Abstract
This report of the European Food Safety Authority and the European Centre for Disease Prevention and Control presents the results of zoonoses monitoring activities carried out in 2017 in 37 European countries (28 Member States (MS) and nine non‐MS). Campylobacteriosis was the commonest reported zoonosis and its EU trend for confirmed human cases increasing since 2008 stabilised during 2013–2017. The decreasing EU trend for confirmed human salmonellosis cases since 2008 ended during 2013–2017, and the proportion of human Salmonella Enteritidis cases increased, mostly due to one MS starting to report serotype data. Sixteen MS met all Salmonella reduction targets for poultry, whereas 12 MS failed meeting at least one. The EU flock prevalence of target Salmonella serovars in breeding hens, laying hens, broilers and fattening turkeys decreased or remained stable compared to 2016, and slightly increased in breeding turkeys. Salmonella results on pig carcases and target Salmonella serovar results for poultry from competent authorities tended to be generally higher compared to those from food business operators. The notification rate of human listeriosis further increased in 2017, despite Listeria seldom exceeding the EU food safety limit in ready‐to‐eat food. The decreasing EU trend for confirmed yersiniosis cases since 2008 stabilised during 2013–2017. The number of confirmed shiga toxin‐producing Escherichia coli (STEC) infections in humans was stable. A total of 5,079 food‐borne (including waterborne) outbreaks were reported. Salmonella was the commonest detected agent with S. Enteritidis causing one out of seven outbreaks, followed by other bacteria, bacterial toxins and viruses. The agent was unknown in 37.6% of all outbreaks. Salmonella in eggs and Salmonella in meat and meat products were the highest risk agent/food pairs. The report further summarises trends and sources for bovine tuberculosis, Brucella, Trichinella, Echinococcus, Toxoplasma, rabies, Coxiella burnetii (Q fever), West Nile virus and tularaemia.
Keywords: zoonoses, monitoring, Salmonella, Campylobacter, Listeria, parasites, food‐borne outbreaks
Introduction
Legal basis of the EU‐coordinated zoonoses monitoring
The EU system for monitoring and collection of information on zoonoses is based on the Zoonoses Directive 2003/99/EC1, which obliges European Union (EU) Member States (MS) to collect relevant and, when applicable, comparable data on zoonoses, zoonotic agents, antimicrobial resistance and food‐borne outbreaks. In addition, MS shall assess trends and sources of these agents, as well as outbreaks in their territory, submitting an annual report each year by the end of May to the European Commission covering the data collected. The European Commission should subsequently forward these reports to the European Food Safety Authority (EFSA). EFSA is assigned the tasks of examining these data and publishing the EU annual Summary Reports. In 2004, the European Commission entrusted EFSA with the task of setting up an electronic reporting system and database for monitoring of zoonoses (EFSA mandate No. 2004‐01782).
The data collection on human diseases from MS is conducted in accordance with Decision 1082/2013/EU3 on serious cross‐border threats to health. This Decision replaced Decision 2119/98/EC on setting up a network for the epidemiological surveillance and control of communicable diseases in the EU in October 2013. The case definitions to be followed when reporting data on infectious diseases to the European Centre for Disease Prevention and Control (ECDC) are described in Decision 2012/506/EU[Link]. ECDC has provided data on zoonotic infections in humans, as well as their analyses, for the EU Summary Reports since 2005. Since 2008, data on human cases have been received via The European Surveillance System (TESSy), maintained by ECDC.
Reporting requirements
According to Annex I of the Zoonoses Directive 2003/99/EC data on animals, food and feed must be reported on a mandatory basis (list A of Annex I of the Zoonoses Directive) for the following eight zoonotic agents: Salmonella, Campylobacter, Listeria monocytogenes, Shiga toxin‐producing Escherichia coli (STEC), Mycobacterium bovis, Brucella, Trichinella and Echinococcus. In addition and based on the epidemiological situations in the MS, data must be reported on the following agents and zoonoses (list B of Annex I of the Zoonoses Directive): (i) viral zoonoses: calicivirus, hepatitis A virus, influenza virus, rabies, viruses transmitted by arthropods; (ii) bacterial zoonoses: borreliosis and their agents, botulism and their agents, leptospirosis and their agents, psittacosis and their agents, tuberculosis other than in M. bovis, vibriosis and their agents, yersiniosis and their agents; (iii) parasitic zoonoses: anisakiasis and their agents, cryptosporidiosis and agents thereof, cysticercosis and agents thereof, toxoplasmosis and their agents; and (iv) other zoonoses and zoonotic agents such as Francisella, Cysticercus and Sarcocystis). Furthermore, MS provide data on certain other microbiological contaminants in foods – histamine, staphylococcal enterotoxins and Cronobacter sakazakii for which food safety criteria are set down in the EU legislation.
According to Article 9 of the Zoonoses Regulation, the MS shall assess trends and sources of zoonoses, zoonotic agents and antimicrobial resistance in their territory and each MS shall send to the European Commission every year by the end of May a report on trends and sources of zoonoses, zoonotic agents and antimicrobial resistance, covering the data collected pursuant to Articles 4, 7 and 8 during the previous year. Reports, and any summaries of them, shall be made publicly available.
The general rules on monitoring of zoonoses and zoonotic agents in animals, food and feed are laid down in Article 4 of Chapter II of the Zoonoses Directive 2003/99/EC. Specific rules for the coordinated monitoring programmes, the food business operators (FBOp), antimicrobial resistance in animals, food and feed are laid down in Articles 5, 6 and 7 of Chapter II of the Zoonoses Directive 2003/99/EC, respectively. The minimum characteristics to be reported are described in Parts A to D of Annex IV of the Zoonoses Directive 2003/99/EC and in Part E for the food‐borne outbreaks.
Terms of reference
In accordance with Article 9 of Directive 2003/99/EC, EFSA shall examine the submitted national reports and data of the EU MS 2017 zoonoses monitoring activities as described above, and publish an EU Summary Report on the trends and sources of zoonoses, zoonotic agents and antimicrobial resistance in the EU.
The 2017 data on antimicrobial resistance in zoonotic agents submitted and validated by the MS are published in a separate EU Summary Report.
General description of methods
Data sources
This EU Summary Report 2017 on zoonoses, zoonotic agents and food‐borne outbreaks (FBOs) was prepared by EFSA in collaboration with the ECDC. Member States (MS), other reporting countries, the European Commission, members of EFSA's Scientific Panels on Biological Hazards (BIOHAZ) and Animal Health and Welfare (AHAW) and the relevant European Union Reference Laboratories (EURLs) were consulted while preparing the report.
The efforts made by MS, the reporting non‐MS and the European Commission in the reporting of zoonoses data and in the preparation of this report are gratefully acknowledged.
The present EU Summary Report on zoonoses and FBOs focuses on the most relevant information on zoonoses and FBOs within the EU in 2017. If substantial changes compared with the previous year were observed, they have been reported.
Human 2017 data collection
The human data analyses in the EU Summary Report for 2017 were prepared by the Food‐ and Waterborne Diseases (FWD) and Zoonoses programme (brucellosis, campylobacteriosis, congenital toxoplasmosis, echinococcosis, listeriosis salmonellosis, STEC infection, trichinellosis, yersiniosis), Emerging and Vector‐borne Diseases (EVD) Programme (Q‐fever, rabies, tularaemia, West Nile virus infection) and Tuberculosis (TB) programme (TB due to M. bovis) at the ECDC. Data were based on the data submitted via The European Surveillance System (TESSy), hosted at ECDC. Please note, as explained above, that the numbers presented in the report may differ from national reports owing to differences in case definitions used at EU and national level or to different dates of data submission and extraction. The latter may also result in some divergence in case numbers presented in different ECDC reports.
TESSy is a software platform that has been operational since April 2008 and in which data on 52 diseases and special health issues are collected. Both aggregated and case‐based data were reported to TESSy. Although aggregated data did not include individual case‐based information, both reporting formats were included where possible to calculate number of cases, country‐specific notification rates and trends in diseases. Human data used in the report were extracted from TESSy as of 20 August 2018 for FWD), as of 10 September 2018 for EVD, and as of 5 October 2018 for TB due to M. bovis. The denominators used for the calculation of the notification rates were the human population data from Eurostat 1 January 2018 update.
Data on human zoonoses cases were received from 28 MS and also from two non‐MS: Iceland and Norway. Switzerland sent its data on human cases directly to EFSA. The human data for Switzerland include data from Liechtenstein.
The data should be interpreted with caution and take into account data quality issues and differences between MS surveillance systems. The reader should refrain from making direct comparisons between countries without taking into account the limitations in the data, which may differ between countries depending on the characteristics of their surveillance systems.
Data collection on food, animals and feed and food‐borne outbreaks
For the year 2017, 28 MS and 4 non‐Member State (non‐MS) European Free Trade Association (EFTA) countries (Iceland, Norway, Lichtenstein, Switzerland) submitted data and national zoonoses reports on monitoring results in food, animals, feed and FBOs. In addition, data and reports were submitted by the four non‐MS: Iceland, Norway, Switzerland and Liechtenstein.4 For some food, animal and feed matrices and FBOs, EFSA received data and reports from preaccession countries Albania, Bosnia and Herzegovina, the Former Yugoslav Republic of Macedonia, Montenegro and Serbia. Data were submitted electronically to the EFSA zoonoses database, through EFSA's Data Collection Framework (DCF). MS could also update data from previous years, before 2017.
The deadline for data submission was 31 May 2018. Two data validation procedures were implemented, by 15 June 2018 and by 13 July 2018. Validated data on food, animals and feed used in the report were extracted from the EFSA zoonoses database on 25 July 2018.
The draft EU Summary Report was sent to MS for consultation on 17 October 2018 and comments were collected by 31 October 2018. The utmost effort was made to incorporate comments and data amendments within the available time frame. The report was finalised by 16 November 2018 and published online by EFSA and ECDC on 12 December 2018.
The detailed description of the terms used in the report is available in the EFSA's manuals for reporting on zoonoses (EFSA, 2018a,b,c,d).
The national zoonoses reports submitted in accordance with Directive 2003/99/EC are published on the EFSA website together with the EU Summary Report. They are available online at http://www.efsa.europa.eu/en/biological-hazards-data/reports.
Data analysis
General principles and presentation
The current summary report for the year 2017 presents a harmonised structure for each chapter, including an abstract with the major findings. In addition, a section explaining the monitoring and surveillance in the EU for the specific disease or for FBOs is summarised. A results section summarises the major findings of 2017 as regards trends and sources. A summary table displaying the data of the last 5 years (2013–2017) for human cases and for major animal and food matrices is presented. Each chapter contains also a discussion and ends with a list of related projects and links with useful information for the specific disease.
As mentioned, for each specific chapter, an overview table presenting all the MS that reported data during 2013–2017 is made available, with key summary statistics. However, for the summary tables, unless stated otherwise, data from industry own‐control programmes and hazard analysis and critical control point (HACCP) sampling as well as data from suspect sampling, selective sampling and outbreak or clinical investigations are excluded. If MS reported only regional data without reporting statistics at the national level, these were not extracted in the summary tables.
Statistical trend analyses were carried out to evaluate the significance of temporal variations in the EU and the specifications of these analyses are explained in each separate chapter. For the human cases trend analyses were covered by data from the EU/European Economic Area (EEA). Also in humans, the implemented general‐use statistical tests must be viewed as hypotheses‐generating, not as confirmatory tests. Analyses other than trend analyses in humans are performed for confirmed and EU cases only (and EEA cases were not included).
Spatial trends in food and animals were visualised using the R software ( http://www.r-project.org); packages ggplot2, lattice and tmap as well as ArcGIS from the Economic and Social Research Institute (ESRI). Choropleth maps with graduated colours over a continuous scale of values were used to map the proportion of positive sample units across the EU and other reporting countries.
The Appendix lists all data summarised in tables and figures for the production of this report, for humans, foods, animals, feed and FBOs.
Comparability and quality of the data
Humans
For data on human infections, please note that the numbers presented in this report may differ from national zoonoses reports due to differences in case definitions used at EU and national level or because of different dates of data submission and extraction. Results are generally not directly comparable between MS and sometimes not even between different years in one country.
Food, animals, feed and food‐borne outbreaks
For data on food, animals and feed please note that the numbers presented in this report may differ from national zoonoses reports due to different dates of data submission and extraction.
The data obtained in the EFSA DCF can vary according the level of data quality and harmonisation. Therefore, the type of data analyses suggested by EFSA strongly depends on this level of harmonisation and can either be a descriptive summary, or trend watching or a full trend analysis of the monitoring data. To make this clear for the reader, EFSA consistently proposed a type of analysis according to Table 1 and adopted from Boelaert et al. (2016). The table shows that the data can be divided into three main categories according to the sampling stage, the matrices collected and the zoonotic agent monitored.
Table 1.
Categorisation of data used in EUSR 2017 (adapted from Boelaert et al., 2016)
| Category | Type of analyses | Type/comparability between MS | Examples |
|---|---|---|---|
| I |
Descriptive summaries at national level and EU levelEU trend watching (trend monitoring) Spatial and temporal trends analyses at the EU level |
Programmed harmonised monitoring or surveillance Comparable between MS; results at EU level are interpretable |
Salmonella national control programmes in poultry; bovine tuberculosis; bovine and small ruminant brucellosis; Trichinella in pigs at slaughterhouse; Echinococcus granulosus at slaughterhouse |
| II |
Descriptive summaries at national level and EU levelEU trend watching (trend monitoring) No trend analysis at the EU level |
Not fully harmonised monitoring or surveillanceNot fully comparable between MS; caution needed when interpreting results at the EU level |
Food‐borne outbreak data. Monitoring of compliance with process hygiene and food safety criteria for L. monocytogenes, Salmonella and E. coli according Reg. No. 2073/2005. Monitoring of Rabies |
| III |
Descriptive summaries at national level and EU levelNo EU trend watching (trend monitoring) No trend analysis at the EU level |
Non‐harmonised monitoring or surveillance data with no (harmonised) reporting requirements Not comparable between MS; extreme caution needed when interpreting results at the EU level |
Campylobacter; Yersinia; Q‐fever; Francisella tularensis; West Nile virus; Taenia spp.; other zoonoses; Toxoplasma |
Summary human zoonoses data, EU, 2017
The numbers of confirmed human cases of 14 zoonoses presented in this report are summarised in Figure 1. In 2017, campylobacteriosis was the most commonly reported zoonosis as it has been since 2005, representing alone almost 70% of all the reported cases. Campylobacteriosis was followed by other bacterial diseases; salmonellosis, yersiniosis and STEC infections in being the most frequently reported. Severity of the diseases was analysed based on hospitalisation and outcome of the reported cases (Table 2). Based on data on severity, listeriosis was the most severe zoonoses with the highest hospitalisation and mortality rate followed by West Nile fever infection. Almost all confirmed cases with data available on hospitalisation for these two diseases were hospitalised. One out of every seven and one out of nine confirmed listeriosis and West Nile fever cases, respectively, with known data were fatal.
Figure 1.
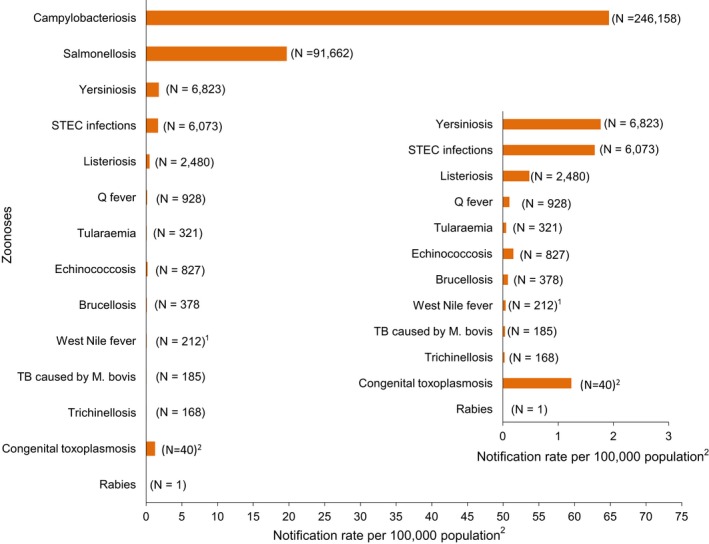
- Note: Total number of confirmed cases is indicated in parenthesis at the end of each bar.
- 1Exception: West Nile fever where total number of cases were used.
- 2Exception: congenital toxoplasmosis notification rate per 100,000 live births.
Table 2.
Reported hospitalisation and case fatalities due to zoonoses in confirmed human cases in the EU, 2017
| Disease | Number of confirmeda | Hospitalisation | Deaths | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Human cases | Status available (%) | Number of reporting MSb | Reported hospitalised cases | Proportion hospitalised (%) | Outcome available (%) | Number of reporting MSb | Reported | Case | |
| Deaths | Fatality (%) | ||||||||
| Campylobacteriosis | 246,158 | 27.6 | 17 | 20,810 | 30.5 | 72.8 | 16 | 45 | 0.04 |
| Salmonellosis | 91,662 | 43.1 | 14 | 16,796 | 42.5 | 67.8 | 17 | 156 | 0.25 |
| Yersiniosis | 6,823 | 27.1 | 14 | 616 | 33.4 | 65.5 | 15 | 3 | 0.07 |
| STEC infections | 6,073 | 41.0 | 18 | 933 | 37.5 | 66.1 | 21 | 20 | 0.50 |
| Listeriosis | 2,480 | 40.4 | 16 | 988 | 98.6 | 65.8 | 18 | 225 | 13.8 |
| Q‐fever | 928 | NAc | NA | NA | NA | 56.0 | 10 | 7 | 1.35 |
| Echinococcosis | 827 | 31.2 | 14 | 140 | 54.3 | 30.1 | 14 | 1 | 0.40 |
| Brucellosis | 378 | 45.8 | 10 | 104 | 60.1 | 33.9 | 10 | 1 | 0.78 |
| Tularaemia | 321 | 38.3 | 9 | 76 | 61.8 | 51.1 | 9 | 1 | 0.6 |
| West Nile fever a | 212 | 72.2 | 8 | 134 | 87.6 | 98.6 | 9 | 25 | 12.0 |
| Trichinellosis | 168 | 44.6 | 9 | 56 | 74.7 | 40.5 | 9 | 0 | 0.0 |
| Congenital toxoplasmosis | 40 | 57.9 | 3 | 18 | NA | 63.2 | 3 | 0 | 0.0 |
| Rabies | 1 | NAc | NA | NA | NA | 0.0 | 0 | NA | NA |
Exception: West Nile fever where total number of cases were included.
Not all countries observed cases for all diseases.
NA: Not applicable as the information is not collected for this disease.
1. Campylobacter
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1475841
1.1. Abstract
In 2017, Campylobacter was the most commonly reported gastrointestinal bacterial pathogen in humans in the EU and has been so since 2005. The number of reported confirmed cases of human campylobacteriosis was 246,158 with an EU notification rate of 64.8 per 100,000 population. This represents a slight decrease compared with 2016. There was a significantly increasing trend over the period 2008–2017; however, in the last 5 years (2013–2017), the EU/EEA trend has not shown any statistically significant increase or decrease. Half of the MS reported significantly increasing trends in the long term (2008–2017) and one‐third in the short term (2013–2017). Despite the high number of human campylobacteriosis cases, their severity in reported case fatality was low (0.04%), even though this was the third most common cause of mortality among the pathogens considered.
From food and animals, about two‐thirds of MS reported Campylobacter monitoring data for the year 2017. Eighteen and 10 MS reported monitoring results of Campylobacter in fresh meat from broilers and turkeys, respectively. In fresh meat, the occurrence of Campylobacter is still high ranging from 37.4% to 31.5% in broilers and turkeys, respectively. Up to nine MS reported on Campylobacter in milk and milk products (including cheeses) with an occurrence lower than 2%. For the year 2017, one MS, Spain, reported on Campylobacter contamination levels from chilled broiler carcasses and 66 (44%) out of 150 tested carcasses were carrying more than 1,000 colony forming units per gram (CFU/g) of Campylobacter. Few MS reported 2017 monitoring data on Campylobacter in animals and most samples originated from broilers (6 MS, 12.3% positive units). None of the MS reported monitoring data from turkeys. The highest proportion positive sampled units (29.3%) was reported in cats and dogs from 7 MS followed by pigs (17.6%) by 10 MS. In addition to the low volumes of food and animal monitoring data reported from investigations on Campylobacter, the sampling and reporting rules are not harmonised, so precluding trend analyses and trend watching. Together these deficiencies prevent inferences being made, beyond the sample statistics, on trends or sources of Campylobacter in foods or animals.
1.2. Surveillance and monitoring of Campylobacter in the EU
1.2.1. Humans
The notification of campylobacteriosis is mandatory in most EU MS, Iceland, Norway and Switzerland, except for six EU MS, where notification is based on a voluntary system (Belgium, France, Italy, Luxembourg and the Netherlands) or other systems (the United Kingdom). No surveillance system exists in Greece. The surveillance systems for campylobacteriosis cover the whole population in all MS except four (France, Italy, the Netherlands and Spain). The coverage of the surveillance system is estimated to be 20% in France and 52% in the Netherlands. These proportions of populations were used in the calculation of notification rates for these two MS. No estimate of population coverage in Italy and Spain was provided, so notification rates were not calculated for these two MS.
In Belgium, full national coverage was established in 2015 and rates before this date are not displayed. All countries report case‐based data except Belgium and Bulgaria, which reported aggregated data. Both reporting formats were included to calculate numbers of cases, notification rates and disease trends.
Diagnosis of human infection is generally based on culture from human stool samples and both culture and non‐culture methods (polymerase chain reaction (PCR)) are used for confirmation. Biochemical tests or molecular methods are used for species determination of isolates submitted to the National Reference Laboratory.
1.2.2. Food and animals
Monitoring data on Campylobacter from food and animals and submitted to EFSA (according to Chapter II (‘monitoring of zoonoses and zoonotic agents’) of the Zoonoses Directive 2003/99/EC) are collected without harmonised design. These data allow for descriptive summaries at the EU level to be made. They preclude trend analyses and trend watching at the EU level (Table 3).
Table 3.
The surveillance and monitoring of Campylobacter in food and animals according to the sampling stage, the sampler and the objective of the sampling
| Preharvest (animals) | Harvest and processing (food) | Retail (food) | |
|---|---|---|---|
| Sampler and context | Official sampling by CA. Private sampling by veterinarians. Monitoring and surveillance; surveys; clinical investigations | Official sampling by CA; industry sampling by FBOp.Monitoring and surveillance; surveys; surveillance for process hygiene criteria foreseeing the compliance with Regulation No. 2017/1495 | Official sampling by CA; industry sampling by FBOp.Monitoring and surveillance; surveys |
| Samples | Detection of Campylobacter from animal faeces Animal faeces, organs, tissues, preputial lavages (artificial insemination centres) | Detection and quantification of Campylobacter in food‐producing animals at the slaughterhousea, and processing and cutting plants | Detection of Campylobacter at retail, catering, hospital care facilities and automatic distribution for consumers (self‐service machines) |
| Objective of the sampling |
Assess the occurrence or prevalence in animals, livestock, zoo animals and pets. Clinical diagnosis or exclusion of campylobacteriosis |
Compliance with own checks and HACCP systems (food management system). Compliance with Regulation No. 2017/1495 (process hygiene criterion) |
Compliance with own checks and HACCP systems (food management system) |
CA: competent authorities; FBOp: food business operators; HACCP: Hazard Analysis and Critical Control Point;
Commission Regulation (EU) 2017/14955 of 23 August 2017 amending Regulation (EC) No. 2073/2005 as regards Campylobacter in broiler carcasses.
Sampling of animals at slaughterhouses can also be used to reflect prevalence at preharvest (although sampling is performed at abattoir level.
In 2017, data on food reported to EFSA by MS and non‐MS were mainly derived from official, industry and private sampling in the context of national monitoring and surveillance and/or organised surveys. Other monitoring data on poultry meat were collected in 2017 according to the process hygiene criterion described in Regulation (EC) No. 2017/14955 amending Regulation (EC) No. 2073/2005 and in force since 1 January 2018. The criterion is relevant for FBOp and a limit of (< 1,000 CFU/g) applies. This new Regulation aims to keep Campylobacter in broiler carcasses under control and to reduce the number of human campylobacteriosis cases attributable to the consumption of poultry meat. The reporting of monitoring data collected by the competent authorities (CA) and verifying the compliance with the new Campylobacter process hygiene criterion becomes mandatory from 2020 onwards.
Monitoring data from animals provided by MS and non‐MS to EFSA are mainly derived from non‐harmonised official, industry and private sampling in the context of national monitoring and surveillance and/or organised surveys. Other reported samples were from clinical investigations by private veterinarians and industry (artificial insemination centres).
Detection of Campylobacter in food and animals is generally based on culture. Biochemical, molecular methods (PCR) and mass spectrometry (such as matrix‐assisted laser desorption/ionisation, time‐of‐flight mass spectrometry (MALDI‐TOF‐MS)), are used for confirmation.
1.2.3. Food‐borne outbreaks of human campylobacteriosis
The reporting of FBO of human campylobacteriosis is mandatory according the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
1.3. Results
1.3.1. Overview of key statistics along the food chain, EU, 2013–2017
Table 4 summarises EU level statistics related to human campylobacteriosis, and to Campylobacter occurrence and prevalence in foods and animals, respectively, in the EU, during 2013–2017. A more detailed description of these statistics is in the results section of this chapter and in the chapter on FBO.
Table 4.
Summary of Campylobacter statistics related to humans and major food categories in the EU, 2013–2017
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 246,158 | 246,917 | 232,134 | 236,818 | 214,710 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 64.8 | 66.3 | 62.9 | 66.5 | 61.4 | ECDC |
| Number of reporting MS | 27 | 27 | 27 | 26 | 26 | ECDC |
| Infection acquired in the EU | 122,242 | 122,781 | 142,536 | 135,822 | 120,521 | ECDC |
| Infection acquired outside the EU | 6,580 | 5,963 | 6,430 | 6,817 | 6,786 | ECDC |
| Unknown travel status or unknown country of infection | 117,336 | 118,173 | 83,168 | 94,179 | 87,403 | ECDC |
| Number of outbreak‐related cases | 1,445 | 4,655 | 1,488 | 2,082 | 1,836 | EFSA |
| Total number of outbreaks | 395 | 476 | 399 | 454 | 417 | EFSA |
| Food a | ||||||
| Meat and meat products b | ||||||
| Number of sampled units | 20,287 | 18,048 | 16,134 | 15,758 | 21,383 | EFSA |
| Number of reporting MS | 21 | 19 | 18 | 20 | 20 | EFSA |
| Milk and milk products c | ||||||
| Number of sampled units | 2,154 | 1,896 | 2,126 | 2,708 | 3,324 | EFSA |
| Number of reporting MS | 11 | 10 | 10 | 10 | 10 | |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member State.
The summary statistics, referring to Member States, were obtained by summing all sampling units (single, batch, slaughter batch), sampling stage (farm, packing centre, automatic distribution system for raw milk, processing plant, cutting plant, slaughterhouse, catering, hospital or medical care facility, restaurant or cafe or pub or bar or hotel or catering service, retail, wholesale, unspecified), sampling strategies (census, convenience sampling, objective sampling, selective sampling, suspected sampling, unspecified) and sampler (industry sampling, official and industry sampling, official sampling, private sampling, unspecified, not applicable).
Meat/meat products refer to carcasses and fresh meat/RTE, cooked and fermented products.
Milk/milk products refer to raw milk/dairy products including cheeses.
Food data of interest reported were classified into the major categories ‘Meat and meat products’ and ‘Milk and milk products’, and aggregated by year over the period 2013–2017 to get an annual overview of the data submitted. In the summary table, data from suspect and selective sampling and from industry own‐control programmes and HACCP sampling were excluded. The number of sampled units reported for 2017 for these two major categories as well as the number of reporting MS increased compared with 2016.
1.3.2. Human campylobacteriosis
For 2017, human campylobacteriosis data were reported by 27 EU MS with 246,158 confirmed cases, resulting in an EU notification rate of 64.8 cases per 100,000 population (Table 5). This was a slight decrease compared with 2016 (66.3 cases per 100,000 population).
Table 5.
Reported human cases of campylobacteriosis and notification rates per 100,000 population in the EU/EFTA, by country and year, 2013–2017
| Country | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 7,204 | 7,204 | 82.1 | 7,083 | 81.5 | 6,258 | 73.0 | 6,514 | 76.6 | 5,731 | 67.8 |
| Belgium | Y | A | 8,649 | 8,649 | 76.2 | 10,055 | 88.9 | 9,066 | 80.7 | 8,098 | – | 8,148 | – |
| Bulgaria | Y | A | 196 | 195 | 2.7 | 202 | 2.8 | 227 | 3.2 | 144 | 2.0 | 124 | 1.7 |
| Croatia | Y | C | 1,694 | 1,686 | 40.6 | 1,524 | 36.4 | 1,393 | 33.0 | 1,647 | 38.8 | 0 | 0.0 |
| Cyprus | Y | C | 20 | 20 | 2.3 | 21 | 2.5 | 29 | 3.4 | 40 | 4.7 | 56 | 6.5 |
| Czech Republic | Y | C | 24,508 | 24,326 | 230.0 | 24,084 | 228.2 | 20,960 | 198.9 | 20,750 | 197.4 | 18,267 | 173.7 |
| Denmark | Y | C | 4,255 | 4,255 | 74.0 | 4,712 | 82.6 | 4,327 | 76.5 | 3,773 | 67.0 | 3,772 | 67.3 |
| Estonia | Y | C | 347 | 285 | 21.7 | 298 | 22.6 | 318 | 24.2 | 285 | 21.7 | 382 | 28.9 |
| Finland | Y | C | 4,289 | 4,289 | 77.9 | 4,637 | 84.5 | 4,588 | 83.8 | 4,889 | 89.7 | 4,066 | 74.9 |
| Franceb | N | C | 6,579 | 6,579 | 49.1 | 6,698 | 50.2 | 6,074 | 45.7 | 5,958 | 45.2 | 5,198 | 39.6 |
| Germany | Y | C | 69,414 | 69,178 | 83.8 | 73,663 | 89.6 | 69,829 | 86.0 | 70,571 | 87.4 | 63,280 | 78.6 |
| Greecec | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Hungary | Y | C | 7,840 | 7,807 | 79.7 | 8,556 | 87.0 | 8,342 | 84.6 | 8,444 | 85.5 | 7,247 | 73.5 |
| Ireland | Y | C | 2,788 | 2,779 | 58.1 | 2,511 | 53.1 | 2,453 | 53.0 | 2,593 | 56.3 | 2,288 | 49.8 |
| Italyd | N | C | 1,060 | 1,060 | – | 1,057 | – | 1,014 | – | 1,252 | – | 1,178 | – |
| Latvia | Y | C | 61 | 59 | 3.0 | 90 | 4.6 | 74 | 3.7 | 37 | 1.8 | 9 | 0.4 |
| Lithuania | Y | C | 993 | 990 | 34.8 | 1,225 | 42.4 | 1,186 | 40.6 | 1,184 | 40.2 | 1,139 | 38.3 |
| Luxembourg | Y | C | 613 | 613 | 103.8 | 518 | 89.9 | 254 | 45.1 | 873 | 158.8 | 675 | 125.7 |
| Malta | Y | C | 231 | 231 | 50.2 | 212 | 48.8 | 248 | 57.8 | 288 | 67.7 | 246 | 58.4 |
| Netherlandse | N | C | 2,890 | 2,890 | 32.5 | 3,383 | 38.3 | 3,778 | 43.0 | 4,159 | 47.5 | 3,702 | 42.4 |
| Poland | Y | C | 874 | 874 | 2.3 | 773 | 2.0 | 653 | 1.7 | 650 | 1.7 | 552 | 1.4 |
| Portugal | Y | C | 602 | 596 | 5.8 | 359 | 3.5 | 271 | 2.6 | – | – | – | – |
| Romania | Y | C | 479 | 467 | 2.4 | 517 | 2.6 | 311 | 1.6 | 256 | 1.3 | 218 | 1.1 |
| Slovakia | Y | C | 7,057 | 6,946 | 127.8 | 7,623 | 140.5 | 6,949 | 128.2 | 6,744 | 124.5 | 5,845 | 108.0 |
| Slovenia | Y | C | 1,408 | 1,408 | 68.2 | 1,642 | 79.5 | 1,328 | 64.4 | 1,184 | 57.4 | 1,027 | 49.9 |
| Spaind | N | C | 18,860 | 18,860 | – | 15,542 | – | 13,227 | – | 11,481 | – | 7,064 | – |
| Sweden | Y | C | 10,608 | 10,608 | 106.1 | 11,021 | 111.9 | 9,180 | 94.2 | 8,288 | 85.9 | 8,114 | 84.9 |
| United Kingdom | Y | C | 63,304 | 63,304 | 96.2 | 58,911 | 90.1 | 59,797 | 92.2 | 66,716 | 103.7 | 66,382 | 103.9 |
| EU Total | – | – | 246,823 | 246,158 | 64.8 | 246,917 | 66.3 | 232,134 | 62.9 | 236,818 | 66.5 | 214,710 | 61.4 |
| Iceland | Y | C | 119 | 119 | 35.2 | 128 | 38.5 | 119 | 36.2 | 142 | 43.6 | 101 | 31.4 |
| Norway | Y | C | 3,884 | 3,884 | 73.9 | 2,317 | 44.5 | 2,318 | 44.9 | 3,386 | 66.3 | 3,291 | 65.2 |
| Switzerlandf | Y | C | 7219 | 7219 | 85.4 | 7,980 | 94.4 | 7,070 | 84.5 | 7,571 | 91.5 | 7,480 | 92.6 |
Y: yes; N: no; A: aggregated data; C: case‐based data; –: no report.
Sentinel surveillance; no information on estimated coverage. So, notification rate cannot be estimated.
Sentinel surveillance; notification rates calculated with estimated coverage of 20%.
No surveillance system.
Sentinel surveillance; notification rates calculated with estimated coverage 52%.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
The highest country‐specific notification rates in 2017 were observed, as in previous years, in the Czech Republic (230.0 cases per 100,000), Slovakia (127.8), Sweden (106.1) and Luxembourg (103.8). The lowest rates in 2017 were observed in Bulgaria, Cyprus, Latvia, Poland, Portugal and Romania (≤ 5.8 per 100,000).
The majority (94.9%) of the campylobacteriosis cases reported with known origin were infected in the EU (Table 4). The highest proportions of domestic cases (> 94%) were reported in the Czech Republic, Hungary, Latvia, Malta, Poland, Portugal, Romania and Slovakia. The highest proportions of travel‐associated cases with known data about importation were reported by the Nordic countries: Finland (78.5%), Denmark (46.9%), Sweden (41.5%), Iceland (67.4%) and Norway (53.5%). Among 14,258 travel‐associated cases with known probable country of infection, more than half (53.9%) of the cases were linked to travel within the EU, with most of the cases linked to travel to Spain, Greece and Bulgaria (17.0, 4.1 and 3.9%, respectively). Thailand, Turkey and Morocco were most often reported as the probable country of infection outside EU (11.0, 4.1 and 3.7%, respectively).
Between 2013 and 2017, there was a clear seasonality in the number of confirmed campylobacteriosis cases reported in the EU/EEA, with peaks in the summer months. Annual winter peaks, albeit with lower numbers compared with summer, were also observed in January starting from 2012. In 2017, the winter peak continued until March. Over the period from 2008 to 2017, a significant increasing trend was observed in EU/EEA (p < 0.05); however, the trend did not show any significant increase or decrease in the period 2013–2017 (Figure 2).
Figure 2.
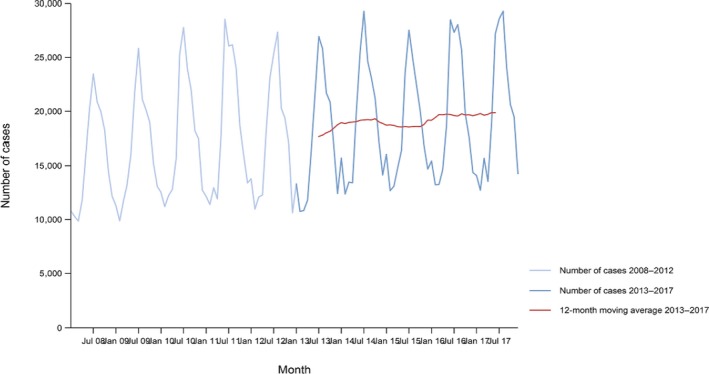
- Source(s): Austria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and United Kingdom. Belgium, Bulgaria, Croatia and Portugal did not report data to the level of detail required for the analysis. In Greece, campylobacteriosis is not under surveillance.
At country level, 14 MS (Austria, the Czech Republic, Estonia, France, Hungary, Ireland, Italy, Lithuania, Malta, Poland, Slovakia, Slovenia, Spain and Sweden) reported significantly increasing trends between 2008 and 2017. Cyprus was the only MS that reported decreasing (p < 0.01) trends, both in 2008–2017 and 2013–2017.
In 2013–2017, nine MS continued to report increasing trends (Austria, the Czech Republic, France, Hungary, Latvia, Poland, Slovenia, Spain and Sweden). In four MS (Estonia, Ireland, Italy and Malta), no significant change was observed.
Information on hospitalisation status was provided for 27.6% of all campylobacteriosis cases by 17 MS in 2017. Of cases with known hospitalisation status, 30.5% were hospitalised. The highest hospitalisation rates (80–100%) were reported in Cyprus, Latvia, Poland, Romania and the United Kingdom.
The outcome was reported for 72.8% of all cases by 16 MS. The number of reported deaths attributed to campylobacteriosis increased from 25 deaths in 2014 to 72 deaths in 2017, resulting in an EU case fatality of 0.04%. This was similar to the average percentage of fatal outcome observed over the last 5 years.
Campylobacter species information was provided by all MS for 54.1% of confirmed cases reported in the EU, which was at the same level as in 2016 (53.2%). Of these, 84.4% were Campylobacter jejuni, 9.2% Campylobacter coli, 0.1% Campylobacter lari, 0.1% Campylobacter fetus and 0.1% Campylobacter upsaliensis. ‘Other’ Campylobacter species accounted for 6.2%, but the large majority of those cases was reported at the national level as ‘C. jejuni/C. coli/C. lari not differentiated’.
Human campylobacteriosis cases associated with food‐borne outbreaks
Campylobacter was identified in 33 strong‐evidence and 362 weak‐evidence food‐borne (including waterborne) outbreaks that together affected 1,445 people (notified FBO cases) in EU, with 207 hospitalised and one death, as reported to EFSA. Overall, for the year 2017, there were 114,564 domestic (acquired within the reporting country) cases reported to the TESSy (Table 6), which was 93.7% of the number of reported human campylobacteriosis cases infected domestically and through travel within EU during 2017 (122,242, Table 4). Table 6 shows data reported by countries to TESSy managed by ECDC and to the FBOs database managed by EFSA. It is important to clarify that the case classification for reporting is different between these two databases. In TESSy, the cases reported are classified based on the EU case definition. All these cases visited a doctor, and are either confirmed by laboratory test (confirmed case) or not (probable case and classification is based on the clinical symptoms and epidemiological link). Cases that never visited a doctor are not reported to TESSy. Moreover, probable cases may be missing in TESSy, as these data are not analysed or published and there is no incentive for reporting such cases. Information on which case is linked to an outbreak ‐ and which not ‐ is not systematically collected. In practice, the cases reported to TESSy are considered mostly sporadic cases. In food‐borne disease outbreak situations cases are also classified into confirmed or probable outbreak cases, but currently these data are not collected by EFSA.
Table 6.
Statistics related to the proportions of human food‐borne outbreak cases caused by Campylobacter (including waterborne outbreaks), EU/EFTA, 2017
| Country | ECDC | EFSA | ||||
|---|---|---|---|---|---|---|
| Confirmed human | Food‐borne outbreaks (including waterborne outbreaks) | |||||
| Total | Travel related | Domestic | Unknown or missing | Human cases (illnesses) | FBO | |
| N | N | N | N | N | N | |
| Austria | 7,204 | 657 | 6,516 | 31 | 61 | 24 |
| Belgium | 8,649 | – a | – | 8,649 | 18 | 4 |
| Bulgaria | 195 | – | – | 195 | –b | – |
| Croatia | 1,686 | 1 | 113 | 1,572 | 44 | 6 |
| Cyprus | 20 | – | – | 20 | – | – |
| Czech Republic | 24,326 | 314 | 24,012 | 0 | 17 | 1 |
| Denmark | 4,255 | 1,097 | 1,242 | 1,916 | 72 | 2 |
| Estonia | 285 | 20 | 265 | 0 | – | – |
| Finland | 4,289 | 2,351 | 643 | 1,295 | 13 | 3 |
| France | 6,579 | – | – | 6,579 | 207 | 40 |
| Germany | 69,178 | 5,989 | 34,244 | 28,945 | 552 | 147 |
| Greece | – | – | – | – | – | – |
| Hungary | 7,807 | 7 | 7,800 | 0 | – | – |
| Ireland | 2,779 | 18 | 120 | 2,641 | 20 | 4 |
| Italy | 1,060 | 46 | 144 | 870 | 2 | 1 |
| Latvia | 59 | 0 | 59 | 0 | 6 | 3 |
| Lithuania | 990 | 13 | 752 | 225 | 15 | 7 |
| Luxembourg | 613 | – | – | 613 | – | – |
| Malta | 231 | 5 | 223 | 3 | 17 | 8 |
| Netherlands | 2,890 | 299 | 2,450 | 141 | 12 | 5 |
| Poland | 874 | 1 | 827 | 46 | 2 | 1 |
| Portugal | 596 | 6 | 558 | 32 | – | – |
| Romania | 467 | 0 | 467 | 0 | – | – |
| Slovakia | 6,946 | 42 | 6,904 | 0 | 133 | 117c |
| Slovenia | 1,408 | 19 | 3 | 1,386 | – | – |
| Spain | 18,860 | 7 | 8,063 | 10,790 | 110 | 11 |
| Sweden | 10,608 | 4,279 | 6,028 | 301 | 8 | 4 |
| United Kingdom | 63,304 | 1,564 | 13,131 | 48,609 | 146 | 9 |
| EU Total | 246,158 | 16,735 | 114,564 | 114,859 | 1,445 | 395 |
| Iceland | 119 | 66 | 32 | 21 | 0 | 1 |
| Norway | 3,884 | 1,713 | 1,489 | 682 | 19 | 3 |
| Switzerland | 7,219 | – | – | 7,219 | 20 | 1 |
No importation data reported.
No food‐borne outbreaks caused by Campylobacter reported.
In case the number of illnesses is less than twice the number of FBO (one FBO at least involves two affected people), the MS reported a number of FBO with an unknown number of illnesses to EFSA.
The highest number of Campylobacter strong‐ or weak‐evidence FBOs (excluding strong‐evidence waterborne outbreaks) was reported by Germany (147 outbreaks, 37.4%) with 552 cases (38.5%) followed by Slovakia (117 outbreaks, 29.8%) with 133 cases (9.3%) and one reported death case after hospitalisation. Two weak‐evidence waterborne outbreaks were also reported affecting 10 people. The highest number of 2017 strong‐evidence outbreaks caused by Campylobacter spp. (excluding strong‐evidence waterborne outbreaks) originated from milk and from broiler meat, with 18 and 8 reported outbreaks out of 33 strong‐evidence outbreaks, respectively. Broiler meat and milk are a significant source of human infection due to Campylobacter (Table 7).
Table 7.
Distribution of strong‐evidence outbreaks caused by Campylobacter (excluding strong‐evidence waterborne outbreaks), by food vehicle, EU, 2017
| Food vehicle | Number of strong‐evidence FBO | % of total |
|---|---|---|
| Milk | 18 | 54.5 |
| Dairy products (other than cheeses) | 2 | 6.1 |
| Broiler meat (Gallus gallus) and their products | 8 | 24.2 |
| Other or mixed red meat and their products | 2 | 6.1 |
| Other, mixed or unspecified poultry meat and their products | 2 | 6.1 |
| Meat and meat products | 1 | 3.0 |
| Total | 33 | 100.0 |
FBO: food‐borne outbreak.
Note: Data from 33 outbreaks are included: Denmark (1), Finland (2), France (3), Germany (16), Slovakia (2), Spain (1) and United Kingdom (8).
1.3.3. Campylobacter in foods
Table 8 summarises the reported occurrence of Campylobacter in the most important food categories in 2017. Few MS reported data on Campylobacter in food: 18 MS and 10 MS reported data on fresh meat from broilers and turkeys, respectively. Highest occurrence was observed in fresh meat from broilers (37.4%) followed by fresh meat from turkeys (31.5%). Very few MS (1–5) reported on RTE meat products with occurrence ranging between 0 and 1.1%.
Table 8.
Summary of Campylobacter statistics related to major food categories and animal species, reporting Member States and non‐Member States, EU, 2017
| Food category | Animal species | Number of reporting (MS/non‐MS) | Number of tested unitsa, EU | Proportion (%) of positive units, EU |
|---|---|---|---|---|
| Fresh Meat | Broilers | 18/1 | 13,445 | 37.4 |
| Turkeys | 10/1 | 1,028 | 31.5 | |
| Poultry (other than Broilers and Turkey) | 8/0 | 1,425 | 27.7 | |
| Pigs | 6/0 | 843 | 6.9 | |
| Bovine animals | 6/0 | 1,456 | 1.4 | |
| Meat products, RTE | Broilers | 3/1 | 101 | 0 |
| Turkeys | 1/0 | 11 | 0 | |
| Pigs | 5/0 | 178 | 1.1 | |
| Bovine animals | 2/0 | 16 | 0 | |
| Unspecified | 5/0 | 74 | 0 | |
| Milk and milk products | Milk | 9/0 | 1,554 | 1.9 |
| Cheese | 8/0 | 522 | 0.5 | |
| Animals | Broilers | 6/2 | 10,077 | 12.3 |
| Turkeys | 0/1 | 0 | 0 | |
| Pigs | 10/2 | 3,817 | 17.6 | |
| Bovine animals | 11/2 | 9,147 | 6.9 | |
| Cats and dogs | 7/2 | 1,176 | 29.3 | |
| Other animalsb | 8/2 | 5,817 | 6.3 |
RTE: ready‐to‐eat; MS: Member State.
From 640 Campylobacter samples from broilers, 94% were documented as C. jejuni and the remaining 6% as C. coli.
The summary statistics were obtained summing all sampling units (single and batch samples).
Sheep, goat, other ruminants, birds, wild animals, other pets including exotic animals, rodents, zoo animals.
Spain was the only MS that reported quantitative monitoring data collected according to the process hygiene criterion described in Regulation (EC) No. 2017/1495 (see Section 1.2). Of the 150 neck skin samples from chilled broiler carcasses, 66 (44%) exceeded the limit and tested ≥ 1,000 CFU/g of which 53 (84%) ranged between 1,000 and 10,000 CFU/g and 13 tested > 10,000 CFU/g. Overall, 56 samples out of the 66 that exceeded the limit of 1,000 CFU/g were reported as C. jejuni.
Campylobacter in milk and cheeses was reported for the year 2017 by nine and eight MS, respectively. The overall occurrence was lower than 2%. One‐third of the collected milk samples (cows’ milk) originated from Germany. The only positive cheese samples, three sheep cheeses out of 522, were reported by Slovakia and were from the retail level.
None of the foods of non‐animal origin (fruit and vegetables) reported by seven MS tested positive for Campylobacter.
Campylobacter species information was provided by MS and non‐MS for fresh meat and meat products from broiler (n = 1,201): 73.6% were C. jejuni and 26.3% were C. coli. Only one strain was serotyped as C. lari and reported by Germany. From fresh meat and meat products from turkeys (n = 65) 60% were C. jejuni strains and 40% C. coli; and for milk and milk products (n = 21) C. jejuni was mostly reported (95%) followed by C. coli.
1.3.4. Campylobacter in animals
In 2017, few MS and non‐MS reported monitoring data on Campylobacter in animals. Most samples originated from broilers and from bovine animals (Table 8). Two‐thirds of reported monitoring data from bovine animals and pigs originated from the Netherlands.
Only Iceland reported on the occurrence and prevalence of Campylobacter in turkeys (2 positive batches out of 71 from fattening turkeys).
1.4. Discussion
Campylobacteriosis has been the most commonly reported zoonosis in humans in the EU since 2005. There has been a significantly increasing trend in the number of cases at EU/EEA level and at country level in half of the MS between 2008 and 2017. The EU notification rate however, did not change significantly over the last 5 years. One‐third of the MS had increasing trends also in the period 2013–2017. The increase in reported cases in some countries may not only reflect changes in exposure, but also improvements in MS surveillance systems. In Poland, the increase of human cases may relate to a better coverage of routine diagnostics across the country, requirement for medical laboratories to report positive test results, and better knowledge and awareness among physicians. In the Czech Republic, testing and diagnostics for campylobacteriosis has improved since 2013. In Spain, coverage of the surveillance system for campylobacteriosis has improved and the number of reported confirmed cases has more than doubled since 2013. In Sweden, an outbreak of Campylobacter starting from 2016 until mid‐June 2017 resulted in almost the double number of domestic human cases compared with previous years (Folkhalsomyndigheten, 2017).
Campylobacter has a characteristic seasonality with a sharp increase of cases in the summer and early autumn. Evidence has shown that Campylobacter tends to be more prevalent during warmer times of the year; however, a smaller but distinct winter peak has become apparent in the past few years, including 2017. The peak of cases was mainly seen in five MS (Austria, Belgium, Germany, Luxembourg and the Netherlands) covering more than 45% of all cases reported in January. The observed winter peak in Campylobacter infections in Switzerland has been partly attributed to a traditional meal, meat fondue, especially if served with chicken meat (Bless et al., 2014). In 2017, the winter peak continued until March. This was due to the outbreak in Sweden with higher number of cases throughout the winter and spring. The outbreak was linked to the increase of Campylobacter in a major domestic broiler abattoir (Dryselius, 2017).
In some countries, the surveillance is known to focus mainly on severe cases. The proportion of hospitalised campylobacteriosis cases was higher than expected in some MS, which also reported the lowest notification rates. In others, hospitalisation status is ascertained and reported for a higher fraction of cases by hospitals, while for cases reported from other sources, e.g. laboratories, hospitalisation status is often missing. Both factors result in an overestimation of the proportion of hospitalised cases.
From food and animals, about two‐thirds to one‐third of MS reported Campylobacter monitoring data on some major categories of food and animals for the year 2017. In addition to the low volume of data reported, sampling and reporting rules are not harmonised, precluding trend analyses and trend watching. These deficiencies prevent inference being made, beyond the sample statistics, on trends or sources of Campylobacter in foods or animals (Boelaert et al., 2016). Despite this, reports from monitoring data with the aim to understand trends and sources of Campylobacter along the food chain remains essential to the overall goal of reducing campylobacteriosis, whether food‐borne or sporadic. Since 1 January 2018, a new process hygiene criterion for Campylobacter is laid out in Regulation (EC) No. 2017/1495. The criterion is relevant for FBOp and the limit of < 1,000 CFU/g applies to samples taken for official control to verify whether the criterion has been met. This new Regulation aims to keep Campylobacter in broiler carcasses under control and to reduce the number of human campylobacteriosis cases attributable to the consumption of poultry meat. The reporting of monitoring data collected by the CA and verifying the compliance with the new Campylobacter process hygiene criterion becomes mandatory from year 2020 onwards. For the year 2017, one MS, Spain, reported on Campylobacter contamination levels from chilled broiler carcasses and nearly half of the tested carcasses were carrying more than 1,000 CFU/g of Campylobacter. In comparison, the latest retail figures of contamination levels in UK6 showed that, on average, across the major retailers, 3.7% of carcasses tested positive for the highest level of contamination, which is more than 1,000 CFU/g; the corresponding figure for the previous set of results (January–March 2018) was 3.8%, while for the first publication (July–September 2017), it was 4.6%.
1.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Fact sheet on Campylobacter | https://www.cdc.gov/foodsafety/diseases/campylobacter/index.html |
| Surveillance Atlas | http://atlas.ecdc.europa.eu/public/index.aspx | |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Water‐borne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| World Health Organization – Campylobacter Fact Sheet | http://www.who.int/mediacentre/factsheets/fs255/en/ | |
| Food | European Union Reference Laboratory (EURL) for Campylobacter | http://www.sva.se/en/service-and-products/eurl-campylobacter |
| Scientific Opinion on Quantification of the risk posed by broiler meat to human campylobacteriosis in the EU | http://www.efsa.europa.eu/en/efsajournal/pub/1437 | |
| Scientific Opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain | https://www.efsa.europa.eu/en/efsajournal/pub/2105 | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports | |
| Bad Bug Book (Second Edition), Food‐borne Pathogenic Microorganisms and Natural Toxins Handbook, Center for Food Safety and Applied Nutrition, Food and Drug Administration (FDA), USA | https://www.fda.gov/food/foodborneillnesscontaminants/causesofillnessbadbugbook/ |
2. Salmonella
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1475841
2.1. Abstract
In 2017, 91,662 confirmed human salmonellosis cases were reported in the EU by all the MS. The EU notification rate was 19.7 cases per 100,000 population and was slightly (2.9% decrease) below the value of 2016 (20.4 cases per 100,000 population). A statistically significant decreasing trend of confirmed salmonellosis cases has been observed in the EU/EEA between 2008 and 2017 considering the 25 countries that reported consistently during this period; however, during the last 5 years (2013–2017), the overall EU/EEA trend has not shown any statistically significant increase or decrease. Seven MS reported an increasing trend and four MS a decreasing trend over the period 2013–2017.
The top five most commonly reported serovars in human cases acquired in the EU during 2017 were, in decreasing order: S. Enteritidis, S. Typhimurium, monophasic S. Typhimurium, S. Infantis and S. Newport. The proportion of human salmonellosis illnesses due to S. Enteritidis continued to increase in 2017, whether considering all cases or only cases infected in EU. This was mainly due to one large MS starting to report case‐based serovar data. When excluding this MS, the proportion was at the same level as in 2016. The data reported on food and animals showed that S. Enteritidis was mainly associated with laying hens, and next also from broiler meat. Between 2012 and 2017 a similar trend was observed in the proportion of S. Enteritidis illnesses in humans acquired in the EU and the EU flock prevalence of S. Enteritidis in laying hens. The proportions of human salmonellosis illnesses acquired within the EU due to S. Typhimurium, monophasic S. Typhimurium and S. Infantis decreased compared with 2016, whereas remained unchanged for S. Newport. S. Typhimurium was isolated from almost all food‐animal sources considered. For the monophasic variants of S. Typhimurium a strong association with the pig chain was confirmed and this group was also related to the broiler chain. S. Infantis was markedly associated with broiler flocks and meat. Finally, S. Newport was associated with turkey and broiler sources.
From food monitoring data reported by MS according to Regulation (EC) No. 2073/2005 on microbiological criteria, as opposed to previous years, only 2017 single sample results collected by CA and labelled as objective sampling were summarised since these data guarantee a satisfactory level of harmonisation. However, data were too scarce and unrepresentative to describe the EU level situation. In general, the highest levels of proportions of Salmonella‐positive units were reported for meat categories intended to be eaten cooked. Process hygiene criterion monitoring data related to Salmonella on pig carcasses were reported by eight MS with samples reported both by CA (official control samples) and by the FBOp (self‐monitoring). For seven of these MS, the estimated occurrence of Salmonella‐positive samples from self‐monitoring was significantly lower than from official control samples.
At the primary production level, in the context of the National Control Programmes (NCP), the EU level flock prevalence of target Salmonella serovars in breeding hens, laying hens, broilers and fattening turkeys decreased or remained unchanged compared with 2016, whereas in breeding turkeys it slightly increased due to S. Typhimurium. This last finding seems to be related to the situation in few MS. The analyses of the time trends, since the implementation of the NCP from 2007 to 2010, showed an overall decreasing prevalence of flocks positive to target Salmonella serovars in all poultry species, except for breeding turkeys, where a stationary trend with minor fluctuations was observed. Moreover, an increasing prevalence of Salmonella‐positive flocks for all poultry categories was noted. In the context of NCP (broilers, fattening and breeding turkeys) the flock prevalence of target Salmonella serovars based on official control samples taken by the CA was generally higher than that resulting from sampling by FBOp. These differences were more evident for some MS.
2.2. Surveillance and monitoring of Salmonella in the EU
2.2.1. Humans
The notification of non‐typhoidal salmonellosis in humans is mandatory in most MS, Iceland, Norway and Switzerland, except for five MS where reporting is based on a voluntary system (Belgium, France Luxembourg and the Netherlands) or other systems (the United Kingdom). In the United Kingdom, although the reporting of food poisoning is mandatory, isolation and species identification of the organism is voluntary. The surveillance systems for salmonellosis cover the whole population in all MS except France, the Netherlands and Spain. The coverage of the surveillance system is estimated to be 48% in France and 64% in the Netherlands. These proportions of populations were used in the calculation of notification rates for these two MS. No estimation for population coverage in Spain was provided, so the notification rate was not calculated. In Belgium, full national coverage was established in 2015 and rates before this date are not displayed. All countries report case‐based data except Bulgaria, which reports aggregated data. Both reporting formats were included to calculate numbers of cases, notification rates and disease trends.
Diagnosis of human Salmonella infections is generally performed by culture from human stool samples. All countries, except Bulgaria, perform serotyping of isolates.
2.2.2. Food, animals and feed
Monitoring of food according to Regulation (EC) No. 2073/2005 on microbiological criteria
Monitoring of Salmonella in foods is mainly based on data collected according to Regulation (EC) No. 2073/2005 on microbiological criteria (Figure 3), which lays down Salmonella food safety criteria (FSC) and Salmonella process hygiene criteria (PHC). Compliance with these criteria ought to be legally verified by the individual FBOp, through self‐monitoring. The Salmonella FSC prescribe that Salmonella must be ‘absent in 25 or 10 grams’ at the retail stage, which means when products are placed on the market, during their shelf life. Absence is defined by testing five or, depending on the food category, 30 sampling units per batch, for specified food categories. Moreover, according to Regulation (EC) No. 1086/20117 compliance with ‘absence in 25 grams’ is required for S. Enteritidis and S. Typhimurium (including monophasic S. Typhimurium strains) in batches of fresh poultry meat, which is meat from fowl breeding hens, laying hens, broilers and turkey breeding hens and fattening turkeys. Salmonella PHC are regulated for carcasses of pigs, cattle, sheep, goats, horses and broilers and turkeys. Specifically, for Salmonella on pig carcasses the PHC is met by the presence of a maximum three positive out of 50 tested carcasses where three is a suggested number that should be changed according to the previous results of the MS. The Competent Authority verifies whether the FBOp correctly implements and checks (through self‐monitoring) this PHC on pig carcasses and verification and sampling schemes are laid down in point G (a) of Annex I, Section IV, Chapter IX of the Regulation (EC) No. 854/2004.
Figure 3.
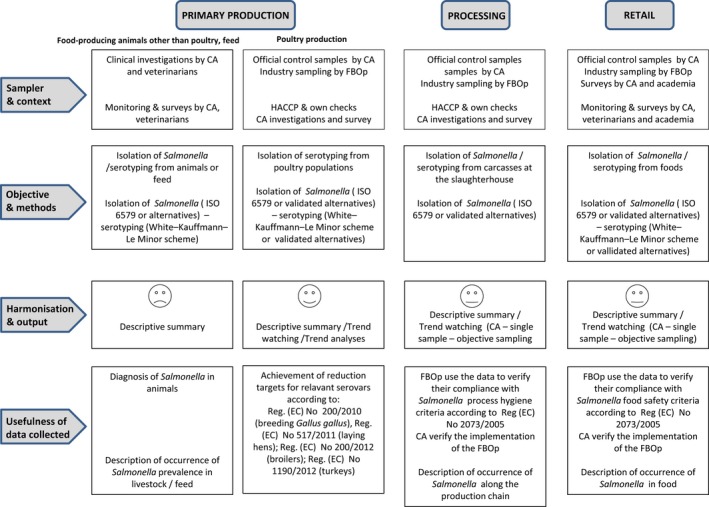
The surveillance and monitoring of Salmonella in food, food‐producing animals and feed according to the sampling stage, the sampler, the objective of the sampling, the quality of data and the degree of harmonisation
In the present annual report EFSA implemented for the first time new rules for summarising data sent by MS according to Regulation (EC) No. 2073/2005, as follows:
-
For trend watching data used were those labelled by the MS as:
sampling context: Surveillance, based on Regulation (EC) No. 2073/2005;
sampling unit type: Single;
sampling strategy: Objective sampling;
sampler: Official sampling, except for pig carcasses where the sampler has to be labelled as ‘official, based on Regulation 854/2004’ and Industry sampling and HACCP and own check (self‐monitoring).
Other food data sets, having other specified options for the different data aspects, were only descriptively summarised as they cannot serve the purpose of trend watching or trend analyses.
Data sent by MS labelled with specified options for the different data aspects from single samples taken by the CA (classified as official sampling) are considered suitable for trend watching at EU and MS level. Other Salmonella monitoring data submitted to EFSA according to Regulation (EC) No. 2073/2005 allow for descriptive summaries at the EU level to be made, but cannot serve the purpose of trend watching or trend analyses (Table 1).
Monitoring data of compliance with the Salmonella National Control Programmes in poultry
According to EU Regulation (EC) No. 2160/2003 and its following amendments, EU MS have to set up Salmonella NCP aimed at reducing the prevalence of Salmonella serovars, which are considered relevant for public health, in certain animal populations. Currently, prevalence targets have been defined for breeding flocks of Gallus gallus, laying hens, broilers and breeding and fattening turkeys and correspond to the maximum annual percentage of flocks positive for relevant serovars (S. Enteritidis and S. Typhimurium, including its monophasic variant, except for breeding flocks of Gallus gallus, where S. Infantis, S. Virchow and S. Hadar are considered to be relevant as well). In particular, the prevalence target is equal to 1% or less for breeding flocks of Gallus gallus, broilers and breeding and fattening turkeys and to 2% or less, generally, for laying hens (for this last animal category the prevalence reduction to be obtained annually has to be calculated according to the prevalence in the preceding year, as described in Regulation (EU) No. 517/20111). For Salmonella NCP monitoring data for broiler flocks, breeding and fattening turkeys, it is compulsory for MS to report investigational results separately for CA and for FBOp.
Salmonella monitoring data originating from the Salmonella NCP in poultry are collected and reported to EFSA in a fully harmonised way and is a census sampling. Therefore, these data allow data analysis like assessing spatial and temporal trends at the EU level. They also allow for descriptive summaries at the EU level to be made, and allow EU trends to be monitored (Table 1).
Other monitoring data of foods, animals and feed
Food, animal and feed monitoring data different from those described above are not collected in a harmonised way because there are no requirements for sampling strategy, sampling methods, analytical tests and reporting (Figure 3). Still, the CA needs to report on those according to Directive 2003/99/EC on the monitoring of zoonoses, at the most appropriate stage of the food chain. There are no harmonised rules on how to report these data to EFSA.
Salmonella monitoring data submitted to EFSA and collected without harmonised design allows only for descriptive summaries at the EU level to be made. They preclude trend analyses and trend watching at the EU level (Table 1).
Within this category, Salmonella serovar data should also be included. Member States are obliged to report the target serovars as part of NCP in poultry populations, whereas for the remaining production categories serotyping is not mandatory. Also, for the food sector, the FSC are the absence of Salmonella spp. with the exception of fresh poultry meat, for which the criterion is limited to absence of the target serovars. Therefore, some MS could decide to not report the presence of non‐target serovars, which could lead to a possible bias in the reporting of target serovars for poultry populations and for fresh poultry meat. Hence, the mandatory reporting of target serovars in the context of NCP and in the context of the FSC for fresh poultry meat guarantees the consistency of such data over many years and among MS, but could result in an overestimation of these target serovars compared with the other serovars. For the remaining matrices, serovar data collected could be strongly biased by what each MS actually serotyped and notified. Also, in this context, it is clear that detection of Salmonella serovars other than those covered by the reduction targets does not in any way equal a ‘Salmonella free’ finding.
2.2.3. Food‐borne outbreaks of human salmonellosis
The reporting of FBO of human salmonellosis is mandatory according to the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
2.3. Data analyses
2.3.1. Comparison between Competent Authority and Food Business Operator sampling results
Comparison of test results between CA and FBOp was carried out by the one‐tailed Fisher's Exact probability test if the expected values in any of the cells of a contingency table were below 5; otherwise the z‐statistic one‐tailed test was calculated. A p‐value < 0.10 (Clayton and Hills, 1993) was considered significant to take account of every possible evidence of differences between FBOp and CA. Differences in official control sampling results by CA and self‐monitoring results by FBOp were expressed by exact binomial confidence interval (95% level).
STATA 12.1 software (StataCorp. 2001. Statistical Software: Release 12. College Station, TX: Stata Corporation) was used to conduct the above‐mentioned analyses.
2.3.2. Statistical trend analyses (methods) of poultry monitoring data
Statistical trend analyses were carried out with the objective of evaluating the significance of temporal variations in the EU level flock prevalence of Salmonella spp. and Salmonella target serovars in poultry, since the start of the implementation of NCP.
As the temporal variations of Salmonella spp. prevalence were difficult to model during the whole period 2007–2017, the analyses concentrated on the last 5 years, except for laying hens for which – in the light of the results of the previous years – the entire period of implementation of NCP was considered. Moreover, the trends during the last 3 years were verified in detail for outcomes of target serovars and of Salmonella spp. The tested flocks could be positive or negative for target serovars and Salmonella spp., and so, the state of the flocks is a dichotomous outcome variable. Therefore, the binomial probability distribution for the response variable was assumed and the logit link function was computed in the model for the trend analysis. The logit is defined as the logarithm of p/(1 – p), where p/(1 – p) is the odds of being positive for the outcome.
According to the temporal change of the prevalence in the MS, polynomial models for the logit of the probability of flocks being positive were fitted for the different poultry categories. Marginal and conditional generalised linear models for repeated measures were used to perform these trend analyses (EFSA, 2009a, 2011). Details about the estimated parameters of the models, odds ratio, prevalence and graphical analysis (conditional and marginal) are reported in the Appendix.
To investigate the EU level prevalence considering the relevant heterogeneity among MS for flock prevalence of Salmonella spp. and target serovars over time, the results obtained using the conditional generalised mixed model for longitudinal binary data were summarised and are discussed in the report, for all poultry categories. To take into account the different levels (baselines) of risk of MS having positive flocks, but similar patterns over time, a random MS‐specific intercept effect was included in the model. To consider the trend over time, the variable ‘time’ was included in the model as fixed effect.
The correlation among repeated observations in the same MS in subsequent years was considered using a first autoregressive or exchangeable structure of the correlation matrix for the residuals (EFSA and ECDC, 2017b).
To evaluate the significance of the overall effect of fixed factors specified in the model, Type III F‐tests were applied, whereas the receiver operating characteristic (ROC) curve was used to assess the goodness of the model. A p‐value < 0.10 (Clayton and Hills, 1993) was considered significant for both random and fixed effects.
GLIMMIX and SGPLOT procedures in SAS 9.4 software were used to fit the models and to produce the graphical outputs, respectively.
2.3.3. Descriptive analyses of Salmonella serovars
With the aim to evaluate the distribution of Salmonella serovars along the food chain and identify the potential sources for human infections, descriptive analyses were made from data on food and food‐producing animals of the five most commonly reported Salmonella serovars from human cases acquired within the EU (domestically or during travel within EU). For animal categories covered by NCP, only serovar data reported in the context of these programmes were presented. For cattle meat‐producing animals were considered, whereas for pigs data from fattening animals were used. To interpret serovar data, it must be kept in mind that for NCP the mandatory reporting is limited to target serovars, and this could lead to a possible bias towards the reporting of these regulated serovars to the detriment of non‐regulated ones. For all the other animal species‐food matrices the reporting of serovar data is carried out on a voluntary basis by the MS. Apart from possible reporting bias as regards serovars, also the reporting on animal or food categories may be unbalanced and certain sources (e.g. cattle) may be underrepresented. Monophasic variants of S. Typhimurium have been reported by MS by using different designations, generally as the generic denomination ‘monophasic S. Typhimurium’. From the epidemiological point of view, all the isolates of the monophasic S. Typhimurium group have the same significance. So, in this report, the isolates belonging to the group of monophasic variants of S. Typhimurium and reported by MS with different designations (S. Typhimurium monophasic, S. 1,4,[5],12:i:‐, S. 1,4,5,12:i:‐, S. 1,4,12:i:‐, S. 4,[5],12:i:‐, S. 4,5,12:i:‐ and S. 4,12:i:‐) were merged into the same group and named ‘monophasic variants of S. Typhimurium’.
Sankey diagrams of the most reported Salmonella serovars from humans in relation to their food and animal sources and in relation to the MS reporting them (geographical provenance) were produced in HTML format and Google Chart libraries ( http://developers.google.com/chart/).
Pyramid plots for each of the serovars of interest were prepared to show for each source the frequency of notification in animal and food sources using the R software ( http://www.r-project.org).
2.4. Results
2.4.1. Overview of key statistics along the food chain, EU, 2013–2017
Table 9 summarises EU level statistics related to human salmonellosis and to Salmonella in food and animals, respectively, in the EU during 2013–2017. More detailed descriptions of these statistics are in the results section of this chapter and in the chapter on FBO.
Table 9.
Summary of Salmonella statistics related to humans, major food categories and major animal species, EU, 2013–2017
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 91,662 | 94,425 | 94,477 | 92,012 | 87,753 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 19.7 | 20.5 | 21.0 | 20.7 | 20.3 | ECDC |
| Number of reporting MS | 28 | 28 | 28 | 28 | 28 | ECDC |
| Infection acquired in the EU | 59,657 | 52,850 | 51,898 | 48,451 | 44,706 | ECDC |
| Infection acquired outside the EU | 6,016 | 6,466 | 6,830 | 6,202 | 7,334 | ECDC |
| Unknown travel status or unknown country of infection | 25,989 | 35,109 | 35,749 | 37,359 | 35,713 | ECDC |
| Number of outbreak‐related cases | 9,600 | 11,425 | 6,616 | 9,294 | 8,709 | EFSA |
| Total number of outbreaks | 1,241 | 1,372 | 953 | 1,049 | 1,168 | EFSA |
| Food | ||||||
| Meat and meat products | ||||||
| Number of sampled units | 366,362 | 278,254 | 203,683 | 503,647 | 410,529 | EFSA |
| Number of reporting countries | 28 | 28 | 27 | 25 | 27 | EFSA |
| Milk and milk products | ||||||
| Number of sampled units | 30,980 | 24,509 | 29,170 | 70,464 | 59,234 | EFSA |
| Number of reporting countries | 24 | 25 | 22 | 24 | 23 | EFSA |
| Fish and fishery products | ||||||
| Number of sampled units | 12,215 | 11,191 | 10,274 | 16,080 | 16,258 | EFSA |
| Number of reporting countries | 22 | 22 | 22 | 20 | 19 | EFSA |
| Eggs and egg products | ||||||
| Number of sampled units | 17,315 | 11,137 | 9,768 | 23,536 | 30,283 | EFSA |
| Number of reporting countries | 23 | 21 | 19 | 20 | 19 | EFSA |
| Fruits and vegetables (and juices) | ||||||
| Number of sampled units | 7,613 | 8,013 | 7,370 | 10,652 | 10,684 | EFSA |
| Number of reporting countries | 25 | 21 | 22 | 23 | 23 | EFSA |
| Animals | ||||||
| Fowl | ||||||
| Number of sampled flocks | 695,920 | 703,097 | 528,933 | 511,008 | 481,222 | EFSA |
| Number of reporting countries | 28 | 28 | 28 | 27 | 28 | EFSA |
| Turkeys | ||||||
| Number of sampled flocks | 74,883 | 78,050 | 54,261 | 41,406 | 36,963 | EFSA |
| Number of reporting countries | 26 | 25 | 24 | 24 | 24 | EFSA |
| Ducks and geese | ||||||
| Number of sampled flocks | 5,715 | 2,627 | 2,757 | 3,020 | 2,283 | EFSA |
| Number of reporting countries | 6 | 9 | 7 | 8 | 8 | EFSA |
| Pigs | ||||||
| Number of sampled herds | 1,257 | 8,560 | 12,100 | 11,988 | 9,901 | EFSA |
| Number of reporting countries | 7 | 8 | 7 | 7 | 7 | EFSA |
| Bovine animals | ||||||
| Number of sampled herds | 4,739 | 4,888 | 12,178 | 8,334 | 6,004 | EFSA |
| Number of reporting countries | 5 | 4 | 5 | 4 | 5 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member State.
Humans
In 2017, the number of reported human salmonellosis cases acquired in the EU (i.e. by domestic infection and through travel within the EU) increased compared with 2016 and was highest since 2013. The increase was due to one large country reporting case‐based data for the first time in 2017. The number of outbreak‐related cases and the total number of food‐borne salmonellosis outbreaks were lower in 2017 compared with 2016 and at a higher level compared with 2015 and previous years.
Food categories
The number of sampled units reported in 2017 for the general food category ‘meat and meat products’ was higher compared with the previous 2 years. This was generally also the case with other food categories (‘milk and milk products’, ‘fish and fishery products’, ‘eggs and egg products’) with the exception of ‘fruits and vegetables including juices’. The number of reporting MS was fairly stable or increased during the last years, within these major food groups.
Animal categories
The number of sampled herds reported by MS from Gallus gallus fowl and from turkeys progressively increased during 2013–2017 and the number of reporting MS was high. These statistics are underpinned by data submitted by MS according the NCP in poultry. For the category ‘ducks and geese’, the number of flocks with monitoring data submitted to EFSA increased compared with 2016 but the number of reporting countries decreased, whereas for ‘pigs’ and ‘bovine animals’ during the last 2 years there was a marked reduction in number of herds with monitoring data submitted to EFSA.
2.4.2. Human salmonellosis
In total, 93,583 human salmonellosis cases were reported by 28 EU MS in 2017, with 91,662 confirmed cases resulting in an EU notification rate of 19.7 cases per 100,000 population (Table 10). This was a slight decrease by 2.9% compared with 2016 (20.4 cases per 100,000 population). As in the previous year, the highest notification rates in 2017 were reported by the Czech Republic (108.5 cases per 100,000 population) and Slovakia (106.5 cases per 100,000 population), while the lowest rates were reported by Cyprus, Greece, Italy, Portugal and Romania (< 7.0 cases per 100,000 population).
Table 10.
Reported human cases of salmonellosis and notification rates per 100,000 population in the EU/EFTA, by country and year, 2013–2017
| Country | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 1,672 | 1,667 | 19.0 | 1,415 | 16.3 | 1,544 | 18.0 | 1,654 | 19.4 | 1,404 | 16.6 |
| Belgium | Y | C | 2,298 | 2,298 | 20.2 | 2,699 | 23.9 | 3,050 | 27.1 | 2,698 | – | 2,528 | – |
| Bulgaria | Y | A | 798 | 796 | 11.2 | 718 | 10.0 | 1,076 | 14.9 | 730 | 10.1 | 766 | 10.5 |
| Croatia | Y | C | 1,250 | 1,242 | 29.9 | 1,240 | 29.6 | 1,593 | 37.7 | 1,494 | 35.2 | 0 | 0.0 |
| Cyprus | Y | C | 59 | 59 | 6.9 | 77 | 9.1 | 65 | 7.7 | 88 | 10.3 | 79 | 9.1 |
| Czech Republic | Y | C | 11,705 | 11,473 | 108.5 | 11,610 | 110.0 | 12,408 | 117.7 | 13,255 | 126.1 | 9,790 | 93.1 |
| Denmark | Y | C | 1,067 | 1,067 | 18.6 | 1,081 | 18.9 | 925 | 16.3 | 1,124 | 20.0 | 1,137 | 20.3 |
| Estonia | Y | C | 279 | 265 | 20.1 | 351 | 26.7 | 112 | 8.5 | 92 | 7.0 | 183 | 13.9 |
| Finland | Y | C | 1,535 | 1,535 | 27.9 | 1,512 | 27.6 | 1,650 | 30.2 | 1,622 | 29.8 | 1,984 | 36.6 |
| Franceb | N | C | 7,993 | 7,993 | 24.9 | 8,876 | 27.7 | 10,305 | 32.3 | 8,880 | 28.1 | 8,927 | 28.4 |
| Germany | Y | C | 14,268 | 14,052 | 17.0 | 12,858 | 15.6 | 13,667 | 16.8 | 16,000 | 19.8 | 18,696 | 22.8 |
| Greece | Y | C | 675 | 672 | 6.2 | 735 | 6.8 | 466 | 4.3 | 349 | 3.2 | 414 | 3.7 |
| Hungary | Y | C | 4,103 | 3,922 | 40.0 | 4,722 | 48.0 | 4,894 | 49.7 | 5,249 | 53.1 | 4,953 | 50.2 |
| Ireland | Y | C | 415 | 379 | 7.9 | 299 | 6.3 | 270 | 5.8 | 259 | 5.6 | 326 | 7.1 |
| Italy | Y | C | 3,348 | 3,347 | 5.5 | 4,134 | 6.8 | 3,825 | 6.3 | 4,467 | 7.3 | 5,048 | 7.8 |
| Latvia | Y | C | 234 | 225 | 11.5 | 454 | 23.1 | 380 | 19.1 | 278 | 13.9 | 385 | 19.0 |
| Lithuania | Y | C | 1,004 | 1,004 | 35.3 | 1,076 | 37.3 | 1,082 | 37.0 | 1,145 | 38.9 | 1,199 | 40.4 |
| Luxembourg | Y | C | 118 | 118 | 20.0 | 108 | 18.7 | 106 | 18.8 | 110 | 20.0 | 120 | 22.3 |
| Malta | Y | C | 107 | 107 | 23.2 | 162 | 36.4 | 126 | 29.3 | 132 | 31.0 | 84 | 19.9 |
| Netherlandsc | N | C | 954 | 954 | 8.7 | 1,150 | 10.6 | 974 | 9.0 | 970 | 9.0 | 979 | 9.1 |
| Poland | Y | C | 9,711 | 8,924 | 23.5 | 9,718 | 25.6 | 8,245 | 21.7 | 8,042 | 21.2 | 7,315 | 19.2 |
| Portugal | Y | C | 470 | 462 | 4.5 | 376 | 3.6 | 325 | 3.1 | 244 | 2.3 | 167 | 1.6 |
| Romania | Y | C | 1,270 | 1,154 | 5.9 | 1,479 | 7.5 | 1,330 | 6.7 | 1,512 | 7.6 | 1,302 | 6.5 |
| Slovakia | Y | C | 6,092 | 5,789 | 106.5 | 5,299 | 97.7 | 4,841 | 89.3 | 4,078 | 75.3 | 3,807 | 70.3 |
| Slovenia | Y | C | 275 | 275 | 13.3 | 311 | 15.1 | 401 | 19.4 | 597 | 29.0 | 316 | 15.4 |
| Spaind | N | C | 9,426 | 9,426 | – | 9,818 | – | 9,015 | – | 6,633 | – | 4,537 | – |
| Sweden | Y | C | 2,280 | 2,280 | 22.8 | 2,247 | 22.8 | 2,312 | 23.7 | 2,211 | 22.9 | 2,842 | 29.7 |
| United Kingdom | Y | C | 10,177 | 10,177 | 15.5 | 9,900 | 15.1 | 9,490 | 14.6 | 8,099 | 12.6 | 8,465 | 13.2 |
| EU Total | – | – | 93,583 | 91,662 | 19.7 | 94,425 | 20.5 | 94,477 | 21.0 | 92,012 | 20.7 | 87,753 | 20.3 |
| Iceland | Y | C | 64 | 64 | 18.9 | 39 | 11.7 | 44 | 13.4 | 40 | 12.3 | 48 | 15.2 |
| Norway | Y | C | 992 | 992 | 18.9 | 865 | 16.6 | 928 | 18.0 | 1,118 | 21.9 | 1,361 | 26.9 |
| Switzerland e | Y | C | 1,848 | 1,848 | 21.9 | 1,517 | 17.9 | 1,375 | 16.4 | 1,241 | 15.0 | 1,265 | 15.2 |
Y: yes; N: no; A: aggregated data; C: case‐based data;–: no report.
Sentinel system; notification rates calculated with an estimated population coverage of 48%.
Sentinel system; notification rates calculated with an estimated population coverage of 64%.
Sentinel surveillance; no information on estimated coverage. So, notification rate cannot be estimated.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
The proportion of domestic vs travel‐associated cases varied markedly between countries, but most of the confirmed salmonellosis cases were acquired in the EU (65.1% cases acquired in the EU, 6.6% travel outside EU and 28.4% of unknown origin) (Table 9). Considering all cases regardless the origin, the highest proportions of domestic cases, ranging from 92.8% to 100% were reported by the Czech Republic, Estonia, Hungary, Latvia, Malta, the Netherlands, Portugal, Romania and Slovakia. The highest proportions of travel‐related cases with known data on importation were reported by Nordic countries – Finland (76.3%), Norway (71.2%), Iceland (64.7%) and Sweden (64.3%). Among 7,996 travel‐associated cases with known information on probable country of infection, 75.2% of the cases represented travel outside EU and 24.8% travel within EU. Thailand, Spain, Turkey and India were the most frequently reported travel destinations (13.8%, 8.3%, 8.2% and 6.7%, respectively).
A seasonal trend was observed for confirmed salmonellosis cases in the EU/EEA in 2013–2017, with more cases reported during summer months (Figure 4). There was a significantly (p < 0.05) decreasing trend for salmonellosis in the EU/EEA in 2008–2017, however the trend did not show any significant increase or decrease over the last 5 years (2013–2017) (Figure 4).
Figure 4.
- Source: Austria, Belgium, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Lithuania, Luxembourg, Latvia, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and United Kingdom. Bulgaria and Croatia did not report data to the level of detail required for the analysis.
At the country level, 13 MS (Austria, Belgium, Cyprus, Denmark, Estonia, Finland, Germany, Hungary, Italy, Lithuania, Luxembourg, Slovenia and Sweden) reported decreasing trends from 2008 to 2017, whereas three MS (Finland, Italy and Germany) reported also a decreasing trend in the last 5 years (2013 to 2017).
A significant increasing trend was observed in seven MS (Greece, Estonia, Poland, Portugal, Slovakia, Spain and the United Kingdom) in 2013–2017 compared with only four MS (the Czech Republic, France, Portugal and Spain) in 2008–2017.
Fourteen MS provided information on hospitalisation. The proportion of confirmed cases with known hospitalisation status at the EU level was 43.1% resulting in the proportion of hospitalised cases of 42.5%, which was an increase compared with 2016 (37.9%). This increase was due to Poland reporting case‐based hospitalisation data for the first time in 2017. The highest proportions of hospitalised cases (71.5–92.2%) were reported, as in previous years, in Cyprus, Greece, Lithuania, Portugal and the United Kingdom. Three of these countries (60%) also reported the lowest notification rates of salmonellosis, which indicates that the surveillance systems in these countries primarily capture the more severe cases.
Seventeen MS provided data on the outcome of salmonellosis and, among these, 11 MS reported 156 fatal cases. The EU case fatality was 0.25%. Fifty‐seven fatal cases (36.5%) were reported by the United Kingdom.
Human serovar data are described in Section 2.4.6.
Human salmonellosis cases associated with food‐borne outbreaks
Salmonella was identified in 1,241 FBOs affecting 9,600 people (notified FBO cases) in 25 MS, as reported to EFSA. Overall, for the year 2017, there were 57,682 domestic (acquired within the country) cases reported to the TESSy (Table 11), which was 96.7% of the number of reported human salmonellosis cases infected domestically and through travel within EU during 2017 (59,657, Table 9). Table 11 shows data reported by countries to TESSy managed by ECDC and to the FBOs database managed by EFSA. It is important to clarify that the case classification for reporting is different between these two databases. In TESSy, the cases reported are classified based on the EU case definition. All these cases visited a doctor, and are either confirmed by laboratory test (confirmed case) or not (probable case and classification is based the clinical symptoms and epidemiological link). Cases that never visited a doctor are not reported to TESSy. Moreover, probable cases may be missing in TESSy, as these data are not analysed or published and there is no incentive for reporting such cases. Information on which case is linked to an outbreak ‐ and which not‐ is not systematically collected. In practice, the cases reported to TESSy are considered mostly sporadic cases. In food‐borne disease outbreak situations cases are also classified into confirmed or probable outbreak cases, but currently these data are not collected by EFSA.
Table 11.
Statistics related to the proportions of human food‐borne outbreak cases caused by Salmonella, EU/EFTA, 2017
| Country | ECDC | EFSA | ||||
|---|---|---|---|---|---|---|
| Confirmed human | Food‐borne outbreaks (including waterborne outbreaks) | |||||
| Total | Travel related | Domestic | Unknown or missing | Human cases (illnesses) | FBO | |
| N | N | N | N | N | N | |
| Austria | 1,667 | 307 | 1,355 | 5 | 106 | 32 |
| Belgium | 2,298 | 91 | 0 | 2,207 | 14 | 2 |
| Bulgaria | 796 | – | – | – | 35 | 4 |
| Croatia | 1,242 | 2 | 73 | 1,167 | 190 | 28 |
| Cyprus | 59 | 0 | 0 | 59 | –a | – |
| Czech Republic | 11,473 | 266 | 11,207 | 0 | 475 | 22 |
| Denmark | 1,067 | 368 | 377 | 322 | 193 | 25 |
| Estonia | 265 | 19 | 246 | 0 | 34 | 6 |
| Finland | 1,535 | 1,004 | 312 | 219 | 55 | 2 |
| France | 7,993 | 825 | 737 | 6,431 | 814 | 132 |
| Germany | 14,052 | 2,220 | 11,832 | 0 | 817 | 133 |
| Greece | 672 | 26 | 533 | 113 | 114 | 5 |
| Hungary | 3,922 | 7 | 3,915 | 0 | 283 | 14 |
| Ireland | 379 | 140 | 173 | 66 | 2 | 1 |
| Italy | 3,347 | 0 | 0 | 3,347 | 142 | 27 |
| Latvia | 225 | 8 | 217 | 0 | 49 | 12 |
| Lithuania | 1,004 | 17 | 832 | 155 | 186 | 29 |
| Luxembourg | 118 | 20 | 7 | 91 | 7 | 2 |
| Malta | 107 | 1 | 106 | 0 | 7 | 3 |
| Netherlands | 954 | 87 | 867 | 0 | 89 | 14 |
| Poland | 8,924 | 41 | 8,741 | 142 | 2,683 | 253 |
| Portugal | 462 | 8 | 446 | 8 | – | – |
| Romania | 1,154 | 0 | 1,101 | 53 | 147 | 4 |
| Slovakia | 5,789 | 40 | 5,749 | 0 | 979 | 301 |
| Slovenia | 275 | 0 | 0 | 275 | – | – |
| Spain | 9,426 | 21 | 5,422 | 3,983 | 1,326 | 171 |
| Sweden | 2,280 | 1,439 | 799 | 42 | 165 | 5 |
| United Kingdom | 10,177 | 2,927 | 2,635 | 4,615 | 688 | 14 |
| EU Total | 91,662 | 9,884 | 57,682 | 23,300 | 9,600 | 1,241 |
| Iceland | 64 | 33 | 18 | 13 | 8 | 1 |
| Norway | 992 | 623 | 253 | 116 | 25 | 2 |
| Switzerland | 1,848 | – | – | – | 30 | 1 |
| Albania | – | – | – | – | 204 | 2 |
| Former Yugoslav Republic of Macedonia, the | – | – | – | – | 93 | 2 |
| Serbia | – | – | – | – | 281 | 50 |
No food‐borne outbreaks caused by Salmonella reported.
Salmonella was the causative agent most frequently detected in FBO. No waterborne outbreaks caused by Salmonella were reported. The 1,241 Salmonella FBO for 2017 were notified by 25 MS, and these Salmonella FBO were 24.4% of the total number of outbreaks. Twenty MS reported 269 Salmonella FBO with strong‐evidence on the implicated food vehicle. ‘Eggs and egg products’ still remain a significant source of human infection due to Salmonella and accounted for 36.8% of strong‐evidence Salmonella FBO (Table 12). Various meat and meat product subcategories totalled together 16.8% and bakery products 16.7%. Further details and statistics on the salmonellosis food‐borne (including waterborne) outbreaks reported by 25 MS for 2017 are in Chapter 16 on FBO. Eighteen MS reported 147 FBO caused by S. Enteritidis with strong‐evidence on the implicated food vehicle. ‘Eggs and egg products’ accounted for 31.3% of strong‐evidence FBO caused by S. Enteritidis, followed by ‘Bakery products’, 25.2% (Table 13). Further details and statistics on the salmonellosis food‐borne (including waterborne) outbreaks reported by 25 MS for 2017 are in Section 16 on FBO.
Table 12.
Distribution of strong‐evidence outbreaks caused by Salmonella, by food vehicle, EU, 2017
| Food vehicle | Number of strong‐evidence FBO | % of total |
|---|---|---|
| Eggs and egg products | 99 | 36.8 |
| Bakery products | 45 | 16.7 |
| Mixed food | 34 | 12.6 |
| Meat and meat products | 22 | 8.2 |
| Other foods | 15 | 5.6 |
| Pig meat and their products | 12 | 4.5 |
| Broiler meat (Gallus gallus) and their products | 6 | 2.2 |
| Cheese | 5 | 1.9 |
| Sweets and chocolate | 5 | 1.9 |
| Dairy products (other than cheeses) | 4 | 1.5 |
| Fish and fish products | 4 | 1.5 |
| Other, mixed or unspecified poultry meat and their products | 4 | 1.5 |
| Vegetables and juices and other their products | 3 | 1.1 |
| Buffet meals | 2 | 0.7 |
| Crustaceans, shellfish, molluscs and their products | 2 | 0.7 |
| Unknown | 2 | 0.7 |
| Cereal products including rice and seeds/pulses (nuts, almonds) | 1 | 0.4 |
| Herbs and spices | 1 | 0.4 |
| Milk | 1 | 0.4 |
| Other or mixed red meat and their products | 1 | 0.4 |
| Sheep meat and their products | 1 | 0.4 |
| Total | 269 | 100.0 |
Note: Data from 269 strong‐evidence outbreaks are included reported by 20 MS: Poland, 102; Spain, 59; France, 20; Germany, 14; Italy, 14; Slovakia, 13; United Kingdom, 8; Austria, 5; Denmark, 5; Croatia, 4; Czech Republic, 4; Lithuania, 4; Romania, 4; Finland, 2; Greece, 2; Hungary, 2; Luxembourg, 2; Netherlands, 2; Sweden, 2; and Belgium, 1.
Table 13.
Distribution of strong‐evidence outbreaks caused by Salmonella Enteritidis, by food vehicle, EU, 2017
| Food vehicle | Number of strong‐evidence FBO | % of total |
|---|---|---|
| Eggs and egg products | 46 | 31.3 |
| Bakery products | 37 | 25.2 |
| Mixed food | 20 | 13.6 |
| Meat and meat products | 18 | 12.2 |
| Other foods | 6 | 4.1 |
| Sweets and chocolate | 4 | 2.7 |
| Cheese | 3 | 2.0 |
| Dairy products (other than cheeses) | 3 | 2.0 |
| Other, mixed or unspecified poultry meat and their products | 3 | 2.0 |
| Buffet meals | 2 | 1.4 |
| Vegetables and juices and other their products | 2 | 1.4 |
| Broiler meat (Gallus gallus) and their products | 1 | 0.7 |
| Pig meat and their products | 1 | 0.7 |
| Unknown | 1 | 0.7 |
| Total | 147 | 100.0 |
Note: Data from 147 strong‐evidence outbreaks are included reported by 18 MS: Poland, 83; Slovakia, 12; France, 9; Spain, 8; Germany, 7; Austria, 4; Lithuania, 4; Croatia, 3; Czech Republic, 3; Romania, 3; United Kingdom, 3; Hungary, 2; Belgium, 1; Denmark, 1; Finland, 1; Luxembourg, 1; Netherlands, 1; Sweden, 1.
2.4.3. Salmonella in foods
Data collected according to Regulation (EC) No. 2073/2005 on microbiological criteria
The 2017 data that serve the purpose of trend watching (sampling context: Surveillance, based on Regulation 2073/2005; sampling unit type: Single; sampling strategy: Objective sampling; and sampler: Official sampling) were too scarce and unrepresentative to describe the situation at the EU level, because they were reported by very few MS. At the level of those reporting MS, the highest proportions of Salmonella‐positive single samples from official control investigations by CA were reported from foods of meat origin intended to be cooked before consumption; respectively, 6.4% and 3.3% of ‘minced meat and meat preparations from poultry’ and of ‘minced meat and meat preparations from other species than poultry’ were positive for Salmonella. From single samples of ‘minced meat and meat preparations intended to be eaten raw’, 1.09% were Salmonella positive. From ‘fresh poultry meat’ 0.11% of single samples were positive to target serovars. Considering food products other than meat, 0.84% of single samples of RTE pre‐cut fruits and vegetables were positive to Salmonella. All the other tested food categories were negative to Salmonella.
As regards Salmonella PHC monitoring data from pig carcasses, the proportions of Salmonella‐positive single samples from official control by CA and from self‐monitoring by FBOp were, respectively, 2.15% (n = 26,802, 15 MS and one non‐MS) and 1.85% (n = 98,386, 17 MS). Eight MS (Belgium, Bulgaria, Greece, Italy, the Netherlands, Poland, Slovakia and Spain) provided data collected by CA and as well by FBOp. For all these MS except Bulgaria the occurrence of Salmonella‐positive samples from official control samples was significantly higher than self‐monitoring results (Table 14).
Table 14.
Comparisons of proportions (%) of Salmonella‐positive single samples from pig carcasses, by sampler, based on eight reporting Member States, EU, 2017
| Country | Competent authorities (CA) | Food Business Operator (FBOp) | p‐valuea | Interpretation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample weight | Tested | Positive | % | CI95 | Sample weight | Tested | Positive | % | CI95 | |||
| Belgium | 600 cm2 | 1,048 | 57 | 5.44 | [4.15; 6.99]a | 600 cm2 | 4,774 | 112 | 2.35 | [1.94; 2.82] | *** | CA > FBOp |
| Bulgaria | 400 cm2 | 734 | 2 | 0.27 | [0.03; 0.98] | 400 cm2 | 425 | 2 | 0.47 | [0.06; 1.69] | NS | |
| 25 g | 101 | 0 | 0 | [0; 3.59] a | 25 g | 51 | 0 | 0 | [0; 6.98] a | NS | ||
| tot | 835 | 2 | 0.24 | [0.03; 0.86] | tot | 476 | 2 | 0.42 | [0.05; 1.51] | NS | ||
| Greece | 400 cm2 | 64 | 1 | 1.56 | [0.04; 8.4] | 400 cm2 | 955 | 0 | 0 | [0; 0.39] a | + | CA > FBOp |
| Italy | 4 cm2 | 5,790 | 227 | 3.92 | [3.44; 4.45] | 4 cm2 | 14,186 | 221 | 1.56 | [1.36; 1.78] | *** | CA > FBOp |
| Netherlands | 400 cm2 | 150 | 23 | 15.33 | [9.98; 22.11] | |||||||
| 100 cm2 | 5,308 | 413 | 7.78 | [7.07; 8.53] | ||||||||
| tot | 150 | 23 | 15.33 | [9.98; 22.11] | tot | 5,308 | 413 | 7.78 | [7.07; 8.53] | ** | CA > FBOp | |
| Poland | 400 cm2 | 2,720 | 37 | 1.36 | [0.96; 1.87] | 400 cm2 | 3,128 | 0 | 0 | [0; 0.12] a | *** | CA > FBOp |
| Slovakia | 400 cm2 | 2,299 | 22 | 0.96 | [0.6; 1.45] | 400 cm2 | 4,509 | 0 | 0 | [0; 0.08] a | *** | CA > FBOp |
| Spain | 400 cm2 | 384 | 45 | 11.72 | [8.68; 15.37] | 400 cm2 | 2,746 | 176 | 6.41 | [5.52; 7.39] | *** | CA > FBOp |
| Total (MS) | 13,290 | 414 | 3.12 | [2.82; 3.42] | 36,082 | 924 | 2.56 | [2.04; 2.73] | *** | CA > FBOp | ||
a One‐sided, 97.5% confidence interval; p‐ value interpretation: NS: not significant; + < 0.10; **< 0.01; ***< 0.001.
Finland, Sweden and Norway, which are countries with special guarantees in relation to Salmonella on pig carcasses (according to Regulation (EU) No 853/2004), reported no single positive carcase out of 12,302 tested.
Other data submitted to EFSA according to Regulation (EC) No. 2073/2005 are summarised descriptively and these summaries are included for information in the Appendix.
Occurrence in food
Food monitoring data reported below are presented by merging investigations from all the sampling stages (retail, slaughterhouse, processing, border inspection activities and unspecified) and from all the sampling units (single and batch).
A summary of monitoring results is found in Table 15. Monitoring activities and control programmes for Salmonella in fresh broiler and turkey meat are based on sampling at the slaughterhouse, where mainly neck skin samples are taken, and/or at processing or cutting plants and at retail, where meat samples are usually collected. Data from the testing of fresh pig and bovine meat mainly originate from surveillance programmes, in which samples were mainly collected at slaughterhouses.
Table 15.
