Abstract
The spatial and temporal patterns of lumpy skin disease (LSD) epidemics were analysed based on the data collected from affected and at‐risk countries in southeastern Europe in 2016 and 2017. The reported outbreaks decreased from 7,483 in 2016 to 385 in 2017. Those were reported mainly in Albania in areas where vaccination was not completed. Only two and four outbreaks were reported in Greece and in the former Yugoslav Republic of Macedonia in 2017, respectively, where the herd immunity achieved by vaccination significantly reduced the further spread of the disease. However, this showed that the virus was still circulating and may re‐emerge in not fully immunised animals. No further outbreaks were reported in the other countries that were affected in 2016, thus providing field evidence about the effectiveness of the regional vaccination campaign. The mathematical model fit to the Albanian data showed that the LSD spread is mostly up to 4 km with some longer distance transmission. The inclusion of relative vector abundance improves the model fit and supports that the abundance of potential LSD vectors is one of the major risk factors for LSD spread. This should be confirmed by field surveys on potential LSD vectors. The vaccination effectiveness in Albania, Bulgaria and Greece was estimated by survival analysis and Cox regression model to be 62%, 96% and 84%, respectively, and these results were validated by the mathematical model. This highlighted that the high coverage vaccination with the live homologous vaccine is the most effective measure for reducing lumpy skin disease virus (LSDV) spread. The housing type of animals was explored as risk factor in Greece, and the risk in farms with outdoor access was six times higher than in farms where animals are kept indoors, independently of vaccination status.
Keywords: lumpy skin disease, spread, vaccine, mathematical model
Summary
The European Commission requested the European Food Safety Authority (EFSA) to perform an epidemiological analysis of the lumpy skin disease (LSD) epidemics based on the data collected from the affected and at‐risk Member States and non‐European Union (EU) countries in southeastern Europe. In particular, temporal and spatial patterns of LSD and risk factors involved in the occurrence, spread and persistence of the LSD virus among the cattle population should be considered. This is the second report produced in the framework of this mandate, and it is based on the data received by the countries involved in this project, namely Albania, Bulgaria, Croatia, the former Yugoslav Republic of Macedonia, Greece, Kosovo,1 Montenegro, Serbia and Turkey. Collected information included data about LSD outbreaks, distribution and size of cattle farms, and on vaccinations.
The comparison of the LSD situation between 2016 and 2017 shows that 7,483 LSD outbreaks were reported in the Balkan region in 2016 with 12,330 affected animals, while only 385 outbreaks with 850 affected animals were notified in 2017. These were mainly in Albania (379 outbreaks), in areas where vaccination was not completed and where the cattle population was most susceptible, and very few in Greece (two outbreaks) and the former Yugoslav Republic of Macedonia (four outbreaks). No further outbreaks were reported in all other countries that were affected in 2016. This reduction in the number of outbreaks reported in 2017, particularly in Bulgaria, Serbia, Montenegro and Kosovo where none was reported, provides field evidence of the effectiveness of the mass vaccination campaigns conducted at regional level.
In 2017, most outbreaks were reported between May and July, confirming the seasonality of LSD previously observed in the Balkans, an important aspect when planning the start of a vaccination campaign. In the outbreak reported in Albania in 2017, the morbidity has a median value of 0.8% with values up to 7.2% while mortality median value was 0.3% with values up to 2.9%, thus in similar ranges as reported in 2016, when morbidity median value was 0.7% up to 4.8% and the mortality upper value was 0.7%.
The vaccination coverage by live‐attenuated homologous vaccine up to 100% and the consequent well‐established herd immunity achieved in infected countries other than Albania, like Greece or the former Yugoslav Republic of Macedonia, has strongly reduced the further spread of the disease in 2017 to few sporadic outbreaks. Nevertheless, the latter show that the virus is still present in the environment and/or in the cattle population and may re‐emerge again when susceptible or not fully immunised animals are exposed.
An improved version of a kernel‐based spread model was built on the Albanian data and used to estimate the force of the LSD infection. This was compared with the previous model developed using Israeli data. The improved model was used to explore the spatial (i.e. kernel shape) and temporal (i.e. seasonality) disease dynamics and to estimate the vaccination effectiveness. The model showed the probability of LSD spread is mostly (95%) up to 4 km (by e.g. vectors), but with some transmission occurring over much longer distances (by e.g. animal movements). Proximity to affected farms can be therefore considered a further risk factor for LSD spread. The kernel shape estimated for Albania is similar to the one for Israel, providing evidence on similar transmission parameters for LSD. Therefore, this aspect further supports the conclusions drawn in previous risk assessment (EFSA AHAW Panel, 2015, 2016). Furthermore, adding seasonality, in particular through modelled relative vector abundance (Stomoxys calcitrans), improves the model fit and supports that the abundance of potential LSD vectors is one of the major risk factors contributing to LSD spread.
An update on the progression of the vaccination campaign and vaccination coverage up to 2017 is shown in the present report and an evaluation of vaccination effectiveness using survival analysis is presented for Albania, Bulgaria, Greece, Montenegro and Serbia. The protective effect of vaccination is supported by the results obtained which showed median vaccination effectiveness values of 62%, 96% and 84% in Albania, Bulgaria and Greece, respectively. These results highlight that high coverage vaccination with live homologous vaccine is the most effective measure for reducing lumpy skin disease virus (LSDV) spread.
In the case of Greece, further risk factors could be explored with the survival model, such as the animals’ housing type (indoor vs outdoor). The risk in farms with outdoor access is six times higher than in farms where animals are kept indoors, independent of vaccination status, possibly because of higher exposure to vectors bite.
The vaccination effectiveness estimated with the mathematical model for a few districts in Albania is similar to that estimated with the survival model, validating the results.
It should be remarked how the commitment and collaboration of the veterinary services from the countries involved in LSD data collection has been consistently at a very high level, showing an excellent spirit of regional cooperation among each other and with EFSA. This was one of the main elements that allowed the achievement of the results presented in this report and the successful control of the disease in the field.
The work done in the framework of this mandate suggests some recommendations that may be useful for both research purposes and control strategies. Firstly, the importance of collecting and validating high quality data as a cornerstone for epidemiological analysis of an animal disease on a regional basis with multiple countries involved. This should be constantly improved and, where possible, automated.
Secondly, data at animal level and within‐farm follow‐up are desirable as they would increase the precision of the estimation and allow use of different analytical methods. These data should be collected particularly during the in‐depth standardised outbreak investigations that should be performed for all new LSD cases.
Thirdly, since the mathematical model presented in this report supports the importance of vector‐borne transmission of LSD, field surveys to explore presence and abundance of potential LSD vectors should be gathered by, e.g. targeting a number of farms experiencing LSD outbreaks and followed up during the entire LSD season.
Finally, from the point of view of disease control, the epidemiological situation observed in 2017 confirms that vaccination is a key tool for LSD control. In particular, achieving the highest vaccination coverage in the shortest period of time is cornerstone to rapidly control of LSD outbreaks. This should be coupled with good clinical surveillance for immediate notification of suspected clinical cases that should be confirmed in the laboratory to differentiate LSD field virus from the vaccine strain.
Future work on LSD should be supported by the development of scenarios based on the elements mentioned in the conclusions above. In particular, to increase the reliability of those scenarios, experimental evidence should be sought about the duration of immunity conferred by the homologous LSD vaccine.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Lumpy Skin Disease (LSD), is a viral infection affecting cattle which is transmitted primarily by blood feeding insects (vectors) and to a lesser extent through direct contact between animals. Mortality due to LSD is not very high (up to 10%), however occurrence of the disease is associated with a drop in production and serious trade restrictions.
LSD is endemic in most African countries. Since 2012, LSD has been spreading on an unusually large scale throughout the Middle East, including Egypt and Israel, into Turkey (reported steadily since 2013) where it is now considered endemic.
By November 2014, shortly before the publication of EFSA's opinion on LSD (January 2015), the disease was confirmed in the island of Cyprus (in the areas not under the effective control of the Republic of Cyprus). In the months that followed LSD also gradually progressed from Anatolia (Turkey) where it is endemic, into the East Thrace area of Turkey (May 2015) and from there to Greece (Evros, August 2015) where it continued to spread westwards, producing new outbreaks as far as the regional units of Thessaloniki and Chalkidiki.
In 2016 the disease reappeared in Greece, close to the border with Bulgaria, in the region of Serres where vaccine coverage was relatively low. Thus, the decision was taken to expand the vaccination zone further to the west (procedure ongoing). Shortly after the first outbreaks in Greece in 2015, in 2016 the disease occurred for the first time Bulgaria, Albania, Serbia, the former Yugoslav Republic of Macedonia, Montenegro, Kosovo.
EU legislation imposes culling and destruction of all cattle present in the affected holdings. This is followed by the establishment of a protection zone (3 km radius) and a surveillance zone (10 km radius) with special restrictions for cattle and products thereof.
Additional Commission protection measures, namely regionalisation, apply in the affected areas and the vaccinated areas (specific Commission Implementing Decision are in place for Greece and Bulgaria).
Similar measures are envisaged for all areas where vaccination is applied to prevent the spread of the disease to previously unaffected areas through the movement of potentially infected cattle.
The Standing Group of Experts on Lumpy skin disease (LSD) for South‐East Europe under the GF‐TADs umbrella,2 in their first meeting (Brussels 4–5 July 2016) proposed, among other recommendations, that: ‘The collection of surveillance data and scientific information that maybe relevant (e.g. incidence, weather conditions) be coordinated for purposes of better risk assessment and management’ (Final recommendations, available at http://web.oie.int/RR-Europe/eng/Regprog/docs/docs/LSD1/LSD%20SGE1%20(Brussels%20%20July2016)%20-%20Conclusions%20and%20recommendations%20(Final).pdf).
In the light of the above the Commission needs an updated epidemiological analysis based on the data collected from the Member States affected by LSD. The use of the EFSA Data Collection Framework is encouraged as it promotes the harmonisation of data collection. Any data that is available from neighbouring non‐EU countries should be used as well.
This analysis should consider and develop the findings of the EFSA scientific opinion on LSD adopted in January 2015. The data to be used should include all the available epidemiological data from 2014 onwards.
Therefore, in the context of Article 31 of Regulation (EC) No 178/2002, EFSA should provide technical and scientific assistance to the Commission based on the following Terms of Reference:
To analyse the epidemiological data on LSD from Cyprus, Greece, Bulgaria and any other Member States or non‐EU countries that might be affected by LSD.
To include an analysis of the temporal and spatial patterns of LSD.
To include an analysis of the risk factors involved in the occurrence, spread and persistence of the LSD virus among the cattle population.
1.2. Introduction and interpretation of the Terms of Reference
In this second report produced in the framework of this mandate, an analysis is presented with the data received by the countries involved in this project, namely Albania, Bulgaria, Croatia, the former Yugoslav Republic of Macedonia, Greece, Kosovo, Montenegro, Serbia and Turkey. The collection of data from the affected countries includes data about LSD outbreaks, the structure and distribution of cattle farms, and on vaccinations. To guarantee harmonisation, the EFSA Data Collection Framework was used as much as possible along the project.
This second report analyses and compares the LSD situation in 2016 and 2017 in term of the disease occurrence and the effectiveness of the control measures applied (i.e. the vaccination campaign).
An analysis of the epidemiological situation in 2017 is presented. This analysis included a description of temporal and spatial patterns of LSD epidemics in four countries (Albania, the former Yugoslav Republic of Macedonia, Greece and Turkey) that reported outbreaks.
An improved version of a kernel‐based spread model was built on the Albanian data and used to estimate the force of the LSD infection. This was compared with the previous model developed using Israeli data. The improved model was used to explore the spatial (i.e. kernel shape) and temporal (i.e. seasonality) disease dynamics and to estimate the vaccination effectiveness (VE).
