Abstract
Following a request from the European Commission, the EFSA Panel on Plant Health performed a pest categorisation of the seed‐borne bacterium Curtobacterium flaccumfaciens pv. flaccumfaciens. The pest is regulated in Council Directive 2000/29/EC (Annex IIB) as a harmful organism whose introduction into, and spread within, the protected zones (PZ) of Greece, Portugal and Spain shall be banned if present on seeds of Phaseolus vulgaris and of Dolichos. The bacterium is widely distributed outside the EU and causes a systemic vascular disease (bacterial wilt of bean) as well as bacterial tan spot disease on soybean. The pest was sporadically recorded in several EU Member States in the past, but is currently not known to occur in the EU. The identity of the bacterium is well established and identification methods are available. The major host is common bean (Phaseolus vulgaris), but other crops and weeds are, or may be, hosts or play a role as reservoirs, with uncertainties. Seed transmission remains uncertain for minor and alternative host species. The main pathway for entry is seed. The role of other pathways (e.g. irrigation water and infected residues) is uncertain. Should the bacterium enter the EU (including the PZ), it may establish, spread and have an impact on its host crops. The use of healthy seeds is the most effective control measure. Curtobacterium flaccumfaciens pv. flaccumfaciens fits all the criteria assessed by EFSA to be regarded as a Union quarantine pest.
Keywords: bacterial wilt of bean, European Union, pest risk, plant health, plant pest, quarantine, systemic vascular disease
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorizations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/20023, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pests categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 5) Potato virus T |
| 2) Andean potato mottle virus | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| 3) Arracacha virus B, oca strain | |
| 4) Potato black ringspot virus | |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Curtobacterium flaccumfaciens pv. flaccumfaciens is one of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest for the area of the European Union (EU) excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
Since C. flaccumfaciens pv. flaccumfaciens is regulated in protected zones (PZ) only, the scope of the categorisation is the territory of the PZ (Greece, Portugal and Spain), thus the criteria refer to the PZ instead of the EU territory.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on C. flaccumfaciens pv. flaccumfaciens was conducted at the beginning of the categorisation, using ISI Web of Science and Scopus databases, for the period 1922–2017. As search terms, the scientific name of this plant pathogen (i.e. full name, or limited to species or pathovar) as well as of the disease it causes were used. Relevant papers were then selected and reviewed. Further references and information were obtained from experts, from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the EPPO Global Database (EPPO, 2018).
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU were obtained from EUROSTAT.
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTE), and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States (MS) and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for C. flaccumfaciens pv. flaccumfaciens, following guiding principles and steps presented in the EFSA guidance on the harmonised framework for pest risk assessment (EFSA PLH Panel, 2010) and as defined in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
In accordance with the guidance on a harmonised framework for pest risk assessment in the EU (EFSA PLH Panel, 2010), this work was started following an evaluation of the EU's plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union regulated non‐quarantine pest in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required as per the specific terms of reference received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a regulated non‐quarantine pest. If one of the criteria is not met, the pest will not qualify. A pest that does not qualify as a quarantine pest may still qualify as a regulated non‐quarantine pest which needs to be addressed in the opinion. For the pests regulated in the PZ only, the scope of the categorisation is the territory of the PZ, thus the criteria refer to the PZ instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a PZ quarantine organism | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pest must be present in the risk assessment area) |
| Regulatory status (Section 3.3 ) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future |
The PZ system aligns with the pest free area system under the International Plant Protection Convention (IPPC). The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. PZ) |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the PZ areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the PZ areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6 ) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the PZ areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential PZ quarantine pest were met, and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, while addressing social impacts is outside the remit of the Panel, in agreement with the EFSA guidance on a harmonised framework for pest risk assessment (EFSA PLH Panel, 2010).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but, following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
Yes, the identity of the pest is established.
C. flaccumfaciens pv. flaccumfaciens (Hedges) Collins & Jones (Hedges, 1922) is a well‐defined Gram‐positive bacterium of the family Microbacteriaceae.
According to the current classification (Collins and Jones, 1984; Young et al., 1996, 2004; Bull et al., 2010), the species C. flaccumfaciens includes the following pathovars (pv.) that have distinct host ranges and that can easily be distinguished: pv. flaccumfaciens, pv. betae, pv. oortii, pv. poinsettiae and pv. ilicis. The pathovars basellae (Chen et al., 2000) and beticola (Chen et al., 2007) have also been described and proposed, but so far they have not been officially accepted by the International Society for Plant Pathology, Committee on the Taxonomy of Plant Pathogenic bacteria.
3.1.2. Biology of the pest
C. flaccumfaciens pv. flaccumfaciens causes a systemic vascular disease, called bacterial wilt of bean (Hedges, 1922, 1926a). It also causes bacterial tan spot disease on soybean (Dunleavy, 1983). Leaves of infected bean plants are flaccid, as well as the entire plant, particularly during the hottest hours of the day or under moisture stress. This is due to bacterial plugging of the vascular system, blocking the physiological acropetal water movement. Other foliar symptoms consist of interveinal necrotic lesions (‘firing’), with irregular margins and sometimes surrounded by yellow borders and haloes. In susceptible cultivars, wilting occurs 7–9 days after infection while firing appears about 7 days later. On bean seedlings and young plants, disease severity and mortality are higher than on adult plants. Generally, plant death occurs around 18–23 days after infection. The time course of the disease is most rapid above 27–30°C and under water stress, because these conditions seem to promote C. flaccumfaciens pv. flaccumfaciens multiplication and host systemic colonisation, as well as to enhance the negative effect of bacterial‐suppressed water supply (Hedges, 1926a).
