Abstract
Avian influenza viruses infect domestic poultry and wild birds as well as humans. In poultry, depending on whether these viruses are of high pathogenicity (HPAI) or low pathogenicity (LPAI), the infection can cause different clinical signs, with HPAI causing high mortality in poultry flocks. In order to ensure early detection of avian influenza viruses, surveillance in poultry and wild birds is considered essential. In 2010, the European Commission provided some guidelines to Member States (MSs) on how this surveillance should be carried out, both in poultry and wild birds. EFSA received a mandate from the Commission to collate, validate, analyse, and summarise in an annual report the data resulting from the ongoing avian influenza surveillance programmes established in the different MSs. To deliver on this mandate, EFSA, in collaboration with the Standing Working Group on AI, initiated its activities with the drafting of a scientific report where the future vision of this collection framework was presented. Initial and later drafts of this report were shared with MS representatives in order to get feedback on the practicalities concerning the collection and submission of avian influenza surveillance data to EFSA. In the present report, the data that MSs are legally requested to submit to EFSA (‘mandatory’) and also the data that would be important to collect in order to optimise the outputs (‘desirable’) are described. A number of actions that would lead to the optimal data collection are also presented and the added value to MSs is discussed. A step‐by‐step implementation of the outlined actions is anticipated, with a description of the initial collection framework for 2019 being included in this report.
Keywords: avian influenza, HPAI/LPAI, surveillance, poultry, wild birds
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
EU legislation on avian influenza1 requires Member States to carry out compulsory surveillance programmes in poultry and wild birds. The objectives are to detect the prevalence of infections with avian influenza virus subtypes H5 and H7 in different species of poultry and to contribute, on the basis of a regularly updated risk assessment, to the knowledge on the threats posed by wild birds in relation to any influenza virus of avian origin in birds.
Surveillance in poultry and wild birds is crucial in order to early detect the occurrence of new avian influenza viruses in the EU that pose a risk to animal and potentially to human health.
In particular, the recent couple of years have shown how important continuous surveillance of domestic and wild bird populations is. Collection of data on avian influenza surveillance results and their timely analysis from a laboratory and epidemiological point of view are essential tools to contribute to awareness and preparedness in order to face avian influenza epidemics.
The surveillance programmes must comply with guidelines laid down by the Commission.2 These describe the strategies and methods for surveillance in poultry and wild birds, as well as sampling and testing procedures.
Member States report via an online system the results of their surveillance activities to the Commission. The results are then analysed by the EU reference laboratory for avian influenza which issues annual reports that can be consulted on the Commission's website.3
In order to harmonise and streamline data collection and analysis of results of surveillance in poultry and wild birds, EFSA shall be mandated to carry out the above tasks. It shall start its work as of 1 January 2019.
Therefore, in the context of Article 31 of Regulation (EC) No. 178/2002, EFSA should provide the technical and scientific assistance to the Commission based on the following Terms of Reference:
-
–
Collate, validate, analyse and summarise in an annual report the results from avian influenza surveillance carried out by Member States in poultry and wild birds.
1.2. Interpretation of the Terms of Reference
Following Decision 2010/367/EU, Member States (MSs) implement surveillance programmes for avian influenza (AI) in poultry and wild birds. All MSs have the obligation to report data on active serological surveillance in poultry, and on passive surveillance of dead and moribund wild birds (as described in their national surveillance programmes), to the European Union (EU). Norway and Switzerland report these data to the EU on a voluntary basis. Several MSs also report voluntarily data on active surveillance of healthy wild birds.
The main objective of the regulatory active current surveillance programme for AI in poultry is to inform the competent authority of circulating avian influenza virus (AIV) in order to implement knowledge‐based control measures against the disease in accordance with Directive 2005/94/EC. The programme in poultry encompasses active surveillance for low and highly pathogenic avian influenza viruses (LPAI and HPAI, respectively):
-
–
LPAI viruses of subtypes H5 and H7 in galliformes and ratites, and
-
–
LPAI viruses of subtypes H5 and H7 and HPAI viruses in domestic waterfowl.
Also, complementing the active surveillance programme in poultry, and in accordance with Commission Implementing Decision (EU) 2018/11364, MSs shall ensure that an early detection system for AIV is in place in poultry establishments.
The main objective of the current surveillance programme in wild birds, as per Decision 2010/367, is early detection of HPAI infection in wild birds, in order to protect poultry and safeguard veterinary public health. By implementing a testing regime of moribund or dead wild birds (passive surveillance), the surveillance programme aims to allow detection of HPAI viruses in wild bird populations prior to viral transmission to poultry/poultry establishments. Also, following guidelines described in Decision 2018/1136/EU, MSs are requested to identify and review the areas of their territory that are at particular risk for the introduction of HPAI viruses into poultry establishments and ensure that increased passive surveillance of wild bird populations is carried out in these ‘higher risk areas’.
From 1 January 2019, EFSA will take on the task to collate, validate, analyse and summarise the data generated by the surveillance activities on AI as regards of poultry and wild birds and to produce annual reports.
Here, a number of actions to be considered in order to optimise the collection, validation, analysis and summarisation of AI surveillance data in poultry and wild birds are described. This report represents a vision for the future of the collection and reporting of AI surveillance data to EFSA, with the actions mentioned in the report aimed to be implemented in a step‐by‐step manner. In the report, a distinction is made between the data that MSs will be legally requested to submit to EFSA (and referred to as ‘mandatory’) and those data considered important to be collated but for which no legal requirement exist, and that would therefore be submitted to EFSA by MSs on a voluntary basis; the later type of data are referred to as ‘desirable’ data. Further, and subsequent to consultation with MS representatives, a subsection describing the expected framework for the data collection that will take place in 2019 was added to the report for most of the sections (‘Expected framework for the 2019 data collection’). In this subsection, the data that EFSA would aim to receive in the first phase of the collection framework commencing in July 2019 are described (data collected in 2018 will still be submitted to the European Commission, with the annual report being produced by EFSA). Nonetheless and as mentioned above, it will be up to each MS to submit any non‐mandatory data that appears in this subsection. The data submitted by MSs will then be analysed by EFSA and the results from the analysis of the surveillance data will be summarised in a scientific annual report. Because of the ‘mandatory and voluntary’ aspect of this data collection, a non‐harmonised data set is expected in the initial phase of the collection framework. Because of this variability, and in order to avoid biases, only a descriptive analysis of the data provided will be presented initially in the annual report. Nonetheless, further analyses might be possible (via a number of pilot studies) in collaboration with a small number of MSs that are willing to submit more data than that requested in the legislation. The protocols to be used in these pilot projects will be developed in collaboration with these MSs. These projects will help to achieve some of the outcomes that are listed in Table 1 and further explained throughout the document. This framework has been developed by the EFSA standing working group on AI involving representatives from all Member States, Switzerland and Norway.
Table 1.
