Abstract
Parasites are important food‐borne pathogens. Their complex lifecycles, varied transmission routes, and prolonged periods between infection and symptoms mean that the public health burden and relative importance of different transmission routes are often difficult to assess. Furthermore, there are challenges in detection and diagnostics, and variations in reporting. A Europe‐focused ranking exercise, using multicriteria decision analysis, identified potentially food‐borne parasites of importance, and that are currently not routinely controlled in food. These are Cryptosporidium spp., Toxoplasma gondii and Echinococcus spp. Infection with these parasites in humans and animals, or their occurrence in food, is not notifiable in all Member States. This Opinion reviews current methods for detection, identification and tracing of these parasites in relevant foods, reviews literature on food‐borne pathways, examines information on their occurrence and persistence in foods, and investigates possible control measures along the food chain. The differences between these three parasites are substantial, but for all there is a paucity of well‐established, standardised, validated methods that can be applied across the range of relevant foods. Furthermore, the prolonged period between infection and clinical symptoms (from several days for Cryptosporidium to years for Echinococcus spp.) means that source attribution studies are very difficult. Nevertheless, our knowledge of the domestic animal lifecycle (involving dogs and livestock) for Echinoccocus granulosus means that this parasite is controllable. For Echinococcus multilocularis, for which the lifecycle involves wildlife (foxes and rodents), control would be expensive and complicated, but could be achieved in targeted areas with sufficient commitment and resources. Quantitative risk assessments have been described for Toxoplasma in meat. However, for T. gondii and Cryptosporidium as faecal contaminants, development of validated detection methods, including survival/infectivity assays and consensus molecular typing protocols, are required for the development of quantitative risk assessments and efficient control measures.
Keywords: food‐borne parasites, Cryptosporidium, Toxoplasma gondii, Echinococcus, public health risk, detection, control
Summary
The Panel on Biological Hazards initiated a self‐tasking mandate following the requirement of the European Food Safety Authority (EFSA) in order to provide information on the occurrence and control of three parasites that may be transmitted via food, namely Cryptosporidium spp., Toxoplasma gondii, and Echinococcus spp. The diseases caused by these parasites are cryptosporidiosis, toxoplasmosis, and alveolar echinococcosis (AE) and cystic echinococcosis (CE), respectively. The human burden associated with these diseases is substantial.
There are many parasites that may be transmitted via food, but these three parasites have been selected as being the focus of this Opinion due to their recent evaluation as being of particular importance in Europe. Additionally, there are currently no routine controls for these parasites in food. This Opinion is a critical evaluation of the available information on these three parasites, the methodologies for their detection, characterisation and tracing, their occurrence and survival in relevant food matrices, and the importance of food as a vehicle of infection. The Opinion draws conclusions on the four terms of reference requested: (1) to critically review current methods for the detection, identification, characterisation and tracing of the three parasites in foods that may be likely vehicles of infection; (2) to evaluate the available information to determine the relative importance of food‐borne pathways for transmission of the three parasites to humans; (3) to examine the available information on the occurrence and survival of the selected parasites in foods, and consumer habits that contribute to infection; and finally, (4) to evaluate possible control measures along the food chain, from farm to consumption.
A literature search and critical review process were used to gather scientific publications, reports, and official documents relevant for this Opinion. The qualitative evaluations were augmented by the knowledge and expertise of the members of the working group. Information about mandatory notification of these parasites was collected through a questionnaire sent to the members and observers of EFSA's Scientific Network for Zoonoses Monitoring Data.
The sources of infection for the three parasites differ widely, and are associated with their distinct lifecycles and specific hosts. In brief, Cryptosporidium spp. oocysts are mainly shed in the faeces of infected young animals, particularly ruminants, and humans; these may contaminate food. T. gondii oocysts are shed in the faeces of infected felids, particularly kittens, and may also contaminate food; in addition, tissue cysts of T. gondii in meat from infected food animals and tachyzoites shed in milk may also be a source of infection. Echinococcus eggs are shed in the faeces of infected canids and may contaminate food. According to information obtained from the authorities, reporting of these parasites, in animals, humans and food, varies between Member States, and not all information supplied was found to be accurate by experts in the working group. This means that the extent of infection or contamination based on notification data is not comparable across Europe.
Generally, and even for parasite–food combinations for which techniques have been developed and published, methods for analysing food as a vehicle of infection for these three parasites have not been well established, standardised, or validated. One of the parasites, T. gondii, infects host tissue and can be transmitted by consuming meat from infected animals that has not been sufficiently thermally treated. The likelihood that meat animals are infected varies by species and the animal husbandry and management practices. Although methods to identify the parasite in meat have been published, they are largely used for research projects and outbreak investigation; there is no routine inspection at abattoirs to ensure the safety of meat with regards to this parasite. All three parasites can also be transmitted as faecal contaminants via their robust oocyst or egg stages that facilitate environmental transmission (e.g., on fresh produce products that are not cooked before consumption). No standard methods for their detection in all relevant foods have been developed; culture is not a feasible option for routine detection. Although there is an ISO method detection of for Cryptosporidium oocysts on fresh produce, it has only been applied to a few product types, and provides no information on species, viability, or infectivity. It is not used for the routine inspection of fresh produce. For T. gondii and Echinococcus as faecal contaminants, detection methods have not been standardised or validated for routine use.
The incubation period, from infection until manifestation of symptoms, ranges from a few days for Cryptosporidium to years or decades for Echinococcus spp., This presents challenges and a lack of data when trying to determine the relative importance of food‐borne transmission (vs transmission via other routes, such as water or soil, or directly from other infected people or infected animals). For Echinococcus, in particular, relevant data are scarce, and the information available is often derived from expert elicitation studies, with their accompanying uncertainties. Nevertheless, approximately 40–0% of T. gondii infections are considered to be food‐borne and approximately 10% of Cryptosporidium infections. For Echinococcus, the data are uncertain, but range from around 4% to 40% for CE and 12% to 80% for AE.
Data on the occurrence of these three parasites on fresh produce are very limited. For Cryptosporidium, for which the most data are available, only six surveys using a reliable method have been conducted and indicate occurrence in 1–70% of samples; most large surveys indicate a contamination rate of around 8%. For Cryptosporidium, a large range of hosts may be infected and shed oocysts in their faeces. For T. gondii and Echinococcus spp., the range of hosts shedding faecal contamination stages is more limited (felids and canids, respectively). Thus, the potential for contamination of fresh produce may be greater for Cryptosporidium. Information on the survival of these parasites as contaminants is largely lacking, due to methodological limitations, but the transmission stages of all three parasites are known to be very robust, particularly for Echinococcus eggs that can, for example, survive heating to + 65°C for 120 min and freezing at −18°C for several months. Although the oocyst transmission stages of Cryptosporidium and Toxoplasma are inactivated by pasteurisation, Cryptosporidium oocysts survive in moist environments at ambient temperatures for many months and Toxoplasma oocysts survive for many months in the environment, including for weeks at freezing temperatures.
There are data on the contamination of molluscan shellfish for both Cryptosporidium and T. gondii, indicating occurrence rates of 20–40% and 10–20%, respectively. However, the absence of documented transmission of these parasites to humans from eating molluscan shellfish means that the relevance of these occurrence data to food‐borne transmission is unclear.
Generally, consumer preferences for raw, fresh produce may contribute to increasing the likelihood of infection; cooking inactivates all parasite transmission stages. Although omitting to wash fresh produce may contribute to an increased likelihood of ingesting viable parasites, industrialised washing of fresh produce, particularly with the reuse of washwater, may spread localised contamination throughout a batch. Consumer preferences for “ready‐to‐eat” produce may therefore increase the likelihood of ingesting viable parasites. With respect to meat, consumer preferences for animals raised with access to outdoor conditions, for not freezing meat prior to consumption, and for eating meat raw or rare may increase the likelihood of exposure to infective T. gondii tissue cysts.
There are many gaps in our knowledge of food‐borne transmission of the three parasites in focus, and acquisition of more and relevant data would assist the definition of targeted control strategies. In order to achieve this, development of robust and reliable methods for the detection of the three parasites on different types of fresh produce is particularly relevant. For T. gondii, the development and implementation of an assay that could be used to distinguish between meatborne infection and infection via oocysts would be useful. Methods for the assessment of viability and infectivity of the three parasites should be developed and validated for use in survival studies to evaluate the efficacy of particular food treatment conditions and disinfectants. Documentation of the fresh‐produce supply chain, would improve knowledge of how, when, and where contamination occurs. Better knowledge on the spread or removal of contaminant parasites in salad‐processing plants may be particularly relevant. When contamination is detected, determination of its origin is hampered by the lack of suitable molecular markers. Although whole genome sequencing may provide a solution in some circumstances, it is not necessarily appropriate for low numbers of parasites (that are hard to amplify) in a contamination situation; identification of appropriate diagnostic markers and validation for their use would improve our knowledge regarding the sources and routes of contamination and infection. As the food‐borne route may be overlooked (for example, Cryptosporidium is more often thought of as a waterborne parasite), public health providers could be encouraged to include questions on food consumption within a relevant time span when investigating cases or outbreaks of infection.
On‐farm measures that reduce the likelihood of contamination may be a more effective control method than post‐harvest interventions. These include: the use of irrigation water of potable or high quality, and, if wastewater is used for irrigation, it being treated sufficiently to inactivate or remove parasite transmission stages; controlling animal access to areas of cultivation; and appropriate storage and application of farm waste. Heat treatment, such as pasteurisation of fruit juice and milk, adequately steaming shellfish and cooking meat thoroughly are all effective at inactivating parasite transmission stages that may be present. The freezing of meat will inactivate T. gondii tissue cysts, but the effect of freezing on faecal contaminant transmission stages on fresh produce is less obvious, and Echinococcus eggs are particularly resistant to inactivation by freezing. Exclusion from work of food handlers with diarrhoea, including for 48 h after cessation of symptoms, may reduce the likelihood of contamination with infective Cryptosporidium oocysts. For T. gondii, vaccination of sheep and pigs will reduce persistent infection in these species, and limiting contamination with cat faeces, either by reducing cat populations or restricting their access, may decrease on‐farm contamination.
Control of Echinococcus granulosus, the lifecycle of which is based on dogs and livestock, is feasible (and has been demonstrated to be successful) without further research, by a multipronged approach that includes the vaccination of sheep, slaughter supervision, routine anthelmintic treatment of dogs and control of stray dogs. Several control programmes have achieved the elimination or a reduction of transmission of this parasite, and prioritisation and initiation of such actions in targeted areas has the potential to achieve control of this parasite within Europe. For E. multilocularis, control is more difficult as the transmission cycle involves wildlife (foxes and rodents). Nevertheless, control or significant reduction of transmission may be achieved in targeted areas (e.g. where there is the potential for close fox–human interaction) by the use of praziquantel bait. However, this requires long‐term commitment and dedicated resources.
These food‐borne parasites, currently considered as being of the highest relevance in Europe, are problematic both clinically and environmentally, presenting challenges for monitoring, prevention, and control. Public health could be benefited by an improved understanding of the role of food‐borne transmission, and the development of assays to understand the routes of that transmission and the efficacy of potential controls.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
A plethora of parasites may be transmitted by contaminated food, and criteria for prioritising which parasites should be considered to be of major concern are not always immediately obvious.
Whereas Taenia solium infections (cause of cysticercosis and neurocysticercosis, as well as taeniosis) are considered to be of the greatest importance on a global basis (resulting in the greatest number of disability‐adjusted life years (DALYs)), in Europe this parasite is not considered to have a substantial impact, largely due to modernised pig husbandry such that pigs farmed in Europe are unlikely to have access to human excrement.
A Europe‐focused ranking exercise was conducted in 2016 as part of the activities of a COST Action on food‐borne parasites.1 This exercise used multi‐criteria decision analysis, such that the parasite prioritisation was based on multiple aspects that compose the risk, and resulted in the following food‐borne parasites being ranked as being of greatest importance (Bouwknegt et al., 2018): Echinococcus multilocularis, Toxoplasma gondii, Trichinella spiralis, Echinococcus granulosus s.l., Cryptosporidium spp., other Trichinella spp. However, distinct regional differences were observed, with Echinococcus granulosus s.l. and Echinococcus multilocularis considered to be most important in south‐eastern Europe (Albania, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, the former Yugoslav Republic of Macedonia, Greece, Kosovo, Montenegro, Serbia, Slovenia, Turkey), south‐western Europe (Andorra, Italy, Malta, Monaco, Portugal, San Marino, Spain) and eastern Europe (Czechia, Estonia, Hungary, Latvia, Lithuania, Moldova, Poland, Romania, Slovakia); E. multilocularis and Cryptosporidium were considered to be the most important and second most important, respectively, in northern Europe (Denmark, Finland, Iceland, Norway, Sweden), and Toxoplasma and Cryptosporidium considered to be the most important and second most important, respectively, in western Europe (Austria, Belgium, France, Germany, Ireland, Liechtenstein, Luxembourg, the Netherlands, Switzerland, United Kingdom). The criterion of probability of a particular parasite becoming established in a particular country led to high rankings for some parasites that currently may be considered of marginal importance in that area; for example, E. multilocularis ranking highest in northern Europe, despite not being present in three of the five countries in that region.
Other food‐borne parasites considered to be of importance in Europe include the Anisakidae, Giardia duodenalis, and Toxocara spp.
Of those parasites judged as being of highest relevance in Europe, Echinococcus spp., and Cryptosporidium spp. can be transmitted via the food‐borne route as faecal contaminants on fresh produce and potentially on other food such as molluscan shellfish. Trichinella spp. is a meatborne parasite, and Toxoplasma can be transmitted as both a faecal contaminant (for example, on fresh produce) and from the consumption of inadequately cooked meat from infected animals.
The biology of these different parasites also varies widely. Cryptosporidium and Toxoplasma are protozoa (as is G. duodenalis), Echinococcus spp. are cestodes (tapeworms) for which humans may act as aberrant intermediate hosts in the lifecycle, and Trichinella spp. (along with the Anisakidae and Toxocara spp.) are nematodes.
In addition, there is considerable variation regarding why particular parasites are considered of importance. These reasons are both epidemiological and clinical (prevalence, occurrence of outbreaks, potential clinical severity, etc.) and economic, and, in particular, include:
The potential for having a significant clinical impact, despite relatively few cases and no reported outbreaks (e.g., Echinococcus spp.);
Outbreaks are regularly reported, however the clinical impact per case is relatively low (e.g., Cryptosporidium spp.);
Outbreaks are rarely reported, but there is a considerable burden of disease from individual cases (e.g., T. gondii);
There is a considerable economic burden associated with compulsory testing (e.g., Trichinella spp.).
This wide variation in how the importance of a particular food‐borne parasite is manifest, reflects and is reflected by the fact that comparison between parasites is particularly difficult.
For example, despite Echinococcus spp. being considered to be of importance as a food‐borne parasite in Europe, actual food‐borne transmission is almost impossible to document due to the delay (months; usually years) between human infection and the development of symptoms and diagnosis. Nevertheless, due to the potential severity of infection and the relatively high potential for food to act as a vehicle of infection, they are considered to be important food‐borne parasites. Furthermore, in countries where Echinococcus is not currently endemic, considerable efforts and expense are directed towards efforts to keep it out, and proving to EC that it is not there (and thus that there can be derogations from particular rules regarding movement of animals). It is worth noting that outbreaks of food‐borne infection with Echinococcus spp. have never been recorded; even individual cases are generally considered unusual in Europe.
Similarly, outbreaks of food‐borne toxoplasmosis have seldom been reported in Europe. The genotypes of T. gondii occurring most widely in Europe tend not to cause overt signs of disease except in vulnerable individuals (immunocompromised or in fetuses where the mother has not previously been exposed to infection). However, calculations from the Netherlands indicate that the burden of disease due to this parasite is considerable, due to the potential for high impact effects. Furthermore, the EFSA BIOHAZ Panel identified T. gondii as a relevant hazard to be covered by inspection of meat from swine, sheep, goats, farmed deer and wild boar (EFSA BIOHAZ Panel, 2011, 2013a,b).
Much of the economic burden of Trichinella spp. in Europe is associated with the requirements for testing and validation of testing capabilities. Nevertheless, some outbreaks are reported and the sequelae can be severe; the EFSA BIOHAZ PANEL identified Trichinella spp. as the most relevant biological hazard in the context of meat inspection of domestic solipeds (EFSA BIOHAZ Panel, 2013c).
Similarly, for the Anisakidae, much of the burden is concerned with testing of fish products and products being held at food inspection due to the presence of larvae in fish (the majority of food contamination by parasites reported in “RASFF – Food and Feed Safety Alerts” is due to Anisakidae). In addition, there is a poorly defined, but not inconsiderable, burden of illness associated with allergenicity to anisakid antigens in fish (EFSA BIOHAZ Panel, 2010).
Finally, although cryptosporidiosis is generally associated with acute gastrointestinal symptoms (although serious, life‐threatening disease can develop in severely immunocompromised patients, and there is no licensed specific treatment in the European Union), prolonged gastrointestinal symptoms are commonly reported and there is growing evidence for long‐term sequelae in some cases. Relatively widespread outbreaks associated with contaminated fresh produce have been documented, and smaller, more localised outbreaks that do not become identified as such, let alone make it to reporting and publication stages, are to be expected.
Although these parasites listed represent the tip of the iceberg globally, and are characterised by presenting different challenges and properties, awareness of different parasites within the food industry tends to be low. Furthermore, with the exception of Trichinella spp. and, perhaps, Anisakidae, specific controls within food production and processing are generally ill‐defined and/or it is assumed that those controls directed towards removal or inactivation of bacterial pathogens will be effective.
Thus, with the exception of Trichinella spiralis and possibly Anisakidae, there is a need to consider the risk of parasites from food, with particular emphasis on:
Identifying those foods that are at greatest risk of acting as transmission vehicles to consumers (and, for parasites that may be transmitted by external contamination of food, the foods at greatest risk of contamination), and how these can best be analysed;
Processes that may lead to dissemination of the parasite in food resulting in point‐contamination spreading throughout a batch;
The geographical distribution of food‐borne cases of parasitic infections and epidemiology;
The occurrence and persistence in foods and consumer habits that contribute to infection;
Measures that are suitable or appropriate for prevention (of food being an infection risk) and/or control (dealing with food that is an infection risk) for different food types;
Optimisation of food‐chain investigations following an outbreak, and general promotion of awareness of parasites in all sectors of the food industry.
Due to the challenges of addressing such a diverse group of pathogens, with different types of implicated foods, different biological and epidemiological characteristics, and different impacts, it is suggested that the focus initially could be on those parasites for which routine testing of food products has not been implemented. This would exclude, among other parasites, Trichinella spp., which is classified as being of importance in Europe, but for which standard methods for analysis and identification are in place, and fish‐borne parasites such as the Anisakidae.
However, it would include Cryptosporidium spp., T. gondii, and Echinococcus spp., all of which are considered of importance in Europe.
1.1.1.
Terms of Reference
The Panel on Biological Hazards (BIOHAZ Panel) is requested to issue a scientific Opinion on public health risk associated with parasites as food‐borne pathogens for which routine testing of food products has not been implemented in Europe. In particular, the BIOHAZ Panel is requested:
To critically review current methods for the detection, identification, characterisation and tracing of specific, selected food‐borne parasites (Cryptosporidium spp., Toxoplasma gondii, and Echinococcus spp.). with emphasis on methods applicable to foods that are likely to be a potential source of infection;
To evaluate available information to determine the relative importance of food‐borne pathways for transmission of the selected parasites to humans;
To examine available information on the occurrence and survival of the selected parasites in food and consumer practices contributing to infection;
To evaluate possible control measures from farm to consumption.
1.2. Interpretation of the Terms of Reference
The terms of reference have been interpreted such that for each of the listed parasites, their characteristics (including relevant food vehicles for transmission), methods for detection and characterisation in food, relative importance of food‐borne pathways, occurrence and survival in food, consumer practices that may contribute to food‐borne infection, and current control methods and likely impacts have been described and analysed. Commonalities for all the parasites considered and knowledge gaps are provided separately.
For this assessment, we have used the Food and Agriculture Organization's definition of food for the purposes of the Codex Alimentarius (FAO, 2001)2; thus, in this document ‘food’ means any substance, whether processed, semi‐processed or raw, which is intended for human consumption.
Food‐borne transmission is defined as when the parasite transmission stage is transferred to the human host via a food vehicle that is ingested, with contamination of the food occurring at any stage along the food‐chain, from primary production to preparation for consumption. Thus, for example, contamination of fresh produce may occur via irrigation water or via handling.
1.3. Additional information
In this Opinion, the focus is on food‐borne transmission of three selected parasites, Cryptosporidium spp., T. gondii, and Echinococcus spp. causing cystic echinococcosis (CE) (E. granulosus sensu lato (s.l.)) and alveolar echinococcosis (AE) (E. multilocularis). Although it is not practical to address all food‐borne parasites in a single document, these parasites are considered to be of the greatest importance in Europe at this time (Bouwknegt et al., 2018) as they are responsible for a substantial proportion of the public health and economic burden due to food‐borne parasites in Europe.
The foods that are relevant for each of the parasites depend directly on the lifecycle of each parasite, and are thus considered individually below (summarised in Table 1). All three parasites, Cryptosporidium spp., T. gondii and Echinococcus spp., can be transmitted as contaminants of food. The parasite transmission stages – oocysts for Cryptosporidium spp. and T. gondii, and eggs for Echinococcus spp. – are shed in the faeces of their (definitive) hosts and may contaminate a food product that is subsequently consumed. In addition, T. gondii, which is infectious to all warm‐blooded animals, can be transmitted as an intrinsic part of meat.
Table 1.
Selected parasites and possible food‐borne transmission pathways
| Parasite | Food group | Possible food‐borne transmission pathway |
|---|---|---|
| Cryptosporidium spp. | Fresh produce (fruit, vegetables and herbs that are eaten raw and have not been processed or preserved) |
Faeces of infected animals/humans during cultivation; splash‐up from rain may assist in spreading contamination Contaminated water used under cultivation (spraying, irrigation, etc.) Infected handlers during any stage of the production process Contaminated washwater during processing prior to packaging and sale |
| Fruit and vegetable juice |
Faeces of infected animals (including humans) during cultivation of the crop (including contaminated water used for irrigation, spraying etc.) and possibly by contaminated water used for dilution Infected handlers during any stage of the production process |
|
| Dairy products |
Faeces of infected animals during milking Infected handlers during any stage of the production process |
|
| Molluscan shellfish |
Filtration of contaminated seawater during growing (filter feeding) Cross‐contamination during depuration Infected handlers during any stage of the preparation process |
|
| Meat |
Faeces/intestinal content of infected animals contaminating the environment and meat surface at the abattoir during slaughter Infected handlers during any stage of the production process |
|
| Toxoplasma gondii | Fresh produce (fruit, vegetables and herbs that are eaten raw and have not been processed or preserved) | Faeces of infected felids during cultivation and possibly by contaminated water used for irrigation, spraying, etc., splash‐up from rain may assist in spreading contamination, cross‐contamination via washwater |
| Fruit and vegetable juice | Faeces of infected felids during cultivation of the crop (including contaminated water used for irrigation, spraying, etc.) | |
| Dairy products | Transfer of tachyzoites to milk of lactating infected mammals such as goats | |
| Molluscan shellfish |
Filtration of contaminated seawater during growing (filter feeding) Cross‐contamination during depuration |
|
| Meat | As all warm‐blooded animals may be infected, and predilection sites are varied and include muscle, etc., all meat that is not adequately treated (e.g., frozen, cooked, etc.) prior to consumption has the potential to transmit Toxoplasma | |
| Echinococcus spp. | Fresh produce (fruit, vegetables and herbs that are eaten raw and have not been processed or preserved) | Faeces of dogs, foxes and other canids during cultivation or, for wild‐picked produce under growth, and possibly by contaminated water used for irrigation, spraying, etc., and cross‐contamination via washwater; splash‐up from rain may assist in spreading contamination |
| Fruit and vegetable juice | Faeces of infected canids during cultivation of the crop (including contaminated water used for irrigation, spraying, etc.) and possibly by contaminated water used for dilution |
It is important to note that when the parasite is transmitted as a contaminant of food, as described above, replication of the parasite in the environment or on the food does not occur. Thus, numbers of the parasite cannot increase during food storage. However, the environmental transmission stages of these parasites are relatively robust, and resistant to significant environmental pressures, such as very cold weather conditions, that are likely to prove detrimental to many other types of food‐borne pathogens. Thus, die‐off occurs relatively slowly (commonly weeks to months, depending on storage conditions).
Although the parasite replicates within the live animal, when the animal has been slaughtered, replication no longer continues.
For Cryptosporidium spp., contamination of fresh produce in the field, either directly from faeces or via irrigation water, is a possible route of transmission. Several outbreaks of cryptosporidiosis associated with fresh produce have been reported (Robertson and Chalmers, 2013; Ryan et al., 2018). Processing/washwater may be, or become, contaminated and disperse oocysts through batches of fresh produce. People infected with Cryptosporidium spp. shed infectious oocysts, so contamination of fresh produce may also occur during food handling. Food‐borne outbreaks of cryptosporidiosis have also been associated with fruit juice and dairy products. For fruit juice, contamination is generally considered to have occurred in the field, but could occur during human handling, or from flies carrying oocysts attracted to the product, or from contaminated water used in processing or dilution. For milk and dairy products, contamination from animal faeces may also be of relevance. Meat surfaces also may be contaminated with oocysts, and one outbreak has been reported to be linked to meat. Molluscan shellfish have also been considered as possible food vehicles of transmission for Cryptosporidium. However, although many surveys have indicated that such shellfish may contain viable Cryptosporidium oocysts, evidence of human infection via this route is lacking and no outbreaks have been reported.
For T. gondii, oocysts shed in the faeces of the definitive host (felids) may contaminate food, with fresh produce being the most likely vehicle of infection, and potentially fruit juice. In addition, as with Cryptosporidium, the oocysts of Toxoplasma have been detected in molluscan shellfish, which also have the potential to serve as a transmission vehicle. Although human toxoplasmosis due to consumption of oocysts in shellfish has not been documented, it seems likely that marine molluscs are the source of toxoplasmosis affecting sea otters off the coast of California (Conrad et al., 2005). Although tachyzoites may occur in milk, the available data do not suggest that this is a frequent transmission route to humans in Europe, possibly due to the relative lability of the tachyzoites combined with widespread pasteurisation.
Toxoplasma may infect any warm‐blooded animal and the proliferating tachyzoites encyst in the tissues. These tissue cysts contain the relatively dormant stage, the bradyzoites, and meat from any infected animal that is not treated appropriately (e.g., adequate freezing or cooking) prior to consumption may transmit the infection. Wild animals or domestic animals that are raised outdoors are more likely to be infected because of the greater potential for exposure to infectious oocysts.
For Echinococcus, contamination of food products can only occur from contact with the faeces of infected canids. In Europe, the relevant canids are, in particular, dogs for E. granulosus and foxes for E. multilocularis. For this parasite, the long incubation period in human infections between infection and symptoms (months to years), means that vehicle attribution is exceptionally difficult. However, extrapolation from non‐human primate investigations may provide clues. Although there is one report of a case of human infection with E. multilocularis, in which the transmission vehicle was proposed to be imported Swiss cheese (Cook, 1991), this seems to be a very unusual situation; proposed identification of the transmission vehicle was based on circumstantial evidence and could not be verified, although survival of Taenia taeniaeformis eggs in the cheese‐making process was demonstrated as part of a retrospective investigation. Thus, fresh produce (farmed or gathered) that is contaminated in the field or forest, potentially via irrigation water or splash up from rainwater, seems to be the most likely food‐borne transmission vehicle for this parasite. Although there are no documented cases of human infection with Echinococcus spp. where fresh produce is implicated, several cases of AE in zoo primates in Switzerland have been recorded in which fresh vegetables contaminated with E. multilocularis eggs were considered to be the most probable infection vehicle (Federer et al., 2016). This indicates the potential for this route of infection and the plausibility of fresh produce acting as an infection vehicle for humans.
Consumption of fresh vegetables has dramatically increased globally, partly because of the wide diversity of fresh vegetables and packaging formats available, and also because of the promotion of these foods as important components of a healthy diet. In high‐income regions, including Europe, consumers’ desire for these products is year‐round, and therefore companies source products from all over the world to fulfil this demand. However, fresh and fresh‐cut herbs, vegetables and soft fruits are not processed in ways that will effectively eliminate human pathogens, including parasites (Jung et al., 2014).
Given these known and potential transmission routes for the parasites under consideration, we have focussed upon the following food groups: fresh produce and herbs (this means fruit, vegetables, and herbs that are eaten raw and have not been processed or preserved); fruit and vegetable juice; dairy products (for the purposes of this document, this includes liquid milk, fermented milk products such as yoghurt, and raw milk cheeses), molluscan shellfish, and all types of meat (meat and meat products from different animal species). The possible food‐borne transmission pathways for the selected parasites are summarised in Table 1.
For all parasites, there are clear limits on the adequacy of detection methods, which also differ by food matrix. These are described in greater detail in the relevant sections on each parasite.
In addition, the detection of a parasite (or parts thereof, including molecules such as DNA) in a food matrix does not necessarily indicate infectious potential.
2. Data and methodologies
A literature search was used to gather scientific publications, reports, and official documents relevant for this Opinion. In general, the qualitative evaluation of the literature was based on the knowledge and expertise of the working group members. The experts in the working group selected relevant references starting from review papers, book chapters and peer‐reviewed papers retrieved through non‐systematic searches, and increasing the number of papers through ‘footnote chasing’ (White et al., 1992) until reaching a coverage of the subject considered sufficient by the working group.
In order to better interpret the reported notifications of human cases, animal infections and food contamination due to Cryptosporidium spp., T. gondii, E. multilocularis, and E. granulosus s.l., a questionnaire about mandatory notification of these parasites was sent to the members and observers of EFSA's Scientific Network for Zoonoses Monitoring Data representing 28 Member States and three European Economic Area (EEA) countries (Iceland, Norway and Switzerland). Replies were obtained from 30 (27 Member States and 3 EEA countries)/31 countries. A summary of the questionnaire and the results is shown in Appendix A.
3. Assessment
3.1. Cryptosporidium spp
3.1.1. Characteristics, including relevant food vehicles for transmission
Cryptosporidium spp. are protozoan parasites in the phylum Apicomplexa, and have an intracellular but extracytoplasmic site of infection, usually epithelial cells in the small intestine. The taxonomy of the genus Cryptosporidium is undergoing continuous revision, and novel species are frequently described based on a combination of biological and genetic data. At present, there are at least 37 valid species and about 60 genotypes of undefined taxonomic status. Although as many as 17 species have been associated with human infection, two are responsible for the vast majority of human cases of disease, cryptosporidiosis, namely, C. hominis and C. parvum (Table 2).
Table 2.
List of the most commonly reported Cryptosporidium species infecting humans
| Species | Major host(s) | Occurrence in humans (globally) |
|---|---|---|
| Cryptosporidium hominis | Humans | Most common species |
| Cryptosporidium parvum | Ruminants and humans | Most common species |
| Cryptosporidium meleagridis | Birds and humans | Commonly reported |
| Cryptosporidium ubiquitum | Ruminants, rodents, primates | Commonly reported |
| Cryptosporidium canis | Dogs | Less commonly reported |
| Cryptosporidium cuniculus | Rabbits | Less commonly reported |
| Cryptosporidium felis | Cats | Less commonly reported |
| Cryptosporidium muris | Rodents | Less commonly reported |
| Cryptosporidium viatorum | Humans and the native Australian swamp rat Rattus lutreolus | Less commonly reported |
The lifecycle of Cryptosporidium spp. is completed within a single host, and starts with the ingestion of oocysts, from which four infective parasites (sporozoites) are released in the gastrointestinal tract where they invade epithelial cells. The cycle progresses with several asexual replications until a sexual phase occurs, enabling genetic recombination, and culminates in the production of new oocysts that are shed in the faeces. Oocysts are small (those of most Cryptosporidium species are about 5 μm in diameter), extremely robust stages that can withstand environmental stress and maintain sporozoite infectivity for weeks to months in moist conditions such as surface waters at typical temperatures (Rochelle and Di Giovanni, 2014). Survival in the aquatic environment is influenced mainly by temperature (Nichols et al., 2004) and solar ultraviolet (UV) (King et al., 2008). Due to the large animal reservoir, a lifecycle that does not require a specific vector or intermediate host for transmission, and the ability to persist in the environment, Cryptosporidium spp. are widely distributed. The major hosts of the different species vary (see Table 2 for examples).
Dose–response modelling predicts a probability of human infection following ingestion of a single oocyst ranging from 0.03% to 6%, depending on the strain (Messner et al., 2001), although recent modelling studies suggest that single oocyst infection probabilities could be as high as 72% (Messner and Berger, 2016).
Symptomatic infection (cryptosporidiosis) is characterised by diarrhoea, abdominal pain, nausea or vomiting, mild fever, anorexia, malaise, fatigue and weight loss. Diarrhoea can be of sudden onset and is generally watery and voluminous, with three to six stools passed each day, which may contain mucus. Symptoms usually last for up to 3 weeks, occasionally longer, and, in otherwise healthy people, resolve spontaneously. During clinical episodes, relapse occurs in about one‐third of cases. Individuals with an impaired immune system (e.g., untreated people with AIDS, malnourished children, people with some congenital immunodeficiencies and some transplant recipients) are at risk of developing more severe and protracted symptoms. To date, very few drugs are available for the treatment of cryptosporidiosis, of which only one, nitazoxanide, a broad‐spectrum antiparasitic and antiviral drug, is licensed in the United States, but not in the EU. The burden of disease is noticeably higher in low‐income countries, and young children are the age group most affected by cryptosporidiosis. Recent studies including the Global Enteric Multicenter Study (Kotloff et al., 2013) and the Global Burden of Disease 2015 Study (GBD Diarrhoeal Diseases Collaborators, 2017) have shown that Cryptosporidium is a major cause of moderate‐to‐severe diarrhoeal disease in young children (< 5 years of age) in sub‐Saharan Africa and South East Asia, and a significant cause of death in toddlers.
In Europe, as elsewhere, knowledge about epidemiological trends of cryptosporidiosis is limited by differences in the ascertainment, reporting and surveillance systems that are in place in the different EU/EEA countries (Appendix A). Therefore, the quality and quantity of the available data varies between Member States and direct comparison of numbers of cases and incidence rates between EU/EEA countries may not be possible (Cacciò and Chalmers, 2016). In the ECDC Annual Epidemiological Report for 2015, nine EU countries (Denmark, France, Greece, Italy, the Netherlands, Poland, Portugal, plus Iceland and Lichtenstein) did not report data on cryptosporidiosis at all. The UK reported over half of all cases. Furthermore, of the 23 EU/EEA countries that did report data on cryptosporidiosis, 13 reported only 0–10 cases in 2015 compared with the average reporting rate of 3.1 cases per 100,000 population. It is therefore evident that cryptosporidiosis is under‐ascertained and under‐reported in most EU/EEA countries. Nevertheless, some relevant trends can be inferred from the reported data, in particular regarding age distribution and seasonality of cases. Indeed, as observed in other parts of the world, the highest notification rate in Europe is usually seen in young children (0–4 years old), with 11.2 confirmed cases per 100,000 males and 9.2 confirmed cases per 100,000 females in this age group. In terms of seasonality, a b‐modal distribution, confirming a trend observed in previous years, has been reported with a small peak of cases in the spring and a larger one in late summer and autumn (August–October) (ECDC, 2018). However, the epidemiology varies between countries.
The transmission of human cryptosporidiosis involves both direct (person‐to‐person and animal‐to‐person) and indirect routes (through water, food and fomites contaminated with infectious oocysts) (Figure 1).
Figure 1.
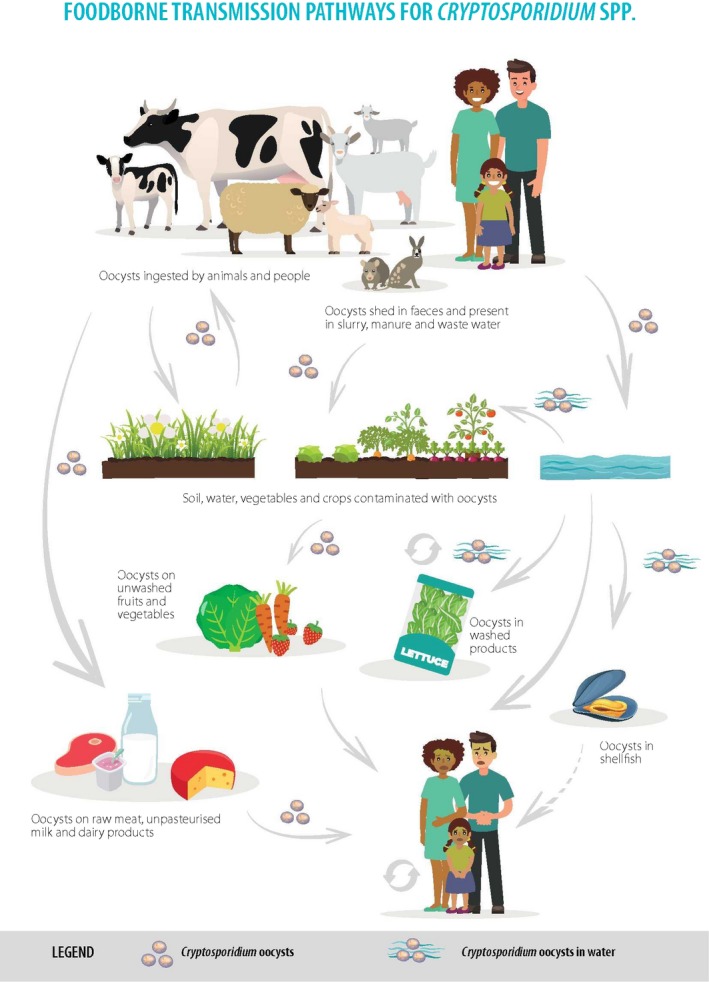
Food‐borne transmission pathways for Cryptosporidium spp.
Cryptosporidiosis occurs as sporadic infections and as outbreaks. Direct person‐to‐person transmission plays a major role in the epidemiology, and cases have been reported between family members, sexual partners, children in day‐care centres, and hospital patients and staff. Contact with people with diarrhoea was identified as a major risk factor for sporadic cryptosporidiosis in the UK, USA, and Australia (Robertson et al., 2002; Hunter et al., 2004; Roy et al., 2004).
Direct zoonotic transmission of C. parvum has been demonstrated in many instances and outbreaks, particularly among veterinary students, other people exposed to livestock, and children and adults visiting open/petting farms (Cacciò and Putignani, 2014), and touching livestock was identified as a significant risk factor in sporadic cases in the UK (Hunter et al., 2004).
The epidemiology and risk factors for the two main species have not only some overlap but also some key differences. For C. hominis, contact with young children (such as changing nappies), or with people having diarrhoea, or ingestion of drinking or recreational water contaminated with human faeces or wastewater represent the main risk factors. In the case of C. parvum, contact with farm animals, especially very young animals, or consumption of water or food contaminated by their faeces are the main risk factors, although this parasite can also be spread between people.
Cryptosporidium has traditionally been regarded as a waterborne parasite, and many studies have demonstrated a widespread occurrence of Cryptosporidium in the aquatic environment. Water plays an important role in the transmission of Cryptosporidium spp. to humans, both from drinking water supplies and recreational waters, and is the most commonly reported vehicle of transmission in outbreaks (Chalmers, 2012). Although both mains and private water supplies have been implicated in outbreaks and may pose an infection risk for sporadic cases, swimming pools are the main setting for outbreaks in which risks from drinking water have been controlled. Oocysts are resistant to the chlorine concentration typically used for chemical disinfection of water and are remarkably stable particularly at low temperature. Water may play an important role in the indirect contamination of food with Cryptosporidium spp.
The role of food in the transmission of cryptosporidiosis is less clear than that for drinking or recreational water (Ryan et al., 2018). This is in part due to the legacy of Cryptosporidium being regarded as a waterborne parasite, but also due to under‐ascertainment and under‐reporting, lack of awareness of food as a vehicle, difficulties in trace‐back to and of food items, and the lack of national and international standards for testing food, in contrast to that of drinking water (Painter et al., 2009; Chalmers, 2012). See Section 3.1.3 for further details.
3.1.2. Food‐borne outbreaks in Europe
In the period 2005–2016, a total of 53 cryptosporidiosis outbreaks (of any cause) in Europe were reported to EFSA, of which seven were attributed to food. Of the 53 outbreaks, 13 were categorised as ‘unknown’ and may also have been food‐borne. However, it is helpful to look at the food‐borne outbreaks of cryptosporidiosis reported globally (Appendix B) to see the range of risky food items and transmission routes, and some of the emerging themes. Between 1984 and 2017, there were 25 reported food‐borne outbreaks of cryptosporidiosis globally. The geographical distribution was skewed towards countries with established surveillance and reporting systems, as well as the resources for outbreak investigation, as discussed by Robertson and Chalmers (2013). Even so, some of the global trends include the following:
Food‐borne outbreaks were mainly linked to fresh produce (n = 11), especially more recently, followed by unpasteurised milk and dairy products (n = 7).
Fruit juice‐related outbreaks (n = 3) have not been reported since 2003.
There has only been one reported outbreak linked to the consumption of meat (although a chicken salad was one of the menu items in another outbreak).
Many of the outbreaks had multiple foods or transmission routes identified through descriptive epidemiology. Unravelling the food‐borne element or precise food item can be hard.
The evidence for association with food was largely descriptive epidemiology – analytical epidemiology (case–control or cohort study) was reported in just three food‐borne outbreaks (two in the UK and one in the USA).
Very large outbreaks (with one hundred or more cases) were linked in 2012 and 2015 to salad leaves in the UK (two outbreaks) and Finland (one outbreak). In the UK outbreaks, food traceability was difficult. In the Finnish outbreak, frisée salad was reported to be traced to production in the Netherlands.
Food‐borne outbreaks affected adults more than children, largely due to the food items (such as salad leaves and vegetables) and settings where they were served (such as workplace canteens and restaurants).
Where the infecting species were identified (15 outbreaks), this was usually C. parvum (13 outbreaks). Both of the C. hominis outbreaks implicated food handlers.
There have been no reported outbreaks linked to the consumption of molluscan shellfish.
The lack of widespread genotyping to identify infecting species and link cases to each other, and lack of efficient and effective methods for testing food and linking contaminating isolates to cases, has hampered investigation and intervention during food‐borne (and other) outbreaks.
3.1.3. Methods of detection in food
The most sensitive methods for the detection of Cryptosporidium in food products (Appendix C) are based on (1) oocyst separation from the sample matrix by a method that minimises co‐concentration of debris (e.g., flotation or immunomagnetic separation (IMS)) and (2) detection by polymerase chain reaction (PCR) or immunofluorescence microscopy (IFM). Other promising methods of oocyst concentration include microfiltration, but some pre‐preparation may be needed to reduce filter clogging. Only IMS‐IFM has been validated in ring trials and then only for iceberg lettuce and raspberries (Cook et al., 2006; Utaaker et al., 2017). This forms the basis of the only relevant standard method, ISO 18744 ‘Microbiology of the food chain — Detection and enumeration of Cryptosporidium and Giardia in fresh leafy green vegetables and berry fruits’.
The relevant commercially available IMS and IFM reagents are broadly specific for the capture and detection of Cryptosporidium spp. oocysts. However, analysts need to be aware that cross‐reactions may occur with other genera and ensure that measures are in place to confirm Cryptosporidium oocysts, by visualisation of confirmatory internal structures. IFM is dependent on the experience and expertise of analysts to identify Cryptosporidium oocysts correctly. In the absence of confirmation, only ‘Cryptosporidium oocyst‐like bodies’ or ‘presumptive Cryptosporidium oocysts’ should be reported.
IFM is desirable for the enumeration of Cryptosporidium oocysts, but as viable and non‐viable oocysts are indistinguishable by this test, species that are not infectious for humans will also be counted, and therefore the human health risk may be overestimated.
Prelabelled, inactivated oocysts can be spiked into samples tested by IFM or IMS‐IFM to monitor test method recovery and detection rates. Recovery rates may vary, depending on the size of the inocula used, the sample matrix and the competency of the analyst, but for drinking water spiked with 100 to 500 oocysts, ≥ 33% is deemed acceptable (US EPA, 2012). For foodstuffs, Utaaker et al. (2015) suggested an acceptable threshold recovery rate of 20% for lettuce spiked with 50 oocysts. During some food sample surveys, oocyst recovery rates of 25–47% have been reported, but these data are rarely provided (Appendix C).
Recovery data from spiked oocysts can be used in risk assessments to estimate the true numbers of oocysts present indicated from oocyst counts.
PCRs for detection have not been well validated for quantification of Cryptosporidium spp. in food, and there have been no ring trials. PCR performance depends on sample preparation, DNA extraction, PCR efficiency, mitigation of inhibitors and the detection system used.
If a validated PCR is applied to IMS concentrates, there is additional assurance that the amplicons derive from oocysts rather than ‘free’ DNA in the sample.
Note that the PCR primers will, in part, determine the specificity of the Cryptosporidium assay. For some foods such as milk, where contamination likely originates from the milk‐producing animal, it may be relevant to target only the Cryptosporidium species from that host likely to be infectious to humans (e.g. cow's milk, C. parvum), whereas for other foods where diffuse sources of contamination are possible, primers with a broader specificity may be useful.
Furthermore, in addition to simply detecting the genus, molecular methods enable the identification of the species (or beyond) of Cryptosporidium and this can be helpful for source attribution and tracking. Sequencing the SSU rRNA gene provides the benchmark for species differentiation. A fragment of the highly polymorphic gene encoding the glycoprotein 60 (gp60) protein is sometimes sequenced to ‘subtype’ Cryptosporidium species, most commonly C. parvum and C. hominis from clinical cases, but a multilocus scheme is desirable to resolve the variation arising from genetic recombination during the sexual phase of the lifecycle (specifically, meiosis). However, there is no standardised multilocus genotyping scheme. Furthermore, the small numbers of oocysts likely to be retrieved from food samples would pose challenges to the application of such a scheme for food, should one be standardised.
ISO 18744 does not include identification of the parasite species/genotypes or a viability assessment. If foods are spiked for recovery data, molecular methods may detect the spike, although this may be reduced if gamma irradiated oocysts are used as their DNA is damaged and less readily detected by PCR.
3.1.3.1. Detection methods in fresh produce
Method development for fresh produce, along with interest from bodies such as Codex, has resulted in the production of an ISO standard for leafy greens and raspberries. It is based on surface elution, centrifugation, IMS and IFM. This has been used in four studies in Europe, of which three had large sample sizes indicating an overall prevalence of Cryptosporidium spp. of up to 8% (Appendix D). The most recent study, which did not use IMS and relied on pooling samples, reported a Cryptosporidium prevalence of < 1% (Caradonna et al., 2017).
