Abstract
The present opinion deals with the re‐evaluation of propane‐1,2‐diol alginate (E 405) when used as a food additive. The Panel noted that absorption, distribution, metabolism and excretion (ADME) data on propane‐1,2‐diol alginate gave evidence for the hydrolysis of this additive into propane‐1,2‐diol and alginic acid. These two compounds have been recently re‐evaluated for their safety of use as food additives (EFSA ANS Panel, 2017, 2018). Consequently, the Panel considered in this opinion the major toxicokinetic and toxicological data of these two hydrolytic derivatives. No adverse effects were reported in subacute and subchronic dietary studies with propane‐1,2‐diol alginate. The available data did not indicate a genotoxic concern for propane‐1,2‐diol alginate (E 405) when used as a food additive. Propane‐1,2‐diol alginate, alginic acid and propane‐1,2‐diol were not of concern with respect to carcinogenicity. The Panel considered that any adverse effect of propane‐1,2‐diol alginate would be due to propane‐1,2‐diol. Therefore, the acceptable daily intake (ADI) of the food additive E 405 is determined by the amount of free propane‐1,2‐diol and the propane‐1,2‐diol released from the food additive after hydrolysis. According to the EU specification, the concentration of free and bound propane‐1,2‐diol amounts to a maximum of 45% on a weight basis. On the worst‐case assumption that 100% of propane‐1,2‐diol would be systemically available and considering the ADI for propane‐1,2‐diol of 25 mg/kg body weight (bw) per day, the Panel allocated an ADI of 55 mg/kg bw per day for propane‐1,2‐diol alginate. The Panel concluded that exposure estimates did not exceed the ADI in any of the population groups from the use of propane‐1,2‐diol alginate (E 405) as a food additive. Therefore, the Panel concluded that there is no safety concern at the authorised use levels.
Keywords: propylene glycol alginate, propane‐1, 2‐diol alginate, (E 405), alginic acid, propane‐1, 2‐diol, food additive
Summary
The present opinion deals with the re‐evaluation of propane‐1,2‐diol alginate (E 405) when used as a food additive.
Propane‐1,2‐diol alginate (E 405) is authorised as a food additive in the European Union (EU) in accordance with Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/20121.
Propane‐1,2‐diol alginate was previously evaluated by the Scientific Committee for Food (SCF) and the Joint FAO/WHO Expert Committee on Food Additives (JECFA), both bodies considering that the acceptable daily intake (ADI) for propane‐1,2‐diol alginate should be limited only by the amount of free propane‐1,2‐diol and propane‐1,2‐diol esterified with alginic acid, hydrolysed after oral exposure and released (JECFA, 1993; SCF, 1994). While the SCF established for propane‐1,2‐diol alginate an ADI of 25 mg/kg body weight (bw) per day, expressed as propane‐1,2‐diol, JECFA allocated an ADI of 70 mg propane‐1,2‐diol alginate/kg bw per day.2
Propane‐1,2‐diol alginate as defined by Commission Regulation (EU) No 231/2012 is the ester of alginic acid with propane‐1,2‐diol. It varies in composition according to its degree of esterification and the percentage of free and neutralised carboxyl groups in the molecule.
In vitro experiments have shown that propane‐1,2‐diol alginate is partially hydrolysed by simulated gastric and intestinal juices to propane‐1,2‐diol and alginic acid. In vivo studies using autoradiography gave also evidence for a partial but not complete hydrolysis to propane‐1,2‐diol and alginic acid. According to previous EFSA assessments of these two hydrolytic moieties, propane‐1,2‐diol is absorbed and its metabolites would enter the normal energy‐forming pathways. However, together with any non‐hydrolysed material, the alginate moiety is not absorbed and excreted in the faeces, confirming the data previously provided in the assessment on the safety of alginic acid and its salts (EFSA ANS Panel, 2017). The Panel noted that absorption, distribution, metabolism and excretion (ADME) data on propane‐1,2‐diol alginate gave evidence for the hydrolysis of this additive into propane‐1,2‐diol and alginic acid. These two compounds have been recently re‐evaluated for their safety of use as food additives E 400 and E 1520 (EFSA ANS Panel, 2017, 2018). Consequently, the Panel considered in this opinion the major toxicokinetic and toxicological data of these two hydrolytic derivatives.
The acute toxicity of propane‐1,2‐diol alginate, alginic acid and its salts, and propane‐1,2‐diol was considered to be low.
Although various subacute and subchronic dietary studies with propane‐1,2‐diol alginate in laboratory animals were available to the Panel, several of these studies were of limited relevance for risk assessment due to the low numbers of animals per group and/or limited reporting. None of these studies demonstrated adverse effects.
Propane‐1,2‐diol alginate was tested in several in vitro assays (point mutation in bacteria, chromosomal aberrations in mammalian cells) and in vivo (host‐mediated assay, dominant lethal and bone marrow chromosome aberration in rat) that, despite some limitations, did not reveal any genotoxic effect. In addition, alginic acid and propane‐1,2‐diol, the two hydrolytic metabolites of propane‐1,2‐diol alginate, were considered by EFSA of no genotoxic concern (EFSA ANS Panel, 2017, 2018). The Panel therefore noted that the available data did not indicate a genotoxic concern for propane‐1,2‐diol alginate (E 405) when used as a food additive.
Propane‐1,2‐diol alginate, alginic acid and propane‐1,2‐diol were not of concern with respect to carcinogenicity. No adverse effects, including neoplastic findings, were reported in a 2‐year study in rats administered 2,500 mg propane‐1,2‐diol/kg bw per day for 2 years (Gaunt et al., 1972). The SCF and JECFA used the study in rats to establish an ADI of 25 mg propane‐1,2‐diol/kg bw per day using an uncertainty factor of 100, the Panel agreed with this ADI (EFSA ANS Panel, 2018).
In limited two‐ or three‐generation reproductive toxicity studies with propane‐1,2‐diol alginate and alginate, no adverse effects were reported up to 2,500 mg/kg bw per day. In prenatal developmental toxicity studies with propane‐1,2–diol alginate in rats, hamsters and rabbits, no developmental effects were observed up to the highest dose tested (~ 700–800 mg propane–1,2‐diol alginate/kg bw per day). No prenatal developmental toxicity studies were available for alginates. Propane‐1,2‐diol induced no treatment‐related effects in two reproductive toxicity studies (mice and rats) and six developmental studies in mice, rats, hamsters and rabbits, no treatment‐related effects were observed at the highest doses tested (1,000 mg propane‐1,2‐diol/kg bw per day and higher).
The Panel considered that any adverse effect of propane‐1,2‐diol alginate would be due to propane‐1,2‐diol. Therefore, the ADI of the food additive E 405 is determined by the amount of free propane‐1,2‐diol and the propane‐1,2‐diol released from the food additive after hydrolysis. According to the EU specification, the concentration of free and bound propane‐1,2–diol amounts to a maximum of 45% on a weight basis. On the worst‐case assumption that 100% of propane‐1,2‐diol (free and bound) would be systemically available and considering the ADI for propane‐1,2‐diol of 25 mg/kg bw per day, the Panel allocated an ADI of 55 mg/kg bw per day for propane‐1,2‐diol alginate (100/45 × 25).
Due to the discrepancies observed between the Mintel Global New Products Databases (GNPDs) and the reported use levels data, the Panel considered that the reported use levels for the refined exposure assessment scenarios are not covering sufficiently the present use of the additive. As a matter of fact, the Panel noted that use levels were reported for only one food category (‘FC 14.1.2 Beer and malt beverages’) out of the 21 in which propane‐1,2‐diol alginate (E 405) is authorised.
Thus, considering that (1) this food category does not cover the uses identified in the Mintel's GNPD, (2) this food category is not relevant to estimate the exposure to the food additive for the younger population groups, (3) use levels were reported only for niche products of beer, (4) low percentage (< 1%) of beers are labelled with the additive according to the Mintel's GNPD, the Panel decided not to perform refined exposure scenarios. Only the regulatory maximum level exposure assessment scenario is presented in the current assessment. As this scenario is not considering the consumption of food supplements and foods for special medical purposes (FSMP), the possible additional exposure from their consumption was also investigated.
Estimates of exposure assessment scenarios did not exceeded the ADI of 55 mg/kg bw per day for propane‐1,2‐diol alginate (E 405) for any population groups.
The Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure to propane‐1,2‐diol alginate (E 405) from its use as a food additive according to Annex II to Regulation (EC) No 1333/2008 in the regulatory maximum level exposure scenario (Section 3.4.1).
The Panel noted that food categories which may contain propane‐1,2‐diol alginate (E 405) due to carry‐over (Annex III, Part 1) were not considered in any of the current exposure assessment scenarios.
The Panel noted that propane‐1,2‐diol available from propane‐1,2‐diol alginate (E 405) would add to the exposure to the food additive propane‐1,2‐diol (E 1520) previously re‐evaluated by the Panel (EFSA ANS Panel, 2018).
Propane‐1,2‐diol may be released from propane‐1,2‐diol alginate (E 405) at a maximum of 45% according to the EU specification. Based on this, and considering the highest P95 exposure level observed, the highest exposure to propane‐1,2‐diol was calculated to be:
in the regulatory maximum level exposure assessment scenario 17.8 mg/kg bw per day of propane‐1,2‐diol in toddlers (released from 39.6 mg/kg bw per day of propane‐1,2‐diol alginate (E 405));
in the regulatory maximum level exposure assessment scenario considering also the consumption of FSMPs, 21.1 mg/kg bw propane‐1,2‐diol per day in infants (released from 46.8 mg/kg bw per day of propane‐1,2‐diol alginate (E 405)).
Considering the overall metabolic and toxicity database, the Panel confirmed the previously established ADI for propane‐1,2‐diol alginate (E 405) of 25 mg/kg bw per day expressed as propane‐1,2‐diol. This corresponds to an ADI for propane‐1,2‐diol alginate (E 405) of 55 mg/kg bw per day, based on the concentration of free and bound propane‐1,2‐diol amounting to a maximum of 45%.
The Panel concluded that exposure estimates did not exceed the ADI in any of the population groups from the use of propane‐1,2‐diol alginate (E 405) as a food additive. Therefore, the Panel concluded that there is no safety concern at the authorised use levels.
The Panel recommended the European Commission to consider:
revising the maximum limits for the impurities of toxic elements (lead, mercury, cadmium and arsenic) in the EU specification for propane‐1,2‐diol alginate (E 405) in order to ensure that propane‐1,2‐diol alginate (E 405) as a food additive will not be a significant source of exposure to these toxic elements in food;
the inclusion of maximum limits for propylene oxide, mono‐ and diethylene glycol, and propylene carbonate in the EU specifications for propane‐1,2‐diol alginate (E 405);
the collection of more data on usage and use levels of propane‐1,2‐diol alginate (E 405) in order to perform a more realistic exposure assessment.
1. Introduction
The present opinion deals with the re‐evaluation of propane‐1,2‐diol alginate (E 405) when used as a food additive.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
Regulation (EC) No 1333/20083 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union. In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the European Union before 20 January 2009 has been set up under the Regulation (EU) No 257/20104. This Regulation also foresees that food additives are re‐evaluated whenever necessary in light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by a group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU5 of 2001. The report ‘Food additives in Europe 2000’6 submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010 the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference
The Commission asks the EFSA to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.1.3. Interpretation of Terms of Reference
This re‐evaluation refers exclusively to the uses of propane‐1,2‐diol alginate (E 405) as a food additive in food, including food supplements, and does not include a safety assessment of other uses of propane‐1,2‐diol l alginate (as described in Section 3.4.2). The Panel noted that the term propylene glycol alginate (PGA) is frequently used as a synonym for propane‐1,2‐diol alginate.
1.2. Information on existing evaluations and authorisations
Propane‐1,2‐diol alginate (E 405) is authorised as a food additive in the EU in accordance with Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/20127.
In the EU, propane‐1,2‐diol alginate (E 405) has been evaluated by the Scientific Committee for Food (SCF) in 1994 (SCF, 1994), who allocated for propane‐1,2‐diol alginate an acceptable daily intake (ADI) of 25 mg/kg body weight (bw) expressed as propane‐1,2‐diol.8 No toxicological data were specified. The SCF recommended a re‐evaluation of propane‐1,2‐diol which was performed in 1996 (SCF, 1997) and an ADI of 25 mg/kg bw per day was assigned. In 2018, the EFSA ANS Panel performed a re‐evaluation of propane‐1,2‐diol and derived an ADI of 25 mg/kg bw per day based on a chronic toxicity study in rats in which a NOAEL of 2,500 mg/kg bw per day was identified and using a safety factor of 100. In 2017, the EFSA Panel on Food Additives and Nutrient Sources added to Food (EFSA ANS Panel, 2017) re‐evaluated the safety of alginic acid and its sodium, potassium, ammonium and calcium salts (E 400–E 404) when used as food additives. The Panel concluded that ‘there was no need for a numerical ADI for alginic acid and its salts (E 400, E 401, E 402, E 403 and E 404)’.
Propane‐1,2‐diol alginate (E 405) was evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1993 (JECFA, 1993) who concluded that the ADI for propane‐1,2‐diol alginate is limited only by the amount of free propane‐1,2‐diol and propane‐1,2‐diol esterified with alginic acid, hydrolysed after oral exposure and released. Propane‐1,2‐diol alginate contains totally up to 36% propane‐1,2‐diol.9 On the assumption that all of the esterified propane‐1,2‐diol is hydrolysed, and ‘taking into account the ADI of 0–25 mg/kg by for propylene glycol, the Committee allocated an ADI of 0–70 mg/kg bw (100/36 x 25) to propylene glycol alginate’. The Committee stated that new toxicological studies are available for propane‐1,2‐diol but these data were not evaluated A re‐evaluation of propane‐1,2‐diol was recommended by JECFA. The ADI for propane‐1,2‐diol alginate should be reconsidered thereafter. In 1973, JECFA evaluated propane‐1,2‐diol E 1520) as a food additive and allocated an ADI of 0–25 mg/kg bw based on the level in rat and dog causing no toxicological effect of 2,500 mg/kg bw and using an uncertainty factor of 100 (JECFA, 1974). In 2001, JECFA evaluated propane‐1,2‐diol as a food flavouring; however, the Committee did not finalise the evaluation since it seeked confirmation that the substance was actually used as food flavouring (JECFA, 2002a,b). The Panel noted that propane‐1,2‐diol is not authorised as a flavouring substance in the EU according to Regulation 1334/2008.
Propane‐1,2‐diol alginate (E 405) has also been reviewed by the Nordic Council of Ministers (TemaNord, 2002a,b). It was concluded that the database is sufficient for evaluation of health hazard although most data are old.
Propane‐1,2‐diol alginate (E 405) belongs to the group of food additives that were found in jelly minicups, which were suspended in 2004 by the European Commission from being to be placed on the market and for import (Commission Decision 2004/37/EC; EC, 2004), following the measures taken and information provided by the different Member States. Jelly minicups are defined as ‘jelly confectionery of a firm consistence, contained in semi‐rigid minicups or minicapsules, intended to be ingested in a single bite by exerting pressure on the minicups or minicapsule to project the confectionery into the mouth.’
