Abstract
A rapid qualitative assessment has been done by performing a theoretical analysis on the transmission of low pathogenic avian influenza (LPAI) via fresh meat from poultry reared or kept in captivity for the production of meat (raw poultry meat) or raw table eggs. A predetermined transmission pathway followed a number of steps from a commercial or non‐commercial poultry establishment within the EU exposed to LPAI virus (LPAIV) to the onward virus transmission to animals and humans. The combined probability of exposure and subsequent LPAIV infection via raw poultry meat containing LPAIV is negligible for commercial poultry and humans exposed via consumption whereas it is very unlikely for non‐commercial poultry, wild birds and humans exposed via handling and manipulation. The probability of LPAIV transmission from an individual infected via raw poultry meat containing LPAIV is negligible for commercial poultry and humans, whereas it is very unlikely for non‐commercial poultry and wild birds. The combined probability of exposure and subsequent LPAIV infection via raw table eggs containing LPAIV is negligible for commercial poultry and humans and extremely unlikely to negligible for non‐commercial poultry and wild birds. The probability of LPAIV transmission from an individual infected via raw table eggs containing LPAIV is negligible for commercial poultry and humans and very unlikely to negligible for non‐commercial poultry and wild birds. Although the presence of LPAIV in raw poultry meat and table eggs is very unlikely to negligible, there is in general a high level of uncertainty on the estimation of the subsequent probabilities of key steps of the transmission pathways for poultry and wild birds, mainly due to the limited number of studies available, for instance on the viral load required to infect a bird via raw poultry meat or raw table eggs containing LPAIV.
Keywords: avian influenza, LPAI, transmission, meat, egg, poultry, wild bird, human
1. Introduction
1.1. Background and terms of reference as provided by the requestor
Avian influenza viruses are classified based on the antigenic properties of their hemagglutinin (HA) and neuraminidase (NA) surface glycoproteins. To date, 16 HA (H1 to H16) and 9 NA (N1 to N9) subtypes have been identified in the majority of possible combinations in wild and domestic birds.
Avian influenza viruses can affect all species of birds. Infection of birds manifests itself depending mainly on the ability of the virus to cause disease (pathogenicity):
Low pathogenic avian influenza viruses (LPAIV) generally cause mild disease in susceptible birds affecting the respiratory and enteric tracts. The HA subtypes H5 and H7 may mutate to highly pathogenic avian influenza viruses.
Highly pathogenic avian influenza viruses (HPAIV) spread rapidly causing serious disease with high mortality up to 100% within 48 h in most Galliformes poultry species. Certain species such as domestic waterfowl and ratites may show little or no clinical signs depending also on factors like age and the virus strain involved. So far only avian influenza viruses of the subtype H5 and H7 have shown potential to mutate to high pathogenicity, which has been taken into account when laying down the definition for that disease and related diagnostic procedures.
EU legislation on avian influenza1 lays down harmonised rules for the control of highly pathogenic avian influenza (HPAI) in light of the possible devastating consequences on poultry health and of low pathogenic avian influenza (LPAI) of the subtypes H5 and H7 due to their potential for mutation to HPAI. The measures are graduated, taking into account the biology of the virus and are proportionate to the risk of virus spread via movements of different commodities such as live poultry, live captive birds, hatching and table eggs, fresh meat and fomites.
Pathogenicity is linked to the fact that LPAI viruses preferentially replicate in the cells of the respiratory and intestinal tracts and cause lesions mainly at these locations, while HPAI viruses can replicate throughout the bird's body and are therefore found in many organs, blood, muscle, brain, oviduct, feathers and other tissues.
Based on these biological differences the risks for LPAI and HPAI virus transmission by different commodities have been assessed for disease control purposes such as trace‐back and trace‐forward investigations in the framework of the epidemiological inquiries during disease outbreaks and the restrictions that are imposed in relation to movements of live birds and their products.
Infected live birds and poultry shed large amounts of virus and therefore pose the highest risk for HPAI and LPAI virus transmission through direct or indirect contact with their excretions to other healthy birds, mammals and possibly humans. Onward virus spread to susceptible bird populations may then lead to disease propagation. Day‐old chicks and hatching eggs pose a much lesser risk due to the way they are produced, in biosecure environments and the risk mitigating measures that can be applied such as sanitising the surface of hatching eggs.
Avian influenza virus transmission through ingestion of raw fresh poultry meat and table eggs sourced from infected poultry may only occur if there is sufficient and viable virus in the commodity able to infect the target susceptible host which must be exposed to the source of infection.2
Fresh poultry meat sourced from HPAI infected birds is very likely to contain virus due to the systemic distribution of the HPAI viruses throughout the bird's body including blood and muscle tissue. HPAI virus can also be found in table eggs laid by infected hens before they die from the disease.
No human case has ever been reported in the EU due to avian influenza viruses of the subtypes A(H5N1), A(H5N6), A(H7N9) or A(H9N2). One single human fatality due to infection with influenza virus A(H7N7) occurred during a large ongoing outbreak in poultry. Human clusters have been identified in countries where these viruses are endemically circulating in poultry, but no sustained human‐to‐human transmission has been observed although sporadic human‐to‐human transmission in nosocomial environments, especially with A(H7N9) have been identified.3 Epidemiological evidence suggests that infection in humans occurs rarely and only after very close contact with infected animals.4 In contrast, human infections through consumption of avian influenza virus‐contaminated food have not been substantiated.5 It is, therefore, likely that a high dose of virus may be needed to initiate an infection and that a readily accessible entry route for avian influenza viruses does not exist in humans. In 2006, an EFSA scientific report concluded that there is no evidence that the lower digestive tract could serve as a portal of entry of avian influenza viruses in humans after consumption of food products from infected animals, despite HPAI A(H5N1) virus titres in muscles being very high. Even if virus uptake in the digestive tract were possible, a minimal infectious dose of the virus must be able to reach the intestinal lumen overcoming the many biological barriers which are present.6 The risk of avian influenza virus transmission to humans via food could increase, if significantly higher virus titres or a change in virus distribution in poultry tissues were reported.
As regards LPAI, several experiments and risk assessments have determined that the probability of transmitting LPAI virus of H5/H7 subtypes through raw chicken meat and eggs from infected poultry to produce infection in naïve bird populations ranges from insignificant to negligible.7
However, there are some experiments suggesting that LPAI virus distribution outside the respiratory and intestinal tracts in birds could be wider than currently assumed which might change the level of risk for a possible virus transmission related to movements and ingestion of fresh meat and table eggs derived from LPAI infected poultry.8 , 9
An evaluation and re‐assessment of existing and new scientific information is therefore necessary in order to ensure that the most appropriate measures are in place for animal disease control to protect animal and public health and for safeguarding movements of these commodities within and between Member States as well as when trading with non‐EU countries.
The Commission, in the context of Article 31 of Regulation (EC) No. 178/2002, therefore asks the EFSA to:
Assess the risk for transmission of low pathogenic avian influenza viruses of subtypes H5 and H7 via raw poultry meat and table eggs to poultry and other captive birds leading to infection and onward virus transmission to animals and humans, by collating, evaluating and reviewing the most recent studies, scientific literature and risk assessments available.
The EFSA is requested to provide a draft report on this risk assessment concerning the animal health aspects by 16 August 2018. By 30 September 2018 the final assessment report shall be provided which then also includes the assessment of the food safety aspects. Publication of that report should be foreseen for 15 October 2018.
1.2. Interpretation of the Terms of Reference
This section of the report provides clarifications on the terms of reference and definitions of key terminology used in the assessment.
‘Fresh meat’ is defined in Regulation (EC) No 853/200410 as ‘meat that has not undergone any preserving process other than chilling, freezing or quick‐freezing, including meat that is vacuum‐wrapped or wrapped in a controlled atmosphere’. The commodity considered in this scientific report is raw poultry meat, i.e. fresh meat from poultry reared or kept in captivity for the production of meat, including the carcass but excluding offal and blood. Animal by‐products are also excluded from the assessment.
Council Directive 2005/94/EC defines poultry as ‘all birds that are reared or kept in captivity for the production of meat or eggs for consumption, the production of other products, for restocking supplies of game birds or for the purposes of any breeding programme for the production of these categories of birds’. Commercial and non‐commercial poultry are both considered and differentiated when relevant. The assessment will exclude captive birds, defined as ‘any bird other than poultry that is kept in captivity for any reason other than those referred to as poultry including those that are kept for shows, races, exhibitions, competitions, breeding or selling’.
‘Eggs’ are defined in Regulation (EC) No 853/200411 as ‘eggs in shell — other than broken, incubated or cooked eggs — that are produced by farmed birds and are fit for direct human consumption or for the preparation of egg products’. Commission Regulation (EC) No 2295/200312, defines 2 grades of eggs (A and B) according to different physical characteristics as follows: (i) Grade A eggs (‘fresh eggs’ or ‘table eggs’) should have a ‘normal, clean and undamaged’ shell and cuticle; they will not be washed or cleaned before or after grading, and will be not chilled or treated for preservation.’; (ii) Grade B eggs, i.e. eggs ‘which do not meet requirements applicable to eggs in grade A’, may only be used by the food or non‐food industries. The commodity considered in this assessment is raw table eggs, i.e. grade A eggs from Galliformes or Anseriformes that have not gone through any processing or thermic treatment. This assessment will assess whether LPAIV can be present inside table eggs and whether time and temperature along the food chain can have any impact on the persistence of the LPAIV until consumption. Grade B eggs, which are often processed for egg products, are not considered in this assessment.
The assessment concerns mainly LPAIV of subtypes H5 and H7 (excluding HPAI and swine influenza viruses), although information from non‐H5 or non‐H7 subtypes is sometimes provided (when considered relevant). For the purpose of this assessment, the species relevant for production of poultry meat include Galliforme and Anseriforme poultry (not Columbiformes) and therefore would include hunted game birds. The animal species considered for the exposure assessment are commercial and non‐commercial poultry and wild birds, and humans, but excluding other animal groups such as mammals. For the wild birds, the assessment focuses on species reported to be infected with LPAIV (i.e. wild water birds) but excluding wild bird species where no LPAIV has been reported (e.g. raptors).
1.3. Scope and limitations of the assessment
This is a rapid qualitative assessment of the probability of transmission performed as a theoretical analysis based on the available scientific evidence complemented with expert opinion. It is restricted in terms of geographical context to the European Union (EU) so the practices common in the EU have been considered, including production and husbandry systems for commercial, non‐commercial and hunted wild birds as well as the processing and consumption practices of poultry meat and eggs. The assessment is based on the assumption of a poultry establishment exposed to LPAIV without the notification of a LPAI outbreak, in which one bird has been infected at any point in time. The estimate of the probability of transmission has not been conducted for a particular period of time, so the qualitative estimates of the probabilities are not accumulated or affected by repeated events over a time period.
From the initial scenario as above described, a transmission pathway has been defined (see Section 2.2). This exercise has not considered the prior probability of this scenario occurring in an EU poultry establishment, taking into account the prevalence of LPAI infection at flock level and the within‐flock prevalence in the EU and the strain(s) of LPAIV present in the EU. Nor has this exercise considered the impact of control and management measures of outbreaks on the probability, for example, of detection of infected carcasses at abattoir or eggs at grading centres. Data on throughputs of poultry meat and other commodities have not been collated to assess the probabilities of subsequent events.
The probabilities of the different steps of the transmission pathway up to the infection step (CP9) have been assessed at an individual level, i.e. the probability of the event occurring in a single individual, independent of the population to which it belongs. By adopting this approach, the probability assessed may result in over‐ or underestimation of the actual probability, depending on the impact of the probability of occurrence of the initial scenario and of the estimation of the probabilities at population level for all steps. The combination of probabilities has been done without applying any mathematical operation but using the rules described in Section 2.2. Given the short timeframe to deliver this assessment, it was agreed that this was the only feasible approach. To do a comprehensive risk assessment, a full quantitative analysis of the probability that an LPAIV could enter, infect and spread in the EU via raw meat and table eggs during a year would be needed, as well as estimation of the consequences of the entry, infection and spread. This was deemed unfeasible not only due to the short deadline to deliver the draft assessment but also to the impossibility of collating and analysing all the data needed, if available.
In summary, this assessment must be considered as a qualitative estimation of the theoretical probability of transmission by assessing the biological plausibility of the events to occur, i.e. the transmission of LPAIV via raw poultry meat and raw table eggs to poultry and other captive birds leading to infection and onward virus transmission to animals and humans, and not the actual probability present in the EU in 2018.
2. Data and methodologies
2.1. Data
Information on the presence of LPAIV in organs, tissue, meat and eggs was retrieved via a search in the Web of Science for the period 2005–2015 using the search terms ‘avian influenza’ and meat or eggs as described in Sections F12 and F13 of the latest EFSA scientific opinion on avian influenza (EFSA AHAW Panel, 2017). An additional search was carried out for the period 1/1/2016–17/7/2018. The PubMed database was searched by using subject index terms and free text terms combined with the appropriate Boolean operators as described in Appendix A. Scientific articles reporting information on the presence or absence of LPAI H5 or H7 virus in the tissues of interest were selected. Reports from other LPAI subtypes were only selected if the information was very relevant for the assessment.
2.2. Methodologies
Two transmission pathways have been determined for raw poultry meat and raw table eggs, respectively, (see Figures 1 and 3) describing all stages in the biological process that lead to the outcome of interest and estimating qualitatively the probability of occurrence in each of the steps. Some probabilities of the different steps have been estimated as independent events, not conditional to the probabilities of the previous step, even though it is acknowledged that they are not independent. These are referred to as ‘independent probabilities (IP)’. On the other hand, ‘combined probabilities (CP)’ were derived for other steps of the transmission pathway by combining the estimated probabilities of two previous steps.
Figure 1.

Transmission pathway used to assess the probability of transmission for LPAIV via raw poultry meat. Key terminology is defined in Sections 1.2 and 2.2
- IP, independent probability; CP, combined probability.
Figure 3.
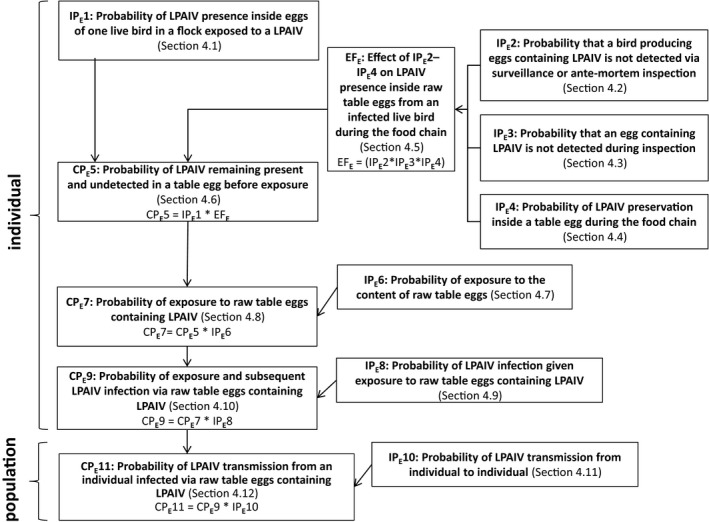
Transmission pathway used to assess the probability for transmission of LPAIV via raw table eggs. Key terminology is defined in Sections 1.2 and 2.2
- IP, independent probability; CP, combined probability.
The starting point of the transmission pathway was a commercial or non‐commercial poultry establishment within the EU that has been exposed to LPAIV and no official notification was made which otherwise would result in disease control measures. The corresponding probability to the first step was the probability of LPAIV presence in muscle tissue or inside eggs of one live bird in a flock exposed to LPAIV (IP1).
The probability of each step in the pathway was assessed separately for Galliformes and Anseriformes, whenever appropriate or possible throughout the pathways, as these poultry types have different susceptibilities to LPAIV infection and therefore different likelihoods of LPAIV being present and detected in muscle tissue or inside raw table eggs. Information on ratites is included in the text when available.
There are three steps involved in determining the presence of LPAIV in raw poultry meat or raw table eggs before an individual is exposed: surveillance and ante‐mortem inspection of live animals (IP2), inspection of meat or table eggs during the food chain (IP3) and the preservation of the virus in raw poultry meat or table eggs during the food chain (IP4). IP2 regards live animals whereas IP3 is related to raw poultry meat or raw table eggs. Therefore, ‘ante‐mortem’ inspection was part of IP2 although it is recognised that it is part of the regular slaughter process. The corresponding probabilities (IP2–IP4) were estimated. The food chain represented by the process as implemented in the EU (including inspection, processing, storage, transport, retail and purchase) was taken into account, which is considered to be similar in countries exporting to the EU. For instance, slaughterhouses, cutting plants and processing facilities need to be approved before they are authorised to export meat to the EU. On‐the‐spot checks are regularly carried out to check the animal health conditions of slaughtered poultry and monitor the hygiene and practices in third‐country slaughterhouses.
The measures assessed in IP2–IP3 and the probability IP4 of LPAIV preservation during the food chain might have an effect on LPAIV presence between infection of the bird and exposure of the derived poultry meat or table eggs containing LPAIV to an individual. Therefore, the corresponding probabilities IP2, IP3 and IP4 were combined to estimate their effect (EF) on LPAIV presence in raw poultry meat and inside raw table eggs from an infected live bird during the food chain that may/may not reduce IP1. Please note that IP2, IP3 and IP4 are expressed in the form of ‘negative’ probabilities (e.g. probability of not being detected during post‐mortem inspection). Thus, the probability (CP5) of LPAIV remaining present and undetected in raw poultry meat or raw table eggs before exposure was estimated by combining the probability IP1 and the effect EF.
The probability of exposure to raw poultry meat or raw table eggs containing LPAIV (CP7) was then determined by the combination of the probability of exposure to raw poultry meat or to the content of raw table eggs (IP6) and the probability of LPAIV remaining present and undetected in raw poultry meat or raw table eggs before exposure (CP5). From this step onwards, an individual could be poultry (from the commercial or non‐commercial sector), a wild bird or a human. Human exposure via manipulation or consumption was separated. Exposure is not the same as infection, as not every exposure event will lead to infection or infectiousness.
The probability of exposure and subsequent LPAIV infection via raw poultry meat or raw table eggs containing LPAIV (CP9) was estimated separately for commercial and non‐commercial poultry, wild birds and humans. The probability CP9 was derived by combining the probability of LPAIV infection given exposure to raw poultry meat or raw table eggs containing LPAIV (IP8) and the probability of exposure to raw poultry meat or raw table eggs containing LPAIV (CP7).
The last step of the assessment addressed the probability (CP11) of LPAIV transmission from an individual infected via raw poultry meat or raw table eggs containing LPAIV to another individual in the same population, differentiating commercial and non‐commercial poultry, wild birds and humans, whenever possible. The probability of LPAIV transmission from an individual infected via raw poultry meat or raw table eggs containing LPAIV (CP11) was estimated by combining the probability of exposure and subsequent LPAIV infection via raw poultry meat containing LPAIV (CP9) with the independent probability of LPAIV transmission from individual to individual (IP10). These last probabilities, IP10 and CP11, are at the level of the population whereas the nine previous probabilities are estimated at the individual level.
Scientific evidence was collected and probabilities were determined first by individual working group members (authors of the report) and subsequently discussed among the working group members to reach consensus via moderated discussion during teleconferences.
It was not possible to develop a questionnaire or use a more structured approach to elicit expert opinion since a draft report was required within one month following acceptance of the mandate. The used probability terms and corresponding subjective probability ranges used are those used in the latest scientific opinion on avian influenza (EFSA AHAW Panel, 2017) and shown in Table 1.
Table 1.
Probability terms and subjective probability ranges used to describe all the steps of the transmission pathways on LPAIV transmission via raw poultry meat and raw table eggs (regardless of the host) (taken from EFSA AHAW Panel, 2017)
| Probability term | Subjective probability range |
|---|---|
| Non‐negligible | > 10% ≤ 100% |
| Unlikely | > 2% ≤ 10% |
| Very unlikely | > 1% ≤ 2% |
| Extremely unlikely | ≤ 1% |
| Negligible | Indistinguishable from 0 |
A set of heuristic rules to combine pairs of probability terms (whether independent or combined) as in Table 1 has been applied throughout the assessment, as follows:
If at least one of the two probabilities is ‘negligible’, the combined probability will be negligible.
Negligible * Very unlikely = Negligible
If the two probabilities are the same, the combined probability will be equal to the individual ones.
Very unlikely * Very unlikely = Very unlikely
If either of the two probabilities is not ‘negligible’ and they have a difference of one grade/score, the combined probability will be the lower value.
Very unlikely * Extremely unlikely = Extremely unlikely
If either of the two probabilities is not ‘negligible’ and they have a difference of two grades/scores, the combined probability will be lie in between.
Unlikely * Extremely unlikely = Very unlikely
If either of the two probabilities is not ‘negligible’ and they have a difference of three grades/scores, there is only one scenario in this situation:
Extremely unlikely * Non‐negligible = Unlikely
The rules to combine the independent probabilities IP2, IP3 and IP4 (IP2*IP3*IP4) into EF are:
If all probabilities are ‘non‐negligible’: ‘no effect’ of the corresponding measures to reduce the probability of LPAIV presence in muscle, raw poultry meat or raw table eggs.
If at least one probability is different from ‘non‐negligible’: ‘reduction’ of the probability of LPAIV presence in muscle, raw poultry meat or raw table eggs due to the corresponding measures.
The rules to combine P1 with EF are:
If EF is ‘no effect’: the combined probability will be the same as probability P1. Very unlikely * No effect = Very unlikely
If EF is ‘reduction’: the combined probability will be one grade/score lower than P1. Unlikely * Reduction = Very unlikely
The levels of uncertainty were identified individually for the independent probabilities and an overall final probability term was elicited from the working group members via a moderated discussion, based on the principles described in the EFSA guidance on uncertainty (EFSA Scientific Committee, 2018). The ratings used to describe the level of uncertainty are provided in Table 2.
Table 2.
Ratings used to describe the level of uncertainty (taken from EFSA AHAW Panel, 2015)
| Name | Explanation |
|---|---|
| Low | No or limited information or data are lacking, incomplete, inconsistent or conflicting. No subjective judgement is introduced. No unpublished data are used |
| Moderate | Some information or data are lacking, incomplete, inconsistent or conflicting. Subjective judgement is introduced with supporting evidence. Unpublished data are sometimes used |
| High |
The majority of information or data are lacking, incomplete, inconsistent or conflicting Subjective judgement may be introduced without supporting evidence. Unpublished data are frequently used |
3. Assessment of LPAIV transmission via raw poultry meat
A general description of the transmission pathway is provided in Section 2.2. The starting point was a commercial or non‐commercial poultry establishment within the EU that has been exposed to LPAIV and no official notification made which otherwise would result in disease control measures. Figure 1 shows the transmission pathway applied to assess the probability of LPAI transmission via raw poultry meat containing LPAIV. The determination of each probability is described in the sections below.
3.1. Probability of LPAIV presence in muscle tissue of one live bird in a flock exposed to a LPAIV (IPM1)
In order for avian influenza viruses to become functional, the virus must produce HA, which is present as a precursor glycoprotein and requires post‐translational cleavage by host proteases, after which the virus particles are infectious. LPAI viruses have a monobasic cleavage site of HA which can only be processed by host proteases such as trypsin‐like enzymes. Therefore, LPAIV replication is mostly restricted to sites in the host where these enzymes are found, i.e. only the respiratory and intestinal tract (Alexander, 2000). Recent reports suggest that the R‐S‐S/R‐R motif in the cleavage site of endemic LPAIV H9N2 can broaden the spectrum of enzymes responsible for cleavage (Baron et al., 2013; Tse et al., 2014). Although viraemic spread of LPAIV has been described, these viruses are to a large extent replication‐incompetent outside the respiratory and intestinal tract. An overview of LPAIV tissue distribution in poultry from studies published between January 2005 and mid‐July 2018 is provided in Appendix B. The most relevant studies are briefly described below whereas details such as the viral strain used are presented in Appendix B.
