Abstract
Sodium saccharin is intended to be used as a sweetener in feed and water for drinking for piglets, pigs for fattening and veal calves. The Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) considers the proposed maximum use level of 150 mg sodium saccharin/kg feed as safe for calves and pigs for fattening. For piglets (sucking and weaned piglets), a lower level of 100 mg sodium saccharin/kg complete feed is considered safe. The corresponding maximum safe concentrations in water for drinking are 30 mg/L for piglets and 50 mg/L for pigs for fattening, respectively. The maximum safe concentrations of sodium saccharin in feed and water for drinking are derived under the premise that only one source, feed or water for drinking, contains the additive. The FEEDAP Panel concludes that no concern for the consumer would result from the use of sodium saccharin in feed and water for drinking at the dose considered safe for the target species. The precautions for handling the product proposed by the applicant are considered to be sufficient to ensure user safety. The FEEDAP Panel concludes that the use of sodium saccharin at the dose considered safe for target species is unlikely to have detrimental effects on the terrestrial and freshwater compartments. The high mobility and relative persistence of saccharin and the high persistency of its degradation product 4‐hydroxysaccharin indicate that groundwater contamination above 0.1 μg/L is likely to occur. Since the function of sodium saccharin in feed for the target species is essentially the same as that in food, the FEEDAP Panel concludes that no demonstration of efficacy is necessary.
Keywords: Sensory additives, flavouring compounds, sodium saccharin, piglets, pigs, veal calves, safety
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7 and, in addition, Article 10(2) of that Regulation also specifies that for existing products within the meaning of Article 10(1), an application shall be submitted in accordance with Article 7, within a maximum of 7 years after the entry into force of this Regulation.
The European Commission received a request from Feed Flavourings Authorisation Consortium European Economic Interest Grouping (FFAC EEIG)2 for authorisation of the product sodium saccharin, when used as a feed additive for piglets (suckling and weaned), pigs for fattening and calves for rearing and fattening (category: sensory additives; functional group: flavouring compounds).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive) and under Article 10(2) (re‐evaluation of an authorised feed additive). EFSA received directly from the applicant the technical dossier in support of this application. The particulars and documents in support of the application were considered valid by EFSA as of 9 December 2010.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5.
EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product sodium saccharin, when used under the proposed conditions of use (see Section 3.1.3).
1.2. Additional information
Saccharin, an artificial sweetener, has been previously assessed for safety by the Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA; WHO, 1993). The Scientific Committee on Food (SCF) expressed an opinion on saccharin and its sodium, potassium and calcium salts (European Commission, 1995). Both Committees established an acceptable daily intake (ADI) of 5 mg/kg body weight (bw) per day for saccharin and its calcium, potassium and sodium salts. The Scientific Panel on Food Additives, Flavouring, Processing Aids and Materials in Contact with Food (AFC) expressed an opinion on the presence of 1,2‐benzisothiazolin‐3‐one as an impurity in saccharin used as a food additive and concluded that it is not of concern at the level detected ranging between 40 and 800 mg/kg (EFSA, 2007). In 2012, the EFSA Panel on Food Contact Materials, Flavourings, Enzymes and Processing Aids (CEF) evaluated sodium salt (saccharin, sodium salt) for use in food contact materials, as additive in polyesters, and concluded that it is not of safety concern for the consumer (EFSA CEF Panel, 2012).
In 1999, the International Agency for Research on Cancer (IARC, 1999) concluded that saccharin and its salts are not classifiable as to their carcinogenicity to humans (Group 3).
Sodium saccharin dihydrate (E 954ii) is authorised as a food additive in the European Union (EU) in accordance with Annex II and Annex III to Regulation (EC) No 1333/20083 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/20124. It is listed in the EU Register of Feed Additives on the basis of the notification procedure and thus authorised for use in feed in the EU. Sodium saccharin has not been previously assessed by EFSA as a feed additive.
Regulation (EC) No 429/20085 allows substances already approved for use in human food to be assessed with a more limited procedure than for other feed additives. However, the use of this procedure is always subject to the condition that food safety assessment is relevant to the use in feed.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier6 in support of the authorisation request for the use of sodium saccharin as a feed additive. The technical dossier was prepared following the provisions of Article 7 of Regulation (EC) No 1831/2003, Regulation (EC) No 429/2008 and the applicable EFSA guidance documents.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts’ knowledge, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the sodium saccharin in animal feed. The Executive Summary of the EURL report can be found in Annex A.7
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of sodium saccharin is in line with the principles laid down in Regulation (EC) No 429/2008 and the relevant guidance documents: Guidance for the preparation of dossiers for sensory additives (EFSA FEEDAP Panel, 2012a), Technical guidance: Tolerance and efficacy studies in target animals (EFSA FEEDAP Panel, 2011), Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012b) and Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012c).
3. Assessment
Sodium saccharin is a sweetener intended for use in feed and water for drinking for piglets (suckling and weaned), pigs for fattening and calves for rearing and fattening as a sensory additive (functional group: flavouring compounds).
3.1. Characterisation
3.1.1. Characterisation of additive
Sodium saccharin (chemical name: 2‐sodium‐1,2‐benzisothiazol‐3(2H)‐one 1,1‐dioxide) is identified by the Chemical Abstracts Service (CAS) number 128‐44‐9 and the European Inventory of Existing Chemical Substances (EINECS) number 204‐886‐1. The structural formula of sodium saccharin is shown in Figure 1. The molecular formula of sodium saccharin is C7H4NO3SNa and the molecular mass is 205.2 (anhydrous), 223.18 (monohydrate) or 241.19 (dihydrate). Sodium saccharin is a solid and is soluble in water. It has an apparent density of 550–650 kg/m3 and a melting point of 226–230°C.
Figure 1.
