Abstract
Background
Multiple blood cultures have been shown to improve pathogen yield and antimicrobial stewardship for adult patients with suspected serious bacterial infection (SBI). For children, the use of multiple blood cultures is less common and volume recommendations are more complicated, often resulting in single cultures with low volume.
Methods
In 2010, Children’s Hospital Colorado instituted electronic medical record (EMR) decision support to recommend collection of 2 blood cultures before administration of antibiotics for suspected SBI. Recommended blood culture volumes were calculated by age rather than weight. We evaluated all children admitted to inpatient units between 2008 and 2009 (pre-intervention) and 2011 and 2013 (postintervention) who received antibiotics in the hospital after having blood cultures drawn in the emergency department, excluding those with a length of stay >8 days. We compared blood culture yield, isolate classification (pathogen vs contaminant), and antimicrobial modifications before and after the interventions.
Results
A total of 3948 children were included in the study. EMR guidelines were associated with a significantly higher number of children with multiple blood cultures drawn before antibiotic administration (88.0% vs 12.3%; P < .001) and an increased percentage of blood cultures with the recommended volume (74.3% vs 15.2%; P < .001), resulting in a significantly higher pathogen isolation rate and improved antimicrobial decisions. Multiple cultures helped define the role of common contaminants in the clinical decision process.
Conclusions
Multiple blood cultures with age-based volumes taken before starting antibiotics increase pathogen isolation rates and appropriate modification of antimicrobial treatment in children.
Keywords: antibiotics, bacteremia, blood culture, sepsis
Blood cultures are of fundamental importance in the diagnosis and treatment of infants and children with suspected sepsis and serious bacterial infection (SBI). Vaccines have reduced the incidence of bacteremia due to previously predictable causes, shifting the epidemiology toward health care–associated pathogens with less predictable antimicrobial susceptibility patterns and more complicated patient scenarios [1].
The number and method of volume determination for pediatric blood cultures is controversial and varies widely [2, 3]. Though multiple blood cultures can be used to differentiate contamination from bacteremia [4–7] and potentially decrease unnecessary antimicrobial use, length of stay, and health care costs, this practice is uncommon in pediatrics [2]. Conflicting recommendations exist for the minimum culture volume, which is based either on the child’s weight (which may not be readily available at the time of blood draw) or, less commonly, on age [2, 8, 9].
This study aimed to determine the impact of an electronic medical record (EMR) decision support and education/compliance feedback intervention on the collection of multiple blood cultures with minimum age-based volumes drawn before initial antibiotic administration and the subsequent impact on pathogen recovery and clinical decision- making.
METHODS
Study Design and Ethical Approval
This is a pre–post cohort study comparing blood culture collection and clinical outcomes at the Children’s Hospital Colorado (CHCO) before and after a blood culture collection intervention. It was approved by the CHCO Organizational Research Risk and Quality Improvement Review Panel as a quality improvement study.
Study Periods
The pre-intervention period was from January 1, 2008, to December 31, 2009, and the postintervention period was from January 1, 2011, to December 31, 2013, with a phase-in period from January 1, 2010, to December 31, 2010, during implementation of the intervention. During the pre-intervention period, the number of blood cultures was determined by the clinician without guidance, and recommended minimum blood volumes were based on Clinical Laboratory Standards Institute (CLSI) weight-based guidelines [10].
Blood Culture Collection Intervention
Two major interventions on blood culture collection were implemented during the phase-in period from September to December 2010. First, the EMR blood culture order was modified by embedding decision support recommendations for 2 blood cultures before starting antibiotics in neonates, immune-compromised patients, acutely ill patients (eg, new-onset fever), patients requiring intensive care, and patients with central lines. In our hospital, blood culture sets were not a standard of care. Instead, the number of individual aerobic blood cultures drawn in the ED was determined at the discretion of the ordering physician, with anaerobic blood cultures only recommended under special circumstances (eg, Lemierre disease). When a single blood culture was ordered, our EMR protocol intervention recommended that at least 2 blood cultures be performed before initiating antibiotics and patient admission. The results of these initial cultures are the focus of this analysis, as antibiotics were begun on all patients included in the study before hospital admission. There was no recommendation for subsequent blood cultures, which would ordinarily be determined by the initial culture results and the patient’s response to antibiotics.
