Abstract
Following a request from the European Commission, the EFSA Panel on Contaminants in the Food Chain (CONTAM) provided a scientific opinion on the assessment of a decontamination process for fish meal. This process entails solvent (hexane) extraction of fish oil from fish meal to remove dioxins (polychlorinated dibenzo‐p‐dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs)) as well as dioxin‐like (DL‐) and non‐dioxin‐like (NDL‐) polychlorinated biphenyls (PCBs) followed by replacement with decontaminated fish oil. All feed decontamination processes must comply with the acceptability criteria specified in the Commission Regulation (EU) 2015/786. The data provided by the feed business operator were assessed with respect to the efficacy of the process, absence of solvent residues, and on information demonstrating that the process does not adversely affect the nature and characteristics of the product. According to data provided, the process was effective in removing PCDD/Fs and DL‐PCBs by approximately 70% and NDL‐PCBs by about 60%. The data showed that it is possible to meet the current EU requirements with respect to these contaminants, provided that the level of contamination of untreated fish meal is within the range of the tested batches. It is unlikely that hazardous substances (i.e. hexane) remain in the final product. The Panel considered that there is no evidence that fish oil extraction followed by replacement with decontaminated fish oil leads to detrimental changes in the nutritional composition of the fish meal, although some beneficial constituents (e.g. lipophilic vitamins) might be depleted. The feed business operator submitted information to demonstrate safe disposal of the waste material. The CONTAM Panel concluded that the proposed decontamination process to remove dioxins (PCDD/Fs) and PCBs from fish meal by means of solvent extraction and fish oil replacement was assessed to be compliant with the acceptability criteria provided for in Commission Regulation (EU) 2015/786 of 19 May 2015.
Keywords: decontamination process, dioxins, PCDD/Fs, dioxin‐like PCBs, non‐dioxin‐like PCBs, fish oil, fish meal
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed1 provides that the use of products intended for animal feed which contain levels of undesirable substances exceeding the maximum levels laid down in Annex I of that Directive is prohibited.
Directive 2002/32/EC provides also that Member States are to ensure that measures are taken to guarantee the correct application of any acceptable detoxification process on products intended for animal feed and the conformity of those detoxified products with the provisions of Annex I of that Directive. In order to ensure a uniform assessment across the European Union of the acceptability of detoxification processes, acceptability criteria for detoxification processes have been established at Union level by Commission Regulation (EU) 2015/786 of 19 May 20152 defining acceptability criteria for detoxification processes applied to products intended for animal feed as provided for in Directive 2002/32/EC of the European Parliament and of the Council.
The acceptability criteria for detoxification processes established by the Regulation shall ensure that the detoxified feed does not endanger animal and public health and the environment and that the characteristics of the feed are not adversely altered by the detoxification process. The Regulation furthermore provides that the compliance of a detoxification process with those criteria shall be scientifically assessed by the European Food Safety Authority (EFSA) on a request from the Commission.
The Commission has received the following application referring to a detoxification process for assessment by EFSA of compliance with the acceptability criteria:
| Feed to be decontaminated | Process | Contaminants of concern |
|---|---|---|
| Fish meal | Hexane extraction with exchange of dioxins contaminated fish oil from fish meal with a filtered fish oil | Dioxins and PCBs |
1.1.2. Terms of Reference
In accordance with Art. 29 (1) of Regulation (EC) No 178/20023, the European Commission asks the European Food Safety Authority for an assessment of this detoxification process for compliance with the acceptability criteria provided for in Commission Regulation (EU) 2015/786 of 19 May 2015.
1.2. Interpretation of the Terms of Reference
EFSA received from the European Commission requests for scientific opinions on the assessment of applications referring to feed detoxification processes to be compliant with acceptability criteria specified in the Commission Regulation (EU) 2015/786 of 19 May 2015. In this context, the term detoxification is interpreted as either decontamination by removing the contaminants or by chemical or biological processes able to reduce the toxicity of the contaminants present. This scientific opinion assesses the decontamination process of fish meal from dioxins (polychlorinated dibenzo‐p‐dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs)), as well as dioxin‐like (DL‐) and non‐dioxin‐like (NDL‐) polychlorinated biphenyls (PCBs) by extracting the contaminated fish oil and replacing with a decontaminated fish oil. Since PCDD/Fs and PCBs mostly occur in the lipid fraction, this process should have the potential to reduce their amount in the final fish meal. The EFSA Scientific Panel on Contaminants in the Food Chain (CONTAM Panel) concluded that the Terms of Reference provided by the European Commission were clear and that the opinion for the assessment of this physical decontamination process should mainly focus on data in order to:
enable the assessment of the efficacy of the process to remove the contaminants from the feed batches to ensure compliance with the requirements of Directive 2002/32/EC, and
demonstrate that the decontamination process does not adversely affect the characteristics and the nature of the feed.