List of proposed actions, outcomes, methodological approaches and add‐on value to risk managers by population of interest: poultry (cells in blue colour), wild birds (cells in pink colour) and both populations (cells in grey colour)
| Proposed actions | Outcome | Methodological approach | Add‐on value to risk managers |
|---|---|---|---|
| EFSA: Collate and merge data on active and passive surveillance for wild birds and poultry (including ADNS data) | a) A greater knowledge of the infection dynamics of AI at population level in wild birds and poultry and the association between the dynamics in both groups | – Comparing results from both types of surveillance activities (passive and active) in wild birds and poultry in time and space |
– Better advice on how to prevent virus spread between wild birds and poultry – Better opportunities for increasing awareness and optimising prevention measures |
| MSs: Provide surveillance data including positive and negative results for poultry not aggregated but at an establishment level providing geo‐coordinates (this is done already for wild birds). If not possible initially, then EFSA would aim to receive data aggregated at monthly level and at NUTS 3* level | b) A description of temporal and spatial trends per MS and across MSs, by poultry production category | – Providing timeline graphs and maps for the surveillance results by poultry production categories | – Better advice provided, taking into account temporal and geographical patterns |
|
MSs: Submit detailed poultry population data at an establishment level on a yearly basis with geo‐locations for all the establishments (if not possible initially, EFSA would aim to receive data on the different poultry categories aggregated at NUTS 3 level) EFSA: In collaboration with MSs, to source/collate the best data set available on wild bird populations |
c) An understanding of the intensity of the surveillance strategies in both poultry and wild birds across MSs. Also, a better knowledge of the relative risk of the different poultry categories |
– Descriptive analysis of surveillance data taking into account the populations at risk – Estimation of the relative risk of infection with/detection of AI viruses in the different poultry production categories |
– Better interpretation of the results obtained from surveillance activities in different MSs as they can be better compared (standardised data) |
|
MSs: Use of updated list of target species when designing their surveillance programmes MSs: Use of new technologies to improve bird identification EFSA: In collaboration with MSs to source/collate data on migration routes of wild birds in each MS |
d) A more efficient surveillance strategy, and a greater knowledge of the entry pathway(s) of HPAI viruses via wild bird migration into the EU and the associated risk factors | – Descriptive analysis of additional data on wild birds provided by MSs (‘desirable’ data, such as migration routes of wild birds in specific MSs) that could lead to further/future risk factor analysis | – Better advice on risk mitigation strategies for the introduction of HPAI viruses via wild birds into poultry establishments |
|
EFSA: Compile data on the criteria used by each MS to determine at risk areas and other criteria used to design their surveillance program, as a once off event with updates when necessary EFSA: In collaboration with MSs to compile spatial information about the ‘at risk areas’ |
e) A better understanding of the criteria used by different MSs to design their poultry risk‐based surveillance and the selection of ‘at risk areas’. Also, a better understanding of the criteria used by different MSs to select the sick/dead wild birds tested for AI |
– Descriptive analysis of the risk factors and criteria used to determine ‘at risk areas/establishments’ where surveillance activities are carried out – Description of the weighting procedure – Quantification of the risk factors (non‐aggregated data) |
– Better assessment and weighting of risk factors associated with AIV infection in poultry – Better interpretation of the results obtained from surveillance activities in different MSs as the results can be standardised and contextualised, allowing comparisons between MSs |
| EFSA: Compile data on the sampling frame and sampling procedure to design the representative surveillance strategy. This will be done as a once off event with updates | f) A better understanding of the sampling frame chosen by MSs to obtain a representative sample for the poultry surveillance strategy | – Descriptive analysis of the methodology used by MSs to obtain a representative sample of poultry establishments | – Better interpretation of the results obtained from surveillance activities in different MSs as the results can be standardised and contextualised, allowing comparisons between MSs |
|
MSs: Provide details on the establishments tested as a result of a serology positive establishment MSs: Provide details on the results of further serology/virology tests carried out in each serology positive farm |
g) An earlier detection of LPAI viruses capable of efficient between‐farm spreading |
– Descriptive analysis of: number of serologically positive establishments, the number of serology positive establishments that are followed up, and the number of followed up establishments that test positive for virus identification – Description of the number of epidemiologically linked establishments (establishments with clear epidemiological link, or located within 1 km radius from the initial serological positive establishment) that are tested as part of follow‐up activities subsequent to the discovery of a specific serology positive result – Description of the number of the above establishments that test positive for serology/virology |
– Better advice on risk mitigation strategies to control spread within affected areas/production system |
| MSs: Provide details on the presence or absence of clinical signs or lesions observed in wild birds that test positive to H5/H7 AIV | h) An evaluation of the adaptation of HPAI virus to wild bird populations | – Descriptive analysis of the presence or absence of clinical signs or lesions observed in both the active and passive surveillance activities in wild birds | – Better advice in outbreak prevention in poultry establishments |
| EFSA: Review the usefulness of collecting and collating all the data above | i) An overall appraisal/qualitative assessment of the surveillance components at EU level | – Review of each of the individual methodological approaches mentioned above | – Assurance that the best scientific advice is provided to maintain the continuous efficacy of the surveillance programme at an EU level. Better contingency planning |
The scientific annual report produced by EFSA will provide an overview of the surveillance activities carried out at an EU level during the year previous to its publication. A descriptive analysis of the data showing the sampling, and the results of this sampling, in poultry and wild birds will be presented. The aim of the annual report will be to inform MSs (and other interested third parties) of the intensity of the AI surveillance taking place in the EU. Also, and following the gradual submission of the data considered ‘desirable’ by MSs to EFSA, this descriptive analysis could also be enhanced with further analysis that could help to provide better guidelines for surveillance, if required, given the constantly changing nature of the AI viruses.
The analysis of AI surveillance data from poultry and wild birds carry out by MSs is essential in order to monitor the AI situation at a MS level. This timely analysis carry out by MSs is crucial to allow risk managers to raise awareness, increase preparedness and ultimately deal with ongoing outbreaks. Likewise, the follow‐up of AI seropositive cases in MSs subsequent to an outbreak is also important; in some instances where the infection is still circulating in the flock or in the neighbouring flocks, epidemiological follow‐up of seropositive cases can allow containment/eradication of the disease/infection, and a proper understanding of the viral spread within the country (resulting in better contingency planning). The framework presented in this report does not aim to substitute the surveillance data analysis done by MSs, rather to complement this analysis by performing an annual analysis of the AI situation at an EU level. These annual reports do not aim to evaluate the mandatory AI surveillance system at MS level or the sensitivity of the surveillance programme (the compliance with the submitted surveillance plans by each MS will not be evaluated either). Although stated in Directive 2005/94/EC, it is not in the remit of this framework to assess AIV prevalence estimates. The estimation of sample size for surveillance purposes, as described in this directive, allows an analysis to be carried out in terms of demonstrating freedom of infection or detection of infection. In order to assess prevalence estimates, a larger number of establishments would need to be sampled.
2. Purpose of report and overview
The aim of this scientific report is to give an overview on how a number of proposed actions related to AI data collection, collation and reporting at a MS level (described in the following sections) could help to provide better guidelines for AIV surveillance and so to improve prevention of AIV outbreaks in poultry in MSs.
To better reflect the current AI situation at an EU level, and as emphasised by EFSA in 2017 (EFSA AHAW Panel, 2017), the reporting and analysing of AI data obtained through passive and active surveillance in poultry and, through passive surveillance in wild birds, should be carried out in conjunction with each other. Currently, passive surveillance data in poultry, collected via the Animal Disease Notification System (ADNS) (European Commission, online) are not presented in the report. Over time, EFSA will aim to incorporate disease outbreak data from ADNS in the annual surveillance reports. Also, with the collaboration of MSs, EFSA will identify the best source of wild bird population data as well as data on migratory routes for the relevant wild bird species. The collation of data currently collected through several data sets (see Section 3) would help to generate a more robust scientific base to underpin risk management actions. These actions are described in Table 1 and in Section 3 of this report. A number of suggested actions related to the way AI data are reported by MSs will also be described in this scientific report, providing also an explanation on how each of these specific actions will help to achieve the desired outcomes.
Although passive surveillance of wild birds is considered the most effective method for early AIV detection associated with high mortality, EFSA (EFSA AHAW Panel, 2017) highlighted the need of targeted active wild bird surveillance through virology testing (swabbing) combined with enhanced passive surveillance at a few priority regions in the EU (and also outside the EU). This would enable to detect the presence of circulating AIVs, when these viruses do not cause massive mortality among wild birds. As the number of wild bird species that can potentially carry AIVs is large, and the overall prevalence of AIV infection in wild birds is low, this targeted surveillance should be designed to take into account AIV epidemiology in wild birds and other ecological factors (most susceptible species, migratory patterns, breeding sites, seasonal trends etc.). The data collected through the addition of targeted serological/virological surveillance carried out at critical locations within EU, would also help provide a much clearer picture of the AI infection within the EU.
In Table 1, the actions described above and a number of proposed actions and outcomes that would result from the addition of data collected/collated by these actions (‘desirable data’) to the currently collected surveillance data, are presented. In this table also, a description of the methodological approaches to achieve each of these outcomes and the add‐on value to risk managers has been provided. A more detailed explanation of these proposed actions and outcomes is provided in Section 4.
3. AI surveillance programme
3.1. Current collection of AI surveillance data
All co‐funded EU surveillance activities are reported to the European Commission database for AI surveillance. This is a separate system to the ADNS. However, there can be overlap between the two databases which could be used to provide additional context to surveillance activities.
AI data on poultry active serological surveillance activities are submitted to the Commission aggregated at NUTS 2 level; the data set includes the number of both positive and negative serological results. Only if/when further epidemiological investigation of poultry serological surveillance discovers active notifiable disease (i.e. in case of a virological positive finding) the outbreak is reported also to the ADNS. No poultry passive surveillance data are included in the Commission surveillance data set. Data on outbreaks detected in poultry establishments (establishments where H5/H7 viruses have been identified) discovered as part of both passive and active surveillance activities are present in the ADNS data set (Figure 1).
Figure 1.
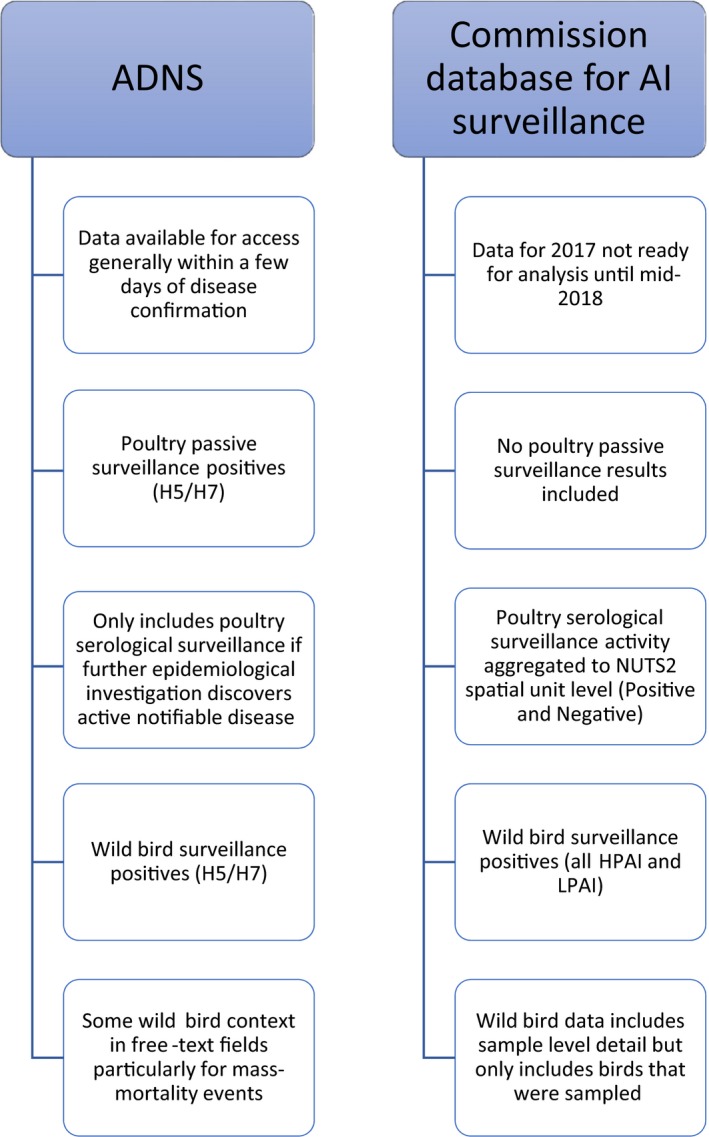
Diagrammatic representation of the data collected by ADNS and the Commission database for AI surveillance
The Commission surveillance database collects wild bird surveillance data on all HPAI and LPAI positives as well as negative results at sample level. However the notification of any LPAI in wild birds is no longer a legal obligation according to the updated list shown in ANNEX 1 to Directive 82/894/EEC on ‘Diseases which are subject to notification’. Only wild bird surveillance data on birds where HPAI H5/H7 virus has been identified, are included in ADNS. The ADNS data set also has some free‐text fields where additional information such as species and data on mass‐mortality events can be reported (Figure 1).