An update on the progression of the vaccination campaign and vaccination coverage up to 2017 is shown and an evaluation of VE using survival analysis and the Cox regression model is presented for Albania, Bulgaria, Greece, Montenegro and Serbia. This is in line with recommendations of the Standing Group of Experts on LSD in southeast Europe under the Global Framework for the progressive control of Transboundary Animal Diseases (GF TADs) umbrella3 to ‘better evidence the positive impact of vaccination on the suppression of LSD outbreaks’.
Risk factors were analysed both by using the model (seasonality and distance between farms) and by controlling the effect of vaccination through the survival analysis. The amount and quality of data allowed to explore the effect of housing type (animals kept indoors/outdoors) in the Serres region of Greece.
Finally, considering the recommendation of the most recent GF TADs meeting (Montenegro, October 2017) on the type of LSD surveillance in the future, a section on update of available diagnostic tools is presented.
2. Data and methodologies
The close collaboration with representatives of the veterinary services from the affected countries in the Balkan region continued into 2017 for the collection of new data and refinement of the earlier data. Bilateral meetings between EFSA WG and representatives from each of the affected country were organised to understand the collection system in each country and to refine the database according to the needs of the analysis. In October 2017, a general meeting was held in Montenegro with all country representatives.
In particular, the importance of connecting databases for different aspects was stressed, as well as providing a unique identifier for the farms (e.g. farm ID) in each database, so the interconnection of information would be assured.
2.1. Data
Data were provided by the competent authorities of Albania, Bulgaria, Croatia, Greece, Kosovo, Montenegro, Serbia, the former Yugoslav Republic of Macedonia.
Data about the cattle population (location of farms and number of animals per farm) were provided by Bulgaria and Croatia as on October 2017, while, for other countries, the population data that were previously provided were considered (EFSA, 2017).
Data on LSD outbreaks from ADNS, EMPRES‐I and national authorities were considered up to November 2017.
Data at farm level on the vaccination campaign against LSD conducted in 2017 were provided by Albania, Bulgaria, Croatia, Greece, Kosovo, Montenegro, Serbia and the former Yugoslav Republic of Macedonia. No information about possible adverse effects of LSD vaccines was available apart from that presented previously (EFSA, 2017).
2.2. Methodologies
Epidemiological patterns of LSD spread were described from the data provided and Geographic Information System software was applied to explore the spatial distribution of the outbreaks, considering farms as epidemiological units. The methodology for estimating the VE and mathematical modelling is explained below.
2.2.1. Estimation of vaccination effectiveness
The LSD live‐attenuated homologous vaccine was used in the Balkan region during the campaigns carried out in 2015–2017. Since the effectiveness of the performance of LSD vaccination under field conditions is a crucial element for the epidemic control and is likely to differ amongst countries, this was assessed in some areas of the Balkan region, i.e. in Albania, Bulgaria (Blagoevgrad region), Greece (Serres regional unit), Montenegro and Serbia (Pcinskj region). These regions were chosen because the data allows performing the analysis at farm level, considered to be the epidemiological unit of interest. A farm was considered infected if at least one animal was reported as clinically affected. The national authorities of the countries where vaccination was performed informed that all animals present at farm were vaccinated at the moment of vaccination. Therefore, a farm was regarded as either vaccinated (i.e. all animals present in the farm vaccinated) or non‐vaccinated (i.e. no animals vaccinated). In a situation in which naive animals are introduced in a vaccinated farm and they become infected, the estimated VE at farm level would represent an underestimation of the true values.
As some of the farms were vaccinated after the epidemic onset within each village, a left truncated survival analysis with right censoring was used to account for the possible change in farm vaccination status (Therneau and Grambsch, 2000). The date of first suspicion in each farm was considered as the date of the outbreak. The end of 2016 (i.e. 31/12/2016) is the end of the follow‐up period if no event occurred prior to this date. Thus, all farms reaching this point without being infected were censored at this date. For each country, the first outbreak in the smallest available administrative unit (village for Albania; province for Bulgaria; district for Greece, Montenegro and Serbia) was used to define the beginning of the follow‐up period. This was to ensure that the most homogeneous hazard exposure to LSD within the spatial resolution used was considered in the follow‐up period. A farm was considered ‘vaccinated’ after a lag period of 21 days after the vaccination date to take into account the development of protective immunity. For these farms, the follow‐up period was left truncated, but it could also be right censored, since the event of interest (outbreak) might or might not have happened by the end of follow‐up period. The follow‐up period for each farm condition was determined according to the following criteria:
In non‐vaccinated farms with no reported outbreak event, the follow‐up period was from the occurrence of the first outbreak in the administrative unit until 31/12/2016;
In non‐vaccinated farms in which an outbreak was reported, the follow‐up period was from the date of occurrence of the first outbreak in the administrative unit until the date of the outbreak event in the farm;
In farms vaccinated after the first outbreak in the administrative unit with no reported outbreak, the follow‐up period was from the date of vaccination plus 21 days until 31/12/2016. If vaccination occurred before the first outbreak in the administrative unit, the follow up began from the occurrence of the first outbreak in the administrative unit until 31/12/2016.
In vaccinated farms in which an outbreak was reported, the follow‐up period was from the first outbreak in the administrative unit until the date of the outbreak event in the farm.
For vaccinated farms, a follow‐up period as non‐vaccinated was included in case the date of vaccination plus 21 days was later than the date of the first outbreak in the administrative unit. In this case, the follow‐up period for this farm under non‐vaccinated status is from the occurrence of the first outbreak event in the administrative unit until the date of vaccination plus 21 days. An additional follow up period as vaccinated farm was included from the date of vaccination plus 21 days until the outbreak event in the farm.
Cumulative occurrence of outbreaks in vaccinated and non‐vaccinated farms was visualised using Kaplan–Meier survival curves using the ggplot package in R. The hazard ratio (HR) for an outbreak in vaccinated vs. non‐vaccinated farms was calculated by fitting a Cox proportional hazards model with the administrative unit as a cluster variable. VE was calculated as 1‐HR.
The type of animal housing (indoor vs. outdoor) was explored as a risk factor in the case of Greece (Serres regional unit); it was included as a covariate in the model after testing for collinearity4 and avoiding separation issues in the modelling exercise. Interaction between covariates and vaccination was tested for each risk factor included in the model. The best‐fitting model was chosen according to the Akaike information criterion (AIC).
A p‐value of 0.05 (Wald test) was considered as indicating statistical significance for all analyses.
Analysis of VE over all possible times between vaccination and protection was performed for the Albanian data as described above and the resultant values plotted against the considered lag time.
2.2.2. Mathematical model for spread between farms
A simple model describing the spread of lumpy skin disease virus (LSDV) between farms was developed in previous opinions, based on transmission parameters derived from published data from Israel (Ben‐Gera et al., 2015; EFSA AHAW Panel, 2015, 2016). Preliminary analyses were carried out using data on reported cases for all affected countries, but the relatively small number of outbreaks and, in particular, the limited availability of location data for unaffected farms meant that it was not possible to obtain robust results for most countries. Accordingly, the modelling focused on Albania for which data on location (centroid of village coordinates) and number of cattle for all farms, on reported outbreaks of LSD (date of suspicion and recovery) and on vaccination (date of vaccination) were available for 2016. In addition, Albania was the most heavily affected country and did not implement stamping out as a control measure.
More specifically, the model was fitted to data for four districts in Albania (Bulqizë, Dibër, Kukës and Mat) individually and for the four districts combined. These districts are contiguous and were among the first and most heavily affected regions in Albania in 2016 (Table 1; Figure 1).
Table 1.
Summary of outbreaks in Albanian districts analysed using the model until end of 2016
| Name | No. of farms | No. of reported outbreaks | Date of first outbreak |
|---|---|---|---|
| Bulqizë | 3,750 | 190 | 28 June 2016 |
| Dibër | 7,445 | 517 | 4 July 2016 |
| Kukës | 7,276 | 193 | 8 July 2016 |
| Mat | 6,157 | 369 | 7 June 2016 |
| All four districts | 24,628 | 1,269 | 7 June 2016 |
| Whole country | 198,105 | 3,585 | – |
Figure 1.
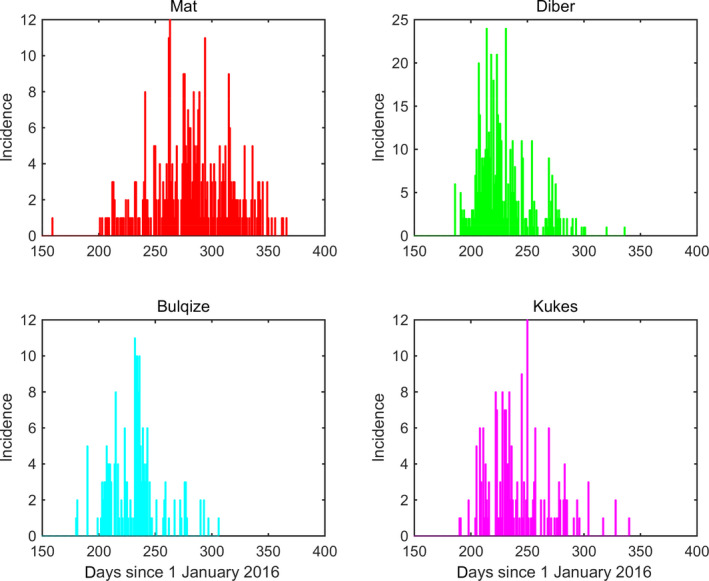
Incidence (number of newly reported infected farms each day) of LSD in four districts of Albania in 2016
Spread between farms was modelled using a kernel‐based approach. In this case, the force of infection (i.e. the rate at which susceptible individuals acquire an infectious disease) for farm j at time t is given by:
where h0 is the baseline risk, Nj(t) is the number of animals on farm j, K(xjk) is the distance kernel (i.e. how the force of infection varies with distance), xjk is the great circle distance between farms j and k, and Ik(t) indicates whether or not farm k is infected at time t (with Ik(t) = 0 if it is not infected and Ik(t) = 1 if it is). The infectious status and the date of suspicion were derived from the outbreak notifications for the four Albanian districts. Three forms for the kernel were tested: fat‐tailed, exponential and Gaussian. These correspond to the density‐dependent forms presented in the earlier EFSA opinion (EFSA AHAW Panel, 2015).
The effect of vaccination was incorporated by assuming it changes force of infection to reflect: (i) the reduced probability of an unaffected farm becoming infected because of fewer susceptible animals being present; (ii) the reduced infectiousness of an affected farm because of fewer animals becoming infected and (iii) the reduced infectiousness of an infected, vaccinated animal. In this case, the herd sizes (Nj and Nk) were replaced by:
where vS(t) and vI(t) are, respectively, the VE for susceptibility and infectiousness at time t and is the time at which affected farm k was infected. VE was assumed to increase linearly from zero to maximum effectiveness (εS and εI) at the time full protection is reached (assumed to be 21 days as per the manufacturer's data sheet5). Four possibilities were considered for vaccination: (i) it has no effect (i.e. vS(t) = 0 and vI(t) = 0); (ii) it affects susceptibility only (i.e. vI(t) = 0); (iii) it affects infectiousness only (i.e. vS(t) = 0) or (iv) it affects both susceptibility and infectiousness).
