Abstract
The Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids of the EFSA was requested to consider evaluations of flavouring substances assessed since 2000 by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) and to decide whether further evaluation is necessary, as laid down in Commission Regulation (EC) No 1565/2000. The present consideration concerns a group of 22 pyridine, pyrrole and quinoline derivatives evaluated by JECFA (63rd meeting). The revision of this consideration is made since additional genotoxicity data have become available for 6‐methylquinoline [FL‐no: 14.042]. The genotoxicity data available rule out the concern with respect to genotoxicity and accordingly the substance is evaluated through the Procedure. For all 22 substances [FL‐no: 13.134, 14.001, 14.004, 14.007, 14.030, 14.038, 14.039, 14.041, 14.042, 14.045, 14.046, 14.047, 14.058, 14.059, 14.060, 14.061, 14.065, 14.066, 14.068, 14.071, 14.072 and 14.164] considered in this Flavouring Group Evaluation (FGE), the Panel agrees with the JECFA conclusion, ‘No safety concern at estimated levels of intake as flavouring substances’ based on the Maximised Survey‐derived Daily Intake (MSDI) approach. Besides the safety assessment of these flavouring substances, the specifications for the materials of commerce have also been evaluated, and the information is considered adequate for all the substances. For the following substances [FL‐no: 13.134, 14.001, 14.030, 14.041, 14.042, 14.058, 14.072], the Industry has submitted use levels for normal and maximum use. For the remaining 15 substances, use levels are needed to calculate the modified Theoretical Added Maximum Daily Intakes (mTAMDIs) in order to identify those flavouring substances that need more refined exposure assessment and to finalise the evaluation.
Keywords: pyridine, FGE.77, pyrrole, quinoline, JECFA, 63rd meeting, FGE.24Rev2
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The use of flavourings is regulated under Regulation (EC) No 1334/2008 of the European Parliament and Council of 16 December 20081 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation, an evaluation and approval are required for flavouring substances.
The Union list of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/20122. The list contains flavouring substances for which the scientific evaluation should be completed in accordance with Commission Regulation (EC) No 1565/20003.
The substance 6‐methylquinoline [FL‐no: 14.042] is one of these substances. New genotoxicity data on it was evaluated in the EFSA opinion FGE.77Rev1 (January 2014) but EFSA concluded that this new data did not clear the concern with respect to genotoxicity in vitro and indicated that studies in vivo would be necessary to address the potential for genotoxicity. EFSA evaluated FGE.77 again in January 2015 (FGE.77Rev2) due to new studies on other substances of this group, although at that time no new data on FL‐no: 14.042 was available.
On 27 January 2016 the Industry submitted a new dossier with the requested studies by EFSA.
1.1.2. Terms of Reference
The European Commission requests the European Food Safety Authority (EFSA) to evaluate this new information and, depending on the outcome, proceed to the full evaluation of this flavouring substance in accordance with Commission Regulation (EC) No 1565/2000.
2. Assessment
The approach used by EFSA for safety evaluation of flavouring substances is referred to in Commission Regulation (EC) No 1565/2000, hereafter named the ‘EFSA Procedure’. This Procedure is based on the opinion of the Scientific Committee on Food (SCF) (SCF, 1999), which has been derived from the evaluation procedure developed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA, 1995, 1996, 1997, 1999), hereafter named the ‘JECFA Procedure’. The Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (the Panel) compares the JECFA evaluation of structurally related substances with the result of a corresponding EFSA evaluation, focussing on specifications, intake estimations and toxicity data, especially genotoxicity data. The evaluations by EFSA will conclude whether the flavouring substances are of no safety concern at their estimated levels of intake, whether additional data are required or whether certain substances should not be evaluated through the EFSA Procedure.
The following issues are of special importance.
Intake
In its evaluation, the Panel as a default uses the Maximised Survey‐derived Daily Intake (MSDI) approach to estimate the per capita intakes of the flavouring substances in Europe.
In its evaluation, JECFA includes intake estimates based on the MSDI approach derived from both European and USA production figures. The higher of the two MSDI figures is used in the evaluation by JECFA. It is noted that in several cases, only the MSDI figures from the USA were available, meaning that certain flavouring substances have been evaluated by JECFA only on the basis of these figures. For Register substances for which this is the case the Panel will need European Union (EU) production figures in order to finalise the evaluation.
When the Panel examined the information provided by the European Flavour Industry on the use levels in various foods, it appeared obvious that the MSDI approach in a number of cases would grossly underestimate the intake by regular consumers of products flavoured at the use level reported by the Industry, especially in those cases where the annual production values were reported to be small. In consequence, the Panel had reservations about the data on use and use levels provided and the intake estimates obtained by the MSDI approach. It is noted that JECFA, at its 65th meeting considered ‘how to improve the identification and assessment of flavouring agents, for which the MSDI estimates may be substantially lower than the dietary exposures that would be estimated from the anticipated average use levels in foods’ (JECFA, 2006b).
In the absence of more accurate information that would enable the Panel to make a more realistic estimate of the intakes of the flavouring substances, the Panel has decided also to perform an estimate of the daily intakes per person using a modified Theoretical Added Maximum Daily Intake (mTAMDI) approach based on the normal use levels reported by Industry.
As information on use levels for the flavouring substances has not been requested by JECFA or has not otherwise been provided to the Panel, it is not possible to estimate the daily intakes using the mTAMDI approach for the substances evaluated by JECFA. The Panel will need information on use levels in order to finalise the evaluation.
Threshold of 1.5 μg/person per day (Step B5) used by JECFA
JECFA uses the threshold of concern of 1.5 μg/person per day as part of the evaluation procedure:
‘The Committee noted that this value was based on a risk analysis of known carcinogens which involved several conservative assumptions. The use of this value was supported by additional information on developmental toxicity, neurotoxicity and immunotoxicity. In the judgement of the Committee, flavouring substances for which insufficient data are available for them to be evaluated using earlier steps in the Procedure, but for which the intake would not exceed 1.5 μg per person per day would not be expected to present a safety concern. The Committee recommended that the Procedure for the Safety Evaluation of Flavouring Agents used at the forty‐sixth meeting be amended to include the last step on the right‐hand side of the original procedure (“Do the condition of use result in an intake greater than 1.5 μg per day?”)’ (JECFA, 1999).
In line with the Opinion expressed by the SCF (SCF, 1999), the Panel does not make use of this threshold of 1.5 μg/person per day.
Genotoxicity
As reflected in the Opinion of SCF (SCF, 1999), the Panel has in its evaluation focussed on a possible genotoxic potential of the flavouring substances or of structurally related substances. Generally, substances for which the Panel has concluded that there is an indication of genotoxic potential in vitro, will not be evaluated using the EFSA Procedure until further genotoxicity data are provided. Substances for which a genotoxic potential in vivo has been concluded, will not be evaluated through the Procedure.
Specifications
Regarding specifications, the evaluation by the Panel could lead to a different opinion than that of JECFA.
Structural relationship
In the consideration of the JECFA evaluated substances, the Panel will examine the structural relationship and metabolism features of the substances within the flavouring group and compare this with the corresponding Flavouring Group Evaluation (FGE).
2.1. History of the evaluation of the substances in the present FGE
JECFA has evaluated a group of 22 flavouring substances consisting of pyridine, pyrrole and quinoline derivatives (JECFA, 2006a).
These 22 substances were considered by EFSA in FGE.77, in which the Panel concluded that additional toxicity data were needed for seven substances [FL‐no: 13.134, 14.001, 14.041, 14.045, 14.046, 14.047 and 14.068] as no adequate toxicity studies were available from which a no observed adverse effect level (NOAEL) could be established, neither on the substances nor on supporting substances. The Panel also concluded, contrary to JECFA, that 6‐methylquinoline [FL‐no: 14.042] should not be evaluated through the Procedure due to concern with respect to genotoxicity in vitro.
In the first Revision of FGE.77, FGE.77Rev1, additional toxicity data were provided for isoquinoline [FL‐no: 14.001], pyrrole [FL‐no: 14.041] and 2‐acetylpyrrole [FL‐no: 14.047]; the toxicity data on 2‐acetylpyrrole also cover 2‐propionylpyrrole [FL‐no: 14.068]. The main studies provided were for each substance a 90‐day study. Furthermore, additional genotoxicity data for 6‐methylquinoline [FL‐no: 14.042] became available. EU production volumes were provided for four substances, [FL‐no: 14.045, 14.058, 14.059 and 14.164] for which the evaluation could not be finalised previously, due to lack of these data. Based on these newly submitted EU production volumes (IOFI, 2013), the substances were already evaluated in FGE.964 (EFSA CEF Panel, 2011), but for the sake of completion, the information was included here as well. Finally, information on solubility was provided for six substances [FL‐no: 13.134, 14.007, 14.030, 14.038, 14.045 and 14.046] since the previous evaluation of FGE.77.
The second revision of FGE.77, FGE.77Rev2, included additional toxicity data, among which an oral 90‐day study provided for 1‐furfurylpyrrole [FL‐no: 13.134]. The data were intended to cover the re‐evaluation of this substance as well as 2‐acetyl‐1‐ethylpyrrole [FL‐no: 14.045] and 2‐acetyl‐1‐methylpyrrole [FL‐no: 14.046]. A search in open literature was conducted for metabolism, genotoxicity and toxicity for 1‐furfurylpyrrole. This search did not reveal any pertinent new information on the substance.
| FGE | Opinion adopted by EFSA | Link | No. of candidate substances |
|---|---|---|---|
| FGE.77 | 31 January 2008 | http://www.efsa.europa.eu/en/efsajournal/pub/936.htm | 22 |
| FGE.77Rev1 | 19 February 2014 | http://www.efsa.europa.eu/en/efsajournal/pub/3586.htm | 22 |
| FGE.77Rev2 | 19 December 2014 | http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/3997.pdf | 22 |
| FGE.77Rev3 | 7 March 2018 | https://www.efsa.europa.eu/it/efsajournal/pub/5226 | 22 |
The present revision of FGE.77, FGE.77Rev3, includes additional genotoxicity and toxicity data for 6‐methylquinoline [FL‐no: 14.042].
3. Presentation of the substances in the JECFA flavouring group
3.1. Description
3.1.1. JECFA status
At the 63rd meeting, JECFA has evaluated a group of 22 flavouring substances consisting of pyridine, pyrrole and quinoline derivatives (JECFA, 2005b, 2006a).
3.1.2. EFSA considerations
The Panel concluded that all the substances in the JECFA flavouring group of pyridine, pyrrole and quinoline derivatives are structurally related to the group of pyridine, pyrrole, indole and quinoline derivatives from chemical group 28 evaluated by EFSA in the Flavouring Group Evaluation 24, Revision 2 (FGE.24Rev2) (EFSA CEF Panel, 2013).
3.2. Isomers
None of the 22 flavouring substances in the group of pyridine, pyrrole and quinoline derivatives has possibility for stereoisomerism.
3.3. Specifications
3.3.1. Status
The JECFA specifications are available for all 22 substances (JECFA, 2005a) (see Table 1).
Table 1.
Summary of specification data (JECFA, 2005a)
| FL‐no JECFA‐no | EU Register name | Structural formula | FEMA no CoE no CAS no | Phys.form Molecular formula Molecular weight | Solubilitya Solubility in ethanolb | Boiling point, °Cc Melting point, °C ID test Assay minimum | Refractive Indexd Specific gravitye |
|---|---|---|---|---|---|---|---|
|
13.134 1310 |
1‐Furfurylpyrrole |

|
3284 2317 1438‐94‐4 |
Liquid C9H9ON 147.18 |
Insoluble Soluble |
76–78 (1 hPa) – NMR 98% |
1.529–1.536 1.078–1.084 |
|
14.001 1303 |
Isoquinoline |

|
2978 487 119‐65‐3 |
Solid C9H7N 129.16 |
Slightly soluble Soluble |
242–243 27–29 NMR 97% |
1.621–1.627 1.097–1.103 |
|
14.004 1304 |
3‐Methylindole |

|
3019 493 83‐34‐1 |
Solid C9H9N 131.18 |
Soluble Soluble |
– 95–97 NMR 97% |
n.a. n.a. |
|
14.007 1301 |
Indole |

|
2593 560 120‐72‐9 |
Solid C8H7N 117.15 |
Insoluble Soluble |
n.a. 51–54 NMR 97% |
n.a. n.a. |
|
14.030 1308 |
2‐Pyridine methanethiol |

|
3232 2279 2044‐73‐7 |
Liquid C6H7NS 125.20 |
Soluble Soluble |
57–58 (0.8 hPa) – NMR 98% |
1.573–1.580 1.150–1.157 |
|
14.038 1309 |
2‐Acetylpyridine |

|
3251 2315 1122‐62‐9 |
Liquid C7H7ON 121.14 |
Insoluble Soluble |
189–193 – IR NMR 97% |
1.518–1.524 1.077–1.084 |
|
14.039 1316 |
3‐Acetylpyridine |

|
3424 2316 350‐03‐8 |
Liquid C7H7ON 121.14 |
Soluble Soluble |
230 – NMR 97% |
1.530–1.540 1.103–1.112 |
|
14.041 1314 |
Pyrrole |

|
3386 2318 109‐97‐7 |
Liquid C4H5N 67.09 |
Slightly soluble Soluble |
130–131 – IR 98% |
1.507–1.510 0.955–0.975 |
|
14.042 1302 |
6‐Methylquinoline |

|
2744 2339 91‐62‐3 |
Liquid C10H9N 143.19 |
Slightly soluble Soluble |
259 – NMR 98% |
1.611–1.617 1.060–1.066 |
|
14.045 1305 |
2‐Acetyl‐1‐ethylpyrrole |

|
3147 11371 39741‐41‐8 |
Liquid C8H11ON 137.18 |
Slightly soluble Soluble |
209–211 – NMR 98% |
1.550–1.556 1.052–1.058 |
|
14.046 1306 |
2‐Acetyl‐1‐methylpyrrole |

|
3184 11373 932‐16‐1 |
Liquid C7H9ON 123.16 |
Slightly soluble Soluble |
200–202 – NMR 98% |
1.539–1.545 1.037–1.043 |
|
14.047 1307 |
2‐Acetylpyrrole |

|
3202 11721 1072‐83‐9 |
Solid C6H7ON 109.13 |
Soluble Soluble |
n.a. 87–93 NMR 97% |
n.a. n.a. |
|
14.058 1311 |
2‐Isobutylpyridine |

|
3370 11395 6304‐24‐1 |
Liquid C9H13N 135.21 |
Insoluble Soluble |
181 – NMR 97% |
1.480–1.486 0.894–0.900 |
|
14.059 1312 |
3‐Isobutylpyridine |

|
3371 11396 14159‐61‐6 |
Liquid C9H13N 135.21 |
Insoluble Soluble |
68–68.5 (10hPa) – NMR 97% |
1.488–1.494 0.898–0.904 |
|
14.060 1313 |
2‐Pentylpyridine |

|
3383 11412 2294‐76‐0 |
Liquid C10H15N 149.24 |
Insoluble Soluble |
102–107 – NMR 97% |
1.485–1.491 0.895–0.901 |
|
14.061 1315 |
3‐Ethylpyridine |

|
3394 11386 536‐78‐7 |
Liquid C7H9N 107.16 |
Slightly soluble Soluble |
166 – NMR 98% |
1.499–1.505 0.951–0.957 |
|
14.065 1317 |
2,6‐Dimethylpyridine |

|
3540 11381 108‐48‐5 |
Liquid C7H9N 107.16 |
Soluble Soluble |
143–145 – MS 99% |
1.495–1.501 0.917–0.923 |
|
14.066 1318 |
5‐Ethyl‐2‐methylpyridine |

|
3546 11385 104‐90‐5 |
Liquid C8H11N 121.18 |
Slightly soluble Soluble |
172–175 – NMR 97% |
1.495–1.502 0.917–0.923 |
|
14.068 1319 |
2‐Propionylpyrrole |

|
3614 11942 1073‐26‐3 |
Solid C7H9ON 123.16 |
Slightly soluble Soluble |
n.a. 43–45 IR NMR 99% |
n.a. n.a. |
|
14.071 1320 |
Methyl nicotinate |

|
3709 93‐60‐7 |
Solid C7H7O2N 137.14 |
Slightly soluble Soluble |
n.a. 38–43 IR NMR MS 98% |
n.a. n.a. |
|
14.072 1321 |
2‐(3‐Phenylpropyl)pyridine |

|
3751 2110‐18‐1 |
Liquid C14H15N 197.28 |
Insoluble Soluble |
142–143 (1 hPa) – IR NMR 97% |
1.558–1.563 1.012–1.018 |
|
14.164 1322 |
2‐Propylpyridine |

