Abstract
According to Article 12 of Regulation (EC) No 396/2005, EFSA has reviewed the maximum residue levels (MRLs) currently established at European level for the pesticide active substance glyphosate. To assess the occurrence of glyphosate residues in plants, processed commodities, rotational crops and livestock, EFSA considered the conclusions derived under Commission Regulation (EU) No 1141/2010 as amended by Commission Implementing Regulation (EU) No 380/2013, the MRLs established by the Codex Alimentarius Commission as well as the import tolerances and European authorisations reported by Member States (including the supporting residues data). Based on the assessment of the available data, MRL proposals were derived and a consumer risk assessment was carried out. Although no apparent risk to consumers was identified, some information required by the regulatory framework was missing. Hence, the consumer risk assessment is considered indicative only and some MRL proposals derived by EFSA still require further consideration by risk managers.
Keywords: glyphosate, MRL review, Regulation (EC) No 396/2005, consumer risk assessment, herbicide, AMPA, N‐acetyl‐AMPA , N‐acetyl‐glyphosate
Short abstract
This publication is linked to the following EFSA Journal articles: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2018.5283/full, http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2019.5862/full
Summary
The active substance glyphosate was included in Annex I to Directive 91/414/EEC on 1 July 2002 by Commission Directive 2001/99/EC and has been deemed to be approved under Regulation (EC) No 1107/2009, in accordance with Commission Implementing Regulation (EU) No 540/2011, as amended by Commission Implementing Regulations (EU) No 541/2011, 2016/1056 and 2016/1313. As the active substance glyphosate was approved before the entry into force of Regulation (EC) No 396/2005 on 2 September 2008, the European Food Safety Authority (EFSA) is required to provide a reasoned opinion on the review of the existing maximum residue levels (MRLs) for that active substance in compliance with Article 12(2) of the aforementioned regulation.
As the basis for the MRL review, on 5 October 2016, EFSA initiated the collection of data for this active substance. In a first step, Member States (MSs) were invited to submit their national Good Agricultural Practices (GAPs) that are authorised in different MSs by 4 November 2016, in a standardised way, in the format of specific GAP forms allowing the rapporteur Member State (RMS), Germany, to identify the critical GAPs, in the format of specific GAP overview files. Subsequently, MSs were requested to provide residue data supporting the critical GAPs, within a period of 1 month, by 7 April 2017. On the basis of all the data submitted by MSs, EFSA asked Germany, the designated RMS, to complete the Pesticide Residues Overview File (PROFile) and to prepare a supporting evaluation report. The PROFile and evaluation report, together with Pesticide Residues Intake Model (PRIMo) calculations and updated GAP overview files were provided by the RMS to EFSA on 13 June 2017. Following a completeness check undertaken by EFSA, a request for further clarifications was forwarded to the RMS on 14 July 2017. After having considered all the information provided, EFSA finalised the completeness check report which was made available to MSs on 9 October 2017.
Based on the information provided by the RMS and MSs and taking into account the conclusions derived by EFSA in the framework of Commission Regulation (EU) No 1141/2010 as amended by Commission Implementing Regulation (EU) No 380/2013, and the MRLs established by the Codex Alimentarius Commission, EFSA prepared, in September 2017, a draft‐reasoned opinion, which was circulated to MSs for consultation via a written procedure. Comments received by 6 November 2017 were considered during the finalisation of this reasoned opinion. In addition, during the finalisation of the assessment, additional information available to the RMS but not submitted to EFSA was identified. The European Commission asked EFSA to request that information and consider it in the final assessment. Therefore, further amendments have become necessary at the final stage. More specifically, EFSA evaluated in this reasoned opinion the import tolerances on glycine N‐phenylacetyltransferase (GAT)‐modified rapeseeds, soybeans and maize, currently not present on the European Union (EU) market but assessed in previous EFSA reasoned opinions. In parallel, in the framework of the evaluation of the impact of glyphosate and its residues in feed on animal health, the toxicological profile of the metabolites N‐acetyl‐AMPA and N‐acetyl‐glyphosate was further considered during the Pesticides Peer Review Experts’ Teleconference 175 (27 February 2018) on the basis of the studies made available to EFSA in January 2018. Furthermore, following late changes reported by certain MSs in the authorised uses on grass, EFSA considered the need to launch a second round of MS consultation for confirmation of these uses, in particular as grass proved to be the main driver for the livestock exposure assessment. The consultation was conducted via a written procedure in February 2018, resulting in changes in the critical uses on grass. Subsequently, livestock dietary burden calculations and exposure assessment were reconsidered accordingly.
It is highlighted that toxicological data were not assessed in the current review and that the present reasoned opinion does not address the toxicological profile of glyphosate and its metabolites. In line with the provisions of Regulation (EC) No 396/2005, this review of MRLs is intended to characterise and quantify the residues of glyphosate in food and feed of plant and animal origin (resulting from the uses of glyphosate currently authorised by MSs), estimate dietary exposure of consumers, compare this dietary exposure to the toxicological reference values derived by EFSA in 2015 (for glyphosate and AMPA) and in 2018 (for N‐acetyl‐glyphosate and N‐acetyl‐AMPA) and propose MRLs in case no concern for consumers is identified, also highlighting the uncertainties due to missing data.
The following conclusions are derived.
The metabolism of glyphosate in primary crops was assessed in conventional and glyphosate tolerant crops containing 5‐enolpyruvylshikimate‐3‐phosphate (EPSP) synthase (EPSPS) and glucose oxidase (GOX) modifications belonging to different crop groups as well as in genetically modified soybean, maize and oilseed rape containing the GAT modification. Additional metabolism studies performed on conventional and EPSPS‐modified soybeans, cotton and maize were submitted by the RMS in the framework of this review. The metabolism in rotational crops (leafy vegetables, root and tuber vegetables and cereals) was investigated following glyphosate application directly to the soil or simulating typical agricultural practices.
In September 2016, during the Standing Committee on Plants, Animals, Food and Feed (SCoPAFF) meeting, the following residue definitions for enforcement were agreed upon by MSs as the basis for the MRL review:
OPTION 1:
for all plant commodities, including plants with glyphosate tolerant genetically modified varieties currently available on the market: sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate;
OPTION 2:
for plants with glyphosate tolerant genetically modified varieties currently available on the market (sweet corn, cotton seeds, sugar beets, rapeseeds, maize and soybeans): sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate;
for all other plant commodities: glyphosate.
For risk assessment, a general residue definition covering both conventional and genetically modified crops was proposed as the sum of glyphosate, AMPA, N ‐acetyl‐glyphosate and N ‐acetyl‐AMPA, expressed as glyphosate.
Although EFSA based this assessment on both residue definitions as agreed by MSs (options 1 and 2), EFSA agrees with the RMS that glyphosate only can be considered a sufficient marker for enforcement in conventional crops. For this reason, in the whole assessment, the option 2 is defined as the ‘main’ residue definition, while the option 1 is reported as ‘optional’.
Sufficiently validated analytical methods are available for the enforcement of glyphosate (relevant for the main residue definition), with a limit of quantification (LOQ) of 0.05 mg/kg in high water, high oil, acidic and dry matrices. Fully validated analytical methods for the enforcement of glyphosate in complex matrices (relevant for the authorisations on conventional tea, coffee beans, carobs, hops, spices and herbal infusions) are missing and are still required. Furthermore, there are indications that AMPA and N‐acetyl‐glyphosate (relevant for the optional residue definition proposed for all plant commodities and for genetically modified crops) can be enforced with a LOQ of 0.05 mg/kg, each. Therefore, the sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate can be enforced at the combined LOQ of 0.2 mg/kg in all matrices. Nevertheless, confirmatory methods for N‐acetyl‐glyphosate (in high water and high fat content matrices and dry commodities) and for AMPA (in all matrices) are still required.
Regarding the residue in primary crops, the available data on conventional crops are considered sufficient to derive (tentative) MRL proposals as well as risk assessment values for all crops under assessment except for cultivated fungi, sunflower seeds, soybeans, mustard seeds, buckwheat, rice (grain and straw), maize straw, millet straw and sorghum stover for which the available data were insufficient to derive MRLs and risk assessment values. Tentative MRLs were also derived for wheat and barley straw, sugar beet tops, fodder beet roots and tops, grass forage, clover forage, alfalfa forage and turnips tops in view of the future need to set MRLs in feed items.
For genetically modified crops, data were sufficient to derive MRL for sweet corn (EPSPS modification) and cotton seed (EPSPS modification), noting that MRLs should be tentative pending on the submission of confirmatory methods for enforcement of AMPA and N‐acetyl‐glyphosate. For sugar beet roots, maize and soybeans (EPSPS modification), soybeans (GAT modification) and rapeseeds (GOX modification), the available data were insufficient to derive MRLs and risk assessment values.
When considering the optional residue definition, in the absence of confirmatory methods for enforcement of AMPA (in all matrices) and N‐acetyl‐glyphosate (in high water content, high fat content and dry matrices), only tentative MRLs could be derived.
Available residue trials also allowed to derive the following conversion factors from enforcement to risk assessment: 1 for all commodities where a no‐residue situation was demonstrated or was tentatively proposed, for crops with glyphosate tolerant genetically modified varieties currently available on the market (sweet corn, cotton seed, sugar beets, rapeseeds, maize and soybeans) and for all MRLs expressed according to the optional residue definition; 2 for dry pulses; 1.1 for linseed; 2.3 for millet and sorghum grain.
According to the results from the confined rotational crop studies performed up to 1.5N the maximum dose rate assessed in the present MRL review, residues of glyphosate or AMPA are not expected in rotational root and leafy crops following annual application of glyphosate, provided that the active substance is used according to the GAPs considered in this review. Residues of glyphosate and its metabolite AMPA above the LOQ of 0.05 mg/kg cannot be excluded in cereals grain (only AMPA), forage and chaff grown in rotation with crops treated with glyphosate. Although these residues can be considered negligible compared to the residues expected according to the most critical GAP for desiccation authorised on cereals, MSs are recommended to implement proper mitigation measures when granting authorisation of plant protection products containing glyphosate, in order to avoid residues to occur in rotated cereals. Moreover, as the available studies do not cover the plateau concentration calculated for AMPA, proper mitigation measures should also be implemented to avoid accumulation of AMPA in soil and possible uptake of AMPA in rotational crops. The plateau concentration calculated for AMPA should be in any case confirmed by an additional study performed in acidic soils (data gap identified in the peer review).
Glyphosate is authorised for use on several crops that might be fed to livestock. Livestock dietary burden calculations were, therefore, performed for different groups of livestock. Considering that livestock may be exposed to residues originating from conventional and genetically modified crops, the calculation of the livestock dietary burden was performed combining the residues originating from the uses authorised on conventional crops and on genetically modified crops. The dietary burden values calculated for all groups of livestock were found to exceed the trigger value of 0.1 mg/kg dry matter (DM), with the residues in conventional crops representing the main contributor to livestock exposure. Behaviour of residues was, therefore, assessed in all commodities of animal origin.
Several livestock metabolism studies on goat and hen using glyphosate and AMPA labelled on the phosphonomethyl‐moiety and conducted with glyphosate, glyphosate‐trimesium or with a 9:1 glyphosate:AMPA mixture were evaluated during the peer review. In addition, in order to address the animal metabolism of residues derived from genetically modified crops, metabolism studies on goat and hen using 14C‐N‐acetyl‐glyphosate were also evaluated during the peer review.
The following residue definitions for animal commodities were agreed upon by MSs at the SCoPAFF meeting in September 2016 as the basis for the MRL review: sum of glyphosate, AMPA and N ‐acetyl‐glyphosate expressed as glyphosate for monitoring, and sum of glyphosate, AMPA, N ‐acetyl‐glyphosate and N ‐acetyl‐AMPA expressed as glyphosate for risk assessment.
During the peer review, a high‐performance liquid chromatography with tandem mass spectrometry (HPLC‐MS/MS) analytical method and its independent laboratory validation (ILV) were assessed for the enforcement of glyphosate and N‐acetyl‐glyphosate at the combined LOQ of 0.05 mg/kg in meat, milk and egg, and 0.1 mg/kg in liver, kidney and fat. A confirmatory gas chromatography with mass spectrometry (GC‐MS) method is, however, only available for glyphosate in milk, eggs and meat. Therefore, a confirmatory method for glyphosate in fat, liver and kidney, as well as a confirmatory method for AMPA and N‐acetyl‐glyphosate in all matrices, are still missing.
Based on available feeding studies and the estimated residue intakes by livestock, MRLs above the LOQ were proposed for all animal commodities, except for cattle, swine and poultry fat, poultry liver, milk and eggs where no residues are expected and the MRLs can be set at the LOQ. Considering that the N‐acetyl compounds are not expected to be present in the animal tissues, a conversion factor from enforcement to risk assessment of 1 has been proposed for all animal commodities. Since confirmatory methods for glyphosate in fat, liver and kidney, and for AMPA and N‐acetyl‐glyphosate in all matrices are still missing, all derived MRLs should be considered tentative only.
Chronic and acute consumer exposure resulting from the authorised uses on conventional and genetically modified crops reported in the framework of this review was calculated using revision 2 of the EFSA PRIMo. For each commodity, risk assessment values obtained for conventional and genetically modified crops were compared and the most critical values were selected for the exposure calculations. Hence, for those commodities where a (tentative) MRL could be derived by EFSA in the framework of this review, input values were derived according to the internationally agreed methodologies. For those plant commodities where data were insufficient to derive (tentative) MRLs, the existing EU MRLs multiplied by the following conversion factors were used for an indicative calculation: for sunflower seeds, soyabeans and mustard seed, the conversion of 1.1 derived from residue trials performed on other oilseeds was considered; for buckwheat and rice grain, the conversion of 2.3 derived from residue trials performed on other cereals was considered. For cultivated fungi, the highest conversion factor of 2.3 derived from all available trials was considered.
The exposure values calculated were compared with the toxicological reference values for glyphosate and its metabolites, derived by EFSA under Commission Regulation (EU) No 1141/2010 as amended by Commission Implementing Regulation (EU) No 380/2013 and in the framework of the evaluation of the impact of glyphosate and its residues in feed on animal health. The highest chronic exposure was calculated for WHO cluster diet B, representing 9.1% of the acceptable daily intake (ADI) and the highest exposure was calculated for dry beans, representing 55.7% of the acute reference dose (ARfD).
Consequently, although major uncertainties remain due to the data gaps identified in the previous sections, the indicative exposure calculations did not indicate a risk to consumers.
Although the residue definition for risk assessment is the same for both options assessed in this review, the MRLs as derived, according to the optional definition and resulting for the summing up of the LOQs of the different compounds included, can be higher than the MRLs as derived according to the main residue definition. For this reason, an additional scenario, based on the optional residue definition, was performed. According to this second scenario, the highest chronic exposure was calculated for WHO cluster diet B, representing 9.9% of the ADI and the highest exposure was calculated for dry beans, representing 55.7% of the ARfD.
Apart from the MRLs evaluated in the framework of this review, internationally recommended codex maximum residue limits (CXLs) have also been established for glyphosate. Additional calculations of the consumer exposure, including these CXLs, were therefore carried out, considering two different scenarios: a first scenario based on the main residue definition and a second scenario based on the optional residue definition.
When considering the main residue definition (scenario 1), the highest chronic exposure was calculated for British toddlers, representing 18.7% of the ADI; the highest acute exposure was calculated for sugar beet roots, representing 91% of the ARfD.
When considering the optional residue definition (scenario 2), the highest chronic exposure was calculated for British toddlers, representing 19% of the ADI; the highest acute exposure was calculated for sugar beet roots, representing 91% of the ARfD.
Background
Regulation (EC) No 396/20051 (hereinafter referred to as ‘the Regulation’) establishes the rules governing the setting and the review of pesticide maximum residue levels (MRLs) at European level. Article 12(2) of that Regulation stipulates that the European Food Safety Authority (EFSA) shall provide by 1 September 2009 a reasoned opinion on the review of the existing MRLs for all active substances included in Annex I to Directive 91/414/EEC before 2 September 2008. As glyphosate was originally included in Annex I to Council Directive 91/414/EEC on 1 July 2002 by means of Commission Directive 2001/99/EC2 and has been deemed to be approved under Regulation (EC) No 1107/20093, in accordance with Commission Implementing Regulation (EU) No 540/20114, as amended by Commission Implementing Regulations (EU) No 541/20115, 2016/10566 and 2016/13137, EFSA initiated the review of all existing MRLs for that active substance. It is noted that the review of MRLs under Article 12 of the Regulation is linked to the first inclusion of the active substance into Annex I and irrespective of the decision on the potential renewal of the approval of the substance.
According to the legal provisions, EFSA shall base its reasoned opinion in particular on the relevant assessment report prepared under Directive 91/414/EEC. It should be noted, however, that, in the framework of Directive 91/414/EEC, only a few representative uses are evaluated, whereas MRLs set out in Regulation (EC) No 396/2005 should accommodate all uses authorised within the European Union (EU), and uses authorised in third countries that have a significant impact on international trade. The information included in the assessment report prepared under Directive 91/414/EEC is therefore insufficient for the assessment of all existing MRLs for a given active substance.
To gain an overview of the pesticide residues data that have been considered for the setting of the existing MRLs, EFSA developed the Pesticide Residues Overview File (PROFile). The PROFile is an inventory of all pesticide residues data relevant to the risk assessment and MRL setting for a given active substance. This includes data on:
the nature and magnitude of residues in primary crops;
the nature and magnitude of residues in processed commodities;
the nature and magnitude of residues in rotational crops;
the nature and magnitude of residues in livestock commodities;
the analytical methods for enforcement of the proposed MRLs.
As the basis for the MRL review, on 5 October 2016, EFSA initiated the collection of data for this active substance. In a first step, MSs were invited to submit their national Good Agricultural Practices (GAPs) that are authorised in MSs by 4 November 2016, in a standardised way, in the format of specific GAP forms. In the framework of this consultation, 20 MSs provided feedback on their national authorisations of glyphosate. Based on the GAP data submitted, the rapporteur Member State (RMS), Germany, was asked to identify the critical GAPs to be further considered in the assessment, within a timeframe of 6 weeks, in the format of specific GAP overview files. Subsequently, in a second step, MSs were requested to provide residue data supporting the critical GAPs, within a period of 1 month, by 7 April 2017. On the basis of the data submitted by MSs, Germany, the designated RMS was asked to complete the PROFile and to prepare a supporting evaluation report for glyphosate (Germany, 2017). The PROFile and the supporting evaluation report, together with the Pesticide Residues Intake Model (PRIMo) calculations and updated GAP overview files following consideration of the residue data provided by MSs, were submitted to EFSA on 13 June 2017. Following a completeness check undertaken by EFSA within a period of 1 month, a request for further clarifications was forwarded to the RMS via a written procedure on 14 July 2017. After having considered all the information provided by the RMS, EFSA finalised the completeness check report which was made available to all MSs on 9 October 2017.
Based on the information provided and taking into account the conclusions derived by EFSA in the framework of Commission Regulation (EU) No 1141/20108 as amended by Commission Implementing Regulation (EU) No 380/20139, and the MRLs established by the Codex Alimentarius Commission (codex maximum residue limit, CXLs), EFSA prepared, in September 2017, a draft reasoned opinion, which was submitted to MSs for commenting via a written procedure. All comments received by 6 November 2017 were evaluated by EFSA and were considered by EFSA during the finalisation of the reasoned opinion. In addition, during the finalisation of the assessment, additional information available to the RMS but not submitted to EFSA was identified. The European Commission asked EFSA to request that information and consider it in the final assessment. Therefore, further amendments have become necessary at the final stage. More specifically, EFSA evaluated in this reasoned opinion the import tolerances on glycine N‐phenylacetyltransferase (GAT)‐modified rapeseeds, soybeans and maize, currently not present on the EU market but assessed in previous EFSA reasoned opinions (Germany, 2009, 2013a; EFSA, 2009, 2013). In parallel, in the framework of the evaluation of the impact of glyphosate and its residues in feed on animal health, the toxicological profile of the metabolites N‐acetyl‐AMPA and N‐acetyl‐glyphosate was further considered during the Pesticides Peer Review Experts’ Teleconference 175 (27 February 2018) on the basis of the studies made available to EFSA in January 2018 (EFSA, 2018b). Furthermore, following late changes reported by certain Member States (MSs) in the authorised uses on grass, EFSA considered the need to launch a second round of MS consultation for confirmation of these uses, in particular as grass proved to be the main driver for the livestock exposure assessment. The consultation was conducted via a written procedure in February 2018, resulting in changes in the critical uses on grass. Subsequently, livestock dietary burden calculations and exposure assessment were reconsidered accordingly.
The evaluation report submitted by the RMS (Germany, 2017) based on the information provided by MSs during the collection of data is considered as a main supporting document to this reasoned opinion and, thus, made publicly available.
In addition, key supporting documents to this reasoned opinion are the completeness check report (EFSA, 2017) and the Member States consultation report (EFSA, 2018a). These reports are developed to address all issues raised in the course of the review, from the initial completeness check to the reasoned opinion. Also, the chronic and acute exposure calculations for all crops reported in the framework of this review performed using the EFSA PRIMo (excel file) and the PROFiles as well as the GAP overview files listing all authorised uses are key supporting documents and made publicly available as background documents to this reasoned opinion. Furthermore, a screenshot of the Report sheet of the PRIMo is presented in Appendix C.
Terms of Reference
According to Article 12 of Regulation (EC) No 396/2005, EFSA shall provide a reasoned opinion on:
the inclusion of the active substance in Annex IV to the Regulation, when appropriate;
the necessity of setting new MRLs for the active substance or deleting/modifying existing MRLs set out in Annex II or III of the Regulation;
the inclusion of the recommended MRLs in Annex II or III to the Regulation;
the setting of specific processing factors as referred to in Article 20(2) of the Regulation.
The active substance and its use pattern
Glyphosate is the ISO common name for N‐(phosphonomethyl)glycine (IUPAC).
Glyphosate can be used as an ester or a salt.
It should be mentioned that the salts glyphosate‐isopropylammonium, glyphosate‐potassium, glyphosate‐monoammonium, glyphosate‐dimethylammonium are the modified ISO common names for iso‐propylammonium N‐(phosphonomethyl)glycinate, potassium N‐[(hydroxyphosphinato)methyl]glycine, ammonium N‐[(hydroxyphosphinato)methyl]glycine and dimethylammonium N‐(phosphonomethyl)glycinate (IUPAC), respectively. Glyphosate‐trimesium is trimethylsulfonium N‐[(hydroxyphosphinato)methyl]glycine (IUPAC). These salts are derivatives of the active substance glyphosate.
Glyphosate is a herbicide which is active against all plants by the inhibition of the shikimate cycle required for the formation of essential amino acids. In principle, it is systemic in plants. However, due to its high potency as a herbicide, the translocation within crops is very limited before withering. Uptake of glyphosate solely occurs via treated leaves.
The chemical structure of the active substance and its main metabolites are reported in Appendix F.
Glyphosate (including glyphosate‐trimesium) was evaluated in the framework of Commission Regulation (EU) No 1141/2010 as amended by Commission Implementing Regulation (EU) No 380/2013, with Germany designated as RMS. The representative uses considered were spraying applications against emerged annual, perennial and biennial weeds in all crops and foliar spraying for desiccation in cereals and oilseeds (preharvest). Following the original peer review, conducted by the European Commission prior to establishment of EFSA, a decision on inclusion of the active substance in Annex I to Directive 91/414/EEC was published by means of Commission Directive 2001/99/EC, which entered into force on 1 July 2002, and has been deemed to be approved under Regulation (EC) No 1107/2009, in accordance with Commission Implementing Regulation (EU) No 540/2011, as amended by Commission Implementing Regulations (EU) No 541/2011. The conditions of the approval were further amended by Regulations (EU) No 2016/1056 and 2016/1313. The original approval is restricted to uses as herbicide only.
The EU MRLs for glyphosate and for trimethyl‐sulfonium (TMS) cation, resulting from the use of glyphosate (including glyphosate‐trimesium) are established in Annexes II and IIIB of Regulation (EC) No 396/2005, as amended by Commission Regulation (EC) No 149/200810 and Commission Regulation (EC) No 839/200811. Codex maximum residue limits (CXLs) for glyphosate were also established by the Codex Alimentarius Commission (CAC). An overview of the MRL changes that occurred since the entry into force of the Regulation mentioned above is provided below (Table 1).
Table 1.
Overview of the MRL changes since the entry into force of Regulation (EC) No 396/2005
| Procedure | Legal implementation | Remarks |
|---|---|---|
| MRL application (EFSA, 2012) | Commission Regulation (EU) No 441/2012a | Modification of the existing MRL for glyphosate in lentils |
| Implementation of CAC 2012 | Commission Regulation (EU) No 293/2013b | Modification of the MRL for glyphosate in sweet corn and sugar beet roots |
Commission Regulation (EU) No 441/2012 of 24 May 2012 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for bifenazate, bifenthrin, boscalid, cadusafos, chlorantraniliprole, chlorothalonil, clothianidin, cyproconazole, deltamethrin, dicamba, difenoconazole, dinocap, etoxazole, fenpyroximate, flubendiamide, fludioxonil, glyphosate, metalaxyl‐M, meptyldinocap, novaluron, thiamethoxam, and triazophos in or on certain products, OJ L 135, 25.5.2012, p. 4–56.
Commission Regulation (EU) No 293/2013 of 20 March 2013 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for emamectin benzoate, etofenprox, etoxazole, flutriafol, glyphosate, phosmet, pyraclostrobin, spinosad and spirotetramat in or on certain products, OJ L 96, 5.4.2013, p. 1–30.
For the purpose of this MRL review, all the uses of glyphosate (as ester and salts) and/or glyphosate‐trimesium on conventional and genetically modified crops (5‐enolpyruvylshikimate‐3‐phosphate (EPSP) synthase (EPSPS),12 glucose oxidase (GOX)13 and GAT14) currently authorised within the EU and in third countries have been collected by the MSs and the RMS and reported in the GAP overview files. The critical GAP identified in the overview files were then summarised in the PROFiles and considered in the assessment. The details of the authorised critical uses (GAPs) for glyphosate are given in Appendix A. Moreover, information available in the EU Register of authorised Genetically Modified Organisms (GMOs)15 was also considered by EFSA.
According to the information received, glyphosate is authorised in conventional crops either on soil or by foliar spray application (Appendix A.1).
Although the cultivation of genetically modified crops is currently not authorised within the EU, glyphosate can be used in genetically modified glyphosate‐tolerant organisms in third countries. In particular, import tolerance GAPs were received for EPSPS modified sweet corn, cotton seeds and sugar beets (Appendix A.2) and for GOX‐modified rapeseeds (Appendix A.3). Furthermore, based on the EU Register of authorised GMOs, the import of genetically modified EPSPS maize and EPSPS soybean is authorised in Europe. Nevertheless, no import tolerances were reported by MSs during the GAP collection phase for these specific genetically modified crops. Regarding GAT‐modified crops, only an import tolerance for rapeseeds was received (Appendix A.4). However, according to the information available in the EU Register of authorised GMOs, GAT genetically modified rapeseed is currently not authorised for placing on the market in the EU. Therefore, although this GAP has been reported for completeness, it has not been considered further in the assessment. It is also noted that, although according to the EU Register of authorised GMOs, the import of genetically modified GAT soybeans is authorised in Europe and import tolerances on soybeans, rapeseeds and maize containing this modification were assessed by EFSA in previous reasoned opinions (EFSA, 2009, 2013), MRLs as derived in these assessments were never legally implemented. Hence, also considering the GAPs notified by MSs, it is concluded that GAT‐modified crops are currently not present on the EU market.
No EU GAPs or import tolerances were reported by MSs for glyphosate‐trimesium.
Assessment
EFSA has based its assessment on the PROFile submitted by the RMS, the evaluation report accompanying the PROFile (Germany, 2017), the renewal assessment report (RAR) and its addenda prepared under Commission Regulation (EU) No 1141/2010 as amended by Commission Implementing Regulation (EU) No 380/2013 (Germany, 2013b, 2015), the conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate (EFSA, 2015), the previous reasoned opinion on borage seeds (EFSA, 2016; United Kingdom, 2015) as well as the Joint Meeting on Pesticide residues (JMPR) Evaluation reports (FAO, 2005, 2011, 2013). The assessment is performed in accordance with the legal provisions of the uniform principles for evaluation and authorisation of plant protection products as set out in Commission Regulation (EU) No 546/201116 and the currently applicable guidance documents relevant for the consumer risk assessment of pesticide residues (European Commission, 1997a, b, c, d, e, f, g, 2000, 2010a,b, 2016; OECD, 2011, 2013).
More detailed information on the available data and on the conclusions derived by EFSA can be retrieved from the list of end points reported in Appendix B.
In order to support risk managers in the decision‐making process, EFSA also evaluated the import tolerances on GAT‐modified rapeseeds, soybeans and maize currently not present on the EU market but assessed in previous EFSA reasoned opinions (Germany, 2009, 2013a; EFSA, 2009, 2013). These uses the derived MRLs and the outcome of the risk assessment are reported in Appendix G.
It is highlighted that toxicological data were not assessed in the current review and that the present reasoned opinion does not address the toxicological profile of glyphosate and its metabolites. In line with the provisions of Regulation (EC) No 396/2005, this review of MRLs is intended to characterise and quantify the residues of glyphosate in food and feed of plant and animal origin (resulting from the uses of glyphosate currently authorised by MSs), estimate dietary exposure of consumers, compare this dietary exposure to the toxicological reference values derived by EFSA in 2015 (for glyphosate and AMPA) and in 2018 (for N‐acetyl‐glyphosate and N‐acetyl‐AMPA) (EFSA, 2018b) and propose MRLs, in case no concern for consumers is identified, also highlighting the uncertainties due to missing data.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of glyphosate in conventional and genetically modified crops (containing EPSPS, GOX and GAT modifications) was assessed during the peer review for the renewal of the approval (Germany, 2015). Additional metabolism studies performed on conventional crops (citrus fruits, soybeans and rice) and on EPSPS genetically modified soybeans, cotton and maize were submitted by the RMS in the framework of this review (Germany, 2017).
During the peer review, the metabolism was investigated in conventional plants belonging to the fruit, root, pulses/oilseeds, cereal and miscellaneous crop groups, using either soil, foliar, hydroponic or local direct (on stem, trunk or into fruit peduncle) application of 14C‐glyphosate and, in some experiments, with 14C‐AMPA. Following soil application, the uptake of glyphosate was very low and mostly amounted to less than 1% of the applied radioactivity (AR) in plant matrices. Limited translocation was also observed after local foliar application, most of the AR (80%) remaining in the treated parts of the plants, except for potatoes, where up to 12.4% of the AR was found in the tubers. Hydroponic studies were, therefore, the key studies to identify the metabolic pattern of glyphosate in conventional plants. Globally, without soil present as substrate, less than 5% of the AR was recovered in the aerial parts, while up to 20% of the AR was recovered in the roots. No significant degradation was observed and unchanged glyphosate was observed as the major component of the residues in most of the samples (ca. 50–80% total radioactive residue (TRR)) with low amounts of AMPA (4–10% TRR) and N‐methyl‐AMPA (0.3–5% TRR in root samples).
The same metabolic pattern was observed in studies representative of the use of glyphosate as desiccant and performed on wheat with foliar application at 6 kg/ha; actually in this study, glyphosate represented the main compound of the TRR (accounting for up to 91% TRR in grain and up to 83% TRR in straw, corresponding to 2.43 mg eq/kg and 103 mg eq/kg, respectively) and AMPA was identified as the only metabolite (accounting for up to 3.9% TRR corresponding to 12.8 mg eq/kg).
Results from the additional metabolism studies on rice (soil application before flooding and transplanting) and on soybean (direct foliar, soil and hydroponic application) received in the framework of this review confirmed the metabolic pattern observed in the previous studies with limited uptake of glyphosate from the roots to the aerial parts in both soybeans and rice plant and limited translocation from the treated leaves into other parts of the soya plant. Low concentrations of glyphosate (max. 3.5% TRR) and AMPA (max. 0.7% TRR) were found in rice plants, while no identification and quantification of the residues was performed in soybeans.
A similar metabolic pattern as observed with glyphosate was depicted when the studies were performed with glyphosate‐trimesium labelled on the PMG‐anion. Metabolism studies conducted with the TMS‐cation labelling demonstrated that the TMS‐cation is not metabolised in plants.