The impact of seasonality was explored in two ways. In the first, the baseline risk h0 was replaced by a function of temperature, so that:
where h0 and h1 are parameters and Tj(t) is the daily mean temperature at farm j on day t and is the annual mean temperature. In the second, the baseline risk was replaced by a function of temperature that captures the seasonality of Stomoxys calcitrans (Diptera: Muscidae), one of the putative vectors of LSDV (Kahana‐Sutin et al., 2017). Evidence for S. calcitrans being a vector for LSDV comes from its ability to transmit other capripox viruses (Kitching and Mellor, 1986) and the strong correlation between abundance of S. calcitrans and LSDV outbreaks observed in Israel (Kahana‐Sutin et al., 2017). Furthermore, S. calcitrans is generally present in Europe (including Albania) considering that is a cosmopolitan species associated to livestock production and well known for transmitting other pathogens apart of LSDV (Baldacchino et al., 2014). Here,
where h0 is the baseline risk, h1 is seasonality parameter and V(t) is the relative vector abundance at time t (normalised so the maximum is equal to one). The relative vector abundance is given by:
where F, E, L and P are temperature‐dependent functions describing fecundity, egg survival, larval survival and pupal survival, respectively, c is the normalising constant and Tm‐1 is the monthly mean temperature for the preceding month. Appropriate functional forms for F, E, L and P were obtained from experiments using laboratory colonies of S. calcitrans (Lysyk, 1998; Kahana‐Sutin et al., 2017).
Temperature data for Albania were obtained from the European Commission Joint Research Centre MARS Meteorological Database,6 which provides daily meteorological data spatially interpolated on a 25 × 25 km grid cell. Specifically, we extracted the daily minimum and maximum temperatures for 2016 and computed the midpoint of these for each of the 70 cells covering Albania. Farms used the temperatures for the cell in which they were located. The data are summarised in Figure 2 and the modelled relative vector abundance is shown in Figure 3.
Figure 2.
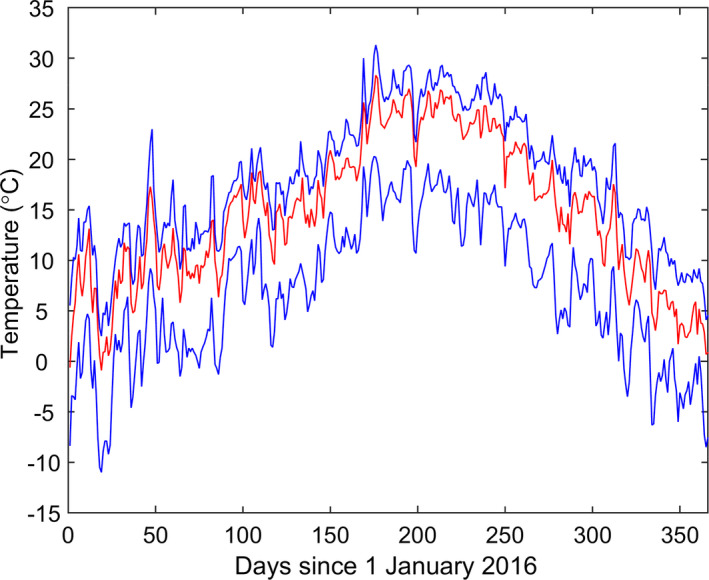
Daily mean temperatures (°C) in Albania for 2016. The red line shows the median and the blue lines show the 5th and 95th percentiles for the temperatures across seventy 25 × 25 km grid cells covering Albania
Figure 3.
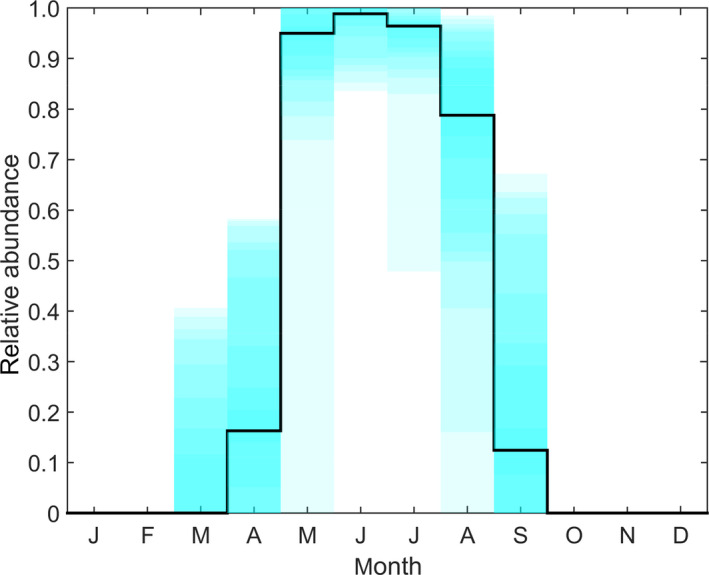
Modelled relative abundance of Stomoxys calcitrans, a putative vector of lumpy skin disease virus, in Albania 2016
- The black line shows the median and the shading shows five percentile bands (up to the 5th and 95th percentiles) for the relative abundance across seventy 25 × 25 km grid cells covering Albania.
Parameters in the force of infection were estimated by fitting the model to each data set (Table 1) using Bayesian Markov Chain Monte Carlo (MCMC) methods. Non‐informative priors (diffuse normal or exponential) were used for the parameters. The different models (i.e. combinations of kernel, VE and seasonality) were compared using the deviance information criterion (DIC) (Spiegelhalter et al., 2002).
3. Assessment
3.1. Overview of LSD situation in affected countries in Europe
Lumpy skin disease (LSD) is a cattle disease caused by a capripoxvirus and characterised by fever and nodules on the skin, mucosal membranes and internal organs. The disease is mainly transmitted mechanically by blood‐feeding arthropod vectors like flies, mosquitoes and ticks. It is characterised by variable levels of case‐fatality rates and can cause a reduction in milk production, sterility in bulls, abortion and damage to hides, leading to significant loss of incomes. Originally affecting cattle across Africa, the disease has spread in recent years outside the African continent with outbreaks in Middle East (Israel, Jordan and Lebanon) in 2012–2013, and further spread into and through Turkey in 2013, where it is now considered endemic.
In August 2015, LSD outbreaks were notified in the European Union (EU) with an incursion in eastern Greece (Tasioudi et al., 2016; FAO, 2017) and further spread over the country. In spring and summer of the following year (2016), LSD spread further over the Balkans to Albania, Bulgaria, Kosovo, Montenegro, Serbia and the former Yugoslavian Republic of Macedonia (Beard, 2016; EFSA, 2016; FAO, 2017). LSD spread also on the eastern side of the Black Sea, to Armenia, Azerbaijan, Georgia, Kazakhstan and the Russian Federation up to 54°N.
In 2017, outbreaks were registered mostly in Albania (379 outbreaks), four in the former Yugoslav Republic of Macedonia, 2 in Greece, 8 in Turkey and 31 in Russia, up to 54°N.
Figure 4 shows the yearly evolution of LSD epidemics in Europe since 2014.
Figure 4.
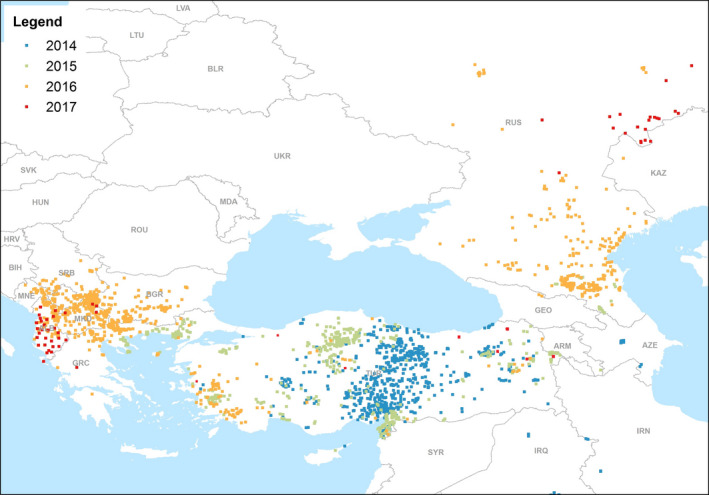
LSD outbreaks notified in Europe and Middle East in 2014–2017 (Data source: Empres‐i)
Figure 4 shows that, compared with the large and rapid transboundary spread of the disease reported over Greece, Bulgaria, Kosovo, the former Yugoslav Republic of Macedonia, Serbia, Albania and Montenegro in 2016, the disease in 2017 was reported only in Albania, Russia and Turkey, with few sporadic outbreaks in Greece (2) and the former Yugoslav Republic of Macedonia (4), and none in any of the other previously affected countries. Given that the outbreaks reported in 2017 in Albania were mostly in areas where the vaccination had not been completed yet, and in Turkey and Russia, heterologous vaccine was mostly used, this shows that in countries of southeast Europe where adequate vaccination coverage was achieved with live‐attenuated homologous vaccine, the occurrence of the disease was effectively prevented. More details are given in the following section.
3.2. Spatial and temporal analysis of LSD outbreaks in 2016–2017 in the Balkans
3.2.1. Descriptive epidemiology of LSD outbreaks in 2017
Figure 5 displays the temporal distribution of outbreaks (number of affected farms) reported per month in 2016–2017 according to the suspicion date from ADNS and country reports.
Figure 5.
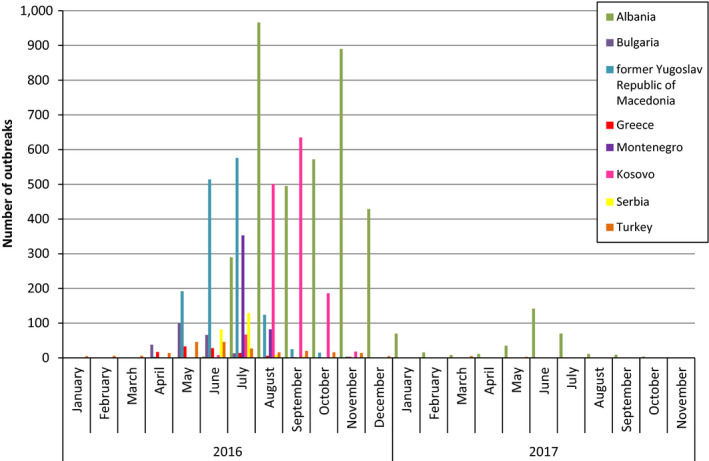
Monthly distribution of LSD outbreaks by country in the Balkans in 2016–2017
From the Figure 5, it is clear that outbreaks in 2016 were observed in most countries of the Balkan region, whereas in 2017, outbreaks were reported only in Albania (379 outbreaks), in addition to four outbreaks in the former Yugoslav Republic of Macedonia (in January, April, May and July) and two in Greece (in February and August). The spatiotemporal distribution of LSD outbreaks in the Balkan region in 2017 and cattle density (animals/km2) is shown in the monthly maps of Figure 6 and in the movie map at this link.
Figure 6.