In 2004, the EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (EFSA AFC Panel, 2004) prepared a scientific opinion on a request from the European Commission related to the use of certain food additives derived from seaweed or non‐seaweed origin, including propane‐1,2‐diol alginate (E 405) in jelly minicups. The AFC Panel concluded that any of these gel‐forming additives or of any other type that gave rise to a confectionery product of a similar size, with similar physical and/or physicochemical properties and that could be ingested in the same way as the jelly minicups, would give rise to a risk for choking (EC, 2004). The use of these additives in jelly minicups is not authorised in the EU.10
In 2006, the EFSA AFC Panel prepared a scientific opinion on the use of formaldehyde as a preservative during the manufacture and preparation of food additives; the Panel estimated that exposure to gelling additives such as alginates containing residual formaldehyde at the levels of 50 mg/kg of additive would be of no safety concern (EFSA, 2006). Maximum limits (not more than 50 mg/kg) are established in the current EC Regulation for formaldehyde in several thickening food additives from algae origin including alginic acid and its salts (E 400–E 404) (EU Regulation No 231/2012).
The Committee for Medicinal Products for Human Use (CHMP) considered the procedure under Article 5(3) of Regulation (EC) No 726/2004 on the excipient, propane‐1,2‐diol in medicines for children as per questions posed (EMA, 2014b). In the final document (Questions & answers on propane‐1,2‐diol and esters in the context of the revision of the guideline on ‘Excipients in the label and package leaflet of medicinal products for human use’ (CPMP/463/00 Rev. 1, November 2014), the European Medicines Agency (EMA) concluded ‘Nevertheless, clinical data showed that in children from the age of 5 years and adult patients, up to 500 mg/kg per day of propane‐1,2‐diol could generally be considered safe. In the absence of compelling data this safety threshold is decreased to 50 mg/kg per day in children less than 5 years old, and even to 1 mg/kg per day in pre‐term and term neonates due to known immaturity of both metabolic and renal clearances of propane‐1,2‐diol in these populations’.
2. Data and methodologies
2.1. Data
The Panel was not provided with a newly submitted dossier. EFSA launched public calls for data,11 , 12 , 13 to collect relevant information from interested parties.
The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up to the last Working Group meeting before the adoption of the opinion.14 Attempts were made at retrieving relevant original study reports on which previous evaluations or reviews were based, however not always these were available to the Panel.
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database15) was used to estimate the dietary exposure.
The Mintel's Global New Products Database (GNPD) is an online database which was used for checking the labelling of products containing propane‐1,2‐diol alginate (E 405) within the EU's food products as the GNPD shows the compulsory ingredient information presented in the labelling of products.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing Guidances from the EFSA Scientific Committee.
The ANS Panel assessed the safety of propane‐1,2‐diol alginate (E 405) as a food additive in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance document: Guidance on submission for food additive evaluations by the Scientific Committee on Food (SCF, 2001) and taking into consideration the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
When the test substance was administered in the feed or in the drinking water, but doses were not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012) for studies in rodents or, in the case of other animal species, by JECFA (2000). In these cases, the daily intake is expressed as equivalent. When in human studies in adults (aged above 18 years) the dose of the test substance administered was reported in mg/person per day, the dose in mg/kg bw per day was calculated by the Panel using a body weight of 70 kg as default for the adult population as described in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012).
Dietary exposure to propane‐1,2‐diol alginate (E 405) from its use as a food additive was estimated combining food consumption data available within the EFSA Comprehensive European Food Consumption Database with the maximum permitted levels (MPLs). Different exposure scenarios were calculated (see Section 3.4.1). Uncertainties on the exposure assessment were identified and discussed.
In the context of this re‐evaluation, the Panel considered the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EC) No 257/2010 (EFSA ANS Panel, 2014).
3. Assessment
3.1. Technical data
3.1.1. Identity of the substances
Propane‐1,2‐diol alginate
Propane‐1,2‐diol alginate as defined by Commission Regulation (EU) No 231/2012 is the ester of alginic acid with propane‐1,2‐diol. It varies in composition according to its degree of esterification and the percentage of free and neutralised carboxyl groups in the molecule.
The substance has the CAS No 9005‐37‐2; the EINECS number is not available. The substance is also known by several synonyms such as hydroxypropyl alginate, 1,2‐propane diol ester of alginic acid and propylene glycol alginate.
Two isomers of the esterification are generally possible. Propane‐1,2‐diol alginate consists of 2‐hydroxypropyl alginate although lesser quantities of the structural isomer 1‐hydroxymethylethyl alginate may be present (Steiner and McNeely, 1951).
The structural formula of propane‐1,2‐diol alginate is presented in Figure 1.
Figure 1.
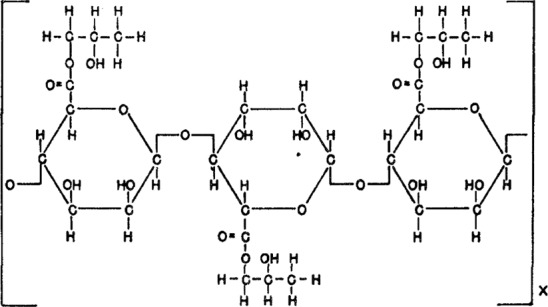
Structural formula of propane‐1,2‐diol alginate (Steiner and McNeely, 1951)
The Panel noted that the propane‐1,2‐diol group contains an asymmetric carbon (C2) atom. As the ester is synthesised by reaction of propylene oxide, two stereoisomers should be formed. On request of EFSA, one of the interested parties replied that the manufacturers of the food additive do not control the stereochemical configuration of the reaction product as this is not a requirement for propane‐1,2‐diol alginate in the EU specifications.
According to Commission Regulation (EU) No 231/2012 and JECFA specifications monograph (JECFA, 2006), the molecular formula of propane‐1,2‐diol alginate is (C9H14O7)n (esterified) and the typical average molecular weight of propane‐1,2‐diol alginate ranges from 10,000 to 600,000. According to JECFA (2006), the theoretical molecular weight of the structural unit is 234.21.
Aqueous solutions of propane‐1,2‐diol alginate are highly viscous at low concentrations, which is characteristic for alginates (Steiner and McNeely, 1951). Viscosity of propane‐1,2‐diol alginate solutions is a function of the degree of polymerisation, the degree of esterification and the presence of polyvalent metal ions like Ca2+. Typically, viscosity is in the range 25–500 mPa·s for a 1% solution and degrees of esterification from about 50% to about 90%. Generally, viscosity is increased by polyvalent metal ions. Propane‐1,2‐diol alginate with less than 60% esterification will react with calcium ions to give enhanced viscosity, while highly esterified propane‐1,2‐diol alginate is not affected by the presence of calcium ions (CRC, 1990; Voragen et al., 2012).
3.1.2. Specifications
The specifications for propane‐1,2‐diol alginate (E 405) as defined in the Commission Regulation (EU) No 231/2012 and by JECFA (2006) are listed in Table 1.
Table 1.
Specifications for propane‐1,2‐diol alginate (E 405) according to Commission Regulation (EU) No 231/2012 and JECFA (2006)
| Commission Regulation (EU) No 231/2012 | JECFA (2006) | |
|---|---|---|
| Definition | Propane‐1,2‐diol ester of alginic acid; varies in composition according to its degree of esterification and the percentage of free and neutralised carboxyl groups in the molecule | Propylene glycol alginate is an ester of alginic acid in which some of the carboxyl groups are esterified with propylene glycol, some neutralised with an appropriate alkali and some remain free |
| Assay | Yields, on the anhydrous basis, not less than 16% and not more than 20% of carbon dioxide | Yields, on the dried basis, not less than 16% and not more than 20% of carbon dioxide (CO2) |
| Description | Nearly odourless, white to yellowish brown fibrous or granular powder | White to yellowish brown filamentous, grainy, granular or powdered forms |
| Identification | ||
| Test for 1,2‐propanediol | Passes test (after hydrolysis) | – |
| Test for alginic acid | Passes test (after hydrolysis) | – |
| Solubility | – | Soluble in water giving a viscous, colloidal solution; soluble in up to 60% aqueous ethanol depending upon degree of esterification |
| Precipitate formation with sulfuric acid | – | To 10 mL of a 1% solution of the sample add 1 mL of sodium hydroxide TS. Heat in a boiling water bath for about 5 min, cool and add 1 mL of dilute sulfuric acid TS. A gelatinous precipitate is formed |
| Precipitate formation with lead acetate | – | To 5 mL of a 1% solution of the sample add 1 mL of lead acetate. A gelatinous precipitate is formed |
| Purity | ||
| Loss on drying | Not more than 20% (105°C, 4 h) | Not more than 20% (105°, 4 h) |
| Total propane‐1,2‐diol content | Not less than 15% and not more than 45% | Not less than 15% and not more than 45% |
| Free propane‐1,2‐diol content | Not more than 15% | Not more than 15% |
| Water‐insoluble matter | Not more than 2% on the anhydrous basis | Not more than 2% on the dried basis |
| Formaldehyde | Not more than 50 mg/kg | – |
| Arsenic | Not more than 3 mg/kg | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
Not more than 5 mg/kg Determine using an atomic absorption technique appropriate to the specified level |
| Mercury | Not more than 1 mg/kg | – |
| Cadmium | Not more than 1 mg/kg | – |
| Total plate count | Not more than 5,000 colonies per gram |
Not more than 5,000 colonies per gram Initially prepare a 10−1 dilution by adding a 50 g sample to 450 mL of Butterfield's phosphate buffered dilution water and homogenising in a high speed blender |
| Yeast and moulds | Not more than 500 colonies per gram | Not more than 500 colonies per gram |
| E. coli | Absent in 5 g | Negative by test |
| Salmonella spp. | Absent in 10 g | Negative by test |
The safety of use of formaldehyde as a processing aid during the storage and manufacturing of thickening agents from algae origin was evaluated by the EFSA (2006) and it was concluded that exposure to gelling additives such as alginic acid and its salts containing residual formaldehyde at the levels of 50 mg/kg of additive would be of no safety concern. The Panel noted that maximum limits (not more than 50 mg/kg) are established in the current EC Regulation for formaldehyde in alginic acid (E 400) and its salts (E 401–E 404) (EU No 231/2012). According to information gathered by the Panel, it is not clear if formaldehyde continues to be used for those needs by some manufacturers, whilst other manufacturers report not using it anymore (EFSA, 2006; Documentation provided to EFSA n. 14).
Formaldehyde has also been shown to be a natural component of most marine algae including brown seaweeds. The levels detected in seaweeds used for alginate extraction were 14 mg/kg for Laminaria digitata, 17 mg/kg for Fucus serratus and 23 mg/kg for Ascophyllum nodosum (Yang et al., 1998). Most of this formaldehyde is expected to be eliminated by drying during the manufacturing process of alginic acid and its salts.
A single‐laboratory validated colorimetric method has been developed for measuring residual formaldehyde in alginate products (Farrell, 2007; Documentation provided to EFSA n. 14). The estimated limit of detection (LOD) and limit of quantification (LOQ) for the method were 0.7 and 2.3 mg/kg, respectively. Residual formaldehyde levels in the five tested alginate products were in the range of 2–3 mg/kg. According to the author, this indicated that the chemical and heat treatments involved in the manufacturing process were efficient at removing formaldehyde from the product.
The Panel considered that it could be appropriate to define in the specifications for propane‐1,2‐diol alginate (E 405) a suitable validated analytical method of appropriate accuracy for the determination of formaldehyde.
Because of the polysaccharidic nature of this compound, it can be a substrate of microbiological contamination during storage. This has been demonstrated recently by the mycotoxin contaminations of gums (Zhang et al., 2014). The Panel noted that the differences in the microbiological criteria for propane‐1,2‐diol alginate between the specifications given by the EU Regulation and those given by JECFA are not decisive.
Pesticides are not used, nor needed in the production of seaweeds and according to industry (Documentation provided to EFSA n. 13), available analytical data did not show the presence of pesticides.
The Panel noted that, according to the EU specifications for propane‐1,2‐diol alginate (E 405) impurities of the toxic elements arsenic, cadmium, lead and mercury are accepted up to concentrations of 3, 1, 5 and 1 mg/kg, respectively. Contamination at such levels could have a significant impact on the exposure to these elements, for which the exposures already are close to the health‐based guidance values or benchmark doses (lower Confidence Limits) established by EFSA (EFSA CONTAM Panel, 2009a,b, 2010, 2012a, 2012b c 2014).
3.1.3. Manufacturing process
Propane‐1,2‐diol alginate is prepared by treatment of partially neutralised alginic acid with propylene oxide (Voragen et al., 2012). The esterification of the carboxylic acid group of alginic acid takes place slowly at room temperature but considerably faster at a slightly elevated temperature with an upper limit of approximately 75°C. The reaction is carried out in equipment suitable for operation under moderate pressure (Steiner and McNeely, 1951).
3.1.4. Methods of analysis in food
A method for the quantitative analysis of propane‐1,2‐diol alginate in beer has been reported by Diepenmaat‐Wolters et al. (1997). The polysaccharide is precipitated from beer using 70% alcohol. The propane‐1,2‐diol residues are saponified with ammonium hydroxide, and then subsequently hydrolysed with methanolic HCl and trifluoroacetic acid. The resulting free guluronic and mannuronic acid can be analysed by high‐performance anion‐exchange chromatography (HPAEC). Propane‐1,2‐diol alginate concentrations of > 10 mg/L can be determined quantitatively, below this level only qualitative detection is obtained. Recovery from spiked beers was found to be 60%.
3.1.5. Stability of the substance, and reaction and fate in food
As a result of the blocking of large fraction of the carboxyl groups with a propane‐1,2‐diol alginate ester, the alginate ester is soluble in acid solutions that will precipitate sodium alginate as alginic acid. In addition to acid resistance, propane‐1,2‐diol alginate has an improved resistance to precipitation by calcium and other metal salts. At alkaline pH, propane‐1,2‐diol alginate is gradually hydrolysed to propane‐1,2‐diol and alginate ion (Steiner and McNeely, 1951). Propane‐1,2‐diol alginate is by a factor of 104–105 more instable against alkaline hydrolysis, compared to the unsubstituted alginate (Draget, 2000).
3.2. Authorised uses and use levels
Maximum levels of propane‐1,2‐diol alginate (E 405) have been defined in Annex II to Regulation (EC) No 1333/200816 on food additives, as amended. These levels are defined by the Panel as ‘maximum permitted levels’ (MPLs) in this document.
Table 2 summarises food categories that are permitted to contain propane‐1,2‐diol alginate (E 405) and the corresponding MPLs as set by Annex II to Regulation (EC) No 1333/2008.
Table 2.
