Abstract
Following a request from the European Commission, the EFSA Panel on Plant Protection Products and their Residues (PPR Panel) prepared a scientific opinion to provide a comprehensive evaluation of pesticide residues in foods for infants and young children. In its approach to develop this scientific opinion, the EFSA PPR Panel took into account, among the others, (i) the relevant opinions of the Scientific Committee for Food setting a default maximum residue level (MRL) of 0.01 mg/kg for pesticide residues in foods for infants and young children; (ii) the recommendations provided by EFSA Scientific Committee in a guidance on risk assessment of substances present in food intended for infants below 16 weeks of age; (iii) the knowledge on organ/system development in infants and young children. For infants below 16 weeks of age, the EFSA PPR Panel concluded that pesticide residues at the default MRL of 0.01 mg/kg for food for infants and young children are not likely to result in an unacceptable exposure for active substances for which a health‐based guidance value (HBGV) of 0.0026 mg/kg body weight (bw) per day or higher applies. Lower MRLs are recommended for active substances with HBGVs below this value. For infants above 16 weeks of age and young children, the established approach for setting HBGVs is considered appropriate. For infants below 16 weeks of age the approach may not be appropriate and the application of the EFSA guidance on risk assessment of substances present in food intended for infants below 16 weeks of age is recommended. The contribution of conventional food to the total exposure to pesticide residues is much higher than that from foods intended for infants and young children. Because of the increased intake of conventional food by young children, these have the highest exposure to pesticide residues, whereas infants 3–6 months of age generally have lower exposure. The impact of cumulative exposure to pesticide residues on infants and young children is not different from the general population and the EFSA cumulative risk assessment methodology is also applicable to these age groups. Residue definitions established under Regulation (EC) No 396/2005 are in general considered appropriate also for foods for infants and young children. However, based on a tier 1 analysis of the hydrolysis potential of pesticides simulating processing, the particular appropriateness of existing residue definitions for monitoring to cover processed food, both intended for infants and young children as well as conventional food, is questionable.
Keywords: infants, young children, pesticide residues, maximum residue level, health‐based guidance values
Summary
The European Commission asked the European Food Safety Authority (EFSA) to provide a comprehensive evaluation of pesticide residues in foods for infants and young children by reviewing the relevant opinions of the Scientific Committee for Food (SCF) of 1997/1998 in the light of scientific progress and provide advice to the Commission on the approach to lay down protective rules on the matter, taking into account the relevant provisions of Regulation (EU) No 609/2013. In the Opinion, developed by the EFSA Panel on Plant Protection Products and their Residues (PPR Panel), the following specific points of the Terms of Reference agreed with the Commission are covered:
The assessment of the appropriateness of the toxicological reference values for pesticides for infants and young children and of the approach to base the maximum residue limits (MRLs) for pesticides for food for infants and young children on the acceptable daily intake (ADI) values (in this context, the assessment of the short‐term dietary risk should also be considered);
The assessment of the contribution of other foods consumed by infants and young children that are not covered by Regulation (EU) No 609/2013;
The impact of a cumulative exposure to pesticides which share a common toxicological effect;
The appropriateness of residue definitions established under Regulation (EU) No 396/2005 for foods for infants and young children.
In addition, the Commission requested to take into consideration in the assessment the experience gained in the assessment of toxicological studies in the framework of the peer review under Regulation (EC) No 1107/2009 (including specific guidelines developed in that context).
The EFSA PPR Panel noted that EFSA, in the context of its duties under Regulation (EC) 1107/2009, has no specific experience with respect to the adequacy for infants and children of the hazard identification and characterisation process for pesticides active substances. Furthermore, a consultation of the Member States through the Pesticide Steering Network revealed that, at a national level, no such specific experience had been gained nor specific guidelines had been developed.
A literature search was performed to identify publications in the areas of toxicokinetics (TK), the physiology of the gut, the nervous system, the immune system, the male and female reproductive systems, the endocrine system in the developing infant and young child. The current evidence indicates that the differences in these areas between infants above the age of 16 weeks and young children and adults are rather limited in comparison with adults. In addition, it was noted that on a body weight basis, therapeutic doses of pharmaceuticals used in infants and young children do not differ much from those used in adults. Based on these findings, it was concluded that the ADI and the acute reference dose (ARfD) can be applied to infants above 16 weeks of age and young children, and that an additional assessment factor is not necessary for these age groups. For infants below the age of 16 weeks, it was concluded that the current approach for setting ADI and ARfD may not be appropriate and the application of the EFSA guidance on risk assessment of substances in food for infants below 16 weeks of age (EFSA Scientific Committee, 2017) is recommended.
For infants below 16 weeks of age, the potential residue intake was calculated to be 0.0026 mg/kg body weight (bw) per day. This calculation is based on an intake of infant formula of approximately 260 g/kg bw per day (EFSA Scientific Committee, 2017) and a default maximum pesticide residue level of 0.01 mg/kg for the infant milk. The EFSA PPR Panel concludes that potential residues at the default MRL of 0.01 mg/kg for food for infants and young children do not result in an unacceptable exposure to infants for any compound for which a health‐based guidance value (HBGV) of 0.0026 mg/kg bw per day or higher applies. For pesticides with HBGVs lower than 0.0026 mg/kg bw after application of the guidance on risk assessment of substances in food for infants (EFSA Scientific Committee, 2017), the default MRL of 0.01 mg/kg for foods intended for infants and young children may not be sufficiently protective, and for these pesticides, the approach to base MRLs on the HBGVs is considered appropriate.
For infants older than 16 weeks of age and young children, new methodologies are proposed to estimate combined exposure to pesticide residues via foods intended for infants and young children, and via other foods not covered by Regulation (EU) No 609/2013 (also referred to as conventional foods). Based on the observed dietary patterns, further distinction was made between infants 3–6 months of age, infants 6–12 months of age and young children (1–3 years of age, also often referred as ‘toddlers’). In order to test the proposed methodologies, calculations were carried out for five case studies. These calculations showed that, generally, exposure was the highest for young children and the lowest for infants from 3 to 6 months old. This increase in exposure with age is correlated to the increasing consumption of conventional foods, which was the main source of exposure in all case studies. The contribution of specific food intended for infants and young children to the overall exposure was low. The commodities driving the exposures depended on the use pattern of the pesticide under assessment. There were also large differences observed between exposures calculated according to a premarketing scenario (i.e. based on residue field trials when a pesticide active substance is evaluated in view of its placing on the market) and exposures calculated according to a post‐marketing scenario (i.e. based on results from monitoring programmes after the pesticide, active substance was placed on the market). Exposure estimates for the premarketing scenarios were 2–100 times higher compared to the post‐marketing scenario.
With respect to the impact of cumulative exposure to pesticide residues on infants and young children the current state of science and developments in the field of cumulative risk assessment were considered. It was concluded that the impact on infants and young children is not different from the general population and that the cumulative risk assessment methodology of EFSA PPR Panel is also applicable to these age groups.
In the European Union (EU), while residue definitions for monitoring are used to survey adequate pesticide applications, they are also intended to allow an indicative, post‐authorisation dietary risk assessment. In this respect, the scope of the maximum residue levels in food for infants and young children is not different to that for the general population. Residue definitions established under Regulation (EC) No 396/2005 are therefore in general considered appropriate also for foods for infants and young children. For monitoring and indicative risk assessment, the particular appropriateness of existing residue definitions established under Regulation (EU) No 396/2005 for infant food as regulated under Commission Directives 2006/125/EC and 2006/141/EC depends on whether potential changes in the nature of residue by food‐processing operations are covered by the actual expression of the residue definition and by appropriate conversion factors if applicable. In the current opinion, an analysis of the hydrolysis potential‐simulating pasteurisation, baking/boiling and sterilisation conditions, was performed on 111 pesticides with a single‐compound residue definition. Under conditions simulating pasteurisation, 4% of active substances considered showed recoveries of parent compound less than 50%. Harsher conditions representative for baking/boiling and for sterilisation showed instability in 12% and 21% of cases, respectively. Based on this analysis, it is concluded that there is doubt on the general appropriateness of existing residue definitions for monitoring to cover processed food, both intended for infants and young children as well as conventional food. Therefore, the EFSA PPR Panel recommends to perform a detailed analysis considering all‐embracing information for pesticide active substances, including the field residue situation of relevant compounds in the starting material as well as realistic industrial‐processing conditions.
EFSA PPR Panel makes the following additional recommendations. In order to address the relevant period of exposure in animal studies, the EFSA PPR Panel notes that the Extended One‐Generation Reproductive Toxicity Study (EOGRTS, OECD TG443) with the developmental neurotoxicity (DNT) and developmental immuno toxicity (DIT) cohorts and the DNT study (OECD TG426) are the only studies that specifically address the postnatal period and this also includes periods similar to the age of 3 years in humans. It is, therefore, recommended that all pesticides should be screened for DNT properties in a DNT in vitro testing battery (to be developed) and triggers for further regulatory studies should be reconsidered. The DNT test guideline (OECD TG426) should be reviewed and improved. Furthermore, if a reproductive study is to be performed, it should be the EOGRTS, including a DIT and DNT cohorts.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Relevant legal framework
Commission Directive 2006/125/EC1 and Commission Directive 2006/141/EC2 set specific requirements on the use of pesticides in products intended for the production of and on pesticide residues in infant formulae, follow‐on formulae, processed cereal‐based foods and baby foods for infants and young children. The requirements of these Directives are based on two opinions given by the Scientific Committee for Food (SCF) on 19 September 19973 and 4 June 1998.4 Because of the scientific uncertainty at that time as to the adequacy of existing acceptable daily intake (ADI) values of pesticides for the protection of health of infants and young children, it was considered appropriate to adopt, on the basis of the precautionary principle, a very low common limit for all pesticides in these foods. This very low common limit was fixed at 0.01 mg/kg which was in practice the limit of quantification (LOQ). More severe limitations were set for pesticides or metabolites of pesticides with an ADI lower that 0.0005 mg/kg body weight per day.
Regulation (EU) No 609/2013 of the European Parliament and of the Council5 requires the Commission to adopt delegated acts laying down specific rules for the foods under its scope by 20 July 2015. In this context, the Regulation requires to lay down (and regularly update) rules on pesticides in foods for infants and young children, taking into account the relevant rules of Directive 2006/125/EC and Directive 2006/141/EC. These rules should, among others, restrict as much as possible pesticides’ use in the production of infant formula, follow‐on formula, processed cereal‐based food, baby food and food for special medical purposes developed to satisfy the nutritional requirements of infants and young children. In addition, maximum residue levels of pesticides in such foods should be set at the lowest achievable level to protect vulnerable population groups.6
Preparation of the delegated acts and previous exchanges with EFSA
Taking into account the obligations of Regulation (EU) No 609/2013 described above and, in particular, the tight deadline for the adoption of delegated acts (20 July 2015), the Commission requested scientific assistance to EFSA on 29 October 20147 in accordance with Article 31(1) of Regulation (EC) No 178/20028. In that request, the Commission explained that it intended to temporarily maintain the existing approach on pesticides in foods for infants and young children based on the precautionary principle (default maximum residue limit (MRL)) and to update the outdated lists of substances originally included in Directive 2006/141/EC and Directive 2006/125/EC for which more severe limitations applied. EFSA was asked to assess whether the specific MRLs proposed by the Commission for these substances at the limit of quantification would lead to an exposure not exceeding the European or internationally agreed ADI taking into account relevant consumption data. The Commission also explained that it intended, after the adoption of the delegated acts, to require EFSA to re‐evaluate the entire existing approach on pesticides in foods for infants and young children in the light of scientific progress.
In its reply of 19 December 2014,9 EFSA accepted the Commission's request. It noted however that the approach envisaged by the Commission would not reflect the state‐of‐the art on the matter from a scientific point of view and that a more comprehensive evaluation should be carried out covering a series of specific elements. EFSA also explained that, in order to take into account recent food consumption data that was in the process of being compiled and due to its limited resources, it could only provide its scientific assistance by 18 June 2015.
After having analysed EFSA's comments and considering that EFSA's proposed timeline was incompatible with the deadline of 20 July 2015 for the adoption of delegated acts on foods for infants and young children, the Commission withdrew its request for scientific assistance with letter of 2 March 2015.10 The existing provisions of Directive 2006/141/EC (which have ensured a sufficient level of protection of infants so far) were included as such in Commission Delegated Regulation (EU) 2016/127 on these foods that was adopted by the Commission on 25 September in line with Article 11 of Regulation (EU) No 609/2013. It is however appropriate, at this stage, to require EFSA to start a comprehensive evaluation on the matter as outlined in the terms of reference below, in view of a future revision of the rules on the basis of the latest relevant scientific advice.
1.1.2. Terms of Reference
In accordance with Article 29(1)(a) of Regulation (EC) No 178/2002, the European Commission asks European Food Safety Authority (EFSA) to provide a comprehensive evaluation of pesticides in foods for infants and young children by reviewing the relevant SCF opinions of 1997/1998 in the light of scientific progress, and provide advice to the Commission on the approach to lay down protective rules on the matter, taking into account the relevant provisions of Regulation (EU) No 609/2013.
The requested Scientific Opinion would also profit from the EFSA guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age (EFSA Scientific Committee, 2017).
When carrying out its assessment, EFSA should address the specific points flagged in its letter to the Commission of 19 December 20149 and cover in particular:
The assessment of the appropriateness of the toxicological reference values for pesticides for infants and young children and of the approach to base the MRLs for pesticides for food for infants and young children on the ADI values (in this context the assessment of the short‐term dietary risk should also be considered);
The assessment of the contribution of other foods consumed by infants and young children that are not covered by Regulation (EU) No 609/2013;
The impact of a cumulative exposure to pesticides which share a common toxicological effect;
The appropriateness of residue definitions established under Regulation (EU) No 396/2005 for foods for infants and young children.
The experience gained in the assessment of toxicological studies in the framework of the peer review under Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market11 (including specific guidelines developed in that context) should also be taken into consideration by EFSA for its assessment.
1.2. Interpretation of the Terms of Reference
The EFSA Panel on Plant Protection Products and their Residues (PPR Panel) will develop a Scientific Opinion on pesticides in foods for infants and young children. Following further clarifications of the Commission, the EFSA PPR panel interpreted the Terms of Reference and the specific tasks as follows:
General considerations
The opinion should address the appropriateness of the currently established health‐based guidance values (HBGVs: ADI and acute reference dose (ARfD)) for the age groups of infants and young children given that, in 1997/1998, the SCF had raised doubts about their applicability for these age groups. The specific tasks were proposed by EFSA in the reply letter to the first Commission mandate received in October 2014.
Different terminologies are used in the legislation12 and by EFSA to define the two subgroups of the population under consideration. In the present opinion, the term ‘infants’ refers to the group 0–1 years of age, while the term ‘young children’ refers to the age group 1–3 years, also often referred to as ‘toddlers13’. In the current opinion, the term ‘young children’ will be used, in accordance to the Regulation (EU) No 609/2013.
All ‘exceptional cases’ will not be considered under this request, such as substances intentionally added to dietetic food and food for infants and young children as mineral (e.g. copper compounds), substances already present in the environment or in the food chain (e.g. CS2, bromide ion, etc.), persistent pesticides such as persistent organic pollutants from Stockholm Convention but also pesticides with DT90 (soil) > 100 days (e.g. boscalide, penthiopyrad, etc.). It is noted that analysis of soil is recommended before producing crops intended to food for infants and young children.
EFSA PPR Panel recognises that exposure of infants to pesticides via breast feeding, as has been demonstrated for substances like persistent organic pollutants, could potentially be significant. However, EFSA PPR Panel considered that this issue is outside the scope of the mandate.
Apart from chemical pesticide active substances, microbial active substances are also used as or in plant protection products. These microbial active substances or their secondary metabolites could remain as residues on edible parts of the plants. It is feasible that these residues have adverse health effects after consumption. However, since, in general, little information is available on the toxicology of, and exposure to these degradation products, the exposure of infants and children to residues from microbial pesticides and their possible adverse effects will not be addressed in this opinion.
Specific considerations on the Terms of Reference
In the first bullet point of the Terms of Reference (ToR 1), EFSA is requested to assess the appropriateness of the approach to base the MRLs for pesticides for foods for infants and young children on the ADI (and if appropriate ARfD) values.
The Commission clarified that in view of the conclusions of the former SCF (1997, 1998) EFSA should discuss whether the existing HBGVs set for the general population are appropriate for infants and young children and if so as from which age, and that EFSA should assess if specific HBGVs and/or additional Uncertainty Factors (UF) are necessary for infants and/or young children (either generally or for any specific age group).
MRLs at the lowest achievable levels to protect vulnerable population groups
Currently, the default MRL for pesticides in food for infants and young children is 0.01 mg/kg, with lower MRLs or a ban for some specific pesticides (Directive 2006/125/EC and Directive 2006/141/EC). Regulation (EC) 609/2013 requires that the EC has to make provisions (in the future) on the use/banning of pesticides according to those of Directive 2006/125/EC and 2006/141/EC. According to recital 21 of Regulation (EU) 609/2013, ‘The maximum residue levels in [infant] food should be set at the lowest achievable level to protect vulnerable population groups, taking into account good agricultural practices as well as other sources of exposure, such as environmental contamination.’
The Commission confirms the following:
the need to set ‘MRL as low as reasonably achievable’ to protect vulnerable population groups, i.e. if necessary and possible lower than the default MRL of 0.01 mg/kg.
-
to let EFSA chose the methodology to check whether:
-
–
current MRLs are safe (particularly default MRLs on foods for infants and young children);
-
–
new MRLs should be derived, and in which case(s);
-
–
real levels of pesticide residues in food (from monitoring programmes) do not present a risk for the infants and young children.
-
–
Contribution of other foods
In the second bullet point of the Terms of Reference (ToR 2), EFSA is requested to assess the contribution of other foods consumed by infants and young children that are not covered by Regulation (EU) No 609/2013.
The Commission clarified that the request is about the contribution of each food category (food for infants and young children and other food, i.e. conventional food) to the total dietary exposure (chronic and acute) for infants and young children.
The Commission specified that approaches/methodologies should be developed for both pre‐ and post‐marketing assessments, i.e. methods to check if MRLs are sufficiently protective for the consumers but also what the contributors are to the exposure considering monitoring results.
The Commission confirmed that EFSA can decide which methods are the best to address this task. Approaches/methodologies should be developed to check the safety of current MRLs of 0.01 mg/kg, of specific MRLs from legislation on infants and young children and from MRLs of Regulation (EU) No 396/2005 on conventional food. Monitoring data should also be used to estimate actual exposure at post‐marketing level. EFSA PPR Panel highlighted the fact that changing the exposure model for young children could also bring about different results than those currently obtained with the regulatory exposure model (PRIMo v2).
Impact of cumulative exposure
In the third bullet point of the Terms of Reference (ToR 3), EFSA is requested to address the impact of a cumulative exposure to pesticides which share a common toxicological effect. In this respect, EFSA PPR Panel will provide its views on whether the opinions and methodologies it delivered from 2008 to 2013 to support the cumulative risk assessment of pesticides apply to the toxicological effects which may affect populations of infants and children and the consequences of the application of cumulative exposure, such as the need for additional UF.
As EFSA PPR Panel is still developing the methodology for cumulative risk assessment, this will not be completely addressed for the specific mandate, but indications for the future applicability of the methodology will be provided in the opinion.
Appropriateness of residue definitions (RD)
In the fourth bullet point of the Terms of Reference (ToR 4), the Commission requests EFSA to address the appropriateness of the residue definitions established under Regulation (EC) No 396/2005 for foods for infants and young children. Regulation (EC) No 396/2005 establishes residue definitions suitable for monitoring which are often used for indicative risk assessments on basis of indicator compound(s) for the pesticide residue. These definitions are fully appropriate for risk assessment only when they are identical to the respective residue definition for risk assessment or, as approximation, converted by appropriate factors to the toxicological relevant residue comprising additional compounds.
Commission clarifies that:
currently, diverging residue definitions have been established for MRLs under Regulation No (EC) 396/2005 and for MRLs in food for infants and young children due to the fact that the legislation for infants and young children was not updated in parallel to Regulation (EC) No 396/2005. EC agreed that it is not necessary to address those residue definitions one by one. Hence, Commission expects EFSA to describe whether and in which cases a difference in residue definitions between both legislations would be justified. These approaches and general considerations could then be applied to substance‐specific assessments in the future. EC also explained that a Refit exercise of pesticides legislation is currently ongoing which will result in a report to Council and Parliament in 2019. Once this review is finalised, steps are foreseen to align the legislation on infants and young children to Regulation (EC) No 396/2005, in particular to address the issue of diverging residue definitions;
the issue is not limited to foods for infants and young children, since the Regulation (EC) No 396/2005 also does not foresee specific residue definitions for processed food;
if relevant metabolites14 are evidenced for processed food for infants and young children, EFSA should present in the opinion the potential issue and possible recommendations should be provided (e.g. to decide on the need for a possible follow‐up mandate).
Experience gained under Regulation (EU) No 1107/2009
EFSA is requested to take into consideration the experience gained in the assessment of toxicological studies in the framework of the peer review under Regulation (EU) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market (including specific guidelines developed in that context). EFSA has no specific experience with respect to the adequacy for infants and children of the hazard identification and characterisation process for pesticides active substances but will invite Member States to share their experience at national level through the Pesticides Steering Network.
2. Data and methodologies
2.1. Literature review
Literature searches were performed in Web of Science (http://wok.mimas.ac.uk/) to identify publications in the public domain in the following areas relevant to the developing infant and young children:
Physiology of the gut
Metabolic and excretory capacities relevant for the elimination of chemicals
The nervous system, including brain and brain barriers
The immune system
The male and female reproductive systems
The endocrine system
All searches were carried out in English. Comprehensive reviews covering the above areas were identified by applying two different protocols for the search of literature on the two subpopulations, as described in Table 1.
Table 1.
Search protocols
| Subpopulation | Information source | Search terms (title) | Timespan |
|---|---|---|---|
| Infants | Web of Science |
1. ‘intestin*’ OR ‘gastro*’ AND ‘development*’ OR ‘infant’ OR ‘neonat*’; 2. metabolism’ OR ‘cytochrome’ OR ‘CYP*’ OR ‘glucuronidation’ OR ‘glucuronosyl*’ OR ‘sulfation’ OR ‘sulphation’ OR ‘sulfonyl*’ OR sulphonyl*’ OR ‘acetylation’ OR ‘conjugation’ OR ‘glutathione OR ‘transporter’ or ‘clearance’ OR ‘ADME’ AND ‘development*’ OR ‘infant’ OR ‘neonat*’; 3. ‘brain’ AND ‘development*’ OR ‘infant’ OR ‘neonat*’; 4. ‘immun*’ AND ‘development*’ OR ‘infant’ OR ‘neonat*’; 5. reproduct* OR gonad* OR sexua* OR endocrin* AND development* OR infant OR neonat* |
May 2016a–May 2017 |
| Young children | Web of Science |
1. ‘intestin*’ OR ‘gastro*’ AND ‘development*’ OR ‘child*’ OR ‘toddler*’; 2. ‘metabolism’ OR ‘cytochrome’ OR ‘CYP*’ OR ‘glucuronidation’ OR ‘glucuronosyl*’ OR ‘sulfation’ OR ‘sulphation’ OR ‘sulfonyl*’ OR sulphonyl*’ OR ‘acetylation’ OR ‘conjugation’ OR ‘glutathione OR ‘transporter’ or ‘clearance’ OR ‘ADME’ AND ‘development*’ OR ‘ontogeny’ OR ‘child*’ OR ‘toddler*’; 3. ‘brain’ AND ‘development*’ OR ‘child*’ OR ‘toddler*’; 4. ‘immun*’ AND ‘development*’ OR ‘child*’ OR ‘toddler*’; 5. ‘reproduct*’ OR ‘gonad*’ OR ‘sexua*’ OR ‘endocrin*’ AND ‘development*’ OR ‘child*’ OR ‘toddler*’ |
January 2012–May 2017 |
To update the literature review reported by the Scientific Committee guidance on the ‘risk assessment of substances present in food intended for infants below 16 weeks’ (EFSA Scientific Committee, 2017).
All titles and abstracts retrieved from the literature search were imported into an EndNoteTM Library and the duplicates removed.
The abstracts of 1,860 retrieved references found for infants (reviews published in 2016–2017) and young children (reviews published from 2012 to 2017) were independently screened in parallel by two members of the WG based on their relevance to the assessment. This was defined by the coverage in the reference of the developing systems in the infant and young children period. Moreover, a scoring system was applied to the retrieved references in the EndNoteTM Library to select relevant, not relevant and possibly relevant references.15 Relevant references identified for the critical review are reported in Table 2.
Table 2.
Relevant references selected for the developing systems
| Subpopulation | Developing systems | Relevant references (from retrieved references) |
|---|---|---|
| Infants |
Physiology of the gut Metabolic and excretory capacities relevant for the elimination of chemicals Nervous system, including brain and brain barriers Immune system Male and female reproductive and endocrine systems |
8 (33) 7 (43) 24 (108) 12 (112) 6 (47) |
| Young children |
Physiology of the gut Metabolic and excretory capacities relevant for the elimination of chemicals Nervous system, including brain and brain barriers Immune system Male and female reproductive and endocrine systems |
17 (169) 30 (155) 50 (426) 21 (476) 18 (291) |
Additional primary references of particular relevance were identified by the working group members. Moreover, publicly available guidance documents and reports produced by committees and international authorities that were relevant to risk assessment of substances in food intended for infants were considered.
2.2. Occurrence data on pesticide residues
The dietary exposure assessment was based on the occurrence data. In order to assess the contribution of foods that are intended for infants and young children as well as the contribution of conventional foods that are not covered by Regulation (EU) No 609/2013, dietary exposure of infants and young children was calculated for five different case studies reported in Appendix A. The criteria for selecting those case studies are also reported in the appendix.
Depending on the exposure scenario, two types of occurrence data were used:
Regulatory data for premarketing scenarios (i.e. results of supervised field trials)
Monitoring data for post‐marketing scenarios (i.e. results of the monitoring programmes)
2.2.1. Regulatory data (premarketing)
When a pesticide active substance is evaluated in view of its placing on the market, pesticide residue concentrations in the raw primary commodities are determined at premarketing level from supervised field trials according to international guidelines (FAO, 2009). Among all pesticide, use patterns that are intended for authorisation, these supervised field trials are designed to reflect the use patterns that lead to the highest possible residues.
In order to assess dietary exposure through conventional foods, available field trials are normally used to derive the following parameters.
-
Supervised Trial Median Residue (STMR)
This parameter is the median residue level estimated from the supervised field trials. It is used as an input value to estimate chronic dietary exposure and acute dietary background exposure.
-
Highest Residue (HR)
This parameter is the highest measured residue level in the supervised field trials. It is used as an input value to estimate acute dietary exposure through a single food item and is therefore only derived for active substances where an ARfD is proposed or established.
-
Maximum residue levels (MRL)
Where the use of pesticide is intended/authorised and supervised trial median residue (STMR) or highest residue (HR) values are not available to EFSA, the MRL value is used as a worst‐case input value for both chronic and acute dietary exposure assessment.
-
Conversion factor (CF) for risk assessment
Where the residue definitions for enforcement and risk assessment purposes differ, the CF refers to the ratio of the residue concentration for risk assessment over the residue concentration for enforcement. Since the STMR and HR values collected in this framework refer to the residue definition for enforcement, STMR and HR values are multiplied by the appropriate CF (when applicable).
-
Peeling factor (PF)
This parameter refers to the ratio of the residue concentration in the peeled commodity over the residue concentration in the unpeeled commodity and is normally only derived for fruits and vegetables that are commonly peeled prior to consumption. Since the STMR and HR values collected in this framework refer to the unpeeled commodity, STMR and HR values are multiplied by the appropriate PF (when available).
For food intended for infants and young children, occurrence data for premarketing assessment during approval or authorisation of a pesticide are not available. However, according to Directives 2006/125/EC16 and 2006/141/EC17 repealed by Regulation (EC) No 609/201318, a default MRL of 0.01 mg/kg is applicable, except for cadusafos, demeton‐S‐methyl, ethoprophos, fipronil, propineb and their metabolites for which lower MRLs have been established. In the absence of specific occurrence data, the MRL is used for both chronic and acute dietary exposure assessment under the premarketing scenario.
Regarding the five case studies reported in Appendix A, the parameters reported above were extracted from EFSA's reasoned opinions on the review of the existing MRLs prepared under article 12 of Regulation (EC) No 396/2005 (EFSA, 2011c,d, 2013c, 2014a, 2015c) and all subsequent reasoned opinions prepared under Article 10 or Article 43 of Regulation (EC) No 396/2005 (EFSA, 2011b,e, 2012a,b,c, 2013a,b, 2014b,c,d, 2015a,b, 2016). In order to ensure consistency with the monitoring data described in Section 2.2.2, only the reasoned opinions issued before 31 December 2015 were considered. All relevant parameters (including those at LOQ) were extracted for those pesticide/commodity combinations where an intended or authorised use was reported to EFSA and where the MRL proposed by EFSA was legally implemented by the European Commission.
A general overview on the number of reasoned opinions and premarketing data considered for the assessment of each case study is provided in Table 3. Further details on the occurrence data used for the premarketing scenario are reported in Annex A – Table 2.
Table 3.
Overview of the number of reasoned opinions and food items considered for assessment of the five case studies under the premarketing scenario
| Pesticide | Year of MRL reviewa | Number of other reasoned opinionsb | Food items | ||
|---|---|---|---|---|---|
| Number of MRLs | Number of STMRs | Number of HRs | |||
| Azoxystrobin | 2013 | 1 | 141 | 140 | n.a. |
| Deltamethrin | 2015 | 0 | 142 | 142 | 142 |
| Fludioxonil | 2011 | 4 | 118 | 118 | n.a. |
| Pyraclostrobin | 2011 | 7 | 131 | 130 | 130 |
| Thiacloprid | 2014 | 2 | 123 | 123 | 123 |
n.a.: not applicable.