There are no shared identifiers between ADNS and the Commission surveillance database. Dates and geospatial information can be used for cross‐referencing in sporadic cases but not during epizootics. There are no existing links to molecular analyses in either database. ADNS reports at an event level while the Commission surveillance database reports at a sample level for wild birds and aggregated at NUTS 2 level for poultry.
3.2. Case definitions
3.2.1. Poultry
For the purpose of the annual EU report on AI surveillance, a poultry establishment is considered positive if at least one sample from that establishment tests positive for H5/H7 AI on either serology (seropositive establishment) or polymerase chain reaction (PCR) or viral isolation (confirmed establishment) (the word ‘holding’ has been replaced throughout the document by the word ‘establishment’ in order to align the phraseology used in this document to Regulation (EU) No 429/20165).
3.2.2. Wild birds
For the purpose of the annual EU report on H5/H7 AI surveillance, a wild bird is considered a case if at least one sample from that bird tested positive on PCR or viral isolation.
3.3. Test methods used for AI surveillance
Laboratory tests used for AI surveillance shall be carried out in accordance with guidance provided in the Diagnostic Manual for AI approved by Decision 2006/437/EC6 . However, if a MS wishes to use laboratory tests not laid down in the Diagnostic Manual nor described in the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of the OIE, those tests must first be deemed fit for purpose by the EURL on AI, based on validated data.
There are three different types of test methods used by MSs in relation to AI surveillance: serology, PCR and virus isolation. Tests that can be reported are: enzyme‐linked immunosorbent assay (ELISA), haemagglutination‐inhibition (HI) test for H5, HI test for H7, agar gel immunodiffusion test (AGID), virus isolation test, M gene reverse transcription polymerase chain reaction (RT‐PCR) test, H5 RT‐PCR test and H7 RT‐PCR test.
With regard to surveillance in wild birds, reporting of findings is done on sample type: cloacal swabs, fresh faeces, tracheal or oropharyngeal swabs, tissue or others. The reporting of these findings is often important in outbreaks as it can give some information into the routes of viral excretion, which can differ between viruses. In poultry outbreaks where the shedding route of a specific virus is known, testing can be reduced to one type of sample (i.e. in large outbreaks when there is reduced laboratory capacity), making the subsequent surveillance more efficient.
4. Relevant elements of the reporting of AI surveillance data
This chapter describes not only the data that MSs are obliged to report based on Commission Decision 2010/367/EC (indicated as ‘mandatory’) but also the additional data that would be desirable to receive from MSs (‘desirable’), in order to optimise the results from the collection, collation and analysis of surveillance data. As the level/specificity of the desirable information to be collected from MSs will vary, this document should also reflect how the outcomes will vary depending on the type of data provided by most MSs. The data classified as desirable in the views of the expert opinion, were discussed with MS representatives in terms of practical aspects and the added administrative burden, and subsequently reviewed. After discussion with MS representatives, a section including the type of data that EFSA would like to receive from MSs starting from 2019 (‘Expected framework for the 2019 data collection’) was then included. Nonetheless and although EFSA will encourage MSs to submit these data, as explained in Section 1.2, it will be up to MSs to provide any data considered desirable.
4.1. Population under surveillance
4.1.1. Poultry
Mandatory
Poultry is defined in Council Directive 2005/94/EC as all birds that are reared or kept in captivity for the production of meat, eggs for consumption, other products and for restocking supplies of game birds, or for the purposes of any breeding programme for the production of these categories of birds. It is relevant to discriminate gallinaceous birds (chickens, turkeys, guinea fowl, pheasants, partridges, quails) and ratites7 from domestic waterfowl (ducks, geese), and mallards8 raised in captivity given their different susceptibility to AI. MSs are requested following Decision 2010/367/EU to carry out and report the results of active surveillance programmes for AI in poultry. Domestic birds kept for production but with no commercial purpose9 are also covered by the AI surveillance if a specific epidemiological reason justifies sampling this population. Testing and reporting data on zoo, pet, or captive birds are not mandatory. For surveillance purposes, reporting common group names of gallinaceous species rather than species’ name is sufficient.
Desirable
Although information on poultry population (description, categories, number of holdings/birds and distribution maps) is requested from MSs when submitting information on their AI surveillance programme (Annex IV of the programmes for eradication, control and surveillance of animal diseases and zoonosis submitted for obtaining EU financial contribution), this information only refers to areas where the sampling takes place (e.g. for MSs carrying out risk‐based surveillance, poultry population data are only reported for the NUTS 2 areas at risk). It would be desirable that MSs provided EFSA (e.g. annually) with an estimate of the total poultry population, by poultry production category aggregated at NUTS 3 level or not aggregated. This estimate of the total poultry population could be based on maximum carrying capacity for each of the poultry establishments if no numbers on the total population were available. If the estimated total poultry population was available for analysis, EFSA would be able to assess the intensity of the surveillance programmes in different MSs (outcome c)), and with that, it would be able to better assess potential risk factors for both entry and spread of AIV into poultry units (outcomes e) and i)). It would be desirable that species‐specific names for domestic ducks were reported, e.g. Muscovy ducks (Cairina moschata), Pekin ducks (Anas platyrhynchos domestica) and mulard (mule ducks: cross breeding of Cairina moschata with Anas platyrhynchos domestica), as there are differences in the way they are bred, resulting in differences in terms of relevant risk factors. As an example, artificial insemination used for cross breeding in mallard ducks has been shown to be a risk factor for AIV spread between breeding duck flocks (Duvauchelle et al., 2013). Also, it has been shown that the pathogenicity of H5N1 AIV strains may differ between Muscovy and Pekin ducks, although similar infection rates were observed (Phuong et al., 2011; Cagle et al., 2012). By having a more detailed description of the species of domestic ducks under surveillance, a greater knowledge of the risk factors associated with AIV infection in poultry (outcome e) in Section 2), as well as a better understanding of the transmission patterns within poultry (outcome a) in Section 2) can be achieved.
Expected framework for the 2019 data collection
EFSA will not aim to receive poultry population data in 2019. From 2020 onwards, a framework for collecting poultry population data will be available; EFSA will then encourage MSs to submit estimated poultry population data at NUTS 3 level (or higher NUTS level) once a year for the different poultry production categories. Also, as part of a pilot project, EFSA will work with a small number of countries that voluntarily want to submit these data (aggregated or not aggregated) to EFSA in 2019, in order to explore the different outputs that will result from the addition of these data. The initial data model for AI surveillance will be able to receive data on species‐specific names of domestic ducks as well as general species names.
4.1.2. Wild birds
Mandatory
MSs are requested to report both the common name and the Latin name of the relevant ‘wild bird populations under surveillance’, as well as the EURING code. As some species of wild birds, in particular migratory water birds, have been shown to be at a higher risk of becoming infected with, and transmitting HPAI viruses, the species to be targeted by MSs surveillance programmes were described in Decision 2010/367/EC (i.e. ‘target species’ (TS)). Also, in Annex IV of the programmes for eradication, control and surveillance of animal diseases and zoonosis submitted for obtaining EU financial contribution (referring to AI surveillance sampling in poultry and wild birds), it is requested that MSs provide an estimation of the local and/or migratory wildlife population relevant to each MS (estimated population numbers).
EURING (http://https://euring.org/) has a standard set of codes for avian species that are used by bird‐ringing programmes throughout Europe. The use of these codes facilitates easy data transfer between programmes and provides researchers with cross‐European data sets in a standard format. Each species code consists of five numerals. For example, the code for mallard (Anas platyrhynchos) is 01860, and for the peregrine falcon (Falco peregrinus) is 03200.
Desirable
As requested by the legislation, MSs submit their surveillance plans based on the target species described in the above Decision. Due to the nature of the surveillance in wild birds (passive surveillance of dead and moribund wild birds), providing very specific surveillance plans is not always feasible, nonetheless providing details such as species to be targeted and thresholds for testing procedures in case of mass mortality events could be within MSs capabilities. An updated list of wild bird species to be targeted was included in the EFSA AI monitoring report for September–November 2017 (Table 2; (EFSA, 2017)). It would be desirable that MSs use this updated list of target species to define their future surveillance programmes. Also, although estimated population numbers of the target species are requested via the forms for financial contribution, this information is not always available within the veterinary services, resulting in a small number of countries providing these numbers (sometimes aggregated data on total number of wintering water birds are provided). It would be important for EFSA to obtain information on the estimated (as these numbers are highly changeable) species population numbers both for resident and migratory birds (preferably, distinguishing between population numbers at autumn migration, wintering and spring migration; available in several MSs from bird counting activities). Collating information on the estimated population of the three suggested categories, would allow EFSA to achieve outcome c) in Section 2 (an assessment of the effort of the surveillance strategies in different MSs). This would also help to design/refine more efficient sampling strategies targeted per wild bird species per MS, as described in outcome i).