|
622‐39‐9 |
Liquid C8H11N 121.20 |
Slightly soluble Soluble |
169–171 – NMR 98% |
1.490–1.496 0.907–0.917 |
n.a.: not applicable.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1013.25 hPa, if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
3.3.2. EFSA considerations
The specifications are considered adequate for all 22 substances.
4. Assessment
4.1. Intake estimation
4.1.1. Status
For all 22 substances, evaluated through the JECFA Procedure, production volumes, based on which MDSI values can be calculated, are available for the EU (see Table D.2).
Table D.2.
Estimated intakes based on the MSDI and the mTAMDIa approach – FGE.77Rev3
| FL‐no | EU Register name | MSDI – EU (μg/capita per day) | MSDI – USA (μg/capita per day) | mTAMDI (μg/person per day) | Structural class | Threshold of concern (μg/person per day) |
|---|---|---|---|---|---|---|
| 14.004 | 3‐Methylindole | 2.4 | 0.07 | Class I | 1,800 | |
| 14.007 | Indole | 26 | 10 | Class I | 1,800 | |
| 14.041 | Pyrrole | 0.11 | 0.01 | 480 | Class I | 1,800 |
| 14.038 | 2‐Acetylpyridine | 50 | 68 | Class II | 540 | |
| 14.039 | 3‐Acetylpyridine | 23 | 0.8 | Class II | 540 | |
| 14.045 | 2‐Acetyl‐1‐ethylpyrrole | 0.12 | 0.009 | Class II | 540 | |
| 14.046 | 2‐Acetyl‐1‐methylpyrrole | 1.2 | 0.02 | Class II | 540 | |
| 14.047 | 2‐Acetylpyrrole | 3.3 | 0.2 | Class II | 540 | |
| 14.059 | 3‐Isobutylpyridine | 0.049 | 0.07 | Class II | 540 | |
| 14.060 | 2‐Pentylpyridine | 0.061 | 0.07 | Class II | 540 | |
| 14.061 | 3‐Ethylpyridine | 9.3 | 3 | Class II | 540 | |
| 14.065 | 2,6‐Dimethylpyridine | 0.26 | 0.007 | Class II | 540 | |
| 14.066 | 5‐Ethyl‐2‐methylpyridine | 0.12 | 0.04 | Class II | 540 | |
| 14.068 | 2‐Propionylpyrrole | 0.012 | 2 | Class II | 540 | |
| 14.071 | Methyl nicotinate | 0.49 | 0.2 | Class II | 540 | |
| 14.164 | 2‐Propylpyridine | 0.61 | 0.9 | Class II | 540 | |
| 14.001 | Isoquinoline | 0.012 | 0.07 | 64 | Class III | 90 |
| 14.042 | 6‐Methylquinoline | 0.32 | 0.01 | 830 | Class III | 90 |
| 14.058 | 2‐Isobutylpyridine | 0.0061 | 0.9 | 64 | Class III | 90 |
| 14.072 | 2‐(3‐Phenylpropyl)pyridine | 1.8 | 0.7 | 65 | Class III | 90 |
| 13.134 | 1‐Furfurylpyrrole | 0.12 | 0.07 | 3000 | Class III | 90 |
| 14.030 | 2‐Pyridine methanethiol | 0.0012 | 0.007 | 520 | Class III | 90 |
For the calculation of mTAMDI – see e.g. Annex II in FGE.03 (EFSA, 2004).
4.1.2. EFSA considerations
For seven substances [FL‐no: 13.134, 14.001, 14.030, 14.041, 14.042, 14.058, 14.072], the Industry has submitted food categories5 and use levels in these for normal and maximum use (EFFA, 2012; DG SANCO, 2014) (see Table D.1, Appendix D). Based on the normal use levels, the mTAMDI value can be calculated for each of these seven substances (see Table D.2, Appendix D). The mTAMDI values for [FL‐no: 14.041] is below the threshold of concern of 1,800 μg/person per day for a structural class I substance. The mTAMDIs for [FL‐no: 14.001, 14.058 and 14.072] are below the threshold of concern of 90 μg/person per day for the structural class III substances. The mTAMDI values for [FL‐no: 13.134, 14.030, 14.042] are above the threshold of concern of 90 μg/person per day for the structural class III substances. For the remaining 15 substances, use levels are needed to calculate the mTAMDIs.
Table D.1.
Normal and Maximum use levels available for substances in FGE.77Rev3
| FL‐no | Food Categories | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal use levels (mg/kg) Maximum use levels (mg/kg) | ||||||||||||||||||
| 01.0 | 02.0 | 03.0 | 04.1 | 04.2 | 05.0 | 06.0 | 07.0 | 08.0 | 09.0 | 10.0 | 11.0 | 12.0 | 13.0 | 14.1 | 14.2 | 15.0 | 16.0 | |
| 13.134 |
0.011 0.027 |
0.13 0.27 |
18 880 |
29 200 |
0.037 – |
0.00073 0.073 |
||||||||||||
| 14.001 |
– 1 |
0.025 – |
0.25 – |
0.09 – |
||||||||||||||
| 14.030 |
1 – |
1 0 |
1 – |
1 – |
||||||||||||||
| 14.041 |
3 3 |
3 3 |
3 3 |
3 3 |
3 3 |
|||||||||||||
| 14.042 |
0.24 19 |
2.9 |
– 9.7 |
2.9 29 |
5 – |
1.5 13 |
1.2 – |
0.00058 – |
||||||||||
| 14.058 |
– 1 |
0.025 – |
0.25 |
0.09 – |
||||||||||||||
| 14.072 |
0.2 – |
|||||||||||||||||
4.2. Biological and toxicological data
4.2.1. Genotoxicity
4.2.1.1. Genotoxicity studies – text taken6 from JECFA (JECFA, 2006a)
In vitro
There was no evidence of mutagenicity in the assay for reverse mutation in bacteria when various strains of Salmonella Typhimurium (TA97, TA98, TA100, TA102, TA104, TA1535, TA1537, TA1538 and TM677) were incubated with indole [FL‐no: 14.007] at a concentration of up to 30 μmol/plate (3,515 μg/plate) (Anderson and Styles, 1978; Kaden et al., 1979; Florin et al., 1980; Ochiai et al., 1986; Vance et al., 1986; Sasagawa and Matsushima, 1991; Fujita et al., 1994), isoquinoline [FL‐no: 14.001] at a concentration of up to 20,000 μg/mL (Sugimura et al., 1976; Nagao et al., 1977; Epler et al., 1979; Kaden et al., 1979; Sideropoulos and Specht, 1984), skatole [FL‐no: 14.004] (4‐methylindole) at a concentration of up to 3 μmol/plate (394 μg/plate) (Florin et al., 1980; Ochiai et al., 1986; Kim et al., 1989; Sasagawa and Matsushima, 1991), pyrrole [FL‐no: 14.041] at a concentration of up to 1.4 mmol/plate (93,926 μg/plate) (Florin et al., 1980; Aeschbacher et al., 1989; Lee et al., 1994) and 3‐ethylpyridine [FL‐no: 14.061] at a concentration of up to 3 μmol/plate (321 μg/plate) (Florin et al., 1980) with and without metabolic activation. Methyl 2‐pyrrolyl ketone [FL‐no: 14.047] (2‐acetylpyrrole) at concentrations of 4 to 100 μmol/plate induced a > 2‐fold increase in the number of revertants/plate compared with the control when tested in S. Typhimurium TA98 in the absence of metabolic activation (Lee et al., 1994). However, negative results were obtained with metabolic activation as well as in S. Typhimurium TA100 (both with and without metabolic activation). Furthermore, no mutagenic activity was reported in either strain when incubated with methyl 2‐pyrrolyl ketone at a concentration of up to 200 μg/plate with and without metabolic activation (Wang et al., 1994). 6‐Methylquinoline [FL‐no: 14.042] at a concentration of 3.3 to 3,600 μg/plate gave uniformly positive results in the presence of metabolic activation (Sugimura et al., 1976; Nagao et al., 1977; Dong et al., 1978; Wild et al., 1983; Takahashi et al., 1988; Debnath et al., 1992; Zeiger et al., 1992). Methylquinolines, tested at a concentration of 400 μg/plate, showed a potent bactericidal or bacteriostatic effect, with only 6% survival of S. Typhimurium TA100 treated with 6‐methylquinoline (Dong et al., 1978).
There was no evidence of mutagenicity when Escherichia coli (strains WP2 uvr4A/pKM101, SD‐4‐73, or B/r HCR+) were incubated with indole [FL‐no: 14.007] at a concentration of up to 0.4 μmol/plate (47 μg/plate) (Sasagawa and Matsushima, 1991), isoquinoline [FL‐no: 14.001] at a concentration of up to 50 μg/mL, skatole [FL‐no: 14.004] (3‐methylindole) at a concentration of up to 0.4 μmol/plate (52 μg/plate) (Szybalski, 1958; Sasagawa and Matsushima, 1991) or 3‐acetylpyridine [FL‐no: 14.039] at a concentration of up to 10,000 mg/plate (Pai et al., 1978).
In non‐standardised assays, 2‐acetylpyridine [FL‐no: 14.038] at 0.50 to 0.87% (54,000 to 93,960 μg/mL) and 3‐acetylpyridine [FL‐no: 14.039] at 0.5 to 1.11% (55,100 to 122,322 μg/mL) caused a dose‐dependent increase in mitotic aneuploidy in strain D61.M of Saccharomyces ceverisiae (Zimmermann et al., 1986). At the higher test concentrations, the growth of D61.M was strongly or completely inhibited. The authors noted that it is generally recognised that there is a threshold dose for induction of aneuploidy in yeast (Zimmermann et al., 1985a,b,c).
Assays in mammalian cell lines have been performed for isoquinoline [FL‐no: 14.001] (Williams, 1984), skatole [FL‐no: 14.004] (3‐methylindole) (Kim et al., 1989) and pyrrole [FL‐no: 14.041] (Williams, 1984). There was no evidence of increased unscheduled DNA synthesis when freshly isolated rat liver cells were incubated with pyrrole or isoquinoline (concentrations not specified) (Williams, 1984). Single‐strand DNA breaks and inhibition of growth were reported when undeuterated or deuterated (at C2 or C3 positions) 3‐methylindole (skatole) at 10 μmol/L to 1 mmol/L (1.31 to 131.18 μg/mL) was incubated with isolated cultured bovine kidney cells. However, there was no evidence of DNA interstrand crosslinks (Kim et al., 1989). These observations are consistent with reports that, at high concentrations, indoles deplete glutathione, leading to increased formation of DNA adducts (Nichols et al., 2000; Regal et al., 2001).
In vivo
There was no evidence for mutation in a standard assay for sex‐linked recessive lethal mutation when adult Drosophila melanogaster were fed 6‐methylquinoline [FL‐no: 14.042] at a concentration of 10 mmol/L (1,432 μg/mL) in a 5% sucrose solution for 3 days (Wild et al., 1983). Furthermore, 6‐methylquinoline did not induce micronucleus formation in bone marrow cells obtained from male and female NMRI mice 30 h after treatment with the test compound as a single intraperitoneal dose at 0, 286, 429 or 572 mg/kg bw (Wild et al., 1983).
JECFA's‐conclusion on genotoxicity
Overall, negative results were reported in assays for reverse mutation in bacteria for six representative pyridine, pyrrole and quinoline derivatives (i.e. indole [FL‐no: 14.007], isoquinoline [FL‐no: 14.001], skatole [FL‐no: 14.004] (3‐methylindole), methyl 2‐pyrrolyl ketone [FL‐no: 14.047] (2‐acetylpyrrole), pyrrole [FL‐no: 14.041] and 3‐ethylpyridine [FL‐no: 14.061]). Although 6‐methylquinoline gave positive results with metabolic activation, it gave negative results in studies in vivo, indicating that there are adequate detoxication mechanisms for the rapid absorption, distribution, biotransformation and elimination of the N‐containing heteroaromatic derivatives. 2‐Acetylpyridine and 3‐acetylpyridine produced positive results in yeast, but this is unlikely to occur at low doses because yeast is generally believed to have a threshold for the induction of aneuploidy. The positive results reported in bacteria for skatole (3‐methylindole) are consistent with observations that, at high concentrations, indoles deplete glutathione, leading to reduced detoxification.
On the basis of the available evidence, the 22 pyridine, pyrrole and quinoline derivatives in this group do not demonstrate genotoxic potential.
For a summary of in vitro/in vivo genotoxicity data considered by JECFA, see Table A.1.
Table A.1.
Genotoxicity data (in vitro/in vivo) JECFA (JECFA, 2006a)
| FL‐no JECFA‐no | EU Register name JECFA name | Structural formula | End‐point | Test system | Concentration | Results | Reference |
|---|---|---|---|---|---|---|---|
| In vitro | |||||||
|
14.007 1301 |
Indole |

|
Reverse mutation | S. Typhimurium TA100 | ≤ 20 μg/plate | Negativea | Ochiai et al. (1986) |
| Reverse mutation | S. Typhimurium TM677 | 4 mmol/L (469 μg/mL)b | Negativec | Kaden et al. (1979) | |||
| Reverse mutation | S. Typhimurium TA98, TA100, TA1535, TA1538 | 4–2,500 μg/plate | Negatived | Anderson and Styles (1978) | |||
| Reverse mutation | S. Typhimurium TA98, TA100 |
≤ 500 nmol/plate (59 μg/plate)b |
Negativea | Vance et al. (1986) | |||
| Reverse mutation | S. Typhimurium TA100, TA1535, TA1537 |
3 μmol/plate (351 μg/plate)b |
Negatived | Florin et al. (1980) | |||
| Reverse mutation | S. Typhimurium TA98 | 0.03–30 μmol/plate (3.5–3515 μg/plate)b,e | Negatived | Florin et al. (1980) | |||
| Reverse mutation | S. Typhimurium TA97, TA102 | 10–1,000 μg/plate | Negatived | Fujita et al. (1994) | |||
| Reverse mutation | S. Typhimurium TA98, TA100 |
≤ 0.4 μmol/plate (47 μg/plate)b |
Negatived | Sasagawa and Matsushima (1991) | |||
| Mutation | E. coli WP2 uvrA/pKM101 |
≤ 0.4 μmol/plate (47 μg/plate)b |
Negatived | Sasagawa and Matsushima (1991) | |||
|
14.042 1302 |
6‐Methylquinoline |

|
Reverse mutation | S. Typhimurium TA100 | 100–600 μg/plate | Positivec | Dong et al. (1978) |
| Reverse mutation | S. Typhimurium TA98, TA100 TA1535, TA1537 and TA1538 | ≤ 3,600 μg/plate | Negativea, Positivec, f | Wild et al. (1983) | |||
| Reverse mutation | S. Typhimurium TA98 and TA100 |
≤ 6 μmol/plate (859 μg/plate)g |
Negativea Positivec | Nagao et al. (1977) | |||
| Reverse mutation | S. Typhimurium TA98 and TA100 | ≤ 1,000 μg/plate | Negativea Positivec | Zeiger et al. (1992) | |||
| Reverse mutation | S. Typhimurium TA98 and TA100 | NR | Negativea Positivec | Sugimura et al. (1976) | |||
| Reverse mutation | S. Typhimurium TA100 |
5 μmol/plate (716 μg/plate)g |
Positivec | Takahashi et al. (1988) | |||
| Reverse mutation | S. Typhimurium TA98 | NR | Negatived | Debnath et al. (1992) | |||
| Reverse mutation | S. Typhimurium TA100 | 3.3–333 μg/plate | Negativea Positivec | Debnath et al. (1992) | |||
|
14.001 1303 |
Isoquinoline |

|
Reverse mutation | S. Typhimurium TA98 and TA100 | 20–50 μg/mL | Negatived | Sideropoulos and Specht (1984) |
| Reverse mutation | S. Typhimurium TM677 |
≤ 8 mmol/L (1,033 μg/mL)h |
Negativec | Kaden et al. (1979) | |||
| Reverse mutation | S. Typhimurium TA98 and TA100 | NR | Negatived | Sugimura et al. (1976) | |||
| Reverse mutation | S. Typhimurium TA98 and TA100 |
1–20 μmol/plate (129–2,583 μg/plate)h |
Negatived | Nagao et al. (1977) | |||
| Reverse mutation | S. Typhimurium TA98 and TA100 | 10,000–20,000 μg/mL | Negatived | Epler et al. (1979) | |||
| Mutation | E. coli B/r HCR+ | 50 μg/mL | Negatived | Sideropoulos and Specht (1984) | |||
| Unscheduled DNA synthesis | Rat hepatocytes | NR | Negative | Williams (1984) | |||
|
14.004 1304 |
3‐Methylindole |

|
Reverse mutation | S. Typhimurium TA100, TA1535 and TA1537 |
3 μmol/plate (394 μg/plate)i |
Negatived | Florin et al. (1980) |
| Reverse mutation | S. Typhimurium TA98 |
0.03–30 μmol/plate (3.9–3,935 μg/plate)i |
Negative(d,j) | Florin et al. (1980) | |||
| Reverse mutation | S. Typhimurium TA98 and TA100 | NR | Negativec | Kim et al. (1989) | |||
| Reverse mutation | S. Typhimurium TA98 and TA100 |
≤ 0.4 μmol/plate (52 μg/plate)i |
Negatived | Sasagawa and Matsushima (1991) | |||
| Reverse mutation | S. Typhimurium TA100 | ≤ 100 μg/plate | Negativea | Ochiai et al. (1986) | |||
| Mutation | E. coli WP2 uvrA/pKM101 |
≤ 0.4 μmol/plate (52 μg/plate)i |
Negatived | Sasagawa and Matsushima (1991) | |||
| Mutation | E. coli Sd‐4‐73 | 0.01–0.025 mL/disk | Negative | Szybalski (1958) | |||
| DNA single‐strand break | Bovine kidney cells |
10 μmol–1 mmol/L (1.31–131.18 μg/mL)i |
Positive | Kim et al. (1989) | |||
|
14.047 1307 |
2‐Acetylpyrrole |

|
Reverse mutation | S. Typhimurium TA98 and TA100 | 12.5–200 μg/plate | Negatived | Wang et al. (1994) |
| Reverse mutation | S. Typhimurium TA98 |
4–100 μmol/plate (437–10,913 μg/plate)j |
Negativec Positivea |
Lee et al. (1994) | |||
| Reverse mutation | S. Typhimurium TA100 |
4–100 μmol/plate (437–10,913μg/plate)j |
Negatived | Lee et al. (1994) | |||
|
14.038 1309 |
2‐Acetylpyridine |

|
Mitotic aneuploidy | S. cerevisiae D61.M |
0.50–0.87% (54,000–939,600 μg/mL)k |
Positive | Zimmermann et al. (1986) |
|
14.041 1314 |
Pyrrole |

|
Reverse mutation | S. Typhimurium TA98, TA100 and TA102 | 14 nmol/plate 1.4 mmol/plate (0.94–93,926 μg/plate)l | Negatived | Aeschbacher et al. (1989) |
| Reverse mutation | S. Typhimurium TA100, TA1535 and TA1537 | 3 μmol/plate (201 μg/plate)k | Negatived | Florin et al. (1980) | |||
| Reverse mutation | S. Typhimurium TA98 |
0.03–30 μmol/plate (2.01–2013 μg/plate)l |
Negatived | Florin et al. (1980) | |||
| Reverse mutation | S. Typhimurium TA98 and TA100 | NR | Negatived | Lee et al. (1994) | |||
| Unscheduled DNA synthesis | Rat hepatocytes | NR | Negative | Williams (1984) | |||
|
14.061 1315 |
3‐Ethylpyridine |

|
Reverse mutation | S. Typhimurium TA98, TA100, TA1535 and TA1537 |
3 μmol/plate (321 μg/plate)m |
Negatived | Florin et al. (1980) |
|
14.039 1316 |
3‐Acetylpyridine |

|
Mutation | E. coli WP2 uvrA | 5,000–10,000 μg/plate | Negative | Pai et al. (1978) |
| Mitotic aneuploidy | S. cerevisiae D61.M |
0.5–1.11% (55,100–122,322 μg/mL)n |
Positive | Zimmermann et al. (1986) | |||
| In vivo | |||||||
|
14.042 1302 |
6‐Methylquinoline |