Among LPAIV, the H7N9 subtype has raised major concerns since its detection in China in 2013 because of the implications for human health. Experimental infections with LPAI H7N9 strain A/Anhui/A/2013 revealed that the virus is highly tropic for the upper respiratory tract in poultry (Pantin‐Jackwood et al., 2014). However, Slomka et al. (2018) have detected systemic dissemination of the LPAIV H7N9 subtype to multiple organs beyond the respiratory and digestive tracts, including skeletal muscles and feather follicles in experimentally infected turkeys. The presence of the virus was detected by immunohistochemistry and mostly in specimens collected from turkeys infected with a high dose of the virus (8 log10 EID50). Uchida et al. (2017) isolated a low concentration of LPAIV H7N9 (0.44 log10 EID50) from the muscle tissue of quail (but not chickens or pigeons) inoculated with 6 log10 EID50 via the intranasal route.
Shibata et al. (2018a) tested chicken, Pekin duck and Muscovy duck meat that had been illegally imported by international flight passengers and detected H5N1 HPAIV and H5N6 HPAIV as well as H9N2 LPAIV and H1N2 LPAIV. These authors further tested the pathogenicity of the isolated viruses experimentally. Chickens and ducks were inoculated via the intranasal route with the obtained H9N2 LPAIV, which could be recovered from the trachea 3 days after the inoculation of the chickens (4/4) but not from inoculated ducks (0/4). For both species, no virus was isolated from muscle, brain, lungs, kidneys or colon (see Appendix B). All chickens (4/4) and ducks (4/4) survived for 14 days without exhibiting any clinical signs. Co‐infection of H9N2 virus with bacteria has been reported to enhance virus replication and extrapulmonary infection in chickens (Kishida et al., 2004), which might explain the isolation of H9N2 LPAIV from poultry meat. Shibata et al. (2018b) recently also detected H7N9 LPAIV when analysing more raw poultry meat illegally imported by travellers. An isolate of H7N9 LPAIV was used to inoculate chickens and ducks intranasally and viable virus was detected in the muscle tissue of one chicken (1/4) but not in the ducks (0/4; ≤ 2.0 log EID50/g). In another study comparing H7N8 LPAI infections between chickens and turkeys, clear differences in pathogenesis were observed between these two poultry species, with not only clinical signs being observed only in turkeys but also LPAIV being detected by qRT‐PCR in heart, brain and muscle from turkeys only. Levels of detected virus in muscle tissue of turkeys were 4.8 log10 EID50/g (as determined by PCR). As mentioned previously, no virus could be detected in muscle tissues of infected chickens (Pantin‐Jackwood et al., 2017).
A comparative pathogenesis study of 12 other H7 LPAIV isolates revealed that turkeys are more susceptible than chickens and ducks (Spackman et al., 2010). Clinical disease was observed in ducks for only 3 out of the 12 H7 LPAIV isolates and disease was generally very mild and did not result in mortality.
Reports on the systemic detection of LPAIV H5N2, H7N1, H7N7 or H9N2 by real‐time reverse transcription PCR (RRT‐PCR) in organs and muscles of experimentally infected chickens were also provided but few of these samples (H7N7 LPAIV from samples with a high viral RNA load (Ct ≤ 35 in qPCR) were confirmed by virus isolation (Post et al., 2013). There are reports on the isolation of LPAIV H7N1 in blood of infected turkeys or detection of LPAIV genome in blood, breast and thigh (by RRT‐PCR) (Toffan et al., 2008). Some LPAIV strains of the H10 subtype have been shown to be able to replicate in the kidneys of infected chickens (Bonfante et al., 2014).
In conclusion, for Galliformes, the probability (IPM1) of LPAIV presence in muscle tissue of one live bird in a flock exposed to LPAIV is deemed very unlikely because LPAIV has only been isolated from muscle tissues of experimentally inoculated chickens and illegally imported meat on rare events. Field observations, results from experimental studies and the molecular characteristics of LPAI viruses indicate that their presence is mainly restricted to the respiratory tract and the intestinal tract. It should be noted that turkeys are more susceptible than chickens and experimentally infected turkeys may exhibit some systemic infection (as seen for H7N9 LPAI).
Studies on LPAIV derived from non‐galliformes are rare (see Appendix B). Pantin‐Jackwood et al. (2017) inoculated mallard ducks with LPAI H7N8 via the intrachoanal route and viral RNA was detected in muscle tissue of one out of three ducks. Daoust et al. (2013) investigated the distribution of LPAIV H2N3 virus in experimentally infected captive‐bred mallards and the virus was mostly found in the respiratory and digestive tracts but sporadically also in the kidneys. The muscles were not tested. Laudert et al. (1993) tested the dissemination of numerous LPAIV subtypes (e.g. H5N1, H5N2 and H7N3) in mallard ducks and found positive results in a broad spectrum of organs (including the brain, kidney and liver) but the route of inoculation was intravenous. No muscles were tested. The presence of LPAIV H10N7 in frozen duck carcasses smuggled from China to Italy was demonstrated (Beato et al., 2006) but the authors tested the tracheas and lungs of the carcass and not the muscles. In the case of ratites, although HPAI H7N1 and HPAI H5N2 are known to occur in multiple organs in ostriches, there is a lack of knowledge about tissue tropism and systemic infection of LPAI strains (Venter et al., 2017).
To summarise, there is scarce evidence for systemic infection and/or presence of LPAIV in the muscles in Anseriformes. It can be concluded that for Anseriformes, the probability (IPM1) is extremely unlikely, since LPAI virus was only isolated from remnants of respiratory organs of illegally imported meat13 and from a mixture of meat and other organs, on rare events.
3.1.1.
Uncertainty
The uncertainty estimate for IPM1 for Galliformes is low since the probability estimate is based on several studies showing that LPAI viraemia is rare and only a few studies could isolate virus from muscle tissue. In the case of Anseriformes, the uncertainty estimate for IPM1 is moderate since there is ample evidence underpinning an extremely low probability that LPAI infection becomes viraemic, but the there is a lack of studies analysing the presence of LPAIV in muscle tissue.
3.2. Probability that a bird with LPAIV in muscle tissue is not detected via surveillance or ante‐mortem inspection (IPM2)
The probability that a bird with LPAIV in muscle tissue is not detected via surveillance or ante‐mortem inspection is dependent on the presence of clinical signs and/or mortality as well as the sensitivity of the implemented surveillance and early warning systems. The text below describes the evidence regarding detection of infection or disease as it is a prerequisite of having LPAIV in muscle tissue.
LPAI viruses are a heterogeneous group of viruses with varying pathogenicity in different species, in general inducing no or only minimal clinical signs (Spickler et al., 2008; Harder et al., 2016). The clinical signs of LPAIV infection are often non‐specific and therefore similar to those of other important poultry diseases such as Newcastle disease, infectious bronchitis, mycoplasmosis, infectious laryngotracheitis, metapneumovirus infections and infectious coryza.
The signs are highly dependent on the strain of the virus and the poultry species. Clinical signs are more likely to occur where there is some co‐morbidity due to, for example, a bacterial infection (which may lead to systemic infection) and more likely in Galliforme poultry than in Anseriforme poultry, as the majority of LPAI viruses are likely to be of waterfowl origin and not poultry‐adapted. Experimental infection studies (which often use high infective doses) showed that inoculation with LPAIV H7N9 does not induce clinical signs in chickens, pigeons, Japanese quail, mallard ducks, Pekin ducks, Muscovy ducks or Embden geese (Pantin‐Jackwood et al., 2014; Spackman et al., 2015). H5N1 and H7N3 LPAIV isolates of wild bird origin were not pathogenic in turkeys and chickens (Ladman et al., 2010). LPAI outbreaks among chickens have been identified based on a decline in egg production without observing clinical signs or mortality, as, for instance, the LPAI H5N2 outbreaks in 2005–2006 in Japan (Okamatsu et al., 2007). On the other hand, there have been LPAI outbreaks where clinical signs and even increased mortality were reported. For instance, in the H7N1 LPAI outbreak in Italy in 1999, the initial signs were depression and decreased feed consumption, followed by a decrease in egg laying of 5–20% in broiler breeders and 3–30% in layers (Mutinelli et al., 2003). Similarly, the first signs in a Canadian outbreak of H7N3 LPAI were a sudden drop in feed consumption and a slight increase in mortality (Bowes et al., 2004). In April 2014, an outbreak of H5N8 LPAI was detected in a commercial flock of Japanese quail (Coturnix coturnix japonica) following a sudden increase in mortality (Carnaccini et al., 2015). In layer flocks infected with H7N2 LPAIV (Pennsylvania, 1997–1998), the mortality rate rose to even 10 times the baseline with a cumulative mortality of 4% (Ziegler et al., 1999; Lu et al., 2004). Turkeys are more susceptible to LPAIV infection than broilers and laying hens (Mutinelli et al., 2003; Slomka et al., 2018). Nevertheless, the severity of clinical signs and the capacity to recover appeared to be primarily age‐related and oldest turkeys recovered within one week of the onset of clinical signs. In ducks, morbidity can be very low with subclinical infection due to localised enteric infection, whereas in chicken layers it may be higher but influenced, for instance, by flock size and density (Gonzales et al., 2012). H5 LPAI viruses circulating in ducks in south‐west France over several months were only detected by serological testing and not by passive surveillance (Ducousso et al., 2018).
Gonzales and Elbers (2018) analysed daily mortality, egg production and feed and water intake records from 61 apparently healthy commercial layer flocks and eight LPAI outbreaks in The Netherlands. They calculated that daily mortalities above 0.08% or 0.13% could be associated with LPAI suspicion for layers kept indoors or with free‐range access, respectively. Combining mortality with a drop in egg‐production at a weekly ratio lower than 0.94 would result in high specificity of early detection, with an expected false alarm rate of between 0.07 and 0.15 false alarms per day in a population of 1,000 layer farms. When mortality is higher, as in the LPAI H6N2 epidemic in 2002 in southern California (mortalities of nearly 2% in just over 2 weeks and egg losses close to 20%), an early warning can be raised using mortality only (Beltran‐Alcrudo et al., 2009). Furthermore, active surveillance in place in the EU can detect some subclinical infections for meat turkeys and game birds but not for broiler chickens as the latter are not by default included in the programmes. LPAIV detection in Anseriformes requires active surveillance.
The national serosurveillance programmes could be effective in tracing clusters of LPAIV‐infected holdings but are unfit for early warning of LPAI outbreaks at holding levels (EFSA AHAW Panel, 2017). Detailed follow‐up of seropositive flocks is important to prevent spread of the virus (Marchée et al., 2014). PCR testing of ducks before transport has been proven to significantly increase the detection of circulating avian influenza viruses in the duck sector (Scoizec et al., 2018). However, the active surveillance sensitivity varies considerably across the Member States and there is a lack of data at an individual bird level. The annual EU poultry survey reports14 that less than 1% of surveyed poultry holdings per year are seropositive, of which only a few are tested positive for virus and consequently reported to the EU's Animal Disease Notification System (ADNS) (European Commission, 2017). Each year, several additional virus positive holdings are detected through passive surveillance and reported to ADNS.
The data in Table 3 are taken from the summary of annual poultry surveys reported by Member States (European Commission, 2017). In addition, the number of cases in poultry reported to the EU ADNS system is included. Some, but not all will be those resulting from the follow‐up of positive poultry survey cases, therefore denominator data are not known. However, it does suggest that there are cases of LPAI occurring in a low percentage of poultry flocks each year which are not detected as clinical cases.
Table 3.
Annual poultry survey data and positive serology for H5 and H7 avian influenza virus and LPAI cases in poultry, reported to ADNS from EU Member States
| Year | Total # holdings sampled | Total # seropos holdings | % of total sampled | Total # H5 seropos holdings | % H5 of total sampled | Total # H7 seropos holdings | % of H7 total sampled | # LPAI cases reported to ADNS |
|---|---|---|---|---|---|---|---|---|
| 2008 | 34,985 | 72 | 0.21 | 52 | 0.15 | 21 | 0.06 | 39 |
| 2009 | 35,016 | 90 | 0.26 | 52 | 0.15 | 38 | 0.11 | 50 |
| 2010 | 29,484 | 59 | 0.2 | 48 | 0.16 | 11 | 0.04 | 13 |
| 2011 | 29,806 | 65 | 0.22 | 50 | 0.17 | 15 | 0.05 | 56 |
| 2012 | 29,404 | 43 | 0.15 | 40 | 0.14 | 4 | 0.01 | 19 |
| 2013 | 25,220 | 63 | 0.25 | 57 | 0.23 | 6 | 0.02 | 27 |
| 2014 | 19,813 | 43 | 0.22 | 38 | 0.19 | 5 | 0.03 | 9 |
| 2015 | 21,867 | 40 | 0.18 | 33 | 0.15 | 7 | 0.03 | 22 |
| 2016 | 18,138 | 134 | 0.74 | 124 | 0.68 | 10 | 0.06 | 45 |
LPAI: low pathogenic avian influenza; ADNS: Animal Disease Notification System.
In conclusion, if LPAIV infections were systemic, there would be clinical manifestations in Galliformes and therefore a high likelihood that the flock would be detected by passive surveillance. However, this is not the case for Anseriformes for which detection only relies on active surveillance. The available data show that it is only a fraction of the LPAIV‐infected birds that might have virus in muscle tissue (see Section 3.1) and although surveillance is identifying birds that could be infected with LPAIV, there is still a possibility that a limited percentage of such birds would not be detected. Although it is difficult to quantify, based on the available data, the probability (IPM2) that a bird with LPAIV in muscle tissue is not detected via surveillance or ante‐mortem inspection, this probability is assumed to be unlikely for commercial Galliformes. In the non‐commercial sector, probability IPM2 is non‐negligible for Galliformes, since these are not included in surveillance programmes and the persons involved likely have less experience to recognise clinical signs compared to a veterinarian or a professional poultry keeper. For Anseriformes, the probability IPM2 is non‐negligible in both the commercial and non‐commercial sectors as the detection of birds with LPAIV in muscle relies only on active surveillance.
Where there is little or no routine serological surveillance or if ante‐mortem detection for avian influenza is masked by the presence of other common avian diseases, the probability of detection of LPAI infection or disease at this step is further reduced. For example, in South‐East Asia where live‐bird markets (LBMs) are a common factor in the pre‐slaughter food chain, H7N9 LPAI is only routinely found in poultry when environmental and poultry samples from the farms of origin are collected or as a result of tracing back from a human case.
3.2.1.
Uncertainty
The uncertainty estimate for IPM2 is moderate in commercial poultry for Galliformes since there is ample evidence that both surveillance and ante‐mortem inspection is detecting birds which could be infected and have LPAIV in muscle tissue although there are limited quantitative data, in particular at the animal level. The uncertainty estimate for IPM2 is high for Anseriformes from the commercial and non‐commercial sectors and for Galliformes from the non‐commercial sector since there is a lack of data on the sensitivity of the passive surveillance and ante‐mortem inspection at individual animal level across the Member States due to high variability in the ability of non‐professionals to identify LPAI‐infected birds.
3.3. Probability that a bird with LPAIV in muscle tissue is not detected during post‐mortem inspection (IPM3)
The probability that a bird with LPAIV in muscle is not detected during slaughter and post‐mortem inspection depends on: (a) the presence of macroscopic lesions (differentiated between Galliformes and Anseriformes in terms of pathology and lesions); and (b) the ability of visual post‐mortem inspection to detect these types of macroscopic lesions in both commercial abattoirs and home slaughter/hunting.
It is acknowledged that the presence of LPAI virus in muscle tissue is not always associated with the presence of macroscopic lesions. For the estimation of IPM3, it has been assumed that all animals showing macroscopic lesions consistent with LPAIV infection during post‐mortem inspection and according to the checks described in the legislation would have the virus present in muscle tissue. Thus, the presence of lesions is considered a proxy for the presence of LPAIV in muscle tissue. The fact that a proportion of the animals with LPAIV in muscle tissue are not showing macroscopic lesions results in an underestimation of IPM3.
3.3.1.
Presence of macroscopic lesions
The expression of gross pathological changes in birds infected with LPAIV is dependent upon the host species, the virus strain, the time to death and the presence of secondary pathogens (Mutinelli et al., 2003; Pantin‐Jackwood and Swayne, 2009). In Galliformes, macroscopic lesions could be found in multiple organs, including rhinitis, sinusitis, congested tracheal mucosa, bronchitis lesions, oedema, congestion, haemorrhage and interstitial pneumonia in the lung and mild enteritis, in particular in the presence of secondary infections and/or adverse environmental conditions such as high ambient temperatures. Only mild lesions (sinusitis) were observed at necropsy of chickens inoculated intrachoanally with LPAI H7N9 (Spackman et al., 2015) and many LPAI viruses do not induce gross lesions in chickens, as, for instance, those reported for isolates derived from 2005–2006 in outbreak of LPAIV from in Japan (Okamatsu et al., 2007). LPAIV H5N1 and H7N3 isolates of wild bird origin were not pathogenic in turkeys and chickens (Ladman et al., 2010). Overall, LPAIV very rarely causes visible lesions on the carcasses of Galliformes birds that can be detected during meat inspection. In non‐galliformes bird species, lesions are rare and, if present, would be mild.
Detection in commercial abattoirs
According to Regulation (EC) No 854/200415, all birds are to undergo post‐mortem inspection in accordance with Sections I and III. In addition, the official veterinarian should personally carry out the following checks: (a) daily inspection of the viscera and body cavities of a representative sample of birds; (b) a detailed inspection of a random sample, from each batch of birds having the same origin, of parts of birds or entire birds declared unfit for human consumption following post‐mortem inspection; and (c) any further investigations necessary when there is reason to suspect that the meat from the birds concerned could be unfit for human consumption.
The EFSA's 2012 scientific opinion on the public health hazards to be covered by inspection of meat (poultry) (EFSA, 2012) in the EU did not include a full assessment of avian influenza virus as a food‐borne biological hazard transmissible to humans through the handling, preparation and/or consumption of poultry meat. It was concluded that ‘the current ante‐mortem and post‐mortem visual inspection are not able to detect any of the public health hazards identified as the main concerns for food safety’…, and that ‘the high speed of the slaughter lines reduces the sensitivity of detection of lesions or faecal carcass contamination by visual inspection and only, at best, a sample of the birds can be thoroughly examined’. There are no data on the sensitivity of the post‐mortem visual inspection for LPAI at abattoir. Although avian influenza virus was not on the list of considered hazards, it is a reasonable assumption to consider the conclusions of the EFSA opinion on post‐mortem visual inspection are also applicable to LPAIV.
There is no reason to differentiate the probability of LPAI not being detected at an abattoir during post‐mortem inspection between Galliformes, Anseriformes and ratites. Differences could only be expected at species level due to bird size. The bigger the bird, the slower the slaughter line, and hence the theoretically better chances of detecting macroscopic lesions. It has been acknowledged that heavy weight flocks of broilers had a significantly higher condemnation rate than standard flocks (Lupo et al., 2008). However, non‐galliformes species are less likely to show macroscopic lesions so despite theoretically increased probability of detection due to slower line speed, no significant differences in the probability of not being detected during post‐mortem inspection should be expected compared to broilers. Only in the case of turkeys could the probability of detection be higher, but there are no data to substantiate this hypothesis in the case of LPAI.
From third countries, it is assumed that abattoirs authorised for exporting raw poultry meat to the EU are subject to similar standards that include post‐mortem inspection at least equal to that conducted in the EU.
Kidneys are usually attached to the carcass and deserve a special attention since they are an organ that can harbour LPAIV (Swayne, 2007), unlike muscle and organs (liver) that harbour very low infectivity titres (Harder et al., 2016). Although full carcasses of poultry can be commercialised together with certain organs, for example giblets (heart, liver and gizzard), organs are not considered a commodity within the scope of the assessment as a potential source of LPAIV transmission to live poultry or humans.
Animal by‐products from LPAI‐infected poultry slaughtered either at home or in commercial abattoirs are not included as a commodity within the scope of this assessment as a potential source of LPAIV transmission to live poultry or humans.
In conclusion, the probability (P3) that a bird with LPAIV in muscle tissue is not detected in an abattoir during post‐mortem inspection is non‐negligible for both Galliformes and Anseriformes due to the low sensitivity of the post‐mortem visual inspection in detecting public health hazards.
Home slaughter and wild game
In the case of the home slaughter of game birds or non‐commercial poultry or backyard flocks without post‐mortem inspection or in the case of hunted wild birds directly consumed by hunters and their relatives or associates, a bird with LPAIV systemic infection, showing macroscopic lesions or not, may pass undetected and follow through the food chain. In fact, carcasses and viscera from hunted wild game birds might not be subject to any official inspection at any stage of processing, especially when the hunter is the handler and also consumer. In terms of volume, this would be negligible compared to the volume of poultry slaughtered in commercial abattoirs in the EU.
If a bird with LPAIV systemic infection (presence in the muscle) was showing macroscopic lesions at post‐mortem inspection, it could be condemned and declared unfit for human consumption. However, condemnation would be more likely to occur in the case of Galliformes than in non‐galliformes species like geese and ducks, which are less likely to show lesions and, if present, they would be mild. Moreover, because most of the macroscopic lesions appear in the organs that are detached from the carcass during evisceration and that are only subject to inspection in a representative sample of birds, a bird showing lesions in affected organs may be missed.
In conclusion, the probability that a bird with LPAIV in muscle tissue is not detected during post‐mortem inspection (IPM3) in home‐slaughtered or hunted birds for both Galliformes and Anseriformes is non‐negligible (more than 10%) given the presumed lack of post‐mortem inspection.
In the hypothetical case that during slaughter in commercial abattoirs systemically LPAIV‐infected birds were contaminated with faecal material, containing LPAIV or not, the probability that a bird with LPAIV in muscle tissue is not detected may be higher if carcasses contaminated with faecal material were more likely to be subject to condemnation. However, the 2012 EFSA opinion (EFSA, 2012) concluded that the sensitivity of detection of faecal contamination in carcasses by visual inspection is limited (EFSA, 2012). If LPAIV infection caused a higher level of faecal contamination (diarrhoea due to enteritis) leading to a higher probability of detection of contamination of carcasses and consequent condemnation, then probability term P3 would need to be changed to unlikely. However, if the level of faecal contamination was the same, the probability term would not change.
Uncertainty
The uncertainty estimate for the probability IPM3 is low as there is sufficient scientific knowledge available to conclude that there is more than a 10% probability of infected birds not being detected by post‐mortem visual inspection due to the mild (if any) lesions, the high speed of the lines in slaughterhouses and the limited ability of non‐professionals to recognise these during slaughter at home. For commercial abattoirs there might be some variability in the sensitivity of the inspection as described by EFSA's (2012) scientific opinion: ‘each slaughterhouse can be viewed as unique, owing to differences in poultry species slaughtered, logistics, processing practices, plant layout, equipment design and performance, standardised and documented procedures, personnel motivation and management, and other factors. These variations individually and in combination lead to between‐slaughterhouse differences in risk‐reduction capacities and, consequently, in the microbiological status of the final carcass’. With regard to the correlation between the presence of LPAIV in muscle tissue and the presence of macroscopic lesions in viscera and carcasses, the uncertainty is high since there are no quantitative data to substantiate it.
3.4. Probability of LPAIV preservation in raw poultry meat during the food chain (IPM4)
It is known that for HPAI viruses, common food preservation processes such as freezing and refrigeration do not substantially reduce the concentration or viability of these viruses in contaminated meat (INFOSAN, 2005). For instance, HPAI H5N1 virus has been isolated from muscle stored at 4°C for a continuous period of up to 160 days and viral loads decreased as a function of temperature and time (Yamamoto et al., 2017). Infectious HPAI H7N1 virus was re‐isolated from chicken, turkey and duck meat kept at 4°C for 135, 90 and 75 days, respectively (Beato et al., 2012). The epidemiological link between HPAI H5N1 virus in frozen duck carcasses and outbreaks in non‐commercial chickens (Harder et al., 2009) suggests that HPAIV could persist at least during some steps of the food chain.