Information concerning the safe disposal of the removed part of the feed was also considered.
Since hexane, an organic solvent, is used in a step of the process, the Panel was of the view that information about presence of its residues in the fish meal should be evaluated.
1.3. Additional information
The feed business operator has provided the European Commission with information referring to the proposed decontamination process and its effectiveness as laid down in Directive 2002/32/EC.
2. Data and methodologies
2.1. Data
The feed business operator has submitted information in support to its claim about the efficacy of the decontamination process consisting in the extraction of fish oil containing high levels of PCDD/Fs and DL‐ and NDL‐PCBs from the fish meal and adding to the defatted fish meal oil with lower level of contamination. This set of documents included information on the decontamination process and its steps, equipment and solvent used for the decontamination procedures, analytical data (certificates of analysis), characteristics of fish meal, on the Hazard Analysis Critical Control Point (HACCP) procedure, and on safe disposal of undesirable substances.
The CONTAM Panel based its assessment on the information provided (see Section ‘Documentation provided to EFSA’) and published literature to address the Terms of Reference.
2.2. Methodologies
The CONTAM Panel evaluated the acceptability of the proposed decontamination process as requested by the relevant regulations, specifically Directive 2002/32/EC and Commission Regulation (EU) 2015/786 with their Annexes. The assessment was conducted in line with the principles described in the EFSA guidance on transparency in the scientific aspects of risk assessment (EFSA, 2009) and following the relevant existing guidance from the EFSA Scientific Committee.
3. Assessment
3.1. Method of analysis
The feed business operator has submitted information on the analysis of PCDD/Fs, DL‐PCBs and NDL‐PCBs performed by an accredited laboratory (Eurofins, Ȍkometric GmbH in Bayreuth, Germany).
The analytical method followed provisions of Commission Regulation (EU) No 709/2014 of 20 June 2014 amending Regulation (EC) No 152/2009 regarding the determination of the levels of PCDD/Fs and polychlorinated biphenyls.4
3.2. Decontamination process
The feed business operator has submitted sufficient information to assess the effectiveness of the procedure, including data on PCDD/Fs, DL‐PCBs and NDL‐PCBs. A scheme and detailed description of the different steps of the decontamination process of the fish meal were also provided.
Solvent extraction has been described in the literature as being effective in extraction of edible oils (Baron et al., 2007; Oterhals, 2011). The applicant uses hexane as a solvent and reports that the residual content of oil in the fish meal following extraction is at most 2%. It is expected that the elimination of PCDD/Fs, DL‐PCBs and NDL‐PCBs should occur to a similar extent as these are all present primarily in the fish oil fraction.
3.2.1. Description of the process
A three‐step process is thoroughly described to ensure sufficient removal of PCDD/Fs from the fish meal (Figure 1). The process is based on the principle that dioxins (PCDD/Fs) and PCBs are lipophilic contaminants found in the lipid fraction; therefore, the defatted fish meal has a considerable lower level of organic (lipophilic) contaminants such as dioxins and PCBs in comparison to the starting material. By reconstituting the fish product adding clean oil the final fish meal is expected to have reduced levels of organic (lipophilic) contaminants whilst maintaining similar original nutritional characteristics.
Figure 1.
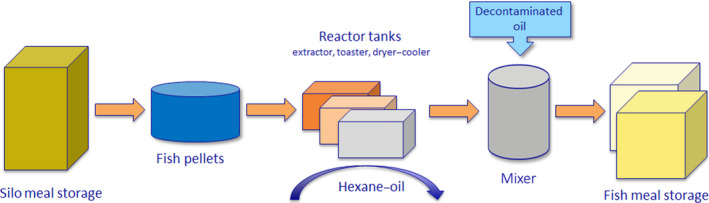
Schematic diagram of the process used by the feed business operator
The different steps of the process are described only in general terms as follows (Figure 1):
oil in fish meal is extracted by hexane (extraction grade; CAS‐no. 64742‐49‐0);
defatted fish meal is heated in the toaster to a temperature and for a time sufficient to remove hexane;
after cooling, a decontaminated fish oil is added to reconstitute the fish meal.