Second, nursing protocols for blood culture collection volume were simplified from the standard, weight-based CLSI recommendations to age-based dosing as follows: 1-mL minimum with the addition of 1 mL per year of age, up to a maximum of 10 mL. This recommendation was made because nurses collecting the blood culture specimens would usually know the age of the child but not necessarily have the weight readily available. When compared with weight-based recommendations, the resulting volumes were quite similar. As part of the new protocol, nurses were requested to recorded the actual volume of blood collected on each blood culture bottle. Nurses and providers were trained on the new protocols in Fall 2010, with audit and feedback on compliance with recommended volumes, based on the nurses’ documentation, provided to hospital units monthly.
Laboratory Practices
Blood culture bottles that accommodated up to 3 mL (BD Bactec Peds Plus) or 10 mL (BD Bactec Plus) were continuously monitored on the Bactec automated system (Becton Dickinson and Co) until identified as positive or for 5 days. Isolates were identified using standard clinical laboratory procedures.
Inclusion/Exclusion Criteria
Children with blood cultures collected in the CHCO emergency department from January 1, 2008, to December 31, 2009, and January 1, 2011, to December 31, 2013, with subsequent admission and receipt of antibacterial medications were included. Only children with direct ED admission on the first ED visit with blood cultures obtained and antibiotics initiated were included. Each patient admission was regarded as a case for analysis. Children with a length of stay >8 days were excluded to remove cases who subsequently developed infections during an extended hospital stay.
Outcomes
The primary outcome of the study was the number of blood cultures initially collected per patient as correlated with the rate of pathogen and contaminant recovery, subsequent antibiotic modifications, and length of stay. A secondary observational outcome was the percentage of blood cultures compliant with recommended age-based blood volumes.
Data Collection
Data on the number and nurse-recorded volume of blood cultures were collected monthly beginning in the intervention period. Cultured organisms (excluding anaerobes) from initial blood cultures were recorded and classified as described below. Additional clinical information, including inpatient antibacterial medications administered and length of stay, was retrospectively extracted from the EMR. Data were entered into a standardized data collection tool in a secure online database (REDCAP).
Definitions
Discharge diagnosis was classified into bacterial infection, nonbacterial infection, or noninfection groups using principal discharge International Classification of Diseases, 9th Revision (ICD-9) diagnosis codes (codes available on request). Cultured organisms were classified as pathogen, possible pathogen, or contaminant (Table 1). For cultures with multiple organisms isolated, organism classifications were assigned based on the most pathogenic organism.
Table 1.
Operational Classification of Bacterial Isolates
| Category | Organisms |
|---|---|
| Absolute pathogens | Staphylococcus aureus, Streptococcus pneumoniae, yeast species, Streptococcus (groups A and B), Salmonella, Shigella, Haemophilus influenzae, Neisseria meningitidis, Mycobacterium, Campylobacter, Listeria |
| Possible pathogens | Abiotrophia, Acinetobacter, Actinomyces, alpha (viridans) Streptococcus, Streptococcus anginosus, Capnocytophaga, Citrobacter, Enterobacteriaceae, Enterococcus, nonfermenting gram-negative rods |
| Common contaminants | Bacillus, Coryneform bacteria, gram-positive rods or gram-positive cocci (unidentified), Kocuria, Leuconostoc, Lactobacillus, Moraxella, Neisseria (not meningitidis or gonorrheae), Rothia, coagulase-negative Staphylococcus |
Antimicrobial modification was classified into no change, modified, or discontinued based on medication changes after culture results. Cases with no change were further classified as appropriate if initial coverage was appropriate for culture results or inappropriate if a patient was continued on antibiotics for ≥3 days despite negative cultures if ICD-9 codes were not indicative of bacterial infection. Cases were classified as inconclusive if they did not match any of the above definitions (usually because they were discharged before 3 days without retrievable information on discharge therapy).