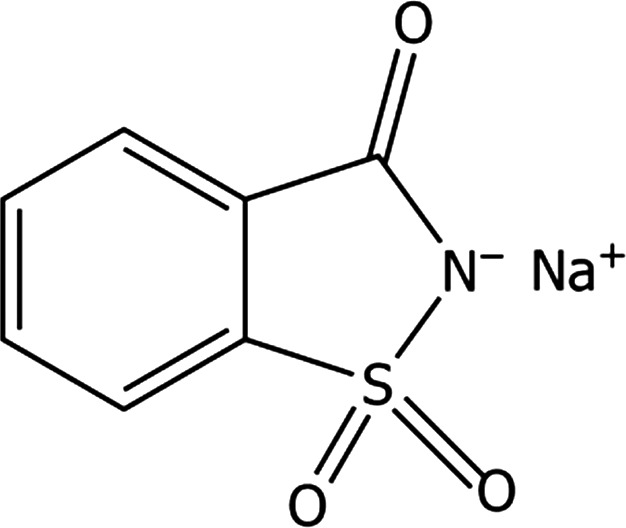
Structural formula of sodium saccharin
Saccharin is chemically synthesised. The manufacturing process described by the applicant uses either phthalic anhydride or methyl anthranilate as starting material. Methyl anthranilate is diazotized to form 2‐carbomethoxybenzene‐diazonium chloride. Sulfonation followed by oxidation yields 2‐carbomethoxybenzenesulfonyl chloride. Amidation of the sulfonylchloride followed by acidification will form insoluble acid saccharin. Subsequent addition of sodium hydroxide produces the soluble sodium salt.
By specification the additive is described as having a purity of 98–101% (on the basis of dry weight), with a loss on drying < 15%, heavy metals < 20 mg/kg, o‐toluene sulfonamide ≤ 10 mg/kg, p‐toluene sulfonamide ≤ 10 mg/kg and benzoic acid p‐sulfonamide ≤ 25 mg/kg.
Analyses ■■■■■■■■■■ showed ■■■■■ compliance with the specifications proposed by the applicant. The variable values for loss on drying ■■■■■ indicate differences in the hydration of the product as used.
In five batches,9 analytical values for o‐toluene sulfonamide (5–8 mg/kg), p‐toluene sulfonamide (5–8 mg/kg), benzoic acid p‐sulfonamide (16–17 mg/kg), arsenic (1–1.86 mg/kg), selenium (6–17 mg/kg) and lead (0.21–0.67 mg/kg) are below the limit values set for food additives by Commission Regulation (EU) No 231/2012.10 The contents of dichloromethane, trichloromethane and trichloroethylene (residual solvents) were each < 1 mg/L and below the thresholds proposed by International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH) (EMA, 2010).11
Five additional batches were analysed for the content of 1,2‐benzisothiazolin‐3‐one, an impurity potentially present in saccharin used as food additive (EFSA, 2007). In three batches, 1,2‐benzisothiazolin‐3‐one was below the limit of detection (LOD, 50 μg/kg), and in the remaining two, the maximum content was 260 μg/kg.12 The FEEDAP Panel considers these levels not of concern, in agreement with the assessment of the AFC Panel (EFSA, 2007).
Five batches of the sodium saccharin were analysed for particle size distributions by laser diffraction. ■■■■■■■■■■ The dusting potential, was measured in the three batches with particles below 100 μm, ranged between 0.5 and 5.46 g/m3.14
3.1.2. Shelf‐life and stability in water
The applicant cited published stability studies which showed that sodium saccharin is stable for up to 5 years (Pearson, 2001), provided that the additive is stored in tightly closed containers under cool, well‐ventilated conditions. Two production batches were monitored over 4 years and no losses in the content of anhydrous sodium saccharin were observed.15
Stability studies in feed and in water for drinking were not provided. However published data investigating the stability of saccharin at different pH and temperature conditions, showed no losses over 2 months at 20°C and 40°C and pH 3 or 4. Hydrolysis of saccharin was measurable only under extreme conditions of high temperature (80°C) and low pH (2) (Pearson, 2001).16 From these results, the FEEDAP Panel concludes that sodium saccharin is stable in feedingstuffs and in water for drinking.
3.1.3. Conditions of use
Sodium saccharin is intended to be used in feed, premixtures and water for drinking for piglets (suckling and weaned piglets), pigs for fattening and calves for rearing up to 4 months and for calves for fattening up to 6 months, with levels up to 150 mg/kg of complete feedingstuffs and water for drinking.
3.2. Safety
The assessment of safety is based on the maximum recommended use level proposed by the applicant (150 mg/kg complete feed or water for drinking).
3.2.1. Absorption, distribution, metabolism and excretion (ADME)
The ADME of saccharin and its salts has been reviewed by JECFA (WHO, 1993). The summary below is based on this JECFA assessment.
Depending on the pH, saccharin may exist in the non‐ionised form (at acidic pH) or in the ionised form (saccharin anion). The non‐ionised form is readily absorbed in the stomach of species with low pH (e.g. guinea pig, rabbit), whereas the saccharin anion is slowly absorbed in the stomach of species with a higher pH (e.g. rat) or in the intestines. In humans and rat, saccharin is slowly absorbed in the intestines and rapidly excreted in the urine. Urinary excretion is considered a measure of gastrointestinal absorption, whereas faecal excretion is an indicator of unabsorbed saccharin (Anderson et al., 1987b; Fisher et al., 1989; Renwick 1985, as referenced in WHO, 1993; FAS 32).
Following a single oral dose to adult rats, saccharin was found to be distributed to most organs with the highest concentrations in the organs of elimination (kidney and bladder) followed by the plasma. There is no evidence of bioaccumulation of saccharin in any tissue (Matthews et al., 1973; Ball et al., 1977; Sweatman and Renwick 1980, as referenced in WHO, 1993). Only when dietary administration to rats exceeded 5% of the diet was there any accumulation of saccharin in plasma and tissues, due to decreased renal clearance.
Based on studies reviewed by Renwick (1986) and summarised by JECFA (WHO, 1993), saccharin does not undergo any detectable metabolism in humans, rats, guinea‐pigs, rabbits or monkeys. Studies in humans and rats indicate that the majority of saccharin administered in the diet (80–85%) is slowly absorbed and rapidly excreted unchanged in the urine. When [3‐14C]‐saccharin was administered orally to rats, 56–87% of the labelled dose was excreted in the urine and 10–40% in the faeces during 7 days. More than 99% of the urinary 14C was unchanged saccharin. Comparative metabolic profiles of a dog, rabbit, guinea pig and hamster indicated that there was little difference in the pattern due to animal species or dose level.17
Considering the similarity of the metabolic profile of dog, rabbit, guinea pig and hamster, it is not expected that sodium saccharin behaves in a different way in pigs and calves.