For the ISO and other suitable methods, variable recovery rates have been reported (Appendix C) both within ring trials where the same type of produce was examined (Cook et al., 2006; Utaaker et al., 2015), and between different types of produce (Robertson and Gjerde, 2001; Hohweyer et al., 2016). The limit of detection (LOD) has only been provided for a quantitative polymerase chain reaction (qPCR)‐based method and the numbers of oocysts used in the inocula were greater than would be expected in natural contamination (Appendix C; Hohweyer et al., 2016). More work needs to be done to investigate food‐type (matrix) effects and whether the ISO standard is suitable for all leafy greens as the validation and ring trials were limited to iceberg lettuce.
The US Food and Drug Administration (FDA) has published a method in the Bacteriological Analysis Manual: BAM 19a ‘Detection of Cyclospora and Cryptosporidium in Fresh Produce: Isolation and Identification by Polymerase Chain Reaction and Microscopic Analysis’ (US FDA, 2004). However, there have been few published studies comparing detection by IFM and PCR, and one study reported lower detection by PCR from lettuce than microscopy (Ripabelli et al., 2004). Providing the sample preparation is appropriate, DNA extraction is efficient, the PCR is well designed and efficient, inhibitors are controlled and performance is validated, these methods should be suitable for detection in fresh produce.
The identification and application of DNA aptamers binding to the oocyst wall of C. parvum, has suggested the ability to detect 100 oocysts spiked on fresh fruit, and could be automated, but more development is needed for practical application (Iqbal et al., 2015).
3.1.3.2. Detection methods in fruit juice
The first investigations of Cryptosporidium in fruit juice were in response to outbreaks in the USA in the early 1990s where the vehicle was identified as unfermented apple cider. Detection methods were based on those used for faeces: ethyl acetate sedimentation or simple sedimentation‐sucrose flotation and IFM (Millard et al., 1994), but no recovery, sensitivity or specificity data were given, although high numbers of oocysts were detected, which was most likely due to high levels of contamination. In an effort to provide comparative recovery data and improve detection, one study (Deng and Cliver, 2000) compared sample preparation by ethyl acetate sedimentation and sucrose flotation with IMS and detection by immunofluorescence microscopy and PCR (Laberge et al., 1996). This established sucrose flotation and IMS with detection by IFM as most sensitive, with 2/3 aliquots positive when spiked with 10 oocysts/100 mL, and therefore suitable for detecting an infectious dose in a portion‐sized sample of the product. In a further development, an alternative IMS system was used and 10 oocysts detected by the same PCR (Laberge et al., 1996; Deng et al., 2000).
As there are no data from Europe, it is necessary to look elsewhere. One study in Canada investigated an apple cider (juice) production process by sampling apples through to finished product and, using the Laberge et al. (1996) and Deng et al. (2000) methods, found material positive for Cryptosporidium at all stages (Garcia et al., 2006). This is probably the most relevant available study for Europe and is included in Appendix C. Other studies have been undertaken elsewhere but their settings (such as juice stalls or farms in Africa) were not considered relevant for risk assessment in Europe.
Frazar and Orlandi (2007) artificially contaminated different food types to compare DNA template preparation for PCR, using Whatman filter paper adsorption with a kit‐based total DNA extraction following IMS. Total DNA extraction provided a more reliable detection of 50 oocysts/10 mL, with the LOD varying by matrix: for apple juice, the LOD was 50 oocysts/10 mL; in high juice pulp orange juice, 1/5 replicates were positive at 5 oocysts/10 mL; and in low pulp orange juice, 2/5 replicates were positive at 5 oocysts/10 mL.
By combining microfiltration with oocyst lysis and spin columns and a real‐time PCR (Guy et al., 2003), nested PCR and single‐tube nested real‐time PCR, consistent detection limits of 10 oocysts per 250 mL have been reported (Minarovičová et al., 2009, 2010).
Recent developments, particularly in PCR detection, may be useful for future work. The US FDA's BAM19a (see above) also includes isolation of Cryptosporidium spp. from juices, unfermented cider and milk by processing a 10‐mL volume of product directly by IMS and detection by PCR or IFM, although no performance data are provided or could be found.
3.1.3.3. Detection methods in milk and dairy products
There have been no prospective sample surveys, or well‐described methods applied during outbreak investigations in Europe. In three outbreaks elsewhere (Australia, Russia and the USA), milk or fermented milk product (kefir) was tested for the presence of Cryptosporidium and oocysts or antigens were detected (Appendix D). In Australia, milk samples were centrifuged and IMS was used to concentrate oocysts, IFM was used for oocyst detection and ELISA for antigen detection. In Russia, microscopy was used for detection in kefir and milk filters at the dairy. In the USA, PCR was used but deemed to provide a false positive result in milk, highlighting the need for validation of molecular methods.
Seeding trials of liquid milk have been reported as part of method development, and PCR‐based methods, particularly the most recent, are more sensitive than other methods used (Appendix C). A key element is the sample preparation prior to PCR. However, none have progressed to sample surveys of milk or dairy products so their application is not known/proven.
3.1.3.4. Detection methods in molluscan shellfish
Molluscan shellfish have been tested for Cryptosporidium for two main purposes: first, as biomonitors of fresh and sea water quality, taking advantage of their filtration capacity, and secondly as food products. The focus is on the latter here.
A variety of molluscan genera have been tested, the most commonly eaten and farmed species being Pacific cupped oysters (Crassotrea gigas). Other main food species are eastern oysters (Crassotrea virginica), European flat oysters (Ostrea edulis), hard clams (Mercenaria mercenaria), soft‐shelled clams (Mya arenaria), common mussels (Mytilus edulis), Mediterranean mussels (Mytilus galloprovincialis) and common cockles (Cerastoderma edule) (Appendix C).
A variety of testing methods have been used, either applied to tissue homogenates or washings of whole shellfish or individual parts (e.g., gills, digestive tract, whole flesh washings, haemolymph), tested separately or pooled, and subjected to concentration or purification through sieving, centrifugation, flotation or IMS before detection by molecular methods or IFM.
Gómez‐Couso et al. (2005) looked at the distribution of Cryptosporidium within 60 clams (Tapes decussatus) on a daily basis following seeding a 20 L tank with 106 C. parvum oocysts (approximately 3.3 × 105 oocysts/specimen). Histological analysis demonstrated the presence of oocysts in siphons, gills, stomach, digestive diverticula and gut, but the frequency of detection was higher in gills and especially gut, where the number of oocysts was greatest on all 10 consecutive days. They recommended that the gills and intestinal tracts should be examined in preference to individual tissues. There is additional evidence that whole tissue homogenates are the most useful sample for testing in occurrence or prevalence surveys (Fayer et al., 1997; Tamburrini and Pozio, 1999; MacRae et al., 2005; Li et al., 2006; Miller et al., 2006; Schets et al., 2007).
Seeding experiments, with detection by IFM, have shown recovery efficiencies ranging from 12% to 50% for mussels (MacRae et al., 2005; Graczyk et al., 2007), 48% to 69.5% for scallops (MacRae et al., 2005) and up to 77.2% from inoculation of sieved and purified mussel and intestinal tract homogenates (Gómez‐Couso et al., 2006). One limitation of the tests applied was that only a small proportion of sample/homogenate could be tested.
In an effort to provide a method that is suitable across a range of shellfish consistencies due to variable protein content, and enabling a larger proportion of material to be tested, Robertson and Gjerde (2008) evaluated a pepsin digestion method that allowed for the examination of 3 g samples by IFM with only a small loss of viability. Recovery efficiencies of 70–80% from blue mussel, horse mussel and oyster homogenates were reported (Robertson and Gjerde, 2008).
Although no study has shown total agreement between detection by IFM and PCR in shellfish, neither method has been shown to be consistently better than the other, probably because of the different detection targets (e.g. empty oocyst‐like bodies may be detected by IFM but sporozoites and hence DNA may not be present) (Fayer et al., 2003; Gómez‐Couso et al., 2004, 2006).
3.1.3.5. Detection methods in meat
The lifecycle of Cryptosporidium usually occurs in the small intestine and meat surfaces might become contaminated with oocysts from faeces, particularly at the slaughterhouse. Very few studies of Cryptosporidium on meat have been undertaken – most of the information comes either from reactive development work for cured meat prepared using possibly contaminated water that caused a drinking waterborne outbreak in Sweden (Robertson and Huang, 2012), or from the EU Fifth Framework Quality of Life and Management of Living Resources programme3 (Appendix C). This EU project focused on the development of new methods to isolate and detect C. parvum in food and water samples. The project investigated meat samples at a commercial beef abattoir in Ireland. The parasite was not detected on carcass meat (Moriarty et al., 2005a). However, oocysts were isolated from 21/288 (7.3%) faecal samples at an estimated 25,000–37,500 per g and in 12/49 water samples (50 L) that had been used to wash beef carcasses at a level of 0.08–9.0 oocysts/L (McEvoy et al., 2005; Moriarty et al., 2005a).
3.1.3.6. Infectivity and viability assessment of Cryptosporidium spp. in food
Cryptosporidium oocysts are already sporulated on excretion in faeces and are therefore immediately capable of infecting another host; additionally, the thick oocyst wall confers protection and they can therefore survive for long periods of time in the environment, particularly in moist conditions. Extremes of temperature, solar UV light, and ammonia reduce their survival, while the effects of biotic antagonism (predation from other organisms) need more investigation (King and Monis, 2007). Additionally, food preservation treatments such as pasteurisation, low‐temperature freezing, low pH and desiccation adversely affect the ability of Cryptosporidium spp. to survive in food (Dawson, 2005). However, the detection methods currently used for Cryptosporidium spp. do not indicate whether the oocysts are viable (alive) or infectious and potentially harmful to humans. They may be dead, or not capable of infection, or belong to a species or genotype non‐infective or pathogenic to humans. Although oocysts processed and detected by ISO 18744 could be subsequently genotyped to identify species or strains, viability or infectivity assessment is not possible once samples have been fixed on microscope slides, and assay modification would be required and applied to additional samples.
The methods to assess infectivity and viability have been recently reviewed (Rousseau et al., 2018). The gold standard is infectivity of a neonatal mouse model, although this is not applicable to C. hominis for which the immunosuppressed Mongolian gerbil provides an infectivity model. However, cell culture infectivity, measured by observation of lifecycle stages or infection foci, has been shown to provide a suitable, more ethical alternative (Johnson et al., 2012). Tests for viability such as those based on the uptake or exclusion of vital dyes, and molecular methods amplifying RNA, may overestimate infectivity and subsequently public health risk.
3.1.3.7. Concluding remarks on detection methods
The most sensitive methods for the detection of Cryptosporidium in food products require oocyst separation from the sample matrix and detection either by PCR or by IFM.
Quantitation of Cryptosporidium in food by PCR needs further development. PCR‐based methods that have been applied to food provide neither an idea of viability or infectivity, for which further tests are needed (see Appendix C). Genotyping, and especially multilocus genotyping, may be difficult to apply to food where small numbers of oocysts might be present.
There is only one standard method: ISO 18744 ‘Microbiology of the food chain — Detection and enumeration of Cryptosporidium and Giardia in fresh leafy green vegetables and berry fruits’. The effectiveness of this method is dependent on the food tested and laboratory staff expertise.
There is a need for standardisation of sampling approaches, experimental design, and outcome measurements to enable comparison between studies, whether they are for detection or survival studies, of food types and processing and control measures.
3.1.4. Occurrence and survival of Cryptosporidium spp. in food
Published surveys for the occurrence of Cryptosporidium spp. in food in Europe (unless stated otherwise) are shown in Appendix D. Of the studies of the foodstuffs considered here, very few reported either the viability or infectivity of the Cryptosporidium oocysts detected; survival data were mainly generated in separate, largely experimental, studies.
The use of indicator organisms as a means to assess the probability of presence of Cryptosporidium remains controversial and often discredited, although the use of indicators for validation of the efficacy of treatment processes is justified. Although spores of Clostridium perfringens are sometimes suggested as an indicator, the rationale is often unclear. Spores of C. perfringens may be as hardy as protozoan parasites, but there are plenty of data that demonstrate a lack of clear correlation between C. perfringens in water and Cryptosporidium spp. (EFSA, 2017).
3.1.4.1. Occurrence and survival of Cryptosporidium spp. in fresh produce
Surveys for the occurrence of Cryptosporidium in fresh produce and herbs in Europe have been published in six papers (Appendix D). Studies of alfalfa, mung bean, radish sprouts and sprout mix, cabbages, leeks, lettuce, spring onions, celery, cauliflower, broccoli, spinach and Brussels sprouts at the point of sale, using methods similar to the ISO standard 18744, and sample sizes > 100 items individually or overall for the study, indicated a prevalence of up to 8% (Robertson and Gjerde, 2001; Rzeżutka et al., 2010). A study in Poland reported that Cryptosporidium‐positive samples were more likely to come from districts with the highest number of cattle herds (Rzeżutka et al., 2010). One small study of cabbages and lettuce irrigated with faecally contaminated water in Spain showed Cryptosporidium oocysts on 2/6 (33.3%) Chinese cabbage, 3/4 (75%) Lollo rosso lettuce and 7/9 (77.8%) Romaine lettuce (Amoros et al., 2010), indicating the risk that is presented by waterborne contamination during cultivation. One study using a test method omitting the IMS step, and using IFM and PCR on pooled samples, reported a prevalence of 0.96% (Caradonna et al., 2017) and although the pooling strategy took into account predicted low prevalence, this may have diluted the concentration of oocysts below the LOD for the assay.
The numbers of oocysts detected on fresh produce were reported in just four papers, from different amounts of initial samples (range 30–100 g; Appendix D) and ranged from 1 to 17 oocysts.
To investigate the survival of Cryptosporidium oocysts, Utaaker et al. (2017) on spiked lettuce leaves and used vital dyes to determine changes in viability under refrigerated and ambient storage conditions. In contrast with Giardia cysts, Cryptosporidium oocysts survived well with little change in viability over a 2‐week period, both when stored refrigerated and at room temperature. Hohweyer et al. (2016) used reverse‐transcriptase qPCR targeting a heat shock protein (hsp70) after heat‐shock induction, and also reported no significant change in the viability of oocysts spiked on to basil leaves throughout storage at 4°C for 8 days.
3.1.4.2. Occurrence and survival of Cryptosporidium spp. in fruit juice
No data were found for the occurrence of Cryptosporidium in fruit juice in Europe. A survey during the processing of apples in Canada reported the presence of Cryptosporidium DNA, detected by PCR, in raw apple juice in 6/113 (5%) samples and in 2/113 (2%) of the same samples following fermentation (Garcia et al., 2006).
The survival of Cryptosporidium in fruit juice has been investigated using vital dyes. One study reported the survival of a significant proportion of oocysts inoculated into orange juice (pH 3.9) stored at 4°C and at 22°C for 24 h (Friedman et al., 1997). When naturally present, oocysts recovered from stored concentrates obtained in Egypt, were tested using vital dyes and 4‐ or 5‐week‐old Swiss albino mouse infectivity. Reduced viability and infectivity were reported from oocysts in lemon and orange juice, but not strawberry, mango or sugar cane juice (Mossallam, 2010).
3.1.4.3. Occurrence and survival of Cryptosporidium spp. in milk and dairy products
No data were found for the occurrence of Cryptosporidium in milk and dairy products in Europe but investigation of two outbreaks elsewhere provided some evidence for the presence of the parasite in reactive sampling, but no enumeration (Appendix D).
Oocyst viability in yoghurt and already pasteurised milk was estimated using spiking experiments and vital dyes to assess viability. Although oocyst viability decreased from a starting point of about 80% at spiking, viability was only reduced to 58% even after 8 days of storage (Deng and Cliver, 1999). The same study also investigated ice cream by inoculating oocysts into the ice cream mix, prior to mixing, freezing and hardening for 24 h at −20°C, after which none were viable, although 8% of oocysts suspended in water as controls were still viable at this point (Deng and Cliver, 1999).
3.1.4.4. Occurrence and survival of Cryptosporidium spp. in molluscan shellfish
Although there has yet to be a cryptosporidiosis outbreak reported that implicates molluscan shellfish (Appendix B), there have been more sample surveys for the occurrence and survival of Cryptosporidium in this foodstuff than for the others considered here (Appendix D). In a review of the potential for marine bivalve shellfish to transmit protozoa, including Cryptosporidium, to humans, Robertson (2007) stated the importance of recognising this potential by those investigating infection routes. Most studies have been in the major mussel‐producing areas of Europe, reflecting the concern about the potential of this food to cause illness and outbreaks.
Field samples in Spain indicated that the depuration process is inefficient for the complete elimination of oocysts (Gómez‐Couso et al., 2003a, 2006), supported by tank experiments (Gomez‐Bautista et al., 2000; MacRae et al., 2005), and the depuration processes may actually spread contamination among shellfish Gómez‐Couso et al., 2003b). Genotyping‐positive samples indicated that oocysts were often C. parvum and linked to agricultural sources. Other Cryptosporidium species pathogenic to humans have also been detected in molluscan shellfish.
Vital dyes were used to assess viability in two studies in Europe. Viable oocysts were detected in 53% of mussel, oyster, clam and cockles samples from Galicia, north‐west Spain and other EU countries (Gómez‐Couso et al., 2003a), and in surface waters that enter the oyster harvesting areas in the Oosterschelde, the Netherlands (Schets et al., 2007).
Elsewhere, one study in the US reported that Cryptosporidium oocysts recovered from commercially harvested Chesapeake Bay oysters were infective for neonatal mice (Fayer et al., 1999). In another US study, a fluorescence in situ hybridisation assay for rRNA was used to determine that 83% of 265 oyster groups contained viable C. parvum oocysts. However, the authors concluded that the numbers of viable oocysts present may be too low to cause infection in healthy individuals (Graczyk et al., 2007).
Mouse infectivity has been demonstrated following experimental contamination of shellfish or tissue to shed more light on Cryptosporidium in molluscan shellfish (Fayer et al., 1997; Tamburrini and Pozio, 1999; Freire‐Santos et al., 2001). Although viability was shown to decline rapidly over the first four days in experimentally contaminated oysters (Ostrea edulis) and clams (Tapes decussatus), 15–25% oocysts remained infective to suckling mice at 31 days post‐contamination (Freire‐Santos et al., 2002). The possibility of transmission between co‐existing shellfish has been demonstrated (Gómez‐Couso et al., 2003b). One study demonstrated maintenance of C. parvum viability in shellfish for 7 days (Sutthikornchai et al., 2016). The study estimated that the greatest risk was from consumption within 72 h of contamination and that at least 3 days of depuration in clean seawater were required to remove oocysts from oysters.
3.1.4.5. Occurrence and survival of Cryptosporidium spp. in meat
When cured meat that had been processed during a drinking water outbreak in Sweden, only a single, putative oocyst was detected (Robertson and Huang, 2012). Oocysts were not detected on beef carcasses sampled at a commercial beef abattoir in Ireland (Moriarty et al., 2005a).
3.1.4.6. Concluding remarks on occurrence and survival of Cryptosporidium spp. in food
There are limited data on the occurrence and survival of Cryptosporidium on fresh produce, and different sampling frames and sample sizes have been used. In large surveys using methods compliant with or similar to ISO 18744, oocysts have been detected in up to 8% of samples. Indications are that oocysts remain viable under refrigerated and ambient storage conditions.
There are no data on the occurrence and survival of Cryptosporidium spp., in fruit juice or milk and dairy products in Europe, but a survey from Canada found Cryptosporidium DNA in raw apple juice.
The only structured, prospective survey of meat in Europe did not detect oocysts.
There are more data for molluscan shellfish indicating that a high proportion of samples may be contaminated and that depuration processes may fail to remove the oocysts. However, the low numbers of oocysts detected may reduce the likelihood of infection among consumers.
Faecal indicators are not reliable predictors of the presence or absence of Cryptosporidium spp.
3.1.5. Relative importance of food‐borne pathways
Cryptosporidium spp. are faecal–oral pathogens for which both humans and animals can act as major hosts. The transmission of human cryptosporidiosis involves both direct routes (person‐to‐person and animal‐to‐person) and indirect routes (through water, food and fomites contaminated with infectious oocysts). Table 3 provides an overview of the current level of application of different source attribution approaches for determining the relative importance of Cryptosporidium spp. food‐borne pathways. Technical details on the considered source attribution approaches are provided in Appendix E.
Table 3.
Different source attribution approaches for Cryptosporidium spp
| Source attribution approach | Comments |
|---|---|
| Epidemiological studies | Several case–control studies of cryptosporidiosis have been conducted, yet the main focus of these studies has been on water; food‐borne outbreaks are relatively rare and not frequently investigated |
| Subtyping | Limited applicability because no sufficiently discriminatory standardised multilocus genotyping scheme exists and because Cryptosporidium spp. are not routinely genotyped |
| Comparative risk assessment | Limited applications despite availability of occurrence data |
| Expert knowledge elicitation | To date the most frequently applied source attribution approach for Cryptosporidium spp. |
3.1.5.1. Epidemiological studies
Several epidemiological studies of cryptosporidiosis have been conducted, yet the main focus of these studies has been water as a risk factor. Majowicz et al. (2001) described a series of 157 sporadic cryptosporidiosis cases reported in Ontario, Canada, during 1996–1997. Waterborne transmission was the most commonly reported probable source of infection (48% of cases), followed by contact with livestock (21%), person‐to‐person contact (15%), food‐borne transmission (8%) and contact with pets (8%).
Case–control studies of sporadic infection in immunocompetent individuals in industrialised countries (the USA, the UK and Australia) did not identify specific foods as risk factors. (Robertson et al., 2002; Hunter et al., 2004).
Globally, food‐borne outbreaks of cryptosporidiosis are rarely reported in high‐income countries (Batz et al., 2012; Painter et al., 2013). In low‐income countries, they are rarely identified (Ryan et al., 2018). Data are therefore lacking to perform outbreak‐based source attribution. Nonetheless, an overview of 25 reported food‐borne outbreaks of cryptosporidiosis globally, showed that the most frequently implicated food source was fresh produce (n = 11) followed by unpasteurised milk and dairy products (n = 7) (Appendix B).
3.1.5.2. Subtyping
Few studies have compared the presence of Cryptosporidium species or genotypes in different animal sources and humans in the same geographical area (Xiao, 2010; Ryan et al., 2014).
Sequencing the Cryptosporidium gp60 gene from stools obtained from cases in outbreaks has revealed C. parvum genotypes suggestive of zoonotic sources in food‐borne outbreaks (Appendix B).
One study in the UK used gp60 sequence comparisons to estimate the proportion of indigenous human C. parvum cases derived directly from livestock and reported 25% for C. parvum and 10% of all reported Cryptosporidium cases in the UK (Chalmers et al., 2011).
However, to date, no sufficiently discriminatory multilocus genotyping scheme has been fully validated or widely adopted by veterinary or public health researchers (Hotchkiss et al., 2015) or reference laboratories in Europe, although an initiative as part of COST Action Euro‐FBP is addressing this issue (Chalmers and Cacciò, 2016). However, in most countries Cryptosporidium is not routinely genotyped, or even the infecting or contaminating species identified, and so point of reservoir source attribution is further hampered.
3.1.5.3. Comparative risk assessment
The use of a comparative risk assessment approach for Cryptosporidium source attribution is limited by a lack of occurrence data. Most Cryptosporidium risk assessment studies have focused on waterborne transmission (e.g., Murphy et al., 2016), although a few examined fresh produce (e.g., Shrestha et al., 2017). Grace et al. (2012) performed a risk assessment of human cryptosporidiosis associated with dairy farming in Kenya, showing that the major exposure pathway was through the consumption of raw and improperly cleaned vegetables.
3.1.5.4. Expert knowledge elicitation
National studies conducted in some North American and European countries have yielded similar food‐borne attribution proportions, ranging from 6% to 12% (Table 4). Other studies conducted in North America, considering only food‐borne transmission, applied similar food‐borne proportions (8–10%; Mead et al., 1999; Scallan et al., 2011; Ravel et al., 2010). Recently, the expert knowledge elicitation conducted by the Food‐borne Disease Burden Epidemiology Reference Group (FERG) in the context of the global burden of food‐borne disease study, yielded food‐borne attribution proportions in the European region that ranged from 9% to 11% (Hald et al., 2016) (Table 4). Waterborne transmission and person‐to‐person contact were estimated to be the dominant transmission routes (36–38% and 28–30%, respectively).
Table 4.
Examples of studies using expert knowledge elicitation for attribution of human cryptosporidiosis to main transmission routes
| Country | Food | Water | Person‐to‐person | Animal contact | Reference |
|---|---|---|---|---|---|
| EUR Aa | 10% (0–39) | 38% (3–70) | 30% (1–65) | 14% (0–44) | Hald et al. (2016) |
| EUR Bb | 11% (0–39) | 37% (2–68) | 28% (1–64) | 16% (0–46) | Hald et al. (2016) |
| EUR Cc | 9% (0–40) | 36% (5–70) | 29% (1–64) | 15% (0–48) | Hald et al. (2016) |
| Canada | 11% (1–37) | 37% (13–68) | 24% (5–61) | 23% (5–57) | Butler et al. (2015) |
| Greece | 6% (6–8) | N/Ad | N/A | N/A | Gkogka et al. (2011) |
| Netherlands | 12% (0–20) | 28% (10–39) | 27% (10–38) | 13% (5–19) | Havelaar et al. (2008)e |
EUR A: Andorra, Austria, Belgium, Croatia, Cyprus, Czechia, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Monaco, the Netherlands, Norway, Portugal, San Marino, Slovenia, Spain, Sweden, Switzerland, the United Kingdom.
EUR B: Albania, Armenia, Azerbaijan, Bosnia and Herzegovina, Bulgaria, Georgia, Kyrgyzstan, Montenegro, Poland, Romania, Serbia, Slovakia, Tajikistan, the former Yugoslav Republic of Macedonia, Turkey, Turkmenistan, Uzbekistan.
EUR C: Belarus, Estonia, Hungary, Kazakhstan, Latvia, Lithuania, Moldova, Russia, Ukraine.
N/A = not available.
Havelaar et al. (2008) also considered travel as an exposure route, with an attribution proportion of 20% (4–29).
Three national studies also provided expert knowledge elicitation estimates of the contribution of specific food groups to food‐borne cryptosporidiosis. Fresh produce emerged as the dominant source in Canada (Davidson et al., 2011) and the USA (Batz et al., 2012), but only as the third most important food source in the Netherlands, after beef and lamb, and (shell)fish (Havelaar et al., 2008). Within the context of the global burden of food‐borne disease study, vegetables were estimated to account for approximately 60% of food‐borne cryptosporidiosis cases across the European region, although the ranges were very wide, followed by the category ‘fruits and nuts’, which accounted for 20–30% of food‐borne cryptosporidiosis cases (Hoffmann et al., 2017) (Figure 2).
Figure 2.
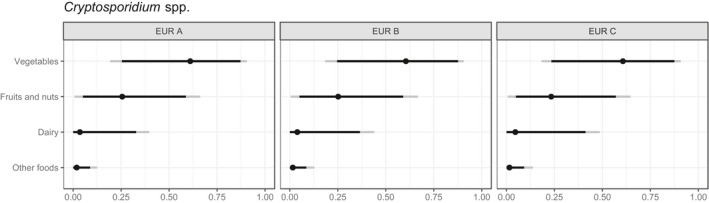
Expert knowledge elicitation estimations of the contribution of specific foods to the disease burden of Cryptosporidium spp. (Hoffmann et al., 2017)
- The dots represent the median estimate; the black line the 90% uncertainty interval; and the grey line the 95% uncertainty interval. EUR A: Andorra, Austria, Belgium, Croatia, Cyprus, Czechia, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Monaco, the Netherlands, Norway, Portugal, San Marino, Slovenia, Spain, Sweden, Switzerland, the United Kingdom; EUR B: Albania, Armenia, Azerbaijan, Bosnia and Herzegovina, Bulgaria, Georgia, Kyrgyzstan, Montenegro, Poland, Romania, Serbia, Slovakia, Tajikistan, the former Yugoslav Republic of Macedonia, Turkey, Turkmenistan, Uzbekistan; EUR C: Belarus, Estonia, Hungary, Kazakhstan, Latvia, Lithuania, Moldova, Russia, Ukraine.
3.1.5.5. Concluding remarks on relative importance of food‐borne transmission pathways
Information on the relative importance of food vs other major transmission pathways for human cryptosporidiosis currently results mainly from expert knowledge elicitation because of limitations with data‐driven approaches. Waterborne transmission, person‐to‐person contact and animal contact are consistently believed to be more important transmission pathways. However, the recent occurrence of very large, widespread outbreaks linked to the consumption of fresh produce in the UK and Finland have raised concerns about food safety. Available estimates suggest that about 10% of human cryptosporidiosis cases may be food‐borne.
From the data that are available, it is probably reasonable to suggest that the predominant baseline/background transmission pathway involves water (drinking water, especially from untreated or small and private supplies, and recreational water), then direct contact with an infected person (person‐to‐person spread) or livestock animal, then other fomites and lastly pets. Variations to this may occur within populations – for example, among young children in day care, person‐to‐person transmission and fomites may be more important. Where food‐borne transmission fits into this hierarchical list is uncertain. Food‐borne outbreaks have been shown to occur, and can be very large and widespread with high impact.
The data from national studies conducted in some North American and European countries, which considered outbreak data (Painter et al., 2013) or expert knowledge elicitations (Hoffmann et al., 2007; Davidson et al., 2011) indicate that food‐borne cryptosporidiosis is mainly associated with fresh produce. Large and widespread outbreaks have been reported linked to the consumption of salad leaves (McKerr et al., 2015; Åberg et al., 2015; Public Health England, 2017). A more recent study estimated the proportion of specific food‐borne diseases attributable to specific food exposure routes, globally, and concluded that contaminated vegetables accounted for somewhere in the range of 15% to 80% of cases of food‐borne cryptosporidiosis (Hoffmann et al., 2017), indicating a high level of uncertainty. Fresh produce has few identifiable controls for Cryptosporidium so is relatively more important than milk and dairy in countries where pasteurisation is commonplace. The importance of fruit juice has declined as controls have been implemented (e.g., pasteurisation).
To date, most estimates of the relative importance of food‐borne pathways have been generated through expert knowledge elicitations, resulting in important uncertainties. Data‐driven approaches have so far been rarely applied because of specific limitations. Specifically, subtyping approaches have shown limited applicability because there is no sufficiently discriminatory standardised multilocus genotyping scheme and because Cryptosporidium spp. are not generally routinely genotyped; comparative risk assessment studies have rarely been performed, despite the availability of occurrence data; and epidemiological studies have rarely focused on food.
3.1.6. Consumer practices contributing to infection
There are limited data about the contribution of consumer practices to Cryptosporidium infection.
Quantitative microbiological risk assessment (QMRA) has shown a high risk of infection from small and private water supplies, and there is a dose–response risk of infection from drinking contaminated water (Gualberto and Heller, 2006; Hunter et al., 2011; Murphy et al., 2016). Additionally, living in a rural area contributes to infection (Pollock et al., 2010). Occupational exposure may occur, for example, among farmworkers, veterinarians, and agricultural and veterinary students, people who work with young children or care for people with diarrhoea. Poor hygiene habits contribute to increased exposure, especially in young children, who also have a lack of immunity and gut immaturity. Recreational exposure occurs among people who visit open/petting farms, especially where hand hygiene is poor (Chalmers and Giles, 2010). Using recreational waters, such as swimming pools, presents a greater risk of infection for children (who tend to drink more pool water) than adults (Suppes et al., 2016). People who continue to use swimming pools when infected have contributed to the spread of cryptosporidiosis.
Changes in consumer habits, including eating outside of the home (food‐borne outbreaks have been reported at coffee shops/restaurants/canteens; Appendix B) and the consumption of more raw and undercooked foods, can certainly influence exposure to, and transmission of, Cryptosporidium spp. Cases of cryptosporidiosis are linked to travel abroad, the exposure being increased where contaminated water or food is consumed, sanitation is poor and where there is increased environmental exposure. The desire for year‐round fresh produce has increased the importation of foods from warmer climes but improved trace‐back is needed to understand where foods involved in outbreaks have come from. Drinking raw milk, or eating dairy products made from raw milk, presents an increased likelihood of exposure to infectious oocysts to consumers, as shown by outbreaks that have occurred (Appendix B). Eating fresh produce, even if it has been washed commercially, has caused widespread outbreaks (Appendix B). Handling food while infected with Cryptosporidium could contaminate items and expose others to infection.
3.1.7. Current control methods for food‐borne transmission and likely impacts
The food‐borne transmission of Cryptosporidium spp. to humans is via oocyst contamination, either directly from animal or human faeces or through the use of faecally contaminated water during production, processing or preparation. Currently, there are no mandatory Cryptosporidium spp. control methods at any of these stages, although the water used in food processing and preparation should be of potable quality.
3.1.7.1. Prevention of contamination
Primary production
Cryptosporidiosis is not only a disease of humans, but also an important disease of livestock and other animals; prevalence of infection can be as high as 100% in young ruminant livestock and large numbers of oocysts are shed in faeces (Santín, 2013). Preventing direct contamination of food therefore requires control of livestock and wild animals, animal and human defecation, and general hygiene measures as described in the Codex Alimentarius Codes of Hygienic Practice for specific food products4 and in the Guidelines on the application of general principles of food hygiene to the control of food‐borne parasites (2016).5
The prevention or reduction of infection among livestock will also reduce the risk of oocyst contamination from manure and slurry, and help to control environmental contamination and subsequently protect water sources.
The control of livestock cryptosporidiosis can be difficult because the oocysts are environmentally stable and resistant to most on‐farm disinfectants. The infective dose is low, high numbers of sporulated, infective oocysts are shed resulting in the rapid spread of infection, there are no vaccines available, and treatment options are limited (Thomson et al., 2017). Measures that reduce Cryptosporidium infection and oocyst shedding in livestock are mainly based on good hygiene and disinfection; for example, by preventing the build‐up of faeces, using steam cleaning or a hydrogen peroxide‐based disinfectant, and allowing surfaces and utensils to dry, providing good hygiene in birthing areas, ensuring adequate uptake of colostrum, low stocking density, using an all‐in all‐out policy, limiting contact between young stock and those of different ages, isolation of scouring animals, quarantine of replacement young stock, rodent control, biosecurity while changing bedding (Hotchkiss et al., 2015). Mass prophylaxis with halofuginone lactate has been reported to resolve persistent cryptosporidiosis successfully on cattle farms (Thomson et al., 2017). Insects may act as mechanical vectors and transfer Cryptosporidium oocysts from dung and slurry to food (Conn et al., 2007).
The EU Water Framework Directive6 requires optimal control of pathogens to protect drinking water supplies. In an extensive review, Kay (2012) identified the best on‐farm management practices for attenuating the transport of livestock‐derived pathogens, including Cryptosporidium, within catchments. These included:
the containment of farm buildings/feedlot sources by reducing the amounts of slurry/contaminated water and applying runoff interception/containment, moving water and feed troughs regularly to prevent a build‐up of excreta;
on‐farm treatment of contaminated water using ponds and constructed farm wetlands and vegetative treatment areas for feedlot runoff;
the control of livestock on farmland and direct voiding of faeces through stream bank fencing and bridging, minimising livestock congregation areas and soil poaching, exclusion from areas at times of high pollution risk, using woodchip corrals and vegetated and riparian buffer strips;
the control of slurry and manure application to land through sufficient slurry storage (ammonia; predation from other organisms), proper composting of manure (heat), location and timing of application and incorporation of manures in soil.
Soil matrix, hydrology, vegetation, and precipitation affect the transfer of oocysts from faeces (King and Monis, 2007). Kay (2012) additionally identified the best on‐farm management practices to attenuate pathogen concentrations within catchments, including grassed waterways (or ‘swales’), interception of track or hard standing runoff, and using in‐stream ponds. Mitigation of other sources of environmental contamination was also identified as important in catchments, including wastewater through enhanced treatment, combination effects of processes, and optimal operation, and the recreational use of catchments (such as the provision of toilets). In a study in Poland, a link was established between high numbers of cattle herds and the Cryptosporidium‐positivity of fresh produce (Rzeżutka et al., 2010). Significant intense rainfall events influenced the presence and persistence of Cryptosporidium oocysts in eastern oysters on Prince Edward Island, Canada (Aguirre et al., 2016).
Protection of water sources and supplies is addressed through adequate risk assessment, and the common occurrence of Cryptosporidium in catchments, its persistence, and resistance to chemical disinfection has led to the critical attention this parasite has received from the drinking water industry. The basic steps and implementation of the microbial risk assessment framework has been described (WHO, 2009).
The use of treated wastewater effluent, e.g., for irrigation of fresh produce, can lead to contamination and a risk of infection with Cryptosporidium (Chaudhry et al., 2017).
Even with some decay in the environment or water sources, viable oocysts can potentially reach and remain on food crops; the critical factors being temperature, desiccation/water activity, solar UV, salinity and biotic antagonism (King and Monis, 2007; Peng et al., 2008). The effects of temperature and water activity have been fairly well characterised and adapted for controls in some food items (see below) but solar UV, salinity and biotic antagonism need further investigation.
Processing
During processing, Cryptosporidium contamination may arise from poor quality water used in washing (for example of fresh produce) or dilution (for example, of fruit juice), or cross‐contamination between food items or water used in processes such as cooling. The water used to wash salad leaves destined for bagged packaging is a recirculating system that may promote the spread of oocysts. Dairy hygiene is important for the prevention of faecal contamination of milk. Meats may be exposed to contaminated water or cross contamination during curing processes (Robertson and Huang, 2012).
Retail and consumers
Hygienic standards and adequate sanitation should be maintained to prevent contamination from infected food handlers, or other infected people they may come into contact with (particularly during toileting or changing nappies) (Hunter et al., 2004).
3.1.7.2. Control
No specific Cryptosporidium testing or control measures are legally required for food. Some foods, such as certain fruits and vegetables, are consumed raw without a cooking or freezing step to kill oocysts and so controls that reduce the parasite hazard to an acceptable level during primary production are especially important.
Efficacy against Cryptosporidium is usually measured as a log‐reduction or percentage reduction in numbers (removal) or viability/infectivity.
There is a lack of hard surface disinfectants and sanitisers that are effective against Cryptosporidium. One study reported that 5 of 35 disinfectants and sanitisers were effective with relatively short contact times: ammonia 5% for 120 min or 50% for 30 min; formalin 10% for 120 min; hydrogen peroxide 3% for 30 min; ‘Exspor’ (a chlorine dioxide‐based disinfectant) working dilution 30 min and ‘Oo‐cide’ (an ammonia‐based disinfectant) 5% for 5 min (O'Donoghue, 1995).
Ozone (25°C, 1 ppm) renders 99.9% of oocysts non‐infective after 10 min (Korich et al., 1990).
Air drying, even at ambient temperatures, can be a useful control with only 5% of oocysts reported viable after 4 h on stainless steel (Deng and Cliver, 1999).
Food workers can reduce the risk of onward spread and contamination if they wash their hands with hot water and soap, and dry them thoroughly after going to the toilet, and before handling food. Hand sanitisers and alcohol gels are not effective against Cryptosporidium.
Control measures for fresh produce
If dropped fruit are used, they may be contaminated with faeces and thus introduce Cryptosporidium. If flies are not controlled, or prevented from landing on picked fruit, they may contaminate the product with faeces. However, although there may be some reduction in numbers, infective Cryptosporidium oocysts remained on experimentally contaminated apples even after washing (Macarisin et al., 2010a).
Furthermore, washing may fail to remove contaminating oocysts on leafy vegetables, where they have been shown to adhere to surfaces and become embedded in stomatal openings (Macarisin et al., 2010a,b). The survival of oocysts in chlorine baths (Duhain et al., 2012) also presents a risk of onward transmission through the recycling of washwater, which is standard industry practice. Viability is retained for weeks on stored produce under chilled conditions (Utaaker et al., 2017).
Control measures for fruit juice
Some of the controls for fresh produce, such as not using dropped fruit and the control of flies, are also relevant control measures for fruit juice (Fetene et al., 2011).
Preservation treatments for fruit juice include UV, ozone, organic acids, H2O2, flash pasteurisation and high hydrostatic pressure. The US FDA requires a 5‐log reduction in fruit juice; there is evidence that this can be achieved using low pressure UV C (e.g. 14.32 mJ/cm2) (Hanes et al., 2002), 0.025% H2O2 (Kniel et al., 2003), regular or flash pasteurisation (Harp et al., 1996; Deng and Cliver, 2001; Kniel et al., 2003), and high hydrostatic pressure, in excess of 30,000 to 45,000 psi (Slifko et al., 2000).
Control measures for milk and dairy
Pasteurisation is an effective control measure for Cryptosporidium in milk (Harp et al., 1996).
After hardening ice cream at −20°C for 24 h, no Cryptosporidium oocysts remained viable, as determined by vital dyes (Deng and Cliver, 1999).
Control measures for molluscan shellfish
Depuration is a key process in shellfish processing, and one study demonstrated that at least 3 days of depuration in clean sea water was required to remove oocysts from oysters (Sutthikornchai et al., 2016), although oocysts have been detected in clams mussels and oysters long after this time period (Freire‐Santos et al., 2000). The efficacy of depuration may be influenced by shellfish species, oocyst load, dissolved oxygen level, water flow, salinity, and temperature (Robertson, 2007).
In 2015, EFSA published an evaluation of alternative heat treatments that could be applied for the control of pathogens in unpurified shellfish produced in lower category production areas (EFSA BIOHAZ Panel, 2015). These alternative processes equivalent to 90°C for 90 s are likely to kill Cryptosporidium.
Control measures for meat
Cryptosporidium might be controlled on cured meats by the salinity and low water activity, but these are assumptions from other investigations as there have been no viability or infectivity studies of cured meat (Robertson and Huang, 2012).
One study investigated the survival of oocysts on beef muscle and reported that heating at 60°C for 45 s and at 75°C for 20 s rendered oocysts non‐infective, indicating that adequate cooking is an effective control measure (Moriarty et al., 2005b). The same study also reported that other controls were also appropriate for Cryptosporidium: washing beef carcasses in water at 85°C (recommended for the decontamination of bacterial pathogens following processing); and using a steam vacuum temperature of at least 82°C; additionally, commercial freezing substantially reduced the proportion of viable oocysts (McEvoy et al., 2004; Moriarty et al., 2005b).
3.1.7.3. Concluding remarks on control measures
Control of Cryptosporidium oocysts as faecal contaminants of food and water will decrease the likelihood of transmission to susceptible hosts. Contamination could be reduced in the field, right through to consumption, through minimising access by animals, providing sanitation and hand hygiene for food workers, and using potable water for irrigation and other processes where there is contact between fresh produce and water. There are few specific treatment or preservation controls for Cryptosporidium in the food chain; heat treatment (pasteurisation, cooking) and freezing at −80°C (−20°C is not sufficiently reliable) may be appropriate for some foods, but for fresh, or minimally treated foods, and those intended to be eaten raw, such as salads and herbs, minimising contamination in the first place will probably be more effective.
To enable sufficiently robust detection of contamination, assuming adequate sampling, and more meaningful survey results, methods for detection need further verification and development to document recovery rates and LOD. The emphasis here should be on the presence or absence, as quantification may be difficult, contamination unevenly distributed, and even small numbers of oocysts may present a risk to consumers. However, quantification data for QMRA are needed. Pinpointing and evaluating control measures would be further improved by identification of contaminating Cryptosporidium species (to indicate the source or origin of contamination) and assessment of viability and infectivity. Improved and standardised methods would contribute not only to occurrence/removal studies, but also to survival studies, allowing robust evaluation of food preservation techniques and control measures aimed at killing the parasite.
3.2. Toxoplasma gondii
3.2.1. Characteristics, including relevant food vehicles for transmission
Toxoplasma gondii is an intracellular protozoan parasite that, like Cryptosporidium, belongs in the phylum Apicomplexa. The parasite has a heterogeneous lifecycle; its sexual multiplication takes place only in Felidae, the definitive hosts; asexual multiplication takes place in possibly all warm‐blooded animals, the intermediate hosts. T. gondii has three different infectious stages: tachyzoites, bradyzoites in tissue cysts and sporozoites in oocysts. T. gondii is considered the most prevalent parasitic zoonotic infection globally (Dubey, 2010), with infection leading to a range of diseases in humans and animals. Studies conducted in animals have shown that cats can be infected with as few as one bradyzoite (Cornelissen et al., 2014) and mice can be infected with only one oocyst (Dubey, 2010). Pathogenesis studies in sheep have shown that 200 oocysts can cause infection and induce protective immunity (McColgan et al., 1988). However, there are no comparable studies to determine the dose–response for human infection.
T. gondii infection often remains asymptomatic in humans, depending on the strain (see later), but if primary infection is acquired during pregnancy, it can cause serious health problems in the fetus and may lead to abortion, stillbirth, or, in live‐born children, chorioretinitis, hydrocephalus or microcephalus, and intracerebral calcifications. Infected children who are healthy at birth may develop chorioretinitis later in life. In immunocompromised hosts with T‐cell defects, such as patients with haematologic malignancies, organ transplant recipients, AIDS patients, and patients receiving immunosuppressive therapy, toxoplasmosis may manifest as potentially fatal encephalitis, pneumonitis, and myocarditis. T. gondii infection in immunocompetent individuals has long been perceived as harmless (depending on the strain of the parasite), as the acute phase of infection usually passes asymptomatically or with symptoms limited to mild fever‐like symptoms (Opsteegh et al., 2015). However, it is now recognised that ocular toxoplasmosis is not necessarily only a (late) sequela of a congenital infection, but may result from postnatally acquired infection (Weiss and Dubey, 2009). It has been estimated that at least two‐thirds of human ocular toxoplasmosis cases in Europe may have acquired the infection postnatally (Gilbert and Stanford, 2000). Moreover, a series of fatal cases of T. gondii infection in immunocompetent individuals was reported from French Guiana (Carme et al., 2009), demonstrating that some strains may be associated with more severe symptoms of acquired toxoplasmosis. In addition, T. gondii infection has been associated with various behavioural changes, including schizophrenia (Yolken et al., 2009), but a causal relationship has not yet been established.
Disease‐burden estimates due to T. gondii infections in various countries have demonstrated the overall high public health impact of toxoplasmosis. T. gondii was the pathogen with the highest disease burden, at both the population and individual levels, based on a calculation of DALYs in the Netherlands (Havelaar et al., 2012), but dropped to third place based on new estimates made for 14 food‐borne diseases in 2016 (Mangen et al., 2017) (dropping from 3,520 to 1,903 DALYs per year), using the newly available European disability weights (Haagsma et al., 2015). T. gondii ranked third in Europe and contributed 17.6% to the total burden of food‐borne diseases in Europe in 2010 (Havelaar et al., 2015). Toxoplasmosis also ranks highly regarding the disease burden in the USA (Batz et al., 2012) and worldwide (Torgerson and Mastroiacovo, 2013).