Summary of Salmonella monitoring results related to major meat and meat products categories, EU, 2017
| Food category | Number of reporting MS | Number of sampling units tested | Percentage Salmonella‐positive (%) |
|---|---|---|---|
| Fresh broiler meat | 26 | 36,079 | 4.85% |
| RTE products from broiler meat a | 14 | 4,215 | 0.14% |
| Fresh turkey meat | 18 | 3,999 | 4.18% |
| RTE products from turkey meat | 12 | 463 | 0% |
| Fresh poultry meat other than broiler meat | 16 | 27,863 | 2.66% |
| Fresh pig meat | 27 | 163,765 | 1.58% |
| RTE minced meat, meat preparations and meat products from pig meat | 20 | 11,087 | 0.50% |
| Fresh bovine meat | 22 | 35,490 | 0.17% |
| RTE minced meat, meat preparations and meat products from bovine meat | 16 | 1,129 | 0.18% |
Six positive samples reported by Poland of RTE meat preparations from broiler meat intended to be eaten raw.
Eggs and egg products
In total, 29 (0.3%) of the 9,700 tested table egg units reported by 15 MS were Salmonella positive and positive eggs were reported by Italy, Slovakia, Spain and Romania.
Live bivalve molluscs
In total, 1,485 samples of live bivalve molluscs were reported by eight MS and overall three (0.2%) were positive for Salmonella. Positive findings were reported by the Netherlands, Portugal and Spain.
Other foodstuffs
Altogether, 1.06% of the 946 samples of dried seeds examined were Salmonella positive in 2017; all of them were collected during border inspection activities from the Netherlands and Cyprus. In 2016, 8.0% positive samples was reported for this matrix.
Out of the 1,302 tested units of sprouted seeds, three samples (0.23%) at retail were reported Salmonella positive by France and the Netherlands.
Of the 4,290 units of vegetables tested, 1.19% was Salmonella positive; most of these (44/51) were collected at retail by the United Kingdom. Among fruits, only one sample out of 1,467 tested units was Salmonella positive. No positive samples were reported among the 740 samples reported as fruit and vegetables.
For spices and herbs, of 2,631 units examined, 0.42% was Salmonella positive. Most positive samples (7/11) were from retail.
Salmonella was found in 0.2% of 27,172 tested samples of other RTE food.
2.4.4. Salmonella in animals
Poultry monitoring data in compliance with the Salmonella National Control Programmes
Achievement of Salmonella reduction targets
Breeding flocks of Gallus gallus
In total, 26 MS and 3 non‐MS reported Salmonella NCP data from fowl breeding flocks. Luxembourg and Malta do not have such flocks. In the EU, Salmonella was found in 1.89% of the flocks (or 297 flocks) compared with 1.47% in 2016. The prevalence of flocks positive for any of the five target serovars (S. Enteritidis, S. Typhimurium including its monophasic variant, S. Virchow, S. Infantis and S. Hadar) was 0.57% (or 90 flocks) compared with 0.54% in 2016. So, 30.3% (90 out of 297) of reported Salmonella‐positive breeding flocks were positive for target serovars. Ten MS and three non‐MS reported no single flock positive for target serovars. All reporting countries except Austria, Belgium, Greece and Slovakia met the flock prevalence target of maximum 1% (Figure 5). Greece did not meet the target for the second year and reported two fowl breeding flocks positive for S. Enteritidis and three for S. Infantis. Austria reported two flocks positive for S. Infantis, Belgium reported three flocks positive for S. Enteritidis, three for S. Typhimurium and four for S. Infantis and Slovakia reported one flock positive for S. Enteritidis and one for S. Typhimurium. The commonest reported target serovar was S. Enteritidis (0.24%), with 16 out of the 37 positive flocks notified by Poland (Figure 6). The number of fowl breeding flocks positive to S. Enteritidis decreased as compared with 2016 when 49 were positive. The next most reported were S. Typhimurium (including monophasic variants) (0.20%, with 10 out of the 32 positive flocks reported by France) (Figure 7) and S. Infantis (0.12%, 19 positive flocks, with more than one positive flock reported by Austria, Belgium, Italy, Spain and Greece) (Figure 8). An increase in the number of positive flocks was seen both for S. Infantis (9 positive flocks notified in 2015 and 2016 and 19 in 2017) and S. Typhimurium, (12 positive flocks in 2015, 24 in 2016 and 32 in 2017). Only two flocks tested positive for S. Virchow (France) and there were no positive flocks for S. Hadar.
Figure 5.
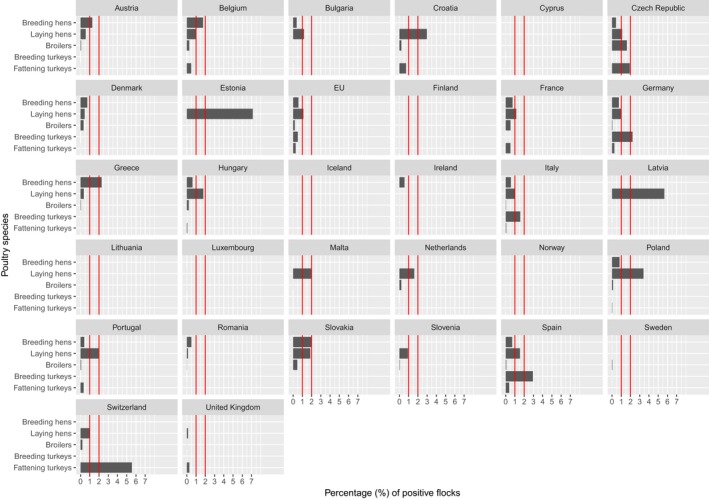
- Red vertical bars indicate the target to be reached, which was fixed at 1% for all categories with the exception of laying hens where it was 2% for all MS with the exception of Poland, for which it was 2.5%.
Figure 6.
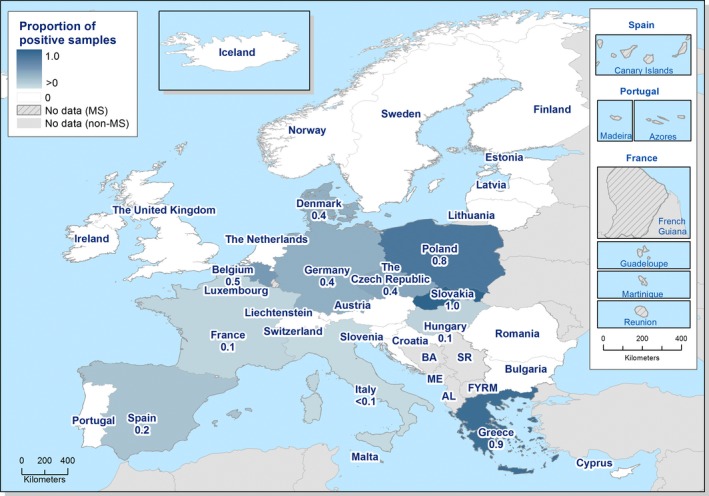
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; SR: Serbia.
Figure 7.
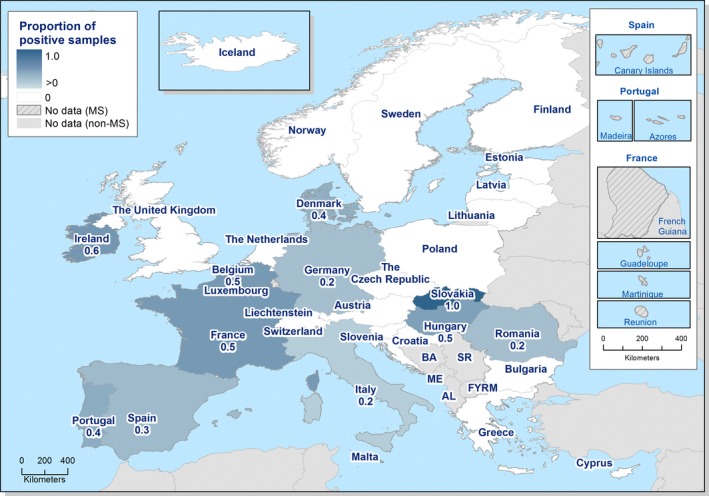
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; SR: Serbia.
Figure 8.
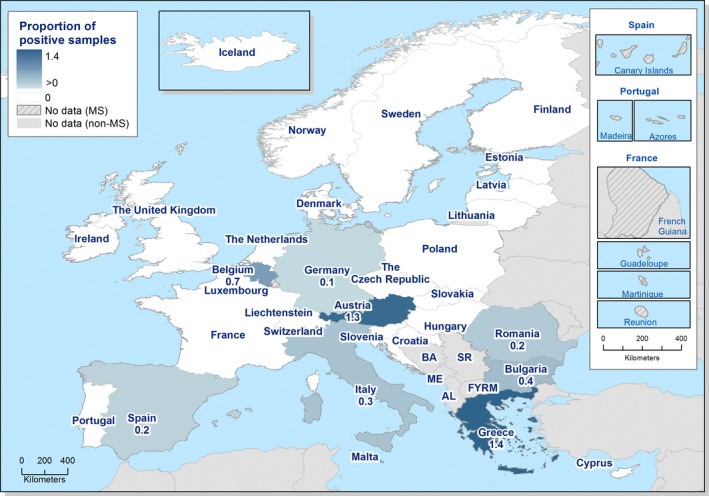
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; SR: Serbia.
Flocks of laying hens
All MS and three non‐MS reported Salmonella NCP data for laying hen flocks. Salmonella was found in 3.70% (or 1,361) of the flocks, compared with 3.71% in 2016. The prevalence of flocks positive for any of the two target serovars was 1.11% (410 flocks), compared with 1.44% in 2016. So, 30.1% (410 out of 1,361) of reported Salmonella‐positive laying hen flocks were positive for target serovars. Six MS and two non‐MS reported no single flock positive for target serovars. Three MS (Croatia, Estonia and Latvia) did not meet their reduction target (Figure 5) and this was mainly due to infection with S. Enteritidis. Croatia and Estonia, Estonia also failed to reach the reduction target in 2016. The flock prevalence was higher for S. Enteritidis (0.89%) as compared with S. Typhimurium (0.22%) (Figures 9 and 10). There was a decrease in the number of laying hen flocks positive for S. Enteritidis (327 in 2017 and 434 in 2016) although the number of tested flocks increased by 2% (36,811 in 2017 and 35,950 in 2016).
Figure 9.
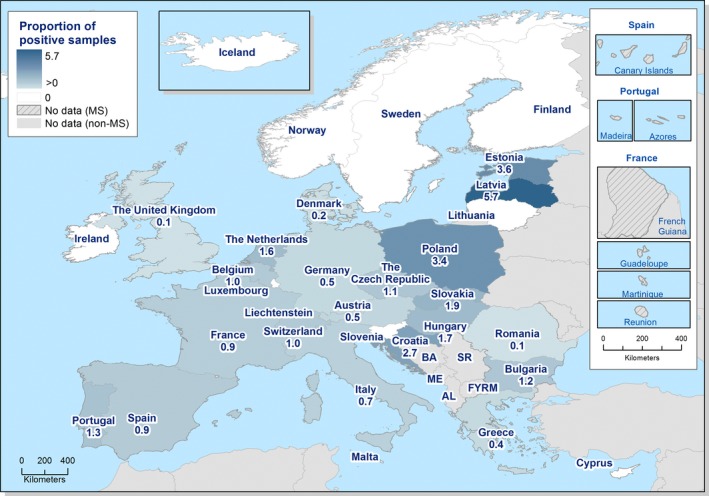
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; SR: Serbia.
Figure 10.
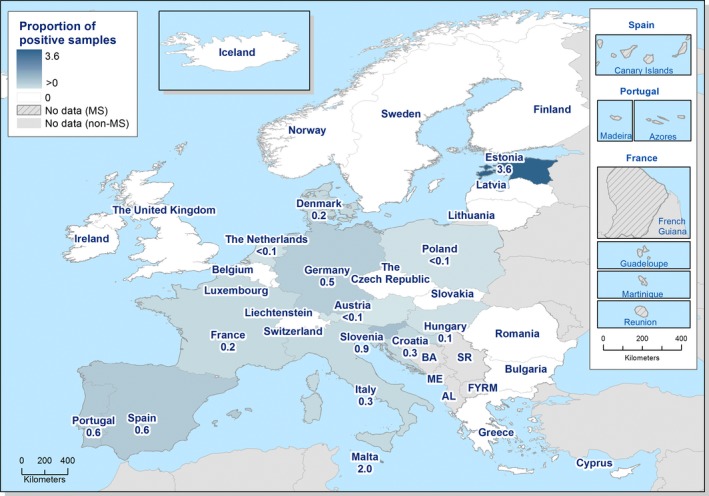
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; SR: Serbia.
Broiler flocks
All MS and three non‐MS reported Salmonella NCP data from broiler flocks. In EU, Salmonella was found in 3.31% of the flocks (or 11,730 flocks) compared with 2.61% in 2016. The prevalence flocks positive to any of the two target Salmonella serovars was 0.19% (or 659 flocks) compared with 0.21% in 2016. So, 5.6% (659 out of 11,730) of reported Salmonella‐positive broiler flocks were positive for target serovars. Eight MS and two non‐MS reported no single flock positive for target serovars. All reporting MS met the target of 1% or less of broiler flocks positive for S. Enteritidis and/or S. Typhimurium, except the Czech Republic (Figure 5). The flock prevalence was higher for S. Typhimurium (0.10%) compared with S. Enteritidis (0.08%) (Figures 11 and 12). Both the number of flocks positive for S. Enteritidis and for S. Typhimurium decreased compared with 2016 (respectively, from 328 in 2016 to 296 in 2017 and from 372 in 2016 to 363 in 2017), although the number of tested flocks reported increased by 6% (354,151 in 2017 and 334,672 in 2016).
Figure 11.
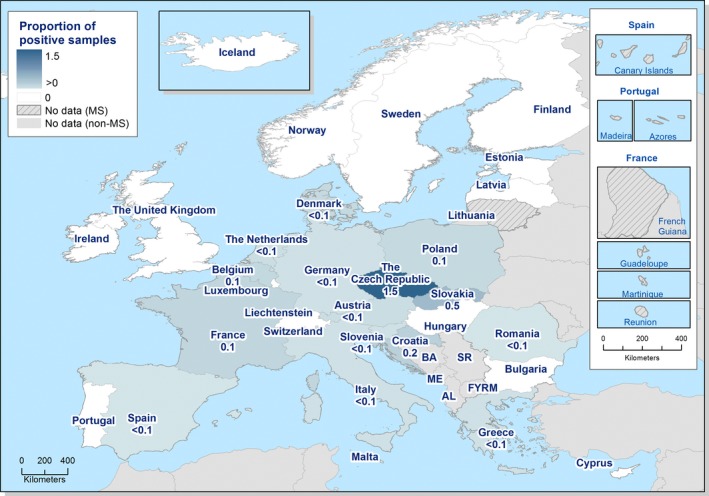
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; SR: Serbia.
Figure 12.
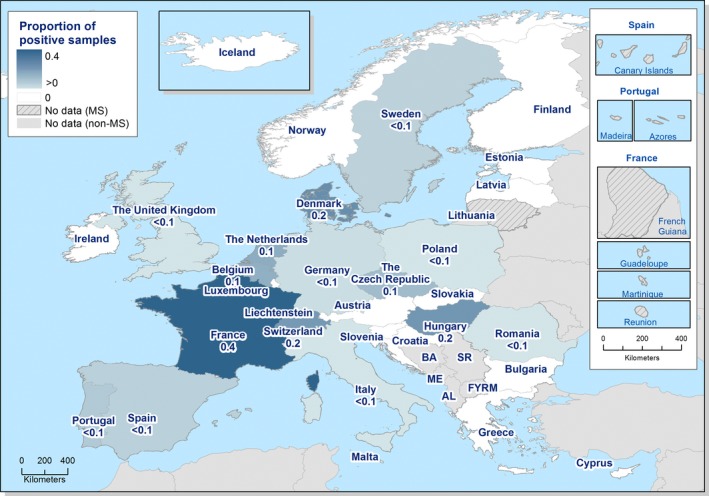
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; SR: Serbia.
Most MS (22) complied with the legal requirement to report separately investigations carried out by CA and by FBOp in broiler flocks. Six MS (Bulgaria, France, Lithuania, Luxembourg and the Netherlands) did not comply and one MS (Croatia) provided exclusively data for FBOp sampling. Also, some inconsistencies between the reported data for the two systems were noted among data provided by some MS. The proportions of Salmonella‐positive flocks from official control samples taken by the CA and from self‐monitoring performed by the FBOp were, respectively, 7.85% (n = 5,454) and 3.31% (n = 260,769), respectively, whereas the results for positivity for any of the Salmonella target serovars were, respectively, 0.81% and 0.09%, respectively. The latter results obtained by the CA were significantly higher than the FBOp's self‐monitoring results and the same finding was also evident for the Czech Republic, Germany, Greece, Poland and Portugal (Table 16). For the remaining reporting MS the differences between the results of both categories of samplers were not significant, the sample size for one or both systems was too low for analyses, or some data were missing.
Table 16.
Comparisons of prevalence of Salmonella target serovar‐positive broiler flocks, by sampler and by reporting Member States, EU, 2017
| Country | Competent authorities (CA) | Food Business Operator (FBOp) | p‐valuea | Interpretation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tested | Positive target | % | CI95 | Tested | Positive target | % | CI95 | |||
| Austria | 66 | 0 | 0.00 | [0.00; 5.43]a | 5,024 | 3 | 0.06 | [0.01; 0.17] | NS | |
| Belgium | 78 | 0 | 0.00 | [0.00; 4.62]a | 10,219 | 27 | 0.26 | [0.17; 3.84] | NS | |
| Croatia | 47 | 0 | 0.00 | [0.00; 7.55]a | ||||||
| Cyprus | 8 | 0 | 0.00 | ‐ ‐ ‐ ‐ | 906 | 0 | 0.00 | [0.00; 0.41]a | ||
| Czech Republic | 37 | 3 | 8.11 | [1.70; 21.91] | 4,801 | 75 | 1.56 | [1.23; 1.95] | * | CA > FBOp |
| Denmark | 253 | 0 | 0.00 | [0; 1.45]a | 4,290 | 14 | 0.33 | [0.19; 0.58] | NS | |
| Estonia | 444 | 0 | 0.00 | [0.00; 0.83]a | 571 | 0 | 0.00 | [0.00; 0.64]a | NS | |
| Finland | 532 | 0 | 0.00 | [0.00; 0.69]a | 3,352 | 0 | 0.00 | [0.00; 0.11]a | NS | |
| Germany | 378 | 8 | 2.12 | [0.92; 4.13] | 24,088 | 10 | 0.04 | [0.02; 0.08] | *** | CA > FBOp |
| Greece | 103 | 3 | 2.91 | [0.60; 8.28] | 7,742 | 0 | 0.00 | [0.00; 0.05]a | *** | CA > FBOp |
| Hungary | 49 | 0 | 0.00 | [0; 7.25]a | 6,632 | 14 | 0.21 | [0.11; 0.36] | NS | |
| Ireland | 41 | 0 | 0.00 | [0; 8.6]a | 3701 | 0 | 0.00 | [0.00; 0.1]a | NS | |
| Italy | 675 | 5 | 0.74 | [0.24; 1.72] | 23,005 | 3 | 0.01 | [0.00; 0.04] | NS | |
| Latvia | 3 | 0 | 0.00 | ‐ ‐ ‐ ‐ | 677 | 0 | 0.00 | [0.00; 0.54]a | ||
| Malta | 4 | 0 | 0.00 | ‐ ‐ ‐ ‐ | 412 | 0 | 0.00 | [0.00; 0.89]a | ||
| Poland | 734 | 20 | 2.72 | [1.67; 4.18] | 40,644 | 31 | 0.08 | [0.052; 0.11] | *** | CA > FBOp |
| Portugal | 126 | 2 | 1.59 | [0.19; 5.62] | 10,934 | 5 | 0.05 | [0.01; 0.11] | ** | CA > FBOp |
| Romania | 940 | 1 | 0.11 | [0.00; 0.59] | 11,622 | 1 | 0.01 | [0.00; 0.05] | NS | |
| Slovakia | 96 | 1 | 1.04 | [0.02; 5.67] | 2,781 | 13 | 0.47 | [0.25; 0.8] | NS | |
| Slovenia | 31 | 0 | 0.00 | [0.00; 11.22]a | 2,452 | 1 | 0.04 | [0.00; 0.23] | NS | |
| Spain | 464 | 1 | 0.22 | [0.00; 1.19] | 39,364 | 25 | 0.06 | [0.04; 0.09] | NS | |
| Sweden | 153 | 0 | 0.00 | [0; 2.38]a | 4,570 | 2 | 0.04 | [0.01; 0.16] | NS | |
| United Kingdom | 192 | 0 | 0.00 | [0.00; 1.90]a | 52,982 | 5 | 0.01 | [0.00; 0.02] | NS | |
| Switzerland | 39 | 0 | 0.00 | [0.00; 9.02]a | 460 | 3 | 0.65 | [0.14; 1.89] | NS | |
| Total (MS) | 5,454 | 44 | 0.81 | [0.59; 1.1] | 26,076 | 229 | 0.09 | [0.08; 0.10] | *** | CA > FBOp |
‐ ‐ ‐ ‐: The confidence interval is not provided because of the small sample size;
a one‐sided, 97.5% confidence interval; p‐value interpretation: NS: not significant; *< 0.05; **< 0.01; ***< 0.001.
Breeding flocks of turkeys
For breeding turkeys, 14 MS and two non‐MS reported Salmonella NCP data. Salmonella was found in 2.63% (or 52) of the flocks tested (n = 1,979), compared with 1.1% in 2016. The prevalence of flocks positive to any of the two target serovars was 0.50% compared with 0.24% in 2016. S. Enteritidis was isolated from one single flock in Germany and S. Typhimurium from nine flocks in Germany, Italy and Spain, in total. Germany (as in 2016), Italy and Spain did not meet the target (Figures 5 and 13), whereas the other reporting MS did not report any breeding turkey flock positive for relevant serovars. So, 19.2% (10 out of 52) of reported Salmonella‐positive flocks were positive for target serovars.
Figure 13.
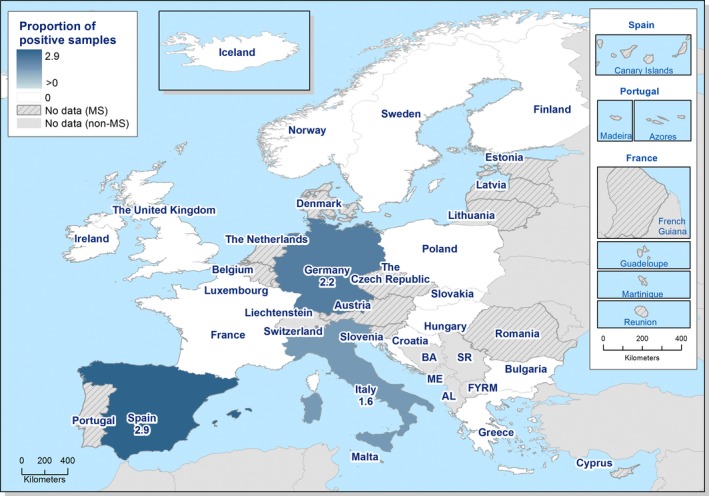
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; SR: Serbia.
Salmonella NCP monitoring data for turkey breeding flocks must be reported separately for samplings performed by CA and by FBOp. Two MS (Bulgaria and France) did not comply with this reporting; 10 MS provided data from FBOp and from CA, and one MS provided data only from CA and another only from FBOp. In some cases, some inconsistencies were present among data notified for the different reporting systems. The proportions of Salmonella‐positive flocks from official control samples taken by the CA and from self‐monitoring performed by the FBOp were, respectively, 2.40% (n = 666) and 2.69% (n = 1,076), whereas the results for Salmonella target serovar positives were, respectively, 1.20% and 0.28%. The comparison among the data reported by CA and by FBOp (Table 17) revealed that at the MS‐group level the prevalence of Salmonella target serovar‐positive flocks from official control samples was significantly higher than self‐monitoring results and the same finding was also evident for Spain.
Table 17.
Comparisons of prevalence of Salmonella target serovar‐positive flocks of breeding turkeys, by sampler and by reporting Member States, EU, 2017
| Country | Competent authorities (CA) | Food Business Operator (FBOp) | Interpretation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tested | Positive target | % | CI95 | Tested | Positive target | % | CI95 | p‐valuea | ||
| Croatia | 1 | 0 | 0.00 | — | ||||||
| Finland | 7 | 0 | 0.00 | — | 7 | 0 | 0.00 | — | ||
| Germany | 73 | 2 | 2.74 | [0.33; 9.55] | 90 | 1 | 1.11 | [0.03; 0.03] | NS | |
| Greece | 2 | 0 | 0.00 | — | ||||||
| Hungary | 36 | 0 | 0.00 | [0.00; 9.74]a | 119 | 0 | 0.00 | [0.00; 3.05]a | NS | |
| Ireland | 6 | 0 | 0.00 | — | 6 | 0 | 0.00 | — | ||
| Italy | 148 | 3 | 2.03 | [0.42; 5.81] | 316 | 2 | 0.63 | [0.08; 2.27] | NS | |
| Poland | 109 | 0 | 0.00 | [0.00; 3.33]a | 161 | 0 | 0.00 | [0.00; 2.27]a | NS | |
| Slovakia | 22 | 0 | 0.00 | [0.00; 15.44]a | 22 | 0 | 0.00 | [0.00; 15.44]a | NS | |
| Spain | 44 | 3 | 6.82 | [1.43; 18.66] | 100 | 0 | 0.00 | [0.00; 3.62]a | * | CA > FBOp |
| Sweden | 4 | 0 | 0.00 | — | 4 | 0 | 0.00 | — | ||
| United Kingdom | 217 | 0 | 0.00 | [0.00; 1.68]a | 251 | 0 | 0.00 | [0.00; 1.46]a | NS | |
| Norway | 3 | 0 | 0.00 | — | ||||||
| Total (MS) | 666 | 8 | 1.20 | [0.52; 2.35] | 1076 | 3 | 0.28 | [0.06; 0.81] | ** | CA > FBOp |
—: The confidence interval is not provided because of the small sample size;
(a): one‐sided, 97.5% confidence interval; p‐value interpretation: NS: not significant; *p < 0.05; **p < 0.01.
Flocks of fattening turkeys
For fattening turkeys, in total, 23 MS and three non‐MS provided data. Salmonella was found in 5.95% of the flocks (or 2,431 flocks) compared with 4.87% in 2016, in the EU. The prevalence flocks positive to any of the two target Salmonella serovars was 0.28% (or 113 flocks) (Figure 5), compared with 0.36% in 2016. So, 4.7% (113 out of 2,431) of reported Salmonella‐positive fattening turkey flocks were positive for target serovars. Twelve MS and two non‐MS reported no single flock positive for target serovars. The Czech Republic did not meet the target (Figure 14) of 1%, as in 2016. The flock prevalence was higher for S. Typhimurium (0.19%) compared with S. Enteritidis (0.08%). Both the number of flocks positive to S. Enteritidis and to S. Typhimurium decreased compared with 2016, respectively, from 43 in 2016 to 34 in 2017 and from 105 in 2016 to 79 in 2017), whereas the number of tested flocks reported was about the same (40,847 in 2017 and 40,831 in 2016). Switzerland reported 1 flock out of 18 tested (5.6%) to be positive for target serovars, notably S. Typhimurium.
Figure 14.
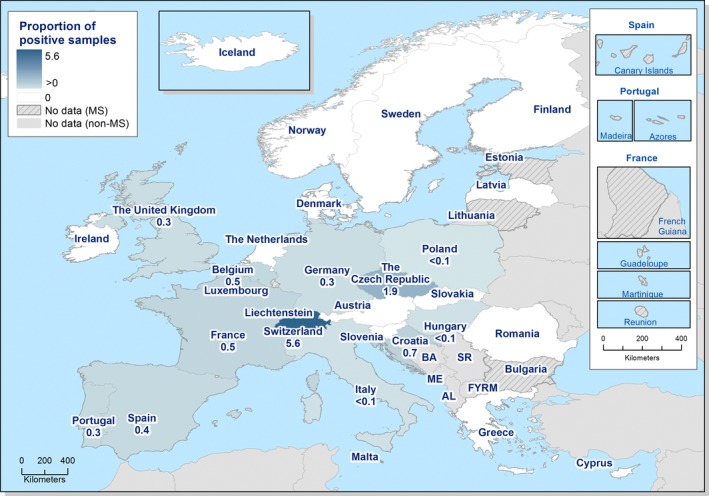
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: the Former Yugoslav Republic of Macedonia; ME: Montenegro; SR: Serbia.
Salmonella NCP monitoring data for turkey fattening flocks must also be reported separately for investigations carried out by CA and by FBOp. Most MS (19) complied with the requirement, while four MS (Croatia, France, Latvia and the Netherlands) did not send separate data from CA and FBOp. Some inconsistencies between the reported data for the two systems were noted among data provided by some MS. The proportions of Salmonella‐positive flocks from official control samples taken by the CA and from self‐monitoring performed by the FBOp were, respectively, 8.46% (n = 898) and 7.54% (n = 27,577), whereas the results for positivity for any of the Salmonella target serovars were, respectively, 1.78% and 0.12%. The latter results obtained by the CA were significantly higher than the FBOp's self‐monitoring results and the same finding was also evident for Germany, Italy and the United Kingdom (Table 18).
Table 18.
Comparisons of prevalence of Salmonella target serovar‐positive flocks of fattening turkeys, by sampler and by reporting Member States, EU, 2017
| Country | Competent authority | Food Business Operator | p‐value | Interpretation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tested | Positive target | % | CI95 | Tested | Positive target | % | CI95 | |||
| Austria | 23 | 0 | 0.00 | [0.00; 14.82]a | 429 | 0 | 0.00 | [0.00; 0.86] | NS | |
| Belgium | 4 | 0 | 0.00 | ‐ ‐ ‐ ‐ | 211 | 1 | 0.47 | [0.01; 2.61] | ||
| Cyprus | 4 | 0 | 0.00 | ‐ ‐ ‐ ‐ | 7 | 0 | 0.00 | — | ||
| Czech Republic | 12 | 0 | 0.00 | ‐ ‐ ‐ ‐ | 254 | 5 | 1.97 | [0.64; 4.53] | ||
| Denmark | 13 | 0 | 0.00 | ‐ ‐ ‐ ‐ | 24 | 0 | 0.00 | [0.00; 14.25]a | ||
| Finland | 49 | 0 | 0.00 | [0.00; 7.25]a | 262 | 0 | 0.00 | [0.00; 1.4]* | NS | |
| Germany | 188 | 12 | 6.38 | [3.34; 10.88] | 4681 | 1 | 0.02 | [0.00; 0.12] | *** | CA > FBO |
| Greece | 6 | 0 | 0.00 | ‐ ‐ ‐ ‐ | 75 | 0 | 0.00 | [0.00; 4.8]a | ||
| Hungary | 28 | 0 | 0.00 | [0.00; 12.34]a | 1717 | 1 | 0.06 | [0.00; 0.32] | NS | |
| Ireland | 22 | 0 | 0.00 | [0.00; 15.44]a | 333 | 0 | 0.00 | [0.00; 1.10]a | NS | |
| Italy | 128 | 2 | 1.56 | [0.19; 5.53] | 5061 | 1 | 0.02 | [0.00; 0.11] | ** | CA > FBO |
| Poland | 176 | 0 | 0.00 | [0.00; 2.07]a | 6687 | 0 | 0.00 | [0.00; 0.05]a | NS | |
| Portugal | 14 | 0 | 0.00 | ‐ ‐ ‐ ‐ | 1196 | 4 | 0.33 | [0.09; 0.85] | ||
| Romania | 49 | 0 | 0.00 | [0.00; 7.25]a | 172 | 0 | 0.00 | [0.00; 2.12]a | NS | |
| Slovakia | 6 | 0 | 0.00 | ‐ ‐ ‐ ‐ | 23 | 0 | 0.00 | [0.00; 14.82]a | ||
| Slovenia | 13 | 0 | 0.00 | ‐ ‐ ‐ ‐ | 131 | 0 | 0.00 | [0.00; 2.78]a | ||
| Spain | 76 | 0 | 0.00 | [0.00; 4.74]a | 3970 | 15 | 0.38 | [0.21; 0.62] | NS | |
| Sweden | 42 | 0 | 0.00 | [0.00; 8.41]a | 236 | 0 | 0.00 | [0.00; 1.55]a | NS | |
| United Kingdom | 45 | 2 | 4.44 | [0.54; 15.15] | 2108 | 5 | 0.24 | [0.08; 0.55] | ** | CA > FBO |
| Switzerland | 18 | 1 | 5.56 | [0.14; 27.29] | ||||||
| Total (MS) | 898 | 16 | 1.78 | [1.02; 2.88] | 27577 | 33 | 0.12 | [0.08; 0.17] | *** | CA > FBO |
‐ ‐ ‐ ‐: The confidence interval is not provided because of the small sample size;
a one‐sided, 97.5% confidence interval; p‐value interpretation: NS: not significant; *< 0.05; **< 0.01; ***< 0.001.
Trends in Salmonella poultry flock prevalence in flocks
The trends in the EU flock prevalence of target Salmonella serovars in poultry flocks since the implementation of EU‐wide NCPs 2007–2017 are displayed in Figure 15. Similar trends at MS level are displayed in the figures in the Appendix. Detailed outputs of the trend analysis are reported in the Appendix.
Figure 15.
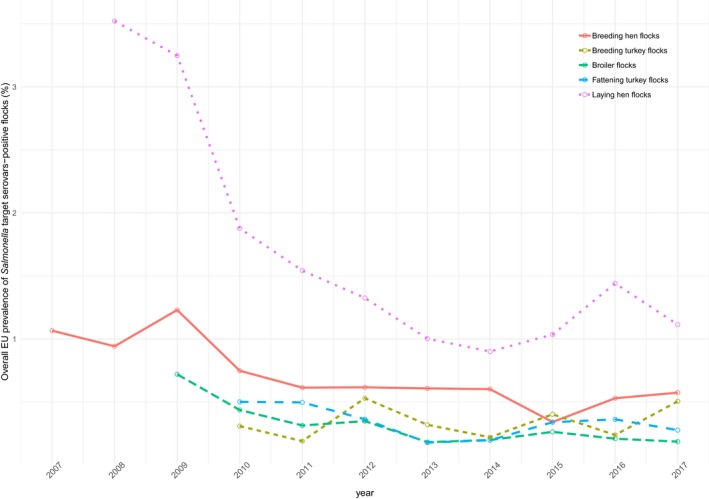
Overall reported prevalence of poultry flocks positive for Salmonella target serovars relevant for public health in different poultry animal populations, among all reporting Member States, EU, 2007–2017
Breeding flocks of Gallus gallus
In breeding flocks of Gallus gallus, S. Enteritidis is the commonest target serovar and its trend over time is nearly identical to that of the target serovars. Moreover, the trends of Salmonella spp. and non‐target serovars are almost similar.
The data used to model the trend in EU Salmonella flock prevalence for target serovars in breeding Gallus gallus for the period 2007–2017 were from 26 MS. Two MS (Estonia and Latvia) reported 0% prevalence for target serovars in their flocks during this entire period.
Since the beginning of NCP, there has been a decreasing overall trend for the prevalence of flocks positive to target serovars (Figures 15 and 17): the estimated prevalence decreased from 0.96% CI95[0.53; 1.74] in 2007 to 0.29% CI95[0.2; 0.43] in 2015. In the next 2 years, there was a slight increase in the prevalence to 0.38% CI95[0.25; 0.59] in 2017. Nevertheless, this prevalence was not significantly different from those of the previous 2 years (2015 and 2016). The wide confidence interval of the estimated prevalence during 2007–2008 reflects the greater variability among MS at the beginning of NCP, for positivity for target serovars. Since the implementation of the NCP, the variability among MS has reduced over time. The estimated EU prevalence of flocks positive to Salmonella spp. decreased from 2.2% CI95[1.1; 4.2] in 2012 to 1.0% CI95[0.61; 1.64] in 2015 and then it increased slightly to 1.3% CI95[0.90; 1.97] in 2017. The latter prevalence was not significantly different from those of the previous 2 years.
Figure 17.
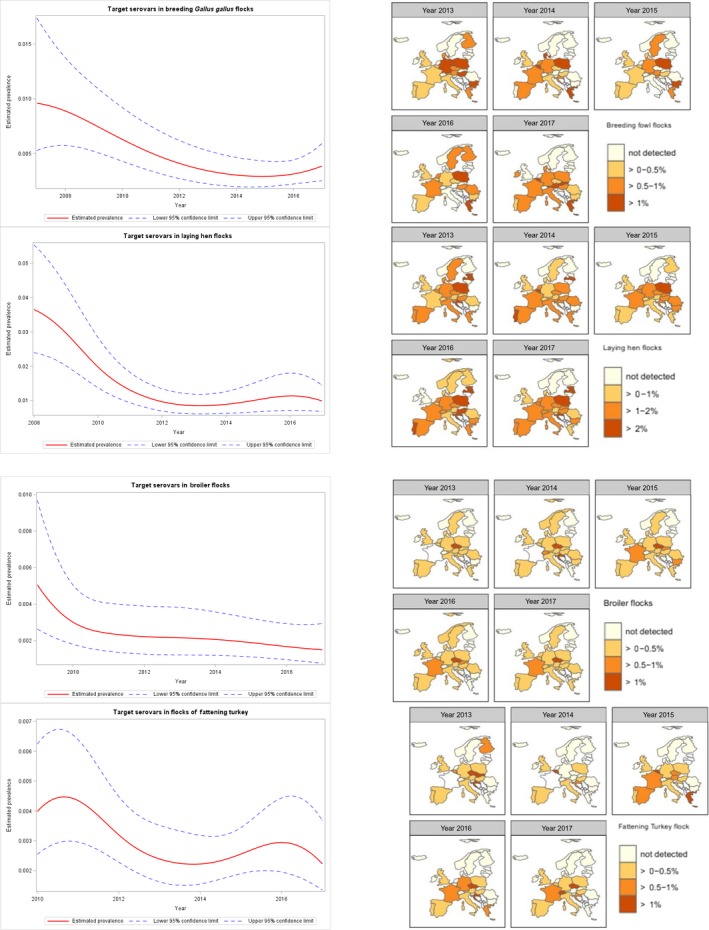
Estimate of the trend prevalence of poultry flocks positive for target Salmonella serovars, at the EU level, in different poultry animal populations, EU, 2007–2017
Flocks of laying hens
In laying hen flocks, the temporal trends for S. Enteritidis, target serovars, non‐target serovars and Salmonella spp. were similar, even though different levels of prevalence occurred.
Data used to model the trend in the EU Salmonella flock prevalence for target serovars in laying hens over the period 2008–2017 were from all MS. No MS reported 0% prevalence for target serovars during this period. Since the beginning of NCP, there has been a decreasing overall trend for the prevalence of flocks positive for target serovars (Figures 15 and 17): the estimated prevalence was 3.65% CI95[2.39; 5.53] in 2008 and decreased to 0.85% CI95[0.62; 1.3] in 2013, with a steep downturn. From 2014 onwards, it increased to 1.1% CI95[0.71; 1.8] in 2016 and then it slowly decreased again in 2017 to 0.99% CI95[0.67; 1.44]. Nevertheless, the 2017 prevalence was not significantly different compared with the previous 3 years. During 2015–2016, different countries (Croatia, the Czech Republic, France, Germany, Poland and Estonia) reported an increased prevalence of laying hen flocks positive for target serovars. For Poland in particular, the laying hen flocks positive for the target Salmonella serovars increased from 2.84% in 2015 to 7.15% in 2016. During 2017, for most of these MS, the prevalence reached again the levels of 2015. As for breeding hens, the wide confidence interval of the estimated prevalence in the first two observation years reflects the greater variability among MS. Since the implementation of the NCP, the variability among MS has reduced over time, with the exception of 2016. The estimated EU prevalence of laying hen flocks positive for Salmonella spp. was 7.16% CI95[4.36; 11.54] in 2008 and it decreased to 2.1% CI95[1.32; 3.34] in 2014, with a steep downturn. During the following years, it increased to 3.1% CI95[1.95; 4.78] in 2017. Nevertheless, the 2017 prevalence was not significantly different compared with the previous 2 years.
Figure 16 displays the EU S. Enteritidis flock prevalence in laying hens and the number of human cases due to S. Enteritidis infection acquired in the EU, without human data from Poland that started to report case‐based serotype data first time in 2017. The EU S. Enteritidis flock prevalence in laying hens decreased from 2012 to 2014, after which it significantly increased during 2015 and 2016. It then decreased again during 2017 to 0.9%. The number of human cases due to S. Enteritidis infection acquired in the EU seemed to follow during 2012–2017 an analogous trend. After a sharp decrease in human cases of S. Enteritidis in 2013 compared with 2012, an increase was observed during the following years. The number of cases due to S. Enteritidis next decreased in 2017 to the same level as in 2015.
Figure 16.
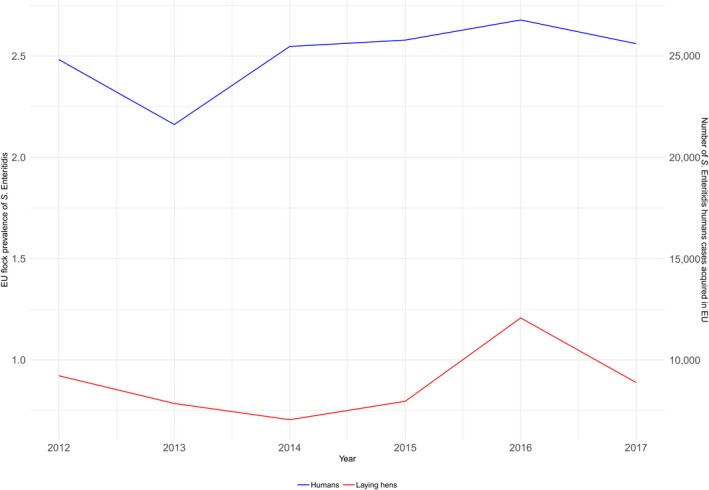
Percentage of laying hen flocks positive for S. Enteritidis and number of human salmonellosis cases due S. Enteritidis infection acquired in the EU, 2012–2017
Broiler flocks
In broiler flocks, the trend over time of S. Enteritidis mimics that of the target serovars. Moreover, the trends over time of Salmonella spp. and non‐target serovars are analogous.
The data from 27 MS were used to model the trend in EU Salmonella flock prevalence for target serovars in broilers for the period 2009–2017. Two MS (Finland and Estonia) reported 0% prevalence for target Salmonella serovars in their broiler flocks during this entire period. Since the beginning of NCP, the estimated flock prevalence for target serovars had a steep decrease in the first time interval (until 2011) and a minor one in the remaining observation time (Figures 15 and 17). The prevalence was 0.51% CI95[0.26; 0.97] in 2009 and decreased to 0.15% CI95[0.08; 0.29] in 2017. This latter prevalence was not significantly different from those of the previous 2 years. The estimated EU prevalence of broiler flocks positive to Salmonella spp. decreased from 1.7% CI95[0.88; 3.12] in 2013 to 1.18% CI95[0.67; 2.09] in 2015 and then it increased slightly to 1.6 CI95[0.88; 3.01] in 2017, reaching the prevalence level of 2013. Nevertheless, the prevalence in 2017 was not significantly different from those of the previous 2 years.
Breeding turkey flocks
In breeding turkeys, the trends over time of S. Enteritidis and target serovar‐positive flocks overlapped, as did those of Salmonella spp. and the non‐target serovars.
The data used to model the trend in the EU Salmonella flock prevalence for target serovars in breeding turkeys for the period 2010–2017 were from 15 MS. Six MS reported 0% prevalence for target Salmonella serovars in their breeding turkey flocks over this entire period (Figure 15). The remaining MS had, from time to time, some positive flocks. The estimated Salmonella serovar prevalence ranged from 0.2% to 0.5% for the entire period. Overall, the prevalence of the target serovars remained constant, although with some fluctuations (Figure 17). The estimated EU Salmonella spp. flock prevalence decreased from 4.6% CI95[1.42; 13.84] in 2013 to 1.14% CI95[0.61; 2.12] in 2016 and then it increased in a significant way (p‐value = 0.035) to 2.84% CI95[1.53; 5.20] in 2017.
Fattening turkeys
In fattening turkeys, the trends over time of S. Enteritidis and the target serovars are different. Conversely, the trends over time of Salmonella spp. and non‐target serovars are very similar.
The data used to model the trend in EU Salmonella flock prevalence for target serovars in fattening turkeys for the period 2010–2017 were from 25 MS. Four MS (the Netherlands, Romania, Slovenia and Sweden) reported 0% prevalence for target Salmonella serovars in their fattening turkey flocks during this entire period. The estimated target serovar flock prevalence was 0.4% CI95[0.25; 0.62] in 2010, it decreased to 0.22% CI95[0.15; 0.32] in 2014, it increased to 0.29% CI95[0.19; 0.44] in 2016 and finally in 2017 it decreased again to the level of 2015 (0.22% CI95[0.13; 0.37]). Overall, the target Salmonella serovars prevalence slightly decreased with small fluctuations over time (Figures 15 and 17). Nevertheless, there were no significant differences among the prevalence in the last 3 years. The estimated EU prevalence of fattening flocks positive to Salmonella spp. decreased from 5% CI95[2.34; 10.59] in 2013 to 2.13% CI95[0.87; 5.1] in 2015 and then it increased to 3.5% CI95[1.71; 6.95] in 2017. Nevertheless, the 2017 prevalence was not significantly different from those of the previous 2 years.
Salmonella monitoring data in other animals
Five MS (Bulgaria, Hungary, Latvia, Poland and Sweden) and one non‐MS (Norway) reported monitoring data on Salmonella flock prevalence in ducks and geese for 2017. Of 5,244 flocks, 2.7% were positive for Salmonella, whereas 1.2% was positive for S. Enteritis and/or S. Typhimurium.
Sixteen MS and two non‐MS (Norway and Switzerland) reported data on Salmonella prevalence in pigs. Overall, 12.7% of the 90,921 reported sampled units were positive for Salmonella. Among these, about 80% (n = 71,860) were collected at the slaughterhouse and 14.2% were positive.
In cattle, based on data reported by 15 MS and three non‐MS, the overall prevalence of Salmonella –positive sampling units was 0.20% with 654,206 sampled units.
2.4.5. Salmonella in feed
The overall prevalence of Salmonella‐positive units in animal‐ and vegetable‐derived feed supplies in 2017 was 1.32% out of 21,868 units reported by 24 MS.
In compound feed (the finished feed for animals), the prevalence of Salmonella‐positive units in 2017 was low for all animal populations: 0.28% of 14,343 tested samples for poultry, 0.43% of 2,808 tested samples for cattle and 0.47% of 3,591 tested samples for pigs.
2.4.6. Salmonella serovars in humans, food and animals
Humans
Serovars among all confirmed salmonellosis cases
For humans, information on Salmonella serovars was available for 86.1% of the total number of confirmed cases (78,949 cases out of 91,662) from 27 MS (Bulgaria did not report case‐based serovar data), Iceland and Norway. Data includes all cases reported with serovar information regardless the importation/travel status. As in previous years, the three most commonly reported Salmonella serovars in 2017 were S. Enteritidis, S. Typhimurium and monophasic S. Typhimurium (1,4,[5],12:i:‐), representing 70.5% of the 78,949 confirmed human cases with known serovar in 2017 (Table 19). The proportion of S. Enteritidis continued to increase in 2017 compared with 2015 and 2016, which was mainly due to one large MS (Poland) starting to report case‐based serotype data for the first time in 2017. The proportions of S. Typhimurium and its monophasic variant strains 1,4,[5],12:i:‐ and S. Infantis that were at same level as in 2015 and 2016. Cases of S. Stanley stayed on the same stable level as before the outbreak in 2013. The fifth most common serovar S. Newport increased 22.8%, and S. Agona increased 61.8% in 2 years since 2015 and replaced S. Derby as a sixth most common serovar. Three ‘new’ serovars (S. Brandenburg, S. Kottbus and S. Coeln), which increased 68.6%, 67.3% and 33.5%, respectively, in 2 years, entered the top 20 list in 2017 and replaced serovars S. Braenderup, S. Panama, S. Weltevreden.
Table 19.
Distribution of reported confirmed cases of human salmonellosis in the EU/EEA, 2015–2017, by the 20 most frequent serovars in 2017
| Serovar | 2017 | 2016 | 2015 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | MS | % | Cases | MS | % | Cases | MS | % | |
| Enteritidis | 38,780 | 27 | 49.1 | 33,325 | 25 | 48.5 | 32,341 | 25 | 44.4 |
| Typhimurium | 10,593 | 27 | 13.4 | 9,789 | 25 | 13.4 | 12,035 | 25 | 16.5 |
| Monophasic Typhimurium 1.4.[5].12:i:‐ | 6,324 | 16 | 8.0 | 5,697 | 15 | 8.4 | 5,786 | 15 | 7.9 |
| Infantis | 1,805 | 28 | 2.3 | 1,658 | 25 | 2.4 | 1,655 | 25 | 2.3 |
| Newport | 925 | 26 | 1.2 | 758 | 18 | 1.1 | 753 | 20 | 1.0 |
| Agona | 647 | 22 | 0.8 | 452 | 17 | 0.8 | 400 | 17 | 0.5 |
| Kentucky | 617 | 20 | 0.8 | 559 | 21 | 0.8 | 545 | 20 | 0.7 |
| Derby | 612 | 24 | 0.8 | 620 | 21 | 0.8 | 697 | 22 | 1.0 |
| Stanley | 554 | 23 | 0.7 | 543 | 21 | 0.7 | 825 | 24 | 1.1 |
| Virchow | 512 | 22 | 0.6 | 509 | 21 | 0.7 | 516 | 23 | 0.7 |
| Bareilly | 427 | 19 | 0.5 | 262 | 16 | 0.6 | 225 | 18 | 0.3 |
| Naples | 406 | 19 | 0.5 | 300 | 16 | 0.6 | 373 | 18 | 0.5 |
| Java | 388 | 17 | 0.5 | 418 | 17 | 0.6 | 436 | 17 | 0.6 |
| Bovismorbificans | 346 | 22 | 0.4 | 393 | 21 | 0.5 | 387 | 21 | 0.5 |
| Hadar | 335 | 20 | 0.4 | 274 | 18 | 0.5 | 251 | 20 | 0.3 |
| Saintpaul | 332 | 22 | 0.4 | 456 | 21 | 0.4 | 292 | 19 | 0.4 |
| Chester | 329 | 19 | 0.4 | 302 | 18 | 0.4 | 294 | 17 | 0.4 |
| Brandenburg | 290 | 20 | 0.4 | 190 | 16 | 0.4 | 172 | 21 | 0.2 |
| Kottbus | 266 | 23 | 0.3 | 121 | 18 | 0.4 | 159 | 22 | 0.2 |
| Coeln | 265 | 22 | 0.3 | 139 | 16 | 0.3 | 200 | 16 | 0.3 |
| Other | 14,196 | – | 18.0 | 13,472 | – | 17.7 | 14,573 | – | 20.0 |
| Total | 78,949 | 27 | 100.0 | 70,237 | 26 | 100.0 | 72,915 | 26 | 100.0 |
MS: Member State.
Source(s): Twenty‐seven MS and two non‐MS; Austria, Belgium, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and United Kingdom, and Iceland and Norway.
Serovars acquired in the EU
To estimate the impact of the Salmonella infections acquired at the EU level, serovar data were analysed for domestic and travel‐associated cases in which the probable country of infection was an EU MS (Table 9). Information on Salmonella serovars with importation/travel data was available from 26 MS, representing 75.0% of cases with known serovar data in 2017. Most cases (81.1%) with known data on importation were infected within the EU. Among the travel‐related cases, the most frequently reported travel destinations were Spain (33.5%), Greece (12.5%), Poland (7.1%) and Italy (6.6%), as in 2016.
From reported cases of human salmonellosis acquired in the EU, S. Enteritidis dominated and almost two in three (61.2%) of the reported cases were infected by this serovar. Together with S. Typhimurium and monophasic S. Typhimurium 1,4,[5],12:i:‐, these three serovars represented 78.1% of the confirmed human cases acquired in the EU in 2017 (Table 20). S. Enteritidis cases were predominantly (94.4%) infected within EU. The proportion of S. Enteritidis continued to increase in 2017 compared with 2015 and 2016. This was mainly due to one large MS (Poland) starting to report case‐based serotype data for the first time in 2017. Without Poland, the proportion of S. Enteritidis was at the same level as in 2016 (57.1%). The proportion of S. Typhimurium continued to decrease in 2017, while its monophasic variant strains 1,4,[5],12:i:‐ and S. Infantis remained at the same level as in 2016 and 2015. The number of cases of S. Newport acquired in the EU, which replaced S. Derby as the fifth serovar in the top five, increased by 21.5% (68 cases) compared with 2016. The majority (82.4%) of the increase of S. Newport cases was reported by one country, the United Kingdom. Fifty‐six per cent of the cases in the United Kingdom were domestically acquired whereas the remaining cases were almost entirely linked to travel in Spain. The decrease of S. Derby was highly influenced by one country (Belgium) not reporting importation status with the data in 2017. When analysed without data from Belgium, there was a decrease of S. Derby by 9.2% (30 cases) compared with 2016.
Table 20.
Distribution of reported cases of human salmonellosis acquired in the EU, 2015–2017, by the five most frequent serovars in 2017
| Serovar | 2017 | 2016 | 2015 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | MS | % | Cases | MS | % | Cases | MS | % | |
| Enteritidis | 32,251 | 25 | 61.2 | 26,781 | 23 | 57.1 | 25,788 | 22 | 54.7 |
| Typhimurium | 6,807 | 25 | 12.9 | 6,725 | 23 | 14.3 | 7,971 | 22 | 16.9 |
| Monophasic Typhimurium 1.4.[5].12:i:‐ | 2,098 | 16 | 4.0 | 2,088 | 16 | 4.5 | 2,303 | 14 | 4.9 |
| Infantis | 1,164 | 22 | 2.2 | 1,099 | 21 | 2.3 | 1,137 | 21 | 2.4 |
| Newport | 384 | 19 | 0.7 | 316 | 16 | 0.7 | 278 | 17 | 0.6 |
| Other | 10,026 | – | 19.0 | 9,909 | – | 21.1 | 9,672 | – | 20.5 |
| Total | 52,730 | 26 | 100.0 | 46,918 | 24 | 100.0 | 47,149 | 22 | 100.0 |
Source(s): Twenty‐six MS; Austria, Belgium, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and United Kingdom.
There was a statistically significant (p < 0.01) decreasing trend for S. Enteritidis acquired in the EU in 2008–2017, however the trend stabilised and did not show any significant increase or decrease between 2013 and 2017 (Figure 18).
Figure 18.
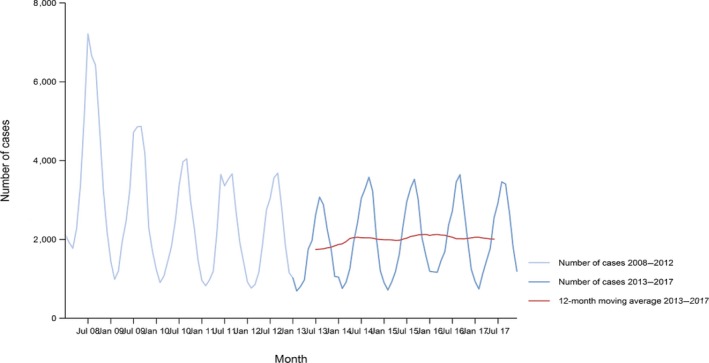
- Source(s): Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Malta, Netherlands, Norway, Portugal, Slovakia, Spain, Sweden and United Kingdom. Austria, Belgium, Bulgaria, Cyprus, Croatia, Lithuania, Luxembourg, Poland, Romania and Slovenia did not report data to the level of detail required for the analysis.
At the country level, nine MS (Denmark, Estonia, Finland, Germany, Hungary, Italy, Latvia, Malta and the Netherlands) reported a decreasing trend of S. Enteritidis cases acquired within the EU in 2008–2017, whereas two MS (the Czech Republic and Portugal) reported an increasing trend over the same period.
In contrast, none of the MS reported a significantly decreasing trend of S. Enteritidis cases acquired within the EU over the last 5 years (2013–2017). A significant increasing trend was observed in six MS (Estonia, Ireland, Portugal, Slovakia, Spain and the United Kingdom) for the last 5 years.
Food and animals
Descriptive analyses were made from food and animal 2017 data of the five most commonly reported Salmonella serovars that were reported from domestic human cases in the EU (including cases that travelled within EU) for 2017 (Table 20). These five most reported serovars were: S. Enteritidis, S. Typhimurium, monophasic S. Typhimurium, S. Infantis and S. Newport. Only isolates related to food‐producing animals and major food categories were aggregated into the following categories: broiler flocks, broiler meat, laying hen flocks, eggs, fattening turkey flocks, turkey meat, pigs, pig meat, cattle and bovine meat (Table SERALLMATRIX in the Appendix). From 14,762 serotyped Salmonella isolates the numbers of reported serovars for the different matrices were in decreasing order from broiler flocks (9,921 isolates, 67.2%), broiler meat (1,664 isolates, 11.3%), turkey flocks (953 isolates, 6.5%), laying hen flocks (948 isolates, 6.4%), pig meat (592 isolates, 4.0%), cattle (235 isolates, 1.6%), pigs (208 isolates, 1.4%), turkey meat (149 isolates, 1.0%), cattle meat (61 isolates, 0.4%) and eggs (31 isolates, 0.2%).
The Sankey diagram in Figure 19 illustrates how the EU top‐five Salmonella serovars in human salmonellosis acquired in the EU are associated with the most important animal species 2017. S. Enteritidis was firstly associated with broiler (57.2% of the S. Enteritidis isolates were from broiler flocks and meat), and secondly with layers (37.1%). A marginal number of S. Enteritidis isolates were obtained from turkey (3.9%) sources. S. Typhimurium was associated with all matrices, in decreasing order: 47.1% of the S. Typhimurium isolates were from broiler sources, 28.3% from pig, 11.1% from turkey, 10.7% from layers and 2.8% from cattle. Monophasic S. Typhimurium was associated mainly with pig (49.7%) and broiler sources (35.3%). S. Infantis was markedly related to broiler sources (94.6%). S. Newport was associated with turkey (65.5%) and broiler (30.5%) sources with, respectively, 100, 41, 33 and 21 isolates from fattening turkey flocks, broiler flocks, turkey meat and broiler meat. As explained in Section 4.2. ‘Surveillance and monitoring of Salmonella in the EU, figures such as Figure 19 can be misleading because the mandatory reporting of target serovars in the context of NCP and in the context of the FSC for fresh poultry meat could result in an overestimation of these target serovars compared with the other serovars. For the remaining matrices, serovar data collected could be strongly biased by what each MS actually serotyped and notified. Moreover, associating S. Enteritidis more with broilers than layers may be misleading because there are far more broiler flocks than laying flocks, the detection sensitivity is highly likely much higher in broiler flocks compared to laying hen flocks, and the impact of broiler meat on human cases might be much less than for eggs in most countries where raw or lightly cooked minced chicken meat (or food‐like liver parfait) is not commonly used. Lastly, Figure 19 is influenced by the contribution of each source within the entire panel of data considered, where broilers (flocks and meat samples) represented the great majority of data.
Figure 19.
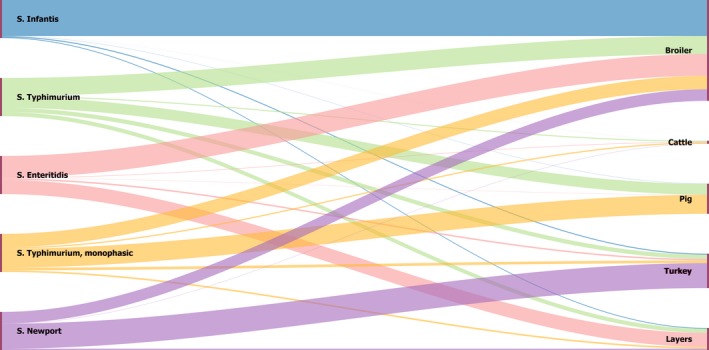
- The left side of the diagram shows the five commonest reported Salmonella serovars from human salmonellosis cases acquired in the EU: S. Infantis (blue), S. Typhimurium (green), S. Enteritidis (pink), monophasic S. Typhimurium (yellow) and S. Newport (violet). Animal and food data from the same source were merged: ‘broiler’ includes isolates from broiler flocks and broiler meat, ‘cattle’ includes isolates from bovines for meat production and bovine meat, ‘pig’ includes isolates from fattening pigs and pig meat, ‘turkey’ includes isolates from fattening turkey flocks and turkey meat and ‘layers’ includes laying hen flocks and eggs. The right side shows the five sources considered (broiler, cattle, pig, turkey and layers). The width of the coloured bands linking sources and serovars is proportional to the percentage of isolation of each serovar from each source.
The Sankey diagram in Figure 20 illustrates how the EU top‐five Salmonella serovars in human salmonellosis acquired in the EU across are distributed across the reporting MS in 2017. Twenty‐six MS reported the top‐five Salmonella serovars from the previous sources. S. Enteritidis was widely reported from most MS, even though Poland and France accounted for most of S. Enteritidis reported (30.7% and 17.3%, respectively). Similarly, S. Typhimurium and monophasic S. Typhimurium isolates were reported from all MS, but the highest percentage of both of these serovars was reported from France, accounting for 30.2% and 29.1%, respectively. S. Infantis isolates were reported mainly from Italy (30.4%), Hungary (20.8%) and Croatia (13.3%). S. Newport, was mostly reported from Hungary, accounting for 58.6% of the total amount of Salmonella isolates belonging to this serovar, and secondly by Poland (15.3%).
Figure 20.
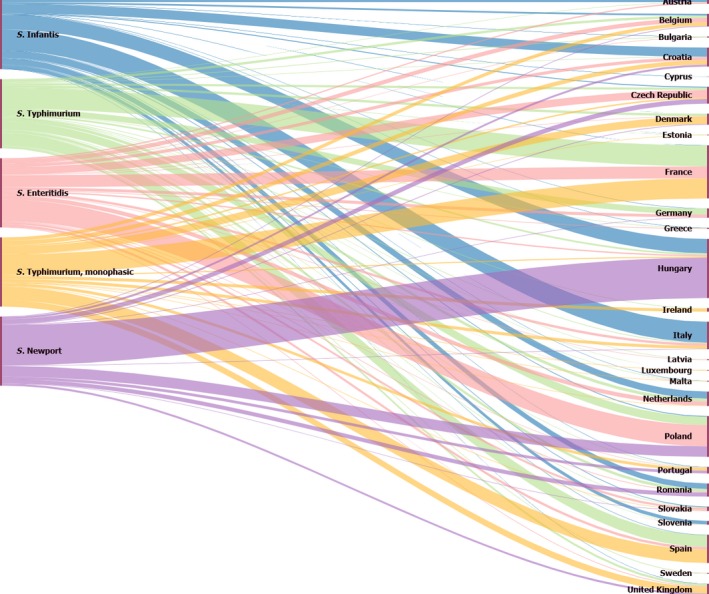
- The left side of the diagram shows the five commonest reported Salmonella serovars from human salmonellosis cases acquired in the EU: S. Infantis (blue), S. Typhimurium (green), S. Enteritidis (pink), monophasic S. Typhimurium (yellow) and S. Newport (violet). The right side shows the reporting Member States. The width of the coloured bands linking Member States and serovars is proportional to the percentage of isolation of each serovar reported from each MS.
Salmonella Enteritidis
Considering all fowl (Gallus gallus, including breeding hens, laying hens and broilers) S. Enteritidis was the fourth most common reported serovar, accounting for 6.7% of the isolates, even though for some MS (the Czech Republic, Germany and Poland) it was the most commonly reported one. In laying hens, S. Enteritidis was the commonest serovar reported. In broiler flocks S. Enteritidis was the seventh commonest reported serovar while from broiler meat it was the second one (14.6%). A negligible number of S. Enteritidis isolates were reported from food and animal sources of turkey, pig and cattle origin. More detailed information can be found in the Appendix (overview tables of distributions of serovars, which include some more data as compared with the data underpinning the pyramid plot because they were extracted using less stringent criteria).
The pyramid plot in Figure 21 displays for each source (animal species and food related to this animal species) the number of isolates and the corresponding percentage. It shows that S. Enteritidis accounted for more than 30% of all Salmonella isolates serotyped from the layers sources while in broiler flocks this is less than 5%. It accounted for about 15% of all isolates from broiler meat. For the other sources, a negligible number of S. Enteritidis isolates was reported by a few MS.
Figure 21.
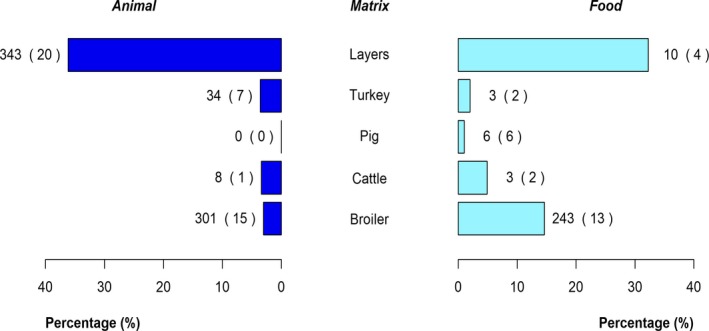
- The percentages were calculated on the total number of isolates serotyped for each animal and food category. The values at the side of each bar are the number of S. Enteritidis isolates and the number in parentheses indicates the number of reporting Member States.
Salmonella Typhimurium
S. Typhimurium accounted for 3.3% and 2.8% of the isolates serotyped from fowl (Gallus gallus, including breeding hens, laying hens and broilers) and broiler flocks, respectively. In broiler meat, it was the fourth most common serovar reported (4.7% out of 1,664 isolates) with Poland reporting 75% of those isolates. In laying hen flocks, S. Typhimurium was the fourth commonest serovar (7.2% of the isolates). In eggs, this serovar accounted for 19.3% of the serotyped isolates. In fattening turkey flocks, 65 S. Typhimurium (6.3%) isolates were reported, mostly by France and Spain. In turkey meat, S. Typhimurium was the third serovar reported (16% out of the 149 isolates) and Poland reported most isolates. In pigs, S. Typhimurium was the second most reported serovar with 92 (20.6% out of 446) isolates. Spain and the United Kingdom reported most of these. In pig meat, S. Typhimurium was the commonest reported serovar and accounted for 161 (27%) out of the 595 Salmonella isolates serotyped. Spain contributed with 30.4% of the S. Typhimurium reported. In cattle herds, S. Typhimurium was the second most common serovar, accounting for 308 out of 1,177 Salmonella isolates (26.2%), whereas for bovine meat S. Typhimurium, with 12 isolates (19.7%) reported out of 61 serotyped, was the most reported one. More detailed information can be found in the Appendix (overview tables of distributions of serovars that include some more data as compared with the data underpinning the pyramid plot because they were extracted using less stringent criteria).
The pyramid plot for S. Typhimurium (Figure 22) shows that 15% and 27% of the isolates serotyped from pig herds and pig meat, respectively, belonged to this serovar. Considering broiler, fattening turkey and laying hen flocks, S. Typhimurium accounted for, respectively, 2.4%, 5.4% and 7.1% of all isolates reported.
Figure 22.
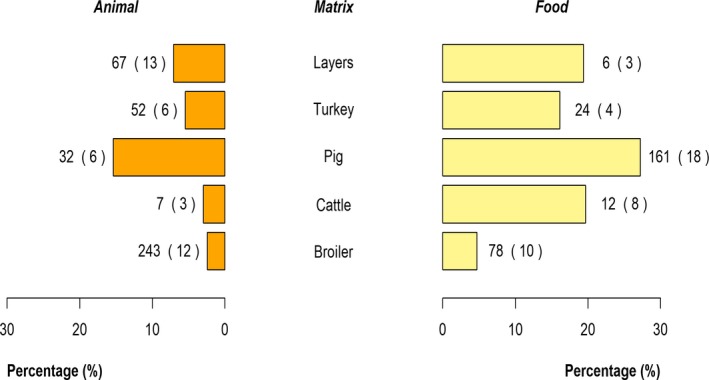
- The percentages were calculated on the total number of isolates serotyped for each animal and food category. The values at the side of each bar are the number of S. Typhimurium isolates and the number in parentheses indicates the number of reporting Member States.
Monophasic variants of Salmonella Typhimurium
In laying hen, broiler and fattening turkey flocks as well as in the corresponding food sources (eggs and meat), serovars reported as ‘monophasic variants of S. Typhimurium’ did not rank among the EU level top‐10 of most reported serovars. The group of monophasic variants of S. Typhimurium was the commonest reported serovar from pigs and pig meat accounting for, respectively, 167 (37.4%) and 129 (22%) isolates. These results confirm that pigs are the main animal reservoir for monophasic variants of S. Typhimurium. In cattle, this group accounted for 1.8% out of 1,177 serotyped Salmonella isolates, whereas in bovine meat it was 16.4% out of 61 serotyped Salmonella isolates. More detailed information can be found in the Appendix (overview tables of distributions of serovars, which include some more data as compared with the data underpinning the pyramid plot because they were extracted using less stringent criteria).
The pyramid plot (Figure 23) shows that about 28% and 20% of all isolates serotyped from pigs and pig meat, respectively, were monophasic variants of S. Typhimurium. The reported percentage of this group of serovars was negligible from other sources with the exception of bovine meat (18%).
Figure 23.
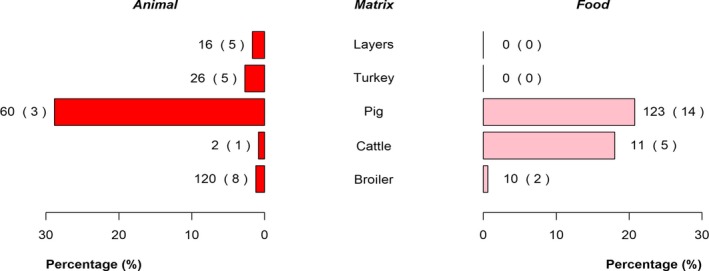
- The percentages were calculated on the total number of isolates serotyped for each animal and food category. The values at the side of each bar are the number of isolates of monophasic variants of S. Typhimurium and the number in parentheses indicates the number of reporting Member States.
Salmonella Infantis
S. Infantis was the commonest reported serovar in fowl (Gallus gallus), accounting for 4,185 out of 9,837 (42.5%) isolates. As in previous years, it accounted for most of the isolates reported by some MS (e.g. Austria, Croatia, Hungary, Italy, Slovakia and Slovenia) for Gallus gallus, while some other MS, which reported large numbers of isolates from fowl (e.g. France and the United Kingdom), hardly reported it. Much more isolates of S. Infantis were reported from fowl (4,185 in 2017 vs 2,399 in 2016). This increase was strongly influenced by the reporting of a few MS. S. Infantis was the commonest reported serovar from broiler flocks as well as broiler meat, accounting for 46.5% and 50.6%, respectively, of all serotyped Salmonella isolates reported from these sources. In laying hen flocks, S. Infantis accounted for 118 (12.7%) of the 932 isolates reported, and in fattening turkey flocks, it accounted for 13.3% of the 953 isolates reported, being the second most reported serovar reported for both categories. Most of S. Infantis isolates from fattening turkey flocks (97.6%) were notified by Hungary. Also, in turkey meat, S. Infantis was the second most reported serovar, accounting for 17.4% of the 149 isolates and mostly reported by Hungary (20 out of 26). S. Infantis was not among the commonest reported serovars from cattle or pig sources (both animals and food). More detailed information can be found in the Appendix (overview tables of distributions of serovars, which include some more data compared with the data underpinning the pyramid plot because they were extracted using less stringent criteria).
Most of the serotyped isolates from broiler flocks and from broiler meat were S. Infantis accounting for 46% and 51% of all serotyped serovars from these sources, respectively (Figure 24). In addition, in laying hen and fattening turkey flocks about 10% of all serotyped isolates were S. Infantis. This serovar was seldom reported from pig and cattle.
Figure 24.
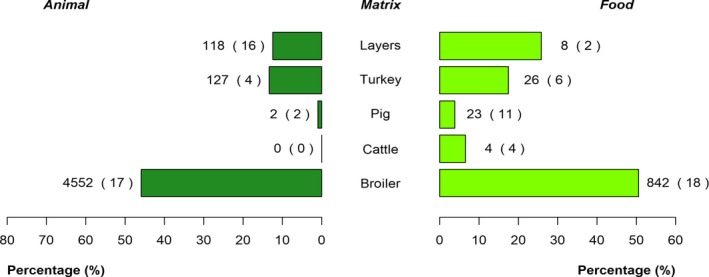
- The percentages were calculated on the total number of isolates serotyped for each animal and food category. The values at the side of each bar are the number of S. Infantis isolates and the number in parentheses indicates the number of reporting Member States.
Salmonella Newport
S. Newport was not among the 10 commonest reported serovars from fowl (Gallus gallus). It was reported in very low numbers by five MS (21 out of 1,664 isolates) from broiler meat. In fattening turkey flocks S. Newport was the third most common serovar accounting for 100 out of 953 isolates (10.5%). The number of reported S. Newport isolates increased compared with 2016 and this was mostly due to the high number (91) of S. Newport reported from Hungary. In turkey, meat samples S. Newport was the most common serovar, accounting for 22.1% of the 149 isolates reported, most of which were by Hungary. S. Newport was not reported from pigs or pig meat, and reports of this isolate from cattle and bovine meat were negligible. More detailed information is in the Appendix (overview tables of distributions of serovars, which include some more data as compared with the data underpinning the pyramid plot because they were extracted using less stringent criteria).
The pyramid plot in Figure 25 shows that S. Newport was mostly associated with the turkey source, accounting for 10% and 22%, respectively (fattening turkey flocks and turkey meat). Overall, this serovar was reported by few MS, for any source.
Figure 25.
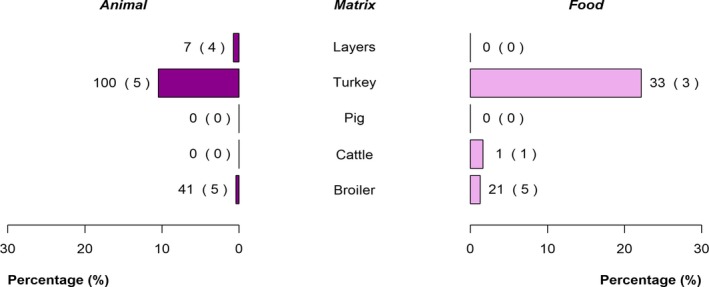
- The percentages were calculated on the total number of isolates serotyped for each animal and food category. The values at the side of each bar are the number of S. Newport isolates and the number in parentheses indicates the number of reporting Member States.
S. Enteritidis was by far the commonest reported serovar in breeding Gallus gallus and laying hens, whereas in broilers it ranked at a lower position. A different picture was seen for S. Infantis. Although during the last years the number of reported S. Infantis isolates increased in breeding Gallus gallus, it remained below S. Enteritidis and S. Typhimurium, whereas in broilers it was the most reported serovar with a marked increase in the number of isolations over several years. S. Mbandaka is another serovar that appeared in the top list of isolated serovars for Gallus gallus, laying hens and broilers. More detailed information is in the Appendix (overview tables of distributions of serovars).
2.5. Discussion
Salmonellosis remains the second most common zoonosis in humans in the EU, despite a significant decreasing EU/EEA trend in confirmed salmonellosis cases since 2008. In the last 5 years (2013–2017), however, the trend has stabilised. In 2017, the number of reported confirmed human cases and the EU notification rate slightly decreased after a 3‐year increase. Almost half of the MS reported a decreasing trend during 2008–2017, but in the majority of those countries the trend has stabilised and the number of MS reporting a significantly increasing trend doubled in 2013–2017. This could be partly attributable to more complete reporting and improvements in the surveillance of salmonellosis in a few countries.
Serovar S. Enteritidis, proportionally continued to increase, particularly in human cases acquired within the EU. S. Enteritidis is predominantly acquired in the EU, more than other serovars. The increase in number in 2017 was mainly due to one new Member State (Poland) starting to report case‐based serovar data for first time. Without Poland, the number of S. Enteritidis cases was at the same level as in 2016. A large multicountry outbreak of S. Enteritidis associated with contaminated eggs from Poland was confirmed in 14 EU/EEA countries in 2016 (EFSA and ECDC, 2017a). Poland implemented control measures and the cases declined in 2017, but started to increase again during the end of the year. It is likely that this multicountry outbreak (ECDC, 2016, 2017a,b; EFSA, 2017a,c) belongs to an epidemic caused by a S. Enteritidis clone already existing since 2012 and still ongoing during 2017, with the most recent case reported from November 2017 (EFSA and ECDC, 2017b).
The number of cases and proportion of the second most common serovar S. Typhimurium continued to decrease in 2017. Together, S. Enteritidis and S. Typhimurium (including monophasic variants) accounted for almost 80% of human cases acquired in the EU. S. Infantis has been consistently the fourth most frequently reported serovar in the domestically acquired and travel‐associated cases.
S. Newport replaced serovar S. Derby as a fifth most common serovar acquired within EU. The increase of S. Newport was mainly (> 80%) due to a higher number of domestic cases in one country (the United Kingdom). The decrease of S. Derby, the previously fifth most common serovar acquired within EU, was greatly affected by one MS, Belgium, which did not report 2017 importation data for serovars and needed being excluded among domestically acquired cases in 2017.
Among reported serovars from all salmonellosis cases and disregarding the travel information S. Agona replaced S. Derby as the sixth most common serovar. This may be due to two S. Agona multicountry outbreaks in the EU that were under investigation during 2017. An outbreak was linked to the consumption of infant formula in France from August 2017 until January 2018 (EFSA and ECDC, 2018a; Jourdan‐da Silva et al., 2018). A multicountry outbreak of S. Agona was possibly linked to RTE food (EFSA and ECDC, 2018c). Overall, 122 outbreak cases were reported by five EU countries (the United Kingdom, Finland, Denmark, Germany and Ireland) from January 2017 to July 2018.
Salmonellosis notification rates for human infections vary between MS, reflecting variations in, for example, quality, coverage and severity focus of the surveillance systems, practices in sampling and testing, disease prevalence in the animal population, food and animal trade between MS, and the proportion of travel‐associated cases. The variation in national surveillance systems is reflected for example by the fact that countries reporting the lowest notification rate for salmonellosis had the highest proportions of hospitalisation, suggesting that the surveillance systems in these countries are focusing on the most severe cases.
From the monitoring data submitted by MS according to Regulation (EC) No. 2073/2005 on microbiological criteria on food samples, the highest proportions of Salmonella‐positive single samples from official control investigations by CA were reported from foods of meat origin intended to be cooked before consumption (minced meat and meat preparations from poultry and minced meat and meat preparations from other species than poultry). These data were, however, too scarce and unrepresentative to describe the situation at the EU level. Still, this observation is consistent with the 2017 notifications in the EU Rapid Alert System for Food and Feed (RASFF), where the highest numbers of non‐compliances were for poultry meat and meat from other species European Commission, 2017.8 As regards Salmonella PHC monitoring data from pig carcasses, from eight MS (Belgium, Bulgaria, Greece, Italy, the Netherlands, Poland, Slovakia and Spain) providing data collected by CA and as well by FBOp, all except one reported the occurrence of Salmonella‐positive samples from official control samples to be significantly higher than self‐monitoring results. These differences can be related to the fact that the CA generally focus their samplings on the most problematic herds/slaughterhouses (risk‐based approach), but it might also be related to different level of sensitivity of the sampling strategies used by CA and FBOp and laboratory analytical methods used (FBOp may use alternative methods). It is advisable to promote further investigations to understand the main reasons explaining these differences.
About Salmonella in animals, for all poultry categories covered by NCP in 2017, the EU prevalence of flocks positive to target Salmonella serovars decreased or stabilised compared with 2016, except in flocks of breeding turkeys where there was an increase in few MS of S. Typhimurium‐positive flocks. For laying hens, after the increase in the prevalence of S. Enteritidis‐positive flocks, documented in 2016 and involving a group of MS, the prevalence decreased during 2017 to a level comparable to 2015. A decrease in the number of S. Enteritidis‐positive flocks was reported for breeding fowl, broiler flocks and for laying hen flocks compared with the previous year. The prevalence of flocks positive to Salmonella spp. increased in 2017 compared with 2016. This increase was for all the poultry categories covered by NCP except for laying hens.
Trend analyses revealed an overall decreasing trend for the prevalence of target serovars in all poultry species, except for breeding turkeys where a stationary but somewhat fluctuating trend was observed for the last 7 years. Nevertheless, in all the poultry species, the prevalence of target serovars over the last 3 years was not significantly different. Conversely, trend analyses of the prevalence of Salmonella spp. showed a generalised increasing trend. This increase may partly be explained by a change in reporting practices and improved surveillance and reporting, brought about through intense collaboration between MS, EFSA and the European Commission. An upward reset of the annual baseline of prevalence of Salmonella spp.‐positive flocks may also be expected due to this.
The data presented here suggest that although the situation related to target serovars was positive for almost all the poultry species covered by NCP, it is pivotal not to underestimate the potential risk posed by the increase in prevalence of Salmonella spp.‐positive flocks. These increases in prevalence could be related to some weaknesses of the measures implemented to control Salmonella in poultry flocks. Among the non‐target serovars for poultry categories other than breeding Gallus gallus, S. Infantis was reported, and it is an important public health concern due to its frequent isolation from humans and the high levels of multidrug‐resistance. For broiler flocks in particular, the increase of prevalence of Salmonella spp.‐positive flocks seem to be related to the massive spread of S. Infantis documented during recent years. This serovar is recognised as by far the commonest serovar in broilers both from animals and meat. Several points must be still clarified to explain its recent success in the broiler production chain. It is the third commonest reported serovar from breeding Gallus gallus as part of NCP, after S. Enteritidis and S. Typhimurium, and an increase in the number of S. Infantis isolates from breeding Gallus gallus was documented in 2017. As already reported in previous years, a heterogeneous situation is described in the EU for this serovar. S. Infantis accounted for most of the isolates from broilers reported by some MS (e.g. Austria, Croatia, Hungary, Italy, Slovakia and Slovenia). For some other MS that reported significant numbers of isolates from broilers (e.g. France and the United Kingdom), this serovar was hardly reported. However, knowledge about the real EU situation on the spread of this serovar is hampered by the fact that the reporting of S. Infantis is only mandatory for breeding flocks of Gallus gallus. Hence, the different situations among the MS for the presence of S. Infantis in poultry flocks could reflect different epidemiological situations or could be simply due to biases related to the different reporting strategies among MS.
When comparing compulsory NCP data reported by CA (official control samples) and FBOp (self‐monitoring) from broilers and turkeys (fattening and breeding) the prevalence data calculated from CA data were significantly higher than those from the FBOp. Analogous observations were described by DG Santé Health and Food Audits and Analysis (European Commission, 2013, 2015). This may relate to the different epidemiological situations on the different farms sampled by CA and FBO, but could also relate to the different sensitivities of the sampling strategies implemented. The latter may be the case in broiler flocks under NCPs, because official samples may be taken in these flocks on a risk basis (see Regulation (EC) No. 200/2012, point 2.1(b) of the Annex), therefore targeting ‘risky’ flocks/holdings and therefore increasing the probability to get a Salmonella‐positive result. However, this may not possibly explain observed differences entirely. This would mean that Salmonella flock prevalence statistics would not capture all infection and would underestimate the true flock prevalence. Reasons for these differences should be seriously investigated, as this may compromise the general effectiveness of the Salmonella NCPs, as they rely mainly on FBOp samplings.
Key findings, Salmonella, EU, 2017
The human salmonellosis trend significantly decreased since 2008 but did not show any decrease since 2013. In 2017, the number of reported confirmed human cases of salmonellosis and the EU notification rate decreased first time after a 3‐year increase.
S. Enteritidis, which was confirmed by far as the most common serovar responsible for human cases, proportionally continued to increase, particularly in cases acquired within the EU. This increase was mainly due to a single Member State starting to report case‐based serovar data for the first time. When excluding this MS, the proportion was at the same level than in 2016.
The highest levels of Salmonella‐positive single samples taken by the CA according to Regulation (EC) No 2073/2005 occurred in foods of meat origin which are intended to be cooked before consumption, more precisely in minced meat and meat preparations from poultry and in minced meat and meat preparations from other species than poultry. However the data were unrepresentative of the overall EU situation.
An overall decreasing trend for the prevalence of flocks positive to target Salmonella serovars was observed for fowl breeding hens, laying hens and broilers and for fattening turkeys but not for breeding turkeys, where the prevalence was constant but with fluctuations over time.
In fowl breeding hens, in broilers, in breeding and in fattening turkeys, but not in laying hens, the prevalence of flocks positive to Salmonella spp. tended to increase.
Numbers of reported S. Infantis isolates increased, which was markedly associated with the broiler production chain, where it was by far the most common serovar isolated both from animals and meat samples.
The comparison of PHC monitoring results from pig carcasses and provided by eight MS indicated that the Salmonella prevalence reported for Competent Authority official control data were significantly higher than those reported by FBOp self‐monitoring data.
The comparison of NCP monitoring results from broiler and turkey flocks indicated that generally the Salmonella target serovars flock prevalence as reported by Competent Authority official control data and by FBOp were highest for the former sampler.
2.6. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Surveillance Atlas | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| World Health Organization – Salmonella (non‐typhoidal) Fact sheet | http://www.who.int/mediacentre/factsheets/fs139/en/ | |
| Food | European Union Reference Laboratory (EURL) for Salmonella | http://www.eurlsalmonella.eu |
| Microbiological criteria | https://ec.europa.eu/food/safety/biosafety/food_hygiene/microbiological_criteria_en | |
| Scientific Opinion on Public health risks of table eggs due to deterioration and development of pathogens | https://www.efsa.europa.eu/en/efsajournal/pub/3782 | |
| Scientific Opinion on the link between Salmonella criteria at different stages of the poultry production chain | https://www.efsa.europa.eu/en/efsajournal/pub/1545 | |
| Bad Bug Book (Second Edition), Food‐borne Pathogenic Microorganisms and Natural Toxins Handbook, Center for Food Safety and Applied Nutrition, Food and Drug Administration (FDA), USA | https://www.fda.gov/food/foodborneillnesscontaminants/causesofillnessbadbugbook/ | |
| Animals | Control of Salmonella in animals | https://ec.europa.eu/food/safety/biosafety/food_borne_diseases/salmonella_en |
| Scientific Opinion on a quantitative estimation of the public health impact of setting a new target for the reduction of Salmonella in laying hens | https://www.efsa.europa.eu/en/efsajournal/pub/1546 | |
| Scientific Opinion on public health impact of new target for the reduction of Salmonella in turkey flocks | https://www.efsa.europa.eu/en/efsajournal/pub/2616 | |
| Scientific Opinion on public health impact new target for the reduction of Salmonella in broiler flocks | https://www.efsa.europa.eu/en/efsajournal/pub/2106 | |
| Scientific Opinion on Salmonella in slaughter and breeder pigs | https://www.efsa.europa.eu/en/efsajournal/pub/1547 |
3. Listeria
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1475841
3.1. Abstract
In 2017, all 28 MS reported 2,480 confirmed invasive human cases of listeriosis. The EU notification rate was 0.48 cases per 100,000 population which was comparable with 2016. There has been a statistically significant increasing trend of confirmed listeriosis cases in the EU/EEA during the period 2008–2017 as well as during the last 5 years (period 2013–2017). Sixteen MS reported 227 deaths due to listeriosis in 2017. The EU case fatality was 13.8% among the 1,633 confirmed cases with known outcome, a slight decrease compared with 2016. Listeria infections were most commonly reported in the elderly population in the age group over 64 years and particularly in the age group over 84 years.
In total, 39 human cases of listeriosis were reported to EFSA due to FBOs by six MS (Austria, Denmark, Germany, Ireland, Italy and Sweden) and one non‐MS (Switzerland). FBOs were due to cheeses, fish and fishery products, meat and meat products and vegetables.
Data on ready‐to‐eat (RTE) food on L. monocytogenes are generated via Regulation 2073/2005 that lays down the microbiological criteria and the implementing rules to be complied with by FBOp when implementing the general and specific hygiene measures. Compliance with the FSC, including for L. monocytogenes shall be verified by the National Competent Authorities at the national level (Regulation No. 852/2004). Data on L. monocytogenes in animals and feed provided by the MS to EFSA are generated by non‐harmonised monitoring schemes across MS and for which no mandatory reporting requirements exist.
In 2017, 26 MS reported data on RTE food categories sampled and tested. The MS were able for the first time in 2017 to report explicitly the data from National Competent Authorities during official sampling (verifying) in the framework of Regulation (EC) No 2073/2005. The number of reporting MS reporting varied considerably according to the RTE food category/type. Non‐satisfactory results in the different RTE food categories were consistently higher at the processing stage compared with retail and highest in fish and fishery products (0.2–3.9%) followed by soft and semi‐soft cheeses (0.1–2.5%) and other dairy products (0–1.5%). Considering data of occurrence in RTE food samples originating from all sampling stages, sampling context, sample unit(single units and batches), in 2017, L. monocytogenes occurrence was highest in fish and fishery products (6%) followed by RTE salads (4.2%), RTE meat and meat products (1.8%), soft and semi‐soft cheeses (0.9%), fruit and vegetables (0.6%) and hard cheeses (0.1%). These occurrence data are, in general, in agreement with the 2016 data. An analysis of trend is not possible because of the variation in the number of tested samples and the number of MS reporting data across years. In 2017, MS increased their sampling for most RTE food categories compared with 2016. However, there is high variation between MS with relation to sampling efforts (sample size) and reporting context (objective sampling and/or suspect sampling). Therefore, the sampling in some MS may not be representative for the estimation of the occurrence of L. monocytogenes in RTE food.
Fourteen MS reported findings of Listeria spp. (mainly L. monocytogenes) in various animal species and mainly in domestic ruminants (cattle, sheep and goats). As data reported on animals originated primarily from clinical (suspect) investigations, they are not suitable for estimating accurate occurrence or trends over time.
3.2. Surveillance and monitoring of Listeria monocytogenes in the EU
3.2.1. Humans
The notification of listeriosis in humans is mandatory in most EU MS, Iceland, Norway and Switzerland, except for three MS, where notification is based on a voluntary system namely Belgium, Luxembourg and the United Kingdom. The surveillance systems for listeriosis cover the whole population in all MS, except in Spain. No estimate for the population coverage was provided for Spain, so the notification rate was not calculated. All countries report case‐based data except Bulgaria, which reported aggregated data. Both reporting formats were included to calculate numbers of cases, notification rates and disease trends.
Surveillance of human listeriosis in the EU is based on invasive forms of L. monocytogenes infection, mostly manifested as septicaemia, meningitis or spontaneous abortion. Diagnosis of human Listeria infections is generally performed by culture from blood, cerebrospinal fluid and vaginal swabs.
3.2.2. Food, animals and feed
Monitoring of L. monocytogenes is conducted along the food chain during preharvest (farm animals and their feed), processing (slaughterhouses, cutting plant) and post‐harvest (retail and catering). The public health risk of L. monocytogenes posed by RTE food also depends on the effectiveness of its control, which include the implementation of Good Agricultural Practices (GAP) at the farm level, the HACCP programme, Good Manufacturing Practices (GMP) and Good Hygiene Practices (GHP) during processing and retail by FBOp. Regulation 2073/2005 (see Section 3.3.1) lays down the microbiological criteria and the implementing rules to be complied with by FBOp when implementing the general and specific hygiene measures of Regulation 852/2002. Compliance with the FSC, including for L. monocytogenes must be verified by the CA (official sampling) at national level.
The rationale for surveillance and monitoring of L. monocytogenes in animals, feed and food at the different stages along the food chain is shown in Figure 26.
Figure 26.
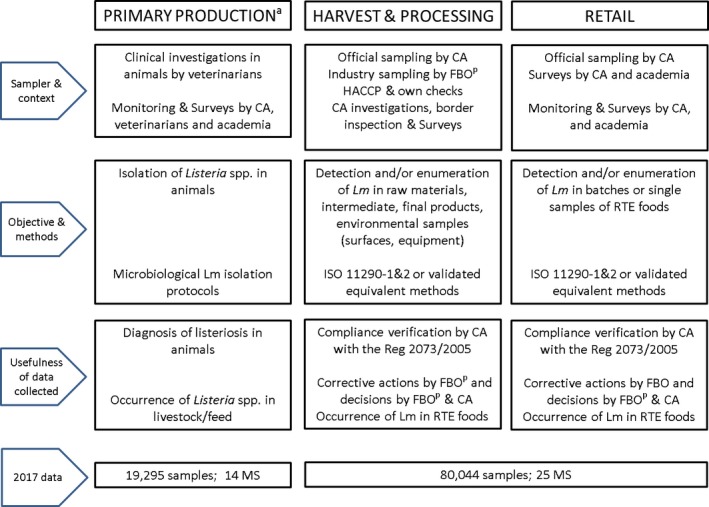
-
CA: competent authorities; FBOp: food business operator; RTE: ready‐to‐eat; Lm: Listeria monocytogenes.(a): Primary production sector: samples from animals and feed.(b): Less than 0.3% of samples correspond to sampling carried out by industry.
Most of the monitoring data on L. monocytogenes in animals and feed provided by the MS to EFSA are generated by non‐harmonised monitoring schemes across MS and for which no mandatory reporting requirements exist. The 2017 data in animals originated primarily from clinical investigations of listeriosis cases from suspect animals. Among several transmission routes, listeriosis in animals can be transmitted via the consumption of contaminated feed such as poor‐quality silage. Data on L. monocytogenes occurrence in feed are only collected as part of clinical investigations in farm animals. Hence, monitoring data on L. monocytogenes in animal feed are rarely available.
Reported data on L. monocytogenes in RTE food are mainly based on samples collected in the scope of the verification by MS of the implementation by FBOp of the FSC for L. monocytogenes in RTE foods which has been in force since January 2006. Data submitted to EFSA within that context only allow a descriptive summary at the EU level and are not harmonised (Table 1). In addition, samples collected for L. monocytogenes not in the context of food safety criteria are mainly from the food categories targeted for the food safety criteria listed in Regulation 2073/2005.
A summary of the number of analysed samples with results reported to EFSA in 2017 is summarised in bottom of Figure 26.
3.2.3. Food‐borne outbreaks of human listeriosis
The reporting of FBOs is mandatory according the EU Zoonoses Directive 2003/99/EC and the reported data represent the most comprehensive set of data available at the EU level for assessing the burden of FBOs – including those caused by L. monocytogenes. Further details are provided in the chapter on FBO.
3.3. Data analyses
Two data streams were distinguished for the reporting of L. monocytogenes in food towards EFSA in 2017: one subset of data is related to data collected by National Competent Authorities as part of verification of implementation of L. monocytogenes food safety criteria listed in Regulation (EC) No. 2073/2005 (official sampling of ‘batches or single units’); another subset of data reported is related to all other monitoring and surveillance activities reported by MS and non‐MS to assess the occurrence L. monocytogenes in different RTE and non‐RTE food categories.
For L. monocytogenes testing in food only reported data obtained from sampling schemes such as ‘census’, ‘convenience’ and ‘objective sampling’ are considered excluding data reported from ‘suspect’ and ‘selective sampling’ context.
Exceptionally, sampling schemes reported as ‘suspect’ and ‘selective sampling’ were also included for the description on the occurrence of Listeria spp. in animals.
3.3.1. Monitoring of food according to Regulation (EC) No 2073/2005 on microbiological criteria
The first stream of data reported to EFSA concerns data from samples (mainly single samples) collected by the CA conducting investigations to verify whether FBOp implement correctly the legal framework of own‐control programmes as well as the analyses as part of HACCP according to the General Food Law principles. L. monocytogenes FSC of the Regulation (EC) No 2073/2005 are different according to the RTE food category and sampling stage (Table 21) and based either on detection (CEN, 2004a) or enumeration (CEN, 2004b) analytical methods.
Table 21.
L. monocytogenes food safety criteria as described in Regulation 2073/2005 for the different food categories across the food chain
| Sampling stage | Foods intended for infants and foods for special medical purpose | Other RTE foods | |
|---|---|---|---|
| Supporting growth of Lm | Not supporting growth of Lm | ||
| Processing stage | NAa | Based on detection method absence of Lm in 25 g of sample (n = 5, c = 0)b | NAa |
| Retail | Based on detection method absence of Lm 25 g of sample (n = 10, c = 0) | Based on enumeration method 100 CFU/g (n = 5, c = 0) | Based on enumeration method 100 CFU/g (n = 5, c = 0) |
NA: not applicable as at processing stage there are no food safety criteria described according Regulation 2073/2005. However it is possible that MS report data on L. monocytogenes are described in Table 26.
n = number of units comprising the sample (number of sample units per food batch that are required for testing); c = the maximum allowable number of sample units yielding unsatisfactory test results. In a two‐class attributes sampling plan defined by n = 10, c = 0 and a microbiological limit of ‘absence in 25 g’, in order for the food batch to be considered acceptable, L. monocytogenes must not be detected in qualitative (detection) analyses of 25‐g food portions obtained from each one of 10 sample units comprising the batch. If even one of the sample units comprising the batch is found to contain L. monocytogenes (presence in 25 g), then the entire batch is deemed unacceptable.