In genetically modified plants, the metabolic pattern of glyphosate is driven by the modifications introduced into the genome of the plant. In the metabolism studies conducted on GM soya bean, cotton and sugar beet containing the EPSPS modification and assessed during the peer review, parent glyphosate was detected as the major component of the residues, accounting for 24–95% TRR in forage, hay, tops and roots and for 12–25% TRR in seeds. AMPA was present in lower amounts (mostly 1–13% TRR) up to 49% TRR in soya bean seeds. Overall, the metabolic pattern was similar to that observed in conventional plants as the EPSPS modification does not affect the metabolism of glyphosate in genetically modified plants. The additional metabolism studies on EPSPS‐modified crops received in the framework of this review mainly confirm the metabolic pattern observed in the previous studies. Glyphosate was the main component of the TRR in soybean forage (99% TRR), soybeans hay (89% TRR), cotton seeds (70% TRR), maize forage (79% TRR), maize foliage (87% TRR) and maize grain (37% TRR) and AMPA was present at much lower amounts (from ‘not detected’ in soybeans forage to 7.1% TRR in soybeans hay). In soybeans seeds, glyphosate and AMPA were present at the same level representing 45% and 48% of the TRR, respectively. An additional study on soybeans was performed with glyphosate‐trimesium radiolabelled at the trimesium cation, without providing information on the fate of the glyphosate moiety, and was therefore not considered further in this review.
The metabolism resulting from the introduction of the GOX modification was investigated in rapeseed and maize in combination with the EPSPS modification. Following two foliar applications, glyphosate was observed in maize forage, silage and fodder (67–83% TRR), but almost not detected in seeds at harvest (7% TRR), where the main component of the residues was identified as AMPA, representing up to 8% TRR in rapeseeds and 60% TRR in maize seeds.
The impact of the GAT modification was investigated in three metabolism studies conducted on genetically modified rapeseed, soya bean and maize, following one pre‐emergence application and three post emergence treatments, up to 7 or 14 days before harvest. Parent glyphosate was detected in the soya bean and maize forage and foliage (9–75% TRR) and in rapeseeds (21%), but was almost absent in soya bean and maize seeds at harvest (0.1–3% TRR). In all plant matrices, the main component of the radioactive residues was identified as the N‐acetyl‐glyphosate, a metabolite formed by the action of the GAT enzyme, and accounting for 51–57% of the TRR in seeds and 18–93% TRR in the other plant parts. In addition, N‐acetyl‐AMPA was also identified as a major metabolite in rape and soya bean seeds, representing 15–24% TRR.
1.1.2. Nature of residues in rotational crops
Glyphosate is authorised for use on crops that can be grown in rotation, and therefore, the possible occurrence of residues in succeeding crops resulting from the use on primary crops has to be assessed. The soil degradation studies demonstrated that the degradation rate of glyphosate is moderate with a maximum field DT90 of 387 days, which exceeds the trigger value of 100 days. In addition, DT90 field value of the soil metabolite AMPA ranged between 958 and > 1,000 days (EFSA, 2015). Thus, further investigation on the nature and magnitude of the residues in rotational crops are required (European Commission, 1997c).
The metabolism of glyphosate was investigated in rotational crops (leafy vegetables, root and tuber vegetables and cereals) (Germany, 2015). In these studies, glyphosate was applied directly to the soil up to 6.5 kg/ha (corresponding to 1.5N the maximum application rate considered in this review) or simulating typical agricultural practices (treatment of primary crops and planting or sowing of the succeeding crops at different plant back intervals (PBIs) after harvest of the treated primary crop).
According to the results from the confined rotational crop studies, it can be concluded that the metabolism in rotational crops is similar to the metabolism in primary crops with higher relative amounts of AMPA expected due to its formation in soil. In fact, glyphosate and AMPA were the only compounds identified in the rotated crops accounting for up to 33% TRR (wheat chaff) and 29% TRR (wheat grain), respectively.
1.1.3. Nature of residues in processed commodities
Standard hydrolysis studies simulating the processing conditions representative of pasteurisation, baking, brewing, boiling and sterilisation were evaluated during the peer review for the renewal (Germany, 2015). Based on the results of these studies, it was possible to conclude that glyphosate and N‐acetyl‐glyphosate are hydrolytically stable under the standard conditions (EFSA, 2015). The effect of processing on the nature of AMPA was not investigated. However, considering the extremely simple structure of AMPA without structural elements capable of hydrolysis, AMPA is expected to be stable following processing and no additional studies are required.
1.1.4. Methods of analysis in plants
Analytical methods for the determination of glyphosate residues in plant commodities were assessed during the peer review for the renewal of approval which concluded that glyphosate and N‐acetyl‐glyphosate can be enforced at the limit of quantification (LOQ) of 0.05 mg/kg for each compound in high water and high oil content, acidic and dry commodities (EFSA, 2015). According to the RMS, the same method has also been sufficiently validated for AMPA in high water and high oil content, acidic and dry matrices, although a confirmatory method for this metabolite is not available (Germany, 2017). A confirmatory method for N‐acetyl‐glyphosate in high water and high fat content matrices and dry commodities was identified as a data gap during the peer review and no additional data were received in the framework of this review. A fully validated analytical method in complex matrices such as hops, spices, tea, coffee, carobs and herbal infusions is not available and it is still required.
According to the information provided by the European Union Reference laboratories (EURLs), the following LOQs can be achieved in the different matrices: 0.02 mg/kg (for glyphosate, AMPA and N‐acetyl‐AMPA) and 0.01 mg/kg (for N‐acetyl‐glyphosate) in high water, high acid content and dry commodities; 0.1 mg/kg (for glyphosate, AMPA and N‐acetyl‐AMPA) and 0.05 mg/kg (for N‐acetyl‐glyphosate) in high oil content commodities (EFSA, 2018a). Nevertheless, detailed information on the analytical methods currently in place for the routine analyses could not be included in this reasoned opinion since they were not reported in an evaluation report. According to the EURLs, analytical standards for glyphosate, AMPA, N‐acetyl‐glyphosate and N‐acetyl‐AMPA are commercially available (EFSA, 2018a).
Analytical methods for the enforcement of TMS‐cation in plant commodities were not assessed during the peer review for renewal and in the MRL review. Nevertheless, according to the information provided by the EURLs, during routine analyses, an LOQ of 0.01 mg/kg can be achieved for the enforcement of TMS‐cation in the four main matrices (EFSA, 2018a).
1.1.5. Stability of residues in plants
During the peer review, residues of glyphosate and AMPA were found to be stable at −18/20°C for at least 24 months in all matrices; except for high protein content commodities where the storage stability of AMPA was not investigated (Germany, 2015). Nevertheless, considering that the storage stability of AMPA has been demonstrated for at least 24 months in the main matrices including dry commodities, a storage stability study in high protein commodities is considered desirable only in the present assessment. Additional storage stability studies were reported in the framework of this review (Germany, 2017). According to the results from these additional studies, at storage temperature of −20°C, metabolite N‐acetyl‐glyphosate is stable for at least 1 year in high oil, high water and dry/starch matrices and N‐acetyl‐AMPA is stable for at least 1 year, 18 months and 23 months in high water, high oil and dry/starch matrices, respectively. Storage stability of N‐acetyl‐glyphosate and N‐acetyl‐AMPA in high protein content and acidic matrices has not been investigated.
1.1.6. Proposed residue definitions
In September 2016, during the Standing Committee on Plants, Animals, Food and Feed (SCoPAFF) meeting, the following residue definitions for enforcement were agreed upon by MSs as the basis for the MRL review:
OPTION 1:
for all plant commodities, including plants with glyphosate tolerant genetically modified varieties currently available on the market: sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate;
OPTION 2:
for plants with glyphosate tolerant genetically modified varieties currently available on the market (sweet corn, cotton seeds, sugar beets, rapeseeds, maize and soybeans): sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate;
for all other plant commodities: glyphosate.
For risk assessment, a general residue definition covering both conventional and genetically modified crops was proposed as the sum of glyphosate, AMPA, N ‐acetyl‐glyphosate and N ‐acetyl‐AMPA, expressed as glyphosate.
Although EFSA based this assessment on both residue definitions as agreed by MSs (options 1 and 2), EFSA agrees with the RMS that glyphosate only can be considered a sufficient marker for enforcement in conventional crops. For this reason, in the whole assessment, the option 2 is defined as the ‘main’ residue definition, while the option 1 is reported as ‘optional’.
Sufficiently validated analytical methods are available for the enforcement of glyphosate (relevant for the main residue definition), with a LOQ of 0.05 mg/kg in high water, high oil, acidic and dry matrices. Fully validated analytical methods for the enforcement of glyphosate in complex matrices (relevant for the authorisations on conventional tea, coffee beans, carobs, hops, spices and herbal infusions) are missing and are still required.
There are indications that AMPA and N‐acetyl‐glyphosate (relevant for the optional residue definition proposed for all plant commodities and for genetically modified crops) can be enforced with a LOQ of 0.05 mg/kg each. Therefore, the sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate can be enforced at the combined LOQ of 0.2 mg/kg in all matrices. The combined LOQ was calculated considering the sum of LOQs and molecular factors of 1.517 to convert AMPA to glyphosate and 0.818 to convert N‐acetyl‐AMPA to glyphosate (combined LOQ = 0.05 +1.5 × 0.05 + 0.8 × 0.05 = 0.165, rounded up to 0.2 mg/kg). Nevertheless, confirmatory methods for N‐acetyl‐glyphosate (in high water and high fat content matrices and dry commodities) and for AMPA (in all matrices) are still required.
Information on the availability of a fully validated analytical method for the enforcement of TMS‐cation in the plant commodities, against illegal uses, is not available to EFSA.
It is highlighted that, since the acetyl compounds are specific for GAT‐modified crops only and GAT‐modified crops are currently not present on the EU market (see also Section on the active substance and its use pattern), the inclusion of N‐acetyl‐glyphosate in the residue definition for enforcement may be reconsidered and a separate residue definition comprising the N‐acetyl‐glyphosate only could be defined. This would allow risk managers to set a lower LOQ for enforcement in all plant commodities and to identify any possible misuse of genetically modified GAT crops by the analysis of the N‐acetyl‐glyphosate.
The metabolism studies conducted with the TMS‐cation labelling demonstrated that the TMS‐cation is not metabolised and remains the relevant marker substance in plants. Analytical methods for the enforcement of TMS‐cation in plant commodities were not assessed during the peer review for renewal and in the MRL review. Nevertheless, according to the information provided by the EURLs, during routine analyses, an LOQ of 0.01 mg/kg can be achieved for the enforcement of TMS‐cation in the four main matrices (EFSA, 2018a).
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
To assess the magnitude of residues resulting from the reported GAPs, EFSA considered all residue trials reported by the RMS in its evaluation report (Germany, 2017), including residue trials evaluated in the framework of the peer review (Germany, 2015) and a previous EFSA reasoned opinion (United Kingdom, 2015). All residue trial samples considered in this framework were stored in compliance with the demonstrated storage conditions, except samples of olives that were stored for up to 32 months and samples of dry peas and beans and borage seeds from northern trials for which the storage conditions were not reported. Although an evaluation report including the summary of the trials on dry beans and peas is still required, considering that the storage stability in the main four matrices was demonstrated for at least 24 months, a significant decline of residues is not expected to have occurred in these samples. The number of residue trials and extrapolations was evaluated in accordance with the European guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs (European Commission, 2016).
Regarding the uses on conventional crops, according to the RMS, a no‐residue situation can be anticipated for all orchards (except olives, since the fruits can be picked from the ground) and for all soil applications done before sowing/planting or as interrow treatment or by wiping or as local treatment by rubbing and dabbing (envelope approach).
It is noted that the envelope approach has been fully supported by EFSA and the MSs in the framework of the peer review. However, EFSA is of the opinion that this approach is not applicable for most of the critical GAPs assessed in the MRL review mainly for the following reasons:
the application rates assessed during the peer review for the early treatments (BBCH 00‐09), were significantly lower (2.16 kg/ha) compared to the most critical uses currently authorised and considered in this review. Moreover, representative uses were supported by residue trials confirming a no‐residue situation while no residue trials, reflecting the most critical application rate authorised, are available.
excluding the uses for desiccation, applications close to the harvest were not assessed during the peer review while in most of the critical uses considered in this review, the active substance is applied close to the harvest, when fruits are already formed and may be exposed to glyphosate. When the edible part is growing close or into the soil, according to EFSA, its exposure to glyphosate should be considered possible also for wiping application, especially if there is little space between the rows. Excluding the trials on orchards, also for this type of application, residue trials reflecting the most critical GAPs are not available.
EFSA acknowledges that for all orchards, contamination of the fruits can be avoided by implementing proper risk mitigation measures (e.g. use of equipment with spray shields). A no residue condition is also confirmed by the available metabolism studies showing that there is no uptake from the soil to the fruits and by available residue trials on tree nuts, apricots, peaches, kiwi and bananas reflecting the most critical GAP assessed in this review. This approach is considered also applicable to the soil treatment of grapes and olives when, according to the authorised use, olives are picked only from the trees. Actually also for these uses, available residue trials performed according to the most critical GAP by using a proper equipment to avoid spray drift, confirm a no‐residue situation.
For applications done close to the harvest (preharvest interval (PHI) of 7–30 days) to all crops other than orchards, grapes and olives, even taking into account the implementation of proper risk mitigation measures to avoid the spray drift of the plant, no residue trials are available to confirm that no residues are taken up from the soil when the application is done close to the harvest. This can be particularly relevant for root crops whose edible parts are formed and are in direct contact with the soil when glyphosate is applied. In all these cases, although the metabolism in primary and rotational crops can give indication that a significant uptake from the soil is not expected to occur, EFSA is still of the opinion that at least two residue trials performed according to the most critical GAP and confirming a no‐residue situation should be submitted.
Similarly, also for soil application done at pre‐emergence or before sowing, planting and after harvest, EFSA is of the opinion that at least two residue trials confirming the no‐residue situation at the critical GAP considered in this review are still required. This approach is aligned to the current guidance document on MRL setting and extrapolation.
Therefore, considering the criteria presented above, EFSA was not in a position to derive MRL and risk assessment values for the following commodities and the corresponding data gaps were identified:
Cultivated fungi: available metabolism studies are not considered representative of the metabolism in fungi and possible uptake from soil cannot be excluded. Therefore, four trials compliant with the northern outdoor GAP and four trials compliant with the southern outdoor GAP are still required. Furthermore, analysis in cereals straw show high residue levels in these matrices and experience with other substances has shown that cultivated fungi (e.g. champignons) may be ‘contaminated’ when cultivated on cereals straw used as substrate. Therefore, in order to avoid cross contamination from straw in cultivated fungi, MSs are recommended to implement proper risk mitigation measures (e.g. do not use straw from cereals treated with glyphosate as substrate for the cultivation of fungi) or to reconsider the existing use on cereals;
Sunflower: only two trials are available to support the northern GAP for desiccation. Moreover, in these trials, residues were analysed for glyphosate only. According to the RMS, additional trials are available. However, since study reports for these trials were not reported to the RMS, they could not be evaluated by the RMS. Therefore, eight trials compliant with the northern outdoor GAP, eight trials compliant with the southern outdoor GAP and eight trials compliant with the import tolerance are required;
Soybeans: eight trials compliant with the northern outdoor GAP, eight trials compliant with the southern outdoor GAP and eight trials compliant with the import tolerance are required;
Mustard seeds: four trials compliant with the northern outdoor GAP and four trials compliant with the southern outdoor GAP are required;
Buckwheat: four trials compliant with the northern outdoor GAP, four trials compliant with the southern outdoor GAP and four trials compliant with the import tolerance are required;
Rice (grain and straw): eight trials compliant with the southern outdoor GAP are required;
Maize stover, millet straw: four trials compliant with the northern outdoor GAP and four trials compliant with the southern outdoor GAP are required;
Sorghum stover: four trials compliant with the northern outdoor GAP and four trials compliant with the southern outdoor GAP are required.
For all other commodities, data were sufficient to derive (tentative) MRL and risk assessment values, taking note of the following considerations:
Citrus fruits, tree nuts, pome fruits, stone fruits, figs, kumquats, kiwi fruits, kaki, litchis, passion fruits, avocados, mango, papayas, pomegranates, cherimoyas: based on residue trials on tree nuts, apricots, peaches, kiwi and bananas compliant with the southern outdoor GAPs, a no‐residue situation can be anticipated for these crops provided that a proper equipment is used to avoid spray drift. Therefore, MRL and risk assessment values can be derived at the LOQ and no additional trials are required;
Table and wine grapes: no residue trials compliant with the northern outdoor GAP for wine grapes are available. Moreover, the number of trials supporting the northern outdoor GAP for table grapes is not compliant with the data requirements for this crop. Nevertheless, considering that residues in the southern and northern outdoor trials available were below the LOQ, a no‐residue situation can be anticipated for this crop, provided that proper equipment is used to avoid spray drift. Therefore, MRL and risk assessment values can be derived at the LOQ and no additional trials are required;
Strawberries: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP, two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are still required;
Cane fruits: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are still required;
Other small fruits and berries: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are still required;
Table olives: although a no‐residue situation can be proposed based on the southern outdoor GAP (tree picked olives only), four trials compliant with the northern outdoor GAP are still required;
Bananas: although a no‐residue situation can be proposed based on the southern outdoor GAP, a drift contamination cannot be excluded according to the import tolerance GAP. Therefore, eight residue trials compliant with the import tolerance GAP are still required;
Potatoes: number of trials is not compliant with the data requirements for this crop. Moreover, results from two northern residue trials performed at longer PHI of 17–18 instead of 7 days and showing higher residues, suggest that longer PHIs may have an effect on the residues in tuber. Although tentative MRL and risk assessment values can be derived from the available data, one additional trial compliant with the northern outdoor GAP is required. Additionally, it should be clarified if the northern GAP identified by the RMS can be considered as the most critical use authorised.
Cassava roots, yams, arrowroots: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials compliant with the southern outdoor GAP are required;
Beetroots, celeriacs, horseradishes, salsifies, swedes and turnips (roots and tops): no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are required;
Sweet potatoes: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials compliant with the southern outdoor GAP are required;
Carrots: although MRL and risk assessment values can be derived from the southern outdoor GAP (no residues are expected in the crops following local treatments by dabbing and rubbing), at least two trials compliant with the northern outdoor GAP are required;
Jerusalem artichokes, parsnips, parsley roots, radishes: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are required;
Garlic, onions, shallots: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials on onions compliant with the northern outdoor GAP, two trials on onions compliant with the southern outdoor GAP and two trials on onions compliant with the indoor GAP are required;
Leeks and spring onions: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials on leek compliant with the northern outdoor GAP, two trials on leek compliant with the southern outdoor GAP and two trials on leek compliant with the indoor GAP are required;
Tomatoes, aubergines: although MRL and risk assessment values can be derived from the southern outdoor GAP (no residues are expected in the crops following local treatments by dabbing and rubbing), at least two trials on tomatoes compliant with the northern outdoor GAP and eight trials on tomatoes compliant with the indoor GAP are required;
Sweet peppers: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials on sweet peppers compliant with the northern outdoor GAP, two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are required;
Okras: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are required;
Cucurbits with edible peel: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials on cucumber/courgettes compliant with the northern outdoor GAP, two trials on cucumber/courgettes compliant with the southern outdoor GAP and two trials on cucumber/courgettes compliant with the indoor GAP are required;
Cucurbits with inedible peel: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials on melons compliant with the northern outdoor GAP, twi trials on melons compliant with the southern outdoor GAP and two trials on melons compliant with the indoor GAP are still required;
Sweet corn: although MRL and risk assessment values can be derived from the northern outdoor GAP, at least two trials compliant with the southern outdoor GAP are still required;
Broccoli, cauliflower: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials compliant with the northern outdoor GAP, two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are still required;
Brussels sprouts: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP, two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are still required;
Head cabbage: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP, two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are still required;
Leafy brassica: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials compliant with the northern outdoor GAP, two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are still required;
Kohlrabies: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP, two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are still required;
Lamb's lettuce: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP, two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are still required;
Lettuces, scaroles, cresses, land cresses, Roman rocket, Red mustards, baby leaf crops (including brassica species), purslane, chards, fresh herbs: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials on lettuce (open‐leaf) compliant with the northern outdoor GAP, two trials on lettuce (open‐leaf) compliant with the southern outdoor GAP and two trials on lettuce (open‐leaf) compliant with the indoor GAP are required;
Spinaches: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP, two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are required;
Grape leaves: no‐residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the southern outdoor GAP are required;
Watercress: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP, two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are still required;
Witloof: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are still required;
Beans and peas (with pods): although MRL and risk assessment values can be derived from the southern outdoor GAP (no residues are expected in the crops following local treatments by dabbing and rubbing), at least two trials on beans/peas (with pods) compliant with the northern outdoor GAP and two trials on beans/peas (with pods) compliant with the indoor GAP are required;
Beans and peas (without pods): although MRL and risk assessment values can be derived from the southern outdoor GAP (local treatments by dabbing and rubbing), at least two trials on beans/peas (without pods) compliant with the northern outdoor GAP and two trials on beans/peas (without pods) compliant with the indoor GAP are required;
Lentils (fresh): although MRL and risk assessment values can be derived from the southern outdoor GAP (no residues are expected in the crops following local treatments by dabbing and rubbing), at least two trials compliant with the northern outdoor GAP and two trials compliant with the indoor GAP are required;
Celeries, cardoons, Florence fennels, rhubarbs: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials on celeries compliant with the northern outdoor GAP, two trials on celeries compliant with the southern outdoor GAP and two trials on celeries compliant with the indoor GAP are required;
Asparagus: although a no‐residue situation can be tentatively proposed for this commodity, at least one additional trial compliant with the northern outdoor GAP, two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are required;
Globe artichokes: although MRL and risk assessment values can be derived from the southern outdoor GAP (no residues are expected in the crops following local treatments by dabbing and rubbing), at least two trials compliant with the northern outdoor GAP are still required;
Bamboo shoots: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP, two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are required;
Palm hearts: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP, two trials compliant with the southern outdoor GAP and two trials compliant with the indoor GAP are required;
Wild fungi: underdosed trials performed on wild fungi (simulating applications on forest and non‐cultivated areas but not compliant with the GAPs received in this review) were reported by the RMS in the evaluation report (Germany, 2017) and show that significant residues can be observed after such treatments. Nevertheless, EFSA is of the opinion that, provided that a proper risk mitigation measure is in place in order to avoid cross‐contamination of wild fungi, a no‐residue situation can be anticipated in this commodity. Therefore, the MRL and risk assessment values are proposed at the LOQ and no additional trials are required.
Beans (dry) and peas (dry): an evaluation report including the summary of the northern residue trials considered to derive the MRL is still required (Germany, 2017); in the meanwhile, MRL and risk assessment values are derived on a tentative basis only. Furthermore, eight trials compliant with the southern outdoor GAP and eight trials compliant with the import tolerance are still required;
Lentils (dry) and lupins (dry): an evaluation report including the summary of the northern residue trials considered to derive the MRL is still required (Germany, 2017); in the meanwhile, MRL and risk assessment values are derived on a tentative basis only;
Rapeseeds and linseeds: although MRL and risk assessment values could be derived from the northern data set, four additional trials on rapeseeds compliant with the southern outdoor GAP are still required;
Peanuts: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are required;
Poppy seeds: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are required;
Sesame seeds, pumpkins seeds, safflower seeds, gold of pleasure seeds, hemp seeds and castor beans: no residue trials are available. Although a no‐residue situation can be tentatively proposed for these commodities, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are required;
Borage seeds: no residue trials supporting the Southern Europe Union (SEU) outdoor GAP are available. Nevertheless, as the Northern Europe Union (NEU) GAP is clearly more critical, no additional trials supporting the SEU outdoor GAP are required;
Cotton seeds: only seven residue trials are available. Nevertheless, since the result of one additional trial is not expected to have significant impact on the derived MRL and risk assessment values, one additional trial compliant with the southern outdoor GAP is only desirable (minor deficiency);
Olives for oil production: residues of AMPA were analysed only in four southern residue trials available. However, as AMPA was never detected at levels above the LOQ, no additional trials are required to support the southern outdoor GAP. Nevertheless, four additional trials compliant with the northern outdoor GAP are still required;
Oil palm kernel: no residue trials are available. Nevertheless, residues are not expected in palm oil kernel after soil treatment on this crop (kernel is not directly exposed to possible spray drift and limited translocation has been observed in the metabolism studies). Therefore, a no‐residue situation can be anticipated for this crop and no additional trials are required.
Oil palm fruits: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodities, at least two trials compliant with the southern outdoor GAP are still required;
Kapok: no residue trials are available. Nevertheless, residues are not expected in fruits after soil treatment on this crop (morphology of kapok trees prevent from drift contamination). Therefore, a no‐residue situation can be anticipated for this crop and no additional trials are required.
Barley and oat (grains and straw): although MRL and risk assessment values can be derived from the northern outdoor GAP, four additional trials compliant with the southern outdoor GAP and eight trials compliant with the import tolerance GAP are still required;
Maize grain: all available trials supporting the import tolerance GAP on conventional maize were performed on EPSPS‐modified maize. Although EPSPS modification is not expected to alter the metabolic pathway of glyphosate in plants, the data were not used to derive an MRL since results were considered questionable (lower residue levels were observed in this data set compared to the trials compliant with the NEU GAP which is significantly less critical). Moreover, no residue trials compliant with the southern outdoor GAP are available and AMPA was analysed only in four of the eight trials compliant with the northern outdoor GAP. Although tentative MRL and risk assessment values can be derived from the northern dataset, four additional trials compliant with the northern GAP, analysing simultaneously AMPA and glyphosate, eight trials compliant with the southern outdoor GAP and eight trials compliant with the import tolerance are still required.
Millet grain: all available trials supporting the import tolerance GAP on conventional millet were performed on EPSPS‐modified maize. Although EPSPS modification is not expected to alter the metabolic pathway of glyphosate in plants, the data were not used to derive an MRL since results were considered questionable (lower residue levels were observed in this data set compared to the trials compliant with the NEU GAP which is significantly less critical). Moreover, no residue trials compliant with the southern outdoor GAP are available. Although MRL and risk assessment values can be derived from the northern data set, four trials compliant with the southern outdoor GAP and four trials compliant with the import tolerance are still required.
Sorghum grain: although MRL and risk assessment values can be derived from the northern data set, eight trials compliant with the southern outdoor GAP and eight trials compliant with the import tolerance are still required.
Wheat and rye (grain): although MRL and risk assessment values can be derived from the northern outdoor GAP, eight trials compliant with the import tolerance are still required;
Teas: no residue trials are available and the GAP for import tolerance is not clear (relevant GAP parameters are missing). Although a no‐residue situation can be tentatively proposed for this commodity based on the southern outdoor GAP, at least two trials compliant with the southern outdoor GAP and eight trials compliant with the import tolerance including a clarification on the authorised GAP (growth stage at last treatment or PHI) are still required;
Coffee beans: no residue trials are available. Nevertheless, since the application is done on soil before seedling, transplanting and after harvest, based on the metabolism study, a no‐residue situation can be anticipated for this crop and no additional residue trials are required;
Herbal infusions (from roots): no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are still required;
Herbal infusions (from flowers), herbal infusions (from leaves and herbs): no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are still required;
Root and rhizome spices: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are still required;
Seed and fruits spices: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are still required;
Bark spices, bud spices, flower pistil spices, aril spices: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the southern outdoor GAP are still required;
Carobs: no residue trials are available. Nevertheless, residues are not expected in fruits after soil treatment on this crop (morphology of carob trees prevent from drift contaminations). Therefore, a no‐residue situation can be anticipated for this crop and no additional trials are required;
Sugar beets (root and leaves): although MRL and risk assessment values can be derived from the northern outdoor GAP, eight residue trials compliant with the southern outdoor GAP are still required.
Sugar canes: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the southern outdoor GAP and eight trials compliant with the import tolerance are still required;
Chicory roots: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP and the southern outdoor GAP are still required;
Hops: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are still required;
Alfalfa forage: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are still required;
Clover forage: no residue trials are available. Although a no‐residue situation can be tentatively proposed for this commodity, at least two trials compliant with the northern outdoor GAP and two trials compliant with the southern outdoor GAP are still required;
Grass forage: although MRL and risk assessment values can be derived from the northern outdoor GAP, two residue trials compliant with the southern outdoor GAP are still required.
It is noted that for the northern uses on fresh legumes, for the southern uses on cassava roots, yams, arrowroots, Jerusalem artichokes, parsnips, parsley, radishes, spring onions, sweet peppers, okra, cucurbits with edible and inedible peel, sweet corn, Chinese cabbages, kales, leafy vegetables and fresh herbs (except lamb's lettuce, spinaches, grape leaves, watercress and witloof), stem vegetables (except globe artichokes) and for the indoor GAPs on bulb vegetables, tomatoes, peppers, aubergines, okra, cucurbits with edible and inedible peel, leafy vegetables and fresh herbs (except watercress) and stem vegetables, the reported PHI of 30 days seems to be inconsistent with the information available in the comment field of the GAP table (application done in‐between production period). Therefore, pending on the confirmation that the soil application is done preplanting, presowing and post‐harvest, EFSA considered the PHI as the most relevant parameter for assessing the GAP.
EFSA highlight that, for most of the crops under assessment, a no‐residue situation is strictly dependent on the risk mitigation measures that MSs will put in place to avoid spray drift. For this reason, MSs are strongly recommended to implement an adequate monitoring programme allowing to verify the appropriateness of the risk mitigation in place.
Regarding the uses on EPSPS genetically modified crops, all available residue trials performed analysing only for glyphosate and AMPA were considered acceptable since N‐acetyl‐glyphosate and N‐acetyl‐AMPA are not expected in EPSPS crops. For most of the crops, available residue trials are sufficient to derive MRL and risk assessment values, taking note of the following considerations:
Sweet corn: trials on sweet corn with three applications at 4, 0.86 and 1.7 kg/ha considered acceptable since the first two applications done at an early growth stage are not expected to have a significant impact on the final residue level.
Cotton seeds: trials on cotton seeds performed with higher dose rate at first application (3.3 instead of 1.7 kg/ha) considered acceptable since the first application done at an early growth stage is not expected to have a significant impact on the final residue level. Residues analysed only for glyphosate and AMPA are acceptable since N‐acetyl‐glyphosate and N‐acetyl‐AMPA are not expected in EPSPS crops.
No residue data were available for sugar beet roots. Therefore, the following data gap was identified:
Eight residue trials compliant with the import tolerance GAP for EPSPS‐modified sugar beets.
Moreover, according to the EU Register of authorised GMOs, the import of EPSPS maize and EPSPS soybeans is authorised in EU. Nevertheless, as no import tolerances on these GM crops were reported by MSs during the GAP collection phase, it was not possible to derive an MRL based on these uses and the following data gaps were identified:
Maize: GAP details and supporting residue trials for the currently authorised import tolerance on EPSPS maize;
Soybeans: GAP details and supporting residue trials for the currently authorised import tolerance on EPSPS soybeans.
Regarding the uses on GOX genetically modified crops, an import tolerance GAP on rapeseed was reported by the RMS. This GAP was not supported by residue trials and the following data gap was identified:
Eight residue trials compliant with the import tolerance GAP for GOX‐modified rapeseeds.
Regarding the uses on GAT genetically modified crops, an import tolerance GAP and the supporting residue trials on rapeseed were reported by the RMS. However, according to the information available in the EU Register of authorised GMOs, GAT genetically modified rapeseed is currently not authorised for placing on the market within the EU.19 Therefore, GAP and supporting residue trials were reported for completeness but not considered further in the assessment.
Considering that the residue definitions for enforcement and risk assessment are different (see Section 1.1.6), EFSA also derived conversion factor (CF) from enforcement to risk assessment. For all commodities other than sweet corn, cotton seed, sugar beets, rapeseeds, maize and soybeans, the proposed residue definition for enforcement is glyphosate only (main proposal) while the residue definition for risk assessment also includes AMPA, N‐acetyl‐glyphosate and N‐acetyl‐AMPA. As none of the MRL derived under this section refer to GAP authorised on GAT genetically modified crop, the metabolites N‐acetyl‐glyphosate and N‐acetyl‐AMPA are not expected to be present. Therefore, CFs for these crops were derived based on the residue data available for metabolite AMPA:
For all commodities where a no‐residue situation was demonstrated (based on residue trials) or tentatively assumed (based on waiver to be confirmed by data), neither glyphosate nor AMPA are expected to be present. Therefore, a CF of 1 could be (tentatively) proposed for these crops;
For all commodities where metabolite AMPA was analysed in the residue trials and demonstrated to remain below LOQ (e.g. wheat grain), a CF of 1 was derived;
Dry pulses (beans, peas, lentils, lupins): based on metabolism studies performed with applications as dessicatant, the potential presence of AMPA cannot be excluded in these crops. However, as the full summary of the residue trials performed on pulses was not available (see above), it was not possible to conclude on the individual levels of AMPA in these commodities. According to comments received during the Member States Consultation (EFSA, 2018a), metabolite AMPA was found in 2 of 10 trials available. However, considering the uncertainty on the storage stability of AMPA in high protein matrices and the data gap for a detailed evaluation of the residue trials performed on dry beans, a conservative CF from enforcement to risk assessment of 2 derived from the available data was tentatively proposed. This CF may be refined in the future when data gaps identified for these crops will be fulfilled;
Linseed: the available residue trials performed on rapeseed and compliant with GAP allow deriving a CF of 1.1 for this commodity. It is noted that residue levels of AMPA above the LOQ was quantified in one trial sample only;
Millet and sorghum grain: four GAP‐compliant trials analysing simultaneously for glyphosate and AMPA were available. These trials indicate AMPA to be present above the LOQ and allow deriving a CF of 2.3 for these commodities.
