Abstract
The present opinion deals with the evaluation of the proposed increase of the currently authorised maximum amounts of ferric sodium ethylenediaminetetraacetic acid (EDTA) as a novel food ingredient used as a source of iron, and its extension of use in processed cereal‐based foods and baby foods. The applicant also provided information on two forms of ferric sodium EDTA, one previously assessed by EFSA and a new one of finer consistency. To support the proposed changes to the uses of ferric sodium EDTA, the applicant proposed a revision of the current acceptable daily intake (ADI) for EDTA, derived from that set for the food additive calcium disodium EDTA (E 385). The Panel confirmed that ferric sodium EDTA is a source from which iron is bioavailable. In assessing the safety of the proposed revision to the existing specifications for the novel food ingredient ferric sodium EDTA, the Panel noted that this would not discriminate between the previously evaluated substance and the one of finer consistency. In particular, the Panel noted that particle size was not one of the proposed parameters for the revised specifications. The Panel noted that it was not possible to determine whether particles of ferric sodium EDTA in the nano range were present in the product with finer consistency in the solid form. The toxicological data submitted did not add any new relevant information to the database on which the current ADI for EDTA is based. Consequently, the Panel concluded that there was no sound scientific justification to increase the ADI for EDTA and hence increase the use levels of ferric sodium EDTA or introduce additional uses as proposed by the applicant. The Panel recommended that additional toxicological data should be provided to address the shortcomings in the available toxicity database prior to the re‐evaluation of calcium disodium EDTA (E 385).
Keywords: ferric sodium EDTA, iron, nutrient source, novel food, food fortification, calcium disodium EDTA, E 385
Summary
Following a request from the European Commission to the European Food Safety Authority (EFSA), the Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to provide a scientific opinion on the proposed changes to the current authorisation of ferric sodium ethylenediaminetetraacetic acid (EDTA) as a novel food ingredient, used as a source of iron. Within the current application, it is proposed to increase the maximum amounts of ferric sodium EDTA that can be added to foods for the general population, food supplements in children, foods for special medical purposes and total diet replacement for weight control. The applicant also proposes the inclusion of ferric sodium EDTA in the list of substances permitted for use in processed cereal‐based foods and baby foods.
In the dossier submitted by the applicant in support of the current application, it is reported that two different products are being commercialised as ferric sodium EDTA: Ferrazone®, which was the subject of the previous evaluation of ferric sodium EDTA by the ANS Panel, and Ferrazone XF®, a product of finer consistency obtained by adding an extra grinding step to the manufacturing process of Ferrazone®.
The applicant has submitted analytical results from three batches of Ferrazone XF® to demonstrate compliance with the proposed update to the existing specifications. The Panel however noted that no parameters have been proposed for the update of the specifications of ferric sodium EDTA with respect to particle size which could be used for the discrimination between the two different products. Furthermore, based on the analytical data provided, the Panel could not exclude the presence of particles of ferric sodium EDTA in the nano‐range in Ferrazone XF® in the solid form. The Panel further noted that according to the applicant, there is no difference in the solubility in water of Ferrazone® and Ferrazone XF®.
To support the proposed increase in the maximum amounts of ferric sodium EDTA in currently authorised uses and to extend the use to processed cereal‐based food and baby food, the applicant proposed a revision of the current acceptable daily intake (ADI) for EDTA on the basis of newly submitted toxicological data on other EDTA salts, previously not available for assessment by the Panel.
Based on the additional absorption, distribution, metabolism and excretion (ADME) studies provided and on its previous assessment, the ANS Panel considered that, following oral ingestion, ferric sodium EDTA would dissociate into its components, EDTA and iron in the gastrointestinal tract. EDTA is not absorbed to a large extent and will be excreted in the faeces. Minor amounts of EDTA may be absorbed but are not metabolised, and may be excreted unchanged in urine. Only a small proportion of the dissociated iron is absorbed; the largest fraction is excreted in an insoluble form in the faeces. The Panel further noted that the ADME studies assessed may be of limited relevance for the newly proposed form of ferric sodium EDTA indicated by the applicant, i.e. Ferrazone XF®, since, due to its reduced particle size, the product may have different characteristics with regard to ADME. With the available data, the Panel was not in the position to draw conclusions on this matter.
In its previous opinion, the ANS Panel reviewed three subchronic studies in rats with dietary exposure to ferric sodium EDTA. In the present dossier, the applicant submitted four additional subchronic studies on EDTA salts other than ferric sodium EDTA. The Panel noted that the design and reporting of some of these studies had limitations, and were not considered suitable for risk assessment. The Panel further noted that these studies did not add any new relevant information to the database available for the previous evaluation of ferric sodium EDTA.
The Panel considered that the two reproductive toxicity studies submitted in the dossier provided insufficient data to evaluate the reproductive toxicity of ferric sodium EDTA.
The Panel noted that the prenatal developmental study provided in the dossier was inadequate for risk assessment but is nevertheless concerned about the effects of ferric sodium EDTA reported. The Panel was also concerned about the adverse developmental effects of EDTA given in the diet seen in other, albeit limited, studies available in the literature.
The Panel has estimated intake levels for iron and exposure to EDTA resulting from the new proposed uses and use levels. The Panel noted that the exposure to EDTA for all population groups from the new proposed uses will significantly exceed the current ADI of 1.9 mg/kg body weight (bw) per day at the mean and 95th percentile.
The Panel confirmed that ferric sodium EDTA is a source from which iron is bioavailable.
In assessing the safety of the proposed revision to the existing specifications for the novel food ingredient ferric sodium EDTA, the Panel noted that this would not discriminate between the previously evaluated substance (marketed as Ferrazone®) and the one of finer consistency (Ferrazone XF®) produced by adding an extra grinding step to the manufacturing process. In particular the Panel noted that particle size was not one of the proposed parameters for the revised specifications. The Panel noted that it was not possible to determine whether particles of ferric sodium EDTA in the nano‐range were present in solid Ferrazone XF®.
The Panel concluded that the exposure assessment based on the proposed extension of uses and use levels would lead to the current ADI for EDTA being exceeded in all population groups at the mean and 95th percentile.
Additionally, the Panel concluded that the toxicological data submitted by the applicant did not provide any new relevant information to the database on which the current ADI was established.
Consequently, the Panel concluded that there was no basis to increase the ADI for EDTA and hence increase the use levels of ferric sodium EDTA or introduce additional uses as proposed by the applicant.
The Panel further noted that in accordance with Regulation (EU) No 257/2010, a full re‐evaluation of the safety of calcium disodium EDTA (E 385) as a food additive is to be performed by EFSA. In this context, the full toxicological database for this substance will be reviewed including the basis for establishing the ADI for EDTA. The Panel recommended that additional toxicological data should be provided to address the shortcomings in the available toxicity database prior to the re‐evaluation of calcium disodium EDTA (E 385) as a food additive.
1. Introduction
The present scientific opinion deals with the evaluation of proposed amendments to the existing specifications and conditions of use of ferric sodium ethylenediaminetetraacetic acid (EDTA) currently authorised as a novel food ingredient; the possible revision of the toxicological database on the basis of which the currently applicable acceptable daily intake (ADI) for EDTA was set, and the evaluation of ferric sodium EDTA as a proposed source of iron in processed cereal‐based foods and baby foods.
In assessing the safety of a nutrient source which dissociates, the Panel evaluates the safety of all products of that dissociation other than the nutrient. Therefore, the safety of iron itself, in terms of amounts that may be consumed, and the consideration of iron as a nutrient are outside the remit of this Panel.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
The European Union legislation lists nutritional substances that may be used for nutritional purposes in certain categories of foods as sources of certain nutrients.
The relevant Union legislative measures are:
Regulation (EC) No 258/97 of the European Parliament and the Council concerning novel foods and novel food ingredients.1
Directive 2002/46/EC of the European Parliament and of the Council on the approximation of the laws of the Member States relating to food supplements.2
Regulation (EC) 1925/2006 on the addition of vitamins and mineral and of certain other substances to foods.3
Regulation (EU) No 609/2013 of the European Parliament and of the Council on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control.4
On 4 September 2006, the company AkzoNobel Chemicals GmbH made a request to the competent authorities of the United Kingdom to place ferric sodium EDTA on the market as a novel food ingredient.
On 26 November 2009, following a request from the Commission, the Scientific Panel on Food Additives and Nutrient Sources added to Food (ANS) adopted an opinion on the safety of ferric sodium EDTA (EFSA ANS Panel, 2010). In the opinion, EFSA concluded that EDTA is of no safety concern as long as the intake does not exceed 1.9 mg EDTA per kg bw per day.
On 14 June 2010, Commission Decision 2010/331/EU authorising the placing on the market of ferric sodium EDTA as a novel food ingredient under Regulation (EC) No 258/97 was published.
On 14 November 2011, Commission Regulation (EU) No 1161/2011 was adopted to allow the use of ferric sodium EDTA as a source of iron in food supplements, fortified foods and certain food for particular nutritional uses.
On 26 July 2017, the company AkzoNobel Business Area Specialty Chemicals made a request to the Commission to amend both Annexes I (specifications) and II (maximum amounts) to Commission Decision 2010/331/EU authorising the placing on the market of ferric sodium EDTA as a novel food ingredient under Regulation (EC) No 258/97, and Annex to Regulation (EU) No 609/2013 for ferric sodium EDTA to be allowed for use as a source of iron also in processed cereal‐based foods and baby foods.
The request concerns the revision of specifications and the maximum use levels of ferric sodium EDTA as an authorised source of iron in certain food categories as well as the authorisation to allow use of ferric sodium EDTA as a source of iron in processed cereal‐based foods and baby foods, based on the new information provided by the applicant.
1.1.2. Terms of Reference
In accordance with Article 29(1)(a) of Regulation (EC) No 178/20025, the European Commission asks the European Food Safety Authority to:
carry out the new assessment for ferric sodium EDTA as a novel food ingredient in the context of Regulation (EC) No 258/97
following the outcome of the novel food assessment, evaluate the safety of ferric sodium EDTA when added for nutritional purposes as a source of iron to food for the general population (including food supplements), total diet replacement for weight control, food for special medical purposes and processed cereal‐based foods and baby foods, and the bioavailability of iron from this source, in the context of Directive 2002/46/EC, Regulation (EC) No 1925/2006 and Regulation (EU) No 609/2013.
