Abstract
The Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to deliver a scientific opinion on the safety of monacolins in red yeast rice (RYR) and to provide advice on a dietary intake of monacolins that does not give rise to concerns about harmful effects to health. The Panel reviewed the scientific evidences available as well as the information provided by interested parties in response of a public ‘Call for data’ launched by EFSA. The Panel considered that monacolin K in lactone form is identical to lovastatin, the active ingredient of several medicinal products authorised for the treatment of hypercholesterolaemia in the EU. On the basis of the information available, the Panel concluded that intake of monacolins from RYR via food supplements, could lead to estimated exposure to monacolin K within the range of the therapeutic doses of lovastatin. The Panel considered that the available information on the adverse effects reported in humans were judged to be sufficient to conclude that monacolins from RYR when used as food supplements were of significant safety concern at the use level of 10 mg/day. The Panel further considered that individual cases of severe adverse reactions have been reported for monacolins from RYR at intake levels as low as 3 mg/day. The Panel concluded that exposure to monacolin K from RYR could lead to severe adverse effects on musculoskeletal system, including rhabdomyolysis, and on the liver. In the reported cases, the product contained other ingredients in addition to RYR. However, these reported effects in particular musculoskeletal effects, have both occurred after ingestion of monacolin K and lovastatin independently. On the basis of the information available and several uncertainties highlighted in this opinion, the Panel was unable to identify a dietary intake of monacolins from RYR that does not give rise to concerns about harmful effects to health, for the general population, and as appropriate, for vulnerable subgroups of the population.
Keywords: monacolin K, red yeast rice, Monascus purpureus, lovastatin, food supplements, musculoskeletal effects, cholesterol
Summary
Following a request from the European Commission, the EFSA Panel on Food Additives and Nutrient Sourced Added to Food (ANS) was asked to deliver a scientific opinion on the evaluation of monacolins in red yeast rice (RYR) in accordance with Article 8 (2) of Regulation (EC) No 1925/2006 on the addition of vitamins and minerals and of certain other substances to foods.
In respect to the approach to be followed for the assessment of monacolins, the ANS Panel was of the view that previous assessments, when relevant to the safety issues that triggered the Article 8 procedures, should be used as starting points for this scientific opinion. The effect of other possible ingredients of food supplements on monacolins bioactivity is not considered in this opinion.
The current evaluation is based on the published scientific literature available up to May 2018, monographs and risk assessment reports by national and international authorities and the data available following the launch of a public ‘Call for data’.
RYR is made by fermentation of rice with yeasts, mainly Monascus purpureus. RYR is traditionally used in China for culinary purposes as a food colouring or as a traditional remedy to promote digestion and blood circulation.
In 2013, the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) issued a scientific opinion on the substantiation of a health claim related to monacolin K from RYR and maintenance of normal blood LDL‐cholesterol concentration. The NDA Panel concluded that a cause and effect relationship had been established between the consumption of 10 mg/day of monacolin K from RYR and the claimed effect.
The ANS Panel considered that monacolin K in lactone form is identical to lovastatin, the active ingredient of several medicinal products authorised for the treatment of hypercholesterolaemia in the European Union (EU).
On the basis of the data received from interested parties in response of a public ‘Call for data’ launched by EFSA, the Panel noted that the recommended daily intake of monacolin K from RYR ranges from 9 to 20 mg. The Panel further noted that the recommended daily intake of 20 mg of monacolin K is reported for one product only that according to the interested party is planned to be delisted.
For the purpose of this scientific opinion, the Mintel Global New Products Database (Mintel GNPD) was used for checking the labelling of products containing monacolin K from RYR within the EU countries’ food products as the Mintel GNPD shows the compulsory ingredient information presented in the labelling of products. According to the Mintel GNPD, monacolin K was labelled on 40 products between January 2012 and February 2018. The food category considered was: ‘Vitamins & Dietary Supplements’. The concentrations of monacolin K were also retrieved from this database, when available. In all these products, the content of monacolin K was clearly stated. The daily consumption in terms of monacolin k for each product was calculated by multiplying the intake unit of monacolin K by the daily number of recommended intakes of the product.
The recommended daily intakes range between 2 and 48 mg of monacolin K, but the majority of food supplements (around 25%) supplies a recommended a daily intake of 10 mg according to the level established by the European Food Safety Authority (EFSA) for supporting the health claim (EFSA NDA Panel, 2013). The Panel noted that only 8 out of 40 products were mono‐ingredient, containing only RYR preparation; in the remaining 32 products, the most frequently associated ingredients were artichoke extract, berberine (Berberis aristata), coenzyme Q10, chromium, policosanol, resveratrol and vitamins.
On the basis of the information retrieved from the Mintel GNPD, the Panel noted that two products reported recommended daily intake of 20 or 48 mg of monacolin K.
Monacolin K and lovastatin are rapidly converted from their lactone to an identical hydroxy acid (HA) form, the latter being responsible for the inhibition of the 3‐hydroxy‐3 methylglutaryl‐coenzyme A (HMG‐CoA) reductase enzyme involved in the biosynthesis of cholesterol. While the acidic form is naturally occurring in RYR, in the case of lovastatin its generation requires conversion from the lactone form.
The bioavailability of lovastatin increases by 30–50% when taken with a standard meal. Due to the involvement of the CYP3A4 isoform in its metabolism, interactions with drugs or food ingredients which are inhibitors of this enzyme have been described, leading to increased plasma levels of statins and possible increased risk of toxic effects. There are indications to support the fact that the monacolin K in RYR may have a different concentration–time profile compared to the corresponding lovastatin. Two studies present conflicting results. In the first one, the serum concentration of monacolin K (in the two forms of lactone and HA) was significantly higher (approximately 4 times) when the subjects were orally administrated with an equivalent intake of monacolin K from RYR or lovastatin (Chen et al., 2013). In this study, an intake of 5–6 mg of monacolin K was considered by the authors bioequivalent to 20–40 mg of lovastatin. The study by Li et al. (2005) indicates instead an opposite result, which could have been influenced by the pretreatment and simultaneous administration of grapefruit juice, a known CYP3A4 inhibitor.
The Panel noted that in some cases, the intake of RYR was associated with other botanicals (either in multi‐ingredient preparations or concomitantly) or other medicinal products, which could be responsible for any side effect(s). However, the reported side effects, in particular musculoskeletal side effects with elevated creatine phosphokinase (CPK) up to several thousand units have occurred after ingestion of monacolin K and lovastatin but not after ingestion of other botanical ingredients.
The therapeutic dose of lovastatin ranges between 10 and 80 mg/day. Considering six clinical trials, where RYR was the only treatment used to reduce hypercholesterolaemia, it was shown that changes in total cholesterolaemia and LDL‐cholesterolaemia were dose‐dependent. The lowest intake of monacolin K, which was effective in reducing total cholesterolaemia (−11.2%) and LDL‐cholesterolaemia (−14.8%) was 3 mg/day.
According to four clinical studies (Dujovne et al., 1991; Furberg et al., 1994; Weintraub et al., 1994; Downs et al., 2001), the published case reports, and EMA Pharmacovigilance, the most frequent adverse effects to lovastatin have as a target the following organs/systems, in decreasing order of frequency: musculoskeletal and connective tissue, including rhabdomyolysis (53.4% of EMA reports), nervous system (20.3%), gastrointestinal tract (14.9%), kidney (11.3%), liver (10%), skin and subcutaneous tissue (8.9%), and other minor targets.
The Panel noted that the profile of adverse effects to RYR is similar to that of lovastatin; through consultation of four sources of case reports (WHO, ANSES, Italian Surveillance system, FDA), the most important targets for adverse events are: (1) musculoskeletal and connective tissue (29.9–37.2% of cases, including 1–5% of rhabdomyolysis); liver (9–32%); nervous system (when reported, 12.8–26.9%); gastrointestinal tract (12–23.1%); skin and subcutaneous tissue (8–17.3%).
The Panel noted that in some cases, the intake of RYR was associated with other botanicals (either in multi‐ingredient preparations or concomitantly) or other medicinal products, which could be responsible for any side effect(s). However, the reported side effects, in particular musculoskeletal side effects with elevated CPK up to several thousand units have occurred after ingestion of monacolin K and lovastatin but not after ingestion of other botanical ingredients.
Case reports specifying the daily intake of monacolin K showed that adverse effects appeared also with the intake of 3 mg/day for period ranging between 2 weeks and 1 year. Cases of rhabdomyolysis, hepatitis and skin disorders occurred and required hospitalisation (Mazzanti et al., 2017).
With respect to the data collected on the safety of use of RYR (and in particular of monacolins), the Panel identified the following uncertainties:
the composition and content of monacolins (and their relative abundance) in food supplements containing RYR;
monacolins in RYR are used in multi‐ingredients botanical preparations, the components of which have not been fully evaluated individually or in combination;
the ratio between monacolin K lactone and monacolin K HA is variable in food supplements containing RYR;
lack of data on the bioactivity of components in RYR other than monacolin K;
due to the lack of data, the safe use of monacolins in certain groups of consumers cannot be evaluated (pregnant women, nursing women, breastfed infants);
the effects of concomitant consumption of RYR‐based food supplements with foods or drugs inhibiting CYP3A4;
in the majority of cases, RYR‐based food supplements are multi‐ingredients products. Interactions with other ingredients on the safety of monacolins are unknown.
The Panel concluded that monacolin K in lactone form is identical to lovastatin, an active ingredient of several medicinal products.
The Panel further concluded that intake of monacolins from RYR via food supplements, could lead to estimated exposure to monacolin K within the range of the therapeutic doses of lovastatin.
The Panel considered that the available information on the adverse effects reported in humans were judged to be sufficient to conclude that monacolins from RYR when used as food supplements were of significant safety concern at the use level of 10 mg/day. The Panel further considered that individual cases of severe adverse reactions have been reported for monacolins from RYR at intake levels as low as 3 mg/day.
The Panel concluded that exposure to monacolin K from RYR could lead to severe adverse effects on musculoskeletal system, including rhabdomyolysis, and on the liver. In the reported cases, the product contained other ingredients in addition to RYR. However, these reported effects, in particular musculoskeletal effects, have both occurred after ingestion of monacolin K and lovastatin independently.
On the basis of the information available and several uncertainties highlighted in this opinion, the Panel was unable to identify a dietary intake of monacolins from RYR that does not give rise to concerns about harmful effects to health, for the general population, and as appropriate, for vulnerable subgroups of the population.
1. Introduction
Following a request from the European Commission to the European Food Safety Authority (EFSA), the Scientific Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to provide a scientific opinion on the safety of monacolins from red yeast rice (RYR).
This risk assessment was carried out in the framework of the procedure under Article 8(2) of Regulation (EC) No 1925/2006 on the addition of vitamins and minerals and of certain other substances to foods, for monacolins from RYR, initiated by the European Commission. Article 8(2) of Regulation (EC) No 1925/2006 is referring to a possible prohibition, restriction or Community scrutiny of a substance or ingredient by placement in Annex III, Part A, B or C of this Regulation.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
In 2011 and 2013, the European Food Safety Authority (EFSA) issued opinions on the scientific substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL‐cholesterol concentrations.1 In these opinions, the EFSA Panel concluded that a cause and effect relationship has been established between the consumption of monacolin K from red yeast rice and maintenance of normal blood LDL‐cholesterol concentrations. In relation to restrictions of use, the EFSA Panel referred to the Summary of Product Characteristics (SmPC) of lovastatin‐containing medicinal products available on the EU Market. In the SmPC for such products, special warnings and precautions for use refer to the risk of myopathy/rhabdomyolysis, which is increased by concomitant use of lovastatin with certain other medicinal products, and discouraged use of lovastatin by pregnant and lactating women.
EFSA and its opinions also made reference to the EFSA CONTAM Panel's opinion on citrinin (a nephrotoxic mycotoxin),2 which can be produced by some strains of Monascus purpureus.
The presence of citrinin in red yeast rice preparations has not been an issue of concern, since the adoption of Commission Regulation (EU) No 212/2014 of 6 March 2014 amending Regulation (EC) No 1881/2006 as regards maximum levels of the contaminant citrinin in food supplements based on rice fermented with red yeast Monascus purpureus.3
Furthermore, the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) in its opinion4 on the risk associated with the presence of “red yeast rice” in food supplements concluded that “due to the composition of red yeast rice and in particular: the presence of monacolin K (also called lovastatin when marketed as a drug) that shares the adverse effects of statins; the presence at varying levels of the other monacolins, compounds whose safety has not been established, consumption of “red yeast rice” exposes some consumers to a health risk”.
On 13 February 2016, the Belgian Superior Health Council adopted a scientific advisory report5 that “provides an evaluation of the supposed beneficial effects and possible toxicity of dietary supplements based on red yeast rice for the Belgian population”, and the report “also provides specific recommendations to the authorities in charge of public health and nutrition policies, healthcare professionals and the general population”. This report refers to the risk associated with the presence of monacolins, in particular monacolin K, in red yeast rice that include adverse effects identical to those observed in patients taking statin drugs. The report concludes that the main toxic effect is muscular with a risk of renal failure in the case of rhabdomyolysis, and that functional digestive disorders are common, however hepatotoxicity is relatively rare.
The report further concludes that certain vulnerable group are at a higher risk of developing toxic effects, including pregnant women, people suffering from liver, kidney and muscle disorders, persons aged over 70 years and children and adolescents.
Other relevant scientific assessments include a “toxicological evaluation of red mould rice” carried out in 2013 by the German research funding organisation DFG.6 This assessment concludes that “red mould rice is not a safe food/food supplement”.
In the light of above opinions, Member States raised concerns during discussions on the possible authorisation of the above‐mentioned health claim, due to the potential risk for consumers linked with the consumption of monacolin K from red yeast rice.
Consequently, the Commission has initiated the procedure under Article 8 (2) of Regulation (EC) No 1925/2006 on the addition of vitamins and minerals and of certain other substances to foods,7 for monacolins from red rice.
1.1.2. Terms of Reference
In accordance with Article 29(1)(a) of Regulation (EC) No 178/20028, the European Commission asks EFSA to:
Review the existing scientific data on the possible link between the intake of monacolins from red yeast rice and harmful effects on health.
Provide advice on a dietary intake of monacolins from red yeast rice that does not give rise to concerns about harmful effects to health, for the general population, and as appropriate, for vulnerable subgroups of the population.
1.2. Interpretation of the Terms of Reference
In respect to the approach to be followed for the assessment of monacolins, the ANS Panel was of the view that previous assessments, when relevant to the safety issues that triggered the Article 8 procedures, should be used as starting points for this scientific opinion.
Monacolin K has been specified as the food constituent in RYR preparations which is responsible for the claimed effect on the maintenance of normal blood LDL‐cholesterol concentrations. In assessing the safety of monacolins, the Panel considered monacolin K and lovastatin identical, the latter being the active ingredients of medicinal product authorised in the EU for the treatment of hypercholesterolaemia. The Panel noted that in the health claim (EFSA NDA Panel, 2013), the intake of monacolin K is 10 mg/day for the maintenance of normal blood LDL‐cholesterol concentrations. This intake of monacolin K overlaps the lowest therapeutic dose of 10 mg/day of lovastatin indicated as the starting dose for the treatment of hypercholesterolaemia (SPC for lovastatin, online).
The effect of other possible ingredients of food supplements on monacolins bioactivity is not considered in this opinion.
1.3. Definition and identification of monacolins from red yeast rice
In its 2013 scientific opinion on the substantiation of health claims, the EFSA NDA Panel describe monacolins as polyketides that are secondary metabolites produced by the fermentation of red rice with the fungus M. purpureus.
Because monacolin K in lactone form is identical to lovastatin,9 data on the latter have also been considered relevant for this opinion (see Figure 1).
Figure 1.
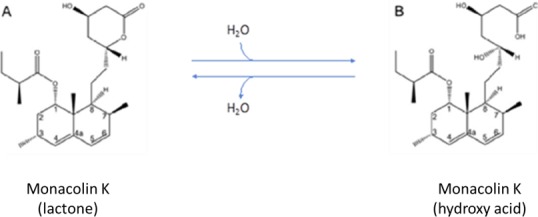
Chemical structures of monacolin K as a lactone (A) and its interconversion to monacolin K hydroxy acid (B) in aqueous media
Although the Panel considered that the lactone form of monacolin K and lovastatin are identical chemical substances, in the current opinion, the two terms ‘monacolin K’ and ‘lovastatin’ have been used when referring to the compound present in RYR and the medicinal product, respectively (i.e. whenever data were generated with RYR, the term monacolin K has been used, in the case of data related to medicinal products, the term lovastatin has been used.
1.4. Data and methodologies
1.4.1. Data
The evaluation is based on the published scientific literature available up to May 2018, monographs and risk assessment reports by national and international authorities and the data available following the launch of a public ‘Call for data’.