As described in EFSA's latest avian influenza scientific opinion (EFSA AHAW Panel, 2017), also LPAI viruses can also survive for a long period at low temperatures. Ejaz et al. (2007) infected 10‐week‐old broiler chickens intranasally with LPAI H9N2 virus. At 10 days post‐inoculation, the animals were euthanised, and their carcasses were cut into small pieces and frozen at −20°C. On a weekly basis, samples were thawed, and the presence of the virus was checked through embryonated egg inoculation. Infectious virus was detected in various parts of chicken meat like legs (until 6 weeks post‐storage), neck and wings (until 4 weeks post‐storage) and breast (until 2 weeks post‐storage). Nazir et al. (2011) analysed at regular intervals, for a maximum of 24 weeks, the residual infectivity on cell culture of duck breast meat spiked with H4N6, H5N1 and H6N8 LPAIVs and incubated at temperatures of 30, 20, 10, and 0°C. A linear regression model was used to analyse the data and to calculate the time required for 90% loss of virus infectivity (T90 values). Incubation of the spiked meat at 20°C resulted in T90 values of 3, 2 and 3 days for H4N6, H5N1 and H6N8 viruses, respectively, whereas incubation of the spiked meat at 0°C resulted in T90 values of 40, 54 and 81 days for H4N6, H5N1 and H6N8 viruses, respectively. The isolation of LPAI H7N9, H9N2 and LPAI H1N2 viruses from illegally imported chicken and Muscovy duck meat (Shibata et al., 2018a) indicates that LPAIV could persist during at least some steps of the food chain.
The lowest chilling temperature that provides the maximum storage life of meat without any surface freezing is −1.5°C +/−0.5°C (James and James, 2002) and the temperatures at which poultry meat must be kept to be considered ‘frozen poultry meat’ (not higher than −12°C at any time) or quick‐frozen meat (not higher than −18°C),16 are all within the range of virus survival, as shown above. However, it is unlikely that there is a significant difference in the infectivity of LPAIV from chilled versus frozen raw poultry meat, based on results of the survival studies of swine influenza virus (H1N1) in experimentally contaminated pig meat (Romijn, 1989).
In the case of hunted wild game birds, the cold chain may not be evenly maintained due to the handling, transport and storage of shot birds at room temperature. LPAIV may suffer decay to an unknown extent during those steps due to higher temperatures (Horigan et al., 2014).
In conclusion, the probability (IPM4) of LPAIV preservation in raw poultry meat in the food chain is non‐negligible.
3.4.1.
Uncertainty
The gap between the temperatures at which raw poultry meat are usually stored and transported and those at which LPAIV can persist is sufficiently large that even if considered at their maximum levels, they are still far from those at which LPAIV persistence could be affected. Consequently, the uncertainty estimate for the probability IPM4 is low.
3.5. Effect of IPM2–IPM4 on the LPAIV presence in raw poultry meat from an infected live bird during the food chain (EFM)
The assessment of probability IPM2 (see Section 3.2) indicated that it is unlikely that a Galliformes bird with LPAIV in muscle tissue would not be detected via surveillance or ante‐mortem inspection in commercial holdings. This means that the probability of LPAIV remaining present and undetected in raw poultry meat (derived from a Galliformes bird originating from an exposed commercial flock) is reduced before its exposure to an individual. Probabilities IPM3 and IPM4 are non‐negligible, meaning that they do not alter the presence of LPAIV during the food chain (see Sections 3.3 and 3.4). Applying the rule as described in Section 2.2, the combined effect of IPM2–IPM4 is ‘reduction’ of the probability of LPAIV presence in raw poultry meat from an infected live bird during the food chain compared to P1 for a commercial Galliformes and ‘no effect’ for non‐commercial Galliformes and both commercial and non‐commercial Anseriformes (Table 4).
Table 4.
Determination of the effect of IPM2–IPM4 on LPAIV presence in raw poultry meat from an infected live bird during the food chain (EFM)
| Bird of origin | EFM – Effect of IPM2–IPM4 on the LPAIV presence in raw poultry meat from an infected live bird during the food chain (IPM2 * IPM3 * IPM4) |
|---|---|
| Commercial Galliformes | Reduction |
| Non‐commercial Galliformes | No effect |
| Commercial Anseriformes | No effect |
| Non‐commercial Anseriformes | No effect |
LPAIV: low pathogenic avian influenza virus.
3.6. Probability of LPAIV remaining present and undetected in raw poultry meat before exposure (CPM5)
The combined probability of LPAIV remaining present and undetected in raw poultry meat before exposure (CPM5) is the result of the combination of the probability of LPAIV presence in muscle tissue of one live bird in a flock exposed to LPAIV (IPM1) (see Section 3.1) and the overall effect EFM of the probabilities IPM2–IPM4 (P2*P3*P4) (Section 3.5). The probabilities are shown in Table 5, based on the set of rules described in Section 2.2.
Table 5.
Determination of the probability of LPAIV remaining present and undetected in raw poultry meat before exposure (CPM5)
| Bird of origin | IPM1 – probability of LPAIV presence in muscle tissue of one live bird in a flock exposed to LPAIV | EFM – effect of IPM2–IPM4 on LPAIV presence in a bird or in poultry meat during the food chain | CPM5 – probability of LPAIV remaining present and undetected in raw poultry meat before exposure |
|---|---|---|---|
| Commercial Galliformes | Very unlikely | Reduction | Extremely unlikely |
| Non‐commercial Galliformes | Very unlikely | No effect | Very unlikely |
| Commercial Anseriformes | Extremely unlikely | No effect | Extremely unlikely |
| Non‐commercial Anseriformes | Extremely unlikely | No effect | Extremely unlikely |
LPAIV: low pathogenic avian influenza virus.
3.7. Probability of exposure to raw poultry meat (IPM6)
3.7.1. Exposure of poultry and wild birds
Deliberate feeding of raw poultry meat to poultry was identified as the only relevant route of exposure since other routes would lead to very low exposure doses (e.g. contact with meat packaging material, contaminated water). The probability of deliberate feeding of raw poultry meat to commercial poultry is assumed to be negligible as it is economically counter‐productive and logistically unfeasible in terms of collection, transport, storage and usage. From the nutritional point of view, commercial poultry are usually fed with well‐balanced dry diets, which make the addition of raw poultry meat on an industrial scale unlikely. Furthermore, it is also not allowed in the EU and its trading partners and it would be in contradiction with biosecurity principles.
It has been speculated that scavenging foxes, for instance, could bring carcasses to a poultry establishment (CVI, 2017 and references therein). The latter may occur in poultry sectors with low levels of biosecurity such as outdoor/free‐range establishments but there are no data underpinning this hypothesis.
The epidemiological link made during investigations into outbreaks of HPAI H5N1 in Germany in 2007, established that uncooked offal from commercial deep‐frozen duck carcasses that entered the food chain before an outbreak caused outbreaks in backyard chickens several months later (Harder et al., 2009). Therefore, it cannot be excluded that feeding raw poultry meat (e.g. kitchen scraps, uncooked meat) to poultry could occur in non‐commercial establishments.
If raw meat was available to non‐commercial poultry holdings, wild birds may also have access to it. Wild birds could also be exposed through consumption of raw meat if it is not disposed of correctly (e.g. at landfill sites or in the surroundings of abattoirs).
The probability of exposure of commercial poultry to raw poultry meat is considered to be negligible whereas the probability of exposure of non‐commercial poultry and wild birds to raw poultry meat is considered to be very unlikely.
Uncertainty
The uncertainty estimate for IPM6 for commercial poultry is low given that the low likelihood of deliberate feeding of raw poultry meat to commercial poultry for economic, logistic, nutritional, and legal reasons combined with the implementation of biosecurity measures in commercial holdings provides sufficient information and there are no conflicting reports.
The uncertainty estimate for IPM6 for non‐commercial poultry and wild birds is high since the probability estimation is mainly based on subjective judgement due to a lack of data on the exposure of non‐commercial poultry and wild birds to raw poultry meat.
3.7.2. Exposure of humans
With regard to the exposure of humans to raw poultry meat, the two main routes in the food chain that have been included in this assessment are (i) the handling and manipulation by professionals (including industrial workers) or consumers, and (ii) consumption.
3.7.2.1. Handling and manipulation
Exposure of professionals (workers at abattoirs and meat processing plants) to raw poultry meat occurs when handling and manipulating carcasses or meat cuts. Exposure to raw poultry meat occurs at household level when consumers handle and manipulate purchased raw poultry meat or, in the case of home‐slaughtered or hunted birds, when raw poultry meat is manipulated and prepared. Therefore, the probability IPM6 for professionals and consumers via handling and manipulation is non‐negligible, although it is recognised that the frequency of exposure by handling and manipulation will be different between professionals and consumers.
Uncertainty
The uncertainty estimate for IPM6 for manipulation and handling by professionals and consumers is high since there are no available data on the exposure of the professional or consumer to raw poultry meat when manipulating or handling.
3.7.2.2. Consumption
Consumption of raw poultry meat is not common in the EU but it is popular in other countries like Japan. Chicken tartare and chicken sashimi (torisashi) are some examples of dishes prepared with raw poultry meat. The average consumption of poultry meat in the EU in 2006 was 22.2 kg/capita (Magdelaine et al., 2008). However, there are no available data on the proportion and frequency of consumption of such raw products, although it is expected to be rare in terms of the number of people consuming these cooked products.
In conclusion, the probability of a human to be exposed to raw poultry meat by consumption has been considered unlikely.
The probability of cross‐contamination of cooked poultry meat with infected raw meat has been widely documented as the source of food‐borne outbreaks due to Salmonella spp. and other food‐borne pathogens (Brown et al., 2013). It is not possible to estimate the frequency of cross‐contamination of cooked poultry meat with raw poultry meat in the EU that could modify the probability of exposure to potentially infected raw poultry meat through consumption. On the other hand, it should also be noted that, in contrast to bacteria, viruses will not amplify in the absence of living cells.
There are extra steps that may affect the probability of exposure through the consumption of hunted wild gamebirds, mostly related to the increased probability of cross‐contamination and the longer time between shooting and consumption (time in the hunt cart/bag, game larder, transport, distribution, hanging, processing and retail), which could result in there being several days during which the cold chain is maintained at all times (Horigan et al., 2014). However, only if game meat infected with LPAIV was consumed uncooked or undercooked, the probability of exposure would be higher. Assuming the good practice in the EU of cooking poultry meat preventing cross‐contamination and consuming cooked poultry meat after cooking at the recommended internal temperature, the probability of exposure of consumers to raw poultry meat is unlikely.
3.7.2.3.
Uncertainty
The uncertainty estimate for IPM6 for human consumption is high due to the lack of data on the intake of raw and undercooked poultry meat. The data available at EU level do not differentiate poultry meat from poultry products and/or do not differentiate raw from processed poultry meat.
3.8. Probability of exposure to raw poultry meat containing LPAIV (CPM7)
The combined probability of exposure to raw poultry meat containing LPAIV (CPM7) is the result of the combination of the probability of exposure to raw poultry meat (IPM6) (Section 3.7) and the probability of LPAIV remaining present and undetected in raw poultry meat before exposure (CPM5) (Section 3.6). The probability terms are shown in Table 6, based on the set of rules described in Section 2.2.
Table 6.
Determination of the probability terms for exposure to raw poultry meat containing LPAIV (CPM7)
| Exposed individual | IPM6 – probability of exposure to raw poultry meat (IPM6) | CPM5 – probability of LPAIV remaining present and undetected in raw poultry meat before exposure | CPM7 – probability of exposure to raw poultry meat containing LPAIV |
|---|---|---|---|
| Commercial poultry | Negligible | Very unlikelya | Negligiblea |
| Commercial poultry | Negligible | Extremely unlikelyb | Negligibleb |
| Non‐commercial poultry and wild birds | Very unlikely | Very unlikelya | Very unlikelya |
| Non‐commercial poultry and wild birds | Very unlikely | Extremely unlikelyb | Extremely unlikelyb |
| Humans (manipulation and handling) | Non‐negligible | Very unlikelya | Unlikelya |
| Humans (manipulation and handling) | Non‐negligible | Extremely unlikelyb | Unlikelyb |
| Humans (consumption) | Unlikely | Very unlikelya | Very unlikelya |
| Humans (consumption) | Unlikely | Extremely unlikelyb | Very unlikelyb |
LPAIV: low pathogenic avian influenza virus.
Raw poultry meat derived from a Galliformes bird of a non‐commercial flock.
Raw poultry meat derived from a Galliformes bird of a commercial flock or an Anseriformes bird of a commercial or non‐commercial flock.
It is important to highlight that the human exposure to LPAIV in raw poultry meat during handling and manipulation could be due to inhalation of aerosols, direct contact with oral, respiratory or conjunctival mucosa (for example by rubbing eyes) or ingestion of the virus. Harder et al. (2016) reviewed the literature on poultry products as the likely source of infection for humans from consumption. They concluded that the majority of human cases of avian influenza were from direct contact of a person with a bird during the slaughtering of inapparently infected or of clinically sick poultry. Occupational health and safety measures applied by the food industry to staff involved in such operations should minimise the probability of exposure of the person with a live bird. However, human exposure to live poultry is excluded from the assessment.
With regard to the oral route, it has been considered in this assessment that human exposure can occur by consuming raw poultry meat, but also by consuming undercooked poultry meat or meat cooked at temperatures not suitable for the inactivation of LPAIV present in meat due to consumption habits or due to the lack of temperature control and monitoring during the cooking process.
3.9. Probability of LPAIV infection given exposure to raw poultry meat containing LPAIV (IPM8)
3.9.1. Commercial and non‐commercial poultry
There are several studies reporting that poultry are much more sensitive to HPAI infection when exposed through the aerosol or intranasal routes compared to the oral and intragastric routes, indicating that alimentary infection requires higher exposure doses to produce infection as compared with intranasal exposure. For instance, chickens were 15 and 300 times more sensitive to HPAI H5N1 strain A/chicken/Suzdalka/Nov‐11/2005 when comparing the intranasal with the oral and intragastric routes, respectively (Sergeev et al., 2013). In another experiment, it was demonstrated that infection of chickens through oral exposure of chickens to meat containing HPAI H5N1 (isolate A/whooper swan/Mongolia/224/05) required a dose 3–4 log10 times higher than through intranasal exposure (Kwon et al., 2010).
Swayne and Beck (2005) reported that specific pathogen‐free (SPF) chickens fed with breast and thigh meat (harvested from chickens 3 days post‐inoculation with H7N2 LPAIV) did not show any clinical signs, neither produced antibodies to the virus nor died. This result is in line with the absence of virus in muscle tissue of the originally inoculated chickens. This is the only study that could be identified where (starved) poultry were exposed to meat from LPAI‐inoculated birds. On the other hand, mortality was observed in 90% of SPF chickens fed with meat containing a high dose of HPAIV (107.8 EID50 per bird), whereas feeding meat containing another HPAIV isolate at a lower dose (103.5−3.6 EID50 per bird) did not produce infection. Yao et al. (2014) assessed the dose–response relationship of chickens challenged intranasally and gastrointestinally with H9N2 LPAIV. These authors showed that the median infection dose for gastrointestinal inoculation was 104.3 TCID50. These results are in line with the study of Swayne and Beck (2005), who described infection of chickens fed with HPAIV only when a dose was available above the median infection dose determined by Yao et al. (2014).
The reported amount of the LPAI virus in muscles of experimentally infected poultry ranged from 0.44 log10 (Uchida et al., 2017) to 1.6–2 log10/g of tissue (Kishida et al., 2004). Shibata et al. (2018a) detected H9N2 virus titres of up to 3.5 log10/g in a mixture of tissues including muscle and other organs. Pantin‐Jackwood et al. (2017) detected viral RNA of H7N8 LPAIV in turkeys (2/2) exposed via the intra‐choanal route. Virus titres of 4.8 log10 EID50/g were determined based on RRT‐PCR, although it is not clear what would be the corresponding titre of viable virus. In the same study, muscle tissue of one out of three mallards tested was positive (2.5 log10 EID50/g), whereas muscle tissue from two infected chickens was negative in RRT‐PCR.
Based on the median infectious dose of 104.3 TCID50 assumed to be required to produce infection following oral inoculation in Galliformes and the low concentrations of viable virus detected in muscle tissue, the probability of infection of a bird (poultry) given exposure to raw poultry meat containing LPAIV is unlikely. The susceptibility data indicate that turkeys are at the higher end of this probability range whereas chickens and Anseriformes are at the lower end.
Uncertainty
The uncertainty estimate for IPM8 is moderate for commercial and non‐commercial poultry since there is only one study on the LPAI dose required to infect chickens through the gastrointestinal route.
3.9.2. Wild birds
For related species such as wild and domestic ducks, it is expected that similar infectious doses would be required for wild birds as for poultry to become infected via the oral route. Experimental infection of wild birds, such as crows and tree sparrows, using an H5N1 non‐pathogenic reassortant virus (H5N2/H7N1) and an H7N9 LPAIV isolate resulted in only limited infection using a high infectious dose (106.0 EID50) (Hiono et al., 2016). These findings suggest that infection is less likely where a low infectious dose is present (such as in poultry meat).
Raptors, such as eagles, hawks and owls, have not been reported positive for any LPAIV. Although several raptor species have tested positive for highly pathogenic H5 viruses after being found dead, this is likely a result of their diet and hunting or scavenging behaviour rather than acting as a natural host for such viruses and therefore are not included in the wild bird assessment.
LPAI is considered endemic in wild water birds although its prevalence varies among species (Hesterberg et al., 2009). Therefore, previous exposure to the same or an antigenically related virus could have induced a partial or full protective immune response (Latorre‐Margalef et al., 2017), but it is not possible to quantify the proportion of wild birds that would be protected against LPAIV infection due to remaining knowledge gaps.
In conclusion, the probability of infection of a wild bird given exposure to raw poultry meat containing LPAIV is unlikely.
Uncertainty
The uncertainty estimate for IPM8 for wild birds is high given the absence of data on the infectious dose through the gastrointestinal route and its variation among different (water) bird species.
3.9.3. Humans
In general, avian influenza viruses lack sufficient adaptation for the efficient infection of, replication in and excretion from human respiratory tissue (Munoz et al., 2016). Avian influenza viruses which cause infection in humans are generally those which have an affinity for human cell surface receptors, and this is driven by several different amino acid substitutions which are involved in the polymerase activity and receptor binding necessary for replication and transmission of avian influenza viruses in mammals (Hill et al., 2015; Xiang et al., 2018). However, the pathogenicity of different AIVs in humans is also driven by cytokine and chemokine responses, by whether there are any neutralising antibodies present for related viruses, and the immune status of the individual. Experimental infection of cynomolgus macaques with H7 viruses through various routes produced clinical infection in those animals infected intranasally or through conjunctiva for only certain viruses, namely H7N7 HPAI and H7N9 LPAI, but not H7N3 or H7N1 HPAI viruses. Clinical scores also varied, but generally severe infection was associated with prolonged infection of the respiratory tract and pro‐inflammatory response (Shichinohe et al., 2016). The only LPAI virus to consistently cause clinical infection in humans is the H7N9 LPAI virus circulating in China (EFSA, ECDC and EURL, 2018). Cowling et al. (2013) reported that of the human cases reporting poultry exposure, contact was defined in the majority of H7N9 cases as due to visiting live poultry markets (43 of 84).
As described in Section 3.7.2, the exposure of humans to raw poultry meat is differentiated for (i) handling and manipulation by professionals (including industrial workers) or consumers and (ii) consumption. Therefore, the probability of human infection is also assessed for these exposure routes.
3.9.3.1. Handling and manipulation
Serology studies could give an indication of the probability of infection given exposure to infectious virus, although the exposure route and the virus source cannot often be determined. Different serological investigations in risk groups like poultry workers, farmers, veterinarians, etc. exposed to infected birds as well as the general population within H7N9 virus‐affected regions in China showed overall low seropositivity indicating a low number of transmission events causing subclinical human infections (Yang et al., 2013; Lin et al., 2016; Liu et al., 2016). Also, the transmission of other avian influenza virus subtypes was studied extensively in China indicating a higher seropositivity in poultry workers and a very low level of seroconversion in the general population particularly for the H5, H7 and H9 subtypes that were present in the poultry population at the time of the study (Huang et al., 2013; Yang et al., 2013, 2016; Chen et al., 2015; Wang et al., 2015b; Li et al., 2016; Lin et al., 2016; Liu et al., 2016; de Bruin et al., 2017; Ma et al., 2018). One study indicated that occupational exposure to chicken flocks was an important risk factor for H9N2 infection (Wang et al., 2014), while others showed seroprevalence estimates against H9 that were significantly higher in live poultry market workers, large‐scale poultry farmers and backyard farmers than in poultry slaughtering factory workers and wild bird habitat workers (Li et al., 2017). In Iran, poultry workers were positive for H9N2 virus while the seroprevalence of non‐exposed individuals for both H9N2 strains were significantly lower (Heidari et al., 2016). Also, in a South African study of human infections during (LPAI) H7N1 and H5N2 outbreaks in ostriches, laboratory workers, abattoir workers and veterinary staff showed antibodies to H7 (Venter et al., 2017). Several of the studies mentioned above suggest that occupational exposure to live birds during slaughter is a risk factor for LPAIV infection; however these studies could not evaluate whether there is an association between handling and manipulating LPAIV‐contaminated raw meat and a person becoming infected.
Data show that the probability of infection with avian influenza virus for occupationally exposed people to poultry is higher than that for the general population due to longer and more direct exposure and to more animals. However, it is not possible to evaluate which route of exposure is mostly responsible for the transmission to humans as poultry workers handle live birds, are involved in practices at slaughter, and have contact with raw poultry meat as well as other slaughter products.
The probability of infection of a human exposed to contaminated raw poultry meat by manipulation or handling is extremely unlikely.
Uncertainty
There is substantial evidence of seroconversion due to occupational exposure but it has not been elucidated to what extent this is due to exposure to live birds or to raw poultry meat. For example, abattoir workers could be exposed by both live birds pre‐slaughter and to raw poultry meat during and post‐slaughter. At the consumer level, there is no evidence of seroconversion due to manipulation and handling of raw poultry meat during preparation of raw poultry meat and cooking. Thus, the uncertainty estimate for the probability of LPAIV infection given exposure to raw poultry meat containing LPAIV via manipulation and handling is moderate.
3.9.3.2. Consumption
Harder et al. (2016) concluded that there are no reports of human infection with avian influenza virus after consumption of properly processed poultry products, which would include recommended time–temperature combinations for different types of raw poultry meat. No H5N1 or H5N2 HPAI viruses could be isolated from poultry meat after it was exposed to 70°C for 1 sec and changed from pink‐tan to a white colour (Swayne, 2006). The meat type does not significantly influence thermal inactivation of H5N1 HPAI virus in chicken meat (Thomas and Swayne, 2007). United States guidelines recommend a minimum internal temperature of 73.9°C for poultry meat (USDA, 2014). Industry standard times and temperatures are suitable for the inactivation of AIVs present in meat. A time of 0.51 sec at 73.9°C achieves a 7‐log kill (Chapter 10.4 of the OIE Terrestrial Code). Zou et al. (2013) reported inactivation of A(H7N9) virus under conditions of 56°C for 15 min or 60°C for 5 min, and the infectivity was completely lost under conditions of 56°C for 30 min, 65°C for 10 min, 70°C, 75°C or 100°C for 1 min.
Assuming the purchase of raw poultry meat contaminated with LPAIV by consumers, exposure via consumption of poultry meat would only be possible if poultry meat was consumed either uncooked, after being cooked at conditions that do not allow the inactivation of LPAIV (undercooking) or in the case of cross‐contamination of cooked poultry meat with infected raw poultry meat. Cross‐contamination events seem to pose a higher risk than undercooking of poultry meat (Luber, 2009).