Statistical Analysis
Outcomes in the postintervention period were compared with the pre-intervention period (data from the phase-in period during implementation were included in figures but excluded in statistical analysis). Categorical variables were compared using the chi-square test or Mann-Whitney test, and continuous variables were compared using the Student t test, with statistical significance set at α < 0.05. Statistical analysis was performed in IBM SPSS Statistics, version 24.
RESULTS
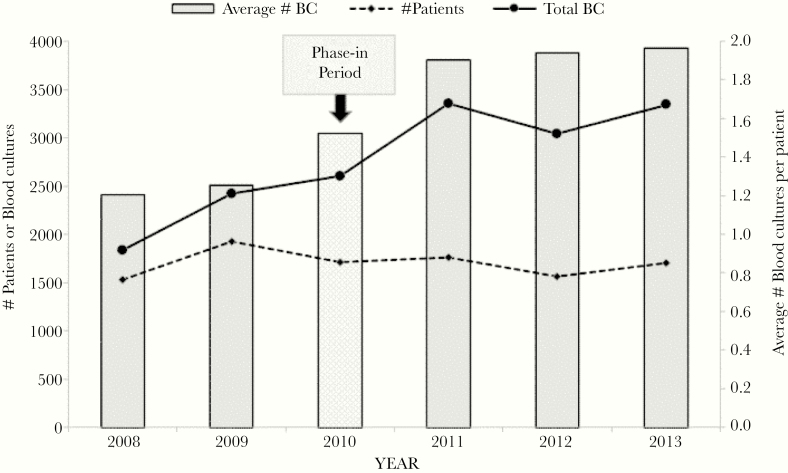
The total number of patients who met inclusion criteria during the study period was 3948. As shown in Figure 1, the number of blood cultures before antibiotic administration increased from 1.20 cultures per patient in 2008 to 1.96 cultures per patient in 2013 (R2 = .8311; P < .001). In the postimplementation group, 88% of patients had ≥2 blood cultures obtained before antibiotics, compared with 12% in the pre-intervention group (P < .001). After the protocol change, we observed that compliance with blood culture volume recording increased rapidly, from 20.8% at the beginning of the intervention period to 83.7% at the end, while obtaining recommended age-based blood volumes increased from 15.2% at the beginning to 74.3% at the end.
Figure 1.
Total number of inpatient cases with blood cultures taken before antimicrobial treatment and average number of blood cultures per patient before initial antibiotic administration, 2008–2013. Abbreviation: BC, blood culture.
Table 2 compares clinical data in the postintervention period (2011–2013) with the pre-intervention period (2008–2009). Overall, 33.9% of cases were determined by ICD-9 discharge codes to have had a clinical bacterial infection, 23.2% to have nonbacterial infection, and 42.9% had no clinical infection identified. The proportion of each diagnostic category did not change between the 2 periods. Of the 1337 children clinically thought to have a bacterial infection (by ICD-9 discharge codes), 13.4% (179/1337) had ≥1 blood cultures growing pathogens (53.3% methicillin-susceptible S. aureus, 20.7% S. pneumoniae, 9.6% group B streptococcus, 8.5% methicillin-resistant S. aureus, 7.8% group A streptococcus) or possible pathogens (59.8% Enterobacteriaceae).
Table 2.