1.4.2. Methodologies
The assessment was conducted in line with the principles described in the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
The risk assessment was performed according to the EFSA Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements (EFSA Scientific Committee, 2009).
1.5. Information on existing assessments
As specified in the Background (Section 1.1) the Superior Health Council of Belgium (‘Documentation provided to EFSA’ n. 1), French Agency for Food, Environmental and Occupational Health and Safety (ANSES) (‘Documentation provided to EFSA’ n. 2) and Joint Expert Committee of Federal Office of Consumer Protection and Food Safety (BVL) and the Federal Institute for Drugs and Medical Devices (BfArM) (‘Documentation provided to EFSA’ n. 3 and 4) have also evaluated the safety assessment of monacolins from RYR.
The Panel is also aware that RYR preparations containing monacolins have been evaluated by a number of Committees or international organisations for their potential beneficial effects and/or for their potential adverse effects as food or as medicinal products (SKLM, 2012; Health Canada, 2014; FDA, 2014; Chinese Ministry of Public Health, 2015).
1.5.1. European Food Safety Authority (EFSA)
In 2011 and 2013, the EFSA NDA Panel issued opinions on the substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL‐cholesterol concentrations.
On the basis of the data available, the NDA Panel in both cases concluded that a cause and effect relationship had been established between the consumption of monacolin K from RYR and maintenance of normal blood LDL‐cholesterol concentrations. According to the NDA Panel in order to obtain the claimed effect, monacolin K from fermented RYR preparations should be consumed at the intake of 10 mg/day. The consumption of monacolin K from RYR is intended in adult population only. In relation to restrictions of use, the NDA Panel referred to the SPC of lovastatin‐containing medicinal products available on the EU market (EFSA NDA Panel, 2011, 2013).
2. Technical data
2.1. Identity and nature of the source material
RYR is traditionally prepared by fermenting rice with the yeast, M. purpureus.
In response of a ‘Call for data’ interested parties submitted data on classifications of Monascus purpureus and on the rice. However, the Panel considered that this information was incomplete (‘Documentation provided to EFSA’ n. 5, 6, 9 and 11). Therefore, the Panel considered retrieving information on the classification of yeast and rice from relevant databases publicly available (Table 1).
Table 1.
Information related to the classification of rice and yeast, as ingredient of RYR
| Plant | Yeast | |
|---|---|---|
| Family | Poaceae | Monascaceae |
| Genus | Oryza L. | Monascus |
| Species | Oryza sativa L. | Monascus purpureus (Went 1895) |
| Variety | – | |
| Synonyms | Red rice koji or Hongqu | – |
| Part used | Rice grain, rice kennel, fruit, seeds | – |
| Geographical origin | Hangzhou, China, Asia | – |
| Growth and harvesting conditions | Standard plain rice | – |
The Panel noted that no information was provided by interested parties on species other than M. purpureus used in the manufacturing of RYR.
2.2. Manufacturing process
Specific request for information on the manufacturing process used for the botanical ingredients used in food supplements containing monacolin K was included in the ‘Call for data’ launched. The information received was complemented by the Panel with data retrieved in the published literature. According to interested parties, red yeast fermented rice is traditionally produced by cultivating the yeast M. purpureus on rice. Rice is sterilised and then inoculated with a M. purpureus starter. A fermentation process at controlled temperature follows for several days. No solvents are used during the manufacturing process (‘Documentation provided to EFSA’ n. 5, 6, 7, 9, 10 and 11).
The rice is first soaked in water until the grains reach a semi‐gelatinous state. Inoculation is done by adding a Monascus starter culture. The mix is then incubated and periodically stirred in a temperature‐controlled chamber for some days. During this period of time, the rice grains turn bright red in their core and reddish purple on the outside (Chen et al., 2005).
Several modifications have been applied to the fermentation process (nitrogen source, pH, temperature, water supply, etc.), which can modulate the resulting final product in term of content in monacolin K, pigments, citrinin, and other minor compounds (Chen et al., 2015a).
According to Zhang et al. (2000), the traditional approach allows the production of RYR derivatives containing low level of monacolin K, while a patent from the same authors describes a new process producing a RYR‐based product with a content of monacolins of at least 0.005% and up to 2% (Zhang et al., 2000).
It has been reported that Monascus purpureus strains (N301 and N310), which are characterised by very high monacolin K and low citrinin production, have been selected and bred to allow manufacture of RYR products on an industrial scale (Wang et al., 2004).
The strain of M. purpureus is critical for the levels of monacolins and citrinin measured in the final products (Chen and Hu, 2005), but also the fermentation processes and growing conditions (medium, temperature) can contribute to the variability (Borresen et al., 2012; Chen et al., 2015a).
2.3. RYR and traditional use
RYR is traditionally used in China as a food colour and for therapeutic purposes (Chinese Medical Herbology and Pharmacology, 2004). It is produced by fermentation of a microscopic fungus grown on cooked rice (Ma et al., 2000; NIH, 2015). Rice fermented in this way becomes red due to the presence of fungal pigments. The fungus used for fermentation belongs to the class Ascomycetes, order Eurotiales, family Monascaceae and genus Monascus. Traditionally prepared, RYR (Hung‐ch'u, Hongqu, Angkak or Beni Koji) is the product of fermentation deriving from a mixture of several species from the genus Monascus, the main one being M. purpureus Went, discovered in 1895. The other species producing similar fermentations are Monascus ruber van Tieghem, Monascus fuliginosus Sato, Monascus pilosus Sato and Monascus albidus Sato (Zhang et al., 2000). Sixty‐five strains are currently deposited at the American Type Culture Collection (ATCC) belonging to three species: M. pilosus, M. purpureus and M. ruber. Non‐traditional RYR preparations are not currently on the market for their uses as ingredients in food supplements (Hawksworth and Pitt, 1983; Lin et al., 2008; ANSES, 2014).
2.4. Chemical composition
2.4.1. Monacolins
Fungi from the genus Monascus produce several molecules called monacolins, among which monacolin K is the most abundant (Ma et al., 2000). In RYR, the monacolin K (hydroxy acid) form is also present (Figure 1). Monacolin K (lactone) and monacolin K (hydroxy acid) exist in equilibrium and their ratio depends mainly on pH. At low pH, monacolin K lactone is the predominant form whereas at neutral and basic conditions, the monacolin K hydroxy acid predominates. The lowest interconversion rate was pH 4.5, there is (Nigović et al., 2013; Mornar et al., 2013). The chemical structure of monacolins identified in RYR is reported in the Appendix A.
According to Mornar et al. (2013), in most tested RYR samples, monacolin K lactone predominates over the monacolin hydroxy acid.
According to information provided by an interested party following a Call for data, before drying, RYR contains more than 80% monacolin K in its acid form. During drying (usually at 50–60°C), some part of acid form loses a molecule of water and converts to the lactone form. The final ratio between acid form and lactone form after drying is usually between 6:4 and 3:7. Both forms of monacolin K (lactone and hydroxy acid) remain stable over time. The lactone form represents approximatively 85% of the total monacolin K content, and the acid form represent approximatively 15% of the total content (‘Documentation provided to EFSA’ n. 11). The Panel noted that there is an inconsistency with the ratio of lactone and acid forms in the information provided by one of the interested parties.
The Panel noted that in response of the public ‘Call for data’ launched by EFSA, no data on the possible presence of monacolins other than monacolin K have been reported by interested parties.
On the basis of the information available in the literature, the Panel noted that different monacolins have been identified in RYR samples. These molecules include monacolin J (lovastatin diol lactone), monacolin L (the precursor of monacolin J), dehydromonacolin K (dehydrolovastatin), compactin (mevastatin). Monacolin K and monacolin K hydroxyacidic forms represent approximately 50% and 25%, respectively, of total monacolins (Ma et al., 2000).
The Panel noted that there seems to be a discrepancy between the information retrieved through the public call for data and the publicly available information from the literature with respect to the occurrence of the chemical forms of monacolin K in RYR.
2.4.2. Other compounds present in RYR
Fatty acids
The fatty acids found in RYR (approximately 3%) can be classified in two groups:
saturated fatty acids including palmitic, stearic and eicosanoic (arachidic) acids;
unsaturated fatty acids including oleic, linoleic and linolenic acids (Ma et al., 2000).
Pigments
Six main pigments have been identified in RYR (Lin et al., 2008; Patakova, 2013; Chen et al., 2017) they are secondary metabolites belonging to the family of azaphilones (Figure 2) and are classified according to their colour: rubropunctatin and monascorubrin are orange; monascin and ankaflavin are yellow; and rubropunctamine and monascorubramine are red.
Figure 2.
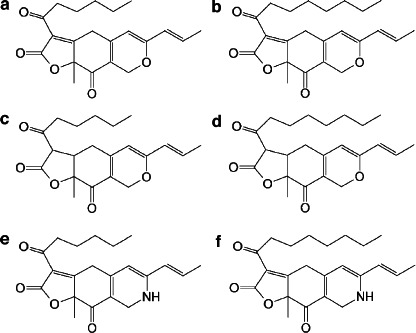
Chemical structures of RYR pigments (modified from Lin et al., 2008). a = rubropunctatin (orange); b = monascorubrin (orange); c = monascin (yellow); d = ankaflavin (yellow); e = rubropunctamine (red); f = monascorubramine (red)
Their content in RYR is approximately 0.3% (Heber et al., 1999).
Citrinin
Under some conditions, Monascus strains produce a secondary toxic metabolite called citrinin, a mycotoxin produced also by the genera Aspergillus and Penicillium (Liao et al., 2014). It may be present in stored grain, as well as in other plant products such as beans, fruits, fruit or vegetable juices, plants used for medicinal or condiment purposes, spices and tainted dairy products (EFSA CONTAM Panel, 2012).
A tolerable daily intake (TDI) of 0.2 μg/kg body weight (bw) per day has been set by the CONTAM Panel for citrinin.
In response of the ‘Call for data’ launched by EFSA, interested parties provided analytical data on the occurrence of citrinin in food supplements containing monacolin K from RYR that were in compliance with the maximum permitted level of 2 mg/Kg in accordance to the Commission Regulation (EU) No 212/2014 (‘Documentation provided to EFSA’ n. 5, 6, 7, 9, 10 and 11).
Other
RYR also contains other components: starch (73.4%), proteins (5.8%), fibres (0.8%), minerals (phosphorus, sodium, calcium, iron, magnesium, aluminium, manganese, copper, silver) (Heber et al., 1999; Li et al., 2004). RYR contains sterols, such as β‐sitosterol, campesterol, stigmasterol and sapogenine (Heber et al., 1999).
2.4.3. Content of monacolins in RYR and sources of variability
According to data reported by several authors, the amounts of monacolins in food supplements containing RYR can vary significantly. Gordon et al. (2010) quantified monacolins and citrinin in 12 RYR food supplements available in the US market. The Panel noted variability both in monacolins and citrinin concentrations (Table 2). However for the latter, no exceedance of the established limit in EU (2 mg/kg) was reported.
Table 2.
Levels of monacolins in 12 commercial products available in the US market (Gordon et al., 2010)
| Product | Daily intake | Total monacolins | MK | MKA | MKA/MK | MJ | MJA | MX | MXA | ML | MLA | MM | MMA | DMK | Citrinin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capsule | mg/600 mg‐capsule | μg/600 mg‐capsule | |||||||||||||
| A | 4 | 5.3 | 2.53 | 1.96 | 0.77 | 4 | 27 | 76 | 59 | 122 | 19 | 29 | NT | 473 | 0 |
| B | 4 | 2.16 | 1.02 | 0.61 | 0.59 | 19 | 12 | 0 | 8 | 55 | 80 | 7 | NT | 212 | 0 |
| C | 1–2 | 4.18 | 1.74 | 1.63 | 0.93 | 32 | 49 | 108 | 24 | 67 | 33 | 18 | 73 | 281 | 0 |
| D | 2 | 1.65 | 1.12 | 0.22 | 0.19 | 0 | 13 | 55 | 0 | 49 | 11 | 0 | 0 | 140 | 14.3 |
| E | 4 | 6.03 | 3.63 | 1.22 | 0.33 | 31 | 169 | 125 | 18 | 88 | 85 | 64 | 31 | 386 | 0 |
| F | 2 | 0.31 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 160 | 51 | 114.2 |
| G | 4 | 6.18 | 2.5 | 2.3 | 0.92 | 43 | 54 | 104 | 20 | 36 | 49 | 19 | 51 | 929 | 0 |
| H | 1 | 11.15 | 10.09 | 0.52 | 0.05 | 0 | 58 | 73 | 0 | 43 | 38 | 45 | 71 | 141 | 0 |
| I | 2 | 1.60 | 0.99 | 0.23 | 0.23 | 0 | 0 | 0 | 0 | 42 | 0 | 210 | 0 | 93 | 57.5 |
| J | 2 | 3.97 | 2.66 | 0.46 | 0.17 | 0 | 25 | 98 | 0 | 126 | 53 | 88 | 41 | 305 | 0 |
| K | 2 | 1.36 | 0.97 | 0.19 | 0.19 | 0 | 14 | 0 | 0 | 39 | 0 | 0 | 0 | 110 | 70.4 |
| L | 2–4 | 6.13 | 3.12 | 2.07 | 0.66 | 42 | 64 | 112 | 16 | 56 | 83 | 59 | 19 | 315 | 0 |
| m ± SD | 4.17 ± 3.00 | 2.54 ± 2.60 | 0.95 ± 0.84 | 14 ± 18 | 40 ± 46 | 63 ± 49 | 12 ± 17 | 60 ± 36 | 38 ± 33 | 40 ± 59 | 45 ± 49 | 286 ± 239 | 21.4 ± 38.2 | ||
| Median | 4.08 | 2.12 | 0.57 | 20 | 26 | 75 | 4 | 52 | 36 | 24 | 36 | 246 | 0 | ||
MK: monacolin K; MKA: monacolin K hydroxyacid; MJ: monacolin J; MJA: monacolin J hydroxyacid; MX: monacolin X; MXA: monacolin X hydroxyacid; ML: monacolin L; MLA: monacolin L hydroxyacid; MM: monacolin M; MMA: monacolin M hydroxyacid; DMK: dehydroxymonacolin K.
Klimek et al. (2009) reported the content of monacolin K, monacolin L and citrinin in nine RYR products recommended for hyperlipidaemia (Table 3). The composition of the unit (capsule) and daily intake were not reported, however no information was available on the weight of the capsule.
Table 3.
Contents of monacolins and citrinin in nine Chinese RYR products sold in US market (Klimek et al., 2009)
| RYR supplements | Monacolin K (mg/capsule) | Monacolin L (mg/capsule) | Citrinin (μg/capsule) |
|---|---|---|---|
| Cholesterex | 1.35 | < 0.006 | 4.87 |
| Cholestene | 2.87 | < 0.006 | 2.22 |
| Cholactive | 1.80 | < 0.006 | 6.06 |
| Cholester‐Reg | 3.37 | < 0.006 | 3.23 |
| Beyond cholesterol | 0.15 | 0.02 | Not available |
| Hongqu | 2.86 | < 0.005 | 11.82 |
| Cholesterol power | 2.51 | < 0.007 | 0.47 |
| Red yeast rice | 1.56 | < 0.006 | 64.7 |
| Cholestin | 2.46 | 0.015 | Not available |
Avula et al. (2014) reported the content of monacolin K in 14 commercial food supplements analysed by liquid chromatography coupled with mass spectrometry: monacolin K content ranged between 0.03 and 2.18 mg and between 0.22 and 5.23 mg in products containing 600 and 1,200 mg of RYR, respectively.
Cohen et al. (2017), analysed monacolin K level in 28 brands of food supplements by ultrahigh‐performance liquid chromatography‐diode array detector quadrupole time‐of‐flight mass spectrometry. Two samples did not contain monacolin K; in the remaining supplements, monacolin K concentrations ranged between 0.05 and 2.74 mg per 600 mg of RYR. According to the serving reported in the label, the amount of monacolin K consumed daily ranged between 0.09 and 10.94 mg.
Li et al. (2004) described the content of monacolins and compactin in 10 commercial products containing RYR; no information on RYR content or daily intake was reported (Table 4).
Table 4.