|
Sex‐linked recessive mutation | Drosophila melanogaster | 10 mmol/L (1432 μg/mL)g | Negative | Wild et al. (1983) |
| Micronucleus formation | NMRI mouse | 0, 286, 429 or 572 mg/kg bw | Negative | Wild et al. (1983) | |||
NR: Not reported.
Without metabolic activation.
Calculated based on relative molecular mass = 117.15.
With metabolic activation.
With and without metabolic activation.
Toxic at concentrations > 3.0 mmol/plate (351 mg/plate).
TA100 and TA1535.
Calculated based on relative molecular mass = 143.19.
Calculated based on relative molecular mass = 129.16.
Calculated based on relative molecular mass = 131.18.
Toxic at concentrations of > 3.0 mmol/plate (394 mg/plate).
Calculated based on relative molecular mass = 109.13.
Calculated based on density = 1.08 g/mL (Sigma‐Aldrich, 2003; available at http://www.sigmaaldrich.com).
Calculated based on relative molecular mass = 67.09.
Calculated based on relative molecular mass = 107.16.
Calculated based on density = 1.102 g/mL (Sigma‐Aldrich, 2003; available at http://www.sigmaaldrich.com).
4.2.1.2. Genotoxicity studies – text taken7 from EFSA FGE.24Rev2 (EFSA CEF Panel, 2013)
In vitro/in vivo
Genotoxicity data were provided for seven of the 24 candidate substances. In in vitro studies on the candidate substances 2‐methylindole [FL‐no: 14.131], 2‐methylpyridine [FL‐no: 14.134], 3‐methylpyridine [FL‐no: 14.135], 4‐methylpyridine [FL‐no: 14.136], 2,4‐dimethylpyridine [FL‐no: 14.104], 3,5‐dimethylpyridine [FL‐no: 14.106] and 4‐acetylpyridine [FL‐no: 14.089] in doses up to 10000 μg/plate, with and without metabolic activation, did not cause reverse mutations in various strains of S. Typhimurium (Table A.2 in the present FGE.77Rev3).
Table A.2.
Genotoxicity data (in vitro) EFSA/FGE.24Rev2 (EFSA CEF Panel, 2013)
| Chemical Name [FL‐no] | Test System | Test Object | Concentration | Result | Reference | Comments |
|---|---|---|---|---|---|---|
| (Pyrrole [14.041]) |
Ames assay (modified preincubation method) |
S. Typhimurium TA98; TA100; TA102 |
1.4 mmol/plate (93,926 μg/plate) |
Negativea | Aeschbacher et al. (1989) | |
|
Ames assay (preincubation method) |
S. Typhimurium TA100; TA1535; TA1537 |
3 μmol/plate (201 μg/plate) |
Negativea | Florin et al. (1980) | ||
|
Ames assay (preincubation method) |
S. Typhimurium TA98 |
30 μmol/plate (2013 μg/plate) |
Negativea | |||
|
Ames assay (plate incorporation method) |
S. Typhimurium TA98; TA100 |
Not reported | Negativec | Lee et al. (1994) | ||
| Rec assay |
B. subtilis H17 (rec+), M45 (rec−) |
4 and 20 mg/disk | Positivec | Kim et al. (1987) | Poor predictive value for mutagenicity. Limited value | |
| Unscheduled DNA synthesis | Rat hepatocytes | Not reported | Negative | Williams (1984) | ||
| 1‐Methylpyrrole |
Ames assay (modified preincubation method) |
S. Typhimurium TA98; TA100; TA102 |
11 nmol–1.1 mmol/plate | Negativea | Aeschbacher et al. (1989) | 6 dose levels. The study is considered valid |
| Rec assay |
B. subtilis H17 (rec+) M45 (rec−) |
2, 4, 20 and 40 mg/disk (500.5 μmol/disk) |
Positivea | Kim et al. (1987) | Poor predictive value for mutagenicity. Limited value | |
| (Indole [14.007]) |
Ames assay (preincubation method) |
S. Typhimurium TA100 |
20 μg/plate | Negativeb | Ochiai et al. (1986) | |
| Ames assay |
S. Typhimurium TM677 |
4 mM (469 μg/mL) |
Negativec | Kaden et al. (1979) | ||
|
Ames assay (plate incorporation method) |
S. Typhimurium TA98; TA100; TA1535; TA1538 |
2,500 μg/plate | Negativea | Anderson and Styles, (1978) | ||
| Ames assay |
S. Typhimurium TA98; TA100 |
500 nmol/plate (59 μg/plate) |
Negativeb | Vance et al. (1986) | ||
|
Ames assay (preincubation method) |
S. Typhimurium TA100; TA1535; TA1537 |
3 μmol/plate (351 μg/plate) |
Negativea | Florin et al. (1980) | ||
|
S. Typhimurium TA98 |
30 μmol/plate (3,515 μg/plate) |
Negativec | ||||
|
S. Typhimurium TA97; TA102 |
1,000 μg/plate | Negativea | Fujita et al. (1994) | |||
|
S. Typhimurium TA98; TA100 E. coli WP2uvrA/pKM101 |
0.4 μmol/plate (47 μg/plate) |
Negativea | Sasagawa and Matsushima (1991) | |||
|
S. Typhimurium TA100 |
500 μg/plate | Negativeb | Hashizume et al. (1991) | |||
| 2‐Methylindole [14.131] |
Ames assay (preincubation method) |
S. Typhimurium TA98; TA100; TA1535; TA1538 |
4, 20, 100, 500 and 2,500 μg/plate | Negativea | Anderson and Styles (1978) | The study is considered valid |
|
S. Typhimurium TA98; TA100; TA1535; TA1537 |
3 μmol/plate (394 μg/plate) |
Negativea | Florin et al. (1980) | Single‐dose study | ||
| Ames assay |
S. Typhimurium TA98 |
3 nmol–30 μmol/plate (12 doses) (3,935 μg/plate) |
Negativea | Curvall et al. (1982) | The study is considered valid. | |
|
S. Typhimurium TM677 |
2 mM (262 μg/mL) |
Negative | Kaden et al. (1979) | Single‐dose study. | ||
| (3‐Methylindole [14.004]) |
Ames assay (preincubation method) |
S. Typhimurium TA100; TA1535; TA1537 |
3 μmol/plate (394 μg/plate) |
Negativea | Florin et al. (1980) | |
|
S. Typhimurium TA98 |
30 μmol/plate (3,935 μg/plate) |
Negativea | ||||
| Ames assay |
S. Typhimurium TA98; TA100 |
Not reported | Negativec | Kim et al. (1989) | ||
|
Ames assay (preincubation method) |
S. Typhimurium TA98; TA100 E. coli WP2uvrA/pKM101 |
0.4 μmol/plate (52 μg/plate) |
Negativea | Sasagawa and Matsushima (1991) | ||
|
S. Typhimurium TA100 |
100 μg/plate | Negativeb | Ochiai et al. (1986) | |||
|
S. Typhimurium TA100 |
Up to 3.33 mM (437 μg/mL) |
Negativec | Reddy et al. (2002) | |||
|
Mutation assay (paper‐disk method) |
E. coli Sd‐4‐73 |
0.025 mL/disk | Negative | Szybalski (1958) | ||
| Chromosomal aberration assay | Chinese hamster ovary cells |
1.3, 1.4 and 1.5 mM (+ S9) 1.4, 1.5 and 1.6 mM (− S9) |
Positivea | Reddy et al. (2002) | Aberrations were only detected at cytotoxic concentrations that showed marked inhibition of DNA synthesis | |
| Alkaline elution assay | Rat primary hepatocytes (uninduced and PB/β‐NF induced) | 0.5, 0.6, 0.7, 0.8, 0.9 and 1 mM | Negative | Reddy et al. (2002) | The study is considered valid | |
| DNA modification assay | Isolated human genomic DNA |
25 and 500 μM (66 μg/mL) |
Positivec Negativeb |
Laws et al. (2001) | Assay demonstrating inhibition of PCR amplification and spots demonstrated by postlabeling. Limited predictive value | |
| DNA Single‐strand break assay | Bovine kidney cells |
10 μM–1 mM (131.2 μg/mL) |
Positive | Kim et al. (1989) | Abstract only. Validity cannot be evaluated | |
| (2‐Acetylpyrrole [14.047]) |
Ames assay (plate incorporation method) |
S. Typhimurium TA98 |
4, 20 and 100 μmol/plate (10913 μg/plate) |
Negativec Positiveb |
Lee et al. (1994) | Positive without S9 only at the two highest concentrations. High concentrations. Technically acceptable, but of limited relevance due to high concentrations. |
|
S. Typhimurium TA100 |
100 μmol/plate (10,913 μg/plate) |
Negativea | ||||
| Ames assay |
S. Typhimurium TA98; TA100 |
Up to 200 μg/plate | Negativea | Wang et al. (1994) | ||
| 2‐Methylpyridine [14.134] |
Ames assay (preincubation method) |
S. Typhimurium TA98; TA100; TA1535; TA1537 |
3 μmol/plate (279 μg/plate) |
Negativea | Florin et al. (1980) | Single‐dose study |
|
Ames assay (modified preincubation method) |
S. Typhimurium TA98; TA100; TA102 |
10 nmol–1 mmol/plate (6 doses) (93 μg/mL) |
Negativea | Aeschbacher et al. (1989) | The study is considered valid | |
|
Ames assay (plate incorporation method) |
S. Typhimurium TA97; TA98; TA100; TA102 |
Up to 5,000 μg/plate (6 doses) |
Negativea | Claxton et al. (1987) | Individual dose levels not reported. The study is considered valid | |
|
S. Typhimurium TA98; TA100; TA1535; TA1537 |
50, 160, 500, 1,600 and 5,000 nL/plate (4,722 μg/plate) |
Negativea | Vleminckx et al. (1993a) | The study is considered valid | ||
| Mitotic aneuploidy assay |
S. cerevisiae D61.M |
0.5–0.74% (6 doses) (6988 μg/mL) |
Positive | Zimmermann et al. (1986) | Very high doses. The effect is considered thresholded. Limited relevance | |
| HGPRT Gene mutation assay | Chinese hamster V79 lung cells |
4.5, 4.75, 5, 5.25 and 5.5 μl/mL (5,194 μg/mL) |
Negativeb | Vleminckx et al. (1993b) | The study is considered valid | |
| Alkaline elution assay | Chinese hamster V79 lung cells |
2, 3, 4, 5 and 6 μL/mL (5,666 μg/mL) |
Negativeb | Schriewer et al. (1993) | The study is considered valid | |
| 3‐Methylpyridine[14.135] |
Ames assay (modified preincubation method) |
S. Typhimurium TA98; TA100; TA1535; TA1537 |
85, 280, 840 and 8,540 μg/plate | Negative | Haworth et al. (1983) | The study is considered valid |
|
Ames assay (plate incorporation method) |
S. Typhimurium TA98; TA100; TA1535; TA1537 |
50, 160, 500, 1,600 and 5,000 nL/plate (4,785 μg/plate) |
Negativea | Vleminckx et al. (1993a) | The study is considered valid | |
| Mutagenicity assay |
E. coli WP2 uvrA |
5–10 mg/plate (5000–10,000 μg/plate) |
Negative | Pai et al. (1978) | Single‐dose study. Very few experimental details. The validity cannot be evaluated | |
| HGPRT Gene mutation assay |
Chinese hamster V79 lung cells |
3, 3.25, 3.5, 3.75 and 4 μL/mL (3,828 μg/mL) |
Negativeb | Vleminckx et al. (1993b) | The study is considered valid | |
| Alkaline elution assay |
Chinese hamster V79 lung cells |
2, 3, 4 and 5 μL/mL (4,785 μg/mL) |
Negativeb | Schriewer et al. (1993) | The study is considered valid. | |
| 4‐Methylpyridine [14.136] |
Ames assay (plate incorporation method) |
S. Typhimurium TA98; TA100; TA1535; TA1537 |
50, 160, 500, 1,600 and 5,000 nL/plate (4779 μg/plate) |
Negativea | Vleminckx et al. (1993a) | The study is considered valid. |
| HGPRT Gene mutation assay |
Chinese hamster V79 lung cells |
3.75, 4, 4.25 and 4.5 μL/mL (4,301 μg/mL) |
Negativeb | Vleminckx et al. (1993b) | The study is considered valid. | |
| Alkaline elution assay |
Chinese hamster V79 lung cells |
3.75, 4, 4.25 and 4.5 μL/mL (4,301 μg/mL) |
Negativeb | Schriewer et al. (1993) | The study is considered valid. | |
| (3‐Ethylpyridine [14.061]) |
Ames assay (preincubation method) |
S. Typhimurium TA98; TA100; TA1535; TA1537 |
3 μmol/plate (321 μg/plate) |
Negativea | Florin et al. (1980) | Single‐dose study. |
| 2,4‐Dimethylpyridine [14.104] | Mitotic aneuploidy assay | S. cerevisiae D61.M |
0.4–0.60% (6 doses) (5,551 μg/mL) |
Positive | Zimmermann et al. (1986) | Very high doses. The effect is considered thresholded. Limited relevance |
| (2,6‐Dimethylpyridine [14.065]) |
Mitotic aneuploidy assay |
S. cerevisiae D61.M |
0.5–0.60% (4 doses) (5,551 μg/mL) |
Positive | Zimmermann et al. (1986) | Very high doses. The effect is considered thresholded. Limited relevance. |
| 3,5‐Dimethylpyridine [14.106] |
Ames assay (preincubation method) |
S. Typhimurium TA98; TA100; TA1535; TA1537 |
3 μmol/plate (321 μg/plate) |
Negativea | Florin et al. (1980) | Single‐dose study |
| (2‐Acetylpyridine [14.038]) |
Ames assay (plate incorporation method) |
S. Typhimurium TA98; TA100; TA1535; TA1537; TA1538 |
100–10,000 μg/plate | Negative | Longfellow (1997) | Very short summary. The results cannot be validated. High doses. |
| Mouse lymphoma assay |
Mouse lymphocytes L5178Y tk+/– |
2,500–4,500 μg/mL (− S9) 1,000–4,000 μg/mL (+ S9) |
Positivea | Very short summary. The results cannot be validated | ||
| Mitotic aneuploidy assay |
S. cerevisiae D61.M |
0.5–0.87% (4 doses) (9,396 μg/mL) |
Positive | Zimmermann et al. (1986) | Very high doses. The effect is considered thresholded. Limited relevance | |
| (3‐Acetylpyridine [14.039]) | Mutation |
E. coli WP2uvrA |
10,000 μg/plate | Negative | Pai et al. (1978) | Single‐dose study. Very few experimental details. The validity cannot be evaluated |
| Mitotic aneuploidy assay |
S. cerevisiae D61.M |
0.5–1.11% (5 doses) (1,223 μg/mL) |
Positive | Zimmermann et al. (1986) | Very high doses. The effect is considered thresholded. Limited relevance | |
| 4‐Acetylpyridine [14.089] |
Ames assay (preincubation method) |
S. Typhimurium TA97; TA98; TA100; TA102; TA104; TA1535; TA1537; TA1538 |
5, 100, 300, 100, 3,000 and 10,000 μg/plate | Negativea | Zeiger et al. (1992) | The study is considered valid |
| Mitotic aneuploidy assay |
S. cerevisiae D61.M |
0.5–1.19% (5 doses) (13,114 μg/mL) |
Positive | Zimmermann et al. (1986) | Very high doses. The effect is considered thresholded. Limited relevance | |
| Mitotic aneuploidy assay |
S. cerevisiae D61.M |
Up to 11 mg/mL | Positive | Whittaker et al. (1989) | Purity 88%. Very high doses. The effect is considered thresholded. Limited relevance |
* Supporting substances are listed in brackets.
With and without metabolic activation.
Without metabolic activation.
With metabolic activation.
Studies on induction of aneuploidy in S. cerevisiae D61.M available for the three candidate substances 2‐methylpyridine [FL‐no: 14.134], 2,4‐dimethylpyridine [FL‐no: 14.104] and 4‐acetylpyridine [FL‐no: 14.089] gave positive results. The positive results were obtained at high doses inhibiting the growth of the yeast. Furthermore, fungal systems for measuring aneuploidy have little relevance compared to the mammalian system.
No in vivo studies on genotoxicity of the candidate substances were available.
Genotoxicity tests are available for the eight supporting substances [FL‐no: 14.004, 14.007, 14.038, 14.039, 14.041, 14.047, 14.061 and 14.065]. 2‐Acetylpyrrole [FL‐no: 14.047] (methyl 2‐pyrrolyl ketone) was positive in TA98 without metabolic activation at the two highest concentrations tested. Negative results were obtained at the lowest concentration as well as with metabolic activation. This study is considered of limited relevance. Pyrrole [FL‐no: 14.041], indole [FL‐no: 14.007], 3‐methylindole [FL‐no: 14.004] (skatole), 3‐ethylpyridine [FL‐no: 14.061] and 2‐acetylpyridine [FL‐no: 14.038] were negative in bacterial mutation assays.
Studies on induction of aneuploidy in S. cerevisiae D61.M are available on three supporting substances, 2,6‐dimethylpyridine [FL‐no: 14.065], 2‐acetylpyridine [FL‐no: 14.038] and 3‐acetylpyridine [FL‐no: 14.039], which gave positive results. However, as for the three candidate substances, the positive results were obtained at high doses inhibiting the growth of the yeast. Furthermore, fungal systems for measuring aneuploidy have little relevance compared to the mammalian system.
In vivo data are available for one supporting substance.
3‐Methylindole (skatole) [FL‐no: 14.004] was reported negative in the MN assay in mice. The validity of this study, however, cannot be evaluated, as only an abstract is available.
Positive results were obtained for some candidate and supporting substances in the Rec, DNA breaking, Chinese hamster ovary (CHO)8 and DNA synthesis assays. These results are, however, not considered valid.
Conclusion on genotoxicity
The genotoxicity data available for the candidate substances do not preclude their evaluation through the Procedure.
For a summary of in vitro/in vivo genotoxicity data considered by EFSA, see Tables A.2 and A.3 (Appendix A).
Table A.3.
Genotoxicity data (in vivo) EFSA/FGE.24Rev2 (EFSA CEF Panel, 2013)
| Chemical Name [FL‐no]* | Test System | Test Object | Route | Dose | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|
| (3‐Methylindole [14.004])* | Micronucleus test | Mouse | Oral | 1,000 mg/kg day | Negative | (Reddy et al. (2003) | Abstract only. The validity cannot be evaluated |
* Supporting substance.
4.2.1.3. Genotoxicity studies evaluated by the panel in FGE.77Rev1
6‐Methylquinoline [FL‐no: 14.042]
6‐Methylquinoline [FL‐no: 14.042] was found to induce chromosome aberrations and sister chromatid exchanges (SCE) in CHO cells (National Toxicology Program (NTP), 1986).
A MN assay was performed by Nakajima (2005) essentially in line with the OECD Guideline 474. No significant increase of micronucleated polychromatic erythrocyte (PCE) frequency was observed in any groups of BDF1 male mice, treated by gavage at 225, 450 and 900 mg/kg bw for two subsequent days, 24 h apart. No significant decrease in the percentage of polychromatic erythrocytes to the analysed total erythrocytes (% PCE) was observed in any treatment group (Nakajima, 2005). The lack of cytotoxicity in the bone marrow cells does not allow a conclusion as to whether the test substance or a metabolite (e.g. an electrophilic epoxide) reached the bone marrow. Therefore, the results of this study have to be considered of limited relevance.
A bone marrow MN assay was performed by Honarvar (2004) on a structurally related substance, 6‐isopropylquinoline, which was in compliance with good laboratory practice (GLP) and OECD test guideline 474. No significant increase of micronucleated PCE frequency was observed in any group of NMRI mice orally treated with 6‐isopropylquinoline at 500, 1,000 and 2,000 mg/kg bw at 24 h after treatment and for the highest dose, 2,000 mg/kg bw also 48 h after treatment (Honarvar, 2004). Slight cytotoxic effects in the bone marrow (less than 10% changes in PCE/normochromatic erythrocyte (NCE) ratio) were observed, only at the high dose. Also, at the high dose group 48 h after treatment, the percentage of micronucleated cells (0.118) was higher than the corresponding vehicle control (0.065). The value was within the historical control range (up to 0.15%). Also in this case, due to the limited cytotoxicity, it is not clear whether the test substance/metabolite reached the target (bone marrow) in sufficient concentrations to elicit genotoxic effects.
The results of the MN assay by Honarvar (2004) on the structurally related substance 6‐isopropylquinoline were considered of limited relevance due to the lack of evidence of target tissue exposure. Therefore, this study could not rule out the concern for genotoxicity for 6‐methylquinoline.
For a summary of in vitro/in vivo genotoxicity data on 6‐methylquinoline, see Tables A.4 and A.5 (Appendix A).
Table A.4.
Genotoxicity data (in vitro) on 6‐Methylquinoline
| Chemical Name [FL‐no] | Test System | Test Object | Concentration | Result | Reference | Comments |
|---|---|---|---|---|---|---|
| 6‐Methylquinoline [14.042] | Chromosomal aberration assay | Chinese hamster ovary cells |
52.7, 69.9, 174.8 and 349.5 μg/mL 50.3, 125.5, 250.9 and 374.5 μg/mL |
Negative (− S9) Positive (+ S9) |
NTP (1986) | |
| Sister chromatid exchanges | Chinese hamster ovary cells |
16.6, 25.1, 33 and 50 μg/mL 16.7, 50.1, 166.9 and 500.7 μg/mL |
Positive (− S9) Positive (+ S9) |
NTP (1986) | ||
| Bacterial reverse mutation assay |
S. Typhimurium TA98 and TA100 |
3, 10, 33, 100, 166, 333, 666, 1,000 μg/plate |
Negative (− S9) Positive (+ S9) |
NTP (1986) |
Table A.5.
Genotoxicity data (in vivo) on 6‐Methylquinoline and 6‐Isopropylquinoline
| Chemical Name [FL‐no] | Test System | Test Object | Route | Dose | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|
| 6‐Methylquinoline [14.042] | Micronucleus test | Male mice | Gavage | 0, 225, 450 and 900 mg/kg bw | Negative | Nakajima (2005) | Limited relevance. |
| Micronucleus test | NMRI mice | i.p. | 0, 286, 429, or 572 mg/kg bw | Negative | Wild et al. (1983) | Single intraperitoneal administration. Limited relevance | |
| Comet assay in liver and duodenum | Male rat | Gavage | 100, 200 and 400 mg/kg bw | Equivocal in liver and Negative in duodenum | Keig‐Shevlin (2016) | The study complies with OECD test guideline 489 and GLP guidelines | |
| Gene mutation in liver and duodenum | Male Muta(™) mice | Gavage | 125, 250, 500 mg/kg bw per day | Negative | Ballantyne (2017) | The study complies with OECD test guideline 488 and GLP guidelines | |
| Micronucleus in peripheral blood reticulocytes | Male Muta(™) mice | Gavage | 125, 250, 500 mg/kg bw per day | Negative | |||
| 6‐Isopropylquinoline | Micronucleus test | NMRI mice | Oral | 0, 500, 1,000 and 2,000 mg/kg bw | Negative | Honarvar (2004) | Limited relevance |
4.2.2. New genotoxicity studies evaluated in FGE.77Rev3
4.2.2.1. 6‐Methylquinoline [FL‐no: 14.042] – in vivo comet assay
6‐Methylquinoline [FL‐no: 14.042] (purity 99.9%) was tested for genotoxicity in vivo in male Han Wistar rats by assessing its ability to induce DNA damage in the liver and duodenum using the Comet assay (Keig‐Shevlin, 2016). An additional separate experiment was conducted in the liver to clarify increases in tail intensity observed in the first experiment. The studies were in compliance with GLP and OECD test guideline 489 (OECD, 2014).
Doses were selected based on a range‐finder experiment, where two doses of 6‐methylquinoline [FL‐no: 14.042] of 400 and 600 mg/kg bw per day were administered at 0 and 21 hrs to both male and female rats. No gender differences were observed for toxicity; therefore, only male rats were treated in the main experiments. The dose of 400 mg/kg bw per day was considered as an estimate of the maximum tolerated dose (MTD), based on clinical signs (ataxia, piloerection, decreased activity) observed. Based on this range‐finder experiment, the following doses were tested in the main experiments: 0, 100, 200 and 400 mg/kg bw per day (six animals per group).
In the main experiments, 6‐methylquinoline was administered twice to male Han Wistar rats by gavage at 0 (day 1) and 21 h (day 2). Vehicle (corn oil) and positive control (ethyl methanesulfonate (EMS), 200 mg/kg bw per day) groups were included. Tissues were sampled on day 2, 24 h after the first treatment.
No clinical signs of toxicity were observed following treatments with vehicle control, 6‐methylquinoline or the positive control. There were no changes in clinical chemistry and no macroscopic or microscopic changes related to administration of 6‐methylquinoline.
Experiment 1 – Comet assay in liver