Data reported by MS were separated in the different categories of RTE food/sampling stages based on the assumptions described in the EUSR of 2016 (EFSA and ECDC, 2017b). Briefly these assumptions are: all sampling units that were collected from ‘cutting plants’, ‘packing centres’ and ‘processing plants’ were considered as units collected at the processing stage while sampling units that were obtained from ‘catering’, ‘hospital or medical care facility’, ‘retail’, ‘wholesale’, ‘unspecified’, ‘restaurant or cafe or pub or bar or hotel or catering service’ and ‘automatic distribution system for raw milk’ and ‘unspecified’ were considered as units collected at retail. Considering the classification of RTE foods, as no obvious data on physicochemical parameters such as pH, aw, levels and types of preservatives are reported to EFSA, it was considered that all RTE foods support the growth of L. monocytogenes. So the criterion applied for samples collected at the processing stage within the context of Regulation 2073/2005 was ‘absence in 25 grams’. Two exceptions were applied for the ‘hard cheeses’ and ‘fermented sausages’, where the criterion of ‘≤ 100 CFU/g’ was applied because these types of RTE foods are generally considered to be unable to support the growth of L. monocytogenes (Table 26). The data reported by some MS from investigations of RTE foods during ‘border inspection activities’ were not taken up in this summary table.
Table 26.
Summary statistics of official sampling for verification of the implementation by food business operators of L. monocytogenes food safety criteria laid down by Regulation (EC) No 2073/2005 (reported as surveillance, according 2073/2005) in the main ready‐to‐eat (RTE) food categories according to sampling stage, analytical method and sampling unit (single units vs batch samples), reporting MS, EU, 2017
| RTE food categoryb | Sampling unit | Processing stagea | Retaila | ||
|---|---|---|---|---|---|
| Analytical methodn | |||||
| Detection | Enumeration | Detection | Enumeration | ||
| Foods intended for infants and food for special purposesc: data reported from BE, CY, DK, EE, EL, NL, SI and SK | Batch | / | 0.0 (n = 5; 1 MS) | ||
| Single | 0.0 (n = 10; 1 MS) | 0.0 (n = 26; 4 MS) | |||
| Fishd and Fishery productse: data reported from AT, BE, CY, CZ, DK, EE, EL, ES, PT, NL, SI and SK | Batch | 0.0 (n = 167; 3 MS) | 0.0 (n = 110; 2 MS) | ||
| Single | 3.9 (n = 129; 6 MS) | 0.2 (n = 422; 7 MS) | |||
| Cheeses, soft and semi‐softf: data reported from AT, BE, CY, CZ, DK, EE, PT, SI and SK | Batch | 0.0 (n = 414; 3 MS) | 5.0 (n = 180; 1 MS) | ||
| Single | 2.5 (n = 1,634; 7 MS) | 0.1 (n = 1,568; 5 MS) | |||
| Cheeses, hardg: data reported from CY, DK, EE, EL, NL, PT and SK | Batch | 0.0 (n = 955; 1 MS) | 0.0 (n = 65; 1 MS) | ||
| Single | 0.0 (n = 58; 2 MS) | 0.0 (n = 1; 1 MS) | |||
| Cheeses, unspecifiedh: data reported from BE, CY, DK, EE, ES, EL, NL, PT and SK | Single | 0.0 (n = 106; 2 MS) | 0.0 (n = 267; 4 MS) | ||
| Batch | 0.0 (n = 112; 2 MS) | / | |||
| Other dairy products (excluding cheeses) – entire categoryi: data reported from AT, BE, CY, CZ, DK, EL, NL, PT, SI and SK | Batch | 0.0 (n = 213; 3 MS) | 0.0 (n = 165; 1 MS) | ||
| Single | 1.5 (n = 194; 6 MS) | 0.0 (n = 822; 5 MS) | |||
| Milkj: data reported from CY, DK and SK | Batch | 0.0 (n = 30; 2 MS) | / | ||
| Single | 0.0 (n = 60; 2 MS) | 1.2 (n = 85; 2 MS) | |||
| Products of meat origin: fermented sausagesk: data reported from BE, CY, DK and EL | Batch | 0.0 (n = 5; 1 MS) | 0.0 (n = 10; 1 MS) | ||
| Single | 0.0 (n = 72; 3 MS) | 0.0 (n = 131; 2 MS) | |||
| Products of meat origin: other than fermented sausagesl: data reported from BE, CY, CZ, DK, EE, ES, PT and SK | Batch | 0.1 (n = 972; 3 MS) | 0.0 (n = 565; 2 MS) | ||
| Single | 4.2 (n = 871; 5 MS) | 0.2 (n = 1,160; 6 MS) | |||
| Other productsm: data reported from BE, BG, CY, CZ, DK, EE, EL, PT, SI and SK | Batch | 0.0 (n = 565; 4 MS) | 0.0 (n = 530; 3 MS) | ||
| Single | 1.9 (n = 310; 7 MS) | 0.0 (n = 1,608; 7 MS) | |||
CFU: colony forming unit; MS: Member State; n: number of sampling units.
Each cell contains the percentage (%) of non‐satisfactory samples (the presence of L. monocytogenes in 25‐g of sample for detection analyses or populations of L. monocytogenes > 100 CFU/g for enumeration analyses) and in parenthesis the number of tested samples (single samples or batches) and the number of reporting MS. Retail includes also data from sampling stage reported as ‘unspecified’.
In the absence of relevant data (pH, aw), EFSA assumes that foods listed under ‘Fish and fishery products’, ‘Soft and semi‐soft cheeses’, ‘Unspecified cheeses’, ‘Milk’, ‘Products of meat origin other than fermented sausages’ and ‘Other products’ belong to the category of foods that are able to support the growth of L. monocytogenes. Foods classified under these categories of RTE products are expected to have near‐neutral or moderately low pH and relatively high water activity (aw) values or can be very heterogeneous in terms of their manufacturing technology and physicochemical characteristics (‘Other products’). EFSA assumes that ‘Fermented sausages’ and ‘Hard cheeses’ belong to the category of foods that are unable to support the growth of L. monocytogenes, because foods classified under these two categories of RTE products undergo ripening/fermentation and are expected to have low pH and moderate aw values. In assessing RTE food category ‘other dairy products’, EFSA is presenting the results in a conservative way by classifying/considering all ‘other dairy products’ as capable of supporting the growth of L. monocytogenes.
Includes ‘Infant formula – dried’, ‘Infant formula – ready‐to‐eat’ and ‘Foodstuffs intended for special nutritional uses – dietary foods for special medical purposes’.
Includes RTE fish which is ‘cooked’, ‘gravad/slightly salted’, ‘marinated’ or ‘smoked’ (cold‐ or hot‐smoked).
Includes cooked crustaceans (shrimps, prawns, unspecified) that were ‘chilled’, ‘frozen’ or ‘shelled and shucked’, cooked molluscan shellfish (‘chilled’, ‘frozen’ or ‘shelled, shucked and frozen’), fishery products unspecified (‘cooked’, ‘cooked and chilled’, ‘ready‐to‐eat chilled or frozen’, ‘seafood pâté’, ‘smoked’).
Includes ‘curd’, ‘fresh’ and ‘soft or semi‐soft’, cheeses made from different milk kinds and types (‘pasteurised’ or ‘raw or low‐heat‐treated’ and from ‘cows’, ‘goats’, ‘sheep’, ‘mixed’, ‘unspecified’ or from other animals’ milk).
Includes ‘hard’ cheeses made from different milk kinds and types (‘pasteurised’ or ‘raw or low‐heat‐treated’ and from ‘cows’, ‘goats’, ‘sheep’, ‘mixed’, ‘unspecified’ or from other animals’ milk).
Includes ‘unspecified’ cheeses made from different milk kinds (‘cows’, ‘goats’, ‘sheep’, ‘mixed’, ‘unspecified’ or from other animals’ milk).
Includes ‘butter’, ‘cream’, ‘dairy desserts’, ‘fermented dairy products’, ‘ice‐cream’, ‘milk‐based drinks’, ‘milk powder and whey powder’, probiotic drink, ‘yoghurt’ and whey.
Includes milk (‘pasteurised’, ‘UHT’, or ‘raw, intended for direct human consumption’) from ‘cows’, ‘goats’, ‘sheep’, ‘unspecified’ or from other animals’ milk. Raw milk and raw milk for the manufacture of raw and low‐heat‐treated products are not included.
Includes fermented sausages made from meat of different animal species (‘bovine animals’, ‘deer’, ‘horse’, ‘pig’, ‘mixed’, ‘other animal species or unspecified’).
Includes ‘meat products’ (‘intended to be eaten raw’ or ready‐to‐eat), meat preparations (‘pâté’) and ‘minced meat’ (‘intended to be eaten raw’ or ‘ready‐to‐eat’) from different animal species (‘bovine animals’, ‘pigs’, poultry (‘broilers’, ‘geese’, ‘ducks’, ‘turkeys’, ‘other poultry species’ or ‘unspecified poultry’), ‘mixed’, ‘goats’, ‘sheep’, ‘horses’, ‘bison’, ‘donkeys’, ‘water buffalos’, ‘wild boar’, ‘farmed game‐land animals’, or ‘other animal species’).
Includes RTE salads, fruits and vegetables (precut or not), processed food products and prepared dishes (sandwiches, ices and frozen desserts, sushi and other ready‐to‐eat foods), spices and herbs, bakery products (bread, cakes, desserts, pastry), vegetables (precut or not, canned, cooked or cooked and chilled), confectionery products and pastes, beverages (non‐alcoholic), chocolate, nuts and nut products, fats and oils (excluding butter), juices (from fruits, vegetables or mixed, pasteurised or unpasteurised), sauces and dressings, cereals and meals, cocoa and cocoa preparations, coffee and tea, sweets, fruits (precut or not, chilled or frozen, canned, dried or fruit puree), coconut, soups, seeds (sprouted or dried), potato chips, egg products (ready‐to‐eat).
The results from qualitative examinations using the detection method were used to assess the criterion of ‘absence in 25 grams’ and the results from quantitative analyses using the enumeration method were used to assess the criterion the criterion of ‘≤ 100 CFU/g’.
3.3.2. Other monitoring data of Listeria monocytogenes in food
Occurrence expresses the proportion of samples of foods in which the presence of L. monocytogenes was detected. To describe the occurrence in food, only the data from countries that took samples that were tested with the detection method were considered. Detection methods are considered to be the most sensitive and appropriate methods to describe the presence of L. monocytogenes in foods. Data from quantitative investigations (using the L. monocytogenes enumeration method) in RTE foods were also submitted to EFSA. However, enumeration data were not used for estimating the occurrence of L. monocytogenes in the different RTE food matrices because of its lower sensitivity compared with the detection method.
All sampling units (single units and batches), sampling stages (processing and retail stages except for border inspections) and sampling contexts (surveillance, monitoring and surveillance – based on Regulation 2073) were considered to describe occurrence of L. monocytogenes in food.
3.3.3. Monitoring data of Listeria monocytogenes in animals and feed
A short description of all data collected by the MS in animals and feed is provided in this report. To describe occurrence of L. monocytogenes in animals, suspect samplings and selective samplings were also considered (Table 2017_LISTANIMALS).
3.4. Results
3.4.1. Overview of key statistics along the food chain, EU, 2013–2017
In 2017, more samples for L. monocytogenes detection were reported by MS compared with 2016 for five out the six main reported RTE food categories (Table 22). The higher number of reported samples in meat and meat products, fish and fishery products, soft and semi‐soft cheese and hard cheese is mainly driven by the higher number of samples reported by Poland, Germany, Bulgaria, the Czech Republic and the Netherlands, respectively. Further information on the sampling effort by each MS (showing the total number of samples collected for both detection and enumeration testing over all sampling stages) is provided in Table 25.
Table 22.
Summary of statistics of human invasive L. monocytogenes infections and L. monocytogenes occurrence in the major RTE food categories in the EU, 2013–2017
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 2,480 | 2,509 | 2,183 | 2,217 | 1,883 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.48 | 0.47 | 0.43 | 0.46 | 0.40 | ECDC |
| Number of reporting MS | 28 | 28 | 28 | 27 | 27 | ECDC |
| Infection acquired in the EU | 1,635 | 1,532 | 1,450 | 1,498 | 1,298 | ECDC |
| Infection acquired outside the EU | 4 | 6 | 7 | 6 | 9 | ECDC |
| Unknown travel status or unknown country of infection | 841 | 971 | 726 | 713 | 576 | ECDC |
| Total number of food borne outbreaks | 10 | 5 | 15 | 13 | 9 | EFSA |
| Number of outbreak‐related cases | 39 | 25 | 233 | 94 | 56 | EFSA |
| RTE food | ||||||
| RTE food – occurrence (%) by detection method (number of tested samples by detection method; number of reporting MS)a | ||||||
| Fish and fishery products | 6.0%(n = 6,730; 22 MS) | 5.1% (n = 2,918; 22 MS) | 3.2% (n = 4,658; 22 MS) | 5.8% (n = 3,436; 16 MS) | 5.1% (n = 3,479; 20 MS) | EFSA |
| Meat and meat products (beef, pork, broiler and turkey meat) | 1.8%(n = 22,544; 19 MS) | 3.3% (n = 15,161; 23 MS) | 2.8% (n = 16,789; 21 MS) | 2.1% (n = 67,215; 18 MS) | 3.4% (n = 44,977; 21 MS) | EFSA |
| Soft and semi‐soft cheeses made from raw or low‐heat‐treated milk | 0.9%(n = 6,117; 17 MS) | 2.6% (n = 853; 15 MS) | 1.4% (n = 730; 13 MS) | 1.0% (n = 2,573; 13 MS) | 4.2% (n = 2,542; 13 MS) | EFSA |
| Hard cheeses made from raw or low‐heat‐treated milk | 0.1%(n = 5,039; 15 MS) | 1.0% (n = 509; 9 MS) | 1.3% (n = 858; 11 MS) | 0.2% (n = 10,175; 9 MS) | 0.7% (n = 1,609; 12 MS) | EFSA |
| Fruit and vegetables | 0.6%(n = 1,773; 17 MS) | 0.7% (n = 1,043; 16 MS) | 2.1% (n = 1,456; 17 MS) | 3.0% (n = 1,503; 17 MS) | 2.1% (n = 1,991; 15 MS) | EFSA |
| Salads | 4.2%(n = 902; 14 MS) | 1.9% (n = 1,042; 14 MS) | 1.9% (n = 1,238; 13 MS) | 1.1% (n = 1,154; 15 MS) | 2.4% (n = 1,822; 14 MS) | EFSA |
For each ready‐to eat (RTE) food category, occurrence estimates (proportion of positive samples (single units and batches)) were obtained from the reporting countries that reported samples that were tested for L. monocytogenes with the detection method and taking into account all sampling stages (processing, retail, border inspections and unspecified), all samplers (industry, official, private and not specified) and the following sampling strategies: census, convenience sampling, objective sampling and not specified.
Table 25.
Number of tested samples by different countries for the main RTE food categories in the EU, 2017
| Country | Soft and semi‐soft cheeses and hard cheeses (Retail & Processing) | RTE fish and fishery products (Processing & Retail) | Meat and Meata products (Processing & Retail) | Fruit and vegetables (Processing & retail) |
|---|---|---|---|---|
| Austria | 406 | 74 | 135 | 30 |
| Belgium | 1,302 | 251 | 852 | 567 |
| Bulgaria | 1,878 | 414 | 4,508 | 257 |
| Croatia | 168 | 32 | 50 | 254 |
| Cyprus | 253 | 32 | 127 | 106 |
| Czech Republic | 2043 | 167 | 7,412 | 95 |
| Denmark | 266 | 648 | 486 | 80 |
| Estonia | 38 | 76 | 126 | 40 |
| Finland | / | / | / | / |
| France | na | na | 429 | na |
| Germany | nab | 1,402 | 393 | 37 |
| Greece | 115 | 215 | na | 10 |
| Hungary | 236 | 169 | 1,489 | 185 |
| Ireland | na | na | 88 | na |
| Italy | na | 631 | / | 1,017 |
| Latvia | 20 | 120 | 145 | / |
| Lithuania | na | 16 | 25 | na |
| Luxembourg | / | / | 243 | / |
| Malta | / | / | / | / |
| Netherlands | 3,388 | 383 | / | 186 |
| Poland | 1,808 | 2,864 | 16,706 | / |
| Portugal | 472 | 40 | 254 | 49 |
| Romania | 107 | 19 | 640 | / |
| Slovakia | 1,587 | 110 | 1,485 | 37 |
| Slovenia | / | 18 | 50 | 60 |
| Spain | 205 | 502 | 381 | 358 |
| Sweden | na | 15 | 8 | na |
| United Kingdom | na | na | 902 | na |
| EU | 14,292 | 8,198 | 36,934 | 3,368 |
| Iceland | / | / | / | / |
| Norwayb | / | / | / | / |
| Serbia | / | / | / | / |
| Switzerland | / | / | / | / |
| Non‐EU | ||||
| Total (EU and non‐EU) | 14,292 | 8,198 | 36,934 | 3,368 |
na: data not presented in this table because of reported sampling strategy (‘selective’ and/or ‘suspect’ sampling);
/: no data reported.
For each food category, the number of samples reported in the table were obtained by extracting the data including the methods (both detection and enumeration method), all sampling stages (processing, retail, border inspections and unspecified), all samplers (industry, official, private and not specified, all sampling context (including ‘monitoring’, ‘surveillance’, ‘surveillance, according 2073/2005’, ‘not specified’), all sampling strategies excluding ‘selective sampling’ and ‘suspect sampling.
Category including: RTE beef, RTE pork, RTE broiler and RTE turkey meat, mixed meat and minced meat intended to be eaten raw.
Germany could not provide information on the type of cheese (‘hard’, ‘soft and semi‐soft cheeses’).
3.4.2. Human listeriosis
In 2017, 28 MS reported 2,480 confirmed human cases of listeriosis (Table 23). The EU notification rate was 0.48 cases per 100,000 population, which was at the same level as in 2016. The highest notification rates were observed for Finland, Denmark, Germany, Luxembourg, Sweden and Belgium with 1.62, 1.01, 0.88, 0.85, 0.81 and 0.80 cases per 100,000 population, respectively. The lowest notification rates were reported by Bulgaria, Croatia, Cyprus, Malta and Romania (≤ 0.2 per 100,000).
Table 23.
Reported cases of human invasive listeriosis and notification rates per 100,000 population in the EU/EFTA, by country and year, 2013–2017
| Country | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 32 | 32 | 0.36 | 46 | 0.53 | 38 | 0.44 | 49 | 0.58 | 36 | 0.43 |
| Belgiumb | Y | C | 73 | 73 | 0.80 | 104 | 0.92 | 83 | 0.74 | 84 | 0.75 | 66 | 0.59 |
| Bulgaria | Y | A | 13 | 13 | 0.18 | 5 | 0.07 | 5 | 0.07 | 10 | 0.14 | 3 | 0.04 |
| Croatia | Y | C | 8 | 8 | 0.19 | 4 | 0.10 | 2 | 0.05 | 4 | 0.09 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.12 |
| Czech Republic | Y | C | 30 | 30 | 0.28 | 47 | 0.45 | 36 | 0.34 | 38 | 0.36 | 36 | 0.34 |
| Denmark | Y | C | 58 | 58 | 1.01 | 40 | 0.70 | 44 | 0.78 | 92 | 1.64 | 51 | 0.91 |
| Estonia | Y | C | 4 | 4 | 0.30 | 9 | 0.68 | 11 | 0.84 | 1 | 0.08 | 2 | 0.15 |
| Finland | Y | C | 90 | 89 | 1.62 | 67 | 1.22 | 46 | 0.84 | 65 | 1.19 | 61 | 1.12 |
| France | Y | C | 370 | 370 | 0.55 | 375 | 0.56 | 412 | 0.62 | 373 | 0.57 | 369 | 0.56 |
| Germany | Y | C | 739 | 726 | 0.88 | 670 | 0.82 | 557 | 0.69 | 573 | 0.71 | 463 | 0.57 |
| Greece | Y | C | 20 | 20 | 0.19 | 20 | 0.19 | 31 | 0.29 | 10 | 0.09 | 10 | 0.09 |
| Hungary | Y | C | 36 | 36 | 0.37 | 25 | 0.25 | 37 | 0.38 | 39 | 0.40 | 24 | 0.24 |
| Ireland | Y | C | 14 | 14 | 0.29 | 13 | 0.28 | 19 | 0.41 | 15 | 0.33 | 8 | 0.17 |
| Italy | Y | C | 165 | 164 | 0.27 | 179 | 0.30 | 153 | 0.25 | 132 | 0.22 | 143 | 0.24 |
| Latvia | Y | C | 3 | 3 | 0.15 | 6 | 0.30 | 8 | 0.40 | 3 | 0.15 | 5 | 0.25 |
| Lithuania | Y | C | 9 | 9 | 0.32 | 10 | 0.35 | 5 | 0.17 | 7 | 0.24 | 6 | 0.20 |
| Luxembourg | Y | C | 5 | 5 | 0.85 | 2 | 0.35 | 0 | 0.00 | 5 | 0.91 | 2 | 0.37 |
| Malta | Y | C | 0 | 0 | 0.00 | 1 | 0.23 | 4 | 0.93 | 1 | 0.24 | 1 | 0.24 |
| Netherlands | Y | C | 108 | 108 | 0.63 | 89 | 0.52 | 71 | 0.42 | 90 | 0.54 | 72 | 0.43 |
| Poland | Y | C | 116 | 116 | 0.31 | 101 | 0.27 | 70 | 0.18 | 87 | 0.23 | 58 | 0.15 |
| Portugal | Y | C | 42 | 42 | 0.41 | 31 | 0.30 | 28 | 0.27 | – | – | – | – |
| Romania | Y | C | 10 | 10 | 0.05 | 9 | 0.05 | 12 | 0.06 | 5 | 0.03 | 9 | 0.05 |
| Slovakia | Y | C | 12 | 12 | 0.22 | 10 | 0.18 | 18 | 0.33 | 29 | 0.54 | 16 | 0.30 |
| Slovenia | Y | C | 13 | 13 | 0.63 | 15 | 0.73 | 13 | 0.63 | 18 | 0.87 | 16 | 0.78 |
| Spainc | N | C | 287 | 284 | – | 362 | – | 206 | – | 161 | – | 140 | – |
| Sweden | Y | C | 81 | 81 | 0.81 | 68 | 0.69 | 88 | 0.90 | 125 | 1.30 | 93 | 0.97 |
| United Kingdom | Y | C | 160 | 160 | 0.24 | 201 | 0.31 | 186 | 0.29 | 201 | 0.31 | 192 | 0.30 |
| EU Total | – | – | 2,498 | 2,480 | 0.48 | 2,509 | 0.47 | 2,183 | 0.43 | 2,217 | 0.46 | 1,883 | 0.40 |
| Iceland | Y | C | 6 | 6 | 1.77 | 0 | 0.00 | 0 | 0.00 | 4 | 1.23 | 1 | 0.31 |
| Norway | Y | C | 16 | 16 | 0.30 | 19 | 0.37 | 18 | 0.35 | 29 | 0.57 | 21 | 0.42 |
| Switzerlandd | Y | C | 45 | 45 | 0.53 | 50 | 0.59 | 54 | 0.65 | 98 | 1.18 | 64 | 0.78 |
Y: yes; N: no; A: aggregated data; C: case‐based data;‐: no report or not applicable.
Sentinel system; notification rates calculated with estimated population coverage of 80% in 2015–2017 and 70% in 2013–2014.
Sentinel surveillance; no information on estimated coverage so notification rate cannot be estimated.
Switzerland provided data directly to EFSA. The human data for Switzerland includes data from Liechtenstein.
The vast majority (99.8%) of listeriosis cases with known origin of infection were reported to be acquired in the EU (Table 24). Eight MS reported 17 travel‐associated listeriosis cases (four cases associated with travel outside EU and 13 cases within EU) in 2017. The proportion of reported listeriosis cases without data about the travel status or unknown country of infection increased and was 38.7% of all confirmed cases in 2017 (Table 24).
Table 24.
Statistics related to the proportions of human food‐borne listeriosis outbreak cases, EU/EFTA, 2017
| Country | ECDC | EFSA | ||||
|---|---|---|---|---|---|---|
| Confirmed human | Food‐borne outbreaks | |||||
| Total | Travel related | Domestic | Unknown or missing | Human cases (illnesses) | FBO | |
| N | N | N | N | N | N | |
| Austria | 32 | 1 | 30 | 1 | 9 a | 2 |
| Belgium | 73 | 1 | 72 | 0 | – ** | – |
| Bulgaria | 13 | – * | – | 13 | – | – |
| Croatia | 8 | 0 | 1 | 7 | – | – |
| Cyprus | 0 | 0 | 0 | 0 | – | – |
| Czech Republic | 30 | 0 | 30 | 0 | – | – |
| Denmark | 58 | – | – | 58 | 18 | 4 |
| Estonia | 4 | 0 | 4 | 0 | – | – |
| Finland | 89 | 3 | 57 | 29 | – | – |
| France | 370 | 0 | 370 | 0 | – | – |
| Germany | 726 | 5 | 324 | 397 | 2 | 1 |
| Greece | 20 | 3 | 14 | 3 | – | – |
| Hungary | 36 | 0 | 36 | 0 | – | – |
| Ireland | 14 | 1 | 9 | 4 | 2 | 1 |
| Italy | 164 | – | – | 164 | 5 | 1 |
| Latvia | 3 | 0 | 3 | 0 | – | – |
| Lithuania | 9 | 0 | 9 | 0 | – | – |
| Luxembourg | 5 | 0 | 0 | 5 | – | – |
| Malta | 0 | – | – | – | – | – |
| Netherlands | 108 | 5 | 100 | 3 | – | – |
| Poland | 116 | 0 | 116 | 0 | – | – |
| Portugal | 42 | 1 | 37 | 4 | – | – |
| Romania | 10 | – | – | 10 | – | – |
| Slovakia | 12 | 0 | 12 | 0 | – | – |
| Slovenia | 13 | 0 | 2 | 11 | – | – |
| Spain | 284 | 0 | 218 | 66 | – | – |
| Sweden | 81 | 3 | 77 | 1 | 3 | 1 |
| United Kingdom | 160 | 10 | 101 | 49 | – | – |
| EU Total | 2,480 | 33 | 1,622 | 812 | 39 | 10 |
| Iceland | 6 | 1 | 2 | 3 | – | – |
| Norway | 16 | 2 | 12 | 2 | – | – |
| Switzerland | 45 | – | – | 45 | 2 | 1 |
FBO: food‐borne outbreak.
Seven of the nine Austrian cases occurred already in 2015 and 2016, but the link to a food‐borne outbreak was recognised during 2017 and therefore these seven cases were reported by Austria for the year 2017.
No importation data reported.
No human food‐borne listeriosis outbreaks reported.
In the period 2008–2017, a seasonal pattern was observed in the listeriosis cases reported in the EU/EEA, with high summer peaks followed by less high winter peaks. Over the same 10‐year period, a statistically significant increasing trend of confirmed listeriosis cases was observed in the EU/EEA (p < 0.01), as well as in the last 5 years (2013–2017) (Figure 27).
Figure 27.
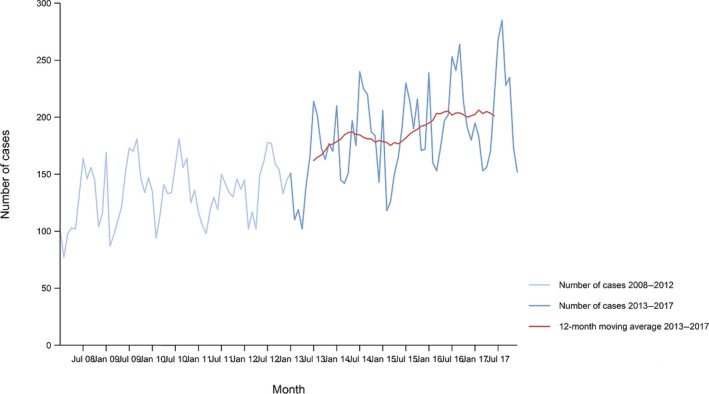
- Source: Austria, Belgium, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Malta, Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and United Kingdom. Bulgaria, Croatia, Luxembourg and Portugal did not report data to the level of detail required for the analysis.
Twelve MS (Belgium, Finland, France, Germany, Greece, Hungary, Italy, the Netherlands, Poland, Slovenia, Spain and Sweden) had a significantly increasing trend of confirmed listeriosis cases (p < 0.01) since 2008. None of the 28 MS reported significantly decreasing trends between 2008–2017 or 2013–2017.
Five MS reported significantly increasing trends (Germany, Italy, the Netherlands, Poland and Spain) in 2013–2017. In seven MS (Belgium, Finland, France, Greece, Hungary, Slovenia and Sweden), which had an increasing overall trend in 2008–2017, no significant increase was observed in the last 5 years (2013–2017).
Information on hospitalisation was provided by 16 MS for 40.4% of all confirmed cases in 2017. Among the cases with information on hospitalisation status, 98.6% were hospitalised. Listeriosis had the highest proportion of hospitalised cases of all zoonoses under EU surveillance.
The outcome was reported for 1,633 confirmed cases (65.8%). Sixteen MS reported 225 deaths due to listeriosis in 2017, while 247 were reported in 2016. There was a steady increase in the annual number of deaths between 2008 and 2016 (annual average: 187). The overall EU case fatality among cases with known outcome was 13.8% and decreased from 15.0% when compared with 2016. France reported the highest number of fatal cases (59) followed by Germany (27).
Listeria infections were most commonly reported in the age group over 64 years. At EU level, the proportion of listeriosis cases in this age group has steadily increased from 54.8% in 2008 to 67.2% in 2017, and especially in the age group over 84 years, with an increase from 7.3% to 14.8%. The case fatality was 15.5% and 24.2% in the age group over 64 years and over 84 years, respectively, in 2017. The proportion of fatal cases in the age group over 84 years of age increased from 7.5% in 2008 to 24.1% in 2016.
Human listeriosis cases associated with food‐borne outbreaks
L. monocytogenes was identified in 10 FBOs affecting 39 people (notified FBO cases) in 6 MS, as reported to EFSA. Overall, for the year 2017, there were 1,622 domestic (acquired within the country) cases reported to the TESSy (Table 24), which was 99.20% of the number of reported human listeriosis cases infected domestically and through travel within EU during 2017 (1,635, Table 22). Table 24 shows the number of human cases reported to TESSy managed by ECDC and those reported from FBOs’ database managed by EFSA. It is important to clarify that the case classification for reporting is different between these two databases. In TESSy, the cases reported are classified based on the EU case definition. All these cases visited a doctor, and are either confirmed by laboratory test (confirmed case) or not (probable case and classification is based on the clinical symptoms and epidemiological link). Cases that never visited a doctor are not reported to TESSy. Moreover, probable cases may be missing in TESSy, as these data are not analysed or published and there is no incentive for reporting such cases. Information on which case is linked or not to an outbreak is not systematically collected. In practice, the cases reported to TESSy are considered mostly sporadic cases. In food‐borne disease outbreak situations, cases are also classified into confirmed or probable outbreak cases, but currently these data are not systematically collected by EFSA.
Four of the 10 L. monocytogenes FBO were reported as strong‐evidence outbreaks by Austria (2), Denmark (1) and Sweden (1). Implicated foods were; ‘cheese’, ‘fish and fish products’, ‘meat and meat products’ and ‘vegetables and juices and other products thereof’. Denmark reported additionally three weak‐evidence FBOs and Germany, Ireland and Italy reported one each.
3.4.3. Listeria monocytogenes in foods
The sampling effort of the MS for 2017 for L. monocytogenes in some major food categories are summarised in Table 25 and concerns samples taken during processing and retail for the major RTE food categories for all type of sampling context except those from selective and suspect sampling.
Of all food samples taken and tested for L. monocytogenes (Table 25), around 80% are obtained from the categories ‘meat and meat products’, ‘cheeses’, ‘fish and fishery products’ and fruit and vegetables. The highest number of samples was taken from ‘meat and meat products’ (mainly by Poland, Bulgaria and the Czech Republic) followed by ‘fish and fishery products’ (mainly by Poland and Germany) and cheeses (by Bulgaria, the Czech Republic, the Netherlands and Poland).
Data for L. monocytogenes on RTE foods according to food safety criteria laid down in Regulation No. 2073/2005
Ten RTE food categories that are targeted in the context of official sampling for verification purposes by the CA in the MS as part of Regulation 2073/2005 (‘surveillance according 2073/2005’) are described in Table 26.
In total, 12 MS (Austria, Belgium, Cyprus, the Czech Republic, Denmark, Estonia, Greece, the Netherlands, Portugal, Slovenia, Slovakia and Spain) reported data.
In general, at retail, depending on the RTE food category, 0% to 1.2% of single units and 0% to 5% of batches were considered as unsatisfactory whereas at processing, these levels (primarily presence in 25 g) ranged from 0% to 4.2% in single samples and batches, respectively.
In ‘fish and fishery products’, a low overall level of unsatisfactory results was noted at retail for single‐unit level (0.2%, 7 MS) compared with processing stage (3.9%, 6 MS).
In ‘Fermented sausages’, a limited number of batches were tested and none was found to be non‐satisfactory.
In ‘meat and meat products other than fermented sausages’, a low level of non‐satisfactory results was noted at retail (respectively, 0.0% and 0.2% for batch and single test‐units), and was higher at the processing stage (4.2% for single test‐units, 5 MS).
All ‘RTE milk’ samples collected at processing were conforming to the FSC. Only a single sample of ‘raw cows’ milk intended for direct human consumption’ sampled at retail was tested positive (1.2%).
In ‘soft and semi‐soft cheeses’ sampled at retail, the level of non‐satisfactory results ranged between 0.1% and 5%. This was due to one MS (PT) reporting positive samples from cheeses made from raw or low‐treated sheep milk.
In ‘hard cheeses’ – which are assumed not to support the growth of L. monocytogenes – all results from batches and single test‐units were conforming to the FSC.
All samples from ‘other dairy products, excluding cheeses’ tested at retail were conforming. At processing, none of the tested batches was positives (0.0%, three MS) whereas 1.5% (six MS) of single units tested was not satisfactory.
Monitoring of occurrence of Listeria monocytogenes in RTE foods
This section on occurrence of L. monocytogenes in foods describes the summary of the data reported by MS and non‐MS from samples tested for L. monocytogenes with the detection method and excludes data reported with sampling context ‘surveillance, according 2073/2005’.
Fish and fishery products, RTE
A summary of the occurrence of L. monocytogenes‐positive units in RTE fish and fishery products in 2017 (reported by Austria, Belgium, Cyprus, Croatia, the Czech Republic, Denmark, Germany, Estonia, Spain, Hungary, Italy, Latvia, Lithuania, the Netherlands, Slovakia, the Netherlands, Slovakia, Slovenia and Sweden) is presented in Figure 28.
Figure 28.
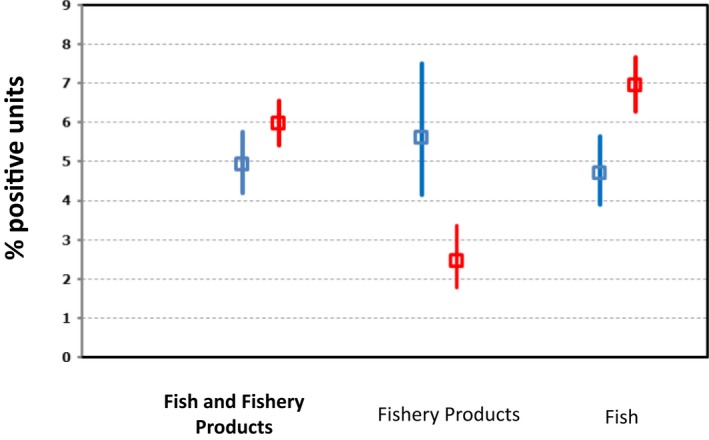
- Only data obtained from detection method are included.
- ‘Fish, RTE’ includes data on ‘Fish’ of the following types: ‘chilled’, ‘cooked’, ‘gravad/slightly salted’, ‘marinated’ and ‘smoked (hot‐ and cold‐smoked)’.
- ‘Fishery products, RTE’ includes the following types: ‘prawns, cooked’, ‘prawns‐shelled, shucked and cooked’, ‘shrimps, cooked’, ‘shrimps, shelled, shucked and cooked’, ‘crustaceans, unspecified, cooked’, ‘crustaceans, unspecified, shelled, shucked and cooked’, ‘molluscan shellfish, cooked’, ‘unspecified’ (cooked, ready‐to‐eat, smoked) and ‘Surimi’.
When combining all sampling stages and all sampling units (‘single’ and ‘batch’) the overall occurrence of L. monocytogenes in RTE fish was 7.0%. Germany and Poland reported the major part of positive samples. This overall occurrence is lower compared with the 2010–2011 EU baseline survey which was 10.4% (EFSA, 2013, 2014a).
The overall occurrence of L. monocytogenes in RTE fishery products was 2.4%.
The prevalence in 2017 by merging RTE fish and fishery products is 6% and is comparable with 2016 (Figure 28).
Meat and meat products, RTE (pork, beef, broilers, turkeys)
Twenty MS reported 2017 data on RTE meat products (93.4% of all samples were obtained from pork followed by RTE meat from broilers (3%), bovine animals (2.3%) and turkeys (1.2%). Combining all RTE meat‐product categories from all sampling stages (‘retail’, ‘processing’, ‘border inspection activities’ and ‘unspecified’) and all sampling units (‘single’ and ‘batch’), the overall occurrence of L. monocytogenes in RTE meat products was 1.8% (400 out of 22,544 samples tested were positive) with no significant differences between the categories. The overall proportion is similar to the proportion reported in the 2010–2011 EU baseline survey (single units of RTE heat‐treated meat products sampled at retail and tested at the end of shelf life) (EFSA, 2013, 2014a). Since data from 2017 were mostly reported by a limited number of MS, the findings presented in this figure may not be representative of the EU level.
Pig meat products, RTE
Sixteen MS (Austria, Belgium, Bulgaria, Cyprus, the Czech Republic, Croatia, Denmark, Estonia, Greece, Hungary, Latvia, Lithuania, Poland, Portugal, Romania and Spain) and one non‐MS (Montenegro) reported 2017 data on RTE pig meat products and, overall, L. monocytogenes was detected in 1.8% of the 20,968 units tested. At processing, almost 70% of the data were obtained from Poland. At retail, L. monocytogenes was detected in 2.5% (91 out of 3,701) of the tested samples, whereas at the processing stage 1.7% (294 out of 17,360 samples) the samples tested positive.
Poultry meat products (broilers and turkeys), RTE
Eleven MS (Austria, Bulgaria, the Czech Republic, Denmark, Estonia, Hungary, Poland, Portugal, Romania, Spain and Slovakia) reported 2017 data on RTE broiler meat products. Overall, L. monocytogenes was detected in 1.6% of the 673 units tested. Ten MS (Austria, Cyprus, the Czech Republic, Estonia, Spain, Greece, Hungary, Poland, Portugal and Slovakia) reported data from RTE turkey meat products. Overall, L. monocytogenes was detected in 0.8% of the 252 units tested.
Bovine meat products, RTE
Eleven MS (Austria, Bulgaria, Cyprus, the Czech Republic, Denmark, Estonia, Spain, Hungary, Poland, Portugal and Romania) and one non‐MS (Montenegro) reported 2017 data on RTE bovine meat products. Overall, L. monocytogenes was detected in 1.7% of the 527 units tested. At retail, L. monocytogenes was detected in 1.2% of the units, whereas at processing, 1.9% of units tested were positive.
A summary of the proportion of L. monocytogenes‐positive units in RTE meat products according to the animal origin is presented in Figure 29.
Figure 29.
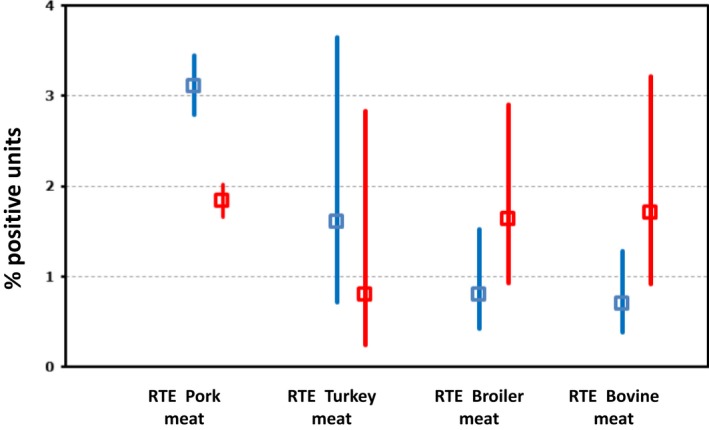
- Only data obtained from detection method are included.
- RTE pig meat products include ‘Meat from pig, meat products’ of the following types: ‘cooked ham (sliced or non‐sliced)’, ‘cooked, RTE’, ‘fermented sausages’, ‘fresh raw sausages’, ‘meat specialities’, ‘pâté’, ‘raw and intended to be eaten raw’, ‘raw ham’, ‘unspecified, ready‐to‐eat’. ‘RTE turkey meat’ includes turkey ‘meat products’ of the following types: ‘cooked, RTE’, ‘preserved’ and ‘raw and intended to be eaten raw’. ‘RTE broiler meat’ broiler ‘meat products’ of the following types: ‘cooked, RTE’ and ‘cooked, RTE, chilled’. ‘RTE bovine meat’ includes ‘Meat from bovine animals, meat products’ of the following types: ‘cooked, RTE’, ‘cooked, RTE, chilled’, ‘fermented sausages’, ‘raw and intended to be eaten raw’, ‘unspecified, RTE’.
Milk and milk products, RTE
Fourteen MS (Austria, Bulgaria, Cyprus, the Czech Republic, Denmark, Germany, Greece, Spain, the Netherlands, Croatia, Italy, Poland, Romania and Slovakia) and one non‐MS (Montenegro) reported 2017 data on RTE milk (‘pasteurised’, ‘UHT’ and ‘raw milk intended for direct human consumption’). Overall, L. monocytogenes was detected in 2.8% of the 2,055 units tested.
In total, two MS (Germany and Italy) found positive values from ‘raw milk intended for direct human consumption’ and from more surprisingly from ‘pasteurised milk’.
Cheeses
Nineteen MS reported 2017 data from L. monocytogenes detection in cheeses. Cheeses made from pasteurised cows’ milk represent more than 50% of samples collected and reported. Overall, considering all sampling stages, all sampling units, all milk origin (cow, goat, sheep and mixed) and all types of cheeses, L. monocytogenes was detected in 0.7% of the 11,156 cheese samples tested. As data were mostly reported by a limited number of MS, the findings presented in this figure may not be presentative of the EU level.
A summary of the proportion of units positive for cheeses is presented in Figure 30.
Figure 30.
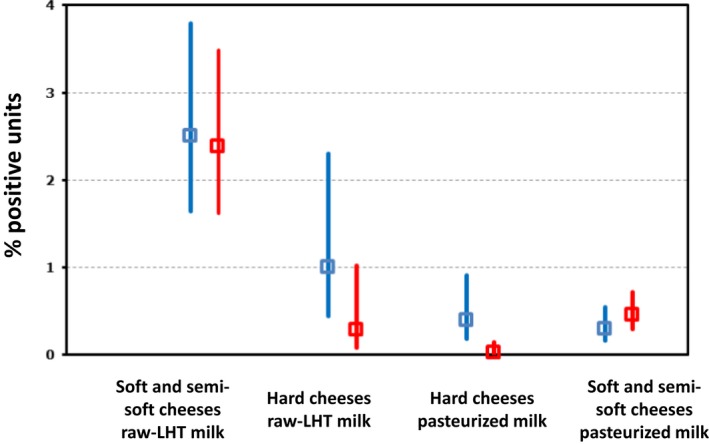
- LHT: low‐heat‐treated. ‘Overall’ and the number of MS correspond to data across all sampling stages (‘retail’ and ‘processing’ + ‘farm’ + ‘border inspection activities’ + ‘unspecified’).For each sampling stage (‘overall’, ‘retail’ and ‘processing’), data are pooled across both types of sampling units (‘single’ and ‘batch’). Soft and semi‐soft cheeses as well as hard cheeses include all cheeses for which Level 2 at matrix level was specified (‘fresh’ or ‘soft’ or ‘semi‐soft’ or ‘hard’).
Soft and semi‐soft cheeses
In 2017, 6,117 units of soft and semi‐soft cheeses (for cow, goat, sheep and mixed species milks) were tested using the detection method and reported by 17 MS.
The occurrence of L. monocytogenes in goat, sheep and cow soft and semi‐soft cheeses made from raw or low‐heat‐treated milk was significantly higher (2.4% of the 1,052 units tested) compared with cheeses made from pasteurised milk (0.5% of the 4,141 units tested). This estimate is comparable with the estimate (0.5%) obtained from the 2010–2011 EU baseline survey (RTE soft and semi‐soft cheeses sampled at retail and tested at the end of shelf life) (EFSA, 2013, 2014a).
Hard cheeses
In 2017, 5,039 units of hard cheeses (for cow, goat, sheep and mixed species milks) were tested using the detection method by 15 MS (but one MS, the Netherlands, provided almost 50% of samples). Similar as in soft and semi‐soft cheeses, hard cheeses produced from pasteurised milk had a significantly lower proportion of positive samples compared with hard cheeses produced from raw milk (Figure 5).
In 2017, overall L. monocytogenes was detected in less than 1% of all samples from hard cheeses.
Other ready‐to‐eat food products
In 2017, results obtained from detection method for other RTE food‐product categories, such as ‘bakery products’, ‘confectionery products and pastes’, ‘egg products’, ‘fruits and vegetables’, ‘salads’, ‘sauces and dressings’, ‘spices and herbs’ and ‘other processed food products and prepared dishes’ were reported.
For ‘bakery products’, most of the data were from single samples collected at retail and were reported by 11 MS. Overall, out of the 3,600 units of bakery products tested, 7.8% were found positive for L. monocytogenes which was higher than in 2016 (0.8%) due to the high number of positive samples reported by Germany that contributed for more than 60% of the total samples tested.
In 2017, 13 MS provided data from investigations of L. monocytogenes on 1,773 units of ‘RTE fruit and vegetables’. The overall prevalence is of 0.6%.
For ‘RTE salads’, 11 MS reported data on 902 units tested. Overall, 4.2% of the units tested were reported as positive.
For ‘sauces and dressings’, 11 MS reported information on 184 units tested and L. monocytogenes was detected only in 1.6% of samples.
For ‘spices and herbs’, ‘confectionery products and pastes’, ‘egg products’, less than 50 samples were analysed and none was found positive.
In ‘other processed food products and prepared dishes’, 12 MS submitted data. Overall, L. monocytogenes was detected in 1.4% of the 646 units tested.
Details on occurrence of L. monocytogenes in main RTE food matrices in 2017 together with 2016 results can be found in Appendix A at the end of this report.
3.4.4. Listeria spp. in animals
In 2017, 14 MS and 1 non‐MS reported data on several animal categories (food‐producing, wild‐, zoo‐ and pet animals, including birds) and animal species tested for Listeria spp. Reported data were mainly at level of animal (97.1%) compared with other unit levels (‘herd/flock’ and ‘holding’). Most animals tested concerned domestic ruminants (cattle, sheep and goats). Among the reporting countries, Italy reported on the highest variety of animal categories and species (Table 2017_LISTERIAANIMALS).
The sample size as well as the sampling strategy and the proportion of positive samples varied considerably among the reporting countries and animal species. Hence, the vast majority of the EU data in animals (90.9% of the total units tested) was reported by two MS (Ireland and the Netherlands).
In total, considering all different sampling units(‘animal’, ‘herd/flock’ or ‘holding’) 19,295 units were tested for Listeria spp. and 247 (1.3%) were found to be positive.
Among the positive units, 146 (59%) were reported as being positive for L. monocytogenes and only limited numbers were reported as Listeria ivanovii (5 units, 2.0%) and Listeria innocua (34 units, 13.8%).
In 2017, a significant proportion – as in 2016 – 62 units (25.1%) were reported as positive under the ‘unspecified Listeria spp.’ or ‘Listeria spp.’ other than L. ivanovii and L. innocua category.
3.4.5. Listeria monocytogenes in feed
Only one MS (Romania) reported data from investigations of L. monocytogenes in feed. In total, 28 samples (mainly silage) were analysed with only one positive sample.
3.5. Discussion
While still relatively rare, human listeriosis is one of the most serious food‐borne diseases under EU surveillance causing high hospitalisation and high mortality, particularly among the elderly. EU surveillance of human listeriosis focuses on severe, invasive forms of the disease, which affects the following risk groups: elderly, immunocompromised people as well as pregnant women and infants. Invasive listeriosis has shown a significant increasing trend since EU surveillance was initiated in 2008. In addition, listeriosis continued to show a significantly increasing trend in the last 5 years (2013–2017), although the number of cases did not increase in 2017. Five MS reported increasing trends over the last 5 years. This is partly attributable to more complete reporting and improvements in the surveillance of listeriosis in a few countries. Most listeriosis cases — when this information is known — have been domestically acquired and a few cases have been linked to travel, within or outside the EU. The number of cases acquired within EU increased slowly compared with the significant increase of listeriosis cases in the EU since 2008 as smaller proportion of cases were reported with information on travel status and country of infection in 2017.
Since the beginning of EU level surveillance, most listeriosis cases have been reported in people over 64 years of age. The number and proportion of cases reported for this age group has increased steadily from 2008 and continued to increase in 2017. Human cases almost doubled in the age group over 84 years in the same time period. As in previous years, almost all (99.8%) reported listeriosis cases were hospitalised. Despite the slight decrease of the fatal cases, listeriosis caused the highest proportion of fatal cases compared with all other zoonotic infections. In addition to the more complete reporting and improvements in surveillance, the increase of Listeria infections may be partially explained by the ageing population in the EU. As ageing of the populations will continue in most MS in the coming years, it is important to raise awareness of listeriosis and the risk, especially to older people, associated with certain types of foods and consumption patterns/habits.
Despite the increasing trend of reported invasive L. monocytogenes, the number of cases reported to EFSA FBO database is rather low (39 cases) compared to the overall reported number of cases reported in TESSy. This may suggest that a substantial amount of human cases are sporadic cases for which we do not know if these might be linked to FBOs. This might be due to reporting bias in the different countries as the number of human cases reported may include FBO cases or not. It is recommended to harmonise this reporting and/or to further investigate the sporadic cases in relation to potential unknown food vehicles.
A wide range of foodstuffs can get occasionally contaminated during various steps of food production and distribution, particularly during the food‐processing stage. In addition, many different RTE food types have been implicated in cases or outbreaks of listeriosis in humans. Some MS focus their sampling effort, especially on those RTE foods supporting the growth of L. monocytogenes and which are stored for extended periods under refrigeration temperatures before consumption. The classical high risk foods tested by MS for L. monocytogenes are RTE meat and meat products, fish and fishery products and cheeses. In recent years, however, listeriosis outbreaks were also caused by foods that were not considered as likely food vehicles, based on previous experience and risk assessments (Buchanan et al., 2018). The recent EU outbreak related to frozen corn (EFSA and ECDC, 2018b) but also in the USA related to cantaloupes (CDC, 2011), ice‐cream (Pouillot et al., 2016), prepacked caramel apples (CDC, 2015a) and sprouts (CDC, 2015b) are illustrations of previously unknown potential vehicles.
In 2017, MS were able to report for the first time under the following specific sampling context ‘Surveillance – based on Regulation 2073’, the results of official sampling carried out by the CA in the context of surveillance of the application of the FSC listed in Regulation (EC) No 2073/2005. Monitoring in this context was focused on 10 RTE food categories. In 2017, for these RTE products at retail the level of non‐satisfactory results was very low as in 2016 (EFSA, 2017b). However, the RTE food categories with non‐compliant samples differ somewhat from those in 2016 and is mainly due to reporting bias (for the first time ‘Surveillance – based on Regulation 2073’ could be reported) and therefore evaluating trends or comparing 2017 with historical data is not possible (EFSA et al., 2018c). In 2017, RTE food categories at processing presented higher levels of non‐compliance than at retail stage. The results for 2017 were in the same range of values as those from 2016 (EFSA and ECDC, 2017b).
L. monocytogenes occurrence ranges from 0.03% for ‘hard cheeses made from pasteurised milk’ to 7.0% for ‘RTE fish’. These occurrence data are in agreement with the median prevalence values gathered in a recently published meta‐analysis for the 1990–2015 period (Jofré et al., 2016). The data are also similar from those of last year zoonosis report (EFSA and ECDC, 2017b).
Fruits and vegetables have been proven to be the cause of listeriosis cases at EU and international level (Buchanan et al., 2018; EFSA and ECDC, 2018b) and therefore MS are encouraged to sample these food categories. In 2017, only 4% of all samples collected were from fruit and vegetables which is substantially lower than RTE food from animal origin). It is worth to notice that in 2017, MS increased their sampling for most RTE food categories compared with 2016. However, there are significant differences between MS with relation to sampling efforts (sample size) and reporting attitude. Indeed some MS report mainly suspect and/or selective sampling which is not representative for objective (official) sampling and therefore cannot be taken up in the analysis of L. monocytogenes occurrence in foods.
The annually reported occurrence results for the different RTE food categories are important indicators of the level of risk of RTE products in EU. In 2017, the overall occurrence in fish and fishery products (6%), meat and meat products (1.8%) and cheeses (< 3% for soft and semi‐soft cheeses and < 1% hard cheeses) are comparable with 2016. Yet, the ability to analyse the trend might be limited because of the annual variation in the number of tested samples at each stage of the production chain as well as the number of MS reporting data across the different years. A more systematic transmission and uniform reporting of data by all the MS for a specific food‐chain stage according a harmonised interpretation of sampling context and sampler would improve the relevance of this annual comparison.
As expected from the results from, MS testing for Listeria spp. in animals, most isolates belong to L. monocytogenes (EFSA, 2017a) and L. ivanovii. A significant proportion of isolates (25.1%) is still reported by the MS as ‘unspecified Listeria spp.’ or ‘Listeria spp.’ and were not identified to the species level. Probably these MS do not further characterise the isolates as it is assumed to be L. monocytogenes.. Therefore, it might be that reported listeriosis in animals is known to be almost exclusively caused by L. monocytogenes and L. ivanovii.
Sequencing of food isolates of L. monocytogenes obtained in the different sampling context would bring a new insight in the analysis of reported data on strain virulence variability among the different food categories. At processing or retail level, clonal complex determination and/or whole sequencing would also bring new insight of level of risk of RTE foods as it was recently shown that virulence of some strains is particularly higher than others (Maury et al., 2017; Fristch et al., 2018). In addition, it has been shown that L. monocytogenes have the ability to survive, multiply and persist under harsh conditions in food processing environments and the re‐isolation of identical L. monocytogenes clones over extended time periods in processing plants shows that L. monocytogenes has the ability to adhere to surfaces and form biofilms (Di Bonaventura et al., 2008; Carpentier and Cerf, 2011; Doijad et al., 2015; Fagerlund et al., 2016; Fagerlund et al., 2017). At animal level, subtyping with MLST or whole genome sequencing (WGS) would help to better characterise the diversity in reservoirs (Nielsen et al., 2017). This knowledge is of particular importance for identifying most virulent strain in animals (Dreyer et al., 2016).
3.6. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Surveillance atlas | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| Humans and food | ||
| Commission Regulation (EC) No. 2073/2005 – Food Safety Criteria for L. monocytogenes in the EU | http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073-20170101&rid=1 | |
| EU Baseline Survey 2010–2011– part A: Listeria monocytogenes prevalence estimates | https://www.efsa.europa.eu/en/efsajournal/pub/3241 | |
| EU Baseline Survey 2010–2011 – Part B: analysis of factors related to prevalence and exploring compliance | https://www.efsa.europa.eu/en/efsajournal/pub/3810 | |
| Scientific opinion – Request for updating the former SCVPH opinion on Listeria monocytogenes risk related to ready‐to‐eat foods and scientific advice on different levels of Listeria monocytogenes in ready‐to‐eat foods and the related risk for human illness | https://www.efsa.europa.eu/en/efsajournal/pub/599 | |
| Draft scientific opinion – L. monocytogenes contamination of RTE foods and the risk for human health in the EU | https://www.efsa.europa.eu/sites/default/files/engage/170724-0.pdf | |
| Quantitative assessment of relative risk to public health from food‐borne Listeria monocytogenes among selected categories of ready‐to‐eat foods | FDA‐CFSAN/USDA‐FSIS 2003, https://www.fda.gov/downloads/Food/FoodScienceResearch/UCM197330.pdf | |
| Risk assessment of Listeria monocytogenes in ready‐to‐eat foods: Technical report | http://www.fao.org/3/a-y5394e.pdf | |
| Risk assessment of Listeria monocytogenes in ready‐to‐eat foods – Interpretive Summary | http://www.fao.org/fileadmin/templates/agns/pdf/jemra/mra4_en.pdf | |
| FSIS comparative risk assessment for Listeria monocytogenes in ready‐to‐eat meat and poultry deli meats | US FDA/FSIS (2010), https://www.fsis.usda.gov/shared/PDF/ | |
| Interagency risk assessment: Listeria monocytogenes in retail delicatessens technical report | US FDA/FSIS (2013), https://www.fsis.usda.gov/shared/PDF/Comparative_RA_Lm_Report_May2010.pdf | |
| Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 1, an extensive literature search and study selection with data extraction on L. monocytogenes in a wide range of RTE food | EFSA External Scientific Report (2016), https://www.efsa.europa.eu/en/supporting/pub/1141e | |
| Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 2, a quantitative risk characterisation on L. monocytogenes in RTE foods; starting from the retail stage | https://www.efsa.europa.eu/en/supporting/pub/1252e | |
| Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 3, the comparison of isolates from different compartments along the food chain, and from humans using whole genome sequencing (WGS) analysis | https://www.efsa.europa.eu/en/supporting/pub/1151e | |
| Surveillance atlas of infectious diseases in humans including listeriosis – Tool for infectious disease data manipulation and presentation | ECDC, https://ecdc.europa.eu/en/surveillance-atlas-infectious-diseases | |
| Guidance document on Listeria monocytogenes shelf‐life studies for ready‐to‐eat foods, under Regulation (EC) No. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs | EC, https://ec.europa.eu/food/sites/food/files/safety/docs/biosafety_fh_mc_guidance_document_lysteria.pdf | |
| EU Reference Laboratory activities and documents on L. monocytogenes for member laboratories | EURL for Listeria monocytogenes, https://eurl-listeria.anses.fr/ | |
| Technical guidance document for conducting shelf‐life studies on Listeria monocytogenes in ready‐to‐eat foods (challenge testing and durability testing) | EURL for Listeria monocytogenes, https://eurl-listeria.anses.fr/en/minisite/listeria/eurl-lm-technical-guidance-document-conducting-shelf-life-studies-listeria | |
| Guidelines on the application of general principles of food hygiene to the control of Listeria monocytogenes in foods | CAC, http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCAC%2BGL%2B61-2007%252FCXG_061e.pdf | |
| A public database of genome sequences, including L. monocytogenes sequences – GenomeTrakr | US FDA and others, https://www.fda.gov/food/foodscienceresearch/wholegenomesequencingprogramwgs/ucm363134.htm | |
| General overview and facts on L. monocytogenes and listeriosis | CDC (US), https://www.cdc.gov/listeria/ | |
| A web‐based platform (‘Listeriomics’) integrating different tools for Listeria ‘omics data analyses | https://listeriomics.pasteur.fr | |
| Bad Bug Book (Second Edition), Food‐borne Pathogenic Microorganisms and Natural Toxins Handbook, Center for Food Safety and Applied Nutrition, Food and Drug Administration (FDA), USA | https://www.fda.gov/food/foodborneillnesscontaminants/causesofillnessbadbugbook/ | |
| Animals | ||
| General overview of listeriosis in animals | Merck Veterinary Manual, http://www.merckvetmanual.com/generalized-conditions/listeriosis/overview-of-listeriosis | |
| Overview and diagnosis of listeriosis in animals | http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.09.06_LISTERIA_MONO.pdf |
4. Shiga toxin‐producing Escherichia coli
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1475841
4.1. Abstract
In 2017, 6,073 confirmed cases of Shiga toxin‐producing Escherichia coli (STEC) infections were reported in the EU. The EU notification rate was 1.66 cases per 100,000 population, which was a 6.2% decrease compared with 2016. Over the last 5‐year‐period from 2013 to 2017, the EU/EEA trend has been stable. In 2017, 20 deaths due to STEC infection were reported, which resulted in an EU case fatality of 0.5%.
As in previous years, the most commonly reported STEC serogroup in confirmed cases of human STEC infections in EU/EEA in 2017 was O157 (31.9%). However, the proportion of this serogroup continued to decrease, whereas that of non‐O157 serogroups increased. This is possibly an effect of increased awareness and of more laboratories testing for other non‐O157 serogroups. Serogroup O157 was followed by O26, O103 and O91. Serogroup O157 was the most frequently reported cause of haemolytic uraemic syndrome (HUS) cases in 2017.
In 2017, 21,574 units of food (batches or single samples) have been tested by 25 MS. Compared with 2016 this was a moderate increase in the number of samples tested and in the number of reporting MS, suggesting an augmented awareness at the EU level of the necessity to monitor this pathogen in food, according to EU Directive 2003/99/EC. In 2017, 2,310 units from animals (animals or herds or flocks) were tested for the presence of STEC, confirming the decrease in the testing of animal samples observed in 2016. A major criticality was represented by the variability in the sampling strategies applied by different MS to the different categories. This variability is likely to introduce a selection bias in the estimates of STEC prevalence or STEC serogroup distribution, hindering spatial and temporal trends analyses. The analysis of the STEC serogroups identified in food and animal samples indicates that those identified in human infections are mostly represented, supporting the importance of food vehicles in the diffusion of STEC infections at the EU level. The analysis of the virulence gene profiles of the isolated STEC strains highlighted the presence in food of STEC with the potential of causing severe disease. This level of characterisation of the isolates, however, was not accomplished for more than half of the STEC isolates from food in 2017. Countries are recommended to report information on the STEC virulence genes as their analysis represents the basis for the molecular risk assessment and the most valuable tool to predict the risk and to infer on the severity of the STEC infections in humans.
4.2. Surveillance and monitoring of Shiga toxin‐producing Escherichia coli in the EU
4.2.1. Humans
The notification of STEC9 infections is mandatory in most EU MS, Iceland, Norway and Switzerland, except for four MS, where notification is based on a voluntary system (France, Luxembourg) or other system (Italy and the United Kingdom). In the United Kingdom, although the reporting of food poisoning is mandatory, isolation and specification of the organism is voluntary. The surveillance systems for STEC infections cover the whole population in all EU MS except for three MS (France, Italy and Spain). The notification rates were not calculated in these three countries for the following reasons: (a) in France, the STEC surveillance in humans is based on paediatric HUS cases; (b) in Italy, STEC surveillance is sentinel and primarily based on the HUS cases reported through the national registry of HUS; (c) no estimation for population coverage of STEC cases was provided by Spain. In Belgium, full national coverage was established in 2015 and rates before this year are not displayed. All countries report case‐based data except Bulgaria, which reported aggregated data. Both reporting formats were included to calculate numbers of cases, notification rates and disease trends.
Diagnosis of human STEC infections is generally performed by culture from stool samples and indirect diagnosis by the detection of antibodies against the E. coli O‐lipopolysaccharides in serum in the event of HUS cases. In addition, diagnosis by direct detection of free faecal Shiga toxin/verocytotoxin by the Vero cell or immune‐assays or the identification of the presence of stx1/vtx1 or stx2/vtx2 genes in stools by PCR without strain isolation is increasing.
4.2.2. Food and animals
STEC data according to Regulation (EC) No. 2073/2005, STEC food safety criterion for sprouts at the retail level
The only existing microbiological criterion for STEC in a food commodity is defined in the Regulation (EC) No. 209/2013 amending Regulation (EC) No. 2073/2005 as regards microbiological criteria for sprouts. This food safety criterion applies to sprouts and the results must be compliant with ‘absence in 25 grams’ of STEC O157, O26, O111, O103, O145 and O104:H4, for sprouts placed on the market during their shelf life (Regulation (EC) 209/2013).
The STEC monitoring data for sprouts submitted to EFSA thus consist of data originating from the reporting obligations of MS under the EU Regulation on microbiological criteria. In spite of the legal framework, the production of these data is not fully harmonised across MS. As a matter of fact, the sampling objectives, the place of sampling and the sampling frequency applied vary or are interpreted differently between MS. Most of these data concerns the food chain control (official monitoring) and data are collected by the National Competent Authorities conducting investigations to verify whether FBOp implement correctly the legal framework of own‐control programmes and, to a lesser extent, they include the analyses carried out as part of the HACCP plans, industry monitoring) according to the General Food Law. In fact, industry data are seldom reported to EFSA because of data ownership sensitivities. In essence, food chain control data are compliance checks and are collected with the aim of installing an early warning and initiate control measures. Although they allow for descriptive summaries to be made at the EU level (Boelaert et al., 2016), these data are not suitable for trends analyses, because a reference (study) population is mostly absent and because the sampling is risk‐based and therefore non‐representative.
In the present annual report, EFSA implemented new aggregation rules – for the first time – for data sent by MS according to Regulation (EC) No. 2073/2005 (STEC microbiological criterion). The summarisation rules were agreed upon with the European Commission and with MS:
-
Data sets usable for trend watching are those with the following specified options for the different data aspects:
Sampling context: Surveillance, based on Regulation 2073;
Sampling unit type: Single;
Sampling stage: as appropriate;
Sampling strategy: Objective sampling;
Sampler: Official sampling.
Other food data (described in the next section), having other specified options for the different data aspects (including sampling context other than based on Regulation 2073/2005), are summarised only and do not serve the purpose of trend watching or trend analyses.
STEC monitoring data reported according to Regulation (EC) No. 2073/2005 (STEC food safety criterion) only allow for descriptive summaries at the EU level to be made highlighting the limitations of these summaries. Specific results from single samples taken by the CA (‘official sampling’) and with an objective sampling strategy allow trends to be monitored at EU and MS level.
Other STEC monitoring data from foods and animals
The monitoring data on STEC in foods other than sprouts and in animals, originate from the reporting obligations of MS under Directive 2003/99/EC, which stipulates that MS must investigate the presence of STEC at the most appropriate stage of the food chain. The Directive is not explicit about the sampling strategy and the data generated by MS are based on investigations with non‐harmonised sampling and they are obtained with different analytical methods. The Directive does not indicate strict details of the mandatory reporting requirements. Therefore, STEC monitoring data according to Directive 2003/99/EC are not comparable between MS and preclude subsequent data analysis like assessing temporal and spatial trends at the EU level. Sampling biases and inaccuracies due to limited numbers of examined samples preclude also the evaluation of the existing prevalence or accurate prevalence estimations. The use by MS of laboratory analytical methods testing for STEC O157 leads to biased STEC prevalence estimations or biased STEC serogroup frequency distributions analysing data at the EU level. Nonetheless, descriptive summaries of sample statistics at the EU level may be made if the relevant limitations of the data set are flagged.
To improve the quality of the data from STEC monitoring in the EU, EFSA issued technical specifications for harmonised monitoring and reporting of STEC in animals and foodstuffs in 2009 (EFSA, 2009b). These guidelines were developed to facilitate the generation of more harmonised data, which would enable a thorough analysis of STEC in food and animals. The EFSA Scientific Opinion encourages MS to extend the monitoring and report data on STEC serogroups.
4.2.3. Food‐borne outbreaks of STEC infections in humans
The reporting of FBOs of human STEC infections is mandatory according to the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
4.3. Data validation and analyses of monitoring data from food and animals
4.3.1.
Data validation
The STEC monitoring data from food and animals reported for the year 2017 to EFSA were verified as regards their plausibility and reliability, in line with the current knowledge. Following this step, the occurrence of STEC in food and animals and the frequency distribution of STEC serogroups were descriptively analysed. Criteria were applied to disclose possible implausible data, which were then reviewed by the MS.
The following plausibility criteria were focused on the level of completion and coherence of the information and on the consistency of the laboratory results with the analytical method reported:
Plausibility of reported occurrence values with respect to the STEC epidemiology based on the updated scientific literature.
Consistency of the reported laboratory results with the purposes of the STEC monitoring data collection. An example of data not consistent with the objective of the data collection, and for this reason excluded from the analysis, is the reporting of E. coli indicators or pathogenic E. coli with negative results for stx‐genes testing.
Consistency of the reported laboratory results with the analytical method used for the analysis. An example may be the reporting of STEC O26 or other non‐O157 STEC serogroups for samples assayed with the standard ISO 16654:2001 (CEN, 2017) or equivalent methods, which can only detect serogroup O157.
A reliability criterion has been used to identify those data that did not match (partly or totally) the current scientific knowledge on STEC epidemiology. An example of reliability criterion was the consistency between 2017 STEC data reported by MS and their recent historical data. Secondly, also the reliability of number of samples reported for STEC was verified. As an example, countries reporting testing of more than 100,000 samples for STEC or unusually high proportions of positive samples would be asked to double‐check their data.
In addition, data or information erroneously reported in free‐text variables were identified in the records provided by two MS (DE and LU) and recoded so as to augment the information value.
Data analysis
To reduce the bias due to the absence of microbiological criteria for STEC, for the description of the proportion of STEC‐positive samples in the different food categories a subset of all validated monitoring data was used. Specifically, the following data were excluded: data reported with a sampler ‘industry sampling’ or ‘HACCP and own checks’, or as sampling strategy; ‘selective sampling’ or ‘suspect sampling’, or having ‘clinical investigations’ as sampling context, or as outbreak data.
The unfiltered entire data set was used instead for any other descriptive analysis on STEC findings in food and animals, including those on the methods used and the virulence genes and serogroups’ frequency distribution, where the interest was to describe the variety and overall distribution of the information reported.
The analysis of the data provided by the reporting countries, on STEC detected in food and animal samples in 2017, has been carried out considering the data grouped according to the methods used for the food testing:
Methods aiming at detecting any STEC. This category includes the method ISO TS 13136:2012 (ISO, 2012) and other PCR‐based methods.
Methods designed to detect only STEC O157, such as the method ISO 16654:2001 (ISO, 2001) and the equivalent methods NMKL 164:2005 (NMKL, 2005) and DIN 1067:2004–03 (DIN, 2004).
One MS used an enzyme‐linked fluorescent assay targeting STEC O157 to test food samples. The related records have been analysed by including these samples into group b).
Such a distinction was necessary when analysing the frequency of the STEC serogroups to minimise the bias introduced by the use of methods directed towards the isolation of STEC O157 only, which would not allow the identification of other STEC possibly present in the samples.
4.4. Results
4.4.1. Overview of key statistics along the food chain, EU, 2013–2017
Table 27 summarises EU level statistics related to human STEC infections, and STEC in food and animals, respectively, in the EU, during 2013–2017. A more detailed description of these statistics can be found in the specific results subsections of this chapter and in the chapter on FBO.
Table 27.
Summary of STEC statistics related to humans, major food categories and major animal species, EU, 2013–2017
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 6,073 | 6,456 | 5,929 | 5,900 | 6,042 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 1.66 | 1.77 | 1.65 | 1.75 | 1.80 | ECDC |
| Number of reporting MS | 28 | 28 | 28 | 27 | 27 | ECDC |
| Infection acquired in the EU | 4,806 | 3,994 | 3,991 | 3,959 | 3,916 | ECDC |
| Infection acquired outside the EU | 528 | 340 | 532 | 474 | 485 | ECDC |
| Unknown travel status or unknown country of infection | 739 | 2,122 | 1,406 | 1,467 | 1,641 | ECDC |
| Total number of food‐borne outbreaks (including waterborne outbreaks) | 48 | 42 | 69 | 67 | 74 | EFSA |
| Number of outbreak‐related cases | 260 | 735 | 674 | 957 | 633 | EFSA |
| Food | ||||||
| Meat and meat products | ||||||
| Number of sampled units | 12,465 | 9,369 | 10,872 | 9,836 | 11,706 | EFSA |
| Number of reporting MS | 20 | 18 | 16 | 16 | 19 | EFSA |
| Milk and milk products | ||||||
| Number of sampled units | 3,637 | 3,848 | 4,370 | 6,788 | 4,388 | EFSA |
| Number of reporting MS | 12 | 12 | 11 | 12 | 13 | EFSA |
| Fruits and vegetables (and juices) | ||||||
| Number of sampled units | 2,325 | 1,518 | 1,821 | 2,015 | 2,498 | EFSA |
| Number of reporting MS | 15 | 21 | 22 | 23 | 23 | EFSA |
| Animals | ||||||
| Bovine animals | ||||||
| Number of sampled herds | 226 | 62 | 49 | 1,178 | 1,307 | EFSA |
| Number of reporting MS | 4 | 2 | 2 | 5 | 4 | EFSA |
| Small ruminants | ||||||
| Number of sampled herds | 10 | 208 | 109 | 44 | 11 | EFSA |
| Number of reporting MS | 1 | 8 | 7 | 7 | 7 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States; STEC: Shiga toxin‐producing Escherichia coli.
Humans
The number of human STEC cases infected domestically and through travel within the EU remained stable since 2013, but increased in 2017, when more cases were reported with data on travel and the probable country of origin. The statistics for FBO due to STEC show that the number of outbreak‐related cases fluctuated around 600–700 with a peak during 2014 (957 cases) and a decrease in 2017. The total number of reported outbreaks decreased since 2012.
Food categories
Data submitted by reporting MS over the period 2013–2017 were aggregated in macrocategories to get an overview, by year, of the data sent for each macro‐category and the respective number of reporting MS.
The food category ‘meat and meat products’ presented the highest number of samples tested in the 5‐year period considered. This may be due to an increase of the number of MS reporting data from the analysis of this food category in 2017 (20 MS). The number of reporting MS was fairly stable for the ‘milk and milk products’ group, while the number of MS reporting data ‘fruit and vegetables’ was lower in 2017 than the previous years, although this category reported the highest number of samples tested in the last 4 years (2014–2017).
For the year 2017, 25 MS provided results from the analysis of 21,574 food units (batches or single samples). The proportion of food samples reported by EU MS and tested for STEC by the different analytical methods is presented in the Table 2017_STECANMETH.
Animal categories
For the year 2017, 2,310 units from animals (animals or herds or flocks), tested for the presence of STEC, were reported by eight MS. This figure reflects the negative trend observed in 2016, when a very noticeable decrease in the numbers of animal samples reported was observed, considering the average of about 6,000 sample units that were reported in the period 2013–2015. The proportion of animal samples reported by EU MS and tested for STEC by the different analytical methods is presented in the Table 2017_STECANMETH.
The animal category ‘bovine animals’, showed a marked increase in the number of sampled herds over the last 2 years. This growth may reflect the parallel increase in the number of reporting MS. The number of sampled herds reported for ‘small ruminants’ was oscillating during 2013–2017, probably due to the variable number of reporting MS (range: one MS in 2017 to eight MS in 2016).
In 2017, about half of the samples were tested using the ISO TS 13136:2012 method, while the remaining samples were assayed using the standard methods ISO 16654:2001 (ISO, 2001), NMKL 164:2005 (NMKL, 2005) and DIN 1067:2004–03 (DIN, 2004), targeting the O157 serogroup only. As all the mentioned methods are intended for testing food and feed, these have been adapted to test animal samples by the reporting countries, following the EFSA recommendations (EFSA, 2009b).
4.4.2. STEC infections in humans
In 2017, 6,260 cases of STEC infections, including 6,073 confirmed cases, were reported in the EU (Table 28). Twenty‐five MS reported at least one confirmed STEC case and three MS reported zero cases. The EU notification rate was 1.66 cases per 100,000 population, which is 6.2% decrease compared with 2016 (1.77 cases per 100,000 population). The highest country‐specific notification rates were observed in Ireland, Sweden, Denmark, Austria and Germany (16.6, 5.0, 4.6, 2.9 and 2.5 cases per 100,000 population, respectively). Nine countries (Bulgaria, Cyprus, Greece, Latvia, Lithuania, Poland, Portugal, Romania and Slovakia) reported ≤ 0.1 cases per 100,000 population.
Table 28.
Reported human cases of STEC infections and notification rates per 100,000 population in the EU/EFTA, by country and year, 2013–2017
| Country | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 250 | 250 | 2.85 | 177 | 2.04 | 107 | 1.25 | 131 | 1.54 | 130 | 1.54 |
| Belgium | Y | C | 123 | 123 | 1.08 | 119 | 1.05 | 100 | 0.89 | 85 | – | 117 | – |
| Bulgaria | Y | A | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 |
| Croatia | Y | C | 7 | 7 | 0.17 | 9 | 0.21 | 0 | 0.00 | 4 | 0.09 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czech Republic | Y | C | 37 | 37 | 0.35 | 28 | 0.27 | 26 | 0.25 | 29 | 0.28 | 17 | 0.16 |
| Denmark | Y | C | 344 | 263 | 4.57 | 210 | 3.68 | 201 | 3.55 | 226 | 4.02 | 191 | 3.41 |
| Estonia | Y | C | 3 | 3 | 0.23 | 5 | 0.38 | 8 | 0.61 | 6 | 0.46 | 8 | 0.61 |
| Finland | Y | C | 124 | 123 | 2.24 | 139 | 2.53 | 74 | 1.35 | 64 | 1.17 | 98 | 1.81 |
| Franceb | N | C | 303 | 260 | – | 302 | – | 262 | – | 221 | – | 218 | – |
| Germany | Y | C | 2098 | 2065 | 2.50 | 1,843 | 2.24 | 1,616 | 1.99 | 1,663 | 2.06 | 1,639 | 2.00 |
| Greece | Y | C | 3 | 3 | 0.03 | 2 | 0.02 | 1 | 0.01 | 1 | 0.01 | 2 | 0.02 |
| Hungary | Y | C | 12 | 12 | 0.12 | 12 | 0.12 | 15 | 0.15 | 18 | 0.18 | 13 | 0.13 |
| Ireland | Y | C | 804 | 795 | 16.62 | 737 | 15.60 | 598 | 12.92 | 572 | 12.42 | 564 | 12.29 |
| Italyb | N | C | 111 | 94 | – | 78 | – | 59 | – | 68 | – | 64 | – |
| Latvia | Y | C | 1 | 1 | 0.05 | 1 | 0.05 | 4 | 0.20 | 0 | 0.00 | 0 | 0.00 |
| Lithuania | Y | C | 0 | 0 | 0.00 | 4 | 0.14 | 3 | 0.10 | 1 | 0.03 | 6 | 0.20 |
| Luxembourg | Y | C | 1 | 1 | 0.17 | 4 | 0.69 | 4 | 0.71 | 3 | 0.55 | 10 | 1.86 |
| Malta | Y | C | 9 | 9 | 1.96 | 4 | 0.92 | 4 | 0.93 | 5 | 1.18 | 2 | 0.48 |
| Netherlands | Y | C | 392 | 392 | 2.29 | 665 | 3.92 | 858 | 5.08 | 919 | 5.46 | 1,184 | 7.06 |
| Poland | Y | C | 6 | 4 | 0.01 | 4 | 0.01 | 0 | 0.00 | 5 | 0.01 | 5 | 0.01 |
| Portugal | Y | C | 2 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | – | – | – | – |
| Romania | Y | C | 11 | 11 | 0.06 | 29 | 0.15 | 0 | 0.00 | 2 | 0.01 | 6 | 0.03 |
| Slovakia | Y | C | 3 | 3 | 0.06 | 2 | 0.04 | 1 | 0.02 | 2 | 0.04 | 7 | 0.13 |
| Slovenia | Y | C | 33 | 33 | 1.60 | 26 | 1.31 | 23 | 1.11 | 29 | 1.41 | 17 | 0.83 |
| Spainc | N | C | 86 | 86 | – | 51 | – | 86 | – | 50 | – | 28 | – |
| Sweden | Y | C | 504 | 504 | 5.04 | 638 | 6.48 | 551 | 5.65 | 472 | 4.89 | 551 | 5.77 |
| United Kingdom | Y | C | 993 | 993 | 1.51 | 1,367 | 2.09 | 1,328 | 2.05 | 1,324 | 2.06 | 1,164 | 1.82 |
| EU Total | – | – | 6,260 | 6,073 | 1.66 | 6,456 | 1.77 | 5,929 | 1.65 | 5,900 | 1.75 | 6,042 | 1.80 |
| Iceland | Y | C | 3 | 3 | 0.89 | 3 | 0.90 | 1 | 0.30 | 3 | 0.92 | 3 | 0.93 |
| Norway | Y | C | 381 | 381 | 7.25 | 239 | 4.59 | 221 | 4.28 | 151 | 2.96 | 103 | 2.04 |
| Switzerlandd | Y | C | 696 | 696 | 8.23 | 463 | 5.47 | 315 | 3.77 | 125 | 1.52 | 82 | 1.53 |
Y: yes; N: no; A: aggregated data; C: case‐based data; –: no report.
Sentinel surveillance; only cases with HUS are notified.
Sentinel surveillance; no information on estimated coverage so notification rate cannot be estimated.
Switzerland provided the data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
Most STEC cases reported were infected in EU (79.1% domestic cases and travel in the EU, 8.7% travel outside EU and 12.2% of unknown importation or unknown country of infection) (Table 28). Three Nordic countries – Finland, Sweden and Norway reported the highest proportion of travel‐associated cases (44.9%, 39.2% and 30.7%, respectively). Among 844 travel‐associated cases with known probable country of infection, 62.6% of the cases travelled outside EU and 37.4% within EU. Turkey was the most frequently reported as the probable country of infection (12.8%), followed by Spain, Egypt, Morocco, Italy and Greece (11.7%, 8.3%, 4.5, 4.0% and 3.4%, respectively).
There was a clear seasonal trend in confirmed STEC cases in the EU/EEA between 2008 and 2017, with more cases reported during the summer months (Figure 31). There was a significantly increasing trend (p < 0.01) for STEC in the EU/EEA in 2008–2017, however results of statistical testing of trends for this period should be interpreted with caution due to a large outbreak in 2011. In the years after this outbreak (2013–2017), the overall EU/EEA trend did not show any significant increase or decrease (Figure 31).
Figure 31.
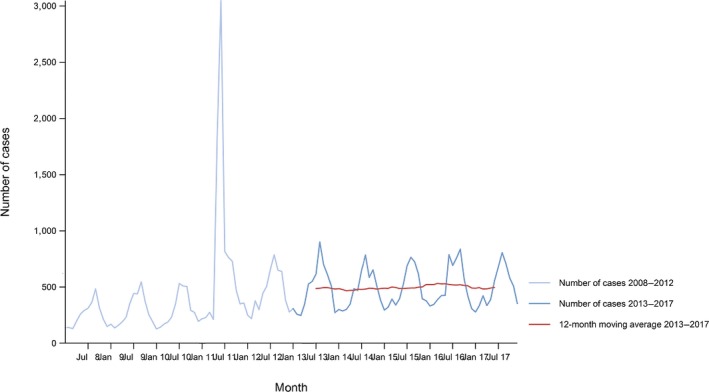
- Source: Austria, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and United Kingdom. Belgium, Bulgaria, Czech Republic, Croatia and Portugal did not report data to the level of detail required for the analysis.
In 2008–2017, a significantly increasing trends (p < 0.01) was observed in 10 MS (Denmark, Finland, France, Greece, Hungary, Ireland, Italy, the Netherlands, Slovenia and Sweden). Two MS (Cyprus and Slovakia) observed decreasing trends.
Over the 5‐year period 2013–2017, eight MS (Austria, Denmark, Finland, Germany, France, Ireland, Malta and Spain) reported significantly increasing trends (p < 0.05), and one MS (the Netherlands) had a significantly decreasing (p < 0.01) trend over the same time period due to a change in notification criteria.
Eighteen MS provided information on hospitalisation for 41.0% of all confirmed STEC cases in the EU in 2017. Out of the 2,487 cases with known hospitalisation status, 37.5% were hospitalised. The highest proportions of hospitalised cases (all cases hospitalised) were reported in Estonia, Greece, Latvia, Portugal and Slovakia. Four hundred and twenty‐nine cases of HUS were reported, which represents an increase by 10.0% (39 cases) compared with 2016. Most HUS patients were in the youngest age‐groups from 0–4 years (266 cases; 62.1%) to 5–14 years (105 cases; 24.5%). The most common serogroups among HUS cases were O157 (37.8%), O26 (26.3%), O145 (7.6%), O111 (6.3%) and O80 (5.6%); while 4.0% were untypeable.
In 2017, 20 deaths due to STEC infection were reported in the EU compared with 10 deaths in 2016. Seven MS reported one to eight fatal cases each, and 14 MS reported no fatal cases. This resulted in an EU case fatality of 0.5% among the 4,014 confirmed cases with known outcome (66.1% of all reported confirmed cases). The serogroup associated with more fatal cases was O157 (seven cases) followed by O145 (two cases). Serogroups O26, O103 and O111 were linked to one fatal case each. For eight fatal cases, the serogroup was not specified.
STEC infections in humans associated with food‐borne outbreaks
STEC was identified in 48 FBOs affecting 206 people (notified FBO cases) in 11 MS, as reported to EFSA. Overall, for the year 2017, there were 4,433 domestic (acquired within the country) cases reported to the TESSy (Table 29), which was 92.2% of the number of reported human STEC infections domestically and through travel within EU during 2017 (4,806, Table 27). Table 29 shows data reported by countries to TESSy managed by ECDC and to the FBOs’ database managed by EFSA. It is important to clarify that the case classification for reporting is different between these two databases. In TESSy, the cases reported are classified based on the EU case definition. All these cases visited a doctor, and are either confirmed by laboratory test (confirmed case) or not (probable case and classification is based on the clinical symptoms and epidemiological link). Cases that never visited a doctor are not reported to TESSy. Moreover, probable cases may be missing in TESSy, as these data are not analysed or published and there is no incentive for reporting such cases. Information on which case is linked to an outbreak – and which not – is not systematically collected. In practice, the cases reported to TESSy are considered mostly sporadic cases. In food‐borne disease outbreak situations cases are also classified into confirmed or probable outbreak cases, but currently these data are not collected by EFSA.
Table 29.
Statistics related to the proportions of human food‐borne outbreak cases caused by STEC (including waterborne outbreaks), EU/EFTA, 2017
| Country | ECDC | EFSA | ||||
|---|---|---|---|---|---|---|
| Confirmed human cases | Food‐borne outbreaks | |||||
| Total | Travel related | Domestic | Unknown or missing | Human cases (illnesses) | FBO | |
| N | N | N | N | N | N | |
| Austria | 250 | 22 | 227 | 1 | 21 | 5 |
| Belgium | 123 | 4 | 54 | 65 | 10 | 2 |
| Bulgaria | 0 | –* | – | 0 | –** | – |
| Croatia | 7 | 0 | 2 | 5 | – | – |
| Cyprus | 0 | 0 | 0 | 0 | – | – |
| Czech Republic | 37 | 3 | 34 | 0 | – | – |
| Denmark | 263 | 55 | 182 | 26 | 24 | 4 |
| Estonia | 3 | 0 | 3 | 0 | – | – |
| Finland | 123 | 48 | 59 | 16 | 3 | 1 |
| France | 260 | – | – | 260 | 54 | 1 |
| Germany | 2,065 | 206 | 1,859 | 0 | 49 | 12 |
| Greece | 3 | 0 | 3 | 0 | – | – |
| Hungary | 12 | 0 | 12 | 0 | – | – |
| Ireland | 795 | 36 | 699 | 60 | 31 | 12 |
| Italy | 94 | 0 | 93 | 1 | 3 | 1 |
| Latvia | 1 | 0 | 1 | 0 | – | – |
| Lithuania | 0 | 0 | 0 | 0 | – | – |
| Luxembourg | 1 | – | – | 1 | – | – |
| Malta | 9 | 1 | 8 | 0 | – | – |
| Netherlands | 392 | 84 | 291 | 17 | – | – |
| Poland | 4 | 0 | 4 | 0 | – | – |
| Portugal | 1 | 0 | 1 | 0 | – | – |
| Romania | 11 | 0 | 11 | 0 | – | – |
| Slovakia | 3 | 0 | 3 | 0 | – | – |
| Slovenia | 33 | 2 | 2 | 29 | – | – |
| Spain | 86 | 3 | 66 | 17 | 4 | 2 |
| Sweden | 504 | 191 | 296 | 17 | 2 | 1 |
| United Kingdom | 993 | 232 | 523 | 238 | 59 | 7 |
| EU Total | 6,073 | 887 | 4,433 | 753 | 260 | 48 |
| Iceland | 3 | 0 | 1 | 2 | – | – |
| Norway | 381 | 99 | 224 | 58 | 10 | 3 |
| Switzerland | 696 | – | – | 696 | – | – |
No importation data reported.
No food‐borne outbreaks caused by STEC (including waterborne outbreaks) reported.
Nine FBOs (notified by seven MS) of the 48 STEC FBOs (notified by 11 MS) were reported with strong‐evidence on the incriminated food vehicle. An overview of these implicated foodstuffs is in Table 30. Further details and statistics on the STEC FBOs reported for 2017 are in Section 16 on FBO.
Table 30.
Distribution of strong‐evidence outbreaks caused by STEC (excluding strong‐evidence waterborne outbreaks), by food vehicle, EU, 2017
| Food vehicle | Number of strong‐evidence FBO | % of total |
|---|---|---|
| Bovine meat and their products | 4 | 44.4 |
| Cheese | 1 | 11.1 |
| Dairy products (other than cheeses) | 1 | 11.1 |
| Meat and meat products | 1 | 11.1 |
| Milk | 2 | 22.2 |
| Total | 9 | 100.0 |
Note: Data from nine outbreaks are included: Belgium (1), Finland (1), Germany (2), Italy (1), Spain (1), Sweden (1) and United Kingdom (2).
4.4.3. STEC in food
Data for STEC on sprouted seeds according to food safety criteria laid down in Regulation No. 2073/2005
In 2017, 12 MS reported STEC monitoring data of sprouted seeds at retail level, for 786 units tested with no positive samples. These figures increased to 14 MS and 984 samples tested when sampling at processing plant and farm were included. This amount of data is far above the average compared with the previous years, either considering the number of units tested or the number of MS reporting these data (Table 31).
Table 31.
STEC sprouted seeds monitoring results at retail, reporting Member States, EU, 2013–2017
| Sprouted seeds | Number of reporting MS | Sample units tested | Sample units positive (%) |
|---|---|---|---|
| 2013 | 6 | 444 | 0 (0.0%) |
| 2014 | 6 | 481 | 0 (0.0%) |
| 2015 | 7 | 576 | 1 (0.2%) |
| 2016 | 8 | 344 | 1 (0.3%) |
| 2017 | 12 | 786 | 0 (0.0%) |
For the year 2017, four non‐compliant batches were reported by one MS in official samples taken at the processing plant. No information on the serogroup, the Shiga toxin type or the presence of the eae gene was provided for the isolated strains.
Out of the total number of samples tested in 2017, 98 official single samples taken both at retail and at processing by the CA of six MS as part of official controls based on Regulation 2073/2005 have been reported, with no positive results. This represents an area of improvement that requires attention to guarantee a wider coverage of the prescriptions of the EU Regulation 2073/2005 on this food matrix. Other 123 samples have been assayed by the FBOp during own checks and HACCP plan testing without recording any positivity for STEC.
Occurrence in food
Meat and meat products
Fresh bovine meat
In 2017, 4,879 units of fresh bovine meat were tested for STEC by 13 MS with 1.0% of them being positive (0.08% for STEC O157). More than half of the reported data were from one MS (Ireland). The proportion of positive units was very low at the processing plant level (0.3%, n = 1,807), and was higher at slaughterhouse (1.0%, n = 2,148) and at retail (2.4%, n = 909). The highest proportion of positive samples has been recorded at the border inspection level, with 6.7% of samples positive for STEC. It has to be noted, however, that only 15 units of fresh bovine meat were tested and reported at the border inspection level and that only one unit tested positive. Only four single samples from the slaughterhouse‐level, reported by Belgium (three samples) and Portugal (one sample), were STEC O157‐positive.
Ground beef is considered an epidemiologically relevant matrix for STEC infections given the production process it undergoes and the high probability of contamination. The process of grinding allows possible superficial contamination of meat within the preparation and the resulting processed food (as an example the hamburgers or patties) need to meet minimal requirements for the cooking step e.g. measuring the temperature at the core of the product for a safe consumption. Eight MS specifically reported on the testing of 764 samples of minced meat from bovine origin in 2017 with nine STEC‐positive records, of which four were STEC O157.
Information on the serogroup was provided for 49 STEC strains isolated from any type of bovine meat. The serogroups most frequently reported in this food commodity were O157 (10 isolates), O103 (6 isolates), O55 and O26 (3 isolates each), O146 and O113 (2 isolates), O145 (1 isolate) and others. Most of the serogroups identified in this food category are also isolated from cases of human disease confirming the importance of this food category in the epidemiology of STEC infections. Of these 49 STEC, 43 were provided with information on the virulence genes asset. In particular, 26 strains possessed the genes encoding the Stx2 and 7 displayed the virulotype stx2+; eae+.
Fresh ovine and goat meat
Five MS reported the results of investigation on 513 sample units of fresh ovine meat tested for STEC with 5.3% of them being positive. Two MS reported on fresh goat meat with no STEC‐positive samples out of the 13 sample units tested.
The analysis of the serogroups, carried out including all the types of ovine meat, indicated that the most frequently isolated STEC strains belonged to the O157, O146 and O38 serogroups (4 isolates each, 14.3% of the 28 isolates with information on the serogroup), followed by O5 (3 strains reported) and O103 (2). Half of the total 16 STEC serogroups identified in fresh ovine and goat meat samples, are included in the list of the 20 most frequent serogroups reported in confirmed cases of human STEC infections in EU/EEA, 2014–2016 (EFSA and ECDC, 2017a,b,c). Of the 28 STEC with information on the serogroup identified when all the data set was used, 15 were positive for the presence of the genes encoding the Stx2, with 5 of them also possessing the eae gene.
Fresh meat from other ruminants
One MS provided information on the presence of STEC in 51 fresh meat samples from deer. Twelve proved positive for non‐O157 STEC. When the entire data set was used for the analyses, 23 STEC‐positive units were reported from 93 samples assayed. All the isolates were non‐O157 serogroups with O146 and O153 being the most represented (2 isolates each). For 15 STEC isolates information on the virulence genes was available, with all of them being positive for stx2 gene and negative for the eae.
Fresh meat from other animal species
Five MS provided information on 164 samples of fresh pig meat tested and five samples (3.0%) were positive for the presence of STEC. No STEC O157 has been isolated in 2017.
Four MS reported on the analyses carried out on 211 samples of food from animal species other than bovine, ovine, goat, pigs and deer. These included samples taken from horses, rabbit, wild boars and unspecified meat. Six samples were STEC positive (2.8%) and all the isolated strains belonged to non‐O157 serogroups. When the entire data set was considered, for this type of meat 1,580 samples were reported with 27 of them positive for STEC. Information on the serogroup of the isolated STEC was provided for 11 strains. These included serogroups O157 and O146 (3 isolates each), O103 (1 isolate) and others. Two of the most represented O‐groups are part of the ‘top‐five’ STEC serogroups, associated with severe disease in humans. Nine STEC isolates were reported with their virulence genes profiles, six were stx2+, two were stx1+ and one stx1+; stx2+, all negative for the presence of the eae gene.
Data on the presence of STEC in meat from broilers and turkeys have been reported by four MS. In total, 53 samples from turkey meat and 249 from broilers were tested with only one STEC O157 reported in fresh meat from turkey.
Meat products and meat preparations from mixed sources
Seven MS reported in 2017 the results of testing of 256 samples of meat preparations and meat products from mixed sources. Seven samples were positive for the presence of STEC non‐O157. The analysis of the entire data set showed that STEC were isolated from 13 out of 588 total samples assayed. The information on the serogroup was provided for four STEC strains only, with two of them being STEC O157. The remaining two isolates belonged to serogroups O54 and O103. Eleven STEC isolates were reported with the information on the stx and adhesion genes. In particular, eight were stx2+; eae‐, two were stx1+; eae+ and one was stx1+; eae‐.
Milk and milk products
In 2017, eight MS reported monitoring results of 498 sample units of raw cow milk with six positive units, all belonging to non‐O157 serogroups. The detected STEC serogroups in raw cow milk samples, considering the full data set (without applying any exclusion criteria), were O103, O146 and O157 (one isolate each). The information on the serogroup was not reported for other eight isolates present in the entire data set, while for other two isolates the only reported information was that they belonged to non‐O157 serogroups. Three isolates expressed the stx2 gene and three the stx1 and stx2 genes, in one case together with the eae gene. Finally, one STEC strain with the virulotype stx1+; eae+ was reported.
For raw milk from goat and sheep, four MS reported monitoring results of 38 sample units of raw goat milk, while one MS reported only one sample of raw sheep milk. The isolation of one non‐O157 STEC was reported from one sample of raw goat milk.
In the entire data set, one MS reported the presence of STEC in 2.5% of 394 samples tested of raw milk from other unspecified animal species. The serogroup and the virulence genes asset of the isolates were not specified.
In total, 2,410 units of ‘milk and dairy products excluding raw milk’ were assayed by seven MS in 2017. About half of the samples were from cheeses (64.9%) followed by treated or fermented milk (29.0%) and other ‘dairy products other than cheese’ (6.0%). In total 49 sample units were positive for STEC. The highest proportion of positive units was reported in treated milk samples (4.0%) followed by cheeses (1.3%). None of the samples of dairy products were positive for STEC O157. The non‐O157 STEC serogroups identified were O111, O113, O126 (1 isolate each). The virulence gene profiles of these isolates were reported as follows: nine were stx1+, in two cases together with the eae gene and six were stx1+and stx2+ with the eae gene in two cases. Finally, three Stx2‐producing strains were reported of which two were also eae‐positive.
Vegetables
Fifteen MS reported data on the testing of 1,803 sample units of vegetables for the presence of STEC. Seven samples were positive, all for STEC non‐O157. From these seven positive units, one STEC O45 and one STEC O63 have been isolated. Both isolates were positive for the presence of eae gene and also harboured the genes encoding the Stx2, subtype f. This particular variant was not considered as being pathogenic until recently. As a matter of fact, it has been reported as a leading cause of diarrhoea in the Netherlands (Friesema et al., 2014) and has also been isolated from some HUS cases (Grande et al., 2016). For the remaining five isolates the information on the serogroup and virulence gene was not provided.