Reasoned opinion on the review of the existing MRLs (prepared under Art. 12 of Regulation (EC) 396/2005).
Number of subsequent reasoned opinions issued before 31 December 2015 (prepared under Art. 10 or Art. 43 of Regulation (EC) 396/2005).
2.2.2. Results from the monitoring programmes (post‐marketing)
Regulation (EC) No 396/2005 imposes on Member States the obligation to carry out controls to ensure that food placed on the market is compliant with MRLs. This regulation establishes both EU‐coordinated control programme (EUCP) and national control programmes (NP). According to Article 31 of Regulation (EC) No 396/2005, Member States are requested to share the results of these monitoring programmes and other relevant information with the European Commission, EFSA and other Member States.
Each year, EFSA publishes the EU Report on pesticide residues in food, based on the results of the latest monitoring programmes. In its report, EFSA analyses the data in view of reporting MRL compliances and assesses the dietary exposure to pesticide residues and the related risk for European consumers.
For the five case studies reported in Appendix A, the results of the 2015 monitoring programmes (EUCP and NP), detailed in the EU report on pesticide residues in food (EFSA, 2017) were extracted for the five pesticides of interest (see Table 4). These data cover more than 296 food products including 291 raw primary commodities and five food categories intended for infants and young children:
Infant formulae
Follow‐on formulae
Processed cereal‐based foods for infants and young children
Baby foods other than processed cereal‐based foods
Food for infants and young children (unspecified)19
Table 4.
Overview of the results from the 2015 monitoring programmes for the five studied pesticides (EFSA, 2017)
| Pesticide | No food items (total)a | No samples analysed | % positive samples (> LOQ) | No baby food itemsa | No baby food samples analysed | % positive baby food samples (> LOQ) |
|---|---|---|---|---|---|---|
| Azoxystrobin | 285 | 63,470 | 5.6 | 5 | 1,232 | 0.0 |
| Deltamethrin | 293 | 66,026 | 1.2 | 5 | 1,198 | 0.0 |
| Fludioxonil | 274 | 59,680 | 6.0 | 5 | 1,089 | 0.09 |
| Pyraclostrobin | 275 | 58,357 | 1.2 | 4 | 1,120 | 0.0 |
| Thiacloprid | 282 | 59,115 | 2.8 | 4 | 1,119 | 0.0 |
| Total | 296 | 306,648 | 4.0 | – | 5,758 | 0.0 |
The available data, therefore, allow estimating exposure through each of the above‐reported food categories separately.
As indicated in Table 4, results of the monitoring programmes also include a large proportion of left‐censored data (results below the LOQ). In order to estimate the uncertainty related to those left‐censored data, the post‐marketing scenario for exposure assessment is further differentiated into a lower bound (LB) and an upper bound (UB) scenario. While the LB scenario leads to an underestimation of the exposure since it postulates that samples with results below the LOQ are completely free of the pertinent pesticide, the UB scenario is a conservative screening which is likely to overestimate the real exposure, since levels below the LOQ should be set to the LOQ if the pesticide is expected in food.
Hence, in view of performing exposure calculations under the LB and UB post‐marketing scenarios, the following parameters were derived for each active substance and food category of interest.
-
Lower bound (LB) mean
The LB mean is the mean residue concentration estimated from the individual sample results, where each result below the LOQ is set at zero. This parameter is used as an input value to estimate both chronic dietary exposure and acute dietary background exposure under the LB scenario. Where regulatory data indicated that the pesticide is not authorised for use on a given primary commodity (see Section 2.2.1), the LB mean is also used for the UB scenario.
-
Upper bound (UB) mean
The UB mean is the mean residue concentration estimated from the individual sample results, where each result below the LOQ is set at the LOQ. This parameter is used as an input value to estimate both chronic dietary exposure and dietary background exposure under the UB scenario, unless regulatory data indicated that the pesticide is not authorised for use on a given primary commodity (see Section 2.2.1).
-
95th Percentile (P95)
The 95th percentile is estimated from the individual sample results, where each result below the LOQ is set at the LOQ. This parameter is used as an input value to estimate acute dietary exposure through a single food item, both under the LB and UB scenarios.
As the samples from the monitoring programmes are normally analysed for the enforcement residue definition without peeling, these parameters were multiplied by a peeling factor and CF for risk assessment when available from the regulatory data (see Section 2.2.1).
Further details on the occurrence data used for both LB and UB post‐marketing scenarios are reported in Annex A – Table 2.
2.3. Consumption data
2.3.1. EFSA Comprehensive Database
The EFSA Comprehensive European Food Consumption Database20 (Comprehensive Database) provides a compilation of existing national information on food consumption at individual level. It was first built in 2010 (EFSA, 2011a; Huybrechts et al., 2011; Merten et al., 2011) and subsequently updated upon reception of new dietary surveys from Member States. Details on how the Comprehensive Database is used are published in the Guidance of EFSA (EFSA, 2011a).
Overall, the food consumption data gathered by EFSA in the Comprehensive Database are the most complete and detailed data currently available in the EU and is already used by EFSA in the other areas, e.g. chemical contaminants, food and feed additives, and nutrition. Consumption data were collected using single or repeated 24‐ or 48‐h dietary recalls or dietary records covering from 3 to 7 days per subject.
For the purpose of this assessment, the Comprehensive Database as of 31 March 2018 was used and only subjects from the following age classes were selected:
Infants (3–6 months): ≥ 3 months to < 6 months old
Infants (6–12 months): ≥ 6 months to < 12 months old
Young children21: ≥ 12 months to < 36 months old
Dietary survey/age class with less than five consumers were disregarded from the assessment because such a data set was not considered robust enough to derive any reliable statistics (EFSA, 2011a). Furthermore, when two different dietary surveys were available for one particular country and age class, only the most recent survey was retained for assessment. Details of the dietary surveys selected for the current assessment are reported in Annex A – Table 1.
Within the Comprehensive Database consumption data for conventional foods are reported separately from those specifically intended for infants and young children. As the occurrence data for pesticides usually refer to Raw Primary Commodities (RPC), the consumption data for conventional foods were subject to a draft conversion model which is currently under development in EFSA. This model disaggregates consumption data for conventional composite foods (e.g. pizza) to primary ingredients (e.g. wheat flour or tomato puree), which are subsequently converted to the RPC (e.g. wheat grain or tomato) by means of reverse yield factors. Through this conversion model, a compilation of RPC consumption data at individual level is obtained where for each consumption event the corresponding amount of primary commodity is recorded. In order to ensure compatibility with the available occurrence data, the consumption data obtained were classified according to Annex I of Regulation (EC) No 396/2005. It should be noted, however, that the conversion of food consumption data to RPCs was still under validation at the time of performing the calculation. This uncertainty should be considered when assessing upper tail exposures obtained with these consumption data.
Regarding foods for infants and young children, consumed amounts were not disaggregated and retained as consumed, except for powdered infant and follow‐on formulae. The latter were converted to the amount of liquid formulae, assuming that one part of powder is diluted in seven parts of water (EFSA Scientific Committee, 2017). These consumption data were then classified according to the same food categories as those reported for the post‐marketing occurrence data:
Infant formulae
Follow‐on formulae
Processed cereal‐based foods for infants and young children
Baby foods other than processed cereal‐based foods
2.3.2. Pesticide Residues Intake Model (PRIMo)
The consumption data reported in the Pesticide Residue Intake Model version 2 (PRIMo v2) currently used in EFSA are detailed in a previous EFSA Opinion (EFSA, 2007a,b). In PRIMo v2, the reported consumption data were converted to raw primary commodities by the Member States and reported as edible portion. The chronic consumption data reported in the PRIMo refer to mean consumption values per survey while the acute consumption data refer to so called ‘large portions’, which mostly corresponds to the 97.5th percentile of the reported consumptions within a single day and survey.
It should be noted, however, that PRIMo v2 does not contain any specific consumption data on baby foods. Moreover, only four surveys were reported for infants and young children and none is adequate for infants below 6 months. Although in PRIMo v3 the number of surveys for these age classes was increased (EFSA, 2018), this latest version of the PRIMo was not yet implemented at the time of the assessment of the case studies.
2.4. Methods for exposure assessment
2.4.1. Exposure assessment for infants below 16 weeks of age
In order to assess exposure of infants below 16 weeks of age to pesticides, the PPR Panel adopted the recommendation of the EFSA Scientific Committee to assume a 95th percentile consumption of infant formula of approximately 260 g/kg body weight (bw) per day, based on 32.5 g/kg bw powder and 227.5 mL/kg bw water (EFSA Scientific Committee, 2017). According to the Scientific Committee, infants from birth up to 16 weeks are expected to be exclusively fed on breast milk and/or infant formula. The recommended approach uses the highest reported consumption of infant formula for the first 16 weeks of age as from the period of 14–27 days of life. The consumption pattern over the 2‐week period requires the assessment for both acute toxicity and other toxicity endpoints.
For premarketing assessments during approval or authorisation of a pesticide, occurrence data for infant formula are not available. The default MRL of 0.01 mg/kg for infant formulae (and follow‐on formulae for older age groups) refers to the product as proposed ready for consumption or as reconstituted according to the instructions of the manufacturer (Article 10 of Directive 2006/141/EC). The potential contribution of relevant residues present in groundwater used for drinking water (< 0.1 μg/L,22 equivalent to < 0.000023 mg/kg bw per day) is therefore covered by the existing MRL. In order to calculate the maximum exposure and to assess the protectiveness of the default value, a theoretical worst‐case exposure was calculated by multiplying the maximum consumption of 260 g/kg bw per day with the default MRL.
In view of the homogeneous nutrition of infants below 16 weeks by infant formula and the limited number of analyses, a simple worst‐case exposure assessment using maximum intake values and virtual residues at the MRL level is considered sufficient (cf. premarketing assessment). No post‐marketing exposure analyses are therefore performed.
A reverse exposure calculation for infants below 16 weeks of age is carried out in order to estimate a trigger value for ADI and ARfD, below which the default MRL of 0.01 mg/kg might not be sufficiently protective. It is noted that in order to ascertain that the HBGVs for a pesticide can be used for infants under the age of 16 weeks the guidance on risk assessment of substances in food for infants, published in 2017 (EFSA Scientific Committee, 2017) should be applied. The ADI is usually the adequate HBGV to cover both acute toxicity and potential periods of high sensitivity for other toxicity endpoints. In particular cases, the ARfD with an additional UF (as indicated upon assessment of the toxicological data package in EFSA Scientific Committee, 2017) might represent a more conservative case: however, this factor is not recommended to be used as default.
It should be noted that the use of the MRL as intake value does not explicitly incorporate the potential occurrence of metabolites. It is, however, assumed that this uncertainty is covered by the conservative assumption of residues at the MRL level.
2.4.2. Exposure assessment for infants above 16 weeks of age and young children
Within the regulatory framework of pesticide residues, dietary exposure is normally calculated by means of the EU PRIMo which uses a fully deterministic methodology (EFSA, 2007a,b). However, to assess the dietary exposure to pesticides for infants above 16 weeks of age and young children, the PPR Panel opted for a methodology that is also deterministic but where, unlike the PRIMo, exposure is calculated for each individual in the consumption surveys. This individual‐based approach was considered more appropriate because it results in a distribution of exposures (rather than a single exposure estimate) and it accounts for variability within a population (e.g. consumer habits and bodyweights). This methodology also provides more flexibility because it allows calculating exposure for any percentile of the exposure distribution that is considered adequate by the risk manager.
Furthermore, the individual‐based approach uses consumption data from the Comprehensive Database. As already discussed in Section 2.3, this database contains data on the consumption of foods for infants and young children, which is currently only partly available in PRIMo. The Comprehensive Database also includes a wider range of surveys and a more accurate classification by age class is possible. The individual‐based approach is, therefore, expected to better address the terms of reference as the exposures will no longer be averaged among different age classes.
Regarding the acute exposure calculations, the individual‐based approach also allows for the estimation of both total acute exposure (assuming a background concentration) and acute exposure per food commodity, while acute exposure in the PRIMo is only calculated per food commodity.
Although processing factors were not included in the current assessment (except peeling), it is noted that the individual‐based model also provides more flexibility compared to PRIMo regarding the possible incorporation of processing factors for a refined exposure assessment.
Nevertheless, all MRLs that are currently in place for the active substance assessed in Appendix A, were previously evaluated on the basis of revision 2 of the PRIMo. In order to compare the outcomes of both methodologies, exposure calculations were also carried out with PRIMo v2.
2.4.2.1. The individual‐based approach
When calculating chronic dietary exposure, consumption data and body weight data from the RPC consumption database were assessed at the individual level. For each individual of the selected dietary surveys, the average daily consumption of each RPC or infant food category was multiplied by the occurrence value of the corresponding food item and scenario (see Table 5), and the resulting exposures per food were summed in order to obtain the total chronic exposure at individual level (standardised by using the individual body weight). Summary statistics of the individual exposures were subsequently calculated for all dietary survey and age class combinations. The 95th and the 97.5th percentile exposures were only calculated for survey and age class combinations with more than 60 and 180 consumers, respectively. Average contributions of the individual foods to the total chronic exposure were calculated for all individuals within a given survey and age class.
Table 5.
Selection of the relevant occurrence input value for each exposure scenario
| Scenario | Data type | Chronic exposure | Acute exposure | |
|---|---|---|---|---|
| Single food | Background | |||
| Premarketing | Regulatory | STMRa | HRb | STMRa |
| Post‐marketing (LB) | Monitoring | LB mean | P95 | LB mean |
| Post‐marketing (UB) | Monitoring | UB meanc | P95 | UB meanc |
HR: highest residue; LB: lower bound; STMR: supervised trial median residue; UB: upper bound; P95: 95th percentile.
Where the STMR is missing, the EU Maximum Residue Level is used instead.
As some of the occurrence data referred to post‐harvest treatments, it was decided to use the HR in all IESTI equations (including the case 3 calculations). Furthermore, where the HR is missing, the EU Maximum Residue Level is used instead.
Where a pesticide is not authorised for use on a given commodity, the LB mean is used instead.
As suggested by the EFSA WG on Food Consumption and Exposure (EFSA, 2011a), dietary surveys with only 1 day per subject were excluded from the chronic exposure assessment because they are not adequate to assess repeated exposure. Similarly, subjects who participated only 1 day in the dietary studies, when the protocol prescribed more reporting days per individual, were also excluded from the chronic exposure assessment. These exclusion criteria did not apply to the acute exposure assessments.
Acute dietary exposure was calculated by assessing the consumption data at the level of the single reporting days. Within each individual's reporting day, the acute exposure was calculated for each infant food category or primary ingredient expressed in amount of RPC (e.g. wheat flour expressed in amount of wheat or apple juice expressed in amount of apple). This calculation was based on the International Estimated Short‐Term Intake (IESTI) as it is currently implemented in the EU (EFSA and RIVM, 2015), although specific adjustments were needed in order to meet the needs of the current assessment.
Case 1 (U < 25 g):
Case 2a (U < Cons):
Case 2b (Cons < U):
Case 3 (processed and bulked food):
Where: Cons = Primary ingredient consumed, expressed in amount of raw primary commodity
Occ = Occurrence value selected for the relevant scenario (see Table 5 )
bw = individual's body weight
U = Unit weight of the raw primary commodity (see Annex A – Table 3)
V = variability factor (see Annex A – Table 3)
In parallel, background exposure resulting from each primary ingredient or infant food category was calculated by multiplying the amount consumed (expressed in amount of RPC and normalised for the individual's body weight) within that day with the relevant occurrence value (see Table 5). Total dietary exposure for each individual's reporting day was subsequently calculated by summing the highest acute exposure observed for a single primary ingredient, and the background exposures for all other foods consumed within that day. Summary statistics for the total acute exposure were derived for each survey and age class combination. Contribution of the background exposure to the total acute exposure within a given survey and age class was calculated for both the full distribution (i.e. all reporting days) and the upper tail distribution (i.e. reporting days where total acute exposure exceeded the 95th percentile exposure within that survey and age class). Acute exposure by individual RPC was calculated by summing the highest acute exposure observed for a single primary ingredient and the background exposures for other primary ingredients derived from the same RPC and consumed within that day. In this case, summary statistics of the acute exposure were generated for each combination of survey, age class and RPC.
For the case studies reported in Appendix A, chronic and acute dietary exposure was assessed according to different scenarios, comprising premarketing (based on regulatory data) and post‐marketing situations (based on monitoring data). For the post‐marketing scenarios, a distinction was made between an UB and a LB scenario. The selection of the relevant occurrence input value for each scenario was discussed in Chapter 2.2.2 and is summarised in Table 5. Where applicable, these occurrence data have been corrected by the use of an additional peeling factor or CF for risk assessment. The calculated exposures were expressed in percent of ADI or ARfD, respectively, while contributions of individual foods or background exposures were expressed in percent of total exposure.
In order to assess exposure through the consumption of specific foods for infants and young children only, an additional scenario was carried out where all conventional foods were excluded from the assessment and both the median and maximum occurrence values in all specific foods were assumed to be at the default MRL of 0.01 mg/kg. In this case, the exposures were expressed in mg/kg bw per day (see Chapter 5.3.1).
2.4.2.2. The EU Pesticide Residues Intake Model (PRIMo)
For the premarketing scenario, EFSA also assessed the short‐term (acute) and long‐term (chronic) dietary exposure to pesticide residues and the related risk for the EU consumers by using PRIMo v2, and occurrence values reported in Table 5. Primo v2 is a deterministic model in which single‐point estimates are used for all input data, i.e. occurrence and consumption data. This calculation tool was originally developed by EFSA for the risk assessment in the context of pesticide authorisations (EFSA, 2007a,b). The model implements the principles of the WHO methodologies for short‐term and long‐term risk assessment (FAO, 2009), based on the food consumption of the European population. The calculations are generally acknowledged as a conservative risk assessment screening. This PRIMo calculation tool is available on the EFSA website.
3. The developing infant and young child: physiological and biochemical considerations
In 2017, the ‘guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age’ developed by the EFSA Scientific Committee was published (EFSA Scientific Committee, 2017). Although the guidance addresses specifically the risk assessment of infants less than 16 weeks of age, the description of physiological development also largely covers infants and young children above 16 weeks of age. For the present opinion, an additional literature search was performed to identify new publications since 2017 and also publications addressing the specific development various subgroups of infants and young children (see Section 2.1). Although the search yielded some new relevant studies, in particular for the nervous system, the findings were in line with those described by the EFSA Scientific Committee (2017). Below a summary of the evaluation of the physiological development of infants and young children is presented. Additional information on the developing nervous system, not described in the guidance of the Scientific Committee is presented in Appendix B.
Generally, the major physiological differences between infants and children as compared to adults are observed in the first weeks after birth, in particular in preterm neonates (WHO, 2006). Maturation of organs and systems occurs at different rates. Some parameters, e.g. certain cytochrome P450 isoforms, reach adult levels within weeks up to months, whereas other functions, organs and physiological systems, such as the brain and the immune system take several years or even decades to reach maturity (EMA, 2009; Pettengill et al., 2014; de Wildt et al., 2014; He et al., 2016).
The development of organs and systems affecting the TK of chemical substances is well studied. At birth, gastric, pancreatic and biliary functions are not fully mature and the absorption of substances in general might be slower, although the amount absorbed is not dependent on the age (Zoppi et al., 1972; Menard et al., 1995; Kearns et al., 2003).
Infants and children have a higher intake of drinking water consumption per kilogramme of body weight. This is estimated to be 1 L of water/day for a 10 kg child against a lifetime average figure of 2 L per day for a 70 kg adult (USA EPA, 2012). This has been taken into account in risk assessment of sub chronic studies. The distribution of the substance may be different from that in adults due to, for instance, differences in body composition, regional blood flow, organ perfusion and cardiac output and plasma protein‐binding capacity between infants and adults (Fredholm et al., 1975; Windorfer et al., 1978; Friis‐Hansen, 1983; Mielke and Gundert‐Remy, 2009). The capacity of enzymes involved in phases I and II metabolism is generally up to two‐ (full term) to threefold (preterm) lower when comparing infants with healthy adults. Likewise, renal function is also reduced at birth and its function increases in the first year of life.
The maturation of the nervous, skeletal and the immune system in children continues well into their teens, although various immune parameters reach adult levels at different rates.
Contrary to previous assumptions, the healthy blood–brain barrier (BBB) in the first 16 weeks, although immature, is considered functional and not leaky (Saunders et al., 2014). However, the expression/activity of transporters and metabolising enzymes appears to vary from adult patterns to meet the needs of the developing brain and apparently give rise to the measured differences in uptake and metabolism rates across the neonatal and infant BBB (Ek et al., 2012; Mann et al., 2016). The developing immune system in foetal and early postnatal life is particularly sensitive to immunotoxicants (DeWitt et al., 2012, Kollmann et al., 2012; Krishnamoorthy et al., 2012). Neonates have specific features in the development and maturation of their immune system, which make their response to an immunogenic/allergenic compound different from that of an adult and therefore, deserve specific studies. The development of the female reproductive system in infants below 16 weeks of age is relatively quiescent (Neal‐Kluever et al., 2014). For the development of the male reproductive organ the testis, however, this is a sensitive period (Camatini et al., 1981; Lemasters et al., 2000). Effects on the development of the reproductive organs generally become apparent later in life. Infants below 16 weeks of age have specific endocrine profiles that are different from those in adults (Grumbach, 2002; Kuiri‐Hänninen et al., 2014).
Thus, effects on the nervous, immune, reproductive, and endocrine systems as well as skeletal system at any early developmental stage may be reflected in deficits at later time points, indicating that the window of exposure, the window of maximum sensitivity and the window of effect require a lifespan approach to generate a fully protective risk assessment.
In infants older than 16 weeks generally, the expression of metabolising enzymes and the renal excretion approaches adult levels. Furthermore, although the maturation of the organ systems in infants above the age of 16 weeks and children may continue well into their teens, the available information indicates that the differences between these age groups and adults are rather limited.
3.1. Appropriateness of animal models and recommendations
The recently adopted Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age (EFSA Scientific Committee, 2017) specifically addresses the testing of the developing immune and nervous system in regulatory animal studies. The discussion of the animal models is not strictly limited to the age below 16 weeks. Further general elaborations can be found in Felter et al., 2015.
The WHO (2006) provides an elaborate comparison between humans and animal test species. Since the standard requirement for pesticides is studies in rodents and nearly always in rats, this will only be discussed below. The reproductive/developmental neurotoxicity (DNT) studies available for pesticides cover at least the period until weaning which is roughly equivalent to the age of 3 years in humans.
To address this period of exposure, according to the data requirements as a minimum (EU, 201323), the two‐generation reproduction toxicity study (OECD TG 416) is available. Alternatively, the extended one‐generation reproductive (EOGRTS; OECD TG 443) may be considered. As observed in guidance on the risk assessment of substances in food intended for infants below the 16 weeks of age (EFSA Scientific Committee, 2017), the EOGRTS with the DNT and developmental immuno toxicity (DIT) cohorts and the DNT study (OECD TG 426) are the only studies that specifically address the postnatal period and this also includes periods similar to the age of 3. However, these two tests (DNT and DIT) are not mandatory for pesticides and are only triggered by observations of neurotoxicity (NT) or immunotoxicity (IT) in other toxicity studies. The EOGRTS including the DNT cohort may be considered as an alternative to development and reproduction studies in rats and could be required for specifically addressing DIT, DNT and endocrine disruptive properties. The DNT TG 426 is triggered when there are signs of adult NT, when the pesticide has a neurotoxic pesticidal mode of action, or if it shares structural similarities to a substance with known DNT properties.
Recent analysis24 of the availability of such studies in pesticide risk assessment particular covering the infant period showed that out of the 485 pesticides approved in Europe, only 35 had DNT studies. Moreover, status is that the EOGRTS has been provided only in very few cases, in particular for pesticides undergoing renewal. The lack of EOGRTS with the DIT/DNT is clearly considered an uncertainty in many regards.
However, in the future, it can be foreseen that the EOGRTS probably would be available in more cases since this study might be triggered in regard to assessing the endocrine disruptive properties of pesticides according to the draft guidance for the identification of endocrine disruptors in the context of Regulation (EU) No 528/2012 and (EC) No 1107/2009. The criteria for identifying the endocrine disruptors require an endocrine mode of action as well as an adverse effect. Hence, for many of the oestrogen‐, androgen‐, thyroid‐ and steroidogenesis‐mediated effects, the EOGRTS will be required to detect the adverse effect.
Regarding identifying DNT properties, it has been recommended that in vitro methods should be developed (EFSA PPR Panel, 2013a,b,c; Fritsche et al., 2017; OECD, 2017). There is consensus that such in vitro assays should cover key processes (neuronal proliferation, migration, differentiation, synaptogenesis, myelin formation and neuronal network formation and function) during the development of the nervous system and should be conducted with all relevant cell types (neurons and glial cells). Recently, the OECD has taken up this activity and will develop a complementary guidance for interpreting and integrating such data into regulatory decision making.
However, as acknowledged, the current DNT (OECD TG 426) protocol has limitations and uncertainties, in particular in regard to sensitivity, reproducibility, relevance of extrapolation from rodent to humans due to kinetics, timing in brain development, use of non‐homologues functional test and that rodents do not capture relevant human diseases like autism/ADHD.
Overall, it should be recommended in the future to (1) screen all pesticides for DNT properties in DNT testing battery, (2) review and improve the DNT (OECD TG 426) and (3) if a reproductive study is to be performed, it should be the EOGRTS with DIT and DNT cohorts (OECD TG 443).
4. The assessment of the appropriateness of the toxicological reference values for pesticides for infants and young children and of the approach to base the MRLs for pesticides for food for infants and young children on the ADI values (ToR1)
4.1. Approach for ADI and ARfD setting for pesticides in infants and young children
It has long been recognised that HBGVs such as the ADI and ARfD do not apply to very young infants (i.e. up to the age of 12–16 weeks). At the request from the European Commission to the European Food Safety Authority (EFSA), EFSA's Scientific Committee prepared a guidance for the risk assessment of substances present in food intended for infants below 16 weeks of age (EFSA Scientific Committee, 2017). The cut‐off of 16 weeks was among others based on the physiological immaturity of many of the organ systems in the first weeks and hence an enhanced vulnerability, nutrition habits up to that age (i.e. mother's milk or infant formulae intended for use as the sole source of nutrition) and since the standard animal testing currently used to assess the toxicity of chemicals do not address this specific age group. In the EFSA Scientific Committee guidance (2017), for substances intentionally added to food, it was recommended to require the EOGRTS if the substance is systemically available, or a neonatal animal study if the substance is not absorbed from the gastrointestinal tract and is not systemically available. If effects are seen with a no observed adverse effect level (NOAEL) below the NOAEL of standard testing results, then this NOAEL will be used to derive the HBGV.
Usually an intraspecies factor of 10 is applied to take the increased vulnerability of sensitive human populations such as infants and children into account. The factor of 10 is generally accepted as being adequate. However, because of reduced activity of most metabolic enzymes, the overall impact on the clearance and half‐life of substances (decrease in clearance, increase in half‐life) is up to two‐ (full‐term) to threefold (preterm) lower when comparing infants below the age of 16 weeks with healthy adults. In addition, the renal function (glomerular filtration rate, tubular reabsorption) is reduced in the first weeks and months resulting in a reduced excretion of substances excreted by the kidney. Hence, the guidance recommends an additional assessment factor for substances not intentionally added to food for infants below 16 weeks of age. If no specific information on the substance is available, the guidance recommends using an additional factor of 3.
The question remains whether infants above 16 weeks of age and children are adequately protected by the ADI and ARfD. As discussed above, the development of organs and systems in infants and children continues well after 4 months, and for certain parameters, adult levels are only reached after several years. However, the differences between infants above 16 weeks of age and young children are rather limited as compared to adults. Concerning the metabolism of xenobiotics, the information which is available indicates that no major differences exist in the age group above 2–4 months (Edginton et al., 2006; de Wildt, 2011; Saghir et al., 2012; Valcke and Krishnan, 2013). In order to further estimate the relative susceptibility to the effects of chemicals of infants and young children as compared to adults, for the present opinion, an inventory was made of the therapeutic doses of pharmaceuticals that are used to treat infants and children as well as adults (see Appendix C for details). Recommended oral doses of 82 medicines used for treatment of infants and young children between about 4 months and up to 3 years of age were compared to the oral doses of these medicines used in adults and the ratio of the doses, expressed as mg/kg bw per day, was calculated. For these 82 medicines, the ratio of the doses in adults to those in infants and young children was 0.95 with a range of 0.23–3.8. This indicates that the mean dose in infants and young children is the same as in adults. The highest value of the ratio of 3.8 means that the dose in adults is 3.8‐fold higher than in the infants and young children, indicating a higher sensitivity in this age group for this particular medicine. It is noted that among the medicines available on the European market, there are some medicines which are contraindicated in infants and young children. In many cases, the contraindication is due to regulatory formalities and is toxicologically not relevant. Otherwise, contraindications for this age group are based on specific toxicological findings, only relevant for the age group of infants (e.g. chinolons). As the testing requirements for pesticides are as strict as the testing requirements for medicines, if approval for this age group is sought, it is expected that age specificities are detected by the required testing.