Representative samples of the final product are then collected on a regular basis to analyse for PCDD/Fs and DL‐ and NDL‐PCBs.
An authorisation from the Danish national authority (Miliø‐og Fødevareministeriet) dated June 2010 was provided for the establishment to carry out the decontamination process.
3.2.2. Efficacy of the process
The feed business operator has provided analytical records referring to three batches of fish meal that underwent the decontamination process and for which laboratory analysis were performed in 2017. Levels of PCDD/Fs, DL‐PCBs and NDL‐PCBs for each fish meal sample were measured. Individual data from these batches with the calculated reduction are reported in Table 1.
Table 1.
Levels of PCDD/Fs and PCBs in samples from three batches of fish meal before and after the decontamination process (final product)
| Contaminant | Batch A | Batch B | Batch C | Mean reduction (%) | Range of reduction (%) | |||
|---|---|---|---|---|---|---|---|---|
| Before process | After process | Before process | After process | Before process | After process | |||
| PCDD/Fs | 1.17 | 0.37 | 1.16 | 0.32 | 1.31 | 0.41 | 70 | 68–72 |
| DL‐PCBs | 1.16 | 0.47 | 1.15 | 0.26 | 1.23 | 0.46 | 66 | 60–77 |
| Sum of above | 2.33 | 0.84 | 2.31 | 0.58 | 2.54 | 0.87 | 68 | 64–75 |
| NDL‐PCBs | 9.12 | 4.33 | 9.13 | 2.37 | 9.87 | 4.39 | 61 | 52–74 |
PCDD/F: polychlorinated dibenzo‐p‐dioxins and polychlorinated dibenzofurans; DL‐PCB: dioxin‐like polychlorinated biphenyls; NDL‐PCB: non‐dioxin‐like polychlorinated biphenyls.
Individual values expressed in ng WHO2005‐TEQ/kg (PCDD/Fs and DL‐PCBs) or μg/kg (NDL‐PCBs).
Data from the above batches showed that the decontamination process decreased the sum of the concentrations of PCDD/Fs and DL‐PCBs (ng WHO2005‐TEQ/kg, expressed as ‘toxic equivalent’) and for PCDD/Fs alone by approximately 70% on average. The efficacy of the removal of NDL‐PCBs was approximately 60%.
Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed and its updates sets action thresholds and maximum limits. Thresholds of action are needed in order to keep the presence of specific undesirable substances in products intended for animal feed as low as possible in order to reduce their presence in the food chain. Where such action thresholds are exceeded, investigations must be carried out to identify the sources of the undesirable substances and steps taken to reduce or eliminate such sources. As shown in Table 2, the action threshold in fish and derived products for dioxins (sum of PCDDs and PCDFs) is 0.75 ng WHO2005‐TEQ/kg and the maximum permitted level is 1.25 ng WHO2005‐TEQ/kg. For DL‐PCBs, the action threshold in fish oil is 2.0 ng WHO2005‐TEQ/kg while the maximum level for the sum of PCDD/Fs and DL‐PCBs is 4.0 ng WHO2005‐TEQ/kg and for NDL‐PCBs 30 μg/kg (ppb).
Table 2.
Action thresholds and maximum levels for PCDD/Fs, DL‐PCBs and NDL‐PCBs in fish products (with exception of fish oil) according to Directive 2002/32/EC and its amendments
| Contaminant | Action thresholds | Maximum levels |
|---|---|---|
| PCDD/Fs | 0.75 | 1.25 |
| DL‐PCBs | 2.0 | – |
| Sum of above | – | 4.0 |
| NDL‐PCBs | – | 30.0 |
PCDD/F: polychlorinated dibenzo‐p‐dioxins and polychlorinated dibenzofurans; DL‐PCB: dioxin‐like polychlorinated biphenyls; NDL‐PCB: non‐dioxin‐like polychlorinated biphenyls; –: not set in regulation.
Values expressed in ng WHO2005‐TEQ/kg (PCDD/Fs and DL‐PCBs) or μg/kg (NDL‐PCBs); moisture content of 12%.
The Panel noted that the absence of hexane in the final product is not analytically verified. However, the hexane used for extraction has a boiling point of 65°C and it is removed from the fishmeal by heating at 80–85°C for an appropriate time (more than one hour) to ensure that residues of hexane are not left in the finished product.