Recently, a pregnant woman with mild illness has been detected as H9N2‐infected in Shenzhen (Guangdong Province, China). The patient did not have any contact with live poultry (IHR report, 2018). There are no clear field observations of human infection with LPAIV via the oral route. Ferrets are considered by many authors to be the most suitable small animal model for influenza research related to humans (ECDC, 2012). Bertran and Swayne (2014) performed an experiment to determine the mean infectious and lethal doses for ferrets following consumption of breast meat from chickens that had been infected with H5N1 HPAI 24 h earlier via the intranasal route. One of the three ferrets became ill when exposed to meat containing 6.8 or 10.92 log10 EID50/g HPAI H5N1 Mong/05 virus, and none of the animals died. Only exposure to meat containing 10.96 log10 EID50/g H5N1 HPAI VN/04 resulted in clinical signs (3/3 animals) and mortality (2/3 animals). In a second experiment, ferrets were fed with the meat of chickens that had been infected with H5N1, H7N3 or H7N7 HPAI viruses. Seroconversion was detected 14 days post‐feeding in the ferrets exposed to meat containing H5N1 or H7N3 HPAI viruses (and virus isolation in nasal or rectal samples of some animals), but not in the H7N7 virus‐exposed animals. However, as described above, LPAI viruses have only been detected at a low dose in the muscle tissue of infected birds.
Human cases of H7N9 LPAI manifest primarily as an infection of the respiratory tract, not the digestive tract, which again points to a respiratory or intranasal route of infection. Infection studies in ferrets showed that direct contact was the most efficient way to transmit the virus, rather than the aerosol route. Risk factors associated with severe infection in humans included increased age and existing medical conditions or immunosuppressive medications, visiting live poultry markets, but not keeping backyard poultry. The live poultry markets will provide an environment with high levels of virus available for direct contact transmission (intranasal, intraconjunctival, respiratory or even oral) (Liu et al., 2013). Xu et al. (2014) reported on an experimental study with ferrets inoculated intranasally either with 106 or 108 TCID50 of influenza A/Anhui/1/2013(H7N9). The animals were observed for clinical signs and were weighed daily as an indicator of disease. Viruses caused sneezing, ruffled fur, lethargy and decreased appetite for food in ferrets. No ferrets inoculated with influenza A(H7N9) died. The mean maximum weight loss during the 14‐day period after inoculation was 10.9% and 9.2% for animals inoculated with 106 or 108 TCID50, respectively, of influenza A(H7N9).
Influenza A viruses are acid‐labile and hence very different from the typical acid‐stable, food‐borne‐ enteric viruses such as hepatitis A virus, norovirus or human enteroviruses. A Scientific Report of the Scientific Panel on Biological Hazards (EFSA, 2006) based on the available evidence at that time concluded that ‘there is no proof that virus replicates in the human intestine. The presence of diarrhoea in several patients, the detection of viral RNA in the intestines of two patients and the demonstration of infectious virus in rectal swabs of one patient do not allow one to conclude that the GI tract is a portal of entry or a target organ’, and that ‘it is unlikely that the lower GI tract (stomach and intestines) could serve as a portal of entry of H5N1 virus in humans after consumption of food products from infected animals’.
It can be concluded that the probability of infection given exposure to raw poultry meat containing LPAIV is negligible based on the low concentration of LPAIV that could be present in raw poultry meat and the acid‐lability of the virus.
Uncertainty
There is sufficient scientific knowledge available to underpin the negligible probability that humans could become infected with LPAIV through the oral route and very limited evidence of seroconversion to LPAIV or disease caused by LPAIV via consumption of raw poultry meat. It is assumed that in order to get infected, meat must be consumed raw or undercooked. In this regard, there is a lack of data on the frequency of human consumption of undercooked poultry meat in the EU that could modify the probability of exposure from consumption of infected or non‐infected raw poultry meat. As pointed out by Horigan et al. (2014), ‘there is a greater tendency to serve game undercooked or ‘pink’ outside the home than when cooked by the consumer in the home environment. Restaurants and catering establishments were more likely to serve game birds undercooked’. For larger birds, this could result in the internal areas of the carcass not reaching sufficiently high temperatures for total pathogen elimination. Taking into account these aspects, the uncertainty estimate for the probability of LPAIV infection given exposure to raw poultry meat containing LPAIV via consumption is moderate.
3.10. Probability of exposure and subsequent LPAIV infection via raw poultry meat containing LPAIV (CPM9)
The probability of exposure and subsequent LPAIV infection via raw poultry meat containing LPAIV (CPM9) is the result of the combination of the probability of LPAIV infection given exposure to raw poultry meat containing LPAIV (IPM8) (Section 3.9) and the probability of exposure to raw poultry meat containing LPAIV (CPM7) (Section 3.8). The probabilities are shown in Table 7, based on the set of rules described in Section 2.2.
Table 7.
Determination of the probability terms for exposure and subsequent LPAIV infection via raw poultry meat containing LPAIV (CPM9)
| Exposed individual | IPM8 – probability of LPAIV infection given exposure to raw poultry meat containing LPAIV | CPM7 – probability of exposure to raw poultry meat containing LPAIV | CPM9 – probability of exposure and subsequent LPAIV infection via raw poultry meat containing LPAIV |
|---|---|---|---|
| Commercial poultry | Unlikely | Negligiblea , b | Negligiblea , b |
| Non‐commercial poultry and wild birds | Unlikely | Very unlikelya | Very unlikelya |
| Non‐commercial poultry and wild birds | Unlikely | Extremely unlikelyb | Very unlikelyb |
| Humans (manipulation and handling) | Extremely unlikely | Unlikelya , b | Very unlikelya , b |
| Humans (consumption) | Negligible | Very unlikelya , b | Negligiblea , b |
LPAIV: low pathogenic avian influenza virus.
Raw poultry meat derived from Galliformes bird of a non‐commercial flock.
Raw poultry meat derived from a Galliformes bird of a commercial flock or an Anseriformes bird of a commercial or non‐commercial flock.
3.11. Probability of LPAIV transmission from individual to individual (IPM10)
3.11.1. Commercial and non‐commercial poultry
LPAIV transmission rates are initially low as an adaptation of the wild bird‐derived virus to poultry host is required to achieve enhanced replication (Giannecchini et al., 2010; Li and Cardona, 2010). Transmission within the flock will depend on the level of virus available to other poultry through direct contact with the exposed birds. If the birds are exposed, but not infected, there is negligible likelihood of spread to other birds. If the bird is exposed and infected but does not produce a high level of virus, this may not result in transmission either. A review on within‐flock transmission made by a group of scientists from Wageningen Bioveterinary Research (NL) and the Animal and Plant Health Agency (UK) showed that the basic reproduction ratio (R0) depends on the LPAIV strain and the poultry species (de Koeijer et al., 2017). Estimated average reproduction ratios for H5 and H7 LPAI viruses isolated from poultry were in general lower in chickens (most estimates between 0.8 and 5.6), than in ducks and turkeys (most estimates between 2.1 and 15.3). Hence, the probability that the virus will be transmitted in the flock (1 − 1/Ro) is expected to be higher in turkey and duck flocks than chickens (de Koeijer et al., 2017).
The data retrieved from the literature indicate that within‐flock transmission of LPAIV is variable (as described above) and it cannot be excluded that the probability of LPAIV transmission amongst poultry is above 10%, in particular in a flock with a high density of birds. Thus the probability of LPAIV transmission within a commercial flock is non‐negligible.
It is considered that transmission under field conditions following exposure of poultry to raw meat containing LPAIV would be even lower than the reported reproductive ratios obtained in controlled environments after experimental infections with high viral doses (Comin et al., 2011; Gonzales et al., 2012; Saenz et al., 2012). Poultry densities are often low in non‐commercial flocks and the virus might be more dispersed in the environment and/or could be more exposed to inactivation (e.g. solar radiation), leading therefore to lower probability of transmission between birds than the probability observed in commercial flocks. The probability of transmission among poultry in a non‐commercial flock is expected to be lower than that expected in commercial flocks, therefore the probability is considered unlikely in non‐commercial flocks.
Uncertainty
The uncertainty for commercial and non‐commercial poultry is high given the possible variation depending on the virus strain, bird species and bird density.
3.11.2. Wild birds
Wild waterfowl are capable of infecting one another, but as the natural hosts for LPAIV, it is not understood how much the natural immunity or other circulating viruses impact on the transmission dynamics. According to EFSA's Scientific Opinion (2017) ‘Experimental infections with LPAIV showed that a transient, low‐level antibody response can be generated, which may be sufficient to provide partial protection against reinfection with viruses of the same subtype; it is less likely that the induced humoral response confers protection against heterologous reinfections…’. The wild bird to wild bird transmission is also affected seasonally as the birds behaviour will change from sedentary, nesting and breeding behaviour in small groups of sometimes single species to more social, foraging behaviour in large groups of mixed species. Prevalence values in wild bird populations were estimated at 0.2%, 2% and 6% in low medium and high prevalence scenarios ('EFSA AHAW Panel, 2017). The probability of LPAIV transmission to a wild waterfowl is therefore considered unlikely.
Uncertainty
The uncertainty for wild birds is moderate as there is ample scientific evidence that wild waterfowl are a reservoir for LPAIV but there is a lack of studies on LPAI transmission dynamics among wild birds.
3.11.3. Humans
Different subtypes of LPAIV have been transmitted to humans, causing infections. Seroepidemiological studies, performed mainly in China (see Section 3.9.3.1), have shown that transmission events to human are in general rare events and most infections are either asymptomatic or cause only mild respiratory symptoms (Kim et al., 2016). However, LPAIV such as H7N9, H6N1, H7N4, H9N2, H10N8 have also caused severe diseases and fatalities in infected people (Wei et al., 2013; Zhang et al., 2015; RahimiRad et al., 2016; EFSA, ECDC and EURL, 2018). Most of these transmission events occur in occupationally‐at‐risk groups that have direct and prolonged contact with potentially infected birds, like poultry workers, veterinarians, workers at live bird markets, etc. The majority of the cases of H7N9 virus infection in humans have been associated with direct or indirect contact with infected live or dead poultry. Small clusters of infections in humans have been documented for H7N9, with possible human‐to‐human transmission (Zhou et al., 2018). No cluster has spread beyond two generations so far nor shown sustained human‐to‐human transmission. Avian influenza viruses do not readily infect people but can do so when people have close contact with infected birds or exposure to high viral loads in untreated products. Therefore, the probability of LPAIV transmission from human‐to‐human is considered negligible.
Uncertainty
There is sufficient scientific knowledge available to underpin the conclusion that the probability that humans will transmit LPAIV to other humans is negligible, given the lack of ability of LPAIV to efficiently cause sustained transmission between humans. It is acknowledged that the reported recent cases of infected humans and the fact that influenza viruses constantly evolve and with every human or mammalian infection with these viruses the possibility that the virus will acquire traits of mammalian transmissibility cannot be excluded. Efforts for early detection and monitoring of every human case are warranted (Uyeki et al., 2017). Despite this mutation potential of influenza viruses, the probability of LPAIV transmission from individual to individual remains low.
3.12. Probability of LPAIV transmission from an individual infected via raw poultry meat containing LPAIV (CPM11)
The combined probability of LPAIV transmission from an individual infected via raw poultry meat containing LPAIV (CPM11) is the result of the combination of the probability of establishment in the population of LPAIV transmission from individual to individual (IPM10) (Section 3.11) and the combined probability of exposure and subsequent LPAIV infection via raw poultry containing LPAIV (CPM9) (Section 3.10). The probabilities scores are shown in Table 8, based on the set of rules described in Section 2.2.
Table 8.
Determination of the probability of LPAIV transmission from an individual infected via raw poultry meat containing LPAIV (CPM11)
| Exposed individual | IPM10 – probability of LPAIV transmission from individual to individual | CPM9‐ probability of exposure and subsequent LPAIV infection via raw poultry meat containing LPAIV | CPM11‐ probability of LPAIV transmission from an individual infected via raw poultry meat containing LPAIV |
|---|---|---|---|
| Commercial poultry | Non‐negligible | Negligiblea , b | Negligiblea , b |
| Non‐commercial poultry and wild birds | Unlikely | Very unlikelya , b | Very unlikelya , b |
| Humans (manipulation) | Negligible | Very unlikelya , b | Negligiblea , b |
| Humans (consumption) | Negligible | Negligiblea , b | Negligiblea , b |
LPAIV: low pathogenic avian influenza virus.
Raw poultry meat derived from Galliformes bird of a non‐commercial flock.
Raw poultry meat derived from a Galliformes bird of a commercial flock or an Anseriformes bird of a commercial or non‐commercial flock.
Transmission between flocks or farms is not considered in this assessment. However, it might be interesting to note that, as described in the latest EFSA opinion (EFSA AHAW Panel, 2017), ‘LPAIV outbreaks often do not result in secondary spread, as it was for example demonstrated for laying hens in the Netherlands (Gonzales et al., 2014); infection fades out without any control measure being implemented. Nevertheless, LPAIV infections may result in secondary spread, as was shown in turkeys in Italy (Comin et al., 2011) and Germany (T Harder, personal communication on September 2017), ducks in France, and laying hens in poultry dense regions (Gonzales et al., 2014)’. Furthermore, several studies have concluded that non‐commercial poultry establishments with no connections to the commercial sector (i.e. no shared workers, equipment, feed delivery or veterinary services, etc.) have little impact on the spread of avian influenza to commercial establishments (Bavinck et al., 2009; Smith and Dunipace, 2011; Gale et al., 2017).
LPAI viruses circulate widely in wild bird populations, particularly in wild waterfowl (Hesterberg et al., 2009). With limited exceptions, there are no requirements in either EU rules or OIE recommendations to report infection with LPAI among wild birds. The only virus which should be reported is one with pandemic potential or public health risk. Therefore, the detection of a LPAI virus in a wild bird as a result of consuming infected raw poultry meat is of no consequence to the overall background risk presented by these birds to the commercial or non‐commercial sector.
3.13. Overall outcome of the assessment
Figure 2 gives an overview of the overall outcome of the assessment of LPAI infection, and transmission via raw poultry meat. The available scientific evidence and the rationale behind each probability have been described in the sections above.
Figure 2.
Main outcomes from the assessment of LPAIV infection, establishment and spread via raw poultry meat (U, uncertainty; COM, commercial; NON‐COM: non‐commercial)
-
Negligible, indistinguishable from 0; extremely unlikely, up to 1%; very unlikely, from 1 up to 2%; unlikely, from 2% up to 10%; non‐negligible, from 10% up to 100%; U, uncertainty; IP, independent probability; CP, combined probability.(a): Raw poultry meat derived from Galliformes bird of a non‐commercial flock; (b) Raw poultry meat derived from a Galliformes bird of a commercial flock or an Anseriformes bird of a commercial or non‐commercial flock.
3.14. Main conclusions
The probability of LPAIV presence in muscle tissue of one live bird in a flock exposed to LPAIV is very unlikely and extremely unlikely for Galliformes and Anseriformes, respectively.
The probability of LPAIV infection given exposure to raw poultry meat containing LPAIV is unlikely for poultry and wild birds whereas for humans it is extremely unlikely via handling/manipulation and negligible via consumption.
The combined probability of exposure and subsequent LPAIV infection given exposure to raw poultry meat containing LPAIV is very unlikely for non‐commercial poultry, wild birds and humans exposed via handling/manipulation of raw poultry meat whereas it is negligible for commercial poultry and humans exposed via consumption.
The probability of LPAIV transmission from an individual infected via raw poultry meat containing LPAIV is negligible for commercial poultry and humans whereas it is very unlikely for non‐commercial poultry and wild birds.
There is in general a high level of uncertainty on the estimation of the probabilities of key steps of the transmission pathway for poultry and wild birds mainly due to the limited number of studies on the presence of LPAIV in meat and on the viral load required to infect a bird via raw poultry meat containing LPAIV.
4. Assessment of LPAIV transmission via raw table eggs
A general description of the transmission pathway is provided in Section 2.2. The starting point was a commercial or non‐commercial poultry establishment within the EU that has been exposed to LPAIV with no official notification made which otherwise would result in disease control measures. Figure 3 shows the pathway applied to assess the probability of LPAIV transmission via raw table eggs containing LPAIV. The determination of each probability is described in the sections below.
4.1. Probability of LPAIV presence inside eggs of one live bird in a flock exposed to LPAIV (IPE1)
It is well known that infection with HPAIV can be detected in albumin and yolk and also in the oviduct, suggesting that the virus also replicates in the reproductive tract and can infect the internal contents of the egg. As a consequence, decrease in egg production and in egg quality can be associated with HPAIV infection and eggs may contain HPAIV in the albumen or yolk (Spickler et al., 2008; Silva et al., 2013). Uchida et al. (2017) showed that H5N8 HPAIV could be detected in both the internal contents and shells of eggs laid by white leghorn hens experimentally infected.
There is less available information regarding the presence of LPAIV in eggs than that available for HPAIV. Infection with LPAI viruses of several subtypes have been reported to induce lesions in the reproductive tract of poultry (see review of Spickler et al., 2008; Pillai et al., 2009, 2010; Pantin‐Jackwood et al., 2012; Wang et al., 2015a; Qi et al., 2016), which can lead to a drop in egg production (as described in Section 3.1). H7N2 LPAI virus has been isolated from the oviduct tissue of naturally infected layers (Ziegler et al., 1999). In LPAI H9N2 inoculated laying hens, the virus was detected in the oviduct tissue via RT‐PCR from the first day after the inoculation (1 day post‐infection (dpi)) to the end of the experiment (7 dpi), with peak17 virus levels detected around 5 dpi (Qi et al., 2016). Furthermore, viral loads showed a close correlation with the changes in the uterus CaBP‐D28k mRNA expression, and abnormal expression of CaBP‐D28k mRNA is associated with declines in egg production and deterioration in eggshell quality. More pores, crevices along the pores and embossment were observed on the external surface of the eggshells from the inoculated hens from 1 dpi to the end of the experiment. Pillai et al. (2009) found LPAI H3N2 virus in the oviduct of experimentally infected turkeys from 3 dpi onwards with peak viral titres around 5 dpi. Also, LPAI H4N8 virus was detected in the ovarian and oviduct tissue of hens infected intratracheally or intravenously (Shalaby et al., 1994).
Despite virus replication in the oviduct, there is very limited scientific evidence on LPAIV presence inside the egg. Cappucci et al. (1985) reported the isolation of a non‐pathogenic avian influenza virus in 2 out of 120 eggs collected from a chicken holding in Virginia that was found through routine virological surveillance to be infected, but which was clinically unaffected while a HPAI H5N2 outbreak was ongoing in Pennsylvania. In contrast to this finding, no virus‐positive albumen samples were identified in eggs tested from several LPAI outbreaks (Lu et al., 2004; Swayne and Beck, 2004). Only one experimental study was found where LPAIV (H3N2) was isolated from inside eggs laid by breeder turkeys inoculated with a dose of 5.13 log10 EID50 through the intrachoanal route (Pillai et al., 2010). The authors of this study performed two experiments (repetitions), in both repetitions they observed drops in egg production of infected turkeys and could detect virus RNA in albumen from eggs laid between 2 and 6 dpi by using RRT‐PCR. In the first experiment, 12 breeder turkeys were initially inoculated and at specified days post‐inoculation (dpi 3, 5, 7 and 9), groups of 3 turkeys were euthanised to assess the presence of virus in the oviduct (see above). Between 2 and 6 dpi (production ceased at 7 dpi) a total of 11 eggs were collected and tested; 3 out of these 11 eggs tested positive by RT‐PCR for the presence of virus in the albumen. During the second experiment using 24 breeder turkeys, a total of 62 albumen samples were tested, 17 samples were found positive by RT‐PCR and live virus could be isolated from three of these samples. The virus strain used in this study was associated with sudden declines in egg production in breeder turkeys and was most closely related to the triple‐reassortant H3N2 swine viruses that have been circulating among pigs in the United States since 1998 (Tang et al., 2005; Pillai et al., 2010). However, white leghorn layer‐type chickens inoculated with an H3N2 virus strain isolated from swine did not cause systemic infection (Thomas et al., 2008). Therefore, the presence of swine influenza H3N2 LPAI virus in turkey eggs cannot be directly extrapolated to the potential presence of other LPAI viruses in table eggs from chickens. In a more recent study, 11 chickens, 11 Japanese quails, 11 pigeons, 11 Pekin ducks, 11 mallard ducks, 7 Muscovy ducks and 9 Embden geese were intranasally inoculated through the choanal cleft with an inoculum containing 6.0 log10 EID50 of A/Anhui/1/2013 (H7N9) in 0.1 mL. The contents of eggs laid by infected chickens were virus‐negative (Pantin‐Jackwood et al., 2014).
Anseriformes are susceptible to LPAIV infection but in general show less clinical signs than Galliformes (see Section 3.1). Significantly less H9N2 viral replication has been detected in magnum organ cultures (MOC) of ducks than of chickens and turkeys (Sid et al., 2017). No other data were retrieved regarding the possibility of LPAIV presence in the reproductive tract or inside eggs.
In conclusion, the probability IPE1 of LPAIV presence inside eggs of one Galliforme bird in a flock exposed to a LPAIV is extremely unlikely since it has only been reported in one naturally exposed chicken holding and in experimentally infected turkeys which were inoculated with a high viral dose. For Anseriformes, probability IPE1 of LPAIV presence inside eggs of one Anseriforme bird in a flock exposed to the virus is negligible since they are less susceptible to (viraemic) LPAI infections than Galliformes and there is no scientific evidence of LPAIV presence inside eggs laid by infected animals.
4.1.1.
Uncertainty
The uncertainty estimate IPE1 is high for Galliformes since several studies analysed LPAIV presence in eggs following natural or experimental exposure, but positive findings were only reported once in natural cases and in one experimental study using breeder turkeys inoculated with a virus strain well adapted to turkeys, and associated with sudden declines in egg production. For Anseriformes, the uncertainty estimate for IPE1 is high since there is an extreme low probability that LPAI infection becomes viraemic but there is a lack of studies analysing virus presence in oviduct or inside eggs.
4.2. Probability that a bird with LPAIV producing eggs containing LPAIV is not detected via surveillance or ante‐mortem inspection (IPE2)
For probability IPE2, the underpinning argumentation and the corresponding uncertainties are identical in the assessments on raw poultry meat and on table eggs. Different detection thresholds are used for instance for turkeys, laying hens and broilers but it is considered that the detection sensitivities are within the same range. It is assumed across this assessment that the presence of infection with/without clinical signs is a precondition for the presence of LPAIV inside eggs. However, it cannot be ruled out that infected birds may lay eggs not containing LPAIV.
As described in Section 3.2, LPAIV‐infected birds in the non‐commercial sector are only identified through the detection by passive surveillance of clinical signs and/or mortality, whereas this is complemented by active surveillance in the commercial sector. LPAI infection can lead to a drop in egg production or the production of thin‐shelled and/or misshapen eggs (see Section 4.1) in Galliformes, whereas this is not described for Anseriformes. As egg production is a measure collected by the commercial sector on a daily basis, any consistent and increasing drop would be a trigger for disease investigation.
In conclusion, probability IPE2 for Galliforme bird, of a non‐commercial flock and for an Anseriformes bird of a commercial or non‐commercial flock is non‐negligible. The probability for a Galliformes bird of a commercial flock is unlikely.
4.2.1.
Uncertainty
The uncertainty estimate for IPE2 for commercial birds is high as described in Section 3.2, plus the added uncertainty of the lack of data about the proportion of eggs laid by infected with LPAIV containing LAPIV. The uncertainty estimate for IPE2 for Anseriformes and for Galliformes in the non‐commercial sector is high due to the lack of data on the detection levels associated with the presence of clinical signs, whereas for Galliformes in the commercial sector it is moderate.
4.3. Probability that an egg containing LPAIV is not detected during inspection (IPE3)
The majority of the table eggs in the EU are sourced from layer hens. Production of table eggs from other species, such as ducks, quails, geese, turkeys, ostriches and seagulls, varies between countries and typically eggs are sold as a small‐scale alternative, niche or luxury commodity. The European egg product industry mainly purchases eggs directly from farms or from packing centres. Commercial production of table eggs usually includes the initial transport of the product from the farm to a grading centre where the eggs are graded. Eggs are selected and stored in a storage area, which may sometimes be chilled, before transfer to the grading/packing centre, which may be on the farm or a separate central facility where grading and inspection take place (EFSA BIOHAZ Panel, 2014). On large farms, eggs may be stored for up to 48 h before they are transported to the grading or packing centre. Eggs downgraded to grade B or eggs showing macroscopic abnormalities (misshapen, cracks, crevices, odd‐shaped, thin‐shelled) are usually diverted for the egg product industry (EFSA BIOHAZ Panel, 2014). Moreover, Regulation (EC) No 853/2004 authorises the processing of eggs downgraded by packing centres (misshapen, dirty or broken eggs; eggs with cracked eggshells) or even from farms contaminated by Salmonella, as long as the eggs are broken upon receipt and the egg products are heat‐treated immediately.