Dose‐related increases in group mean %tail intensity in the liver were observed in all test article treated groups. No dose‐related increase in %clouds was observed. At the intermediate and high doses, the increases in tail intensity were statistically significant and a positive linear trend was also apparent (p ≤ 0.01). At 100 mg/kg bw per day, tail intensity and tail moment values were comparable with the vehicle control animals. At 200 mg/kg bw per day, a two‐fold increase in group mean tail intensity and tail moment was observed. At 400 mg/kg bw per day, the group mean tail intensity and tail moment were three‐fold higher than the vehicle control. The author of the study considered that this result was mainly due to two animals displaying elevated tail intensity and tail moment values which increased the group mean. As all individual animal data were within the laboratory's historical control data and in the absence of any clinical chemistry or pathological changes in the liver which could be considered to be due to toxic effects of the test article, the biological relevance of these statistically significant increases in tail intensity was unclear. Therefore, the experiment in liver was repeated to check the reproducibility of these results.
The Panel considered the results of this experiment as positive taking into account that two criteria for evaluation and interpretation of results as positive (OECD test guideline 489) were fulfilled:
at least one of the test doses exhibits a statistically significant increase compared with the concurrent negative control,
the increase is dose related when evaluated with an appropriate trend test.
The third criterion (‘any of the results are outside the distribution of the historical negative control data for a given species, vehicle, route, tissue, and number of administrations’) is not applicable in this case because the range for historical negative controls is very wide (95% reference range of 0.08–5.08%).
Experiment 1 – Comet assay in duodenum
Following treatment with 6‐methylquinoline at all dose levels, there was no evidence of any induction of DNA damage in cells isolated from duodenum. The group mean %tail intensity and tail moment values were comparable to the concurrent vehicle control group. No dose‐related increase in %clouds in duodenum was reported following treatment with 6‐methylquinoline.
Experiment 2 – Comet assay in liver
Group mean %tail intensity and tail moment values for all groups of animals treated with 6‐methylquinoline were comparable with the group mean vehicle control data. There were no statistically significant differences in %tail intensity between treated and control groups. All individual animal data at all dose levels were generally consistent with the vehicle control animals and fell within the laboratory's historical control data. There was no dose‐related increase in %clouds in liver following treatment with 6‐methylquinoline.
The increases in liver group mean tail intensity observed in experiment 1 were not reproduced in experiment 2. The authors of this study considered that the statistically significant increases in group mean liver tail intensity observed in the first experiment were due to chance occurrence in individual animals increasing the group means, rather than a true genotoxic effect and that there was no reproducible evidence that exposure of animals to 6‐methylquinoline induced DNA damage in cells isolated from the liver.
Conclusion on comet assay
The Panel considered that the in vivo comet assay in rats (Keig‐Shevlin, 2016) reported negative results in duodenum and equivocal results in liver, showing positive results in a first experiment, not reproduced in a second one. The Panel requested the applicant to clarify these results. The transgenic rodent gene mutation assay was considered as appropriate test for the in vivo follow‐up of in vitro positive results in bacteria (EFSA Scientific Committee, 2011) In addition, a combined analysis of MN in peripheral blood in the same animals was requested to confirm the negative in vivo data observed in studies on chromosomal damage (Wild et al., 1983; Nakajima et al., 2005) which were considered by the Panel to be of only limited reliability.
4.2.2.2. 6‐Methylquinoline [FL‐no: 14.042] – in vivo gene mutation assay and micronucleus assay in Muta™ Mice
6‐Methylquinoline (purity 99.9%) was tested for its ability to induce gene mutations in the lacZ transgene in liver and duodenum from treated male Muta™ Mice (CD2‐lacZ80/HazfBR strain). In addition, an assessment of MN induction in peripheral blood reticulocytes from the same animals was included (Ballantyne, 2017).
Based on a range‐finder experiment where no gender difference was observed, the MTD was established (500 mg/kg bw per day) for the main experiment based on clinical signs of toxicity (e.g. hunched posture, piloerection, semi‐closed eyes, raised skin and fur, reduced activity and staggering). Groups of seven male mice were administered 6‐methylquinoline, by oral gavage, at 0, 125, 250 or 500 mg/kg bw per day for 28 days. No positive control was included in the dosing regimen of this study, but was provided from another study carried out in the same laboratory measuring the same endpoints (positive control: ethylnitrosourea animals dosed at 10 mg/kg bw per day). The 28‐day dosing period was followed by 3 days expression time before necropsy at day 31. On day 29, blood samples were collected from the tail vein of all the animals for the MN assay. After a 3‐day expression period following the final dosing (day 31), the animals were sacrificed. All animals were macroscopically examined and the liver and duodenum were collected for the mutant frequency assay.
Mutant frequency in duodenum and liver
DNA was obtained from duodenum and liver tissues from all four treatment groups as well as frozen positive control DNA from animals treated with ethylnitrosourea from another study of the same laboratory for the mutant frequency assay. The DNA was packaged into phage heads ready to transfection in suspensions of E. coli, according to the OECD test guideline 488 (OECD, 2013). The transfected E. coli was plated, incubated and scored for plaques. According to the study report, all accepted packaging reactions resulted in at least 30,000 plaque‐forming units (pfu) and at least one mutant plaque. All reported mutation data were generated for at least 200,000 pfu per tissue per animal, from at least three independent packaging reactions. At least 1 million pfu were obtained per group, per tissue from a minimum of five animals.
No statistically significant increases in mutant frequency were observed either in the liver or duodenum of treated Muta™ Mice, and all study results were within the laboratory's historical control data set.
Micronucleus assay
Blood samples taken on day 29 were analysed for MN frequency by high speed flow cytometry. At least 20,000 reticulocytes from each sample were scored for MN.
No positive control was included in the MN experiment, but positive control blood samples (cyclophosphamide at 20 mg/kg bw per day) from another study of the same laboratory were analysed alongside the study samples. No significant increase in the frequency of micronucleated cells was observed in the peripheral blood reticulocytes of mice from any of the 6‐methylquinoline treated groups compared to the vehicle controls. According to the author of the study report, all data fell within the historical control data set for the laboratory, but no historical control data were provided for animals dosed for more than seven days.
Conclusion on the in vivo gene mutation assay and in vivo micronucleus assay
The Panel concluded that under the conditions of this study, 6‐methylquinoline did not induce mutations in the liver and duodenum of Muta™Mice, following treatment for 28 days up to the dose of 500 mg/kg bw per day (the MTD estimated for this study) and that the substance did not induce micronucleated cells in peripheral blood reticulocytes. In this study, no toxicity in liver and bone marrow was observed that would be considered as a direct evidence of tissue exposure to the tested substance. However, the clinical signs of toxicity observed in the dose range‐finding study such as changes in behaviour (hunched posture, reduced activity and staggering), observed at high and mid doses) suggest that animals were systemically exposed to 6‐methylquinoline, and this is considered as a line of evidence of target tissue exposure (liver and bone marrow), on the basis of the criteria reported in the recent opinion of the EFSA Scientific Committee (EFSA Scientific Commitee, 2017).
The Panel considered this line of evidence, together with the observation that several other quinoline derivatives produce toxicity in liver and other tissue, sufficient to assume that 6‐methylquinoline is systemically bioavailable and that, therefore, the negative results of the gene mutation assay in liver and the MN assay in peripheral blood are conclusive.
For a summary of in vivo genotoxicity data on 6‐methylquinoline, see Appendix A, Table A.5.
4.2.2.3. EFSA considerations on genotoxicity
The Panel concluded that one of the 22 substances evaluated by JECFA, 6‐methylquinoline [FL‐no: 14.042], showed a genotoxic potential in vitro, with consistently positive results in several bacterial mutagenicity tests, after metabolic activation. Increases of chromosomal aberrations and sister chromatid exchanges in CHO cells were reported in a summary table (NTP, 1986), but were considered inadequate for the evaluation due to high level of toxicity at all the concentrations tested and the low number of metaphases examined.
6‐Methylquinoline was reported negative in a test for gene mutations in Drosophila (Wild et al., 1983).
Negative results were also reported in two in vivo MN studies in mice considered of limited relevance. In the first one (Wild et al., 1983), carried out by single ip administration, a single sampling time was used. In a more recent one, by gavage, no toxicity to the bone marrow was observed, although some weak evidence of systemic exposure of the animals was provided by clinical signs (reduction of spontaneous activity, prone position) reported at the highest dose tested (Nakajima, 2005).
The in vivo comet assay in rats reported negative results in duodenum and equivocal results in liver, showing a dose‐related increase in %tail intensity in liver in a first experiment, which was not reproduced in a second one (Keig‐Shevlin, 2016).
A transgenic gene mutation assay in Muta™Mice (Ballantyne, 2017) carried out in liver and duodenum of animals treated with 6‐methylquinoline for 28 days up to 500 mg/kg bw per day, the MTD, did not show any increase of mutant frequency. Therefore, the Panel considered that the concern for gene mutation is ruled out.
The analysis of MN in peripheral reticulocytes of the same animals after 28 days of treatment (Ballantyne, 2017) did not result in any increase in cells with MN compared to the control group. Therefore, the Panel considered that the concern for structural and numerical chromosomal aberrations is ruled out, likewise.
Based on the data available, the Panel concluded that there is no concern for genotoxicity of 6‐methylquinoline [FL‐no: 14.042].
For 21 JECFA evaluated pyridine, pyrrole and quinoline derivatives, the Panel concluded previously that the data available do not preclude evaluation through the Procedure. Based on the new data provided recently, the Panel concluded that also 6‐methylquinoline [FL‐no: 14.042] can now be evaluated through the Procedure.
4.2.3. Repeated dose toxicity studies
Since the publication of FGE.77 (EFSA, 2009), additional toxicity data have been provided for isoquinoline [FL‐no: 14.001], pyrrole [FL‐no: 14.041] and 2‐acetylpyrrole [FL‐no: 14.047], the latter also to cover the evaluation of the structurally related 2‐propionylpyrrole [FL‐no: 14.068]. The main studies provided are for each substance a 90‐day study. Since the publication of FGE.77Rev1 (EFSA CEF Panel, 2014), additional toxicity data were provided for 1‐furfurylpyrrole [FL‐no: 13.134]. The main study provided is a 90‐day study; these data are intended to cover the re‐evaluation of this substance and 2‐acetyl‐1‐ethylpyrrole [FL‐no: 14.045] and 2‐acetyl‐1‐methylpyrrole [FL‐no: 14.046] and have been evaluated in FGE.77Rev2 (EFSA CEF Panel, 2015). In the present revision of FGE.77 (FGE.77Rev3), data from a 90‐day study and from a carcinogenicity study on 6‐methylquinoline [FL‐no: 14.042] are evaluated.
The available toxicity studies are summarised in Appendix B, Table B.1.
Table B.1.
Toxicity data considered by the panel
| Chemical Name [FL‐no] | Species; Sex No/group | Route | Doses (mg/kg bw per day) | Duration (days) | NOAEL (mg/kg bw per day) | Reference | Comments |
|---|---|---|---|---|---|---|---|
|
Isoquinoline [FL‐no: 14.001] |
Rat; M, F 10 |
Oral | 0, 0.03, 0.3 and 3 | 90 | 3 | Kojima (2006) | It is a GLP study performed according to the Japanese ‘Guidelines for designation of food additives and revision of standards for use of food additives, Notification No 29’ of the Environmental Health Bureau, Ministry of Health and Welfare, Japan, 22 March 1996. The requirements of this guideline are very similar to the OECD Guideline 408 |
|
Pyrrole [FL‐no: 14.041] |
Rat; M, F 10 |
Gavage | 0, 0.03, 0.3 and 3 | 90 | 3 | Marumo (2008) | Same as above |
|
2‐Acetylpyrrole [FL‐no: 14.047] |
Rat; M, F 3 |
Diet |
0, 85, 550 and 842 (males) 0, 91, 582 and 949 (females) |
14 | Range finding | Bauter (2012a) | |
|
Rat; M, F 10 |
Diet |
0, 68, 133 and 263 (males) 0, 79, 155 and 298 (females) |
90 | 48 | Bauter (2012b) | Compliant to the OECD test guideline 408 | |
|
1‐Furfurylpyrrole [FL‐no: 13.134] |
Rat; M, F 3 |
Diet |
0, 29, 84 and 211 (males) 0, 27, 81 and 192 (females) |
14 | Range‐finding | Kappeler (2013a) | |
|
Rat; M, F 10 |
Diet |
0, 25, 77 and 154 (males) 0, 25, 75 and 151 (females) |
90 | 25 | Kappeler (2013b) | Compliant to the OECD test guideline 408 | |
|
6‐methylquinoline [FL‐no: 14.042] |
Rat; M,F 10 to 16 |
Diet | 2.2 (males) 2.7 (females) | 90 | 2.2 | Posternak et al. (1969) | The study was performed with a single dose that produced no adverse effects. The report is only a summary of the study. Poor study protocol |
| Rat; M 38, F 37 | Diet | 25 mg/kg bw per day | 2‐year | Fukushima et al. (1981) | Carcinogenicity study. Only one dose was tested | ||
| Mouse; F 30 | Skin painting | 7.5 mg per animal | 18 weeks | La Voie et al. (1984) | Assay for tumour‐initiating activity |
M: Male.
F: Female.
4.2.3.1. Isoquinoline [FL‐no: 14.001]
A 90‐day oral study in rats was performed on isoquinoline according to the Japanese ‘Guidelines for designation of food additives and revision of standards for use of food additives, Notification No 29’ of the Environmental Health Bureau, Ministry of Health and Welfare, Japan, 22 March 1996. The requirements of this guideline are very similar to the OECD Guideline 408. It is a GLP study. Groups (10/sex per dose) of male and female Sprague–Dawley rats were administered 0 (vehicle control), 0.03, 0.3 and 3 mg/kg bw per day of isoquinoline dissolved in corn oil by gavage daily for 90 and 91 days for males and females, respectively (Kojima, 2006). The purity of the test article was 98.5%. Animals were weighed at the start of the study and weekly thereafter. Food consumption and efficiency were measured weekly. The rats were caged individually during the experiment. All animals were subject to ophthalmologic examination prior to the start of the study, and on day 79, five animals of each sex per group were examined again. Urine was analysed on day 82 for five animals from each group. The rats were fasted for 18–21 h prior to blood sampling immediately prior to necropsy. A full haematological and biochemical analysis of blood was performed. At termination of the study, animals were sacrificed and subject to full necropsy. Histopathological examination was performed on all organs (as in the OECD Guideline 408) for the control and high dose group.
No animals died through the course of the study. No clinical signs of toxicity or behavioural changes were observed. Ophthalmological examination revealed no treatment‐related changes. Mean body weights were comparable throughout the study between control and test groups of both sexes. Urine analysis did not reveal any treatment‐related alterations when compared to controls. Haematology and blood chemistry results showed no significant differences between the test groups and controls. There were no organ weight changes or other macroscopic findings attributable to the administration of the test substance.
Histopathological examination did not show differences between controls and treated animals of either sex; some incidental findings occurred in both controls and treated animals, but there was no significant difference in their occurrence or intensity in the various organs when compared to the control groups.
Since there were no statistically significant changes due to the administration of the test material, the NOAEL of isoquinoline was determined to be 3 mg/kg bw per day in male and female rats after 90 days of administration by oral gavage (Kojima, 2006).
4.2.3.2. Pyrrole [FL‐no: 14.041]
In a gavage study (Marumo, 2008), groups (10/dose per sex) of male and female Sprague–Dawley rats were administered 0 (vehicle control), 0.03, 0.30 and 3.00 mg/kg bw of aqueous pyrrole daily, by gavage for 90 days prior to necropsy. This study was performed according to ‘Guidelines for designation of food additives and for revision of standards for use of food additives’, Notification No. 29 of the Environmental Health Bureau, the Ministry of Health and Welfare, Japan, 22 March 1996 which is comparable to an OECD Guideline 408 study. It is a GLP study. Clinical observations were recorded daily and body weights and food consumption were recorded weekly. On day 79, five animals from each group were subject to ophthalmology examination. Urine samples were collected on day 82 for routine clinical chemical analysis. At termination, blood samples were taken for clinical chemistry determinations and haematological examination. At necropsy, organ weights for all organs required for an OECD Guideline 408 study were recorded. Tissues from all organs required in an OECD Guideline 408 study from both sexes of the control and 3.00 mg/kg bw per day groups were fixed and preserved for histopathological examination.
No mortality was observed throughout the course of the study and the general condition of the rats was unremarkable. Mean body weight gains and food consumption were comparable across test and control groups. Ophthalmologic examination revealed that in some animals in all male groups, controls included and some females of the 0.03 and 3.00 mg/kg bw per day groups, corneal clouding was observed.
Urine analysis revealed no toxicologically significant findings except that one male rat out of five in the 3.00 mg/kg bw per day group showed some changes, suggesting a possible kidney effect at that level; however, there were no indications of kidney pathology in the histopathological findings of this rat. In the females, there were no effects observed in urinalysis except that they showed significantly higher concentrations of sodium, potassium and chloride ions, but this was not dose dependent. Males and females in the 3.00 mg/kg bw per day groups showed an increase in ‘urobilinogen concentrations’ in blood, but this was not accompanied by associated histopathology in the liver, spleen, bone marrow or haemolysis; the effect can be attributed to the interference of pyrrole present in urine in the colorimetric assay; it gives the same reaction as urobilinogen in the detection method used.
In female rats, the white blood cell count was lower for all three exposure levels than the control group, but this showed no dose relationship; the values were 4,600 ± 1,500, 3,600 ± 900, 3,400 ± 800 and 3,400 ± 1,100 per μL, respectively. At the two higher dose levels, this was statistically significant (p < 0.05). None of the other haematological parameters was changed as compared to controls. In male rats, there was no difference in white blood cell levels or any other haematological parameter. Small changes in blood biochemical findings in male rats at the highest exposure were considered incidental.
Gross pathology examination revealed some organ weight variations including decreased absolute and relative pituitary gland weights only in low‐dose treated male rats. All groups of male rats showed a somewhat decreased relative seminal vesicle weight due to a combination of increased body weight in the treated rats in combination with a slight decrease in absolute seminal vesicle weight. However, histopathology did not reveal abnormalities, neither in pituitary gland nor in seminal vesicles.
Histopathological examination was performed on all high dose and control animals, along with any tissues with lesions at other doses. In the lungs, alveolar accumulation of foamy cells was observed in eight males and three females at the 3.00 mg/kg bw dose and in four male controls. Mineralisation of the pulmonary arterial wall was reported for five males and two females of the high‐dose group and two male controls. Focal thickening of alveolar septum with neutrophilic infiltration was seen in two high‐dose male rats. Basophilic tubules were noted in the kidney cortex of eight males and five females of the high‐dose group and five females of the control group. Atrophy of the seminiferous tubule was observed in two male in the high dosed group, but the changes were very slight. In female in the high dosed group, single animals showed follicle cysts or retention of the corpus luteum with a marked decrease of eosinophils in the endometrium and myometrium or marked mucification of the vaginal mucosa. Most of these phenomena were observed in both the treated and the control groups, and they are, therefore, considered incidental findings.
The lower white blood cell count in the females is considered an incidental finding and not considered an adverse effect since the count of all other blood cells types was normal in the female treated groups. In the males, no lower blood count for any cell types was observed, the histopathological examination revealed no correlating changes in the haematopoietic tissue and there was no dose–effect relationship (raising the question whether the control value was incidentally too high; the company, unfortunately, did not give an indication of historical control values in their report). The Panel decided, based on the findings, that the NOAEL level was the highest exposure level 3.0 mg/kg bw per day.