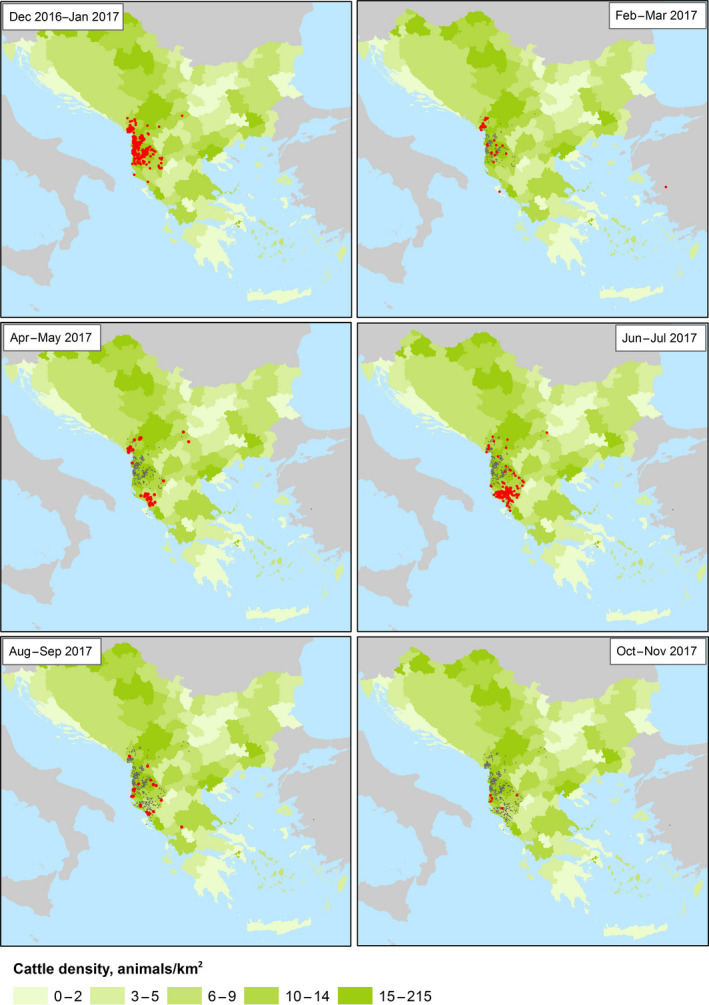
Spatial and temporal (monthly) distribution of LSD outbreaks occurring in Albania, the former Yugoslav Republic of Macedonia and Greece between December 2016 and November 2017 and cattle density (green shade, animals/square km; for Bosnia and Herzegovina data at regional level are not available). Red and grey dots indicate new and past outbreaks, respectively
Outbreaks in Albania in 2017
The epidemiological evolution of LSD infection in 2017 in the Balkan region is related mainly to Albania (379 outbreaks out of 385 outbreaks reported in 2017). By studying the temporal distribution of the outbreaks in Albania in 2017, the epidemic curve could be compared with that of 2016 (Figure 7). The outbreaks reported in January–February 2017 and the re‐emergence of the disease from May to July 2017 are discussed.
Figure 7.
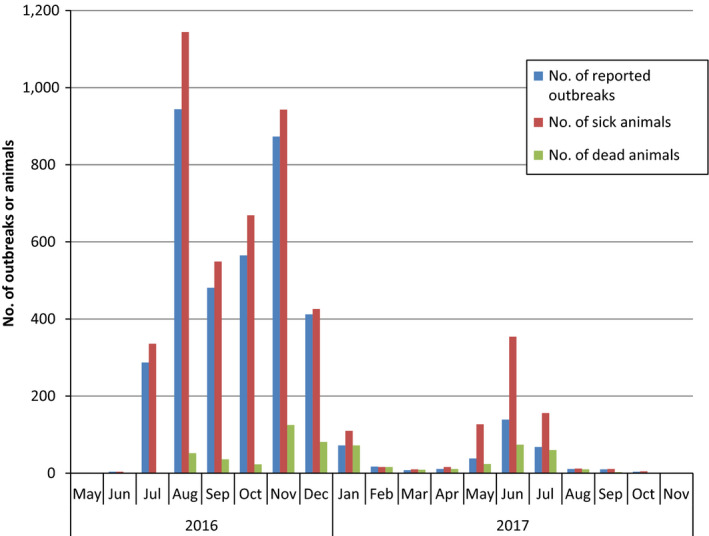
Monthly distribution of LSD outbreaks reported in Albania between May 2016 and November 2017 according to suspicion date, number of farms and animals affected and number of dead animals
From the graph, the 2016 epidemic persisted until January 2017 and then, after a period of lower incidence, a rise in the outbreak number was registered in the period May–July 2017. Compared with the 2016 epidemic, which lasted over six months and affected 3,567 farms and over 4,000 animals, leading to 317 deaths, the 2017 epidemic was shorter, basically lasting only three months (May–July), with 379 affected farms, 818 affected animals and 280 deaths.
Regarding the spatial and temporal occurrence of the outbreaks, those reported in January 2017 were observed mainly in northern (Shkodër and Dibër) and central (Tirana and Elbasan) Albanian counties (see Figure 8). These outbreaks could be spatially and temporally linked to the ones reported in the same counties in November and December 2016, when most outbreaks of the 2016 epidemic were reported. Indeed, the 2016 LSD epidemics in Albania developed mostly in the northern and central part of the country; therefore, the outbreaks in January 2017 can be considered a ‘tail’ of the 2016 epidemics that appears to fade out in winter 2016–2017 (Figure 7).
Figure 8.
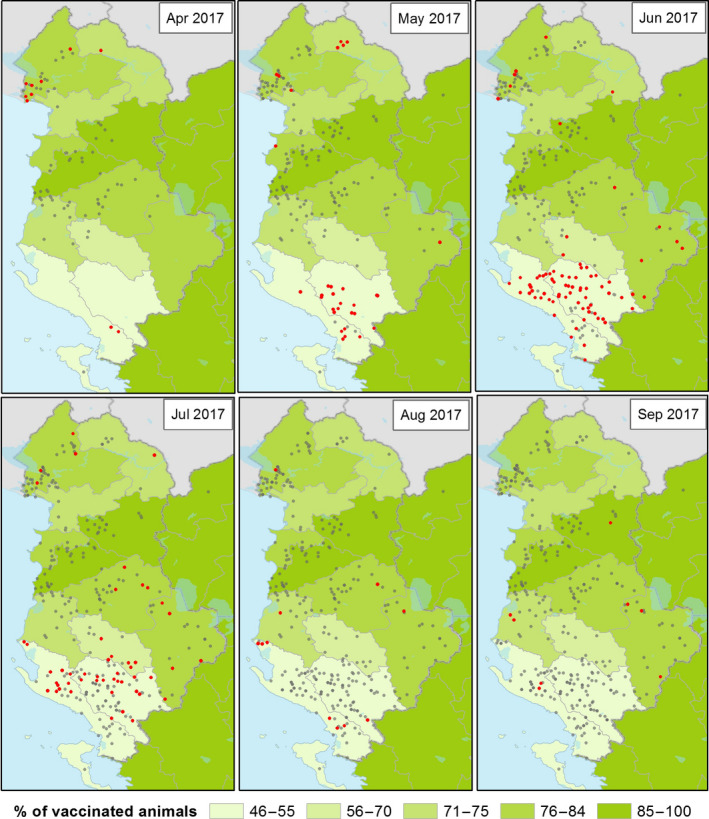
Spatial and temporal distribution of current and past LSD outbreaks (red and grey dots respectively) in Albania in 2017 and percentage of vaccinated animals per county
Nevertheless in winter months, when low temperatures should reduce vector abundance and activity thus hampering virus transmission (in December 2016, the mean average temperature in Albania was 4.1°C and the mean of the maximum temperature was 9.8°C, two degrees less than in 2015), new LSD outbreaks would not be expected. Beyond the possibility of vector‐independent transmission routes that should be still investigated, possible explanations for this could be related to the fact that, since animals are generally kept in cowsheds during winter months in Albania, neither the transmission by vectors present in the stable environment nor that by contaminated needles can be excluded. Moreover, a possible delay in reporting is possible. Thus, the outbreaks reported in January could be actually infections that occurred in the previous month(s). Even in a situation where farmers are aware of the disease and ready to report any suspected clinical sign in their own animals to the veterinary authority, a certain delay in reporting should be considered, because of the individual incubation period and the time needed to have a sufficient number of clinically affected animals in the farms, enough to gain farmer's attention.
Regarding the re‐emergence of LSD in late spring–summer 2017, more than 75% of those were reported in June and July 2017 in Girokaster and Vlore, two counties in southern Albania that were not heavily affected in the summer of 2016, when epidemics mostly spread in the north of the country. These could be explained by the spatial progression of the epidemics towards areas with a high density of susceptible animals in southern Albania, where the vaccination was not complete. In fact, up to September 2017, only 46% and 55% of animals had been vaccinated in the Girokaster and Vlore counties, respectively (Figure 8).
The dynamics of LSD spread in southern Albania is very similar to that observed in 2016 in the Serres regional unit of Greece where the reoccurrence of LSD was registered in April 2016, after the apparent fade‐out of LSD at the end of 2015. In that situation, the vaccination coverage was around 60%.
Unexpectedly, 34% of the outbreaks reported in 2017 in Albania were notified in farms vaccinated between 21 and 300 days before the onset of the outbreak, according to the date of suspicion (Figure 9). For comparison, this proportion was, in 2016, 12% in the former Yugoslavian Republic of Macedonia, 10% in Serbia, 4% in Bulgaria.
Figure 9.
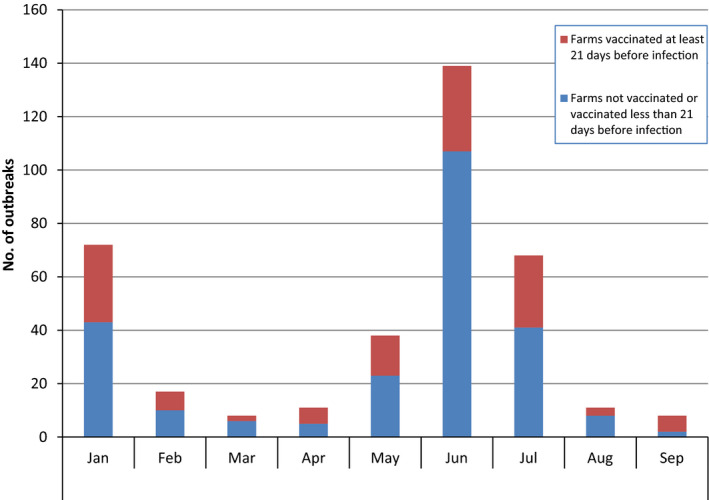
Number of LSD outbreaks in Albania notified each month in 2017, according to the immune status of the herd: farms vaccinated at least 21 days before infection or less than 21 days before infection or not vaccinated
This effect could be explained by considering that, because of the lack of data recorded at animal level, the vaccination status of each individual animal on those farms is not known, in particular the infected ones, and it also cannot be excluded that the infected animals were non‐immune new born or newly introduced animals not yet vaccinated and consequently fully susceptible to the infection.
Regarding the analysis of the morbidity and mortality (number of sick or dead animals reported out of the susceptible, respectively), the box plot comparing these two parameters in 2016 and 2017 is shown in Figure 10. The structure of the cattle population in Albania is characterised by mainly small size farms (median: two animals; 95th percentile: five animals), as already described before (EFSA, 2017). Such small cattle farms in Albania would lead to extreme values of morbidity within farms (e.g. one sick animal in a farm of two animals would lead to 50% morbidity). Moreover, since usually the animals of the farms of the same village are usually pooled together, the animals of a village have similar exposure to the disease, as explained in Section 2.2.1. Therefore, considering the village as epidemiological unit is more informative to report morbidity and mortality. Villages in Albania have a median of 51 farms (interquartile range (IQR): 22–100, 95th percentile: 229) and a median number of animals of 112 (interquartile range: 51–220, 95th percentile: 529).
Figure 10.
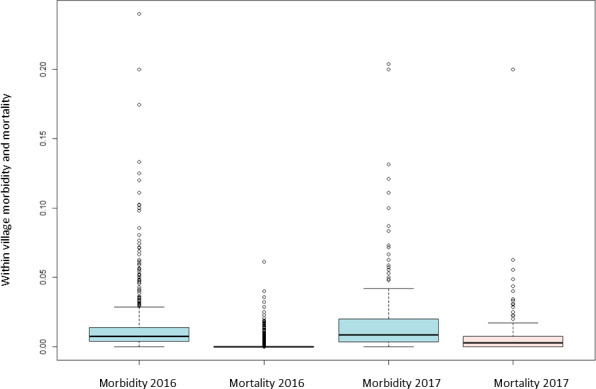
Within village morbidity and mortality due to LSD in Albania in 2016 and 2017
The boxplot shows that the morbidity in 2016 had a median value of 0.7% (IQR: 0.4–1.4%, 95th percentile: 4.8%) and the mortality 0% (95th percentile: 0.7%); while in 2017, the morbidity had a median value of 0.8% (IQR: 0.4%–2.0%, 95th percentile: 7.2%) and mortality 0.3% (IQR: 0.0–0.8%, 95th percentile: 2.9%) (Figure 10). Nevertheless, from statistical and biological point of view, there is no significant difference between the values registered in the two years (the confidence intervals in the data distribution in the two years are overlapping).
