Abstract
The Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to deliver a scientific opinion on the safety of hydroxyanthracene derivatives and to provide advice on a daily intake that does not give rise to concerns about harmful effects to health. Hydroxyanthracene derivatives are a class of chemical substances naturally occurring in different botanical species and used in food to improve bowel function. The ANS Panel reviewed the available scientific data on a possible relationship between hydroxyanthracene derivatives exposure and genotoxic and carcinogenic effects. On the basis of the data currently available, the Panel noted that emodin, aloe‐emodin and the structurally related substance danthron have shown evidence of in vitro genotoxicity. Aloe extracts have also been shown to be genotoxic in vitro possibly due to the presence of hydroxyanthracene derivatives in the extract. Furthermore, aloe‐emodin was shown to be genotoxic in vivo and the whole‐leaf aloe extract and the structural analogue danthron were shown to be carcinogenic. Epidemiological data suggested an increased risk for colorectal cancer associated with the general use of laxatives, several of which contain hydroxyanthracene derivatives. Considering the possible presence of aloe‐emodin and emodin in extracts, the Panel concluded that hydroxyanthracene derivatives should be considered as genotoxic and carcinogenic unless there are specific data to the contrary, such as for rhein, and that there is a safety concern for extracts containing hydroxyanthracene derivatives although uncertainty persists. The Panel was unable to provide advice on a daily intake of hydroxyanthracene derivatives that does not give rise to concerns about harmful effects to health.
Keywords: hydroxyanthracene derivatives, food supplements, genotoxicity, carcinogenicity, bowel function, colorectal cancer, laxatives
Summary
Following a request from the European Commission, the European Food Safety Authority (EFSA) was asked to deliver a scientific opinion on the evaluation of hydroxyanthracene derivatives in accordance with Article 8 (2) of Regulation (EC) No 1925/2006 on the addition of vitamins and minerals and of certain other substances to foods. This request was triggered by the concerns raised by one of the European Union (EU) Member States on the possible harmful effects associated with the consumption of foods containing hydroxyanthracene derivatives and preparations thereof, for example, in food supplements.
In particular, EFSA was requested to review the existing scientific data on the possible link between the intake of hydroxyanthracene derivatives and harmful effects on health, and to provide advice on a daily intake of hydroxyanthracene derivatives that does not give rise to concerns about harmful effects to health, for the general population and for subgroups of the population (including vulnerable groups).
The risk assessment was performed by the EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS Panel) in accordance with the 2009 EFSA Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements.
The ANS Panel considered that the specific issue of concern – the relationship between hydroxyanthracene derivative exposures and colorectal cancer – should be addressed in this opinion.
Plants containing hydroxyanthracene derivatives are numerous and belong to different botanical families and genera. The hydroxyanthracene derivatives considered relevant for this risk assessment were those found in the root and rhizome of Rheum palmatum L. and/or Rheum officinale Baillon and/or their hybrids; leaves or fruits of Cassia senna L. and/or Cassia angustifolia Vahl; bark of Rhamnus frangula L., bark of Rhamnus purshianus D.C. and in leaves of Aloe barbadensis Miller and/or various Aloe species, mainly Aloe ferox Miller and its hybrids and further, that are also indicated as present in the respective EU Pharmacopeia monographs as characteristic active components of these botanicals. Other structurally related compounds have been assessed by the US National Toxicology Program (NTP) (anthraquinone, emodin) and have only been included for the purpose of potential read across.
Preparations containing hydroxyanthracene derivatives have been already evaluated by a number of Committees or international organisations for their potential beneficial effects and/or for their potential adverse effects as food or as medicinal products.
A public call for data was launched by EFSA to gather information from the relevant food business operators, on several specific topics related to food supplements containing hydroxyanthracene derivatives.
Exposure to hydroxyanthracene derivatives from food supplements has been estimated from the recommended daily doses of food supplements as provided by the interested parties and range from 2.25 to 24.83 mg/day for sennoside B, from 1.95 to 78.8 mg/day for rhein, from 13 to 26 mg/day for glucofrangulin A, from 1.2 to 24 mg/day for aloin (barbaloin) and from 17.9 to 51 mg/day for aloin A+B. However, the Panel noted that exposure to aloe‐emodin and emodin is not known due to lack of data on these substances.
In this opinion, data from human and animal studies have been assessed.
Several in vivo studies describing the biological fate of hydroxyanthracene derivatives and the metabolism and pharmacokinetics of anthranoid laxatives are reported in literature. Glycosidic hydroxyanthracene derivatives have been demonstrated to remain intact until they are hydrolysed in the gastrointestinal (GI) tract to their corresponding anthrones (aglycone anthrones). Regarding aglycone hydroxyanthracenes, they may be absorbed intact, however, only rhein is present in the systemic circulation. In the GI tract, remaining hydroxyanthracenes may be reduced back to the corresponding anthrones by the microbiota. After absorption, hydroxyanthracenes such as aloe‐emodin are rapidly and totally oxidised to rhein. In the gut epithelium and liver, absorbed aglycone hydroxyanthracenes are conjugated into corresponding glucuronides and sulfates, which are excreted in bile or urine.
The genotoxicity of hydroxyanthracene derivatives has been evaluated in numerous in vitro and in vivo studies identified from the public literature. The available studies focused mainly on emodin, aloe‐emodin, rhein and sennosides and to lesser extent on aloe vera, senna and fructus sennae extracts. A limited number of studies on danthron and chrysophanol were also available.
Among the extracts, only senna extracts proved to be mutagenic in the TA98 Salmonella Typhimurium tester strain in the presence of S9 metabolism and in S. Typhimurium tester strains TA1537 and TA98 in the absence of S9 metabolism. It also induced DNA damage in the Bacillus subtilis rec‐assay and induced dose‐related increases in chromosomal aberrations in Chinese hamster ovary (CHO) cells in the absence of S9.
No positive results in the bacterial mutation assay for aloe vera gel and for both aloe vera whole leaf extract (WLE) and aloe vera charcoal‐filtered whole leaf extract (decolourised) were observed in S. Typhimurium strains TA98 and TA100, and Escherichia coli strain WP2 uvrA/pKM101, with and without S9 metabolic activation. The 1,8‐dihydroxyanthraquinones emodin, aloe‐emodin and danthron were shown to be mutagenic in the Tk+/− locus in mouse lymphoma L5178Y cells and consistently clastogenic in vitro in mammalian cells interacting via intercalation into DNA with DNA topoisomerase II and consequent inhibition of their catalytic function resulting in genotoxicity and mutagenicity with a ranking potency greater for danthron and lower for emodin. Results of a ‘modified comet assay’ showed that pretreatments of the cells with each of these test compounds reduced the DNA damage induced by etoposide, an inhibitor of topoisomerase II which acts through the stabilisation of the DNA–topoisomerase complex known as the ‘cleavable complex’ in which danthron, aloe‐emodin and emodin showed capability to inhibit the non‐covalent binding of bisbenzimide Hoechst 33342 to isolated DNA and to DNA of intact mouse lymphoma L5178Y cells. On the other hand, emodin, the least potent compound among these three anthraquinones, was also shown to induce DNA double‐strand breaks (DSB) through poisoning of topoisomerase II‐cleavable complex and through inhibiting adenosine triphosphate (ATP) hydrolysis by DNA topoisomerase II. Thus, it is not possible to exclude also a potential mechanism of inhibition of DNA topoisomerase II through poisoning of the DNA–topoisomerase ‘cleavable complex’. Aloe‐emodin was also mutagenic in S. Typhimurium strains TA1537, TA1538, TA97 and TA98 (all frameshift mutant sites) in the absence of S9 metabolism. While these results further support the intercalating capability of aloe‐emodin and consequent catalytic inhibition of DNA topoisomerase II they also show the induction of frameshift mutations which, per se, is a mutagenic event not related to the inhibition of DNA topoisomerase II.
The Panel noted that, beyond frameshift mutations, danthron also induced base‐pair substitutions in bacteria, only after exogenous metabolic activation, and oxidative DNA damage in mammalian cells, highlighting the involvement of multiple mechanisms of genotoxicity.
In addition, aloe‐emodin induced unscheduled DNA synthesis (UDS) in primary rat hepatocytes, and DNA breakage in mouse lymphoma L5178Y cells, in NPC‐039 and NPC‐076 human nasopharyngeal carcinoma cells, and SCC‐4 human tongue cancer cells. These results are also compatible with a mechanism of inhibition of DNA topoisomerase II activities.
However, although individual anthranoids induced gene mutations (frameshift and base‐pair substitutions) in bacteria, frameshift mutations and clastogenic effects in mammalian cells through inhibition of DNA topoisomerase II, for plant extracts containing hydroxyanthracene derivatives, other mutagenic components with different mechanisms of action appear to play a role in extract genotoxicity and carcinogenicity. It has been demonstrated that both aloe vera WLE and aloe vera decolourised whole leaf extract (WLD) – for which the content of hydroxyanthracene derivatives (both glycosidic and free aglycones) was reduced by 99% compared to the WLE – induced statistically significant and biologically relevant increases in mutation frequencies (MF) in the mammalian cell TK+/− mutation assay using the mouse lymphoma L5178Y cells. As revealed by 2′,7′‐dichlorodihydrofluorescein diacetate (DCF‐DA) staining, intracellular reactive oxygen species (ROS) levels were increased about 5‐ and 15‐fold in WLD‐ and WLE‐treated cells, respectively, when compared to the concurrent control groups. This implies that components in the WLE, in addition to the hydroxyanthracene derivatives, could contribute to the mutagenicity of the WLE in part through a ROS‐dependent mechanism. This conclusion is further substantiated by the loss of heterozygosity (LOH) of WLE/WLD‐induced mutants. Results indicated that, while both extracts were clastogenic, their induced mutation spectra were significantly different, thus confirming that different constituents may be responsible for the genetic damage caused by the two preparations. These results indicate that aloe vera and senna extracts and individual hydroxyanthracene derivatives, particularly aloe‐emodin, interact with bacterial and mammalian DNA under certain conditions in vitro.
Overall, the results of genotoxicity testing in vitro indicate that extracts of aloe vera and senna and individual hydroxyanthracene derivatives, particularly aloe‐emodin, interact with bacterial and mammalian DNA under certain conditions in vitro.
There are several in vivo genotoxicity studies available, which include the rodent bone marrow micronucleus assays, the mouse somatic mutation assay in fetal melanoblasts (Mouse Spot Test), the rat bone marrow chromosome aberration assays and the in vivo/in vitro UDS assay in rat liver. The results indicated that senna, fructus sennae extracts, sennosides and the individual 1,8‐dihydroxyanthraquinones tested were uniformly negative. However, the Panel noted that, in all these studies, no evidence of toxicity in the target cells was observed, indicating that target tissues may not have been adequately exposed to the test compounds. This assumption is substantiated by results of an in vivo study in rat, showing a low absorption of [14C]aloe‐emodin and a rapid metabolism to rhein, an anthranoid with no significant genotoxic activity in a battery of in vitro and in vivo assays.
Marked and significant increases in DNA fragmentation have been found in the colon cells of male OF1 mice treated with aloe‐emodin by oral gavage at 2,000, 1,000 and 500 mg/kg body weight (bw) on two occasions 24 h apart in the in vivo rodent comet assay. Slight increases in DNA breakage compared to the concurrent vehicle control values were also observed in the kidney which were dose‐related but reached statistical significance only at the high‐dose level tested. On this basis, the limited absorption of aloe‐emodin and its quick transformation to rhein (a compound devoid of genotoxic capabilities) indicate that bone marrow may be considered as an inadequate tissue to demonstrate a possible in vivo genotoxicity for both emodin and aloe‐emodin.
Overall, the Panel considered that the in vitro genotoxicity of aloe‐emodin was reproduced in the colon in vivo, the target tissue for aloe vera WLE carcinogenicity. The Panel therefore considered that aloe‐emodin represents a genotoxic risk for humans. The Panel also considered that the presence of other mutagenic components, in addition to hydroxyanthracene derivatives, cannot be excluded.
In vivo carcinogenicity studies were also considered relevant by the Panel for this assessment. Overall, the results of carcinogenicity studies indicated a carcinogenic effect of a whole leaf powder of Aloe when given to rats at dietary concentration of 4%, a level associated with diarrhoea or loose stool, of aloe vera WLE in drinking water when given to rats at concentration of 1% or higher, and of chrysazin given in the diet at concentrations of 0.2% and 1% to mice and rats, respectively. Apart from tumours, exposure to these test compounds caused hyperplastic changes in the large intestine of rats and mice. Senna extract was not carcinogenic to rats at doses up to 25 mg/kg bw per day in drinking water. Senna preparation was not carcinogenic to rats at doses amounting to 300 mg/kg bw per day administered by gavage but the rats developed diffuse mucosal epithelial hyperplasia in the large intestine and caecum with an incidence increasing in a dose‐dependent manner. Senna in the diet in doses amounting to 1,260 and 1,520 mg/kg bw per day was not carcinogenic to p53+/‐ males and females, respectively, but induced epithelial hyperplasia in the large intestine.
Epidemiological data suggested an increased risk for colorectal cancer associated with the general use of laxatives, several of which contain hydroxyanthracene derivatives.
Five cohort studies were reviewed by the Panel and an increased risk for colorectal cancer was found in all, however, only in two studies the results were statistically significant. Based on the studies reviewed by the European Medicines Agency (EMA) and the results of more recent large epidemiological studies, the Panel agreed with previous evaluations that the prolonged use of laxatives is a possible risk factor for colorectal cancer. Nevertheless, the Panel was of the view that better designed epidemiological studies (e.g. cohort studies with large sample size and proper control for confounding factors) that investigate the relationship between anthranoids laxatives use and colorectal are needed.
Based on the data currently available, the Panel concluded that hydroxyanthracene derivatives should be regarded as genotoxic and carcinogenic unless there are specific data to the contrary, such as for rhein and that there is a safety concern for extracts containing hydroxyanthracene derivatives although uncertainty persists.
Furthermore, the Panel was unable to provide advice on a daily intake of hydroxyanthracene derivatives that does not give rise to concerns about harmful effects to health, for the general population, and as appropriate, for vulnerable subgroups of the population.
1. Introduction
Following a request from the European Commission to the European Food Safety Authority (EFSA), the Scientific Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to provide a scientific opinion on the safety of hydroxyanthracene derivatives from all sources in foods including preparations such as food supplements and infusions.
This risk assessment was carried out in the framework of the procedure under Article 8(2) of Regulation (EC) No 1925/2006 on the addition of vitamins and minerals and of certain other substances to foods, for hydroxyanthracene derivatives, initiated by the European Commission. Article 8(2) of Regulation (EC) No 1925/2006 is referring to a possible prohibition, restriction or Community scrutiny of a substance or ingredient by placement in Annex III, Part A, B or C of this Regulation.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
In 2013, the European Food Safety Authority (EFSA) issued an opinion on the scientific substantiation of a health claim related to hydroxyanthracene derivatives and improvement of bowel function. In this opinion EFSA concluded that a cause and effect relationship has been established between consumption of hydroxyanthracene derivatives and the improvement of bowel function (EFSA NDA Panel, 2013) and considered it necessary to recommend certain restrictions of use. In particular, the Panel noted that “stimulant laxatives should not be consumed continually for periods longer than one to two weeks” and “the use of stimulant laxatives for more than two weeks requires medical supervision”. Furthermore, EFSA recommended in the scientific opinion that the “long‐term use of stimulant laxatives should be avoided owing to the danger of electrolyte imbalance, impaired function of the intestine, and dependence on laxatives”, and that “stimulant laxative should only be used if an effect on bowel function cannot be achieved by a change of diet or the administration of bulk forming agents”. EFSA based the above‐mentioned recommendations and restrictions on the available evidence obtained mainly from monographs published by the World Health Organisation (WHO) and by the Committee on Herbal Medicinal Products (HMPC) of the European Medicines Agency (EMA), as referenced in the EFSA opinion on the scientific substantiation of a health claim related to hydroxyanthracene derivatives and improvement of bowel function.
In the light of the above, Member States raised concerns during discussions on the possible authorisation of the above‐mentioned health claim regarding a potential risk to consumers linked with the consumption of hydroxyanthracene derivatives in foods.
Consequently, the Commission has initiated the procedure under Article 8 (2) of Regulation (EC) No 1925/2006 on the addition of vitamins and minerals and of certain other substances to foods,1 for hydroxyanthracene derivatives from all sources.
1.1.2. Terms of Reference
In accordance with Article 29(1)(a) of Regulation (EC) No 178/20022, the European Commission asks EFSA to:
Review the existing scientific data on the possible link between the intake of hydroxyanthracene derivatives and a harmful effect on health.
Provide advice on a daily intake of hydroxyanthracene derivatives that does not give rise to concerns about harmful effects to health, for the general population, and as appropriate, for vulnerable subgroups of the population.
1.1.3. Interpretation of the Terms of Reference
In respect to the approach to be followed for the assessment of hydroxyanthracene derivatives, the Panel was of the view that previous assessments, when relevant to the safety issues that triggered the Article 8 procedures, should be used as starting points for this scientific opinion. Since these procedures are triggered by specific safety concerns of either the European Commission or the Member States, in addressing the mandate received, it is the interpretation of the Panel that the specific issue(s) of concern – the relationship between hydroxyanthracene derivative exposures and colorectal cancer (CRC) – should be addressed in this opinion.
1.1.4. Definition and identification of hydroxyanthracene derivatives
In its 2013 scientific opinion, the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) refers to hydroxyanthracene derivatives as those found in the root and rhizome of Rheum palmatum L. and/or Rheum officinale Baillon and/or their hybrids; leaves or fruits of Cassia senna L. and/or Cassia angustifolia Vahl; bark of Rhamnus frangula L., bark of Rhamnus purshianus D.C. and in leaves of Aloe barbadensis Miller and/or various aloe species, mainly Aloe ferox Miller and its hybrids.
In this opinion, the term ‘hydroxyanthracene derivatives’ is restricted to those anthranoid compounds found in the root and rhizome of Rheum palmatum L. and/or Rheum officinale Baillon and/or their hybrids; leaves or fruits of Cassia senna L. and/or Cassia angustifolia Vahl; bark of Rhamnus frangula L., bark of Rhamnus purshianus D.C. and in leaves of Aloe barbadensis Miller and/or various aloe species, mainly Aloe ferox Miller and its hybrids and further, that are also indicated as present in the respective EU Pharmacopeia monographs as characteristic active components of these botanicals.
The substances reported in Appendix A include – but are not limited to – those hydroxyanthracene derivatives indicated in the respective EU Pharmacopeia monographs as characteristic active components of these botanicals (EU Ph, 9th edition). Other structurally related compounds have been assessed by the US National Toxicology Program (NTP) (anthraquinone, emodin) and have only been included for the purpose of potential read across. Data on the structurally related synthetic compound danthron (chrysazin) have also been considered suitable for read‐across.
Due to the continuous revision of botanical classifications and scientific names, some differences can be found between names present in previous assessment documents and the names used in this opinion.
1.1.5. Identification of safety concern triggering Article 8 procedure
In previous evaluations (EMA, 2007a,b,c,d, 2008a,b), the relationship between laxatives use and CRC was examined and it was concluded that ‘The question of a possible carcinogenic risk of long‐term use of anthranoid‐containing laxatives is still open and the results of the more recent studies are inconsistent. Therefore the conditions determined in the pharmacovigilance actions for anthranoid‐containing laxatives have to be maintained’. In the EMA evaluation, seven epidemiological studies and a meta‐analysis were reviewed. Out of the seven epidemiological studies reviewed by EMA (Kune, 1993; Nusko et al., 1993; Siegers et al., 1993; Jacobs and White, 1998; Kune et al., 1988; Nusko et al., 2000; Roberts et al., 2003), two studies showed an increased risk for CRC (Nusko et al., 1993; Siegers et al., 1993). In the study of Siegers et al. (1993), subjects using anthranoids laxatives were three times more likely to develop CRC (relative risk (RR): 3.04; 95% CI: 1.18–4.90) than subjects not using it. Nusko et al. (1993) showed that individuals using laxatives, in which the main ingredient was anthranoid, had an increased risk of CRC (RR (adenomas): 1.72; 95% CI: 1.46–2.01; RR for carcinoma: 1.26; 95% CI: 0.74–2.15). The results of the meta‐analysis that was conducted on 14 case–control studies showed a significant association between CRC and laxatives use (summary risk estimate: 1.46, 95% CI: 1.33–1.61) (Sonnenberg and Müller, 1993). However, most of the above epidemiological studies reviewed – including the ones in the meta‐analysis – had methodological limitations such as the use of a retrospective design, small sample size and lack of adjustment for potential confounding factors.
The Panel is aware that in the current risk assessment a number of aspects have not been considered, as they were deemed to fall outside the remit of the Panel and the scope of the current mandate. The current risk assessment was performed to address questions on specific adverse effects and not as an overall evaluation of hydroxyanthracene derivatives used in food and food supplements.
1.2. Data and methodologies
1.2.1. Data
The Panel was not provided with a newly submitted dossier and based its evaluation on previous evaluations (EMA, 2006, 2008a,b, 2017,b; IARC Monographs 108, 2016), additional literature that became available since then and the data available following public call for data.3
1.2.2. Methodologies
The assessment was conducted in line with the principles described in the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
The risk assessment was performed according to the EFSA Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements (EFSA Scientific Committee, 2009).
When the test substance was administered in the feed or in the drinking water, but doses were not explicitly reported by the authors as mg/kg body weight (bw) per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee (2012) for studies in rodents or, in the case of other animal species, by the Joint FAO/WHO Expert Committee on Food Additives (JECFA, 2000). In these cases, the daily intake is expressed as ‘equivalent to’. When in human studies in adults (aged above 18 years), the dose of the test substance administered was reported in mg/person per day, the dose in mg/kg bw per day was calculated by the Panel using a body weight of 70 kg as default for the adult population as described in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012).
1.3. Information on existing assessments
Preparations containing hydroxyanthracene derivatives have been evaluated by a number of Committees or international organisations for their potential beneficial effects and/or for their potential adverse effects as food or as medicinal products.
1.3.1. Opinion from international bodies or organisations
Evaluation performed by the European Food Safety Authority
Aloe spp., Rheum spp., Cassia spp. and Rhamnus spp. are listed in the EFSA Compendium of Botanicals reported to contain naturally occurring substances of possible concern for human health when used in food and food supplements (EFSA, online).4
The EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) has conducted an assessment on the substantiation of health claims related to the hydroxyanthracene derivatives and improvement of bowel function (EFSA NDA Panel, 2013). A cause and effect relationship has been established for the claimed beneficial effect. The NDA Panel considered that in order to obtain the beneficial effect claimed, a product should provide 10 mg hydroxyanthracene derivatives, in the adult population.
Evaluations performed by the European Medicines Agency
EMA has published some Community herbal monographs on hydroxyanthracene derivatives (Cassia senna L. and Cassia angustifolia Valh, Rhamnus frangula L., Rhei radix, Rhamnus purshianus D.C.; Rhamni purshianae cortex, Aloe barbadensis Mill. and Aloe various species, mainly Aloe ferox Mill. and its hybrids), based on an assessment reports with an associated reference lists (EMA, 2007a–c, 2008a,b, 2017,b).
For all the monographs, EMA concluded that the short‐term use in case of occasional constipation can be regarded as safe. However, the pharmacovigilance actions for anthranoid‐containing laxatives have to be maintained because further investigations are needed in regard of the carcinogenic potential of these substances.
Furthermore, the use in children below 12 years and during pregnancy or lactation is not recommended.
Evaluation performed by the German Federal Institute for Risk Assessment (BfR)
The BfR recently issued an opinion on food supplements containing whole‐leaf Aloe preparations containing anthranoids (BfR, 2017). The BfR concluded that products which contain preparations of unpeeled leaves of Aloe arborescens, and thereby anthranoids, do not belong to the category of botanical food supplements which can be designated as being of ‘no safety concern’ based on current knowledge. The BfR concluded that preparations containing anthranoids are not suitable for use in foods, including food supplements due to the suspicion that plant‐based anthranoids have a carcinogenic effect in humans. Their assessment does not apply to preparations made from anthranoid‐free gel or inner pulp from the leaves of Aloe species (mostly Aloe barbadensis or Aloe vera), which are commonly used in foods and cosmetics in the EU.
Evaluation performed by the International Agency for Research on Cancer (IARC)
In the 2013 monograph on ‘Some drugs and herbal products’, IARC considered that there was inadequate evidence in humans for the carcinogenicity of Aloe vera. However, it was considered that evidence in experimental animals for the carcinogenicity of whole leaf extract (WLE) of Aloe vera was sufficiently detailed. Consequently, the WLE of Aloe vera has been classified as possibly carcinogenic to humans and included in ‘Group 2B’.
The structurally related compound danthron (chrysazin) has also been assessed by IARC and included in ‘Group 2B’ as a possible carcinogen to humans (IARC, 1990).
Evaluations performed by the World Health Organization (WHO)
The WHO has published monographs on the safety, efficacy and quality control of Aloe, Cassia, Frangula and Cascara (WHO, 1999) for their use as medicinal plants.
In the monographs, it is recommended that products containing anthraquinone glycosides should not be used for longer than 1–2 weeks, due to possible incidence of electrolyte imbalance. Furthermore, the use of these substances is contraindicated during pregnancy or lactation, except under medical supervision, after evaluating benefits and risks. Their use is contraindicated in children under 10 years old.
1.3.2. Regulatory status and maximum limits in the EU and USA
On the basis of information gathered from the relevant food sector operators, the ANS Panel was made aware of the regulatory status of hydroxyanthracene derivatives in some of the EU countries (‘Documentation provided to EFSA’ n. 1 and 2).
The regulatory overview of the status of plants containing hydroxyanthracene derivatives are described below.
In addition to the information reported below, the Panel noted that aloin is listed in Annex III, part A (Substances which shall not be added as such to food) to Regulation (EC) No 1334/2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods.5
Belgium
The Belgian Royal Decree6 lists plants and their parts that are prohibited or allowed for use in food supplements. Plants containing hydroxyanthracene derivatives that are explicitly allowed in food supplements are listed in Table 1 (‘Documentation provided to EFSA’ n. 1).
Table 1.
List of plants containing hydroxyanthracene derivatives allowed in food supplements in Belgium (‘Documentation provided to EFSA’ n. 1)
| Plant | Parts of plants | Condition of use |
|---|---|---|
| Senna alexandrina Miller | Leaf, pod |
Recommended daily intake not exceeds 18 mg (expressed as sennoside B); do not administer to children (< 12 years old); consult physician during pregnancy and lactation; no prolonged use without professional advice |
| Senna italic Miller | Fruit, (pod), leaf | |
| Senna obtusifolia (L.) H.S. Irwin & Barneby | Whole plant | |
| Senna occidentalis (L.) Link | Leaf | |
| Senna tora (L.) Roxb. | Leaf, seed | |
| Cassia fistula L. | Fruit, (pod), leaf |
Recommended daily intake not exceeds 18 mg (expressed as sennoside B); do not administered to children (< 12 years old); consult physician during pregnancy and lactation; no prolonged use without professional advice |
| Frangula alnus Mill. | Bark |
Recommended daily intake not exceeds 14 mg (expressed as frangulin A); do not administered to children (< 12 years old); consult physician during pregnancy and lactation; no prolonged use without professional advice |
| Frangula purshiana Cooper | Bark | |
| Rheum austral D. Don | Leaf, rhizome |
Recommended daily intake not exceeds 25 mg (expressed as rhein); do not administered to children (< 12 years old); consult physician during pregnancy and lactation; no prolonged use without professional advice |
| Rheum x hydrodum Murray | Root, rhizome | |
| Rheum officinale Baill. | Root, rhizome | |
| Rheum palmatum L. | Root, rhizome | |
| Rheum rhabarbarum L. | Root, rhizome | |
| Rheum rhaponticum L. | Root, rhizome | |
| Aloe Africana Mill. | Leaf, gel, latex (juice) |
Recommended daily intake not exceeds 14 mg (expressed as barbaloin); do not administered to children (< 12 years old); consult physician during pregnancy and lactation; no prolonged use without professional advice |
| Aloe arborescens Mill. | Leaf, gel | |
| Aloe ferox Mille. | Leaf | |
| Aloe perryi Baker | Leaf, gel | |
| Aloe plicatilis (L.) Mill. | Leaf, gel | |
| Aloe vera (L.) Burm. f. | Leaf |
These plants are also included in the so‐called BELFRIT list, a list of plants elaborated by Belgium, France and Italy as a common list of plants that are permitted for use in food supplements in these countries.
Bulgaria
Ordinance No 477 covers the use of substances other than vitamins and minerals in food supplements. Annex 4 of the Bulgarian Ordinance includes a list of botanicals which are prohibited for use in food supplements.
Cassia acutifolia (Cassia senna), Cassia angustifolia, Frangula alnus, Rheum officinale, Rheum palmatum, Rhamnus cathartica, Rhamnus purshiana (Cascara), Aloe vera and Aloe ferox are not included in the negative list, therefore they can be used in food supplements (‘Documentation provided to EFSA’ n. 1).
Czech Republic
According to the National Decree No. 225/2008 Coll.,8 the use of Cassia acutifolia (Cassia senna), Cassia angustifolia, Frangula alnus, Rheum officinale, Rheum palmatum, Rhamnus cathartica and Rhamnus purshiana species are prohibited in the production of foods in the Czech Republic (‘Documentation provided to EFSA’ n. 1).
Denmark
The national Order No. 729 requires a toxicological evaluation before substances can be included in the positive list of substances that can be used in foods or food supplements. Since hydroxyanthracene derivatives are not listed in this national Order, these compounds cannot be included in food or food supplements.
However, extracts that do not have a purity degree of at least 50% or are not concentrated 40 times or more fall outside the scope of the Order.
The Technical University of Denmark (DTU) has been asked to perform a toxicological evaluation of plants in food supplements. The list contains plants considered as unacceptable, plants with a restriction on daily use (max. level), and plants that are evaluated at a daily dose (‘Drogelisten’ (2000) and later update March 2011).
The following plants are listed in the ‘Drogelisten’ with conditions of use, used alone and in combination:
Cassia acutifolia Delile or Cassia angustifolia Vahl (leaf and fruit): maximum 50 mg/day
Rheum officinale, Rheum palmatum (root stick): maximum 100 mg/day
Rhamnus cathartica L. (dried berries): maximum 30 mg/day
Rhamnus frangula L. (bark): maximum 100 mg/day
Rhamnus purshiana (Cascara) (bark): maximum 200 mg/day
If more than one plant is included in the same products the percentage of the maximum daily dose for each plant should be reduced relatively (‘Documentation provided to EFSA’ n. 1).
Finland
The Finnish Law 989/2007 of the Ministry of Agriculture and Forestry10 allows the use of botanicals and other substances with a nutritional or physiological effect.
There are no legal lists specifying the permission or prohibition of botanicals in food supplements. The evaluation is done on case‐by‐case basis (‘Documentation provided to EFSA’ n. 1).
France
The provisions of the EU Food Supplement Directive are transposed by the French Decree No. 2006‐352,11 which permits the use of botanicals and other bioactive substances.
The French Order of 24 June 201412 completes this Decree and establishes a list of plants that can be used in food supplements. It also requires a toxicological assessment if the mode of preparation is significantly different from the traditional mode of preparation of the plant.
The plants containing hydroxyanthracene derivatives, listed in Table 2, are permitted for use in food supplements, under specific conditions of use. France also accepts under mutual recognition the plants that are included in the BELFRIT list (‘Documentation provided to EFSA’ n. 1).
Table 2.
List of plants containing hydroxyanthracene derivatives permitted for use in food supplements in France (‘Documentation provided to EFSA’ n. 1)
| Plant | Parts of plants | Condition of use |
|---|---|---|
|
Senna alexandrina Miller Senna obtusifolia |
Fruit, leaf Seed |
Hydroxyanthracene derivatives to be monitored. The labelling should include the statement: not recommended for children aged under 12, pregnant or breastfeeding women and not suitable for prolonged use |
| Cassia fistula L. | Fruit, leaf | Hydroxyanthracene derivatives to be monitored. The labelling should include the statement: not recommended for children aged under 12, pregnant or breastfeeding women and not suitable for prolonged use |
|
Frangula alnus Mill. Frangula purshiana Cooper |
Bark | Hydroxyanthracene derivatives to be monitored. The labelling should include the statement: not recommended for children aged under 12, pregnant or breastfeeding women and not suitable for prolonged use |
|
Rheum palmatum L. Rheum officinale Baill. |
Root, rhizome | Hydroxyanthracene derivatives and anthraquinones to be monitored. The labelling should include the statement: not recommended for children aged under 12, pregnant or breastfeeding women and not suitable for prolonged use |
|
Aloe ferox Mill. Aloe vera (L.) Burm. f. |
Leaf, gel, latex (juice) | Hydroxyanthracene derivatives (aloins) to be monitored. The labelling should include the statement: not recommended for children aged under 12, pregnant or breastfeeding women and not suitable for prolonged use |
| Rhamnus cathartica | Bark | Hydroxyanthracene derivatives (aloins) to be monitored. The labelling should include the statement: not recommended for children aged under 12, pregnant or breastfeeding women and not suitable for prolonged use |
The quality of dietary supplements containing hydroxyanthracene derivatives in France in based on the Belgian Regulation and used by the French supervisory authorities (Direction Generale de la Concurrence, de la Consommation et de la Repression des Fraudes (DGCCRF)) (‘Documentation provided to EFSA’ n. 2).
The limits of hydroxyanthracene derivatives are:
10 mg/day for Rhamnus purshianus, Rhamnus frangula and Aloe – expressed as aloin,
15 mg/day for Cassia senna L., Cassia angustifolia Vahland – expressed as sennoside B,
20 mg/day for Rheum palmatum L., Rheum officinale Baillon – expressed as rhein.
Italy
Italy has adopted the BELFRIT list in its legislation.13 , 14 The plants listed in the Belgian Royal Decree also are accepted for the use in food supplements in Italy, without conditions of use (‘Documentation provided to EFSA’ n.1).
Romania
The Health Minister Order 1069/200715 permits the use of botanicals and other bioactive substances in food supplements. The 2005 Common Order of Ministry of Health and Ministry of Agriculture, Forests and Rural Development no. 401/244 regulates the use of botanicals in food supplements and includes a positive and negative list of herbs and plants, and a positive list of cultivated and wild mushrooms. Moreover, the Order 1228/2005 specifies rules on the approval of food supplements containing animal or herbal products (extracts), or in combination with vitamins and minerals.
The following hydroxyanthracene derivatives containing plants are specifically allowed for use in food supplements (‘Documentation provided to EFSA’ n. 1):
Cassia acutifolia (Cassia senna), Cassia angustifolia
Frangula alnus
Rheum officinale, Rheum palmatum
Rhamnus cathartica, Rhamnus purshiana (Cascara)
Aloe vera, Aloe ferox
Spain
The Royal Decree 1478/200916 on food supplements allows the use of botanicals and other ingredients in food supplements. However, the authorities have not issued any list (‘Documentation provided to EFSA’ n.1).
Sweden
The Swedish National Food Administration's Code of Statues17 permits the use of botanicals and other bioactive substances in food supplements. The authorities tolerate the use of other bioactive substances in food supplements as long as they are not classified as medicines or natural remedies by the Medicinal Products Agency.
The following plants are included in the list of the Swedish Medical Products Agency (‘Documentation provided to EFSA’ n. 1):
Cassia angustifolia Senna, Cassia senna. The product is generally a medicinal product. The herb has a laxative effect.
Frangula alnus. The product is generally a medicinal product.
Aloe vera, Aloe ferox. The product is generally not a medicinal product. Aloe products containing anthraquinones can be medicinal products, since these substances have a laxative effect.
The following plants are not included in this list:
Rheum officinale, Rheum palmatum
Rhamnus cathartica, Rhamnus purshiana (Cascara).
United Kingdom
The Food Supplement (England) Regulations 200318 permit the use of substances with nutritional or physiological effect in food supplements. The Regulation does not include positive and/or negative lists of botanicals or other bioactive substances (‘Documentation provided to EFSA’ n.1).
United States
Before 2002, products containing various components of Aloe vera (aloin, aloe‐emodin and barbaloin) were considered as oral over‐the‐counter (OTC) laxatives and regulated by the Food and Drugs Administration (FDA). In 2002, the FDA promulgated a regulation stating that the Aloe vera ingredients present in OTC drugs were not ‘generally recognized as safe and effective’ or were misbranded (FDA, 2002).19 Since the lack of safety data on Aloe vera laxative products, FDA required all OTC Aloe vera laxative products to be withdrawn from the US market or reformulated. However, Aloe vera may be used as a food flavouring as defined in FDA Regulation 21CFR172.510.
1.3.3. Overview of composition from industry
A public call for data was launched to gather information from the relevant food business operators, on the composition of food supplements containing hydroxyanthracene derivatives.20
Based on the information gathered, the Panel noted the no information was received on food supplements containing Cascara preparations as ingredients. Only one product was reported to contain Frangula in combination with the other botanical species and the concentration of hydroxyanthracene derivatives in this product (expressed as glucofrangulin A) was reported to be 7%, corresponding to a dose of 13 mg/tablet.
The Panel further noted that, according to the information provided by relevant food sectors, following the launch of the ‘Call for data’, the majority of the products contain botanical preparations from Cassia, Aloe and Rhubarb, alone or in combinations and that the percentage of hydroxyanthracene derivatives in food supplements, as reported by food business operators, is variable (sennoside B 0.2–24%; rhein 1.6–6%; barbaloin: 15–20%) (‘Documentation provided to EFSA’ n. 3, 4, 5, 6 and 7).
2. Technical data
2.1. Identity and nature of the source material
Plants containing hydroxyanthracene derivatives are numerous and belong to different botanical families and genera. To focus the attention on the most important botanicals for the consumers, plants used in food supplements and traditional medicine have been selected.
For food supplements, the main source of information was the so‐called BELFRIT list, which contains more than 1000 botanicals allowed in this food category.
For herbal medicinal products, the main source of information was the EMA website21 and the European Pharmacopoeia.
Since the botanical classification is complicated and several synonyms are associated with any species, a short description of botanicals is reported here (Table 3), with common names and parts used. However, detailed tables are present in Appendix B.
Table 3.
Botanical classification and short description of Aloe spp., Cassia spp., Frangula spp., Rhamnus spp. and Rheum spp
| Aloe spp., family: Xanthorrhoeaceae, genus: Aloe L. | ||
|---|---|---|
| Species | Main common name | Part/s used |
| Aloe africana Mill | ND | Leaf, leaf gel |
| Aloe arborescens Mill | Candelabra aloe | Leaf, leaf gel |
| Aloe ferox Mill. | Cape aloe | Leaf, leaf gel |
| Aloe perryi Baker | Perry's aloe | Leaf, leaf gel |
| Aloe plicatilis (L.) Mill | ND | Leaf, leaf gel |
| Aloe vera (L.) Burm. F | Barbados aloe | Leaf, leaf gel |
| Cassia spp., family: Leguminosae, genus: Cassia L. or Senna Mill. | ||
| Species | Main common name | Part/s used |
| Cassia fistula L. | Golden shower | Fruit |
| Cassia italica (Mill.) F.W. Andrew | Port Royal senna | Whole plant |
| Cassia mimosoides L. var. nomame Makino | Cassia nomame | Leaf, pod |
| Senna alexandrina Mill. | Alexandrian senna | Leaf, pod |
| Senna obtusifolia (L.) H.S. Irwin & Barneby | Java‐bean | Whole plant |
| Senna occidentalis (L.) Link | Septicweed | Bark, leaf |
| Senna tora (L.) Roxb. | Sickle senna | Leaf, seed |
|
Frangula spp. and Rhamnus spp. spp., family: Rhamnaceae, genus: Frangula Mill. and Rhamnus L. | ||
| Species | Main common name | Part used |
| Frangula alnus Mill. | Glossy buckthorn | Bark |
| Frangula purshiana Cooper | Cascara buckthorn | Bark |
| Rhamnus alpina L. | – | Bark, fruit |
| Rhamnus cathartica L. | Common buckthorn | Whole plant |
| Rheum spp., family: Polygonaceae, genus: Rheum L. | ||
| Species | Main common name | Part used |
| Rheum australe D. Don | ND | |
| Rheum officinale Baill. | Chinese rhubarb | |
| Rheum palmatum L. | Chinese rhubarb | |
| Rheum rhabarbarum L. | Garden rhubarb | |
| Rheum rhaponticum L. | ND | |
| Rheum x hybridum Murray | ND | |
ND: no common name found.
On the basis of the information gathered from the relevant food sector operators, a description of the identity of the botanical sources of hydroxyanthracene derivatives is also provided in Table 4.
Table 4.
Information gathered from the relevant food sector operators related to the identity of the botanical sources of hydroxyanthracene derivatives
| Aloe | Cassia | Rhamnus | Rheum | |
|---|---|---|---|---|
| Botanical family |
Aloaceae – Aloe family Asparagaceae |
Fabaceae/Leguminosae – Pea family | NA | Polygonaceae |
| Genus | Aloe L. | Senna Mill.– senna | NA | NA |
| Species |
Aloe ferox Mill. – Cape aloe Aloe vera L. Burm. F |
Senna alexandrina Mill. | NA | Rheum palmatum L. /Rheum officinale Baillon |
| Variety | NA | NA | NA | NA |
| Synonyms |
Aloe horrida Haw., A. perfoliata Thunberg., A. pseudoferox Salm. Dyck, A. socotrina Masson., A. supralaevis Haw., Pachydendron ferox Humb. & Bonpl., P. supralaeve Haw Aloe barbadensis Mill |
Cassia acutifolia Delile, Cassia senna L., Senna alexandrina, Senna angustifolia (Vahl) Batka Senna alexandrina Mill. |
Frangula dodonei Ard – Rhamnus frangula L. |
Rheum potaninii Losinsk, Rheum qinlingense Y.K. Yang, J.K. Wu & D.K. Zhang = synonyms of Rheum palmatum L., no synonym are recorded for Rheum officinale Baill. (source: The Plant List) Rheum palmatum subsp. dissectum Stapf Rheum palmatum f. rubiflora Stapf |
| Part used | Leaves juice | Leaves, fruit | Bark, rhizome | Leaves, roots |
| Geographical origin |
Continent: Africa, South America Country: South Africa |
Continent: Asia Country: India Area: Rajasthan/Gujarat |
Continent: Europe Country: – Area: Eastern Europe |
Continent: Asia Country: China Area: Shanxi province |
| Growth and harvesting conditions | Wild | Cultivated |
Wild Stage of harvest: before flowering Manual harvesting. drying: natural |
Wild and cultivated Cultivation practices: time of harvest in relation to season. Stage of the plant growth: after flowering |
NA: no information available further to request to food sector operators.