For sweet corn, cotton seed, sugar beets, rapeseeds, maize and soybeans (crops with glyphosate tolerant genetically modified varieties currently available on the market) and for all MRLs expressed according to the optional residue definition, the proposed residue definition for enforcement already includes glyphosate, AMPA and N‐acetyl‐glyphosate. As none of the MRL derived for these commodities refer to GAP authorised on GAT genetically modified crops, metabolite N‐acetyl‐AMPA is not expected to be present. Therefore, CF of 1 was considered appropriate.
1.2.2. Magnitude of residues in rotational crops
Considering the degradation rates of glyphosate and its main soil metabolite AMPA (see Section 1.1.2), the maximum application rate of 4.32 kg/ha per year assessed in this review, a soil density of 1.5 kg/L, soil depth of 15 cm and no crop interception, the plateau concentration in soil (taking into account accumulation over the years) has been calculated as 0.2140 mg/kg for glyphosate and as 1.0359 mg/kg for AMPA. However, it is noted that a data gap for information regarding the degradation/dissipation rate of AMPA in acidic soils (pH 5–6) has been identified during the peer review (EFSA, 2015). Therefore, the plateau calculation for AMPA may need to be reconsidered once the confirmatory data addressing this data gap will be made available.
In the confined rotational crop study by Hattermann (Germany, 2015) performed with a bare soil application at 6.5 kg/ha (representing 1.5N the maximum application rate assessed in this review), samples contained substantial total radioactivity residues equivalent to glyphosate concentrations of up to 4.8 mg eq/kg (radish leaf planted at 30 days PBI following bare soil application at 6.5 kg/ha and sampled 75 days after treatment (DAT)). However, in this sample most of the radioactivity remained unextracted due to the incorporation of 14CO2 from the degradation of glyphosate in soil. In the rotated leafy and root crops (radish leaf and roots and lettuce), absolute levels of glyphosate and AMPA were below the LOQ of 0.05 mg/kg at all PBIs and at all sampling times. In rotated cereals, residues of glyphosate were found at levels above the LOQ only in wheat forage (0.4 mg eq/kg at PBI of 120 days) and chaff (0.3 and 0.06 mg eq/kg at PBIs 120 and 365 days, respectively). Metabolite AMPA was present at absolute amounts of 0.2, 0.4 and 0.3 mg eq/kg in wheat forage, chaff and grain at PBI of 30 days; at absolute amounts of 0.1, 0.2 and 0.2 mg eq/kg in wheat forage, chaff and grain at 120 days PBI, while at the longest PBI of 365 days, AMPA decreased being below the LOQ of 0.05 mg/kg in all wheat parts.
Although in the study by Hattermann, only TRR expressed as mg eq/kg soil were reported, individual levels of glyphosate and AMPA were available in other confined rotational crop studies where a characterisation of the residue in the soil was performed (studies by Spiller and Bowler, 1993 by Nicholls, 1990 reported in Germany, 2015). In particular, in the study by Spillner and Bowler, following application of glyphosate at 3.87 kg/ha, glyphosate accounted for a maximum of 2.11 mg/kg soil (at 0 DAT, immediately after application) and AMPA for a maximum of 0.84 mg/kg soil (34 DAT). In general, in these studies, immediately after the application, glyphosate and AMPA in soil account for an average of 60% and 4% of the TRR, respectively. After soil aging, a degradation of glyphosate to AMPA is observed with glyphosate accounting for an average of 9% of the TRR in soil and AMPA for an average of 44% of the TRR. When considering this information, the maximum concentrations of glyphosate and AMPA in soil from the study by Hattermann could be estimated as 2.4 mg eq/kg soil for glyphosate (60% of the maximum TRR measured in 15 cm soil layer at day 0) and as 0.81 mg eq/kg soil for AMPA (44% of the maximum TRR measured in 15 cm soil layer at PBI of 120 days).
Hence, it can be concluded that the available rotational crop studies cover the plateau concentration in soil calculated for glyphosate and, therefore, the multiannual applications of glyphosate. However, residues estimated in the soil for AMPA are not covering the calculated plateau concentration. As a consequence, following multiannual applications, the accumulation of AMPA and possible uptake by crops grown in rotation cannot be excluded.
In conclusion, according to the results from the confined rotational crop studies performed up to 1.5N the maximum dose rate assessed in the present MRL review, residues of glyphosate or AMPA are not expected in rotational root and leafy crops following annual application of glyphosate, provided that the active substance is used according to the GAPs considered in this review. Residues of glyphosate and its metabolite AMPA above the LOQ of 0.05 mg/kg cannot be excluded in cereals grain (only AMPA), forage and chaff grown in rotation with crops treated with glyphosate. Although these residues can be considered negligible compared to the residues expected according to the most critical GAP for desiccation authorised on cereals, MSs are recommended to implement proper mitigation measures when granting authorisation of plant protection products containing glyphosate, in order to avoid residues to occur in rotated cereals. Moreover, as the available studies do not cover the plateau concentration calculated for AMPA, proper mitigation measures should also be implemented to avoid accumulation of AMPA in soil and possible uptake of AMPA in rotational crops. The plateau concentration calculated for AMPA should be in any case confirmed by an additional study performed in acidic soils (data gap identified in the peer review).
1.2.3. Magnitude of residues in processed commodities
Studies investigating the effect of processing on the magnitude of glyphosate residues in processed commodities from conventional crops were assessed in the conclusion on the peer review for the renewal of the approval (EFSA, 2015). Additional processing studies on conventional grass and GAT‐modified crops were provided in the framework of this review (Germany, 2017). Regarding the conventional crops, robust‐processing factors could be derived for citrus juice, peel, dry pomace and press liquor; crude and refined olive oil; linseed oil and press cake; crude and refined rapeseed oil and rapeseed press cake; crude and refined maize oil and maize meal; rye bran, flour, bread and middlings; wheat bran and flour and grass hay and silage. In all processing studies on conventional crops, residues were analysed for glyphosate and AMPA, allowing to derive CFs from enforcement to risk assessment. When residues of AMPA were below the LOQ, a CF of 1 was proposed for risk assessment.
No robust‐processing factors for enforcement and risk assessment could be derived for soya beans fat, hulls and crude oil; maize flour; wheat wholemeal flour and bread, middlings, semolina and semolina bran, as they were not sufficiently supported by studies; a minimum of three processing studies is normally required. The processing factors reported in Appendix B for these commodities should, therefore, be considered as indicative only.
Further processing studies are not required as they are not expected to affect the outcome of the risk assessment. However, if more robust‐processing factors were to be required by risk managers, in particular for enforcement purposes, additional processing studies would be needed.
1.2.4. Proposed MRLs
MRL and risk assessment values can be derived according to the two different residue definitions proposed in this review (main and optional).
The available data on conventional crops are considered sufficient to derive (tentative) MRL proposals as well as risk assessment values for all crops under assessment except for cultivated fungi, sunflower seeds, soybeans, mustard seeds, buckwheat, rice (grain and straw), maize straw, millet straw and sorghum stover for which the available data were insufficient to derive MRLs and risk assessment values. Tentative MRLs were also derived for wheat and barley straw, sugar beet tops, fodder beet roots and tops, grass forage, clover forage, alfalfa forage and turnips tops in view of the future need to set MRLs in feed items.
For genetically modified crops, data were sufficient to derive MRL for sweet corn (EPSPS modification) and cotton seed (EPSPS modification), noting that MRLs should be tentative pending on the submission of confirmatory methods for enforcement of AMPA and N‐acetyl‐glyphosate. For sugar beet roots, maize and soybeans (EPSPS modification) and rapeseeds (GOX modification), the available data were insufficient to derive MRLs and risk assessment values.
When considering the optional residue definition, in the absence of confirmatory methods for enforcement of AMPA (in all matrices) and N‐acetyl‐glyphosate (in high water content, high fat content and dry matrices), only tentative MRLs could be derived.
2. Residues in livestock
Glyphosate is authorised for use on several crops that might be fed to livestock. Livestock dietary burden calculations were, therefore, performed for different groups of livestock according to OECD guidance (OECD, 2013), which has now also been agreed upon at European level. Considering that livestock may be exposed to residues originating from conventional and genetically modified crops, the calculation of the livestock dietary burden was performed combining the residues originating from the uses authorised on conventional crops and on genetically modified crops. Therefore, for each feed item, risk assessment values obtained for conventional and genetically modified crops were compared and the most critical values selected for the exposure calculation. The input values for all relevant commodities are summarised in Appendix D. The dietary burden values calculated for all groups of livestock were found to exceed the trigger value of 0.1 mg/kg dry matter (DM), with the residues in conventional crops representing the main contributors to livestock exposure. Behaviour of residues was, therefore, assessed in all commodities of animal origin.
It is highlighted that for several feed items, no residue data were available (e.g. sunflowers, soybeans, maize stover, millet straw, rice grain and straw and sorghum stover). The animal intake of glyphosate residues via these commodities has, therefore, not been assessed and may have been underestimated. However, this is not expected to have a major impact on the outcome of the dietary burden considering the overwhelming contribution of grass forage and wheat straw.
2.1. Nature of residues and methods of analysis in livestock
Several livestock metabolism studies on goat and hen using glyphosate and AMPA labelled on the phosphonomethyl‐moiety and conducted with glyphosate, glyphosate‐trimesium or with a 9:1 glyphosate:AMPA mixture were evaluated during the peer review (Germany, 2015). In these studies, parent glyphosate was identified as the major component of the radioactive residues, accounting for 21–99% TRR in all animal matrices and AMPA was detected in significant proportions in liver (up to 36% TRR), muscle and fat (up to 19% TRR) and egg yolk (14% TRR). Additional metabolism studies on goat and hens were also provided in the framework of this review (Germany, 2017). Although these studies can only be used as additional information, due to the poor methodology used for the identification of radioactive residues, they confirmed that glyphosate is not significantly metabolised in ruminants and poultry, accounting for 88–91% TRR. It is noted that all the available metabolism studies on ruminants were performed with a lower dose rate compared to the calculated dietary burdens. Nevertheless, considering that in the available studies, residues were well characterised and the metabolic pattern clearly elucidated, additional metabolism studies are not required.
In addition, in order to address the animal metabolism of residues derived from genetically modified crops, metabolism studies on goat and hen using 14C‐N‐acetyl‐glyphosate were also evaluated during the peer review. In these studies, N‐acetyl‐glyphosate was identified as the major component of the radioactive residues, accounting for 17–77% TRR. Degradation to N‐acetyl‐AMPA was observed in fat (10–15% TRR), to glyphosate in liver (15% TRR), poultry fat (37% TRR) and egg white (11% TRR) and to AMPA in poultry muscle and fat (11–17% TRR).
The following residue definitions were agreed upon by MSs at the SCoPAFF meeting in September 2016 and are considered in this review: sum of glyphosate, AMPA and N ‐acetyl‐glyphosate expressed as glyphosate for monitoring, and sum of glyphosate, AMPA, N ‐acetyl‐glyphosate and N ‐acetyl‐AMPA expressed as glyphosate for risk assessment. No information on the metabolism of the TMS‐cation has been submitted in the framework of this MRL review and in the peer review for the renewal.
During the peer review, a high‐performance liquid chromatography with tandem mass spectrometry (HPLC‐MS/MS) analytical method and its independent laboratory validation (ILV) were assessed for the enforcement of glyphosate, AMPA and N‐acetyl‐glyphosate at the combined LOQs20 of 0.1 mg/kg (corresponding to a LOQ of 0.025 mg/kg for each compound) in meat, milk and egg and 0.2 mg/kg (corresponding to a LOQ of 0.05 mg/kg for each compound) in liver, kidney and fat. A confirmatory gas chromatography with mass spectrometry (GC‐MS) method is, however, only available for glyphosate in milk, eggs and meat. Therefore, a confirmatory method for glyphosate in fat and liver and kidney, as well as a confirmatory method for AMPA and N‐acetyl‐glyphosate in all matrices, are still missing. According to the information provided by the EURLs, sufficient validation data are not currently available for the routine enforcement of the proposed residue definition in animal commodities (EFSA, 2018a).
Information on the availability of a fully validated analytical method for the enforcement of TMS‐cation in the animal commodities, against illegal uses, is not available to EFSA.
During the peer review, the storage stability of glyphosate and AMPA was investigated in all animal commodities and it was concluded that glyphosate and AMPA are stable in meat, fat, liver and kidney for up to 26 months when samples were stored at −20°C. At the same storage temperature, residues of glyphosate and AMPA were found to be stable for 16 and 14 months in milk and eggs, respectively. The storage stability of N‐acetyl‐AMPA and N‐acetyl‐glyphosate was not investigated.
It is noted that, as underlined for plants, since the acetyl compounds are specific for GAT‐modified crops only and GAT‐modified crops are currently not on the EU market, the inclusion of N‐acetyl‐glyphosate in the residue definition for enforcement may be reconsidered and a separate residue definition comprising N‐acetyl glyphosate only could be defined. This would allow risk managers to set a lower LOQ for the enforcement in all animal commodities and to identify any possible misuses of genetically modified GAT crops by the analysis of the N‐acetyl glyphosate.
2.2. Magnitude of residues in livestock
Feeding studies conducted on dairy cows and laying hens fed with either glyphosate, glyphosate‐trimesium or a 9:1 glyphosate:AMPA mixture were evaluated in the framework of the peer review. A feeding study on pig using the glyphosate:AMPA mixture was also provided (Germany, 2015). In all the available feeding studies, residues were analysed for glyphosate and AMPA only while N‐acetyl‐compounds were not analysed. As GAT‐modified crops are currently not on the EU market, all feeding studies can be considered suitable to derive MRL and risk assessment values. Nevertheless, the study on cows dosed with glyphosate‐trimesium at 1.4, 7.38 and 19.4 mg glyphosate equivalent/kg body weight (bw) per day, was considered the most suitable to derive MRL and risk assessment values for ruminants since dose spacing matches the calculated dietary burdens as best as possible. For poultry and pigs, the studies performed with glyphosate and AMPA were considered instead. The results of AMPA from these studies were recalculated as glyphosate considering the molecular factor of 1.5.20 All samples from the livestock feeding studies were stored in compliance with the demonstrated storage stability conditions.
Based on these studies and the estimated residue intakes by livestock, MRLs above the LOQ were proposed for all animal commodities, except for cattle, swine and poultry fat, poultry liver, milk and eggs where no residues are expected and the MRLs can be set at the LOQ. Considering that the N‐acetyl compounds are not expected to be present in the animal tissues, a CF from enforcement to risk assessment of 1 has been proposed for all animal commodities. Since confirmatory methods for glyphosate in fat, liver and kidney, and for AMPA and N‐acetyl‐glyphosate in all matrices are still missing, all derived MRLs should be considered tentative only.
3. Consumer risk assessment
It is highlighted that toxicological data were not assessed in the current review and that the present reasoned opinion does not address the toxicological profile of glyphosate and its metabolites. In line with the provisions of Regulation (EC) No 396/2005, this review of MRLs is intended to characterise and quantify the residues of glyphosate in food and feed of plant and animal origin (resulting from the uses of glyphosate currently authorised by MSs), estimate dietary exposure of consumers, compare this dietary exposure to the toxicological reference values derived by EFSA in 2015 (for glyphosate and AMPA) and in 2018 (for N‐acetyl glyphosate and N‐acetyl‐AMPA) (EFSA, 2018b) and propose MRLs in case no concern for consumers is identified, also highlighting the uncertainties due to missing data.
It is underlined that in the framework of the evaluation of the impact of glyphosate and its residues in feed on animal health, the toxicological profile of the metabolites N‐acetyl‐AMPA and N‐acetyl‐glyphosate was further considered during the Pesticides Peer Review Experts’ Teleconference 175 (27 February 2018) on the basis of the raw studies made available to EFSA in January 2018 and taking into account other international evaluations. On this basis, it was concluded that the toxicological profile of glyphosate would cover those of the N‐acetyl metabolites. Therefore, the same reference values for consumers would be applicable to N‐acetyl compounds (EFSA, 2018b).
In the framework of this review, only the uses of glyphosate reported by the RMS in Appendix A were considered; however, the use of glyphosate was previously also assessed by the JMPR (FAO, 2005, 2011, 2013). The CXLs, resulting from these assessments by JMPR and adopted by the CAC, are now international recommendations that need to be considered by European risk managers when establishing MRLs. To facilitate consideration of these CXLs by risk managers, the consumer exposure was calculated both with and without consideration of the existing CXLs.
3.1. Consumer risk assessment without consideration of the existing CXLs
Chronic and acute exposure calculations for all crops reported in the framework of this review were performed using revision 2 of the EFSA PRIMo (EFSA, 2007). For each commodity, risk assessment values obtained for conventional and genetically modified crops were compared and the most critical values were selected for the exposure calculations. Input values for the exposure calculations were derived in compliance with the decision tree reported in Appendix E. Hence, for those commodities where a (tentative) MRL could be derived by EFSA in the framework of this review, input values were derived according to the internationally agreed methodologies (FAO, 2009).
The CFs derived in Sections 1 and 2 were used to convert the residues from enforcement to risk assessment residue definition. For those plant commodities where data were insufficient to derive MRLs in Section 1, the existing EU MRLs multiplied by the following CFs were used for an indicative calculation: for sunflower seeds, soyabeans and mustard seed, the conversion of 1.1 derived from residue trials performed on other oilseeds was considered; for buckwheat and rice grain, the conversion of 2.3 derived from residue trials performed on other cereals was considered. For cultivated fungi, the highest CF of 2.3 derived from all available trials was considered. All input values included in the exposure calculations are summarised in Appendix D.2.
The exposure values calculated were compared with the toxicological reference values for glyphosate and its metabolites, derived by EFSA (2015) under Commission Regulation (EU) No 1141/2010 as amended by Commission Implementing Regulation (EU) No 380/2013 and in the framework of the evaluation of the impact of glyphosate and its residues in feed on animal health (EFSA, 2018b). The highest chronic exposure was calculated for WHO cluster diet B, representing 9.1% of the acceptable daily intake (ADI) and the highest exposure was calculated for dry beans, representing 55.7% of the acute reference dose (ARfD).
Consequently, although major uncertainties remain due to the data gaps identified in the previous sections, the indicative exposure calculations did not indicate a risk to consumers.
It is noted that MRLs were derived for two different monitoring residue definitions (main and optional). Although the residue definition for risk assessment is the same in both cases, the MRLs as derived according to the optional definition (i.e. including glyphosate, AMPA and N‐acetyl‐glyphosate) can be higher than the MRLs as derived according to the main residue definition (glyphosate only). In particular, with the optional residue definition, a higher LOQ applies to all commodities for which a no‐residue situation can be anticipated. For this reason, an additional scenario, based on the optional residue definition, was performed. According to this second scenario, the highest chronic exposure was calculated for WHO cluster diet B, representing 9.9% of the ADI and the highest exposure was calculated for dry beans, representing 55.7% of the ARfD.
3.2. Consumer risk assessment with consideration of the existing CXLs
To include the CXLs in the calculations of the consumer exposure, CXLs were compared with the EU MRL proposals in compliance with Appendix E and all data relevant to the consumer exposure assessment have been collected from JMPR evaluations. An overview of the input values used for this exposure calculation is also provided in Appendix D.3.
As done in Section 3.1, also for the assessment of the existing CXLs, two different scenarios were considered: a first scenario based on the main residue definition and a second scenario based on the optional residue definition.
When considering the main residue definition, CXLs for bananas, dry beans, dry lentils, dry peas, sunflower seeds, barley, buckwheat, millet, oats, rye, sorghum, wheat and sugar canes, which are defined for glyphosate only, are in line with the residue definition derived by EFSA under this review. Therefore, for these commodities comparison between existing CXLs and the EU MRLs derived according to the main residue definition was possible and these CXLs could be considered in an exposure scenario (scenario 1).
When considering the optional residue definition (sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate also extended to conventional crops), commodities for which residues of AMPA and/or N‐acetyl compounds above the LOQ may occur according to the data available in the JMPR report(s), could not be considered comparable with the EU MRLs. Therefore, CXLs for dry beans, dry lentils, dry peas, sunflower seeds and sugar canes could not be included in the risk assessment (scenario 2).
For commodities where glyphosate tolerant varieties are currently available on the market (sweet corn, cotton seeds, sugar beets, rapeseeds, maize and soybeans) and for the animal commodities, it is noted that the residue definition proposed by EFSA is the same in both scenarios (sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate). Therefore, considerations on the comparability of the EU MRLs and these CXLs are the same in both scenarios. For these commodities, the residue definitions applying to CXLs differ from the residue definition derived by EFSA. The residue definition for monitoring proposed by EFSA is the sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate, while the residue definitions for monitoring for the CXLs are more restrictive. In particular, the CXL residue definition for monitoring does not include AMPA for all commodities and is even more restrictive for sweet corn, cottons seeds, soybeans and sugar beets for which is defined as glyphosate only. Possible inclusion of CXLs in the consumer exposure was assessed on a case‐by‐case basis:
Rapeseed and sugar beets: the absence of AMPA (and N‐acetyl‐glyphosate) in the CXL residue definitions is not considered as an issue since the available data in the JMPR report indicate that these metabolites are not expected in these commodities. Indeed, a CF of 1 for enforcement to risk assessment was considered by the JMPR (FAO, 2011, 2013). Therefore, it was possible to include these CXLs in the risk assessment assuming that the residue definition derived by EFSA can also apply to the CXLs of rapeseed and sugar beets. It should be noted that the CXL for rapeseed is derived from trials compliant with a GAP on GAT‐modified rapeseeds.
Sweet corn, cotton seeds, soybean and maize: the JMPR assessment indicates that significant levels of metabolite AMPA and/or N‐acetyl‐glyphosate may occur (CF > 1 were derived by JMPR). Therefore, these CXLs could not be considered further in the assessment.
Livestock commodities: metabolite AMPA is not included in the CXL residue definition while it was considered relevant in the EU assessment. However, only the CXLs for liver of swine, ruminants and poultry were found to be higher than the MRLs derived in Section 2. Since the dietary burden calculations based on the EU GAPs were found to be higher or comparable with the dietary burden reported in the JMPR assessment (FAO, 2005), this difference is considered linked to different approach in the extrapolation rules between EU and JMPR. Therefore, the MRLs for livestock as derived from the EU uses and import tolerance are expected to cover the residues in livestock derived by the JMPR and no further consideration of these CXLs is necessary.
When considering the main residue definition (scenario 1), the highest chronic exposure was calculated for British toddlers, representing 18.7% of the ADI; the highest acute exposure was calculated for sugar beet roots, representing 91% of the ARfD.
When considering the optional residue definition (scenario 2), the highest chronic exposure was calculated for British toddlers, representing 19% of the ADI; the highest acute exposure was calculated for sugar beet roots, representing 91% of the ARfD.
Based on these calculations, EFSA considers that the CXLs for glyphosate that could be assessed in this review are not expected to pose a risk to European consumers.
Conclusions
It is highlighted that toxicological data were not assessed in the current review and that the present reasoned opinion does not address the toxicological profile of glyphosate and its metabolites. In line with the provisions of Regulation (EC) No 396/2005, this review of MRLs is intended to characterise and quantify the residues of glyphosate in food and feed of plant and animal origin (resulting from the uses of glyphosate currently authorised by MSs), estimate dietary exposure of consumers, compare this dietary exposure to the toxicological reference values derived by EFSA in 2015 (for glyphosate and AMPA) and in 2018 (for N‐acetyl‐glyphosate and N‐acetyl‐AMPA) and propose MRLs, in case no concern for consumers is identified, also highlighting the uncertainties due to missing data.
The metabolism of glyphosate in primary crops was assessed in conventional and glyphosate tolerant crops containing EPSPS and GOX modifications belonging to different crop groups as well as in genetically modified soybean, maize and oilseed rape containing the GAT modification. Additional metabolism studies performed on conventional and EPSPS‐modified soybeans, cotton and maize were submitted by the RMS in the framework of this review. The metabolism in rotational crops (leafy vegetables, root and tuber vegetables and cereals) was investigated following glyphosate application directly to the soil or simulating typical agricultural practices.
In September 2016, during the Standing Committee on Plants, Animals, Food and Feed (SCoPAFF) meeting, the following residue definitions for enforcement were agreed upon by MSs as the basis for the MRL review:
OPTION 1:
for all plant commodities, including plants with glyphosate tolerant genetically modified varieties currently available on the market: sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate;
OPTION 2:
for plants with glyphosate tolerant genetically modified varieties currently available on the market (sweet corn, cotton seeds, sugar beets, rapeseeds, maize and soybeans): sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate;
for all other plant commodities: glyphosate.
For risk assessment, a general residue definition covering both conventional and genetically modified crops was proposed as the sum of glyphosate, AMPA, N ‐acetyl‐glyphosate and N ‐acetyl‐AMPA, expressed as glyphosate.
Although EFSA based this assessment on both residue definitions as agreed by MSs (options 1 and 2), EFSA agrees with the RMS that glyphosate only can be considered a sufficient marker for enforcement in conventional crops. For this reason, in the whole assessment, the option 2 is defined as the ‘main’ residue definition, while the option 1 is reported as ‘optional’.
Sufficiently validated analytical methods are available for the enforcement of glyphosate (relevant for the main residue definition), with a LOQ of 0.05 mg/kg in high water, high oil, acidic and dry matrices. Fully validated analytical methods for the enforcement of glyphosate in complex matrices (relevant for the authorisations on conventional tea, coffee beans, carobs, hops, spices and herbal infusions) are missing and are still required. Furthermore, there are indications that AMPA and N‐acetyl‐glyphosate (relevant for the optional residue definition proposed for all plant commodities and for genetically modified crops) can be enforced with a LOQ of 0.05 mg/kg, each. Therefore, the sum of glyphosate, AMPA and N‐acetyl‐glyphosate expressed as glyphosate can be enforced at the combined LOQ of 0.2 mg/kg in all matrices. Nevertheless, confirmatory methods for N‐acetyl‐glyphosate (in high water and high fat content matrices and dry commodities) and for AMPA (in all matrices) are still required.
Regarding the residue in primary crops, the available data on conventional crops are considered sufficient to derive (tentative) MRL proposals as well as risk assessment values for all crops under assessment except for cultivated fungi, sunflower seeds, soybeans, mustard seeds, buckwheat, rice (grain and straw), maize straw, millet straw and sorghum stover for which the available data were insufficient to derive MRLs and risk assessment values. Tentative MRLs were also derived for wheat and barley straw, sugar beet tops, fodder beet roots and tops, grass forage, clover forage, alfalfa forage and turnips tops in view of the future need to set MRLs in feed items.
For genetically modified crops, data were sufficient to derive MRL for sweet corn (EPSPS modification) and cotton seed (EPSPS modification), noting that MRLs should be tentative pending on the submission of confirmatory methods for enforcement of AMPA and N‐acetyl‐glyphosate. For sugar beet roots, maize and soybeans (EPSPS modification) and rapeseeds (GOX modification), the available data were insufficient to derive MRLs and risk assessment values.
When considering the optional residue definition, in the absence of confirmatory methods for enforcement of AMPA (in all matrices) and N‐acetyl‐glyphosate (in high water content, high fat content and dry matrices), only tentative MRLs could be derived.
Available residue trials also allowed to derive the following CFs from enforcement to risk assessment: 1 for all commodities where a no‐residue situation was demonstrated or was tentatively proposed, for crops with glyphosate tolerant genetically modified varieties currently available on the market (sweet corn, cotton seed, sugar beets, rapeseeds, maize and soybeans) and for all MRLs expressed according to the optional residue definition; 2 for dry pulses; 1.1 for linseed; 2.3 for millet and sorghum grain.
According to the results from the confined rotational crop studies performed up to 1.5N the maximum dose rate assessed in the present MRL review, residues of glyphosate or AMPA are not expected in rotational root and leafy crops following annual application of glyphosate, provided that the active substance is used according to the GAPs considered in this review. Residues of glyphosate and its metabolite AMPA above the LOQ of 0.05 mg/kg cannot be excluded in cereals grain (only AMPA), forage and chaff grown in rotation with crops treated with glyphosate. Although these residues can be considered negligible compared to the residues expected according to the most critical GAP for desiccation authorised on cereals, MSs are recommended to implement proper mitigation measures when granting authorisation of plant protection products containing glyphosate, in order to avoid residues to occur in rotated cereals. Moreover, as the available studies do not cover the plateau concentration calculated for AMPA, proper mitigation measures should also be implemented to avoid accumulation of AMPA in soil and possible uptake of AMPA in rotational crops. The plateau concentration calculated for AMPA should be in any case confirmed by an additional study performed in acidic soils (data gap identified in the peer review).
Glyphosate is authorised for use on several crops that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock. Considering that livestock may be exposed to residues originating from conventional and genetically modified crops, the calculation of the livestock dietary burden was performed combining the residues originating from the uses authorised on conventional crops and on genetically modified crops. The dietary burden values calculated for all groups of livestock were found to exceed the trigger value of 0.1 mg/kg DM, with the residues in conventional crops representing the main contributor to livestock exposure. Behaviour of residues was, therefore, assessed in all commodities of animal origin.
Several livestock metabolism studies on goat and hen using glyphosate and AMPA labelled on the phosphonomethyl‐moiety and conducted with glyphosate, glyphosate‐trimesium or with a 9:1 glyphosate:AMPA mixture were evaluated during the peer review. In addition, in order to address the animal metabolism of residues derived from genetically modified crops, metabolism studies on goat and hen using 14C‐N‐acetyl‐glyphosate were also evaluated during the peer review.
The following residue definitions for animal commodities were agreed upon by MSs at the SCoPAFF meeting in September 2016 and are considered in this MRL review: sum of glyphosate, AMPA and N ‐acetyl‐glyphosate expressed as glyphosate for monitoring, and sum of glyphosate, AMPA, N ‐acetyl‐glyphosate and N ‐acetyl‐AMPA expressed as glyphosate for risk assessment.
During the peer review, a HPLC‐MS/MS analytical method and its ILV were assessed for the enforcement of glyphosate and N‐acetyl‐glyphosate at the combined LOQ of 0.05 mg/kg in meat, milk and egg, and 0.1 mg/kg in liver, kidney and fat. A confirmatory GC‐MS method is, however, only available for glyphosate in milk, eggs and meat. Therefore, a confirmatory method for glyphosate in fat, liver and kidney, as well as a confirmatory method for AMPA and N‐acetyl‐glyphosate in all matrices, are still missing.
Based on available feeding studies and the estimated residue intakes by livestock, MRLs above the LOQ were proposed for all animal commodities, except for cattle, swine and poultry fat, poultry liver, milk and eggs where no residues are expected and the MRLs can be set at the LOQ. Considering that the N‐acetyl compounds are not expected to be present in the animal tissues, a CF from enforcement to risk assessment of 1 has been proposed for all animal commodities. Since confirmatory methods for glyphosate in fat, liver and kidney, and for AMPA and N‐acetyl‐glyphosate in all matrices are still missing, all derived MRLs should be considered tentative only.
Chronic and acute consumer exposure resulting from the authorised uses on conventional and genetically modified crops reported in the framework of this review was calculated using revision 2 of the EFSA PRIMo. For each commodity, risk assessment values obtained for conventional and genetically modified crops were compared and the most critical values were selected for the exposure calculations. Hence, for those commodities where a (tentative) MRL could be derived by EFSA in the framework of this review, input values were derived according to the internationally agreed methodologies. For those plant commodities where data were insufficient to derive (tentative) MRLs, the existing EU MRLs multiplied by the following CFs were used for an indicative calculation: for sunflower seeds, soybeans and mustard seed, the conversion of 1.1 derived from residue trials performed on other oilseeds was considered; for buckwheat and rice grain, the conversion of 2.3 derived from residue trials performed on other cereals was considered. For cultivated fungi, the highest CF of 2.3 derived from all available trials was considered.
The exposure values calculated were compared with the toxicological reference values for glyphosate and its metabolites, derived by EFSA under Commission Regulation (EU) No 1141/2010 as amended by Commission Implementing Regulation (EU) No 380/2013 and in the framework of evaluation of the impact of glyphosate and its residues in feed on animal health. The highest chronic exposure was calculated for WHO cluster diet B, representing 9.1% of the ADI and the highest exposure was calculated for dry beans, representing 55.7% of the ARfD.
Consequently, although major uncertainties remain due to the data gaps identified in the previous sections, the indicative exposure calculations did not indicate a risk to consumers.
Although the residue definition for risk assessment is the same for both options assessed in this review, the MRLs as derived, according to the optional definition and resulting for the summing up of the LOQs of the different compounds included, can be higher than the MRLs as derived according to the main residue definition. For this reason, an additional scenario, based on the optional residue definition, was performed. According to this second scenario, the highest chronic exposure was calculated for WHO cluster diet B, representing 9.9% of the ADI and the highest exposure was calculated for dry beans, representing 55.7% of the ARfD.