4.2.3.3. 2‐Acetylpyrrole [FL‐no: 14.047]
A 14‐day range finding study
In a 14‐day range‐finding dietary study (Bauter, 2012a), groups (3/sex per dietary intake level) of male and female Sprague–Dawley rats were fed a diet designed to provide 0 (dietary control), 1,000, 9,000 and 18,000 mg/kg feed of 2‐acetylpyrrole [FL‐no: 14.047] daily. These estimated dietary levels correspond to the measured intake of 0, 85, 550 and 842 mg/kg bw per day for males and 0, 91, 582 and 949 mg/kg bw per day for females, respectively. Clinical observations were recorded daily and body weights were recorded on days 0, 7, 11 and 12. Individual food consumption was recorded on days 7 and 12. Due to increasing mortality in the high intake groups of both sexes, the study was terminated early at day 12. The results showed that the two higher doses were too toxic for a 90‐day study. A 90‐day study was started at lower exposure levels.
Effect on urinary iron excretion
The company also studied the effect of 2‐acetylpyrrole [FL‐no: 14.047] on urinary excretion of iron because 2‐acetylpyrrole is a strong‐complexing agent of metal ions. At a very high dose gavage study in rats (375 mg/kg bw orally for 10 days), the urinary excretion of total iron was increased six‐fold (Mendes, 2012); no data are provided on absorption of iron from the intestinal tract, which might be influenced by complexation of iron with 2‐acetylpyrrole.
90‐day study
In an OECD test guideline 408 compliant 90‐day study, groups of rats (10/sex per dietary intake level) of male and female Sprague–Dawley CD rats were fed a diet designed to provide 0 (dietary control), 1,050, 2,100 and 4,200 mg 2‐acetylpyrrole [FL‐no: 14.047]/kg feed daily (Bauter, 2012b). These dietary levels correspond to the calculated average daily intakes of 0, 68, 133 and 263 mg/kg bw for males and 0, 79, 155 and 298 mg/kg bw for females, respectively.
The test material was not stable in the diet, and in the report (Bauter, 2012b), it is suggested that part of it was probably not detected by the extraction method employed due to complexation with metal ions in the feed. It is calculated that over the course of the study, the animals received concentrations of 35–40% of the target intake level on average. Therefore, values for exposure levels based on the measured intake are proportionally lower. Based on this analysis of the test diets, the mean daily intakes were calculated to be 367, 754 and 1,705 mg/kg feed. Assuming that the toxicity is only related to the free 2‐acetylpyrrole, these dietary concentrations correspond to average daily intakes of 24, 48 and 107 mg/kg bw for males and 28, 56 and 121 mg/kg bw for females, respectively, over 90 days.
Clinical observations of toxicity were performed on day 0 and weekly until sacrifice. Animals were weighed on day 0 at the start of the study and weekly thereafter. Food consumption and efficiency were measured and calculated weekly. Blood chemistry and haematology were performed on blood drawn via sublingual bleed at day 43 for the controls and high intake groups and at day 86 for all groups after overnight fast. Urine was collected during the 15 h prior to the blood draw. Prior to initiation of the study and on day 91, the eyes of all rats were examined by focal illumination and indirect ophthalmoscopy. At termination of the study, all survivors were sacrificed and subject to full necropsy and histopathology as required by the OECD Guideline.
There were no mortalities or ophthalmological changes associated with the presence of 2‐acetylpyrrole in the diet. Most other findings, generally also noted in control animals, were not considered adverse effects of test substance administration and were regarded as incidental. Statistically significant concentration‐dependent reductions in body weight, body weight gain, food consumption (males and females) and food efficiency (females) at the highest dietary level (1,705 mg/kg feed measured concentration) during the study were attributed to the possible decrease in test substance palatability at high dietary levels.
Haematology parameters for both males and females were mostly unchanged during treatment. Although incidentally reaching a statistically significant difference when compared to concurrent controls, the values were in general within the range of historical controls and without associated histopathology correlate; they were, therefore, considered to be incidental and not related to the test material. However, statistically significantly (p < 0.05) decreased total white blood cell counts, erythrocyte counts, haemoglobin concentrations, haematocrit, absolute lymphocyte counts, absolute monocyte counts and absolute basophil counts and increased red cell distribution width were reported in the high intake group females on day 86. These parameters are outside of historical control levels although the variations are low in magnitude. There were no meaningful differences in coagulation parameters between test and control groups of both sexes.
Variations in clinical chemistry parameters were considered incidental and unrelated to the presence of 2‐acetylpyrrole in the diet due to lack of concentration dependence or correlated pathology.
Organ weight measurements, absolute and relative brain weight, for males were comparable to controls, with some isolated exceptions; these were without histologic correlate and were considered unrelated to test substance in the diet.
Female rats of the high intake groups displayed minimal to moderate dark bilateral thyroid glands. Microscopic changes were slight thyroid hypertrophy/hyperplasia among 4/10 and 10/10 high intake group males and females, respectively. This was characterised by enlarged subgross tall columnar appearance of the follicular epithelial cells which appeared with fine cytoplasmic vacuolation with intermittent focally piled papillary projections into the follicular lumen. The company did not provide a clear (mechanistic) explanation for this finding.
In conclusion, although some haematology and clinical parameter changes were observed in mid‐ and high‐dose groups, in the mid dose group were considered incidental and not of concern (not dose related and/or very small in magnitude and/or within historic controls and without histopathology correlation). However, the thyroid effects at the exposure level are of concern as well as the haematological changes in the high dose group females. Therefore, a NOAEL for 2‐acetylpyrrole is derived from the middle dose 48 mg/kg bw per day in males and 56 mg/kg bw per day in females. The NOAEL value of 48 mg/kg bw per day is used in calculating the margin of safety.
Metabolites of 2‐acetylpyrrole
Mendes (Mendes, 2012) analysed the urine obtained in metabolism cages from rats dosed with 2‐acetylpyrrole [FL‐no: 14.047] at 375 mg/kg by oral gavage as described above. Based on gas chromatography‐mass spectroscopy (GC‐MS) analysis, three major components were identified in the urine of both males and females treated with 2‐acetylpyrrole. Unchanged 2‐acetylpyrrole and pyrrol‐2,5‐dione were detected; the structure of another main metabolite detected in the urine is proposed to be 1,5‐dihydropyrrol‐2‐one; however, further experiments have yet to be performed to confirm this.
4.2.3.4. 1‐Furfurylpyrrole [FL‐no: 13.134]
A 14‐day range finding study
In a 14‐day palatability and range‐finding dietary study (Kappeler, 2013a), 1‐furfurylpyrrole [FL‐no: 13.134] was administered to male and female Crl:CD(SD) rats (3/sex per group) for 14 consecutive days in the diet to provide 0, 25, 75 and 200 mg/kg bw per day (Kappeler, 2013a). Observations for mortality and morbidity were performed twice daily and clinical examinations were performed once daily. Detailed physical examinations including body weight measurements were performed within 4 days of receipt, on the day of randomisation, prior to dosing on study day 0 (body weights, only) and weekly during the study. Food consumption was recorded 1 week prior to randomisation, on the day of randomisation and weekly throughout the study. Based on food consumption measurements, the calculated doses of 1‐furfurylpyrrole were 0, 29, 84 and 211 mg/kg bw per day, respectively, for males and 0, 27, 81 and 192 mg/kg bw per day, respectively, for females over the entire study. All animals survived the 14 days of diet administration.
There were no overt adverse effects from 1‐furfurylpyrrole administration. The only differences from control group animals were body weight reductions of 18% and 16% for males and females, respectively, in the 200 mg/kg bw per day group, that were associated with lower food consumption levels. It was concluded that 1‐furfurylpyrrole was well tolerated in Crl:CD(SD) rats at dietary levels of up to 84 mg/kg bw per day and 81 mg/kg bw per day for males and females, respectively.
90‐day study
In an OECD test guideline 408 compliant 90‐day dietary study (GLP), 1‐furfurylpyrrole [FL‐no:13.134] (purity 99.6%) was administered to individually housed Crl:CD(SD) rats (10/sex per group) at levels calculated to provide nominal doses of 0, 25, 75 and 150 mg/kg bw per day (Kappeler, 2013b). All animals were subject to observations for mortality and morbidity twice daily, daily clinical examinations and weekly detailed physical examinations, including body weight and food consumption measurements. Ophthalmic examinations were performed before the start of the study and in week 12. Clinical pathology parameters (haematology, coagulation, serum chemistry and urinalysis) were evaluated for all animals at the scheduled necropsy, after 13 weeks of treatment. Blood for haematology and serum chemistry was collected at necropsy after overnight fast and urine was collected overnight prior to necropsy. Coagulation was measured in blood collected from anaesthetised animals prior to sacrifice. All tissues were examined microscopically for animals of the 150 mg/kg bw per day groups. In addition, liver, spleen and nasal tissues were also examined for animals of the two lower intake level groups.
Based on food consumption data, the mean intake of 1‐furfurylpyrrole was calculated to be 25, 77 and 154 mg/kg bw per day for males and 25, 75 and 151 mg/kg bw per day for females. All animals survived to the end of the study. There were no differences between treated and control groups noted in clinical observations, macroscopic findings or urinalysis, except for an increase in urobilinogen concentration.
By the time of necropsy, body weights were lower in the 150 mg/kg bw per day dose group for both males (−17%) and females (−11%) and in the 75 mg/kg bw per day dose group of females (−8%), relative to the control group. Decreased food consumption was also observed. These decreases were statistically significant for the male group at 150 mg/kg bw per day beginning on the first week and throughout the length of the study; for the female groups, the decrease was not statistically significant, although the weight gain in these females was decreased statistically significant. Slightly lower body weights were noted in the 25 and 75 mg/kg bw per day males and the 25 mg/kg bw per day females (4%, 5% and 6%, respectively).
Reductions in red blood cell counts (0.6%, 7.0*% and 9.3**%), haemoglobin levels (3.6%, 5.4*% and 9.6**%) and haematocrit values (3.7%, 7.2*% and 9.9**%) and slightly higher red blood cell distribution widths (4.1%, 4.9**% and 4.1**%) were observed in all treated groups (25, 75 and 150 mg/kg bw per day, respectively) of males but were not observed in females. The effect at the lower exposure level was considered minor. Histopathology showed no changes in bone marrow at the highest dose level. Pigment deposits in the red pulp of the spleen were minimally increased only at the highest dose level in eight out of 10 males; for females, this was one out of 10 at the middle dose and four out of 10 at the highest dose.
In males, dose‐dependent changes in serum chemistry values included higher total bilirubin (0.01 ± 0.03, 0.02 ± 0.04, 0.07 ± 0.05** and 0.08 ± 0.04** mg/dL, respectively), which might be related to the reduced red blood cell counts and cholesterol (39*, 84** and 91**%, respectively). In females, similar increases were not dose dependent: 0.04 ± 0.05, 0.09 ± 0.03**, 0.11 ± 0.03** and 0.10 ± 0.00** mg/dL) for total bilirubin and 41**, 33* and 66**% for cholesterol. In parallel with the higher total bilirubin levels, the urobilinogen concentration in urine was increased at the higher dose levels. Only at the highest dose, sorbitol dehydrogenase (SDH) values in both sexes were increased by 111** and 33% in males and females, respectively.
The increases in total bilirubin and cholesterol suggest effects on the liver. Higher mean liver weights relative to final body weight were noted in the male (6, 16** and 32**%) and female groups (12**, 17** and 32**% increase based on body weight). Centrilobular hepatocellular vacuolation was observed in the 75 and 150 mg/kg bw per day male groups and the 150 mg/kg bw per day group females only. The male groups scored 5/10 minimal and 5/10 mild at the highest dose; 4/10 minimal and 1/10 mild at the middle dose; 0/10 at the lowest dose; against 0/10 in the controls; the females showed only at the highest dose in 7/10 centrilobular vacuolisation against 0/10 in the control and both lower dose groups.
Other microscopic changes were identified in nasal sections and spleen. In the spleen, findings were limited to a very minimal increase in pigment deposits that were mainly restricted to the 150 mg/kg bw per day dose group. Microscopically, changes in nasal tissue included olfactory mucosa degeneration, depleted mucous secretion by goblet cells and thin deposits of a hyaline material along the surface of the olfactory epithelial cells. Olfactory mucosa degeneration was observed in both sexes at the 75 and 150 mg/kg bw per day intake levels and was slightly more prevalent and severe in males. The findings in the olfactory mucosa were considered to be adverse. Depleted mucous secretion by goblet cells was especially prominent in Nasal Section I and was observed in most rats administered 1‐furfurylpyrrole.
It is concluded that exposure to 1‐furfurylpyrrole [FL‐no: 13.134] in the diet for 90 days resulted in adverse effects in the 75 and 150 mg/kg bw per day groups of males and females and consisted of effects on liver, red blood cells and olfactory mucosa. Therefore, the NOAEL of [FL‐no: 13.134] was 25 mg/kg bw per day for male and female Crl:CD(SD) rats.
4.2.3.5. 6‐Methylquinoline
6‐Methylquinoline [FL‐no: 14.042] was tested in a single‐dose 90‐day dietary study in Charles River CD rats (10–16 animals/sex per group) (Posternak et al., 1969). This publication is a summary of 90‐day studies on 42 flavouring compounds. The publication states that ‘of these, 29 were at least 98% pure (..) the purity of the remainder was 90% or more’; the purity of 6‐methylquinoline is not specified. The only available information on that study is a brief description of the method employed and a few general remarks. No study report or any details are available. The study results were submitted to a FEMA Expert Panel (before 1969).
A control and a single treatment group of the same sex were housed in pairs and given ad libitum access to water and food. The concentration of the test material in the diet was adjusted during the study to maintain a dietary intake of 2.2 mg/kg bw per day and 2.7 mg/kg bw per day in males and females, respectively. Food consumption and body weights were determined weekly. Limited haematology and blood urea analyses were conducted during the weeks 7 and 13 of the study, on half of the animals. At the end of the study, animals were sacrificed, liver and kidney weights were measured and ‘gross and histological examinations were carried out on a wide range of organs’.
The authors claim that at the exposure of 2.2 mg/kg bw per day in males and 2.7 mg/kg bw per day in females, 6‐methylquinoline did not induce any effects in the parameters measured. However, the procedure of the 90‐day study is very deficient compared to the current requirements for 90‐day study according to OECD test guideline 408; moreover, the reporting is extremely deficient.
Carcinogenicity study on 6‐methylquinoline
Fukushima et al. (1981) reported results from a 2‐year oral carcinogenicity study on 6‐methylquinoline in the rat. These data are part of a study which investigated the carcinogenicity of six quinoline derivatives including 8‐nitroquinoline as positive control. In the publication by Fukushima et al. (1981), few study details are reported.
The compounds were mixed with the diet at 0.05%, except for the positive compound, 8‐nitroquinoline, which was added at 0.10%. Thus, only one exposure level was tested in groups consisting of 43–46 animals per sex per group. Since pneumonia occurred during the last 12 weeks of the experiment, many rats died prematurely. The surviving animals were necropsied after 104 weeks and ‘all organs’ were examined histologically. No details about the tissues examined are given.
In the 8‐nitroquinoline‐treated animals, extensive changes in the forestomach were observed in both males (n = 30) and females (n = 37): hyperplasia, papilloma and squamous cell carcinoma were seen in 97, 93 and 67%, respectively, of the males, and 97, 97 and 65% of the females. In addition, 30% (males) and 38% (females) had urinary bladder tumours and 8% of the females had a uterus carcinoma; in controls (n = 31 and 44 for males and females, respectively), the incidence of these tumours was 0%. Other tumours were of similar frequency as in controls.
For 6‐methylquinoline (n = 38 and 37 for males and females, respectively, dose level: 500 mg/kg feed which is equivalent to 25 mg/kg bw per day), no differences with controls were observed; only the frequency of liver hyperplastic nodules seemed increased compared to controls: 10% vs. 0% in males and 13% vs. 7% in females.
These results show that the study did pick up signs of carcinogenicity for 8‐nitroquinoline, while 6‐methylquinoline was negative. The exposure level to these two substances was different: 0.10% in the feed for 8‐nitroquinoline vs. 0.05% in the feed for 6‐methylquinoline.
4.3. Other studies
Assay for tumour initiation
Quinoline and seven monomethylated derivatives, including 6‐methylquinoline, were tested for tumour‐initiating activity on SENCAR female mice skin with promotion by tetradecanoyl phorbol acetate (La Voie et al., 1984). The total initiation dose of either quinoline or the isomeric methylquinolines was 7.5 mg per mouse.
Quinoline induced tumours in 53% of the mice (on average 0.73 skin tumours per animal); a similar tumorigenic potential was observed for 4‐ and 8‐methylquinoline (45% of the mice, with an average skin tumours per animal of 0.90 and 0.66, respectively). No statistically significant increases of skin tumours were observed for 2‐, 3‐, 5‐, 6‐ and 7‐methylquinoline.
These results suggest that 6‐methylquinoline has no tumour‐initiating properties in skin, under the conditions of this study. This would support the lack of carcinogenicity as reported in the study by Fukushima et al. (1981).
The Panel considered the data on 6‐methylquinoline sufficient to disregard the observations with quinoline and other methylquinolines.
4.4. Application of the procedure
4.4.1. Application of the procedure to 22 pyridine, pyrrole and quinoline derivatives by JECFA (JECFA, 2005b, 2006a)
According to JECFA, three of the substances belong to structural class I, 13 to structural class II and six to structural class III, using the decision tree approach presented by Cramer et al. (1978).
JECFA concluded 20 pyridine, pyrrole and quinoline derivatives at step A3 in the JECFA Procedure, i.e. the substances are expected to be metabolised to innocuous products (step 2) and the intakes for all substances are below the thresholds for their structural classes I, II and III (step A3).
Two substances, 1‐furfurylpyrrole [FL‐no: 13.134] and 2‐pyridine methanethiol [FL‐no: 14.030], were evaluated via the B‐side of the Procedure as the substances could not be anticipated to be metabolised to innocuous products. For these substances, the intake is below the threshold for the structural class III (step B3) and a NOAEL exists to provide an adequate margin of safety to the estimated intake as flavouring substances (step B4). For 1‐furfurylpyrrole [FL‐no: 13.134], a NOAEL of 12 mg/kg bw per day from a 90‐day feeding study in rats (Morgareidge, 1971) is > 1,000,000 times greater than the estimated current intake of this substance as a flavouring substance. For 2‐pyridine methanethiol [FL‐no: 14.030], the NOAEL of 3.4 mg/kg bw per day from a 90‐day feeding study in rats (Posternak et al., 1969) is > 20,000,000 times higher than the estimated current intake of this substance as a flavouring substance.
In conclusion, JECFA evaluated all 22 substances as to be of no safety concern at the estimated levels of intake as flavouring substances based on the MSDI approach.
The evaluations of the 22 pyridine, pyrrole and quinoline derivatives are summarised in Appendix C, Table C.1.
Table C.1.
Summary of Safety Evaluation by JECFA (JECFA, 2005b)
| FL‐no JECFA‐no | EU Register name | Structural formula | EU MSDIa US MSDI (μg/capita per day) | Classb Evaluation procedure pathc | Outcome on the named compound [d or e] | EFSA conclusion on the named compound (Procedure steps, intake estimates, NOAEL, genotoxicity) | EFSA conclusion on the material of commerce |
|---|---|---|---|---|---|---|---|
|
14.004 1304 |
3‐Methylindole |

