Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on vitamin B2 in the form of riboflavin 5′‐phosphate ester monosodium salt as an additive for all animal species for use in water for drinking. The additive under assessment is obtained from ■■■■■ a source of riboflavin produced by Ashbya gossypii. No information was provided on the identity and characterisation of the production strain, and on whether or not it is a genetically modified microorganism. Therefore, in the absence of adequate information, it is not possible to perform an assessment of the safety of the production strain. Riboflavin 5′‐phosphate ester monosodium salt ‘per se’ is considered safe for the target animals, consumers and the environment. In the absence of data regarding the identity and characterisation of the production strain, the FEEDAP Panel cannot conclude on the safety for the target species, consumers, users and the environment of the riboflavin 5′‐phosphate ester monosodium salt under assessment. Similarly, in the absence of data, no conclusion can be reached on the safety of the product for the users. Riboflavin 5′‐phosphate ester monosodium salt is regarded as effective in covering the animal's requirement when administered via water for drinking.
Keywords: nutritional additive, vitamins and provitamins, vitamin B2, riboflavin, riboflavin 5′‐phosphate ester monosodium salt
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 10(2) of that Regulation also specifies that for existing products within the meaning of Article 10(1), an application shall be submitted in accordance with Article 7, at the latest one year before the expiry date of the authorisation given pursuant to Directive 70/524/EEC for additives with a limited authorisation period, and within a maximum of 7 years after the entry into force of this Regulation for additives authorised without a time limit or pursuant to Directive 82/471/EEC.
The European Commission received a request from SINTOFARM2 for the re‐evaluation of vitamin B2 in the form of riboflavin 5′‐phosphate ester monosodium salt, when used as a feed additive for all animal species (category: nutritional additive; functional group: vitamins, provitamins and chemically well‐defined substances having similar effect).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 10(2) (re‐evaluation of an authorised feed additive). EFSA received directly from the applicant the technical dossier in support of this application. The particulars and documents in support of the application were considered valid by EFSA as of 11 February 2014.3
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of vitamin B2 in the form of riboflavin 5′‐phosphate ester monosodium salt, when used under the proposed conditions of use (see Section 3.1.5).
1.2. Additional information
Riboflavin is the generic name for the water‐soluble vitamin B2. Riboflavin is primarily found as an integral component of the coenzymes, flavin adenine dinucleotide and flavin mononucleotide. Flavocoenzymes participate in redox reactions of carbohydrates, fats and proteins from which living organisms derive most of their energy.
The EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) issued two opinions on the safety and efficacy of vitamin B2 (80%) as riboflavin produced by Bacillus subtilis KCCM‐10445 for all animal species (EFSA FEEDAP Panel, 2014, 2018a), an opinion on the safety and efficacy of vitamin B2 as riboflavin and riboflavin‐5′‐phosphate ester monosodium salt, produced by either Bacillus subtilis DSM 17339 or Bacillus subtilis DSM 23984 (EFSA FEEDAP Panel, 2016), and another opinion on the safety and efficacy of vitamin B2 (riboflavin) produced by Ashbya gossypii (EFSA FEEDAP Panel, 2018b).
The Scientific Committee on Food (SCF) expressed an opinion on riboflavin as a colouring matter authorised for use in foodstuffs produced by fermentation using genetically modified B. subtilis (European Commission, 1998) and another opinion on the tolerable upper intake level of vitamin B2 (European Commission, 2000). The EFSA Panel on Food Additives and Nutrient Sources added to food (ANS) issued a statement on the inability to assess the safety of riboflavin‐enriched yeast added for nutritional purposes as a source of riboflavin in food supplements and the bioavailability of riboflavin from this source, based on the supporting dossier (EFSA, 2009). The EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) issued several opinions on the substantiation of several health claims related to riboflavin (EFSA NDA Panel, 2010, 2012, 2013a,b). The ANS Panel issued an opinion on the re‐evaluation of riboflavin (E 101(i)) and riboflavin‐5′‐phosphate (E 101(ii)) as part of the food additives re‐evaluation programme specified under Regulation (EU) No 257/20104 (EFSA ANS Panel, 2013).
Riboflavin sodium phosphate is included in the current issue (9th edition) of the European Pharmacopeia (PhEur), Monograph (MG) 0786 (European Pharmacopeia (PhEur), 2016).
The additive vitamin B2 in the form of riboflavin and riboflavin‐5′‐phosphate ester monosodium salt has been authorised in the European Union (EU) for all animal species without a time limit (Commission list of the authorised additives in feedingstuffs published in application of Article 9t (b) of Council Directive 70/524/EEC)5 and is included in the European Union Register of Feed Additives6 pursuant to Regulation (EC) No 1831/2003 and foreseen for re‐evaluation.
Vitamin B2 is listed as a pharmacologically active substance in veterinary medicinal products. It is not subject to maximum residue levels when used in food‐producing animals.7 The use of riboflavin/lactoflavin as colourant is authorised in cosmetic products.8 Vitamin B2 is authorised for use in food9 and food supplements,10 for addition for specific nutritional purposes to foods for particular nutritional uses,11 and for addition to processed cereal‐based foods and baby foods for infants and young children12 and to infant formulas and follow‐on formulas.13 Riboflavin and riboflavin‐5′‐phosphate are also authorised as a colouring for use in foodstuffs under number E 101 (i) and E 101 (ii), respectively. Specifications for identification and purity are defined in Commission Regulation (EU) No 231/201214.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier15 in support of the authorisation request for the use of vitamin B2 in the form of riboflavin 5′‐phosphate ester monosodium salt as a feed additive.