Level of monacolins in 10 commercial products (Li et al., 2004)
| Product | Reference | Total monacolins | MK | MKA | MKA/MK | MJ | MJA | MX | MXA | ML | MLA | MM | MMA | DMK | PI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RYRP | μg/g | 622.65 | 362.37 | 103.23 | 0.28 | 12.64 | 7.08 | 4.74 | 3.64 | 23.32 | 15.36 | 5.19 | 0.72 | 72.22 | 12.14 |
| CP1 | μg/capsule | 307.08 | 302.48 | 3.57 | 0.01 | ND | ND | ND | ND | ND | ND | ND | ND | 1.03 | ND |
| CP2 | μg/capsule | 98.20 | 93.65 | 2.81 | 0.03 | ND | ND | ND | ND | ND | ND | ND | ND | 1.74 | ND |
| CP3 | μg/capsule | 142.34 | 112.76 | 6.9 | 0.06 | ND | ND | ND | ND | ND | ND | ND | ND | 22.98 | ND |
| CP4 | μg/capsule | 135.68 | 126.61 | 3.44 | 0.03 | ND | ND | ND | ND | ND | ND | ND | ND | 5.63 | ND |
| CP5 | μg/tablet | 17.27 | 10.52 | 6.75 | 0.64 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| CP6 | μg/tablet | 13.91 | ND | 12.8 | ND | ND | 1.11 | ND | ND | ND | ND | ND | ND | ND | ND |
| CP7 | μg/tablet | 155.68 | 112.00 | 19.60 | 0.18 | ND | ND | ND | ND | 4.48 | ND | 0.91 | ND | 15.05 | 1.75 |
| CP8 | μg/capsule | 18.69 | 34.19 | 11.55 | 0.34 | ND | ND | ND | ND | ND | ND | ND | ND | 7.14 | ND |
| CP9 | μg/capsule | 357.76 | 198.65 | 63.48 | 0.32 | 7.22 | 4.33 | 2.75 | 2.08 | 13.47 | 8.86 | 3.01 | 0.44 | 46.5 | 6.97 |
| CP10 | μg/tablet | 461.56 | 259.32 | 82.66 | 0.32 | 8.56 | 3.62 | 2.94 | 3.12 | 18.46 | 9.23 | 3.22 | 0.52 | 59.68 | 8.23 |
| Averagea | μg/unit | 170.82 | 138.91 | 21.36 | 0.15 | 15.98 | ND | ||||||||
| St Deva | 155.25 | 97.39 | 28.10 | 21.11 | ND | ||||||||||
| Mediana | μg/unit | 139.01 | 119.68 | 9.23 | 6.39 | ND |
RYRP: RYR powder; CP: Commercial product: MK: monacolin K; MKA: monacolin K acid form; MJA: monacolin J acid form; MJ: monacolin J; MXA: monacolin X acid form; MLA: monacolin L acid form; MX: monacolin X; ML: monacolin L; MMA: monacolin M acid form; MM: monacolin M; DMK: dehydroxymonacolin K; PI: compactin, ND: not detectable.
Products C1–C6 were from US store market; C7 and C8 from Taiwan; and C9 and C10 from China marketing.
Calculated by the Panel on CP values, where ND was considered 0.01.
The Panel noted that there is a large variability and discrepancy in both the content of monacolin K and the ratio between monacolin K lactone and its hydroxyacid form in the 10 commercial products considered (Table 4). Some samples showed a clear predominance of monacolin K lactone, which seems not usual in natural RYR products (see ratio in RYR powder (RYRP)).
The Panel calculated the mean, SD and the median of the monacolin K contents and recommended daily intake of 26 food supplements containing RYR where monacolin K was detectable and marketed in the US (as reported by Cohen et al., 2017). The Panel noted that no information was provided on monacolin K hydroxy acid form. The mean monacolin K contents of food supplements containing RYR (m ± SD) was 1.22 ± 1.85 mg/600 mg RYR, ranging between 0.05 and 2.74 mg/600 mg; the recommended daily intake 2.4±2.88 mg/day (m±SD), ranging between 0.09 and 10.94 mg/day. The medians were 0.92 mg/600 mg RYR and 1.23 mg/day, respectively.
Chen and Hu (2005) evaluated the production of monacolin K and citrinin by 29 Monascus strains: the concentrations in RYR ranged between <0.08 and 0.22 mg/g, and between <40 and >1,000 ng/g, respectively.
The Panel noted that information on the presence of monacolins other than monacolin K was included in some of the studies described above (Li et al., 2004; Klimek et al., 2009; Gordon et al., 2010). Furthermore, the Panel noted that in some cases the recommended daily intake of monacolin K in food supplements as reported in the studies, overlap the therapeutic dose (10 mg/day) (Gordon et al., 2010; Cohen et al., 2017). However, in other cases, the recommended daily intakes of monacolin K or of RYR are not reported by the authors ( Li et al., 2004; Klimek et al., 2009).
2.5. Specifications
There are no specifications for RYR used as food including food supplements in EU Regulations. No specifications for RYR were found on European, Japanese, Canadian, US Pharmacopeia or in the WHO monographs.
The Panel noted that specifications exist for lovastatin in the EU Pharmacopeia when used as a medicinal product.
On the basis of the information gathered from the relevant food sector operators following the launch of a public ‘Call for data’, the Panel was made aware of certain maximum levels for contaminants and of results of microbiological analysis that are applicable to the food supplements containing monacolin K from RYR (‘Documentation provided to EFSA’ n. 5, 6, 7, 9 and 11).
2.6. Stability of the botanical or botanical preparation used as ingredients in food supplements
Specific request for information on the stability of botanical ingredients used in food supplements containing monacolin K was included in the ‘Call for data’ launched. The information received was complemented by the Panel with data retrieved in the published literature. The Panel noted that most of the interested parties provided stability data on the final products rather than on the botanical ingredients. According to data submitted by one interested party, the monacolin K content is stable over a period of 2 years (‘Documentation provided to EFSA’ n. 7).
In the scientific literature, there are only few studies reporting the effects of storage on the levels of monacolins in RYR‐based products. Lin et al. (2005) performed a study using accelerated storage conditions, where the role of temperature, humidity and sunlight exposure on RYR preparations was assessed.
The results of testing the stability under the accelerated storage conditions of 40°C and 75% relative humidity are given in Table 5.
Table 5.
Changes in monacolins levels (mg/g) in RYR during an accelerated testing at 40°C and 75% of relative humiditya
| Molecule | Day 0 | Day 10 | Day 30 | Day 90 | Changes at 90 days (%) |
|---|---|---|---|---|---|
| Monacolin K hydroxy acid | 1.048 | 1.055 | 1.064 | 1.092 | +4.2 |
| Monacolin K | 3.351 | 3.223 | 3.128 | 3.006 | −10.3 |
| Dehydromonacolin K | 0.493 | 0.494 | 0.49 | 0.481 | −2.4 |
| Total monacolins | 5.326 | 5.212 | 5.125 | 5.021 | −5.9 |
Modified from Lin et al. (2005).
2.7. Use and use levels
2.7.1. Content of monacolins in RYR products as provided by interested parties following the launch of a public ‘Call for data’
In October 2017, a request was sent to relevant business operators, asking for information on recommended daily intake of food supplements containing monacolins (monacolin K) from RYR.
On the basis of the data received, the Panel noted that the recommended daily intake of monacolin K from RYR ranges from 9 to 20 mg (‘Documentation provided to EFSA’ n. 5, 6, 8, 9 and 10). The Panel further noted that the recommended daily intake of 20 mg of monacolin K is reported for one product only that according to the interested party is planned to be delisted.
The Panel noted that no data were provided on the occurrence levels of other monacolins in RYR.
Summarised data extracted from the Mintel GNPD
The Mintel Global New Products Database (Mintel GNPD) is an online database which monitors product introductions in consumer packaged goods markets worldwide. It contains information of over 2 million food and beverage products of which more than 800,000 are or have been available on the food market of the Member States. Mintel started covering the EU countries’ food markets in 1996, currently having 20 out of its 28 member countries presented in the Mintel GNPD.
For the purpose of this Scientific Opinion, the Mintel GNPD was used for checking the labelling of products containing monacolin K from RYR within the EU countries’ food products as the Mintel GNPD shows the compulsory ingredient information presented in the labelling of products. According to the Mintel GNPD, monacolin K was labelled on 40 products between January 2012 and February 2018. The food category considered was: ‘Vitamins & Dietary Supplements’. The concentrations of monacolin K were also retrieved from this database, when available. In all these products, the content of monacolin K was clearly stated.
The daily consumption in terms of monacolin k for each product was calculated by multiplying the intake unit of monacolin K by the daily number of recommended intakes of the product. The daily intake of monacolin K for these products ranged from 2 to 48 mg/day, for adult population (Table 6).
Table 6.
Summary of data on food supplements containing RYR, collected by consulting MINTEL GNPD
| Country | No. of products | Intake of monacolin K (mg/day) | Comments | ||
|---|---|---|---|---|---|
| Mean ± SD | Median | Range | |||
| Austria | 1 | 10 | 10 | 10 | One product at 10 mg/day |
| Croatia | 4 | 10 | 10 | 10 | Four products all at 10 mg/day |
| Czech Rep. | 1 | 3 | 3 | 3 | One product at 3 mg/day |
| Finland | 1 | 10 | 10 | 10 | One product at 10 mg/day |
| France | 10 | 10.5 ± 13.6 | 7.45 | 2–48 | Wide range of daily intake |
| Germany | 2 | 7.1 | 4–10.2 | ||
| Ireland | 1 | 10 | 10 | 10 | One product at 10 mg/day |
| Italy | 9 | 6.1 ± 3.7 | 3 | 3–10 | Five products at 3 mg/day; four products at 10 mg/day |
| Netherland | 5 | 10.5 ± 6.2 | 10 | 2.6–20 | Three products at 10 mg/day |
| Spain | 4 | 8.6 ± 3.7 | 10.2 | 3–10.9 | Three products at values close to 10 mg/day |
| UK | 2 | 10 | 10 | 10 | |
| Total | 40 | 10.5 ± 6.2 | 10 | 2–48 | More than 50% at values close to 10 mg/day |
The Panel noted that the number of RYR products retrieved from the Mintel GNPD is only a part of those on the European market. However, the Panel considered that it would be possible to draw sufficiently reliable conclusions on the average intake of monacolin K according to the recommended daily intake reported on the label. The recommended daily intakes range between 2 and 48 mg of monacolin K, but the majority of food supplements (around 25%) supplies a recommended daily intake of 10 mg according to the level established by EFSA for supporting the health claim (EFSA NDA Panel, 2013). The Panel noted that only 8 out of 40 products were mono‐ingredient, containing only RYR preparation; in the remaining 32 products the most frequently associated ingredients were: artichoke extract, berberine (Berberis aristata), coenzyme Q10, chromium, policosanol, resveratrol and vitamins.
On the basis of the information retrieved from the Mintel GNPD database, the Panel noted that two products reported recommended a daily intake of 20 or 48 mg of monacolin K.
3. Biological and toxicological data
3.1. Preclinical studies
3.1.1. Lovastatin
The Panel considered that information included in the SPC, based on the assessments performed by regulatory authorities for medicinal products containing lovastatin, is current and relevant for this assessment.
The Panel noted data from preclinical studies are reported in the SPCs for lovastatin tablets (SPC, online). While genotoxicity studies in vitro and in vivo did not indicate a genotoxic potential, results of long‐term studies (21 months) involving oral administration of lovastatin at 500 mg/kg bw per day in mice revealed an increase in tumour incidences (e.g. hepatocellular carcinomas and adenomas) (TOXNET, online).10 According to the SPC, the relevance of this data for the risk assessment in humans is unclear.
Fetal skeletal malformations have been described in rodents following exposure to high doses (800 mg/kg bw per day) and impaired fertility has been observed in dogs at doses of 20 mg/kg bw per day and above (MacDonald and Halleck, 2004; SPC, online).
The Panel considered that the available preclinical data do not allow to derive a safe intake for monacolins in RYR.
3.1.2. Other compounds
Several biological activities were associated with the RYR pigments in in vitro studies: antifungal, antiviral, antioxidant, cytotoxic, nematicidal and anti‐inflammatory (Osmanova et al. 2010). Moreover, a RYR fraction containing only red Monascus pigments was associated with the induction of cellular senescence of highly proliferating HepG2 cancer cells (Wei and Popovich, 2013). In 3‐day‐old chicken embryos, incubation at 38.5°C for 9 days with the pigments monascorubrin, rubropunctatin, monascin and ankaflavin, which were purified from the mycelium of M. purpureus, led to malformations and lethality. From the sum of dead and malformed embryos, the ED50 (dose causing these effects in 50% of the embryos) were calculated. The means ED50 were 4.3 μg/embryo, 8.3 μg/embryo, 9.7 μg/embryo and 28 μg/embryo, respectively (Martínková et al., 1999).
Citrinin is hepatotoxic and nephrotoxic in different animal species and in humans (EFSA CONTAM Panel, 2012). Citrinin as such did not show mutagenic activity in the Ames test, but hepatocyte‐derived metabolites were positive in this test (Sabater‐Vilar et al., 1999; Patakova, 2013).
3.2. Pharmacology
3.2.1. Statins
The therapy with statins is aimed at reducing the level of circulating cholesterol in subjects suffering from dyslipidaemia, who are at risk for cardiovascular diseases. The target of statins is the enzyme 3‐hydroxy‐3 methylglutaryl‐coenzyme A (HMG‐CoA) reductase (EC 1.1.1.34), which converts HMG‐CoA into mevalonate, the precursor of cholesterol. The site of statin action is shown in Figure 3 (Rotta Bonfim et al., 2015).
Figure 3.

Biosynthetic pathway of cholesterol and statin target (Rotta Bonfim et al., 2015)
Statins reduce the cellular cholesterol concentration through two different mechanisms: (1) the inhibition of the enzyme HMG‐CoA reductase with the associated reduction of cholesterol biosynthesis, and (2) the reduction of cholesterol concentration in liver. As a consequence, there is an increase of the expression of LDL‐receptors in hepatic cell membranes, producing a more efficient clearance of LDL‐cholesterol from blood (Stancu and Sima, 2001; Schachter, 2004; Sirtori, 2014).
Lovastatin hydroxy acid shows its inhibitory activity on HMG‐CoA reductase both in humans and other animal species (Goswami et al., 2012).
3.2.2. Dose range for pharmacological effect of lovastatin
According to the SPC of medicinal products containing lovastatin as an active ingredient, the recommended therapeutic dose of lovastatin for the treatment of hypercholesterolaemia ranges between 10 and 80 mg/day, where the dose of 10 mg/day is mainly considered as the starting dose.
3.2.3. RYR and monacolin K
Effects on cholesterolaemia
Most clinical studies performed with RYR focused on the cholesterolemia reduction and, in particular, of the serum concentration of total cholesterol (TC) and LDL‐cholesterol (LDL‐C).
There are some trials where RYR mono‐ingredient products were used (Heber et al., 1999; Lin et al., 2005; Burke, 2015).; two of them were considered in the EFSA opinion (Heber et al., 1999; Lin et al., 2005 cited in EFSA NDA Panel, 2011).
In 2014, Li and co‐workers published a meta‐analysis collecting clinical data on the effects and safety of RYR (Li et al., 2014). Considering all the papers describing clinical studies published at that time in PubMed, Cochrane Library, EBSCO and other databases, the authors selected 13 papers, which were considered relevant. These publications were selected considering, as an inclusion factor, the quality of clinical studies so that most of them were randomised, placebo‐controlled trials. All papers reported data of cholesterolemia before and after the consumption of products containing RYR. The Panel noted that only in 4 out of 13 trials, RYR was the only ingredient of the products received by the intervention group. For these reasons, only the clinical studies where the supplementation was based on mono‐ingredient products will be considered in this opinion (Table 5). The paper by Lin et al. (2005) is not included in the meta‐analysis by Li et al. (2014) but it is added in the table since it was reported by EFSA in its opinion on the ‘health claim related to the effect of monacolin K from RYR on the maintenance of normal blood cholesterolemia’ (EFSA NDA Panel, 2011). Another more recent study, also included in Table 5, demonstrated that also an intake dose of 3 mg of monacolin K from RYR (with 200 μg of folic acid) is active in reducing significantly the level of total cholesterol and LDL‐cholesterol (Heinz et al., 2016).
All studies were randomised, double‐blind and placebo‐controlled; the dose used ranged from 200 to 3,600 mg of RYR, corresponding to 3–11.4 mg of monacolin K; the trials lasted from 8 to 16 weeks. Data are summarised in Table 7.
Table 7.