3.3. Characteristics and nature of the fish meal
The characteristics and nature of the fish meal from decontaminated batches were not described. Therefore, a direct comparison of the same batches before and after the decontamination by means of evaluation of the fatty acids profile and other key characteristics was not possible. The feed operator included technical sheets with specifications from commercially available ‘prime quality fish meal’; however, the CONTAM Panel noted that the information reported in these sheets were limited to crude protein, crude fat, moisture and chloride contents, which were judged insufficient for a complete assessment of the possible impact of the decontamination process.
3.4. Disposal of the removed materials
The hexane used for oil extraction is recovered by distillation and reused.
The feed business operator submitted information to demonstrate safe disposal of the waste material. Specifically, the approval from Danish authorities (Miliø‐ og Fødevareministeriet) to carry out the decontamination process has been provided. The operator reported that solid waste is collected and handled by an authorised external company. The extracted oil is decontaminated by a separate authorised process.
3.5. Discussion
The CONTAM Panel assessed the information made available in the documents submitted by the feed business operator and was of the view that sufficient information was available to assess the proposed decontamination process for PCDD/Fs and DL‐ and NDL‐PCBs from the fish meal.
A detailed description of the process with its different steps was provided. The CONTAM Panel also considered that a clear description of the proposed process and its performances was available. Based on the data made available to EFSA, the process is able to remove PCDD/Fs and DL‐PCBs by approximately 70% and NDL‐PCBs by about 60%. The concentrations of PCDD/Fs and DL‐ and NDL‐PCBs remaining in the feed after the decontamination process, complied with the levels reported in the Annex I of Directive 2002/32/EC, falling below the legal limits including the action thresholds (Table 2). The feed business operator stated that regular monitoring of the finished product occurs by analysis of PCDD/Fs and DL‐ and NDL‐PCBs on representative samples (every 300–1,000 tonnes of produced fish meal).
The CONTAM Panel concluded that it is possible to meet the current European Union (EU) requirements for quality of fish meal with respect to these contaminants after removal of oil with PCDD/Fs and DL‐ and NDL‐PCBs and replacement with decontaminated fish oil. This assessment is based on the assumption that the levels of PCDD/Fs and DL‐ and NDL‐PCBs in untreated fish meal would be within the range of the batches listed in Table 1.
Although insufficient experimental evidence was available for the maintained characteristics of the treated fish meal, the CONTAM Panel was of the view that in principle the use of lipid extraction with organic solvent (hexane) followed by replacement with decontaminated fish oil should not lead to any detrimental changes in the quality of the fish meal as long as it was reconstituted with fish oil with similar fatty acid profile. This view is supported by information from literature (Baron et al., 2007). It is possible that the decontaminated oil used to reconstitute the fish meal could be depleted of some beneficial constituents (e.g. lipid soluble vitamins).
According to the information provided to EFSA about the decontamination process, there is no analytical confirmation that the solvent (hexane) used to extract fish oil has been removed from the final product. However, before reconstitution with decontaminated fish oil, the defatted fish meal is heated to a temperature well above the boiling point of hexane for at least an hour. This should ensure that residues of hexane are not present in the final product.
The CONTAM Panel noted that it is the responsibility of the Member State to ensure that measures are taken to guarantee the correct application of any acceptable decontamination process on products intended for animal feed and the conformity of those decontaminated products with the provisions included in the Commission Regulation (EU) 2015/786 and its Annexes. The feed business operator already holds an authorisation from the national competent authority for the plant to carry out the decontamination process according to the described method.
3.6. Uncertainty analysis
According to the interpretation of the Terms of Reference, the assessment of a physical decontamination process should mainly focus on the evaluation of the efficacy of the process to remove the contaminants and on the evidence that the characteristics and the nature of the product are not adversely affected.
Efficacy of the process: the method used relies on the ability of an organic solvent (hexane) to extract the oil fraction from the fish meal. Based on the limited evidence provided by the business operator, it appears that the process will be effective in removing lipophilic contaminants such as PCDD/Fs and PCBs from the product. The efficacy is checked by regular monitoring of the levels of PCDD/Fs and PCBs in the final product. The level of uncertainty with regards to the efficacy of the process to reduce levels of PCDD/Fs and DL‐ and NDL‐PCBs is therefore low.