The FEEDAP Panel has sought to use the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers and experts’ knowledge, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of riboflavin in animal feed. The Executive Summary of the EURL report can be found in Annex A.16
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of vitamin B2, in the form of riboflavin 5′‐phosphate ester monosodium salt is in line with the principles laid down in Regulation (EC) No 429/200817 and the relevant guidance documents: Guidance for the preparation of dossiers for the re‐evaluation of certain additives already authorised under Directive 70/524/EEC (EFSA, 2008a), Guidance on nutritional additives (EFSA FEEDAP Panel, 2012a), Technical guidance: Tolerance and efficacy studies in target animals (EFSA FEEDAP Panel, 2011), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012b), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012c), Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008b) and Technical Guidance: Microbial Studies (EFSA, 2008c).
3. Assessment
The additive under assessment is a mixture containing riboflavin 5′‐phosphate ester monosodium salt as the main component and other riboflavin monophosphates, diphosphates and free riboflavin. The additive ■■■■■ of a riboflavin source obtained by fermentation. It is intended to be used as a nutritional additive (functional group: vitamins, pro‐vitamins and chemically well‐defined substances having similar effect) in water for drinking in all animal species (except fish).
3.1. Characterisation
3.1.1. Characterisation of the production organism
According to the applicant, the riboflavin used in the production of the additive under assessment is obtained by fermentation with Ashbya gossypii. However, no information on the characterisation of the production organism was submitted by the applicant.
In the absence of this information, the FEEDAP Panel is unable to conclude on the identity and the characterisation of the production strain.
3.1.2. Manufacturing process
■■■■■18
3.1.3. Characterisation of the additive
Riboflavin 5′‐phosphate ester monosodium salt (IUPAC name: sodium[(2S,3R,4R)‐5‐(7,8‐dimethyl‐2,4‐dioxobenzo[g]pteridin‐10‐yl)‐2,3,4‐trihydroxypentyl] hydrogen phosphate; synonyms: vitamin B2, lactoflavin) is identified by the Chemical Abstracts Service (CAS) number 130‐40‐5 and the European Inventory of Existing Chemical Substances (EINECS) number 201‐988‐6. The molecular formula of riboflavin 5′‐phosphate ester monosodium salt is C17H20N4O9PNa and its molecular weight is 478.3. Solutions deteriorate on exposure to light, especially in the presence of alkali. The structural formula of riboflavin 5′‐phosphate monosodium salt is shown in Figure 1.
Figure 1.
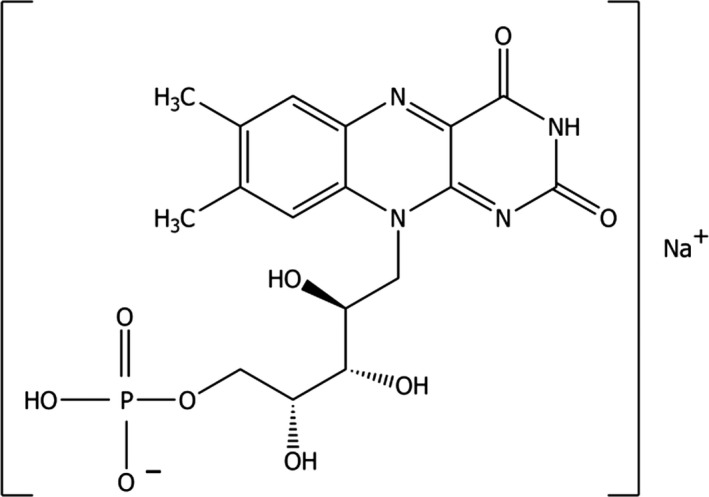
Structural formula of riboflavin 5′‐phosphate ester monosodium salt (the main component is shown)
The specification proposed by the applicant for the product under assessment are based on the description of riboflavin 5′‐phosphate sodium in the European Pharmacopoeia monograph 0786 (PhEur, 2010) and on the purity criteria for the food colour E 101 (ii) riboflavin‐5′‐phosphate set in Commission Regulation (EU) No 231/2012. By specification, the product under assessment contains not less than 95% of total colouring matters calculated as C17H20N4O9P.2H2O, 73–79% riboflavin determined by absorption spectrophotometry in the dried substance (on dry matter (DM)), < 6% riboflavin on DM (impurity D), < 6% total riboflavin diphosphates DM (impurities A, B and C corresponding to riboflavin 3′,4′‐diphosphate, 3′,5′‐diphosphate and 4′,5′‐diphosphate, respectively), inorganic phosphate < 1%. Specifications are also set for loss on drying < 7.5%, residue on ignition < 25%, pH between 5.0 and 6.5, specific optical rotation between +38° and +42° and impurities, i.e. primary aromatic amines < 70 mg/kg, arsenic < 3 mg/kg, lead < 2 mg/kg, mercury < 1 mg/kg, cadmium < 1 mg/kg, heavy metals (expressed as lead) < 10 mg/kg.