Table 2.
Current reporting of the poultry production categories at NUTS 2 level as defined in Decision 2010/367/EU (dark grey) and proposed extra types to accommodate for differences across MSs (light grey)
| Poultry production categories | |
|---|---|
| Defined in decision 2010/367/EU | Laying hens |
| Free range laying hens | |
| Chicken breeders | |
| Turkey breeders | |
| Duck breeders | |
| Geese breeders | |
| Fattening turkeys | |
| Fattening ducks | |
| Fattening geese | |
| Farmed game birds (gallinaceous) | |
| Farmed game birds (waterfowl) | |
| Ratites | |
| Broilers* | |
| Backyard flocks* | |
| Proposed | Growers |
| Muscovy ducks breeders | |
| Muscovy ducks fattening | |
| Pekin ducks breeders | |
| Pekin ducks fattening | |
| Mallard ducks (breeders and/or fattening) | |
| Ducks for foie‐gras (mule ducks) | |
| Geese for foie‐gras | |
| Others | |
* These poultry production categories may be included under specified exceptional circumstances.
A number of methods that MSs could avail to enhance their abilities to correctly identify wild birds, are listed below. The correct identification of bird species sampled under passive surveillance could be increased by taking pictures of birds during the necropsy and submitting them to ornithologists. Also, the use of mobile phone applications submitting pictures of sampled wild birds would help to increase the reporting of the correct species name. The Merlin Bird ID App (http://merlin.allaboutbirds.org/) is an example on assistance in determining the wild bird species, designed by the Cornell Laboratory of Ornithology. An interesting system for including and identifying wild bird species has been developed in the Danish Veterinary and Food Administration (DVFA), called FugleinfluenzaTip (‘Bird flu Tip’). This mobile application allows taking pictures of wild birds (moribund or found dead) that are automatically re‐directed to the animal health authorities which will follow‐up that event. Finally, for birds that are difficult to identify to species visually, DNA barcoding techniques on tissue and faecal samples could be used for host species identification (Lee et al., 2010). Correct species identification which is problematic, given the large number of unspecified wild birds reported in the database on AI surveillance can help in assessing which wild bird species are involved in the epidemiology of AI, both in the entry pathway of AI viruses via wild birds in the EU (outcome d), and in the possible adaptation of HPAI to wild bird populations (outcome h)).
Expected framework for the 2019 data collection
MSs are encouraged to adapt their surveillance programmes for wild birds in accordance with the updated list of target species mentioned above, despite this list not having been updated yet in the legislation. In 2019, EFSA aims to initiate a project to collate data available on population numbers for resident and migratory birds. Identifying the key relevant national organisations in each MS would be essential; for that EFSA will aim to get MSs’ support in identifying these organisations. EFSA will also assess the possibility of availing existing data from other international organisations. An evaluation of the type of data to be collected and the best methodology for collating and analysing these data will be carry out by EFSA.
4.2. Timeframe of the surveillance data
4.2.1. Time unit
Mandatory
MSs need to report AI surveillance data on poultry and wild birds every 6 months, with the first data submission compiling data collected from January to June and the second data submission from July to December. The surveillance data for poultry are aggregated over the 6 months of the relevant reporting period. In terms of wild birds, MSs are requested to provide data on wild birds with specific dates of sampling (no aggregation on time/animal level is allowed). Also, MSs are requested to indicate whether or not they plan to enhance surveillance of wild birds during specific sampling periods, e.g. during autumn migration. In poultry, positive serological and virological findings of H5/H7 have to be reported at an establishment level.
Desirable
From an epidemiological analysis perspective, it would be ideal that the surveillance data regarding poultry are not reported at an aggregated level, but provided with dates of individual sampling. Nonetheless, and as this might not be feasible for a large proportion of MSs (at least at the start of this data collection framework), EFSA will encourage MSs to submit data that are not aggregated more than at a monthly level. Although the added value of moving from reporting data aggregated at 6‐month interval to a 1‐month interval will vary depending on the poultry production category (life span), for short‐lived poultry production categories such as fattening ducks, it would be quite useful. The prevalence of AI fluctuates across seasons; hence, providing data aggregated at a monthly level, would allow studying more accurately the relationship between AI infections in domestic birds and the AI wild bird surveillance data. Having access to this level of detail would improve the quality of the data analysis, which would help in providing better guidance on making AI surveillance more efficient as described in outcomes a), b), and d) in Section 2.
EFSA has initiated a project, the SIGMA project10 , which aims to automate data submission from MSs to EFSA. On a voluntary basis, MSs that agree to be part of this project will be able to report data on AI surveillance (as well as data on many other diseases/species) in an automated manner. Linking MSs’ databases to EFSA's Data Collection Framework would make feasible to report the exact sampling day without putting an additional burden on to the MSs once the system is implemented. The product of this project will be available to all MSs but it will be up to each MS to decide, on a voluntary basis, whether or not they want to take on this approach. By collecting these automated data it would be possible to link surveillance data with environmental data, e.g. the weather conditions directly influencing the migration of wild birds (Ottaviani et al., 2010; Reperant et al., 2011) and the survival of AIV in the environment (EFSA AHAW Panel, 2017). This would help EFSA to attain outcome a) in Section 2, and contribute to achieving outcomes b) and d) of the referred section.
In wild birds, it would be useful to have information on the number of birds of each species during the biologically relevant periods: breeding period (summer), autumn migration, wintering period and spring migration. This is part of the information requested via the forms for financial contributions, but not all MSs give specific details. If this information was available from all MSs, it would allow EFSA to better predict at risk periods of AIV introduction into poultry flocks.
Expected framework for the 2019 data collection
The EFSA data model for collecting AI surveillance data in 2019 will be ready to receive non‐aggregated negative surveillance data for both poultry and wild birds (data provided with precise sampling dates), but also aggregated data from countries that are not in a position to submit the non‐aggregated poultry data to EFSA. EFSA will encourage these MSs to submit negative poultry surveillance data aggregated at monthly level.
4.2.2. Submission frequency
Mandatory
MSs have to submit the AI surveillance data covering the first 6 months of the year by the 31 of July and data covering the second half of the year by the 31 of January of the following calendar year with confirmation of accuracy of data uptake in the Commission database via the online ‘infoview tool’ (validation process) until the 15 of March of that year.
Desirable
Although MSs will not be required to submit data more than twice a year, the implementation of the SIGMA project will give MSs the possibility to automate the submission of the surveillance data (as described in Section 4.2.1). Also, if MSs choose to do so, they will be allowed to make partial submissions (as it is the case currently), as long as the full data set referring to the six month period is submitted by the required deadlines.
4.3. Relevant epidemiological unit of the surveillance system
4.3.1. Poultry
Mandatory
The relevant epidemiological unit to report surveillance results is the establishment. Regulation (EU) 2016/429 defines an establishment as any premises, structure, or, in the case of open‐air farming, any environment or place, where animals or germinal products are kept, on a temporary or permanent basis, except for: (a) households where pet animals are kept; (b) veterinary practices or clinics. This definition does not include slaughterhouses, means of transport, quarantine facilities, border inspection posts and laboratories authorised by the competent authority to hold AIV.
Epidemiological data collected on the establishment aggregated at NUTS 2 level such as poultry production categories, are defined in Decision 2010/367/EU. Based on this decision, poultry establishments are divided into 14 categories (12, plus backyard and broilers under exceptional circumstances) as shown in Table 2 in dark grey colour.
Desirable
It would be ideal that MSs report data on AI active surveillance in a non‐aggregated manner (time or space) by providing specific geo‐coordinates for individual establishments (the advantages associated with providing specific geo‐coordinates for individual establishments are discussed in Section 4.4.1). If data were to be provided at an establishment level, then the field ‘Poultry Production Categories’ could be divided into two separate fields: ‘Species’ and ‘Production category’ (the categories within these two fields will be described in a ‘Data Dictionary’ that will be circulated with the initial data model for the AI surveillance data collection). Although these two fields would be ‘new’ in the surveillance data set, they are already used to report HPAI poultry outbreaks to EFSA as reflected in the monitoring reports produced by EFSA (EFSA, 2018). Having the same fields in both data sets would ensure that epidemiological analysis could integrate data from, active surveillance, and passive surveillance (outbreak reports), helping to achieve outcome a) in Section 2. Also, by separating poultry production category into two fields, EFSA would be able to collect data on species not specified in this field ‘(e.g. instead of having ‘ratites’ as a group, it would be possible to distinguish between establishments containing ostrich, rhea, etc). This could be relevant in order identify other plausible risk factors associated with AIV introduction into poultry units (outcome e)).
Nonetheless, as data on AI active surveillance are likely to be reported initially in an aggregated manner by some MSs (ideally aggregated monthly and a minimum of NUTS 3 level11), the field defined by legislation as ‘Poultry Production Categories’ (Table 2, dark grey colour) will still be the most appropriate field to be used by MSs when reporting aggregated data. Within this field, a number of extra categories are included (as shown in light grey colour cells) to the categories already described in the legislation, in order to allow for differences in terms of poultry production systems in the different MSs. New fields added to the data model will be defined in collaboration with MSs and described fully in the Data Dictionary accompanying the data model. For instance, by the term ‘growers’ we refer to poultry establishments (different species) in which birds are reared for only part of their productive cycle, to be then sold to other farms belonging to the rural sector where birds will end their production cycle for meat/eggs. This definition will lately be refined following communication with MSs. MSs will be able to report on the categories presented in light grey colour in Table 2 when relevant. By including these extra categories, MSs will be able to report data in a way that mirrors their national data collection framework.