|
2.4 0.07 |
Class I A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.007 1301 |
Indole |

|
26 10 |
Class I A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.041 1314 |
Pyrrole |

|
0.11 0.01 |
Class I A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.038 1309 |
2‐Acetylpyridine |

|
50 68 |
Class II A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.039 1316 |
3‐Acetylpyridine |
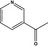
|
23 0.8 |
Class II A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.045 1305 |
2‐Acetyl‐1‐ethylpyrrole |

|
0.12 0.009 |
Class II A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.046 1306 |
2‐Acetyl‐1‐methylpyrrole |

|
1.2 0.02 |
Class II A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.047 1307 |
2‐Acetylpyrrole |

|
3.3 0.2 |
Class II A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.059 1312 |
3‐Isobutylpyridine |

|
0.049 0.07 |
Class II A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.060 1313 |
2‐Pentylpyridine |

|
0.061 0.07 |
Class II A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.061 1315 |
3‐Ethylpyridine |

|
9.3 3 |
Class II A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.065 1317 |
2,6‐Dimethylpyridine |

|
0.26 0.007 |
Class II A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.066 1318 |
5‐Ethyl‐2‐methylpyridine |

|
0.12 0.04 |
Class II A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.068 1319 |
2‐Propionylpyrrole |

|
0.012 2 |
Class II A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.071 1320 |
Methyl nicotinate |

|
0.49 0.2 |
Class II A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.164 1322 |
2‐Propylpyridine |

|
0.61 0.9 |
Class II A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.001 1303 |
Isoquinoline |

|
0.012 0.07 |
Class III A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.042 1302 |
6‐Methylquinoline |

|
0.32 0.01 |
Class III A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.058 1311 |
2‐Isobutylpyridine |

|
0.0061 0.9 |
Class III A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.072 1321 |
2‐(3‐Phenylpropyl)pyridine |

|
1.8 0.7 |
Class III A3: Intake below threshold |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
13.134 1310 |
1‐Furfurylpyrrole |

|
0.12 0.07 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
14.030 1308 |
2‐Pyridine methanethiol |

|
0.0012 0.007 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists |
d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
EU MSDI: Amount added to food as flavour in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita per day. EU MSDIs may deviate from those reported in the JECFA evaluation because for several substances new data were available.
Thresholds of concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
No safety concern based on intake calculated by the MSDI approach of the named compound.
Data must be available on the substance or closely related substances to perform a safety evaluation.
4.4.2. Application of the procedure to 24 pyridine, pyrrole, indole and quinoline derivatives from chemical group 28 evaluated by EFSA in FGE.24Rev2 (EFSA CEF Panel, 2013)
Twenty‐four candidate substances were evaluated in FGE.24Rev2. Twenty‐two of the 24 candidate substances are classified into structural class II and two substances into structural class III using the decision tree approach presented by Cramer et al. (1978).
Two of the substances, ethyl nicotinate [FL‐no: 14.110] and isopropyl nicotinate [FL‐no: 14.120], were concluded at step A3, i.e. the substances are expected to be metabolised to innocuous products (step 2) and the estimated daily intake is below the threshold for the structural class (step A3).
The remaining 22 substances were concluded at step B4, i.e. the substances could not be anticipated to be metabolised to innocuous products (step 2) and the estimated daily intake is below the threshold for the structural class (step B3). For the 22 substances, NOAELs could be derived to provide adequate margins of safety to the estimated levels of intake as flavouring substance (step B4).
For the candidate substance 2‐acetyl‐5‐methylpyrrole [FL‐no: 14.085], a NOAEL of 48 mg/kg bw per day for the supporting substance 2‐acetylpyrrole [FL‐no: 14.047] is derived. The estimated daily per capita intake of 0.0012 μg for 2‐acetyl‐5‐methylpyrrole [FL‐no: 14.085] corresponds to 0.02 ng/kg bw per day at a body weight of 60 kg. Thus, a margin of safety of 2.4 × 109 can be calculated. 2‐Acetyl‐5‐methylpyrrole is accordingly not expected to be of safety concern at the estimated level of intake.
In an oral 37 weeks feeding study in rats on indole‐3‐carbinole, a substance structurally related to the two indole derivatives in this FGE (FGE.24Rev2), a NOAEL of 50 mg/kg bw per day could be derived. The combined estimated daily per capita intake of 0.0024 μg for 1‐acetylindole [FL‐no: 14.088] and 2‐methylindole [FL‐no: 14.131] corresponds to 0.04 ng/kg bw per day at a body weight of 60 kg. Thus, a margin of safety of 1.3 × 109 can be calculated. 1‐Acetylindole [FL‐no: 14.088] and 2‐methylindole [FL‐no: 14.131] are accordingly not expected to be of safety concern at the estimated level of intake.
A 90‐day oral feeding study in rats is available for the supporting substance 2‐acetylpyridine [FL‐no: 14.038]. The NOAEL derived is 37 mg/kg bw per day. The MSDI values for the 19 pyridine derivatives in this FGE (EFSA CEF Panel, 2013) are between 0.012 and 0.21 μg/capita per day. The combined estimated daily per capita intake of these 19 derivatives is 1.5 μg, corresponding to 0.025 μg/kg bw per day. Thus, a margin of safety of 1.5 × 106 can be calculated using the NOAEL of 37 mg/kg bw per day. The 19 pyridine derivatives in this flavouring group are accordingly not expected to be of safety concern at the estimated level of intake.
In conclusion, the Panel evaluated the 24 substances as to be of no safety concern at the estimated levels of intake as flavouring substances based on the MSDI approach.
The stepwise evaluations of the 24 substances are summarised in Appendix C, Table C.2 of the present opinion.
Table C.2.
Summary of safety evaluation by the EFSA (FGE.24Rev2) (EFSA CEF Panel, 2013)
| FL‐no | EU Register name | Structural formula | MSDIa (μg/capita per day) | Classb Evaluation procedure pathc | Outcome on the named compound [d ore] | Outcome on the material of commerce [f , g , or h] | Evaluation remarks |
|---|---|---|---|---|---|---|---|
| 14.110 | Ethyl nicotinate |

|
0.013 |
Class II A3: Intake below threshold |
d | f | |
| 14.120 | Isopropyl nicotinate |

|
0.0012 |
Class II A3: Intake below threshold |
d | f | |
| 14.023 | 1‐Methylpyrrole |

|
0.3 |
Class II B3: Intake below threshold, B4: No adequate NOAEL |
Additional data required | i | |
| 14.085 | 2‐Acetyl‐5‐methylpyrrole |

|
0.0012 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.089 | 4‐Acetylpyridine |

|
0.0073 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.092 | 2‐Butylpyridine |

|
0.012 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.093 | 3‐Butylpyridine |

|
0.061 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.103 | 2,3‐Dimethylpyridine |

|
0.037 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
|
14.104 2151 |
2,4‐Dimethylpyridine |

|
0.024 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.105 | 3,4‐Dimethylpyridine |

|
0.13 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.106 | 3,5‐Dimethylpyridine |

|
0.073 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.107 | 2,5‐Dimethylpyrrole |

|
0.061 |
Class II B3: Intake below threshold, B4: No adequate NOAEL |
Additional data required | i | |
| 14.115 | 2‐Ethylpyridine |

|
0.027 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.116 | 4‐Ethylpyridine |

|
0.027 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.117 | 2‐Hexylpyridine |

|
0.012 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.118 | 2‐Hydroxypyridine |

|
0.024 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.124 | 2‐Isopropylpyridine |

|
0.021 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.125 | 4‐Isopropylpyridine |

|
0.012 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.134 | 2‐Methylpyridine |

|
0.21 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.135 | 3‐Methylpyridine |

|
0.027 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.136 | 4‐Methylpyridine |

|
0.73 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.140 | 3‐Pentylpyridine |

|
0.0012 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.143 | 3‐Propylpyridine |

|
0.0012 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.145 | Pyrrole‐2‐carbaldehyde |

|
0.12 |
Class II B3: Intake below threshold, B4: No adequate NOAEL |
Additional data required | j | |
| 14.150 | 2,4,6‐Trimethylpyridine |

|
0.012 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
|
14.169 2150 |
1‐Ethyl‐2‐pyrrolecarboxaldehyde |

|
0.12 |
Class II B3: Intake below threshold, B4: No adequate NOAEL |
Additional data required | j | |
| 13.100 | 2‐Acetyl‐1‐furfurylpyrrole |

|
0.091 |
Class III B3: Intake below threshold, B4: No adequate NOAEL |
Additional data required | i | |
| 14.088 | 1‐Acetylindole |

|
0.0012 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
| 14.131 | 2‐Methylindole |

|
0.0012 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists |
d | f | |
|
14.163 2152 |
1‐Methylpyrrole‐2‐carboxaldehyde |

|
0.0024 |
Class III B3: Intake below threshold, B4: No adequate NOAEL |
Additional data required | j | |
| 14.002 | 4‐Methylquinoline |