MPLs of propane‐1,2‐diol alginate (E 405) in foods according to the Annex II to Regulation (EC) No 1333/2008
| Food category number | Food category name | E‐number | Restrictions/exception | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|---|
| 02.2.2 | Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions | E 405 | 3,000 | |
| 03 | Edible ices | E 405 | Only water‐based edible ices | 3,000 |
| 04.2.4.1 | Fruit and vegetable preparations excluding compote | E 405 | 5,000 | |
| 05.2 | Other confectionery including breath freshening microsweets | E 405 | Only sugar confectionary | 1,500 |
| 05.3 | Chewing gum | E 405 | 5,000 | |
| 05.4 | Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | E 405 | 1,500 | |
| Only fillings, toppings and coatings for fine bakery wares and desserts | 5,000 | |||
| 07.2 | Fine bakery wares | E 405 | 2,000 | |
| 12.6 | Sauces | E 405 | 8,000 | |
| 13.1.5.1 | Dietary foods for infants for special medical purposes and special formulae for infants | E 405 | From 12 months onwards in specialised diets intended for young children who have cow's milk intolerance or inborn errors of metabolism | 200 |
| 13.1.5.2 | Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC | E 405 | From 12 months onwards in specialised diets intended for young children who have cow's milk intolerance or inborn errors of metabolism | 200 |
| 13.2 | Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5) | E 405 | 1,200 | |
| 13.3 | Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet) | E 405 | 1,200 | |
| 14.1.4 | Flavoured drinks | E 405 | 300 | |
| 14.2.1 | Beer and malt beverages | E 405 | 100 | |
| 14.2.3 | Cider and perry | E 405 | Excluding cidre bouché | 100 |
| 14.2.6 | Spirit drinks as defined in Regulation (EC) No 110/2008 | E 405 | Only emulsified liqueurs | 10,000 |
| 14.2.8 | Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol (14.2.8) | E 405 | Only in fermented grape must‐based drinks | 100 |
| 15.1 | Potato‐, cereal‐, flour‐ or starch‐based snacks | E 405 | Only cereal‐ and potato‐based snacks | 3,000 |
| 17.1a | Food supplements supplied in a solid form including capsules and tablets and similar forms, excluding chewable forms | E 405 | 1,000 | |
| 17.2a | Food supplements supplied in a liquid form | E 405 | 1,000 | |
| 17.3a | Food supplements supplied in a syrup‐type or chewable form | E 405 | 1,000 |
MPL: maximum permitted level.
17 refers to food supplements as defined in Directive 2002/46/EC of the European Parliament and of the Council excluding food supplements for infants and young children.
According to Annex III, Part 1, of Regulation (EC) No 1333/2008 propane‐1,2‐diol alginate (E 405) is also authorised as carrier in all food additives with a maximum level at quantum satis (QS).
3.3. Exposure data
3.3.1. Reported use levels or data on analytical levels of propane‐1,2‐diol alginate (E 405)
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued public calls17 , 18 for occurrence data (usage level and/or concentration data) on propane‐1,2‐diol alginate (E 405).
In response to the first call, Marinalg International provided some information (Documentation provided to EFSA n. 3) on the use of propane‐1,2‐diol alginate (E 405) in foods.
In addition, in response to the public call of year 2015, information on the actual use levels of propane‐1,2‐diol alginate (E 405) in foods was made available to EFSA by industry. No analytical data on the concentration of propane‐1,2‐diol alginate (E 405) in foods were made available by the Member States.
Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with data on use levels (n = 12) of propane‐1,2‐diol alginate (E 405) in foods for 3 out of the 21 food categories in which propane‐1,2‐diol alginate (E 405) is authorised.
Updated information on the actual use levels of propane‐1,2‐diol alginate (E 405) in foods was made available to EFSA by FoodDrinkEurope (FDE) (Documentation provided to EFSA n. 16) and Marinalg International (Marinalg) (Documentation provided to EFSA n. 17).
The Panel noted that Marinalg (which submitted three use levels) is not directly using gums in food products but is a food additive producer. Use levels reported by food additive producers are not considered at the same level as those provided by food industry. Food additive producers might recommend use levels to the food industry but the final levels might, ultimately, be different. Therefore, unless food additive producers confirm that the recommended levels are used by food industry, they are not considered in the refined exposure scenarios. Data from food additive producers will only be used in the maximum level exposure assessment scenario in case of QS authorisation when no data are available from food industry. In this way, the most complete exposure estimates are calculated. In the current assessment, all food categories are authorised with a numerical MPL, and maximum reported use levels of Marinalg equalled the MPLs.
The Panel noted that the remaining nine use levels on beer and malt beverages referred to niche products. Since no other use levels were available from food industry, the Panel decided not to carry out refined assessment, as the available dataset was considered insufficient to provide a solid basis for it.
Appendix A provides data on the use levels of propane‐1,2‐diol alginate (E 405) in foods as reported by industry.
3.3.2. Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information of over 2.5 million food and beverage products of which more than 1,000,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 20 out of its 28 member countries and Norway presented in the Mintel GNPD.19
For the purpose of this Scientific Opinion, the Mintel's GNPD20 was used for checking the labelling of food and beverages products and food supplements for propane‐1,2‐diol alginate (E 405) within the EU's food market as the database contains the compulsory ingredient information on the label.
According to the Mintel's GNPD, propane‐1,2‐diol alginate (E 405) was labelled on 226 products mainly of ‘Beer’, ‘Dairy Based Ice Cream & Frozen Yogurt’ and ‘Table Sauces’ between January 2013 and January 2018.
Appendix B lists the number and percentage of the food products labelled with propane‐1,2‐diol alginate (E 405) out of the total number of food products per food subcategories according to the Mintel's GNPD food classification. The percentages ranged from less than 0.1% in many food subcategories to 1.5% in Mintel's GNPD food sub‐category ‘Water Based Ice Lollies, Pops & Sorbets’. The average percentage of foods labelled to contain propane‐1,2‐diol alginate (E 405) was 0.13%.
The Panel noted that propane‐1,2‐diol alginate (E 405) was listed as ingredient in 11 food products of the Mintel's GNPD subcategories ‘Fish Products’, ‘Instant Noodles’, ‘Meal Kits’, ‘Chocolate Tablets’, ‘Pasta’ where it is not authorised as such.
3.3.3. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). Consumption surveys added in the Comprehensive database in 2015 were also taken into account in this assessment.15
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database includes the currently best available food consumption data across Europe.
Food consumption data from the following population groups: infants, toddlers, children, adolescents, adults and the elderly were used for the exposure assessment. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 3).
Table 3.
Population groups considered for the exposure estimates of propane‐1,2‐diol alginate (E 405)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlersa | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrenb | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlyb | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Netherlands, Sweden, UK |
The term ‘toddlers’ in the EFSA Comprehensive Database corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013.
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure estimates. In practice, the FoodEx food codes were matched to the FCS food categories.
Food categories considered for the exposure assessment of propane‐1,2‐diol alginate (E 405)
The food categories in which the use of propane‐1,2‐diol alginate (E 405) is authorised were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
Some food categories or their restrictions/exceptions are not referenced in the EFSA Comprehensive Database. For the following food categories, the restrictions/exceptions which apply to the use of propane‐1,2‐diol alginate (E 405) could not be taken into account, and therefore the whole food category was considered in the exposure assessment. This applies to five food categories (three of them considered in the MPL scenario, while FCs 13.1.5.1 and 13.1.5.2 are taken into account in the calculations concerning foods for special medical purposes (FSMPs)) (Appendix C) and may have resulted in an overestimation of the exposure:
5.4 Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4. In this food category the additive is authorised with an MPL of 1,500 mg/kg in general, but for ‘only fillings, toppings and coatings for fine bakery wares and desserts’ has an MPL of 5,000 mg/kg. As these products cannot be clearly distinguished in the Comprehensive Database, in order to be conservative, an MPL of 5,000 mg/kg was applied for the whole category.
13.1.5.1 Dietary foods for infants for special medical purposes and special formulae for infants and 13.1.5.2 Dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC, ‘from 12 months onwards in specialised diets intended for young children who have cow's milk intolerance or inborn errors of metabolism’. The restriction could not be taken into account and the whole food categories were considered.
14.2.3 Cider and perry, ‘excluding cidre bouché’. Restriction could not be taken into account as this specific information is not included in the Comprehensive Database. Thus, the whole food category was taken into account.
14.2.8 Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol (14.2.8), ‘only in fermented grape must‐based drinks’. Restriction could not be taken into account as this specific information is not included in the Comprehensive Database. The whole food category was taken into account.
For the FCs 17.1/17.2/17.3 Food supplements, in solid, liquid, syrup‐type or chewable form, the form cannot be differentiated and the same level was applied to the whole FC 17.
Propane‐1,2‐diol alginate (E 405) is also allowed in FCs 13.2 and 13.3. Food items under FCs 13.2, 13.3 consumed by population groups‐children, adolescents, adults and the elderly‐ may be very diverse and, in addition, there is very limited information on their consumption. Therefore, eating occasions belonging to the FCs 13.2, 13.3 were reclassified under food categories in accordance to their main component.
The MPLs available for FCs 13.2 and 13.3 were not considered for the exposure assessment.
Considering that the FC 18 (Processed foods not covered by categories 1 to 17, excluding foods for infants and young children) is extremely unspecific (e.g. composite foods), processed foods, prepared or composite dishes belonging to the FC 18 were reclassified under food categories in accordance to their main component.
The Panel considered the data received insufficient to carry out refined exposure scenarios therefore, only the regulatory maximum level exposure scenario was performed including 14 food categories in the present exposure assessment to propane‐1,2‐diol alginate (E 405). As this scenario is not considering the consumption of food supplements and FSMP, the possible additional exposure from their consumption was also investigated. (Appendix C).
3.4. Exposure estimates
3.4.1. Exposure to propane‐1,2‐diol alginate (E 405) from its use as a food additive
The Panel estimated the chronic dietary exposure to propane‐1,2‐diol alginate (E 405) for the following population groups: infants; toddlers, children, adolescents, adults and the elderly. Dietary exposure to propane‐1,2‐diol alginate (E 405) was calculated by multiplying MPLs of propane‐1,2‐diol alginate (E 405) per food category (Appendix C) with their respective consumption amount per kilogram body weight for each individual in the Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only 1 day per subject were excluded as they are considered as not adequate to assess repeated exposure.
This was carried out for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 3). On the basis of these distributions, the mean and 95th percentile of exposure were calculated per survey and per population group. The 95th percentile of exposure was only calculated for those population groups with a sufficiently large sample size (EFSA, 2011a). Therefore, in the present assessment, the 95th percentile of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain were not estimated.
Exposure assessment to propane‐1,2‐diol alginate (E 405) was carried out by the ANS Panel based on the MPLs as set down in the EU legislation, defined as the regulatory maximum level exposure assessment scenario.
Regulatory maximum level exposure assessment scenario does not consider the consumption of food supplements and FSMP. However, the possible exposure from their consumption was also investigated using their MPLs in addition to the food categories considered in the regulatory maximum level exposure assessment scenario.
Regarding the food supplements, dietary exposure was estimated only for the four older population groups as food supplements for infants and young children are excluded from FC 17.
A possible additional exposure from the use of propane‐1,2‐diol alginate (E 405) as carrier in all food additives in accordance with Annex III to Regulation (EC) No 1333/2008 (Part 1) was not considered in any of the exposure assessment scenarios.
Regulatory maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario is based on the MPLs as set in Annex II to Regulation (EC) No 1333/2008. For propane‐1,2‐diol alginate (E 405), the MPLs used in the assessment are listed in Table 2 (Appendix C).
The Panel considers the exposure estimates derived following this scenario as the most conservative since it is assumed that that the population will be exposed to the food additive present in food at the MPL over a longer period of time.
Dietary exposure to propane‐1,2‐diol alginate (E 405)
Table 4 summarises the estimated exposure to propane‐1,2‐diol alginate (E 405) from its use as a food additive in six population groups (Table 3) according to the regulatory maximum level exposure assessment scenario. Detailed results per population group and survey are presented in Appendix D.
Table 4.
Summary of dietary exposure to propane‐1,2‐diol alginate (E 405) from its use as food additive in the regulatory maximum level exposure assessment scenario in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants | Toddlers | Children | Adolescents | Adults | The elderly | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (12 weeks–11 months) | (12–35 months) | (3–9 years) | (10–17 years) | (18–64 years) | (≥ 65 years) | |||||||
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | |
| Mean | 0.5 | 8.5 | 3.6 | 19.0 | 6.0 | 18.9 | 3.5 | 13.2 | 1.8 | 8.2 | 1.3 | 6.0 |
| P95th | 7.4 | 35.1 | 11.7 | 39.6 | 13.9 | 38.2 | 7.6 | 27.7 | 4.9 | 21.8 | 3.2 | 14.5 |
From the regulatory maximum level exposure assessment scenario, the mean exposure to propane‐1,2‐diol alginate (E 405) from its use as a food additive ranged from 0.5 mg/kg bw per day in infants to 19.0 mg/kg bw per day in toddlers. The 95th percentile of exposure to propane‐1,2‐diol alginate (E 405) ranged from 3.2 mg/kg bw per day in elderly to 39.6 mg/kg bw per day in toddlers.
Taking into account, for consumers only, also the exposure from food supplements with the MPLs of the corresponding categories (FCs 17.1, 17.2, 17.3), added to categories considered for the regulatory maximum level exposure assessment scenario, the highest mean and 95th percentile exposure was estimated in children, with levels of 29.1 mg/kg bw per day and 38.1 mg/kg bw per day respectively.
Taking into account the consumption of foods for special medical purposes for the whole population of infants and toddlers based on MPLs of the corresponding categories (FCs 13.1.5.1, 13.1.5.2) and added to food categories considered for the regulatory maximum level exposure assessment scenario, the highest mean exposure was estimated in toddlers, with 21.4 mg/kg bw per day while the highest 95th percentile exposure was estimated in infants with a level of 46.8 mg/kg bw per day.
Main food categories contributing to exposure to propane‐1,2‐diol alginate (E 405) in the regulatory maximum level exposure assessment scenario
From the regulatory maximum level exposure assessment scenario, the main contributing food categories to the total mean exposure estimates for every population groups were fine bakery wares and sauces. In addition, for infants, processed fruits and vegetables (FC 4.2.4.1 Fruit and vegetable preparation excluding compote) contributed significantly, while for toddlers, children, adolescents and adults, flavoured drinks are considered as an important source of exposure. In the elderly group, fat and oil emulsions are contributing significantly as well. (Appendix E).
Uncertainty analysis
Uncertainties in the exposure assessment of propane‐1,2‐diol alginate (E 405) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 5.
Table 5.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/– |
| Use of data from food consumption surveys covering only a few days to estimate high percentiles (95th) long‐term (chronic) exposure | + |
|
Concentration data:
|
+ |
| Foods which may contain the food additive according to Annex III to Regulation (EC) No 1333/2008 not taken into account | – |
|
Regulatory maximum level exposure assessment scenario:
|
+ + |
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
For all scenarios, propane‐1,2‐diol alginate (E 405) present in foods as carry‐over (Annex III to Regulation No 1333/2008) was not considered which would also lead to an underestimation of exposure to propane‐1,2‐diol alginate (E 405).
Overall, the Panel considered that the uncertainties identified indicated an overestimation of the exposure to propane‐1,2‐diol alginate (E 405) as a food additive according to Annex II, in European countries considered in the EFSA European Comprehensive database in the regulatory maximum level exposure scenario.
Based on the Mintel's GNPD information, the Panel noted that propane‐1,2‐diol alginate (E 405) may not be used in all food categories in which it is authorised, as confectionary, chewing gums, cider and perry, spirits drinks are not listed in the Mintel GNPD's. In many other food categories, only few food products are labelled with the food additive (such as other fat and oil emulsions, snacks, etc. (Appendix B)).
The main uncertainty identified by the Panel raised from the unavailability of use levels for many of the 21 food categories for which propane‐1,2‐diol alginate (E 405) is authorised as a food additive.
The Panel noted that use levels were reported on niche products of one food category listed in the Mintel GNPD. ‘Beer’ is the main food category labelled with propane‐1,2‐diol alginate (E 405), as it represented approximately 30% of the food products labelled with propane‐1,2‐diol alginate (E 405) with however only a few percentage of beers labelled with the food additive (less than 1%). Thus, the Panel considered that these uncertainties were too large to enable to propose an accurate refined exposure assessment of propane‐1,2‐diol alginate (E 405) as a food additive.