In non‐commercial poultry, eggs may be collected and consumed immediately or shortly after lay and the criteria for grading or discarding may not be applied and eggs may be discarded or used as feed only in the case of gross abnormalities.
The probability that a table egg containing LPAIV is not detected at inspection in the grading/packing centre depends on: (a) the probability that LPAIV‐containing eggs arriving at the grading/packing centre and (b) the probability that LPAIV‐containing table eggs show macroscopic deficiencies resulting in the downgrade or discard at the grading/packing centre or by handlers in the non‐commercial sector after inspection.
The sensitivity of the inspections (a) and (b) is directly associated with the presence of abnormalities in the egg or eggshell detected on the farm before transport (a) or at the grading/packing centre during inspection (b).
As mentioned in Section 4.1, there is evidence of a decrease in egg quality due to LPAIV infection. Qi et al. (2016) reported the correlation of viral loads of H9N2 subtype avian influenza virus with a decline in egg production and deterioration in eggshell quality in H9N2 avian influenza virus‐infected laying hens. The main findings were a significantly decreased level of calcium content during 3~7 dpi, and the observation of more pores, crevices along the pores, and embossment on the external surface of the eggshells from hens inoculated with avian influenza virus for the period from 1 dpi to the end of the experiment. Evident embossment as well as more small holes on the shell surface were detected on eggs from infected hens at 3~5 dpi. This study did not analyse H9N2 virus presence inside the eggs.
Despite the fact that egg quality may be affected in outbreaks of LPAIV, there are no data on the proportion of eggs laid by LPAIV‐infected birds that show macroscopic abnormalities conducive to downgrading or discarding. The probability that a table egg from both Galliformes and Anseriformes containing LPAIV is not detected at inspection is considered non‐negligible (more than 10%) for both the commercial and non‐commercial sectors, since it cannot be excluded that less than 90% of LPAIV‐containing eggs might be deformed.
4.3.1.
Faecal contamination on egg surface
Transmission via the faecal–oral route is considered to be the primary mode of LPAIV transmission in many bird species, although transmission via respiratory secretions may also be relevant for particular land‐based bird species (Ellstrom et al., 2008). Influenza viruses are shed from the body in secretions and excretions, particularly faeces. The surfaces of eggs laid by hens in flocks infected with AIVs may be contaminated by infective faeces (Spickler et al., 2008). Duck eggs are usually dirtier than hens’ eggs when collected, increasing the chance of faecal contamination of shells by other commonly occurring intestinal organisms, including Campylobacter and Listeria (Adzitey et al., 2012), and, if present, of LPAIV.
The persistence of LPAI in poultry faeces has been studied by Hauck et al. (2017) by spiking bedding material and faeces obtained from turkey, broiler, and egg‐layer commercial productive units with HPAIV and LPAIV. Live HPAIV particles persisted for 96 h, the last time point measured, in layer faeces and less than 60 h in broiler and turkey bedding. In contrast, LPAIV persisted for less than 24 h after having been spiked in all the different substrates kept at room temperatures ranging from 19 to 21°C in biosafety level 2 and between 21.5 and 22.5°C in the biosafety level 3. Stephens and Spackman (2017) measured inactivation of poultry litter infected LPAIV. At 1 day at 26.7°C or above, LPAIV was inactivated. At 10.0, 15.6 and 21.1°C, inactivation times increased to 2–5 days.
One method to remove faecal contamination from the eggshell is by washing. According to Commission Regulation (EC) No 589/200818, as amended, grade A eggs are not to be washed or cleaned before or after grading, and if washed, even where they fulfil the criteria applicable to grade A eggs, must be marked ‘washed egg’. For economic and technical purposes and to avoid problems associated with ineffective washing, egg washing is not a current practice in European egg‐processing plants (EFSA BIOHAZ Panel, 2014). Washing removes the cuticle, a natural coating, which provides a natural barrier to bacteria and pathogens. Therefore, washing is not considered in the estimation of the probability. In fact, table eggs may contain some faecal contamination on the surface and follow through the food chain until retail.
One way to inactivate viruses on the egg surface is by radiation with ultraviolet (UV) light. Experiments in water have shown that the most persistent organisms after UV disinfection are viruses, specifically adenoviruses, and bacterial spores (Hijnen et al., 2006), and UV radiation is only able to inactivate microorganisms on eggshells directly exposed to the rays (EFSA, 2005; De Reu et al., 2006). It is uncertain whether UV‐light is able to inactivate viruses inside the egg.
It is not clear whether the presence of faecal contamination containing LPAIV in the egg surface may result in the introduction of the LPAIV into the egg, as can occur for other spoilage and food poisoning bacteria (Al‐Bahry et al., 2012). However, the low ability of the LPAIV to persist in faeces at room or storage temperature precludes any possibility of entering the egg. In summary, faecal contamination of the eggshell of raw table eggs does not affect the overall probability that a raw table egg containing LPAIV is not detected at inspection. The presence of LPAIV on the egg surface is not included in the assessment (see section 1.2).
Uncertainty
The uncertainty estimate for IPE3 is high given the lack of data on the proportion of eggs laid by LPAIV‐infected birds that show macroscopic abnormalities conducive to downgrading or discarding whether at grading centres or by handlers of non‐commercial poultry. There is also uncertainty as to whether LPAIV, if present on the egg surface, can enter the egg. Inactivation methods like irradiation with UV‐light are effective in inactivating bacteria, but it is not known whether they can inactivate all types of virus, especially those that are inside the egg.
4.4. Probability of LPAIV preservation inside a raw table egg during the food chain (IPE4)
The probability that LPAIV inside a table egg is preserved during the food chain depends on the combinations of time and temperature in the different steps of the food chain.
In commercial egg production, eggs may be transported from the grading centre to a classification/packing centre or directly to a wholesale/distribution centre prior to the transport to retail. According to Commission Regulation (EC) 1020/200819, ‘eggs must be stored and transported until sale to the final consumer at a temperature, preferably constant, that is best suited to assure optimal conservation of their hygiene properties, unless the competent authority imposes national temperature requirements for egg storage facilities and for vehicles transporting eggs between such storage facilities’. However, the temperatures that assure optimal conservation of the egg hygienic properties are not specified, hence empirical data may be more appropriate to assess the potential preservation of LPAIV, should they be present in a table egg.
Table eggs are not required to be refrigerated prior to sale. However, according to Commission regulation 589/2008, ‘class (table) A eggs shall not be treated for preservation or chilled in premises or plants where the temperature is artificially maintained at less than 5°C. Eggs which have been kept at a temperature below 5°C during transport for not more than 24 h or on retail premises or in annexes thereto for not more than 72 h shall not be considered as chilled’.
Time and temperature of storage of eggs in the EU, from the ‘on farm’ to the ‘transport to retail’ stages were derived from expert opinion among industry experts, as part of the expert knowledge elicitation exercise to feed the modified Australian egg storage model (MAESM) (EFSA BIOHAZ Panel, 2014) (see Table 9).
Table 9.
Summary of time and temperature of storage of eggs in the EU, from the ‘on farm’ to the ‘transport to retail’ stages as derived from expert opinion (industry experts) (EFSA BIOHAZ Panel, 2014)
| Stage | Time (h) | Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| Minimum | Most likely | Maximum | Minimum | Most likely | Maximum | |
| On farm | 0 | 45 | 168 | 4 | 15 | 30 |
| Transport to grading | 0 | 6 | 48 | 4 | 15 | 30 |
| Grading | 0 | 18 | 168 | 5 | 15 | 30 |
| Transport to wholesale | 0 | 5 | 48 | 0.1 | 14 | 30 |
| Wholesale/distribution centre | 0 | 23 | 336 | 0.1 | 13 | 28 |
| Transport to retail | 0 | 7.5 | 36 | 0 | 14 | 30 |
Taking the combinations of time–temperature at the maximum values, they would not be able to influence/reduce the preservation of LPAIV inside a table egg in any of the steps of the food chain. Thus, the probability of LPAIV preservation inside a table egg from Galliformes and Anseriformes in the commercial sector during the food chain is non‐negligible. In the non‐commercial sector, the food chain could be very short with no intermediate steps that might reduce the probability of preservation of LPAIV. Therefore, that probability is also non‐negligible.
Uncertainty
The gap between the temperatures at which eggs are usually stored and transported and those at which LPAIV can persist is sufficiently large that even if considered at their maximum levels, they are still far from those at which LPAIV persistence could be affected. Consequently, the uncertainty estimate for IPE4 is low.
4.5. Effect of IPE2–IPE4 on the LPAIV presence inside raw table eggs from an infected live bird during the food chain (EFE)
The assessment of probability IPE2 (see Section 3.2) indicated that it is unlikely that a Galliformes bird laying eggs with LPAIV inside would not be detected via surveillance or ante‐mortem inspection in commercial holdings. This means that the probability of LPAIV remaining present and undetected in raw table eggs (derived from a Galliformes bird originating from an exposed commercial flock) is reduced before its exposure to an individual. Probabilities IPE3 and IPE4 are non‐negligible, meaning that they do not ensure any reduction in the presence of LPAIV inside raw table eggs during the food chain (see Sections 3.3 and 3.4). Taken together, the combined effect of IPE2–IPE4 is a ‘reduction’ of the probability of LPAIV presence inside raw table eggs from an infected live bird during the food chain compared to IP1 for a Galliformes bird from a commercial holding (Table 10).
Table 10.
Determination of the effect of IPE2–IPE4 on LPAIV presence in raw table eggs from an infected live bird during the food chain (EFE)
| Bird of origin | EFE – Effect of IPE2–IPE4 on the LPAIV presence inside raw table eggs from an infected live bird during the food chain (IPE2 * IPE3 * IPE4) |
|---|---|
| Commercial Galliformes | Reduction |
| Non‐commercial Galliformes | No effect |
| Commercial and non‐commercial Anseriformes | No effect |
LPAIV: low pathogenic avian influenza virus.
For Galliformes in non‐commercial holdings and Anseriformes (both in commercial and non‐commercial holdings), probabilities IPE2–IPE4 are non‐negligible. This means that there is ‘no effect’ of the probability of LPAIV presence in a bird producing eggs or inside a table egg during the food chain compared to IPE1 for these birds.
4.6. Probability of LPAIV remaining present and undetected in a table egg before exposure (CPE5)
The probability of LPAIV remaining present and undetected in a table egg before exposure (CPE5) builds on probability IPE1 (see Section 4.1) by combining it with the effect EFE of the probabilities IPE2–IPE4 (see Section 4.5). The probabilities are shown in Table 11, based on the set of rules described in 2.2.
Table 11.
Determination of the probability terms of LPAI virus remaining present and undetected in raw table eggs before exposure
| Bird of origin | IPE1 – probability of LPAIV presence in eggs of one live bird in a flock exposed to a LPAIV | EFE – effect of IPE2‐IPE4 on the LPAIV presence inside raw table eggs from an infected live bird during the food chain | CPE5 – probability of LPAIV remaining present and undetected in a table egg before exposure (IPE1 * EFE) |
|---|---|---|---|
| Commercial Galliformes | Extremely unlikely | Reduction | Negligible |
| Non‐commercial Galliformes | Extremely unlikely | No effect | Extremely unlikely |
| Commercial and non‐commercial Anseriformes | Negligible | No effect | Negligible |
LPAIV: low pathogenic avian influenza virus.
4.7. Probability of exposure to the content of raw table eggs (IPE6)
4.7.1. Exposure of poultry and wild birds
It is known that poultry can eat their own eggs and that wild birds (e.g. crows) rob poultry eggs. In a survey of backyard poultry keepers in the USA in 2014 (Elkhoraibi et al., 2014), 18.2% of the keepers reported egg‐eating by the chickens in the previous 12 months. However, in this section, only exposure to raw table eggs derived from another flock is considered, since the assessment is looking into the potential spread of LPAIV via this commodity.
The probability of deliberate feeding of raw table eggs to commercial poultry is negligible as it is economically counter‐productive and logistically unfeasible in terms of collection, transport, storage and usage. From the nutritional point of view, commercial poultry are usually fed with well‐balanced dry diets, which make the addition of raw table eggs on an industrial scale negligible.
It is possible that non‐commercial poultry are accidentally exposed to raw eggs, if they eat their own or other birds’ eggs but, if that was the case, these eggs would not leave the flock and would not be table eggs. The only actual exposure to raw table eggs would be if handlers disposed of kitchen waste containing uncooked table eggs by feeding backyard poultry. There are no data available on the proportion of households in the EU performing this practice. However, the chain of events necessary for non‐commercial poultry to be exposed to raw table eggs makes the probability higher than for commercial poultry, but still very unlikely.
In the case of wild birds, the only opportunity to be exposed to contaminated raw table eggs would be by having access to kitchen or catering waste containing uncooked table eggs, which is considered similar the situation for to non‐commercial poultry.
Uncertainty
The uncertainty estimate for the probability of exposure to the content of raw table eggs (IPE6) is low for commercial poultry given the absence of an incentive for feeding raw table eggs to commercial poultry. On the other hand, the uncertainty estimate for IPE6 is high for non‐commercial poultry and wild birds due to a lack of data.
4.7.2. Exposure of humans
4.7.2.1. Handling and manipulation
Following the rationale of Section 3.7.2.1, exposure to the content of raw table eggs occurs mostly at household level and catering industry when handling and manipulating prior to cooking. Unlike in the case of raw poultry meat, professional exposure of content of raw table eggs at food processing plants is not relevant since most of the industries process pasteurised egg and/or egg products. Even if raw table eggs are used by a professional or consumer, there is a low probability of exposure to the content since egg content is immediately processed or cooked minimising the opportunities for direct contact.
Therefore, the probability IPE6 for professionals and consumers via handling and manipulation is negligible.
Uncertainty
The uncertainty associated to the probability IPE6 for professionals and consumers via handling and manipulation is low given the current practices of using raw table eggs for cooking in European countries.
4.7.2.2. Consumption
Consumption of raw table eggs is not common in the EU. It is acknowledged that good hygienic practices during food preparation and the consumption of only cooked eggs ensure a low risk of exposure to any pathogen in general. High‐risk practices include consumption of raw products (e.g. drinking of duck blood or eating uncooked embryonated eggs – as practised in some regions of South‐East Asia) obtained from infected poultry (Harder et al., 2016). Another risky practice is the consumption of raw eggs or the production of sauces or dishes that require raw eggs like Hollandaise sauce, mayonnaise, Caesar salad dressing, deserts (e.g. tiramisu, zabaglione), homemade ice cream or beverages (milkshakes, smoothies, eggnog), and some dishes from Asian cuisine (e.g. Japan, Korea). In these cases, it is increasingly recommended to be made with pasteurised eggs. The use of pasteurised egg products has grown enormously in all variants (liquid, dried, frozen or cooked), mainly in the catering industry for their convenience, for example to the equivalent of 2.6 billion eggs in 2014 in Great Britain (ACMSF, 2016). That leaves home cooking as the most likely scenario for exposure of humans to in the content of raw table eggs. If food is made with raw eggs, infected eggs could result in exposure via the oral route through the ingestion of such products.
European egg consumption amounts to 240 eggs per person per year, with strong variations among countries (Magdelaine, 2011). There are no data on the proportion of eggs that are used in meals that include raw eggs in their preparation or are consumed raw, but it is expected to be a very small fraction of the total number of table eggs consumed in the EU. The United States food safety guidelines specify that eggs should be cooked until ‘yolk and white are firm’ (USDHS, online). Hard‐cooked eggs should be cooked at a temperature between 80°C and 85°C (McGee, 1984). The United States Department of Agriculture recommends cooked above the recommended temperature before the consumer considers them done (Godwin et al., 2016).
Given the fact that the majority of the consumed eggs are cooked at temperature that ensure the inactivation of the LPAIV, exposure of one individual through consumption to raw table eggs is extremely unlikely.
Uncertainty
The uncertainty estimate for IPE6 for humans via consumption is high due to the lack of data on consumption of raw eggs and meals containing raw eggs. There are no data on the proportion of eggs consumed that are undercooked or below the recommended temperatures for cooking egg mixtures.
4.8. Probability of exposure to raw table eggs containing LPAIV (CPE7)
Probability CPE7 combines the Probability of LPAIV remaining present and undetected in raw a table eggs before exposure (CPE5) (Section 4.6) with the probability exposure to the content of raw table eggs (Section 4.7). An overview is provided in Table 12, based on the methodology described in Section 2.2.
Table 12.
Determination of probability terms for exposure to raw table eggs containing LPAIV (CPE7)
| Exposed individual | IPE6 – probability of exposure to the content of raw table eggs | CPE5 – probability of LPAIV remaining present and undetected in a table eggs before exposure | CPE7 – probability of exposure to raw table eggs containing LPAIV |
|---|---|---|---|
| Commercial poultry | Negligible | Extremely unlikelya | Negligiblea |
| Commercial poultry | Negligible | Negligibleb | Negligibleb |
| Non‐commercial poultry and wild birds | Very unlikely | Extremely unlikelya | Extremely unlikelya |
| Non‐commercial poultry and wild birds | Very unlikely | Negligibleb | Negligibleb |
| Humans (manipulation and handling) | Negligible | Extremely unlikelya | Negligiblea |
| Humans (manipulation and handling) | Negligible | Negligibleb | Negligibleb |
| Humans (consumption) | Extremely unlikely | Extremely unlikelya | Extremely unlikelya |
| Humans (consumption) | Extremely unlikely | Negligibleb | Negligibleb |
LPAIV: low pathogenic avian influenza virus.
Raw table eggs derived from a Galliformes bird of a non‐commercial flock.
Raw table eggs derived from a Galliformes bird of a commercial flock or an Anseriformes bird of a commercial or non‐commercial flock.
There is no virus amplification in unfertilised chicken eggs. In general, eggs produced in the commercial sector are unfertilised whereas the simultaneous presence of hens and cocks in non‐commercial flocks could lead to the presence of fertilised eggs. There is no reason in Europe to justify different habits with regard to the consumption of eggs purchased from the commercial sector and those purchased from the non‐commercial sector. The fact that virus amplification occurs in embryonated eggs would only affect the probability of infection given exposure due to higher viral loads, i.e. consumption of embryonated eggs, which is extremely unlikely anyway.
Disposal of uncooked whole table eggs (e.g. that are beyond the ‘sell by’ date) to birds would give the highest exposure to LPAIV in egg white and yolk, compared to already broken or processed eggs, should they be infected.
4.9. Probability of LPAIV infection given exposure to raw table eggs containing LPAIV (IPE8)
4.9.1. Poultry and wild bird
Only one study was found which attempted to quantify, though only by PCR, LPAIV loads inside albumin from eggs laid by infected breeder turkeys (Pillai et al., 2010). In this study, two experiments (repetitions) were performed where 5.13 log10 TCID50 of LPAIV H3N2 was inoculated through the intrachoanal route to turkey breeders. Some turkeys were euthanised at predefined days post‐inoculation to assess the presence of virus in the reproductive track. The presence of virus in albumen has been described in Section 4.1. Egg samples were collected daily and the presence and level of LPAIV in shell and albumen was assessed by RT‐PCR. In the first experiment, a total of 11 eggs collected between 3 and 6 days post‐inoculation (dpi) were tested and 3 eggs were found positive. The average viral titre in these eggs was reported to be 3.68 log10 EID50/mL with a maximum titre of 4.03 log10 EID50/mL. In the second experiment, a total of 62 eggs collected between 2 and 6 dpi for testing. Seventeen out of these 62 eggs were found positive. Four of these positive eggs had no egg shell and PCR‐quantified virus titres in albumin of these eggs were higher than 5.6 log10 EID50/mL. The authors argued that these high titres could be associated with contamination of the albumen due to the absence of shell in these eggs. Reported average titres in albumen of ‘normal eggs’, measured at days when no shell‐less eggs were collected, ranged between 3.3 and 4.39 log10 EID50/mL. As described in Section 4.1, viable virus was isolated in only three out the 17 positive egg samples. This indicates that the reported virus titres as determined by RRT‐PCR in this study have only a limited correlation with viable virus, with viable viral titres likely to be lower than the titres estimated by PCR. Furthermore, it is not specified whether the viable virus was isolated from the shell‐less or normal eggs.
As described in Section 3.9.1, the median infective dose reported for gastrointestinal inoculation of chickens was 104.3 TCID50. It is expected that similar infective doses would be required for wild birds as for poultry, in particular for related species such as wild and domestic ducks. The available scientific evidence both on possible LPAIV concentrations inside a raw table egg and the infectious dose for LPAIV infection via the oral route is limited to one study. Taken these studies together, and assuming that these measures apply to all poultry species, it can be concluded that the probability of infection of one commercial, non‐commercial or wild bird given exposure to raw table eggs containing LPAIV is extremely unlikely.
Uncertainty
The uncertainty estimate for IPE8 for poultry is high given the difficulties in the interpretation of the limited available data. Equally, there is uncertainty on whether the viral load inside of infected raw table eggs is sufficiently high to infect other birds by consumption. However, it is clear that infection of eggs would be a rare event (even more in chickens than in turkeys) since the proportion of normal eggs being infected is already very low (see Section 4.1) and so is the concentration of LPAIV in raw table eggs that contain LPAIV. The uncertainty estimate for IPE8 is high for wild birds given the general lack of data.
4.9.2. Human
The probability of infection of one human given exposure to raw table eggs containing LPAIV via consumption is negligible based on the assumed low concentration of LPAIV inside the egg and the acid‐lability of the virus. The corresponding argumentation described in Section 3.9.3 is also relevant in relation to raw table eggs.
Uncertainty
The uncertainty estimate for IEP8 for humans is low since there is sufficient scientific knowledge available to underpin the view that it is negligible that humans could become infected with LPAIV through the oral route.
4.10. Probability of exposure and subsequent LPAIV infection via raw table eggs containing LPAIV (CPE9)
The probability of exposure and subsequent LPAIV infection via raw table eggs containing LPAIV (CPE9) is the results combination of the probability (CPE7) of exposure to raw table eggs containing LPAIV (Section 4.8) with the probability (IPE8) of LPAIV infection given exposure to raw table eggs containing LPAIV (Section 4.9). An overview is provided in Table 13, based on the methodology described in section 2.2.
Table 13.
Determination of probability terms for exposure and subsequent LPAIV infection via raw table eggs containing LPAIV (CPE9)
| Exposed individual | IPE8 – probability of LPAIV infection given exposure to raw table eggs containing LPAIV | CPE7 – probability of exposure to raw table eggs containing LPAIV | CPE9 – probability of exposure and subsequent LPAIV infection given exposure to raw table eggs containing LPAIV |
|---|---|---|---|
| Commercial poultry | Extremely unlikely | Negligiblea , b | Negligiblea , b |
| Non‐commercial poultry and wild birds | Extremely unlikely | Extremely unlikelya | Extremely unlikelya |
| Non‐commercial poultry and wild birds | Extremely unlikely | Negligibleb | Negligibleb |
| Humans (manipulation and handling) | Negligible | Negligiblea , b | Negligiblea , b |
| Humans (consumption) | Negligible | Extremely unlikelya | Negligiblea |
| Humans (consumption) | Negligible | Negligibleb | Negligibleb |
LPAIV: low pathogenic avian influenza virus.
Raw table eggs derived from a Galliformes bird of a non‐commercial flock
Raw table eggs derived from a Galliformes bird of a commercial flock or an Anseriformes bird of a commercial or non‐commercial flock.
4.11. Probability of LPAIV transmission from individual to individual (IPE10)
The same arguments and uncertainty scores apply as those described in Section 3.11.