Changes in total cholesterol and LDL‐Cholesterol reported in controlled studies where RYR was the only treatment to reduce hypercholesterolaemia (modified from Li et al., 2014)
| Reference | Type of study (period) | Number of patients | Sex age | Daily intake of RYR (monacolin K) | Total cholesterol in RYR group | LDL‐Cholesterol in RYR group | ||
|---|---|---|---|---|---|---|---|---|
| Change (%) | Statistical significance vs placebo | Change (%) | Statistical significance vs placebo | |||||
| Heinz et al. (2016, b) | R, DB, PC (12 weeks) |
C = 72 T = 70 |
M/F 18–70 years |
200 mg (3 mg) | −11.2 | p < 0.001 | −14.8 | p < 0.001 |
| Becker et al. (2009) | R, PC (24 weeks) |
C = 31 T = 31 |
M/F 21–80 years |
3,600 mg (6.12 mg) | −14.9 | p < 0.016 | −21.3 | p < 0.011 |
| Bogsrud et al. (2010) | R, DB, PC (16 weeks) |
C = 22 T = 20 |
M/F 18–75 years |
1,200 mg (7.2 mg) | −15.0 | p < 0.001 | −23.0 | p < 0.001 |
| Heber et al. (1999, a) | R, DB, PC (12 weeks) |
C = 41 T = 42 |
M/F 34–78 years |
2,400 mg (7.2 mg) | −16.1 | p < 0.05 | −21.9 | p < 0.05 |
| Huang et al. (2007) | R, DB, PC (8 weeks) |
C = 40 T = 39 |
M/F 18–65 years |
1,200 mg (11.4 mg) |
−20.4 | p < 0.001 | −26.3 | p < 0.001 |
| Lin et al. (2005, c) | R, DB, PC (8 weeks) |
C = 38 T = 37 |
M/F 23–65 years |
1,200 mg (11.4 mg) | −20.4 | p < 0.001 | −26.3 | p < 0.001 |
The reduction in total cholesterolaemia and LDL‐cholesterolaemia showed a dose‐dependent trend. The treatment for 8 weeks with the highest dose was sufficient to reach the mean reduction of 20% in both total and LDL‐cholesterol. Longer periods (12–24 months) were necessary to obtain statistically significant reduction of total and LDL‐cholesterol at the lowest doses (3–6.12 mg) of monacolin K.
The Panel noted that the data from the clinical trials above show a dose‐dependent pattern.
3.3. Absorption, distribution, metabolism and excretion (ADME)
3.3.1. Lovastatin
Lovastatin is a lactone prodrug that is converted by esterases to its active form, lovastatin hydroxy acid (HA) which inhibits the enzyme HMG‐CoA reductase, the rate‐limiting enzyme in the cholesterol biosynthetic pathway in the liver (Alberts et al., 1980). These esterases, also known as lactonases, are known to be present in the plasma and liver and according to pH, to catalyse also lactonisation of the corresponding HAs (Fishbein et al., 1966). Due to this extensive first‐pass metabolism and its low solubility (1.3 g/mL in water, Serajuddin et al., 1991), whereas the absorption is about 30%, intact lovastatin exhibits a poor oral absolute bioavailability (< 5%) due to the CYP3A4 dependent high first pass (Schachter, 2004).
3.3.1.1. In vitro studies
Greenspan et al. (1988) investigated the hepatic metabolism of lovastatin and lovastatin HA, by using rat and human liver microsomes. In human liver samples, the conversion of lovastatin to its 3‐ and 6‐hydroxy derivatives was observed. No differences were observed between the microsomes derived from males and females. The open acid form of lovastatin was not metabolised by the human microsomes, as observed with rat liver microsomes.
Similar results were obtained by Vyas et al. (1990a) using rat and mice liver microsomes. Microsomal metabolites were described to be active inhibitors of HMG‐CoA reductase and their relative enzyme inhibitory activities were 1, 0.6, 0.5 and 0.15 for the hydroxy acid forms of lovastatin, 6′‐β‐hydroxy‐, 6′‐exomethylene‐ and 3″‐hydroxy‐lovastatin, respectively (Figure 4). In another study, Vyas et al. (1990b) confirmed that hydroxylation at the 6′‐position was the major pathway of lovastatin biotransformation in human liver microsomes, whereas hydroxylation at the 3″‐position of the side chain was a minor pathway. The conversion of lovastatin to an additional 6′‐exomethylene metabolite was shown to be catalysed by cytochrome P450.
Figure 4.
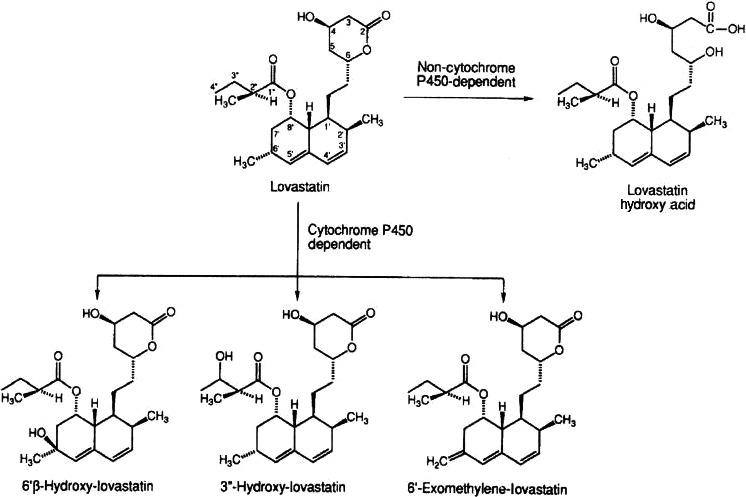
Major liver metabolic pathways of lovastatin in humans (from Wang et al., 1991)
Wang et al. (1991) reported that using human liver microsomes, that both anti‐rat P450 3A and anti‐human P450 3A antibodies inhibited lovastatin metabolism (formation of 6′‐beta‐hydroxy‐, 6′‐exomethylene‐ and 3″‐hydroxy‐lovastatin). There was a correlation between lovastatin oxidation and the P450 3A content in human liver microsomes. In addition, preincubation of human liver microsomes with the suicide substrate troleandomycin and NADPH inhibited lovastatin metabolism by 60%. These results clearly indicate that cytochrome P450 3A enzymes are primarily responsible for the oxidative metabolism of lovastatin in rat and human liver microsomes.
3.3.1.2. In vivo studies
In rat, dog, monkey and human studies, Duggan et al. (1989) used 14C‐lovastatin labelled on the methylbutyryl side chain, or its acid metabolite 14C‐HA prepared by hydrolysis of lovastatin at pH 11. In all animal studies, intravenous and oral doses were 0.8 and at 8.0 mg/kg, respectively. Human doses were 100 mg lovastatin given as a capsule or 40 mg HA infused over 120 min. In dog and human plasma, the bioavailable (albumin‐free) fraction of HA (4–4.6%) was about twice that of lovastatin (1.4–1.8%) at pharmacologically relevant concentrations. Lovastatin was absorbed to a lower extent in rats and dogs compared to the same dose of lovastatin acid (HA) given per se, but the absorbed fraction reached the portal circulation largely unchanged and was more efficiently extracted by the liver. In the liver, lovastatin was reversibly transformed to HA and irreversibly to other biologically active metabolites. Metabolites such as HA, were maintained at high hepatic levels when compared to all tissues examined in the rat. According to the authors, the low systemic levels of HA are attributable in part to the metabolic equilibrium, lovastatin/HA equilibrium, the opposing reactions for which appeared to be present in most tissues. In rats, dogs, monkeys and humans, urinary and faecal recoveries of total radioactivity were measured over 4 days after either oral or intravenous doses of radiolabelled lovastatin or HA. Following oral administration of lovastatin in humans, 9.6% and 83.2% of the administered radioactivity were excreted in urine and faeces, respectively. Similar results were obtained in dogs and monkeys. The excretion was largely biliary as measured in rats and dogs. The authors concluded that comparisons of ADME profiles presented in their study indicate the dog as the most appropriate animal model for studying human lovastatin bioavailability.
Similar results were reported by Halpin et al. (1993) investigating differences in the in vivo disposition of lovastatin (same radiolabelled material as used in Duggan et al. 1989) in mouse, rat, dog and human. Lovastatin underwent extensive and complex metabolism in animals and humans, with the metabolites excreted predominantly in bile. Radiochromatograms of bile from three human subjects and of bile and liver homogenates from mouse, rat and dog indicated species differences in metabolism. Three major metabolic pathways of lovastatin were described: hydrolysis of the lactone ring to yield the pharmacologically active HA, cytochrome P450‐mediated oxidation of the fused‐ring system, and beta‐oxidation of the dihydroxy acid side chain. The first two reactions occurred in all four species, but the last one was observed only in mouse and rat. Human bile contained predominantly polar metabolites. According to the authors, cytochrome P450 oxidation is the primary route of phase I metabolism for lovastatin in human and dog whereas beta‐oxidation plays a major role in rodents.
Additional pharmacokinetic data of lovastatin in man were reported in the review of Schachter (2004). The conclusion by the Authors was that lovastatin has a short elimination half‐life of 3 h, whereas it is more effectively absorbed when taken with food. This is in line with the indication that lovastatin tablets should be given with meals, as it increases lovastatin by 30–50% when taken with a standard meal (Schmidt and Dalhoff, 2002). Moreover, due to the involvement of the CYP3A4 isoform in its metabolism, the authors concluded that could be a risk for interactions between drugs that are metabolised or inhibit CYP450, resulting in increased plasma levels of statins and possible consequent increased risk of toxic effects.
Statins including lovastatin are usually administered as oral formulations; their absorption from the small and large intestine depends on several parameters (dose, dissolution rate, intestinal transit and absorption across the intestinal mucosa, etc.). In humans up to 31% of the dose is absorbed. Intact lovastatin exhibits a low systemic bioavailability of 5% due to the CYP3A4 dependent high first pass. Both lovastatin and lovastatin HA are bound to human plasma proteins. When lovastatin enters the enterocytes, it is metabolised by different enzyme systems, which are more abundant in small intestine (Lennernäs and Fager, 1997).
According to the information reported in the SPC for medicinal product containing lovastatin, ‘It is not known whether lovastatin or its metabolites are excreted into breast milk. As many drugs are excreted into breast milk, and as there is a potential risk of serious undesirable effects, women taking lovastatin should not breast‐feed’ (SPC, online).
Moreover, ‘on the basis of results from animal studies, lovastatin crosses the blood/brain barrier and the placental barrier’ (SPC, online).
3.3.2. RYR and Monacolin K
Li et al. (2005) compared the pharmacokinetics of a Chinese red yeast rice preparation (CRYR) with lovastatin. CRYR is a botanical dietary supplement consisting mainly of rice, and by‐products of fermentation. The complete composition of this preparation was provided. The most abundant ingredient was starch, constituting over 73% of the bulk and the protein content was 6%. Total monacolins content corresponded to 0.4% of the preparation. Eleven healthy volunteers were randomised to a crossover study taking either 20 mg of lovastatin or 2,400 mg CRYR (corresponding to 4.6 mg monacolin K). In both arms, the participants received 200 mL of ‘double‐strength grapefruit juice’ three times/day for 2 days prior to the treatment; on the third day, treatments were administered with 200 mL of the same juice. The Panel noted that the simultaneous administration of grapefruit juice with lovastatin has been described to inhibit CYP3A4, and hence in this study, there may have been a limitation in the presystemic elimination of lovastatin. Lovastatin and lovastatin acid (HA) were measured in plasma of volunteers by a high‐performance liquid chromatography with tandem mass spectrometry (HPLC–MS/MS) method. When lovastatin was administered, Cmax were 23.67 and 40.94 ng/mL for lovastatin and HA, respectively. Following administration of CRYR, the corresponding values were 1.25 and 4.31 ng/mL, respectively. Comparisons of area under the curves (AUCs) demonstrated that AUC of lovastatin was 26‐fold higher after lovastatin administration, whereas AUC of HA was 9.7‐fold higher after administration of lovastatin by comparison to the intake of CRYR preparation. As reported in the paper, after normalisation for dose, the AUC of lovastatin was calculated to be 7.21 greater than that of CRYR. According to the authors, this study demonstrated that plasma concentrations of lovastatin and lovastatin hydroxy acid are much higher after ingestion of lovastatin than of CRYR. The mixture of monacolins and other substances present in the RYR may have some inhibitory effect on cholesterol biosynthesis. Moreover, in the current study, the lovastatin to HA ratio of AUC was 1.72 for lovastatin and 3.48 for CRYR, which could suggest possible differences in metabolism between the two modes of administration. Overall, these results would suggest that the effect of CRYR on the cholesterol concentration might be caused by the additive and/or synergistic effects of monacolin K with other monacolins and substances present in CRYR.
Chen et al. (2013) compared the dissolution rate, physical state and oral bioavailability in humans of lovastatin in three RYR products (LipoCol Forte, Cholestin or Xuezhikang) to those of two lovastatin tablets (Mevacor or Lovasta) (Chen et al., 2013). According to the authors, the dissolution rate of lovastatin in various dissolution media in the registered RYR products was faster and higher than that of lovastatin tablets. Powder X‐ray diffraction and differential scanning calorimetry patterns showed that the crystallinity of lovastatin was reduced in RYR products. In a first human pharmacokinetic study, a single intake of either four LipoCol Forte capsules (22.8 mg of monacolin K) and one Mevacor tablet (20 mg of lovastatin) were compared in 14 volunteers. The results showed that plasma levels of lovastatin and lovastatin hydroxy acid were significantly higher in subjects given the LipoCol Forte capsules. In these subjects, increases in AUC and Cmax values for both lovastatin (203% and 345%, respectively) and lovastatin HA (366% and 515%, respectively) were observed by comparison to corresponding data obtained in volunteers receiving lovastatin tablets. Shorter and less variable Tmax values were also observed in the volunteers taking LopoCol Forte capsules. In a second pharmacokinetic study (12 volunteers), 2,400 mg of powder from four LipoCol Forte capsules (22.8 mg of lovastatin) and the powder from one ground Lovasta tablet (20 mg of lovastatin) were used in order to reduce the effect of disintegration. In subjects taking LipoCol Forte powder, plasma concentration, Cmax and AUC for both lovastatin and lovastatin acid were also significantly higher (AUC increased by 1.9 and 6.2 fold, respectively) than in those taking the Lovasta tablet powder. According to the authors, the results of this study suggest that (1) the formulation of lovastatin (intact tablet vs. ground tablet) is not an important factor in differences between pharmacokinetic properties of lovastatin and lovastatin HA in RYR products compared to lovastatin tablets, (2) absorption rate and systemic exposure of lovastatin are both increased in RYR products, (3) the oral bioavailability of lovastatin is significantly improved in RYR products as a result of a higher dissolution rate and reduced crystallinity. In this paper, the authors discussed the differences in pharmacokinetic data when compared to those obtained by Li et al. (2005). For them, lower serum lovastatin levels described following CRYR preparation would be attributable to the simultaneous intake of lovastatin or CRYR with grapefruit juice, a well‐known CYP3A4 inhibitor. This could significantly increase plasma levels of lovastatin and HA as demonstrated by Kantola et al. (1998) and ‘the results may not truly reflect the pharmacokinetic properties of lovastatin in RYR products’.
The Panel noted as suggested by Chen et al. (2013) that differences observed in the plasma concentration of lovastatin (and lovastatin HA) from RYR and the conventional medicinal product is possibly due to the difference between the dissolution rates of the two formulations. The Panel further noted that the complete composition of RYR extract used by Chen et al. (2013) was not provided, particularly regarding the possible presence of other monacolins. From these studies, the Panel considered that divergent data could be obtained in the pharmacokinetics of lovastatin and lovastatin acid when RYR extracts or preparations are administered to humans.
3.3.2.1. Metabolic interaction between monacolin K from RYR with medicinal products or foods
An interaction between a preparation containing RYR and cyclosporine was postulated in a 28‐year old kidney transplantation female patient (Prasad et al., 2002). This patient received several drugs in the post‐transplant period and developed rhabdomyolysis several months after starting the use of RYR. The muscle disorder reversed when the intake of RYR product was ceased. The authors hypothesised that the bioavailability of monacolin K was increased due to the interference of cyclosporine with the metabolism of monacolins by mediated by CYP3A4, isoenzyme.
Chen et al. (2012) observed that three registered RYR‐based products (LipoCol Forte, Cholestin, and Xuezhikang) were more active than pure lovastatin in the in vitro inhibition of cytochrome P450 enzymes and P‐glycoprotein. In an additional clinical study conducted in 14 volunteers, the concomitant use of gemfibrozil with a RYR extract (LipoCol Forte) resulted in a significant increase in the plasma concentration and AUC of lovastatin acid (HA), without any effect on the pharmacokinetic parameters of lovastatin lactone.
3.3.3. Conclusion of ADME
Overall, from the available database, the Panel considered that:
orally administered lovastatin rapidly converts by esterases to its active form, lovastatin HA,
due to its extensive first‐pass metabolism and its low solubility, intact lovastatin exhibits low systemic bioavailability of 5%
lovastatin and lovastatin HA are bound to human plasma proteins,
the cytochrome P4503A‐dependent oxidation at the 6′‐position is the major pathway of phase I metabolism for lovastatin in humans whereas hydroxylation at the 3″‐position of the side chain is a minor pathway,
hepatic metabolites of lovastatin are active inhibitors of HMG‐CoA reductase,
there is biliary excretion of unidentified polar metabolites of lovastatin,
following oral administration of 14C‐lovastatin in humans, 9.6% and 83.2% of the administered radioactivity were excreted in urine and faeces, respectively,
the bioavailability of lovastatin increases by 30–50% when taken with a standard meal,
due to the involvement of the CYP3A4 isoform in monacolin metabolism, there is a risk of adverse effects due to interactions with other medicinal products and foods,
in subjects given RYR extract (LipoCol Forte), plasma levels of lovastatin and lovastatin acid were two‐ to fivefold higher than in volunteers receiving a similar dose administered as lovastatin tablets, but divergent data have been obtained in other pharmacokinetic studies in humans.