Seeds produced by plants infected by C. flaccumfaciens pv. flaccumfaciens are systemically infected via the vascular system (Schuster and Smith, 1983; Hsieh et al., 2006). Generally, infected seeds appear asymptomatic, as well as infected pods, while sometime infected seeds are discoloured or yellow, orange or purple irregularly pigmented on their surface or on the hilus (Schuster and Christiansen, 1957; Schuster et al., 1968; Huang et al., 2006; Harveson and Vidaver, 2008; Harveson et al., 2015; Osdaghi and Lak, 2015; Osdaghi et al., 2016). Secondary (i.e. non‐seed‐borne) infections occur through wounds, made by rain and hailstorms, and infrequently through stomata (Evtushenko and Takeuchi, 2006).
C. flaccumfaciens pv. flaccumfaciens is a seed‐borne pathogen for common bean (Hedges, 1926b; Hsieh et al., 2006; Camara et al., 2009). It can overwinter, survive and remain viable in seeds, even up to 24 years under laboratory conditions, while on infected bean residues may survive under field conditions for about 8 months, depending on soil type, moisture content, and climatic conditions (Silva Júnior et al., 2012). Infected seeds are considered the most important source of inoculum and means for the pathogen spread over long and short distances (Hedges, 1926b; Zaumeyer, 1932; Zaumeyer and Thomas, 1957; Hsieh et al., 2006; Camara et al., 2009; Bastas and Sahin, 2017).
However, the biology and the epidemiology of the bacterium are still incompletely understood, for instance regarding the role of irrigation water on secondary infections (Harveson et al., 2015). No limiting environmental conditions for the disease are expected, provided host plants may grow. More important, evidence is accumulating about C. flaccumfaciens pv. flaccumfaciens associated with other crops as alternative hosts, including some grown in rotation with dry beans (e.g. wheat, corn, sunflower, alfalfa, barley, black oat, white oat, canola and ryegrass) and several Solanaceous plants (Harveson et al., 2015; Gonçalves et al., 2017; Osdaghi et al., 2018). However, seed transmission in other minor or alternative hosts has not been demonstrated yet.
3.1.3. Intraspecific diversity
The species C. flaccumfaciens shows intraspecific diversity leading to the distinction of various pathovars that infect different crops (Section 3.1.1). These pathovars can be easily discriminated through laboratory tests (Tegli et al., 2002; Guimaraes et al., 2003).
Within the pathovar flaccumfaciens, variants have been described based on pigments that may be produced in vitro on agarised media or in vivo in seeds (Schuster and Christiansen, 1957; Schuster et al., 1968; Huang et al., 2006; Harveson and Vidaver, 2008; Harveson et al., 2015; Osdaghi and Lak, 2015; Osdaghi et al., 2016). Those variants may also differ in their virulence (Harveson and Vidaver, 2008; Osdaghi et al., 2016).
3.1.4. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, the organism can be identified in vitro or in planta by various techniques.
Symptoms caused by C. flaccumfaciens pv. flaccumfaciens on plants may be sometimes confused with those caused by Xanthomonas axonopodis pv. phaseoli (Harveson et al., 2015). Infected seeds can be very often asymptomatic (Tegli, 2011).
Semi‐selective media for in vitro growth of C. flaccumfaciens pv flaccumfaciens are available (Mizuno and Kawai, 1993; Tegli et al., 1998; Maringoni and Camara, 2006; Maringoni et al., 2006), but methods based on isolation are quite time‐consuming and insufficiently specific (Tegli, 2011).
Polymerase chain reaction (PCR) tests with two different sets of primers have been designed for the identification of C. flaccumfaciens pv. flaccumfaciens isolated colonies and for its detection from bean seeds (Guimaraes et al., 2001; Tegli et al., 2002). However, only one of them was demonstrated to reliably detect all the strains of C. flaccumfaciens pv. flaccumfaciens, including the different pigmented variants found so far (Tegli et al., 2002; Osdaghi et al., 2018). Accordingly, an EPPO diagnostic standard exists for C. flaccumfaciens pv. flaccumfaciens (Tegli, 2011).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
C. flaccumfaciens pv. flaccumfaciens is present in North and South America, Africa, Asia and Australia (Figure 1 and Table 2).
Figure 1.
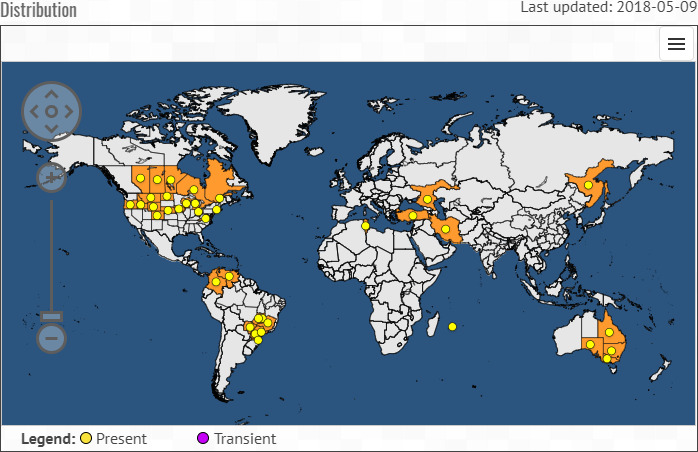
Global distribution map for Curtobacterium flaccumfaciens pv. flaccumfaciens (from the EPPO Global Database, accessed on 14 May 2018)
Table 2.