Apart from the MRLs evaluated in the framework of this review, internationally recommended CXLs have also been established for glyphosate. Additional calculations of the consumer exposure, including these CXLs, were therefore carried out, considering two different scenarios: a first scenario based on the main residue definition and a second scenario based on the optional residue definition.
When considering the main residue definition (scenario 1), the highest chronic exposure was calculated for British toddlers, representing 18.7% of the ADI; the highest acute exposure was calculated for sugar beet roots, representing 91% of the ARfD.
When considering the optional residue definition (scenario 2), the highest chronic exposure was calculated for British toddlers, representing 19% of the ADI; the highest acute exposure was calculated for sugar beet roots, representing 91% of the ARfD.
In order to support risk managers in the decision‐making process, EFSA also evaluated the import tolerances on GAT‐modified rapeseeds, soybeans and maize currently not present on the EU market but assessed in previous EFSA reasoned opinions. Based on the results of the studies on the magnitude of residues in plant and animal commodities, the MRLs proposed in the MRL review for plant and animal commodities are expected to cover the intended uses on GAT crops. Therefore, the consumer risk assessment performed in the MRL review does not need to be reconsidered and it can be concluded that the short‐term and long‐term intake of residues resulting from the intended uses on GAT soybeans, maize and rapeseeds is unlikely to present a risk to consumer health.
Recommendations
Considering that two separate residue definitions were derived for enforcement purposes, two lists of MRLs are proposed:
Main residue definition: MRL recommendations were derived in compliance with the decision tree reported in Appendix D of the reasoned opinion (see Table 2). All MRL values listed as ‘Recommended’ in the table are sufficiently supported by data and are therefore proposed for inclusion in Annex II to the Regulation. The remaining MRL values listed in the table are not recommended for inclusion in Annex II because they require further consideration by risk managers (see Table 2 footnotes for details).
Optional residue definition: MRLs derived for this residue definition take into account AMPA and N‐acetyl‐glyphosate in all plant and animal commodities and are listed in Table 3. Due to the major data gaps identified in the assessment, MRL values listed in this table are not recommended for inclusion in Annex II because they require further consideration by risk managers (see Table 3 for details). The indicative risk assessment for this optional residue definition showed similar outcome compared to the main proposal. It is also noted that glyphosate only is a sufficient marker in all commodities other than sweet corn, cotton seeds, rapeseeds, maize, soybeans and sugar beets. However, if risk managers consider that enforcement of AMPA and N‐acetyl‐glyphosate in all commodities is necessary, the optional list of MRLs is available below.
Table 2.
Summary table – main residue definition
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
| Enforcement residue definition: glyphosate | |||||
| 110010 | Grapefruits | 0.1* | – | 0.05* | Recommendeda |
| 110020 | Oranges | 0.5 | – | 0.05* | Recommendeda |
| 110030 | Lemons | 0.1* | – | 0.05* | Recommendeda |
| 110040 | Limes | 0.1* | – | 0.05* | Recommendeda |
| 110050 | Mandarins | 0.5 | – | 0.05* | Recommendeda |
| 120010 | Almonds | 0.1* | – | 0.05* | Recommendeda |
| 120020 | Brazil nuts | 0.1* | – | 0.05* | Recommendeda |
| 120030 | Cashew nuts | 0.1* | – | 0.05* | Recommendeda |
| 120040 | Chestnuts | 0.1* | – | 0.05* | Recommendeda |
| 120050 | Coconuts | 0.1* | – | 0.05* | Recommendeda |
| 120060 | Hazelnuts/cobnuts | 0.1* | – | 0.05* | Recommendeda |
| 120070 | Macadamias | 0.1* | – | 0.05* | Recommendeda |
| 120080 | Pecans | 0.1* | – | 0.05* | Recommendeda |
| 120090 | Pine nut kernels | 0.1* | – | 0.05* | Recommendeda |
| 120100 | Pistachios | 0.1* | – | 0.05* | Recommendeda |
| 120110 | Walnuts | 0.1* | – | 0.05* | Recommendeda |
| 130010 | Apples | 0.1* | – | 0.05* | Recommendeda |
| 130020 | Pears | 0.1* | – | 0.05* | Recommendeda |
| 130030 | Quinces | 0.1* | – | 0.05* | Recommendeda |
| 130040 | Medlars | 0.1* | – | 0.05* | Recommendeda |
| 130050 | Loquats/Japanese medlars | 0.1* | – | 0.05* | Recommendeda |
| 140010 | Apricots | 0.1* | – | 0.05* | Recommendeda |
| 140020 | Cherries (sweet) | 0.1* | – | 0.05* | Recommendeda |
| 140030 | Peaches | 0.1* | – | 0.05* | Recommendeda |
| 140040 | Plums | 0.1* | – | 0.05* | Recommendeda |
| 151010 | Table grapes | 0.5 | – | 0.05* | Recommendeda |
| 151020 | Wine grapes | 0.5 | – | 0.05* | Recommendeda |
| 152000 | Strawberries | 0.1* | – | 0.05* | Further consideration neededb |
| 153010 | Blackberries | 0.1* | – | 0.05* | Further consideration neededb |
| 153020 | Dewberries | 0.1* | – | 0.05* | Further consideration neededb |
| 153030 | Raspberries (red and yellow) | 0.1* | – | 0.05* | Further consideration neededb |
| 154010 | Blueberries | 0.1* | – | 0.05* | Further consideration neededb |
| 154020 | Cranberries | 0.1* | – | 0.05* | Further consideration neededb |
| 154030 | Currants (black, red and white) | 0.1* | – | 0.05* | Further consideration neededb |
| 154040 | Gooseberries (green, red and yellow) | 0.1* | – | 0.05* | Further consideration neededb |
| 154050 | Rose hips | 0.1* | – | 0.05* | Further consideration neededb |
| 154060 | Mulberries (black and white) | 0.1* | – | 0.05* | Further consideration neededb |
| 154070 | Azaroles/Mediterranean medlars | 0.1* | – | 0.05* | Further consideration neededb |
| 154080 | Elderberries | 0.1* | – | 0.05* | Further consideration neededb |
| 161020 | Figs | 0.1* | – | 0.05* | Recommendeda |
| 161030 | Table olives | 1 | – | 0.05* | Recommendeda |
| 161040 | Kumquats | 0.1* | – | 0.05* | Recommendeda |
| 161060 | Kaki/Japanese persimmons | 0.1* | – | 0.05* | Recommendeda |
| 162010 | Kiwi fruits (green, red, yellow) | 0.1* | – | 0.05* | Recommendeda |
| 162020 | Litchis/lychees | 0.1* | – | 0.05* | Recommendeda |
| 162030 | Passion fruits/maracujas | 0.1* | – | 0.05* | Recommendeda |
| 163010 | Avocados | 0.1* | – | 0.05* | Recommendeda |
| 163020 | Bananas | 0.1* | 0.05* | 0.05* | Recommendede |
| 163030 | Mangoes | 0.1* | – | 0.05* | Recommendeda |
| 163040 | Papayas | 0.1* | – | 0.05* | Recommendeda |
| 163050 | Granate apples/pomegranates | 0.1* | – | 0.05* | Recommendeda |
| 163060 | Cherimoyas | 0.1* | – | 0.05* | Recommendeda |
| 211000 | Potatoes | 0.5 | – | 1 | Further consideration neededb |
| 212010 | Cassava roots/manioc | 0.1* | – | 0.05* | Further consideration neededb |
| 212020 | Sweet potatoes | 0.1* | – | 0.05* | Further consideration neededb |
| 212030 | Yams | 0.1* | – | 0.05* | Further consideration neededb |
| 212040 | Arrowroots | 0.1* | – | 0.05* | Further consideration neededb |
| 213010 | Beetroots | 0.1* | – | 0.05* | Further consideration neededb |
| 213020 | Carrots | 0.1* | – | 0.05* | Recommendeda |
| 213030 | Celeriacs/turnip rooted celeries | 0.1* | – | 0.05* | Further consideration neededb |
| 213040 | Horseradishes | 0.1* | – | 0.05* | Further consideration neededb |
| 213050 | Jerusalem artichokes | 0.1* | – | 0.05* | Further consideration neededb |
| 213060 | Parsnips | 0.1* | – | 0.05* | Further consideration neededb |
| 213070 | Parsley roots/Hamburg roots parsley | 0.1* | – | 0.05* | Further consideration neededb |
| 213080 | Radishes | 0.1* | – | 0.05* | Further consideration neededb |
| 213090 | Salsifies | 0.1* | – | 0.05* | Further consideration neededb |
| 213100 | Swedes/rutabagas | 0.1* | – | 0.05* | Further consideration neededb |
| 213110 | Turnips | 0.1* | – | 0.05* | Further consideration neededb |
| 220010 | Garlic | 0.1* | – | 0.05* | Further consideration neededb |
| 220020 | Onions | 0.1* | – | 0.05* | Further consideration neededb |
| 220030 | Shallots | 0.1* | – | 0.05* | Further consideration neededb |
| 220040 | Spring onions/green onions and Welsh onions | 0.1* | – | 0.05* | Further consideration neededb |
| 231010 | Tomatoes | 0.1* | – | 0.05* | Recommendeda |
| 231020 | Sweet peppers/bell peppers | 0.1* | – | 0.05* | Further consideration neededb |
| 231030 | Aubergines/eggplants | 0.1* | – | 0.05* | Recommendeda |
| 231040 | Okra/lady's fingers | 0.1* | – | 0.05* | Further consideration neededb |
| 232010 | Cucumbers | 0.1* | – | 0.05* | Further consideration neededb |
| 232020 | Gherkins | 0.1* | – | 0.05* | Further consideration neededb |
| 232030 | Courgettes | 0.1* | – | 0.05* | Further consideration neededb |
| 233010 | Melons | 0.1* | – | 0.05* | Further consideration neededb |
| 233020 | Pumpkins | 0.1* | – | 0.05* | Further consideration neededb |
| 233030 | Watermelons | 0.1* | – | 0.05* | Further consideration neededb |
| 241010 | Broccoli | 0.1* | – | 0.05* | Further consideration neededb |
| 241020 | Cauliflowers | 0.1* | – | 0.05* | Further consideration neededb |
| 242010 | Brussels sprouts | 0.1* | – | 0.05* | Further consideration neededb |
| 242020 | Head cabbages | 0.1* | – | 0.05* | Further consideration neededb |
| 243010 | Chinese cabbages/pe‐tsai | 0.1* | – | 0.05* | Further consideration neededb |
| 243020 | Kales | 0.1* | – | 0.05* | Further consideration neededb |
| 244000 | Kohlrabies | 0.1* | – | 0.05* | Further consideration neededb |
| 251010 | Lamb's lettuces/corn salads | 0.1* | – | 0.05* | Further consideration neededb |
| 251020 | Lettuces | 0.1* | – | 0.05* | Further consideration neededb |
| 251030 | Escaroles/broadleaved endives | 0.1* | – | 0.05* | Further consideration neededb |
| 251040 | Cresses and other sprouts and shoots | 0.1* | – | 0.05* | Further consideration neededb |
| 251050 | Land cresses | 0.1* | – | 0.05* | Further consideration neededb |
| 251060 | Roman rocket/rucola | 0.1* | – | 0.05* | Further consideration neededb |
| 251070 | Red mustards | 0.1* | – | 0.05* | Further consideration neededb |
| 251080 | Baby leaf crops (including brassica species) | 0.1* | – | 0.05* | Further consideration neededb |
| 252010 | Spinaches | 0.1* | – | 0.05* | Further consideration neededb |
| 252020 | Purslanes | 0.1* | – | 0.05* | Further consideration neededb |
| 252030 | Chards/beet leaves | 0.1* | – | 0.05* | Further consideration neededb |
| 253000 | Grape leaves and similar species | 0.1* | – | 0.05* | Further consideration neededb |
| 254000 | Watercresses | 0.1* | – | 0.05* | Further consideration neededb |
| 255000 | Witloofs/Belgian endives | 0.1* | – | 0.05* | Further consideration neededb |
| 256010 | Chervil | 0.1* | – | 0.05* | Further consideration neededb |
| 256020 | Chives | 0.1* | – | 0.05* | Further consideration neededb |
| 256030 | Celery leaves | 0.1* | – | 0.05* | Further consideration neededb |
| 256040 | Parsley | 0.1* | – | 0.05* | Further consideration neededb |
| 256050 | Sage | 0.1* | – | 0.05* | Further consideration neededb |
| 256060 | Rosemary | 0.1* | – | 0.05* | Further consideration neededb |
| 256070 | Thyme | 0.1* | – | 0.05* | Further consideration neededb |
| 256080 | Basil and edible flowers | 0.1* | – | 0.05* | Further consideration neededb |
| 256090 | Laurel/bay leave | 0.1* | – | 0.05* | Further consideration neededb |
| 256100 | Tarragon | 0.1* | – | 0.05* | Further consideration neededb |
| 260010 | Beans (with pods) | 0.1* | – | 0.05* | Recommendeda |
| 260020 | Beans (without pods) | 0.1* | – | 0.05* | Recommendeda |
| 260030 | Peas (with pods) | 0.1* | – | 0.05* | Recommendeda |
| 260040 | Peas (without pods) | 0.1* | – | 0.05* | Recommendeda |
| 260050 | Lentils (fresh) | 0.1* | – | 0.05* | Recommendeda |
| 270010 | Asparagus | 0.1* | – | 0.05* | Further consideration neededb |
| 270020 | Cardoons | 0.1* | – | 0.05* | Further consideration neededb |
| 270030 | Celeries | 0.1* | – | 0.05* | Further consideration neededb |
| 270040 | Florence fennels | 0.1* | – | 0.05* | Further consideration neededb |
| 270050 | Globe artichokes | 0.1* | – | 0.05* | Recommendeda |
| 270060 | Leeks | 0.1* | – | 0.05* | Further consideration neededb |
| 270070 | Rhubarbs | 0.1* | – | 0.05* | Further consideration neededb |
| 270080 | Bamboo shoots | 0.1* | – | 0.05* | Further consideration neededb |
| 270090 | Palm hearts | 0.1* | – | 0.05* | Further consideration neededb |
| 280010 | Cultivated fungi | 0.1* | – | 0.1 | Further consideration neededc |
| 280020 | Wild fungi | 50 | – | 0.05* | Recommendeda |
| 300010 | Beans (dry) | 2 | 2 | 15 | Further consideration neededd |
| 300020 | Lentils (dry) | 10 | 5 | 15 | Further consideration neededd |
| 300030 | Peas (dry) | 10 | 5 | 15 | Further consideration neededd |
| 300040 | Lupins/lupine beans (dry) | 10 | – | 15 | Further consideration neededb |
| 401010 | Linseeds | 10 | – | 15 | Recommendeda |
| 401020 | Peanuts/groundnuts | 0.1* | – | 0.05* | Further consideration neededb |
| 401030 | Poppy seeds | 0.1* | – | 0.05* | Further consideration neededb |
| 401040 | Sesame seeds | 0.1* | – | 0.05* | Further consideration neededb |
| 401050 | Sunflower seeds | 20 | 7 | 20 | Further consideration neededd |
| 401080 | Mustard seeds | 10 | – | 10 | Further consideration neededc |
| 401100 | Pumpkin seeds | 0.1* | – | 0.05* | Further consideration neededb |
| 401110 | Safflower seeds | 0.1* | – | 0.05* | Further consideration neededb |
| 401120 | Borage seeds | 0.1 | – | 10 | Recommendeda |
| 401130 | Gold of pleasure seeds | 0.1 | – | 0.05* | Further consideration neededb |
| 401140 | Hemp seeds | 0.1* | – | 0.05* | Further consideration neededb |
| 401150 | Castor beans | 0.1 | – | 0.05* | Further consideration neededb |
| 402010 | Olives for oil production | 1 | – | 30 | Recommendeda |
| 402020 | Oil palms kernels | 0.1 | – | 0.05* | Recommendeda |
| 402030 | Oil palms fruits | 0.1 | – | 0.05* | Further consideration neededb |
| 402040 | Kapok | 0.1 | – | 0.05* | Recommendeda |
| 500010 | Barley grains | 20 | 30 | 30 | Recommendede |
| 500020 | Buckwheat and other pseudo‐cereal grains | 0.1* | 30 | 30 | Recommendedf |
| 500040 | Common millet/proso millet grains | 0.1* | 30 | 30 | Recommendedg |
| 500050 | Oat grains | 20 | 30 | 30 | Recommendede |
| 500060 | Rice grains | 0.1* | – | 0.1 | Further consideration neededc |
| 500070 | Rye grains | 10 | 30 | 30 | Recommendedg |
| 500080 | Sorghum grains | 20 | 30 | 30 | Recommendedg |
| 500090 | Wheat grains | 10 | 30 | 30 | Recommendedg |
| 610000 | Teas | 2 | – | 0.05* | Further consideration neededb |
| 620000 | Coffee beans | 0.1 | – | 0.05* | Further consideration neededb |
| 631000 | Herbal infusions from flowers | 2* | – | 0.05* | Further consideration neededb |
| 632000 | Herbal infusions from leaves and herbs | 2* | – | 0.05* | Further consideration neededb |
| 633000 | Herbal infusions from roots | 2* | – | 0.05* | Further consideration neededb |
| 650000 | Carobs/Saint John's breads | 0.1* | – | 0.05* | Further consideration neededb |
| 700000 | Hops | 0.1* | – | 0.05* | Further consideration neededb |
| 810000 | Seed spices | 0.1* | – | 0.05* | Further consideration neededb |
| 820000 | Fruit spices | 0.1* | – | 0.05* | Further consideration neededb |
| 830000 | Bark spices | 0.1* | – | 0.05* | Further consideration neededb |
| 840000 | Root and rhizome spices | 0.1* | – | 0.05* | Further consideration neededb |
| 850000 | Bud spices | 0.1* | – | 0.05* | Further consideration neededb |
| 860000 | Flower pistil spices | 0.1* | – | 0.05* | Further consideration neededb |
| 870000 | Aril spices | 0.1* | – | 0.05* | Further consideration neededb |
| 900020 | Sugarcanes | 0.1* | 2 | 2 | Recommendedl |
| 900030 | Chicory roots | 0.1* | – | 0.05* | Further consideration neededb |
| – | Other commodities of plant origin | – | – | Further consideration neededh | |
|
Enforcement residue definition (existing): glyphosate Enforcement residue definition (proposed): sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate | |||||
| 234000 | Sweet corn | 3 | 3 | 3 | Further consideration neededi |
| 401060 | Rapeseeds/canola seeds | 10 | 30 | 30 | Further consideration neededj |
| 401070 | Soyabeans | 20 | 20 | 20 | Further consideration neededk |
| 401090 | Cotton seeds | 10 | 40 | 60 | Further consideration neededi |
| 500030 | Maize/corn grains | 1 | 5 | 3 | Further consideration neededi |
| 900010 | Sugar beet roots | 15 | 15 | 15 | Further consideration neededj |
| 1011010 | Swine muscle | 0.05* | 0.05* | 0.2 | Further consideration neededi |
| 1011020 | Swine fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededi |
| 1011030 | Swine liver | 0.05* | 0.5 | 0.4 | Further consideration neededi |
| 1011040 | Swine kidney | 0.5 | 0.5 | 3 | Further consideration neededi |
| 1012010 | Bovine muscle | 0.05* | 0.05* | 0.2 | Further consideration neededi |
| 1012020 | Bovine fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededi |
| 1012030 | Bovine liver | 0.2 | 5 | 0.7 | Further consideration neededi |
| 1012040 | Bovine kidney | 2 | 5 | 7 | Further consideration neededi |
| 1013010 | Sheep muscle | 0.05* | 0.05* | 0.2 | Further consideration neededi |
| 1013020 | Sheep fat tissue | 0.05* | 0.05* | 0.3 | Further consideration neededi |
| 1013030 | Sheep liver | 0.05* | 5 | 0.9 | Further consideration neededi |
| 1013040 | Sheep kidney | 0.05* | 5 | 10 | Further consideration neededi |
| 1014010 | Goat muscle | 0.05* | 0.05* | 0.2 | Further consideration neededi |
| 1014020 | Goat fat tissue | 0.05* | 0.05* | 0.3 | Further consideration neededi |
| 1014030 | Goat liver | 0.05* | 5 | 0.9 | Further consideration neededi |
| 1014040 | Goat kidney | 0.05* | 5 | 10 | Further consideration neededi |
| 1015010 | Equine muscle | 0.05* | 0.05* | 0.2 | Further consideration neededi |
| 1015020 | Equine fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededi |
| 1015030 | Equine liver | 0.05* | 5 | 0.7 | Further consideration neededi |
| 1015040 | Equine kidney | 0.05* | 5 | 7 | Further consideration neededi |
| 1016010 | Poultry muscle | 0.05* | 0.05* | 0.2 | Further consideration neededi |
| 1016020 | Poultry fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededi |
| 1016030 | Poultry liver | 0.05* | 0.5 | 0.2* | Further consideration neededi |
| 1020010 | Cattle milk | 0.05* | 0.05 | 0.1* | Further consideration neededi |
| 1020020 | Sheep milk | 0.05* | 0.05 | 0.1* | Further consideration neededi |
| 1020030 | Goat milk | 0.05* | 0.05 | 0.1* | Further consideration neededi |
| 1020040 | Horse milk | 0.05* | 0.05 | 0.1* | Further consideration neededi |
| 1030000 | Birds eggs | 0.05* | 0.05* | 0.1* | Further consideration neededi |
| – | Other commodities of animal origin | – | – | Further consideration neededh | |
MRL: maximum residue level; CXL: codex maximum residue limit.
*Indicates that the MRL is set at the limit of quantification.
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available (combination G‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; no CXL is available (combination E‐I in Appendix E).
GAP evaluated at EU level is not supported by data, but no risk to consumers was identified for the existing EU MRL; no CXL is available (combination C‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; existing CXL is covered by the tentative MRL (combination E‐III in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL; existing CXL is covered by the existing EU MRL (combination C‐III in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; existing CXL is covered by the recommended MRL (combination G‐III in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level is not supported by data, but the existing EU MRL is lower than the existing CXL (combination C‐VII in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level, which is also fully supported by data, leads to a lower MRL (combination G‐VII in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; CXL is not compatible with EU residue definitions (combination E‐II in Appendix E).
MRL is derived from the existing CXL, which is not sufficiently supported by data but for which no risk to consumers is identified; GAP evaluated at EU level, which is also not fully supported by data, would lead to a lower tentative MRL (combination E‐V in Appendix E).
GAP evaluated at EU level is not supported by data, but no risk to consumers was identified for the existing EU MRL; CXL is not compatible with EU residue definitions (combination C‐II in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level, which is not fully supported by data, leads to a lower tentative MRL (combination E‐VII in Appendix E).
Table 3.
Summary table – optional residue definition
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
|
Enforcement residue definition (existing): glyphosate Enforcement residue definition (proposed – optional): sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate | |||||
| 110010 | Grapefruits | 0.1* | – | 0.2* | Further consideration neededa |
| 110020 | Oranges | 0.5 | – | 0.2* | Further consideration neededa |
| 110030 | Lemons | 0.1* | – | 0.2* | Further consideration neededa |
| 110040 | Limes | 0.1* | – | 0.2* | Further consideration neededa |
| 110050 | Mandarins | 0.5 | – | 0.2* | Further consideration neededa |
| 120010 | Almonds | 0.1* | – | 0.2* | Further consideration neededa |
| 120020 | Brazil nuts | 0.1* | – | 0.2* | Further consideration neededa |
| 120030 | Cashew nuts | 0.1* | – | 0.2* | Further consideration neededa |
| 120040 | Chestnuts | 0.1* | – | 0.2* | Further consideration neededa |
| 120050 | Coconuts | 0.1* | – | 0.2* | Further consideration neededa |
| 120060 | Hazelnuts/cobnuts | 0.1* | – | 0.2* | Further consideration neededa |
| 120070 | Macadamias | 0.1* | – | 0.2* | Further consideration neededa |
| 120080 | Pecans | 0.1* | – | 0.2* | Further consideration neededa |
| 120090 | Pine nut kernels | 0.1* | – | 0.2* | Further consideration neededa |
| 120100 | Pistachios | 0.1* | – | 0.2* | Further consideration neededa |
| 120110 | Walnuts | 0.1* | – | 0.2* | Further consideration neededa |
| 130010 | Apples | 0.1* | – | 0.2* | Further consideration neededa |
| 130020 | Pears | 0.1* | – | 0.2* | Further consideration neededa |
| 130030 | Quinces | 0.1* | – | 0.2* | Further consideration neededa |
| 130040 | Medlars | 0.1* | – | 0.2* | Further consideration neededa |
| 130050 | Loquats/Japanese medlars | 0.1* | – | 0.2* | Further consideration neededa |
| 140010 | Apricots | 0.1* | – | 0.2* | Further consideration neededa |
| 140020 | Cherries (sweet) | 0.1* | – | 0.2* | Further consideration neededa |
| 140030 | Peaches | 0.1* | – | 0.2* | Further consideration neededa |
| 140040 | Plums | 0.1* | – | 0.2* | Further consideration neededa |
| 151010 | Table grapes | 0.5 | – | 0.2* | Further consideration neededa |
| 151020 | Wine grapes | 0.5 | – | 0.2* | Further consideration neededa |
| 152000 | Strawberries | 0.1* | – | 0.2* | Further consideration neededa |
| 153010 | Blackberries | 0.1* | – | 0.2* | Further consideration neededa |
| 153020 | Dewberries | 0.1* | – | 0.2* | Further consideration neededa |
| 153030 | Raspberries (red and yellow) | 0.1* | – | 0.2* | Further consideration neededa |
| 154010 | Blueberries | 0.1* | – | 0.2* | Further consideration neededa |
| 154020 | Cranberries | 0.1* | – | 0.2* | Further consideration neededa |
| 154030 | Currants (black, red and white) | 0.1* | – | 0.2* | Further consideration neededa |
| 154040 | Gooseberries (green, red and yellow) | 0.1* | – | 0.2* | Further consideration neededa |
| 154050 | Rose hips | 0.1* | – | 0.2* | Further consideration neededa |
| 154060 | Mulberries (black and white) | 0.1* | – | 0.2* | Further consideration neededa |
| 154070 | Azaroles/Mediterranean medlars | 0.1* | – | 0.2* | Further consideration neededa |
| 154080 | Elderberries | 0.1* | – | 0.2* | Further consideration neededa |
| 161020 | Figs | 0.1* | – | 0.2* | Further consideration neededa |
| 161030 | Table olives | 1 | – | 0.2* | Further consideration neededa |
| 161040 | Kumquats | 0.1* | – | 0.2* | Further consideration neededa |
| 161060 | Kaki/Japanese persimmons | 0.1* | – | 0.2* | Further consideration neededa |
| 162010 | Kiwi fruits (green, red, yellow) | 0.1* | – | 0.2* | Further consideration neededa |
| 162020 | Litchis/lychees | 0.1* | – | 0.2* | Further consideration neededa |
| 162030 | Passion fruits/maracujas | 0.1* | – | 0.2* | Further consideration neededa |
| 163010 | Avocados | 0.1* | – | 0.2* | Further consideration neededa |
| 163020 | Bananas | 0.1* | 0.05* | 0.2* | Further consideration neededb |
| 163030 | Mangoes | 0.1* | – | 0.2* | Further consideration neededa |
| 163040 | Papayas | 0.1* | – | 0.2* | Further consideration neededa |
| 163050 | Granate apples/pomegranates | 0.1* | – | 0.2* | Further consideration neededa |
| 163060 | Cherimoyas | 0.1* | – | 0.2* | Further consideration neededa |
| 211000 | Potatoes | 0.5 | – | 1 | Further consideration neededa |
| 212010 | Cassava roots/manioc | 0.1* | – | 0.2* | Further consideration neededa |
| 212020 | Sweet potatoes | 0.1* | – | 0.2* | Further consideration neededa |
| 212030 | Yams | 0.1* | – | 0.2* | Further consideration neededa |
| 212040 | Arrowroots | 0.1* | – | 0.2* | Further consideration neededa |
| 213010 | Beetroots | 0.1* | – | 0.2* | Further consideration neededa |
| 213020 | Carrots | 0.1* | – | 0.2* | Further consideration neededa |
| 213030 | Celeriacs/turnip rooted celeries | 0.1* | – | 0.2* | Further consideration neededa |
| 213040 | Horseradishes | 0.1* | – | 0.2* | Further consideration neededa |
| 213050 | Jerusalem artichokes | 0.1* | – | 0.2* | Further consideration neededa |
| 213060 | Parsnips | 0.1* | – | 0.2* | Further consideration neededa |
| 213070 | Parsley roots/Hamburg roots parsley | 0.1* | – | 0.2* | Further consideration neededa |
| 213080 | Radishes | 0.1* | – | 0.2* | Further consideration neededa |
| 213090 | Salsifies | 0.1* | – | 0.2* | Further consideration neededa |
| 213100 | Swedes/rutabagas | 0.1* | – | 0.2* | Further consideration neededa |
| 213110 | Turnips | 0.1* | – | 0.2* | Further consideration neededa |
| 220010 | Garlic | 0.1* | – | 0.2* | Further consideration neededa |
| 220020 | Onions | 0.1* | – | 0.2* | Further consideration neededa |
| 220030 | Shallots | 0.1* | – | 0.2* | Further consideration neededa |
| 220040 | Spring onions/green onions and Welsh onions | 0.1* | – | 0.2* | Further consideration neededa |
| 231010 | Tomatoes | 0.1* | – | 0.2* | Further consideration neededa |
| 231020 | Sweet peppers/bell peppers | 0.1* | – | 0.2* | Further consideration neededa |
| 231030 | Aubergines/eggplants | 0.1* | – | 0.2* | Further consideration neededa |
| 231040 | Okra/lady's fingers | 0.1* | – | 0.2* | Further consideration neededa |
| 232010 | Cucumbers | 0.1* | – | 0.2* | Further consideration neededa |
| 232020 | Gherkins | 0.1* | – | 0.2* | Further consideration neededa |
| 232030 | Courgettes | 0.1* | – | 0.2* | Further consideration neededa |
| 233010 | Melons | 0.1* | – | 0.2* | Further consideration neededa |
| 233020 | Pumpkins | 0.1* | – | 0.2* | Further consideration neededa |
| 233030 | Watermelons | 0.1* | – | 0.2* | Further consideration neededa |
| 234000 | Sweet corn | 3 | 3 | 3 | Further consideration neededc |
| 241010 | Broccoli | 0.1* | – | 0.2* | Further consideration neededa |
| 241020 | Cauliflowers | 0.1* | – | 0.2* | Further consideration neededa |
| 242010 | Brussels sprouts | 0.1* | – | 0.2* | Further consideration neededa |
| 242020 | Head cabbages | 0.1* | – | 0.2* | Further consideration neededa |
| 243010 | Chinese cabbages/pe‐tsai | 0.1* | – | 0.2* | Further consideration neededa |
| 243020 | Kales | 0.1* | – | 0.2* | Further consideration neededa |
| 244000 | Kohlrabies | 0.1* | – | 0.2* | Further consideration neededa |
| 251010 | Lamb's lettuces/corn salads | 0.1* | – | 0.2* | Further consideration neededa |
| 251020 | Lettuces | 0.1* | – | 0.2* | Further consideration neededa |
| 251030 | Escaroles/broad‐leaved endives | 0.1* | – | 0.2* | Further consideration neededa |
| 251040 | Cresses and other sprouts and shoots | 0.1* | – | 0.2* | Further consideration neededa |
| 251050 | Land cresses | 0.1* | – | 0.2* | Further consideration neededa |
| 251060 | Roman rocket/rucola | 0.1* | – | 0.2* | Further consideration neededa |
| 251070 | Red mustards | 0.1* | – | 0.2* | Further consideration neededa |
| 251080 | Baby leaf crops (including brassica species) | 0.1* | – | 0.2* | Further consideration neededa |
| 252010 | Spinaches | 0.1* | – | 0.2* | Further consideration neededa |
| 252020 | Purslanes | 0.1* | – | 0.2* | Further consideration neededa |
| 252030 | Chards/beet leaves | 0.1* | – | 0.2* | Further consideration neededa |
| 253000 | Grape leaves and similar species | 0.1* | – | 0.2* | Further consideration neededa |
| 254000 | Watercresses | 0.1* | – | 0.2* | Further consideration neededa |
| 255000 | Witloofs/Belgian endives | 0.1* | – | 0.2* | Further consideration neededa |
| 256010 | Chervil | 0.1* | – | 0.2* | Further consideration neededa |
| 256020 | Chives | 0.1* | – | 0.2* | Further consideration neededa |
| 256030 | Celery leaves | 0.1* | – | 0.2* | Further consideration neededa |
| 256040 | Parsley | 0.1* | – | 0.2* | Further consideration neededa |
| 256050 | Sage | 0.1* | – | 0.2* | Further consideration neededa |
| 256060 | Rosemary | 0.1* | – | 0.2* | Further consideration neededa |
| 256070 | Thyme | 0.1* | – | 0.2* | Further consideration neededa |
| 256080 | Basil and edible flowers | 0.1* | – | 0.2* | Further consideration neededa |
| 256090 | Laurel/bay leave | 0.1* | – | 0.2* | Further consideration neededa |
| 256100 | Tarragon | 0.1* | – | 0.2* | Further consideration neededa |
| 260010 | Beans (with pods) | 0.1* | – | 0.2* | Further consideration neededa |
| 260020 | Beans (without pods) | 0.1* | – | 0.2* | Further consideration neededa |
| 260030 | Peas (with pods) | 0.1* | – | 0.2* | Further consideration neededa |
| 260040 | Peas (without pods) | 0.1* | – | 0.2* | Further consideration neededa |
| 260050 | Lentils (fresh) | 0.1* | – | 0.2* | Further consideration neededa |
| 270010 | Asparagus | 0.1* | – | 0.2* | Further consideration neededa |
| 270020 | Cardoons | 0.1* | – | 0.2* | Further consideration neededa |
| 270030 | Celeries | 0.1* | – | 0.2* | Further consideration neededa |
| 270040 | Florence fennels | 0.1* | – | 0.2* | Further consideration neededa |
| 270050 | Globe artichokes | 0.1* | – | 0.2* | Further consideration neededa |
| 270060 | Leeks | 0.1* | – | 0.2* | Further consideration neededa |
| 270070 | Rhubarbs | 0.1* | – | 0.2* | Further consideration neededa |
| 270080 | Bamboo shoots | 0.1* | – | 0.2* | Further consideration neededa |
| 270090 | Palm hearts | 0.1* | – | 0.2* | Further consideration neededa |
| 280010 | Cultivated fungi | 0.1* | – | 0.2* | Further consideration neededf |
| 280020 | Wild fungi | 50 | – | 0.2* | Further consideration neededa |
| 300010 | Beans (dry) | 2 | 2 | 30 | Further consideration neededc |
| 300020 | Lentils (dry) | 10 | 5 | 30 | Further consideration neededc |
| 300030 | Peas (dry) | 10 | 5 | 30 | Further consideration neededc |
| 300040 | Lupins/lupini beans (dry) | 10 | – | 30 | Further consideration neededa |
| 401010 | Linseeds | 10 | – | 15 | Further consideration neededa |
| 401020 | Peanuts/groundnuts | 0.1* | – | 0.2* | Further consideration neededa |
| 401030 | Poppy seeds | 0.1* | – | 0.2* | Further consideration neededa |
| 401040 | Sesame seeds | 0.1* | – | 0.2* | Further consideration neededa |
| 401050 | Sunflower seeds | 20 | 7 | 20 | Further consideration neededd |
| 401060 | Rapeseeds/canola seeds | 10 | 30 | 30 | Further consideration needede |
| 401070 | Soyabeans | 20 | 20 | 20 | Further consideration neededd |
| 401080 | Mustard seeds | 10 | – | 10 | Further consideration neededf |
| 401090 | Cotton seeds | 10 | 40 | 60 | Further consideration neededc |
| 401100 | Pumpkin seeds | 0.1* | – | 0.2* | Further consideration neededa |
| 401110 | Safflower seeds | 0.1* | – | 0.2* | Further consideration neededa |
| 401120 | Borage seeds | 0.1 | – | 10 | Further consideration neededa |
| 401130 | Gold of pleasure seeds | 0.1 | – | 0.2* | Further consideration neededa |
| 401140 | Hemp seeds | 0.1* | – | 0.2* | Further consideration neededa |
| 401150 | Castor beans | 0.1 | – | 0.2* | Further consideration neededa |
| 402010 | Olives for oil production | 1 | – | 30 | Further consideration neededa |
| 402020 | Oil palms kernels | 0.1 | – | 0.2* | Further consideration neededa |
| 402030 | Oil palms fruits | 0.1 | – | 0.2* | Further consideration neededa |
| 402040 | Kapok | 0.1 | – | 0.2* | Further consideration neededa |
| 500010 | Barley grains | 20 | 30 | 30 | Further consideration neededb |
| 500020 | Buckwheat and other pseudo‐cereal grains | 0.1* | 30 | 30 | Further consideration neededg |
| 500030 | Maize/corn grains | 1 | 5 | 3 | Further consideration neededc |
| 500040 | Common millet/proso millet grains | 0.1* | 30 | 30 | Further consideration needede |
| 500050 | Oat grains | 20 | 30 | 30 | Further consideration neededb |
| 500060 | Rice grains | 0.1* | – | 0.2* | Further consideration neededf |
| 500070 | Rye grains | 10 | 30 | 30 | Further consideration needede |
| 500080 | Sorghum grains | 20 | 30 | 30 | Further consideration needede |
| 500090 | Wheat grains | 10 | 30 | 30 | Further consideration needede |
| 610000 | Teas | 2 | – | 0.2* | Further consideration neededa |
| 620000 | Coffee beans | 0.1 | – | 0.2* | Further consideration neededa |
| 631000 | Herbal infusions from flowers | 2* | – | 0.2* | Further consideration neededa |
| 632000 | Herbal infusions from leaves and herbs | 2* | – | 0.2* | Further consideration neededa |
| 633000 | Herbal infusions from roots | 2* | – | 0.2* | Further consideration neededa |
| 650000 | Carobs/Saint John's breads | 0.1* | – | 0.2* | Further consideration neededa |
| 700000 | Hops | 0.1* | – | 0.2* | Further consideration neededa |
| 810000 | Seed spices | 0.1* | – | 0.2* | Further consideration neededa |
| 820000 | Fruit spices | 0.1* | – | 0.2* | Further consideration neededa |
| 830000 | Bark spices | 0.1* | – | 0.2* | Further consideration neededa |
| 840000 | Root and rhizome spices | 0.1* | – | 0.2* | Further consideration neededa |
| 850000 | Bud spices | 0.1* | – | 0.2* | Further consideration neededa |
| 860000 | Flower pistil spices | 0.1* | – | 0.2* | Further consideration neededa |
| 870000 | Aril spices | 0.1* | – | 0.2* | Further consideration neededa |
| 900010 | Sugar beet roots | 15 | 15 | 15 | Further consideration needede |
| 900020 | Sugar canes | 0.1* | 2 | 0.2* | Further consideration neededc |
| 900030 | Chicory roots | 0.1* | – | 0.2* | Further consideration neededa |
| 1011010 | Swine muscle | 0.05* | 0.05* | 0.2 | Further consideration neededc |
| 1011020 | Swine fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededc |
| 1011030 | Swine liver | 0.05* | 0.5 | 0.4 | Further consideration neededc |
| 1011040 | Swine kidney | 0.5 | 0.5 | 3 | Further consideration neededc |
| 1012010 | Bovine muscle | 0.05* | 0.05* | 0.2 | Further consideration neededc |
| 1012020 | Bovine fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededc |
| 1012030 | Bovine liver | 0.2 | 5 | 0.7 | Further consideration neededc |
| 1012040 | Bovine kidney | 2 | 5 | 7 | Further consideration neededc |
| 1013010 | Sheep muscle | 0.05* | 0.05* | 0.2 | Further consideration neededc |
| 1013020 | Sheep fat tissue | 0.05* | 0.05* | 0.3 | Further consideration neededc |
| 1013030 | Sheep liver | 0.05* | 5 | 0.9 | Further consideration neededc |
| 1013040 | Sheep kidney | 0.05* | 5 | 10 | Further consideration neededc |
| 1014010 | Goat muscle | 0.05* | 0.05* | 0.2 | Further consideration neededc |
| 1014020 | Goat fat tissue | 0.05* | 0.05* | 0.3 | Further consideration neededc |
| 1014030 | Goat liver | 0.05* | 5 | 0.9 | Further consideration neededc |
| 1014040 | Goat kidney | 0.05* | 5 | 10 | Further consideration neededc |
| 1015010 | Equine muscle | 0.05* | 0.05* | 0.2 | Further consideration neededc |
| 1015020 | Equine fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededc |
| 1015030 | Equine liver | 0.05* | 5 | 0.7 | Further consideration neededc |
| 1015040 | Equine kidney | 0.05* | 5 | 7 | Further consideration neededc |
| 1016010 | Poultry muscle | 0.05* | 0.05* | 0.2 | Further consideration neededc |
| 1016020 | Poultry fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededc |
| 1016030 | Poultry liver | 0.05* | 0.5 | 0.2* | Further consideration neededc |
| 1020010 | Cattle milk | 0.05* | 0.05 | 0.1* | Further consideration neededc |
| 1020020 | Sheep milk | 0.05* | 0.05 | 0.1* | Further consideration neededc |
| 1020030 | Goat milk | 0.05* | 0.05 | 0.1* | Further consideration neededc |
| 1020040 | Horse milk | 0.05* | 0.05 | 0.1* | Further consideration neededc |
| 1030000 | Birds eggs | 0.05* | 0.05* | 0.1* | Further consideration neededc |
| – | Other commodities of animal origin | – | – | Further consideration neededh | |
MRL: maximum residue level; CXL: codex maximum residue limit.