3.4. Adverse effects in humans
3.4.1. RYR Preparations
3.4.1.1. Adverse effects reported by international and national institutions
Several adverse effects associated with the intake of RYR‐based products have been reported; the nature of symptoms and target organs/systems are similar to those reported for lovastatin (Chen et al., 2015b). Data from case reports (ICSRs) have been retrieved from (1) the Vigibase database maintained by the World Health Organization (WHO) Collaborating Centre for International Drug Monitoring (online); (2) two publications reporting cases collected through national systems of nutrivigilance, the French Phytovigilance (ANSES, 2014), Italian Surveillance System of Natural Health Products (Mazzanti et al., 2017), and (3) data collected by the FDA. Appendix B illustrates these data together with the reported level of causality.
In the period 2002–May 2018, 82 cases of adverse effects to RYR were reported to the WHO Vigibase. The geographical distribution was as follows: 37 (45%) of them were from Americas; 2 (3%) from Asia; and 43 (52%) from Europe. The cases occurred in 51 females (62%) and 30 males (37%); in one case, the sex was unknown. One adverse event was reported in a child (28 days–23 month), 7 (9%) in subjects aged 18–44 years, 31 (38%) in subjects aged 45–64 years, 20 (24%) in subjects aged 65–74 years and 3 (4%) in subjects aged > 75 years. For the remaining 19 cases (24%), the age was unknown.
The more frequently reported adverse events were musculoskeletal and connective tissue disorders (myalgia was the most frequently reported reaction) (39%), followed by general disorders and administration site conditions (e.g. drug interaction, pain, fatigue) (32.9%), gastrointestinal disorders (e.g. diarrhoea, nausea and abdominal pain) (18%).
Between April 2002 and September 2015, the Italian Surveillance System of Natural Health Products (Mazzanti et al., 2017) collected 52 reports concerning 55 adverse reactions to food supplements containing RYR. The majority of the cases (71%) occurred in women. The distribution of cases among the different age groups was: 7 cases (13.5%) between 18 and 44 years; 21 cases (40.4%) between 45 and 64 years; 13 subjects (25%) between 65 and 74 years; and 8 cases (15.4%) in subjects > 75 years old. For three patients (13, 46 and 48), the age was unknown. The adverse effects reported to the system were associated with 16 different food supplements. Composition of three food supplements indicated the quantity of monacolin K with the daily dose (3 mg/day). These food supplements whose composition was indicated were responsible (with different grade of causality) for 34 (65%) out of 52 clinical events. In more detail, 33 cases were referred to two products (Armolipid and Armolipid Plus) and one case to the third one (Colest 500). The most frequently reported ‘system organ classes’ were ‘Musculoskeletal and connective tissue disorders’ (20 reactions, 36%), ‘Gastrointestinal disorders’ (12, 22%), ‘Hepatobiliary disorders’ (10, 18%), ‘Skin and subcutaneous tissue disorders’ (9, 16%). After the cases were reviewed for causality, the association was classified as certain (1 reaction of rhabdomyolisis in a 48‐year‐old woman who had experienced the same reaction after being treated with statins), probable (31 reactions, 56%), possible (18 reactions, 34%), unlikely (3 reactions) or unassessable (2 reactions).
In 14 cases (27%), the reaction was fulfilling the criteria for seriousness, including 13 cases that required hospitalisation. The hepatic reactions reported were classified as serious (acute hepatitis) in six out of ten cases (60%) (see Appendix B).
The report published by ANSES (2014) contains reactions reported to the French nutrivigilance system between its creation in 2009 and 31 May 2013. A total of 30 reports of adverse reactions potentially associated with the consumption of food supplements containing RYR were included, however only 25 had sufficient information to complete the causality assessment. Also, in this case, the majority of the cases concerned women (n = 15, 60%). The median age for the reported cases was 59 years.
Causality was considered very likely for 2 cases, likely for 10 cases and possible for 8 cases. The most frequently reported reactions were musculoskeletal (nine cases of muscular damage, all of them classified as likely and very likely) and liver‐related (eight cases) (see Appendix B).
Adverse effects to RYR have been reported also by FDA, through the CFSAN Adverse Event Reporting System (CAERS), which is a post‐market surveillance system, collecting information on the adverse events for food, dietary supplements and cosmetics. Data regarding the period between January 2004 and September 2017 can be downloaded from the FDA website (FDA, online).11 For this opinion, the search was performed using the term ‘yeast’ and selecting cases for ‘red yeast rice’. The few cases of reaction to yeast as such were excluded. The Panel noted that data reflect information as reported by healthcare professionals, industries, agencies and consumers, so that there is no conclusion on causality. People reporting reactions to FDA should classify them as: (1) suspected adverse reaction (suspected) or (2) possible result of concurrent condition or activity or co‐consumption of other products (concomitant) (see Appendix B).
In the period considered, FDA collected 164 cases of possible adverse effects to RYR; 67% of them occurred in women; 30.6% in males and in 4 cases (2.4%) the sex was not indicated. The distribution of cases among the different age groups was: 6 cases (3.7%) between 36 and 44 years; 54 cases (32.9%) between 45 and 64 years; 42 subjects (25.6%) between 65 and 74 years; 29 cases (17.7%) in subjects ≥ 75 years old. For 33 subjects, the age was unknown.
Considering the organs/systems involved in the adverse effects reported by the 4 national/international sources, the Panel noted that:
The most frequent adverse effects were reported for the musculoskeletal and connective tissues: from 29.9% of cases of FDA to 38.4% of Italian Phytovigilance cases. In all the four registries, cases of rhabdomyolysis were reported and, when assessed for causality, they were considered to be in the highest ranks;
The gastrointestinal system was frequently involved from 12% of cases of ANSES to 23.1% of Italian cases;
Skin and subcutaneous adverse effects were registered in approximately 12% of cases (from 8% of ANSES to 17.3% of Italian cases);
Hepatobiliary system was the target of a significant percentage of cases: from 8.5% of WHO cases to 32% of ANSES ones.
3.4.1.2. Adverse effects reported by interested parties following a public ‘Call for data’
Following the launch of a public ‘Call for data’, the Panel was provided with post‐marketing data related to certain food supplements containing monacolin K from RYR with a daily recommended intake ranges from 10 to 20 mg monacolin K/day. Eighty case reports encompassing 142 adverse reactions have been collected. Most of them were reported under the ‘system organ classes’ ‘Gastrointestinal disorders’ (24%), followed by ‘Musculoskeletal and connective tissue disorders’ (15%) and ‘Nervous system disorders’ (15%). Eight cases involved 13 serious adverse events (impaired gastric emptying, arrhythmia, splegia, abdominal pain upper, oropharyngeal discomfort, pharyngeal erosion, respiratory distress, toxic epidermal necrolysis, hepatitis acute, seizure, abdominal discomfort, dyspepsia, hepatitis). All these serious cases were spontaneous and six of them were medically confirmed. Of these six medically confirmed cases, confounding factors such as advanced age and/or concomitant medication were reported in three cases. Two of these patients recovered after withdrawn of the product while the outcome is unknown for the remaining case. Little information is available for the remaining three serious medically confirmed cases. Patients involved in the two serious non‐medically confirmed cases recovered after product withdrawal.
3.4.2. Adverse effects from clinical trials
Gerards et al. (2015) performed a systematic literature review and meta‐analysis of randomised clinical trials in which a known dose of monacolin K from RYR based‐products was tested against a control (either placebo or active treatment, including statins). A total of 20 clinical trials were included in the meta‐analysis, including a total of 6,663 patients, most of them from China. The dose of monacolin K reported in the included studies ranged between 4.8 and 24 mg/day; subjects were followed‐up from 6 to 168 weeks. Despite the low number of adverse effects reported in the studies, the authors conducted a meta‐analysis elaborated the results and compared the incidence of health problems in RYR‐treated vs control groups (Table 8).
Table 8.
Adverse effects reported in 36 papers (20 clinical trials) (modified from Gerards et al., 2015)
| Organ/system | RYR‐treated group | Control group |
|---|---|---|
| Gastrointestinal disorders (diarrhoea, GI discomfort, other symptoms) | 51 | 20 |
| Muscoloskeletal (arthralgia, weakness) | 15 | 9 |
| Laboratory value alterations (LDL, leucocytosis, leukopenia, hyperglycaemia) | 3 | 2 |
| Infectious problems (influenza, urinary tract, pneumonia) | 10 | 5 |
| Immunologic problems (rash, alopecia, allergic reactions) | 7 | 4 |
| General problems (dizziness, malaise, fatigue) | 6 | 6 |
| CNS disorders (headache) | 5 | 5 |
| Cardiovascular disorders (QT prolongation, uncontrolled hypertension, oedema, erectile dysfunction) | 2 | 6 |
| Miscellaneous problems (breast cancer, unspecified) | 23 | 29 |
RYR: red yeast rice; LDL: low‐density lipoprotein; CNS: central nervous system.
The authors extracted information from the studies reporting data on kidney injury (8 studies), liver abnormalities (14 studies reporting data on liver transaminases) and muscle symptoms (10 studies reporting creatinine kinase (CK) values or information) obtained from a validated questionnaire. There were no significant differences between the incidence of the events in the patients treated with RYR compared to the controls, for any of the three endpoints. However, the authors stated that ‘risk of bias was evident in the assessment of adverse reactions which could have led to the underestimation of the incidence of adverse reactions in RYR groups’. The authors noted that safety assessment was not included in all studies; some studies did not provide quantitative data and the methods for evaluating, defining and reporting adverse reactions were unclear. They specified that studies in which the criterion was unclear the prevalence of adverse reactions was very low (0–9%) in comparison to studies that reported all adverse reactions (30–76%).
Other adverse reactions and patient reported symptoms were classified by organ system (Table 10).
Table 10.
Case reports of liver function alteration associated with intake of food supplements containing monacolin K from RYR preparations, published in the scientific literature
| Patient data | Daily intake of monacolin Ka | Period of intake | Adverse effects | Reference |
|---|---|---|---|---|
| Female, 63 years old | 15–30 mg | 6 months | Severe lobular necroinflammatory changes at liver biopsy; previous mild hepatotoxicity during therapy with lovastatin | Grieco et al. (2009) |
| Female, 53 years old | 3 mg | 60 days | Increased level of ALT and AST, GGT | Lapi et al. (2008) |
| Male, 49 years old | 5 mg | 60 days | Increased level of ALT and AST | Lapi et al. (2008) |
| Female, 42 years old | 3 mg | 30 days | Acute hepatitis with hospitalisation | Mazzanti et al. (2017) |
| Female, 46 years old | 3 mg | 50 days | Acute hepatitis with hospitalisation | Mazzanti et al. (2017) |
| Female, 58 years old | 3 mg | 60 days | Increased level of AST (2x normal level) | Mazzanti et al. (2017) |
| Female, 68 years old | 3 mg | 15 days | Increased level of pancreas and hepatic enzymes | Mazzanti et al. (2017) |
| Female, 68 years old | 3 mg | 1 year | Increased level of transaminases; previous statin intolerance | Mazzanti et al. (2017) |
| Male, 35 years old | 3 mg | 60 days | Toxic acute hepatitis with hospitalisation | Mazzanti et al. (2017) |
| Male, 36 years old | 3 mg | 76 days | Acute hepatitis with hospitalisation | Mazzanti et al. (2017) |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma‐glutamyl transpeptidase.
Ingredients other than monacolin K may be present in the products.
Following the launch of a public ‘Call for data’, the Panel was aware of a study performed in 123 patients affected by hypercholesterolaemia (‘Documentation provided to EFSA’ n. 9). The patients received two capsules per day of a RYR preparation containing 6 mg of monacolin K per capsule (corresponding to a total daily intake of monacolin K of 12 mg) for 6 weeks. Measurements of blood level of cholesterol and CPK were performed before starting the treatment and after the 6 weeks. Among the 123 patients enrolled in the study, 86 never took statins before, 37 took statins irregularly due to intolerance. No statistically significant changes in the CK levels were observed after treatment with monacolin K in all the 123 patients. Out of the 37 patients who had exhibited intolerance to statins, four patients (11%) complained myalgia in the lower back (Descamps, 2013).
3.4.3. Case reports from the scientific literature
Some case reports have been published in the scientific literature. The following sections list the published cases, where causality was assessed or where clinical data were sufficiently detailed.
3.4.3.1. Musculoskeletal disorders
Myopathy and rhabdomyolysis are the most frequent adverse effects associated with RYR. Most cases were collected by ANSES (Philibert et al., 2016), and the Italian Surveillance System (Mazzanti et al., 2017).
The most significant data on musculoskeletal disorders have been selected and summarised in Table 9. Other case reports describing similar adverse effects, but with intake of monacolin K unknown, are listed below:
an increased level of CK and muscle pain were described in two females 43‐ and 60‐year‐old, receiving 400 and 200 mg of RYR, respectively, for 6 months (Lapi et al., 2008);
a group of eight cases of increased level of CK, myopathy, and/or other muscle disorders, where the intake of monacolin K was not specified, were reported by Mazzanti et al., 2017.
after 90 days of treatment with RYR, a female (61 years old) showed diffuse myalgia and increased level of serum CK. Both problems were solved after elimination of RYR. The same adverse effects were previously suffered by the patient during a therapy with simvastatin (Mueller, 2006);
rhabdomyolysis and increased level of CK were observed in a male (37 years old) after the intake of RYR for 60 days (Philibert et al., 2016);
myalgia appeared in a woman (53 years old; thyroidectomy) receiving RYR (Philibert et al., 2016);
A case in a man (46 years old) developing body aches, increased level of serum CK (10 times) as a consequence of the intake of 600 mg of RYR has been reported by Polsani et al. (2008). The patient suffered similar problems during a previous treatment with simvastatin;
a case of rhabdomyolysis was observed in a 70‐year‐old woman in polytherapy, when she started consuming a dietary supplement containing RYR. The adverse effect was probably due to the interaction between monacolin K and the medicinal products sertraline and rosuvastatin (Russo et al., 2016);
the association between RYR intake and myopathy was observed in a middle‐aged man after intake of a herbal preparation. A mild increase of creatine phosphokinase (CPK) level was detected during laboratory testing; after discontinuation of the product, the CPK level returned to normal, but when the product was resumed some months after, the increase in the CPK level reoccurred (Smith and Olive, 2003);
muscle weakness and increased level of CK (previously appeared with atorvastatin) were shown by a diabetic male (76 years old) after 90 days of RYR intake (Vercelli et al., 2006);
a case of myalgia, regurgitation of food, appetite absence, fatigue and upper abdominal pain was observed in a 53‐year‐old woman, who used a RYR supplement (10 mg of monacolin K) bought from online web shop (Venhuis et al., 2017).
Table 9.
Case reports of myopathy and rhabdomyolysis associated with intake of food supplements containing monacolin K from RYR preparations, published in the scientific literature
| Patient data | Daily intake of monacolin K | Period of intake | Adverse effects | Reference |
|---|---|---|---|---|
| Female, 52 years old | 2.6 mg | 90 days | Increase level of serum CK; Myalgia | Philibert et al. (2016) |
| Female, 53 years old | 3 mg | 60 days | Increased level of serum CK | Lapi et al. (2008) |
| Sex unknown, 48 years old | 3 mg | 60 days | Rhabdomyolysis (hospitalisation) | Mazzanti et al. (2017) |
| Female, 45 years old | 3 mg | 24 days | Nocturnal leg muscle cramps | Mazzanti et al. (2017) |
| Female, 45 years old | 3 mg | 54 days | Myalgia in the leg | Mazzanti et al. (2017) |
| Female, 53 years old | 3 mg | 97 days | Generalised muscle aches | Mazzanti et al. (2017) |
| Female, 57 years old | 3 mg | 60 days | Increased level of serum CK (10 times). | Mazzanti et al. (2017) |
| Female, 65 years old | 3 mg | 31 days | Cramps and myalgia of lower extremities | Mazzanti et al. (2017) |
| Female, 67 years old | 3 mg | 31 days | Increased level of serum CK, myopathy, asthenia. Previous myopathy by statins | Mazzanti et al. (2017) |
| Female, 68 years old | 3 mg | 62 days | Localised muscle pain; increased level of serum CK | Mazzanti et al. (2017) |
| Female, 69 years old | 3 mg | 25 days | Myalgia of lower extremities | Mazzanti et al. (2017) |
| Female, 70 years old | 3 mg | 90 days | Myalgia, increased level of serum CK, Previous statin intolerance | Mazzanti et al. (2017) |
| Female, age unknown | 3 mg | > 365 days | Increased level of serum CK, previously observed with statins | Mazzanti et al. (2017) |
| Male, 60 years old | 3 mg | 165 days | Increased level of serum CK | Mazzanti et al. (2017) |
| Female, | 4–8 mg | 4 months | Myalgia | Venhuis et al. (2016) |
| Male, 49 years old | 5 mg | 60 days | Increased level of serum CK | Lapi et al. (2008) |
| Female, 64 years old | 10.2 mg | 30 days | Increase level of serum CK | Philibert et al. (2016) |
| Female, 51 years old | 19.8 mg | 30 days | Myalgia | Philibert et al. (2016) |
| Male, 37 years old | 19.2 mg | 48 days | Rhabdomyolysis, increased level of serum CK | Philibert et al. (2016) |
| Female, 51 years old | 19.8 mg | 30 days | Myalgia | Philibert et al. (2016) |
CK: creatine kinase.