1.2. Interpretation of the Terms of Reference
The Panel is requested to evaluate the safety of the proposed changes to the specifications of the novel food ingredient ferric sodium EDTA. With the present dossier the applicant intends to cover two different forms of ferric sodium EDTA: the previously assessed form, i.e. Ferrazone®, and a new form with finer consistency, i.e. Ferrazone XF®.
To address the evaluation of the safety of the proposed changes to the maximum amounts listed in Annex II to Commission Decision 2010/331/EU and the proposed inclusion in the list of substances permitted for use in processed cereal‐based foods and baby foods, the Panel considered the information provided by the applicant in the dossier, including new toxicological data submitted in support of a revision of the current ADI for EDTA (see Section 1.3) and that were used as a basis to set maximum amounts for the authorisation of the novel food ingredient ferric sodium EDTA. The evaluation involved an assessment of ferric sodium EDTA and its constituents iron and EDTA.
1.3. Information on existing evaluations and authorisations
Ferric sodium EDTA is currently authorised as a novel food ingredient to be used in fortified foods, dietetic foods, foods for specific groups (foods for special medical purposes, total diet replacement for weight control, processed cereal‐based foods and baby foods) and food supplements.
The safety of ferric sodium EDTA and the bioavailability of iron from this source added for nutritional purposes to foods for the general population and to foods for particular nutritional uses have been previously evaluated by EFSA (EFSA ANS Panel, 2010). In its 2010 opinion, the Panel concluded that the use of ferric sodium EDTA for the proposed uses is of no safety concern as long as the intake of EDTA does not exceed 1.9 mg EDTA/kg body weight (bw) per day.
The conclusions from the ANS Panel were similar to the conclusions reached by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 2007, when it had also evaluated the safety of sodium iron EDTA as to its use for iron fortification (JECFA, 2007). JECFA had established an ADI for EDTA of 1.9 mg EDTA/kg bw per day, based on the ADI of 2.5 mg/kg bw per day established for the food additive calcium disodium EDTA by JECFA in 1974 (JECFA, 1974).
The ADI for EDTA established by JECFA in 1974 had been previously endorsed by the Scientific Committee for Food (SCF) in 1977 and in 1990, when calcium disodium EDTA was evaluated for use as an antioxidant food additive (SCF, 1977, 1990).
Currently, calcium disodium EDTA (E 385) is an authorised food additive in the European Union (EU) according to Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives. It is permitted for use in several food categories, with maximum levels ranging from 75 to 250 mg calcium disodium EDTA/kg. Currently, its re‐evaluation as a food additive, as foreseen in Regulation (EC) No 257/20106, is still ongoing.7
1.3.1. Iron
No Tolerable Upper Intake Level (UL) has been set for iron by EFSA (EFSA NDA Panel, 2004). Adverse gastrointestinal effects have been reported after short‐term ingestion of non‐haem iron preparations at doses of 50–60 mg/day, particularly if taken without food. The NDA Panel considered that these adverse gastrointestinal effects were not a suitable basis to establish a UL for iron from all sources. The NDA Panel also considered that a UL cannot be established for iron based on iron overload, because there were inadequate data to enable the construction of reliable response curves between intake, body burden, homoeostatic adaptations and adverse health effects, including increased risk of chronic diseases such as cardiovascular disease, diabetes and cancer.
The NDA Panel has defined dietary reference values for iron (EFSA NDA Panel, 2015) providing Average Requirements and Population Reference Intake values that are summarised in Table 1.
Table 1.
Summary of dietary reference values for iron
| Age | Average requirement (mg/day) | Population reference Intake (mg/day) |
|---|---|---|
| 7–11 months | 8 | 11 |
| 1–6 years | 5 | 7 |
| 7–11 years | 8 | 11 |
| 12–17 years (M) | 8 | 11 |
| 12–17 years (F) | 7 | 13 |
| ≥ 18 years (M) | 6 | 11 |
|
≥ 18 years (F) Premenopausal Postmenopausal |
7 6 |
16a 11 |
| Pregnancy | As for non‐pregnant premenopausal women | As for non‐pregnant premenopausal women |
| Lactation | As for non‐lactating premenopausal women | As for non‐lactating premenopausal women |
F, females; M, males.
The PRI covers the requirement of approximately 95% of premenopausal women.
2. Data and methodologies
2.1. Data
The present evaluation is based on the data on ferric sodium EDTA in a newly submitted dossier by the applicant (Documentation provided to EFSA n. 1) and on the additional information that was sought from the applicant during the assessment process (Documentation provided to EFSA n. 2, Documentation provided to EFSA n. 3).
2.2. Methodologies
The assessment was conducted in line with the principles described in the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA, 2009) and following the relevant existing Guidance from the EFSA Scientific Committee.
The ANS Panel assessed the safety of ferric sodium EDTA in line with the principles contained in the latest existing guidance on the safety evaluation of food additives, namely the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
The evaluation of bioavailability of the nutrient (iron) from the source ferric sodium EDTA was conducted in line with the principles contained in the ‘Guidance on submissions for safety evaluation of nutrients or of other ingredients proposed for use in the manufacture of foods’ (SCF, 2001).
Dietary exposure to ferric sodium EDTA from the intended use as a source of iron added to food and the relative iron intake derived from this intended use was estimated using the food consumption data available within the EFSA Comprehensive European Food Consumption Database (EFSA, 2011a).
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
According to the applicant, Ferrazone® and Ferrazone XF® are the trihydrate form of ferric sodium EDTA with CAS Number 18154‐32‐0. The anhydrous form of ferric sodium EDTA has the CAS number 15708‐41‐5 and the EINECS number 239‐802‐2 (Documentation provided to EFSA n. 1).
The molecular formula of the trihydrate form is C10H12N2O8FeNa · 3H2O and it has a molecular weight of 421.1 g/mol; the molecular weight of the anhydrous form is 367.047 g/mol.
The structural formula for the trihydrate form is shown in Figure 1.
Figure 1.
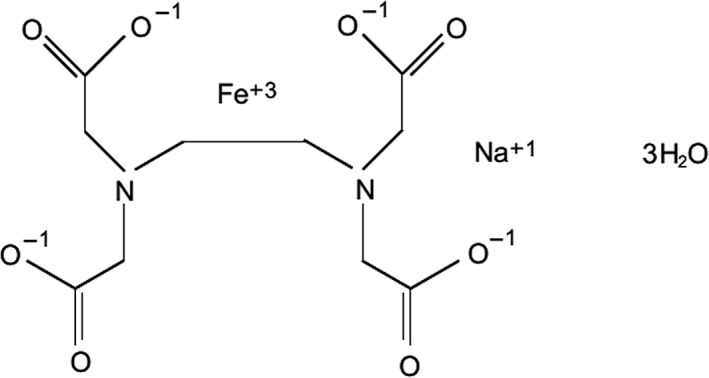
Structural formula of the novel food ingredient ferric sodium EDTA trihydrate according to Commission Decision 2010/331/EU
The chemical names provided by the applicant are the followings:
Ferrate(1‐), [[N,N’‐1,2‐ethanediylbis[N‐[(carboxy‐kO)methyl]glycinato‐kN,kO]] (4)]‐, sodium, (OC‐6‐21) and ferrate(1‐), [[N,N’‐1,2‐ethanediylbis[N‐(carboxymethyl)glycinato]](4‐)‐N,N’,O,O’,ON,ON’]‐, sodium, (OC‐6‐21).
The synonyms provided by the applicant for ferric sodium EDTA are the following:
Ferrate(1‐), [(ethylenedinitrilo)tetraacetato]‐, sodium; Ferric ethylenediaminetetraacetic acid, sodium salt; Ferric sodium edetate; Ferric sodium ethylenediaminetetraacetate; Iron EDTA; Iron monosodium EDTA; Iron sodium ethylenediaminetetraacetate; Iron sodium ethylenediaminetetraacetate (1:1:1); Monosodium ferric EDTA; Sodium (ethylenediaminetetraacetato)ferrate(1‐); Sodium [(ethylenedinitrilo)tetraacetato]ferrate(III); Sodium (N,N,N’,N’‐ethylenediaminetetraacetato)ferrate(1‐); Sodium feredetate; Sodium ferric EDTA; Sodium ferric ethylenediaminetetraacetate; Sodium iron EDTA; Sodium iron(III) EDTA; Sodium iron(III) ethylenediaminetetraacetate; Sodium [(ethylenedinitrilo)tetraacetatato]ferrate(III); Sodium [(ethylenedinitrilo)tetraacetato]ferrate(III).
3.1.2. Specifications
The applicant is proposing to update the current specifications for ferric sodium EDTA (Documentation provided to EFSA n. 1) (Table 2).
Table 2.
Current specifications for ferric sodium EDTA as laid down in Annex 1 of Commission Decision of 14 June 2010a and proposed changes by the applicant
| Parameter | Current specification | New proposed specifications |
|---|---|---|
| Appearance | Yellow to brown powder | Light‐yellow to yellow‐brown powder |
| Odour | Odourless | Odourless |
| pH (in 1% solution) | 3.5–5.5 | 4.5–5.5 |
| Iron (%) | 12.5–13.5 | 13–13.5 |
| Sodium (%) | 5.5 | NA |
| Water (%) | 12.8 | NA |
| Organic matter (CHNO) (%) | 68.4 | NA |
| EDTA (%) | 65.5–70.5 | 67.5–71.5 |
| Water‐insoluble matter (%) | Not more than 0.1 | ≤ 1 |
| Nitrilo‐triacetic acid (%) | Not more than 0.1 | < 1 |
| Identification by IR | NA | – |
| Arsenic (mg/kg) | NA | ≤ 1 |
| Lead (mg/kg) | NA | ≤ 1 |
| Chloride (mg/kg) | NA | ≤ 600 |
| Loss on drying (%) | NA | 12.5–13.5 |
| Sulfate (%) | NA | ≤ 0.06 |
| Free iron (%) | NA | ≤ 0.05 |
| Absorbance (AU) | NA | ≤ 0.24 |
AU: absorbance unit; NA: not applicable.
Commission Decision of 14 June 2010 authorising the placing on the market of Ferric Sodium EDTA as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council.
While at the time of the previous EFSA opinion on ferric sodium EDTA (EFSA ANS Panel, 2010), the assessment focussed on Ferrazone®, which at the time was the only ferric sodium EDTA product produced by the applicant, the applicant now commercialises also a second product, Ferrazone XF®, which has a finer consistency due to an extra grinding step in the manufacturing process. The updated specifications are intended to be applicable to both products. The Panel however noted that the proposed update to the specifications did not contain any information on the particle size of either Ferrazone® or Ferrazone XF®.