Based on the information received in response to a public call for data, the ANS Panel noted that the identity of the botanical species used in food supplements is in line with the information presented above, with the exception of cascara preparations, for which no response has been received (‘Documentation provided to EFSA’ n. 3, 4, 5, 6 and 7).
2.2. Chemical composition
The EFSA Compendium of Botanicals (EFSA, online) was consulted to identify bioactive substances present in the botanical species that are the subject to this opinion.
Information on composition, as reported in the respective EU Pharmacopoeia monographs, is also reported.
Rheum spp. Rhubarb
According to the EFSA Compendium of Botanicals (EFSA, online), roots and other underground parts of Rheum palmatum L and Rheum officinale Baillon, contain anthraquinones at levels ranging from 2.2% to 6.0%. The compounds identified are emodin, palmidin C, rhein, sennoside A, sennoside B; however, their levels are not quantified.
No other substances of possible concern for human health when present in food are reported to be present.
Rhein is the substance used as a reference for the standardisation of rhubarb preparations included in the EU Pharmacopeia (Reference to be added).
The whole or cut, dried underground parts of Rheum palmatum L or of Rheum officinale Baillon or of hybrids of these two species or of a mixture must have a minimum content of 2.2% of hydroxyanthracene derivatives, expressed as rhein (dried drug), in order to comply with the specifications given in the EU Pharmacopoeia for the drug ‘rhubarb’. Rhubarb is used as a starting material for the preparation of the dry standardised extract produced by ethanol extraction. In order to comply with the EU Pharmacopoeia requirements, the standardised extract shall have a content of 15.0–30.0% of glucofrangulins.
Cassia spp. and Senna spp.
According to the EFSA Compendium of Botanicals (EFSA, online), the leaves and the fruit of Cassia fistula L., contain chrysophanol, physcion and rhein; however their levels are not quantified.
Aloe‐emodin, emodin, emodin anthrone, physcion are reported, albeit not quantified, in the seed of Senna occidentalis (L.), alongside other phytotoxins (not further specified) (EFSA, online).
Sennoside B is the substance used as a reference for the standardisation of senna preparations included in the EU Pharmacopeia (Reference to be added).
Dried leaflets of Cassia senna L. (synonym Cassia acutifolia Delile), known as Alexandrian or Khartoum senna, or Cassia angustifolia Vahl, known as Tinnevelly senna or a mixture of the two species must have a minimum content of 2.5% of hydroxyanthracene glycosides, expressed as sennoside B (dried drug) in order to comply with the specifications given in the EU Pharmacopoeia for the drug ‘senna leaf’. Senna leaf is used as a starting material for the preparation of the dry standardised extract produced by ethanol extraction. In order to comply with the EU Pharmacopoeia requirements, the standardised extract shall have a content of 5.0–8.0% of hydroxyanthracene glycosides, expressed as sennoside B.
In addition to the leaf, also the dried fruit of Cassia senna L. are used for the herbal drug ‘senna pods, alexandrian’, which must contain a minimum of 3.4% hydroxyanthracene glycosides, expressed as sennoside B, in order to comply with the specifications given in the EU Pharmacopoeia. The drug ‘senna pods, tinnevelly’ is instead obtained by the dried fruit of Cassia angustifolia Vahl and must contain a minimum of 2.2% hydroxyanthracene glycosides, expressed as sennoside B in order to comply with the specifications given in the EU Pharmacopoeia.
Aloe spp.
According to the EFSA Compendium of Botanicals (EFSA, online), the leaves of Aloe vera (L) contain aloin A and aloin B; however, their levels are not quantified. The same substances are reported also to be present in the ‘live plants’ of Aloe ferox Mill.
Anthraquinones, aloe‐emodin, aloenin, aloin A and aloin B are reported to be present in the leaves of Aloe arborescens Mill., alongside the following other substances: esculetin, fructose, glucose, glutamic acid, glycine, malic acid, scopoletin, serine, sucrose and vanillic acid. The levels of these substances are not quantified. The Panel noted that these data refers to a publication (Olennikov et al., 2010) on aloe juice, a term which should be used to describe the latex material of the pericycle while ‘gel’ refers to the inner leaf liquid material (IARC, 2016).
Barbaloin is the substance used as a reference for the standardisation of aloe preparations included in the EU Pharmacopeia (Reference to be added).
The concentrated and dried juice of the leaves of various species of Aloe (mainly Aloe ferox Miller and its hybrids must have a minimum content of 18% hydroxyanthracene derivatives, expressed as barbaloin (dried drug), to comply with the specifications given in the EU Pharmacopoeia for the drug ‘Cape aloes’.
The drug ‘Barbados aloes’, refers instead to the concentrated and dried juice of the leaves of Aloe barbadensis Mill. and, in order to comply with the specifications given in the EU Pharmacopoeia, it should contain a minimum of 28% of hydroxyanthracene derivatives, expressed as barbaloin (dried drug).
Both ‘Cape aloes’ and ‘Barbados aloes’, individually or in combination are used in the EU Pharmacopeia, as starting materials for the preparation of the dry standardised extract using boiling water. In order to comply with the EU Pharmacopoeia requirements, the standardised dry extract shall have a content of 19.0–21.0% of hydroxyanthracene derivatives, expressed as barbaloin.)
Frangula spp.
According to the EFSA Compendium of Botanicals (EFSA, online), the bark of Frangula alnus Mil. (Rhamnus frangula Mil.) contains emodin anthrone, glucofrangulin A, glucofrangulin B and palmidin C; however, their levels are not quantified.
No other substances of possible concern for human health when present in food are reported to be present.
Glucofrangulin A is the substance used as a reference for the standardisation of frangula preparations included in the EU Pharmacopeia (Reference to be added).
The dried bark (whole or fragmented) of the stems and branches of Rhamnus frangula L. (Frangula alnus Miller) must have a minimum content of 7.0% of glucofrangulins, expressed as frangulin A (dried drug), to comply with the specifications given in the EU Pharmacopoeia for the drug ‘Frangula bark’. Frangula bark is used as a starting material for the preparation of the dry standardised extract produced by ethanol extraction. In order to comply with the EU Pharmacopoeia requirements, the standardised extract shall have a content of 15.0–30.0% of glucofrangulins.
Rhamnus spp.
According to the EFSA Compendium of Botanicals (EFSA, online), the bark of Frangula purshiana Cooper (Rhamnus purshiana DC.) contains cascarosides, aloe‐emodin and emodin at levels not quantified.
No other substances of possible concern for human health when present in food are reported to be present.
Cascaroside A is the substance used as a reference for the standardisation of cascara preparations included in the EU Pharmacopeia (Reference to be added).
The dried bark (whole or fragmented) of Rhamnus purshiana DC. must have a minimum content of 8.0% of hydroxyanthracene glycosides, of which at least 60% consists as cascarosides, both expressed as cascaroside A (dried drug), to comply with the specifications given in the EU Pharmacopoeia for the drug ‘Cascara’. Cascara is used as a starting material for the preparation of the dry standardised extract produced by either boiling water or hydroalcoholic extraction. In order to comply with the EU Pharmacopoeia requirements, the standardised extract shall have a nominal content of 8.0–25.0% of hydroxyanthracene glycosides, and at least 60% of which must be cascarosides, expressed as cascaroside A.
2.3. Specifications
There are no specifications for Aloe, Rhubarb, Cassia, Frangula and Cascara preparations used as food including food supplements in EU Regulations.
Specifications, however, are available for Aloe, Rhubarb, Cassia, Frangula and Cascara when used as medicinal plants, from the European Pharmacopeia and WHO monographs.
The Panel noted that the specifications given for medicinal plants may not be relevant or applicable to the same botanicals when used in food, including food supplements.
On the basis of the information gathered from the relevant food sector operators following the launch of a public call for data, the Panel was made aware of certain maximum levels for contaminants that are applicable to the food supplements containing hydroxyanthracene derivatives (‘Documentation provided to EFSA’ n. 3, 4, 5, 6 and 7).
No information was received on food supplements containing Rhamnus purshiana (Cascara) as ingredient.
2.4. Manufacturing process
Specific request for information on the manufacturing process used for the preparation of the botanical ingredients used in food supplements containing hydroxyanthracene derivatives was included in the call for data launched. The information received was complemented by the Panel with data retrieved in the published literature.
Extraction methods
According to the information provided by the interested parties, there is no standardised method for the extraction of hydroxyanthracene derivatives. In some cases, the extraction is performed by using ethanol or water (at different percentages); in other cases, an extraction solvent is not used since the plants are naturally dried (‘Documentation provided to EFSA’ n. 4, 5, 6 and 7).
The aloe vera industry produces different commercial raw materials including bitters, gels, WLE powders and so‐called jelly. There are two distinct layers in aloe leaves: the green outer leaf rind and the soft, colourless inner gel parenchyma (Figure 1). Processing techniques vary according to what part of the leaf is required for a particular product/use, especially with regard to the bitters and gels. Some products comprise pure gel, while others are based on various mixtures of gel and bitters, depending on of the product (Boudreau et al., 2013).
Figure 1.
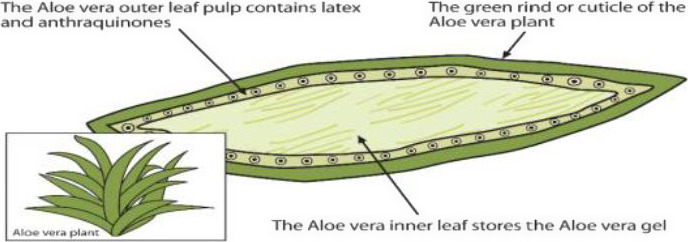
Aloe leaf cross section. Anthraquinones are mainly located in the outer leaf pulp (from Rahman et al., 2017; Copyright © 2017 by the authors, CC BY)
Hydroxyanthracene derivatives are primarily found in the aloe latex, the exudate found in between the inner leaf, i.e. the clear, central parenchymatous tissues of the aloe leaf, and the rind.
The inner leaf is the part of the plant typically used to derive the aloe gel, a liquid product with wide application in the food industry. Aloe gel differs from aloe juice, the latter being obtained from the leaf without the removal of the rind (IASC, 2009).
Different commercial preparations are obtained from aloe vera, depending on the parts of the plant used and the processes applied. The terminology used to name the different preparations does not always allow a clear identification of the material and the processes applied. Owing to their laxative properties, the presence of hydroxyanthracene derivatives in the preparations in‐tended for use in foods is controlled and limited by adding purification steps in the manufacturing process of products intended for use in foods. The International Aloe Science Council has established a quality standard for their certification program of not more than 10 ppm (10 mg/kg) aloin A and B for all aloe vera leaf juice ingredients for use in products intended for oral consumption (IASC, online).
In the whole leaf juice process, the entire aloe vera leaf is cut and ground into a slurry which is subsequently pressed to obtain a liquid. Hydroxyanthracene derivatives are removed by passing through a series of press filters with carbon coated plates. This step is also known as decolourisation process.
The aloe vera juice is continually passed through a filter press until 99.9% or more of the aloin is removed (IASC, online).
The presence of hydroxyanthracene derivatives in the aloe juice can be minimised by a second manufacturing process which uses the inner leaf as the starting material. In this method, the outer leaf rind is separated and discarded from the inner pulp before expressing the juice. A further decolourisation step with activated carbon may also be implemented even in this process.
There is no single method that is regarded as standard for the extraction of bioactive compounds from aloe (Chandegara and Varshney, 2013; Choche et al., 2014). Leaves show losses of biological activity beginning at 6 h following harvest and most biological activities are completely lost after 24 h when stored at ambient temperatures. The processing must be completed within 36 h of harvesting the leaves (Chandegara and Varshney, 2013; Choche et al., 2014). Exudate from the cut leaf base of Aloe spp. contains a high concentration of anthraquinone compounds. The maximum recoverable aloin was estimated to be 24 h after harvest by Soxhlet extraction (SE) of dry gel particles of size 0.42–0.841 mm using methanol 5% (w/w) as a solvent (Choche et al., 2014; Dhodare et al., 2015). Anthraquinones are usually removed from the Aloe commercial products used in food applications. In the whole leaf processing method, the aloe liquid is passed through several filters in order that aloin and aloe‐emodin can be removed (Ahlawat and Khatkar, 2011), as well as any microscopic traces of leaves, sand or other particles. A comparison of various processing methods and the effect on aloin concentration was presented by Chandegara and Varshney (2013). The highest content of aloin (32 mg/kg) is obtained by a roller method, followed by a leaf splitter method (18 mg/kg) and a hand‐filleting method (6 mg/kg), while the lowest content of aloin is obtained by a whole leaf method (1 mg/kg).
In 2003, Cohen patented a method for extracting aloin from aloe juice or other products of industrial utility. In his patent (EP‐A‐374 890), Cohen describes a procedure that consists of two steps. In the first step, an aliphatic diol or triol is added to the yellow aloe juice or other aloin‐containing derivative of the juice. Following the first step, a concentration is performed. The second step includes the extraction that is followed by recrystallisation from an alcohol. The solvent used for the extraction can be either ethyl acetate or acetone. According to the author, this method leads to a greater yield (60%) compared to other usual methods of extraction of aloin.
According to Zhao et al. (2011), the most common methods for the extraction of anthraquinone compounds in rhubarb are maceration extraction (ME), heat reflux extraction (HRE), SE and microwave‐assisted extraction (MAE). In his study, response surface methodology (RSM) was used to optimise ultrasonic‐assisted extraction (UAE). RSM is a statistical technique that investigates the different variables (simultaneously) and their interactions. Here, three variables were investigated: time and temperature of the extraction and percentage concentration of the solvent used (methanol). The software predicted that the optimum methanol concentration, extraction time and extraction temperature were 83.6%, 33.2 min and 67.1°C, respectively. All three factors contributed to the extraction of the anthraquinones.
Conventional extraction techniques such as ME or SE are essentially based on the extracting power of different solvents and the application of heat and/or mixing. These methods are still much in use as they are simple and inexpensive. However, they are often time‐consuming and require large solvent volumes. In contrast, some of the most recently developed methods have definite advantages (Duval et al., 2016).
Many authors compared conventional and novel methods for extraction efficiency of the anthraquinones. Arvindekar et al. (2015) used various solvents like petroleum ether, chloroform, ethyl acetate and ethanol in the extraction of two different kinds of samples (powder and acid hydrolysed) from Rheum emodi. Various sample‐to‐solvent ratios were examined (1:4, 1:8, 1:10, 1:15, 1:20, 1:25, 1:32). The best solvent appeared to be ethanol, indicating maximum solubility for both kinds of samples. The best sample‐to‐solvent ratio was 1:20. In general, the selection of the solvent offers the ability to control the selectivity of the method (Duval et al., 2016).
Different conventional methods for extraction of hydroxyanthracene derivatives are described in the literature. Among these are maceration‐assisted extraction, reflux‐assisted extraction, sublimation‐assisted extraction and SE.
According to Duval et al. (2016), maceration is the most popular method for the extraction of anthraquinones. Arvindekar et al. (2015) studied the extraction of anthraquinones from R. emodi. Solid R. emodi and its acid hydrolysate were suspended in ethanol, separately. After intermittent shakings and a resting period, the extracts were centrifuged, diluted in methanol (1:100) and injected for high‐performance liquid chromatography (HPLC) analyses. Locatelli et al. (2012), Sakulpanich and Gritsanapan (2008) and Zhao et al. (2015), as referred by Duval, mention the use of water and protic solvents, as well as acidic hydrolysis to release aglycone forms or ethyl acetate to achieve the extraction of anthraquinone dimers (Donfack et al., 2014 as referred by Duval et al., 2016).
Pulverised and hydrolysed samples of R. emodi were transferred to an ethanol‐containing round‐bottom flask (RBF) and refluxed for respective time intervals on water bath. The extracts were centrifuged, diluted in methanol (1:100, except t0 1:10) and injected for HPLC analysis. In order to extract the 1,8‐dihydroxyanthraquinones by sublimation process, 1 g of thoroughly dried powder and the acid hydrolysate were transferred in a 50‐mL RBF attached to a reflux condenser (length > 1 m) with cold water circulation and placed in a heating mantle. The flask was strongly heated (8–10 min), until yellow fumes ceased from the sample. On cooling to room temperature, divided portions of 100 mL ethanol were used to dissolve the sublimed 1,8‐dihydroxy anthraquinone (DHAQ) adhered the walls of condenser and volume was made to 100 mL of which 0.1 mL was diluted with methanol (1:100) and subjected to HPLC (Arvindekar et al., 2015).
In the study of Arvindekar, the same two kinds of samples were soxhleted in 100 mL of ethanol for three time intervals. The extracts were centrifuged, diluted in methanol (1:100, except t0 1:10) and injected for HPLC analysis (Arvindekar et al., 2015). According to Duval et al. (2016), in SE the solvent is heated to boil; this is the reason it has to be replaced continuously, until the plant material is completely exhausted. The method is considered very effective. The use of SE for the extraction of aloin from aloe vera was also studied by Choche et al. (2014).
Novel methods are also reported in the literature, such as ultrasound‐assisted extraction (UAE), MAE, pressurised liquid extraction (PLE), super/subcritical fluid extraction and use of ionic liquids (ILs).
Zhao et al. (2011) realised ultrasonic extraction of anthraquinone compounds in rhubarb at a frequency of 40 kHz for a certain time and at different temperatures. Methanol was used as a solvent and the solid to solvent ratio was 1/15. The material size was 0.2–0.5 m. The quantification was achieved by HPLC with ultraviolet detection (HPLC‐UV), where the temperature and wavelength were set at 30°C and 254 nm, respectively.
In the method described by Arvindekar et al. (2015), two samples (one powdered and one hydrolysed) were suspended in ethanol and immersed in an ultrasonic bath. After 0, 15, 30 and 45 min sonication, the extracts were centrifuged, diluted in methanol (1:100, except t0 1:10) and injected for HPLC analysis.
According to Duval et al. (2016), the temperature, in UAE, varies from 55 to 67°C and the time from 30 to 60 min. As a solvent, ethanol (or ethanol–water mixture) is used due to its low cost and non‐toxicity. However, acetone and methanol appeared to be more effective. According to Duval, there are several studies that show the greater efficiency of this method compared to the conventional methods of extraction of anthraquinones.
Bhosle et al. (2015) also investigated the use of UAE and its efficiency compared to the classical solvent extraction techniques used for the extraction of bioactive compounds from natural sources. The extraction took place at 25°C, 15.7 W for 45 min. As a solvent, acetone was used based on the polarity of anthraquinones. However, the use of an ethanol–water mixture as a solvent provided greater anthraquinone extraction yield. According to the author, UAE method of extraction provides increased yield, compared to other methods, such as stirred batch and soxhlet extraction. A temperature of 40°C for 30 min as the optimum parameters for UAE was reported in another publication (Jawade and Chavan, 2013).
Wang (2008) developed a differentiated method called ultrasonic nebulisation extraction (UNE) for the extraction of anthraquinones from Rheum palmatum L. In contrast to the general application of ultrasonic baths, in this study, an ultrasonic humidifier was used for the extraction. As a solvent, ethanol was used, although methanol provided better yields. After the extraction, the sample was dried and re‐dissolved using ethanol A capillary electrophoresis system (sodium dodecyl sulfate micellar electrokinetic chromatography (SDS‐MEKC) system) equipped with a UV detector was used for the separation and determination of anthraquinones. Comparing the different methods of extraction (maceration, reflux extraction, stirring and UAE), the author concluded that UAE displays greater efficiency, while it minimises the energy cost and time of extraction.
According to Duval et al. (2016), the MAE takes place in temperatures between 60 and 120°C for a very short time (7.5–15 min). Srikanth et al. (2011) noticed that MAE led to higher yield of calcium sennosides compared to conventional heating. It is also indicated that increasing the power of the microwaves increases yield, while the solvent consumption and the time required are reduced.
A temperature of 75–150°C is commonly used for pressurised hot water extraction (PHWE). Vázquez et al. (2015) and Pongnaravane et al. (2006), as referred by Duval et al. (2016), used temperatures from 170 to 200°C. According to Vasquez, the increase of temperature from 120 to 170°C provides better extraction. The pressure also varies but is not considered a critical parameter. The extraction can be static or dynamic. An increase of the water flow rate up to 5 mL/min can increase the extraction yield of total anthraquinones (Duval et al., 2016).
Super/subcritical fluid extraction (SFE) mainly uses supercritical carbon dioxide as an extraction solvent. The best conditions were: 30% ethanol in SF at 200 bar and 60°C. The main advantage of SFE compared to the other extraction methods is the use of low temperatures that prevents degradation or oxidation of specific compounds due to high temperature (Duval et al., 2016). Anthraquinones such as aloe‐emodin, physcion, emodin or rhein that have hydroxyl or carboxylic acid groups are not good candidates for SFE using pure CO2 (Shamsipur et al., 2008 as referred by Duval et al., 2016).
In a study, the extraction of five anthraquinones from rhubarb using ionic liquid‐based ultrasonic/microwave‐assisted extraction (IL‐UMAE) was investigated. According to the author, IL‐UMAE showed greater efficacy and decreased extraction time, when compared to conventional methods of extraction of anthraquinones, such as UAE, MAE and HRE (Lu et al., 2011).
Tan et al. (2012) also investigated the use of ILs in the extraction of anthraquinones from aloe. Ionic liquid‐based aqueous two‐phase systems (ILATPS) are considered more efficient and environmentally friendly, compared to other liquid–liquid extraction methods (Tan et al., 2012).
In another study, the same author used an alcohol/salt ATPS to purify anthraquinones extracted from aloe vera. In this study, 5.0 mL distilled water, 2.0 mL alcohol, a given amount of salt (NH4)2SO4 was chosen as the phase‐forming salt) and 0.1 mL aloe anthraquinones solution were mixed. After complete dilution of the salt, the mixture separated in two phases and the anthraquinone rich phase was removed by a syringe and treated with magnesium acetate–methanol solution. Under weakly basic conditions, the anthraquinone solution turns to red. The total anthraquinone concentration was determined using a UV–Vis spectrophotometer. The concentration of anthraquinones in the salt‐rich phase was determined by mass balance. The author concluded that the alcohol/salt system is an efficient method in the purification of active compounds in natural plant or biomolecules (Tan et al., 2013).
Other methods of extraction were retrieved from the literature and are summarised below.
A method described by Su and Ferguson (1973) to extract and separate anthraquinone aglycones and glycosides from cascara (Frangula spp.) and senna (Cassia spp. and Senna spp.) is based on the ability of the aglycones, unlike glycosides, to dissolve in chloroform, as well as on the fact that the hydroxyanthracene derivatives and their glycosides can be found in the plant material free or combined as salts. The procedure started with maceration of the ground plant. After drying, several extractions followed, using chloroform, alcohol and ethanol. The separation was realised using two different chromatographic techniques for glycosides and aglycones, because of their different solubility. This basically means that the aglycones had to be hydrolysed from glycosides prior to the chromatographic separation.
Shi et al. (2007) proposed cloud point extraction (CPE) as a simple and effective method for the extraction and preconcentration of anthraquinones, prior to the use of high‐performance liquid chromatography with diode‐array detection (HPLC‐DAD) for their determination. As an organic solvent for the extraction, a non‐ionic surfactant, oligoethylene glycol monoalkyl ether (Genapol X‐080), was used. Shi used rhubarb which was pulverised and sieved prior the manufacture. UAE was used to extract the hydroxyanthracene derivatives from the plant powder. The extracts were then centrifuged and the supernatant was filtered and analysed by HPLC. In comparison to the other organic solvents, Genapol X‐080 showed greatest efficiency. The CPE method showed good reproducibility and is also considered as an environmental friendly and simplified method.
The method of decoction applied by Sakulpanich and Gritsanapan (2008) includes only the use of distilled water for the extraction of anthraquinones from Cassia fistula, for several times. In his study, Sakulpanich used also percolation, where the pulp was moistened with ethanol and then transferred in a percolator adding alcohol, as well as other conventional methods of extraction (maceration and soxhlet extraction). The author concluded that decoction is the ideal method for the extraction of anthraquinones from C. fistula, according to its yield performance.
Mehta and Laddha (2009) proposed a simple method for the extraction of rhein from senna (Cassia angustifolia) leaves. According to this method, the leaves were powdered and a mixture of ethanol–water was added to the powder. The mixture was warmed and HCl was added. Subsequently, a biphasic system was produced by the use of toluene and was boiled for 6 h. After cooled and filtered, the liquid and organic phases were separated. Another treatment with toluene was applied and 10% aqueous sodium hydrogen carbonate solution was applied until the aqueous layer ceased to show the characteristic pink colour. The aqueous layer was acidified and the precipitate was collected. A dark yellow compound was obtained. Borntrager's reagent was used for the detection of anthraquinones (also referred by Sakulpanich and Gritsanapan, 2008). The compound was identified as rhein after chemical tests and spectral studies. For use in large scale extraction, the method needs further optimisation.
Yang et al. (2014) proposed a new gas‐assisted three‐liquid‐phase extraction system (GATE) for the separation of emodin and rhein from herbal extract. In this study, the system consists of butyl acetate, PEG4000 and ammonium sulfate. In contrast to traditional three‐liquid‐phase extraction, where mechanical or magnetic agitation are used, solutes with surface activity are absorbed on or dissolved in the surface of ascending bubbles in the aqueous phase. According to the author, GATE provides better separation with lower consumption of polymer and organic solvent.
In another study, Shi et al. (2014) proposed an improved liquid–liquid extraction for the extraction of anthraquinones from herbal mixtures. In this improved method, the mixture is extracted, also with ethyl acetate–butanol (1:2, v/v), but this time under stirring and then allowed to separate. The solvent is again evaporated by reduced pressure. The yellow residue is then dissolved in acetone and the procedure followed is the same as for the conventional method. The improved liquid–liquid extraction provided higher yield and lower variability.
According to the author, among different solvents tested (ethyl acetate, ethyl acetate–butanol, chloroform, dichloromethane and chloroform–butanol), the ethyl acetate–butanol (1:2, v/v) solution showed higher extraction efficiency. The method used for the identification and quantification of the target components in the mixture was reverse phase‐high‐performance liquid chromatography (RP‐HPLC) coupled with a photodiode array (PDA) detector.
Cao et al. (2015) investigated the use of a magnetic solid‐phase extraction (MSPE) method combined with ultra‐high‐performance liquid chromatography coupled with quadrupole time‐of‐flight tandem mass spectrometry (UHPLC‐Q‐TOF/MS) for the preconcentration of five active compounds in rhubarb (Rheum palmatum L). The method was based in the use of chemically modified graphene sheets, combined with Fe3O4 nanoparticles and a large delocalised two‐electron system, which serves as a good absorbent for aromatic analytes, like anthraquinones. According to the author, the method displayed high sensitivity and selectivity, as well as high recovery.
Other extraction methods referred by the different authors are high‐speed counter current technology (HSCCC) and solid‐phase extraction (SPE) (Shi et al., 2014), enzyme digestion and pulsed electric field (Arvindekar et al., 2015). As well as, microextraction methods like dispersive liquid–liquid microextraction (DLLME), single drop microextraction (SDME), solid‐phase microextraction (SPME) and its coupled technologies (Shi et al., 2014).
Purification and identification methods that have been used for the target anthraquinones, include HPLC, ultra‐pressure liquid chromatography (UPLC), capillary zone electrophoresis (CZE), electrochromatography, microemulsion electrokinetic chromatography (MEC), HPLC with mass spectrometry (HPLC–MS), gas chromatography with MS (GC–MS) and thin‐layer chromatography (TLC) (Shi et al., 2014).
2.5. Stability of the botanical or botanical preparation used as an ingredient in food supplements
Specific request for information on the stability of the botanical or botanical preparation used as an ingredient in food supplements was included in the call for data launched. The information received was complemented by the Panel with data retrieved in the published literature.
According to the information provided by the interested parties, food supplements containing hydroxyanthracene derivatives have a shelf‐life ranging from 24 to 36 months. The products should be kept closed (5–25°C), away from sources of heat, light and moisture (‘Documentation provided to EFSA’ n. 3, 4, 5, 6 and 7).
The effect of thermal treatments (50, 60, 70, 80 and 90°C) on polysaccharides and barbaloin content in gel juice from Aloe vera was evaluated by Chang et al. (2006). The highest decrease in the content of barbaloin was observed after 10 h treatment at 90°C. No effects were reported at 60°C for 1 h of thermal process. The highest decrease in the content of polysaccharides was demonstrated after 10 h treatment at 50°C and 90°C. Significant effects (p < 0.05) were also showed at 60°C while samples treated at 70 and 80°C did not report significant changes compared to the initial samples used as controls. A similar trend was noted in a different experiment with an extended period of treatment (until 70 h). Samples treated at 50°C showed the greatest effects following by 60°C and 90°C. It has been also noted that during the thermal treatments, the colour of aloe vera gel juice changed from whitish to slight yellow to brownish.
The effects of pH, temperature and light conditions on the stability of aloin A were evaluated by Ding et al. (2014). Thermal treatment experiments were performed at 4, 30, 50 and 70°C at a constant pH value (pH = 7.0). The highest loss (90%) in the content of aloin A was showed at 70°C within 6 h. The amount of aloin A decreased by 90% after treatment at 50°C within 12 h while at 30°C more than 50% of aloin A decreased within 1 day. At 4°C, aloin A was relatively stable for 1 day showing 10% of loss; however, within 14 days more than 60% were degraded. Light had no effects on the stability of aloin A during the experiment period (14 days). The effects of pH on aloin A stability were also evaluated. A series of pH values (2.0, 3.0, 5.0, 7.0 and 8.0) were applied to the degradation experiment. A significant decrease in stability was observed at higher pHs. Aloin A showed good stability at pH 2.0 for 14 days with only 6% loss. At pH 8.0, the content of aloin A decreased after 6 h to 93% and after 12 h only 2% of aloin A remained. In general, it has been demonstrated a remarkable decrease in aloin A content with the increase of pH values while acid condition ensured excellent stability of aloin A. The degradation products of aloin A were also analysed in this study by HPLC system. Aloe‐emodin, elgonica‐dimers A and B were found to be the major degradation products of aloin A at pH 5.0 or below. 10‐Hydroxyaloins A and B were formed under any condition except at pH 2.0 and 3.0, and they were mainly formed under high temperature and at any light conditions.
Pellizzoni et al. (2011) investigated the stability during storage at different conditions of anthraquinones and acetylated mannans (acemannans) in prepared and commercial gel and whole leaf homogenates from A. barbadensis and A. arborescens and in common household preparations from traditional medicine (Father Zago's recipe). The aloin content in the household preparation stored at +4°C resulted more stable (degradation time, DT50 = 108 days) than in all the whole leaf homogenates tested, including those with antimicrobial or antioxidant agents. Additionally, the samples at +4°C were more stable than those at room temperature. The aloin content in whole leaf‐based commercial products was stable for the whole 70 days of testing. In all cases, aloe‐emodin was detected in traces and sporadically, suggesting that it is not the direct oxidative or microbial degradation product of aloin. Generally, aloin and β‐polysaccharides showed poor stability in most of the conditions tested with the exception of samples prepared according to traditional medicine (Father Zago's recipe) that were found to be stable at 4°C. Concerning the colour of aloe gel, it changed slightly from whitish to brownish after 2 days of storage at room temperature. When aloe gel was stored at 4°C, the colour change took 7 days to occur. Stabilised gel commercial preparations stored at room temperature showed colour change after 22 days of storage. The whole leaf homogenate changed colour from dark green to light green in all samples analysed, while the colour of the whole leaf‐based commercial product and that of the household preparation remained stable when stored at 4°C.
The shelf‐life and colour change kinetics of Aloe vera gel powder packed in three different packaging materials has been evaluated by Ramachandra and Rao (2013): biaxially oriented polypropylene (BOPP), polypropylene (PP) and laminated aluminium foil (AF). The study demonstrated that during storage under accelerated storage conditions (38 ± 1°C, 90 ± 1% relative humidity), the predicted shelf‐life of dehumidified air‐dried aloe vera gel was 33.87, 42.58 and 51.05 days in BOPP, PP and AF pouches. The colour change of powder during storage followed first‐order reaction kinetics with a rate constant of 0.0444 per day for AF, 0.075 per day for BOPP and 0.0498 per day for PP. The results of this study suggested that AF is better suited than BOPP and PP for packaging of dried Aloe vera gel powder.
Vargas et al. (2002) demonstrated that ethanol solutions of aloe‐emodin, emodin and rhein are photolabile by visible light (390–500 nm) under aerobic conditions. The photolysis was followed by monitoring the changes in ultraviolet–visible (UV–Vis) and fluorescence spectra at regular interval of 20 min of irradiation. In particular, the results showed that photolability of the ethanol solutions of aloe‐emodin and emodin is higher than for rhein.
Gulia et al. (2010) studied the effects of convective drying on various parameters (yield, ash content, pH, crude fat, crude protein, crude fibre, wettability and water absorption capacity) of Aloe vera leaves powder. Aloe vera leaves were treated at different temperatures (50, 60, 70, 80°C) in hot air oven and powdered. No significant difference (p < 0.5) for tested physicochemical parameters was observed at different processing temperatures. The HPLC analysis demonstrated that aloin content was temperature‐dependent since aloin decreased from 10.6 to 1.7 ppm as the temperature increased from 50 to 80°C. The study showed that the aloe powder obtained at 70°C had better physicochemical and functional properties than the samples obtained at other different temperatures selected for the study.
Miranda et al. (2009) investigated the effects of temperature (50, 60, 70, 80 and 90°C) on a series of parameters using Aloe vera gel such as proximal composition, water activity (aw), pH, acidity, non‐enzymatic browning, surface colour, vitamin content (C and E) and mineral content. The stability of tested nutritional and functional quality parameters in rehydrated A. vera gel depended mostly on the temperature used during the drying process, with increased nutrient loss observed at 80 and 90°C. According to the authors, drying temperatures for A. vera gel between 60 and 70°C would ensure acceptable commercial quality with features as similar as possible to those of fresh product.
Goppel and Franz (2004) investigated the degradation pathways of known constituents of powdered Senna spp. leaves and commercial methanolic Senna spp. leaf extract depending on different storage conditions and packaging. The analysis was performed using HPLC‐PDA detection. In the crude plant material, sennosides were degraded to sennidine monoglycosides, while rhein 8‐O‐glucoside was hydrolysed to rhein by enzymatic processes. Degradation of the anthranoid compounds was not due to the same pathways in the investigated commercial extracts, where only unspecific alterations of all compounds were observed. Forced decomposition of tested preparations under high temperature caused oxidative decomposition of the sennosides to rhein‐8‐O‐glucoside. Furthermore, flavonoid glucosides decomposition was observed with an apparent increase in the content of flavone aglycones. According to the authors, this may due to the fact that industrial processes like alcoholic extraction denature enzymes needed for the degradation reactions.
Lainonen et al. (1988) investigated the effect of different storage conditions on the chemical stability of sennoside A and B in water solutions, measured by the HPLC method. The variables in storage conditions were pH, time and temperature (room temperature or 100°C). It has been demonstrated that the chemical stability of sennosides in aqueous solutions was low and pH‐dependent (best stability at pH 6.5 with t90% = 8.4 months and the poorest at pH 8.0 with t90% = 2.5 months) and temperature‐dependent (t50% = 2 h; t90% = 0.31 h after heating at 100°C under reflux. Note t50% and t90% is the time interval needed to decrease the content of testing substance to 50% and 90% of the starting concentration, respectively. t90% value is commonly used as an expiration time for drug products). There was also a clear difference in the disappearance rate between sennoside A and sennoside B. The initial ratio of sennoside A to sennoside B was 1.90 and the final ratio after 3.5 years was 1.66 at pH 5.5 and 2.42 at pH 8.0. The pharmacopoeial assay methods for sennoside in senna products based on colorimetric measurement was also performed and no decline in the drug content is observed during the 4.6 years of storage. The authors concluded that the transformation products also have light absorption at the same wavelength as sennosides.
Chewchinda et al. (2013) evaluated the effects of different storage conditions on the content of rhein in Cassia fistula pod pulp decoction extracts, using the HPLC method. Three batches of C. fistula pod pulp decoction extract were stored in glass vials and AF bags at the accelerated (40°C ± 2°C/75% RH ± 5% RH) and real‐time storage conditions (30°C ± 2°C /75% RH 5% RH) for 6 months. The rhein contents remained more than 95% when compared with the initial amount. Moreover, the finding showed no significant differences on the chemical stability of the extracts kept in glass vials and in aluminium foil bags.
2.6. Use and use levels
Food supplements
The EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), in its 2013 Scientific Opinion on the ‘substantiation of a health claim related to hydroxyanthracene derivatives and improvement of bowel function’, noted the established effects of a daily dose of 10 mg/day hydroxyanthracene derivatives per day on the short‐term alleviation of occasional constipation and the mechanism by which hydroxyanthracene derivatives exert an effect on bowel function (i.e. stimulation of colonic motility through the enhancement of colonic transit which reduces fluid absorption from the faecal mass and increases in the permeability across the colonic mucosa which results in an increase in the water content in the large intestine), either from the root and rhizome of Rheum palmatum L. and/or Rheum officinale Baillon and/or their hybrids, and/or from the leaves or fruits of Cassia senna L. and/or Cassia angustifolia Vahl, and/or from the bark of Rhamnus frangula L and/or from the bark of Rhamnus purshianus D.C. and/or from Aloe barbadensis Miller and/or various aloe species, mainly Aloe ferox Miller and its hybrids. The target population of these products is adults.
A request was sent to relevant food business operators, asking for information on the recommended doses of food supplements containing hydroxyanthracene derivatives marketed in the EU.
The Panel noted that the recommended daily doses when used as a food supplement are extremely variable, ranging from 2.25 to 24.83 mg/day for sennoside B (information retrieved for a total of 16 products; in 9 products, sennoside B was used as the unique ingredient); from 1.95 to 78.8 mg/day for rhein (information retrieved for a total of 37 products; in 24 products, rhein was used as the unique ingredient), from 13 to 26 mg/day for glucofrangulin A (information retrieved for a total of 2 products; in 1 product, frangulin A was used as the unique ingredient), from 1.2 to 24 mg/day for aloin (barbaloin) (information retrieved for a total of 4 products, where barbaloin was used as the unique ingredient), from 17.9 to 51 mg/day for aloin A+B (information retrieved for 1 product, where aloins A and B where used in combination with other hydroxyanthracene derivatives) (‘Documentation provided to EFSA’ No. 3, 4, 5, 6, 7).
Medicinal products
According to the EMA monographs, the maximum daily dosage should not exceed 30 mg of hydroxyanthracene derivatives in medicinal products used as a laxative for adults, elderly and adolescents over 12 years. According to the EMA monographs, the patient has to be informed that the correct individual dose is the smallest required producing a comfortable soft‐formed motion.
Normally, it is sufficient to take an anthranoid‐containing laxative up to two to three times a week. The use for more than 1–2 weeks requires medical supervision (Konsensuspapier expertenforum, 1999).
2.7. Exposure
2.7.1. Exposure via food supplements
Exposure to hydroxyanthracene derivatives from food supplements has been estimated from the recommended daily doses of food supplements as provided by the interested parties following the launch of a public ‘Call for data’ (see Section 2.6).
As suggested by the Scientific Committee of EFSA (EFSA Scientific Committee, 2012) a body weight of 70 kg has been used in this opinion as the default value to express the exposure results in mg/kg bw per day in adults.
Maximum daily exposure to hydroxyanthracene derivatives is reported below:
Exposure to sennoside B was estimated up to 0.35 mg/kg bw per day, corresponding to 24.83 mg/person per day for a 70‐kg adult.
Exposure to rhein was estimated up to 1.12 mg/kg bw per day, corresponding to 78.8 mg/person per day for a 70‐kg adult.
Exposure to glucofrangulin A was estimated up to 0.37 mg/kg bw per day, corresponding to 26 mg/person per day for a 70‐kg adult.
Exposure to barbaloin was estimated up to 0.34 mg/kg bw per day, corresponding to 24 mg/person per day for a 70‐kg adult.
Exposure to aloin A+B was estimated up to 0.72 mg/kg bw per day, corresponding to 51 mg/person per day for a 70‐kg adult.
The Panel noted that the recommended daily doses provided by the interested parties are extremely variable. In order to calculate the worst‐case scenario, the highest number in the range was used for the calculation. The Panel further noted that the concentrations of single hydroxyanthracene derivatives used in the food supplements vary also depending whether used alone or in combination with other hydroxyanthracene derivatives.
The Panel noted that exposure to aloe‐emodin and emodin is not known due to lack of data provided by the interested parties.
Exposure in young children was not performed since the use of food supplements containing hydroxyanthracene derivatives is not recommended in this population category.
2.7.2. Exposure via normal diet
The Panel noted that parts of plants containing hydroxyanthracene derivatives may be part of the normal diet. However, no data on the concentrations of hydroxyanthracene derivatives present in the parts of the plants consumed were made available to the Panel by interested parties following a call for data.