T. gondii strains vary in virulence for mice, and the introduction of molecular typing methods has shown that mice‐virulent strains could be linked to a single clonal genotype, whereas less virulent T. gondii strains were genetically more diverse. Most human and livestock strains belonged to three clonal lineages (type I, II and III). In Europe, type II strains dominate in humans and animals (Ajzenberg et al., 2005), whereas T. gondii strains from South America seem to be more diverse and linked to greater virulence in humans (Frazão‐Teixeira et al., 2011). Moreover, in French Guiana, severe and fatal cases of respiratory syndromes have been described in immunocompetent human cases caused by a T. gondii (Demar et al., 2007). The highly virulent atypical strains, although not circulating in Europe yet – as far as we know – have also been the cause of congenital and fatal cases in Europe. One of these European patients consumed raw horse meat imported from South America (Pomares et al., 2011). The import of non‐frozen, vacuum‐packed fresh meat products of beef and horse from South America to Europe could be considered to be an emerging threat for public health, because beef and horse meat are sometimes consumed undercooked or even raw, and more virulent strains of T. gondii could be introduced to Europe this way.
Food‐borne transmission is considered to be the main mode for transmission of T. gondii to humans. Tissue cysts and tachyzoites of T. gondii are responsible for infections via meat and milk, respectively (Jones and Dubey, 2010), and sporulated oocysts in the environment can contaminate fresh produce, shellfish, and water (Dubey, 2010; Opsteegh et al., 2014) and infect humans after consumption (Figure 3).
Figure 3.
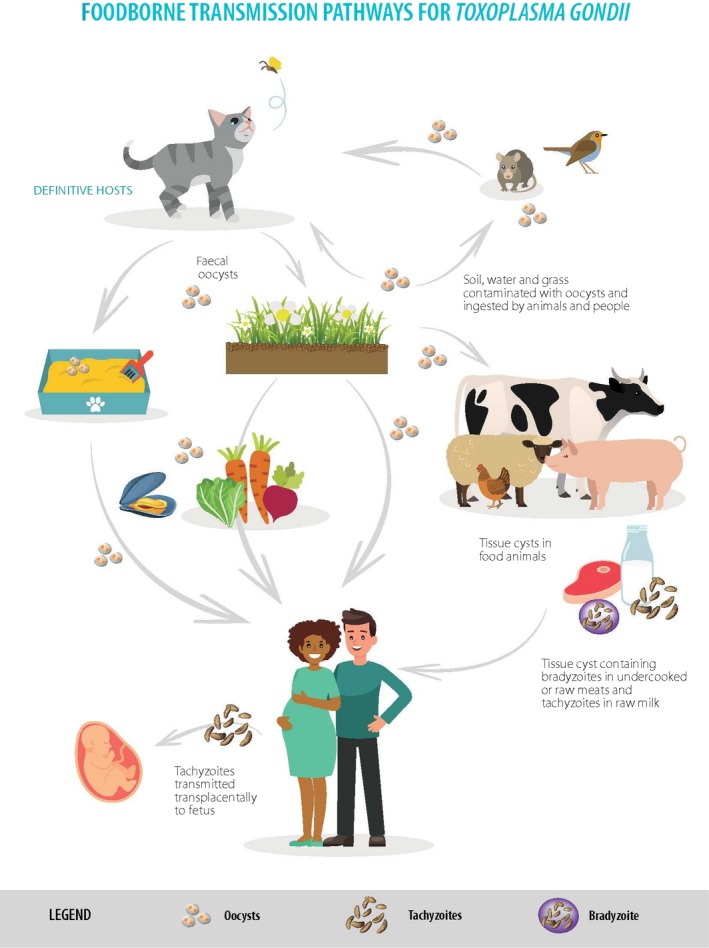
Food‐borne transmission pathways for Toxoplasma gondii
3.2.2. Methods of detection in food
There are several methods available to detect T. gondii tachyzoites, bradyzoites, and oocysts in or on food products. Most of these methods involve an isolation and concentration stage prior to applying the direct detection methods on the test samples. Molecular‐based assays are very commonly used to show the presence of T. gondii DNA in samples, whereas information on viability and infectivity can be determined using mouse or cat bioassays and in vitro culture methods. Table 5 contains a summary overview of the main detection methods (direct and indirect) used in food products, with further information on these assays given in Appendix F and in the specific sections below.
Table 5.
Summary overview of the main detection methods for Toxoplasma gondii in food products
| Detection Method | Type of food | Direct/indirect | Demonstration of viability/infectivity | Comments |
|---|---|---|---|---|
| Cat bioassay | Meat, shellfish, milk products | Direct | Yes | Seronegative cats fed test samples of food and their faeces checked for oocysts, and blood for seroconversion. Cats can be fed large quantities of food |
| Mouse bioassay | Meat, shellfish, milk products | Direct | Yes | Homogenates of food samples are inoculated into mice followed by clinical monitoring and demonstration of T. gondii in body tissues and seroconversion |
| PCR | Meat, fresh produce, shellfish and milk products | Direct | No | The B1 gene and the 529 bp repeat element are the most common targets. Various systems are used; conventional, nested and real‐time PCR. A magnetic capture‐based PCR technique detects 1 tissue cyst in 100 g of meat. In addition, sporulated oocysts can be identified using RT‐PCR |
| Loop‐mediated isothermal amplification (LAMP) | Meat, fresh produce | Direct | No | Unlike PCR, amplification products from LAMP cannot be sequenced. Recent adaptation to a lateral‐flow dipstick method for rapid results |
| Microscopy | Meat, fresh produce | Direct | No | Detection based on morphology and staining using specific conjugated antibodies. Limited sensitivity for direct use on food samples, but useful to confirm infection on mouse and cat bioassays. Technique is labour intensive and requires an experienced technician |
| In vitro culture | Liquid samples where tachyzoites or bradyzoites may be present, e.g. meat homogenates or milk samples | Direct | Yes | Tachyzoites and bradyzoites (tissue cysts) may be cultured in a wide variety of cell lines with vero cells being commonly used. In vitro cultures are mostly used to prepare antigen or for strain isolation after bioassays. Not common to use directly on food samples |
| Specific antibodies | Liquid samples from meat juices where antibodies may be present. Blood samples from food animals | Indirect | No | The detection of specific antibodies in food animals confirms the animal has been infected with T. gondii and has had an immune response to the parasite. The correlation of seropositivity and the presence of tissue cysts vary according to different livestock species |
3.2.2.1. Detection methods in fresh produce
T. gondii oocysts shed by the definitive hosts, felines, are spread in the environment. Food matrices that are exposed to the environment (e.g., fresh produce) can be contaminated by oocysts and these foods are another source of human infections. A few studies have linked acute human toxoplasmosis outbreaks to the ingestion of oocysts (Teutsch et al., 1979; Stagno et al., 1980; Benenson et al., 1982; Bowie et al., 1997; Ekman et al., 2012), and one of them identified green vegetables as the probable infection vehicle (Ekman et al., 2012).
Detection of T. gondii oocysts in food and environmental samples is very challenging because of difficulties in separating and concentrating them from complex matrices, such as raw vegetables. An immunomagnetic separation assay (IMS Toxo) targeting the cell wall of T. gondii oocysts has been developed by Hohweyer et al. (2016) but is not commercially available. Based on the methods used for other protozoa, possible methods for T. gondii detection in water, soil and food (fruit and vegetables) have been proposed by Dumètre and Dardé (2003). In addition to conventional microscopy, PCR or quantitative PCR methods, a LAMP assay has been developed and used to detect T. gondii in experimentally contaminated ready‐to‐eat baby lettuce. The detection limit of this method was approximately 25 oocysts per 50 g of lettuce leaves (Lalle et al., 2018).
To date, there are no standard detection methods for T. gondii oocysts in fresh produce.
3.2.2.2. Detection methods in milk and dairy products
Tachyzoites of T. gondii can be shed in the milk of acutely infected animals; thus, raw milk and raw milk products may pose a risk of infection to consumers.
The main detection methods used on raw milk samples involve the detection of T. gondii DNA using PCR‐based tests, most commonly targeting the 529 bp repeat element (Bezerra et al., 2015; de Santana Rocha et al., 2015; da Silva et al., 2015; Vismarra et al., 2017) or the B1 gene (Dehkordi et al., 2013).
Detection of T. gondii DNA using PCR‐based methods does not provide evidence of parasite viability, and hence risk of infection following consumption of the milk products. Other techniques have been used to investigate parasite viability in milk and dairy products, including: cell culture viability assay measuring the cytopathic effect of T. gondii tachyzoites on HEp‐2 cells (Koethe et al., 2017); mouse bioassay where mice are inoculated with samples and then checked for seroconversion and or presence of parasites; and cat bioassay where cats are fed samples of milk and or cheese and then their faeces tested for the presence of oocysts (Dehkordi et al., 2013; Dubey et al., 2014).
A commercial ELISA test has been used to evaluate the presence of T. gondii antibodies in bulk milk samples from goats. The analysis showed that milk samples were a useful alternative to serological tests and could be more easily applied to look for seroprevalence of T. gondii (Gazzonis et al., 2018), but were less useful for detecting T. gondii tachyzoites shed in milk.
3.2.2.3. Detection methods in molluscan shellfish
Molluscan shellfish, including clams, mussels, oysters and scallops, are filter feeders that trap phytoplankton in their gills. The process of filter feeding may also result in the concentration of waterborne pathogens such as T. gondii oocysts that can survive for long periods of time in both fresh and salt water (Lindsay and Dubey, 2009). Consumption of T. gondii oocyst‐contaminated, undercooked, molluscan shellfish may pose a risk to consumers.
Samples of whole tissues or organs (e.g., gills or mantle), may be used to sample the bivalves, and pools of individual samples are often used for the analysis. DNA is extracted from homogenised tissues and used for molecular detection of T. gondii DNA using PCR‐based tests, with the majority of investigations targeting the B1 gene (Putignani et al., 2011; Aksoy et al., 2014; Marquis et al., 2015; Cong et al., 2017; Ghozzi et al., 2017). There is little information concerning whether it is better to sample particular organs or use whole tissue; therefore, it may be best to recommend sampling of whole tissue. It would also be helpful to have better validation data for the techniques employed, to investigate recovery rates and limits of detection, and to harmonise the tissue samples being tested. A recent paper described the first report of the presence of T. gondii sporulated oocysts in samples taken from commercially sourced green lipped mussels (Perna canaliculus) (Coupe et al., 2018). T. gondii sporozoite‐specific mRNA was detected using a reverse transcriptase‐PCR (RT‐PCR) assay targeting a 71‐bp fragment of the SporoSAG gene. Detection of sporulated oocysts in this context is important as, if the oocysts detected are sporulated, they can be infectious for other hosts.
3.2.2.4. Detection methods in meat
T. gondii tissue cysts in meat are an important source of human infection. Different techniques are available for detecting the presence of T. gondii tissue cysts. Depending on the characteristics of the method (e.g., discrimination between viable and non‐viable parasites), and the performance (i.e., sensitivity and specificity) of the method, the results obtained with different methods should be evaluated differently. Moreover, these methods are not suitable for routine testing.
Mouse bioassay and PCR are the most commonly used direct detection methods, followed by microscopy and cat bioassay (Opsteegh et al., 2016a). Direct detection methods can be used to detect T. gondii in various food samples (meat, milk, fresh produce, oysters, water), although validation of methods for food other than meat has rarely been described.
3.2.2.5. Infectivity and viability assessment of Toxoplasma gondii in food
The infectivity and viability of T. gondii in food can be assessed by bioassays (cat or mouse), or, in principle, by in vitro culture techniques (Appendix F). Bioassays are a sensitive technique to determine the infectivity of T. gondii, although there are ethical concerns with using animals for these tests. Swiss‐Webster and severe combined immunodeficiency (SCID) mice are most commonly used to assess the infectivity and viability of T. gondii, with the mice monitored for seroconversion and for the presence of the parasite in their tissues.
Cell culture methods are another alternative to assess T. gondii viability. Microscopic examination, looking for changes in oocysts morphology and evidence of intact sporozoites, may also be applied to assess the viability of individual oocysts.
Excystation techniques, where the T. gondii oocyst wall is opened in vitro using sonication or bead beating and then the sporocyst wall disrupted by incubation in a digestive solution containing bile salts to release the sporozoites, may be used to assess viability (Rousseau et al., 2018).
Moreover, tests for the viability and virulence of recovered oocysts must be developed. A recent RT‐PCR assay has been developed that targets the sporozoite surface marker (SporoSAG) indicating that the oocysts were sporulated and therefore capable of infecting other hosts (Coupe et al., 2018).
3.2.2.6. Concluding remarks on detection methods
The most commonly used detection methods for meat are mouse bioassay, followed by cat bioassay and PCR‐based methods (Opsteegh et al., 2016a). The bioassays have the advantage that they can detect viable and infective T. gondii in contrast to PCR‐based methods, but have the disadvantage that they require the use of experimental animals and are expensive. In 2010, a more sensitive PCR‐based method was published that can detect one tissue cyst in 100 g of meat (Opsteegh et al., 2010). In contrast to meat, only limited detection methods are available for fresh produce, milk, and other food products, and these have not been validated. Indirect assays, based on the detection of T. gondii‐specific antibodies, are only applicable to animals and not food products.
3.2.3. Occurrence and survival of T. gondii in food
3.2.3.1. Occurrence and survival of T. gondii in fresh produce
In Europe, Lass et al. (2012) reported the presence of T. gondii on vegetables from shops and home gardens in Poland with a contamination rate of 9.7%. In this study, the fresh produce was washed and the eluate concentrated using a flocculation method. Afterwards, real‐time PCR targeting the B1 gene was used for specific T. gondii detection. Caradonna et al. (2017) investigated the prevalence of T. gondii in ready‐to‐eat packaged mixed salads by microscopy examination and PCR detection. Although Toxoplasma oocysts were not detected by microscopy, PCR results revealed that 0.8% of pooled ready‐to‐eat salads were T. gondii positive, and a high oocyst burden was found in those pooled samples (ranging from 62 to 554 per gram of vegetable, based on extrapolation from a qPCR standard curve). In these studies, the general approach of recovery and detection method followed three steps: (i) wash the samples; (ii) concentrate the parasites (e.g., filtration and centrifugation); and (iii) PCR detection or microscopy examination.
In conclusion, although only a few studies have investigated the occurrence of T. gondii in fresh produce, the parasite has been detected on vegetables and fruit. More research is needed to evaluate the recovery of oocysts (de Souza et al., 2016) and the sensitivity and specificity of the detection methods.
Survival in fresh produce
Sporulated oocysts are very resistant to environmental conditions, including freezing (28 days in water at −21°C), and can survive for up to 54 months in cold water and up to 18 months after deposition in soil (Jones and Dubey, 2010), indicating that oocysts can also survive for long periods on fresh produce.
3.2.3.2. Occurrence and survival of T. gondii in milk and dairy products
Consumption of raw milk, whey and fresh cheese prepared from animals infected with T. gondii may provide a route for transmission to people (Dubey et al., 2014; Boughattas, 2017), and consumption of raw milk from infected goats has been linked to human infection and disease (Sacks et al., 1982; Chiari and Neves, 1984).
The stage of the parasite likely to be present in milk is the tachyzoite, which is directly shed in the milk and comparatively fragile compared with the other lifecycle stages. T. gondii tachyzoites are not thought to survive pasteurisation (Dubey, 2010) and would also be vulnerable to the low pH in gastric secretions (Pocock et al., 2013).
T. gondii has been detected in raw milk from infected animals using a variety of techniques, mainly using PCR, tissue culture, and in vivo bioassays (Dehkordi et al., 2013). A recent study sampled 21 milk samples taken from three different sheep farms in southern Italy and found one milk sample to be positive using a PCR assay (Vismarra et al., 2017). A previous study in Italy looking at goat milk found 13% of 77 samples to be positive using a T. gondii‐specific PCR test (Mancianti et al., 2013). Milk samples collected from 80 ewes 1 month after term in Slovakia were tested using a T. gondii‐specific PCR and 9 samples were found to be positive (Luptakova et al., 2015). T. gondii DNA was detected in 43–65% of milk samples collected from goats (n = 60) in southwest Poland (Sroka et al., 2017). Lower detection levels were reported in another study in eastern Poland where T. gondii DNA was detected in raw milk samples from 10 cattle (n = 63), 1 sheep (n = 27) and 1 goat (n = 29) (Cisak et al., 2017).
Survival in milk and dairy products
Detection of T. gondii using molecular techniques will confirm the presence of specific DNA and this is the technique reported in the majority of studies looking at the occurrence of T. gondii in milk. Tissue culture or in vivo bioassay can be used to determine the viability and infectivity of the parasite and therefore the potential risk to consumers. Although tachyzoites are considered to be relatively fragile compared with other transmission stages, a recent study (Koethe et al., 2017), showed that T. gondii tachyzoites were able to survive for at least 1 h in gastric fluids that were mixed with different volumes of experimentally spiked cow's milk samples. The mixture of milk and gastric fluids increased the overall pH, enabling survival of the tachyzoites, potentially long enough to enable passage through the stomach and gain entry to the intestine where they could infect the host.
Further studies have looked at the survival of T. gondii tachyzoites in spiked milk samples and shown that tachyzoites could remain viable for several days (Walsh et al., 1999; Kalani et al., 2016). Milk samples collected from experimentally infected goats were found to contain viable T. gondii using bioassays. Similar results were obtained for fresh cheese made from this milk by cold enzyme treatment (Dubey et al., 2014).
3.2.3.3. Occurrence and survival of T. gondii in molluscan shellfish
Oocysts shed from the faeces of infected cats can be washed into fresh water and, thus, to the sea. Due to the ability of molluscan shellfish, such as clams, oysters, mussels, scallops and cockles, to filter large volumes of water (10–15 L/h) they can concentrate oocysts within their tissues.
Consumption of raw shellfish products was recognised as a risk factor for T. gondii infection in the USA (Jones et al., 2009). A survey of Mediterranean mussels (Mytilus galloprovincialis) collected from eight different sites on the west coast of Turkey found 9.4% (n = 795) to be positive for T. gondii using a PCR assay (Aksoy et al., 2014). A study looking at the presence of T. gondii in a range of different farmed shellfish in Italy (Putignani et al., 2011) found the presence of positive DNA samples using a nested PCR assay and a fluorescent amplicon generation real‐time PCR assay using the B1 target in 17% of Crassostrea gigas and 4% of Tapes decussates.
Survival in molluscan shellfish
The oocyst stage of T. gondii has a very tough outer shell and can survive for long periods of time in the environment outside the host. T. gondii oocysts can survive and remain viable at 4°C in fresh water for up to 54 months (Dubey, 1998) and in seawater at for 24 months (Lindsay and Dubey, 2009).
T. gondii oocysts were found to be viable in eastern oysters (Crassostrea virginica) for up to 85 days (Lindsay et al., 2001) and in mussels (Mytilus galloprovincialis) for 3 days (Arkush et al., 2003). T. gondii DNA was found in California mussels (Mytilus californicus) near to where cases of toxoplasmosis had been identified in Californian sea otters (Miller et al., 2008).
A recent study looked at the uptake of T. gondii oocysts by migratory feeding fish such as northern anchovies (Engraulis mordax) and Pacific sardines (Sardinops sagax), and found that in controlled experimental conditions, both species of fish were able to filter T. gondii oocysts out of seawater with the parasite persisting inside the fish alimentary canals (Massie et al., 2010). Therefore, these fish could help to transmit this parasite from near shore to pelagic marine environments.
3.2.3.4. Occurrence and survival of T. gondii in meat
The main livestock species, such as cattle, small ruminants, pigs, poultry and horses, are sources for meatborne toxoplasmosis. The prevalence of Toxoplasma in meat‐producing animals can therefore be an indication of the risk to humans. Many studies (Tenter et al., 2000) use indirect detection methods (serology) to estimate the seroprevalence to T. gondii, but serology can only be used to estimate risk of human infection if there is a correlation between seroprevalence and the presence of tissue cysts in meat. Opsteegh et al. (2016a) assessed this correlation for the main livestock species in an extensive literature review. The probability of detecting parasites in seropositive animals was highest for pigs (58.8%), followed by chickens (53.4%), sheep and goats (39.4% and 35.0%), and was lowest in horses and cattle. These data suggest that the correlation between the detection of antibodies to T. gondii and direct detection of the parasite is high in pigs, small ruminants, and chickens. For these species, the use of serology can help to identify a risk to the consumer, but serology may not be so useful with other animal species, such as horses and cattle. Moreover, due to the occurrence of serological non‐responders, with tissue cysts were also described in sero‐negative pigs (4.9%), sheep and goats (1.8% and 2.0%), and chickens (1.8%), a seronegative result does not necessarily mean that the meat is free of T. gondii. Therefore, serology cannot be used for individual carcass control in pigs, chickens and small ruminants.
The similar rates of detection of T. gondii in seropositive cattle (3.6%) and horses (8.8–13.8%) and seronegative cattle (2.4%) and horses (2.4–32.0%) implies that, for these species, detection of antibodies does not reflect the public health risk. From a public health perspective, the lack of information on the prevalence of T. gondii tissue cysts in horses and cattle was an important data gap as beef is a major meat source in many European countries and horse meat in some (e.g. France and Italy). Furthermore, beef and horse meat are more often consumed undercooked or raw than pork or poultry (Opsteegh et al., 2016a).
This information is of importance to evaluate the studies using serology to detect T. gondii in meat samples. Many serological studies have been published (e.g. reviewed by Tenter et al., 2000) and seroprevalence can rank between a few to more than 80% in pigs and small ruminants, depending on the husbandry system. This indicates that pork and mutton are important meat sources of Toxoplasma infection for humans. In the Netherlands, a quantitative risk assessment was performed for meatborne toxoplasmosis, and this revealed that beef (rather than pork or mutton) contributed to 67% of the predicted human cases. Although the prevalence of Toxoplasma in cattle was only 2%, as detected by magnetic capture (MC)‐PCR using 100 g of heart, the amount of beef eaten ‐and eaten raw ‐was much higher than that of mutton, according to food consumption data for the Dutch population. Moreover, pork in the Netherlands mostly originated from farms with controlled housing and is usually eaten well cooked (Opsteegh et al., 2011). The viability of bradyzoites in PCR‐positive cattle is of relevance, but Opsteegh et al. (2016b) confirmed that 1.6% of cattle (6/385) were bioassay positive, indicating the presence of viable tissue cysts and thus a potential risk to consumers.
Survival in animals and meat
In the intermediate host, little information is available about the reactivation of tissue cysts. Intact tissue cysts probably persist for the life of the host (Dubey, 2010) and in livestock animals this means that tissue cysts can survive until slaughter. Opsteegh et al. (2016a,b) showed that viable T. gondii were detected by bioassay in slaughtered pigs, laying hens, cattle and horses.
3.2.3.5. Concluding remarks on occurrence and survival of T. gondii in food
T. gondii can infect all warm‐blooded animals and the parasite will persist within tissue cysts, in most animals, for the lifetime of the host. Meat‐producing animals may harbour T. gondii cysts in their tissues and can pose a risk to consumers of rare and undercooked meat from those animals. Assessing the infection risk from meat‐producing animals involves the application of direct detection methods using molecular‐based diagnostics and the use of bioassays on samples of the tissues. The prevalence of exposure to T. gondii in livestock species can be determined indirectly through assessment of T. gondii‐specific antibodies in blood samples. The correlation between detection of specific antibodies to T. gondii and direct detection of the parasite in tissues is variable between different food‐animal species and thus, may not always be a useful indicator of consumer risk. Consumer preferences for consumption of rare meat from particular animal species (e.g., cattle) will increase the public health risk from this animal species compared with other food‐animal species, such as chicken or pork that are not usually consumed rare. Husbandry practices will also influence the likelihood of T. gondii infection in food animals; e.g. pigs reared outdoors are more likely to come into contact with T. gondii than those reared in controlled housing. Viable tissue cysts of T. gondii can be found at slaughter in tissues from T. gondii‐infected meat‐producing animals. T. gondii tissue cysts in meat can be inactivated though cooking (> 66°C) or by freezing (< −12°C).
T. gondii oocysts have a very tough outer shell wall and can survive for long periods in the environment outside the host, including in water (54 months) and in the soil (18 months). Molecular diagnostics have been used to show the occurrence of T. gondii as a faecal contaminant of fresh produce and also in the tissues of molluscan shellfish and migratory fish that filter water containing T. gondii oocysts. T. gondii oocysts can remain viable in the tissues of eastern oysters (Crassostrea virginica) for up to 85 days.
Raw milk, whey and fresh cheese made from animals infected with T. gondii may act as a vehicle of transmission through tachyzoites shed in the milk. The majority of studies in this area have shown the presence of T. gondii in raw milk samples from infected goats and sheep, although T. gondii has also been found in cows’ milk. Studies looking at the survival of T. gondii in milk have shown that the parasite can remain viable for several days. T. gondii tachyzoites in spiked milk samples remained viable for at least 1 h in gastric fluids, thus enabling them to survive long enough to pass through the stomach and into the intestine where they could establish infection. Pasteurisation or ultraheat treatment will inactivate T. gondii tachyzoites and therefore these types of dairy products will be unlikely to be transmission vehicles for T. gondii.
3.2.4. Relative importance of food‐borne pathways
The complex lifecycle of T. gondii results in specific transmission characteristics. The parasite has different infectious stages that have different pathways; i.e. bradyzoites transmitted via meat, oocysts transmitted via environmental contamination (including fresh produce), and tachyzoites transmitted via milk consumption, congenital transmission, and, to a lesser extent, blood donation. Any warm‐blooded animal can be a reservoir for the parasite, and even molluscan shellfish may pose a risk to consumers as mechanical vectors.
Table 6 provides an overview of different methods to perform T. gondii source attribution.
Table 6.
Different source attribution approaches for Toxoplasma gondii
| Source attribution approach | Comments |
|---|---|
| Epidemiological studies | Several case–control studies on toxoplasmosis have been conducted; outbreaks are relatively rare or, at least, rarely noticed and documented |
| Subtyping | So far, subtyping methods have shown that there is insufficient genetic heterogeneity in Europe for using subtyping to discriminate infection routes. This might change when more discriminative methods are used, as was recently demonstrated in France |
| Comparative risk assessment | Several comparative risk assessments provided evidence on the relative contribution of different meat products, but did not enable a definition of the relative contribution of meatborne toxoplasmosis vs other transmission routes |
| Expert knowledge elicitation | To date, the most frequently applied approach for attributing T. gondii to major transmission routes |
In addition to the methods summarised in Table 6, a discriminative serological method has been developed that, in the acute phase, can determine whether an infection has been due to sporozoites from T. gondii oocysts, and can therefore distinguish between human infections transmitted from oocysts in the environment and meatborne infections. The methods have not been successfully implemented in epidemiological studies in Europe; only in North and South America (Boyer et al., 2011; Hill et al., 2011; Robert‐Gangneux and Dardé, 2012).
3.2.4.1. Epidemiological studies
Due to the public health relevance of the parasite, extensive epidemiological research on T. gondii has been conducted in recent decades (Petersen et al., 2010). In a systematic review and meta‐analysis of 11 case–control studies, Belluco et al. (2017) identified three risk factors significantly associated with acute T. gondii infection in humans; consumption of raw/undercooked meat, raw/undercooked beef and raw/undercooked sheep meat. Consumption of raw/undercooked pork, raw eggs and unpasteurised milk proved to be non‐significant risk factors. In England and Wales, Said et al. (2017) found a significant association between beef consumption and acute toxoplasmosis. In the USA, Jones et al. (2009) found exposure to certain raw or undercooked foods (raw ground beef; rare lamb; locally produced cured, dried, or smoked meat; and unpasteurised goats’ milk) and exposure to kittens to be significant risk factors for T. gondii infection. Studies that also include the prevalence of exposure to food products can be used to extrapolate to the population‐attributable fraction, as reported by Cook et al. (2000). In this European case–control study, depending on the centre, 30–63% of new infections in pregnant women were attributed to meat and 6–17% to soil.
T. gondii outbreaks are rare, or, at least, rarely noticed and documented, because acute illness often involves aspecific clinical signs such as fever, headache, and enlarged lymph nodes, although more T. gondii‐specific clinical signs may be observed later, such as retinochoroiditis (Batz et al., 2012; Painter et al., 2013). Documented outbreaks are mostly waterborne because this transmission route will probably result in a larger and more easily identified outbreak. One of the best documented outbreaks occurred among more than 100 individuals in British Columbia, Canada, and was associated with municipal drinking water (Bowie et al., 1997). An outbreak in 2001 in Santa Isabel do Ivaí, Brazil, was also attributed to a contaminated municipal reservoir (Vaudaux et al., 2010; Silveira et al., 2015). A toxoplasmosis outbreak, assumed to be waterborne, in Coimbatore, Tamil Nadu, India resulted in around 250 patients with acquired retinitis in around 2003 (Balasundaram et al., 2010). The source of a more recent outbreak among 171 boarding school students in Izmir, Turkey, remained unidentified (Doganci et al., 2006).
An additional method that is available to study source attribution for Toxoplasma is based on the specific identification of T. gondii infections from oocysts. T. gondii embryogenesis‐related protein (TgERP) elicits an antibody response in humans (and also mice and pigs), who have previously been exposed to sporulated oocysts, therefore identifying individuals that have been infected with this stage of the parasite (Hill et al., 2011; Burrells et al., 2016). Among 176 individuals with unknown infection route and within 6–8 months of an initial oocyst‐acquired infection, antibody to TgERP was detected in 31 of them (17.6%) indicating that this assay was useful to identify oocyst‐derived infections (i.e. excreted from felids). None of the controls was positive.
Boyer et al. (2011) applied an oocyst‐specific antibody assay to quantify the risk of acquiring T. gondii from environmental sources vs meat. Among 76 pregnant women in the USA with a recent T. gondii infection, 78% had oocyst‐specific antibodies, indicating that environmental contamination plays an important role in the transmission of this parasite. A further study conducted on samples collected from blood donors in Scotland looked at those blood donors that had shown evidence of recent seroconversion to T. gondii and found that only one out of the 10 samples was positive for specific antibodies to sporozoites using the T. gondii sporozoite specific antigen assay (Burrells et al., 2016). Both studies indicate that oocyst‐derived infections from cats are of importance.
3.2.4.2. Subtyping
In Europe, clonal lineage type II strains dominate in humans and animals (Su et al., 2012). The use of subtyping data for source attribution is therefore currently less feasible than for other parasites. However, a discriminatory microsatellite‐based method has been described that can differentiate T. gondii type II isolates (Verma et al., 2015) and in a French study it was shown that these microsatellite‐types differed in rural and urban areas (Ajzenberg et al., 2015). Moreover, the introduction of atypical more virulent T. gondii strains originating from South America could be identified by molecular typing tools. Subtyping is useful to identify more virulent strains from South America, but for distinguishing among the subtypes most commonly occurring in Europe, more sensitive methods are required.
3.2.4.3. Comparative risk assessment
Facilitated by the large amount of occurrence data on T. gondii presence in different meat animals and meat products, several comparative risk assessments have been conducted. These studies provided evidence on the relative contribution of different meat products, but did not allow the relative contribution of meatborne toxoplasmosis vs other transmission routes to be defined.
Opsteegh et al. (2011) quantified the relative contribution of sheep, beef, and pork products to human T. gondii infections in the Netherlands. Despite a low prevalence of infection in cattle, consumption of beef was found to be the most important source of meatborne infection, followed by a near equal contribution of pork and sheep. Guo et al. (2015) performed a qualitative risk assessment of meatborne toxoplasmosis in the United States, suggesting that exposure associated with meats from free‐range chickens, and non‐confinement‐raised pigs, goats, and lamb were higher than those from caged chickens and confinement‐raised pigs and cattle. Finally, Belluco et al. (2018) compared the relative risk of T. gondii exposure through bovine meat vs pork in Italy. As in the Netherlands, bovine meat was found to be a more likely route of transmission to consumers than pig meat.
3.2.4.4. Expert knowledge elicitation
To date, expert knowledge elicitation remains the most frequently applied approach for attributing T. gondii to major transmission routes (i.e., transmission via food, water, soil, person‐to‐person contact, or animal contact). For T. gondii, food transmission would mostly occur via oocyst‐contaminated fresh produce or shellfish or via the meat of tissue cyst‐infected food‐producing animals. National studies conducted in some North American and European countries assumed the food‐borne attribution proportion to be approximately 50% (Table 7). Other studies conducted in North America, and only considering food‐borne transmission, applied similar food‐borne proportions (Mead et al., 1999; Scallan et al., 2011). Havelaar et al. (2008) in the Netherlands estimated environmental contamination to be the second most important transmission route (36% (95% UI 6–66%) vs 56% (95% UI 26–88%) food‐borne transmission), whereas Butler et al. (2015) in Canada estimated animal contact to be the second most important transmission route (34% (95% UI 7–81%) vs 51% (95% UI 9–83%) food‐borne transmission). The expert knowledge elicitation conducted by FERG in the context of the global burden of food‐borne disease study, yielded food‐borne attribution proportions within the European region ranging from 45% (95% UI 23–76%) to 61% (95% UI 35–82%) (Hald et al., 2016). Soil contact (18% (95% UI 0–40%) to 37% (95% UI 1–58%)) and waterborne transmission (15% (95% UI 2–35%) to 23% (95% UI 3–41%)) were estimated to be the other major transmission routes.
Table 7.
Examples of studies using expert knowledge elicitation for attribution of human toxoplasmosis to main transmission routes
| Country | Food | Water | Person‐to‐person | Animal contact | Soil | Reference |
|---|---|---|---|---|---|---|
| EUR Aa | 61% (35–82) | 19% (2–36) | N/Ad | 1% (0–21) | 18% (0–40) | Hald et al. (2016) |
| EUR Bb | 45% (23–76) | 15% (2–35) | N/A | 1% (0–20) | 37% (1–58) | Hald et al. (2016) |
| EUR Cc | 53% (31–78) | 23% (3–41) | N/A | 1% (0–20) | 22% (1–41) | Hald et al. (2016) |
| Canada | 51% (9–83) | 9% (1–26) | 3% (0–11) | 34% (7–81) | N/A | Butler et al. (2015) |
| Greece | 50% (30–63) | N/A | N/A | N/A | N/A | Gkogka et al. (2011) |
| Netherlands | 56% (26–88) | N/A | 1% (0–1) | 3% (0–3) | N/A | Havelaar et al. (2008, e) |
EUR A: Andorra, Austria, Belgium, Croatia, Cyprus, the Czech Republic, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Monaco, the Netherlands, Norway, Portugal, San Marino, Slovenia, Spain, Sweden, Switzerland, the United Kingdom.
EUR B: Albania, Armenia, Azerbaijan, Bosnia and Herzegovina, Bulgaria, Georgia, Kyrgyzstan, Montenegro, Poland, Romania, Serbia, Slovakia, Tajikistan, The Former Yugoslav Republic of Macedonia, Turkey, Turkmenistan, Uzbekistan.
EUR C: Belarus, Estonia, Hungary, Kazakhstan, Latvia, Lithuania, Republic of Moldova, Russian Federation, Ukraine.
N/A = not available.
Havelaar et al. (2008) also considered ‘environment’ as a transmission route, defined as transmission through contaminated water (drinking water, recreational water), soil, air or other environmental media, and accounting for 36% (6–66) of total transmission.
Two national studies also provided expert knowledge elicitation estimates of the contribution of specific food groups to food‐borne toxoplasmosis. In the United States, pork was estimated to be the major food source (41%), followed by beef (23%), game (20%) and fresh produce (7%). In the Netherlands, the major food sources were estimated to be pork (50%), beef and lamb (23%), and fruit and vegetables (6%). Within the context of the global burden of food‐borne disease study, the major food sources in the European region were estimated to be beef (25–38%), vegetables (17–22%), pork (12–13%) and meat from small ruminants (9–18%) (Hoffmann et al., 2017) (Figure 4).
Figure 4.
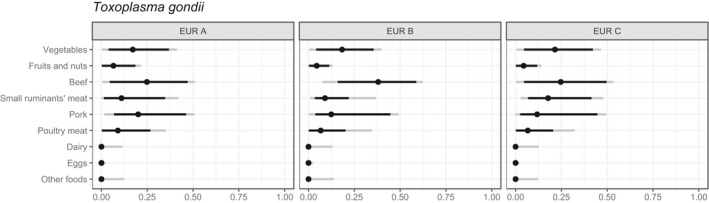
Expert knowledge elicitation estimations of the contribution of specific foods to the disease burden of Toxoplasma gondii (Hoffmann et al., 2017)
- The dots represent the median estimate; the black line the 90% uncertainty interval; and the grey line the 95% uncertainty interval. EUR A: Andorra, Austria, Belgium, Croatia, Cyprus, the Czech Republic, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Monaco, the Netherlands, Norway, Portugal, San Marino, Slovenia, Spain, Sweden, Switzerland, the United Kingdom; EUR B: Albania, Armenia, Azerbaijan, Bosnia and Herzegovina, Bulgaria, Georgia, Kyrgyzstan, Montenegro, Poland, Romania, Serbia, Slovakia, Tajikistan, the former Yugoslav Republic of Macedonia, Turkey, Turkmenistan, Uzbekistan; EUR C: Belarus, Estonia, Hungary, Kazakhstan, Latvia, Lithuania, Moldova, Russia, Ukraine.
3.2.4.5. Concluding remarks on relative importance of food‐borne transmission pathways
Available information suggests that food‐borne transmission accounts for 40–60% of the T. gondii infections. The major contributing food sources are meat (beef, pork, and small ruminant meat) and vegetables.
Subtyping methods have shown limited applicability due to insufficient genetic heterogeneity in Europe, although some more recent studies using different molecular methods, for example, targeting those genes with variable number tandem repeats, have been able to distinguish between European strains (Moretta et al., 2018). Several comparative risk assessments have provided evidence on the relative contribution of different meat products, but did not enable the definition of the relative contribution of meatborne toxoplasmosis vs other transmission routes. Several case–control studies on toxoplasmosis have been conducted; outbreaks are relatively rare, or, at least, rarely noticed and documented. When it comes to attributing T. gondii to major transmission routes, expert knowledge elicitation is the most applied methodology.
The relative importance of the main transmission routes, meaning via the environment (oocyst‐borne) or via meat (tissue cyst‐borne) is still a major issue and can currently only be answered with expert knowledge elicitation, which has the drawback that this is not data‐driven. Another approach with high potential is to use population‐based studies where patients with acute infections are tested by the sporozoite‐specific assay. When the test results are positive, this indicates that the infection has been derived via oocysts. So far, limited studies have been published in the USA and Latin America, but none from Europe.
QMRA‐based methods have been described for meatborne toxoplasmosis. Different meat products have been identified, and, based on food consumption data and cultural habits, raw beef is considered to be the most important meat source in the Netherlands and Italy. Nevertheless, QMRAs describing the environmental pathway (fresh produce) are lacking, and therefore the relative attribution of the different pathways using the risk assessment approach is still unclear.
3.2.5. Consumer practices contributing to infection
Some consumer preferences may increase the likelihood of infection with T. gondii, such as consumption of raw, very rare or undercooked meat. In the Netherlands, a QMRA model study showed that more than 30% of all meatborne infections were attributable to the consumption of a raw beef product (filet americain) (Opsteegh et al., 2011).
Consumer preferences for buying meat from animals raised outside, particularly pork, may increase the likelihood of exposure to infectious bradyzoites in tissue cysts if consumed raw/undercooked, due to the greater probability that these animals will be infected with T. gondii.
Consumer practices of drinking raw milk, particularly milk from goats, or eating fresh cheeses made from raw milk may also increase the likelihood of risk to consumers. The consumption of fresh produce that cannot be properly washed, or consumption of raw shellfish (e.g., oysters), may increase the likelihood of exposure to infectious oocysts.
3.2.6. Current control methods for food‐borne transmission and likely impacts
Two main transmission routes to humans can be extrapolated from the T. gondii lifecycle: (1) via tissue‐cysts in meat and meat products from food‐producing animals such as beef, pork, mutton, chicken and horse meat or wild/game meat and (2) via oocyst‐contaminated food products such as fresh produce, raw oysters/mussels and drinking water. In addition, tachyzoites can be found in raw milk. Knowledge of the transmission routes of T. gondii and the survival of the different stages of the parasite in the environment and in different food products will help to devise strategies to reduce and prevent the infection of people.
3.2.6.1. Control of meatborne transmission
Prevention of infection
Risk factors for T. gondii seropositivity in food‐producing animals have been identified (Opsteegh et al., 2015) and measures to prevent infection in food‐producing animals include measures such as: keeping the animals indoors; keeping cats away from farms, feed, and bedding production and storage; providing clean drinking water and blocking access to surface water; implementing strict rodent control; and refraining from feeding offal and raw goat whey are considered important, although intervention studies are rare. In industrialised pig‐production systems, these measures are generally implemented and indoor farming has drastically reduced the prevalence of T. gondii infection in pigs (Dubey, 2009) which is considered to be an important factor in the decrease of seroprevalences observed in human populations. With the current tendency towards welfare‐friendly outdoor reared systems, animals are more likely to become infected with T. gondii, thus increasing the likelihood of exposure of the consumer. Pigs and chickens with outdoor access have a higher risk of being T. gondii positive (van der Giessen et al., 2007; Schares et al., 2017). For outdoor‐reared animals (e.g., pigs, horses, cattle, and sheep), it is unlikely that prevention measures can substantially reduce the prevalence of infection. For these animals, vaccination would be a more feasible option. However, a vaccine aimed at preventing tissue cyst formation is currently not on the market (Opsteegh et al., 2015). The only veterinary vaccine available for T. gondii (Toxovax®) is licensed for the prevention of T. gondii abortions in sheep. As the vaccine prevents dissemination of parasites to the placenta, it has been also shown to prevent dissemination to other tissues and thereby to reduce tissue cyst development (Katzer et al., 2014). The same vaccine has also been found to reduce T. gondii tissue cysts significantly in experimentally infected pigs (Burrells et al., 2015). Nevertheless, a different type of vaccine would be preferable, as the current sheep vaccine is based on an attenuated live strain of T. gondii (S48) with a short shelf life. Moreover, it is potentially hazardous to the person administering the vaccine (Opsteegh et al., 2015).
To increase the feasibility of preventing infection in food animals, large‐scale screening to identify high‐risk farms (identifying farms with a high number of seropositive animals) could be implemented, after which the preventive measures could be limited to seropositive farms. This strategy could be particularly useful in pig production, as seropositive pigs are often only found on a limited number of farms (Opsteegh et al., 2015). This strategy can be adjusted depending on animal species and prevalence.
Control measures
There are currently no control methods for Toxoplasma available during meat inspection. Visual meat inspection cannot identify tissue cysts in the tissues of infected animals as they are normally only identifiable by microscopy. Therefore, serological testing of slaughtered animals could be used to identify T. gondii infected animals. However, this may only be useful when there is a good correlation between seropositivity and the presence of tissue cysts in the meat (see also in Section 3.2.3.4). So far, although the correlation is high in pigs and small ruminants, tissue cysts are also found in 5% of seronegative pigs. Therefore, serology cannot be used to identify individual animals and screening at the primary production level might be a better option for pigs and broilers in order to identify high‐risk flocks and herds. If specific risk factors could be identified for high‐risk herds (pigs and broilers), control options could be implemented to reduce the seropositivity. Furthermore, pigs from high‐risk farms at the slaughterhouse could be treated with validated cyst‐inactivating methods, whereas carcasses from seronegative herds would not need treatment (EFSA BIOHAZ Panel, 2011). This possibility needs to be further evaluated by cost‐benefit analyses (van Asseldonk et al., 2017). In the case of small ruminants, where the risk was identified as high, identifying risk factors at the farm level will not lead to a reduction of the risk (EFSA BIOHAZ Panel, 2013a). In cattle, the risk was identified as undetermined by EFSA (EFSA BIOHAZ Panel, 2013d) because only limited data were available for assessment, but recently more studies showed that cattle can harbour viable tissue cysts in their meat (Opsteegh et al., 2016b) and quantitative risk assessment studies in the Netherlands and Italy have shown that beef contributes substantially to human infections (Opsteegh et al., 2011; Belluco et al., 2018). Inactivation of tissues cysts within meat products might be the only practical possibility for beef. At the slaughtering plant, hygienic measures during slaughter should be taken to minimise exposure to abattoir workers.
Several methods can be applied to decontaminate meat containing T. gondii bradyzoites (Franssen et al., 2018). Heating and freezing meat at −12°C for 2 days will render tissue cysts nonviable (Kotula et al., 1991), as will γ‐irradiation (effective doses varying from 0.4 to 0.7 kGy have been reported), and high‐pressure processing at 300 MPa or more (Lindsay et al., 2006). Meat should be cooked thoroughly until the internal temperature has reached 66°C. Cooking times will vary with thickness and type of meat (Dubey et al., 1990). Cooking temperature and T. gondii bradyzoite concentration in muscle impacted most on the risk of transmission to humans (Condoleo et al., 2018). Microwave cooking is considered unreliable for inactivating viable tissue cysts because of hot and cold spots due to the physics of microwaves (Lundén and Uggla, 1992). Consumer acceptance may be a problem because of actual or perceived effects on colour, texture, and taste of the meat. In addition, the use of γ‐irradiation and high‐pressure processing may be restricted by legislation, and may incur high costs (Opsteegh et al., 2015). The feasibility of decontamination can be improved by limiting measures to high‐risk meat products. In a QMRA for meatborne T. gondii infections, including 50 meat products, nine unheated meat products contributed 40% of the predicted infections (Opsteegh et al., 2011). Opsteegh et al. (2015) defined high‐risk meat products as: (1) meat destined for preparation of raw meat products (such as raw sausages, carpaccio, or steak tartare) and products that are more likely to be eaten undercooked (e.g., beef steak, lamb chops); (2) meat from animals with outdoor access; or, after implementation of screening on the animal or farm level, (3) meat from animals infected with T. gondii or; (4) meat from animals originating from farms with a high T. gondii prevalence. These definitions could also be combined, e.g. focusing on decontamination of meat to be eaten raw or partially undercooked (1) from high‐risk farms (4). Salting, curing and smoking can reduce the viability of tissue cysts, but there is too much variability under household conditions that these methods are not considered safe (Dubey, 2010). There are reports that salt cured meat will reduce the risk of T. gondii infectivity despite the variability of the manufacturing parameters, with the risk reduction being related to a longer curing time (Pott et al., 2013). Another study has found viable T. gondii in dry cured ham samples (Gomez‐Samblas et al., 2015).
3.2.6.2. Control of oocyst‐contaminated food
Besides basic hygiene measures when preparing food, including washing hands carefully especially after gardening or emptying the cat litter tray, washing raw vegetables, and cleaning utensils and knives that have come into contact with raw meat in hot soapy water to help kill T. gondii bradyzoites and not using surface water for irrigation of fresh produce, there are no specific testing or control measures described to produce T. gondii‐safe fresh vegetables. In addition, there are also no specific guidelines on safe depuration times for shellfish to clear them of T. gondii oocysts.
Recently, the Codex Alimentarius amended the general guidelines for the control of food‐borne parasites in food, describing some basic concepts of food hygiene throughout the food chain, but guidelines for testing food‐producing animals or specific food products for T. gondii are not yet in place.