To demonstrate compliance with the proposed specifications, the applicant provided analytical data of three independent batches of Ferrazone XF® (Documentation provided to EFSA n. 1). All the analyses were carried out according to respective methods described in the Food Chemical Codex. The Panel noted that for one proposed parameter (identification by IR) the applicant did not provide supporting analytical information to demonstrate that the product Ferrazone XF® complies with the proposed specifications.
To demonstrate microbiological safety the applicant provided microbiological analysis of five batches of ferric sodium EDTA complying with ISO standards (Documentation provided to EFSA n. 1).
The Panel noted that no information on the solubility of the material was mentioned in either the current or the proposed revised specifications, despite the applicant has stated that both Ferrazone® and Ferrazone XF® have a solubility in water of 90 g/L at 20°C (Documentation provided to EFSA n. 3). The Panel noted that according to the applicant, there is no difference in the solubility in water of the two materials.
3.1.2.1. Particle size
Upon request from EFSA, the applicant provided information on particle size distribution for three commercial batches of Ferrazone XF® obtained by laser diffraction (Documentation provided to EFSA n. 3). The information provided indicated that the particle size corresponding to 10% of the cumulative undersize distribution by volume was around 3 μm. However, the Panel noted that the data provided did not follow the recommendation from the EFSA Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed (EFSA Scientific Committee, 2011, 2018) where information on particle size, number based size distribution and mass based size distribution of the material is requested to be measured by more than one independent technique, one being electron microscopy (EM) and if EM cannot be applied, the use of a different imaging technique is suggested. Therefore, based on the information provided, the Panel cannot exclude the presence of particles of ferric sodium EDTA in the nano range in Ferrazone XF® in the solid form.
3.1.3. Manufacturing process
The applicant provided description of the manufacturing process for the novel food ingredient. The product under assessment is obtained by crystallisation following the addition of an aqueous solution of FeCl3 to an aqueous solution of tetrasodium EDTA (Documentation provided to EFSA n. 1).
The Panel noted that, compared to the assessment performed in the previous evaluation (EFSA ANS Panel, 2010), the applicant is now commercialising also another ferric sodium EDTA product, Ferrazone XF®, which is produced by adding an extra grinding step in the manufacturing process for Ferrazone®, which results in a product with finer consistency. The Panel however noted that in the proposed specifications for ferric sodium EDTA, particle size is not listed as a parameter to characterise the material and to discriminate between Ferrazone® and Ferrazone XF®.
3.1.4. Methods of analysis in food
The applicant provided reference to the Food Chemical Codex for the analytical methods used to verify that the batches conformed to the proposed specifications. (Documentation provided to EFSA n. 1).
The Panel noted that no methods for the analysis of ferric sodium EDTA in the food were provided.
3.1.5. Stability of the substance and reaction and fate in food
For the stability of ferric sodium EDTA, the applicant refers to the previous EFSA opinion (EFSA ANS Panel, 2010) and did not provide any new information.
The Panel noted that no information was provided on whether the extra grinding step applied to produce Ferrazone XF® has an impact on the stability of the final product.
3.2. Proposed uses and use levels
The applicant proposes to revise the current maximum amounts of ferric sodium EDTA authorised in fortified foods, food supplements, foods for special medical purposes and total diet replacement for weight control; the applicant is also requesting the extension of use of ferric sodium EDTA to processed cereal‐based foods and baby foods. According to the applicant, the intake of iron derived from the currently authorised uses of ferric sodium EDTA would be equal to 16% of the PRI established by EFSA for iron in infants.
3.2.1. Use in foods for the general population according to Regulation (EC) No 1925/2006
The applicant proposes to increase the current maximum amount of ferric sodium EDTA, expressed as anhydrous EDTA, authorised in fortified foods under Regulation (EC) No 1925/2006, i.e. 12 mg EDTA per 100 g of final food. The proposed uses and use levels for ferric sodium EDTA in fortified foods are reported in Table 3 (Documentation provided to EFSA n. 2).
Table 3.
Current maximum amounts of ferric sodium EDTA and proposed uses and use levels for ferric sodium EDTA, and corresponding iron levels
| Food category number | Food category name | Proposed use levels (mg/kg) of ferric sodium EDTA expressed as
|
Corresponding level of iron (mg/kg) |
|---|---|---|---|
| Current maximum amounts a | |||
| Not specified | Fortified foods according to Reg (EC) 1925/2006 | 120b | 23 |
| Proposed uses and use levels | |||
| 1 | Dairy products and analogues | ||
| 1.1 | Unflavoured pasteurised and sterilised (including UHT) milk | 350/300/240 | 46 |
| 1.2 | Unflavoured fermented milk products, including natural unflavoured buttermilk (excluding sterilised buttermilk) non heat treated after fermentation | 350/300/240 | 46 |
| 1.3 | Unflavoured fermented products, heat‐treated after fermentation | 350/300/240 | 46 |
| 1.4 | Flavoured fermented milk products including heat treated products | 350/300/240 | 46 |
| 3 | Edible ices | ||
| 3 | Edible ices | 350/300/240 | 46 |
| 4 | Fruit and vegetables | ||
| 4.2 | Processed fruit and vegetables | 350/300/240 | 46 |
| 6 | Cereals and cereal products | ||
| 6.1 | Whole, broken, or flaked grain | 350/300/240 | 46 |
| 6.2 | Flours and other milled products and starches | 450/390/310 | 60 |
| 6.3 | Breakfast cereals | 1150/1000/800 | 150 |
| 6.4 | Pasta | 350/300/240 | 46 |
| 6.5 | Noodles | 350/300/240 | 46 |
| 6.6 | Batters | 350/300/240 | 46 |
| 6.7 | Pre‐cooked or processed cereals | 350/300/240 | 46 |
| 7 | Bakery wares | ||
| 7.1 | Bread and rolls | 350/300/240 | 46 |
| 7.2 | Fine bakery wares | 350/300/240 | 46 |
| 10 | Eggs and egg products | ||
| 10.2 | Processed eggs and egg products | 350/300/240 | 46 |
| 12 | Salts, spices, soups, sauces, salads and protein products | ||
| 12.5 | Soups and broths | 350/300/240 | 46 |
| 12.6 | Sauces | 350/300/240 | 46 |
| 12.7 | Salads and savoury based sandwich spreads | 350/300/240 | 46 |
| 12.8 | Yeast and yeast products | 350/300/240 | 46 |
| 12.9 | Protein products, excluding products covered in category 1.8 | 350/300/240 | 46 |
| 13 | Foods intended for particular nutritional uses as defined by Directive 2009/39/EC | ||
| 13.4 | Foods suitable for people intolerant to gluten | 350/300/240 | 46 |
| 14 | Beverages | ||
| 14.1 | Non‐alcoholic beverages | 175/150/120 | 23 |
| 15 | Ready‐to‐eat savouries and snacks | ||
| 15.1 | Potato‐, cereal‐, flour‐ or starch‐based snacks | 350/300/240 | 46 |
| 15.2 | Processed nuts | 350/300/240 | 46 |
| 16 | Desserts excluding products covered in category 1, 3 and 4 | ||
| 16 | Desserts excluding products covered in category 1, 3 and 4 | 350/300/240 | 46 |
| 18 | Processed foods not covered by categories 1 to 17, excluding foods for infants and young children | ||
| 18 | Processed foods not covered by categories 1 to 17, excluding foods for infants and young children | 350/300/240 | 46 |
EDTA: ethylenediaminetetraacetic acid.
Commission Decision of 14 June 2010 authorising the placing on the market of Ferric Sodium EDTA as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council.
Expressed as anhydrous EDTA.
3.2.2. Use in food supplements according to Directive 2002/46/EC
Currently, ferric sodium EDTA, expressed as anhydrous EDTA, is allowed for use in food supplements at doses up to 18 and 75 mg EDTA/day for children and adults respectively. The applicant is proposing a single dose of ferric sodium EDTA up to 75 mg EDTA/day for both adults and children, corresponding to intake levels of iron from food supplements up to 14 mg/day.
3.2.3. Use in foods for specific groups (FSG) according to Commission Regulation (EU) No 609/2013
Currently, ferric sodium EDTA, expressed as anhydrous EDTA, is allowed for use in foods for special medical purposes and total diet replacement for weight control at maximum amounts of 12 mg EDTA/100 g of final food.
The applicant proposed changes to increase the current maximum amount of ferric sodium EDTA, expressed as anhydrous EDTA, allowed in foods for special medical purposes and total diet replacement for weight control to 24 mg EDTA per 100 g of final food or per individual serving of final food, when (according to the label) smaller than 100 g. The applicant also requested to extend the authorisation to also include processed cereal‐based foods and baby foods at the same proposed use levels. With the new maximum amounts of ferric sodium EDTA proposed by the applicant, the corresponding levels of iron from FSG would be up to 4.6 mg per 100 g of final product. The proposed use levels for ferric sodium EDTA in FSG and the corresponding level of iron are reported in Table 4 (Documentation provided to EFSA n. 2).
Table 4.
Current maximum amounts of ferric sodium EDTA and proposed changes to uses and use levels for ferric sodium EDTA in FSG and corresponding iron levels
| Food category number | Food category name | Proposed use levels (mg/kg) of ferric sodium EDTA expressed as
|
Corresponding level of iron (mg/kg) |
|---|---|---|---|
| Current authorised uses a | |||
| – | Foods for special medical purposes | 120b | 23 |
| – | Total diet replacement for weight control | 120b | 23 |
| Proposed new uses | |||
| 13 | Foods intended for particularnutritional uses as defined by Directive 2009/39/EC | ||
| 13.1.3 | Processed cereal‐based foods and baby foods for infants and young children as defined by Directive 2006/125/EC | 350/300/240 | 46 |
| 13.2 | Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5) | 350/300/240 | 46 |
| 13.3 | Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet) | 350/300/240 | 46 |
EDTA: ethylenediaminetetraacetic acid.
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009.
Expressed as anhydrous EDTA.
3.3. Exposure estimate
3.3.1. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (EFSA, 2011a). New consumption surveys added in the Comprehensive database were also taken into account in this assessment.
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database represents the best available source of food consumption data across Europe at present.
Food consumption data from the following population groups: infants, toddlers, children, adolescents, adults and the elderly were used for the exposure assessment. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 5).
Table 5.
