on host plants for planting. | None |
| Conclusion on pest categorisation (Section 4 ) | F. oxysporum f. sp. albedinis meets all the criteria assessed by EFSA above for consideration as potential Union quarantine pest | F. oxysporum f. sp. albedinis is not known to occur in the EU. Therefore, it does not meet at least one of the criteria assessed by EFSA for consideration as Union regulated non‐quarantine pest. | None |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | None | ||
Abbreviations
- CDA
Czapek dox agar
- DG SANCO
Directorate General for Health and Consumers
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- MS
Member State
- PCR
polymerase chain reaction
- PDA
potato dextrose agar
- PLH
EFSA Panel on Plant Health
- RFLP
Restriction Fragment Length Polymorphism
- SNA
synthetic nutrient poor agar
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
- UNESCO
United Nations Educational, Scientific and Cultural Organization
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Grégoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Armengol Forti J, Vloutoglou I, Bottex B and Rossi V, 2018. Scientific Opinion on the pest categorisation of Fusarium oxysporum f. sp. albedinis . EFSA Journal 2018;16(3):5183, 24 pp. 10.2903/j.efsa.2018.5183
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00371
Panel members: Claude Bragard, David Caffier, Thierry Candresse, Elisavet Chatzivassiliou, Katharina Dehnen‐Schmutz, Gianni Gilioli, Jean‐Claude Grégoire, Josep Anton Jaques Miret, Michael Jeger, Alan MacLeod, Maria Navajas Navarro, Björn Niere, Stephen Parnell, Roel Potting, Trond Rafoss, Vittorio Rossi, Gregor Urek, Ariena Van Bruggen, Wopke Van der Werf, Jonathan West and Stephan Winter.
Adopted: 31 January 2018
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © EPPO; Figure 2: © CABI
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
References
- Azouaou‐Ait Kettout T, Boucenna B, Amgoud M and Rahmania F, 2010. Essai de Lutte in vitro par le Glyphosate Contre des champignons telluriques phytopathogènes: Fusarium et Pythium . Sciences et Technologie C – N°26, 75–80. [Google Scholar]
- Bengyella L, Pranab R, Yekwa E and Waikhom S, 2012. The farmers cry: impact of heat stress on Fusarium oxysporum f. sp. dianthi, interaction with fungicides. Asian Journal of Plant Pathology, 6, 19–24. [Google Scholar]
- Benzohra IE, Megateli M and Berdja R, 2015. Bayoud disease of date palm: history, epidemiology and integrated disease management. African Journal of Biotechnology, 14, 542–550. 10.5897/AJBX2014.14292 [DOI] [Google Scholar]
- Benzohra IE, Megateli M, Elayachi BA, Zekraoui M, Djillali K, Bouafia A, Benouis S, Benaziza A and Rekis A, 2017. Integrated management of Bayoud disease on date palm (Phoenix dactylifera L.) caused by Fusarium oxysporum f. sp. albedinis in Algeria. Journal Algérien des Régions Arides, 14, 93–100. [Google Scholar]
- Bounaga N, 1975. Germination de microconidies et macroconidies de Fusarium oxysporum f. sp. albedinis . Bulletin de la Société D'Histoire Naturelle de L'Afrique du Nord, 66, 39–44. [Google Scholar]
- Brac de la Perriére RA, Amir H and Bounaga N, 1995. Prospects for integrated control of “bayoud” (Fusarium wilt of the date palm) in Algerian plantations. Crop Protection, 14, 227–235. [Google Scholar]
- Bulit J, Bouhot D, Louvet J and Toutain G, 1967. Recherches sur les fusarioses. I. Travaux sur le bayoud fusariose vasculaire du palmier dattier en Afrique du Nord. Annales des Epiphyties, 18, 213–239. [Google Scholar]
- Djerbi M, 1982. Bayoud disease in North Africa: history distribution, diagnosis and control. Date Palm Journal, 1, 153–197. [Google Scholar]
- Djerbi M, 1983. Diseases of the date palm Phoenix dactylifera. FAO, Baghdad, Iraq. [Google Scholar]
- Djerbi M, 1990. Méthodes de diagnostic du Bayoud du palmier dattier. Bulletin OEPP/EPPO Bulletin, 20, 607–613. [Google Scholar]
- Djerbi M, El Ghorfi A and El Idrissi Ammari MA, 1985. Etude du comportement du henné Lawsonia inermis et de la luzerne Medicago sativa et quelques espèces de palmacées vis‐à‐vis du Fusarium oxysporum f.sp. albedinis, agent causal du bayoud. Annales de L'Institut National de la Recherche Agronomique de Tunisie, 58, 1–11. [Google Scholar]
- Djerbi M, Aouad L, Filali H, Saaidi M, Chtioui A, Sedra MH, Allaoui M, Hamdaoui T and Oubrich M, 1986. Preliminary results of selection of high‐quality bayoud‐resistant clones among natural date palm population in Morocco. In: Proceedings of the Second Symposium on the Date Palm, Saudi Arabia, March 3‐6, 1986, Jumada, pp. 383–399.