*Indicates that the MRL is set at the limit of quantification.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; no CXL is available (combination E‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; existing CXL is covered by the tentative MRL (combination E‐III in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; CXL is not compatible with EU residue definitions (combination E‐II in Appendix E).
GAP evaluated at EU level is not supported by data, but no risk to consumers was identified for the existing EU MRL; CXL is not compatible with EU residue definitions (combination C‐II in Appendix E).
MRL is derived from the existing CXL, which is not sufficiently supported by data but for which no risk to consumers is identified; GAP evaluated at EU level, which is also not fully supported by data, would lead to a lower tentative MRL (combination E‐V in Appendix E).
GAP evaluated at EU level is not supported by data, but no risk to consumers was identified for the existing EU MRL; no CXL is available (combination C‐I in Appendix E).
MRL is derived from the existing CXL, which is not sufficiently supported by data but for which no risk to consumers is identified; GAP evaluated at EU level is not supported by data, but the existing EU MRL is lower than the CXL (combination C‐V in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Tentative MRLs and existing EU MRLs need to be confirmed by the following data:
additional residue trials on strawberries, cane fruits, other small fruits and berries, potatoes, tropical roots and tuber vegetables, beetroots, celeriacs, horseradishes, Jerusalem artichokes, parsnips, parsley roots, radishes, salsifies, swedes, turnips, sweet potatoes, bulb vegetables, sweet peppers, cultivated fungi, okras, cucurbits with edible and inedible peel, brassica vegetables, leafy vegetables and fresh herbs, asparagus, leeks, celeries, cardoons, Florence fennels, rhubarbs, bamboo shoots, palm hearts, peanuts, poppy seeds, sesame seeds, sunflower seeds, soybeans, mustard seeds, pumpkin seeds, safflower seeds, borage seeds, gold of pleasure seeds, hemp seeds, castor beans, oil palm fruits, rice, maize, teas, herbal infusions and spices, hops and chicory roots (relevant for main and optional residue definition);
Fully validated analytical methods for the enforcement of glyphosate in complex matrices (relevant for the authorisations on hops, tea, coffee beans, carobs, spices and herbal infusions);
Confirmatory methods for N‐acetyl‐glyphosate (in high water and high fat content matrices and dry commodities) and for AMPA (in all matrices) (relevant for all commodities when considering the optional residue definition and for the authorisations on genetically modified crops currently on the market: sweet corn, cotton seeds, rapeseeds, maize, soybeans and sugar beets);
Summaries of the trials supporting the northern outdoor GAP for dry pulses (relevant for main and optional residue definition);
A confirmatory method for glyphosate in fat and liver and kidney as well as a confirmatory method for AMPA and N‐acetyl‐glyphosate in all animal matrices (relevant for main and optional residue definition).
It is highlighted that some of the MRLs derived result from a CXL or from a GAP in one climatic zone only, whereas other GAPs reported by the RMS were not fully supported by data. EFSA, therefore, identified the following data gaps which are not expected to impact on the validity of the MRLs derived but which might have an impact on national authorisations:
additional residue trials on bananas, table olives, carrots, tomatoes, aubergines, sweet corn, beans and peas (with pods), beans and peas (without pods), lentils (fresh), globe artichoke, dry pulses, rapeseeds, linseeds, olives for oil production, barley, oats, millet, sorghum, wheat, rye, buckwheat, sugar beets root and leaves, sugar cane, alfalfa, clover and grass forage, rapeseed (import tolerance for GOX), sugar beets (import tolerance for EPSPS) (relevant for main and optional residue definition);
GAP details and supporting residue trials for the currently authorised import tolerance on the following genetically modified crops: ESPSP soybeans and ESPSP maize (relevant for main and optional residue definition).
If the above‐reported data gaps are not addressed in the future, MSs are recommended to withdraw or modify the relevant authorisations at national level.
Minor deficiencies were also identified in the assessment, but these deficiencies are not expected to impact either on the validity of the MRLs derived or on the national authorisations. The following data are, therefore, considered desirable but not essential:
Studies investigating the storage stability of AMPA in high protein content matrices (relevant for the authorisations on dry pulses; relevant for main and optional residue definition);
One additional trial compliant with the southern outdoor GAP for cotton seeds (relevant for main and optional residue definition).
The RMS is also asked to verify if the northern GAP for potatoes considered in this review is reflecting the most critical use currently authorised.
It is also noted that for the northern uses on fresh legumes, for the southern uses on cassava roots, yams, arrowroots, Jerusalem artichokes, parsnips, parsley, radishes, spring onions, sweet peppers, okra, cucurbits with edible and inedible peel, sweet corn, Chinese cabbages, kales, leafy vegetables and fresh herbs (except lamb's lettuce, spinaches, grape leaves, watercress and witloof), stem vegetables (except globe artichokes) and for the indoor GAPs on bulb vegetables, tomatoes, peppers, aubergines, okra, cucurbits with edible and inedible peel, leafy vegetables and fresh herbs (except watercress) and stem vegetables, the reported PHI of 30 days seems to be inconsistent with the information available in the comment field of the GAP table (application done in‐between production period). Therefore, the confirmation that the soil application is done preplanting, presowing and post‐harvest, is still required from MSs authorising these GAPs. For the time being, EFSA considered the PHI as the most relevant parameter for assessing these GAPs.
When granting plant protection products containing glyphosate, MSs are recommended to implement proper risk mitigation measures, in order to avoid the spray drift and cross‐contamination in the primary crops, residues to occur in rotated cereals and possible uptake of AMPA by rotational crops.
EFSA emphasises that for most of the crops under assessment, a no‐residue situation is strictly dependent on the risk mitigation measures that MSs will put in place. For this reason, MSs are strongly recommended to implement an adequate monitoring programme allowing to verify the appropriateness of the risk mitigation in place.
Furthermore, analysis in cereals straw shows high residue levels in these matrices and experience with other substances has shown that cultivated fungi (e.g. champignons) may be ‘contaminated’ when cultivated on cereals straw used as substrate. Therefore, in order to avoid cross‐contamination from straw in cultivated fungi, MSs are recommended to implement proper risk mitigation measures (e.g. do not use straw from cereals treated with glyphosate as substrate for the cultivation of fungi), or to reconsider the more critical uses currently authorised on cereals.
It is highlighted that GAT‐modified crops are currently not present on the EU market. As a consequence, the inclusion of N‐acetyl‐glyphosate in the residue definitions for enforcement in plant and animal may be reconsidered and a separate residue definition comprising N‐acetyl glyphosate only could be defined. This would allow risk managers to set a lower LOQ for the enforcement in genetically modified crops and in animal commodities and to identify any possible misuses of genetically modified GAT crops by the analysis of the N‐acetyl‐glyphosate. It is noted that, in case risk managers wish to restrict the residue definition to glyphosate and AMPA only, this is not expected to have an impact on the risk assessment performed in the present review.
Furthermore, according to the information received in this review, glyphosate‐trimesium is currently not authorised for use and existing EU MRLs for TMS‐cation higher than the LOQ are in principle no longer required. Considering that the enforcement against potential illegal uses falls under the remit of risk managers, EFSA is not in a position to recommend whether the default MRL of 0.01 mg/kg, as defined by Regulation (EC) No 396/2005, should apply or whether the setting of a specific LOQ is necessary. Available data indicate that TMS is the most relevant indicator for enforcement against potential illegal uses in primary crops. The metabolism of the TMS‐cation in livestock and the analytical methods for the enforcement of this compound in plant and animal commodities were not assessed during the peer review for renewal and in the MRL review. Nevertheless, according to the information provided by the EURLs, during routine analyses, an LOQ of 0.01 mg/kg can be achieved for the enforcement of TMS‐cation in the four main matrices of plant origin.
Abbreviations
- a.i.
active ingredient
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CAC
Codex Alimentarius Commission
- CF
conversion factor for enforcement residue definition to risk assessment residue definition
- CXL
codex maximum residue limit
- DALA
days after last application
- DAR
draft assessment report
- DAT
days after treatment
- DM
dry matter
- DT90
period required for 90% dissipation (define method of estimation)
- EMS
evaluating Member State
- EPSPS
5‐enolpyruvylshikimate‐3‐phosphate (EPSP) synthase
- EURLs
European Union Reference Laboratories for Pesticide Residues (former CRLs)
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- GAT
glycine N‐phenylacetyltransferase
- GC‐MS
gas chromatography with mass spectrometry
- GMO
Genetically Modified Organism
- GOX
glucose oxidase
- HPLC‐MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- Mo
monitoring
- MRL
maximum residue level
- MS
Member States
- MS/MS
tandem mass spectrometry detector
- NEU
Northern European Union
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- PROFile
(EFSA) Pesticide Residues Overview File
- Rber
statistical calculation of the MRL by using a non‐parametric method
- Rmax
statistical calculation of the MRL by using a parametric method
- RA
risk assessment
- RAC
raw agricultural commodity
- RAR
renewal assessment report
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SCoPAFF
Standing Committee on Plants, Animals, Food and Feed (formerly: Standing Committee on the Food Chain and Animal Health; SCFCAH)
- SEU
southern European Union
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- TRR
total radioactive residue
- WHO
World Health Organization
Appendix A – Summary of authorised uses considered for the review of MRLs
A.1. Authorised uses on conventional crops
| Critical outdoor GAPs for Northern Europe | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conventional crops | ||||||||||||||||||||
| Crop | Region | Outdoor/indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Grapefruits | Citrus paradisi | NEU | Outdoor | SI | Weeds | sl | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | During the intensive growth of weeds | ||
| Oranges | Citrus sinensis | NEU | Outdoor | SI | Weeds | sl | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | During the intensive growth of weeds | ||
| Lemons | Citrus limon | NEU | Outdoor | SI | Weeds | sl | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | During the intensive growth of weeds | ||
| Mandarins | Citrus reticulata, syn: Citrus deliciosa | NEU | Outdoor | SI | Weeds | sl | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | During the intensive growth of weeds | ||
| Almonds | Amygdalus communis, syn: Prunus dulcis | NEU | Outdoor | SI | weeds | sl | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | During the intensive growth of weeds | ||
| Brazil nuts | Bertholletia excelsa | NEU | Outdoor | CZ | Weeds | SL | 360.0 | g/L | Soil treatment – spraying | 95 | 0 | 1 | 2 | 0.72 | 2.16 | kg a.i./ha | n.a. | Up to 2,160 g a.i./ha per season | ||
| Cashew nuts | Anacardium occidentale | NEU | Outdoor | CZ | Weeds | SL | 360.0 | g/L | Soil treatment – spraying | 95 | 0 | 1 | 2 | 0.72 | 2.16 | kg a.i./ha | n.a. | up to 2,160 g a.i./ha per season | ||
| Chestnuts | Castanea crenata; Castanea dentata; Castanea mollissima; Castanea sativa | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | EW | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | After emergence of weeds, spring bis summer | ||||||
| Coconuts | Cocos nucifera | NEU | Outdoor | CZ | Weeds | SL | 360.0 | g/L | Soil treatment – spraying | 95 | 0 | 1 | 2 | 0.72 | 2.16 | kg a.i./ha | n.a. | Up to 2,160 g a.i./ha per season | ||
| Hazelnuts | Corylus avellana | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | EW | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | After emergence of weeds, spring bis summer | ||||||
| Macadamias | Macadamia ternifolia, syn: Macadamia integrifolia; Macadamia tetraphylla | NEU | Outdoor | CZ | Weeds | SL | 360.0 | g/L | Soil treatment – spraying | 95 | 0 | 1 | 2 | 0.72 | 2.16 | kg a.i./ha | n.a. | Up to 2,160 g a.i./ha per season | ||
| Pecans | Carya illinoinensis | NEU | Outdoor | CZ | Weeds | SL | 360.0 | g/L | Soil treatment – spraying | 95 | 0 | 1 | 2 | 0.72 | 2.16 | kg a.i./ha | n.a. | Up to 2,160 g a.i./ha per season | ||
| Pine nut kernels | Pinus pinea | NEU | Outdoor | CZ | Weeds | SL | 360.0 | g/L | Soil treatment – spraying | 95 | 0 | 1 | 2 | 0.72 | 2.16 | kg a.i./ha | n.a. | Up to 2,160 g a.i./ha per season | ||
| Pistachios | Pistacia vera | NEU | Outdoor | CZ | Weeds | SL | 360.0 | g/L | Soil treatment – spraying | 95 | 0 | 1 | 2 | 0.72 | 2.16 | kg a.i./ha | n.a. | Up to 2,160 g a.i./ha per season | ||
| Walnuts | Juglans nigra; Juglans regia | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | EW | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | After emergence of weeds, spring bis summer | ||||||
| Apples | Malus domestica | NEU | Outdoor | SI | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | During the intensive growth of weeds | ||
| Pears | Pyrus communis | NEU | Outdoor | SI | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | During the intensive growth of weeds | ||
| Quinces | Cydonia oblonga | NEU | Outdoor | AT | Monocotyledonous and dicotyledonous weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 2 | 1.08 | 3.60 | kg a.i./ha | 35 | 2 applications as split application 1.8 kg a.i./ha | ||||
| Medlars | Mespilus germanica | NEU | Outdoor | AT | Monocotyledonous and dicotyledonous weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 2 | 1.08 | 3.60 | kg a.i./ha | 35 | 2 applications as split application max. 1.8 kg a.i./ha | ||||
| Loquats | Eriobotrya japonica | NEU | Outdoor | AT | Monocotyledonous and dicotyledonous weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 2 | 1.08 | 3.60 | kg a.i./ha | 35 | 2 applications as split application max. 1.8 kg a.i./ha | ||||
| Apricots | Armeniaca vulgaris, syn: Prunus armeniaca | NEU | Outdoor | SI | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | During the intensive growth of weeds | ||
| Cherries | Cerasus avium, syn: Prunus avium | NEU | Outdoor | SI | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | During the intensive growth of weeds | ||
| Peaches | Persica vulgaris, syn: Prunus persica | NEU | Outdoor | SI | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | During the intensive growth of weeds | ||
| Plums | Prunus domestica | NEU | Outdoor | SI | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 3 | 0.72 | 3.60 | g a.i./ha | 35 | During the intensive growth of weeds | ||
| Table grapes | Vitis vinifera | NEU | Outdoor | CZ | Weeds | SG | 720.0 | g/kg | Soil treatment – spraying | 1 | 2 | 7 | 14 | 0.72 | 2.88 | g a.i./ha | 14 | Up to 3,600 g a.i./ha per season | ||
| Wine grapes | Vitis vinifera | NEU | Outdoor | SI | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | During the intensive growth of weeds | ||
| Strawberries | Fragaria × ananassa | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Blackberries | Rubus sect. Rubus | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Dewberries | Rubus caesius | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Raspberries | Rubus idaeus | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Blueberries | Vaccinium angustifolium; Vaccinium corymbosum; Vaccinium formosum; Vaccinium virgatum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | EW | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | From 3rd year after planting, after emergence of weeds, spring bis summer | ||||||
| Cranberries | Vaccinium macrocarpon | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Currants | Ribes nigrum; Ribes rubrum | NEU | Outdoor | DE | monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Gooseberries | Ribes uva‐crispa | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Rose hips | Rosa canina; Rosa majalis; Rosa rugosa | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Mulberries | Morus alba; Morus nigra | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Azaroles | Crataegus azarolus | NEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.20 | kg a.i./ha | 21 | One application per year max. dose is expressed as ground area (as opposed to ‘treated area’). | ||||||||||
| Elderberries | Sambucus nigra | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Table olives | Olea europaea | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Potatoes | Solanum tuberosum subsp. tuberosum | NEU | Outdoor | NL | 360.0 | g/l | Foliar treatment – broadcast spraying | 97 | 1 | 0.72 | 2.16 | kg a.i./ha | 7 | Envision | ||||||
| Beetroots | Beta vulgaris var. vulgaris | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Carrots | Daucus carota subsp. sativus | NEU | Outdoor | DE | monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Celeriacs | Apium graveolens var. rapaceum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Horseradishes | Armoracia rusticana | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Jerusalem artichokes | Helianthus tuberosus | NEU | Outdoor | DE | monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Parsnips | Pastinaca sativa | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Parsley roots | Petroselinum crispum convar. radicosum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Radishes | Raphanus sativus Radish Group | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Salsifies | Tragopogon porrifolius | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Swedes | Brassica napus subsp. napobrassica | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | SG | 720.0 | g/kg | Soil treatment – spraying | 85 | 85 | 1 | 1.44 | kg a.i./ha | 7 | Except for seed production | ||||
| Turnips | Brassica rapa subsp. rapa | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | SG | 720.0 | g/kg | Soil treatment – spraying | 85 | 85 | 1 | 1.44 | kg a.i./ha | 7 | Except for seed production | ||||
| Garlic | Allium sativum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Onions | Allium cepa Common Onion Group | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Shallots | Allium cepa Aggregatum Group, syn: Allium ascalonicum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Spring onions | Allium cepa Common Onion Group; Allium fistulosum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Tomatoes | Lycopersicon esculentum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During grwoing season, wiping. Maximum application range per crop and year 3 | |||||||
| Sweet peppers | Capsicum annuum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During grwoing season, wiping. Maximum application range per crop and year 3 | |||||||
| Aubergines | Solanum melongena | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Cucumbers | Cucumis sativus | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Gherkins | Cucumis sativus | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Courgettes | Cucurbita pepo Zucchini Group | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Melons | Cucumis melo | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Pumpkins | Cucurbita maxima | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Watermelons | Citrullus vulgaris, syn: Citrullus lanatus | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Sweet corn | Zea mays convar. Saccharata | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds, self‐sown crops | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 1 | 1.08 | kg a.i./ha | n.a. | Up to 2 days before sowing | |||||
| Broccoli | Brassica oleracea var. italica | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Cauliflowers | Brassica oleracea var. botrytis | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Brussels sprouts | Brassica oleracea var. gemmifera | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Head cabbages | Brassica oleracea var. capitata | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Chinese cabbages | Brassica rapa subsp. pekinensis | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Kales | Brassica oleracea var. sabellica; Brassica oleracea var. viridis | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | SG | 720.0 | g/kg | Soil treatment – spraying | 85 | 85 | 1 | 1.44 | kg a.i./ha | 7 | except for seed production | ||||
| Kohlrabies | Brassica oleracea var. gongylodes | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Lamb's lettuces | Valerianella locusta | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Lettuces | Lactuca sativa | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Escaroles | Cichorium endivia var. latifolia | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Cresses | Lepidium sativum subsp. sativum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Land cresses | Barbarea verna | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Roman rocket | Eruca sativa | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Red mustards | Brassica juncea var. rugosa | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Baby leaf crops | Not specified | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Spinaches | Spinacia oleracea | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Purslanes | Portulaca oleracea | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Chards | Beta vulgaris var. flavescens | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Watercresses | Nasturtium officinale | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Witloofs | Cichorium intybus Foliosum group | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | Application refers to the field phase (root production). No application is performed during the forcing phase (witloof production). Wiping application. Maximum application range per crop and year 3 | |||||||
| Chervil | Anthriscus cerefolium | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Chives | Allium schoenoprasum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Celery leaves | Apium g raveolens var. secalinum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Parsley | Petroselinum crispum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Sage | Salvia officinalis | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Rosemary | Rosmarinus officinalis | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Thyme | Thymus vulgaris | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Basil | Ocimum basilicum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Laurel | Laurus nobilis | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Tarragon | Artemisia dracunculus | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Beans (with pods) | Phaseolus vulgaris | NEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Beans (without pods) | Phaseolus vulgaris | NEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Peas (with pods) | Pisum sativum | NEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Peas (without pods) | Pisum sativum | NEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Lentils (fresh) | Lens culinaris, syn: Lens esculenta | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | SL | 480.0 | g/L | Soil treatment – general (see also comment field) | 2 | 21 | 1.44 | kg a.i./ha | 21 | After emergence of weeds | |||||
| Asparagus | Asparagus officinalis | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Cardoons | Cynara cardunculus Cardoon group | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Celeries | Apium graveolens var. dulce | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Florence fennels | Foeniculum vulgare var. azoricum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Globe artichokes | Cynara cardunculus Globe artichoke group | NEU | Outdoor | DE | monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Leeks | Allium ampeloprasum ampeloprasum Leek Group, syn: Allium porrum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Rhubarbs | Rheum rhabarbarum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Bamboo shoots | Bambusa vulgaris; Phyllostachys edulis | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Palm hearts | Bactris gasipaes; Cocos nucifera; Daemonorops jenkinsiana; Euterpe edulis; Euterpe oleracea | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Cultivated fungi | Not specified | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Wild fungi | Not specified | NEU | Outdoor | AT | Stump shooting; forest | SL | 360.0 | g/L | Local treatment – dabbing or rubbing | 1 | 5.40 | kg a.i./hL | n.a. | label restrictions to exclude possible contamination from forest use | ||||||
| Beans (dry) | Phaseolus vulgaris | NEU | Outdoor | NL | 360.0 | Foliar treatment – broadcast spraying | 89 | 1 | 0.72 | 2.16 | kg a.i./ha | 7 | Envvision, Roundup+, Etna Next, Roundup Force, Roundup Evolution, Panic Free | |||||||
| Lentils (dry) | Lens culinaris, syn: Lens esculenta | NEU | Outdoor | NL | 360.0 | Foliar treatment – broadcast spraying | 89 | 1 | 0.72 | 2.16 | kg a.i./ha | 7 | ||||||||
| Peas (dry) | Pisum sativum | NEU | Outdoor | NL | 360.0 | Foliar treatment – broadcast spraying | 89 | 1 | 0.72 | 2.16 | kg a.i./ha | 7 | ||||||||
| Lupins (dry) | Lupinus albus subsp. albus; Lupinus angustifolius; Lupinus luteus; Lupinus mutabilis | NEU | Outdoor | NL | 360.0 | Foliar treatment – broadcast spraying | 89 | 1 | 0.72 | 2.16 | kg a.i./ha | 7 | ||||||||
| Linseeds | Linum usitatissimum | NEU | Outdoor | EE, CZ, LT, LV, DE, BE | Foliar treatment – broadcast spraying | 1 | 1.44 | g a.i./ha | 14 | before harvest | ||||||||||
| Peanuts | Arachis hypogaea | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Poppy seeds | Papaver somniferum subsp. somniferum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Sesame seeds | Sesamum indicum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Sunflower seeds | Helianthus annuus | NEU | Outdoor | HU | Desiccation | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 82 | 1 | 0.72 | 1.80 | kg a.i./ha | 14 | |||||
| Rapeseeds | Brassica napus subsp. napus | NEU | Outdoor | EE, CZ, LT, LV, DE, BE | Foliar treatment – broadcast spraying | 1 | 1.44 | g a.i./ha | 14 | Before harvest | ||||||||||
| Soyabeans | Glycine max | NEU | Outdoor | HU | Desiccation | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 82 | 1 | 1.08 | 1.80 | kg a.i./ha | 14 | |||||
| Mustard seeds | Brassica juncea; Brassica nigra; Sinapis alba | NEU | Outdoor | NL | 720.0 | Foliar treatment – broadcast spraying | 89 | 1 | 1.80 | 1.80 | kg a.i./ha | 7 | Roundup Record | |||||||
| Cotton seeds | Gossypium barbadense; Gossypium herbaceum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Pumpkin seeds | Cucurbita pepo Styrian Hulless Group | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Safflower seeds | Carthamus tinctorius | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Borage seeds | Borago officinalis | NEU | Outdoor | UK | 480 | g/L | Foliar treatment – broadcast spraying | 1 | 1.44 | kg a.i./ha | 14 | Dessicant use on corn gromwell seeds (EFSA, 2016) | ||||||||
| Gold of pleasure seeds | Camelina sativa | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Hemp seeds | Cannabis sativa subsp. Sativa; Cannabis sativa subsp. spontanea | NEU | Outdoor | UK | SL | 450.0 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 1 | Application methods – via rotary atomisers, weedwiper | |||||||
| Castor beans | Ricinus communis | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Olives for oil production | Olea europaea var. europaea | NEU | Outdoor | SI | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | |||
| Barley | Hordeum vulgare | NEU | Outdoor | FR | Foliar treatment – general (see also comment field) | 1 | 2.16 | kg a.i./ha | 7 | Dessicant use | ||||||||||
| Buckwheat | Fagopyrum esculentum | NEU | Outdoor | NL | 480.0 | Foliar treatment – broadcast spraying | 89 | 1 | 0.72 | 2.16 | kg a.i./ha | 7 | ||||||||
| Maize | Zea mays | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 89 | 1 | 1.80 | kg a.i./ha | 14 | Lodging cereal except seed and brewer's cereal | |||||
| Common millet | Panicum miliaceum | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 89 | 1 | 1.80 | kg a.i./ha | 14 | Lodging cereal except seed and brewer's cereal | |||||
| Oat | Avena sativa | NEU | Outdoor | NL | 480.0 | Foliar treatment – broadcast spraying | 89 | 1 | 0.72 | 2.16 | kg a.i./ha | 7 | ||||||||
| Rye | Secale cereale | NEU | Outdoor | NL | 480.0 | Foliar treatment – broadcast spraying | 89 | 1 | 0.72 | 2.16 | kg a.i./ha | 7 | ||||||||
| Sorghum | Sorghum bicolor | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 89 | 1 | 1.80 | kg a.i./ha | 14 | Lodging cereal except seed and brewer's cereal | |||||
| Wheat | Triticum aestivum | NEU | Outdoor | NL | 480.0 | Foliar treatment – broadcast spraying | 89 | 1 | 0.72 | 2.16 | kg a.i./ha | 7 | ||||||||
| Herbal infusions from flowers | Not specified | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Herbal infusions from leaves and herbs | Not specified | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Herbal infusions from roots | Not specified | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Hops | Humulus lupulus | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Seed spices | Not specified | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Fruit spices | Not specified | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Root and rhizome spices | Not specified | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | 7.2 | g/L | Soil treatment – general (see also comment field) | 1 | 1.44 | kg a.i./ha | 7 | During growing season, wiping. Maximum application range per crop and year 3 | |||||||
| Sugar beets | Beta vulgaris ssp. vulgaris var. altissima | NEU | Outdoor | DE | Monocotyledonous weeds, dicotyledonous weeds | SL | 360.0 | g/L | Soil treatment – spraying | 00 | 00 | 1 | 1.80 | g a.i./ha | n.a. | Up to 2 days before sowing | ||||
| Chicory roots | Cichorium intybus; Sativum group | NEU | Outdoor | FR | Soil treatment – general (see also comment field) | 0 | 0 | 1 | 2.52 | kg a.i./ha | n.a. | 1 application per year (in‐between crop production periods) | ||||||||
| Alfalfa (for forage) | Medicago sativa | NEU | Outdoor | FR | Soil treatment – general (see also comment field) | 0 | 0 | 1 | 2.52 | kg a.i./ha | n.a. | 1 application per year (in‐between crop production periods) | ||||||||
| Clover (for forage) | Trifolium spp. | NEU | Outdoor | FR | Soil treatment – general (see also comment field) | 0 | 0 | 1 | 2.52 | kg a.i./ha | n.a. | 1 application per year (in‐between crop production periods) | ||||||||
| Grass (for forage) | Not specified | NEU | Outdoor | SI | Weeds | SL | 360.0 | g/L | Foliar treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 0.72 | 2.52 | kg a.i./ha | 7 | Local or broadcast | ||
| Fodder beets | Beta vulgaris spp. vulgaris var. crassa | NEU | Outdoor | AT | Beet proliferation and cirsium arvense | SL | 360.0 | g/L | Local treatment – dabbing or rubbing | 1 | 11.80 | kg a.i./hL | 60 | |||||||
GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient.