4.12. Probability of LPAIV transmission from an individual infected via raw table eggs containing LPAIV (CPE11)
The probability of LPAIV transmission from an individual infected via raw poultry meat (CPE11) is the result of the combination of the probability of LPAIV transmission from individual to individual via raw table eggs (IPE10) (Section 4.11) and the combined probability of exposure and subsequent LPAIV infection given exposure to raw table eggs containing LPAIV (CPE9) (Section 4.10). The probabilities scores are shown in Table 14, based on the set of rules described in Section 2.2.
Table 14.
Determination of probability terms for LPAIV transmission from an individual infected via raw table eggs containing LPAIV (CPE11)
| Exposed individual | IPE10 – probability of LPAIV transmission from individual to individual | CPE9 – probability of exposure and subsequent LPAIV infection via raw table eggs containing LPAIV | CPE11 – probability of LPAIV transmission from an individual infected via table eggs containing LPAIV |
|---|---|---|---|
| Commercial poultry | Non‐negligible | Negligiblea , b | Negligiblea , b |
| Non‐commercial poultry and wild birds | Unlikely | Extremely unlikelya | Very unlikelya |
| Non‐commercial poultry and wild birds | Unlikely | Negligibleb | Negligibleb |
| Humans (manipulation and handling) | Negligible | Negligiblea , b | Negligiblea , b |
| Humans (consumption) | Negligible | Negligibleb | Negligibleb |
LPAIV: low pathogenic avian influenza virus.
Raw table eggs derived from Galliformes bird of a non‐commercial flock
Raw table eggs derived from a Galliformes bird of a commercial flock or an Anseriformes bird of a commercial or non‐commercial flock.
4.13. Overview outcomes of the assessment
Figure 4 gives an overview of the main outcomes from the assessment of LPAI infection, establishment and spread via raw table eggs. The available scientific evidence and the rationale behind each probability are described in the sections above.
Figure 4.
Main outcomes from the assessment of LPAIV infection, establishment and spread via raw table eggs (U, uncertainty; COM, commercial; NON‐COM, non‐commercial)
-
Negligible, indistinguishable from 0; extremely unlikely, up to 1%; very unlikely, from 1 up to 2%; unlikely, from 2% up to 10%; non‐negligible, from 10% up to 100%; U, uncertainty; IP, independent probability; CP, combined probability.(a): Raw table eggs derived from Galliformes bird of a non‐commercial flock; (b) Raw table eggs derived from a Galliformes bird of a commercial flock or an Anseriformes bird of a commercial or non‐commercial flock.
4.14. Main conclusions
The probability of LPAIV presence in eggs of one live bird in a flock exposed to LPAIV is extremely unlikely and negligible for Galliformes and Anseriformes, respectively.
The probability of LPAIV infection given exposure to raw table eggs containing LPAIV is extremely unlikely for poultry and wild birds and negligible for humans.
The combined probability of exposure and subsequent LPAIV infection given exposure to raw table eggs containing LPAIV is negligible for commercial poultry and humans and extremely unlikely to negligible for non‐commercial poultry and wild birds.
The probability of LPAIV transmission from an individual infected via raw table eggs containing LPAIV is negligible for commercial poultry and humans and very unlikely to negligible for non‐commercial poultry and wild birds.
There is in general a high level of uncertainty on the estimation of the probabilities of key steps of the transmission pathway for poultry and wild birds mainly due to the limited number of studies analysing the presence of LPAIV in the content of eggs of different species and on the viral load required to infect a bird via raw table eggs containing LPAIV.
Abbreviations
- ADNS
Animal Disease Notification System
- CP
combined probabilities
- dpi
Days post‐inoculation
- EID50
50% egg infectious dose
- HA
hemagglutinin
- HPAI
highly pathogenic avian influenza
- IP
independent probabilities
- LBM
live‐bird market
- LPAI
low pathogenic avian influenza
- LPAIV
low pathogenic avian influenza virus
- MAESM
modified Australian egg storage model
- MOC
magnum organ cultures
- NA
Neuraminidase
- RRT‐PCR
Real‐time reverse transcription – polymerase chain reaction
- SPF
Specific pathogen‐free
- UV
ultraviolet
Appendix A – Literature search on LPAI virus in meat and eggs
1.
Review question:
Demonstrate the presence/absence of LPAI H5 or H7 viruses in organs, tissues, meat and eggs for the period 1/1/2016–17/7/2018 (information on other LPAI subtypes to be selected if very relevant for the assessment).
Search:
The PubMed database was searched by using subject index terms and free text terms combined with the appropriate Boolean operators. The search was run on 17 July 2018. Information regarding the search strategy (terms and search string) is provided in Tables A.1 and A.2.
Table A.1.
Search terms
| Concept | Terms |
|---|---|
| Virus presence | Tissue Distribution, organ, organs, tissue*, Meat, Eggs, meat, egg, eggs |
| Avian influenza and LPAI | Influenza in Birds, Influenzavirus A, avian[tiab] AND (influenza*[tiab]OR flu[tiab])) OR “influenza in bird”[tiab] OR “influenza in birds”[tiab] OR “bird flu”[tiab] OR “birds flu”[tiab] OR bird influenza*[tiab] OR “birds influenza”[tiab] OR ((influenza*[tiab] OR flu[tiab]) AND (“virus A” OR “viruses A”)), influenza A, flu A, influenzavirus A, fluvirus A, LPAI, LPAIV, AIV, AIVs, virus* |
| Virus subtype | H5, H5N*, H7, H7N* |
Table A.2.
Search strings (Pubmed)
| Search | Query | Items found |
|---|---|---|
| #5 | Search (#4) AND (“2016”[Date ‐ Publication] : “3000”[Date ‐ Publication]) Filters: Publication date from 2016/01/01 | 359 |
| #4 | Search (#1 AND #2 AND #3) | 2,083 |
| #3 | Search “Tissue Distribution”[Mesh] OR organ[tiab] OR organs[tiab] OR tissue*[tiab] OR “Meat”[Mesh] OR “Eggs”[Mesh] OR meat[tiab] OR egg[tiab] OR eggs[tiab] | 2,181,892 |
| #2 | Search (“Influenza in Birds”[Mesh] OR “Influenzavirus A”[Mesh] OR (avian[tiab] AND (influenza*[tiab]OR flu[tiab])) OR “influenza in bird”[tiab] OR “influenza in birds”[tiab] OR “bird flu”[tiab] OR “birds flu”[tiab] OR bird influenza*[tiab] OR “birds influenza”[tiab] OR ((influenza*[tiab] OR flu[tiab]) AND (“virus A” OR “viruses A”)) OR “influenza A”[tiab] OR “flu A”[tiab] OR “influenzavirus A”[tiab] OR “fluvirus A”[tiab] OR LPAI [tiab] OR LPAIV[tiab] OR AIV[tiab] OR AIVs[tiab] OR virus*[tiab]) | 679,788 |
| #1 | Search H5[tiab] OR H5N*[tiab] OR H7[tiab] OR H7N*[tiab] | 21,087 |
Relevance criteria:
Scientific articles added to the database from 1/1/2016 to 17/7/2018 and reporting information on the presence/absence of LPAI virus in organs, tissue, meat and eggs.
Eligibility criteria:
Host species domestic or wild birds;
Studies conducted in vivo;
Diagnosis conducted via virus isolation or RNA detection;
Virus subtype reported as H5 or H7 (other LPAI subtypes were only selected if very relevant for the assessment).
Results:
The search retrieved 359 papers. Title and abstract of these articles were subsequently screened against the relevance and eligibility criteria and 330 were excluded. The full text of the selected 29 papers were further screened and 23 were excluded because they were not conducted in birds (7), or were not about LPAI (5), or virus identification were not conducted via virus isolation or RNA detection (7), or not conducted in tissues, organs, meat or eggs (3), or because of more than one of the listed aspect (1).
Appendix B – LPAIV tissue distribution in poultry
1.
Table B.1.
Overview LPAIV tissue distribution in poultry from studies published from January 2005 to mid‐July 2018
| LPAIV HxNx (isolate name) | Inoculation | Test | DPI | Organ (positive/total animals tested) | Reference | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species (age) | Route | Dose | Lung | Trachea | Turbinates | Air sacs | Muscle | Brain | Intestine | Blood | Heart | Liver | Kidney | {Spleen | Pancreas | Bone | Oviduct | Bursa of Fabricius | ||||
| H5N1 (A/Mallard/Italy/3401/05) | Chicken (1 month) | IN and IT | 2 x 105 EID50 | RNA detection | 2 | 2/6 | – | – | – | – | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | – | – | – | – | Post et al. (2013) |
| 4 | 2/6 | – | – | – | – | 0/6 | 0/5 | 2/6 | 0/6 | 0/6 | 0/6 | 0/6 | – | – | – | – | ||||||
| H7N1 (A/chicken/Italy/1067/99) | Chicken (1 month) | IN and IT | 2 x 105 EID50 | RNA detection | 2 | 6/6 | – | – | – | – | 0/6 | 1/6 | 3/6 | 0/6 | 1/6 | 3/6 | 6/6 | – | – | – | – | |
| 4 | 6/6 | – | – | – | – | 1/6 | 4/6 | 2/6 | 3/6 | 1/6 | 6/6 | 6/6 | – | – | – | – | ||||||
| H7N7 (A/Chicken/Netherlands/06022003/06) | Chicken (1 month) | IN and IT | 2 x 105 EID50 | Virus isolation | 4 | 4/6 | – | – | – | – | 0/6 | 3/6 | ND | 2/6 | 4/6 | 1/6 | 5/6 | – | – | – | – | |
| RNA detection | 2 | 5/6 | – | – | – | – | 1/6 | 5/6 | 1/6 | 3/6 | 1/6 | 2/6 | 5/6 | – | – | – | – | |||||
| 4 | 5/6 | – | – | – | – | 0/6 | 4/6 | 3/6 | 2/6 | 5/6 | 1/6 | 6/6 | – | – | – | – | ||||||
| H5N2 (A/Chicken/Pennsylvania/21525/83) | Chicken (1 month) | IN and IT | 2 x 105 EID50 | RNA detection | 2 | 4/5 | – | – | – | – | 3/5 | 3/5 | 4/5 | 4/5 | 4/5 | 4/5 | 5/5 | – | – | – | – | |
| 4 | 5/5 | – | – | – | – | 5/5 | 2/5 | 5/5 | 4/5 | 2/5 | 4/5 | 5/5 | – | – | – | – | ||||||
| H9N2 (A/Chicken/Saudi Arabia/SP0525/3AAV/2000) | Chicken (1 month) | IN and IT | 2 x 105 EID50 | RNA detection | 2 | 4/5 | – | – | – | – | 4/5 | 2/5 | 3/5 | 0/5 | 1/5 | 0/5 | 5/5 | – | – | – | – | |
| 4 | 4/5 | – | – | – | – | 3/5 | 4/5 | 5/5 | 4/5 | 4/5 | 3/5 | 4/5 | – | – | – | – | ||||||
| H5N2 (Ck/Ibaraki/1/05) | Chicken (4 weeks) | IV | 1 x 105.7 EID50 | Virus isolation | 3 | 0/3 | 1/3 | – | – | 0/3 | 0/3 | 1/3 | – | – | – | 0/3 | 0/3 | 0/3 | – | – | – | Okamatsu et al. (2007) |
| 5 | 1/3 | 0/3 | – | – | 0/3 | 0/3 | 2/3 | – | – | – | 1/3 | 0/3 | 0/3 | – | – | – | ||||||
| 7 | 2/3 | 0/3 | – | – | 0/3 | 0/3 | 1/3 | – | – | – | 1/3 | 0/3 | 1/3 | – | – | – | ||||||
| H7N2 (A/Turkey/Virginia/158512/2002) | Chicken (3–4 weeks) | IN | 1 x 106 EID50 | Virus isolation | 1 | 0/5 | 0/5 | – | – | 0/5a | – | – | 0/5b | – | – | – | – | – | 0/5 | – | – | Swayne and Beck (2005) |
| 3 | 3/5 | 3/5 | – | – | 0/5a | – | – | 0/5b | – | – | – | – | – | 0/5 | – | – | ||||||
| 5 | 3/5 | 3/5 | – | – | 0/5a | – | – | 0/5b | – | – | – | – | – | 0/5 | – | – | ||||||
| 7 | 1/5 | 1/5 | – | – | 0/5a | – | – | 0/5b | – | – | – | – | – | 0/5 | – | – | ||||||
| 10 | 0/5 | 0/5 | – | – | 0/5a | – | – | 0/5b | – | – | – | – | – | 0/5 | – | – | ||||||
| H7N2 (A/Chicken/New York/21586‐8/99) | Chicken (3–4 weeks) | IN | 1 x 106 EID50 | Virus isolation | 1 | 0/5 | 0/5 | – | – | 0/5a | – | – | 0/5b | – | – | – | – | – | 0/5 | – | – | |
| 3 | 2/5 | 2/5 | – | – | 0/5a | – | – | 0/5b | – | – | – | – | – | 0/5 | – | – | ||||||
| 5 | 3/5 | 3/5 | – | – | 0/5a | – | – | 0/5b | – | – | – | – | – | 0/5 | – | – | ||||||
| 7 | 1/5 | 0/5 | – | – | 0/5a | – | – | 0/5b | – | – | – | – | – | 0/5 | – | – | ||||||
| 10 | 0/5 | 0/5 | 0/5a | – | – | 0/5b | – | – | – | – | – | 0/5 | – | – | ||||||||
| H7N9 (A/Anhui/1/13) | Chicken (3 weeks) | OcN | 1 x 108 EID50 | RNA detection | 4 | 0/2 | 0/2 | 2/2 | 0/2 | – | – | – | – | – | – | – | 2/2 | – | – | – | – | Slomka et al. (2018) |
| R1 (chicken) | 0 | RNA detection | 7 | 0/2 | 0/2 | 1/2 | 0/2 | – | – | 1/2 | – | – | – | – | 0/2 | – | – | – | – | |||
| R2 (turkey) | 0 | RNA detection | 15 | 0/2 | 0/2 | 0/2 | 0/2 | – | – | 0/2 | – | – | – | 0/2 | 0/2 | 0/2 | – | – | – | |||
| Turkey (3 weeks) | OcN | 1 x 108 EID50 | RNA detection | 4 | 2/2 | 2/2 | 2/2 | 2/2 | – | – | 0/2 | – | – | – | ND | 2/2 | – | – | – | – | ||
| R1 (turkey) | 0 | RNA detection | 7 | 2/2 | 2/2 | 2/2 | 2/2 | – | – | 2/2 | – | – | – | ND | 2/2 | – | – | – | – | |||
| R2 (turkey) | 0 | RNA detection | 5–9 | 3/3 | 3/3 | 3/3 | 3/3 | – | – | 2/2 | – | – | – | 3/3 | 3/3 | 3/3 | – | – | – | |||
| R2 (turkey) | 0 | RNA detection | 15 | 1/1 | 0/1 | 1/1 | 1/1 | – | – | 3/3 | – | – | – | 1/1 | 0/1 | 1/1 | – | – | – | |||
| Turkey (3 weeks) | OcN | 1 x 106EID50 | RNA detection | 4 | 2/2 | 1/2 | 0/2 | 1/2 | – | – | 1/1 | – | – | – | – | 0/2 | – | – | – | – | ||
| R2 (turkey) | 0 | RNA detection | 5–8 | 1/3 | 3/3 | 3/3 | 3/3 | – | – | 0/2 | – | – | – | 3/3 | 3/3 | 3/3 | – | – | – | |||
| Turkey (3 weeks) | R2 (turkey) | 0 | RNA detection | 15 | 0/1 | 0/1 | 0/1 | 0/1 | – | 0/1 | 2/3 | – | – | – | 1/1 | 0/1 | 0/1 | – | – | – | ||
| H7N9 (A/chicken/Guangdong/110/2013) | Chicken (6 weeks) | IN | 1 x 108 EID50 | Virus isolation | 3 | Yes | Yes | – | – | – | Yes | 0/1 | – | No | No | No | No | No | – | – | – | Jiao et al. (2018) |
| 5 | Yes | Yes | – | – | – | Yes | no | – | Yes | Yes | Yes | No | Yes | – | – | – | ||||||
| H7N9 (A/chicken/Guangdong/134/2013) | Chicken (6 weeks) | IN | 1 x 108 EID50 | Virus isolation | 3 | Yes | Yes | – | – | – | Yes | no | – | No | No | No | No | No | – | – | – | |
| 5 | Yes | Yes | – | – | – | Yes | Yes | – | Yes | Yes | Yes | Yes | Yes | – | – | – | ||||||
| H9N2 (A/chicken/Egypt/S4456B/2011) | Chicken (4 weeks) | IN, IT, C | 0.5 x 106 EID50 | Virus isolation | 2 | 0/3 | – | – | – | – | – | Yes | – | – | 0/3 | 0/3 | – | – | Moatasim et al. (2017) | |||
| RNA detection | 2 | 0/3 | – | – | – | – | – | 0/3 | – | – | 0/3 | 0/3 | – | – | ||||||||
| H9N2 (A7duck/Japan/AQ‐HE5/2015) | Chicken (4 weeks) | IN | 1 x 106 EID50 | Virus isolation | 4 | 0/4 | 4/4 | – | – | 0/4 | 0/4 | 0/4 | – | – | – | 0/4 | – | – | – | – | – | Shibata et al. (2018a) |
| Ducks (6 weeks) | IN | 1 x 106 EID50 | Virus isolation | 4 | 0/4 | 0/4 | – | – | 0/4 | 0/4 | 0/4 | – | – | – | 0/4 | – | – | – | – | – | ||
| H7N9 A/Anhui/1/2013‐derived strain 10E4 | Chicken (4 weeks) | IN | 1 x 106 EID50 | Virus isolation | 2 | 0/3 | 0/3 | – | – | 0/3 | 0/3 | 0/6 | – | – | – | – | 0/3 | – | – | – | Uchida et al. (2017) | |
| 4 | 0/3 | 0/3 | – | – | 0/3 | 0/3 | 0/6 | – | – | – | – | 0/3 | – | – | – | |||||||
| 6 | 0/3 | 0/3 | – | – | 0/3 | 0/3 | 1/6 | – | – | – | – | 0/3 | – | – | – | |||||||
| Quail (4 weeks) | IN | 1 x 106 EID50 | Virus isolation | 2 | 0/3 | 1/3 | – | – | 0/3 | 1/3 | 0/6 | – | – | – | – | 0/3 | – | – | 0/3 | |||
| 4 | 2/3 | 1/3 | – | – | 1/3 | 2/3 | 1/6 | – | – | – | – | 2/3 | – | – | 2/3 | |||||||
| 6 | 0/3 | 1/3 | – | – | 0/3 | 0/3 | 0/6 | – | – | – | – | 1/3 | – | – | 0/3 | |||||||
| Pigeon (2 years) | IN | 1 x 106 EID50 | Virus isolation | 2 | 0/3 | 0/3 | – | – | 0/3 | 0/3 | 0/6 | – | – | – | – | 0/3 | – | – | 0/3 | |||
| 4 | 0/3 | 0/3 | – | – | 0/3 | 0/3 | 0/6 | – | – | – | – | 0/3 | – | – | 0/3 | |||||||
| 6 | 0/3 | 0/3 | – | – | 0/3 | 0/3 | 0/6 | – | – | – | – | 0/3 | – | – | 0/3 | |||||||
| H9N2 (A/chicken/aq‐Y‐55/01) | Chicken (4 weeks) | IN | 1 x 107 EID50 | Virus isolation | 3 | 4/4 | 4/4 | – | – | 2/4 | – | 0/4 | – | – | 0/2 | 1/2 | 2/2 | – | 2/4 | – | – | Kishida et al. (2004) |
| H9N2 (A/chicken/aq‐Y‐135/01) | Chicken (4 weeks) | IN | 1 x 107 EID50 | Virus isolation | 3 | 1/4 | 3/4 | – | – | 1/4 | – | 1/4 | – | – | 0/2 | 0/2 | 0/2 | – | 1/4 | – | – | |
| H9N2 (A/chicken/Beijing/2/97) | Chicken (4 weeks) | IN | 1 x 107 EID50 | Virus isolation | 3 | 1/4 | 2/4 | – | – | 0/4 | – | 1/4 | – | – | 1/4 | 0/4 | 2/4 | – | 3/4 | – | – | |
| H9N2 (A/duck/Hokkaido/9/99) | Chicken (4 weeks) | IN | 1 x 107 EID50 | Virus isolation | 3 | 0/2 | 0/2 | – | – | 0/2 | – | 0/2 | – | – | – | – | – | – | 0/2 | – | – | |
| H10N1 (A/mallard/Italy/4518/2007) | Chicken (3 weeks) | IN | 1 x 106 EID50 | RNA detection | 4 | 1/1 | 1/1 | – | – | – | – | 1/1 | – | – | – | 1/1 | – | – | – | – | – | Bonfante et al. (2014) |
| Chicken (6 weeks) | IN | 1 x 106 EID50 | RNA detection | 4 | 1/1 | 0/1 | – | – | – | 0/1 | 0/1 | – | – | 1/1 | 1/1 | – | – | – | – | – | ||
| 5 | 3/3 | 2/3 | – | – | – | 2/3 | 3/3 | – | – | 3/3 | 3/3 | – | – | – | – | – | ||||||
| 6 | 2/2 | 1/1 | – | – | – | 2/2 | 2/2 | – | – | 2/2 | 2/2 | – | – | – | – | – | ||||||
| 12 | 1/1 | 1/1 | – | – | – | 0/1 | 1/1 | – | – | 1/1 | 1/1 | – | – | – | – | – | ||||||
| H7N1 (A/turkey/Italy/3675/1999) | Turkey (50 days) | OrN | 1 x 106 EID50 | Virus isolation | 1 | 0/3 | – | – | – | 0/6a | – | – | 0/15 | – | – | – | – | – | – | – | – | Toffan et al. (2008) |
| 2 | 2/3 | – | – | – | 0/6a | – | – | 1/12 | – | – | – | – | – | – | – | – | ||||||
| 3 | 3/3 | – | – | – | 0/6a | – | – | 0/9 | – | – | – | – | – | – | – | – | ||||||
| 4 | 0/3 | – | – | – | 0/6a | – | – | 0/6 | – | – | – | – | – | – | – | – | ||||||
| 5 | 0/3 | – | – | – | 0/6a | – | – | 0/3 | – | – | – | – | – | – | – | – | ||||||
| RNA detection | 1 | 0/3 | – | – | – | 0/6a | – | – | 0/15 | – | – | – | – | – | – | – | – | |||||
| 2 | 3/3 | – | – | – | 5/6a | – | – | 2/12 | – | – | – | – | – | – | – | – | ||||||
| 3 | 3/3 | – | – | – | 4/6a | – | – | 0/9 | – | – | – | – | – | – | – | – | ||||||
| 4 | 0/3 | – | – | – | 0/6a | – | – | 0/6 | – | – | – | – | – | – | – | – | ||||||
| 5 | 0/3 | – | – | – | 0/6a | – | – | 0/3 | – | – | – | – | – | – | – | – | ||||||
| H9N2 A/chicken/Iran/772/1998(H9N2) | Chicken (35 days) | IN | 1 x 106.5 EID50 | Virus isolation | 1 | 0/5 | 0/5 | – | – | – | – | – | 0/5 | – | – | 0/5 | 0/5 | 0/5 | – | – | – | Mosleh et al. (2009) |
| 2 | 0/1 | 0/1 | – | – | – | – | – | – | – | – | 1/1 | 0/1 | 0/1 | – | – | – | ||||||
| 3 | 1/5 | 2/5 | – | – | – | – | – | 0/5 | – | – | 2/5 | 1/5 | 0/5 | – | – | – | ||||||
| 6 | 4/5 | 2/6 | – | – | – | – | – | 0/5 | – | – | 3/6 | 3/6 | 0/6 | – | – | – | ||||||
| 9 | 0/5 | 0/5 | – | – | – | – | – | 0/5 | – | – | 5/5 | 0/5 | 0/5 | – | – | – | ||||||
| H7N1 A/chicken/Italy/1067/99 | Chicken (3 weeks) | IN, IT | 1 x 106 EID50 | RNA detection | 1 | 6/6 | – | – | – | – | 5/6 | 3/6 | – | – | – | – | 3/6 | – | – | – | – | Post et al. (2012) |
| 2 | 6/6 | – | – | – | – | 4/6 | 2/6 | – | – | – | – | 4/6 | – | – | – | – | ||||||
| 4 | 6/6 | – | – | – | – | 5/6 | 4/6 | – | – | – | – | 6/6 | – | – | – | – | ||||||
| 7 | 4/6 | – | – | – | – | 1/6 | 1/6 | – | – | – | – | 0/6 | – | – | – | – | ||||||
| H7N9 A/duck/Japan/A‐Q‐HE28‐3/2016 | Chicken (4 weeks) | IN | 1 x 106 EID50 | Virus isolation | 3 | 3/4 | 4/4 | – | – | 1/4 | 0/4 | 3/4 | – | – | – | 1/4 | – | – | – | – | – | Shibata et al. (2018b) |
| Duck (4 weeks) | IN | 1 x 106 EID50 | Virus isolation | 3 | 2/4 | 1/4 | – | – | 0/4 | 0/4 | 0/4 | – | – | – | 0/4 | – | – | – | – | – | ||
| H7N8 A/turkey/Indiana/16‐001571‐6/2016 | Chicken (3 weeks) | IC | 1 x 106 EID50 | RNA detection | 2 | 0/2 | – | – | – | 0/2 | 0/2 | – | – | 0/2 | – | – | 1/2 | – | – | – | – | Pantin‐Jackwood et al. (2017) |
| Turkey (3 weeks) | IC | 1 x 106 EID50 | RNA detection | 2 | 2/2 | – | – | – | 2/2 | 2/2 | – | – | 2/2 | – | – | 2/2 | – | – | – | – | ||
| Duck (2 weeks) | IC | 1 x 106 EID50 | RNA detection | 3 | 0/3 | – | – | – | 1/3 | 1/3 | – | – | 0/3 | – | – | 0/3 | – | – | – | – | ||
| H3N2 A/turkey/Ohio/313053/04 | Turkey (26 weeks) | IC | 1 x 10513 TCID50 | RNA detection | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2/3 | – | Pillai et al. (2010) |
| 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3/3 | – | ||||||
| 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3/3 | – | ||||||
| 9 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3/3 | – | ||||||
| H9N2 A/chicken/Shaanxi/01/2011 | Chicken (30 weeks) | IN, IO | 1 x 106 EID50 | RNA detection | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | Yes | – | Qi et al. (2016) |
| 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | Yes | – | ||||||
| 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | Yes | – | ||||||
| 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | Yes | – | ||||||
| 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | Yes | – | ||||||
DPI: days post‐inoculation; EID: embryo infectious dose; TCID50: median tissue culture infectious dose; IN: intranasal; IT: intratracheal; IV: intravenous; C: conjunctival; OcN: ocular/nasal; R1: first contact co‐housed with directly inoculated birds; R2: second contact co‐housed with R1 birds; OrN: oronasal; IC: intrachoanal; IO: intraocular; Yes: positive result; No: negative result; –: not determined.