Global distribution of Curtobacterium flaccumfaciens pv. flaccumfaciens (extracted from the EPPO Global Database accessed on 14 May 2018)
| Continent | Country | Status – EPPO GD |
|---|---|---|
| Africa | Mauritius | Present, no details |
| Africa | Tunisia | Present, restricted distribution |
| Africa |
Brazil Distrito Federal, Goias, Mato Grosso do Sul, Minas Gerais, Parana, Santa Catarina, Sao Paulo |
Present, restricted distribution Present, no details |
| America |
Canada Alberta, Manitoba, Ontario, Québec, Saskatchewan |
Present, restricted distribution Present, no details |
| America | Colombia | Present, no details |
| America | Mexico | Absent, unreliable record |
| America |
United States of America Colorado, Connecticut, Idaho, Iowa, Michigan, Montana, Nebraska, North Dakota, Ohio, Oregon, Virginia, Wisconsin, Wyoming |
Present, restricted distribution Present, no details |
| America | Venezuela | Present, no details |
| Asia | Iran | Present, restricted distribution |
| Europe | Albania | Absent, unreliable record |
| Europe/Asia | Russia (Southern Russia and Far East) | Present, restricted distribution |
| Europe | Switzerland | Absent, invalid record |
| Europe | Serbia | Absent, pest no longer present |
| Europe | Ukraine | Absent, pest no longer present |
| Europe | Turkey | Present, few occurrences |
| Oceania |
Australia New South Wales Queensland South Australia Victoria |
Present, restricted distribution Present, no details Present, no details Present, few occurrences Present, no details |
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
No, the pest was sporadically recorded in EU MS in the past, but is currently not known to occur in the EU.
Is the pest present in Protected Zones? (Greece, Portugal and Spain)
NO, the pest is not known to occur in the Protected Zones.
The pest was sporadically recorded in several EU MS in the past, but is currently not known to occur in the EU (Table 3).
Table 3.
Distribution of Curtobacterium flaccumfaciens pv. flaccumfaciens in the EU (extracted from the EPPO Global Database accessed on 14 May 2018
| Continent | Country | Status ‐ EPPO GD |
|---|---|---|
| Europe | Belgium | Absent, pest no longer present |
| Europe | Bulgaria | Absent, pest no longer present |
| Europe | France | Absent, invalid record |
| Europe | Greece | Absent, pest no longer present |
| Europe | Germany | Absent, eradicated |
| Europe | Hungary | Absent, pest no longer present |
| Europe | Italy | Absent, confirmed by survey |
| Europe | Netherlands | Absent, confirmed by survey |
| Europe | Poland | Absent, pest no longer present |
| Europe | Portugal | Absent, confirmed by survey |
| Europe | Romania | Absent, pest no longer present |
| Europe | Spain | Absent, pest eradicated |
A restricted distribution was reported for Romania with uncertainty (see below), but the Romanian NPPO replied upon inquiry that there are ‘no recent data confirming the presence of C. flaccumfaciens pv. flaccumfaciens in Romania’ (pers. comm., Florica Gogu, Romanian Phytosanitary Authority, 2 May 2018). The bacterium is now considered to be absent, eradicated in Germany (pers. comm., Ernst Pfeilstetter, Julius Kühn‐Institut, 24 April 2018).
Regarding the situation of C. flaccumfaciens pv. flaccumfaciens in Romania, strong uncertainties existed on the reliability of the previous record of presence, which was based on invalid references, not related to C. flaccumfaciens pv. flaccumfaciens but to its close relative C. flaccumfaciens pv. oortii (Marinescu and Hatisi, 1984), or to data of susceptibility tests on common bean to its phytopathogenic bacteria including C. flaccumfaciens pv. flaccumfaciens (Phang et al., 1974).
In Germany, C. flaccumfaciens pv. flaccumfaciens was found and identified on soybean in 2011, and was then eradicated. Its origin was attributed to imported soybean seeds (Sammer and Reiher, 2012).
For the other EU MS where the disease occurred in the past (Belgium, Bulgaria, Greece, Hungary and Poland), no detailed information was found.
C. flaccumfaciens pv. flaccumfaciens has occasionally been reported in Spain, which is a PZ for this pest, where currently the pest is considered as eradicated. The pathogen was first isolated in Spain from bean seeds in 2001, and subsequently found in 2005 in a bean field (cultivar Donna) in South Eastern Spain (González et al., 2005). Seed lots of some local bean varieties preserved in a germplasm bank were also found to be infected, 10 years after their production in north‐western Spain (Galicia), which the authors suggested to be probably due to contaminated foreign seeds (Trapiello and González, 2012).