Exposure to propane‐1,2‐diol from propane‐1,2‐diol alginate (E 405)
The Panel noted that propane‐1,2‐diol available from propane‐1,2‐diol alginate (E 405) would add to the exposure to the food additive propane‐1,2‐diol (E 1520) previously re‐evaluated by the Panel (EFSA ANS Panel, 2018).
The total maximum content of propane‐1,2‐diol in propane‐1,2‐diol alginate (E 405) amounts up to a maximum of 45% by weight according to the EU specification. The Panel considered a maximum release of 45% as a worst case on which exposure was estimated.
Based on the highest P95 exposure level observed in the regulatory maximum level exposure assessment scenario for propane‐1,2‐diol alginate (E 405) (39.6 mg/kg bw per day for toddlers), the highest exposure to propane‐1,2‐diol was calculated to be 17.8 mg/kg bw per day.
For infants in the regulatory maximum level exposure assessment scenario considering also the consumption of FSMPs, the highest P95 exposure to propane‐1,2‐diol alginate (E 405) was 46.8 mg/kg bw per day, an exposure of 21.1 mg propane‐1,2‐diol/kg bw per day was calculated.
Overall, the Panel considered that accurate exposure assessment for propane‐1,2‐diol released from propane‐1,2‐diol alginate was hampered by the same uncertainties as mentioned above.
3.4.2. Exposure via other uses
3.4.2.1. Pharmaceutical uses
Information on pharmaceutical uses was obtained by searches of the literature, the websites of national competent authorities for medicinal products and publicly available SmPC (summary of product characteristics) on the nationally available authorised products indicated to EFSA by the EMA communication (Documentation provided to EFSA, n. 15).
Propane‐1,2‐diol alginate is also used as an excipient in medicinal products authorised in centralised procedures as seen from the answer of EMA. The documented daily dosage is approximately 63 mg/day for adults.
Propane‐1,2‐diol alginate is used as suspending and thickening agents but also as a stabiliser for oil‐in‐water emulsions and as binding and disintegrating agents in pharmaceutical industry (Martindale, 2014).
There are no data available indicating the use of propane‐1,2‐diol alginate as an active ingredient in medicinal products.
Nevertheless alginates are known to be given as an active ingredient, sometimes formulated in combination with carbonates and other antacids (aluminium hydroxide), in the management of gastroesophageal reflux disease (GERD). Alginic acid or its salts react with gastric acid to form a viscous gel acting as a mechanical barrier to reduce reflux of gastric content (Mandel et al., 2000; Kapadia and Mane, 2007; Martindale, 2014; Reimer et al., 2016).
Propane‐1,2‐diol is used as humectant, solvent and preservative in a wide range of medicinal products (EMA, 2014a).
As no data were available to the Panel, exposure to propane‐1,2‐diol alginate via medicinal products were not considered in this opinion.
3.5. Biological and toxicological data
The Panel also considered in this opinion the major toxicokinetic and toxicological data on alginic acid and propane‐1,2‐diol, the two hydrolytic derivatives of propane‐1,2‐diol alginate. These two compounds have been recently re‐evaluated for their safety of use as food additives E 400 and E 1520 (EFSA ANS Panel, 2017, 2018).
3.5.1. Absorption, distribution, metabolism and excretion (ADME)
The Panel noted that certain high‐molecular‐weight dietary polysaccharides could be partially broken down by the microbiota in the large intestine of humans. In addition to intermediate metabolites such as lactic, acrylic or fumaric acids, the main end products of this colonic anaerobic digestive process are short‐chain fatty acids (SCFA), such as acetic, propionic and butyric acids, that are absorbed from the colon (Cummings and Englyst, 1987).
3.5.1.1. Propane‐1,2‐diol alginate (E 405)
3.5.1.1.1. In vitro studies
As reported by JECFA (1993), ‘in vitro hydrolysis studies with propylene glycol alginate in simulated gastric juice and simulated intestinal juice showed no hydrolysis in simulated gastric juice, while intestinal juice hydrolyzed 25% in 4 h, 65% in 12 h and 80% in 24 h’ (McNeely and Shepherd, 1966, unpublished report).
3.5.1.1.2. In vivo study
The absorption, distribution throughout the body tissues and excretion of propane‐1,2‐diol alginate were followed in mice after oral administration, using the technique of whole‐body autoradiography (Sharratt and Dearn, 1972). The authors used two different samples of propane‐1,2‐diol alginate. One labelled with 14C in the alginate moiety was prepared from seaweed of Laminaria hyperborean cultivated in sea‐water containing 14C‐carbonate. The other 14C‐labelled in the propane‐1,2‐diol was prepared by esterification of alginic acid from Ascophyllum nodosum with 14C‐labelled propylene oxide.
In case of radiolabelling on alginate, CF1 mice (n = 8; 3–4 weeks old; no data about sex) received an oral dose of approximately 5,000 mg/kg bw propane‐1,2‐diol alginate (as a 10% aqueous solution). One mouse was killed every 1, 2, 4, 6 or 8 h and 1, 3 or 5 days after gavage.
A similar experiment was performed with radiolabelled propane‐1,2‐diol alginate on propane‐1,2‐diol; 7 mice received a dose of approximately 1,000 mg/kg bw and one mouse was killed every 2, 4, 6 or 8 h and 1, 3 or 5 days after gavage. All animals were sectioned (100‐μm thick sections) for whole body autoradiography. The sections were in contact with X‐ray film for 6 or 60 days prior to film processing.
When propane‐1,2‐diol alginate labelled on the alginate moiety was given to mice, the autoradiographs indicated that the whole radioactivity (100%) was confined to the contents of the gastrointestinal tract at all times whereas no radioactivity was detected in other organs. Five days after the oral administration, nearly complete excretion of radioactivity was observed and only traces were present in the rectum.
When propane‐1,2‐diol alginate labelled on propane‐1,2‐diol moiety was administered, the radioactivity was absorbed to some extent, distributed rapidly over the whole body, particularly concentrated in the liver and was completely removed from all tissues in 3–4 days. The Panel calculated that the part of radioactivity present in extra digestive tissues would represent approximately 25% of the administered radioactivity. The unabsorbed or non‐hydrolysed portion was excreted in the faeces within 3 days.
According to the authors, while hydrolysis of propane‐1,2‐diol alginate to propane‐1,2‐diol and alginate was only partial at a dose of 1,000 mg/kg bw in mice, the hydrolysis is likely to be more complete at lower levels, such as those likely to be ingested by humans.
3.5.1.2. Alginic acid and derived salts (E 400–E 404)
In its previous assessment of the safety of alginic acid and its salts (EFSA ANS Panel, 2017), the ANS Panel considered that the in vitro degradation and the in vivo studies demonstrated that the biological fate of alginic acid and its salts was similar. Alginic acid and its salts would not be absorbed intact regardless of the form administered; they would not be metabolised by enzymes present in the gastrointestinal tract. However, considering in vitro studies using alginic acid, they could be partially fermented during their passage through the large intestine by the action of the intestinal microbiota. The rate of hydrolysis in the gastrointestinal tract in humans is unknown, but it is expected that the limited extent of hydrolysis of alginic acid and its salts would lead to the production of fermentation products such as SCFAs. Based on the available knowledge on the role of SCFA as end products of the fermentation of dietary fibres by the anaerobic intestinal microbiota (Topping and Clifton, 2001; Den Besten et al., 2013), the Panel considered that their potential formation as fermentation products from alginic acid and its salts did not raise a safety concern.
3.5.1.3. Propane‐1,2‐diol (E 1520)
As reported in its previous evaluation of propane‐1,2‐diol (EFSA ANS Panel, 2018), the ANS Panel considered that this compound is readily absorbed from the gastrointestinal tract in experimental animals and in humans and is expected to be widely distributed to organs and tissues. The major route of metabolism is oxidation to lactic acid and pyruvic acid. An alternative route of metabolism of propane‐1,2‐diol to lactic acid is via phosphorylated glycol. Lactate is mainly metabolised via the citric acid cycle and excreted as carbon dioxide via exhalation. At high concentrations, free propane‐1,2‐diol is excreted in the urine as the metabolism of propane‐1,2‐diol is saturated at dose levels higher than 20,000 mg/day in humans. The Panel noted that ADME data on the hydrolysis product propane‐1,2‐diol are presented in the OECD evaluation (2001). Absorption of orally applied propane‐1,2‐diol and its removal from the body follows first order kinetics. Clearance from blood in humans is rapid, with a mean half‐life of 2 h. Absorbed propane‐1,2‐diol is readily converted into lactic acid and pyruvic acid and enters the general metabolic pool.
Overall, in vitro experiments have shown that propane‐1,2‐diol alginate is partially hydrolysed by simulated gastric and intestinal juices to propane‐1,2‐diol and alginic acid. In vivo studies using autoradiography gave also evidence for a partial but not complete hydrolysis to propane‐1,2‐diol and alginic acid. According to previous EFSA assessment propane‐1,2‐diol is absorbed and its metabolites would enter the normal energy‐forming pathways. However, together with any non‐hydrolysed material, the alginate moiety was not absorbed intact but excreted in the faeces, confirming the data previously provided in the assessment on the safety of alginic acid and its salts (EFSA ANS Panel, 2017).
3.5.2. Acute toxicity
3.5.2.1. Propane‐1,2‐diol alginate (E 405)
Several acute toxicity studies were performed with propane‐1,2‐diol alginate. LD50s of propane‐1,2‐diol alginate were reported to be approximately 7,000 or 7,800 mg/kg bw in mice (Bailey et al., 1976; FDRL, 1976 as cited in JECFA, 1993) greater than 5,000 mg/kg (WRC, 1972; Documentation provided to EFSA n. 12), 7,200 mg/kg bw (FDRL, 1976; cited in JECFA, 1993) or greater than 10,000 mg/kg bw (Documentation provided to EFSA n. 4) in rats, approximately 7,000 mg/kg bw in hamsters (Bailey et al., 1976; FDRL, 1976 as cited in JECFA, 1993), and approximately 7,000 mg/kg bw or 7,600 mg/kg bw in rabbits (Bailey et al., 1976; FDRL, 1976 as cited in JECFA, 1993).
The Panel considered propane‐1,2‐diol alginate to be of low acute oral toxicity.
3.5.2.2. Alginic acid and derived salts (E 400–404)
The Panel considered alginic acid and its salts (E 400–E 404) of low acute oral toxicity (EFSA ANS Panel, 2017).
3.5.2.3. Propane‐1,2‐diol (E 1520)
The Panel considered that the data from acute toxicity studies in mice, rats, hamsters and rabbits indicated low acute toxicity of propanediol‐1,2‐diol (EFSA ANS Panel, 2018).
Overall, the Panel considered the acute toxicity of propane‐1,2‐diol alginate, alginic acid and propane‐1,2‐diol to be low.
3.5.3. Short‐term and subchronic toxicity
3.5.3.1. Propane‐1,2‐diol alginate (E 405)
Rats
No adverse effects (no further details) were observed in five male Sprague–Dawley rats gavaged with 5,000 mg propane‐1,2‐diol alginate/kg bw per day for 5 days (vehicle corn oil, origin not further specified). Data on control rats were not available (Documentation provided to EFSA n. 4).
In another study, two female rats per dose (strain not stated) received a diet containing 0% or 21.5% propane‐1,2‐diol alginate (not further specified) (equivalent to 0 or 21,500 mg/kg bw per day) for 4 weeks (no further details). In the treated rats, diarrhoea was noted. No effects were detected in histopathology of the liver, kidneys and intestine (no further data; MRCL, 1951; cited in JECFA, 1993). In a second study, four female rats per dose received 0 or 21.5% propane‐1,2‐diol alginate in the diet for 4 weeks followed by a 4‐week post‐exposure observation period. Treated rats showed diarrhoea and a slight decrease in body weight gain but appearance and behaviour were normal (no further data; MRCL, 1951; cited in JECFA, 1993). The Panel considered the relevance of these studies for risk assessment as limited due to the low number of animals per group, and limited number of parameters investigated, and that the only dose tested was very high.
In a study performed by Anderson et al. (1991), a group of 15 male rats (strain not stated) received 5% propane‐1,2‐diol alginate (not further specified) in the diet (5,000 mg/kg bw per day) for 30 days; the control was not specified. No clinical signs were observed. Ten treated rats had soft/‐ill formed faecal pellets in comparison to a stool of the controls. The standard urinalysis did not reveal any effects but necropsy showed distended ileum (n = 5), caecum (n = 15) and colon (n = 12) (no further data).
Guinea pigs
Guinea pigs (strain and sex not stated, n = 1–3/group) were fed 0%, 5%, 10%, 15% propane‐1,2‐diol alginate (Kelcoloid) in the diet (equivalent to 0, 2,000, 4,000 or 6,000 mg/kg bw per day, JECFA, 2000) for 26 weeks (Nilson and Wagner, 1951). Mortality occurred in controls and in the low‐dose group (2 guinea pigs died within 8 weeks in the control and 5% groups, respectively). No effects on body weight were reported after addition of vitamins. As the evaluation of this study was hampered by the insufficient reporting and low number of animals, the Panel considered the relevance of this study for risk assessment as limited.
Cats
In the same publication (Nilson and Wagner, 1951), a study in cats (strain, sex and number per group not stated) fed diets containing 0%, 5%, 10% or 15% of propane‐1,2‐diol alginate (Kelcoloid) for periods of varying duration (from 14 to 110 days) was described. The mean consumption of propane‐1,2‐diol alginate was reported to be from 5.0 to 15.8 g/animal per day depending on the dose level. Body weight loss was recorded for all cats on diet added propane‐1,2‐diol alginate. According to the authors this could be related to lower feed intake due to physical texture of the diet. The cats receiving ‘higher’ levels of propane‐1,2‐diol alginate (no information about the concentration) passed 2–3 stools of soft texture per day. There were no indications of toxicity and no findings, which could be attributed to treatment, were reported at necropsy and by histology. As the evaluation of this study was hampered by the insufficient reporting and low number of animals, the Panel considered the relevance of this study for risk assessment as limited.
Dogs
Groups of three male and three female Beagle Dogs received daily a diet containing 0%, 5%, or 15% propane‐1,2‐diol alginate (equal to 0, 1,000 or 3,000 mg/kg bw per day) for 12 months (WRC, 1959; Documentation provided to EFSA n. 11). Dogs were observed daily for clinical signs. The body weight was recorded once weekly. Haematology (parameters: haemoglobin, packed cell volume, white blood cell count, differential blood count) was performed at weeks –1, 0, 2, 6, 13, 26, 39 and 52, and clinical chemistry (parameters: urea nitrogen, alkaline phosphatase, glucose) at termination as well as urinalysis (parameters: sugar, albumin, microscopy, occult blood). Necropsy was performed and organ weights were measured (including liver, kidney, heart, spleen, testis, ovary, uterus, prostate, adrenals, thyroid gland). Organs and tissues were examined microscopically for histopathological changes (investigated organs: cerebrum, pons, midbrain, pituitary, heart, salivary gland, heart, liver, lung, kidney, spleen, pancreas, stomach, ileum, jejunum, duodenum, large bowel, testis, ovary, urinary bladder, prostate, uterus, lymph nodes). No clinical signs except soft stool consistency in the high‐dose group and no treatment‐related effects on feed consumption and body weight gain were noted. Haematology, clinical chemistry and urinalysis were within the normal limits. No treatment‐related effects were detected at necropsy and by microscopy. The Panel considered 3,000 mg/kg bw per day as the NOAEL, the highest dose tested.