Characteristics of the product: the nutritional characteristics of the treated fish meal are based on literature information and there is therefore some uncertainty as to whether this can be extended to the process under assessment. There is little chance that hazardous substances (i.e. hexane) remain in the final product, but some beneficial constituents (e.g. lipophilic vitamins) might be removed. Although no analytical evidence has been provided to demonstrate the absence of adverse effects of the process on the nutritional quality of the fish meal, circumstantial evidence (i.e. consumer satisfaction) indicates that the product is suitable for use in animal feed.
4. Conclusions
In relation to the Terms of Reference, the CONTAM Panel concluded:
on the basis of the information submitted by the feed business operator the proposed decontamination process is effective in reducing dioxins (PCDD/Fs) and DL‐ and NDL‐PCBs in fish meal;
there is no evidence that fish oil extraction followed by replacement with decontaminated fish oil leads to detrimental changes in the nutritional composition of the fish meal;
the proposed decontamination process to remove dioxins (PCDD/Fs) and DL‐ and NDL‐PCBs from fish meal, was assessed to be compliant with the acceptability criteria provided for in Commission Regulation (EU) 2015/786 of 19 May 2015.
Documentation provided to EFSA
Information provided by Polar Omega A/S (TripleNine group A/S) ‐ Denmark to support the effectiveness of a process to “exchange dioxin contaminated fish oil from fish meal to a dioxin filtered oil”; June 2016.
Additional information submitted by Polar Omega A/S (TripleNine group A/S) ‐ Denmark in response to a request from the EFSA CONTAM Panel; July 2017.
Abbreviations
- CAS
Chemical Abstracts Service
- CONTAM Panel
EFSA Panel on Contaminants in the Food Chain
- DL‐PCBs
dioxin‐like polychlorinated biphenyls
- HACCP
Hazard Analysis Critical Control Point
- NDL‐PCBs
non‐dioxin‐like polychlorinated biphenyls
- PCBs
polychlorinated biphenyls
- PCDDs
polychlorinated dibenzo‐p‐dioxins
- PCDD/Fs
polychlorinated dibenzo‐p‐dioxins and polychlorinated dibenzofurans
- PCDFs
polychlorinated dibenzofurans
- TEQ
toxic equivalents
- WHO
World Health Organization
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl‐Kraupp B, Hoogenboom LR, Nebbia CS, Oswald IP, Petersen A, Rose M, Roudot A‐C, Schwerdtle T, Vleminckx C, Vollmer G, Wallace H, Lundebye A‐K, Metzler M, Colombo P and Hogstrand C, 2018. Scientific Opinion on the assessment of a decontamination process for dioxins and PCBs from fish meal by hexane extraction and replacement of fish oil. EFSA Journal 2018;16(2):5173, 9 pp. 10.2903/j.efsa.2018.5173
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00796
Panel members: Jan Alexander, Lars Barregård, Margherita Bignami, Beat Brueschweiler, Sandra Ceccatelli, Bruce Cottrill, Michael Dinovi, Lutz Edler, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Helle Katrine Knutsen, Carlo Stefano Nebbia, Isabelle P Oswald, Annette Petersen, Martin Rose, Alain‐Claude Roudot, Tanja Schwerdtle, Christiane Vleminckx, Günter Vollmer and Heather Wallace.
Adopted: 23 January 2018
Notes
Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. OJ L 140, 30.5.2002, p. 10–22.
Commission Regulation (EU) 2015/786 of 19 May 2015 defining acceptability criteria for detoxification process applied to products intended for animal feed as provided for in Directive 2002/32/EC of the European Parliament and of the Council. OJ L 125, 21.5.2015, p. 10–14.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Commission Regulation (EU) No 709/2014 of 20 June 2014 amending Regulation (EC) No 152/2009 as regards the determination of the levels of dioxins and polychlorinated biphenyls. OJ L 188, 27.6.2014, p. 1–18.
References
- Baron CP, Børresen T and Jacobsen C, 2007. Comparison of methods to reduce dioxin and polychlorinated biphenyls contents in fishmeal: extraction and enzymatic treatments. Journal of Agricultural and Food Chemistry, 55, 1620–1626. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessment carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(5):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- Oterhals Å, 2011. Decontamination of persistent organic pollutants in fishmeal and fish oil. PhD Thesis. University of Bergen, Norway. Dissertation date: 9 March 2011, 94 pp. Available online: http://bora.uib.no/handle/1956/5129