As the dose of a medicine considers and encompasses kinetic differences as well as dynamic differences, the factor of 3.8 as the greatest difference found in the database is well within the default uncertainty factor of 10 (kinetic plus dynamic) used to account for differences in intraspecies susceptibility. Based on the observation that the differences between infants above 16 weeks of age and young children as compared to adults are rather limited, as illustrated by an analysis of the metabolic pathways and an additional analysis of the ratios of therapeutic doses of medicines, it was concluded that the ADI and ARfD can be applied to infants above 16 weeks of age and young children, and that an additional assessment factor is not necessary for these age groups.
The guidance document on the risk assessment of substances present in food intended for infants below 16 weeks of age (EFSA Scientific Committee, 2017) establishes that the EOGRTS with DNT/DIT cohorts or, if justified the two‐generation reproductive toxicity study (OECD TG 416) with postnatal studies including IT and NT investigations, should be provided for substances intentionally added to food for infants. For approved pesticides in EU, there would be data equivalent to EOGRTS (i.e. current OECD TG 416 protocol would be adequate but older protocols not) and postnatal studies are only triggered by signals (NT/IT) in other studies conducted in adult animals. These data are always taken into account when establishing the ADI and ARfD with the standard UFs accounting for inter‐ and intraspecies differences. Therefore, in conclusion, the already established approach for setting ADI and ARfD is appropriate for infants above 16 weeks of age and young children (see case studies in Appendix A). For infants below 16 weeks of age, the current approach for setting ADI and ARfD may not be appropriate and the application of the EFSA guidance on risk assessment of substances in food for infants (EFSA Scientific Committee, 2017) is recommended.
4.2. Appropriateness of the approach to base the MRLs for pesticides for food for infant and young children on the ADI (and if appropriate ARfD) values
In order to guarantee the consumer safety of MRLs in food (and infant food in particular), a risk assessment for all MRLs is generally necessary based on maximum expected levels of residue intake and appropriate toxicological reference values.
For infant food, a standard level of 0.01 mg/kg (based on the validated LOQ level for the monitoring residue definition) is set as regulatory standard. Where the residue intake at such levels does not allow to conclude on an acceptable dietary intake below the established HBGVs, an appropriate lower LOQ of the analytical method needs to be available for risk assessment and to survey the safety of food specifically dedicated to children.
The approach to base MRLs for pesticides for foods for infants and young children on the HBGVs is considered as appropriate, where the default MRL (standard LOQ of 0.01 mg/kg) is not indicating a safe level of dietary intake. For this approach, it is recommended to apply the monitoring residue definitions as harmonised to Regulation (EU) No 396/2005 for both monitoring and indicative risk assessment.
5. Dietary exposure of infants and young children to pesticide residues (ToR2)
5.1. Characteristics of food consumption by infants and young children
During the first years of life dietary patterns change rapidly, more than at any other period later in life. This may implicate challenges and uncertainties for a precise and valid food consumption assessment to be used as a basis for exposure calculations to substances in food. Basically, food diversity increases from essentially exclusive milk feeding in the first months of life to a more and more varied food selection starting with the introduction of complementary food which is recommended not to start before the age of 4 months (EFSA NDA Panel, 2009; EFSA Scientific Committee, 2017; Fewtrell et al., 2017) and is followed by a gradual transition to family food around the end of the first year of life.
5.1.1. Introduction of complementary feeding
In practice, infants are sometimes not fed in line with the recommendations of EFSA (EFSA NDA Panel, 2009). They may thus be exposed to food other than milk, i.e. complementary food, well before the age of 4 months. Indeed, consumption of complementary food already at an age of 3 months was reported in up to 30% of formula fed infants and up to 8% in breastfed infants on average in five European countries (Schiess et al., 2010). Moreover, infant feeding practices differ across European countries (Schiess et al., 2010; de Lauzon‐Guillain et al., 2013). Untimely introduction of complementary feeding even before the age of 3 months occurred ′twice as often in English and French cohorts as in Greek and Portuguese cohorts (10% vs. < 5%). In the majority of infants per cohort, complementary feeding was introduced in England between 3 and 4 months of age, in the Netherlands at 4 months and in Greece at 5 months (de Lauzon‐Guillain et al., 2013).
As to the type of complementary food used, commercial complementary food products were more popular in formula fed infants than in breast fed infants throughout the complementary feeding period (Schiess et al., 2010).
5.1.2. Interindividual variability in eating skills
Attainment of specific eating skills may impact on what types of food parents offer to the child. There is considerable variation in the ages at which infants and young children achieve new feeding skills depending on differences in their psychomotor development, interaction with the environment and how often these skills are promoted by their parents. For instance, the mean age at which infants use the tongue to move food to the back of the tongue to swallow was observed as 4.95 months, with a standard deviation of 1.27 months and a range of 2.0–7.5 months (Carruth and Skinner, 2002). Similar interindividual variations are observable in eating capabilities in older infants and young children, for instance a median (10, 90th percentile) age of around 9 (11, 17) months for holding the bottle and of around 17 (13, 18) months for self‐feeding with a spoon (Largo, 2008).
5.1.3. Selective food intake
During the course of decreasing energy requirements (per kg bw) in the second year of life, children often show a decrease in appetite and get selective and erratic in their food choice, so‐called ‘fuzzy’ or ‘picky’ eating. The terms are generally used to characterise children who eat a limited amount of food, have strong food preferences, a low‐dietary variety and restricted intake particularly of vegetables and fruit. Picky/fuzzy eating is usually a temporal behaviour and does not have a significant effect on growth (Mascola et al., 2010; Cardona Cano et al., 2015).
Food neophobia, the avoidance of new foods, is a distinct but related characteristic of young children's dietary habits, associated with a low vegetable and fruit acceptance as well (Harris and Mason, 2017). Even foods that have been accepted prior to the onset of the neophobic stage might be refused if their appearance changes on subsequent presentations. It is thought that food neophobia is an evolutionary defence against the possibility of ingesting poisons by consuming unknown substances.
Food fussiness and food neophobia both show considerable heritability, but shared environmental factors, for example, the home environment, are of greater influence on interindividual differences in their expression (Smith et al., 2017).
Unfortunately, due to a missing uniform definition and due to differences in data collection in the existing studies, no definite data on occurrence of selective food intake in young children can be given (Taylor et al., 2015).
5.1.4. Food consumption assessment
Assessment of dietary intake in infants and young children is especially challenging, requires special methodology to consider age‐specific food consumption habits and is further complicated by the rapid growth changes that occur during a relatively short period of time. Not all food served to an infant or young child is necessarily consumed, some may be wasted. This is expected to lead to overestimations of exposure. If the child attends day care, adults other than the parents may be involved in dietary assessment (Gondolf et al., 2012).
Nevertheless, misreporting in the form of underreporting of energy intake which is a common phenomenon in dietary assessments is much less frequent in young children than for instance in female adolescents (1% vs. 20%) (Sichert‐Hellert et al., 1998) or in another study in 3–10‐year‐old children and adolescents aged 11–17 years (4.9% vs. 26.0%) (Lioret et al., 2011).
Diet records over several days have been proven to be a valuable tool for measuring energy and food intake in infants in the second half of the first year of life and young children when compared to total energy expenditure. Repeated 24‐h recalls are suggested to be the most accurate method to estimate total energy intake in children aged 4–11 years (Burrows et al., 2010; Gondolf et al., 2012). In both methods, parents need to be instructed how to quantify food consumption and consider leftovers. Fewer days are needed for reasonably accurate dietary assessments in infants and young children as compared with adults and older children (Lanigan et al., 2004).
5.1.5. Conclusions
Based on the findings above, the EFSA PPR Panel concluded that exposure assessment for the following age classes should be performed:
infants below 16 weeks of age
infants from 3 to 6 months
infants from 6 to 12 months
young children (or toddlers; from 12 to 36 months).
As infants below 16 weeks of age will be expected to be exclusively fed on breast milk and/or infant formula, the exposure assessment for this age class can be carried out according to the recommendations of the EFSA Scientific Committee (see Section 5.2). For older infants and young children, exposure assessment can be carried out on the basis of the dietary surveys included in the EFSA Comprehensive Database (see Section 2.3.1). These surveys were carried out using either a dietary record or a 24 h‐recall method, which are considered appropriate for assessing food consumption in infants and young children.
The EFSA PPR Panel noted the overlap between infants below 16 weeks of age and infants from 3 to 6 months. This overlap was considered acceptable as it addresses uncertainty regarding infants that are exposed early to complementary feeding at the age of 3 months (see Section 5.1.1).
5.2. Exposure of infants below 16 weeks of age
The residue intake via ready‐to‐feed milk during the peak consumption period 2–4 weeks after birth (260 g/kg bw per day; EFSA Scientific Committee, 2017) containing residues at the maximum level of 0.01 mg/kg is calculated as 0.0026 mg/kg bw per day. The higher residue intake compared to the calculation of the SCF (0.0005 mg/kg bw per day; SCF, 1997) is mainly caused by the commodity to which the MRL applies milk after reconstitution in the current Scientific Opinion (according to Article 10 of Directive 2006/141/EC) vs. milk powder before reconstitution in SCF opinion (SCF, 1997).
It is concluded that the default MRL of 0.01 mg/kg for infant formulae does not result in an unacceptable exposure to infants for all compounds to which a HBGV of 0.0026 mg/kg bw per day or higher applies after application of the guidance on risk assessment of substances in food for infants (EFSA Scientific Committee, 2017). Lower MRLs, however, are recommended for active substances with HBGV for infants below 0.0026 mg/kg bw per day.
For the five case studies reported in Appendix A, the HBGVs were above the value 0.0026 mg/kg bw per day and the default MRL of 0.01 mg/kg for infant formula is considered sufficiently protective for these substances. The consumer safety of the threshold of 0.0026 mg/kg bw per day is connected with uncertainties as regards the pesticide‐specific degradation under processing conditions, the potential relevance of metabolites and the composition of the formula.
It is, therefore, recommended to perform a dietary risk assessment for foods intended for infants and young children during a regulated procedure.
5.3. Exposure of infants above 16 weeks of age and young children
For assessing exposure of infants above 16 weeks of age and young children, the EFSA PPR Panel evaluated a new methodology (see Section 2.4.2.1) by means of several case studies reported in Appendix A that studied age classes 3–6 months, 6–12 month and 12–36 months (young children).
In the Appendix A, both the 95th and the 97.5th percentile exposures are presented, but it is noted that, in other regulatory frameworks, the 95th percentile is the most frequently used reference point (e.g. chemical contaminants and food and feed additives).
In general, exposures were found to be the highest for young children and the lowest for infants from 3 to 6 months of age (although some exceptions were also identified). This increase of exposure with age is correlated to the increasing consumption of conventional foods, which was the main source of exposure in all case studies. The contribution of specific foods for infants and young children to the overall exposure was low.
5.3.1. Contribution of foods intended for infants and young children
For the five case studies reported in Appendix A, assuming that all foods specifically dedicated to infants and young children (including infant and follow‐on formulae) contain residues at the maximum level of 0.01 mg/kg, contribution of these food items mostly represented less than 5% of the total chronic exposure. For cereal‐based foods and infant formulae, however, these contributions may go up to around 50% in certain surveys, in particular for infants between 3 and 6 months of age. This finding is consistent with the expectation that some infants within this age class will still be fed mainly with infant formulae.
The highest contributions, however, were usually identified for the surveys with the lowest total exposures. Furthermore, when exposure through foods for infants and young children is calculated in absolute amounts, the mean chronic exposure of infants between 3 and 6 months ranges from 0.0005 to 0.0008 mg/kg bw per day. Total acute exposure (95th percentile) for this age class is estimated to be between 0.0014 and 0.0020 mg/kg bw which is in any case lower than the exposure calculated for infants below 16 weeks of age. Hence, any default MRL derived for infants below 16 weeks of age (based on the consumption of infant formula, see Section 5.2), will also provide adequate protection as regards the exposure of other infants and young children through the consumption of all specifically designed foods.
Table 6.
Summary statistics of total chronic dietary exposure across European dietary surveys when assuming a default MRL of 0.01 mg/kg in all infant food categories (excluding conventional foods)
| Level of exposurea | Age class | N | Total chronic dietary exposure (mg/kg bw per day) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Mean | Infants (3–6 months) | 3 | 0.0005 | 0.0008 | 0.0008 |
| Infants (6–12 months) | 6 | 0.0003 | 0.0005 | 0.0006 | |
| Toddlers | 10 | 0.0000 | 0.0001 | 0.0004 | |
| 95th percentile | Infants (3–6 months) | 2 | 0.0014 | 0.0017 | 0.0020 |
| Infants (6–12 months) | 5 | 0.0009 | 0.0011 | 0.0012 | |
| Toddlers | 6 | 0.0002 | 0.0005 | 0.0008 | |
| 97.5th percentile | Infants (3–6 months) | 1 | 0.0020 | 0.0020 | 0.0020 |
| Infants (6–12 months) | 4 | 0.0011 | 0.0012 | 0.0012 | |
| Toddlers | 2 | 0.0009 | 0.0009 | 0.0009 | |
N: number of surveys.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
Table 7.
Summary statistics of total acute dietary exposure across European dietary surveys when assuming a default MRL of 0.01 mg/kg in all infant food categories (excluding conventional foods)
| Level of exposurea | Age class | N | Total acute dietary exposure (mg/kg bw) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Mean | Infants (3–6 months) | 3 | 0.0006 | 0.0008 | 0.0009 |
| Infants (6–12 months) | 6 | 0.0004 | 0.0005 | 0.0006 | |
| Toddlers | 10 | 0.0001 | 0.0002 | 0.0004 | |
| 95th percentile | Infants (3–6 months) | 3 | 0.0014 | 0.0015 | 0.0020 |
| Infants (6–12 months) | 5 | 0.0010 | 0.0011 | 0.0012 | |
| Toddlers | 8 | 0.0002 | 0.0006 | 0.0008 | |
| 97.5th percentile | Infants (3–6 months) | 3 | 0.0015 | 0.0016 | 0.0021 |
| Infants (6–12 months) | 5 | 0.0011 | 0.0012 | 0.0013 | |
| Toddlers | 5 | 0.0003 | 0.0009 | 0.0010 | |
N: number of surveys.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations respectively may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
5.3.2. Contribution of conventional foods
Within the group of conventional foods, the commodities driving the exposures will be very dependent on the use pattern of the pesticide under assessment. Typically, chronic exposure will mainly be driven by the most frequently consumed food commodities (e.g. potatoes, cereals, citrus fruits and pome fruits) whereas acute exposure may also be driven by less frequently consumed foods and seasonal products (e.g. peaches, table grapes etc.). While cereals were found to be the main drivers for the exposure to deltamethrin, exposure to the other pesticides investigated was mainly driven by fruits and potatoes. It should be noted, however, that some of these contributors are either consumed peeled (e.g. citrus fruits) or processed (e.g. cereals). If data on the effect of processing would be readily available, a more refined exposure calculation could be carried out for these food items and their actual contribution to the exposure is likely to be much lower.
5.3.3. Other considerations
Another important finding is the large difference between exposures calculated from premarketing and post‐marketing data. Premarketing scenarios have the implicit assumption that all foods were treated according to the most critical authorised uses for a given pesticide (i.e. conditions of use assumed for deriving the MRLs in conventional foods). The available post‐marketing data show, however, that pesticide concentrations in food are generally lower than those anticipated from the premarketing data, hereby resulting in a lower exposure for the post‐marketing scenario. The magnitude of this difference between premarketing and post‐marketing exposure is again very much depending on the use pattern of a compound. Among the five case studies investigated, the smallest difference was observed for deltamethrin and thiacloprid (two to fivefold difference) and the largest difference was observed for azoxystrobin (10‐ to 100‐fold difference). Post‐marketing assessments have additional uncertainty associated to the high proportion of left‐censored data (reflected by the differences between the LB and UB results), which is also varying significantly depending on the active substance assessed. Hence, while it is very likely that actual exposure calculated from post‐marketing data will be lower than the exposure anticipated from premarketing data, it is not possible to predict the magnitude of this difference.
Calculations reported in Appendix A also show that exposure estimates obtained through the individual‐based approach (IBA) are higher than those obtained with PRIMo v2. From the 655 MRLs assessed in Appendix A, exceedances of the ARfD were identified for 14 MRLs that have not been identified with PRIMo v2 for these MRLs. Furthermore, while the mean chronic exposure estimates obtained with the new methodology are in the same range as chronic exposure estimates obtained with PRIMo v2, the new method also allows calculating exposures for different percentiles of the chronic exposure distribution, which may be of interest for risk managers. The main reasons for these differences were already discussed in Sections 2.3.2 and 2.4.2. Meanwhile, these outcomes should be considered with caution because the conversion of EFSA's Comprehensive Database into consumption data for raw primary commodities is still under validation and exposure calculations might still be refined by means of processing factors. It would therefore at this stage not be appropriate to take any MRL‐specific measures on the basis of the current calculations. These figures are mainly intended to provide risk managers with an idea on how the individual‐based methodology compares to the reference method that is currently in place for exposure assessment in the area of pesticides.
6. Impact of cumulative exposure (ToR3)
Regulation (EC) No 396/2005 on MRLs provides that decisions on applications concerning MRLs of pesticides must take account of their cumulative and synergistic effects when methodologies to do so will be available.
In 2006, EFSA organised a Scientific Colloquium on the topic where more than 100 experts evaluated the state of knowledge in the field and proposed a strategy for implementation of cumulative risk assessment for Pesticides in Europe.25 Key conclusions of this international event were that
a methodology should be developed starting from already existing approaches,
criteria for grouping substances and a guidance for probabilistic exposure modelling needed to be conceived and that
EFSA PPR Panel should be tasked with these activities.
As recommended, the EFSA PPR Panel initiated a programme of activities to develop the needed methodologies.
In 2008, a first Scientific Opinion was adopted, evaluating the suitability of existing methodologies to assess cumulative and synergistic risks from pesticides to human health with a view to set MRLs for those pesticides in the frame of Regulation (EC) 396/2005 (EFSA PPR Panel, 2008). In this opinion, the three main forms of combined toxicity of chemicals were reviewed:
Dose addition (also referred to as similar action), which takes place where chemicals in a mixture act in the same way, by the same mechanism/mode of action and differ only in their potencies;
Response addition (also referred to as dissimilar action), which takes place where the modes of action and possibly, but not necessarily, the nature and sites of toxic effects differ between the chemicals in a mixture, and one chemical does not influence the toxicity of another;
Interaction, which embraces all forms of joint action that deviates from the two classes of combined toxicity described above. This includes for example synergistic, potentiating, antagonistic, inhibitive effects.
The main conclusion of the Panel was that only the first of these forms of combined toxicity had a major relevance for pesticides, because their residues are present in food at very low concentrations. However, the panel noted that certain endocrine disruptors showed dose additivity even when they do not share the same primary molecular target. An issue was, therefore, identified with respect to the concept of common mechanism/mode of action and the Panel suggested considering the commonality of a phenomenological effect as a criterion for grouping chemicals.
As it considered dose addition as the most relevant form of combined toxicity, the Panel reviewed the existing methods characterising cumulative risks based on this principle. These methods include calculations of (adjusted) hazard indexes, reference point indexes, combined margins of exposure, cumulative risk indexes or require first the identification of an index compounds and normalisation of the potencies of the chemicals in the mixture. The Panel further recommended that tiered approach for both toxicological evaluation and intake estimation be developed in order to make the most efficient use of the available resources. In this approach, all identifiable assumptions and uncertainties should be evaluated qualitatively and those, which are potentially critical to the outcome of the assessment, should be examined quantitatively (either by sensitivity analysis or probabilistic modelling).
In a second Scientific Opinion adopted in 2009 (EFSA PPR Panel, 2009a,b), the Panel tested a tiered approach in a case study dedicated to triazole pesticides, using progressive steps of refinement in the hazard characterisation of mixtures and in the exposure assessment. Based on this exercise, the panel recommended to establish cumulative assessment groups of pesticides as refined as the data allow and to restrict the exposure assessment to one deterministic and one probabilistic tiers.
In June 2013, the Scientific Opinion on the identification of pesticides to be included in cumulative assessment groups on the basis of their toxicological profile (EFSA PPR Panel, 2013a,b,c) was adopted by the Panel. This opinion has developed a generic methodology to establish CAGs of pesticides. This methodology comprises four main steps as follows:
Identification of specific effects (based on their adversity, human relevance, specificity in the nature and site of occurrence…);
Characterisation of the identified specific effects;
Collection of appropriate data;
Grouping of pesticides in a cumulative assessment group related to the specific effects.
In this opinion, the proposed methodology was illustrated for the effects of pesticides on the nervous system and on the thyroid function. It must be noted that, in this methodology, the driving principle to establish cumulative assessment groups is not the observation of a common mode or mechanism of action, but the common capacity of active substances to produce a certain phenomenological effect. This approach was followed due to a lack of data on the mode or mechanism of action; therefore further refinement of the CAGs could not be carried out.
In order to consolidate this strategy, the Panel considered the relevance of dissimilar mode of action for cumulative risk assessment. In a Scientific Opinion (EFSA PPR Panel, 2013a,b,c), the Panel reviewed the scientific data and evidence which emerged since the first opinion in 2008 and concluded that the empirical evidence available now suggest that the distinctions between similar and dissimilar modes of action are fraught with great conceptual and practical difficulties and are of limited practical relevance in cumulative risk assessment. The PPR Panel therefore confirmed the recommendation to group pesticides producing common adverse outcomes and further recommended to use the methods based on the concept of dose addition as a pragmatic and conservative default approach for the purpose of assessing cumulative risks.
Conducting cumulative exposure assessment to pesticides belonging to the same cumulative assessment group also requires specific techniques. Probabilistic modelling is essential in this context because it informs on the distribution of cumulative exposure between all the individuals in a population following their respective consumption profiles. Furthermore, only probabilistic modelling allows quantifying the frequency of peaks of acute exposure resulting from the simultaneous exposure to residues of different pesticides present in different commodities and consumed by chance during one day or eating event.
For this reason, the Panel adopted in 2012 a Guidance on the Use of Probabilistic Methodology for Modelling Dietary Exposure to Pesticide Residues (EFSA PPR Panel, 2012). This guidance proposes a strategy for producing basic and refined modelling of probabilistic exposure assessments under different regulatory scenarios (MRL setting/authorisation and actual exposure) and evaluating the sources of uncertainties through sensitivity analyses.
In 2014, EFSA adopted an implementation plan for the cumulative risk assessment of pesticides which will deliver in 2018 the first cumulative risk assessments at European level resulting from pesticides residues in food. These assessments will concern their effects on the nervous system and the thyroid.
Based on the assumption that adequate hazard data to determine human health‐based reference values that also cover infants and young children must be available for approval of each active substance, consequently the data and the derived health‐based reference values should also be considered adequate in regard to cumulative risk. Also, the methodology developed for addressing cumulative risk assessment as such is considered to be applicable for infants and young children as well. Thus, the grouping methodology based on phenomenological effects as a first tier and likewise the exposure assessment applying probabilistic approaches would be similar.
In conclusion, the already established methodology to address cumulative exposure is also applicable for infants and young children.
7. Appropriateness of the residue definitions established under Regulation (EC) No 396/2005 for foods for infants and young children (ToR4)
In the EU, the maximum permitted pesticide residue levels in food of plant and animal origin are set in the Annexes II to V of Regulation (EC) No 396/2005. They are specific for every pesticide–commodity combination and are based on the expected residue levels of one or more indicator compounds, which are included in the particular residue definition for monitoring. The appropriate indicator (or set of such marker compounds) is identified in nature of residue studies and ideally the transformation of residues under industrial and household‐processing conditions to encompass the entire chain between harvested commodity and marketed end product. MRLs are set before authorisation at the lowest achievable level consistent with good agricultural practice for each pesticide with a view to protecting vulnerable groups such as infants, children and the unborn. If no residues are expected for a pesticide–commodity combination, the MRL is set at the respective analytical limit of quantification.
While residue definitions for monitoring shall be appropriate for an adequate surveillance of pesticide applications, they are also intended to allow an indicative, post‐authorisation dietary risk assessment in the framework of Art.32 of Regulation (EC) No 396/2005. However, such an assessment only allows a more profound conclusion on the dietary risks, if the monitoring residue definition is identical to the corresponding residue definition for risk assessment or, as approximation, if it is convertible by appropriate factors to the toxicological relevant residue comprising additional compounds. Apart from these restrictions, such dietary risk assessments rely on information from the most recent of repetitive active substance assessments, ensuring qualitative and quantitative state‐of‐the‐art evaluation of pesticide residues as well as of their toxicological profile.
The production of food for infants and young children is governed by specific requirements as regards pesticide residues. According to Commission Directives 2006/125/EC and 2006/141/EC, infant food shall not contain residues of individual pesticides at levels exceeding 0.01 mg/kg in the ready to use product or after reconstitution according to the instructions of the manufacturer. MRLs below this standard level of analytical detection were set for few active substances, which were identified based on the toxicological characterisation of that time (ADI < 0.0005 mg/kg bw per day). No considerations were made on the appropriateness of residue definitions to cover the nature of residues in the (processed) product in terms of the residue in the starting material for processing as well as the potential change in the nature and level of residue during processing. In addition, no update of the legislation for infants and young children was done since then to account for changes in the agricultural practices, new active substances, toxicological characterisations of pesticides or analytical developments.
Maximum residue levels refer to different positions in the production chain for infant food (processed commodities) and for conventional food (raw commodities). In both cases, the underlying residue definitions for monitoring should equally allow the surveillance of residue levels post‐harvest and post‐processing and should also enable an indicative risk assessment post‐MRL setting for all consumer groups including infants (supported by consumption data and suitable reference values). Since the scope of the maximum residue levels in food for infants and young children is not different to those for the general population regulated under Regulation (EU) No 396/2005 (which explicitly includes vulnerable consumer groups such as children), since adequate food consumption data are available, and since the tools and methods to assess the dietary risks pre‐ and post‐authorisation are identical, no difference between legislations is justified, unless a particular higher or lower protection goal is expressed by COM for any one consumer group.
The appropriateness of existing residue definitions established under Regulation (EU) No 396/2005 for infant food as regulated under Commission Directives 2006/125/EC and 2006/141/EC depends on whether potential changes in the nature of residue by food‐processing operations is covered in the actual expression of the residue definition. While, theoretically, the nature of residues in processed commodities is included in the considerations of setting the residue definition for monitoring, its practical implementation has not yet been proven. In order to quantitatively assess the appropriateness of existing residue definitions for monitoring according to Regulation (EC) No 396/2005 to processed commodities including food for infants and young children, the residue definitions are scrutinised in a tiered approach.
The potential issue of only limited coverage of processing residues by the monitoring residue definition is assessed by analysing the impact of relevant processing operations on the marker residue compounds. Regulatory standard hydrolysis studies for approved active substances indicate how much of the residue in processed products is covered by the existing residue definition.
At tier 1, which is included in this opinion, the appropriateness of the currently established single‐compound residue definitions (SCRD) for processed food is addressed. The aim is to quantify the degradation potential of the residue of concern under conditions representing processes such as pasteurisation, cooking of vegetables and cereals, fruit and vegetable preservation, or fruit juice production, which are relevant processes in the preparation of infant food. This screening assessment identifies the potential for degradation of residues under harsh, artificial conditions. A tier 1 assessment is not sufficient to propose any changes in the residue definitions, since neither the appropriateness (in terms of analytical approach) nor the amount of particular alternative marker compounds is investigated under this tier. In view of the MRLs being set at the LOQ, the requirement for a change of the residue definitions would have been triggered by higher amounts of a particular degradate in the processed product compared to parent, and thus, a higher probability of unintentional misuse of a pesticide being discovered. The sine qua non for further assessment is therefore degradation of parent in hydrolysis studies of at least 50%.
It is emphasised that the regulatory task of how to combine residue definitions for raw (Regulation (EU) No 396/2005) and processed commodities (Directives 2006/125/EC and 2006/141/EC) is a management issue. The quantitative results might trigger the need to further assess degradation under realistic processing conditions.
A tier 2 assessment of degradation under realistic conditions, which might be conducted in a follow‐up mandate, should comprise:
Substances having a SCRD and proven degradation potential of at least 50% under artificial conditions (identified in tier 1);
Substances having multicompound residue definitions (MCRD) and unclear degradation potential. The level of coverage of the residue definition of these compounds needs to be analysed under refined conditions considering information related to the field residue situation of all compounds in the starting material, appropriate industrial‐processing conditions, matrix effects etcetera;
Substances of high toxicity included in the specific residue definitions of Directive 2006/125/EC and Directive 2006/141/EC considering updated information on toxicity and occurrence.
Tier 1 assessment
This assessment is performed for active substances with SCRDs and aims in estimating how much of the residue in processed products is actually covered by the existing residue definition, and in how many cases, a refined tier 2 assessment is triggered.
The potential for changes in the nature of pesticide residues under food processing is analysed by mimicking representative chemical hydrolysis conditions. The standard regulatory model study (OECD TG 507, formerly EU Guidance 7035/VI/95 rev.5) requires a range of hydrolytic conditions in buffered solutions that is employed to the radiolabelled residue in order to simulate normal processing practices, i.e. pasteurisation (90°C, 20 min, pH 4), baking, brewing, boiling (100°C, 60 min, pH 5), and sterilisation (120°C, 20 min, pH 6).
For the compilation of active substances in the test data set, compounds need to fulfil all of the following criteria:
Active substance is approved under Regulation (EU) No 1107/2009 (n = 494)26
Active substance has a single compound residue definition for products of plant and animal origin (n = 233)
-
Active substance does not fall under the following exclusion criteria:
Multicompound residue definition for plant and/or animal (n = 73)
Inorganic compound OR no residue definition OR default MRL OR compound included in Annex IV of Reg. (EU) 396/2005 (n = 189)
Among the approved active substances, 233 active substances have a single compound residue definition and do not fall in an exclusion category. Of this group, 111 active substances are covered by studies on the nature of processing. The remaining 122 compounds are approved with no processing information required, e.g. due to a no‐residue situation, low water solubility or alternative information showing residue stability on hydrolysis.