3.2.6.3. Concluding remarks on control measures
Specific control measures to prevent human exposure to T. gondii are still not in place. The relative importance of the main transmission routes remains to be determined and the cultural habits and consumer preferences will affect the likelihood of consumer exposure and will influence the effectiveness of control measures. So far, in Europe, only Cook et al. (2000) presented the population‐attributable fractions in different countries and suggested that meat contributed to 30–61% to human infections. Control measures to reduce meatborne infections might be relevant. However, more knowledge of the oocyst‐driven pathways is also relevant.
In food‐producing animals, serological screening of livestock might be useful to identify Toxoplasma‐positive farms. These farms then need to take measures to reduce exposure of the food‐producing livestock to T. gondii. For swine and small ruminants, serology can be useful to identify T. gondii‐positive animals, but this is not useful for cattle since there is no correlation between seropositivity and the presence of tissue cysts. In addition, control measures at the farm level to reduce the exposure of ruminants are very difficult.
Another approach is vaccination, which has been shown to be a feasible approach for sheep and pigs, with tissue cyst formation reduced or absent after vaccination.
Another possibility is decontamination of meat and meat products, such as freezing meat intended for raw consumption.
Heating is also an effective control measure; e.g., pasteurisation and ultra‐heat treatment of milk or cooking meat products.
A further measure could be to develop a vaccine for use in cats that would prevent or reduce the shedding of oocysts into the environment although the feasibility of such an approach should be studied first. Keeping cats away from areas and water sources used for the production of fresh produce would also help to reduce the likelihood of oocyst contamination.
Knowledge of the key transmission routes is important for the development of education programmes to help inform high‐risk groups of people, particularly pregnant women and immunocompromised individuals.
3.3. Echinococcus spp
3.3.1. Characteristics, including relevant food vehicles for transmission
The tapeworm genus Echinococcus is composed of a minimum of nine, probably more, species, some of which contain distinctive genotypes or strains (Nakao et al., 2013; Lymbery, 2017). All are transmitted in two‐host lifecycles (Figures 5 and 6), where the adult (worm) stages are intestinal parasites of mammals of the order Carnivora (definitive hosts), and the larval stages are tissue parasites of a wide range of mammal species (intermediate hosts and dead‐end hosts). Echinococcus spp. are trophically transmitted: definitive hosts shed parasite eggs, each containing an infectious larva (oncosphere), with their faeces into the environment, where they may contaminate food or feed and be inadvertently ingested by the (mainly herbivorous) intermediate hosts. Following ingestion, the oncospheres hatch in the small intestine of the intermediate host, invade the circulatory system, and are transported into various organs. In suitable locations (often the liver and the lungs), they develop into metacestodes, which take the form of large cysts or conglomerates of small vesicles (depending on the species of Echinococcus). Inside the metacestodes, embryonic tapeworm heads (protoscolices) are produced in large numbers. Following ingestion of metacestode‐infected organs by the definitive hosts (together with the intermediate host or its infected organs), the protoscolices grow into adult worms in the small intestine of the definitive host. After a few weeks, the adult worms start producing eggs (Thompson et al., 2017).
Figure 5.
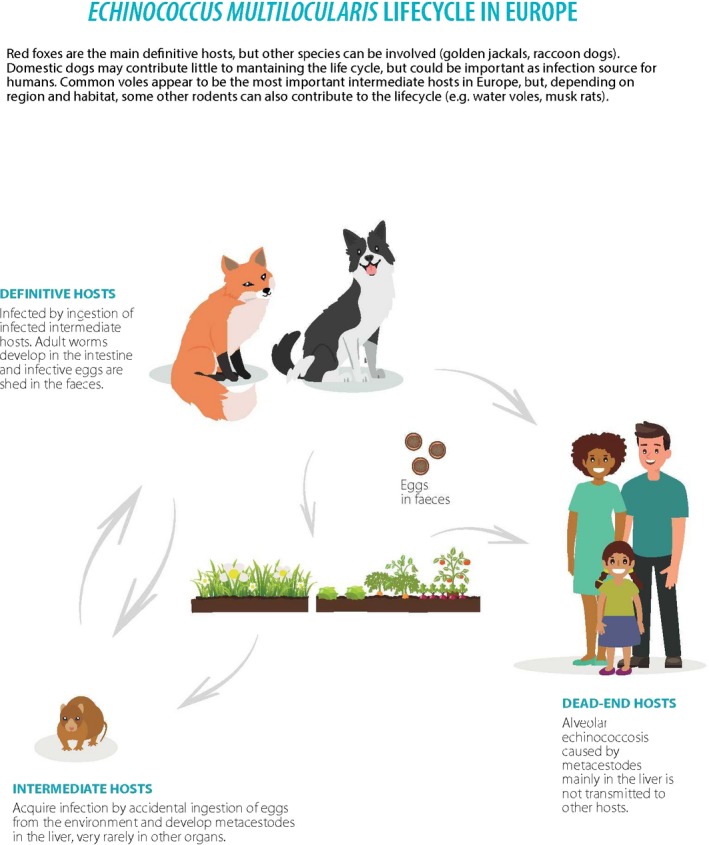
Echinococcus multilocularis lifecycle in Europe
Figure 6.
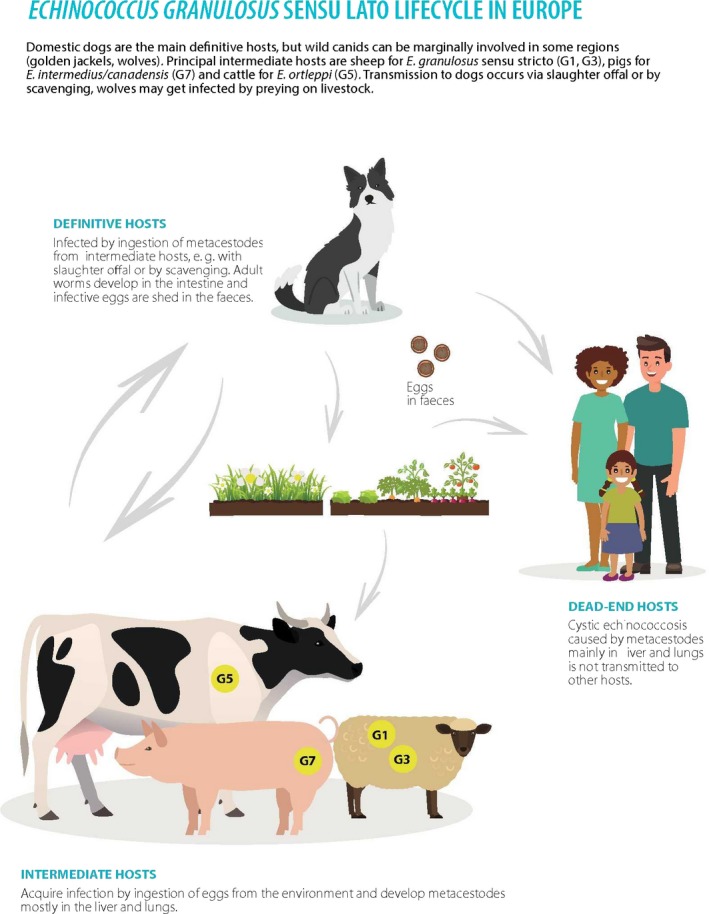
Echinococcus granulosus s.l. lifecycle in Europe
All Echinococcus species are transmitted either in predator–prey systems (for wildlife cycles) or by domestic dogs feeding on offal from slaughtered livestock or on animal carcasses (Romig et al., 2017). Definitive hosts are mainly canids (members of the dog family, including domestic dogs), but for some Echinococcus spp., felids (members of the cat family) may also act as definitive hosts. The intermediate hosts are species that are frequently preyed upon or eaten by the definitive hosts. Some Echinococcus spp. have wide intermediate host ranges and infect a large number of unrelated mammals (including humans), but others are restricted to a few host species and are probably not zoonotic.
The adult stages of the parasites do not cause disease in the definitive hosts, are comparatively short‐lived (majority < 3 months, though some worms can persist longer with a relative low egg production), and are highly susceptible to anthelmintic treatment (praziquantel, epsiprantel). In contrast, metacestodes develop in the organs of the intermediate hosts, may grow (expansive or invasive) to considerable dimensions, and cause disease due mainly to the occupation of space that leads to organ failure. Size, growth pattern and pathogenicity vary greatly according to the species of Echinococcus and the host species. Treatment options of metacestode infection include invasive surgical interventions, percutaneous therapy, and long‐term treatment with benzimidazoles (usually albendazole) that have a parasitostatic effect. There are limited data about the incubation times for the various Echinococcus spp. in human patients. It varies significantly between patients, with estimates ranging from several months to many years, depending on the immune competence, organ location, Echinococcus species, and unknown factors (Kern et al., 2017).
Humans acquire Echinococcus infection by oral uptake of infective eggs. Transmission has been hypothetically linked to water or food‐borne sources (vegetables/fruit/berries), but any source attribution is uncertain due to the long incubation time (see Section 3.3.4.5). Hand‐to‐mouth is a putative route of transmission, after contact with Echinococcus eggs in the environment. Echinococcus eggs can be dispersed from carnivore faeces with water or by adhering to objects (e.g., hooves of sheep, shoes and tyres). It has been speculated that birds and flies could be possible vectors (Lawson and Gemmell, 1990). As Echinococcus eggs can adhere to the coats of infected dogs or foxes, there is an obvious risk originating from direct contact with definitive hosts. Furthermore, dogs rolling in faeces can be externally contaminated without being infected. Proglottids of E. multilocularis have been found and documented in the peri‐anal region of a naturally infected dog (Deplazes et al., 2004), and examination of the hair coat of 46 foxes revealed taeniid eggs in 11 animals, with three cases confirmed to be E. multilocularis (Nagy et al., 2011). These data indicate the variety of potential infection routes to humans.
Human echinococcosis can be caused by different species (strains or genotypes) of Echinococcus (Table 8), which lead to drastically diverging diseases: AE is caused by E. multilocularis, and CE by various species and genotypes of E. granulosus s.l. Both diseases require different clinical management (diagnosis, treatment options), which is a fact that is often not recognised in health systems (Kern et al., 2017). In addition, the lifecycles of the causative parasites show significant differences in terms of host animals (wild or domestic species) and geographical occurrence, which directly relate to significantly different risks for either disease in any one area.
Table 8.
Echinococcus spp., strains and genotypes in Europe and their occurrence and/or severity of disease
| Echinococcus species | Echinococcus strains or E. granulosus s.l. genotypes (G) | Definitive hosts | Intermediate hosts | Main European endemic areas | Human disease clinical severity, (+, ++, +++) |
|---|---|---|---|---|---|
| E. multilocularis | European genotypea | Fox, dog, raccoon dog, (cat)a | Arvicolids and other rodents | Central and eastern Europe, from southern Sweden to the Alps and the Balkan peninsula |
Alveolar echinococcosis +++ |
| E. granulosus sensu stricto (s.s.) | Sheep strains (G1, G3) | Dog (fox)b | Sheep, cattle,c pigs and other herbivoresc | Southern and eastern Europe, sporadic elsewhere |
Cystic echinococcosis +++ |
| E. ortleppi | Cattle strain (G5) | Dog | Cattle | Sporadic in central and southern Europe |
Cystic echinococcosis +d |
| E. canadensis | Cervid strains (G8, 10) | Wolf (dog) | Cervids | Northern and north‐eastern Europe |
Cystic echinococcosis +d |
| E. intermedius (proposed species, synonym, E. canadensis group) | Pig strain (G7) | Dog (wolf) | Pigs, goats | Baltic states, Poland, Corsica, Sardinia; Greece, sporadic elsewhere |
Cystic ECHINOCOCCOSIS +++ |
| E. equinus | Horse strain (G4) | Dog | Equines | Sporadic in all European regions | The first human case recently reported (Timur et al., 2018) |
Genotype assemblages based on mitochondrial gene sequences.
Mostly low worm numbers with very low egg production.
Mostly with strongly reduced protoscolex formation in the cysts often resulting in infertile cysts.
Very few, mostly historic cases.
3.3.1.1. Alveolar echinococcosis
AE is caused by the metacestode of E. multilocularis. The parasite is widely distributed in the temperate and cold regions of the northern hemisphere (Deplazes et al., 2017), where it exploits predator–prey systems, largely between wild canids and small mammals (mainly rodents). E. multilocularis is not divided into any subspecific taxa, although different genotypes have been described and geographical correlations have been proposed. The highly unequal impact on public health in different regions worldwide has been hypothetically linked to different genotypes, but data are insufficient and partially contradictory (Romig et al., 2017).
In most temperate regions, red foxes (Vulpes vulpes) are the main definitive hosts, but coyotes (Canis latrans), jackals (C. aureus), wolves (C. lupus) and raccoon dogs (Nyctereutes procyonoides) may contribute to the lifecycle in some regions. Arctic foxes (Vulpes lagopus) are the main definitive hosts in Arctic and sub‐Arctic regions (Romig et al., 2017). Domestic dogs are susceptible to intestinal infection and, although the prevalence of infection in dogs in Europe is generally low, they may contribute locally to the lifecycle due to their large population size. The principal impact of domestic dogs is, however, associated with transmission of the parasite to humans due to close contact (Hegglin and Deplazes, 2013). Domestic cats are not well suited as definitive hosts as they do not shed large numbers of eggs (Kapel et al., 2006). They are not important for the lifecycle, but some contribution to human infection by cats cannot be excluded.
A large number of rodents and other small mammals has been recorded as intermediate hosts, although the epidemiological importance of the individual species is only clear in a few regions (Romig et al., 2017). In Europe, common voles (Microtus arvalis) are key intermediate hosts, but a number of other rodents are also susceptible (e.g., water voles, bank voles, muskrats). Apart from these lifecycle‐appropriate intermediate hosts, E. multilocularis infects a number of dead‐end hosts, where metacestode growth ranges from abortive without causing disease (e.g. pigs, horses) to progressive, eventually being fatal (e.g., humans, other primates, dogs) (Romig et al., 2017).
Compared with other species of Echinococcus, E. multilocularis metacestodes have a deviant morphology: rather than forming a fluid‐filled cyst, they grow in the form of small vesicles, which are densely packed with protoscolices and have the potential to infiltrate the surrounding host tissue continuously throughout life. The primary location for metascestodes is almost always the liver. Macroscopically, the appearance and pathology of the metacestodes are comparable to slow‐growing malignant tumours, which include their capacity to form metastases in distant organs at a progressed stage of the disease. Curative treatment for human patients is restricted to surgical removal of the metacestode at an early stage of development (when the parasite is usually still non‐symptomatic). At later stages of the disease (around 50% of cases at first diagnosis), further metacestode growth can be prevented in most patients by continuous (life‐long) treatment with benzimidazoles (usually albendazole) (Kern et al., 2017). Survival analyses of AE patient cohorts in France and Switzerland documented that nowadays the patient survival after first diagnosis is reduced by only 2–3 years as compared with the unaffected general population (Torgerson et al., 2008; Piarroux et al., 2011). However, the survival time of patients is still much shorter in Lithuania; in 34.4% of AE cases, survival was less than 1 year from diagnosis, due to the initial diagnosis being at an advanced stage of the disease (Marcinkut≐ et al., 2015).
Based on published data, Torgerson et al. (2015) estimated that 18,451 true (including underdiagnoses and underreporting) incidence of AE cases occurred in 2010, resulting in a global disease burden of 687,823 DALYs. The high number of DALYs per patient (37) reflects the severity and limited treatment options of AE (as compared with CE with < 1 DALY per case – see Section 3.3.1.2). As DALYs are strongly dependent on medical infrastructure and treatment facilities, the high global average per patient is caused by the fact that > 90% of cases occur in (western) China. In contrast, for Switzerland, the number of DALYs per patient was estimated at 3.7, ten times less than the global estimate (Torgerson et al., 2008).
In Europe, the most recent EUSR on zoonoses and food‐borne outbreaks listed 415 laboratory‐confirmed cases of CE and 104 cases of AE in (EFSA and ECDC, 2017). However, the reported number of cases is difficult to interpret and it is not possible to compare between countries as AE is not notifiable in all European countries or in all EU Member States, in addition to diverging systems of diagnostic effort and reporting. In Germany, an evaluation of the reporting system found that the notification system failed to detect 67% of AE cases over a 3‐year period (Jorgensen et al., 2008). Therefore, in many areas (e.g., France, Germany), reliable baseline data are missing for a statistically based documentation of increased AE incidence (Schmidberger et al., 2018). However, there is convincing evidence for an emergence of AE in the last decade in some European regions (Gottstein et al., 2015). In Switzerland, the incidence of infection increased from 0.1 to 0.26 per 100,000 per annum between 1993 and 2005 (Schweiger et al., 2007). In Austria, the total new cases per year varied between 2.4 and 2.8 in the period 2001–2010. However, 13 new cases were registered in 2011 (Schneider et al., 2013). A steady increase in cases was reported from Poland between 1990 and 2011 (Deplazes et al., 2017). In Lithuania, the AE incidence increased from 0.03 per 100,000 in 2004 to 0.74 per 100,000 in 2012 (Marcinkut≐ et al., 2015). One reason for the increase of AE incidence may be the urbanisation of the E. multilocularis cycle and the contamination of highly populated areas, thereby significantly increasing the human population exposure to E. multilocularis eggs (Deplazes et al., 2004). Even if humans are regarded to be highly resistant to the development of AE (Gottstein et al., 2015), such ecological changes could be responsible for the relatively low (2‐ to 3‐fold) increase of AE incidence in some areas. Reports of sporadic human AE cases from southern and eastern Europe are available, but older cases are particularly difficult to verify because the diagnostic criteria used in those days may not have differentiated between AE and CE. No autochthonous cases have been reported from the UK or Scandinavia, nor from most Mediterranean countries.
3.3.1.2. Cystic echinococcosis
CE in humans is caused by various cryptic species of the E. granulosus (s.l.) complex (Table 9). Taxa within this complex were formerly identified as genotypes (G1–10) or strains of E. granulosus, but the substantial differences between these forms (e.g., host species, infectivity/pathogenicity to humans, morphology, developmental parameters, geography) have led to a subdivision into at least five species. For the European situation, this complex comprises E. granulosus sensu stricto (G1, G3 ‐ ‘sheep strains’), E. equinus (G4, ‘horse strain’), E. ortleppi (G5, ‘cattle strain’), E. canadensis (G7, G8, G10, ‘pig/cervid strains’). E. canadensis may be further subdivided in the future, and the name E. intermedius has been used by some authors for the pig and camel strains (G6/7) (Romig et al. 2015; Romig et al., 2017).
Table 9.
Different source attribution approaches for Echinococcus spp
| Source attribution approach | Comments |
|---|---|
| Epidemiological studies | Limited because of the long incubation period of the disease |
| Subtyping | Several studies have genotyped E. granulosus s.l. in livestock and human hosts; source attribution to reservoirs for E. multilocularis is at present not possible to perform as the various known reservoirs are not characterised by different subtypes |
| Comparative risk assessment | Limited by a lack of occurrence data, particularly on contamination of foodstuffs with Echinococcus eggs |
| Expert knowledge elicitation | May appear to be the most feasible approach, but results indicate wide uncertainty |
Globally, E. granulosus s.l. is responsible for the great majority of human CE worldwide, with close to 90% of surgical samples analysed worldwide belonging to this species (Alvarez Rojas et al., 2014). It is mainly transmitted in a domestic lifecycle, with domestic dogs as the definitive hosts and sheep as the principal intermediate hosts. However, the range of potential intermediate hosts is very wide, and other livestock species (pigs, camels, goats, and, to a lesser degree, cattle) may also become infected and contribute to transmission. In parts of its worldwide range (Deplazes et al., 2017), wild animals can also, to various degrees, be involved (wild canids as definitive hosts, various large wild herbivores as intermediate hosts). E. ortleppi is also zoonotic, but very few cases of human infection have been reported. The lifecycle is mainly domestic, involving domestic dogs and cattle, but other livestock and wild animal species have also been recorded as hosts. The E. canadensis ‘cervid strains’ (G8, G10) are basically northern wildlife parasites (involving wolves, moose, and other cervids), although dogs and domesticated reindeer can also become infected. Few human cases are on record, and infections were described as benign. The ‘pig strain’ of G6/7 (E. intermedius) occurs worldwide in mainly domestic lifecycles involving dogs and pigs (in Europe, mainly in the Baltic States, Poland, and further east). G6 and G7 have the second highest impact of all CE agents on human health, being responsible for more than 10% of the human CE cases worldwide, but may predominate regionally, e.g., in the Baltic countries and Poland (Marcinkut≐ et al., 2015).
Metacestodes of all species of the E. granulosus s.l. complex grow as fluid‐filled cysts in a large number of organs, most frequently the liver or lungs (Kern et al., 2017). In humans, cysts can become very large and contain several litres of fluid. Pathology is due to the occupation of space and is highly variable, depending on the location. The multi‐layered cyst wall provides a clear demarcation between parasite and host tissue, and surgical removal is usually possible, even in advanced stages of the disease. Percutaneous treatment and antiparasitic medication with benzimidazoles are alternative or accompanying treatment options. There are clear indications that clinical parameters (e.g., cyst location and size) vary between CE caused by the different Echinococcus species. However, most clinical data collected in the past do not differentiate between the causative genotypes or species, and recent data are too scarce to draw firm conclusions.
The latest estimate of the global public health impact of CE is 188,079 new cases per year, with a disease burden of 183,573 DALYs (Torgerson et al., 2015). The far lower health burden per patient (compared with AE) is due to the low mortality. In contrast to AE, there is an economic impact caused by CE in livestock, which is often difficult to quantify.
In Europe, data on human CE are fragmentary due to the lack of dedicated reporting and documentation systems. A European register is in the process of being established (Rossi et al., 2016). Sporadic CE cases, often imported, can be expected anywhere in Europe, but substantial numbers of cases are limited to southern and eastern Europe (Deplazes et al., 2017). The annual surgical incidence (per 100,000) can be as high as 9 (Northern Cyprus), 7 (Sardinia), and 4 (Sicily). In Europe, there is a general trend to reduced incidences, e.g., in Greece from 12.9 in 1984 to 0.25 in 2010 (Sotiraki et al., 2003). In the context of the recently completed EU project, HERACLES, high CE prevalence (based on abdominal ultrasound screening surveys) was found among inhabitants of rural villages in south‐eastern Europe. Prevalence adjusted for age and sex was 0.41% (95% CI 0.26–0.65) in Bulgaria, 0.41% (0.26–0.65) in Romania, and 0.59% (0.19–1.85) in Turkey (Tamarozzi et al., 2018).
3.3.2. Methods of detection in food
3.3.2.1. Morphological and molecular identification of Echinococcus eggs
Echinococcus eggs, shed by definitive hosts, represent the infectious agent and therefore are the target for detection in food, mainly fresh produce that might be irrigated with contaminated water or have direct or indirect contact with faeces from infected carnivores. Eggs are dispersed from the carnivore faeces by water or by adhering to objects (e.g., hooves of sheep, shoes and tyres). It has also been speculated that birds and flies could be possible vectors (Lawson and Gemmell, 1990).
There is no standardised method for the isolation of Echinococcus eggs present on vegetables for subsequent detection, and standardisation is difficult due to the heterogeneity of substrates (food matrices) involved. Methods for the isolation of taeniid eggs from faecal samples (Conraths and Deplazes, 2015) can be adopted for food samples. In general, after a washing stage using low concentrations of detergents (SDS (1%) and/or Tween 80 (0.1%)), the parasites eluted in the washwater can be concentrated using flotation, sedimentation, and/or sieving steps, and then detected by microscopy. Published reports on the presence of parasite eggs on vegetables have used samples of between 100 and 300 g. So far two strategies have been used for the analysis of vegetables for contamination with E. multilocularis (see Appendix G).
Detection of Echinococcus eggs isolated from any matrix is very difficult due to the similarities in morphology among all taeniid eggs, which hamper microscopy‐based genus‐specific or species‐specific identification. Therefore, microscopy‐based detection of taeniid eggs should be accompanied by PCR and sequencing using species‐specific primers. Technical challenges (cross‐contamination during sampling/processing of samples and DNA amplification) need to be addressed, especially if the eggs are not visualised in the procedure. Further, the specificity of amplification of DNA from environmental samples has to be critically validated. The sensitivity of PCR amplification is high and claimed to detect theoretically one single egg. This is convincing, as taeniid eggs contain between 18 and 56 cells (Alvarez Rojas et al., 2018) and it has been estimated that a single taeniid egg (based on Taenia hydatigena) contains around 7,000 mitochondrial targets, while the detection limit of PCRs targeting the mitochondrial DNA was estimated at 33 copies (Trachsel et al., 2007). A critical point that needs to be addressed is the fact that the detection of DNA from Echinococcus spp. isolated from eggs that have been detected in food sources does not imply that these eggs are viable (see below).
3.3.2.2. Infectivity and viability assessment of Echinococcus spp. in food
Various methods have been used to assess the viability of tapeworm eggs. Detection of eggs by any in vitro method does not necessarily indicate infectivity. Hatching of taeniid eggs using artificial gastric fluid was developed as a method to assess viability in the 1950s (Silverman, 1954), and has been extensively used. However, variability in egg activation was noted between samples of the same batch of eggs and the infectivity of eggs was consistently overestimated (Coman and Rickard, 1977). The same authors also described that as taeniid eggs age, first they lose their ability to infect, and subsequently they lose their ability to hatch and activate in vitro. This means that not all viable eggs are infective; hence, in vitro test results may overestimate infectivity. Other in vitro and in vivo methodologies used to assess the viability of taeniid eggs include the use of vital stains, oncosphere culture, and infection of intermediate hosts and laboratory animals (Williams and Colli, 1970; Wang et al., 1997; Minozzo et al., 2002; Kyngdon et al., 2006; Chapalamadugu et al., 2008). An elegant in vitro technique to assess the viability of taeniid eggs freshly collected from proglottids is their treatment with 2% sodium hypochlorite (SH‐RT) for a few minutes to dissolve their embryophore. Immature eggs are destroyed by this procedure while viable oncospheres are protected by a resistant membrane (Lightowlers et al., 1984). However, the results of the SH‐RT do not correlate with the in vitro activation and development rate of taeniids, including E. multilocularis (Deplazes et al., 2005; Moazeni and Rakhshandehroo, 2012; Federer et al., 2015). In vivo studies yield the most reliable results, provided that the intermediate and definitive hosts are available for the target species or an appropriate surrogate species is selected.
3.3.2.3. Concluding remarks on detection methods
There is no standardised method for the detection of infectious eggs of Echinococcus spp. present in food. However, in principle, there are established methods for egg isolation and genetic characterisation. As food can be contaminated with non‐viable eggs persisting in the environment, specific DNA identification does not mean that viable eggs were present in the food investigated. Therefore, there is an urgent need for the standardisation and validation of robust diagnostic strategies and for the estimation of the infection risk of Echinococcus spp. associated with food.
3.3.3. Occurrence and survival of Echinococcus spp. in food
3.3.3.1. Determination of food contamination
The scientific literature provides several reports on microscopy‐based findings of taeniid eggs on vegetables, mainly in Asia and Africa, with contamination rates ranging between 0.9 and 18.3% (Alvarez Rojas et al., 2018). Studies performed in Norway (Robertson and Gjerde, 2001), France (Hohweyer et al., 2016), and Italy (Caradonna et al., 2017) did not detect taeniid eggs.
One study in an E. multilocularis‐endemic area in Poland used PCR to assess food contamination with E. multilocularis eggs (Lass et al. 2015). In this study, 103 samples (fruit, vegetables, mushrooms) were subjected to a procedure developed for soil samples (Szostakowska et al., 2014), and almost 25% of samples were found to be positive. This publication triggered some discussion in the literature, including questioning the finding of positive raspberries collected from plants at some distance from the ground (Robertson et al., 2016), although such contamination cannot be excluded, as flies could transport such eggs (Lawson and Gemmell, 1990). An investigation into the presence of cestode eggs in the washwater of fresh produce (vegetables, fruit) used as feed (Federer et al., 2016) was triggered by frequent cases AE in primates kept in captivity at a Swiss zoo. Egg‐DNA PCR using multiplex PCR/sequencing (Trachsel et al., 2007) on filtered samples of elution water revealed non‐zoonotic Taenia spp. of dogs, foxes or cats in 14 of the total 95 samples (each consisting of the washing of around 40 heads of lettuce in addition to a day ration of fruit and vegetables) originating from an endemic area of Switzerland. Taeniid‐DNA was further detected in 13 (28%) of 46 samples of vegetables and fruit originating from different parts of Europe, including E. granulosus s.l. (2), Taenia crassiceps (1), T. hydatigena (2), Taenia multiceps/serialis (2), Taenia saginata (1) and Taenia taeniaeformis (5). Although E. multilocularis DNA was not identified in this study, the detection of DNA of other taeniids of foxes revealed that fresh produce as feed is a potential source of E. multilocularis eggs for zoo primates, and thus, also, potentially, for humans.
3.3.3.2. Survival of Echinococcus eggs
Earlier reports showed that Echinococcus eggs remain viable at temperatures below zero for long periods of time, but that very low temperatures (−70 to −80°C for 96 and 48 h, respectively), inactivate the eggs (reviewed in Eckert et al., 2001). More specifically, E. multilocularis eggs are able to survive temperatures of 4°C and −18°C for 478 and 240 days, respectively (Veit et al., 1995) and were still infective to voles after storage for 54 days at −27°C (Schiller, 1955). On the other hand, E. multilocularis eggs are sensitive to higher temperatures and also to desiccation in in vitro conditions (Laws, 1968; Veit et al., 1995). Although they can survive temperatures of +65°C for 2 h, they were killed after 3 h +65°C at or at 70, 75 and 80°C for 30, 15 or 7.5 min, respectively (Federer et al., 2015).
Eggs were more resistant to elevated temperatures when suspended in water than in environments with 70% relative humidity (Federer et al., 2015). This is relevant, since eggs can be in water droplets on vegetables and this would extend their lifespan. Although high temperature is a lethal factor, the temperature of the soil surface rarely reaches the temperatures used in in vitro experiments; thus, given the Holarctic area distribution of E. multilocularis, these data might not be particularly relevant to its transmission. Indeed, E. multilocularis eggs remained viable in their natural environment in Germany for 240 days in autumn/winter and for 78 days in summer (Veit et al., 1995). E. granulosus s.l. eggs remained viable up to 41 months in the environment of Argentinian Patagonia (Sánchez Thevenet et al., 2005) and for only four winter months in New Zealand (Sweatman and Williams, 1963). Survival of the eggs in the environment is obviously vital for completion of the lifecycle. Furthermore, detailed knowledge on the physical resistance of taeniid eggs is relevant when assessing their inactivation in food, for modelling of transmission dynamics, and for the appropriate design of control measures. However, information on the mechanisms that allow the eggs to survive in such conditions is lacking. No data exist on the potential differences in egg tenacity between different Echinococcus spp., or between populations of the same species that exist in different climatic zones.
3.3.3.3. Concluding remarks on occurrence and survival in food
Classical methods based on egg isolation and morphological identification have only rarely documented the presence of taeniid eggs in food and without differentiation between Taenia and Echinococcus spp. Only recently, the detection of Echinococcus eggs/DNA in food using methods for egg isolation and genetic characterisation (PCR, sequencing) has documented the presence of specific Echinococcus DNA in food. Echinococcus eggs are known to survive for months in the environment, especially at low temperatures. Therefore, it can be assumed that the eggs can survive on food (fruit or vegetables) for months during storage at low temperatures or frozen (as only freezing at −80°C or heating above 70°C guarantees that the eggs are killed).
3.3.4. Relative importance of food‐borne pathways
Echinococcus spp. are faecal–oral pathogens with red foxes and domestic dogs (E. multilocularis) and domestic dogs (E. granulosus) as major definitive hosts. The transmission of human echinococcosis hypothetically involves both direct (animal‐to‐person) and indirect routes, i.e. through hand‐to‐mouth contact after contact with the contaminated environment, or through water and food contaminated with infectious eggs. Table 9 provides an overview of the different source attribution approaches for Echinococcus spp.
3.3.4.1. Epidemiological studies
A number of case–control and cross‐sectional studies identifying risk factors have been published, which have recently undergone systematic reviews and meta‐analyses for AE and CE separately (Possenti et al., 2016; Conraths et al., 2017). The results of these studies should, however, be interpreted with caution and in the light of the long incubation period of the disease.
E. multilocularis
For AE, a meta‐analysis for potential risk factors has been conducted, including 6 case–control and 11 cross‐sectional studies (Conraths et al., 2017). The authors conclude that ‘the chance of AE transmission through the ingestion of food and water contaminated with E. multilocularis eggs does indeed exist, but it is important to note that food‐ and waterborne potential risk factors do not significantly increase the risk of infection’. The case–control studies originated from central Europe (4), Alaska (1) and China (1), the cross‐sectional studies came predominantly from China (10), only one from central Europe. As the epidemiology of AE in China and Alaska differs from Europe, the results from the European studies included in the meta‐analysis are listed separately:
-
Case–control studies. Four European studies are included in the meta‐analysis, of which three address food‐related potential risk factors:
-
–
Kreidl et al. (1998). A study from Austria based on 21 AE patients. Only cat ownership (OR 6.47, 95% CI 1.54–27.29) and hunting (OR 7.83, 95% CI 1.16–52.77) emerged as significant risk factors (although with extremely large OR confidence intervals). Food‐related factors (eating mushrooms and/or berries) were not identified as risks.
-
–
Kern et al. (2004). A study from Germany based on 40 AE patients. High ORs were related to dog ownership and farming activities (OR from 4.7 to 18.0). Any food‐related factors (eating raw produce) gave OR < 2.5 except ‘chewing grass’ (OR 4.4, 95% CI 1.7–11.2).
-
–
Piarroux et al. (2013). A study from France based on 180 AE patients, 164 of which lived in known AE‐endemic regions. The dominant risk factor for the latter was agricultural occupation (OR 7.33, 95% CI 3.13–20.00). Minor or no risks were associated with food (eating raw wild salads OR 1.80 95% CI 0.98–3.31); eating raw wild berries OR 0.95, 95% CI 0.33–2.50), although ‘having a kitchen garden’ had an OR of 5.50 (95% CI 2.52–12.66).
-
–
Cross‐sectional studies. Only one study originates from Europe (Romig et al., 1999): 2,540 inhabitants of a rural town in southern Germany were screened with ultrasound and antibody serology. One AE patient was identified by ultrasound; nine persons were seropositive without detectable lesions. Food‐related potential risk factors (eating raw garden produce or wild berries and herbs) were less or equally prevalent among the specific seropositives compared with the seronegative inhabitants (raw garden produce: 5/10 positives and 1,790/2,530 negatives; berries and herbs: 1/10 positives and 281/2,539 negatives).
E. granulosus s.l
For CE, 8 case–control studies and 29 cross‐sectional studies were included in a meta‐analysis (Possenti et al., 2016). Case–control studies originated from South America (2), North Africa/Middle East (4), Turkey (1) and Spain (1). Cross‐sectional studies included in this meta‐analysis came from China/central Asia (10), the Middle East/North Africa (8), sub‐Saharan Africa (1), South America (4), India (1), Canada (1), Turkey (3) and Greece (1). The authors conclude that ‘Results from case‐control studies […] do not provide significant evidence that CE is a strictly food‐ or waterborne disease’ and, for the cross‐sectional studies, ‘[…] that the risk of CE transmission through the ingestion of food and water contaminated with E. granulosus s.l. eggs was not evidence based and is potentially anecdotal’. Results from the European studies are:
-
Case–control studies:
-
–
One study from Spain (Campos‐Bueno et al., 2000), based on 127 CE cases, identified risk factors related to the size of the town/village, the number of owned dogs over the lifetime and length of time having contact with a dog (> 30 years with family dogs: OR 5.23, 95% CI 1.91–14.34), and the feeding of dogs with raw viscera (OR 5.50, 95% CI 2.80–10.79). No significant correlation was found with the eating of raw vegetables: highest ORs were found for ‘ingestion of lettuce > 3 days per week’ (OR 1.64, 95% CI 0.78–3.45) and ‘> 10 years eating produce from family vegetable garden’ (OR 1.22, 95% CI 0.55–2.28).
-
–
One study from Turkey (Kiper et al., 2010) tested genetic predisposition in humans, not food‐related factors.
-
–
-
Cross‐sectional studies:
-
–
One study from Greece (Fotiou et al., 2012), screened blood samples from 542 persons using ELISA, with six seropositives. Old age and rural residence were associated with positivity, and no food‐related factors were tested.
-
–
An ultrasound survey of 6,093 Turkish school children (Ok et al., 2007) revealed nine CE cases. The only food‐related question was ‘eating unwashed vegetables’, which applied to 5 of 9 positives, and 2012 of 6,093 negatives.
-
–
A seroprevalence study in Turkey (Akalin et al., 2014) identified 78 ELISA seropositives among 1,133 rural inhabitants. Farming activity had high ORs, but no food‐related factors were tested.
-
–
A seroprevalence study from Turkey (Cetinkaya et al., 2005) screened 611 individuals with ELISA, finding 89 seropositives. No significant differences regarding potential risk factors were found between seropostives and seronegatives; no food‐related factors were tested.
-
–
3.3.4.2. Subtyping
E. multilocularis
Nuclear and mitochondrial genes have been used for subtyping of E. multilocularis. Microsatellite analysis, using repetitive sequences in the nuclear genome, have documented high variability. The use of mitochondrial genes for subtyping has clearly distinguished at least four genotype assemblages (Asian, Mongolian, North American, and European). However, mitochondrial and microsatellite analyses have not, so far, produced consensual results (Knapp et al., 2007; Jastrzembski, 2017) and the global genetic structure of E. multilocularis is still largely unclear. As examples, ‘Asian’ genotypes were recently found in Poland (Karamon et al., 2017), ‘North American’ genotypes in Russia (Konyaev et al., 2013), and ‘European’ genotypes in Canada (Gesy and Jenkins, 2015), so these approaches are currently still of doubtful value in Europe until more comprehensive surveys are done. Consequently, source attribution to reservoirs for E. multilocularis is currently not possible.
E. granulosus s.l
Point‐of‐reservoir source‐attribution studies for echinococcosis focus on the intermediate hosts that harbour parasitic cysts (reservoir). This is particularly relevant for CE, given the role of livestock as intermediate hosts and the consequent opportunity for intervention. In recent years, many molecular studies investigating Echinococcus genotypes in humans and animals have been undertaken worldwide (Deplazes et al., 2017). Recently, Alvarez Rojas et al. (2014) reviewed the published genotyped cases of E. granulosus s.l. globally. Although genotyping has not been performed systematically, and therefore the data available are not fully representative, the genotype G1, which is mostly transmitted with a dog‐sheep cycle, was found to be responsible for the majority of human CE worldwide (88%). The genotype G7 is the major genotype in the Baltic States and Poland, but only a limited number of cases have been typed (Marcinkut≐ et al., 2015).
3.3.4.3. Comparative risk assessment
The use of a comparative risk assessment approach on Echinococcus source attribution is limited by a lack of occurrence data, particularly of contamination of foodstuffs with Echinococcus eggs.
3.3.4.4. Expert knowledge elicitation
As data on the sources of infection and risk factors for infection are scarce, the only point of exposure source attribution done is based on expert knowledge elicitation performed by FERG in the context of the global burden of food‐borne disease study (Devleesschauwer et al., 2015). FERG considered five main transmission routes: food, animal contact (domestic and wild), water, soil, and air (Hald et al., 2016).
For E. multilocularis, the uncertainties for the proportion of food transmission in these studies (EUR A7: 15–79%; EUR B,8 EUR C9: 12–72%; Hald et al., 2016) are large. The same applies to subsequent analyses that attributed food‐borne disease burden to specific food groups; the contribution of, e.g. vegetables had uncertainty ranges of 9–86%, 20–88% and 21–87%, respectively (Hoffmann et al., 2017).
For E. granulosus s.l., the proportion of food‐borne transmission was estimated for EUR A, B, and C at 4–40%, 6–39% and 6–39%, respectively (Hald et al., 2016), while the contribution of vegetables in these European regions was estimated to be 40–97%, 48–96%, and 47–97%, respectively (Hoffmann et al., 2017).
3.3.4.5. Concluding remarks on relative importance of food‐borne pathways
Humans acquire Echinococcus infection by the oral uptake of infective eggs, but the exact mechanisms and vehicles of transmission remain controversial, with quantification based solely on expert knowledge elicitation. Transmission vehicles might vary both between and within endemic areas, based on sociocultural and economic factors. However, it is clear that although the extent of food‐borne transmission may not be apparent, the potential for this transmission route is incontrovertible. Transmission could partially be linked to a typical food‐borne mechanism, after ingestion of viable eggs present on unwashed vegetables, fruit, and berries.
In Europe, the association of human cystic and alveolar echinococcosis, with contaminated food has to be considered based on the few preliminary data concerning food contamination with taeniid eggs and or parasite DNA. Standardised methods and more studies are needed to document the risk of food being contaminated with viable Echinococcus eggs (Alvarez Rojas et al., 2018). Expert knowledge elicitation indicates wide uncertainty for both CE (4–40% of cases) and AE (12–79% of cases) (Hald et al., 2016), and discussions on risk factors based on systematic review and meta‐analysis indicate the need for more primary studies. These, however, can be a challenge due to the long period between infection and the appearance of clinical signs.
Diagnosis of both AE and CE is usually made at an advanced stage of the disease, after a non‐symptomatic period of several years. This delayed diagnosis makes identification of the source of infection very difficult. It is therefore very challenging to link food exposures to disease (Torgerson, 2014). Furthermore, due to the long incubation period for disease, outbreaks of echinococcosis cannot be expected. Point‐of‐exposure source attribution studies for this parasite is therefore limited.
3.3.5. Consumer practices contributing to infection
There are no data specifying the contribution of food consumer practices. Certain consumer behaviour, life styles, or working conditions may increase exposure to Echinococcus spp. For example, regular close contact with dogs or activities in agriculture or in private gardens have been claimed to be associated with an increased infection risk. Risks based on contamination of food in production or in the kitchen with egg‐contaminated substrates or surfaces have, so far, not been identified. Furthermore, inappropriate washing of vegetables or fruit may contribute to a higher exposure to Echinococcus eggs; but, again, no data are available documenting that, for example, a higher exposure to E. multilocularis eggs will result in a higher incidence of disease.
3.3.6. Current control methods for food‐borne transmission and likely impact
3.3.6.1. Prevention of contamination
Attempts to control AE and CE are through measures that aim to interrupt the transmission cycle between definitive hosts and intermediate hosts.
Current concepts for control are different for AE and CE, as AE is caused by a parasite with a predominantly wild animal lifecycle, whereas CE is caused (mainly) by parasites of domesticated animals.
E. multilocularis
Control of E. multilocularis is currently not being conducted as a routine measure. However, numerous experimental studies have shown that effective control can be achieved, at least temporarily, by reducing infection in wild foxes. This can be achieved through the application of bait containing anthelmintic agents (praziquantel). Protocols have been published with variable parameters concerning bait density (up to 50 per km2), baiting frequency (1 per month to 2 per year), methods of distribution (by aircraft, by car, by hand), and the inclusion or exclusion of human settlements. As treatment is not preventive, numerous consecutive baiting campaigns are needed to reduce reinfection of foxes via rodents. Control by praziquantel baiting has been tested in large rural areas (up to 5,000 km2), as well as in small, circumscribed urban areas. Tools for control have been developed, are ready for use, and can be adapted to different environments (Hegglin and Deplazes, 2013; Craig et al., 2017), but the high cost of control contrasts with comparatively low numbers of AE patients in Europe, requiring risk‐management decisions (Hegglin and Deplazes, 2013). On the other hand, instigating such control measures is cheaper and easier when the infection is less widespread. Regular anthelmintic treatment of dogs is proposed as an additional measure, in particular to decrease the probability of transmission to humans (Deplazes et al., 2011).
E. granulosus s.l
Domestically transmitted species of the E. granulosus s.l. complex are, in theory, far easier to control. As transmission depends on dogs having access to slaughter offal, complete supervision of slaughtering practices by qualified personnel, such that dogs do not have access to slaughter offal, should be sufficient to interrupt the lifecycle. This has been achieved through improved general slaughtering hygiene (and without any specific control measures implemented) in large parts of central and western Europe, where CE is now reduced to sporadic occurrences only (Deplazes et al., 2017). Where a high‐standard of slaughtering is difficult to achieve, where home slaughtering is common, or where owned or stray dogs have access to dead animals on pastures, alternative measures can be applied (registration and regular deworming of dogs, education of stakeholders). By such approaches, CE has been eradicated under island conditions (Iceland, New Zealand, and Tasmania). For such targeted, long‐term control programmes, detailed protocols are available, providing recommendations for consecutive project phases spanning more than 30 years (planning, attack, consolidation, and maintenance phase) (Craig et al., 2017). Immunisation of livestock (which is available) has been experimentally tested for efficacy as a control measure (Craig et al., 2017). Depending on the region, the control situation can be complicated by the additional contribution of wild animals as hosts (e.g., wolves, jackals). On mainland Australia, the lifecycle of E. granulosus s.l. has switched from domestic transmission to a sylvatic cycle that includes wild dogs and marsupials, and cannot yet be contained by conventional control concepts.
3.3.6.2. Control measures
Due to the uncertainty about important routes of transmission to humans (see Section 3.3.4), there are no scientifically based recommendations or measures to prevent Echinococcus transmission via food or water. General recommendations (e.g., Eckert et al., 2001) include washing any produce that is eaten uncooked (e.g., vegetables, berries) and that originate either from the wild (e.g., blueberries) or from production sites with ineffective barriers against fox or dog access. However, although Taenia and Echinococcus eggs could be detected in the washwater after intense washing of vegetables, the proportion of contaminating eggs that had been removed by this process remains unknown (Federer et al., 2016). Alternatively, any physical conditions that are known to deactivate eggs and that are suitable for the food item in question are recommended: heating of food (see Section 3.1.3.2) for at least 3 h at 65°C (Federer et al., 2015)., as well as deep freezing at −80°C for a minimum of 24 h (Eckert et al., 2001).
3.3.6.3. Concluding remarks on control measures
Prevention of food contamination with Echinococcus eggs is best achieved by controlling parasite transmission. Strategies are available and evaluated in the case of CE. Especially for Europe, where E. granulosus s.s. and E. intermedius are overwhelmingly only present in domesticated animals, which facilitates the application of control measures, a strong reduction of parasite transmission and, in certain areas, even elimination is feasible. For AE, largely transmitted by wildlife (e.g., foxes), regional elimination would be difficult, but methods for control at a local level (e.g., in areas of close human‐wildlife contact) are available. Accompanying measures would be the restriction of the access of animals that may be shedding Echinococcus eggs to food production areas.
3.4. Commonalities for all parasites
Although the three parasites included in this Opinion differ in many respects, there are several commonalities regarding their food‐borne transmission potential and the methodologies available for investigating food‐borne transmission.