3.2.2. Toxicological studies
The data available on the toxicity of saccharin have been reviewed by JECFA (WHO, 1993) and the SCF (European Commission, 1995).
Two‐generation study in rat
JECFA based the assessment on a two‐generation feeding study in rats with sodium saccharin at 1%, 3%, 4%, 5%, 6.25% and 7.5% of the diet (Schoenig et al., 1985). Although no adverse effect on survival was observed up to the highest dose (7.5%), at levels of 3% and higher the animals showed a marked disturbance in homeostasis, with a dose‐related decrease in body weight gain despite increased food consumption. This effect was related to inhibitory effects of saccharin on carbohydrate and protein digestion. No relevant toxicological effects were observed at 1%, which was taken as the no observed effect level (NOEL). On the basis of this NOEL of 1% (corresponding to 500 mg/kg bw per day) and the application of an uncertainty factor (UF) of 100, JECFA allocated a group ADI of 5 mg/kg bw to saccharin and its calcium, potassium and sodium salts (WHO, 1993). Bladder tumours induced by sodium saccharin were observed in male rats only. According to JECFA, ‘it would be inappropriate to consider the bladder tumours induced in male rats by sodium saccharin to be relevant to the assessment of a toxicological hazard in humans’. In addition, epidemiological studies on saccharin did not show any evidence that saccharin ingestion increased the incidence of bladder tumours in human populations.
The same two‐generation, long‐term study in rat was assessed by the SCF (Schoenig et al., 1985; European Commission, 1995). The SCF considered the bladder tumours induced by saccharin to be specific for the male rat and not equally relevant for female rats and mice, hamsters and monkeys, and not relevant for humans. As the increase in the tumours incidence at 1% supplementation level was not statistically significant and there were no compound‐related effects in either the grade or incidence of any lesions in the 1% group, the SCF concluded that a dose of 1% sodium saccharin in the diet should be taken as the NOEL for bladder tumours in the male rat. Based on this NOEL of 1% in the diet (500 mg/kg bw per day) and the application of a 100‐fold UF, an ADI of 5 mg/kg bw was established (SCF, 1995).18
The FEEDAP Panel concludes that the NOEL of 500 mg/kg bw per day applied for the derivation of the ADI for humans is equally relevant for the target species (piglets, pigs for fattening and calves for rearing and for fattening).
Genotoxicity and carcinogenicity
Subsequent to the JECFA and SCF evaluations, IARC reviewed human and animal carcinogenicity data together with the available information on mutagenicity and genotoxicity of saccharin and its salts (IARC, 1999).19
Based upon this analysis and review of all available studies, IARC reached the following overall conclusions:
there is inadequate evidence in humans for the carcinogenicity of saccharin salts used as sweeteners;
there is inadequate evidence in experimental animals for the carcinogenicity of saccharin (acid form) and calcium saccharin;
there is sufficient evidence in experimental animals for the carcinogenicity of sodium saccharin.
IARC concluded that ‘sodium saccharin produces urothelial bladder tumours in rats by a non‐DNA‐reactive mechanism that involves the formation of a urinary calcium phosphate‐containing precipitate, cytotoxicity and enhanced cell proliferation. This mechanism is not relevant to humans because of critical interspecies differences in urine composition’.
3.2.3. Safety for the target species
The first approach to the safety assessment for target species takes account of the intended use levels in animal feed relative to the maximum reported exposure of humans on the basis of the metabolic body weight (kg bw0.75). The data for the human exposure in the EU ranges from 0.1 to 2.5 mg/kg bw (equivalent to 278–6,957 μg/kg bw0.75).20 The calculated intake by the target species (veal calves 9,494, piglets 15,789 and pigs for fattening 14,241 μg/kg0.75 per day)21 from the proposed maximum feed concentration exceeds that of humans.
Therefore, safety for the target species cannot be derived from the risk assessment for food use. As an alternative, the maximum feed concentration which can be considered safe for the target animals can be derived from the lowest NOEL if suitable data are available. Toxicological data derived from a two‐generation chronic study are available for sodium saccharin (see Section 3.2.2). From this study, a NOEL was identified both by JECFA and the SCF to be 500 mg/kg bw per day.
Applying an UF of 100 to the NOEL, the maximum safe intake for different target species was derived for sodium saccharin following the EFSA Guidance for sensory additives (EFSA FEEDAP Panel, 2012a), and thus the maximum safe feed concentration was calculated (Table 1).
Table 1.
Maximum safe concentration in feed for different target animals for sodium saccharin
| Target animal | Default values | Maximum safe intake/feed concentration | ||
|---|---|---|---|---|
| BW (kg) | FI (g/day)a | Intake (mg/day) | Concentration (mg/kg feed)b | |
| Piglets | 20 | 1,000 | 100 | 100 |
| Pigs for fattening | 100 | 3,000 | 500 | 167 |
| Veal calves (milk replacer) | 100 | 2,000 | 500 | 250 |
BW: body weight; FI: feed intake.
Complete feed with 88% dry matter (DM), except milk replacer for veal calves (94.5% DM).
Complete feed containing 88% DM, milk replacer 94.5% DM.
Sodium saccharin in water for drinking can only be administered if the combined intake from both oral routes does not exceed the amount which is given from the intake of feed containing the maximum content. The maximum content of water for drinking is derived from the maximum content in feed assuming that no feed containing sodium saccharin is given simultaneously. Under these conditions, water for drinking for piglets and calves may contain 30 mg sodium saccharin/L and for pigs for fattening 50 mg/L (EFSA FEEDAP Panel, 2010).