C. flaccumfaciens pv. flaccumfaciens is currently not known to occur in the PZ (Greece, Portugal and Spain) (EPPO, 2018).
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
Curtobacterium flaccumfaciens pv. flaccumfaciens is listed in Council Directive 2000/29/EC only for seeds of Phaseolus vulgaris and of Dolichos for the PZ of Greece, Portugal and Spain.
Details are presented in Tables 4 and 5.
Table 4.
Curtobacterium flaccumfaciens pv. flaccumfaciens in Council Directive 2000/29/EC and Implementing Regulation 2016/873/EC
| Annex II, Part B | Harmful organisms whose introduction into, and spread within, certain protected zone shall be banned if they are present on certain plants or plant products | ||
|---|---|---|---|
| (b) | Bacteria | ||
| Species | Subject of contamination | Protected zone | |
| 1. | Curtobacterium flaccumfaciens pv. flaccumfaciens | Seeds of Phaseolus vulgaris L. and Dolichos Jacq. | Greece, Portugal and Spain |
Table 5.
Regulated hosts and commodities that may involve C. flaccumfaciens pv. flaccumfaciens in Annex V of Council Directive 2000/29/EC
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the Community, before being moved within the Community—in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community |
|---|---|
| Part A | Plants, plant products and other objects originating in the Community |
| Section II | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for certain protected zones, and which must be accompanied by a plant passport valid for the appropriate zone when introduced into or moved within that zone |
| 1.8. | Seeds of […] Dolichos Jacq. and Phaseolus vulgaris L. |
| Part B. | Plants, plant products and other objects originating in territories, other than those territories referred to in part A |
| I. Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community | |
| Section I | Plants, intended for planting, other than seeds but including seeds of […] Phaseolus L. |
| Section II | Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for certain protected zones |
| 5. | Seeds of Dolichos Jacq […] and Phaseolus vulgaris L. |
3.3.2. Legislation addressing the hosts of Curtobacterium flaccumfaciens pv. flaccumfaciens
3.3.3. Other legislation addressing the bean seed production marketed within the Community
Vegetable seed should be allowed to be marketed only if it has been officially examined and certified, according to Council Directive 2002/55/EC.
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
The major host of C. flaccumfaciens pv. flaccumfaciens is Phaseolus vulgaris (Tegli, 2011). Other Leguminosae crops are reported as hosts of this pest (EPPO, 2018), specifically several other species belonging to the genus Phaseolus, to the genera Vigna and Dolichos, including hyacinth bean (Lablab purpureus, syn. D. lablab), plus pea (Pisum sativum) and soybean (Glycine max).
The scientific literature also mentions as Leguminosae hosts the species Lupinus polyphyllus (Schuster and Sayre, 1967), Cicer arietinum, Vicia faba, Vicia villosa, Lens culinaris (Osdaghi et al., 2016) and Zornia spp. (Lenné et al., 1985), in addition to the Amaranthaceae species Amaranthus retroflexus and Chenopodium album (Schuster, 1959), and to Ipomoea lonchophylla (“cowvine morning glory”) (Condè and Diatloff, 1991).
Moreover, the host range of C. flaccumfaciens pv. flaccumfaciens may even be wider, with the bacterium able to adapt to new hosts. C. flaccumfaciens pv. flaccumfaciens was recently detected and isolated from several crops often grown in rotation with beans, such as wheat, corn, sunflower, alfalfa, barley, black oat, white oat, canola, ryegrass, and Solanaceous plants (Harveson et al., 2015; Gonçalves et al., 2017; Osdaghi et al., 2018). This makes the extent of the host range uncertain.
Among the regulated hosts of the bacterium (P. vulgaris and Dolichos), there are currently few common bean genotypes resistant or tolerant to C. flaccumfaciens pv. flaccumfaciens, that so far have been identified outside the EU. Among them, only some are considered suitable for commercial production (Maringoni, 2002; Souza et al., 2006; Valentini et al., 2011; Urrea and Harveson, 2014).
Host plants are widely grown throughout the EU (Section 3.4.3) and surfaces are increasing (EUROSTAT, 2016).
3.4.2. Entry
Is the pest able to enter into the protected zone?
Yes, the pest could enter the EU (including the PZ) on infected host seed.
C. flaccumfaciens pv. flaccumfaciens could enter the EU (including the PZ) with:
infected host seeds.
This pathway is closed by regulation for Phaseolus vulgaris and Dolichos spp. destined to the EU PZ, where C. flaccumfaciens pv. flaccumfaciens may still enter through infected seeds of non‐regulated host plants (see Section 3.4.1), similarly to the rest of the EU.
No records of interception of C. flaccumfaciens pv. flaccumfaciens were registered in the Europhyt database between 2005 and October 2017. Nevertheless, the other reports from Spain and Germany suggest that introduction could be linked to the import of infected seeds. Although the origin of these seeds was not reported, this hypothesis is supported by the observation that just one or two cultivars of the host were found to be affected (Sammer and Reiher, 2012; Trapiello and González, 2012).
3.4.3. Establishment
Is the pest able to become established in the protected zones?
Yes, the pest could established in the EU (including the PZ), as host plants are present and climatic conditions are favourable.