3.5.3.2. Alginic acid and derived salts (E 400–404)
One short‐term toxicity study performed in rats with alginic acid up to 24,000 mg/kg bw per day, seven short‐term toxicity studies performed in rats with sodium alginate up to 12,000 mg/kg bw per day, one subchronic toxicity study performed in rats with sodium alginate up to 13,500 mg/kg bw per day and one subchronic study in dogs with sodium alginate up to 3,000 mg/kg bw per day were available. No adverse effects were observed. In the rat studies, the caecal enlargement described by the authors was considered by the Panel as an adaptive process related to the high doses tested (EFSA ANS Panel, 2017).
3.5.3.2.1. Propane‐1,2‐diol (E 1520)
No treatment‐related effects were observed in subchronic toxicity studies in which propane‐1,2‐diol was administered by gavage (1,000 mg/kg bw per day) to mice, rats, dogs and monkeys for 92–97 days (Thackaberry et al., 2010) (EFSA ANS Panel, 2018).
Overall, although various dietary studies with propane‐1,2‐diol alginate in laboratory animals were available to the Panel, several of these studies were of limited relevance for risk assessment due to the low numbers of animals per group and/or limited reporting. In these studies with dietary concentrations up to 25% of propane‐1,2‐diol alginate, loose stools were observed. Reliable studies comprised one short‐term toxicity study performed in rats with alginic acid up to 24,000 mg/kg bw per day, seven short‐term toxicity studies in rats with sodium alginate up to 12,000 mg/kg bw per day, one subchronic toxicity study performed in rats with sodium alginate up to 13,500 mg/kg bw per day and two subchronic studies in dogs, one with sodium alginate and another one with propane‐1,2‐diol both up to 3,000 mg/kg bw per day, and a subchronic study in monkeys exposed to 1,000 mg propane‐1,2‐diol/kg bw per day, the highest dose tested. None of these studies demonstrated adverse effects.
3.5.4. Genotoxicity
3.5.4.1. Propane‐1,2‐diol alginate (E 405)
3.5.4.1.1. In vitro studies
In the study by Litton Bionetics (1975; Documentation provided to EFSA n. 8), propane‐1,2‐diol alginate (not further specified) was assessed for its mutagenicity in the reverse mutation assay using Salmonella Typhimurium strains TA1535, TA1537, TA1538 according to the method of Ames, at concentrations of 0.15%, 0.3% or 0.6% (1.5, 3 or 6 mg/mL) and for mitotic recombination in Saccharomyces cerevisiae (strain D‐4) at concentrations of 2.5%, 5% and 10% (25, 50 or 100 mg/mL) both in the absence and presence of S9 metabolism. Negative results were reported for both mutagenic and mitotic recombination capabilities of propane‐1,2‐diol alginate. However, the Panel noted that the results from the bacteria gene mutation assay are limited due to the inadequate number of S. Typhimurium tester strains used and that only one dose level was tested in the plate incorporation assay (0.3%). In addition, the Panel noted that the gene conversion assay with Saccharomyces cerevisiae has not been validated and is no longer used for risk assessment.
In the study by Ishidate et al. (1984) propane‐1,2‐diol alginate (not further specified) was not mutagenic up to 10 mg/plate in S. Typhimurium strains TA92, TA1535, TA100, TA1537, TA94 and TA98 both in the absence and presence of S9 metabolic activation. Propane‐1,2‐diol alginate was not tested in the TA102 or Escherichia coli WP2 tester strains and only the pre‐incubation method was applied. However, the Panel considered that since oxidising or cross‐linking properties of propane‐1,2‐diol alginate is not expected and that the pre incubation method in the absence of cytotoxicity at the highest recommended dose levels, as in the present case, was the most sensitive method, the results obtained were sufficient.
In the study by Fujita et al. (1988), propane‐1,2‐diol alginate (not further specified) was assessed for its mutagenicity in the reverse mutation assay using S. Typhimurium strains TA97 and TA102 both in the absence and presence of S9 metabolism, at concentrations of 0.1, 0.5, 1, 5 and 10 mg/plate (vehicle not stated). No mutagenic effects were reported in the absence and presence of metabolic activation. However, the Panel noted that reliability of the study was limited due to an invalid positive control in tester strain TA97 in the absence of metabolic activation and incomplete set of S. Typhimurium tester strains.
Propane‐1,2‐diol alginate (not further specified) was assessed for its capability to induce chromosomal aberrations in anaphase in human embryonic lung cells (WI‐38) at dose levels up to 1,000 μg/mL without S9 mix (SRI, 1972; Documentation provided to EFSA n. 4). No cytogenetic effects were reported by authors. However, the Panel noted that this assay has not been validated and does not belong to the assays recommended for regulatory purposes (EFSA Scientific Committee, 2011).
In a comparative analysis of data on chromosomal aberration on 951 chemical substances, propane‐1,2‐diol alginate was assayed for its clastogenic properties in a Chinese hamster lung (CHL) cell line. Treatments were performed for 48 h at three different dose levels. The maximum dose level employed for propane‐1,2‐diol alginate was 1 mg/mL, selected in a preliminary toxicity test as the dose causing 50% cell‐growth inhibition only without metabolic activation. Results obtained indicated that sodium alginate did not induce polyploidy or clastogenic effects. However, the Panel noted that treatments were not performed in the presence of S9 metabolic activation (Ishidate et al., 1988).
3.5.4.1.2. In vivo studies
In a host‐mediated assay, groups of 5–10 male mice were administered propane‐1,2‐diol alginate (not further specified) by oral gavage at three dose levels (not clearly specified, but presumably up to 5 g/kg bw per day). Administration was performed once (acutely) or on five consecutive days 24 h apart at the same dose levels employed in the single administration regime using the S. Typhimurium tester strains TA 1530 and G‐46 for mutagenicity and S. cerevisiae (strain D4) for mitotic recombination. Negative and positive control animal groups were also included. Results obtained in both experiments indicated that propane‐1,2‐diol alginate did not show genotoxic activity in any of the indicator organisms and dose levels employed (SRI, 1972; Documentation provided to EFSA n. 4). The Panel noted that the host‐mediated assay has not received further validation and it is presently considered obsolete.
In an in vivo cytogenetic assay, the induction by propane‐1,2‐diol alginate (not further specified) of chromosomal aberrations in bone marrow cells of rats was investigated. Groups of five male albino rats were administered with test substance by oral gavage acutely at 30, 2,500 or 500 mg/kg bw or subacutely on five consecutive days, 24 h apart, at the same dose levels employed for the acute treatment. Negative and positive control animal groups were also included. For the acute treatment, sampling of bone marrow cells was performed at 6, 24 and 48 h from the last administration, while in the subacute study sampling was only performed at 6 h from the last administration. Results obtained indicated that propane‐1,2‐diol alginate induced no increases in the incidence of chromosomal aberrations in the bone marrow cells following both acute and subacute administration, at any of dose‐level employed. A slight reduction of mitotic indices in the bone marrow treatment group cells, compared with the negative control values was consistently observed, possibly indicating that the target tissue was exposed, at least to the propane‐1,2‐diol hydrolysed moiety (SRI, 1972; Documentation provided to EFSA n. 4). The Panel noted that this study, essentially complied with the OECD Guideline 475 requirements, although it was performed much earlier in the 1974.
In a dominant lethal assay, propane‐1,2‐diol alginate (not further specified) was administered by oral gavage to groups of ten male albino rats acutely at 30, 2,500 or 5,000 mg/kg bw or subacutely on 5 consecutive days, 24 h apart, at the same dose‐levels employed for the acute treatment. Negative and positive control animal groups were also included. Following treatment, the males were sequentially mated to two females per week for 8 weeks (7 weeks in the subacute study) and housed separately until sacrifice. Fertility index, total implants (live fetuses plus early and late foetal deaths), total dead (early and late fetal deaths), dead implants per total implants and preimplantation loss (calculated as the difference between the total corpora lutea and total implant counts) were evaluated. The results obtained were considered by the authors of no genotoxic concern (SRI, 1972; Documentation provided to EFSA n. 4). The Panel agreed with this conclusion.
3.5.4.2. Alginic acid and derived salts (E 400‐404)
According to a recent re‐evaluation of EFSA (EFSA ANS Panel, 2017), alginic acid and sodium alginate were tested in several in vitro assays and in one in vivo assay that, despite some limitations, did not reveal any genotoxic effect for alginic acid and sodium alginate. No studies were available for calcium alginate, potassium alginate and ammonium alginate. However, the Panel considered that a read‐across approach can be applied to calcium alginate, potassium alginate and ammonium alginate to exclude a potential genotoxicity also for these compounds. The Panel also noted that alginic acid would not be absorbed unchanged and would not be metabolised by enzymes present in the gastrointestinal tract but partially fermented during its passage through the large intestine by the action of the intestinal tract microflora leading to the production of its fermentation products such as SCFA which do not raise concerns for genotoxicity (OECD Toolbox 4.0). On this basis, the Panel concluded that there was no concern with respect to the genotoxicity for alginic acid and its salts (E 400–E 404).
3.5.4.3. Propane‐1,2‐diol (E 1520)
According to a recent re‐evaluation of EFSA (EFSA ANS Panel, 2018), propane‐1,2‐diol did not raise concern with respect to genotoxicity when used as a food additive.
Overall, propane‐1,2‐diol alginate was tested in several in vitro assays (point mutation in bacteria, chromosomal aberrations in mammalian cells) and in vivo (host‐mediated assay, dominant lethal and bone marrow chromosome aberration in rat) that, despite some limitations, did not reveal any genotoxic effect. In addition, alginic acid and propane‐1,2‐diol, the two hydrolytic metabolites of propane‐1,2‐diol alginate, were evaluated by EFSA of no genotoxic concern (EFSA ANS Panel, 2017, 2018). Overall, the Panel concluded that the available data did not indicate a genotoxic concern for propane‐1,2‐diol alginate (E 405) when used as a food additive.
3.5.5. Chronic toxicity and carcinogenicity
3.5.5.1. Propane‐1,2‐diol alginate (E 405)
Rats
Groups of 10–15 male and female rats (strain not stated) received 0%, 5%, 15% or 25% propane‐1,2‐diol alginate in the diet (equivalent to 0, 2,500, 7,500 or 12,500 mg/kg bw per day, respectively) for a lifelong exposure period (Nilson and Wagner, 1951). At a dose level of 2,500 mg/kg bw per day, no effect on final body weight, survival or food consumption occurred. No treatment‐related effects were detected at any dose level in histopathology (examined organs: brain, heart, lung, stomach, liver, spleen, kidney, intestine, ovaries, testis). Haematology, clinical chemistry and urinalysis were not performed. At ≥ 7,500 mg/kg bw per day, loose and smeary faeces were reported. At the mid‐dose level the food intake and the final body weight were decreased and survival reduced; the water intake was increased. Further enhancement of effects was seen at the high dose level of 25% in the diet: the food intake was reduced to 51% of the control value (water intake unchanged) and the final body weight was only 44% of the control value. Inanition was reported at this dose and reduced survival. The authors of the study considered these effects a consequence of the increased water absorbing and bulking capacity of the unabsorbed test material (alginate) which caused enough bulk to limit food intake. Therefore, these effects were not considered to be of toxicological relevance. The Panel agreed with the interpretations of the authors.
In a further experiment in rats (strain not stated, 10/sex), propane‐1,2‐diol alginate was also mixed with dog chow at a concentration of 15%; (equivalent to 7,500 mg/kg bw per day) for 37 weeks (Nilson and Wagner, 1951). No laxative effects were found but food and water intake was increased. The body weight gain was not altered. However, no concurrent control was available and the comparison was presumably related to a control group receiving a basal diet.
Data on chronic oral toxicity were available from the parent generation in a multigeneration study (Georgetown University Medical School, 1959; Documentation provided to EFSA n. 10). Groups of 20 male and 20 female Sprague–Dawley rats were exposed to 0% or 5% propane‐1,2‐diol alginate in the diet (equivalent to 0 or 2,500 mg/kg bw per day, respectively) for 2 years. The clinical signs were scored daily and the body weight was measured weekly (first 4 months, then monthly). Haematology (n = 4/group) on months 5, 8, 12 and 23 included red and white blood cell counts, haematocrit and differential blood counts. After final sacrifice, necropsy and histopathology of the lung, liver, kidney, small intestine, pancreas and stomach were performed. In the treatment group, no clinical signs were observed and no effects on body weight gain. Haematology, gross pathology and histopathology were without treatment‐related effects. Further investigations revealed no significant effects on the intestinal microflora. Although limited parameters were investigated, this chronic study gave no evidence for adverse effects at a dose level of 2,500 mg/kg bw per day, the only dose tested.
Overall, based on the results of the long‐term toxicity/carcinogenicity studies in rats, the Panel considered that propane‐1,2‐diol alginate was not of concern with respect to carcinogenicity.
3.5.5.2. Alginic acid and derived salts (E 400–404)
The chronic exposure of mice to 25% sodium alginate (equivalent to 37,500 mg/kg bw per day) for 89 weeks resulted in nephrotoxic effects that were considered to be due to high sodium intake followed by extremely high water intake and not related to the alginate moiety. According to the results of long‐term toxicity studies in mice and rats, the Panel considered that alginic acid and its salts were not of concern with respect of carcinogenicity (EFSA ANS Panel, 2017).
3.5.5.3. Propane‐1,2‐diol (E 1520)
No adverse effects were observed in a 2‐year study in dogs administered propane‐1,2‐diol in the diet (2,000 or 5,000 mg/kg bw per day), except for haematological changes suggestive of an increased red blood cell destruction with a compensatory increased rate of haematopoiesis at the highest dose level (5,000 mg/kg bw per day) (Weil et al., 1971). Similarly, no adverse effects, including neoplastic findings, were reported in a 2‐years study in rats administered 2,500 mg propane‐1,2‐diol/kg bw per day for 2 years (Gaunt et al., 1972). The SCF and JECFA used the study in rats to derive an ADI of 25 mg propane‐1,2‐diol/kg bw per day using an uncertainty factor of 100. The Panel agreed with this ADI (EFSA ANS Panel, 2018).
Overall, propane‐1,2‐diol alginate, alginic acid and its salts and propane‐1,2‐diol were not of concern with respect to carcinogenicity.
3.5.6. Reproductive and developmental toxicity
3.5.6.1. Propane‐1,2‐diol alginate (E 405)
3.5.6.1.1. Reproductive toxicity
In a three‐generation reproductive toxicity study, groups of 20 male and 20 female Sprague–Dawley rats received 0% or 5% propane‐1,2‐diol alginate in their diet (equivalent to 0 or 2,500 mg/kg bw per day) (Georgetown University Medical School; Documentation provided to EFSA n. 10). After 5–6 months, some of these F0 animals were mated (no further details) to produce a F1 generation. The F1 control group (7 males and 7 females) and the F1 test group (10 males and 10 females) were mated after 4 months to produce the F2 generation (9 male and 10 female controls and 9 male and 10 female test animals). All generations were kept on the same diets. The F0 generation survived 761 days; the F1 and F2 generation were sacrificed after 202 and 212 days, respectively. No differences from controls were noted regarding survival, clinical signs (daily scored), body weight (measured weekly during the first 4 months, then monthly), fertility and lactation (no details available) for all three generations. Haematology, including red and white blood cell counts, haematocrit and differential blood counts, was performed in the P (blood sampling at study month 5, 8, 12 and 23) and F2 generation (sampling after 3 and 6 months postnatal) and did not reveal any differences compared to controls. No effects were found at necropsy of P rats after 2 years of treatment and in F1 and F2 rats at the conclusion of the rapid growth period (organ weights not determined). Histopathology of the liver, lung, kidney, stomach and pancreas (no further details) did not show abnormalities. The study was too limited for risk assessment as no details about reproduction were described and the number of animals per group was too low.