Comparison of Clinical and Blood Culture Characteristics for Children With Blood Cultures Taken and Antibiotics Started, 2008–2013
| 2008–2009 | 2011–2013 | ||||
|---|---|---|---|---|---|
| No. | % | No. | % | Probability | |
| Cases via the emergency department | 1598 | 2350 | |||
| Clinical diagnosis (ICD-9) | 0.462a | ||||
| Noninfectious | 669 | 41.9 | 1028 | 43.7 | |
| Infection, bacterial | 547 | 34.2 | 790 | 33.6 | |
| Infection, nonbacterial | 382 | 23.9 | 532 | 22.6 | |
| Age group | |||||
| Newborn (0–28 d) | 239 | 15.0 | 243 | 10.3 | <0.001a |
| Infant (29 d–11 mo) | 342 | 21.4 | 430 | 18.3 | |
| Toddler (12–35 mo) | 278 | 17.4 | 377 | 16.0 | |
| Preschool (3–5 y) | 179 | 11.2 | 260 | 11.1 | |
| School age (6–12 y) | 337 | 21.1 | 607 | 25.8 | |
| Teen/adult (≥13 y) | 223 | 14.0 | 433 | 18.4 | |
| Length of stay (d) | |||||
| Median | 3.0 | 3.0 | 0.143b | ||
| Average | 2.95 | 3.07 | 0.034c | ||
| No. of blood cultures before antibiotics | <0.001a | ||||
| 1 | 1,401 | 87.7 | 282 | 12.0 | |
| ≥2 | 197 | 12.3 | 2068 | 88.0 | |
| Result of blood cultures in each case | <0.001a | ||||
| Absolute pathogen ≥1 | 29 | 1.8 | 70 | 3.0 | |
| Possible pathogens | 30 | 1.9 | 101 | 4.3 | |
| Common contaminants | 36 | 2.3 | 90 | 3.8 | |
| All no growth | 1503 | 94.1 | 2089 | 88.9 | |
| Antibiotic outcome (excluding inconclusive) | <0.001a,d | ||||
| Continue antibiotics (appropriate) | 451 | 28.2 | 627 | 26.7 | |
| Modify antibiotics | 433 | 27.1 | 689 | 29.3 | |
| Discontinue antibiotics | 182 | 11.4 | 201 | 8.6 | |
| Continue antibiotics (inappropriate) | 52 | 3.3 | 10 | 0.4 | |
Abbreviation: ICD-9, International Classification of Diseases, 9th Revision.
aChi-square test.
bMann-Whitney test.
cStudent t test.
dInconclusives excluded (2008–2009: n = 385, 25.6%; 2011–2013: n = 562, 26.9%).
The rate of positive cultures increased (11.1% vs 5.9%, P < .001), as well as the rate with ≥1 pathogens or possible pathogens (7.3% vs 3.7%, P < .001) postintervention. There was a small yet significant (P < .001) percent shift in the distribution between antimicrobial decision-making categories (excluding those considered inconclusive: 2008–2009: n = 385, 25.6%; 2011–2013: n = 562, 26.9%) in the postintervention period, with an increased percentage of cases with modified antimicrobials after blood culture results and a decrease in the continuation of inappropriate antibiotics. The mean length of stay postintervention increased minimally to 3.07 days from 2.95 days (P = .034), whereas the median length of stay remained the same (3.0 days).
Table 3 shows the influence of blood culture results on subsequent antimicrobial treatment in cases with ≥2 blood cultures (excluding inconclusive cases for whom antimicrobial therapy could not be evaluated and those for whom antimicrobial therapy was continued inappropriately). Cases with growth on any blood culture were significantly more likely (P = .006) to have antibiotics continued appropriately or modified than discontinued. Those who grew an absolute or possible pathogen were more likely to have appropriate therapy if ≥2 of the cultures were positive (P = 0.03). Those with only 1 of multiple blood cultures positive for a contaminant were significantly less likely (P = .04) to have antimicrobials continued inappropriately.
Table 3.
Treatment Response to Organism Isolation in Cases With ≥2 Blood Cultures (Excluding Inconclusive and Inappropriate Cases)
| Absolute or Possible Pathogen, No. (%) | Common Contaminant, No. (%) | |||||
|---|---|---|---|---|---|---|
| Antimicrobial Response | No Growth, No. (%) | Growth, No. (%) | 1 Positive | ≥2 Positive | 1 Positive | ≥2 Positive |
| Continue (appropriate) or modify | 1076 (86.4) | 137 (93.8) | 23 (88.5) | 64 (100) | 23 (79.3) | 27 (100) |
| Discontinue | 169 (13.6) | 9 (6.2) | 3 (11.5) | 0 | 6 (20.7) | 0 |
| Probability | P = .006a | P = .03a | P = .04a | |||
aChi-square test.