| Critical outdoor GAPs for Southern Europe | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conventional crops | ||||||||||||||||||||
| Crop | Region | Outdoor/indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments (max. 250 characters) | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Grapefruits | Citrus paradisi | SEU | Outdoor | HR | Perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 1.44 | 3.60 | kg a.i./ha | 7 | In orchards older than 2 years (3 years after planting), BBCH not specified Spraying with sprayer | ||
| Oranges | Citrus sinensis | SEU | Outdoor | HR | Perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 1.44 | 3.60 | kg a.i./ha | 7 | In orchards older than 2 years (3 years after planting), BBCH not specified Spraying with sprayer | ||
| Lemons | Citrus limon | SEU | Outdoor | HR | Perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 1.44 | 3.60 | kg a.i./ha | 7 | In orchards older than 2 years (3 years after planting) BBCH not specified Spraying with sprayer | ||
| Limes | Citrus aurantiifolia | SEU | Outdoor | EL | Annual and perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 2 | 0.70 | 3.60 | kg a.i./ha | 7 | 1 application for Perennial weeds and 2 applications for annual weeds Uniform application of weed leaves with 200–400 L water/ha | ||||
| Mandarins | Citrus reticulata, syn: Citrus deliciosa | SEU | Outdoor | HR | Perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 1.44 | 3.60 | kg a.i./ha | 7 | In orchards older than 2 years (3 years after planting) BBCH not specified Spraying with sprayer | ||
| Almonds | Amygdalus communis, syn: Prunus dulcis | SEU | Outdoor | PT | SL | 360.0 | g/L | Soil treatment – spraying | 1 | 0.54 | 4.32 | kg a.i./ha | 7 | Isopropylammonium salt | ||||||
| Brazil nuts | Bertholletia excelsa | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 99 | 1 | 1 | 3.60 | kg a.i./ha | 7 | ||||||||
| Cashew nuts | Anacardium occidentale | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 99 | 1 | 1 | 3.60 | kg a.i./ha | 7 | ||||||||
| Chestnuts | Castanea crenata; Castanea dentata; Castanea mollissima; Castanea sativa | SEU | Outdoor | PT | SL | 360.0 | g/L | Soil treatment – spraying | 1 | 0.72 | 3.60 | kg a.i./ha | 7 | Isopropylammonium salt | ||||||
| Coconuts | Cocos nucifera | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 99 | 1 | 1 | 3.60 | kg a.i./ha | 7 | ||||||||
| Hazelnuts | Corylus avellana | SEU | Outdoor | PT | SL | 360.0 | g/L | Soil treatment – spraying | 1 | 0.54 | 4.32 | kg a.i./ha | 7 | Isopropylammonium salt | ||||||
| Macadamias | Macadamia ternifolia, syn: Macadamia integrifolia; Macadamia tetraphylla | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 99 | 1 | 1 | 3.60 | kg a.i./ha | 7 | ||||||||
| Pecans | Carya illinoinensis | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 99 | 1 | 1 | 3.60 | kg a.i./ha | 7 | ||||||||
| Pine nut kernels | Pinus pinea | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 99 | 1 | 1 | 3.60 | kg a.i./ha | 7 | ||||||||
| Pistachios | Pistacia vera | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 99 | 1 | 1 | 3.60 | kg a.i./ha | 7 | ||||||||
| Walnuts | Juglans nigra; Juglans regia | SEU | Outdoor | PT | SL | 360.0 | g/L | Soil treatment – spraying | 1 | 0.54 | 4.32 | kg a.i./ha | 7 | Isopropylammonium salt | ||||||
| Apples | Malus domestica | SEU | Outdoor | IT | Annual and Perennial broadleaved weeds and annual and perennial grasses | SG | 680.0 | g/kg | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 3 | 0.54 | 4.28 | kg a.i./ha | 7 | Do not spray the trunk, particularly if not hardened. Applications between plants with hydraulic sprayers, rotary atomisers or knapsacks. Max. dose/rate per year: 4.28 kg/ha a.i. | ||
| Pears | Pyrus communis | SEU | Outdoor | IT | annual and perennials weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 3 | 0.36 | 4.32 | kg a.i./ha | 7 |
Preplanting: overall spraying Post‐planting: overall spraying over weeds under trees and shielded spraying for selective treatments |
||||
| Quinces | Cydonia oblonga | SEU | Outdoor | EL | Annual weeds (broadleaved and grasses) perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | |||||
| Medlars | Mespilus germanica | SEU | Outdoor | EL | Annual weeds (broadleaved and grasses) perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | |||||
| Loquats | Eriobotrya japonica | SEU | Outdoor | EL | Annual weeds (broadleaved and grasses) perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 2 | 0.54 | 3.60 | kg a.i./ha | 7 | |||||
| Apricots | Armeniaca vulgaris, syn: Prunus armeniaca | SEU | Outdoor | HR | Perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2 | 1.44 | 3.60 | kg a.i./ha | 7 | In orchards older than 2 years (3 years after planting) BBCH not specified Spraying with sprayer | ||
| Cherries | Cerasus avium, syn: Prunus avium | SEU | Outdoor | IT | Annual and perennials weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 3 | 0.36 | 4.32 | kg a.i./ha | 7 | Preplanting: overall spraying Post‐planting: overall spraying over weeds under trees and shielded spraying for selective treatments | ||||
| Peaches | Persica vulgaris, syn: Prunus persica | SEU | Outdoor | IT | Annual and Perennial broadleaved weeds and annual and perennial grasses | SG | 680.0 | g/kg | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 3 | 0.54 | 4.28 | kg a.i./ha | 7 | Do not spray the trunk, particularly if not hardened. Stone fruit can show signs of phytotoxicity if the trunk is sprayed. Applications between plants with hydraulic sprayers, rotary atomisers or knapsacks. Max. dose/rate per year: 4.28 kg/ha a.i. | ||
| Plums | Prunus domestica | SEU | Outdoor | IT | Annual and Perennial broadleaved weeds and annual and perennial grasses | SG | 680.0 | g/kg | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 3 | 0.54 | 4.28 | kg a.i./ha | 7 | Do not spray the trunk, particularly if not hardened. Stone fruit can show signs of phytotoxicity if the trunk is sprayed. Applications between plants with hydraulic sprayers, rotary atomisers or knapsacks. Max. dose/rate per year: 4.28 kg/ha a.i. | ||
| Table grapes | Vitis vinifera | SEU | Outdoor | IT | Annual and Perennial broadleaved weeds and annual and perennial grasses | SG | 680.0 | g/kg | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 3 | 0.54 | 4.28 | kg a.i./ha | 7 | Do not spray the trunk, particularly if not hardened. Applications between plants with hydraulic sprayers, rotary atomisers or knapsacks. Max. dose/rate per year: 4.28 kg/ha a.i. | ||
| Wine grapes | Vitis vinifera | SEU | Outdoor | IT | Annual and Perennial broadleaved weeds and annual and perennial grasses | SG | 680.0 | g/kg | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 3 | 0.54 | 4.28 | kg a.i./ha | 7 | Do not spray the trunk, particularly if not hardened. Applications between plants with hydraulic sprayers, rotary atomisers or knapsacks. Max. dose/rate per year: 4.28 kg/ha a.i. | ||
| Strawberries | Fragaria × ananassa | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | |||||||||||
| Blackberries | Rubus sect. Rubus | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.20 | kg a.i./ha | 21 | One application per year max. dose is expressed as ground area (as opposed to ‘treated area’) | ||||||||||
| Dewberries | Rubus caesius | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.20 | kg a.i./ha | 21 | One application per year max. dose is expressed as ground area (as opposed to ‘treated area’) | ||||||||||
| Raspberries | Rubus idaeus | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.20 | kg a.i./ha | 21 | One application per year max. dose is expressed as ground area (as opposed to ‘treated area’) | ||||||||||
| Blueberries | Vaccinium angustifolium; Vaccinium corymbosum; Vaccinium formosum; Vaccinium virgatum | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.20 | kg a.i./ha | 21 | One application per year max. dose is expressed as ground area (as opposed to ‘treated area’) | ||||||||||
| Cranberries | Vaccinium macrocarpon | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.20 | kg a.i./ha | 21 | One application per year max. dose is expressed as ground area (as opposed to ‘treated area’) | ||||||||||
| Currants | Ribes nigrum; Ribes rubrum | SEU | Outdoor | EL | Idem as above | 360.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 2 | 2160.00 | g a.i./ha | 7 | ||||||
| Gooseberries | Ribes uva‐crispa | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.20 | kg a.i./ha | 21 | One application per year max. dose is expressed as ground area (as opposed to ‘treated area’) | ||||||||||
| Rose hips | Rosa canina; Rosa majalis; Rosa rugosa | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.20 | kg a.i./ha | 21 | One application per year max. dose is expressed as ground area (as opposed to ‘treated area’) | ||||||||||
| Mulberries | Morus alba; Morus nigra | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.20 | kg a.i./ha | 21 | One application per year max. dose is expressed as ground area (as opposed to ‘treated area’) | ||||||||||
| Azaroles | Crataegus azarolus | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.20 | kg a.i./ha | 21 | One application per year max. dose is expressed as ground area (as opposed to ‘treated area’) | ||||||||||
| Elderberries | Sambucus nigra | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.20 | kg a.i./ha | 21 | One application per year max. dose is expressed as ground area (as opposed to ‘treated area’) | ||||||||||
| Figs | Ficus carica | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.20 | kg a.i./ha | 21 | One application per year max. dose is expressed as ground area (as opposed to ‘treated area’) | ||||||||||
| Table olives | Olea europaea | SEU | Outdoor | EL | Perenial weeds | SL | 36.0 | % (v/v) | Soil treatment – spraying | 1 | 1.80 | 2.70 | kg a.i./ha | 7 | To be harvested only from the tree | |||||
| Kumquats | Fortunella japonica; Fortunella margarita | SEU | Outdoor | ES | Weeds | Soil treatment – general (see also comment field) | 1 | 2 | 1.90 | kg a.i./ha | 7 | |||||||||
| Kaki | Diospyros kaki | SEU | Outdoor | ES | Weeds | Soil treatment – general (see also comment field) | 1 | 2 | 1.90 | kg a.i./ha | 7 | |||||||||
| Kiwi fruits | Actinidia deliciosa; Actinidia chinensis | SEU | Outdoor | ES | Weeds | Soil treatment – general (see also comment field) | 1 | 2 | 1.90 | kg a.i./ha | 7 | |||||||||
| Litchis | Litchi chinensis | SEU | Outdoor | ES | Weeds | Soil treatment – general (see also comment field) | 1 | 2 | 1.90 | kg a.i./ha | 7 | |||||||||
| Passionfruits | Passiflora edulis, syn: Passiflora laurifolia | SEU | Outdoor | ES | Weeds | Soil treatment – general (see also comment field) | 1 | 2 | 1.90 | kg a.i./ha | 7 | |||||||||
| Avocados | Persea americana | SEU | Outdoor | ES | Weeds | Soil treatment – general (see also comment field) | 1 | 2 | 1.90 | kg a.i./ha | 7 | |||||||||
| Bananas | Musa acuminata; Musa balbisiana; Musa acuminata × Musa balbisiana | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 99 | 1 | 2 | 3.60 | kg a.i./ha | 1 | Application directed to soil | |||||||
| Mangoes | Mangifera indica | SEU | Outdoor | ES | Weeds | Soil treatment – general (see also comment field) | 1 | 2 | 1.90 | kg a.i./ha | 7 | |||||||||
| Papayas | Carica papaya | SEU | Outdoor | ES | Weeds | Soil treatment – general (see also comment field) | 1 | 2 | 1.90 | kg a.i./ha | 7 | |||||||||
| Granate apples | Punica granatum | SEU | Outdoor | ES | Weeds | Soil treatment – general (see also comment field) | 1 | 2 | 1.90 | kg a.i./ha | 7 | |||||||||
| Cherimoyas | Annona cherimola | SEU | Outdoor | ES | Weeds | Soil treatment – general (see also comment field) | 1 | 2 | 1.90 | kg a.i./ha | 7 | |||||||||
| Potatoes | Solanum tuberosum subsp. tuberosum | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Local treatment – dabbing or rubbing | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | 21 | ||||
| Cassava roots | Manihot esculenta | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Sweet potatoes | Ipomoea batatas | SEU | Outdoor | IT | Annual and perennials weeds | SL | 0.0 | g/L | Soil treatment – general (see also comment field) | 9 | 1 | 1 | 0.36 | 4.32 | kg a.i./ha | n.a. | Post‐planting (within 3 days) – Pre‐emergence | |||
| Yams | Dioscorea spp. | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Arrowroots | Maranta arundinacea | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Beetroots | Beta vulgaris var. vulgaris | SEU | Outdoor | IT | Weeds | SC | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, trasplanting or after harvest at the end of the crop cultivation | ||||
| Carrots | Daucus carota subsp. sativus | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Local treatment – dabbing or rubbing | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | 21 | ||||
| Celeriacs | Apium graveolens var. rapaceum | SEU | Outdoor | IT | Weeds | SC | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, trasplanting or after harvest at the end of the crop cultivation | ||||
| Horseradishes | Armoracia rusticana | SEU | Outdoor | IT | weeds | SC | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, trasplanting or after harvest at the end of the crop cultivation | ||||
| Jerusalem artichokes | Helianthus tuberosus | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Parsnips | Pastinaca sativa | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Parsley roots | Petroselinum crispum convar. radicosum | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Radishes | Raphanus sativus Radish Group | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Salsifies | Tragopogon porrifolius | SEU | Outdoor | IT | Weeds | SC | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, trasplanting or after harvest at the end of the crop cultivation | ||||
| Swedes | Brassica napus subsp. napobrassica | SEU | Outdoor | IT | Annual and perennial broadleaved weeds and annual and perennial grasses | SL | 450.0 | g/L | Soil treatment – general (see also comment field) | n.a. | 0 | 1 | 3 | 0.54 | 4.32 | kg a.i./ha | n.a. | Fields for sowing or planting or after harvesting. Sowing and planting must be conducted at least 48 h following treatment. Max. dose/rate per year: 4.32 kg/ha a.i. | ||
| Turnips | Brassica rapa subsp. rapa | SEU | Outdoor | IT | Weeds | SC | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, trasplanting or after harvest at the end of the crop cultivation | ||||
| Garlic | Allium sativum | SEU | Outdoor | IT | Weeds | SC | 480.0 | g/L | Soil treatment – spraying | 9 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before crop emergence | ||||
| Onions | Allium cepa Common Onion Group | SEU | Outdoor | IT | Weeds | SC | 480.0 | g/L | Soil treatment – spraying | 9 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before crop emergence | ||||
| Shallots | Allium cepa Aggregatum Group, syn: Allium ascalonicum | SEU | Outdoor | IT | Weeds | SC | 480.0 | g/L | Soil treatment – spraying | 9 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before crop emergence | ||||
| Spring onions | Allium cepa Common Onion Group; Allium fistulosum | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Tomatoes | Lycopersicon esculentum | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Local treatment – dabbing or rubbing | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | 21 | ||||
| Sweet peppers | Capsicum annuum | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Aubergines | Solanum melongena | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Local treatment – dabbing or rubbing | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | 21 | ||||
| Okra | Abelmoschus esculentus | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Cucumbers | Cucumis sativus | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Gherkins | Cucumis sativus | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Courgettes | Cucurbita pepo Zucchini Group | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Melons | Cucumis melo | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Pumpkins | Cucurbita maxima | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Watermelons | Citrullus vulgaris, syn: Citrullus lanatus | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Sweet corn | Zea mays convar. Saccharata | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Broccoli | Brassica oleracea var. italica | SEU | Outdoor | IT | Annual and perennial broadleaved weeds and annual and perennial grasses | SL | 450.0 | g/L | Soil treatment – general (see also comment field) | n.a. | 0 | 1 | 3 | 0.54 | 4.32 | kg a.i./ha | n.a. | Fields for sowing or planting or after harvesting. Sowing and planting must be conducted at least 48 h following treatment. Max. dose/rate per year: 4.32 kg/ha a.i. | ||
| Cauliflowers | Brassica oleracea var. botrytis | SEU | Outdoor | IT | Annual and perennial broadleaved weeds and annual and perennial grasses | SL | 450.0 | g/L | Soil treatment – general (see also comment field) | n.a. | 0 | 1 | 3 | 0.54 | 4.32 | kg a.i./ha | n.a. | Fields for sowing or planting or after harvesting. Sowing and planting must be conducted at least 48 h following treatment. Max. dose/rate per year: 4.32 kg/ha a.i. | ||
| Brussels sprouts | Brassica oleracea var. gemmifera | SEU | Outdoor | IT | Annual and perennial broadleaved weeds and annual and perennial grasses | SL | 450.0 | g/L | Soil treatment – general (see also comment field) | n.a. | 0 | 1 | 3 | 0.54 | 4.32 | kg a.i./ha | n.a. | Fields for sowing or planting or after harvesting. Sowing and planting must be conducted at least 48 h following treatment. Max. dose/rate per year: 4.32 kg/ha a.i. | ||
| Head cabbages | Brassica oleracea var. capitata | SEU | Outdoor | IT | Annual and perennial broadleaved weeds and annual and perennial grasses | SL | 450.0 | g/L | Soil treatment – general (see also comment field) | n.a. | 0 | 1 | 3 | 0.54 | 4.32 | kg a.i./ha | n.a. | Fields for sowing or planting or after harvesting. Sowing and planting must be conducted at least 48 h following treatment. Max. dose/rate per year: 4.32 kg/ha a.i. | ||
| Chinese cabbages | Brassica rapa subsp. pekinensis | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Kales | Brassica oleracea var. sabellica; Brassica oleracea var. viridis | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Kohlrabies | Brassica oleracea var. gongylodes | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | n.a. | Treatments before or after cultivation | ||
| Lamb's lettuces | Valerianella locusta | SEU | Outdoor | IT | Annual and perennial broadleaved weeds and annual and perennial grasses | SL | 450.0 | g/L | Soil treatment – general (see also comment field) | n.a. | 0 | 1 | 3 | 0.54 | 4.32 | kg a.i./ha | n.a. | Fields for sowing or planting or after harvesting. Sowing and planting must be conducted at least 48 h following treatment. Max. dose/rate per year: 4.32 kg/ha a.i. | ||
| Lettuces | Lactuca sativa | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Escaroles | Cichorium endivia var. latifolia | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Cresses | Lepidium sativum subsp. sativum | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Land cresses | Barbarea verna | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Roman rocket | Eruca sativa | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Red mustards | Brassica juncea var. rugosa | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Baby leaf crops | Not specified | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Spinaches | Spinacia oleracea | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 1 | 1 | 0.72 | 4.30 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, trasplanting or after harvest at the end of the crop cultivation | |||
| Purslanes | Portulaca oleracea | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Chards | Beta vulgaris var. flavescens | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Grape leaves | Vitis vinifera | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 3.60 | kg a.i./ha | n.a. | |||||||||
| Watercresses | Nasturtium officinale | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 0 | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | n.a. | Treatments before or after cultivation | |
| Witloofs | Cichorium intybus Foliosum group | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | n.a. | Treatments performed before or after cultivation. Applications refer to the field phase (root production). No application is performed during the forcing phase (witloof production). | ||
| Chervil | Anthriscus cerefolium | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Chives | Allium schoenoprasum | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Celery leaves | Apium g raveolens var. secalinum | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Parsley | Petroselinum crispum | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Sage | Salvia officinalis | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Rosemary | Rosmarinus officinalis | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Thyme | Thymus vulgaris | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Basil | Ocimum basilicum | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Laurel | Laurus nobilis | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Tarragon | Artemisia dracunculus | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Beans (with pods) | Phaseolus vulgaris | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Local treatment – dabbing or rubbing | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | 21 | Broad bean (Vicia faba). Not present in the available choices | |||
| Beans (without pods) | Phaseolus vulgaris | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Local treatment – dabbing or rubbing | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | 21 | Broad bean (Vicia faba). Not present in the available choices | |||
| Peas (with pods) | Pisum sativum | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Local treatment – dabbing or rubbing | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | 21 | ||||
| Peas (without pods) | Pisum sativum | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Local treatment – dabbing or rubbing | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | 21 | Broad bean (Vicia faba). Not present in the available choices | |||
| Lentils (fresh) | Lens culinaris, syn: Lens esculenta | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Local treatment – dabbing or rubbing | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | 21 | Broad bean (Vicia faba). Not present in the available choices | |||
| Asparagus | Asparagus officinalis | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Cardoons | Cynara cardunculus Cardoon group | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Celeries | Apium graveolens var. dulce | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Florence fennels | Foeniculum vulgare var. azoricum | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Globe artichokes | Cynara cardunculus Globe artichoke group | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Local treatment – dabbing or rubbing | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | 21 | ||||
| Leeks | Allium ampeloprasum ampeloprasum Leek Group, syn: Allium porrum | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Rhubarbs | Rheum rhabarbarum | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Bamboo shoots | Bambusa vulgaris; Phyllostachys edulis | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Palm hearts | Bactris gasipaes; Cocos nucifera; Daemonorops jenkinsiana; Euterpe edulis; Euterpe oleracea | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Cultivated fungi | Not specified | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 3.60 | kg a.i./ha | n.a. | |||||||||
| Wild fungi | Not specified | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 3.60 | kg a.i./ha | n.a. | |||||||||
| Beans (dry) | Phaseolus vulgaris | SEU | Outdoor | HR | Perennial weeds | SL | 360.0 | g/L | Foliar treatment – general (see also comment field) | n.a. | n.a. | 1 | 1.44 | 1.44 | kg a.i./ha | 7 |
Field bean Beans (MRL code number 0300010 SANCO 600/2010) Preharvest BBCH not specified Spraying with sprayer |
|||
| Lentils (dry) | Lens culinaris, syn: Lens esculenta | SEU | Outdoor | IT | Annual and perennial broadleaved weeds and annual and perennial grasses | SL | 450.0 | g/L | Soil treatment – spraying | 0 | 0 | 1 | 3 | 0.54 | 4.32 | kg a.i./ha | n.a. | Fields for sowing or planting or after harvesting. Sowing and planting must be conducted at least 48 h following treatment. Max. dose/rate per year: 4.32 kg/ha a.i. | ||
| Peas (dry) | Pisum sativum | SEU | Outdoor | HR | Perennial weeds | SL | 360.0 | g/L | Foliar treatment – general (see also comment field) | n.a. | n.a. | 1 | 1.44 | 1.44 | kg a.i./ha | 7 |
Combining peas is the crop Peas (MRL code number 0300030 SANCO 600/2010) Preharvest BBCH not specified Spraying with sprayer |
|||
| Lupins (dry) | Lupinus albus subsp. albus; Lupinus angustifolius; Lupinus luteus; Lupinus mutabilis | SEU | Outdoor | IT | Annual and perennial broadleaved weeds and annual and perennial grasses | SL | 450.0 | g/L | Soil treatment – spraying | 0 | 0 | 1 | 3 | 0.54 | 4.32 | kg a.i./ha | n.a. | Fields for sowing or planting or after harvesting. Sowing and planting must be conducted at least 48 h following treatment. Max. dose/rate per year: 4.32 kg/ha a.i. | ||
| Linseeds | Linum usitatissimum | SEU | Outdoor | HR | Perennial weeds | SL | 360.0 | g/L | Foliar treatment – general (see also comment field) | n.a. | n.a. | 1 | 1.44 | 1.44 | kg a.i./ha | 14 |
Preharvest BBCH not specified Spraying with sprayer |
|||
| Peanuts | Arachis hypogaea | SEU | Outdoor | IT | Weeds | SC | 360.0 | g/L | Soil treatment – spraying | 0 | 0 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, transplanting or after harvest at the end of the crop cultivation | |||
| Poppy seeds | Papaver somniferum subsp. somniferum | SEU | Outdoor | IT | Annual and perennials weeds | SL | 0.0 | g/L | Soil treatment – general (see also comment field) | 0 | 9 | 1 | 1 | 0.36 | 4.32 | kg a.i./ha | n.a. | Post‐planting (within 3 days) – Pre‐emergence | ||
| Sesame seeds | Sesamum indicum | SEU | Outdoor | IT | Weeds | SC | 360.0 | g/L | Soil treatment – spraying | 0 | 0 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, transplanting or after harvest at the end of the crop cultivation | |||
| Sunflower seeds | Helianthus annuus | SEU | Outdoor | HR | Desiccation | SL | 360.0 | g/L | Foliar treatment – general (see also comment field) | n.a. | n.a. | 1 | 0.72 | 1.80 | kg a.i./ha | 14 |
BBCH not specified Spraying with sprayer |
|||
| Rapeseeds | Brassica napus subsp. napus | SEU | Outdoor | HR | Perennial weeds | SL | 360.0 | g/L | Foliar treatment – general (see also comment field) | n.a. | n.a. | 1 | 1.44 | 1.44 | kg a.i./ha | 14 |
Preharvest BBCH not specified Spraying with sprayer |
|||
| Soyabeans | Glycine max | SEU | Outdoor | HR | Desiccation | SL | 360.0 | g/L | Foliar treatment – general (see also comment field) | n.a. | n.a. | 1 | 1.08 | 1.80 | kg a.i./ha | 14 |
BBCH not specified Spraying with sprayer |
|||
| Mustard seeds | Brassica juncea; Brassica nigra; Sinapis alba | SEU | Outdoor | HR | Perennial weeds | SL | 360.0 | g/L | Foliar treatment – general (see also comment field) | n.a. | n.a. | 1 | 1.44 | 1.44 | kg a.i./ha | 8 |
Preharvest BBCH not specified Spraying with sprayer |
|||
| Cotton seeds | Gossypium barbadense; Gossypium herbaceum | SEU | Outdoor | EL | Annual & perennial weeds | SL | 360.0 | g/L | Foliar treatment – general (see also comment field) | 1 | 0.70 | 1.80 | kg a.i./ha | 7 | This use exists also in the GAP of Glyphosate 540 SL Directed spraying | |||||
| Pumpkin seeds | Cucurbita pepo Styrian Hulless Group | SEU | Outdoor | IT | Weeds | SC | 360.0 | g/L | Soil treatment – spraying | 0 | 0 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, transplanting or after harvest at the end of the crop cultivation | |||
| Safflower seeds | Carthamus tinctorius | SEU | Outdoor | IT | Weeds | SC | 360.0 | g/L | Soil treatment – spraying | 0 | 0 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, trasplanting or after harvest at the end of the crop cultivation | |||
| Borage seeds | Borago officinalis | SEU | Outdoor | IT | Weeds | SC | 360.0 | g/L | Soil treatment – spraying | 0 | 0 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, trasplanting or after harvest at the end of the crop cultivation | |||
| Gold of pleasure seeds | Camelina sativa | SEU | Outdoor | IT | Weeds | SC | 360.0 | g/L | Soil treatment – spraying | 0 | 0 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, trasplanting or after harvest at the end of the crop cultivation | |||
| Hemp seeds | Cannabis sativa subsp. Sativa; Cannabis sativa subsp. spontanea | SEU | Outdoor | IT | Weeds | SC | 360.0 | g/L | Soil treatment – spraying | 0 | 0 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, trasplanting or after harvest at the end of the crop cultivation | |||
| Castor beans | Ricinus communis | SEU | Outdoor | IT | Weeds | SC | 360.0 | g/L | Soil treatment – spraying | 0 | 0 | 1 | 0.72 | 4.32 | kg a.i./ha | n.a. | Soil with emerged weeds, but before seeding, trasplanting or after harvest at the end of the crop cultivation | |||
| Olives for oil production | Olea europaea var. europaea | SEU | Outdoor | HR | Convolvulus arvensis | SL | 450.0 | g/L | Soil treatment – general (see also comment field) | n.a. | n.a. | 1 | 2.70 | 2.70 | g a.i./ha | 7 |
Spraying with sprayer BBCH not specified In orchards older than 3 years |
|||
| Oil palms kernels | Attalea maripa; Elaeis guineensis; Elaeis oleifera | SEU | Outdoor | IT | SL | 450.0 | g/L | Soil treatment – spraying | 1 | 1.80 | kg a.i./ha | 21 | ||||||||
| Oil palms fruits | Attalea maripa; Elaeis guineensis; Elaeis oleifera | SEU | Outdoor | IT | SL | 450.0 | g/L | Soil treatment – spraying | 1 | 1.80 | kg a.i./ha | 21 | ||||||||
| Kapok | Ceiba pentandra | SEU | Outdoor | IT | SL | 450.0 | g/L | Soil treatment – spraying | 1 | 1.80 | kg a.i./ha | 21 | ||||||||
| Barley | Hordeum vulgare | SEU | Outdoor | HR | Perennial broadleaf weeds | SL | 360.0 | g/L | Foliar treatment – general (see also comment field) | n.a. | n.a. | 1 | 1.44 | 2.16 | kg a.i./ha | 7 | Spraying with sprayer BBCH not specified preharvest | |||
| Buckwheat | Fagopyrum esculentum | SEU | Outdoor | ES | Foliar treatment – broadcast spraying | 1 | 2.16 | kg a.i./ha | 7 | Apply before harvesting to dry the crop | ||||||||||
| Maize | Zea mays | SEU | Outdoor | ES | Foliar treatment – broadcast spraying | 1 | 2.16 | kg a.i./ha | 7 | Apply before harvesting to dry the crop | ||||||||||
| Common millet | Panicum miliaceum | SEU | Outdoor | ES | Foliar treatment – broadcast spraying | 1 | 2.16 | kg a.i./ha | 7 | Apply before harvesting to dry the crop | ||||||||||
| Oat | Avena sativa | SEU | Outdoor | ES | Foliar treatment – broadcast spraying | 1 | 2.16 | kg a.i./ha | 7 | Apply before harvesting to dry the crop | ||||||||||
| Rice | Oryza sativa | SEU | Outdoor | ES | Foliar treatment – broadcast spraying | 1 | 2.16 | kg a.i./ha | 7 | Apply before harvesting to dry the crop | ||||||||||
| Rye | Secale cereale | SEU | Outdoor | ES | Foliar treatment – broadcast spraying | 1 | 2.16 | kg a.i./ha | 7 | Apply before harvesting to dry the crop | ||||||||||
| Sorghum | Sorghum bicolor | SEU | Outdoor | ES | Foliar treatment – broadcast spraying | 1 | 2.16 | kg a.i./ha | 7 | Apply before harvesting to dry the crop | ||||||||||
| Wheat | Triticum aestivum | SEU | Outdoor | ES | Foliar treatment – broadcast spraying | 1 | 2.16 | kg a.i./ha | 7 | Apply before harvesting to dry the crop | ||||||||||
| Teas | Camellia sinensis | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Coffee beans | Coffea arabica; Coffea canephora, syn: Coffea robusta; Coffea liberica | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Herbal infusions from flowers | Not specified | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Herbal infusions from leaves and herbs | Not specified | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Herbal infusions from roots | Not specified | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Carobs | Ceratonia siliqua | SEU | Outdoor | PT | SL | 360.0 | g/L | Soil treatment – spraying | 1 | 0.72 | 3.60 | kg a.i./ha | 28 | Isopropylammonium salt | ||||||
| Hops | Humulus lupulus | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 2 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Seed spices | Not specified | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 2 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Fruit spices | Not specified | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Bark spices | Not specified | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Root and rhizome spices | Not specified | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Bud spices | Not specified | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Flower pistil spices | Not specified | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 2 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Aril spices | Not specified | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Sugar beets | Beta vulgaris ssp. vulgaris var. altissima | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | 21 | ||||
| Sugar canes | Saccharum officinarum | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 2 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Chicory roots | Cichorium intybus; Sativum group | SEU | Outdoor | ES | Soil treatment – spraying | 0 | 0 | 2 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Alfalfa (for forage) | Medicago sativa | SEU | Outdoor | IT | Weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 2 | 60 | 0.72 | 4.30 | kg a.i./ha | 21 | ||||
| Clover (for forage) | Trifolium spp. | SEU | Outdoor | FR | Soil treatment – general (see also comment field) | 0 | 0 | 1 | 2.52 | kg a.i./ha | n.a. | 1 application per year (in‐between crop production periods) | ||||||||
| Grass (for forage) | Not specified | SEU | Outdoor | IT | Annual and Perennial broadleaved weeds and annual and perennial grasses | SL | 450.0 | g/L | Soil treatment – general (see also comment field) | 0 | 0 | 1 | 3 | 0.54 | 4.32 | kg a.i./ha | n.a. | Fields for sowing or planting or after harvesting. Sowing and planting must be conducted at least 48 h following treatment. Max. dose/rate per year: 4.32 kg/ha a.i. | ||
| Fodder beets | Beta vulgaris spp. vulgaris var. crassa | SEU | Outdoor | HR | Weeds (grass and broadleaf) | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 0 | 1 | 1.08 | 1.08 | kg a.i./ha | n.a. | Before sowing BBCH not specified Spraying with sprayer | |||
GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient.