3. Biological and toxicological data
3.1. Absorption, distribution, metabolism and excretion (ADME)
EMA (2008a,b) reported several studies describing the biological fate of hydroxyanthracene derivatives and the metabolism and pharmacokinetics of anthranoid laxatives. According to this report, glycosidic hydroxyanthracenes such as barbaloins, sennosides are not absorbed in the upper gut. In humans, they pass into the colon unmodified after oral ingestion. Human intestinal flora is able to break down O‐glycosides easily but only to some extent C‐glycosides of most anthranoids. Aloe‐emodin‐9‐anthrone is the main active metabolite, which acts specifically on the colon. In comparison to anthrones, anthraquinones are absorbed to a much larger extent in the upper gut. After absorption, aglycones are distributed over the different tissues in the body and excreted in urine and bile as glucuronides and sulfates. The Panel noted that several studies, carried out on the biological fate of hydroxyanthracene derivatives and metabolism and pharmacokinetics of anthranoid laxatives, have been reported by EMA (2008a,b). However, the Panel further noted that many studies have been become available since then and are reported in this opinion.
3.1.1. Administration of preparations and extracts
Animal studies
The pharmacokinetics of rhein was determined in rats orally administered an oriental herbal medicine Onpi‐to that included 1.24% of rhein equivalents derived from sennoside A, sennoside B, rhein 8‐O‐glucopyranoside and rhein. Plasma, urinary and biliary (in bile duct cannulated animals) levels of rhein were determined. Following oral doses of 125, 250 and 500 mg Onpi‐to/kg bw, the plasma rhein levels peaked at 8.3–20.0 min. The area under the curve (AUC; 0–48 h) values were dose‐dependent. As determined 48 h after administration, cumulative urinary excretion of unconjugated and conjugated rhein was 3.14% and 38.21% of the dose, respectively. The cumulative biliary excretion of unconjugated rhein accounted for 1.34% of the dose (Takizawa et al., 2003).
Shia et al. (2011) investigated the pharmacokinetics and tissue distribution of anthraquinones following twice daily administration of 2.0 mL rhizome of Rheum palmatum decoction/kg bw to rats reaching a total of seven doses (0.5 g of rhizome/mL decoction). In this decoction, the most abundant anthraquinone (free and conjugated) was rhein (910 μg/mL, mainly in the free form) and the least was physcion (200 μg/mL). Serum and tissue specimens were assayed for contents of anthraquinones by HPLC before and after hydrolysis with β‐glucuronidase or sulfatase. In serum, rhein free form was present in all specimens, emodin free form existed only transiently in the early phase after dosing, whereas the free forms of aloe‐emodin, chrysophanol and physcion were not detected. Glucuronides/sulfates of aloe‐emodin, rhein, emodin and chrysophanol predominantly existed in bloodstream, while no trace of physcion glucuronides/sulfates were detected. The tissue contents of each anthraquinone free form ranged as follows: kidney > liver > lung for rhein (generally 10‐ to 15‐fold higher concentrations than other anthraquinones), kidney and liver > lung for aloe‐emodin; liver > lung for emodin; only traces of chrysophanol in kidney and liver. In investigated organs, the concentration of rhein free form in kidney was the highest (24 μg/g) and even much higher than serum concentration (14 μg/mL). Considering conjugate concentrations, no traces of them were found in liver. Only traces of rhein, aloe‐emodin, emodin sulfates were found in lung. Both glucuronides and sulfates of rhein, aloe‐emodin, emodin and chrysophanol were present in kidney, but in much lower concentrations. In brain, neither unconjugated forms nor their glucuronides/sulfates were detected.
The study of Zhang et al. (2013) compared the pharmacokinetics of aloe‐emodin, rhein and emodin in plasma of rats after oral administration (10 mL/kg bw) of a rhubarb extract (the concentrations were not reported). A liquid chromatography with mass spectrometry (LC–MS) method was developed and validated for the determination of the plasma concentrations of the three analytes. Following administration of rhubarb extract, area under the plasma concentration–time curve (AUC 0–12 h) values clearly indicated that rhein was the most abundant circulating hydroxyanthracene derivatives, by comparison to aloe‐emodin (77 times lower) and emodin (103 times lower).
A liquid chromatography with tandem mass spectrometry (LC–MS/MS) method was developed for simultaneous determination of aloe‐emodin, rhein, emodin, chrysophanol and physcion and their conjugates in plasma (Wu et al., 2014a). Rats (n = 6) received a rhubarb extract at a dose of 10.0 mL/kg (5.0 g extract/kg bw) by gavage. One gram of extract contained, respectively, 1.24, 3.16, 0.86, 2.85 and 2.30 mg rhein, chrysophanol, physcion, emodin and aloe‐emodin. The concentrations of the total circulating forms (free and respective conjugates) of each anthraquinone were measured. AUC (0–36 h) of total aloe‐emodin, emodin, chrysophanol and physcion in rat plasma were lower (by 11.3, 1.9, 4.7 and 6.1 times, respectively) than those of rhein (14.5 μM h). According to the authors, conjugates were the dominant in vivo circulating forms of hydroxyanthracene derivatives from rhubarb and rhein appeared to be the most abundant anthraquinone in rat plasma.
Overall, the Panel noted that in rats receiving rhubarb preparations or extracts, free and conjugated forms of aloe‐emodin, rhein, emodin and chrysophanol were detected in plasma, with rhein being by far the major circulating hydroxyanthracene. Among the organs, kidneys, liver and lungs contained the highest concentrations of free and conjugated rhein, aloe‐emodin, emodin and chrysophanol. Rhein was mainly excreted as free form in urine and as conjugates in bile.
Human studies
As reported in EMA (2008a,b), Krumbiegel and Schulz (1993) investigated the kinetics of rhein and aloe‐emodin from senna laxatives in man. Therapeutic doses of the two laxatives Agiolax (four single doses of 6.3 g granulate containing 13.23 mg anthranoids including 7.62 mg rhein and 0.38 mg aloe‐emodin) and Sennatin (two tablets containing 20.36 mg anthranoids including 13.06 mg rhein and 0.4 mg aloe‐emodin) were daily administered to 10 volunteers for 4 days. Rhein plasma concentrations showed the highest level of 156 ng/mL (after Agiolax) and peak maxima at 3–5 h and 10–11 h after dosing likely due to absorption of free rhein and then to rhein released from prodrugs (e.g. sennosides) by intestinal bacterial metabolism, respectively. Aloe‐emodin though co‐administered in measurable amounts was not detectable in any plasma sample.
Lee et al. (2003) identified the main active compounds of Rhei undulati Rhizoma and determined the pharmacokinetic parameters of anthraquinones absorbed in man. Each volunteer (9 men/3 women) was administered 100 mg of Rhei undulati Rhizoma extract/kg bw. The amounts of aloe‐emodin, rhein, emodin and chrysophanol in the extract were 1,179, 167, 2,257 and 8,494 μg/g of the dried herb, respectively. The anthraquinone levels in plasma were determined using TLC, HPLC and LC–MS analysis without previous deconjugative hydrolysis. Rhein was the only hydroxyanthracene derivative detected in plasma and its elimination half‐life was estimated as 3.38 h.
Overall, in humans receiving senna laxatives or rhubarb extract, rhein was the main hydroxyanthracene detectable in plasma. Rhein was also observed in volunteers treated with a rhubarb extract containing 7‐ to 50‐fold higher concentrations of aloe‐emodin, emodin and chrysophanol than that of rhein.
3.1.2. Administration of aglycone hydroxyanthracenes
Rhein
Absorption
As reported in EMA (2008a,b), animal experiments using 14C‐rhein or 14C‐rhein anthrone administered into the caecum of rats demonstrated absorption lower than 10% of the dose (De Witte and Lemli, 1988a,b).
The pharmacokinetics of 14C‐rhein was determined after intravenous (0.16 mg/kg bw), oral or intracaecal administration (0.78 mg/kg bw) to male rats (Lang, 1988). Radioactivity from 14C‐rhein was absorbed after oral or intracaecal administration by about 50–60%. In most organs, the radioactivity was lower than in plasma with very low levels in testes and brain, the highest values were found in the kidneys. In bile, there were mainly two conjugated metabolites of rhein, whereas in urine larger amounts of rhein and one or two additional metabolites (conjugates) were detected.
Sund and Elvegård (1988) investigated the intestinal absorption and metabolism of rhein. Everted sacs of rat jejunum and colon were filled with Krebs–Henseleit solution on the serosal side, and bathed at the mucosal side with the same solution containing either danthron or rhein. After 60 min, incubation at 37°C, solutions and gut tissues were analysed by HPLC for parent rhein and metabolites. Only small amounts of unchanged drug were present on the serosal side of jejunum and colon while in both tissues, danthron was present as glucuronide or sulfate conjugates. In jejunum, conjugates were mainly secreted into the lumen. In the colon, glucuronoconjugates were absorbed and remained in the tissue, whereas sulfoconjugates were secreted into the lumen. Rhein was more slowly taken up and metabolised, but seemed otherwise to behave as danthron.
Distribution
The in vitro interactions of serum albumins such as human serum albumin (HSA) and bovine serum albumin (BSA) with emodin, rhein, aloe‐emodin and aloin were assessed using fluorescence quenching and absorption spectroscopic techniques (Bi et al., 2005). The results obtained revealed that there were strong binding affinities of these anthraquinones to HSA and BSA.
Metabolism
Dahms et al. (1997) elucidated the oxidative and conjugative metabolism of 14C‐labelled or ‐unlabelled rhein, using in vitro and in vivo studies in dogs, rabbits, rats and human volunteers. In vitro experiments with subcellular liver fractions of rats and rabbits revealed the presence of three monohydroxylated metabolites of rhein, a bishydroxylated derivative of rhein and quinoid metabolites. HPLC–MS/MS analysis of urine samples of all investigated species detected the hydroxylated metabolites as glucuronides. Two isomeric phenolic glucuronides and sulfates or glucosides of rhein were found as major conjugates in urine of all species. Furthermore, acyl glucuronides of rhein and monohydroxylated rhein were identified in human urine whilst two phenolic glucosides were found in dog and human urines.
The in vitro glucuronidation of purified rhubarb anthraquinones (aloe‐emodin, emodin, chrysophanol, physcion, rhein) was investigated by using liver and intestinal microsomes from rats and humans and human recombinant uridine 5′‐diphospho‐glucuronosyltransferases (UDP‐glucuronosyltransferases) (Wu et al., 2014b). All anthraquinones formed monoglucuronides. Glucuronidation showed an absolute preference towards β‐OH, followed by α‐OH and β‐alcoholic OH (aloe‐emodin) while the activity was decreased greatly with a β‐COOH (rhein). Only slight differences between organs and species were observed in the overall glucuronidation activity and regioselectivity of anthraquinones tested. Human recombinant UGT1A9 (UGT=UDP‐glucuronosyltransferase) showed the highest activity and similar regioselectivity to that of human liver microsomes while UGT2B7 and UGT2B15 isoforms showed high activity towards β‐OH.
Song et al. (2009) compared the metabolism of rhein, emodin, aloe‐emodin, chrysophanol and physcion in rat liver microsomes. Different metabolic reactions for anthraquinones were dependent on the substituent group in the skeleton. Hydrogenation and methyl substitution on the benzene ring (e.g. to produce 2‐methylrhein) were the major metabolic reactions for rhein.
Regarding the metabolism by bacteria of rhein, De Witte et al. (1992) observed that in caecal contents of rats, rhein was reduced into rhein anthrone, by 23.5%. The in vitro reduction capacity of the caecal content of conventional rats was drastically decreased (to 0.2% and 5.2%) by oral administration to rats of antibiotics. According to the authors, rhein is reduced in caecal content to the highly reactive and labile rhein anthrone, accounting for the in vivo disappearance of dihydroxy‐anthranoid equivalents in routine analysis after oral administration of anthraquinones to animals.
Excretion
As above mentioned in pharmacokinetic studies (De Witte and Lemli, 1988a,b; Lang, 1988), orally administered 14C‐rhein was mainly excreted in urine and bile as radiolabelled conjugates. After intracaecal administration, 14C‐rhein was recovered as conjugates in faeces and urine (53% and 37% of the dose, respectively).
The transfer of rhein in milk was investigated in the rhesus monkey by Cameron et al. (1988). An HPLC method was developed to measure rhein in milk and plasma. Samples from two lactating rhesus monkeys were taken over 48 h after oral administration of sennosides (1 mg/kg bw). Detectable rhein levels were found in plasma (25–144 ng/mL) between 2 and 12 h and in milk (225–829 ng/mL) between 4 and 12 h after administration. However, rhein excretion in milk seems to be far below the dose necessary to elicit a laxative effect in the suckling offspring.
EMA (2008a,b) reported the study of Faber and Strenge‐Hesse (1988) investigating the excretion of rhein into human breast milk. Twenty post‐partum women received daily doses of 5 g of a standardised senna laxative (Agiolax containing 15 mg sennosides) for 3 days. The concentration of rhein in milk samples from every lactation during 24 h post‐dose varied between 0 and 27 ng/mL with values below 10 ng/mL in 94%. Based on median values, 0.007% of the dose of sennoside would be excreted in breast milk. None of the breast‐fed infants had an abnormal stool consistency. According to the authors, assuming a complete conversion of sennosides to rhein in the mother, the amount of rhein delivered to the infant is by the factor 10−3 below the rhein intake of the mother.
Overall, when administered orally to animals, rhein may be reduced to rhein‐anthrone in the large intestine. Around 50–60% of the dose of rhein is absorbed intact and then conjugated in intestine and liver.
Emodin
Absorption, distribution and excretion
Absorption, tissue distribution, metabolism and excretion of 14C‐emodin were studied after a single oral administration (approx. 50 mg/kg) to rats (Bachmann and Schlatter, 1981). Radioactivity in most organs decreased significantly between 3 and 5 days. In kidneys, however, the 14C activity was still equivalent to 4.33 mg/kg emodin after 5 days. Urinary excretion amounted to 18% and 22% of the dose in 24 and 72 h, respectively. Metabolites in pooled urine (0–72 h) were mostly free emodin and emodic acid (16% dose). In faeces, 48% and 68% dose was excreted after 24 and 120 h, mostly as free anthraquinone form. In two bile duct cannulated rats, biliary excretion amounted to 49% of the dose within 15 h; 70% of the biliary radioactivity was in the form of conjugated emodin.
Intravenous or oral administration (10 mg/kg bw) of emodin to rabbits resulted in a serum profile described by a two‐compartment model (Liang et al., 1995). The elimination half‐life was 227 min. Oral administration of emodin resulted in negligible serum concentration. The authors considered the bioavailability of emodin very low. Emodin was found to be highly bound (99.6%) to serum protein.
Metabolism
Song et al. (2009) compared the metabolism of five anthraquinone derivatives from rhubarb (rhein, emodin, aloe‐emodin, chrysophanol and physcion) by using rat liver microsomes. Monohydroxylation in the exocyclic methyl group was the major metabolic modification for emodin.
Liu et al. (2010) determined the metabolism of emodin by using in vitro and in situ disposition models of the intestine and liver. Liver microsomes (mice, rats, guinea pigs, dogs and humans), rat intestinal microsomes and a rat intestinal perfusion model were used. A mixed system of oxidation and glucuronidation reaction was used to determine the main pathway of metabolism of emodin by using male rat liver microsomes with both oxidation and glucuronidation reaction cofactors. Emodin glucuronidation in liver microsomes was species‐dependent. In the rat intestine, excretion rates of emodin‐3‐O‐glucuronide were significantly higher in duodenum and jejunum than in ileum and colon. According to the authors, emodin was considered rapidly glucuronidated and this would be the major reason of its poor bioavailability. Glucuronidation rates obtained using liver microsomes from various experimental animals correlated well with those in human liver microsomes.
As above mentioned, the glucuronidation of aloe‐emodin, emodin, chrysophanol, physcion, rhein was investigated in liver and intestinal microsomes from rats and humans (Wu et al., 2014b). Emodin was rapidly glucuronidated, with an absolute positional preference at the β‐OH, leading to a β‐glucuronide accounting for > 95% of three glucuronides formed in rat and human microsomes.
Overall, the bioavailability of emodin is low, probably because of a rapid glucuronidation occurring in both intestinal wall and liver.
Aloe‐emodin
The intestinal uptake and metabolism of aloe components were determined by using in vitro intestinal absorption models (Park et al., 2009). Caco‐2 cell and rat everted gut sac were incubated with aloe‐emodin ranging from 5 to 50 μM. As quantified using HPLC, the percentage of absorption of aloe‐emodin ranged from 6.60 to 11.32%. Up to 18–38% of aloe‐emodin was absorbed as glucuronidated or sulfated form. The absorption rates were generally similar in the two models. These results would suggest that a significant amount is conjugated during the absorption process.
As reported in EMA report (2008), Lang (1993) investigated the general metabolism of aloe‐emodin in rats orally administered with 4.5 mg 14C‐aloe‐emodin/kg bw. As evaluated by TLC in plasma collected 1.5, 3 and 6 h post‐administration, only 4.5% of the radioactivity was identified as free aloe‐emodin with maximum plasma values reached at 1.5–3 h following the administration. Free rhein and various conjugates represented 21.6% and 72.3% of the radioactivity. Half‐life of the radioactivity in blood was about 50 h. Maximum concentrations of radioactivity in plasma were about three times higher than those in ovaries and 10 times higher than those in testes, whereas liver, kidney and intestinal tract showed higher concentrations than plasma. Twenty to thirty per cent of the administered radioactivity was excreted in urine and the rest in faeces. According to the authors, following oral administration, aloe‐emodin is quickly oxidised to rhein and further conjugated.
Song et al. (2009) compared the metabolism of five anthraquinone derivatives from rhubarb (rhein, emodin, aloe‐emodin, chrysophanol and physcion) by using rat liver microsomes. Different metabolic reactions for anthraquinone derivatives were dependent on the substituent group in the skeleton. Aloe‐emodin was mainly metabolised through a methylation reaction.
The pharmacokinetics and metabolism of aloe‐emodin were investigated in intravenously (5.0 mg/kg bw) or orally (40 mg/kg bw) administered rats (Yu, 2016). The serum concentrations of aloe‐emodin and its metabolite rhein were determined by the HPLC method prior to and after hydrolysis with β‐glucuronidase and sulfatase/β‐glucuronidase. When aloe‐emodin was given intravenously, the major molecules in blood were aloe‐emodin glucuronides and rhein sulfates. When aloe‐emodin was administered orally, only traces of aloe‐emodin (below limit of quantification (LOQ)) were detected in the serum at the very early phase, whereas aloe‐emodin glucuronides and rhein glucuronides were the major circulating molecules. The authors concluded that aloe‐emodin was not absorbed per se and the Panel agreed with this conclusion.
As mentioned above, the glucuronidation of rhubarb anthraquinones (aloe‐emodin, emodin, chrysophanol, physcion and rhein) was investigated in liver and intestinal microsomes from rats and humans (Wu et al., 2014b). In the case of aloe‐emodin, the presence of a β‐alcoholic OH group leads to a unique glucuronidation occurring at the α‐OH.
Overall, following oral absorption, aloe‐emodin is quickly oxidised to rhein and further conjugated. In plasma, only traces of free aloe‐emodin were detected whereas aloe‐emodin glucuronides and rhein glucuronides were the major circulating molecules.
3.1.3. Administration of glycosidic hydroxyanthracenes
As mentioned by EMA (2008a,b) and according to Witte and Lemli (1990), anthrone‐glycosides remain intact to the large intestine, where aglycones are released by bacterial hydrolysis of the sugar. The intestinal bacterial flora also accounts for the reduction of anthraquinone aglycones to the corresponding anthrones. After absorption, anthranoids are mainly conjugated into glucuronide and sulfate derivatives, which appear in urine and bile.
Sennosides
The glycosidic sennosides are not absorbed, only the β‐glycosidase of the bacteria of the large intestine is able to hydrolyse them to sennidins. These sennidins are further cleaved to the active metabolite (rhein anthrone) by the bacteria. Aglycones are absorbed in the upper gut and glucuronidated in the liver. Excretion of sennosides and their known metabolites is mainly via the faeces up to 92.8%. In experimental animal studies, up to 6% of orally administered doses of sennosides anthranoids could be found intact in the urine and faeces (EMA, 2008a,b).
Absorption
Using the Caco‐2 human colonic cell line as an in vitro model, the bioavailability of dianthrones (sennosides A and B and their aglycones sennidins A and B) was studied in apical to basolateral (absorptive) and basolateral to apical (secretive) directions (Waltenberger et al., 2008). The absorption in apical to basolateral direction was poor, whereas the transport was higher in the secretory direction, indicating a significant efflux of the (poorly) absorbed compounds in the intestinal lumen.
Metabolism
Hattori et al. (1988) isolated microbial enzymes from Peptostreptococcus intermedius that catalysed the electron transfer from NADH to FAD, FMN or benzyl viologen, which reduced sennosides and sennidins to 8‐glucosyl‐rhein anthrone and rhein anthrone, respectively.
Aloins
As reported above (section on aloe‐emodin), Park et al. (2009) determined the intestinal uptake and metabolism of aloe components including aloin using two in vitro intestinal absorption models. The percentage of absorption of aloin ranged from 5.51% to 6.60%, with 18% of this was present as glucuronidated or sulfated metabolites. The absorption rate of aloin was generally similar in the two models.
A strictly anaerobic bacterium, Eubacterium sp. BAR, was isolated by Che et al. (1991) from human faeces as one of the intestinal bacteria capable of metabolising aloin (barbaloin) to aloe‐emodin anthrone).
Overall, glycosidic hydroxyanthracenes are negligibly absorbed intact. Glycosidic hydroxyanthracenes are hydrolysed in the gastrointestinal (GI) tract into the corresponding aglycones which are absorbed and subject to conjugation in the intestine and liver and excreted in bile and urine.
3.1.4. Conclusion on ADME
Glycosidic hydroxyanthracene derivatives remain intact and until they are hydrolysed in the GI tract to their corresponding anthrones (aglycone anthrones). Regarding aglycone hydroxyanthracenes, as demonstrated in animals, may be absorbed intact, however only rhein is present in the systemic circulation.
In the GI tract, remaining hydroxyanthracenes may be reduced back to the corresponding anthrones by the microbiota.
After absorption, hydroxyanthracenes such as aloe‐emodin are rapidly and totally oxidised to rhein. In the gut epithelium and liver, absorbed aglycone hydroxyanthracenes are conjugated into corresponding glucuronides and sulfates, which are excreted in bile or urine. An overview of the metabolism of aglycone and glycosidic hydroxyanthracenes is reported in Figure 2.
Figure 2.
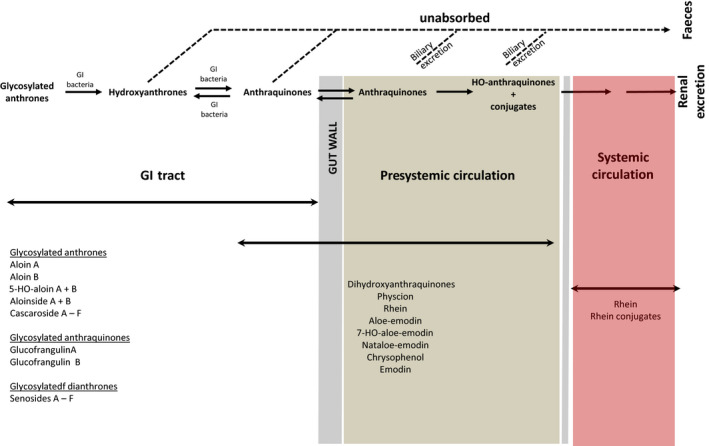
Metabolism of aglycone and glycosidic hydroxyanthracenes
The Panel considered that animal models in general reflect the fate of hydroxyanthracenes in humans.
3.2. Toxicological data
3.2.1. Genotoxicity
In vitro studies
Aloe vera extracts and/or gels
In the study by Morimoto et al. (1982), crude extracts of Aloe ferox Mill. in water or methanol were assessed for their genotoxicity in a bacterial reverse mutation test (Ames test) using the Salmonella Typhimurium tester strains TA98 and TA100 and in the Bacillus subtilis rec‐assay (DNA repair test) using the mutant strain M45 rec‐ (unable to repair DNA damage) and the wild‐type strain H17 rec+ as control at dose levels of 10 mg/plate and 6 mg/disk, respectively. The Ames test was performed both in the absence and presence of an exogenous rat liver S9 metabolism. The results obtained indicated that only water extracts of Aloe ferox Mill. induced a genotoxic effect in the Bacillus subtilis rec‐assay. However, the Panel noted that the set of S. Typhimurium tester strains was insufficient to detect potential genotoxins. On this basis, the Ames test performed is of limited relevance for risk assessment of water or methanol Aloe ferox Mill extracts.
Extracts from the Aloe vera leaf pulp (3 g in 5 mL physiological solution) were not able to significantly decrease survival of wild‐type Escherichia coli or defective repair mutants (recA, xthA, uvrA, wild‐type/rfa, uvrA/rfa) tester strains both in the absence and presence of UV‐A radiation. However, DNA single strand breaks were observed in the pUC 9.1 plasmid when incubated with 3, 30 and 300 μg/mL aloe vera leaf pulp extract (Paes‐Leme et al., 2005). The Panel noted that chemical characterisation of the extracts was not performed and that the assays employed are not validated and are not included in the current OECD guidelines for genotoxicity testing.
In the study by Sehgal et al. (2013), the genotoxicity of a commercial decolourised WLE that contained approximately aloin A at 0.9 ppm, aloin B at 1.3 ppm and aloe‐emodin at 0.2 ppm was evaluated in the S. Typhimurium reverse mutation assays with tester strains TA98 and TA100 and E. coli SOS DNA damage‐repair assay both in the absence and presence of S9 metabolic activation. Concentrations applied ranged from 21‐fold concentrated extract to 20‐fold diluted and negative results were observed in both assays. However, the Panel noted that both studies are of limited relevance since in the reverse mutation assay only two S. Typhimurium tester strains were used and that SOS DNA damage‐repair assay is not currently used for genetic toxicology testing.
In the study by Williams et al. (2010), a tan powder derived from mucilaginous parenchymal cells found in the inner central area of the Aloe vera (L.) leafs manufactured using a proprietary extraction process in which the aloe latex contaminant (containing anthraquinones) was substantially reduced (including aloin, aloeresin A) and referred to as Qmatrix®, was assessed for its genotoxicity in a bacterial reverse mutation test (Ames test) using the S. Typhimurium TA98, TA100, TA1535, TA1537 and the E. coli WP2 uvrA tester strains and in a chromosomal aberration assay in Chinese hamster lung (CHL) cells. Both studies were performed in the absence and presence of rat liver S9 metabolic activation. Concentrations of 185, 556, 1,667, 5,000 and 10,000 μg/plate and 2,500, 5,000 and 10,000 μg/mL were used in the two assays, respectively, and negative results were observed. However, the Panel noted that the outcome of these studies is only relevant for Aloe vera extracts deprived of anthranoid compounds.
In the study by Boudreau et al. (2013), two different samples of Aloe vera gel were tested over a dose range of 100–10,000 μg/plate in S. Typhimurium strains TA97, TA98, TA100 and TA1535, with and without 10% or 30% induced rat or hamster liver S9 mix and up to the limit dose of 6,000 μg/plate in S. Typhimurium strains TA98 and TA100, or E. coli strain WP2 uvrA/pKM101, with or without 10% induced rat liver S9, respectively, and no evidence of mutagenicity was seen. Similarly, aloe vera WLE (native) and aloe vera charcoal‐filtered whole leaf extract (decolourised) were assayed for mutagenicity up to concentrations of 6,000 μg/plate in S. Typhimurium strains TA98 and TA100, and E. coli strain WP2 uvrA/pKM101, with and without 10% induced rat liver S9; no evidence of mutagenicity was observed in any of the three strains, with or without S9.
In the study by Guo (2014), the mutagenicity of the aloe vera WLE and aloe vera WLD was investigated using the mouse lymphoma assay. The intracellular reactive oxygen species (ROS) levels in the mouse lymphoma cells treated with the extracts were evaluated. In order to identify the mode of action for mutation induction, the loss of heterozygosity (LOH) in induced mutants was also evaluated. The objective of this investigation aimed to provide insights on the identity and mechanisms of potential mutagenic components of the aloe vera extracts, since in a 2‐year cancer bioassay in rodents with a preparation of aloe vera WLE administered in drinking water showed clear evidence of carcinogenic activity. The WLD extract was obtained through activated carbon adsorption to remove the phenolic components of aloe vera WLE such as anthraquinone C‐glycosides, anthrones and free anthraquinones. HPLC analysis revealed that the major difference between WLE and WLD was the content of anthraquinones and anthrones. WLE was reported to contain approximately 14–15 mg/g aloin A, the principal anthraquinone in aloe latex, while WLD contained approximately 0.01–0.05 mg/g of it. Both WLE and WLD extracts showed dose‐related cytotoxic and biologically relevant mutagenic effects in mouse lymphoma cells after treatment for 24 h in the absence of S9 metabolism, but in different concentration ranges with WLE showing a positive response at lower concentrations than WLD.
The concentration at which WLD induced significant increases in mutation frequency compared to the concurrent negative control was less than twice that required for WLE‐induced mutagenicity. Since the anthraquinone component was reduced by 99% relative to WLE, this indicates that other components in the mixtures, in addition to hydroxyanthracene derivatives, have a mutagenic capability (at least, in the near complete absence of hydroxyanthracene derivatives) This conclusion is further substantiated by the molecular analysis of induced mutations by assessing the LOH involving the TK and the D11Mit42 loci which indicate that, while both extracts were clastogenic, their induced mutation spectra were significantly different, thus confirming that different constituents may be responsible for the genetic damage caused by the two preparations. In this respect, the intracellular ROS levels were also determined using 2′,7′‐dichlorodihydrofluorescein diacetate (DCF‐DA) staining. After 4‐h exposure, the ROS levels of WLE and WLD groups were increased about 5‐ and 15‐fold, respectively, when compared to the concurrent control groups, thus indicating that WLD generated much more ROS than WLE.
The authors concluded that components other than the hydroxyanthracene derivatives (e.g. pro‐oxidant) were contributing to the genotoxicity of Aloe vera extract in vitro.
Senna extracts
In the study by Morimoto et al. (1982), crude extracts from senna (Cassia angustifolia Vahl) in water or methanol were assessed for their genotoxicity in a bacterial reverse mutation test (Ames test) using the S. Typhimurium TA98 and TA100 and in the Bacillus subtilis rec‐assay (DNA repair test) using the mutant strain M45 rec‐ (unable to repair DNA damage) and the wild type strain H17 rec+ as control at dose levels of 5 mg/plate and 6 mg/disk, respectively. The Ames test was performed both in the absence and presence of an exogenous rat liver S9 metabolism. The results obtained indicated that methanol senna extracts induced DNA damage in the Bacillus subtilis rec‐assay and were mutagenic in the S. Typhimurium TA98 tester strain in the presence of S9 metabolism. The Panel noted that, despite the set of S. Typhimurium tester strains was limited a genotoxic effect was anyway detected. The Panel also noted that the Bacillus subtilis rec‐assay is not adequately validated and not currently used in genotoxicity testing.
In the study by Heidemann et al. (1993), senna extracts (10.1% content of hydroxyanthracene derivatives) were tested for their capability to induce gene mutation in a bacterial reverse mutation test (Ames test) using the S. Typhimurium TA98, TA100, TA1535, TA1537 and the E. coli WP2 uvrA tester strains test and in the mammalian cell hypoxanthine‐guanine phosphoribosyl transferase (HPRT) mutation assay using V79 cells. Induction of chromosomal aberrations in Chinese hamster ovary (CHO) cells was also assessed. All assays were performed both in the absence and presence of S9 metabolic activation. In the bacterial mutation test and mammalian cell HPRT mutation assay, treatments were performed up to concentration of 5,000 μg/plate and 5,000 μg/mL, respectively. In the chromosome aberration assay, no indication about dose level employed was reported. However, according to the authors, the studies were performed according to the relevant OECD TG guideline no 471, no. 476 and no. 473, respectively, valid at the time and according to the Good Laboratory Practice (GLP) requirements. The results obtained indicated that senna extract was positive for the induction of gene mutation in S. Typhimurium TA1537 tester strain in the absence of S9 metabolism and in tester strain TA98 in the absence and presence of S9 metabolism. The induction of gene mutation was dose‐dependent. In the V79 gene mutation, HPRT assay negative results were observed in the absence of S9 metabolism up to cytotoxic concentrations while equivocal results were observed in its presence since some mutants were outside the historical control range values but only at cytotoxic concentrations. In the chromosome aberration test, positive results for the induction of structural chromosome aberrations were observed both in the absence and presence of S9 metabolism. In the first case, the increases were also dose‐dependent, and in the latter, the increases were only observed at the highest concentration.
Emodin
In the study by Mueller et al. (1996), emodin was tested for its capability to induce gene mutation and micronuclei in the mouse lymphoma L5178Y cells locus TK+/−. In both cases, treatment with emodin was performed for 4 h with dose levels of 37, 55.5, 74 and 111 μM in the absence of S9 metabolic activation and post‐treatment cells were collected at appropriate sampling times. Results obtained indicated that emodin induced dose‐related increases of gene mutation frequency and micronuclei compared to the concurrent negative controls. Although the authors did not perform statistical evaluation of data, the Panel noted that increases observed over concurrent negative controls for both gene mutation and micronuclei were sufficiently high to conclude for a genotoxic outcome. For the induction of micronuclei, the authors further analysed the presence of stainable kinetochore protein (CREST serum) in order to distinguish between clastogenicity and aneugenicity mode of action. The results obtained indicated that the majority of emodin‐induced micronuclei did not provide kinetochore signals (94%) indicating that the compound acted as a clastogen. The Panel agreed with this conclusion. Furthermore, based on the assumption that the genotoxicity of emodin might have been related to its ability to intercalate DNA like many known DNA topoisomerase II inhibitors, the authors evaluated this hypothesis, accordingly, in a cell‐free decatenation assay and in the comet assay in mouse lymphoma L5178Y cells. The results obtained indicated that emodin inhibited catalytically DNA topoisomerase II rather than poisoning them through the formation of the ‘cleavable complex’. The Panel agreed also with this conclusion.
In the study by Chen et al. (2010), emodin was evaluated for its capability to induce DNA damage by comet assay in human tongue cancer (SCC‐4) cells following treatments at dose levels of 20, 30 and 40 μM for 24 h, in the absence of S9 metabolic activation only. The authors showed significant increases in DNA fragmentation at the two higher dose levels (30 and 40 μM). However, the Panel noted that the study bears shortcomings which include the use of a long treatment (24 h) only and, a marked reduction of cell viability and consequent elevated cytotoxicity linked to excessive DNA damage that could have interfered with Comet analysis.
Aloe‐emodin
In the study by Heidemann et al. (1996), aloe‐emodin was assessed for its genotoxicity in a bacterial reverse mutation test (Ames test) using the S. Typhimurium TA98, TA100, TA1535, TA1537 and the E. coli WP2 uvrA tester strains test and in the mammalian cell HPRT mutation assay using V79 cells. Induction of chromosomal aberrations in CHO cells was also assessed. All assays were performed in the absence and presence of S9 metabolic activation. In the bacterial mutation assay, no indication about dose levels employed was reported. However, according to the authors, the studies were compliant with the relevant OECD TG guideline no 471, no. 476 and no. 473, respectively, valid at the time and the GLP requirements. The results obtained indicated that aloe‐emodin was positive for the induction of gene mutation, in three independent experiments, in S. Typhimurium TA1537, TA1538 and TA98 tester strains in the absence of S9 metabolism and in tester strain TA1538 in its presence. However, in the mammalian cell HPRT mutation assay in V79 cells, no induction of gene mutation was observed. In one independent experiment, at an intermediate dose level both in the absence and presence of S9 metabolic activation slight increases in the number of mutants compared to the concurrent negative controls. These increases, which were not reproduced in two additional independent experiments, were considered by the authors as not biologically relevant. The Panel agreed with this conclusion. In the chromosome aberration test, positive results for the induction of structural chromosome aberrations were observed both in the absence and presence of S9 metabolism at dose levels of 18.7–75.0 and 37.5–75.0 μg/mL, respectively, where the increases were also dose‐dependent.
In the study by Mueller et al. (1996), aloe‐emodin was tested for its capability to induce gene mutation and micronuclei in the mouse lymphoma L5178Y cells locus TK+/‐. In both cases, treatment with aloe‐emodin was performed for 4 h with dose levels of 37, 55.5, 74 and 111 μM in the absence of S9 metabolic activation and post‐treatment cells were collected at appropriate sampling times. Results obtained indicated that emodin induced dose‐related increases of gene mutation frequency and micronuclei compared to the concurrent negative controls. Although the authors did not perform statistical evaluation of data, the Panel noted that increases observed over concurrent negative controls for both gene mutation and micronuclei were sufficiently high to conclude for a genotoxic outcome. For the induction of micronuclei, the authors further analysed the presence of stainable kinetochore protein (CREST serum) in order to distinguish between clastogenicity and aneugenicity mode of action. The results obtained indicated that the majority of aloe‐emodin‐induced micronuclei did not provide kinetochore signals (94%) indicating that the compound acted as a clastogen. The Panel agreed with this conclusion. Furthermore, based on the assumption that the genotoxicity of aloe‐emodin might have been related to its ability to intercalate DNA like many known DNA topoisomerase II inhibitors, the authors evaluated this hypothesis, accordingly, in a cell‐free decatenation assay and in the comet assay in mouse lymphoma L5178Y cells. The results obtained indicated that aloe‐emodin inhibited catalytically DNA topoisomerase II rather than poisoning them through the formation of the ‘cleavable complex’. The Panel agreed also with this conclusion.
In the study by Chen et al. (2010), aloe‐emodin was evaluated for its capability to induce DNA damage by comet assay in human tongue cancer (SCC‐4) cells following treatments with aloe‐emodin at dose levels 25, 50 and 100 μM for 24 h in the absence of S9 metabolic activation only. The authors showed significant increases in DNA fragmentation at the higher dose level (100 μM) only. However, the Panel noted that the study bears significant shortcomings which include an inappropriate long treatment (24 h), a marked reduction of cell viability and consequent elevated cytotoxicity linked to excessive DNA damage that could have interfered with Comet analysis at higher dose levels. In addition, the ‘comet length’ used to measure the extent of DNA fragmentation in this study is usually not recommended (OECD TG. 489). On this basis, the results obtained cannot be considered reliable.
Danthron
Danthron was reported to be mutagenic to the S. Typhimurium frameshift strain TA1537, with and without metabolic activation (Brown and Brown, 1976; Liberman et al., 1982; as reported in IARC Monograph vol. 50, 1990). In the presence of exogenous metabolic activation, it was also mutagenic to the S. Typhimurium base‐pair substitution detecting strains TA2627 (Tikkanen et al., 1983), TA102 (Levin et al., 1984) and TA104 (Chesis et al., 1984) (as reported in IARC, 1990).
In the study by Mueller et al. (1996), danthron was tested for its capability to induce gene mutation and micronuclei in the mouse lymphoma L5178Y cells locus TK+/−. In both cases, treatment with danthron was performed for 4 h with dose levels of 21, 42, 63, 84 and 111 μM in the absence of S9 metabolic activation and post‐treatment cells were collected at appropriate sampling times. Results obtained indicated that emodin induced dose‐related increases of gene mutation frequency and micronuclei compared to the concurrent negative controls. Although the authors did not perform statistical evaluation of data, the Panel noted that increases observed over concurrent negative controls for both gene mutation and micronuclei were sufficiently high to conclude for a genotoxic outcome. For the induction of micronuclei, the authors further analysed the presence of stainable kinetochore protein (CREST serum) in order to distinguish between clastogenic and aneugenic modes of action. The results obtained indicated that the majority of danthron‐induced micronuclei did not provide kinetochore signals (94%) indicating that the compound acted as a clastogen. The Panel agreed with this conclusion. Furthermore, based on the assumption that the genotoxicity of danthron might have been related to its ability to intercalate DNA like many known DNA topoisomerase II inhibitors, the authors evaluated this hypothesis, accordingly, in a cell‐free decatenation assay and in the comet assay in mouse lymphoma L5178Y cells. The results obtained indicated that danthron inhibited catalytically DNA topoisomerase II rather than poisoning them through the formation of the ‘cleavable complex’. The Panel agreed also with this conclusion.
The in vitro genotoxicity of danthron was further investigated by Zhang et al. (2011). In this study, danthron was tested in an Ames test, a cytokinesis‐block micronucleus assay and a comet assay in Balb/c 3T3 cells. In the Ames test, danthron was only mutagenic towards strain TA102, specifically sensitive to oxidative mutagens, in the presence of exogenous metabolic activation system (S9 mix). When NADP was substituted with NAD in the S9 mix, the mutagenicity towards TA102 was unaffected. The addition of dicumarol, a DT‐diaphorase inhibitor, significantly suppressed the mutagenicity of danthron in TA102, indicating the involvement of the quinones dehydrogenase DT‐diaphorase in its metabolic activation. In Balb/c 3T3 cells, danthron increased both the frequency of micronucleated cells and comet tail length and Olive tail moment in a dose‐dependent manner in the dose range 25–100 μg/mL, with and without S9. The authors concluded that 3T3 cells were capable of endogenous activation of danthron. The increases in ROS and in 8‐hydroxydeoxyguanosine levels and the reduction in glutathione (GSH) levels in 3T3 cells were dose dependent. According to the authors, these results indicate that oxidative stress may be a major contributing pathway in the genotoxicity of danthron.
Overall, the available experimental data indicate that danthron is genotoxic through multiple mechanisms, such as DNA topoisomerase II poisoning, intercalation into DNA and ROS generation.