DISCUSSION
This study shows that blood culture collection can be effectively changed using simple implementation strategies including: EMR decision support, simplified age-based instructions for blood culture volume, provider/nurse education, and audit and compliance feedback. In this study, increased blood culture number and volume taken prior to starting antibiotics increased pathogen isolation rates leading to more appropriate antimicrobial treatment. Additionally, 2 negative blood cultures or only 1 of 2 blood cultures growing a typical contaminant prompted clinicians to discontinue antibiotics [4, 5]. With the introduction or more rapid molecular organism identification platforms, earlier differentiation of pathogen vs contaminant may further decrease unnecessary antibiotic initiation and expedite antibiotic discontinuation for children with contaminants [11].
Blood culture volume is a critically important factor in the detection of true bacteremia in pediatric patients and especially in neonates [9, 12–14]. Volume guidelines can be difficult to implement due to complex weight-based formulas and lack of patient weights at the time of blood draw. By simplifying age-based volume guidelines and providing relevant and timely audit and feedback to unit leaders, pediatric blood culture volumes increased in our study with a resultant increase in pathogen yield. Collection of two such cultures prior to initiating empiric antimicrobial therapy, increases volumes with further added benefit.
There are important limitations to this study and to the concept of ruling out sepsis or serious bacterial infection with blood cultures alone. Our observation that blood culture volumes increased was based on nurse self-reporting rather than more optimal pre-intervention and postintervention measurement of collection bottle mass. Our assessment of appropriateness of antimicrobial therapy was based on available electronic data and not extensive chart review. Even with the optimal number and volume of cultures, many bacterial infections may not have detectable bacteremia, as evidenced by only 13.4% of patients in this study ultimately having a discharge diagnosis of bacterial infection who had positive blood cultures. Even when positive with a common contaminant, clinicians may be hesitant to discontinue antibiotics in vulnerable children (eg, newborns). [15] The utility of multiple blood cultures before initiating antimicrobial therapy as shown in this study will be an adjunct to antimicrobial stewardship efforts to educate clinicians on appropriate management of children with suspected SBI [11]. Because not all SBI patients are bacteremic, it is also important to consider the infectious disease version of Sutton”s Law—to “go where the infection is” and obtain an appropriate culture from any focal infection site as well as blood cultures before initiation of empiric antimicrobial treatment, especially in high-risk patients or ones begun on broad-spectrum antimicrobial agents [16, 17].
In our study, only 2.5% of children admitted to the hospital with antibiotic treatment who had blood cultures drawn in the ED had an absolute pathogen, compared with 3.2% with contaminants, suggesting that more targeted patient selection for blood culturing may be indicated. An example of a low-yield population for blood culture collection is children with uncomplicated community-acquired pneumonia, where blood cultures are significantly more likely to yield a contaminant organism than a pathogenic organism, leading to an antibiotic change [18–20]. At our institution, quality improvement efforts are now targeting decreased collection of blood cultures in such low-yield populations, where blood cultures have little decision-making value.
With these caveats, it is important to recognize that blood culture results play an important role in the diagnosis and treatment of children with serious bacterial illness. Multiple blood cultures with appropriate blood volume obtained before initiation of empiric antimicrobial therapy can be successfully encouraged with EMR order sets. Coupled with rapid organism identification technology, they can lead to higher pathogen isolation rates and more timely antimicrobial decision-making.