3.2.3.1. Conclusions on safety for the target species
The FEEDAP Panel concludes that sodium saccharin is safe at the proposed use level of 150 mg/kg feed pigs for fattening, calves for rearing and calves for fattening. Only a lower level of 100 mg sodium saccharin/kg complete feed is considered safe for piglets (suckling and weaned piglets).
The corresponding maximum safe concentrations in water for drinking are 30 mg/L for piglets and calves, and 50 mg/L for pigs for fattening, respectively.
The maximum safe concentrations of sodium saccharin in feed and water for drinking are derived under the premise that only one source, feed or water for drinking, contains the additive.
3.2.4. Safety for the consumer
A group ADI of 5 mg saccharin/kg bw per day was allocated by JECFA (WHO, 1993) and the SCF (European Commission, 1995) for saccharin and its calcium, potassium and sodium salts when used in food. Therefore, it is necessary to consider the potential contribution of residues in tissues from animals administered saccharin in feed to the overall exposure of consumers. Although no studies on residues in food from animal origin were submitted, the metabolic data (see Section 3.2.1) suggest that there is no evidence of accumulation in any tissue and that tissue accumulation can occur but only at doses far in excess of those proposed for animal feed. The possibility that residues will be present in tissues from animals fed with sodium saccharin cannot be excluded; however, it is considered unlikely that any residues would cause consumer exposure to saccharin to exceed the ADI, even considering that some consumers may be exposed up to 2.5 mg/kg bw (half of the ADI) using saccharin as a sweetener.20
3.2.4.1. Conclusions on safety for the consumer
No concern for the consumer would result from the use of sodium saccharin in feed and water for drinking at the dose considered safe for the target species.
3.2.5. Safety for the user
There are no concerns regarding systemic toxicity from previous safety evaluations by JECFA (WHO, 1993) and the SCF (European Commission, 1995).
No data were provided on user safety other than particle size and dusting potential. Approximately 50% of volume of the additive was of inhalable size and the dusting potential indicates a likelihood of exposure by respiratory route.
The applicant regards saccharin as being potentially harmful to users exposed by inhalation or by contact with skin or eyes and this is reflected in the material safety data sheet.22
3.2.6. Safety for the environment
Sodium saccharin is a synthetic chemical which is not found in nature. Saccharin can also be formed as a metabolite from various pesticides, i.e. metsulfuron methyl23 (EFSA, 2015), propoxycarbazone24 (EFSA, 2016), oxasulfuron25 (EFSA, 2017a), tribenuron26 (EFSA, 2005) and tribenuron methyl27 (EFSA, 2017b). 4‐Hydroxysaccharin has also been identified as a soil metabolite of the pesticide propoxycarbazone (EFSA, 2016). Sodium saccharin is excreted efficiently by livestock via urine as saccharin anion. Once in the environment, saccharin may be oxidised into 4‐hydroxysaccharin under aerobic conditions.
Saccharin is not a physiological/natural substance of established safety for the environment. It is also not intended for companion animals. Consequently, according to Regulation (EC) No 429/2008 the Phase I assessment has to be performed to determine the predicted environmental concentrations (PECs).
In Phase I and II initially, a total residues approach will be taken, meaning that the PECs will be calculated, based on the assumption that the additive is excreted 100% as parent compound.
3.2.6.1. Phase I assessment
Physicochemical properties
The physicochemical properties of saccharin anion are summarised in Table 2.
Table 2.
Physicochemical properties of saccharin anion28
| Property | Value | Units |
|---|---|---|
| Octanol/water partition coefficient (log Kow 25°C) | 0.91 | – |
| Water solubility (20°C) | 4,000 | mg/L |
| Vapour pressure | 1.03 × 10−7 | Pa |
| Dissociation constant pKa | 1.3 | – |
Fate and behaviour
Fate in manure
Experimental studies demonstrated the stability of saccharin under anaerobic conditions in pig manure for 62 days of storage at 20°C (Buerge et al., 2011). Thus, the saccharin excreted can be assumed to end up on arable land.
Fate in soil
Adsorption
The sorption characteristics of saccharin were investigated in a variety of soils. ■■■■■■■■■■
Degradation
The degradation of saccharin in soil is highly variable ■■■■■
Fate in water
Saccharine is highly soluble (4,000 mg/L) and not expected to adsorb to suspended solids and sediment or to volatilise from water surfaces (vapour pressure 1.03 × 10−7 Pa).
Saccharin is stable in aqueous solution for most normal food applications.28
Predicted environmental concentrations
The methodology for the calculation of the maximum PECs in soil, groundwater, surface water and sediment are described in the technical guidance for assessing the safety of feed additives for the environment (EFSA, 2008a). The input values used for saccharin were: 134 mg saccharin/kg feed (corresponding to the use level of 150 mg sodium saccharin/kg feed), molecular weight 183.18, vapour pressure 1.03 × 10−7 Pa, solubility 4,000 mg/L, ■■■■■ The calculated values for pigs for fattening and veal calves are given in Table 3.
Table 3.
Initial predicted environmental concentration of saccharine anion in soil (μg/kg), groundwater, surface water and sediment (μg/L)
| Compartment | Units | PEC | |
|---|---|---|---|
| Pigs for fattening | Veal calves | ||
| Soil | μg/kg | 2,406 | 2,126 |
| Ground water | μg/L | 2,169 | 1,917 |
| Surface water | μg/L | 2,892 | 2,555 |
| Sediment | μg/kg | 5,668 | 5,010 |
PEC: predicted environmental concentration.
The Phase I PEC trigger values are exceeded; therefore, a Phase II assessment is considered necessary.
The calculated application rate was 1.8 and 1.6 kg/ha for pigs for fattening and calves, respectively (EFSA, 2008).
3.2.6.2. Phase II assessment
Exposure assessment
PEC refinement
A FOCUS scenario considering a single pre‐emergence application of manure on winter cereals in Jokioinen (Finland) yielded a PEC of 64 μg/L in groundwater (according to FOCUS‐PEARL 4.4.4, Table 4).30 This PEC is considerably higher than the value of 0.1 μg/L, identified by the EU as a quality standard for pesticides and their metabolites in groundwater.31
Table 4.