Based on earlier reports and the information retrieved (EPPO, 2018), C. flaccumfaciens pv. flaccumfaciens can establish in the EU including the PZ. Host plants are widely grown throughout the EU. In 2015, dry pulses were grown on 2.2 million hectares in the EU (about 2% of the total arable land), with a production of about 5 million tonnes. France and the UK were the largest producer (both countries account for about 18% of the total production of the EU in tonnes), followed by Poland (about 14%). Additionally, Spain and Poland covered more than two‐fifths of the grown area of dry pulses (over 0.5 million hectares) in the EU (EUROSTAT, 2016). The production of soybean has been recently increasing in Europe.
Provided that host plants are present, there is no evidence suggesting that climatic conditions might limit the establishment of C. flaccumfaciens pv. flaccumfaciens, both in the EU and in the PZ.
3.4.4. Spread
Is the pest able to spread within the protected zone following establishment?
Yes, the pest could spread within the EU (including the PZ) via movement of infected seed and other plant parts.
Regulated Non‐Quarantine Pests (RNQP): Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects?
Yes, plants for planting (i.e. seed) are the main means of spread of the pathogen.
The bacterium can spread at short and long distances mainly through movement of infected seeds (Hedges, 1926b; Zaumeyer, 1932; Zaumeyer and Thomas, 1957; Hsieh et al., 2006; Camara et al., 2009; Bastas and Sahin, 2017).
However, any part of an infected plant or its residues can be a potential source of inoculum (Silva Júnior et al., 2012; Gonçalves et al., 2017). Several irrigation methods are reported to enhance survival and dispersion of C. flaccumfaciens pv. flaccumfaciens within fields where infected plants and/or their residues are present (Harveson and Yonts, 2007). Nevertheless, the importance of those pathways for spread is poorly documented.
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact in the protected zones?
Yes, with uncertainties.
RNQP: Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? 4
Yes, with uncertainties.
In countries where the disease occurs, its incidence is variable. Values higher than 90% have been recorded in those USA bean‐growing regions where C. flaccumfaciens pv. flaccumfaciens is endemic (Harveson et al., 2015). The recent C. flaccumfaciens pv. flaccumfaciens epidemics in North America, after about 20 years of absence, with the emergence of new pigmented variants, seem to be related to an increase in the virulence of the pathogen, which is attributed to jumps from alternative hosts (Harveson and Vidaver, 2008; Osdaghi et al., 2016).
However, no information is available on the yield losses observed in the EU and in the PZ where the pathogen has occasionally been reported to be present in the past.
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zones such that the risk becomes mitigated?
Yes, see Section 3.6.2.
The only effective mitigation measure to prevent the entry, establishment and spread of the pest is to rely on healthy seeds (e.g. ban of import of seeds, seed production in pest free areas, pest free places of production or pest free sites of production).
Chemical treatments with pesticides or copper are not efficient against C. flaccumfaciens pv. flaccumfaciens. The use of antibiotics against plant pathogens is forbidden in the EU.
The broader use of resistant cultivars could also be an efficient mitigation measure provided that resistant commercial cultivars are available.
3.6.1. Phytosanitary measures
C. flaccumfaciens pv. flaccumfaciens is listed in Council Directive 2000/29/EC only for seeds of Phaseolus vulgaris and of Dolichos, for the EU PZ (Greece, Portugal and Spain). However, the host range of the pathogen encompasses many other plant species (Section 3.4.1), for which similar measures (e.g. ban of importing seeds, seed production in pest free areas, pest free places of production or pest free sites of production) could be introduced.
An EPPO diagnostic standard exists for C. flaccumfaciens pv. flaccumfaciens (Tegli, 2011), whose wide and systematic application would be essential to limit its entry and spread both in the EU including the PZ.
Measures given by the Council Directive 2002/55/EC on certification and inspections aim to guarantee the health status of seeds before marketing.
3.6.1.1. Biological or technical factors limiting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
Absence of information on genetic resources for resistance in cultivars commonly grown in Europe;
Infected seeds are generally asymptomatic.
3.6.1.2. Biological or technical factors limiting the ability to prevent the presence of the pest on plants for planting
Limitations related to the analysis of seed (e.g. sensitivity, threshold).
3.6.2. Control methods
Use of healthy and certified seed;
Use of resistant cultivars;
Cultural practices (e.g. proper crop rotation and ploughing of infested plant residues).
3.7. Uncertainty
Information on the natural distribution of cultivated and wild hosts other than P. vulgaris;
Role of reservoirs and pathways other than seeds (e.g. irrigation water and infected residues);
Status of the pest in the EU, as many records date back to a long time ago;
Seed transmission by other minor and alternative hosts.
4. Conclusions
All criteria assessed by EFSA for consideration as potential Union quarantine pest are met (Table 6).