Population groups considered for the exposure estimates of ferric sodium EDTA
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finlandb, Germanyb, Italyb, UKb |
| Toddlers | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finlandb, Germanyb, Italyb, Netherlands, Spain, UKb |
| Childrena | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finlandb, Franceb, Germanyb, Greece, Italyb, Latvia, Netherlandsb, Spain, Sweden, UKb |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finlandb, Franceb, Germanyb, Italyb, Latviab, Netherlandsb, Spain, Swedenb, UKb |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finlandb, Franceb, Germany, Hungary, Irelandb, Italyb, Latviab, Netherlandsb, Romania, Spain, Swedenb, UKb |
| The elderlya | From 65 years of age and Older | Austria, Belgium, Denmark, Finlandb, Franceb, Germany, Hungary, Irelandb, Italyb, Netherlandsb, Romania, Swedenb, UKb |
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Dietary surveys considered by the NDA Panel in its opinion on Dietary Reference Values for iron (EFSA NDA Panel, 2015).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure estimates. In practice, the FoodEx food codes were matched to the FCS food categories.
3.3.2. Exposure to ferric sodium EDTA from its proposed uses
The previous EFSA opinion on ferric sodium EDTA (EFSA ANS Panel, 2010), concluded that if ferric sodium EDTA was consumed from all three of the intended sources (PARNUTS, fortified foods and supplements), the combined mean and 95th percentile EDTA intakes would be 8.6 and 9.5 mg/kg bw per day, respectively, for children and 4.2 and 4.8 mg/kg bw per day, respectively, for adults at the 95th percentile. The Panel noted that this would exceed the ADI established by JECFA for calcium disodium EDTA of 2.5 mg/kg bw per day (1.9 mg EDTA/kg bw per day) (JECFA, 2007). The Panel also noted that when ferric sodium EDTA is used in PARNUTS or food supplements at levels which provide 22.3 mg iron/day for an adult and 11.1 mg iron/day for a child, the corresponding exposure to EDTA would be 1.9 mg EDTA/kg bw per day for adults and 3.9 mg EDTA/kg bw per day for children.
Estimate of exposure based on the Food Additives Intake Model (FAIM) template
The applicant did not provide an estimate of the exposure to ferric sodium EDTA in the context of the present dossier. The Panel therefore decided to perform a new estimate exposure using the FAIM tool (version 2, June 2018). For the estimation of the exposure, the Panel considered the uses proposed by the applicant (Documentation provided to EFSA n. 2) and reported in Sections 3.2.1, 3.2.2 and 3.2.3, with the exceptions listed below because data were not available in the FAIM tool for the following food categories: 1.3 – Unflavoured fermented products, heat‐treated after fermentation; 6.6 – Batters; 6.7 – Pre‐cooked or processed cereals; and 13.4 ‐ Foods suitable for people intolerant to gluten.
The food category 13.1 as proposed by the applicant (Documentation provided to EFSA n. 2) was interpreted as referring to 13.1.3 – Processed cereal‐based foods and baby foods for infants and young children as defined by Directive 2006/125/EC, which was not included in the table of proposed uses provided by the applicant, but is part of the extension of use requested.
For the food category 14.1 ‐ Non‐alcoholic beverages, in the absence of further specifications from the applicant it was assumed that the proposed uses included the corresponding subcategories 14.1.2.1, 14.1.2.2, 14.1.3, 14.1.4.1, 14.1.4.2, 14.1.5, and would exclude water as defined in subcategory 14.1.1.
Considering that the food category 18 (Processed foods not covered by categories 1–17, excluding foods for infants and young children) is extremely unspecific (e.g. composite foods), processed foods, prepared or composite dishes belonging to the food category 18 were reclassified under food categories in accordance to their main component. Therefore, food category 18, although included in the list of proposed uses by the applicant is not taken into account as contributor to the total exposure estimates.
The results of the estimated intake of iron and exposure to EDTA calculated from the proposed uses of ferric sodium EDTA are reported in Table 6.
Table 6.
Summary of anticipated intake of iron and exposure to EDTA from the proposed uses of ferric sodium EDTA. Between brackets: number of surveys
| Estimated exposure | Infants (4–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) |
|---|---|---|---|---|---|---|
| A. Dietary intake of iron (EFSA NDA Panel, 2015 ) a (mg/day) | ||||||
| 2.6–6.0 (4) | 5.0–7.0 (5) | 7.5–11.5 (7) | 9.2–14.7 (7) | 9.4–17.9 (8) | 9.4–17.9 (8) | |
| B. Population reference intake for iron (EFSA NDA Panel, 2015 ) a (mg/day) | ||||||
| 11 | 7 | 11 | 13(F) 11(M) | 11–16(F)b 11(M) | 11(F) 11(M) | |
| C. Estimated iron intake from ferric sodium EDTA in fortified foods, and foods for specific groups (mg/day) c | ||||||
| Mean | 3.5–18 (6) | 23.2–35.9 (10) | 28.9–54.3 (18) | 24.9–73.2 (17) | 24.3–60.4 (17) | 26.6–61.3 (14) |
| D. Estimated iron intake from ferric sodium EDTA in food supplements (mg/day) | ||||||
| – | – | 14 | 14 | 14 | 14 | |
| E. Estimated combined iron intake from all proposed uses of ferric sodium EDTA (mg/day) | ||||||
| Mean | 3.5–18 | 23.2–35.9 | 42.9–68.3 | 38.9–87.2 | 38.3–74.4 | 40.6–75.3 |
| F. Estimated exposure to EDTA from ferric sodium EDTA in fortified foods, and foods for specific groups (mg/kg bw per day) | ||||||
| Mean | 3.7–18.8 (6) | 10.1–15.6 (10) | 6.5–12.3 (18) | 3.0–6.2 (17) | 1.8–4.5 (17) | 2.0–4.6 (14) |
| High‐level (P95) | 8.1–23.3 (5) | 17.7–26.0 (7) | 11.0–19.4 (18) | 4.6–10.3 (17) | 3.3–8.1 (17) | 3.5–8.8 (14) |
| G. Estimated exposure to EDTA from ferric sodium EDTA in food supplements (mg/kg bw per day) | ||||||
| – | – | 3 | 1–2 | 1 | 1 | |
| H. Estimated combined exposure to EDTA from all proposed uses of ferric sodium EDTA (mg/kg bw per day) | ||||||
| Mean | 3.7–18.8 | 10.1–15.6 | 9.5–15.3 | 4.0–8.2 | 2.8–5.5 | 3.0–5.6 |
| High‐level (P95) | 8.1–23.3 | 17.7–26.0 | 14.0–22.4 | 5.6–12.3 | 4.3–9.1 | 4.5–9.8 |
| I. Estimated exposure to EDTA from [13.1.3] Processed cereal‐based foods and baby foods (mg/kg bw per day) | ||||||
| Mean | 1.2–8.5 (5) | 0.2–1.4 (8) | – | – | – | – |
EDTA: ethylenediaminetetraacetic acid; M: male; F: female; bw: body weight.
Although the classification into age groups used by the NDA Panel in its 2015 opinion was slightly different from the one used by the ANS Panel, it was assumed that estimates of iron intake would still be comparable. The following age groups were used by the NDA Panel: < 1 year; 1 to < 3 years; 3 to < 10 years; 10 to < 18 years; > 18 years.
For detail on women's Population Reference Intake values, refer to Table 1.
Calculated using default body weights as defined by the EFSA Scientific committee (2012).
The Panel noted that the estimated ranges of combined exposure to EDTA from the uses and use levels of ferric sodium EDTA proposed by the applicant (Table 6, row H) will exceed the current ADI established for EDTA (1.9 mg/kg bw per day) in all population groups at the mean and 95th percentile.
The Panel further noted that, with respect to the proposed extension of use to include Processed cereal based foods and baby foods, the estimated ranges of exposure to EDTA from this source would be 1.2–8.5 mg/kg bw per day for infants and 0.2–1.4 mg/kg bw per day for toddlers (Table 6, row I).
Table 7 reports the main food categories contributing to total exposure to EDTA and iron intake from the proposed uses of ferric sodium EDTA.
Table 7.
Main food categories contributing to total exposure to EDTA and iron intake calculated from the proposed use levels of ferric sodium EDTA in fortified foods, and FSG, across dietary surveys using FAIM (version 2). Results are shown as range of contribution (%) and number of surveys (> 5% to the total mean exposure)
| Food category number | Food category name | Infants (4–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥65 years) |
|---|---|---|---|---|---|---|---|
| 1.1 | Unflavoured pasteurised and sterilised (including UHT) milk | 7.4–68.0 (6) | 14–50.6 (10) | 10.3–52.1 (18) | 7.2–45.8 (17) | 7.6–42.0 (15) | 5.8–22.2 (14) |
| 1.2 | Unflavoured fermented milk products, including natural unflavoured buttermilk (excluding sterilised buttermilk) non heat treated after fermentation | 36.1 (1) | 5.3–30.1 (2) | 19.2 (1) | 5.3 (1) | 5.2–6.2 (3) | 5.9–7.9 (5) |
| 1.4 | Flavoured fermented milk products including heat treated products | 5.3 (1) | 5.8–13.6 (5) | 7.8–15.2 (8) | 5.9–7.1 (5) | 5.3 (1) | – |
| 4.2 | Processed fruit and vegetables | 12.7–38.8 (3) | 6.2–13.6 (9) | 7.3–16.7 (14) | 5.6–16.3 (14) | 5.4–18.3 (15) | 6.0–19.8 (14) |
| 6.1 | Whole, broken, or flaked grain | – | – | 5.6 (1) | – | – | – |
| 6.2 | Flours and other milled products and starches | 13.8 (1) | 6.7 (1) | 7.7 (1) | 5.6–10.2 (3) | 5.4–7.5 (4) | 7.4 (1) |
| 6.3 | Breakfast cereals | 8.7–21.5 (4) | 7.0–14.9 (5) | 6.0–10.6 (8) | 5.2–8.5 (7) | 7.3–17.2 (3) | 5.6–29.2 (4) |
| 6.4 | Pasta | – | 6.7 (1) | 6.9–9.0 (2) | 8.2–8.4 (2) | 8.7 (1) | 9.2 (1) |
| 7.1 | Bread and rolls | 8.2 (1) | 5.1–11.9 (6) | 5.7–16.0 (17) | 7.3–17.3 (16) | 6.9–21.7 (17) | 5.9–22.1 (14) |
| 7.2 | Fine bakery wares | – | 8.7 (1) | 5.1–11.4 (14) | 5.6–10.9 (14) | 5.7–9.5 (10) | 5.3–10.5 (7) |
| 12.5 | Soups and broths | – | 7.7 (1) | 6.5–14.1 (2) | 5.7–12.5 (2) | 7.1–13.6 (3) | 6.0–14.4 (5) |
| 12.7 | Salads and savoury based sandwich spreads | – | – | – | – | 5.7–6.1 (2) | – |
| 13.1.3 | Processed cereal‐based foods and baby foods for infants and young children as defined by Directive 2006/125/EC | 13.1–65 (5) | 5.2–11.4 (4) | – | – | – | – |
| 14.1.2.1 | Fruit juices as defined by Directive 2001/112/EC | 5.1 (1) | 6.2–11.4 (5) | 5.1–12.0 (13) | 5.2–16.0 (11) | 5.6–10.0 (2) | 6.4 (1) |
| 14.1.4.1 | Flavoured drinks with sugar | – | 5.5–11.6 (3) | 5.3–14.7 (13) | 5.2 ‐20.1 (15) | 5.4–11.5 (10) | 6.8 (1) |
| 14.1.4.2 | Flavoured drinks with sweetener | – | – | 7.2 (1) | 8.0 (1) | 5.1 (1) | – |
| 14.1.5 | Coffee, tea, herbal and fruit infusions, chicory; tea, herbal and fruit infusions and chicory extracts; tea, plant, fruit and cereal preparations for infusions, as well as mixes and instant mixes of these products | – | 6.2 (1) | 5.4–11.3 (5) | 5.1–13.6 (6) | 5.7–34.2 (16) | 11.4–39.7 (14) |
| 16 | Desserts excluding products covered in category 1, 3 and 4 | – | 5.2 (1) | – | – | – | – |
EDTA: ethylenediaminetetraacetic acid; FAIM: Food Additives Intake Model; FSG: foods for specific groups.