In line with the above, for MSs reporting aggregated surveillance data, the total number of poultry establishments of the different poultry production categories for each of the NUTS levels could be provided. For MSs reporting non‐aggregated surveillance and total population data, the species and production category present in each establishment (in the total population) could be reported.
In both instances, for aggregated and non‐aggregated data, it would also be useful that MSs report on the accessibility of poultry to outdoor facilities. If reported data are not aggregated, this would be reported for each establishment as ‘yes/no’, while for aggregated data, the total number of each of the poultry production categories that had outdoor access could be reported (this is already requested by legislation for laying hens12 , 13).
Expected framework for the 2019 data collection
The data model for collection of AI surveillance data for poultry in 2019 will be ready to collect non‐aggregated data at a sample or establishment level, as well as aggregated data. In both instances, when data are submitted in an aggregated or non‐aggregated manner, the field ‘poultry production categories’ will have to be filled in by MSs as requested by the legislation (Table 2, dark grey). Aside, those MSs aiming to submit information using the extra types shown in light grey will be able to do so. The types of poultry production categories in the proposed section will be further reviewed with MSs. MSs will be encouraged to start collecting/submitting data on the indoor/outdoor poultry access.
4.3.2. Wild birds
Mandatory
The most practical epidemiological unit to report wild bird surveillance is the animal level. The information on the animals tested for AI under the surveillance framework are provided at sample level (specifying the sample matrix), but subsequently aggregated at animal level which is the epidemiological unit. A unique bird identifier has to be provided for each individual bird.
Desirable
Having data on the negative and positive results as already requested by legislation facilitates a better understanding of the susceptibility of the different species. The ratio of the number of positive over the number of tested wild birds will be used to review and update the list of wild bird species targeted for AI surveillance. This information could also be used to guide species‐specific sampling efforts (outcome c) in Section 2). It would also help in the evaluation of the adaptation of HPAI virus to wild bird populations (outcome h)) as well as the identification of transmission patterns (outcome a)) as described in Section 2.
Expected framework for the 2019 data collection
MSs will be able to continue submitting data as they do to the Commission currently.
4.4. Geographic coverage
4.4.1. Geographic unit for poultry
Mandatory
MSs have to report poultry surveillance data at the minimal requested level of NUTS 214. These highly spatially aggregated data make interpretation of data analysis results across MSs very difficult, especially when different surveillance strategies are used. Only an analysis of trends in time per MS can be done. An interpretation of poultry surveillance data at EU level can best be done when reporting is done at higher resolution (preferably establishment level or aggregated at a maximum NUTS3 level) and denominator data (i.e. total poultry population data) are available.
Desirable
Reporting specific coordinates of the sampled establishments would be the ideal scenario from an epidemiological perspective, as it would allow a deep analysis of the data provided by MSs. Providing data aggregated at NUTS 3 level for all establishments tested, would be the second best alternative. Reporting AI surveillance data at NUTS 3 level would allow comparison of surveillance results in poultry and wild birds (outcome a) in Section 2), also a better assessment of the risk factors and criteria used for risk‐based surveillance (also for representative surveillance) in poultry (e.g. factors related to the proximity of wetlands or to the density of poultry population around the sampled establishment) helping to achieve (outcomes e) and f) in Section 2). Also, it would be important that total poultry population for each of the poultry production categories in each MS are reported either non‐aggregated or aggregated (at least) at the same level of aggregation (e.g. NUTS 3) as that used for reporting surveillance data; this would allow a better description of temporal trends (outcome b)) and a better understanding of the intensity of the surveillance programme (outcome c)). EFSA, by putting in place adequate data sharing agreements, will ensure confidentiality of the submitted data based on EU legislation.
Expected framework for the 2019 data collection
EFSA will encourage MSs to submit data non‐aggregated or aggregated using NUTS 3 (or NUTS 4 if more convenient for the MS) geolocation data. Nonetheless, MSs that are not in a position to report NUTS 3 level data will be identified, and modifications to the EFSA data model will be made to be able to report NUTS 2 location data (as the minimum legal requirement).
4.4.2. Geographic unit for wild birds
Mandatory
The location of negative and positive wild bird samples is requested and already reported by most MSs (see Section 4.3.2), nonetheless, this is done in a heterogeneous manner, as different level of aggregation can be selected of the sampling event (MSs can include Lat/Long coordinates, NUTS up to 4/5 (LAU 1/2) level or Town name) and in some instances is difficult to estimate the location with precision.
Desirable
MSs will be encouraged to continue providing geographical information on both positive and negative samples, enabling in this way the identification of the most at‐risk areas for AI in wild birds (outcome e) in Section 2). Improving species identification and standardisation of the way geographical information are reported would facilitate data analysis and would improve quality of results. Also, it would be useful that information on the geographical range of the wintering area/spring migration route/autumn migration route or (summer) breeding area from which the sampled birds were collected was provided by MSs, as this information cannot be inferred from the data already collected. This information would only have to be submitted once. This information will allow EFSA to obtain greater knowledge of AI dynamics in wild birds (a)), a better understanding of the intensity of surveillance in wild birds (c)), greater knowledge of the entry pathways of HPAI viruses via wild bird migration into the EU, and associated risk factors (d)), better understanding of the criteria used by MSs to design their wild bird surveillance activities (e)), and overall evaluation of surveillance methodologies (i)).
Expected framework for the 2019 data collection
It would be important that MSs provide information on the precision of the geo‐location submitted to EFSA (whether the coordinates refer to the exact location where the bird was found, the centroid of the NUTS 4/5 value, etc).
4.5. Types of surveillance
4.5.1. Passive surveillance
4.5.1.1. Poultry
Mandatory
AI poultry outbreaks detected under passive (or active) surveillance are notified via ADNS.
Desirable
It is desirable that the designed framework for AI data surveillance allows all information regarding passive and active surveillance to be collated together. Adding passive surveillance data to the data collected from active surveillance, would give a much clearer picture of the epidemiology of AIV. Also, it would be desirable that MSs differentiate whether specific outbreaks were detected as a result of active or passive surveillance or as a follow‐up from a previous outbreak. This information could help to assess the intensity of the surveillance activities (outcome c) in Section 2). This will also help to understand and quantify the follow‐up activities carried out by MSs, helping to achieve outcome g) in Section 2.
Expected framework for the 2019 data collection
EFSA will investigate the alternatives to collate data obtained from ADNS into the surveillance annual report. The EFSA data model for collection of surveillance data will then be able to differentiate between the different types of surveillance.
4.5.1.2. Wild birds
Mandatory
Passive surveillance of moribund wild birds or wild birds found dead is deemed compulsory in all MSs. Outbreaks of HPAI in wild birds detected under passive or voluntary active surveillance are notified via ADNS (where details on mass mortalities could be reported) and through the surveillance data set (where species information and laboratory results are reported). See also Section 4.5.3.2 on risk‐based surveillance in wild birds.
Desirable
It would be desirable that MSs select the wild bird species targeted in their surveillance programme to match the species in the ‘updated list of target species’. Understanding the underlying population of the target species would be also important in order to assess the intensity of MSs’ surveillance programmes (outcome c) in Section 2). This would also help to improve the early warning as better details of the entry pathways and ‘areas at risk’ would be available (outcome d)). MSs could use comprehensive pathological examination in order to properly assess the pathogenicity of HPAI virus in the different wild bird species. Detecting HPAI in a dead bird does not necessarily mean that the bird died from HPAI: a comprehensive pathological examination is needed to reach this conclusion. If several birds of one species are found dead together on the same day, it is recommended to perform such an examination and rule out other causes of mortality. This would help to determine the pathogenicity of the virus and the susceptibility of species for HPAI.
It would also be advisable to report the age categories (juvenile, adult) of birds sampled under the surveillance framework, as juveniles are more susceptible to be ill after AI infection. Most bird identification books (e.g. Birds of Europe: Second Edition by Lars Svensson, Dan Zetterström, and Killian Mullarney; Princeton University Press, 2010) provide information on how juvenile birds can be distinguished from older birds. Determination of the age category of a bird is species‐dependent, and best performed in consultation with an experienced birder. For example, the age category of ducks can be assessed via inspection of plumage (including wing and tail feathers), eyes, and beak (http://www.oncfs.gouv.fr/Espace-Presse-Actualites-ru16/Guide-de-determination-du-sexe-et-de-lage-des-canards-ar1790). Reporting the results from the pathological examination and the age categories will help to understand the adaptation of the virus to the species (outcome i) in Section 2).
Expected framework for the 2019 data collection
As described in Section 4.1.2, MSs are encouraged to adapt their surveillance programmes for wild birds in accordance with the updated list of target species mentioned above, despite this list not having been updated yet in the legislation. Also in 2019, EFSA will initiate a project to collate data available on species population numbers for resident and migratory birds.
4.5.2. Active surveillance
4.5.2.1. Poultry
Mandatory
All MSs are requested to submit information on the active surveillance carried out in their respective countries under their national surveillance programme to the European Commission twice a year as explained in Section 4.2.2. The information is submitted aggregated at least at the NUTS 2 level and for each poultry production category. The total number of establishments, total number of establishments sampled and the number of establishments that tested sero‐positive and sero‐negative (respectively) at the screening tests are reported. For those establishments that turn out positive at the screening test, further information is collected (establishment identifier, NUTS code level, poultry production category, serology, PCR and virology results and status of the epidemiological follow‐up).