For all three parasites, the available data lead to an under‐estimate of the burden of infection in Europe. Furthermore, because of the differences across and between countries regarding mandatory reporting, it is difficult to ascertain whether regional variations with relation to infections reflect actual epidemiological differences or rather diagnostic effort or reporting differences.
Incubation period from infection until manifestation of symptoms ranges from a few days for Cryptosporidium to years or decades for Echinococcus spp. Toxoplasma infection may not demonstrate symptoms at all unless triggered by an event such as immunosuppression. Furthermore, symptoms of ocular toxoplasmosis often only manifest decades after infection occurred. This means that source attribution is difficult – and may even be impossible for those parasites with the longest incubation period.
Although Toxoplasma can also be transmitted by ingestion of the meat of an infected animal, all three parasites can be transmitted via their robust external transmission stages (eggs or oocysts), which are shed into the environment in relatively high numbers by infected definitive hosts. These environmental transmission stages may contaminate fresh produce (and, potentially, other food stuffs, e.g., shellfish), and can remain viable and infective on or in such food for prolonged periods (weeks or longer), even under relatively harsh conditions. None of these transmission stages proliferate in the environment, and thus, the potential for contaminated foodstuffs to act as a vehicle of infection decreases over time due to die‐off. However, given the short shelf‐life for fresh produce, die‐off is not expected to be considerable.
Although a considerable amount of research has been conducted on developing methods for determining whether meat contains Toxoplasma bradyzoites, and is therefore potentially a route of transmission to consumers, method development for detection and assessment of viability and infectivity of all three parasites as faecal contaminants on fresh produce is less well developed. Sensitivity of detection and quantification are desirable for risk assessment, and LOD data are important to understand negative results from sample surveys. Thus, these data are also needed for risk management and consumer protection. However, in the absence of standard methods for most foods, experiments to establish LOD have not been done for all food types. It is desirable that portion‐sized amounts of food are spiked with test parasites for LOD studies.
Molecular methods to amplify nucleic acids (e.g., DNA) have been used to assess contamination of fresh produce with all three parasites. It is worth noting that the parasites reviewed in this Opinion contain different amounts of DNA in the lifecycle stages present and used as detection targets in food detected as faecal contaminants on food; for example, one embryonated Echinococcus egg contains tens of cells whereas one Cryptosporidium oocyst contains only four.
Unlike with culture methods for the detection of bacteria, detection of parasites on food, either by microscopy to detect oocysts or eggs, or by methods to detect nucleic acids, e.g. DNA, provides no indication of the viability or infectivity of any parasites detected. Methods to assess the viability or infectivity of the three parasites are, at best, expensive, cumbersome, and ethically questionable (bioassays), or the results may overestimate viability (molecular methods, vital dye methods). Therefore, assessment of viability and infectivity during surveys may be inappropriate. However, such assays would be of value and should be developed for the investigation of post‐harvest treatments.
3.5. Knowledge gaps in answering the terms of reference
3.5.1.
Cryptosporidium spp.
The relative importance of food‐borne infections (food versus other routes or vehicles) is not known.
The relative importance of different sources of contamination of food (e.g., direct handling by people, livestock, irrigation water, wastewater, etc.) is not known.
The likelihood of transmission from food overall or from specific foods is not known; data for QMRA are not yet available.
The robustness and LOD of detection methods are not known for most foods.
More sensitive methods for genotyping Cryptosporidium oocysts from foods need to be developed so that they can be applied to samples containing small numbers of oocysts.
Sample surveys/sampling frames have yet to be established for adequate studies of food, whether for the presence/absence of oocysts or for their quantification.
Survival studies with optimal methods for assessing viability and infectivity, to provide improved efficacy data for control measures and food preservation/treatment conditions, need to be undertaken.
Food trace‐back is currently poor; methods to interrogate the supply chain for Cryptosporidium contamination are not well developed.
Toxoplasma gondii
The relative importance of food‐borne infections (food versus other sources) is not known, but due to the meatborne transmission route, likely to be higher than for the other two parasites considered here (Cryptosporidium and Echinococcus spp.).
The relative contributions of meatborne (via tissue cysts) transmission and environmental (via oocysts) transmission has not yet been clarified. This will vary according to region and consumer habits.
Diagnostic methods to detect T. gondii in fresh produce are currently poorly developed.
Diagnostic methods to detect T. gondii in cattle are currently poorly developed.
QMRAs of combined transmission routes (meatborne and via food contaminated with Toxoplasma oocysts) have not been developed.
Dose–response data in humans that are necessary to translate exposure into human infection risk are lacking.
Data on inactivation of tissue cysts in meat products (the necessary concentrations of salt and other preservatives) have not been collected systematically.
Methods, other than animal bioassays, to assess the infectivity/viability of T. gondii are lacking.
The efficacy of intervention strategies to reduce infection in food‐producing animals based on risk factors has not been evaluated.
The feasibility of cat vaccination (development and coverage needed) has not yet been addressed.
Data on the prevalence of different T. gondii genotypes in food are lacking, as are standardised methods to assess this, particularly in Europe where genotype II predominates.
Depuration times required for shellfish to clear themselves of T. gondii oocysts are currently unknown.
Survival times of T. gondii in dairy products made from raw milk derived from infected goats and sheep are unknown.
The effectiveness of intervention strategies for reducing contamination of fresh produce with infective oocysts has not been evaluated.
Echinococcus spp.
The relative importance of food‐borne infections (food versus hand‐to‐mouth contact or other vehicles) is not known.
Validated methods to detect Echinococcus eggs in fresh produce and data for QMRA‐based are not yet available.
Dose–response data in humans that are necessary to translate exposure in human infection risk are lacking.
Factors determining the susceptibility of humans to infection, especially for AE, are not understood.
The efficacy of intervention strategies (anthelmintic treatment of canids) to reduce the environmental contamination with eggs and their effective contribution towards minimising the likelihood of infection have not been investigated.
The correlation of infectivity, pathogenicity and treatment response with the different causative Echinococcus spp. of CE (and their genotypes) has not been determined.
Ecological parameters that favour persistence of the E. multilocularis lifecycle, including the impact of climate change, have not been elucidated.
4. Conclusions
4.1.
4.1.1.
Answers to TOR 1
To critically review current methods for the detection, identification, characterisation and tracing of specific, selected food‐borne parasites ( Echinococcus spp., Toxoplasma gondii , and Cryptosporidium spp.), with emphasis on methods applicable to foods that are likely to be a potential source of infection
Meatborne transmission: of the parasites under consideration, only T. gondii can be transmitted as an intrinsic part of meat. Methods to detect, identify, characterise and trace T. gondii in meat are relatively well‐developed; mouse bioassay and PCR are the most commonly used direct detection methods, followed by microscopy and cat bioassay. These methods tend to be used largely in research projects or for investigating interventions or investigating outbreaks, rather than as a routine procedure for checking the safety of meat. There are currently no regulatory requirements to conduct meat inspection or analysis for T. gondii in Europe. Interlaboratory validation for these methods is not routinely performed.
-
Transmission as faecal contaminants (e.g., on fresh produce): all three parasites may be transmitted via their robust environmental stages as faecal contaminants of food, but standard methods for their detection in all possible matrices have not been developed.
-
−
For Cryptosporidium oocysts, an ISO standard method is available for detection on some fresh produce types (fresh leafy green vegetables and berry fruits), although it should be noted that it has been validated only for relatively few produce types within this category. The ISO method specifies microscopy for detection; this provides no information on species, viability or infectivity.
-
−
For T. gondii and Echinococcus spp., methods for analysing fresh produce types (see above) have not been standardised, and methods have to be further validated for routine use in analytical laboratories. Microscopy lacks sensitivity and does not distinguish between genotypes, and, for taeniid eggs, between species or even genera. For both these parasites, DNA amplification after oocyst or egg concentration is necessary to obtain this information.
-
−
In general, and even for those parasite‐food combinations for which techniques have been developed and published, methods for analysing foods as vehicles of infection for these three parasites are not well established, standardised, or validated.
The use of methods that have been validated by inter‐laboratory ring trials have the potential to provide results from surveys, which allow comparison on a temporal and spatial scale. Although molecular‐based studies for detection of faecal contaminant parasites may be more sensitive, it should be noted that detection of DNA molecules does not necessarily indicate the presence of an intact transmission stage, although partial purification of the parasites from the sample prior to application of molecular methods may indicate whether this is the case. If transmission stages are detected by microscopy methods (i.e., Echinococcus eggs, or Toxoplasma or Cryptosporidium oocysts), whether they are infective and thereby represent a public health risk cannot be determined. Thus, methods that indicate infectivity as well as occurrence would be of interest.
Faecal indicators are not reliable indicators for presence or absence of these parasites.
Answers to TOR 2
To evaluate available information to determine the relative importance of food‐borne pathways for transmission of the selected parasites to humans
The source of infection for the three parasites differs widely, and depends upon the distinct lifecycles and the hosts of the three parasites. In brief, Cryptosporidium oocysts are shed in the faeces of infected animals, particularly young ruminants, and humans and may contaminate food. Toxoplasma oocysts shed in the faeces of infected felids may also contaminate food, but tissue cysts in meat animals are also a source of infection, as are tachyzoites shed in milk. Echinococcus spp. eggs shed in the faeces of infected canids may contaminate food.
Across all three food‐borne parasites, there is a general lack of data that limits the application of data‐driven methods to identify the relative importance of major transmission routes (i.e., food‐borne transmission vs transmission via water, soil, person‐to‐person contact or animal contact). This is particularly so for Echinococcus. Much information is therefore derived from expert knowledge elicitation studies and is typically characterised by the associated important uncertainties.
Available information suggests that food‐borne infection is responsible for approximately 40–60% of T. gondii infections and approximately 10% of human Cryptosporidium infections. The relative importance of food‐borne transmission for Echinococcus spp. infections remains largely uncertain, ranging from around 4–40% for CE to 12–80% for AE, indicating the insurmountable limitations in trying to determine source attribution for a pathogen for which the incubation period between infection and diagnosis is generally measured in years.
Knowledge of lifecycles and identified outbreaks and cases provides a better basis for identifying those food categories that are of greater importance in food‐borne transmission. However, although more data are available to ascertain the different food groups that contribute to food‐borne transmission of the three parasites, many studies still rely on expert knowledge elicitation.
Food‐borne transmission of Toxoplasma gondii is possible via a range of routes, including consumption of undercooked meat or, to a lesser extent, unpasteurised milk, from an infected animal, or as a faecal contaminant. Although meat is considered to be the more usual source of food‐borne infection in Europe, based on risk factor studies, the exact contribution of different food‐borne routes is still a major research question.
In contrast, food‐borne transmission of both Cryptosporidium and Echinococcus occurs solely with the parasites as faecal contaminants. However, whereas for Echinococcus, the source of contamination is infected canids (in Europe, largely dogs and foxes), for Cryptosporidium the range of potential hosts shedding oocysts in their faeces is much broader, and includes livestock animals, especially young ruminants, and people.
For Cryptosporidium, fresh produce is considered to be the main route for food‐borne transmission, on the basis of outbreak data, national studies, and expert knowledge elicitation, especially where dairy hygiene standards are high and milk pasteurisation is the norm. Although some surveys of fresh produce have been conducted and provided some information on the extent of contamination, lack of routine genotyping, along with a paucity of exploration of infection sources, has limited our knowledge on transmission routes.
For Toxoplasma, there is restricted ability to determine transmission routes. This is largely due to the usual prolonged period between infection and clinical presentation. In addition, there is a lack of methods to detect oocyst‐contaminated food.
For Echinococcus (E. granulosus s.l. and E. multilocularis), the relative importance of food‐borne transmission is uncertain. This is due to the long latent period between infection and clinical signs and the very limited data on the occurrence of Echinococcus contamination of food.
Answers to TOR 3
To examine available information on the occurrence and survival of the selected parasites in food and consumer practices contributing to infection
All three parasites can be transmitted by ingestion of contaminated foods that are often not cooked before consumption, such as some types of fresh produce. For Toxoplasma and Echinococcus, survey data are very limited. For Cryptosporidium, only six surveys have been conducted using a reliable method and these suggest contamination rates from 1% to 70% with most large surveys indicating a contamination rate of 8% of samples. There are many hosts of Cryptosporidium and therefore greater potential for contamination in comparison with T. gondii, for which only cats, nearly always kittens, shed oocysts for a limited period, or Echinococcus, for which only infected canids shed eggs in their faeces.
Toxoplasma can be transmitted via the ingestion of raw or inadequately cooked meat from infected animals. The likelihood that meat animals are infected varies by species and animal husbandry/animal management practices.
Information on survival of contaminating parasites is largely lacking due to absence of available and validated methods to determine this.
Cryptosporidium oocysts survive in moist environments at ambient temperatures for many months, but are less resistant to freezing; Toxoplasma oocysts are more robust, surviving for many months in the environment and also surviving freezing temperatures for weeks; experimental data indicate prolonged survival of Echinococcus eggs in the environment, including freezing at −18°C for several months.
The oocyst transmission stages of Cryptosporidium and Toxoplasma are inactivated by pasteurisation; E. multilocularis eggs survive heating to + 65°C for 120 min.
Imported foods also present a risk to consumers. For Toxoplasma, strains from South America have greater genetic diversity and virulence than those currently circulating in Europe. The import of fresh, vacuum‐packed beef or horse meat from these countries may be considered as an emerging threat to European consumers as these products are sometimes consumed undercooked or raw.
Fresh produce may present a threat for all three parasites, as the transmission stages are likely to survive well under the refrigerated conditions of transport. However, trace‐back is currently inadequate and relevant information is generally lacking.
For both Cryptosporidium and Toxoplasma, survey studies have investigated contamination of molluscan shellfish, and data accrued indicated occurrence of 10–20% (Toxoplasma) and 20–40% (Cryptosporidium). However, in the absence of documented infection with either of these parasites via molluscan shellfish, the relevance of these data to food‐borne transmission remains unclear.
In general, consumer preferences for raw fresh produce may contribute towards an increased likelihood of infection, as cooking for an adequate time at a specified temperature inactivates all parasite transmission stages. Furthermore, a lack of general hygiene measures, including not washing fresh produce prior to consumption, may increase the likelihood of ingesting viable parasites. However, there is evidence that significant numbers of transmission stages may remain even after washing. Industrialised washing, with reuse of water, may result in the spread of contamination throughout a batch, and consumer preference for ready‐to‐eat fresh produce may increase the likelihood of ingesting infective contaminant parasites. Consumer preferences for not freezing produce or meat prior to consumption, and for eating more raw or rare products (vegetables, meat, milk, and dairy products), may also increase the likelihood of exposure to infective parasites.
Data on the efficacy of preservation methods (salting, conservatives, curing) at inactivating T. gondii bradyzoites in meat products are limited.
Answers to TOR 4
To evaluate possible control measures from farm to consumption
-
For all three parasites (as faecal contaminants of fruit and vegetables)
-
−
On‐farm measures that reduce the likelihood of faecal contamination may be more effective than post‐harvest interventions.
-
−
Use of irrigation water of potable standard or good quality will reduce the likelihood of contamination of fresh produce.
-
−
Faecal indicators are not reliable indicators for the presence or absence of any other parasites, including Cryptosporidium.
-
−
If wastewater is used for irrigation, it is important that it is tertiary‐treated, and includes treatments that are adequate against parasite oocysts and eggs, and not only against bacteria.
-
−
Washing fresh produce prior to consumption may remove a proportion of adherent parasites, but relevant data are few, and the presence of Cryptosporidium oocysts remaining on fresh produce after energetic washing has been demonstrated. Industrial‐scale washing may spread localised contamination throughout a batch, and some fresh produce (e.g., strawberries) may not be appropriate for vigorous washing.
-
−
-
For Toxoplasma and Cryptosporidium
-
−
Pasteurisation will inactivate both Cryptosporidium oocysts and Toxoplasma tachyzoites.
-
−
Sufficient heat treatment that reaches the internal flesh and inactivates parasite oocysts in shellfish would prevent transmission.
-
−
-
For Cryptosporidium
-
−
Control of animal access, particularly by young ruminants, to areas of cultivation of fresh produce reduces the probability of contamination.
-
−
Proper composting of manure or storage of liquid slurry before application to crops reduces the likelihood of contamination with viable oocysts.
-
−
Providing adequate sanitation and hygiene facilities (e.g., toilets and hand washing facilities) for food production and processing workers reduces the potential for contamination from infected workers.
-
−
Enabling exclusion from work of food handlers with diarrhoea, and for 48 h after symptoms cease, reduces the chances of transmitting infection to co‐workers and contaminating produce.
-
−
-
For Toxoplasma
-
−
For transmission via meat from housed animals, reduction of their exposure to infection via appropriate biosecurity measures (e.g., preventing access of cats to pig pens or poultry barns) will reduce transmission potential. Reducing cat numbers at farms and neutering cats to limit new kittens annually has been suggested to reduce on farm contamination with infective oocysts.
-
−
For transmission via meat from grazing animals, reduction of exposure is more difficult, but vaccination of sheep and pigs is a relevant option for reducing infection.
-
−
For transmission via meat, freezing the meat prior to consumption or sale will inactivate any bradyzoites.
-
−
For fresh produce, limiting access of cats to areas where produce is grown, packed, and transported, would be relevant and ensuring that irrigation water is of an appropriate quality.
-
−
-
For Echinococcus
-
−
Control of E. granulosus s.l. is feasible by an integrated approach at the regional or national level, including control of stray dogs, meat inspection and slaughter supervision, public education campaigns, routine anthelmintic treatment of dogs, and vaccination of sheep.
-
−
For both, E. granulosus s.l. and E. multilocularis, regular treatment of dogs in endemic areas will limit the potential for contamination of food such as fresh produce.
-
−
Similarly, regular treatment of foxes using praziquantel baits in endemic areas, or even locally in areas where fresh produce is cultivated, will reduce the prevalence of E. multilocularis, and thereby the potential for environmental contamination, and thus the potential for fresh produce being contaminated.
-
−
Implementation of measures that reduce the population of foxes and stray dogs, or the access of foxes and dogs to fresh produce areas, may also reduce the potential for contamination of fresh produce.
-
−
5. Recommendations
It is clear that there are many data gaps in our knowledge of food‐borne transmission of parasites, compared with the transmission of other pathogens. This Opinion focuses on three parasites considered to be of highest importance in Europe at the time of writing; however, it is important to note that other food‐borne parasites may also become of increasing public health relevance. These recommendations suggest not only areas where more data should be gathered, but also indicate where we already have sufficient data to reach concrete recommendations.
In order to define targeted control strategies, generally we need to acquire more knowledge on the relative importance of food‐borne transmission for each of the three parasites. This will require a multifaceted approach, and should be directed towards obtaining sufficient data such that robust QMRA can be performed for each of the parasites. Recommendations on how further essential data collection can be facilitated are listed below, as are potential approaches towards reducing the food‐borne transmission of the three parasites included in this Opinion.
-
Recommendations for further data collection
-
−
Robust and reliable methods for detection of the three parasites on different foods need to be developed and validated (interlaboratory validation). For all three parasites, detection of parasites on different types of fresh produce (lettuce, other leafy greens, fruits) is particularly important. It should be borne in mind that different fresh produce may have different properties that affect detection methodologies (e.g., saponins in spinach leaves, cyanins in blueberries). One method does not necessarily suit all matrices or parasites.
-
−
For Toxoplasma, development, validation and use of a sporozoite‐targeted assay that could be used to distinguish transmission of T. gondii by oocysts (e.g., via contamination of fresh produce, shellfish) from other forms of transmission (e.g., by bradyzoites in meat) should be prioritised.
-
−
Methods for viability/infectivity assessment for each of the three parasites need to be developed, validated, and applied in survival/efficacy studies of food storage and treatment conditions. Although bioassays are available, these are not appropriate or ethically supportable for such studies.
-
−
Improved documentation of the fresh produce supply chain would provide better knowledge regarding how and where contamination occurs. In particular, the role of water as a vehicle of contamination of fresh produce with parasite transmission stages is relevant.
-
−
Our knowledge of the origins of contamination when parasites are detected is hampered by the lack of suitability and/or use of molecular markers for subtyping. Although whole genome sequencing (WGS) may provide a solution, it is not necessarily appropriate for small numbers of parasites (that cannot be amplified) in a contamination situation. Development and validation of the use of appropriate molecular markers would improve our knowledge on sources and routes of contamination and infection.
-
−
Public health professionals should be encouraged to include questions regarding food consumption in a relevant time‐span when investigating cases or outbreaks of infection.
-
−
The wide uncertainty regarding the contribution of food‐borne transmission to cases of both CE and AE indicate the need for more primary studies. However, due to the long latent period between infection and clinical signs, these can be a challenge.
-
−
The relative importance of these and other food‐borne parasites should be re‐evaluated as further data become available.
-
−
-
Recommendations for potential approaches to reducing the potential for food‐borne transmission
-
−
In order for the fresh produce industry to understand where the risks of parasite contamination lie and develop reduction/intervention strategies, investigation of parasite contamination along the fresh‐produce food chain and through produce‐processing plants is necessary.
-
−
Inactivation technologies should be developed and optimised against parasite transmission stages to provide post‐harvest control options that are evidence based.
-
−
Following relevant data collection, risk assessments regarding the food‐borne transmission of toxoplasmosis should be extended to include the environmental (oocyst) pathway.
-
−
The feasibility of applying the current commercial T. gondii live vaccine (S48 strain) to reduce T. gondii cyst development in food animals should be explored.
-
−
Development of methods for reliable detection of T. gondii in cattle should be prioritised.
-
−
Development of education programmes based on key transmission routes could be used to help inform high‐risk groups of people, particularly pregnant women and immunocompromised individuals, regarding both toxoplasmosis and cryptosporidiosis (the immunocompromised), as these groups may account for a relatively high burden of disease.
-
−
For Cryptosporidium and Toxoplasma, the feasibility of whether vaccination may decrease oocyst shedding and thus environmental contamination for key animal species (such as cattle for Cryptosporidium and cats for Toxoplasma) should be explored.
-
−
CE, caused by Echinococcus granulosus s.l. in Europe, is largely transmitted during lifecycles that involve domestic animals (dogs and livestock). Therefore, CE is a preventable and controllable disease, and scientific evidence exists for the efficacy of control measures. The persistence of human morbidity in Europe is not due to knowledge gaps, but lack of prioritisation to implement the control measures. Successful elimination or considerable reductions in transmission of E. granulosus have been achieved in several control programmes, and the initiation of such actions in endemic areas of Europe is recommended if the intention is to control this parasite.
-
−
For AE, caused by Echinococcus multilocularis, the practicality of implementing effective control actions is less obvious as transmission is largely wildlife‐based (foxes and rodents). In areas of high egg contamination and in areas of close human‐wildlife contact (e.g., periurban areas with high fox populations), the use of praziquantel bait for foxes may be effective, even locally, but requires long‐term commitment and resources.
-
−
Abbreviations
- AE
alveolar echinococcosis
- AIDS
acquired immune deficiency syndrome
- CE
cystic echinococcosis
- COST
European Cooperation in Science and Technology
- DALY
disability‐adjusted life year
- ECDC
European Centre for Disease Prevention and Control
- EEA
European Economic Area
- ELISA
enzyme‐linked immunosorbent assay
- FERG
Food‐borne Disease Burden Epidemiology Reference Group
- IFM
immunofluorescence microscopy
- IHC
immunohistochemistry
- IMS
immunomagnetic separation
- ISO
International Organization for Standardization
- LAMP
loop‐mediated isothermal amplification
- LOD
limit of detection
- (MC)‐PCR
magnetic capture
- PAF
population attributable fractions
- PCR
polymerase chain reaction
- QMRA
Quantitative microbiological risk assessment
- qPCR
quantitative polymerase chain reaction
- SCID
severe combined immunodeficiency
- SH‐RT
sodium hypochlorite
- s.l.
sensu lato
- s.s.
sensu stricto
- US FDA
United States Food and Drug Administration
- UV
ultraviolet
- WGS
whole genome sequencing
- WHO
World Health Organization
Glossary
- Bradyzoite
a slowly multiplying stage in the lifecycle of Toxoplasma, that usually develops in cells of the central nervous system, eye, or striated muscle, forming tissue cysts
- Host
the organism within or on which a parasite lives, potentially deriving nutrients or other benefits from it, at the expense of that organism
- Intermediate host
a host organism that is essential in the lifecycle of the parasite and in which development occurs, but sexual reproduction does not occur
- Definitive host
the host organism in which a parasite reaches sexual maturity and reproduces sexually
- Dead‐end host
an aberrant or incidental host of the parasite, in which development may be similar to that in an intermediate host, but without contributing to the perpetuation of the lifecycle
- Excystation
the process of hatching of an oocyst (Toxoplasma or Cryptosporidium) to release the sporozoites
- Molecular detection
use of methods that amplify nucleic acids to determine the presence of a target parasite
- Oocyst
the external transmission stage of both Cryptosporidium and Toxoplasma, consisting of an encysted zygote from sexual reproduction, and that may contain infective sporozoites or may develop (sporulation) to contain infective sporozoites
- Sporozoite
a motile stage that is contained within the oocysts of Cryptosporidium (4 sporozoites per oocyst) and Toxoplasma (eight sporozoites per oocyst), and that invade the host cells following excystation of ingested oocysts by an appropriate host
- Sporulation
the development of sporozoites within oocysts; for Cryptosporidium, oocyst sporulation occurs prior to shedding in the faeces, whereas for Toxoplasma sporulation of oocysts occurs in the environment after 1–5 days, depending on temperature and humidity. Oocysts that are not sporulated are not infective
- Tachyzoite
a rapidly multiplying motile stage in the lifecycle of Toxoplasma, and that multiplies asexually in almost any cell of the host (not intestinal epithelial cells or erythrocytes), bursting the cells and moving to a new cell
- Tissue cyst
the cyst containing bradyzoites; these vary in size depending on the number of bradyzoites
Appendix A – Replies from EU/EEA countries to Questionnaire on notification systems for selected parasites
1.
In April 2018, a questionnaire about mandatory notification of Cryptosporidium spp., T. gondii, E. multilocularis and E. granulosus s.l. was sent to the 28 Member States and 3 European Economic Area (EEA) countries (Iceland, Norway, and Switzerland) through the members and observers of EFSA's Scientific Network for Zoonoses monitoring data. Replies were obtained from 30 (27 Member States and 3 EEA countries)/31 countries.
A summary of the questionnaire and the results is shown below. It should be noted that the results obtained are those provided from the questionnaire and they have not been validated.
A.1. Mandatory notifications in Animals in EU/EEA countries
Questions for animals:
Is notification of alveolar echinococcosis (AE in intermediate and dead‐end hosts and infection with E. multilocularis in definitive hosts) mandatory in your country?
Is notification of cystic echinococcosis (CE in intermediate and dead‐end hosts and infection with E. granulosus in definitive hosts) mandatory in your country?
Is notification of toxoplasmosis (infection with Toxoplasma gondii) mandatory in your country?
Is notification of cryptosporidiosis (infection with Cryptosporidium spp.) mandatory in your country?
Table A.1.
Summary of replies on notification of detection in animals
| Country | E. multilocularis | E. granulosus | Toxoplasma gondii | Cryptosporidium spp. | |
|---|---|---|---|---|---|
| Austria | AT | ○ | ○ | ○ | ○ |
| Belgium | BE | ● | ● | ● | ● |
| Bulgaria | BG | V | ○ | V | V |
| Croatia | HR | ● | ● | ○ | ○ |
| Cyprus | CY | ● | ● | ○ | ○ |
| Czech Republic | CZ | ● | ● | ○ | ○ |
| Denmark | DK | ● | ● | ○ | ○ |
| Estonia | EE | ● | ● | ● | V |
| Finland | FI | ● | ● | ● | ○ |
| France | FR | ○ | ○ | / | / |
| Germany | DE | ● | ● | ● | ○ |
| Greece | EL | V | V | V | V |
| Hungary | HU | ● | ● | V | V |
| Iceland | IS | ● | ● | ● | ● |
| Ireland | IS | ● | ○ | V | V |
| Italy | IT | ● | ● | ○ | ○ |
| Latvia | LV | ● | ● | ● | ● |
| Lithuania | LT | – | – | – | – |
| Luxembourg | LU | V | V | V | V |
| Malta | MT | V | V | V | V |
| Netherlands | NL | ● | ● | ● | ○ |
| Norway | NO | ○ | ○ | ○ | ○ |
| Poland | PL | ● | ● | ● | ○ |
| Portugal | PT | ○ | V | ○ | ○ |
| Romania | RO | ● | ● | ● | ○ |
| Slovak Republic | SK | ● | ● | ○ | ○ |
| Slovenia | SI | ● | ● | ○ | ○ |
| Spain | ES | V | V | V | V |
| Sweden | SE | ● | ● | ○ | ○ |
| Switzerland | CH | ● | ● | ● | ● |
| United Kingdom | UK | / | ○ | ○ | ○ |
●: Yes; ○: No; V: Voluntary; /: no surveillance; – : no information.
A.2. Mandatory notifications in Food in EU/EEA countries
Questions:
Is detection of E. multilocularis in food notifiable in your country?
Is detection of E. granulosus in food notifiable in your country?
Is detection of Toxoplasma gondii in food notifiable in your country?
Is detection of Cryptosporidium spp. in food notifiable in your country?
Table A.2.
Summary of replies on notification of detection in food
| Country | E. multilocularis | E. granulosus | Toxoplasma gondii | Cryptosporidium spp. | |
|---|---|---|---|---|---|
| Austria | AT | ○ | ○ | ○ | ○ |
| Belgium | BE | ● | ● | ● | ● |
| Bulgaria | BG | V | V | V | V |
| Croatia | HR | / | / | / | / |
| Cyprus | CY | ○ | ○ | ○ | ○ |
| Czech Republic | CZ | V | V | ○ | ○ |
| Denmark | DK | ○ | ○ | ○ | ○ |
| Estonia | EE | ● | ● | ● | ○ |
| Finland | FI | V | V | V | V |
| France | FR | ○ | ○ | / | / |
| Germany | DE | ● | ● | ● | ● |
| Greece | EL | V | V | V | V |
| Hungary | HU | ● | ● | V | V |
| Iceland | IS | / | / | / | / |
| Ireland | IE | ● | V | V | V |
| Italy | IT | ● | ● | ○ | ○ |
| Latvia | LV | ● | ● | ○ | ○ |
| Lithuania | LT | – | – | – | – |
| Luxembourg | LU | ● | ● | ● | ● |
| Malta | MT | ○ | V | V | V |
| Netherlands | NL | ○ | V | V | V |
| Norway | NO | ○ | ○ | ○ | ○ |
| Poland | PL | ● | ● | V | V |
| Portugal | PT | V | V | ○ | V |
| Romania | RO | ● | ● | V | ○ |
| Slovak Republic | SK | ● | ● | ○ | ○ |
| Slovenia | SI | ○ | ● | ○ | ○ |
| Spain | ES | ● | ● | V | V |
| Sweden | SE | ○ | ○ | ○ | ○ |
| Switzerland | CH | ● | ● | ● | ● |
| United Kingdom | UK | ○ | ○ | ○ | ○ |
●: Yes; ○: No; V: Voluntary; /: no surveillance; – : no information.
A.3. Mandatory notifications in Humans in EU/EEA countries
Member States, CH, IS and NO, were asked to verify the information summarised from ECDC (https://ecdc.europa.eu/en/surveillance-atlas-infectious-diseases).
If notification of Echinococcosis in humans is mandatory: is this notification mandatory for alveolar echinococcosis (E. multilocularis) and cystic echinococcosis (E. granulosus)?
Table A.3.
Summary of replies on notification of human cases
| Country | E. multilocularis | E. granulosus | Toxoplasma gondii | Cryptosporidium spp. | |
|---|---|---|---|---|---|
| Austria | AT | ● | ● | / | / |
| Belgium | BE | V | V | / | V |
| Bulgaria | BG | ○ | ● | ● | ● |
| Croatia | HR | ● | ● | ● | ● |
| Cyprus | CY | ● | ● | ● | ● |
| Czech Republic | CZ | ● | ● | ● | ● |
| Denmark | DK | / | / | / | / |
| Estonia | EE | ● | ● | ● | ● |
| Finland | FI | ● | ● | ● | ● |
| France | FR | – | / | V | / |
| Germany | DE | ● | ● | ● | ● |
| Greece | EL | ● | ● | / | / |
| Hungary | HU | ● | ● | ● | ● |
| Iceland | IS | ● | ● | ● | ● |
| Ireland | IE | ● | ● | ● | ● |
| Italy | IT | ● | ● | / | / |
| Latvia | LV | ● | ● | ● | ● |
| Lithuania | LT | – | – | – | – |
| Luxembourg | LU | ● | ● | ● | ● |
| Malta | MT | ● | ● | ● | ● |
| Netherlands | NL | V | V | / | V |
| Norway | NO | ● | ● | ● | ● |
| Poland | PL | ● | ● | ● | ● |
| Portugal | PT | ○ | ● | ● | ● |
| Romania | RO | ● | ● | ● | ● |
| Slovak Republic | SK | ● | ● | ● | ● |
| Slovenia | SI | ● | ● | ● | ● |
| Spain | ES | ● | ● | ● | ‐ |
| Sweden | SE | ● | ● | / | ● |
| Switzerland | CH | – | – | – | – |
| United Kingdom | UK | V | V | V | ● |
●: Yes; ○: No; V: Voluntary; /: no surveillance; – : no information.
A.4. Identification of Cryptosporidium spp. for humans in EU/EEA countries
Additional question on Cryptosporidium:
During investigation of cryptosporidiosis (infection with Cryptosporidium spp.) in humans, is the species of Cryptosporidium determined (e.g., Cryptosporidium hominis, Cryptosporidium parvum, etc.)?
Table A.4.
Summary of replies of identification of Cryptosporidium spp. in humans
| Country | Answer | |
|---|---|---|
| Austria | AT | – |
| Belgium | BE | – |
| Bulgaria | BG | No, never |
| Croatia | HR | Yes, always |
| Cyprus | CY | No, never |
| Czech Republic | CZ | Sometimes |
| Denmark | DK | Sometimes |
| Estonia | EE | Sometimes |
| Finland | FI | Sometimes |
| France | FR | – |
| Germany | DE | Sometimes |
| Greece | EL | No, never |
| Hungary | HU | – |
| Iceland | IS | – |
| Ireland | IE | Sometimes |
| Italy | IT | No, never |
| Latvia | LV | No, never |
| Lithuania | LT | – |
| Luxembourg | LU | Yes, always |
| Malta | MT | Sometimes |
| Netherlands | NL | Sometimes |
| Norway | NO | Yes, always |
| Poland | PL | Sometimes |
| Portugal | PT | Yes, always |
| Romania | RO | Sometimes |
| Slovak Republic | SK | Sometimes |
| Slovenia | SI | Yes, always |
| Spain | ES | Sometimes |
| Sweden | SE | Yes, always |
| Switzerland | CH | – |
| United Kingdom | UK | Yes, always |
– : no information.
Appendix B – Food‐borne outbreaks of cryptosporidiosis
1.
Table B.1.
Documented food‐borne outbreaks of cryptosporidiosis globally, highlighting those in Europe
| Country | Year | Implicated food type | No. of cases (lab confirmed) | Species and gp60 genotype in cases | Age/population affected | Strength of evidence for food‐borne transmission and food type | Suspected source of contamination | Other information | References |
|---|---|---|---|---|---|---|---|---|---|
| UK | 2015 | Fresh produce: salad leaves in products at a coffee shop chain | 424 confirmed cases from national surveillance data | C. parvum IIdA24G1 | Mostly adults; mostly females | Analytical epidemiology | Not known | Public Health England (2017) | |
| USA | 2014 | Milk and dairy products: unpasteurised goat milk | 11 (6) | C. parvum IIaA16G3R1 | Children and adults | Descriptive epidemiology | Not known | PCR tests on milk samples in a commercial lab considered false positive; importance of validating PCR protocols | Rosenthal et al. (2014) |
| Finland | 2012 | Fresh produce: Frisee salad | 264 (18) | C. parvum IIdA17G1 | Adults | Descriptive epidemiology; five linked outbreaks at restaurants | Traceback identified vegetable processor and wholesaler in Finland, production in The Netherlands | Authors suggested methods to analyse frozen samples should be developed | Åberg et al. (2015) |
| UK | 2012 | Fresh produce: bagged ready‐to‐eat salad | > 300 excess confirmed cases from national surveillance data | C. parvum IIaA15G2R1 | Mostly adults; mostly females | Analytical epidemiology (case‐control study) | Not known; difficulties in product traceability | McKerr et al. (2012) | |
| Sweden | 2010 | Not known | 16 (2) | C. parvum IIdA20G1e | Adults | Assumed to be a food handler | Gherasim et al. (2010) | ||
| Sweden | 2010 | Fresh produce: salad garnish | 89 (10) | C. parvum IIdA24G1 (6 cases) | Adults | Descriptive epidemiology outbreak investigation | Not known | Gherasim et al. (2010) | |
| USA | 2009 | Most likely fresh produce (lettuce, possibly tomatoes or onions) | 46 (12) | C. parvum, IIaA17G2R1 (7 cases) | Adults and children at a youth summer camp | Descriptive epidemiology | Possibly livestock at the camp farm (calves, goat kids, piglets) contaminated the fresh produce. C. parvum, gp60 subtypea IIaA17G2R1 from most animals | Common consumption of sandwich bar ingredients | Collier et al. (2011) |
| Sweden | 2008 | Fresh produce: Arugula salad | 18 (15) | C. parvum, four subtypes | Descriptive epidemiology; outbreak investigation | Unknown | Insulander et al. (2013) | ||
| Finland | 2008 | Fresh produce: Lettuce mixture | 72 (12) | C. parvum | Adults | Outbreak investigation | Unknown | Packed in Sweden and originating from 5 different European. No oocysts were detected on salad remains | Pönka et al. (2009) |
| Sweden | 2008 | Fresh produce: Béarnaise sauce containing freshly chopped parsley | 21 (16) | C. parvum | Adults | Outbreak investigation | Unknown, but parsley was suspected as source of food contamination rather than a food handler at the premises | Bagged parsley imported from Italy and added to sauce shortly before serving (after heating) | Insulander et al. (2008) |
| Japan | 2006 | Meat: Yukke, a Korean‐style beef tartar and/or raw liver | 4 (4) | C. parvum IIa | Adults | Unknown | Yoshida et al. (2007) | ||
| Germany | 2006 | Possibly milk and dairy products | Unknown; two outbreaks reported – but numbers infected not provided | Unknown | Unknown | Descriptive epidemiology; outbreak investigation | Unknown | Insufficient information to assess whether these were food‐borne outbreaks | EFSA and ECDC (2007) |
| Denmark | 2005 | Fresh produce: Salad bar items (particularly whole carrots, grated carrots, red peppers) | 99 (12) | C. hominis | Adults | Outbreak investigation | Speculated to be an infected food handler (but not involved in food preparation) contaminated the buffet | Ethelberg et al. (2009) | |
| USA | 2003 | Fruit juice: Ozonated apple cider | 144 (23) | C. parvum IIaA15G2R1 and IIaA17G2R1 identified and 1 C. ubiquitum | Adults and children | Analytical epidemiology | Unknown | C. parvum IIaA17G2R1 also found in a jug of cider | Blackburn et al. (2006) |
| Australia | 2001 | Milk and dairy: Unpasteurised milk | 8 (8) | Children | Outbreak investigation | Unknown | It is illegal to sell unpasteurised cows milk for human consumption in Queensland. This milk was labelled as unpasteurised pet milk; milk tested Cryptosporidium‐positive by ELISA | Harper et al. (2002) | |
| USA | 1998 | Not identified | 152 (25) | C. hominis (reported as C. parvum genotype 1) | College students, canteen setting | Descriptive epidemiology | Food handler also had C. hominis | Quiroz et al. (2000) | |
| USA | 1997 | Not identified but maybe green onions | 54 | Adults | Food handler or unwashed green onions | CDC (1998) | |||
| USA | 1996 | Fruit juice: Apple cider | 31 | Adults and children | Descriptive epidemiology; outbreak investigation community setting | Water from faecally contaminated well that was used for washing apples | CDC (1997) | ||
| UK | 1995 | Milk and dairy: Cow's milk | 50 (16) | School children | Pasteurisation failure at commercial, on‐farm dairy supplying a local school | Gelletlie et al. (1997) | |||
| USA | 1995 | Food handler or fresh produce: Chicken salad including, pasta, hard‐boiled eggs, celery, and grapes in a seasoned mayonnaise dressing | 15 (2) | Not stated; attendees of a social event | Descriptive epidemiology | Food handler. Food prepared in domestic kitchen of a licenced daycare home | Food handler changed a nappy before preparing salad, but reported washing hands | CDC (1996) | |
| USA | 1993 | Fruit juice: Apple cider | 160 primary cases (50) | Students and staff attending a school agricultural fair | Descriptive epidemiology; outbreak investigation | Apples collected from the ground in an orchard grazed by infected calves | Oocysts detected in apple cider, on the press, and in calves from the farm | Millard et al. (1994) | |
| Russia | 1990 | Milk and dairy: Kefir (a yoghurt type drink) | 13+ | At least 13 infants from nursery and orphanage | All children with Cryptosporidium had eaten kefir prepared in the same premises | Although some evidence that food‐borne transmission occurred, person‐to‐person spread may also have been responsible for at least some of the cases. Oocysts were detected in milk filters | Romanova et al. (1992) | ||
| Mexico | 1985 | Milk and dairy: Unpasteurised cow's milk | 22 (22) | High school students and teachers visiting from Canada | Descriptive Epidemiology | Postulated food‐borne outbreak, but other possible transmission routes noted | Elsser et al. (1986) | ||
| Australia | 1983 | Milk and dairy: Unpasteurised goat milk | 2 (2) | Mother and infant | Descriptive epidemiology; outbreak investigation | Information scanty. Postulated food‐borne outbreak | Anonymous (1984) |
PCR: polymerase chain reaction.
References
Anonymous, 1984. Cryptosporidiosis surveillance. Weekly Epidemiological Record, 59, 72–73.
Åberg R, Sjöman M, Hemminki K, Pirnes A, Räsänen S, Kalanti A, Pohjanvirta T, Caccio SM, Pihlajasaari A, Toikkanen S, Huusko S, Rimhanen‐Finne R, 2015. Cryptosporidium parvum caused a large outbreak linked to Frisée salad in Finland, 2012. Zoonoses Public Health, 62, 618–624.
Blackburn BG, Mazurek JM, Hlavsa M, Park J, Tillapaw M, Parrish M, Salehi E, Franks W, Koch E, Smith F, Xiao L, Arrowood M, Hill V, da Silva A, Johnston S, Jones JL, 2006. Cryptosporidiosis associated with ozonated apple cider. Emerging Infectious Diseases, 12, 684–686.
Centers for Disease Control and Prevention (CDC), 1996. Foodborne outbreak of diarrheal illness associated with Cryptosporidium parvum–Minnesota, 1995. MMWR. Morbidity and mortality weekly report, 45, 783.
Centers for Disease Control and Prevention (CDC), 1997. Outbreaks of Escherichia coli 0157:H7 infection and cryptosporidiosis associated with drinking unpasteurized apple cider, Connecticut and New York, October 1996. Morbidity and Mortality Weekly. MMWR, 46, 4–8.
Centers for Disease Control and Prevention (CDC), 1998. Foodborne Outbreak of Cryptosporidiosis – Spokane, Washington, Morb. Mortal. Wkly. MMWR, 47, 565–567.
Collier SA, et al. 2011. Cryptosporidiosis Outbreak at a Summer Camp — North Carolina. Morbidity Mortality Weekly MMWR, 60, 918–922.
EFSA and ECDC, 2007. The community summary report on trends and sources of zoonoses, zoonotic agents, antimicrobial resistance and food‐borne outbreaks in the European Union in 2006. EFSA Journal 2007; 130, 1–352.
Elsser K, Moricz M, Proctor EM, 1986. Cryptosporidium infections: a laboratory survey CMAJ, 135, 211–213.
Ethelberg S, Lisby M, Vestergaard LS, Enemark HL, Olsen KE, Stensvold CR, Nielsen HV, Porsbo LJ, Plesner AM, Mølbak K, 2009. A food‐borne outbreak of Cryptosporidium hominis infection. Epidemiology and Infection, 137, 348–536.
Gelletlie R, Stuart J, Soltanpoor N, Armstrong R, Nichols G, 1997. Cryptosporidiosis associated with school milk. Lancet, 350, 1005–1006.
Gherasim A, Lebbad M, Insulander M, Decraene V, Kling A, Hjertqvist M, Wallensten A, 2012. Two geographically separated food‐borne outbreaks in Sweden linked by an unusual Cryptosporidium parvum subtype, October 2010. Euro Surveillance, 17. https://doi.org/10.2807/ese.17.46.20318-en
Harper CM, Cowell NA, Adams BC, Langley AJ, Wohlsen TD, 2002. Outbreak of Cryptosporidium linked to drinking unpasteurised milk. Communicable Diseases Intelligence, 26, 449–450.
Insulander M, Silverlås C, Lebbad M, Karlsson L, Mattsson J and Svenungsson B, 2013. Molecular epidemiology and clinical manifestations of human cryptosporidiosis in Sweden. Epidemiology and Infection, 141, 1009–1020. https://doi.org/10.1017/s0950268812001665
Insulander M, De Jong B and Svenungsson B, 2008. A food‐borne outbreak of cryptosporidiosis among guests and staff at a hotel restaurant in Stockholm county, Sweden, September 2008. Eurosurveillance, 13, 19071.
Quiroz ES, Bern C, MacArthur JR, Xiao L, Fletcher M, Arrowood MJ, Shay DK, Levy ME, Glass RI, Lal A, 2000. An outbreak of cryptosporidiosis linked to a food handler. The Journal of Infectious Diseases, 181, 695–700.
McKerr C, Adak GK, Nichols G, Gorton R, Chalmers RM, Kafatos G, Cosford P, Charlett A, Reacher M, Pollock KG, Alexander CL, Morton S, 2015. An Outbreak of Cryptosporidium parvum across England and Scotland associated with consumption of fresh pre‐cut salad leaves, May 2012. PLoS One, 10, e0125955.
Millard PS, Gensheimer KF, Addiss DG, Sosin DM, Beckett GA, Houck‐Jankoski A, Hudson A, 1994. An outbreak of cryptosporidiosis from fresh‐pressed apple cider. JAMA, 272, 1592–1596.
Pönka A, Kotilainen H, Rimhanen‐Finne R, Hokkanen P, Hänninen ML, Kaarna A, Meri T, Kuusi M, 2009. A food‐borne outbreak due to Cryptosporidium parvum in Helsinki, November 2008. Eurosurveillance, 14, 28.
Public Health England, 2017. National increase of C. parvum 2015. Final outbreak investigation report.
Romanova TV, et al. 1992. Gruppovye zabolevaniia kriptosporidiozom detei. [Group cryptosporidiosis morbidity in children]. Med. Parazitol. (Mosk). 3, 50–52.