Fruits
No STEC‐positive units were detected by six MS who reported information on fruit samples in 2017.
Other foodstuffs
This category contains miscellaneous food commodities, which included cereals and meals, bakery products, non‐alcoholic beverages, juices, live bivalve molluscs, eggs, fish and fishery products, RTE salads, sauces and dressing, dried seeds and fresh and dried spices and herbs, infant formula and foodstuffs intended for special nutritional uses, chocolate, coconuts, mushrooms and others.
For the whole category, 1,665 samples were analysed by 13 MS with seven positive samples reported by five MS. The serogroups identified were one STEC O8 and one STEC O78, representing the only two isolates belonging to these serogroups. One STEC possessed the stx2 gene. Two were stx1+ stx2+ and one was stx1+. All the strains were negative for the presence of the eae gene or this information was missing.
4.4.4. STEC in animals
Overall, the presence of STEC was reported in 10.6% out of the 2,310 sample units from animals (animals or herds or flocks) tested in 2017, considering the entire data set without applying any exclusion criteria.
As observed in the previous years, high proportions of STEC‐positive sample units have been reported in deer with 20.1% positive samples. This animal category was followed by the ‘other animals’ group (17.2%), cattle (8.3%) goat and sheep (2.9%). Half of STEC‐positive units were reported from samples belonging to the animal category ‘pigs’ for which only 10 samples were reported by one MS in 2017.
The most relevant results on the animal categories are detailed below.
Cattle
Six MS reported 1,680 sample units of cattle tested for the presence of STEC. In total, 137 samples (8.1%) were positive for STEC and 4.0% of the total samples tested were positive for STEC O157. Interestingly, 40 out of the 68 STEC O157 positive samples were reported by one MS, which declared to have used the ISO TS 13136:2012 method aiming at detecting any STEC present in the sample.
When the analyses on the serogroups were carried out considering the entire data set with no restrictions on the sampling context or the methods used, three additional STEC O157 were identified for 140 positive samples. The analysis of serogroups returned a figure of 46 non‐O157 STEC strains out of the 117 with serogroup information reported. These included O103, O26, O113, O121 and O91 among others, all serogroups involved in human cases of infections. Forty‐five non‐O157 strains were also provided with the information on the virulence genes. Twenty‐one strains harboured the stx1 gene, with six of them also positive for the eae gene. Five out the 20 stx2+ isolates also had the eae gene. Finally, four STEC displayed the stx1+; stx2+ toxin genes profile, in one case together with the eae gene.
Sheep and goats
Two MS reported on the analysis of 50 samples of goats and 11 of sheep taken at the farm with two positive results, all from goats, in one MS.
By analysing the data regardless their sampling context or the methods used for the tests, 68 samples from sheep and goats were reported from the same two MS. The two positive samples yielded one STEC O26 and one STEC of unspecified serogroup. No information on the virulence genes of these isolates was provided.
Pigs and other animal species
Pigs were tested by one MS (Italy) that reported three positive results from the eight samples assayed, all belonging to non‐specified serogroups. When the entire data set was analysed, two supplementary samples were reported (10 samples in total) together with the isolation of two additional STEC O157.
In 2017, two MS reported on the presence of STEC in birds, Cantabrian chamois, cats, chinchillas, deer, dogs, ferrets, Gallus gallus, gerbils, hedgehogs, monkeys, rabbits, solipeds, water buffalos, wild boars and wolves. As a whole 526 samples have been analysed with 95 (18.1%) of them positive for STEC, of which four were O157. The analysis of the STEC serogroups, conducted using the entire data set, indicated that out of the 95 STEC isolates, information on the serogroup was provided only for seven strains. In particular, besides the four STEC O157, two belonged to O145 serogroup and one was a STEC O26. Interestingly, all the STEC with the information on the serogroup, with the exception of two O157 from rabbits, were isolated from dogs. The remaining 88 STEC reported were isolated in one MS from water buffalos, deer and Cantabrian chamois. No information on the virulence genes was provided for the STEC isolated from this animal category.
4.4.5. Serogroups in humans, food and animals
Humans
Data on STEC serogroups (based on O antigen) were reported in 2017 by 25 MS, Iceland and Norway. As in previous years, the most commonly reported serogroup was O157 accounting for 31.9% of the cases in humans with known serogroup, although it has been steadily decreasing since 2012. The proportion of the second most common serogroup O26 also decreased in 2017, these two serogroups, however represented almost half (46.2%) of the total number of confirmed human cases with known serogroups in 2017 (Table 32). Serogroup O157 and O26 were followed by serogroup O103, O91, O145, O146 and O111. A new serogroup O76 was added and three serogroups (O5, O182 and O27) were dropped from the top 20 list in 2017. Serogroups other than O157, increased by 35.8%, whereas the proportion of O157 decreased by 16% in 2 years from 2015 to 2017. The proportion of untypeable STEC strains increased in 2017 to the highest level since 2012 representing 12.1% of the reported cases with known serogroup.
Table 32.
Distribution of the 20 most frequent serogroups reported in confirmed cases of human STEC infections in EU/EEA, 2015–2017
| Serogroup | 2017 | 2016 | 2015 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | MS | % | Cases | MS | % | Cases | MS | % | |
| O157 | 1,304 | 24 | 31.9 | 1,552 | 22 | 38.6 | 1,510 | 21 | 42.1 |
| O26 | 582 | 18 | 14.3 | 671 | 19 | 16.7 | 537 | 16 | 15.0 |
| NTa | 493 | 11 | 12.1 | 335 | 12 | 8.3 | 397 | 10 | 11.1 |
| O103 | 245 | 14 | 6.0 | 218 | 18 | 5.4 | 172 | 14 | 4.8 |
| O91 | 179 | 14 | 4.4 | 149 | 11 | 4.0 | 114 | 12 | 3.2 |
| O145 | 150 | 14 | 3.7 | 121 | 12 | 3.7 | 95 | 12 | 2.6 |
| O146 | 140 | 10 | 3.4 | 158 | 11 | 3.0 | 75 | 10 | 2.1 |
| O111 | 94 | 18 | 2.3 | 57 | 14 | 1.6 | 42 | 11 | 1.2 |
| O113 | 56 | 8 | 1.4 | 60 | 11 | 1.5 | 25 | 7 | 0.7 |
| NON‐O157 | 48 | 4 | 1.2 | 25 | 5 | 1.4 | 29 | 3 | 0.8 |
| O128 | 46 | 12 | 1.1 | 65 | 13 | 1.0 | 49 | 12 | 1.4 |
| O80 | 42 | 7 | 1.0 | 42 | 8 | 0.8 | 24 | 4 | 0.7 |
| O‐roughb | 37 | 3 | 0.9 | 26 | 4 | 0.7 | 44 | 8 | 1.2 |
| O128ab | 33 | 2 | 0.8 | 9 | 1 | 0.7 | 2 | 6 | 0.1 |
| O76 | 31 | 7 | 0.8 | 20 | 6 | 0.6 | 31 | 9 | 0.9 |
| O121 | 30 | 7 | 0.7 | 24 | 5 | 0.6 | 17 | 4 | 0.5 |
| O55 | 30 | 9 | 0.7 | 34 | 10 | 0.6 | 28 | 8 | 0.8 |
| O63 | 30 | 6 | 0.7 | 24 | 4 | 0.6 | 8 | 4 | 0.2 |
| O117 | 29 | 4 | 0.7 | 28 | 7 | 0.6 | 23 | 7 | 0.6 |
| O8 | 28 | 7 | 0.7 | 25 | 10 | 0.5 | 20 | 9 | 0.6 |
| Other | 455 | – | 11.1 | 369 | – | 7.8 | 348 | – | 9.7 |
| Total | 4,082 | 25 | 100.0 | 4,012 | 25 | 100.0 | 3,590 | 21 | 100.0 |
Untypeable STEC include those strains where the laboratory tried, but was not able to define the O‐serogroup. This depends on how many sera/molecular tools are included in the typing panel.
O‐rough strains lack the O‐chains in the lipopolysaccharide, leading to autoagglutination in the agglutination tests used to determine serogroup or serotype.
Source: 25 MS and two non‐MS: Austria, Belgium, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and United Kingdom, and Iceland and Norway.
Food
The proportion of food samples positive for the so‐called top‐five STEC serogroups, being O157, O26, O103, O111 and O145, was estimated by considering only the reported STEC monitoring results obtained using the analytical method ISO TS 13136:2012 (ISO 2012). As a matter of fact, the scope of this standard is to detect any STEC, and additionally, it allows identifying the ‘top 5’ serogroups. This subset of data can so be considered homogeneous and may facilitate a more comparable estimation of the level of contamination of the different food categories with these STEC serogroups. In the previous years, an increasing trend in the adoption of this standard by the MS for food testing was observed, with a proportion of food samples tested using the ISO TS 13136:2012 standard (ISO 2012) in 2016 of 91.5% (EFSA and ECDC, 2017a,b,c). In 2017, this figure increased to 97.4%. The remaining 2.6% of the assays have been carried out using methods targeting STEC O157 only.
In 2017, 23 MS provided data on the detection of STEC in food obtained using the method ISO TS 13136:2012 (ISO 2012) on 21,011 out of the total 21,574 samples analysed. Four hundred samples resulted positive for the presence of STEC (1.9%) (Table 33). The STEC belonging to the top‐five serogroups accounted for 10.7% of the whole population of the STEC isolated from food (43 out of the 400 isolates reported).
Table 33.
Proportion of positive samples for any STEC and STEC belonging to the ‘top‐five’ serogroups in food categories, in reporting Member States, 2017a
| Food categoryb | Samples tested by ISO TS 13136c | Samples positive for | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any STEC | O157 | O26 | O145 | O103 | O111 | ||||||||
| nd | % | n | % | n | % | n | % | n | % | n | % | ||
| Bovine meat | 8,059 | 134 | 1.7 | 10 | 0.1 | 2 | 0.0 | 1 | 0.0 | 6 | 0.1 | 0 | 0.0 |
| Ovine and goat meat | 579 | 39 | 6.7 | 4 | 0.7 | 0 | 0.0 | 0 | 0.0 | 2 | 0.3 | 0 | 0.0 |
| Other ruminants meate | 93 | 23 | 24.7 | 0 | 0.0 | 1 | 1.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Pig meat | 1,363 | 60 | 4.4 | 5 | 0.4 | 1 | 0.1 | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 |
| Other meatf | 1,466 | 27 | 1.8 | 3 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 | 0 | 0.0 |
| Mixed meat | 587 | 13 | 2.2 | 2 | 0.3 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 |
| Milk and dairy productsg | 2,322 | 57 | 2.5 | 1 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.0 |
| Raw milkh | 1,094 | 23 | 2.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 | 0 | 0.0 |
| Fruit and vegetable | 2,280 | 7 | 0.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Seedsi | 1,565 | 10 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Other food | 1,603 | 7 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total | 21,011 | 400 | 1.9 | 25 | 0.1 | 4 | 0.0 | 2 | 0.0 | 11 | 0.1 | 1 | 0.0 |
STEC: Shiga toxin‐producing Escherichia coli.
The different meat categories presented in this table include all type of meat (not only fresh).
Only samples tested by the ISO TS 13136 method are included.
n: number of samples.
Includes meat from deer.
Includes meat from other animals (other than ruminants).
Includes any type of dairy product, cheese and milk other than raw milk.
Includes raw milk from different species, but most of the tested and all the positive samples were from cows.
Includes only sprouted seeds.
The proportions of the top‐five serogroups reported in food in 2017 were: O157 (0.12% of 21,011 samples tested and 6.2% of the positive samples), O103 (0.05% of 21,011 samples tested and 2.7% of the positive samples), O26 (0.02% of 21,011 samples tested), O111 and O145 (< 0.01%).
The relative frequency of all the STEC serogroups identified in the reported food sample units for 2017 was estimated by considering all the reported results regardless the specified analytical method. Overall, 401 STEC isolates were obtained from the 21,574 samples analysed (1.9%). For 48 isolates, the only information reported was that the strain did not belong to O157 serogroup, while for 234 no information on the serogroup was provided. The STEC isolated from the remaining 119 positive samples were serotyped and the related information was reported. These included 26 STEC O157, mainly isolated from bovine meat (10 isolates), pig meat (5 strains) ovine and goat meat (4 strains), other meat (3), mixed meat (2) milk and dairy products including raw milk (2 isolates).
As for the 93 STEC non‐O157 detected in 2017 (Table 34), the main serogroup identified was O146 (3.0% of the total 401 STEC isolates, 10% of the 119 strains with an identified serogroup). This STEC serogroup was mainly detected in meat samples of different origin and from raw milk. STEC O103 was the third serogroup reported (2.7% of the total 401 STEC isolates, 9.2% of the 119 strains with an identified serogroup) and was identified in samples of different origin, mainly bovine meat and raw milk. Other STEC serogroups identified included O26 (3.3% of the 119 strains with an identified serogroup), O5 (3.3%), O113 (2.5%), O145 (1.7%) and O111 (0.8%). These STEC serogroups are all among the 15 most commonly reported in human infections in the EU in the period 2014–2016 (EFSA and ECDC, 2017a,b,c).
Table 34.
Frequency distribution of non‐O157 STEC serogroups in food categories in reporting Member States, 2017a
| Food categoryb | STEC isolates with serogroup reported | STEC serogroups | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of total STEC isolates with serogroup reported in the specific food category | ||||||||||||||||
| nc | O26 | O103 | O145 | O111 | O146 | O38 | O76 | O113 | O5 | O174 | O8 | O116 | O6 | Other serogroups (list) | ||
| Bovine meat | 39 | 5.1 | 15.4 | 2.6 | 0.0 | 5.1 | 0.0 | 2.6 | 5.1 | 2.6 | 5.1 | 0.0 | 2.6 | 0.0 | 53.8 | (O126, O136, O139, O15, O168, O171, O187, O2, O23, O3, O55, O88, O98) |
| Ovine and goat meat | 24 | 0.0 | 8.3 | 0.0 | 0.0 | 16.7 | 16.7 | 4.2 | 0.0 | 12.5 | 0.0 | 4.2 | 0.0 | 4.2 | 33.3 | (O128, O15, O166, O176, O181, O187, O21, O9) |
| Other ruminants meatd | 8 | 12.5 | 0.0 | 0.0 | 0.0 | 25.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 12.5 | 50.0 | (O142, O148, O153) |
| Pig meat | 3 | 33.3 | 0.0 | 33.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 33.3 | 0.0 | 0.0 | 0.0 | |
| Other meate | 8 | 0.0 | 12.5 | 0.0 | 0.0 | 37.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 50.0 | (O18, O19, O54) |
| Mixed meat | 2 | 0.0 | 50.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 50.0 | (O54) |
| Milk and dairy productsf | 3 | 0.0 | 0.0 | 0.0 | 33.3 | 0.0 | 0.0 | 0.0 | 33.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 33.3 | (O126) |
| Raw milkg | 2 | 0.0 | 50.0 | 0.0 | 0.0 | 50.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Fruit and vegetable | 2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | (O45, O63) |
| Seedsh | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Other food | 2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 50.0 | 0.0 | 0.0 | 50.0 | (O78) |
| Total | 93 | 4.3 | 11.8 | 2.2 | 1.1 | 12.9 | 4.3 | 2.2 | 3.2 | 4.3 | 2.2 | 3.2 | 1.1 | 2.2 | 45.2 | (O126, O128, O136, O139, O142, O148, O15, O153, O166, O168, O171, O176, O18, O181, O187, O19, O2, O21, O23, O3, O45, O54, O55, O63, O78, O88, O9, O98) |
Note: Data originating from any analytical method are included.
STEC: Shiga toxin‐producing Escherichia coli. Non‐O157 STEC serogroups are listed according to their public health relevance as a cause of human infections in the EU (EFSA, 2009b).
The different meat categories presented in this table include all types of meat (not only fresh).
n: number of samples.
Includes meat from deer.
Includes meat from animals other than ruminants and pigs.
Includes any type of dairy product, cheese and milk other than raw milk.
Includes raw milk from different species, but most of tested samples and all the positive samples were from cows.
Includes sprouted seeds and dried seeds.
In 2017, the decrease in the reporting of STEC O157 over the previous years was confirmed with a stabilised proportion of STEC belonging to this serogroup on the total number of samples analysed of 1.2‰. This scenario is correlated with the wide adoption of the ISO TS 13136:2012 analytical method, which aims at detecting any STEC in contrast to the use of those specific for STEC O157 serogroup, commonly used in the previous years.
As a whole, the 119 STEC serotyped isolates reported belonged to 42 serogroups, of which 12 are included in the top 20 STEC serogroups causing human disease in the 2014–2016 period (EFSA and ECDC, 2016a, b, 2017b), confirming the importance of food sources as vehicles of STEC infections.
Only less than one‐third of the STEC isolated from food in 2017 have been provided with information on the serogroup and this figure equals that of the previous years. This situation reflects the current methodological limitation of the necessity to assess each single serogroup individually, strengthening the importance of more holistic approaches such as the WGS for an extensive characterisation of the isolates.
Only 180 out of the 401 STEC strains isolated were characterised with information on the genes encoding the Shiga toxins and the accessory adhesion gene eae (virulotype). In particular, 90 of the characterised strains (50%) carried the genes encoding the Stx2, 13 of which were also positive for the presence of the gene eae. As a whole, 7.2% of the virulotyped STEC strains displayed the virulence genes profile (stx2+; eae+) associated with the STEC strains causing HUS. Additionally, eight more strains presented a virulotype stx1+; stx2+; eae+, also associated with the isolates causing severe disease in humans.
The analysis of the virulence genes content of the STEC strains represents the basis for the molecular risk assessment and is the most valuable tool to carry out a deep analysis of the STEC circulating in the possible food vehicles for human infections and the related inference on their impact on public health. Therefore, the reporting of this information should be encouraged.
Animals
In total, 244 positive samples out of the 2,310 tested were reported, with information on serogroup for 127 isolates. Seventy‐seven STEC O157 (31.6% of the total number of STEC‐positive samples) were detected, with 71 of them from cattle and the remaining reported in dogs, pigs and rabbits.
As regards the non‐O157 serogroups, the most reported ones were O136 (2.9% of the total number of STEC‐positive samples and 14% of the non‐O157 STEC with information on the serogroup) followed by O26 (2.5% of the total number of STEC‐positive samples and 12% of the non‐O157 STEC with information on the serogroup) O116 and O168 (both 1.2% of the total number of STEC‐positive samples and 6% of the non‐O157 STEC with information on the serogroup) (Table 35). Other 17 STEC serogroups have been reported in animal samples, all below 1.0% of the total number of STEC‐positive samples. The latter included O103, O121, O113, O145, O91 and O8, all serogroups that have been implicated in human infection with STEC.
Table 35.
Frequency distribution of non‐O157 STEC serogroups in animals in reporting Member States, 2017a
| Animal category | STEC isolates with serogroup reported | STEC serogroups (g) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of total STEC isolates with serogroup reported in the specific animal category | ||||||||||||||||
| nb | O26 | O103 | O145 | O112 | O136 | O91 | O121 | O113 | O168 | O15 | O150 | O182 | O116 | Other serogroups (list) | ||
| Cattle | 46 | 8.7 | 4.3 | 0.0 | 2.2 | 15.2 | 4.3 | 4.3 | 4.3 | 6.5 | 4.3 | 4.3 | 10.9 | 6.5 | 23.9 | (O117, O171, O177, O187, O3, O8, O9, O93) |
| Goat and sheep | 1 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0 |
| Other ruminantsc | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0 |
| Pigs | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0 |
| Other animalsd | 3 | 33.3 | 0.0 | 66.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0 |
| Total | 50 | 12.0 | 4.0 | 4.0 | 2.0 | 14.0 | 4.0 | 4.0 | 4.0 | 6.0 | 4.0 | 4.0 | 10.0 | 6.0 | 22.0 | (O117, O171, O177, O187, O3, O8, O9, O93) |
Note: Data originating from any analytical method are included.
STEC: Shiga toxin‐producing Escherichia coli. Non‐O157 STEC serogroups are listed according to their occurrence in the animal samples tested.
n: number of samples.
Includes deer and Cantabrian chamois.
Includes birds, cats, chinchillas, dogs, ferrets, gallus, gerbils, hedgehogs, monkeys, rabbits, solipeds, water buffalos, wild boar and wolves.
Atlases of STEC serogroups: food and animals
All data provided by the reporting countries were used to generate an analysis of the STEC serogroups’ frequencies in the different food and animal categories for the period 2012–2017 (Figure 32) as well as for the year 2017 separately for food (Figure 33) and animals (Figure 34). The relative presence of STEC serogroups reported in 2017 in food and animals by reporting country is presented in Figure 35. It has to be emphasised that the differences in the sampling strategies, and to a lesser extent the analytical methods, applied by reporting countries do not allow confirmation of the existence of specific trends in the geographical distribution of STEC serogroups.
Figure 32.
- Note: The presence (red boxes) and absence (white boxes) of STEC serogroups in food (left) and animals (right). The E. coli O104:H4 stx2+ eae‐ was isolated from sprouted seeds in 2015. No information was provided on the H type and genotype of the E. coli O104 strains isolated from food in 2012.
Figure 33.
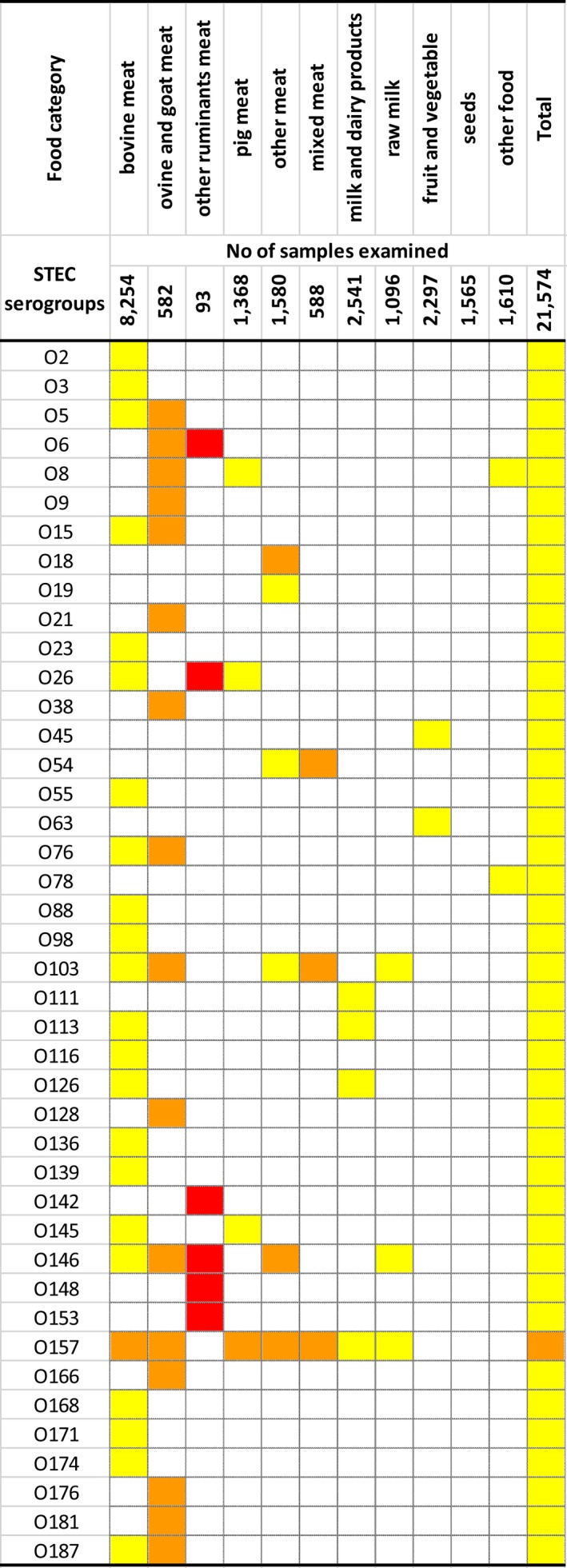
- Proportions of STEC serogroups: red boxes > 1%, orange boxes > 0.1% and ≤ 1%, yellow boxes > 0.0001% and ≤ 0.1% of positive samples. White boxes indicate absence of the serogroup.
- Other ruminants’ meat includes meat from deer.
- Other meat includes meat from animals other than ruminants.
- Milk and dairy products include any type of dairy product, cheese and milk other than raw milk.
- Raw milk includes raw milk from different species, but most of the tested and all the positive samples were from cows.
- Seeds category includes mostly sprouted seeds, but dry seeds are also included.
- Sources: 25 Member States.
Figure 34.
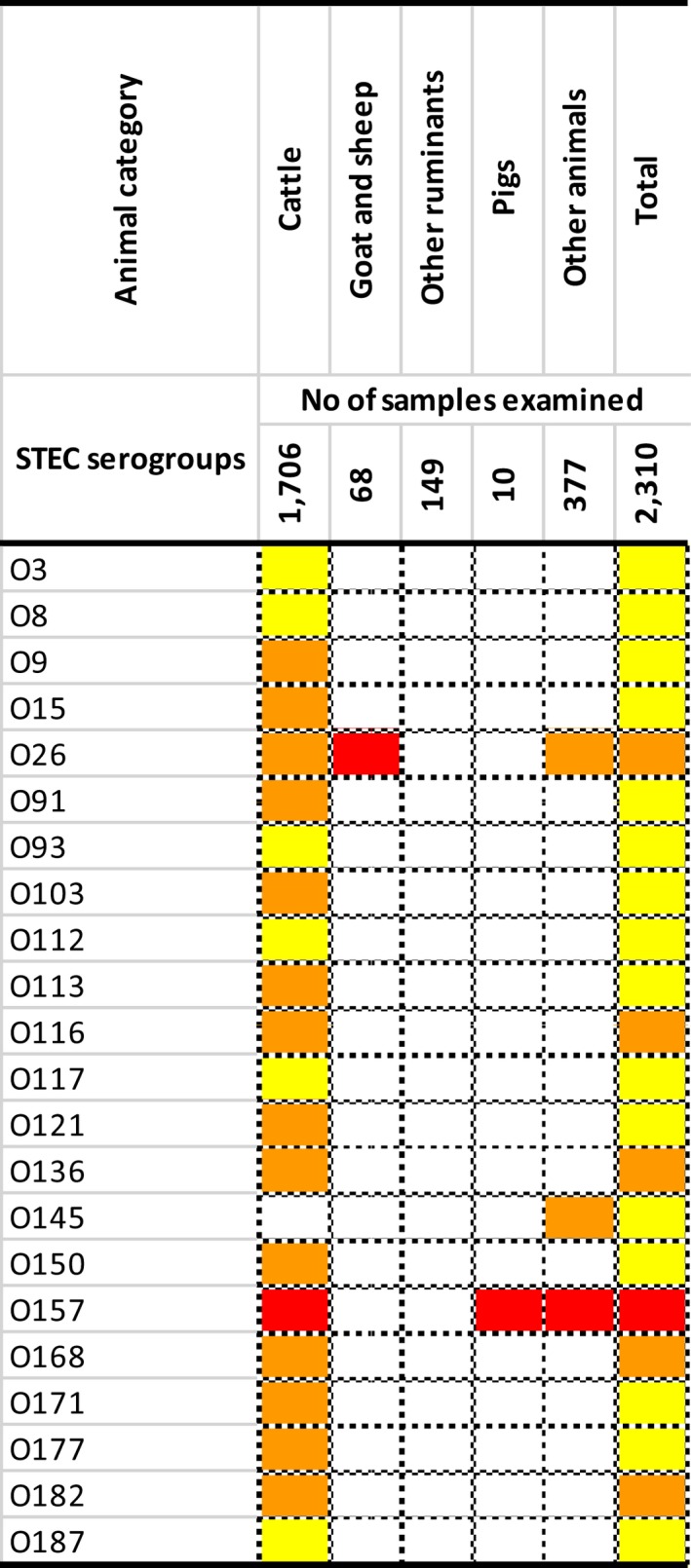
- Proportions of STEC serogroups: red boxes > 1%, orange boxes > 0.1% and ≤ 1%, yellow boxes > 0.0001% and ≤ 0.1% of positive samples. White boxes indicate absence of the serogroup.
- The animal category ‘other ruminants’ includes deer. The ‘other animal’ category comprises birds, Cantabrian chamois, cats, chinchillas, dogs, ferrets, gallus, gerbils, hedgehogs, monkeys, rabbits, solipeds, water buffalos, wild boar and wolves.
- Sources: Eight Member States.
Figure 35.
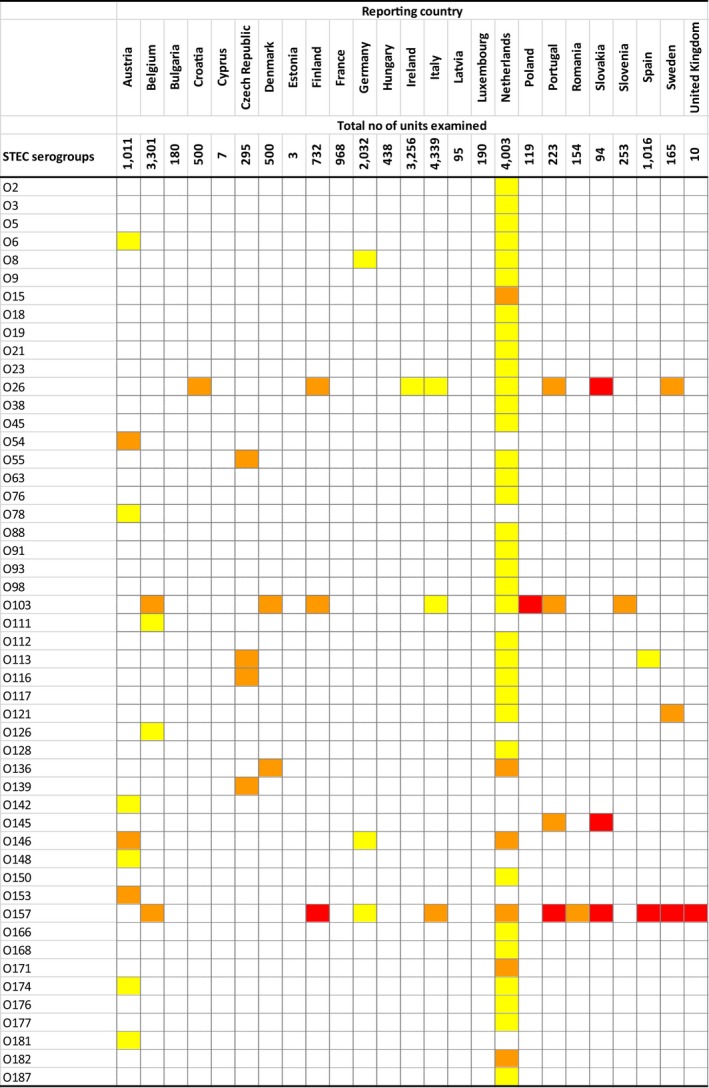
- Proportions of STEC serogroups: red boxes > 1%, orange boxes > 0.1% and ≤ 1%, yellow boxes > 0.0001% and ≤ 0.1% of positive samples. White boxes indicate absence of the serogroup.
- Includes data from both animals and food samples.
4.5. Discussion
STEC was the fourth most commonly reported zoonosis in the EU in 2017, and the trend for human STEC infections increased from 2008 to 2017, which was mainly due to the large STEC outbreak in 2011. The overall trend of reported cases increased immediately after the outbreak, but has remained stable 2012–2017. The notification rate stayed at a markedly higher level after the outbreak than before the outbreak. Part of the increase may be explained by improved general awareness of STEC detection following the reported STEC outbreak. Other contributing factors probably are the changes in laboratory techniques such as using molecular‐based assays including multiplex PCR and direct DNA extraction from a specimen to detect STEC presence.
Of the STEC cases with known hospitalisation status, more than one‐third was hospitalised. Some countries reported very high proportions of hospitalised cases, but had notification rates that were among the lowest, indicating that the surveillance systems in these countries primarily capture the most severe cases. The age group most affected by STEC were infants and children up to 4 years of age, who accounted for two‐thirds of the cases of HUS. As in previous years, the most commonly reported serogroup in human cases was O157, followed by O26. The proportion of serogroup O157 continued to decrease, whereas the proportion of non‐O157 STEC serogroups increased. An increasing number of laboratories tested for serogroups other than O157 and there has been a shift in diagnostic methods, with PCR being more commonly used for detection of STEC cases in several Member States.
In 2017, data on the presence of STEC in food and animals were reported by 25 MS. This represents an improvement over the last years indicating an increased awareness of the necessity to monitor the pathogens in food with the highest priority, as laid down in EU Directive 2003/99/EC.
In spite of the large proportion of MS (89.3%) reporting monitoring activities on food, the distribution of these varies when the different food categories are considered, from four MS reporting data in meat from other ruminants up to 18 MS testing bovine meat. This observation should be not neglected and the testing and reporting on at least the more epidemiologically relevant food commodities should be improved. This would be necessary to obtain data suitable for making inference on the existence of specific trends in the geographical distribution of STEC and their serogroups.
As already observed in the previous years, the amount of data on animal samples tested continued to decrease in 2017, configuring a negative trend although the number of reporting countries remained approximately stable (EFSA and ECDC, 2015a,b, 2016a,b, 2017a,b,c).
The analysis of the use of the ISO TS 13136:2012 to test food confirmed the wide adoption of the standard method at the EU level. In 2017, 97.4% of the food samples were tested using this method, bringing this figure very close to the goal of 100% of food samples assayed with this approach.
A major critical factor in the data collection remains and is represented by the number of samples tested by the reporting countries for each food and animal category, which is highly variable and such an unequal distribution is likely to introduce selection bias in the estimates of STEC prevalence or STEC serogroup distribution, hindering spatial and temporal trends analyses or even comparison across MS.
Nevertheless, the descriptive summaries made on occurrence of STEC in food showed that, the presence of STEC was reported in 1.8% of the 21,574 food samples tested and in 10.6% of the 2,310 animal samples tested. The highest proportion of positive food samples was reported from meat samples particularly from small ruminants, followed by milk and dairy products. Such a finding witnesses the importance of these food commodities as vehicles of STEC infections. Conversely, the analysis of fruits and vegetables confirmed the low level of contamination with STEC of these food commodities, as observed in the previous years (EFSA and ECDC, 2015a,b, 2016a,b, 2017a,b,c). For sprouts, the sole food commodity for which a microbiological criterion for STEC has been established in the EU, in 2017 the number of reporting MS and of samples tested increased considerably over the last 5 years. Conversely, only 6 MS provided information on 98 single samples taken as part of the official controls. This figure reflects the real application of the EU Regulation 2073/2005 at the EU level for this food commodity and reveals that a broader adoption of this regulation by MS would be necessary to ensure an appropriate level of control of sprouts and sprouted seeds.
Forty‐two different STEC serogroups were reported in food samples in 2017. STEC O157, O146 and O103 were the three most reported serogroups. STEC O26, which was the most reported non‐O157 serogroup in food in 2016 (EFSA and ECDC, 2017a,b,c), was the fourth STEC serogroup reported in 2017. All these four STEC serogroups are included among those most commonly found as cause of human infections in the EU/EEA in 2016 and in the preceding years (EFSA and ECDC, 2017a,b,c). It is important to stress that only less than one‐third of the STEC isolated from food in 2017 have been provided with information on the serogroup. This configures a strong limitation when attempting to investigate the overlapping of the STEC types found in human disease and those occurring in food and represents an area of improvement of the data collection.
The profile of the virulence genes from STEC strains represents an invaluable tool to predict the risk and the severity of the STEC infections in humans (JEMRA report. See 1.6, Internet sources). This information, however, is still largely missing in the data provided by MS. As a matter of fact, less than half of the STEC strains reported were characterised for the presence of the stx and eae genes and only a few records reported the identification of the stx‐genes subtypes. Countries are recommended to report information on the STEC virulence genes as their analysis represents the basis for the molecular risk assessment and is the most valuable tool to predict the risk and to infer the severity of the STEC infections in humans.
The same situation was observed for the animal samples. Twenty‐two STEC serogroups were reported in the unfiltered, entire data set of animal samples, but only half of the STEC isolated from animals were provided with the information on the serogroup. STEC O157 was the most reported serogroup, mainly from cattle. Other serogroups as the O103, O121, O113, O145, O91 and O8, all implicated in human infection with STEC, have been detected in animals. Conversely, less than one‐third of them were provided with the virulotypes, making the inference on the presence and distribution of virulence genes de facto unusable.
When interpreting the data on STEC in food and animals, it is important to note that results from different investigations may be not directly comparable owing to differences in sampling strategies and the analytical methods applied. Monitoring criteria and analytical methods for STEC are not yet fully harmonised across the different countries. Therefore, a non‐uniform distribution of sampled units per country or the use of analytical methods selecting one specific STEC serogroup may have introduced artefacts in the calculation of STEC prevalence or STEC serogroup distribution when data were analysed at the EU level. To reduce the possible biases linked to the type of sampling and the analytical methods applied, specific choices were made in the use and analysis of the data, as described in Sections 1.2 and 1.3 of the present chapter.
4.6. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Surveillance Atlas | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| World Health Organization – E. coli Fact sheet | http://www.who.int/mediacentre/factsheets/fs125/en/ | |
| National Center for Emerging and Zoonotic Infectious Diseases (NCEZID) | https://www.cdc.gov/ncezid/ | |
| Food, animals | EFSA Scientific Opinion of the Panel on Biological Hazards (BIOHAZ)‐ Monitoring of verotoxigenic Escherichia coli (VTEC) and identification of human‐pathogenic VTEC types | http://www.efsa.europa.eu/en/efsajournal/pub/579 |
| Scientific Opinion of the Panel on Biological Hazards (BIOHAZ)‐ Monitoring of verotoxigenic Escherichia coli (VTEC) and identification of human‐pathogenic VTEC types | http://www.efsa.europa.eu/en/efsajournal/pub/579 | |
| VTEC seropathotype and scientific criteria on pathogenicity assessment | http://www.efsa.europa.eu/en/efsajournal/pub/3138 | |
| JEMRA FAO/WHO report: Shiga toxin‐producing Escherichia coli (STEC) and food: attribution, characterisation and monitoring | http://www.fao.org/documents/card/en/c/CA0032EN | |
| Public health advice on prevention of diarrhoeal illness with special focus on Shiga toxin‐producing Escherichia coli (STEC), also called verotoxin‐producing E. coli (VTEC) or enterohaemorrhagic E. coli (EHEC) | http://www.efsa.europa.eu/en/press/news/110611 | |
| Reg. (EC 209/2013) | http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32013R0209 | |
| EURL VTEC webpage: laboratory methods for VTEC/STEC detection and typing | http://www.iss.it/vtec/index.php?lang=2&anno=2017&tipo=3 | |
| EURL VTEC webpage: Focus on STEC facts | http://www.iss.it/vtec/index.php?lang=2&anno=2017&tipo=20# |
5. Yersinia
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1475841
5.1. Abstract
Twenty‐six MS reported 6,823 confirmed cases of yersiniosis in 2017, making it the third most reported zoonosis in the EU. The EU notification rate was 1.77 cases per 100,000 population, which was 2.8% lower than in 2016. There was a decreasing trend in reported confirmed human cases of yersiniosis in the EU/EEA from 2008 to 2017, but the trend did not show any significant increase or decrease in the past 5 years (2013–2017). The highest country‐specific notification rates were observed in MS in north‐eastern Europe. Yersinia enterocolitica was the most common reported species from human cases. The most common bioserotype was 4/O:3 followed by 2/O:9 and 2/O:5,27. Three fatal cases were reported among the 4,467 confirmed yersiniosis cases for which this information was available.
Yersinia was identified in 12 FBOs affecting 147 people in 6 MS. Eleven of them were caused by Y. enterocolitica, including a large outbreak involving 80 patients in Denmark. Two outbreaks were general and reported as strong‐evidence outbreak and the incriminated food was ‘mixed foods’. This number of reported outbreaks was comparable to the previous annual monitoring results.
Very few MS reported food and animal data on Yersinia in 2017, as during previous years. These scarce data preclude meaningful observations at the EU level. Despite this, documenting findings with the aim of understanding trends and sources of Yersinia along the food chain, including reporting of information on the biotype of each Y. enterocolitica isolate and also serotyping data, is essential to the overall goal of reducing yersiniosis.
5.2. Surveillance and monitoring of Yersinia in the EU
5.2.1. Humans
Notification of yersiniosis in humans is mandatory in most EU MS, Iceland, Norway, except for five MS where notification is based on a voluntary system (Belgium, France, Italy and Luxembourg) or other system (the United Kingdom). No surveillance system exists in Greece and the Netherlands. The surveillance systems for Yersinia infections cover the whole population in all MS, except three (France, Italy and Spain). The latter countries did not provide an estimate for population coverage so no notification rates could be calculated. In Belgium, full national coverage was established in 2015 and rates before this date are not displayed. All countries report case‐based data except Belgium and Bulgaria, which reported aggregated data. Both reporting formats were included to calculate numbers of cases, notification rates and disease trends.
Diagnosis of human gastrointestinal infections is generally based on culture from human stool samples.
5.2.2. Food and animals
Although the reporting of Yersinia occurrence or prevalence in food and animals is not mandatory, MS can report monitoring data on Yersinia to the European Commission in accordance with the Zoonoses Directive 2003/99/EC. The Directive specifies that, in addition to the number of zoonoses and zoonotic agents, for which monitoring is mandatory, zoonoses such as yersiniosis and their agents shall also be monitored when the epidemiological situation so warrants. At present, there is no harmonised surveillance of Yersinia in the EU for food or animals and Yersinia food and animal monitoring data submitted by the MS to EFSA are collected without harmonised design. These data allow for descriptive summaries at the EU level to be made but they preclude trend analyses and trend watching at the EU level (Table 1). A scientific report of EFSA suggested technical specifications for the harmonised monitoring and reporting of Y. enterocolitica in slaughter pigs in the EU (EFSA, 2009c).
5.2.3. Food‐borne outbreaks of human yersiniosis
The reporting of FBO of human yersiniosis is mandatory according the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
5.3. Results
5.3.1. Overview of key statistics along the food chain, EU, 2013–2017
Table 36 summarises EU level statistics related to human yersiniosis, and to Yersinia occurrence in food and animals, respectively, in the EU, during 2013–2017. More detailed descriptions of these statistics are in the results section of this chapter and in the FBO.
Table 36.
Summary of Yersinia statistics related to humans, major food categories and major animal species, EU, 2013–2017
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 6,823 | 6,888 | 6,928 | 6,435 | 6,352 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 1.77 | 1.82 | 1.91 | 1.83 | 1.92 | ECDC |
| Number of reporting MS | 26 | 26 | 26 | 25 | 25 | ECDC |
| Infection acquired in the EU | 4,033 | 3,197 | 3,336 | 3,314 | 3,263 | ECDC |
| Infection acquired outside the EU | 82 | 79 | 78 | 79 | 84 | ECDC |
| Unknown travel status or unknown country of infection | 2,708 | 3,612 | 3,514 | 3,042 | 3,005 | ECDC |
| Number of outbreak‐related cases | 147 | 41 | 54 | 208 | 16 | EFSA |
| Total number of outbreaks | 12 | 8 | 13 | 11 | 8 | EFSA |
| Food | ||||||
| Meat and meat products | ||||||
| Number of sampled units | 1,187 | 961 | 1,234 | 1,505 | 2,213 | EFSA |
| Number of reporting countries | 6 | 5 | 5 | 4 | 7 | EFSA |
| Milk and milk products | ||||||
| Number of sampled units | 2 | 4 | 48 | 121 | 203 | EFSA |
| Number of reporting countries | 1 | 1 | 4 | 2 | 4 | EFSA |
| Animals | ||||||
| Pigs | ||||||
| Number of sampled units | 369 | 100 | 2,050 | 2,447 | 5,892 | EFSA |
| Number of reporting countries | 1 | 1 | 3 | 3 | 8 | EFSA |
| Cattle | ||||||
| Number of sampled units | 124 | 47 | 2,707 | 6,482 | 6,646 | EFSA |
| Number of reporting countries | 1 | 1 | 2 | 3 | 4 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
Humans
The number of human yersiniosis cases for which information related to the travel status was available was higher in 2017 (60%) compared with the previous years (around 50%). Considering the cases with known travel status, almost all cases (97–98%) were infected domestically or through travel within the EU. The total numbers of reported food‐borne yersiniosis outbreaks in the EU varied around 10 during 2013–2017, and outbreak‐related cases were below 50 with peaks of more than 100 cases in 2017 and 2014.
Food and animal categories
For 2017, as for the previous years, very few MS reported food and animal monitoring data on investigations on Yersinia.
5.3.2. Human yersiniosis
In total, 6,823 confirmed cases of yersiniosis were reported in the EU for 2017 by 26 MS (Table 37). The number of confirmed cases slightly decreased compared with 2016. The EU notification rate was 1.77 cases per 100,000 population which was 2.8% lower than in 2016, and the lowest notification rate in the last 5 years. The highest country‐specific notification rates were observed in Finland, Lithuania, the Czech Republic, Slovakia and Denmark (7.69, 6.11, 5.78, 4.45 and 3.58 cases per 100,000 population, respectively).
Table 37.
Reported human cases of yersiniosis and notification rates in the EU/EFTA, by country and year, 2013–2017
| Country | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 95 | 95 | 1.08 | 86 | 0.99 | 118 | 1.38 | 107 | 1.26 | 158 | 1.87 |
| Belgium | Y | A | 317 | 317 | 2.79 | 355 | 3.14 | 350 | 3.11 | 309 | – | 350 | – |
| Bulgaria | Y | A | 17 | 17 | 0.24 | 10 | 0.14 | 12 | 0.17 | 20 | 0.28 | 22 | 0.30 |
| Croatia | Y | C | 29 | 29 | 0.70 | 22 | 0.52 | 16 | 0.38 | 20 | 0.47 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.12 |
| Czech Republic | Y | C | 611 | 611 | 5.78 | 608 | 5.76 | 678 | 6.43 | 557 | 5.30 | 526 | 5.00 |
| Denmark | Y | C | 206 | 206 | 3.58 | 278 | 4.87 | 273 | 4.82 | 250 | 4.44 | 225 | 4.02 |
| Estonia | Y | C | 43 | 43 | 3.27 | 45 | 3.42 | 53 | 4.04 | 62 | 4.71 | 72 | 5.45 |
| Finland | Y | C | 423 | 423 | 7.69 | 407 | 7.42 | 582 | 10.64 | 579 | 10.62 | 549 | 10.12 |
| Franceb | N | C | 738 | 738 | – | 735 | – | 624 | – | 574 | – | 430 | – |
| Germany | Y | C | 2586 | 2579 | 3.13 | 2,763 | 3.36 | 2,741 | 3.38 | 2,470 | 3.06 | 2,579 | 3.15 |
| Greecec | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Hungary | Y | C | 30 | 30 | 0.31 | 70 | 0.71 | 41 | 0.42 | 43 | 0.44 | 62 | 0.63 |
| Ireland | Y | C | 6 | 6 | 0.13 | 3 | 0.06 | 13 | 0.28 | 5 | 0.11 | 4 | 0.09 |
| Italyb | N | C | 8 | 8 | – | 9 | – | 7 | – | 18 | – | 25 | – |
| Latvia | Y | C | 47 | 47 | 2.41 | 47 | 2.39 | 64 | 3.22 | 28 | 1.40 | 25 | 1.24 |
| Lithuania | Y | C | 174 | 174 | 6.11 | 155 | 5.37 | 165 | 5.65 | 197 | 6.69 | 262 | 8.82 |
| Luxembourg | Y | C | 15 | 15 | 2.54 | 12 | 2.08 | 15 | 2.66 | 19 | 3.46 | 15 | 2.79 |
| Malta | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlandsc | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Poland | Y | C | 191 | 191 | 0.50 | 167 | 0.44 | 172 | 0.45 | 212 | 0.56 | 199 | 0.52 |
| Portugal | Y | C | 35 | 35 | 0.34 | 14 | 0.14 | 24 | 0.23 | – | – | – | – |
| Romania | Y | C | 36 | 36 | 0.18 | 40 | 0.20 | 25 | 0.13 | 32 | 0.16 | 43 | 0.22 |
| Slovakia | Y | C | 247 | 242 | 4.45 | 200 | 3.69 | 224 | 4.13 | 172 | 3.18 | 164 | 3.03 |
| Slovenia | Y | C | 18 | 18 | 0.87 | 31 | 1.50 | 10 | 0.48 | 19 | 0.92 | 26 | 1.26 |
| Spainb | N | C | 585 | 585 | – | 514 | – | 432 | – | 436 | – | 243 | – |
| Sweden | Y | C | 243 | 236 | 2.36 | 230 | 2.33 | 245 | 2.51 | 248 | 2.57 | 313 | 3.28 |
| United Kingdom | Y | C | 143 | 142 | 0.22 | 87 | 0.13 | 44 | 0.07 | 58 | 0.09 | 59 | 0.09 |
| EU Total | – | – | 6,843 | 6,823 | 1.77 | 6,888 | 1.82 | 6,928 | 1.91 | 6,435 | 1.83 | 6,352 | 1.92 |
| Iceland | Y | C | 0 | 0 | 0.00 | 1 | 0.30 | 1 | 0.30 | 3 | 0.92 | 0 | 0.00 |
| Norway | Y | C | 67 | 67 | 1.30 | 57 | 1.09 | 76 | 1.47 | 211 | 4.13 | 55 | 1.09 |
Y: yes; N: no; A: aggregated data; C: case‐based data; –: no report.
Sentinel surveillance; no information on estimated coverage, so notification rate cannot be estimated.
No surveillance system.
Most (98.0%) of the yersiniosis cases acquired the infection in the EU; however, 39.7% of the cases were reported to be of unknown origin or unknown travel status (Table 38). The highest proportions of domestic cases (> 95%) were reported in Austria, the Czech Republic, France, Hungary, Latvia, Portugal and Slovakia. The highest proportions of travel‐related cases were reported by Finland (38.7%), and Sweden (24.0%). Among the 248 travel‐associated cases with known information on probable country of infection, 48.0% of the cases represented travel within EU. Spain, Italy and Greece were the most frequently reported travel destinations within the EU (17.4%, 8.0% and 7.5%, respectively). Cuba and Thailand were the most common probable countries of infection outside the EU representing 10.0% and 2.5% of the travel‐associated cases, respectively.
Table 38.
Statistics related to the proportions of human food‐borne yersiniosis outbreak cases (including waterborne outbreaks), EU/EFTA, 2017
| Country | ECDC | EFSA | ||||
|---|---|---|---|---|---|---|
| Confirmed human | Food‐borne outbreaks | |||||
| Total | Travel related | Domestic | Unknown or missing | Human cases (illnesses) | FBO | |
| N | N | N | N | N | N | |
| Austria | 95 | 6 | 88 | 1 | –a | – |
| Belgium | 317 | –b | – | 317 | – | – |
| Bulgaria | 17 | – | – | 17 | – | – |
| Croatia | 29 | 0 | 1 | 28 | – | – |
| Cyprus | 0 | 0 | 0 | 0 | – | – |
| Czech Republic | 611 | 12 | 599 | 0 | – | – |
| Denmark | 206 | 11 | 50 | 145 | 80 | 1 |
| Estonia | 43 | 2 | 41 | 0 | – | – |
| Finland | 423 | 43 | 68 | 312 | – | – |
| France | 738 | – | – | 738 | 4 | 2 |
| Germany | 2,579 | 112 | 1,052 | 1,415 | 9 | 4 |
| Greece | – | – | – | – | – | – |
| Hungary | 30 | 0 | 30 | 0 | – | – |
| Ireland | 6 | 0 | 2 | 4 | – | – |
| Italy | 8 | 0 | 0 | 8 | – | – |
| Latvia | 47 | 0 | 47 | 0 | – | – |
| Lithuania | 174 | 2 | 107 | 65 | – | – |
| Luxembourg | 15 | 0 | 0 | 15 | – | – |
| Malta | 0 | 0 | 0 | 0 | – | – |
| Netherlands | – | – | – | – | – | – |
| Poland | 191 | 1 | 181 | 9 | 13 | 1 |
| Portugal | 35 | 0 | 34 | 1 | – | – |
| Romania | 36 | 0 | 18 | 18 | – | – |
| Slovakia | 242 | 0 | 242 | 0 | 4 | 2 |
| Slovenia | 18 | 1 | 4 | 13 | – | – |
| Spain | 585 | 2 | 367 | 216 | – | – |
| Sweden | 236 | 54 | 171 | 11 | 37 | 2 |
| United Kingdom | 142 | 2 | 74 | 66 | – | – |
| EU Total | 6,823 | 248 | 3,176 | 3,399 | 147 | 12 |
| Iceland | 0 | 0 | 0 | 0 | – | – |
| Norway | 67 | 21 | 29 | 17 | 11 | 1 |
| Switzerland | – | – | – | – | – | – |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority.
No yersiniosis food‐borne outbreaks reported.
No importation data reported.
No clear seasonality was shown of the case reports in the EU/EEA. There was a decreasing trend in reported confirmed human cases of yersiniosis in the EU/EEA from 2008 to 2017 (p < 0.01), but the trend did not show any significant increase or decrease in the past 5 years (2013–2017) (Figure 36).
Figure 36.
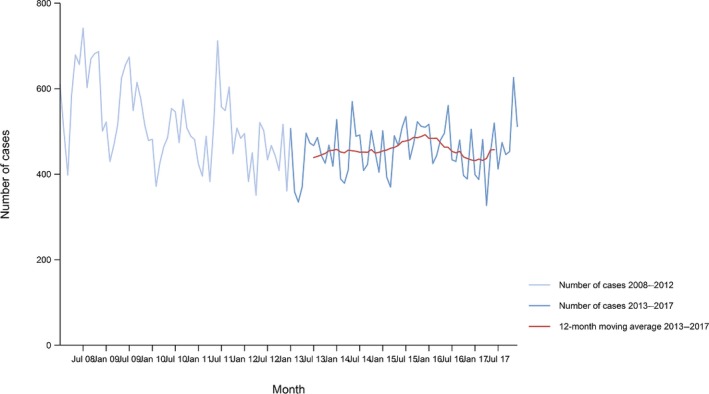
- Source(s): Austria, Cyprus, Czech Republic, Denmark, Estonia, Finland, Germany, Hungary, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, Norway, Poland, Romania Slovakia, Slovenia, Spain, Sweden and United Kingdom. Belgium, Bulgaria, Croatia, France, Italy and Portugal did not report data to the level of detail required for the analysis. Greece and the Netherlands do not have any formal surveillance system for the disease.
Among 17 MS with data available for the whole period 2008–2017, the Czech Republic, Slovakia, Spain and the United Kingdom reported significantly increasing trends (p < 0.01), while Finland, Germany and Sweden reported decreasing trends (p < 0.01) from 2008 to 2017.
In 2013–2017, the Czech Republic, Slovakia, Spain and the United Kingdom continued to report increasing trends (p < 0.01). Four MS (Austria, Estonia, Lithuania and Sweden) observed decreasing trends among the 20 MS having data available for the whole period.
Species information was reported by 22 countries for 6,223 (95.1%) of the confirmed yersiniosis cases in the EU in 2017. Y. enterocolitica was by far the most common species reported in all countries, with the isolation percentage being 99.3% at the EU level. Information about the Y. enterocolitica serotypes was provided for 3,438 (55.7%) of confirmed Y. enterocolitica cases by 16 countries. The most common serotype was O:3 (86.5%), followed by O:9 (9.3%) and O:5,27 (1.9%). Biotype information was provided for 1,040 (16.8%) confirmed cases by six countries (Austria, Denmark, Finland, France, Lithuania and Poland) resulting in threefold increase of biotyped cases compared with 2016. The most commonly reported biotypes in 2017 were biotype 4 (86.8%) followed by biotype 2 (11.6%) and biotype 1B (1.0%).
Nine countries (Austria, the Czech Republic Finland, France, Lithuania, Slovakia, Spain, Sweden and the United Kingdom) reported Y. pseudotuberculosis cases representing 0.7% of all EU yersiniosis cases. Sweden and the United Kingdom reported the highest proportion of Y. pseudotuberculosis infections, representing 5.5% and 5.3% of all their confirmed yersiniosis cases, respectively.
Fourteen MS provided information on hospitalisation. Of 1,847 cases (27.1%) with known hospitalisation status, 33.4% were hospitalised, about the same level as in 2016 (31.5%). The highest hospitalisation rates (64.4–100% of cases) were reported in Ireland, Latvia, Lithuania, Poland, Romania, Slovenia and the United Kingdom. Three fatal cases were reported in 2017 among 4,467 (65.5%) reported cases with known outcome; the case fatality was 0.07%.
Human yersiniosis cases associated with food‐borne outbreaks
Yersinia was identified in 12 FBOs affecting 147 people (notified FBO cases) in six MS, as reported to EFSA. Overall, for the year 2017, there were 3,176 domestic (acquired within the country) cases reported to the TESSy (Table 38), which was 97.0% of the number of reported human yersiniosis cases infected domestically and through travel within EU during 2017 (4,033, Table 36). Table 38 shows data reported by countries to TESSy managed by ECDC and to the FBOs’ database managed by EFSA. It is important to clarify that the case classification for reporting is different between these two databases. In TESSy, the cases reported are classified based on the EU case definition. All these cases visited a doctor, and are either confirmed by laboratory test (confirmed case) or not (probable case and classification is based the clinical symptoms and epidemiological link). Cases that never visited a doctor are not reported to TESSy. Moreover, probable cases may be missing in TESSy, as these data are not analysed or published and there is no incentive for reporting such cases. Information on which case is linked to an outbreak ‐ and which not ‐ is not systematically collected. In practice, the cases reported to TESSy are considered mostly sporadic cases. In food‐borne disease outbreak situations cases are also classified into confirmed or probable outbreak cases, but currently these data are not collected by EFSA.
Of the 12 FBOs, 11 were caused by Y. enterocolitica, including a large outbreak involving 80 patients in Denmark. Two outbreaks were general ones (not household outbreaks) were and reported as strong‐evidence outbreak. The incriminated food in these two outbreaks was ‘mixed foods’. One of these two was the mentioned Danish outbreak, which involved boarding school pupils attending a sport event during a weekend and which were served dinner at the school consisting of pork chops with tomato sauce prepared in the oven and served with boiled rice, bread and salad. Insufficient heat treatment was considered a contributing factor. Six students needed hospitalisation. Y. pseudotuberculosis was the causative agent reported in one outbreak, in Norway. This was comparable to the previous annual monitoring results. Further details and statistics on the yersiniosis FBOs reported for 2017 are in Chapter 16 on FBO.
5.3.3. Yersinia in food and in animals
As reported in Table 36, very few MS reported food and animal monitoring data on investigations on Yersinia in 2017. Results are shown in Table 39 and more detailed summarising results are available in the tables in the Appendix.
Table 39.
Summary of Yersinia statistics related to food categories and animal species, reporting Member States and non‐Member States, EU, 2017
| Food category and animal species | Number of reporting (MS/non‐MS) | Number of tested unitsa, EU | Proportion (%) Yersinia‐positive units, EU | |
|---|---|---|---|---|
| Food | ||||
| Fresh Meat | Pigs | 5/0 | 529 | 8.3 |
| Meat products, RTE | Sheep | 1/0 | 25 | 16.0 |
| Bovine animals | 3/0 | 32 | 6.3 | |
| Milk and milk products | Yoghurt | 1/0 | 1 | 0 |
| Cheese | 1/0 | 1 | 0 | |
| Foods other than meat and meat products and milk and milk products | Bakery products, vegetables, seeds, fruits, eggs, cereals, fishery products, sauce and dressings, mushrooms, other processed foods | 5/0 | 166 | 2.4 |
| Animals | ||||
| Pigs | 5/1 | 3,135 | 4.4 | |
| Domestic livestock other than pigsb | 7/1 | 15,707 | 0.9 | |
| Other animal speciesc | 4/1 | 2,407 | 3.5 | |
RTE: ready‐to‐eat; MS: Member State.
The summary statistics were obtained summing all sampling units (single and batch samples).
Bovine animals, sheep, goats, farmed alpacas, farmed rabbits, farmed reindeers, donkeys, horses.
Cats and dogs, exotic pet animals, wildlife, zoo animals.
Estonia reported 39 (15.6%) out of a total of 250 pig meat samples to be positive to Yersinia enterocolitica whereof 34, 4 and 1 were, respectively, positive to biotype 1A, biotype 4 and biotype 3.
5.4. Discussion
Yersiniosis remains the third most commonly reported bacterial food‐borne zoonosis in the EU in 2017. There was a significant decreasing trend in yersiniosis cases in the EU/EEA between 2008 and 2017, but in the last 5 years (2013–2017) the trend did not show any significant increase or decrease. The highest notification rates were reported in MS in north‐eastern Europe. According to the EU case definition, only human‐pathogenic Y. enterocolitica or Y. pseudotuberculosis cases should be reported.10 Y. enterocolitica was by far the dominating species in all countries.
To assess the public health significance and pathogenicity of Y. enterocolitica for humans, it is recommended to report information on the biotype of each human isolate and preferably also serotyping data. Y. enterocolitica represents six biotypes (1A, 1B, 2, 3, 4 and 5), which are considered pathogenic for human, except biotype 1A. Species and serotype information is provided frequently and markedly increased due to two additional Member States (Croatia and France) starting to report case‐based yersiniosis data in 2017. Reporting of biotypes increased more than threefold compared with the previous years, however information is still only available for a small fraction of the yersiniosis cases. Pathogenicity of the isolates can also be confirmed by using more advanced methods e.g. molecular typing, though currently this information cannot be reported into the EU TESSy. Twelve yersiniosis outbreaks (2 strong‐evidence outbreak and 10 weak‐evidence outbreaks) were reported by six MS and this was comparable to the previous annual monitoring results.
As for the previous years, very few MS reported food and animal data on Yersinia in 2017. This may be explained by the non‐compulsory reporting on Yersinia. In addition to the scarcity of the reported data, the sampling and reporting rules are not harmonised, precluding trend analyses and trend watching, or inference beyond the sample statistics on trends or sources of Yersinia in food or animals. A scientific report of EFSA suggested technical specifications for the harmonised monitoring and reporting of Y. enterocolitica in slaughter pigs in the EU (EFSA, 2009a,bc). Documenting trends and sources of Yersinia along the food chain, including reporting of information on the biotype of each Y. enterocolitica isolate and preferably also serotyping data, remains essential to the overall goal of reducing yersiniosis, whether food‐borne or sporadic.
5.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Fact sheet yersiniosis | https://www.cdc.gov/yersinia/faq.html |
| Surveillance Atlas | http://atlas.ecdc.europa.eu/public/index.aspx | |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| Food animals | Monitoring and identification of human enteropathogenic Yersinia spp. – Scientific Opinion of the Panel on Biological Hazards | https://www.efsa.europa.eu/en/efsajournal/pub/595 |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sauces of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports | |
| Bad Bug Book (Second Edition), Food‐borne Pathogenic Microorganisms and Natural Toxins Handbook, Center for Food Safety and Applied Nutrition, Food and Drug Administration (FDA), USA | https://www.fda.gov/food/foodborneillnesscontaminants/causesofillnessbadbugbook/ |
6. Tuberculosis due to Mycobacterium bovis
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1475841
6.1. Abstract
Tuberculosis due to Mycobacterium bovis is a rare infection in humans in the EU, with 185 confirmed human cases reported in 2017. The EU notification rate has increased from 0.03 cases per 100,000 population in 2013 to 0.04 cases per 100,000 population in 2017. The notification rate of M. bovis in humans was higher in MS not officially bovine tuberculosis free (non‐OTF) compared with MS that were OTF in cattle (OTF) in 2017.
The 2017 monitoring data on bovine tuberculosis in EU cattle demonstrate that the current situation in Europe on bovine tuberculosis infection, detection and control is heterogeneous. Bovine tuberculosis was reported by 16 MS and was much spatially clustered with the herd prevalence ranging from absence to 13.5% within the United Kingdom in the non‐OTF region England‐Wales.
In the OTF regions of 22 MS, the detection during 2017 of bovine tuberculosis‐infected herds remained a rare event, as in the previous years. As compared with 2016, two more MS, Malta and Portugal detected bovine tuberculosis infection in their OTF regions.
All 10 non‐OTF MS, except Cyprus, reported having detected bovine tuberculosis in their non‐OTF regions. The total reported number of positive cattle herds in non‐OTF regions increased by 8% compared with 2016 and also the prevalence of bovine tuberculosis‐positive cattle herds increased to 1.8% as compared with 1.6% during 2016. This increase is due to the United Kingdom and Ireland. The United Kingdom reported for 2017 for Wales and for England, as well as for Northern Ireland, an increasing herd prevalence of 10% and higher, such as in recent years. Ireland reported a low herd prevalence, between 1% and 5%, which increased moderately, compared with recent years. Spain reported a low herd prevalence that decreased moderately. Greece reported, for 2017, 2.3% bovine tuberculosis test‐positive cattle herds while during 2004–2017 test‐positive cattle herds reported ranged from 1.9% in 2008 to 5.2% in 2015. Italy and Portugal reported very low (0.1–1%) herd prevalence.
6.2. Surveillance and monitoring of tuberculosis due to M. bovis in the EU
6.2.1. Humans
The notification of tuberculosis in humans is mandatory in all EU MS, Iceland, Norway, Liechtenstein and Switzerland. The surveillance covers the whole population. France did not report species‐specific data within the M. tuberculosis complex for the human tuberculosis cases; therefore, no human M. bovis data are available for France.
As tuberculosis is a chronic disease with a long incubation period, it is not possible to assess travel‐associated cases in the same way as diseases with acute onset. Instead, the distinction is made between individuals with the disease originating from an EU MS (cases of EU origin), and those originating from outside the EU (case originating outside of EU). In the analyses, origin is mainly defined by the reported birthplace, except for cases from Austria, Belgium, Greece, Hungary and Poland, whose origin is mainly defined by their reported nationality.
The treatment outcome for tuberculosis cases due to M. bovis is assessed 1 year (12 months) after the case notification, since the shortest duration for treatment completion is 6 months according to the international treatment guidelines of tuberculosis.
6.2.2. Animals
Bovine tuberculosis monitoring data from bovine animals originating from the National Control and Eradication Programmes and/or Officially Free status
According to the Zoonoses Directive 2003/99/EC, MS must report bovine tuberculosis annual monitoring data. These data originate from national control and surveillance programmes implemented by the MS in accordance with EU legislation. The reports submitted by the MS are based on Council Directive 64/432/EEC and subsequent legislation, and are essential for the assessment of the epidemiological situation in MS and MS’ regions, whether declared OTF or not yet declared OTF. Annual surveillance programmes are carried in OTF regions to confirm freedom from bovine tuberculosis, whereas in all non‐OTF regions control and eradication programmes for bovine tuberculosis are in place. These data are comparable across MS because the monitoring schemes are harmonised, and the data collected and reported to EFSA originate from the census as sampling frame. In addition to trend analysis both at the EU level and at MS level, and to trend watching and descriptive summaries, these data may also be used to assess the impact of control and eradication programmes, (Table 1).
EU MS also need to notify outbreaks of bovine tuberculosis in terrestrial animals from OTF regions to the EU Animal Disease Notification System (ADNS)11 and regular summaries are posted online.
For bovine tuberculosis cases, all tuberculosis cases irrespective of their causative agent (i.e. also including those caused by Mycobacterium caprae) are included in the statistics provided by MS, as opposed to what happens in the above‐mentioned statistics for humans, which only include cases by M. bovis). Based on the definition recommended by the bovine tuberculosis subgroup of the task force on monitoring animal disease eradication of the EU (SANCO/10200/2006), who made it explicit that all cases of tuberculosis in cattle due to a disease‐causing member of the M. tuberculosis complex is to be considered as a case of bovine tuberculosis, all available information on the specific bacterial species part of the M. tuberculosis complex recovered from cattle was taken into account to summarise the EU situation on bovine tuberculosis. A distinction is made descriptively, whenever possible, of reporting by MS on Mycobacterium tuberculosis complex, M. bovis and M. caprae.
Mycobacterium monitoring data from animals other than bovine animals
Mycobacterium monitoring data from animals other than bovine animals submitted to EFSA and collected without harmonised design allow for descriptive summaries at the EU level, but are not suitable for trend analyses and trend watching (Table 1).
6.2.3. Food‐borne outbreaks of human tuberculosis due to M. bovis
The reporting of FBOs of human tuberculosis due to M. bovis is mandatory according the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
6.3. Results
6.3.1. Overview of key statistics along the food chain, EU, 2013–2017
Table 40 summarises EU level statistics of human tuberculosis due to M. bovis, and of bovine tuberculosis, in the EU, during 2013–2017. The statistics displayed are the numbers of OTF MS, the numbers of non‐OTF MS, and the number of cattle herds positive for bovine tuberculosis. Further descriptions of findings can be found in the Section 6.3.3.
Table 40.
Summary of tuberculosis due to M. bovis statistics related to humans, major food categories and animal species, EU, 2013–2017
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 185 | 182 | 176 | 159 | 144 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.04 | 0.04 | 0.03 | 0.03 | 0.03 | ECDC |
| Number of EU MS that reported data on M. bovis cases | 27 | 27 | 27 | 27 | 27 | ECDC |
| M. bovis cases in individuals of EU origin | 115 | 102 | 108 | 100 | 97 | ECDC |
| M. bovis cases in individuals originating outside of EU | 70 | 80 | 68 | 59 | 47 | ECDC |
| M. bovis cases in individuals of unknown origin | 0 | 0 | 0 | 0 | 0 | ECDC |
| Total number of food‐borne outbreaks | 0 | 0 | 0 | 0 | 0 | EFSA |
| Number of outbreak‐related cases | 0 | 0 | 0 | 0 | 0 | EFSA |
| Animals | ||||||
| Bovine animals | ||||||
| Number of positive herds in OTF regions | 134 | 147 | 155 | 139 | 197 | EFSA |
| Number of reporting OTF MS | 18 | 18 | 18 | 16 | 15 | EFSA |
| Number of positive herds in non‐OTF regions | 18,857 | 17,421 | 17,441 | 17,122 | 18,059 | EFSA |
| Number of reporting non‐OTF MS | 10 | 10 | 10 | 12 | 13 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States; OTF: Officially bovine tuberculosis free (status on freedom from bovine tuberculosis, in cattle).
6.3.2. Tuberculosis due to M. bovis in humans
In 2017, 185 confirmed cases of tuberculosis due to M. bovis in humans were reported by 27 EU MS (Table 41). According to preliminary data, human M. bovis cases represented 0.4% of all confirmed tuberculosis cases that were reported from the 27 EU MS that reported on M. bovis in humans in 2017. Twelve MS reported at least one confirmed case and 15 MS did not report any cases. The EU notification rate was 0.04 cases per 100,000 population, the same as the previous year, but higher than in the years 2015, 2014 and 2013. The highest notification rate in 2017 was reported by Spain (0.12 per 100,000), followed by the Netherlands and the United Kingdom (0.06 per 100,000). Fifteen EU MS have OTF status in cattle. In 2017, the notification rate of human M. bovis cases in EU MS with OTF status was 0.03 per 100,000 population, whereas it was 0.05 per 100,000 population in non‐OTF EU MS.
Table 41.
Reported human cases of tuberculosis due to M. bovis and notification rates per 100,000 population in the EU/EFTA, by country and year, 2013–2017
| Country | National coveragea | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Data formatb | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | |||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | |||
| Austria (OTF)c | Y | C | 1 | 0.01 | 1 | 0.01 | 3 | 0.03 | 1 | 0.01 | 1 | 0.01 |
| Belgium (OTF) | Y | C | 6 | 0.05 | 14 | 0.12 | 9 | 0.08 | 10 | 0.09 | 10 | 0.09 |
| Bulgaria | Y | C | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 |
| Croatia | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czech Republic (OTF) | Y | C | 0 | 0.00 | 1 | 0.01 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 |
| Denmark (OTF) | Y | C | 1 | 0.02 | 2 | 0.04 | 0 | 0.00 | 1 | 0.02 | 0 | 0.00 |
| Estonia (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Finland (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.02 |
| Franced (OTF) | – | – | – | – | – | – | – | – | – | – | – | – |
| Germany (OTF) | Y | C | 43 | 0.05 | 54 | 0.07 | 49 | 0.06 | 44 | 0.05 | 44 | 0.05 |
| Greece | Y | C | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Hungary (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Ireland | Y | C | 2 | 0.04 | 3 | 0.06 | 5 | 0.11 | 3 | 0.06 | 6 | 0.13 |
| Italye | Y | C | 21 | 0.03 | 13 | 0.02 | 17 | 0.03 | 18 | 0.03 | 14 | 0.02 |
| Latvia (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Lithuania (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Luxembourg (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Malta (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlands (OTF) | Y | C | 10 | 0.06 | 14 | 0.08 | 9 | 0.05 | 8 | 0.05 | 10 | 0.06 |
| Poland (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Portugalf | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Romania | Y | C | 2 | 0.01 | 2 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Slovakia (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Slovenia (OTF) | Y | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Spain | Y | C | 55 | 0.12 | 35 | 0.08 | 38 | 0.08 | 33 | 0.07 | 28 | 0.06 |
| Sweden (OTF) | Y | C | 3 | 0.03 | 5 | 0.05 | 6 | 0.06 | 4 | 0.04 | 0 | 0.00 |
| United Kingdomg | Y | C | 40 | 0.06 | 38 | 0.06 | 38 | 0.06 | 37 | 0.06 | 30 | 0.05 |
| EU Total | – | – | 185 | 0.04 | 182 | 0.04 | 176 | 0.03 | 159 | 0.03 | 144 | 0.03 |
| Icelandh, * | Y | C | 0 | 0.00 | – | – | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Liechtenstein (OTF) | – | C | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway (OTF) | Y | C | 3 | 0.06 | 5 | 0.10 | 1 | 0.02 | 3 | 0.06 | 0 | 0.00 |
| Switzerland (OTF)i | – | C | 3 | 0.00 | 5 | 0.00 | 6 | 0.00 | 1 | 0.00 | 2 | 0.00 |
*Not reporting species of the M. tuberculosis complex in 2017.
Y: Yes; N: No; –: No report.
A: Aggregated data; C: Case‐based data; –: No report.
OTF: Officially bovine tuberculosis free (status on freedom from bovine tuberculosis, in cattle).
Does not report species of the M. tuberculosis complex.
In Italy, seven regions and 14 provinces are OTF.
In Portugal, all administrative regions within the superior administrative unit of the Algarve are OTF.
In the United Kingdom, Scotland and the Isle of Man are OTF (in cattle).
In Iceland that has no special agreement for animal health (status) with the EU, the last outbreak of bovine tuberculosis was in 1959.
Switzerland provided data directly to EFSA.
Most cases reported in the EU in 2017 (62.2%, 115/185) were of EU origin (native cases and/or cases originating from other EU MS), and 37.8% (70/185) originated from outside the EU (Table 40). Among the reported M. bovis cases, there was a larger proportion (66.1%) of cases of EU origin in non‐OTF EU MS than in OTF EU MS (54.7%).
Treatment outcome after 12 months of treatment was reported for 169 (92.9%) of 182 human M. bovis cases reported in 2016. Successful treatment was reported for 119 cases (70.4%), while 14 cases (8.3%) died during the treatment, 17 cases (10.1%) were still on treatment, and four cases (2.4%) were lost to follow‐up. The treatment outcome was not evaluated for 15 cases (8.9%).
Drug resistance to isoniazid and rifampicin among human tuberculosis cases due to M. bovis was low in 2017; among 112 cases with test results reported for both isoniazid and rifampicin, nine were isoniazid‐resistant (8.0%). No multidrug resistant (resistance to rifampicin and isoniazid) cases were reported.
Figure 37.
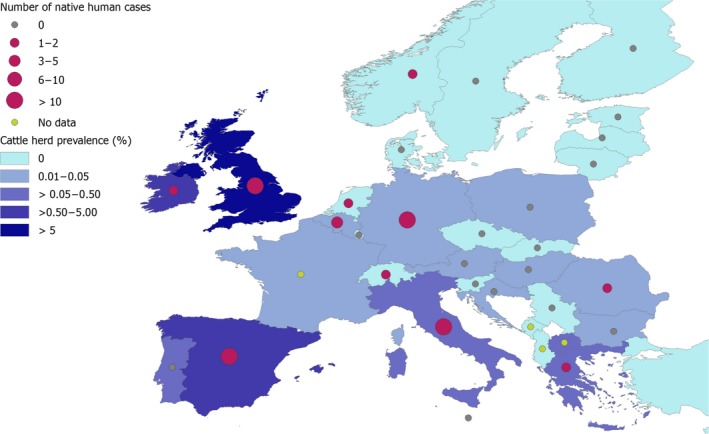
- No human data were obtained from France, Albania, Bosnia and Herzegovina, Former Yugoslav Republic of Macedonia, Montenegro and Serbia.
6.3.3. Bovine tuberculosis in animals
The status of freedom from bovine tuberculosis is displayed in Table 42. Eighteen MS were OTF during 2017. Four MS were non‐OTF with OTF regions. The OTF regions of these four MS are:
in Italy: 9 regions and 13 provinces;
in Portugal: all administrative regions within the superior administrative unit of the Algarve;
in Spain: the Canary Islands;
in the United Kingdom: Scotland and the Isle of Man.
Table 42.
Status of countries on bovine tuberculosis, EU, 2017
| Member State (MS) | Officially free of bovine tuberculosis (OTF) |
|---|---|
| Austria | |
| Belgium | |
| Bulgaria | |
| Croatia | |
| Cyprus | |
| Czech Republic | |
| Denmark | |
| Estonia | |
| Finland | |
| France | |
| Germany | |
| Greece | |
| Hungary | |
| Ireland | |
| Italy | |
| Latvia | |
| Lithuania | |
| Luxembourg | |
| Malta | |
| Netherlands | |
| Poland | |
| Portugal | |
| Romania | |
| Slovakia | |
| Slovenia | |
| Spain | |
| Sweden | |
| United Kingdom |
 All regions of the MS are OTF.
All regions of the MS are OTF.
 Not all regions of the MS are OTF.
Not all regions of the MS are OTF.
 No region of the MS is OTF.
No region of the MS is OTF.
Finally, six MS were non‐OTF without OTF regions: Bulgaria, Croatia, Cyprus, Greece, Ireland and Romania.
Norway and Switzerland were OTF, in accordance with EU legislation. Liechtenstein has the same status (OTF) as Switzerland. In Iceland, which has no special agreement for animal health status with the EU, the last outbreak of bovine tuberculosis was reported in 1959.
During 2017, the overall EU proportion of cattle herds infected with, or positive for, bovine tuberculosis, considering all OTF and non‐OTF regions, remained very low (0.9%). Figure 38 displays the herd prevalence (infected or positive cattle herds out of the total number of herds) at region or national levels in EU/EEA. It shows that bovine tuberculosis is reported by 16 MS and that the current situation in Europe on bovine tuberculosis infection in cattle is heterogeneous and much spatially clustered with herd prevalence ranging from absence to 13.5% within the United Kingdom in the non‐OTF region England‐Wales.
Figure 38.
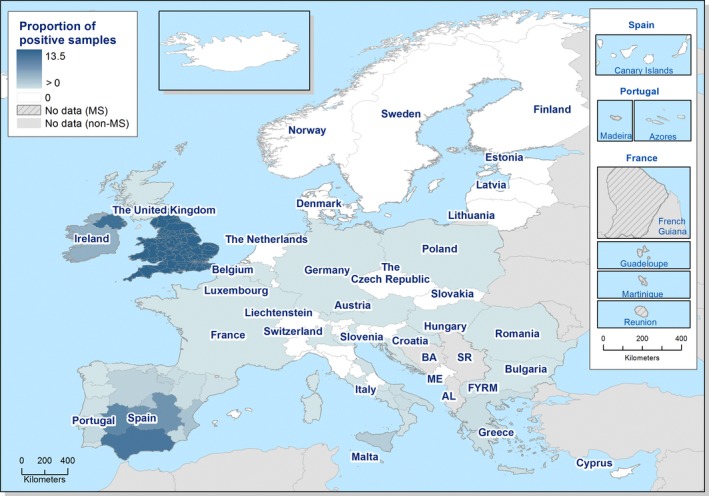
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: Former Yugoslav Republic of Macedonia; ME: Montenegro; SR, Serbia.
In the EU OTF regions, there were in total 1,195,660 cattle herds during 2017. Ten MS reported 134 bovine tuberculosis‐infected herds in OTF regions; nine MS reported infection with M. bovis (Belgium, five herds; France, 95 herds; Germany, 3; Hungary, 2; Italy, 2; Malta, 1; Poland, 12; Portugal, one and UK, 5), whereas Austria12 reported 8 herds infected with M. caprae.
Bovine tuberculosis was not detected in 2017 in the non‐MS Iceland, Norway, Switzerland and Liechtenstein.
From 2010 to 2017, the annual number (prevalence) of cattle herds reported infected in the EU OTF regions decreased from 227 (0.016%) to 134 (0.011%), respectively (Figure 39). Concomitantly, the total number of cattle herds decreased from 1,439,899 in 2010 to 1,195,660 in 2017.
Figure 39.
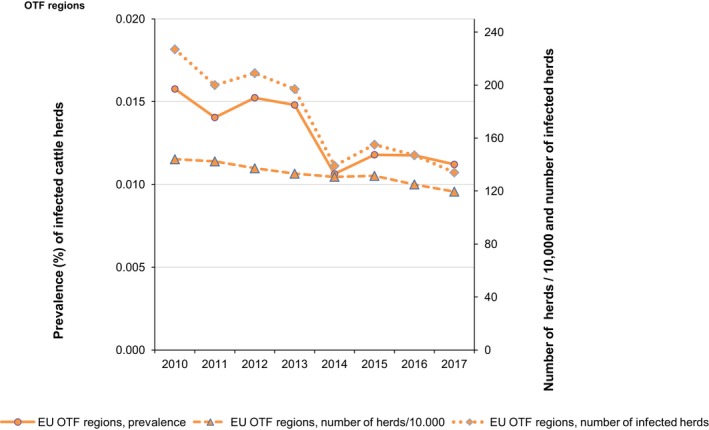
- OTF: Officially bovine tuberculosis free in cattle.
During 2017, the 10 non‐OTF MS had 1,022,664 cattle herds in their non‐OTF regions. Five of these MS (Ireland, Italy, Portugal, Spain and the United Kingdom) had their eradication programmes cofinanced by the EU. The number of positive herds reported by these MS was 5,472 in Ireland (4,047 in 2016), 312 in Italy (335 in 2016), 87 in Portugal (77 in 2016), 2,461 in Spain (3,048 during 2016) and 10,334 in the United Kingdom (9,694 in 2016). Reports concerned M. bovis except for Spain reporting M. tuberculosis complex. Of the five non‐cofinanced non‐OTF MS, Cyprus did not report any infected herds for the year 2017 (like during previous years). Croatia reported 1 M. tuberculosis complex‐infected herd (2 in 2016), whereas 28 M. bovis‐infected herds were reported by Bulgaria (10 in 2016), and 93 by Greece (147 in 2016). Romania reported 69 (61 in 2016) infected herds with M. bovis or M. caprae.
From 2010 to 2017, the annual number (prevalence) of reported test‐positive cattle herds in the EU non‐OTF regions increased by 6% (76.0% for prevalence) from 17,814 (1.1%) to 18,857 (1.8%), respectively, whereas compared with 2016 the increase amounted to 8% (18.5% for prevalence). Concomitantly, from 2010 to 2017, the total number of cattle herds decreased importantly from 1,638,694 in 2010 to 1,022,664 in 2017 (Figure 40).
Figure 40.
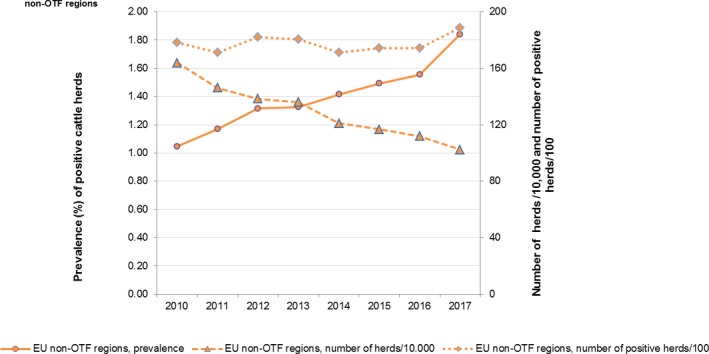
- OTF: Officially bovine tuberculosis free in cattle.
Figure 41 displays trends in the reported prevalence of bovine tuberculosis test‐positive cattle herds during 2004–2017 in non‐OTF regions of five non‐OTF cofinanced Member States and of one non‐OTF not funded Member State Greece, 2004–2017. The United Kingdom reported for 2017 for Wales and for England, as well as for Northern Ireland, an increasing prevalence of 10% and higher, such as in recent years. Ireland and Spain reported a low prevalence between 1% and 5%, which increased and decreased moderately, respectively, compared with recent years. Greece reported, for 2017, 2.3% (93 of 3,969) bovine tuberculosis test‐positive cattle herds while during 2004–2017 reported test‐positive cattle herds ranged from 1.9% in 2008 to 5.2% in 2015. Italy and Portugal reported very low (0.1–1%) prevalence.
Figure 41.
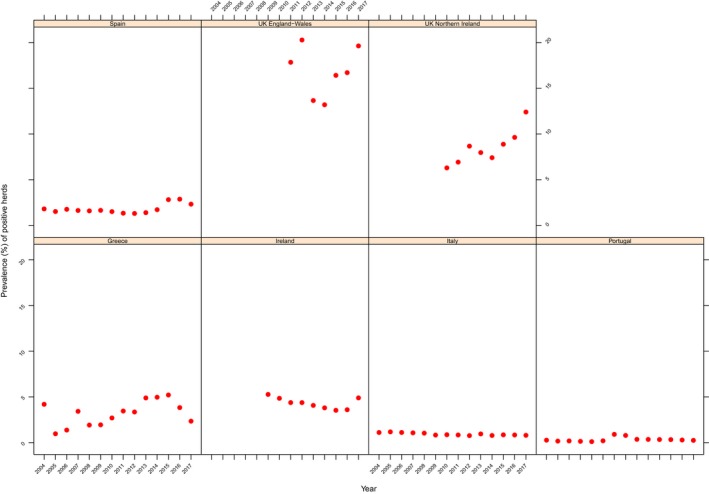
Prevalence of bovine tuberculosis test‐positive cattle herds, in non‐OTF regions of five non‐OTF cofinanced Member States and of one non‐OTF not funded Member State Greece, 2004–2017
Complementary to 2017 reports from cattle, M. bovis was reported by countries in: domestic breeding pigs, sheep and goats, pets (cats and dogs), farmed water buffalos, farmed alpacas, wild boars, badgers, wild and farmed deer, and fallow deer and antelopes in zoos. France reported 6% of 188 foxes to be positive for M. bovis. M. caprae was reported in wild boars and wild red deer.
In food, Spain also reported isolations of M. bovis from meat from bovine animals (carcasses), M. caprae from meat from goats (carcasses) and unspecified Mycobacterium spp. from carcasses from bovine animals, pigs, sheep and goats, deer (venison) and wild boars.
6.4. Discussion
Tuberculosis due to M. bovis is a rare disease in humans in the EU because of decades of disease control in cattle and routine pasteurisation of cow's milk. According to preliminary data, human M. bovis cases represented only a small proportion (0.4%) of all confirmed tuberculosis cases that were reported from the 27 EU MS that reported on M. bovis in humans in 2017. The EU notification rate of M. bovis has been increasing between 2013 and 2017. In 2017, the notification rate of M. bovis in humans was higher in non‐OTF EU MS than OTF EU MS.
The 2017 monitoring data on bovine tuberculosis in EU cattle demonstrate that the current situation in Europe on bovine tuberculosis infection, detection and control is heterogeneous, as substantiated by EFSA (EFSA AHAW Panel, 2014). Bovine tuberculosis was reported by 16 MS but the infection was much clustered spatially within Europe, with the prevalence ranging from absence to 13.5% within the United Kingdom in the non‐OTF region England‐Wales.
In the OTF regions of 22 MS, the detection during 2017 of bovine tuberculosis‐infected herds remained a rare event, as in the previous years. As compared with 2016, two more MS, Malta and Portugal detected bovine tuberculosis infection in their OTF regions.
All 10 non‐OTF MS except Cyprus reported detecting bovine tuberculosis during 2017 in their non‐OTF regions. The total reported number of positive cattle herds in non‐OTF regions increased by 8% compared with 2016, and the prevalence of bovine tuberculosis‐positive cattle herds also increased from 1.6% in 2016 to 1.8%. Actually, the number of positive cattle herds and the prevalence in non‐OTF regions has been increasing since 2010. The increase in prevalence can be partly explained by the 6% increase in test‐positive cattle herds being detected in these regions along with an important decrease in the total number of cattle herds due to the gradual declaration of OTF status in regions within non‐OTF MS over this period. The overall increase can be further explained by the MS‐specific trends reported by the United Kingdom, which reported an increasing prevalence of above 10% for Wales, England and Northern Ireland during recent years, and by Ireland, who also reported an increasing prevalence albeit at a lower level. Greece and Spain reported a decreasing prevalence. Other non‐OTF MS reported stable low to very low prevalence, to rare detection.
Stagnating or increasing trends in prevalence of bovine tuberculosis‐positive cattle herds demonstrate that control and eradication of this disease is a challenge, owing to the complex interactions between the pathogen, hosts and the local environments (EFSA AHAW Panel, 2014). MS‐specific evaluations of status, trends and of the relevance of bovine tuberculosis as a source of disease for humans can be found in the 2017 Annual national zoonoses country reports referenced in Section 6.5.
In 2017, M. bovis was reported to be isolated from a wide range of animal species, both domestic and wild, reflecting this causative agent of tuberculosis in cattle has a broad host range. M. bovis was reported to be recovered from cats and dogs in Ireland and France, respectively, and also in United Kingdom, where M. bovis is now quite commonly found in cats, pigs, South American camelids (llamas and alpacas) and deer (Gunn‐Moore, 2014). Still, tuberculosis due to M. bovis is diagnosed in fewer than 40 people in the United Kingdom each year (Davidson et al., 2017). Other infected mammals such as cats, deer, wild boars and badgers can become infectious too, as are infected humans. France reported M. bovis‐positive foxes. This is line with findings published recently by Michelet et al. (2018), detecting M. bovis‐infection of wild red foxes in southern France, suggesting a possible role of the red fox in the epidemiology of bovine tuberculosis. M. caprae, recognised to cause bovine tuberculosis, was reported in cattle but also in wild boar and wild red deer. Species of the M. tuberculosis complex were also reported from meat from meat production animals and from deer and wild boar.
6.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Surveillance Atlas | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| European Tuberculosis Surveillance Network | http://ecdc.europa.eu/en/healthtopics/Tuberculosis/european_tuberculosis_surveillance_network/Pages/index.aspx | |
| Food/Animals | European Union Reference Laboratory for Bovine Tuberculosis | https://www.visavet.es/bovinetuberculosis/ |
| Summary Presentations on the situation as regards Bovine Tuberculosis control and eradication programmes in Member States; | https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en#20180919 | |
| Bovine tuberculosis – Austria | https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20180712_pres_bov-bruc-erad_aut.pdf | |
| Bovine tuberculosis – Croatia | https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20160705_bov-tb_croatia.pdf | |
| Bovine tuberculosis – Ireland | https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20180712_pres_bov-tub_irl.pdf | |
| Bovine tuberculosis – Italy | https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20180712_pres_bov-tub_ita.pdf | |
| Bovine tuberculosis – Malta | https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20180228_tb_mlt.pdf | |
| Bovine tuberculosis – Portugal | https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20180712_pres_bov-tub_por.pdf | |
| Bovine tuberculosis – Spain | https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20180712_pres_bov-tub_esp.pdf | |
| Bovine tuberculosis – United Kingdom | https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20180712_pres_bov-tub_gbr.pdf | |
| General information on EU Food Chain Funding | https://ec.europa.eu/food/funding_en | |
| 2003/467/EC: Commission Decision of 23 June 2003 establishing the official tuberculosis, brucellosis, and enzootic‐bovine‐leukosis‐free status of certain Member States and regions of Member States as regards bovine herds (Text with EEA relevance) (notified under document number C(2003) 1925) | https://eur-lex.europa.eu/eli/dec/2003/467/oj/eng | |
| General information on National Veterinary Programmes, in EU | https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en | |
| EU approved and cofinanced veterinary programmes for Bovine Tuberculosis carried out by the MS | http://ec.europa.eu/dgs/health_food-safety/funding/cff/animal_health/vet_progs_en.htm | |
| World Organisation for Animal health, Summary of Information on Bovine tuberculosis | http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/Disease_cards/BOVINE-TB-EN.pdf | |
| 2016 National Veterinary Programmes funded (cofinanced) by the EU for bovine tuberculosis (approved programmes and type of measures approved) | https://ec.europa.eu/food/sites/food/files/safety/docs/cff_animal_vet-progs_working_doc_12114_rev2_2016.pdf | |
| More information on EU approved and cofinanced eradication programmes for bovine tuberculosis in cattle carried out by the MS is available online at | http://ec.europa.eu/dgs/health_food-safety/funding/cff/animal_health/vet_progs_en.htm | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports | |
| Scientific Opinion of the EFSA Panel on Animal Health and Welfare (AHAW): Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No. 2016/429): bovine tuberculosis | https://www.efsa.europa.eu/en/efsajournal/pub/4959 | |
| EU Task Force on the eradication of animal diseases – bovine tuberculosis subgroup reports | https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en | |
| Bad Bug Book (Second Edition), Food‐borne Pathogenic Microorganisms and Natural Toxins Handbook, Center for Food Safety and Applied Nutrition, Food and Drug Administration (FDA), USA | https://www.fda.gov/food/foodborneillnesscontaminants/causesofillnessbadbugbook/ |
7. Brucella
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1475841
7.1. Abstract
Brucellosis is a rare infection in humans in the EU with similar notification rates over the last 5 years. It is a severe disease with most infected cases hospitalised and with one death reported in 2017. The highest notification rates and most domestic brucellosis cases were reported from four MS that are not officially brucellosis free in cattle, sheep or goats (Greece, Italy, Portugal and Spain). These MS reported the most Brucella‐positive or ‐infected herds of these ruminant species in the EU. From food, only Italy reported one investigation during 2017 with Brucella‐positive findings in pasteurised milk from a processing plant. Greece, Italy, Portugal and Spain reported very few monitoring data from milk and milk products, in particular those destined to be consumed raw and that are the main food sources of brucellosis in human. Non‐food‐borne transmission of brucellosis to humans also happens by direct contact with infected animals.
In livestock, bovine brucellosis and ovine and caprine brucellosis have been eradicated by most MS. As a result, reported food‐borne disease outbreaks due to Brucella have become rare in large areas of the EU and, for the year 2017, only one FBO due to Brucella was reported, by Germany, with two illnesses, but the incriminated food was unknown.
The overall situation in animals in the EU in 2017 further improved with the total number of Brucella‐positive or ‐infected cattle herds, sheep flocks and goat herds further decreasing in the MS regions that are not officially free of brucellosis by 20% and by 6%, respectively. The temporal trends of bovine brucellosis and of ovine and caprine brucellosis in not officially free regions in Italy, Portugal and Spain showed a decrease in recent years. Brucellosis in cattle herds, in sheep flocks and in goat herds was also reported by Greece, as during previous years.
These findings underline the situation that brucellosis is still an animal health problem with public health relevance in few EU MS.
7.2. Surveillance and monitoring of Brucella in the EU
7.2.1. Humans
Notification of brucellosis in humans is mandatory in all MS, Iceland, Norway and Switzerland, except in Belgium and in the United Kingdom that have another (not specified) system. Denmark has no surveillance system is in place for brucellosis and the disease is not notifiable nor reported at the EU level. The surveillance systems for brucellosis cover the whole population in all MS where surveillance system is in place. In Belgium, full national coverage was set up in 2015, so notification rates before this date are not displayed. All countries reported case‐based data except Bulgaria, which reported aggregated data. Both reporting formats were included to calculate numbers of cases, notification rates and disease trends.
7.2.2. Food and animals
Brucella monitoring data from bovine animals, and sheep and goats originating from the National Control and Eradication Programmes and/or Officially Free status
According to the Zoonoses Directive 2003/99/EC, MS must report bovine brucellosis and sheep and goat brucellosis annual monitoring data. These data originate from national control and surveillance programmes implemented by the MS in accordance with EU legislation. The reports submitted by the MS are based on Council Directive 64/432/EEC and subsequent legislation, and are essential for the assessment of the epidemiological situation in MS and MS’ regions, whether declared officially brucellosis free in cattle (OBF) and/or officially B. melitensis free in sheep and goats (ObmF). Annual surveillance programmes are carried out in OBF regions to confirm freedom from bovine brucellosis and in ObmF regions freedom from B. melitensis in sheep and goats, whereas in all non‐OBF and non‐ObmF regions control and eradication programmes for brucellosis in cattle and in sheep and goats are in place. These data are comparable across MS because the monitoring schemes are harmonised, and the data collected and reported to EFSA originate from the census as sampling frame. In addition to trend analysis both at the EU level and at MS level, and to trend watching and descriptive summaries, these data may also be used to assess the impact of control and eradication programmes (Table 1).