Rhein
In the study by Heidemann et al. (1993) rhein (stated purity 98.5‐99.7%) was tested for its capability to induce gene mutation in a bacterial reverse mutation test (Ames test) using the S. Typhimurium TA98, TA100, TA1535, TA1537 and the E. coli WP2 uvrA tester strains test and in the mammalian cell TK+/− mutation assay using the mouse lymphoma L5178Y cells. Induction of chromosomal aberrations in Chinese hamster ovary (CHO) cells was also assessed. All assays were performed both in the absence and presence of S9 metabolic activation. In the bacterial mutation test, treatments were performed up to concentration of 5,000 μg/plate while in the chromosomal aberration up to the limit of solubility, but no dose levels are reported as in the mammalian cell TK+/‐ mutation assay. However, according to the authors, the studies were performed in compliance with the relevant OECD TG guideline no 471, no. 476 and no. 473, respectively, valid at the time and according to GLP requirements. The results obtained indicated that rhein did not show genotoxic capabilities in any of the assays performed in all treatment conditions. A significant increase in the number of mutants at the intermediate dose level in the presence of S9 metabolic activation in the mammalian cell TK+/− mutation assay was not reproduced in an independent experiment and was within the historical control range values and therefore considered of no biological relevance. The Panel agreed with this conclusion.
In the study by Chen et al. (2010), rhein was evaluated for its capability to induce DNA damage by comet assay in human tongue cancer (SCC‐4) cells following treatments with emodin at dose levels 25, 50 and 100 μM for 24 h in the absence of S9 metabolic activation only. The authors showed significant increases in DNA fragmentation at the two higher dose levels (50 and 100 μM). However, the Panel noted that the study bears significant shortcomings which include a long treatment (24 h), a marked reduction of cell viability and consequent elevated cytotoxicity linked to excessive DNA damage that could have interfered with Comet analysis. On this basis, the results obtained are considered to be of limited reliability.
Sennosides
In the study by Heidemann et al. (1993), sennosides (a mixture of sennosides A + B in the range of 75.0–82.2% and sennosides C + D in the range of 3.7–4.3%) were tested for their capability to induce gene mutation in a bacterial reverse mutation test (Ames test) using the S. Typhimurium TA98, TA100, TA1535, TA1537 and the E. coli WP2 uvrA tester strains test and in the mammalian cell TK+/− mutation assays using the mouse lymphoma L5178Y cells. Induction of chromosomal aberrations in CHO cells was also assessed. All assays were performed both in the absence and presence of S9 metabolic activation. In the bacterial mutation test and in the chromosomal aberration assay in CHO cells, treatments were performed up to concentration of 5,000 μg/plate and 9,000 μg/mL, respectively. In the mammalian cell TK+/‐ mutation assay using the mouse lymphoma L5178Y cells, no indication about dose level employed was reported. However, according to the authors, the studies were compliant with the relevant OECD TG guideline no 471, no. 476 and no. 473, respectively, valid at the time and the GLP requirements. The results obtained indicated that sennosides did not induce gene mutation in the bacterial reverse mutation test (Ames test) and the mammalian cell TK+/‐ mutation assay and chromosomal aberration in CHO cells both in the absence and presence of S9 metabolism when tested up to cytotoxic concentrations.
Chrysophanol
In the study by Mengs et al. (2001), chrysophanol was tested for its ability to induce chromosomal aberrations in CHO cells both in the absence and presence of S9 metabolic activation up to concentration of 30 μg/mL, the solubility limit. The results obtained indicated that chrysophanol did not induce any significant increase in the frequency of aberration‐bearing cells, compared to the concurrent solvent control values. The Panel noted that only a short treatment (2 h) was used in the absence of S9 metabolism and judged the results as limited.
In vivo studies
Aloe vera extracts and/or gels
In the study by Williams et al. (2010), a tan powder derived from mucilaginous parenchymal cells found in the inner central area of the Aloe vera (L.) leafs manufactured using a proprietary extraction process in which the aloe latex contaminant (containing anthraquinones) was substantially reduced (including aloin, aloeresin A) and referred to as Qmatrix®, was assessed for its genotoxicity in a mouse bone marrow micronucleus test. Qmatrix® dissolved in sterile distilled water was administered to groups of six male ICR mice by oral gavage at doses of 1,250, 2,500 and 5,000 mg/kg per day once daily for two consecutive days. Animals were administered the Qmatrix® in sterile distilled water via gavage. The animals were sacrificed 24 h following the second test compound administration. For each animal, a minimum number of 2,000 polychromatic erythrocytes (PCE) was scored for the induction of micronuclei. The results obtained indicated that Qmatrix® did not induce any statistically significant increases in the incidence of micronucleated PCE and that the PCE/(PCE + NCE) ratio in the test substance treatment groups was not substantially changed compared to the concurrent control group indicating that test compound was not systemically available nor did it exert a sufficient bone marrow toxicity to elicit a potential genotoxic response. On this basis, the Panel noted that the study is of limited relevance for risk assessment, since the potentially DNA reactive compounds were reduced in the manufacturing process, being the content of aloin reported in the final preparation to be less than 10 μg/g ratio. The Panel considered the results of this study reliable but limited to extracts that are devoid and/or purified of anthranoids.
Fructus sennae
In the study by Heidemann et al. (1993), fructus sennae (2.51% content of hydroxyanthracene derivatives) was assayed for its ability to induce gene mutation in NMRI mice by the Mouse Spot Test and micronuclei and structural chromosomal aberrations in the bone marrow cells of Wistar rats. Groups of 4–8 male and female animals were treated with suspensions of fructus sennae in 0.3–0.5% tragacanth by oral gavage at 100 or 1,000 mg/kg bw for the Mouse Spot Test and at dose levels up to 1,000 mg/kg in the bone marrow chromosome aberration assay in rat and at 1,500 mg/kg in the bone marrow micronucleus test in rats. The studies included concurrent solvent and positive control animal groups. Micronuclei and chromosomal aberrations were determined at 24, 48 and 72 h and 6, 24 and 48 h from the beginning of treatment, respectively. In the latter case, intraperitoneal injections of colchicine at 2 mg/kg bw, 2.5 h before sacrifice were also performed. For the Mouse Spot Test, female animals were treated on day 9 of pregnancy and the F1 offspring was checked for colour spots 3 weeks after birth. A minimum number of 50 metaphases or 1,000 PCE per animal were scored for induction of chromosomal aberrations and micronuclei, respectively. The results obtained indicated that fructus sennae did not induce any significant increase in the genetically relevant spots compared to the concurrent control in the Mouse Spot Test nor micronuclei. No induction of chromosomal aberration was observed up to dose level of 1,000 mg/kg and no toxicity was observed. The Panel noted that the studies, though performed according to the standard protocols at the time bear some shortcomings which include the scoring of limited number of PCE in the micronucleus test (1,000 PCE/animal) and no evaluation of the ratio between PCE and normochromatic erythrocytes (NCE) to assess bone marrow toxicity was reported. In addition the maximum dose level of 1,000 mg/kg used in the chromosome aberration assay which did not elicit any toxicity in the target tissue appears to be low since in the micronucleus test a higher dose level (1,500 mg/kg bw) with no reported bone marrow toxicity was employed. This suggests that the bone marrow was not sufficiently exposed to the test compound. Furthermore, the Panel noted that the Mouse Spot Test is not currently used/recommended in genotoxicity testing for induction of gene mutation in vivo and that the relevant OECD Guideline 484 has been permanently withdrawn on 2 April 2014. Overall, the Panel considered these studies to be of insufficient reliability for risk assessment.
Senna extracts
In the study by Mengs et al. (1999), dried aqueous extracts from Tinnevelly senna pods were assessed for the induction of micronuclei in the bone marrow of NMRI mice. The main anthranoids contained in the extracts were rhein, aloe‐emodin and emodin present at 5.96%, 0.287% and 0.014% respectively. Extracts were administered once by oral gavage at a dose level of 2,000 mg/kg (rhein, aloe‐emodin and emodin equal to 119, 5.74 and 0.28 mg/kg bw, respectively) and animals were sacrificed at 24 and 48 h from treatment. Plasma levels of the different anthranoids were also determined in a satellite experiment following administration of aqueous extracts at 2,000 mg/kg bw to six male and six female NMRI mice. Three animals each of both genders were sacrificed at 1 and 3 h from treatment. For all compounds detected, plasma maximum levels were found after 1 h. The highest values were 3.4 μg/mL for rhein and 0.065 μg/mL for aloe‐emodin in female mice. The concentrations of emodin were in all cases below the limit of quantification. No induction of micronuclei was observed at any experimental test point. However, the Panel noted the low levels of anthranoids administered and the absence of any reduction of the ratio between PCE and NCE erythrocytes in the treated animal groups compared to the concurrent control animal groups.
Sennosides
In the study by Heidemann et al. (1993), sennosides (a mixture of sennosides A + B in the range of 75.0–82.2% and sennosides C+D in the range of 3.7–4.3%) were assessed for their potential induction of micronuclei in the bone marrow of male and female NMRI mice in two independent experiments following treatment by oral gavage. In the first experiment, animals received a single dose of 1,500 mg/kg bw while in the second experiment animals received two administration of 2,500 mg/kg bw 24 h apart. In both cases, sampling of bone marrow cell was performed at 24, 48 and 72 h from the last administration and 1,000 PCE per animal were analysed for the presence of micronuclei. The ratio of PCE to NCE was also carried out. The results obtained indicated that sennosides did not increase the frequencies of micronuclei in the test substance treatment groups compared to the concurrent negative controls and no toxicity was observed. However, the Panel noted that no raw data are reported in the publication and that at present the study appears to be inadequate for assessment of genotoxicity since only 1,000 PCE per animal were scored for the presence of micronuclei (against 4,000 currently requested) and no proof of absorption or target tissue exposure were present. On this basis, the Panel considered the results obtained to be of insufficient reliability for risk assessment of sennosides.
Emodin
In the study by Mengs et al. (1997), emodin (purity 99%) was assessed for the induction of micronuclei in the bone marrow of NMRI mice following a single administration by oral gavage at a dose level of 2,000 mg/kg as a suspension (in 0.3% aqueous tragacanth). The study included solvent and positive control animal groups and sacrifice of animals was performed at 24 and 48 h from treatment. A minimum number 2,000 PCE per animal were scored for micronuclei. The ratio between PCE and NCE to assess bone marrow toxicity was also calculated. Plasma levels of emodin was also determined in a satellite experiment following administration of test substance at 2,000 mg/kg bw to four male and four female NMRI mice. Animals were sacrificed at 1 and 3 h from treatment. Plasma levels of emodin ranged from 73 to 190 μg/mL within 3 h. No induction of micronuclei was observed at any experimental test point. However, the Panel noted that despite the presence of emodin in plasma (73–190 μg/mL within 3 h) the mean number of NCE in the emodin treatment group was not increased as compared to the mean values of NCE of the concurrent solvent controls, indicating that emodin had no cytotoxic effects on bone marrow cells and no proof of exposure of target issue were present. Overall, the Panel considered these studies to be of insufficient reliability for risk assessment.
Aloe‐emodin
In the study by Heidemann et al. (1996), aloe‐emodin (purity 99.4–99.9%) was assayed for its ability to induce gene mutation in NMRI mice by the Mouse Spot Test and micronuclei and structural chromosomal aberrations in the bone marrow cells of Wistar rats. An in vivo/vitro unscheduled DNA synthesis (UDS) study in primary hepatocytes of Wistar rats was also performed. Groups of 4–8 male and female animals were treated with aloe‐emodin by oral gavage at 200 and 2,000 mg/kg bw for the Mouse Spot Test and at dose levels up to 2,000 mg/kg in the bone marrow chromosome aberration assay in rat and at 1,500 mg/kg in the bone marrow micronucleus test in rats. For UDS, dose levels of 100 and 1,000 mg/kg were used. The studies included concurrent solvent and positive control animal groups. Micronuclei and chromosomal aberrations were determined at 24, 48 and 72 h and at 6, 24 and 48 h from the beginning of the treatment, respectively. In the latter case, intraperitoneal injections of colchicine at 2 mg/kg bw, 2.5 h before sacrifice were also performed. For the Mouse Spot Test, female animals were treated on day 9 of pregnancy and the F1 offspring was checked for colour spots 3 weeks after birth. For UDS, after a treatment period of 4 and 16 h, animals were anaesthetised and during liver perfusion were sacrificed. Primary hepatocyte cultures were set up and 3H‐thymidine was added for 4 h in order to detect UDS by autoradiography. A minimum number of 50 metaphases or 1,000 PCE per animal were scored for induction of chromosomal aberrations and micronuclei, respectively, and 100 cells per animal were analysed for UDS. The results obtained indicated that aloe‐emodin did not induce any significant increase in the genetically relevant spots in the F1 offspring (210 and 458 animals at 200 and 2,000 mg/kg bw) compared to the concurrent control in the Mouse Spot Test. No micronuclei, chromosomal aberrations or UDS were observed at any dose level or sampling time assayed. No reduction of PCE/NCE ratio or mitotic indices were observed at any experimental point in the micronucleus and chromosomal aberration assays, respectively, indicating that target tissue exposure could not be demonstrated. The Panel noted that the studies, although performed according to the standard protocols at the time bear some shortcomings which include the scoring of limited number of PCE in the micronucleus test (1,000 PCE/animal against 4.000 currently requested) and only one or two dose levels were used. Furthermore, the Panel noted that the Mouse Spot Test is not a validated test and is presently considered obsolete and that no raw data were reported. Overall, the Panel considered these studies to be of insufficient reliability for risk assessment.
In the study by Nesslany et al. (2009), aloe‐emodin (purity 95.59%, Extrasynthese, Genay, France) was assayed for its capability to induce DNA fragmentation in the colon and kidney of male OF1 mice. Groups of five male mice were treated with aloe‐emodin by oral gavage on two occasions 24‐h apart at dose levels of 500, 1,000 and 2,000 mg/kg bw as a suspension in 0.5% carboxymethylcellulose (CMC 0.5%). The study included concurrent solvent and positive control animal groups. Negative control received the vehicle only and the positive control groups (four animal each) were dosed once, either intravenously with streptozotocin at 20 mg/kg (kidney) or orally with dimethylhydrazine at 20 mg/kg bw (colon). Between 3 and 6 h from the last treatment, animals were anesthetised and maintained under deep anaesthesia before collecting cells from each target organ. The viability of cells in the resulting cell suspensions was evaluated using the trypan blue exclusion technique and preparation of slides to be assessed for DNA breakage followed standard recommendations (Tice et al., 2000; Hartmann et al., 2003; Burlinson et al., 2007). At least 150 randomly selected cells per animal (50 cells per slide) were analysed at 250x magnification using a fluorescence microscope connected to the Comet Image‐analysis System (Kinetic Imaging Ltd., Liverpool, UK). The Olive tail moment (OTM) was used to evaluate DNA damage. OTM values from the three replicate slides were pooled and the median was calculated. The mean of medians and standard errors of the mean were calculated for each group and log‐transformed before statistical analyses. The results obtained indicated that aloe‐emodin induced marked and statistically significant increases in OTM values in the colon at all dose levels used compared to the concurrent vehicle control values. The observed increases were 2.2, 2.4 and 2.1 greater than the concurrent vehicle control value at the low‐, intermediate‐ and high‐dose level, respectively. Increases in OTM values compared to the concurrent vehicle control were also observed in kidney. These increases were dose‐related but statistical significance was only reached at the highest dose level tested. The Panel noted that the study complied with the international recommendations at the time and essentially lined up to the current OECD TG 489 ‘In vivo mammalian alkaline Comet Assay’.
The main figures of in vitro and in vivo genotoxicity studies are reported in Tables 5 and 6.
Table 5.
Summary of in vitro genotoxicity studies evaluated in the present opinion
| Test material | Mutagenicity assay | Test object | Reported result | Reference | Comments |
|---|---|---|---|---|---|
| Aloe vera extracts and/or gels | Bacterial reverse mutation | Salmonella Typhimurium TA98 and TA100 | Negativea | Morimoto et al. (1982) |
Limited reliability. Set of S. Typhimurium strains not complete |
| DNA repair | Bacillus subtilis rec‐assay |
Positivec (only for water extracts) |
Insufficient reliability. Test system not adequately validated and not currently used/recommended in genotoxicity testing |
||
| DNA repair | E. coli wild‐type or defective repair mutants (recA, xthA, uvrA, wild‐type/rfa, uvrA/rfa) tester strains | Negativec | Paes‐Leme et al. (2005) |
Insufficient reliability. Test system not adequately validated and not currently used/recommended in genotoxicity testing |
|
| Bacterial reverse mutation | S. Typhimurium TA98 and TA100 | Negativea | Sehgal et al. (2013) |
Limited reliability. Set of S. Typhimurium strains not complete |
|
| DNA damage | E. coli strain containing a transgene for β‐galactosidase downstream of the SOS‐DNA repair promoter system | Negativec |
Insufficient reliability. Test system not adequately validated and not currently used/recommended in genotoxicity testing |
||
| Bacterial reverse mutation | S. Typhimurium TA1535, TA1537, TA98, TA100 and E. coli WP2 uvrA | Negativea | Williams et al. (2010) | Reliable, but limited to extracts which are devoid and/or purified of anthranoids | |
| Bacterial reverse mutation | S. Typhimurium TA1535, TA97, TA98, TA100 and E. coli WP2 uvrA | Negativea | Boudreau et al. (2013) | Reliable. | |
| Chromosome aberrations | Chinese hamster lung (CHL) cells | Negativea | Williams et al. (2010) | Reliable, but limited to extracts which are devoid and/or purified of anthranoids | |
| Mammalian cell gene mutation | Mouse lymphoma L5178Y cells (TK locus) | Positivec | Guo (2014) | Reliable. | |
| Senna extracts | Bacterial reverse mutation | S. Typhimurium TA98 and TA100 | Positivea | Morimoto et al. (1982) |
Reliable. Although the set of S. Typhimurium strains was not complete, a positive effect was detected |
| DNA repair | Bacillus subtilis rec‐assay | Positiveb |
Insufficient reliability. Test system not adequately validated and not currently used/recommended in genotoxicity testing |
||
| Emodin | Bacterial reverse mutation |
S. Typhimurium TA98, TA100, TA1535, TA1537 E. coli WP2 uvrA |
Positive in TA1537c and TA98a | Heidemann et al. (1996) | Reliable. |
| Mammalian cell gene mutation |
Chinese hamster (V79) cells (HPRT locus) |
Negativec Equivocalb | Heidemann et al. (1996) | Reliable. | |
| Valid | Chinese hamster ovary (CHO) cells | Positivea | Reliable. | ||
| Gene mutation and micronuclei | Mouse lymphoma L5178Y cells (TK locus) | Positivec | Mueller et al. (1996) | Reliable. | |
| Micronucleus induction | Mouse lymphoma L5178Y cells | Positivec | Reliable. | ||
| Alkaline comet assay | Human tongue cancer (SCC‐4) cells | Positivec | Chen et al. (2010) |
Limited reliability. Only a long treatment (24 h) performed with possible elevated cytotoxicity associated. |
|
| Aloe‐emodin | Bacteria reverse mutation |
S. Typhimurium TA98, TA100, TA1535, TA1537 E. coli WP2 uvrA |
Positive |
Heidemann et al. (1996) | Reliable. |
| Mammalian cell gene mutation |
Chinese hamster (V79) cells (HPRT locus) |
Negativea | Reliable. | ||
| Chromosomal aberrations | Chinese hamster ovary (CHO) cells | Positivea | Reliable. | ||
| Mammalian cell gene mutation | Mouse lymphoma L5178Y cells (TK locus) | Positivec | Mueller et al. (1996) | Reliable. | |
| Micronucleus induction | Mouse lymphoma L5178Y cells | Positive | Reliable. | ||
| Alkaline comet assay | Human tongue cancer (SCC‐4) cells | Positivec | Chen et al. (2010) |
Limited reliability. Only a long treatment (24 h) performed with possible elevated cytotoxicity associated |
|
| Danthron | Mammalian cell gene mutation | Mouse lymphoma L5178Y cells (TK locus) | Positivec | Mueller et al. (1996) | Reliable. |
| Micronucleus induction | Mouse lymphoma L5178Y cells | Reliable | |||
| Bacterial reverse mutation | S. Typhimurium TA97, TA98, TA100, TA102 | Positive (TA102)b | Zhang et al. (2011) |
Reliable. Though the set of S. Typhimurium strains was not complete, a positive effect was detected |
|
| Micronucleus induction | Balb/c 3T3 cell line | Positivea |
Reliable. Though treatments were only performed for 24 h, a positive effect was anyway found |
||
| Comet assay | Balb/c 3T3 cell line | Positivea |
Limited reliability. Only a long treatment (24 h) performed with possible elevated cytotoxicity associated |
||
| Rhein | Bacterial reverse mutation | S. Typhimurium TA98, TA100, TA1535, TA1537 and E. coli WP2 uvrA | Negativea | Heidemann et al. (1993) | Reliable |
| Mammalian cell gene mutation |
Mouse lymphoma L5178Y cells (TK locus) |
Negativea | Reliable. | ||
| Chromosomal aberrations | Chinese hamster ovary (CHO) cells | Negativea | |||
| Alkaline comet assay | Human tongue cancer (SCC‐4) cells | Positivec | Chen et al. (2010) |
Limited reliability. Only a long treatment (24 h) performed with possible elevated cytotoxicity associated |
|
| Sennosides | Bacterial reverse mutation | S. Typhimurium TA98, TA100, TA1535, TA1537 and E. coli WP2 uvrA | Negativea | Heidemann et al. (1993) | Reliable. |
| Mammalian cell gene mutation | Mouse lymphoma L5178Y cells | Negativea | Reliable. | ||
| Chromosomal aberrations | Chinese hamster ovary (CHO) | Negativea | Reliable. | ||
| Chrysophanol | Chromosomal aberrations | Chinese hamster ovary (CHO) cells | Negativea | Mengs et al. (2001) | Mengs et al. (2001) |
With and without metabolic activation.
With metabolic activation.
Without metabolic activation.
Reliability of genotoxicity studies:
- Limited reliability (e.g. if certain aspects are not in accordance with OECD guidelines or current standards and/or limited documentation).
- Insufficient reliability (e.g. if main aspects are not in accordance with any recognised guidelines (e.g. OECD) or current standards and/or inappropriate test system).
- Reliability cannot be evaluated (e.g. insufficient documentation, short abstract only, too little experimental details provided).
Table 6.
Summary of in vivo genotoxicity studies evaluated in the present opinion
| Test material | Mutagenicity assay | Test object | Route | Dose | Reported result | Reference | Commentsa |
|---|---|---|---|---|---|---|---|
| Aloe vera extracts and/or gels | Micronucleus formation | ICR, male mouse bone marrow cells | Oral gavage/ | 1,250, 2,500, 5,000 mg/kg bw once daily on two consecutive days | Negative | Williams et al. (2010) |
Insufficient reliability. No evidence or demonstration that target tissue was sufficiently exposed |
| Fructus sennae |
Mouse spot test (Induction of different mutations in target genes which control the pigmentation of the coat hairs in melanoblasts) |
NMRI mice melanoblasts in developing embryos | Oral gavage |
100 and 1,000 mg/kg bw (single administration) |
Negative | Heidemann et al. (1996) |
Insufficient reliability. No evidence or demonstration that target tissues were sufficiently exposed. The Mouse Spot Test is not currently used/recommended in genotoxicity testing for induction of gene mutation in vivo. The relevant OECD Guideline 484 has been permanently withdrawn on 2 April 2014 |
| Micronucleus formation | Wistar male and female rat bone marrow cells | Oral gavage |
15,00 mg/kg bw (single administration) |
Negative |
Insufficient reliability. No evidence or demonstration that target tissue was sufficiently exposed. A limited number of PCE in the micronucleus test (1,000 PCE/animal) and no evaluation of the ratio between polychromatic and normochromatic erythrocytes to assess bone marrow toxicity |
||
| Chromosome aberrations | Wistar male and female rat bone marrow cells | Oral gavage |
Up to 1,000 mg/kg bw (single administration) |
Negative |
Insufficient reliability. No evidence or demonstration that target tissue was sufficiently exposed. No bone marrow toxicity observed. Selection of the highest dose level (1,000 mg/kg bw) not consistent with the highest dose level used for the micronucleus test (1,500 mg/kg bw) |
||
| Senna extracts | Micronucleus formation | NMRI male and female mouse bone marrow cells | Oral gavage |
2,000 mg/kg bw (single administration) |
Negative | Mengs et al. (1999) |
Insufficient reliability. No evidence or demonstration that target tissue was sufficiently exposed. No bone marrow toxicity observed. Maximum plasma levels of aloe‐emodin and emodin, two components genotoxic in vitro were 0.065 μg/mL and below the limit of detection (LOD), respectively. Only 2,000 PCE/animal) were scored for evaluation of micronuclei induction |
| Sennosides | Micronucleus formation | Bone marrow of male and female NMRI mice | Oral gavage |
2,500 mg/kg bw (twice 24 h apart) |
Negative | Heidemann et al. (1996) |
Insufficient reliability. No evidence or demonstration that target tissue was sufficiently exposed. No bone marrow toxicity observed. A limited number of PCE (1,000 PCE/animal) was scored |
| Emodin |
Micronucleus formation |
NMRI male and female mouse bone marrow cells | Oral gavage |
2,000 mg/kg bw (single administration) |
Negative | Mengs et al. (1997) |
Insufficient reliability. No evidence or demonstration that target tissue was sufficiently exposed. No bone marrow toxicity observed. Only 2,000 PCE/animal were scored for evaluation of micronuclei induction. Only one dose level |
| Aloe‐emodin |
Mouse spot test (Induction of different mutations in target genes which control the pigmentation of the coat hairs in melanoblasts) |
NMRI mice melanoblasts in developing embryos | Oral gavage |
200 and 2,000 mg/kg bw (single administration) |
Negative | Heidemann et al. (1996) |
Insufficient reliability. No evidence or demonstration that target tissues were sufficiently exposed. The Mouse Spot Test is not currently used/recommended in genotoxicity testing for induction of gene mutation in vivo. The relevant OECD Guideline 484 has been permanently withdrawn on 2 April 2014 |
| Micronucleus formation | Wistar male and female rat bone marrow cells | Oral gavage |
1,500 mg/kg bw (single administration) |
Negative |
Insufficient reliability. No evidence or demonstration that target tissue was sufficiently exposed. A limited number of PCE (1,000 PCE/animal) were scored for evaluation of micronuclei induction. Only one dose level administered. No bone marrow toxicity observed |
||
| Chromosome aberrations | Wistar male and female rat bone marrow cells | Oral gavage |
200, 666 and 2,000 mg/kg bw (single administration) |
Negative |
Insufficient reliability. No evidence or demonstration that target tissue was sufficiently exposed. Only two dose level administered. No bone marrow toxicity observed |
||
| In vivo/vitro unscheduled DNA synthesis | Primary hepatocytes of Wistar rats | Oral gavage |
100 and 1,000 mg/kg bw (single administration) |
Negative |
Insufficient reliability. No evidence or demonstration that target tissue was sufficiently exposed. Only two dose level administered. Not recommended as an adequate in vivo follow‐up study in genotoxicity testing (EFSA, 2017) |
||
| DNA fragmentation | Colon and kidney cells of male OF1 mice | Oral gavage | 500, 1,000 and 2,000 mg/kg (twice 24 h apart) |
Positive (colon); Weakly positive (kidney) |
Nesslany et al. (2009) |
Reliable. Essentially compliant with the current OECD Guideline 489 ‘in vivo mammalian alkaline comet assay’ |
bw: body weight; PCE: polychromatic erythrocytes.
- Reliable.
- Limited reliability (e.g. if certain aspects are not in accordance with OECD guidelines or current standards and/or limited documentation).
- Insufficient reliability (e.g. if main aspects are not in accordance with any recognised guidelines (e.g. OECD) or current standards and/or inappropriate test system).
- Reliability cannot be evaluated (e.g. insufficient documentation, short abstract only, too few experimental details provided).
Discussion on genotoxicity
In this opinion, the genotoxicity of hydroxyanthracene derivatives has been evaluated in numerous in vitro and in vivo studies identified from the public literature. The available studies focused mainly on emodin, aloe‐emodin, rhein and sennosides and to lesser extent on aloe vera, senna and fructus sennae extracts. A limited number of studies on danthron and chrysophanol were also available.
Among the extracts, only senna extracts proved to be mutagenic in the TA98 S. Typhimurium tester strain in the presence of S9 metabolism (Morimoto et al., 1982), in S. Typhimurium tester strain ta 1537 and TA98 in the absence of S9 metabolism (Heidemann et al., 1993). It also induced DNA damage in the Bacillus subtilis rec‐assay (Morimoto et al., 1982) and induced dose‐related increases in chromosomal aberrations in CHO cells in the absence of S9 (Heidemann et al., 1993).
No positive results in the bacterial mutation assay for aloe vera gel and for both aloe vera WLE and aloe vera charcoal‐filtered whole leaf extract (decolourised) were observed in S. Typhimurium strains TA98 and TA100, and E. coli strain WP2 uvrA/pKM101, with and without S9 metabolic activation (Boudreau et al., 2013). The 1,8‐dihydroxyanthraquinones emodin, aloe‐emodin and danthron were shown to be mutagenic in the Tk+/− locus in mouse lymphoma L5178Y cells (Mueller et al., 1996) and consistently clastogenic in vitro in mammalian cells interacting via intercalation into DNA with DNA topoisomerase II and consequent inhibition of their catalytic function resulting in genotoxicity and mutagenicity with a ranking potency greater for danthron and lower for emodin.
This was demonstrated by the results of a ‘modified comet assay’ which showed that pretreatments of the cells with each of these test compounds reduced the DNA damage induced by etoposide, an inhibitor of topoisomerase II which acts through the stabilisation of the DNA–topoisomerase complex known as the ‘cleavable complex’ (Mueller et al., 1996) and following findings by Mueller and Stopper (1999) in which danthron, aloe‐emodin and emodin showed capability to inhibit the non‐covalent binding of bisbenzimide Hoechst 33342 to isolated DNA and to DNA of intact mouse lymphoma L5178Y cells. On the other hand, emodin, the least potent compound among these three anthraquinones, was also shown to induce DNA double‐strand breaks (DSB) through poisoning of topoisomerase II‐cleavable complex and through inhibiting adenosine triphosphate (ATP) hydrolysis by DNA topoisomerase II (Li et al., 2010). Thus, it is not possible to exclude also a potential mechanism of inhibition of DNA topoisomerase II through poisoning of the DNA–topoisomerase ‘cleavable complex’. Aloe‐emodin was also mutagenic in S. Typhimurium strains TA1537, TA1538, TA97 and TA98, (all frameshift mutant sites) in the absence of S9 metabolism. While these results further support the intercalating capability of aloe‐emodin and consequent catalytic inhibition of DNA topoisomerase II they also show the induction of frameshift mutations which, per se, is a mutagenic event not related to the inhibition of DNA topoisomerase II.
In this respect, the Panel noted that, beyond frameshift mutations, danthron also induced base‐pair substitutions in bacteria, only after exogenous metabolic activation, and oxidative DNA damage in mammalian cells, highlighting the involvement of multiple mechanisms of genotoxicity.
In addition aloe‐emodin induced UDS in primary rat hepatocytes (Westendorf et al., 1990), and DNA breakage in mouse lymphoma L5178Y cells (Mueller et al., 1996), in NPC‐039 and NPC‐076 human nasopharyngeal carcinoma cells (Lin et al., 2007) and SCC‐4 human tongue cancer cells (Chen et al., 2010). These results are also compatible with a mechanism of inhibition of DNA topoisomerase II activities.
However, although individual anthranoids induced gene mutations (frameshift and base‐pair substitutions) in bacteria, frameshift mutations and clastogenic effects in mammalian cells through inhibition of DNA topoisomerase II, for plant extracts containing hydroxyanthracene derivatives, other mutagenic components with different mechanisms of action appear to play a role in extract genotoxicity and carcinogenicity as reported in the study by Guo (2014). In this study, both aloe vera WLE and aloe vera WLD – for which the content of hydroxyanthracene derivatives (both glycosidic and free aglycones) was reduced by 99% compared to the WLE induced statistically significant and biologically relevant increases in mutation frequencies (MF) in the mammalian cell TK+/‐ mutation assay using the mouse lymphoma L5178Y cells. As revealed by DCF‐DA staining, intracellular ROS levels were increased about 5‐ and 15‐fold in WLD‐ and WLE‐treated cells, respectively, when compared to the concurrent control groups. This implies that components in the WLE, in addition to the hydroxyanthracene derivatives, could contribute to the mutagenicity of the WLE in part through a ROS‐dependent mechanism. This conclusion is further substantiated by the LOH of WLE/WLD‐induced mutants. Results indicated that, while both extracts were clastogenic, their induced mutation spectra were significantly different, thus confirming that different constituents may be responsible for the genetic damage caused by the two preparations. These results indicate that aloe vera and senna extracts and individual hydroxyanthracene derivatives, particularly aloe‐emodin, interact with bacterial and mammalian DNA under certain conditions in vitro.
However, for risk assessment, in vivo studies are requested to conclude on potential genotoxic hazard and in this respect, a number of in vivo studies were available which included rodent bone marrow micronucleus assays, mouse somatic mutation assay in fetal melanoblasts commonly identified as Mouse Spot Test, rat bone marrow chromosome aberration assays and in vivo/in vitro UDS in rat liver performed by oral gavage for aloe‐emodin and fructus sennae extracts (Heidemann et al., 1996). Data from rodent bone marrow micronucleus tests were also available for emodin (Mengs et al., 1997), senna extracts (Mengs et al., 1999) and sennosides (Heidemann et al., 1993) following oral administration. The results obtained indicated that senna, fructus sennae extracts, sennosides and individual 1,8‐dihydroxyanthraquinones tested were uniformly negative in any of the different endpoints analysed, following high acute exposure by oral gavage. However, in all cases, no evidence of toxicity in the target cells was observed, indicating that target tissues could have not been adequately exposed to the test compounds. This assumption is substantiated by results of an in vivo study in rats (Lang, 1993), where [14C]aloe‐emodin, was shown to be hardly absorbed and quickly metabolised to rhein which was found to be non‐mutagenic in a battery of in vitro and in vivo assays (Westendorf et al., 1990; Heidemann et al., 1996; Mengs and Heidemann, 1993).
On the other hand, Nesslany et al. (2009) found marked and significant increases in DNA fragmentation in the colon cells of male OF1 mice treated with aloe‐emodin by oral gavage at 2000, 1000 and 500 mg/kg bw on two occasions 24 h apart in the in vivo rodent comet assay. Slight increases in DNA breakage compared to the concurrent vehicle control values were also observed in the kidney which were dose‐related but reached statistical significance only at the high‐dose level tested. On this basis, the limited absorption of aloe‐emodin and its quick transformation to rhein (a compound devoid of genotoxic capabilities) indicate that bone marrow may be considered as an inadequate tissue to demonstrate a possible in vivo genotoxicity for both emodin and aloe‐emodin. Overall, the Panel considered that the in vitro genotoxicity of aloe‐emodin was reproduced in the colon in vivo (Nesslany et al., 2009), the target tissue for aloe vera WLE carcinogenicity (Boudreau et al., 2013). The Panel therefore considered that aloe‐emodin represents a genotoxic risk for man. The Panel also considered that the presence of other mutagenic components, in addition to hydroxyanthracene derivatives, cannot be excluded.
3.2.2. Short‐term and subchronic toxicity
Aloe Vera
Short‐term (16‐day) and subchronic (14‐week) toxicity studies have been conducted in two rodent species (mice and rats) within the US NTP on WLE of Aloe barbadensis Miller (Aloe Vera) (NTP, 2013).
Mice
Groups of B6C3F1/Ncrt mice (4/sex per group, 7 weeks old) received for 14 days 0, 0.5%, 1.0%, 1.5%, 2% or 3.0% (wt/wt) of aloe vera gel (malic acid content of 116–212 mg/g, aloin A content of 1.1–1.4 mg/g), aloe vera decolourised whole leaf (malic acid content of 215–258 mg/g, aloin A content of 0.06–0.2 mg/g no further information provided on chemical composition) or aloe vera non‐decolourised WLE (malic acid content of 188–197 mg/g, aloin A content of 14.1–15.9 mg/g) in drinking water (NTP, 2013). Additional groups (4/sex per group) received the same concentrations of the Aloe vera extracts for the same duration period and were used to study metabolism. According to EFSA ‘Guidance on default values to be used in the absence of measured data’ (EFSA Scientific Committee, 2012), these dietary concentration were equivalent to 0, 11.25, 22.5, 33.75, 45, 67.5 mg/aloin A/kg bw per day for aloe vera gel; 0, 1.17, 2.34, 3.51, 4.68, 7.02 mg/aloin A/kg bw per day for aloe vera decolourised whole leaf and 0, 135, 270, 405, 540, 810 mg/aloin A/kg bw per day for aloe vera non‐decolourised WLE.22
All mice survived to the scheduled termination. Dose‐related decreases in the GI transit time were recorded at week 1 in males receiving aloe vera WLE. No treatment‐related changes were observed in the examined organs.
Groups of B6C3F1 mice (12/sex per group, 6 to 7 weeks old) received 0, 1%, 2% or 3% (wt/wt) of aloe vera non‐decolourised whole leaf extract (the bulk material malic acid content of 170.7–192.9 mg/g, aloin A content of 12.6–14.4 mg/g) in drinking water for 13 consecutive weeks (NTP, 2013). These concentrations provided (as reported by the authors of the study) 0, 3,700, 7,300 and 9,100 mg aloe vera non‐decolourised whole leaf extract/kg bw per day for males and 0, 3,700, 7,600, and 9,500 mg aloe vera non‐decolourised whole leaf extract/kg bw per day for females, corresponding to 0, 49.95, 98.55 and 122.85 mg aloin A/kg bw per day for males and 0, 49.95, 102.6 and 128.25 mg aloin A/kg bw per day for females.23 Additional groups (12/sex per group) received 0% or 3% of the aloe vera WLE and were used for metabolism study. All mice survived to the scheduled termination. Body weights and feed intake of male and female mice from the treated groups were comparable to controls. Water intake was statistically significantly higher than that of controls for male and female mice from 1%‐ and 2% groups on day 30, and in 2% group on day 60. Significant dose‐related increases in water intake were reported only for females on day 90. Water intake of males and females in the metabolism study was did not differ from that in controls. The haematology and clinical chemistry results in the treated groups were within the reference values for laboratory mice. GI transit times of 3% group males and females were similar to those of controls with exception of a transient increase in transit time at week 8 for males from 3%‐dose group. The absolute and relative organ weights of males and females exposed to Aloe vera non‐decolourised whole leaf extract were similar to those in controls with exception slightly but statistically significantly increased relative liver weight, absolute and relative spleen and kidney weights in the 1%‐dose group females, and of relative kidney weight in the 3%‐dose group females. Although within physiological ranges, the 24‐h urine levels of creatinine and microprotein were statistically significantly increased compared with controls. The increased incidences and severities of goblet cell hyperplasia in the caecum and large intestine were reported for males and females from groups exposed to the test material as compared to controls.
Rats
Groups of F344/N rats (4/sex per group, 7 weeks old) received for 14 days 0, 0.5%, 1.0%, 1.5%, 2% or 3.0% (wt/wt) of aloe vera gel (malic acid content of 116‐212 mg/g, aloin A content of 1.1 to 1.4 mg/g), Aloe vera decolourised whole leaf (malic acid content of 215–258 mg/g, aloin A content of 0.06–0.2 mg/g) or aloe vera non‐decolourised WLE (malic acid content of 188–197 mg/g, aloin A content of 14.1–15.9 mg/g) in drinking water (NTP, 2013). According to EFSA ‘Guidance on default values to be used in the absence of measured data’ (EFSA Scientific Committee, 2012), these dietary concentration were equivalent to 7.56, 15.12, 22.68, 30.24, 45.36 mg/aloin A/kg bw per day for aloe vera gel; 0, 0.78, 1.56, 2.34, 3.12, 4.68 mg/aloin A/kg bw per day for aloe vera decolourised whole leaf and 0, 90, 180, 270, 360, 540 mg/aloin A/kg bw per day for aloe vera non‐decolourised WLE.24
Additional groups (4/sex per group) received the same concentrations of the aloe vera extracts for the same duration period and were used to study metabolism. At necropsy, the heart, liver, right kidney, lungs/bronchi, spleen, right testis and thymus were weighed. Microscopic examination was performed on thyroid, parathyroid glands, liver, thymus, lung and kidney from the control group and high‐dose groups receiving the aloe vera WLE and on all gross lesions. All rats survived to the scheduled termination. No treatment related to non‐neoplastic lesions was reported.