Refined predicted environmental concentrations of saccharine anion in soil (μg/kg), groundwater, surface water and sediment (μg/L) for pigs for fattening
| Compartment | Units | PEC | Derivation of PEC |
|---|---|---|---|
| Soil | μg/kg | 2,406 | Initial PEC |
| Ground water | μg/L | 64 | FOCUS refinement |
| Surface water | μg/L | 31 | FOCUS refinement |
| Sediment | μg/kg | 5,668 | Initial PEC |
PEC: predicted environmental concentration.
Generally, even non‐relevant metabolites of pesticides (Laabs et al., 2015) are not admitted at concentrations above 10 μg/L in groundwater in the European Member States.
Saccharin has been detected in groundwater from a pumping station in Switzerland at concentrations up to 0.26 μg/L, likely due to the application of manure from pigs fed with saccharin (Buerge et al., 2011).
The exposure of sodium saccharin was performed by using FOCUS‐PRZM, FOCUS‐MACRO and FOCUS‐TOXSWA model calculations to estimate the peak PEC and the time weighted in the surface water. The highest peak PEC is 31 μg/L (scenario R3 stream runoff for swine, Table 4).29
Ecotoxicity studies
Toxicity to soil organisms
Effects on plants
The effect of sodium saccharin on the emergence and growth of seedlings of three plant species, ■■■■■ according to OECD guideline 208 (GLP compliant).■■■■■■■■■■ The lowest EC50 and EC10 for growth was determined for B. oleracea (286 and 106 mg sodium saccharin/kg soil dw, respectively).
Effect on earthworms
The effect of sodium saccharine dihydrate ■■■■■■■■■■■■■■■ and was not further considered in the assessment.
A 56‐day earthworm reproduction study was performed with sodium saccharin, following the OECD 222 guideline (GLP compliant).■■■■■■■■■■ No statistically significant adverse effects on mortality, biomass and reproduction of the earthworm E. andrei in artificial soil were determined ■■■■■ The no observed effect concentration (NOEC) for mortality, biomass and reproduction was determined to be 100 mg sodium saccharin/kg soil dw. The EC50 value for reproduction was estimated to be > 100 mg sodium saccharin/kg soil dw.
Effects on the soil microorganisms
In a GLP study, the effect of sodium saccharin on nitrification was studied ■■■■■ according to OECD 216.35 ■■■■■ on the soil nitrogen transformation ■■■■■ were observed at the maximum dose tested.
Toxicity to aquatic compartment
Effect on algae
The acute toxicity of saccharin to Scenedesmus subspicatus was determined ■■■■■■■■■■■■■■■ The EC50 for growth inhibition was > 100 mg/L and the NOEC was 46 mg/L.
Effects on crustaceans
The acute toxicity of saccharin to the crustacean Daphnia magna was determined in a limit test after 48 h under static conditions at a measured concentration of 118 mg/L according OECD 202 and in compliance with GLP. The 48‐h EC50 was 118 mg/L.27 However, the full report of the study was not available.
Effect on fish
In keeping with its low toxicity to mammals, saccharin has a low acute toxicity to fish with a reported 96‐h LC50 for rainbow trout (Oncorynchus mykiss) of 124 mg/L.26 However, the full report of the study was not available.
Effect on sediment dwelling organisms
The toxicity of sodium saccharin dihydrate was assessed in a sediment‐water Lumbriculus variegatus toxicity test under laboratory conditions using five test ■■■■■■■■■■■■■■■ The worms in the test vessels reacted normal and showed no abnormal behaviour compared to the controls. The individual biomass of the living worms in the test vessels showed no significant difference to the control.
Conclusions on the ecotoxic effect on soil, water and sediment
The applicant submitted GLP studies which followed OECD guidelines as proposed in the technical guidance for assessing the safety of feed additives for the environment (EFSA, 2008a). Tests are valid and the test results can be accepted and used for determination of predicted no effect concentrations (PNECs) and to establish the safe values for exposed environmental compartments.
For the terrestrial compartment, data are available for microorganisms, earthworms and plants. The lowest L(E)C50 is 100 mg sodium saccharin/kg found for earthworms.
For the aquatic compartment, data are available for algae, aquatic invertebrates and fish. The lowest toxicity value for the aquatic compartment was found for algae. The 72‐h effect concentration measured as 50% reduction in growth rate in algae tests (ErC50) was 100 mg saccharin/L.
Ecotoxicological data for sediment‐dwelling invertebrates are provided for the sediment compartment. No effects were seen at the highest concentration tested (1,000 mg/kg soil).
Risk characterisation (PEC/PNEC ratio)
The risk characterisation ratios for terrestrial, freshwater and sediment compartments are reported in Tables 5, 6 and 7, respectively.
Table 5.
Risk characterisation (PEC/PNEC ratio) for the terrestrial compartment
| Taxa | PECsoil (μg/kg) | E(L)C50 /NOEC (mg/kg) | UF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
| Earthworm | 2,406 | 100 | 10 | 10,000 | 0.24 |
| Plants | 106 | 10 | 10,600 | 0.23 |
PEC: predicted environmental concentration; E(L)C50: half‐maximal effective (lethal) concentration; NOEC: no observed effect concentration; UF: uncertainty factor; PNEC: predicted no effect concentration.
Table 6.
Risk characterisation (PEC/PNEC ratio) for the freshwater compartment
| Taxa | PECsurfacewater (μg/L) | E(L,r)C50/NOEC (mg/L) | UF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
|
Algae Scenedesmus subspicatus |
31 | 100 | 1,000 | 100 | 0.31 |
|
Aquatic invertebrates Daphnia magna |
118 | 1,000 | 118 | 0.26 | |
|
Fish Oncorynchus mykiss |
124 | 1,000 | 124 | 0.25 |
PEC: predicted environmental concentration; E(L)C50: half‐maximal effective (lethal) concentration; ErC50: effect concentration measured as 50% reduction in growth rate in algae tests; NOEC: no observed effect concentration; UF: uncertainty factor; PNEC: predicted no effect concentration.
Table 7.