The stability of pesticide active substances under industrial‐ and household‐processing conditions is shown in Figure 1 for the set of 111 compounds, which are covered by hydrolysis data provided in Appendix D. A recovery of less than 50% of parent is considered as triggering further analysis under tier 2. On conditions simulating pasteurisation, 4% of active substances considered show recoveries of parent < 50%. Harsher conditions representative for baking/boiling and for sterilisation show instability in 12% and 21% of cases, respectively.
Figure 1.
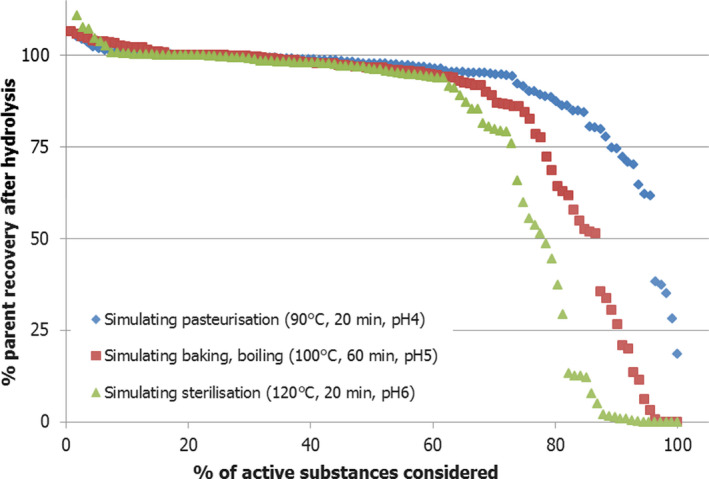
Degradation of pesticide active substances with single‐compound residue definitions under simulated hydrolysis conditions representative of industrial and household processing (n = 111)
Based on the analysis of the hydrolysis potential of pesticides with a single‐compound residue definition, there is doubt on the general appropriateness of existing residue definitions for monitoring to cover processed food, both intended for infants and children as well as conventional food.
The assessment is connected with some inherent limitations:
Real industrial‐processing conditions may vary to those applied in the model studies of tier 1; e.g. conditions of temperature and time that might be found in the preparation of meat and fish are not represented by the standard study design.
No consideration is made on the effective field residue situation in treated crops as source of exposure to infants and young children.
No consideration is made on matrix effects during processing. For instance, residues bound to the sample matrix may be more stable to hydrolysis.
No consideration is made on toxicological properties of the active substances as well as of undetected hydrolysis products.
Limited consideration is made on analytical methods for monitoring, which may be capable of detecting hydrolysis products.
No consideration is made on MCRD. Conclusions of SCRD should not be generalised to MCRD, as certain molecule instability, indicated by the breakdown of the parent structure in plants and animals, is already proven.
-
Uncertainties remain as regards deviations from standard study design and reporting of the data:
-
–
In few cases, untypical study designs were chosen. Such studies are compared to industrial conditions and assessed for exclusion or inclusion to the test data set, taking into account the indicative character of the assessment.
-
–
Some studies report only percent of parent recovery of the total applied radioactivity in the tests (losses are considered as potential degradation), others report percent of chromatogram (losses are considered irrelevant for closed vessels). The numbers were taken from the report, i.e. both reporting types are considered in parallel; a typical mass balance of 100 ± 10% is considered acceptable.
-
–
Results of duplicate sample analyses were averaged
-
–
Results of two labels were reported separately and the worst case taken.
-
–
It is recommended to perform a detailed tier 2 analysis considering all‐embracing information for pesticide active substances, including the field‐residue situation of relevant compounds in the starting material as well as realistic industrial‐processing conditions.
8. Summary of uncertainty considerations
The procedure for uncertainty analysis will follow the pattern laid down in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA Scientific Committee, 2007) using a qualitative tier 1 approach. Before entering the analysis process, it is helpful to summarise the general concerns about health risk assessment resulting from early life exposures and briefly to describe current views relevant to this Opinion.
Understanding of human physiological and developmental differences in infants and young children from adults as summarised here supports the view that sensitivity to chemical exposure in infants below the age of 4 months is in some cases greater than at other life stages. For risk assessment of infants older than 4 months, there is still uncertainty in extrapolating from adult information. Any differential life‐stage sensitivity can reflect the TK of a xenobiotic exposure and/or the toxicodynamic (TD) response. Traditional use of UFs designed to protect against short comings in the inter‐ and intraspecies variation during the conventional toxicological risk assessment studies are based on factors of 10. These have been subdivided. For intraspecies variation, allowances of 3.2‐fold for TK and for TD are made. For interspecies differences fourfold for TK and 2.5‐fold for TD are the defaults. These assumptions about the extent of uncertainty have been shown to be broadly adequate for most cases where detailed knowledge of particular chemicals is available. Such data generally show the application of default uncertainty factors to allow sufficiently protective conclusions. The purpose in splitting these UFs into subcomponents is to allow chemical‐specific quantitative toxicity information that affects either TK or TD to be introduced in the appropriate setting. Therefore, replacing assumption with evidence as it becomes available for the assessment of a specific chemical's toxicity will make the assessment more accurate and reliable. Although overall there is similarity between laboratory species used for developmental toxicity testing and human, the greatest uncertainty in interspecies extrapolation arises from differences in timing of relatively similar events that may be targets for toxicity at sensitive stages of development.
Concerning the biological development and the influence on TK and TD the guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age (EFSA Scientific Committee, 2017) has extensively described the differences between infants and adults. Concerning the TK an extensive body of evidence exists which allows to understand and to take into consideration the differences in a quantitative way. In addition, further work carried out for this opinion elucidated the situation for infants above 16 weeks. However, the approach which can be taken is based on a knowledge base of substances not identical with the pesticide under evaluation and brings with it an uncertainty which cannot be quantified but can be towards an estimate too high and an estimate too low. Concerning both the TK and TD in the human species, the Scientific Committee was of the opinion, despite knowledge gaps, that the existing testing paradigms in animals would address the differences appropriately. For pesticide substances, the regulatory requirements are such that the most critical function in the infant age is addressed with the two generation reproduction toxicity test and, as recommended in the guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age (EFSA Scientific Committee, 2017), the EOGRTS with DNT and DIT cohorts. However, the use of testing paradigms is based on our current level of knowledge which means that with changes in the knowledge gaps might be detected. The uncertainty in this respect cannot be estimated neither qualitatively nor quantitatively.
In producing the opinion, several steps have been performed which may contribute to the uncertainty surrounding the opinion. Whereas it might be that the restrictions set to find and select relevant publications concerning the biological part of the opinion could result in failure to detect information, this possibility is judged to be minor and the uncertainty derived from this source is likely to be extremely low.
For both chronic and acute exposure, there is uncertainty arising from the limited sources of contemporary food consumption data in all EU Member States.
The regulatory data on RPCs were drawn from premarketing studies of field trials leading to STMR, HR and MRLs were determined using the WHO standard procedures. As the estimates are based on usage designed to elicit the highest possible residue levels, this procedure causes a consistent bias towards overestimation of general residue levels. Based on the post‐marketing scenario, it was estimated that premarketing data may overestimate actual exposure by a factor of 2–100 depending on the active substance. The procedures used to summarise monitoring data favour a bias towards overestimation of chronic exposure. This bias may be high. For the acute exposure assessments, there are uncertainties owing to imprecision of the input data that favour a high estimate. The overestimate of residue levels is greatest when unit variability factors (VFs) of five‐ to sevenfold are applied to account for variability in residue levels among individual units.
In addition, losses of residues during household or industrial preparation were not considered in the current assessment which is expected to overestimate even further the exposure estimates.
The conversion of EFSA's Comprehensive Database into consumption data for raw primary commodities is still under validation and exposure calculations might still be refined by means of processing factors.
In the end, limitations for the tier 1 assessment on the appropriateness of residue definitions established under Regulation (EC) 396/2005 for foods for infants and young children are listed in chapter 7. Therefore, a more complete (tier 2) analysis is recommended. From the first assessment, sources of inaccuracy are founded by potential changes of the molecular structure by processing operations, which may not be covered by the residue definitions under Regulation (EU) 396/2005. The other inaccuracy is based on the often divergent residue definitions of monitoring and risk assessment, of which the latter may include additional metabolites (see chapter 5.2).
9. Conclusions
Based on the assessment reported in the previous chapters, the following conclusions are provided by the EFSA PPR Panel:
ToR 1 – The assessment of the appropriateness of the toxicological reference values for pesticides for infants and young children and of the approach to base the MRLs for pesticides for food for infants and young children on the ADI values (in this context, the assessment of the short‐term dietary risk should also be considered):
The established approach for setting ADI and ARfD is appropriate for infants above 16 weeks of age and young children. For infants below the age of 16 weeks, the established approach may not be appropriate and the application of the EFSA guidance on risk assessment of substances in food for infants (EFSA Scientific Committee, 2017) is recommended.
It is concluded that potential residues at the default MRL of 0.01 mg/kg for food intended for infants and young children are not likely to result in an unacceptable exposure to infants below 16 weeks of age for all active substances to which a HBGV of 0.0026 mg/kg bw per day or higher applies. Lower MRLs, however, are recommended for active substances with HBGV below 0.0026 mg/kg bw per day, taking into account the guidance on risk assessment of substances in foods for infants (EFSA Scientific Committee, 2017). For this approach, it is recommended to apply the monitoring residue definitions as harmonised with Regulation (EU) 396/2005 for both monitoring and indicative risk assessment. For active substances with HBGVs equal or above 0.0026 mg/kg bw per day, confirmation of the acceptability of potential exposure levels at the default MRL of 0.01 mg/kg should be provided in a detailed assessment. The assessment should make use of compound‐specific information on the expected nature and magnitude of residues in processed food for infants and young children as well as on toxicological properties of the residue of concern.
ToR 2 – The assessment of the contribution of other foods consumed by infants and young children that are not covered by Regulation (EU) No 609/2013:
The contribution to the total dietary exposure to pesticides residues of conventional food is much higher than of foods intended for infants and young children. Contribution of these food items are generally expected to be less than 5% of the total chronic exposure. For cereal‐based foods and infant formulae, these contributions may go up to around 50% in certain surveys, in particular for infants between 3 and 6 months of age, but these high contributions usually refer to surveys with a low total exposure.
Because of the increased intake of conventional food by young children, these have the highest exposure to pesticide residues in the investigated populations on a mg/kg bw basis, whereas infants 3–6 months of age generally have lower exposure.
Exposure assessments using data for individual daily consumption from EFSA Comprehensive Database indicate that acute exposure estimates are higher under the chosen boundary conditions compared to PRIMo v2 calculations. Mean chronic exposure estimates are in the same range for both models.
ToR 3 – The impact of a cumulative exposure to pesticides which share a common toxicological effect:
The impact of cumulative exposure to pesticide residues on infants and young children is not different from the general population and the cumulative risk assessment methodology of EFSA is also applicable to these age groups.
ToR 4 – The appropriateness of residue definitions established under Regulation (EU) No 396/2005 for foods for infants and young children:
Differences in the residue definitions for monitoring between Regulation No (EC) 396/2005 and for MRLs in food for infants and young children are not considered justified.
The appropriateness of the existing residue definitions under Regulation No (EC) 396/2005 for foods for infants and young children depends on whether potential changes in the nature of residue by food‐processing operations is covered in the actual expression of the residue definition. Based on a tier 1 analysis of the hydrolysis potential of pesticides simulating processing, the general appropriateness of existing residue definitions for monitoring to cover processed food, both intended for infants and young children as well as conventional food is questionable.
10. Recommendations
Based on the assessment reported in the previous chapters, the following recommendations are provided by the EFSA PPR Panel:
ToR 1 – The assessment of the appropriateness of the toxicological reference values for pesticides for infants and young children and of the approach to base the MRLs for pesticides for foods for infants and young children on the ADI values (in this context, the assessment of the short‐term dietary risk should also be considered):
Overall, it should be recommended in the future to:
Screen all pesticides for DNT properties in DNT in vitro testing battery (OECD TG 426) to be developed and triggers for further regulatory studies should be reconsidered;
Review and improve the DNT (OECD TG426) taking into account considerations discussed in the approach proposed by Vorhees et al. (2018);
If a reproductive study is to be performed, it should be the EOGRTS (OECD TG443) with DIT and DNT cohort.
For pesticides with allocated HBGVs below 0.0026 mg/kg bw per day, the default MRL of 0.01 mg/kg for foods intended for infants and young children may not be sufficiently protective and therefore lowering could be considered. Since the consumer safety of the trigger of 0.0026 mg/kg bw per day is connected with uncertainties as regards the pesticide‐specific degradation under processing conditions, the appropriateness of HBGV to infants and the relevance of metabolites and degradates, it is recommended to perform a dietary risk assessment for potential residues of pesticide active substances in food for infants and young children during a regulated procedure. Moreover, for the risk assessment of potential residues of pesticide active substances, it is recommended to apply the EFSA PPR guidance on the establishment of the residue definition for dietary risk (EFSA PPR Panel, 2016).
ToR 2 – The assessment of the contribution of other foods consumed by infants and young children that are not covered by Regulation (EU) No 609/2013:
Clarification should be provided on the applicability of residue definitions for monitoring in case of composite baby food products containing ingredients of animal and plant origin.
Obtain a contemporary and robust database on food consumption patterns in Europe, and to improve the overall consumption data quality, the available database should be updated and expanded to include consumption habits in further countries, in particular for the age classes addressed under the current mandate. This activity is currently ongoing in the framework of EFSA's EU Menu project.27
Further validation and finalisation of the RPC model that is used for converting food consumption data in corresponding amounts of RPC.
EFSA to define in consultation with risk managers a strategy for the possible implementation of the newly proposed methodologies in a regulated procedure. Implementation of the proposed changes may also be considered in a step‐wise approach.
ToR 3 – The impact of a cumulative exposure to pesticides which share a common toxicological effect:
As the cumulative risk assessment methodology currently under development in EFSA is also applicable to these infants and young children, no specific recommendations were identified. However, since as discussed in this opinion, there are certain effects which are regarded as critical for infants and young children for which, for example, an acute exposure might have permanent detrimental effects, this would suggest that cumulative risk assessment encompassing these effects could be prioritised.
ToR 4 – The appropriateness of residue definitions established under Regulation (EU) No 396/2005 for foods for infants and young children:
It is recommended to perform detailed tier 2 analyses on the appropriateness of residue definitions for monitoring to cover, besides raw commodities, also processed foods. The assessment should consider all‐embracing information for pesticide active substances and their relevant metabolites in food intended for infants and young children, taking into account realistic processing conditions.
Abbreviations
- ADI
Acceptable daily intake
- AOP
Adverse outcome pathways
- ARfD
Acute reference dose
- BBB
Blood–brain barrier
- CF
Conversion factor
- CSF
cerebrospinal fluid
- DHA
Docosahexaenoic acid
- DIT
Developmental immunotoxicity
- DNT
Developmental neurotoxicity
- EOGRTS
Extended one generation reproductive toxicity study
- EUCP
EU‐coordinated control programme
- FAO/WHO
Food and Agriculture Organization of the United Nations/World Health Organization
- HBGV
Health‐based guidance value
- HR
Highest residue
- IBA
individual‐based approach
- IESTI
International estimated short‐term intake
- JMPR
Joint Meetings on Pesticide Residues
- IT
Immunotoxicity
- LB
Lower bound
- LCPUFA
long chain poly‐unsaturated fatty acids
- MCRD
Multicompound residue definition
- MRL
Maximum residue level
- NOAEL
No observed adverse effect level
- NP
National control programmes
- NT
Neurotoxicity
- OECD
Organisation for Economic Co‐operation and Development
- P95
95th percentile
- PF
Peeling factor
- PRIMo
Pesticide residues intake model
- RD
Residue definition
- RPC
Raw processed commodity
- SCF
Scientific Committee for Food
- SCRD
Single‐compound residue definition
- STMR
Supervised trials median residue
- TD
Toxicodynamics
- TK
Toxicokinetics
- TG
Test guideline
- UB
Upper bound
- VFs
Variability factors
Appendix A – Case studies
A.1. Introduction
In this scientific opinion, new methodologies are proposed for the assessment of pesticide residues in foods for infants and young children, in particular (1) for assessing the appropriateness of toxicological reference values and (2) for calculating dietary exposure in this population group. In order to test the proposed methodologies, the PPR Panel decided to carry out case studies for five active substances, which were selected according to the following criteria:
all substances should be approved under Regulation (EC) 1107/2009 at the time of publication;
the MRL review under Article 12 of Regulation (EC) No 396/2005 should be finalised for all active substances (in order to ensure availability to EFSA of all premarketing data);
at least 100 MRLs should have been assessed/proposed for each active substance under Article 12 of Regulation (EC) No 396/2005 (hereby ensuring a wide coverage of foods contributing to the diets under assessment).
for each active substance more than 50,000 food samples (including the four main categories of foods for infants and young children) should be analysed in the latest monitoring program of 2015;
substances should be quantified in more than 1% of the above‐mentioned samples (to reduce as much as possible the uncertainties around left‐censored data).
Based on these criteria, eight potential substances were initially presented to the Working Group, where it was decided to exclude substances producing common metabolites with other compounds (e.g. tebuconazole/triazoles metabolites, carbendazim) on the grounds that post‐marketing data would require additional assumptions and thereby increasing the uncertainties. Substances under peer review (e.g. spinosad) at the time of the selection (September 2017) were also excluded. Hence, the following five active substances were selected for assessment: azoxystrobin, deltamethrin, fludioxonil, pyraclostrobin and thiacloprid.
This appendix summarizes the outcome of the case studies where for each active substance, scenario and age class, the minimum, median and maximum exposure estimates among dietary surveys are provided. These estimates refer not only to the mean exposure but also to the higher exposure levels within those surveys. These higher percentile exposures represent consumers that within a given country and age class are subject to higher exposure compared to the majority of consumers within the same population (e.g. due to their personal habits and preferences). For example, looking at Table A.1, it can be seen that among the six surveys available for the infants between 6 and 12 months of age, mean chronic exposure within a survey may range from 7.9% to 16.2% of the acceptable daily intake (ADI). Considering an exposure level at the 95th percentile for each survey, the chronic exposure among surveys from the same age class represents between 22.2% and 45.2% of the ADI. At this exposure level, it can also be seen that only five dietary surveys are considered because one dietary survey did not have sufficient subjects in order derive a reliable 95th percentile. This interpretation can be applied to all tables reporting total chronic and acute exposure. Detailed results of the exposure calculations for each dietary survey can also be retrieved from Annex A. This annex also provides an overview of the input data, which were selected according to the methodologies described in Section 2 of the scientific opinion.
Table A.1.
Summary statistics of total chronic dietary exposure to azoxystrobin across European dietary surveys (premarketing scenario)
| Level of exposurea | Age class | N | Total chronic dietary exposure (% ADI) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Mean | Infants (3–6 months) | 3 | 2.1 | 3.9 | 7.2 |
| Infants (6–12 months) | 6 | 7.9 | 9.7 | 16.2 | |
| Toddlers | 10 | 15.2 | 21.4 | 56.5 | |
| 95th percentile | Infants (3–6 months) | 2 | 7.1 | 11.4 | 15.7 |
| Infants (6–12 months) | 5 | 22.2 | 25.8 | 45.2 | |
| Toddlers | 7 | 35.2 | 54.4 | 74.2 | |
| 97.5th percentile | Infants (3–6 months) | 1 | 29.3 | 29.3 | 29.3 |
| Infants (6–12 months) | 4 | 28.4 | 31.9 | 58.9 | |
| Toddlers | 7 | 43.9 | 72.2 | 97.4 | |
N: number of surveys.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
It should also be noted that the five case studies selected by the Working Group are primarily intended for testing of the new methodologies proposed in this scientific opinion. They are not intended to support risk managers in taking any substance‐specific measures because these calculations are still subject to uncertainties that may be further improved in the future:
At the time of performing the exposure calculations, the conversion of food consumption data to Raw Primary Commodities (RPC) was still under finalisation, and inaccuracies in the RPC consumption data used for exposure assessment cannot be excluded. Previous experience has shown that such inaccuracies tend to generate extreme consumption values, which is mainly expected to impact on high percentile exposures. Mean exposure estimates were shown to be less sensitive for such inaccuracies.
Except for the effect of peeling observed for some active substances, losses of residues during household or industrial preparation were not considered in the current assessment, mainly due to time constraints and the current lack of a database on processing factors in EFSA. However, activities on the collection of processing factors are currently ongoing in EFSA and incorporation of such processing factors in the exposure assessments may significantly reduce some exposure estimates.
Meanwhile, the Working Group is confident that these calculations are robust enough to inform risk managers on the impact of the newly proposed methodologies.
For the assessment of the appropriateness of the toxicological reference values for selected substances, the Working Group evaluated summary information on the toxicity endpoints available in the relevant assessment documents (EFSA conclusions, EFSA scientific opinions and European Commission review reports). The Working Group also took into consideration extracts of the two‐generation reproductive toxicity studies of the five active substances as special attention may be needed for young infants.
A.2. Azoxystrobin
A.2.1. Appropriateness of the toxicological reference values
In a dietary two‐generation reproductive toxicity study in rats, minor reductions in pup and parental body weight gain were observed at 170 mg azoxystrobin/kg bw per day (highest dose tested). In addition, an increase in liver weight was observed in the parental females and pups at this dose; no histopathological changes were observed in the liver. No effects on reproduction were observed. The no observed adverse effect level (NOAEL)for reproductive effects was 170 mg azoxystrobin/kg bw per day. The NOAEL for maternal and developmental effects identified was 32 mg azoxystrobin/kg bw per day.
The ADI was based on the 2‐year rat study and it was set at 0.2 mg azoxystrobin/kg bw per day, applying an assessment factor of 100. No acute reference dose (ARfD) is allocated (not necessary) (EFSA, 2010).
The ADI (0.2 mg azoxystrobin/kg bw per day) was based on a NOAEL (using an uncertainty factor, UF of 100) which was slightly lower than the 32 mg azoxystrobin/kg bw per day the NOAEL for developmental effects in the two‐generation reproductive toxicity study, which was based on minor changes in pup weight, increased liver weight (in the absence of histopathological findings). Therefore, an additional factor for infants above 16 weeks and children up to 3 year is considered not to be applicable.
A.2.2. Exposure of infants below 16 weeks of age
Assuming a default MRL of 0.01 mg/kg (SCF, 1997) and an intake of infant formulae of 260 mL/kg bw at the highest, this will result in a exposure of approximately 0.0026 mg/kg bw per day. This exposure is below the ADI of 0.2 mg azoxystrobin/kg bw per day and the default MRL of 0.01 mg/kg is considered sufficiently protective for azoxystrobin.
A.2.3. Exposure of infants above 16 weeks of age and toddlers
A.2.3.1. Chronic exposure assessment
A.2.3.1.1. Premarketing scenario
Based on the premarketing data, chronic exposure to azoxystrobin is expected to be the highest for toddlers, with mean estimates ranging from 15% to 57% of the ADI (see Table A.1). Depending on the selected percentiles of exposure, highly exposed toddlers may reach chronic exposures up to 74% and 97% of the ADI (95th and 97.5th percentiles, respectively). When calculating exposure with PRIMo v2, mean chronic dietary intakes range from 9% to 17% of the ADI, respectively, for infants (UK) and toddlers (FR).
The primary commodities contributing the most to the calculated exposure are citrus fruits (oranges, lemons and mandarins) and potatoes (see Figure A.1). It is noted, however, that a peeling factor for azoxystrobin in citrus fruits was not reported to EFSA and therefore not included in the exposure calculation. Hence, the actual exposure through citrus fruits is most likely lower than calculated.
Figure A.1.

Mean chronic dietary exposure to azoxystrobin across European dietary surveys, highlighting the contribution of individual food commodities (premarketing scenario)
The anticipated chronic exposure for infants is significantly lower compared to the exposure calculated for toddlers and, although the main contributing commodities are the same, the contribution of potatoes is more important for infants. Another notable contributor identified for some infant surveys is the group of ‘Basil and edible flowers’. According to Annex I of Regulation (EC) No 396/2005, this group also covers mints which may be used as ingredient of certain herbal infusions.
Assuming a default MRL of 0.01 mg/kg, contribution of specific foods for infants and young children to the chronic exposure is expected to be low, except for infants from 3 to 6 months where the average contribution of infant formulae ranges between 2% and 16% of the total chronic exposure.
A.2.3.1.2. Post‐marketing scenario
Based on the upper bound (UB) estimates, the chronic exposure to azoxystrobin was found to be at least 10–100 times lower compared to the chronic exposure anticipated from the premarketing scenario. Furthermore, compared to the other active substances assessed, the uncertainty resulting from left‐censored data is high because an approximate 20‐fold difference was observed between lower bound and upper bound estimates (see Table A.2).
Table A.2.
Summary statistics of total chronic dietary exposure to azoxystrobin across European dietary surveys (post‐marketing scenario)
| Level of exposurea | Age class | N | Total chronic dietary exposure (% ADI) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Mean | Infants (3–6 months) | 3 | 0.00 | 0.33 | 0.01 | 0.40 | 0.01 | 0.49 |
| Infants (6–12 months) | 6 | 0.01 | 0.41 | 0.02 | 0.46 | 0.04 | 0.59 | |
| Toddlers | 10 | 0.02 | 0.44 | 0.03 | 0.53 | 0.05 | 0.66 | |
| 95th percentile | Infants (3–6 months) | 2 | 0.02 | 0.66 | 0.03 | 0.80 | 0.04 | 0.94 |
| Infants (6–12 months) | 5 | 0.03 | 0.68 | 0.04 | 0.80 | 0.11 | 0.89 | |
| Toddlers | 7 | 0.05 | 0.76 | 0.06 | 0.80 | 0.11 | 0.89 | |
| 97.5th percentile | Infants (3–6 months) | 1 | 0.07 | 0.98 | 0.07 | 0.98 | 0.07 | 0.98 |
| Infants (6–12 months) | 4 | 0.03 | 0.78 | 0.05 | 0.89 | 0.14 | 0.96 | |
| Toddlers | 7 | 0.06 | 0.82 | 0.08 | 0.89 | 0.13 | 0.97 | |
LB: lower bound; N: number of surveys; UB: upper bound.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
Oranges, together with a number of other various fruits, were found to be the most important contributors to the chronic exposure (see Figure A.2). While from the premarketing scenario, potatoes were also expected to be a significant contributor to the exposure, contribution of potatoes to the actual chronic exposure is low.
Figure A.2.

Mean chronic dietary exposure to azoxystrobin across European dietary surveys, highlighting the contribution of individual food commodities (lower bound post‐marketing scenario)
As for the premarketing scenario, the exposures were found to be the highest for toddlers and contribution of specific foods for infants and young children (including formulae) to the chronic exposure is low.
A.2.3.2. Acute exposure assessment
Acute exposure calculations were not carried out because an ARfD was not deemed necessary for this active substance.
A.3. Deltamethrin
A.3.1. Appropriateness of the toxicological reference values
In a dietary two‐generation reproductive toxicity, study in rats increased pup mortality and increased pup weight at a parental toxic dose of 320 mg deltamethrin/kg diet was observed. No effects on reproductive performance were observed at this dose. The NOAEL for parental and developmental effects was 80 mg deltamethrin/kg diet, 4.2 mg deltamethrin/kg bw per day.
The ADI of 0.01 mg deltamethrin/kg bw per day was based on the 90‐day and 1‐year dog studies. The ARfD was set at 0.01 mg deltamethrin/kg bw per day (European Commission, 2002). UF's of 100 were used.
In 2009, PPR Panel issued a scientific opinion on developmental neurotoxicity (EFSA PPR Panel, 2009a,b). No developmental toxicity was observed at the highest dose tested (7 mg deltamethrin/kg bw per day) in a DNT study complying with OECD guideline 426. The PPR Panel concluded in that opinion that the ADI derived of the NOAEL of 1 mg deltamethrin/kg bw per day detected in the 90‐day and 1‐year dog studies provides adequate protection for neurodevelopmental effects.
The ADI and ARfD (0.01 mg deltamethrin/kg bw per day) were based on a NOAEL of 1 mg/kg bw per day, which was lower than the NOAEL of 4.2 mg deltamethrin/kg bw per day for developmental effects in the two‐generation toxicity reproductive toxicity study, which was based on increased pup mortality and increased pup weight at parental toxic dose. Therefore, an additional factor for infants > 16 weeks and children up to 3 year is considered not to be applicable.
A.3.2. Exposure of infants below 16 weeks of age
Assuming a default MRL of 0.01 mg/kg (SCF, 1997) and an intake of infant formulae of 260 mL/kg bw at the highest, this will result in a exposure of approximately 0.0026 mg/kg bw per day. This exposure is below the ADI of 0.01 mg deltamethrin/kg bw per day and the default MRL of 0.01 mg/kg is considered sufficiently protective for deltamethrin.
A.3.3. Exposure of infants above 16 weeks of age and toddlers
A.3.3.1. Chronic exposure assessment
A.3.3.1.1. Premarketing scenario
Based on the premarketing data, chronic exposure to deltamethrin is expected to be the highest for toddlers, with mean estimates ranging from 66% to 100% of the ADI (see Table A.3). Depending on the selected percentiles of exposure, highly exposed toddlers may reach chronic exposures up to 150% and 158% of the ADI (95th and 97.5th percentiles, respectively). Although the anticipated chronic exposure for infants is generally lower compared to the exposure calculated for toddlers, one survey for infants results in higher exposure estimates representing 175% and 234% of the ADI (95th and 97.5th percentiles, respectively). With PRIMo v2, mean chronic dietary intakes range from 14% to 44% of the ADI, respectively, for French and UK infants. For toddlers, the highest exposure is 41% of the ADI (UK toddlers).