Outbreaks in the former Yugoslav Republic of Macedonia and Greece in 2017
The first event in the former Yugoslav Republic of Macedonia was reported at the beginning of January 2017 in the northwestern region of the country, close to Gostivar, around 30 km from the Albanian border. This outbreak might be connected to those reported in Albania in December 2016–January 2017. The other outbreaks in the former Yugoslav Republic of Macedonia were reported in April, June and July, in the northeastern area of the country, close to border with Bulgaria and Serbia, and just one affected animal was recorded for each. No epidemiological links are known to explain these outbreaks.
The two outbreaks reported in Greece in 2017 were observed in western Greece, where, in some areas, the first vaccination campaign was still in progress.7 The outbreak reported in the Greek island of Kerkyra (Corfu) at the beginning of February was in an unvaccinated dairy farm of 28 animals; 12 animals showed clinical symptoms and 3 died. This outbreak could be epidemiologically linked to those occurring in southern Albania in Vlorë county, around 20 km from Corfu, which is only 2 km from the Albanian coast. The last outbreak reported in Greece in 2017 was in Karditsa, in Thessaly region, reported in August. This was reported in a vaccinated beef farm of 200 ranging cattle; 15 animals were affected and 3 died. As prescribed by the EC Directive 92/119, the farm was depopulated few days later.
Conclusions
In 2017, the number of reported LSD outbreaks was substantially less (385) than in 2016 (7,483), and the vast majority were limited to areas of Albania, in areas where vaccination was still incomplete and where the cattle population was most susceptible. In contrast, Bulgaria, Serbia, Montenegro and Kosovo, which had all been affected in 2016, reported no outbreaks.
In areas where vaccination had not been completed in 2016, further outbreaks were observed in the following high risk season in 2017, indicating that the virus may have overwintered and circulated in the area.
In 2017, most outbreaks were reported between May and August, confirming the typical seasonality of LSD.
In the outbreak reported in Albania in 2017, the morbidity has a median value of 0.8% with values up to 7.2% while mortality median value was 0.3% with values up to 2.9%, thus in similar ranges as reported in 2016, when morbidity median value was 0.7% up to 4.8% and the mortality upper values was 0.7%.
The few outbreaks occurred in Greece and the former Yugoslav Republic of Macedonia in 2017 confirm that it is difficult for LSD to further spread in a population with well‐established herd immunity (vaccination coverage above 80%, see Section 3.3).
In general, compared with the 2016 situation, the low number of outbreaks reported in 2017 in Greece and the former Yugoslav Republic of Macedonia (6), could be considered as sporadically arising in a solidly immunised population (see Section 3.3), but showing that the virus is still present in the environment and/or in the cattle population and could re‐emerge when susceptible or not fully immunised animals are exposed.
3.2.2. Modelling the spread of LSDV between farms in Albania in 2016
The best‐fit models for the districts of Dibër and Mat and for Bulqizë, Dibër, Kukës and Mat combined were those that included a fat‐tailed kernel (ΔDIC > 10 for models including a Gaussian or exponential kernel). The best‐fit kernels are shown in Figure 11 and are compared to that estimated previously for Israel (EFSA AHAW Panel, 2015). The kernels are broadly comparable with similar distance scaling of around 1 km (or lower) and kernel powers of around 1.5–2 (Table 2). This shape of kernel is consistent with a majority of transmissions occurring over short distances (< 5 km) (e.g. via vectors), but with some transmission occurring over longer distances (e.g. via animal movements).
Figure 11.
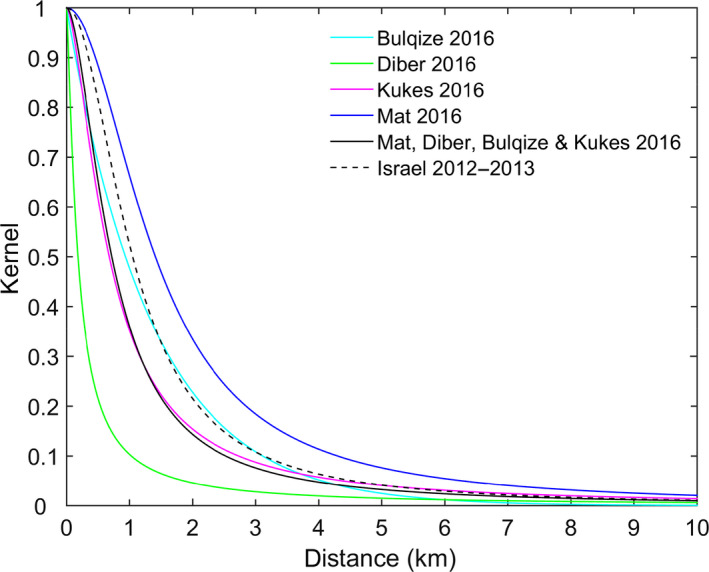
Estimated kernels (relative probability) for the transmission of LSDV between farms in Albania in 2016
- Results show the kernel for the posterior median estimates for Bulqizë (cyan), Dibër (green), Kukës (magenta) and Mat (blue) and for Bulqizë, Dibër, Kukës and Mat combined (black solid line). For comparison, the fat‐tailed kernel estimated previously for the spread of LSDV between farms in Israel in 2012–2013 is also shown (black dashed line).
Table 2.
Estimates (posterior median) and 95% credible intervals (CI) for fat‐tailed kernel parameters for the transmission of LSDV in Albania in 2016
| District | Kernel powera | Distance scaling (km)a | ||
|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |
| Dibër | 1.26 | 0.95–1.53 | 0.18 | 0.05–0.34 |
| Kukës | 1.58 | 0.86–2.27 | 0.68 | 0.08–1.52 |
| Mat | 1.97 | 1.31–2.72 | 1.41 | 0.63–2.30 |
| Bulqizë, Dibër, Kukës and Mat | 1.75 | 1.59–1.90 | 0.72 | 0.55–0.91 |
| Israel (2012–2013) | 2.01 | 1.76–2.33 | 1.05 | 0.57–1.87 |
The kernel has the form K(x) = 1/(1 + (x/d0)α) where α is the kernel power, which controls how fast the kernel decays with distance, and d0 is the distance scaling, which is the distance at which the force of infection is reduced by 50%.
Parameters could not be estimated for the fat‐tailed kernel when fitting to data for Bulqizë district. However, Gaussian and exponential kernels could be fitted to the data, with an exponential kernel providing a significantly better fit than the Gaussian (ΔDIC > 70) (Figure 11). The estimated kernel parameter was 0.74 (95% credible interval [CI]: 0.61–0.88).
The key difference between the fat‐tailed and exponential kernels relates to the probability of transmission over longer distances. This is much higher for the fat‐tailed compared to the exponential kernels (Figure 11) suggesting that longer distance transmission plays a less important role in Bulqizë compared with Dibër, Kukës and Mat. In order to compare the results from the previous risk assessment (EFSA AHAW Panel, 2015, 2016), the kernel obtained from the model based on transmission parameters derived from Israeli data is also shown.
The impact of seasonality (so that the force of infection depends on temperature or on relative vector abundance) was explored by incorporating it in the best‐fit model for the kernel and impact of vaccination in each district (i.e. a fat‐tailed (Dibër, Kukës and Mat) or exponential (Bulqizë) kernel and vaccine affecting susceptibility only), as in Table 4 Section 3.3.2 and in Figure 12.
Table 4.
Vaccination effectiveness estimated by the mathematical model in the four Albanian districts considered
| District | VE (%) | |
|---|---|---|
| Estimate | 95% credible interval | |
| Bulqizë | 75.7 | 55.2–88.2 |
| Dibër | 48.3 | 33.0–61.2 |
| Kukës | 59.3 | 35.8–76.0 |
| Mat | 84.7 | 62.8–96.0 |
| Bulqizë, Dibër, Kukës and Mat (combined) | 64.9 | 57.3–71.4 |
Figure 12.
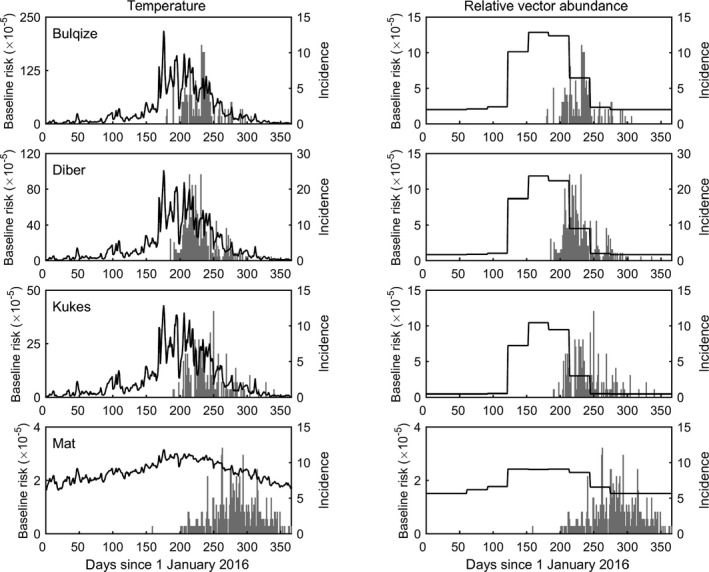
Estimated seasonal variation in the baseline risk of transmission of LSDV between farms for four districts in Albania in 2016. Seasonality is incorporated either via temperature dependence (left‐hand column) or relative abundance of S. calcitrans, a putative vector of LSDV (right hand column). Each plot shows the posterior median for the baseline risk over time (black line, left‐hand y axis) and the daily incidence of newly reported farms (grey bars, right hand y axis)
Including seasonality significantly improved model fit (ΔDIC > 2) for all districts, with a model incorporating seasonality via relative vector abundance providing a better fit than one in which seasonality is incorporated via temperature. However, the evidence for seasonality is much stronger for Bulqizë, Dibër and Kukës than for Mat.
Comparing the inferred seasonally varying baseline risk with the incidence of newly reported cases shows that the highest incidence coincides with the highest baseline risk, especially for the model in which seasonality reflects relative vector abundance (Figure 12). The magnitude of the seasonal variation in baseline risk is high for Bulqizë and Kukës, but relatively small for Mat. This is reflected in the strength of evidence for seasonality, which is weakest for Mat. Whether this is linked to a possible reporting bias or to any biological reasons is not known.
From the graphs in Figure 13, the peak risk of infection (black line) precedes the peak of the incidence (grey bars), most likely due to the incubation period and the time to disease detection.
Figure 13.