Analysis of eight production batches showed compliance with the proposed specifications, resulting in an average content (on DM basis) of 73.5% riboflavin (range 73.1–74.4%), free riboflavin (impurity D) 5.3% (4.7–5.8%), riboflavin diphosphates (sum of impurities A, B and C) 4.5% (3.8–6.0%), inorganic phosphate 0.8% (0.7–1.0%), loss on drying 5.5% (3.6–7.0%).19 In the same batches, lumiflavine20 was < 0.025% and ash was < 25% (as sulfated ash). The additive under assessment also complies with the description and the purity criteria set by PhEur and by Commission Regulation (EU) No 231/2012 the food colour E 101 (ii), as shown by the total colouring matters (97.3–97.5%) in five batches. In the same five batches, the specific optical rotation ranged from +38.5 to +38.7°, the pH between 5.7 and 6.2.21
Besides to the specifications of the PhEur, the applicant proposed additional specification for the relative proportion of riboflavin (5′‐, 4′‐ and 3′‐)monophosphates and other components contributing with riboflavin activity on ‘as is’ basis: > 57.8% riboflavin 5′‐phosphate sodium, < 14.4% riboflavin 4′‐phosphate sodium, < 5.8% riboflavin 3′‐phosphate sodium, < 5% of total riboflavin diphosphates (sum of impurities A, B and C), < 5.5% riboflavin.22 Analytical data, determined by high‐performance liquid chromatography (HPLC) with ultraviolet (UV) detection, were provided for three batches of the additive and showed levels (on ‘as is’ basis) of riboflavin 5′‐phosphate sodium ranging from 65.1% to 67.8%; riboflavin 4′‐phosphate sodium ranging from 11% to 13%, riboflavin 3′‐phosphate sodium ranging from 4.7% to 5.2%, total riboflavin diphosphates ranging from 3.7% to 4.2%, riboflavin from 4.0% to 4.3%.23 The corresponding certificates of analysis were not provided. Other components, included in the specifications proposed by the applicant are < 2% riboflavin dehydrated on ribose, ≤ 1% inorganic phosphate, < 1.0% sodium chloride and ≤ 7.5% moisture. The complete characterisation accounting for 100% of the composition was provided for one of the three batches: riboflavin 5′‐, 4′‐ and 3′‐phosphate 67.8%, 11.2%, and 4.7%, respectively, riboflavin diphosphates 3.7%, riboflavin 4%, riboflavin dehydrated on ribose 1.8%, inorganic phosphate 0.7%, sodium chloride 0.7% and moisture 5.4%.
3.1.3.1. Impurities
The applicant states that chemical and microbial contamination is regularly monitored as part of the Hazard Analysis and Critical Control Points (HACCP) programme.24 In five batches of the additive volatile organic solvents were absent, aromatic amines, heavy metals (mercury cadmium and lead) and arsenic levels were below the corresponding limits of detection (LOD).25 Salmonella spp., Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Listeria monocytogenes were absent in 25 g sample, total microbial aerobic counts ranged from < 10 to 120 colony forming units (CFU)/g. Total combined yeast and mould counts ranged from < 10 to 220 CFU/g sample. Coliforms and Enterobacteriaceae were < 10 CFU/g sample in five batches of the additive.26
No information was provided on the possible presence of viable cells of the production strain and on the presence of substances with antimicrobial activity.
3.1.3.2. Physico‐chemical characteristics
The additive under assessment is a yellow or orange‐yellow, practically odourless crystalline powder. It has a density of 800–900 kg/m3, a tapped density of 0.9–0.95 g/mL, a dissociation constant (pKa) of 8. The pH of a 1% solution is 5.0–6.5. It is soluble in water (37 g/L at 25°C).27
Three batches of the additive were analysed for particle size distribution by laser diffraction.28 The fraction of particles below 10 and 52 μm were in the ranges 7.0–7.2% and 69–70% by volume, respectively (v/v). In the same batches the dusting potential measured according to Stauber–Heubach ranged between 0.79 and 1.02 g/m3.29
3.1.4. Stability and homogeneity
Three batches of the additive riboflavin 5′‐phosphate sodium were stored in three‐layer bag thermo‐welded containers at 25°C and 60% relative humidity for 36 months or at 40°C and 75% relative humidity for 6 months.30 No losses were observed under both conditions.
The stability of riboflavin 5′‐phosphate sodium was tested when incorporated into a powder solid premixture and a liquid premixture (containing 10,000 mg choline chloride/kg) intended to be used in water for drinking (one batch each).31 Both premixtures contained 2,584 mg riboflavin 5′‐phosphate sodium/kg. The packaging was not described. In both cases, a loss of < 5% was observed after 6‐month storage at 25°C (60% relative humidity (RH)) or 1‐month storage at 45°C (70% RH).
At practical use levels, the additive (from both premixtures described above) was stable in water when added at 4.0 mg riboflavin/L and kept for 48 h at room temperature.32 Losses were < 8% in both cases.
In the presence of light, riboflavin is degraded in alkaline solution rapidly to lumiflavin and in neutral or acidic solutions to lumichrome (EFSA ANS Panel, 2013).
In order to demonstrate the ability of the additive to distribute homogeneously in solid or liquid premixtures, 10 subsamples of each premixture described above were analysed for riboflavin 5′ phosphate.33 The coefficient of variation (CV) was 2.1% for the solid premixture and 2.3% for the liquid premixture.
3.1.5. Physico‐chemical incompatibilities in feed
No physico‐chemical incompatibilities or interactions have been reported between riboflavin and feed materials, carriers, other approved additives or medicinal products when the additive was added to premixtures and water. No such incompatibilities or interactions are expected.
3.1.6. Conditions of use
Riboflavin 5′‐phosphate ester monosodium salt is intended for use in all animal species and categories without maximum limit and withdrawal period. The active substance is to be administered only via water for drinking. Because of the solubility properties of riboflavin 5′‐phosphate ester monosodium salt in water, the applicant recommends not to exceed the concentration of 3%.
3.2. Safety
3.2.1. Safety of the production strain
No information was provided on the identity and characterisation of the production strain, and on whether or not it is a genetically modified microorganism. Therefore, in the absence of adequate information it is not possible to perform an assessment of the safety of the production strain.