Desirable
In some MSs, apart from the surveillance activities performed under the national surveillance programme, parallel surveillance activities might be carried out by private companies and/or governments. Nonetheless, the accessibility of these data might be difficult, even for MSs (i.e. data from private industries, monitoring programmes of ‘local authorities’, and research activities). Ideally, access to the data collected from these extra surveillance activities could help to have a better understanding of temporal trends. Also a good understanding of the intensity of the surveillance programmes in MSs, helping to achieve the objectives listed in Section 2: b), c) and f).
Expected framework for the 2019 data collection
The data collection model for AI surveillance data will be able to receive data on active parallel surveillance activities ongoing in some MSs. EFSA will encourage the submission of these data on a voluntary basis.
4.5.2.2. Wild birds
Mandatory
Some MSs, apart from carrying out the requested passive surveillance programme in moribund and dead wild birds, also have an active surveillance programme in place (i.e. testing of cloacal and oropharyngeal swabs of wild birds with no clinical signs of disease for the presence of AIV). Reporting of active surveillance results is done by MSs on a voluntary basis. A positive case (HPAI virus or H5/H7 LPAI virus) has to be reported, independently on whether the positive case was discovered as part of the active or passive surveillance programme.
Desirable
Currently, there are limited data on the detection of HPAI in apparently healthy wild birds to guide sampling under active surveillance. As explained in Section 1.2, because the number of wild bird species that carry AI is high and the overall prevalence of AI infection in wild birds is low, it is necessary to make full use of the knowledge of AI epidemiology in wild bird populations in order to increase the chance of AI detection. In the same way a target list of wild bird species for passive surveillance has been developed (see Section 4.1.2), available information from active surveillance and research on AI in infected wild birds that do not show any symptomatology, should make possible to develop a similar list of wild bird species for active surveillance. Besides species’ names, this list also should include age category, season, and locations where the sampling took place. As for passive surveillance (see Section 4.5.1.2), the reporting of the age category of sampled animals (juvenile, adult) would allow a better analysis of the surveillance results. Besides testing oropharyngeal and cloacal swabs for AIV, there are some programmes where sera are collected from apparently healthy wild birds for detection of anti‐AIV antibodies. The results of this serological analysis can provide information on past contact of wild birds with AI viruses.
For the reasons above, it would be recommended that results of active surveillance conducted in healthy wild birds continue to be reported on voluntary basis. Also, data collected by other research institutions could be retrospectively submitted to EFSA in order to have historical analysis of larger data sets.
Expected framework for the 2019 data collection
The data collection model for AI surveillance data will be able to receive data on active parallel surveillance activities ongoing in some MSs. EFSA will encourage the submission of these data on a voluntary basis.
4.5.3. Risk‐based surveillance
4.5.3.1. Poultry
Mandatory
Active surveillance in poultry can be either based on a representative sample of establishments in a MS (see Section 4.5.4) or region thereof, or risk based. The advantage of risk based sampling is that it focuses on those farms that are at higher risk for the introduction of AIV, and consequently, while including the same number of farms as in representative sampling, the likelihood of detecting antibodies against AIV is higher. Phrased differently, in case risk based sampling fails to identify antibodies against AIV, the likelihood that this region has been free from introductions is higher than in case of a representative sample. Decision 2018/1136/EU describes several criteria and risk factors for introduction (e.g. proximity to water bodies) or spread (e.g. area with high density of poultry) to be used by MSs in the design of a risk based surveillance programme. However, MSs can also use criteria and risk factors beyond the ones mentioned in the decision, as long as they are duly indicated and justified in the surveillance programme. Furthermore, the level of targeting must reflect the number and local weighting of risk factors present on the poultry establishment. Information on the criteria and risk factors considered by each MS when designing their risk‐based surveillance is requested via the forms for obtaining EU financial contribution.
Desirable
Ideally, the weighting of risk factors would be based on quantitative scientific evidence (Bouwstra et al., 2017; Gonzales et al., 2017), however as indicated in the 2017 EFSA opinion, such evidence is sparse across Europe. In 2016, the Annual Report on surveillance for AI in poultry and in wild birds in Member States of the European Union showed that 10/28 MSs performed risk based surveillance (Animal and Plant Health Agency, 2017). However, as shown in this report (table 20), hardly any MS provided the weighting of these risk factors, and none gave the number of individual establishments with and without these risk factors. This makes comparison of outcomes in terms of prevalence/occurrence or evidence of absence, between these 10 MSs, very difficult. Assuming that the criteria and risk factors used by these MSs are valid (meaning that they are indeed associated with a higher risk of introduction or spread), all that can be said is that the observed prevalence/occurrence is an overestimation of the prevalence in the overall population, or the likelihood of freedom of AI in case of absence of positives findings is higher than in case a similar representative sample had been collected. Assuming that MSs will use the similar risk based surveillance procedure from year to year, results can be compared within each MS from year to year to identify trends. However, comparing results between MSs would lead to a biased conclusion.
It would be important that a general description of the methodology used to determine the presence/absence of a given risk factor (e.g. poultry dense area, proximity to a water body) in each MS carrying out risk‐based surveillance was available to EFSA. This would allow EFSA to compare and understand better the results from different risk‐based surveillance strategies across MSs (description of risk factors).
Also, it would be ideal that for MSs reporting non‐aggregated data, information on the risk factors present at establishment level were made available to EFSA. If that information was available, EFSA would be able to provide MSs with a quantification of the risk factors involved in AIV transmission from wild birds to poultry, or between poultry units, allowing a weighting of these risk factors (assuming data from farms with and without the risk factors are available). This information would allow EFSA to provide a better understanding of the intensity of the surveillance programme, and an assessment and weighting of risk factors associated with AIV infection in poultry, helping to achieve outcomes c) and e), respectively.
As non‐aggregated data for AI active surveillance might not be available in the short future, another alternative in order to be able to provide a weighting of risk factors, would be for MSs to provide the total number of each poultry production category establishments with and without a specific risk factor (e.g. at NUTS 3 level). Then, quantification and weighting of these risk factors would also be feasible.
Expected framework for the 2019 data collection
Information on the risk factors used for the design of risk‐based programmes is reported by MSs to the Commission via the forms for obtaining EU financial contribution. This information, if standardised, could be useful to EFSA in order to allow better interpretations of the results from the surveillance data analysis. EFSA will aim to assess the best way of collating this information in a standardised manner. For that, EFSA will engage with MSs’ representatives to seek clarification (if needed) on the information provided in the financial forms. Once this information is gathered, EFSA would aim to receive updates from MSs on an annual basis, if changes are made to their risk‐based programme in terms of risk factors and the weighting of these risk factors.
4.5.3.2. Wild birds
Mandatory
A risk‐based surveillance (RBS) shall be implemented as a ‘passive’ surveillance system by laboratory investigation of moribund wild birds or birds found dead and it shall be specifically directed towards the ‘target species’ (TS). A new target list of wild bird species for HPAI passive surveillance of H5 HPAI viruses in the EU has been produced to guide operators involved in passive wild bird surveillance for early warning of H5 HPAI in their region (Table 2 in (EFSA, 2017)). As explained in this monitoring report (also in several sections in this document), the purpose of this list is to provide information on which bird species to focus in order to achieve the most effective testing of dead birds for detection of H5 HPAI viruses. It should be noted that the programmes within countries should be modulated according to demographics of local wild bird populations. Also, this list does not imply that only the carcasses of wild bird species on this list should be examined for H5 HPAI virus; the carcasses of other wild bird species may also be examined for H5 HPAI virus, but only if there are valid reasons to do so. The analysis of wild bird surveillance data will be used to review and update the target list in the future.
The ‘status of wild birds’ sampled under passive surveillance is reported as follows: found dead, injured, live with or without clinical signs and hunted with or without clinical signs.
Desirable
Given that resources are limited, testing should focus on the species that are mentioned in the updated list of target species, as the chance of detecting HPAI in these species is higher than in non‐target species, such as feral pigeons or blackbirds.
Strategic sampling programmes that take into account key migratory pathways into the EU, important wild bird species, and timing of breeding and migratory activities, can both help to assess the risk of HPAI virus incursion and to improve the efficacy of the active surveillance programmes in the future (outcomes a) and c) in Section 2). For this reason, it is important that MSs report in detail the criteria used to define their risk‐based surveillance programmes always focusing on the species provided in the new target list. These criteria should also specify the cut‐off values for mortality events testing selected by MSs and the reasoning behind. Also, based on past outbreaks of HPAI, the seasonal intensity of MSs’ surveillance activities could be matched to the risk observed in wild birds in Europe, being the highest risk observed in the following descending order: autumn, winter, spring, and summer.
Expected framework for the 2019 data collection
It would be important that MSs implement changes in their respective surveillance programmes for wild birds with the aim to focus their sampling on the updated list of target species mentioned above (despite this list not having been updated yet in the legislation). EFSA will engage with MSs in order to understand the cut‐off values used for testing in cases of mortality events.
As mentioned in Section 4.1.2, EFSA will also embark on a project to gather data on wild birds across Europe. Apart from collating data on species population numbers for resident and migratory birds, EFSA aims to collate data on migratory pathways.
4.5.4. Representative surveillance in poultry
Mandatory
If a MS is not in a position to carry out a sufficiently evidence based assessment of the risk pathways for infection of poultry flocks on its territory, it shall implement surveillance based on a representative sampling scheme (as described in Decision 2010/367/EU). Sample size is calculated depending on the poultry sector (see Section 4.6).