Table 6.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of the pest is clear | The identity of the pest is clear | None |
| Absence/presence of the pest in the EU territory (Section 3.2 ) | The pest was sporadically recorded in several EU Member States in the past, but is currently not known to occur in the EU | The pest was sporadically recorded in several EU Member States in the past, but is currently not known to occur in the EU | Not all notifications are updated |
| Regulatory status (Section 3.3 ) |
Currently regulated in Directive 2000/29/EC on seed of Phaseolus vulgaris and Dolichos spp. in protected zones only (Greece, Portugal and Spain). Phaseolus vulgaris seed is also regulated in the marketing Directive 2002/55/EC |
Currently regulated in Directive 2000/29/EC on seed of Phaseolus vulgaris and Dolichos spp. in protected zones only (Greece, Portugal and Spain). Phaseolus vulgaris seed is also regulated in the marketing Directive 2002/55/EC |
None |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | The pest is able to enter the EU, the main pathway being seed. Should it enter, it may establish. It may then spread through seeds and other minor pathways (irrigation) | Plants for planting (i.e. seed) are the main means of spread of the pathogen |
There is uncertainty on the extent of the host range. Seed transmission by minor and alternative hosts has not been demonstrated |
| Potential for consequences in the EU territory (Section 3.5 ) | Yes, should the pest be introduced in the EU, it may have economic impacts | Yes, should the pest be introduced in the EU, it may have an impact on the intended use of plants for planting. | No information was found on the yield losses in the EU MS where presence was declared |
| Available measures (Section 3.6 ) | Use and release of healthy seeds (e.g. pest free production, certification) |
Use and release of healthy seeds (e.g. pest free production, certification) Cultural practices, (e.g. proper crop rotation and ploughing of infested plant residues) |
None |
| Conclusion on pest categorisation (Section 4 ) | All criteria assessed by EFSA for consideration of C. flaccumfaciens pv. flaccumfaciens as potential Union quarantine pest are met | The criterion on pest presence in the EU is not met | |
| Aspects of assessment to focus on/scenarios to address in future if appropriate |
The main knowledge gaps concern:
|
||
Abbreviations
- DG SANTE
Directorate General for Health and Food Safety
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- MS
Member State
- PCR
polymerase chain reaction
- PLH
EFSA Panel on Plant Health
- PZ
Protected Zone
- RNQP
Regulated Non‐Quarantine Pest
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Grégoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Tegli S, Hollo G and Caffier D, 2018. Scientific Opinion on the pest categorisation of Curtobacterium flaccumfaciens pv. flaccumfaciens . EFSA Journal 2018;16(5):5299, 22 pp. 10.2903/j.efsa.2018.5299
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00625
Panel members: Claude Bragard, David Caffier, Thierry Candresse, Elisavet Chatzivassiliou, Katharina Dehnen‐Schmutz, Gianni Gilioli, Jean‐Claude Grégoire, Josep Anton Jaques Miret, Michael Jeger, Alan MacLeod, Maria Navajas Navarro, Björn Niere, Stephen Parnell, Roel Potting, Trond Rafoss, Vittorio Rossi, Gregor Urek, Ariena Van Bruggen, Wopke Van der Werf, Jonathan West and Stephan Winter.
Adopted: 17 May 2018
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © EPPO
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
See Section 2.1 on what falls outside EFSA's remit.
References
- Bastas KK and Sahin F, 2017. Evaluation of seedborne bacterial pathogens on common bean cultivars grown in central Anatolia region. Turkey. European Journal of Plant Pathology, 147, 239–253. [Google Scholar]
- Bull CT, De Boer SH, Denny TP, Firrao G, Fischer‐Le Saux M, Saddler GS, Scortichini M, Stead DE and Takikawa Y, 2010. Comprehensive list of names of plant pathogenic bacteria, 1980‐2007. Journal of Plant Pathology, 92, 551–592. [Google Scholar]
- Camara RC, Vigo SC and Maringoni AC, 2009. Plant‐to‐seed transmission of Curtobacterium flaccumfaciens pv. flaccumaciens in a dry bean cultivar. Journal of Plant Pathology, 91, 549–554. [Google Scholar]
- Chen YF, Guo JH and Fang ZD, 2000. A new pathovar of Curtobacterium flaccumfaciens on Malabar spinach. Acta Phytopathologica Sinica, 30, 171–175. [Google Scholar]
- Chen YF, Yin YN, Zhang XM and Guo JH, 2007. Curtobacterium flaccumfaciens pv. beticola, a new pathovar of pathogens in sugar beet. Plant Disease, 91, 677–684. [DOI] [PubMed] [Google Scholar]
- Collins MD and Jones D, 1984. Reclassification of Corynebacterium flaccumfaciens, Corynebacterium betae, Corynebacterium oortii and Corynebacterium poinsettiae in the genus Curtobacterium, as Curtobacterium flaccumfaciens comb. nov. Journal of General Microbiology, 129, 3545–3548. [Google Scholar]