Risk characterisation (PEC/PNEC ratio) for the sediment compartment
| Taxa | PECsediment (μg/kg) | EC10 (mg/kg) | UF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
|
Sediment‐dwelling invertebrates Lumbriculus variegatus |
5,668 | 1,000 | 10 | 100,000 | 0.057 |
PEC: predicted environmental concentration; EC10: the concentration that will have an effect on 10% of the population of test organisms; NOEC: no observed effect concentration; UF: uncertainty factor; PNEC: predicted no effect concentration.
Risk for bioaccumulation
No experimentally determined bioconcentration factors (BCF) for earthworm and fish have been submitted. Since the log Kow for saccharine is 0.91,28 the estimated potential for bioaccumulation is low.
Risk for secondary poisoning
The log Kow for sodium saccharine is 0.91.28 The estimated risk for secondary poisoning is considered to be unlikely.
Assessment of the metabolite 4‐hydroxysaccharin
4‐Hydroxysaccharin is a recognised major metabolite of saccharin in soil and is known to be more persistent than saccharin.24
Fate in soil
Adsorption
The sorption characteristics of 4‐hydroxysaccharin were investigated in sorption studies with five soils. ■■■■■■■■■■
Degradation
A normalised geometric mean of 143.5 days was calculated for 4‐hydroxysaccharin in the assessment of propoxycarbazone sodium. However, the exact number of soils considered in the calculation of the DT50 was not reported. The DT50 of 4‐hydroxysaccharin ranged from 178.4 to higher than 953.8,24 but these values were extrapolations since the experiment lasted 123 days.
Calculation of the PEC groundwater
A FOCUS scenario ■■■■■ PEC of 38.7 μg/L in groundwater for 4‐hydroxysaccharin (according to FOCUS‐PEARL 3.3.3.).
Although the FEEDAP Panel has reservation on the selection of the appropriate Koc and DT50 values used in the FOCUS calculations, nevertheless this PEC is considerably higher than the value of 0.1 μg/L, identified by the EU as a quality standard for pesticides and their metabolites in groundwater.
3.2.6.3. Conclusions on safety for the environment
An environmental risk assessment of saccharin indicates that the proposed use level of 150 mg sodium saccharin/kg feed for pigs for fattening and veal calves is unlikely to have detrimental effects on the terrestrial and freshwater compartments.
The high mobility and relative persistence of saccharin and the high persistency of its degradation product 4‐hydroxysaccharin indicate that groundwater contamination above 0.1 μg/L is likely to occur.
3.3. Efficacy
Sodium saccharin is widely used in food as a sweetener. There is evidence that sweet taste receptors of animals (pigs and bovines) also respond to saccharin (Hellekant et al., 1994; Moran et al., 2010). Since the function of sodium saccharin in feed is essentially similar to that in food, the FEEDAP Panel concludes that no further demonstration of efficacy is necessary.
4. Conclusions
The FEEDAP Panel considers the proposed maximum use level of 150 mg sodium saccharin/kg feed is safe pigs for fattening, calves for rearing and calves for fattening. For piglets (sucking and weaned piglets), a lower level of 100 mg sodium saccharin/kg complete feed is considered safe. The corresponding maximum safe concentrations in water for drinking are 30 mg/L for piglets and calves, and 50 mg/L for pigs for fattening, respectively. The maximum safe concentrations of sodium saccharin in feed and water for drinking are derived under the premise that only one source, feed or water for drinking, contains the additive.
The FEEDAP Panel concludes that no concern for the consumer would result from the use of sodium saccharin in feed and water for drinking at the dose considered safe for the target species.
Sodium saccharin is considered to be potentially harmful by inhalation or by contact to skin and eyes.
The FEEDAP Panel concludes that the use of sodium saccharin at the dose considered safe for target species is unlikely to have detrimental effects on the terrestrial and freshwater compartments. The high mobility and relative persistence of saccharin and the high persistency of its degradation product 4‐hydroxysaccharin indicate that groundwater contamination above 0.1 μg/L is likely to occur.
Since the function of sodium saccharin in feed for the target species is essentially the same as that in food, the FEEDAP Panel concludes that no demonstration of efficacy is necessary.
Documentation provided to EFSA
Sodium saccharin for pigs, piglets (suckling and weaned), pigs for fattening, calves for rearing, calves for fattening. October 2010. Submitted by Feed Flavourings Authorisation Consortium European Economic Interest Grouping (FFAC EEIG).
Sodium saccharin for pigs, piglets (suckling and weaned), pigs for fattening, calves for rearing, calves for fattening. Supplementary information. April 2013. Submitted by Feed Flavourings Authorisation Consortium European Economic Interest Grouping (FFAC EEIG).
Sodium saccharin for pigs, piglets (suckling and weaned), pigs for fattening, calves for rearing, calves for fattening. Supplementary information. September 2013. Submitted by Feed Flavourings Authorisation Consortium European Economic Interest Grouping (FFAC EEIG).
Sodium saccharin for pigs, piglets (suckling and weaned), pigs for fattening, calves for rearing, calves for fattening. Supplementary information. June 2017. Submitted by Feed Flavourings Authorisation Consortium European Economic Interest Grouping (FFAC EEIG).
Evaluation report of the European Union Reference Laboratory for Feed Additives on the Methods(s) of Analysis for sodium saccharin.
Comments from Member States.