Table A.3.
Summary statistics of total chronic dietary exposure to deltamethrin across European dietary surveys (premarketing scenario)
| Level of exposurea | Age class | N | Total chronic dietary exposure (% ADI) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Mean | Infants (3–6 months) | 3 | 12.4 | 19.1 | 47.0 |
| Infants (6–12 months) | 6 | 37.6 | 49.3 | 79.1 | |
| Toddlers | 10 | 66.0 | 81.1 | 100.0 | |
| 95th percentile | Infants (3–6 months) | 2 | 26.8 | 36.6 | 46.4 |
| Infants (6–12 months) | 5 | 84.6 | 111.4 | 175.3 | |
| Toddlers | 7 | 106.2 | 122.6 | 149.8 | |
| 97.5th percentile | Infants (3–6 months) | 1 | 61.2 | 61.2 | 61.2 |
| Infants (6–12 months) | 4 | 97.2 | 117.9 | 234.0 | |
| Toddlers | 7 | 122.8 | 136.8 | 157.8 | |
N: number of surveys.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
The primary commodities contributing the most to the calculated exposure are cereal grains, but the contribution of the individual grain species (maize, oat, rice, rye and wheat) is very much depending on the diet (see Figure A.3). Another important contributor throughout all surveys is milk. These observations are mainly resulting from the fact that deltamethrin is authorised for post‐harvest treatment of cereal grains (which may also be used as a feed item for dairy cattle). However, cereal grains are mainly consumed in a processed form and it is likely that residues will be lost during such processing steps. Therefore, inclusion of processing factors in the exposure calculation may significantly reduce the exposure estimates.
Figure A.3.
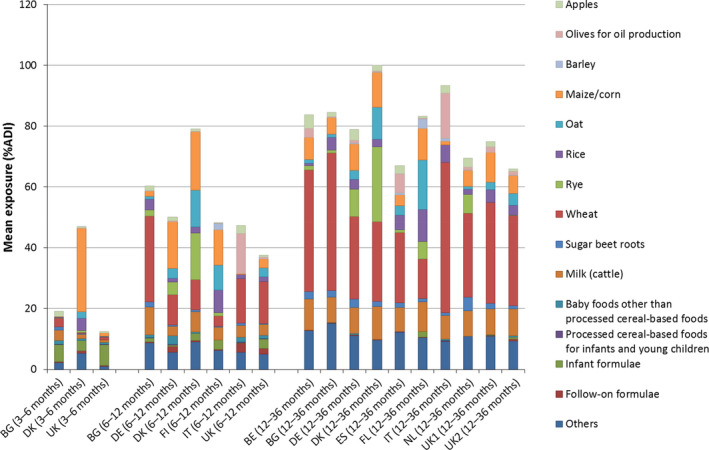
Mean chronic dietary exposure to deltamethrin across European dietary surveys, highlighting the contribution of individual food commodities (premarketing scenario)
Overall, assuming a default MRL of 0.01 mg/kg, contribution of specific foods for infants and young children to the chronic exposure was found to be relatively high for processed foods (other than cereals) and infant formulae where the average contribution in infants may represent up to 7% and 54% of the total chronic exposure, respectively. These high contributions, however, usually refer to the surveys with the lowest total exposures.
A.3.3.1.2. Post‐marketing scenario
Based on the UB estimates, the chronic exposure to deltamethrin was found to be only 1–10 times lower compared to the chronic exposure anticipated from the premarketing scenario. However, compared to the other active substances assessed, the uncertainty resulting from left‐censored data are high because an approximate 25‐fold difference was observed between lower bound and upper bound estimates (see Table A.4).
Table A.4.
Summary statistics of total chronic dietary exposure to deltamethrin across European dietary surveys (post‐marketing scenario)
| Level of exposurea | Age class | N | Total chronic dietary exposure (% ADI) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Mean | Infants (3–6 months) | 3 | 0.04 | 8.69 | 0.07 | 8.85 | 0.55 | 11.96 |
| Infants (6–12 months) | 6 | 0.32 | 9.45 | 0.51 | 11.97 | 0.63 | 14.56 | |
| Toddlers | 10 | 0.62 | 12.40 | 0.77 | 14.65 | 0.99 | 19.74 | |
| 95th percentile | Infants (3–6 months) | 2 | 0.20 | 15.21 | 0.27 | 18.93 | 0.34 | 22.65 |
| Infants (6–12 months) | 5 | 0.93 | 18.36 | 1.51 | 20.09 | 1.89 | 23.70 | |
| Toddlers | 7 | 1.12 | 19.31 | 1.32 | 22.42 | 1.69 | 30.41 | |
| 97.5th percentile | Infants (3–6 months) | 1 | 0.57 | 24.22 | 0.57 | 24.22 | 0.57 | 24.22 |
| Infants (6–12 months) | 4 | 1.09 | 20.51 | 1.64 | 21.77 | 2.58 | 26.38 | |
| Toddlers | 7 | 1.25 | 21.34 | 1.52 | 24.86 | 1.93 | 36.48 | |
LB: lower bound; N: number of surveys; UB: upper bound.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
Overall, the main contributing commodities are cereal grains, which is consistent with those observed for the premarketing scenario (see Figure A.4). Nevertheless, while from the premarketing scenario, milk was also expected to be a significant contributor to the exposure, its contribution to the actual chronic exposure is limited.
Figure A.4.
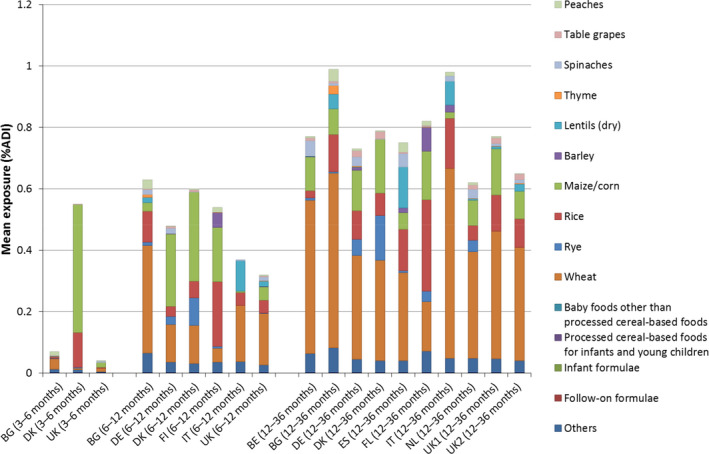
Mean chronic dietary exposure to deltamethrin across European dietary surveys, highlighting the contribution of individual food commodities (lower bound post‐marketing scenario)
As for the premarketing scenario, the exposures were found generally to be the highest for toddlers and contribution of specific foods for infants and young children (including formulae) to the chronic exposure is low.
A.3.3.2. Acute exposure assessment
A.3.3.2.1. Premarketing scenario
Total acute exposure to deltamethrin is generally expected to be the highest in toddlers where, based on the premarketing data, upper tail exposures may range between 162% and 254% of the ARfD (depending on the survey and percentile of interest, see Table A.5). Nevertheless, some surveys for infants leading to even higher exposure were also identified (up to 408% of the ARfD).
Table A.5.
Summary statistics of total acute dietary exposure to deltamethrin across European dietary surveys (premarketing scenario)
| Level of exposurea | Age class | N | Total acute dietary exposure (% ARfD) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Mean | Infants (3–6 months) | 3 | 22.8 | 28.8 | 73.9 |
| Infants (6–12 months) | 6 | 57.8 | 83.6 | 118.3 | |
| Toddlers | 11 | 88.4 | 113.5 | 147.3 | |
| 95th percentile | Infants (3–6 months) | 3 | 74.6 | 102.5 | 281.5 |
| Infants (6–12 months) | 5 | 138.5 | 180.7 | 279.8 | |
| Toddlers | 10 | 162.0 | 201.2 | 247.8 | |
| 97.5th percentile | Infants (3–6 months) | 3 | 104.7 | 125.6 | 368.2 |
| Infants (6–12 months) | 5 | 166.1 | 200.2 | 407.7 | |
| Toddlers | 7 | 175.6 | 236.0 | 254.2 | |
N: number of surveys.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
In several surveys, the contribution of background exposure may reach more than 50% of the total acute exposure, even for the upper tail exposures (see Table A.6). This indicates that, for deltamethrin, the contribution of background exposures is high compared to other active substances. As for the chronic exposure calculations, this effect is mainly due to the post‐harvest treatment in cereals, which are widely consumed throughout all surveys and age classes.
Table A.6.
Average contribution of the background exposure to the total acute exposure of deltamethrin across European dietary surveys (premarketing scenario)
| Distribution | Age class | N | Average contribution (% of total exposure) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Fulla | Infants (3–6 months) | 3 | 20.0 | 22.0 | 27.8 |
| Infants (6–12 months) | 6 | 36.8 | 42.2 | 45.0 | |
| Toddlers | 11 | 46.8 | 52.0 | 55.8 | |
| Upper tailb | Infants (3–6 months) | 3 | 12.2 | 19.1 | 31.9 |
| Infants (6–12 months) | 5 | 10.3 | 32.6 | 39.8 | |
| Toddlers | 10 | 31.5 | 43.7 | 58.0 | |
N: number of surveys.
Average contribution refers to the full distribution of acute exposures calculated within a survey and age class.
Average contribution refers to the acute exposures exceeding the 95th percentile within a survey and age class. Surveys with less than 61 observations were excluded because the 95th percentile estimates obtained on these dietary surveys/age classes were not considered statistically robust (EFSA, 2011a).
Across surveys, the main RPCs driving total acute exposures are maize, apples, oats and pears, also noting that wheat is an important contributor to the background exposure. Furthermore, when assessing the acute exposure per RPC (consumers only), exceedances of the ARfD may also be expected for less frequently consumed RPCs such as rye, millet, buckwheat, aubergines and pumpkins (see Table A.6). It should be noted however that, for pumpkin, a peeling factor was not available and therefore not included in the exposure calculations. The high exposure identified for maize is due to the consumption of maize oil which requires 25 g of corn to produce only 1 g of oil.
Assuming a default MRL of 0.01 mg/kg, acute exposure through specific foods for infants and young children generally ranges from 0.1% to 7.8% of the ARfD, but may reach up to 20.8% of the ARfD for infant and follow‐on formulae (see Annex A – Table 8).
Acute exposure was also calculated with PRIMo v2. For toddlers, highest exposure levels reach 99% of the ARfD for celery leaves, 98% ARfD for lettuces, 96% ARfD for leeks. For infants (6–12 months), it was estimated a maximum acute exposure of 98% ARfD for apples and 93% ARfD for maize.
Table A.7.
Summary statistics of the acute dietary exposure to deltamethrin across European dietary surveys arising from individual raw primary commodities that may lead to an exceedance of the ARfD (premarketing scenario)
| Age class | Raw primary commodity | N | Acute dietary exposurea (% ARfD) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Infants (3–6 months) | Maize/corn | 3 | 0.8 | 24.6 | 339.2 |
| Infants (6–12 months) | Apples | 5 | 50.6 | 81.7 | 126.8 |
| Pears | 5 | 69.8 | 117.3 | 173.5 | |
| Maize/corn | 5 | 51.8 | 109.0 | 607.7 | |
| Common millet/proso millet | 2 | 105.7 | 198.9 | 292.0 | |
| Oat | 5 | 110.6 | 164.9 | 201.1 | |
| Rye | 5 | 12.4 | 63.6 | 101.1 | |
| Toddlers | Apples | 11 | 46.7 | 83.2 | 100.5 |
| Pears | 9 | 63.5 | 83.3 | 108.9 | |
| Aubergines/eggplants | 4 | 9.5 | 55.7 | 142.2 | |
| Pumpkins | 1 | 103.6 | 103.6 | 103.6 | |
| Buckwheat and other pseudocereals | 4 | 4.9 | 43.5 | 115.1 | |
| Maize/corn | 11 | 10.5 | 74.0 | 120.4 | |
| Oat | 6 | 72.4 | 150.1 | 180.4 | |
| Rye | 9 | 1.5 | 52.6 | 101.7 | |
| Wheat | 11 | 50.0 | 100.3 | 143.9 | |
N: number of surveys.
The acute dietary exposure refers to the highest percentile that is considered statistically robust for a given dietary survey, age class and food, considering that a minimum of 12, 30, 61 and 181 observations are, respectively, required to derive 75th, 90th, 95th and 97.5th percentile estimates (EFSA, 2011a). Estimates with less than 12 observations were not included in this table.
A.3.3.2.2. Post‐marketing scenario
Total acute exposure to deltamethrin calculated according to the post‐marketing scenario (see Table A.8) is approximately 2–5 times lower than the exposure estimates anticipated by the premarketing scenario. This ratio is similar to the one observed for the chronic exposure. Regarding the contribution of the background exposure, the large difference between lower bound (LB) and upper bound (UB) estimates makes it difficult to assess the contribution of background exposure in the current scenario (see Table A.9). Nevertheless, compared to the chronic exposure calculations, the impact of this uncertainty on the total acute exposure estimates is smaller (1.5‐fold difference between LB and UB estimates). Furthermore, background exposure is significantly lower than for the premarketing scenario.
Table A.8.
Summary statistics of total acute dietary exposure to deltamethrin across European dietary surveys (post‐marketing scenario)
| Level of exposurea | Age class | N | Total acute dietary exposure (% ARfD) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Mean | Infants (3–6 months) | 3 | 13.6 | 18.9 | 15.3 | 19.1 | 16.3 | 21.8 |
| Infants (6–12 months) | 6 | 21.7 | 29.0 | 25.4 | 35.3 | 44.7 | 53.5 | |
| Toddlers | 11 | 23.0 | 34.5 | 28.4 | 41.9 | 39.2 | 57.1 | |
| 95th percentile | Infants (3–6 months) | 3 | 29.5 | 42.9 | 42.2 | 52.7 | 43.7 | 57.8 |
| Infants (6–12 months) | 5 | 48.8 | 58.0 | 52.8 | 67.0 | 76.8 | 91.1 | |
| Toddlers | 10 | 46.7 | 61.2 | 52.8 | 72.6 | 63.9 | 98.0 | |
| 97.5th percentile | Infants (3–6 months) | 3 | 36.7 | 50.3 | 56.9 | 64.1 | 61.7 | 74.0 |
| Infants (6–12 months) | 5 | 53.4 | 62.2 | 61.2 | 75.6 | 106.9 | 121.6 | |
| Toddlers | 7 | 53.0 | 68.2 | 57.5 | 73.2 | 69.0 | 87.8 | |
LB: lower bound; N: number of surveys; UB: upper bound.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
Table A.9.
Average contribution of the background exposure to the total acute exposure of deltamethrin across European dietary surveys (post‐marketing scenario)
| Distribution | Age class | N | Average contribution (% of total exposure) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Fulla | Infants (3–6 months) | 3 | 0.3 | 16.9 | 0.4 | 25.2 | 2.3 | 25.6 |
| Infants (6–12 months) | 6 | 0.9 | 25.0 | 2.4 | 28.9 | 3.2 | 36.0 | |
| Toddlers | 11 | 2.6 | 30.6 | 3.5 | 37.5 | 4.4 | 39.3 | |
| Upper tailb | Infants (3–6 months) | 3 | 0.1 | 14.2 | 0.5 | 21.1 | 5.9 | 30.8 |
| Infants (6–12 months) | 5 | 0.6 | 12.4 | 1.0 | 18.2 | 1.4 | 22.0 | |
| Toddlers | 10 | 1.1 | 22.2 | 1.3 | 27.6 | 2.2 | 39.9 | |
LB: lower bound; N: number of surveys; UB: upper bound.
Average contribution refers to the full distribution of acute exposures calculated within a survey and age class.
Average contribution refers to the acute exposures exceeding the 95th percentile within a survey and age class. Surveys with less than 61 observations were excluded because the 95th percentile estimates obtained on these dietary surveys/age classes were not considered statistically robust (EFSA, 2011a).
The total acute exposure under this scenario is mainly driven by the consumption of bananas, apples, pumpkins and pears. Also, when assessing the acute exposure for consumers of these RPCs individually, an exceedance of the ARfD is identified for pears and pumpkins (see Table A.10). As for the premarketing scenario, it should be noted that for pumpkin and bananas, a peeling factor was not available and therefore not included in the exposure calculations. Contribution of cereals, however, is lower than anticipated from the premarketing scenario.
Table A.10.
Summary statistics of the acute dietary exposure to deltamethrin across European dietary surveys arising from raw primary commodities that may lead to an exceedance of the ARfD (post‐marketing scenario)
| Age class | Raw primary commodity | N | Acute dietary exposurea (% ARfD) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Infants (6–12 months) | Pears | 5 | 43.6 | 73.3 | 108.4 |
| Pumpkins | 2 | 137.3 | 330.7 | 524.0 | |
| Toddlers | Pumpkins | 1 | 573.3 | 573.3 | 573.3 |
N: number of surveys.
The acute dietary exposure refers to the highest percentile that is considered statistically robust for a given dietary survey, age class and food, considering that a minimum of 12, 30, 61 and 181 observations are, respectively, required to derive 75th, 90th, 95th and 97.5th percentile estimates (EFSA, 2011a). Estimates with less than 12 observations were not included in this table.
Acute exposure to through specific foods for infants and young children ranged from 0.5% to 13.8% of the ARfD and exposures up to 54.6% of the ARfD were noted for infant formulae (see Annex A – Table 8). These estimates were, however, derived from left‐censored monitoring data.
A.4. Fludioxonil
A.4.1. Appropriateness of the toxicological reference values
In a dietary two‐generation reproductive toxicity study in rats, decreased pup weight and in the parental animals decreased body weight and food consumption were observed at 3,000 mg fludioxonil/kg diet, the highest dose tested. At 300 mg fludioxonil/kg diet, no effects were observed. No effects on reproductive performance were observed at 3,000 mg fludioxonil (212 mg fludioxonil/kg bw per day). The NOAEL for parental and developmental effects was 300 mg fludioxonil/kg diet (21 mg fludioxonil/kg bw per day).
The ADI of 0.37 mg fludioxonil/kg bw per day was based on a 2‐year rat study, applying an assessment factor of 100 (EFSA, 2007a,b). No ARfD was allocated (not necessary).
The ADI (0.37 mg fludioxonil/kg bw per day) was based on a NOAEL which was higher than or comparable to the NOAEL of 21 mg fludioxonil/kg bw per day for developmental effects in the two‐generation toxicity reproductive toxicity study, which was based on decreased pup weight. The justification for the NOAEL chosen for setting ADI was based on dose spacing in the studies, and in addition, there were no reproductive effects.
A.4.2. Exposure of infants below 16 weeks of age
Assuming a default MRL of 0.01 mg/kg (SCF, 1997) and an intake of infant formulae of 260 mL/kg bw at the highest, this will result in a exposure of approximately 0.0026 mg/kg bw per day. This exposure is below the ADI of 0.37 mg fludioxonil/kg bw per day and the default MRL of 0.01 mg/kg is considered sufficiently protective for fludioxonil.
A.4.3. Exposure of infants above 16 weeks of age and toddlers
A.4.3.1. Chronic exposure assessment
A.4.3.1.1. Premarketing scenario
Based on the premarketing data, chronic exposure to fludioxonil is expected to be the highest for toddlers, with mean estimates ranging from 8% to 38% of the ADI (see Table A.11). Depending on the selected percentiles of exposure, highly exposed toddlers may reach chronic exposures up to 50% and 62% of the ADI (95th and 97.5th percentiles, respectively). When calculating exposure with PRIMo v2, mean chronic dietary intakes range from 5% to 10% of the ADI, respectively, for UK infants and French toddlers.
Table A.11.
Summary statistics of total chronic dietary exposure to fludioxonil across European dietary surveys (premarketing scenario)
| Level of exposurea | Age class | N | Total chronic dietary exposure (% ADI) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Mean | Infants (3–6 months) | 3 | 2.4 | 3.9 | 5.3 |
| Infants (6–12 months) | 6 | 5.3 | 6.8 | 11.0 | |
| Toddlers | 10 | 8.3 | 15.8 | 38.0 | |
| 95th percentile | Infants (3–6 months) | 2 | 10.2 | 14.1 | 17.9 |
| Infants (6–12 months) | 5 | 13.5 | 17.6 | 27.6 | |
| Toddlers | 7 | 19.2 | 39.6 | 49.9 | |
| 97.5th percentile | Infants (3–6 months) | 1 | 19.9 | 19.9 | 19.9 |
| Infants (6–12 months) | 4 | 15.8 | 19.0 | 35.5 | |
| Toddlers | 7 | 27.6 | 50.0 | 62.6 | |
N: number of surveys.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
The primary commodities contributing the most to the calculated exposure are citrus fruits (oranges, lemons and mandarins), apples and potatoes (see Figure A.5). It is noted, however, that a peeling factor for fludioxonil in citrus fruits was not reported to EFSA and therefore not included in the exposure calculation. Hence, the actual exposure through citrus fruits is most likely lower than calculated.
Figure A.5.

Mean chronic dietary exposure to fludioxonil across European dietary surveys, highlighting the contribution of individual food commodities (premarketing scenario)
Although the anticipated chronic exposure for infants is significantly lower compared to the exposure calculated for toddlers, the main contributors are the same.
Overall, assuming a default MRL of 0.01 mg/kg, contribution of specific foods for infants and young children to the chronic exposure is expected to be low, except for infants from 3 to 6 months where the average contribution of infant formulae ranges between 2% and 8% of the total chronic exposure.
A.4.3.1.2. Post‐marketing scenario
Based on the UB estimates, the chronic exposure to fludioxonil was found to be at least 10–50 times lower compared to the chronic exposure anticipated from the premarketing scenario. However, compared to the other active substances assessed, the uncertainty resulting from left‐censored data is low because an approximate sixfold difference was observed between lower bound and upper bound estimates (see Table A.12).
Table A.12.
Summary statistics of total chronic dietary exposure to fludioxonil across European dietary surveys (post‐marketing scenario)
| Level of exposurea | Age class | N | Total chronic dietary exposure (% ADI) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Mean | Infants (3–6 months) | 3 | 0.03 | 0.26 | 0.04 | 0.28 | 0.07 | 0.38 |
| Infants (6–12 months) | 6 | 0.05 | 0.38 | 0.07 | 0.41 | 0.09 | 0.58 | |
| Toddlers | 10 | 0.07 | 0.48 | 0.10 | 0.59 | 0.15 | 0.69 | |
| 95th percentile | Infants (3–6 months) | 2 | 0.23 | 0.60 | 0.29 | 0.72 | 0.34 | 0.84 |
| Infants (6–12 months) | 5 | 0.16 | 0.69 | 0.19 | 0.79 | 0.35 | 0.93 | |
| Toddlers | 7 | 0.20 | 0.79 | 0.25 | 0.93 | 0.30 | 1.01 | |
| 97.5th percentile | Infants (3–6 months) | 1 | 0.27 | 0.99 | 0.27 | 0.99 | 0.27 | 0.99 |
| Infants (6–12 months) | 4 | 0.23 | 0.87 | 0.31 | 0.91 | 0.49 | 1.02 | |
| Toddlers | 7 | 0.26 | 0.88 | 0.31 | 1.02 | 0.42 | 1.22 | |
LB: lower bound; N: number of surveys; UB: upper bound.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
Overall, fruits (including apples) were found to be the most important contributors to the chronic exposure and, in a few surveys, sweet potatoes were the major contributor (see Figure A.6). Although from the premarketing scenario, citrus fruits and potatoes were also expected to be significant contributors to the exposure, their contribution to the actual exposure is low.
Figure A.6.

Mean chronic dietary exposure to fludioxonil across European dietary surveys, highlighting the contribution of individual food commodities (lower bound post‐marketing scenario)
As for the premarketing scenario, the exposures were found to be the highest for toddlers and contribution of specific foods for infants and young children (including formulae) to the chronic exposure is low.
A.4.3.2. Acute exposure assessment
Acute exposure calculations were not carried out because an ARfD was not deemed necessary for this active substance.
A.5. Pyraclostrobin
A.5.1. Appropriateness of the toxicological reference values
In a dietary two‐generation reproductive toxicity study in rats, decreased pup weight gain, decreased thymus and spleen weights at weaning in the presence of parental toxicity were observed at 300 mg pyraclostrobin/kg diet the highest dose tested. At 75 mg pyraclostrobin/kg diet (8.2 mg pyraclostrobin/kg bw per day), no effects were observed. No effects on reproductive performance were observed at 300 mg pyraclostrobin/kg diet (32.6 mg pyraclostrobin/kg bw per day). The NOAEL for parental and developmental effects was 75 mg pyraclostrobin/kg diet (8.2 mg pyraclostrobin/kg bw per day). The lowest NOAEL identified was found in the prenatal developmental toxicity study in rabbits at 3 mg pyraclostrobin/kg bw per day, and in the same study, the NOAEL for developmental toxicity was 5 mg pyraclostrobin/kg bw per day in this study.
The ADI and ARfD (0.03 mg pyraclostrobin/kg bw per day) were based on the NOAEL (3 mg pyraclostrobin/kg bw per day) of a chronic study in rats and maternal toxicity in a prenatal developmental toxicity study in rabbits (European Coimmission, 2004). The NOAEL for parental and developmental effects in the two‐generation reproductive toxicity study was 75 mg pyraclostrobin/kg diet (8.2 mg pyraclostrobin/kg bw per day. Therefore, an additional factor for infants > 16 weeks and children up to 3 years are considered not to be applicable.
A.5.2. Exposure of infants below 16 weeks of age
Assuming a default MRL of 0.01 mg/kg (SCF, 1997) and an intake of infant formulae of 260 mL/kg bw at the highest, this will result in a exposure of approximately 0.0026 mg/kg bw per day. This exposure is below the ADI of 0.03 mg pyraclostrobin/kg bw per day and the default MRL of 0.01 mg/kg is considered sufficiently protective for pyraclostrobin.
A.5.3. Exposure of infants above 16 weeks of age and toddlers
A.5.3.1. AChronic exposure assessment
A.5.3.1.1. Premarketing scenario
Based on the premarketing data, chronic exposure to pyraclostrobin is expected to be the highest for toddlers, with mean estimates ranging from 13% to 20% of the ADI (see Table A.13). Depending on the selected percentiles of exposure, highly exposed toddlers may reach chronic exposures up to 39% and 47% of the ADI (95th and 97.5th percentiles, respectively). The anticipated chronic exposure for infants is significantly lower compared to the exposure calculated for toddlers. When calculating exposure with PRIMo v2, mean chronic dietary intakes range from 5.5% to 8% of the ADI, respectively, for infants and toddlers (FR diets).
Table A.13.
Summary statistics of total chronic dietary exposure to pyraclostrobin across European dietary surveys (premarketing scenario)
| Level of exposurea | Age class | N | Total chronic dietary exposure (% ADI) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Mean | Infants (3–6 months) | 3 | 4.6 | 4.9 | 8.1 |
| Infants (6–12 months) | 6 | 9.1 | 10.3 | 12.7 | |
| Toddlers | 10 | 13.1 | 17.5 | 20.5 | |
| 95th percentile | Infants (3–6 months) | 2 | 9.7 | 14.6 | 19.6 |
| Infants (6–12 months) | 5 | 19.0 | 22.9 | 25.8 | |
| Toddlers | 7 | 26.7 | 34.5 | 39.3 | |
| 97.5th percentile | Infants (3–6 months) | 1 | 21.9 | 21.9 | 21.9 |
| Infants (6–12 months) | 4 | 23.2 | 26.3 | 29.9 | |
| Toddlers | 7 | 32.6 | 38.6 | 47.2 | |
N: number of surveys.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
The primary commodities contributing the most to the calculated exposure are in this case difficult to identify because they are very dependent on the dietary survey, but the most frequently reported commodities include various citrus fruits, pome fruits, berry fruits, milk and sugar beets (see Figure A.7). The exposure from sugar beets is driven by the consumption of sugar, but the current assessment does not consider the possible loss of residues during sugar production. Exposure estimates are therefore likely to be overestimated.
Figure A.7.

Mean chronic dietary exposure to pyraclostrobin across European dietary surveys, highlighting the contribution of individual food commodities (premarketing scenario)
Overall, assuming a default MRL of 0.01 mg/kg, contribution of specific foods for infants and young children to the chronic exposure was found to be relatively high for processed foods (other than cereals) and infant formulae where the average contribution in infants may represent up to 10% and 49% of the total chronic exposure, respectively. These high contributions, however, usually refer to the surveys with the lowest total exposures.
A.5.3.1.2. Post‐marketing scenario
Based on the UB estimates, the chronic exposure to pyraclostrobin was found to be only 3–6 times lower compared to the chronic exposure anticipated from the premarketing scenario. However, compared to the other active substances assessed, the uncertainty resulting from left‐censored data is high because an approximate 20‐fold difference was observed between lower bound and upper bound estimates (see Table A.14).
Table A.14.