- Condè BD and Diatloff A, 1991. Diseases in mungbean. In: Imrie BC, Lawn RJ (eds.). Mungbean: The Australian Experience. Proceedings of the first Australian mungbean workshop. CSIRO Division of Tropical Crops and Pastures, Brisbane. pp. 73–77. [Google Scholar]
- Dunleavy JM, 1983. Bacterial tan spot, a new foliar disease of soybeans. Crop Science, 23, 473–476. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2010. PLH Guidance on a harmonised framework for pest risk assessment and the identification and evaluation of pest risk management options by EFSA. EFSA Journal 2010;8(2):1495, 66 pp. 10.2903/j.efsa.2010.1495 [DOI] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 2018. EPPO Global Database. Accessed May 2018. Available online: https://gd.eppo.int
- EUROSTAT , 2016. Agriculture, forestry and fishery statistics, 2016th edition. Publications Office of the European Union, Luxembourg. [Google Scholar]
- Evtushenko L and Takeuchi M, 2006. The family Microbacteriaceae. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH and Stackebrandt E (eds.), The prokaryotes, 3rd ed. Springer Science, New York, USA. pp. 1020–1098. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online:https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online:https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- Gonçalves RM, Schipanski CA, Koguishi L, Soman JM, Sakate RK, Silva Júnior TAF and Maringoni AC, 2017. Alternative hosts of Curtobacterium flaccumfaciens pv. flaccumfaciens, causal agent of bean bacterial wilt. European Journal of Plant Pathology, 148, 357–365. [Google Scholar]
- González AJ, Tello JC and Rodicio MR, 2005. Bacterial wilt of beans (Phaseolus vulgaris) caused by Curtobacterium flaccumfaciens in Southern Spain. Plant Disease, 89, 1361. [DOI] [PubMed] [Google Scholar]
- Guimaraes PM, Palmano S, Smith JJ, De Sá MF and Saddler GS, 2001. Development of a PCR test for the detection of Curtobacterium flaccumfaciens pv. flaccumfaciens . Antonie van Leeuwenhoek, 80, 1–10. [DOI] [PubMed] [Google Scholar]
- Guimaraes PM, Smith JJ, Palmano S and Saddler GS, 2003. Characterisation of Curtobacterium flaccumfaciens pathovars by AFLP, rep‐PCR and Pulsed‐Field Gel Electrophoresis. European Journal of Plant Pathology, 109, 817–825. [Google Scholar]
- Harveson RM and Vidaver AK, 2008. A new color variant of the dry bean bacterial wilt pathogen (Curtobacterium flaccumfaciens pv. flaccumfaciens) found in western Nebraska. Plant Health Progress, Available online: 10.1094/php-2008-0815-01-br [DOI]
- Harveson RM and Yonts CD, 2007. Influence of irrigation method on incidence and severity of bacterial wilt of dry beans in Nebraska. Phytopathology, 97, S45. [Google Scholar]
- Harveson RM, Schwartz HF, Urrea CA and Yonts CD, 2015. Bacterial wilt of dry‐edible beans in the central high plains of the U.S.: past, present, and future. Plant Disease, 99, 1665–1677. [DOI] [PubMed] [Google Scholar]
- Hedges F, 1922. A bacterial wilt of the bean caused by Bacterium flaccumfaciens nov. sp. Science, 55, 433–434. [DOI] [PubMed] [Google Scholar]
- Hedges F, 1926a. Bacterial wilt of beans (Bacterium flaccumfaciens Hedges), including comparisons with Bacterium phaseoli . Phytopathology, 16, 1–22. [Google Scholar]
- Hedges F, 1926b. Bean wilt traceable to infected seed. The Year Book of Agriculture. US Department of Agriculture, Washington, USA. pp. 165–166. [Google Scholar]
- Hsieh TF, Huang HC and Erickson RS, 2006. Bacterial wilt of common bean: effect of seed borne inoculum on disease incidence and seedling vigour. Seed Science and Technology, 34, 57–67. [Google Scholar]
- Huang HC, Erickson RS, Yanke LJ, Chelle CD and Mündel HH, 2006. First report of the purple variant of Curtobacterium flaccumfaciens pv. flaccumfaciens, causal agent of bacterial wilt of bean, in Canada. Plant Disease, 90, 1262. [DOI] [PubMed] [Google Scholar]
- Lenné JM, Chavarro A and López C, 1985. Effect of Corynebacterium flaccumfaciens on yield of Zornia glabra and Phaseolus vulgaris in Colombia. Phytopathology, 75, 1288. [Google Scholar]
- Marinescu G and Hatisi A, 1984. Bacterial canker of tulip caused by Corynebacterium oortii (Saaltink & Maas Geesteranus). Productia Vegetala Horticultura, 33, 37–38. [Google Scholar]
- Maringoni AC, 2002. Behaviour of dry bean cultivars to bacterial wilt. Fitopatologia Brasileira, 27, 151–156. [Google Scholar]
- Maringoni AC and Camara RC, 2006. Curtobacterium flaccumfaciens pv. flaccumfaciens detection in bean seeds using a semi‐selective culture medium. Brazilian Journal of Microbiology, 37, 451–455. [Google Scholar]
- Maringoni AC, Camara RC and Souza VL, 2006. Semi‐selective culture medium for Curtobacterium flaccumfaciens pv. flaccumfaciens isolation from bean seeds. Seed Science and Technology, 34, 117–124. [Google Scholar]
