Abstract
The coccidiostat amprolium hydrochloride from COXAM ® is considered safe for chickens for fattening at 125 mg/kg complete feed. The margin of safety is at least 5. This conclusion is extended to chickens reared for laying. Amprolium hydrochloride does not possess any significant antibacterial activity. The applicant provided no information on the absorption, distribution, metabolism and excretion (ADME) and on the toxicology of the additive or active substance. Reference was made to the Committee for Medicinal Products for Veterinary Use (CVMP) summary reports from 1999 and 2001 citing studies used for the establishment of maximum residue limits (MRLs) for amprolium. However, the original data used in these assessments were not provided and the literature review covering the subsequent period was not made. Thus, the FEEDAP Panel cannot independently evaluate all data relevant to the current application and is therefore unable to conclude on the safety for the consumer of amprolium when used as a feed additive in chickens for fattening and chickens reared for laying. COXAM ® is considered to be a skin and respiratory sensitiser. Inhalation exposure to dust from COXAM ® may present a risk for the user. The use of amprolium hydrochloride from COXAM ® in feed for chickens for fattening up to 125 mg/kg complete feed does not pose a risk for the environment. This conclusion can be extended to chickens reared for laying because of the lower predicted concentration in soil. COXAM ® was effective as a coccidiostat in three floor pen studies and in two anticoccidial sensitivity tests. Since three anticoccidial sensitivity tests showing positive effects of the treatment are required, the FEEDAP Panel is not in the position to conclude on the efficacy of COXAM ® for chickens for fattening under EU farming conditions. Consequently, a conclusion on the efficacy for chickens reared for laying is also not possible.
Keywords: coccidiostat, COXAM ® , amprolium hydrochloride, safety, efficacy, chickens for fattening, chickens reared for laying
Summary
Following a request from European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on COXAM® (amprolium hydrochloride) for chickens for fattening and chickens reared for laying.
The coccidiostat amprolium hydrochloride (amprolium HCl) from COXAM® is considered safe for chickens for fattening at the highest applied concentration of 125 mg/kg complete feed. The margin of safety is at least 5. This conclusion is extended to chickens reared for laying (up to 12 weeks of age). Amprolium HCl does not possess any significant antibacterial activity.
The applicant provided no information on the absorption, distribution, metabolism and excretion (ADME) and on the toxicology of the additive or active substance. Reference was made to the summary reports of the Committee for Medicinal Products for Veterinary Use (CVMP) of the European Medicines Agency (EMA) from 1999 and 2001 citing studies used for the establishment of maximum residue limits (MRLs) for amprolium. However, the original data used in these assessments were not provided and the literature review covering the subsequent period was not made. Thus, the FEEDAP Panel cannot independently evaluate all data relevant to the current application and is therefore unable to conclude on the safety for the consumer of amprolium when used as feed additive in chickens for fattening and chickens reared for laying.
COXAM® is considered to be a skin and respiratory sensitiser. Inhalation exposure to dust from COXAM® may present a risk for the user.
The use of amprolium HCl from COXAM® in feed for chickens for fattening up to 125 mg/kg complete feed does not pose a risk for the environment. This conclusion can be extended to chickens reared for laying because of the lower predicted concentration in soil.
Amprolium HCl from COXAM® was effective as a coccidiostat in three floor pen studies and in two anticoccidial sensitivity tests. Since three anticoccidial sensitivity tests showing positive effects of the treatment with coccidiostat under application are required, the FEEDAP Panel is not in the position to conclude on the efficacy of COXAM® for chickens for fattening under the European Union (EU) farming conditions. Consequently, a conclusion on the efficacy of COXAM® for chickens reared for laying is also not possible.
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from Huvepharma N.V.2 for authorisation of the product COXAM® (amprolium hydrochloride), when used as a feed additive for chickens for fattening and chickens reared for laying (category: coccidiostats and histomonostats).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 1 June 2016.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product COXAM® (amprolium hydrochloride), when used under the proposed conditions of use (see Section 3.1.5).
1.2. Additional information
The feed additive COXAM® (amprolium hydrochloride) is not authorised in the European Union (EU). The active substance amprolium, an antagonist of thiamine, had been included in Annex I of Directive 70/524/EEC3. It was subject of re‐evaluation according to Article 9 g of the same Directive. The dossier presented, however, did not comply with the applicable guidelines Council Directive 87/153/EEC4; therefore, the authorisation was withdrawn by Regulation EC (No) 2205/20015.
The Committee for Medicinal Products for Veterinary Use (CVMP) of the European Medicines Agency (EMA) carried out the assessment of the maximum residue limits (MRLs) of the active substance amprolium (EMA CVMP, 1999, 2001). The CVMP concluded that there is no need to establish an MRL for amprolium. According to the recommendation of the CVMP, Annex II of Council Regulation (EEC) No 2377/90 of 26 June 1990 laying down a Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin was updated and contains amprolium for poultry without MRLs.6
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier7 in support of the authorisation request for the use of COXAM® (amprolium hydrochloride) as a feed additive. The technical dossier was prepared following the provisions of Article 7 of Regulation (EC) No 1831/20038 Regulation (EC) No 429/20089 and the applicable EFSA guidance documents.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, and other scientific reports, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the COXAM® (amprolium hydrochloride) in animal feed. The Executive Summary of the EURL report can be found in Annex A.10
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of COXAM® (amprolium hydrochloride) is in line with the principles laid down in Regulation (EC) No 429/2008 and the relevant guidance documents: Guidance for the preparation of dossiers for coccidiostats and histomonostats (EFSA FEEDAP Panel, 2011a), Technical guidance: Tolerance and efficacy studies in target animals (EFSA FEEDAP Panel, 2011b), Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008a), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012a), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012b) and Technical Guidance: Microbial Studies (EFSA, 2008b).
3. Assessment
The present opinion assesses the safety and efficacy of the coccidiostat COXAM®, containing amprolium hydrochloride (amprolium HCl) as an active substance, when used as a feed additive for the prevention of coccidiosis caused by Eimeria spp. in chickens for fattening and chickens reared for laying. The Panel noted that amprolium HCl is currently not authorised as a feed additive in the EU.
3.1. Characterisation
3.1.1. Characterisation of the additive
The additive is a mixture of amprolium HCl (250 g/kg) with liquid paraffin (30 g/kg) and rice hulls as carrier up to 1,000 g.
The analysis of six batches of COXAM® indicated product consistency; the mean amprolium HCl content was 254.5 g/kg (range: 250.0–260.4 g/kg).11
Data for impurities were provided for three batches of the additive.12 Results showed concentrations of arsenic between 0.069 and 0.348 mg/kg, cadmium between 0.021 and 0.023 mg/kg, lead between 0.17 and 0.18 mg/kg, and mercury < 0.005 mg/kg. Levels of aflatoxins B1, B2, G1 and G2 were < 1 μg/kg each (total < 1.5 μg/kg). Dioxins (polychlorinated dibenzo‐p‐dioxins and dibenzofurans (PCDD/F)) amounted to 0.127 ng WHO‐PCDD/F‐TEQ per kg, and the sum of dioxins and dioxin‐like polychlorinated biphenyls (DL‐PCBs) was 0.257 ng WHO‐PCDD/F‐DL‐PCB‐TEQ per kg. Salmonella was absent in 25 g. None of these impurities is considered to be of concern.
COXAM® is a light brown to brown powder with an average bulk density of 357 kg/m3 and an average tapped density of 393 kg/m3.13 Three batches were analysed for particle size (sieve analysis). COXAM® consisted of 98.3% and 1.8% particles (w/w) with a diameter < 800 μm and < 100 μm, respectively.13 Dusting potential (measured by the Stauber–Heubach method in three batches) was between 0.041 and 0.116 g/m3.14 Dust from the Stauber–Heubach test was collected and analysed for particle size and amprolium HCl content. The dust contained about 48% (v/v) particles of respirable size (< 10 μm).15 The concentration of amprolium HCl in the dust was 55.5–94.8%, indicating a considerable enrichment of the active substance compared to the additive.16
3.1.2. Characterisation of the active substance
Amprolium hydrochloride (1‐[(4‐amino‐2‐propyl‐5‐pyrimidinyl)methyl]‐2‐methylpyridinium chloride monohydrochloride, C14H19ClN4 · HCl, molecular weight 315.24, Chemical Abstracts Service No 137‐88‐2) is a synthetic compound. Its structural formula is given in Figure 1.
Figure 1.
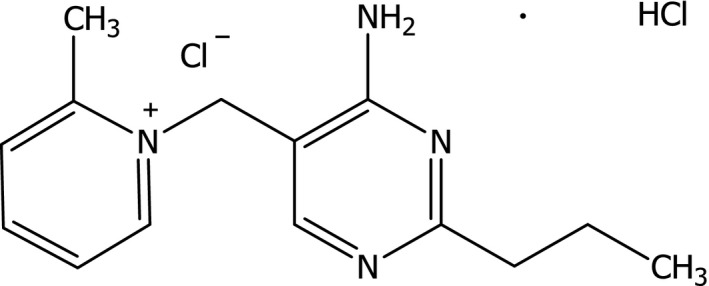
Structural formula of amprolium HCl
■■■■■
■■■■■■■■■■■■■■■
■■■■■
3.1.3. Manufacturing
The manufacturing process of the active substance is fully described in the dossier. ■■■■■
■■■■■
3.1.4. Stability and homogeneity
3.1.4.1. Shelf‐life of the additive
The stability of amprolium HCl from COXAM® was investigated in three batches at 25°C/60% relative humidity (RH) up to 36 months, at 30°C/65% RH up to 12 months and at 40°C/75% RH up to 6 months.18 After 36 months, amprolium HCl concentrations remained within the specification (256, 252 and 257 g/kg, respectively, in the three batches). After 12 months at 30°C/65% RH, a slight decrease in the amprolium HCl concentration was detected (242, 239 and 241 mg/kg). Results at 40°C/75% RH showed a rapid decline of the amprolium HCl concentrations with values of 239–252, 220–224 and 212–216 g/kg at 1, 2 and 3 months respectively.