- Djerbi M, Frederix MJJ, Den Braber K, Cheikh Aissa A and Skouti S, 1991. Mise au point sur I'tradication du bayoud: efficacite du bromure de methyle seul ou associe à la chloropicrine (50%‐50%) sur Fusarium oxysporum f. sp. albedinis en plein champ. Bull. du Reseau Maghrebin de Recherche sur la Phomiciculture et la Protection du Palmier Dattier, 3, 25–38. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2010. PLH Guidance on a harmonised framework for pest risk assessment and the identification and evaluation of pest risk management options by EFSA. EFSA Journal 2010;8(2):1495, 66 pp. 10.2903/j.efsa.2010.1495 [DOI] [Google Scholar]
- El Modafar C, 2010. Mechanisms of date palm resistance to Bayoud disease: current state of knowledge and research prospects. Physiological and Molecular Plant Pathology, 74, 287–294. [Google Scholar]
- Elliot LL, 2011. First Report of Fusarium Wilt Caused by Fusarium oxysporum f. sp. palmarum on Canary Island Date Palm in Florida. Plant Disease, 95, 356. 10.1094/PDIS-11-10-0851 [DOI] [PubMed] [Google Scholar]
- Elliott ML, Des Jardin EA, O'Donnell K, Geiser DM, Harrison NA and Broschat TK, 2010. Fusarium oxysporum f. sp. palmarum, a novel forma specialis causing a lethal disease of Syagrus romanzoffiana and Washingtonia robusta in Florida. Plant Disease, 94, 31–38. [DOI] [PubMed] [Google Scholar]
- Elliott ML, Des Jardin EA, Harmon CL and Bec S, 2017. Confirmation of Fusarium Wilt Caused by Fusarium oxysporum f. sp. palmarum on × Butyagrus nabonnandii (mule palm) in Florida. Plant Disease, 101 (In press) [Posted online on 14 Nov 2016 at 10.1094/pdis-08-16-1099-pdn] [DOI]
- EPPO (European and Mediterranean Plant Protection Organization), 2002. Diagnostic protocols for regulated pests: PM 7/16(1)‐Fusarium oxysporum f. sp. albedinis . Bulletin OEPP/EPPO Bulletin, 33, 265–269 [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), online. EPPO Global Database. Available online: https://gd.eppo.int [Accessed: 06/12/2017].
- Europhyt , online. The European Network of Plant Health Information System. EUROPHYT database. Available online: https://europhyt.ec.europa.eu [Accessed: 08/12/2017]
- EUROSTAT , Online. http://ec.europa.eu/eurostat/web/agriculture/data/database
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013.ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- FAOSTAT , 2015. FAO food and agriculture data. Available online: www.fao.org/faostat/
- Fernandéz M, Lourd M, Ouinten M, Tantaoui A and Geiger JP, 1995. Le Bayoud du palmier dattier, une maladie qui menace la phoeniciculture. Phytoma, 469, 36–39. [Google Scholar]
- Fernandéz D, Ouinten M, Tantaoui A, Geiger J‐P, Daboussi M‐J and Langin T, 1998. Fot 1 insertions in the Fusarium oxysporum f. sp. albedinis genome provide diagnostic PCR targets for detection of the date palm pathogen. Applied and Environmental Microbiology, 64, 633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederix MJJ and Den Brader K. 1989. Résultats des essais de désinfection des sols contenant des échantillons de Fusarium oxysporum f.sp. albedinis. FAO/PNUD/RAB/88/024. Ghardaia, Algeria. [Google Scholar]
- Ghaemi A, Rahimi A and Banihashemi Z, 2011. Effects of water stress and Fusarium oxysporum f. sp. lycopersici on growth (leaf area, plant height, shoot dry matter) and shoot nitrogen content of tomatoes under greenhouse conditions. Iran Agricultural Research, 29, 51–62. [Google Scholar]
- Killian C and Maire R, 1930. Le Bayoud, maladie du dallier. Bulletin de la Société D'Histoire Naturelle de L'Afrique du Nord, 21, 89–101. [Google Scholar]
- Koulla L and Saaidi M, 1985. Etude du rôle des inflorescences et de fruits du palmier dattier dans la dissémination du Bayoudh. Séminaire National sur l'Agronomie Saharienne, pp. 67–70. INRA, Marrakech, Morocco.
- Laurence M, Burgess L, Summerell B and Liew E, 2012. High levels of diversity in Fusarium oxysporum from non‐cultivated ecosystems in Australia. Fungal Biology, 116, 289–297. [DOI] [PubMed] [Google Scholar]
- Loth M, Meddah B, Tir Touil A, Len C and Verstraete W, 2015. Antifungal activity of synthesized dithiocarbamate derivatives on Fusarium oxysporum f. sp. albedinis in Algeria. Journal of Chemical and Pharmaceutical Research, 7, 49–54. [Google Scholar]
- Louvet J, 1977. Observations sur la localisation des chlamydospores de Fusarium oxysporum dans les tissus des plantes parasites. Travaux de Diesil G. Viennot‐Bourgin, INRA Societe Française de Phytopathologic, pp. 193–197
- Louvet J, Bulit J, Toutain G and Rieuf P, 1970. Le bayoud, fusariose vasculaire du palmier dattier, symptômes et nature de la maladie, moyens de lutte. Al‐Awamia, 35, 161–182. [Google Scholar]
- Macheix JJ, 1992. Les polyphénols, marqueurs potentiels de la résistance du palmier dattier (Phoenix dactylifera L.) au Fusarium oxysporum f. sp. albedinis. In: XVI International Conference in Association with the Royal Society of Chemistry. Groupe Polyphenol, Lisboa, Portugal, pp. 346–349.