3.5.6.1.2. Developmental toxicity
In all studies performed by the Food and Drug Res. Lab. (FDRL, 1972a,b,c, 1974; Documentation provided to EFSA n. 5,6,7 and 9) described below, body weights were recorded at regular intervals during gestation and all animals were observed daily for appearance and behaviour. All dams were subjected to caesarean section, and the numbers of implantation sites, resorption sites, live and dead fetuses, and body weights of live fetuses were recorded. All fetuses were examined grossly for sex distribution and for external abnormalities (one‐third detailed visceral examination and two‐third stained and examined for skeletal defects). For the rabbits, all live pups were placed in an incubator for 24 h to evaluate the postnatal survival prior to sacrifice. All pups were examined for external, visceral and skeletal abnormalities.
Mice
Groups of 20–21 pregnant CD‐1 mice received on gestation day (GD) 6–15 propane‐1,2‐diol alginate at dose levels of 0, 8, 36, 170 or 780 mg/kg bw per day by gavage (suspension in corn oil; dose volume 1 mL/kg bw). Body weight was determined at GD 0, 6, 11, 15 and 17 (FDRL, 1972a; Documentation provided to EFSA n. 5). All dams were subjected to Caesarean section at GD 17. No maternal or developmental toxicity was detected up to 170 mg/kg bw per day. At 780 mg/kg bw per day, increased mortality of dams (3/20 deaths versus 0/21 in controls) was reported as well as decreased final body weight (13% lower than in control dams) indicating maternal toxicity (no data about clinical signs). In this group also, a lower number of live litters (13 vs 20 in controls) and live fetuses/litter (8.2 vs 10.3 in controls) were found as well as increased number of resorptions (52 vs 26 in control). There was no treatment‐related increase in abnormalities in either soft or skeletal tissues (FDRL, 1972b; Documentation provided to EFSA n. 6). The Panel identified 170 mg propane‐1,2‐diol alginate/kg bw per day as the NOAEL for maternal and developmental toxicity.
Rats
Groups of 20–23 pregnant Wistar rats received propane‐1,2‐diol alginate on GD 6–15, via gavage at dose levels of 0, 7, 33, 155 or 720 mg/kg bw per day (suspension in corn oil; dose volume 1 mL/kg bw) (FDRL, 1972b; Documentation provided to EFSA n. 6). Body weight was determined at GD 0, 6, 11, 15 and 20. On GD 20, a caesarean section was carried out. No treatment‐related maternal or developmental toxicity was detected. The Panel identified 720 mg propane‐1,2‐diol alginate/kg bw per day as the NOAEL for maternal and developmental toxicity.
Hamsters
Groups of 20–21 pregnant golden hamsters received via gavage 0, 7, 33, 150 or 700 mg propane‐1,2‐diol alginate/kg bw per day (suspension in corn oil; dose volume 1 ml/kg bw) from GDs 6 to 10 (FDRL, 1972c; Documentation provided to EFSA n. 7). On GD 14, a caesarean section was carried out. There was no evidence for maternal or developmental toxicity. The Panel identified 700 mg propane‐1,2‐diol alginate/kg bw per day as the NOAEL for maternal and developmental toxicity.
Rabbits
Groups of 19–20 Dutch belted inseminated rabbits (pregnant n = 10–15/group) received propane‐1,2‐diol alginate by gavage at dose levels of 0, 8, 37, 173 or 800 mg/kg bw per day (suspension in corn oil; dose volume 1 mL/kg bw except for the high‐dose group a dose volume of 4 mg/kg bw) on GD 6–18 (FDRL, 1974: Documentation provided to EFSA n. 9). No maternal toxicity was reported. The study was terminated by the caesarean section on GD 29; 9‐13 pregnant rabbits per group were evaluated at termination, 3 controls and 0–2 treated doses per group aborted or died during gestation. No treatment‐related effects on the number of corpora lutea, live litters, implantation sites, resorptions, live or dead fetuses or on the fetal weights were detected. Examination of all the fetuses did not reveal a treatment‐related increase in abnormalities. However, it should be noted that the number of dams with live litters was low (11, 10, 13, 10 or 9 in the 0, 8, 37, 173 or 800 mg/kg bw per day groups, respectively).
Overall, there was only one limited three‐generation reproductive toxicity study with propane‐1,2‐diol alginate in rats in which no adverse effects were observed. Prenatal developmental toxicity studies with propane‐1,2‐diol alginate in mice, rats, hamsters and rabbits were available. The reporting of the prenatal developmental studies was limited. No maternal and no developmental toxicity were found at the high dose levels of 720 mg/kg bw per day in rats (FDRL, 1972b; Documentation provided to EFSA n. 6), 700 mg/kg bw per day in hamsters (FDRL, 1972c; Documentation provided to EFSA n. 7) and 800 mg/kg bw per day in rabbits (FDRL, 1974; Documentation provided to EFSA n. 9). In mice (FDRL, 1972b; Documentation provided to EFSA n. 6), the high dose of 780 mg/kg bw per day resulted in maternal toxicity such as decreased body weight and mortality; no data were given on clinical signs. Effects are suggested to be related to the bulking capacity of the unabsorbed test material. As a consequence of this maternal toxicity, developmental effects such as decreased number of live litters and fetuses and increased total resorptions but no fetal visceral or skeletal abnormalities, were reported. No such effects were observed at doses which did not induce maternal toxicity.
3.5.6.2. Alginic acid and derived salts (E 400‐404)
In a two‐generation reproductive study, male and female Sprague–Dawley rats were fed diets containing 0 or 5% sodium alginate (equivalent to 0 and 2,500 mg/kg bw per day) for a period of 2 years. The Panel noted that the data presented in this study were insufficient for hazard characterisation. However, the Panel noted that in the 90‐day study in rats (TNO, 1967; as cited in EFSA ANS Panel, 2017), no effects were observed on testes and ovary weights and also no histopathological changes were observed in testes, ovaries and uteri. In the chronic study in mice (Til et al., 1986), no effect was observed on testes weights and no histopathological changes were observed on testes, epididymides, prostate, seminal vesicles, ovaries and uteri. No prenatal developmental toxicity studies were available (EFSA ANS Panel, 2017).
3.5.6.3. Propane‐1,2‐diol (E 1520)
In two reproductive toxicity studies (mice and rats) and six developmental studies in mice, rats, hamsters and rabbits, no treatment‐related effects were observed at the highest doses tested (≥ 1,000 mg propane‐1,2‐diol/kg bw per day) (EFSA ANS Panel, 2018).
Overall, in limited two‐ or three‐generation reproductive toxicity studies with propane‐1,2‐diol alginate and alginate, no adverse effects were reported up to 2,500 mg/kg bw per day. In prenatal developmental toxicity studies with propane‐1,2‐diol alginate in rats, hamsters and rabbits no developmental effects were observed up to the highest dose tested (approx. 700–800 mg propane‐1,2‐diol alginate/kg bw per day). No prenatal developmental toxicity studies were available for alginates. Propane‐1,2‐diol induced no treatment‐related effects in two reproductive toxicity studies (mice and rats) and six developmental studies in mice, rats, hamsters and rabbits, these no treatment‐related effects were observed at the highest doses tested (1,000 mg propane‐1,2‐diol/kg bw and higher).
3.5.7. Hypersensitivity, allergenicity and food intolerance
3.5.7.1. Propane‐1,2‐diol alginate (E 405)
A study on skin sensitisation in humans was published by Ouer (1949). Fifty patients known to be allergic to numerous substances (no further details) were tested intradermally with various dilutions of propane‐1,2‐diol alginate (no further details). In the control group, 50 individuals without an allergic history or family history of allergy were used (no further details). Only very slight skin reaction occurred in three subjects of the control group. In the test group, very slight skin reaction in six patients, three cases of slight skin reaction (presumably allergic), and two cases of moderate skin reaction were reported. Slight and moderate skin reactions were judged by the author to be ‘significant skin reactions’ in five persons. They were further tested in the passive transfer test: all five persons gave positive results (no further details). A mild degree of an ‘usual allergic reaction’ was observed after ingestion of an amount of propane‐1,2‐diol alginate ‘greater than would normally be ingested as food’ in 3 out of these 5 patients; the effect was reproducible (no further details). The authors concluded that propane‐1,2‐diol alginate is of low allergenicity (Ouer, 1949).
The Panel noted that the study could not be considered for evaluation of the endpoint allergy and/or food intolerance due to the route of exposure, which was irrelevant for the food additive use, and to limited information.
3.5.7.2. Alginic acid and derived salts (E 400–404)
It was reported that under certain conditions, alginates have cytokine and nitric oxide inducing activities (Uno et al., 2006; Ueno and Oda, 2014). However, from the publications available, the Panel considered that there was no indication for immunotoxicity or for an allergenic potential of alginic acid and its salts when used as food additives.
3.5.7.3. Propane‐1,2‐diol (E 1520)
The EFSA CONTAM Panel did not consider propane‐1,2‐diol as a possible allergen (EFSA CONTAM Panel, 2011). In its opinion (EFSA ANS Panel, 2018), the ANS Panel considered that given the large body of evidence available, it was very unlikely that propane‐1,2‐diol could be an allergen for human when used as a food additive.
Overall, the Panel considered that there were no indication for a possible concern regarding intolerance or immunotoxicity, including allergenicity, for propane‐1,2‐diol alginate (E 405) used as a food additive.
3.5.8. Other studies with propane‐1,2‐diol alginate (E 405)
Animal data
The effects on the intestinal microflora was tested in two rats fed a control diet during 6 months followed by 5% propane‐1,2‐diol alginate in the diet for 3 weeks and further weeks on control diet. Microflora analysis of the intestinal content showed lower counts of lactobacilli and aerobe bacteria but an increased count of coliforms compared to controls, whereas anaerobic counts were not altered (WRC, 1959; cited in JECFA, 1993).
Human data
Five healthy male volunteers (23–43 years old; body weight: 68–88 kg) received propane‐1,2‐diol alginate in orange juice at a dose level of 175 mg/kg bw per day for 7 days (approximately 14 g/person per day), followed by 200 mg/kg bw per day (approximately 16 g/person per day) for further 16 days (Anderson et al., 1991). The daily doses were consumed in three portions. The portions were added to 220 mL water and the resulting hydrocolloid was then allowed to hydrate for 24 h to a gel to which each volunteer added orange juice prior to consumption. At day 3 of the initial control period, on the last day of the 23‐day treatment period, and at day 7 of the 1 week post‐exposure observation period, the following parameters were examined: glucose tolerance test, plasma insulin, exhaled hydrogen concentrations during the glucose tolerance test, from fasting blood samples haematological parameters (haemoglobin, haematocrit, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, red and white blood cell count, platelets, differential blood cell count, red blood cell distribution width) and clinical chemistry parameters (cholesterol, high‐density lipoprotein, triglyceride, sodium, chloride, potassium, calcium, phosphate, CO2, urea, lactate dehydrogenase, aspartate aminotransferase, bilirubin, alkaline phosphatase, protein, albumin, creatinine, urate, lipids). Routine urinalysis of 24‐h samples was carried out during the initial control week and during the third week of treatment (no details available). Complete 5‐day faecal collections were made during days 2–6 of the initial control period and during days 16–20 of the treatment period. Faecal transit time (after ingestion of a marker), stool wet weight and dry weight, water content, pH, occult blood, neutral sterols, fat, volatile fatty acids and bile acids in faeces were determined. No differences in the composition of the diet were found during control and treatment period. None of the volunteers reported allergic reactions, gastro‐intestinal disturbances or discomfort. Haematology, clinical chemistry and urinalysis did not show significant and treatment‐related changes; all parameters were within the normal range. No changes were seen in parameters of faecal analysis except a slight decrease in faecal pH from 6.57 to 6.31 and a slight decrease in total neutral sterols from 2.1 to 1.4 mmol/24 h. The authors concluded that under the conditions of this study, propane‐1,2‐diol alginate did not induce adverse or physiological effects at dose levels up to 200 mg/kg bw per day (approximately 16 g per day per person) for 23 days.
For human data on alginic acid and alginates and propane‐1,2‐diol, the Panel referred to the EFSA ANS Panel (2017, 2018) opinions.
4. Discussion
Propane‐1,2‐diol alginate was previously evaluated by the SCF and JECFA, both bodies considering that the ADI for propane‐1,2‐diol alginate should be limited only by the amount of free propane‐1,2‐diol and propane‐1,2‐diol esterified with alginic acid, hydrolysed after oral exposure and released (JECFA, 1993; SCF, 1994). While the SCF established for propane‐1,2‐diol alginate an ADI of 25 mg/kg bw per day, expressed as propane‐1,2‐diol, JECFA allocated an ADI of 70 mg propane‐1,2‐diol alginate/kg bw per day.7
In vitro experiments have shown that propane‐1,2‐diol alginate is partially hydrolysed by simulated gastric and intestinal juices to propane‐1,2‐diol and alginic acid. In vivo studies using autoradiography gave also evidence for a partial but not complete hydrolysis to propane‐1,2‐diol and alginic acid. According to previous EFSA assessments of these two hydrolytic moieties, propane‐1,2‐diol is absorbed and its metabolites would enter the normal energy‐forming pathways. However, together with any non‐hydrolysed material, the alginate moiety is not absorbed and excreted in the faeces, confirming the data previously provided in the assessment on the safety of alginic acid and its salts (EFSA ANS Panel, 2017). The Panel noted that ADME data on propane‐1,2‐diol alginate gave evidence for the hydrolysis of this additive into propane‐1,2‐diol and alginic acid. These two compounds have been recently re‐evaluated for their safety of use as food additives E 400 and E 1520 (EFSA ANS Panel, 2017, 2018). Consequently, the Panel considered in this opinion the major toxicokinetic and toxicological data of these two hydrolytic derivatives.
The acute toxicity of propane‐1,2‐diol alginate, alginic acid and its salts and propane‐1,2‐diol was considered to be low.
Although various subacute and subchronic dietary studies with propane‐1,2‐diol alginate in laboratory animals were available to the Panel, several of these studies were of limited relevance for risk assessment due to the low numbers of animals per group and/or limited reporting. In these studies with dietary concentrations up to 25% of propane‐1,2‐diol alginate, loose stools were observed. Reliable studies comprised one short‐term toxicity study performed in rats with alginic acid up to 24,000 mg/kg bw per day, seven short‐term toxicity studies in rats with sodium alginate up to 12,000 mg/kg bw per day, one subchronic toxicity study performed in rats with sodium alginate up to 13,500 mg/kg bw per day and two subchronic studies in dogs, one with sodium alginate and another one with propane‐1,2‐diol both up to 3,000 mg/kg bw per day, and a subchronic study in monkeys exposed to 1,000 mg propane‐1,2‐diol/kg bw per day, the highest dose tested. None of these studies demonstrated adverse effects.