3.1.4.2. Stability of the additive in premixtures and feedingstuffs
The stability of amprolium HCl (three bathes of COXAM®) was studied in a vitamin–mineral premixture (with choline chloride; intended concentration 25 g amprolium/kg premixture)19 and in three batches of mash and pelleted complete feed for chickens for fattening (wheat, soya, maize; intended concentration 125 mg amprolium HCl/kg).20 Pelleting of the mash feed was carried out at 85°C. Premixture samples were stored for 6 and 3 months under standard (25°C/60% RH) and accelerated (40°C/75% RH) conditions, respectively. Complete feed samples were stored for 3 months and 4 weeks under standard (25°C/60% RH) and accelerated (40°C/75% RH) conditions, respectively. The recovery rates in the premixture under standard conditions were 97% and 95% after 3 and 6 months, respectively; they were 95% and 93% after 3 and 6 months, respectively, under accelerated conditions. Amprolium HCl in the mash feed was stable for 3 months under standard conditions (recovery 101%). Losses of 12% were observed when stored under accelerated conditions for 4 weeks. The effect of pelleting was not constant, the recovery rates varying between 87% and 100%. The storage stability of amprolium in pelleted feed under both conditions was not different to that of mash feed.
3.1.4.3. Homogeneity
Samples from the premixture and the feed taken for the stability studies were used to investigate the homogeneous distribution of COXAM®. Amprolium HCl was analysed in 10 subsamples of the premixture and complete feed (mash and pelleted). The coefficient of variation (CV) of amprolium HCl concentration in the premixture was 7%, in the mash feed 5% and in the pelleted feed 2%.
3.1.5. Conditions of use
COXAM® is intended for the prevention of coccidiosis in chickens for fattening and chickens reared for laying up to 12 weeks of age at a dose of 125 mg amprolium HCl/kg complete feed. The proposed withdrawal period is 0 days.
3.2. Safety
3.2.1. Safety for the target species
3.2.1.1. Tolerance study in chickens for fattening
A total of 224 one‐day‐old male and female chickens for fattening (Ross 308) was randomly allocated to four groups with four replicates per sex (five birds + two spare birds for the first week/replicate) each fed mash diets containing 0, 125 (1x proposed maximum use level), 375 (3x) and 625 (5x) mg amprolium HCl/kg complete feed, respectively, for 43 days.21 The starter diet, fed for the first 14 days, consisted mainly of wheat, soybean meal, maize, barley and wheatfeed; the grower diet, fed until study completion, consisted mainly of wheat bulk, soybean bulk and wheatfeed bulk. The starter formulation was calculated to contain 23.6% crude protein (CP), 0.54% methionine (met) and 12.8 MJ metabolisable energy (ME)/kg; the grower formulation 20.7% CP, 0.58% met and 13.1 MJ ME/kg.22 The birds had ad libitum access to feed and water. The intended concentrations of amprolium HCl in the starter and grower diets were analytically confirmed.
Clinical observations were made daily; body weight and feed intake were recorded at weekly intervals. During week 6, blood samples were taken from one bird per replicate (four males and four females per treatment) for haematology23 and clinical blood biochemistry.24 On day 43/44, one bird/replicate was killed and subjected to necropsy, organ weights were determined for heart, liver, kidneys and spleen. Histopathology was performed in duodenum, ileum, caeca, colon, crop, gall bladder, liver, kidneys, spleen, heart and lungs.
The data were statistically analysed by parametric methods (Williams test when monotonicity of dose response was given or Dunnet's test when monotonicity for dose response was not given), if the Bartlett's test for variance homogeneity was not significant at the 1% level. Covariance analysis was applied to organ weights, and the Fisher's exact test to clinical pathology. For other data, non‐parametric tests (Shirley's or Steel's test) were applied. The statistical analysis was made separately for males and females, both with the individual animal as an experimental unit, the applicant was requested to re‐analyse the data set consisting of both male and female data using the pen as experimental unit for the zootechnical parameters. This second analysis considered for the zootechnical endpoints the pen as a source of variation and was again based on the individual animal as experimental unit for the performance data. This statistical output for the zootechnical data was not considered further. All other endpoints, not considering the pen as source of variation, were analysed using one‐way ANOVA. Normality was tested using the Shapiro–Wilk test, and in case the normality test failed, Kruskal–Wallis one‐way ANOVA on ranks was used. Group differences were tested by the Dunnett's method.
Three male birds died, one each in the control, the intermediate and the high‐dose group without preceding clinical symptoms. One female bird died in the high‐dose group. The main results are summarised in Table 1.
Table 1.
Main results of a six week tolerance study in chickens for fattening with amprolium HCl from COXAM®
| Gender | Amprolium HCl (mg/kg feed) | Final body weight (g) | Feed intake (g/bird and day) | Feed to gain ratio |
|---|---|---|---|---|
| Male | 0 | 2,466 | 99 | 1.61 |
| 125 | 2,284 | 93 | 1.63 | |
| 375 | 2,424 | 96 | 1.60 | |
| 625 | 2,649 | 107 | 1.63 | |
| Female | 0 | 2,396 | 100 | 1.65 |
| 125 | 2,176 | 90 | 1.66 | |
| 375 | 2,176 | 95 | 1.65 | |
| 625 | 2,368 | 96 | 1.63 |
No significant differences were observed for the body weight of male and female birds, based on individual data of 20 birds per treatment for each sex. Feed to gain ratio was of the same magnitude for all groups without differences between sexes.
No significant differences were observed for the haematological endpoints (except for higher MCH values in the amprolium HCl‐treated males) and the clinical chemical endpoints (except for lower serum Na and K concentrations for the amprolium HCl‐treated females (except the high‐dose group for serum K)). The (second) statistical analysis based on all animals did not detect any differences in haematological and clinical biochemical parameters. All parameters remained in the physiological range and no dose‐related effects were observed.
No significant differences in organ weights were found. Macro‐ and histopathology did not indicate any potentially relevant treatment effect, all microscopic changes were considered incidental.
3.2.1.2. Microbial studies
In its assessment, the CVMP (EMA CVMP, 2001) noted that ‘Amprolium is not considered to have antimicrobial activity other than its action on coccidia’.
The FEEDAP Panel supports the view of the CVMP.
3.2.1.3. Conclusions on safety for the target species
Amprolium hydrochloride from COXAM® is considered safe for chickens for fattening at the highest applied concentration of 125 mg/kg complete feed. The margin of safety is at least 5. This conclusion is extended to chickens reared for laying (up to 12 weeks of age).
Amprolium hydrochloride does not possess any significant antibacterial activity.
3.2.2. Safety for the consumer
According to Article 8(e) of Regulation (EC) 1831/20032, a proposal for the establishment of MRLs is not necessary when MRLs have already been established. Currently no MRLs are foreseen for amprolium for poultry (Regulation (EC) No 37/201025).
In the present application, no new studies were presented by the applicant concerning safety for the consumer, and the only information available are the summary reports for the establishment of MRLs made by CVMP in 1999 and 2001. The FEEDAP Panel considered these CVMP assessments and asked the applicant to provide the full CVMP report, including the original reports of all studies referenced. The applicant was further asked to perform a literature search for toxicity and residue studies with amprolium performed since 2000. The requested data were not provided.
The summary reports by CVMP, which established MRLs, were made prior to 2000 and finally concluded in 2001. These reports were based upon studies conducted many years earlier, which were acknowledged at the time to have deficiencies, giving rise to uncertainty in the risk assessment. The studies assessed almost certainly do not comply with current standards, although this can only be finally judged with access to the full data.
In their summary reports, the CVMP established a toxicological acceptable daily intake (ADI) for amprolium of 100 μg/kg body weight (bw), based on a no observed adverse effect level (NOAEL) of 20 mg/kg bw per day for effects on body weight in a 2‐year study with rats applying an uncertainty factor of 200 (i.e. 6 mg/person based on a 60‐kg person). In the first assessment of 1999, provisional MRLs for chicken, turkey and eggs were established; the applicant was asked to provide a final report concerning the total and the marker residue depletion study in chickens, turkey and eggs and the validation of the proposed routine analytical method. In the 2001 re‐evaluation of the residue depletion in chicken and turkey tissues and in eggs (from animals treated in the majority of the studies with 240 mg amprolium/L for 7 days followed by 60 mg amprolium/L for 14 days, in water for drinking corresponding to about 480 mg amprolium/kg and 120 mg amprolium/kg feed, respectively), the total amount of residues likely to be ingested by consumers 6 h after treatment were estimated to be less than 20% of the ADI. CVMP concluded that there is no need to establish an MRL for amprolium.
3.2.2.1. Conclusions on safety for the consumer
The applicant provided no information on the absorption, distribution, metabolism and excretion (ADME) and on the toxicology of the additive or active substance. Reference was made to the CVMP summary reports from 1999 and 2001 citing studies used for the establishment of MRLs for amprolium. However, the original data used in these assessments were not provided and the literature review covering the subsequent period was not made. Thus, the FEEDAP Panel cannot independently evaluate all data relevant to the current application and is therefore unable to conclude on the safety for the consumer of amprolium when used as a feed additive in chickens for fattening and chickens reared for laying.
3.2.3. Safety for the user
3.2.3.1. Effects on the respiratory system
No inhalation toxicity study in laboratory animals has been provided.
Exposure of workers is calculated based on the results of the Stauber–Heubach test, chemical analysis of the generated dust and the EFSA inhalation exposure model as follows.
A calculation made using the data submitted for dusting potential (0.116 g/m3) and amprolium concentration in the dust (mean: 75.6%), identified that workers in a premixture factory handling the COXAM® may be exposed to a concentration of 87 mg amprolium HCl/m3 resulting in an inhalation exposure of 12 mg/person during a 8‐h working day (see Appendix A), of which about 50% are in the respirable fraction (6 mg/8 h working day). This may present a risk for the user by inhalation.
3.2.3.2. Effects on the eyes and skin
A skin irritation test was carried out according to OECD 404.26 A 0.5 g sample of the additive containing 25% amprolium was applied on rabbit skin. This did not provoke local or general changes within an observation period of 72 h, yielding an irritation index (Draize) of 0.00. It is concluded that amprolium is non‐irritant to the skin.
Acute dermal toxicity of COXAM® was tested in rats according to OECD 402.27 Dose applied was 2,000 mg/kg bw. After a 14‐day observation period, no clinical or local adverse signs were noted, and no changes were seen at necropsy, indicating no dermal toxicity.