Groups of F344/N rats (12/sex per group in the core study, 6 weeks old) received 0, 1%, 2% or 3% (wt/wt) of aloe vera non‐decolourised WLE (the bulk material malic acid content of 170.7–192.9 mg/g, aloin A content of 12.6–14.4 mg/g) in drinking water for 13 consecutive weeks. These concentrations provided (as reported by the authors of the study) 0, 1.1, 2.7 and 3.8 g aloe vera non‐decolourised whole leaf extract/kg bw per day for males and 0, 1.3, 4.0, and 3.2 g aloe vera non‐decolourised whole leaf extract/kg bw per day for females, corresponding to 0, 14.8, 36.45 and 51.3 mg aloin A/kg bw per day for males and 0, 18.72, 54 and 43.2 mg aloin A/kg bw per day for females.23 Additional groups (12/sex per group) received 0 or 2% of the aloe vera WLE and were used for metabolism study. Two males and four females from 2% and five males and eight females from the 3% aloe vera non‐decolourised whole extract groups died or were euthanised due to morbidity before the scheduled termination. Mean final body weights and body weight gains of males from all dose groups and of females from 2%‐ and 3% groups were statistically significantly lower than the controls. Statistically significantly decreased feed intake for males and females from all dose groups was recorded in the 4th week and for males from the 2% and 3% groups in the 13th week. Mean water intake in all male groups and in 2% females on days 60 and 90 was statistically significantly increased as compared to the controls. Despite higher water intake in Aloe vera non‐decolourised whole leaf extract exposed groups decreased urine production was recorded in the 2% group in males on days 30 and 60 and on day 90 in females only. Urinary protein and 24‐h glucose levels were increased in males and females at 30 days and at 60 days in males only. Urine 24‐h creatinine levels were statistically significantly decreased at 30, 60 and 90 days in males and at 30 and 60 days in females. Haematology examination revealed increased erythrocyte counts and a twofold increase in leukocytes counts in 2% group of males and females compared to the controls. The increase in leukocytes was due to increase of neutrophils. Cholesterol and albumin levels were significantly lower than those in the control group. GI transit times were decreased in aloe vera non‐decolourised WLE exposed male and female rats. For 2% group, transit time of male rats was 4.3 vs 11.5 h in the controls and of females 6.2 vs 11 h. Statistically significant lower absolute weights of brain, liver, heart, spleen and thymus were recorded for males and female from 2%‐ and 3% groups compared to the controls. At necropsy, increased incidence of mesenteric lymph node enlargement was recorded in the 2% and 3% groups of both sexes but no information on statistical significance was given. According to the authors, microscopic examination showed lymphoid hyperplasia of the mesenteric lymph nodes in all treated groups with the severity markedly increased in the 2%‐ and 3%‐dose groups but no information on statistical significance was given. The incidence of Goblet cell hyperplasia of the large intestine was increased in males and females from the treated groups compared to controls, but no information on statistical significance was given.
Emodin
Short‐term (16‐day) and subchronic (14‐week) toxicity studies have been conducted in two rodent species (mice and rats) within the US NTP on emodin (NTP, 2001).
Mice
Groups of B6C3F1 mice (5/sex per group, 6 weeks old) received for 15 (males) or 16 (females) days 0, 600, 2000, 5,500, 17,000 or 50,000 mg emodin/kg diet (equal to daily doses of 0, 120, 400, 1,200 or 3,800 mg emodin/kg bw per day for males and to 0, 140, 530, 1,600 or 5,000 mg emodin/kg bw per day for females; no calculation of the dose was done for the highest dietary concentration because of high mortality in this group) (NTP, 2001). Discolouration (yellow or orange) of the urine was observed in all groups and of the fur in groups receiving 5,500 mg emodin/kg diet or greater. Two males from 17,000 mg emodin/kg diet group and one female each from groups of 5,500 mg emodin/kg diet or greater 20,000 mg emodin/kg diet, manifested diarrhoea. None of mice from the 50,000 mg emodin/kg diet survived to the end of the study. Body weights of males and females from 17,000 mg emodin/kg diet group were significantly lower than in controls. Macroscopic changes were seen in gallbladder and kidneys. The authors reported the following statistically significant differences in relative organs weights compared to the control group: (a) lower absolute and thymus weights of males and females from 5,500 and 17,000 mg emodin/kg diet groups, (b) increased relative weights of testis of males from 17,000 mg emodin/kg diet group, and (c) increased relative weights of kidneys of females from 17,000 mg emodin/kg diet group. There were also differences in relative weights of other organs but the authors attributed these to body weight differences. The microscopic changes observed in males and females were: inflammation of gallbladder from 5,500 mg emodin/kg diet or higher groups, golden yellow crystalline material in gallbladders in 17,000 mg emodin/kg diet group, and renal changes such as crystalline material and associated inflammation, necrosis, and fibrosis from 5,500 mg emodin/kg diet or higher groups.
In another study, groups of B6C3F1 mice (10/sex per group, 6 weeks old) received for 14 consecutive weeks 0, 312.5, 625, 1,250, 2,500 or 5,000 mg emodin/kg diet (equal to daily doses of 0, 50, 100, 190, 400 or 800 mg emodin/kg bw per day for males and to 0, 60, 130, 240, 500, or 1,100 mg emodin/kg bw per day for females) (NTP, 2001). All mice survived until termination. Statistically significantly lower body weights and body weight gains were recorded for males from 2,500 and 5,000 mg emodin/kg diet groups as compared to controls. Discolouration (yellow or orange) of urine, skin and fur was seen in all groups. Coloured faeces were seen in males from groups receiving dietary doses of 321.5, 2,500 or 5,000 mg emodin/kg and in females treated with 1,250 mg emodin/kg diet or greater. Diarrhoea was seen in the two high‐dose groups of males and in females treated with 1,250 mg emodin/kg diet or greater. Sperm motility and vaginal cytology results from all treatment groups were not statistically significantly different from the controls. The authors reported the following differences in relative organs weights compared to the control group: (a) increased relative kidney weights of males from 1,250 mg emodin/kg diet group, (b) increased relative lung weights of males from 625 mg emodin/kg diet or greater group, (c) increased relative liver weights of females treated with 625 mg emodin/kg diet or greater. There were also differences in relative weights of other organs but the authors attributed these to body weight differences. According to the authors, the microscopic change related to treatment with emodin was nephropathy. The incidence and severity of nephropathy were statistically significantly increased in male and female mice treated with 1,250 mg emodin/kg diet or higher dietary doses. The incidence of renal tubule pigmentation was statistically significantly increased in males exposed to 625 mg emodin/kg diet or higher dietary doses and in females exposed to a dietary dose of 1,250 mg/kg diet or higher.
Rats
Groups of F344/N rats (5/sex per group, 6 weeks old) received for 15 (males) or 16 (females) days 0, 600, 2,000, 5,500, 17,000 or 50,000 mg emodin/kg diet (equal to daily doses of 0, 50, 170, 480, 1,400 or 3,700 mg/kg bw per day for males and to 0, 50, 160, 460, 1,250 or 2,000 mg/kg bw per day for females) (NTP, 2001). Three females from the highest dose group died before termination. Emaciation and inactivity were recorded for females from the highest dose group. All males and most females from 17,000 and 50,000 mg emodin/kg diet had diarrhoea. Discolouration of fur (pink to yellow) was seen in males and females from dietary dose groups of 2,000 mg emodin/kg diet or greater, of anus area (reddish‐brown) in males and females from dietary dose groups of 17,000 and 50,000 mg emodin/kg diet, and of urine (pink to yellow) in females from all treated groups. Feed intake in all treated groups of either sex was lower compared to controls. Body weighs and body weight gains were statistically significantly lower in males and females receiving dietary doses of 5,500 mg emodin/kg diet or greater. The reported statistically significant differences in organs weights compared to the control group were for males: (a) decreased absolute and increased relative kidney weights from 17,000 and 50,000 mg emodin/kg diet groups, (b) increased relative testis weight from 5,500 mg emodin/kg diet group or greater, (c) decreased absolute thymus weights from 5,500 mg emodin/kg diet group or greater, and decreased relative thymus weight from 17,000 and 50,000 mg emodin/kg diet groups. For females, differences in organ weights included (a) increased relative kidney weights from 17,000 and 50,000 mg emodin/kg diet groups, (b) decreased absolute thymus weight 17,000 and 50,000 mg emodin/kg diet groups, and decreased relative thymus weight in 50,000 mg emodin/kg diet group. At necropsy, kidneys from some of the animals from two highest dose groups were mottled and contained yellowish foci. Microscopic changes in the kidneys, recorded in one male and two females from 17,000 mg emodin/kg diet group and two males and five females from 50,000 mg emodin/kg diet group were suppurative inflammation, fibrosis, renal tubule epithelial hyperplasia, renal tubule dilatation and necrosis. The severity of these changes appeared to increase in a dose‐dependent manner. In some renal tubules, elongated clefts were seen presumably after the presence of crystals of emodin or a metabolite.
In another study, groups of F344/N rats (10/sex per group in the core study and 10/sex per group in a special study; 6 weeks old) received for 14 consecutive weeks 0, 312.5, 625, 1,250, 2,500 or 5,000 mg emodin/kg diet (equal to daily doses of 0, 20, 40, 80, 170 or 300 mg emodin/kg bw per day for males and females) (NTP, 2001). Statistically significantly decreased feed intake was recorded only in the first week for males in the two high‐dose groups and for females in the highest dose group. Statistically significantly lower body weights and body weight gains were recorded for males from 2,500 and 5,000 mg emodin/kg diet groups and for females from 1,250 mg emodin/kg diet group or greater. Discolouration of fur (yellow to red) was observed in all groups. Coloured faeces were observed only in one male from 5,000 mg emodin/kg diet group. Diarrhoea was observed in all exposed groups at various times. Statistically significantly increased platelet counts were recorded for males and females from 2,500 mg emodin/kg diet group and in segmented neutrophils counts in females from 2,500 and 5,000 mg emodin/kg diet groups. The oestrus cycle length was increased in females from groups exposed to 1,250 mg emodin/kg diet or 5,000 mg emodin/kg diet. The sperm motility was not statistically significantly different in the treated males as compared to controls. The statistically significant differences in organs weights compared to the control group were for males: (a) increased relative kidney weights from 1,250 mg emodin/kg diet group or greater, (b) increased relative lung weights from 625 mg emodin/kg diet or greater, (c) increased relative testis weights from 2,500 and 5,000 mg emodin/kg diet groups. For females, differences in organ weights included (a) increased relative kidney weights from 625 mg emodin/kg diet group or higher, and of absolute kidney weights in the 5,000 mg emodin/kg diet group, (b) increased relative liver weights from 625 mg emodin/kg diet group or higher, (c) increased absolute thymus weights of females from 2,500 and 5,000 mg emodin/kg diet groups. The differences in other organs weights were considered by the authors of the study as incidental and not related to treatment. According to the authors, treatment‐related microscopical changes were in the kidneys. These included (a) pigment in renal tubules in males and females from 1,250 mg emodin/kg diet group or greater, and the severity of pigmentation increased with the dietary doses, and (b) increased incidences of hyaline droplets in the cortical epithelial cytoplasm in all male groups and in females from 312.5, 625 or 1,250 mg emodin/kg diet groups.
Sennosides
Mice
Groups of male and female C57BL/6NTac mice (N = 5/sex per group, 6–7 weeks old at the start) received in basal diet 0, 625, 1250, 2500, 5000 or 10000 mg senna/kg diet (equal to 0, 115, 245, 490, 975 and 2,075 mg/kg bw per day for males and 0, 160, 310, 625, 1,190 and 2,570 mg/kg bw per day for females) for 29 days (Surh et al., 2013). The content of active components of the used senna was reported as: 0.7% (wt/wt) sennoside A, 1.3% sennoside B, 0.06% sennidin A and 0.03% sennidin B. All mice survived to the scheduled termination. Body weight was comparable to that in controls and feed intake was similar between all groups. No apparent treatment‐related clinical symptoms were reported. Absolute heart weights in all male groups and relative heart weights in males treated with 115, 975 and 2,075 mg senna/kg bw per day were lower than in the control group. Epithelial hyperplasia was recorded in the caecum and colon of all treated groups of males and in the caecum, colon and rectum of females receiving 625 mg senna/kg bw per day or higher.
3.2.3. Chronic toxicity and carcinogenicity
Aloe vera extracts
Feeding studies were in two rodent species (mice and rats) within the US NTP on aloe vera WLE (NTP, 2013; Boudreau et al., 2013).
Mice
Groups of B6C3F1 mice (48/sex per group, 6 weeks old) received aloe vera non‐decolourised WLE (the bulk material malic acid content of 186–203 mg/g, aloin A content of 5.7–7.2 mg/g) in drinking water at concentrations of 0, 1%, 2% or 3% (wt/wt) for 104 weeks. These concentrations provided (as reported by the authors of the study) 0, 2.94, 6.95 and 11.76 g aloe vera non‐decolourised whole leaf extract/kg bw per day for males and 0, 2.18, 6.28 and 11.83 g aloe vera non‐decolourised whole leaf extract/kg bw per day for females, corresponding to 0, 18.7, 44.82 and 75.85 mg aloin A/kg bw per day for males and 0, 14.06, 40.5 and 76.3 mg aloin A/kg bw per day for females.25
The survival of the controls and treated groups of either sex was comparable. The body weights of males in the treated groups were lower than in the controls through the study but the differences did not exceed 10% of the control body weights. The mean body weights of all treated male groups were statistically significantly lower compared to the controls (the 1% group 40.9 g (p < 0.01); the 2% group 40.7 g. (p < 0.01); the 3% group 40.3 g (p < 0.001) vs 43.2 g). The body weights of treated female groups throughout the study and the mean for the study period were not statistically significantly lower compared to the controls. Feed intake of the controls and treated groups of either sex was comparable. Polydipsia was observed in all male and female treated groups compared to the controls throughout the study. Mean daily water intake (g/day) in the 1%, 2% and 3% groups was for males 12.0 (p < 0.05), 14.2 (p < 0.01), 15.8 (p < 0.001) vs 7.8 in the control group, and for females 8.3 (p < 0.001), 11.7 (p < 0.001), 14.1 (p < 0.001). When expressed in g/kg bw per day, although markedly increased in a dose‐dependent manner the water intake of males and females was not statistically different from the controls (males: 181, 294, 348 and 392; females: 147, 218, 314 and 394 in the controls, 1%, 2% and 3% groups). The treatment‐related statistically significantly increased incidence of goblet cell hyperplasia of the large intestine was recorded for males and females. The incidences in the control, 1%, 2% and 3% groups were: (a) in the ascending colon of males 2/44 (4%), 16/44 (36%), 20/45 (44%), 19/42 (45%) and of females 1/44 (2%), 15/43 (35%), 20/44 (46%), 25/43 (58%); (b) in the transverse colon of males 4/47 (9%), 14/44 (32%), 21/45 (47%), 22/43 (51%) and of females 2/42 (5%), 18/42 (43%)23/44 (52%), 26/43 (61%); (c) in descending colon of males 0/47 (0%), 7/44 (16%), 12/45 (27%), 17/43 (40%) and of females 0/43 (0%), 4/43 (9%), 7/44 (16%), 17/43 (40%); (d) large intestine all site goblet cell hyperplasia of males 4/47 (9%), 17/44 (39%), 22/45 (49%), 22/44 (50%) and of females 3/43 (7%), 19/43 (44%), 24/44 (55%), 28/43 (65%). The incidence of mesenteric lymph node cellular infiltration was statistically significantly increased only in males from 3% group (6/43 (14%) vs 0/48 (0%) in the controls). No treatment‐related neoplastic changes were recorded in this study. The Panel considered hyperplastic changes in the large intestine as attributable to the treatment with Aloe vera non‐decolourised WLE.
Rats
Groups of F344/N rats (48/sex per group, 6 to 7 weeks old) received aloe vera non‐decolourised whole leaf extract (the bulk material malic acid content of 186–203 mg/g, aloin A content of 5.7–7.2 mg/g) in drinking water at concentrations of 0, 0.5%, 1% or 1.5% (wt/wt) for 104 weeks. These concentrations provided (as reported by the authors of the study) 0, 0.25, 0.62 and 1.136 g aloe vera non‐decolourised whole leaf extract/kg bw per day for males and 0, 0.33, 0.73, and 1.28 g Aloe vera non‐decolourised whole leaf extract/kg bw per day for females, corresponding to 0, 0.25, 0.65 and 1.136 mg aloin A/kg bw per day for males and 0, 0.33, 0.73 and 1.28 mg aloin A/kg bw per day for females.25
The survival of the controls and treated groups of either sex was comparable except for females from the 1.5% group for which the survival was statistically significantly reduced (91.9 vs 95.7 weeks in the control group). Mean body weights of males and females showed statistically significantly dose‐related decreases throughout the study. Mean body weights for the study in 0%, 0.5%, 1% and 1.5% groups were 457.5 g, 452.2 g, 436.6 g (p < 0.001) and 412.7 g (p < 0.001) for males and 276.7 g, 276.4 g, 260.1 (p < 0.001) and 238.5 g (p < 0.001) for females. Feed intake was statistically significantly decreased in the high‐dose males and in mid‐ and high‐dose females (g/day for males: 18.9, 18.5, 18.6, 17.8 p < 0.001 and for females 13.5, 13.3, 13.0 p < 001, 12.3 p < 0.001). Polydipsia was notable in males from 1% and 1.5% groups and in females from 1.5% group. Mean daily water intake (g/day) in the 0.5%, 1% and 1.5% groups was for males 22.5, 27.0 (p < 0.001), 31.0 (p < 0.001) vs 21.7 in the control group, and for females 18.2, 19.1, 20.4 (p < 0.01). When expressed in g/kg bw per day, although increased in a dose‐dependent manner the water intake of males and females was not statistically different from the controls (males: 47.6, 49.7, 61.8 and 75.1; females: 64.8, 65.7, 73.4 and 85.3 in the controls, 0.5%, 1% and 1.5% groups). Noteworthy microscopic non‐neoplastic changes were reported in the large intestine and associated mesenteric lymph nodes of the treated groups of both sexes. Statistically significant dose‐related increases in the incidences of mucosal hyperplasia of the proximal, ascending, transverse and descending colon and caecum were reported for males and females. The incidences in the control, 0.5%, 1%, and 1.5% male groups were: (a) for the proximal colon: 0/44 (0%), 29/44 (65.9%), 36/46 (78.3%), 32/41 (78.0%); (b) for the caecum: 0/47 (0%), 4/48. (8.3%), 17/47 (36.2%), 27/48 (56.3%); (c) for the ascending colon: 0/47 (0%), 40/48 (83.3%), 35/46 (76.1%), 39/46 (84.8%); (d) for the transverse colon: 0/47 (0%), 40/48 (83.3%), 33/46 (71.8%), 42/46 (91.3%) and (e) for the descending colon: 0/47 (0%), 17/48 (35.4%), 18/46 (39.1%), 27/47 (57.4%). The incidences in the control, 0.5, 1, 1.5% female groups were: (a) for the proximal colon: 0/43 (0%), 30/45 (66.7%), 33/42 (78.6%), 32/39 (82.1%); (b) for the caecum 0/47 (0%), 4/48 (8.3%), 17/47 (36.2%), 27/48 (56.3%); (c) for the ascending colon: 0/47 (0%), 40/48 (83.3%), 35/46 (76.1%), 39/46 (84.4%); (d) for the transverse colon: 0/47 (0%), 40/48 (83.3%),33/46 (71.1%) and (e) for the descending colon: 0/47 (0%), 17/48 (35.4%), 18/46 (39.1%), 27/47 (57.4%). Furthermore, statistically significant dose‐related increases in the incidences of mucosal hyperplasia were reported for the forestomach, glandular stomach, ileum, and rectum of females and for the glandular stomach, jejunum and rectum of males. In mesenteric lymph nodes, significant dose‐trend incidence increases were reported for cystic degeneration for both sexes. The incidences were 8/47 (17%), 11/48 (22.9%), 42/48 (87.5%), 41/48 (85.4%) for males and 0/46 (0%), 16/47 (34%), 40/48 (83.3%), 43/47 (91.5%) in the control, 0.5%, 1% and 1.5% groups. For males also, a significant dose‐trend increased incidences were reported for hyperplasia in mesenteric lymph nodes: 0/47 (0%), 0/48 (0%), 1/48 (2.1%), 4/48 (8.3%) (linear trend test p < 0.01). In the caecum, significant dose‐trend increases in the incidences of dilatation (linear trend test p < 0.001) were reported for males and females.
The neoplastic changes, considered by the authors as treatment‐related, were observed in the large intestine. These were adenomas and carcinomas confined within the mucosal wall and they did not metastasize to regional mesenteric lymph nodes or more distal sites. For males, statistically significant dose‐related increases in the incidence of adenomas were reported in the proximal, ascending and transverse colon, and in the caecum and the incidences were statistically significantly higher in the 1% and 1.5% groups compared to the control group. The incidences of adenomas in the control, 0.5%, 1%, and 1.5% male groups were: (a) for the proximal colon: 0/44 (0%), 0/44 (0%), 7/46 (15%, p < 0.05), 10/41 (24%, p < 0.01); (b) for caecum: 0/46 (0%), 0/45 (0%), 8/48 (17%, p < 0.01), 8/48 (17%, p < 0.001); (c) for ascending colon: 0/47 (0%), 0/47 (0%), 19/48 (40%, p < 0.001, 8/46 (17%, p < 0.01) and (d) for transverse colon: 0/47 (0%), 0/47 (0%), 6/47 (13%, p < 0.05), 3/47 (6%). Statistically significant dose‐related increases in the incidence of carcinomas were reported in the proximal and ascending colon of males but the statistical significance in the incidence was recorded only in the ascending colon in the 1.5% group. The incidences of carcinomas in the control, 0.5%, 1%, and 1.5% male groups were: (a) for the proximal colon: 0/44 (0%), 0/44 (0%), 4/46 (9%), 4/41 (10%); (b) for caecum: 0/46 (0%), 0/45 (0%), 1/48 (2%), 2/48 (4%); (c) for ascending colon: 0/47 (0%), 0/47 (0%), 4/48 (8%), 8/46 (17%) p < 0.01; and (d) for transverse colon: 0/47 (0%), 0/47 (0%), 1/47 (2%), 1/47 (2%). Significant dose‐related increases in the incidences for all adenomas, all carcinomas or all adenomas and all carcinomas of the large intestine, and statistically significantly higher incidences for the 1% and 1.5% male groups compared to the control group were reported. The incidences of tumours of the large intestine (all sites) in the control, 0.5%, 1% and 1.5% male groups were (a) for all adenomas: 0/47 (0%), 0/48 (0%), 26/48 (54%, p < 0019) and 23/48 (48%, p < 0.001); (b) for all carcinomas: 0/47 (0%), 0/48 (0%), 10/48 (21%, p < 0.001), 14/48 (29%, p < 0.001) and (c) for all adenomas and all carcinomas: 0/47 (0%), 0/48 (0%), 28/48 (58%, p < 0.001), 31/48 (65%, p < 0.001).
For females, statistically significant dose‐related increases in the incidence of adenomas were reported in the proximal and ascending colon, and in the caecum. The incidences were statistically significantly higher in the proximal colon in the 1% and 1.5% groups and in the ascending colon and the caecum in the 1% group compared to the control group. The incidences of adenomas in the control, 0.5%, 1%, and 1.5% female groups were: (a) for the proximal colon: 0/43 (0%), 0/45 (0%), 4/42 (10%, p < 0.05), 5/39 (13%, p < 0.05); (b) for caecum: 0/47 (0%), 0/48 (0%), 1/47 (2%), 6/48 (13%, p < 0.05) and (c) for ascending colon: 0/47 (0%), 0/48 (0%), 1/46 (2%), 5/46 (11%, p < 0.05). Carcinomas were observed in the proximal colon only. There was a statistically significant dose‐related increase in their incidences and a statistically significantly higher incidence was reported in the 1.5% group compared to the control group. The incidences of carcinomas in the proximal colon were 0/43 (0%), 0/45 (0%), 2/42 (5%), 4/39 (10%, p < 0.05) in the control, 0.5%, 1% and 1.5% female groups, respectively. For all adenomas, all carcinomas, or all adenomas or carcinomas of the large intestine significant dose trend increases in their incidences were reported and the incidence of all adenomas or all adenomas or all carcinomas were statistically significantly higher in the 1% and 1.5% groups compared to the control group. For all carcinomas a statistically significantly higher incidence was recorded only in the 1.5% group compared to the control. The incidences of tumours of the large intestine (all sites) in the control, 0.5%, 1%, and 1.5% female groups were (a) for all adenomas: 0/48 (0%), 0/48 (0%), 6/48 (13%, p < 0.05), and 13/48 (27%, p < 0.001), (b) for all carcinomas: 0/48 (0%), 0/48 (0%), 3/48 (6%), 4/48 (8%, p < 0.05), and for all adenomas and all carcinomas: 0/48 (0%), 0/48 (0%), 8/48 (17%, p < 0.01), 15/48 (31%, p < 0.001). The authors considered that laboratory rodents did not develop spontaneous neoplasms of the colon and therefore that ‘the combined results from this study suggest that the long‐term exposure of the rats to the Aloe vera whole‐leaf extract induced insult to the intestinal tract and caused a progression of lesion types from goblet cell hyperplasia to mucosal hyperplasia to adenoma and carcinoma in the large intestine’ (Boudreau et al., 2013). The authors concluded that under conditions of this bioassay ‘there was a clear evidence of carcinogenic activity of nondecolorized a whole leaf extract of aloe vera in male and female F344/N rats based upon increased incidences of adenomas and carcinomas of the large intestine’. The Panel considered that the long‐term exposure of rats to Aloe vera non‐decolourised whole leaf extract at concentrations of 1% and 1.5% (wt/wt) in drinking water resulted in development of adenomas and carcinomas in the large intestine.
In a study by Matsuda et al. (2008), four groups of Wistar Hanover rats (10–14/sex per group, 6 week of age at the start of the study) received 0%, 0.16%, 0.8% or 4% of whole leaf powder of Aloe (Aloe arborescens Miller var. natalensis Berger, content of aloin 0.83% and of 1.91% of aloenin) in the diet for 1 year. These dietary concentrations of the whole leaf powder of Aloe provided, according to the authors, the test compound in mean daily doses of 87.70 ± 6.08, 431.35 ± 33.76 and 2,212.17 ± 141.65 mg/kg bw for males and of 109.57 ± 5.92, 540.57 ± 31.16 and 2,681.39 ± 149.33 mg/kg bw for females from the low‐, mid‐ and high‐dose groups. The concentrations of aloin were 0.0009%, 0.0039%, and 0.0179%, and of aloenin 0.0022%, 0.0133% and 0.0663% in the diet containing 0.16%, 0.8% and 4% of the whole leaf powder of Aloe, respectively, corresponding to 0, 0.00078, 0.016, 0.395 mg aloin/kg bw per day for males and 0, 0.00098, 0.021, 0.479 mg aloin/kg bw per day for females. The content of aloin and aloenin was not determined in the control diet. All animals survived to the termination but the authors reported that several samples were lost (the type of the samples and the reason for the loss was not informed) and therefore the number of animals evaluated for the various parameters ranged from 6 to 14. In the high‐dose group, diarrhoea was observed in males and loose stool in females through the experimental period, body weights of females were statistically significantly lower compared to the controls and body weights of males were reported to show a tendency to decrease. The feed intake was similar in all groups. From results of haematology analyses, only a statistically significant increase in leucocytes in the high‐dose male group and statistically significant decrease in haemoglobin in the mid‐ and high‐dose female groups as compared to the controls were considered treatment related by the authors of the study. From results of clinical chemistry analyses, only treatment‐related changes compared to the controls were, according to the authors, a statistically significant decrease in inorganic phosphorus in the mid‐ and high‐dose male groups and of inorganic phosphorus and calcium in the high‐dose female group. For males, no toxicologically significant changes in absolute and relative organ weights as compared to controls were recorded, as the only statistically significant change was the increase in the relative liver weight in the mid‐dose group as compared to the control. The Panel noted lack of a dose response and of histological changes in the liver. For females, no toxicologically significant absolute organ weight changes as compared to controls were recorded, as the only statistically significant change was the decrease in the absolute adrenal weight in the high‐dose group without a dose response or histological changes in adrenals. The changes in relative organ weights of females as compared to controls were statistically significant higher relative brain and heart weights in the mid‐ and high‐dose groups, and a statistically significantly higher relative weight of the kidneys in the high‐dose group. According to the authors, these changes were of no toxicological significance as they were the results of lower body weights of these groups compared with controls. The Panel agreed that the increases in the relative weights of these organs were secondary to the decreased body weight. At necropsy, swelling of ileocaecal lymph nodes was observed in 70% males and 77.8% females from the high‐dose group as compared to none in the controls. Microscopical findings considered by the authors as toxicologically significant and with incidences statistically significantly higher than those in the controls were: (a) a severe dilatation of lymph sinus in the swelled ileocaecal lymph nodes (60% for males and 77.8% in females vs 0% in the controls), (b) yellow‐brown pigmentation in ileocaecal lymph nodes (for males 90% vs 8.3%, and for females 55.6% vs 0%), (c) sinus dilatation in mesenteric lymph nodes in the high‐dose females (80% vs 33.3%), (d) yellow‐brown pigmentation in renal tubules in the high‐dose group of either sex as compared to the controls (for males 90% vs 8.3%, and for females 80.0% vs 28.6%). There was no statistically significant differences in the incidences of the tumours between the treated groups and the controls of the same genders. No hyperplastic or neoplastic changes were found in the GI system of the treated and controls animals. The authors concluded that the diarrhoea, lower body weight, morphological changes in ileocaecal lymph nodes and yellowish pigmentation in renal tubules in both sexes from the high‐dose group might have been due to anthranoid in Aloe. Considering that changes in some haematological and clinical chemistry parameters were recorded also in the mid‐dose group of males and females, the authors identified a no‐observed‐adverse‐effect‐level (NOAEL) of 0.16% in the diet of the whole leaf powder of Aloe, equal to 87.70 and 109.7 mg whole leaf powder of aloe/kg bw per day for males and females, respectively. The Panel concurred with the conclusions of the authors.
Three groups of Wistar Hanover rats (52–58/sex per group, 6 week of age at the start of the study) received 0, 0.8% or 4% of whole leaf powder of Aloe (Aloe arborescens Miller var. natalensis Berger, content of aloin 0.83% and of 1.91% of aloenin) in the diet for 2 years (Yokohira et al., 2009). These dietary concentrations of the whole leaf powder of Aloe provided, according to the authors, the test compound in mean daily doses of 374.5 ± 152.7 and 2,073.9 ± 731.9 mg/kg bw for males and in 462.9 ± 138.8 and 2,509.6 ± 701.7 mg/kg bw for females from the low‐ and the high‐dose groups. The concentrations of aloin were 0.0009% and 0.0179%, and of aloenin 0.0022% and 0.0663% in the diet containing 0.8% and 4% of the whole leaf powder of Aloe, respectively, corresponding to 0, 0.0033, 0.37 mg aloin/kg bw per day for males and to 0, 0041, 0.449 mg/kg bw per day for females. The content of aloin and aloenin was not determined in the control diet. The Panel noted that the same dietary preparations were used in the 1‐year chronic toxicity study (Matsuda et al., 2008) and the 2 year carcinogenicity study (Yokohira et al., 2009) but that the dietary levels of aloin and aloenin reported present in the 0.8% carcinogenicity study diet is that given for the 0.16% chronic toxicity study diet. The levels of aloin were also reported to be approximately 20 fold lower in the 0.8% diet than the levels in the 4% diet in the carcinogenicity study, rather than the expected approximate 5 fold lower level. The Panel therefore considered that the dietary aloin and aloenin content in the 0.8% group in the carcinogenicity study were incorrect and presumed the dietary data given in the chronic toxicity study (Matsuda et al., 2008) was correct and applicable for both the chronic toxicity and carcinogenicity studies. The survival was 67%, 77%, 79% for males and 59%, 67% and 77% for females from 0, 0.8% and 4% groups. The difference in the survival rate was statistically significant in the high‐dose females as compared to the control and to the low‐dose groups. Diarrhoea was observed in the high‐dose males and loose stool in the high‐dose females through the whole experimental period. Body weights of males and females in the high‐dose group were statistically significantly lower compared to the controls but the feed intake was similar in all groups. From haematology parameters the only differences to controls were significant increases in haemoglobin (13.4 ± 1.8 vs 11.7 ± 3.5 control, p < 0.05) and platelet count (135 ± 24.6 vs 114.3 ± 24.8, p < 0.05) of 4% group males. Clinical chemistry analyses revealed significant decreases in aspartate transaminase (AST), Na in the 4% male group and of Cl in the 0.8% and 4% male groups. Albumin/globulin ratio was statistically significantly increased in the 4% female group and Cl was significantly decreased in 0.8% female group. Statistically significant differences to the controls in organ weights were: increased relative liver weight of 4% males, increased absolute and relative spleen weights of 0.8% and 4% males (but without a dose response), and increased relative weight of uterus in 4% females. Noteworthy microscopic non‐neoplastic changes as compared to the controls were for males statistically significantly increased incidences of dilatation of sinus in mesenteric lymph nodes in 0.8% and 4% groups (14/39, 31/42 vs 5/38 in the control group, p < 0.05 and p < 0.01), of thickening of colon epithelium in 0.8% and 4% groups (10/39, 34/42 vs 0/38, p < 0.01); of yellow‐brown pigmentation of renal tubules (18/39, 36/42 vs 5/38, p < 0.01). Noteworthy microscopic non‐neoplastic changes as compared to the controls were for females statistically significantly increased incidences of dilatation of sinus in mesenteric lymph nodes (38/45 vs 3/40, p < 0.01), and thickening of colon epithelium (31/45 vs 0/40, p < 0.01) in the 4% group and of yellow‐brown pigmentation of renal tubules in 0.8% and 4% groups (33/42, 43/45 vs 18/40, p < 0.01). In addition atypical hyperplasia in colon was recorded in the 4% male group (5/42 vs 0/38, p < 0.05) and in the 4% female group (3/45 vs 0/40, p > 0.05). Noteworthy microscopic neoplastic changes were adenomas (3/42) and a carcinoma (1/42) in colon of males and adenomas in colon of females (3/45) from 4% group, one adenocarcinoma in caecum (1/42) and one adenoma (1/42) in rectum in the 4% male group compared to none in the control and 0.8% groups. There were no significant differences in the incidences of other tumours between the treated groups of males and females as compared to controls. The authors concluded that the whole leave powder of Aloe ‘exerted equivocal carcinogenic potential at 4% high dose level in the colon in the 2‐year carcinogenicity study in rats. Aloe is not carcinogenic at non‐toxic dose levels and that carcinogenic potential at 4% high‐dose on colon is probably due to irritation of the intestinal tract by diarrhoea’. The Panel considered that the long‐term exposure of rats to whole leaf powder of Aloe at concentration of 4% (wt/wt) in the diet resulted in development of adenomas and carcinomas in the large intestine. The Panel agreed with the authors that irritation of the intestinal tract manifested clinically as diarrhoea could play a role in the aetiology of the tumours observed in the large intestine.
Emodin
Feeding studies were conducted in two rodent species (mice and rats) within the US NTP on emodin (NTP, 2001).
Mice
Groups of B6C3F1 mice (60/sex per group, 6 weeks old) received for 105 weeks dietary doses of emodin which were for males 0, 160, 312, or 625 mg/kg diet and for females 0, 312, 625 or 1,250 mg/kg diet (equal to daily doses of 0, 15, 35, or 70 mg emodin/kg bw per day for males and 0, 30, 60, or 120 mg emodin/kg bw per day for females) (NTP, 2001). Clinical appearance, survival, feed intake and body weights of treated and control males and females were similar during the study.
The noteworthy non‐neoplastic changes were reported in kidneys. The statistically significantly increased incidences of renal tubule pigmentation were recorded after 12 and 24 months in all treated male groups. The incidence of renal tubule pigmentation of females was statistically significantly increased in 625 and 1,250 mg emodin/kg diet groups after 12 months and in all treated groups after 24 months. Severities increased in dose related manner. The incidences of nephropathy were statistically significantly increased only in females in 1,250 mg emodin/kg diet group after 12 months and in all treated groups after 24 months. Severities of nephropathy appeared to increase with increasing concentrations.
Neoplastic changes in kidneys were observed in males only. These were renal tubular adenomas and adenocarcinomas. The incidences of these tumours were not statistically significantly increased compared to the controls but the incidence of adenomas or carcinomas combined in the mid‐dose male group exceeded the historical control range. As renal tubular neoplasms are rare in male mice, the authors considered the presence of these tumours as suggestive of a possible association with exposure to emodin. The Panel noted that renal tubular hyperplasia, adenomas, and carcinomas are considered to represent a morphologic continuum of proliferative lesions. Therefore, in case of treatment relationship of these tumours, one could expect increased incidence of renal tubule hyperplasia in the emodin treated groups. However, the incidences of renal tubule hyperplasia were not statistically significantly increased in the treated groups. On the other hand, because the data on incidence of adenomas or carcinomas combined in the mid‐dose male group exceeded the historical control range, the Panel considered that treatment dependency of these tumours could not be excluded.
Other neoplastic changes were malignant lymphomas and alveolar/bronchiolar carcinomas in males. The incidences of both tumours were decreased as compared to the control group. According to the authors, the incidence of malignant lymphomas in the study was at the lower end of the historical control range. As the incidences of alveolar epithelial hyperplasia in the lungs were not decreased in the treated males and no decreases were observed in female mice, the decrease in the incidences of alveolar/bronchiolar carcinoma in males was not considered by the authors to be treatment‐related. The Panel concurred with this view.
Rats
Groups of F344/N rats (65/sex per group, 6 weeks old) received for 105 weeks 0, 280, 830 or 2,500 mg emodin/kg diet (equal to daily doses of 0, 110, 320 or 1,000 mg emodin/kg bw per day for males and 120, 370 or 1,100 mg emodin/kg bw per day for females) (NTP, 2001). Clinical appearance, survival, feed intake and body weights of treated and control males and females were similar during the study. The statistically significantly increased incidences of renal tubular hyaline droplets (a non‐neoplastic kidney change which was also seen in a 14‐week study) were recorded after 6, 12 and 24 months in males and females from all treatment groups and of renal tubular pigmentation after 2 years in all treatment groups of males. The noteworthy neoplastic changes were Zymbal gland (sebaceous gland) carcinomas, squamous cell carcinomas of the nose, pituitary gland adenomas and mononuclear cell leukaemia. The incidences of Zymbal gland carcinomas were in males 0/50; 1/50, 0/50, 1/50 and in females 0/50, 0/50; 0/50, 3/50 for dietary dose groups of 0, 280, 830 and 2,500 mg emodin/kg diet, respectively. The incidences in males from the low and the high‐dose groups were within the historical controls. The incidence in the female high‐dose group exceeded the historical control values. The latter could, according to the Panel, indicate relation to treatment, as Zymbal gland carcinoma is not a common type of tumour in rats.
Squamous cell carcinomas were recorded in the nasal cavity of males from 2,500 mg emodin/kg diet group (0/50, 0/50, 0/50, 2/50). These tumours are rare in F344/N rats (the range in historical controls is 0‐2%). However, the absence of preneoplastic lesions or other treatment‐related lesions in the nasal cavity indicated, according to the authors, that the squamous cell carcinomas were unrelated to treatment. The Panel concurred with these considerations.
Another neoplastic finding, increased incidence of pituitary gland adenoma (pars distalis) in females in 830 and 2,500 mg emodin/kg diet groups (15/49, 21/50, 25/49, 25/49) was considered by the authors of the study as not treatment related because (a) the incidences in the treated groups were similar to the historical control incidence, (b) the incidence in concurrent control was lower than in the historical controls, (c) there were no increases in incidence of hyperplasia in the pituitary gland of females and (d) there was no evidence of an increase in the incidence of proliferative lesions in male rats. The Panel concurred with the considerations of the authors.
The fourth type of neoplastic lesion was mononuclear cell leukaemia (MNCL) with incidence in the males and females from 2,500 mg emodin/kg diet group significantly less than in the controls and lower than in historical control females and at the lower end of the historical control range for males. The Panel noted that MNCL (a) is a unique and common finding in the Fischer rat strain used by the US NTP program, (b) the incidence of MNCL in the F344 rats is around 50% for males and around 30% for females, with large variation from study to study, (c) that exposure to some genotoxic carcinogens did not lead to an increase in MNCL in F344 rats, while a number of substances, which are believed to be non‐carcinogens, caused increase and (d) based on this MNCL in the F344 rats has been considered of limited relevance for humans (Caldwell, 1999).
Overall, the Panel noted that in carcinogenicity studies with emodin in the diet by NTP (2001) no treatment‐related non‐neoplastic or neoplastic changes in the GI system were reported at doses up to 70 or 120 mg/kg bw per day for male and females mice, respectively, and at doses up to 1000 or 1100 mg/kg bw per day for male and female rats, respectively.
Sennosides
Rats
Four groups of Sprague–Dawley rats (50/sex per group) received 0, 5, 15 or 25 mg/kg bw per day of purified senna extract (total content of sennosides 94.7% by fluorimetry USP method, or 35.7% of total sennosides and 5.1% of other anthranoids by a reversed‐phase HPLC with UV detection) in drinking water for 104 weeks (Lydén‐Sokolowski et al., 1993). Slight to moderate loose stool (referred by the authors’ laxative effect of the sennosides preparation) was observed through the clinical phase of the study in the high‐dose group and it was more pronounced in males compared to females. Loose stool was also occasionally recorded in the mid‐dose males. The survival of the controls and the treated was similar (23/5, 22/50, 25/50, 23/50 and 26/50, 29/49, 31/50, 27/50 for males and females from the control, low‐, mid‐ and high‐dose groups, respectively). Body weight gain and body weights of the high‐dose males were statistically significantly lower than those of the controls. Body weight and body weight gains of females from all treated group were comparable to those of the controls. Water intake of mid‐ and high‐dose females was about 15% less than in the controls. No difference in water intake was reported for treated males compared to controls. No treatment‐related changes haematology, clinical chemistry and urinalysis parameters were observed. Kidney weights of females from mid‐ and high‐dose groups were statistically significantly increased compared to the controls (absolute: 11% and 16%, relative 15% and 23% higher in mid‐ and high‐dose groups). In males the relative kidney weight was 14% higher in the mid‐dose group only compared to the control. A higher incidence of reactive mesenteric lymph node hyperplasia without dose‐response relationship was reported for treated rats sacrificed before the scheduled termination but the incidence of this lesion was similar in the control and high‐dose group at the termination. Therefore, the authors considered reactive mesenteric lymph node hyperplasia of no biological relevance. There was no increase in the incidence of neoplastic lesions between the control and high‐dose group. According to the authors the results did not indicate any relationship between long‐term exposure to purified senna extract and development of tumours in kidney, adrenal, liver or GI system. The high dose which was associated with moderate laxative effect in the rat was 20–25 times the recommended clinical dose according to the authors. The Panel considered that the purified senna extract used in this study was not carcinogenic to rats under condition of this bioassay. The Panel noted that incidence of mesenteric lymph node hyperplasia and of neoplastic lesions was not presented separately for each sex.