Rosenthal M, Pederson R, Leibsle S, Hill V, Carter K, Roellig DM, 2015. Notes from the Field: Cryptosporidiosis associated with consumption of unpasteurized goat milk‐Idaho, 2014. MMWR Morbidity Mortality Weekly Report, 64, 194–195.
Yoshida H, Matsuo M, Miyoshi T, Uchino K, Nakaguchi H, Fukumoto T, Teranaka Y, Tanaka T, 2007. An outbreak of cryptosporidiosis suspected to be related to contaminated food, October 2006, Sakai City, Japan. Japanese Journal of Infectious Diseases, 60, 405–407.
Appendix C – Detection methods for Cryptosporidium in food
1.
Table C.1.
Detection methods for Cryptosporidium in food
| Type of food | Methods and their validation | |||||||
|---|---|---|---|---|---|---|---|---|
| Standard method | Other suitable methods | |||||||
| Fresh produce and herbs |
ISO 18744, 2016 Microbiology of the food chain — Detection and enumeration of Cryptosporidium and Giardia in fresh leafy green vegetables and berry fruits. Based on elution from the surface of the leaf or berry, concentration of oocysts from the eluate by immunomagnetic separation (IMS), and enumeration by immune fluorescence microscopy (IFM) LOD: no data Validated in a ring trial (8 data points/labs): |
Alternative of ISO according Utaaker et al. (2015) Method is using a smaller volume of magnetic beads in the IMS step and buffers made in‐house LOD: no data Validated in a ring trial (7 data points/labs): |
Oocyst elution and concentration by ISO or Utaaker et al. (2015) and detection by PCR (Hohweyer et al., 2016) Using PCR for detection LOD: 3 oocysts/g basil (30 g seeded with xx oocysts) < 1 oocyst/g raspberries Validation in one laboratory study |
|||||
| Lettuce | Raspberries | Leafy greens | Basil | Raspberries | ||||
| Mean recovery rate (100 oocysts) | 30.4% | 44.3% | Mean Recovery rate (50 oocysts) | 53.0% | Mean recovery rate (408 oocysts) | 11.0% (6–23%) | 14% (1–45%) | |
| Sensitivity | 89.6% | 95.8% | Sensitivity | 87.5% | ||||
| Specificity | 85.4% | 83.3% | Specificity | 87.5% | ||||
| Accordance | 82.4% | 92.1% | Accordance | n/a | ||||
| Concordance | 81% | 91.8% | Concordance | 80.0% | ||||
|
Advantages: Quantitative Microscopy slides amenable to onward testing by PCR for species and genotype Disadvantages: Expensive Time consuming Species non‐infective for humans will be counted Further processing is needed for molecular characterisation Recovery of oocysts from sample matrix can be low No viability or infectivity assessment is possible |
Advantages: Significantly cheaper method than ISO Disadvantages: As for ISO, apart from cost |
Performance will depend on DNA extraction and PCR efficiency and specificity Advantages: High throughput detection Potential for species identification Disadvantages: Not quantitative Sample preparation remains time consuming |
||||||
| Fruit and Vegetable juice | No |
IMS‐IFM Orange juice LOD 5–50 oocysts/10 mL (Frazar and Orlandi, 2007) IMS‐IFM Apple juice LOD 5–50/10 mL (Frazar and Orlandi, 2007) IMS‐IFM Apple juice LOD 10/100 mL (Deng and Cliver, 2000) IMS‐PCR LOD Apple juice 30/100 mL (Deng and Cliver, 2000) No ring trials |
Microfiltration‐PCR LOD 10/250 mL apple juice (Minarovičová et al., 2010) | |||||
| Dairy: Milk | No |
IMS‐PCR LOD Homogenised milk 10/100 mL (Deng and Cliver, 2000) IMS‐PCR Raw and pasteurised whole milk 10/50 mL (Di Pinto et al., 2002) IMS‐nPCR whole milk 5–50/10 mL (Frazar and Orlandi, 2007) Centrifugation‐PCR raw milk 1–10/20 mL (Laberge et al., 1996) |
Microfiltration‐single‐tube nested real‐time PCR LOD 10/100 mL milk (Minarovičová et al., 2011) | |||||
| Dairy: Fermented products | No | No data | ||||||
| Cheese | No | No data | ||||||
| Molluscan shellfish | No |
Sieved, pooled tissue homogenates (a method most commonly used), processed by IMS and detection by IFM or PCR LOD: no data Validation: no ring trials Data from systematic study (MacRae et al., 2005): known numbers of oocysts seeded into a 20‐L tank of seawater and circulated for 20 min to disperse before either 10 mussels, 2 oysters or 2 scallops were placed in the tank for 4 h at 5°C Shellfish removed and tissue homogenates (30 s in a Waring blender) were pooled for each sample and processed (5 tests per sample, aggregate 0.5‐mL tissue) by centrifugation, IMS and IFM Recovery rates were: Mussels: 34% of 20,000, 12% of 2,000, 20% of 200 oocysts spiked into the tank Oyster 69.5% of 20,000, 60.5% of 2,000, 48% of 200 oocysts spiked into the tank Scallop 32.5% of 20,000, 30% of 2,000, 65% of 200 oocysts spiked into the tank Gómez‐Couso et al. (2006) compared IFM and PCR detection, using 10% of spiked mollusc sediments containing 0, 10, 50, 100, 500 and 1,000 C. parvum oocysts. Average numbers of oocysts detected in three replicates by IFM were 0, 0.7, 3.6, 6.7, 37.6 and 77.2, respectively; the average percentage of recovery was approximately 70.0% and did not differ by spiking dose DNA was extracted and 10 amplification reactions were performed for each spiking dose. The theoretical numbers of oocysts present in the volume used in the PCR technique were 0, 1, 2, 4 and 40 oocysts. PCR positive results diminished as spiking dose decreased, ranging from 90% for 40 oocysts to 10% for 1 oocyst |
Pepsin digestion of 3 g pooled homogenate using IFM for detection (Robertson and Gjerde, 2007) LOD: no data Validation: no ring trials Data from developing laboratory: 68–79% of 179 oocysts (horse mussel and oyster homogenates) This method has been used by the developing laboratory and there is one published report of a sample survey that has used it (Aguirre et al., 2016) |
|||||
| Meat | No |
Surface elution centrifugation, IMS, IFM (Robertson and Huang, 2012) LOD, not stated Validation: No ring trials Recovery rates for ~ 100 oocysts were 63.5% (CI 54.6–70.2) |
||||||
ISO: International Organization for Standardization; LOD: limit of detection; PCR: polymerase chain reaction.
Sensitivity (=true positive rate): the ability of a test to correctly identify positive samples (spiked or contaminated samples).
Specificity (=true negative rate): the ability of a test to correctly identify negative samples (non‐inoculated samples)
Accordance or repeatability: Accordance is the percentage (ratio) that two identical test materials analyzed by the same laboratory under standard repeatability conditions will both be given the same result (i.e. both found positive or both found negative) (Langton et al., 2002).
Concordance or reproducibility: Concordance is the percentage (ratio) that two identical test materials sent to different laboratories will both be given the same result (i.e. both found positive or both found negative result) (Langton et al., 2002).
References
Aguirre J, Greenwood SJ, McClure J, Davidson J and Sanchez J, 2016. Effects of rain events on Cryptosporidium spp. levels in commercial shellfish zones in the Hillsborough River, Prince Edward Island, Canada. Food and Waterborne Parasitology, 5, 7–13.
Deng MQ and Cliver DO, 2000. Comparative detection of Cryptosporidium parvum oocysts from apple juice. International Journal of Food Microbiology, 54, 155–162.
Di Pinto A and Tantillo MG, 2002. Direct detection of Cryptosporidium parvum oocysts by immunomagnetic separation‐polymerase chain reaction in raw milk. Journal of Food Protection, 65, 1345–1348.
Frazar CD and Orlandi PA, 2007. Evaluation of Two DNA Template Preparation Methods for Post‐Immunomagnetic Separation Detection of Cryptosporidium parvum in Foods and Beverages by PCR. Applied and Environmental Microbiology, 73, 7474–7476.
Gómez‐Couso H, Méndez‐Hermida F and Ares‐Mazás E, 2006. Levels of detection of Cryptosporidium oocysts in mussels (Mytilus galloprovincialis) by IFA and PCR methods. Veterinary Parasitology, 141, 60–65.
Hohweyer J, Cazeaux C, Travaillé E, Languet E, Dumètre A, Aubert D, Terryn C, Dubey JP, Azas N and Houssin M, 2016. Simultaneous detection of the protozoan parasites Toxoplasma, Cryptosporidium and Giardia in food matrices and their persistence on basil leaves. Food Microbiology, 57, 36–44.
Laberge I, Griffiths MW and Griffiths M, 1996. Prevalence, detection and control of Cryptosporidium parvum in food. International Journal of Food Microbiology, 32, 1–26.
Langton SD, Chevennement R, Nagelkerke N and Lombard B, 2002. Analysing collaborative trials for qualitative microbiological methods: accordance and concordance. International Journal of Food Microbiology, 79, 175–181.
MacRae M, Hamilton C, Strachan NJ, Wright S and Ogden ID, 2005. The detection of Cryptosporidium parvum and Escherichia coli O157 in UK bivalve shellfish. Journal of Microbiology Methods, 60, 395–401.
Minarovičová J, Kaclíková E and Kuchta T, 2010. A method for the detection of Cryptosporidium parvum in apple juice based on microfiltration and real‐time polymerase chain reaction. Journal of Food and Nutrition Research, 49, 160–164.
Minarovičová J, Lopašovská J, Valík Ľ et al, 2011. Food Analysis Methods, 4, 116. https://doi.org/10.1007/s12161-010-9141-9
Robertson LJ and Huang Q, 2012. Analysis of cured meat products for Cryptosporidium oocysts following possible contamination during an extensive waterborne outbreak of cryptosporidiosis. Journal of Food Protection, 75, 982–988.
Utaaker KS, Huang Q and Robertson LJ, 2015. A reduced‐cost approach for analyzing fresh produce for contamination with Cryptosporidium oocysts and/or Giardia cysts. Food Research International, 77, 326–332.
Appendix D – Occurrence of Cryptosporidium in food
1.
Table D.1.
Occurrence of Cryptosporidium in food
| Food type | Source | Country | Year | Sampling frame | Method used | Number % contaminated samples | Enumeration | Comments | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Fresh Produce and Herbs | |||||||||
| Ready to eat packaged mixed salads | Retailers | Italy | Prospective sample survey | Elution, centrifugation, immunofluorescence microscopy (IFM) and semi‐nested PCR COWP gene | 6 pools, 0.96% (95% CI 0.35–2.08) | Not done | Pooling took into account predicted low prevalence but may have diluted oocysts below LOD | Caradonna et al. (2017) | |
| Cabbages, leeks, lettuce, spring onions, celery, cauliflower, broccoli, spinach, Brussels sprouts, raspberries, strawberries | Locally grown, sampled at market | Poland | Prospective sample survey | Elution, centrifugation, IMS, IFM | 12/163 (7.4%) overall 6/128 (4.7%) leek /celery/cabbage; one leek sample, one celery sample, four cabbage samples 0/35 berries | 4–17 oocysts/30 g |
Cryptosporidium‐positive samples came from districts with the highest number of cattle herds Oocyst recovery data from ColorSeed 4–47% |
Rzeżutka et al. (2010) | |
| Chinese cabbage, Lollo rosso and romaine lettuce | Grower | Spain | Prospective sample survey | Elution, centrifugation, IMS, IFM |
2/6 (33.3%) Chinese cabbage 3/4 (75%) Lollo rosso lettuce 7/9 (77.8%) Romaine lettuce |
2–15 oocysts/50 g |
Irrigation water at the same site was contaminated with faeces Oocyst recovery data from ColorSeed 24.5 ± 3.5% |
Amoros et al. (2010) | |
| Alfalfa/mung bean/radish sprouts, dill, lettuce (diverse varieties), mushrooms, parsley, precut salad mixes, raspberries, strawberries | Commercial distributors of imported and locally produced produce | Norway | Prospective sample survey | Elution, concentration, IMS, IFM |
19/475 (4%) Lettuce 4% Mung bean sprouts 9% Cryptosporidium oocysts not detected in other samples |
1–6/100 g |
Recovery of oocysts from sprouted seeds is impaired by sample‐related factors Oocyst recovery data from EasySeed 42% (average) |
Robertson and Gjerde (2001) | |
| Alfalfa, mung bean, radish sprouts and sprout mix | Retail shops | Norway | Prospective sample survey | Elution, concentration, IMS, IFM | 14/171 (8%) | 2–6/100 g | Oocyst recovery data from EasySeed 25–35% | Robertson et al. (2002) | |
| Fruit and Vegetable Juice (No European Data With Sufficiently Well‐Described Methods) | |||||||||
| Raw and fermented apple juice | During processing | Canada | Prospective sample survey | Sucrose gradient‐Laberge PCR |
Raw: 6/113 (5%) Fermented: 2/113 (2%) |
N/A | Garcia et al. (2006) | ||
| Milk and Dairy Products (No European Data With Sufficiently Well‐Described Methods) | |||||||||
| Raw cows’ milk | Point of sale | Australia, Queensland | Reactive sampling of suspected vehicle in a community outbreak | Centrifugation, IMS, IFM and ELISA | Oocysts not detected by IFM but positive ELISA reactions in milk; | Unpasteurised cow's milk sold as ‘unpasteurised pet milk’ unsatisfactory bacterial counts | Cowell et al. (2002) | ||
| Raw goats’ milk | Producer, retail or household | Idaho, USA | Reactive sampling of suspected vehicle in a community outbreak | Preparation method not stated, PCR | False positive PCR; oocysts not detected by IFM | Sequencing revealed amplicons were from goat DNA | Rosenthal et al. (2015) | ||
| Fermented products: | Kefir prepared in a nursery kitchen | Russia | Reactive sampling of suspected vehicle in an outbreak at a hospital nursery and local community | To be confirmed | Oocysts detected on milk filters in dairy | Romanova et al. (1992) | |||
| Bivalve Molluscs | |||||||||
| Mussels | Mediterranean mussels (Mytilus galloprovincialis) | Italy | May–December 2012 | Prospective sample survey: markets in Foggia, South Eastern Italy | Samples comprised pools from 15 mussels: gills and digestive glands homogenised and pooled. Haemolymph sucrose gradient flotation DNA extraction freeze‐thaw, spin column (Nucleospin, Machery‐Nagel); gp60 conventional PCR | 34/60 (56.7%) | No data | Varied by season: > twice as many positives in May–Sept compared with Oct–Dec C. parvum gp60 subtypes detected: IIaA15G2R1, IIa15G2 and IIaA14G3R1 Most positives were in haemolymph | Giangaspero et al. (2014) |
| Clams and mussels | Clams (Ruditapes decussatus) and Mediterranean mussels (Mytilus galloprovincialis) | Italy | February 2006–January 2007 | Prospective sample survey: Varano Lagoon, Apulia region, southern Italy Class A water | 60 shellfish sampled per month (total 1,385; 665 clams and 720 mussels tested in 2 pools of haemolymph from 30 shellfish. Prepared by sucrose flotation and tested by IF microscopy and DNA extraction by QIAampDNAMini Kit and semi‐nested COWP PCR | 0/47 pools of haemolymph | 0 | Oocysts not detected in shellfish, although the parasite was detected in lagoon waters | Giangaspero et al. (2009) |
| Mussels | Blue mussels (Mytilus edulis) | Norway | February–July 2007 | Commercial harvesting sites, Norwegian coast | 1.36–3 g homogenate of pooled flesh from several shellfish, pepsin digestion, IMS, IFM | 6/14 (43%) blue mussels 0/12 horse mussels 0/13 oysters | 1 or 2 oocysts in each of the positive samples | Pepsin digestion method, which showed highest recoveries, used in this study | Robertson and Gjerde (2008) |
| Clams | Ruditapes philippinarum clams | Italy | January–December 2004 | Prospective sample survey/surveillance: north‐eastern Italian Adriatic coast, three clam farms 2,160 specimens (180 clams per month) were from 36 pools (i.e. one pool of 60 clams per month per site) | Pooled hemolymph, sucrose floatation, freeze‐thaw, Quantum Prep AquaPure Genomic DNA Kit (Bio‐Rad Laboratories), semi‐nested COWP PCR | 7/36 (19.4%) pools C. parvum and C. hominis detected | No data | Molini et al. (2007) | |
| Oysters | Pacific cupped oyster (Crassostrea gigas) | The Netherlands (the Oosterschelde) | Prospective sample survey | 6 per site Gill washings and digestive tract homogenates sieved, IFM | 9/179 (5%) oysters | No data | Schets et al. (2007) | ||
| Mussels | Duck mussel (Anodonta anatine) Painter's mussel (Unio pictorum) Asian clam (Corbicula fluminea) | Portugal | May 2003 to June 2005 | Prospective sample survey Guadiana river hydrographic basin in south‐east Portugal 55 samples in 16 areas, 24 U. pictorium, 19 C. fluminea, 12 A. anatina | Pooled gills and the gastrointestinal tracts 3–15 bivalves of each species pooled Homogenised, sucrose flotation, IFM. For DNA preparation, Proteinase k digestion and Qiagen extraction nested 18s PCR of 47 samples | 6/16 sampling areas positive 18/55 (33.3%) samples by IFM7/24 (29%) U. pictorum samples, 4/12 (33%) A. anatina samples, 7/19 (37%) C. fluminea samples. PCR was negative | 1–8 oocysts | Melo et al. (2006) | |
| Mussels | Cultured mussels (Mytilus edulis) | France | 1999 | Prospective sample survey/surveillance: north‐western coastal area of Normandy Collected seasonally during 1 year from three sites | Pools made of preps from each mussel: (a) inner‐shell water extract; (b) gill washing; (c) gill homogenate; (d) flesh washing; (e) flesh homogenate. Filtered, sucrose flotation and gradients. 10 mL to IMS and then IFM. From 1 site in January, 1 mL to DNA extraction by Tris/SDS/EDTA and phenol chloroform, 18s PCR (Guyot et al., 2001) | Flesh homogenates showed all sites IFM positive on each sampling occasion |
Estimated numbers of oocysts/mussel ranged from 0.05 to 0.90 Oocyst numbers in gill washings were > gill homogenate. In contrast, oocyst numbers in flesh homogenate were > flesh washings |
One sample infected a mouse. C. parvum detected in mussels | Li et al. (2006) |
| Mussels | Mussels (Mytilus galloprovincialis) | Galician coast, Spain | Jan–June 2004 | Prospective sample survey/surveillance: four Galician estuaries, known collectively as Rı′as Baixas, on the Atlantic coast of north‐western Spain Harvested from floating rafts, no depuration Each sample consisted of the pooled gills and gastrointestinal tracts from six to eight mussels | Test method as per Gomez‐Couso et al. (2003): homogenised, sieved, diethyl ether concentrated by centrifugation and IFM for detection. Genotyping by Bead‐beating, boom, nested COWP PCR | Cryptosporidium was detected in 13 (27.7%) of the 47 samples of mussels from category A water and in 41 (29.9%) of the 137 samples of mussels from category B water | 25–275 oocysts per sample of 6–8 mussels | C. parvum was detected. Although the percentage of positive samples increased as the bacteriological quality decreased, there was no significant association between Cryptosporidium detection and bacteriological quality | Gómez‐Couso et al. (2006b) |
| Mussels | Mussels (Mytilus galloprovincialis) | Galician coast, Spain | Not stated |
Prospective sample survey: 222 non‐depurated cultured mussels |
Pools of gills and gastrointestinal tracts from 6 to 8 mussels, homogenised, sieved, diethyl ether concentration, IFM and guanidinium thiocyanate‐bead beating DNA extraction and nested COWP PCR‐RFLP | 69 (31.1%) of 222 samples were positive, of which 41 by IFM only, 15 by PCR only, and 13 by both assays | Only provided for the IFM and PCR positive samples: 6 to 300 oocysts | IFA detected more positives (if true) but not statistically significant | Gómez‐Couso et al. (2006a) |
| Clams | Clams (Chamelea gallina) | Italy | March–July 2003 | Prospective sample survey: 960 specimens Adriatic coast (Abruzzo region) | Tissues pooled, homogenised, sieved, sedimentation by overnight settlement and IFM were performed on pools of 30 clams (n = 32) of samples. Haemolymph also tested by IFM. Genotyping IFM‐positive samples by salt float, freeze‐thaw, enzyme digestion, DNeasy Kit, Qiagen and COWP PCR | 23/32 pools | Concentrations ranging from 8 to 45 oocysts/g in the tissue and from 18 to 200 oocysts/mL in the haemolymph | 2/23 pools C. parvum | Giangaspero et al. (2005) |
| Mussels, clams, oysters and cockles | Mussels (Mytilus galloprovincialis), clams (Ruditapes decussatus, Ruditapes philippinarum, Venerupis pulastra, Dosinia exoleta), oysters (Ostrea edulis) and cockles (Cerastoderma edule) | Spain | Cultured shellfish in Galicia | Pooled gills and intestinal tracts of 6 to 12 molluscs homogenised, filtered, PBS centrifugation and ethyl acetate concentration. IFM detection. DNA extraction by Boom and PVP and multiplex nested COWP PCR‐RFLP (Amar et al., 2003) | 28/49 (56%) molluscan shellfish specimens (18 clam, 22 mussel and 9 oyster) samples 56% by immunofluorescence microscopy, and in 44% by ABC‐PCR | No data | A significant association, but not total agreement, between IFM and PCR results. In 26 Cryptosporidium IFM‐positive samples, C. hominis was detected in 1, C. parvum in 22, and the remaining three samples contained either sequences similar to C. parvum genotype 2 or heterogeneous mixtures of Cryptosporidium species. There was no significant association between Escherichia coli levels and the presence of Cryptosporidium by PCR | Gómez‐Couso et al. (2004) | |
| Mussels, clams, oysters and cockles | Mussels (Mytilus galloprovincialis), clams (Ruditapes decussatus, Ruditapes philippinarum, Venerupis pulastra, Dosinia exoleta), oysters (Ostrea edulis) and cockles (Cerastoderma edule) | Spain mostly, and other EU countries | October 2000–September 2001 | Prospective sample survey: 241 samples from different parts of the Galician coast (30 from Italy, 5 from the United Kingdom, 2 from Ireland and 1 from Portugal) | Pooled gills and intestinal tracts of 7 molluscs homogenised, sieved, ethyl ether concentration and IFM and PI | 83/241 (34.4%) of all samples | No data | Use of PI showed 53% positives viable No relation between species of shellfish, month of sampling or MPN for faecal coliforms Depuration process was ineffective in totally removing oocyst contamination or significantly reducing viability | Gómez‐Couso et al. (2003) |
| Mussels | Mussel Mytilus edulis | UK, Northern Ireland | July and August 1999 | Prospective sample survey: Marine mussels harvested for human consumption | Individual mussels homogenised, IMS‐PCR (TRAP‐C2 RFLP and seq) and IFM DNA extraction by Proeinase K digest, freeze‐thaw and High Pure PCR Template Preparation Kit (Boehringer Mannheim | 0/16 by IFM, 2/16 (12.5%) by PCR | 0/no data | C. hominis detected (reported as C. parvum genotype 1) | Lowery et al. (2001) |
| Mussels and cockles |
Mediterranean mussel (Mytilus galloprovincialis) Common cockle (Cerastoderma edule) |
Spain | February 1999 | Prospective study: 9 sites Gallician coast | Up to 25 molluscs in tank and entire water changed and sampled at 24 h intervals, processed by centrifugation; after 72 h, whole tissue homogenised IFM or IMS‐IFM, also mouse infectivity. PCR after lysis buffer, glass bead beating, silica, wash buffer, acetone, COWP PCR‐RFLP Direct testing: 172 cultured mussels, 8 wild mussels, 6 cockles | Tissue homogenates and water after 72 h negative. Tank water positive Direct testing used to estimate oocyst loads | An approximation of the parasite load of shellfish collected in El Burgo indicates that each shellfish transported about 5 x 103 oocysts | Oocyst detections in aquaria declined from 24 h after collection and were not detected at 72 h. Viable oocysts of C. parvum were confirmed by mouse infectivity and PCR. Oocysts taken up by mussels are released within 48 h | Gómez‐Bautista et al. (2000) |
| Wild harvested Common mussel (Mytilus edulis) | Ireland | May–August 1996 | Prospective study: in Sligo west coast of Ireland. Pools of 10 | Whole tissue homogenised (100 mL), IFM | 3/26 (11.5%) | No data | Chalmers et al. (1997) | ||
| Meat | |||||||||
| Processed meat | Cured meats | Sweden | Prospective study / risk assessment | None confirmed, one possible oocyst | Robertson and Huang (2012) | ||||
| Fresh meat | Beef carcases at slaughter | Ireland | Prospective study | Matched samples | 0/288 matched carcases | Moriarty et al. (2005) | |||
PCR: polymerase chain reaction; IMS: immunomagnetic separation.
References
Amoros I, Alonso JL and Cuest G, 2010. Cryptosporidium oocysts and Giardia cysts on salads products irrigated with contaminated water. Journal of Food Protection, 73, 1138e1140.
Caradonna T, Marangi M, Del Chierico F, Ferrari N, Reddel S, Bracaglia G, Normanno G, Putignani L and Giangaspero A, 2017. Detection and prevalence of protozoan parasites in ready‐to‐eat packaged salads on sale in Italy. Food Microbiology, 67, 67–75.
Chalmers R, Sturdee A, Mellors P, Nicholson V, Lawlor F, Kenny F and Timpson P, 1997. Cryptosporidium parvum in environmental samples in the Sligo area, Republic of Ireland: a preliminary report. Letters in Applied Microbiology, 25, 380–384. https://doi.org/10.1046/j.1472-765x.1997.00248.x
Cowell NA, Wohlsen TD, Harper CM, Langley AJ and Adams BC, 2002. Outbreak of Cryptosporidium linked to drinking unpasteurised milk. Communicable diseases intelligence quarterly report, 26, 449.
Garcia L, Henderson J, Fabri M and Oke M, 2006. Potential sources of microbial contamination in unpasteurized apple cider. Journal of Food Protection, 69, 137–144.
Giangaspero A, Cirillo R, Lacasella V, Lonigro A, Marangi M, Cavallo P, Berrilli F, Di Cave D and Brandonisio O, 2009. Giardia and Cryptosporidium in inflowing water and harvested shellfish in a Lagoon in Southern Italy. Parasitology International, 58, 12–17.
Giangaspero A, Molini U, Iorio R, Traversa D, Paoletti B and Giansante C, 2005. Cryptosporidium parvum oocysts in seawater clams (Chamelea gallina) in Italy. Preventive Veterinary Medicine, 69, 203–212.
Giangaspero A, Papini R, Marangi M, Koehler AV and Gasser RB, 2014. Cryptosporidium parvum genotype IIa and Giardia duodenalis assemblage A in Mytilus galloprovincialis on sale at local food markets. International Journal of Food Microbiology, 171, 62–67.
Gomez‐Bautista M, Ortega‐Mora LM, Tabares E, Lopez‐Rodas V and Costas E, 2000. Detection of infectious Cryptosporidium parvum oocysts in mussels (Mytilus galloprovincialis) and cockles (Cerastoderma edule). Applied and Environmental Microbiology, 66, 1866–1870.
Gómez‐Couso H, Freire‐Santos F, Martınez‐Urtaza J, Garcıa‐Martın O and Ares‐Mazas M, 2003. Contamination of bivalve molluscs by Cryptosporidium oocysts: the need for new quality control standards. International Journal of Food Microbiology, 87, 97–105.
Gómez‐Couso H, Freire‐Santos F, Amar C, Grant K, Williamson K, Ares‐Mazas M and McLauchlin J, 2004. Detection of Cryptosporidium and Giardia in molluscan shellfish by multiplexed nested‐PCR. International Journal of Food Microbiology, 91, 279–288.
Gómez‐Couso H, Méndez‐Hermida F and Ares‐Mazás E, 2006a. Levels of detection of Cryptosporidium oocysts in mussels (Mytilus galloprovincialis) by IFA and PCR methods. Veterinary parasitology, 141, 60–65.
Gomez‐Couso H, Mendez‐Hermida F, Castro‐Hermida JA and Ares‐Mazás E, 2006b. Cryptosporidium contamination in harvesting areas of bivalve molluscs. Journal of Food Protection, 69, 185–190.
Li X, Guyot K, Dei‐Cas E, Mallard J‐P, Ballet JJ and Brasseur P, 2006. Cryptosporidium oocysts in mussels (Mytilus edulis) from Normandy (France). International Journal of Food Microbiology, 108, 321–325.
Lowery C, Nugent P, Moore J, Millar B, Xiru X and Dooley J, 2001. PCR–IMS detection and molecular typing of Cryptosporidium parvum recovered from a recreational river source and an associated mussel (Mytilus edulis) bed in Northern Ireland. Epidemiology and Infection, 127, 545–553. https://doi.org/10.1017/s0950268801006276
Melo PC, Teodosio J, Reis J, Duarte A, Costa JC and Fonseca IP, 2006. Cryptosporidium spp. in freshwater bivalves in Portugal. Journal of Eukaryotic Microbiology, 53, S28.
Molini U, Traversa D, Ceschia G, Iorio R, Boffo L, Zentilin A, Capelli G and Giangaspero A, 2007. Temporal occurrence of Cryptosporidium in the Manila clam Ruditapes philippinarum in northern Adriatic Italian lagoons. Journal of Food Protection, 70, 494–499.
Moriarty EM, McEvoy JM, Lowery CJ, Thompson HP, Finn M, Sheridan JJ, Blair IS, McDowell DA and Duffy G, 2005. Prevalence and characterisation of Cryptosporidium species in cattle faeces and on beef carcases at slaughter. Veterinary Record, 156, 165–168.
Robertson LJ and Gjerde B, 2001. Occurrence of Parasites on Fruits and Vegetables in Norway. Journal of Food Protection, 64, 1793–1798.
Robertson L and Gjerde B, 2008. Development and use of a pepsin digestion method for analysis of shellfish for Cryptosporidium oocysts and Giardia cysts. Journal of Food Protection, 71, 959–966.
Robertson LJ, Johannessen GS, Gjerde BK and Loncarevic S, 2002. Microbiological analysis of seed sprouts in Norway. International Journal of Food Microbiology, 75, 119–126.
Robertson LJ and Huang Q, 2012. Analysis of cured meat products for Cryptosporidium oocysts following possible contamination during an extensive waterborne outbreak of cryptosporidiosis. Journal of Food Protection, 75, 982–988.
Romanova TV, Shkarin VV, Khazenson LB and Parazitol M, 1992. Group morbidity with cryptosporidiosis in infants. Med Parazitol, 3, 50–52.
Rosenthal M, Pederson R, Leibsle S, Hill V, Carter K and Roellig DM, 2015. Notes from the Field: Cryptosporidiosis associated with consumption of unpasteurized goat milk‐Idaho, 2014. MMWR Morbidity Mortality Weekly Report, 64, 194–195.
Rzeżutka A, Nichols RAB, Connelly L, Kaupke A, Kozyra I, Cook N, Birrell S and Smith HV, 2010. Cryptosporidium oocysts on fresh produce from areas of high livestock production in Poland. International Journal of Food Microbiology, 139, 96–101.
Schets FM, van den Berg HH, Engels GB, Lodder WJ and de Roda Husman AM, 2007. Cryptosporidium and Giardia in commercial and non‐commercial oysters (Crassostrea gigas) and water from the Oosterschelde, The Netherlands. International Journal of Food Microbiology, 113, 189–194.
Appendix E – Attributing the burden of food‐borne disease to specific foods, food groups or reservoirs
E.1. Introduction
To identify and prioritise food safety intervention strategies to prevent and reduce the burden of diseases in a population, knowledge on the most important sources of the causative food‐borne hazards is needed. Several source attribution methods are available, including approaches based on analysis of data from occurrence of hazards in foods and humans, epidemiological studies, intervention studies, and expert knowledge elicitations (Table E.1). All methods present both advantages and limitations, and their utility and applicability depends on the public health questions being addressed and on characteristics and distribution of the hazard. For instance, epidemiological studies may be useful for source attribution of disease by parasites such as Cryptosporidium that lead mostly to acute disease and thus enable an association of exposure to specific contaminated foods with the onset of symptoms; on the contrary, they are usually insufficient to attribute disease by parasites such as Echinococcus that is typically chronic and symptoms appear a long time after exposure. Additionally, methods have different data requirements and attribute human illness at either the point of production (reservoir) or of exposure to the food, and therefore their utility will vary depending on the hazard and/or the country or region in question (Pires, 2013).
Table E.1.
Definition of source attribution approaches
| Source attribution approach | Definition | Application level |
|---|---|---|
| Epidemiological studies | Case–control studies of sporadic cases and analyses of data from outbreak investigations | Point of exposure |
| Subtyping | Probabilistic comparison of the relative occurrence of specific subtypes/strains of the pathogen in animal reservoirs and human cases; ideally based on a collection of representative isolates from all (major) sources | Point of reservoir |
| Comparative risk assessment | Bottom‐up modelling of the number of human illnesses resulting from different routes of exposure, including several routes from the same reservoir |
Point of reservoir Point of exposure |
| Expert knowledge elicitation | Use of expert judgements as an alternative source of information when data‐driven methods are not applicable |
Point of reservoir Point of exposure |
E.2. Overview of source attribution methods
Approaches to source attribution can be grouped broadly into four categories: epidemiological studies, subtyping, comparative risk assessment, and expert knowledge elicitation (Pires et al., 2009; Batz et al., 2005).
Epidemiological studies comprise case–control studies of sporadic cases and analyses of data from outbreak investigations. Case–controls studies are useful to identify sources and risk factors for a disease, as well as the fraction of human cases that can be attributable to these (by estimating population attributable fractions (PAF)). Although if case–control studies are not often conducted and are insufficient to extrapolate source attribution estimates at national level, a meta‐analysis of several case–control studies (i.e. combining studies conducted in several countries) can be used to estimate the number of illnesses attributable to each exposure at the regional and global levels. In contrast, food‐borne outbreak data are widely available from most world regions. Outbreak investigations are often able to identify the contaminated source or ingredient that caused infections, and an analysis of these data can show the relative contribution of the most important sources of disease. These analyses can be done at national, regional, and global levels and, if the limitations of assuming that outbreak data are representative of all cases in the population (i.e. also of sporadic cases of disease), outbreak attribution analyses are useful evidence for source prioritisation.
Subtyping approaches for source attribution compare the occurrence of food‐borne hazards in humans versus animals, food and/or environmental sources. These data are ideally available from surveillance or monitoring programmes in a country, but may also be obtained through, e.g., targeted projects or literature review. The subtyping approach was designed to attribute human cases to the reservoir level, i.e. the closest possible to the origin of the pathogen, and gives no information on the relative contribution of different exposure routes to humans.
Comparative risk assessment combines information on the occurrence of the food‐borne hazards in animals, food and/or environmental sources, with dose–response functions modelling the probability of infection (or illness, death) in function of the ingested dose. The comparative risk assessment approach may estimate the relative importance of different routes of exposure, including several routes from the same reservoir. The approach is, however, more data intensive, which may lead to large uncertainties.
Expert knowledge elicitation may be used to fill data gaps, and to combine the data from different studies and scientific approaches into a single estimate, or as an alternative source attribution method when remaining methods are not applicable or useful to address a public health question (Andreoletti et al., 2008). The source attribution approach by expert knowledge elicitations can be used to estimate the proportion of illnesses that are attributed to food‐borne, environmental, contact with animals, or human‐to‐human transmission pathways (Hald et al., 2016). There are numerous methods used for expert knowledge elicitation, and these different methods may be more or less appropriate depending on the hazard; some methods (e.g., the Delphi method) are based upon iteration and finding consensus among a small group of experts, whereas other methods are based on large panel surveys. Expert judgements are subjective by nature and may be biased by the specific background and scientific expertise of the respondents, and several methods to evaluate an individual expert's performance have been described. Structured expert knowledge elicitations define criteria for the selection of experts, and include protocols for the experts’ inquires and statistical analysis of obtained answers.
Source attribution can take place at different points along the food chain (points of attribution), including at the origin of the pathogen, i.e. the point of reservoir, such as the animal production stage, or at the point of exposure, such as the food consumption stage. The different source attribution methods attribute disease at different points, and will depend on the availability of data and on the risk management question being addressed (Table E.1).
E.3. Attribution to main types of transmission
The first step in the source attribution process is to estimate the overall proportion of the burden of disease that can be attributed to the four main transmission routes, i.e., food‐borne, environmental, direct contact with animals or person‐to‐person. For most food‐borne hazards, data‐driven methods, based, for example, on surveillance and monitoring data, would require an exhaustive review and inclusion of all potential sources and pathways within these main routes, and consequently are not the most appropriate tool for this initial step when applied individually. A combination of epidemiological methods could provide a more adequate picture of the relative importance of the types of transmission, namely a combination of an analysis of outbreak data and of studies of sporadic cases. For hazards that are transmitted through a limited number of routes (e.g., Brucella spp.), the application of one epidemiological approach for source attribution may be sufficient. Alternatively, two methods are currently available to attribute disease to these main routes: expert knowledge elicitations and intervention studies.
Attribution of food‐borne disease to food and other transmission routes could be undertaken for individual food‐borne hazards or for syndromic groups, e.g., diarrhoeal disease. In both cases, expert knowledge elicitations can be conducted at a country or regional level, whereas interventions are optimally designed as small scale population‐based studies. Additionally, the latter are expensive and difficult to apply.
The Food‐borne Disease Burden Epidemiology Reference Group (FERG) has undertaken a large‐scale expert elicitation to attribute disease by 19 food‐borne hazards to main transmission groups at a global, regional, and subregional level (Hald et al., 2016). The study applied structured expert judgement using Cooke's Classical Model (Cooke, 1991) to obtain estimates for the relative contributions of different transmission pathways for 11 diarrheal diseases, seven other infectious diseases, and one chemical (lead). Experts were selected based on their experience, including international working experience, and included in 10 global panels or 9 subregional panels. This study presented the first worldwide estimates of the proportion of specific diseases attributable to food and other major transmission routes. Other expert elicitations have been conducted to deliver similar estimates but at a national level, specifically in the Netherlands and in Canada (Havelaar et al., 2008; Davidson et al., 2011; Lake et al., 2010; Vally et al., 2014). Similar country‐specific initiatives will be useful to improve estimates and reduce uncertainties.
In general, determining the relative importance of different transmission pathways in causing human illness, generally known as source attribution, can take place at different points along the food chain and can be based on different approaches. Source attribution at the point of reservoir represents attribution at the original source of the hazard, whereas source attribution at the point of exposure represents attribution at the final stage of the transmission pathway, such as the food consumption stage. Subtyping approaches, typically based on molecular genotyping, may permit identification of the most important reservoirs of the hazard, while epidemiological approaches based on case–control studies of sporadic cases or outbreak analyses allow identifying specific exposure pathways. Comparative exposure assessment and expert knowledge elicitations, allow source attribution both at the point of reservoir and the point of exposure (Table E.1). Expert opinion may be used to fill gaps when scientific or epidemiologic data are lacking, sparse, or highly uncertain (Batz et al., 2005), or to combine and weigh data from the different approaches for which no analytical methods currently exist (EFSA BIOHAZ Panel, 2008).
E.4. Attribution to specific foods and exposure routes
Attribution of human illness to specific sources requires categorisation of the sources. Harmonisation of the categorisation scheme is necessary to compare and integrate the results from various data sources, models, approaches, or hazards. Such a system should be hierarchical, to accommodate different levels of detail required for different purposes. A hierarchical approach allows for several subcategories to be specified within one category (e.g., poultry can be subdivided into layers (eggs), chickens, ducks, and turkeys), and the source attribution study may consider either a category or subcategories, depending on the purpose or data availability. Additionally, other subcategories may be included in the main one, even if not specified (e.g., poultry could also include other poultry types like geese and pigeons). Ideally, the food categorisation scheme should be in alignment with food consumption data and be internationally standardised. FERG adopted a scheme for the categorisation of the food sources, adapted from Painter et al. (2009) (Figure E.1).
Figure E.1.
Categorisation scheme of food commodities
The type of reservoir of the hazard will influence the applicability of some source attribution methods, particularly the subtyping approach. This approach applies to hazards with one or more animal reservoirs, to which disease can be traced back and where the hazard can potentially be controlled. All other approaches are, in principle, applicable regardless of the origin of the hazard, since they focus on routes of transmission or the point of exposure.
There may also be differences in the utility of methods for regional or national level. In general, epidemiological approaches, specifically analysis of outbreak data and systematic review and meta‐analysis of case control studies of sporadic infections, are useful for source attribution at a regional level when data are not available at country‐level.
References
Andreoletti O, Budka H, Buncic S, Colin P, Collins JD, De A, Koeijer JG, Havelaar A, Hope J, Klein G and Kruse H, 2008. Overview of methods for source attribution for human illness from food‐borne microbiological hazards Scientific Opinion of the Panel on Biological Hazards. EFSA Journal 2008; 764.
Batz MB, Doyle MP, Morris Jr JG, Painter J, Singh R, Tauxe RV, Taylor MR, Wong DM and Food Attribution Working Group, 2005. Attributing illness to food. Emerging Infectious Diseases, 11, 993.
Cooke R, 1991. Experts in uncertainty: opinion and subjective probability in science. Oxford University Press on Demand.
Davidson VJ, Ravel A, Nguyen TN, Fazil A and Ruzante JM, 2011. Food‐specific attribution of selected gastrointestinal illnesses: estimates from a Canadian expert elicitation survey. Foodborne Pathogens and Disease, 8, 983–995.
EFSA BIOHAZ Panel, 2008. Scientific Opinion of the Panel on Biological Hazards on a request from EFSA on Overview of methods for source attribution for human illness from food borne microbiological hazards. EFSA Journal 2008; 764, 1–43.
Hald T, Aspinall W, Devleesschauwer B, Cooke R, Corrigan T, Havelaar AH, Gibb HJ, Torgerson PR, Kirk MD, Angulo FJ and Lake RJ, 2016. World Health Organization estimates of the relative contributions of food to the burden of disease due to selected food‐borne hazards: a structured expert elicitation. PLoS One, 11, e0145839.
Havelaar AH, Galindo AV, Kurowicka D and Cooke RM, 2008. Attribution of food‐borne pathogens using structured expert elicitation. Foodborne Pathogens and Disease, 5, 649–659.
Lake RJ, Cressey PJ, Campbell DM and Oakley E, 2010. Risk ranking for food‐borne microbial hazards in New Zealand: burden of disease estimates. Risk Analysis, 30, 743–752.
Painter JA, Ayers T, Woodruff R, Blanton E, Perez N, Hoekstra RM, Griffin PM and Braden C, 2009. Recipes for food‐borne outbreaks: a scheme for categorizing and grouping implicated foods. Foodborne Pathogens and Disease, 6, 1259–1264.
Pires SM, Evers EG, van Pelt W, Ayers T, Scallan E, Angulo FJ, Havelaar A and Hald T, 2009. Attributing the human disease burden of food‐borne infections to specific sources. Foodborne Pathogens and Disease, 6, 417–424.
Pires SM, 2013. Assessing the applicability of currently available methods for attributing food‐borne disease to sources, including food and food commodities. Foodborne Pathogens and Disease, 10, 206–213.
Pires SM, Vieira AR, Hald T and Cole D, 2014. Source attribution of human salmonellosis: an overview of methods and estimates. Foodborne Pathogens and Disease, 11, 667–676.
Vally H, Glass K, Ford L, Hall G, Kirk MD, Shadbolt C, Veitch M, Fullerton KE, Musto J and Becker N, 2014. Proportion of illness acquired by food‐borne transmission for nine enteric pathogens in Australia: an expert elicitation. Foodborne Pathogens and Disease, 11, 727–733.
Table E.2.
Strengths and limitations of source attribution methods (adapted from Pires et al., 2014)
| SA approach | Strengths | Limitations |
|---|---|---|
| Epidemiological studies | ||
| Case–control studies (including systematic review) |
|
|
| Analysis of data from outbreaks |
|
|
| Subtyping |
|
|
| Comparative Risk Assessment |
|
|
| Expert knowledge elicitations |
|
|
Appendix F – Detection methods for Toxoplasma gondii in food
Cat bioassay
To perform a cat bioassay, cats without previous exposure of T. gondii must be used and usually obtained by demonstrating the absence of antibodies using a serological assay (often modified agglutination test). The cats are then fed with up to 500 g of the meat or tissue to be tested for the presence of T. gondii tissue cysts. Subsequently, the cats’ faeces are tested for the presence of T. gondii oocysts (microscopy, PCR or bioassay in mice) for up to 3 weeks after exposure and serum samples tested for the presence of T. gondii ‐specific antibodies 3 weeks or longer post‐exposure. For further confirmation of infection, T. gondii can be demonstrated in cat tissues (e.g., PCR or mouse bioassay) (Dubey, 2010).
Cat bioassay demonstrates oral infectivity and viability of tissue cysts (bradyzoites) through production of oocysts in the faeces of the cat and seroconversion, and enables testing of large portions of meat. Cats may become infected following ingestion of all stages of T. gondii (tachyzoite, bradyzoite, or oocyst), although the time to oocyst shedding and the frequency of shedding is related to the parasite stage used to initiate the infection. Less than 50% of cats shed oocysts after ingesting tachyzoites or oocysts, whereas nearly 100% of cats shed oocysts after ingesting tissues cysts. Cats are very sensitive to infection with bradyzoites, and infection could be achieved using only one bradyzoite (Dubey, 2010). Isolated oocysts can be used for strain isolation and genotyping. Cat bioassays can be ethically undesirable and costly.
Mouse bioassay
Depending on the preference and type of sample, homogenates or artificially digested meat tissues are inoculated (usually intraperitoneally or subcutaneously) into mice (Dubey, 2010). For digestion, either acid‐pepsin solution or trypsin is used, and differences in survival of bradyzoites and tachyzoites in these solutions have been suggested (Dubey, 1998). Usually, 50–200 g of tissue is digested and a fraction of the pellet is inoculated into mice. Normally, between two and five mice per sample are used. Mice may also be infected orally with oocysts of T. gondii. Different mouse strains are used and immunosuppressive drugs may be administered to increase sensitivity. The mice are monitored clinically and, when mice die or need to be euthanised, or at the end of the experiment, samples (e.g. brain, peritoneal fluid) are examined for the presence of T. gondii by microscopy or PCR. Usually the mice are also tested for T. gondii‐specific antibodies.
Mouse bioassay demonstrates the infectivity of T. gondii, but does not necessarily provide an indication of oral infectivity. In particular, survival of trypsin digestion by tachyzoites, which are assumed to be less infective after oral ingestion, is a point of discussion. Mouse bioassays can also be used for strain isolation. Mouse bioassays are usually less costly than cat bioassays, but are also undesirable for ethical reasons. The size of the sample that can be investigated is smaller than that used for cat bioassay, particularly as only a fraction of the digest is inoculated.