Acute eye irritation was studied according to OECD 405.28 Test animals received 0.1 g powdered COXAM® in the test eye. Observations included initial eye reactions which disappeared shortly thereafter. Therefore, the additive is not considered an eye irritant.
Skin sensitisation was tested by the local lymph node assay in mice according to OECD 429.29 Based on a preliminary irritation/toxicity test, the maximum dose of COXAM® was determined at 10% w/v in dimethylformamide; test doses were 2.5%, 5% and 10%. No clinical, local signs or body weight changes were noted during the observation period. The stimulation index showed a dose response (1.5, 2.5 and 3.9) with the positive control indicating a valid test. The results indicate that the additive is a potential skin sensitiser.
3.2.3.3. Case reports from occupational exposure
Two cases of adverse effects in humans from occupational exposure to amprolium have been reported in the literature, namely one case of asthma30 and one case of allergic contact dermatitis.31
3.2.3.4. Conclusions on user safety
Based on the studies provided and literature data, COXAM® is not an irritant to skin and eye but should be considered a skin and respiratory sensitiser. Calculations on inhalation exposure to dust from COXAM® indicate that a risk for the user cannot be excluded.
3.2.4. Safety for the environment
The active substance is not a physiological/natural substance of established safety for the environment. Consequently, according to Regulation (EC) No 429/200832 the Phase I assessment has to be continued to determine the predicted environmental concentration.
In Phase I a total residues approach is taken, meaning that the predicted environmental concentrations (PECs) is calculated, based on the assumption that the additive is excreted 100% as parent compound.
3.2.4.1. Phase I
Physicochemical properties of amprolium
The physicochemical properties of amprolium are summarised in Table 2. The FEEDAP Panel noted that water solubility was determined for the acid cation but was not determined for the neutral form. Although the data set is limited, the value of 540,320 mg/L was considered appropriate for the assessment.
Table 2.
Physicochemical properties of amprolium
| Property | Value | Unit |
|---|---|---|
|
Molecular weight Amprolium hydrochloride Amprolium |
315.24 278.78 | |
| Octanol/water partition coefficient (log Kow)1 |
pH 5: −3.133 pH 7: −2.529 pH 9: −2.239 |
– |
| Water solubility (20°C pH between 2 and 3)1 | 540,320 | mg/L |
| Dissociation constant pKa 2 | 4.65 | – |
| Vapour pressure1 |
20°C 4.928 × 10−15 25°C 1.769 × 10−14 |
Pa |
Technical dossier/Section III/Reference III.20.
Technical dossier/Section III Reference III.20.
Fate and behaviour
No information is available on the hydrolysis or biodegradation of amprolium in water.
Fate in soil
Adsorption
A Good Laboratory Practice (GLP)‐compliant soil adsorption/desorption study following OECD guideline 106 batch equilibrium method was performed with five soils of differing properties using non‐radiolabelled amprolium.33 The liquid chromatography with tandem mass spectrometry (LC–MS/MS) analysis method showed recovery ranging from 95% up to 111%. The mass balance requirement of the study (> 70%) was met for one soil of the two soils assessed. A mass balance of 75.62% was achieved for a loamy sand soil (Soil 2.1). However, a mass balance of 45.43% was achieved for a clay soil (Soil 6S). Adsorption/desorption isotherms were performed at five concentrations spanning two orders of magnitude. Freundlich adsorption coefficients (Kf) normalised to the organic carbon content (Kfoc) for amprolium are provided in Table 3. Since amprolium has a pKa of 4.65, it indicates that the acid cation is converted to a neutral molecule at higher pH values. The values in Table 3 do not show a clear pH dependence of the sorption since soil 2.1 with pH 5.2 has a similar Koc compared to soil 2.4 with pH 7.2. The highest sorption was found in the clay soil 6S with pH 7.1. The high Koc from the clay soil might be attributed to sorption to clay since organic cations show considerable sorption to clay minerals (Droge and Goss, 2013) but at pH 7.1 only a small proportion of the molecule in the aqueous phase is in the cationic form. Since the sorption behaviour of amprolium in soil is complicated the lowest Koc of 1,006 is selected as a reasonable worst‐case estimate.
Table 3.
Adsorption of amprolium in different soils1
| Soil (code) | %OC | pH (CaCl2) | Kf (mL/g) | 1/n | Koc (mL/g) |
|---|---|---|---|---|---|
| Sand (2.1) | 0.66 | 5.2 | 8.780 | 0.7053 | 1,330 |
| Loamy sand (2.2) | 1.77 | 5.5 | 25.645 | 0.8785 | 1,449 |
| Sandy loam (2.3) | 0.94 | 6.8 | 9.462 | 0.7596 | 1,006 |
| Loam (2.4) | 2.26 | 7.2 | 31.254 | 0.7084 | 1,382 |
| Clay (6S) | 1.66 | 7.1 | 74.148 | 0.7805 | 4,466 |
| Arithmetic mean | 29.86 | 0.77 | 1,928 | ||
| Geometric mean | 21.81 | 0.76 | 1,643 |
%OC: % of organic carbon; Kf: Freundlich constant; 1/n: adsorption Freundlich exponent; Koc: adsorption or desorption coefficient corrected for soil organic carbon content.
Technical dossier/Section III/Reference III.28.
Degradation
A GLP‐compliant soil biodegradation study following OECD guideline 307 was performed with four soils of differing properties using non‐radiolabelled amprolium.34 The rate of biodegradation was determined in all four soils; however, the route of degradation was not assessed due to the lack of a radiolabel. The study was performed at an application rate of 650 μg/kg dry weight soil. Amprolium demonstrated a single first order dissipation rate. In the clay soil 6S, the degradation was too slow to be measured accurately in 120 days. The FEEDAP Panel assumes that the higher sorption in clay soils slows down the degradation. The geometric mean DT50 from four soils was 54.3 days (Table 4).35 When the soil DT50 is adjusted to an incubation temperature of 12°C using the Arrhenius equation,36 the geometric mean DT50 is 116 days. The FEEDAP Panel noted that a low degradation is shown in one specific soil, therefore, a worst‐case DT50 of 1,000 days at 12°C is used for the assessment.
Table 4.
Half‐life (DT50) of amprolium in different soils1
| Soil | %OC | pH (CaCl2) | DT50 at 12°C (days) | DT50 at 20°C (days) | DT90 at 20°C (days) |
|---|---|---|---|---|---|
| Sand (2.1) | 0.66 | 5.2 | 88.42 | 41.35 | 137.35 |
| Loamy sand (2.2) | 1.74 | 5.5 | 61.33 | 28.68 | 86.82 |
| Sandy loam (2.3) | 1.0 | 6.8 | 80.53 | 37.66 | 125.12 |
| Clay (6S) | 1.66 | 7.1 | 417.35 | 195.18 | 648.37 |
| Arithmetic mean | 161.9 | 75.7 | 249.4 | ||
| Geometric mean | 116.2 | 54.3 | 176.4 |
Technical dossier/Section III/Reference III.27.
Conclusion on fate and behaviour
The Koc of 1,006 L/kg is used for the further risk assessment.
A DT50 for transformation of amprolium of 1,000 days at 12°C is used for further calculations.
Predicted environmental concentrations
The PECs were calculated according to the FEEDAP technical guidance for assessing the safety of feed additives for the environment (EFSA, 2008a).
The input values used for initial PEC calculations were: 125 mg/kg feed, molecular weight (MW) = 315.24, vapour pressure (VP(Pa)) = 4.928E‐15, Solubility (SOL) = 540,320 mg/L, DT50 = 1,000 days (at 12°C) and Koc = 1,006 L/kg. The calculated values are given in Table 5.
Table 5.
Initial predicted environmental concentrations (PECs) of amprolium in soil (μg/kg), groundwater (μg/L), surface water (μg/L) and sediment (μg/kg dry weight)
| Compartment | PEC |
|---|---|
| Soil | 649 |
| Ground water | 36 |
| Surface water | 12 |
| Sediment | 629 |
The Phase I PEC trigger values were exceeded. Therefore, a Phase II assessment is considered necessary.
3.2.4.2. Phase II
Exposure assessment
PECs calculation refined in Phase II
No data were provided for the refinement of the PEC plateau based on metabolism in animal, and degradation in manure. The following refinements were done.
Refinement of PECs for persistent compounds
According to EFSA guidance (EFSA, 2008a), if a high persistence in soil is anticipated (DT90 > 1 year), the potential for residues to accumulate in soil should be considered. This is the case of amprolium and PECs refined at steady state were calculated. The input values used for plateau PEC calculations were: 125 mg/kg feed, MW = 315.24, VP(Pa) = 4.928E‐15, SOL = 540,320 mg/L, DT50 = 1,000 days (at 12°C) and Koc = 1,006 L/kg. The calculated values are given in Table 6.
Table 6.
Plateau predicted environmental concentrations (PECs) of amprolium in soil (μg/kg), groundwater (μg/L), surface water (μg/L) and sediment (μg/kg)
| Compartment | PEC |
|---|---|
| Soil | 2,905 |
| Ground water | 162 |
| Surface water | 54 |
| Sediment | 2,813 |
PEC groudwater refined with FOCUS
Leaching occurs mainly in sandy soils. Since reliable DT50s were provided for the sandy soils, the geometric mean of DT50 of 54 days was considered a reliable endpoint to check whether the additive is a leacher or not. This was checked using the metamodel described in the EFSA guidance (EFSA, 2008a,b), considering a Koc of 1,006 (Kom = Koc/1.7 = 585). The results from the metamodel suggest that an application rate of 1.9 kg/ha is not expected to leach into groundwater at concentrations higher than 0.1 μg/L.
The above results were confirmed by the applicant who simulated the leaching of amprolium to groundwater using the FOCUS‐recommended leaching model PEARL (FOCUS Version 4.4.4.). The 80th percentile annual average concentrations for amprolium in leachate at 100 cm depth were all below 0.001 μg/L for all relevant scenarios as identified by FEEDAP guidance (EFSA, 2008a) and treatments (Table 7). The results indicate that PECgw are below the value of 0.1 μg/L identified by the EU as quality standard.37
Table 7.