| Critical indoor GAPs for Northern and Southern Europe (including post‐harvest treatments) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conventional crops | ||||||||||||||||||||
| Crop | Region | Outdoor/indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments (max. 250 characters) | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Strawberries | Fragaria × ananassa | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | |||||||||||
| Blackberries | Rubus sect. Rubus | NEU/SEU | Indoor | ES | Soil treatment – spraying | 0 | 0 | 2 | 3.60 | g a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Dewberries | Rubus caesius | NEU/SEU | Indoor | ES | Soil treatment – spraying | 0 | 0 | 2 | 3.60 | g a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Raspberries | Rubus idaeus | NEU/SEU | Indoor | ES | Soil treatment – spraying | 0 | 0 | 2 | 3.60 | g a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Garlic | Allium sativum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Onions | Allium cepa Common Onion Group | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Shallots | Allium cepa Aggregatum Group, syn: Allium ascalonicum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Spring onions | Allium cepa Common Onion Group; Allium fistulosum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Tomatoes | Lycopersicon esculentum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Sweet peppers | Capsicum annuum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Aubergines | Solanum melongena | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Okra | Abelmoschus esculentus | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Cucumbers | Cucumis sativus | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Gherkins | Cucumis sativus | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Courgettes | Cucurbita pepo Zucchini Group | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Melons | Cucumis melo | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Pumpkins | Cucurbita maxima | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Watermelons | Citrullus vulgaris, syn: Citrullus lanatus | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Broccoli | Brassica oleracea var. italica | NEU/SEU | Indoor | IT | Emerged annual, biannual and perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 2 | 3.60 | g a.i./ha | n.a. | Application presowing or preplanting, or after harvest, end of cycle | |||||
| Cauliflowers | Brassica oleracea var. botrytis | NEU/SEU | Indoor | IT | Emerged annual, biannual and perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 2 | 3.60 | kg a.i./ha | n.a. | Application presowing or preplanting, or after harvest, end of cycle | |||||
| Brussels sprouts | Brassica oleracea var. gemmifera | NEU/SEU | Indoor | IT | Emerged annual, biannual and perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 2 | 3.60 | kg a.i./ha | n.a. | Application presowing or preplanting, or after harvest, end of cycle | |||||
| Head cabbages | Brassica oleracea var. capitata | NEU/SEU | Indoor | IT | Emerged annual, biannual and perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 2 | 3.60 | kg a.i./ha | n.a. | Application presowing or preplanting, or after harvest, end of cycle | |||||
| Chinese cabbages | Brassica rapa subsp. pekinensis | NEU/SEU | Indoor | IT | Emerged annual, biannual and perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 2 | 3.60 | kg a.i./ha | n.a. | Application presowing or preplanting, or after harvest, end of cycle | |||||
| Kales | Brassica oleracea var. sabellica; Brassica oleracea var. viridis | NEU/SEU | Indoor | ES | Soil treatment – spraying | 0 | 0 | 2 | 3.60 | kg a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | ||||||||
| Kohlrabies | Brassica oleracea var. gongylodes | NEU/SEU | Indoor | IT | Emerged annual, biannual and perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 2 | 3.60 | kg a.i./ha | n.a. | Application presowing or preplanting, or after harvest, end of cycle | |||||
| Lamb's lettuces | Valerianella locusta | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Lettuces | Lactuca sativa | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Escaroles | Cichorium endivia var. latifolia | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Cresses | Lepidium sativum subsp. sativum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Land cresses | Barbarea verna | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Roman rocket | Eruca sativa | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Red mustards | Brassica juncea var. rugosa | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Baby leaf crops | Not specified | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Spinaches | Spinacia oleracea | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Purslanes | Portulaca oleracea | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Chards | Beta vulgaris var. flavescens | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Watercresses | Nasturtium officinale | NEU/SEU | Indoor | IT | Emerged annual, biannual and perennial weeds | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 0 | 0 | 2 | 3.60 | g a.i./ha | n.a. | Application presowing or preplanting, or after harvest, end of cycle | ||||
| Chervil | Anthriscus cerefolium | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Chives | Allium schoenoprasum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Celery leaves | Apium g raveolens var. secalinum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Parsley | Petroselinum crispum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Sage | Salvia officinalis | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Rosemary | Rosmarinus officinalis | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Thyme | Thymus vulgaris | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Basil | Ocimum basilicum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Laurel | Laurus nobilis | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Tarragon | Artemisia dracunculus | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Beans (with pods) | Phaseolus vulgaris | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | g a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Beans (without pods) | Phaseolus vulgaris | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | g a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Peas (with pods) | Pisum sativum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | g a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Peas (without pods) | Pisum sativum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | g a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Lentils (fresh) | Lens culinaris, syn: Lens esculenta | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | g a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Asparagus | Asparagus officinalis | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Cardoons | Cynara cardunculus Cardoon group | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Celeries | Apium graveolens var. dulce | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Florence fennels | Foeniculum vulgare var. azoricum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Leeks | Allium ampeloprasum ampeloprasum Leek Group, syn: Allium porrum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Rhubarbs | Rheum rhabarbarum | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | g a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Bamboo shoots | Bambusa vulgaris; Phyllostachys edulis | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Palm hearts | Bactris gasipaes; Cocos nucifera; Daemonorops jenkinsiana; Euterpe edulis; Euterpe oleracea | NEU/SEU | Indoor | FR | Soil treatment – general (see also comment field) | 1 | 2.52 | kg a.i./ha | 30 | 1 application per year (in‐between crop production periods) | ||||||||||
| Beans (dry) | Phaseolus vulgaris | NEU/SEU | Indoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 2 | 3.60 | g a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | |||||||
| Lentils (dry) | Lens culinaris, syn: Lens esculenta | NEU/SEU | Indoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 2 | 3.60 | g a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | |||||||
| Peas (dry) | Pisum sativum | NEU/SEU | Indoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 2 | 3.60 | g a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | |||||||
| Lupins (dry) | Lupinus albus subsp. albus; Lupinus angustifolius; Lupinus luteus; Lupinus mutabilis | NEU/SEU | Indoor | ES | Soil treatment – spraying | 0 | 0 | 1 | 2 | 3.60 | g a.i./ha | n.a. | Treatments only in presowing/preplanting of crop | |||||||
GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient.
| Critical GAPs for import tolerances (non‐European indoor, outdoor or post‐harvest treatments) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conventional crops | ||||||||||||||||||||
| Crop | Region | Outdoor/indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments (max. 250 characters) | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Bananas | Musa acuminata; Musa balbisiana; Musa acuminata × Musa balbisiana | non‐EU | Outdoor | US | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 1 | 0.18 | 4.30 | kg a.i./ha | 30 | |||||||
| Beans (dry) | Phaseolus vulgaris | non‐EU | Outdoor | US | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 1 | 0.90 | 2.16 | kg a.i./ha | 7 | ||||
| Lentils (dry) | Lens culinaris, syn: Lens esculenta | non‐EU | Outdoor | US | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 1 | 0.90 | kg a.i./ha | 7 | |||||
| Peas (dry) | Pisum sativum | non‐EU | Outdoor | US | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 1 | 0.90 | 2.16 | kg a.i./ha | 7 | ||||
| Sunflower seeds | Helianthus annuus | non‐EU | Outdoor | US | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 0.72 | 1.80 | kg a.i./ha | 21 | |||||
| Soyabeans | Glycine max | non‐EU | Outdoor | US | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 1 | 4.20 | kg a.i./ha | 7 | |||||
| Barley | Hordeum vulgare | non‐EU | Outdoor | US | SL | 360.0 | g/kg | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 1 | 0.54 | 2.16 | kg a.i./ha | 7 | ||||
| Buckwheat | Fagopyrum esculentum | non‐EU | Outdoor | US | SL | 360.0 | g/kg | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 1 | 0.54 | 2.16 | kg a.i./ha | 7 | ||||
| Maize | Zea mays | non‐EU | Outdoor | US | SL | 360.0 | g/kg | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 1 | 2.50 | kg a.i./ha | 7 | |||||
| Common millet | Panicum miliaceum | non‐EU | Outdoor | US | SL | 360.0 | g/kg | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 1 | 0.54 | 2.16 | kg a.i./ha | 7 | ||||
| Oat | Avena sativa | non‐EU | Outdoor | US | SL | 360.0 | g/kg | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 1 | 0.54 | 2.16 | kg a.i./ha | 7 | ||||
| Rye | Secale cereale | non‐EU | Outdoor | US | SL | 360.0 | g/kg | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 1 | 0.54 | 2.16 | kg a.i./ha | 7 | ||||
| Sorghum | Sorghum bicolor | non‐EU | Outdoor | US | SL | 360.0 | g/l | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 1 | 1.70 | kg a.i./ha | 7 | |||||
| Wheat | Triticum aestivum | non‐EU | Outdoor | US | SL | 360.0 | g/kg | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 1 | 0.54 | 2.16 | kg a.i./ha | 7 | ||||
| Teas | Camellia sinensis | non‐EU | Outdoor | US | SL | 360.0 | g/L | Soil treatment – general (see also comment field) | 3 | 3 | 0.90 | 2.30 | kg a.i./ha | |||||||
| Sugar canes | Saccharum officinarum | non‐EU | Outdoor | US | 360.0 | g/kg | Foliar treatment – broadcast spraying | 85 | 89 | 1 | 1 | 0.50 | 0.84 | kg a.i./ha | 21 | |||||
GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient.
A.2. Authorised uses on EPSPS genetically modified crops
| Critical GAPs for import tolerances (non‐European indoor, outdoor or post‐harvest treatments) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPSPS genetically modified crops | ||||||||||||||||||||
| Crop | Region | Outdoor/indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Sweet corn | Zea mays convar. Saccharata | non‐EU | Outdoor | US | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 9 | 18 | 1 | 3 | 10 | 0.63 | 1.70 | kg a.i./ha | 30 | |||
| Cotton seeds | Gossypium barbadense; Gossypium herbaceum | non‐EU | Outdoor | US | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 9 | 89 | 1 | 3 | 1.70 | kg a.i./ha | 7 | |||||
| Sugar beets | Beta vulgaris ssp. vulgaris var. altissima | non‐EU | Outdoor | US | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 9 | 39 | 1 | 4 | 10 | 0.90 | 1.30 | kg a.i./ha | 30 | |||
GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient.
A.3. Authorised uses on GOX genetically modified crops
| Critical GAPs for import tolerances (non‐European indoor, outdoor or post‐harvest treatments) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GOX genetically modified crops | ||||||||||||||||||||
| Crop | Region | Outdoor/ Indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Rapeseeds | Brassica napus subsp. napus | non‐EU | Outdoor | US | SL | 540.0 | g/L | Foliar treatment – broadcast spraying | 9 | 31 | 1 | 2 | 60 | 0.43 | 0.87 | kg a.i./ha | n.a. | Directions for winter canola varieties. One fall application up to 0.87 kg/ha, and one spring application up to 0.87 kg/ha up to bolting, for a combined post‐emergence total of 1.7 kg/ha | ||
GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient.
A.4. Uses on GAT genetically modified crops reported by MSs
| Critical GAPs for import tolerances (non‐European indoor, outdoor or post‐harvest treatments) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GAT genetically modified crops | ||||||||||||||||||||
| Crop | Region | Outdoor/indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Rapeseeds | Brassica napus subsp. napus | non‐EU | Outdoor | US | SL | 360.0 | g/L | Foliar treatment – broadcast spraying | 16 | 89 | 1 | 2 | 0.30 | 0.90 | kg a.i./100 kg | 7 | According to the information available to EFSA, GAT GM rapeseed is currently not authorised for placing on the market within the EU (currently under assessment in the framework of Regulation (EC) No 1829/2003) | |||
GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crops | Applications | Sampling (DAT) |
|---|---|---|---|---|
| Conventional crops | ||||
| Fruit crops | Mandarins | Soil or foliar, 1 × 2.24 kg/ha | 119 | |
| Hydroponic, 10 mg/L solution | 7, 14 | |||
| Foliar, 1 × 4 mg/leaf | 7–56 | |||
|
Almonds Walnuts Pecans |
Soil, 1 × 5.1 kg/ha | 112 | ||
| Foliar, 1 × 0.1 mg/leaf | 14, 35 | |||
| Apples | Soil, 1 × 3.4 kg/ha glyphosate or 1.7 kg/ha AMPA | 42, 84 | ||
| Trunk, 1 × 0.09 mg/plant | 8, 42 | |||
| Foliar, 1 × 0.005 mg/4–5 leaves | 7 to 70 | |||
| Grapes | Soil spraying, 8 kg/ha split in 2 applications (glyphosate‐trimesium) | 14, 365 | ||
| Foliar, 0.03 g/ha split in 2 applications | 14 | |||
| Soil drench, 1 × 8 kg/ha (glyphosate‐trimesium) | 7 | |||
| Soil, 1 × 3.4 kg/ha (glyphosate) or 1.7 kg/ha (AMPA) | 42, 84 | |||
| Trunk, 1 × 0.04 mg/plant | 42, 84 | |||
| Hydroponic, 5, 10, 20 or 40 mg/L solution | 10, 21, 42 | |||
| Foliar, 1 × 0.01, 0.06 or 0.12 mg/plant | 7 to 70 | |||
| Avocados | Onto the leaf, rate not reported | 10 | ||
| Into fruit peduncle, a 453000 cpm solution | Not reported | |||
| Root crops | Potatoes | Soil, 1 × 5.75 × 108 dpm | 9–128 | |
| Foliar, 1 × 0.1 mg/plant | 1–34 | |||
| Sugar beet | Soil, 1 × 4.5 kg/ha | 28, 49, 56 | ||
| Cereals/grass crops | Barley, Oat, Rice, Sorghum | Soil, 1 × 4.5 kg/ha | 28, 49, 56 | |
| Hydroponic, 0.183 mg/L solution | 7, 14, 28 | |||
| Maize, Wheat | Soil, 1 × 4.5 kg/ha glyphosate or 1.7 kg/ha AMPA | 28, 49, 56 | ||
| Hydroponic (solution or substrate), equivalent to 2.24 kg/ha | 4, 10, 18 | |||
| Hydroponic, 0.6–2.4 mg/L solutions | 6 to 28 | |||
| Wheat | Foliar, 1 × 6 kg/ha (glyphosate‐trimesium) | 7 | ||
| Maize |
Soil, 1 × 5.1 kg/ha (glyphosate‐trimesium) Study informative only |
33, 48, 154 | ||
| Rice | Soil, 1 × 2.5 kg/ha | 31, 47, 73, 122 | ||
| Pulses/oilseeds | Cotton, Soya bean | 1 × 4.5 kg/ha (glyphosate) or 1.7 kg/ha (AMPA) | 28, 49, 56 | |
| Hydroponic (solution or substrate), equivalent to 2.24 kg/ha | 4, 10, 18 | |||
| Hydroponic, 2.4 to 2.65 mg/L solutions | 6–28 | |||
| Soya bean | Soil drench, 1 × 8.4 kg/ha (glyphosate‐trimesium) | 31, 97 | ||
| Foliar, not reported. Study informative only | 0–14 | |||
| Soil, 1 × 4.35 kg/ha. Study informative only | Not reported | |||
|
Hydroponic, 4.4 mg/L solution Study informative only |
9 | |||
| Miscellaneous | Coffee | Soil, 1 × 4.5 kg/ha (glyphosate) or 4.5 kg/ha (AMPA) | 28, 49, 56 | |
| Hydroponic, 1.1, 3.6 or 11.1 mg/L solution | 21 | |||
| Stem application, 700 g solution | 35 | |||
| Foliar, 7.7 × 106 to 1.5× 107 dpm | 21 to 35 | |||
| Sugarcane | Soil, 1 × 11.2 kg/ha, preplanting | 195, 354 | ||
| Soil, 1 × 3.4 or 6.7 kg/ha, post‐planting | 0, 91, 83 | |||
| Foliar, 1 × 5.6 or 11.2 kg/ha, post‐emergence | 40, 42, 44, 47 | |||
| Pasture | Soil, 1 × 4.5 kg/ha. Study informative only | 42, 84, 126, 168 and 224 | ||
|
Preplanting weed spraying, 1.7 kg/ha Study informative only |
42, 84, 126 168 | |||
|
Foliar, 1.1 kg/ha Study informative only |
63, 105 and 161 | |||
| Foliar, 1.1 kg/ha. Study informative only | 7 | |||
| EPSPS & GOX tolerant crops | ||||
| Pulses/oilseeds | Oilseed rape (EPSPS and GOX) | Foliar, 1 × 0.455 kg/ha, BBCH 14 | 87 | |
| Foliar, 2 × 0.9 kg/ha, 14 and 22 days after planting | 79 | |||
| Soya bean (EPSPS) | Soil, 1× 5.4 kg/ha | 56, 84, 104 | ||
| Foliar, 1× 0.84 kg/ha (BBCH 23) | 35, 63, 83 | |||
| Foliar, 0.84 (BBCH 23) + 1.68 kg/ha (BBCH 51) | 13, 41, 61 | |||
| 4.2 kg/ha (presowing) + 1.26 kg/ha (BBCH 13) | 14 | |||
| 4.2 kg/ha (presowing) + 1.26 kg/ha (BBCH 13) + 1.26 kg/ha (BBCH 65) | 0, 60 | |||
| Cotton (EPSPS) | Foliar, 930 (BBCH 14) + 1260 g/ha, (BBCH 16) | 27, 158 | ||
| 2.5 kg/ha (pre‐emergence) + 2× 1.7 kg/ha (BBCH 15 and 19) + 0.84 (7 days preharvest) | 168 | |||
| Root | Sugar beet (EPSPS) | 1× 0.9 kg/ha (pre‐emergence) + 1.08 kg/ha (BBCH 19) | 160 | |
| Foliar, 1× 1.08 kg/ha (BBCH 14) + 1.08 kg/ha (BBCH 19) | 92 | |||
| Cereal | Maize (EPSPS and GOX) | Foliar, 1× 0.9 kg/ha (BBCH 16) + 0.8 kg/ha (BBCH 19) | 3, 49–53, 83 | |
| Maize (EPSPS) | 1× 4.2 kg/ha (after sowing) + 3× 0.84 kg/ha | 65, 96, 131 | ||
| GAT tolerant crops | ||||
| Crop groups | Crops | Applications | Sampling | |
| Pulses/oilseeds | Oilseed rape | 4.5 kg/ha (pre‐emergence) + 3× 1.9 kg/ha (BBCH 12 and 15 and 7 days preharvest) | At BBCH 69, 87, 89 (7 DALA) | |
| Soya bean | 3.4 kg/ha (pre‐emergence) + 1.5 (BBCH 61) + 2.4 (BBCH 65) + 0.9 kg/ha (14 days preharvest) | 36 DATsoil, 82 DAT2, 14 DALA | ||
| Cereal | Maize | 4.3 kg/ha (pre‐emergence) + 3× 1.1 kg/ha (at BBCH 31, 39 and 87) | 48 DATsoil, 59 DAT2, 7 DALA | |
| Sources: Germany (2015, 2017) | ||||
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) |
|---|---|---|---|---|
| Root/tuber crops | Beets | Soil before sowing soybeans or wheat (primary), 4.5 kg/ha | 120 | |
| Soil before sowing cabbages (primary), 4.5 kg/ha | 360 | |||
| Soil before sowing soybeans (primary), 2 × 4.5 kg/ha | 30 | |||
| Carrots | Foliar on rye grass, 4.2 kg/ha | 30, 120, 365 | ||
| Foliar on peas (primary), 4.5 kg/ha | 1–23 | |||
| Foliar on cabbages (primary), 4.5 kg/ha | 1–23 | |||
| Radishes | Bare soil, 6.5 kg a.s./ha | 30, 120, 365 | ||
| Foliar on soybean (primary), 4.4 kg/ha + bare soil 1.4 kg/ha + 0.75 kg ha (glyphosate‐trimesium) | 63, 308 | |||
| Foliar on soybean (primary), 1 × 3.87 kg/ha (glyphosate‐trimesium) | 35 | |||
| Leafy crops | Cabbages | Foliar on peas (primary), 4.5 kg/ha | 1–23 | |
| Foliar on carrots (primary), 4.5 kg/ha | 1–23 | |||
| Soil before sowing beets (primary), 4.5 kg/ha | 120 | |||
| Soil before sowing soybeans (primary), 4.5 kg/ha | 360 | |||
| Soil before sowing cabbages (primary), 2 × 4.5 kg/ha | 30 | |||
| Lettuces | Foliar on rye grass, 4.2 kg/ha. | 30, 120, 365 | ||
| Bare soil, 6.5 kg a.s./ha | 30, 120, 365 | |||
| Foliar on soybean (primary), 4.4 kg/ha + bare soil 1.4 kg/ha + 0.75 kg ha (glyphosate‐trimesium) | 63, 308 | |||
| Foliar on soybean (primary), 1 × 3.87 kg/ha (glyphosate‐trimesium) | 35 | |||
| Cereal (small grain) | Barley | Foliar on rye grass, 4.2 kg/ha | 30, 120, 365 | |
| Maize | Foliar on beans (primary), 4.5 kg/ha | 1–23 | ||
| Wheat | bare soil, 6.5 kg a.s./ha | 30, 120, 365 | ||
| Soil before sowing cabbages (primary), 4.5 kg/ha | 120 | |||
| Soil before sowing beets (primary), 4.5 kg/ha | 360 | |||
| Soil before sowing wheat (primary), 2 × 4.5 kg/ha | 30 | |||
| Foliar on soybean (primary), 4.4 kg/ha + bare soil 1.4 kg/ha + 0.75 kg ha (glyphosate‐trimesium) | 63, 308 | |||
| Foliar on soybean (primary), 1× 3.87 kg/ha (glyphosate‐trimesium) | 35 | |||
| Other | Legumes beans and legumes peas | Foliar on carrots (primary), 4.5 kg/ha | 1–23 | |
| foliar on cabbages (primary), 4.5 kg/ha | 1–23 | |||
| Source: Germany, 2015 | ||||
| Processed commodities (hydrolysis study) | Conditions | Investigated? | ||
| Pasteurisation (20 min, 90°C, pH 4) | Yes | |||
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | |||
| Sterilisation (20 min, 120°C, pH 6) | Yes | |||
|
Parent and N‐acetyl‐glyphosate were found to be stable. AMPA was not investigated Source: Germany, 2015 | ||||
| Can a general residue definition be proposed for primary crops? | No |
| Rotational crop and primary crop metabolism similar? | Yes |
| Residue pattern in processed commodities similar to residue pattern in raw commodities? | Yes |
| Plant residue definition for monitoring (RD‐Mo) |
Main RD‐enforcement: – For plants with glyphosate tolerant genetically modified varieties currently available on the market (sweet corn, cotton seeds, sugar beets, rapeseeds, maize and soybeans): sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate – For all other plant commodities: glyphosate Optional RD‐enforcement: – For all plant commodities (including plants with glyphosate tolerant genetically modified varieties currently available on the market): sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate |
| Plant residue definition for risk assessment (RD‐RA) | Sum of glyphosate, AMPA, N‐acetyl‐glyphosate and N‐acetyl‐AMPA, expressed as glyphosate |
| Conversion factor (monitoring to risk assessment) | See Appendix B.1.2 |
| Methods of analysis for monitoring of residues (analytical technique, crop groups, LOQs) | HPLC‐MS/MS; high water and high oil content, acidic and dry commodities; LOQ = 0.05 mg/kg each for glyphosate, AMPA and N‐acetyl‐glyphosate; ILV available for glyphosate (Germany, 2017; EFSA, 2015). Confirmatory methods for AMPA (in all matrices) and N‐acetyl‐glyphosate (in high water and high fat content matrices and dry commodities) not available. A fully validated analytical method in complex matrices is not available. |
a.i.: active ingredient; DAT: days after treatment; DATsoil: days after soil treatment; DAT2: days after second treatment; DALA: days after last treatment; PBI: plant‐back interval; HPLC–MS/MS: high‐performance liquid chromatography with tandem mass spectrometry; LC–MS/MS: liquid chromatography with tandem mass spectrometry; LOQ: limit of quantification; ILV: independent laboratory validation.