Sprague–Dawley rats (60/sex per group, body weight of 145–218 g for males and 120–165 for females at the start of the study) received by gavage 0, 25, 100 or 300 mg senna preparation/kg bw for 104 weeks (Mitchell et al., 2006). The senna preparation consisted of powdered Tinnevelly senna pods containing 1.829% of sennosides A–D, 1.596% of potential rhein, 0.111% of potential aloe‐emodin, 0.014% of total emodin and 0.004% of total chrysophanol (sum of potential hydroxyanthraquinones 1.725%). The survival of all treated groups of females and the low‐dose group of males was comparable to controls while it was lower in the mid‐ and high‐dose group males. The differences to controls in several parameters were recorded solely in the high‐dose group: (a) body weights were decreased and for females attained statistical significance on several occasions, (b) water intake was increased in both sexes and attained statistical significance on several occasions, (c) serum levels of potassium and chloride were increased, (d) urinary levels of sodium, potassium and chloride were decreased and the urine was darker, (e) the faeces were reported as ‘mucoid’. At necropsy dark discolouration of kidneys was observed in some animals of all treated groups with the highest incidence in the high‐dose group. By microscopy, a treatment‐related and dose‐dependent pigment deposition within cortical tubular epithelia along with diffuse swelling and basophilia were reported in the kidneys. In some kidneys, vacuolisation of tubular cells and increased fat deposition were reported. In the large intestine and caecum, a treatment‐related diffuse mucosal epithelial hyperplasia with incidence increasing in a dose‐dependent manner was reported. No treatment‐related tumours were observed in GI tract or other organs. The authors concluded that senna was not carcinogenic at doses up to 300 mg/kg bw given for 2 years to SD rats. The Panel concurred that the powdered senna pods was not carcinogenic under conditions of this bioassay.
Nine groups of male Wistar rats (body weight of 120–140 g at the start of the study) received the following treatment in an initiation–promotion study: group 1 (N = 10) served as a vehicle control; group 2 (N = 20) received 15 mg azoxymethane (AOM)/kg bw; group 3 (N = 10) 10 mg/kg bw senna pod extract from Cassia angustifolia containing about 50% sennoside B (SE) by gavage, group 4 (N = 10) 100 mg SE/kg bw; group 5 (N = 10) 15 mg AOM/kg bw and 10 mg SE/kg bw; group 6 (N = 10) 15 mg AOM/kg bw and 100 mg SE/kg bw; group 7 (N = 10) 15 mg AOM/kg bw; group 8 (N = 10) 10 mg SE/kg bw; group 9 (N = 10) 15 mg AOM/kg bw and 10 mg SE/kg bw (Mascolo et al., 1999). The Panel noted that there is no explanation in the study on the why ‘group 2′ has twice the number of animals compared to the other groups. AOM was dissolved in saline and given intraperitoneally on day 1 and 5. SE was dissolved in water and given by gavage 6 times per week. Groups 2–6 were terminated after 13 weeks, and groups 1 and 7–9 after 28 weeks. According to the authors, the doses of SE were chosen to induce laxation (10 mg/kg bw) or diarrhoea (100 mg/kg bw). No aberrant crypt foci (ACF) were observed in control rats or those treated with a low dose of SE for 13 or 28 weeks or with a high dose SE for 13 weeks (groups 1, 3, 4 and 8). In rats treated solely with AOM, ACF in the colon were recorded both after 13 weeks (group 2) and 28 weeks (group 7) of treatment. In these groups, a single adenoma and carcinoma in the colon were recorded after 13 weeks (incidence 10.5%) and 1 adenoma and 12 adenocarcinomas (incidence 55.5%) were reported after 28 weeks. In rats treated with AOM and a low dose of SE (group 5), the number of ACF and tumours were similar to those in group 2 (initiated with AOM) after 13 weeks and not statistically significantly increased after 28 weeks (group 9 compared to group 7). In group 6 treated with AOM and a high dose of SE for 13 weeks, statistically significant increases compared to group 2 (initiated with AOM) were recorded in number of ACF which consisted of 4 or more crypt (43.8 ± 11.7 vs 29.3 ± 17.4 in group 2), number of crypt per ACF (4.2 ± 0.8 vs 2.5 ± 0.3 in group 2), incidence of tumours (90% vs 10% in group 2), number of tumours in colon/rat (3.20 ± 2.4 vs 0.11 ± 0.3) and number of carcinomas (19 vs 1). According to the authors the results indicated that SE did not cause ACF in the colon nor had a tumour promoting effect at a dose, which caused laxation while at a dose which caused diarrhoea SE increased number of colonic tumours in rats. The Panel agreed with the authors and noted that the diarrhoeagenic dose appeared to promote development of tumours initiated by AOM.
Mice
Groups of male and female C3B6.129F1‐Tr53 tm1BrdN12 haploinsufficient (p53+/−) mice received in basal diet 0, 100, 300, 1,000, 3,000 or 10000 mg senna/kg diet (equal to 0, 12, 36, 120, 365 and 1,260 mg/kg bw per day for males and 0, 14, 42, 140, 435, and 1,520 mg/kg bw per day for females) for 40 weeks (Surh et al., 2013). The content of active components of the used senna was reported as: 0.7% (wt/wt) sennoside A, 1.3% sennoside B, 0.06% sennidin A and 0.03% sennidin B. Body weights, feed intake and clinical findings were recorded weekly. Survival of all treated groups was similar to that of controls. Differences in body weight in dosed groups did not exceed 10% of the weight in the control group throughout the study. The absolute and relative liver weights of males from the highest dose group were statistically significantly lower than in the control group. Microscopic examination revealed epithelial hyperplasia in the large intestine: (a) in males from the highest dose group the incidence of epithelial hyperplasia in caecum (22/25) and colon (25/25) was statistically significantly higher than in the control group (0/25 in both locations); (b) in females a statistically significantly higher incidence was reported in the colon of the group receiving 435 mg senna/kg bw per day and in the caecum and colon of the group receiving 1,520 mg senna/kg bw per day, the highest dose tested. Neoplastic findings included osteosarcomas in males (0/25, 0/25, 0/25, 3/25, 2/25 and 0/25 in groups exposed to 0, 12, 36, 120, 365 and 1,260 mg senna/kg bw per day, respectively), and in females (2/25, 2/25, 1/25, 3/25, 0/25 and 2/25 in the groups exposed to 0, 14, 42, 140, 435 and 1,520 mg senna/kg bw per day, respectively), and hepatocellular adenomas in males (1/25, 0/25, 5/25, 0/25, 2/25 and 1/25 in groups exposed to 0, 12, 36, 120, 365 and 1260 mg senna/kg bw per day, respectively). The incidence of osteosarcomas in males did not exceed the range in historical controls (0–12%). In females exposed to 140 mg senna/kg bw per day the incidence of osteosarcoma exceeded the historical control range of 0–12% but the difference to the concurrent control was not statistically significant. The incidences of hepatocellular adenoma exceeded the historical control range (4–11%) only in the males exposed to 36 mg senna/kg bw per day (20%) and this incidence was not statistically significantly higher than that in the control group (4%). The authors considered increased incidences of osteosarcoma and hepatocellular adenoma as not treatment‐related finding. The Panel concurred with this conclusion.
Danthron
Mice
Two groups of C3H/HeN male mice (20/group, 2 months old) received either basal diet or diet containing 0.2% chrysazin (1,8‐dihydroxy‐9, 10‐anthracenedione=danthron), a synthetic anthraquinone (N = 18) for 540 days (Mori et al., 1986). This dietary concentration was equivalent to 300 mg/chrysazin/kg bw per day according to EFSA ‘Guidance on default values to be used in the absence of measured data’ (EFSA Scientific Committee, 2012). Nineteen mice from the control group survived until the scheduled termination. One control mouse died of pneumonia soon after the start of experimental feeding (no exact information on the day of death). In the 0.2% chrysazin group, 16 mice survived to the scheduled termination, 3 mice died of pneumonia during the first 100 days of the clinical phase of the study and one mouse died on day 510. The Panel noted that this mouse has to be subjected to the necropsy and the samples must have been taken for microscopic examination as the effective number of the mice in this group was reported as 17. Terminal body weights of the mice were similar in both groups (27.6 g ± 2.9 (SD) and 27.5 g ± 3.0 in the control and the treated groups, respectively). The mean absolute liver weight of the treated mice was statistically significantly increased compared to that in the control group (1.7 g ± 0.1 (SD) and 2.1 g ± 0.5, p < 0.05). At necropsy, the caecal wall in all mice from the 0.2% chrysazin group was thickened and multiple polyps were visible. In the liver, single or multiple nodules were seen in 5/19 control mice and in 9/17 treated mice. The nodules in the controls were smaller. Microscopic examination revealed that in the caeca with polyps all layers of the caecal wall were frequently penetrated with cystic epithelium–lined glands. Penetration of the Peyer's patches with these cystic glands was also frequently seen. Fibrosis with infiltration of inflammatory cells was associated with these changes. Goblet cells were increased in number and excess mucin was present intracellularly and in the dilated lumen of the glands. Similar changes were recorded in the upper 1/3 part of the colon. These changes were referred by the authors of the study as adenomatous hyperplasia; the incidences were 17/17 in caecum and 5/17 in the colon vs 0/19 in the control group. Microscopic examination of the livers revealed that 5/19 control mice had adenomas but no carcinomas while 9/17 treated had adenomas and 4/19 had carcinomas. According to the authors, the adenomatous hyperplasia in the large intestine indicated ‘a certain carcinogenic potency of chrysazin in mice’ and the liver tumour findings indicated that chrysazin ‘enhanced the progression of spontaneously occurring hepatocarcinogenesis’. The Panel noted the limitation in the study design (e.g. low number of animals/group, only one sex) and the limited reporting (e.g. no data on housing and environmental conditions, no data on body weight changes, feed intake and calculated intake of the test compound, or on incidence of non‐neoplastic changes other than adenomatous hyperplasia in caecum and colon in GI system or other organs/tissues). The Panel considered that the presence of hyperplastic lesions in the large intestine and increased number of liver adenomas (the most common hepatic neoplasm in laboratory mouse, Harada et al., 1999) and carcinoma (a common spontaneous hepatic neoplasm in mice; Harada et al., 1999, Doull et al., 1983; as cited by Mori et al., 1986) in the treated group indicated that chrysazin was carcinogenic to C3H/HeN male mice under conditions of this limited bioassay.
Rats
Two groups of male ACI rats (2 months old) received either basal diet (N = 15) or this diet added 1% chrysazin (1,8‐dihydroxy‐9, 10‐anthracenedione = danthron), a synthetic anthraquinone (N = 18) for 16 months (Mori et al., 1985). This dietary concentration was equivalent to 520 mg/chrysazin per kg bw per day according to EFSA ‘Guidance on default values to be used in the absence of measured data’ (EFSA Scientific Committee, 2012). Fourteen rats from the control group and 12 from 1% chrysazin group survived to the schedules termination. One rat in the control group died of pneumonia before one year of the study. In the treated group, two rats manifesting diarrhoea and diagnosed with anaemia died during the first 3 months of the study and additional four were lost before the 12 months. According to the authors of the study, the feed intake and body weights of control and treated rats were similar during the study. The non‐neoplastic changes were recorded in the treated group only and included focal hyperplasia of the glandular epithelium of the colon and caecum (which was recorded both in the animals which had not or had intestinal tumours; exact incidence not reported) and focal necrosis, fibrosis, cystic lesions and bile duct proliferation in the liver (exact incidences not reported). The neoplastic changes were recorded in colon and caecum of 7 from 12 survivors in 1% chrysazin group. Three animals had single adenomas of the colon, four animals had single adenocarcinomas of the colon and one of these animals had in addition to adenomas in the caecum. No tumours were recorded in the control group. The authors ascribed the tumours in the large intestine to the treatment of chrysazin (referred by them as ‘pure anthraquinone’’ because (a) untreated ACI rats did not develop spontaneous intestinal tumours as previously reported (Maekawa and Odashima, 1975, as cited by the authors of the study), (b) the rats in the present study in the chrysazin group developed mucosal hyperplasia in the colon and the caecum, a preneoplastic lesion, and (c) both tumours and mucosal hyperplasia in the colon and the caecum were not recorded in the concurrent control group. The Panel concurred with the authors’ interpretation of the results but noticed the limitation in the study design (e.g. low number of animals/group, only one sex, shorter duration of the clinical phase as compared to the recommendation in the internationally recognised guidelines for a carcinogenicity study) and the limited reporting (e.g. no data on housing conditions, no data on body weight changes, feed intake and calculated intake of the test compound, no exact information on time of death/prescheduled termination due to moribund condition, or on incidence of non‐neoplastic changes).
3.2.4. Human Data
Colon cancer and laxatives use
The Panel is aware that evaluations of epidemiological studies addressing the potential association between CRC and the use of laxatives have been done previously by EMA, (2007a,b,c,d, 2008a,b). It is not the purpose of the Panel to re‐examine in this opinion all available information on this topic and therefore only studies published after 2006, or some older studies not mentioned in the past evaluations will be discussed in detail below. The interested reader is thus advice to consult EMA for details on all studies taken into account.
The findings of three epidemiological studies on laxatives use and CRC that were not reviewed by EMA (2007a,b,c,d, 2008a,b) (Dukas et al., 2000; Watanabe et al., 2004; Kojima et al., 2004) and studies after 2006 are described below (Park et al., 2009; Zhang et al., 2013; Citronberg et al., 2014).
Park et al. (2009), within the EPIC‐Norfolk prospective study (UK) conducted a nested case–control study to investigate the association between CRC and bowel habit and laxative use. Among the 25,663 subjects enrolled in the study aged between 45 and 79 years, 299 CRC invasive cases were identified after 12 years of follow‐up. Data on health and lifestyle characteristics such as height, weight, smoking, physical exercise and diet were collected at baseline while data on bowel movement and laxative use were collected after three years from the first health examination. In the study, 159 cases and 771 controls were included. In this study, laxative use (odds ratio (OR): 0.92; 95% CI: 0.44–1.93) was not associated with CRC, after controlling for waist to hip circumference, body mass index (BMI), smoking, energy intake, alcohol intake, dietary fibre and total meat intake. Having loose stools compared to soft stools was associated with an increased risk (OR: 2.80; 95% CI: 1.41–5.56). The limitation of the study was the low response rate of cases (53%), the low number of cases and the high number of missing data on laxative frequency (91% among cases and 89% among controls). The strength of the study was the good control for confounding factors.
Zhang et al. (2013) examined the association between bowel movement frequency, laxative use and the risk of colorectal cancer in two prospective cohort studies in the US. Data on demographic, lifestyle factors and medical history was collected from 88,173 women aged 30–55 years (The Nurses’ Health Study (NHS)) and from 23,722 men aged 40–75 years (Health Professionals Follow‐up Study (HPFS)). Participants were asked how often they use laxatives including softeners, bulking agents, and suppositories. In the follow‐up period (8 years for the NHS and 10 years for HPFS), 2,012 incident colorectal cancer cases were identified. Weekly and daily use of laxative vs never use was associated with an increased risk for colorectal cancer, although not statistical significant, in men (HR: 1.41; 95%CI: 0.96–2.06, p trend = 0.07) but not in women (HR: 0.98; 95% CI: 0.81–1.20, p trend = 0.79), after controlling for age, history of CRC, history of endoscopy, aspirin use, BMI, physical activity, post‐menopausal hormone use, processed meat intake, alcohol consumption, calcium, folate, vitamin D and total energy. No association was found for infrequent bowel movement. The strength of the study was the large sample size and the high completeness of follow‐up (> 90%). The limitations of the study were as follow: the lack of control in the multivariable analysis for fruit and vegetable intake and smoking; no subdivision of laxatives by type and the method of cases identification (self‐report).
Citronberg et al. (2014), investigated the use of non‐fibre and fibre containing laxative in relation to risk of CRC in a prospective cohort study (N = 364,418) conducted in Washington state (Vitamins and lifestyle study (VITAL). Baseline standardised questionnaires on socio‐demographic and lifestyle characteristics and medical history including history of constipation, bowel movement frequency and the use of non‐fibre laxatives (e.g. Ex‐Lax, Correctol) and fibre laxatives (e.g. Metamucil, Citrucel) were mailed to all participants between 2000 and 2002. Among these, 72,214 subjects, aged 50–76 years, with no CRC, ulcerative colitis or Crowns diseases and intestinal polyposis answered the questionnaire and participated in the study. In 2008, 558 incidence cases of invasive CRC were identified via linkage to state cancer registries. After controlling for age, sex, race, education, physical activity, BMI, fibre, calcium, fruit and vegetable intake, red and processed meat intake, alcohol consumption, caloric intake, smoking, NSAID use, aspirin, family history of CRC, colonoscopy/sigmoidoscopy screening history, polyp removal, hormonal replacement therapy use (for women), bowel movement and constipation, an increased risk was found for 10‐year use of non‐fibre laxative vs none or less than once a year (1–4 times yearly, HR: 1.49; 95% CI: 1.04–2.14; 5 times or more yearly, HR: 1.43; 95% CI: 0.82–2.48, p trend = 0.05) and CRC in both man and women. Regarding fibre laxative use over the past 10 years, a protective effect was found HR: 0.44; 95% CI: 0.21–0.95). Constipation and bowel movement frequency were not associated with CRC risk. The strength of the study was the good control for confounding factors and the subdivision of non‐fibre and fibre laxatives while the limitations of the study was the lack of description of the subjects lost from follow‐up.
Dukas et al. (2000), within the Nurses’ Health Study, studied the association between bowel movement frequency, laxative use and the risk of colorectal cancer among 84,577 women, 36–61 years of age and free of cancer. After 12 years of follow‐up, 611 incident cases of colorectal cancer were documented. The information on cancer incidence was obtained by asking participants to report a diagnosis of cancer of the colon or rectum every two years. Participants were also asked for the permission to obtain relevant hospital records and pathology reports. After controlling for age, BMI, fibre intake, physical activity, post‐menopausal status and hormone use and bowel movement frequency, no association was found between weekly to daily laxative use and colorectal cancer (HR = 1.00; 95% CI = 0.72, 1.40). However, for distal colon cancer, monthly laxative use was associated with an increased risk (HR = 1.67, 95% CI = 0.95, 2.94, p trend = 0.55), although not statistically significant. The strength of the study was the large sample size while the limitations of the study were the method of cases identification (self‐report), the lack of control for important confounding factors (e.g. smoking and fruit and vegetable intake) and no information on types of laxatives.
Watanabe et al. (2004) conducted a cohort study to investigate the association between constipation and laxative use and the risk of colorectal cancer in subjects of 40–64 years old living in 14 municipalities in Miyagi Prefecture in rural northern Japan. Out of the 41,670 enrolled in the study, 20,044 were men and 21,626 were women. Information on health, diet, alcohol consumption, smoking and physical activity was collected in the study. After seven years of follow‐up, 251 incident cases of colorectal cancer were identified by record linkage with the Miyagi Prefectural Cancer Registry. After the exclusion of colorectal cancer cases diagnosed in the first 3 years of follow‐up and after controlling for confoundings (education, smoking, alcohol consumption, BMI, family history of colorectal cancer, pork intake, green vegetables intake, orange intake, walking), an increased risk of colorectal cancer was found for subjects using two times weekly or more laxatives (HR: 2.40; 95% CI: 1.11–5.18) in comparison to non‐users. The risk of colorectal cancer for laxative users vs non‐users was 1.31 (95% CI: 0.88–1.95). In this study, constipation was associated with an increased risk for colorectal cancer (HR: 1.35, 95% CI: 0.99–1.84). The strength of this study was the high response rate (91.7%) and the good control for confounding factors. The limitation of the study was the lack of data on types of laxatives.
Kojima et al. (2004) investigated the association between bowel movement frequency, laxatives use and the risk of colorectal cancer in a large cohort of 25,731 men and 37,198 women, living in 24 communities in Japan, aged 40–79 years. A self‐administrated questionnaire that covered demographic characteristics and lifestyle factors that included diet, tobacco smoking, alcohol consumption and physical activity, bowel movement frequency and laxative use, was completed by each participant. After the follow‐up (average 7.6 years), 649 cases of colorectal cancer were identified from population cancer registries. After controlling for age, education, BMI, intake frequency of green leafy vegetables, alcohol, smoking status, walking and family history of colorectal cancer, an increased risk, although not statistically significant, was found for laxative use and the risk of colorectal cancer in both men (HR: 1.28; 95% CI: 0.89–1.86) and women (HR: 1.20; 95% CI: 0.85–1.69). Women reporting bowel movement every 6 days or less had an increased risk of developing colorectal (HR: 2.47, 95% CI: 1.01–6.01) and colon cancer (HR: 2.52, 95% CI: 0.93–6.82). The limitations of this study were the criteria of defining laxatives use (use of it only over the past year), the lack of data on frequency, duration and type of laxatives. Moreover, they did not control for aspirin use and hormone replacement therapy. The strength of the study was the large sample size and low number of subjects lost in the follow‐up period (3.3%).
3.2.4.1. Interactions
Chronic use or abuse of laxatives including hydroxyanthracene derivatives preparations may lead to hypokalaemia due to increased loss of potassium. This hypokalaemia, may increase the activity of cardiac glycosides and interfere with the action of antiarrythmic agents (Haverkamp et al. 2002). Concomitant use with medicinal products inducing hypokalaemia (e.g. diuretics, corticosteroids and liquorice root) may aggravate electrolyte imbalance (EMA, 2007a,b,c,d, 2008a,b, 2017,b).
Senna may cause mild abdominal discomfort such as colic or cramps. Prolonged use or overdosage can result in diarrhoea with excessive loss of water and electrolytes, particularly potassium; there is also the possibility of developing an atonic non‐functioning colon. Anthraquinone derivatives may colour the urine yellowish‐brown at acid pH, and red at alkaline pH. Reversible melanosis coli has been reported after chronic use. Similar adverse effects are reported also for aloe, even though with a more drastic and irritant action (Martindale, online).26
Theoretically, all laxatives, can accelerate intestinal transit, and may modulate the normal absorption of intestinally absorbed drugs. However, drug absorption in the small intestine is less likely to be affected than absorption in the colon since intestinal transit changes with anthranoid laxatives occur mainly in the colon.
Forty healthy premenopausal volunteers were randomly allocated into one of three groups. The first group took senna (dose not stated) for two menstrual cycles then, after a washout period, took wheat bran for two further menstrual cycles. The second group did the reverse. The third group took loperamide (dose not stated) for two menstrual cycles. A 4‐day dietary record was kept; whole‐gut transit time was measured; stools and blood were taken at the beginning and end of each intervention. Senna and increased intestinal transit and serum oestrone sulfate and total‐ and non‐protein‐bound oestrone concentrations fell with senna. No significant change in serum oestrogens was seen with loperamide. The authors concluded that speeding up intestinal transit can lower serum oestrogen concentrations (Lewis et al., 1997).
As reported in EMA (2008a,b), certain aglycone hydroxyanthracenes have been described to be metabolised by liver cytochromes P450 whereas a rhubarb extract was able to inhibit MDR1 function in tumour cells. However, the Panel considered that data available concerning such putative pharmacokinetic interactions are too weak to draw some conclusions.
3.2.4.2. Clinical studies in special populations
Use in children
In the open‐label controlled trial conducted by Nolan et al. (1991), 169 children with encopresis were randomly allocated to receive multimodal (MM) therapy, which includes laxatives plus behaviour modification (n = 83) or behaviour modification (BM) only (n = 86). Treatment protocol in the MM group consisted of using different laxatives throughout two phases. Laxatives used in the first phase included Microlax® (sodium citrate 90 mg, sodium lauryl sulfoacetate 9 mg, sorbic acid 5 mg, glycerol, sorbitol, distilled water) and bisacodyl. In the maintenance phase, patients received Agarol® (liquid paraffin, phenolphthalein, benzoic acid, sorbic acid), senna granules, and/or bisacodyl tablets. After 12 months of follow‐up, 42 (51%) of the MM group vs 31 (36%) of the BM group (p = 0.079) had achieved remission, while 52 (63%) vs 37 (43%) (p = 0.016) had achieved at least partial remission. Laxative treatments safety or tolerability data were not evaluated and limited information concerning senna were provided in the publication. Moreover, conclusions lack generalisability as they are applicable to children with encopresis rather than constipated children in general.
In a randomised, single‐blinded study conducted by Dahshan et al. (1999), the safety and the efficacy of three different bowel preparations (Group A: Magnesium citrate with X‐Prep® and clear liquid diet; Group B: Dulcolax® and Fleet® enema without dietary restriction. Group C: Golytely with clear liquid diet) were assessed in children (aged between 3 and 20) undergoing colonoscopy. While colon cleansing was superior in groups A and C compared to group B, tolerance and compliance were significantly better in groups A and B. Patients in group C reported adverse effects more frequently compared to groups A and B. Although magnesium citrate and X‐Prep® treatment combination was efficacious and well‐tolerated, the efficacy and tolerability of senna cannot be inferred since it was given in combination with magnesium citrate. Several reports have indicated the occurrence of skin irritations, which were bullous and comparable to skin irritations caused by scalds, with X‐Prep® on skin in children wearing napkins (Sitzmann et al., 1979).
In a cross‐over study conducted by Perkin (1977), children with chronic constipation under 15 years of age received lactulose (10–15 mL daily) or senna (10–20 mL daily). The latter was shown to be less effective and it has been associated with higher frequency of side effects (no details were provided on specific adverse events). This study has been reviewed by Gordon et al. (2013). According to the authors, no severe or significant adverse effects were reported in the two groups (lactulose vs senna). Minor adverse events such as colic or diarrhoea, were reported in patients included in the senna group.
In an open‐label randomised study, Sondheimer and Gervaise (1982) compared the efficacy of mineral oil (1.5–5 mL/kg per day) or standardised senna concentrate (Senokot, in tablet or syrup form in doses sufficient to induce at least one bowel movement daily, no further details available) in 37 children (aged 3–12 years) with chronic functional constipation. Mineral oil demonstrated more beneficial outcomes in terms of symptom control. Safety outcomes were not evaluated.
Berg et al. (1983) compared senna (one tablet of Senokot increased to two, if there was no improvement, no further details available) to placebo and reported no significant differences in the number of soiling episodes per week. No safety outcomes were reported.
In a prospective, randomised, single blinded study, patients ages 6–21 years were assigned to a 2‐day cleanout regimen of polyethylene glycol‐based solution (PEG‐P) at a dose of 1.5 g/kg bis in die (BID ‐ twice daily) for 2 days or to senna 15 mL daily (ages 6–12) or 30 mL daily (ages 12–21) for 2 days. No significant difference in the serum electrolyte and creatine levels between the two treatment groups has been reported. No significant adverse events were recorded (Terry et al., 2013). In the study conducted by Kierkus et al. (2013), 240 patients (ages 8–18) were randomly allocated to one of the three groups and treated with three different methods for bowel preparation for paediatric colonoscopy. In the first group, patients received high‐volume polyethylene glycol (PEG) (60 kg/day for 2 days), in the second low‐volume PEG (30 kg/day) combined with bisacodyl (BPEG) (10–15 mg/day for 2 days) and in the third group, patients received sennosides (2 mg/kg day for 2 days). Adverse events, such as abdominal pain, nausea, vomiting, headache, dizziness and fatigue, were reported in 133 patients. There were no significant differences in the incidence of the adverse events among the treatment groups, except for nausea, which was statistically more frequent in the BPEG group (22/80) compared to the PEG group (11/80) but not to the sennosides group (13/80). No serious adverse event was recorded.
To assess the incidence of functional constipation, the factors involved and the efficacy of the treatment, a prospective study was performed on 62 children using a standard questionnaire in addition to physical and anthropometric assessments. Treatments included demystification, behavioural changes and drugs (mineral oil and senna). Senna (20–30 mg/dose) combined with mineral oil showed good results on 16 (26%) patients. Maintenance treatment with mineral oil (15–30 mL/day) and senna at 5–15 mg/day had satisfactory results in 32% of the patients (1 month later), in 71% (3–6 months later), and in 85% (6–12 months later). No safety outcomes were reported by the authors (Martínez‐Costa et al., 2005).
The aforementioned data do not provide sufficient evidence to conclude the efficacy and safety of senna leaves when used for the treatment of constipated children
No clinical studies were available to assess the effects of cascara, frangula, aloe and rhubarb as laxatives in children.
According to the ESCOP monograph, the use of laxatives in children under 10 years of age cannot be recommended. According to the ‘Note for guidance on clinical investigation of medicinal products in the paediatric population’ (CPMP/ICH/2711/99) of 27 July 2000, the age limit between ‘children’ and ‘adolescents’ is set to 12 years of age.
According to the updated EMA monographs on Aloe and Senna (EMA, 2017,b), children younger than 12 years should not use products containing hydroxyanthracene derivatives to treat constipation.
Use during pregnancy and lactation
The EMA Committee on herbal medicinal products has evaluated the use of a number of herbal extracts for medicinal use (EMA, 2007a,c,d, 2008b).
Limited data were available but it was reported that no teratogenic or fetotoxic effects were seen in rats after oral treatment with aloe extract up to 1000 mg/kg bw or aloin A up to 200 mg/kg bw (EMA, 2007a).
EMA (2007c) report a study in 95 pregnant women suffering constipation and administered Laxariston® for an average of 61.4 days and a daily dose of 3.9 g (3 g contained 0.3 g frangula bark (13.5 mg hydroxyanthracene derivatives), 0.3 g senna leaves (7.5 mg hydroxyanthracene derivatives), 0.15 g rhubarb root (6.75 mg hydroxyanthracene derivatives) and 0.015 g achillea extract. Four patients (4.2%) were reported to suffer adverse reactions, but no further details were provided.
In summary, it is generally concluded for hydroxyanthracene derivate laxatives that reflex stimulation might occur not only in the colon, but also uterine muscles, which might lead to the development of hyperaemia in the pelvic region and to miscarriage as a result of neuromuscular stimulation of uterine muscles. In addition, it was concluded by EMA that there was insufficient data on the excretion of hydroxyanthracene derivate metabolites in breast milk and use during lactation was not recommended.
According to the updated EMA monographs on Aloe and Senna, the safety of hydroxyanthracene derivatives has not been established during pregnancy and lactation. Due to the lack of sufficient data and because of concerns on genotoxic and cancerogenic potential of these substances, the use during pregnancy and lactation is contraindicated (EMA, 2017,b).
4. Discussion
The present opinion, following a request from the European Commission to EFSA, in accordance with Article 29(1)(a) of Regulation (EC) No 178/2002 provides a scientific assessment of the hydroxyanthracene derivatives in foods, including preparations such as food supplements and infusions.
The safety of hydroxyanthracene derivatives is limited only to those anthranoid compounds extracted from the plants of the Rheum, Rhamnus, Senna and Aloe groups, which include (as also detailed in Section 2.3 of this opinion) the glycosides such as sennosides (A + B), aloins (A + B), cascarosides (A + B + C + D), frangulins (A + B), aloe‐emodin and emodin glycosides and small amounts of free aglycone anthrones, dianthrones and aglycone anthraquinones.
This procedure was triggered by Member States concerns during discussions on the possible authorisation of health claim related to hydroxyanthracene derivatives and improvement of bowel function previously substantiated in an EFSA scientific opinion (EFSA NDA Panel, 2013).
The EFSA NDA Panel considered as necessary to recommend certain restrictions when hydroxyanthracene derivatives are used as herbal medicinal products for their laxative properties.
In particular, the NDA Panel noted that ‘stimulant laxatives should not be consumed continually for periods longer than one to two weeks’ and ‘the use of stimulant laxatives for more than two weeks requires medical supervision’ (EFSA NDA Panel, 2013). Furthermore, EFSA NDA Panel recommended in the scientific opinion that the ‘long‐term use of stimulant laxatives should be avoided owing to the danger of electrolyte imbalance, impaired function of the intestine, and dependence on laxatives’, and as a second line treatment option (e.g. ‘stimulant laxative should only be used if an effect on bowel function cannot be achieved by a change of diet or the administration of bulk forming agents’) (EFSA NDA Panel, 2013). The EFSA NDA Panel based the above‐mentioned recommendations and restrictions on the available evidence obtained mainly from monographs published by the WHO and by the HMPC of EMA. In addition, the ANS Panel noted that aloe vera whole leaf extract has been classified by the International Agency for Research on Cancer as a possible (Group 2B) human carcinogen (IARC, 2016).
The current risk assessment was performed to address questions on specific concerns on the potential genotoxicity and carcinogenicity and not as an overall evaluation of hydroxyanthracene derivatives used in food and food supplements.
Glycosidic hydroxyanthracene derivatives remain intact until they are hydrolysed in the GI tract to their corresponding anthrones (aglycone anthrones). Regarding aglycone hydroxyanthracenes, as demonstrated in animals, they may be absorbed intact, however only rhein is present in the systemic circulation. In the GI tract, remaining hydroxyanthracenes may be reduced back to the corresponding anthrones by the microbiota. After absorption, hydroxyanthracenes such as aloe‐emodin are rapidly and totally oxidised to rhein. In the gut epithelium and liver, absorbed aglycone hydroxyanthracenes are conjugated into corresponding glucuronides and sulfates, which are excreted in bile or urine. The Panel considered that animal models in general reflect the fate of hydroxyanthracenes in humans.
The genotoxicity of hydroxyanthracene derivatives has been evaluated in numerous in vitro and in vivo studies identified from the public literature. The available studies focused mainly on emodin, aloe‐emodin, rhein and sennosides and at lesser extent on aloe vera, senna and fructus sennae extracts. A limited number of genotoxicity studies on chrysazin (danthron) and chrysophanol was also available.
In the in vitro studies, among the extracts, only senna extracts proved to be mutagenic in bacteria, in the TA 1537 and TA98 S. Typhimurium tester strain. Senna extract also induced DNA damage in Bacillus subtilis and dose‐related increases in chromosomal aberrations in CHO cells. Negative results were reported for mutagenicity in bacteria for aloe vera gel and for both aloe vera WLE and aloe vera charcoal‐filtered whole leaf extract (decolourised). The 1,8‐dihydroxyanthraquinones emodin, aloe‐emodin and danthron were shown to induce moderate increases in the frequency of gene mutation and dose‐related increases in micronuclei by a clastogenic mechanism interacting via intercalation into DNA with DNA topoisomerase II. The genotoxic and mutagenic potency were greater for danthron and lower for emodin in mouse lymphoma cells. Aloe‐emodin was also mutagenic in S. Typhimurium (all frameshift mutant sites). While these results further support the intercalating capability of aloe‐emodin and consequent catalytic inhibition of DNA topoisomerase II, they also show the induction of frameshift mutations which per se, is a mutagenic event not related to the inhibition of DNA topoisomerase II. Emodin, the least potent compound among these three anthraquinones, was also shown to induce DNA DSB through poisoning of Topo II‐cleavable complex and by inhibiting ATP hydrolysis of DNA topoisomerase II. Thus, a potential mechanism of inhibition of DNA topoisomerase II through poisoning of the DNA–topoisomerase ‘cleavable complex’ cannot be excluded. In addition, aloe‐emodin‐induced UDS in various mammalian cells. These results are also compatible with a mechanism of inhibition of DNA topoisomerase II activities.
However, in addition to the induction of frameshift and base‐pair substitution mutations and clastogenic effects through inhibition of DNA topoisomerase II identified for individual anthranoids, plant extracts containing hydroxyanthracene derivatives (glycosidic and aglycones) have been demonstrated to contain also other mutagenic components with different mechanisms of action which appear to play a role on their genotoxicity and carcinogenicity.
Thus, both aloe vera WLE and aloe vera WLD for which the content of hydroxyanthracene derivatives was reduced by 99% compared to the WLE, induced statistically significant and biologically relevant increases in the MF in the mammalian cell TK+/‐ mutation assay. These results indicate that other components in the mixtures, in addition to the hydroxyanthracene derivatives may contribute to the induced mutagenicity in vitro.
Overall, the results of genotoxicity testing in vitro indicate that extracts of aloe vera and senna and individual hydroxyanthracene derivatives, particularly aloe‐emodin, interact with bacterial and mammalian DNA under certain conditions in vitro. However, for risk assessment, in vivo studies are necessary to conclude on potential genotoxic hazard. In this respect, there are several in vivo studies available, which include the rodent bone marrow micronucleus assays, the mouse somatic mutation assay in fetal melanoblasts (Mouse Spot Test), the rat bone marrow chromosome aberration assays and the in vivo/in vitro UDS assay in rat liver. The results indicated that senna, fructus sennae extracts, sennosides and the individual 1,8‐dihydroxyanthraquinones tested were uniformly negative. However, the Panel noted that, in all these studies, no evidence of toxicity in the target cells was observed, indicating that target tissues may not have been adequately exposed to the test compounds. This assumption is substantiated by results of an in vivo study in rat, showing a low absorption of [14C]aloe‐emodin and a rapid metabolism to rhein, an anthranoid with no significant genotoxic activity in a battery of in vitro and in vivo assays.
On the other hand, significant increases in DNA fragmentation in the colon cells was demonstrated mice treated with aloe‐emodin by oral gavage at 500, 1,000 and 2,000 mg/kg bw on two occasions 24 h apart. Slight increases in DNA breakage compared to the concurrent vehicle control values were also observed in the kidney. These increases were dose‐related but reached statistical significance only at the high dose. On this basis, the limited absorption of aloe‐emodin and its quick transformation to rhein (a compound devoid of genotoxic capabilities) indicate that bone marrow may be considered as an inadequate tissue to demonstrate possible in vivo genotoxicity of both emodin and aloe‐emodin.
Overall, based on the available data, the Panel considered that the in vitro genotoxicity of aloe‐emodin was also reproduced in vivo, mainly in the colon, the target tissue for carcinogenicity of the WLE of aloe vera with a content of aloe‐emodin of 0.071 mg/g of whole leaf. In addition, the presence of mutagenic components other than hydroxyanthracene derivatives cannot be excluded based on the results obtained by Guo (2014) indicating that decolourised aloe vera extracts (WLD) are genotoxic in vitro through different mechanisms of action.
In subchronic studies with 0%, 1%, 2% and 3% aloe vera non‐decolourised leaf extract in drinking water by NTP (2013) GI transit time was decreased in male and female rats. Goblet cell hyperplasia in caecum and large intestine and lymphoid cell hyperplasia of mesenteric lymph nodes were reported in mice and rats from all exposed groups (doses for mice from 3,700 to 9,100 (males) or 9,700 (females) mg/kg bw per day, and for rats from 1,100 to 3,800 mg/kg bw per days for males and 1,300 to 3,200 g/kg bw per day for females. Epithelial hyperplasia was reported in caecum and colon of mice kept on diet providing daily doses of 625 mg senna/kg bw or higher for 29 days. In short‐term studies (15 or 16 days) with emodin in feed, diarrhoea was reported in some male mice receiving 1,200 mg/kg bw per day, in some female mice receiving 530–5,000 mg/kg bw per day, and in all male and most female rats administered with 17,000 or 50,000 mg emodin/kg diet (1,400 or 3,700 mg/kg bw for males and 1,250 or 2,000 mg/kg bw for females) (NTP, 2001).
In 13‐week studies (NTP, 2001), diarrhoea was reported in male mice kept administered 400 and 800 mg emodin/kg bw per day) and in female mice receiving 40–1,100 mg emodin/kg bw per day). In male and female rats, diarrhoea was reported at various times in all emodin exposed groups i.e. dose of 20–300 mg emodin/kg bw per day). In short‐term and subchronic studies with emodin. no treatment‐related histological changes in the GI system were reported. Treatment‐related non‐neoplastic changes were reported in kidneys (NTP, 2001).
In a 1‐year chronic toxicity study in rats with a whole leave powder of Aloe (Aloe arborescens Miller var. natalensis Berger) added to the diet at concentrations up to 2,212.17 mg extract/kg bw per day for males and 2,681.39 mg extract/kg bw per day for females, no hyperplastic or neoplastic changes in the GI system were seen. The low incidences of tumours found in other tissues were similar in the control and the treated groups (Matsuda et al., 2008).
In a carcinogenicity study in rats with whole leaf powder of Aloe added to the diet at concentrations of 0.8% or 4%, hyperplasia in the colon was recorded in some animals in the high‐dose group but not in the controls of the same gender (males: 5/42 vs 0/38, p < 0.05; females: 3/45 vs 0/40, p > 0.05). Furthermore, neoplastic changes were seen in the large intestine of some animals solely in the high‐dose group (adenomas (3/42) and a carcinoma (1/42) in colon of males and adenomas in colon of females (3/45), one adenocarcinoma in caecum (1/42) and one adenoma (1/42) in rectum in males compared to none in the control and 0.8% groups). As laboratory rats do not develop spontaneous neoplasm in the large intestine the occurrence of hyperplasia in the colon and of tumours in the caecum, colon and rectum in some of the rats from the high‐dose group was considered by the Panel as indication of a carcinogenic potential of the whole leaf powder of Aloe when given in a dose associated with diarrhoea in this study.