Predicted environmental concentration (PEC) in groundwater in leachate at 100 cm depth (μg/L) following the use of COXAM® in chickens for fattening
| Application rate (kg/ha) | FOCUS scenario | PECgw Winter cereals (μg/L) |
|---|---|---|
| 1.722 | Jokioinen | < 0.001 |
| 1.722 | Piacenza | < 0.001 |
FOCUS: FOrum for the Co‐ordination of pesticide fate models and their Use.
Conclusions on PECs used for calculation
The following values are used for the assessment of amprolium: a PECsoil of 2,905 μg/kg, a PECsurface water of 54 μg/L and a PECsediment of 2,813 μg/kg.
Ecotoxicity studies
Toxicity to terrestrial compartment
Effects on plants
A GLP‐compliant terrestrial plant seedling emergence and growth study following OECD 208 was performed with six plant species; four dicotyledons (Phaseolus vulgaris, Raphanus sativus, Cucumis sativus and Solanum lycopersicum) and two monocotyledons (Triticum aestivum and Alium cepa) in natural soil (sandy loam).38 Based on results of a range‐finding experiment, a limit test was performed for P. vulgaris, and R. sativus species. A dose–response test was performed for, C. sativus, S. lycopersicum, T. aestivum and A. cepa species. Control seedling emergence for all species was ≥ 70% (lowest 83.3% for P. vulgaris), the mean survival of emerged control seedlings was ≥ 90% (lowest 96.7% for R. sativus) and the control seedlings did not exhibit any visible phytotoxic effects. Seedling emergence, seedling survival, visual injury, shoot length and growth (as fresh and dry weight biomass) were measured. Amprolium had a positive effect on some of the measured end points when compared to controls and only limited negative effects were observed. Median effect concentration (EC50) values could not be established for any species tested.
Ecotoxicity data (EC50) for the effects of amprolium on terrestrial plants were not available for any of the six species tested. Due to the positive effects observed and lack of negative effects observed during the study no observed adverse effect levels (NOAEL) were established rather than no observed effect concentration (NOEC) values (Table 8). R. sativus was the most sensitive species with a NOAEL of 127.0 mg amprolium/kg for both endpoints of shoot length and fresh weight biomass.
Table 8.
Amprolium ecotoxicological effects data (NOAEL) for terrestrial plants (mg/kg)
| Endpoints | R. sativus | T. aestivum | A. cepa | C. sativus | P. vulgaris | S. lycopersicum |
|---|---|---|---|---|---|---|
| Shoot length | 127.0 | 493.9 | 493.9 | 493.9 | 493.9 | 254.0 |
| Fresh weight biomass | 127.0 | 493.9 | 493.9 | 493.9 | 493.9 | 254.0 |
| Dry weight biomass | 254.0 | 493.9 | 493.9 | 493.9 | 493.9 | 254.0 |
Effect on earthworms
A GLP‐compliant earthworm reproduction study following OECD 222 was performed with the earthworm Eisenia fetida in an artificial soil.33 According to this guideline, the substance is mixed into the soil and the earthworms are fed with clean manure without any toxicant. This is different from the situation in the field where the substance is present in the manure at much higher concentrations than the final concentration in soil. Based on the results of a range‐finding experiment,39 the study was performed as a limit test at a concentration of 88.4 mg amprolium/kg (equivalent 100 mg amprolium hydrochloride/kg). Adult mortality in the controls over the initial 4 weeks of the test was < 10% (actual 0%), all control replicates produced > 30 juvenile worms (mean 364.6) and the CV of reproduction was < 30% (actual 9.2%). In both treatments, the soil moisture was more than 10% higher at the end of the test than the initial soil moisture (13.6–26.5%). Since all the validity criteria mentioned above were fulfilled and the worms showed no effects on behaviour (e.g. avoidance of the substrate), these deviations are considered to have had no impact on the outcome of this study. No mortality was observed in the control and treated replicates and there was no significant difference in juvenile worm production between the control and treated groups. Therefore, it was concluded that the NOEC for reproduction was 88.4 mg amprolium/kg.
Nitrogen transformation
A GLP‐compliant nitrogen transformation study following OECD 216 was performed in a natural sandy loam soil.40 Amprolium was applied to soil at a level slightly higher than the PECsoil dw calculated by the applicant (652.0 μg amprolium/kg (equivalent to 745.5 μg amprolium hydrochloride/kg)) and 10 times that value (6,520.1 μg amprolium/kg (equivalent to 7,454.8 μg amprolium hydrochloride/kg)). Nitrogen transformation rates were calculated using two methods: an incremental method calculating rates based on the difference between sequential time points and an overall method, calculating rates based on the difference between time points and the initial concentration.
The soil nitrogen transformation rate was determined at 7, 14, and 28 days after application. The variation in soil nitrate concentration for replicate control samples was < 15% for all time points (actual ≤ 9.96%). However, one time point (7 day) demonstrated a CV of 16.3%. As the conclusion of the study was based on the formation rates between 0 and 28 days the study was considered valid.
Nitrate formation rate deviations from the controls at 28 day after treatment were less than 25% for the PECsoil dw concentration and the 10x PECsoil dw treatment (Table 9). It is concluded that amprolium when applied at about 10 times the maximum PECsoil dw has no long‐term influence on nitrogen transformation in soils.
Table 9.
Mean soil nitrate formation rate and percentage deviation in PECsoil dw and 10x PECsoil dw compared to control, up to 28 day after treatment
| Days after treatment | Soil nitrate concentration (mg/kg dry weight soil) | ||||
|---|---|---|---|---|---|
| Control | PEC2 | Deviation1 | PEC x102 | Deviation1 | |
| Incremental rates | |||||
| 0–7 | 5.67 ± 1.35 | 5.52 ± 0.63 | −2.76 | 5.46 ± 1.31 | −3.76 |
| 7–14 | 4.58 ± 1.63 | 3.72 ± 0.63 | −18.6 | 5.38 ± 2.35 | 17.59 |
| 14–28 | 3.46 ± 1.00 | 3.57 ± 0.19 | 3.38 | 2.87 ± 1.03 | −16.90 |
| Overall rates | |||||
| 0–7 | 5.67 ± 1.35 | 5.52 ± 0.63 | −2.76 | 5.46 ± 1.31 | −3.76 |
| 0–14 | 5.12 ± 0.44 | 4.62 ± 0.22 | −9.84 | 5.42 ± 1.32 | 5.78 |
| 0–28 | 4.29 ± 0.28 | 4.10 ± 0.05 | −4.52 | 4.15 ± 0.32 | −3.36 |
1 percentage deviation from the control.
2 PEC calculated by the applicant.
Toxicity to aquatic organisms
Effect on algae
A GLP‐compliant study following OECD 201 was performed to investigate the effect of amprolium on green algae.41 Based on the results of a non‐GLP range‐finding test, the green algal species (Pseudokirchneriella subcapitata) was exposed to a concentration range of 0.22, 0.73, 2.56, 10.0, 27.56, 83.25 and 258.42 mg amprolium hydrochloride/L (equivalent to 0.19, 0.65, 2.27, 8.85, 24.39, 73.67 and 228.69 mg amprolium/L) for up to 72 h. The stability of the test item was assessed determining the concentration of amprolium in the test media at the start and end of the exposure period (all concentrations). The mean biomass increase in the control cultures was at least a factor of 16 within the 72‐h test period (actual 163.0), mean CV for section‐by‐section specific growth rates in the control cultures was < 35% (actual 20.4%) and the CV of average specific growth rates during test period in replicate control cultures were < 10% (actual 1.76%). The measured concentrations at the start and end of the exposure period exceeded 20% of nominal concentrations and therefore the evaluation of biological endpoints was performed using calculated geometric mean measured concentrations (Table 10).
Table 10.
Effect concentrations for growth rate of Pseudokirchneriella subcapitata for amprolium (mg/L)
| EC10 | EC20 | EC50 | NOEC | |
|---|---|---|---|---|
| Growth rate | 9.07 | > 228.69 | > 228.69 | 0.19 |
Effect on crustaceans
A GLP‐compliant study following OECD 202 was performed to investigate the effect of amprolium on aquatic invertebrates.42 Based on the results of a non‐GLP range‐finding test, the aquatic invertebrate Daphnia magna were exposed to two concentrations, 100 and 10 mg amprolium hydrochloride/L (equivalent to 88.4 and 8.8 mg amprolium/L) for up to 48 h. To assess the stability of the test item, the concentration of amprolium in the test media was determined at the start and end of the exposure period (both concentrations). Immobilised daphnids in the control are ≤ 10% (actual 0%) and dissolved oxygen concentration in control and test vessels at the end of the test is ≥ 3 mg/L (actual ≥ 8.2 mg/L). Since the measured concentrations at the end of the exposure period were within 20% of nominal concentrations (actual within 6%), the evaluation of biological endpoints was performed using nominal concentrations. At the highest concentration tested (88.4 mg/L), 16.7% of the test animals were immobile after 48 h; the 48‐h EC50 for immobilisation was determined to be > 88.4 mg amprolium/L.
A GLP‐compliant study following OECD 211 was performed to investigate the chronic effect of amprolium on the reproductive output of aquatic invertebrates.43 Based on the results of a non‐GLP range‐finding test, the aquatic invertebrate D. magna were exposed for 21 days to one concentration as a limit test, nominal concentration of 10 mg amprolium hydrochloride/L (equivalent to 8.8 mg amprolium/L). To assess the stability of the test item, the concentration of amprolium in the test media was determined at the start and end of exposure at media renewal. The mortality of the parent animals did not exceed 20% at the end of the test (actual 0%) and the mean number of living offspring produced per parent animal surviving at the end of the test is ≥ 60 (actual 113). Since in some of the analytical measurements of the media amprolium hydrochloride concentrations were outside ± 20% of nominal values the evaluation of biological endpoints was performed using a time weighted mean concentration. Since there was no significant difference in the offspring produced in the negative control and those treated with amprolium hydrochloride, the NOEC was determined to be 11.93 mg amprolium hydrochloride/L (equivalent to 10.56 mg amprolium/L).