Abbreviations
- 1/n
slope of Freundlich isotherm
- ADI
acceptable daily intake
- ADME
absorption, distribution, metabolism and excretion
- AFC
EFSA Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food
- BCF
bioconcentration factor
- BW
body weight
- CAS
Chemical Abstracts Service
- CEF
EFSA Panel on Food Contact Materials, Flavourings, Enzymes and Processing Aids
- DM
dry matter
- DT50
degradation half‐time
- dw
Dry weight
- EC10
the concentration that will have an effect on 10% of the population of test organisms
- EC50
half‐maximal effective concentration
- EINECS
European Inventory of Existing Chemical Substances
- ErC50
effect concentration measured as 50% reduction in growth rate in algae tests
- EURL
European Union Reference Laboratory
- FAO
Food Agricultural Organization
- FFAC
Feed Flavourings authorisation Consortium of FEFANA (EU Association of Specialty Feed Ingredients and their Mixtures)
- FI
feed intake
- FOCUS
FOrum for the Co‐ordination of pesticide fate models and their USe
- GLP
good laboratory practice
- HPLC‐UV
high‐performance liquid chromatography with UV detection
- IARC
International Agency for Research on Cancer
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- Koc
organic carbon sorption constant
- Kow
octanol–water partition coefficient
- LC50
lethal concentration 50
- LOD
limit of detection
- LOQ
limit of quantification
- Log Kow
logarithm of octanol‐water partition coefficient
- NOEC
No observed effect concentration
- NOEL
no observed effect level
- OECD
Organisation for Economic Co‐operation and Development
- PEC
predicted environmental concentration
- PNEC
predicted no effect concentration
- RSDint
relative standard deviation of repeatability
- RSDr
relative standard deviation for intermediate precision
- RRec
recovery rate
- SCF
Scientific Committee on Food
- UF
uncertainty factor
- WHO
World Health Organization
Annex A – Executive summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for sodium saccharin
1.
In the current application authorisation is sought under articles 4(1) and 10(2) for sodium saccharin under the category/functional group 2(b) “sensory additives”/“flavouring compounds”, according to the classification system of Annex I of Regulation (EC) No 1831/2003. According to the Applicant, the active substance of the feed additive is sodium saccharin with purity above 98%. Specifically, authorisation is sought for the use of the feed additive for suckling and weaned piglets, pigs for fattening, bovines and calves for rearing and fattening. The feed additive is intended to be used via premixtures in feedingstuffs and water at a maximum level of 150 mg/kg.
For the determination of sodium saccharin in the feed additive, the Applicant proposed the internationally recognised European Pharmacopoeia method 01/2008:0787, based on potentiometric titration with 0.1 M perchloric acid. No performance characteristics of this method are provided. However, the EURL considers this method suitable to be used within the frame of official control.
For the determination of sodium saccharin in feedingstuffs and water the Applicant proposed a single laboratory validated and further verified High‐Performance Liquid Chromatography with UV detection (HPLC‐UV) method. The following performance characteristics were reported for feedingstuffs:
-
–
a relative standard deviation of repeatability (RSDr) ranging from of 2.7 to 11%;
-
–
a relative standard deviation for intermediate precision (RSDint) ranging from 6.6 to 18%;
-
–
a recovery rate (RRec) ranging from 82 to 100%; and
-
–
a limit of detection (LOD) and a limit of quantification (LOQ) of 0.1 and 0.3 mg/kg, respectively.
Furthermore the Applicant applied the method, upon request from the EURL, to determine the method performance characteristics in water and reported an RSDr ranging from 0.1 to 1.1%. Based on the performance characteristics presented, the EURL recommends for official control, the single laboratory validated and further verified HPLC‐UV method, submitted by the Applicant, to determine sodium saccharin in feedingstuffs and water.
For the determination of sodium saccharin in premixtures the Applicant did not provide sufficient validation data, therefore the EURL cannot evaluate nor recommend any method for official control to determine sodium saccharin in premixtures.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Brantom P, Dusemund B, Van Beelen P, Westendorf J, Gregoretti L, Manini P and Chesson A, 2018. Scientific Opinion on the safety and efficacy of sodium saccharin when used as a feed flavour for piglets, pigs for fattening, calves for rearing and calves for fattening. EFSA Journal 2018;16(3):5208, 18 pp. 10.2903/j.efsa.2018.5208
Requestor: European Commission
Question number: EFSA‐Q‐2010‐01228
Panel members: Gabriele Aquilina, Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Georges Bories, Andrew Chesson, Pier Sandro Cocconcelli, Gerhard Flachowsky, Jürgen Gropp, Boris Kolar, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Alberto Mantovani, Baltasar Mayo, Fernando Ramos, Guido Rychen, Maria Saarela, Roberto Edoardo Villa, Robert John Wallace and Pieter Wester.
Legal Notice: Relevant information or parts of this scientific output have been blackened in accordance with the European Commission decision on the confidentiality requests formulated by the applicant. A previous, provisional version of this output which had been made publicly available pending the adoption of the decision has been replaced by this version. The full output has been shared with the European Commission, EU Member States and the applicant.
Acknowledgements: The EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) wishes to thank the following for the support provided to this scientific output: Rosella Brozzi, Jaume Galobart, Jordi Tarres‐Call and Maria Vittoria Vettori.
Adopted: 21 February 2018
Amended: 15 June 2018
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Feed Flavourings Authorisation Consortium European Economic Interest Grouping (FFAC EEIG), Avenue Louise 130A, B‐1050, Brussels, Belgium.
Commission Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
FEED dossier reference: FAD‐2010‐0157.
The full report is available on the EURL website: http://irmm.jrc.ec.europa.eu/SiteCollectionDocuments/FinRep-FAD-2010-0157.pdf
■■■■■
Technical dossier/Supplementary information September 2013/Annexes_Qiii_CoA 1‐5.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annex II and III of Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83 22.03.2012, p. 1.
Technical dossier/Section II/Annex_24_Residual solvents.
Technical dossier/Supplementary information September 2013/ Annex_Qiii_Impurity 1‐5.
■■■■■
Technical dossier/Supplementary information September 2013/Annexes Qii_dust_pot 1‐3.
Technical dossier/Section II/Annex_25_Stability.
Technical dossier/Section II/Annex II_18_Pearson_2001.
Technical Dossier/Section III/Annex_III_7_Lethco and Wallace, 1975.
Technical dossier/Supplementary information April 2013/Annex_SIn_11_Echemportal_2012_Saccharin.
See also Guidance for the preparation of dossiers for sensory additives.
Technical Dossier/ Section II/ Annex_3_MSDS_Na_Sacch_Generic.
Technical Dossier/Supplementary Information April 2013/Annex_SIn_06_EC_2000_Metsulfuron‐methyl‐s.
Technical Dossier/Supplementary Information April 2013/Annex_SIn_01_BBA_2001_DAR_Propoxycarbazone.