Detection of DNA using PCR methods
Several different targets are available for PCR‐based detection of T. gondii, the B1 gene and the 529‐bp repeat element are the most common targets (Su and Dubey, 2009). Various systems (conventional, nested, semi‐nested, and real‐time PCR) have been described. In general, all these methods can detect low concentrations of T. gondii DNA and the methods perform well on spiked samples or in cases of disseminated toxoplasmosis. However, tissue cysts are sparse and commercial DNA isolation methods are usually designed for 25 mg of samples; the chance of detecting T. gondii in such a small sample is low. For that reason, the main factor limiting the sensitivity of PCR‐based detection of tissue cysts is the DNA isolation method. To enable testing of large samples, and thereby increase the sensitivity of detection, methods based on artificial digestion, homogenisation and isolation over Percoll gradients and sequence‐based magnetic capture have been described.
Detection of T. gondii DNA does not necessarily provide an indication of oral infectivity, as non‐viable parasites or tachyzoites, which appear to be less infective after oral ingestion, can also be detected. Development of molecular methods for determining viability is ongoing for various pathogens, however such approaches to assessment of the viability of T. gondii bradyzoites in meat have not been published to date.
Detection of DNA using LAMP
In addition to PCR, loop‐mediated isothermal amplification (LAMP)‐based DNA detection methods have been described (Zhang et al., 2009; Lin et al., 2012; Liu et al. 2015) as a highly sensitive alternative to PCR. An adaptation of the LAMP technique to include a lateral flow dipstick chromatographic detection system to speed up visualisation of results has been applied to detect oocysts in fresh produce (Lalle et al., 2018). On spiked samples, performance of these methods is often comparable to PCR. Amplification products from LAMP cannot be sequenced and the method has the same drawbacks as PCR regarding sample size and viability.
In vitro isolation
T. gondii tachyzoites can be cultivated in a wide range of cell lines. In vitro cultivation is commonly used to maintain or multiply parasites, e.g. for antigen preparation (Dubey, 2010). After several passages in cell culture or mice, strains may lose their ability to form tissue cysts or oocysts (non‐cystogenic strains such as RH and S48) (O'Connell et al., 1988).
Diagnostic usage of tissue culture‐based methods is limited and mainly described for fluid samples in which tachyzoites can be expected (e.g., liquor, peritoneal exudate, amniotic fluid). Meat homogenates or sediments from artificial digestion have been tested with variable success rates (Warnekulasuriya et al., 1998).
A tissue culture‐based assay was described for milk samples from a range of livestock species where 10% of caprine (n = 180); 7% of ovine (n = 185) and 4% of bovine (n = 200) milk samples were found to be positive using a tissue culture‐based assay with vero cells (Dehkordi et al., 2013).
Microscopy
T. gondii tachyzoites (approximately 2 by 6 μm crescent‐shaped organisms) or tissue cysts (an intracellular cluster of bradyzoites of up to 100 μm contained by a tissue cyst wall) cannot be detected by macroscopic inspection of meat, but can be identified by microscopy (Hall and Dubey, 2002). Although parasites are visible using non‐specific stains such as Giemsa or haemotoxylin and eosin, the use of specific stains with enzymes (IHC) or fluorescent‐conjugated antibodies will help differentiation from other apicomplexan parasites or structures, and increases sensitivity. Cross‐reactivity of conjugated antibody, especially polyclonal antisera, may reduce the specificity and should be determined. Microscopy is labour‐intensive and requires an experienced technician. The main disadvantage is the size of the sample that can be examined. The use of microscopy directly on meat samples is limited, but it is often used secondarily to demonstrate infection in bioassay mice (tachyzoites in peritoneal fluid in acute infections or tissue cysts in brain in chronic infections) (Dubey, 2010).
In summary, T. gondii cat bioassay, mouse bioassay or a PCR‐based method that allows processing of large samples (e.g. by processing many replicates or by performing DNA isolation after artificial digestion or using sequence‐based magnetic capture) are recommended. Cat and mouse bioassays demonstrate the infectivity of detected parasites, whereas PCR does not. To limit the use of mice or cats, a first screening with PCR or indirect assays can be applied and positive samples could be selected for bioassay. This strategy is useful only when there is a good correlation between indirect and direct detection. Development of sensitive viability assays that do not require animal testing would be preferable.
References
Dehkordi FS, Haghighi Borujeni MR, Rahimi E and Abdizadeh R, 2013. Detection of Toxoplasma gondii in raw caprine, ovine, buffalo, bovine, and camel milk using cell cultivation, cat bioassay, capture ELISA, and PCR methods in Iran. Foodborne Pathogens and Disease, 10, 120–125.
Dubey JP, 1998. Refinement of pepsin digestion method for isolation of Toxoplasma gondii from infected tissues. Veterinary Parasitology, 74, 75–77.
Dubey JP, 2010. Toxoplasmosis of Animals and Humans.
CRC Press. Second edition. 2010. 336 pages ISBN 978‐1‐4200‐9236‐3.
Hill D and Dubey JP, 2002. Toxoplasma gondii: transmission, diagnosis and prevention. Clinical Microbiology and Infection, 8, 634–640.
Lalle M, Possenti A, Dubey JP and Pozio E, 2018. Loop‐Mediated Isothermal Amplification‐Lateral‐Flow Dipstick (LAMP‐LFD) to detect Toxoplasma gondii oocyst in ready‐to‐eat salad. Food Microbiology, 70, 137–142.
Lin Z, Zhang Y, Zhang H, Zhou Y, Cao J and Zhou J, 2012. Comparison of loop‐mediated isothermal amplification (LAMP) and real‐time PCR method targeting a 529‐bp repeat element for diagnosis of toxoplasmosis. Veterinary Parasitology, 185, 296–300.
Liu Q, Wang ZD, Huang SY and Zhu XQ, 2015. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites & Vectors, 8, 292.
O'Connell E, Wilkins MF and Te Punga WA, 1988. Toxoplasmosis in sheep II. The ability of a live vaccine to prevent lamb losses after an intravenous challenge with Toxoplasma gondii. New Zealand Veterinary Journal, 36, 1–4.
Su C and Dubey JP, 2009 Toxoplasma gondii. In: Molecular detection of food‐borne pathogens. D Liu (ed). CRC Press. pp. 741–753. ISBN 9781420076431.
Warnekulasuriya MR, Johnson JD and Holliman RE, 1998. Detection of Toxoplasma gondii in cured meats. International Journal of Food Microbiology, 45, 211–215.
Zhang H, Thekisoe OM, Aboge GO, Kyan H, Yamagishi J, Inoue N, Nishikawa Y, Zakimi S and Xuan X, 2009. Toxoplasma gondii: sensitive and rapid detection of infection by loop‐mediated isothermal amplification (LAMP) method. Experimental Parasitology, 122, 47–50.
Appendix G – Detection methods for Echinococcus spp. in food
Federer et al. (2016)
The total mass of vegetables (around 50 kg) and fruits (around 10 kg) represented one sample. Each sample was washed using tap water.
First, all fruits and vegetables were checked, dirty spots were removed and the fruits and vegetables were submerged as a whole and washed in a sink filled with tap water for around 1 min;
Heads of lettuce were cut in two to facilitate subsequent rinsing of the internal leaves;
Vegetables were then placed in a large meshed plastic‐container and thoroughly rinsed with a dish sprinkler;
All the washwater (a volume of 240 L collected in smaller containers of 60 L) was collected and sieved through filters with different mesh sizes. For this purpose, a tube system (diameter of 16 cm) with two filters (aperture sizes: 50 μm and 21 μm) was built;
Debris bigger than 21 μm was retained in the smallest filter. Subsequently, the filter was turned upside down and washed again with tap water and placed inside two 1.5‐L bottles. Finally, the sediment was concentrated through a series of centrifugation steps and collected in a flat tube of 10 mL volume to allow examination for the presence of eggs using an inverted microscope.
Molecular analysis: DNA of each sample was extracted according to Štefanic′ et al. (2004). A multiplex PCR for the discrimination of E. granulosus and E. multilocularis from other cestodes was used (Trachsel et al., 2007).
Lass et al. (2015)
This method is based on a strategy developed for the detection of Echinococcus multilocularis in soil by the same group (Szostakowska, 2014).
One sample consisted of 0.3–0.5 kg of fruits (raspberries, cranberries, blueberries, cowberries) or mushrooms, 0.5 kg of vegetables (carrot, parsley, beets, celery, radishes), or one lettuce or two bunches of dill or chives.
For the concentration of the eggs, a flotation method using saturated ZnCl2 solution was used. The following steps were applied:
One sample of fruit, vegetables, or mushrooms was placed in a glass vessel, mixed slightly with 2 L 0.05% Tween 80 solution;
Shaken for 30 min at 120 rpm;
Washing solution transferred to another vessel;
0.5 L of 0.05% Tween 80 solution added to first vessel (step 1);
Washings mixed (from steps 3 and 4) and left overnight at 4°C;
The next day, supernatant removed using an automatic pipette filler;
Sediment (approximately 100 mL) filtered through a set of sieves (pore size of last sieve 50 μm), and the retained sediment placed in a 200‐mL conical tube and centrifuged for 15 min at 200×g;
Supernatant removed, and the pellet placed in a 50‐mL Falcon tube and frozen at −70°C;
Thawed pellet suspended in 30 mL of saturated ZnCl2 solution, mixed thoroughly, and centrifuged for 3 min at 200×g;
20 mL of saturated ZnCl2 solution is added and centrifuged for 3 min at 200×g;
Each tube is placed in a holder and topped up carefully with saturated ZnCl2 solution;
The surface of the liquid is covered with a cover glass slide for 15 min;
Finally, the slide is washed with distilled water, and the material retrieved into a 2.0‐mL Eppendorf tube.
To concentrate the content, tube centrifuged for 1 min at 200×g, and any excess water is removed carefully. Finally, the suspension obtained frozen at −20°C for further analysis.
Molecular analysis:
DNA extracted using a commercial kit (Sherlock AX Kit (A&A Biotechnology, Gdynia, Poland) and samples treated with an Anti‐inhibitor Kit (A&A Biotechnology, Gdynia, Poland).
Nested PCR targeting the mitochondrial 12S ribosomal RNA (rRNA) gene:
First PCR using the primers p60 forward primer (5′‐TTAAGATATATGTGGTACAGGATTAGATACCC‐3′) and p375 reverse primer (5′‐AACCGAGGGTGACGGGCGGTGTGTACC‐3′) (von Nickisch‐Rosenegk et al. 1999). Second PCR using primers E. multilocularis. Nest/forward primer (5′‐GTGAGTGATTCTTGTTAGGGGAAGA‐3′) and E. multilocularis nest/reverse primer. DNA isolated from adult E. multilocularis was used as a positive control and distilled water as a negative control.
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Cacciò S, Chalmers R, Deplazes P, Devleesschauwer B, Innes E, Romig T, van der Giessen J, Hempen M, Van der Stede Y and Robertson L, 2018. Scientific Opinion on the public health risks associated with food‐borne parasites. EFSA Journal 2018;16(12):5495, 113 pp. 10.2903/j.efsa.2018.5495
Requestor: European Food Safety Authority
Question number: EFSA‐Q‐2017‐00460
Panel members: Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Kostas Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Acknowledgements: The BIOHAZ Panel wishes to thank the following for the support provided to this scientific output: Matilde Garcia Gomez. The BIOHAZ Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 25 October 2018
Notes
European Cooperation in Science and Technology. FA1408 ‐ A European Network for Foodborne Parasites (Euro‐FBP). Available from: http://www.cost.eu/COST_Actions/fa/FA1408
FAO (2001). ‘Definitions for the Purposes of the Codex Alimentarius’. http://www.fao.org/docrep/005/y2200e/y2200e07.htm
A risk assessment of Cryptosporidium parvum, an emerging pathogen in the food and water chain in Europe, CARAFE, project no. QLK1 CT 1999 00775, https://cordis.europa.eu/project/rcn/51475_en.html
Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. OJ L 327, 22.12.2000, p. 1–73.
EUR A: Andorra, Austria, Belgium, Croatia, Cyprus, the Czech Republic, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Monaco, the Netherlands, Norway, Portugal, San Marino, Slovenia, Spain, Sweden, Switzerland, the United Kingdom.
EUR B: Albania, Armenia, Azerbaijan, Bosnia and Herzegovina, Bulgaria, Georgia, Kyrgyzstan, Montenegro, Poland, Romania, Serbia, Slovakia, Tajikistan, The Former Yugoslav Republic of Macedonia, Turkey, Turkmenistan, Uzbekistan.
EUR C: Belarus, Estonia, Hungary, Kazakhstan, Latvia, Lithuania, Republic of Moldova, Russian Federation, Ukraine.
References
- Åberg R, Sjöman M, Hemminki K, Pirnes A, Räsänen S, Kalanti A, Pohjanvirta T, Caccio SM, Pihlajasaari A, Toikkanen S, Huusko S and Rimhanen‐Finne R, 2015. Cryptosporidium parvum caused a large outbreak linked to Frisée Salad in Finland, 2012. Zoonoses Public Health, 62, 618–624. 10.1111/zph.12190 [DOI] [PubMed] [Google Scholar]
- Aguirre J, Greenwood SJ, McClure J, Davidson J and Sanchez J, 2016. Effects of rain events on Cryptosporidium spp. levels in commercial shellfish zones in the Hillsborough River, Prince Edward Island, Canada. Food and Waterborne Parasitology, 5, 7–13. [Google Scholar]
- Ajzenberg D, Collinet F, Aubert D, Villena I, Dardé ML and Devillard S, 2015. The rural–urban effect on spatial genetic structure of type II Toxoplasma gondii strains involved in human congenital toxoplasmosis, France, 2002–2009. Infection, Genetics and Evolution, 36, 511–516. [DOI] [PubMed] [Google Scholar]
- Ajzenberg D, Dumètre A and Dardé ML, 2005. Multiplex PCR for typing strains of Toxoplasma gondii . Journal of Clinical Microbiology, 43, 1940–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akalin S, Kutlu SS, Caylak SD, Onal O, Kaya S and Bozkurt AI, 2014. Seroprevalence of human cystic echinococcosis and risk factors in animal breeders in rural communities in Denizli, Turkey. The Journal of Infection in Developing Countries, 8, 1188–1194. [DOI] [PubMed] [Google Scholar]
- Aksoy U, Marangi M, Papini R, Ozkoc S, Delibas SB and Giangaspero A, 2014. Detection of Toxoplasma gondii and Cyclospora cayetanensis in Mytilus galloprovincialis from Izmir Province coast (Turkey) by real time PCR/High‐Resolution Melting analysis (HRM). Food Microbiology, 44, 128–135. [DOI] [PubMed] [Google Scholar]
- Alvarez Rojas CA, Romig T and Lightowlers MW, 2014. Echinococcus granulosus sensu lato genotypes infecting humans–review of current knowledge. International Journal for Parasitology, 44, 9–18. [DOI] [PubMed] [Google Scholar]
- Alvarez Rojas CA, Mathis A and Deplazes P, 2018. Assessing the contamination of food and the environment with Taenia and Echinococcus eggs and their zoonotic transmission. Current Clinical Microbiology Reports, 5, 154–163. [Google Scholar]
- Amoros I, Alonso JL and Cuest G, 2010. Cryptosporidium oocysts and Giardia cysts on salads products irrigated with contaminated water. Journal of Food Protection, 73, 1138e1140. [DOI] [PubMed] [Google Scholar]
- Arkush KD, Miller MA, Leutenegger CM, Gardner IA, Packham AE, Heckeroth AR, Tenter AM, Barr BC and Conrad PA, 2003. Molecular and bioassay‐based detection of Toxoplasma gondii oocyst uptake by mussels (Mytilus galloprovincialis). International Journal for Parasitology, 33, 1087–1097. [DOI] [PubMed] [Google Scholar]
- van Asseldonk M, van Wagenberg CPA and Wisselink HJ, 2017. Break‐even analysis of costs for controlling Toxoplasma gondii infections in slaughter pigs via a serological surveillance program in the Netherlands. Preventive Veterinary Medicine, 138, 139–146. [DOI] [PubMed] [Google Scholar]
- Balasundaram MB, Andavar R, Palaniswamy M and Venkatapathy N, 2010. Outbreak of acquired ocular toxoplasmosis involving 248 patients. Archives of Ophthalmology, 128, 28–32. 10.1001/archophthalmol.2009.354 [DOI] [PubMed] [Google Scholar]
- Batz MB, Hoffmann S and Morris JG Jr, 2012. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. Journal of Food Protection, 75, 1278–1291. [DOI] [PubMed] [Google Scholar]
- Belluco S, Simonato G, Mancin M, Pietrobelli M and Ricci A, 2017. Toxoplasma gondii infection and food consumption: a systematic review and meta‐analysis of case‐controlled studies. Critical Reviews in Food Science and Nutrition, 11, 1–12. [DOI] [PubMed] [Google Scholar]
- Belluco S, Patuzzi I and Ricci A, 2018. Bovine meat versus pork in Toxoplasma gondii transmission in Italy: A quantitative risk assessment model. International Journal of Food Microbiology, 269, 1–11. [DOI] [PubMed] [Google Scholar]
- Benenson MW, Takafuji ET, Lemon SM, Greenup RL and Sulzer AJ, 1982. Oocyst‐transmitted toxoplasmosis associated with ingestion of contaminated water. New England Journal of Medicine, 307, 666–669. [DOI] [PubMed] [Google Scholar]
- Bezerra M, Kim P, Moraes ÉP, Sá S, Albuquerque P, Silva J, Alves B and Mota R, 2015. Detection of Toxoplasma gondii in the milk of naturally infected goats in the Northeast of Brazil. Transboundary and Emerging Diseases, 62, 421–424. [DOI] [PubMed] [Google Scholar]
- Boughattas S, 2017. Toxoplasma infection and milk consumption: meta‐analysis of assumptions and evidences. Critical Reviews in Food Science and Nutrition, 57, 2924–2933. [DOI] [PubMed] [Google Scholar]
- Bouwknegt M, Devleesschauwer B, Graham H, Robertson LJ and dervan Giessen JW , The Euro‐Fbp Workshop Participants , 2018. Prioritisation of food‐borne parasites in Europe, 2016. Eurosurveillance Weekly 23, 17‐00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie WR, King AS, Werker DH, Isaac‐Renton JL, Bell A, Eng SB and Marion SA, 1997. Outbreak of toxoplasmosis associated with municipal drinking water. The Lancet, 350, 173–177. [DOI] [PubMed] [Google Scholar]
- Boyer K, Hill D, Mui E, Wroblewski K, Karrison T, Dubey JP, Sautter M, Noble AG, Withers S, Swisher C, Heydemann P, Hosten T, Babiarz J, Lee D, Meier P and McLeod R; Toxoplasmosis Study Group , 2011. Unrecognized ingestion of Toxoplasma gondii oocysts leads to congenital toxoplasmosis and causes epidemics in North America. Clinical Infectious Diseases, 53, 1081–1089. 10.1093/cid/cir667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrells A, Benavides J, Cantón G, Garcia JL, Bartley PM, Nath M, Thomson J, Chianini F, Innes EA and Katzer F, 2015. Vaccination of pigs with the S48 strain of Toxoplasma gondii – Safer meat for human consumption. Veterinary Research, 46, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrells A, Opsteegh M, Pollock KG, Alexander CL, Chatterton J, Evans R, Walker R, McKenzie CA, Hill D, Innes EA and Katzer F, 2016. The prevalence and genotypic analysis of Toxoplasma gondii from individuals in Scotland, 2006–2012. Parasites and Vectors, 9, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AJ, Thomas MK and Pintar KD, 2015. Expert elicitation as a means to attribute 28 enteric pathogens to food‐borne, waterborne, animal contact, and person‐to‐person transmission routes in Canada. Foodborne Pathogens and Disease, 12, 335–344. [DOI] [PubMed] [Google Scholar]
- Cacciò SM and Chalmers RM, 2016. Human cryptosporidiosis in Europe. Clinical Microbiology and Infection, 22, 471–480. 10.1016/j.cmi.2016.04 [DOI] [PubMed] [Google Scholar]
- Cacciò SM and Putignani L, 2014. Epidemiology of human cryptosporidiosis In: Cacciò SM. and Widmer G. (eds). Cryptosporidium: Parasite and Disease. Springer, Vienna: pp. 43–79. [Google Scholar]
- Campos‐Bueno AN, López‐Abente G and Andres‐Cercadillo AM, 2000. Risk factors for Echinococcus granulosus infection: a case‐control study. The American Journal of Tropical Medicine and Hygiene, 62, 329–334. [DOI] [PubMed] [Google Scholar]
- Caradonna T, Marangi M, Del Chierico F, Ferrari N, Reddel S, Bracaglia G, Normanno G, Putignani L and Giangaspero A, 2017. Detection and prevalence of protozoan parasites in ready‐to‐eat packaged salads on sale in Italy. Food Microbiology, 67, 67–75. [DOI] [PubMed] [Google Scholar]
- Carme B, Demar M, Ajzenberg D and Dardé ML, 2009. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerging Infectious Diseases, 15, 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetinkaya Z, Ciftci IH, Demirel R, Altindis M and Ayaz E, 2005. A sero‐epidemiologic study on cystic echinococcosis in midwestern region of Turkey. Saudi Medical Journal, 26, 350. [PubMed] [Google Scholar]
- Chalmers R, 2012. Waterborne outbreaks of cryptosporidiosis. Annali dell'Istituto Superiore di Sanità, 48, 429–446. [DOI] [PubMed] [Google Scholar]
- Chalmers R and Cacciò S, 2016. Towards a consensus on genotyping schemes for surveillance and outbreak investigations of Cryptosporidium, Berlin, June 2016. Eurosurveillance, 21, pii=30338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers RM and Giles M, 2010. Zoonotic cryptosporidiosis. Journal of Applied Microbiology, 109, 1487–1497. 10.1111/j.1365-2672.2010.04764.x [DOI] [PubMed] [Google Scholar]
- Chalmers R, Smith R, Elwin K, Clifton‐Hadley F and Giles M, 2011. Epidemiology of anthroponotic and zoonotic human cryptosporidiosis in England and Wales, 2004–2006. Epidemiology & Infection, 139, 700–712. [DOI] [PubMed] [Google Scholar]
- Chapalamadugu KC, Busboom JR, Nelson ML, Hancock DD, Tang J and Jasmer DP, 2008. Taenia taeniaeformis: effectiveness of staining oncospheres is related to both temperature of treatment and molecular weight of dyes utilized. Veterinary Parasitology, 151, 203–211. [DOI] [PubMed] [Google Scholar]
- Chaudhry RM, Hamilton KA, Haas CN and Nelson KL, 2017. Drivers of microbial risk for direct potable reuse and de facto reuse treatment schemes: the impacts of source water quality and blending. International Journal of Environmental Research and Public Health, 14, pii: E635. 10.3390/ijerph14060635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiari CdA and Neves DP, 1984. Toxoplasmose humana adquirida através da ingestão de leite de cabra. Memorias do Instituto Oswaldo Cruz, 79, 337–340. [DOI] [PubMed] [Google Scholar]
- Cisak E, Zając V, Sroka J, Sawczyn A, Kloc A, Dutkiewicz J and Wójcik‐Fatla A, 2017. Presence of pathogenic rickettsiae and protozoan in samples of raw milk from cows, goats, and sheep. Foodborne Pathogens and Disease, 14, 189–194. [DOI] [PubMed] [Google Scholar]
- Coman BJ and Rickard MD, 1977. A comparison of in vitro and in vivo estimates of the viability of Taenia pisiformis eggs aged under controlled conditions, and their ability to immunise against a challenge infection. International Journal for Parasitology, 7, 15–20. [DOI] [PubMed] [Google Scholar]
- Condoleo R, Rinaldi L, Sette S and Mezher Z, 2018. Risk assessment of human toxoplasmosis associated with the consumption of Pork Meat in Italy. Risk Analysis, 38, 1202–1222. 10.1111/risa.12934 [DOI] [PubMed] [Google Scholar]
- Cong W, Ju HL, Zhang XX, Meng QF, Ma JG, Qian AD and Zhu XQ, 2017. First genetic characterization of Toxoplasma gondii infection in common quails (Coturnix coturnix) intended for human consumption in China. Infection, Genetics and Evolution, 49, 14–16. [DOI] [PubMed] [Google Scholar]
- Conn DB, Weaver J, Tamang L and Graczyk TK, 2007. Synanthropic flies as vectors of Cryptosporidium and Giardia among livestock and wildlife in a multispecies agricultural complex. Vector‐Borne and Zoonotic Diseases, 7, 643–652. [DOI] [PubMed] [Google Scholar]
- Conrad PA, Miller MA, Kreuder C, James ER, Mazet J, Dabritz H, Jessup DA, Gulland F and Grigg ME, 2005. Transmission of Toxoplasma: clues from the study of sea otters as sentinels of Toxoplasma gondii flow into the marine environment. International Journal for Parasitology, 35, 1155–1168. [DOI] [PubMed] [Google Scholar]
- Conraths FJ and Deplazes P, 2015. Echinococcus multilocularis: epidemiology, surveillance and state‐of‐the‐art diagnostics from a veterinary public health perspective. Veterinary Parasitology, 213, 149–161. [DOI] [PubMed] [Google Scholar]
- Conraths FJ, Probst C, Possenti A, Boufana B, Saulle R, La Torre G, Busani L and Casulli A, 2017. Potential risk factors associated with human alveolar echinococcosis: systematic review and meta‐analysis. PLoS Neglected Tropical Diseases, 11, e0005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook BR, 1991. Echinococcus multilocularis infestation acquired in UK. Lancet, 337, 560–561. [DOI] [PubMed] [Google Scholar]
- Cook AJC, Holliman R, Gilbert RE, Buffolano W, Zufferey J, Petersen E, Jenum PA, Foulon W, Semprini AE and Dunn DT, 2000. Sources of toxoplasma infection in pregnant women: European multicentre case‐control study Commentary: congenital toxoplasmosis—further thought for food. BMJ, 321, 142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N, Paton CA, Wilkinson N, Nichols RAB, Barker K and Smith HV, 2006. Towards standard methods for the detection of Cryptosporidium parvum on lettuce and raspberries. Part 2: validation. International Journal of Food Microbiology, 109, 222–228. [DOI] [PubMed] [Google Scholar]
- Cornelissen JBWJ, van der Giessen JWB, Takumi K, Teunis PFM and Wisselink HJ, 2014. An Experimental Toxoplasma gondii Dose. Response Challenge Model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupe A, Howe L, Burrows E, Sine A, Pita A, Velathanthiri N, Vallée E, Hayman D, Shapiro K and Roe WD, 2018. First report of Toxoplasma gondii sporulated oocysts and Giardia duodenalis in commercial green‐lipped mussels (Perna canaliculus) in New Zealand. Parasitology Research, 117, 1453–1463. [DOI] [PubMed] [Google Scholar]
- Craig PS, Hegglin D, Lightowlers MW, Torgerson PR and Wang Q, 2017. Echinococcosis: control and prevention In Advances in parasitology (Vol. 96, pp. 55‐158). Academic Press. [DOI] [PubMed] [Google Scholar]
- Davidson VJ, Ravel A, Nguyen TN, Fazil A and Ruzante JM, 2011. Food‐specific attribution of selected gastrointestinal illnesses: estimates from a Canadian expert elicitation survey. Foodborne Pathogens and Disease, 8, 983–995. [DOI] [PubMed] [Google Scholar]
- Dawson D, 2005. Foodborne protozoan parasites. International Journal of Food Microbiology, 103, 207–227. [DOI] [PubMed] [Google Scholar]
- Dehkordi FS, Haghighi Borujeni MR, Rahimi E and Abdizadeh R, 2013. Detection of Toxoplasma gondii in raw caprine, ovine, buffalo, bovine, and camel milk using cell cultivation, cat bioassay, capture ELISA, and PCR methods in Iran. Foodborne Pathogens and Disease, 10, 120–125. [DOI] [PubMed] [Google Scholar]
- Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, Punwasi W, Valery N, Peneau C, Daigre JL, Aznar C, Cottrelle B, Terzan L, Darde ML and Carme B, 2007. Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clinical Infectious Diseases, 45, e88–e95. [DOI] [PubMed] [Google Scholar]
- Deng MQ and Cliver DO, 1999. Cryptosporidium parvum studies with dairy products. International Journal of Food Microbiology, 46, 113–121. [DOI] [PubMed] [Google Scholar]
- Deng MQ and Cliver DO, 2000. Comparative detection of Cryptosporidium parvum oocysts from apple juice. International Journal of Food Microbiology, 54, 155–162. [DOI] [PubMed] [Google Scholar]
- Deng MQ and Cliver DO, 2001. Inactivation of Cryptosporidium parvum oocysts in cider by flash pasteurization. Journal of Food Protection, 64, 523–527. [DOI] [PubMed] [Google Scholar]
- Deng MQ, Lam KM and Cliver DO, 2000. Immunomagnetic separation of Cryptosporidium parvum oocysts using MACS MicroBeads and high gradient separation columns. Journal of Microbiological Methods, 40, 11–17. [DOI] [PubMed] [Google Scholar]
- Deplazes P, Hegglin D, Gloor S and Romig T, 2004. Wilderness in the city: the urbanization of Echinococcus multilocularis . Trends in Parasitology, 20, 77–84. [DOI] [PubMed] [Google Scholar]
- Deplazes P, Grimm F, Sydler T, Tanner I and Kapel CMO, 2005. Experimental alveolar echinococcosis in pigs, lesion development and serological follow up. Veterinary Parasitology, 130, 213–222. [DOI] [PubMed] [Google Scholar]
- Deplazes P, van Knapen F, Schweiger A and Overgaauw PA, 2011. Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Veterinary Parasitology, 182, 41–53. [DOI] [PubMed] [Google Scholar]
- Deplazes P, Rinaldi L, Rojas CA, Torgerson PR, Harandi MF, Romig T, Antolova D, Schurer JM, Lahmar S, Cringoli G and Magambo J, 2017. Global distribution of alveolar and cystic echinococcosis In Advances in parasitology (Vol. 95, pp. 315‐493). Academic Press. [DOI] [PubMed] [Google Scholar]
- Devleesschauwer B, Haagsma JA, Angulo FJ, Bellinger DC, Cole D, Döpfer D, Fazil A, Fèvre EM, Gibb HJ, Hald T and Kirk MD, 2015. Methodological framework for World Health Organization estimates of the global burden of food‐borne disease. PLoS ONE, 10, e0142498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doganci L, Tanyuksel M, Araz ER, Besirbellioglu BA, Erdem U, Ozoguz CA, Yucel N and Ciftcioglu A, 2006. A probable outbreak of toxoplasmosis among boarding school students in Turkey. Clinical Microbiology and Infection, 12, 672–674. [DOI] [PubMed] [Google Scholar]
- Dubey JP, 1998. Toxoplasma gondii oocyst survival under defined temperatures. The Journal of parasitology, 862–865. [PubMed] [Google Scholar]
- Dubey J, 2009. Toxoplasmosis in pigs—the last 20 years. Veterinary Parasitology, 164, 89–103. [DOI] [PubMed] [Google Scholar]
- Dubey J, 2010. Toxoplasmosis of Animals and Humans. CRC Press, Boca Raton, FL: 336 pp. [Google Scholar]
- Dubey JP, Kotula AW, Sharar A, Andrews CD and Lindsay DS, 1990. Effect of high temperature on infectivity of Toxoplasma gondii tissue cysts in pork. The Journal of Parasitology, 76, 201–204. [PubMed] [Google Scholar]
- Dubey J, Verma S, Ferreira L, Oliveira S, Cassinelli A, Ying Y, Kwok O, Tuo W, Chiesa O and Jones J, 2014. Detection and survival of Toxoplasma gondii in milk and cheese from experimentally infected goats. Journal of Food Protection, 77, 1747–1753. [DOI] [PubMed] [Google Scholar]
- Duhain G, Minnaar A and Buys E, 2012. Effect of chlorine, blanching, freezing, and microwave heating on Cryptosporidium parvum viability inoculated on green peppers. Journal of Food Protection, 75, 936–941. [DOI] [PubMed] [Google Scholar]
- Dumètre A and Dardé ML, 2003. How to detect Toxoplasma gondii oocysts in environmental samples? FEMS Microbiology Reviews, 27, 651–661. [DOI] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control), 2018. Cryptosporidiosis In: ECDC. Annual epidemiological report for 2015. ECDC, Stockholm. [Google Scholar]
- Eckert J, Gottstein B, Heath D and Liu F‐J, 2001. Prevention of echinococcosis in humans and safety precautions In: Eckert J, Gemmell MA, Meslin F‐X. and Pawlowski ZS. (eds.). WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France: 238–247. [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010. Scientific Opinion on risk assessment of parasites in fishery products. EFSA Journal 2010;8(4):1543, 91 pp. 10.2903/j.efsa.2010.1543 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011. Scientific Opinion on the public health hazards to be covered by inspection of meat (swine). EFSA Journal 2011;9(10):2351, 198 pp. 10.2903/j.efsa.2011.2351 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013a. Scientific Opinion on the public health hazards to be covered by inspection of meat from sheep and goats. EFSA Journal 2013;11(6):3265, 186 pp. 10.2903/j.efsa.2013.3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013b. Scientific Opinion on the public health hazards to be covered by inspection of meat from farmed game. EFSA Journal 2013;11(6):3264, 181 pp. 10.2903/j.efsa.2013.3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013c. Scientific Opinion on the public health hazards to be covered by inspection of meat (solipeds). EFSA Journal 2013;11(6):3263, 161 pp. 10.2903/j.efsa.2013.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013d. Scientific Opinion on the public health hazards to be covered by inspection of meat (bovine animals). EFSA Journal 2013;11(6):3266, 261 pp. 10.2903/j.efsa.2013.3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2015. Scientific opinion on the evaluation of heat treatments, different from those currently established in the EU legislation, that could be applied to live bivalve molluscs from B and C production areas, that have not been submitted to purification or relaying, in order to eliminate pathogenic microorganisms. EFSA Journal 2015;13(12):4332, 76 pp. 10.2903/j.efsa.2015.4332 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2016. EFSA Journal 2017;15(12):5077, 228 pp. 10.2903/j.efsa.2017.5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Allende A, Barceló Culleres D, Gironés Llop R, Laval A, Robertson L, da Silva Felício MT, Gervelmeyer A, Ramos Bordajandi L and Liebana E, 2017. Request for scientific and technical assistance on proposed EU minimum quality requirements for water reuse in agricultural irrigation and aquifer recharge. EFSA supporting publication 2017;14(7):EN‐1247, 19 pp. 10.2903/sp.efsa.2017.EN-1247 [DOI] [Google Scholar]
- Ekman CCJ, Chiossi MFdV, Meireles LR, Andrade Júnior HFd, Figueiredo WM, Marciano MAM and Luna EJdA, 2012. Case‐control study of an outbreak of acute toxoplasmosis in an industrial plant in the state of São Paulo, Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 54, 239–244. [DOI] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the World Health Organization), 2001. Codex Alimentarius Commission ‐ Procedural Manual. 12th Ed. Secretariat of the Joint FAO/WHO Food Standards Programme, FAO, Rome. Available online: http://www.fao.org/docrep/005/y2200e/y2200e00.htm#Contents
- Fayer R, Farley CA, Lewis EJ, Trout JM and Graczyk TK, 1997. Potential Role of the Eastern Oyster, Crassostrea virginica, in the Epidemiology of Cryptosporidium parvum . Applied and Environmental Microbiology, 63, 2086–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R, Lewis EJ, Trout JM, Graczyk TK, Jenkins MC, Higgins J, Xiao L and Lal AA, 1999. Cryptosporidium parvum in oysters from commercial harvesting sites in the Chesapeake Bay. Emerging Infectious Diseases, 5, 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R, Trout J, Lewis E, Santin M, Zhou L, Lal A and Xiao L, 2003. Contamination of Atlantic coast commercial shellfish with Cryptosporidium. Parasitology Research, 89, 141–145. [DOI] [PubMed] [Google Scholar]
- Federer K, Armua‐Fernandez MT, Hoby S, Wenker C and Deplazes P, 2015. In vivo viability of Echinococcus multilocularis eggs in a rodent model after different thermo‐treatments. Experimental Parasitology, 154, 14–19. [DOI] [PubMed] [Google Scholar]
- Federer K, Armua‐Fernandez MT, Gori F, Hoby S, Wenker C and Deplazes P, 2016. Detection of taeniid (Taenia spp., Echinococcus spp.) eggs contaminating vegetables and fruits sold in European markets and the risk for metacestode infections in captive primates. International journal for parasitology. Parasites and wildlife, 5, 249–253. 10.1016/j.ijppaw.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetene T, Worku N, Huruy K and Kebede N, 2011. Cryptosporidium recovered from Musca domestica, Musca sorbens and mango juice accessed by synanthropic flies in Bahirdar, Ethiopia. Zoonoses Public Health, 58, 69–75. 10.1111/j.1863-2378.2009.01298.x [DOI] [PubMed] [Google Scholar]
- Fotiou V, Malissiova E, Minas A, Petinaki E and Hadjichristodoulou C, 2012. Seroprevalence of IgG antibodies against Echinococcus granulosus in the population of the region of Thessaly. Central Greece. PloS one, 7, e37112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen F, Gerard C, Cozma‐Petruţ A, Vieira‐Pinto M, Režek Jambrak A, Rowan N, Paulsen P, Rozycki M, Tysnes K, Rodriguez‐Lazaro D and Robertson L, 2018. Inactivation of parasite transmission stages: efficacy of treatments on food of animal origin. Trends in Food Science & Technology, 10.1016/j.tifs.2018.11.009 [DOI] [Google Scholar]
- Frazão‐Teixeira E, Sundar N, Dubey JP, Grigg ME and De Oliveira FCR, 2011. Multi‐locus DNA sequencing of Toxoplasma gondii isolated from Brazilian pigs identifies genetically divergent strains. Veterinary Parasitology, 175, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazar CD and Orlandi PA, 2007. Evaluation of two DNA template preparation methods for post‐immunomagnetic separation detection of Cryptosporidium parvum in foods and beverages by PCR. Applied and Environmental Microbiology, 73, 7474–7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire‐Santos F, Oteiza‐López AM, Vergara‐Castiblanco CA, Ares‐Mazás E, Alvarez‐Suárez E and García‐Martín O, 2000. Detection of Cryptosporidium oocysts in bivalve molluscs destined for human consumption. Journal of Parasitology, 86, 853–854. [DOI] [PubMed] [Google Scholar]
- Freire‐Santos F, Oteiza‐Lopez AM, Castro‐Hermida JA, Garcia‐Martin O and Ares‐Mazas ME, 2001. Viability and infectivity of oocysts recovered from clams, Ruditapes philippinarum, experimentally contaminated with Cryptosporidium parvum . Parasitology Research, 87, 428–430. [DOI] [PubMed] [Google Scholar]
- Freire‐Santos F, Gómez‐Couso H, Ortega‐Inarrea MR, Castro‐Hermida JA, Oteiza‐Lopez AM, Garcia‐Martin O and Ares‐Mazas ME, 2002. Survival of Cryptosporidium parvum oocysts recovered from experimentally contaminated oysters (Ostrea edulis) and clams (Tapes decussatus). Parasitology Research, 88, 130–133. [DOI] [PubMed] [Google Scholar]
- Friedman DE, Patten KE, Rose JB and Barney MC, 1997. The potential for Cryptosporidium parvum oocyst survival in beverages associated with contaminated tap water. Journal of Food Safety, 17, 125–132. [Google Scholar]
- Garcia L, Henderson J, Fabri M and Oke M, 2006. Potential sources of microbial contamination in unpasteurized apple cider. Journal of Food Protection, 69, 137–144. [DOI] [PubMed] [Google Scholar]
- Gazzonis AL, Zanzani SA, Stradiotto K, Olivieri E, Villa L and Manfredi MT, 2018. Toxoplasma gondii antibodies in bulk tank milk samples of caprine dairy herds. Journal of Parasitology. 10.1645/17-44 [DOI] [PubMed] [Google Scholar]
- GBD Diarrhoeal Diseases Collaborators , 2017. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infectious Diseases, 17, 909–948. 10.1016/S1473-3099(17)30276-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesy KM and Jenkins EJ, 2015. Introduced and native haplotypes of Echinococcus multilocularis in wildlife in Saskatchewan, Canada. Journal of wildlife Diseases, 51, 743–748. [DOI] [PubMed] [Google Scholar]
- Ghozzi K, Marangi M, Papini R, Lahmar I, Challouf R, Houas N, Dhiab RB, Normanno G, Babba H and Giangaspero A, 2017. First report of Tunisian coastal water contamination by protozoan parasites using mollusk bivalves as biological indicators. Marine Pollution Bulletin, 117, 197–202. [DOI] [PubMed] [Google Scholar]
- van der Giessen J, Fonville M, Bouwknegt M, Langelaar M and Vollema A, 2007. Seroprevalence of Trichinella spiralis and Toxoplasma gondii in pigs from different housing systems in The Netherlands. Veterinary Parasitology, 148, 371–374. [DOI] [PubMed] [Google Scholar]
- Gilbert RE and Stanford MR, 2000. Is ocular toxoplasmosis caused by prenatal or postnatal infection? British Journal of Ophthalmology, 84, 224–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkogka E, Reij MW, Havelaar AH, Zwietering MH and Gorris LG, 2011. Risk‐based estimate of effect of food‐borne diseases on public health, Greece. Emerging Infectious Diseases, 17, 1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Bautista M, Ortega‐Mora L, Tabares E, Lopez‐Rodas V and Costas E, 2000. Detection of infectious Cryptosporidium parvum oocysts in mussels (Mytilus galloprovincialis) and cockles (Cerastoderma edule). Applied and Environmental Microbiology, 66, 1866–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Couso H, Freire‐Santos F, Martınez‐Urtaza J, Garcıa‐Martın O and Ares‐Mazas M, 2003a. Contamination of bivalve molluscs by Cryptosporidiumoocysts: the need for new quality control standards. International Journal of Food Microbiology, 87, 97–105. [DOI] [PubMed] [Google Scholar]
- Gómez‐Couso H, Freire‐Santos F, Ortega‐Inarrea MR, Castro‐Hermida JA and Ares‐Mazas ME, 2003b. Environmental dispersal of Cryptosporidium parvum oocysts and cross transmission in cultured bivalve molluscs. Parasitology Research, 90, 140–142. [DOI] [PubMed] [Google Scholar]
- Gómez‐Couso H, Freire‐Santos F, Amar C, Grant K, Williamson K, Ares‐Mazas M and McLauchlin J, 2004. Detection of Cryptosporidium and Giardia in molluscan shellfish by multiplexed nested‐PCR. International Journal of Food Microbiology, 91, 279–288. [DOI] [PubMed] [Google Scholar]
- Gómez‐Couso H, Freire‐Santosa F, Hernández‐Córdovab GA and Ares‐Mazás ME, 2005. A histological study of the transit of Cryptosporidium parvum oocysts through clams (Tapes decussatus). International Journal of Food Microbiology, 102, 57–62. [DOI] [PubMed] [Google Scholar]
- Gómez‐Couso H, Méndez‐Hermida F and Ares‐Mazás E, 2006. Levels of detection of Cryptosporidium oocysts in mussels (Mytilus galloprovincialis) by IFA and PCR methods. Veterinary Parasitology, 141, 60–65. [DOI] [PubMed] [Google Scholar]
- Gomez‐Samblas M, Vílchez S, Racero JC, Fuentes MV and Osuna A, 2015. Quantification and viability assays of Toxoplasma gondii in commercial “Serrano” ham samples using magnetic capture real‐time qPCR and bioassay techniques. Food Microbiology, 46, 107–113. [DOI] [PubMed] [Google Scholar]
- Gottstein B, Stojkovic M, Vuitton DA, Millon L, Marcinkute A and Deplazes P, 2015. Threat of alveolar echinococcosis to public health–a challenge for Europe. Trends in Parasitology, 31, 407–412. [DOI] [PubMed] [Google Scholar]
- Grace D, Monda J, Karanja N, Randolph TF and Kang'ethe EK, 2012. Participatory probabilistic assessment of the risk to human health associated with cryptosporidiosis from urban dairying in Dagoretti, Nairobi, Kenya. Tropical Animal Health and Production, 44, 33–40. [DOI] [PubMed] [Google Scholar]
- Graczyk TK, Lewis EJ, Glass G, Dasilva AJ, Tamang L, Girouard AS and Curriero FC, 2007. Quantitative assessment of viable Cryptosporidium parvum load in commercial oysters (Crassostrea virginica) in the Chesapeake Bay. Parasitology Research, 100, 247. [DOI] [PubMed] [Google Scholar]
- Gualberto FA and Heller L, 2006. Endemic Cryptosporidium infection and drinking water source: a systematic review and meta‐analyses. Water Science and Technology, 54, 231–238. [DOI] [PubMed] [Google Scholar]
- Guo M, Buchanan RL, Dubey JP, Hill DE, Lambertini E, Ying Y, Gamble HR, Jones JL and Pradhan AK, 2015. Qualitative assessment for Toxoplasma gondii exposure risk associated with meat products in the United States. Journal of Food Protection, 78, 2207–2219. [DOI] [PubMed] [Google Scholar]
- Guy RA, Payment P, Krull UJ and Horgen PA, 2003. Real‐time PCR for quantification of Giardia and Cryptosporidium in environmental water samples and sewage. Applied and Environmental Microbiology, 69, 5178–5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagsma JA, De Noordhout CM, Polinder S, Vos T, Havelaar AH, Cassini A, Devleesschauwer B, Kretzschmar ME, Speybroeck N and Salomon JA, 2015. Assessing disability weights based on the responses of 30,660 people from four European countries. Population Health Metrics, 13, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald T, Aspinall W, Devleesschauwer B, Cooke R, Corrigan T, Havelaar AH, Gibb HJ, Torgerson PR, Kirk MD and Angulo FJ, 2016. World Health Organization estimates of the relative contributions of food to the burden of disease due to selected food‐borne hazards: a structured expert elicitation. PLoS ONE, 11, e0145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes D, Worobo R, Orlandi P, Burr D, Miliotis M, Robl M, Bier J, Arrowood M, Churey J and Jackson G, 2002. Inactivation of Cryptosporidium parvum oocysts in fresh apple cider by UV irradiation. Applied and Environmental Microbiology, 68, 4168–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harp JA, Fayer R, Pesch BA and Jackson GJ, 1996. Effect of pasteurization on infectivity of Cryptosporidium parvum oocysts in water and milk. Applied and Environmental Microbiology, 62, 2866–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelaar AH, Galindo AV, Kurowicka D and Cooke RM, 2008. Attribution of food‐borne pathogens using structured expert elicitation. Foodborne Pathogens and Disease, 5, 649–659. [DOI] [PubMed] [Google Scholar]