- Nardi S, Ricci E, Lozzi R, Marozzi F, Ladurner E, Chiabrando F, Isidoro N and Riolo P, 2009. Use of entomopathogenic nematodes for the control of Paysandisia archon Burmeister. IOBC/Wprs Bulletin, 45, 375–378. [Google Scholar]
- Peel MC, Finlayson BL and McMahon TA, 2007. Updated world map of the Köppen‐Geiger climate classification. Hydrology and Earth System Sciences, 11, 1633–1644. [Google Scholar]
- Rivera D, Obón C, Alcaraz F, Carreño E, Laguna E, Amorós A, Johnson DV, Díaz G and Morte A, 2015. Date Palm Status and Perspective in Spain. In: Al‐Khayri JM, Jain SM and Johnson DV (eds.), Date Palm Genetic Resources and Utilization: Volume 2: Asia and Europe, 10.1007/978-94-017-9707-8_15 [DOI]
- Saaidi M, 1979. Contribution à la lutte contre le Bayoud, fusariose vasculaire du palmier dattier. Thèse d'université, Dijon, France. [Google Scholar]
- Sedra M, 2015. Date palm status and perpective in Mauritania. In: Al‐Khayri JM, Jain SM and Johnson DV (eds.). Date Palm Genetic Resources and Utilization: Volume 1: Africa and the Americas, Springer Science+Business Media, Dordrecht. pp. 325–368. [Google Scholar]
- Sedra MH and Djerbi M, 1986. Comparative study of morphological characteristics and pathogenicity of two Fusarium oxysporum causing respectively the vascular wilt disease of date palm (Bayoudh) and Canary Island palm. In: Proceedings of the Second Symposium on the Date Palm, Saudi Arabia, March 3‐6, 1986, Jumada. pp. 359–365.
- Shabani F and Kumar L, 2013. Risk levels of invasive Fusarium oxysporum f. sp. in areas suitable for date palm (Phoenix dactylifera) cultivation under various climate change projections. PLoS ONE, 8, e83404. 10.1371/journal.pone.0083404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani F, Kumar L and Esmaeili A, 2014. Future distributions of Fusarium oxysporum f. spp. in European, Middle Eastern and North African Agricultural Regions under Climate Change. Agriculture Ecosystems and Environment, 197, 96–105. 10.1016/j.agee.2014.08.005 [DOI] [Google Scholar]
- Sneh B, 1998. Use of non‐pathogenic or hypovirulent fungal strains to protect plants against closely related fungal pathogens. Biotechnology Advances, 16, 1–32. [DOI] [PubMed] [Google Scholar]
- Summerell B, Salleh B and Leslie JF, 2003. A Utilitarian Approach to Fusarium Identification. Plant Disease, 87, 117–128. 10.1094/PDIS.2003.87.2.117 [DOI] [PubMed] [Google Scholar]
- Sutherland R, Viljoen A, Myburg AA, Van Den Berg N. 2013. Pathogenicity associated genes in Fusarium oxysporum f.sp. cubense race 4. Siuth Afr. J. Sci. 109, 1–10. [Google Scholar]
- Tantaoui A, 1989. Contribution à l’étude de l’écologie du Fusarium oxysporum f.sp. albedinis agent causal du bayoudh. Densité et répartition de l'inoculum au sein du peuplement fusarien. D.E.S., University Cadi Ayyad, Marrakech, Morocco.
- Tantaoui A, Ouinten M, Geiger J‐P and Fernandez D, 1996. Characterization of a single clonal lineage of Fusarium oxysporum f. sp. albedinis causing bayoud disease of date palm in Morocco. Phytopathology, 86, 787–792. [Google Scholar]
- Toutain G, 1965. Note sur l’ épidémiologie du Bayou den Afrique du Nord. Al Awamia, 15, 37–45. [Google Scholar]
- Toutain G, 1970. Observations sur Ia progression d'un foyer actif de Bayoud ans une plantation reguliere de Palmier dattier. AI Awamia, 35, 155–161. [Google Scholar]
- Toutain G, 1972. Progression du Bayoud en palmeraies etablies sur terrain sales. AI‐Awamia, 42, 65–75. [Google Scholar]
- Toutain G and Louvet J, 1972. Resistance to Bayoud in varieties of date palm. First International Seminar and Workshop on Bayoud, Algiers, October 1972, I.T.A.S. Alger, pp. 208‐210.
- Toutain G and Louvet J, 1974. Lutte contre le bayoud. IV. Orientations de la lutte au Maroc. Al‐Awamia, 53, 114–162. [Google Scholar]