In carcinogenicity study (104 weeks) in mice exposed to aloe vera non‐decolourised WLE in drinking water at concentrations from 1% to 3% (wt/wt) (from 2,940 to 11,760 mg/kg bw for males and from 2,180 to 11,830 mg/kg bw per day for females), no treatment‐related neoplastic changes were reported. However, the incidence of goblet cell hyperplasia of large intestine was statistically significantly increased in all treated groups as compared to controls and the incidence of mesenteric lymph node cellular infiltration was statistically significantly increased in the high‐dose males (NTP, 2013, Boudreau et al., 2013). In a carcinogenicity study (104 weeks), rats were exposed to the same aloe vera non‐decolourised WLE in drinking water at concentrations from 0.5% to 1.5% (wt/wt) (from 250 to 1136 mg/kg bw per day for males and from 330 to 1280 mg/kg bw per day for females). There was a statistically significant dose‐trend increases in (a) incidence of mucosal hyperplasia of the caecum and colon for males and females, (b) of mucosal hyperplasia in forestomach, glandular stomach, ileum and rectum of females, and of glandular stomach, jejunum and rectum of males, (c) of mesenteric lymph nodes hyperplasia for males, and (d) of cystic degeneration in mesenteric lymph nodes hyperplasia for males and females. Moreover, statistically significant dose‐dependent increases in the incidences of large intestine adenomas and carcinomas were reported for male and female rats. The incidence of large intestine adenoma was statistically significantly higher in 1% and 1.5% groups of males and females and of carcinoma of males from 1% and 1.5% groups and of females from 1.5% group. The large intestine tumours were not seen in the control and 0.5% groups of male and female rats. The Panel noted that laboratory rodents do not develop spontaneous neoplasms in the large intestine. Therefore, the Panel considered the presence of hyperplastic changes in the large intestine of mice and rats and of neoplastic changes in GI system of rats in the NTP study as indication of carcinogenic effect of aloe vera non‐decolourised WLE. The Panel noted that the authors of the NTP studies suggested that lack of the neoplastic changes in the large intestine of mice exposed to aloe vera non‐decolourised WLE could be explained by the fact that the intestinal bacteria in mice are less efficient than the intestinal bacteria of rats in converting aloin A and aloin B to aloe‐emodin anthrone and subsequently to aloe‐emodin which bear genotoxic capabilities. In addition, mice have shorter gastrointestinal tracts and faster gastrointestinal transit times than rats, which could contribute to the lack of a tumour response in this species (Boudreau et al., 2013).
In carcinogenicity studies with emodin administered to mice or rats in the diet, no tumours of large intestine were reported (doses amounting to 625 or 1,250 mg/kg bw per day for male and female mice, and to 1,000 or 1,100 mg/kg bw for male and female rats) (NTP, 2001).
No treatment‐related neoplasms were found in carcinogenicity study in rats exposed to purified senna extract in drinking water providing doses up to 25 mg/kg bw per day. Also, in another carcinogenicity study with senna preparation administered to rats by gavage in doses amounting to 300 mg/kg bw per day, no treatment‐related tumours were found in GI tract or other organs but diffuse mucosal epithelial hyperplasia in the large intestine with incidence increasing in dose‐dependent manner was reported. Furthermore, no treatment‐related neoplasms were reported in p53+/‐ mice kept on diet containing senna up to 10,000 mg/kg diet (equal to 1,260 and 1,560 mg/kg bw per day for males and females, respectively) for 40 weeks. In an initiation–promotion study in rats, senna pod extract administered by gavage for 13 or 28 weeks did not induce ACF, did not affect incidence of tumours induced by AOM when given in a low dose (10 mg senna pod extract/kg bw per day), but the high and diarrhoeagenic dose of senna pod extract (100 mg/kg bw per day) appeared to promote development of tumours initiated by AOM in the colon (Mascolo et al., 1999). The Panel speculated whether irritation of the intestinal tract by diarrhoea could play a role in promoting the development of the tumours initiated by AOM. The Panel also noted that senna extracts contain sennosides A, B, C and D, which are glycosidic di‐anthrones converted to rhein by gut microbiota, one of the major metabolites of hydroxyanthracene derivatives, practically devoid of any genotoxic activity.
In male mice, a dietary exposure to 0.2% chrysazin (a synthetic anthraquinone, also known as danthron) for 540 days was associated with adenomatous hyperplasia in the caecum and colon and higher incidence of liver adenomas and carcinomas than in the control group. In male rats, 1% chrysazin in the diet was associated with focal hyperplasia of glandular epithelium of caecum and colon and with occurrence of adenomas, adenocarcinomas in the colon and caecum. The Panel considered that both studies indicated carcinogenic effect of chrysazin. The Panel noted the limitations of both studies (small number of animals per group, use of one sex, one dose of the test compound and limited reporting).
Overall, the Panel considered that the results of carcinogenicity studies indicated a carcinogenic effect of a whole leaf powder of aloe when given to rats at dietary concentration of 4%, a level associated with diarrhoea or loose stool, of aloe vera whole leaf extract in drinking water when given to rats at concentration of 1% or higher, and of chrysazin given in the diet at concentrations of 0.2% and 1% to mice and rats, respectively. Apart of tumours exposure to these test compounds caused hyperplastic changes in the large intestine of rats and mice. Senna extract was not carcinogenic to rats at doses up to 25 mg/kg bw per day in drinking water. Senna preparation was not carcinogenic to rats at doses amounting to 300 mg/kg bw per day administered by gavage but the rats developed diffuse mucosal epithelial hyperplasia in the large intestine and caecum with an incidence increasing in a dose‐dependent manner. Senna in the diet in doses amounting to 1,260 and 1,520 mg/kg bw per day was not carcinogenic to p53+/‐ males and females, respectively, but induced epithelial hyperplasia in the large intestine.
In previous evaluations (EMA, 2007a,b,c,d, 2008a,b), the relation between laxatives use and colorectal cancer was reported and it was concluded that ‘The question of a possible carcinogenic risk of long‐term use of anthranoid‐containing laxatives is still open and the results of the more recent studies are inconsistent. Therefore the conditions determined in the pharmacovigilance actions for anthranoid‐containing laxatives have to be maintained’.
An increased risk for colorectal cancer was found in all five cohort studies reviewed but only the study by Watanabe et al. (2004) (HR: 2.40; 95% CI: 1.11–5.18) and the study by Citronberg et al. (2014) showed statistical significance (1–4 times yearly vs none or less than once a year, HR: 1.49; 95% CI: 1.04–2.14; 5 times or more yearly vs none or less than once a year, HR: 1.43; 95% CI: 0.82–2.48, p trend = 0.05). Based on the studies reviewed by EMA and the results of more recent large epidemiological studies, the Panel agreed with previous evaluations that the prolonged use of laxatives is a possible risk factor for colorectal cancer. Nevertheless, better designed epidemiological studies (e.g. cohort studies with large sample size and proper control for confounding factors) that investigate the relationship between anthranoids laxatives use and colorectal are needed.
The Panel noted that parts of plants containing hydroxyanthracene derivatives may be part of the normal diet. However, no data on the concentrations of hydroxyanthracene derivatives present in the parts of the plants consumed were made available to the Panel by interested parties following a call for data.
Exposure to hydroxyanthracene derivatives from food supplements has been estimated from the recommended daily doses of food supplements as provided by the interested parties following the launch of a public ‘Call for data’ (see Section 2.6).
The Panel noted that exposure to aloe‐emodin and emodin is not known due to lack of data provided by the interested parties.
Maximum daily exposure to hydroxyanthracene derivatives is reported below:
Exposure to sennoside B was estimated up to 0.35 mg/kg bw per day, corresponding to 24.83 mg/person per day for a 70‐kg adult.
Exposure to rhein was estimated up to 1.12 mg/kg bw per day, corresponding to 78.8 mg/person per day for a 70‐kg adult.
Exposure to glucofrangulin A was estimated up to 0.37 mg/kg bw per day, corresponding to 26 mg/person per day for a 70‐kg adult.
Exposure to barbaloin was estimated up to 0.34 mg/kg bw per day, corresponding to 24 mg/person per day for a 70‐kg adult.
Exposure to aloin A+B was estimated up to 0.72 mg/kg bw per day, corresponding to 51 mg/person per day for a 70‐kg adult.
The Panel noted that the recommended daily doses provided by the interested parties are extremely variable. In order to calculate the worst‐case scenario the highest number in the range was used for the calculation. The Panel further noted that the concentrations of single hydroxyanthracene derivatives used in the food supplements vary also depending whether used alone or in combination with other hydroxyanthracene derivatives.
Exposure in young children was not performed since the use of food supplements containing hydroxyanthracene derivatives is not recommended in this population category.
The dose of hydroxyanthracene derivatives used in herbal medicinal products for the treatment of constipation should not exceed 30 mg/day for a maximum period of 2 weeks, in adults’ only (excluding pregnant and lactating women).
5. Conclusion
Epidemiological data suggest an increased risk for colorectal cancer associated with the general use of laxatives, several of which contain hydroxyanthracene derivatives.
Based on the data currently available, the Panel concluded that the hydroxyanthracenes, emodin, aloe‐emodin and the structurally related substance danthron, have been shown to be genotoxic in vitro.
Aloe extracts have also been shown to be genotoxic in vitro and the Panel concluded this was most likely due – at least in part – to hydroxyanthracene derivatives present in the extract. However, the Panel further noted that Aloe extracts depleted of hydroxyanthracenes, contained an additional genotoxic component(s).
Furthermore, aloe‐emodin was shown to be genotoxic in mice, the whole leaf aloe extract was carcinogenic to rats and there was evidence of carcinogenicity of the structural analogue danthron in both rodent species. Given that aloe‐emodin and emodin may be present in the extracts, the Panel concluded that hydroxyanthracene derivatives should be regarded as genotoxic and carcinogenic unless there are specific data to the contrary, such as for rhein, and that there is a safety concern for extracts containing hydroxyanthracene derivatives although uncertainty persists.
The Panel was unable to provide advice on a daily intake of hydroxyanthracene derivatives that does not give rise to concerns about harmful effects to health, for the general population, and as appropriate, for vulnerable subgroups of the population.
Documentation provided to EFSA
FSE (Food Supplement Europe), July 2017. Response to EFSA request for information on the use of hydroxyanthracene derivatives.
Synadiet, June 2017. Response to EFSA request for information on the use of hydroxyanthracene derivatives.
Aboca, June 2017. Response to EFSA request for information on the use of hydroxyanthracene derivatives.
ORTIS Laboratoires, June 2017. Response to EFSA request for information on the use of hydroxyanthracene derivatives.
Laboratoires Superdiet, June 2017. Response to EFSA request for information on the use of hydroxyanthracene derivatives.
Laboratoires Juvasante’, June 2017. Response to EFSA request for information on the use of hydroxyanthracene derivatives.
EHPM (European Federation of Associations of Health Food Products Manufacturers), June 2017. Response to EFSA request for information on the use of hydroxyanthracene derivatives.
Abbreviations
- ACF
aberrant crypt foci
- ADME
absorption, distribution, metabolism and excretion
- AG
aluminium foil
- ANS Panel
Panel on Food Additives and Nutrient Sources Added to Food
- AST
aspartate transaminase
- ATP
adenosine triphosphate
- AUC
area under the curve
- aw
water activity
- BfR
German Federal Institute for Risk Assessment
- BID
bis in die (twice daily)
- BM
behaviour modification
- BMI
body mass index
- BOPP
biaxially oriented polypropylene
- BPEG
bisacodyl polyethylene glycol
- BSA
bovine serum albumin
- CAS
Chemical Abstracts Service
- CHL
Chinese hamster lung
- CHO
Chinese hamster ovary
- CI
cumulative incidence
- CPE
cloud point extraction
- CRC
colorectal cancer
- CZE
capillary zone electrophoresis
- DAD
diode‐array detection
- DCF‐DA
2′,7′‐dichlorodihydrofluorescein diacetate
- DGCCRF
Direction Generale de la Concurrence, de la Consommation et de la Repression des Fraudes
- DHAQ
1,8‐dihydroxy anthraquinone
- DLLME
dispersive liquid–liquid microextraction
- DSB
double‐strand breaks
- DT
degradation time
- DTU
Technical University of Denmark
- EFSA SC
EFSA Scientific Commettee
- EMA
European Medicines Agency
- FAD
flavin adenine dinucleotide
- FDA
Food and Drugs Administration
- FMN
flavin mononucleotide
- GATE
gas‐assisted three‐liquid‐phase extraction system
- GC
gas chromatography
- GI
gastrointestinal
- GLP
Good Laboratory Practice
- GSH
glutathione
- HMPC
Committee on Herbal Medicinal Products
- HPFS
Health Professionals Follow‐up Study
- HPLC
high‐performance liquid chromatography
- HPRT
hypoxanthine‐guanine phosphoribosyl transferase
- HRE
heat reflux extraction
- HSA
human serum albumin
- HSCCC
high‐speed counter current technology
- IARC
International Agency for Research on Cancer
- IL
ionic liquid
- ILATPS
Ionic liquid‐ based aqueous two‐phase systems
- IL‐UMAE
ionic liquid‐based ultrasonic/microwave‐assisted extraction
- IUPAC
International Union of Pure and Applied Chemistry
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LC
liquid chromatography
- LOD
limit of detection
- LOQ
limit of quantification
- LOH
loss of heterozygosity
- MAE
microwave‐assisted extraction
- ME
maceration extraction
- MEC
microemulsion electrokinetics chromatography
- MF
mutation frequencies
- MM
multimodal
- MNCL
mononuclear cell leukaemia
- MS
mass spectrometry
- MSPE
magnetic solid‐phase extraction
- MS/MS
tandem mass spectrometry
- NAD
nicotinamide adenine dinucleotide
- NCE
normochromatic erythrocytes
- NDA Panel
Panel on Dietetic Products, Nutrition and Allergies
- NHS
Nurses’ Health Study
- NOAEL
no‐observed‐adverse‐effect‐level
- NTP
US National Toxicology Program
- OR
odds ratio
- OTM
Olive tail moment
- OTC
over‐the‐counter
- PCE
polychromatic erythrocytes
- PDA
photodiode array
- PEG
polyethylene glycol
- PEG‐P
polyethylene glycol‐based solution
- PHWE
pressurised hot water extraction
- PLE
pressurised liquid extraction
- PP
polypropylene
- RBF
round‐bottom flask
- ROS
reactive oxygen species
- RP‐HPLC
reverse phase‐high‐performance liquid chromatography
- RSM
response surface methodology
- SDME
single drop microextraction
- SDS‐MEKC
sodium dodecyl sulfate micellar electrokinetic chromatography
- SE
Soxhlet extraction
- SFE
super/subcritical fluid extraction
- SPE
solid‐phase extraction
- SPME
solid‐phase microextraction
- TLC
thin‐layer chromatography
- UAE
ultrasound‐assisted extraction
- UDP
uridine 5′‐diphospho
- UDS
unscheduled DNA synthesis
- UGT
UDP‐glucuronosyltransferase
- UHPLC‐Q‐TOF/MS
ultra‐high‐performance liquid chromatography coupled with quadrupole time‐of‐flight tandem mass spectrometry
- UNE
ultrasonic nebulisation extraction
- UPLC
ultra‐pressure liquid chromatography
- UV
ultraviolet
- UV–Vis
ultraviolet–visible
- WHO
World Health Organization
- WLD
decolourised whole leaf extract
- WLE
whole leaf extract
Appendix A – Complete list of hydroxyanthracene derivativesa
1.
| Name |
Structural formula IUPAC Name |
CAS Registry Number | Chemical formula | Molecular weight |
Botanical Source |
|---|---|---|---|---|---|
| Parent compounds | |||||
| Anthracene |
 Anthracene Anthracene |
120‐12‐7 | C14H10 | 178.234 | |
| Anthraquinone |
 Anthracene‐9,10‐dione Anthracene‐9,10‐dione |
84‐65‐1 | C14H8O2 | 208.216 | |
| Emodin |
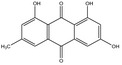 1,3,8‐trihydroxy‐6‐methylanthracene‐9,10‐dione 1,3,8‐trihydroxy‐6‐methylanthracene‐9,10‐dione |
518‐82‐1 | C15H10O5 | 270.24 | |
| Dihydroxyanthraquinones | |||||
| Chrysophanol |
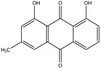 1,8‐dihydroxy‐3‐methylanthracene‐9,10‐dione 1,8‐dihydroxy‐3‐methylanthracene‐9,10‐dione |
481‐74‐3 | C15H10O4 | 254.241 | Rhubarb |
| Physcion |
 1,8‐dihydroxy‐3‐methoxy‐6‐methylanthracene‐9,10‐dione 1,8‐dihydroxy‐3‐methoxy‐6‐methylanthracene‐9,10‐dione |
521‐61‐9 | C16H12O5 | 284.267 | |
| Rhein |
 4,5‐dihydroxy‐9,10‐dioxo‐9,10‐dihydroanthracene‐2‐carboxylic acid 4,5‐dihydroxy‐9,10‐dioxo‐9,10‐dihydroanthracene‐2‐carboxylic acid |
478‐43‐3 | C15H8O6 | 284.223 | |
| Rhein anthrone |
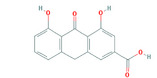
|
480‐09‐1 | C15H10O5 | 270.24 | |
| Aloe‐emodin |
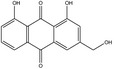 1,8‐dihydroxy‐3‐(hydroxymethyl)anthracene‐9,10‐dione 1,8‐dihydroxy‐3‐(hydroxymethyl)anthracene‐9,10‐dione |
481‐72‐1 | C15H10O5 | 270.24 | Aloe |
| Emodin anthrone |
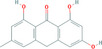
|
491‐60‐1 | C15H12O4 | 256.257 | |
| 7‐Hydroxy aloe emodin |
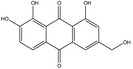 1,2,8‐trihydroxy‐6‐(hydroxymethyl)anthracene‐9,10‐dione 1,2,8‐trihydroxy‐6‐(hydroxymethyl)anthracene‐9,10‐dione |
156547‐97‐6 | C15H10O6 | 286.239 | |
| Nataloe‐emodin |
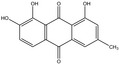 1,2,8‐trihydroxy‐6‐methylanthracene‐9,10‐dione 1,2,8‐trihydroxy‐6‐methylanthracene‐9,10‐dione |
478‐46‐6 | C15H10O5 | 270.24 | |
| Nataloe‐emodin 8‐methyl ether |
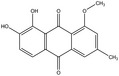 1,2‐dihydroxy‐8‐methoxy‐6‐methylanthracene‐9,10‐dione 1,2‐dihydroxy‐8‐methoxy‐6‐methylanthracene‐9,10‐dione |
125708‐14‐7 | C16H14O5 | 286.282 | |
| Anthrone glycosides | |||||
| Aloin A |
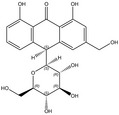 (10S)‐1,8‐dihydroxy‐3‐(hydroxymethyl)‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐10H‐anthracen‐9‐one (10S)‐1,8‐dihydroxy‐3‐(hydroxymethyl)‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐10H‐anthracen‐9‐one |
1415‐73‐2 | C21H22O9 | 418.398 | Aloe |
| Aloin B |
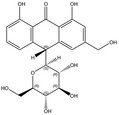 (10R)‐1,8‐dihydroxy‐3‐(hydroxymethyl)‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐10H‐anthracen‐9‐one (10R)‐1,8‐dihydroxy‐3‐(hydroxymethyl)‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐10H‐anthracen‐9‐one |
28371‐16‐6 | C21H22O9 | 418.398 | |
| 5‐Hydroxyaloin A |
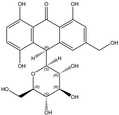 (10S)‐1,5,8‐trihydroxy‐3‐(hydroxymethyl)‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐10H‐anthracen‐9‐one (10S)‐1,5,8‐trihydroxy‐3‐(hydroxymethyl)‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐10H‐anthracen‐9‐one |
138373‐23‐6 | C21H22O10 | 434.39 | |
| 5‐Hydroxyaloin B |
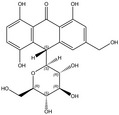 (10R)‐1,5,8‐trihydroxy‐3‐(hydroxymethyl)‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐10H‐anthracen‐9‐one (10R)‐1,5,8‐trihydroxy‐3‐(hydroxymethyl)‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐10H‐anthracen‐9‐one |
138373‐24‐7 | C21H22O10 | 434.39 | |
| Aloinoside A |
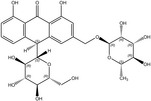 (10S)‐1,8‐dihydroxy‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐3‐[[(2R,3R,4R,5R,6S)‐3,4,5‐trihydroxy‐6‐methyloxan‐2‐yl]oxymethyl]‐10H‐anthracen‐9‐one (10S)‐1,8‐dihydroxy‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐3‐[[(2R,3R,4R,5R,6S)‐3,4,5‐trihydroxy‐6‐methyloxan‐2‐yl]oxymethyl]‐10H‐anthracen‐9‐one |
56645‐88‐6 | C27H32O13 | 564.54 | |
| Aloinoside B |
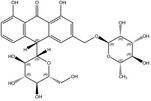 (10R)‐1,8‐dihydroxy‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐3‐[[(2R,3R,4R,5R,6S)‐3,4,5‐trihydroxy‐6‐methyloxan‐2‐yl]oxymethyl]‐10H‐anthracen‐9‐one (10R)‐1,8‐dihydroxy‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐3‐[[(2R,3R,4R,5R,6S)‐3,4,5‐trihydroxy‐6‐methyloxan‐2‐yl]oxymethyl]‐10H‐anthracen‐9‐one |
11006‐91‐0 | C27H32O13 | 564.54 | |
| Cascaroside A |
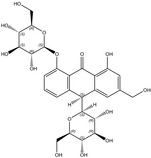 (10S)‐1‐hydroxy‐3‐(hydroxymethyl)‐10‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐8‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐10H‐anthracen‐9‐one (10S)‐1‐hydroxy‐3‐(hydroxymethyl)‐10‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐8‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐10H‐anthracen‐9‐one |
53823‐08‐8 | C27H32O14 | 580.539 | Cascara |
| Cascaroside B |
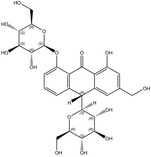 (10R)‐1‐hydroxy‐3‐(hydroxymethyl)‐10‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐8‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐10H‐anthracen‐9‐one (10R)‐1‐hydroxy‐3‐(hydroxymethyl)‐10‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐8‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐10H‐anthracen‐9‐one |
53861‐34‐0 | C27H32O14 | 580.539 | |
| Cascaroside C |
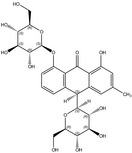 (10S)‐1‐hydroxy‐3‐methyl‐10‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐8‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐10H‐anthracen‐9‐one (10S)‐1‐hydroxy‐3‐methyl‐10‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐8‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐10H‐anthracen‐9‐one |
53823‐09‐9 | C27H32O13 | 564.54 | |
| Cascaroside D |
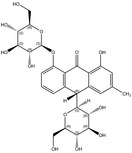 (10R)‐1‐hydroxy‐3‐methyl‐10‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐8‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐10H‐anthracen‐9‐one (10R)‐1‐hydroxy‐3‐methyl‐10‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐8‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐10H‐anthracen‐9‐one |
53861‐35‐1 | C27H32O13 | 564.54 | |
| Cascaroside E |
 (10R)‐1,6‐dihydroxy‐3‐methyl‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐8‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐10H‐anthracen‐9‐one (10R)‐1,6‐dihydroxy‐3‐methyl‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐8‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐10H‐anthracen‐9‐one |
164178‐32‐9 | C27H32O14 | 580.539 | |
| Cascaroside F |
 (10S)‐1,6‐dihydroxy‐3‐methyl‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐8‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐10H‐anthracen‐9‐one (10S)‐1,6‐dihydroxy‐3‐methyl‐10‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]‐8‐[(2S,3R,4R,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐10H‐anthracen‐9‐one |
164322‐83‐2 | C27H32O14 | 580.539 | |
| Glucofrangulin A |
 1‐hydroxy‐3‐methyl‐8‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐6‐[(2S,3R,4R,5R,6S)‐3,4,5‐trihydroxy‐6‐methyloxan‐2‐yl]oxyanthracene‐9,10‐dione 1‐hydroxy‐3‐methyl‐8‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐6‐[(2S,3R,4R,5R,6S)‐3,4,5‐trihydroxy‐6‐methyloxan‐2‐yl]oxyanthracene‐9,10‐dione |
21133‐53‐9 | C27H30O14 | 578.523 | Frangula |
| Glucofrangulin B |
 6‐[(3R,4R)‐3,4‐dihydroxy‐4‐(hydroxymethyl)oxolan‐3‐yl]oxy‐1‐hydroxy‐3‐methyl‐8‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxyanthracene‐9,10‐dione 6‐[(3R,4R)‐3,4‐dihydroxy‐4‐(hydroxymethyl)oxolan‐3‐yl]oxy‐1‐hydroxy‐3‐methyl‐8‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxyanthracene‐9,10‐dione |
14062‐59‐0 | C26H28O14 | 564.493 | |
| Dianthrones | |||||
| Palmidin C |
 10‐(4,5‐dihydroxy‐2‐methyl‐10‐oxo‐9H‐anthracen‐9‐yl)‐1,3,8‐trihydroxy‐6‐methyl‐10H‐anthracen‐9‐one 10‐(4,5‐dihydroxy‐2‐methyl‐10‐oxo‐9H‐anthracen‐9‐yl)‐1,3,8‐trihydroxy‐6‐methyl‐10H‐anthracen‐9‐one |
17177‐86‐5 | C30H22O7 | 494.499 | |
| Dianthrone glycosides (sennosides) | |||||
| Sennoside A |
 (9R)‐9‐[(9R)‐2‐carboxy‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracen‐9‐yl]‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid (9R)‐9‐[(9R)‐2‐carboxy‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracen‐9‐yl]‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid |
81‐27‐6 | C42H38O20 | 862.746 | Cassia |
|
Sennoside A1 (Sennoside G) (Sennoside A&B) |
 9‐[2‐carboxy‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracen‐9‐yl]‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid 9‐[2‐carboxy‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracen‐9‐yl]‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid |
66575‐30‐2 | C42H38O20 | 862.746 | |
| Sennoside B |
 (9S)‐9‐[(9R)‐2‐carboxy‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracen‐9‐yl]‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid (9S)‐9‐[(9R)‐2‐carboxy‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracen‐9‐yl]‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid |
128‐57‐4 | C42H38O20 | 862.746 | |
| Sennoside C |
 (9R)‐4‐hydroxy‐9‐[(9R)‐4‐hydroxy‐2‐(hydroxymethyl)‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracen‐9‐yl]‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid (9R)‐4‐hydroxy‐9‐[(9R)‐4‐hydroxy‐2‐(hydroxymethyl)‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracen‐9‐yl]‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid |
37271‐16‐2 | C42H40O19 | 848.763 | |
| Sennoside D |
 (9R)‐4‐hydroxy‐9‐[(9S)‐4‐hydroxy‐2‐(hydroxymethyl)‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracen‐9‐yl]‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid (9R)‐4‐hydroxy‐9‐[(9S)‐4‐hydroxy‐2‐(hydroxymethyl)‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracen‐9‐yl]‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid |
37271‐17‐3 | C42H40O19 | 848.763 | |
| Sennoside E |
 9‐[2‐carboxy‐5‐[(2S,3R,4S,5S,6R)‐4,5‐dihydroxy‐6‐(hydroxymethyl)‐3‐oxalooxyoxan‐2‐yl]oxy‐4‐hydroxy‐10‐oxo‐9H‐anthracen‐9‐yl]‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid 9‐[2‐carboxy‐5‐[(2S,3R,4S,5S,6R)‐4,5‐dihydroxy‐6‐(hydroxymethyl)‐3‐oxalooxyoxan‐2‐yl]oxy‐4‐hydroxy‐10‐oxo‐9H‐anthracen‐9‐yl]‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid |
11137‐63‐6 | C44H38O23 | 934.765 | |
| Sennoside F |
 (9S)‐9‐[(9R)‐2‐carboxy‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(oxalooxymethyl)oxan‐2‐yl]oxy‐9H‐anthracen‐9‐yl]‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid (9S)‐9‐[(9R)‐2‐carboxy‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(oxalooxymethyl)oxan‐2‐yl]oxy‐9H‐anthracen‐9‐yl]‐4‐hydroxy‐10‐oxo‐5‐[(2S,3R,4S,5S,6R)‐3,4,5‐trihydroxy‐6‐(hydroxymethyl)oxan‐2‐yl]oxy‐9H‐anthracene‐2‐carboxylic acid |
1037532‐47‐0 | C44H38O23 | 934.765 | |
IUPAC: International Union of Pure and Applied Chemistry; CAS: Chemical Abstracts Service.
Chemical structures from SciFinder, available at: https://www.cas.org/products/scifinder.
Appendix B – Identity and nature of source materials
1.
The plant synonyms included in the Appendix B refers to the ‘The Plant List’ website (http://www.theplantlist.org); in this opinion, tables list only synonyms having high or medium confidence levels (see below). When the number of synonyms is very large, only high confidence level is considered.
Confidence Levels:
For each name record, The Plant List offers an indication of the confidence that the Status of the name record is correct: Our confidence assessments are based primarily on the nature and taxonomic integrity of the source data.
 High Confidence level
High Confidence level
is applied to the Status of name records derived from taxonomic datasets which treat the whole of the taxonomic group in question on a global basis and have been peer reviewed (e.g. ILDIS, WCSP, see collaborators).
 Medium Confidence level
Medium Confidence level
is applied to the Status of name records derived from:
Either national or regional databases via a rules‐based automated process, reflecting the challenges inherent in resolving taxonomic differences between different name data sets for the same species for different geographic areas. Regional datasets used as sources for The Plant List are primarily those stored within Tropicos (see collaborators for details).
Or taxonomic data sets which treat the whole of the taxonomic group in question on a global basis but which have not yet undergone peer review (e.g. GCC and WCSP (in review) see Collaborators).
Aloe spp. Family Xanthorrhoeaceae
| Species | Synonyma | Common name | Parts used | EU Ph. | EMA | The Plant List | USDA | Mansf. | EFSA Comp. | BELFRIT | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aloe africana Mill |
Aloe angustifolia Haw. Aloe bolusii Baker Aloe pseudoafricana Salm‐Dyck Pachidendron africanum (Mill.) Haw Pachidendron angustifolium (Haw.) Haw. |
African aloe |
Leaf, leaf gel |
– | X | X | spp | X |
World Health Organization, 1999 Japan Ph US Ph |
||
| Aloe arborescens Mill |
A. arborea Medik. A. frutescens Salm‐Dyck A. fruticosa Lam. A. fulgens Tod. A. natalensis J.M. Wood & M.S. Evans A. perfoliatum Meyen A. principis (Haw) Stearn A. salm‐dyckiana Schult. & Schult.f A. sigmoidea Baker. Catevala arborescens (Mill.) Medik. Pachidendron principis Haw |
Candelabra aloe Octopus plant Torch plant |
Leaf, leaf gel |
X | X | X | spp | X | |||
| Aloe ferox Mill |
A. galpinii Baker A. horrida Haw A. muricata Haw. A. pseudoferox Salm‐Dyck A. subferox Spreng A. supralaevis Haw. Pachidendron ferox (Mill.) Haw. Pachidendron pseudoferox (Salm‐ Dyck) Haw Pachidendron supralaeve (Haw.) Haw |
Cape aloe Uganda aloe |
Leaf, leaf gel |
8.0 (2014) 8.3 (2015) |
X | X | X | X | spp | X |
Germany WHO Mon. 1999 Japan Ph US Ph |
| Aloe perryi Baker | – |
Perry's aloe Socotra aloe West indian aloe Zanzibar aloe |
Leaf, leaf gel |
X | X | X | spp | X | |||
| Aloe plicatilis (L.) Mill |
A. lingua Thunb. A. tripetala Medik. Kumara disticha Medik Rhipidodendum distichum (Medik.) Willd. Rhipidodendrum plicatile (L.) Haw. |
Leaf, leaf gel |
X | spp | X | ||||||
| Aloe vera (L.) Burm. f |
A. barbadensis Mill A. chinensis Steud. ex Baker A. elongata Murray A. flava Pers. A. indica Royle A. lanzae Tod. A. rubescens DC A. vulgaris Lam |
Barbados aloe Curaçao aloe Indian aloe Mediterranean aloe |
Leaf, leaf gel |
8.3 (2015) | X | X | X | X | spp | X |
Germany WHO Mon. 1999 Japan Ph US Ph |
Only resolved names and with confidence level 3. spp = Aloe spp (cited as a group); Ph = Pharmacopoeia; EU Ph = European Pharmacopoeia; EMA = European Medicines Agency; The plant List = The Plant List (2013). Version 1.1. Published on the Internet; http://www.theplantlist.org/ USDA = United States Department of Agriculture, Natural Resources Conservation Service Available at: https://plants.usda.gov/java/; Mansf. = Mansfeld's World Database of Agricultural and Horticultural Crops. Available at: http://mansfeld.ipk-gatersleben.de/apex/f?p=185:3::::::#; EFSA Comp. = Compendium of botanicals reported to contain naturally occurring substances of possible concern for human health when used in food and food supplements (2012). Available at https://www.efsa.europa.eu/it/efsajournal/pub/2663; BELFRIT = Harmonised list of Plants allowed in Food supplements by Belgium, France and Italy; WHO Mon. = WHO Monographs on selected medicinal plants (http://apps.who.int/medicinedocs/en/)
Genus Cassia and Senna – Family Leguminosae
| Species | Synonym(a) | Common name | Parts used | EU Ph. | EMA | The Plant List | USDA | Mansf. | EFSA Comp. | BELFRIT | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cassia fistula L. |
Bactyrilobium fistula Willd. Cassia bonplandiana DC Cassia excelsa Kunth Cassia fistuloides Collad. Cassia rhombifolia Roxb. Cathartocarpus excelsius G.Don. Cathartocarpus fistula Pers. Cathartocarpus fistuloides (Collad.) G.Don. Cathartocarpus rhombifolius G.Don |
Golden shower Indian laburnum Purging cassia |
Fruit | X | X | X | spp | X | |||
| Cassia italica (Mill.) F.W. Andrew | Senna italica (Mill.) F.W.Andrew |
Port Royal senna Mecca senna Senegal senna Tripoli senna |
Whole plant | X | as Senna Italica | X | spp | X | |||
| Cassia mimosoides L. var Nomame (Siebold) Makino |
Chamaecrista nomame (Sieber) H. Ohashi |
Cassia nomame |
Leaf, pod |
Italian List | |||||||
| Senna alexandrina Mill. |
Cassia acutifolia Delile Cassia alexandrina (Garsault) Thell. Cassia angustifolia M. Vahl. Cassia senna L. Senna acutifolia (Delile) Batka Senna alexandrina Garsault Senna angustifolia (Vahl) Batka |
Alexandrian senna |
Leaf, pod |
8.3 (2015) | X | X | X | X | spp | X |
China Ph US Ph |
| Senna obtusifolia (L.) H.S. Irwin & Barneby |
Cassia humilis Collad. Cassia obtusifolia L. Cassia toroides Roxb. Cassia toroides Raf. Diallobus falcatus Raf. Diallobus uniflorus Raf. Senna toroides Roxb. |
Java‐bean Charamazca |
Whole plant | X | X | X | spp | X | |||
| Senna occidentalis (L.) Link |
Cassia caroliniana Walter Cassia ciliata Raf. Cassia falcata L. Cassia foetida Pers. Cassia macradenia Collad. Cassia obliquifolia Schrank. Cassia occidentalis L. Cassia occidentalis (L.) Rose Cassia planisiliqua L. Ditramexa occidentalis Britton & Rose Ditremexa occidentalis (L.) Britton & Wilson |
Septicweed Coffee senna Negro coffee |
Bark, leaf |
X | X | X | spp | X | |||
| Senna tora (L.) Roxb. |
Cassia borneensis Miq Cassia gallinaria Collad. Cassia numilis Collad. Cassia tora L. Emelista tora Britton & Rose |
Sickle senna Foetid cassia Wild senna |
Leaf, seed | X | X | X | spp | X |
Genus Frangula Mill. and Rhamnus L.‐ Family Rhamnaceae
| Species | Synonym a | Common name | Parts used | EU Ph. | EMA | The Plant List | USDA | Mansf | EFSA Comp. | BELFRIT | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frangula alnus Mill. |
Frangula atlantica Grubov Frangula frangula H. Karst. Frangula nigra Samp. Frangula pentapetala Gilib. Frangula vulgaris Hill Girtanneria frangula Neck. Rhamnus frangula L. |
Glossy buckthorn Alder buckthorn Frangula Persian bark |
Bark | 8.0 (2014) | X | X | X | X | spp | X |
WHO Mon. 2004 |
| Frangula purshiana Cooper |
Cardiolepis obtusa Raf. Frangula anonifolia (Greene) Grubov Rhamnus annonifolia Greene Rhamnus purshiana DC. |
Cascara buckthorn Pursh's buckthorn Bearberry Sacred bark |
Bark | 8.0 (2014) | X | X | X | X | spp | X |
WHO Mon. 2004 US Ph |
| Rhamnus alpina L. | Frangula latifolia Mill. | – |
Bark, fruit |
– | – | X | – | – | spp | X | – |
| Rhamnus cathartica L. |
Cervispina cathartica (L.) Moench |
Common buckthorn | Whole plant | – | – | X | X | X | spp | X | – |
Only resolved names and with confidence level 3. spp. = Cassia spp. (cited as a group); Ph = Pharmacopoeia; EU Ph = European Pharmacopoeia; EMA = European Medicines Agency; The plant List = The Plant List (2013). Version 1.1. Published on the Internet; http://www.theplantlist.org/; USDA = United States Department of Agriculture, Natural Resources Conservation Service Available at: https://plants.usda.gov/java/. Mansf. = Mansfeld's World Database of Agricultural and Horticultural Crops. Available at: http://mansfeld.ipk-gatersleben.de/apex/f?p=185:3::::::#; EFSA Comp. = Compendium of botanicals reported to contain naturally occurring substances of possible concern for human health when used in food and food supplements (2012). Available at: https://www.efsa.europa.eu/it/efsajournal/pub/2663; BELFRIT = Harmonized list of Plants allowed in Food supplements by Belgium, France and Italy. WHO Mon. = WHO Monographs on selected medicinal plants (http://apps.who.int/medicinedocs/en/).
Genus Rheum L. – Family Polygonaceae
| Species | Synonym | Common name | Parts used | EU Ph. | EMA | The Plant List | USDA | Mansf. | EFSA Comp. | BELFRIT | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rheum australe D. Don | R. emodii Wall ex Meisn. | – |
Leaf, rhizome |
X | X | spp | X | India Ph | |||
| Rheum officinale Baill. | – |
Chinese rhubarb Medicinal rhubarb |
Root, rhizome |
8.0 (2014) | X | X | X | X | spp | X |
WHO Mon. 1999 Japan Ph |
| Rheum palmatum L. |
R. potaninii Losinsk R. qinlingense Y.K.Yang, DK Zhang & JK Wu |
Chinese rhubarb |
Root, rhizome |
8.0 (2014) | X | X | X | X | spp | X |
WHO Mon. 1999 Japan Ph |
| Rheum rhabarbarum L. |
R. franzenbachii Münter R. undulatum L. |
Garden rhubarb |
Rhizome, stalk |
X | X | spp | X | ||||
| Rheum rhaponticum L. | – | – |
Leaf, rhizome |
8.0 (2014) | X | spp | X | ||||
|
Rheum x hybridum Murray |
– | – |
Leaf, rhizome |
spp | X |
EU Ph = European Pharmacopoeia; EMA = European Medicines Agency; The plant List = The Plant List (2013). Version 1.1. Published on the Internet; http://www.theplantlist.org/; USDA = United States Department of Agriculture, Natural Resources Conservation Service Available at: https://plants.usda.gov/java/; Mansf. = Mansfeld's World Database of Agricultural and Horticultural Crops. Available at: http://mansfeld.ipk-gatersleben.de/apex/f?p=185:3::::::#; EFSA Comp. = Compendium of botaniclas reported to contain naturally occurring substances of possible concern for human health when used in food and food supplements (2012). Available at https://www.efsa.europa.eu/it/efsajournal/pub/2663; BELFRIT = Harmonized list of Plants allowed in Food supplements by Belgium, France and Italy; WHO Mon. = WHO Monographs on selected medicinal plants (http://apps.who.int/medicinedocs/en/).
Suggested citation: EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Younes M, Aggett P, Aguilar F, Crebelli R, Filipič M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Lambré C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Andrade RJ, Fortes C, Mosesso P, Restani P, Pizzo F, Smeraldi C, Papaioannou A and Wright M, 2018. Scientific Opinion on the safety of hydroxyanthracene derivatives for use in food. EFSA Journal 2018;16(1):5090, 97 pp. 10.2903/j.efsa.2018.5090
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00562
Panel members: Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipič, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Gunter Georg Kuhnle, Claude Lambré, Jean‐Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Competing interests: In line with EFSA's policy on declarations of interest, Panel member Birgit Dusemund did not participate in the development and adoption of this scientific output.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Rita Sousa. The Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 22 November 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: from Rahman et al., 2017; © 2017 by the authors
Notes
Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. OJ L 404, 30.12.2006, p. 26–38.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Call for data on new scientific information as regards the use of hydroxyanthracene derivatives. Published: 10 April 2017. Available online: https://www.efsa.europa.eu/en/data/call/170410-0
Available online: https://www.efsa.europa.eu/en/data/compendium-botanicals
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC In force OJ L 354, 31.12.2008, p. 34–50.
Belgian Royal Decree of 29 August 1997 on the manufacture and trade of foods composed of or containing plants or plant preparations. Available at: http://ec.europa.eu/growth/tools-databases/tris/en/index.cfm/search/?trisaction=search.detail&year=2015&num=162&mLang=EN (accessed on November 2017).