3.2.2. Metabolic and residue studies
Riboflavin 5′‐phosphate sodium is rapidly dephosphorylated to riboflavin in the intestinal mucosa (Christensen, 1969).34 Riboflavin absorption, its metabolic fate and its potential accumulation in edible tissues and eggs have been described in detail in previous opinions (EFSA FEEDAP Panel, 2014, 2016).
3.2.3. Toxicological studies
The EFSA ANS Panel assessed the safety of riboflavin and riboflavin 5′‐phosphate of different origin (EFSA ANS Panel, 2013) and concluded that riboflavin ‘per se’ has a low toxicity. This conclusion also applies to riboflavin 5′‐phosphate. In its previous opinions on vitamin B2, the FEEDAP Panel supported the conclusions of the ANS Panel concerning the vitamin ‘per se’ (EFSA FEEDAP Panel, 2014, 2016, 2018b).
No toxicological studies were submitted with the product under assessment.
3.2.4. Safety for the target species
The nutrient requirements and recommendations for the target species, their tolerance limits to riboflavin excess and the toxic effect of riboflavin depending on the administration route were discussed in previous opinions (EFSA FEEDAP Panel, 2014, 2016). According to the National Research Council (NRC), requirements for vitamin B2 are in the range of 1.7–4.0 mg/kg feed for poultry (NRC, 1994), 2–4 mg/kg for pigs (NRC, 2012), 2.7–25 mg/kg for fish (NRC, 2011) and 2.0–4.2 mg/kg for companion animals (NRC, 2006, 2007a). Similar ranges for vitamin B2 requirements have been proposed by the German Society of Nutrition Physiology (Gesellschaft für Ernährungsphysiologie, GfE): 2.8–4.5 mg/kg feed for poultry (GfE, 1999, 2004), 2.3–4.4 mg/kg for pigs (GfE, 2008) and 2.2 mg/kg for horses (GfE, 2014). Owing to microbial synthesis of riboflavin in the rumen, no dietary requirements have been established for ruminants (GfE, 1995, 2001, 2003; NRC, 1996, 2001, 2007b). For young calves, the requirement of vitamin B2 in milk replacer is 6.5 mg/kg dry matter (NRC, 2001). Requirements for laboratory animals are in the range 2–4 mg/kg diet for rats and 7 mg/kg diet for mice (NRC, 1995).
Vitamin B2 supplementation of commercial compound feed is mostly oriented towards recommendations, which are in the range of 3–8 mg/kg feed for pigs, 4–10 mg/kg for poultry, 10–30 mg/kg for fish and 3–10 mg/kg for pets (German economic association of manufacturers of feed additives – Arbeitsgemeinschaft für Wirkstoffe in der Tierernährung e.V. (AWT), 2002). A survey on vitamin supplementation of commercial feeds for pigs and poultry in Europe (Belgium, Denmark, Germany, Italy, the Netherlands, Portugal, Spain and the United Kingdom) identified a range of 0–17.5 mg riboflavin as commercial use levels (Gropp, 1994; Whittemore et al., 2002). Comparable data are not available for vitamin B2 supplementation in water for drinking.
Available data with rats suggest that dietary levels between 10 and 20 (possibly up to 100) times the requirement are tolerated (NRC, 1987). The FEEDAP Panel concluded that supplementation levels of riboflavin and riboflavin 5′‐phosphate sodium are safe for the target animals with a wide margin of safety of about 20–60 (EFSA FEEDAP Panel, 2014, 2016). Since no specific information on the commercial supplementation levels in water for drinking is available, the FEEDAP Panel considered that the intake of the additive via water would be two to three times higher than the intake via feed for poultry, pigs and rabbits (EFSA FEEDAP Panel, 2010).
The FEEDAP Panel concludes that the use of riboflavin 5′‐phosphate ester monosodium salt is safe for all animal species with a margin of safety of about 10–20 compared to the supplementation level in water for drinking. This conclusion applies to 5′‐phosphate ester monosodium salt ‘per se’.
The additive under assessment is obtained by fermentation and contains less than 1% of unidentified material. However, no information was provided on the identity and characterisation of the production strain, on whether or not it is a genetically modified microorganism, on the possible presence of viable cells of the production strain and on the presence of substances with antimicrobial activity.
Therefore, the FEEDAP Panel cannot conclude on the safety of the product under assessment for the target species.
3.2.4.1. Conclusions on safety for the target species
The use of vitamin B2 in the form of riboflavin 5′‐phosphate ester monosodium salt as nutritional additive in water for drinking is considered safe for the target animals with a wide margin of safety, when considering riboflavin 5′‐phosphate ester monosodium salt ‘per se’.
As the production strain was not characterised, the FEEDAP Panel cannot conclude on the safety of the product under assessment for the target species.
3.2.5. Safety for the consumer
The EFSA ANS Panel (2013) concluded that riboflavin and riboflavin‐5′‐phosphate sodium are unlikely to be of safety concern at the currently authorised uses and use levels as food additives.
In its previous opinions on vitamin B2, the EFSA FEEDAP Panel concluded that:
supplementation of animal feed with riboflavin has been a common practice for decades and it can be assumed that the exposure estimates made by ANS Panel from non‐supplemented foods already include the potential influence of riboflavin supplementation of feed at the practical use levels;
differences in use levels of feed supplementation do not significantly alter tissue/product deposition (EFSA FEEDAP Panel, 2014, 2016);
the supplementation of feed with riboflavin would not modify the current consumer exposure to riboflavin.
The FEEDAP Panel considers that the use of the vitamin B2 in the form of riboflavin 5′‐phosphate ester monosodium salt in water for drinking is not of safety concern for consumers when considering riboflavin 5′‐phosphate ester monosodium salt ‘per se’.