‘Intestine’ contains results reported as intestine, duodenum, ileum, colon or rectum.
Results from breast and thigh muscle.
Results from plasma, white blood cells and red blood cells.
Suggested citation: EFSA (European Food Safety Authority) , Gonzales JL, Roberts H, Smietanka K, Baldinelli F, Ortiz‐Pelaez A and Verdonck F, 2018. Scientific report on the assessment of low pathogenic avian influenza virus transmission via raw poultry meat and raw table eggs. EFSA Journal 2018;16(10):5431, 53 pp. 10.2903/j.efsa.2018.5431
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00571
Acknowledgements: EFSA wishes to thank the involved members of the standing working group on avian influenza, Jose Luis Rojas, Helen Roberts and Krzysztof Smietanka, for their input in performing the assessment; the hearing experts Farouk El Allaki, Ingrid Kotowski*, and Joseph Todd Weaver* who provided comments, suggestions and additional scientific evidence, but did not contribute to the methods, results, and conclusions; Dominique Bicout and Preben Willeberg for reviewing the document; the ECDC staff members Cornelia Adlhoch and Pasi Pentinen for reviewing the human related aspects of the assessment and EFSA staff members Francesca Baldinelli, Angel Ortiz Pelaez, Irene Pilar Muñoz Guajardo and Frank Verdonck for their support provided in performing the assessment and collecting scientific evidence.
Approved: 28 September 2018
* The Findings and Conclusions in this publication have not been formally disseminated by the U. S. Department of Agriculture (USDA) and should not be construed to represent any USDA determination or policy.
Notes
Council Directive 2005/94/EC of 20 December 2005 on Community measures for the control of avian influenza and repealing Directive 92/40/EEC (OJ L 10, 14.1.2006, p. 16).
Beato MS et al.; Avian influenza viruses in poultry products: a review; Avian Pathology (June 2009) 38(3), 193200.
EFSA (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control), EURL (European Reference Laboratory on Avian Influenza), Brown I, Kuiken T, Mulatti P, Smietanka K, Staubach C, Stroud D, Therkildsen OR, Willeberg P, Baldinelli F, Verdonck F and Adlhoch C, 2017. Scientific report: Avian influenza overview September ‐ November 2017. EFSA (Journal 2017;15(12):5141, 70 pp. https://doi.org/10.2903/j.efsa.2017.5141).
Zhou L, Tan Y, Kang M, Liu F, Ren R, Wang Y, et al. Preliminary epidemiology of human infections with highly pathogenic avian influenza A (H7N9) virus, China, 2017. Emerging infectious diseases. 2017; 23 (8):1355. https://doi.org/10.3201/eid2308.170640 PMID: 28580900.
Harder TC, Buda S, Hengel H, Beer M, Mettenleiter TC. Clin Microbiol Infect. 2016; Poultry food products‐‐a source of avian influenza virus transmission to humans? Feb;22(2):141‐146. https://doi.org/10.1016/j.cmi.2015.11.015.
The EFSA Journal (2006) 74, 1‐29, “Food as a possible source of infection with highly pathogenic avian influenza viruses for humans and other mammals.
Swayne DE; Trade and food safety aspects for animal influenza viruses Animal Influenza, Second Edition. Edited by David E. Swayne. Published 2016 by John Wiley & Sons, Inc.
Post et al. Virology Journal 2012, 9:61, http://www.virologyj.com/content/9/1/61
Post et al. Virology Journal 2013, 10:23, http://www.virologyj.com/content/10/1/23
Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. OJ L 139, 30.4.2004, p. 55–205.
Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin.
Commission Regulation (EC) No 2295/2003 of 23 December 2003 introducing detailed rules for implementing Council Regulation (EEC) No 1907/90 on certain marketing standards for eggs. OJ L 340, 24.12.2003, p. 16–34.
From travellers so probably small quantities for direct consumption.
Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption.
Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007. OJ L 347, 20.12.2013, p. 671–854.
Viral load around 3,000 copies per 0.1 g of magnum tissue and between 500 and 1,500 copies per 0.1 g of infundibulum, isthmus, uterus or vagina tissue.
Commission Regulation (EC) No 589/2008 of 23 June 2008 laying down detailed rules for implementing Council Regulation (EC) No 1234/2007 as regards marketing standards for eggs. OJ L 163, 24.6.2008, p. 6–23.
COMMISSION REGULATION (EC) No 1020/2008 of 17 October 2008 amending Annexes II and III to Regulation (EC) No 853/2004 of the European Parliament and of the Council laying down specific hygiene rules for food of animal origin and Regulation (EC) No 2076/2005 as regards identification marking, raw milk and dairy products, eggs and egg products and certain fishery products. OJ L 277, 18.10.2008, p. 8–14.
References
- ACMSF (Advisory Committee on the Microbiological Safety of Food), 2016. Ad Hoc Group on Eggs. An update on the microbiological risk from shell eggs and their products. Food Standards Agency (FSA). Available online: https://acmsf.food.gov.uk/sites/default/files/acmsf-egg-reportv1.pdf
- Adzitey F, Huda N and Ali GRR, 2012. Prevalence and antibiotic resistance of Campylobacter, Salmonella, and L. monocytogenes in Ducks: a review. Foodborne Pathogens and Disease, 9, 498–505. [DOI] [PubMed] [Google Scholar]
- Al‐Bahry SN, Mahmoud IY, Al‐Musharafi SK and Al‐Ali MA, 2012. Penetration of spoilage and food poisoning bacteria into fresh chicken egg: a public health concern. Global Journal of Bioscience and Biotechnology (GJBB), 1, 33–39. [Google Scholar]
- Alexander DJ, 2000. A review of avian influenza in different bird species. Veterinary Microbiology, 74, 3–13. [DOI] [PubMed] [Google Scholar]
- Sergeev ARA, Demina OK, Pyankov OV, Pyankova OG, Agafonov AP, Kiselev SA, Agranovski IE, AlA Sergeev, Shikov AN, Shishkina LN, Safatov AS and Sergeev AN, 2013. Infection of chickens caused by avian influenza virus A/H5N1 delivered by aerosol and other routes. Transboundary and Emerging Diseases, 60, 159–165. [DOI] [PubMed] [Google Scholar]
- Baron J, Tarnow C, Mayoli‐Nussle D, Schilling E, Meyer D, Hammami M, Schwalm F, Steinmetzer T, Guan Y, Garten W, Klenk HD and Bottcher‐Friebertshauser E, 2013. Matriptase, HAT, and TMPRSS2 activate the hemagglutinin of H9N2 influenza A viruses. Journal of Virology, 87, 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavinck V, Bouma A, van Boven M, Bos MEH, Stassen E and Stegeman JA, 2009. The role of backyard poultry flocks in the epidemic of highly pathogenic avian influenza virus (H7N7) in the Netherlands in 2003. Preventive Veterinary Medicine, 88, 247–254. [DOI] [PubMed] [Google Scholar]
- Beato MS, Terregino C, Cattoli G and Capua I, 2006. Isolation and characterization of an H10N7 avian influenza virus from poultry carcasses smuggled from China into Italy. Avian Pathology, 35, 400–403. [DOI] [PubMed] [Google Scholar]
- Beato MS, Capua I and Alexander DJ, 2009. Avian influenza viruses in poultry products: a review. Avian Pathology, 38, 193–200. [DOI] [PubMed] [Google Scholar]
- Beato MS, Mancin M, Bertoli E, Buratin A, Terregino C and Capua I, 2012. Infectivity of H7 LP and HP influenza viruses at different temperatures and pH and persistence of H7 HP virus in poultry meat at refrigeration temperature. Virology, 433, 522–527. [DOI] [PubMed] [Google Scholar]
- Beltran‐Alcrudo D, Carpenter TE and Cardona C, 2009. A flock‐tailored early warning system for low pathogenic avian influenza (LPAI) in commercial egg laying flocks. Preventive Veterinary Medicine, 92, 324–332. [DOI] [PubMed] [Google Scholar]
- Bertran K and Swayne DE, 2014. High doses of highly pathogenic avian influenza virus in chicken meat are required to infect ferrets. Veterinary Research, 45, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante F, Fusaro A, Zanardello C, Patrono LV, De Nardi R, Maniero S and Terregino C, 2014. Lethal nephrotropism of an H10N1 avian influenza virus stands out as an atypical pathotype. Veterinary Microbiology, 173, 189–200. [DOI] [PubMed] [Google Scholar]
- Bowes VA, Ritchie SJ, Byrne S, Sojonky K, Bidulka JJ and Robinson JH, 2004. Virus characterization, clinical presentation, and pathology associated with H7N3 avian influenza in British Columbia broiler breeder chickens in 2004. Avian Diseases, 48, 928–934. [DOI] [PubMed] [Google Scholar]
- Brown LG, Khargonekar S and Bushnell L and Environmental Health Specialists Network Working Group , 2013. Frequency of inadequate chicken cross‐contamination prevention and cooking practices in restaurants. Journal of Food Protection, 76, 2141–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin E, Zhang X, Ke C, Sikkema R and Koopmans M, 2017. Serological evidence for exposure to avian influenza viruses within poultry workers in Southern China. Zoonoses and Public Health, 64, e51–e59. [DOI] [PubMed] [Google Scholar]
- Cappucci DT, Johnson DC, Brugh M, Smith TM, Jackson CF, Pearson JE and Senne DA, 1985. Isolation of avian influenza‐virus (subtype‐H5N2) from chicken eggs during a natural outbreak. Avian Diseases, 29, 1195–1200. [PubMed] [Google Scholar]
- Carnaccini S, Crossley B, Breitmeyer R, Charlton BR, Bland M, Fowler K, De La Torre F, Torchetti MK, Wong SS, Wilson D, Jones A and Senties‐Cue CG, 2015. Diagnosis and control of a LPAI H5N8 outbreak in a Japanese Quail (Coturnix coturnix japonica) Commercial Flock in the Central Valley of California. Avian Diseases, 59, 344–348. [DOI] [PubMed] [Google Scholar]
- CVI (Central Veterinary Institute (NL)), 2017. Risk factors of primary introduction of highly pathogenic and low pathogenic avian influenza virus into European poultry holdings, considering at least material contaminated by wild birds and contact with wild birds. EFSA supporting publication 2017:EN‐1282, 24 pp. 10.2903/sp.efsa.2017.EN-1282 [DOI] [Google Scholar]
- Chen JD, Ma J, White SK, Cao ZP, Zhen Y, He SY, Zhu WJ, Ke CW, Zhang YB, Su S and Zhang GH, 2015. Live poultry market workers are susceptible to both avian and swine influenza viruses, Guangdong Province, China. Veterinary Microbiology, 181, 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comin A, Klinkenberg D, Marangon S, Toffan A and Stegeman A, 2011. Transmission dynamics of low pathogenicity avian influenza infections in Turkey flocks. PLoS ONE, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling BJ, Jin LM, Lau EHY, Liao QH, Wu P, Jiang H, Tsang TK, Zheng JD, Fang VJ, Chang ZR, Ni MY, Zhang Q, Ip DKM, Yu JX, Li Y, Wang LP, Tu WX, Meng L, Wu JT, Luo HM, Li Q, Shu YL, Li ZJ, Feng ZJ, Yang WZ, Wang Y, Leung GM and Yu HJ, 2013. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population‐based study of laboratory‐confirmed cases. Lancet, 382, 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoust PY, van de Bildt M, van Riel D, van Amerongen G, Bestebroer T, Vanderstichel R, Fouchier RAM and Kuiken T, 2013. Replication of 2 subtypes of low‐pathogenicity avian influenza virus of duck and gull origins in experimentally infected mallard ducks. Veterinary Pathology, 50, 548–559. [DOI] [PubMed] [Google Scholar]
- De Reu K, Grijspeerdt K, Herman L, Heyndrickx M, Uyttendaele M, Debevere J, Putirulan FF and Bolder NM, 2006. The effect of a commercial UV disinfection system on the bacterial load of shell eggs. Letters in Applied Microbiology, 42, 144–148. [DOI] [PubMed] [Google Scholar]
- Ducousso J, Duval RE and Le Faou A, 2018. Avian influenza: back to the French epizootic (2015–2017). Revue De Medecine Veterinaire, 169, 38–51. [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control), 2012. Ferrets as experimental models of influenza in humans. ECDC comment 5 March 2012. Available online: https://ecdc.europa.eu/en/news-events/ferrets-experimental-models-influenza-humans [Accessed: 9 August 2018]
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Panel on Biological Hazards on the request from the Commission related to the Microbiological risks on washing of Table Eggs. EFSA Journal 2005;3(10):269, 39 pp. 10.2903/j.efsa.2005.269 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006. Scientific Report of the Scientific Panel on Biological Hazards on: food as a possible source of infection with highly pathogenic avian influenza viruses for humans and other mammals March 2006 ‐ 21st Plenary meeting. EFSA Journal 2006;74, 1–29. Available online: http://www.adiveter.com/ftp_public/biohaz_report_ej74_avian_influenza_en2.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Scientific Opinion on the public health hazards to be covered by inspection of meat (poultry). EFSA Journal 2012;10(6):2741, 179 pp. 10.2903/j.efsa.2012.2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2015. Scientific opinion on the survival, spread and establishment of the small hive beetle (Aethina tumida). EFSA Journal 2015;13(12):4328, 77 pp. 10.2903/j.efsa.2015.4328 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2017. Scientific opinion on avian influenza. EFSA Journal 2017;15(10):4991, 233 pp. 10.2903/j.efsa.2017.4991 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2014. Scientific Opinion on the public health risks of table eggs due to deterioration and development of pathogens. EFSA Journal 2014;12(7):3782, 147 pp. 10.2903/j.efsa.2014.3782 [DOI] [Google Scholar]
- EFSA ECDC and EURL (European Food Safety Authority, European Centre for Disease Prevention and Control and European Reference Laboratory on Avian Influenza), 2017. Scientific report: Avian influenza overview September ‐ November 2017. EFSA Journal 2017;15(12):5141, 70 pp. 10.2903/j.efsa.2017.5141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA, ECDC and EURL (European Food Safety Authority, European Centre for Disease Prevention and Control, European Reference Laboratory for Avian Influenza), Adlhoch C, Brouwer A, Kuiken T, Mulatti P, Smietanka K, Staubach C, Muñoz Guajardo I, Verdonck F, Amato L and Baldinelli F, 2018. Scientific report: Avian influenza overview February ‐ May 2018. EFSA Journal 2018;16(6):5358, 50 pp. 10.2903/j.efsa.2018.5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz R, Ahmed Z, Siddique N and Naeem K, 2007. Chicken meat as a source of avian influenza virus persistence and dissemination. International Journal of Poultry Science, 6, 871–874. [Google Scholar]
- Elkhoraibi C, Blatchford RA, Pitesky ME and Mench JA, 2014. Backyard chickens in the United States: a survey of flock owners. Poultry Science, 93, 2920–2931. [DOI] [PubMed] [Google Scholar]
- Ellstrom P, Latorre‐Margalef N, Griekspoor P, Waldenstrom J, Olofsson J, Wahlgren J and Olsen B, 2008. Sampling for low‐pathogenic avian influenza A virus in wild Mallard ducks: Oropharyngeal versus cloacal swabbing. Vaccine, 26, 4414–4416. [DOI] [PubMed] [Google Scholar]
- European Commission , 2017. Annual Report on surveillance for avian influenza in poultry and wild birds in Member States of the European Union in 2016. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/ad_control-measures_ai_surv-rslt_pltry-wld-brds_2016.pdf Accessed 07/08/2018.