Effect on fish
A GLP‐compliant study following OECD 203 was performed to investigate the effect of amprolium on fish.44 Based on the results of a non‐GLP range‐finding test, the fish species Danio rerio was exposed to 100 mg amprolium hydrochloride/L (equivalent to 88.4 mg amprolium/L) for up to 96 h within a static system. To assess the stability of the test item, the concentration of amprolium in the test media was determined at the start and end of the exposure period. Mortality in the controls was ≤ 10% (actual 0%) and dissolved oxygen in control and test vessels ranged between 7.9 and 8.2 mg/L. Concentrations of the test item determined in the test media were 106% of nominal values and the conditions were within acceptable limits throughout the duration of the test, therefore the evaluation of biological endpoints was performed using nominal concentration. No mortalities were observed during the exposure period, the 96‐h median lethal concentration (LC50) was determined to be > 88.4 mg amprolium/L.
Additional information on aquatic toxicity
Another GLP‐compliant study following OECD 201 was performed to investigate the effect of amprolium on cyanobacteria (Anabaena flos‐aquae).45 Based on the results of a non‐GLP range‐finding test, the tested species was exposed to a concentration range of 9.5, 17.1, 30.9, 55.6 and 100 mg amprolium hydrochloride/L (equivalent to 8.4, 15.1, 27.3, 49.2 and 88.4 mg amprolium/L) for up to 72 h. To assess the stability of the test item, the concentration of amprolium in the test media was determined at the start and end of the exposure period (all concentrations). The mean biomass increase in the control cultures was at least a factor of 16 within the 72 h test period (actual 185.0), mean CV for section‐by‐section specific growth rates in the control cultures was ≤ 35% (actual 24.9%) and the CV of average specific growth rates during test period in replicate control cultures were ≤ 10% (actual 1.1%). Since the measured concentrations at the end of the exposure period were within 20% of nominal concentrations (actual within 7%), the evaluation of biological endpoints was performed using nominal concentrations (Table 11). Yield was the most sensitive endpoint. EC20 and EC50 value for growth rate could not be established due to the extent of the extrapolation outside the study concentration range that would be required.
Table 11.
Effects concentrations for growth rate of Anabaena flos‐aquae for amprolium (mg/L)
| EC10 | EC20 | EC50 | NOEC | |
|---|---|---|---|---|
| Growth rate | 123.0 | nd | nd | ≥ 88.4 |
nd: not determined.
Effect on sediment dwelling organisms
A GLP‐compliant study following OECD guideline 218 was performed to investigate the effect of amprolium on the sediment‐dwelling larvae of Chironomus riparius.46 The chironomid larvae were exposed to 50.5, 101.1, 202.1, 404.1 and 808.1 mg amprolium hydrochloride/kg sediment dry weight (dw) basis (equivalent to 44.7, 89.4, 178.7, 357.3 and 714.5 mg amprolium/kg sediment (dw) for up to 28 days. To assess the stability of the test item, the concentration of amprolium in the test media (sediment, porewater and overlying water) was determined at the start and end of the exposure period in the lowest and highest exposure concentrations. The emergence in the controls was > 70% at the end of the test, the emergence of adults in the control vessels occurred between 12 and 28 days, the oxygen concentration was > 60 the air saturation value, the pH of the overlying water was in the range pH 6–9 and the water temperature did not differ by more than 1.0°C. The evaluation of biological endpoints was performed using nominal concentrations since the concentrations were within 20% of nominal concentrations (actual within 6%). Emergence was the most sensitive endpoint and the NOEC was determined as 89.4 mg amprolium/kg sediment (dw).
Conclusions on the ecotoxic effect on soil, water and sediment
For the terrestrial compartment, data are available for microorganisms, earthworms and plants. Risk for terrestrial compartment was evaluated based on earthworm chronic study resulting in the NOEC of 88.4 mg/kg. For the aquatic compartment, data are available for algae, aquatic invertebrates and fish. The lowest toxicity value of EC10 of 9.1 mg/L for the aquatic compartment was found in a study on the effect on green algae Pseudokirchneriella subcapitata.
Ecotoxicological data for sediment‐dwelling invertebrate Chironomus riparius is provided for the sediment compartment resulting in NOEC of 89.4 mg/kg.
Risk characterisation (PEC/PNEC ratio)
The risk characterisation ratios for terrestrial, freshwater and sediment compartments are reported in the tables below.
Table 12.
Risk characterisation (PEC/PNEC ratio) of amprolium for terrestrial compartment
| Taxa | PECsoil (μg/kg) | NOEC/NOAEL (mg/kg) | AF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
| Earthworm 1 | 2,905 | 88.4 | 10 | 8,840 | 0.33 |
| Plants 2 | 127 | 10 | 12,700 | 0.23 |
PNEC: predicted no effect concentration; AF: assessment factor; PEC: predicted environmental concentration.
1 NOEC.
2 NOAEL.
Table 13.
Risk characterisation (PEC/PNEC ratio) of amprolium for freshwater compartment
| Taxa | PECsurfacewater (μg/L) | EC10/EC50/NOEC/LC50 (mg/L) | AF | PNEC (μg/L) | PEC/PNEC |
|---|---|---|---|---|---|
|
Algae 1 Pseudokirchneriella subcapitata |
54 | 9.1 | |||
|
Aquatic invertebrates Daphnia magna acute2 reproductive3 |
88.4 11.9 |
50 | 182 | 0.30 | |
|
Fish 4 Brachydanio rerio |
> 84.4 |
1 EC10.
2 48‐h EC50.
3 NOEC.
4 96‐h LC50.
Table 14.
Risk characterisation (PEC/PNEC ratio) of amprolium for sediment
| Taxa | PECsediment (μg/kg) | NOEC (mg/kg) | AF | PNEC (μg/kg) | PEC/PNEC |
|---|---|---|---|---|---|
| Sediment‐dwelling invertebrates Chironomus riparius | 2,813 | 89.4 | 10 | 8,940 | 0.31 |
Bioaccumulation and secondary poisoning
Since the log Kow is estimated as −2.5, the bioaccumulation and the risk for secondary poisoning is considered to be low.
3.2.4.3. Conclusions on safety for the environment
The use of amprolium from COXAM® in feed for chickens for fattening up to 125 mg/kg complete feed does not pose a risk for the environment.
This conclusion can be extended to chickens reared for laying because of the lower predicted concentration in soil.
3.3. Efficacy
Efficacy data for coccidiostats should derive from three types of target animal experiments: (a) dose–titration studies; (b) natural/artificial infection to simulate use conditions (e.g. floor pen studies with poultry), at least one of the locations should be in the EU; and (c) actual use conditions in field trials, all should be done in the EU within the last 5 years. Anticoccidial sensitivity tests (AST) could replace field trials provided they follow the criteria mentioned in the relevant guidance document on coccidiostats and histomonostats (EFSA FEEDAP Panel, 2011a).47
For the current assessment, the applicant provided three floor pen studies, five anticoccidial sensitivity tests and a field study. In all studies, the endpoints of an infected untreated control (IUC) group were compared with an uninfected untreated control (UUC) group and one or more infected treated (IT) group/s. Intestinal lesions were scored following the method of Johnson and Reid (1970) (0 = no lesion, 1 = very mild, 2 = mild, 3 = moderate and 4 = severe).
3.3.1. Floor pen studies
Three floor pen studies in chickens for fattening, conducted between 2012 and 2013, were submitted.48 In each study male chickens (Ross 308) were penned and distributed into three treatment groups: UUC, IUC and IT (see Table 15). The IT group received feed containing 125 mg amprolium hydrochloride from COXAM®/kg feed, the dose was analytically confirmed. The experimental diets were fed for the whole duration of the study, i.e. 35/35/42 days in trials 1, 2 and 3, respectively. In trials 1 and 2, all birds of the infected groups were inoculated orally on day 14 with recent field isolates of pathogenic Eimeria species. In trial 3, seeder birds (8 of 25) were either inoculated in the infected groups or sham‐inoculated (water only) in the UUC group. Animal health and mortality were monitored daily. Feed intake and body weight of the animals were measured, feed to gain ratio was calculated. Samples of excreta were analysed for oocyst excretion. Intestinal lesions were scored on three birds per pen.
Table 15.
Experimental design of floor pen studies with chickens for fattening using COXAM®
| Trial | Replicates per treatment (birds per replicate) | Inoculum characteristics | Feed analysis (mg/kg feed)1 Amprolium hydrochloride | |||
|---|---|---|---|---|---|---|
| Month/year and country of isolation | Intended dose per bird | Day and mode of inoculation | ||||
| 1 | 14 (31) |
03/2012 Spain |
100,000 | E. acervulina | Day 14 via feed | 123/125/124 |
| 10,000 | E. tenella | |||||
| 50,000 | E. maxima | |||||
| 2 | 10 (35) |
09/2012 France |
29,280 | E. acervulina | Day 14 orally via syringe | 122/123/125 |
| 10,920 | E. tenella | |||||
| 3,880 | E. maxima | |||||
| 3,480 | E. mitis | |||||
| 3 | 10 (25) |
03/2012 Belgium |
104,800 | E. acervulina | Day 12 (8 of 25 animals per pen) orally via syringe | 123/119‐126/122‐128 |
| 18,400 | E. tenella | |||||
| 28,000 | E. maxima | |||||
| 3,200 | E. praecox | |||||
| 8,000 | E. mitis | |||||
1 In trial 1, birds received starter diet from day 1 to 14, grower diet from day 14 to 28 and finisher diet from day 28 to 35; in trial 2, birds received starter diet from day 1 to 14, grower diet from day 14 to 26 and finisher diet from day 26 to 35; in trial 3, birds received starter diet from day 1 to 12, grower diet from day 12 to 35 and finisher diet from day 35 to day 42.
In all trials, an ANOVA was done with the data, with exception of lesion scores and oocyst counts which were analysed with a non‐parametric test. Group means were compared with least significant difference (LSD) tests. In trial 1, data were considered on pen basis with the exception of lesion score; in trials 2 and 3, the statistical unit for feed intake and oocyst count was the pen while for lesions score, body weight and weight gain was the bird.
Oocyst excretion was significantly reduced in the IT group compared to the IUC group on day 21 in trial 1 and trial 2 and on day 35 in trial 3 (Table 16). In trials 2 and 3, in the UUC the animals became infected.
Table 16.