In the absence of information on the identity and characterisation of the production strain and on the manufacturing process, the FEEDAP Panel cannot conclude on the safety of the product under assessment for consumers.
3.2.6. Safety for the user
No data were provided on the effects of riboflavin 5′‐phosphate ester monosodium salt on the respiratory system. The dusting potential of the product (Section 3.1.3.2) indicates a risk of exposure by inhalation for people handling the additive.
No data were provided on the potential skin and eye irritation or skin sensitisation of the product under assessment.
Photoallergenic skin reaction has been shown with riboflavin on guinea pigs (Joshi and Pathak, 1987). In addition, riboflavin is a recognised photosensitiser inducing oxidative damage to light‐exposed tissues; therefore, it may elicit skin and eye photoallergic reactions (Cardoso et al., 2012).
In the absence of information on the identity and characterisation of the production strain, the FEEDAP Panel cannot conclude on the safety of the product under assessment for the user.
3.2.6.1. Conclusions on safety for the user
In absence of data, the FEEDAP Panel cannot conclude on a possible risk by inhalation, skin and eye irritation and skin sensitisation. Riboflavin is a known photosensitiser.
3.2.7. Safety for the environment
Riboflavin 5′‐phosphate sodium is dephosphorylated in the intestinal mucosa to riboflavin, a naturally occurring substance. Its use in animal nutrition is not expected to substantially increase the concentration in the environment. Therefore, a risk for the environment resulting from the use of riboflavin 5′‐phosphate ester monosodium salt ‘per se’ in animal nutrition is not foreseen.
Since no information was provided on the identity and characterisation of the production strain, and on whether or not it is a genetically modified microorganism, the FEEDAP Panel cannot conclude on the safety of the product under assessment for the environment.
3.3. Efficacy
Vitamin B2 has been used world‐wide in animal nutrition for decades. Dietary requirements are set for domestic animals except for ruminants, owing to microbial synthesis of riboflavin in the rumen (GfE, 1995, 2001, 2003; NRC, 1996, 2001, 2007b). Owing to the long history of use and its established nutritional role in domestic animals, riboflavin 5′‐phosphate ester monosodium salt is regarded as effective in covering the animal's requirement. Data on requirement, allowances and recommendations for feed supplementation are easily accessible in the standard literature on animal nutrition.
The FEEDAP Panel considers that riboflavin 5′‐phosphate ester monosodium salt is effective in covering the animal's requirement when administered via water for drinking.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation35 and Good Manufacturing Practice.
4. Conclusions
No information was provided on the identity and characterisation of the production strain, and on whether or not it is a genetically modified microorganism. Therefore, in the absence of adequate information, it is not possible to perform an assessment of the safety of the production strain.
Riboflavin 5′‐phosphate ester monosodium salt ‘per se’ is considered safe for the target animals, consumers and the environment. No conclusion can be reached on the safety of the product for the users. In the absence of data regarding the identity and characterisation of the production strain, the FEEDAP Panel cannot conclude on the safety for the target species, consumers, users and the environment of the riboflavin 5′‐phosphate ester monosodium salt under assessment.
Riboflavin 5′‐phosphate ester monosodium salt is regarded as effective in covering the animal's requirement when administered via water for drinking.
Documentation provided to EFSA
Riboflavin sodium phosphate for all animal species. November 2012. Submitted by Sintofarm SpA.
Riboflavin sodium phosphate for all animal species. Supplementary information. June 2014. Submitted by Sintofarm SpA.
Riboflavin sodium phosphate for all animal species. Supplementary information. June 2018. Submitted by Sintofarm SpA.
Evaluation report of the European Union Reference Laboratory for Feed Additives on the methods(s) of analysis for Vitamin B2.
Comments from Member States.
Chronology
| Date | Event |
|---|---|
| 20/12/2011 | Dossier received by EFSA |
| 12/11/2012 | Reception mandate from the European Commission |
| 11/2/2014 | Application validated by EFSA – Start of the scientific assessment |
| 26/3/2014 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation, safety for the user |
| 8/5/2014 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 12/5/2014 | Comments received from Member States |
| 5/6/2014 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 26/7/2016 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended Issues: Characterisation |
| 8/6/2018 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 27/11/2018 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- ANS
EFSA Panel on Food Additives and Nutrient Sources added to food
- AWT
Arbeitsgemeinschaft für Wirkstoffe in der Tierernährung e.V.
- CAS
Chemical Abstracts Service
- CFU
colony forming unit
- CV
coefficient of variation
- DM
dry matter
- EURL
European Union Reference Laboratory
- EINECS
European Inventory of Existing Chemical Substances
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- FL
fluorimetric detection
- GfE
Gesellschaft für Ernährungsphysiologie
- HACCP
Hazard Analysis and Critical Control Points
- HPLC
high‐performance liquid chromatography
- IUPAC
International Union of Pure and Applied Chemistry
- MG
monograph
- MIC
minimum inhibitory concentration
- NDA
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA
- NRC
National Research Council
- OECD
Organisation for Economic Co‐operation and Development
- pKa
dissociation constant
- PCR
polymerase chain reaction
- PhEur
European Pharmacopoeia
- RH
relative humidity
- SCF
Scientific Committee on Food
- UV
ultraviolet
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for Vitamin B2 (Riboflavin Sodium Phosphate)
1.