The Panel noted that unflavoured pasteurised and sterilised (including UHT) milk [1.1] is one of the major contributors in all the population groups. With lower contributions the same applies to processed fruit and vegetables [4.2]. Unflavoured fermented milk products [1.2] are highly contributing to the intake of EDTA in younger population groups; while breakfast cereals [6.3], bread and rolls [7.1] and coffee, tea, herbal and fruit infusions, chicory [14.1.5] highly contribute to the intake of EDTA in older population groups.
The Panel further noted that the contribution of processed cereal‐based foods and baby foods [13.1.3] from the proposed extension of use, would range from approximately 13% to 65% of the total exposure to EDTA in infants and from 5 to 11% in toddlers.
3.3.2.1. Uncertainty analysis
Uncertainties in the exposure assessment of ferric sodium EDTA as a source of iron have been introduced in the previous section. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the sources of uncertainties have been considered and summarised in Table 8.
Table 8.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/– |
| Use of data from food consumption surveys covering only a few days to estimate high percentiles (95th) long‐term (chronic) exposure | + |
| Food categories selected for the exposure assessment: exclusion of food categories due to missing FoodEx linkage (5/out of 34 categories) | – |
| Proposed maximum level exposure assessment scenario: proposed use levels considered for all items within the food category | + |
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
Overall, the Panel considered that the uncertainties identified would, in general, result in an overestimation of both the real exposure to ferric sodium EDTA from its proposed use as a source of iron added for nutritional purposes to foods for the general population, food supplements and foods for special medical purposes, total diet replacement for weight control and processed cereal‐based foods and baby foods, and to the total exposure to EDTA and iron intake from the proposed uses of ferric sodium EDTA.
3.4. Biological and toxicological data
The safety of ferric sodium EDTA as a source of iron and the bioavailability of iron from this source have been previously evaluated by the ANS Panel (EFSA ANS Panel, 2010). The previous conclusions on ferric sodium EDTA were reached by the Panel on the basis of the evaluation of its components and in particular of the ADI for EDTA established by JECFA (JECFA, 2007).
In support to the present application, the applicant has not provided any new data generated with ferric sodium EDTA but has submitted data from studies conducted on other EDTA salts.
Ferric sodium EDTA, like other EDTA‐metal complexes, dissociates in the gut to iron and EDTA; hence, the applicant deemed toxicological studies of other EDTA salts also relevant when considering the safety of ferric sodium EDTA. The Panel agreed that data generated with other EDTA salts could be considered relevant for the safety assessment of ferric sodium EDTA.
3.4.1. Absorption, distribution, metabolism and excretion (ADME)
3.4.1.1. Bioavailability of iron from ferric sodium EDTA
In its previous opinion (EFSA ANS Panel, 2010), the Panel noted that information on the bioavailability of iron from ferric sodium EDTA based on iron fortification studies in humans was available. From those studies, the Panel deduced that iron was liberated from the complex and that it was bioavailable despite the presence of inhibitory factors in the diet that might form insoluble complexes with iron. The Panel also noted data indicating that iron in the form of ferric sodium EDTA was two to three times more bioavailable than iron in the form of ferrous sulphate and that it was efficiently incorporated into haemoglobin.
The Panel also noted that the absorption of iron from ferric sodium EDTA was regulated physiologically by the body's iron status, in a manner similar to that for other iron compounds and that dietary iron fortification with ferric sodium EDTA was not expected to result in iron overload in iron‐repleted individuals.
In 2010, the Panel reviewed a study of iron absorption from ferric sodium EDTA in pigs (Candela et al., 1984) demonstrating that the iron in ferric sodium EDTA dissociates from the chelate and is released into the luminal inorganic iron pool. The majority of the excreted iron was found in the faeces, in an insoluble form, which was not identified by the authors. Less than 1% of ferric‐EDTA complex‐ion and not more than 5% of the EDTA moiety was absorbed after dissociation from iron (likely bound to other metals). The iron and the EDTA that were found in the faeces were not associated with each other. The majority of the EDTA remained in the soluble pool, while the Fe was in an insoluble fraction. The authors did not determine the nature of this iron. The authors reported that the iron that was absorbed was incorporated into haemoglobin.
In the context of this application, the applicant has provided three new field trials aimed at demonstrating efficacy of a food fortification regimen with ferric sodium EDTA. All three studies were controlled, double‐blinded, randomised trials on children using different food vehicles fortified with ferric sodium EDTA: porridge prepared with maize flour supplemented with 28 or 56 mg iron/kg flour (Andang'o et al., 2007), chapattis prepared with wheat flour supplemented with 60 mg iron/kg flour (Muthayya et al., 2012) and biscuits prepared with wheat flour supplemented with 3.6 mg iron/biscuit (Bouhouch et al., 2016) for a duration of 5–7 months. The Panel noted these new publications provided by the applicant as part of the dossier and considered them as supporting evidence for the bioavailability of iron from ferric sodium EDTA.
The Panel noted that no data on the potential difference in the bioavailability of Ferrazone® and Ferrazone XF® were provided.
3.4.1.2. Studies with other EDTA salts
In addition to the studies previously evaluated by the Panel, the applicant has submitted further information on the ADME of other EDTA salts.
In a rat study (Foreman et al., 1953), an oral dose of 50 mg/kg bw of 14C‐labelled calcium disodium EDTA was poorly absorbed in the gastrointestinal tract. Absorption after 24 h was between 2% and 4% with 80–95% of the dose appearing in the faeces within 24 h; absorption was still apparent at 48 h. In animals given intraperitoneal injections, the EDTA was rapidly excreted in the urine (85% by 1.5 h, 95% in 25 h). According to the authors in the stomach, the calcium‐EDTA complex‐ion dissociated at the low pH, which was subsequently followed by precipitation of the acid form of EDTA and slow re‐dissolution in the intestines.
Srbová and Teisinger (1957) injected 200 mg of calcium disodium EDTA into the duodenum of rats, and 2 h later observed that the amount of complex not recovered ranged from 6.5% to 26%.
In support of the current application, the applicant has provided full study reports from two studies (Yang, 1952; Chan, 1956), which were part of PhD theses and were available to the Panel only in summary format at the time of the previous assessment of ferric sodium EDTA.
In rats fed disodium EDTA in the diet at levels of 0.5%, 1.0% or 5.0% (Yang, 1952), approximately 82.2%, 44.5% and 45.4% of the ingested EDTA, respectively, were excreted, primarily in faeces (99.4%, 98.2% and 97.5%) and in small amounts in the urine.
Chan (1956) conducted a similar experiment, in which he observed that 32 h after a single dose of 95 mg disodium EDTA per rat administered by gavage, 93% of the EDTA was recovered from the colon. After doses of 47.5, 95, and 142.5 mg disodium EDTA, the amount of EDTA recovered in the urine was directly proportional to the dose given, suggesting that EDTA was absorbed from the gastrointestinal tract by passive diffusion.
In a human study (Foreman and Trujillo, 1954), an administered dose of 1.5 mg of 14C‐labelled calcium disodium EDTA was absorbed to an extent of 5%.
In a human study in which a single dose of 3 g of calcium disodium EDTA was administered orally (Srbová and Teisinger, 1957), only 2.5% of the complex was excreted in the urine.
3.4.2. Genotoxicity
In its previous assessment (EFSA ANS Panel, 2010), the Panel concluded that from the information available at the time there was no safety concern with respect to genotoxicity of ferric sodium EDTA as a source of iron added for nutritional purposes to food.
No further genotoxicity studies on ferric sodium EDTA were provided in the context of the present application.
3.4.3. Acute toxicity
3.4.3.1. Studies on ferric sodium EDTA
In its previous opinion (EFSA ANS Panel, 2010), the Panel noted that ferric sodium EDTA has a low acute oral toxicity with oral LD50 values of 2,710–10,000 mg/kg bw (equivalent to approximately 359–1,326 mg iron/kg bw, respectively) in male and female Sprague–Dawley rats, and 794 mg/kg bw (approximately 105 mg iron/kg bw) in male and female Kunming mice (Sichuan Provincial Sanitary and Anti‐epidemic Station, 1993; Whittaker et al., 2002).
3.4.3.2. Studies on other EDTA salts
In support of the present application, the applicant submitted additional information on acute toxicity studies with other EDTA salts.
Based on the new information provided in the dossier, the Panel noted that also calcium disodium EDTA was of low acute toxicity as the oral LD50 was reported to be 10,000 mg/kg bw in the rat (strain and species not specified), 7,000 mg/kg bw in the rabbit (strain and species not specified) and 12,000 mg/kg bw in the dog (Oser et al., 1963).