3.4.3.2. Effects on the liver
Effects on the liver are among the most frequent and severe adverse effects reported in association with the intake of RYR. As for musculoskeletal disorders, Table 10 summarised the case reports where the intake of monacolin K, the symptomatology, and a convincing assessment of causality was specified. Mazzanti et al. (2017) described 10 cases of hepatobiliary disorders associated with the intake of RYR; two of them were considered unassessable/unlikely. The remaining cases are included in the Table 10, apart from one of them where the quantity of monacolin K was not specified. In the latter case, the patient (male, 51 years old) was hospitalised for an acute cholestatic hepatitis appeared after 28 days of RYR intake.
Grieco et al. (2009) described the case of a 63‐year‐old woman, who received chronically RYR extract (Equisterol®, containing 15 mg of monacolin K) to treat hypercholesterolaemia. The product was prescribed to replace a medicinal product containing statins, as the patient had developed hepatotoxicity. The patient after a few weeks of intake of the RYR product showed severe elevation of liver transaminases, and a severe lobular hepatic necrosis and inflammation with eosinophilic infiltrate observed in liver biopsy. The case was regarded as an adverse effect to RYR, after exclusion of other possible causes of hepatotoxicity. Lapi et al. (2008) reported three cases of patients showing increased levels of biomarkers of liver disorders (such as alanine aminotransferase (ALT), glutaryl aminotransferase and gamma glutamyl transpeptidase) after the use for few months of food supplements containing RYR; two of them are included in Table 11.
Table 11.
Frequency, organ class and adverse effects of lovastatin as reported in the SPC on the basis of clinical studies and post‐marketing data (SCP, online)
| Frequency | Organ class | Adverse effect |
|---|---|---|
| Common (≥ 1/100 to < 1/10) | Metabolism and nutrition disorders | Digestive disorders |
| Nervous system disorders | Vertigo, cephalagia | |
| Eye disorders | Blurred vision | |
| Gastrointestinal disorders | Flatulence, diarrhoea, constipation, nausea, dyspepsia, abdominal pains | |
| Skin and subcutaneous tissue disorders | Rash | |
| Musculoskeletal and connective tissue disorders | Muscle cramps and myalgia | |
| Uncommon (≥ 1/1,000 to < 1/100) | Psychiatric disorders | Insomnia, sleeping difficulties |
| Nervous system disorders | Dysgeusia | |
| Skin and subcutaneous tissue disorders | Pruritus, xerostomia | |
| General disorders and administration site conditions | Tiredness | |
| Rare (≥ 1/10,000 to < 1/1,000) | Immune system disorders | Hypersensitivity syndrome associated with one or more of the following symptoms: anaphylaxis, angioedema, lupus‐like syndrome, polymyalgia rheumatica, dermatomyositis, vasculitis, thrombocytopenia, leucopenia, eosinophilia, haemolytic anaemia, positive antinuclear antibodies (ANA), increased sedimentation rate, arthritis, arthralgia, urticaria, asthenia, photosensitivity, fever, flushes, chills, dyspnoea and malaise |
| Metabolism and nutrition disorders | Anorexia | |
| Psychiatric disorders | Psychological disturbances including restlessness/anxiety | |
| Nervous system disorders | Peripheral neuropathy, in particular if used for a long period of term, paraesthesia | |
| Gastrointestinal disorders | Vomiting | |
| Hepatobiliary disorders | Hepatitis, cholestatic jaundice | |
| Skin and subcutaneous tissue disorders | Hair loss, toxic epidermal necrolysis and erythema multiforme including Stevens‐Johnson syndrome | |
| Musculoskeletal and connective tissue disorders | Myopathia and rhabdomyolysis, erectile dysfunction | |
| Investigations |
Marked and persistent increases in serum transaminase concentrations Other irregularities in liver function tests, including elevated alkaline phosphatase and bilirubin have been reported. Increases in the serum concentration of CK (which may be attributed to the non‐cardiac fraction of CK) have been seen. These have usually been slight and transient; marked increases have only occurred in rare cases |
|
| Not known | Psychiatric disorders | Depression |
A case of liver injury was described in a 62‐year‐old woman, who showed a 10‐week history of flu‐like symptoms (Roselle et al., 2008). She suffered chronic diseases (asthma, allergic rhinitis, and depression and took (in addition to RYR 600 mg/twice a day, level of monacolin K unknown) montelukast sodium and fluoxetine. Results from laboratory analysis showed an increased level of aspartate amino transferase (4.6 times the highest normal level), ALT (14.4 times the highest normal level), and of the erythrocyte sedimentation rate (three times the highest normal value). Further tests performed on the liver biopsy supported a possible drug‐induced hepatitis. Clinical pattern was totally solved with cessation of RYR product.
3.4.3.3. Gastrointestinal disorders
Mazzanti et al. (2017) collected 12 cases of gastrointestinal disorders associated with the intake of RYR‐based products; one of them was classified as unassessable. Among the 11 cases considered probable/possible, one required hospitalisation (female, 43 years old) for severe vomiting and one (male, 55 years old) showed severe symptoms.
3.4.3.4. Skin and subcutaneous tissue disorders
Mazzanti et al. (2017) described nine cases of skin disorders associated with the intake of RYR‐based products; two of them were classified as unlikely. Among the probable/possible cases, two required hospitalisation: one female, 66 years old, for generalised urticaria and itching, and one female (age unknown) for drug eruption with eosinophilia and systemic symptoms.
3.4.3.5. Other reported effects
A case of peripheral neuropathy has been described in a 60‐year‐old male patient, who received contemporary imatinib to treat a metastatic gastrointestinal cancer and a food supplement containing RYR (Kumari et al., 2013). The causality was demonstrated by the total remission of symptoms after withdrawal of RYR preparation.
A case of erectile dysfunction was described in a 39‐year‐old subject, who self‐prescribed a RYR preparation to reduce his hyperlipidaemia (Liu and Chen, 2018). Symptoms disappeared after 5 weeks of wash out from RYR.
It has been described a case of IgE‐mediated allergy to RYR in a 26‐year‐old man, who was preparing sausage handling raw meats and spices (including RYR), used as preservatives (Wigger‐Alberti et al., 1999).
3.4.4. Lovastatin
The safety profile lovastatin is described in the Summary of Product Characteristics (SPC) of the medicinal products containing it as an active ingredient. In its opinion on the substantiation of health claims, the NDA Panel referred to the information contained in the SPC of lovastatin with respect to the safety profile of monacolin K.
According to the SPC for medicinal products containing lovastatin, the use of lovastatin is contraindicated in the following cases (SCP, online):
Hypersensitivity to lovastatin or to any of the excipients of the medicinal product
Active liver disease or unexplained persistently elevated serum transaminase levels
Cholestasis
Myopathy
Concomitant administration of potent CYP3A4 inhibitors (itraconazole, ketoconazole, HIV protease inhibitors, erythromycin, clarithromycin, telithromycin and nefazodone).
Concomitant treatment with delavirdine, verapamil and amiodarone.
Pregnancy and lactation
Alcoholism.
As stated in the SPC, the use of lovastatin in children and adolescents (below 18 years) ‘is not recommended as safety and efficacy studies have not been established’.
Special warnings and precautions for use are reported for the occurrence of myopathy, an adverse effect common to inhibitors of HMG‐CoA reductase, which is manifested as muscle pain, tenderness or weakness with increase in the values of CK above 10 times the normal upper limit (ULN). In the most severe cases, myopathy takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. According to the information reported in the SPC, the risk of myopathy is increased by high levels of HMG‐CoA reductase inhibitory activity in plasma. The risk is also increased by the concomitant use of lovastatin with potent inhibitors of CYP3A4, other lipid‐lowering medicines that can cause myopathy and in certain subgroups of the population.
In the initial clinical trials, marked (to more than three times the ULN) increases in transaminases occurred in a few patients, usually appearing 3–12 months after the start of therapy with lovastatin, but without the development of jaundice or other clinical signs or symptoms. There was no evidence of hypersensitivity. As a precaution, the SPC recommends performing liver function tests before initiation of therapy in patients with a history of liver disease, or when otherwise clinically indicated, and also periodically later (e.g. every half year), particularly in patients whose results are abnormal and/or who consume high quantities of alcohol and/or who receive doses > 40 mg lovastatin/day.
It is further recommended to test liver function in all patients prior to use of 40 mg or more daily and thereafter when clinically indicated.
Continuation of the treatment should be reconsidered in the event of serum transaminase level rising above three times the upper limit of normal values and the analysis should be performed again after discontinuation of the treatment.
As with other lipid‐lowering agents, moderate (less than three times the ULN) elevations of serum transaminases have been reported during therapy with lovastatin, appearing soon after initiation of therapy with lovastatin but usually of a transient nature and not accompanied by any symptoms. Such mild effects were not considered to deserve interruption of treatment.
Lovastatin should be used with caution in patients who consume substantial quantities of alcohol and/or have a past history of liver disease. Active liver disease or unexplained persistent elevations of serum transaminases is a contraindication to the use of lovastatin.
3.4.4.1. Frequency of side effects to lovastatin
Table 11 lists the most frequent adverse effects based on data derived from clinical studies and from post‐marketing experience with lovastatin (SPC, online).
The Panel noted the reported adverse effects for lovastatin are consistent with those reported for monacolin K from RYR.
3.4.4.2. Case of adverse effects collected by European Pharmacovigilance
The European Medicines Agency (EMA) is responsible for the collection of suspected adverse reactions to human and veterinary medicines, including herbal medicines for human use. Data are published in the Eudravigilance (http://www.adrreports.eu/en/index.html), the European database, which can be accessed online. The Eudravigilance database exists since December 2001; the information is regularly updated and the current status is February 2018.
Information on the adverse effects to lovastatin is shown in Tables 12. Reports from countries of the European Economic Area are approximately the 25% of total cases. The highest numbers of case reports were from Germany (12.1%), Italy (8.3%), and Spain (2.9%). Cases were distributed among males and females for 50.4 and 42.9%, respectively. Adverse effects involving the musculoskeletal and connective tissues were more than 50% (53.4%); the other systems, which were frequently target of the adverse symptomatology, were: nervous system (20.3%); gastrointestinal system (14.9%); renal system (11.3%); hepatobiliary system (10%); skin and subcutaneous tissue (8.9%); and cardiac system (8.3%).
Table 12.
Distribution of suspected adverse effects to lovastatin reported to EMA according to the ‘system organ classes’
| Target of the adverse effect | Males | Females | UN | Total | Prevalence (%)a |
|---|---|---|---|---|---|
| Musculoskeletal and connective tissue disorders | 436 | 367 | 63 | 866 | 53.4 |
| Investigations | 237 | 183 | 26 | 446 | 27.5 |
| General disorders and administration site conditions | 206 | 206 | 22 | 434 | 26.8 |
| Nervous system disorders | 168 | 145 | 7 | 329 | 20.3 |
| Gastrointestinal disorders | 106 | 125 | 11 | 242 | 14.9 |
| Renal and urinary disorders | 113 | 63 | 8 | 184 | 11.3 |
| Hepatobiliary disorders | 82 | 74 | 6 | 162 | 10.0 |
| Skin and subcutaneous tissue disorders | 69 | 68 | 7 | 144 | 8.9 |
| Cardiac disorders | 76 | 56 | 3 | 135 | 8.3 |
| Injury, poisoning and procedural complications | 58 | 65 | 5 | 128 | 7.9 |
| Psychiatric disorders | 70 | 42 | 2 | 114 | 7.0 |
| Respiratory, thoracic and mediastinal disorders | 52 | 51 | 4 | 107 | 6.6 |
| Metabolism and nutrition disorders | 42 | 42 | 3 | 87 | 5.4 |
| Eye disorders | 42 | 29 | 3 | 74 | 4.6 |
| Vascular disorders | 37 | 33 | 2 | 72 | 4.4 |
| Infections and infestations | 30 | 32 | 1 | 63 | 3.9 |
| Blood and lymphatic system disorders | 22 | 22 | 5 | 49 | 2.7 |
| Neoplasm benign, malignant and unspecified | 25 | 23 | 1 | 49 | 3.0 |
| Reproductive system and breast disorders | 18 | 9 | 1 | 28 | 1.7 |
| Ear and labyrinth disorders | 10 | 15 | – | 25 | 1.5 |
| Immune system disorders | 10 | 12 | 2 | 24 | 1.5 |
| Social circumstances | 7 | 14 | 1 | 22 | 1.4 |
| Product issues | 7 | 11 | 1 | 19 | 1.2 |
| Congenital, familial, genetic disorders | 3 | 11 | 4 | 18 | 1.1 |
| Endocrine disorders | 5 | 4 | – | 9 | 0.6 |
| Pregnancy, puerperium and perinatal conditions | – | 4 | 2 | 6 | 0.4 |
Number of subjects with symptoms in a target organ or system/total subjects (1,622).
Prevalence has been calculated according to the following equation.
4. Discussion
RYR is made by fermentation of rice with yeasts, mainly M. purpureus. RYR is traditionally used in China for culinary purposes as a food colouring or as a traditional remedy to promote digestion and blood circulation.
In 2013, the NDA Panel issued a scientific opinion on the substantiation of a health claim related to monacolin K from RYR and maintenance of normal blood LDL‐cholesterol concentration. The NDA Panel concluded that a cause and effect relationship has been established between the consumption of 10 mg/day of monacolin K from RYR and the claimed effect.
Monacolin K in lactone form is identical to lovastatin, the active ingredient of several medicinal products authorised for the treatment of hypercholesterolaemia in the EU.
Due to the wide variability in composition of food supplements containing RYR and to the lack of data on the safety of these mixtures, the possible role of other ingredients of food supplements on monacolins bioactivity was not considered in this opinion.
Monacolin K and lovastatin are rapidly converted from their lactone to an identical HA form, the latter being responsible for the inhibition of the HMG‐CoA reductase enzyme involved in the biosynthesis of cholesterol. While the acidic form is naturally occurring in RYR, in the case of lovastatin its generation requires conversion from the lactone form.
The bioavailability of lovastatin increases by 30–50% when taken with a standard meal. Due to the involvement of the CYP3A4 isoform in its metabolism, interactions with drugs or food ingredients which are inhibitors of this enzyme have been described, leading to increased plasma levels of statins and possible increased risk of toxic effects. There are indications to support the fact that the monacolin K of the RYR may have a different concentration–time profile compared to the corresponding lovastatin. Two studies present conflicting results. In the first one, the serum concentration of monacolin K (in the two forms of lactone and HA) would be significantly higher (approximately four times) when the subjects are treated with equivalent dose of monacolin K from RYR or lovastatin (Chen et al., 2013). In this study, a dose of 5–6 mg of monacolin K was considered by the authors bioequivalent to 20–40 mg of lovastatin. The study by Li et al. (2005) indicates instead an opposite result, which could have been influenced by the pre‐treatment and simultaneous administration of grapefruit juice, a known CYP3A4 inhibitor.
Through the use of the Mintel GNPD it is possible to monitor the products containing monacolin K in the European market over a specific period of time. The Panel conducted a search related to the introduction of products containing monacolin K from RYR from January 2012 to February 2018. According to the Mintel GNPD in 2012, four products containing monacolin K have been placed in the European market, eight products in 2013, five in 2014, five in 2015, six in 2016, nine in 2017 and two in 2018.
However, the Panel noted that the search conducted with the Mintel GNPD, was not informative with regard to the extent of use of food supplements containing monacolin K from RYR.
The Panel noted that in some cases, the intake of RYR was associated with other botanicals (either in multi‐ingredient preparations or concomitantly) or other medicinal products, which could be responsible for any side effect. However, the reported side effects, in particular musculoskeletal side effects with elevated CPK up to several thousand units have occurred after ingestion of monacolin K and lovastatin but not after ingestion of other botanical ingredients.
The therapeutic dose of lovastatin ranges between 10 and 80 mg/day. Considering six clinical trials, where RYR was the only treatment used to reduce hypercholesterolaemia, it was shown that changes in total cholesterolaemia and LDL‐cholesterolaemia were dose‐dependent. The lowest intake of monacolin K, which was effective in reducing total cholesterolaemia (−11.2%) and LDL‐cholesterolaemia (−14.8%) was 3 mg/day.