EU MS also need to notify outbreaks in terrestrial animals of bovine brucellosis and of caprine and ovine brucellosis (excluding Brucella ovis) in their OBF and/or ObmF regions to the EU ADNS11 and regular summaries are posted online.
Brucella monitoring data from food and animals other than bovine animals, and sheep and goats
Brucella monitoring data from food and from animals other than bovine animals, and sheep and goats, submitted to EFSA according to Chapter II (‘monitoring of zoonoses and zoonotic agents’) of the Zoonoses Directive 2003/99/EC, and collected without harmonised design, allow for descriptive summaries at the EU level to be made. They preclude trend analyses and trend watching at the EU level (Table 1).
7.2.3. Food‐borne outbreaks of human brucellosis
The reporting of FBOs of human brucellosis is mandatory according the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
7.3. Results
7.3.1. Overview of key statistics along the food chain, EU, 2013–2017
Table 43 summarises EU level statistics of human and animal brucellosis, and of food investigated for Brucella, in the EU, during 2013–2017. A more detailed description of these statistics is in the results section of this chapter and in the FBOs.
Table 43.
Summary of Brucella statistics related to humans, major food categories and animal species, EU, 2013–2017
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 378 | 530 | 437 | 460 | 498 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.09 | 0.11 | 0.09 | 0.09 | 0.10 | ECDC |
| Number of reporting MS | 26 | 27 | 27 | 27 | 27 | ECDC |
| Infection acquired in the EU | 247 | 194 | 281 | 325 | 375 | ECDC |
| Infection acquired outside the EU | 46 | 39 | 38 | 43 | 41 | ECDC |
| Unknown travel status or unknown country of infection | 85 | 297 | 118 | 92 | 82 | ECDC |
| Number of outbreak‐related cases | 2 | 0 | 2 | 7 | 10 | EFSA |
| Total number of outbreaks | 1 | 0 | 1 | 2 | 4 | EFSA |
| Food | ||||||
| Milk and milk products | ||||||
| Number of sampled units | 1,338 | 283 | 282 | 1,030 | 778 | EFSA |
| Number of reporting MS | 3 | 2 | 2 | 3 | 2 | EFSA |
| Animals | ||||||
| Bovine animals | ||||||
| Number of positive herds in OBF regions in | ||||||
| OBF or non‐OBF MS | 0 | 2 | 4 | 2 | 2 | EFSA |
| Number of reporting OBF MS | 20 | 19 | 19 | 18 | 16 | EFSA |
| Number of positive herds in non‐OBF regions in non‐OBF MS | 648 | 808 | 938 | 879 | 1,019 | EFSA |
| Number of reporting non‐OBF MS | 8 | 9 | 9 | 10 | 12 | EFSA |
| Small ruminants | ||||||
| Number of positive flocks/herds in ObmF regions | 7 | 2 | 10 | 3 | 4 | EFSA |
| Number of reporting ObmF MS | 20 | 20 | 20 | 19 | 19 | EFSA |
| Number of positive flocks/herds in non‐ObmF regions | 809 | 870 | 1,094 | 1,133 | 1,440 | EFSA |
| Number of reporting non‐ObmF MS | 8 | 8 | 8 | 9 | 9 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States; OBF/ObmF: Officially brucellosis free in cattle/Officially B. melitensis free in sheep and goats.
Reported food data of interest were categorised in the major category ‘Milk and milk products’, and aggregated by year over the period 2013 to 2017 to get an overview, by year, of data sent. The numbers of sampled units reported and the number of reporting MS are extremely low. The annual animal data statistics displayed include the numbers of Officially Free (OF) MS and non‐OF MS, and the number of flocks and herds remaining Brucella‐positive, during in the period 2013–2017.
7.3.2. Humans brucellosis
In 2017, 26 MS provided data and information on brucellosis in humans. In total, 387 cases were reported in the EU. They included 378 confirmed cases, a decrease by 28.7% compared with 530 during 2016. The notification rate was 0.09 cases per 100,000 population (Table 44) and this represents a slight decrease compared with 2016, which was the highest notification rate in the last 5 years. Seven MS (Cyprus, Estonia, Hungary, Latvia, Lithuania, Luxembourg and Malta) and Iceland reported no human cases.
Table 44.
Reported human cases of brucellosis and notification rates per 100,000 population in the EU/EFTA, by country and year, 2013–2017
| Country | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria (OBF/ObmF)b | Y | C | 6 | 6 | 0.07 | 4 | 0.05 | 1 | 0.01 | 1 | 0.01 | 7 | 0.08 |
| Belgium (OBF/ObmF) | Y | C | 8 | 8 | 0.07 | 4 | 0.04 | 9 | 0.08 | 0 | – | 0 | – |
| Bulgaria | Y | A | 2 | 2 | 0.03 | 0 | 0.00 | 36 | 0.50 | 2 | 0.03 | 0 | 0.00 |
| Croatia | Y | C | 0 | 1 | 0.00 | 2 | 0.05 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Cyprus (OBF/ObmF) | Y | C | 1 | 0 | 0.02 | 0 | 0.00 | 0 | 0.00 | 1 | 0.02 | 0 | 0.00 |
| Czech Republic (OBF/ObmF) | Y | C | 1 | 1 | 0.01 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Denmarkc (OBF/ObmF) | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Finland (OBF/ObmF) | Y | C | 1 | 1 | 0.02 | 0 | 0.00 | 0 | 0.00 | 1 | 0.02 | 0 | 0.00 |
| Franced :(OBF) | Y | C | 23 | 21 | 0.03 | 19 | 0.03 | 17 | 0.03 | 14 | 0.02 | 19 | 0.03 |
| Germany (OBF/ObmF) | Y | C | 41 | 41 | 0.05 | 36 | 0.04 | 44 | 0.05 | 45 | 0.06 | 26 | 0.03 |
| Greece | Y | C | 95 | 94 | 0.87 | 119 | 1.10 | 109 | 1.00 | 135 | 1.24 | 159 | 1.44 |
| Hungary (ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Ireland (OBF/ObmF) | Y | C | 2 | 2 | 0.04 | 2 | 0.04 | 0 | 0.00 | 3 | 0.07 | 1 | 0.02 |
| Italye : | Y | C | 99 | 99 | 0.16 | 211 | 0.35 | 105 | 0.17 | 121 | 0.22 | 141 | 0.24 |
| Latvia (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.05 |
| Lithuania (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 2 | 0.07 |
| Luxembourg (OBF/ObmF) | Y | C | 0 | 0 | 0.00 | 1 | 0.17 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Malta (OBF) | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.24 |
| The Netherlands (OBF/ObmF) | Y | C | 2 | 2 | 0.01 | 5 | 0.03 | 7 | 0.04 | 1 | 0.01 | 5 | 0.03 |
| Poland (OBF/ObmF) | Y | C | 2 | 2 | 0.01 | 3 | 0.01 | 4 | 0.01 | 1 | 0.00 | 1 | 0.00 |
| Portugalf | Y | C | 16 | 16 | 0.16 | 50 | 0.48 | 46 | 0.44 | 50 | 0.48 | 22 | 0.21 |
| Romania (OBF, ObmF) | Y | C | 3 | 3 | 0.02 | 1 | 0.01 | 0 | 0.00 | 2 | 0.01 | 0 | 0.00 |
| Slovakia (OBF/ObmF) | Y | C | 1 | 1 | 0.02 | 1 | 0.02 | 1 | 0.02 | 0 | 0.00 | 1 | 0.02 |
| Slovenia (OBF/ObmF) | Y | C | 1 | 1 | 0.05 | 1 | 0.05 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Spaing | Y | C | 68 | 63 | 0.14 | 37 | 0.08 | 33 | 0.07 | 56 | 0.12 | 87 | 0.19 |
| Sweden (OBF/ObmF) | Y | C | 15 | 14 | 0.14 | 19 | 0.19 | 13 | 0.13 | 16 | 0.17 | 10 | 0.11 |
| United Kingdomh (ObmF) | Y | C | – | – | – | 14 | 0.02 | 12 | 0.02 | 11 | 0.02 | 15 | 0.02 |
| EU Total | – | – | 387 | 378 | 0.09 | 530 | 0.11 | 437 | 0.09 | 460 | 0.09 | 498 | 0.10 |
| Icelandi | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway (OBF/ObmF) | Y | C | 3 | 3 | 0.01 | 4 | 0.08 | 2 | 0.04 | 2 | 0.04 | 2 | 0.04 |
| Switzerlandj : (OBF/ObmF) | Y | C | 9 | 9 | 0.11 | 7 | 0.08 | 1 | 0.01 | 3 | 0.04 | 4 | 0.04 |
Y: yes; N: no; A: aggregated data; C: case‐based data;–: no report.
OBF/ObmF: Officially Brucellosis free in cattle/Officially B. melitensis free in sheep and goats.
No surveillance system.
In France, all but one of the 96 metropolitan departments (due to Rev.1 vaccination against Brucella ovis) are ObmF and no cases of brucellosis have been reported in small ruminants since 2003.
In Italy, 11 regions and nine provinces are OBF and also 13 regions and four provinces are ObmF.
In Portugal, six islands of the Azores and the superior administrative unit of Algarve are OBF whereas all nine Azores islands are ObmF.
In Spain, Asturias, the Balearic Islands, the Canary Islands, Castile‐La Mancha, four provinces of Castile and Leon, Catalonia, Basque Country, Galicia, Murcia, La Rioja and Navarre are OBF and Aragon, Asturias, the Balearic Islands, the Canary Islands, Cantabria, three provinces of Castile‐La Mancha, Castile and Leon, Catalonia, Extremadura, Galicia, La Rioja, Navarre, Basque Country and Valencia in Spain are ObmF.
In the United Kingdom, Great Britain (England, Scotland and Wales), the Isle of Man and Northern Ireland are OBF, and the whole of the United Kingdom is ObmF.
In Iceland, which has no special agreement on animal health (status) with the EU, brucellosis (B. abortus, B. melitensis and B. suis) has never been reported.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
As in previous years the highest notification rates of brucellosis were reported in four MS that were non‐OBF and/or non‐ObmF (Table 44): Greece (0.87 cases per 100,000 population), Italy (0.16), Portugal (0.16) and Spain (0.14) together accounting for 72% of all confirmed cases reported in 2017. The lowest notification rates were observed in OBF and ObmF MS where brucellosis cases were mainly travel‐associated. Sweden, which has the status OBF/ObmF and had a relatively high notification rate (0.14 cases per 100,000 population) reported all confirmed brucellosis cases as travel associated.
Most cases of brucellosis (84.3%) with known data on importation and travel destination were reported to be acquired in the EU (Table 43). Among the 54 travel‐associated cases with known probable country of infection, 46 (85.2%) travelled outside EU. The most common travel destinations were Iraq 15 (27.8%), Turkey 9 (16.7%) and Syria 5 (9.3%) of the imported cases, respectively. Eight travel‐associated brucellosis cases (14.8%) were infected within the EU. Greece, Italy and Romania were each reported as probable country of infection for two travel‐associated cases, whereas one case was linked to travel, respectively, in Bulgaria and France.
A clear seasonality was observed in the number of confirmed brucellosis cases in the EU/EEA with more cases reported from April to September. There was a significantly (p < 0.01) declining EU/EEA trend from 2008 to 2017. Three MS (Greece, Portugal and Spain) reported decreasing trends (p < 0.01), whereas Germany and Sweden reported an increasing trend over the same period. During 2013–2017, the EU/EEA trend was not decreasing or increasing (Figure 42). One MS (Greece) continued to report a decreasing trend (p < 0.05) during 2013–2017. None of the countries observed an increasing trend from 2013 to 2017.
Figure 42.
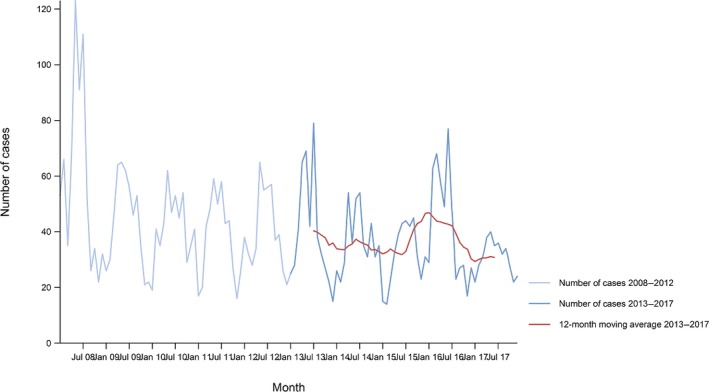
- Source: Austria, Cyprus, Czech Republic, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain and Sweden. Belgium, Bulgaria, Croatia, Luxembourg and the United Kingdom did not report data to the level of detail required for the analysis. Denmark does not have a surveillance system for this disease.
Ten MS provided data on hospitalisation, accounting for 45.8% of confirmed cases in the EU. On average, 60.2% of the confirmed brucellosis cases with known status were hospitalised. In 7 of the 10 countries reporting hospitalisation, the proportion of hospitalised cases ranged between 50% and 100%. One case with fatal outcome was reported in 2017, among 128 confirmed cases reported with outcome by ten MS (33.9% of all confirmed cases in the EU), in a patient with comorbidities and co‐infection with pseudomonas.
Brucella species information was missing for 69.6% of the 378 confirmed cases reported in the EU. Of the 115 cases with known species, 100 (87%) were infected by B. melitensis, 12 (10.4%) by B. abortus and 3 (2.6%) by B. suis.
Figure 43 shows, for the year 2017, the number of domestically acquired confirmed brucellosis cases in humans overlaid with the prevalence of Brucella‐positive sheep flocks and cattle and goat herds. The map shows that Greece, Italy, Portugal and Spain have a higher number of domestically acquired confirmed brucellosis cases in humans and a higher prevalence of Brucella‐positive ruminant flocks and herds.
Figure 43.
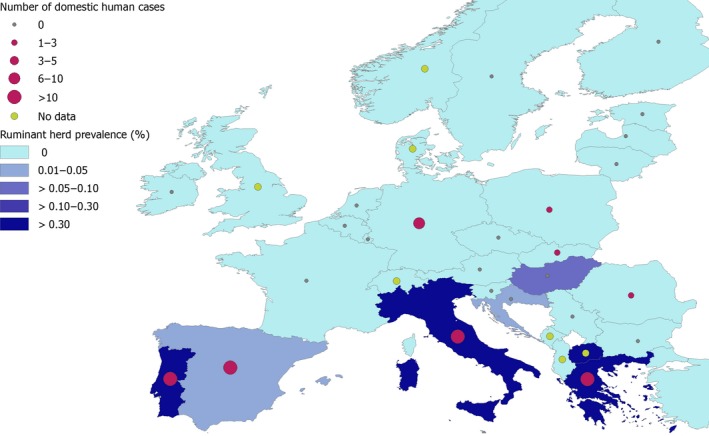
Number of domestically acquired confirmed brucellosis cases in humans, and prevalence of Brucella test‐positive cattle, sheep and goat herds, EU, 2017
Human brucellosis cases associated with food‐borne outbreaks
One FBO due to Brucella was reported in 2017, by Germany, with two affected people, but the incriminated food was unknown.
7.3.3. Brucella in food
Three MS (Italy, Portugal and Spain) provided 2017 Brucella monitoring data in food, from 5,845,208 samples. Italy and Portugal submitted data from raw milk from cows, sheep and goats, from milk from other animal species, from cheese, and from ‘dairy products excluding cheeses’, whereas Spain from fresh meat from cattle, pigs, sheep and goats and horses. Nine samples of pasteurised milk ‘from other animal species or unspecified’ taken by Italy in a processing plant tested positive and all other samples were negative.
7.3.4. Brucella in animals
Cattle
The status of freedom from bovine brucellosis (OBF) is displayed in Table 45. Twenty MS were OBF in 2017. Four MS were non‐OBF with OBF regions. The OBF regions of these four MS are:
in Italy: 11 regions and nine provinces;
in Portugal: all administrative regions within the superior administrative unit of the Algarve as well as six of the nine islands of the Azores;
in Spain: Asturias, the Balearic Islands, the Canary Islands, Castile‐La Mancha, four provinces of Castile and Leon, Catalonia, Basque Country, Galicia, Murcia, La Rioja and Navarre;
in the United Kingdom: England, Scotland, Wales, Northern Ireland and the Isle of Man in the United Kingdom (Channel Islands Jersey and Guernsey are not yet OBF).
Table 45.
Status of countries on bovine brucellosis, EU, 2017
| Member State (MS) | Officially free of bovine brucellosis (OBF) |
|---|---|
| Austria | |
| Belgium | |
| Bulgaria | |
| Croatia | |
| Cyprus | |
| Czech Republic | |
| Denmark | |
| Estonia | |
| Finland | |
| France | |
| Germany | |
| Greece | |
| Hungary | |
| Ireland | |
| Italy | |
| Latvia | |
| Lithuania | |
| Luxembourg | |
| Malta | |
| Netherlands | |
| Poland | |
| Portugal | |
| Romania | |
| Slovakia | |
| Slovenia | |
| Spain | |
| Sweden | |
| United Kingdom |
 All regions of the MS are officially free.
All regions of the MS are officially free.
 Not all regions of the MS are officially free.
Not all regions of the MS are officially free.
 No region of the MS is officially free.
No region of the MS is officially free.
Finally, four MS were non‐OBF without OBF regions: Bulgaria, Croatia, Greece and Hungary.
Norway, Switzerland and Liechtenstein were OBF in accordance with EU legislation and. Iceland, which has no special agreement on animal health (status) with the EU, has never reported brucellosis due to B. abortus, B. melitensis or B. suis.
Figure 44 displays the herd prevalence of brucellosis in cattle at region or national levels for MS and non‐MS during 2017. It shows that the infection is not reported by most MS and regions of the EU, whereas it is present still in few MS in southern Europe with Sicily, in Italy, having the highest reported regional prevalence with 2.8% of positive herds.
Figure 44.
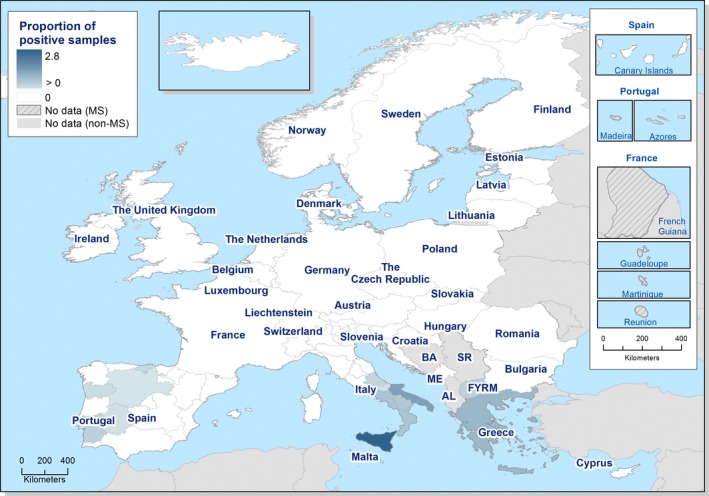
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: Former Yugoslav Republic of Macedonia; ME: Montenegro; SR, Serbia.
In the EU OBF regions, there were in total 1,961,231 cattle herds in 2017. Bovine brucellosis was not reported in any of the OBF regions in the reporting year. Bovine brucellosis was not detected either in 2017 in the non‐MS: Iceland, Norway, Switzerland and Liechtenstein. In the years 2012–2016, there had been, respectively, 9, 2, 2, 4 and 2 cattle herds reported infected in OBF regions, meaning it was an extremely rare event.
In 2017, in the eight non‐OBF MS, there were 243,030 cattle herds in their non‐OBF regions. Three of these MS (Italy, Portugal and Spain) had their eradication programmes cofinanced by the EU. The number of positive herds reported by these MS was: 457 in Italy (510 in 2016), 62 in Portugal (64 in 2016) and 21 in Spain (26 in 2016). Of the five non‐cofinanced non‐OBF MS, only Greece reported infected herds: 108 (208 in 2016), whereas Bulgaria, Croatia, Hungary and the United Kingdom did not report positive herds in 2017.
From 2012 to 2017, the annual number of reported Brucella test‐positive cattle herds in the EU non‐OBF regions decreased by 45% from 1,181 to 648, whereas the decrease between 2017 and the previous year was 20%. Concomitantly, from 2012 to 2017, the herd prevalence slightly increased from 0.10% to 0.21%, mainly due to the drastic decrease in the total number of cattle herds from 1,162,978 to 311,874 during the same period (Figure 45).
Figure 45.
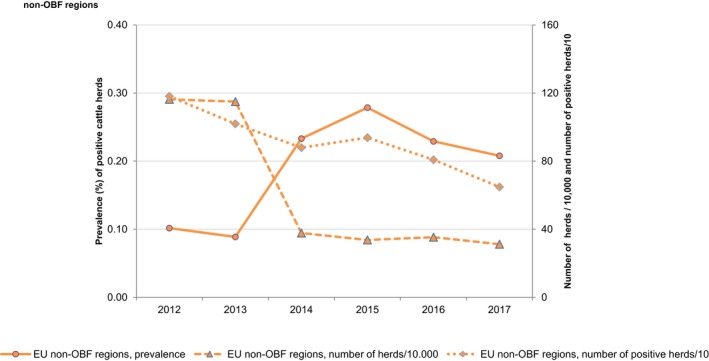
- Non‐OBF: Non‐officially brucellosis free in cattle.
Figure 46 displays the proportion of Brucella test‐positive cattle herds in the non‐OBF regions of three MS (Italy, Spain and Portugal) with EU cofinanced eradication programmes for bovine brucellosis, and in one non‐OBF MS, Greece – that had no EU funded eradication programme – by year in the period 2004–2017. The prevalence in Greece showed a huge variation across years from a minimum 2% in 2008 to a maximum 12% in 2012. Portugal and Spain showed a prevalence consistently decreasing from about 2% to below 0.5% during recent years, whereas Italy showed a prevalence consistently decreasing from about 4% to below 2% during recent years.
Figure 46.
Prevalence of Brucella test‐positive cattle herds, in Greece, Italy, Portugal and Spain, 2004–2017
Two preaccession countries, namely the Former Yugoslav Republic of Macedonia and Montenegro, submitted monitoring data on bovine brucellosis for the second consecutive year. The Former Yugoslav Republic of Macedonia reported 78 positive out of 22,194 herds (0.35%) compared with 82 (0.31%) reported in 2016, whereas Montenegro did not report any positive herd in the last 2 years, out of 24,076 cattle herds present in the country.
Sheep and goats
The status on freedom from ovine and caprine brucellosis caused by B. melitensis (ObmF) by country is displayed in Table 46. Twenty MS had the ObmF status in 2017; four were non‐ObmF with ObmF regions. The ObmF regions of these four MS are:
in France: all but one of the 96 metropolitan departments in France (Perrin et al., 2016);
in Italy: 13 regions and four provinces;
in Portugal: the Azores islands;
in Spain: Aragon, Asturias, the Balearic Islands, the Canary Islands, Cantabria, three provinces of Castile‐La Mancha, Castile and Leon, Catalonia, Extremadura, Galicia, La Rioja, Navarre, Basque Country and Valencia.
Table 46.
Status of countries on ovine and caprine brucellosis, EU, 2017
| Member State (MS) | Officially free of ovine and caprine brucellosis (ObmF) |
|---|---|
| Austria | |
| Belgium | |
| Bulgaria | |
| Croatia | |
| Cyprus | |
| Czech Republic | |
| Denmark | |
| Estonia | |
| Finland | |
| France | |
| Germany | |
| Greece | |
| Hungary | |
| Ireland | |
| Italy | |
| Latvia | |
| Lithuania | |
| Luxembourg | |
| Malta | |
| Netherlands | |
| Poland | |
| Portugal | |
| Romania | |
| Slovakia | |
| Slovenia | |
| Spain | |
| Sweden | |
| United Kingdom |
 All regions of the MS are officially free.
All regions of the MS are officially free.
 Not all regions of the MS are officially free.
Not all regions of the MS are officially free.
 No region of the MS is officially free.
No region of the MS is officially free.
Finally, four MS were non‐ObmF without ObmF regions: Bulgaria, Croatia, Greece and Malta.
Norway, Switzerland and Liechtenstein were ObmF in accordance with EU legislation. Iceland, which has no special agreement on animal health (status) with the EU, has never reported brucellosis due to B. abortus, B. melitensis or B. suis.
Figure 47 displays the herd prevalence of brucellosis in sheep and goats at region or national levels for MS and non‐MS in 2017. It shows that the infection is not reported in most MS and regions of the EU, whereas it is present still in few MS in southern Europe with Sicily, in Italy, having the highest reported regional prevalence with 2.9% of positive flocks/herds. In the EU ObmF regions, there were in total 1,052,955 sheep and goat flocks/herds, in 2017. B. melitensis was only reported from two sheep and goat flocks/herds in ObmF regions: one in Italy and one in Spain. B. melitensis was not detected either in 2017 in sheep and goat flocks/herds in the non‐MS: Iceland, Norway, Switzerland and Liechtenstein. In the years 2012–2016, there had been, respectively, 5, 4, 3, 10 and 2 sheep and goat flocks/herds reported infected in these ObmF regions, meaning it was an extremely rare event.
Figure 47.
- AL: Albania; BA: Bosnia and Herzegovina; FYRM: Former Yugoslav Republic of Macedonia; ME: Montenegro; SR, Serbia.
In 2017, in the eight non‐ObmF MS there were 256,154 sheep and goat flocks/herds in their non‐ObmF regions. Four of these MS (Croatia, Italy, Portugal and Spain) had their eradication programmes cofinanced by the EU. The number of positive flocks/herds reported by these MS was: 5 in Croatia (8 in 2016), 362 in Italy (447 in 2016), 396 in Portugal (325 in 2016) and 18 in Spain (49 in 2016). Of the four non‐cofinanced non‐ObmF MS, Bulgaria reported 6 infected flocks/herds (0 in 2016) and Greece 27 (41 in 2016), whereas France and Malta did not report any in 2017.
From 2012 to 2017, the annual number of reported B. melitensis‐positive sheep and goat flocks/herds in the EU non‐ObmF regions decreased by 52% from 1,693 to 814, whereas the decrease between 2017 and the previous year was 7%. Concomitantly, from 2012 to 2017, the flock/herd prevalence decreased from 0.45% to 0.24% while the total number of sheep flocks and goat herds decreased by 9% from 377,690 to 345,353 (Figure 48).
Figure 48.
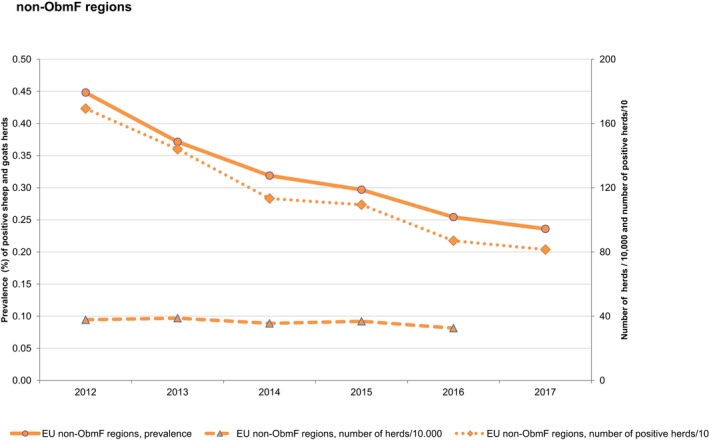
- Non‐ObmF: Non‐officially B. melitensis free in sheep and goats.
Figure 49 displays the reported prevalence of B. melitensis test‐positive sheep flocks and goat herds in the non‐ObmF regions of the four cofinanced MS, and in one non‐OBF MS, Greece – that had no EU funded eradication programme during 201713 – by year in the period 2004–2017. To note that in 2016 and 2017, only vaccination was cofinanced in Greece. Also for Greece, it is of note that the monitoring data reported on brucellosis in sheep and goats are exclusively from the eradication programme that runs in the Greek islands. The prevalence in Greece varied across years from a minimum 0.4% in 2015 to a maximum of 8.6% in 2012.
Figure 49.
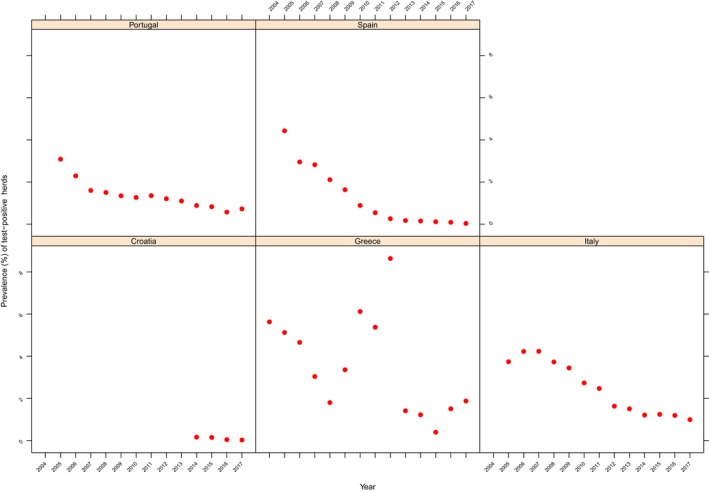
Prevalence of Brucella melitensis test‐positive sheep and goat herds, in Croatia, Greece, Italy, Portugal and Spain, 2004–2017
Italy and Portugal reported a low (> 1–10%) to very low (0.1–1%) prevalence during this period, decreasing for both MS. The 2017 prevalence in Portugal was at a slightly higher level compared with 2016. Croatia and Spain reported a very low prevalence (0.1–1%) to a rare detection (< 0.1%), and both decreasing. The Greek statistics display huge variation across years.
Two pre‐accession countries, namely the Former Yugoslav Republic of Macedonia and Montenegro submitted monitoring data on ovine and caprine brucellosis for the second consecutive year. The Former Yugoslav Republic of Macedonia reported 97 positive out of 8,246 herds (1.18%) compared to 129 (1.51%) in 2016, whereas Montenegro did not report any positive flock or herd in the last 2 years, out of 7,968 sheep flocks and goat herds present in the country.
Complementary to 2017 reports from cattle and from sheep and goats, Brucella species were reported from a wide range of animal species; Brucella spp. from wild seals and farmed water buffalos; B. abortus from dogs (pets), dolphins, pigs, wild boar and wild deer; B. melitensis from sheep and goats, breeding pigs and from wild mountain goats in France; B. ovis from sheep; B. suis from (breeding) pigs, wild boars, hares and wild roe deer; B. canis from dogs and B. ceti from dolphins; and unspecified Brucella species from badgers, bears, wild roe deer, dolphins, foxes, wild boar and wild wolves.
7.4. Discussion
Brucellosis is a rare, although severe disease in the EU, with most of the human cases hospitalised. The EU notification rate of brucellosis in humans declined in 2017 and the number of reported cases of brucellosis in humans in 2017 was the lowest in the last 5 years. During 2017, the highest notification rates and most of the domestic brucellosis cases were reported from four MS (Greece, Italy, Portugal and Spain) that are not officially brucellosis free in cattle, sheep or goats. The number of confirmed cases declined over 50% in Italy, after the high increase of cases reported in 2016, mainly in one region in the country. Greece and Portugal also reported decreased numbers of confirmed cases. In Greece, the trend has been decreasing significantly since 2008, and it is the only MS having a declining trend in 2013–2017. Spain reported the fourth highest notification rate in 2017 and cases increased again after a decline in the last 2 years. These four MS, which are not officially brucellosis free in cattle, sheep or goats, consistently report the highest notification rates within EU.
Bovine brucellosis and ovine and caprine brucellosis have been eradicated by most EU MS. As a result, human brucellosis has become quite rare in Northern and Western Europe, where most cases are travel associated. Imported cases are mainly in travellers, although an increased disease incidence may occur in recently arrived migrants in some MS (Norman et al., 2016, and Georgi et al., 2017). Since 2014, a significant increase of imported infections caused by B. melitensis has been noticed in Germany in patients predominantly originated from Middle East including Turkey and Syria (Georgi et al., 2017). The human B. melitensis cases that occurred in Sweden during the period 2008–2012 reflected the Swedish migration trends of groups from Iraq, Afghanistan and Somalia (Garofolo et al., 2016).
In food, very few monitoring data are reported during these last years by few non‐OF MS Italy, Portugal and Spain. For 2017, Brucella‐positive findings were reported with nine samples of pasteurised milk ‘from other animal species or unspecified’ at processing plant found positive for Brucella in Italy. The other two MS (Portugal and Spain) reported no positive surveillance results in food. Non‐food‐borne transmission of brucellosis to humans also happens by direct contact with infected animals.
In livestock, bovine brucellosis and ovine and caprine brucellosis have been eradicated by most MS. As a result, reported food‐borne disease outbreaks due to Brucella have become rare in large areas of the EU and, only one FBO due to Brucella was reported in 2017, by Germany, with two affected people, but the incriminated food was unknown. In the EU regions officially free from bovine brucellosis and ovine and caprine brucellosis, no infected herds were reported during 2017, except for two B. melitensis‐infected sheep and goat herds (one in Italy and one in Spain). In the EU non‐OBF regions, the overall prevalence of bovine brucellosis was 0.21% in 2017, continuing the slight increase observed since years, whereas the prevalence of ovine and caprine brucellosis in the non‐ObmF regions was 0.24% in 2017 and was reduced by 50% compared with 2012 when prevalence was 0.45%.
Brucellosis in cattle and sheep and goat herds was reported by Greece during 2017, as previous years, at low prevalence levels (> 1–10%). The situation in Greece is however peculiar as vaccination programmes run against both brucellosis in cattle (in mountainous areas) and sheep and goats (on the mainland and some bigger islands). The reported Greek data pertain to unvaccinated areas and varied considerably from one year to another. As such, these data are less comparable with data from officially free regions from Italy, Portugal and Spain, because these MS apply the same monitoring programme with an exhaustive coverage of the national herd.
These findings underline that brucellosis is still an animal health problem with public health relevance in few EU MS.
In‐depth information on the specific prevalence situations and their trends in the MS – and of brucellosis in cattle and sheep and goat as a source for humans – can be found in the 2017 Annual national zoonoses country reports referenced in Section 7.5.
In 2017, Brucella spp. were reported to be isolated from a wide range of animal species, both domestic and wild, reflecting the broad host range, primarily in mammals. France, bovine brucellosis officially free since 2005 with no cases reported in domestic/wild ruminants since 2003, reported isolations of B. melitensis from wild mountain goats. Previously, Mick et al. (2014) reported on the risk of transmission to livestock and spillover of B. melitensis from wildlife to domestic ruminants and the sustainability of the infection in Alpine ibex in the French Alps. In France, it may be furthermore underlined that seven human cases were identified between 2004 and 2016, all confirmed by the isolation of B. suis biovar 2 in clinical specimens (Mailles et al., 2017). All patients had direct contact with wild boars while hunting or preparing wild boar meat for consumption. Five patients had chronic medical conditions possibly responsible for an increased risk of infection.
7.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Surveillance Atlas | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| Animals | EURL for Brucella | https://sites.anses.fr/en/minisite/lrue-brucellose/brucellosis-home |
| Summary Presentations on the situation as regards Bovine Brucellosis and Brucellosis in Sheep and Goats control and eradication programmes in Member States | https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en#20180919 | |
| Brucellosis eradication – Croatia | https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20180613_brucellosis_eradication_hrv.pdf | |
| Brucellosis eradication – Greece | https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20180613_brucellosis_eradication_grc.pdf | |
| Brucellosis eradication – Italy | https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20180613_brucellosis_eradication_ita.pdf | |
| Brucellosis eradication – Portugal | https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20180613_brucellosis_eradication_por.pdf | |
| Brucellosis eradication – Spain | https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20180613_brucellosis_eradication_esp.pdf, and https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20171130_brucellosis-melitensis_esp.pdf | |
| General information on EU Food Chain Funding | https://ec.europa.eu/food/funding_en | |
| 2003/467/EC: Commission Decision of 23 June 2003 setting up the official tuberculosis, brucellosis, and enzootic‐bovine‐leukosis‐free status of certain Member States and regions of Member States as regards bovine herds (Text with EEA relevance) (notified under document number C(2003) 1925) | https://eur-lex.europa.eu/eli/dec/2003/467/oj/eng | |
| 93/52/EEC: Commission Decision of 21 December 1992 recording the compliance by certain Member States or regions with the requirements on brucellosis (B. melitensis) and according them the status of a Member State or region officially free of the disease | https://eur-lex.europa.eu/eli/dec/1993/52/oj/eng | |
| General information on National Veterinary Programmes, in EU | https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en | |
| EU approved and cofinanced veterinary programmes for Bovine Brucellosis and Brucellosis in Sheep and Goats carried out by the MS | http://ec.europa.eu/dgs/health_food-safety/funding/cff/animal_health/vet_progs_en.htm | |
| World Organisation for Animal health, Summary of Information on Brucellosis | http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/Disease_cards/BCLS-EN.pdf | |
| 2016 National Veterinary Programmes funded (cofinanced) by the EU for bovine brucellosis and in ovine and caprine animals brucellosis (approved programmes and type of measures approved) | https://ec.europa.eu/food/sites/food/files/safety/docs/cff_animal_vet-progs_working_doc_12114_rev2_2016.pdf | |
| EU Task Force on the eradication of animal diseases – Brucellosis subgroup reports | https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en | |
| Bad Bug Book (Second Edition), Food‐borne Pathogenic Microorganisms and Natural Toxins Handbook, Center for Food Safety and Applied Nutrition, Food and Drug Administration (FDA), USA | https://www.fda.gov/food/foodborneillnesscontaminants/causesofillnessbadbugbook/ |
8. Trichinella
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at https://doi.org/10.5281/zenodo.1475841
8.1. Abstract
In 2017, 224 trichinellosis cases in humans were reported in the EU. The EU notification rate was 0.03 cases per 100,000 population, which represented an increase of 50.0% compared with 2016. Bulgaria reported the highest notification rate, followed by Croatia, Lithuania and Romania. The EU trend for trichinellosis was greatly influenced by a number of smaller and larger outbreaks with peaks often occurring in January–February. The most commonly reported species in human cases was Trichinella spiralis followed by Trichinella britovi. Trichinellosis cases in humans were related to FBOs and mainly linked to the consumption of meat and meat products from pigs and/or wild boar.
In 2017, Trichinella infections in domestic animals in the EU were not observed in 71,625,597 fattening pigs, 1,677,660 breeding pigs and 103,444 slaughtered batches from pigs kept under controlled housing conditions, confirming that the farming conditions are the key factor to prevent this zoonosis. In total, 224 (< 0.01%) out of 121,957,976 tested fattening pigs and 132 out of 17,799 farmed wild boars not kept under controlled housing conditions, were positive. Romania accounted for most positive pigs followed by Spain, Croatia, Poland, France and Bulgaria. In total, 1,228 (0.08%) hunted wild boars tested positive. As in 2015 and 2016, no Trichinella infections were observed in solipeds in the EU in 2017. In the red fox, which can be considered as an indicator animal, the prevalence of Trichinella was 1.2%. In the last 5 years (2013–2017), the trend of Trichinella infections in domestic animals was stable, with infections documented only in a few hundred of free‐ranging and backyard pigs and farmed wild boar reared in rural EU regions. During the last 5 years, the reported EU prevalence of Trichinella was reduced in the wild‐boar population (by threefold) and in the red fox population (by twofold).
8.2. Surveillance and monitoring of Trichinella in the EU
8.2.1. Humans
The notification of Trichinella infections in humans is mandatory in all EU MS, Iceland, Norway and Switzerland, except in three MS (Belgium, France and the United Kingdom) having voluntary surveillance systems. No surveillance system for trichinellosis exists in Denmark. The surveillance systems for trichinellosis cover the whole population in all MS except in Belgium. All countries report case‐based data except Belgium and Bulgaria, which reported aggregated data. Both reporting formats were included to calculate numbers of cases, notification rates and disease trends.
In humans, diagnosis of Trichinella infections is primarily based on clinical symptoms and serology (indirect enzyme‐linked immunosorbent assay (i‐ELISA) and Western blot). Histopathology on muscle biopsies is rarely performed.
8.2.2. Animals
Trichinella monitoring data from domestic pigs (both fattening and breeding animals), farmed wild boar and solipeds
According to the Commission Regulation 2015/1375/,14 all Trichinella susceptible animals intended for human consumption in the EU, i.e. domestic pigs (both fattening and breeding animals), farmed wild boar and solipeds, should be tested for presence of parasite larvae in the muscles unless carcasses are appropriately frozen to inactivate the parasite. It follows that data on Trichinella infections in these animals are comparable across MS because the monitoring schemes are harmonised and the data collected and reported to EFSA originate from a census sampling. Domestic pigs, farmed and hunted wild boar, and other wild animals (e.g. bears) that are not placed on the EU market (e.g. intended for own consumption) are exempted from the Commission Regulation 1375/2015 and their control falls under the national legislation. Commission Regulation (EU) No. 1375/2015 states that the reporting of data on domestic swine shall, at least, provide specific information related to number of animals tested that were raised under controlled housing conditions as well as the number of breeding sows, boars and fattening pigs tested. Further, the Regulation states that a negligible risk status for a country or region is no longer recognised. Belgium and Denmark have had such a status since 2011, and the holdings and compartments of domestic swine in those two MS complied with the conditions for controlled housing at the date when this Regulation came into force.
Trichinella monitoring data from animals other than domestic pigs, farmed wild boar and solipeds
MS should monitor the circulation of these nematodes in the main natural reservoir hosts (carnivore and omnivore animals) to acquire information on the risk of transmission to domestic animals and from them to humans, and on the introduction of new Trichinella species from non‐EU countries. However, sampling biases and imprecision due to limited numbers of specimens examined preclude extending findings to reflect real prevalence or accurate prevalence estimations. So, these are monitoring data provided by the MS to EFSA and that are generated by non‐harmonised monitoring schemes across MS and for which no mandatory reporting requirements exist. Since the main reservoir hosts of Trichinella are wild animals, their biology and ecology vary from one MS to another and from one region or habitat in the same MS to another due to the human and environmental impact on the ecosystems, resulting in different transmission patterns and prevalence of infection. It follows that data from Trichinella in wild animals are not fully comparable between MS and the reported findings must therefore be interpreted with caution. These data allow descriptive summaries at the EU level but preclude subsequent data analysis such as assessing temporal and spatial trends.
8.2.3. Food‐borne outbreaks of human trichinellosis
The reporting of FBO of human trichinellosis is mandatory according the Zoonoses Directive 2003/99/EC. Further details are provided in the chapter on FBO.
8.3. Results
8.3.1. Trichinellosis in humans
In 2017, 224 cases of trichinellosis, including 168 confirmed cases, were reported in 27 MS (Table 47). The EU notification rate was 0.03 cases per 100,000 population, which represented an increase of 50.0% compared with 2016 (0.02 cases per 100,000 population) and mainly due to an increased number of cases in Romania (+38) and Bulgaria (+20). In 2017, Bulgaria had the highest notification rate in the EU (0.77 cases per 100,000), followed by Croatia, Lithuania and Romania with 0.51, 0.32 and 024 cases per 100,000 population, respectively. Together, these four countries accounted for 79.2% of all confirmed cases reported at the EU level in 2017. Thirteen MS reported zero confirmed cases in 2017 including four MS (Cyprus, Finland, Luxembourg and Malta) that have never reported any trichinellosis cases. Portugal reported the first trichinellosis case since the beginning of the surveillance in 2007. Three other countries (the Czech Republic, the Netherlands and the United Kingdom) have reported only one case each since the beginning of EU level surveillance in 2007.
Table 47.
Reported human cases of trichinellosis and notification rates per 100,000 population in the EU/EFTA, by country and year, 2013–2017
| Country | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 3 | 3 | 0.03 | 2 | 0.0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Belgiumb | Y | A | 2 | 0 | – | 0 | – | 0 | – | 16 | – | 1 | – |
| Bulgaria | Y | A | 55 | 55 | 0.77 | 35 | 0.49 | 22 | 0.31 | 60 | 0.83 | 36 | 0.49 |
| Croatia | Y | C | 37 | 21 | 0.51 | 5 | 0.12 | 3 | 0.07 | 3 | 0.07 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czech Republic | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Denmarkc | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 2 | 0.15 | 0 | 0.00 | 0 | 0.00 |
| Finland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| France | Y | C | 8 | 8 | 0.01 | 3 | 0.00 | 3 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Germany | Y | C | 2 | 2 | 0.00 | 4 | 0.00 | 3 | 0.00 | 1 | 0.00 | 14 | 0.02 |
| Greece | Y | C | 1 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Hungary | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Ireland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Italy | Y | C | 4 | 4 | 0.01 | 5 | 0.01 | 36 | 0.06 | 4 | 0.01 | – | – |
| Latvia | Y | C | 1 | 1 | 0.05 | 1 | 0.05 | 4 | 0.20 | 5 | 0.25 | 11 | 0.54 |
| Lithuania | Y | C | 9 | 9 | 0.32 | 1 | 0.03 | 21 | 0.72 | 5 | 0.17 | 6 | 0.20 |
| Luxembourg | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Malta | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlands | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Poland | Y | C | 9 | 9 | 0.02 | 4 | 0.01 | 1 | 0.00 | 6 | 0.02 | 4 | 0.01 |
| Portugal | Y | C | 1 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Romania | Y | C | 86 | 48 | 0.24 | 26 | 0.13 | 55 | 0.28 | 221 | 1.11 | 116 | 0.58 |
| Slovakia | Y | C | 1 | 1 | 0.02 | 1 | 0.02 | 1 | 0.02 | 0 | 0.00 | 5 | 0.09 |
| Slovenia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.05 |
| Spain | Y | C | 5 | 5 | 0.01 | 12 | 0.03 | 3 | 0.01 | 1 | 0.00 | 23 | 0.05 |
| Sweden | Y | C | 0 | 0 | 0.00 | 2 | 0.02 | 1 | 0.01 | 1 | 0.01 | 0 | 0.00 |
| United Kingdom | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.00 | 0 | 0.00 |
| EU Total | – | – | 224 | 168 | 0.03 | 101 | 0.02 | 155 | 0.03 | 324 | 0.06 | 217 | 0.04 |
| Iceland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0.0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0.0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Switzerlandd | Y | C | 1 | 1 | 0.01 | 0 | 0.00 | 2 | 0.02 | 0 | 0.00 | 1 | 0.01 |
Y: yes; N: no; A: aggregated data; C: case‐based data; –: no report.
Sentinel surveillance, disease not under formal surveillance. Notification not calculated.
No surveillance system.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
The vast majority (> 97.6%) of trichinellosis cases with known travel status was reported to be domestically acquired. Five MS reported 14 travel‐associated trichinellosis cases of which four cases (28.6%) were infected within EU and two cases (14.3%) infected outside EU. In eight cases (57.1%) or travel status or destination was unknown (Table 48).
Table 48.
Summary of Trichinella statistics related to humans and most important animal species, EU, 2013–2017
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 168 | 101 | 156 | 324 | 217 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.03 | 0.02 | 0.03 | 0.06 | 0.04 | ECDC |
| Number of reporting MS | 27 | 27 | 27 | 27 | 27 | ECDC |
| Domestic EUa | 81 | 53 | 126 | 40 | 170 | ECDC |
| Non‐EU countriesb | 2 | 1 | 0 | 0 | 0 | ECDC |
| Unknown travel/importation | 85 | 47 | 30 | 284 | 47 | ECDC |
| Total number of outbreaks | 11 | 5 | 15 | 17 | 22 | EFSA |
| Number of outbreak‐related cases | 199 | 14 | 119 | 187 | 174 | EFSA |
| Animals | ||||||
| Domestic pigs NRCHCc: number of animals | 121,962,787 | 121,232,589 | 50,645,975 | 69,466,211 | Not available | EFSA |
| Domestic pigs NRCHC: % positive animals | < 0.01% | < 0.01 | < 0.01 | < 0.01 | < 0.01 | EFSA |
| Domestic pigs NRCHC: number of MS | 25 | 25 | 19 | 14 | Not available | EFSA |
| Farmed wild boar: % positive animals | 0.74% | 0.3% | 0% | 0.24% | 0.025% | EFSA |
| Hunted wild boar: % positive animals | 0.08% | 0.04% | 0.08% | 0.1% | 0.1% | EFSA |
| Red foxes: % positive animals | 1.2% | 1.1% | 1.6% | 1.3% | 2% | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
Infections acquired in EU.
Infections acquired outside EU.
NRCHC: not raised under controlled housing conditions.
The EU/EEA trend from 2008 to 2017 in confirmed cases of trichinellosis was substantially influenced by a number of smaller and larger outbreaks, often with peaks in January and February (Figure 50).
Figure 50.
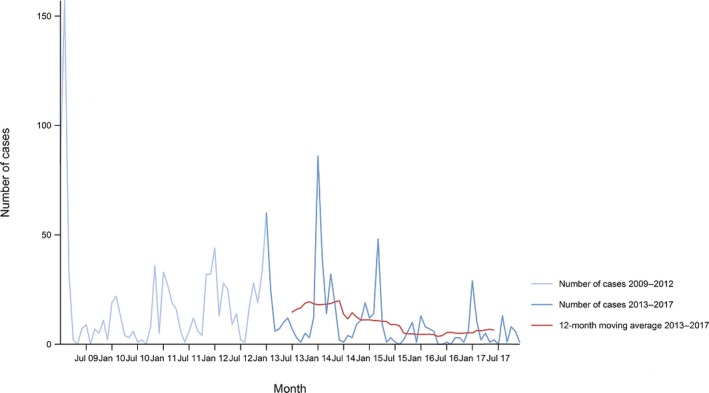
- Source: Austria, Cyprus, Czech Republic, Estonia, Finland, Germany, France, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and United Kingdom. Belgium, Bulgaria, Croatia and Iceland did not report data to the level of detail required for the analysis. Denmark does not have any formal surveillance system for the disease.
The EU trend was significantly decreasing (p < 0.01) in 2013–2017. Between 2008 and 2017, Romania was the only country reporting a significantly decreasing trend. From 2013 to 2017, a decreasing trend was reported in Romania and Latvia. No significant increasing trends were observed for any country during 2008–2017 and during 2013–2017.
Of the 14 MS reporting confirmed cases for 2017, nine provided information on hospitalisation (44.6% of all confirmed cases reported in the EU). On average 74.7% of these cases were reported as having been hospitalised. In five of the nine countries, over 85.0% of the confirmed cases were hospitalised. Nine MS provided information on the outcome of their cases (40.5% of all confirmed cases). No deaths due to trichinellosis were reported in 2017 among the 68 confirmed cases for which this information was available.
Species information was available for 40.5% of the reported confirmed cases from 14 MS. The most commonly reported species was T. spiralis (50.0%) followed by T. britovi (48.5%) and Trichinella nativa (1.5%). Bulgaria reported all cases to be infected by T. britovi.
Table 48 summarises the occurrence of Trichinella in humans as well as in the most significant animal species between 2013 and 2017 and shows that the prevalence in farmed wild boar is higher than that in hunted wild boar.
Table 49 and Figure 51 show for 2017 for each country the number human confirmed cases combined with the number of FBOs due to Trichinella spp. as well as the number of positive animals found in domestic pigs that were raised under not controlled housing conditions, wild boar and other wild life. Human cases are almost entirely linked to FBOs with pig and wild‐boar meat identified as an important vehicle in Eastern European countries. In addition, other wild life species harbour Trichinella spp. in many countries.
Table 49.
Statistics related to the proportions of human food‐borne outbreak cases caused by Trichinella, EU/EFTA, 2017
| Country | ECDC | EFSA | ||||
|---|---|---|---|---|---|---|
| Confirmed human | Food‐borne outbreaks | |||||
| Total | Travel related | Domestic | Unknown or missing | Human cases (illnesses) | FBO | |
| N | N | N | N | N | N | |
| Austria | 3 | 1 | 1 | 1 | – a | – |
| Belgium | 0 | 0 | 0 | 0 | – | – |
| Bulgaria | 55 | – b | – | 55 | 12 | 1 |
| Croatia | 21 | – | – | 21 | 34 | 4 |
| Cyprus | 0 | 0 | 0 | 0 | – | – |
| Czech Republic | 0 | 0 | 0 | 0 | – | – |
| Denmark | – | – | – | – | – | – |
| Estonia | 0 | 0 | 0 | 0 | – | – |
| Finland | 0 | 0 | 0 | 0 | – | – |
| France | 8 | 8 | 0 | 0 | 21 | 1 |
| Germany | 2 | 1 | 1 | 0 | – | – |
| Greece | 1 | 0 | 1 | 0 | – | – |
| Hungary | 0 | 0 | 0 | 0 | – | – |
| Ireland | 0 | 0 | 0 | 0 | – | – |
| Italy | 4 | 0 | 4 | 0 | 4 | 1 |
| Latvia | 1 | 0 | 1 | 0 | – | – |
| Lithuania | 9 | 0 | 9 | 0 | 9 | 1 |
| Luxembourg | 0 | 0 | 0 | 0 | – | – |
| Malta | 0 | 0 | 0 | 0 | – | – |
| Netherlands | 0 | 0 | 0 | 0 | – | – |
| Poland | 9 | 0 | 9 | 0 | 8 | 1 |
| Portugal | 1 | 1 | 0 | 0 | – | – |
| Romania | 48 | 0 | 48 | 0 | 111 | 2 |
| Slovakia | 1 | 0 | 1 | 0 | – | – |
| Slovenia | 0 | 0 | 0 | 0 | – | – |
| Spain | 5 | 3 | 2 | 0 | – | – |
| Sweden | 0 | 0 | 0 | 0 | – | – |
| United Kingdom | 0 | 0 | 0 | 0 | – | – |
| EU Total | 168 | 14 | 77 | 77 | 199 | 11 |
| Iceland | 0 | 0 | 0 | 0 | – | – |
| Norway | 0 | 0 | 0 | 0 | – | – |
| Switzerland | 1 | – | – | – | – | – |
| Bosnia and Herzegovina | – | – | – | – | 22 | 1 |
| Serbia | – | – | – | – | 17 | 2 |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority.
No trichinellosis food‐borne outbreaks reported.
No importation data reported.
Figure 51.
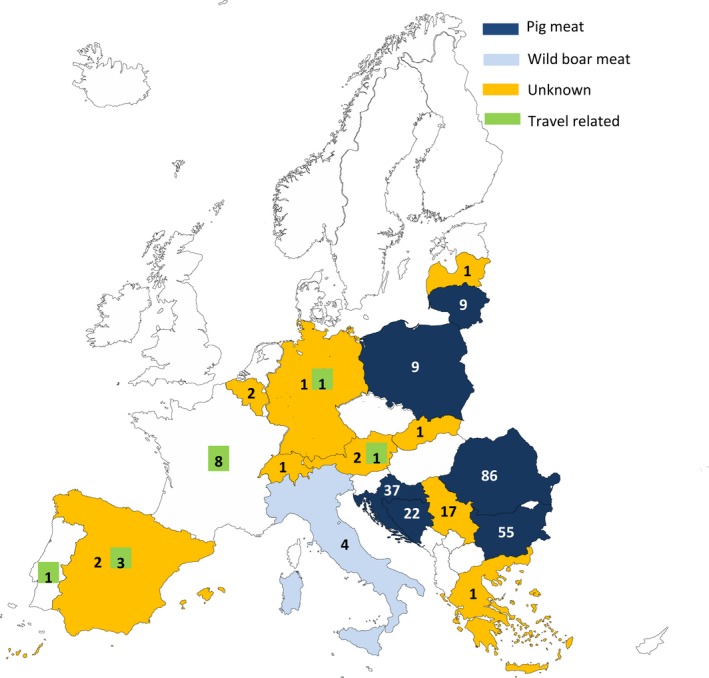
- Countries in which human cases were reported due to food‐borne outbreaks (EFSA data) are in colour according the food vehicle causing the outbreaks (‘pigmeat’, ‘wild‐boar meat’ or ‘unknown’ food vehicle). The number of cases in each country indicates domestic trichinellosis cases (ECDC data); numbers in green box indicate the travel‐related trichinellosis human cases.
8.3.1.1. Human trichinellosis cases associated with food‐borne outbreaks
Trichinella was identified in 11 FBOs affecting 199 people (notified FBO cases) in seven MS, as reported to EFSA. Overall, for the year 2017, there were 77 domestic (acquired within the country) cases reported to the TESSy (Table 49), whereas the number of reported human trichinellosis cases infected domestically and through travel within EU during 2017 was 81 (Table 48) shows data reported by countries to TESSy managed by ECDC and to the FBOs’ database managed by EFSA. It is important to clarify that the case classification for reporting is different between these two databases. In TESSy, the cases reported are classified based on the EU case definition. All these cases visited a doctor, and are either confirmed by laboratory test (confirmed case) or not (probable case and classification is based the clinical symptoms and epidemiological link). Cases that never visited a doctor are not reported to TESSy. Moreover, probable cases may be missing in TESSy, as these data are not analysed or published and there is no incentive for reporting such cases. Information on which case is linked or not to an outbreak is not systematically collected. In practice, the cases reported to TESSy are considered mostly sporadic cases. In food‐borne disease outbreak situations cases are also classified into confirmed or probable outbreak cases, but currently these data are not collected by EFSA.
Nine of the 11 Trichinella FBO were reported as strong‐evidence outbreaks and reported by Croatia (4), Romania (2), and France, Lithuania and Poland reporting one outbreak each. Bulgaria and Italy each reported one weak‐evidence Trichinella FBO. Strong‐evidence outbreaks accounted for 183 cases, of which 119 needed hospitalisation. This high number of reported hospitalisations due to ‘Meat and meat products’ were mainly attributable to a single large general outbreak by Trichinella spiralis that occurred in household setting in Romania. Further details and statistics on the trichinellosis FBOs reported by seven MS for 2017 are in Chapter 16 on FBO.
8.3.2. Trichinellosis in animals
The data reported by MS and non‐MS on the occurrence of Trichinella in pigs raised under controlled housing conditions, pigs and farmed wild boar not raised under controlled housing conditions has been summarised in Table 50 and data for wild animals are presented in Table 51.
Table 50.
Number of Trichinella‐positive/tested (% positive) domestic pigs and farmed wild boar in reporting MS and non‐MS, by housing conditions, EU, 2017
| Country | Not controlled housing conditions or not specifieda | Controlled housing conditions | |||
|---|---|---|---|---|---|
| Farmed wild boar | Fattening pigs | Breeding pigs | Fattening pigs | Breeding pigs | |
| AT | 0/1,544 | 0/5,038,213 | 0/85,794 | 0 | 0 |
| BE | 0 | 0 | 0/879,180 | 0/9,590,067 | 0 |
| BG | 0 | 1/28,009 (< 0.01)a | 0 | 0/479,916b | 0/4,001c |
| HR | 0 | 5/338,564 (< 0.01)d | 0/5,289 | 0/854,187e | 0/8,505 |
| CY | 0 | 0/558,441 | 0/10,406 | 0 | 0 |
| CZ | 0 | 0/2,374,022 | 0 | 0 | 0 |
| DK | 0/437 | 0/675,561 | 0/299,979 | 0/16,171,408 | 0/181,492 |
| EE | 0 | 0/468,899 | 0 | 0/4,134 | 0 |
| FI | 4/361 (1.1) | 0/1,928,570 | 0/34,931 | 0/482 | 0/9 |
| FR | 0/427 | 2/334,067 (< 0.01) | 0 | 0/203,901 | 0 |
| DE | 0 | 0/56,894,081 | 0 | 0 | 0 |
| EL | 0/1,286 | 0/1,013,604f | 0/19,672 | 0 | 0 |
| ES | 0 | 93/20,915,620 (< 0.01)g | 0/1,903 | 0 | 0 |
| HU | 0 | 0/4,529,124 | 0/118,509 | 0 | 0 |
| IE | 0 | 0 | 0 | 0/3,265,789h | 0/92,522 |
| IT | 0/2,037 | 0/121,938i | 0 | 0/9,626,373 | 0/119,728 |
| LV | 0 | 0/447,034 | 0 | 0 | 0 |
| LT | 0 | 0/1,581,740 | 0 | 0 | 0 |
| LU | 0 | 0/154,398 | 0 | 0 | 0 |
| MT | 0 | 0/54,376 | 0/1,031 | 0 | 0 |
| NL | 0 | 0 | 0 | 0/15,241,457 | 0 |
| PL | 0 | 3/22,278,957 (< 0.01)j | 0 | 0 | 0 |
| PT | 0 | 0/181,909 | 0/2,246 | 0/4,180,901 | 0/18,358 |
| RO | 128/10,968 (1.16) | 120/128,054 (0.09) | 0 | 0/4,482,024 | 0/42,008 |
| SE | 0 | 0/397,783 | 0/26,230 | 0/1,037,112 | 0/26,129 |
| SI | 0 | 0/238,557 | 0/6,659 | 0 | 0 |
| SK | 0 | 0/727,797 | 0/10,860 | 0 | 0 |
| UK | 0/739 | 0/448,658 | 0/412,145 | 0/6,487,846k | 0/6,171 |
| EU Total | 132/17,799 (0.74) | 224/121,857,976 (< 0.01) | 0/1,914,834 | 0/71,625,597 | 0/498,923 |
| IS | 0 | 0 | 0 | 0/76,011 | 0 |
| ME | 0 | 0 | 0 | 0/10,896 | 0 |
| NO | 0 | 0/1,648,100 | 0 | 0 | 0 |
| CH | 0 | 0/2,476,085 | 0/32,613 | 0 | 0 |
| Total non‐MS | 0 | 0/4,124,185 | 0/32,613 | 0/86,907 | 0 |
| TOTAL EU + 4 non‐MS | 132/17,799 (0.74) | 224/125,982,161 (< 0.01) | 0/1,947,447 | 0/71,712,504 | 0/498,923 |
Including 342 pigs reported from mixed herds (containing both breeding and fattening pigs).
BG reported 102,554 batches of fattening pigs; including 8,380 pigs from mixed herds (containing both breeding and fattening pigs).
BG reported 890 batches of breeding pigs.
Including 132,171 pigs from mixed herds five of which tested positive.
Including 341 mixed herds (containing both breeding and fattening pigs).
Including 295 piglets from mixed herds (both breeding and fattening pigs).
In addition, Spain reported also 2,498,364 slaughtered batches of fattening pigs all tested negative; of these slaughtered batches, 136,590 originated from mixed herds.
Including 62,682 pigs from mixed herds (both breeding and fattening pigs).
Including 28 wild pigs.
All pigs originated from mixed herds (both breeding and fattening pigs).
Of which 1,223 pigs originated from mixed herds.
Table 51.
Number of Trichinella‐positive/tested (% positive) hunted wild boar and other wild animals in reporting MS and non‐MS, EU, 2017
| Country | Positive/tested (% positive) | |||
|---|---|---|---|---|
| Hunted or not specified wild boar | Brown bears | Red foxes | Other wild animals | |
| AT | 0/22,769 | 0 | 0/11 | 0/7a |
| BE | 0/17,094 | 0 | 0 | 0 |
| BG | 116/11,997 (0.96) | 0 | 0 | 0 |
| HR | 15/29,740 (0.05) | 1/109 (0.91) | 9/152 (5.92) | 0 |
| CY | 0 | 0 | 0/117 | 0 |
| CZ | 1/230,791 (< 0.01) | 0 | 3/2,942 (0.1) | 1/98a |
| DK | 0 | 0 | 0/28 | 0/54b |
| EE | 9/1,515 (0.59) | 6/36 (16.6) | 0 | 0 |
| ES | 435/153,259 (0.28)c | 0 | 0 | 0 |
| FI | 4/717 (0.56) | 10/247 (4.0) | 49/110 (44.5) | 116/478 (24.26)d |
| FR | 3/47,168 (< 0.01) | 0 | 0 | 0 |
| DE | 20/276,255 (< 0.01) | 0 | 0 | 0 |
| HU | 9/87,088 (0.01) | 0 | 0 | 0 |
| IT | 6/133,784 (< 0.01) | 0/2 | 12/2,489 (0.5) | 20/555 (3.6)e |
| LV | 21/5,194 (0.4) | 0 | 0 | 1/16 (6.25)f |
| LU | 0/4,348 | 0 | 0/146 | 0 |
| NL | 0/5,169 | 0 | 0 | 0 |
| PL | 576/240,071 (0.24) | 0 | 0 | 0/107g |
| PT | 3/275 (1.09) | 0 | 0 | 0 |
| RO | 0 | 2/73 (2.7) | 0 | 0 |
| SK | 3/16,522 (0.02) | 0/16 | 6/211 (2.8) | 0 |
| SI | 0/2,174 | 0/89 | 0 | 0 |
| SE | 7/111,845 (< 0.01) | 0/180 | 0/1 | 5/151 (3.3)h |
| UK | 0/614 | 0 | 0/279 | 0 |
| EU Total | 1,228/1,398,389 (0.08) | 19/752 (2.52) | 79/6,486 (1.21) | 143/1,466 (9.75) |
| CH | 0/6,176 | 0 | 0/2 | 4/26 (15.4)i |
| Total EFTA | 0/6,176 | 0 | 0/2 | 4/26 (15.4) |
| Total EU + EFTA | 1,228/1,404,565 (0.08) | 19/752 (2.52) | 79/6,488 (1.21) | 147/1,492 (9.90) |
Badgers.
0/34 minks, 0/15 raccoon dogs, 0/1 seal, 0/4 dolphins.
In addition, 257 slaughtered batches tested negative.
91/226 (40.3%) raccoon dogs, 0/57 wolves, 0/2 wolverines, 12/39 (30.8%) lynxes, 5/16 (31.2%) badgers, 2/8 martens, 4/51 (7.8%) otters, 0/2 beavers, 0/7 minks, 0/2 polecats, 0/2 seals, 0/23 eagles, 0/16 owls and 2/27 (7.4%) falcons.
18/142 (12.6%) wolves, 0/229 badgers, 2/82 (2.4%) stone martens, 0/95 pine marten, 0/1 raccoon dog, 0/2 wild cats, 0/2 polecats, 0/1 weasel, 0/1 lynx.
Beavers.
Synanthropic rats.
1/45 (2.2%) wolves, 0/3 beavers, 0/2 birds, 0/12 seals, 0/5 carnivores from zoo, 0/3 badgers, 4/80 (5.0%) lynxes, 0/1 otter.
0/1 wolf, 0/4 badgers, 4/21 lynxes (19.0%).
In 2017, 32 countries (all 28 MS and four non‐MS) provided information on Trichinella in domestic animals (pigs and/or farmed wild boar) and seven MS (Bulgaria, Croatia, Finland, France, Spain, Poland and Romania) reported positive findings in farmed wild boar and domestic pigs not raised under controlled housing conditions.
Fifteen MS and two non‐MS reported data on breeding and fattening pigs raised under controlled housing conditions, no positive findings were reported.
Data on 71,625,597 fattening pigs, 498,923 breeding pigs and 103,444 slaughtered batches from pigs kept under controlled housing conditions were tested for Trichinella spp. in 15 MS. None of these animals tested positive. Iceland and Montenegro tested 86,907 fattening pigs kept under controlled housing conditions, and all were negative.
Twenty‐five MS and two non‐MS reported data on breeding and fattening pigs or farmed wild boar that were not raised under controlled housing conditions and seven MS reported positive findings among fattening or breeding pigs or farmed wild boar (Table 50). In total, 224 (< 0.01%) fattening pigs were positive. Romania accounted for most positive pigs followed by Spain, Croatia, Poland, France and Bulgaria. Two MS (Romania and Finland) reported Trichinella‐positive farmed wild boars: in total 132 farmed wild boars out of 17,799 (0.74%) tested animals from eight MS. Norway and Switzerland tested 4,124,185 fattening pigs from not controlled housing conditions and all were tested negative (Table 50).
As shown in Figure 52 from 2012 to 2016 (5‐year period), Trichinella spp. were not documented in domestic pigs and farmed wild boar in 16 MS (Austria, Belgium, Cyprus, the Czech Republic, Denmark, Estonia, Finland, Hungary, Ireland, Luxembourg, Malta, the Netherlands, Portugal, Slovenia, Sweden and the United Kingdom) while this was the case in the other 12 MS (Bulgaria, Croatia, France, Germany, Greece, Italy, Latvia, Lithuania, Poland, Romania, Slovakia and Spain).
Figure 52.
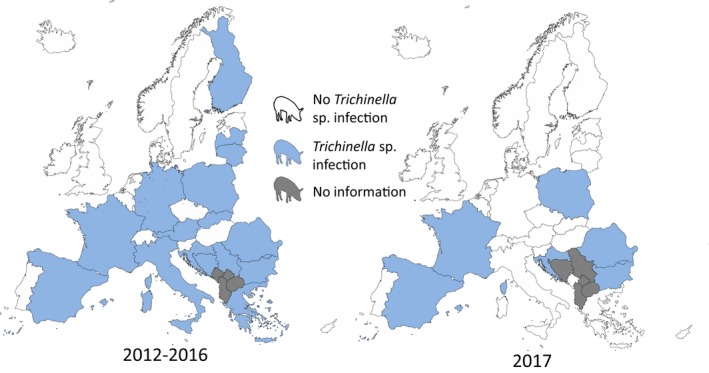
In total in the EU, more than 199 million animals (breeding pigs, fattening pigs and unspecified pigs kept under controlled or not controlled housing conditions and farmed wild boar) were tested for Trichinella and 356 were positive (1.8 per million tested). Most (69.6%) of the positive findings were reported by Romania followed by Spain (26.1%), Croatia (1.4%), Finland (1.1%), Poland (0.8%), France (0.6%) and Bulgaria (0.3%). All Trichinella spp. infected pigs and farmed wild boar originated from animals not kept under controlled housing conditions. Most (93.3%) Trichinella spp. isolates from swine were identified at the species level; 167 (74.5%) as T. spiralis, 41 (18.3%) as T. britovi and 1 (0.4%) as T. pseudospiralis.
As in 2015 and 2016, no positive findings were reported in domestic solipeds (144,912 animals tested and reported by 22 MS (Austria, Belgium, Bulgaria, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Luxembourg, Malta, the Netherlands, Portugal, Romania, Slovenia, Spain, Sweden and the United Kingdom) and in two non‐MS (Iceland and Switzerland).
Twenty‐one MS and one non‐MS provided data on hunted wild boar (Table 51). Fifteen MS reported 1,228 positive findings out of 1,398,389 animals tested (0.08%). Most of the positive animals were reported by Poland, Spain, Bulgaria, Latvia, Germany and Hungary. Most of the findings were reported as Trichinella spp. (92.4%) followed by T. britovi (3.6%), T. spiralis (3.2%), T. pseudospiralis (0.5%) and T. nativa (0.16%).
Eleven MS and one non‐MS reported data on Trichinella in red foxes (Vulpes vulpes) with 79 (1.1%) positive out of 6,486 tested animals in five MS. Eight MS reported data on Trichinella in brown bears (Ursus arctos) with 19 (2.52%) positive out of 752 tested animals in four MS. Eight MS and one non‐MS reported data on Trichinella in other wild animals. Positive findings were detected in eight species (lynx, raccoon dog, wolf, badger, marten, otter, beaver and falcons) of five MS and in one non‐MS (Table 51). The highest number of positive animals was seen in lynxes, raccoon dogs and wolves.
8.4. Discussion
Trichinellosis is a rare but serious human disease, which is still present in the EU. Almost half of the MS reported zero cases including four MS (Cyprus, Finland, Luxembourg and Malta) that have never reported any trichinellosis cases since the beginning of the EU level surveillance in 2007.
Most human cases were reported from a few MS mainly in countries in the eastern part of Europe and were domestically acquired. The EU/EEA trend for trichinellosis has been greatly affected by the number and size of disease outbreaks. Number of cases and EU notification rate has, however, been steadily decreasing in the last 5 years since 2012, and in 2016, the lowest rate (0.02) was reported since the beginning of the EU level surveillance. Despite the increase of cases and notification rate again in 2017, the 5‐year trend from 2012–2017 was significantly decreasing. The decrease was mainly due to a markedly reduced number of trichinellosis cases over the same period reported from Romania, which had experienced most Trichinella outbreaks in previous years. The main reason of this reduction was the increasing number of pigs raised under controlled housing conditions and the reduction of pigs not raised under controlled housing conditions, farmer's education and increased control at slaughtering of pigs not raised under controlled housing conditions. These measures strongly reduced the parasite biomass in the domestic habitat and therefore the risk of acquiring the infection for humans. Despite the reduced numbers of human cases since the beginning of the trichinellosis EU level surveillance in 2007, two MS (Bulgaria and Romania) still reported more than half of the confirmed cases and outbreaks in 2017. The recurring peak in trichinellosis cases in January and February may reflect the higher consumption of various pork products during winter as well as wild boar hunting season; almost 80% of the confirmed human trichinellosis cases were hospitalised with no fatal outcomes. Trichinella infections in humans are mainly linked to FBOs and it is striking that in 2017, more human cases were reported in the data collection of FBOs (n = 199, Table 49) then those confirmed and shown Figure 48 (n = 168). This gap could be explained by different data collection among countries and different case definitions. Moreover, data from hospitalised cases and data from epidemiological investigations carried out in the course of the outbreaks might be reported differently across the different data collections.
In 2017, 11 Trichinella outbreaks were reported by seven MS (reporting rate < 0.01 outbreak per 100.000 population) and three by two non‐MS. In total, 199 patients were affected in the EU and 39 in the non‐EU MS of which 125 were hospitalised. Nine of the outbreaks were reported with strong evidence, and all of these were associated with ‘pig meat and their products’ (including four outbreaks involving meat from hunted wild boar).
In the EU, most pigs are subject to official meat inspection at slaughter in accordance with Regulation (EC) No. 2015/1375; only pigs slaughtered for own consumption are not covered by the Regulation. About 200 million pigs were tested for Trichinella in MS and non‐MS in 2017, out of about 246 million reared pigs in the EU (Marquer et al., 2014), with only 224 positive animals, about 0.9 per million reared pigs. Only 6 out of 28 MS reported Trichinella in pigs in 2017, with an overall prevalence of 0.00011%. All positive findings were from pigs not raised under controlled housing conditions. In the EU, infected pigs are clustered in five MS (Bulgaria, Croatia, Poland, Romania and Spain) and sporadic infections are documented in other MS in 2017. The number of Trichinella‐positive domestic pigs is an underestimation since most pigs at risk for this infection are slaughtered at home without any veterinary control and recording. For example, only five pigs and one pig were detected as positive at the slaughterhouse in Croatia and Bulgaria, respectively, but at least other five pigs, which caused five human outbreaks in these two countries, were positive.
In 2017, Trichinella spp. were detected in farmed wild boar of Romania and Finland, which are assumed to be reared as pigs not raised under controlled conditions. EFSA has identified that non‐controlled housing condition is a main risk factor for Trichinella infections in domestic pigs, and the risk of Trichinella infection in pigs from well‐managed officially recognised controlled housing conditions is considered negligible (EFSA BIOHAZ Panel, EFSA CONTAM Panel and EFSA AHAW Panel, 2011; EFSA and ECDC, 2011).
No positive findings were reported from solipeds in 2017. In the last 11 years, only four horses tested positive out of more than one million tested animals in 2008, 2010 and 2012. This extremely low (< 0.001%) prevalence could be related the effective control (EFSA BIOHAZ Panel, 2013a) and reduction of Trichinella infections in domestic pigs, since pig scraps and offal were the main source of infection for horses. Indeed, a risk ranking process identified Trichinella spp. as the most relevant biological hazard in the context of meat inspection of domestic solipeds. Without a full and reliable solipeds traceability system, it is considered that either testing all slaughtered solipeds for Trichinella spp., or inactivation meat treatments should be used to maintain the current level of safety (EFSA BIOHAZ Panel, 2013a).
These zoonotic parasites circulate among wild animals in large parts of Europe and only Cyprus, Luxembourg and Malta had never reported any findings. In 2017, 16 MS and 1 non‐MS reported positive findings in wild animals. The lack of positive findings or confirmation of previous findings in other MS during 2017 is simply due to the lack of surveys, inadequacy of sample sizes, or investigation in regions where the environmental conditions do not favour the transmission of these zoonotic nematodes among wildlife.
In addition to domestic pigs, hunted wild boar is the second source of trichinellosis infections for humans. However, the prevalence of Trichinella spp. infections in this animal species has declined over the years due to the increased control for these pathogens in the domestic habitat. In the last 6 years (2012–2017), the prevalence of infection is reduced by threefold up to 2016 (from 0.13% in 2012 to 0.04% in 2016), but increased up to 0.08% in 2017 in the wild‐boar population.
The red foxes, with a large and widespread population, can be considered as the main natural reservoir of Trichinella in Europe. The prevalence decreased by twofold the last 5 years (from 2.4% in 2012 to 1.1% in 2016) and remained stable in 2017 (1.2%). In 2017, only 11 MS monitored Trichinella spp. infection in only 6,486 red foxes, and positive animals were detected in five MS. The proportion of positive samples from wildlife was higher in bears, lynxes, raccoon dogs and wolves, but their population size and distribution in Europe is generally limited to a few countries.
Identification of Trichinella larvae at the species level carried out in 2017, confirms that T. spiralis is more prevalent than T. britovi in swine and the opposite occurs in wild boar and carnivores (Pozio, 2014). However, since T. spiralis is patchy distributed, T. britovi and in one case T. pseudospiralis, were detected in swine in some countries. T. nativa has been documented in wild carnivores of Estonia, Finland and Sweden. T. pseudospiralis was documented in hunted (#6) and farmed (#2) wild boars and two falcons confirming its low frequency in target animals (Pozio, 2016).
In conclusion, there is a vicious cycle between unawareness and low income population, rural areas, inadequacy of local veterinary services and the occurrence of Trichinella in domestic animals in the EU and non‐EU countries (Pozio, 2014). The European and national institutions should provide free information and courses on the prevention of trichinellosis targeted to people rearing backyard and free‐ranging pigs and to hunters. The increasing number of wild boar and red foxes and the spread of the raccoon dog from eastern to western Europe and of the jackal from southern‐eastern to northern‐western Europe may increase the prevalence of Trichinella circulating among wild animals (Alban et al., 2011; Széll et al., 2013). Therefore, it is important to continue educating hunters and others eating wild game about the risk of eating undercooked game meat.
8.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Surveillance Atlas of trichinellosis in humans | http://ecdc.europa.eu/en/data-tools/atlas/Pages/atlas.aspx |
| FAO/WHO/OIE Guidelines for the surveillance, management, prevention and control of trichinellosis | http://www.trichinellosis.org/uploads/FAO-WHO-OIE_Guidelines.pdf | |
| International Trichinella Reference Center | http://www.iss.it/site/Trichinella/scripts/ | |
| International Commission on Trichinellosis | http://www.trichinellosis.org/ | |
| European Union Reference Laboratory for Parasites (humans and animals) | http://www.iss.it/crlp/ | |
| Animals | World Organisation for Animal health, Summary of Information on Trichinellosis | http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/Disease_cards/TRICHI-EN.pdf |
| FAO/WHO/OIE Guidelines for the surveillance, management, prevention and control of trichinellosis | http://www.trichinellosis.org/uploads/FAO-WHO-OIE_Guidelines.pdf | |
| International Trichinella Reference Center | https://www.iss.it/site/Trichinella/ | |
| International Commission on Trichinellosis | http://www.trichinellosis.org/ | |
| Development of harmonised schemes for the monitoring and reporting of Trichinella in animals and foodstuffs in the European Union | http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/35e.pdf | |
| OIE Manual Chapter 2.1.16. Trichinellosis | https://web.oie.int/eng/normes/MMANUAL/2008/pdf/2.01.16_TRICHINELLOSIS.pdf | |
| Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015 laying down specific rules on official controls for Trichinella in meat | http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32015R1375 | |
| Pig farming in the European Union: considerable variations from one Member State to another | http://ec.europa.eu/eurostat/statistics-explained/index.php/Pig_farming_sector_-_statistical_portrait_2014 |
9. Echinococcus
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable at https://doi.org/10.5281/zenodo.1475841
9.1. Abstract
Alveolar (AE) and cystic echinococcosis (CE) are zoonotic parasitic diseases transmitted to humans through the ingestion of eggs shed by the tapeworms Echinococcus multilocularis and Echinococcus granulosus sensu lato (s.l.), respectively, in the faeces of canid definitive hosts. Even if human AE and CE are notifiable in some MS, in practice, these parasitic diseases are largely underreported in Europe. In 2017, 827 confirmed human echinococcosis cases were reported in the EU. The EU notification rate was 0.19 cases per 100,000 population, a decrease by 13.6% compared with 2016. Species information was provided for the majority (71.4%) of cases and E. multilocularis and E. granulosus accounted, respectively, for 146 cases (26.3%) and 409 cases (53.3%). A high proportion (> 75%) of the human echinococcosis cases were reported without information on importation and travel destination. The proportion of cases who were hospitalised continued to decrease, with higher hospitalisation rates for AE compared to CE. One fatal case (species not specified) was reported in 2017.
Twenty‐four MS provided 2017 monitoring data on Echinococcus in animals. Eleven MS reported data on 7,148 foxes examined for E. multilocularis, and nine MS reported positive findings with an overall prevalence of 16.9%. Data of 2017 from Finland, Ireland, Malta, the United Kingdom and Norway confirmed the free status of these countries for E. multilocularis in the context of Regulation (EU) 1152/2011. For E. granulosus, 21 MS reported data from around 111 million animals of which mainly domestic livestock animals. Nine MS reported positive samples with an overall prevalence of 0.24%.
9.2. Surveillance and monitoring of cystic and alveolar echinococcosis in humans and animals in the EU
9.2.1. Humans
Cases of both AE by E. multilocularis and cystic echinococcosis (CE) caused by E. granulosus are reported jointly as echinococcosis, as the EU case definition does not distinguish between these two forms of the disease. Countries can, however, report their cases by species into the TESSy database, and ECDC can differentiate between the two forms of the diseases based on that data. The notification of echinococcosis in humans is mandatory in most MS, Iceland and Norway, except for Belgium, France, the Netherlands and the United Kingdom where reporting is based on a voluntary surveillance system. Denmark and Italy have no surveillance system for echinococcosis. In Switzerland, echinococcosis in humans is not notifiable. The surveillance systems for echinococcosis cover the whole population in all MS. Belgium changed surveillance in 2015 and notification rates before this date are not displayed. All countries report case‐based data except Belgium, Bulgaria and the Netherlands, which reported aggregated data. Both reporting formats were included to calculate numbers of cases, notification rates and disease trends.
An attempt to collect harmonised clinical data in the EU on a voluntary basis is currently represented by the European Register of Cystic Echinococcosis (ERCE) (Rossi et al., 2016; http://www.heracles-fp7.eu/erce.html) and in the past with the European (Alveolar) Echinococcosis Registry (EurEchinoReg) (Kern et al., 2003).
9.2.2. Animals
E. multilocularis in Europe is mainly transmitted to humans by a sylvatic cycle that is wildlife based. Intermediate hosts (IHs) for E. multilocularis are small rodents (microtine or arvicolid), while definitive hosts (DHs) are mainly red foxes, raccoon dogs and, to a lesser extent, dogs and wolves.
E. granulosus sensu lato (s.l.) is a complex of species of cestode causing CE, in animals and humans. E. granulosus s.l. in Europe is mainly transmitted to humans by a pastoral cycle. IHs for E. granulosus s.l. are mainly livestock species (sheep, cattle, goats and secondarily pigs), while DHs are shepherd dogs (rarely wild canids). As mentioned before, people become infected with AE and CE through the ingestion of eggs of the tapeworm prevalent in these DHs.
Surveillance for E. multilocularis in Europe is usually carried out on a voluntary basis, with the exception of the five reporting countries claiming to be free from this parasite according to Regulation (EU) No. 1152/2013. Surveillance is carried out in the main European DHs, the red fox (Vulpes vulpes). Four MS (Finland, Ireland, Malta and the United Kingdom) have demonstrated the absence of E. multilocularis through the implementation of an annual surveillance programme required in accordance with Regulation (EU) No. 1152/2011. One EEA State, mainland Norway (Svalbard excluded), also implements a surveillance programme in line with Regulation (EU) No. 1152/2011. In all other MS, data on E. multilocularis rely on whether findings are notifiable and if monitoring is in place or if studies on E. multilocularis are performed. As data on E. multilocularis in animals vary geographically (also within countries) and over time, reported cases of E. multilocularis are difficult to compare within and between countries. According to a recent meta‐analysis, based on studies published between 1900 and 2015, E. multilocularis has been documented in red foxes from 21 countries (Oksanen et al., 2016; Figure 53). Starting from 2015, this parasite has been found in foxes and golden jackals from Croatia (Beck et al., 2018; Sindičić et al., 2018).
Figure 53.
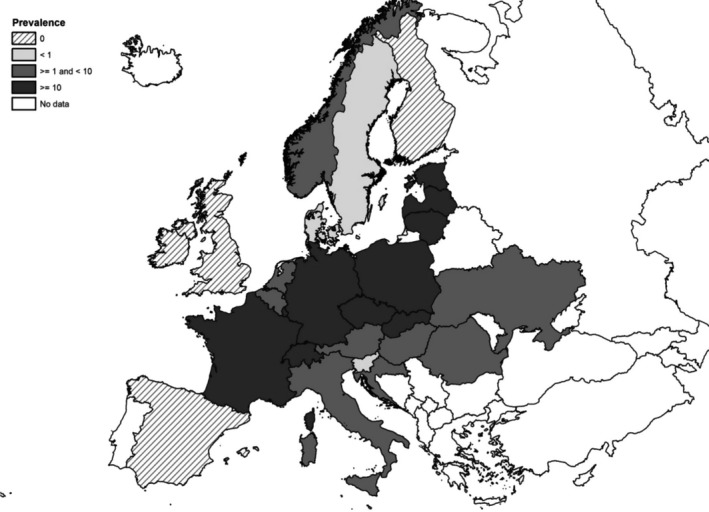
Pooled prevalence of Echinococcus multilocularis in red and Arctic foxes within the European Union and adjacent countries at national level depicting current epidemiological situation in Europe (Oksanen et al., 2016)
Surveillance of E. granulosus s.l. is usually carried out in livestock IHs during slaughterhouse inspections. In particular, necroscopy on sheep liver is used to detect the presence of parasitic cysts, while molecular PCR‐based methods are used to confirm and to identify genotype/species belonging to the Echinococcus genus (Siles‐Lucas et al., 2017). Although Commission Regulation (EU) No. 1152/2011 is present for E. multilocularis, no specific EU Regulation is in place for detecting E. granulosus in animals or humans, therefore surveillance for the latter parasite depends on national regulations.
E. granulosus s.l. monitoring data from livestock (IHs, sheep and pigs) are based on programmed surveillance/monitoring. They are collected in a fully harmonised way and with harmonised reporting rules, and therefore allow descriptive summaries at the EU level, trend watching and subsequent data analysis such as assessing spatial and temporal trends at the EU level.
9.3. Results
9.3.1. Overview of key statistics, EU, 2013–2017
Table 52 summarises EU level statistics related to human echinococcosis and to occurrence and prevalence in animals, respectively, in the EU, during 2013–2017.
Table 52.
Summary of Echinococcus granulosus sensu lato and Echinococcus multilocularis/cystic and alveolar echinococcosis in humans and most significant animal species, 2013–2017 in the EU
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 827 | 840 | 887 | 820 | 805 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.19 | 0.22 | 0.20 | 0.19 | 0.18 | ECDC |
| Number of reporting MS | 26 | 26 | 26 | 26 | 26 | ECDC |
| Infection acquired in the EU | 193 | 145 | 163 | 99 | 195 | ECDC |
| Infection acquired outside the EU | 12 | 33 | 29 | 23 | 14 | ECDC |
| Unknown travel status or unknown country of infection | 622 | 662 | 695 | 698 | 596 | ECDC |
| Animals | ||||||
| Echinococcus multilocularis in red foxes | ||||||
| Number of samples tested | 7,148 | 4,561 | 7,353 | 8,243 | 5,994 | EFSA |
| Proportion of positive samples | 16.9% | 19.5% | 13% | 8.3% | 10.9% | EFSA |
| Number of reporting MS | 11 | 12 | 11 | 14 | 12 | EFSA |
| Echinococcus multilocularis in raccoon dogs | ||||||
| Number of samples tested | 342 | 483 | 477 | 409 | 515 | EFSA |
| Proportion of positive samples | 0% | 0% | 0% | 0.2% | 0% | EFSA |
| Number of reporting MS | 3 | 2 | 4 | 5 | 3 | EFSA |
| Echinococcus granulosus s.l. in dogs | ||||||
| Number of samples tested | 2,536 | 2,183 | 3,478 | 2,759 | 1,469 | EFSA |
| Proportion of positive samples | 0% | 0.4% | 0.2% | 0.3% | 0% | EFSA |
| Number of reporting MS and associated countries | 6 | 5 | 8 | 7 | 5 | EFSA |
| Echinococcus granulosus s.l. in cattle | ||||||
| Number of samples tested | 9,834,374 | 6,885,353 | 5,636,424 | 5,263,603 | 7,591,851 | EFSA |
| Proportion of positive samples | 0.2% | 0.1% | 0.1% | 0.2% | 0.3% | EFSA |
| Number of reporting MS and associated countries | 15 | 19 | 17 | 15 | 13 | EFSA |
| Echinococcus granulosus s.l. in small ruminants | ||||||
| Number of samples tested | 38,444,352 | 9,617,700 | 5,281,192 | 13,335,803 | 29,135,951 | EFSA |
| Proportion of positive samples | 0.4% | 1.3% | 1.1% | 1.3% | 0.4% | EFSA |
| Number of reporting MS and associated countries | 16 | 14 | 13 | 11 | 13 | EFSA |
MS: Member State.
9.3.2. Human echinococcosis
In 2017, 827 laboratory‐confirmed echinococcosis cases were reported in the EU by 26 MS (Table 53). Twenty‐three MS reported at least one confirmed case and three MS reported zero cases. The EU notification rate was 0.19 cases per 100,000 population, which was a decrease by 13.6% compared with 2016 (0.22 cases per 100,000 population). The highest notification rates were observed in Bulgaria with 3.07 cases per 100,000 population, followed by Lithuania and Austria with 1.86 and 0.57 cases per 100,000 population, respectively. Bulgaria reported for 2017 the lowest number of cases and notification rate compared with the previous 4 years.
Table 53.
Reported human cases of cystic and alveolar echinococcosis and notification rates per 100,000 population in the EU/EFTA, by country and year, 2013–2017
| Country | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 50 | 50 | 0.57 | 26 | 0.30 | 8 | 0.09 | 14 | 0.17 | 11 | 0.13 |
| Belgium | Y | A | 12 | 12 | 0.11 | 17 | 0.15 | 9 | 0.08 | 15 | – | 15 | – |
| Bulgaria | Y | A | 218 | 218 | 3.07 | 269 | 3.76 | 313 | 4.35 | 302 | 4.17 | 278 | 3.82 |
| Croatia | Y | C | 15 | 15 | 0.36 | 9 | 0.21 | 7 | 0.17 | 20 | 0.47 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 2 | 0.24 | 0 | 0.00 | 0 | 0.00 |
| Czech Republic | Y | C | 1 | 1 | 0.01 | 4 | 0.04 | 3 | 0.03 | 6 | 0.06 | 2 | 0.02 |
| Denmarkb | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 1 | 1 | 0.08 | 0 | 0.00 | 0 | 0.00 | 1 | 0.08 | 3 | 0.23 |
| Finlandc | Y | C | 5 | 5 | 0.09 | 4 | 0.07 | 2 | 0.04 | 0 | 0.00 | 4 | 0.07 |
| France | Y | C | 48 | 48 | 0.07 | 38 | 0.06 | 48 | 0.07 | 32 | 0.05 | 34 | 0.05 |
| Germany | Y | C | 123 | 123 | 0.15 | 177 | 0.22 | 157 | 0.19 | 131 | 0.16 | 132 | 0.16 |
| Greece | Y | C | 15 | 15 | 0.14 | 18 | 0.17 | 13 | 0.12 | 13 | 0.12 | 10 | 0.09 |
| Hungary | Y | C | 14 | 14 | 0.14 | 5 | 0.05 | 2 | 0.02 | 2 | 0.02 | 5 | 0.05 |
| Irelandc | Y | C | 0 | 0 | 0.00 | 2 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.00 |
| Italyb | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Latvia | Y | C | 6 | 6 | 0.31 | 11 | 0.56 | 10 | 0.50 | 13 | 0.65 | 7 | 0.35 |
| Lithuania | Y | C | 53 | 53 | 1.86 | 26 | 0.90 | 33 | 1.13 | 22 | 0.75 | 23 | 0.77 |
| Luxembourg | Y | C | 2 | 2 | 0.34 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Maltac | Y | C | 0 | 0 | 0.00 | 1 | 0.23 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlands | Y | A | 38 | 38 | 0.22 | 33 | 0.19 | 64 | 0.00 | 30 | 0.18 | 33 | 0.20 |
| Poland | Y | C | 75 | 75 | 0.20 | 64 | 0.17 | 47 | 0.12 | 48 | 0.13 | 39 | 0.10 |
| Portugal | Y | C | 2 | 2 | 0.02 | 2 | 0.02 | 4 | 0.04 | 4 | 0.04 | 3 | 0.03 |
| Romania | Y | C | 14 | 14 | 0.07 | 13 | 0.07 | 18 | 0.09 | 31 | 0.16 | 55 | 0.28 |
| Slovakia | Y | C | 7 | 7 | 0.13 | 4 | 0.07 | 5 | 0.09 | 8 | 0.15 | 20 | 0.37 |
| Slovenia | Y | C | 7 | 7 | 0.34 | 3 | 0.15 | 7 | 0.34 | 5 | 0.24 | 6 | 0.29 |
| Spain | Y | C | 83 | 83 | 0.18 | 87 | 0.19 | 83 | 0.18 | 77 | 0.17 | 94 | 0.20 |
| Sweden | Y | C | 34 | 34 | 0.34 | 27 | 0.27 | 26 | 0.27 | 21 | 0.22 | 16 | 0.17 |
| United Kingdomc | Y | C | 4 | 4 | 0.01 | 0 | 0.00 | 26 | 0.04 | 25 | 0.04 | 14 | 0.02 |
| EU Total | – | – | 827 | 827 | 0.19 | 840 | 0.22 | 887 | 0.20 | 820 | 0.19 | 805 | 0.18 |
| Iceland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway | Y | C | 5 | 5 | 0.10 | 3 | 0.06 | 2 | 0.04 | 0 | 0.00 | 2 | 0.04 |
Y: yes; N: no; A: aggregated data; C: case‐based data;–: no report.
No surveillance system.
Finland, Ireland, Malta, the United Kingdom and mainland Norway have been declared free of E. multilocularis.
A high proportion (> 75%) of echinococcosis cases were reported without data about the travel status or unknown country of infection (Table 52). Most cases (94.2%) with known travel status were domestically acquired. Six MS (the Czech Republic, Hungary, Latvia, Lithuania, Portugal and Slovakia) of the 15 MS reporting information on importation in 2017 notified all their Echinococcus cases as being domestically acquired. The highest proportion of travel‐related cases was reported by Luxembourg (100%), Sweden (76.5%), Finland (60%) and Norway (100%). Among 18 travel‐associated cases with known origin of infection, the majority (66.7%) was reported as originating from outside the EU. Afghanistan and Turkey were the most frequently reported probable countries of infection, representing 38.9% of the imported cases in 2017.
In 2017, species information was provided for 555 confirmed echinococcosis cases (71.4%) by 15 MS. E. multilocularis accounted for 146 cases (26.3%), which was an increase of 49.1% compared with 2016. This was mainly due to an increase of reported cases in France, Lithuania and Poland. There was a significant increasing (p < 0.01) trend of E. multilocularis in 2008–2017 but the trend did not show any significant increase or decrease in 2013–2017 (Figure 54). For 10 MS with available data for the whole period 2008–2017, one country (Poland) reported an increasing trend (p < 0.01) since 2008. Other MS reported no decreasing or increasing trends, either long‐term (2008–2017) or short‐term (2013–2017).
Figure 54.
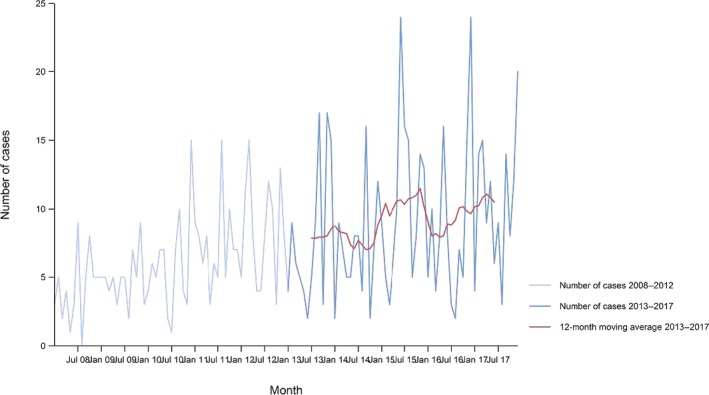
- Source: Austria, Estonia, France, Germany, Hungary, Latvia, Lithuania, Poland, Sweden, Slovakia and Slovenia. Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Finland, Greece, Iceland, Italy, Ireland, Luxembourg, Malta, Netherlands, Norway, Portugal, Romania, Spain and the United Kingdom did not report data to the level of detail required for the analysis.