MSs should report the total number of establishments sampled in each poultry production category in the NUTS regions where sampling took place (refer to section 4.6 for details).
Desirable
In case a strict random sample is selected and the number of establishments in each poultry production category and surveillance region is known, results are comparable between countries and regions that apply representative sampling. Nevertheless, also in these cases it is important to know the way the representative sample has been collected. Different methods to collect the sampling units may be associated with different biases and this is important to know for comparisons between MSs. It is therefore desirable that MSs report the sampling frame (i.e. the formal selection procedure indicating how establishments to be tested have been selected). This would allow a better interpretation of the results obtained from surveillance activities in different MSs, as the results could be standardised and contextualised, helping to deliver on outcome f).
Expected framework for the 2019 data collection
Information on the sampling frame used by MSs is submitted to the Commission via the forms for obtaining EU financial contribution. This information, if standardised, could be useful to EFSA in order to allow better interpretations of the results from the surveillance data analysis. EFSA, as a first step, will aim to review the information already submitted by MSs in these forms in order to have a better understanding of the methodologies used by MSs and engage with them for clarification if needed.
4.6. Sample size
4.6.1. Number of poultry establishments to be tested
Mandatory
For each MS, the surveillance plan is divided into subchapters corresponding to the poultry production categories targeted. The number of poultry establishments to be sampled shall be defined so as to ensure the identification of at least one infected poultry establishment where the prevalence of infected poultry establishments is at least 5%, with a 95% confidence interval. The number of domestic duck, goose and mallard establishments to be sampled shall be defined to ensure the identification of at least one infected establishment where the prevalence of infected establishments is at least 5%, with a 99% confidence interval. The information is provided per NUTS 2 region (where sampling has taken place) and for each poultry production category each year (http://https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en) on:
-
–
Total number of establishments;
-
–
Number of establishments sampled.
The number of establishments to be sampled for representative and risk based surveillance, shall be determined according to Tables 1 and 2 provided in the Commission Decision 2010/367/EU.
4.6.2. Number of birds within a establishment to be tested
Mandatory
The numbers of birds to be sampled in the poultry establishment shall be defined so as to ensure 95% probability of identifying at least one bird that tests seropositive for AI, if the prevalence of sero‐positive birds is ≥ 30% (Decision 2010/367/EU). For domestic water birds (ducks, geese), and mallards, at least 20 blood samples have to be collected for each establishment. When several categories of poultry and/or several poultry species are present in the same establishment, samples have to be taken from at least 5–10 birds per category (20 for water birds). Samples have to be taken in all sheds when there is more than one shed in the targeted establishment. Information on the number of establishments tested of each of the poultry production categories, as well as the number of samples taken from each establishment and the number of tests carried out per sample (at NUTS 2 level) are requested via the forms for obtaining financial contribution.
Desirable
The number of establishments tested is provided at NUTS2 level, which is not detailed enough to allow relevant epidemiological analysis (see Section 4.4.1). Also, providing the number of birds tested, the number of tests performed and the number of positive tests per establishment may improve the surveillance analysis. In particular, this information would be useful for adjusting the number of samples to be taken in an establishment according to poultry production category.
Expected framework for the 2019 data collection
MSs choosing to submit non‐aggregated data in 2019 will be able to submit information on the number of establishments and birds tested as well as on the number of tests carried out per bird. For other MSs reporting aggregated data, the reporting of these data will be similar to the way they are currently reported to the Commission. EFSA nonetheless will continue to encourage MSs to submit non‐aggregated data.
4.6.3. Wild birds
MSs estimate every year the number of wild birds that will be sampled under the passive surveillance framework in the reporting year. Programmes are available at: http://https://ec.europa.eu/food/funding/animal-health/national-veterinary-programmes_en. Information to be reported are NUTS code/region (often country), total number of birds to be sampled, estimated total number of samples to be taken for passive surveillance, type of test, number of tests.
Desirable
The total number of birds to be sampled per MS ranges largely; nonetheless, there is not always a clear relationship between sample size and population numbers of wild birds belonging to target species. It would be important that the number of dead birds of a particular wild bird species to be sampled in a MS was roughly correlated to population size. Also, it would be important that the historic rate of HPAI virus detection was taken into account (expressed in the recently updated target species list) when determining target sample size. This would ensure a better understanding of surveillance strategies selected by MSs (outcome c)) and a greater knowledge of the entry pathway(s) of HPAI viruses and associated risk factors (outcome d)).
Expected framework for the 2019 data collection
As described in Section 4.1.2, MSs are encouraged to adapt their surveillance programmes for wild birds in accordance with the updated list of target species mentioned above, despite this list not having been updated yet in the legislation. In addition, EFSA will initiate a project to collate data available on species population numbers for resident and migratory birds.
4.7. Epidemiological follow‐up
Mandatory
All positive serological H5/H7 findings must be followed up at the poultry establishment by epidemiological investigations and further sampling for testing by virological methods in order to determine, if active infection of AIV is present on the poultry establishment as per Decision 2010/367/EU. So, it is mandatory to go back to the farm and test for the presence of virus; it is, however, not prescribed when this testing should take place.
Desirable
From an epidemiological point of view, it is particularly relevant to timely identify LPAI virus that is capable of efficient between farm transmission (Rh >1). As indicated in the EFSA opinion (EFSA AHAW Panel, 2017), it is currently not possible to predict when an LPAI virus H5/7 will mutate to HPAI virus. Furthermore, the current active and passive surveillance programmes are not capable of early detection of LPAI virus infections or HPAI virus infections masking LPAI virus in waterfowl. The passive surveillance is reliant on disease notifications and because the course of LPAI virus infections is usually only mild and can easily be misinterpreted for non‐notifiable poultry diseases (such as infectious bronchitis, infectious laryngotracheitis, etc.), there is considerable under‐reporting. The latter is corroborated by the results of active surveillance in EU (many seropositive findings in surveillance that have not been previously notified as clinical suspicions). The active surveillance in itself is not capable of early detection, because it includes only a small sample of the farms (unsampled = undetected) and because of the sampling frequency (once a year) and sample size, infected farms will only be detected when the sero‐prevalence is high and most birds have already cleared infection. Consequently, only a minority of the serological findings turns out virus positive, because the infection within a farm has already faded out.
Despite the above, epidemiological follow‐up of H5/7 positive findings is very useful, because it is important to identify whether or not between‐farm transmission of LPAI virus has taken place. Although we know that most LPAI viruses do not efficiently transmit from farm to farm, from time to time this does happen. Each newly infected farm increases the number of virus replications tremendously and with it the number of mutations. As a consequence, LPAI viruses capable of efficient between farm transmission will likely mutate to HPAI virus sooner or later, as has been observed for example in Pennsylvania (1983), Italy (1999), France (2015) and Germany (2015; (Dietze et al., 2018)). For that reason epidemiological follow‐up should include collecting serum samples and/or tracheal/cloacal swabs of clinically suspect birds or domestic water birds on epidemiologically linked farms that have been exposed to virus from those farms found seropositive in active surveillance (close proximity, e.g. 1 km radius, or epidemiological link). These samples should be linked to the case originating the specific follow‐up. This enables identification of LPAI virus transmission chains and creates the opportunity to take risk mitigating measures based on risk assessment, achieving outcomes a), g) and i).
Expected framework for the 2019 data collection
Although the follow‐up on epidemiologically linked farms is not explicitly mentioned in Decision 2010/367/EU, for the reasons explained above it would be desirable that MSs carry out and report this information to EFSA. The new EFSA data collection model will allow the reporting of this type of information given MSs the opportunity to report the dummy identification of the serological positive establishment that initiated the follow‐up actions.
4.8. Pathotype and subtype test results
Mandatory
For poultry and wild birds, it is mandatory to report all H5 and H7 viruses and their pathotype (HPAI, LPAI). MSs should also report H and N subtypes when that information is available.
Desirable
For poultry, although not mandatory, reporting of all different subtypes (including non H5/H7 viruses) identified during follow‐up activities could be of value, as these types could be of interest in the future. The same can be said about the reporting of the N types. The analysis of these data should take into account the origin of the sample (amplified isolate, RNA extract from field, individual or pooled samples, etc). Due to the occurrence of mixed infections (not so rare in waterfowl), the association between H and N subtypes should be interpreted with care. This would help to have a better understanding of the AIV pathotypes circulating in poultry establishments, helping to achieve outcome g) in Section 2. The collection and distribution of this information can be also useful for research purposes.
It is also desirable that MSs report the pathotype information of positive wild bird samples. Often, MSs carry out pathotyping on the first samples taken, but might not continue doing so for the rest of samples obtained from epidemiologically linked outbreaks. It is up to each MSs to decide on whether the samples taken at a later stage can be considered or not to have the same pathotype.
Aside, the collection of viral sequence data (if linked to an accession number or genbank ID) could be of value as an add‐on in epidemiological investigations, in particular for research purposes. Nonetheless, no standards are available for the collection of viral sequence data.
These data would be able to allow a better understanding of the transmission patterns between and within poultry and wild birds, and associated risk factors, helping to achieve objectives a), b) and d).
Expected framework for the 2019 data collection
MSs will be able to continue submitting data as they currently do to the Commission.