Total number of Eimeria oocysts per gram of excreta (OPG) in floor pen studies
| Trial 1 (1) | Day 21 | Day 28 | Day 35 | |
| UUC | 129 | 79 | nd | |
| IUC | 234,000 | 955 | 447 | |
| IT | 27,500* | 525 | 380 | |
| Trial 2 | Day 21 | Day 28 | Day 35 | |
| UUC | 148,140a | 308,100b | 25,280 | |
| IUC | 545,800b | 49,420a | 8,880 | |
| IT | 249,000a | 21,720a | 12,160 | |
| Trial 3 | Day 20 | Day 26 | Day 35 | Day 42 |
| UUC | 0b | 20b | 87,260a | 55,340a |
| IUC | 68,580a | 382,600a | 26,660b | 6,020b |
| IT | 14,240a | 642,000a | 14,560c | 3,800b |
nd: not detected.
* IT means with * are significantly different to IUC (p ≤ 0.05).
1 Only IUC and IT group means were compared.
a,b,c Means in a column within a study with different superscript are significantly different (p ≤ 0.05).
Table 17 shows the intestinal lesion scores observed in the three trials. A significant reduction of lesion scores was seen in IT birds compared to the IUC groups in trial 1 at day 20, in trial 2 at days 20 and 26 in the upper intestine and on day 20 in caeca. In trial 3 total lesion scores at days 18 and 35 in IT birds were significantly reduced compared with IUC birds.
Table 17.
Eimeria infection‐related intestinal lesion scores in floor pen studies
| Day 20 | Day 26 | |||||
|---|---|---|---|---|---|---|
| Upper intestine | Middle intestine | Caecum | Upper intestine | Middle intestine | Caecum | |
| Trial 1 | ||||||
| UUC | 0 | 0 | 0 | 0 | 0 | 0 |
| IUC | 2.0 | 1.0 | 2.4 | 0 | 0 | 0 |
| IT | 0* | 0* | 0* | 0 | 0 | 0 |
| Day 20 | Day 26 | |||||||
|---|---|---|---|---|---|---|---|---|
| Upper intestine | Middle intestine | Lower intestine | Caecum | Upper intestine | Middle intestine | Lower intestine | Caecum | |
| Trial 2 | ||||||||
| UUC | 0.77b | 0.50b | 0.30 | 0.00a | 1.27c | 1.03b | 0.67b | 0.03 |
| IUC | 0.90b | 0.17a | 0.33 | 0.93b | 0.67b | 0.40a | 0.30a | 0.07 |
| IT | 0.10a | 0.13a | 0.20 | 0.30a | 0.20a | 0.40a | 0.27a | 0.00 |
* IT means with * are significantly different to IUC (p ≤ 0.05).
1 Intestinal lesions were scored on seeder birds on day 18 and on contact birds on days 26 and 35. The values reported represent the sum of lesion scores specific to Eimeria acervulina, tenella and maxima.
a,b,c, Means in a column within a study with different superscript are significantly different (p ≤ 0.05).
Table 18 summarises the results concerning mortality and zootechnical endpoints. Mortality after Eimeria inoculation was low in all three trials. Significant difference was found between IUC and IT birds in trial 1.
Table 18.
Performance parameters and mortality of chickens for fattening in the floor pen studies
| Feed Intake (g)1 | Body Weight (g) | Weight Gain (g)2 | Feed to gain ratio | Mortality (%) | |
|---|---|---|---|---|---|
| Trial 1 | |||||
| UUC | 97.1* | 2,210* | 61.9* | 1.57* | 2.3 |
| IUC | 91.5* | 1,899* | 53.1* | 1.72* | 6.7* |
| IT | 94.4 | 2,068 | 57.9 | 1.63 | 0.5 |
| Trial 2 | |||||
| UUC | 3,760b | NR | 2,238b | 1.68b | 0.8 |
| IUC | 3,685a | NR | 2,119a | 1.73a | 0.5 |
| IT | 3,701a | NR | 2,140a | 1.73a | 0.2 |
| Trial 3 | |||||
| UUC | 133 | 3,254a | 76a | 1.75b | 2.5 |
| IUC | 136 | 3,011b | 71b | 1.92a | 5.0 |
| IT | 137 | 3,054b | 72b | 1.92a | 3.6 |
NR: not reported.
* Means within a column with * are significantly different to IT group (p ≤ 0.05).
1 Results of trials 1 and 3 refer to daily feed intake; results of trial 2 refer to cumulative feed intake (day 0–35).
2 Results of trials 1 and 3 refer to daily gain; results of trial 2 refer to cumulative weight gain (day 0–35).
a,b,c Means in columns with different superscript are significantly different (p ≤ 0.05).
In trial 1 feed intake, body weight and daily weight gain were significantly higher in the IT group compared with IUC group. In all three trials the body weight gain of the UUC groups exceeded significantly that of IT groups. Feed to gain ratio was significantly improved in IT compared to IUC only in trial 1.
3.3.2. Anticoccidial sensitivity tests
Five studies called AST were submitted. Two of them were not considered since the inoculation with sporulated Eimeria oocysts did not provoke coccidiosis‐related adverse effects.49 The studies considered were performed in 2013, 2015 and 2017.50 Study design and conduct of AST‐1 and AST‐2 identified both trials as floor pen studies which do not comply with the typical procedure applied to ASTs. The differences are mainly due to the period for which the coccidiostat is administered (although in relation to the date of inoculation), the route of administration of the inoculum and study duration. In both ASTs, feed supplemented with COXAM® was given from day 1 (typical for AST day 12) and in AST 1 inoculation was provided via feed (typical for AST via syringe). However, other criteria applied to ASTs like origin and treatment of the inoculum, measurement of Eimeria specific endpoints about 1 week after inoculation and coccidiosis‐related mortality in this period were all provided in the study report. The results of these floor pen studies were consequently described for the critical period applied to a typical AST and considered in the assessment as ASTs.
In all three ASTs, feed of the IT group was supplemented with amprolium from COXAM® at an intended concentration of 125 mg/kg, which was analytically confirmed (see Table 19). The birds (Ross 308) were randomly allocated to the groups. Birds were artificially infected with sporulated oocysts from recent field isolates. Animal health and mortality were monitored. Feed intake and body weight of the animals were measured, feed to gain ratio was calculated. Samples of excreta were analysed for oocyst excretion. Intestinal lesions were scored on three birds per pen in AST‐1, on nine birds per pen in AST‐2 and on all five birds of the individual pens in AST‐3.
Table 19.
Experimental design of ASTs with chickens for fattening using COXAM®
| AST | Replicates per treatment (birds per replicate) | Inoculum characteristics | Start of anti‐coccidial treatment (day of life) | Feed analysis1 amprolium hydrochloride (mg/kg feed) | |||
|---|---|---|---|---|---|---|---|
| Month/year and country of isolation | Intended dose per bird | Day of inoculation | |||||
| 1 | 9 (22) |
03/2013 Spain |
50,000 | E. acervulina | 15 via feed | 1 | 140/118/102/137 |
| 17,000 | E. tenella | ||||||
| 2 | 14 (15) | 07/2015 The Netherlands | 66,150 | E. acervulina | 14 via syringe | 1 | 121/119/125 |
| 7,844 | E. tenella | ||||||
| 5,111 | E. maxima | ||||||
| 3 | 6 (5) |
2016 Russia |
105,097 | E. acervulina | 16 via syringe | 14 | 119 |
| 3,419 | E. tenella | ||||||
| 16,000 | E. maxima | ||||||
| 8,710 | E. mitis | ||||||
1 In trial 1, birds received starter diet from day 1 to 12, grower I diet from day 13 to 21, grower II diet from day 22 to 35 and finisher diet from day 36 to 45; in trial 2, birds received starter diet from day 1 to 14, grower diet from day 14 to 26 and finisher diet from day 26 to 35; in trial 3, birds received starter diet from day 1 to 12, grower diet from day 12 to 35 and finisher diet from day 35 to day 42.
In AST‐1, the statistical unit was the pen for all endpoints except lesion scores for which the individual bird data were used. In AST‐2 and AST‐3, the statistical unit for feed intake, feed to gain ratio and oocyst excretion was the pen, for body weight, body weight gain and lesion score the individual bird.
In all ASTs, ANOVA was used as the main statistical tool, in AST‐2 and 3 together with a linear mixed regression model with treatment group as a categorical fixed effect and pen as a random effect. Group comparisons were done in AST‐1 for the means by LSD. In ASTs 2 and 3, the least square means of IUC were tested against UUC and IT separately.
Table 20 summarises the results of the three ASTs. In AST‐1 and AST‐3, Eimeria inoculation increased coccidiosis‐related mortality in the week period after inoculation from 0 for UUC to about 6% for the IUC groups. No data for this period were given for AST‐2 and no statistics were applied to the data of AST‐1 and AST‐3. The mortality rate of the IT groups in AST‐1 was near to UUC, but not different to IUC in AST‐3.
Table 20.
Results of anticoccidial sensitivity tests1
| Group | bw | ADG | ADFI | F/G | Mortality2 | OPG | Lesion score | |
|---|---|---|---|---|---|---|---|---|
| AST‐1 | D21 | D16−D21 | D13−D21 | D22 | D21 Duodenum | D21 Caecum | ||
| UUC | 831a | 66.8a | 97.3 | 1.46 | 0 | 15,663 | 0.52a | 0.07a |
| IUC | 789b | 58.5b | 91.3 | 1.57 | 6.6 | 66,390 | 1.48b | 1.89b |
| IT | 820ab | 65.8a | 98.1 | 1.49 | 0.5 | 21,170 | 0.37a | 0.07a |
| AST‐2 | D21 3 | D15−D21 | D20−D23 | D20−D22 4 | ||||
| UUC | 919* | 72* | 100* | 1.42* | nd | 1,096b | 0.4* | |
| IUC | 779 | 45 | 82 | 1.86 | nd | 147,266a | 5.0 | |
| IT | 821* | 51* | 86 | 1.70* | nd | 179,871a | 3.5* | |
| AST‐3 | D22 | D14−D22 | Total | D22 | D22 | |||
| UUC | 886* | 67* | 96.1* | 1.43* | 0 | 581* | 0.3* | |
| IUC | 776 | 54 | 87.5 | 1.63 | 6.7 | 458,166 | 2.6 | |
| IT | 840* | 61* | 94.7 | 1.57 | 6.7 | 264,740 | 2.0 | |
nd: not determined in this period.
a,b,c Means within a column within a study with different superscript are significantly different (p ≤ 0.05).