E. granulosus s.l. accounted for 73.7% (409 cases) of those with species information available (505 confirmed cases). Most cases (53.3%; 218) were from Bulgaria. There was a decreasing trend of E. granulosus s.l. (p < 0.01) in the EU/EEA in 2008–2017, but the trend increased (p < 0.01) in 2013–2017 (Figure 55). For 16 countries with available data for the whole period 2008–2017, two countries (Latvia and Spain) reported significantly (p < 0.01) decreasing trends. Spain was the only country reporting a decreasing trend in 2008–2017 and in 2013–2017. Finland, Germany and Poland reported increasing trends, in 2008–2017 and 2013–2017. Austria and Lithuania reported an increasing trend from 2013 to 2017 and Slovakia reported a decreasing trend for the same period. Bulgaria, which reported the most cases in the EU in 2008–2017 (all cases were E. granulosus s.l.) was not included in the EU trend calculations as no monthly data were available. Cases from Bulgaria decreased by 43.5%, i.e. from 386 cases to 218 cases, in 2008–2017.
Figure 55.
- Source: Austria, Estonia, Finland, Germany, Greece, Hungary, Ireland, Latvia, Lithuania, Malta, Netherlands, Norway, Malta, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and United Kingdom. Belgium, Bulgaria, Croatia, Cyprus, the Czech Republic, Denmark, France, Iceland, Italy and Luxembourg did not report data to the level of detail required for the analysis.
Fourteen MS provided information on hospitalisation, covering 31.2% of all confirmed cases of echinococcosis in the EU in 2017. The overall hospitalisation rate was 54.3%, a continuous decrease during the last 7 years from 80% in 2011. In 2017, the highest proportions of hospitalised cases (80–100%) were reported in the Czech Republic, Estonia, Luxembourg, Poland, Portugal and Romania. The proportion of hospitalised E. multilocularis cases was 62.9%, and for E. granulosus, it was 44.9%, based on reporting by six and eight MS, respectively.
Information on the outcome of the cases was provided by 14 MS. One fatal case (species not specified) was reported in Lithuania. This resulted in an EU case fatality of 0.4% among the 249 cases for which this information was reported (30.1% of all confirmed cases) in 2017.
9.3.3. Echinococcosis in animals
Eleven MS and two non‐MS (Norway and Switzerland) reported 2017 monitoring data on 7,850 foxes examined for E. multilocularis, and nine MS reported positive findings with a total prevalence of 16.6%. Switzerland (45.9%), France (34.7%), the Czech Republic (26.3%), Luxembourg (25.2%) and Slovakia (10.7%), reported the highest proportion of positive samples.
In addition to foxes, E. multilocularis has been reported in 3 dogs, 137 pigs from Switzerland and 1 beaver (reintroduction of this host species from endemic to non‐endemic countries can cause E. multilocularis introduction). Poland reported 33,006 (0.14%) positive pigs with Echinococcus spp., while Hungary reported one positive pig out of 75 tested. In these last cases, it was not possible to confirm the Echinococcus species as in these countries this IH (pigs) can potentially harbour both E. multilocularis and E. granulosus s.l. Such uncertainty in species identification in co‐endemic countries for E. multilocularis and E. granulosus s.l. also applies to dogs and wolves. These findings are similar to those of recent years. Findings from most of the endemic countries fluctuated between years but, in most years, they reported positive findings. Fluctuations in reported numbers of infected animals are probably associated with investigational efforts performed in a particular year, rather than reflecting a change in true prevalence. Table 54 summarises the most relevant DH and IH species tested for Echinococcus multilocularis, such as foxes, raccoon dogs, dogs, wolves, cats, beaver, voles and pigs by MS and adjacent countries in 2017. In accordance with the Commission Regulation (EU) No. 1152/2011, surveillance of E. multilocularis is mainly focused on red foxes as definitive host.
Table 54.
Echinococcus multilocularis positive/tested (%) animals (wild and domestic) in EU/EEA, 2017
| Country | Foxes | Raccoon dogs | Dogsa | Wolvesa | Cats | Beaver | Voles | Pigsa |
|---|---|---|---|---|---|---|---|---|
| Czech Republic | 772/2,931 (26.3%) | |||||||
| Denmark | 1/1 | 0/16,987,437 | ||||||
| Estonia | 0/514,861 | |||||||
| Finland | 0/217 | 0/339 | 0/2 | 0/371 | 0/1,964,669 | |||
| France | 355/1,023 (34.7%)b | 0/179 | 0/25 | 5/482 (1.03%) | ||||
| Germany | 15/1,761 (0.9%) | |||||||
| Hungary | 1/5 | 1/75 (1.3%) | ||||||
| Ireland | 0/405 | |||||||
| Italy | 0/15 | |||||||
| Latvia | 0/447,034 | |||||||
| Luxembourg | 35/139 (25.2%) | 0/157,504 | ||||||
| Netherlands | 2/146 (1.4%) | |||||||
| Poland | 33,006/22,278,957 (0.1%) | |||||||
| Slovakia | 26/243 (10.7%) | 0/2 | 0/1,912 | 0/593 | 0/10,860 | |||
| Slovenia | 0/245,216 | |||||||
| Sweden | 0/1 | 0/8 | 0/60 | 0/2,576,290 | ||||
| United Kingdom | 0/277 | |||||||
| Total EU | 1,207/7,148 (16.9%) | 0/342 | 0/2,116 | 0/60 | 0/618 | 0 | 5/853 (0.6%) | 33,007/45,182,903 (0.07%) |
| Norway | 0/495 | 0/60 | 0/1,648,100 | |||||
| Switzerland | 95/207 (45.9%) | 3/21 (14.3%) | 0/5 | 1/1 | 137/662 (20.69%) | |||
| Total EFTA | 95/702 (13.5%) | 0 | 3/21 (14.3%) | 0/11 | 0/5 | 1/1 | 0 | 137/1,648,762 (< 0.01%) |
| Total EU + EFTA | 1,302/7,850 (16.6%) | 0/342 | 3/2,137 (0.1%) | 0/71 | 0/623 | 1/1 | 5/853 (0.6%) | 33,144/46,831,665 (0.07%) |
Dogs, wolves and pigs for which the species level of Echinococcus was not specified were allocated in this table for those MS for which is known there is circulation of E. multilocularis. Reported samples as ‘Slaughter batch’ data and ‘animals from zoo’ were not included in the table.
For the prevalence on foxes results of different studies with different protocols have been merged, therefore this prevalence should be taken cautiously.
Belgium, Cyprus, Estonia, Latvia, Malta, Norway and Sweden did not report any finding of E. granulosus or E. multilocularis.
In total, 23 countries (21 MS and two non‐MS) reported data from around 115 million domestic and wild animals tested for E. granulosus s.l. of which 99.8% were domestic animals (sheep, cattle, goats, pigs, horses, water buffalos, dogs and cats) (Table 55). These data were obtained mainly from the meat inspection performed at the slaughterhouse. Wild animals tested included mouflons, deer, water buffalo, wild boar, moose, wolves and foxes.
Table 55.
Echinococcus granulosus sensu lato positive/tested (%) animals (domestic and wild), EU/EEA, 2017
| Country | Sheep | Sheep and goats | Goats | Cattle | Pigsa | Mouflons | Solipeds, domestic | Deer | Water buffalos | Wild boars | Moose | Dogsa | Wolvesa | Cats |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgium | 0/922,797 | |||||||||||||
| Croatia | 11/12 | |||||||||||||
| Cyprus | 0/14 | |||||||||||||
| Denmark | 0/475,700 | 0/16,987,437 | ||||||||||||
| Estonia | 0/8,899 | 0/108 | 0/35,408 | 0/514,861 | 0/16 | |||||||||
| Finland | 0/57,600 | 0/329 | 0/272,671 | 0/1,964,669 | 0/6 | 0/1,252 | 0/640 | 0/365 | 2/383 (0.52%) | 10/57 (17.54%) | 0/2 | |||
| France | 0/179 | 0/25 | ||||||||||||
| Greece | 8,620/817,368 (1.05%) | 1,491/337,114 (0.44%) | 702/51,501 (1.36%) | 8/167,045 (> 0.001) | ||||||||||
| Hungary | 2/11 | 0/2 | 0/1 | 28/75 (37.33%) | 0/1 | |||||||||
| Italy | 62,044/1,278,549 (4.85%) | 500/55,459 (0.9%) | 5,127/1,715,855 (0.29%) | 361/691,744 (0.05%) | 3/2,860 (0.1%) | 0/676 | 69/33,315 (0.2%) | 16/24,384 (0.06%) | 0/15 | 1/1 | ||||
| Latvia | 0/27,127 | 0/251 | 0/85,677 | 0/447,034 | 0/87 | |||||||||
| Luxembourg | 0/26,173 | 0/157,504 | ||||||||||||
| Malta | 0/383 | |||||||||||||
| Netherlands | 0/1 | |||||||||||||
| Poland | 151/44,012 (0.34%) | 33,006/22,278,957 (0.14%) | ||||||||||||
| Romania | 1/13 | 0/3 | 268/299 (89.63%) | 5/59 (8.47%) | 0/1 | 0/39 | ||||||||
| Slovakia | 0/86,955 | 0/5 | 0/72,196 | 0/738,569 | 0/1,912 | 0/593 | ||||||||
| Slovenia | 0/11,529 | 0/865 | 1/118,235 (> 0.001) | 0/245,216 | 0/1,688 | |||||||||
| Spain | 42,492/7,727,221 (0.55%) | 0/1,967 | 13,597/946,876 (1.43%) | 14,236/1,975,193 (0.72%) | 2,677/17,548,560 (0.01%) | 0/687 | 0/106,068 | 6/19,111 (0.03%) | ||||||
| Sweden | 0/299,240 | 0/1,144 | 0/406,030 | 0/2,576,290 | 0/2,270 | 0/6,709 | 0/16,130 | 0/8 | 0/60 | |||||
| United Kingdom | 23,596/26,569,918 (0.08%) | 13/7,705 (0.16%) | 1,315/3,676,638 (0.03%) | |||||||||||
| Total EU | 136,755/36,884,421 (0.37%) | 151/45,979 (0.32%) | 15,601/109,050 (14.3%) | 21,649/9,834,374 (0.22%) | 36,096/64, 318,032 (0.05%) | 0/20 | 3/8,860 (0.03%) | 0/114,095 | 69/33,315 (0.2%) | 22/59,990 (0.03%) | 2/383 (0.52%) | 0/2,536 | 10/117 (8.5%) | 1/621 (0.16%) |
| Norway | 0/1,376,300 | 0/28,600 | 0/298,000 | 0/1,648,100 | 0/11 | |||||||||
| Switzerland | 0/2 | 0/2 | 3/21 (14.28%) | |||||||||||
| Total EFTA | 0/1,376,302 | 0 | 0/28,600 | 0/298,000 | 0/1,648,100 | 0 | 0/2 | 0 | 0 | 0 | 0 | 3/21 (14.28%) | 0/11 | 0 |
| Total EU + EFTA | 136,755/38,260,723 (0.35%) | 151/45,979 (0.32%) | 15,601/137,650 (11.3%) | 21,649/10,132,374 (0.2%) | 36,096/65,966,132 (0.05%) | 0/20 | 3/8,862 (0.03%) | 0/114,095 | 69/33,315 (0.2%) | 22/59,990 (0.03%) | 2/383 (0.52%) | 3/2,557 (0.11%) | 10/128 (7.8%) | 1/621 (0.16%) |
Dogs, wolves and pigs for which the species level of Echinococcus was not specified were allocated in this table for those MS for which is known there is circulation of E. multilocularis. Meat from sheep, cattle and pigs (single food samples) were included. Slaughter batch data and animals from zoo were not included in the table.
In 2017, 10 MS reported 210,356 positive samples mainly from domestic animals (TABLE_2017_ECHINO_INTERMED). These were mainly reported from small ruminants (sheep and goats with a prevalence ranging from 0.08% to 18%) in Spain, Italy, Greece, Spain and the United Kingdom. Also in cattle (mainly from Italy, Spain and Greece) and in pigs, positive animals (Poland and Switzerland) and herds were reported.
Figures 56 and 57 show, respectively, the proportion of positive samples (cumulative for the period between 2013 and 2017) for different intermediate hosts of E. granulosus s.l., (small ruminants, cattle, solipeds, deer and wild boar) and its geographical distribution in the EU. It is clear in small ruminants (sheep and goats) contribute for more than 75% of all positive samples and that these are reported from a few countries with large populations (Greece, Italy, Spain and the United Kingdom).
Figure 56.
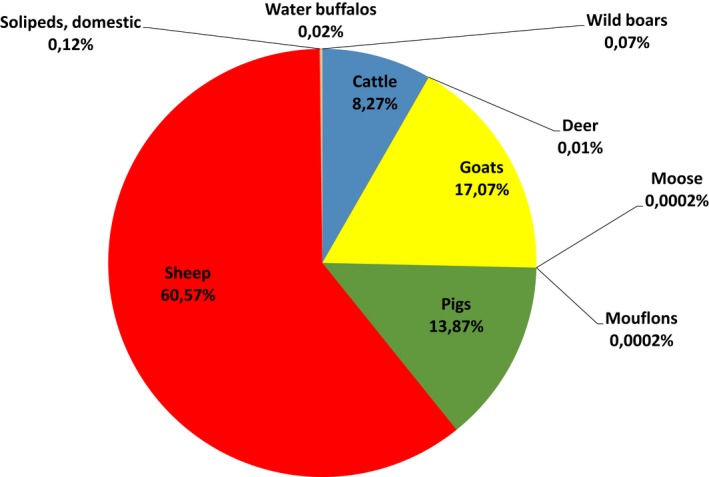
- Number of positive animals: cattle (n = 77,722), deer (n = 126), goats (n = 160,398), moose (n = 2), mouflons (n = 2), pigs (n = 130,359), sheep (n = 569,179), domestic solipeds (n = 1,108), water buffalos (n = 157), wild boars (n = 666). The total number of positive animals for E. granulosus s.l. reported in this reported period was 939,719. Positive pigs could be overestimated in co‐endemic countries with E. multilocularis.
Figure 57.
- Intermediate hosts included in map are: cattle, deer, goats, moose, mouflons, sheep, horses, water buffalos and wild boars. Pigs were excluded from Poland and Germany because of the co‐endemicity with E. multilocularis.
- Colours legend: black > 10,000 positive cases; dark blue < 1,000 positive cases; light blue < 100 cases; yellow: 0 cases reported; white: data not reported.
In pigs, it was not possible to differentiate E. multilocularis from E. granulosus s.l.; therefore, this IH species was excluded from the map (Figure 57). Finland, Italy, Spain and Switzerland reported findings of E. granulosus s.l. in moose, wild boar, wolves and cats.
9.4. Discussion
The EU case definition does not differentiate between the two clinical forms of the disease in humans, CE and AE, caused by E. granulosus s.l. and E. multilocularis, respectively. These two species can, however, be reported separately to ECDC. Most MS reported species information through TESSy from 2007 to 2017. Since the beginning of the surveillance of human echinococcosis in the EU, E. granulosus s.l. has been more frequently reported than E. multilocularis. The EU notification rate of confirmed human echinococcosis cases was stable, and the trends for E. multilocularis did not show any significant increase or decrease in the last 5 years since 2013. In a few countries, the increase in the number of cases in 2017 could be explained by intensified surveillance and improved notification system for echinococcosis. The awareness of the disease among clinicians and the migration (people from endemic countries) may also have influenced the number of diagnosed cases in some countries.
The EFSA Panel on Animal Health and Welfare (AHAW Panel) has stated in a scientific opinion that in many human cases, the diagnosis is established only as echinococcosis, and the aetiological agent of the disease, E. multilocularis or E. granulosus s.l., is not determined (EFSA AHAW Panel, 2007). Distinction between infection with E. granulosus s.l. and E. multilocularis is needed because the two diseases require different clinical management and strategies to control them. It should be also stressed that the true prevalence of these diseases is extremely difficult to estimate due to the long incubation period (AE and CE), the high proportion of asymptomatic or paucisymptomatic carriers who never seek medical attention (CE) and the underreporting/misdiagnosed cases (AE and CE), factors, which contribute to their neglected status. For these reasons, the patchy data on the number of people affected by ‘echinococcosis’ currently reported by MS, represents the tip of the iceberg. The invisible portion includes asymptomatic carriers of CE and misdiagnosed cases of AE especially in recently discovered foci where physicians do not have experience with these diseases.
As an example for this underreporting, data recently published in peer review journals reported around 34,000 hospitalisations of CE from Italy, France and Spain in 12‐, 16‐ and 12‐year period, respectively (Brundu et al., 2015; van Cauteren et al., 2016; Herrador et al., 2016). More recently, an extended study conducted in Italy (which is currently not reporting any human CE cases to the EU annual zoonoses monitoring data collection) identified 21,050 hospital discharge records with CE diagnosis from 2001 to 2014 related to 12,619 patients (Piseddu et al., 2017). The median of CE hospitalisations per year in Italy was 848, which is equal to the total number of CE and AE cases reported by all the MS in the EU annual zoonoses monitoring data collection.
Population surveys provide more reliable and partly complementary data, enabling a more accurate estimate of infection burden. In 2014–2015, the biggest research‐based abdominal ultrasound screenings were conducted on almost 25,000 people in 50 villages of rural Bulgaria, Romania and Turkey, in the context of the European FP7 Project HERACLES (Human cystic Echinococcosis ReseArch in CentraL and Eastern Societies’; http://www.heracles-fp7.eu/index.html). The sampling methodology and the use of rigorous case definition and staging allowed, for the first time, a reliable estimate of the prevalence of abdominal cystic echinococcosis and of the number of infected people living in the rural areas of these Eastern European countries. The authors of this study estimated that about 151,000 people living in these areas (7,872 in Bulgaria, 37,229 in Romania, 106,237 in Turkey) might be infected with abdominal cystic echinococcosis, a third of whom having active infection (Tamarozzi et al., 2018). This cross‐sectional study, screening 0.1% of the total rural population living in Romania, identified double the number of CE cases compared to those notified at national level in Romania during the same time period (EFSA and ECDC, 2017b). So, estimates from hospital data might underestimate the true value by 700‐fold for Romania. These published data and findings give an indication of the true magnitude of human CE as a public health problem and related costs in Europe.
In animals, in 2017, E. granulosus s.l., aetiological agent of cystic echinococcosis, and E. multilocularis, aetiological agent of alveolar echinococcosis, have been reported in 10 MS. The highest number of animals infected with E. granulosus s.l. was reported in Spain, Greece and Italy and mainly observed in small ruminants (Figures 56 and 57).
The highest numbers of animals (mainly foxes) infected with E. multilocularis and reported to EFSA was noted in Switzerland, France, the Czech Republic, Luxembourg and Slovakia. The surveillance of E. multilocularis in foxes is important to assess the prevalence in Europe, as the geographical distribution of E. multilocularis seems to be enlarged in the last decades. Whether the increased geographical distribution of E. multilocularis is due to an increased fox population in Europe (Oksanen et al., 2016), or the expansion of their habitat to urban areas (Deplazes et al., 2004) or it reflects an increased surveillance effort is difficult to disentangle, since there is a general lack of baseline data. Possibly, the parasite had already been present, but undetected, in small foci, which can rapidly expand with increasing red fox population (EFSA AHAW Panel, 2015) and/or changing habitat.
In addition, the occurrence of E. multilocularis identified in 2017 in 11 countries (MS and non‐MS) must be interpreted with caution as many variables such as temperature, rainfall, humidity levels and soil have been identified as relevant factors that partially explain the distribution of the parasite. These factors may vary considerably, leading to local foci within MS. Also, in animals, notification is a requirement for reliable data and information on parasite speciation is very important for risk management efforts as E. granulosus s.l. and E. multilocularis have a different epidemiology and pose different health risks for humans. For E. granulosus s.l., a notification requirement would ensure that comparable data between MS is obtained from meat inspection of food producing animals. For E. multilocularis, a general notification requirement for all MS can be questioned but it is required in countries free from this parasite, according to EU Regulation 1152/2011. In countries where the parasite is endemic, reporting each case gives no additional valuable information. Therefore, repeated surveys, as surveillance for E. multilocularis, can be a basis for follow‐up and monitoring (EFSA AHAW Panel, 2015). A recent questionnaire organised by EFSA (EFSA, 2018d) asked MS to report the mandatory notification of E. multilocularis and E. granulosus s.l. in animals in the EU. For E. multilocularis, five countries reported voluntary notification (Bulgaria, Greece, Luxembourg, Malta and Spain), four countries no notification (Austria, France, Norway and Portugal) and all the other countries reported a mandatory notification for this parasite. For E. granulosus s.l., five countries reported voluntary notification (Greece, Luxembourg, Malta, Portugal and Spain), six countries no notification (Austria, Bulgaria, France, Ireland, Norway and the United Kingdom) and all other countries have a mandatory notification for this parasite (LT did not provide information on the questionnaire).
More recently, E. multilocularis was detected in south‐western Italian Alps with eggs of this parasite molecularly identified in four faecal samples from two shepherd dogs, and in five wolf faecal samples (Massolo et al., 2018). Such findings in dogs could pose a serious hazard due to its zoonotic potential.
Finally, it is noteworthy that, in general, reported data on animals and humans represent a substantial underestimation of the real burden of these two diseases in Europe considering that around 200 human cases and a few thousand human cases are expected annually for AE and CE, respectively (Conraths and Deplazes, 2015; A. Casulli, personal communication, International Congress for Parasitology, Daegu, South Korea, 19–24 August 2018).
9.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Surveillance Atlas of echinococcosis in humans | http://ecdc.europa.eu/en/data-tools/atlas/Pages/atlas.aspx |
| EU case definitions (all diseases) | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| Food‐ and waterborne diseases and zoonoses Programme | https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/food-and-waterborne-diseases-and-zoonoses-programme | |
| European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) | https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/fwd-net | |
| CDC – Echinococcosis – Centers for Disease Control and Prevention | https://www.cdc.gov/parasites/echinococcosis/index.html | |
| World Health Organization – Echinococcosis | http://www.who.int/echinococcosis/en/ | |
| New approach needed to tackle parasitic liver disease in Europe and Turkey | http://www.who.int/neglected_diseases/news/new-approach-needeed-to-tackle-echinococcosis-europe/en/ | |
| Prevalence of abdominal cystic echinococcosis in rural Bulgaria, Romania, and Turkey: a cross‐sectional, ultrasound‐based, population study | https://www.sciencedirect.com/science/article/pii/S1473309918302214?via%3Dihub | |
| Human cystic Echinococcosis ReseArch in CentraL and Eastern Societies (HERACLES Project) | http://www.heracles-fp7.eu/index.html | |
| European Register of Cystic Echinococcosis (ERCE) | http://www.heracles-fp7.eu/erce.html | |
| Humans and animals | ||
| WHO/OIE Manual on Echinococcosis in Humans and Animals: a Public Health Problem of Global Concern | http://apps.who.int/iris/bitstream/10665/42427/1/929044522X.pdf | |
| OIE Manual, Chapter 2.1.6. Echinococcosis (infection with Echinococcus granulosus and with E. multilocularis) | http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.06_ECHINOCOCCOSIS.pdf | |
| COMMISSION DELEGATED REGULATION (EU) No. 1152/2011 (preventive health measures for the control of Echinococcus multilocularis infection in dogs) | http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1152 | |
| European Union Reference Laboratory for Parasites (humans and animals) | http://www.iss.it/crlp/ | |
| Animals | Scientific Opinion of the Animal health and welfare (AHAW) Panel of EFSA : Echinococcus multilocularis infection in animals | https://www.efsa.europa.eu/en/efsajournal/pub/4373 |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports | |
10. Toxoplasma gondii
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at http://doi.org/10.5281/zenodo.1475841
10.1. Abstract
Toxoplasma gondii is widely prevalent in humans and animals world‐wide. Virtually all warm‐blooded animals can act as intermediate hosts but the life cycle is only completed in the DHs: cats and other felines, including lynx which is present in Europe. In 2017, 40 cases of congenital toxoplasmosis were reported in the EU by 20 MS. The EU notification rate was 1.31 cases per 100,000 live births. The number of cases reported in 2017 is comparable to the annual number of cases reported between 2013 and 2016, after excluding France, which report data with a 2‐year delay and represent over 80% of the annual cases in the EU. It is not possible to make a good estimate of the prevalence of congenital toxoplasmosis in the EU, as only three MS have an active surveillance system of congenital cases.
Thirteen MS and two non‐MS reported 2017 monitoring data on Toxoplasma infections in animals. The highest overall prevalence of Toxoplasma infections in animals was detected in small ruminants (sheep and goats; 13.1%; 12 MS reported data) and pigs (15%; four MS reported) followed by cattle (10.5%; seven MS reported). Most samples were obtained from clinical investigations. It is not possible to make a good estimate of the prevalence of Toxoplasma infections in animals due to the use of different diagnostic methods (indirect methods detecting antibodies vs direct methods), the different sampling schemes in the MS and the lack of information on the animals’ age and rearing conditions.
10.2. Surveillance and monitoring of Toxoplasma gondii in the EU
10.2.1. Humans
National surveillance systems for toxoplasmosis differ from each other between countries. Only congenital toxoplasmosis is reported to ECDC. In 19 MS and Iceland, a compulsory surveillance system is implemented, while the United Kingdom has a voluntary system. The surveillance systems for toxoplasmosis have full national coverage in these 19 MS, except in Spain. France reports their cases with a 2‐year delay. Surveillance systems in some countries focus on severe cases in all age groups. Only three MS (France, Slovakia and Slovenia) have active surveillance of congenital cases with compulsory screening of pregnant women. No surveillance system for toxoplasmosis exists in eight MS (Austria, Belgium, Denmark, Greece, Italy, the Netherlands, Portugal and Sweden), Norway and Switzerland. All countries report case‐based data except Bulgaria, which reports aggregated data. Both reporting formats were included to calculate numbers of cases, notification rates and disease trends.
10.2.2. Animals
No EU Regulation exists with relation to the surveillance and monitoring of Toxoplasma gondii in animals. Therefore, the available and reported information is strictly determined by national legislation and whether the countries have a mandatory reporting system after the detection of Toxoplasma gondii. The main animal species tested are small ruminants (goat and sheep), cattle, pigs and pet animals (cats and dogs) using samples from aborted animals (ruminants) or clinically suspected animals. Mainly blood samples but also sample from tissue and organs are analysed with either indirect methods to detect antibodies (ELISA, Latex agglutination test (LAT), complement fixation test (CFT) and immunofluorescence assay (IFA)) or direct methods (PCR and immunohistochemistry (IHC)). As the surveillance of Toxoplasma in animals is not harmonised, data on Toxoplasma only allow descriptive summaries to be made, at the EU level. This is because the results submitted by different countries, and from different regions within a country, are mostly not directly comparable due to differences in sampling strategy, testing methods, as well as different sampling schemes. Both age of animals and also production systems at farm level may influence the occurrence of Toxoplasma.
10.2.3. Food‐borne outbreaks of human toxoplasmosis
The reporting of FBOs of human toxoplasmosis is mandatory according the Zoonoses Directive 2003/99/EC. No FBOs due to Toxoplasma were reported in the EU for 2017.
10.3. Results
10.3.1. Overview of key statistics, EU, 2013–2017
Table 56 summarises EU level statistics related to congenital toxoplasmosis related to humans and to occurrence of Toxoplasma spp. detected in major animal species, respectively, in the EU, during 2013–2017
Table 56.
Summary of congenital toxoplasmosis related to humans and Toxoplasma spp. detected in major animal species, EU, 2013–2017
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 40 | 242 | 288 | 258 | 213 | ECDC |
| Total number of confirmed cases/100,000 live birth (notification rates) | 1.31 | 6.72 | 8.27 | 7.40 | 6.20 | ECDC |
| Number of reporting MS | 20 | 19 | 20 | 20 | 20 | ECDC |
| Infection acquired in the EU | 30 | 34 | 24 | 28 | 28 | ECDC |
| Infection acquired outside the EU | 0 | 0 | 0 | 1 | 0 | ECDC |
| Unknown travel status or unknown country of infection | 8 | 208 | 264 | 229 | 185 | ECDC |
| Animals | ||||||
| Small ruminants (animal level) | ||||||
| Number of sampled units | 5,421 | 5,561 | 3,139 | 4,694 | 4,813 | EFSA |
| Proportion of positive units (%)a | 13.1 | 18.7 | 38.8 | 26.8 | 42.4 | EFSA |
| Number of reporting MS | 12 | 12 | 11 | 12 | 12 | EFSA |
| Cattle (animal level) | ||||||
| Number of sampled units | 2,163 | 451 | 1,177 | 1,000 | 1,078 | EFSA |
| Proportion of positive units (%)a | 10.5 | 3.3 | 4.2 | 6.2 | 13.8 | EFSA |
| Number of reporting MS | 7 | 8 | 7 | 9 | 5 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
For the summary statistics, indirect and direct diagnostic methods were taken together to calculate the proportion of positive units.
10.3.2. Human toxoplasmosis
In 2017, 40 cases of congenital toxoplasmosis were reported in the EU by 19 MS (Table 57). Seven MS (Bulgaria, the Czech Republic, Germany, Poland, Slovenia, Spain and the United Kingdom) reported at least one confirmed congenital toxoplasmosis case and 12 MS reported zero cases. The EU notification rate was 1.31 per 100,000 live birth. This is not comparable with notification rates from previous years as France, which data represented over 80% of the annual cases in the EU in 2013–2016, reports their data with a 2‐year delay. Excluding the French congenital toxoplasmosis data, the number of cases reported by 19 MS in 2017 is comparable to the annual number of cases (an average of 40 cases/year) and EU notification rate of 1.48 cases per 100,000 live births in 2013–2016.
Table 57.
Reported human cases of congenital toxoplasmosis and notification rates per 100,000 live births in the EU/EFTA, by country and year, 2013–2017
| Country | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Belgium | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Bulgaria | Y | A | 2 | 2 | 3.08 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Croatia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czech Republic | Y | C | 2 | 2 | 1.78 | 0 | 0.00 | 1 | 0.90 | 1 | 0.90 | 0 | 0.00 |
| Denmark | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Estonia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Finland | Y | C | 0 | 0 | 0.00 | 1 | 1.80 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Franceb | Y | C | – | – | – | 195 | 24.9 | 246 | 30.8 | 216 | 26.4 | 179 | 22.0 |
| Germany | Y | C | 6 | 6 | 0.76 | 10 | 1.36 | 15 | 2.03 | 6 | 0.80 | 10 | 1.50 |
| Greece | – | – | 0 | – | 0.00 | – | – | – | – | – | – | – | – |
| Hungary | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 1.10 | 3 | 3.20 | 0 | 0.00 |
| Ireland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 1.50 | 0 | 0.00 | 1 | 1.50 |
| Italy | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Latvia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Lithuania | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 3.30 | 0 | 0.00 | 1 | 3.30 |
| Luxembourg | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Malta | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlands | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Poland | Y | C | 18 | 18 | 4.71 | 20 | 5.42 | 15 | 4.00 | 20 | 5.30 | 18 | 4.90 |
| Portugal | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Romania | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.50 | 0 | 0.00 |
| Slovakia | Y | C | 0 | 0 | 0.00 | 2 | 3.60 | 0 | 0.00 | 0 | 0.00 | 2 | 3.60 |
| Slovenia | Y | C | 2 | 2 | 9.83 | 1 | 4.84 | 1 | 4.80 | 0 | 0.00 | 0 | 0.00 |
| Spainc | N | C | 3 | 3 | – | 5 | – | 0 | – | 0 | – | 0 | – |
| Sweden | – | – | – | – | – | – | – | – | – | – | – | – | – |
| United Kingdom | Y | C | 7 | 7 | 0.90 | 8 | 1.03 | 7 | 0.90 | 11 | 1.40 | 2 | 0.30 |
| EU Total | – | – | 40 | 40 | 1.31 | 242 | 6.72 | 288 | 8.27 | 258 | 7.40 | 213 | 6.20 |
| Iceland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Switzerlandd | – | – | – | – | – | – | – | – | – | – | – | – | – |
Y: yes; N: no; A: aggregated data; C: case‐based data; –: no report.
France: 2017 data not reported as there is a 2‐year delay in reporting of congenital toxoplasmosis in France.
Sentinel surveillance; no information on estimated coverage. So, notification rate cannot be estimated.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
In 2017, the highest country‐specific notification rates were observed in Slovenia and Poland (9.8 and 4.7 cases per 100,000 live births, respectively). Data from Poland alone accounted for 47.4% of all confirmed cases reported at the EU level in 2017 and showed the second highest notification rates after France, over the 4‐year period in 2013–2016.
Four MS provided data on outcome, accounting for 60.0% of confirmed cases in the EU (≥ 88% in 2013–2016). No fatal cases due to congenital toxoplasmosis were reported in 2017 among 24 confirmed cases from four MS reporting outcome.
10.3.3. Toxoplasma in animals
Thirteen MS (Austria, Finland, Germany, Greece, Hungary, Ireland, Italy, Latvia, the Netherlands, Romania, Slovakia, Spain and the United Kingdom) and two non‐MS (Norway and Switzerland) provided monitoring data on Toxoplasma in livestock (small ruminants, cattle, solipeds and pigs).
In small ruminants (sheep and goats), 12 MS (Austria, Finland, Germany, Greece, Hungary, Ireland, Italy, Latvia, the Netherlands, Romania, Slovakia and Spain) and two non‐MS (Norway, Switzerland) reported data. The overall prevalence was 13.1% but much higher (around 30%) using indirect tests such as ELISA, CFT, LAT or IFA compared with direct tests (3%) using staining, PCR and/or IHC.
In cattle, seven MS (Austria, Ireland, Italy, Latvia, Slovakia, Spain and the United Kingdom) reported data on Toxoplasma‐specific antibodies. At animal level about 10.5% was tested seropositive as the main proportion of samples (95%) was tested by indirect diagnostic methods. Italy performed a national survey (around 2,000 samples) by ELISA and 10% were seropositive.
From pigs, four MS (Austria, Italy, Latvia and the United Kingdom) reported monitoring data: in total 689 animals were tested and 105 (15%) were detected as positive by two MS (Austria and Italy).
In pet animals (cats and dogs), nine MS (Finland, Germany, Ireland, Italy, Latvia, the Netherlands, Poland, Romania and Slovakia) and one non‐MS (Switzerland) tested in total 2,623 animals (690 cats and 1,933 dogs) of which 188 were positive (7%) and obtained mainly from suspected animals and clinical investigations.
In 2017, six MS (Austria, Finland, Italy, the Netherlands, Slovakia and the United Kingdom) and one non‐MS reported on testing of Toxoplasma in wildlife. In total, 859 animals (mainly from Italy) were tested and 46 were positive (5.3%).
10.4. Discussion
Toxoplasma gondii is a zoonotic protozoan parasite that can cause serious disease in humans, especially when primary infection is acquired during pregnancy. Based on the reported data for the year 2017, congenital toxoplasmosis in the EU shows a stable number of confirmed cases and notification rates from 2013 to 2017, but remains a rare disease overall. The decrease in notifications of cases in 2017, compared with previous years, is a surveillance artefact due to France (reporting over 80% of the cases in EU) not reporting toxoplasmosis data at the time of data collection for this report. France regularly reports the highest number of cases, most likely due to its sensitive surveillance system, which includes screening of pregnant women, follow‐up of those that are negative to detect infection during pregnancy and laboratory confirmation of any congenital toxoplasmosis cases detected during this process, including asymptomatic cases.
National surveillance systems differ from each other and so does case underascertainment between countries. Very few EU countries have active surveillance for congenital toxoplasmosis. A quarter of the EU countries do not have any surveillance for toxoplasmosis and most countries having surveillance systems reported zero cases. Therefore, it is not possible to provide a good estimate of the prevalence of this disease in the EU.
In 2015, WHO reported that food‐borne toxoplasmosis, spread through undercooked or raw meat and fresh produce, may cause up to 20% of the total food‐borne disease burden in the EU and affects more than 1 million people in the European Region each year (WHO, 2015). The EFSA BIOHAZ Panel identified T. gondii as one of two most relevant biological hazards for meat inspection of sheep and goats (EFSA BIOHAZ Panel, 2013b) and of medium relevance in pork (EFSA BIOHAZ Panel, EFSA CONTAM Panel and EFSA AHAW Panel, 2011).
The 2017 monitoring data reported by MS from animals show that Toxoplasma is present in most livestock species across the EU. A reduction of the prevalence of Toxoplasma infection in small ruminants and a fluctuating prevalence in cattle occurred during the last 5 years. However the limitations of these surveillance data preclude any trend watching or trend analysis.
The current surveillance system of Toxoplasma in animals of EU is strongly affected by several significant limitations: i. small amount of tested animals; ii. the use of different indirect and direct detection methods, which, in most cases have been not validated by an independent body; iii. unknown age of tested animals; and iv. no information on type of breeding. Furthermore, there is no relationship between the presence of anti‐Toxoplasma antibodies and infecting parasites in cattle and horses (Aroussi et al., 2015; Opsteegh et al., 2016). For pigs, poultry and small ruminants, serological methods could be useful for the detection of high risk animals/herds but not as an indicator of infection in individual animals, since the concordance between direct and indirect methods was estimated as low to moderate. All these limitations result in the lack of any scientific value of data provided by MS, and therefore of their use by the European Commission, MS and stakeholders. The data are mostly not directly comparable across MS.
10.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | ECDC Surveillance Atlas of Infectious | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definitions | https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | |
| European Union Reference Laboratory for Parasites | http://www.iss.it/crlp/ | |
| Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV‐Infected Adults and Adolescents | https://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-oi-prevention-and-treatment-guidelines/322/toxo | |
| Animals | European Union Reference Laboratory for Parasites | http://www.iss.it/crlp/ |
| Scientific Opinion of the Panel on Biological Hazards (BIOHAZ) of EFSA: Surveillance and monitoring of Toxoplasma in humans, food and animals | https://www.efsa.europa.eu/en/efsajournal/pub/583 | |
| EFSA External Scientific Report: Relationship between seroprevalence in the main livestock species and presence of Toxoplasma gondii in meat (GP/EFSA/BIOHAZ/2013/01) An extensive literature review | https://www.efsa.europa.eu/en/supporting/pub/en-996 | |
| EFSA Supporting publication: Experimental studies on Toxoplasma gondii in the main livestock species (GP/EFSA/BIOHAZ/2013/01) Final report. M. Opsteegh, G. Schares, R. Blaga and J. van der Giessen | http://www.efsa.europa.eu/it/supporting/pub/en-995 | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports | |
| OIE Manual Chapter 2.9.9. Toxoplasmosis | http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.09.09_TOXO.pdf | |
| Bad Bug Book (Second Edition), Food‐borne Pathogenic Microorganisms and Natural Toxins Handbook, Center for Food Safety and Applied Nutrition, Food and Drug Administration (FDA), USA | https://www.fda.gov/food/foodborneillnesscontaminants/causesofillnessbadbugbook/ |
11. Rabies
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at http://doi.org/10.5281/zenodo.1475841
11.1. Abstract
For 2017, one imported human case of rabies was reported in France. For 2016, France had also reported one imported case. For 2015, no human cases of rabies were reported in the EU, while for 2014 and 2013 three and one human cases of rabies were reported, respectively. All these six cases had been exposed outside the EU (i.e. Sri Lanka, Pakistan, Haiti, India, Mali and Morocco).
The 2017 monitoring data from animals from the Eastern European countries showed that rabies persisted in sylvatic reservoirs such as foxes (Poland, Hungary and Serbia) and was transmitted to dogs (a stray dog in Romania), which are the main source for human infection, and to cats (Poland). Also domestic livestock was infected (two goats in Hungary and one cow in Romania). These findings demonstrate that the risk of infection for humans’ remains in eastern Europe and therefore vaccination of people at higher risk of infection should be considered, in line with the relevant national and international recommendations. Continued monitoring of rabies is therefore necessary in those MS in target animals in wildlife (foxes, bats, raccoon dogs) and in domestic animals (farmed livestock, dogs, cats). The reported 2017 animal monitoring results in EU were very favourable with only two foxes found positive and all other non‐flying terrestrial wild animals testing negative, like during 2016. This is without doubt due to the impact of the large‐scale vaccination programmes in foxes and dogs. Still, it appeared that the numbers of reported tests vary across MS and non‐MS and that during the last 5 years the number of reported tested foxes decreased, which warrants caution with the assessment. The number of reported tested raccoon dogs remained about the same, while the number of tested wild animals (other than foxes and raccoon dogs), bats and farmed animals increased.
In Western Europe, rabies is rare due to its elimination in non‐flying terrestrial animals. This may explain why 2017 monitoring data from bats were mainly reported by Western and Northern European MS countries and why during the last 5 years the number of reported tested bats in central and Western Europe increased. Nineteen MS and three non‐MS reported monitoring data on bats. In total 2,079 bats were investigated in EU, which was an increase by 48.0% compared with 2016, and 39 bats (1.9%) were found positive by seven MS. These findings are in line with previous years’ findings confirming bats to be a reservoir for rabies and reaffirm the public recommendation to handle bats with utmost caution, if any. A tentative novel member of the genus Lyssavirus, designated as Kotalahti bat lyssavirus (KBLV), was detected in a Brandt's bat (Myotis brandtii) in Finland. In France, one case was identified as Lleida bat lyssavirus (LLEBV). Five other MS (Belgium, Germany, the Netherlands, Poland and the United Kingdom) and Switzerland reported positive cases in bats.
Altogether 41 cases of rabies or Lyssavirus were found in wild animals (foxes and bats) in 2017 in EU. Therefore, as in 2016, the EU target (maximum 80 rabies cases in wild animals) was achieved.
The surveillance of rabies in humans, wildlife and domestic animals in the endemic areas remains therefore important as there is a continuous risk of reintroduction of the virus from endemic areas to free zones in the EU.
11.2. Surveillance and monitoring of rabies in the EU
11.2.1. Humans
The notification of rabies in humans is mandatory in most MS, Iceland, Norway and Switzerland. The United Kingdom has another unspecified system. Most countries use the EU case definition apart from Denmark, Finland, France, Germany and Italy, which use other/non‐specified case definitions.
Most countries examine saliva and neck skin biopsy for ante mortem diagnosis of rabies. For post mortem examinations, the central nervous system is sampled. Identification is mostly based on antigen detection, viral genome detection by real‐time RT‐PCR and/or isolation of virus. Serum and spinal fluid are used to test for the presence of antibodies to rabies virus.
11.2.2. Animals
Rabies surveillance in animals is performed by testing indicator animals at risk such as (hunted) foxes, jackals, raccoon dogs (Nyctereutes procyonoides), bats and other wildlife. The aim of rabies surveillance is mainly to demonstrate the absence of disease or to identify its presence or distribution to allow timely dissemination of information for integrated action among different sectors such as public health and veterinary sectors.
According to the Regulation (EU) No. 652/201415, multiannual programmes for eradication of rabies may be cofinanced by the EU. In 2017, 12 MS (Bulgaria, Croatia, Estonia, Finland, Greece, Hungary, Latvia, Lithuania, Poland, Romania, Slovakia and Slovenia) had approved eradication, control and surveillance programmes for rabies. The eradication programmes involve mainly assessing the prevalence of the disease in animals that are more at risk of being infected. Therefore, rabies is mainly monitored in wildlife using indicator animals that are found dead in their natural habitat and/or suspected animals from target species (foxes, badgers, raccoon dogs, etc.). Financial contribution is planned for active and passive surveillance/monitoring, purchase of oral vaccine baits (foxes and raccoon dogs) and its distribution and awareness campaigns.
In addition, the monitoring of rabies also relies on the analysis of routine clinical investigations in domestic animals (cattle, sheep, goats, rabbits, etc.) showing neurological clinical signs compatible with rabies and on the evaluation of vaccination (titres) in imported or travel‐related companion animals (mainly dogs and cats) from territories and non‐EU countries not included in Annex II of Regulation (EC) No. 577/201316.
Nineteen MS (Austria, Belgium, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Luxembourg, the Netherlands, Poland, Romania, Slovakia, Slovenia, Spain and the United Kingdom) and three non‐MS (Norway, Serbia and Switzerland) reported bat rabies 2017 monitoring data, from passive (bats found dead or clinically affected bats) or active surveillance (random survey). Rabies in European bats is caused by at least five different Lyssavirus species that seem to have be biologically related to certain bat species such as the Serotine bat (European bat lyssavirus 1 (EBLV‐1)), the Daubenton's and Pond bat (European bat lyssavirus 2 (EBLV‐2)), Schreiber's long‐fingered bat (West Caucasian bat virus (WCBV) and LLEBV) and the Natterer's bat (Bokelogh bat lyssavirus (BBLV), Fisher et al., 2018).
EU MS also need to notify outbreaks of infection with rabies virus in terrestrial animals to the EU ADNS11 and regular summaries are posted online.
11.3. Data analyses
Results of surveillance activities for rabies in wildlife were summarised for the major indicator/target species such as foxes, raccoon dogs and raccoons (Procyon lotor) and other wild species (badgers, deer, marten, rodents, jackals, lynx, bears, hares, hedgehogs, minks, wolverine, wild boar, squirrels, ferrets, otter, polecat, etc.).
Lastly, also separate tables for dogs, cats and farmed domestic animals (cattle, small ruminants, solipeds, pigs, rabbits, ferrets) were produced for summarising the data obtained from surveillance activities in the different MS for canine/domestic rabies. All data are summarised (aggregated) at MS level and if MS reported data at regional level or for only some regions, the total number of tested animals are not integrated in the summary tables because it was not clear whether all regions in the MS were tested or not.
11.4. Results
11.4.1. Overview of key statistics, EU, 2013–2017
Table 58 summarises EU level statistics related to human cases of rabies, and to rabies/Lyssavirus occurrence and prevalence in major animal species in the EU, during 2013–2017.
Table 58.
Summary of rabies/Lyssavirus statistics related to humans and main animal species, EU, 2013–2017
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 1 | 1 | 0 | 3 | 1 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ECDC |
| Number of reporting countries | 28 | 27 | 28 | 28 | 28 | ECDC |
| Infection acquired in the EU | 0 | 0 | – | 0 | 0 | ECDC |
| Infection acquired outside the EU | 1 | 1 | – | 3 | 1 | ECDC |
| Unknown travel status or unknown country of infection | 0 | 0 | – | 0 | 0 | ECDC |
| Animals | ||||||
| Foxes | ||||||
| Number of tested animals | 30,485 | 37,296a | 49,958 | 41,854 | 49,190 | EFSA |
| Proportion of positive animals | 0.007% | 0.04% | 0.20% | 0.25% | 1.11% | EFSA |
| Number of reporting MS | 20 | 22 | 21 | 22 | 23 | EFSA |
| Raccoons and raccoon dogs | ||||||
| Number of tested animals | 1,004 | 1,172 | 725 | 795 | 1,040 | EFSA |
| Proportion of positive animals | 0.0% | 0.09% | 0.28% | 0.13% | 0.0% | EFSA |
| Number of reporting MS | 9 | 7 | 7 | 10 | 12 | EFSA |
| Dogs | ||||||
| Number of tested animals | 2,334 | 2,469 | 2,974 | 2,943 | 3,326 | EFSA |
| Proportion of positive animals | 0.04% | 0.1% | 0.5% | 0.3% | 2.2% | EFSA |
| Number of reporting MS | 22 | 24 | 22 | 22 | 24 | EFSA |
| Bats | ||||||
| Number of tested animals | 2,079 | 1,405 | 1,747 | 1,969 | 1,442 | EFSA |
| Proportion of positive animals | 1.9% | 3.5% | 1.9% | 1.7% | 1.3% | EFSA |
| Number of reporting MS | 19 | 19 | 17 | 16 | 19 | EFSA |
MS: Member State.
Lithuania (regional data) and Slovenia were reporting suspect and selective sampled foxes.
11.4.2. Rabies in humans
For 2017, all EU MS reported data on rabies in humans. For 2017 and 2016, one human case was reported for each of the years (France in both cases), respectively. No cases were reported in 2015, while for 2014 and 2013, three and one human cases were reported, respectively. All these six cases had been exposed outside the EU (i.e. Sri Lanka, Pakistan, Haiti, India, Mali and Morocco).
11.4.3. Rabies in animals
Wildlife rabies
In 2017, 20 MS reported monitoring data on red foxes (Vulpes vulpes). In total, 30,485 foxes were investigated, which was a decrease by 18.3% compared with 2016. Seventy‐five % of the sampled foxes however originated from six MS: mainly from Romania, followed by Poland, the Czech Republic, Hungary, Slovenia and Slovakia. Two rabies cases in foxes were reported: one in Hungary and one in Poland. Serbia, pre‐accession country, reported one rabies case out of 163 tested foxes (Figure 58).
Figure 58.
The geographical distribution of the reported rabies cases in foxes and the number of tested foxes, by reporting country, EU, 2017
Investigations (n = 1,004) from raccoons and raccoon dogs were carried out by nine MS (Austria, the Czech Republic, Estonia, Finland, Latvia, Lithuania, Luxembourg, Poland and Slovakia) and none was found positive.
Seventeen MS reported and tested approximately 3,000 wild animals (an increase of 47% compared with 2016 when 2,036 were tested) other than foxes, raccoons and raccoon dogs (badgers, bears, buffalos, deer, ferrets, hedgehogs, lynx, marten, mice, minks, moles, otter, owl, polecat, rabbit, rodents, squirrel, weasel, wolverine and wolves) and no animal tested positive, like in 2016.
In 2017, 19 MS and 3 non‐MS reported monitoring data on bats. In total, 2,079 bats were investigated in EU, which was an increase by 48.0% compared with 2016. Eighty per cent of all the sampled bats in 2017 originated from five MS: France followed by the United Kingdom, Germany, Poland and Spain. There were 39 positive bats (1.9% of 2,079 tested) reported by seven MS: Belgium (1, EBLV‐1b), Finland (1, KBLV), France (3, EBLV‐1, and 1, LLEBV)), Germany (15, unknown species), the Netherlands (9, EBLV‐1), Poland (8, EBLV‐1), the United Kingdom (1, unknown species) and Switzerland (1, EBLV‐1) (Figure 59). In Finland, a tentative novel member of the genus Lyssavirus, designated as KBLV was detected in a Brandt's bat (Myotis brandtii) for the first time in 2017. In France, one case was identified as LLEBV, another novel tentative species in bats.
Figure 59.
The geographical distribution of reported rabies cases (EBLV‐1 or EBLV‐2 or other species) in bats and the number of tested bats, by reporting country, EU, 2017
Domestic/canine rabies
Seventeen MS reported 831 samples from domestic farmed animals (mainly cattle, small ruminants and domestic solipeds). In total, three cases (0.4%) were detected that originated from clinically suspect sampling: one cow (Romania) and two goats in Hungary. The number of samples taken from samples from domestic farmed animals in 2017 was higher compared with 2016 (714 with nine positive cows in RO and one positive horse in PL).
Twenty‐three MS reported monitoring results from more than 2,700 cats and 2,334 dogs. Poland reported one positive cat and Romania one positive dog, both from suspect sampling.
11.5. Discussion
Rabies is a zoonosis which causes nearly 60,000 deaths a year ( http://www.oie.int/infographic/rabies/) world‐wide, is found all over the world except in certain areas with favourable geographical characteristics, such as Australia, Antarctica and Britain and Ireland, and in regions which have eliminated the virus through oral animal vaccination programmes (Central and Western Europe).
The 2017 monitoring data from animals from the Eastern European countries showed that rabies persisted in sylvatic reservoirs such as foxes (Poland, Hungary and Serbia) and was transmitted to dogs (a stray dog in Romania), which are the main source for human infection, and to cats (Poland). Also, domestic livestock was infected (two goats in Hungary and one cow in Romania). These findings demonstrate that the risk of infection for humans’ remains in eastern Europe and therefore vaccination of people at higher risk of infection should be considered, in line with the relevant national and international recommendations. Continued monitoring of rabies is therefore necessary in those MS in target animals in wildlife (foxes, bats, raccoon dogs) and in domestic animals (farmed livestock, dogs, cats). The reported 2017 animal monitoring results in EU were very favourable with only two foxes found positive and all other non‐flying terrestrial wild animals testing negative, like during 2016. This is without doubt due to the impact of the large‐scale vaccination programmes in foxes and dogs. Still, it appeared that the numbers of reported tests vary across MS and non‐MS and that during the last 5 years the number of reported tested foxes decreased, which warrants caution with the assessment. The number of reported tested raccoon dogs remained about the same, while the number of tested wild animals (other than foxes and raccoon dogs), bats and farmed animals increased. It may be the case that detection of rabies may in those MS depend on the sampling effort and so sizing the sampling efforts is key to produce reliable situational updates. The most cost‐effective strategy for preventing rabies in people is by eliminating the disease in dogs and wildlife through animal vaccinations and dog and fox population management.
In Western Europe, rabies is rare due to its elimination in non‐flying terrestrial animals, first in dogs at the beginning of the 20th century, and then progressively in foxes since the 1980s (Mueller et al., 2015). Therefore, the epidemiology of rabies in Western Europe has changed during the past 22 years. As such, Parize et al. (2018) recently reported that in France, which eliminated rabies in non‐flying terrestrial mammals in 2001, the risk of rabies is now limited to contact with bats, rabid animals illegally imported from rabies‐enzootic countries, and traveller exposure in enzootic areas. This may explain why 2017 monitoring data from bats were mainly reported by Western and Northern European MS countries and why during the last 5 years the number of reported tested bats in Central and Western Europe increased. Nineteen MS and three non‐MS reported monitoring data on bats. In total, 2,079 bats were investigated in EU, which was an increase by 48.0% compared with 2016, and 39 bats (1.9%) were found positive by seven MS. In Finland and France new species of the genus Lyssavirus were detected in 2017: a tentative novel member of the genus Lyssavirus, designated as KBLV was detected in a Brandt's bat (Myotis brandtii) in Finland (Nokireki et al., 2018) and LLEBV was detected in France (Arechiga‐Ceballos et al., 2013). These findings are in line with previous years’ findings confirming bats to be a reservoir for rabies and reaffirm the public recommendation to handle bats with utmost caution, if any. The public health hazard of bat rabies in Europe ought to not be underestimated. Altogether, 41 cases of rabies or Lyssavirus were found in wild animals in 2017 in EU. The majority of these were found in bats and indicates an improved surveillance while in foxes the number of cases is low. This shows that the implemented control programmes (vaccination strategy in different MS) in foxes is successful. In 2017, an increased number of wild animals – other than foxes and raccoon dogs – were tested by 17 MS and none of them was reported positive. As in 2016, the EU target (maximum 80 rabies cases in wild animals) was achieved.17
Surveillance for rabies among humans and in domestic animals should be pursued even in countries that have successfully eliminated animal rabies as there is a continuous risk of reintroduction of the virus via illegally imported rabid companion animals from endemic areas (Lardon et al., 2010).
11.6. Related projects and internet sources
12. Q fever
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at http://doi.org/10.5281/zenodo.1475841
12.1. Abstract
Following an increasing trend in confirmed Q fever cases in humans observed over the period 2012–2016 in the EU, case numbers have decreased in 2017. For 2017, 928 confirmed cases of Q fever were reported by 18 EU MS. Spain reported the most cases (n = 379, more than one‐third of all cases) for the year 2017, followed by France and Germany (194 and 107, respectively). The EU notification rate was 0.12 per 100,000 population, which is lower than in the previous 4 years. Compared with 2016, case numbers increased in Bulgaria, Croatia, Portugal and Spain, while they remained stable or decreased in other MS.
In animals, sampling is mainly performed in cattle, sheep and goats and wild ruminants for clinical investigations of animals suspected of being infected by Coxiella burnetii (passive surveillance), or for ‘abortion protocol’ in domestic ruminants (programmed surveillance to exclude the presence of Coxiella burnetii from aborted animals), or for animals for trade or travel (export/import/fairs/licensing purposes). There is an active and planned monitoring of sheep and goats by frequently sampling and analysing the presence of C. burnetii‐specific antibodies in bulk milk samples in few MS. Seventeen MS and four non‐MS reported 2017 data for Coxiella burnetii, mainly from cattle, sheep and goats. In sheep and goats Belgium, Bulgaria, Italy and Poland organised a national survey or had an active national monitoring programme in place using ELISA, fluorescence in situ hybridisation (FISH), CFT and PCR methods to detect Coxiella burnetii or its antibodies at animal or herd/flock level. Belgium, the Czech Republic, Italy, Poland and Slovakia performed an active monitoring and together accounted for 90% of submitted samples from cattle. In 2017, the overall animal‐level seroprevalence was 8.6% in cattle and 9.2% in sheep and goats. National differences in seroprevalence could be due to differences in sampling strategy and monitoring efforts.
12.2. Surveillance and monitoring of Coxiella burnetii in the EU
12.2.1. Humans
Q fever in humans is a mandatory notifiable disease at the EU level and cases are reported through TESSy. For 2017, 27 EU MS, Iceland, Norway and Switzerland provided information on Q fever in humans. Twenty EU countries used the EU case definition, whereas Denmark, France, Germany, Italy and Romania used another case definition. Belgium and Finland did not specify the case definition used.
Reporting is mandatory in 25 EU countries and voluntary in France and the UK. Disease surveillance is comprehensive and mostly passive except in the Czech Republic and Slovakia. Data reporting is case‐based except from Belgium and Bulgaria.
12.2.2. Animals
The main pillar of surveillance for Q fever in animals implemented by most MS is passive monitoring. At EU level, there is no harmonised active surveillance in place. The main animal species tested are small ruminants (goats and sheep) and cattle using samples from aborted animals, animals suspected of being infected by C. burnetii or from animals in connection with trade or travel (export/import/fairs/licensing purposes). Also wild ruminants are tested. Few MS (Belgium, Germany, Slovakia, the Netherlands and the United Kingdom) and Norway implement an active and planned monitoring of milk sheep and of milk goats, by regularly sampling and analysing the presence of C. burnetii‐specific antibodies in bulk milk samples.
Systematic surveys are performed occasionally to estimate the national seroprevalence or to confirm the presence of C. burnetii in bovine or small ruminant livestock regionally or even at herd level. Mainly milk samples followed by blood samples, tissue samples (e.g. from fetus/stillbirth/organs) or placentae are analysed and indirect methods used are ELISA, CFT (for detection of antibodies) and/or FISH or PCR and real‐time PCR (for the direct detection of C. burnetii).
As the surveillance in animals is mainly based on case reporting and passive surveillance at national level, and data reported by MS to EFSA are generated by non‐harmonised monitoring schemes across MS with no mandatory reporting requirements, the data on C. burnetii are only useful to make descriptive summaries at the EU level. They preclude additional data analysis such as assessing EU level temporal and spatial trends. This is because the results submitted by MS are mostly not directly comparable due to differences in sampling strategy, testing methods, coverage of the monitoring and sensitivity of the surveillance for C. burnetii.
12.3. Results
12.3.1. Overview of key statistics, EU, 2013–2017
Table 59 summarises EU level statistics related to Q fever in humans and to Q fever occurrence and prevalence in major animal species, respectively, in the EU, during 2013–2017.
Table 59.
Summary of Coxiella burnetii statistics related to humans and major animal species, EU, 2013–2017
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 928 | 1,056 | 822 | 780 | 647 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.12 | 0.16 | 0.18 | 0.18 | 0.15 | ECDC |
| Number of reporting EU MS | 27 | 27 | 26 | 25 | 25 | ECDC |
| Infection acquired in the EU | 702 | 730 | 550 | 518 | 516 | ECDC |
| Infection acquired outside the EU | 8 | 29 | 8 | 21 | 16 | ECDC |
| Unknown travel status or unknown country of infection | 218 | 297 | 264 | 241 | 115 | ECDC |
| Animals | ||||||
| Sheep and goats (animal level) | ||||||
| Number of sampled units | 4,245 | 7,545 | 15,819 | 9,005 | 9,057 | EFSA |
| Proportion of positive unitsa | 9.2% | 12.8% | 10.3% | 6% | 1.1% | EFSA |
| Number of reporting MS | 11 | 16 | 14 | 18 | 14 | EFSA |
| Cattle (animal level) | ||||||
| Number of sampled units | 16,272 | 17,480 | 62,335 | 48,141 | 36,757 | EFSA |
| Proportion of positive unitsa | 8.6% | 6.3% | 13% | 9.1% | 8.3% | EFSA |
| Number of reporting MS | 16 | 16 | 15 | 18 | 16 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority; MS: Member States.
For the summary statistics indirect and direct diagnostic methods were taken together to calculate proportion of positive units.
Humans
The number of Q fever cases in humans who acquired the infection in the EU decreased compared with 2016 but was higher than during 2015 and before.
Animal categories
The year 2017 was the year since 2013 for which least samples were submitted from sheep and goats, and from cattle, by, respectively, about one‐third and half of the MS. Those numbers of submitted samples tend to decrease during recent years. The proportion of positive samples ranged from 1.1% to 9.2% for sheep and goats and from 6.3% to 13% in cattle, during 2013–2017.
12.3.2. Coxiella burnetii in humans
Overall, 928 confirmed cases of Q fever were reported by 18 EU MS, four cases were reported by Norway and 42 cases were reported by Switzerland (Table 60). In 2017, Spain was the country that reported most cases (n = 379), followed by France and Germany (194 and 107, respectively).
Table 60.
Reported human cases of Q fever and notification rates per 100,000 population in the EU/EFTA, by country and year, 2013–2017
| Country | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austriab | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Belgium | Y | A | 15 | 7 | 0.06 | 16 | 0.14 | 8 | 0.07 | 3 | 0.03 | 5 | 0.04 |
| Bulgaria | Y | A | 30 | 28 | 0.39 | 17 | 0.24 | 15 | 0.21 | 15 | 0.21 | 23 | 0.32 |
| Croatia | Y | C | 29 | 23 | 0.55 | 8 | 0.19 | 14 | 0.33 | 21 | 0.49 | 0 | 0.00 |
| Cyprus | Y | C | 4 | 3 | 0.35 | 2 | 0.24 | 4 | 0.47 | 1 | 0.12 | 3 | 0.35 |
| Czech Republic | Y | C | 0 | 0 | 0.00 | 2 | 0.02 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 |
| Denmark | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | – | – | – | – |
| Estonia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Finland | Y | C | 4 | 4 | 0.07 | 2 | 0.04 | 3 | 0.05 | 0 | 0.00 | 5 | 0.09 |
| France | Y | C | 194 | 194 | 0.29 | 251 | 0.38 | 250 | 0.38 | 209 | 0.32 | 158 | 0.24 |
| Germany | Y | C | 107 | 107 | 0.13 | 270 | 0.33 | 310 | 0.38 | 238 | 0.29 | 114 | 0.14 |
| Greece | Y | C | 4 | 4 | 0.04 | 9 | 0.08 | 10 | 0.09 | 15 | 0.14 | 11 | 0.10 |
| Hungary | Y | C | 29 | 29 | 0.30 | 39 | 0.40 | 35 | 0.36 | 59 | 0.60 | 135 | 1.36 |
| Ireland | Y | C | 2 | 2 | 0.04 | 6 | 0.13 | 4 | 0.09 | 0 | 0.00 | 0 | 0.00 |
| Italy | Y | C | 7 | 7 | 0.01 | 3 | 0.00 | – | – | – | – | – | – |
| Latvia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 0.05 | 3 | 0.15 | 1 | 0.05 |
| Lithuania | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Luxembourg | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 0.18 | 0 | 0.00 | 0 | 0.00 |
| Malta | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 2 | 0.47 |
| Netherlands | Y | C | 22 | 22 | 0.13 | 14 | 0.08 | 20 | 0.12 | 26 | 0.15 | 20 | 0.12 |
| Poland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.00 | 0 | 0.00 |
| Portugal | Y | C | 49 | 48 | 0.47 | 17 | 0.16 | 20 | 0.19 | 25 | 0.24 | 21 | 0.20 |
| Romania | Y | C | 48 | 46 | 0.23 | 32 | 0.16 | 3 | 0.02 | 21 | 0.11 | 24 | 0.12 |
| Slovakia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.02 | 0 | 0.00 |
| Slovenia | Y | C | 3 | 3 | 0.15 | 1 | 0.05 | 1 | 0.05 | 3 | 0.15 | 1 | 0.05 |
| Spain | Y | C | 449 | 379 | – | 330 | – | 97 | – | 77 | – | 75 | – |
| Sweden | Y | C | 2 | 1 | 0.01 | 3 | 0.03 | 4 | 0.04 | 2 | 0.02 | 3 | 0.03 |
| United Kingdom | Y | C | 21 | 21 | 0.03 | 34 | 0.1 | 21 | 0.03 | 60 | 0.09 | 46 | 0.07 |
| EU Total | – | – | 1,019 | 928 | 0.12 | 1,056 | 0.16 | 822 | 0.18 | 780 | 0.18 | 647 | 0.15 |
| Iceland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway | Y | C | 4 | 4 | 0.08 | 2 | 0.04 | 1 | 0.02 | 1 | 0.02 | 4 | 0.08 |
| Switzerlandc | Y | C | 42 | 42 | 0.50 | 47 | 0.56 | 40 | 0.48 | 43 | 0.52 | 26 | 0.53 |
Y: yes; N: no; A: aggregated data; C: case‐based data; –: no report.
Not notifiable, no surveillance system exists.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
The EU notification rate was 0.12 per 100,000 population, which is lower than the previous 4 years. In 2017, the highest notification rate (0.55 cases per 100,000 population) was observed in Croatia, followed by Portugal (0.47), Bulgaria (0.39), Cyprus (0.35), and Hungary (0.30). An increasing trend in confirmed Q fever cases was observed over the period 2013–2016 in the EU with a decrease in 2017 (Figure 60).
Figure 60.
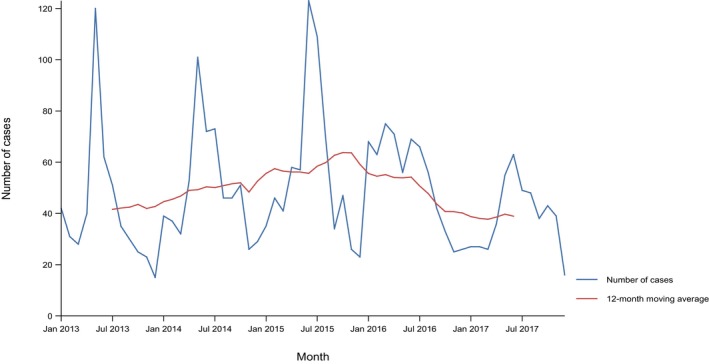
- Source(s): Cyprus, Czech Republic, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Sweden.
- Austria, Belgium, Bulgaria, Croatia, Denmark, Italy, Spain, Switzerland and the United Kingdom did not report data to the level of detail required for the analysis.
Ten countries (the Czech Republic, Denmark, Estonia, Iceland, Latvia, Lithuania, Luxembourg, Malta, Poland and Slovakia) reported no human cases. The large majority (75.6%) of Q fever cases in the EU was domestically acquired. In total, eight travel‐associated cases were reported, which had travelled to Bolivia, Japan, Morocco, Mauritius, Namibia, Tunisia and South Africa.
Cases occurred during the whole year but with a seasonal increase between April and July where 43% of the cases reported in 2017 occurred.
Seven deaths due to Q fever were reported for 2017 in the EU (two cases in Germany, Spain and Portugal each and one case in Hungary), resulting in an EU case fatality of 1.35% among the 517 confirmed cases with reported outcome.
12.3.3. Coxiella burnetii in animals
Twelve MS and three non‐MS provided data for sheep and goats for 2017. In total, 6,359 holdings/flocks and 4,245 individual animals were tested of which 4.2% and 9.2%, respectively, tested positive for C. burnetii. Samples were mainly taken in Belgium, Bulgaria, Italy, Poland and Spain (Table 2017_COXOVINEGOAT).
Fifteen MS and four non‐MS provided data for cattle for 2017. In total, 1,885 holdings and flocks and 16,272 animals were tested of which 13% and 8.6% were positive, respectively. Belgium, the Czech Republic, Italy, Poland and Slovakia performed an active monitoring (by testing systematically aborted animals or serological monitoring via national survey) and tested about 90% of the samples (Table 2017_COXCATTLE).
Five MS and two non‐MS reported on animals other than sheep, goats and cattle. In total, 703 different domestic and wild animal species (alpaca's, buffalos, Cantabrian chamois, cats, deer, dogs, dolphin, elephant, foxes, hares, lamas, pigs, squirrels, steinbock, water buffalos, wild boar, wolves) were tested and only 0.9% out of 703 were found positive (Table 2017_COXOTHERAN).
12.4. Discussion
Following an increasing trend in confirmed Q fever cases observed over the period 2012–2016 in the EU, case numbers have decreased in 2017. After several consecutive years of increase in France and Germany, the case numbers reported for 2017 are lower than in the previous 3 and 4 years, respectively. While France and Germany reported the most confirmed human cases since 2013, in 2017 Spain accounted for more than a third of the overall number of cases. Since 2013, the number of human cases reported by Spain has continuously increases and is mostly likely explained by a change in their reporting system: from voluntary to mandatory.
Between 2007 and 2010, the Netherlands experienced a large outbreak with more than 4 000 human cases (Schneeberger et al., 2014). The number of cases in the Netherlands returned to the preoutbreak level in 2013 and remained low since then.
Although the number of cases increased between 2015 and 2016, the EU rate decreased. This is due to the fact that Italy started to report data in 2016 which impacted the overall population and the EU notification rate. Besides the change in reporting system in some MS, there is no clear and identified explanation for the increasing trend in the EU between 2013 and 2016.
The results obtained from animals — mainly from small ruminants and cattle — prevent the following or analysis of trends for Q fever at the EU level because the results submitted by MS are mostly not directly comparable due to differences in sampling strategy, testing methods, coverage of the monitoring and sensitivity of the surveillance for C. burnetii. The regional variability within Europe highlights the importance of understanding risk factors which may operate at a local scale and may be subtle (Georgiev et al., 2013).
12.5. Related projects and internet sources
13. West Nile virus
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at http://doi.org/10.5281/zenodo.1475841
13.1. Abstract
For 2017, 212 West Nile virus (WNV) infections in humans were reported by 11 Member States. Most infections were reported by Romania, Italy and Greece, with 31%, 26% and 23% of the total EU, respectively. The overall EU notification rate per 100,000 population was 0.05 compared to 0.06 in 2016. For 2017, Austria, Bulgaria, Croatia, France, Greece, Hungary, Italy and Romania reported locally acquired infections. No locally acquired infections had been reported by Greece for 2015 and 2016, and by France for 2016.
The number of MS sending animal surveillance data increased in 2017 compared with previously. During 2017, as in recent years, WNV circulation, and subsequent outbreaks and positive animals have been reported by countries in Central and Eastern Europe and in the Mediterranean basin; Austria, Bulgaria, Croatia, Cyprus, the Czech Republic, France, Greece, Hungary, Italy, Portugal, Serbia, Slovakia and Spain.
The risk of WNV transmission is complex and multifactorial; it concerns the virus, the vectors, the animal reservoirs, the environmental conditions and human behaviour. Preventing or reducing of WNV transmission depends mainly on successful controlling of the abundance of the vector abundance or interruption of human‐vector contact. Human, animal and entomological West Nile fever (WNF) surveillance is crucial to allow for the early detection of WNV infections in humans and the recording of the geographical distribution of WNV, in order to take timely preventive measures.
13.2. Surveillance and monitoring of West Nile virus infections in the EU
WNF, also known as ‘West Nile virus disease’, is an arboviral disease transmitted in natural conditions to humans and animals via infected female mosquito bites (Diptera; Culicidae). The transmission period is typically in the summer and early autumn when mosquitoes (typically Culex spp.) are most active and more abundant. The mosquitoes, in which the WNV replicates, acquire infection by feeding on viraemic birds. WNV is maintained in a bird‐mosquito cycle, with birds acting as amplifying hosts. Apart from humans, the virus can also emerge in equine species, which, as humans, are accidental hosts and which cannot in turn transmit the virus to the vectors. MS with areas where infected birds and competent mosquitoes co‐exist may be affected by both human cases as well as outbreaks in animals.
13.2.1. Humans
Human WNV infections data are collected through two complementary data collection processes. During the period of high mosquito abundance and activity (June–November), the MS report human infections timely to TESSy at ECDC. Complementary to this real‐time data collection, an annual data collection is carried out. Countries who did not detect any infections during the year are asked to report ‘zero cases’; all other countries are encouraged to report complementary data on detected infections if considered relevant.
For 2017, 26 EU Member States, Iceland and Norway provided information on WNV infections in humans to TESSy. The EU case definition was used by 25 countries, Finland did not specify which case definition was used and France and the United Kingdom used an alternative case definition. All reporting countries had a comprehensive surveillance system. Reporting is compulsory in 26 EU/EEA countries and voluntary in France and the United Kingdom. Surveillance is passive, except in the Czech Republic, Greece, Portugal, Slovakia and the United Kingdom. All countries have a national coverage of reporting and mainly case based reporting (except Belgium).
13.2.2. Animals
Although the reporting of WNV infections in animals is not mandatory, MS can report annually WNV monitoring data from animals according the Zoonoses Directive 2003/99/EC, which are next presented in the annual EUSR. The Directive specifies that, in addition to the number of zoonoses and zoonotic agents for which monitoring is mandatory, others shall also be monitored when the epidemiological situation so warrants. These WNV animal monitoring and surveillance data submitted to EFSA are typically outbreak results, from European countries regularly experiencing outbreaks. Owing to heterogeneity in study design and the variety of analytical methods used, the reported WNV prevalence in animals from different countries is not directly comparable. These data allow descriptive summaries at the EU level to be made (Table 1). Proposals for harmonised schemes for monitoring and reporting of WNV in animals can be found in an External Scientific Report submitted to EFSA (Mannelli et al., 2012).
EU MS also need to report weekly notifications of outbreaks of WNV encephalomyelitis in horses to the EU ADNS11 and regular summaries are posted online. Moreover, animal WNF outbreak data reported to the OIE are publically available on the World Animal Health Information Database (WAHIS) interface.18
13.3. Results
13.3.1. Overview of key statistics, EU, 2013–2017
Table 61 summarises EU level statistics related to human WNV infections, and to the occurrence of WNV in birds and solipeds, respectively, in the EU, during 2013–2017. More detailed descriptions of these statistics are in the results section of this chapter.
Table 61.
Summary of WNV infections statistics related to humans, birds and solipeds, EU, 2013– 2017
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of cases | 212 | 240 | 128 | 76 | 331 | ECDC |
| Total number of cases/100,000 population (notification rates) | 0.05 | 0.06 | 0.03 | 0.02 | 0.08 | ECDC |
| Number of reporting MS | 26 | 26 | 26 | 24 | 25 | ECDC |
| Infection acquired in the EU | 207 | 226 | 121 | 74 | 274 | ECDC |
| Infection acquired outside the EU | 2 | 3 | 0 | 2 | 1 | ECDC |
| Unknown travel status or unknown country of infection | 3 | 11 | 7 | 0 | 56 | ECDC |
| Animals | ||||||
| Birds | ||||||
| Number of sampled units | 11,525 | 8,258 | 8,594 | 10,378 | 8,937 | EFSA |
| Number of reporting countries | 8 | 4 | 7 | 7 | 6 | EFSA |
| Solipeds | ||||||
| Number of sampled animals | 11,670 | 9,949 | 13,075 | 15,273 | 12,278 | EFSA |
| Number of reporting countries | 12 | 10 | 11 | 12 | 12 | EFSA |
ECDC: European Centre for Disease Prevention and Control; EFSA: European Food Safety Authority.
Animal categories
Table 61 indicates that more MS submitted data for the year 2017 compared with 2016, likely indicating increased surveillance and reporting efforts.
13.3.2. West Nile virus infections in humans
Table 62 summarises EU reported WNV infections in humans. WNV infections occur seasonally with most occurring in the summer and early autumn. In total, 212 infections were reported by 11 MS, of which 72% were confirmed. Most infections were reported from Romania, Italy and Greece, with 31%, 26% and 23% of the total European Union cases, respectively. The overall EU notification rate per 100,000 population in 2017 was 0.05 compared to 0.06 in 2016.
Table 62.
Locally acquired and travel‐related reported human WNV infections and notification rates per 100,000 population in the EU/EFTA, by country and year, 2013–2017
| Country | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Confirmed cases | Total cases & rates | Total cases & rates | Total cases & rates | Total cases & rates | Total cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 7 | 7 | 0.08 | 5 | 0.06 | 7 | 0.08 | 1 | 0.01 | 0 | 0.00 |
| Belgium | Y | A | 0 | 2 | 0.02 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Bulgaria | Y | C | 0 | 1 | 0.01 | 2 | 0.03 | 2 | 0.03 | 0 | 0.00 | 0 | 0.00 |
| Croatia | Y | C | 5 | 5 | 0.12 | 2 | 0.05 | 1 | 0.02 | 20 | 0.47 | ||
| Cyprus | Y | C | 0 | 0 | 0.00 | 1 | 0.12 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czech Republic | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 |
| Denmarkb | – | – | – | – | – | – | – | – | – | ||||
| Estonia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Finland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| France | Y | C | 2 | 2 | 0.00 | 3 | 0.00 | 1 | 0.00 | 0 | 0.00 | 1 | 0.00 |
| Germany | – | – | – | – | – | – | – | – | – | ||||
| Greece | Y | C | 10 | 48 | 0.45 | 0 | 0.00 | 0 | 0.00 | 15 | 0.14 | 86 | 0.78 |
| Hungary | Y | C | 8 | 23 | 0.23 | 48 | 0.49 | 22 | 0.22 | 10 | 0.10 | 36 | 0.36 |
| Ireland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.02 |
| Italy | Y | C | 56 | 56 | 0.09 | 81 | 0.13 | 62 | 0.10 | 24 | 0.04 | 160 | 0.27 |
| Latvia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Lithuania | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Luxembourg | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Malta | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlands | Y | C | 0 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Poland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Portugal | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 | ||||
| Romania | Y | C | 64 | 66 | 0.34 | 93 | 0.47 | 32 | 0.16 | 24 | 0.12 | 24 | 0.12 |
| Slovakia | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Slovenia | Y | C | 0 | 1 | 0.05 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.05 |
| Spain | Y | C | 0 | 0 | 0.00 | 4 | 0.01 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Sweden | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 |
| United Kingdom | Y | C | 1 | 1 | 0.00 | 0 | 0.0 | 0 | 0.00 | 2 | 0.00 | 0 | 0.00 |
| EU Total | – | – | 153 | 212 | 0.05 | 240 | 0.06 | 128 | 0.03 | 76 | 0.02 | 331 | 0.08 |
| Iceland | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Norway | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Switzerlandc | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 0.00 |
Y: yes; N: no; A: aggregated data; C: case‐based data; –: no report.
Not notifiable, no surveillance system exists.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
In 2017, 98% of the total WNV infections were locally acquired or acquired during travel within the EU. Infections acquired outside the EU were reported with information about exposure in Serbia and South Africa.
For 2017, Austria, Bulgaria, Croatia, France, Greece, Hungary, Italy and Romania reported locally acquired infections. No locally acquired infections had been reported by Greece for 2015 and 2016, and by France for 2016.
There was a no significant (p < 0.05) increase or decrease over the last five (2013–2017) or 10 (2008–2017) years for West Nile virus infections in the EU/EEA (Figure 61). At the country level, Austria and Romania reported significantly (p < 0.05) increasing trends in the past 10 years (2008–2017), and Austria also reported an increasing trend in the last 5 years (2013–2017).
Figure 61.
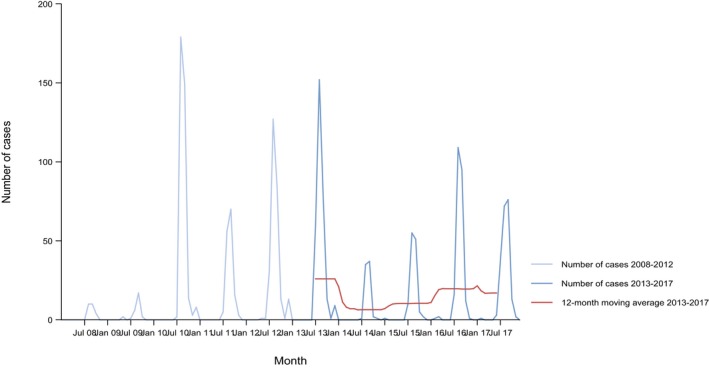
- Source: Austria, Cyprus, Czech Republic, Estonia, Finland, France, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and United Kingdom.
- Belgium, Bulgaria, Croatia, Denmark, Germany, Iceland and Portugal did not report data to the level of detail required for the analysis.
Eight EU MS provided data on the hospitalisation status of their cases. Among the cases with known hospitalisation status (72% of total infections) in 2017, 88% were hospitalised. Among the infections with known clinical manifestations (99% of total infections), 71% (n = 149) were neuroinvasive and 8.6% (n = 18) of infections were asymptomatic blood donors compared to 75% and 9.7% in 2016, respectively. Data on the outcome of infections was provided by nine EU MS. In 2017, 25 deaths were reported due to WNV infections, compared to 28 in 2016.
13.3.3. West Nile fever infections in animals
13.3.3.1. Annual monitoring and surveillance data reported to EFSA
In 2017, the WNV testing results of 11,525 birds, mostly wild birds but also fowl on farms, have been reported by eight MS: Austria (129), Bulgaria (20), Denmark (660), France (55), Hungary (98), Italy (6,830), Spain (3,457) and the United Kingdom (276), and two non‐MS: Serbia (390) and Switzerland (349). Italy and Spain provided for 89.2% of the data. Positive birds were reported by all countries except France and Switzerland. The analytical methods used to report positive results were almost all direct diagnostic tests such as the PCR method, which detects viral genetic material, except for Denmark reporting the birds to be positive to an ELISA (serological, indirect) test.
Furthermore, the results of 11,670 solipeds, almost all horses, were reported by 12 MS: Austria (8), Cyprus (157), the Czech Republic (783), France (303), Greece (1,139), Hungary (167), Italy (7,392), Portugal (50), Romania (208), Slovakia (102), Spain (1,359) and the United Kingdom (2) and two non‐MS: Serbia (2,495) and Switzerland (7). Italy and Spain provided for 75.0% of the data. Positive horses were reported by all countries except Romania, the United Kingdom and Switzerland. Countries reported the horses (and for Italy one donkey) to be confirmatory test‐positive specifically to the immunoglobulin M (IgM)‐capture ELISA (MAC‐ELISA), real‐time PCR, neutralising antibody testing, except for Cyprus, Greece19 and Portugal reporting test‐positivity to immunoglobulin G (IgG) ELISA20 or ELISA. Positive animals were unvaccinated (Cyprus, the Czech Republic and Portugal) or having an unknown vaccination.
13.3.3.2. WNV equine cases reported to the EU Animal Disease Notification System
Figure 62 displays trends during 2003–2017 in the reported annual numbers of WNV‐affected equines, as reported to ADNS by MS and at the EU level. During 2017 there were 127 affected equines reported, due to 84 outbreaks. At EU level the annual median number of reported affected equines and outbreaks during 2013–2017 was, respectively, 111 and 88. The ranges were, respectively, 36–188 and 31–173. The highest annual median number of affected equines and outbreaks over this period, respectively, 43 and 33, was reported by Italy. These numbers indicate that each outbreak only involved few animals.
Figure 62.
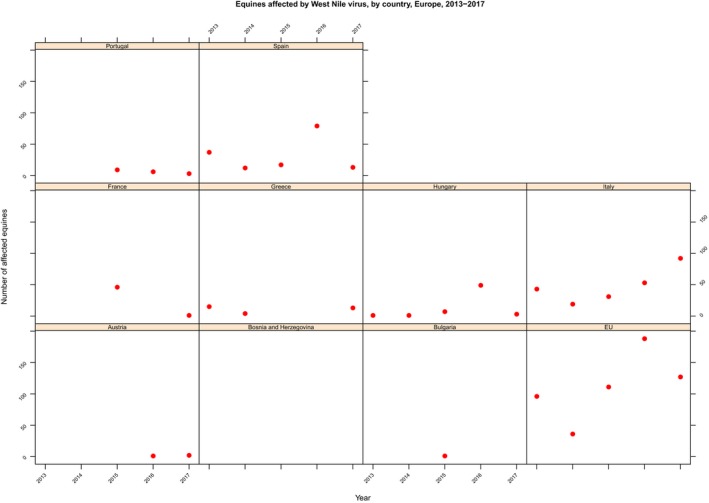
Number of affected equines reported to the EU Animal Disease Notification System (ADNS), by reporting MS, EU, 2013–2017
An interactive overview map for both the EU and neighbouring countries, including the regional level, is published on the https://ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc (ECDC, 2018) with an epidemiological update summarising the WNV transmission season, historical maps and the weekly updates of the ECDC West Nile risk map including three types of maps: (1) human WNF cases; (2) equine WNF cases; and (3) combined human and equine WNF cases. The map with combined 2017 human and equine WNF cases is in Figure 63. In the table in the https://ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc, one finds the number of outbreaks among equids per area (at NUTS three level). During the 2017 transmission season, 212 human cases and 127 equine cases were reported in the European Union.
Figure 63.
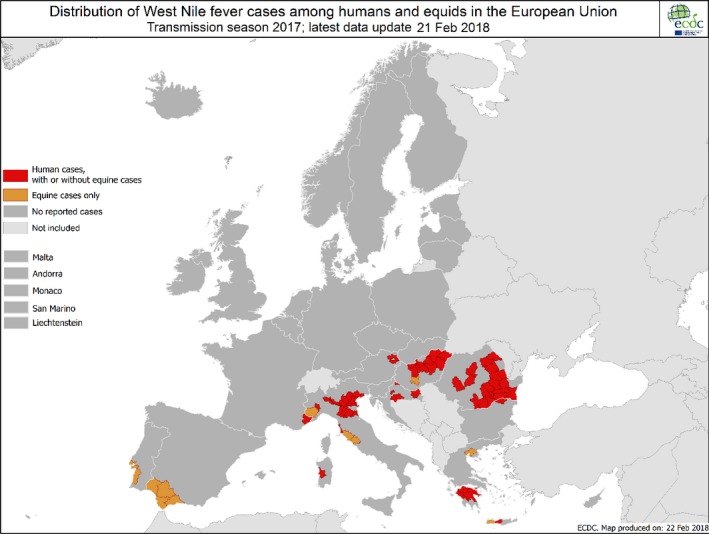
Distribution of human and equine West Nile fever cases by affected areas, EU/EEA region, transmission season 2017 (Source: TESSy and ADNS)
13.3.3.3. Member States’ evaluation of status on WNV and trends
More information on the evaluation of the status as regards WNV and trends are in the national zoonoses reports submitted in accordance with Directive 2003/99/EC, which are published on the EFSA website together with the EU Summary Report (available online at http://www.efsa.europa.eu/en/biological-hazards-data/reports). Short extracts are provided here.
The Czech Republic
‘… In 2017, 783 horses from the entire Czech Republic were tested for the presence of antibodies against West Nile virus. Of the total number of sera tested, 116 sera (14.8%) responded positively to cELISA with WNV antigen. Of the 116 samples of sera tested with virus neutralisation tests (VNT) for the presence of 57 antibodies against WNV, 11 samples were positively responded, one sample responded dubiously. Most seropositive horses were imported into the Czech Republic. …’
France
‘… The status of the disease is stable with long absences of virus circulation and outbreaks in horse populations. During the last 5 years virus circulation has only been detected in 2015 and 2017 in animal and human populations.’
In 2015, 39 equine outbreaks have been confirmed in three counties surrounding the Camargue area: Bouches‐du‐Rhône, Gard and Hérault departments. In total, 49 equines were found to be infected (positive in WNV competition and MAC‐ELISAs); among them, 41 exhibited neuroinvasive forms and three showed febrile forms. Camargue is known to be the highest risk zone where horse owners and veterinarians are aware of the existence of the disease and its clinical signs. It is not clear if the virus circulation is maintained over the time at a very low level or if virus is regularly reintroduced in Camargue through bird migrations.
Greece
‘…In the following map one can notice the regions where IgM antibodies were detected in equines (Figure 64). This fact implies that these equines were recently infected and that the virus is circulating in these areas. As shown in the map, the total number of WNV outbreaks in 2017 was 12.’
Figure 64.
Greek regions where outbreaks in equines were detected, Greece, 2017
Italy
‘…During the last epidemic season, 2017, infected wild birds were collected in Emilia Romagna, Sardinia and Veneto regions. Genetic analyses of WNV strain confirmed the circulation of Lineage 2. Infected birds among the resident species were collected in Emilia Romagna, Piedmonts and Sardinia and Lombardy regions. Genetic analyses of WNV strain confirmed the circulation of Lineage 2. From 2012 to date 11 outbreak of WND were reported in poultry. During 2017, 93 infected horses were identified and six of them in Piedmont, Tuscany and Sardinia regions developed neurological symptoms. Genetic analyses of the WNV strain identified in a dead horse have been clustered in viral Lineage 2. Furthermore during 2017 79 positive mosquitoes pool were collected between June and October in Lombardy, Emilia Romagna, Veneto, Tuscany, Sardinia, Piedmonts regions. Genetic analyses of WNV strain confirmed the circulation of Lineage 2 in most of the mosquito population with the exception of a pool collected in Piacenza province (Emilia Romagna region) infected by WNV belonging to Lineage 1.’
Romania
‘… At present West Nile disease is considered endemic in the susceptible animal population from the entire territory of Romania. As a result of this research evidence was gathered that a high proportion of the horse population proved to be seropositive for West Nile virus antibodies. Following this find measures were taken to implement an active surveillance programme at national level to detect the prevalence of the disease in the horse population, by detecting IgG and IgM antibodies. During 2009–2011 sufficient data were gathered to demonstrate that West Nile disease is endemic at least in the local horse population. As a consequence active surveillance was reduced to only two counties (Constanța and Brăila) in three localities (Esna, Polizești and Nuntași) where outbreaks were declared to OIE in 2010. Information gathered during the active surveillance was shared with the Institute for Public Health, to help decision making on the control of the disease in humans. Although during the last 10 years of active and passive surveillance no animal clinical case was confirmed by laboratory diagnosis, one cannot conclude that the disease has a declining trend. Arguments to sustain this are the natural immunity of the horse population which leads to low clinical expression, the presence of migratory birds that transport the virus each year on the national territory and the human outbreaks registered almost every year. …’
Slovakia
‘…The results of serological monitoring in the horses population in Slovakia indicate indirectly the presence of the virus in our territory and indicate that the situation in our country is similar to other European countries in the Central European region. West Nile Fever virus in horses was never isolated. Presence of virus was detected only serologically. In 2017 one Northern Goshawk (Accipiter gentilis) was positive for WNV.’
13.4. Discussion
In 2017, the notification rate of WNV infections in humans in the EU/EEA slightly decreased compared to 2016. The highest number of WNV infections was reported by Romania, followed by Italy and Greece. No locally acquired infections had been reported by Greece for 2015 and 2016, and by France for 2016. The clinical manifestations as well as the case‐fatality ratio in 2017 were comparable to 2016.
Gossner et al. (2017) described examples that can support European countries to strengthen their WNV surveillance or preparedness, and that also serve as a model for surveillance and monitoring of other (vector‐borne) zoonotic infections.
The number of MS sending animal surveillance data increased in 2017 compared with previously. During 2017, as in recent years, WNV circulation, and subsequent outbreaks and positive animals has been reported by countries in Central and Eastern Europe and in the Mediterranean basin; Austria, Bulgaria, Croatia, Cyprus, the Czech Republic, France, Greece, Hungary, Italy, Portugal, Serbia, Slovakia and Spain. These reported observations are consistent with the OIE's conclusion that the occurrence of WNF in humans and animals along with bird and mosquito surveillance for WNV activity demonstrates that the virus range has dramatically expanded including North, Central and South America as well as Europe and countries facing the Mediterranean basin (OIE Terrestrial Manual).
The risk of WNV transmission is complex and multifactorial; it concerns the virus, the vectors, the animal reservoirs, the environmental conditions and human behaviour. Preventing or reducing of WNV transmission depends on successful controlling vector's abundance or interruption of human‐vector contact. Human, animal and entomological WNF surveillance is crucial to allow the early detection of WNV infections in humans and take timely preventive measures. In horses, the spread of WNV is preventable with proper vaccination and protection against mosquito bites.
13.5. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Humans | Surveillance Atlas | http://atlas.ecdc.europa.eu/public/index.aspx |
| EU case definitions | ||
| https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions | ||
| Emerging and Vector‐borne Diseases Programme | ||
| https://ecdc.europa.eu/en/about-us/who-we-are/disease-programmes/emerging-and-vector-borne-diseases-programme | ||
| Emerging Viral Diseases‐Expert Laboratory Network (EVD‐LabNet) | ||
| https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/evd-labnet | ||
| ECDC – Surveillance and disease data for West Nile fever | https://ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data | |
| World Health Organization – West Nile virus Fact sheet | ||
| http://www.who.int/mediacentre/factsheets/fs354/en/ | ||
| Animals | World Organisation for Animal health, Summary of Information on West Nile fever | http://www.oie.int/animal-health-in-the-world/animal-diseases/west-nile-fever/ |
| OIE Reference Laboratory for West Nile Fever | http://www.izs.it/IZS/Centres_of_excellence/International_Centres/OIE_Reference_Laboratory_for_West_Nile_Fever | |
| Annual national zoonoses country reports (reports of reporting countries on national trends and sources of zoonoses) | http://www.efsa.europa.eu/en/biological-hazards-data/reports | |
| EU Animal Disease Notification system (ADNS) | https://ec.europa.eu/food/animals/animal-diseases/not-system_en#proc | |
| Summary Presentations on the situation as regards Bovine Tuberculosis control and eradication programmes in Member States | https://ec.europa.eu/food/animals/health/regulatory_committee/presentations_en#20160705 | |
| Scientific Opinion of the EFSA Panel on Animal Health and Welfare (AHAW): Vector‐borne diseases | http://www.efsa.europa.eu/en/efsajournal/pub/4793 | |
| VectorNet, a joint initiative of the European Food Safety Authority (EFSA) and the European Centre for Disease Prevention and Control (ECDC), which started in May 2014. The Project supports the collection of data on vectors and pathogens in vectors, related to both animal and human health | https://vectornet.ecdc.europa.eu/ | |
| WNV, a story map | https://efsa.maps.arcgis.com/apps/MapJournal/index.html?appid=512a03aa8df84d54a51bcb69d1b62735 | |
| Scientific Opinion of the EFSA Panel on Animal Health and Welfare (AHAW): Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation(EU) No. 2016/429): West Nile fever, Vector‐borne diseases | http://www.efsa.europa.eu/en/efsajournal/pub/4955 |
14. Tularaemia
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at http://doi.org/10.5281/zenodo.1475841
14.1. Abstract
For 2017, 355 cases of tularaemia in humans were reported in 17 MS and, 321 (90%) were confirmed. This is a decrease of more than 30% in case numbers compared to 2015 and 2016. This decrease is mainly due to a large decrease in case numbers in Finland and Sweden. The overall EU notification rate was 0.06 per 100,000 population in 2017, less than a third of the rate reported in 2016 (0.21 per 100,000 population). In 2017, Sweden had the highest notification rate with 0.84 per 100,000 followed by Finland and the Czech Republic, with 0.58 and 0.48 per 100,000 population, respectively.
Tularaemia is a seasonal and cyclical disease with a complex ecological cycle. The high number of human cases in Finland in 2016 followed a peak in the number of voles in 2015 and climatic conditions favouring the abundance of mosquitoes transmitting the bacteria to humans.
Tularaemia in animals is rarely reported in EU and the submission of the data to EFSA is on voluntary basis. In 2017, as for the year 2016, only one Member State (Sweden) reported data on the occurrence of Francisella tularensis. Seven brown hares out of 39 tested animals (17.9%) were found to be positive. Also, Switzerland reported on the occurrence of F. tularensis in five out of 11 tested animals mainly from natural habits and zoo. The number of positive tested animals in 2017 is comparable to the previous 2016 with no reported outbreaks. However the detection of F. tularensis in brown hares in Sweden during 2017 suggests that the bacterium is still present and outbreaks may occur in the future, in particular in northern European Countries.
To predict outbreaks and to avoid them whenever possible, greater efforts are needed to assess the extent of the true animal reservoir population of F. tularensis and to assess the occurrence of this zoonotic pathogen in the EU animal reservoir populations including the environment. Francisella spp. are widely present in the environment and a wide range of wild animals (e.g. hares, rabbits) but also vectors (e.g. ticks) could be used to enforce the passive surveillance in EU as they can be sources of infections for humans.
14.2. Surveillance and monitoring of tularaemia in the EU
14.2.1. Humans
For 2017, 27 EU MS, Iceland, Norway and Switzerland provided information on tularaemia in humans.
All reporting EU countries have a comprehensive surveillance system. Twenty‐three EU countries used the EU case definition. Germany and Italy used an alternative case definition. Finland and France did not specify the case definition used. The reporting is compulsory in all countries, except in the United Kingdom where it is voluntary. The surveillance is mostly passive except in the Czech Republic, Portugal and Slovakia where it is active. Belgium and Bulgaria reported aggregated data while all other countries reported case‐based data.
14.2.2. Animals
Among EU MS, tularaemia in animals is not a reportable disease according to Council Directive 82/894/EEC on the notification of animal diseases within the EU amended and consolidated version 2013 01 01 but it is reportable to OIE if a new disease event occurs in a country. However the notification is mandatory by national law in the Netherlands, Sweden, Iceland and Switzerland. The monitoring data from animals on F. tularensis are voluntary submitted by MS and EFTA countries to EFSA. The data are collected without harmonised design at the EU level and only allowing for descriptive summaries and not for trend analyses and trend watching (Boelaert et al., 2016).
14.3. Results
14.3.1. Overview of key statistics, EU, 2013–2017
Table 63 summarises EU level statistics related to human tularaemia, and to tularaemia occurrence and prevalence in major animal species, respectively, in the EU, during 2013–2017.
Table 63.