Similarly, the acute oral toxicity of disodium EDTA was low as the reported LD50 was from 2,000 to 2,200 mg in the rat (strain and species not specified) (Yang, 1952) and 2,300 mg/kg bw in the rabbit (Shibata, 1956).
3.4.4. Short‐term and subchronic toxicity
3.4.4.1. Studies on ferric sodium EDTA
In its previous opinion (EFSA ANS Panel, 2010), the ANS Panel reviewed three subchronic studies in rats with dietary exposure to ferric sodium EDTA.
One of these studies was a 90‐day study by Sichuan Provincial Sanitary and Anti‐epidemic Station (1993) where the authors identified a no observed adverse effect level (NOAEL) of 160 mg ferric sodium EDTA/kg bw per day.
The second was a 90‐day study where the dose tested amounted to 2,500 mg/kg bw per day (Su et al., 1999). Based on the results, the ANS Panel had identified a NOAEL of 250 mg ferric sodium EDTA/kg bw per day.
In the third study of 61 days in rats (Appel et al., 2001), a NOAEL of 84 mg ferric sodium EDTA/kg bw per day was proposed by the authors. When expressed as iron, this NOAEL corresponded to 11.2 mg iron/kg bw per day. In its opinion (EFSA ANS Panel, 2010), the ANS Panel noted, that JECFA concluded in 2000, based on this study, that administration of ferric sodium EDTA in the diet would not result in a greater uptake of iron once nutritional requirements for iron were met (JECFA, 2007).
3.4.4.2. Studies on other EDTA salts
In addition to the studies previously evaluated, the applicant has submitted four other subchronic studies, which were not available to the ANS Panel in 2010. The studies are summarised below.
Thirty‐three albino rats were divided into five groups and received a standard rodent diet (Purina Fox Chow) added with either 0 (group 1, 3 males and 5 females; and group 5, 1 males and 2 females), 0.5 (group 2, 3 males and 5 females), 1.0 (group 3, 3 males and 5 females) or 5% (group 4, 1 males and 5 females) of disodium EDTA8 (Yang, 1952, 1964) for 12 weeks. These doses were equivalent to 0, 250, 500 and 2,500 mg/kg bw per day of disodium EDTA. The author noted that the rats from groups 4 and 5 were littermates born from dams kept on diet added 0.5% disodium EDTA for 8 months. The only parameters monitored were clinical conditions, body weight and feed intake. All animals survived to the end of week 12. According to the authors, the only differences to controls were a continuing diarrhoea and lower feed intake in the high‐dose group. The Panel noted that male and female rats receiving 5% of disodium EDTA had lower body weights than their littermates control (e.g. on day 84: for males 132 g vs 300 g in the control and for females 133 g vs 180 g in the controls). The animals were not killed but continued for a 2‐year period as ‘a chronic toxicity experiment’ since, according to the author, the results of this subchronic toxicity study did not show any marked toxic effect of the test substance (see Section 3.4.5 for further description). Based on the limitations in the study design and reporting, the Panel considered this study not suitable for risk assessment of ferric sodium EDTA.
In a second study, 50 weanling albino rats (Wistar, initial body weight of 50–70 g) of both sexes were divided into five groups and received either a basal low mineral diet (control group, 7 males and 6 females) or this diet added 0.5% or 1% of disodium calcium EDTA (4 males and 4 females, and 6 males and 2 females, respectively) or 0.5% or 1% disodium EDTA9 (5 males and 6 females, and 4 males and 6 females, respectively) for 90 days (Chan, 1956, 1964). The only parameters monitored were clinical conditions, body weight and feed intake. As reported by the author at the end of the study period, body weights and feed intake in groups receiving 0.5% and 1% disodium calcium EDTA were comparable to those in the control group. A slight increase in body weight was reported for the 0.5% disodium EDTA group. In the 1% disodium EDTA group, a slight decrease in body weight, anaemic appearance and diarrhoea were reported. The author concluded that there was no apparent toxicity in all test groups and the study therefore continued for 205 days (see below).
The rats from the above 90‐day study continued on the respective diets up to 205 days (Chan, 1956, 1964). The survival was not different between the experimental and control groups. In the 1% disodium EDTA group, animals appeared anaemic and manifested slight diarrhoea, while clinical appearance of other test groups was similar to that of controls. The author considered that the diarrhoea was most probably due ‘to the action of EDTA on the intestinal tract influencing its absorption and secretory function’. Final body weight, body weight gain and feed intake of all test groups were not statistically significantly different from the controls. Statistical analyses of data from limited haematological and clinical chemistry examinations demonstrated a decrease (p < 0.01) in erythrocyte and leucocyte counts, increase (p < 0.01) in coagulation time and serum calcium content in males and females from the 1% disodium EDTA group as compared to controls. Furthermore, erythrocyte count was decreased (p < 0.05) and leucocyte count was increased (p < 0.01) in the 0.5% disodium calcium EDTA and 0.5% disodium EDTA groups, and erythrocyte count was also decreased (p < 0.01) in 1% disodium EDTA group. Histopathological examination which was limited to liver, kidney and spleen did not reveal any appreciable differences between the treated and the control groups. The author noted, however, sinusoid dilatation and slight increase in number of Kupffer cells in the livers and occasionally indistinct appearance of tubules in the kidney in 1% disodium EDTA group as compared to controls. The author considered that for both EDTA salts there was absence of adverse effects at 0.5% and 1% concentrations in the diet. The Panel noted that feeding 1% disodium EDTA was associated with changes in some haematological parameters and with slight osmotic diarrhoea, which is regarded as an undesirable effect. The Panel further noted that the relevance of the study was limited because of the low number of animals and the limited number of parameters measured.
The applicant also provided an additional toxicity study in the rat performed with disodium EDTA,8 which is a part of another PhD thesis work (Krum, 1948). Weanling male and female Wistar rats (mean body weight 60 g) received either 0% (control, n = 6, number per sex not informed) or 0.5% (n = 9, number per sex not informed) of disodium EDTA in the diet for 44 weeks. This dietary concentration was equivalent to 250 mg disodium EDTA/kg bw per day or equal to 196 mg/kg bw per day based on recorded feed intake. Body weight and feed intake (the only parameters monitored), were recorded once weekly throughout the study and were not different between the groups. All animals survived to the termination except one from the 0.5% group (sex not reported) which died in week 38 from respiratory infection. The Panel noted that this study was performed on a low number of animals, with one dose level, no haematology, clinical chemistry examinations or urinalysis were performed in the study, the reporting was limited to body weight and feed intake and no pathology reporting was included in the thesis. Based on the limitations in the study design and reporting, the Panel considered this study not suitable for risk assessment of ferric sodium EDTA.
In a 12‐month study, 16 mongrel dogs (6 months of age, 2 males and 2 females in the control and 1 male and 3 females in each of the test groups) received either a control diet (30 g ration/kg bw per day) or the control diet supplemented with 50, 100 or 250 mg calcium disodium EDTA/kg bw per day (equal to 58, 130 and 338 mg/kg bw per day) (Oser et al., 1963). No statistically significant differences were reported in body weight, clinical pathology parameters or organ weights between the calcium disodium EDTA‐treated groups and controls. No evidence of osteoporosis or other osseous changes was reported and there were no notable macroscopic or microscopic changes.
3.4.5. Chronic toxicity and carcinogenicity
3.4.5.1. Studies on other EDTA salts
In its previous opinion (EFSA ANS Panel, 2010), the Panel noted that no chronic toxicity or carcinogenicity studies had been conducted with ferric sodium EDTA; however, several studies were presented that had been conducted with other EDTA salts such as trisodium EDTA (NCI, 1977), calcium disodium EDTA (Oser et al., 1963) and disodium EDTA (Yang, 1964). From these studies, the Panel concluded that EDTA salts do not raise concern with respect to carcinogenicity.
The applicant has now provided the full study report of a chronic toxicity study that was available only in summary form at the time of the previous EFSA assessment of ferric sodium EDTA. The study was a 2‐year toxicity study in the rat with disodium EDTA, which was the follow up of the 12‐week rats study by Yang (1952, 1964) described in Section 3.4.4. The five groups of Wistar rats previously fed 0%, 0.5%, 1% or 5% of disodium EDTA in their diets for 12 weeks continued on their respective diets for a total period of 2 years. The survival (based on combined mortality for males and females reported by the author) was 37.5%, 62.5%, 75% and 100% in the control, 0.5%, 1% and 5% groups, respectively. Pneumonia was reported as the cause of the death. According to the author, the body weights of males and females in all treated groups were not statistically significantly different from those in the respective controls. Feed intake, erythrocyte counts and blood coagulation times (recorded at the end of the study period), ash content of bones and histological examination of several organs and tissues did not reveal any differences which could be attributed to treatment. The Panel noted low number of animals per group (see Section 3.4.4) and that no information was given on the clinical condition of the animals and whether the continuous diarrhoea in the 5% group reported in the subchronic phase of the study was also present during the 2‐year study period. With regard to results on body weight the Panel noted that: (1) the data on mean body weight of control males (group 1) were not available from week 88 to 104 because all males were dead (controls for group exposed to 0.5% and 1% of the test compound), (2) the body weights of males and females from 5% group tended to be lower than in their litter mate control (group 5) through whole experimental period. The Panel considered this study not suitable for risk assessment of ferric sodium EDTA because of the low number of animals and limited reporting.
3.4.6. Reproduction and Developmental Toxicity
Reproductive toxicity studies with other EDTA salts
In its 2010 assessment, the Panel considered one dietary multigenerational study with calcium disodium EDTA (Oser et al., 1963), and noted that no compound‐related mortality, reproductive, or teratogenic effects were reported. At the time, the Panel identified a NOAEL of 195 mg EDTA/kg bw per day (EFSA ANS Panel, 2010).
In the context of the current dossier the applicant has provided a new reproductive toxicity study, which was part of the PhD thesis from Yang (1952), assessed in Sections 3.4.4 and 3.4.5. Twenty‐four Wistar rats divided into four groups (2 males and 4 females each) were administered a control diet supplemented with 0%, 0.5%, 1% and 5% disodium EDTA,8 respectively. Animals were mated at 100 days of age. In order to obtain second litters, mating was repeated 10 days after weaning of the first litters. The author reported that the tests did not give consistent results. The animals fed 5% disodium EDTA in the diet did not produce any litters after 2 months of mating. However, the results of the 0.5% and 1% disodium EDTA groups were satisfactory in that the test animals all gave normal first and second litters. All litters were normal in size, growth, and health. The Panel considered the study too limited due to the very low number of animals used per group and the very limited reporting and therefore the Panel could draw no conclusions regarding reproductive toxicity effects.