Technical Dossier/Supplementary Information April 2013/Annex_SIn_07_EC_2002_Oxasulfuron.
Technical Dossier/Supplementary Information April 2013/Annex_Sin13_EFSA_2004b_Tribenuron.
Technical Dossier/Supplementary Information April 2013/Annex_SIn_28_KEMI_2004_DAR_Tribenuron methyl.pdf/ Tribenuron methyl_DAR_05_Vol3_B8‐B9 + appendices‐public.
Technical Dossier/Supplementary Information April 2013/Annex_SIn_26_HSDB_2012_Saccharin.
■■■■■
■■■■■
Council Directive 98/83/EC on the quality of water intended for human consumption, OJ L 330, 5.12.1998, p. 32.
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
References
- Buerge IJ, Keller M, Buser HR, Muller MD and Poiger T, 2011. Saccharin and other artificial sweeteners in soils: estimated inputs from agriculture and households, degradation, and leaching to groundwater. Environmental Science & Technology, 45, 615–621. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Conclusions regarding the peer review of the pesticide risk assessment of the active substance tribenuron. EFSA Journal 2005;3(3):1–52, 52 pp. 10.2903/j.efsa.2005.15r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on the presence of 1,2‐benzisothiazolin‐3‐one as an impurity in saccharin used as food additive. EFSA Journal 2007;5(1):416, 7 pp. 10.2903/j.efsa.2007.416 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Conclusion on the peer review of the pesticide risk assessment of the active substance metsulfuron‐methyl. EFSA Journal 2015;13(1):3936, 106 pp. 10.2903/j.efsa.2015.3936 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. Conclusion on the peer review of the pesticide risk assessment of the active substance propoxycarbazone (variant evaluated propoxycarbazone‐sodium). EFSA Journal 2016;14(10):4612, 25 pp. 10.2903/j.efsa.2016.4612 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017a. Conclusion on the peer review of the pesticide risk assessment of the active substance oxasulfuron. EFSA Journal 2017;15(3):4722, 25 pp. 10.2903/j.efsa.2017.4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Flavourings, Enzymes and Processing Aids), 2012. Scientific Opinion on the safety evaluation of the substance, 1,2‐benzisothiazol‐3(2H)‐one 1,1‐dioxide, sodium salt, CAS No. 128‐44‐9, for use in food contact materials. EFSA Journal 2012;10(3):2640, 9 pp. 10.2903/j.efsa.2012.2640 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2010. Scientific Opinion on the use of feed additives authorised/applied for use in feed when supplied via water. EFSA Journal 2010;8(12):1956, 9 pp. 10.2903/j.efsa.2010.1956 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011. Technical guidance: Tolerance and efficacy studies in target animals. EFSA Journal 2011;9(5):2175, 15 pp. 10.2903/j.efsa.2011.2175 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534, 26 pp. 10.2903/j.efsa.2012.2534 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance for establishing the safety of additives for the consumer. EFSA Journal 2012;10(1):2537, 12 pp. 10.2903/j.efsa.2012.2537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Arena M, Auteri D, Barmaz S, Bellisai G, Brancato A, Brocca D, Bura L, Byers H, Chiusolo A, Court Marques D, Crivellente F, De Lentdecker C, De Maglie M, Egsmose M, Erdos Z, Fait G, Ferreira L, Goumenou M, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Magrans JO, Medina P, Miron I, Molnar T, Nougadere A, Padovani L, Parra Morte JM, Pedersen R, Reich H, Sacchi A, Santos M, Serafimova R, Sharp R, Stanek A, Streissl F, Sturma J, Szentes C, Tarazona J, Terron A, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017b. Conclusion on the peer review of the pesticide risk assessment of the active substance tribenuron‐methyl. EFSA Journal 2017;15(7):4912, 32 pp. 10.2903/j.efsa.2017.4912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA (European Medicines Agency), 2010. VICH GL 18 residual solvents in new veterinary medicinal products, active substances and excipients, revision. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/06/WC500091533.pdf
- European Commission , 1995. Opinion of the Scientific Committee of Food on Saccharin and its sodium, potassium and calcium salts. Available online: http://ec.europa.eu/food/fs/sc/oldcomm7/out26_en.pdf
- Hellekant G, Hard af Segerstad C and Roberts TW, 1994. Sweet taste in the calf: III. Behavioral responses to sweeteners. Physiology & Behaviour, 56, 555–562. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on cancer), 1999. Saccharin and its salts. Vol. 73 (1999), p. 517. Available online: http://www.inchem.org/documents/iarc/vol73/73-19.html
- Laabs V, Leake C, Botham P and Melching‐Kollmuss S, 2015. Regulation of non‐relevant metabolites of plant protection products in drinking and groundwater in the EU: Current status and way forward. Regulatory Toxicology & Pharmacology, 73, 276–286. [DOI] [PubMed] [Google Scholar]
- Moran AW, Al‐Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Daly K, Ionescu C, Bravo D and Shirazi‐Beechey SP, 2010. Expression of Na+/glucose co‐transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. The British Journal of Nutrition, 104, 637–646. [DOI] [PubMed] [Google Scholar]
- Pearson RL, 2001. Saccharin. In: Nabors LO (ed.). Alternative Sweeteners, Vol. 112. Food Science and Technology. M. Dekker, New York. pp. 147–165. [Google Scholar]
- Renwick AG, 1986. The metabolism of intense sweeteners. Xenobiotica, 16, 1057–1071. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Commitee on food), 1995. Saccharin and its sodium, potassium and calcium salts. Opinion expressed on 2 June 1995. Published by Commission of the European Communities. Brussels. [Google Scholar]
- Schoenig GP, Goldenthal EI, Geil RG, Frith CH, Richter WR and Carlborg FW, 1985. Evaluation of the dose response and in utero exposure to saccharin in the rat. Food Chemical Toxicology, 23, 475–490. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 1993. Evaluation of certain food additives and contaminants. Forty‐first report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 9‐18 February 1993. WHO Technical Report Series, no. 837, Geneva. [PubMed]