- Gale P, Horrigan V and Kelly L, 2017. Risk Assessment for the transmission of Highly Pathogenic Avian Influenza from infected smallholdings to commercial poultry premises. Biomathematics and Risk Research Workgroup, Department of Epidemiological Sciences, Animal and Plant Health Agency, Weybridge. [Google Scholar]
- Giannecchini S, Clausi V, Di Trani L, Falcone E, Terregino C, Toffan A, Cilloni F, Matrosovich M, Gambaryan AS, Bovin NV, Delogu M, Capua I, Donatelli I and Azzi A, 2010. Molecular adaptation of an H7N3 wild duck influenza virus following experimental multiple passages in quail and turkey. Virology, 408, 167–173. [DOI] [PubMed] [Google Scholar]
- Godwin S, Maughan C and Chambers E, 2016. Food safety: recommendations for determining doneness in consumer egg dish recipes and measurement of endpoint temperatures when recipes are followed. Foods, 5, pii: E45. 10.3390/foods5030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales JL and Elbers ARW, 2018. Effective thresholds for reporting suspicions and improve early detection of avian influenza outbreaks in layer chickens. Scientific Reports, 8, Article number: 8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales JL, Elbers ARW, van der Goot JA, Bontje D, Koch G, de Wit JJ and Stegeman JA, 2012. Using egg production data to quantify within‐flock transmission of low pathogenic avian influenza virus in commercial layer chickens. Preventive Veterinary Medicine, 107, 253–259. [DOI] [PubMed] [Google Scholar]
- Gonzales JL, Boender GJ, Elbers ARW, Stegeman JA and de Koeijer AA, 2014. Risk based surveillance for early detection of low pathogenic avian influenza outbreaks in layer chickens. Preventive Veterinary Medicine, 117, 251–259. [DOI] [PubMed] [Google Scholar]
- Harder TC, Teuffert J, Starick E, Gethmann J, Grund C, Fereidouni S, Durban M, Bogner KH, Neubauer‐Juric A, Repper R, Hlinak A, Engelhardt A, Nockler A, Smietanka K, Minta Z, Kramer M, Globig A, Mettenleiter TC, Conraths FJ and Beer M, 2009. Highly pathogenic avian influenza virus (H5N1) in frozen duck carcasses, Germany, 2007. Emerging Infectious and Diseases, 15, 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder TC, Buda S, Hengel H, Beer M and Mettenleiter TC, 2016. Poultry food products–a source of avian influenza virus transmission to humans? Clinical Microbiology and Infection, 22, 141–146. [DOI] [PubMed] [Google Scholar]
- Hauck R, Crossley B, Rejmanek D, Zhou H and Gallardo RA, 2017. Persistence of highly pathogenic and low pathogenic avian influenza viruses in footbaths and poultry manure. Avian Diseases, 61, 64–69. [DOI] [PubMed] [Google Scholar]
- Heidari A, Mancin M, Nili H, Pourghanbari GH, Lankarani KB, Leardini S, Cattoli G, Monne I and Piccirillo A, 2016. Serological evidence of H9N2 avian influenza virus exposure among poultry workers from Fars province of Iran. Virology Journal, 13, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg U, Harris K, Stroud D, Guberti V, Busani L, Pittman M, Piazza V, Cook A and Brown I, 2009. Avian influenza surveillance in wild birds in the European Union in 2006. Influenza and Other Respiratory Viruses, 3, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijnen WAM, Beerendonk EF and Medema GJ, 2006. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Research, 40, 3–22. [DOI] [PubMed] [Google Scholar]
- Hill AA, Dewe T, Kosmider R, Von Dobschuetz S, Munoz O, Hanna A, Fusaro A, De Nardi M, Howard W, Stevens K, Kelly L, Havelaar A and Stark K, 2015. Modelling the species jump: towards assessing the risk of human infection from novel avian influenzas. Royal Society Open Science, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiono T, Okamatsu M, Yamamoto N, Ogasawara K, Endo M, Kuribayashi S, Shichinohe S, Motohashi Y, Chu DH, Suzuki M, Ichikawa T, Nishi T, Abe Y, Matsuno K, Tanaka K, Tanigawa T, Kida H and Sakoda Y, 2016. Experimental infection of highly and low pathogenic avian influenza viruses to chicken's, ducks, tree sparrows, jungle crows, and black rats for the evaluation of their roles in virus transmission. Veterinary Microbiology, 182, 108–115. [DOI] [PubMed] [Google Scholar]
- Horigan V, Davies RH, Kelly LA, Mead GC, Irvine RM and Simons RRL, 2014. A qualitative risk assessment of the microbiological risks to consumers from the production and consumption of uneviscerated and eviscerated small game birds in the UK. Food Control, 45, 127–137. [Google Scholar]
- Huang R, Wang AR, Liu ZH, Liang W, Li XX, Tang YJ, Miao ZM and Chai TJ, 2013. Seroprevalence of avian influenza H9N2 among poultry workers in Shandong Province, China. European Journal of Clinical Microbiology & Infectious Diseases, 32, 1347–1351. [DOI] [PubMed] [Google Scholar]
- IHR report , 2018. with the following two data sources: http://www.who.int/influenza/vaccines/virus/201809_zoonotic_vaccinevirusupdate.pdf?ua=1 http://www.promedmail.org/direct.php?id=20180801.5940642
- INFOSAN (International Food Safety Authorities Network), 2005. Highly pathogenic H5N1 avian influenza outbreaks in poultry and in humans: Food safety implications. Information Note No. 7/2005 ‐ Avian influenza. World Health Organization. Available online: http://www.who.int/foodsafety/fs_management/No_07_AI_Nov05_en.pdf
- James SJ and James B, 2002. Chapter 1.1: factors affecting the refrigerated shelf‐life of meat. IN: Meat Refrigeration. Woodhead. Bristol, UK. 360 pp. [Google Scholar]
- Jiao PR, Song YF, Huang JN, Xiang CW, Cui J, Wu SY, Qu N, Wang NC, Ouyang GW and Liao M, 2018. H7N9 avian influenza virus is efficiently transmissible and induces an antibody response in chickens. Frontiers in Immunology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Kim YI, Pascua PNQ and Choi YK, 2016. Avian influenza A viruses: evolution and zoonotic infection. Seminars in Respiratory and Critical Care Medicine, 37, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida N, Sakoda Y, Eto M, Sunaga Y and Kida H, 2004. Co‐infection of Staphylococcus aureus or Haemophilus paragallinarum exacerbates H9N2 influenza A virus infection in chickens. Archives of Virology, 149, 2095–2104. [DOI] [PubMed] [Google Scholar]
- Kwon YK, Thomas C and Swayne DE, 2010. Variability in pathobiology of South Korean H5N1 high‐pathogenicity avian influenza virus infection for 5 species of migratory waterfowl. Veterinary Pathology, 47, 495–506. [DOI] [PubMed] [Google Scholar]
- Ladman BS, Driscoll CP, Pope CR, Slemons RD and Gelb J Jr, 2010. Potential of low pathogenicity avian influenza viruses of wild bird origin to establish experimental infections in turkeys and chickens. Avian Diseases, 54, 1091–1094. [DOI] [PubMed] [Google Scholar]
- Latorre‐Margalef N, Brown JD, Fojtik A, Poulson RL, Carter D, Franca M and Stallknecht DE, 2017. Competition between influenza A virus subtypes through heterosubtypic immunity modulates re‐infection and antibody dynamics in the mallard duck. Plos Pathogens, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudert E, Halvorson D, Sivanandan V and Shaw D, 1993. Comparative‐evaluation of tissue trophism characteristics in turkeys and mallard ducks after intravenous inoculation of type‐A influenza‐viruses. Avian Diseases, 37, 773–780. [PubMed] [Google Scholar]
- Li JL and Cardona CJ, 2010. Adaptation and transmission of a Wild Duck Avian influenza isolate in chickens. Avian Diseases, 54, 586–590. [DOI] [PubMed] [Google Scholar]
- Li S, Zhou YF, Song WG, Pang QH and Miao ZM, 2016. Avian influenza virus H9N2 seroprevalence and risk factors for infection in occupational poultry‐exposed workers in Tai'an of China. Journal of Medical Virology, 88, 1453–1456. [DOI] [PubMed] [Google Scholar]
- Li X, Tian B, Zhou JF, Chen YK, Li XD, Zhu WF, Yan L, Jing T, Guo JF, Tao C, Gao RB, Wang DY and Shu YL, 2017. A comprehensive retrospective study of the seroprevalence of H9N2 avian influenza viruses in occupationally exposed populations in China. PLoS ONE, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Yang ZF, Liang Y, Li ZT, Bond HS, Chua HY, Luo YS, Chen Y, Chen TT, Guan WD, Lai JCC, Siu YL, Pan SH, Peiris JSM, Cowling BJ and PunMok CK, 2016. Population seroprevalence of antibody to influenza A(H7N9) virus, Guangzhou, China. BMC Infectious Diseases, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Yang K, Qi X, Xu K, Ji H, Ai J, Ge A, Wu Y, Li Y, Dai Q, Liang Q, Bao C, Bergquist R, Tang F and Zhu Y, 2013. Spatial and temporal analysis of human infection with avian influenza A(H7N9) virus in China, 2013. Eurosurveillance, 18. pii: 20640. [DOI] [PubMed] [Google Scholar]
- Liu SL, Li MX, Su ZY, Deng F and Chen JJ, 2016. A 3‐year follow‐up study of the seroprevalence of antibodies to avian influenza A H5, H6, H7 and H10 viruses among the general population of Wuhan, China. Journal of Clinical Virology, 77, 109–110. [DOI] [PubMed] [Google Scholar]
- Lu HG, Dunn PA, Wallner‐Pendleton EA, Hertzler DJ, Kradel DC, Liu JB, Shaw DP and Miller P, 2004. Investigation of H7N2 avian influenza outbreaks in two broiler breeder flocks in Pennsylvania, 2001–02. Avian Diseases, 48, 26–33. [DOI] [PubMed] [Google Scholar]
- Luber P, 2009. Cross‐contamination versus undercooking of poultry meat or eggs ‐ which risks need to be managed first? International Journal of Food Microbiology, 134, 21–28. [DOI] [PubMed] [Google Scholar]
- Lupo C, Chauvin C, Balaine L, Petetin I, Peraste J, Colin P and Le Bouquin S, 2008. Postmortem condemnations of processed broiler chickens in Western France. Veterinary Record, 162, 709–713. [DOI] [PubMed] [Google Scholar]
- Ma MJ, Zhao T, Chen SH, Xia X, Yang XX, Wang GL, Fang LQ, Ma GY, Wu MN, Qian YH, Dean NE, Yang Y, Lu B and Cao WC, 2018. Avian influenza A virus infection among workers at live poultry markets, China, 2013–2016. Emerging Infectious Diseases, 24, 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdelaine P, 2011. Egg and egg product production and consumption in Europe and the rest of the world In: Nys Y, Bain M. and Van Immerseel F. (eds.). Improving the Safety and Quality of Eggs and Egg Products, Vol 1: Egg Chemistry, Production and Consumption. Woodhead Publishing, Cambridge: pp. 3–16. [Google Scholar]
- Magdelaine P, Spiess MP and Valceschini E, 2008. Poultry meat consumption trends in Europe. Worlds Poultry Science Journal, 64, 53–63. [Google Scholar]
- Marchée S, Van Borm S, Lambrecht B, Houdart P and van den Berg T, 2014. Chasing notifiable avian influenza in domestic poultry: a case report of low‐pathogenic avian influenza H5 viruses in two Belgian holdings. Transboundary and Emerging Diseases, 61, 526–536. [DOI] [PubMed] [Google Scholar]
- McGee H, 1984. On Food and Cooking. Scribner, New York: 898 pp. [Google Scholar]
- Moatasim Y, Kandeil A, Mostafa A, Abd Elghaffar SK, El Shesheny R, Elwahy AHM and Ali MA, 2017. Single gene reassortment of highly pathogenic avian influenza A H5N1 in the low pathogenic H9N2 backbone and its impact on pathogenicity and infectivity of novel reassortant viruses. Archives of Virology, 162, 2959–2969. [DOI] [PubMed] [Google Scholar]
- Mosleh N, Dadras H and Mohammadi A, 2009. Evaluation of H9N2 avian influenza virus dissemination in various organs of experimentally infected broiler chickens using RT‐PCR. Iranian Journal of Veterinary Research, 10, 12–20. [Google Scholar]
- Munoz O, De Nardi M, van der Meulen K, vanReeth K , Koopmans M, Harris K, von Dobschuetz S, Freidl G, Meijer A, Breed A, Hill A, Kosmider R, Banks J, Stark KDC, Wieland B, Stevens K, van der Werf S, Enouf V, Dauphin G, Dundon W, Cattoli G and Capua I; FLURISK Consortium , 2016. Genetic adaptation of influenza A viruses in domestic animals and their potential role in interspecies transmission: a literature review. EcoHealth, 13, 171–198. [DOI] [PubMed] [Google Scholar]
- Mutinelli E, Capua I, Terregino C and Cattoli G, 2003. Clinical, gross, and microscopic findings in different avian species naturally infected during the H7N1 low‐ and high‐pathogenicity avian influenza epidemics in Italy during 1999 and 2000. Avian Diseases, 47, 844–848. [DOI] [PubMed] [Google Scholar]
- Nazir J, Haumacher R, Ike AC and Marschang RE, 2011. Persistence of avian influenza viruses in lake sediment, duck feces, and duck meat. Applied and Environmental Microbiology, 77, 4981–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamatsu M, Saito T, Yamamoto Y, Mase M, Tsuduku S, Nakamura K, Tsukamoto K and Yamaguchi S, 2007. Low pathogenicity H5N2 avian influenza outbreak in Japan during the 2005–2006. Veterinary Microbiology, 124, 35–46. [DOI] [PubMed] [Google Scholar]
- Pantin‐Jackwood MJ and Swayne DE, 2009. Pathogenesis and pathobiology of avian influenza virus infection in birds. Revue scientifique et technique, 28, 113–136. [PubMed] [Google Scholar]
- Pantin‐Jackwood MJ, Smith DM, Wasilenko JL and Spackman E, 2012. Low pathogenicity avian influenza viruses infect chicken layers by different routes of inoculation. Avian Diseases, 56, 276–281. [DOI] [PubMed] [Google Scholar]
- Pantin‐Jackwood MJ, Miller PJ, Spackman E, Swayne DE, Susta L, Costa‐Hurtado M and Suarez DL, 2014. Role of poultry in the spread of novel H7N9 influenza virus in China. Journal of Virology, 88, 5381–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin‐Jackwood MJ, Stephens CB, Bertran K, Swayne DE and Spackman E, 2017. The pathogenesis of H7N8 low and highly pathogenic avian influenza viruses from the United States 2016 outbreak in chickens, Turkeys and Mallards. PLoS ONE, 12, e0177265 10.1371/journal.pone.0177265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai SPS, Pantin‐Jackwood M, Jadhao SJ, Suarez DL, Wang L, Yassine HM, Saif YM and Lee CW, 2009. Pathobiology of triple reassortant H3N2 influenza viruses in breeder turkeys and its potential implication for vaccine studies in turkeys. Vaccine, 27, 819–824. [DOI] [PubMed] [Google Scholar]
- Pillai SPS, Saif YM and Lee CW, 2010. Detection of influenza A viruses in eggs laid by infected turkeys. Avian Diseases, 54, 830–833. [DOI] [PubMed] [Google Scholar]
- Post J, Burt DW, Cornelissen J, Broks V, van Zoelen D, Peeters B and Rebel JMJ, 2012. Systemic virus distribution and host responses in brain and intestine of chickens infected with low pathogenic or high pathogenic avian influenza virus. Virology Journal, 9, 61 10.1186/1743-422X-9-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post J, de Geus ED, Vervelde L, Cornelissen J and Rebel JMJ, 2013. Systemic distribution of different low pathogenic avian influenza (LPAI) viruses in chicken. Virology Journal, 10, 23 10.1186/1743-422X-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XF, Tan D, Wu CQ, Tang C, Li T, Han XY, Wang J, Liu CH, Li RQ and Wang JY, 2016. Deterioration of eggshell quality in laying hens experimentally infected with H9N2 avian influenza virus. Veterinary Research, 21, 51 10.4103/1735-1995.187253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RahimiRad S, Alizadeh A, Alizadeh E and Hosseini SM, 2016. The avian influenza H9N2 at avian‐human interface: a possible risk for the future pandemics. Journal of Research in Medical Sciences, 21, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn PC, 1989. Studies on porcine influenza viruses. Doctoral dissertation, ProQuest Dissertations & Theses. Available online: https://core.ac.uk/download/pdf/159072518.pdf
- Saenz RA, Essen SC, Brookes SM, Iqbal M, Wood JLN, Grenfell BT, McCauley JW, Bron IH and Gog JR, 2012. Quantifying transmission of highly pathogenic and low pathogenicity H7N1 avian influenza in turkeys. PLoS ONE, 7, e45059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoizec A NE, Schmitz A, Huneau‐Salaün A, Troyano‐Groux A, Briand F‐X, Martenot C, Cherbonnel‐Pansart M, Bronner A and Le Bouquin S, 2018. Bilan de la campagne de depistage de l'influenza aviaire chez les palmipedes gras avant mouvement vers un autre site d’élevage hiver 2017‐2018 (entre le 01/12/2017 et le 31/03/2018). Platforme Epidémiosurveillance Santé Animale. Available online: https://www.plateforme-esa.fr/sites/default/files/Bilan%20autocontrole%20PAG%20%20IA-hiver2017-2018-note%20plateforme.pdf
- Shalaby AA, Slemons RD and Swayne DE, 1994. Pathological‐studies of a chicken Alabama 7395/75 (H4N8) influenza‐virus in specific‐pathogen‐free laying hens. Avian Diseases, 38, 22–32. [PubMed] [Google Scholar]
- Shibata A, Hiono T, Fukuhara H, Sumiyoshi R, Ohkawara A, Matsuno K, Okamatsu M, Osaka H and Sakoda Y, 2018a. Isolation and characterization of avian influenza viruses from raw poultry products illegally imported to Japan by international flight passengers. Transboundary and Emerging Diseases, 65, 465–475. [DOI] [PubMed] [Google Scholar]
- Shibata A, Okamatsu M, Sumiyoshi R, Matsuno K, Wang ZJ, Kida H, Osaka H and Sakoda Y, 2018b. Repeated detection of H7N9 avian influenza viruses in raw poultry meat illegally brought to Japan by international flight passengers. Virology, in review. [DOI] [PubMed]
- Shichinohe S, Itoh Y, Nakayama M, Ozaki H, Soda K, Ishigaki H, Okamatsu M, Sakoda Y, Kida H and Ogasawara K, 2016. Comparison of pathogenicities of H7 avian influenza viruses via intranasal and conjunctival inoculation in cynomolgus macaques. Virology, 493, 31–38. [DOI] [PubMed] [Google Scholar]
- Sid H, Hartmann S, Winter C and Rautenschlein S, 2017. Interaction of influenza A viruses with oviduct explants of different avian species. Frontiers in Microbiology, 8, 1338 10.3389/fmicb.2017.01338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MSE, Rissi DR, Pantin‐Jackwood M and Swayne DE, 2013. High‐pathogenicity avian influenza virus in the reproductive tract of chickens. Veterinary Pathology, 50, 956–960. [DOI] [PubMed] [Google Scholar]
- Slomka MJ, Seekings AH, Mahmood S, Thomas S, Puranik A, Watson S, Byrne AMP, Hicks D, Nunez A, Brown IH and Brookes SM, 2018. Unexpected infection outcomes of China‐origin H7N9 low pathogenicity avian influenza virus in turkeys. Scientific Reports 8. Article number: 7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G and Dunipace S, 2011. How backyard poultry flocks influence the effort required to curtail avian influenza epidemics in commercial poultry flocks. Epidemics, 3, 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E, Gelb J, Preskenis LA, Ladman BS, Pope CR, Pantin‐Jackwood MJ and McKinley ET, 2010. The pathogenesis of low pathogenicity H7 avian influenza viruses in chickens, ducks and turkeys. Virology Journal, 7, 331 10.1186/1743-422X-7-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E, Pantin‐Jackwood M, Swayne DE, Suarez DL and Kapczynski DR, 2015. Impact of route of exposure and challenge dose on the pathogenesis of H7N9 low pathogenicity avian influenza virus in chickens. Virology, 477, 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spickler AR, Trampel DW and Roth J, 2008. The onset of virus shedding and clinical signs in chickens infected with high‐pathogenicity and low‐pathogenicity avian influenza viruses. Avian Pathology, 37, 555–577. [DOI] [PubMed] [Google Scholar]
- Stephens CB and Spackman E, 2017. Thermal inactivation of avian influenza virus in poultry litter as a method to decontaminate poultry houses. Preventive Veterinary Medicine. 145, 73–77. [DOI] [PubMed] [Google Scholar]
- Swayne DE, 2006. Occupational and consumer risks from avian influenza viruses. Developments in Biologicals, 124, 85–90. [PubMed] [Google Scholar]
- Swayne DE, 2007. Understanding the complex pathobiology of high pathogenicity avian influenza viruses in birds. Avian Diseases, 51, 242–249. [DOI] [PubMed] [Google Scholar]
- Swayne DE, 2016. Trade and food safety aspects for animal influenza viruses In: Swayne DE. (ed.). Animal Influenza. 2nd Edition Wiley Blackwell, Ames, IA: pp. 74–91. [Google Scholar]
- Swayne DE and Beck JR, 2004. Heat inactivation of avian influenza and Newcastle disease viruses in egg products. Avian Pathology, 33, 512–518. [DOI] [PubMed] [Google Scholar]
- Swayne DE and Beck JR, 2005. Experimental study to determine if low‐pathogenicity and high‐pathogenicity avian influenza viruses can be present in chicken breast and thigh meat following intranasal virus inoculation. Avian Diseases, 49, 81–85. [DOI] [PubMed] [Google Scholar]
- Tang Y, Lee CW, Zhang Y, Senne DA, Dearth R, Byrum B, Perez DR, Suarez DL and Saif YM, 2005. Isolation and characterization of H3N2 influenza A virus from turkeys. Avian Diseases, 49, 207–213. [DOI] [PubMed] [Google Scholar]
- Thomas C and Swayne DE, 2007. Thermal inactivation of H5N1 high pathogenicity avian influenza virus in naturally infected chicken meat. Journal of Food Protection, 70, 674–680. [DOI] [PubMed] [Google Scholar]
- Thomas C, Manin TB, Andriyasov AV and Swayne DE, 2008. Limited susceptibility and lack of systemic infection by an H3N2 swine influenza virus in intranasally inoculated chickens. Avian Diseases, 52, 498–501. [DOI] [PubMed] [Google Scholar]
- Toffan A, Beato MS, De Nardi R, Bertoli E, Salviato A, Cattoli G, Terregino C and Capua I, 2008. Conventional inactivated bivalent H5/H7 vaccine prevents viral localization in muscles of turkeys infected experimentally with low pathogenic avian influenza and highly pathogenic avian influenza H7N1 isolates. Avian Pathology, 37, 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse LV, Hamilton AM, Friling T and Whittaker GR, 2014. A novel activation mechanism of avian influenza virus H9N2 by furin. Journal of Virology, 88, 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Kanehira K, Takemae N, Hikono H and Saito T, 2017. Susceptibility of chickens, quail, and pigeons to an H7N9 human influenza virus and subsequent egg‐passaged strains. Archives of Virology, 162, 103–116. [DOI] [PubMed] [Google Scholar]
- USDA (United States Department of Agriculture), 2014. Food safety information. Chicken from farm to table. Available online: https://www.fsis.usda.gov/wps/wcm/connect/ad74bb8d-1dab-49c1-b05e-390a74ba7471/Chicken_from_Farm_to_Table.pdf?MOD=AJPERES
- USDHS (US Department of Health and Human Services), online. Safe Minimum Cooking Temperatures. Available online: https://www.foodsafety.gov/keep/charts/mintemp.html
- Uyeki TM, Katz JM and Jernigan DB, 2017. Novel influenza A viruses and pandemic threats. Lancet, 389, 2172–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter M, Treurnicht FK, Buys A, Tempia S, Samudzi R, McAnerney J, Jacobs CA, Thomas J and Blumberg L, 2017. Risk of human infections with highly pathogenic H5N2 and low pathogenic H7N1 avian influenza strains during outbreaks in ostriches in South Africa. Journal of Infectious Diseases, 216, S512–S519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fang SS, Lu X, Xu CL, Cowling BJ, Tang XJ, Peng B, Wu WH, He JF, Tang YJ, Xie X, Mei SJ, Kong DF, Zhang RL, Ma HW and Cheng JQ, 2014. Seroprevalence to avian influenza A(H7N9) virus among poultry workers and the general population in southern china: a longitudinal study. Clinical Infectious Diseases, 59, E76–E83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Chen ZL, Li CS, Cao XL, Wang R, Tang C, Huang JJ, Chang CD and Liu HJ, 2015a. The distribution of sialic acid receptors of avian influenza virus in the reproductive tract of laying hens. Molecular and Cellular Probes, 29, 129–134. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ju L, Liu P, Zhou J, Lv X, Li L, Shen H, Su H, Jiang L and Jiang Q, 2015b. Serological and virological surveillance of avian influenza A virus H9N2 subtype in humans and poultry in Shanghai, China, between 2008 and 2010. Zoonoses and Public Health, 62, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SH, Yang JR, Wu HS, Chang MC, Lin JS, Lin CY, Liu YL, Lo YC, Yang CH, Chuang JH, Lin MC, Chung WC, Liao CH, Lee MS, Huang WT, Chen PJ, Liu MT and Chang FY, 2013. Human infection with avian influenza A H6N1 virus: an epidemiological analysis. Lancet Respiratory Medicine, 1, 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang D, Shen XJ, Pu ZQ, Irwin DM, Liao M and Shen YY, 2018. Convergent evolution of human‐isolated H7N9 avian influenza A viruses. Journal of Infectious Diseases, 217, 1699–1707. [DOI] [PubMed] [Google Scholar]
- Xu LL, Bao LL, Deng W, Dong LB, Zhu H, Chen T, Lv Q, Li FD, Yuan J, Xiang ZG, Gao K, Xu YF, Huang L, Li YH, Liu JN, Yao YF, Yu P, Li XY, Huang WJ, Zhao X, Lan Y, Guo JF, Yong WD, Wei Q, Chen HL, Zhang LF and Qin C, 2014. Novel avian‐origin human influenza A(H7N9) can be transmitted between ferrets via respiratory droplets. Journal of Infectious Diseases, 209, 551–556. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Nakamura K and Mase M, 2017. Survival of highly pathogenic avian influenza H5N1 virus in tissues derived from experimentally infected chickens. Applied and Environmental Microbiology, 83 10.1128/AEM.00604-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SG, Chen Y, Cui DW, Yao HP, Lou JZ, Huo ZX, Xie GL, Yu F, Zheng SF, Yang YD, Zhu YX, Lu XQ, Liu XL, Lau SY, Chan JFW, To KKW, Yuen KY, Chen HL and Li LJ, 2013. Avian‐origin influenza A(H7N9) infection in influenza A(H7N9)‐affected areas of china: a serological study. Journal of Infectious Diseases, 209, 265–269. [DOI] [PubMed] [Google Scholar]
- Yang P, Ma CN, Cui SJ, Zhang DT, Shi WX, Pan Y, Sun Y, Lu GL, Peng XM, Zhao JC, Liu YM and Wang QY, 2016. Avian influenza A(H7N9) and (H5N1) infections among poultry and swine workers and the general population in Beijing, China, 2013–2015. Scientific Reports, 6, 33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao ML, Lv J, Huang R, Yang Y and Chai TJ, 2014. Determination of infective dose of H9N2 avian influenza virus in different routes: aerosol, intranasal, and gastrointestinal. Intervirology, 57, 369–374. [DOI] [PubMed] [Google Scholar]
- Zhang H, de Vries RP, Tzarum N, Zhu XY, Yu WL, McBride R, Paulson JC and Wilson IA, 2015. A human‐infecting H10N8 influenza virus retains a strong preference for avian‐type receptors. Cell Host & Microbe, 17, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Tan Y, Kang M, Liu F, Ren R, Wang Y, Chen T, Yang Y, Li C, Wu J, Zhang H, Li D, Greene CM, Zhou S, Iuliano AD, Havers F, Ni D, Wang D, Feng Z, Uyeki TM and Li Q, 2017. Preliminary epidemiology of human infections with highly pathogenic avian influenza A (H7N9) virus, China, 2017. Emerging Infectious Diseases, 23, 1355 10.3201/eid2308.170640. PMID: 28580900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chen EF, Bao CJ, Xiang NJ, Wu JB, Wu SG, Shi J, Wang XJ, Zheng YX, Zhang Y, Ren RQ, Greene CM, Havers F, Iuliano AD, Song Y, Li C, Chen T, Wang YL, Li D, Ni DX, Zhang YP, Feng ZJ, Uyeki TM and Li Q, 2018. Clusters of human infection and human‐to‐human transmission of avian influenza A(H7N9) virus, 2013–2017. Emerging Infectious Diseases, 24, 397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Guo J, Gao R, Dong L, Zhou J, Zhang Y, Dong J, Bo H, Qin K and Shu Y, 2013. Inactivation of the novel avian influenza A (H7N9) virus under physical conditions or chemical agents treatment. Virol J., 10, 289 10.1186/1743-422X-10-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler AF, Davison S, Acland H and Eckroade RJ, 1999. Characteristics of H7N2 (nonpathogenic) avian influenza virus infections in commercial layers, in Pennsylvania, 1997–98. Avian Diseases, 43, 142–149. [PubMed] [Google Scholar]