Propane‐1,2‐diol alginate was tested in several in vitro assays (point mutation in bacteria, chromosomal aberrations in mammalian cells) and in vivo (host‐mediated assay, dominant lethal and bone marrow chromosome aberration in rat) that, despite some limitations, did not reveal any genotoxic effect. In addition, alginic acid and propane‐1,2‐diol, the two hydrolytic metabolites of propane‐1,2‐diol alginate, were considered by EFSA of no genotoxic concern (EFSA ANS Panel, 2017, 2018). The Panel therefore noted that the available data did not indicate a genotoxic concern for propane‐1,2‐diol alginate (E 405) when used as a food additive.
Propane‐1,2‐diol alginate, alginic acid and propane‐1,2‐diol were not of concern with respect to carcinogenicity. No adverse effects, including neoplastic findings, were reported in a 2‐year study in rats administered 2,500 mg propane‐1,2‐diol/kg bw per day for 2 years (Gaunt et al., 1972). The SCF and JECFA used the study in rats to establish an ADI of 25 mg propane‐1,2‐diol/kg bw per day using an uncertainty factor of 100, the Panel agreed with this ADI (EFSA ANS Panel, 2018).
In limited two‐ or three‐generation reproductive toxicity studies with propane‐1,2‐diol alginate and alginate, no adverse effects were reported up to 2,500 mg/kg bw per day. In prenatal developmental toxicity studies with propane‐1,2‐diol alginate in rats, hamsters and rabbits, no developmental effects were observed up to the highest dose tested (~ 700–800 mg propane‐1,2‐diol alginate/kg bw per day). No prenatal developmental toxicity studies were available for alginates. Propane‐1,2‐diol induced no treatment‐related effects in two reproductive toxicity studies (mice and rats) and six developmental studies in mice, rats, hamsters and rabbits, no treatment‐related effects were observed at the highest doses tested (1,000 mg propane‐1,2‐diol/kg bw and higher).
There were no indications for a possible concern regarding intolerance or immunotoxicity, including allergenicity, for propane‐1,2‐diol alginate (E 405) used as a food additive.
In a human study with five volunteers exposed orally for 23 days to propane‐1,2‐diol alginate at doses of 175–200 mg/kg bw per day (14–16 g/person per day), no allergic reactions, gastrointestinal disturbances or discomfort were noted and no adverse effects were detected in haematology, clinical chemistry and urinalysis. No relevant changes were seen in parameters of faecal analysis.
The Panel considered that any adverse effect of propane‐1,2‐diol alginate would be due to propane‐1,2‐diol. Therefore, the ADI of the food additive E 405 is determined by the amount of free propane‐1,2‐diol and the propane‐1,2‐diol released from the food additive after hydrolysis. According to the EU specification, the concentration of free and bound propane‐1,2‐diol amounts to a maximum of 45% on a weight basis. On the worst‐case assumption that 100% of propane‐1,2‐diol (free and bound) would be systemically available and considering the ADI for propane‐1,2‐diol of 25 mg/kg bw per day, the Panel allocated an ADI of 55 mg/kg bw per day for propane‐1,2‐diol alginate (100/45 × 25).
To assess the dietary exposure to propane‐1,2‐diol alginate (E 405) from its use as a food additive, the exposure was calculated based on the MPLs set out in the EU legislation (defined as the regulatory maximum level exposure assessment scenario).
Due to the discrepancies observed between the Mintel GNPDs and the reported use levels data, the Panel considered that the use levels data for the refined exposure assessment scenarios are not covering sufficiently the present use of the additive. As a matter of fact, the Panel noted that use levels were reported for only one food category (‘FC 14.1.2 Beer and malt beverages’) out of the 21 in which propane‐1,2‐diol alginate (E 405) is authorised.
Thus, considering that (1) this food category does not cover the uses identified in the Mintel's GNPD, (2) this food category is not relevant to estimate the exposure to the food additive for the younger population groups, (3) use levels were reported only for niche products of beer, (4) low percentage (< 1%) of beers are labelled with the additive according to the Mintel's GNPD, the Panel decided not to perform refined exposure scenarios. Only the regulatory maximum level exposure assessment scenario is presented in the current assessment. As this scenario is not considering the consumption of food supplements and FSMP, the possible additional exposure from their consumption was also investigated.
Estimates of exposure assessment scenarios did not exceeded the ADI of 55 mg/kg bw per day for propane‐1,2‐diol alginate (E 405) for any population groups.
The Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure to propane‐1,2‐diol alginate (E 405) from its use as a food additive according to Annex II to Regulation (EC) No 1333/2008 in the regulatory maximum level exposure scenario (Section 3.4.1).
The Panel noted that food categories which may contain propane‐1,2‐diol alginate (E 405) due to carry‐over (Annex III, Part 1) were not considered in any of the current exposure assessment scenarios.
The Panel noted that propane‐1,2‐diol available from propane‐1,2‐diol alginate (E 405) would add to the exposure to the food additive propane‐1,2‐diol (E 1520) previously re‐evaluated by the Panel (EFSA ANS Panel, 2018).
Propane‐1,2‐diol may be released from propane‐1,2‐diol alginate (E 405) at a maximum of 45% according to the EU specification. Based on this, and considering the highest P95 exposure level observed, the highest exposure to propane‐1,2‐diol was calculated to be:
in the regulatory maximum level exposure assessment scenario 17.8 mg/kg bw per day of propane‐1,2‐diol in toddlers (released from 39.6 mg/kg bw per day of propane‐1,2‐diol alginate (E 405));
in the regulatory maximum level exposure assessment scenario considering also the consumption of FSMPs, 21.1 mg/kg bw propane‐1,2‐diol per day in infants (released from 46.8 mg/kg bw per day of propane‐1,2‐diol alginate (E 405)).
5. Conclusions
Considering the overall metabolic and toxicity database, the Panel confirmed the previously established ADI for propane‐1,2‐diol alginate (E 405) of 25 mg/kg bw per day expressed as propane‐1,2‐diol. This corresponds to an ADI for propane‐1,2‐diol alginate (E 405) of 55 mg/kg bw per day, based on the concentration of free and bound propane‐1,2‐diol amounting to a maximum of 45%.
The Panel concluded that exposure estimates did not exceed the ADI in any of the population groups from the use of propane‐1,2‐diol alginate (E 405) as a food additive. Therefore, the Panel concluded that there is no safety concern at the authorised use levels.
6. Recommendations
The Panel recommended the European Commission to consider:
revising the maximum limits for the impurities of toxic elements (lead, mercury, cadmium and arsenic) in the EU specification for propane‐1,2‐diol alginate (E 405) in order to ensure that propane‐1,2‐diol alginate (E 405) as a food additive will not be a significant source of exposure to these toxic elements in food;
the inclusion of maximum limits for propylene oxide, mono‐ and diethylene glycol, and propylene carbonate in the EU specifications for propane‐1,2‐diol alginate (E 405);
the collection of more data on usage and use levels of propane‐1,2‐diol alginate (E 405) in order to perform a more realistic exposure assessment.
Documentation provided to EFSA
Pre‐evaluation document on Propylene glycol alginate (E 405). Frauenhofer ITEM. Submitted in June‐October 2012.
Ursapharm, 2010. German Association of Pharmaceutical Industry. Working Group of Ophthalmic Manufactures. Reply to EFSA: Call for data on emulsifiers, stabilisers and gelling agents. Statement on the safety of emulsifiers, stabilisers and gelling agents in ophthalmic products. Submitted on 21 May 2010.
Marinalg International, 2010. Reply to EFSA: Call for data on emulsifiers, stabilisers and gelling agents. Information on alginic acid and its salts (E 400–404) and propane 1, 2 diol alginate (E 405) on present usage, ADME (Metabolism and Toxicokinetics), subchronic toxicity, reproduction and developmental toxicity and other study. Submitted on 18 November 2010.
SRI (Stanford Research Institute), 1972. Study of mutagenic effects of propylene glycol alginate (71–18). Report PB‐221826. 102 p. Submitted by Marinalg on 18 November 2010.
FDRL (Food and Drug Research Laboratories), 1972a. Teratologic Evaluation of FDA 71‐18 (Propylene glycol alginate) in rats. Unpublished. 43 p. Submitted by Marinalg on 18 November 2010.
FDRL (Food and Drug Research Laboratories), 1972b. Teratologic Evaluation of FDA 71‐18 (Propylene glycol alginate) in mice. Unpublished. 43 p. Submitted by Marinalg on 18 November 2010.
FDRL (Food and Drug Research Laboratories), 1972c. Teratologic Evaluation of FDA 71‐18 (Propylene glycol alginate) in hamsters. Unpublished. 43 p. Submitted by Marinalg on 18 November 2010.
LBI (Litton Bionetics Incorporated), 1975. Mutagenic evaluation of compound FDA 71‐18, propylene glycol alginate. PB‐245497. 43 p. Submitted by Marinalg on 18 November 2010.
FDRL (Food and Drug Research Laboratories), 1974. Report on teratologic evaluation of compound FDA 71–18 propylene glycol alginate in rabbits. PB‐267196. 18 p. Submitted by Marinalg on 18 November 2010.
Georgetown University Medical School, 1959. The effects of algin products on the rat. Unpublished report. Department of Physiology, Georgetown University Medical School. Submitted by Marinalg on 18 November 2010.
Woodard Research Corporation, 1959. Feeding Kelgin or Kelcoloid to dogs for one year. 87 p. Submitted by Marinalg on 18 November 2010.
WRC (Woodard Research Corporation), 1972. SS‐3428 and SS‐3429. Acute oral toxicity to rats. 7 p. Submitted by Marinalg on 18 November 2010.
Marinalg International, 2015. Reply to EFSA: Call for technical data on certain thickening agents permitted as food additives in the EU. Information on technical data including specifications on alginic acid and its salts (E 400–E 404). Submitted on 22 December 2015.
Marinalg International, 2016. Reply to EFSA letter of 8 February 2016: request of information on the methods used to analyse and measure formaldehyde. Technical report of the development of the Farrell method (2007). Submitted on 4 March 2016.
EMA (European Medicines Agency), 2016. Communication to EFSA request for information on a certain group of substances used as food additives. Submitted on 4th January 2016.
FoodDrinkEurope (FDE), 2016. Data on usage levels on propane‐1,2‐diol alginate (E 405) in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 4). Submitted to EFSA 31 May 2016.
Marinalg International, 2016. Data on usage levels on propane‐1,2‐diol alginate (E 405) in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 4). Submitted to EFSA by 31 May 2016.
Abbreviations
- ADI
acceptable daily intake
- AFC
Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food
- ALT
Alanine transaminase
- ANS
Panel on Food Additives and Nutrient Sources added to Food
- AOAC
Association of Analytical Communities
- AST
aspartate transaminase
- CAS
Chemical Abstracts Service
- CHL
Chinese hamster lung
- CHMP
Committee for Medicinal Products for Human Use
- EINECS
European Inventory of Existing Commercial Chemical Substances
- EMA
European Medicines Agency
- FAO/WHO
Food and Agriculture Organization/World Health Organization
- FC
Food Category
- FCS
Food Classification System
- FDA
Food and Drug Administration
- FDE
FoodDrinkEurope
- FDRL
Food and Drug Research Laboratories
- FEEDAP
Panel on Additives and Products or Substances used in Animal Feed
- FSMP
Foods for special medical purposes
- GD
gestational day
- GERD
gastroesophageal reflux disease
- GNPD
Global New Products Database
- HPAEC
high‐performance anion‐exchange chromatography
- HPLC
high‐performance liquid chromatography
- INS
International Numbering System for Food Additives
- IOM
Institute of Medicine
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LD50
lethal dose, 50% i.e. dose that causes death among 50% of treated animals
- LOD
limit of detection
- LOQ
limit of quantification
- MPL
maximum permitted levels
- NDA
Panel on Dietetic Products, Nutrition and Allergies
- NOAEL
no‐observed‐adverse effect
- OECD
Organisation for Economic Co‐operation and Development
- PGA
propylene glycol alginate
- QS
quantum satis
- SCE
sister chromatid exchange
- SCF
Scientific Committee for Food
- SCFA
short‐chain fatty acids
- SIDS
Screening Information Dataset
- SmPC
Summary of product characteristics
- TNO
Netherlands Organisation for Applied Scientific Research
- WHO
World Health Organization
Appendix A – Summary of the reported use levels (mg/kg or mg/L as appropriate) of propane‐1,2‐diol alginate (E 405) provided by industry
Appendix B – Number and percentage of the food products labelled with propane‐1,2‐diol alginate (E 405) between 2013 and 2018, out of the total number of food products per food subcategories according to the Mintel GNPD food classification
Appendix C – Concentration levels of propane‐1,2‐diol alginate (E 405) used in the regulatory maximum level exposure scenarios (mg/kg or mL/kg as appropriate)
Appendix D – Summary of total estimated exposure of propane‐1,2‐diol alginate (E 405) from its use as a food additive for the regulatory maximum level exposure scenario per population group and survey: mean and 95th percentile (mg/kg bw per day)
Appendix E – Main food categories contributing to exposure to propane‐1,2‐diol alginate (E 405) using the regulatory maximum level exposure scenario (> 5% to the total mean exposure)
Appendix Appendix A – Summary of the reported use levels (mg/kg or mg/L as appropriate) of propane‐1,2‐diol alginate (E 405) provided by industry, Appendix B – Number and percentage of the food products labelled with propane‐1,2‐diol alginate (E 405) between 2013 and 2018, out of the total number of food products per food subcategories according to the Mintel GNPD food classification, Appendix C – Concentration levels of propane‐1,2‐diol alginate (E 405) used in the regulatory maximum level exposure scenarios (mg/kg or mL/kg as appropriate), Appendix D – Summary of total estimated exposure of propane‐1,2‐diol alginate (E 405) from its use as a food additive for the regulatory maximum level exposure scenario per population group and survey: mean and 95th percentile (mg/kg bw per day)–E can be found in the online version of this output (‘Supporting information’ section).
Supporting information
Summary of the reported use levels (mg/kg or mg/L as appropriate) of propane‐1,2‐diol alginate (E 405) provided by industry
Number and percentage of the food products labelled with propane‐1,2‐diol alginate (E 405) between 2013 and 2018, out of the total number of food products per food subcategories according to the Mintel GNPD food classification
Concentration levels of propane‐1,2‐diol alginate (E 405) used in the regulatory maximum level exposure scenarios (mg/kg or mL/ kg as appropriate)
Summary of total estimated exposure of propane‐1,2‐diol alginate (E 405) from its use as a food additive for the regulatory maximum level exposure scenario per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to propane‐1,2‐diol alginate (E 405) using the regulatory maximum level exposure scenario (> 5% to the total mean exposure)
Suggested citation: EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Younes M, Aggett P, Aguilar F, Crebelli R, Filipič M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Lambré C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Brimer L, Mosesso P, Christodoulidou A, Horváth Zs, Lodi F, Tard A and Dusemund B, 2018. Scientific Opinion on the re‐evaluation of propane‐1,2‐diol alginate (E 405) as a food additive. EFSA Journal 2018;16(7):5371, 38 pp. 10.2903/j.efsa.2018.5371
Requestor: European Commission
Question number: EFSA‐Q‐2011‐00506
Panel members: Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipič, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Gunter Georg Kuhnle, Claude Lambré, Jean‐Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Oliver Lindtner and Jacqueline Wiesner. The ANS Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 27 June 2018
Notes
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1.