In the current application authorisation is sought under articles 10(2) for Vitamin B2 (Riboflavin sodium phosphate) under the category/functional group 3(a) ‘nutritional additives’/’vitamins, pro‐vitamins and chemically well‐defined substances having similar effect’ according to Annex I of Regulation (EC) No 1831/2003. Authorisation is sought for the use of the feed additive for all animal species and categories. The feed additive is a yellow orange, crystalline hygroscopic powder produced by chemical synthesis consisting mainly of the riboflavin sodium phosphate (73‐79% of riboflavin). The feed additive is intended to be used in water for drinking through liquid or soluble powder premixtures. While no maximum dosage in water is provided, the Applicant recommends not to exceed the 3% of Vitamin B2 in the final solution, due to its solubility.
For the characterisation of Riboflavin sodium phosphate in the feed additives, the Applicant proposed the European Pharmacopoeia method and the FAO JECFA monograph recommended by Commission Regulation EU No 231/2012, where identification is based on specific optical rotation and ultraviolet and visible absorption spectrophotometry, while quantification of the total colouring matter content of the Riboflavin sodium phosphate is based on spectrophotometry at 444 nm. Even though no performance characteristics are provided, the EURL recommends for official control the European Pharmacopoeia and the FAO JECFA methods to characterise the Riboflavin sodium phosphate salt.
For the quantification of Riboflavin sodium phosphate in the liquid and soluble powder form premixtures the Applicant proposed an in‐house developed High Performance Liquid Chromatography coupled to an UV detector (HPLC‐UV) method, derived from the method described in the European Pharmacopoeia. Based on the satisfactory performance characteristics provided, the EURL considers the HPLC‐UV method submitted by the Applicant suitable for official control to quantify riboflavin sodium phosphate in liquid and soluble powder form premixtures.
The Applicant did not present experimental data of analysis of the riboflavin 5′‐phosphate sodium in water, however, the EURL considers the method derived from the ring‐trial validated CEN method (EN 14152) suitable for the quantification of riboflavin sodium phosphate in water, based on acid hydrolysis followed by enzymatic dephosphorylation and using HPLC with fluorimetric detection (HPLC‐FL).
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Suggested citation: EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Costa L, Dierick N, Flachowsky G, Mantovani A, Wallace RJ, Manini P and Tarres‐Call J, 2018. Safety and efficacy of vitamin B2 (riboflavin 5′‐phosphate ester monosodium salt) for all animal species when used in water for drinking. EFSA Journal 2018;16(12):5531, 15 pp. 10.2903/j.efsa.2018.5531
Requestor: European Commission
Question number: EFSA‐Q‐2012‐00955
Panel members: Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Maryline Kouba, Mojca Kos Durjava, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Acknowledgements The EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) wishes to thank the following for the support provided to this scientific output: Montserrat Anguita, Gloria López‐Galvez, Lucilla Gregoretti, Jaume Galobart and Orsolya Holczknecht.
Adopted: 27 November 2018
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
SINTOFARM S.p.A., via Togliatti 5, 42016 Guastalla (RE), Italy. During the assessment, the company Sintofarm S.p.A. was taken over by Eigenmann and Veronelli.
A new mandate was received in EFSA on 12/11/2012.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 29.
2004/C 50/01. OJ C 50 25.2.2004, 144 pp. Council Directive of 23 November 1970 concerning additives in feedingstuffs 70/524/EEC. OJ L 270, 14.12.70.
European Union Register of Feed Additives pursuant to Regulation (EC) No 1831/2003. Available online: http://ec.europa.eu/food/food/animalnutrition/feedadditives/comm_register_feed_additives_1831-03.pdf
Commission Regulation (EU) 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 15, 20.1.2010, p. 1.
Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetics product (recast). OJ L 342, 22.12.2009, p. 59.
Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. OJ L 404, 30.12.2006, p. 26, last amended by Commission Regulation (EC) No 1170/2009 of 30 November 2009 amending Directive 2002/46/EC of the European Parliament and of Council and Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards the lists of vitamin and minerals and their forms that can be added to foods, including food supplements. OJ L 314, 1.12.2009, p. 36.
Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. OJ L 183 12.7.2002, p. 51.
Commission Regulation (EC) 953/2009 of 13 October 2009 on substances that may added for specific nutritional purposes in foods for particular nutritional uses. OJ L 269, 14.10.2009, p. 9.
Commission Directive 2006/125 EC of 5 December 2006 on processed cereal‐based foods and baby foods for infants and young children. OJ L 339, 6.12.2006, p. 16.
Commission Directive 2006/141 EC of 22 December 2006 on infant formulas and follow‐on formulas and amending Directive 1999/21/EC. OJ L 401, 30.12.2006, p. 1.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, 295 pp.
FEED dossier reference: FAD‐2011‐0051.
The full report is available on the EURL website. https://ec.europa.eu/jrc/sites/default/files/finrep2_fad-2011-0051_riboflavin%20sodium%20phosphate.pdf
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section 2.3.
Technical dossier/Section II/Annex II_1 and Annex II_2.
Lumiflavine (7,8,10‐trimethylbenzo[g] pteridine‐2,4(3H,10H)‐dione) is a toxic yellow photoderivative of riboflavin produced by ultraviolet irradiation in alkaline solution.
Technical dossier/Section II/Annex II.1.
Technical dossier/Section 2.1.3 page 7 and Section 2.2.2.1.16 page 22.
Technical dossier/Section 2.1.3, page 7; and Supplementary information June 2018/Answer to Q4. No certificates of analyses were provided.
Technical dossier/Section II Annex II_4.
Technical dossier/Section II/Annex II_6. Limits of detection (LOD): aromatic amines (aniline, o‐anisidine, o,m‐anisidine, diphenylamine, p‐toluidine and morpholine) 5 mg/kg (either individually or as their sum), arsenic and lead 1 mg/kg, mercury and cadmium 0.5 mg/kg.