Summary statistics of total chronic dietary exposure to pyraclostrobin across European dietary surveys (post‐marketing scenario)
| Level of exposurea | Age class | N | Total chronic dietary exposure (% ADI) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Mean | Infants (3–6 months) | 3 | 0.04 | 2.33 | 0.07 | 2.71 | 0.10 | 3.42 |
| Infants (6–12 months) | 6 | 0.09 | 2.72 | 0.13 | 3.18 | 0.21 | 3.98 | |
| Toddlers | 10 | 0.14 | 3.20 | 0.23 | 3.73 | 0.32 | 4.18 | |
| 95th percentile | Infants (3–6 months) | 2 | 0.25 | 4.49 | 0.32 | 5.52 | 0.38 | 6.54 |
| Infants (6–12 months) | 5 | 0.30 | 4.82 | 0.35 | 5.43 | 0.52 | 5.89 | |
| Toddlers | 7 | 0.38 | 5.16 | 0.58 | 5.55 | 0.75 | 6.22 | |
| 97.5th percentile | Infants (3–6 months) | 1 | 0.46 | 6.90 | 0.46 | 6.90 | 0.46 | 6.90 |
| Infants (6–12 months) | 4 | 0.34 | 5.25 | 0.40 | 6.06 | 0.65 | 6.30 | |
| Toddlers | 7 | 0.45 | 5.50 | 0.75 | 6.19 | 0.87 | 6.80 | |
LB: lower bound; N: number of surveys; UB: upper bound.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
The most important contributors to the chronic exposure are apples, pears and currants (see Figure A.8). Although from the premarketing scenario, milk was also expected to be a significant contributor to the exposure, its contribution to the actual exposure is low.
Figure A.8.

Mean chronic dietary exposure to pyraclostrobin across European dietary surveys, highlighting the contribution of individual food commodities (lower bound post‐marketing scenario)
As for the premarketing scenario, the exposures were found to be the highest for toddlers and contribution of specific foods for infants and young children (including formulae) to the chronic exposure is low.
A.5.3.2. Acute exposure assessment
A.5.3.2.1. Premarketing scenario
Total acute exposure to pyraclostrobin is expected to be the highest in toddlers where, based on the premarketing data, upper tail exposures may range between 67 and 109% of the ARfD (depending on the survey and percentile of interest, see Table A.15). While the contribution of background exposure is generally 20–40% of the total acute exposure, background exposure only accounts for 10–35% in the upper tail exposures (see Table A.16). This indicates that high acute exposures to pyraclostrobin are very much driven by specific food items.
Table A.15.
Summary statistics of total acute dietary exposure to pyraclostrobin across European dietary surveys (premarketing scenario)
| Level of exposurea | Age class | N | Total acute dietary exposure (% ARfD) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Mean | Infants (3–6 months) | 3 | 11.7 | 12.0 | 15.1 |
| Infants (6–12 months) | 6 | 23.9 | 25.5 | 34.4 | |
| Toddlers | 11 | 30.2 | 36.4 | 46.6 | |
| 95th percentile | Infants (3–6 months) | 3 | 31.5 | 41.1 | 48.5 |
| Infants (6–12 months) | 5 | 52.0 | 75.3 | 82.4 | |
| Toddlers | 10 | 67.2 | 79.1 | 103.4 | |
| 97.5th percentile | Infants (3–6 months) | 3 | 34.0 | 50.3 | 59.8 |
| Infants (6–12 months) | 5 | 61.3 | 84.0 | 98.0 | |
| Toddlers | 7 | 80.4 | 90.3 | 108.8 | |
N: number of surveys.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
Table A.16.
Average contribution of the background exposure to the total acute exposure of pyraclostrobin across European dietary surveys (premarketing scenario)
| Distribution | Age class | N | Average contribution (% of total exposure) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Fulla | Infants (3–6 months) | 3 | 17.0 | 25.0 | 26.8 |
| Infants (6–12 months) | 6 | 28.1 | 31.5 | 37.0 | |
| Toddlers | 11 | 29.5 | 36.9 | 41.2 | |
| Upper tailb | Infants (3–6 months) | 3 | 13.8 | 13.9 | 19.2 |
| Infants (6–12 months) | 5 | 16.2 | 19.3 | 29.5 | |
| Toddlers | 10 | 21.3 | 25.1 | 35.3 | |
N: number of surveys.
Average contribution refers to the full distribution of acute exposures calculated within a survey and age class.
Average contribution refers to the acute exposures exceeding the 95th percentile within a survey and age class. Surveys with less than 61 observations were excluded because the 95th percentile estimates obtained on these dietary surveys/age classes were not considered statistically robust (EFSA, 2011a).
Across surveys, the main RPCs driving total acute exposures are table grapes, onions, currants and apricots. Furthermore, when assessing the acute exposure per RPC (consumers only), an exceedance of the ARfD may also be expected for less frequently consumed RPCs such as apricots and rose hips (see Table A.17).
Table A.17.
Summary statistics of the acute dietary exposure to pyraclostrobin across European dietary surveys arising from raw primary commodities that may lead to an exceedance of the ARfD (premarketing scenario)
| Age class | Raw primary commodity | N | Acute dietary exposurea (% ARfD) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Toddlers | Rose hips | 3 | 35.0 | 51.5 | 107.4 |
N: number of surveys.
The acute dietary exposure refers to the highest percentile that is considered statistically robust for a given dietary survey, age class and food, considering that a minimum of 12, 30, 61 and 181 observations are, respectively, required to derive 75th, 90th, 95th and 97.5th percentile estimates (EFSA, 2011a). Estimates with less than 12 observations were not included in this table.
Assuming a default MRL of 0.01 mg/kg, acute exposure through specific foods for infants and young children generally ranges from 0.1% to 2.6% of the ARfD, but may reach up to 6.9% of the ARfD for infant and follow‐on formulae (see Annex A – Table 8).
Using PRIMo v2, the highest exposure levels are 157% of the ARfD for toddlers (table grapes and kale) with no other ARfD exceedance. For infants (6–12 months), the highest exposure is 95% of the ARfD for apples.
A.5.3.2.2. Post‐marketing scenario
Total acute exposure to pyraclostrobin calculated according to the post‐marketing scenario (see Table A.18) is approximately 4–9 times lower than the exposure estimates anticipated by the premarketing scenario. This ratio is similar to the one observed for the chronic exposure. Regarding the contribution of the background exposure, the large difference between LB and UB estimates makes it difficult to assess the contribution of background exposure in the current scenario (see Table A.19). Nevertheless, compared to the chronic exposure calculations, the impact of this uncertainty on the total acute exposure estimates is smaller (twofold difference between LB and UB estimates).
Table A.18.
Summary statistics of total acute dietary exposure to pyraclostrobin across European dietary surveys (post‐marketing scenario)
| Level of exposurea | Age class | N | Total acute dietary exposure (% ARfD) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Mean | Infants (3–6 months) | 3 | 2.3 | 3.5 | 2.8 | 3.6 | 3.1 | 4.2 |
| Infants (6–12 months) | 6 | 2.5 | 4.4 | 2.7 | 5.0 | 3.9 | 5.9 | |
| Toddlers | 11 | 2.3 | 4.9 | 2.9 | 5.6 | 3.6 | 7.1 | |
| 95th percentile | Infants (3–6 months) | 3 | 4.8 | 7.0 | 5.5 | 7.6 | 6.6 | 7.8 |
| Infants (6–12 months) | 5 | 4.9 | 8.3 | 5.6 | 9.5 | 6.6 | 11.1 | |
| Toddlers | 10 | 4.8 | 9.0 | 5.8 | 9.9 | 6.9 | 11.7 | |
| 97.5th percentile | Infants (3–6 months) | 3 | 5.2 | 9.0 | 6.2 | 9.7 | 7.0 | 10.2 |
| Infants (6–12 months) | 5 | 6.4 | 9.9 | 6.6 | 10.9 | 9.2 | 13.9 | |
| Toddlers | 7 | 6.2 | 10.2 | 6.6 | 10.7 | 7.7 | 11.7 | |
LB: lower bound; N: number of surveys; UB: upper bound.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
Table A.19.
Average contribution of the background exposure to the total acute exposure of pyraclostrobin across European dietary surveys (post‐marketing scenario)
| Distribution | Age class | N | Average contribution (% of total exposure) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Fulla | Infants (3–6 months) | 3 | 0.8 | 17.5 | 1.9 | 25.7 | 1.9 | 29.3 |
| Infants (6–12 months) | 6 | 1.5 | 39.5 | 2.8 | 43.8 | 3.6 | 51.7 | |
| Toddlers | 11 | 2.9 | 46.9 | 5.0 | 54.3 | 7.0 | 60.4 | |
| Upper tailb | Infants (3–6 months) | 3 | 1.3 | 37.4 | 1.5 | 37.5 | 2.3 | 45.9 |
| Infants (6–12 months) | 5 | 1.1 | 29.4 | 2.0 | 37.3 | 2.8 | 45.9 | |
| Toddlers | 10 | 1.8 | 35.9 | 3.3 | 43.3 | 5.0 | 48.8 | |
LB: lower bound; N: number of surveys; UB: upper bound.
Average contribution refers to the full distribution of acute exposures calculated within a survey and age class.
Average contribution refers to the acute exposures exceeding the 95th percentile within a survey and age class. Surveys with less than 61 observations were excluded because the 95th percentile estimates obtained on these dietary surveys/age classes were not considered statistically robust (EFSA, 2011a).
The total acute exposure under this scenario is mainly driven by the consumption of pears. When assessing the acute exposure per RPC (consumers only), the highest exposure was also observed for pears, up to 18% of the ARfD (see Annex A – Table 8). Other important drivers to the acute exposure are apples, currants and peaches. Apricots, rose hips and onions, which on the basis of the premarketing scenario were expected to be important drivers of the acute exposure, resulted in low exposure under the current scenario because concentrations in the monitoring data were much lower than those retrieved from the regulatory data, or not available in the case of rose hips.
Acute exposure to through specific foods for infants and young children (mainly derived from left‐censored monitoring data) were similar to those observed for the premarketing scenario.
A.6. Thiacloprid
A.6.1. Appropriateness of the toxicological reference values
In a dietary two‐generation reproductive toxicity study in rats, decreased pup weight (300 and 600 mg thiacloprid/kg diet) and viability (600 mg thiacloprid/kg diet) were observed at maternally toxic dose levels (300 and 600 mg thiacloprid/kg diet). At these dose levels also, dystocia was observed in the dams. In the high‐dose group (600 mg thiacloprid/kg diet), decreased body weights were observed in both sexes. In addition, increased macroscopical and histopathological changes were observed in the liver and thyroid of the parental animals in the 300 and 600 mg thiacloprid/kg diet groups. The low‐dose group 50 mg thiacloprid/kg diet (2.7 mg thiacloprid/kg bw per day) was identified as the NOAEL. The lowest relevant NOAEL for developmental toxicity of 2 mg thiacloprid/kg bw was detected in the prenatal developmental toxicity study in rabbits.
The ADI of 0.01 mg thiacloprid/kg bw per day was based on the 2‐year rat study, applying an UF of 100 (European Commission, 2004a,b). The ARfD was set on 0.03 mg thiacloprid/kg bw per day based on the acute neurotoxicity study rat using an UF of 100.
The ADI of 0.01 mg thiacloprid/kg bw per day was based on the NOAEL of a chronic 2‐year study in rats of 1 mg thiacloprid/kg bw per day. The ARfD was set on 0.03 mg thiacloprid/kg bw per day based on the acute neurotoxicity study rat. In the two‐generation reproductive toxicity study in rats, a NOAEL of 2.7 mg thiacloprid/kg bw per day for developmental effects identified. This NOAEL was approximately three times higher than the NOAEL identified from the chronic 2‐year study in rats. Therefore, an additional factor for infants > 16 weeks and children up to 3 years is considered not to be applicable.
A.6.2. Exposure of infants below 16 weeks of age
Assuming a default MRL of 0.01 mg/kg (SCF, 1997) and an intake of infant formulae of 260 mL/kg bw at the highest, this will result in a exposure of approximately 0.0026 mg/kg bw per day. This exposure is below the ADI of 0.01 mg thiacloprid/kg bw per day and the default MRL of 0.01 mg/kg is considered sufficiently protective for thiacloprid.
A.6.3. Exposure of infants above 16 weeks of age and toddlers
A.6.3.1. Chronic exposure assessment
A.6.3.1.1. Premarketing scenario
Based on the premarketing data, chronic exposure to thiacloprid is expected to be the highest for toddlers, with mean estimates ranging from 36% to 68% of the ADI (see Table A.20). Depending on the selected percentiles of exposure, highly exposed toddlers may reach chronic exposures up to 79% and 90% of the ADI (95th and 97.5th percentiles respectively). The anticipated chronic exposure for infants is lower compared to the exposure calculated for toddlers. When calculating exposure with PRIMo v2, mean chronic dietary intakes range from 16% to 23% of the ADI, respectively, for infants and toddlers (FR diets).
Table A.20.
Summary statistics of total chronic dietary exposure to thiacloprid across European dietary surveys (premarketing scenario)
| Level of exposurea | Age class | N | Total chronic dietary exposure (% ADI) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Mean | Infants (3–6 months) | 3 | 12.3 | 13.0 | 22.7 |
| Infants (6–12 months) | 6 | 23.6 | 27.2 | 53.7 | |
| Toddlers | 10 | 36.2 | 44.1 | 68.2 | |
| 95th percentile | Infants (3–6 months) | 2 | 23.9 | 40.3 | 56.6 |
| Infants (6–12 months) | 5 | 48.3 | 51.2 | 78.7 | |
| Toddlers | 7 | 60.7 | 70.4 | 78.5 | |
| 97.5th percentile | Infants (3–6 months) | 1 | 72.5 | 72.5 | 72.5 |
| Infants (6–12 months) | 4 | 53.4 | 55.6 | 85.1 | |
| Toddlers | 7 | 67.9 | 80.9 | 89.9 | |
N: number of surveys.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
The primary commodities contributing the most to the calculated exposure are milk, apples and tomatoes (see Figure A.9). Also, olives for oil production are expected to be an important contributor in certain surveys.
Figure A.9.

Mean chronic dietary exposure to thiacloprid across European dietary surveys, highlighting the contribution of individual food commodities (premarketing scenario)
Overall, assuming a default MRL of 0.01 mg/kg, contribution of specific foods for infants and young children to the chronic exposure was found to be relatively high for processed foods (other than cereals) and infant formulae where the average contribution in infants may represent up to 10% and 55% of the total chronic exposure, respectively. These high contributions, however, usually refer to the surveys with the lowest total exposures.
A.6.3.1.2. Post‐marketing scenario
Based on the UB estimates, the chronic exposure to thiacloprid was found to be only 2–5 times lower compared to the chronic exposure anticipated from the premarketing scenario. However, compared to the other active substances assessed, the uncertainty resulting from left‐censored data is very high because an approximate 50‐fold difference was observed between lower bound and upper bound estimates (see Table A.21).
Table A.21.
Summary statistics of total chronic dietary exposure to thiacloprid across European dietary surveys (post‐marketing scenario)
| Level of exposurea | Age class | N | Total chronic dietary exposure (% ADI) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Mean | Infants (3–6 months) | 3 | 0.05 | 9.18 | 0.09 | 11.64 | 0.14 | 14.43 |
| Infants (6–12 months) | 6 | 0.14 | 10.57 | 0.22 | 12.47 | 0.33 | 16.17 | |
| Toddlers | 10 | 0.22 | 13.97 | 0.39 | 14.68 | 0.54 | 17.86 | |
| 95th percentile | Infants (3–6 months) | 2 | 0.30 | 20.29 | 0.41 | 24.50 | 0.51 | 28.70 |
| Infants (6–12 months) | 5 | 0.43 | 17.37 | 0.53 | 24.23 | 0.99 | 25.23 | |
| Toddlers | 7 | 0.60 | 21.81 | 1.13 | 23.16 | 1.46 | 28.06 | |
| 97.5th percentile | Infants (3–6 months) | 1 | 0.67 | 31.07 | 0.67 | 31.07 | 0.67 | 31.07 |
| Infants (6–12 months) | 4 | 0.54 | 21.96 | 0.64 | 26.37 | 1.14 | 27.96 | |
| Toddlers | 7 | 0.79 | 24.11 | 1.53 | 25.06 | 2.08 | 29.37 | |
LB: lower bound; N: number of surveys; UB: upper bound.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
The most important contributors to the chronic exposure are apples, pears and currants (see Figure A.10). Although from the premarketing scenario milk was also expected to be a significant contributor to the exposure, its contribution to the actual exposure is low.
Figure A.10.

Mean chronic dietary exposure to thiacloprid across European dietary surveys, highlighting the contribution of individual food commodities (lower bound post‐marketing scenario)
As for the premarketing scenario, the exposures were found to be the highest for toddlers and contribution of specific foods for infants and young children (including formulae) to the chronic exposure is low.
A.6.3.2. Acute exposure assessment
A.6.3.2.1. Premarketing scenario
Total acute exposure to thiacloprid is expected to be the highest in toddlers where, based on the premarketing data, upper tail exposures may range between 58% and 133% of the ARfD (depending on the survey and percentile of interest, see Table A.22). While the contribution of background exposure generally ranges between 18% and 41% of the total acute exposure, background exposure only accounts for 8–30% in the upper tail exposures (see Table A.23). This indicates that high acute exposures to thiacloprid are very much driven by specific food items.
Table A.22.
Summary statistics of total acute dietary exposure to thiacloprid across European dietary surveys (premarketing scenario)
| Level of exposurea | Age class | N | Total acute dietary exposure (% ARfD) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Mean | Infants (3–6 months) | 3 | 9.9 | 10.5 | 15.2 |
| Infants (6–12 months) | 6 | 19.6 | 25.3 | 55.7 | |
| Toddlers | 11 | 24.4 | 34.1 | 65.5 | |
| 95th percentile | Infants (3–6 months) | 3 | 25.6 | 39.5 | 61.1 |
| Infants (6–12 months) | 5 | 50.2 | 66.5 | 105.9 | |
| Toddlers | 10 | 57.7 | 68.9 | 132.8 | |
| 97.5th percentile | Infants (3–6 months) | 3 | 30.2 | 47.9 | 93.2 |
| Infants (6–12 months) | 5 | 60.6 | 79.3 | 120.4 | |
| Toddlers | 7 | 68.6 | 80.5 | 118.9 | |
N: number of surveys.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
Table A.23.
Average contribution of the background exposure to the total acute exposure of thiacloprid across European dietary surveys (premarketing scenario)
| Distribution | Age class | N | Average contribution (% of total exposure) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Fulla | Infants (3–6 months) | 3 | 18.1 | 22.4 | 27.3 |
| Infants (6–2 months) | 6 | 22.2 | 34.8 | 38.4 | |
| Toddlers | 11 | 22.1 | 37.9 | 41.3 | |
| Upper tailb | Infants (3–6 months) | 3 | 9.7 | 10.0 | 27.2 |
| Infants (6–12 months) | 5 | 12.4 | 14.1 | 22.2 | |
| Toddlers | 10 | 10.6 | 21.9 | 29.5 | |
N: number of surveys.
Average contribution refers to the full distribution of acute exposures calculated within a survey and age class.
Average contribution refers to the acute exposures exceeding the 95th percentile within a survey and age class. Surveys with less than 61 observations were excluded because the 95th percentile estimates obtained on these dietary surveys/age classes were not considered statistically robust (EFSA, 2011a).
Across surveys, the main RPCs driving total acute exposures are peaches, raspberries, olives for oil production and apples. Also when assessing the acute exposure for consumers of these RPCs individually, an exceedance of the ARfD may be expected, except for apples (see Table A.24).
Table A.24.
Summary statistics of the acute dietary exposure to thiacloprid across European dietary surveys arising from raw primary commodities that may lead to an exceedance of the ARfD (premarketing scenario)
| Age class | Raw primary commodity | N | Acute dietary exposurea (% ARfD) | ||
|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||
| Infants (3–6 months) | Peaches | 1 | 107.2 | 107.2 | 107.2 |
| Infants (6–12 months) | Peaches | 5 | 65.9 | 94.3 | 112.6 |
| Toddlers | Peaches | 7 | 28.2 | 85.4 | 119.4 |
| Raspberries (red and yellow) | 7 | 34.7 | 45.8 | 115.2 | |
| Olives for oil production | 11 | 3.8 | 26.8 | 126.0 | |
N: number of surveys.
The acute dietary exposure refers to the highest percentile that is considered statistically robust for a given dietary survey, age class and food, considering that a minimum of 12, 30, 61 and 181 observations are respectively required to derive 75th, 90th, 95th and 97.5th percentile estimates (EFSA, 2011a). Estimates with less than 12 observations were not included in this table.
Assuming a default MRL of 0.01 mg/kg, acute exposure through specific foods for infants and young children generally ranges from 0.1% to 2.6% of the ARfD, but may reach up to 6.9% of the ARfD for infant and follow‐on formulae (see Annex A – Table 8).
With PRIMo v2, the highest acute levels are 52% of the ARfD for apples and infants (UK), and 50% of the ARfD for celery leaves and toddlers (BE).
A.6.3.2.2. Post‐marketing scenario
Total acute exposure to thiacloprid calculated according to the post‐marketing scenario (see Table A.25) is approximately 2–8 times lower than the exposure estimates anticipated by the premarketing scenario. This ratio is similar to the one observed for the chronic exposure. Regarding the contribution of the background exposure, the large difference between LB and UB estimates makes it difficult to assess the contribution of background exposure in the current scenario (see Table A.26). Nevertheless, compared to the chronic exposure calculations, the impact of this uncertainty on the total acute exposure estimates is smaller (twofold difference between LB and UB estimates).
Table A.25.
Summary statistics of total acute dietary exposure to thiacloprid across European dietary surveys (post‐marketing scenario)
| Level of exposurea | Age class | N | Total acute dietary exposure (% ARfD) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Mean | Infants (3–6 months) | 3 | 4.1 | 5.6 | 5.1 | 5.8 | 5.4 | 6.6 |
| Infants (6–12 months) | 6 | 3.5 | 5.9 | 4.0 | 6.9 | 4.4 | 8.0 | |
| Toddlers | 11 | 3.3 | 7.0 | 3.8 | 7.6 | 5.3 | 10.3 | |
| 95th percentile | Infants (3–6 months) | 3 | 9.0 | 10.7 | 9.4 | 12.0 | 13.0 | 13.7 |
| Infants (6–12 months) | 5 | 7.6 | 12.0 | 7.9 | 12.6 | 8.6 | 14.1 | |
| Toddlers | 10 | 6.3 | 11.9 | 7.4 | 13.0 | 8.9 | 16.5 | |
| 97.5th percentile | Infants (3–6 months) | 3 | 9.7 | 11.3 | 10.1 | 13.0 | 13.5 | 14.7 |
| Infants (6–12 months) | 5 | 8.6 | 13.9 | 9.1 | 14.8 | 10.7 | 16.2 | |
| Toddlers | 7 | 7.2 | 12.9 | 8.7 | 14.5 | 10.0 | 16.9 | |
LB: lower bound; N: number of surveys; UB: upper bound.
The 95th and 97.5th percentile estimates obtained on dietary surveys/age classes with less than 61 and 181 observations, respectively, may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
Table A.26.
Average contribution of the background exposure to the total acute exposure of thiacloprid across European dietary surveys (post‐marketing scenario)
| Distribution | Age class | N | Average contribution (% of total exposure) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Fulla | Infants (3–6 months) | 3 | 0.3 | 12.9 | 0.6 | 21.1 | 1.2 | 24.1 |
| Infants (6–12 months) | 6 | 1.1 | 35.1 | 1.5 | 42.7 | 2.3 | 47.6 | |
| Toddlers | 11 | 1.5 | 47.6 | 2.8 | 51.8 | 3.8 | 58.0 | |
| Upper tailb | Infants (3–6 months) | 3 | 0.1 | 19.2 | 0.2 | 19.8 | 0.4 | 38.2 |
| Infants (6–12 months) | 5 | 0.4 | 30.1 | 0.9 | 40.5 | 1.2 | 48.1 | |
| Toddlers | 10 | 0.8 | 43.5 | 1.8 | 50.0 | 2.3 | 54.6 | |
LB: lower bound; N: number of surveys; UB: upper bound.
Average contribution refers to the full distribution of acute exposures calculated within a survey and age class.
Average contribution refers to the acute exposures exceeding the 95th percentile within a survey and age class. Surveys with less than 61 observations were excluded because the 95th percentile estimates obtained on these dietary surveys/age classes were not considered statistically robust (EFSA, 2011a).
The total acute exposure under this scenario is mainly driven by the consumption of pears and potatoes. When assessing the acute exposure per RPC (consumers only), the highest exposure were also observed for pears and potatoes, up to 17% and 11% of the ARfD, respectively (see Annex A – Table 8). Peaches, raspberries and olives for oil production, which on the basis of the premarketing scenario were expected to be important drivers of the acute exposure, resulted in low exposure under the current scenario because concentrations in the monitoring data were much lower than those retrieved from the regulatory data.
Acute exposure to through specific foods for infants and young children ranged from 0.1% to 2.5% of the ARfD and exposures up to 13.8% of the ARfD were noted for infant formulae (see Annex A – Table 8). These estimates were, however, derived from left‐censored monitoring data.
Appendix B – Recent literature review on developing brain and brain barriers
B.1. Brain and brain barriers
Since the closing of the literature review before the composition of the recent EFSA report a number of reports have appeared that enrich our comprehension of the possible targets for pesticides which may be developmental neurotoxicants. These and their possible implications for the vulnerability of infants are considered in this section.
B.2. Neuronal plasticity
Recent work on brain development and brain barriers has underscored the temporal importance of functional stimuli during brain development highlighting neuronal plasticity in the sensory systems and linking this to autism, fragile ‐X and Rett syndromes (Chaudhury et al., 2015). Plasticity is also important in brain development and specifically in the context of learning in the amygdala where brain‐derived neurotrophic factor, and other molecules work together to transduce extracellular signals (Ehrlich and Josselyn, 2016). Autism has also been linked to disruption of usually continuous and significant neural‐network structural development in infancy. This advance has occurred through observing changes that have been detected by connectomics analysis (Cao et al., 2016). Further progress is also being made using transcriptomics to understand the timing of some of the large number of key steps in cortical development. These include healthy neuronal development in the prenatal and early postnatal period, stem‐cell division, progenitor cell proliferation, migration of neurons, architectural folding of the cortex into lobes and gyri, synaptogenesis, neural circuit development, gliogenesis and myelination. A limitation of these molecular methods is that they are point in time averages of events taking place at different times in several different brain regions (Jiang and Nardelli, 2016).
B.3. Cellular and molecular neurodevelopmental biology
The level of cellular and molecular detail in descriptions of developmental processes such as neuronal migration is increasing. Needed for correct positioning of cells in developing networks the key events involved are being explained in terms of their functional components. For example axon extension, nuclear approximation to centrosomes and the cytoskeletal and motor proteins involved in the differentiation and correct positioning of neurons are now partly understood (Dantas et al., 2016). These are of interest as some of the key molecules such as the motor proteins dynein and KIF1A when inhibited experimentally cause different forms of microcephaly and lissencephaly conditions which are sometimes observed in risk assessments where teratogenesis of pesticides has been a cause for concern. It is likely that this level of understanding will be necessary to elaborate Adverse Outcome Pathways (AOPs) for some of the health outcomes in the area of DNT.
Despite these advances, it is still the case that precise evidence is lacking for where and when in human brain and brain barrier (BB) development the most potentially pesticide sensitive events are occurring, although it is clear that there are many of these still to be described scientifically throughout the early post‐natal period and infancy.
B.4. Haemodynamics of the brain
Recent advances in non‐invasive MRI techniques have allowed neonatal and infant brain haemodynamics to be observed and quantitated in vivo (De Vis et al., 2016). Such studies are at an early stage but offer the prospect of directly measuring effects on infant brain such as haemodynamic‐induced encephalopathy. Whilst apparently no case studies have yet been carried out on infants that are reported to have been exposed to environmental factors such as pesticides (Richmond et al., 2016) there will possibly be opportunities for advances in knowledge in future as these techniques develop.
B.5. Microbiome effects on brain development
The increasing interest in the gut microbiome has revealed that the establishment of microbiota at birth can be disturbed with consequences for brain development and behaviour although without clear understanding of linking mechanisms (Diaz Heijtz et al., 2011; O'Mahony et al., 2017). Some of these are thought to be mediated through glial mechanisms (Tay et al., 2017).
B.6. DHA
Amongst long‐chain poly‐unsaturated fatty acids (LCPUFA), a component specific to cell membranes in the CNS is docosahexaenoic acid (DHA), an omega‐3 fatty acid. It is synthesised at a low rate but accumulates rapidly during the third trimester of pregnancy (via placental transport) and during the 2 years after birth from breast milk or formula. It is important for neurone growth, differentiation and signalling in infancy. Its beneficial effects on cognition are inconsistently observed at lower levels of supplementation but seem to be consistent at supplementation levels above 1.4%. At this level, three studies with high doses of DHA yielded some short‐term beneficial effects, two for cognition (i.e., 1.4% fatty acid DHA) and one for vision (Isaacs et al., 2008). However, the long‐term sustainability of these effects remains to be demonstrated (Schneider and Garcia‐Rodenas, 2017) and the level of DHA may affect early development with some effects of supplementation suggested to affect behaviour. Its action is genotype and gender specific. This physiological difference of the foetal and infant brain from that of the adult and ageing brain is of interest should its accumulation be affected by a toxin (Lauritzen et al., 2016).
B.7. Neurovesicles in brain development
Recently, attention has been drawn to two populations of small vesicles (exosomes and microvesicles) that shuttle proteins, lipids and RNA between donor and recipient cells in the developing brain. The fact that these cells include neurones, glia, neuroepithelial and choroid plexus cells and that amongst the contents of the vesicles are known morphogens shows that they are clearly relevant to neurodevelopment (Morton and Feliciano, 2016). As yet nothing is known about the response of these organelles to toxic effects of pesticides.