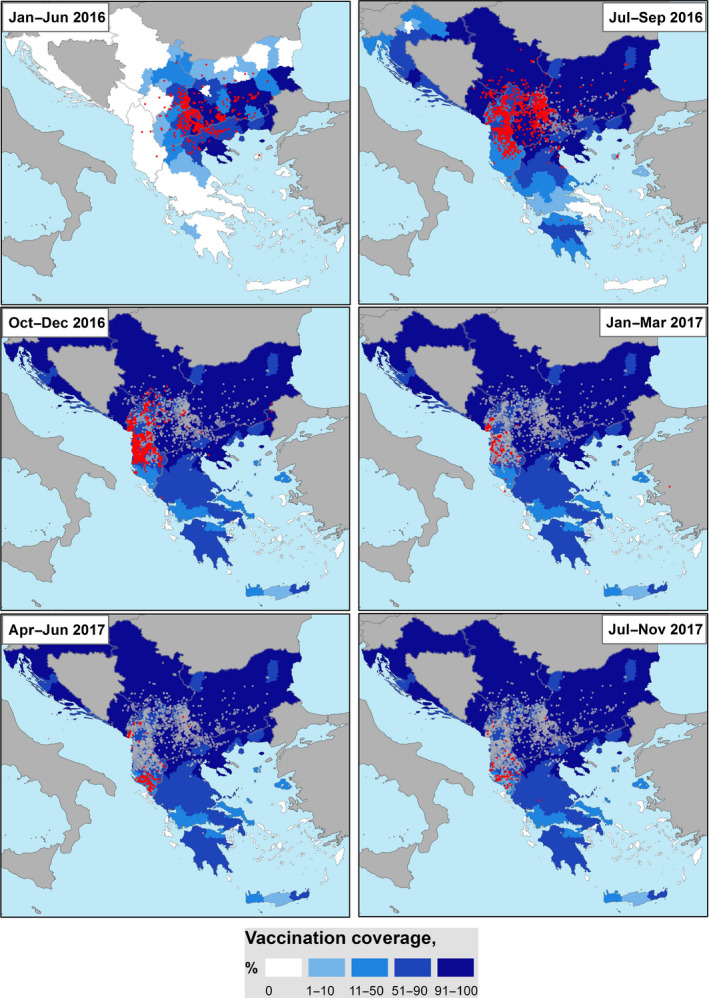
Month‐to‐month proportion of LSD‐vaccinated animals at regional level (NUTS3) (January 2016–November 2017) and reported outbreaks (red dots: new outbreaks; grey dots: past outbreaks). For Bosnia and Herzegovina, data were not available
Overall, these results suggest that there are seasonal factors influencing the transmission of LSDV in Albania, potentially related to temperature and vector abundance, but there are other, as yet undetermined, factors that also play a role.
Conclusions
The mathematical model fitted to the Albanian data showed that LSD spreads mostly up to 4 km (e.g. via vectors), but with some transmission occurring over much longer distances (e.g. via animal movement).
The kernel shape estimated for Albania is similar to the one for Israel, providing evidence on similar transmission parameters for LSD. Therefore, this further supports the conclusions drawn in previous risk assessment (EFSA, 2015, EFSA 2016).
Adding seasonality, in particular through modelled relative vector abundance, improves the model fit.
3.3. Vaccination against LSD
The increasing proportion of vaccinated animals in the course of the vaccination campaigns (% of vaccinated animals vaccinated in 2016 and revaccinated in 2017 plus the animals vaccinated in 2017 for the first time) carried out in the Balkan region compared with the available data on total cattle population is shown in the monthly map in Figure 13 and in the movie map that can be viewed at this link. The sequence starts in April 2016 when LSD reoccurred in Greece after the winter of 2015–2016. At that time, vaccination had already started in Greece in some regional units.
The map above provides an indication of the overall level of population immunity achieved in different areas compared with the epidemic spread (% of animals immunised and in the immunity period, i.e. 1 year after one vaccination, as indicated by some manufacturers). Nevertheless, it should be taken into account that the estimation of the vaccination coverage may be affected by some limitations because the number of vaccinated animals changed continuously over time following the progress of the vaccination campaign and because of the continuous variation in the animal population. Furthermore, there are some issues linked to the data that may lead to a certain degree of bias in the estimates, either for the denominator (total cattle population) or for the numerator (vaccinated animals). For example, as pointed out at the GF TADs meeting on LSD in October 2017,8 in some countries, the number of animals registered in the cattle population database can be higher than the number actually present. This supports the importance of having updated cattle population data. Furthermore, the number of vaccinations registered is less than the real number of vaccinated animals because of a variable delay in registering the vaccinations in the databases. Finally, the total bovine population also includes calves younger than 4 months born to vaccinated cattle but which are not themselves vaccinated and are not included in the number of vaccinated animals. In Greece, for example, the number of these animals could be estimated as 4% of the total bovine population.
3.3.1. Estimation of vaccination effectiveness by survival analysis
The VE estimated as in Section 2.2.1 is presented for case studies of Albania, Bulgaria, Greece, Montenegro and Serbia, in relation to 2016 and according to follow‐up time as described in Section 2.2.1. In the case of Greece, an analysis of other risk factors is also presented. For Kosovo and the former Yugoslav Republic of Macedonia, the information provided did not allow estimation of VE using the survival model at farm level, further considerations need to be taken into account.
Bulgaria
For Bulgaria, for which the district of Blagoevgrad was considered; Figure 14 shows the survival analysis graph comparing the cumulative proportion of vaccinated and unvaccinated farms, according to follow‐up time and starting with the occurrence of the first outbreak notified in the district of Blagoevgrad. This shows a higher proportion of non‐vaccinated farms experienced an outbreak, compared with the vaccinated farms. The VE, estimated by the Cox model, was equal to 95.6% (CI: 86.6–98.5). This is the probability of vaccinated farms remaining uninfected compared with the non‐vaccinated. This confirms that the vaccine was very effective, something that is also supported by the field evidence; no outbreaks were reported in Bulgaria in 2017.
Figure 14.
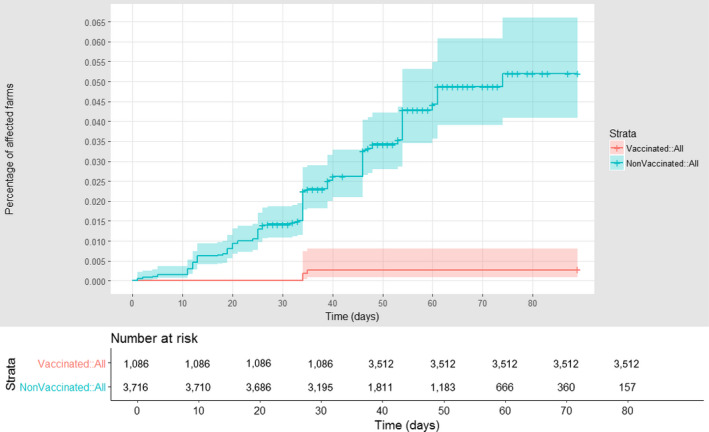
Cumulative proportion of LSD vaccinated (red) and unvaccinated (blue) farms affected in Bulgaria, according to follow‐up time starting with the occurrence of the index case in the Blagoevgrad district. Shown under the graph are the numbers of farms at risk of infection in each of the two groups (vaccinated and non‐vaccinated) at different times after the first reported infection in each village
Montenegro
Figure 15 shows the graph of the survival analysis of the cumulative proportion of vaccinated and unvaccinated affected farms in Montenegro, according to follow‐up time, starting with the occurrence of the first outbreak notified in the country. It demonstrates high effectiveness of the vaccination, in that a higher proportion of non‐vaccinated farms experienced an outbreak, compared to the vaccinated.
Figure 15.
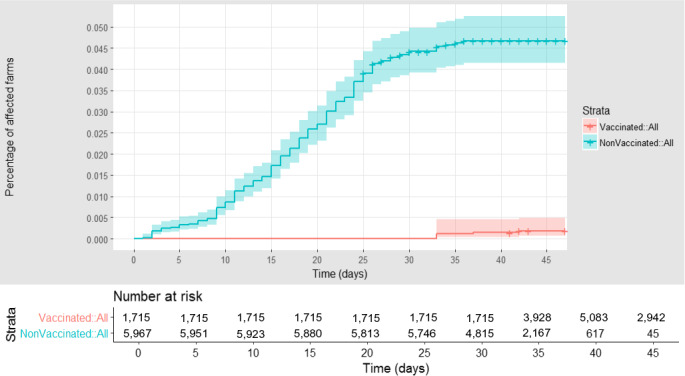
Cumulative proportion of LSD vaccinated (red) and unvaccinated (blue) farms affected in Montenegro, according to follow‐up time starting with the occurrence of the index case. Shown under the graph are the numbers of farms at risk of infection in each of the two groups (vaccinated and non‐vaccinated) at different time after the first reported infection in each village
For Montenegro, the estimation of VE by the Cox model using the available data was affected by high uncertainty, the median is therefore not informative of the actual VE in this country. The reason for this lies in the data: (i) the limited number of outbreaks in the groups; (ii) until day 40, no infected farm was observed in the vaccinated group, and from that moment, there were very few outbreak cases in the non‐vaccinated farms.
Serbia
For Serbia, where the region of Pcinskij is considered, the survival analysis graph comparing vaccinated and unvaccinated affected farms according to follow‐up time, starting with the occurrence of the first outbreak notified in the Region of Pcinskij, shows a higher proportion of non‐vaccinated farms having experienced an outbreak, compared to the vaccinated ones (Figure 16) thus confirming that vaccination was effective. This was also confirmed from the field evidence; no outbreaks were reported in Serbia in the following season (in 2017).
Figure 16.
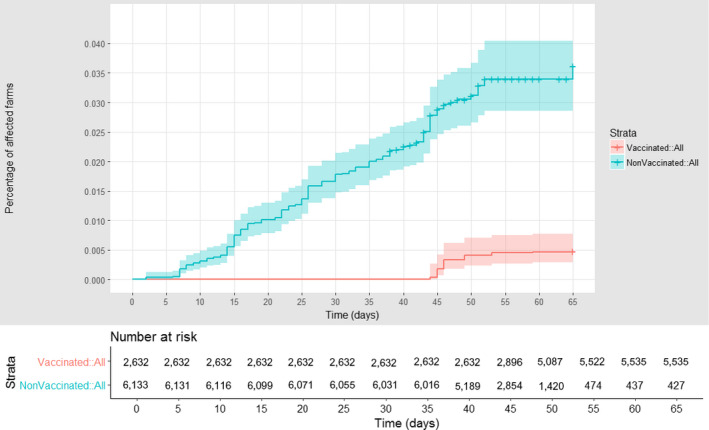
Cumulative proportion of vaccinated (red) and unvaccinated (blue) farms affected in Serbia according to follow‐up time, starting with the occurrence of the index case in the Pcinjski district. Shown under the graph are the numbers of farms at risk of infection in each of the two groups (vaccinated and non‐vaccinated) at different times after the first reported infection in each village
Nevertheless, similarly to the case of Montenegro, because of the scarcity of data in the different categories (i.e. vaccinated vs. non‐vaccinated farms and affected vs non‐affected farms), the value of VE for Serbia estimated by Cox model results in very wide confidence intervals, indicating high uncertainty as to the actual VE in the country.
Greece
The estimation of VE in Greece (Serres regional unit) considering the district as epidemiological unit was presented previously (EFSA AHAW Panel, 2016). A similar analysis was conducted for the same area considering the village as epidemiological unit. The cumulative proportion of vaccinated and unvaccinated affected farms in the Serres regional unit (Greece), according to follow‐up time, starting with the occurrence of the first outbreak notified in the region, is reported in Figure 17. The VE was estimated to be 84% (CI: 73–91).
Figure 17.
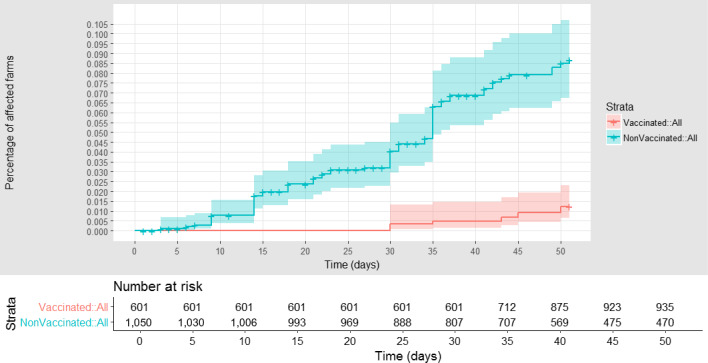
Cumulative proportion of LSD vaccinated (red) and unvaccinated (blue) affected farms in Greece (Serres), according to follow‐up time starting with the occurrence of the index case in the first village affected. Shown under the graph are the numbers of farms at risk of infection in each of the two groups (vaccinated and non‐vaccinated) at different time after the first infection reported in each village
The data available for Serres also allowed analysis of other risk factors, in particular the type of housing (animals with access to outdoors compared with animals kept indoors), was considered and included as fixed factors in the model. The graph is shown in Figure 18.