Acknowledgments
Financial support. This work was supported by the Jules Amer Community Pediatrics Fund, Children’s Hospital Colorado.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Irwin AD, Drew RJ, Marshall P, et al. . Etiology of childhood bacteremia and timely antibiotics administration in the emergency department. Pediatrics 2015; 135:635–42. [DOI] [PubMed] [Google Scholar]
- 2. Dien Bard J, McElvania TeKippe E. Diagnosis of bloodstream infections in children. J Clin Microbiol 2016; 54:1418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buttery JP. Blood cultures in newborns and children: optimising an everyday test. Arch Dis Child Fetal Neonatal Ed 2002; 87:F25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flournoy DJ, Catron TL, Stalling FH. A comparison of pathogens and contaminants based on the number of positive blood cultures in a set. Zentralbl Bakteriol Mikrobiol Hyg A 1983; 255:336–9. [PubMed] [Google Scholar]
- 5. Hitzenbichler F, Simon M, Salzberger B, Hanses F. Clinical significance of coagulase-negative staphylococci other than S. epidermidis blood stream isolates at a tertiary care hospital. Infection 2017; 45:179–86. [DOI] [PubMed] [Google Scholar]
- 6. Pavlovsky M, Press J, Peled N, Yagupsky P. Blood culture contamination in pediatric patients: young children and young doctors. Pediatr Infect Dis J 2006; 25:611–4. [DOI] [PubMed] [Google Scholar]
- 7. Hossain B, Islam MS, Rahman A, et al. . Understanding bacterial isolates in blood culture and approaches used to define bacteria as contaminants: a literature review. Pediatr Infect Dis J 2016; 35:S45–51. [DOI] [PubMed] [Google Scholar]
- 8. Gonsalves WI, Cornish N, Moore M, et al. . Effects of volume and site of blood draw on blood culture results. J Clin Microbiol 2009; 47:3482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Connell TG, Rele M, Cowley D, et al. . How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children’s hospital. Pediatrics 2007; 119:891–6. [DOI] [PubMed] [Google Scholar]
- 10. Wilson M, Mitchell M, Morris A, et al. Principles and Procedures for Blood Cultures: Approved Guideline. Wayne, PA: Clinical and Laboratory Standards Institute: 2007; 27:60. [Google Scholar]
- 11. Messacar K, Hurst AL, Child J, et al. . Clinical impact and provider acceptability of real-time antimicrobial stewardship decision support for rapid diagnostics in children with positive blood culture results. J Pediatric Infect Dis Soc 2017; 6(3):267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schelonka RL, Chai MK, Yoder BA, et al. . Volume of blood required to detect common neonatal pathogens. J Pediatr 1996; 129:275–8. [DOI] [PubMed] [Google Scholar]
- 13. Kellogg JA, Ferrentino FL, Goodstein MH, et al. . Frequency of low level bacteremia in infants from birth to two months of age. Pediatr Infect Dis J 1997; 16:381–5. [DOI] [PubMed] [Google Scholar]
- 14. Kellogg JA, Manzella JP, Bankert DA. Frequency of low-level bacteremia in children from birth to fifteen years of age. J Clin Microbiol 2000; 38:2181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cantey JB, Farris AC, McCormick SM. Bacteremia in early infancy: etiology and management. Curr Infect Dis Rep 2016; 18:1. [DOI] [PubMed] [Google Scholar]
- 16. Wheeler AM, Heizer HR, Todd JK. Influence of culture results on management and outcome of pediatric osteomyelitis and/or septic arthritis. J Pediatric Infect Dis Soc 2012; 1:152–6. [DOI] [PubMed] [Google Scholar]
- 17. Todd JK. Office laboratory diagnosis of skin and soft tissue infections. Pediatr Infect Dis 1985; 4:84–7. [DOI] [PubMed] [Google Scholar]
- 18. McCulloh RJ, Koster MP, Yin DE, et al. . Evaluating the use of blood cultures in the management of children hospitalized for community-acquired pneumonia. PLoS One 2015; 10:e0117462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis TR, Evans HR, Murtas J, et al. . Utility of blood cultures in children admitted to hospital with community-acquired pneumonia. J Paediatr Child Health 2017; 53:232–6. [DOI] [PubMed] [Google Scholar]
- 20. Driscoll AJ, Deloria Knoll M, Hammitt LL, et al. ; PERCH Study Group The effect of antibiotic exposure and specimen volume on the detection of bacterial pathogens in children with pneumonia. Clin Infect Dis 2017; 64:368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]