- Mizuno A and Kawai A, 1993. Studies on the diagnosis of foreign bacterial diseases of quarantine significance, VI: Curtobacterium flaccumfaciens pv. flaccumfaciens . Research Bulletin of the Plant Protection Service of Japan, 29, 27–36. [Google Scholar]
- Osdaghi E and Lak MR, 2015. Occurrence of a new orange variant of Curtobacterium flaccumfaciens pv. flaccumfaciens, causing common bean wilt in Iran. Journal of Phytopathology, 163, 867–871. [Google Scholar]
- Osdaghi E, Taghavi SM, Hamzehzarghani H, Fazliarab A, Harveson RM and Lamichhane JR, 2016. Occurrence and characterization of a new red‐pigmented variant of Curtobacterium flaccumfaciens, the causal agent of bacterial wilt of edible dry beans in Iran. European Journal of Plant Pathology, 146, 129–145. [Google Scholar]
- Osdaghi E, Taghavi SM, Hamzehzarghani H, Fazliarab A, Harveson RM, Tegli S and Lamichhane JR, 2018. Epiphytic Curtobacterium flaccumfaciens strains isolated from symptomless solanaceous vegetables are pathogenic on leguminous but not on solanaceous plants. Plant Pathology, 67, 388–398. [Google Scholar]
- Phang PD, Gutenmaher P and Molea I, 1974. The resistance of some new lines of bean to bacterial diseases. Lucrari Stiintifice, 17, 45–48. [Google Scholar]
- Sammer U and Reiher K, 2012. Curtobacterium flaccumfaciens pv. flaccumfaciens on soybean in Germany – a threat for farming. Journal of Phytopathology, 160, 314–316. [Google Scholar]
- Schuster ML, 1959. Relation of root‐knot nematodes and irrigation water to the incidence and dissemination of bacterial wilt of bean. Plant Disease Report, 43, 25–32. [Google Scholar]
- Schuster ML and Christiansen DW, 1957. An orange‐colored strain of Corynebacterium flaccumfaciens causing bean wilt. Phytopathology, 47, 51–53. [Google Scholar]
- Schuster ML and Sayre RM, 1967. A Coryneform bacteria induces purple‐coloured seed and leaf hypertrophy of Phaseolus vulgaris and other Leguminosae. Phytopathology, 57, 1064–1066. [Google Scholar]
- Schuster ML and Smith CC, 1983. Surveillance and seed transmission of three strains of Corynebacterium flaccumfaciens in beans (Phaseolus vulgaris L.). Fitopatologia Brasileira, 8, 87–91. [Google Scholar]
- Schuster ML, Vidaver AK and Mandel M, 1968. A purple pigment producing bean wilt bacterium Corynebacterium flaccumfaciens var. violaceum n. var. Canadian Journal of Microbiology, 14, 423–427. [DOI] [PubMed] [Google Scholar]
- Silva Júnior TAF, Negrão DR, Itako AT, Soman JM and Maringoni AC, 2012. Survival of Curtobacterium flaccumfaciens pv. flaccumfaciens in soil and bean crop debris. Journal of Plant Pathology, 94, 331–337. [Google Scholar]
- Souza VL, Maringoni AC, Carbonell SA and Ito MF, 2006. Genetic resistance to Curtobacterium flaccumfaciens pv. flaccumfaciens in bean genotypes. Summa Phytopathologica, 32, 339–344. [Google Scholar]
- Tegli S, 2011. Curtobacterium flaccumfaciens pv. flaccumfaciens . EPPO Bulletin, 41, 320–328. [Google Scholar]
- Tegli S, Surico G and Esposito A, 1998. Studi sulla diagnosi di Curtobacterium flaccumfaciens pv. flaccumfaciens nei semi di fagiolo. Notiziario sulla Protezione delle Piante, 9, 63–71. [Google Scholar]
- Tegli S, Sereni A and Surico G, 2002. PCR‐based assay for the detection of Curtobacterium flaccumfaciens pv. flaccumfaciens in bean seeds. Letters in Applied Microbiology, 35, 331–337. [DOI] [PubMed] [Google Scholar]
- Trapiello E and González AJ, 2012. Diversity of culturable bacteria and occurrence of phytopathogenic species in bean seeds (Phaseolus vulgaris L.) preserved in a germplasm bank. Genetic Resources and Crop Evolution, 59, 1597–1603. [Google Scholar]
- Urrea CA and Harveson RM, 2014. Identification of sources of bacterial wilt resistance in common bean (Phaseolus vulgaris). Plant Disease, 98, 973–976. [DOI] [PubMed] [Google Scholar]
- Valentini G, da Cruz Baldissera JN, da Rocha F, de Almeida CB, Heidemann JC, Guidolin AF and Coimbra JLM, 2011. Sources of resistance to Curtobacterium flaccumfaciens pv. flaccumfaciens in common bean accessions. Crop Breeding and Applied Biotechnology, 11, 257–262. [Google Scholar]
- Young JM, Saddler GS, Takikawa Y, De Boer SH, Vauterin L, Gardan L, Gvozdyak RI and Stead DE, 1996. Names of plant pathogenic bacteria 1864–1995. Review of Plant Pathology, 75, 721–763. [Google Scholar]
- Young JM, Watson DRW and Dye DW, 2004. Reconsideration of Arthrobacter ilicis as a plant‐pathogenic species. Proposal to amend the authority and description of the species. Request for an opinion. International Journal of Systematic and Evolutionary Microbiology, 54, 303–305. [DOI] [PubMed] [Google Scholar]
- Zaumeyer WJ, 1932. Comparative pathological history of three bacterial diseases of bean. Journal of Agricultural Research, 44, 605–632. [Google Scholar]
- Zaumeyer WJ and Thomas HR, 1957. A monographic study of bean diseases and methods for their control. US Department of Agriculture, Technical Bulletin Nr 868. Washington, DC, USA.