Developmental toxicity studies with ferric sodium EDTA
In its previous opinion (EFSA ANS Panel, 2010), the Panel identified a NOAEL of 200 mg ferric sodium EDTA trihydrate/kg bw per day for developmental effects from a prenatal developmental toxicity study in rats (Sichuan Provincial Sanitary and Anti‐epidemic Station, 1993). The ANS Panel revisited the prenatal developmental study of the Sichuan Provincial Sanitary and Anti‐epidemic Station (1993) and noted that the study had limitations. It was unclear from the information available whether the compound was given in the diet or by gavage. The Panel considered that the NOAEL of the prenatal toxicity study should be 50 mg ferric sodium EDTA/kg bw per day (corresponding to 34.7 mg EDTA/kg bw per day). The Panel noted that this study cannot be used for risk assessment.
Developmental toxicity studies with other EDTA salts
In its previous opinion (EFSA ANS Panel, 2010), the Panel considered a developmental study conducted with several EDTA salts (EDTA, disodium EDTA dehydrate, trisodium EDTA monohydrate, calcium disodium EDTA dehydrate and tetrasodium EDTA dehydrate) (Schardein et al., 1981), administered by gavage from gestation day 7 to 14 to CD rats. The Panel considered that the NOAEL for developmental effects in this study was approximately 900 mg EDTA/kg bw per day.
3.5. Discussion
The present opinion deals with the evaluation of proposed changes to the current authorisation of ferric sodium EDTA as a novel food ingredient used as a source of iron. Within the current application, it is proposed to increase the maximum amounts of ferric sodium EDTA that can be added to foods for the general population, food supplements in children, foods for special medical purposes and total diet replacement for weight control. The applicant also proposed inclusion of ferric sodium EDTA in the list of substances permitted for use in processed cereal‐based foods and baby foods.
In the dossier submitted by the applicant in support of the current application, it was reported that two different products are being commercialised as ferric sodium EDTA: Ferrazone®, which was the subject of the previous evaluation by the ANS Panel (EFSA ANS Panel, 2010) and Ferrazone XF®, a product of finer consistency obtained by adding an extra grinding step to the manufacturing process of Ferrazone®.
The applicant has submitted analytical results from three batches of Ferrazone XF® to demonstrate compliance with the proposed update to the existing specifications.
The Panel however noted that no parameters have been proposed for the update of the specifications of ferric sodium EDTA with respect to particle size which could be used for the identification of the two different products. Furthermore, based on the analytical data provided, the Panel could not exclude the presence of particles of ferric sodium EDTA in the nano range in Ferrazone XF® in the solid form.
The Panel further noted that according to the applicant, there was no difference in the solubility in water of Ferrazone® and Ferrazone XF®.
To support the proposed increase in the maximum amounts of ferric sodium EDTA in currently authorised uses and to extend the use to processed cereals‐based food and baby food, the applicant proposed a revision of the current ADI for EDTA on the basis of newly submitted toxicological data on other EDTA salts, previously not available for assessment by the Panel.
The Panel noted that the currently authorised amounts of ferric sodium EDTA have been set such that the EDTA intake should not exceed the ADI of 1.9 mg/kg bw per day (EFSA ANS Panel, 2010). This ADI for EDTA of 1.9 mg/kg bw per day, established by JECFA from the ADI for calcium disodium EDTA (E 385) based on the study of Oser et al. (1963) (JECFA, 2007), was used by the ANS Panel in 2010.
Based on the additional studies provided and on the previous assessment (EFSA ANS Panel, 2010), the Panel considered that following oral ingestion, ferric sodium EDTA would dissociate in the gastrointestinal tract into its components, EDTA and iron. EDTA was not absorbed to a large extent and was excreted in the faeces. Minor amounts of EDTA may be absorbed but are not metabolised, and may be excreted unchanged in urine. Only a small proportion of the dissociated iron was absorbed; the largest fraction was excreted in an insoluble form in the faeces.
The Panel further noted that the ADME studies assessed may be of limited relevance for the newly proposed form of ferric sodium EDTA indicated by the applicant, i.e. Ferrazone XF®, since, due to its reduced particle size, the product may have different characteristics with regard to ADME. With the available data, the Panel was not in the position to draw conclusions on this matter.
In its previous opinion (EFSA ANS Panel, 2010), the ANS Panel reviewed three subchronic studies in rats with dietary exposure to ferric sodium EDTA. In the present dossier the applicant submitted four additional subchronic studies on EDTA salts other than ferric sodium EDTA. The Panel noted that the design and reporting of some of these studies had limitations, and were not considered suitable for risk assessment. The Panel further noted that these studies did not add any new relevant information to the database available for the previous evaluation of ferric sodium EDTA (EFSA ANS Panel, 2010).
The Panel considered that the two reproductive toxicity studies submitted in the dossier (Yang, 1952; Oser et al., 1963) provided insufficient data to evaluate the reproductive toxicity of ferric sodium EDTA.
The Panel noted that the prenatal developmental study of the Sichuan Provincial Sanitary and Anti‐epidemic Station (1993) was inadequate for risk assessment, but is nevertheless concerned about the effects of ferric sodium EDTA. The Panel was also concerned about the effects of EDTA given in the diet seen in other, albeit limited, studies. The difference in adverse developmental effects following different routes of administration (gavage vs dietary) were observed by Kimmel (1977). The effect in the dietary studies may be caused by binding of other metal ions to EDTA (e.g. zinc). Zinc deficiency can be the reason for the developmental effects (Hurley and Swenerton, 1971; Swenerton and Hurley, 1971). Furthermore, NOAELs of approximately 900 mg EDTA/kg bw per day were identified in prenatal developmental studies in rats with various forms of EDTA (EDTA, disodium EDTA dehydrate, trisodium EDTA monohydrate, calcium disodium EDTA dehydrate and tetrasodium EDTA dehydrate), when administered by gavage from gestational day 7 to 14 (EFSA ANS Panel, 2010). The Panel noted that a dietary prenatal developmental toxicity and a reproductive toxicity studies according to the current OECD guidelines are required.
On the basis of the information provided in the current dossier, the Panel considered that there was no sound scientific justification to increase the current ADI for EDTA and change the previous conclusion on ferric sodium EDTA.
The Panel has estimated intake levels for iron and exposure to EDTA resulting from the new proposed uses and use levels. The Panel noted that the exposure to EDTA for all population groups from the new proposed uses will significantly exceed the current ADI of 1.9 mg/kg bw per day at the mean and 95th percentile.
The Panel further noted that in accordance with Regulation (EU) No 257/2010, a full re‐evaluation of the safety of calcium disodium EDTA (E 385) as a food additive is to be performed by EFSA. In this context, the full toxicological database for this substance will be reviewed including the basis for establishing the ADI for EDTA. The Panel noted that additional toxicity studies will be needed for this re‐evaluation.
4. Conclusions
The Panel confirmed that ferric sodium EDTA is a source from which iron is bioavailable.
In assessing the safety of the proposed revision to the existing specifications for the novel food ingredient ferric sodium EDTA, the Panel noted that this would not discriminate between the previously evaluated substance (marketed as Ferrazone®) and the one of finer consistency (Ferrazone XF®) produced by adding an extra grinding step to the manufacturing process. In particular, the Panel noted that particle size was not one of the proposed parameters for the revised specifications. The Panel noted that it was not possible to determine whether particles of ferric sodium EDTA in the nano range were present in solid Ferrazone XF®.
The Panel concluded that the exposure assessment based on the proposed extension of uses and use levels would lead to the current ADI for EDTA being exceeded in all population groups at the mean and 95th percentile.
Furthermore, the Panel concluded that the toxicological data submitted by the applicant did not add any new relevant information to the database on which the current ADI was established.
Consequently, the Panel concluded that there was no basis to increase the ADI for EDTA and hence increase the use levels of ferric sodium EDTA or introduce additional uses as proposed by the applicant.
5. Recommendations
The Panel recommended that additional toxicological data should be provided to address the shortcomings in the available toxicity database prior to the re‐evaluation of calcium disodium EDTA (E 385) as a food additive.
Documentation provided to EFSA
Dossier ‘Application to Amend Annexes I and II of the Commission Decision of 14 June 2010 Authorising the Placing on the Market of Ferric Sodium EDTA as a Novel Food Ingredient [Under Regulation (EC) No 258/97 of the European Parliament and of the Council of 27th January 1997 Concerning Novel Foods and Novel Food Ingredients] and to Amend the Union List in the Annex of Regulation (EU) No 609/2013 of 12 June 2013 on Food Intended for Infants and Young Children, Food for Special Medical Purposes, and Total Diet Replacement for Weight Control’. February 2014. Submitted by AkzoNobel Business Area Specialty Chemicals.
Additional data provided on 8 November 2017. Submitted by AkzoNobel Business Area Specialty Chemicals in response to a request from EFSA.
Additional data provided on 10 April 2018. Submitted by AkzoNobel Business Area Specialty Chemicals in response to a request from EFSA.
Abbreviations
- ADI
acceptable daily intake
- ADME
absorption, distribution, metabolism and excretion
- ANS Panel
Panel on Food Additives and Nutrient Sources added to Food
- AR
average requirement
- AU
absorbance unit
- BW
body weight
- CAS
Chemical Abstracts Service
- EDTA
ethylenediaminetetraacetic acid
- EINECS
European List of Notified Chemical Substances
- EM
electron microscopy
- FAIM
Food additive intake model
- FCS
food categorisation system
- FSG
foods for specific groups
- IR
infrared radiation
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LD
lethal dose
- NDA Panel
Panel on Dietetic Products, Nutrition and Allergies
- NOAEL
no observed adverse effect level
- PARNUTS
Foodstuffs intended for particular nutritional uses
- PRI
Population Reference Intake
- SCF
Scientific Committee on Food
- UHT
ultra‐high temperature processing
- UL
Upper level
Suggested citation: EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gundert‐Remy U, Kuhnle GG, Lambré C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Tobback P, Mcardle H, Germini A and Gott D, 2018. Scientific opinion on the evaluation of authorised ferric sodium EDTA as an ingredient in the context of Regulation (EC) 258/97 on novel foods and Regulation (EU) 609/2013 on food intended for infants and young children, food for special medical purposes and total diet replacement for weight control. EFSA Journal 2018;16(8):5369, 27 pp. 10.2903/j.efsa.2018.5369
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00696
Panel members: Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipič, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Gunter Georg Kuhnle, Claude Lambré, Jean‐Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The Panel wishes to thank the members of the Working Group on Applications for the preparatory work on this scientific output, and EFSA staff members Fabiola Pizzo, Ana Maria Rincon and Camilla Smeraldi for the support provided to this scientific output.