* Means within a column with * are significantly different to IUC group (p ≤ 0.05).
1 Average daily feed intake (ADFI) in g, body weight (bw) in grams, average daily gain (ADG) in g/day, feed to gain ratio (F/G), mortality: %, total oocysts per g excreta (OPG).
2 No statistical analysis was applied to the periods reported.
3 Average of days 20 and 21 (3 birds each/pen killed for lesion scoring) and of day 22 (remaining birds).
4 Average total intestinal lesion scores of the three scoring days (sum of LS due to E. acervulina, E. maxima and E. tenella).
Oocyst excretion about 1 week after inoculation indicated successful Eimeria infection. OPG was not significantly reduced by the COXAM® treatment in all ASTs, however, there was a numerical reduction to the level of the UUC group in AST‐1.
A significant reduction of intestinal lesion scores (duodenum and caecum) by the COXAM® treatment compared to the IUC groups was observed in AST‐1. In AST‐2 statistical analysis was performed on the total lesion score calculated as a sum of lesion scores specific to E. acervulina maxima and E. tenella. The total lesion score in the IT group was found significantly lower than in IUC group; however, the significance of is only due to differences in E. tenella lesion score (no statistical analysis was performed on Eimeria specific lesion scores). It appears that E. acervulina and E. maxima in the inoculum of AST‐2 were resistant to amprolium since approximately equal lesion scores were observed. No difference in lesion score between IUC and IT was seen in AST‐3.
Average daily gain in the IT group was significantly improved by the anticoccidial treatment as seen by a comparison of the IUC and IT groups in all three ASTs. This difference is related to reduced intestinal lesions in AST‐1 and ‐2 and is therefore considered as a measure of the anticoccidial efficacy of COXAM® in these two ASTs but not in AST‐3.
3.3.3. Field trial
The applicant provided a trial carried out in 2014 under controlled field conditions.51 Two groups with different dietary coccidiostats were compared on a total of 89,700 one‐day‐old male and female chickens for fattening in three houses (two houses with amprolium hydrochloride, one house with a shuttle program as control). The test diet contained 125 mg amprolium hydrochloride/kg. The control diet was supplemented with 50 + 50 mg of a polyether+a synthetic coccidiostat for the starter phase followed by 60 mg of another polyether coccidiostat/kg for the grower phase. Study duration was 35 days followed by a 1‐week withdrawal period. Weekly records of body weight were done on a basis of 50 animals per house. Lesion scores were examined four times on the basis of 10 animals per house. Faecal samples to determine OPG were taken weekly in each house.
The field trial was not considered further due to weaknesses in study design and conduct. The influence of poultry houses may overlap the effect of the coccidiostat. Body weight measured on 50 birds randomly selected from about 30,000 chickens in one house cannot be considered as representative. The same argument is applied to 10 chickens per house used for lesion scoring. Coccidiosis‐related mortality was not examined, only total mortality.
Synopsis on efficacy studies
In three floor pen studies, treatment with COXAM® significantly reduced oocyst excretion on day 21 in trial 1 and trial 2, and on day 35 in trial 3. A significant reduction of lesion scores was seen in IT birds compared to the IUC groups in trial 1 at day 20, in trial 2 at days 20 and 26 in the upper intestine and on day 20 in caeca. In trial 3 total lesion scores at days 18, 26, 35 were significantly reduced in IT birds compared with IUC birds. Mortality after Eimeria inoculation was low in all three trials; it was significantly lower in IT birds compared to IUC birds in trial 1. In trial 1 feed intake, body weight, daily weight gain and feed to gain ratio were significantly improved in the IT group compared with IUC group. In all three trials the body weight of the UUC groups exceeded significantly that of IT groups.
Two floor pen studies were assessed on the basis of endpoints obtained in a study design of a typical AST. AST‐1 showed a significant reduction of lesion scores in the small intestine and the hind gut related with better weight gain by the COXAM® treatment, which also significantly reduced mortality, compared to the IUC group. In AST‐2 a significant reduction of the total lesion score was reported, however, the numerical differences could be traced back to differences by E. tenella lesion scores only, while E. acervulina and E. maxima appeared to be resistant to amprolium. Body weight and body weight gain were higher in the IT group compared to the IUC group, this improvement is considered to be related to the anticoccidial effect seen in this study.
In a third AST, none of the coccidiosis‐related endpoints showed a significant improvement in the IT group compared to IUC, a significantly higher body weight of the IT group could not be considered as an effect of the anticoccidial efficacy.
Conclusions on efficacy for the target species
Amprolium from COXAM® was effective as a coccidiostat in three floor pen studies and in two anticoccidial sensitivity tests. Since three anticoccidial sensitivity tests showing positive effects of the treatment with coccidiostat under application are required, the FEEDAP Panel is not in the position to conclude on the efficacy of COXAM® for chickens for fattening under EU farming conditions. Consequently, a conclusion on the efficacy of COXAM® for chickens reared for laying is also not possible.
3.4. Post‐market monitoring
Field monitoring of Eimeria spp. resistant to amprolium should be undertaken, preferably during the latter part of the authorisation period.
4. Conclusions
Amprolium hydrochloride from COXAM® is considered safe for chickens for fattening at the highest applied concentration of 125 mg/kg complete feed. The margin of safety is at least 5. This conclusion is extended to chickens reared for laying (up to 12 weeks of age). Amprolium HCl does not possess any significant antibacterial activity.
The applicant provided no information on the ADME and on the toxicology of the additive or active substance. Reference was made to the CVMP summary reports from 1999 and 2001 citing studies used for the establishment of MRLs for amprolium. However, the original data used in these assessments were not provided and the literature review covering the subsequent period was not made. Thus, the FEEDAP Panel cannot independently evaluate all data relevant to the current application and is therefore unable to conclude on the safety for the consumer of amprolium when used as feed additive in chickens for fattening and chickens reared for laying.
COXAM® is not an irritant to skin and eye but should be considered a skin and respiratory sensitiser. Inhalation exposure to dust from COXAM® may present a risk for the user.
The use of amprolium HCl from COXAM® in feed for chickens for fattening up to 125 mg/kg complete feed does not pose a risk for the environment. This conclusion can be extended to chickens reared for laying because of the lower predicted concentration in soil.
Amprolium HCl from COXAM® was effective as a coccidiostat in three floor pen studies and in two anticoccidial sensitivity tests. Since three anticoccidial sensitivity tests showing positive effects of the treatment with coccidiostat under application are required, the FEEDAP Panel is not in the position to conclude on the efficacy of COXAM® for chickens for fattening under EU farming conditions. Consequently, a conclusion on the efficacy of COXAM® for chickens reared for laying is also not possible.
Documentation provided to EFSA
COXAM® (amprolium) for chickens for fattening and chickens reared for laying. March 2016. Submitted by Huvepharma N.V.
COXAM® (amprolium) for chickens for fattening and chickens reared for laying. Supplementary information. June 2017. Submitted by Huvepharma N.V.
Evaluation report of the European Union Reference Laboratory for Feed Additives on the Methods(s) of Analysis for COXAM®.
Comments from Member States.
Chronology
| Date | Event |
|---|---|
| 11/3/2016 | Dossier received by EFSA |
| 14/4/2016 | Reception mandate from the European Commission |
| 1/6/2016 | Application validated by EFSA – Start of the scientific assessment |
| 18/7/2016 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation, safety for the target species and safety for the consumer |
| 1/9/2016 | Comments received from Member States |
| 17/8/2016 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 28/6/2017 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 16/10/2017 | Request of clarification to the applicant on the supplementary information via e‐mail |
| 3/11/2017 | Reception of clarification from the applicant via e‐mail |
| 13/6/2018 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- AF
assessment factor
- ADI
acceptable daily intake
- ADME
absorption, distribution, metabolism and excretion
- AST
anticoccidial sensitivity test
- bw
body weight
- CAS
Chemical Abstracts Service
- CP
crude protein
- CV
coefficient of variation
- CVMP
Committee for Medicinal Products for Veterinary Use
- DM
dry matter
- DT50
Disappearance Time 50 (the time within which the concentration of the test substance is reduced by 50%)
- DT90
Disappearance Time 90 (the time within which the concentration of the test substance is reduced by 90%)
- dw
dry weight
- EC50
median effective concentration
- EMA
European Medicines Agency
- ErC10
median effective concentration which results in a 10% reduction in growth rate
- ErC50
median effective concentration which results in a 50% reduction in growth rate
- EURL
European Union Reference Laboratory
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- FOCUS
FOrum for the Co‐ordination of pesticide fate models and their Use
- GLP
Good Laboratory Practice
- Kf
Freundlich adsorption coefficient
- Koc
adsorption or desorption coefficient corrected for soil organic carbon content
- Kom
Koc expressed in organic matter (Koc/1.7)
- LC50
median lethal concentration
- LOQ
limit of quantification
- log Kow
logarithm of octanol–water partition coefficient
- LSD
least significant difference
- MIC
minimum inhibitory concentration
- MRL
maximum residue limit
- MW
molecular weight
- NOAEL
no observed adverse effect level
- NOEC
no observed effect concentration
- OECD
Organisation for Economic Co‐operation and Development
- OPG
oocysts per gram of excreta
- pKa
dissociation constant
- PEC
predicted environmental concentration
- PNEC
predicted no effect concentration
- RH
relative humidity
- SOL
solubility
- VP
vapour pressure
Appendix A – Estimation of user exposure to amprolium HCl from the additive COXAM®, including consideration of using a filter mask FF P2 or FF P3 as a preventative measure
1.
| Calculation | Identifier | Description | Amount | Source |
|---|---|---|---|---|
| a | Amprolium HCl concentration in dust (mg/kg) | 756,000 | Technical dossier | |
| b | Dusting potential (g/m3) | 0.116 | Technical dossier | |
| a × b | c | Amprolium HCl in the air (mg/m3) | 87.7 | |
| d | No of premixture batches made/working day | 10 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012b) | |
| e | Time of exposure (s) per production of one batch | 20 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012b) | |
| d × e | f | Total duration of daily exposure/worker (s) | 200 | |
| g | Uncertainty factor | 2 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012b) | |
| f × g | h | Refined total duration of daily exposure/worker (s) | 400 | |
| h/3 600 | i | Refined total duration of daily exposure (h) | 0.11 | |
| j | Inhaled air (m3) per hour | 1.25 | EFSA Guidance on user safety (EFSA FEEDAP Panel, 2012b) | |
| j/8 × i | k | Inhaled air during exposure (m3) | 0.14 | |
| c × k | l | Amprolium HCl (mg) inhaled during exposure per eight‐hour working day | 12 | |
| l/10 | Amprolium HCl inhaled (mg) per eight‐hour working day reduced by filter mask FF P2 (reduction factor 10) | 1 | ||
| l/20 | Amprolium HCl inhaled (mg) per eight‐hour working day reduced by filter mask FF P3 (reduction factor 20) | 0.6 |
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for COXAM®
1.