According to four clinical studies (Dujovne et al., 1991; Furberg et al., 1994; Weintraub et al., 1994; Downs et al., 2001), the published case reports, and EMA Pharmacovigilance, the most frequent adverse effects to lovastatin have as a target the following organs/systems, in decreasing order of frequency: musculoskeletal and connective tissue, including rhabdomyolysis (53.4% of EMA reports), nervous system (20.3%), gastrointestinal tract (14.9%), kidney (11.3%), liver (10%), skin and subcutaneous tissue (8.9%), and other minor targets.
The Panel noted that the profile of adverse effects to RYR is similar to that of lovastatin; through consultation of four sources of case reports (WHO, ANSES, Italian Surveillance system, FDA), the most important targets of adverse events are: 1) musculoskeletal and connective tissue (29.9–37.2% of cases, including 1–5% of rhabdomyolysis); liver (9–32%); nervous system (when reported, 12.8–26.9%; gastrointestinal tract (12–23.1%); skin and subcutaneous tissue (8–17.3%).
Case reports specifying the daily intake of monacolin K showed that adverse effects appeared also with the intake of 3 mg/day for period ranging between 2 weeks and 1 year. Cases of rhabdomyolysis, hepatitis and skin disorders occurred and required hospitalisation (Mazzanti et al., 2017).
With respect to the data collected on the safety of use of RYR (and in particular of monacolins), the Panel identified the following uncertainties:
The composition and content of monacolins (and their relative abundance) in food supplements containing RYR.
Monacolins in RYR are used in multi‐ingredients botanical preparations, the components of which have not been fully evaluated individually or in combination.
The ratio between monacolin K lactone and monacolin K HA is variable in food supplements containing RYR.
Lack of data on the bioactivity of components in RYR other than monacolin K.
Due to the lack of data, the safe use of monacolins in certain groups of consumers cannot be evaluated (pregnant women, nursing women, breast‐fed infants).
The effects of concomitant consumption of RYR‐based food supplements with foods or drugs inhibiting CYP3A4.
In the majority of cases, RYR‐based food supplements are multi‐ingredients products. Interactions with other ingredients on the safety of monacolins are unknown.
5. Conclusion
The Panel concluded that monacolin K in lactone form is identical to lovastatin, an active ingredient of several medicinal products.
The Panel further concluded that intake of monacolins from RYR via food supplements, could lead to estimated exposure to monacolin K within the range of the therapeutic doses of lovastatin.
The Panel considered that the available information on the adverse effects reported in humans were judged to be sufficient to conclude that monacolins from RYR when used as food supplements were of significant safety concern at the use level of 10 mg/day. The Panel further considered that individual cases of severe adverse reactions have been reported for monacolins from RYR at intake levels as low as 3 mg/day.
The Panel concluded that exposure to monacolin K from RYR could lead to severe adverse effects on musculoskeletal system, including rhabdomyolysis, and on the liver. In the reported cases, the product contained other ingredients in addition to RYR. However, these reported effects, in particular musculoskeletal effects, have both occurred after ingestion of monacolin K and lovastatin independently.
On the basis of the information available and several uncertainties highlighted in this opinion, the Panel was unable to identify a dietary intake of monacolins from RYR that does not give rise to concerns about harmful effects to health, for the general population, and as appropriate, for vulnerable subgroups of the population.
Documentation provided to EFSA
Opinion No 9312 of the Superior Health Council of Belgium on “Food supplements based on red yeast rice”. February 2017.
Opinion of the French Agency for Food, Environmental and Occupational Health and Safety on the risk associated with the presence of “red yeast tice” in food supplements. February 2017.
Opinion of the Joint Expert Committee BVL/BfArM on “Classification of red yeast rice products”. February 2017.
Germany Joint Expert Commission, December 2017. Response to EFSA request for information monacolins from red yeast rice.
EHPM, January 2018. Response to EFSA request for information monacolins from red yeast rice.
FederSalus, January 2018. Response to EFSA request for information monacolins from red yeast rice.
Natural, December 2017. Response to EFSA request for information monacolins from red yeast rice.
Interested Party #1, December 2017. Response to EFSA request for information monacolins from red yeast rice.
Facobel, December 2017. Response to EFSA request for information monacolins from red yeast rice.
PonRoy, December 2017. Response to EFSA request for information monacolins from red yeast rice.
Zhejiang Sanhe Bio‐tech Co.,Ltd. December 2017. Response to EFSA request for information monacolins from red yeast rice.
Bachi, December 2017. Response to EFSA request for information monacolins from red yeast rice.
Abbreviations
- ADME
absorption, distribution, metabolism and excretion
- ALT
alanine aminotransferase
- ANA
antinuclear antibodies
- ANS Panel
EFSA Panel on Food Additives and Nutrient Sources Added to Food
- ANSES
Agency for Food, Environmental and Occupational Health & Safety
- AST
aspartate aminotransferase
- ATCC
American Type Culture Collection
- AUC
area under the curve
- BfArM
Federal Institute for Drugs and Medical Devices
- BVL
Expert Committee of Federal Office of Consumer Protection and Food Safety
- Cmax
maximum concentration
- CAERS
CFSAN Adverse Event Reporting System
- CK
creatinine kinase
- CNS
central nervous system
- CP
commercial product
- CPK
creatine phosphokinase
- CRYR
Chinese red yeast rice
- DMK
dehydroxymonacolin K
- ED50
Median effective dose
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- GGT
gamma ‐glutamyl transferase
- HA
hydroxy acid
- HMG‐CoA
3‐hydroxy‐3 methylglutaryl‐coenzyme A
- HPLC–MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- LDL
low‐density lipoprotein
- LDL‐C
low‐density lipoprotein‐cholesterol
- Mintel GNPD
Mintel Global New Products Database
- MJ
monacolin J
- MJA
monacolin J hydroxyacid
- MK
monacolin K
- MKA
monacolin K hydroxyacid
- ML
monacolin L
- MLA
monacolin L hydroxyacid
- MM
MM monacolin M
- MMA
monacolin M hydroxyacid
- MX
monacolin X
- MXA
monacolin X hydroxyacid
- NDA Panel
EFSA Panel on Dietetic Products, Nutrition and Allergies
- PI
compactin
- RYR
red yeast rice
- RYRP
RYR powder
- SD
standard deviation
- SmPC
Summary of Product Characteristics
- SPC
Summary of Product Characteristics
- TC
total cholesterol
- TDI
tolerable daily intake
- ULN
Upper Limit of Normal
- US
United States
- WHO
World Health Organization
Appendix A – List of monacolins identified in RYR
1.
Appendix B – Case collected by FDA and European phytovigilance Units with assessment of level of causalitya
1.
| Organ/system involved | ANSES (2009–May 2013) | Italian surveillance system (Apr 2002–Sep 2015) | FDA (CAERS) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number cases for symptomb | % Total cases | Causality | Number cases for symptomb | % total cases | Causality | Number cases for symptomb | % Total cases | Causality | |
| Musculoskeletal and connective tissue | 8 | 32.0 |
VL = 1 L = 7 |
19 | 36.5 |
L = 11 P = 8 |
47 | 28.7 |
SP = 31 CM = 16 |
| Rhabdomyolysis | 1 | 4.0 | L = 1 | 1 | 1.9 | C = 1 | 2 | 1.2 | SP = 2 |
| Nervous system (including psychiatric disorders) | 0 | 0 | 0 | 0 | 21 | 12.8 |
SP = 9 CM = 12 |
||
| Gastrointestinal system | 3 | 12.0 |
L = 2 UN = 1 |
12 | 23.1 |
L = 6 P = 5 UN = 1 |
31 | 18.9 |
SP = 21 CM = 10 |
| Skin and subcutaneous tissue | 2 | 8.0 |
VL = 1 P = 1 |
9 | 17.3 |
L = 3 P = 4 UN = 2 |
20 | 12.2 |
SP = 10 CM = 10 |
| Hepatobiliary system | 8 | 32.0 |
L = 3 P = 4 UN = 1 |
10 | 19.2 |
L = 7 P = 1 UN = 2 |
25 | 15.2 |
SP = 8 CM = 17 |
| Other | 7 | 28.0 |
P = 3 UN = 3 EX = 1 |
4 | 7.7 | L = 4 | 56 | 34.1 |
SP = 41 CM = 15 |
| TOTAL CASES | 25 | – | 52 | 164 | |||||
NR: Not Reported; C: Certain; VL: Very Likely; L: Likely; P: Possible; UN: unassessable, unlikely; EX: Excluded; SP: Suspected; CM: concomitant.
http://www.vigiaccess.org, https://www.fda.gov; ANSES; 2014; Mazzanti et al., 2017.
Symptoms for each subject can be more than one.
Suggested citation: EFSA ANS Panel (EFSA Panel Food Additives and Nutrient Sources added to Food) , Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Lambré C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Andrade RJ, Fortes C, Mosesso P, Restani P, Pizzo F, Smeraldi C and Wright M, 2018. Scientific opinion on the safety of monacolins in red yeast rice. EFSA Journal 2018;16(8):5368, 46 pp. 10.2903/j.efsa.2018.5368
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00138
Panel members: Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipič, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Gunter Georg Kuhnle, Claude Lambré, Jean‐Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 25 June 2018
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 3: Rotta Bonfim et al., 2015; Figure 4: Wang et al., 1991
Notes
EFSA Journal 2011;9(7):2304; EFSA Journal 2013;11(7):3327; EFSA Journal 2013;11(2):3084.
EFSA Journal 2012; 10(3):2605.
OJ l 67, 7.3.2014, p. 3.
Avis du Conseil Superieur de la Santé No 9312: Complements alimentaires a base de ‘levure de riz rouge’.
“Toxicological evaluation of red mould rice: un updated”, December 18th 2012, DFG Permanent Senate Commission on Food Safety, Deutsche Forschungsgemeinschaft DFG.
OJ L 404, 30.12.2006, p. 26.
OJ. L 031, 1.2.2002, p. 1–24.
From Eu Pharmacopeia: (1S,3R,7S,8S,8aR)‐8‐[2‐[(2R,4R)‐4‐Hydroxy‐6‐oxotetrahydro‐2H‐pyran‐2‐yl]ethyl]‐3,7‐dimethyl‐1,2,3,7,8,8a‐hexahydronaphthalen‐1‐yl (2S)‐2‐methylbutanoate.
References
- Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffmant C, Rothrockt J, Lopezt M, Joshuat H, Harrist E, Patchettt A, Monaghan R, Currie S, Stapleyt E, Albers‐Schonbergy O, Hirshfieldt HJ, Hoogsteent K, Liescht J and Springeri J, 1980. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl‐coenzyme A reductase and a cholesterol‐lowering agent [fungal metabolites/crystal structure/absolute configuration/cholesterol synthesis/ML‐236B (compactin)]. Proceedings of the National Academy of Sciences of the United States of America, 77, 3957–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANSES , 2014. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety. Request n. 2012‐SA‐0228. Available online: https://www.anses.fr/en/system/files/NUT2012sa0228EN.pdf [Accessed: 14 February 2014]
- Avula B, Cohen PA, Wang Y‐H, Sagi S, Feng W, Wang M, Zweigenbaum J, Shuangcheng M and Khan IA, 2014. Chemical profiling and quantification of monacolins and citrinin in red yeast rice commercial raw material and dietary supplements using liquid chromatography‐accurate QToF mass spectrometry: chemometrics application. Journal of Pharmaceutical and Biomedical Analysis, 100, 243–253. [DOI] [PubMed] [Google Scholar]
- Becker DJ, Gordon RY, Halbert SC, French B, Morris PB and Rader DJ, 2009. Red yeast rice for dyslipidemia in statin‐intolerance patients: a randomized trial. Annals of Internal Medicine, 150, 830–839, W147–W149. [DOI] [PubMed] [Google Scholar]
- Bogsrud MP, Ose L, Langslet G, Ottestad I, Strøm EC, Hagve TA and Retterstøl K, 2010. HypoCol (red yeast rice) lowers plasma cholesterol – a randomized placebo controlled study. Scandinavian Cardiovascular Journal, 44, 197–200. [DOI] [PubMed] [Google Scholar]
- Borresen EC, Henderson AJ, Kumar A, Weir TL and Ryan EP, 2012. Fermented foods: patented approaches and formulations for nutritional supplementation and health promotion. Recent Patents on Food, Nutrition and Agriculture, 4, 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MF, 2015. Red yeast rice for the treatment of dyslipidemia. Current Atherosclerosis Reports, 17, 495. [DOI] [PubMed] [Google Scholar]
- Chen F and Hu X, 2005. Study on red fermented rice with high concentration of monacolin K and low concentration of citrinin. International Journal of Food Microbiology, 103, 331–337. [DOI] [PubMed] [Google Scholar]
- Chen C‐H, Uang Y‐S, Wang S‐T, Yang J‐C and Lin C‐J, 2012. Interaction between Red Yeast Rice and CYP450 enzymes/P‐Glycoprotein and its implication for clinical pharmacokinetics of lovastatin. Evidence‐Based Complementary and Alternative Medicine, 2012, 127043 10.1155/2012/127043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C‐H, Yang J‐C, Uang Y‐S and Lin C‐J, 2013. Improved dissolution rate and oral bioavailability of lovastatin in red yeast rice products. The International Journal of Pharmaceutics, 444, 18–24. [DOI] [PubMed] [Google Scholar]
- Chen W, He Y, Zhou Y, Shao Y, Feng Y, Li M and Chen F, 2015a. Edible filamentous fungi from the species Monascus: early traditional fermentations, modern molecular biology, and future genomics. Comprehensive Reviews in Food Science and Food Safety, 14, 555–567. [Google Scholar]
- Chen H‐HS, Neher J and Safranek S, 2015b. Is red‐yeast rice a safe and effective alternative to ststins? The Journal of Family Practice, 64, 128–129. [PubMed] [Google Scholar]
- Chen W, Chen R, Liu Q, He Y, He K, Ding X, Kang L, Guo X, Xie N, Zhou Y, Lu Y, Cox RJ, Molnár I, Li M, Shao Y and Chen F, 2017. Orange, red, yellow: biosynthesis of azaphilone pigments in Monascus fungi. Chemical Science, 8, 4917–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Medical Herbology and Pharmacology , 2004. Hong Qu (Monascus) In: Chen JK. and Chen TT. (eds.). Art of Medicine Press Inc. Chap. 9. [Google Scholar]
- Chinese Ministry of Public Health , 2015. Chinese standards for food Additives ‐ GB 2760‐2015. Translated by: USDA Foreign Agricultural Services. “8 April 20165. Available online: https://gain.fas.usda.gov/Recent%20GAIN%20Publications/Standard%20for%20Food%20Additive%20Use%20-%20GB2760-2015_Beijing_China%20-%20Peoples%20Republic%20of_4-28-2015.pdf
- Cohen PA, Avula B and Khan IA, 2017. Variability in strength or red yeast rice supplements purchased from mainstream retailers. European Journal of Preventive Cardiology, 24, 1431–1434. [DOI] [PubMed] [Google Scholar]
- Descamps ÓS, 2013. Étude Mona Lisa Effet d'une préparation de levure rouge de riz (Artéchol®) dans une cohorte de patients hypercholestérolémiques traités par des médecins généralistes. Louvain Medical, 132, 149–154. [Google Scholar]
- Downs JR, Clearfield M, Tyroler A, Whitney EJ, Kruyer W, Langendorfer A, Zagrebelsky V, Weis S, Shapiro DR, Beere PA and Gatto AM, 2001. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): additional perspectives on tolerability of long‐term treatment with lovastatin. American Journal of Cardiology, 87, 1074–1079. [DOI] [PubMed] [Google Scholar]
- Duggan DE, Chen IW, Bayne WF, Halpin RA, Duncan CA, Schwartz MS, Stubbs RJ and Vickers S, 1989. The physiological disposition of lovastatin. Drug Metabolism and Disposition, 17, 166–173. [PubMed] [Google Scholar]