Adopted: 26 June 2018
Notes
OJ L 43, 14.2.97, p. 1.
03 L 183, 12.7.2002, p. 5I.
01 L 404, 30.12.2006, p. 26.
OJ L 181, 29.6.2013, p. 35.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
Referred to as Sequestrene Na2 in the original PhD thesis.
Disodium calcium EDTA and disodium EDTA were referred to as Sequestrene Na2Ca and Sequestrene Na2 in the original PhD thesis.
References
- Andang'o PEA, Osendarp SJM, Ayah R, West CE, Mwaniki DL, De Wolf CA, Kraaijenhagen R, Kok FJ and Verhoef H, 2007. Efficacy of iron‐fortified whole maize flour on iron status of schoolchildren in Kenya: a randomised controlled trial. Lancet, 369, 1799–1806. [DOI] [PubMed] [Google Scholar]
- Appel MJ, Kuper CF and Woutersen RA, 2001. Disposition, accumulation and toxicity of iron fed as iron (II) sulphate or as sodium iron EDTA in rats. Food and Chemical Toxicology, 39, 261–269. [DOI] [PubMed] [Google Scholar]
- Bouhouch RR, El‐Fadeli S, Andersson M, Aboussad A, Chabaa L, Zeder C, Kippler M, Baumgartner J, Sedki A and Zimmermann MB, 2016. Effects of wheat‐flour biscuits fortified with iron and EDTA, alone and in combination, on blood lead concentration, iron status, and cognition in children: a double‐blind randomized controlled trial. American Journal of Clinical Nutrition, 104, 1318–1326. [DOI] [PubMed] [Google Scholar]
- Candela E, Camacho MV, Martinez‐Torres C, Perdomo J, Mazzarri G, Acurero G and Layrisse M, 1984. Iron absorption by humans and swine from Fe(III)‐EDTA. Further studies. Journal of Nutrition, 114, 2204–2211. [DOI] [PubMed] [Google Scholar]
- Chan MS, 1956. Some toxicological and physiological studies of ethylenediamine tetraacetic acid in the albino rat. PhD Thesis, University of Massachusetts, Amherst, MA, USA. [Google Scholar]
- Chan MS, 1964. “Some toxicological and physiological studies of ethylenediaminetetraacetic acid in the albino rat” ‐ Summaries of toxicology data. Toxicology of EDTA. Food and Cosmetics Toxicology, 2, 765–767. [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Scientific opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Scientific Opinion of the Panel on Food Additives and Nutrient Sources added to Food on choline‐stabilised orthosilicic acid added for nutritional purposes to food supplements following a request from the European Commission. EFSA Journal 2009;7(2):948, 23 pp. 10.2903/j.efsa.2009.948 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2010. Scientific Opinion on the use of ferric sodium EDTA as a source of iron added for nutritional purposes to foods for the general population (including food supplements) and to foods for particular nutritional uses. EFSA Journal 2010;8(1):1414. 10.2903/j.efsa.2010.1414 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance forsubmission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2004. Scientific opinion on a request from the Commission related to the Tolerable Upper Intake Level of iron. EFSA Journal 2004;2(11):125, 34 pp. 10.2903/j.efsa.2004.125 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015. Scientific Opinion on Dietary Reference Values for iron. EFSA Journal 2015;13(10):4254, 115 pp. 10.2903/j.efsa.2015.4254 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. EFSA Journal 2011;9(5):2140. 10.2903/j.efsa.2011.2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Chaudhry Q, Cubadda F, Gott D, Oomen A, Weigel S, Karamitrou M, Schoonjans A and Mortensen A, 2018. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: part 1, human and animal health. EFSA Journal 2018;16(7):5327, 114 pp. 10.2903/j.efsa.2018.5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman H and Trujillo TT, 1954. The metabolism of C14 ‐ethylenediaminetetraacetic acid in human beings. Journal of Laboratory and Clinical Medicine, 43, 566–571. [PubMed] [Google Scholar]
- Foreman H, Vier M and Magee M, 1953. The metabolism of C14‐labeled ethylenediaminetetraacetic acid in the rat. Journal of Biological Chemistry, 203, 1045–1053. [PubMed] [Google Scholar]
- Hurley LS and Swenerton H, 1971. Lack of mobilization of bone and liver zinc under teratogenic conditions of zinc deficiency in rats. The Journal of Nutrition, 101, 597–603. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1974. Annex 4: Acceptable daily intakes [Ethylenediaminetetraacetate, disodium and calcium disodium salts]. In: Toxicological Evaluation of Certain Food Additives With a Review of General Principles and of Specifications. Prepared by the 17th Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Geneva (Switz.): World Health Organization (WHO); WHO Technical Report Series No. 539, pp. 35–38.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Sodium iron ethylenediamine tetraacetic acid (EDTA). In: Toxicological Evaluation of Certain Food Additives and Contaminants. Prepared by the 53rd Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Geneva (Switz.): World Health Organization (WHO); WHO Food Additives Series, No. 44, pp. 105–111.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2007. Sixty‐eighth meeting, Geneva, 19‐28 June 2007. Summary and Conclusions. Toxicological recommendations and information on specifications. 1. Food additives and ingredients evaluated toxicologically or assessed for dietary exposure. Issued 12 July, 2007. Available online: http://www.who.int/ipcs/food/jecfa/summaries/summary68.pdf
- Kimmel CA, 1977. Effect of route of administration on the toxicity and teratogenicity of EDTA in the rat. Toxicology and Applied Pharmacology, 40, 299–306. [DOI] [PubMed] [Google Scholar]
- Krum JK, 1948. Toxicological and food usage studies of ethylenediamine tetraacetic acid. PhD Thesis, University of Massachusetts, Amherst, MA. [Google Scholar]
- Muthayya S, Thankachan P, Hirve S, Amalrajan V, Thomas T, Lubree H, Agarwal D, Srinivasan K, Hurrell RF, Yajnik CS and Kurpad AV, 2012. Iron fortification of whole wheat flour reduces iron deficiency and iron deficiency anemia and increases body iron stores in Indian school‐aged children. Journal of Nutrition, 142, 1997–2003. [DOI] [PubMed] [Google Scholar]
- NCI (National Cancer Institute), 1977. Bioassay of trisodium ethylenediaminietetraacetate trihydrate (EDTA) for possible carcinogenicity: CAS No. 150‐38‐9. National Cancer Institute (NCI), Division of Cancer Cause and Prevention, Carcinogenesis Program, Carcinogen Bioassay and Program Resource Branch, Bethesda, MD. NCI Carcinogenesis Technical Report Series, no 11; NCI‐CG‐TR‐11; DREW Publication No. (NIH) 77‐811. [PubMed]
- Oser BL, Oser M and Spencer HC, 1963. Safety evaluation studies of calcium EDTA. Toxicology and Applied Pharmacology, 5, 142–162. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food), 1977. Report of the Scientific Committee for Food on calcium disodium ethylenediamine tetra‐acetate. Opinion expressed 24 June, 1977. Available online: http://ec.europa.eu/food/fs/sc/scf/reports/scf_reports_04.pdf
- SCF (Scientific Committee for Food), 1990. Report of the Scientific Committee for Food on a second series of food additives of various technological functions. Opinion expressed 19 October 1990. Available online: http://ec.europa.eu/food/fs/sc/scf/reports/scf_reports_26.pdf
- SCF (Scientific Committee for Food), 2001. Guidance on submissions for safety evaluation of nutrients or of other ingredients proposed for use in the manufacture of foods. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out100_en.pdf
- Schardein JL, Sakowski R, Petrere J and Humphrey RR, 1981. Teratogenesis studies with EDTA and its salts in rats. Toxicology and Applied Pharmacology, 61, 423–428. [DOI] [PubMed] [Google Scholar]
- Shibata S, 1956. Toxicological studies of EDTA salt (disodium ethylenediaminetetraacetate). Nippon Yakurigaku Zasshi. Folia Pharmacologica Japonica, 52, 113–119. [Google Scholar]
- Sichuan Provincial Sanitary and Anti‐epidemic Station , 1993. Acute toxicity, mutagenicity and teratogenicity of NaFeEDTA in rats and mice [unpublished study]. Chengdu (China): Sichuan Provincial Sanitary and Anti‐epidemic Station. [Translation provided by Junshi Chen, Beijing; Chinese Center for Disease Control and Prevention, Institute of Nutrition and Food Safety].
- Srbová J and Teisinger J, 1957. Über die Resportion des Cacliumdinatriumsalzes der Äthylendiamintetraessigsäure bei der peroralen Verabreichung zur Therapie der Bleivergiftung [Absorption of CaNa2‐EDTA after oral administration for lead poisoning treatment]. Archiv für Gewerbepathologie und Gewerbehygiene, 15, 572–580. [PubMed] [Google Scholar]
- Su YL, Chen ZL, Zhang J and Jiang ZC, 1999. A 90‐day rat study on subchronic toxicity of NaFeEDTA [unpublished study]. Beijing; Capital Medical University. [Translation provided by Junshi Chen, Beijing; Chinese Center for Disease Control and Prevention, Institute of Nutrition and Food Safety].
- Swenerton H and Hurley LS, 1971. Teratogenic effects of a chelating agent their prevention by zinc. Science, 173, 62–64. [DOI] [PubMed] [Google Scholar]
- Whittaker P, Ali SF, Imam SZ and Dunkel VC, 2002. Acute toxicity of carbonyl iron and sodium iron EDTA compared with ferrous sulfate in young rats. Regulatory Toxicology and Pharmacology: RTP, 36, 280–286. [DOI] [PubMed] [Google Scholar]
- Yang S‐S, 1952. Toxicological investigation of ethylenediaminetetraacetic acid in the rat. PhD Thesis, University of Massachusetts, Amherst, MA, USA. [Google Scholar]
- Yang S‐S, 1964. “Toxicological investigation of ethylenediaminetetraacetic acid” in the rat ‐ Summaries of toxicology data. Toxicology of EDTA. Food and Cosmetics Toxicology, 2, 763–765. [Google Scholar]