Technical dossier/Section II/Annex II_5.
Technical dossier/Sections II.2.1.3, II.2.1.5, II 2.2.2.1 and Annex II.18.
Technical dossier/Supplementary information June 2014/Annexes 7 and 8.
Technical dossier/Supplementary information June 2014/Annex 9.
Technical dossier/Section II.2.4.1.
Technical dossier/Section II.2.4.1.3 and Supplementary information June 2014/Annex 5.
Technical dossier/Supplementary information June 2014/Annex 5.
Technical dossier/Section II.2.4.2.
Technical dossier/Section 3.1.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- AWT (Arbeitsgemeinschaft für Wirkstoffe in der Tierernährung e.V.), 2002. Vitamins in animal nutrition, Agrimedia GmbH, ISBN 3‐86037‐167‐3. [Google Scholar]
- Cardoso DR, Libardi SH and Skibsted LH, 2012. Riboflavin as a photosensitizer. Effects on human health and food quality. Food & Function, 3, 487–502. [DOI] [PubMed] [Google Scholar]
- Christensen S, 1969. Studies on Riboflavin metabolism in the rat. I. Urinary and faecal excretion after oral administration of Riboflavin‐5′‐phosphate. Acta Pharmacologica et Toxicologica, 27, 34–40. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for the preparation of dossiers for the re‐evaluation of certain additives already authorised under Directive 70/524/EEC. EFSA Journal 2008;6(9):779, 9 pp. 10.2903/j.efsa.2008.779 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008b. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008c. Technical Guidance: microbial studies. EFSA Journal 2008;6(10):836, 3pp. 10.2903/j.efsa.2008.836 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Opinion of the Scientific Panel on Food Additive and Nutrient Sources added to food on the inability to assess the safety of riboflavin‐enriched yeast added for nutritional purposes as a source of riboflavin in food supplements and the bioavailability of riboflavin from this source, based on the supporting dossier. EFSA Journal 2009;7(6):1135, 6 pp. 10.2903/j.efsa.2009.1135 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additive and Nutrient Sources added to food), 2013. Scientific Opinion on the re‐evaluation for riboflavin (E 101(i)) and Riboflavin‐5′‐phosphate (E 101(ii)). EFSA Journal 2013;11(10):3357, 49 pp. 10.2903/j.efsa.2013.3357 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2010. Statement on the use of feed additives authorised/applied for use in feed when supplied via water. EFSA Journal 2010;8(12):1956, 9 pp. 10.2903/j.efsa.2010.1956 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011. Technical guidance: Tolerance and efficacy studies in target animals. EFSA Journal 2011;9(5):2175, 15 pp. 10.2903/j.efsa.2011.2175 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for the preparation of dossiers for nutritional additives. EFSA Journal 2012;10(1):2535, 14 pp. 10.2903/j.efsa.2012.2535 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance for establishing the safety of additives for the consumer. EFSA Journal 2012;10(1):2537, 12 pp. 10.2903/j.efsa.2012.2537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014. Scientific Opinion on the safety and efficacy of vitamin B2 (80%) as riboflavin produced by Bacillus subtilis for all animal species, based on a dossier submitted by VITAC EEIG. EFSA Journal 2014;12(1):3531, 2 pp. 10.2903/j.efsa.2014.3531 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016. Scientific Opinion on Safety and efficacy of vitamin B2 (riboflavin and riboflavin 5′‐phosphate ester monosodium salt) produced by Bacillus subtilis for all animal species based on a dossier submitted by DSM. EFSA Journal 2016;14(1):4349, 26 pp. 10.2903/j.efsa.2016.4349 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Herman L, Glandorf B, Kärenlampi S, Aguilera J and Cocconcelli PS, 2018a. Scientific Opinion on the safety of vitamin B2 (80%) as riboflavin produced by Bacillus subtilis KCCM‐10445 for all animal species. EFSA Journal 2018;16(3):5223, 8 pp. 10.2903/j.efsa.2018.5223 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wester P, Costa L, Dierick N, Glandorf B, Herman L, Kärenlampi S, Leng L, Tebbe C, Aguilera J, Manini P, Tarres‐Call J and Wallace RJ, 2018b. Scientific Opinion on the safety and efficacy of vitamin B2 (riboflavin) produced by Ashbya gossypii for all animal species based on a dossier submitted by BASF SE. EFSA Journal 2018;16(7):5337, 19 pp. 10.2903/j.efsa.2018.5337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2010. Scientific Opinion on the substantiation of health claims related to riboflavin (vitamin B2) and contribution to normal energy‐yielding metabolism (ID 29, 35, 36, 42), contribution to normal metabolism of iron (ID 30, 37), maintenance of normal skin and mucous membranes (ID 31, 33), contribution to normal psychological functions (ID 32), maintenance of normal bone (ID 33), maintenance of normal teeth (ID 33), maintenance of normal hair (ID 33), maintenance of normal nails (ID 33), maintenance of normal vision (ID 39), maintenance of normal red blood cells (ID 40), reduction of tiredness and fatigue (ID 41), protection of DNA, proteins and lipids from oxidative damage (ID 207), and maintenance of the normal function of the nervous system (ID 213) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2010;8(10):1814, 28 pp. 10.2903/j.efsa.2010.1814 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2012. Scientific Opinion on the substantiation of a health claim related to a combination of thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, D‐biotin and pumpkin seed oil and maintenance of normal hair pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA Journal 2012;10(7):2807, 8 pp. 10.2903/j.efsa.2012.2807 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013a. Scientific Opinion on the substantiation of a health claim related to the combination of artichoke leaf dry extract standardised in caffeoylquinic acids, monacolin K in red yeast rice, sugar‐cane derived policosanols, OPC from French maritime pine bark, garlic dry extract standardised in allicin, d‐α‐tocopheryl hydrogen succinate, riboflavin and inositol hexanicotinate in Limicol® and reduction of blood LDL‐cholesterol concentrations pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA Journal 2013;11(7):3327, 16 pp. 10.2903/j.efsa.2013.3327 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013b. Scientific Opinion on the substantiation of a health claim related to riboflavin (vitamin B2) and contribution to normal energy‐yielding metabolism pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA Journal 2013;11(10):3410, 10 pp. 10.2903/j.efsa.2013.3410 [DOI] [Google Scholar]
- European Commission , 1998. Opinion on Riboflavin as a colouring matter authorized for use in foodstuffs produced by fermentation using genetically modified Bacillus subtilis. Available online: http://ec.europa.eu/food/fs/sc/scf/out18_en.html
- European Commission , 2000. Opinion of the Scientific Committee on Food on the Upper Tolerable Intake of Vitamin B2 . Available online: http://ec.europa.eu/food/fs/sc/scf/out80i_en.pdf
- GfE (Gesellschaft für Ernährungsphysiologie), 1995. Recommendations for the supply of energy and nutrients to beef cattle (in German), DLG‐Verlag, Frankfurt am Main, 85 pp.