Bulgarian Ordinance Nr. 47/28.12.2004 on the requirements for food additives. Food Law, Promulgated SG No. 90 of 15 October 1999, Amended SG No. 80 of 12 October 2010, Amended SG No. 98 of 14 December 2010, Amended SG, No. 8 of 25 January 2011 (in Bulgarian).
Decree No. 225/2008 Coll., laying down requirements on food supplements and enrichment of foodstuffs, prohibits, within labelling, attributing qualities related to preventing, treating or curing human diseases to food supplements or to refer to these qualities. Available at: http://eagri.cz/public/web/mze/legislativa/ostatni/Legislativa-ostatni_uplna-zneni_vyhlaska-2008-225.html (accessed on November 2017).
Order No. 72 on the addition to food of certain substances other than vitamins and minerals (2013). Available at: http://www.fao.org/faolex/results/details/en/?details=LEX-FAOC120080 (accessed on November 2017).
Finnish Law 989/2007 of the Ministry of Agriculture and Forestry. Available at: http://www.finlex.fi/fi/laki/alkup/2007/20070989 (accessed on November 2017).
French Decree No 2006‐352 of 20 March 2006 on food supplements. Available at: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000000638341&fastPos=1&fastReqId=487973546&categorieLien=cid&oldAction=rechTexte (accessed on November 2017).
French Order of 24 June 2014. Establishing the list of plants authorized in the food complements and the conditions of their employment. Available at: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000029254516&fastPos=1&fastReqId=279617386&categorieLien=cid&oldAction=rechTextehttps://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000029254516&fastPos=1&fastReqId=279617386&categorieLien=cid&oldAction=rechTexte (accessed on November 2017).
Italian Ministry of Health. Disciplina dell'impiego negli integratori alimentari di sostanze e preparati vegetali. Gazzetta Ufficiale della Repubblica Italiana 2012, 169. Available at: http://www.salute.gov.it/imgs/C_17_pagineAree_1268_listaFile_itemName_2_file.pdf (accessed on November 2017).
Italian Ministry of Health. D.M. 27 marzo 2014 ‘Aggiornamento del DM 9 luglio 2012 sulla Disciplina dell'impiego negli integratori alimentari di sostanze e preparati vegetali’. Available online: http://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=48636 (accessed on November 2017).
Health Minister Order No 1069/2007 on food supplements (Norma din 19/06/2007 ‘privind suplimentele alimentare’). Available at: http://www.ms.ro/2017/01/11/notificari-suplimente-alimentare/ (accessed on November 2017).
Real Decreto 191/2011, de 18 de febrero, sobre Registro General Sanitario de Empresas Alimentarias y Alimentos. Available at: http://www.boe.es/boe/dias/2011/03/08/pdfs/BOE-A-2011-4293.pdf (accessed on November 2017).
Swedish National Food Administration's Code of Statues (LIVSFS 2003:9). Available at: https://www.livsmedelsverket.se/globalassets/om-oss/lagstiftning/berikn---kosttillsk---livsm-spec-gr-fsmp/livsfs-2003-09-kons-tom-2014-10.pdf (accessed on November 2017).
The Food Supplement (England) Regulations 2003 S.I. 2003 No. 1387. Available at: http://www.legislation.gov.uk/uksi/2003/1387/pdfs/uksi_20031387_en.pdf (accessed on November 2017).
Available at: https://www.fda.gov/ohrms/dockets/98fr/050902a.htm
For these calculations, the mean content of aloin A was calculated as follows: 1.25 mg/g for aloe vera gel, 0.13 mg/g for aloe vera decolourised whole leaf and 15 mg/g for aloe vera non‐decolourised whole leaf extract.
For these calculations, the mean content of aloin A (13.5 mg/g) was used.
For these calculations, the mean content of aloin A was calculated as follows: 1.26 mg/g for aloe vera gel, 0.13 mg/g for aloe vera decolourised whole leaf and 15 mg/g for aloe vera non‐decolourised whole leaf extract.
For these calculations, the mean content of aloin A (6.45 mg/g) was used.
References
- Ahlawat KS and Khatkar BS, 2011. Processing, food applications and safety of aloe vera products: a review. Journal of Food Science and Technology, 48, 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvindekar AU, Pereira GR and Laddha KS, 2015. Assessment of conventional and novel extraction techniques on extraction efficiency of five anthraquinones from Rheum emodi . Journal of Food Science and Technology, 52, 6574–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M and Schlatter C, 1981. Metabolism of [14C]emodin in the rat. Xenobiotica, 11, 217–225. [DOI] [PubMed] [Google Scholar]
- Berg I, Forsythe I, Holt P and Watts J, 1983. A controlled trial of ‘Senokot’ in faecal soiling treated by behavioural methods. Journal of Child Psychology and Psychiatry, 24, 543–549. [DOI] [PubMed] [Google Scholar]
- BfR , 2017. . Food supplements with whole‐leaf Aloe preparations containing anthranoids are associated with health risks. BfR Opinion No. 032/2017 of 2 November 2017. 10.17590/20171108-091347 [DOI]
- Bhosle D, Janghel A, Deo S, Raut P, Verma C, Kumar SS, Agrawal M, Amit N, Sharma M, Giri T and Tripathi DK, 2015. Emerging Ultrasound Assisted Extraction (UAE) Techniques as Innovative Green Technologies for the effective extraction of the active phytopharmaceuticals. Research Journal of Pharmacy and Technology, 8, 963–970. [Google Scholar]
- Bi S, Song D, Kan Y, Xu D, Tian Y, Zhou X and Zhang H, 2005. Spectroscopic characterization of effective components anthraquinones in Chinese medicinal herbs binding with serum albumins. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy., 62, 203–212. [DOI] [PubMed] [Google Scholar]
- Boudreau MD, Mellick PW, Olson GR, Felton RP, Thorn BT and Beland FA, 2013. Clear evidence of carcinogenic activity by a whole‐leaf extract of Aloe barbadensis miller (aloe vera) in F344/N rats. Toxicological sciences, 131, 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP and Brown RJ, 1976. Mutagenesis by 9,10‐anthraquinone derivatives and related compounds in Salmonella typhmurium . Mutation Research/Genetic Toxicology, 40, 203–224. [DOI] [PubMed] [Google Scholar]
- Burlinson B, Tice RR, Speit G, Agurell E, Brendler‐Schwaab SY, Collins AR, Escobar P, Honma M, Kumaravel TS, Nakajima M, Sasaki YF, Thybaud V, Uno Y, Vasquez M and Hartmann A; In Vivo Comet Assay Workgroup, part of the Fourth International Workgroup on Geno , 2007. Fourth International Workgroup on Genotoxicity testing: results of the in vivo comet assay workgroup (in Nesslany et al., 2009 Aloe‐emodin‐induced DNA fragmentation in the mouse in vivo comet assay). Mutation research, 627:31–35. [DOI] [PubMed] [Google Scholar]
- Caldwell DJ, 1999. Review of mononuclear cell leukemia in F‐344 rat bioassays and its significance to human cancer risk: A case study using alkyl phthalates. Regulatory toxicology and pharmacology, 30, 45–53. [DOI] [PubMed] [Google Scholar]
- Cameron BD, Phillips MW and Fenerty CA, 1988. Milk transfer of rhein in the rhesus monkey. Pharmacology, 36(Suppl 1), 221–225. [DOI] [PubMed] [Google Scholar]
- Cao W, Yi L, Ye LH, Cao J, Hu SS, Xu JJ, Peng LQ, Zhu QY and Zhang QY, 2015. Application of a highly sensitive magnetic solid phase extraction for phytochemical compounds in medicinal plant and biological fluids by ultra‐high performance liquid chromatography coupled with quadrupole time‐of‐flight tandem mass spectrometry. Electrophoresis, 36, 2404–2412. [DOI] [PubMed] [Google Scholar]
- Chandegara VK and Varshney AK, 2013. Aloe vera L. processing and products: a review. International Journal of Medicinal Aromatic Plants, 3, 492–506. [Google Scholar]
- Chang XL, Wang C, Feng Y and Liu Z, 2006. Effects of heat treatments on the stabilities of polysaccharides substances and barbaloin in gel juice from Aloe vera Miller. Journal of Food Engineering, 75, 245–251. [Google Scholar]
- Che QM, Akao T, Hattori M, Tsuda Y, Namba T and Kobashi K, 1991. Barbaloin stimulates growth of Eubacterium sp. strain BAR, a barbaloin‐metabolizing bacterium from human feces. Chemical and pharmaceutical bulletin, 39, 757–760. [DOI] [PubMed] [Google Scholar]
- Chen YY, Chiang SY, Lin JG, Yang JS, Ma YS, Liao CL, Lai TY, Tang NY and Chung JG, 2010. Emodin, aloe‐emodin and rhein induced DNA damage and inhibited DNA repair gene expression in SCC‐4 human tongue cancer cells. Anticancer research, 30, 945–951. [PubMed] [Google Scholar]
- Chesis PL, Levi DE, Smith ML, Emster L and Ames BN, 1984. Mutagenicity of quinones: pathways of metabolic activation and detoxication. Proceedings of the National Academy of Sciences, 81, 1696–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chewchinda S, Wuthi‐udomlert M and Gritsanapan W, 2013. HPLC quantitative analysis of rhein and antidermatophytic activity of Cassia fistula pod pulp extracts of various storage conditions. BioMed research international, 19, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choche T, Shende S and Kadu P, 2014. Extraction and identification of bioactive components from Aloe barbadensis Miller. Journal of Pharmacognosy and Phytochemistry, 2, 14–23. [Google Scholar]
- Citronberg J, Kantor ED, Potter JD and White E, 2014. A prospective study of the effect of bowel movement frequency, constipation, and laxative use on colorectal cancer risk. American Journal of Gastroenterology, 109, 1640–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms M, Lotz R, Lang W, Renner U, Bayer E and Spahn‐Langguth H, 1997. Elucidation of phase I and phase II metabolic pathways of rhein: species differences and their potential relevance. Drug metabolism and disposition, 25, 442–452. [PubMed] [Google Scholar]
- Dahshan A, Lin CH, Peters J, Thomas R and Tolia V, 1999. A randomized, prospective study to evaluate the efficacy and acceptance of three bowel preparations for colonoscopy in children. The American Journal of Gastroenterology, 94, 3497–3501. [DOI] [PubMed] [Google Scholar]
- De Witte P and Lemli J, 1988a. 1988b Excretion and distribution of [14C]rhein and [14C]rhein anthrone in rat. Journal of pharmacy and pharmacology, 40, 652–655. [DOI] [PubMed] [Google Scholar]
- De Witte P and Lemli J, 1988b. Metabolism of 14C‐rhein and 14C‐rhein anthrone in rats. Pharmacology, 36(Suppl. 1), 152–157. [DOI] [PubMed] [Google Scholar]
- De Witte P, Cuveele J and Lemli J, 1992. In vitro deterioration of rhein anthraquinone in cecal content of rats. Pharmaceutica acta Helvetiae, 67, 198–203. [PubMed] [Google Scholar]
- Dhodare GD, Wandhare GD, Jirapure MN and Dumore NS, 2015. Extraction of aloin from aloevera by using soxhlet apparatus. International Journal For Engineering Applications and Technology, ISSN: 2321‐8134 [Google Scholar]
- Ding WJ, Wu XF, Zhong JS and Wan JZ, 2014. Effects of temperature, pH and light on the stability of aloin A and characterisation of its major degradation products. International journal of food science & technology, 49, 1773–1779. [Google Scholar]
- Donfack AR, Tala MF, Wabo HK, Jerz G, Zeng GZ, Winterhalter P, Tan NH and Tane P, 2014. Two new anthraquinone dimers from the stem bark of Pentas schimperi (Rubiaceae). Phytochemistry Letters, 31, 55–58. [Google Scholar]
- Doull J, Bridges BA, Kroes R, Golberg L, Munro IC, Paynter OE, Pitot HC, Squire R, Williams GM and Darby WJ, 1983. The Relevance of Mouse Liver Hepatoma to Human Carcinogenic Risk: A Report of the International Expert Advisory Committee to the Nutrition Foundation. The Nutrition Foundation, Washington. [Google Scholar]
- Dukas L, Willett WC, Colditz GA, Fuchs CS, Rosner B and Giovannucci EL, 2000. Prospective study of bowel movement, laxative use, and risk of colorectal cancer among women. American Journal of Epidemiology, 151, 958–964. [DOI] [PubMed] [Google Scholar]
- Duval J, Pecher V, Poujol M and Lesellier E, 2016. Research advances for the extraction, analysis and uses of anthraquinones: A review. Industrial Crops and Products, 94, 812–833. [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: General Principles. EFSA Journal 2009;5(5):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013. Scientific Opinion on the substantiation of a health claim related to hydroxyanthracene derivatives and improvement of bowel function pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA Journal 2013;11(10):3412, 12 pp. 10.2903/j.efsa.2013.3412 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements, on request of EFSA. EFSA Journal 2009;7(9):1249, 19 pp. 10.2093/j.efsa.2009.1249 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EMA (European Medicines Agency), 2006. Community Herbal Monograph on Aloe barbadensis Miller and on Aloe (Various Species, Mainly Aloe Ferox Miller and Its Hybrids). EMA, London. [Google Scholar]
- EMA (European Medicines Agency), 2007a. Committee on herbal medicinal products. Assessment report on Aloe barbadensis miller and aloe (various species, mainly aloe ferox Miller and its hybrids). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2009/12/WC500017830.pdf
- EMA (European Medicines Agency), 2007b. Committee on herbal medicinal products. Assessment report on Rhamnus frangula l., cortex. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2009/12/WC500018613.pdf
- EMA (European Medicines Agency), 2007c. Committee on herbal medicinal products. Assessment report on Cassia senna L. and Cassia angustifolia Vahl, folium. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2009/12/WC500018219.pdf
- EMA (European Medicines Agency), 2007d. Committee on herbal medicinal products. Assessment report on Cassia senna L., fructus and Cassia angustifolia Vahl, fructus. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2009/12/WC500018206.pdf
- EMA (European Medicines Agency), 2008a. Assessment report for Rhubarb (Rhei radix). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2009/12/WC500018404.pdf
- EMA (European Medicines Agency), 2008b. Assessment Report: Rhamnus purshianus d.c.; Rhamni purshianae cortex; Cascara and herbal preparation(s) thereof with well‐established use and traditional use. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2009/12/WC500018426.pdf
- EMA (European Medicines Agency) , 2017. a. Committee on Herbal Medicinal Products (HMPC). Assessment report on Aloe barbadensis Mill. and on Aloe (various species, mainly Aloe ferox Mill. and its hybrids), folii succus siccatus. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2009/12/WC500017830.pdf
- EMA (European Medicines Agency) , 2017b. EMA, Committee on Herbal Medicinal Products (HMPC). European Union herbal monograph on Senna alexandrina Mll. (Cassia senna L.; Cassia angustifolia Vahl), fructus. Revised draft document, currently under consultation. Available on line: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2017/10/WC500236598.pdf
- Faber P and Strenge‐Hesse A, 1988. Relevance of rhein extraction into breast milk. Pharmacology, 36(Suppl 1), 212–220. [DOI] [PubMed] [Google Scholar]
- Goppel M and Franz G, 2004. Stability control of senna leaves and senna extracts. Planta Medica, 70, 432–436. [DOI] [PubMed] [Google Scholar]
- Gordon M, Naidoo K, Akobeng AK and Thomas AG, 2013. Osmotic and stimulant laxatives for the management of childhood constipation (Review). Evidence‐Based Child Health: A Cochrane Review Journal, 8, 57–109. [DOI] [PubMed] [Google Scholar]
- Gulia A, Sharma HK, Sarkar BC, Upadhyay A and Shitandi A, 2010. Changes in physico‐chemical and functional properties during convective drying of aloe vera (Aloe barbadensis) leaves. Food and Bioproducts Processing, 88, 161–164. [Google Scholar]
- Guo X, Zhang S, Dial SL, Boudreau MD, Xia Q, Fu PP, Levy DD, Moore MM and Mei N, 2014. In vitro investigation of the mutagenic potential of Aloe vera extracts. Toxicology Research, 3, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Enomoto A, Boorman GA and Maronpot RR, 1999. Chapter 7: Liver and gallbladder. In: Maronpot RR (ed.). Pathology of the Mouse. Reference and Atlas, 1st Edition. Library of Congress Catalog Card Number 98‐74814. ISBN 1‐889899‐03‐8. [Google Scholar]
- Hartmann A, Agurell E, Beevers C, Brendler‐Schwaab S, Burlinson B, Clay P, Collins A, Smith A, Speit G, Thybaud V and Tice RR, 2003. Recommendations for conducting the in vivo alkaline comet assay (in Nesslany et al., 2009 Aloe‐emodin‐induced DNA fragmentation in the mouse in vivo comet assay). Mutagenesis, 18, 45–51. Available here: https://www.ncbi.nlm.nih.gov/pubmed/12473734 [DOI] [PubMed] [Google Scholar]
- Hattori M, Namba T, Akao T and Kobashi K, 1988. Metabolism of sennosides by human intestinal bacteria. Pharmacology, 36, 172–179. [DOI] [PubMed] [Google Scholar]
- Haverkamp W, Monnig G, Schulze‐Bahr E, Haverkamp F and Breithardt G, 2002. Physician‐induced torsade de pointes—therapeutic implications. Cardiovascular Drugs and Therapy, 16, 101–109. [DOI] [PubMed] [Google Scholar]
- Heidemann A, Miltenburger HG and Mengs U, 1993. The genotoxicity status of senna. Pharmacology, 47, 178–186. [DOI] [PubMed] [Google Scholar]
- Heidemann A, Voelkner W and Mengs U, 1996. Genotoxicity of aloeemodin in vitro and in vivo. Mutation Research/Genetic Toxicology, 367, 123–133. [DOI] [PubMed] [Google Scholar]
- IARC , 1990. IARC Monograph on DANRON (CHRYSAZIN;1,8‐DIHYROXYANTHRQUINONE) 1990, 50, pp. 265–275. [PMC free article] [PubMed]
- IARC , 2016. IARC MONOGRAPHS: Some Drugs and Herbal Products, Vol. 108. IARC, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- IASC , 2009. IASC Labeling Guidance and Definitions document. Available online: http://www.iasc.org/Portals/19/Documents/Members/10_0405_IASC_Labeling_Guidance_Definitions.pdf
- IASC (International Aloe Science Council), online. Available online: http://www.iasc.org/Portals/19/Documents/Scientific/16_0531_Decolorization_statement_final.pdf?ver=2016-05-31-163902-973
- Jacobs EJ and White E, 1998. Constipation, laxative use, and colon cancer among middle‐aged adults. Epidemiology, 9, 385–391. [PubMed] [Google Scholar]
- Jawade NR and Chavan AR, 2013. Ultrasonic‐assisted extraction of aloin from aloe vera gel. Procedia Engineering, 51, 487–493. doi: 10.1016/j.proeng.2013.01.069 [DOI] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
- Kierkus J, Horvath A, Szychta M, Woynarowski M, Wegner A, Wiernicka A, Dadalski M, Teisseyre M and Dziechciarz P, 2013. High‐versus low‐volume polyethylene glycol plus laxative versus sennosides for colonoscopy preparation in children. Journal of pediatric gastroenterology and nutrition, 57, 230–235. [DOI] [PubMed] [Google Scholar]
- Kojima M, Wakai K, Tokudome S, Tamakoshi K, Toyoshima H, Watanabe Y, Hayakawa N, Suzuki K, Hashimoto S, Ito Y and Tamakoshi A; JACC Study Group , 2004. Bowel movement frequency and risk of colorectal cancer in a large cohort study of Japanese men and women. British Journal of Cancer, 90, 1397–13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsensuspapier expertenforum , 1999. Obstipation und Laxanzien.
- Krumbiegel G and Schulz HU, 1993. Rhein and aloe‐emodin kinetics from senna laxatives in man. Pharmacology, 47, 120–124. [DOI] [PubMed] [Google Scholar]
- Kune GA, 1993. Laxative use not a risk for colorectal cancer: data from the Melbourne Colorectal Cancer Study. Zeitschrift fur Gastroenterologie, 31, 140–143. [PubMed] [Google Scholar]
- Kune GA, Kune S, Field B and Watson LF, 1988. The role of chronic constipation, diarrhea, and laxative use in the etiology of large‐bowel cancer. Diseases of the colon & rectum, 31, 507–512. [DOI] [PubMed] [Google Scholar]
- Lainonen H, Marvola M, Hietala P and Parviainen T, 1988. The effect of different storage conditions on the chemical stability, laxative effect and acute toxicity of sennoside solutions. Pharmacology and Toxicology, 63, 37–41. [DOI] [PubMed] [Google Scholar]
- Lang W, 1988. Pharmacokinetics of 14C‐labelled rhein in rats. Pharmacology, 36(Suppl 1), 158–171. [DOI] [PubMed] [Google Scholar]
- Lang W, 1993. Pharmacokinetic‐metabolic studies with 14C‐aloe emodin after oral administration to male and female rats. Pharmacology, 47(Suppl 1), 110–119. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim JM and Kim C. Pharmacokinetic analysis of rhein in Rheum undulatum L. Journal of ethnopharmacology 2003;84:5–9. [DOI] [PubMed] [Google Scholar]
- Levi DE, Hollstein M, Chritman ME and Ames RN, 1984. Detection of oxidative mutagens with a new Salmonella tester strain (fA102). Methods in Enzymology, 105, 249–254. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Heaton KW, Oakey RE and McGarrigle HH, 1997. Lower serum oestrogen concentrations associated with faster intestinal transit. British Journal of Cancer, 76, 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luan Y, Qi X, Li M, Gong L, Xue X, Wu X, Wu Y, Chen M, Xing G and Yao J, 2010. Emodin triggers DNA double‐strand breaks by stabilizing topoisomerase II‐DNA cleavage complexes and by inhibiting ATP hydrolysis of topoisomerase II. Toxicological Sciences, 118, 435–443. [DOI] [PubMed] [Google Scholar]
- Liang J, Hsiu S, Wu P and Chao P, 1995. Emodin pharmacokinetics in rabbits. Planta Medica, 61, 406–408. [DOI] [PubMed] [Google Scholar]
- Liberman DE, Fink RC, Schaefer EL, Mulcahy RJ and Stark A‐A, 1982. Mutagenicity of anthraquinone and hydroxylated anthraquinones in the Ames/Salmonella microsome system. Applied and Environment Microbiology, 43, 1354–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ML, Chen SS, Lu YC, Liang RY, Ho YT, Yang CY and Chung JG, 2007. Rhein induces apoptosis through induction of endoplasmic reticulum stress and Ca2 + ‐dependent mitochondrial death pathway in human nasopharyngeal carcinoma cells. Anticancer Research, 27, 3313–3322. [PubMed] [Google Scholar]
- Liu W, Tang L, Ye L, Cai Z, Xia B, Zhang J, Hu M and Liu Z, 2010. Species and gender differences affect the metabolism of emodin via glucuronidation. The AAPS Journal, 12, 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli M, Genovese S, Carlucci G, Kremer D, Randic M and Epifano F, 2012. Development and application of high‐performance liquid chromatography for the study of two new oxyprenylated anthraquinones produced by Rhamnus. Journal of Chromatography A, 1225, 113–120. [DOI] [PubMed] [Google Scholar]
- Lu C, Wang H, Lv W, Ma C, Xu P, Zhu J, Xie J and Zhou Q, 2011. Ionic liquid‐based ultrasonic/microwave‐assisted extraction combined with UPLC for the determination of anthraquinones in Rhubarb. Chromatographia, 74, 139–144. [Google Scholar]
- Lydén‐Sokolowski A, Nilsson A and Sjöberg P, 1993. Two‐year carcinogenicity study with sennosides in the rat: Emphasis on gastro‐intestinal alterations. Pharmacology, 47(Suppl 1), 209–215. [DOI] [PubMed] [Google Scholar]
- Maekawa A and Odashima S, 1975. Spontaneous tumors in ACI/Nrats. Journal of the National Cancer Institute, 55, 1437–1445. [DOI] [PubMed] [Google Scholar]
- Martínez‐Costa C, Palao Ortuño MJ, Alfaro Ponce B, Núñez Gómez F, Martínez‐Rodríguez L, Ferré Franch I and Brines Solanes J, 2005. Functional constipation: prospective study and treatment response. Anales de Pediatría, 63, 418–425. [DOI] [PubMed] [Google Scholar]
- Mascolo N, Mereto E, Borrelli F, Orsi P, Sini D, Izzo AA, Massa B, Boggio M and Capasso F, 1999. Does senna extract promote growth of aberrant crypt foci and malignant tumors in rat colon? Digestive Diseases and Sciences, 44, 2226–2230. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Yokohira M, Suzuki S, Hosokawa K, Yamakawa K, Zeng Y, Ninomiya F, Saoo K, Kuno T and Imaida K, 2008. One‐year chronic toxicity study of Aloe arborescens Miller var. natalensis Berger in Wistar Hannover rats. A pilot study. Food and Chemical Toxicology, 46, 733–739. [DOI] [PubMed] [Google Scholar]
- Mehta N and Laddha KS, 2009. A modified method for isolation of rhein from senna. Indian Journal of Pharmaceutical Sciences, 71, 128–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengs U and Heidemann A, 1993. Genotoxicity of sennosides and rhein in vitro and in vivo. Medical science research, 21, 749–750. [Google Scholar]
- Mengs U, Krumbiegel G and Völkner W, 1997. Lack of emodin genotoxicity in the mouse micronucleus assay. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 393, 289–293. [DOI] [PubMed] [Google Scholar]
- Mengs U, Grimminger W, Krumbiegel G, Schuler D, Silber W and Völkner W, 1999. No clastogenic activity of a senna extract in the mouse micronucleus assay. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 444, 421–426. [DOI] [PubMed] [Google Scholar]
- Mengs U, Schuler D and Marshall RR, 2001. No induction of chromosomal aberrations in Chinese hamster ovary cells by chrysophanol. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 492, 69–72. [DOI] [PubMed] [Google Scholar]
- Miranda M, Maureira H, Rodríguez K and Vega‐Gálvez A, 2009. Influence of temperature on the drying kinetics, physicochemical properties, and antioxidant capacity of Aloe Vera (Aloe Barbadensis Miller) gel. Journal of Food Engineering, 91, 297–304. [Google Scholar]
- Mitchell JM, Mengs U, McPherson S, Zijlstra J, Dettmar P, Gregson R and Tigner JC, 2006. An oral carcinogenicity and toxicity study of senna (Tinnevelly senna fruits) in the rat. Archives of toxicology, 80, 34–44. [DOI] [PubMed] [Google Scholar]
- Mori H, Sugie S, Niwa K, Takahashi M and Kawai K, 1985. Induction of intestinal tumours in rats by chrysazin. British Journal of Cancer, 52, 781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Sugie S, NIwA K, Yoshimi N, Tanaka T and Hirono I, 1986. Carcinogenicity of chrysazin in large intestine and liver of mice. Japanese Journal of Cancer Research Gann, 77, 871–876. [PubMed] [Google Scholar]
- Morimoto I, Watanabe F, Osawa T, Okitsu T and Kada T, 1982. Mutagenicity screening of crude drugs with Bacillus subtilis rec‐assay and Salmonella/microsome reversion assay. (in Williams et al., 2010 Safety studies conducted on a proprietary high‐purity aloe vera inner leaf fillet preparation, Qmatrix). Mutation Research/Environmental Mutagenesis and Related Subjects, 97, 81–102. [DOI] [PubMed] [Google Scholar]
- Mueller SO and Stopper H, 1999. Characterization of the genotoxicity of anthraquinones in mammalian cells. Biochimica et Biophysica Acta, 1428, 406–414. [DOI] [PubMed] [Google Scholar]
- Mueller SO, Eckert I, Lutz WK and Stopper H, 1996. Genotoxicity of the laxative drug components emodin, aloe‐emodin and danthron in mammalian cells: Topoisomerase II mediated? Mutation Research, 371, 165–173. [DOI] [PubMed] [Google Scholar]
- Nesslany F, Simar‐Meintières S, Ficheux H and Marzin D, 2009. Aloe‐emodin‐induced DNA fragmentation in the mouse in vivo comet assay. Mutation Research, 678, 13–19. [DOI] [PubMed] [Google Scholar]
- Nolan T, Debelle G, Oberklaid F and Coffey C, 1991. Randomised trial of laxatives in treatment of childhood encopresis. Lancet, 338, 523–527. [DOI] [PubMed] [Google Scholar]
- NTP , 2001. NTP technical report on the toxicology and carcinogenesis studies of emodin (CAS NO. 518‐82‐1) in F344/N rats and B6C3F1 mice (feed studies). [PubMed]
- NTP , 2013. NTP technical report on the toxicology and carcinogenesis studies of a nondecolorized whole leaf extract of aloe barbadensis miller (aloe vera) in F344/N rats and B6C3F1 mice (drinking water studies). [PubMed]
- Nusko G, Schneider B, Müller G, Kusche J and Hahn EG, 1993. Retrospective study on laxative use and melanosis coli as risk factors for colorectal neoplasma. Pharmacology, 47(Suppl 1):234–241. [DOI] [PubMed] [Google Scholar]
- Nusko G, Schneider B, Schneider I, Wittekind C and Hahn EG, 2000. Anthranoid laxative use is not a risk factor for colorectal neoplasia: results of a prospective case control study. Gut, 46, 651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olennikov DN, Zilfikarov IN, Ibragimov TA, Toropova AA and Tankhaeva LM, 2010. Chemical composition and antioxidative activity (in vitro) of arborescent aloe juice (Aloe Arborescens Mill. Chemistry, 3, 83–90. [Google Scholar]
- Paes‐Leme AA, Motta ES, De Mattos JC, Dantas FJ, Bezerra RJ and Caldeira‐de‐Araujo A, 2005. Assessment of Aloe vera (L.) genotoxic potential on Escherichia coli and plasmid DNA. Journal of Ethnopharmacology, 102, 197–201. [DOI] [PubMed] [Google Scholar]
- Park JY, Mitrou PN, Luben R, Khaw KT and Bingham SA, 2009. Is bowel habit linked to colorectal cancer? ‐ Results from the EPIC‐Norfolk study. European Journal of Cancer, 45, 139–145. [DOI] [PubMed] [Google Scholar]
- Pellizzoni M, Molinari GP and Lucini L, 2011. Stability of the main Aloe fractions and Aloe‐based commercial products under different storage conditions. Agrochimica, 55, 288–296. [Google Scholar]
- Perkin J, 1977. Constipation in childhood: A controlled comparison between lactulose and standard senna. Current Medical Research and Opinion, 4, 540–543. [DOI] [PubMed] [Google Scholar]
- Pongnaravane B, Goto M, Sasaki M, Anekpankul T, Pavasant P and Shotipruk A, 2006. Extraction of anthraquinones from roots of Morinda citrifolia by pressurized hot water: Antioxidant activity of extracts. The Journal of supercritical fluids, 37, 390–396. [Google Scholar]
- Rahman S, Carter P and Bhattarai N, 2017. Aloe vera for tissue engineering applications. Journal of Functional Biomaterials, 8, pii: E6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra CT and Rao PS, 2013. Shelf‐life and colour change kinetics of Aloe vera gel powder under accelerated storage in three different packaging materials. Journal of food science and technology, 50, 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MC, Millikan RC, Galanko JA, Martin C and Sandler RS, 2003. Constipation, laxative use, and colon cancer in a North Carolina population. American Journal of Gastroenterology, 98, 857–864. [DOI] [PubMed] [Google Scholar]
- Sakulpanich A and Gritsanapan W, 2008. Extraction method for high content of anthraquinones from Cassia fistula pods. Journal of Health Research, 22, 167–172. [Google Scholar]
- Sehgal I, Winters WD, Scott M, David A, Gillis G, Stoufflet T, Nair A and Kousoulas K, 2013. Toxicologic assessment of a commercial decolorized whole leaf aloe vera juice, lily of the desert filtered whole leaf juice with aloesorb. Journal of Toxicology, 11, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsipur M, Fasihi J, Khanchi A, Yamini Y, Valinezhad A and Sharghi H, 2008. Solubilities of some 9, 10‐anthraquinone derivatives in supercritical carbon dioxide: A cubic equation of state correlation. The Journal of Supercritical Fluids, 47, 154–160. [Google Scholar]
- Shi Z, Zhu X, Cheng Q and Zhang H, 2007. Micellar Extraction and Preconcentration of Anthraquinone Derivatives from Rhubarb Prior to Their HPLC‐DAD Determination. Journal of liquid chromatography & related technologies, 30, 255–271. [Google Scholar]
- Shi YB, Li HL, Wang HQ, Yang YB, Zhang XY, Wang H, Zhu ZJ, Zhang ZY and Zhang CA, 2014. Simultaneous determination of five anthraquinones in a Chinese traditional preparation by RP‐HPLC using an improved extraction procedure. Journal of Integrative Medicine, 12, 455–462. [DOI] [PubMed] [Google Scholar]
- Shia CS, Tsai SY, Lin JC, Li ML, Ko MH, Chao PD, Huang YC and Hou YC, 2011. Steady‐state pharmacokinetics and tissue distribution of anthraquinones of Rhei Rhizoma in rats. Journal of ethnopharmacology, 137, 1388–1394. [DOI] [PubMed] [Google Scholar]
- Siegers CP, Siemers J and Baretton G, 1993. Sennosides and aloin do not promote dimethylhydrazine‐induced colorectal tumors in mice. Pharmacology, 47(Suppl. 1), 205–208. [DOI] [PubMed] [Google Scholar]
- Sitzmann FC, 1979. Lokale Nebenwirkungen an der Haut durch Anthraglykoside. Pädiatr Prax, 21, 355–358. [Google Scholar]
- Sondheimer J and Gervaise E, 1982. Lubricant versus laxative in the treatment of chronic functional constipation of children: A comparative study. Journal of Pediatric Gastroenterology and Nutrition, 1, 223–226. [DOI] [PubMed] [Google Scholar]
- Song R, Lin H, Zhang Z, Li Z, Xu L, Dong H and Tian Y, 2009. Profiling the metabolic differences of anthraquinone derivatives using liquid chromatography/tandem mass spectrometry with data‐dependent acquisition. Rapid Communications in Mass Spectrometry, 23, 537–547. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A and Müller AD, 1993. Constipation and cathartics as risk factors of colorectal cancer: a meta‐analysis. Pharmacology, 47(Suppl. 1), 224–233. [DOI] [PubMed] [Google Scholar]
- Srikanth S, Kumar VS, Kiran RS, Prasad MV and Krishnamohan G, 2011. Microwave assisted extraction of calcium sennosides from senna leaflets. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2, 137–145. [Google Scholar]
- Su SC and Ferguson NM, 1973. Extraction and separation of anthraquinone glycosides. Journal of pharmaceutical sciences, 62, 899–901. [DOI] [PubMed] [Google Scholar]
- Sund RB and Elvegård SO, 1988. Anthraquinone laxatives: metabolism and transport of danthron and rhein in the rat small and large intestine in vitro. Pharmacology, 36(Suppl. 1), 144–151. [DOI] [PubMed] [Google Scholar]
- Surh I, Brix A, French JE, Collins BJ, Sanders JM, Vallant M and Dunnick JK, 2013. Toxicology and carcinogenesis study of senna in C3B6. 129F1‐Trp53 tm1Brd N12 haploinsufficient mice. Toxicologic pathology, 41, 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa Y, Morota T, Takeda S and Aburada M, 2003. Pharmacokinetics of rhein from Onpi‐to, an oriental herbal medicine, in rats. Biological and Pharmaceutical Bulletin, 26, 613–617. [DOI] [PubMed] [Google Scholar]
- Tan Z, Li F and Xu X, 2012. Isolation and purification of aloe anthraquinones based on an ionic liquid/salt aqueous two‐phase system. Separation and Purification Technology, 19, 150–157. [Google Scholar]
- Tan ZJ, Li FF and Xu XL, 2013. Extraction and purification of anthraquinones derivatives from Aloe vera L. using alcohol/salt aqueous two‐phase system. Bioprocess and biosystems engineering, 36, 1105–1113. [DOI] [PubMed] [Google Scholar]
- Terry NA, Chen‐Lim ML, Ely E, Jatla M, Ciavardone D, Esch S, Farace L, Jannelli F, Puma A, Carlow D and Mamula P, 2013. Polyethylene glycol powder solution versus senna for bowel preparation for colonoscopy in children. Journal of pediatric gastroenterology and nutrition, 56, 215–219. [DOI] [PubMed] [Google Scholar]
- Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC and Sasaki YF, 2000. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing (in Nesslany et al., 2009 Aloe‐emodin‐induced DNA fragmentation in the mouse in vivo comet assay). Environmental and molecular mutagenesis, 35, 206–221. [DOI] [PubMed] [Google Scholar]
- Tikkanen L, Matsushima T and Natori S, 1983. Mutagenicity of anthraquinones in the Salmonella preincuabation test. Mutation Research, 116, 297–304. [DOI] [PubMed] [Google Scholar]
- Vargas F, Fraile G, Velasquez M, Correia H, Fonseca G, Marin M, Marcano E and Sanchez Y, 2002. Studies on the photostability and phototoxicity of aloe‐emodin, emodin and rhein. Die Pharmazie, 57, 399–404. [PubMed] [Google Scholar]
- Vázquez MB, Comini LR, Milanesio JM, Montoya SN, Cabrera JL, Bottini S and Martini RE, 2015. Pressurized hot water extraction of anthraquinones from Heterophyllaea pustulata Hook f. (Rubiaceae). The Journal of Supercritical Fluids, 30, 170–175. [Google Scholar]
- Waltenberger B, Avula B, Ganzera M, Khan IA, Stuppner H and Khan SI, 2008. Transport of sennosides and sennidines from Cassia angustifolia and Cassia senna across Caco‐2 monolayers–an in vitro model for intestinal absorption. Phytomedicine, 15, 373–377. [DOI] [PubMed] [Google Scholar]
- Wang L, Li D, Bao C, You J, Wang Z, Shi Y and Zhang H, 2008. Ultrasonic extraction and separation of anthraquinones from Rheum palmatum L. Ultrasonics sonochemistry, 15, 738–746. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakaya N, Kurashima K, Kuriyama S, Tsubono Y and Tsuji I, 2004. Constipation, laxative use and risk of colorectal cancer: the Miyagi Cohort Study. European Journal of Cancer, 40, 2109–2115. [DOI] [PubMed] [Google Scholar]
- Westendorf J, Marquardt H, Poginsky B, Dominiak M, Schmidt J and Marquardt H, 1990. Genotoxicity of naturally occurring hydroxyanthraquinones. Mutation Research/Genetic Toxicology, 240, 1–2. [DOI] [PubMed] [Google Scholar]
- Williams LD, Burdock GA, Shin E, Kim S, Jo TH, Jones KN and Matulka RA, 2010. Safety studies conducted on a proprietary high‐purity aloe vera inner leaf fillet preparation, Qmatrix®. Regulatory Toxicology and Pharmacology, 57, 90–98. [DOI] [PubMed] [Google Scholar]
- Witte P and Lemli L, 1990. The metabolism of anthranoid laxatives. Hepato‐gastroenterology, 37, 601–605. [PubMed] [Google Scholar]
- World Health Organization , 1999. WHO Monographs On Selected Medicinal Plants, Vol. 1. World Health Organization, Geneva. [Google Scholar]
- Wu W, Yan R, Yao M, Zhan Y and Wang Y, 2014a. Pharmacokinetics of anthraquinones in rat plasma after oral administration of a rhubarb extract. Biomedical Chromatography, 28, 564–572. [DOI] [PubMed] [Google Scholar]
- Wu W, Hu N, Zhang Q, Li Y, Li P, Yan R and Wang Y, 2014b. In vitro glucuronidation of five rhubarb anthraquinones by intestinal and liver microsomes from humans and rats. Chemico‐biological interactions, 5, 18–27. [DOI] [PubMed] [Google Scholar]
- Yang X, Liang X, Yang L, Pan F, Deng F and Liu H, 2014. Novel gas‐assisted three‐liquid‐phase extraction system for simultaneous separation and concentration of anthraquinones in Herbal Extract. Chinese Journal of Chemical Engineering, 22, 968–973. [Google Scholar]
- Yokohira M, Matsuda Y, Suzuki S, Hosokawa K, Yamakawa K, Hashimoto N, Saoo K, Nabae K, Kuno T and Imaida K, 2009. Equivocal colonic carcinogenicity of aloe arborescens miller var. natalensis berger at high‐dose level in a wistar Hannover rat 2‐y study. Journal of food science, 74, 24–30. [DOI] [PubMed] [Google Scholar]
- Yu, et al., 2016. Species and gender differences affect the metabolism of emodin via glucuronidation. [DOI] [PMC free article] [PubMed]
- Yu C‐P, Shia C‐S, Lin H‐J, Hsieh Y‐W, Lin S‐P and Hou Y‐C, 2016. Analysis of the pharmacokinetics and metabolism of aloe‐emodin following intravenous and oral administrations in rats. Biomedical Chromatography, 30, 1641–1647. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Fu J, Yao B, Zhang X, Zhao P and Zhou Z, 2011. In vitro genotoxicity of danthron and its potential mechanism. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 722, 39–43. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu K, Cho E, Ma J, Chan AT, Gao X, Willett WC, Fuchs CS and Giovannucci EL, 2013. Prospective cohort studies of bowel movement frequency and laxative use and colorectal cancer incidence in US women and men. Cancer Causes & Control, 24, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LC, Liang J, Li W, Cheng KM, Xia X, Deng X and Yang GL, 2011. The use of response surface methodology to optimize the ultrasound‐assisted extraction of five anthraquinones from Rheum palmatum L. Molecules, 16, 5928–5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Zhao S, Han JT, Wang YF, Wang YN and Wang CH, 2015. Antiviral anthraquinones from the roots of Knoxia valerianoides. Phytochemistry Letters, 31, 57–60. [Google Scholar]