Figure 18.
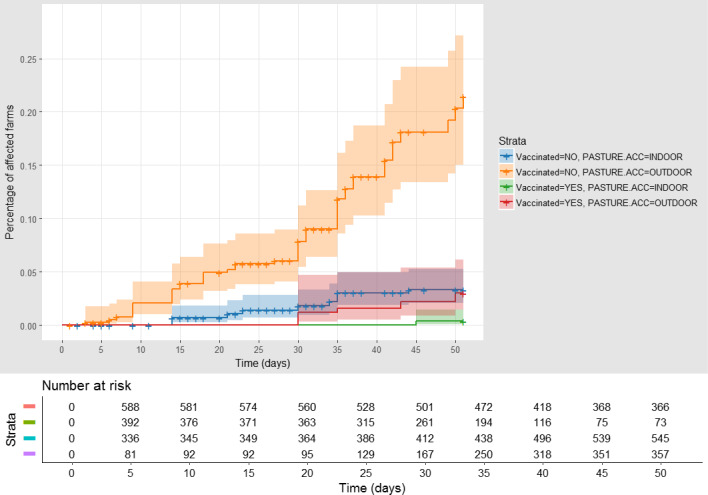
Cumulative proportion of affected farms in Greece (Serres) (vaccinated herds with outdoor access (red), vaccinated kept indoors (green), unvaccinated with outdoor access (orange) and unvaccinated kept indoors (blue)), according to follow‐up time starting with the occurrence of the index case in the first village affected
- Shown under the graph are the numbers of farms at risk of infection in each of the two groups (vaccinated, non‐vaccinated, outdoor/indoor) at different time after the first infection reported in each village.
The highest proportion of affected farms is among those unvaccinated and with outdoor access. The Cox regression results are displayed in the Table 3.
Table 3.
Cox regression for LSD vaccinated vs. non‐vaccinated farms and outdoor access vs. kept indoors
| Coef | Exp(coef) | SE(coef) | Z | Pr(> |z|) | |
|---|---|---|---|---|---|
| Vaccinated YES | −1.9267 | 0.1456 | 0.3556 | −6.762 | 1.36e‐11* |
| Access OUTDOOR | 1.8824 | 6.5695 | 0.2676 | 6.270 | 3.62e‐10* |
n = 1,908, number of events = 76; Significance code: *p < 0.001.
In this case, the VE is 85.5% (74.5, 91.7), and the risk in farms with outdoor access is 6.57 times higher (CI: 3.64–11.83) than in farms where animals are kept indoors.
Albania
The estimation of VE in Albania considering the district as epidemiological unit was presented in the first report (EFSA, 2017). A similar analysis was conducted considering the village as epidemiological unit. Figure 19 shows the cumulative proportion of vaccinated and unvaccinated affected farms in Albania, according to follow‐up time, starting with the occurrence of the first outbreak notified. The VE is estimated to be 62.5% (CI: 56.9–67.4).
Figure 19.
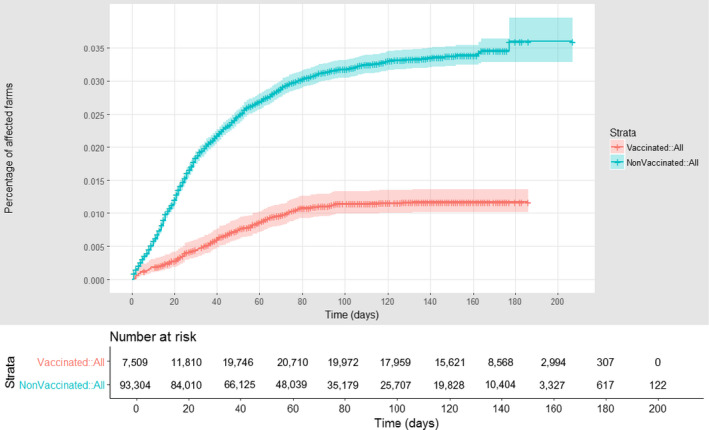
Cumulative proportion of LSD vaccinated (red) and unvaccinated (blue) affected farms in Albania
3.3.2. Estimation of vaccination effectiveness in Albania explored by the mathematical model
Only the models in which vaccination affected susceptibility produced the best fit to the data. More specifically, these models produced a significantly better fit than models in which vaccination either had no effect or only affected infectiousness (ΔDIC > 309). The models in which vaccination affected both susceptibility and infectiousness did not produce a significantly worse fit than those in which it only affected susceptibility (ΔDIC < 2), but the simpler model (i.e. susceptibility only) was preferred as it had the smaller number of parameters. In addition, the VE for infectiousness was not well identified (reflected in very wide CIs for maximum VE, εI). The VE estimated for the four Albanian regions (Bulqizë, Dibër, Kukës and Mat) using the transmission model (Table 4) were broadly similar to those obtained using the survival analysis method (Figure 14, 15, 16, 17, 18–19).
3.3.3.
Conclusions
The VE estimated with the survival model ranges was 62% in Albania, 96% in Bulgaria and 84% in Greece (median values), confirming the positive effect of vaccination in controlling the LSD spread.
Mathematical models assuming the vaccine only affects susceptibility produces the best fit.
The VE estimated with the mathematical model is similar to that estimated with the survival model, validating the results.
3.4. Update on available diagnostic tools for LSD
Since a vaccination by homologous live‐attenuated vaccine has been applied in the whole region, the need of differentiating field virus strains from vaccine strains in the current and future outbreaks could be a relevant component of active surveillance, and thus, diagnostic tools fit for this purpose would be of benefit. Furthermore, under the recently adopted rules of Chapter 11.9 of OIE Terrestrial Animal Health Code about LSD,10 virological and serological testings are part of the procedures for a country's recognition or recovery of LSD freedom. Therefore, in this section, an update of the currently available diagnostic test for LSD is presented.
There are several polymerase chain reaction (PCR)‐based commercial diagnostic kits for the detection of capripoxvirus (e.g. Techne® quantitative PCR (qPCR) test, Genesig® Standard and Advanced Kit, Tetracore®, Biosellal®), but the need to differentiate LSDV field strains from vaccine strains in areas where vaccination against LSD is performed with live‐attenuated vaccines, has led some research groups to develop DIVA diagnostic protocols. These are particularly important owing to the possibility of mild or systemic post‐vaccination reactions in vaccinated animals (EFSA, 2017), It is therefore important to be able to apply diagnostic procedures that will rapidly and specifically differentiate LSD field virus from vaccine strains. These assays include gel‐based PCR protocols to differentiate wild‐type LSDV from vaccine strains, and some to distinguish sheep pox virus from vaccine strains. Among the former, PCR‐RFLP methods have been developed in Greece (Agianniotaki et al., 2016) and in Israel (Menasherow et al., 2014).
Real‐time PCR assays have also been developed to differentiate between wild‐type LSDV and vaccine strains; these are in general faster and cheaper than gel‐based PCR methods. Israeli researchers developed a method based on a high‐resolution melting (HRM) assay that distinguishes between Israeli field and Neethling vaccine LSDV (Menasherow et al., 2016). In Serbia, two real‐time TaqMan‐PCR assays for detection of field LSDV strain currently circulating in the Balkan Peninsula were developed by Vidanovic et al. (2016) and validated on 111 field samples from both infected and vaccinated animals. The assays were considered more sensitive than the gel‐based PCR developed by Menasherow et al. (2014) but less sensitive than the qPCR set of (Bowden et al., 2008)11 Because it is based on the absence of signal rather than a vaccine‐specific signal, it needs to be used in parallel with other real‐time PCR methods for capripoxvirus detection to exclude vaccine strains.
Agianniotaki et al. (2017) recently developed and validated a duplex quantitative real‐time PCR method targeting the viral G protein‐coupled receptors (GPCR) gene, for the concurrent detection and differentiation of LSDV wild‐type and vaccine strains. The method was evaluated in three laboratories. The amplification efficiencies were 91.3% and 90.7%, for LSD virus wild‐type and vaccine strains, respectively; the limit of detection was eight DNA copies for both targets. The diagnostic performance was assessed using 163 LSDV‐positive samples, including field specimens and samples from experimentally vaccinated/infected animals. The diagnostic sensitivity and specificity for the wild‐type virus were 100% (95% CI: 96.67–100%) and 100% (95% CI: 97.14–100%), respectively, and for the vaccine virus, they were 98.18% (95% CI: 90.28–99.95%) and 100% (95% CI: 97.99–100%), respectively (Agianniotaki et al., 2017).
A novelty in LSD diagnostics is the recently marketed commercial enzyme‐linked immunosorbent assay (ELISA) (ID vet) for the detection of antibodies against capripoxviruses (LSDV, SPPV and goat pox virus (GTPV)).12 The producer indicates that the test allows the detection of antibodies from approximately 20 days until 7 months post‐vaccination. Sensitivity is therefore better than virus neutralisation test and specificity is indicated as 99.7% in the capripoxvirus population. Nevertheless, individual animals with low antibody levels – such as those in the early stage of infection, or with mild disease or a low antibody response to vaccine – may not be detected.13
4. Conclusions
Given the results of the epidemiological analysis, it can be concluded that:
Excluding Turkey, since the beginning of the epidemic in southeastern Europe in 2015, over 7,900 LSD outbreaks with around 13,650 affected animals have been reported.
In 2016, 7,483 LSD outbreaks were reported with 12,330 affected animals in the Balkan region, while in 2017, only 385 outbreaks with 850 affected animals were notified, mainly in Albania (379 outbreaks), in areas where vaccination was not completed and where the cattle population was most susceptible, and very few in Greece (two outbreaks) and the former Yugoslav Republic of Macedonia (four outbreaks). No further outbreaks were reported in all other countries that were affected in 2016. This reduction of the number of outbreaks reported in 2017, particularly in Bulgaria, Serbia, Montenegro and Kosovo where none was reported, provides field evidence of the effectiveness of the mass vaccination campaigns conducted at regional level
In 2017, most outbreaks were reported between May and July, confirming the seasonality of LSD previously observed in the Balkans, an important aspect when planning the start of a vaccination campaign.
In the outbreak reported in Albania in 2017, the morbidity has a median value of 0.8% with values up to 7.2% while mortality median value was 0.3% with values up to 2.9%, thus in similar ranges as reported in 2016, when morbidity median value was 0.7% up to 4.8% and the mortality upper values was 0.7%.
The vaccination coverage by live‐attenuated homologous vaccine up to 100% and the consequent well‐established herd immunity achieved in infected countries other than Albania, like Greece or the former Yugoslav Republic of Macedonia, has strongly reduced the further spread of the disease in 2017 to few sporadic outbreaks. Nevertheless, the latter show that the virus is still present in the environment and/or in the cattle population and may re‐emerge again when susceptible or not fully immunised animals are exposed.
The mathematical model that best fitted to the Albanian data was the density‐dependent fat‐tailed kernel. This showed the probability of LSD spread is mostly (95%) up to 4 km (by e.g. vectors), but with some transmission occurring over much longer distances (by e.g. animal movements). Proximity to affected farms and animal density, assuming an homogeneous spatial distribution of vectors, can be therefore considered as further risk factors for LSD spread.