|
0.12 |
Class III No evaluation |
i | ||
| 14.094 | 4‐Butylquinoline |

|
0.0012 |
Class III No evaluation |
i | ||
| 14.138 | 2‐Methylquinoline |

|
0.012 |
Class III No evaluation |
i |
EU MSDI: Amount added to food as flavour in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita per day.
Thresholds of concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
No safety concern based on intake calculated by the MSDI approach of the named compound.
Data must be available on the substance or closely related substances to perform a safety evaluation.
No safety concern at the estimated level of intake of the material of commerce meeting the specification requirement (based on intake calculated by the MSDI approach).
Tentatively regarded as presenting no safety concern (based on intake calculated by the MSDI approach) pending further information on the purity of the material of commerce and/or information on stereoisomerism.
No conclusion can be drawn due to lack of information on the purity of the material of commerce.
No longer supported by Industry (DG SANCO, 2012).
No longer supported by Industry (DG SANCO, 2013).
4.4.3. EFSA consideration
The Panel agrees with the way the application of the Procedure has been applied by JECFA for four of the 22 substances. Methyl nicotinate [FL‐no: 14.071], indole [FL‐no: 14.007] and 3‐methylindole [FL‐no: 14.004] were evaluated via the A‐side of the Procedure as they were anticipated to be metabolised to innocuous products. For these three substances, EFSA agreed no safety concern at step A3 of the Procedure, as the intake is below the threshold of the structural class (Cramer et al., 1978). 1‐Furfurylpyrrole [FL‐no: 13.134] and 2‐pyridine methanethiol [FL‐no: 14.030] were the only two substances evaluated through the B‐side of the Procedure as the substances were not anticipated to be metabolised to innocuous products by JECFA. For 1‐furfurylpyrrole [FL‐no: 13.134],9 EFSA disagreed with JECFA, as the 90‐day feeding study in rats (Morgareidge, 1971) was considered a poorly reported old study, the quality of which cannot be assessed, as stated in FGE.24 (EFSA, 2006). For 2‐pyridine methanethiol [FL‐no: 14.030], EFSA agrees with JECFA.
For 6‐methylquinoline [FL‐no: 14.042], contrary to JECFA, the Panel concluded in FGE.77, that this substance should not be evaluated using the Procedure until adequate in vivo genotoxicity data become available. Additional genotoxicity data have become available for 6‐methylquinoline after the publication of FGE.77, which have been evaluated in Revision 1 of FGE.77; however, the data were not sufficient to rule out the concern on the genotoxic potential of 6‐methylquinoline. New in vivo genotoxicity data have been provided for 6‐methylquinoline, a Comet assay in duodenum and liver of rats and a combined gene mutation assay and MN assay in Muta™Mice. Based on these studies, the genotoxicity concern for 6‐methylquinoline [FL‐no: 14.042] can be ruled out.
The carcinogenicity study in rats on 6‐methylquinoline (Fukushima et al., 1981) reports few study details and only one single dose of 6‐methylquinoline was tested. Despite these limitations, this study suggests that 6‐methylquinoline is not carcinogenic in rats under condition of the experiment. It induced an increase of liver hyperplastic nodules (not statistically significant) compared to controls. In addition, 6‐methylquinoline did not induce skin tumours in a tumour‐initiating test in mice (La Voie et al., 1984).
The only 90‐day study in rats on 6‐methylquinoline (Posternak et al., 1969) is of very limited quality, but was acceptable at the time the study was performed; it suggests that at a level around 2 mg/kg bw per day, no changes in a number of parameters measured occurred. If this dose is considered as a NOAEL, which is supported by the absence of strong indications of toxicity at higher exposure and longer duration in the study by Fukushima et al. (1981), an adequate margin of safety of approximately 400,000 can be calculated based on the MSDI.
For the remaining 16 substances, the Panel, in contrast to JECFA, did not anticipate that they will be metabolised to innocuous products due to concern with respect to N‐oxidation of pyridines and for the pyrroles concerns about N‐oxidation and epoxidation and accordingly concluded that they should be evaluated along the B‐side. However, in FGE.77, for 10 [FL‐no: 14.038, 14.039, 14.058, 14.059, 14.060, 14.061, 14.065, 14.066, 14.072 and 14.164] of these 16 substance, a NOAEL could be derived to provide adequate margins of safety to the estimated level of intakes as flavouring substance (step B4). A 90‐day oral‐feeding study in rats is available for 2‐acetylpyridine [FL‐no: 14.039]. The NOAEL derived is 37 mg/kg bw per day (Til and van der Meulen, 1971). The MSDI values for the 10 pyridine derivatives in this FGE are between 0.06 and 50 μg/capita per day. The combined estimated daily per capita intake of the 10 pyridine derivatives evaluated through the B‐side is 57 μg corresponding to 0.95 μg/kg bw per day. Thus, a margin of safety of approximately 39,000 can be calculated using the NOAEL of 37 mg/kg bw per day. The 10 pyridine derivatives in this flavouring group evaluated through the B‐side are accordingly not expected to be of safety concern at the estimated levels of intake.
For pyrrole [FL‐no: 14.041] and the five pyrrole derivatives [FL‐no: 13.134, 14.045, 14.046, 14.047 and 14.068] as well as for isoquinoline [FL‐no: 14.001], NOAELs could not be derived as such or for structurally related substances in FGE.77. Accordingly, additional toxicological data were required for these seven substances (step B4) in FGE.77.
Additional toxicity data have become available, after the publication of FGE.77, for isoquinoline [FL‐no: 14.001], pyrrole [FL‐no: 14.041] and 2‐acetylpyrrole [FL‐no: 14.047] (Kojima, 2006, Marumo, 2008 and Bauter, 2012b), the latter also to cover the evaluation of the structurally related 2‐propionylpyrrole [FL‐no: 14.068].
Based on the data for isoquinoline [FL‐no: 14.001] (Kojima, 2006), a NOAEL of 3 mg/kg bw per day could be established. When comparing this NOAEL at step B4 in the Procedure to the estimated exposure based on the MSDI (0.012 μg/capita per day, corresponding to 0.0002 μg/kg bw per day), an adequate margin of safety of 15 × 106 can be calculated.
Based on the data for pyrrole [FL‐no: 14.041] (Marumo, 2008), a NOAEL of 3 mg/kg bw per day could be established. When comparing this NOAEL at step B4 in the Procedure to the estimated exposure based on the MSDI (0.11 μg/capita per day, corresponding to 0.0018 μg/kg bw per day), an adequate margin of safety of 16 × 105 can be calculated.
Based on the data for 2‐acetylpyrrole [FL‐no: 14.047] (Bauter, 2012b), a NOAEL of 48 mg/kg bw per day could be established. When comparing the NOAEL at step B4 in the Procedure to the estimated exposure based on the MSDI (3.3 μg/capita per day, corresponding to 0.055 μg/kg bw per day), an adequate margin of safety of 87 × 104 can be calculated. For 2‐propionylpyrrole [FL‐no: 14.068], supported by 2‐acetylpyrrole [FL‐no: 14.047], the MSDI is 0.012 μg/capita per day, which is well below the MSDI of 2‐acetylpyrrole and accordingly not expected to be of safety concern at the estimated levels of intake.
Additional toxicity data have become available, after the publication of FGE.77Rev1, for 1‐furfurylpyrrole [FL‐no: 13.134] (Kappeler, 2013b). The data are intended to cover the re‐evaluation of this substance and 2‐acetyl‐1‐ethylpyrrole [FL‐no: 14.045] and 2‐acetyl‐1‐methylpyrrole [FL‐no: 14.046]. The main study provided is a 90‐day study.
Based on the data submitted for 1‐furfurylpyrrole [FL‐no: 13.134] (Kappeler, 2013b), a NOAEL of 25 mg/kg bw per day could be established. When comparing this NOAEL at step B4 in the Procedure to the estimated exposure based on the MSDI (0.12 μg/capita per day, corresponding to 0.002 μg/kg bw per day), an adequate margin of safety of 12.5 × 106 can be calculated. For 2‐acetyl‐1‐ethylpyrrole [FL‐no: 14.045], supported by 1‐furfurylpyrrole [FL‐no: 13.134], the MSDI is also 0.12 μg/capita per day and accordingly not expected to be of safety concern at the estimated levels of intake. For 2‐acetyl‐1‐methylpyrrole [FL‐no: 14.046], the MSDI is 1.2 μg/capita per day which is 10 times the figure for the MSDI of 1‐furfurylpyrrole resulting in an adequate margin of safety of 12.5 × 105; 2‐acetyl‐1‐methylpyrrole [FL‐no: 14.046] is accordingly not expected to be of safety concern at the estimated levels of intake.
5. Conclusions
The present Revision of FGE.77, FGE.77Rev3, includes the assessment of additional genotoxicity data for 6‐methylquinoline [FL‐no: 14.042]. The studies provided are an in vivo Comet assay in rats and a combined gene mutation and MN assay in Muta™Mice. The applicant also submitted toxicity data for 6‐methylquinoline.
The Panel concluded that the 22 substances in the JECFA flavouring group of pyridine, pyrrole and quinoline derivatives are structurally related to the group of pyridine, pyrrole, indole and quinoline derivatives from chemical group 28 evaluated by EFSA in the Flavouring Group Evaluation 24, Revision 2 (FGE.24Rev2).
JECFA evaluated two substances [FL‐no: 13.134 and 14.030] via the B‐side of the Procedure and 20 substances via the A‐side.
The Panel agrees with the way that the application of the Procedure has been applied by JECFA for four of the 22 substances. Three of these four substances, methyl nicotinate [FL‐no: 14.071], indole [FL‐no: 14.007] and 3‐methylindole [FL‐no: 14.004], were evaluated by JECFA on the A‐side of the Procedure, as they were anticipated to be metabolised to innocuous products. For these three substances, the Panel agreed no safety concern at step A3 of the Procedure, as their intake estimates (MSDI) were below the threshold of their structural class. For the fourth substance, 2‐pyridine methanethiol [FL‐no: 14.030], the Panel agreed with JECFA that it should be evaluated through the B‐side of the Procedure, as the substance was not anticipated to be metabolised to innocuous products. However, a NOAEL was derived from a 90‐day study, which provided an adequate margin of safety.
For 1‐furfurylpyrrole [FL‐no: 13.134], EFSA disagreed with JECFA, as the 90‐day feeding study in rats was considered a poorly reported old study, the quality of which cannot be assessed. However, for this substance, additional toxicity data were submitted and evaluated in FGE.77Rev2. Based on these data, adequate margins of safety could be calculated for [FL‐no: 13.134, 14.045 and 14.046].
For 16 substances, the Panel, in contrast to JECFA, did not anticipate that they will be metabolised to innocuous products and accordingly concluded that they should be evaluated along the B‐side of the Procedure. However, in FGE.77, for 10 [FL‐no: 14.038, 14.039, 14.058, 14.059, 14.060, 14.061, 14.065, 14.066, 14.072 and 14.164] of these 16 JECFA‐evaluated pyridine derivatives evaluated via the B‐side of the Procedure by EFSA, NOAELs could be derived to provide adequate margins of safety and the Panel agrees with the JECFA conclusion ‘no safety concern at estimated levels of intake as flavouring substances’ based on the MSDI approach.
In FGE.77Rev1, three 90‐day studies were evaluated for isoquinoline [FL‐no: 14.001], pyrrole [FL‐no: 14.041] and 2‐acetylpyrrole [FL‐no: 14.047] and NOAELs to provide adequate margin of safety were derived to cover these three substances as well as the structurally related 2‐propionylpyrrole [FL‐no: 14.068].
In FGE.77Rev2, one 90‐day study was evaluated for 1‐furfurylpyrrole [FL‐no: 13.134]. A NOAEL to provide adequate margin of safety was derived to cover this substance as well as the structurally related 2‐acetyl‐1‐ethylpyrrole [FL‐no: 14.045] and 2‐acetyl‐1‐methylpyrrole [FL‐no: 14.046].
In previous revisions of this FGE, the Panel concluded, contrary to JECFA, that 6‐methylquinoline [FL‐no: 14.042] (evaluated via the A‐side by JECFA) should not be evaluated through the Procedure due to concern with respect to genotoxicity in vitro.
Based on the available data and the new in vivo genotoxicity studies on 6‐methylquinoline [FL‐no: 14.042], the Panel concluded that, for 6‐methylquinoline [FL‐no: 14.042], there is no concern with respect to genotoxicity. Therefore, 6‐methylquinoline [FL‐no: 14.042] can now be evaluated through the Procedure.
The carcinogenicity study in rats on 6‐methylquinoline (Fukushima et al., 1981) reports few study details and only one single dose (0.05% in diet which is equivalent to 25 mg/kg bw per day) of 6‐methylquinoline was tested. Despite these limitations, this study suggests that 6‐methylquinoline is not carcinogenic in rats under the condition of the experiment. In addition, 6‐methylquinoline did not induce skin tumours in a tumour‐initiating test in mice (La Voie et al., 1984).
The only 90‐day study in rats on 6‐methylquinoline (Posternak et al., 1969) is of very limited quality, but was acceptable at the time the study was performed; it suggests that at a level around 2 mg/kg bw per day, no changes in a number of parameters measured occurred. If this dose is considered as a NOAEL, an adequate margin of safety of approximately 400,000 can be calculated based on the MSDI.
From the group of 22 substances evaluated by JECFA, the Panel evaluated three substances via the A‐side [FL‐no: 14.004, 14.007, 14.071] and 19 substances via the B side: [FL‐no: 13.134, 14.001, 14.030, 14.038, 14.039, 14.041, 14.042, 14.045, 14.046, 14.047, 14.058, 14.059, 14.060, 14.061, 14.065, 14.066, 14.068, 14.072 and 14.164]. For these 19 substances, NOAELs could be derived, which provided adequate margins of safety.
For the following substances [FL‐no: 13.134, 14.001, 14.030, 14.041, 14.042, 14.058, 14.072], the Industry has submitted use levels for normal and maximum use. For the remaining 15 substances, use levels are needed to calculate the mTAMDIs in order to identify those flavouring substances that need more refined exposure assessment and to finalise the evaluation.
In order to determine whether the conclusion for the 22 JECFA evaluated substances can be applied to the materials of commerce, it is necessary to consider the available specifications. Adequate specifications including complete purity criteria and identity tests are available for the 22 JECFA‐ evaluated substances.
Thus, for the 22 JECFA evaluated pyridine, pyrrole and quinoline derivatives [FL‐no: 13.134, 14.001, 14.004, 14.007, 14.030, 14.038, 14.039, 14.041, 14.042, 14.045, 14.046, 14.047, 14.058, 14.059, 14.060, 14.061, 14.065, 14.066, 14.068, 14.071, 14.072 and 14.164], the Panel agrees with the JECFA conclusion ‘no safety concern at estimated levels of intake as flavouring substances’ based on the MSDI approach.
Documentation provided to EFSA
Ballantyne, 2017. 6‐Methylquinoline: Transgenic gene mutation assay in Muta(™) mice. Covance Laboratories Ltd. Study no. 8361973. 09 November 2017. Unpublished report submitted by Firmenich to EFSA.
Bauter MR, 2012a. 2‐Acetylpyrrole: palatability/toxicity study: a 14‐day dietary study in rats. Product Safety Labs. Study no. 31097. January 9, 2012. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Bauter MR, 2012b. Draft Report. 2‐Acetylpyrrole: A 90‐day dietary study in rats. Product Safety Labs. Study no. 33327. Marts 2, 2012. Unpublished report submitted by EFFA to FLAVIS Secretariat.
DG SANCO (Directorate General for Health and Consumer Affairs), 2012. Information from DG SANCO 07/02 2012, concerning two lists of 85 and 15 non‐supported substances and one list of 30 substances for which no data have been submitted or which are duplicates. FLAVIS.2.23rev1.
DG SANCO (Directorate General for Health and Consumer Affairs), 2013. Information from DG SANCO 14/05 2013, concerning a list of 18 non‐supported substances. FLAVIS.2.26.
DG SANCO (Directorate General for Health and Consumer Affairs), 2014. Information from DG SANCO concerning use levels and poundage data of flavouring substances. Unpublished communication submitted from DG SANCO to EFSA.
EFFA (European Flavour Association), 2012. Addendum of Additional Data Relevant to the Flavouring Group Evaluation of the Chemical Group 28 (Annex I of 1565/2000/EC) Pyridine, Pyrrole, Indole and Quinoline Derivatives [JECFA/WHO FAS 63] Used as Flavouring Substances. June 2012. FLAVIS/8.163.
Honarvar N, 2004. Final report. Micronucleus assay in bone marrow cells of the mouse with BASE 3. RCC‐Cytotest Cell Research GmbH. Study no. 809103. February 23, 2004. Unpublished report submitted by EFFA to FLAVIS Secretariat.
IOFI (International Organization of the Flavor Industry), 2013. Global poundage survey 2010. IOFI Global Poundage Survey Committee, Geneva, Switzerland 2013, pp. 1‐291.
Kappeler KV, 2013a. A 14‐Day Oral (Dietary) Toxicity Study of 1‐Furfurylpyrrole in Rats. WIL Research. Study no. WIL‐968003. 23 July 2013. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Kappeler KV, 2013b. A 90‐Day Oral (Dietary) Toxicity Study of 1‐Furfurylpyrrole in Sprague Dawley Rats. WIL Research. Study no. WIL‐968004. 15 April 2013. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Keig‐Shevlin Z, 2016. 6‐methylquinoline: Rat alkaline Comet assay. Covance Laboratories Ltd. Study no. 8302669. 25 January 2016. Unpublished final report submitted by EFFA to DG SANTE.
Kojima K, 2006. Studies or tests for the standards for foods, foods additives, etc. 90‐Day repeated oral dose toxicity study of isoquinoline in rats. Hatano Research Institute, Food and Drug Safety Center, Japan. Study No. C‐06‐010. Unpublished report submitted by EFFA to FLAVIS Secretariat. [In Japanese ‐ English translation included]
Marumo H, 2008. 90‐day repeated oral dose toxicity study of pyrrole in rats. Final report. Hatano Research Institute, Food and Drug Safety Center. Study no. C‐06‐009. January 25, 2008. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Mendes O, 2012. Draft Report. 2‐Acetylpyrrole: A 10‐day oral metabolism/toxicity study in rats. Product Safety Labs. Study no. 34496. June 11‐21, 2012. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Morgareidge K, 1971. 90‐Day feeding study with N‐furfurylpyrrole in rats. Food and Drug Research Laboratories. February 12, 1971. Unpublished incomplete report submitted by EFFA to FLAVIS Secretariat.
Nakajima Y, 2005. In vivo micronucleus study in mice. 6‐Methylquinoline. Experiment No: 8818‐ 079‐254. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Schriewer L, Vleminckx C, Rigaux G, Ottogali M and Lakhanisky T, 1993. Evaluation of the genotoxic potential of pyridine and methylated pyridines. Pyridine, 2‐methylpyridine, 3‐methylpyridine, 4‐methylpyridine. C. DNA single‐stranded breaks measurement in V79 cells. Reilly Industries. Institute of Hygiene and Epidemiology. Vleminckx, C. Study no. IHE‐TOX‐1003. EPA Doc 86‐930000182, microfiche no. OTS0538163. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Til HP and van der Meulen HC, 1971. Subchronic (90‐day) toxicity study with 2‐acetylpyridine in albino rats. Centraal Instituut Voor Voedingsonderzioek, Netherlands. Report no. R3373. February, 1971. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Vleminckx C, Ottogali M, Schriewer L, Rigaux G and Lakhanisky T, 1993a. Evaluation of the genotoxic potential of pyridine and methylated pyridines. Pyridine, 2‐methylpyridine, 3‐methylpyridine, 4‐methylpyridine. A. Salmonella/microsome test. Reilly Industries with cover letter dated 03/29/93. Study no. IHE‐TOX‐1003. Date 3/29/93. EPA Doc. 86‐930000180, microfiche no. OTS0538163. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Vleminckx C, Rigaux G, Schriewer L, Ottogali M and Lakhanisky T, 1993b. Evaluation of the genotoxic potential of pyridine and methylated pyridines. Pyridine, 2‐methylpyridine, 3‐methylpyridine, 4‐methylpyridine. B. HGPRTgene mutation assay in V79 cells. Reilly Industries. Study no. IHE‐TOX‐1003. EPA Doc. 86‐930000181, microfiche no. OTS0538163. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Abbreviations