- Havelaar AH, Haagsma JA, Mangen MJJ, Kemmeren JM, Verhoef LP, Vijgen SM, Wilson M, Friesema IH, Kortbeek LM, van Duynhoven YT and van Pelt W, 2012. Disease burden of food‐borne pathogens in the Netherlands, 2009. International Journal of Food Microbiology, 156, 231–238. [DOI] [PubMed] [Google Scholar]
- Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, Praet N, Bellinger DC, De Silva NR, Gargouri N and Speybroeck N, 2015. World Health Organization global estimates and regional comparisons of the burden of food‐borne disease in 2010. PLoS Medicine, 12, e1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegglin D and Deplazes P, 2013. Control of Echinococcus multilocularis: strategies, feasibility and cost–benefit analyses. International Journal for Parasitology, 43, 327–337. [DOI] [PubMed] [Google Scholar]
- Hill D, Coss C, Dubey J, Wroblewski K, Sautter M, Hosten T, Muñoz‐Zanzi C, Mui E, Withers S and Boyer K, 2011. Identification of a sporozoite‐specific antigen from Toxoplasma gondii . The Journal of Parasitology, 97, 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S, Fischbeck P, Krupnick A and McWilliams MI, 2007. Using expert elicitation to link food‐borne illnesses in the United States to foods. Journal of Food Protection, 70, 1220–1229. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Devleesschauwer B, Aspinall W, Cooke R, Corrigan T, Havelaar A, Angulo F, Gibb H, Kirk M and Lake R, 2017. Attribution of global food‐borne disease to specific foods: findings from a World Health Organization structured expert elicitation. PLoS ONE, 12, e0183641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohweyer J, Cazeaux C, Travaillé E, Languet E, Dumètre A, Aubert D, Terryn C, Dubey JP, Azas N and Houssin M, 2016. Simultaneous detection of the protozoan parasites Toxoplasma, Cryptosporidium and Giardia in food matrices and their persistence on basil leaves. Food Microbiology, 57, 36–44. [DOI] [PubMed] [Google Scholar]
- Hotchkiss EJ, Gilray JA, Brennan ML, Christley RM, Morrison LJ, Jonsson NN, Innes EA and Katzer F, 2015. Development of a framework for genotyping bovine‐derived Cryptosporidium parvum, using a multilocus fragment typing tool. Parasites & Vectors, 8, 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter PR, Hughes LS, Woodhouse S, Syed Q, Verlander N, Chalmers RM and members of the project steering committee , 2004. Case‐control study of sporadic cryptosporidiosis with genotyping. Emerging Infectious Diseases, 10, 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter PR, de Sylor MA, Risebro HL, Nichols GL, Kay D and Hartemann P, 2011. Quantitative microbial risk assessment of Cryptosporidiosis and Giardiasis from very small private water supplies. Risk Analysis, 31, 228–236. [DOI] [PubMed] [Google Scholar]
- Iqbal A, Labib M, Muharemagic D, Sattar S, Dixon BR and Berezovski MV, 2015. Detection of Cryptosporidium parvum oocysts on fresh produce using DNA aptamers. PLoS ONE, 10, e0137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrzembski S, 2017. Genetische Diversität von Echinococcus multilocularis. WFA Medien Verlag. 200 pp. ISBN‐13: 978‐3946589129. [Google Scholar]
- Johnson AM, Di Giovanni GD and Rochelle PA, 2012. Comparison of assays for sensitive and reproducible detection of cell culture‐infectious Cryptosporidium parvum and Cryptosporidium hominis in drinking water. Applied and Environmental Microbiology, 78, 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J and Dubey J, 2010. Waterborne toxoplasmosis–recent developments. Experimental Parasitology, 124, 10–25. [DOI] [PubMed] [Google Scholar]
- Jones JL, Dargelas V, Roberts J, Press C, Remington JS and Montoya JG, 2009. Risk factors for Toxoplasma gondii infection in the United States. Clinical Infectious Diseases, 49, 878–884. [DOI] [PubMed] [Google Scholar]
- Jorgensen P, An der Heiden M, Kern P, Schöneberg I, Krause G and Alpers K, 2008. Underreporting of human alveolar echinococcosis, Germany. Emerging Infectious Diseases, 14, 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Jang H and Matthews KR, 2014. Vegetable microbial safety. Microbial Biotechnology, 7, 517–527. 10.1111/1751-7915.12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani H, Daryani A, Sharif M, Ahmadpour E, Alizadeh A, Nasrolahei M, Sarvi S, Kalani F and Faridnia R, 2016. Comparison of eight cell‐free media for maintenance of Toxoplasma gondii tachyzoites. Iranian Journal of Parasitology, 11, 104. [PMC free article] [PubMed] [Google Scholar]
- Kapel CM, Torgerson PR, Thompson RCA and Deplazes P, 2006. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. International Journal for Parasitology, 36, 79–86. [DOI] [PubMed] [Google Scholar]
- Karamon J, Stojecki K, Samorek‐Pieróg M, Bilska‐Zajac E, Rózycki M, Chmurzyńska E, Sroka J, Zdybel J and Cencek T, 2017. Genetic diversity of Echinococcus multilocularis in red foxes in Poland: the first report of a haplotype of probable Asian origin. Folia Parasitologica, 64, 007. [DOI] [PubMed] [Google Scholar]
- Katzer F, Canton G, Burrells A, Palarea‐Albaladejo J, Horton B, Bartley PM, Pang Y, Chianini F, Innes EA and Benavides J, 2014. Immunization of lambs with the S48 strain of Toxoplasma gondii reduces tissue cyst burden following oral challenge with a complete strain of the parasite. Veterinary Parasitology, 205, 46–56. [DOI] [PubMed] [Google Scholar]
- Kay, D , 2012. Effectiveness of best management practices for attenuating the transport of livestock‐derived pathogens within catchments In: Dufour A. and Bartram J. (eds.). Animal Waste Water Quality and Human Health. IWA Publishing, London: pp. 195–255. [Google Scholar]
- Kern P, Ammon A, Kron M, Sinn G, Sander S, Petersen LR, Gaus W and Kern P, 2004. Risk factors for alveolar echinococcosis in humans. Emerging Infectious Diseases, 10, 2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P, da Silva AM, Akhan O, Müllhaupt B, Vizcaychipi KA, Budke C and Vuitton DA, 2017. The echinococcoses: diagnosis, clinical management and burden of disease In Advances in parasitology (Vol. 96, pp. 259‐369). Academic Press. [DOI] [PubMed] [Google Scholar]
- King B and Monis P, 2007. Critical processes affecting Cryptosporidium oocyst survival in the environment. Parasitology, 134, 309–323. [DOI] [PubMed] [Google Scholar]
- King BJ, Hoefel D, Daminato DP, Fanok S and Monis PT, 2008. Solar UV reduces Cryptosporidium parvum oocyst infectivity in environmental waters. Journal of Applied Microbiology, 104, 1311–1323. [DOI] [PubMed] [Google Scholar]
- Kiper N, Gerçeker F, Utine E, Yalçın E, Pekcan S, Çobanoğlu N, Aslan A, Köse M, Doğru D, Özçelik U and Özgüç M, 2010. TAP1 and TAP2 gene polymorphisms in childhood cystic echinococcosis. Parasitology International, 59, 283–285. [DOI] [PubMed] [Google Scholar]
- Knapp J, Bart JM, Glowatzki ML, Ito A, Gerard S, Maillard S, Piarroux R and Gottstein B, 2007. Assessment of use of microsatellite polymorphism analysis for improving spatial distribution tracking of Echinococcus multilocularis . Journal of Clinical Microbiology, 45, 2943–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniel KE, Sumner SS, Lindsay DS, Hackney CR, Pierson MD, Zajac AM, Golden DA and Fayer R, 2003. Effect of organic acids and hydrogen peroxide on Cryptosporidium parvum viability in fruit juices. Journal of Food Protection, 66, 1650–1657. [DOI] [PubMed] [Google Scholar]
- Koethe M, Schade C, Fehlhaber K and Ludewig M, 2017. Survival of Toxoplasma gondii tachyzoites in simulated gastric fluid and cow's milk. Veterinary Parasitology, 233, 111–114. [DOI] [PubMed] [Google Scholar]
- Konyaev SV, Yanagida T, Nakao M, Ingovatova GM, Shoykhet YN, Bondarev AY, Odnokurtsev VA, Loskutova KS, Lukmanova GI, Dokuchaev NE and Spiridonov S, 2013. Genetic diversity of Echinococcus spp. Russia. Parasitology, 140, 1637–1647. [DOI] [PubMed] [Google Scholar]
- Korich D, Mead J, Madore M, Sinclair N and Sterling CR, 1990. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Applied and Environmental Microbiology, 56, 1423–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque ASG, Zaidi AKM, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins‐Browne RM and Levine MM, 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case‐control study. Lancet, 382, 209–222. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- Kotula AW, Dubey JP, Sharar AK, Andrews CD, Shen SK and Lindsay DS, 1991. Effect of freezing on infectivity of Toxoplasma gondii tissue cysts in pork. Journal of Food Protection, 54, 687–690. [DOI] [PubMed] [Google Scholar]
- Kreidl P, Allerberger F, Judmaier G, Auer H, Aspöck H and Hall AJ, 1998. Domestic pets as risk factors for alveolar hydatid disease in Austria. American Journal of Epidemiology, 147, 978–981. [DOI] [PubMed] [Google Scholar]
- Kyngdon CT, Gauci CG, Rolfe RA, Velásquez Guzmán JC, Farfán Salazar MJ, Verástegui Pimentel MR, Gonzalez AE, Garcia HH, Gilman RH, Strugnell RA and Lightowlers MW, 2006. In vitro oncosphere‐killing assays to determine immunity to the larvae of Taenia pisiformis, Taenia ovis, Taenia saginata, and Taenia solium . Journal of Parasitology, 92, 273–281. [DOI] [PubMed] [Google Scholar]
- Laberge I, Griffiths MW and Griffiths M, 1996. Prevalence, detection and control of Cryptosporidium parvum in food. International Journal of Food Microbiology, 32, 1–26. [DOI] [PubMed] [Google Scholar]
- Lalle M, Possenti A, Dubey JP and Pozio E, 2018. Loop‐mediated isothermal amplification‐lateral‐flow dipstick (LAMP‐LFD) to detect Toxoplasma gondii oocyst in ready‐to‐eat salad. Food Microbiology, 70, 137–142. [DOI] [PubMed] [Google Scholar]
- Lass A, Pietkiewicz H, Szostakowska B and Myjak P, 2012. The first detection of Toxoplasma gondii DNA in environmental fruits and vegetables samples. European Journal of Clinical Microbiology & Infectious Diseases, 31, 1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Szostakowska B, Myjak P and Korzeniewski K, 2015. The first detection of Echinococcus multilocularis DNA in environmental fruit, vegetable, and mushroom samples using nested PCR. Parasitology Research, 114, 4023–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws GF, 1968. Physical factors influencing survival of taeniid eggs. Experimental Parasitology, 22, 227–239. [DOI] [PubMed] [Google Scholar]
- Lawson JR and Gemmell MA, 1990. Transmission of taeniid tapeworm eggs via blowflies to intermediate hosts. Parasitology, 100, 143–146. [DOI] [PubMed] [Google Scholar]
- Li X, Guyot K, Dei‐Cas E, Mallard J‐P, Ballet JJ and Brasseur P, 2006. Cryptosporidium oocysts in mussels (Mytilus edulis) from Normandy (France). International Journal of Food Microbiology, 108, 321–325. [DOI] [PubMed] [Google Scholar]
- Lightowlers MW, Rickard MD, Honey RD, Obendorf DL and Mitchell GF, 1984. Serological diagnosis of Echinococcus granulosus infection in sheep using cyst fluid antigen processed by antibody affinity chromatography. Australian Veterinary Journal, 61, 101–108. [DOI] [PubMed] [Google Scholar]
- Lindsay DS and Dubey JP, 2009. Long‐term survival of Toxoplasma gondii sporulated oocysts in seawater. Journal of Parasitology, 95, 1019–1020. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Phelps KK, Smith SA, Flick G, Sumner SS and Dubey JP, 2001. Removal of Toxoplasma gondii oocysts from sea water by eastern oysters (Crassostrea virginica). Journal of Eukaryotic Microbiology., 1, 197s–198s. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Collins MV, Holliman D, Flick GJ and Dubey JP, 2006. Effects of high‐pressure processing on Toxoplasma gondii tissue cysts in ground pork. Journal of Parasitology, 92, 195–196. [DOI] [PubMed] [Google Scholar]
- Lundén A and Uggla A, 1992. Infectivity of Toxoplasma gondii in mutton following curing, smoking, freezing or microwave cooking. International Journal of Food Microbiology, 15, 357–363. [DOI] [PubMed] [Google Scholar]
- Luptakova L, Benova K, Rencko A and Petrovova E, 2015. DNA detection of Toxoplasma gondii in sheep milk and blood samples in relation to phase of infection. Veterinary Parasitology, 208, 250–253. [DOI] [PubMed] [Google Scholar]
- Lymbery AJ, 2017. Phylogenetic pattern, evolutionary processes and species delimitation in the genus Echinococcus In: Advances in parasitology (Vol. 95, pp. 111‐145). Academic Press. [DOI] [PubMed] [Google Scholar]
- Macarisin D, Santín M, Bauchan G and Fayer R, 2010a. Infectivity of Cryptosporidium parvum oocysts after storage of experimentally contaminated apples. Journal of Food Protection, 73, 1824–1829. [DOI] [PubMed] [Google Scholar]
- Macarisin D, Bauchan G and Fayer R, 2010b. Spinacia oleracea L. leaf stomata harboring Cryptosporidium parvum oocysts: a potential threat to food safety. Applied and Environmental Microbiology, 76, 555–559. 10.1128/AEM.02118-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae M, Hamilton C, Strachan NJ, Wright S and Ogden ID, 2005. The detection of Cryptosporidium parvum and Escherichia coli O157 in UK bivalve shellfish. Journal of Microbiological Methods, 60, 395–401. [DOI] [PubMed] [Google Scholar]
- Majowicz SE, Michel P, Aramini JJ, McEwen SA and Wilson JB, 2001. Descriptive analysis of endemic cryptosporidiosis cases reported in Ontario, 1996–1997. Canadian Journal of Public Health, 92, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancianti F, Nardoni S, D'Ascenzi C, Pedonese F, Mugnaini L, Franco F and Papini R, 2013. Seroprevalence, Detection of DNA in Blood and Milk, and Genotyping of Toxoplasma gondii in a Goat Population in Italy. BioMed Research International, 2013, 6 pp. 10.1155/2013/905326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangen MJ, Friesema IHM, Haagsma JA and Van Pelt W, 2017. Disease burden of food‐related pathogens in the Netherlands, 2016. [DOI] [PubMed]
- Marcinkut≐ A, Šarkūnas M, Moks E, Saarma U, Jokelainen P, Bagrade G, Laivacuma S, Strupas K, Sokolovas V and Deplazes P, 2015. Echinococcus infections in the Baltic region. Veterinary Parasitology, 213, 121–131. [DOI] [PubMed] [Google Scholar]
- Marquis ND, Record NR and Robledo JAF, 2015. Survey for protozoan parasites in Eastern oysters (Crassostrea virginica) from the Gulf of Maine using PCR‐based assays. Parasitology International, 64, 299–302. [DOI] [PubMed] [Google Scholar]
- Massie GN, Ware MW, Villegas EN and Black MW, 2010. Uptake and transmission of Toxoplasma gondii oocysts by migratory, filter‐feeding fish. Veterinary Parasitology, 169, 296–303. [DOI] [PubMed] [Google Scholar]
- McColgan C, Buxton D and Blewett DA, 1988. Titration of Toxoplasma gondii oocysts in non‐pregnant sheep and the effects of subsequent challenge during pregnancy. The Veterinary Record, 123, 467–470. [DOI] [PubMed] [Google Scholar]
- McEvoy JM, Moriarty EM, Duffy G, Sheridan JJ, Blair IS and McDowell DA, 2004. Effect of a commercial freeze/tempering process on the viability of Cryptosporidium parvum oocysts on lean and fat beef trimmings. Meat Science, 67, 559–564. 10.1016/j.meatsci.2003.12.007 [DOI] [PubMed] [Google Scholar]
- McEvoy JM, Duffy G, Moriarty EM, Lowery CJ, Sheridan JJ, Blair IS and McDowell DA, 2005. The prevalence and characterisation of Cryptosporidium spp. in beef abattoir water supplies. Water Research, 39, 3697–3703. [DOI] [PubMed] [Google Scholar]
- McKerr C, Adak GK, Nichols G, Gorton R, Chalmers RM, Kafatos G, Cosford P, Charlett A, Reacher M, Pollock KG and Alexander CL, 2015. An outbreak of Cryptosporidium parvum across England & Scotland associated with consumption of fresh pre‐cut salad leaves, May 2012. PLoS ONE, 10, e0125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM and Tauxe RV, 1999. Food‐related illness and death in the United States. Emerging Infectious Diseases, 5, 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner MJ and Berger P, 2016. Cryptosporidium infection risk: results of new dose‐response modeling. Risk Analysis, 36, 1969–1982. [DOI] [PubMed] [Google Scholar]
- Messner MJ, Chappell CL and Okhuysen PC, 2001. Risk assessment for Cryptosporidium: a hierarchical Bayesian analysis of human dose response data. Water Research, 35, 3934–3940. [DOI] [PubMed] [Google Scholar]
- Millard PS, Gensheimer KF, Addiss DG, Sosin DM, Beckett GA, Houck‐Jankoski A and Hudson A, 1994. An outbreak of cryptosporidiosis from fresh‐pressed apple cider. JAMA, 272, 1592–1596. [PubMed] [Google Scholar]
- Miller WA, Gardner IA, Atwill ER, Leutenegger CM, Miller MA, Hedrick RP, Melli AC, Barnes NM and Conrad PA, 2006. Evaluation of methods for improved detection of Cryptosporidium spp. in mussels (Mytilus californianus). Journal of Microbiological Methods, 65, 367–379. [DOI] [PubMed] [Google Scholar]
- Miller MA, Miller WA, Conrad PA, James ER, Melli AC, Leutenegger CM, Dabritz HA, Packham AE, Paradies D, Harris M and Ames J, 2008. Type X Toxoplasma gondii in a wild mussel and terrestrial carnivores from coastal California: new linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters. International Journal for Parasitology, 38, 1319–1328. [DOI] [PubMed] [Google Scholar]
- Minarovičová J, Kaclíková E, Krascsenicsová K, Siekel P and Kuchta T, 2009. A single‐tube nested real‐time polymerase chain reaction for sensitive contained detection of Cryptosporidium parvum . Letters in Applied Microbiology, 49, 568–572. [DOI] [PubMed] [Google Scholar]
- Minarovičová J, Kaclíková E and Kuchta T, 2010. A method for the detection of Cryptosporidium parvum in apple juice based on microfiltration and real‐time polymerase chain reaction. Journal of Food and Nutrition Research, 49, 160–164. [Google Scholar]
- Minozzo JC, Gusso RLF, Castro EAD, Lago O and Soccol VT, 2002. Experimental bovine infection with Taenia saginata eggs: recovery rates and cysticerci location. Brazilian Archives of Biology and Technology, 45, 451–455. [Google Scholar]
- Moazeni M and Rakhshandehroo E, 2012. In vitro viability test for the eggs of Echinococcus granulosus: a rapid method. Parasitology Research, 110, 925–930. [DOI] [PubMed] [Google Scholar]
- Moretta R, Sanchez VR, Fenoy IM, Goldman A, Ruybal P and Martin V, 2018. Genotyping of Toxoplasma gondii: The advantages of variable number tandem repeats within coding regions. Infection, Genetics and Evolution, 65, 226–230. [DOI] [PubMed] [Google Scholar]
- Moriarty EM, McEvoy JM, Lowery CJ, Thompson HP, Finn M, Sheridan JJ, Blair IS, McDowell DA and Duffy G, 2005a. Prevalence and characterisation of Cryptosporidium species in cattle faeces and on beef carcases at slaughter. Veterinary Record, 156, 165–168. [DOI] [PubMed] [Google Scholar]
- Moriarty EM, Duffy G, McEvoy JM, Caccio S, Sheridan JJ, McDowell D and Blair IS, 2005b. The effect of thermal treatments on the viability and infectivity of Cryptosporidium parvum on beef surfaces. Journal of Applied Microbiology, 98, 618–623. [DOI] [PubMed] [Google Scholar]
- Mossallam SF, 2010. Detection of some intestinal protozoa in commercial fresh juices. Journal of the Egyptian Society of Parasitology, 40, 135–149. [PubMed] [Google Scholar]
- Murphy H, Thomas M, Schmidt P, Medeiros D, McFadyen S and Pintar K, 2016. Estimating the burden of acute gastrointestinal illness due to Giardia, Cryptosporidium, Campylobacter, E. coli O157 and norovirus associated with private wells and small water systems in Canada. Epidemiology & Infection, 144, 1355–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Ziadinov I, Schweiger A, Schnyder M and Deplazes P, 2011. Hair coat contamination with zoonotic helminth eggs of farm and pet dogs and foxes. Berliner und Munchener Tierarztliche Wochenschrift, 124, 503–511. [PubMed] [Google Scholar]
- Nakao M, Lavikainen A, Yanagida T and Ito A, 2013. Phylogenetic systematics of the genus Echinococcus (Cestoda: Taeniidae). International Journal for Parasitology, 43, 1017–1029. [DOI] [PubMed] [Google Scholar]
- Nichols RAB, Paton CA and Smith HV, 2004. Survival of Cryptosporidium parvum oocysts after prolonged exposure to still natural mineral waters. Journal of Food Protection, 67, 517–523. [DOI] [PubMed] [Google Scholar]
- O'Donoghue PJ, 1995. Cryptosporidium and cryptosporidiosis in man and animals. International Journal for Parasitology, 25, 139–195. [DOI] [PubMed] [Google Scholar]
- Ok ÜZ, Özkol M, Kilimcioğlu AA, Dinç G, Bayındır P, Östan İ, Pabuşçu Y, Özcan C, Korkmaz M, Coşkun Ş and Yüksel H, 2007. A province‐based study using sampling method to investigate the prevalence of cystic echinococcosis among primary school children in Manisa, Turkey. Acta Tropica, 103, 116–122. [DOI] [PubMed] [Google Scholar]
- Opsteegh M, Langelaar M, Sprong H, Den Hartog L, De Craeye S, Bokken G, Ajzenberg D, Kijlstra A and Van Der Giessen J, 2010. Direct detection and genotyping of Toxoplasma gondii in meat samples using magnetic capture and PCR. International Journal of Food Microbiology, 139, 193–201. [DOI] [PubMed] [Google Scholar]
- Opsteegh M, Prickaerts S, Frankena K and Evers EG, 2011. A quantitative microbial risk assessment for meatborne Toxoplasma gondii infection in The Netherlands. International Journal of Food Microbiology, 150, 103–114. [DOI] [PubMed] [Google Scholar]
- Opsteegh M, Kortbeek TM, Havelaar AH and van der Giessen JW, 2014. Intervention strategies to reduce human Toxoplasma gondii disease burden. Clinical Infectious Diseases, 60, 101–107. [DOI] [PubMed] [Google Scholar]
- Opsteegh M, Kortbeek TM, Havelaar AH and van der Giessen JW, 2015. Intervention strategies to reduce human Toxoplasma gondii disease Burden. Clinical Infectious Diseases, 60, 101–107. 10.1093/cid/ciu721 [DOI] [PubMed] [Google Scholar]
- Opsteegh M, Maas M, Schares G and Giessen J, 2016a. Relationship between seroprevalence in the main livestock species and presence of Toxoplasma gondii in meat (GP/EFSA/BIOHAZ/2013/01) An extensive literature review, Final report. EFSA Supporting Publications, 13, 294. [Google Scholar]
- Opsteegh M, Schares G, Blaga R and Giessen J, 2016b. Experimental studies of Toxoplasma gondii in the main livestock species (GP/EFSA/BIOHAZ/2013/01) Final report. EFSA Supporting Publications, 13, 161. [Google Scholar]
- Painter JA, Ayers T, Woodruff R, Blanton E, Perez N, Hoekstra RM, Griffin PM and Braden C, 2009. Recipes for food‐borne outbreaks: a scheme for categorizing and grouping implicated foods. Foodborne Pathogens and Disease, 6, 1259–1264. [DOI] [PubMed] [Google Scholar]
- Painter JA, Hoekstra RM, Ayers T, Tauxe RV, Braden CR, Angulo FJ and Griffin PM, 2013. Attribution of food‐borne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerging Infectious Diseases, 19, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Murphy T and Holden N, 2008. Evaluation of the effect of temperature on the die‐off rate for Cryptosporidium parvum oocysts in water, soils, and feces. Applied and Environmental Microbiology, 74, 7101–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E, Vesco G, Villari S and Buffolano W, 2010. What do we know about risk factors for infection in humans with Toxoplasma gondii and how can we prevent infections? Zoonoses and Public Health, 57, 8–17. 10.1111/j.1863-2378.2009.01278.x [DOI] [PubMed] [Google Scholar]
- Piarroux M, Piarroux R, Giorgi R, Knapp J, Bardonnet K, Sudre B, Watelet J, Dumortier J, Gérard A, Beytout J and Abergel A, 2011. Clinical features and evolution of alveolar echinococcosis in France from 1982 to 2007: results of a survey in 387 patients. Journal of Hepatology, 55, 1025–1033. [DOI] [PubMed] [Google Scholar]
- Piarroux M, Piarroux R, Knapp J, Bardonnet K, Dumortier J, Watelet J, Gerard A, Beytout J, Abergel A, Bresson‐Hadni S and Gaudart J, 2013. Populations at risk for alveolar echinococcosis, France. Emerging Infectious Diseases, 19, 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock G, Richards CD and Richards DA, 2013. Human physiology, 4th Edition Oxford University Press, Oxford: 944 pp. [Google Scholar]
- Pollock KGJ, Ternent HE, Mellor DJ, Chalmers RM, Smith HV, Ramsay CN and Innocent GT, 2010. Spatial and temporal epidemiology of sporadic human cryptosporidiosis in Scotland. Zoonoses and Public Health, 57, 487–492. [DOI] [PubMed] [Google Scholar]
- Pomares C, Ajzenberg D, Bornard L, Bernardin G, Hasseine L, Dardé M‐L and Marty P, 2011. Toxoplasmosis and horse meat, France. Emerging Infectious Diseases, 17, 1327–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possenti A, Manzano‐Román R, Sánchez‐Ovejero C, Boufana B, La Torre G, Siles‐Lucas M and Casulli A, 2016. Potential risk factors associated with human cystic echinococcosis: systematic review and meta‐analysis. PLoS Neglected Tropical Diseases, 10, e0005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott S, Koethe M, Bangoura B, Zöller B, Daugschies A, Straubinger RK, Fehlhaber K and Ludewig M, 2013. Effects of pH, sodium chloride, and curing salt on the infectivity of Toxoplasma gondii tissue cysts. Journal of Food Protection, 76, 1056–1061. [DOI] [PubMed] [Google Scholar]
- Public Health England , 2017. National increase of Cryptosporidium parvum, 2015. Final outbreak investigation report. Public Health England, London. [Google Scholar]
- Putignani L, Mancinelli L, Del Chierico F, Menichella D, Adlerstein D, Angelici MC, Marangi M, Berrilli F, Caffara M, di Regalbono DF and Giangaspero A, 2011. Investigation of Toxoplasma gondii presence in farmed shellfish by nested‐PCR and real‐time PCR fluorescent amplicon generation assay (FLAG). Experimental Parasitology, 127, 409–417. [DOI] [PubMed] [Google Scholar]
- Ravel A, Davidson VJ, Ruzante JM and Fazil A, 2010. Foodborne proportion of gastrointestinal illness: estimates from a Canadian expert elicitation survey. Foodborne Pathogens and Disease, 7, 1463–1472. [DOI] [PubMed] [Google Scholar]
- Ripabelli G, Leone A, Sammarco ML, Fanelli I, Grasso GM and McLauchlin J, 2004. Detection of Cryptosporidium parvum oocysts in experimentally contaminated lettuce using filtration, immunomagnetic separation, light microscopy, and PCR. Foodborne Pathogens and Disease, 1, 216–222. [DOI] [PubMed] [Google Scholar]
- Robert‐Gangneux F and Dardé ML, 2012. Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical Microbiology Reviews, 2, 264–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L, 2007. The potential for marine bivalve shellfish to act as transmission vehicles for outbreaks of protozoan infections in humans: a review. International Journal of Food Microbiology, 120, 201–216. [DOI] [PubMed] [Google Scholar]
- Robertson LJ and Chalmers RM, 2013. Foodborne cryptosporidiosis: is there really more in Nordic countries? Trends in Parasitology, 29, 3–9. 10.1016/j.pt.2012.10.003 [DOI] [PubMed] [Google Scholar]
- Robertson LJ and Gjerde B, 2001. Occurrence of parasites on fruits and vegetables in Norway. Journal of Food Protection, 64, 1793–1798. [DOI] [PubMed] [Google Scholar]
- Robertson L and Gjerde B, 2008. Development and use of a pepsin digestion method for analysis of shellfish for Cryptosporidium oocysts and Giardia cysts. Journal of Food Protection, 71, 959–966. [DOI] [PubMed] [Google Scholar]
- Robertson LJ and Huang Q, 2012. Analysis of cured meat products for Cryptosporidium oocysts following possible contamination during an extensive waterborne outbreak of cryptosporidiosis. Journal of Food Protection, 75, 982–988. [DOI] [PubMed] [Google Scholar]
- Robertson B, Sinclair MI, Forbes AB, Veitch M, Kirk M, Cunliffe D, Willis J and Fairley CK, 2002. Case‐control studies of sporadic cryptosporidiosis in Melbourne and Adelaide, Australia. Epidemiology and Infection, 128, 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LJ, Troell K, Woolsey ID and Kapel CM, 2016. Fresh fruit, vegetables, and mushrooms as transmission vehicles for Echinococcus multilocularis in Europe: inferences and concerns from sample analysis data from Poland. Parasitology Research, 115, 2485–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochelle PA and Di Giovanni GD, 2014. Cryptosporidium oocysts in drinking water and recreational water In: Cryptosporidium: parasite and disease (pp. 489‐513). Springer, Vienna. [Google Scholar]
- Romig T, Kratzer W, Kimmig P, Frosch M, Gaus WI, Flegel WA, Gottstein B, Lucius R, Beckh K and Kern P, 1999. An epidemiologic survey of human alveolar echinococcosis in southwestern Germany. Römerstein Study Group. The American Journal of Tropical Medicine and Hygiene, 61, 566–573. [DOI] [PubMed] [Google Scholar]
- Romig T, Deplazes P, Jenkins D, Giraudoux P, Massolo A, Craig PS, Wassermann M, Takahashi K and De La Rue M, 2017. Ecology and life cycle patterns of Echinococcus species In: Advances in parasitology (Vol. 95, pp. 213–314). Academic Press. [DOI] [PubMed] [Google Scholar]
- Romig T, Ebi D and Wassermann M, 2015. Taxonomy and molecular epidemiology of Echinococcus granulosus sensu lato. Veterinary Parasitology, 213, 76–84. [DOI] [PubMed] [Google Scholar]
- Rossi P, Tamarozzi F, Galati F, Pozio E, Akhan O, Cretu CM, Vutova K, Siles‐Lucas M, Brunetti E and Casulli A, 2016. The first meeting of the European Register of Cystic Echinococcosis (ERCE). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau A, La Carbona S, Dumètre A, Robertson LJ, Gargala G, Escotte‐Binet S, Favennec L, Villena I, Gérard C and Aubert D, 2018. Assessing viability and infectivity of food‐borne and waterborne stages (cysts/oocysts) of Giardia duodenalis, Cryptosporidium spp., and Toxoplasma gondii: a review of methods. Parasite, 25, 14. 10.1051/parasite/2018009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SL, DeLong SM, Stenzel SA, Shiferaw B, Roberts JM, Khalakdina A, Marcus R, Segler SD, Shah DD, Thomas S, Vugia DJ, Zansky SM, Dietz V and Beach MJ; Emerging Infections Program FoodNet Working Group , 2004. Risk factors for sporadic cryptosporidiosis among immunocompetent persons in the United States from 1999 to 2001. Journal of Clinical Microbiology, 42, 2944–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U, Fayer R and Xiao L, 2014. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology, 141, 1667–1685. [DOI] [PubMed] [Google Scholar]
- Ryan U, Hijjawi N and Xiao L, 2018. Foodborne cryptosporidiosis. International Journal for Parasitology, 48, 1–12. [DOI] [PubMed] [Google Scholar]
- Rzeżutka A, Nichols R, Connelly L, Kaupke A, Kozyra I, Cook N, Birrell S and Smith H, 2010. Cryptosporidium oocysts on fresh produce from areas of high livestock production in Poland. International Journal of Food Microbiology, 139, 96–101. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Roberto RR and Brooks NF, 1982. Toxoplasmosis infection associated with raw goat's milk. JAMA, 248, 1728–1732. [DOI] [PubMed] [Google Scholar]
- Said B, Halsby KD, O'connor CM, Francis J, Hewitt K, Verlander NQ, Guy E and Morgan D, 2017. Risk factors for acute toxoplasmosis in England and Wales. Epidemiology & Infection, 145, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Thevenet P, Jensen O, Drut R, Cerrone GE, Grenóvero MS, Alvarez HM, Targovnik HM and Basualdo JA, 2005. Viability and infectiousness of eggs of Echinococcus granulosus aged under natural conditions of inferior arid climate. Veterinary Parasitology, 133, 71–77. [DOI] [PubMed] [Google Scholar]
- de Santana Rocha D, de Sousa Moura R, Maciel B, Guimarães L, O'dwyer H, Munhoz A and Albuquerque G, 2015. Detection of Toxoplasma gondii DNA in naturally infected sheep's milk. Genetics and Molecular Research, 14, 8658–8662. [DOI] [PubMed] [Google Scholar]
- Santín M, 2013. Clinical and subclinical infections with Cryptosporidium in animals. New Zealand Veterinary Journal, 61, 1–10. [DOI] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL and Griffin PM, 2011. Foodborne illness acquired in the United States—major pathogens. Emerging Infectious Diseases, 17, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schares G, Bangoura B, Randau F, Goroll T, Ludewig M, Maksimov P, Matzkeit B, Sens M, Bärwald A, Conraths FJ and Opsteegh M, 2017. High seroprevalence of Toxoplasma gondii and probability of detecting tissue cysts in backyard laying hens compared with hens from large free‐range farms. International Journal for Parasitology, 47, 765–777. [DOI] [PubMed] [Google Scholar]
- Schets FM, van den Berg HH, Engels GB, Lodder WJ and de Roda Husman AM, 2007. Cryptosporidium and Giardia in commercial and non‐commercial oysters (Crassostrea gigas) and water from the Oosterschelde, The Netherlands. International Journal of Food Microbiology, 113, 189–194. [DOI] [PubMed] [Google Scholar]
- Schiller EL, 1955. Studies on the helminth fauna of Alaska. XXVI. Some observations on the cold‐resistance of eggs of Echinococcus sibiricensis Rausch and Schiller, 1954. The Journal of Parasitology, 41, 578–582. [PubMed] [Google Scholar]
- Schmidberger J, Weimer H, Schlingeloff P, Kratzer W and Grüner B and Echinococcosis Working Group , 2018. Health‐related quality of life in patients with alveolar echinococcosis: a cross‐sectional study. Infection, 1–9. [DOI] [PubMed] [Google Scholar]
- Schneider R, Aspöck H and Auer H, 2013. Unexpected increase of alveolar echincoccosis, Austria, 2011. Emerging Infectious Diseases, 19, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger A, Ammann RW, Candinas D, Clavien PA, Eckert J, Gottstein B, Halkic N, Muellhaupt B, Prinz BM, Reichen J and Tarr PE, 2007. Human alveolar echinococcosis after fox population increase, Switzerland. Emerging Infectious Diseases, 13, 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Haramoto E and Shindo J, 2017. Assessing the infection risk of enteropathogens from consumption of raw vegetables washed with contaminated water in Kathmandu Valley, Nepal. Journal of Applied Microbiology, 123, 1321–1334. [DOI] [PubMed] [Google Scholar]
- da Silva JG, Alves BHL, Melo RPB, Kim PCP, Neto OLS, Bezerra MJG, Sá SG and Mota RA, 2015. Occurrence of anti‐Toxoplasma gondii antibodies and parasite DNA in raw milk of sheep and goats of local breeds reared in Northeastern Brazil. Acta Tropica, 142, 145–148. [DOI] [PubMed] [Google Scholar]
- Silveira C, Muccioli C, Holland GN, Jones JL, Yu F, de Paulo A and Belfort R Jr, 2015. Ocular involvement following an epidemic of Toxoplasma gondii infection in Santa Isabel do Ivaí, Brazil. American Journal of Ophthalmology, 159, 1013–1021.e3. 10.1016/j.ajo.2015.02.017 [DOI] [PubMed] [Google Scholar]
- Silverman PH, 1954. Studies on the biology of some tapeworms of the Genus Taenia: I.—Factors Affecting Hatching and Activation of Taeniid Ova, and Some Criteria of their Viability. Annals of Tropical Medicine & Parasitology, 48, 207–215. [PubMed] [Google Scholar]
- Slifko TR, Raghubeer E and Rose JB, 2000. Effect of high hydrostatic pressure on Cryptosporidium parvum infectivity. Journal of Food Protection, 63, 1262–1267. [DOI] [PubMed] [Google Scholar]
- Sotiraki S, Himonas C and Korkoliakou P, 2003. Hydatidosis–echinococcosis in Greece. Acta Tropica, 85, 197–201. [DOI] [PubMed] [Google Scholar]
- de Souza CZ, Rafael K, Sanders AP, Tiyo BT, Marchioro AA, Colli CM, Gomes ML and Falavigna‐Guilherme AL, 2016. An alternative method to recover Toxoplasma gondii from greenery and fruits. International Journal of Environmental Health Research, 26, 600–605. [DOI] [PubMed] [Google Scholar]
- Sroka J, Kusyk P, Bilska‐Zając E, Karamon J, Dutkiewicz J, Wójcik‐Fatla A, Zając V, Stojecki K, Różycki M and Cencek T, 2017. Seroprevalence of Toxoplasma gondii infection in goats from the south‐west region of Poland and the detection of T. gondii DNA in goat milk. Folia Parasitologica, 64. [DOI] [PubMed] [Google Scholar]
- Stagno S, Dykes AC, Amos CS, Head RA, Juranek DD and Walls K, 1980. An outbreak of toxoplasmosis linked to cats. Pediatrics, 65, 706–712. [PubMed] [Google Scholar]
- Su C, Khan A, Zhou P, Majumdar D, Ajzenberg D, Dardé M‐L, Zhu X‐Q, Ajioka JW, Rosenthal BM and Dubey JP, 2012. Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proceedings of the National Academy of Sciences, 109, 5844–5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppes LM, Canales RA, Gerba CP and Reynolds KA, 2016. Cryptosporidium risk from swimming pool exposures. International Journal of Hygiene and Environmental Health, 219, 915–919. [DOI] [PubMed] [Google Scholar]
- Sutthikornchai C, Popruk S, Chumpolbanchorn K, Sukhumavasi W and Sukthana Y, 2016. Oyster is an effective transmission vehicle for Cryptosporidium infection in human. Asian Pacific Journal of Tropical Medicine, 9, 562–566. [DOI] [PubMed] [Google Scholar]
- Sweatman GK and Williams RJ, 1963. Comparative studies on the biology and morphology of Echinococcus granulosus from domestic livestock, moose and reindeer. Parasitology, 53, 339–390. [DOI] [PubMed] [Google Scholar]
- Szostakowska B, Lass A, Kostyra K, Pietkiewicz H and Myjak P, 2014. First finding of Echinococcus multilocularis DNA in soil: preliminary survey in Varmia‐Masuria Province, northeast Poland. Veterinary Parasitology, 203, 73–79. [DOI] [PubMed] [Google Scholar]
- Tamarozzi F, Akhan O, Cretu CM, Vutova K, Akinci D, Chipeva R, Ciftci T, Constantin CM, Fabiani M, Golemanov B and Janta D, 2018. Prevalence of abdominal cystic echinococcosis in rural Bulgaria, Romania, and Turkey: a cross‐sectional, ultrasound‐based, population study from the HERACLES project. The Lancet Infectious Diseases, 18, 769–778. [DOI] [PubMed] [Google Scholar]
- Tamburrini A and Pozio E, 1999. Long‐term survival of Cryptosporidium parvum oocysts in seawater and in experimentally infected mussels (Mytilus galloprovincialis). International Journal for Parasitology, 29, 711–715. [DOI] [PubMed] [Google Scholar]
- Tenter AM, Heckeroth AR and Weiss LM, 2000. Toxoplasma gondii: from animals to humans. International Journal for Parasitology, 30, 1217–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teutsch SM, Juranek DD, Sulzer A, Dubey J and Sikes RK, 1979. Epidemic toxoplasmosis associated with infected cats. New England Journal of Medicine, 300, 695–699. [DOI] [PubMed] [Google Scholar]
- Thompson RCA, Deplazes P, Lymbery AJ, Editors, 2017. Echinococcus and Echinococcosis, Part A. Volume 95, Advances in Parasitology 95. Academic Press. 525 pages, ISBN 978‐0‐12‐8114711 (hardcover); 9780128114728 (eBook). [Google Scholar]
- Thomson S, Hamilton CA, Hope JC, Katzer F, Mabbott NA, Morrison LJ and Innes EA, 2017. Bovine cryptosporidiosis: impact, host‐parasite interaction and control strategies. Veterinary Research, 48, 42 10.1186/s13567-017-0447-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timur M, Suvonkulov U, Zafar S, Olesya A, Kim H‐J, Yong T, Lee G‐J, Park G‐M, Shin M‐H and Yu HS, 2018. Genotypic and phylogenetic characteristics of Echinococcus granulosus sensu lato in Uzbekstan. Proceedings of the 14th International Congress of Parasitology, Daegu, Korea. Available online: https://guidebook.com/guide/135457/poi/10545427/?pcat=741072
- Torgerson PR, 2014. Helminth‐Cestode: Echinococcus granulosus and Echinococcus mutilocularis. Encyclopedia of food safety. AcademicPress, Waltham: pp. 63–69. [Google Scholar]
- Torgerson PR and Mastroiacovo P, 2013. The global burden of congenital toxoplasmosis: a systematic review. Bulletin of the World Health Organization, 91, 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson PR, Schweiger A, Deplazes P, Pohar M, Reichen J, Ammann RW, Tarr PE, Halkik N and Müllhaupt B, 2008. Alveolar echinococcosis: from a deadly disease to a well‐controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. Journal of Hepatology, 49, 72–77. [DOI] [PubMed] [Google Scholar]
- Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, Rokni MB, Zhou XN, Fèvre EM, Sripa B and Gargouri N, 2015. World Health Organization estimates of the global and regional disease burden of 11 food‐borne parasitic diseases, 2010: a data synthesis. PLoS Medicine, 12, e1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel D, Deplazes P and Mathis A, 2007. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology, 134, 911–920. [DOI] [PubMed] [Google Scholar]
- US EPA (United States Environmental Protection Agency), 2012. Method 1623.1: Cryptosporidium and Giardia in Water by Filtration/IMS/FA. Cincinnati, OH: 83 pp. [Google Scholar]
- US FDA (United States Food and Drug Administration), 2004. Bacteriological Analytical Manual. Chapter 19a: detection of Cyclospora and Cryptosporidium from Fresh Produce: Isolation and Identification by Polymerase Chain Reaction (PCR) and Microscopic analysis. Silver Spring, MD. Available online: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm073638.htm
- Utaaker KS, Huang Q and Robertson LJ, 2015. A reduced‐cost approach for analyzing fresh produce for contamination with Cryptosporidium oocysts and/or Giardia cysts. Food Research International, 77, 326–332. [Google Scholar]
- Utaaker KS, Skjerve E and Robertson LJ, 2017. Keeping it cool: survival of Giardia cysts and Cryptosporidium oocysts on lettuce leaves. International Journal of Food Microbiology, 255, 51–57. [DOI] [PubMed] [Google Scholar]
- Vaudaux JD, Muccioli C, James ER, Silveira C, Magargal SL, Jung C, Dubey JP, Jones JL, Doymaz MZ, Bruckner DA, Belfort R Jr, Holland GN and Grigg ME, 2010. Identification of an atypical strain of Toxoplasma gondii as the cause of a waterborne outbreak of toxoplasmosis in Santa Isabel do Ivai, Brazil. Journal of Infectious Diseases, 202, 1226–1233. 10.1086/656397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit P, Bilger B, Schad V, Schäfer J, Frank W and Lucius R, 1995. Influence of environmental factors on the infectivity of Echinococcus multilocularis eggs. Parasitology, 110, 79–86. [DOI] [PubMed] [Google Scholar]
- Verma S, Ajzenberg D, Rivera‐sanchez A, Su C and Dubey J, 2015. Genetic characterization of Toxoplasma gondii isolates from Portugal, Austria and Israel reveals higher genetic variability within the type II lineage. Parasitology, 142, 948–957. [DOI] [PubMed] [Google Scholar]
- Vismarra A, Barilli E, Miceli M, Mangia C, Bacci C, Brindani F and Kramer L, 2017. Toxoplasma gondii and pre‐treatment protocols for polymerase chain reaction analysis of milk samples: a field trial in sheep from Southern Italy. Italian Journal of Food Safety, 6, 6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CP, Hammond SE, Zajac AM and Lindsay DS, 1999. Survival of Toxoplasma gondii tachyzoites in goat milk: potential source of human toxoplasmosis. The Journal of Eukaryotic Microbiology, 46, 73S–74S. [PubMed] [Google Scholar]
- Wang IC, Ma YX, Kuo CH and Fan PC, 1997. A comparative study on egg hatching methods and oncosphere viability determination for Taenia solium eggs. International Journal for Parasitology, 27, 1311–1314. [DOI] [PubMed] [Google Scholar]
- Weiss LM and Dubey JP, 2009. Toxoplasmosis: a history of clinical observations. International Journal for Parasitology, 39, 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HD, Bates MJ and Wilson P, 1992. For Information Specialists: Interpretations of Reference and Bibliographic Work. Ablex Publishing Corporation, Norwood, NJ: 320 pp. [Google Scholar]
- WHO (World Health Organization), 2009. Risk assessment of Cryptosporidium in drinking water. World Health Organization, Geneva: 143 pp. [Google Scholar]
- Williams JF and Colli CW, 1970. Primary cystic infection with Echinococcus granulosus and Taenia hydatigena in Meriones unguiculatus . The Journal of Parasitology, 56, 509–513. [PubMed] [Google Scholar]
- Xiao L, 2010. Molecular epidemiology of cryptosporidiosis: an update. Experimental Parasitology, 124, 80–89. [DOI] [PubMed] [Google Scholar]
- Yolken R, Dickerson F and Fuller Torrey E, 2009. Toxoplasma and schizophrenia. Parasite Immunology, 31, 706–715. [DOI] [PubMed] [Google Scholar]