B.8. Links between the development of the CNS and the immune system
-
i
Cancer metastasis
A study of cancer metastasis to the cerebrospinal fluid (CSF) through the leptomeninges28 (which is frequently fatal) has relevance (Ghannam et al., 2008). The ability of cancer cells to infiltrate and grow in CSF has not been easy to understand as CSF is generally an acellular, mitogen‐poor microenvironment unsuitable for metastatic spread. The authors discovered that in the model disease process complement component 3 (C3) was upregulated. This allowed cancer growth within the rat leptomeningeal space where CSF is located. In human disease, cancer cells within the CSF also synthesise C3. Furthermore, it was found that C3 concentration increased as the disease increased in severity. C3 expression in primary tumours was also found to be predictive of leptomeningeal relapse. It appears from these studies that C3 activates the C3a receptor in the choroid plexus epithelium causing disruption of the blood‐CSF barrier. This effect allows plasma components such as mitogens to enter the CSF and stimulation of tumour growth. Consistent with this interpretation, inhibition of C3 signalling suppressed leptomeningeal metastasis in the rat preclinical models.
This article is particularly interesting as it indicates in human adults and rats a link between a component of the complement cascade and the opening of one of the brain barriers (the blood choroid plexus barrier). This suggests that the effect of neurotoxicants can be controlled by substances that are part of the immune system providing a potential adverse outcome pathway for developmental neurotoxicity following changes in the immune status of the very young. There is as yet no evidence for the developmental age or stage at which this opening system becomes functional in humans. However, C3 is present in humans and is consistently synthesised in the liver from 14 weeks of age, and in some instances possibly earlier. This draws attention to a data gap, present for humans, that could be filled by research. The fact that the process appears also to occur in rats is helpful as an animal model for the study of the process is available.
-
ii
Anaphylatoxins (Ats)
The effects of the complement C3 pathway at this site as part of the innate immune system are of interest as one small cleavage product of its proteolytic digestion is C3a that binds to receptors throughout the brain, particularly on astrocytes and microglia (Saunders et al., 2000; Klos et al., 2009). These anaphylotoxins, such as C3a and C5a, are bacteriocidal and cause inflammation.
As potent mediators of inflammation, they control many responses that include vasodilation, small blood vessel permeability and smooth muscle contraction. They trigger oxidative bursts of neutrophils, macrophages, basophils and eosinophils, and cause histamine degranulation of mast cells, eosinophil migration and attachment and serotonin release from platelets. They are a chemoattractant for macrophages and modulate IL‐6 and TNF‐alpha synthesis by B cells and monocytes (Klos et al., 2009). Beyond their inflammatory roles, they appear to regulate tissue regeneration, haematopoietic stem cell homing and fibrosis. Importantly, the C3a receptor has been suggested to have a role in the development of the cerebellum (Bérnard et al., 2004). Receptors for these anaphylatoxins C3aR and C5aR are expressed in cerebellar granule neurons reaching their highest concentration in the 12‐day‐old rat. It is difficult to translate the timescale into human development terms but four rat days per human year might allow a very rough estimate of equivalence of 3 years in humans (Eason et al., 2004).
This additional evidence‐linking components of the innate immune system with opening of the blood–brain barrier and with developmental effects on the cerebellum can be viewed as potential parts of developmental neurotoxicity adverse outcome pathways.
-
iii
Microglia
The roles of the glial system include the regulation of brain development and homeostasis via microglial–neuronal interactions. These comprise but are not restricted to synaptic modelling, cellular immune functions, removal of cellular debris, participation in BBs function and production of trophic factors. The variety of roles, which includes effects on cognitive functions, may depend on subpopulations of microglia which first originate in the yolk sac (but later arise from haematopoietic tissue) and come to colonise different brain niches by crossing leptomeninges. It is suggested that the variety of forms are influenced by their local interactions with populations of neurones that have different functions. One key location is the subventricular zone where chemoattractant processes cause one group to coalesce. A recently described group of ‘dark microglia’ found in mouse hippocampus, amygdala, hypothalamus and cortex shows evidence of oxidative stress and coalesce at synapses where an observed role in a specialised form of synapse removal has been linked with neurodegenerative conditions. This work is of interest but much further study will be required to explore possible relevance to potential effects of pesticides in developing human infant brain (Tay et al. 2017). As with the differential gender effects of DHA supplementation, it is of interest to note the possession of oestrogen and progesterone receptors on microglia. These properties are expected to expose the brain to harm from endocrine active substances. Also, there are reports of disruption of microglial function linked to the absence of a gut microbiome and alterations in gut microbiota (Tay et al. 2017). It is worth pointing out that astroglia and microglia share a considerable repertoire of functions and the roles of glia are considered ever more important in brain function and development (Reemst et al., 2016). Functions shared by these two disparate forms of glia include roles in neuro‐, angio‐ and glio‐genesis, axon growth, synaptogenesis and the pruning of synapses. Dysfunction may result in neurodevelopmental conditions and possibly delayed onset neuropathology.
To summarise we are at a stage where a great deal of detail is now available concerning canonical functions of the cellular, organellar and molecular components of the developing brain. There is, however, a gulf to be crossed and a need for greater descriptive detail concerning the stages of differentiation of brain regions with different functional roles and with adequate time resolution. With this information, AOPs can be developed that will in future allow stage‐specific experimental designs to determine the sensitivity of the developing systems to be assessed in risk assessment.
Appendix C – Therapeutic doses of pharmaceuticals in infants and young children and comparison with doses in adults
1.
List of medicines in clinical use with doses (p.o.) in infants above 16 weeks up to children of 3 years of age and corresponding doses (p.o.) in adults.
When doses are not provided in the references in mg/kg bw per day they were calculated by assuming a weight of 10 kg for the paediatric dose and a weight of 70 kg for the adult dose. When a range was given in the references, the mean value was used to calculate the ratio adult/infant.
| Medicine (INN) | Dose (p.o.) infants > 4 months up to children 3 years old (mg/kg bw per day) | Dose (p.o.) adult (mg/kg bw per day) | Ratio adult/infant (value > 1 indicates that infant is more sensitive) |
|---|---|---|---|
| Weight (used for calculation) | 10 kg | 70 kg | 7 |
| Acetycystein | 10–15 | 8.6 | 0.715 |
| Acetylsalicylic acid | 30–45 | 21.4–42.8 | 0.95 |
| Albendazol | 15 | 11.4 | 0.76 |
| Amlodipine | 0.05 | 0.07–0.14 | 1.2 |
| Amoxicillin | 24–48 | 21.4–28.6 | 0.7 |
| Azithromycin | 10 | 7.14 | 0.714 |
| Captopril | 2 | 6 | 3 |
| Carbamazepine | 35 | 22.8 | 0.65 |
| Carvedilol | 0.05 | 0.178 | 3.6 |
| Cefaclor | 40 | 42.85 | 1.1 |
| Cefadroxil | 100 | 57.1 | 0.57 |
| Cefazolin | 100 | 171 | 1.7 |
| Cefepim | 150 | 85 | 0.57 |
| Cefixim | 8–12 | 5.7 | 0.7 |
| Cefpodoxime | 8–10 | 11.4 | 1.1 |
| Cefuroxime | 20–30 | 14.3 | 0.57 |
| Cephalexin | 40 | 42.85 | 1.1 |
| Chloroquine | 25–30 | 25–30 | 1 |
| Cimetidine | 15–30 | 28.5 | 0.95 |
| Clarithromycin | 15 | 14.3 | 0.95 |
| Clindamycin | 30 | 25.7 | 0.86 |
| Clobazam | 0.5–1 | 1.14 | 1.5 |
| Clonidine | 0.025 | 0.01285 | 0.5 |
| Desloratadine | 0.1 | 0.07 | 0.7 |
| Diclofenac | 1–3 | 1.4–2.8 | 1 |
| Digoxin | 0.01 | 0.0036 | 0.36 |
| Dimenhydrinate | 5 | 5.7 | 1.1 |
| Dimetindenmaleat | 0.15–0.225 | 0.08 | 0.53 |
| Doxylamine succinate | 0.625–1.25 | 0.35–0.7 | 0.57 |
| Enalapril | 0.5 | 0.6 | 1.1 |
| Ethosuximide | 15–40 | 14.3 | 0.5 |
| Flucloxacillin | 40–50 | 171.4 | 3.8 |
| Fluconazol | 6–12 | 5.7 | 0.63 |
| Fluconazole | 3–12 | 5.7 | 0.76 |
| Fosfomycin | 100–300 | 285 | 1.43 |
| Furosemid | 1–2 | 1.7 | 1.1 |
| Hydrochlorothiazide | 1–3 | 1.42 | 0.71 |
| Ibuprofen | 30–60 | 45.7 | 1.01 |
| Isoniacid | 8–10 | 8 | 0.9 |
| Ketotifen | 0.1 | 0.11 | 1.1 |
| Lacosamide | 10 | 8.6 | 0.86 |
| Levetiracetam | 20–60 | 42.9 | 1.1 |
| Linezolid | 30 | 17.1 | 0.58 |
| Loracarbef | 15–30 | 11.4 | 0.5 |
| Lorazepam | 0.4 | 0.14 | 0.36 |
| Meropenem | 30–60 | 42 | 0.95 |
| Metamizol | 32–64 | 57.1 | 1.2 |
| Metildogoxin | 0.008 | 0.004 | 0.5 |
| Metolazone | 0.2–0.4 | 0.07–0.14 | 0.36 |
| Metronidazol | 20–30 | 28 | 1.14 |
| Morphin | 1.8 | 1.8 | 1 |
| Naproxen | 15 | 17.85 | 1.2 |
| Neostigminmethylsulfat | 0.6 | 0.14 | 0.23 |
| Niclosamid | 25 | 14.2 | 0.57 |
| Nitrofurantoin | 5 | 5 | 1 |
| Omeprazol | 3.5 | 1.7 | 0.5 |
| Oxacillin | 80–160 | 171 | 1.43 |
| Paracetamol | 40–60 | 40–60 | 1 |
| Penicillin V | 15–63 | 50.6 | 2.1 |
| Pethidin | 3–12 | 2.1–12.8 | 1 |
| Phenobarbital | 5–10 | 1–3 | 0.27 |
| Pipamperon | 2‐4‐6 | 1.7–5.1 | 1 |
| Propafenon | 10–20 | 12.8 | 0.96 |
| Propiverin | 0.7–0.9 | 0.21–0.63 | 0.52 |
| Propranolol | 8 | 4.6 | 0.57 |
| Ranitidin | 4–8 | 4.3–12.9 | 1.6 |
| Rifampicin | 15 | 17 | 1.1 |
| Roxithromycin | 5–7.5 | 4.3 | 0.7 |
| Spironolactone | 1–3 | 1.4–2.8 | 1.15 |
| Sulfamethazol | 60 | 34.3 | 0.57 |
| Sultamicillin | 50 | 21.4 | 0.43 |
| Sultiam | 5–10 | 5–10 | 1 |
| Terbutalin | 0.15–0.45 | 0.21 | 0.7 |
| Theophyllin | 18 | 13 | 0.7 |
| Topiramate | 5–9 | 7.14 | 1.0 |
| Tramadol | 12 | 8.6 | 0.7 |
| Trimethoprim | 12 | 6.8 | 0.57 |
| Ursodesoxycholic acid | 10–15 | 10–15 | 1 |
| Valproic acid | 30–60 | 14.3–35.7 | 0.56 |
| Vancomycin | 40 | 28.6 | 0.7 |
| Verapamil | 4 | 6.8 | 1.7 |
| Vigabatrin | 40 | 14.3 | 0.35 |
| Mean value (n = 82) | 0.95 | ||
| SD | 0.61 | ||
| Range | 0.23–3.8 | ||
Information extracted from:
MacPeds Formulary, a document used in McMaster Children's Hospital (MCH), McMaster and St Joseph's Healthcare, Canada.
Medicine Base, Wissenschaftliche Verlagsgesellschaft, Stuttgart, Germany. www.drugbase.de last accessed 09 January 2018.
Appendix D – Hydrolysis studies for residue definition
1.
Hydrolysis data of 111 approved active substances having a single compound residue definition (SCRD), used for the assessment of the appropriateness of residue definition established under Regulation (EC) No 396/2005 (chapter 7).
| Substance | Minimum recovery per a.s. (90°C, 20 min, pH 4) | Minimum recovery per a.s. (100°C, 60 min, pH 5) | Minimum recovery per a.s. (120°C, 20 min, pH 6) | |
|---|---|---|---|---|
| 1 | Aclonifen | 96.7 | 96.8 | 98.0 |
| 2 | Amisulbrom | 89.9 | 49.3 | 11.5 |
| 3 | Azoxystrobin | 100.9 | 96.2 | 100.1 |
| 4 | Beflubutamid | 104.4 | 95.4 | 98.5 |
| 5 | Alpha‐Cypermethrin (aka alphamethrin) | 99.7 | 97.6 | 54.7 |
| 6 | Benalaxyl | 101.0 | 102.0 | 98.0 |
| 7 | Benthiavalicarb | 94.8 | 96.5 | 93.9 |
| 8 | Benzovindiflupyr | 100.0 | 100.0 | 100.0 |
| 9 | Beta‐Cyfluthrin | 106.5 | 99.7 | 12.1 |
| 10 | Bifenthrin | 49.0 | 31.0 | 81.3 |
| 11 | Bupirimate | 94.9 | 95.6 | 91.1 |
| 12 | Buprofezin | 28.2 | 30.5 | 76.0 |
| 13 | Chlorantraniliprole | 98.4 | 85.9 | 96.2 |
| 14 | Chlormequat | 101.0 | 99.1 | |
| 15 | Chlorpyrifos | 74.6 | 19.8 | 0.8 |
| 16 | Chromafenozide | 94.4 | ||
| 17 | Clopyralid | 99.3 | 96.9 | 97.1 |
| 18 | Clothianidin | 100.0 | 100.0 | 100.0 |
| 19 | Cyantraniliprole | 98.6 | 98.8 | 100.2 |
| 20 | Cyazofamid | 18.6 | 0.0 | 0.0 |
| 21 | Cyflumetofen | 69.3 | 5.0 | 0.0 |
| 22 | Cymoxanil | 11.8 | ||
| 23 | Cypermethrin | 95.2 | 96.9 | 54.7 |
| 24 | Cyproconazole | 96.6 | 101.0 | 97.0 |
| 25 | Cyromazine | 99.9 | 99.1 | 100.6 |
| 26 | Deltamethrin | 94.8 | 94.0 | 25.6 |
| 27 | Desmedipham | 81.8 | 0.0 | 0.0 |
| 28 | Dicamba | 100.7 | 105.1 | 107.6 |
| 29 | Diethofencarb | 98.8 | 98.7 | 98.2 |
| 30 | Difenoconazole | 95.6 | 96.0 | 98.6 |
| 31 | Dimethenamid‐P | 96.3 | 98.6 | 95.1 |
| 32 | Dimethomorph | 98.1 | 93.0 | 99.0 |
| 33 | Dimoxystrobin | 97.7 | 94.4 | 0.0 |
| 34 | Dithianon | 28.1 | 1.0 | 0.0 |
| 35 | Dodine | 97.1 | 91.8 | 94.7 |
| 36 | Emamectin | 84.4 | 85.9 | 79.8 |
| 37 | Epoxiconazole | 92.5 | 97.6 | 97.6 |
| 38 | Ethephon | 80.5 | 0.0 | 0.0 |
| 39 | Etofenprox | 95.6 | 93.0 | 94.3 |
| 40 | Etoxazole | 72.2 | 62.8 | 59.9 |
| 41 | Famoxadone | 96.2 | 66.0 | 43.3 |
| 42 | Fenamidone | 90.1 | 100.1 | 102.6 |
| 43 | Fenazaquin | 35.0 | 61.6 | 79.4 |
| 44 | Fenbuconazole | 100.7 | 97.7 | 104.6 |
| 45 | Fenhexamid | 99.7 | 95.7 | 100.2 |
| 46 | Fenoxycarb | 92.2 | 94.7 | 95.3 |
| 47 | Fenpyrazamine | 95.1 | 99.8 | 89.1 |
| 48 | Flubendiamide | 101.0 | 100.9 | 99.8 |
| 49 | Fluopicolide | 98.9 | 103.8 | 100.4 |
| 50 | Fluoxastrobin | 95.3 | 102.6 | 98.2 |
| 51 | Flupyradifurone | 100.0 | 100.0 | 100.0 |
| 52 | Fluquinconazole | 99.0 | 91.2 | |
| 53 | Fluxapyroxad | 100.0 | 100.0 | 100.0 |
| 54 | Formetanate | 88.5 | 0.6 | 1.4 |
| 55 | Fosthiazate | 82.6 | 79.2 | |
| 56 | Hymexazol | 100.0 | 100.0 | 100.0 |
| 57 | Imazamox | 102.2 | 103.5 | 95.2 |
| 58 | Imidacloprid | 97.7 | 99.2 | 98.1 |
| 59 | Indoxacarb | 88.0 | 69.0 | |
| 60 | Iprovalicarb | 98.1 | 97.2 | 96.9 |
| 61 | Isofetamid | 99.2 | 97.8 | 96.8 |
| 62 | Isopyrazam | 96.1 | 98.6 | 98.4 |
| 63 | Lambda‐Cyhalothrin | 87.8 | 82.6 | 7.8 |
| 64 | Lufenuron | 100.3 | 95.4 | 107.2 |
| 65 | Mandestrobin | 94.2 | 99.8 | 99.6 |
| 66 | Mandipropamid | 104.9 | 103.4 | 109.1 |
| 67 | Mepanipyrim | 101.1 | 105.7 | 97.9 |
| 68 | Mepiquat | 95.1 | 96.6 | 94.9 |
| 69 | Metaflumizone | 77.0 | 62.1 | 89.4 |
| 70 | Metalaxyl‐M | 98.6 | 100.6 | 99.3 |
| 71 | Methoxyfenozide | 97.7 | 95.9 | 96.1 |
| 72 | Methomyl | 93.7 | 86.5 | 58.2 |
| 73 | Metrafenone | 100.5 | 101.4 | 94.8 |
| 74 | Metribuzin | 97.6 | 97.9 | 97.8 |
| 75 | Oxamyl | 100.0 | 57.8 | 0.0 |
| 76 | Oxathiapiprolin | 94.8 | 94.7 | 93.8 |
| 77 | Penconazole | 97.3 | 97.6 | 97.8 |
| 78 | Pendimethalin | 99.4 | 99.9 | 99.2 |
| 79 | Penflufen | 100.9 | 106.5 | 99.2 |
| 80 | Penthiopyrad | 101.5 | 102.1 | 99.8 |
| 81 | Phenmedipham | 87.3 | 0.0 | 0.0 |
| 82 | Pinoxaden | 86.2 | 72.3 | 53.5 |
| 83 | Propiconazole | 98.0 | 99.9 | 99.6 |
| 84 | Propineb | 62.1 | 26.4 | 1.6 |
| 85 | Proquinazid | 95.2 | 95.9 | 93.9 |
| 86 | Prosulfocarb | 91.5 | 94.1 | 94.6 |
| 87 | Prothioconazole | 99.4 | 99.9 | 100.3 |
| 88 | Pyraclostrobin | 98.1 | 0.0 | 2.5 |
| 89 | Pyridaben | 97.8 | 90.0 | 85.2 |
| 90 | Pyridalyl | 103.4 | 92.5 | 95.7 |
| 91 | Pyriofenone | 98.6 | 98.2 | 98.3 |
| 92 | Quinmerac | 97.2 | 103.3 | 96.2 |
| 93 | Quizalofop‐P | 100.0 | ||
| 94 | Sedaxane | 98.9 | 99.4 | 99.3 |
| 95 | Spinetoram | 94.5 | 92.1 | 85.0 |
| 96 | Spinosad | 96.9 | 95.6 | 77.5 |
| 97 | Spirodiclofen | 99.1 | 35.4 | 37.3 |
| 98 | Spiromesifen | 70.8 | 13.4 | 0.9 |
| 99 | Sulfoxaflor | 99.6 | 100.0 | 100.4 |
| 100 | Tau‐Fluvalinate | 90.9 | 40.8 | 1.7 |
| 101 | Tebufenozide | 100.0 | 100.0 | 100.0 |
| 102 | Tebufenpyrad | 100.0 | 102.4 | 115.4 |
| 103 | Tetraconazole | 98.7 | 97.5 | 97.5 |
| 104 | Thiacloprid | 98.1 | 96.5 | 97.0 |
| 105 | Thiamethoxam | 100.0 | 100.0 | 98.3 |
| 106 | Thiencarbazone | 36.4 | 2.6 | 0.0 |
| 107 | Thiram | 80.3 | 20.8 | 0.3 |
| 108 | Tolclofos‐methyl | 61.7 | 33.6 | 12.6 |
| 109 | Triadimenol | 96.2 | 94.4 | 93.2 |
| 110 | Triflumuron | 98.4 | 88.9 | 51.4 |
| 111 | Trinexapac (aka cimetacarb ethyl) | 52.5 | 58.5 | 50.9 |
Appendix E – List of uncertainties
1.
Detailed list of uncertainties summarised under Chapter 8:
ToR 1 – The assessment of the appropriateness of the toxicological reference values for pesticides for infants and young children and of the approach to base the MRLs for pesticides for foods for infants and young children on the acceptable daily intake (ADI) values (in this context, the assessment of the short‐term dietary risk should also be considered):
No. 1: Literature search was restricted to review articles published in WEB of Science for specific timespans and carried out in English. Relevant references were identified by two independent WG members based on inclusion criteria.
No. 2: Age and stage of development. Estimates of developmental stage equivalence between animals and humans are not precise and a further source of uncertainty. There are also significant interindividual differences in humans in the stage of development at any time after parturition. This evidence introduces uncertainty into the classification of infants and toddlers for risk assessment based on time after birth.
No. 3: In the newborn the brain, liver and kidney are relatively larger than in the adult and the fraction of the body of an infant consisting of intracellular and extracellular water and of fat alters over the first year. Protein, fat and water compartments in the body change at different rates adding uncertainty to predictions of local concentrations in the target organ, and hence, the extent of toxic effects extrapolated from adults.
No. 4: Smaller surface areas in infant than adult as a result of a less elaborate convolution of the gut lining may restrict absorption in the infant to an unknown, but limited degree for nutrients.
No. 5: Other intestinal maturational differences are expected to be the basis of physiological differences. These include motility, transit time of food, gastric emptying and volume, pH of gut contents and the expression of metabolizing enzymes and transporter molecules (Nicolas et al., 2017). The variety of potential differences and the paucity of data add uncertainty to the risk assessment. However, studies show that gastric emptying is slower and the rate of absorption reduced whereas the extent of absorption is not. However, it is uncertain whether these observations of a small number of substances are fully applicable to pesticides residues.
No. 6: Bile salts metabolism is active in the neonate, but the extent may change over the first 8 months. There is therefore a contribution to uncertainty because of this reduced capacity in the infant as compared with the adult (de Belle et al., 1979).
No. 7: Infants and children have a higher drinking water consumption per kilogram of bodyweight than adults. This adds uncertainty to estimations of the uptake of water soluble residues.
No. 8: Closure is a stage in gut development that occurs after birth in some laboratory animals; but in humans, this is functionally complete by 4 days after the start of oral feeding so will add uncertainty in extrapolations from animal studies to humans.
No. 9: The human microbiome is important to health and physiology. Microbiota differ in their species composition from one part of the body to another. In the GI tract, microorganisms produce and modify substances with a variety of biological activities affecting host metabolism including metabolism of bile acids and vitamins. Obesity, inflammatory bowel disorders and intestinal cancer have been linked to alterations in the microbiome in early life. Particular bacterial species are required during specific developmental stages to ensure a healthy pattern of host immunity. In infancy, it is developed to a stage where it can influence health and development. This is in some cases a known effect of diet, but there is a small amount of evidence that it could be affected by pesticide residues (Derrick et al., 2017; Claus et al., 2016). Further research is needed to establish the role of currently approved pesticides and to determine if they contribute to the risk assessment by effects mediated by the developing microbiome.
No. 10: The main toxicokinetic differences concern reduced clearance from the body. This leads to a higher internal exposure at the same external exposure. These differences can be quantified at a general level and if necessary corrected by an additional default uncertainty factor. For individual substances, the additional default factor might be too high or too low.
No. 11: The lack of EOGRTS with the DIT/DNT is considered an important uncertainty that leads to a recommendation.
ToR 2 – The assessment of the contribution of other foods consumed by infants and young children that are not covered by Regulation (EU) No 609/2013:
No. 12: Assessment of dietary intake in infants and young children is especially challenging. It requires special methodology to consider age‐specific food consumption habits and is further complicated by the rapid growth changes that occur during a relatively short period of time. Not all food served to an infant or young child is necessarily consumed, a significant amount may be wasted. This is expected to lead to overestimations of exposure. In addition, food consumption surveys may not always be actual enough to reflect recent changes in infant and young child‐feeding practices that result from the changes in dietary recommendations from relevant authorities (Fewtrell et al., 2017). As systematically repeated food consumption surveys, e.g. every 5 years as recommended for breastfeeding monitoring (WHO UNICEF 2017), are mostly not available in infants and young children at country level, there remains uncertainty about the timeliness of the food consumption data in the EFSA Database.
No. 13: The conversion of EFSA's Comprehensive Database into food consumption data for Raw Primary Commodities (RPC) assumes the same proportions and probabilities of composite food ingredients for all EU Member States while these are expected to vary according to cultural and even individual practices. Furthermore, this conversion model was still under finalisation at the time of performing the calculations. Previous experience has shown that inaccuracies in the conversion model will tend to generate extreme consumption values, hereby mainly overestimating high‐percentile exposures. Mean exposure estimates were shown to be less sensitive for such inaccuracies.
No. 14: Premarketing scenarios have the implicit assumption that all foods were treated according to the most critical conditions of use for a given pesticides (i.e. conditions of use assumed for deriving the MRLs in conventional foods). Post‐marketing data have demonstrated, however, that pesticide concentrations in food are generally lower than those anticipated from the premarketing data. Premarketing scenarios may overestimate actual exposure by a factor of 2–100 depending on the active substance and dietary survey.
No. 15: Post‐marketing data used for the exposure assessment may be obtained using different analytical methods resulting in different LOQs. This fact together with the large proportion of samples with left‐censored results introduces uncertainty to the overall dietary exposure estimates for the post‐marketing scenario. While the lower bound (LB) values tend to underestimate the dietary exposure to pesticides, UB values tend to overestimate it.
No. 16: Except for the effect of peeling observed for some active substances, losses of residues during household or industrial preparation were not considered in the current assessment. Incorporation of such processing factors in the exposure assessments may significantly reduce the exposure estimates.
ToR 3 – The impact of a cumulative exposure to pesticides which share a common toxicological effect:
No. 17: No specific uncertainties relevant to the section of this opinion were identified as additional to those already discussed in the references to the EFSA methodologies.
ToR 4‐The appropriateness of residue definitions established under Regulation (EU) No 396/2005 for foods for infants and young children:
No. 18: Sources of inaccuracy are founded by potential changes of the molecular structure by processing operations (see Chapter 7), which may not be covered by the residue definitions under Reg. (EU) 396/2005. The other inaccuracy is based on the often divergent residue definitions of monitoring and risk assessment, of which the latter may include additional metabolites (see chapter 5.2).
No. 19: The use of the ADME study conducted in rodent species is a relevant source of uncertainties in regard to the approach for the residue definition, particularly when dealing with metabolites observed in different species and in particular in the pups.
Annex A – Calculations for exposure assessment: tables (xls)
1.
Annex A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2018.5286
Supporting information
Calculations for exposure assessment: tables (xls)
Suggested citation: EFSA Panel on Plant Protection Products and their Residues (PPR) , Ockleford C, Adriaanse P, Hougaard Bennekou S, Berny P, Brock T, Duquesne S, Grilli S, Hernandez‐Jerez AF, Klein M, Kuhl T, Laskowski R, Machera K, Pelkonen O, Pieper S, Smith R, Stemmer M, Sundh I, Teodorovic I, Tiktak A, Topping CJ, Gundert‐Remy U, Kersting M, Waalkens‐Berendsen I, Chiusolo A, Court Marques D, Dujardin B, Kass GEN, Mohimont L, Nougadère A, Reich H and Wolterink G, 2018. Scientific opinion on pesticides in foods for infants and young children. EFSA Journal 2018;16(6):5286, 75 pp. 10.2903/j.efsa.2018.5286
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00702
Acknowledgements: EFSA would like to thank the Scientific Committee for the revision of the Opinion and the input provided.
Adopted: 3 May 2018
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © Stockphoto; Figure 5: © WHO
Notes
OJ L 339, 6.12.2006, p. 16.
OJ L 401, 30.12.2006, p. 1.
Scientific Opinion on a maximum residue limit (MRL) of 0.01 mg/kg for pesticides in foods intended for infants and young children (1997).
Further advice on the Scientific Opinion on a maximum residue limit (MRL) of 0.01 mg/kg for pesticides in foods intended for infants and young children (1998).
OJ L 181, 29.6.2013, p. 35.
During the negotiations on Regulation (EU) No 608/2013, the following statement was made by the Commission: “In implementing [the rules on pesticides of the Regulation], the Commission will pay particular attention to pesticides containing active substances, safeners or synergists classified in accordance with Regulation (EC) No 1272/2008 as mutagen category 1A or 1B, carcinogen category 1A or 1B, toxic for reproduction category 1A or 1B, or considered to have endocrine disrupting properties that may cause adverse effects on humans, or which are very toxic, or which cause critical effects such as developmental neurotoxic or immunotoxic effects, with the objective to ultimately avoid their use”. COM(2013) 241.