Summary of tularaemia statistics related to humans and major animal species (brown hares) EU MS, 2013–2017
| 2017 | 2016 | 2015 | 2014 | 2013 | Data source | |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Total number of confirmed cases | 321 | 1,056 | 1,080 | 482 | 280 | ECDC |
| Total number of confirmed cases/100,000 population (notification rates) | 0.06 | 0.21 | 0.23 | 0.10 | 0.06 | ECDC |
| Number of reporting EU MS | 27 | 26 | 25 | 26 | 26 | ECDC |
| Infection acquired in EU MS | 253 | 326 | 902 | 396 | 248 | ECDC |
| Infection acquired outside EU MS | 2 | 5 | 4 | 6 | 2 | ECDC |
| Unknown travel status or unknown country of infection | 66 | 725 | 174 | 80 | 30 | ECDC |
| Animals (hares) | ||||||
| Total number of animals tested | 39 | 41 | 65 | 31 | 37 | EFSA |
| Proportion of positive animals | 17.9% | 14.6% | 47.7% | 6.5% | 29.7% | EFSA |
| Number of reporting EU MS | 1a | 1a | 1 | 1 | 1 | EFSA |
MS: Member State.
Reporting MS is Sweden.
14.3.2. Tularaemia in humans
In total, 355 cases of tularaemia in humans were reported in 17 EU MS. Among those cases, 321 (90%) were confirmed. This marks a decrease of more than 30% in the number of cases compared to 2015 and 2016. This decrease is mainly due to a large decrease in the number of cases reported by Finland and Sweden. The highest number of cases was reported by Sweden, the Czech Republic and Germany, with 84, 51 and 50 confirmed cases, respectively (Table 64). Ten EU MS (Cyprus, Estonia, Greece, Ireland, Latvia, Luxembourg, Malta, Portugal, Romania and the United Kingdom) did not report any cases. Norway reported 92 confirmed cases. The overall EU notification rate was 0.06 per 100,000 population in 2017, less than a third of the rate reported in 2016 (0.21 per 100,000 population). In 2017, Sweden had the highest notification rate with 0.84 per 100,000 population followed by Finland and the Czech Republic, with 0.58 and 0.48 per 100,000 population, respectively.
Table 64.
Reported human cases of tularaemia and notification rates per 100,000 population in the EU/EFTA, by country and year, 2013–2017
| Country | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| National coveragea | Data formata | Total cases | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | Confirmed cases & rates | ||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | ||||
| Austria | Y | C | 13 | 13 | 0.15 | 9 | 0.10 | 4 | 0.05 | 0 | 0.00 | 2 | 0.02 |
| Belgium | Y | A | 5 | 5 | 0.04 | 1 | 0.01 | 1 | 0.01 | 2 | 0.02 | 1 | 0.01 |
| Bulgaria | Y | A | 1 | 1 | 0.01 | 2 | 0.03 | 17 | 0.24 | 1 | 0.01 | 1 | 0.01 |
| Croatia | Y | C | 3 | 3 | 0.07 | 2 | 0.05 | 13 | 0.31 | 2 | 0.05 | 2 | 0.05 |
| Cyprus | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Czech Republic | Y | C | 51 | 51 | 0.48 | 59 | 0.56 | 56 | 0.53 | 48 | 0.46 | 36 | 0.34 |
| Denmarkb | – | – | – | – | – | – | – | – | – | – | – | – | |
| Estonia | Y | C | 0 | 0 | 0.00 | 1 | 0.08 | 0 | 0.00 | 1 | 0.08 | 1 | 0.08 |
| Finland | Y | C | 32 | 32 | 0.58 | 699 | 12.74 | 104 | 1.90 | 9 | 0.17 | 15 | 0.28 |
| France | Y | C | 48 | 19 | 0.03 | 47 | 0.07 | 28 | 0.04 | 19 | 0.03 | 21 | 0.03 |
| Germany | Y | C | 50 | 50 | 0.06 | 41 | 0.05 | 34 | 0.04 | 21 | 0.03 | 20 | 0.02 |
| Greece | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Hungary | Y | C | 11 | 11 | 0.11 | 22 | 0.22 | 35 | 0.36 | 140 | 1.42 | 48 | 0.48 |
| Ireland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Italy | Y | C | 2 | 2 | 0.00 | 0 | 0.00 | – | – | 0 | 0.00 | 1 | 0.00 |
| Latvia | Y | C | 0 | 0 | 0.00 | 1 | 0.05 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Lithuania | Y | C | 5 | 5 | 0.18 | 2 | 0.07 | 4 | 0.14 | 4 | 0.14 | 4 | 0.13 |
| Luxembourg | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Malta | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Netherlands | Y | C | 1 | 1 | 0.01 | 5 | 0.03 | 1 | 0.01 | 5 | 0.03 | 0 | 0.00 |
| Poland | Y | C | 30 | 30 | 0.08 | 18 | 0.05 | 9 | 0.02 | 11 | 0.03 | 8 | 0.02 |
| Portugal | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | – | – | – | – |
| Romania | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 1 | 0.01 | 0 | 0.00 | 1 | 0.00 |
| Slovakia | Y | C | 2 | 2 | 0.04 | 7 | 0.13 | 28 | 0.52 | 6 | 0.11 | 9 | 0.17 |
| Slovenia | Y | C | 1 | 1 | 0.05 | 3 | 0.15 | 0 | 0.00 | 1 | 0.05 | 2 | 0.10 |
| Spain(d) | Y | C | 13 | 11 | 0.02 | 3 | 0.01 | 22 | 0.05 | 62 | 0.13 | 0 | 0.00 |
| Sweden | Y | C | 87 | 84 | 0.84 | 134 | 1.36 | 722 | 7.41 | 150 | 1.56 | 108 | 1.13 |
| United Kingdom | Y | C | 0 | 0 | 0.00 | 0 | 0.0 | 1 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| EU Total | – | – | 355 | 321 | 0.06 | 1,056 | 0.21 | 1,080 | 0.24 | 482 | 0.10 | 280 | 0.06 |
| Iceland | Y | C | 0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Norway | Y | C | 92 | 92 | 1.75 | 40 | 0.77 | 42 | 0.81 | 46 | 0.90 | 28 | 0.55 |
| Switzerlandc | Y | C | 130 | 130 | 1.54 | 55 | 0.65 | 48 | 0.57 | 39 | 0.47 | 29 | 0.48 |
Y: yes; N: no; A: aggregated data; C: case‐based data; –: no report.
Not notifiable, no surveillance system exists.
Switzerland provided data directly to EFSA. The human data for Switzerland include data from Liechtenstein.
Travel information was available for 79% of the confirmed cases reported by EU MS. Eight travel‐associated cases were reported, of which two cases with place of infection in non‐EU countries (Gambia and Ukraine, one case each).
Between 2008 and 2016 (Figure 65), three peaks in number of cases were observed in 2012, 2015 and 2016. These peaks were due to high numbers of reported cases in Finland and Sweden. Tularaemia shows a seasonal pattern, with most cases occurring between July and October, but some cases also occur during the winter.
Figure 65.
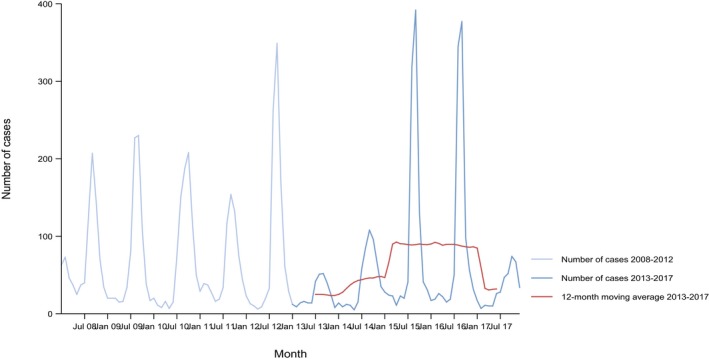
- Source: Cyprus, Czech Republic, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Latvia, Luxembourg, Malta, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and United Kingdom.
- Austria, Belgium, Bulgaria, Croatia, Denmark, Italy, Lithuania, Netherlands and Portugal did not report data to the level of detail required for the analysis.
Nine MS provided data on hospitalisation status of their cases, representing 38% of the confirmed cases. Of the confirmed cases for which information is available, 62% were hospitalised. Ten MS provided information on the outcome of their cases, representing 51% of the confirmed cases. One death due to tularaemia was reported in 2017.
14.3.3. Tularaemia in animals
In 2017, only one EU MS (Sweden) reported to EFSA data on the occurrence of F. tularensis in animals. In total, 39 hare samples from 31 European brown hares (Lepus europaeus) and eight mountain hares (Lepus timidus) were submitted and tested within the context of passive surveillance.
Switzerland reported on tularaemia from wild animals (hares, lynx and squirrels), zoo animals (hares and monkeys), with, overall, five positives (from monkeys, hares and squirrels) out of 11 collected samples.
14.4. Discussion
In 2017, the number of human cases observed in the EU decreased compared to the two previous years and was in the range of case numbers reported in 2013 and 2014.
Notification rates of tularaemia vary considerably among MS and over time. Between 2013 and 2015, Sweden showed the highest notification rate while in 2016, Finland had the highest notification rate which was also the highest notification rate observed among EU MS in the past years. In Finland, tularaemia outbreaks are preceded one year earlier by a peak in the vole population (Rossow et al., 2015). In 2017, the highest notification rate was again reported by Sweden.
Tularaemia has terrestrial and aquatic ecological cycles with an extensive host range among animals including vertebrates and invertebrates. Lagomorphs of the genus Lepus and small rodents are considered reservoirs, but antibodies against F. tularensis have been detected in other wild animals, such as red fox and wild boar, and domestic animals such as cat and dog (Hestvik et al., 2015; Maurin and Gyuranecz, 2016). As for humans, the animal species susceptible to tularaemia may be infected either through the terrestrial or the aquatic cycle. A study performed in the Netherlands during an outbreak in hares in 2015 to assess potential reservoirs and transmission routes of F. tularensis showed the importance of the environmental surveillance of water and its valuable use to monitor this pathogen (Janse et al., 2015). In 2016 and 2017 only Sweden reported data on hares obtained from passive surveillance. The proportion of positive hares decreased compared to 2015. Data of Sweden shows that F. tularensis is still present in the wildlife and that hares (genus Lepus) are good indicator animals to monitor the occurrence. Wildlife may continue to play a role in the maintenance of F. tularensis in the ecological cycle and the occurrence of human cases.
In 2017, only Sweden and Switzerland submitted data to EFSA on the occurrence of F. tularensis in animals: Sweden reported 17.9% positive hares obtained from passive surveillance and Switzerland reported positive wild animals (hares, monkeys and squirrels) sampled from their natural habitat and zoos. It is clear that Francisella spp. are widely present in the environment and a wide range of wild animals (such as hares), but also vectors (e.g. ticks as illustrated in the previous chapter) could be used to enforce the passive surveillance in EU as they can be sources of infections in humans (WHO, 2007).
The tularaemia monitoring data on animals are generated by non‐EU harmonised monitoring schemes, reported to EFSA from very few Member States and originate from investigations with small sample sizes. They preclude trend watching and trend analysis and they only allow descriptive summaries to be made. No inference on the occurrence and prevalence of F. tularensis at animal level in the EU can be made on the basis of these data.
14.5. Related projects and internet sources
15. Other zoonoses and zoonotic agents
In 2017, among others, data on Bacillus, Calicivirus, Chlamydia, Clostridium, Cryptosporidium, Cysticercus, Enterococcus spp., Erysipelothrix, hepatitis A virus, Klebsiella, Leptospira, marine biotoxins, Proteus, Sarcocystis, coagulase‐positive Staphylococcus spp., tick‐borne encephalitis virus and Anisakis and other parasites were reported to EFSA.
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at http://doi.org/10.5281/zenodo.1475841
15.1. Bacillus and B. cereus enterotoxins in foods
Four MS (Bulgaria, Greece, Lithuania and Spain) submitted data on the prevalence of Bacillus in food and animals in 2017. All samples tested by Bulgaria (n = 3, from bakery products) and Lithuania (n = 6, from infant formula and other food) were negative. Greece found nine positive cases in the 28 animals tested (cattle, goats and sheep). Spain tested 113 samples from cow's or goat's milk or cheese (n = 32), infant formula (n = 20) and other processed products/prepared dishes (n = 61). Only three units of the latter were positive. One sample tested B. cereus enterotoxins was negative.
15.2. Calicivirus
In 2017, four MS (Portugal, Romania, Slovenia and Spain) reported on the occurrence of Calicivirus (mainly Norovirus) in live bivalve molluscs and fruit and vegetables. All samples tested by Portugal and Romania (fruits) were collected during border inspections (n = 23) and processing plant (n = 5) and were negative.
In Slovenia and Spain, 5 out of 9 and 10 out of 49 samples (collected at processing plant and retail), respectively, from seafood (live bivalve molluscs mainly) were positive while all samples from fruits and vegetables tested in both countries were negative.
15.3. Chlamydia spp
In 2017, Greece took 59 blood samples from sheep and goat in the context of clinical investigations, and detected 17 positive samples for C. caviae.
Denmark also reported 18 samples from clinical suspected birds and three of them were positive for C. psittaci.
15.4. Clostridium spp. and Clostridium botulinum toxin
Four MS (Bulgaria, Greece, Lithuania and Spain) provided information on Clostridium, in various animals, feed and food products taken at retail and processing plants.
Greece tested 55 animals in the context of clinical investigations (cattle, goats, sheep and rabbits) and 30 of these samples were positive (12 from cattle, 11 from goats and seven from sheep).
Spain tested 222 samples from processed food and prepared dishes – and one from potable water – of which nine were positive. In Bulgaria and Lithuania, all samples tested for Clostridium were negative (85 feed samples and six fishery and other food samples, respectively).
15.5. Pathogenic and non‐pathogenic Enterococcus
Spain was the only MS which reported data on non‐pathogenic Enterococcus in 2017. None of the samples (infant formula, n = 20) taken at retail level were positive.
For pathogenic Enterococcus, Greece reported nine positive cases from 148 milk samples and Spain reported two positive samples taken at retail (one potable water sample and one sample was unspecified).
15.6. Erysipelothrix
In 2017, Spain submitted data on the occurrence of Erysipelothrix in 5,120,487 pigs inspected at slaughterhouse, and 230 (0.004%) were found with signs of swine erysipelas (mainly from breeding pigs not raised under controlled housing conditions). This is comparable with 2016, when data on Erysipelothrix were reported to EFSA for the first time by Spain. Greece reported two suspected sheep but microbiological results were negative.
15.7. Proteus
In 2017, Greece provided data on 189 milk samples (from cattle, goat and sheep) tested for Proteus and 10 (5.3%) of them were positive.
15.8. Coagulase‐positive Staphylococcus spp
In 2017, six MS (Bulgaria, Croatia, Greece, Italy, Poland and Spain) and one non‐MS (Bosnia and Herzegovina) reported data on coagulase‐positive Staphylococcus spp. (S. aureus, S. intermedius, S. hyicus and unspecified) in various animals and food products.
Bulgaria, Greece, Italy and Spain tested different food matrices, such as bakery products, cheese, milk and meat from different species, vegetables, fish and seafood and prepared dishes and other processed food. Four (0.31%) out of 1,271 samples from Bulgaria, 602 (4.3%) out of 14,082 samples from Italy and 49 (1.9%) out of 2,570 samples from Spain were positive. All food samples from Greece (n = 126) were negative.
In addition, Greece and Italy submitted data on the presence of Staphylococcus in animals. From the farm sampling performed by Greece under clinical investigations, 24 (29.6%) of the 81 cattle samples, 25 (49%) of the 51 goat samples and 58 (62.4%) of the 93 sheep samples were positive. Italy submitted 4,600 samples from a wide variety of animals (livestock, pets, wild and zoo animals). 1,326 (28.8%) of the samples were positive. A significant prevalence was detected in sheep (37.4%), dogs (31.3%), goats (29.5%) and cats (27.3%).
Croatia tested five cheeses during border inspection activities. Poland tested 302 samples from cheese (239 samples), other dairy products (58) and smoked fish (5). All samples in both MS were negative.
In total, 655 (3.6%) of the 18,361 food samples tested and 1,425 (29.6%) of the 4,812 animal samples tested were positive. One non‐MS (Bosnia and Herzegovina) tested a substantial number of prepared dishes and processed food (n = 7,830) for the presence of Staphylococcus spp. and 0.04% was positive.
15.9. Tick‐borne encephalitis virus (TBE)
Slovenia tested 20 raw milk samples (goat and sheep) taken at retail level for the presence of TBE and none were positive. This is in accordance with their results from milk samples tested in the previous years.
15.10. Anisakis, Cysticercus, Sarcocystis and other parasites
In 2017, two MS (Spain and France) reported data on raw fish or fishery products tested for Anisakis at retail: 101 out of the 366 samples tested were positive and reported by Spain (raw fish).
In 2017, six MS (Belgium, Greece, Luxembourg, Slovenia, Spain and Sweden) submitted data on Cysticercus mainly based on reports from slaughterhouse surveillance, active monitoring or clinical investigations. In Belgium, 1,375 out of the 922,797 cattle (0.14%) inspected at the slaughterhouse showed bovine cysticercosis, caused by Taenia saginata. Slovenia and Sweden reported data on both bovine and porcine cysticercosis. In Slovenia, none of the 245,216 inspected pig carcasses were found positive, whereas eight out of the 118,235 (0.007%) inspected carcasses in cattle were positive for T. saginata. Sweden inspected 2,576,290 and 406,030 pig and cattle carcasses and all of them were negative. Spain provided data on the prevalence of Cysticercus in various animals (cattle, small ruminants, pigs, deer and wild boar) and the various contexts stated above. One (0.0009%) out of the 107,419 cattle, 154 (0.005%) out of the 2,993,124 pigs, 111,968 (6.3%) out of the 1,761,093 sheep and 13,908 (6.1%) out of the 226,606 goats were positive for Cysticercus spp. Finally, 18,854 wild boars and 42,943 deer were inspected during hunting (clinical investigations) and six (0.03%) and 18 (0.12%) were positive for Cysticerci spp., respectively. Results from Belgium, Sweden, Slovenia and Spain are similar to those reported in 2016. In addition, in 2017, Greece reported one clinical suspected sheep and Luxembourg reported 19 (0.07%) positive cases from 26,173 inspected cattle at slaughterhouse.
In 2017, Belgium reported 922,797 bovine carcasses from slaughterhouse inspection for the presence of Sarcocystis and 99 (0.011%) were positive.
In 2017, Spain reported 12 (0.71%) positive samples for Ascaris in 1,678 pigs tested and none for Cryptosporidium and Giardia (11 samples).
15.11. Other
All reported samples for Leptospira (10,070, Bulgaria), Shigella (three from Spain, 60 from Slovenia), Vibrio (30, Spain) and other viruses (seven, Spain) were negative. Out of the 168 samples tested for hepatitis A virus (Romania, Slovenia, Spain), one sample from mussels in Slovenia was positive. Out of the 144 milk samples tested for Klebsiella (Greece), two were positive. Out of the 69 molluscs samples tested for marine biotoxins (Spain), two were positive.
15.12. Related projects and internet sources
| Subject | For more information see | |
|---|---|---|
| Food | Bad Bug Book (Second Edition), Food‐borne Pathogenic Microorganisms and Natural Toxins Handbook, Center for Food Safety and Applied Nutrition, Food and Drug Administration (FDA), USA | https://www.fda.gov/food/foodborneillnesscontaminants/causesofillnessbadbugbook/ |
16. Food‐borne outbreaks
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at http://doi.org/10.5281/zenodo.1475841
16.1. Abstract
In 2017, 27 MS reported in total 5,079 food‐borne and waterborne outbreaks (372 less than 2016), which correspond to 97.7 outbreaks per week, on average, at the EU level. Another 149 outbreaks were notified by eight non‐MS. In EU, among the 43,400 involved cases (13,519 less than 2016), 4,541 hospitalisations (119 more than in 2016) and 33 deaths (one more than in 2016) were reported in MS. The number of outbreaks reported by each MS varied importantly, with few MS accounting for most of the events. There was also a huge variability in the types of causative agents reported to be linked to outbreaks, and in the types and mean size of these incidents reported to EFSA. These differences depict an extremely heterogeneous geography of FBOs across the EU. Apart from true epidemiological differences, variations in reporting between MS may be due to differences in the approach and the sensitivity of the surveillance of FBOs.
The number of FBOs reported in 2017 did not substantially change compared with 2016, for most MS. At the MS level, the most reported causative agent in food‐borne (including waterborne) outbreaks was Salmonella for 15 MS (Austria, Croatia, the Czech Republic, Denmark, Estonia, Greece, Hungary, Italy, Latvia, Lithuania, Luxembourg, Poland, Slovakia, Spain, United Kingdom), bacterial toxins other than C. botulinum for four MS (Bulgaria, France, Portugal, Romania), Campylobacter for three MS (Belgium, Germany, Malta), norovirus including other caliciviruses for three MS (Finland, Netherlands, Sweden) and Shiga toxin‐producing E. coli for one MS (Ireland).
Salmonella was the most frequently reported causative agent in the EU (1,241 FBOs and no waterborne outbreaks; 24.4% of total outbreaks, 25 MS). Outbreaks of salmonellosis had the highest impact on human cases (9,600, 22.1% of all outbreak cases), hospitalisations (2,227, 49.0% of all hospitalisations) and deaths (11, 33.3% of all deaths). S. Enteritidis was by far the most frequently reported Salmonella serovar and totalled 61.1% (n = 758, 23 MS) of Salmonella FBO, corresponding to 14.9% – about one in seven – of all reported FBO at the EU level. Two MS (Poland and Slovakia) accounted together for the 63.3% of all outbreaks caused by this serovar, in the EU.
Listeria and C. botulinum were associated with the highest case fatality among FBO cases (5.1% and 7.7%, respectively). Compared with 2016, a marked decrease (−100 outbreaks) was observed in the number of outbreaks due to Norovirus, Campylobacter (−79 outbreaks) while for histamine an increase was observed (+22 outbreaks). In the last 4 years, the number of histamine poisoning outbreaks reported by France and Spain increased significantly. These findings deserve attention since there is an increased habit in the EU of consuming raw fish. Outbreaks of hepatitis E were reported by Germany, for the first time since the beginning of the reporting of FBOs. This is a significant finding since hepatitis E is considered an emerging problem in the EU and interest of public health.
Analysis of strong‐evidence outbreaks (643 outbreaks, 12.7% of total outbreaks) revealed that in 2017 60% of strong‐evidence FBOs were associated with food of animal origin; ‘Meat and meat products’ (i.e. including meat from poultry, pork, bovine, sheep, and other unspecified red meats and their products) was the food group most frequent involved (121 outbreaks), followed by ‘Fish and fishery products’ (106 outbreaks), ‘Eggs and egg products’ (105 outbreaks) and ‘Milk and milk products’ (49 outbreaks). Compared with previous years, no important changes were observed for any of the food items being implicated in the strong‐evidence FBOs. Outbreaks by Salmonella implicating ‘Eggs and egg products’, ‘Bakery products’ and ‘Meat and meat products’ had the highest impact on number of outbreaks, cases, hospitalisations and deaths. In particular, Salmonella in ‘Eggs and egg products’ caused the highest number of strong‐evidence outbreaks (99 outbreaks). Other critical pathogen/food pairs were bacterial toxins other than C. botulinum in ‘Meat and meat products’, ‘Mixed food’ or ‘Other foods’ and Campylobacter in ‘Milk and milk products’ and ‘Meat and meat products’.
‘Household’ was the most frequent reported place of exposure of cases to contaminated foods and one in three outbreaks occurring in this setting. The diversity of causative agents reported in the ‘Household’ setting was the largest one compared to other settings with Salmonella being most frequently reported (61.4%). In addition, outbreaks by C. botulinum, Trichinella and mushrooms toxins were only reported as causative agents in the setting ‘Household’. The frequent occurrence of FBO in household setting and the peculiarity of causative agents reaffirm the need to deliver recommendations to consumers on correct behaviours and practices for food handling and preservation. FBOs in ‘Canteen or catering to workplace, school, hospital etc.’ were predominantly caused by bacterial toxins other than C. botulinum, as well as by norovirus and other caliciviruses (together 58%). In the settings ‘Restaurant, pub, street vendors, take away’ and ‘Other settings and multiple settings’ more than half of the FBO were reported to be caused by either Salmonella or bacterial toxins other than C. botulinum.
An important limitation of the FBO data analysis is that in 2017 more than 33% of the outbreaks reported to EFSA lack information on the causative agent and in many cases, also on the suspected food vehicle and on the FBO setting and the contributory factors. This informative gap hampers a comprehensive understanding of the epidemiology of FBOs at both the EU and MS level.
16.2. Surveillance and monitoring of food‐borne and waterborne outbreaks in the EU
The annual reporting of information on food‐borne and waterborne outbreaks is mandatory for EU MS since 2003, according to Directive 2003/99/EC. The aim of the data collection and analysis is to provide information on the causative agents and the foodstuffs implicated in the outbreaks, as well as the circumstances, the events and the potential risk factors that may underlie the contamination of foodstuffs and the occurrence of the outbreaks.
Information reported to EFSA by MS on FBO include data on the causative agents, the numbers of human cases (illnesses), of hospitalisations and of deaths, the type of FBO (i.e. general/household), the implicated type of food, and the place of consumption/exposure (setting). Moreover information on the place of origin of the problem leading to contamination of food and on factors that may have contributed (e.g. cross‐contamination, inadequate heat treatment, etc.) are also collected.
Since the chain of the events leading to FBO may be very complex, the opportunity to describe and analyse jointly all these factors contributes importantly to the epidemiological characterisation of FBO in the EU and to the understanding of the most relevant sources.
The reporting system for FBO includes any bacterium, virus, parasite, alga, fungus and their products, such as toxins and biological amines (e.g. histamine), not just zoonotic agents. The data collection focuses any outbreaks for which the implication of food is suspected. As a consequence, the reporting does not limit to the causative agents whose transmission to humans occur primarily through food (e.g. Salmonella, Listeria), but also includes agents for which the food‐borne transmission is possible but usually accidental. Outbreaks caused by ingestion of drinking water are also deemed food‐borne as drinking water is defined as a food, in Regulation 178/2002/EC.
FBO data reporting is based on harmonised specifications which have been increasingly applied in the EU since 2007. The current system is known as European Union Food‐borne reporting System (EU‐FORS) and has been implemented since 2010. Outbreaks are categorised as having ‘strong evidence’ or ‘weak evidence’ based on the strength of proofs implicating a suspected food vehicle as the cause of the outbreak (EFSA, 2014b). For the former, it is compulsory to report a detailed data set, while for the latter type of outbreaks this is not mandatory but voluntary. This categorisation is therefore important to represent the level of uncertainty associated with the identification of the potential implicated vehicle, contributory factors and source. The evaluation of the strength of evidence implicating a suspected food vehicle in FBO as being strong or weak, is based on the assessment of all available types of evidence related to illness and exposure information (i.e. microbiological, epidemiological, descriptive, environmental, based on tracing‐back of the investigated foodstuffs) and according to the EU‐FORS guidance and the last published manual for reporting on FBO (EFSA, 2014b).
FBOs surveillance activities and criteria are not fully harmonised among MS although the data reporting rules follow the same standard EFSA harmonised specifications (EFSA, 2014b). Therefore, differences in sensitivity and type of outbreaks under surveillance may exist. For this reason the difference in the numbers and types of reported outbreaks, as well as in the causative agents, type of outbreak, etc. may not necessarily reflect the level of food safety among MS.
16.3. Data analyses
All reported food‐borne and waterborne outbreaks are summarised in tables and figures. Data on reported FBO in the EU MS and non‐MS are separately and descriptively analysed for ‘strong‐evidence’ and for ‘weak‐evidence’ outbreaks.21
In Section 16.4.1, outbreaks are generally summarised according to the associated health impact on the total number of cases, hospitalisations and deaths and also according the causative agent. In Section 16.4.2, the distributions of the implicated food vehicles and the places of exposure are described based on the reported strong‐evidence outbreaks only. However, as MS are allowed since 2014 to report detailed information on the suspected vehicles also in weak‐evidence outbreaks, the most significant agent/food combinations have been also described considering all the outbreaks with available information.
Details on FBO by causative agent, excluding waterborne outbreaks, are provided in the tables in the Appendix. All waterborne outbreaks are described separately.
Causative agents, food vehicles and outbreak settings are grouped to facilitate the understanding of the epidemiological picture at the EU level. Causative agents are described using a two‐level categorisation by type of agent (i.e. bacteria, bacterial toxins, parasites, viruses, other causative agents) and priority according with Directive 99/2003/EC. Outbreaks by pathogens listed under Annex IA (Brucella, Salmonella, Campylobacter, Listeria, Shiga toxin‐producing E. coli and Trichinella) have been described separately. Vibrio, C. botulinum toxin, norovirus including other caliciviruses, hepatitis A, Cryptosporidium, are also been described separately, given their importance as causative agents in FBO. Histamine, marine biotoxins and mushrooms toxins are also described separately given their increasing importance and diversity. Any other causative agent is described as ‘other agents’.
Food vehicles have been uniformly grouped following the general criteria adopted by EFSA for presenting data in this report. Place of exposures have been grouped so as to basically represent the different characteristics and level of risk connected to the setting and the process behind food preparation.
In tables and figures, sums and proportions (%) are the basic statistics used to describe the reported counts (numbers) of outbreaks. The rate of reported outbreaks per 100,000 population (‘outbreak reporting rate’) is calculated to compare MS independently on demographic size and variations. For estimations of the ‘Reporting rate’ at supranational or EU level, the overall population has been calculated by summing the populations of those MS that provided data on the specific reported FBO. Data on resident population at 1 January 2018 from Eurostat have been used for this purpose.
At the MS level, temporal (yearly) variations in the number of reported FBO (time trends) were tested for statistical significance using the autoregressive integrated moving average (ARIMA) model. p value ≤ 0.05 was considered to identify a statistical significant trend, beyond chance. The distribution of FBO (including waterborne outbreaks) over years by causative agent and by MS has been analysed for two different time‐frames, short period (2014–2017) and long period (2010–2017). This approach allows describing the general long‐term trend of occurrence of different causative agents involved in outbreaks, which may be useful if control programmes of specific pathogens in the food chain are implemented, but also for investigating the more recent trends. It is important to underline that due to major changes in the reporting specifications for FBO applied since 2014 (EFSA, 2014b), long‐period trends should be interpreted with caution as data may be not fully comparable along years.
Historical data used for temporal (2010–2017) descriptions and comparability may differ from data already published in previous EU Summary Report, as they may include information that were not available at the end of the reporting period but were only provided afterwards. These data have been included in the current analysis of the historical data set that was run again also to take into account possible changes in the value recoding.
16.4. Results
16.4.1. General overview
Impact on health
Data on food‐borne and waterborne outbreaks were provided in 2017 by 27 MS and seven non‐MS.22 Cyprus did not report any FBO data. In total, 5,079 FBO (including waterborne outbreaks) were reported by MS, including 643 strong‐evidence and 4,436 weak‐evidence outbreaks (Figure 66 and Table 65). This is 6.8% less compared to outbreaks reported in 2016 (n = 5,451). Strong‐evidence outbreaks were 12.7% of total outbreaks, similarly as in 2016. The EU level trend in reported outbreaks during 2010–2017 was fairly stable (Figure 67). In 2017, eight non‐MS including Norway, Iceland, Switzerland and the preaccession countries Albania, Bosnia and Herzegovina, the Former Yugoslav Republic of Macedonia, Montenegro and Serbia reported another 149 outbreaks.
Figure 66.
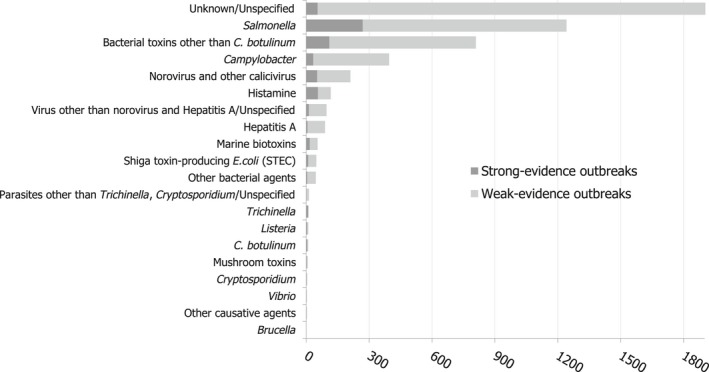
Distribution of strong‐evidence and weak‐evidence food‐borne and waterborne outbreaks, per causative agent, EU, 2017
- Other bacterial agents include Aeromonas hydrophila, enteroaggregative E. coli (EAEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), Shigella flexneri, Shigella sonnei, Yersinia enterocolitica other unspecified bacteria. Bacterial toxins other than C. botulinum include toxins produced by Bacillus, Clostridium other than C. botulinum and Staphylococcus and other unspecified bacterial toxins. Virus other than norovirus and hepatitis A include adenovirus, flavivirus, hepatitis E, rotavirus and other unspecified viruses. Marine biotoxins include ciguatoxin and other unspecified toxins. Other causative agents include scombrotoxin. Parasites other than Trichinella, Cryptosporidium include Giardia and other unspecified parasites.
Table 65.
Number of food‐borne (including waterborne) outbreaks, human cases, hospitalisations and deaths in reporting Member States and non‐Member States, 2017
| Country | Strong‐evidence outbreaks | Weak‐evidence outbreaks | Total outbreaks | % of total | Reporting rate per 100,000 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Cases | Hospitalised | Deaths | n | Cases | Hospitalised | Deaths | 2017 | 2010–2016 (mean) | |||
| Austria | 9 | 53 | 19 | 2 | 60 | 174 | 37 | 0 | 69 | 1.4 | 0.79 | 1.58 |
| Belgium | 9 | 275 | 18 | 0 | 295 | 1,134 | 31 | 0 | 304 | 6.0 | 2.68 | 2.72 |
| Bulgaria | 0 | 0 | 0 | 0 | 20 | 267 | 102 | 0 | 20 | 0.4 | 0.28 | 0.14 |
| Croatia | 13 | 179 | 23 | 0 | 44 | 325 | 67 | 0 | 57 | 1.1 | 1.37 | 1.23 |
| Czech Republic | 4 | 92 | 16 | 0 | 25 | 788 | 68 | 1 | 29 | 0.6 | 0.27 | 0.23 |
| Denmark | 23 | 767 | 16 | 0 | 39 | 400 | 12 | 0 | 62 | 1.2 | 1.08 | 1.11 |
| Estonia | 0 | 0 | 0 | 0 | 9 | 200 | 11 | 0 | 9 | 0.2 | 0.68 | 0.99 |
| Finland | 12 | 264 | 13 | 0 | 27 | 296 | 11 | 2 | 39 | 0.8 | 0.71 | 0.86 |
| France | 105 | 2,262 | 130 | 1 | 1,273 | 11,557 | 537 | 5 | 1,378 | 27.1 | 2.06 | 1.94 |
| Germany | 49 | 987 | 169 | 4 | 340 | 1,290 | 243 | 0 | 389 | 7.7 | 0.47 | 0.50 |
| Greece | 2 | 82 | 37 | 0 | 4 | 45 | 13 | 0 | 6 | 0.1 | 0.06 | 0.11 |
| Hungary | 20 | 805 | 10 | 0 | 20 | 496 | 47 | 3 | 40 | 0.8 | 0.41 | 1.25 |
| Ireland | 1 | 8 | 1 | 0 | 23 | 89 | 6 | 0 | 24 | 0.5 | 0.50 | 0.53 |
| Italy | 39 | 472 | 37 | 2 | 60 | 454 | 62 | 0 | 99 | 1.9 | 0.16 | 0.36 |
| Latvia | 3 | 50 | 4 | 0 | 19 | 72 | 32 | 0 | 22 | 0.4 | 1.13 | naa |
| Lithuania | 5 | 43 | 22 | 0 | 44 | 233 | 111 | 0 | 49 | 1.0 | 1.72 | 3.96 |
| Luxembourg | 2 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0.0 | 0.34 | 0.48 |
| Malta | 0 | 0 | 0 | 0 | 37 | 330 | 11 | 0 | 37 | 0.7 | 8.04 | 8.42 |
| Netherlands | 11 | 396 | 10 | 0 | 654 | 2,545 | 6 | 0 | 665 | 13.1 | 3.89 | 1.90 |
| Poland | 134 | 2,028 | 496 | 2 | 351 | 3,693 | 848 | 1 | 485 | 9.5 | 1.28 | 1.19 |
| Portugal | 10 | 195 | 117 | 0 | 8 | 128 | 28 | 0 | 18 | 0.4 | 0.17 | 0.15 |
| Romania | 10 | 351 | 147 | 0 | 2 | 74 | 64 | 0 | 12 | 0.2 | 0.06 | 0.09 |
| Slovakia | 18 | 287 | 39 | 0 | 444 | 1,527 | 323 | 1 | 462 | 9.1 | 8.50 | 9.38 |
| Slovenia | 0 | 0 | 0 | 0 | 2 | 45 | 4 | 0 | 2 | 0.0 | 0.10 | 0.33 |
| Spain | 133 | 1,189 | 151 | 4 | 282 | 2,358 | 206 | 1 | 415 | 8.2 | 0.89 | 1.04 |
| Sweden | 8 | 410 | 7 | 0 | 336 | 2,248 | 9 | 0 | 344 | 6.8 | 3.44 | 3.06 |
| United Kingdom | 23 | 906 | 66 | 1 | 18 | 524 | 103 | 3 | 41 | 0.8 | 0.06 | 0.11 |
| EU Total | 643 | 12,108 | 1,549 | 16 | 4,436 | 31,292 | 2,992 | 17 | 5,079 | 100.0 | 0.99 | 1.08 |
| Albania | 3 | 218 | 55 | 0 | 0 | 0 | 0 | 0 | 3 | – | 0.10 | – |
| Bosnia and Herzegovina | 3 | 40 | 16 | 0 | 2 | 25 | 7 | 0 | 5 | – | 0.14 | – |
| the Former Yugoslav Republic of Macedonia | 6 | 300 | 16 | 0 | 6 | 897 | 19 | 0 | 12 | – | 0.58 | – |
| Iceland | 4 | 186 | 0 | 0 | 5 | 182 | 1 | 0 | 9 | – | 2.66 | – |
| Montenegro | 6 | 112 | 12 | 0 | 3 | 11 | 4 | 0 | 9 | – | 1.45 | – |
| Norway | 2 | 68 | 0 | 0 | 34 | 428 | 0 | 0 | 36 | – | 0.68 | 1.07 |
| Serbia | 57 | 575 | 94 | 0 | 0 | 0 | 0 | 0 | 57 | – | 0.65 | – |
| Switzerland | 11 | 124 | 35 | 0 | 7 | 241 | 36 | 0 | 18 | – | 0.21 | 0.11 |
Due to changes in the FBO reporting system over years the mean annual reporting rate was not calculated. Latvia reported the mean value for 2015 and 2016 to be 5.35 food‐borne outbreaks (per 100,000 population).
Outbreak reporting rate for 2017 and mean value for the seven previous years (2010–2016) is also provided.
Figure 67.
Number of food‐borne (including waterborne) outbreaks in the reporting Member States, EU, 2010–2017
The number of outbreaks reported by various countries differed importantly and few MS reported most of the outbreaks.23 France notified, as previously, the largest number of outbreaks and accounted for more than a quarter of all outbreaks reported in 2017 in the EU. Outbreaks reported by other seven MS (Belgium, Germany, the Netherlands, Poland, Slovakia, Spain and Sweden) accounted altogether for more than 60% of total outbreaks, whereas the remaining 19 MS (Austria, Bulgaria, Croatia, the Czech Republic, Denmark, Estonia, Finland, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Portugal, Romania, Slovenia and the United Kingdom) reported about 13% of all outbreaks in EU.
Overall in 2017, FBO (including waterborne outbreaks) caused 43,400 illnesses (13,519 less than in 2016 corresponding to a 23.8% reduction), 4,541 hospitalisations (119 more than in 2016; corresponding to a 2.7% increase) and 33 deaths (1 more than in 2016), in the 27 MS.24 Overall, in the EU, the reporting rate of FBO (including waterborne outbreaks) per 100,000 was 0.9923 which represents a slight decrease compared with 2016 (1.08 outbreaks per 100,000).
At the MS level, outbreak reporting rates per 100,000 population varied importantly in 2017, ranging from 0.06 (Greece, Romania, United Kingdom) to 8.50 (Slovakia) (median: 0.71 outbreaks per 100,000 population). Variations compared with 2016 are reported in Figure 68. For 17 MS the outbreak reporting rate decreased or remained quite stable (i.e. below 20% increase), while for three MS (Denmark, Estonia and Lithuania) an increase over 20% was observed.
Figure 68.
Food‐borne (including waterborne) outbreaks reporting rate (per 100,000 population) in 2017 (in brackets), by EU Member State and % of difference compared with 2016 (green bars)
Over the period 2010–2017, the outbreaks reporting rate had a statistically significant increase for Portugal while conversely for Austria, Denmark, Hungary and United Kingdom the trend was decreasing. No specific trends were observed for the other MS.
The reporting rate of human illnesses due to FBO varied importantly among countries and ranged from 1.2 (Greece) to 71.7 (Malta) cases per 100.000 (median: 8.3 outbreak cases per 100,000 population). Also the mean number of cases involved in single outbreaks, 8.54 cases/FBO) had a wide range of variation in different MS. In 15 MS, the mean FBO average size was ≤ 10 cases/outbreak (minimum 3.5 cases/outbreak observed for Austria). For the other 12 MS, this number was higher and up to 35.4 cases/outbreak (Romania) (Figure 69). Interestingly, while the former group includes MS with the highest values of outbreak reporting rate, the latter include MS which do not systematically collect or report household outbreaks.25 The mean outbreak size varied importantly also according to the type of outbreaks. General outbreaks involved 15.2 cases per outbreak on average, while those involving a single households just 3.7 cases/outbreak, on average. In outbreaks lacking of information on the type of FBO the mean size was 4.5 cases/outbreak.
Figure 69.
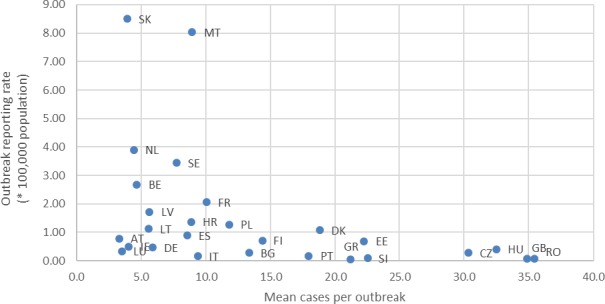
Mean number of human cases per outbreak (food‐borne including waterborne outbreaks) and outbreak reporting rate (per 100,000 population), by reporting Member State, EU, 2017
In the MS, FBO involving cases from a single household numbered 608 (12.0% of total outbreaks) in 2017, those with cases from more than one household, general outbreaks, were 1,964 (38.7% of total outbreaks). The level of uncertainty of this estimation is high, given that this information was lacking for 2,507 outbreaks (49.4% of total outbreaks) and that not all MS systematically collect or report information on household outbreaks.
Causative agents in food‐borne (including waterborne) outbreaks
During 2017
In 2017, FBO (including waterborne outbreaks) with a known causative agent were 3,170, corresponding to 62.4% of all outbreaks, meaning that for more than one‐third of the total of these incidents reported at the EU level, no information on the agents involved in the outbreaks was available. Identification of causative agent was much more frequent in strong‐evidence outbreaks (91.6% of all strong‐evidence outbreaks) than in weak‐evidence outbreaks (58.8% of all weak‐evidence outbreaks) (Table 66).
Table 66.
Number of food‐borne (including waterborne) outbreaks, human cases, hospitalisations and deaths, per causative agents in the reporting Member States, EU, 2017
| Type of agent | Outbreaks | Cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strong‐evidence outbreaks | Weak‐evidence outbreaks | Total outbreaks | % of total | Reporting rate per 100,000 | Human cases | Mean number per outbreak | Hospitalised | Deaths | ||||
| n | n | n | n | n | % of cases | n | % of cases | |||||
| Bacteria | Brucella | 0 | 1 | 1 | < 0.1 | < 0.01 | 2 | 2.0 | 1 | 50.0 | 0 | 0 |
| Campylobacter | 33 | 362 | 395 | 7.8 | 0.08 | 1,445 | 3.7 | 207 | 14.3 | 1 | 0.1 | |
| Listeria | 4 | 6 | 10 | 0.2 | < 0.01 | 39 | 3.9 | 22 | 56.4 | 2 | 5.1 | |
| Salmonella | 269 | 972 | 1,241 | 24.4 | 0.24 | 9,600 | 7.7 | 2,227 | 23.2 | 11 | 0.1 | |
| Shiga toxin‐producing E. coli (STEC) | 9 | 39 | 48 | 0.9 | 0.01 | 260 | 5.4 | 65 | 25.0 | 2 | 0.8 | |
| Vibrio | 0 | 3 | 3 | 0.1 | < 0.01 | 59 | 19.7 | 7 | 11.9 | 0 | 0 | |
| Other bacterial agents/Unspecified | 5 | 41 | 46 | 0.9 | 0.01 | 816 | 17.7 | 67 | 8.2 | 0 | 0 | |
| Subtotal | 320 | 1,424 | 1,744 | 34.3 | 0.34 | 12,221 | 7.0 | 2,596 | 21.2 | 16 | 0.1 | |
| Bacterial toxins | Clostridium botulinum | 5 | 4 | 9 | 0.2 | < 0.01 | 26 | 2.9 | 26 | 100.0 | 2 | 7.7 |
| Other bacterial toxins | 110 | 699 | 809 | 15.9 | 0.16 | 8,442 | 10.4 | 577 | 6.8 | 5 | < 0.1 | |
| Subtotal | 115 | 703 | 818 | 16.1 | 0.16 | 8,468 | 10.4 | 583 | 6.9 | 7 | 0.1 | |
| Viruses | Norovirus and other caliciviruses | 52 | 159 | 211 | 4.2 | 0.04 | 6,550 | 31.0 | 153 | 2.3 | 2 | 0 |
| Hepatitis A | 6 | 84 | 90 | 1.8 | 0.02 | 591 | 6.6 | 452 | 76.5 | 2 | 0.3 | |
| Other viruses/unspecified | 12 | 85 | 97 | 1.9 | 0.02 | 1,379 | 14.2 | 107 | 7.8 | 0 | 0 | |
| Subtotal | 70 | 328 | 398 | 7.8 | 0.08 | 8,520 | 21.4 | 712 | 8.4 | 4 | < 0.1 | |
| Parasites | Cryptosporidium | 0 | 5 | 5 | 0.1 | < 0.01 | 15 | 3.0 | 0 | 0.0 | 0 | 0 |
| Trichinella | 9 | 2 | 11 | 0.2 | < 0.01 | 199 | 18.1 | 125 | 62.8 | 0 | 0 | |
| Other parasites/unspecified | 0 | 13 | 13 | 0.3 | < 0.01 | 28 | 2.2 | 1 | 3.6 | 0 | 0 | |
| Subtotal | 9 | 20 | 29 | 0.6 | 0.01 | 242 | 8.3 | 126 | 52.1 | 0 | 0 | |
| Other causative agents | Histamine | 56 | 61 | 117 | 2.3 | 0.02 | 572 | 4.9 | 51 | 8.9 | 0 | 0 |
| Marine biotoxins | 17 | 37 | 54 | 1.1 | 0.01 | 170 | 3.1 | 14 | 8.2 | 0 | 0.0 | |
| Mushroom toxins | 2 | 5 | 7 | 0.1 | < 0.01 | 22 | 3.1 | 16 | 72.7 | 0 | 0 | |
| Other/Unspecified | 0 | 3 | 3 | 0.1 | < 0.01 | 6 | 2.0 | 0 | 0.0 | 0 | 0.0 | |
| Subtotal | 75 | 106 | 181 | 3.6 | 0.04 | 770 | 4.3 | 81 | 10.5 | 0 | 0 | |
| Unknown | Unknown | 54 | 1,828 | 1,882 | 37.1 | 0.37 | 12,794 | 6.8 | 423 | 3.3 | 6 | < 0.1 |
| Unspecified | 0 | 27 | 27 | 0.5 | 0.01 | 385 | 14.3 | 20 | 5.2 | 0 | 0.0 | |
| Subtotal | 54 | 1,855 | 1,909 | 37.6 | 0.37 | 13,179 | 6.9 | 443 | 3.4 | 6 | < 0.1 | |
| Total (EU) | 643 | 4,436 | 5,079 | 100.0 | 0.99 | 43,400 | 8.5 | 4,541 | 10.5 | 33 | < 0.1 | |
In Sweden, a large domestic campylobacteriosis outbreak continued in 2017 with hundreds of cases in 2017. As the outbreak had been reported in 2016 data, it was not repeatedly reported in 2017.
Other bacterial agents include Aeromonas hydrophila, enteroaggregative E. coli (EAEC), Enterotoxigenic E. coli (ETEC), Enteroinvasive E. coli (EIEC), Enteropathogenic E. coli (EPEC), Shigella flexneri, Shigella sonnei, Yersinia enterocolitica and other unspecified bacteria. Other bacterial toxins include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins. Other viruses include adenovirus, flavivirus, hepatitis E virus, rotavirus and other unspecified viruses. Marine biotoxins include ciguatoxin and other unspecified toxins. Other toxins include scombrotoxin. Other parasites include Giardia and other unspecified parasites.
In 2017, the most frequently implicated causative agents in FBO (including waterborne outbreaks) reported in the EU were bacterial agents. The proportion of outbreaks caused by bacteria (34.3% of all outbreaks) was more than twofold that of bacterial toxins (16.1% of all outbreaks) and more than fourfold that of viruses (7.8% of all outbreaks). FBO implicating other causative agents and parasites were less frequently reported (3.6% and 0.6% of all outbreaks, respectively). This overall ranking in the EU was the same as in 2016. Variations within MS in the proportion of outbreaks compared with 2016, by agent's group are shown in Figure 70. The same graphic is used in Figure 71 to describe variations for the most significant single causative agents.
Figure 70.
Food‐borne (including waterborne outbreaks) reported in EU in 2017, by reporting Member States and by type of pathogen and % of difference compared with 2016
Figure 71.
Food‐borne (including waterborne) outbreaks reported in the EU in 2017, by Member State and by type causative agent and % of difference compared with 2016
- Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins.
- Only causative agents with more than 100 outbreaks reported in the EU, are shown.
A detailed overview of the distribution of numbers of FBO (including waterborne outbreaks) and of involved human cases, by country and by causative agent, is in Figure 72. These MS‐specific graphics allow visualising the important differences between countries in reported causative agents detected in outbreaks and numbers of outbreaks and involved cases. To help readers understand how single MS contribute to the overall outbreak data collection, by causative agent, Figure 73 summarises all MS‐specific reported 2017 FBO (including waterborne outbreaks) and their relative contribution (%) to the total number of outbreaks reported at the EU level.
Figure 72.
Frequency distribution of food‐borne (including waterborne) outbreaks (internal circle) and human cases involved in outbreaks (external circle), by reporting EU Member States and non‐Member States (bottom figure), by causative agent, 2017
Figure 73.
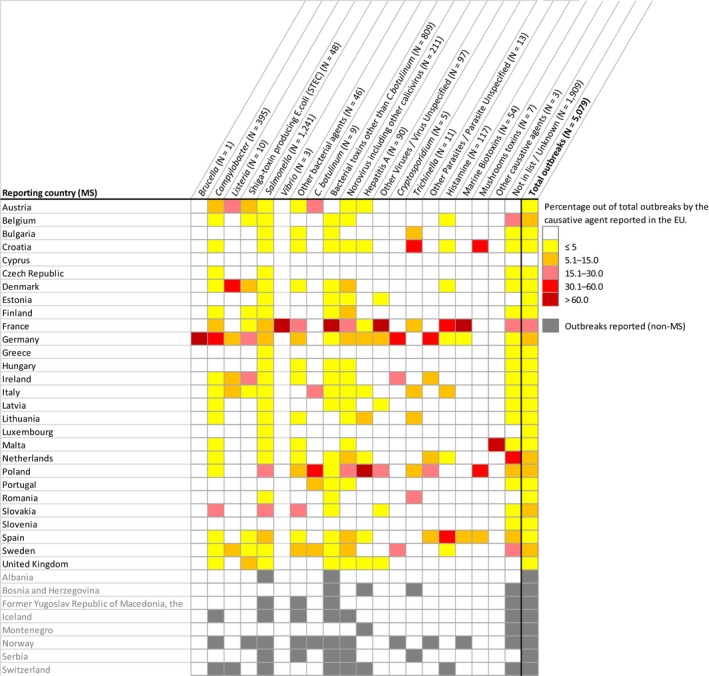
Reporting of food‐borne (including waterborne) outbreaks, by causative agent and by reporting country, 2017
- Other bacterial agents include Aeromonas hydrophila, enteroaggregative E. coli (EAEC), Enterotoxigenic E. coli (ETEC), Enteroinvasive E. coli (EIEC), Enteropathogenic E. coli (EPEC), Shigella flexneri, Shigella sonnei, Yersinia enterocolitica, Yersinia pseudotuberculosis and other unspecified bacteria. Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins. Other viruses include adenovirus, flavivirus, hepatitis E virus, rotavirus and other unspecified viruses. Marine biotoxins include ciguatoxin and other unspecified toxins. Other toxins include scombrotoxin. Other parasites include Giardia and other unspecified parasites.
At the country level, the most reported causative agent in FBO (including waterborne outbreaks) was Salmonella for 15 MS and two non‐MS (Albania, Austria, Croatia, the Czech Republic, Denmark, Estonia, Greece, Hungary, Italy, Latvia, Lithuania, Luxembourg, Poland, Serbia, Slovakia, Spain and the United Kingdom), bacterial toxins other than C. botulinum for four MS and three non‐MS (Bosnia and Herzegovina, Bulgaria, Former Yugoslav Republic of Macedonia, France, Portugal, Romania, Switzerland), Campylobacter for three MS (Belgium, Germany and Malta), norovirus including other caliciviruses for three MS and one non‐MS (Finland, Netherlands, Norway and Sweden), Shiga toxin‐producing E. coli for one MS (Ireland), hepatitis A for one non‐MS (Montenegro) and Aeromonas for one non‐MS (Iceland).
Salmonella was the causative agent most frequently detected in FBO and no Salmonella waterborne outbreaks were reported. Twenty‐five MS reported 1,241 Salmonella FBO for 2017,26 which was 24.4% of the total number of outbreaks, almost one in four outbreaks. In addition, the health impact connected to outbreaks of salmonellosis was the most important given that this pathogen was responsible of the highest number of reported cases, hospitalisations and deaths (Table 66). Compared with 2016 the numbers of Salmonella FBO and of human salmonellosis cases decreased, respectively, by 9.5% (131 less outbreaks) and by 16.0% (1,825 less cases). Outbreaks by Salmonella were reported by 25 MS (no Salmonella outbreaks were reported by Portugal and Slovenia, and Cyprus did not report any 2017 FBO data) (Table 67). For eight MS (the Czech Republic, Estonia, Greece, Latvia, Lithuania, Luxembourg, Poland, Slovakia), Salmonella accounted for more than 50% of total outbreaks reported. Conversely, for seven MS (Belgium, France, Finland, Ireland, Malta, the Netherlands and Sweden), Salmonella was reported in a minority of outbreaks (< 10% of total outbreaks). At the MS level, 2017/2016 proportional differences in the number of Salmonella outbreaks are described in Figure 71. The marked decrease (> 30%) in S. Enteritidis FBO for 10 MS is a favourable signal. Conversely, an increase higher than 25% in Salmonella FBO was observed for seven MS (Bulgaria, Denmark, the Netherlands, Estonia, Germany, Lithuania and the Czech Republic). A similar increase was also reported in the number of involved cases for the same MS, except for the Czech Republic, Estonia and the Netherlands. At the EU level, the mean number of cases involved in general Salmonella outbreaks (n = 365) was 18.0 cases per outbreak. This statistic varied importantly in the different MS and was not correlated with the reporting rate of general outbreaks of salmonellosis (Figure 74).
Table 67.
Overview of countries reporting data on food‐borne outbreaks (including waterborne outbreaks), 2017
| Causative agent | Total number of reporting MS | Countries |
|---|---|---|
| Salmonella | 25 | All MS except: CY, PT, SI; Non‐MS: AL, CH, IS, MK, RS |
| Campylobacter | 19 | MS: AT, BE, CZ, DE, DK, ES, FI, FR, GB, HR, IE, IT, LT, LV, MT, NL, PL, SE, SK; Non‐MS: CH, IS, NO |
| Shiga toxin‐producing E. coli (STEC) | 11 | MS: AT, BE, DE, DK, ES, FI, FR, GB, IE, IT, SE; Non‐MS: NO |
| Listeria | 6 | MS: AT, DE, DK, IE, IT, SE; Non‐MS: CH |
| Brucella | 1 | MS: DE |
| Vibrio | 1 | MS: FR |
| Other bacterial agents a | 15 | MS: AT, BG, DE, DK, ES, FR, HR, HU, IE, LT, MT, NL, PL, SE, SK; Non‐MS: IS, MK, NO, RS |
| Clostridium botulinum | 5 | MS: AT, IT, PL, PT, SE; Non‐MS: NO |
| Bacterial toxins other than Clostridium botulinum b | 20 | All MS except: AT, CY, CZ, GR, LT, LU, MT, SI; Non‐MS: AL, BA, CH, IS, MK, NO, RS |
| Norovirus including other caliciviruses | 20 | All MS except: BG, CY, CZ, GR, LU, RO, SI, SK; Non‐MS: AL, BA, CH, IS, ME, MK, NO, RS |
| Hepatitis A | 10 | MS: AT, DE, ES, FR, GB, HR, IT, LT, NL, PL; Non‐MS: BA, CH, ME |
| Other Viruses/Virus Unspecified c | 7 | MS: DE, EE, FR, GB, LV, PL, SK; Non‐MS: BA, RS |
| Trichinella | 7 | MS: BG, FR, HR, IT, LT, PL, RO; Non‐MS: BA, RS |
| Cryptosporidium | 3 | MS: DE, IE, SE; Non‐MS: NO |
| Other Parasite/Parasite Unspecified d | 5 | MS: DE, ES, IE, NL, PL; Non‐MS: NO |
| Histamine | 9 | MS: BE, DE, DK, ES, FR, HR, IT, NL, SE; Non‐MS: CH |
| Marine biotoxins e | 3 | MS: DE, ES, FR; Non‐MS: NO |
| Mushroom toxins | 3 | MS: ES, HR, PL |
| Unknown | 22 | All MS except: AT, CY, EE, GB, LU, RO; Non‐MS: AL, BA, CH, IS, ME, MK, NO, RS |
Note: The overview table contains all data reported by MS. Cyprus did not report any 2017 FBO data.
Other bacterial agents include Aeromonas hydrophila, enteroaggregative E. coli (EAEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), Shigella flexneri, Shigella sonnei, Yersinia enterocolitica, Yersinia pseudotuberculosis and other unspecified bacteria.
Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins.
Other viruses include flavivirus, Rotavirus and other unspecified viruses.
Other parasites include Giardia and other unspecified parasites.
Marine biotoxins include ciguatoxin and other unspecified toxins.
Figure 74.
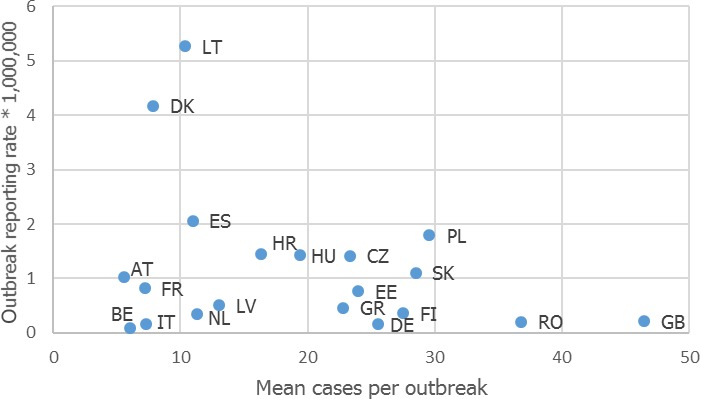
Mean number of cases per outbreak and reporting rate of general food‐borne (including waterborne) outbreaks of salmonellosis (per million population) reported in the EU, by reporting Member State, 2017
Details of the serovars reported in Salmonella FBO are described in the Appendix.27 This information was lacking in 27.5% of the reported salmonellosis outbreaks and six MS either failed to report this information (Ireland, Malta) or detailed the serovar only for half (or less) of their Salmonella outbreaks (Spain, the Netherlands, Italy and France). Six non‐MS also notified 58 outbreaks of food‐borne salmonellosis for 2017, of which 50 were reported by Serbia that reported 46 FBO to be caused by S. Enteritidis.
S. Enteritidis was reported in 61.1% (n = 758) of Salmonella FBO, by 23 MS,28 corresponding to 14.9% – about one in seven – of all reported FBO at the EU level. This was a decrease by 11.9% (102 less outbreaks) compared to 2016. For 10 MS (the Czech Republic, Slovakia, Lithuania, Estonia, Latvia, Luxembourg, Poland, Hungary, Croatia and Austria) S. Enteritidis accounted for more than 30% of the outbreaks reported, while only two MS (Malta and Ireland) of those reporting Salmonella FBO did not report any outbreak by this serovar. Altogether, Poland and Slovakia accounted for 63.6% and 56.1% of total outbreaks and cases by S. Enteritidis reported in the EU in 2017, respectively. At the MS level FBO by S. Enteritidis decreased by more than 25% in 10 MS (France, Hungary, Ireland, Italy, Latvia, Netherlands, United Kingdom, Greece, Belgium and Spain) compared with 2016, while an increase over 25% in both number of outbreaks and of involved cases was reported by six MS (Estonia, Romania, Denmark, the Czech Republic, Lithuania and Germany) (Figure 71).
S. Typhimurium including monophasic variants was reported in 7.9% (n = 98) of Salmonella FBO, by 15 MS.29 Most of the outbreaks (n = 61) were reported by three MS (France, Germany and Slovakia). An increase by more than 25% in numbers of outbreaks by this serovar group was observed for five MS (Slovakia, Croatia, Germany, Denmark and France) compared to 2016. The same trend was also observed for the number of outbreak cases in these MS except for France. A remarkable decrease by 81% (n = 17) in the number of outbreaks by this serovar group and in the involved human cases was reported by Spain. Three outbreaks by S. Typhimurium including monophasic variants were also reported by three non‐MS (Serbia, Iceland and Norway).
Campylobacter was during 2017 the second most frequently reported (n = 393) bacterial causative agent in the EU in FBO, by 18 MS. One MS, Ireland, reported two Campylobacter waterborne outbreaks. FBO were mostly caused by C. jejuni (170 outbreaks) while C. coli was reported in 14 outbreaks only (for 53.4% of the outbreaks information on the species was lacking). Compared with 2016 the number of Campylobacter FBO30 decreased by 16.7% (79 less outbreaks). Outbreaks declined in most reporting MS (Figure 71). In five of them (Austria, Germany, Malta, Slovakia, United Kingdom) incidents implicating this pathogen accounted for more than 20% of total reported FBO. In Germany, in particular, this proportion peaked up to 38.8% and Campylobacter was the most frequently identified causative agent in FBO. In Sweden, the large general outbreak of Campylobacter in meat from broilers (Gallus gallus) which started in 2016 finished during 2017. The number of notified cases increased up to 4,000, in total. In 2017, two non‐MS (Iceland and Norway) reported three outbreaks of campylobacteriosis.
Outbreaks by L. monocytogenes 31 (n = 10) and STEC32 (n = 37) were reported only by 6 and 11 MS, respectively. For the latter pathogen, Ireland reported 11 waterborne outbreaks. Both pathogens were associated with high proportions of hospitalisations and deaths. In Ireland, STEC represented during 2017 the most reported causative agent and was identified in 50% of total FBO (including waterborne outbreaks) reported. Among non‐MS, Norway was the only country that notified outbreaks by STEC infection (3 outbreaks). It is worth mentioning that during 2017 a long‐lasting outbreak of listeriosis continued linked to cold‐smoked salmon involving cases in three MS (Denmark, Germany and France in 2016) and that there was a large multicountry outbreak of invasive listeriosis (serogroup IVb) linked to frozen vegetables.
Other bacterial agents were less reported as causative agent for FBO, by MS,33 and no waterborne outbreaks were reported caused by this pathogen group. Brucella was only identified in a single FBO in Germany.34 Shigella was reported by eight MS in 22 outbreaks, including S. flexneri (11 outbreaks) and S. sonnei (six outbreaks). Nine outbreaks were associated with infection by pathogenic Escherichia coli 35 including enteroaggregative E. coli (EAEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), reported by five MS. Yersinia 36 was identified in 12 outbreaks. Eleven of them were caused by Y. enterocolitica, including a large outbreak involving 80 patients in Denmark. All these agents were also detected in outbreaks notified by non‐MS which also reported incidents caused by Coxiella burnetii (1 outbreaks notified by Serbia) and Yersinia pseudotuberculosis (1 outbreak, by Norway).
Outbreaks by bacterial toxins were reported by 20 MS and were predominantly associated with toxins by Clostridium perfringens,37 Staphylococcus 38 and Bacillus cereus.39 No waterborne outbreaks were reported caused by bacterial toxins. Similarly to previous years, the vast majority of these incidents and human cases associated with these agents in 2017 (84.4% and 70.1%, respectively) was reported by France. During 2017 bacterial toxins other than Clostridium botulinum were the first cause of FBO in four MS (Bulgaria, France, Portugal and Romania) and accounted in each of them for more than 30% of total outbreaks. Variations at the MS level over 2016 are shown in Figure 71. Outbreaks by bacterial toxins, mostly by Staphylococcus, were also reported by six non‐MS.
FBOs of botulism were reported by five MS40 in smaller number than 2016 (24 outbreaks), and also by one non‐MS.
A marked decrease in the number of outbreaks by viruses was observed in 2017 compared with 2016. This was due to norovirus for which a decrease by 50% was observed in the number of reported outbreaks (204 outbreaks less than 2016). Twenty MS reported norovirus and other caliciviruses41 as causative agent of FBOs. Three MS (Finland, Spain and Sweden) also reported each one waterborne outbreak due to norovirus and other caliciviruses. In almost all these countries, the numbers of reported outbreaks and human cases were less than in 2016 (Figure 71). So, the health impact of norovirus outbreaks at the EU level on human cases and hospitalisation was milder than in 2016 (43.7% of cases less and 62.2% of hospitalisations less). In addition, four non‐MS reported 10 norovirus FBOs and one norovirus waterborne outbreak. Conversely, outbreaks by hepatitis A increased remarkably in 2017, from 17 outbreaks in 2016 to 90 outbreaks. This increase was mainly due to reporting by Poland (99.4% increase, 64 more outbreaks compared with 2016). Hepatitis A outbreaks were reported by 11 MS42 and 3 non‐MS. Viruses other than norovirus and hepatitis A were identified in 91 FBOs by seven MS in 2017. For the first time since the beginning of the outbreak data collection, six outbreaks of hepatitis E were reported,43 all by Germany and involving altogether 12 cases. Other viruses implicated in FBO44 were rotavirus (20 outbreaks from 2 MS), flavivirus including tick‐borne encephalitis virus (3 outbreaks from three MS), adenovirus (1 outbreak) and other unspecified viruses (65 outbreaks; four MS).
The number of FBO by parasites was stable in 2017 compared with previous years. A remarkable increase in the number of trichinellosis cases was reported by Romania (111 cases in 2017; 4 cases in 2016) due to one general outbreak involving 109 cases. FBO of trichinellosis (n = 11), including T. spiralis (7 outbreaks) and T. britovi (1 outbreak) were reported by seven MS.45 FBO of cryptosporidiosis (n = 4) were reported by two MS.46 Giardia was responsible of 12 of 13 outbreaks caused by ‘other parasites’ which were notified by five MS47 and one of these was waterborne. Outbreaks by Trichinella, Cryptosporidium and Giardia were also notified by two, one and one non‐MS, respectively.
FBO incidents (n = 117) by histamine poisoning were notified by nine MS48 and increased by 22% compared with 2016 (21 more outbreaks). Remarkably, histamine was also the third most common agent identified in strong evidence outbreaks in the EU. Outbreaks by marine biotoxins including ciguatoxin49 also increased by 42% compared to 2016 and the increase was mainly due to a higher reporting (+50% than in 2016) by France, which was the MS that contributed most to this data collection. Outbreaks by Histamine and Marine biotoxins were also reported by two non‐MS.
FBOs lacking information on causative agents were reported by 22 MS.50 Slovenia only reported FBO caused by unknown agents and in six MS (Belgium, Finland, Italy, Malta, the Netherlands and Sweden) these outbreaks outnumbered those for which the causative agent was reported. Large outbreaks with unknown causative agent were also reported, in particular five events involving each more than 100 cases, by France, Hungary and Italy. Two of these were reported as strong‐evidence outbreaks.
Apart from the general Campylobacter outbreak that occurred in Sweden during 2016 and 2017 and involving 4,000 cases, other large (> 100 cases) or very large (> 200 cases) outbreaks occurred in 2017 in 12 MS (Belgium, Estonia, France, Germany, Hungary, Italy, the Netherlands, Poland, Romania, Spain, Sweden, United Kingdom). Most of them were caused by norovirus (10 outbreaks) followed by Salmonella (4 outbreaks), Clostridium perfringens (3 outbreaks), Bacillus cereus, Staphylococcus aureus toxins and Shigella flexneri (1 outbreak each). Moreover, Sweden reported one strong‐evidence outbreak associated with EAEC infection involving hundred cases. Both patients and the implicated food, however, tested positive also for ETEC. Among non‐MS, the Former Yugoslav Republic of Macedonia reported a single waterborne outbreak with unknown aetiology involving 777 cases. Three large outbreaks by Salmonella, Staphylococcus and norovirus were reported by Albania, the Former Yugoslav Republic of Macedonia and Switzerland, respectively.
High proportion of hospitalisation (> 50% of outbreak cases) was reported in outbreak of listeriosis, hepatitis A, botulism, trichinellosis and intoxication by mushroom toxins. A twofold increase in hospitalisation rate was observed among cases of hepatitis A, and illness due to bacterial toxins other than Clostridium botulinum compared with to 2016, and a mild increase was also observed in cases of salmonellosis. The high number of hospitalisations reported in outbreaks by Trichinella in ‘Meat and meat products’ were mainly attributable to a single large general outbreak by Trichinella spiralis that occurred in household setting in Romania.
Deaths were reported among cases involved in FBO associated with eight different causative agents either bacterial (n = 16), viral (n = 4) or by bacterial toxins (n = 7). Fatal cases were also reported in outbreaks connected to undetectable causative agents. Most deaths (n = 11) were reported among Salmonella outbreak cases, although fatal cases decreased importantly compared with 2016 (n = 18). Listeria (n = 2) and Clostridium botulinum (n = 2) were the agents characterised by the highest case fatality rate, similarly to previous years.
Temporal trends 2010–2017
Figure 75 displays the temporal distribution of numbers of reported food‐borne (including waterborne) outbreaks by the causative agent during 2010–2017. The EU trends depicted should be interpreted with much caution because they may reflect the trends and statistics of MS that contributed more to the outbreak data collection. Therefore, the temporal distribution of reported food‐borne (including waterborne) outbreaks by causative agent has been analysed separately for each MS, over the short (2014–2017) and the long time period (2010–2017) (Figures 76 and 77 displaying trends for several MS, only statistically significant trend are shown). It is important to underline that due to major changes in the reporting specifications for FBO in 2014, long‐period trends should be interpreted with caution as data may be not fully comparable along years.
Figure 75.
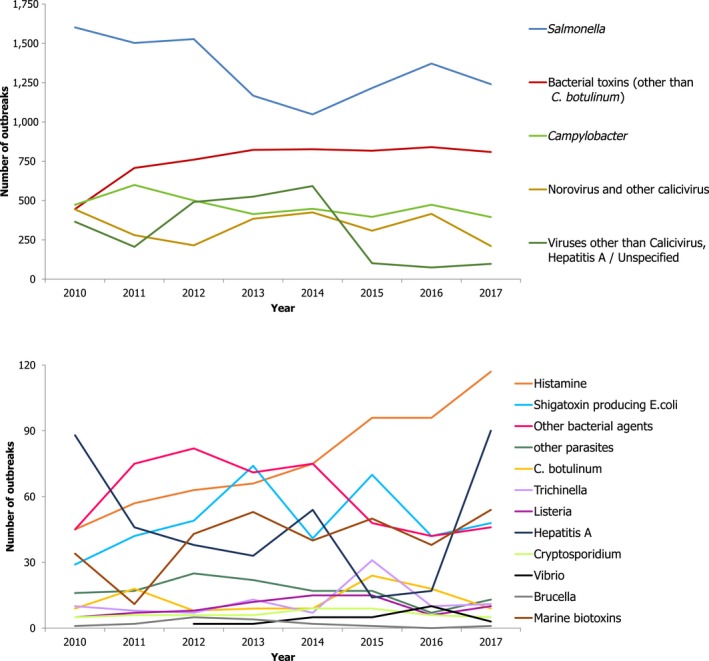
Number of food‐borne (including waterborne) outbreaks in reporting Member States and non‐Member States, by causative agent, 2010–2017
- Other bacterial agents include Aeromonas hydrophila, enteroaggregative E. coli (EAEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), Shigella flexneri, Shigella sonnei, Yersinia enterocolitica and other unspecified bacteria. Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins. Other viruses include adenovirus, flavivirus, hepatitis E, rotavirus and other unspecified viruses. Marine biotoxins include ciguatoxin and other unspecified toxins. Other toxins include scombrotoxin and other unspecified toxins. Other parasites include Giardia and other unspecified parasites.
Figure 76.
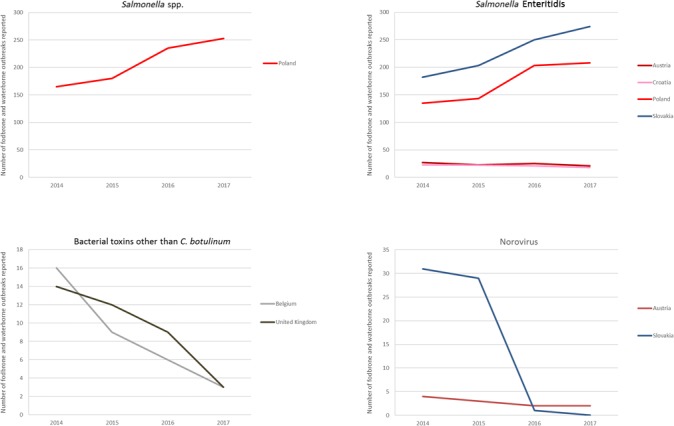
Number of food‐borne (including waterborne) outbreaks, by reporting Member States and by causative agents, 2014–2017
- Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins.
- Only Member States with statistically significant trends (either increasing or decreasing) over years are shown.
Figure 77.
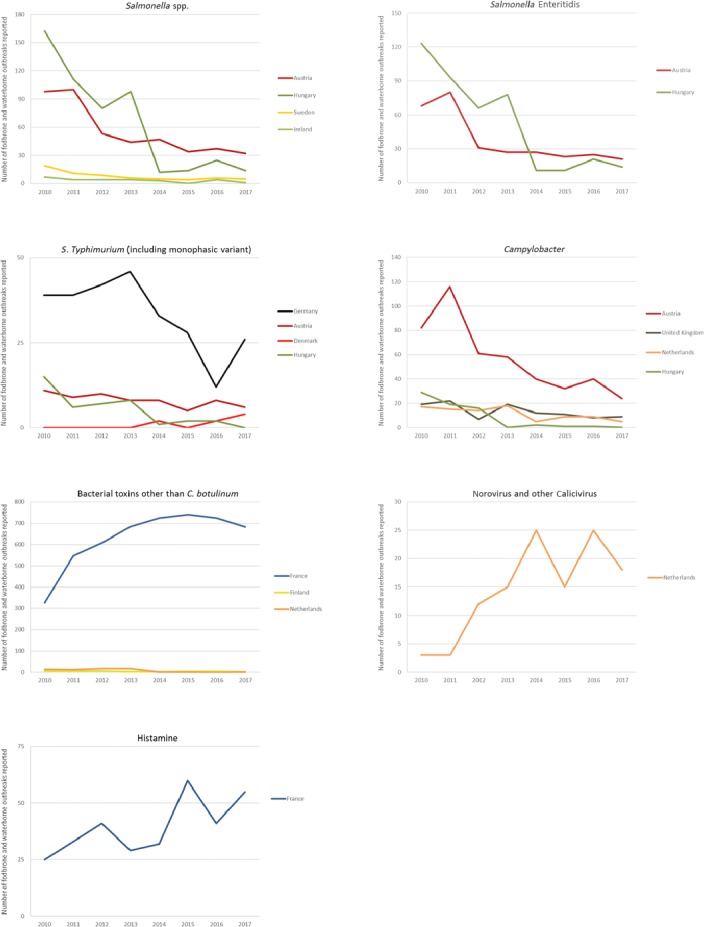
Number of food‐borne (including waterborne) outbreaks, by reporting Member States and causative agents, 2010–2017
- Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins.
- Only Member States with statistically significant trends (either increasing or decreasing) over years are shown.
Salmonella: The assessment of the temporal trend over the short period 2014–2017 indicated that there was no statistically significant decreasing trend in the reported numbers of Salmonella FBO in any MS. Poland however reported a significant increase during 2014–2017 (+88 outbreaks) corresponding to a 53% increase and this trend was primarily attributable to an increase in S. Enteritidis outbreaks. In 2017, this serovar caused 82% of the total Salmonella outbreaks in Poland. A similar increase over recent years was also observed for Slovakia (92 outbreaks corresponding to a 50% increase all over the period), where this serovar accounted in 2017 for 91.0% of all outbreaks of salmonellosis. Over the long time period (2010–2017), a statistically significant decrease in salmonellosis FBO was observed for Austria, Hungary, Sweden and Ireland. In Austria and Hungary, the decrease was linked to a significant reduction in FBO due to S. Enteritidis. In 2017, this serovar accounted for 66% and 100% of all salmonellosis FBO in Austria and Hungary, respectively. In Germany, a significant decrease in salmonellosis FBO was observed from 2010 to 2016 (EFSA and ECDC, 2017b) that was reversed suddenly during 2017 in both the numbers of FBO (42 outbreaks more than 2016; 46% increase) and of human cases (466 cases more than in 2016; 133% increase). This rise was mainly due to an increase in FBO by S. Typhimurium and its monophasic variant (24 outbreaks more than 2016; 117% increase) and by S. Enteritidis (20 outbreaks more than 2016; 34% increase).
No statistically significant decrease was observed in the reported numbers of salmonellosis FBO during the short (2014–2017) or the long (2010–2017) period by any of the nine MS having the highest rate of reporting of salmonellosis FBO (> 0.40 outbreaks per 100,000) (Slovakia, Lithuania, Croatia, Poland, Malta, Latvia, Estonia, Denmark and Spain). Similarly, no statistically significant decrease over the long (2010–2017) or short (2014–2017) period was observed for the nine MS where salmonellosis FBO accounted for more than 50% of total outbreaks reported in 2017 (Luxembourg, Greece, the Czech Republic, Estonia, Slovakia, Lithuania, Latvia and Poland).
Campylobacter: No significant trends were reported on the short (2014–2017) period for any MS. On the long‐term 2010–2017 a significant decrease was reported for four MS. The increase in reported numbers of campylobacteriosis FBO by Germany from 2010 to 2016 (EFSA and ECDC, 2017b) was discontinued during 2017. For the other MS the reporting of campylobacteriosis FBO was quite stable over those years.
Bacterial toxins other than Clostridium botulinum: no significant trends were reported on the short (2014–2017) period by any MS, except by Belgium and United Kingdom, which reported a significant decrease in numbers of FBO. Over the long period (2010–2017) a statistically significant increasing trend was reported by France, although the number of FBO caused by these pathogens decreased progressively over the last 3 years (741 outbreaks reported in 2015; 724 in 2016; 683 in 2017).
Norovirus and other caliciviruses: no significant trends were reported on the short (2014–2017) period by any MS, except by Austria and Slovakia, which reported a significantly decreasing trend. The significant increase in numbers of reported FBO due to norovirus and other caliciviruses during 2010–2016 in France (EFSA and ECDC, 2017b) was discontinued in 2017 due to a marked drop (100 outbreaks less than 2016; 68% drop). Over the long period (2010–2017), a statistically significant increasing trend was reported by the Netherlands, although the number of norovirus outbreaks during 2017 was lower than in 2016 (7 outbreaks less; 28% drop).
Histamine: No significant trend in outbreaks due to histamine poisoning was reported over the short (2014–2017) period by MS. Over the long period (2010–2017) the reported number of histamine FBO increased significantly in France (+30 outbreaks; 120% increase).
No statistically significant trend was observed in the numbers of FBO reported by MS with agents being indicated as ‘Other causative agents’.
16.4.2. Detailed descriptions of strong‐evidence food‐borne outbreaks
Implicated food vehicles
Twenty‐three MS reported in total 643 strong‐evidence food‐borne and waterborne outbreaks, which was 12.7% of all outbreaks (Table 65). The highest numbers of strong‐evidence outbreaks were reported by Poland, Spain and France and together these totalled more than half (57.9%) of the strong‐evidence outbreaks. Therefore, at the EU level, the description of food vehicles implicated in 2017 in strong evidence outbreaks and the place of exposure (epidemic setting) should be interpreted with caution because they may mostly reflect the trends and statistics of these MS. The type of evidence supporting the link between outbreaks and the implicated foods are described in the Appendix.51 Food vehicles implicated in strong‐evidence FBO (excluding the four strong‐evidence waterborne outbreaks are described in Table 68). Sixty per cent (59.6%, 381 out of 639) of strong‐evidence FBOs were associated with food of animal origin; ‘Meat and meat products’, ‘Eggs and egg products’, ‘Fish and Fisheries’ and ‘Milk and milk products’. ‘Mixed food’ and ‘Buffet meals’ and ‘Other foods’ were together reported in almost one‐fourth of all strong‐evidence outbreaks (24.7%). For single reported types of food vehicles, ‘eggs and egg products’ (n = 105) and ‘Meat and meat products, unspecified’ (n = 39) were the items most frequently reported. Compared with previous years, no important changes were observed for any of the food items being implicated in the strong‐evidence FBO. Compared with 2016, fish was implicated in more outbreaks (+26 outbreaks), while there was a decrease for ‘Crustaceans, shellfish, molluscs and their products’ (−68 outbreaks) and ‘Poultry meat’ (−35 outbreaks).
Table 68.
Frequency distribution of strong‐evidence food‐borne (excluding waterborne) outbreaks, by food vehicle, in reporting Member States, EU, 2017
| Food vehicle | Strong‐evidence outbreaks | Reporting rate per 100,000 | |||||
|---|---|---|---|---|---|---|---|
| Number of outbreaks | % of total outbreaks | Number of cases | % of total cases | 2017 | 2010–2016 (mean) | ||
| Meat and meat products (and their products) | Poultry meat | 30 | 4.7 | 613 | 5.2 | 0.006 | 0.010 |
| Meat and meat products, unspecified | 39 | 6.1 | 681 | 5.7 | 0.008 | 0.002 | |
| Pigmeat | 27 | 4.2 | 821 | 6.9 | 0.005 | 0.008 | |
| Bovine meat | 13 | 2.0 | 350 | 3.0 | 0.003 | 0.004 | |
| Sheep meat | 2 | 0.3 | 110 | 0.9 | < 0.001 | < 0.001 | |
| Other or mixed red meat and their products | 10 | 1.6 | 313 | 2.6 | 0.002 | 0.003 | |
| Subtotal | 121 | 18.9 | 2,888 | 24.4 | 0.024 | 0.028 | |
| Mixed food | Mixed food | 90 | 14.1 | 1,828 | 15.4 | 0.018 | 0.017 |
| Buffet meals | Buffet meals | 10 | 1.6 | 368 | 3.1 | 0.002 | 0.004 |
| Other foods | Canned food products | 1 | 0.2 | 2 | 0.0 | < 0.001 | < 0.001 |
| Cereal products and legumes | 8 | 1.3 | 82 | 0.7 | 0.002 | 0.002 | |
| Other foods/Unspecified | 49 | 7.7 | 1,759 | 14.9 | 0.010 | 0.009 | |
| Subtotal | 58 | 9.1 | 1,843 | 15.6 | 0.011 | 0.011 | |
| Food of non‐animal origin | Confections | 6 | 0.9 | 74 | 0.6 | 0.001 | 0.003 |
| Fruits (and juices) | 7 | 1.1 | 84 | 0.7 | 0.001 | 0.002 | |
| Herbs and spices | 2 | 0.3 | 109 | 0.9 | < 0.001 | < 0.001 | |
| Vegetables (and juices) | 24 | 3.8 | 601 | 5.1 | 0.005 | 0.007 | |
| Subtotal | 39 | 6.1 | 868 | 7.3 | 0.008 | 0.012 | |
| Bakery products | Bakery products | 56 | 8.8 | 901 | 7.6 | 0.011 | 0.006 |
| Eggs and egg products | Eggs and egg products | 105 | 16.4 | 1,035 | 8.7 | 0.021 | 0.022 |
| Fish and fishery products | Crustaceans, shellfish, molluscs and their products | 24 | 3.8 | 243 | 2.1 | 0.005 | 0.009 |
| Fish and fishery products | 82 | 12.8 | 471 | 4.0 | 0.016 | 0.010 | |
| Subtotal | 106 | 16.6 | 714 | 6.0 | 0.021 | 0.018 | |
| Milk and milk products | Cheese | 14 | 2.2 | 755 | 6.4 | 0.003 | 0.004 |
| Milk | 26 | 4.1 | 380 | 3.2 | 0.005 | 0.002 | |
| Dairy products (other than cheeses) | 9 | 1.4 | 38 | 0.3 | 0.002 | 0.001 | |
| Subtotal | 49 | 7.7 | 1,173 | 9.9 | 0.010 | 0.007 | |
| Unknown | Unknown | 5 | 0.8 | 226 | 1.9 | 0.001 | < 0.001 |
| Total (EU) | 639 | 100.0 | 11,844 | 100.0 | 0.125 | 0.128 | |
Distribution of causative agents by implicated food vehicle
The distribution of causative agents by type of food, in strong‐evidence food‐borne and waterborne outbreaks is shown in Figure 78. ‘Meat and meat products’, ‘Other foods’, ‘Milk and milk products’ and ‘Food of non‐animal origin’ were associated with a large variety of causative agents. On the contrary, the other foods were implicated in FBO caused predominantly by a single or a restricted number of causative agents: ‘Eggs and egg products’ and ‘Bakery products’ were associated mainly with Salmonella; ‘Fish and fishery products’ with Histamine and Marine biotoxins’ and ‘Buffet meals’ with norovirus and with bacterial toxins other than Clostridium botulinum.
Figure 78.
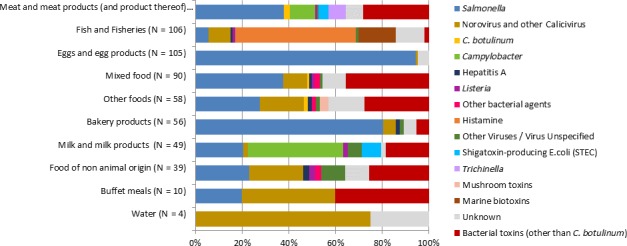
Frequency distribution of causative agents associated with strong evidence food‐borne and waterborne outbreaks, by food vehicle, in reporting Member States, EU, 2017
- Five strong‐evidence outbreaks with food vehicle ‘unknown’ are not shown in the figure.
- Meat and meat products include ‘Bovine meat’, ‘Pigmeat’, ‘Poultry meat’, ‘Sheep meat’, ‘Other or mixed red meat and their products’, ‘Meat and Meat products unspecified’. Fish and fishery products include: ‘Fish’, ‘Crustaceans, shellfish, molluscs and their products’. Food of non‐animal origin includes ‘Confections, ‘Fruits (and juices)’, ‘Herbs and spices’, ‘Vegetables (and juices)’. Milk and milk products include ‘Cheese’, ‘Dairy products (other than cheeses)’ and ‘Milk’. Other foods include ‘Canned food products’, ‘Cereal products and legumes’, ‘Other foods (Unspecified)’.
- Other bacterial agents include enteroaggregative E. coli (EAEC), enteroinvasive E. coli (EIEC), Shigella flexneri, Yersinia enterocolitica. Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins. Other viruses include adenovirus, flavivirus (TBE virus), rotavirus and other unspecified viruses. Marine biotoxins include ciguatoxin and other unspecified toxins. Strong‐evidence FBO by ‘other causative agents have been detailed in the graph into the three classes: histamine, mushroom and marine toxins.
The distribution of causative agents in ‘Mixed food’ and ‘Other foods’ was similar and characterised by Salmonella, norovirus and other caliciviruses and bacterial toxins other than C. botulinum. Most of the strong‐evidence outbreaks implicating ‘Milk and milk products’ were caused by Campylobacter, as during recent years. They were mainly reported by Germany where this food/agent combination accounted for almost a third of total strong‐evidence outbreaks. Several large campylobacteriosis FBO implicating the consumption of raw unpasteurised and unheated milk were reported by Germany and Denmark. Among strong‐evidence outbreaks implicating ‘Fish and fisheries’, a decrease in outbreaks by norovirus and other caliciviruses was observed (80%), compared with 2016. The drop was mostly due to a reduction in the number of outbreaks implicating ‘Crustaceans, shellfish, molluscs and their products’.
Top‐10 combinations of causative agents and food vehicles associated with the highest health impact in strong‐evidence food‐borne (including waterborne) outbreaks
Tables 69–72 aim to provide insight into the causative agent/food pairs that in 2017 were associated with the highest impact on public health on numbers of outbreaks (Table 69), of cases (Table 70), of hospitalisations (Table 71) and of deaths (Table 72). In each of these tables the 10 most reported causative agent/food pairs, at the EU level, are listed and ranked. Rank position occupied by the same combination in previous years (2010–2016), is also reported to provide rapid information on its trend of occurrence, over time. Salmonella in eggs and egg products and Salmonella in meat and meat products were the highest risk agent/food pairs.
Table 69.
Top‐10 pathogen/food vehicle pair causing the highest number of strong‐evidence food‐borne (including waterborne) outbreaks, in reporting Member States, EU, 2017
| 2010–2016 | Evolution (2017 vs 2010–2016)* | |||||||
|---|---|---|---|---|---|---|---|---|
| Rank | Causative agenta | Food vehicleb | Number of outbreaks | Number of reporting MS | Rank | Number of outbreaks (mean/year) | Number of reporting MS | |
| 1 | Salmonella | Eggs and egg products | 99 | 13 | 1 | 98.9 | 18 | Stable |
| 2 | Histamine | Fish and fishery products | 55 | 8 | 5 | 31.0 | 12 | ↑↑ |
| 3 | Salmonella | Meat and meat products | 46 | 12 | 2 | 56.3 | 21 | Stable |
| 4 | Salmonella | Bakery products | 45 | 5 | 8 | 21.6 | 11 | ↑↑ |
| 5 | Bacterial toxins other than Clostridium botulinum | Meat and meat products | 34 | 9 | 3 | 33.0 | 14 | Stable |
| 6 | Bacterial toxins other than Clostridium botulinum | Mixed food | 32 | 10 | 4 | 31.9 | 17 | Stable |
| 7 | Campylobacter | Milk and milk products | 20 | 4 | 22 | 7.1 | 9 | ↑↑ |
| 8 | Marine biotoxins | Fish and fishery products | 17 | 3 | 17 | 11.1 | 5 | ↑↑ |
| 9 | Bacterial toxins other than Clostridium botulinum | Other foods | 16 | 6 | 9 | 19.1 | 13 | Stable |
| 10 | Campylobacter | Meat and meat products | 13 | 5 | 10 | 18.1 | 12 | ↓ |
*Single arrow indicates variations between 25% and 50%; double arrows indicate variations > 50%; ‘stable’ value indicates variations between −25% and 25%.
Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins. Marine biotoxins include ciguatoxin and other unspecified toxins.
Meat and meat products include ‘Bovine meat’, ‘Pigmeat’, ‘Poultry meat’, ‘Sheep meat’, ‘Other or mixed red meat and their products’, ‘Meat and Meat products unspecified’. Milk and milk products include ‘Dairy products (other than cheeses)’, ‘Milk’. Other foods include ‘Cereal products and legumes’ and ‘Other foods (Unspecified)’.
Table 72.
Top‐10 pathogen/food vehicle pair causing the highest number of deaths, in strong‐evidence food‐borne (including waterborne) outbreaks, in reporting Member States, EU, 2017
| 2017 | 2010–2016 | Evolution (2017 vs 2010–2016)* | ||||||
|---|---|---|---|---|---|---|---|---|
| Rank | Causative agent | Food vehiclea | Number of deaths | Number of reporting MS | Rank | Number of deaths (mean/year) | Number of reporting MS | |
| 1 | Salmonella | Eggs and egg products | 3 | 13 | 13 | 1.6 | 18 | ↑↑ |
| 2 | Unknown | Fish and fishery products | 3 | 2 | 2 | 0 | 7 | na |
| 3 | Clostridium botulinum | Other foods | 2 | 1 | 1 | 0.1 | 11 | ↑↑ |
| 4 | Salmonella | Meat and Meat products | 1 | 12 | 12 | 1.9 | 21 | ↓ |
| 4 | Salmonella | Mixed foods | 1 | 9 | 9 | 1.4 | 20 | ↑ |
| 4 | Unknown | Mixed foods | 1 | 5 | 5 | 0 | 15 | na |
| 4 | Shiga toxin‐producing E. coli (STEC) | Meat and meat products | 1 | 5 | 5 | 0 | 8 | na |
| 4 | Shiga toxin‐producing E. coli (STEC) | Milk and milk products | 1 | 4 | 4 | 0.1 | 7 | ↑↑ |
| 4 | Listeria | Meat and Meat products | 1 | 1 | 1 | 1.4 | 7 | ↓ |
| 4 | Hepatitis A | Food of non‐animal origin | 1 | 1 | 1 | 0 | 7 | na |
| 4 | Listeria | Food of non‐animal origin | 1 | 1 | 1 | 0.1 | 2 | ↑↑ |
*Single arrow indicates variations between 25% and 50%; double arrows indicate variations > 50%; na: not available.
Fish and fishery products include ‘Fish’ and ‘Crustaceans, shellfish, molluscs and their products’. Meat and meat products include ‘Bovine meat’, ‘Pigmeat’, ‘Poultry meat’, ‘Sheep meat’, ‘Other or mixed red meat and their products’, ‘Meat and Meat products unspecified’. Milk and milk products include ‘Cheese’, ‘Dairy products (other than cheeses)’ and ‘Milk’. Food of non‐animal origin includes, ‘Fruits (and juices)’, ‘Herbs and spices’.
Table 70.
Top‐10 pathogen/food vehicle pair causing the highest number of cases, in strong‐evidence food‐borne (including waterborne) outbreaks, in reporting Member States, EU, 2017
| 2017 | 2010–2016 | Evolution (2017 vs 2010–2016)* | ||||||
|---|---|---|---|---|---|---|---|---|
| Rank | Causative agenta | Food vehicleb | Number of human cases | Number of reporting MS | Rank | Number of human cases (mean/year) | Number of reporting MS | |
| 1 | Salmonella | Meat and meat products | 1365 | 12 | 4 | 1053.1 | 21 | ↑ |
| 2 | Salmonella | Eggs and egg products | 964 | 13 | 3 | 1067.6 | 18 | Stable |
| 3 | Norovirus and other caliciviruses | Other Foods | 943 | 3 | 16 | 712.9 | 8 | ↑ |
| 4 | Bacterial toxins other than Clostridium botulinum | Meat and meat products | 870 | 9 | 5 | 979.9 | 14 | Stable |
| 5 | Bacterial toxins other than Clostridium botulinum | Mixed foods | 719 | 10 | 7 | 864.3 | 17 | Stable |
| 6 | Salmonella | Bakery products | 621 | 5 | 18 | 267.3 | 11 | ↑↑ |
| 7 | Bacterial toxins other than Clostridium botulinum | Milk and milk products | 487 | 5 | 34 | 96.9 | 11 | ↑↑ |
| 8 | Salmonella | Mixed foods | 411 | 9 | 12 | 452.7 | 20 | Stable |
| 9 | Norovirus and other caliciviruses | Food of non‐animal origin | 392 | 7 | 2 | 2155.7 | 13 | ↓↓ |
| 10 | Salmonella | Other Foods | 391 | 6 | 14 | 337.1 | 14 | Stable |
*Single arrow indicates variations between 25% and 50%; double arrows indicate variations > 50%; ‘stable’ value indicates variations between −25% and 25%.
Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins.
Meat and meat products include ‘Bovine meat’, ‘Pigmeat’, ‘Poultry meat’, ‘Sheep meat’, ‘Other or mixed red meat and their products’, ‘Meat and Meat products unspecified’. Food of non‐animal origin includes ‘Fruits (and juices)’, ‘Vegetables (and juices)’. Milk and milk products include ‘Cheese’, ‘Dairy products (other than cheeses)’ and ‘Milk’. Other foods include ‘Cereal products and legumes’, ‘Other foods (Unspecified)’.
Table 71.