In its allocation of the ADI, JECFA considered that propane‐1,2‐diol alginate contains totally up to 36% propane‐1,2‐diol which could be released. The Panel noted that according to the last JECFA specifications (JECFA, 2006) propane‐1,2‐diol alginate contains totally up to 45% propane‐1,2‐diol.
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, pp. 16–33.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, pp. 19–27.
COM(2001) 542 final.
Food Additives in Europe 2000, Status of safety assessments of food additives presently permitted in the EU, Nordic Council of Ministers, TemaNord, 2002a,b, 560.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p 1.
The Panel is using the synonym of propylene glycol being propane‐1,2‐diol.
The Panel noted that according to the recent JECFA specifications (JECFA, 2006) propane‐1,2‐diol alginate contains totally up to 45% propane‐1,2‐diol.
Annex II of Regulation (EC) No 1333/2008 on food additives for use in foodstuffs.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents Deadline: 23 May 2010. Available from: http://www.efsa.europa.eu/en/dataclosed/call/ans091123
Call for technical data on certain thickening agents permitted as food additives in the EU ‐ extended deadline: 31 December 2015. Available online: http://www.efsa.europa.eu/it/data/call/141219
Call for food additives usages level and/or concentration data in food and beverages intended to human consumption. Deadline for submission: 31 May 2016. Available online: https://www.efsa.europa.eu/en/data/call/151012
14/6/2018.
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, pp. 16–33.
Missing Bulgaria, Cyprus, Estonia, Latvia, Lithuania, Luxembourg, Malta and Slovenia.
http://www.gnpd.com/sinatra/home/ accessed on 17/1/2018.
References
- Anderson DMW, Brydon WG, Eastwood MA and Sedgwick DM, 1991. Dietary effects of propylene glycol alginate in humans. Food Additives and Contaminants, 8, 225–236. [DOI] [PubMed] [Google Scholar]
- Bailey DE, Morgreidge K and Collins TX, 1976. Comparative acute oral toxicity of 12 food grade gums in the mouse rat hamster and rabbit. Abstracts of papers for the Fifteenth Annual Meeting of the Society of Toxicology, Atlanta, Georgia March 14–18, 1976. Toxicology and Applied Pharmacology, 37, 143. [Google Scholar]
- CRC , 1990. Gum Tragacanth. In: Furia TE (ed.). CRC Handbook of Food Additives, 2nd Edition. CRC Press, Cleveland. pp. 295–359. [Google Scholar]
- Cummings JH and Englyst HN, 1987. Fermentation in the human large intestine and the available substrates. American Journal of Clinical Nutrition, 45, 1243–1255. [DOI] [PubMed] [Google Scholar]
- Den Besten G, vanEunen K , Groen A, Venema K, Reijngoud DJ and Bakker M, 2013. The role of short‐chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. The Journal of Lipid Research, 54, 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepenmaat‐Wolters MGE, Angelino SAGF, Verbeek C and Douma AC, 1997. High‐performance anion‐exchange chromatography method for analysis of propylene glycol alginate in beer. Journal of the American Society of Brewing Chemists, 55, 147–152. [Google Scholar]
- Draget KI, 2000. Alginates. In: Phillips GO and Williams PA (eds.). Handbook of hydrocolloids. CRC Press, Boca Raton, FL. pp. 379–395. [Google Scholar]
- EC (European Commission), 2004. Commission Decision of 13 April 2004 suspending the placing on the market and import of jelly mini‐cups containing the food additives E 400, E 401, E 402, E 403, E 404, E 405, E 406, E 407, E 407s, E 410, E 412, E 413, E 414, E 415, E 417 and/or E 418 (2004/37/EC). Official Journal of the European Union, L118/70, 23.4.2004.
- EFSA AFC Panel (EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food), 2004. Opinion on a request from the Commission related to the use of certain food additives in jelly mini cups. EFSA Journal 2004;82(2):8, 11 pp. 10.2903/j.efsa.2004.82 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to Use of formaldehyde as a preservative during the manufacture and preparation of food additives. Question N° EFSA‐Q‐2005‐032. EFSA Journal, 415, 1–10. 10.2903/j.efsa.2007.415 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA ANS Panel on Food Additives and Nutrient Sources added to Food), 2014. Statement on a conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No. 257/2010. EFSA Journal 2014;12(6):3697, 11 pp. 10.2903/j.efsa.2014.3697 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Younes M, Aggett P, Aguilar F, Crebelli R, Filipic M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Lambre C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Brimer L, Lindtner O, Mosesso P, Christodoulidou A, Horvath Z, Lodi F and Dusemund B, 2017. Scientific opinion on the re‐evaluation of alginic acid and its sodium, potassium, ammonium and calcium salts (E 400–E 404) as food additives. EFSA Journal 2017;15(11):5049, 57 pp. 10.2903/j.efsa.2017.5049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food),Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipic M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Boon P, Chrysafidis D, G€urtler R, Mosesso P, Parent‐Massin D, Tobback P, Rincon AM, Tard A and Lambre C, 2018. Scientific Opinion onthe re‐evaluation of propane‐1,2‐diol (E 1520) as a food additive. EFSA Journal 2018;16(4):5235, 40 pp. 10.2903/j.efsa.2018.5235 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009a. Scientific opinion on cadmium in food. EFSA Journal 2009;7(10):980, 139 pp. 10.2903/j.efsa.2009.98 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009b. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351, 199 pp. 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2011. Scientific Opinion on the evaluationof the substances currently on the list in the Annex to Commission Directive 96/3/EC as acceptable previouscargoes for edible fats and oils–Part I of III. EFSA Journal 2011;9(12):2482, 61 pp. 10.2903/j.efsa.2011.2482 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012a. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 241 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012b. Scientific Opinion on lead dietary exposure in the European population. EFSA Journal 2012;10(7):2831, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012c. Scientific Opinion on cadmium dietary exposure in the European population. EFSA Journal 2012;10(1):2551, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on dietary exposure to inorganic arsenic in the European population. EFSA Journal 2014;12(3):3597, 68 pp. 10.2903/j.efsa.2014.3597 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal, 1051, 1–22. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feedsafety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EMA (European Medicines Agency), 2014a. Assessment report. Propylene glycol in medicinal products for children. EMA/175205/2014. Committee for Medicinal Products for Human Use (CHMP). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2014/03/WC500163989.pdf
- EMA (European Medicines Agency), 2014b. Questions & answers on propylene glycol and esters in the context of the revision of the guideline on ‘Excipients in the label and package leaflet of medicinal products for human use’ (CPMP/463/00 Rev. 1). November 2014.
- FDRL (Food and Drug Research Laboratories), 1972a. Teratologic Evaluation of FDA 71‐18 (Propylene glycol alginate) in mice. unpublished. 43 p.
- FDRL (Food and Drug Research Laboratories), 1972b. Teratologic Evaluation of FDA 71‐18 (Propylene glycol alginate) in rats. unpublished. 43 p.
- FDRL (Food and Drug Research Laboratories), 1972c. Teratologic Evaluation of FDA 71‐18 (Propylene glycol alginate) in hamsters. unpublished. 43 p.
- FDRL (Food and Drug Research Laboratories), 1974. Report on teratologic evaluation of compound FDA 71‐18 propylene glycol alginate in rabbits. PB‐267196. 18 p.
- FDRL (Food and Drug Research Laboratories), 1976. cited in JECFA, 1993.
- Fujita H, Nakano M and Sasaki M, 1988. Mutagenicity test of food additives with Salmonella typhimurium TA 97 and TA 102. III. Kenkyu Nenpo ‐ Tokyo‐toritsu Eisei Kenkyusho, 39, 343–350. [Google Scholar]
- Gaunt IF, Carpanini FMB, Grasso P and Lansdown ABG, 1972. Long‐term toxicity of propylene glycol in rats. Foodand Cosmetics Toxicology, 10, 151–162. [DOI] [PubMed] [Google Scholar]
- Ishidate M Jr, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M and Matsuoka A, 1984. Primary mutagenicity screening of food additives currently used in Japan. Food and Chemical Toxicology, 22, 623–636. [DOI] [PubMed] [Google Scholar]
- Ishidate M Jr, Harnois MC and Sofuni T, 1988. A comparative analysis of data on the clastogenicity of 951 chemical substances tested in mammalian cell cultures. Mutation Research, 195, 151–213. [DOI] [PubMed] [Google Scholar]
- JECFA (Join FAO/WHO expert Committee on Food Additives), 1974. Toxicological evaluation of certain food additives with a review of general principles and of specifications. WHO Technical Report Series 539, 17th report of JECFA 1974. 42 p. [PubMed]
- JECFA (Join FAO/WHO expert Committee on Food Additives), 1993. 794. Propylene glycol alginate. WHO Food Additives Series 32, 6 p.
- JECFA (Join FAO/WHO expert Committee on Food Additives), 2000. 976. Lead. WHO Food Additives Series 44, 39 p.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002a. Aliphatic acyclic diols, triols, and relatedsubstances. Safety evaluation of certain food additives and contaminants. Prepared by thefifty‐seventhmeeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additives Series, 48.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2002b. Evaluation of certain food additives andcontaminants. Fifty‐seventh Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, 1–171.
- JECFA (Join FAO/WHO expert Committee on Food Additives), 2006. Propylene glycol alginate. Combined Compendium of Food Additive Specifications ‐ all Specifications Monographs from the 1st to the 65th Meeting (1956‐2005), FAO JECFA Monographs 1‐3, 3 p.
- Kapadia CJ and Mane VB, 2007. Raft‐forming agents: Antireflux formulations. Drug development and industrial pharmacy, 33, 1350–1361. [DOI] [PubMed] [Google Scholar]
- Martindale , 2014. The Complete Drug Reference, 38th edition. Pharmaceutical Press, London, UK. [Google Scholar]
- Mandel KG, Daggy BP, Brodie DA and Jacoby HI, 2000. Review article: alginate‐raft formulations in the treatment of heartburn and acid reflux. Alimentary Pharmacology & Therapeutics, 14, 669–690. [DOI] [PubMed] [Google Scholar]
- McNeely WH and Shepherd VM, 1966. Report to Kelco Co. Labs. cited in JECFA, 1993.
- MRCL , 1951. Medical Research Council Laboratories. Unpublished report.; cited in JECFA, 1993.
- Nilson HW and Wagner JA, 1951. Feeding tests with some algin products. Proceedings of the Society for Experimental Biology and Medicine, 76, 630–635. [DOI] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2001. 1,2‐Dihydroxypropane. Screening Information Dataset (SIDS). SIAM 11, USA. 166 p.
- Ouer RA, 1949. Sensitivity to kelcoloid; preliminary study. Annals of Allergy, 7, 681. [PubMed] [Google Scholar]
- Reimer C, Lødrup AB, Smith G, Wilkinson J and Bytzer P, 2016. Randomised clinical trial: alginate (Gaviscon Advance) vs. placebo as add‐on therapy in reflux patients with inadequate response to a once daily proton pump inhibitor. Alimentary Pharmacology & Therapeutics, 43, 899–909. 10.1111/apt.13567 [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food), 1994. Revision of previous opinions on alginates. Food science and techniques. Reports from the Scientific Committee for Food, 32nd series, 37 p.
- SCF (Scientific Committee for Food), 1997. Opinion on propane‐1,2‐diol (Opinion expressed on 20 September1996). Reports of the Scientific Committee for Food, Fortieth series, 63–64.
- SCF (Scientific Committee for Food), 2001. Guidance on submissions for food additive evaluations by the Scientific Committee on Food. Opinion expressed on 11 July 2001. Available online: http://ec.europa.eu/food/fs/sc/scf/out98_en.pdf
- Sharratt M and Dearn P, 1972. Autoradiographic study of propylene glycol alginate in the mouse. Food and Cosmetics Toxicology, 10, 35–40. [Google Scholar]
- Steiner AB and McNeely WH, 1951. Organic derivatives of alginic acid. Industrial and Engineering Chemistry, 43, 2073–2077. [Google Scholar]
- TemaNord , 2002a. E 400‐4 Alginic acid and alginates. In: Food Additives in Europe 2000 ‐ Status of safety assessments of food additives presently permitted in the EU. Ed Nordic Council of Ministers, 5 p. [Google Scholar]
- TemaNord , 2002b. E405 Propane‐ 1,2‐doil alginate. In: Food Additives in Europe 2000 ‐ Status of safety assessments of food additives presently permitted in the EU. Ed Nordic Council of Ministers, 3 p.
- Thackaberry EA, Kopytek S, Sherratt P, Trouba K and McIntyre B, 2010. Comprehensive investigation ofhydroxypropyl methylcellulose, propylene glycol, polysorbate 80, and hydroxypropyl‐beta‐cyclodextrin for usein general toxicology studies. Toxicological Sciences, 117, 485–492. [DOI] [PubMed] [Google Scholar]
- Topping DL and Clifton PM, 2001. Short‐chain fatty acids and human colonic function: roles and resistant starch and nonstarch polysaccharides. Physiological Reviews, 81, 1031–1064. [DOI] [PubMed] [Google Scholar]
- Til HP, Feron VJ, Immel HR and Vogel WF, 1986. Chronic (89‐week) feeding study with hydroxypropyl distarch phosphate, starch acetate, lactose and sodium alginate in mice. Food and Chemical Toxicology, 24, 825–834. [DOI] [PubMed] [Google Scholar]
- Ueno M and Oda T, 2014. Biological Activities of Alginate. In: Kim SE (ed.). Advances in Food and Nutrition Research, Volume 72. Elsevier Inc., New York. pp. 95–112. [DOI] [PubMed] [Google Scholar]
- Uno T, Hattori M and Yoshida T, 2006. Oral administration of alginic acid oligosaccharide suppresses IgE production and inhibits the induction of oral tolerance. Bioscience, Biotechnology, and Biochemistry, 70, 3054–3057. [DOI] [PubMed] [Google Scholar]
- Voragen ACJ, Rolin C, Marr BU, Challen I, Riad A, Lebbar R and Knutsen SH, 2012. Polysaccharides. In: Ullmann′s Encyclopedia of Industrial Chemistry. Online version. Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim, 58 p. [Google Scholar]
- Weil CS, Woodside MD, Smyth HFJ and Carpenter CP, 1971. Results of feeding propylene glycol in the diet to dogsfor two years. Food and Cosmetics Toxicology, 9, 479–490. [DOI] [PubMed] [Google Scholar]
- Yang MH, Blunden G and Tyihak E, 1998. Formaldehyde from marine algae. Biochemical Systematics and Ecology, 26, 117–123. [Google Scholar]
- Zhang K, Wong JW, Zhengwei J, Vaclavikova M, Trucksess MW and Begley TH, 2014. Screening multimycotoxins in food‐grade gums by stable isotope dilution and liquid chromatography/tandem mass spectrometry. Journal of the Association of Analytical Chemists International, 97, 889–895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the reported use levels (mg/kg or mg/L as appropriate) of propane‐1,2‐diol alginate (E 405) provided by industry
Number and percentage of the food products labelled with propane‐1,2‐diol alginate (E 405) between 2013 and 2018, out of the total number of food products per food subcategories according to the Mintel GNPD food classification
Concentration levels of propane‐1,2‐diol alginate (E 405) used in the regulatory maximum level exposure scenarios (mg/kg or mL/ kg as appropriate)
Summary of total estimated exposure of propane‐1,2‐diol alginate (E 405) from its use as a food additive for the regulatory maximum level exposure scenario per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to propane‐1,2‐diol alginate (E 405) using the regulatory maximum level exposure scenario (> 5% to the total mean exposure)