The kernel shape estimated for Albania is similar to the one for Israel, providing evidence on similar transmission parameters for LSD. Therefore, this aspect further supports the conclusions drawn in previous risk assessment (EFSA AHAW Panel, 2015, 2016).
Adding seasonality, in particular through modelled relative vector abundance (Stomoxys calcitrans), improves the model fit and supports that the abundance of potential LSD vectors is one of the major risk factors contributing to LSD spread.
The protective effect of vaccination is supported by the analysis of the VE performed with the survival model and Cox model in the case study of Albania, Bulgaria and Greece, with median VE values of 62%, 96% and 84%, respectively. These results highlight that high coverage vaccination with live homologous vaccine is the most effective measure for reducing LSDV spread.
In the case of Greece, further risk factors could be explored with the survival model, such as the animals’ housing type (indoor vs. outdoor). The risk in farms with outdoor access is six times higher than in farms where animals are kept indoors, independent of vaccination status, possibly because of higher exposure to vectors bite.
The VE estimated with the mathematical model for a few districts in Albania is similar to that estimated with the survival model, validating the results.
The commitment and collaboration of the veterinary services from the countries involved in LSD data collection have been consistently at a very high level, showing an excellent spirit of regional cooperation among each other and with EFSA. This was one of the main elements that allowed the achievement of the results presented in this report and the successful control of the disease in the field.
5. Recommendations
Quality and validation of collected data are a cornerstone for epidemiological analysis of an animal disease on a regional basis (multiple countries involved) and should be constantly improved and, where possible, automated.
Data at animal level and within‐farm follow‐up are desirable as they would increase the precision of the estimation and allow use of different analytical methods. These data should be collected particularly during the in‐depth standardised outbreak investigations that should be performed for all new LSD cases.
Since the mathematical model presented in this report supports the importance of vector‐borne transmission of LSD, field data about relative abundance of potential LSD vectors should be gathered by targeting a number of farms experiencing LSD outbreaks and followed up during the entire LSD season, from the first LSD cases in spring until the last ones in autumn.
The epidemiological situation observed in 2017 confirms that vaccination is a key tool for LSD control. Achieving the highest vaccination coverage in the shortest period of time should therefore be sought to rapidly control LSD outbreaks, coupled with good clinical surveillance for immediate notification of suspected clinical cases that should be confirmed in the laboratory to differentiate LSD field virus from the vaccine strain.
Future work on LSD should be supported by the development of scenarios based on the elements mentioned in the conclusions above. In particular, to increase the reliability of those scenarios, experimental evidence should be sought about the duration of immunity conferred by the homologous LSD vaccine.
Abbreviations
- AIC
Akaike information criterion
- CI
credible interval
- DIC
deviance information criterion
- ELISA
enzyme‐linked immunosorbent assay
- GF TADs
Global Framework for the progressive control of Transboundary Animal Diseases
- GPCR
G protein‐coupled receptors
- GTPV
goat pox virus
- HR
hazard ratio
- HRM
high‐resolution melting
- IQR
interquartile range
- LSD
lumpy skin disease
- LSDV
lumpy skin disease virus
- MCMC
Markov Chain Monte Carlo
- PCR
polymerase chain reaction
- qPCR
quantitative PCR
- SPPV
sheep pox virus
- VE
vaccination effectiveness
Glossary
- Vaccination coverage
The proportion of animals that are vaccinated in a target population.
- Vaccination effectiveness
The proportion of vaccinated animals which are protected from infection under field conditions.
- Vaccine efficacy
The proportion of vaccinated animals which are protected from infection under ideal conditions (experimental study), usually expressed as a percentage.
Suggested citation: EFSA (European Food Safety Authority) , 2018. Scientific report on lumpy skin disease II. Data collection and analysis. EFSA Journal 2018;16(2):5176, 33 pp. 10.2903/j.efsa.2018.5176
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00625
Acknowledgements: EFSA wishes to thank the members of the Working Group on lumpy skin disease: Paolo Calistri, Simon Gubbins, Eyal Klement and Jan Arend Stegeman, for the preparatory work on this scientific output and the hearing experts Ledi Pite, Aleksandra Miteva, Brigita Hengl, Marijan Glavina, Sotiria‐Eleni Antoniou, Bafti Murati, Tatjana Labus, Drago Maroejvic, Zorana Mehmedbasic, Srgjan Meshterovikj and Esra Satir for the data collection; Klaus Depner, Bruno Garin‐Bastuji, Margaret Good, Miguel Miranda, Soeren Saxmose Nielsen, Liisa Sihvonen, for reviewing the document and EFSA staff members: Alessandro Broglia, Josè Cortiñas Abrahantes, Andrey Gogin, Lisa Kohnle, Mario Monguidi, Jane Richardson and Jelena Vracar for the support provided to this scientific output. A special thanks is due to: Ledi Pite from Ministry of Agriculture, Rural Development and Water Administration, Albania; Aleksandra Miteva from Food Safety Agency, Bulgaria; Brigita Hengl from Croatian Food Agency and Marijan Glavina from Ministry of Agriculture, Croatia; Sotiria‐Eleni Antoniou and Chrysoula Dile of the Animal Health Directorate of the Greek Ministry of Rural Development and Food and to the veterinary staff of the Greek NRL of Capripoxviruses and Regional Veterinary Authorities, Greece; Bafti Murati from the Food and Veterinary Agency, Kosovo; Drago Marojevic from the Ministry of Agriculture and Rural Development, Montenegro; Srgjan Meshterovikj, Food and Veterinary Agency, former Yugoslav Republic of Macedonia; Tatjana Labus and Budimir Plavsic from the Ministry of Agriculture and Environmental Protection, Republic of Serbia and Esra Satir from Pendik Veterinary Control Institute, Turkey for the timely provision of the epidemiological data for this report.
Approved: 29 January 2018
Notes
This designation is without prejudice to positions on status and is in line with UNSCR 1244 and the ICJ Opinion on the Kosovo Declaration of Independence. This footnote is applied whenever ‘Kosovo’ is mentioned in the present report.
In statistics, multicollinearity (also collinearity) is a phenomenon in which one predictor variable in a multiple regression model can be linearly predicted from the others with a substantial degree of accuracy.
The deviance information criterion (DIC) is a hierarchical modelling generalization of the Akaike information criterion (AIC) and the Bayesian information criterion (BIC).
References
- Agianniotaki EI, Tasioudi KE, Chaintoutis SC, Iliadou P, Mangana‐Vougiouka O, Kirtzalidou A, Alexandropoulos T, Sachpatzidis A, Plevraki E and Dovas CI, 2016. Lumpy skin disease outbreaks in Greece during 2015–16, implementation of emergency immunization and genetic differentiation between field isolates and vaccine virus strains. Veterinary Microbiology, 201, 78–84. [DOI] [PubMed] [Google Scholar]
- Agianniotaki EI, Chaintoutis SC, Haegeman A, Tasioudi KE, De Leeuw I, Katsoulos PD, Sachpatzidis A, De Clercq K, Alexandropoulos T, Polizopoulou ZS, Chondrokouki ED and Dovas CI, 2017. Development and validation of a TaqMan probe‐based real‐time PCR method for the differentiation of wild type lumpy skin disease virus from vaccine virus strains. Journal of Virological Methods, 249, 48–57. [DOI] [PubMed] [Google Scholar]
- Baldacchino F, Desquesnes M, Mihok S, Foil LD, Duvallet G and Jittapalapong S, 2014. Tabanids: neglected subjects of research, but important vectors of disease agents!. Infection Genetics Evolution, 28, 596–615. [DOI] [PubMed] [Google Scholar]
- Beard PM, 2016. Lumpy skin disease: a direct threat to Europe. Veterinary Record, 178, 557–558. [DOI] [PubMed] [Google Scholar]
- Ben‐Gera J, Klement E, Khinich E, Stram Y and Shpigel NY, 2015. Comparison of the efficacy of Neethling lumpy skin disease virus and x10RM65 sheep‐pox live attenuated vaccines for the prevention of lumpy skin disease ‐ the results of a randomized controlled field study. Vaccine, 33, 4837–4842. [DOI] [PubMed] [Google Scholar]
- Bowden TR, Babiuk SL, Parkyn GR, Copps JS and Boyle DB, 2008. Capripoxvirus tissue tropism and shedding: a quantitative study in experimentally infected sheep and goats. Virology, 371, 380–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. Strengthening regional cooperation in South East Europe and Middle East for prevention and control of Lumpy Skin Disease (LSD). 13, EFSA supporting publication 2016:EN‐1059, 2019 pp. [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Lumpy skin disease: I. Data collection and analysis. EFSA Journal 2017;15(4):4773, 54 pp. 10.2903/j.efsa.2017.4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (Panel on Animal Health Welfare), 2015. Scientific Opinion on lumpy skin disease. EFSA Journal 2015;13(1):3986, 73 pp. 10.2903/j.efsa.2015.3986 [DOI] [Google Scholar]
- EFSA AHAW Panel (Panel on Animal Health Welfare), 2016. Statement: urgent advice on lumpy skin disease. EFSA Journal 2016;14(8):4573 27 pp. 10.2903/j.efsa.2016.4573 [DOI] [Google Scholar]
- FAO (Food and Agriculture Organisations of the United Nations), 2017. EMPRES‐Animal Health 360, No. 347. Rome.
- Kahana‐Sutin E, Klement E, Lensky I and Gottlieb Y, 2017. High relative abundance of the stable fly Stomoxys calcitrans is associated with lumpy skin disease outbreaks in Israeli dairy farms. Medical Veterinary Entomology, 31, 150–160. [DOI] [PubMed] [Google Scholar]
- Kitching RP and Mellor PS, 1986. Insect transmission of capripoxvirus. Research in Veterinary Science, 40, 255–258. [PubMed] [Google Scholar]
- Lysyk T, 1998. Relationships between temperature and life‐history parameters of Stomoxys calcitrans (Diptera: Muscidae). Journal of Medical Entomology, 35, 107–119. [DOI] [PubMed] [Google Scholar]
- Menasherow S, Rubinstein‐Giuni M, Kovtunenko A, Eyngor Y, Fridgut O, Rotenberg D, Khinich Y and Stram Y, 2014. Development of an assay to differentiate between virulent and vaccine strains of lumpy skin disease virus (LSDV). Journal of Virological Methods, 199, 95–101. [DOI] [PubMed] [Google Scholar]
- Menasherow S, Erster O, Rubinstein‐Giuni M, Kovtunenko A, Eyngor E, Gelman B, Khinich E and Stram Y, 2016. A high‐resolution melting (HRM) assay for the differentiation between Israeli field and Neethling vaccine lumpy skin disease viruses. Journal of Virological Methods, 232, 12–15. [DOI] [PubMed] [Google Scholar]
- Spiegelhalter DJ, Best NG, Carlin BP and Van Der Linde A, 2002. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 64, 583–639. [Google Scholar]
- Tasioudi KE, Antoniou SE, Iliadou P, Sachpatzidis A, Plevraki E, Agianniotaki EI, Fouki C, Mangana‐Vougiouka O, Chondrokouki E and Dile C, 2016. Emergence of lumpy skin disease in Greece, 2015. Transboundary and Emerging Diseases, 63, 260–265. [DOI] [PubMed] [Google Scholar]
- Therneau TM and Grambsch PM, 2000. Modeling survival data: extending the Cox model. Springer Science & Business Media, pp. 349. [Google Scholar]
- Vidanovic D, Sekler M, Petrovic T, Debeljak Z, Vaskovic N, Matovic K and Hoffmann B, 2016. Real‐time PCR assays for the specific detection of field Balkan strains of lumpy skin disease virus. Acta Veterinaria‐Beograd, 66, 444–454. [Google Scholar]