Top‐10 pathogen/food vehicle pair causing the highest number of hospitalisations, in strong‐evidence food‐borne (including waterborne) outbreaks, in reporting Member States, EU, 2017
| 2017 | 2010–2016 | Evolution (2017 vs 2010–2016)* | ||||||
|---|---|---|---|---|---|---|---|---|
| Rank | Causative agenta | Food vehicleb | Number of hospitalised cases | Number of reporting MS | Rank | Number of hospitalised cases (mean/year) | Number of reporting MS | |
| 1 | Salmonella | Meat and Meat products | 301 | 12 | 3 | 214.3 | 21 | ↑ |
| 2 | Salmonella | Eggs and egg products | 224 | 13 | 2 | 271.0 | 18 | Stable |
| 3 | Salmonella | Bakery products | 148 | 5 | 6 | 69.3 | 11 | ↑↑ |
| 4 | Bacterial toxins other than Clostridium botulinum | Mixed foods | 126 | 10 | 9 | 52.7 | 17 | ↑↑ |
| 5 | Trichinella | Meat and Meat products | 119 | 5 | 5 | 75.7 | 9 | ↑ |
| 6 | Salmonella | Mixed foods | 99 | 9 | 4 | 96.1 | 20 | Stable |
| 7 | Salmonella | Other Foods | 93 | 6 | 10 | 47.1 | 14 | ↑↑ |
| 8 | Salmonella | Food of non‐animal origin | 47 | 5 | 8 | 63.3 | 14 | ↓ |
| 9 | Salmonella | Milk and milk products | 38 | 4 | 12 | 25.4 | 12 | ↑ |
| 10 | Histamine | Fish and fishery products | 36 | 8 | 16 | 15.1 | 12 | ↑↑ |
*Single arrow indicates variations between 25% and 50%; double arrows indicate variations > 50%; ‘stable’ value indicates variations between −25% and 25%.
Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins.
Meat and meat products include ‘Bovine meat’, ‘Pigmeat’, ‘Poultry meat’, ‘Sheep meat’, ‘Other or mixed red meat and their products’, ‘Meat and Meat products unspecified’. Other foods include ‘Cereal products and legumes’, ‘Other foods (Unspecified)’. Food of non‐animal origin includes ‘Confections, ‘Fruits (and juices)’, ‘Herbs and spices’, ‘Vegetables (and juices)’. Milk and milk products include ‘Cheese’, ‘Dairy products (other than cheeses)’ and ‘Milk’.
Places of exposure
Information on the place of exposure (epidemic setting) was provided for 596 strong‐evidence food‐borne (including waterborne) outbreaks (92.7% of all strong‐evidence outbreaks) (Table 73). As mentioned before, these descriptions should be interpreted with caution at the EU level because they mainly may reflect the trends and statistics of Poland, France and Spain that contributed with more data. One in three FBOs happened at home because the ‘Household’ setting was the most frequent reported place of exposure of cases to the implicated food (34.2% out of 643 strong‐evidence food‐borne (including waterborne) outbreaks), followed by ‘Restaurants, pubs, street vendors and take away’ (30.0%) and ‘Canteen or catering to workplace, school, hospital’ (16.0%) that are settings where food was prepared and/or served by catering services. ‘Other settings’ such as farms, fairs and festivals, and other undefined places were reported less frequently (12.4%). This pattern seemed to be stable over years.
Table 73.
Frequency distribution of strong‐evidence food‐borne (including waterborne) outbreaks, by place of exposure (setting), in reporting Member States, EU, 2017
| Type of setting | Strong‐evidence outbreaks | Reporting rate per 100,000 | |||||
|---|---|---|---|---|---|---|---|
| Number of outbreaks | % of total | Number of human cases | % of total | 2017 | 2010–2016 (mean) | ||
| Household | Household | 220 | 34.2 | 1,807 | 14.9 | 0.043 | 0.046 |
| Restaurant, pub, street vendors, take away | Restaurant or Cafe or Pub or Bar or Hotel or Catering service | 182 | 28.3 | 3,225 | 26.6 | 0.036 | 0.030 |
| Mobile retailer or market/street vendor | 9 | 1.4 | 77 | 0.6 | 0.002 | 0.001 | |
| Take‐away or fast‐food outlet | 2 | 0.3 | 19 | 0.2 | < 0.001 | 0.001 | |
| Subtotal | 193 | 30.0 | 3,321 | 27.4 | 0.038 | 0.032 | |
| Canteen or Catering to Workplace, school, hospital | School or kindergarten | 34 | 5.3 | 1,830 | 15.1 | 0.007 | 0.009 |
| Residential institution (nursing home or prison or boarding school) | 19 | 3.0 | 382 | 3.2 | 0.004 | 0.004 | |
| Canteen or workplace catering | 35 | 5.4 | 1,554 | 12.8 | 0.007 | 0.005 | |
| Hospital or medical care facility | 14 | 2.2 | 351 | 2.9 | 0.003 | 0.002 | |
| Subtotal | 103 | 16.0 | 4,119 | 34.0 | 0.020 | 0.020 | |
| Other settings | Others | 36 | 5.6 | 875 | 7.2 | 0.007 | 0.008 |
| Multiple places of exposure in one country | 20 | 3.1 | 687 | 5.7 | 0.004 | 0.001 | |
| Camp or picnic | 5 | 0.8 | 63 | 0.5 | 0.001 | 0.002 | |
| Farm | 13 | 2.0 | 164 | 1.4 | 0.003 | 0.001 | |
| Multiple places of exposure in more than one country | 4 | 0.6 | 76 | 0.6 | 0.001 | < 0.001 | |
| Subtotal | 80 | 12.4 | 1,918 | 15.8 | 0.016 | 0.002 | |
| Unknown | Unknown | 47 | 7.3 | 943 | 7.8 | 0.009 | 0.014 |
| Total (EU) | 643 | 100.0 | 12,108 | 100.0 | 0.126 | 0.128 | |
Distribution of causative agents by place of exposure
The distribution of causative agents by known place of exposure (of cases to the implicated food), in the strong‐evidence food‐borne (including waterborne) outbreaks is shown in Figure 79. In the setting ‘Household’, the diversity of causative agents was largest and Salmonella was more frequently reported compared to other settings (61.4% out of 220). In addition, outbreaks by C. botulinum, Trichinella and mushrooms toxins were only reported as causative agents in the setting ‘Household’. Conversely, outbreaks by bacterial toxins other than C. botulinum, which are frequently associated with wrong procedures of food preservations, were less frequently reported in domestic setting.
Figure 79.
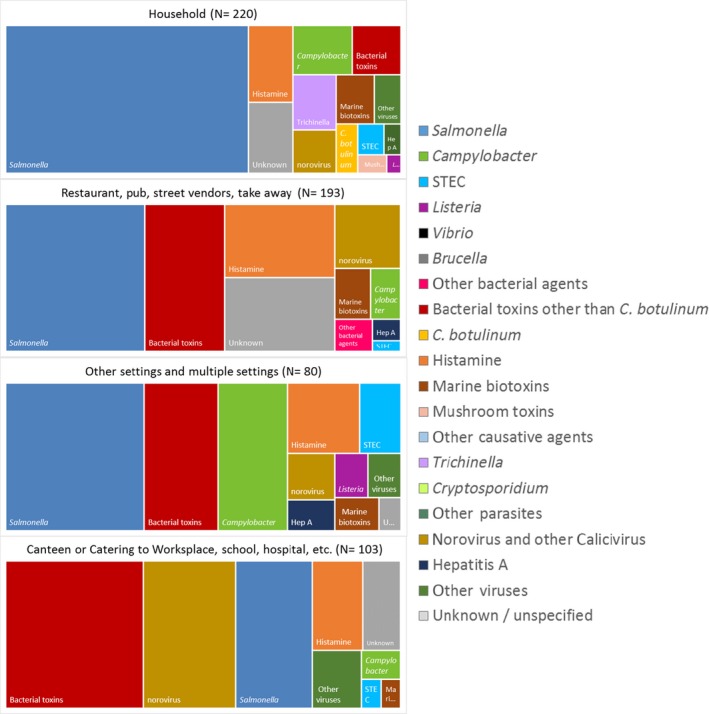
Distribution of strong‐evidence food‐borne (including waterborne) outbreaks, by place of exposure (setting) and by causative agent, in reporting Member States, EU, 2017
- Forty‐seven food‐borne‐outbreaks with setting ‘unknown’ are not shown in the figure.
- Other bacterial agents include enteroaggregative E. coli (EAEC), Enteroinvasive E. coli (EIEC), Shigella flexneri, Yersinia enterocolitica. Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins. Other viruses include adenovirus, flavivirus (TBE virus), rotavirus and other unspecified viruses. Marine biotoxins include ciguatoxin and other unspecified toxins.
- Restaurant, pub, street vendors, take away, etc. include ‘Mobile retailer or market/street vendor’, ‘Restaurant or Cafe or Pub or Bar or Hotel or Catering service’, ‘Take‐away or fast‐food outlet’.
- Other settings and multiple settings include ‘Camp or picnic’, ‘Disseminated cases’, ‘Farm’, ‘Multiple places of exposure in more than one country’, ‘Multiple places of exposure in one country’, ‘Others’, ‘Temporary mass catering (fairs or festivals)’.
- Canteen or Catering to Workplace, school, hospital include ‘School or kindergarten’, ‘Residential institution (nursing home or prison or boarding school)’, ‘Canteen or workplace catering’, ‘Hospital or medical care facility’.
FBO linked to ‘Restaurant, pub, street vendors, take away’ as well as ‘Other settings and multiple settings’ had a similar pattern of distribution of causative agents, at least for the most frequently reported agents. More than half of the FBO involving these settings were caused by both Salmonella and bacterial toxins other than C. botulinum. Outbreaks by Campylobacter ranked third (18% out of 80) among incidents occurred in ‘Other settings and multiple settings’ due to the high number of Campylobacter outbreaks reported in farms.
FBO in ‘Canteen or catering to workplace, school, hospital etc.’ were predominantly caused by bacterial toxins other than C. botulinum, as well as by norovirus and other caliciviruses, which together totalled 58% (out of 103) of total outbreaks in these settings. Interestingly among outbreaks occurring during 2017 in ‘Canteen or Catering to Workplace, school, hospital etc.’ the proportion of infected food handlers as contributory factor was higher than in other settings.52
Although many factors may contribute to the occurrence of strong‐evidence outbreaks in the different settings (Brown et al., 2017; Jones et al., 2017), in the reported 2017 FBO data information on the contributory factors was lacking for 61.1% of strong‐evidence outbreaks (Figure 80).
Figure 80.
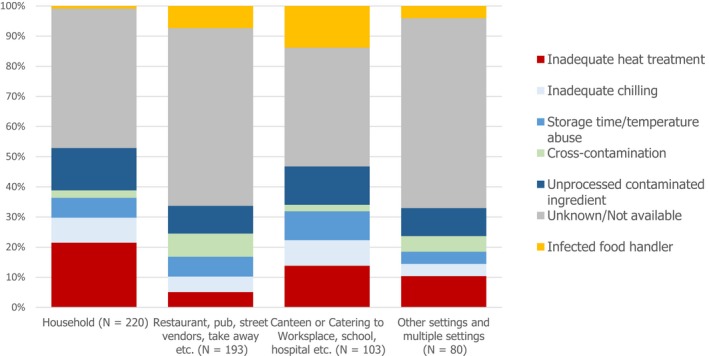
Frequency distribution of contributory factors in strong‐evidence food‐borne (including waterborne) outbreaks, by place of exposure (setting), in reporting Member States, EU, 2017
- Canteen or Catering to Workplace, school, hospital, etc., include ‘Canteen or workplace catering’, ‘Catering on aircraft or ship or train’, ‘Hospital or medical care facility’, ‘Residential institution (nursing home or prison or boarding school)’, ‘School or kindergarten’.
- Other settings and multiple settings include ‘Camp or picnic’, ‘Disseminated cases’, ‘Farm’, ‘Multiple places of exposure in more than one country’, ‘Multiple places of exposure in one country’, ‘Others’, ‘Temporary mass catering (fairs or festivals)’. Restaurant, pub, street vendors, take away, etc., include ‘Mobile retailer or market/street vendor’, ‘Restaurant or Cafe or Pub or Bar or Hotel or Catering service’, ‘Take‐away or fast‐food outlet’.
16.4.3. Temporal trends in numbers of food‐borne outbreaks, by causative agent and by food vehicle, 2014–2017
Strong‐evidence outbreaks were 12.7% of all food‐borne and waterborne outbreaks reported by MS for the year 2017, similarly to previous years. When aggregating data from the last 4 years (2014–2017), they accounted for the 12.1% of all reported outbreaks in the EU (n = 20,647). Since 2014, MS have the possibility to provide detailed information on the suspected food vehicle also for weak‐evidence outbreaks and for the year 2017 1,953 (38.5% of total reported FBO) weak‐evidence outbreaks had this information. The temporal distributions of outbreaks caused by the most important food/agent combinations are reported in Figure 81. Much caution is needed, however, in interpreting such data as the correlation between food items and causative agent is only ‘suspect’ for most of the outbreaks. Moreover, as explained above, these supranational trends may reflect the trends and statistics of the MS that contributed more to the data collection.
Figure 81.
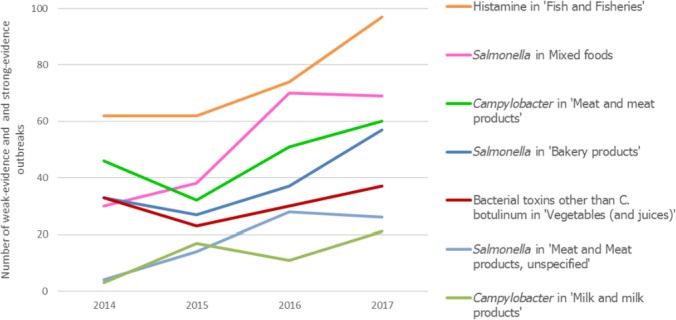
Number of strong‐ and weak‐evidence food‐borne (including waterborne) outbreaks associated with the most frequently reported combination of causative agent and implicated food vehicle, in reporting Member States, EU, 2014–2017
- Bacterial toxins other than Clostridium botulinum include toxins produced by Bacillus, Clostridium other than Clostridium botulinum and Staphylococcus and other unspecified bacterial toxins.
- Meat and meat products include ‘Bovine meat’, ‘Pigmeat’, ‘Poultry meat’, ‘Sheep meat’, ‘Other or mixed red meat and their products’, ‘Meat and Meat products unspecified’. Milk and milk products include ‘Dairy products (other than cheeses)’, ‘Milk’.
- Only food/agent combinations that progressively increased over years among those most frequently reported are shown.
A significant increasing trend over several years was identified for Salmonella FBO implicating ‘Mixed foods’ (n = 69 of which 34 strong evidence and 35 weak evidence; 5.6% of total Salmonella outbreaks). This pattern seems to be primarily attributable to S. Enteritidis, which accounted for almost half of the Salmonella outbreaks in ‘Mixed foods’. For food vehicle implicated in Salmonella outbreaks, it is worth mentioning that the positive trend reported until 2016 for outbreaks due to ‘Eggs and egg products’ (EFSA and ECDC, 2017b) was halted in 2017, due to a marked drop in strong and weak‐evidence outbreaks implicating this food item (116 outbreaks less than 2016; 35.0% reduction).
No other statistically significant trend resulted for the most frequent combinations of causative agents and food, meaning that, during the years 2014–2017, the trends were stable or that the data were too scarce to disclose any increase or decrease, with statistical significance. However, it is worth mentioning that the number of FBO by Salmonella in ‘Bakery products’, Campylobacter in ‘Milk and milk products’ and Histamine in ‘Fish and fish products’ increased over the years but without statistical significance. Distributions over years of strong‐evidence and weak‐evidence outbreaks implicating these combinations are visualised in Figure 81.
16.4.4. Waterborne outbreaks
Seven MS reported 27 waterborne outbreaks for the year 2017, meaning outbreaks with ‘Tap water, including well water’ as the incriminated vehicle and with ‘Drinks, including bottled water’ not being included into this category. The latter food vehicle was reported by Belgium and France in, respectively, 3 and 1 outbreaks. Four of the 27 waterborne outbreaks were reported as strong‐evidence outbreaks by three MS and involved 264 cases with no hospitalisations. Agents detected in three of the four strong‐evidence outbreaks were norovirus and other caliciviruses while the causative agent in the remaining outbreak was unknown (Table 74). In addition, one non‐MS, the Former Yugoslav Republic of Macedonia, reported three strong evidence outbreaks.53
Table 74.
List of reported strong‐evidence waterborne outbreaks, in reporting Member States, EU, 2017
| Causative agent | Country | Setting | Additional information | Strong‐evidence outbreaks | Reporting rate (per 100,000) | |||
|---|---|---|---|---|---|---|---|---|
| n | Human cases | Hospitalised | Deaths | |||||
| Norovirus including other caliciviruses | Finland | Household | Not available | 1 | 58 | 0 | 0 | < 0.001 |
| Spain | Restaurant or Cafe or Pub or Bar or Hotel or Catering service | Not available | 1 | 80 | 0 | 0 | < 0.001 | |
| Sweden | Others | Not available | 1 | 100 | 0 | 0 | < 0.001 | |
| Unknown | Spain | Camp or picnic | Not available | 1 | 26 | 0 | 0 | < 0.001 |
| Total (EU) | 4 | 264 | 0 | 0 | < 0.001 | |||
Six MS reported 23 weak‐evidence waterborne outbreaks caused by Campylobacter (n = 2), Cryptosporidium (n = 1), hepatitis virus (‘Viruses other than hepatitis A virus and Calicivirus’) (n = 1), Shiga toxin‐producing E. coli (n = 11) and parasite (other than Cryptosporidium and Trichinella) (n = 1). Other seven outbreaks were caused by unknown agents.
Among non‐MS, the Former Yugoslav Republic of Macedonia, Norway and Switzerland reported four outbreaks caused by Campylobacter, norovirus and other caliciviruses and unknown agent. Further details on the number of weak‐evidence outbreaks and human cases, including information on the causative agents, and reporting countries are available in the Appendix.54
16.5. Discussion
16.5.1. Overview of results
In 2017, 27 MS reported 5,079 food‐borne and waterborne outbreaks which correspond to a mean number of 97.7 outbreaks per week, at the EU level. Another 149 outbreaks were notified by eight non‐MS. Compared with 2016, the numbers of reported outbreaks and cases of illnesses decreased whereas the hospitalisations and deaths slightly increased. The number of outbreaks reported by each MS varied importantly, with few MS accounting for most of the events. There was also a huge variability in the types of causative agents reported to be linked to outbreaks, and in the types and mean size of these incidents reported to EFSA. These differences depict an extremely heterogeneous geography of FBOs across the EU. Apart from true epidemiological differences, variations in reporting between MS may be due to differences in the approach and the sensitivity of the surveillance of FBOs.
The number of FBOs reported in 2017 did not substantially change compared with 2016, for most MS. However, major variations (> 25%) towards either increase or decrease were identified in 10 MS. These variations appeared to be primarily driven by changes in the number of Salmonella FBO. Variations towards decrease were also observed for some MS in outbreaks by Campylobacter, bacterial toxins other than Clostridium botulinum and Norovirus. Reasons why the number of FBOs did not substantially changed in 2017 could be multiple; no change in level of contamination of food at the consumer level, better reporting practices, as well as improvement in the detection of outbreaks thanks to the implementation of WGS for routine typing of pathogens.
Outbreaks of salmonellosis had the highest impact on human cases, hospitalisations and deaths. In particular Salmonella in ‘Eggs and egg products’ caused the highest number of strong‐evidence outbreaks and deaths, and ranked second among the most frequently reported pathogen/food pairs as for number of cases and hospitalisations. Salmonella was also detected in the several multicountry outbreaks identified in 2017 and investigated at the supranational level. Listeria and Clostridium botulinum caused the highest case fatality among FBO cases. The health impact of norovirus outbreaks on human cases and hospitalisations was reduced in 2017 as a consequence of the decrease in the number of outbreaks compared with 2016. Also, FBO by Campylobacter had an important drop in 2017.
More than one‐third of these incidents reported to EFSA in 2017 lacked information on the causative agent. In many cases, also descriptive information on the suspected food vehicle and epidemic context (i.e. the setting, whether single or multiple households were involved) was not reported. This is a critical gap in FBOs’ data collection since almost one in five reported outbreaks in the EU had this information missing. In many MS the proportion of events with no information accounted for most reported outbreaks. Several reasons may explain the reporting of unknown agents, including late reporting of illness by consumers, failure to detect causative agents in patients or in the food, unavailability of clinical or of food samples, delay in sample collection, etc. This lack of information hampers a comprehensive understanding of the epidemiology of FBOs at both the EU and MS level.
Despite this uncertainty, the 2017 FBO data collection indicated that in the EU the ‘household’ was the most frequent place of exposure of cases to contaminated foods, with one in three outbreaks occurring in this setting. FBO in households may probably even be underestimated as not all MS systematically collect and report data on such events occurring at home. In this context, data analyses showed that higher outbreak reporting rates were reported by MS also reporting smaller average numbers of cases per outbreak, which may be related to the household setting. Causative agents associated with household outbreaks had quite peculiar distribution with a large variability of causative agents. This finding suggests, beyond chance due to a higher number of FBO reported, that incidents leading to food contamination and outbreaks in domestic kitchen were probably different than those occurring in other settings. This finding reaffirms the need to deliver recommendations to consumers on correct behaviours and practices for food handling and preservation.
Trends
Variations over years in the number of Salmonella outbreaks seem to be a major determinant of the overall trend of FBO over years. This is a significant finding, since Salmonella is the only causative agent in the EU subjected to specific NCP, at the primary production level. In 2017, Salmonella was the leading causative agent of FBO in 15 MS. In these countries, comparing with 2016, the number of reported Salmonella FBO decreased importantly only in Hungary, Latvia and Spain, while no substantial change or even an increase was noted for the other MS. In Poland, the increase observed in 2017 consolidated the significant increasing trend observed in recent years, which appears to be mainly attributable to an increase in FBO due to S. Enteritidis. A similar positive trend in the same period was also observed for Slovakia. The progressive increase in S. Enteritidis outbreaks in these MS, despite NCP having been implemented for many years, is a matter of concern given that together Poland and Slovakia reported almost two‐thirds of total outbreaks by S. Enteritidis in the EU. Assuming no specific changes in outbreak detection capability by the MS (e.g. change in case definition, implementation of molecular‐based surveillance for Salmonella infection), these findings may suggest that Salmonella NCP in animal reservoirs and food safety interventions are becoming less successful in these MS. Conversely, the marked decrease in S. Enteritidis FBO observed in 2017 for 10 MS is a favourable signal. In Denmark, the increase of Salmonella outbreaks in recent years was partly attributable to a better clustering of cases thanks to the application of WGS for routine typing of all human isolates (Gymoese et al., 2017). In Germany and the Czech Republic, the reporting of Salmonella outbreaks increased in 2017 after years of progressive drop. This countertrend was mainly attributable to a rise in outbreaks by S. Enteritidis in both country and also in incidents implicating S. Typhimurium including monophasic variant, in Germany.
For the other causative agents identified in FBOs, formulating robust hypotheses what is driving the trends in occurrence is challenging, given the absence of specific control programmes at the EU level. The general drop in outbreaks by norovirus suggests a possible reduction of the level of contamination of food vehicles more often implicated (e.g. oysters and other crustacean, shellfish, molluscs and their products, vegetables and berries). Conversely, it is well known that the introduction of new genetic variants influences importantly the spread of norovirus infection into the general population (including food handlers) which is favoured by the large proportion of susceptible individuals. According to the information provided by the Noronet network, norovirus genotypes circulating in the EU have been quite stable since 2012, except for variant GII.17 which emerged in 2015 and 2016. This genetic stability could partly explain the decrease in the occurrence of norovirus outbreaks in recent years. The reporting of listeriosis outbreaks was quite stable over years, however it is important to mention that in 2017 a large multicountry outbreak of invasive listeriosis (serogroup IVb) linked to frozen corn and possibly other frozen vegetables was identified in several MS (EFSA and ECDC, 2018b). The epidemic, which was ongoing since 2015, came to the attention of general public due to the unexpected implicated food vehicle.
Reported FBO due to histamine poisoning increased in recent years. This finding suggests that incidents due to inadequate conditions of chilling, storage, processing of fish and fish products leading to intoxication by histamine, may have increased over time. This is therefore a matter of concern given the increasing habits of eating raw fish. The situation for histamine is under surveillance at the EU level and a recent EFSA assessment on histamine intoxication in some MS has been published (EFSA, 2017b).
For the first time since the beginning of the outbreak data collection, outbreaks of hepatitis E were reported by Germany, in 2017. This is a significant finding as hepatitis E is considered an emerging problem in the EU and the interest of public health and food safety sectors have remarkably increased in recent years. A summary of hepatitis E outbreaks in the EU between 2005 and 2015 has been published in a recent EFSA opinion (EFSA BIOHAZ Panel et al., 2018d), which stated food‐borne transmission of hepatitis E appearing to be a major route in Europe with pigs and wild boars as the main source of hepatitis E.
Sources
Food vehicles involved in strong‐evidence FBOs in 2017 were mostly of animal origin. ‘Meat and meat products’ (i.e. including meat from poultry, pork, bovine, sheep and other unspecified red meats and their products) was the food group most implicated in outbreaks, even if less frequently than in 2016. The implication of ‘Poultry meat’ decreased compared with 2016. Outbreaks by ‘Eggs and egg products’ increased in 2017 and this food item were implicated in almost one every six strong‐evidence outbreaks. ‘Fish’ were also reported in a much higher number of strong evidence outbreaks, compared with 2016. Conversely, outbreaks implicating ‘Crustaceans, shellfish, molluscs and their products’ decreased remarkably, due to a drop in outbreaks by norovirus implicating this foodstuff.
‘Eggs and eggs products’ are a well‐known vehicle of Salmonella, in particular of S. Enteritidis. Most of the strong‐evidence Salmonella FBO in the EU implicating ‘Eggs and egg products’ were reported by Poland and Spain. In this context, it is of note that Slovakia reported the highest number of S. Enteritidis outbreaks but a very low proportion of strong‐evidence outbreaks. In 2017, ‘Eggs and egg products’ were implicated in large multicountry Salmonella outbreaks which involved several MS, similarly to previous years. Fine‐tuned molecular characterisation by WGS allowed disclosing close genetic relationships among S. Enteritidis clones implicated in many of these epidemic incidents which were also linked to historical outbreak cases occurred in previous years. These findings reaffirm the need to strictly monitor microbiological contamination at both the primary production level and at other FBOp downstream in the food chain. Among other foodstuffs implicated in Salmonella outbreaks causing a high health impact ‘Bakery products’ had the highest increase in total outbreaks, human cases and hospitalisations.
In 2017, impact of outbreaks by bacterial toxins other than C. botulinum in combination with ‘Meat and meat products’, ‘Mixed food’ or ‘Other foods’ were also important. Trend over years were stable and mirrors quite exclusively the pattern of France, which contributed more to the data collection.
It is worth underlining that most of the food/agent pairs having the highest public health impact in 2017 are regulated through specific microbiological food safety criteria, which are implemented in the EU to give guidance on the acceptability of foods and their manufacturing processes at certain points of the farm‐to fork chain. The analysis of 2017 outbreak data and historical trends increases the importance of these criteria to manage the epidemic risk connected with these food/agent pairs. Other important food/agent pairs that had an important health impact in 2017 but are not subjected to similar regulations include Campylobacter in ‘Meat and meat products’ and ‘Milk and milk products’, norovirus and other caliciviruses in ‘Food on non‐animal origin (Vegetable, fruits and juices’)’ as well as in ‘Crustaceans, shellfish, molluscs and their products’, Marine biotoxins in ‘Fish and fisheries’ and bacterial toxins other than Clostridium botulinum in different food matrices.
Among reported serovars from all salmonellosis cases and disregarding the travel information, S. Agona replaced S. Derby as a sixth most common serovar. This may be due to two S. Agona multicountry outbreaks in the EU that were under investigation during 2017. An outbreak was linked to the consumption of infant formula in France from August 2017 until January 2018 (EFSA and ECDC, 2018a and Jourdan‐da Silva et al., 2018). A multicountry outbreak of S. Agona was possibly linked to RTE food (EFSA and ECDC, 2018c). Overall, 122 outbreak cases were reported by five EU countries (the United Kingdom, Finland, Denmark, Germany and Ireland) from January 2017 to July 2018.
Multicountry outbreaks by Salmonella in the EU, 2017
Several large prolonged multicountry outbreaks by Salmonella Enteritidis have been reported in 2017 and involved a large number of EU MS plus Norway. Evidence from epidemiological, microbiological, environmental and tracing investigations identified either eggs, or poultry products and meat as the food vehicles implicated. In all these incidents, the characterisation of the S. Enteritidis clinical isolates by WGS made it possible to establish a link among the cases scattered all over the involved countries, to recognise the supranational dimension of the outbreaks and to investigate the genetic relatedness with historical cases of S. Enteritidis previously. One of these outbreaks was linked to eggs from Poland and the tracing of the outbreak source allowed identifying farms and packing centres located in Poland as being implicated in the 2017 outbreak, similarly to previous year (EFSA and ECDC, 2017a).
A prolonged multicountry outbreak of infection with a new Salmonella serotype (antigenic formula 11:z41:enz15) involved five MS between 2016 and 2017 and caused at least 47 cases (EFSA and ECDC, 2017c). The common source of Salmonella infection was traced back to the contamination of sesame seeds and sesame paste. The involvement of several MS in the outbreaks was due to the long shelf‐life of the implicated food item which were distributed and processed over a long time period and wide geographical area in the EU.
From August 2017 until January 2018, an outbreak by Salmonella Agona caused 39 cases of infection in infants (children < 1 year of age) in three MS (France, Greece, Spain) (EFSA and ECDC, 2018a and Jourdan‐da Silva et al., 2018). The event was the consequence of a contamination of different brands of infant formula (powdered milk) all produced in a single processing company in France. The products potentially contaminated, including products other than infant formula, were recalled and/or withdrawn, as a precautionary measure. The information was delivered to EU MS and non‐EU countries through the (RASFF and the INFOSAN (WHO). Moreover, a multicountry outbreak of S. Agona was possibly linked to RTE food (EFSA and ECDC, 2018c) and reported by the United Kingdom, Finland, Denmark, Germany and Ireland from January 2017 to July 2018
Key findings, food‐borne outbreaks, EU, 2017
In 2017, 27 EU Member States reported 5,079 food‐borne and waterborne outbreaks and 43,400 cases which correspond to a 6.8% and 23.8% decrease, compared with 2016, respectively. Large differences were observed among MS in the number of outbreaks reported, with few MS accounting for most.
At the MS level, the reporting rate of food‐borne and waterborne outbreaks was quite stable or show only small variations for most of the MS (n = 22), over recent years (2014–2017).
Bacteria, in particular Salmonella, were the most common causative agent detected in food‐borne and waterborne outbreaks in the EU (34.3% of all outbreaks), followed by bacterial toxins (16.1%), viruses (7.8%) in particular Norovirus, other causative agents (3.6%) in particular histamine and parasites (0.6%). In 37.6% of the outbreaks, the causative agent was not reported.
Salmonella caused the highest number of outbreaks, cases, hospitalisations and deaths. Listeria and Clostridium botulinum were associated with the highest case fatality. Impact of norovirus outbreaks was greatly reduced in 2017 due to a marked decrease in the number of outbreaks. Also the number of outbreaks by Campylobacter decreased.
In 2017, for the first time since the beginning of the outbreak data collection, six outbreaks of hepatitis E were reported by Germany.
Causative agents implicated in FBO differed importantly at the MS level. The agent most frequently reported was Salmonella for 15 MS, bacterial toxins other than Clostridium botulinum for four MS, Campylobacter for three MS, norovirus for three MS and Shiga toxin‐producing E. coli for one MS.
The geography of Salmonella FBO was highly variable across EU with few MS accounting for most of the outbreaks. S. Enteritidis was by far the most frequently identified serovar, even if most of the outbreaks were reported by two MS only. In these MS a significant increasing trend of S. Enteritidis outbreaks resulted in the most recent years (2014–2017).
Outbreaks by histamine were increasingly reported in the EU over recent years and a statistical significant positive trend was observed for France.
There were 643 strong‐evidence FBO (12.7% of total outbreaks). Sixty per cent of strong‐evidence FBOs were associated with food of animal origin; ‘Meat and meat products’ (i.e. including meat from poultry, pork, bovine, sheep and other unspecified red meats and their products) was the food category most frequent involved, followed by ‘Fish and fishery products’, ‘Eggs and egg products’ and ‘Milk and milk products’. Compared with previous years, no important changes were observed for any of the food items being implicated in the strong‐evidence FBOs.
In 2017, FBO by Salmonella implicating ‘Eggs and egg products’, ‘Bakery products’ and ‘Meat and meat products’ had the highest impact. Other critical pathogen/food pairs were bacterial toxins other than Clostridium botulinum in ‘Meat and meat products’, ‘Mixed food’ or ‘other foods’, Histamine in ‘Fish’ and Campylobacter in ‘Milk and milk products’ and ‘Meat and meat products’.
Important differences were observed for causative agents implicated in outbreaks, in different setting. ‘Household’ outbreaks were characterised by the largest variety of causative agents, with events by Clostridium botulinum, Trichinella and mushrooms toxins only reported in this type of setting. Outbreaks by bacterial toxins other than Clostridium botulinum, and norovirus were more frequently reported in settings such as ‘Restaurant, pub, street vendors, take away’ and ‘Canteen or catering to workplace, school, hospital, etc.’
Household was the most frequent place of exposure to contaminated foods with one every three outbreaks occurring at home in this setting. Contribution of domestic setting to total FBOs is probably even underestimated as not all MS systematically collect and report data on events.
16.5.2. Food‐borne outbreaks EU surveillance data: use and limitations
Structural and functional resources for the integrated surveillance of FBOs vary importantly among MS. The lack of harmonisation hampers data comparability among countries and trend analysis at the supranational level. For this reason in recent years the approach to data analysis and interpretation has been focused in particular on single MS. Figure 72 has been added to depict these differences at a glance and to make clear why results and trends at the EU level should be interpreted with caution. Aggregated estimates may reflect the different relative ‘weights’ of single MS, rather than representing a true EU picture. As an example, data on FBO by bacterial toxins other than Clostridium botulinum are almost exclusively due to the trend of a single MS. To support a proper data interpretation, Table 73 has been added to allow rapidly visualising the relative contribution of each MS to the 2017 FBO data collection, by causative agent.
The healthcare system organisation influences critically the likelihood of identifying and reporting FBOs and tracing successfully the sources. The capability of detecting, investigating and reporting FBO depends on the overall architecture and components of the surveillance system (e.g. case definition, type of outbreak under surveillance, diagnostic methods, food testing strategies), and on the availability of laboratory methods harmonised between public health and food safety sectors. As significant differences exist among MS, the degree of underascertainment and underreporting of outbreaks and cases moving through the different steps of the food‐borne surveillance pyramid (Haagsma et al., 2013), may hugely vary. As a consequence, the outcomes of the FBO data analysis may be affected by different degree of uncertainty and bias, in particular at the EU level.
Biases limiting the use of data are not only connected to the structural aspects of FBO surveillance but also to peculiarity of the epidemiology of causative agents. As an example, for outbreaks caused by agents that have a restricted range of foodstuffs potentially implicated, such as Trichinella or histamine, the relationship with the implicated foodstuff is probably easier to establish than for more ubiquitous causative agents. As a result, the importance of these items may be overestimated in the EFSA analysis as it focuses primarily on strong‐evidence outbreaks. As a matter of fact, in 2017, the proportion of strong‐evidence outbreaks among epidemic incidents by Trichinella, Clostridium botulinum and histamine was more than twofolds higher compared to outbreaks by Salmonella and Norovirus.
Characterisation of food‐borne pathogens up to the optimal discriminatory level by molecular typing methods is very important to link dispersed cases to the same epidemic incident and to trace the implicated food sources. This may be easily achieved, if a functional collaborative network involving peripheral, regional and national reference laboratories is set up (Schjørring et al., 2017), especially in case of multicountry outbreak (Mylius et al., 2018). In the EU, this integrated approach is deemed as critically important and the implementation of intersectoral databases such as the molecular‐based Joint ECDC–EFSA database (Rizzi et al., 2017) is encouraged. In recent years, the implementation of innovative methods for WGS allowed significant advancements in the detection and investigation of FBOs (ECDC, 2016). Many multicountry outbreaks that challenged importantly the EU in recent years (e.g. Listeria in food of non‐animal origin (2017/2018); S. Enteritidis in eggs and egg products (2016/2017)) could only be detected and investigated thanks to the routine application of WGS (Inns et al., 2017). Surveillance and control of FBOs is probably the sector that benefits most and most quickly of the implementation of such an approach. Nevertheless, in 2017, while some MS have definitively completed the switch to WGS routine characterisation of causative agents, others have just started this transition. This lack of harmonisation has consequences on the reporting and interpretation of 2017 FBO data as they may affect both the capability to detect FBOs and the pattern of causative agents detected. Conversely, the increasing use of culture‐independent molecular methods to detect pathogens, especially in peripheral laboratories implies that causative agents may be not available for further typing, given that these methods only allow achieving a diagnosis by the detection of specific molecular markers. This approach may represent another critical element for outbreak surveillance (Huang et al., 2016) especially for food‐borne pathogens (i.e. E. coli or Yersinia) that need to be extensively characterised to establish their pathogenicity.
16.6. Related projects and internet sources
17. Microbiological contaminants (for which food safety criteria are laid down in EU legislation)
This chapter summarises the information provided on the non‐zoonotic microbiological contaminants histamine, Cronobacter sakazakii and staphylococcal enterotoxins in food, in 2017.
Tables and figures that are not presented in this section are published as supporting information to this report and are available in downloadable files at http://doi.org/10.5281/zenodo.1475841
17.1. Histamine
Histamine is an endogenous compound of the human body that can also be added from external sources such as contaminated food. If histamine reaches a critical threshold, it can lead to symptoms such as skin flushing, rash, gastrointestinal complaints and throbbing headache. The Commission Regulation (EC) No. 2073/2005 on microbiological criteria for foodstuffs defines food safety criteria for histamine in food, at retail level, in two major food categories: ‘fish, fishery products from fish species associated with a high amount of histidine’ (Food category 1.25: n = 9; c = 2; m = 100 mg/kg; M = 200 mg/kg) and ‘fish, fishery products which have undergone enzyme maturation treatment in brine’ (Food category 1.26: n = 9; c = 2; m = 200 mg/kg; M = 400 mg/kg).
In 2017, the presence of histamine in ‘fish, fishery products from fish species associated with a high amount of histidine’ was reported at retail by 10 MS (Austria, Belgium, Bulgaria, the Czech Republic, Greece, Portugal, Romania, Slovakia, Slovenia and Spain). In total, 205 batches and 686 single samples were tested of which, respectively, five (2.4% reported by Spain) and 23 (4.1% reported by Austria, Belgium, Greece and Spain) had levels of histamine above 200 mg/kg. At processing plant level, eight MS (Belgium, the Czech Republic, Denmark, Poland, Portugal, Romania, Slovakia and Spain) reported data and only four out of the 1,174 tested samples (batch and single samples) had levels of histamine above 200 mg/kg (from Belgium, Portugal and Spain). In total, 607 samples were taken during border inspection activities by Belgium, Denmark, Romania, Slovenia and the non‐MS (Iceland) and one sample (originating from Vietnam) was reported with levels higher than 200 mg/kg by Denmark.
Four MS (Austria, Poland, Romania and Spain) reported data for the food category ‘fish, fishery products which have undergone enzyme maturation treatment in brine’ sampled at retail, processing plant and/or border inspection level. In total, 25 batch samples and 60 single samples were investigated and one single sample from Austria taken at retail level had a level of histamine above 200 mg/kg.
Member States did not report any data for the category ‘fish sauce produced by fermentation of fishery products’. Only Iceland submitted one negative sample.
Eleven MS (Austria, Belgium, Bulgaria, Croatia, Estonia, France, Ireland, Italy, Latvia, Portugal and Spain) reported on the presence of histamine in other food category than those mentioned above. Out of the 3,899 samples tested, 238 (6.1%) samples (batches and single samples) were tested positive – mainly reported by Italy and from fishery products.
17.2. Staphylococcal enterotoxins
In 2017, single samples of milk, cheese and other dairy products taken within the framework of Regulation 2073/2005 were reported by four MS (the Czech Republic, Portugal, Slovenia and Slovakia). In total 120 samples were tested and none were positive. Most samples were taken at processing stage and mainly milk and whey powder and/or soft or semisoft cheeses made from raw or low heat‐treated milk. Besides the reporting within the framework of Regulation 2073/2005 around 1,800 samples (cheeses and dairy products) were tested in the context of national monitoring and surveillance and or surveys by Cyprus, the Czech Republic, Italy, Romania and Spain. In total, 23 samples were tested positive (1.2%) mainly from Italy (cheese and pasteurised milk) and Spain (milk).
Data on staphylococcal enterotoxins in other food were submitted by five MS (Bulgaria, the Czech Republic, Italy, Slovakia and Spain). Out of the 645 samples tested, 40 were positive. These included two samples from Bulgaria (potato based dishes and RTE pigmeat), seven from Italy (meat preparation, other processed food), 12 from Slovakia (from sandwiches, RTE food, confectionery products and frozen desserts) and 19 from Spain (in bakery products, sauce and dressings, meat‐based dishes, vegetable‐based dishes, RTE salads, other prepared dishes).
Suspect samples were collected by Ireland (ice‐cream, precut vegetables, prepared dishes) and Hungary (noodles and fermented sausages). Only Ireland reported positive samples.
17.3. Cronobacter sakazakii
Cronobacter sakazakii in infant formula and dietary foods for special medical purposes was reported by 14 MS (Austria, Belgium, Cyprus, Croatia, the Czech Republic, Estonia, Germany, Hungary, Ireland, the Netherlands, Portugal, Slovakia, Slovenia and Spain). In total, 1,401 batch (65%) or single samples (35%) were examined and obtained mainly from retail (72%). As in the previous year, at retail level, out of the 1,014 samples only one – a single sample of follow‐on formulas with origin of the Czech Republic – was reported positive. At processing plant level, out of the 387 samples tested 16 were positive: 1 from the Czech Republic, 4 from the Netherlands and 11 from Ireland.
Two EU MS (Austria and Spain) submitted data on Cronobacter in other foods at retail level. No positive samples were found in 40 tested. The Czech Republic and Ireland submitted data on Cronobacter sakazakii in other foods at processing plant level. Out of the 126 samples tested – dairy products (excluding cheeses), milk powder and whey powder – the Czech Republic reported 38 positive batches out of 123 (31%) tested.
Abbreviations
- ADNS
Animal Disease Notification System
- AE
alveolar echinococcosis
- AHAW
EFSA Panel on Animal Health and Welfare
- ARIMA
autoregressive integrated moving average
- BBLV
Bokelogh bat lyssavirus
- BIOHAZ
EFSA Panel on Biological Hazards
- CA
Competent Authorities
- CE
cystic echinococcosis
- cELISA
complement‐enzyme linked immuno sorbent assay
- CFT
complement fixation test
- CFU
colony forming unit
- CI
confidence interval
- CONTAM
EFSA Panel on Contaminants in the Food Chain
- DCF
Data Collection Framework
- DH
definitive host
- EAEC
enteroaggregative E. coli
- EBLV
European bat lyssavirus
- ECDC
European Centre for Disease Prevention and Control
- EEA
European Economic Area
- EFSA
European Food Safety Authority
- EFTA
European Free Trade Association
- EIEC
enteroinvasive E. coli
- ELISA
Enzyme‐linked immunosorbent assay
- EPEC
enteropathogenic E. coli
- ERCE
European Register of Cystic Echinococcosis
- ESRI
Economic and Social Research Institute
- ETEC
enterotoxigenic E. coli
- EU
European Union
- EU‐FORS
European Union Food‐borne reporting System
- EURL
European Union Reference Laboratory
- EVD
Emerging and Vector‐borne Diseases
- EVD‐LabNet
Emerging Viral Diseases‐Expert Laboratory Network
- FAT
fluorescent antibody test
- FBO
food‐borne outbreak
- FBOp
food business operator
- FDA
Food and Drug Administration
- FoodNet
Food‐borne Diseases Active Surveillance Network
- FSC
food safety criteria
- FWD‐Net
European Food‐ and Waterborne Diseases and Zoonoses Network
- GAP
Good Agricultural Practices
- GHP
Good Hygiene Practices
- GMP
Good Manufacturing Practices
- HACCP
hazard analysis and critical control point
- HUS
haemolytic uraemic syndrome
- i‐ELISA
indirect enzyme‐linked immunosorbent assay
- IFA
immunofluorescence assay
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IH
intermediate host
- IHC
immunohistochemistry
- ISO
International Organization for Standardization
- LAT
Latex agglutination test
- LHT
low heat‐treated
- KBLV
Kotalahti bat lyssavirus
- LLEBV
Lleida bat lyssavirus
- MLST
multilocus sequence typing
- MALDI‐TOF‐MS
matrix‐assisted laser desorption/ionisation, time‐of‐flight mass spectrometry
- MS
Member State
- NCEZID
National Center for Emerging and Zoonotic Infectious Diseases
- NCP
National Control Programmes
- NMKL
Nordic Committee on Food Analysis
- NRCHC
not raised under controlled housing conditions
- NT
not typeable
- OBF
official brucellosis free in cattle
- ObmF
official Brucella melitensis free in sheep and goats
- OF
Officially Free
- OIE
World Organisation for Animal Health
- OTF
official tuberculosis‐free in cattle
- PCR
polymerase chain reaction
- PHC
process hygiene criteria
- RTE
ready‐to‐eat
- RASFF
EU Rapid Alert System for Food and Feed
- RT‐PCR
reverse transcriptase‐polymerase chain reaction
- STEC
Shiga toxin‐producing Escherichia coli
- TB
tuberculosis
- TBE
tick‐borne encephalitis virus
- TESSy
The European Surveillance System
- UHT
Ultra‐high Temperature
- VNT
virus neutralisation test
- VTEC
verotoxigenic Escherichia coli
- WAHIS
World Animal Health Information Database
- WCBV
West Caucasian bat virus
- WGS
whole genome sequencing
- WHO
World Health Organization
- WNF
West Nile fever
- WNV
West Nile virus
Country codes
- Albania
AL
- Austria
AT
- Belgium
BE
- Bosnia and Herzegovina
BA
- Bulgaria
BG
- Croatia
HR
- Cyprus
CY
- Czech Republic
CZ
- Denmark
DK
- Estonia
EE
- Finland
FI
- The Former Yugoslav Republic of Macedonia
MK
- France
FR
- Germany
DE
- Greece
EL
- Hungary
HU
- Iceland
IS
- Ireland
IE
- Italy
IT
- Latvia
LV
- Liechtenstein
LI
- Lithuania
LT
- Luxembourg
LU
- Montenegro
ME
- Malta
MT
- Netherlands
NL
- Norway
NO
- Poland
PL
- Portugal
PT
- Romania
RO
- Serbia
RS
- Slovakia
SK
- Slovenia
SI
- Spain
ES
- Sweden
SE
- Switzerland
CH
- United Kingdom
UK
Appendix A – Details on occurrence of Listeria monocytogenes in main ready‐to‐eat (RTE) food matrices in 2017
1.
Occurrence of L. monocytogenes in main ready‐to‐eat (RTE) food matrices in 2017 and 2016. For each food category the number of tested samples (using the detection method) and the percentage (%) of positive samples are shown. The total number of tested samples as well as total number of positive samples is calculated over all reported sampling stages.
| RTE food category | Food subcategories | Sampling unit | 2016 | 2017 | ||
|---|---|---|---|---|---|---|
| Number of tested samples | % of positive samples | Number of tested samples | % of positive samples | |||
| Fish and fishery products | Fish | Batch | 373 | 4.0 | 549 | 0.9 |
| Single | 1,845 | 4.8 | 4,706 | 7.6 | ||
| Fishery products | Batch | 441 | 7.0 | 284 | 0.0 | |
| Single | 288 | 3.5 | 1,191 | 3.0 | ||
| Milk | Pasteurised | Batch | 123 | 0 | 1,852 | 3.0 |
| Single | 568 | 0 | ||||
| UHT a | Batch | 15 | 0 | 8 | 0.0 | |
| Single | 14 | 0 | ||||
| Raw, intended for direct human consumption | Batch | 10 | 0 | 199 | 2.0 | |
| Single | 238 | 2.9 | ||||
| Hard cheeses from pasteurised milk | From cow milk | Batch | 466 | 0 | 3,011 | 0.0 |
| Single | 608 | 0.8 | 732 | 0.03 | ||
| From goat milk | Batch | 67 | 0 | 5 | 0.0 | |
| Single | 11 | 0 | 46 | 0.0 | ||
| From sheep milk | Batch | 114 | 0 | 15 | 0.0 | |
| Single | 5 | 0 | 4 | 0.0 | ||
| Hard cheeses from raw milk | From cow milk | Batch | 193 | 0 | 615 | 0.0 |
| Single | 231 | 2.2 | 90 | 2.2 | ||
| From goat milk | Batch | 2 | 0 | – | – | |
| Single | 5 | 0 | 5 | 0.0 | ||
| From sheep milk | Batch | 1 | 0 | 3 | 0.0 | |
| Single | 50 | 0 | 7 | 14.3 | ||
| Soft and semi‐soft cheeses from pasteurised milk | From cow milk | Batch | 779 | 0.6 | 1,124 | 0.0 |
| Single | 1,852 | 0.2 | 2,487 | 0.78 | ||
| From goat milk | Batch | 60 | 0 | 181 | 0.0 | |
| Single | 88 | 0 | 410 | 0.0 | ||
| From sheep milk | Batch | 113 | 0 | – | – | |
| Single | 74 | 1.4 | – | – | ||
| Soft and semi‐soft cheeses from raw milk | From cow milk | Batch | 43 | 2.3 | 72 | 0.0 |
| Single | 416 | 2.9 | 514 | 1.7 | ||
| From goat milk | Batch | 30 | 0 | 2 | 0.0 | |
| Single | 37 | 0 | 71 | 0.0 | ||
| From sheep milk | Batch | 170 | 4.7 | – | – | |
| Single | 111 | 0 | 843 | 3.1 | ||
| Meat products | From bovine animals | Batch | 379 | 0.5 | 267 | 3.0 |
| Single | 1,067 | 0.8 | 260 | 0.4 | ||
| From broilers | Batch | 207 | 0 | 243 | 0.0 | |
| Single | 891 | 1.0 | 430 | 2.6 | ||
| From turkeys | Batch | 27 | 0 | 27 | 0.0 | |
| Single | 294 | 1.7 | 250 | 0.8 | ||
| From pigs | Batch | 1,214 | 3.6 | 1,488 | 3.0 | |
| Single | 9,747 | 3.0 | 19,579 | 1.7 | ||
| Other RTE products | Salads | Single + Batch | 1,042 | 2.0 | 902 | 4.2 |
| Bakery products | Single + Batch | 1,984 | 0.8 | 3,600 | 7.8 | |
| Fruits and Vegetables | Single + Batch | 1,772 | 0.5 | 1,773 | 0.6 | |
| Sauces and dressings | Single + Batch | 299 | 0.3 | 184 | 1.6 | |
| Egg products | Single + Batch | 72 | 0 | 3 | 0.0 | |
| Confectionery products and pastes | Single + Batch | 154 | 0.6 | 10 | 0.0 | |
| Spices and herbs | Single + Batch | 48 | 0 | 45 | 0.0 | |
| Prepared dishes | Single + Batch | 646 | 0.3 | 441 | 1.4 | |
Ultra‐high temperature processing.
Suggested citation: EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) , 2018. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2017. EFSA Journal 2018;16(12):5500, 262 pp. 10.2903/j.efsa.2018.5500
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00751
Acknowledgements: EFSA and the ECDC wish to thank the members of the EFSA Scientific Network for Zoonoses Monitoring Data and of the ECDC Food and Waterborne Diseases and Zoonoses Network, the ECDC Emerging and Vector‐borne Diseases Network and the ECDC Tuberculosis Network, who provided the data and reviewed the report; the members of the Scientific Network for Zoonoses Monitoring Data for their endorsement of this scientific report; the EFSA staff members (Frank Boelaert, Yves Van der Stede, Anca Stoicescu, Giusi Amore, Krisztina Nagy, Valentina Rizzi, Maria Teresa Da Silva Felicio, Winy Messens, Angel Ortiz Pelaez, Michaela Hempen, Eleonora Sarno, Daniel Thomas and Frank Verdonck), the ECDC staff members (Taina Niskanen, Joana Haussig, Hanna Merk and Joana Gomes Dias) and the EFSA contractors: the Istituto Zooprofilattico Sperimentale delle Venezie, Italy (and staff members: Lisa Barco, Marzia Mancin, Ilaria Patuzzi, Antonia Anna Lettini, Alessandra Longo, Carmen Losasso and Antonia Ricci), the Istituto Superiore di Sanita, Italy (and staff members: Stefano Morabito, Gaia Scavia, Arnold Knijn, Rosangela Tozzoli, Ornella Moro, Monica Gianfranceschi, Elisabetta Suffredini, Ilaria Di Bartolo, Elisabetta Delibato, Fabrizio Anniballi, Giovanni Ianiro and Antonella Maugliani), the European Union Reference Laboratory for Parasites (and staff members: Edoardo Pozio and Adriano Casulli), the WHO Collaborating Centre for the Epidemiology, Detection and Control of Cystic and Alveolar Echinococcosis (and staff member: Adriano Casulli), and the European Union Reference Laboratory for Listeria monocytogenes (the French agency for food, environmental and occupational health safety (ANSES) and staff members: L. Guillier, B. Felix and B. Lombard), for the support provided to this scientific report.
Approved: 19 November 2018
Amended: 18 February 2019
Notes
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L 325, 12 December 2003, p. 31–40.
EFSA Registry of Questions: http://raw-app.efsa.eu.int:8080/raw-war/wicket/page?2
Decision No. 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross‐border threats to health and repealing Decision No. 2119/98/EC. OJ L 293, 5 November 2013, p. 1–15.
Commission Decision 2012/506/EU amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the European Union network under Decision No. 2119/98/EC of the European Parliament and of the Council. OJ L 262, 27 September 2012, p. 1–57.
Based on the customs union treaty of the Principality of Liechtenstein with Switzerland, Liechtenstein is part of the Swiss customs territory. Due to the tight connection between the veterinary authorities of Liechtenstein and Switzerland as well as Liechtenstein's integration into the Swiss system in the veterinary field, in principle, all legislation, rules and data on contagious diseases are identical for both Switzerland and Liechtenstein. If not mentioned otherwise, the Swiss data include also the data from Liechtenstein.
Commission Regulation (EU) 2017/1495 of 23 August 2017 amending Regulation (EC) No. 2073/2005 as regards Campylobacter in broiler carcases. OJ L 218, 24.8.2017, p. 1–6.
Commission Regulation (EU) No 1086/2011 of 27 October 2011 amending Annex II to Regulation (EC) No 2160/2003 of the European Parliament and of the Council and Annex I to Commission Regulation (EC) No 2073/2005 as regards salmonella in fresh poultry meat. OJ L 281, 28.10.2011, p. 7–11.
Also known as verotoxigenic, verocytotoxigenic, verotoxin‐producing, verocytotoxin‐producing E. coli (VTEC).
Decision 2012/506/EU. Commission implementing Decision of 8 August 2012 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the European Union network under Decision No 2119/98/EC of the European Parliament and of the Council. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:262:0001:0057:EN:PDF
ADNS, the EU Animal Disease Notification System, see http://ec.europa.eu/food/animals/animal-diseases/not-system_en
During 2017, Austria was an OTF MS for all its regions and also covered by an EU cofinanced eradication programme for some single regions.
During 2009, 2011, 2012, 2013, 2014 and 2015, Greece had a cofinanced programme.
Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015 laying down specific rules on official controls for Trichinella in meat (Text with EEA relevance). OJ L 212, 11.8.2015, pp. 7–34.
Regulation (EU) No. 652/2014 of the European Parliament and of the Council of 15 May 2014 laying down provisions for the management of expenditure relating to the food chain, animal health and animal welfare, and relating to plant health and plant reproductive material, amending Council Directives 98/56/EC, 2000/29/EC and 2008/90/EC, Regulations (EC) No. 178/2002, (EC) No. 882/2004 and (EC) No. 396/2005 of the European Parliament and of the Council, Directive 2009/128/EC of the European Parliament and of the Council and Regulation (EC) No. 1107/2009 of the European Parliament and of the Council and repealing Council Decisions 66/399/EEC, 76/894/EEC and 2009/470/EC OJ L 189, 27.6.2014, pp. 1–32.
Commission Implementing Regulation (EU) No. 577/2013 of 28 June 2013 on the model identification documents for the non‐commercial movement of dogs, cats and ferrets, the establishment of lists of territories and non‐EU countries and the format, layout and language requirements of the declarations attesting compliance with certain conditions provided for in Regulation (EU) No. 576/2013 of the European Parliament and of the Council.OJ L 178, 28.6.2013, pp. 109–148.
Working document SANCO/10181/2014 Rev7 Guidelines for the Union cofunded programmes of eradication, control and surveillance of animal diseases and zoonoses for the years 2015–2017: https://ec.europa.eu/food/sites/food/files/safety/docs/cff_animal_vet-progs_guidance_progs_erad_2017.pdf
World Organisation for Animal Health (OIE). World Animal Health Information Database (WAHIS) Interface. Available from: http://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home
Greece reported also to perform for West Nile laboratory analyses; competition multispecies ELISA, IgM‐capture ELISA (MAC‐ELISA) and real‐time PCR.
Low specificity of IgG ELISA kits low specificity of IgG ELISA kits meant that it could detect animals infected with other flaviviruses (Beck C, Lowenski S, Durand B, Bahuon C, Zientara S, Lecollinet S (2017) Improved reliability of serological tools for the diagnosis of West Nile fever in horses within Europe. PLoS Negl Trop Dis 11(9): e0005936).
Table FBOEVID2017 of the Appendix.
Table 2017_FBOOVERVIEW.
Table: 2017_NOOUTHUM.
Table 2017_NOFBOAGENT.
Figure 2017_FBO _RR_CASES.
Table 2017_FBOSALM.
Table 2017_NOFBOSALMSEROV.
Table: 2017_FBOSALMSE.
Table: 2017_FBOSALMSTM.
Table: 2017_FBOCAMP.
Table: 2017_FBOLISTERIA.
Table: 2017_FBOSTEC.
Tables: 2017_FBOOTHERBACT; 2017_FBOSTROTHBACT and 2017_FBOWEAKOTHBACT.
Table : 2017_FBOBRUC.
Table: 2017_FBOSTEC.
Table: 2017_FBOYERSIN.
Table: 2017_FBOCLOSTOX.
Table: 2017_FBOSTAPH.
Table: 2017_FBOBACIL.
Table: 2017_FBOBOT.
Table: 2017_FBOCALICIVIRUS.
Table: 2017_FBOHEPA.
Table : 2017_FBOHEPE.
Table: 2017_FBOOTHERVIRUS.
Table: 2017_FBOTRICH.
Table: 2017_FBOCRYPT.
Table: 2017_FBOWEAKPARAS and 2017_FBOSTRPARAS.
Table: 2017_FBOHIST.
Table: 2017_FBOSTROTHER and 2017_FBOWEAKOTHER.
Table : 2017_FBOUNKNOWN.
2017_FBOEVID.
Figure 2017_FBO_FACTOR.
2017_WATER.
Table 2017_FBOWATERWEAK.
References
- Alban L, Pozio E, Boes J, Boireau P, Boue F, Claes M, Cook AJC, Dorny P, Enemark HL, van der Giessen J, Hunt KR, Howell M, Kirjusina M, Noeckler K, Rossi P, Smith GC, Snow L, Taylor MA, Theodoropoulos G, Vallee I, Viera‐Pinto MM and Zimmer IA, 2011. Towards a standardised surveillance for Trichinella in the European Union. Preventive Veterinary Medicine, 99, 148–160. 10.1016/j.prevetmed.2011.02.008 [DOI] [PubMed] [Google Scholar]
- Arechiga‐Ceballos N, Vazquez Moron S, Berciano JM, Nicolas O, Aznar Lopez C, Juste J, Rodriguez Nevado C, Aguilar Setien A and Echevarria JE, 2013. Novel lyssavirus in bat, Spain. Emerging Infectious Diseases, 19, 793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroussi A, Vignoles P, Dalmay F, Wimel L, Darde M‐L, Mercier A and Ajzenberg D, 2015. Detection of Toxoplasma gondii DNA in horse meat from supermarkets in France and performance evaluation of two serological tests. Parasite, 22, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck R, Mihaljevic Z, Brezak R, Bosnic S, Jankovic IL and Deplazes P, 2018. First detection of Echinococcus multilocularis in Croatia. Parasitology Research, 117, 617–621. 10.1007/s00436-017-5732-3 [DOI] [PubMed] [Google Scholar]
- Bless PJ, SchmutzKathrin C, Suter K, Jost M, Hattendorf J, Mäusezahl‐Feuz M and Mäusezah D, 2014. A tradition and an epidemic: determinants of the campylobacteriosis winter peak in Switzerland. European Journal of Epidemiology, 29, 527 10.1007/s10654-014-9917-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert F, Amore G, Van der Stede Y and Hugas M, 2016. EU‐wide monitoring of biological hazards along the food chain: achievements, challenges and EFSA vision for the future. Current Opinion in Food Science, 12, 52–62. 10.1016/j.cofs.2016.08.004 [DOI] [Google Scholar]
- Brown LG, Hoover ER, Selman CA, Coleman EW and Rogers HS, 2017. Outbreak characteristics associated with identification of contributing factors to foodborne illness outbreaks. Epidemiology and Infection, 145, 2254–2262. 10.1017/s0950268817001406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundu D, Piseddu T, Stegel G, Masu G, Ledda S and Masala G, 2015. Retrospective study of human cystic echinococcosis in Italy based on the analysis of hospital discharge records between 2001 and 2012 (vol. 140, p. 91, 2014). Acta Tropica, 146, 158–158. 10.1016/j.actatropica.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Buchanan RL, Gorris LGM, Hayman MM, Jackson TC and Whiting RC, 2018. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose–response, ecology, and risk assessments (vol. 75, p. 1, 2017). Food Control, 88, 236–236. 10.1016/j.foodcont.2018.01.004 [DOI] [Google Scholar]
- Carpentier B and Cerf O, 2011. Review — Persistence of Listeria monocytogenes in food industry equipment and premises. International Journal of Food Microbiology, 145, 1–8, ISSN 0168‐1605. 10.1016/j.ijfoodmicro.2011.01.005 ( http://www.sciencedirect.com/science/article/pii/S0168160511000122) [DOI] [PubMed] [Google Scholar]
- van Cauteren D, Millon L, de Valk H and Grenouillet F, 2016. Retrospective study of human cystic echinococcosis over the past decade in France, using a nationwide hospital medical information database. Parasitology Research, 115, 4261 10.1007/s00436-016-5204-1 [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention), 2011. Multistate outbreak of listeriosis linked to whole cantaloupes from Jensen farms, Colorado (final update). Available online: http://www.cdc.gov/listeria/outbreaks/cantaloupes-jensen-farms/index.html
- CDC (Centers for Disease Control and Prevention), 2015a. Multistate outbreak of listeriosis linked to commercially produced, prepackaged caramel apples made from bidart bros. Apples (final update). Available at: http://www.cdc.gov/listeria/outbreaks/caramel-apples-12-14/index.html
- CDC (Centers for Disease Control and Prevention), 2015b. Sprouts and investigation of human listeriosis cases (final update). Wholesome Soy Products, Inc.. Available at: http://www.cdc.gov/listeria/outbreaks/bean-sprouts-11-14/index.html
- CEN (Comité Européen de Normalisation), 2004a. Microbiology of food and animal feeding stuffs. Horizontal method for the detection and enumeration of Listeria monocytogenes. Part 1. Detection method. Amendment 1. Modification of the isolation media and the haemolysis test, and inclusion of precision data. ISO 11290–1:1996/Amd 1:2004. European Standard EN ISO 11290–1/A1, 15 pp.
- CEN (Comité Européen de Normalisation), 2004b. Microbiology of food and animal feeding stuffs. Horizontal method for the detection and enumeration of Listeria monocytogenes. Part 2. Enumeration method. Amendment 1. Modification of the enumeration medium. ISO 11290–2:1998/FDAM 1:2004. European Standard EN ISO 11290–2/A1. 7 pp.
- CEN (Comité Européen de Normalisation), 2017. Microbiology of food and animal feeding stuffs – horizontal method for the detection of Escherichia coli O157 – amendment 1: annex B: result of interlaboratory studies (ISO 16654:2001/Amd 1:2017). European Standard EN ISO 16654/A1, 3 pp.
- Clayton C and Hills M, 1993. Statistical Models in Epidemiology. ISBN 0‐19‐852221‐5. Oxford University Press, New York, 367 pp. [Google Scholar]
- Conraths FJ and Deplazes P, 2015. Echinococcus multilocularis: epidemiology, surveillance and state‐of‐the‐art diagnostics from a veterinary public health perspective. Veterinary Parasitology, 213, 149–161. 10.1016/j.vetpar.2015.07.027 [DOI] [PubMed] [Google Scholar]
- Davidson JA, Loutet MG, O'Connor C, Kearns C, Smith RMM, Lalor MK, Thomas HL, Abubakar I and Zenner D, 2017. Epidemiology of Mycobacterium bovis disease in humans in England, Wales, and Northern Ireland, 2002–2014. Emerging Infectious Diseases, 23, 377–386. 10.3201/eid2303.161408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplazes P, Hegglin D, Gloor S and Romig T, 2004. Wilderness in the city: the urbanization of Echinococcus multilocularis . Trends in Parasitology, 20, 77–84. 10.1016/j.pt.2003.11.011 [DOI] [PubMed] [Google Scholar]
- Di Bonaventura G, Piccolomini R, Paludi D, D'Orio V, Vergara A, Conter M and Ianieri A, 2008. Influence of temperature on biofilm formation by Listeria monocytogenes on various food‐contact surfaces: relationship with motility and cell surface hydrophobicity. Journal of Applied Microbiology, 104, 1552–1561. 10.1111/j.1365-2672.2007.03688.x [DOI] [PubMed] [Google Scholar]
- DIN (Deutsches Institut Fur Normung E.V. ‐German National Standard‐), 2004. DIN 10167:2004 ‐03. Nachweis von Escherichia coli O157 in Fleisch und Fleischerzeugnissen. Detection of Escherichia coli O157 in meat and meat products.
- Doijad SP, Barbuddhe SB, Garg S, Poharkar KV, Kalorey DR, Kurkure NV, et al., 2015. Biofilm‐Forming Abilities of Listeria monocytogenes Serotypes Isolated from Different Sources. PLoS ONE, 10, e0137046 10.1371/journal.pone.0137046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer M, Aguilar‐Bultet L, Rupp S, Guldimann C, Stephan R, Schock A, Otter A, Schüpbach G, Brisse S, Lecuit M, Frey J and Oevermann A, 2016. Listeria monocytogenes sequence type 1 is predominant in ruminant rhombencephalitis. Scientific Reports, 6, 36419 10.1038/srep36419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryselius R, 2017. Extensive spread of Campylobacter infection in Sweden 2016‐2017. EURL ‐ Campylobacter workshop 20170914‐15, Nantes, France. Available online: https://www.sva.se/globalassets/redesign2011/pdf/om_sva/eurl-campylobacter/camp-sweden-rd-2017.pdf
- ECDC (European Centre for Disease Prevention and Control), 2016. Expert opinion on whole genome sequencing for public health surveillance. ECDC, Stockholm. [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control), 2017a. Re‐emerging multi‐country WGS‐defined outbreak of Salmonella Enteritidis, MLVA type 2‐12‐7‐3‐2 and 2‐14‐7‐3‐2, 3 February 2017. 6 pp. Available online: https://ecdc.europa.eu/en/publications-data/re-emerging-multi-country-wgs-defined-outbreak-salmonella-enteritidis-mlva-type-2
- ECDC (European Centre for Disease Prevention and Control), 2017b. Multi‐country outbreak of Salmonella Enteritidis phage types 56 and 62, MLVA profile 2‐11‐3‐3‐2 and 2‐12‐3‐3‐2 infections 20 July 2017. 7 pp. Available online: https://ecdc.europa.eu/sites/portal/files/documents/rapid-risk-assessment-multi-country-outbreak-Salmonella-Enteritidis.pdf
- ECDC (European Centre for Disease Prevention and Control), 2018. West Nile fever data 2017. Available online: https://ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc
- EFSA (European Food Safety Authority), 2007. Scientific Opinion of the Panel on Biological Hazards on a request from EFSA on Surveillance and monitoring of Toxoplasma in humans, foods and animals. EFSA Journal 2007;5(12):583, 64 pp. 10.2903/j.efsa.2007.583 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009a. Statistical analysis of temporal and spatial trends of zoonotic agents in animals and food Part I: critical review of the statistical analysis carried out on the Community Summary Report 2006 data. EFSA Journal 2009;7(5):RN‐253, 77 pp. 10.2903/j.efsa.2009.253r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009b. Technical specifications for the monitoring and reporting of verotoxigenic Escherichia coli (VTEC) on animals and food (VTEC surveys on animals and food). EFSA Journal 2009;7(11):1366, 43 pp. 10.2903/j.efsa.2009.1366 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. The Community Summary Report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in the European Union in 2008. EFSA Journal 2010;8(1):1496, 410 pp. 10.2903/j.efsa.2010.1496 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Statistical analysis of temporal and spatial trends of zoonotic agents in animals and food Part II: applications of spatial analysis and further developments of temporal analysis. EFSA Journal 2011;9(8):2331, 72 pp. 10.2903/j.efsa.2011.2331 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. Analysis of the baseline survey on the prevalence of Listeria monocytogenes in certain ready‐to‐eat (RTE) foods in the EU, 2010‐2011 Part A: Listeria monocytogenes prevalence estimates. EFSA Journal 2013;11(6):3241, 75 pp. 10.2903/j.efsa.2013.324 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014a. Analysis of the baseline survey on the prevalence of Listeria monocytogenes in certain ready‐to‐eat foods in the EU, 2010‐2011. Part B: analysis of factors related to prevalence and exploring compliance. EFSA Journal 2014;12(8):3810, 73 pp. 10.2903/j.efsa.2014.381 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014b. Update of the technical specifications for harmonised reporting of food‐borne outbreaks through the European Union reporting system in accordance with Directive 2003/99/EC. EFSA Journal 2014;12(3):3598, 25 pp. 10.2903/j.efsa.2014.3598 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017a. Manual for reporting on zoonoses and zoonotic agents, within the framework of Directive 2003/99/EC, and on some other pathogenic microbiological agents for information deriving from the year 2016. EFSA supporting publication 2017:14(1):EN‐1175, 98 pp. 10.2903/sp.efsa.2017.en-1175 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017b. Assessment of the incidents of histamine intoxication in some EU countries. EFSA supporting publication 2017;14(9):EN‐1301, 37 pp. 10.2903/sp.efsa.2017.en-1301 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017c. Stakeholder meeting on the draft Scientific Opinion on Listeria monocytogenes contamination of ready‐to‐eat foods and the risk for human health in the EU. EFSA supporting publication 2017;14(12):EN‐1343, 18 pp. 10.2903/sp.efsa.2017.en-1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Bocca V, Boelaert F, Riolo F, Rizzi V, van der Stede Y and Stoicescu AV, 2018a. Guidelines for reporting 2017 prevalence sample‐based data in accordance with SSD2 data model. EFSA supporting publication 2018;15(3):EN‐1391, 38 pp. 10.2903/sp.efsa.2018.en-1391 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Boelaert F, Rizzi V, dervan Stede Y and Stoicescu AV, 2018b. Manual for reporting on zoonoses and zoonotic agents, within the framework of Directive 2003/99/EC, and on some other pathogenic microbiological agents for information derived from the year 2017. EFSA supporting publication 2018;15(1):EN‐1370, 61 pp. 10.2903/sp.efsa.2018.en-1370. [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Mulligan K and Stoicescu AV, 2018c. User manual for mapping Member State zoonoses standard terminology to EFSA standard terminology for information derived from the year 2017. EFSA supporting publication 2018;15(1):EN‐1371, 25 pp. 10.2903/sp.efsa.2018.en-1371 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Bocca V, Boelaert F, Beloeil P‐A, Guerra B, derVan Stede Y and Stoicescu A‐V, 2018d. Data dictionaries—guidelines for reporting 2017 data on zoonoses, antimicrobial resistance and food‐borne outbreaks. EFSA supporting publication 2018;EN‐1368, 107 pp. 10.2903/sp.efsa.2018.en-1368 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2007. Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) regarding the assessment of the risk of Echinococcosis introduction into the UK, Ireland, Sweden, Malta and Finland as a consequence of abandoning national rules. EFSA Journal 2007;5(1):441, 59 pp. 10.2903/j.efsa.2007.441 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2014. Statement on a conceptual framework for bovine tuberculosis. EFSA Journal 2014;12(5):3711, 59 pp. 10.2903/j.efsa.2014.3711 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2015. Scientific opinion on Echinococcus multilocularis infection in animals. EFSA Journal 2015;13(12):4373, 129 pp. 10.2903/j.efsa.2015.4373 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013a. Scientific opinion on the public health hazards to be covered by inspection of meat (solipeds). EFSA Journal 2013;11(6):3263, 161 pp. 10.2903/j.efsa.2013.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013b. Scientific Opinion on the public health hazards to be covered by inspection of meat from sheep and goats. EFSA Journal 2013;11(6):3265, 186 pp. 10.2903/j.efsa.2013.3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel, EFSA CONTAM Panel and EFSA AHAW Panel (EFSA Panel on Biological Hazards EFSA Panel on Contaminants in the Food Chain and EFSA Panel on Animal Health and Welfare), 2011. Scientific Opinion on the public health hazards to be covered by inspection of meat (swine). EFSA Journal 2011;9(10):2351, 198 pp. 10.2903/j.efsa.2011.2351 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernandez Escamez PS, Herman L, Koutsoumanis K, Lindqvist R, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlström H, Di Bartolo I, Johne R, Pavio N, Rutjes S, van der Poel W, Vasickova P, Hempen M, Messens W, Rizzi V, Latronico F and Girones R, 2017. Scientific Opinion on the public health risks associated with hepatitis E virus (HEV) as a food‐borne pathogen. EFSA Journal 2017;15(7):4886, 89 pp. 10.2903/j.efsa.2017.4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Allende A, Alvarez‐Ordonez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Caccio S, Chalmers R, Deplazes P, Devleesschauwer B, Innes E, Romig T, van der Giessen J , Hempen M, Van der Stede Y and Robertson L, 2018d. Public health risks associated with foodborne parasites. EFSA Journal 2018;16(11):5495, 110 pp. 10.2903/j.efsa.2018.5495 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2011. The European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2009. EFSA Journal 2014;9(3):2090, 378 pp. 10.2903/j.efsa.2011.2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2015a. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2013. EFSA Journal 2015;13(1):3991, 165 pp. 10.2903/j.efsa.2015.3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2015b. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2014. EFSA Journal 2015;13(12):4329, 191 pp. 10.2903/j.efsa.2015.4329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2016a. Multi‐country outbreak of Salmonella Enteritidis phage type 8, MLVA type 2–9‐7–3‐2 and 2–9‐6–3‐2 infections. EFSA supporting publication 2016;13(10):EN‐1110, 20 pp. 10.2903/sp.efsa.2016.en-1110. [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2016b. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2015. EFSA Journal 2016;14(12):4634, 231 pp. 10.2903/j.efsa.2016.4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017a. Multi‐country outbreak of Salmonella Enteritidis infections linked to Polish eggs. EFSA supporting publication 2017;14(12):EN‐1353, 21 pp. 10.2903/sp.efsa.2017.en-1353 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017b. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2016. EFSA Journal 2017;15(12):5077, 228 pp. 10.2903/j.efsa.2017.5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority European Centre for Disease Prevention and Control), 2017c. Multi‐country outbreak of new Salmonella enterica 11:z41:e,n,z15 infections associated with sesame seeds. EFSA supporting publication 2017;14(6):EN‐1256, 10 pp. 10.2903/sp.efsa.2017.EN-1256 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018a. Multi‐country outbreak of Salmonella Agona infections linked to infant formula. EFSA supporting publication 2018;15(1):EN‐1365, 11 pp. 10.2903/sp.efsa.2018.en-1365 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018b. Multi‐country outbreak of Listeria monocytogenes serogroup IVb, multi‐locus sequence type 6, infections linked to frozen corn and possibly to other frozen vegetables – first update. EFSA supporting publication 2018;15(7):EN‐1448, 22 pp. 10.2903/sp.efsa.2018.en-1448 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018c. Multi‐country outbreak of Salmonella Agona infections possibly linked to ready‐to‐eat food. EFSA supporting publication 2018;15(7):EN‐1465, 15 pp. 10.2903/sp.efsa.2018.en-1465 [DOI] [Google Scholar]
- European Commission , 2013. Overview report of a series of audits carried out in the Member States in order to evaluate the implementation of measures taken to detect and control Salmonella in specific poultry populations 2006–2012. Available online: https://ec.europa.eu/food/fvo/specialreports/act_getPDF.cfm?PDF_ID=135
- European Commission , 2015. Final report of an audit carried out in Slovakia from 24 November 2015 to 03 December 2015 in order to evaluate the Salmonella national control programmes for particular poultry populations (breeders, laying hens, broilers and turkeys. Available online: https://ec.europa.eu/food/audits-analysis/act_getPDF.cfm?PDF_ID=12631
- European Commission , 2018. RASFF – the rapid alert system for food and feed: 2017 annual report. 60 pp. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/rasff_annual_report_2017.pdf
- Fagerlund A, Langsrud S, Schirmer BC, Møretrø T and Heir E, 2016. Genome analysis of Listeria monocytogenes sequence type 8 strains persisting in Salmon and poultry processing environments and comparison with related strains. PLoS ONE, 11, e0151117 10.1371/journal.pone.0151117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlund A, Møretrø T, Heir E, Briandet R and Langsrud S, 2017. Cleaning and disinfection of biofilms composed of Listeria monocytogenes and background microbiota from meat processing surfaces. Applied and Environmental Microbiology, pii: AEM.01046‐17. 10.1128/AEM.01046-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CR, Streicker DG and Schnell MJ, 2018. The spread and evolution of rabies virus: conquering new frontiers. Nature Reviews Microbiology, 16, 241–255. 10.1038/nrmicro.2018.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkhalsomyndigheten , 2017. https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistikdatabaser-och-visualisering/sjukdomsstatistik/campylobacterinfektion/arsrapporter-och-kommentarer/2017/
- Friesema I, van der Zwaluw K, Schuurman T, Kooistra‐Smid M, Franz E, van Duynhoven Y and van Pelt W, 2014. Emergence of Escherichia coli encoding Shiga toxin 2f in human Shiga toxin‐producing E. coli (STEC) infections in the Netherlands, January 2008 to December 2011. EuroSurveillance, 19, 26–32. 10.2807/1560-7917.es2014.19.17.20787 [DOI] [PubMed] [Google Scholar]
- Fritsch F, Mauder N, Williams T, Weiser J, Oberle M and Beier D, 2011. The cell envelope stress response mediated by the LiaFSR Lm three‐component system of Listeria monocytogenes is controlled via the phosphatase activity of the bifunctional histidine kinase LiaS. Microbiology, 157, 373–386. 10.1099/mic.0.044776-0 [DOI] [PubMed] [Google Scholar]
- Garofolo G, Fasanella A, Di Giannatale E, Platone I, Sacchini L, Persiani T, Boskani T, Rizzardi K and Wahab T, 2016. Cases of human brucellosis in Sweden linked to Middle East and Africa. BMC Research Notes, 9, 277–277. 10.1186/s13104-016-2074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi E, Walter MC, Pfalzgraf M‐T, Northoff BH, Holdt LM, Scholz HC, Zoeller L, Zange S and Antwerpen MH, 2017. Whole genome sequencing of Brucella melitensis isolated from 57 patients in Germany reveals high diversity in strains from Middle East. PLoS ONE, 12 10.1371/journal.pone.0175425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev M, Afonso A, Neubauer H, Needham H, Thiery R, Rodolakis A, Roest H, Stark K, Stegeman J, Vellema P, dervan Hoek W and More S, 2013. Q fever in humans and farm animals in four European countries, 1982 to 2010. EuroSurveillance, 18. [PubMed] [Google Scholar]
- Gossner CM, Marrama L, Carson M, Allerberger F, Calistri P, Dilaveris D, Lecollinet S, Morgan D, Nowotny N, Paty M, Pervanidou D, Rizzo C, Roberts H, Schmoll F, Van Bortel W and Gervelmeyer A, 2017. West Nile virus surveillance in Europe: moving towards an integrated animal‐human‐vector approach. EuroSurveillance, 22, 10–19. 10.2807/1560-7917.es.2017.22.18.30526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande L, Michelacci V, Bondi R, Gigliucci F, Franz E, Badouei MA, Schlager S, Minelli F, Tozzoli R, Caprioli A and Morabito S, 2016. Whole‐genome characterization and strain comparison of VT2f‐producing Escherichia coli causing hemolytic uremic syndrome. Emerging Infectious Diseases, 22, 2078–2086. 10.3201/eid2212.160017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn‐Moore D, 2014. Feline tuberculosis caused by Mycobacterium bovis . Veterinary Record, 174, 322–323. 10.1136/vr.g2338 [DOI] [PubMed] [Google Scholar]
- Gymoese P, Sorensen G, Litrup E, Olsen JE, Nielsen EM and Torpdahl M, 2017. Investigation of outbreaks of Salmonella enterica serovar Typhimurium and its monophasic variants using whole‐genome sequencing, Denmark. Emerging Infectious Diseases, 23, 1631–1639. 10.3201/eid2310.161248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagsma JA, Geenen PL, Ethelberg S, Fetsch A, Hansdotter F, Jansen A, Korsgaard H, O'Brien SJ, Scavia G, Spitznagel H, Stefanoff P, Tam CC, Havelaar AH and Med Vet Net Working G, 2013. Community incidence of pathogen‐specific gastroenteritis: reconstructing the surveillance pyramid for seven pathogens in seven European Union Member States. Epidemiology and Infection, 141, 1625–1639. 10.1017/s0950268812002166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrador Z, Siles‐Lucas M, Aparicio P, Lopez‐Velez R, Gherasim A, Garate T and Benito A, 2016. Cystic echinococcosis epidemiology in Spain based on hospitalization records, 1997–2012. PLoS Neglected Tropical Diseases, 10 10.1371/journal.pntd.0004942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestvik G, Warns‐Petit E, Smith LA, Fox NJ, Uhlhorn H, Artois M, Hannant D, Hutchings MR, Mattsson R, Yon L and Gavier‐Widen D, 2015. The status of tularemia in Europe in a one‐health context: a review. Epidemiology and Infection, 143, 2137–2160. 10.1017/s0950268814002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JY, Henao OL, Griffin PM, Vugia DJ, Cronquist AB, Hurd S, Tobin‐D'Angelo M, Ryan P, Smith K, Lathrop S, Zansky S, Cieslak PR, Dunn J, Holt KG, Wolpert BJ and Patrick ME, 2016. Infection with pathogens transmitted commonly through food and the effect of increasing use of culture‐independent diagnostic tests on surveillance – Foodborne Diseases Active Surveillance Network, 10 US sites, 2012–2015. MMWR‐Morbidity and Mortality Weekly Report, 65, 368–371. 10.15585/mmwr.mm6514a2 [DOI] [PubMed] [Google Scholar]
- Inns T, Ashton PM, Herrera‐Leon S, Lighthill J, Foulkes S, Jombart T, Rehman Y, Fox A, Dallman T, De Pinna E, Browning L, Coia JE, Edeghere O and Vivancos R, 2017. Prospective use of whole genome sequencing (WGS) detected a multi‐country outbreak of Salmonella Enteritidis . Epidemiology and Infection, 145, 289–298. 10.1017/s0950268816001941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO (International Organization for Standardization), 2001. Microbiology of food and animal feeding stuffs –Horizontal method for the detection of Escherichia coli O157. ISO 16654:2001. [DOI] [PubMed]
- ISO (International Organization for Standardization), 2012. Microbiology of food and animal feed. Real‐time polymerase chain reaction (PCR)‐based method for the detection of food‐borne pathogens. Horizontal method for the detection of shiga toxin‐producing Escherichia coli (STEC) and the determination of O157, O111, O26, O103 and O145 serogroups. ISO/TS 13136:2012. 22 pp.
- Janse I, Maas M, Rijks JM, Koene M, van der Plaats RQ, Engelsma M, van der Tas P, Braks M, Stroo A, Notermans DW, de Vries MC, Reubsaet F, Fanoy E, Swaan C, Kik MJ, IJzer J, Jaarsma RI, van Wieren S, de Roda‐Husman AM, van Passel M, Roest HJ and van der Giessen J, 2015. Environmental surveillance during an outbreak of tularaemia in hares, the Netherlands. Eurosurveillance Weekly, 22, pii: 30607 10.2807/1560-7917.es.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofré A, Garriga M, Aymerich T, Perez‐Rodriguez F, Valero A, Carrasco E and Bover‐Cid S, 2016. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 1, an extensive literature search and study selection with data extraction on L. monocytogenes in a wide range of RTE food. EFSA supporting publication 2016;13(12):EN‐1141, 184 pp. 10.2903/sp.efsa.2016.en-1141 [DOI] [Google Scholar]
- Jones AK, Cross P, Burton M, Millman C, O'Brien SJ and Rigby D, 2017. Estimating the prevalence of food risk increasing behaviours in UK kitchens. PLoS ONE, 12 10.1371/journal.pone.0175816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan‐da Silva N, Fabre L, Robinson E, Fournet N, Nisavanh A, Bruyand M, Mailles A, Serre E, Ravel M, Guibert V, Issenhuth‐Jeanjean S, Renaudat C, Tourdjman M, Septfons A, de Valk H and Le Hello S, 2018. Ongoing nationwide outbreak of Salmonella Agona associated with internationally distributed infant milk products, France, December 2017. EuroSurveillance, 23, 4–8. 10.2807/1560-7917.es.2018.23.2.17-00852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P, Bardonnet K, Renner E, Auer H, Pawlowski Z, Ammann RW, Vuitton DA and Kern P Registry European Echinococcsis , 2003. European echinococcosis registry: human alveolar echinococcosis, Europe, 1982–2000. Emerging Infectious Diseases, 9, 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardon Z, Watier L, Brunet A, Bernede C, Goudal M, Dacheux L, Rotivel Y, Guillemot D and Bourhy H, 2010. Imported episodic rabies increases patient demand for and physician delivery of antirabies prophylaxis. PLoS Neglected Tropical Diseases, 4 10.1371/journal.pntd.0000723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailles A, Ogielska M, Kemiche F, Garin‐Bastuji B, Brieu N, Burnusus Z, Creuwels A, Danjean MP, Guiet P, Nasser V, Tourrand B, Valour F, Maurin M, O'Callaghan D, Mick V, Vaillant V, Jay M, Lavigne JP and deValk H , 2017. Brucella suis biovar 2 infection in humans in France: emerging infection or better recognition? Epidemiology and Infection, 145, 2711–2716. 10.1017/S0950268817001704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli A, Martello E, Tomassone L, Calzolari M, Casalone C, De Meneghi D, Dottori M, Estrada‐Peña A, Fabbi M, Ferreri L, Ferroglio E, Luini M, Nicolau Solano S, Ortega C, Pautasso A, Prati P and Vesco U, 2012. Inventory of available data and data sources and proposal for data collection on vector‐borne zoonoses in animals. EFSA Supporting Publication 2012; 9(3):EN‐234, 189 pp. 10.2903/sp.efsa.2012.EN-234 [DOI] [Google Scholar]
- Marquer P, Rabade T and Forti R, 2014. Pig Farming in the European Union: Consider‐able Variations From One Member State to Another. Statistics in Focus. Eurostat, Kirchberg.
- Massolo A, Valli D, Wassermann M, Cavallero S, D'Amelio S, Meriggi A, Torretta E, Serafini M, Casulli A, Zambon L, Boni CB, Ori M, Romig T and Macchioni F, 2018. Unexpected Echinococcus multilocularis infections in shepherd dogs and wolves in south‐western Italian Alps: a new endemic area?. International Journal for Parasitology Parasites and Wildlife, 7, 309–316. 10.1016/j.ijppaw.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurin M and Gyuranecz M, 2016. Tularaemia: clinical aspects in Europe. Lancet Infectious Diseases, 16, 113–124. [DOI] [PubMed] [Google Scholar]
- Maury MM, Tsai Y‐H, Charlier C, Touchon M, Chenal‐Francisque V, Leclercq A, Criscuolo A, Gaultier C, Roussel S, Brisabois A, Disson O, Rocha EPC, Brisse S and Lecuit M, 2017. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity (vol. 48, p. 308, 2016). Nature Genetics, 49, 970 10.1038/ng0617-970d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet L, De Cruz K, Hénault S, Tambosco J, Richomme C, Réveillaud É, Gares H, Moyen JL and Boschiroli ML, 2018. Mycobacterium bovis Infection of Red Fox, France. Emerging Infectious Diseases, 24, 1150–1153. 10.3201/eid2406.180094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick V, Le Carrou G, Corde Y, Game Y, Jay M and Garin‐Bastuji B, 2014. Brucella melitensis in France: persistence in wildlife and probable spillover from Alpine ibex to domestic animals. PLoS ONE, 9, e94168–e94168. 10.1371/journal.pone.0094168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T, Freuling CM, Wysocki P, Roumiantzeff M, Freney J, Mettenleiter TC and Vos A, 2015. Terrestrial rabies control in the European Union: historical achievements and challenges ahead. Veterinary Journal, 203, 10–17. 10.1016/j.tvjl.2014.10.026 [DOI] [PubMed] [Google Scholar]
- Mylius M, Dreesman J, Pulz M, Pallasch G, Beyrer K, Claußen K, Allerberger F, Fruth A, Lang C, Prager R, Flieger A, Schlager S, Kalhöfer D and Mertens E, 2018. Shiga toxin‐producing Escherichia coli O103:H2 outbreak in Germany after school trip to Austria due to raw cow milk, 2017 ‐ The important role of international collaboration for outbreak investigations. International Journal of Medical Microbiology, 308, 539–544. [DOI] [PubMed] [Google Scholar]
- Nielsen EM, Björkman JT, Kiil K, Grant K, Dallman T, Painset A, Amar C, Roussel S, Guillier L, Félix B, Rotariu O, Perez‐Reche F, Forbes K and Strachan N, 2017. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 3, the comparison of isolates from different compartments along the food chain, and from humans using whole genome sequencing (WGS) analysis. EFSA supporting publication 2017:EN‐1151. 170 pp. 10.2903/sp.efsa.2017.en-1151 [DOI] [Google Scholar]
- NMKL (Nordisk Metodikkomite for Næringsmidler – Nordic Committee on Food Analysis), 2005. Escherichia coli O157. Detection in food and feeding stuffs. NMKL No. 164, 2 Edition. 2005. Available online: http://www.nmkl.org/index.php?option=com_zoo&task=item&item_id=337&Itemid=319&lang=en
- Nokireki T, Tammiranta N, Kokkonen U‐M, Kantala T and Gadd T, 2018. Tentative novel lyssavirus in a bat in Finland. Transboundary and Emerging Diseases., 65, 593–596. 10.1111/tbed.12833 [DOI] [PubMed] [Google Scholar]
- Norman FF, Monge‐Maillo B, Chamorro‐Tojeiro S, Pérez‐Molina J‐A and López‐Vélez R, 2016. Imported brucellosis: A case series and literature review. Travel Medicine and Infectious Disease, 14, 182–199. ISSN 1477‐8939, 10.1016/j.tmaid.2016.05.005 ( http://www.sciencedirect.com/science/article/pii/S1477893916300382) [DOI] [PubMed] [Google Scholar]
- Oksanen A, Siles‐Lucas M, Karamon J, Possenti A, Conraths FJ, Romig T, Wysocki P, Mannocci A, Mipatrini D, LaTorre G, Boufana B and Casulli A, 2016. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: a systematic review and meta‐analysis. Parasites and Vectors, 9, 519 10.1186/s13071-016-1746-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opsteegh M, Maas M, Schares G and Giessena J, 2016. Relationship between seroprevalence in the main livestock species and presence of Toxoplasma gondii in meat (GP/EFSA/BIOHAZ/2013/01) an extensive literature review. Final report. EFSA Supporting Publication 2016;13(2):EN‐996, 294 pp. 10.2903/sp.efsa.2016.en-996 [DOI]
- Parize P, Dacheux L, Larrous F and Bourhy H, The French Network Of Antirabies Clinics , 2018. The shift in rabies epidemiology in France: time to adjust rabies post‐exposure risk assessment. Eurosurveillance Weekly, 23, 1700548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin J‐B, Rautureau S, Bronner A, Hosteing S, Jaÿ M, Garin‐Bastuji B and Dufour B, 2016. Brucellosis in small ruminants in 2014: 95 départements of metropolitan France are now officially disease‐free. Bull Epidémiol Santé Anim Alim Focus on Regulated and Emerging Animal Diseases (REDs) – 2014 Review.
- Piseddu T, Brundu D, Stegel G, Loi F, Rolesu S, Masu G, Ledda S and Masala G, 2017. The disease burden of human cystic echinococcosis based on HDRs from 2001 to 2014 in Italy. PLoS Neglected Tropical Diseases, 11, e0005771 10.1371/journal.pntd.0005771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouillot R, Klontz KC, Chen Y, Burall LS, Macarisin D, Doyle M, Bally KM, Strain E, Datta AR, Hammack TS and Van Doren JM, 2016. Infectious Dose of Listeria monocytogenes in Outbreak Linked to Ice Cream, United States, 2015. Emerging Infectious Diseases, 22, 2113–2119. 10.3201/eid2212.160165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozio E, 2014. Searching for Trichinella: not all pigs are created equal. Trends in Parasitology, 30, 4–11. https:// doi.org/10.1016/j.pt.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Pozio E, 2016. Trichinella pseudospiralis an elusive nematode. Veterinary Parasitology, 231, 97–101. 10.1016/j.vetpar.2016.03.021 [DOI] [PubMed] [Google Scholar]
- Rizzi V, Da Silva Felicio T, Felix B, Gossner CM, Jacobs W, Johansson K, Kotila S, Michelon D, Monguidi M, Mooijman K, Morabito S, Pasinato L, Torgny Björkman J, Torpdahl M, Tozzoli R and Van Walle I, 2017. The g datECDC‐EFSA molecular typinabase for European Union public health protection Euroreference 2 – March 2017, 2–12.
- Rossi P, Tamarozzi F, Galati F, Pozio E, Akhan O, Cretu CM, Vutova K, Siles‐Lucas M, Brunetti E and Casulli A Extended Network Heracles , 2016. The first meeting of the European Register of Cystic Echinococcosis (ERCE). Parasites and Vectors, 9, 243 10.1186/s13071-016-1532-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow H, Ollgren J, Hytonen J, Rissanen H, Huitu O, Henttonen H, Kuusi M and Vapalahti O, 2015. Incidence and seroprevalence of tularaemia in Finland, 1995 to 2013: regional epidemics with cyclic pattern. Eurosurveillance, 20, 13–22. [DOI] [PubMed] [Google Scholar]
- Schjørring S, Gillesberg Lassen S, Jensen T, Moura A, Kjeldgaard JS, Müller L, Thielke S, Leclercq A, Maury MM, Tourdjman M, Donguy MP, Lecuit M, Ethelberg S and Nielsen EM, 2017. Cross‐border outbreak of listeriosis caused by cold‐smoked salmon, revealed by integrated surveillance and whole genome sequencing (WGS), Denmark and France, 2015 to 2017. Eurosurveillance Weekly 22, 17–00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger PM, Wintenberger C, Van der Hoek W and Stahl JP, 2014. Q fever in the Netherlands‐2007‐2010: what we learned from the largest outbreak ever. Medecine et Maladies Infectieuses, 44, 339 –353. 10.1016/j.medmal.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Siles‐Lucas M, Casulli A, Conraths FJ and Muller N, 2017. Laboratory diagnosis of Echinococcus spp. in human patients and infected animals. Advances in Parasitology, 96, 159–257. 10.1016/bs.apar.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Sindičić M, Bujanić M, Štimac I, Martinković F, Tuškan N, Špehar M and Konjević D, 2018. First identification of Echinococcus multilocularis in golden jackals in Croatia. Acta Parasitologica, 63, 000–000; ISSN 1230‐2821. 10.1515/ap-2018-00 [DOI] [PubMed] [Google Scholar]
- Szell Z, Marucci G, Pozio E and Sreter T, 2013. Echinococcus multilocularis and Trichinella spiralis in golden jackals (Canis aureus) of Hungary. Veterinary Parasitology, 197, 393–396. 10.1016/j.vetpar.2013.04.032 [DOI] [PubMed] [Google Scholar]
- Tamarozzi F, Akhan O, Cretu CM, Vutova K, Akinci D, Chipeva R, Ciftci T, Constantin CM, Fabiani M, Golemanov B, Janta D, Mihailescu P, Muhtarov M, Orsten S, Petrutescu M, Pezzotti P, Cosmin Popa A, Gabriela Popa L, Ioan Popa M, Velev V, Siles‐Lucas M, Brunetti E and Casulli A, 2018. Prevalence of abdominal cystic echinococcosis in rural Bulgaria, Romania, and Turkey: a cross‐sectional, ultrasound‐based, population study from the HERACLES project. The Lancet Infectious Diseases, 18, 769–778. ISSN 1473‐3099, 10.1016/s1473-3099(18)30221-4 [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2015. The World Health Organization Estimates of the Global Burden of Foodborne Dieseases: FERG Project Report. Available online: http://www.who.int/foodsafety/areas_work/foodborne-diseases/ferg/en/