- GfE (Gesellschaft für Ernährungsphysiologie), 1999. Recommendations for the supply of energy and nutrients to laying hens and chicken for fattening (broilers; in German). DLG‐Verlag, Frankfurt am Main, 185 pp.
- GfE (Gesellschaft für Ernährungsphysiologie), 2001. Recommendations for the supply of energy and nutrients to dairy cows and heifers (in German). DLG‐Verlag, Frankfurt am Main, 136 pp.
- GfE (Gesellschaft für Ernährungsphysiologie), 2003. Recommendations for the supply of energy and nutrients to goats. DLG‐Verlag, Frankfurt am Main, 121 pp.
- GfE (Gesellschaft für Ernährungsphysiologie), 2004. Recommendations for the supply of energy and nutrients to fattening turkeys (in German). Proceedings of the Society of Nutrition Physiology, 13, 199–233. [Google Scholar]
- GfE (Gesellschaft für Ernährungsphysiologie), 2008. Recommendations for the supply of energy and nutrients to pigs. DLG‐Verlag, Frankfurt am Main, 245 pp.
- GfE (Gesellschaft für Ernährungsphysiologie), 2014. Recommendations to the supply of energy and nutrients to horses (in German), DLG‐Verlag, Frankfurt am Main, 190 pp.
- Gropp JM, 1994. Vitamin fortification levels in European commercial poultry and swine diets. Proceedings of the Arkansas Nutrition Conference, Fayetteville, Arkansas, 105–134. [Google Scholar]
- Joshi PC and Pathak MA, 1987. Skin photosensitizing effects of riboflavin and its photodegradation products. Photochemistry and Photobiology, 45, 99S. [Only an abstract of this study was available]. [Google Scholar]
- NRC (National Research Council), 1987. Vitamin tolerance of animals. The National Academies Press, Washington, DC, USA, pp. 53–57. [Google Scholar]
- NRC (National Research Council), 1994. Nutrient requirements of poultry. 9th revised Edition. The National Academies Press, Washington DC, USA, 176 pp. [Google Scholar]
- NRC (National Research Council), 1995. Nutrient requirements of laboratory animals. 4th revised Edition. The National Academies Press, Washington DC, USA, 173 pp. [Google Scholar]
- NRC (National Research Council), 1996. Nutrient requirements of beef cattle. 7th revised Edition. The National Academies Press, Washington DC, USA, 232 pp. [Google Scholar]
- NRC (National Research Council), 2001. Nutrient requirements of dairy cattle. 7th revised Edition. The National Academies Press, Washington DC, USA, 381 pp. [Google Scholar]
- NRC (National Research Council), 2006. Nutrient requirements of dogs and cats. The National Academies Press, Washington DC, USA, 398 pp. [Google Scholar]
- NRC (National Research Council), 2007a. Nutrient requirements of horses. The National Academies Press, Washington DC, USA, 341 pp. [Google Scholar]
- NRC (National Research Council), 2007b. Nutrient requirements of small ruminants. Sheep, goats, cervids, and new world camelids. The National Academies Press, Washington DC, USA, 362 pp. [Google Scholar]
- NRC (National Research Council), 2011. Nutrient requirements of fish and shrimp. The National Academies Press, Washington DC, USA, 376 pp. [Google Scholar]
- NRC (National Research Council), 2012. Nutrient requirements of swine. 11th revised Edition. The National Academies Press, Washington DC, USA, 400 pp. [Google Scholar]
- PhEur (European Pharmacopeia), 2010. Riboflavin sodium phosphate, Monograph (MG) 0786. 7th Edition Strasbourg, France: Council of Europe (COE)—European Directorate for the Quality of Medicines. [Google Scholar]
- PhEur (European Pharmacopeia), 2016. Riboflavin sodium phosphate, Monograph (MG) 0786. 9th Edition. Strasbourg, France. Council of Europe (COE)—European Directorate for the Quality of Medicines.
- Whittemore CT, Close WH and Hazzledine MJ, 2002. The need for nutrient requirement standards for pigs. A report of the British Society of Animal Science nutritional standards working group: pigs. Pig News and Information, 23, 67N–74N. [Google Scholar]