In the current application authorisation is sought for Coxam ®, under article 4(1), for the category “coccidiostats and histomonostats”, according to the classification system of article 6 of Regulation (EC) No 1831/2003. Authorisation is sought for chickens for fattening and reared for laying. Coxam ® consists of 250 g/kg of amprolium hydrochloride complemented by liquid paraffin as antidusting agent, and rice hulls as carrier. Coxam ® is intended to be incorporated in feedingstuffs through premixtures at a content of amprolium hydrochloride of 125 mg/kg feedingstuffs.
For the quantification of amprolium in the feed additive (Coxam ®), the Applicant submitted a single‐laboratory validated and further verified method based on Reversed‐Phase High Performance Liquid Chromatography coupled to Ultraviolet detection (RP‐HPLC‐UV). The following performance characteristics were reported: ‐ a precision ranging from 0.8 to 1.0%; and ‐ a recovery rate (RRec) ranging from 99 to 100%.
For the quantification of amprolium in the premixtures and feedingstuffs the Applicant submitted the ring trial validated Community method (Commission Regulation (EC) No 152/2009) based on Cation Exchange High Performance Liquid Chromatography coupled to Ultraviolet detection IE‐HPLC‐UV. The following performance characteristics were reported: ‐ a relative standard deviation for repeatability (RSDr) ranging from 1.9 to 5.0% and a relative standard deviation for reproducibility (RSDR) ranging from 3.0 to 6.5%, ‐ RRec ranging from 91 to 103% and ‐ a limit of quantification (LOQ) of 5 mg/kg feedingstuffs. In addition, the Applicant provided experimental evidence demonstrating the applicability of the Community method for determining amprolium in premixtures and feedingstuffs samples containing Coxam ®.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Brantom P, Halle I, van Beelen P, Holczknecht O, Vettori MV and Gropp J, 2018. Scientific Opinion on the safety and efficacy of COXAM® (amprolium hydrochloride) for chickens for fattening and chickens reared for laying. EFSA Journal 2018;16(7):5338, 28 pp. 10.2903/j.efsa.2018.5338
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00290
Panel members: Gabriele Aquilina, Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Georges Bories, Andrew Chesson, Pier Sandro Cocconcelli, Gerhard Flachowsky, Jürgen Gropp, Boris Kolar, Maryline Kouba, Secundino López Puente, Marta López‐Alonso, Alberto Mantovani, Baltasar Mayo, Fernando Ramos, Guido Rychen, Maria Saarela, Roberto Edoardo Villa, Robert John Wallace and Pieter Wester.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the European Commission decision on the confidentiality requests formulated by the applicant. A previous, provisional version of this output which had been made publicly available pending the adoption of the decision has been replaced by this version. The full output has been shared with the European Commission, EU Member States and the applicant.
Acknowledgements: The EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) wishes to thank the following for the support provided to this scientific output: Jaume Galobart, Rosella Brozzi, Lucilla Gregoretti and Paola Manini.
Adopted: 13 June 2018
Amended: 2 Jul 2020
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Huvepharma NV, Uitbreidingstraat 80, 2600 Antwerp, Belgium.
Council Directive 70/524/EEC concerning additives in feedingstuffs. OJ L 270, 14.12.70, p. 1.
Council Directive of 16 February 1987 fixing guidelines for the assessment of additives in animal nutrition (87/153/EEC). OJ L 64, 7.3.87, p. 19.
Commission Regulation (EC) No 2205/2001 of 14 November 2001 amending Council Directive 70/524/EEC concerning additives in feedingstuffs as regards withdrawal of the authorisation of certain additives. OJ L 297, 15.11.2001, p. 3.
Commission Regulation (EC) No 1478/2001 of 18 July 2001 amending Annexes I, II and III of Council Regulation (EEC) No 2377/90 laying down a Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. OJ L 195, 19.7.2001, p. 32.
FEED dossier reference: FAD‐2016‐0017.
OJ L 268, 18.10.2003, p. 29.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
The full report is available on the EURL website: https://ec.europa.eu/jrc/en/eurl/feed-additives/evaluation-reports/fad-2016-0017?search&form-return
Technical dossier/Section II/Reference II.04.
Technical dossier/Section II/Reference II.06. and Reference II.07.
Technical dossier/Section II/Reference II.08.
Technical dossier/Section II/Reference II.09.
Technical dossier/Section II/Reference II.11.
Technical dossier/Section II/Reference II.10.
■■■■■
Technical dossier/Supplementary information June 2017/Annex 1 and clarification received in November 2017.
Technical dossier/Section II/Reference II.19.
Technical dossier/Section II/Reference II.20.
Technical dossier/Section III/Reference III.1.
Technical dossier/Supplementary information June 2017/Answers to questions.
RBC (red blood cell count), Hct (haematocrit), Hb (haemoglobin), MCH (mean corpuscular haemoglobin), MCV (mean corpuscular volume), MCC (mean corpuscular haemoglobin concentration), THBC (thrombocyte – Avian platelets), WBC (white blood cell count: Het (heterophils), Eos (eosinophils), Bas (basophils), Mon (monocytes) and Lym (lymphocytes)).
AST (aspartate aminotransferase), ALT (alanine aminotransferase), ALP (alkaline phosphatase), LDH (lactate dehydrogenase), CK (creatine kinase), total protein, total bilirubin, BIAC (bile acids), total cholesterol, glucose, calcium, inorganic phosphorus, magnesium, sodium, potassium, chloride, uric acid, creatinine.
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 15, 20.1.2010, p. 1.
Technical dossier/Section III/Reference III.9.
Technical dossier/Section III/Reference III.10.
Technical dossier/Section III/Reference III.12.
Technical dossier/Section III/Reference III.4.
Technical dossier/Section III/Reference III.14.
Technical dossier/Section III/Reference III.13.
OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section III/Reference III.28.
Technical dossier/Section III/Reference III.27.
Technical dossier/Section III/Reference III.24.
The temperature correction was performed according to the scientific opinion of the Panel on Plant Protection Products and their Residues on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil (EFSA, 2007).
Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the protection of groundwater against pollution and deterioration. OJ L 372, 27.12.2006, p. 19.
Technical dossier/Supplementary information June 2017/Annex 7.
Technical dossier/Section III/Reference III.23.
Technical dossier/Section III/Reference III.25.
Technical dossier/Supplementary information June 2017/Annex 8.
Technical dossier/Section III/Reference III.19.
Technical dossier/Supplementary information June 2017/Annex 9.
Technical dossier/Section III/Reference III.21.
Technical dossier/Section III/Reference III.22.
Technical dossier/Section III/Reference III.18.
The FEEDAP Panel stated in its guidance for the preparation of dossiers for coccidiostats and histomonostats (EFSA FEEDAP Panel, 2011a) that studies with artificial infection would be preferred over field trials due to their inherent weaknesses. These short term studies should use field strains of Eimeria, recently confirmed as pathogenic/resistant by a sensitivity test or recognised problems in the poultry operation (confirmed by veterinary certificate). The Eimeria field strains should ideally undergo one, but in any case not more than two passage(s) before use in such trials.
Trial 1: Technical dossier/Section IV/Reference IV.5. Trial 2: Technical dossier/Section IV/Reference IV.6. Trial 3: Technical dossier/Section IV/Reference IV.7.
AST 1: Technical dossier/Section IV/Reference 8 and 9.
AST 1: Technical dossier/Supplementary information June 2017/Annex 10. AST 2: Technical dossier/Supplementary information June 2017/Annex 11. AST 3: Technical dossier/Supplementary information June 2017/Annex 12. and clarification received in November 2017.
Technical dossier/Section IV/Annex IV.10.
References
- Droge STJ and Goss KU, 2013. Development and evaluation of a new sorption model for organic cations in soil: contributions from organic matter and clay minerals. Environmental Science and Technology, 47, 14233–14241. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Scientific opinion of the Panel on Plant Protection Products and their Residues on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. EFSA Journal 2007;5(12):622, 32 pp. 10.2903/j.efsa.2007.622 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008b. Technical Guidance: microbial studies. EFSA Journal 2008;6(10):836, 3 pp. 10.2903/j.efsa.2008.836 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011a. Guidance for the preparation of dossiers for coccidiostats and histomonostats. EFSA Journal 2011;9(5):2174, 12 pp. 10.2903/j.efsa.2011.2174 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011b. Technical guidance: tolerance and efficacy studies in target animals. EFSA Journal 2011;9(5):2175, 15 pp. 10.2903/j.efsa.2011.2175 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for establishing the safety of additives for the consumer. EFSA Journal 2012;10(1):2537, 12 pp. 10.2903/j.efsa.2012.2537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EMA CVMP (European Medicines Agency Committee for Medicinal Products for Veterinary Use), 1999, Amprolium Summary Report (1). EMEA/MRL/721/99‐FINAL. December 1999.
- EMA CVMP (European Medicines Agency Committee for Medicinal Products for Veterinary Use), 2001. Amprolium Summary Report (2). EMEA/MRL/767/00‐FINAL. January 2001.
- Johnson J and Reid WM, 1970. Anticoccidial drugs: lesion scoring techniques in battery and floor pen experiments with chickens. Experimental Parasitology, 28, 30–36. [DOI] [PubMed] [Google Scholar]