Ref. Ares(2014)3592505 – 29/10/2014.
OJ L31, 1.2.2002, p. 1.
Ref. Ares(2015)54901– 8/1/2015.
Ref. Ares(2015)890257– 2/3/2015.
OJ L 309, 24.11.2009, p. 1.
Regulation (EU) No 609/2013.
The term ‘toddler’ is used in the EFSA Comprehensive Food Consumption Database; therefore, it will be used to present data on dietary exposure assessment in Appendix A and Annex A.
OECD (2007), Test No. 507: Nature of the Pesticide Residues in Processed Commodities – High Temperature Hydrolysis, https://doi.org/10.1787/9789264067431-en
Full text was evaluated for identified ‘possibly relevant’ references and finalise the assessment (relevant/not relevant).
Commission Directive 2006/125/EC of 5 December 2006 on processed cereal‐based foods and baby foods for infants and young children. OJ L 339, 6.12.2006, p. 16–35.
Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow‐on formulae and amending Directive 1999/21/EC. OJ L 401, 30.12.2006, p. 1–33.
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009. OJ L181, 29.6.2013, p. 35–56.
This food category was not considered in the exposure assessment because consumption data were only available for the specific food categories mentioned above.
In the Comprehensive Database this age class is referred to as'toddlers’.
Directive 98/83/EC (EU Drinking Water Directive). OJ L 330, 5.12.1998.
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, OJ L 93, 3.4.2013, pp. 1–84.
OECD/EFSA Workshop on Developmental Neurotoxicity (DNT): the use of non‐animal test methods for regulatory purposes (https://www.efsa.europa.eu/it/events/event/161018b).
The EFSAs 7th Scientific Colloquium Report ‐ Cumulative Risk Assessment of pesticides to human health: The Way forward: https://www.efsa.europa.eu/it/supporting/pub/117e
As of 8 November 2017.
The arachnoid and pia are together called the leptomeninges, literally ‘thin meninges’ whilst the dura mater is thicker.
References
- de Belle RC, Vaupshas V, Vitullo BB, Haber LR, Shaffer E, Mackie GG, Owen H, Little JM and Lester R, 1979. Intestinal absorption of bile salts: immature development in the neonate. Journal of Pediatrics 94, 472–476. [DOI] [PubMed] [Google Scholar]
- Bénard M, Gonzalez BJ, Schouft MT, Falluel‐Morel A, Vaudry D, Chan P, Vaudry H and Fontaine M, 2004. Characterization of C3a and C5a receptors in rat cerebellar granule neurons during maturation. Neuroprotective effect of C5a against apoptotic cell death. Journal of Biological Chemistry, 279, 43487–96. [DOI] [PubMed] [Google Scholar]
- Burrows TL, Martin RJ and Collins CE, 2010. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. Journal of the American Dietetic Association, 110, 1501–1510. [DOI] [PubMed] [Google Scholar]
- Camatini M, Franchi E and DeCurtis I, 1981. Differentiation of inter‐sertoli junctions in human testis. Cell Biology International Reports, 5, 109. [Google Scholar]
- Cao M, Huang H, Peng Y, Dong Q and He Y, 2016. Toward developmental connectomics of the human brain. Frontiers in Neuroanatomy, 10, 25. 10.3389/fnana.2016.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona Cano S and Tiemeier H, Van Hoeken D, Tharner A, Jaddoe VW, Hofman A, Verhulst FC, Hoek HW, 2015. Trajectories of picky eating during childhood: a general population study. International Journal of Eating Disorders, 48, 570–579. [DOI] [PubMed] [Google Scholar]
- Carruth BR and Skinner JD, 2002. Feeding behaviors and other motor development in healthy children (2–24 months). Journal of the American College of Nutrition, 21, 88–96. [DOI] [PubMed] [Google Scholar]
- Chaudhury S, Sharma V, Kumar V, Nag TC and Wadhwa S, 2015. Activity‐dependent synaptic plasticity modulates the critical phase of brain development. Development, 38, 355–363. [DOI] [PubMed] [Google Scholar]
- Claus SP, Guillou H and Ellero‐Simatos S, 2016. The gut microbiota: a major player in the toxicity of environmental pollutants? npj Biofilms and Microbiomes 2, 16003; 10.1038/npjbiofilms.2016.3; published online 4 May 2016 Available online: https://www.nature.com/articles/npjbiofilms20163.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas TJ, Carabalona A, Jun‐Kit Hu D and Vallee RB, 2016. Emerging Roles for Motor Proteins in Progenitor Cell Behavior and Neuronal Migration During Brain Development Cytoskeleton. 73, 566–576. 10.1002/cm.21293 [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H and Pettersson S, 2011. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America, 108, 3047–3052. 10.1073/pnas.1010529108. Epub 2011 Jan 31. PMID:21282636 Free PMC Article [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC, Peden‐Adams MM, Keller JM and Germolec DR, 2012. Immunotoxicity of perfluorinated compounds: recent developments. Toxicologic Pathology, 40, 300–311. 10.1177/0192623311428473 [DOI] [PubMed] [Google Scholar]
- De Vis JB, Alderliesten T, Hendrikse J, Petersen ET and Benders MJLN, 2016. Magnetic resonance imaging based noninvasive measurements of brain hemodynamics in neonates: a review. Pediatric Research, 80. [DOI] [PubMed] [Google Scholar]
- Derrick MC, Ma J, Prince AL, Antony KM, Seferovic MD and Aagaard KM, 2017. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nature Medicine, 23, 314–326. https://www.nature.com/articles/nm.4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eason RR, Velarde MC, Chatman L, Till SR, Geng Y and Ferguson M, 2004. Dietary exposure to whey proteins alters rat mammary gland proliferation, apoptosis, and gene expression during postnatal development. Journal of Nutrition, 134, 3370–3377. [DOI] [PubMed] [Google Scholar]
- Edginton AN, Schmitt W, Voith B and Willmann S, 2006. A mechanistic approach for the scaling of clearance in children. Clinical Pharmacokinetics, 45, 683–704. 10.2165/00003088-200645070-00004 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2007a. Reasoned opinion on the potential chronic and acute risk to consumers’ health arising from proposed temporary EU MRLs. EFSA Journal 2007;5(3):32r, 1141 pp. 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007b. Conclusion regarding the peer review of the pesticide risk assessment of the active substance fludioxonil. EFSA Journal 2007;5(8):110r, 85 pp. 10.2903/j.efsa.2007.110r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Conclusion on the peer review of the pesticide risk assessment of the active substance azoxystrobin. EFSA Journal 2010;8(4):1542, 110 pp. 10.2903/j.efsa.2010.1542 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Reasoned opinion on the modification of the existing MRLs for pyraclostrobin in various crops. EFSA Journal 2011;9(3):2120, 41 pp. 10.2903/j.efsa.2011.2120 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011c. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for fludioxonil according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2011;9(8):2335, 86 pp. 10.2903/j.efsa.2011.2335 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011d. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for pyraclostrobin according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2011;9(8):2344, 92 pp. 10.2903/j.efsa.2011.2344 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011e. Reasoned opinion on the modification of the existing MRLs for fludioxonil in various leafy crops. EFSA Journal 2011;9(12):2487, 27 pp. 10.2903/j.efsa.2011.2487 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012a. Reasoned opinion on the modification of the existing MRLs for pyraclostrobin in leafy brassica and various cereals. EFSA Journal 2012;10(3):2606, 36 pp. 10.2903/j.efsa.2012.2606 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012b. Scientific support for preparing an EU position in the 44th Session of the Codex Committee on Pesticide Residues (CCPR). EFSA Journal 2012;10(7):2859, 155 pp. 10.2903/j.efsa.2012.2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2012c. Reasoned opinion on the modification of the existing MRLs for fludioxonil in celery, celery leaves and radishes. EFSA Journal 2012;10(12):3014, 26 pp. 10.2903/j.efsa.2012.3014 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013a. Reasoned opinion on the modification of the existing MRLs for pyraclostrobin in cucumbers and Jerusalem artichokes. EFSA Journal 2013;11(2):3109, 27 pp. 10.2903/j.efsa.2013.3109 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013b. Reasoned opinion on the modification of the existing MRLs for fludioxonil in cucurbits inedible peel and radishes. EFSA Journal 2013;11(2):3113, 25 pp. 10.2903/j.efsa.2013.3113 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013c. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for azoxystrobin according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2013;11(12):3497, 97 pp. 10.2903/j.efsa.2013.349 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014a. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for thiacloprid according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2014;12(3):3617, 111 pp. 10.2903/j.efsa.2014.3617 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014b. Reasoned opinion on the modification of the existing MRL for pyraclostrobin in chicory roots. EFSA Journal 2014;12(5):3685, 23 pp. 10.2903/j.efsa.2014.3685 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014c. Scientific support for preparing an EU position in the 46th Session of the Codex Committee on Pesticide Residues (CCPR). EFSA Journal 2014;12(7):3737, 182 pp. 10.2903/j.efsa.2014.3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014d. Reasoned opinion on the modification of the existing MRLs for pyraclostrobin in swedes and turnips. EFSA Journal 2014;12(10):3872, 19 pp. 10.2903/j.efsa.2014.3872 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015a. Reasoned opinion on the modification of the existing maximum residue level for thiacloprid in Jerusalem artichokes. EFSA Journal 2015;13(7):4191, 18 pp. 10.2903/j.efsa.2015.4191 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015b. Scientific support for preparing an EU position in the 47th Session of the Codex Committee on Pesticide Residues (CCPR). EFSA Journal 2015;13(7):4208, 178 pp. 10.2903/j.efsa.2015.4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2015c. Reasoned opinion on the review of the existing maximum residue levels for deltamethrin according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2015;13(11):4309, 104 pp. 10.2903/j.efsa.2015.4309 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. Reasoned opinion on the modification of the existing maximum residue level for thiacloprid in honey. EFSA Journal 2016;14(3):4418, 21 pp. 10.2903/j.efsa.2016.4418 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. The 2015 European Union report on pesticide residues in food. EFSA Journal 2017;15(4): 4791, 134 pp. 10.2903/j.efsa.2017.4791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2009. Scientific Opinion on the appropriate age for introduction of complementary feeding of infants. EFSA Journal 2009;7(12):1423, 38 pp. 10.2903/j.efsa.2009.1423 [DOI] [Google Scholar]
- EFSA PPR Panel (European Food Safety Authority Panel on Plant Protection Products and their Residues), 2008. Scientific Opinion to evaluate the suitability of existing methodologies and, if appropriate, the identification of new approaches to assess cumulative and synergistic risks from pesticides to human health with a view to set MRLs for those pesticides in the frame of Regulation (EC) 396/2005. EFSA Journal 2008;6(5):705, 84 pp. 10.2903/j.efsa.2008.705 [DOI] [Google Scholar]
- EFSA PPR Panel (European Food Safety Authority Panel on Plant Protection Products and their Residues), 2009a. Scientific Opinion on the potential developmental neurotoxicity of deltamethrin. EFSA Journal 2009;7(1):921, 34 pp. 10.2903/j.efsa.2009.921 [DOI] [Google Scholar]
- EFSA PPR Panel (European Food Safety Authority Panel on Plant Protection Products and their Residues), 2009b. Scientific Opinion for a selected group of pesticides from the triazole group to test possible methodologies to assess cumulative effects from exposure through food from these pesticides on human health. EFSA Journal 2009;7(9);1167, 187 pp. 10.2903/j.efsa.2009.1167 [DOI] [Google Scholar]
- EFSA PPR Panel (European Food Safety Authority Panel on Plant Protection Products and their Residues), 2012. Guidance on the use of probabilistic methodology for modelling dietary exposure to pesticide residues. EFSA Journal 2012;10(10):2839. 10.2903/j.efsa.2012.2839 [DOI] [Google Scholar]
- EFSA PPR Panel (European Food Safety Authority Panel on Plant Protection Products and their Residues), 2013a. Scientific Opinion on the developmental neurotoxicity potential of acetamiprid and imidacloprid. EFSA Journal 2013;11(12):3471. 10.2903/j.efsa.2013.3471 [DOI] [Google Scholar]
- EFSA PPR Panel (European Food Safety Authority Panel on Plant Protection Products and their Residues), 2013b. Scientific Opinion on the identification of pesticides to be included in cumulative assessment groups on the basis of their toxicological profile. EFSA Journal 2013;11(7):3293, 131 pp. 10.2903/j.efsa.2013.3293 [DOI] [Google Scholar]
- EFSA PPR Panel (European Food Safety Authority Panel on Plant Protection Products and their Residues), 2013c. Scientific Opinion on the relevance of dissimilar mode of action and its appropriate application for cumulative risk assessment of pesticides residues in food. EFSA Journal 2013;11(12):3472, 40 pp. 10.2903/j.efsa.2013.3472 [DOI] [Google Scholar]
- EFSA PPR Panel (European Food Safety Authority Panel on Plant Protection Products and their Residues), 2016. Guidance on the establishment of the residue definition for dietary risk assessment. EFSA Journal 2016;14(12):4549. 10.2903/j.efsa.2016.454 [DOI] [Google Scholar]
- EFSA and RIVM (European Food Safety Authority and the Dutch National Institute for Public Health and the Environment), 2015. EFSA Scientific Workshop, co‐sponsored by FAO and WHO: revisiting the International Estimate of Short‐Term Intake (IESTI equations) used to estimate the acute exposure to pesticide residues via food. EFSA supporting publication 2015:EN‐907, 81 pp. 10.2903/sp.efsa.2015.en-907 [DOI] [Google Scholar]
- EFSA Scientific Committee (European Food Safety Authority Scientific Committee), 2007. Opinion of the Scientific Committee related to Uncertainties in Dietary Exposure Assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.4382 [DOI] [Google Scholar]
- EFSA Scientific Committee (European Food Safety Authority Scientific Committee), 2017. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. EFSA Journal 2017;15(5):4849, 58 pp. 10.2903/j.efsa.2017.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DE and Josselyn SA, 2016. Plasticity‐related genes in brain development and amygdala‐dependent learning. Genes, Brain and Behavior, 15, 125–143, 135. [DOI] [PubMed] [Google Scholar]
- Ek CJ, Dziegielewska KM, Habgood MD and Saunders NR, 2012. Barriers in the developing brain and neurotoxicology. Neurotoxicology, 33, 586–604. 10.1016/j.neuro.2011.12.009 [DOI] [PubMed] [Google Scholar]
- EMA (European Medicines Agency), 2009. Guideline on the investigation of medicinal products in the term and preterm neonate (Committee for medicinal products for human use (CHMP) and paediatric committee (PDCO)).
- European Commission , Standing Committee on the Food Chain and Animal Health, 2002. Review report for the active substance deltamethrin. 6504/VI/99‐final. 17 October 2002.
- European Commission , Standing Committee on the Food Chain and Animal Health, 2004a. Review report for the active substance thiacloprid. SANCO/4347/2000 – Final. 13 May 2004.
- European Commission , Standing Committee on the Food Chain and Animal Health, 2004b. Review report for the active substance pyraclostrobin. SANCO/1420/2001‐Final. 8. September 2004.
- FAO (Food and Agriculture Organization of the United Nations), 2009. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 2nd Edition. FAO Plant Production and Protection Paper 197, 2009.
- Felter SP, Daston GP, Euling SY, Piersma AH and Tassinari MS, 2015. Assessment of health risks resulting from early‐life exposures: are current chemical toxicity testing protocols and risk assessment methods adequate? Critical Reviews in Toxicology, 45, 219–244. 10.3109/10408444.2014.993919 [DOI] [PubMed] [Google Scholar]
- Fewtrell M, Bronsky J, Campoy C, Domelöff M, Embleton N, Fidler N, Hojsak JM, Indrio F, Lapillone A and Moolgaard C, 2017. Complementary feeding: a position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. JPGN, 64, 119–132. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Rane A and Persson B, 1975. Diphenylhydantoin binding to proteins in plasma and its dependence on free fatty‐acid and bilirubin concentration in dogs and newborn‐infants. Pediatric Research, 9, 26–30. 10.1203/00006450-197509010-00005 [DOI] [Google Scholar]
- Friis‐Hansen B, 1983. Water distribution in the fetus and newborn‐infant. Acta Paediatrica Scandinavica, 305, 7–11. [DOI] [PubMed] [Google Scholar]
- Fritsche E, Crofton KM, Hernandez AF, Hougaard Bennekou S, Leist M, Bal‐Price A, Reaves E, Wilks MF, Terron A, Solecki R, Sachana M and Gourmelon A, 2017. OECD/EFSA workshop on developmental neurotoxicity (DNT): the use of non‐animal test methods for regulatory purposes. Altex, 34, 311–315. 10.14573/altex.1701171 [DOI] [PubMed] [Google Scholar]
- Ghannam A, Pernollet M, Fauquert JL, Monnier N, Ponard D, Villiers MB, Péguet‐Navarro J, Tridon A, Lunardi J, Gerlier D and Drouet C, 2008. Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. Journal of Immunology, 181, 5158–5166. [DOI] [PubMed] [Google Scholar]
- Gondolf U, Tetens I, Hills P, Michaelsen KF and Trolle E, 2012. Validation of a pre‐coded food record for infants and young children. European Journal of Clinical Nutrition, 66, 91–96. [DOI] [PubMed] [Google Scholar]
- Grumbach MM, 2002. The neuroendocrinology of human puberty revisited. Hormone Research, 57, 2–14. 10.1159/000058094 [DOI] [PubMed] [Google Scholar]
- Harris G and Mason S, 2017. Are there sensitive periods for food acceptance in infancy? Current Nutrition Reports, 6, 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Nie YL, Li JF, Meng XG, Yang WH, Chen YL, Wang SJ, Ma X, Kan QC and Zhang LR, 2016. Developmental regulation of CYP3A4 and CYP3A7 in Chinese Han population. Drug Metabolism and Pharmacokinetics, 31, 433–444. [DOI] [PubMed] [Google Scholar]
- Huybrechts I, Sioen I, Boon PE, Ruprich J, Lafay L, Turrini A, Amiano P, Hirvonen T, De Neve M, Arcella D, Moschandreas J, Westerlund A, Ribas‐Barba L, Hilbig A, Papoutsou S, Christensen T, Oltarzewski M, Virtanen S, Rehurkova I, Azpiri M, Sette S, Kersting M, Walkiewicz A, Serra‐Majem L, Volatier JL, Trolle E, Tornaritis M, Busk L, Kafatos A, Fabiansson S, De Henauw S and Van Klaveren JD, 2011. Dietary exposure assessments for children in Europe (the EXPOCHI project): rationale, methods and design. Archives of Public Health, 69, 4. 10.1186/0778-7367-69-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs EB, Gadian DG, Sabatini S, Chong WK, Quinn BT, Fischl BR and Lucas A, 2008. The effect of early human diet on caudate volumes and I.Q. Pediatric Research, 63, 308–314. [DOI] [PubMed] [Google Scholar]
- Jiang X and Nardelli J, 2016. Cellular and molecular introduction to brain development. Neurobiology of Disease, 92, 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns GL, Abdel‐Rahman SM, Alander SW, Blowey DL, Leeder JS and Kauffman RE, 2003. Developmental pharmacology ‐ drug disposition, action, and therapy in infants and children. New England Journal of Medicine, 349, 1157–1167. [DOI] [PubMed] [Google Scholar]
- Klos A, Tenner AJ, Johswich K‐O, Ager RR, Reis ES and Köhl J, 2009. The role of the anaphylatoxins in health and disease. Molecular Immunology, 46, 2753–2766. 10.1016/j.molimm.2009.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann TR, Levy O, Montgomery RR and Goriely S, 2012. Innate immune function by Toll‐like receptors: distinct responses in newborns and the elderly. Immunity, 37, 771–783. 10.1016/j.immuni.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, Wenzel SE, Moore ML, Peebles RS, Ray A and Ray P, 2012. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nature Medicine, 18, 1525–1530. 10.1038/nm.2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiri‐Hänninen T, Sankilampi U and Dunkel L, 2014. Activation of the hypothalamic‐pituitary‐gonadal axis in infancy: minipuberty. Hormone Research in Paediatrics, 82, 73–80. 10.1159/000362414 [DOI] [PubMed] [Google Scholar]
- Lanigan JA, Wells JCK, Lawson MS, Cole TJ and Lucas A, 2004. Number of days needed to assess energy and nutrient intake in infants and young children between 6 months and 2 years of age. European Journal of Clinical Nutrition, 58, 745–750. [DOI] [PubMed] [Google Scholar]
- Largo R. Growth and development. In: Lentze MJ, Schaub KJ, Schulte F and Spranger J (eds.). Pediatrics. Springer, Berlin, 2008. (in German). [Google Scholar]
- Lauritzen L, Brambilla P , Mazzocchi A, Harsløf LBS, Ciappolino V and Agostoni C, 2016. DHA effects in brain development and function. Nutrients, 8, 6. 10.3390/nu8010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lauzon‐Guillain B, Jones L, Oliveira A, Moschonis G, Betoko A, Lopes C, Moreira P, Manios Y, Pap adopoulos N, Emmett P and Charles MA, 2013. The influence of early feeding practices on fruit and vegetable intake among preschool children in 4 European birth cohorts. American Journal of Clinical Nutrition, 98, 804–812. [DOI] [PubMed] [Google Scholar]
- Lemasters GK, Perreault SD, Hales BF, Hatch M, Hirshfield AN, Hughes CL, Kimmel GL, Lamb JC, Pryor JL, Rubin C and Seed JG, 2000. Workshop to identify critical windows of exposure for children's health: reproductive health in children and adolescents work group summary. Environmental Health Perspectives, 108, 505–509. 10.2307/3454542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioret S, Touvier M, Balin M, Huybrechts I, Dubuisson C, Dufour A, Bertin M, Maire B and Lafay L, 2011. Characteristics of energy under‐reporting in children and adolescents. British Journal of Nutrition, 105, 1671–1680. [DOI] [PubMed] [Google Scholar]
- Mann A, Han H and Eyal S, 2016. Imaging transporters: transforming diagnostic and therapeutic development. Clinical Pharmacology and Therapeutics, 100, 479–488. 10.1002/cpt.416 [DOI] [PubMed] [Google Scholar]
- Mascola AJ, Bryson SW and Agras WS, 2010. Picky eating during childhood: a longitudinal study to age 11 years. Eating Behaviors, 11, 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard D, Monfils S and Tremblay E, 1995. Ontogeny of human gastric lipase and pepsin activities. Gastroenterology, 108, 1650–1656. 10.1016/0016-5085(95)90125-6 [DOI] [PubMed] [Google Scholar]
- Merten C, Ferrari P, Bakker M, Boss A, Hearty A, Leclercq C, Lindtner O, Tlustos C, Verger P, Volatier JL and Arcella D, 2011. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food Additives and Contaminants. Part A, 28, 975–995. 10.1080/19440049.2011.576440 [DOI] [PubMed] [Google Scholar]
- Mielke H and Gundert‐Remy U, 2009. Bisphenol A levels in blood depend on age and exposure. Toxicology Letters, 190, 32–40. 10.1016/j.toxlet.2009.06.861 [DOI] [PubMed] [Google Scholar]
- Morton MC and Feliciano DM, 2016. Neurovesicles in brain development. Cellular and Molecular Neurobiology, 36, 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal‐Kluever A, Aungst J, Gu Y, Hatwell K, Muldoon‐Jacobs K, Liem A, Ogungbesan A and Shackelford M, 2014. Infant toxicology: state of the science and considerations in evaluation of safety. Food and Chemical Toxicology, 70, 68–83. 10.1016/j.fct.2014.05.003 [DOI] [PubMed] [Google Scholar]
- Nicolas JM, Bouzom F, Hugues C and Ungell AL, 2017. Oral drug absorption in pediatrics: the intestinal wall, its developmental changes and current tools for predictions. Biopharmaceutics and Drug Disposition, 38, 209–230. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5516238/pdf/BDD-38-209.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD , 2017. Report of the OECD/EFSA workshop on developmental neurotoxicity (DNT): the use of non‐animal test methods for regulatory purposes. Series on Testing and Assessment No. 261. ENV/JM/MONO(2017)4.
- O'Mahony SM, Clarke G, Dinan TG and Cryan JF, 2017. Early‐life adversity and brain development: is the microbiome a missing piece of the puzzle? Neuroscience, 7, 37–54. 10.1016/j.neuroscience.2015.09.068 [DOI] [PubMed] [Google Scholar]
- Pettengill MA, van Haree SD and Levy O, 2014. Soluble mediators regulating immunity in early life. Frontiers in Immunology, 5, 547. 10.3389/fimmu.2014.00457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reemst K, Noctor SC, Lucassen PJ and Hol EM, 2016. The indispensable roles of microglia and astrocytes during brain development. Frontiers in Human Neurosciences, 10, 566. 10.3389/fnhum.2016.00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond S, Johnson KA, Seal ML and Allen NB, 2016. Sarah Whittle Development of brain networks and relevance of environmental and genetic factors: a systematic review. Neuroscience and Biobehavioral Reviews, 71, 215–239. [DOI] [PubMed] [Google Scholar]
- Saghir SA, Khan SA and McCoy AT, 2012. Ontogeny of mammalian metabolizing enzymes in humans and animals used in toxicological studies. Critical Reviews in Toxicology, 42, 323–357. 10.3109/10408444.2012.674100 [DOI] [PubMed] [Google Scholar]
- Saunders NR, Knott GW and Dziegielewska KM, 2000. Barriers in the immature brain. Cellular and Molecular Neurobiology, 20, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders NR, Dreifuss JJ, Dziegielewska KM, Johansson PA, Habgood MD, Mollgard K and Bauer HC, 2014. The rights and wrongs of blood‐brain barrier permeability studies: a walk through 100 years of history. Frontiers in Neuroscience, 8, 404. 10.3389/fnins.2014.00404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiess S, Grote V, Scaglioni S, Luque V, Martin F, Stolarczyk A, Vecchi F, Koletzko B and for the European Childhood Obesity Project . 2010. Introduction of complementary feeding in 5 European countries. JPGN 50, 92–98. [DOI] [PubMed] [Google Scholar]
- SCF , 1997. Scientific Opinion on a maximum residue limit (MRL) of 0.01 mg/kg for pesticides in foods intended for infants and young children.
- SCF , 1998. Further advice on the Scientific Opinion on a maximum residue limit (MRL) of 0.01 mg/kg for pesticides in foods intended for infants and young children.
- Schneider N and Garcia‐Rodenas CL, 2017. Early nutritional interventions for brain and cognitive development in preterm infants: a review of the literature. Nutrients, 9, 187; 10.3390/nu9030187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sichert‐Hellert W, Kersting M and Schöch G, 1998. Underreporting of energy intake in 1 to 18 year old German children and adolescents. Zeitschrift fur Ernahrungswissenschaft, 37, 242–251. [DOI] [PubMed] [Google Scholar]
- Smith AD, Herle M, Fildes A, Cooke L, Steinsbekk S and Llewellyn CH, 2017. Food fussiness and food neophobia share a common etiology in early childhood. Journal of Child Psychology and Psychiatry, 58, 189–196. 10.1111/jcpp.126472016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CM, Wernimont SM, Northstone K and Emmett PM, 2015. Picky/fussy eating in children: review of definitions, assessment, prevalence and dietary habits. Appetite, 95, 349–359. [DOI] [PubMed] [Google Scholar]
- Tay TL, Savage JC, Hui CW, Bisht K and Tremblay MÈ, 2017. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. Journal of Physiology, 595.6, 1929–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USA EPA , 2012. 2012 Edition of the drinking water standardsand Health Advisories EPA‐822‐S‐12‐001. Office of Water US Environmental Protection Agency, Washington, DC. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/dwstandards2012.pdf
- Valcke M and Krishnan K, 2013. Assessing the impact of child/adult differences in hepatic first‐pass effect on the human kinetic adjustment factor for ingested toxicants. Regulatory Toxicology and Pharmacology, 65, 126–134. 10.1016/j.yrtph.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Sprowles GN, Regan SL and Williams MT, 2018. A better approach to in vivo developmental neurotoxicity assessment: alignment of rodent testing with effects seen in children after neurotoxic exposures. Toxicology and Applied Pharmacology. Available online: 10.1016/j.taap.2018.03.012 [Accessed: 12 March 2018] [DOI] [PubMed] [Google Scholar]
- WHO , 2006. Principles for evaluating health risks in children associated with exposure to chemicals. Environmental Health Criteria 237.
- WHO UNICEF , 2017. Global Monitoring Framework: operational guidance for tracking progress in meeting targets for 2025. Available online: http://www.who.int/nutrition/publications/operational-guidance-GNMF-indicators/en/
- de Wildt SN, 2011. Profound changes in drug metabolism enzymes and possible effects on drug therapy in neonates and children. Expert Opinion on Drug Metabolism and Toxicology, 7, 935–948. 10.1517/17425255.2011.577739 [DOI] [PubMed] [Google Scholar]
- de Wildt SN, Tibboel D and Leeder JS, 2014. Drug metabolism for the paediatrician. Archives of Disease in Childhood, 99, 1137–1142. 10.1136/archdischild-2013-305212 [DOI] [PubMed] [Google Scholar]
- Windorfer A, Karitzky D, Gasteiger U and Stehr K, 1978. Investigations on salicylate protein‐binding in newborns and infants. European Journal of Pediatrics, 127, 163–172. 10.1007/bf00442057 [DOI] [PubMed] [Google Scholar]
- Zoppi G, Andreotti G, Pajno‐Ferrara F, Njai DM and Gaburro D, 1972. Exocrine pancreas function in premature and full term neonates. Pediatric Research, 6, 880–886. 10.1203/00006450-197212000-00005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calculations for exposure assessment: tables (xls)