- Dujovne CA, Chremos AN, Pool JL, Schnaper H and Reagan H, 1991. Expanded clinical evaluation of lovastatin (EXCEL) study results: IV. Additional perspectives on the tolerability of lovastatin. American Journal of Medicine, 91, S25–S30. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Transparency in Risk Assessment – Scientific Aspects Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: General Principles. EFSA Journal 2009;7(5):1051, 1–22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA CONTAM panel (EFSA Panel on Contaminants in the Food Chain), 2012. Scientific Opinion on the risks for public and animal health related to the presence of citrinin in food and feed1, EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA Journal 2012;10(3):2605 10.2903/j.efsa.2012.2605 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2011. Scientific Opinion on the substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL‐cholesterol concentrations (ID 1648, 1700) pursuant to Article 13(1) of Regulation (EC) No. 1924/2006. EFSA Journal 2011;9(7):2304, 16 pp. 10.2903/j.efsa.2011.2304. Available online: http://www.efsa.europa.eu [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013. Scientific Opinion on the substantiation of a health claim related to monacolin K in SYLVAN BIO red yeast rice and maintenance of normal blood LDL‐cholesterol concentrations pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA Journal 2013;11(2):3084 10.2903/j.efsa.2013.3084 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance on Safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements. EFSA Journal 2009;7(9):1249, 25 pp. 10.2903/j.efsa.2009.1249 [DOI] [Google Scholar]
- FDA , 2014. Warning letter FLA‐14‐10 dated April, 2014. Available online: https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm396822.htm. [Accessed: 12‐2017]
- Fishbein WN and Bessman SP, 1966. Purification and properties of an enzyme in human blood and rat liver microsomes catalyzing the formation and hydrolysis of gammalactones. II. Metal ion effects, kinetics, and equilibra. The Journal of Biological Chemistry, 241, 4842–4847. [PubMed] [Google Scholar]
- Furberg CD, Adams HP, Applegate WB, Byington RP, Espeland MA, Hartwell T, Hunninghake DB, Lefkowitz DS, Probstfield J, Riley WA and Young B, for the ACAPS Research Group , 1994. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Circulation, 90, 1679–1687. [DOI] [PubMed] [Google Scholar]
- Gerards MC, Terlou RJ, Yu H, Koks CHW and Gerdes VEA, 2015. Traditional Chinese lipid‐lowering agent red yeast rice results in significant LDL reduction but safety is uncertain ‐ A systematic review and meta‐analysis. Atherosclerosis, 240, 415–423. [DOI] [PubMed] [Google Scholar]
- Gordon RY, Cooperman T, Obermeyer W and Becker DJ, 2010. Market variability of monacolin levels in commercial red yeast rice products. Archives of Internal Medicine, 170, 1722–1727. [DOI] [PubMed] [Google Scholar]
- Goswami S, Vidyarthi AS, Bhinia B and Mandal T, 2012. A review on lovastatin and its production. Journal of Biochemical Technology, 4, 581–587. [Google Scholar]
- Greenspan MD, Yudkovitz JB, Alberts AW, Argenbright LS, Arison BH and Smith JL, 1988. Metabolism of lovastatin by rat and human liver microsomes in vitro. Drug Metabolism and Disposition, 16, 678–682. [PubMed] [Google Scholar]
- Grieco A, Miele L, Pompili M, Biolato M, Vecchio FM, Grattagliano I and Gasbarrini G, 2009. Acute hepatitis caused by a natural lipid‐lowering product: When “alternative” medicine is no “alternative at all. Journal of Hepatology, 50, 1273–1277. [DOI] [PubMed] [Google Scholar]
- Halpin RA, Ulm EH, Till AE, Kari PH, Vyas KP, Hunninghake DB and Duggan DE, 1993. Biotransformation of lovastatin. V. Species differences in in vivo metabolite profiles of mouse, rat, dog, and human. Drug Metabolism and Disposition, 21, 1003–1011. [PubMed] [Google Scholar]
- Hawksworth DL and Pitt JI, 1983. A new taxonomy for Monascus species based on cultural and microscopical characters. Australian Journal of Botany, 31, 51–61. [Google Scholar]
- Health Canada , 2014. Notice. Prescription drug list (PDL): Lovastatin. File number 14‐105859‐93, dated June 4, 2014. Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/public-involvement-consultations/drug-products/notice-prescription-drug-list-lovastatin.html?_ga=2.175840228.773901738.1501236921-557739232.1501236921
- Heber D, Yip I, Asley JM, Elashoff RM and Go VLW, 1999. Cholesterol‐lowering effects of a proprietary Chinese red‐yeast‐rice dietary supplement. The American Journal of Clinical Nutrition, 69, 231–236. [DOI] [PubMed] [Google Scholar]
- Heinz T, Schuchardt JP, Möller K, Hadji P and Hahn A, 2016. Low daily dose of 3 mg monacolin k from RYR reduces the concentration of LDL‐C in a randomized‐controlled intervention. Nutrition Research, 36, 1162–1170. [DOI] [PubMed] [Google Scholar]
- Huang C‐F, Li T‐C, Lin C‐C, Liu C‐S, Shih H‐C and Lai M‐M, 2007. Efficacy of Monascus purpureus Went rice on lowering lipid ratios in hypercholesterolemic patients. The European Journal of Cardiovascular Prevention & Rehabilitation, 14, 438–440. [DOI] [PubMed] [Google Scholar]
- Kantola T, Kivistö KT and Neuvonen PJ, 1998. Grapefruit juice greatly increases serum concentrations of lovastatin and lovastatin acid. Clinical Pharmacology and Therapeutics, 63, 397–402. [DOI] [PubMed] [Google Scholar]
- Klimek M, Wang S and Ogunkanmi A, 2009. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative theraphy for hyperlipidemia. Pharmacy and Therapeutics, 34, 313–327. [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Sherriff M, Spooner D and Beckett R, 2013. Peripheral neuropathy induced by red yeast rice in a patient with a known small bowel gastrointestinal tumour. BMJ Case Reports, 10.1136/bcr-2013-009060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapi F, Gallo E, Bernasconi Vietri M, Mennoto‐Ippolito Raschetti R, Gori L, Firenzuoli F, Mugelli A and Vannacci A, 2008. Myopathies associated with red yeast rice and liquorice: spontaneous reports from the Italian Surveillance System of Natural Health Products. British Journal of Clinical Pharmacology, 66, 572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennernäs H and Fager G, 1997. Pharmacodynamics and pharmacokinetics of the HMN‐CoA reductase inhibitors. Clinical Pharmacokinetics, 32, 403–423. [DOI] [PubMed] [Google Scholar]
- Li YG, Zhang F, Wang ZT and Hu ZB, 2004. Identification and chemical profiling of monacolins in red yeast rice using high‐performance liquid chromatography with photodiode array detector and mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 35, 1101–1112. [DOI] [PubMed] [Google Scholar]
- Li Z, Seeram NP, Lee R, Thames G, Minutti C, Wang H‐J and Heber D, 2005. Plasma clearance of lovastatin versus Chinese Red Yeast Rice in healthy volunteers. Journal of Alternative and Complementary Medicine, 11, 1031–1038. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang L, Jia Z, Xin W, Yang S, Yang Q and Wang L, 2014. A meta‐analysis of red yeast Rice: an effective and relatively safe alternative approach for dyslipidemia. PLoS ONE, 9, e98611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C‐D, Chen Y‐C, Lin H‐Y, Chiueh L‐C and Shih DY‐C, 2014. Incidence of citrinin in red yeast rice and various commercial Monascus products in Taiwan from 2009 to 2012. Food Control, 38, 178–183. [Google Scholar]
- Lin CC, Li TC and Lai MM, 2005. Efficacy and safety of Monascus purpureus Went rice in subjects with hyperlipidemia. European Journal of Endocrinology, 153, 679–686. [DOI] [PubMed] [Google Scholar]
- Lin Y‐L, Wang T‐H, Lee M‐H and Su N‐W, 2008. Biologically active components and nutraceuticals in the Monascus‐fermented rice: a review. Applied Microbiology and Biotechnology, 77, 965–973. [DOI] [PubMed] [Google Scholar]
- Liu Z and Chen P, 2018. A case of erectile dysfunction induced by red yeast rice in lipid‐lowering therapy. Phytotherapy Research, 32, 953–954. 10:1002/ptr.6025 [DOI] [PubMed] [Google Scholar]
- Ma J, Li Y, Ye Q, Li J, Hua Y, Ju D, Zhang D, Cooper R and Chang M, 2000. Constituents of red yeast rice, a traditional Chinese food and medicine. Journal of Agricultural and Food Chemistry, 48, 5220–5225. [DOI] [PubMed] [Google Scholar]
- Macdonald JS and Halleck MM, 2004. The toxicology of HMG–CoA reductase inhibitors: prediction of human risk toxicologic. Pathology, 32(Suppl. 2), 26–41. [DOI] [PubMed] [Google Scholar]
- Martínková L, Patáková‐Jůzlová P, Krent V, Kucerová Z, Havlícek V, Olsovský P, Hovorka O, Ríhová B, Veselý D, Veselá D, Ulrichová J and Prikrylová V, 1999. Biological activities of oligoketide pigments of Monascus purpureus. Food Additives and Contaminants, 16, 15–24. [DOI] [PubMed] [Google Scholar]
- Mornar A, Sertic M and Nigovic B, 2013. Development of a rapid LC/DAD/FLD/MSn method for the simultaneous determination of monacolins and citrinin in red fermented rice products. Journal of Agricultural and Food Chemistry, 61, 1072–1080. [DOI] [PubMed] [Google Scholar]
- Mazzanti G, Moro PA, Raschi E, Da Cas R and Menniti‐Ippoliti F, 2017. Adverse reactions to dietary supplements containing red yeast rice: assessment of cases from the Italian surveillance system. British Journal of Clinical Pharmacology, 83, 894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PS, 2006. Symptomatic myopathy due to red yeast rice. Annals of Internal Medicine, 145, 474–475. [DOI] [PubMed] [Google Scholar]
- Nigović B, Sertić M and Mornar A, 2013. Simultaneous determination of lovastatin and citrinin in red yeast rice supplements by micellar electrokinetic capillary chromatography. Food Chemistry, 138, 531–538. [DOI] [PubMed] [Google Scholar]
- NIH , 2015. Red Yeast Rice. National Center for Complementary and Integrative Health, NIH. Available online: https://nccih.nih.gov/sites/nccam.nih.gov/files/Red_Yeast_Rice_11-30-2015.pdf [Accessed: 12‐2017]
- Osmanova N, Schultze W and Ayoub N, 2010. Azaphilones: a class of fungal metabolites with diverse biological activities. Phytochemistry Reviews, 9, 315–342. [Google Scholar]
- Patakova P, 2013. Monascus secondary metabolites: production and biological activity. Journal of Industrial Microbiology and Biotechnology, 40, 169–181. [DOI] [PubMed] [Google Scholar]
- Philibert C, Bres V, Jea‐Pastor M‐J, Guy C, Lebrun‐Vignes B, Robin P, Pinzani V and Hillaire‐Buys D, 2016. Atteites musculaires et levure de riz rouge: analyse des données de la base national de pharmacovigilance et revue de la littérature. Thérapie, 10.2515/therapie/2015053 [DOI] [PubMed] [Google Scholar]
- Polsani VR, Jones PH, Ballantyne CM and Nambi V, 2008. A case report from consumption of red yeast rice. Journal of Clinical Lipidology, 2, 60–62. [DOI] [PubMed] [Google Scholar]
- Prasad GVR, Wong T, Meliton G and Bhaloo S, 2002. Rhabdomyolysis due to red yeast rice (Monascus purpureus) in a renal transplant recipient. Transplantation, 74, 1200–1201. [DOI] [PubMed] [Google Scholar]
- Roselle H, Ekatan A, Tzeng J, Sapienza M and Kocher J, 2008. Symptomatic hepatitis associated with the use of herbel red yeast rice. Annals of Internal Medicine, 149, 516–517. [DOI] [PubMed] [Google Scholar]
- Rotta Bonfim M, Souza Bulle Oliveira A, do Amaral SL and Monteiro HL, 2015. Treatment of dyslipidemia with statins and physical exercises. Recent findings of skeletal muscle responses. Arquivos Brasileiros de Cardiologia 104, 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Gallelli L, Cannataro R, Perri M, Calignano A, Citraro R, Rusoo E, Gareri P, Corsonello A and De Sarro G, 2016. When nutraceuticals reinforce drugs side effects: a case report. Current Drug Safety, 11, 264–266. [DOI] [PubMed] [Google Scholar]
- Sabater‐Vilar M, Maas RFM and Fink Gremmels J, 1999. Mutagenicity of commercial Monascus fermentation products and the role of citrinin contamination. Mutation Reserach, 444, 7–16. [DOI] [PubMed] [Google Scholar]
- Schachter M, 2004. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundamental and Clinical Pharmacology, 19, 117–125. [DOI] [PubMed] [Google Scholar]
- Schmidt LE and Dalhoff K, 2002. Food‐Drug Interactions. Drug Metabolism and Disposition, 62, 1481–1502. [DOI] [PubMed] [Google Scholar]
- Serajuddin AT, Ranadive SA and Mahoney EM, 1991. Relative lipophilicities, solubilities, and structure‐pharmacological considerations of 3‐hydroxy‐3‐methylglutaryl‐coenzyme a (HMG‐COA) reductase inhibitors pravastatin, lovastatin, mevastatin, and simvastatin. Journal of Pharmaceutical Sciences, 80, 830–834. [DOI] [PubMed] [Google Scholar]
- Sirtori CR, 2014. The pharmacology of statins. Pharmacological Research, 88, 3–11. [DOI] [PubMed] [Google Scholar]
- SKLM (Permanent Senate Commission on Food Safety), 2012. Toxicological evaluation of red mould rice: An update. Issued on December 18th 2012. Available at: http://www.dfg.de/download/pdf/dfg_im_profil/reden_stellungnahmen/2013/131206_sklm_red_mould_rice_update.pdf.
- Smith DJ and Olive KE, 2003. Chinese red rice‐induced myopathy. Southern Medical Journal, 96, 1265. [DOI] [PubMed] [Google Scholar]
- SPC (Summary of Product Characteristic) for lovastatin, Available online: https://mri.cts-mrp.eu/download/DK_H_0744_001_FinalSPC.pdf
- Stancu C and Sima A, 2001. Statins: mechanism of action and effects. Journal of Cellular and Molecular Medicine, 5, 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venhuis BJ, van Hunsel F, van de Koppel S, Keizers PHJ, Jeurissen SM and De Kaste D, 2016. Pharmacologically effective red yeast rice preparations marketed as dietary supplements illustrated by a case report. Drug Testing and Analysis, 8, 315–318. [DOI] [PubMed] [Google Scholar]
- Vercelli L, Mongini T, Olivero N, Rudolico C, Musumeci O and Palmucci L, 2006. Chinese red rice depletes muscle coenzyme Q10 and maintains muscle damage after discontinuation of statin treatment. Journal of the American Geriatrics Society, 54, 718–720. [DOI] [PubMed] [Google Scholar]
- Vyas KP, Kari PH, Prakash SR and Duggan DE, 1990a. Biotransformation of lovastatin. I. Structure elucidation of in vitro and in vivo metabolites in the rat and mouse. Drug Metabolism and Disposition, 18, 203–211. [PubMed] [Google Scholar]
- Vyas KP, Kari PH, Prakash SR and Duggan DE, 1990b. Biotransformation of lovastatin. II. In vitro metabolism by rat and mouse liver microsomes and involvement of cytochrome P‐450 in dehydrogenation of lovastatin. Drug Metabolism and Disposition, 18, 218–222. [PubMed] [Google Scholar]
- Wang RQ, Kari PH, Lu AY, Thomas PE, Guengerich FP and Vyas KP, 1991. Biotransformation of lovastatin: IV. Identification of cytochrome P450 3A proteins as the major enzymes responsible for the oxidative metabolism of lovastatin in rat and human liver microsomes. Archives of Biochemistry and Biophysics, 290, 355–361. [DOI] [PubMed] [Google Scholar]
- Wang J‐J, Lee C‐L and Pan T‐M, 2004. Modified method for screening low‐citrinin‐producing strains of Monascus purpureus on rice culture. Journal of Agricultural and Food Chemistry, 52, 6977–6982. [DOI] [PubMed] [Google Scholar]
- Wei Y and Popovich DG, 2013. Red azaphilone pigments extracted from red yeast rice induces cellular senescence and reduces viability in HepG2 cells. Biomedicine Preventive Nutrition, 3, 331–337. [Google Scholar]
- Weintraub WS, Boccuzzi SJ, Klein JL, Kosinski AS, King SBIII, Ivanhoe R, Cedarholm JC, Stillabower ME, Talley JD, DeMaio SJ, O'Neill WW, Frazier JII, Cohen‐Bernstein CL, Robbins DC, Brown CL and Alexande RW, and the LRT Study Group , 1994. Lack of effect of lovastatin on restenosis after coronary angioplasty. The New England Journal of Medicine 331, 1331–1337. [DOI] [PubMed] [Google Scholar]
- Wigger‐Alberti W, Bauer A, Hipler U‐C and Eisner P, 1999. Anaphylaxis due to Monascus purpureus‐fermented rice (red yeast rice). Allergy, 54, 1330–1331. [DOI] [PubMed] [Google Scholar]
- Zhang ML, Peng C‐X and Zhou Y‐F, 2000. Methods and compositions employing red rice fermentation products. US Patent n 6,046,022, dated April 4th, 2000.