- bw
Body Weight
- CAS
Chemical Abstract Service
- CEF
Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CHO
Chinese hamster ovary (cells)
- CoE
Council of Europe
- DNA
Deoxyribonucleic acid
- EPA
United States Environmental Protection Agency
- FAO
Food and Agriculture Organization of the United Nations
- FEMA
Flavor and Extract Manufacturers Association
- FGE
Flavouring Group Evaluation
- FLAVIS (FL)
Flavour Information System (database)
- GLP
Good Laboratory Practise
- GC‐MS
Gas chromatography‐mass spectrometry
- ID
Identity
- i.p.
Intraperitoneal
- IR
Infrared spectroscopy
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- MN
micronucleus
- MSDI
Maximised Survey‐derived Daily Intake
- mTAMDI
Modified Theoretical Added Maximum Daily Intake
- NCE
Normochromatic erythrocyte
- No
Number
- NOAEL
No observed adverse effect level
- NTP
National Toxicology Program
- OECD
Organisation for Economic Co‐operation and Development
- PCE
Polychromatic erythrocyte
- PCR
Polymerase Chain Reaction
- pfu
plaque‐forming units
- SCE
Sister chromatic exchange
- SCF
Scientific Committee on Food
- SDH
sorbitol dehydrogenase
- WHO
World Health Organization
Appendix A – Summary of genotoxicity data
1.
Appendix B – Summary of toxicity data
1.
Appendix C – Summary of safety evaluations
1.
Appendix D – Exposure data
1.
Suggested citation: EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , Silano V, Bolognesi C, Castle L, Chipman K, Cravedi J‐P, Engel K‐H, Fowler P, Franz R, Grob K, Gürtler R, Husøy T, Kärenlampi S, Milana MR, Pfaff K, Riviere G, Srinivasan J, Tavares Poças MF, Tlustos C, Wölfle D, Zorn H, Benigni R, Binderup M‐L, Brimer L, Marcon F, Marzin D, Mosesso P, Mulder G, Oskarsson A, Svendsen C, van Benthem J, Anastassiadou M, Carfì M and Mennes W, 2018. Scientific opinion on flavouring group evaluation 77, revision 3 (FGE.77Rev3): consideration of pyridine, pyrrole and quinoline derivatives evaluated by JECFA (63rd meeting) structurally related to pyridine, pyrrole, indole and quinoline derivatives evaluated by EFSA in FGE.24Rev2. EFSA Journal 2018;16(4):5226, 51 pp. 10.2903/j.efsa.2018.5226
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00159
Panel members: Claudia Bolognesi, Laurence Castle, Kevin Chipman, Jean‐Pierre Cravedi, Karl‐Heinz Engel, Paul Fowler, Roland Franz, Konrad Grob, Rainer Gürtler, Trine Husøy, Sirpa Kärenlampi, Wim Mennes, Maria Rosaria Milana, Karla Pfaff, Gilles Riviere, Jannavi Srinivasan, Maria de Fátima Tavares Poças, Vittorio Silano, Christina Tlustos, Detlef Wölfle and Holger Zorn
Acknowledgements: The Panel wishes to thank the hearing experts: Vibe Beltoft and Karin Nørby and the EFSA staff Annamaria Rossi for the support provided to this scientific output.
Adopted: 7 March 2018
Notes
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Commission Regulation No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
Consideration of 88 flavouring substances considered by EFSA for which EU production volumes/anticipated production volumes have been submitted on request by DG SANCO.
Annex III, Commission Regulation No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. Official Journal of the European Communities 19.7.2000, L 180, p. 8–16.
The text is taken verbatim from the indicated reference source, but text related to substances not included in the present FGE has been removed.
The text is taken verbatim from the indicated reference source, but text related to substances not included in the present FGE has been removed.
This refers to a chromosomal aberration assay using CHO cells, where positive results were obtained at concentrations that show marked inhibition of DNA synthesis (Table A.2).
* significant at 0.05 according to Dunnett's test.
** significant at 0.01 according to Dunnett's test.
[FL‐no: 13.134] has been removed from FGE.24 Revision 2.
References
- Aeschbacher HU, Wolleb U, Loliger J, Spadone JC and Liardon R, 1989. Contribution of coffee aroma constituents to the mutagenicity of coffee. Food and Chemical Toxicology, 27, 227–232. [DOI] [PubMed] [Google Scholar]
- Anderson D and Styles JA, 1978. An evaluation of 6 short‐term tests for detecting organic chemical carcinogens. Appendix 2. The bacterial mutation test. British Journal of Cancer, 37, 924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton LD, Dearfield KL, Spanggord RJ, Riccio ES and Mortelmans K, 1987. Comparative mutagenicity of halogenated pyridines in the Salmonella typhimurium/mammalian microsome test. Mutation Research, 176, 185–198. [DOI] [PubMed] [Google Scholar]
- Cramer GM, Ford RA and Hall RL, 1978. Estimation of toxic hazard ‐ a decision tree approach. Food and Cosmetics Toxicology, 16, 255–276. [DOI] [PubMed] [Google Scholar]
- Curvall M, Florin I and Jansson T, 1982. Mutagenicity of some indoles and related compounds in the Ames test. Toxicology, 23, 1–10. [DOI] [PubMed] [Google Scholar]
- Debnath AK, Lopez de Compadre RL and Hansch C, 1992. Mutagenicity of quinolines in Salmonella typhimurium TA100. A QSAR study based on hydrophobicity and molecular orbital determinants. Mutation Research, 280, 55–65. [DOI] [PubMed] [Google Scholar]
- Dong M, Schmeltz I, La Voie E and Hoffmann D, 1978. Aza‐arenes in the respiratory environment analysis and assays for mutagenicity In: Jones PW. and Freudenthal RI. (eds.). Carcinogenesis ‐ A comprehensive survey. Vol 3 Raven Press, New York: pp. 97–108. [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Scientific Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with food (AFC) on a request from the Commission related to Flavouring Group Evaluation 03 (FGE.03): acetals of branched‐ and straight‐chain aliphatic saturated primary alcohols and branched‐ and straight‐chain saturated aldehydes, and an orthoester of formic acid, from chemical groups 1 and 2 (Commission Regulation (EC) No 1565/2000 of 18 July 2000). EFSA Journal 2004;2(11):107, 59 pp. 10.2903/j.efsa.2004.107 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 24 (FGE.24): pyridine, pyrrole, indole and quinoline derivatives from chemical group 28 (Commission Regulation (EC) No 1565/2000 of 18 July 2000). EFSA Journal 2006;4(2):372, 63 pp. 10.2903/j.efsa.2006.372 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Scientific Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with food (AFC) related to Flavouring Group Evaluation 77: consideration of Pyridine, Pyrrole and Quinoline Derivatives evaluated by JECFA (63rd meeting) structurally related to Pyridine, Pyrrole, Indole and Quinoline Derivatives evaluated by EFSA in FGE.24Rev1 (2008) (Commission Regulation (EC) No 1565/2000 of 18 July 2000). EFSA Journal 2009;7(1):936, 4pp. 10.2903/j.efsa.2009.936 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2011. Scientific Opinion on Flavouring Group Evaluation 96 (FGE.96): consideration of 88 flavouring substances considered by EFSA for which EU production volumes/anticipated production volumes have been submitted on request by DG SANCO. Addendum to FGE. 51, 52, 53, 54, 56, 58, 61, 62, 63, 64, 68, 69, 70, 71, 73, 76, 77, 79, 80, 83, 84, 85 and 87. EFSA Journal 2011;9(12):1924, 60 pp. 10.2903/j.efsa.2011.1924 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2013. Scientific Opinion on Flavouri2ng Group Evaluation 24, Revision 2 (FGE.24Rev2): pyridine, pyrrole, indole and quinoline derivatives from chemical group 28. EFSA Journal 2013;11(11):3453, 64 pp. 10.2903/j.efsa.2013.3453 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2014. Scientific Opinion on Flavouring Group Evaluation 77, Revision 1 (FGE.77Rev1): consideration of Pyridine, Pyrrole and Quinoline Derivatives evaluated by JECFA (63rd meeting) structurally related to Pyridine, Pyrrole, Indole and Quinoline Derivatives evaluated by EFSA in FGE.24Rev2. EFSA Journal 2014;12(2):3586, 50pp. 10.2903/j.efsa.2014.3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2015. Scientific Opinion on Flavouring Group Evaluation 77, Revision 2 (FGE.77Rev2): consideration of Pyridine, Pyrrole and Quinoline Derivatives evaluated by JECFA (63rd meeting) structurally related to Pyridine, Pyrrole, Indole and Quinoline Derivatives evaluated by EFSA in FGE.24Rev2 (2015). EFSA Journal 2015;13(1):3997, 58 pp. 10.2903/j.efsa.2015.3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger M, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Younes M, Aquilina G, Crebelli R, Gürtler R, Hirsch‐Ernst KI, Mosesso P, Nielsen E, van Benthem J, Carfì M, Georgiadis N, Maurici D, Parra Morte J and Schlatter J, 2017. Scientific Opinion on the clarification of some aspects related to genotoxicity assessment. EFSA Journal 2017;15(12):5113, 25 pp. 10.2903/j.efsa.2017.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epler JL, Rao TK and Guerin MR, 1979. Evaluation of feasibility of mutagenic testing of shale oil products and effluents. Environmental Health Perspectives, 30, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin I, Rutberg L, Curvall M and Enzell CR, 1980. Screening of tobacco smoke constituents for mutagenicity using the Ames’ test. Toxicology, 18, 219–232. [DOI] [PubMed] [Google Scholar]
- Fujita H, Aoki N and Sasaki M, 1994. Mutagenicity test of food additives with Salmonella typhimurium TA97 and TA102 (IX*). Annual Report of Tokyo Metropolitan Research Laboratory of Public Health, 45, 191–199 (In Japanese). [Google Scholar]
- Fukushima S, Ishihara Y, Nishio O, Ogiso T, Shirai T and Ito N, 1981. Carcinogenicities of quinoline derivatives in F344 rats. Cancer Letters, 14, 115–123. [DOI] [PubMed] [Google Scholar]
- Hashizume T, Santo H, Tsujisawa H, Kosaka K, Ozawa T, Yamashita M and Kinae N, 1991. Mutagenic activities of tryptophan metabolites before and after nitrite treatment. Food and Chemical Toxicology, 29, 839–844. [DOI] [PubMed] [Google Scholar]
- Haworth S, Lawlor T, Mortelmans K, Speck W and Zeiger E, 1983. Salmonella mutagenicity test results for 250 chemicals. Environmental Mutagenesis, 5(Suppl. 1), 3–142. [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1995. Evaluation of certain food additives and contaminants. Forty‐fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives. 14–23 February 1995. WHO Technical Report Series, no. 859. Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1996. Toxicological evaluation of certain food additives. Forty‐fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives and contaminants. WHO Food Additives Series: 35. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1997. Evaluation of certain food additives and contaminants. Forty‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 6–15 February 1996. WHO Technical Report Series, no. 868. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1999. Evaluation of certain food additives and contaminants. Forty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. Rome, 17–26 June 1997. WHO Technical Report Series, no. 884. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2005a. Compendium of food additive specifications. Addendum 12. Joint FAO/WHO Expert Committee of Food Additives 63rd session. Rome, 8–17 June 2004. FAO Food and Nutrition paper 52 Add. 12.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2005b. Evaluation of certain food additives. Sixty‐third report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 928. Geneva, 8–17 June 2004.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006a. Safety evaluation of certain food additives and contaminants. Sixty‐third Meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 54. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006b. Sixty‐seventh Meeting. Rome, 20‐29 June 2006, Summary and Conclusions. Issued 7 July 2006.
- Kaden DA, Hites RA and Thilly WG, 1979. Mutagenicity of soot and associated polycyclic aromatic hydrocarbons to Salmonella typhimurium . Cancer Research, 39, 4152–4159. [PubMed] [Google Scholar]
- Kim E‐H, Shinohara K, Murakami H and Omura H, 1987. Pyrrole compounds as food mutagens. Journal of the Faculty of Agriculture, Kyushu University, 31, 279–285. [Google Scholar]
- Kim HY, Hincks JR, Huie JM, Yost GS and Coulombe RA Jr, 1989. Deuterated 3‐methylindole is less genotoxic than 3‐methylindole. Toxicologist, 9, 154. [Google Scholar]
- La Voie EJ, Shigematsu A, Adams EA, Rigotty J and Hoffmann D, 1984. Tumor‐initiating activity of quinoline and methylated quinolines on the skin of SENCAR mice. Cancer Letters, 22, 269–273. [DOI] [PubMed] [Google Scholar]
- Laws GM, Skopek TR, Reddy MV, Storer RD and Glaab WE, 2001. Detection of DNA adducts using a quantitative long PCR technique and the fluorogenic 5’ nuclease assay (TaqMan). Mutation Research, 484, 3–18. [DOI] [PubMed] [Google Scholar]
- Lee H, Bian SS and Chen YL, 1994. Genotoxicity of 1,3‐dithiane and 1,4‐dithiane in the CHO/SCE assay and the Salmonella/microsomal test. Mutation Research, 321, 213–218. [DOI] [PubMed] [Google Scholar]
- Longfellow D, 1997. Mutagenicity studies. Benzothiazole. Short‐term test program sponsored by the Division of Cancer Etiology, National Cancer Institute.
- Nagao M, Yahagi T, Seino Y, Sugimura T and Ito N, 1977. Mutagenicities of quinoline and its derivatives. Mutation Research, 42, 335–342. [DOI] [PubMed] [Google Scholar]
- Nichols WK, Bossio JI and Yost GS, 2000. 3‐Methylindole (3MI) causes both apoptosis and necrosis in cultured human lung cells. Toxicologist, 54, 114. [Google Scholar]
- NTP (National Toxicology Program), 1986. Genetic toxicology (6‐Methylquinoline). Study no. 741114.
- Ochiai M, WakabaYashi K, Sugimura T and Nagao M, 1986. Mutagenicities of indole and 30 derivatives after nitrite treatment. Mutation Research, 172, 189–197. [DOI] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2013. Test No. 488: Transgenic Rodent Somatic and Germ Cell Gene Mutation Assays. OECD Guidelines for the Testing of Chemicals, Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 2014. Test No. 489: In Vivo Mammalian Alkaline Comet Assay. OECD Guidelines for the Testing of Chemicals, Section 4.
- Pai V, Bloomfield SF, Jones J and Gorrod JW, 1978. Mutagenicity testing of nitrogenous compounds and their N‐oxidised products using Trp+ reversion in E. coli In: Gorrod JW. (ed.). Biological Oxidation of Nitrogen in organic compounds. Elsevier North Holland Biomedical Press, New York: pp. 375–382. [Google Scholar]
- Posternak NM, Linder A and Vodoz CA, 1969. Summaries of toxicological data. Toxicological tests on flavouring matters. Food and Cosmetics Toxicology, 7, 405–407. [DOI] [PubMed] [Google Scholar]
- Reddy MV, Storer RD, Laws GM, Armstrong MJ, Barnum JE, Gara JP, McKnight CG, Skopek TR, Sina JF, DeLuca JG and Galloway SM, 2002. Genotoxicity of naturally occurring indole compounds: correlation between covalent DNA binding and other genotoxicity tests. Environmental and Molecular Mutagenesis, 40, 1–17. [DOI] [PubMed] [Google Scholar]
- Reddy MV, Laws GM, Armstrong MJ, Galloway SM and Storer RD, 2003. In vivo genotoxicity of 3‐methylindole and melatonin: formation of DNA adducts but not micronuclei. Proceedings of the American Association for Cancer Research, 44, 291–292. [Google Scholar]
- Regal KA, Laws GM, Yuan C, Yost GS and Skiles GL, 2001. Detection and characterization of DNA adducts of 3‐methylindole. Chemical Research in Toxicology, 14, 1014–1024. [DOI] [PubMed] [Google Scholar]
- Sasagawa C and Matsushima T, 1991. Mutagen formation on nitrile treatment of indole compounds derived from indole‐glucosinolate. Mutation Research, 250, 169–174. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food), 1999. Opinion on a programme for the evaluation of flavouring substances (expressed on 2 December 1999). Scientific Committee on Food. SCF/CS/FLAV/TASK/11 Final 6/12/1999. Annex I to the minutes of the 119th Plenary meeting. European Commission, Health & Consumer Protection Directorate‐General.
- Sideropoulos AS and Specht SM, 1984. Evaluation of microbial testing methods for mutagenicity of quinoline and its derivatives. Current Microbiology, 11, 59–65. [Google Scholar]
- Sugimura T, Sato S, Nagao M, Yahagi T, Matsushima T, Seino Y, Takeuchi M and Kawachi T, 1976. Overlapping of carcinogens and mutagens. In: Magee PN, Takayama S, Sugimura T and Matsushima T (eds.). Proceedings of the International Symposium of the Princess Takamatsu Cancer Research Fund, Tokyo, 1975. Fundamentals In Cancer Prevention. Vol. 6. University Par Press, Baltimore, pp. 191–215.
- Szybalski W, 1958. Special microbiological systems. II. Observations on chemical mutagenesis in microorganisms. Annals of the New York Academy of Sciences, 76, 475–489. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kamiya M, Sengoku Y, Kohda K and Kawazoe Y, 1988. Deprivation of the mutagenic property of quinoline: inhibition of mutagenic metabolism by fluorine substitution. Chemical and Pharmaceutical Bulletin, 36, 4630–4633. [DOI] [PubMed] [Google Scholar]
- Vance WA, Okamoto HS and Wang YY, 1986. Structure‐activity relationships of nitro and methyl‐nitro derivatives of indoline, indole, indazole and benzimidazole in Salmonella typhimurium . Mutation Research, 173, 169–176. [DOI] [PubMed] [Google Scholar]
- Wang CJ, Lin YL and Lin JK, 1994. Mutagenicity and cytotoxicity of nitropyrrole compounds derived from the reaction of 2‐acetyl pyrrole with nitrite. Food and Chemical Toxicology, 32, 839–844. [DOI] [PubMed] [Google Scholar]
- Whittaker SG, Zimmermann FK, Dicus B, Piegorsch WW, Fogel S and Resnick MA, 1989. Detection of induced mitotic chromosome loss in Saccharomyces cerevisiae ‐ an interlaboratory study. Mutation Research, 224, 31–78. [DOI] [PubMed] [Google Scholar]
- Wild D, King MT, Gocke E and Eckhard K, 1983. Study of artificial flavouring substances for mutagenicity in the Salmonella/microsome, BASC and micronucleus tests. Food and Chemical Toxicology, 21, 707–719. [DOI] [PubMed] [Google Scholar]
- Williams GM, 1984. DNA damage and repair tests for the detection of genotoxic agents. Food Additives and Contaminants, 1, 173–178. [DOI] [PubMed] [Google Scholar]
- Zeiger E, Anderson B, Haworth S, Lawlor T and Mortelmans K, 1992. Salmonella mutagenicity tests: V. Results from the testing of 311 chemicals. Environmental and Molecular Mutagenesis, 19, 2–141. [DOI] [PubMed] [Google Scholar]
- Zimmermann FK, Mayer VW, Scheel I and Resnick MA, 1985a. Acetone, methyl ethyl ketone, ethyl acetate, acetonitrile and other polar aprotic solvents are strong inducers of aneuploidy in Saccharomyces cerevisiae . Mutation Research, 149, 339–351. [DOI] [PubMed] [Google Scholar]
- Zimmermann FK, Groschel‐Stewart U, Scheel I and Resnick MA, 1985b. Genetic change may be caused by interference with protein‐protein interactions. Mutation Research, 150, 203–210. [DOI] [PubMed] [Google Scholar]
- Zimmermann FK, Mayer VW, Taylor‐Mayer RE, Groschel‐Stewart U and Scheel I, 1985c. Induction of mitotic aneuploidy in yeast with aprotic polar solvents In: Zimmermann PK. and Taylor RE. (eds.). Mutagenicity Testing in Environmental Control. Ellis Horwood, Chichester: pp. 166–179. [Google Scholar]
- Zimmermann FK, Henning JH, Schell I and Oehler M, 1986. Genetic and anti‐tubulin effects induced by Pyridine derivatives. Mutation Research, 163, 23–31. [DOI] [PubMed] [Google Scholar]