Abbreviations
- ADNS
Animal Disease Notification System
- AGID
Agar gel immunodiffusion test
- AI
avian influenza
- AIV
avian influenza virus
- DVFA
Danish Veterinary and Food Administration
- ELISA
enzyme‐linked immunosorbent assay
- EURL
European Union Reference Laboratory
- HI test
haemagglutination‐inhibition test
- HPAI
highly pathogenic avian influenza
- LPAI
low pathogenic avian influenza
- MS
Member State
- NUTS
Nomenclature of Territorial Units for Statistics
- OIE
World Organisation for Animal Health
- PCR
polymerase chain reaction
- RBS
risk‐based surveillance
- RT‐PCR
reverse transcription polymerase chain reaction
- TS
target species
Suggested citation: EFSA (European Food Safety Authority) , Brouwer A, Huneau A, Kuiken T, Staubach C, Stegeman A, Baldinelli F, Verdonck F and Aznar I, 2018. Scientific report: Reporting Avian Influenza surveillance. EFSA Journal 2018;18(11):5493, 26 pp. 10.2903/j.efsa.2018.5493
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00829
Acknowledgements: In addition to the listed authors, EFSA wishes to thank all MS representatives that provided support to this scientific output either at the AI workshop and/or by sending comments to the circulated drafts: Austria (Andrea Hoeflechner‐Poeltl), Croatia (Tihana Miškić) Cyprus (Giorgos Krasias), the Czech Republic (Richard Wallo), Denmark (Pernille Nielsen, Charlotte Hjulsager), Estonia (Kärt Jaarma), Finland (Niina Tammiranta), France (Marie Grandcollot‐Chabot, Eric Niqueux), Germany (Timm Harder), Greece (Sokratis Perdikaris), Hungary (Márk Hóvári), Ireland (Laura Garza‐Cuartero), Italy (Calogero Terregino), Latvia (Mara Uzule‐Springe), Lithuania (Vilija Grigaliūnien≐), Norway (Siri Kulberg Sjurseth), Poland (Edyta Swieton), Portugal (Renata Carvalho), Romania (Theodora Chesnoiu Vasile), Slovakia (Vilem Kopriva), Slovenia (Aleksandra Hari), Spain (Elena García Villacieros), Sweden (Linda Ernholm), the Netherlands (Marcel Spierenburg) and the United Kingdom (Helen Roberts).
Approved: 6 November 2018
Notes
Council Directive 2005/94/EC of 20 December 2005 on Community measures for the control of avian influenza and repealing Directive 92/40/EEC (OJ L 10, 14.1.2006, p. 16).
Commission Decision 2010/367/EU of 25 June 2010 on the implementation by Member States of surveillance programmes for avian influenza in poultry and wild birds (OJ L 166, 1.7.2010, p.122).
Commission Implementing Decision (EU) 2018/1136 of 10 August of 2018 on risk mitigating and reinforced biosecurity measures and early detection systems in relation to the risks posed by wild birds for the transmission of highly pathogenic avian influenza viruses to poultry.
Regulation (EC) No 429/2016 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). OJ L 84, 31.3.2016, p. 1–208.
Commission Decision 2006/437/EC approving a Diagnostic Manual for avian influenza as provided for in Council Directive 2005/94/EC.
Ratites are considered poultry species in Commission Decision 2010/367/EU.
Wild mallards raised in captivity, not domestic.
According to the definition provided in Article 2 of the Council Directive 2005/94/EC ‘Commercial holding’ means a holding where poultry are kept for commercial purposes; ‘Non‐commercial holding’ means a holding where poultry or other captive birds are kept by their owners: (a) for their own consumption or use; or (b) as pets.
A brief project description is available at http://www.efsa.europa.eu/en/supporting/pub/1428e
This categorization is already used by ADNS and TRACES, is well defined and relatively stable overtime in all MSs compared to NUTS 4/5, and the geographical resolution still allows statistical analysis to test hypotheses at EU level.
Commission Directive 2002/4/EC of 30 January 2002 on the registration of establishments keeping laying hens and (EC) covered by Council Directive 1999/74/EC.
Commission Regulation (EC) No 589/2008 of 23 June 2008 laying down detailed rules for implementing Council Regulation (EC) No 1234/2007 as regards marketing standards for eggs.
References
- Animal and Plant Health Agency , 2017. Annual Report on surveillance for avian influenza in poultry and wild birds in Member States of the European Union in 2016. 164 pp. Available online: http://https://ec.europa.eu/food/sites/food/files/animals/docs/ad_control-measures_ai_surv-rslt_pltry-wld-brds_2016.pdf
- Bouwstra R, Gonzales JL, de Wit S, Stahl J, Fouchier RAM and Elbers ARW, 2017. Risk for Low Pathogenicity Avian Influenza Virus on Poultry Farms, the Netherlands, 2007–2013. Emerging Infectious Diseases, 23, 1510–1516. 10.3201/eid2309.170276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle C, Wasilenko J, Adams SC, Cardona CJ, To TL, Nguyen T, Spackman E, Suarez DL, Smith D, Shepherd E, Roth J and Pantin‐Jackwood MJ, 2012. Differences in pathogenicity, response to vaccination, and innate immune responses in different types of ducks infected with a virulent H5N1 highly pathogenic avian influenza virus from Vietnam. Avian Diseases, 56, 479–487. 10.1637/10030-120511-Reg.1 [DOI] [PubMed] [Google Scholar]
- Dietze K, Graaf A, Homeier‐Bachmann T, Grund C, Forth L, Pohlmann A, Jeske C, Wintermann M, Beer M, Conraths FJ and Harder T, 2018. From low to high pathogenicity: characterization of H7N7 avian influenza viruses in two epidemiologically linked outbreaks. Transboundary and Emerging Diseases. 10.1111/tbed.12906 [DOI] [PubMed] [Google Scholar]
- Duvauchelle A, Huneau‐Salaün A, Balaine L, Rose N and Michel V, 2013. Risk factors for the introduction of avian influenza virus in breeder duck flocks during the first 24 weeks of laying. Avian Pathology, 42, 447–456. 10.1080/03079457.2013.823145 [DOI] [PubMed] [Google Scholar]
- EFSA AHAW Panel (Animal Health and Welfare), More S, Bicout D, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortazar‐Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Thulke HH, Velarde A, Willeberg P, Winckler C, Breed A, Brouwer A, Guillemain M, Harder T, Monne I, Roberts H, Baldinelli F, Barrucci F, Fabris C, Martino L, Mosbach‐Schulz O, Verdonck F, Morgado J and Stegeman JA, 2017. Scientific opinion on avian influenza. EFSA Journal 2017;15(10):4991, 233 pp. 10.2903/j.efsa.2017.4991 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control), EURL (European Union Reference Laboratory for Avian influenza), Brown I, Kuiken T, Mulatti P, Smietanka K, Staubach C, Stroud D, Therkildsen OR, Willeberg P, Baldinelli F, Verdonck F and Adlhoch C, 2017. Scientific Report: Avian influenza overview September–November 2017. EFSA Journal 2017;15(12):5141, 70 pp. 10.2903/j.efsa.2017.5141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control), EURL (European Union Reference Laboratory for Avian influenza), Adlhoch C, Brouwer A, Kuiken T, Mulatti P, Smietanka K, Staubach C, Willeberg P, Barrucci F, Verdonck F, Amato L and Baldinelli F, 2018. Scientific Report: Avian influenza overview November 2017–February 2018. EFSA Journal 2018;16(3):5240, 55 pp. 10.2903/j.efsa.2018.5240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , online. Animal Disease Notification System (ADNS). Available online: http://https://ec.europa.eu/food/animals/animal-diseases/not-system_en [Accessed: 19 March 2018].
- Gonzales JL, Elbers ARW and Beerens N, 2017. Risk factors of primary introduction of highly pathogenic and low pathogenic avian influenza virus into European poultry holdings, considering at least material contaminated by wild birds and contact with wild birds. EFSA Supporting Publications, 14, 1282E 10.2903/sp.efsa.2017.EN-1282 [DOI] [Google Scholar]
- Lee DH, Lee HJ, Lee YJ, Kang HM, Jeong OM, Kim MC, Kwon JS, Kwon JH, Kim CB, Lee JB, Park SY, Choi IS and Song CS, 2010. DNA barcoding techniques for avian influenza virus surveillance in migratory bird habitats. Journal of Wildlife Diseases, 46, 649–654. 10.7589/0090-3558-46.2.649 [DOI] [PubMed] [Google Scholar]
- Ottaviani D, de la Rocque S, Khomenko S, Gilbert M, Newman SH, Roche B, Schwabenbauer K, Pinto J, Robinson TP and Slingenbergh J, 2010. The cold European winter of 2005–2006 assisted the spread and persistence of H5N1 influenza virus in wild birds. EcoHealth, 7, 226–236. 10.1007/s10393-010-0316-z [DOI] [PubMed] [Google Scholar]
- Phuong DQ, Dung NT, Jørgensen PH, Handberg KJ, Vinh NT and Christensen JP, 2011. Susceptibility of Muscovy (Cairina moschata) and mallard ducks (Anas platyrhynchos) to experimental infections by different genotypes of H5N1 avian influenza viruses. Veterinary Microbiology, 148, 168–174. 10.1016/j.vetmic.2010.09.007 [DOI] [PubMed] [Google Scholar]
- Reperant LA, van de Bildt MWG, van Amerongen G, Buehler DM, Osterhaus ADME, Jenni‐Eiermann S, Piersma T and Kuiken T, 2011. Highly pathogenic avian influenza virus H5N1 infection in a long‐distance migrant shorebird under migratory and non‐migratory states. PLoS ONE, 6, e27814 10.1371/journal.pone.0027814 [DOI] [PMC free article] [PubMed] [Google Scholar]