B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability (months) | Source |
|---|---|---|---|---|---|
| Glyphosate | |||||
| High water content | Tomatoes | –18 | 31 months | Germany (2015) | |
| High oil content | Soybeans | –20 | 24 months | Germany (2015) | |
| Dry/high protein | Dry beans | –18 | 18 months | Germany (2015) | |
| Dry/high starch | Sorghum grain | –20 | 48 months | Germany (2015) | |
| High acid content | Oranges | –18 | 24 months | Germany (2015) | |
| Other | Rye straw | –20 | 45 months | Germany (2015) | |
| AMPA | |||||
| High water content | Soybean forage | –18 | 24 months | Germany (2015) | |
| High oil content | Soybean | –20 | 24 months | Germany (2015) | |
| Dry/high protein | – | – | – | – | |
| Dry/high starch | Maize grain | −18 | 31 months | Germany (2015) | |
| High acid content | Oranges | −18 | 24 months | Germany (2015) | |
| Other | Soybeans straw | −20 | 24 months | Germany (2015) | |
| N ‐acetyl‐glyphosate | |||||
| High water content | Soybean forage, maize green plant and forage | −20 | 12 months | Germany (2015), Germany (2017) | |
| High oil content | Soybean seeds | −20 | 12 months | Germany (2015), Germany (2017) | |
| Dry/high protein | – | – | – | – | |
| Dry/high starch | Maize grain | −20 | 12 months | Germany (2015), Germany (2017) | |
| High acid content | – | – | – | – | |
| Other |
Soybean hay Soybean hay, maize stover |
−20 | 12 months | Germany (2015), Germany (2017) | |
| N ‐acetyl‐AMPA | |||||
| High water content |
Maize stover Soybean forage, maize green plant and forage |
−20 | 12 months | Germany (2015), Germany (2017) | |
| High oil content | Soybean seeds | −20 | 18 months | Germany (2017) | |
| Dry/high protein | – | – | – | – | |
| Dry/high starch | Maize grain | −20 | 23 months | Germany (2017) | |
| High acid content | – | – | – | – | |
| Other | Soybean hay, maize stover | −20 | 12 months | Germany (2017) | |
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials on conventional crops
| Crop | Region/indoora | Residue levels observed in the supervised residue trials relevant to the supported GAPs (mg/kg) | Recommendations/comments (OECD calculations) | MRL proposals (mg/kg) | HRMo (mg/kg)b | STMRMo (mg/kg)c | CFd |
|---|---|---|---|---|---|---|---|
|
Main RD‐enforcement 1: glyphosate Values into parentheses refer to the optional RD‐enforcement: sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosatee | |||||||
|
Citrus fruits Almonds Chestnuts Hazelnuts/cobnuts Walnuts Pome fruits Stone fruits |
NEU | – | A no‐residue situation can be anticipated based on metabolism study and southern trials, provided that proper equipment is used to avoid spray drift. No GAP authorised for limes in northern zone |
0.05* |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
| SEU |
Mo: 14× < 0.05 RA: 14 × < 0.125 |
Combined data set on tree nuts (2), apricots (4), peaches (2), kiwi (2) and bananas (4), showing no residue in orchard trees (Germany, 2015, 2017) |
0.05* |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
|
|
Brazil nuts Cashew nuts Coconuts Macadamias Pecans Pine nut kernels Pistachios |
NEU | – | Soil treatment performed at BBCH 00, i.e. before sowing, transplanting or after harvest; no residues are expected at harvest |
0.05* |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
| SEU |
Mo: 14× < 0.05 RA: 14 × < 0.125 |
Combined data set on tree nuts (2), apricots (4), peaches (2), kiwi (2) and bananas (4), showing no residue in orchard trees (Germany, 2015, 2017) |
0.05* |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
|
| Table grapes | NEU |
Mo: 3 × < 0.05 RA: 3 × < 0.125 |
Trials on grapes compliant with GAP (considering 25% tolerance on PHI, 10 d instead of 14 d) (Germany, 2015). Single positive finding from NEU disregarded as may be avoided provided that proper equipment is used |
0.05* |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
| SEU |
Mo: 8 × < 0.05 RA: 8 × < 0.125 |
Trials on grapes compliant with GAP for table and wine grapes (Germany, 2017) |
0.05* |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
|
| Wine grapes | NEU | – | A no‐residue situation can be anticipated based on metabolism study and southern trials, provided that proper equipment is used to avoid spray drift |
0.05* |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
| SEU |
Mo: 8 × < 0.05 RA: 8 × < 0.125 |
Trials on grapes compliant with GAP for table and wine grapes (Germany, 2017) |
0.05* |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
|
| Strawberries | NEU | – | A no‐residue situation can be anticipated based on metabolism study, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials |
(tentative) |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism study, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials |
(tentative) |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
|
| EU | – | A no‐residue situation can be anticipated based on metabolism study, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials |
(tentative) |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
|
| Cane fruits | NEU | – | A no‐residue situation can be anticipated based on metabolism study, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials |
(tentative) |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism study, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials |
(tentative) |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
|
| EU | – | Soil treatment performed at BBCH 00, i.e. before sowing, transplanting or after harvest; no residues are expected at harvest. However, this should be confirmed by at least two residue trials |
(tentative) |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
|
| Other small fruits and berries | NEU | – | A no‐residue situation can be anticipated based on metabolism study, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials |
(tentative) |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism study, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials |
(tentative) |
< 0.05 (< 0.2) |
< 0.05 (< 0.2) |
1g (1)h |
|
| Table olives | NEU | – | No data available. As olives can be picked from the soil, residue trials compliant with GAP are required | – | – | – | – |
| SEU | Mo: 10 × < 0.05RA: 4 × < 0.125 | Trials on olives compliant with GAP (Germany, 2015; Germany, 2017). Only samples from tree picked olives were considered, as specified in the GAP. Two positive findings (0.05 and 0.23 mg/kg) were disregared as it is considered that they could be avoided if proper equipment is used (as for orchards). Some samples were stored up to 32 months, but no degradation is expected to have occurred. | 0.05*(0.2*)f | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
|
Figs Kumquats Kiwi fruits (green, red, yellow) Kaki/Japanese persimmons Litchis/lychees Passionfruits/maracujas Avocados Mango Papayas Pomegranates Cherimoyas |
SEU | Mo: 14× < 0.05RA: 14 × < 0.125 | Combined data set on tree nuts (2), apricots (4), peaches (2), kiwi (2) and bananas (4), showing no residue in orchard trees (Germany, 2015, 2017) | 0.05*(0.2*)f | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| Bananas | SEU | Mo: 14× < 0.05RA: 14 × < 0.125 | Combined data set on tree nuts (2), apricots (4), peaches (2), kiwi (2) and bananas (4), showing no residue in orchard trees (Germany, 2015, 2017) | 0.05*(0.2*)f | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| Import (US) | – | No data available. Drift contamination cannot be excluded for the authorised GAP. | – | – | – | – | |
| Potatoes | NEU | Mo: < 0.05; < 0.05; < 0.05; 0.07; 0.09; 0.21; 0.59RA: < 0.125; < 0.125; < 0.125; 0.145; 0.165; 0.285; 0.665 | Trials on potatoes (Germany, 2017). Last 2 values are derived from trials with residues analysed at a longer PHI of 17–18 days. According to these results, it seems that longer PHIs may have an effect on the residues in tuber. It should be clarified if the northern GAP identified by the RMS can be considered as the most critical authorised.MRLOECD = 0.95 | 1i(1)f , i(tentative) | 0.59(0.71) | 0.07(< 0.2) |
1g (1)h |
| SEU | – | No data available. However, for local treatments by dabbing and rubbing, a no‐residue situation can be anticipated. | 0.05*(0.2*)f | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Sweet potatoes | SEU | – | Soil treatment performed at early growth stage (BBCH 09). A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| Yams Arrowroots Cassava roots/manioc | SEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
Beetroots Celeriacs/turnip rooted celeries Horseradishes Salsifies Swedes/rutabagas Turnips roots |
NEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Soil treatment performed at early growth stage (BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. It is noted that GAP compliant trials were available but could not be considered further since generated by using an analytical method not properly validated (2 × < 0.05; 0.07; Germany, 2015). | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Carrots | NEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | No data available. However, for local treatments by dabbing and rubbing, a no‐residue situation can be anticipated. | 0.05*(0.2*)f | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
|
Jerusalem artichokes Parsnips Parsley roots/Hamburg roots Radishes |
NEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Turnip tops | NEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Soil treatment performed at BBCH 00. Metabolism studies in primary and rotational crops indicate that no traslocation from roots to leaves is expected. A no‐residue situation can be anticipated. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| GarlicOnionsShallots | NEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Soil treatment performed at early growth stage (BBCH 09). A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Spring onions/green onions and Welsh onions Leeks | NEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| TomatoesAubergines/eggplants | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | No data available. However, for local treatments by dabbing and rubbing, a no‐residue situation can be anticipated. | 0.05*(0.2*)f | < 0.05(< 0.2) | < 0.05(< 0.2) | 1g(1)h | |
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) | 1g(1)h | |
| Sweet peppers/bell peppers | NEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Okra/lady's fingers | SEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Cucurbits with edible and inedible peel | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| BroccoliCauliflowers | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
|
Brussels sprouts Head cabbages |
NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
|
Chinese cabbages/pe‐tsai Kale |
NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Kohlrabies | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Lamb's lettuces/corn salads | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
|
Lettuces Escaroles/broadleaved endives Cresses and other sprouts and shoots Land cresses Roman rocket/rucola Red mustardsBaby leaf crops (including brassica species) Fresh herbs PurslanesChards/beet leaves |
NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Spinaches | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Grape leaves and similar species | SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| Watercresses | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Witloofs/Belgian endives | NEU | – | Application during the field phase (root production) is not expected to lead to significant residues in harvested roots (based on metabolism studies in primary and rotational crops and provided that proper equipment is used to avoid spray drift). As only limited transfer from roots to leaves is expected, significant residues in witloof (after forcing phase) are unlikely. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application during the field phase (root production) before seeding (BBCH 00). Significant residues are not expected, neither in roots (at harvest) nor in witloof (after forcing phase). However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
|
Beans (with pods) Beans (without pods) Peas (with pods) Peas (without pods) Lentils (fresh) |
NEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | No data available. However, for local treatments by dabbing and rubbing, a no‐residue situation can be anticipated. | 0.05*(0.2*)f | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| CardoonsCeleriesFlorence fennelsRhubarbs | NEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Asparagus | NEU | Mo: < 0.05RA: < 0.125 | Trial on asparagus compliant with GAP (Germany, 2017). A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least one additional trial. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Globe artichokes | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | No data available. However, for local treatments by dabbing and rubbing, a no‐residue situation can be anticipated. | 0.05*(0.2*)f | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Bamboo shoots | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Palm hearts | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| EU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Cultivated fungi | NEU | – | No data available. Uptake from the soil and/or cross‐contamination cannot be excluded for the authorised GAP (metabolism studies are not representative for fungi). | – | – | – | – |
| SEU | – | No data available. Uptake from the soil and/or cross contamination cannot be excluded for the authorised GAP (metabolism studies are not representative for fungi). | – | – | – | – | |
| Wild fungi | NEU | – | Authorised GAP is on forestry. A no‐residue situation can be anticipated for this GAP, provided that adequate risk mitigation measures are in place to avoid cross‐contamination in wild fungi. | 0.05*(0.2*)f | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Authorised GAP is on forestry. A no‐residue situation can be anticipated for this GAP, provided that adequate risk mitigation measures are in place to avoid cross‐contamination in wild fungi. | 0.05*(0.2*)f | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Beans (dry)Peas (dry) | NEU | Mo: < 0.05; < 0.05; < 0.05; < 0.05; 0.06; 0.08; 0.14; 0.23; 2.5; 7.62RA: < 0.125; < 0.125; < 0.125; < 0.125; 0.135; 0.155; 0.215; 0.305; 2.6; 7.79 | Combined data set on beans and peas (Germany, 2017).MRLOECD = 10.76 | 15j(30)f , j(tentative) | 7.62(15.24) | 0.07(< 0.2) |
2.0 (1)h |
| SEU | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| EU | – | No data available. However, application on soil before seedling, transplanting and after harvest (i.e. BBCH 00) is expected to be less critical than the northern outdoor GAP. | – | – | – | – | |
| Import (US) | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Lentils (dry) | NEU | Mo: < 0.05; < 0.05; < 0.05; < 0.05; 0.06; 0.08; 0.14; 0.23; 2.5; 7.62RA: < 0.125; < 0.125; < 0.125; < 0.125; 0.135; 0.155; 0.215; 0.305; 2.6; 7.79 |
Direct extrapolation from combined data set on beans and peas (Germany, 2017). MRLOECD = 10.76 |
15j(30)f , j(tentative) | 7.62(15.24) | 0.07(< 0.2) |
2.0 (1)h |
| SEU | – | No data available. However, application on soil before seedling, transplanting and after harvest (i.e. BBCH 00) is expected to be less critical than the northern outdoor GAP. | – | – | – | – | |
| EU | – | No data available. However, application on soil before seedling, transplanting and after harvest (i.e. BBCH 00) is expected to be less critical than the northern outdoor GAP. | – | – | – | – | |
| Import (US) | Mo: < 0.05; < 0.05; 1.4; 3.02RA: < 0.125; < 0.125; 1.48; 3.1 |
Trials on lentils performed in USA/Canada compliant with GAP for desiccation (Germany, 2017). Storage stability not covered for AMPA (deemed as minor deficiency). MRLOECD = 6.78 |
7(15)f | 3.02(5.4) | 0.73(1.46) |
1.8 (1)h |
|
| Lupins/lupini beans (dry) | NEU | Mo: < 0.05; < 0.05; < 0.05; < 0.05; 0.06; 0.08; 0.14; 0.23; 2.5; 7.62RA: < 0.125; < 0.125; < 0.125; < 0.125; 0.135; 0.155; 0.215; 0.305; 2.6; 7.79 |
Direct extrapolation from combined data set on beans and peas (Germany, 2017). MRLOECD = 10.76 |
15j(30)f , j(tentative) | 7.62(15.24) | 0.07(< 0.2) |
2.0 (1)h |
| SEU | – | No data available. However, application on soil before seedling, transplanting and after harvest (i.e. BBCH 00) is expected to be less critical than the northern outdoor GAP. | – | – | – | – | |
| EU | – | No data available. However, application on soil before seedling, transplanting and after harvest (i.e. BBCH 00) is expected to be less critical than the northern outdoor GAP. | – | – | – | – | |
| Linseeds | NEU | Mo: 0.06; 0.21; 0.23; 0.28; 0.35; 0.40; 0.40; 0.40; 0.48; < 0.5; < 0.5; 0.57; 0.60; 0.60; 0.70; 0.90; 0.96; < 1.0; 1.0; 1.3; 1.5; 2.0; 2.0; 2.0; 2.8; 4.1; 4.6; 8.6; 11.6RA: –; –; 0.29; 0.31; –; –; 0.42; 0.48; –; –; 0.68; 0.68; 0.78; < 0.8; < 0.8; 0.98; 1.0; 1.1; < 1.3; 1.3; 1.4; 1.8; 2.3; 2.5; 3.1; 4.6; 4.7; 8.5; 11.9 | Trials on rapeseed compliant with GAP for desiccation (Germany, 2017). Extrapolation from rapeseed to linseed is applicable.MRLOECD = 12.13 | 15(15)f | 11.60(11.94) | 0.70(1.14) |
1.1 (1)h |
| SEU | Mo: 0.23; 0.93; 1.4; 5.6RA: 0.31; 1.0; 1.48; 5.7 |
Trials on rapeseed compliant with GAP for desiccation (2) or performed with a shorter PHI of 10 days (2) (Germany, 2015). Extrapolation to linseeds is applicable. AMPA above LOQ was quantified in one sample only (0.07 mg/kg). MRLOECD = 11.73 |
15i(15)f , i(tentative) | 5.60(5.74) | 1.17(1.28) |
1.1 (1)h |
|
| Peanuts/groundnuts | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Poppy seeds | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Soil treatment performed at early growth stage (BBCH 09). No residues are expected. Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Sunflower seeds | NEU | Mo: 2 × <0.5RA: – | Trials on sunflower seed compliant with GAP for desiccation, but not sufficient to derive an MRL (Germany, 2017). According to the RMS, additional trials are available. However, since study reports were not reported to the RMS, they could not be evaluated. | – | – | – | – |
| SEU | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Import (US) | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Mustard seeds | NEU | – | No data compliant with GAP for desiccation. No extrapolation possible from rapeseed as the GAP reported for mustard seed is more critical (PHI 7 days instead of 14 days). | – | – | – | – |
| SEU | – | No data compliant with GAP for desiccation. No extrapolation possible from rapeseed as the GAP reported for mustard seed is more critical (PHI 8 days instead of 14 days). | – | – | – | – | |
|
Sesame seeds Pumpkin seedsSafflower seeds Gold of pleasure seedsHemp seedsCastor beans |
NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Borage seeds | NEU | Mo: 0.06; 0.3; 0.3; 2 × 0.4; 0.04; 0.6; 0.7; 0.9; 1.0; 1.3; 2.8; 5.1; 6.8 RA: 0.11; 0.35; 0.35; 2 × 0.45; 0.045; 0.65; 0.75; 0.95; 1.05; 1.35; 2.85; 5.15; 6.85 | Trials on rapeseeds compliant with the GAP for borage seeds (United Kingdom, 2015).Underlined values: samples with no information on storage conditions. Since results were in the range of the other trials, the lack of information is considered a minor deficiency and accepted. Only five trials analysed for AMPA (5 × < 0.05) which is expected to remain < LOQ.MRLOECD: 9.6 | 10(10)f | 6.80(6.85) | 0.65(0.70) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP and this should be in principle confirmed by at least two residue trials. Nevertheless, as the NEU is clearly more critical, no additional trials supporting the SEU GAP are required. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Olives for oil production | NEU | – | No data available. As olives can be picked from the soil, residue trials compliant with GAP are required. | – | – | – | – |
| SEU | Mo: < 0.05; < 0.05; < 0.05; 0.11; 0.14; 0.30; 0.53; 0.93; 1.7; 3.3; 7.2; 16RA: –; –; –; 0.185; 0.215; –; 0.605; 1.0; –; –; –; – |
Trials on olives compliant with GAP for soil applications (Germany, 2015, 2017). Samples from ground picked olives were considered (in accordance with possible practices). In all trials analysing for AMPA, this metabolite is < LOQ. Samples stored for up to 32 months, but no degradation is expected to have occurred. MRLOECD = 21.45 |
30(30)f | 16.00(16.1) | 0.42(0.53) |
1g (1)h |
|
| Oil palms kernels | SEU | – | Residues are not expected in palm oil kernel after soil treatment on this crop (kernel is not directly exposed to possible spray drift and limited translocation has been observed in the metabolism studies). | 0.05*(0.2*)f | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| Oil palms fruits | SEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| Kapok | SEU | – | Residues are not expected in fruits after soil treatment on this crop (morphology of kapok trees prevent from drift contaminations). | 0.05*(0.2*)f | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| Barley grainsoat grains | NEU | Mo: 1.2; 1.5; 2.0; 2.1; 2.1; 2.2; 2.4; 2.5; 2.6; 2.6; 2.8; 3.95; 4.3; 4.4; 4.5; 4.6; 4.8; 5.1; 5.2; 5.2; 5.2; 5.3; 5.4; 5.5; 5.5; 5.7; 5.9; 5.9; 6.2; 6.5; 6.7; 7.4; 7.7; 7.8; 8.0; 8.1; 8.4; 9.8; 10; 10.3; 12.4; 12.5; 14; 15.5; 16.5; 17; 17.5; 18.4; 21; 21.4RA: 1.3; 1.5; 2.1; 2.2; 2.2; 2.3; 2.5; 2.5; 2.7; 2.9; 3.2; 4.2; 4.4; 4.6; 4.9; 5.0; 5.1; 5.2; 5.3; 5.3; 5.3; 5.5; 5.5; 5.6; 5.8; 5.8; 5.9; 6.2; 6.2; 6.6; 6.9; 7.5; 7.9; 8.0; 8.2; 8.3; 8.4; 10; 10.3; 10.4; 12.4; 12.8; 14.4; 16; 16.6; 17.2; 17.8; 18.4; 21.4; 21.6 |
Trials on barley compliant with GAP for desiccation (Germany, 2015); covered by RAR representative use, some trials did not involve analysis of AMPA, but its contribution is considered insignificant. Extrapolation to oats is applicable. MRLOECD = 28.57 |
30(30)f | 21.40(21.64) | 5.60(5.84) |
1g (1)h |
| SEU | Mo: 6.0; 7.8; 13.5; 19RA: 6.0;7.9;13.7;19.3 |
Trials on barley compliant with GAP for desiccation (Germany, 2015). Extrapolation to oats is applicable. MRLOECD = 35.15 |
30i , k(30)f , i(tentative) | 19.00(19.34) | 10.65(10.84) |
1g (1)h |
|
| Import (US) | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Barley strawoats straw | NEU | Mo: 4.6; 6.9; 9.6; 10.5; 11; 11.5; 12.8; 12.8; 14.5; 16; 17; 18; 22; 24; 26; 26.3; 26.5; 27; 27.3; 28.4; 32.2; 33.3; 36.9; 37; 41.5; 44; 49.7; 54; 56; 60.5; 69.6; 80.5; 86; 90.2; 109; 115; 117; 136; 140RA: 4.7; 6.9; 10; 10.6; 11.3; 12.1; 13.1; 13.2; 14.6; 16.3; 17.7; 18; 22; 24.5; 26.7; 27.1; 27.6; 28.6; 28.7; 29.3; 29.6; 32.7; 33.9; 37.8; 38; 42.1; 44.4; 51.3; 56;60.8; 61.9; 70.7; 83.6; 89.8; 92; 109; 115; 119; 140; 142 |
Trials on barley compliant with GAP for desiccation (Germany, 2015); covered by RAR representative use, some trials did not involve analysis of AMPA, but its contribution is considered insignificant. Extrapolation to oats is applicable. MRLOECD = 195.54 |
200l(200)f , l(tentative) | 140.00(142) | 28.40(29.5) |
1g (1)h |
| SEU | Mo: 34; 49.5; 66; 102RA: 34.9; 51; 68.1; 105 |
Trials on barley compliant with GAP for desiccation (Germany, 2015). Extrapolation to oats is applicable. MRLOECD = 188.62 |
200l(200)f , l(tentative) | 102.00(105) | 57.75(59.5) |
1g (1)h |
|
| Import (US) | – | Cereals straw not relevant for import tolerance GAP. | – | – | – | – | |
| Buckwheat and other pseudo‐cereal grains | NEU | – | No data available to support the GAP for desiccation. | – | – | – | – |
| SEU | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Import (US) | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Common millet/proso millet grains | NEU | Mo: 0.229; 0.27; 0.279; 0.319; 0.452; 0.558; 0.7; 0.753RA: 0.72; 0.43; 0.82; 0.48; –; –; –; – |
Trials on maize compliant with GAP for desiccation (Germany, 2017). Only four trials analysed for AMPA. Residues of AMPA were reconverted to glyphosate using respective molecular weights, assuming that they were expressed as AMPA in the evaluation report. Applicable extrapolation to millet. MRLOECD = 1.34 |
1.5(3)f | 0.75(1.77) | 0.39(0.94) |
2.3 (1)h |
| SEU | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Import (US) | Mo: < 0.05; < 0.05;< 0.05; < 0.05; 0.058; 0.063; 0.1; 0.11RA: < 0.125; < 0.125; < 0.125; < 0.125; 0.19; 0.133; 0.14; 0.18 | Conventional GAP supported by trials performed on EPSPS maize. Although EPSPS modification is not expected to alter the metabolic pathway of glyphosate in plants, the data were not used to derive an MRL since results were considered questionable (lower residue levels were observed in this data set compared to the trials compliant with the NEU GAP which is significantly less critical). Outlier of 3.2 mg/kg was disregarded (Germany, 2017). | – | – | – | – | |
| Common millet straw | NEU | – | No data available to support the GAP for desiccation. | – | – | – | – |
| SEU | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Import (US) | – | Cereals straw not relevant for import tolerance GAP. | – | – | – | – | |
| Sorghum grains | NEU | Mo: 0.229; 0.27; 0.279; 0.319; 0.452; 0.558; 0.7; 0.753RA: 0.72; 0.43; 0.82; 0.48; –; –; –; – | Direct extrapolation from common millet grain (Germany, 2017).MRLOECD = 1.34 | 1.5(3)f | 0.75(1.77) | 0.39(0.94) |
2.3 (1)h |
| SEU | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Import (US) | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Sorghum stover | NEU | – | No data available to support the GAP for desiccation. | – | – | – | – |
| SEU | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Import (US) | – | Cereals straw not relevant for import tolerance GAP. | – | – | – | – | |
| Rice grains | SEU | – | No data available to support the GAP for desiccation. | – | – | – | – |
| Rice straw | SEU | – | No data available to support the GAP for desiccation. | – | – | – | – |
|
Wheat grains Rye grains |
NEU |
Mo: 0.05; 0.11; 0.16; 0.19; 0.22; 0.23; 0.23; 0.26; 0.33; 0.5; 0.5; 0.6; 0.64; 0.67; 0.7; 0.7; 0.7;0.7;0.7; 0.71; 0.74; 0.75; 0.75; 0.77; 0.85; 1.3; 1.4; 1.5; 1.55; 1.6; 1.7; 1.7; 1.75; 2.2; 2.4; 2.9; 3.1; 3.45; 3.5; 3.7; 3.85; 4.7; 4.8; 4.85; 5.4; 9.5; 12.4; 17.5 RA: 0.125; 0.18; 0.24; 0.26; 0.27; 0.27; 0.28; 0.29; 0.36; 1.1; 0.58; 0.64; 0.7; 0.74; 0.74; 0.75; 0.77; 0.78; 0.78; 0.78; 0.78; 0.83; 0.83; 0.84; 0.93; 1.3; 1.5; 1.6; 1.6; 1.6; 1.7; 1.8; 1.9; 2.3; 2.4; 2.9; 3.1; 3.5; 3.6; 3.8; 3.9; 4.9; 5.0; 5.0; 5.4; 9.5; 13.3; 18.1 |
Trials on wheat compliant with GAP for desiccation (Germany, 2015); covered by RAR representative use. Applicable extrapolation to rye. MRLOECD = 17.5 |
20(20)f | 17.50(18.14) | 0.81(1.06) |
1g (1)h |
| SEU |
Mo: 0.07; 0.38; 0.4; 0.4; 0.47; 0.6; 0.95; 1.2; 2.8 RA: 0.15; 0.45; 0.48; 0.48; 0.55; 0.68; 1.0; 1.3; 3.0 |
Trials on wheat compliant with GAP for desiccation (Germany, 2015). Applicable extrapolation to rye. MRLOECD = 4.08 |
4(4)f | 2.80(3.04) | 0.47(0.59) |
1g (1)h |
|
| Import (US) | – | No data available to support the GAP for desiccation. | – | – | – | – | |
|
Wheat straw Rye straw |
NEU |
Mo: 1.4; 5.3; 8.4; 9.5; 10.3; 10.6; 11.4; 14.7; 14.9; 17.3; 18.5; 19.1; 19.7; 21.5; 24.8; 26.9; 27.4; 27.5; 29.6; 31.4; 34.8; 42; 43.2; 43.8; 44.5; 46; 52.8; 63.3; 68; 70.5; 84.5; 85; 95.3; 95.5; 95.7; 96.5; 99; 175 RA: 1.5; 5.4; 9.3; 10.5; 10.9; 11; 12.6; 15.7; 15.7; 17.6; 19.2; 19.4; 19.9; 22.1; 25.5; 28; 28.2; 28.9; 29.6; 31.8; 35.9; 42.6; 43.2; 44.2; 45.4; 46; 52.8; 64.3; 68; 71.4; 87.5; 88.5; 96.5; 97.3; 97.6; 98; 103; 179 |
Trials on wheat compliant with GAP for desiccation (Germany, 2015); covered by RAR representative use. Applicable extrapolation to rye. MRLOECD = 193.56 |
200l(200)f , l(tentative) | 175(179) | 30.5(30.7) |
1g (1)h |
| SEU | Mo: 3.4; 15.5; 16; 20; 22; 28; 28.5; 55.5; 98RA: 3.5; 16.9; 18.6; 20.9; 23.2; 29.6; 29.7; 56.5; 99 |
Trials on wheat compliant with GAP for desiccation (Germany, 2015). Applicable extrapolation to rye. MRLOECD = 146.13 |
150l(150)f , l(tentative) | 98(99) | 22(23.2) |
1g (1)h |
|
| Import (US) | – | Cereals straw not relevant for import tolerance GAP. | – | – | – | – | |
| Teas | SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i , m(0.2*)f , i , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| Import (US) | – | No residue trials on tea available. Moreover, relevant GAP parameters are missing (growth stage at last treatment or PHI). | – | – | – | – | |
| Coffee beans | SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies indicate that a no‐residue situation can be anticipated for this GAP. | 0.05*, m(0.2*)f , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
Herbal infusions (from flowers Herbal infusions (from leaves and herbs) |
NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i , m(0.2*)f , i , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Soil treatment performed at BBCH 00, i.e. before sowing, transplanting or after harvest; Studies on rotational crops indicate that no residues uptake occurs in leafy and in roots crops. No residues are expected at harvest. | 0.05*, i , m(0.2*)f , i , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Herbal infusions (from roots) | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i , m(0.2*)f , i , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. It is noted that GAP compliant trials were available but could not be considered further since generated by using an analytical method not properly validated (2 × < 0.05; 0.07; Germany, 2015). | 0.05*, i , m(0.2*)f , i , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Carobs/Saint John's breads | SEU | –– | Residues are not expected in fruits after soil treatment on this crop (morphology of carob trees prevent from drift contaminations). | 0.05*, m(0.2*)f , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| Hops | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i , m(0.2*)f , i , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i , m(0.2*)f , i , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
|
Seed spices Fruit spices |
NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i , m(0.2*)f , i , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i , m(0.2*)f , i , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Root and rhizome spices | NEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i , m(0.2*)f , i , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i , m(0.2*)f , i , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Bark spicesBud spicesFlower pistil spicesAril spices | SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i , m(0.2*)f , i , m(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| Sugar canes | SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| Import (US) | – | No data available. | – | – | – | – | |
| Chicory roots | NEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However this should be confirmed by at least two residue trials. | 0.05*, i(0.2*)f , i(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Alfalfa forage | NEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i , l(0.2*)f , i , l(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.05*, i , l(0.2*)f , i , l(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Clover forage | NEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However this should be confirmed by at least two residue trials. | 0.05*, i , l(0.2*)f , i , l(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However, this should be confirmed by at least two residue trials. | 0.05*, i , l(0.2*)f , i , l(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Grass forage | NEU |
Mo: 3.2; 3.9; 7.4; 8.7; 9.6; 15; 16; 21; 29; 40; 42; 45; 139 RA: 3.5; –; –; 9; –; 15; –; 22; –; –; 43; 46; – |
Trials on grass/pasture compliant with GAP for desiccation (within the 25% deviation). Means of analytical replicates were considered (Germany, 2017). MRLOECD = 178.56 |
200l(200)l(tentative) | 139(139) | 16(16) |
1g (1)h |
| SEU | – | Application on soil before seedling, transplanting and after harvest (i.e. BBCH 00). Available metabolism studies in primary and rotational crops indicate that a no‐residue situation can be anticipated for this GAP. However this should be confirmed by at least two residue trials. | 0.05*, i , l(0.2*)f , i , l(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Fodder beet roots | NEU | – | No data available. However, for local treatments by dabbing and rubbing, a no‐residue situation can be anticipated. | 0.05*, l(0.2*)f , l(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | Mo: 2 × < 0.05RA: 2 × < 0.125 | A no‐residue situation can be anticipated for this GAP (application on soil at BBCH 00), which is confirmed by 2 southern residue trials performed on sugar beet and performed with a more critical GAP (Germany, 2017). | 0.05*, l(0.2*)f , l(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| Fodder beet tops | NEU | – | No data available. However, for local treatments by dabbing and rubbing, a no‐residue situation can be anticipated. | 0.05*, l(0.2*)f , l(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
| SEU | Mo: 2 × < 0.05RA: 2 × < 0.125 | A no‐residue situation can be anticipated for this GAP (application on soil at BBCH 00), which is confirmed by 2 southern residue trials performed on sugar beet and performed with a more critical GAP (Germany, 2017). | 0.05*, l(0.2*)f , l(tentative) | < 0.05(< 0.2) | < 0.05(< 0.2) |
1g (1)h |
|
| RD‐enforcement main = RD‐enforcement optional: sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate | |||||||
| Sweet corn | NEU | Mo: 4 × < 0.2RA: – | Trials on maize (sampling on immature maize, 30 days before maturity) (Germany, 2017). Glyphosate and AMPA are below LOQ. N‐acetyl‐glyphosate is not expected in conventional crops. | 0.2*, f(tentative) | < 0.2 | < 0.2 | 1n |
| SEU | – | A no‐residue situation can be anticipated based on metabolism studies in primary and rotational crops, provided that proper equipment is used to avoid spray drift. However, this should be confirmed by at least two residue trials. | 0.2*, i , f(tentative) | < 0.2 | < 0.2 | 1n | |
| Cotton seeds | NEU | – | No data available, but this GAP is expected to be less critical than the southern outdoor GAP (dessication). A no‐residue situation can be anticipated based on metabolism study in primary and rotational crops, provided that proper equipment is used to avoid spray drift. | – | – | – | – |
| SEU | Mo: 0.14; 0.30; 0.34; 0.38; 0.49; 0.58; 0.92RA: – | Trials on cotton seeds compliant with GAP for desiccation, with 25% tolerance on the application rate (Germany, 2017). Residue levels are expressed for the sum of glyphosate and AMPA, expressed as glyphosate (AMPA < LOQ). N‐acetyl‐glyphosate is not expected in conventional crop.MRLOECD = 1.45 | 1.5f(tentative) | 0.92 | 0.38 | 1n | |
| Rapeseed/ canola seed | NEU | Mo: 0.29; 0.31; 0.42; 0.48; 0.68; 0.68; 0.78; < 0.8; < 0.8; 0.98; 1.0; 1.1; < 1.3; 1.3; 1.4; 1.8; 2.3; 2.5; 3.1; 4.6; 4.7; 8.5; 11.9RA: – | Trials on rapeseed compliant GAP (Germany, 2017). Residue levels are expressed for the sum of glyphosate and AMPA, expressed as glyphosate. N‐acetyl‐glyphosate is not expected in conventional crop.MRLOECD = 13.6 | 15f(tentative) | 11.9 | 1.10 | 1n |
| SEU | Mo: 0.31; 1.0; 1.48; 5.7RA: – | Trials on rapeseed compliant with GAP (2) or performed with a shorter PHI of 10 days (2) (Germany, 2015). Residue levels are expressed for the sum of glyphosate and AMPA, expressed as glyphosate. N‐acetyl‐glyphosate is not expected in conventional crop.MRLOECD = 11.9 | 15i , f(tentative) | 5.70 | 1.24 | 1n | |
| Soybeans | NEU | – | No data available to support the GAP for desiccation. | – | – | – | – |
| SEU | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Import (US) | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Maize/corn grain | NEU |
Mo: Trials analysing for sum of glyphosate and AMPA, expressed as glyphosate: 0.43; 0.48; 0.72; 0.82 Trials analysing for glyphosate, recalculated for the sum of glyphosate and AMPA, expressed as glyphosate considering a CF of 2.3: 1.04; 1.28; 1.61; 1.73 RA: – |
Trials on maize compliant with GAP (Germany, 2017). Four trials analysed for glyphosate and AMPA (AMPA residues were reconverted to glyphosate using respective molecular weights, assuming that they were expressed as AMPA in the evaluation report). Four other trials analysed for glyphosate only (0.45; 0.56; 0.7; 0.75) were reconverted to the sum of glyphosate and AMPA, using the CF of 2.3. MRLOECD = 3.0 |
3i , f(tentative) | 1.73 | 0.93 | 1n |
| SEU | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Import (US) | Mo: < 0.125; < 0.125; < 0.125; < 0.125; 0.19; 0.133; 0.14; 0.18RA: – | Conventional GAP supported by trials performed on EPSPS maize. Although EPSPS modification is not expected to alter the metabolic pathway of glyphosate in plants, the data were not used to derive an MRL since results were considered questionable (lower residue levels were observed in this data set compared to the trials compliant with the NEU GAP which is significantly less critical). Outlier of 3.2 mg/kg was disregarded (Germany, 2017). | – | – | – | – | |
| Maize/corn stover | NEU | – | No data available to support the GAP for desiccation. | – | – | – | – |
| SEU | – | No data available to support the GAP for desiccation. | – | – | – | – | |
| Import (US) | – | Cereals straw not relevant for import tolerance GAP. | – | – | – | – | |
| Sugar beet roots | NEU | Mo: 8 × < 0.2RA: – | Trials on sugar beets compliant with GAP (Germany, 2017). Glyphosate and AMPA are below LOQ. N‐acetyl‐glyphosate is not expected in conventional crops. | 0.2*, f(tentative) | < 0.2 | < 0.2 | 1n |
| SEU | – | No data available. | – | – | – | – | |
| Sugar beet tops | NEU | Mo: 8 × < 0.2RA: – | Trials on sugar beets compliant with GAP (Germany, 2017). Glyphosate and AMPA are below LOQ. N‐acetyl‐glyphosate is not expected in conventional crops. | 0.2*, f , l(tentative) | < 0.2 | < 0.2 | 1n |
| SEU | – | No data available. | – | – | – | – | |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level.
*Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue according to the residue definition for monitoring.
Supervised trials median residue according to the residue definition for monitoring.
Conversion factor for risk assessment; median of the individual conversion factors at the supported PHI for each residues trial (unless otherwise specified).
Values calculated for the optional residue definition correspond to the value calculated for glyphosate, plus residue levels of AMPA (from the trials), plus the LOQ of N‐acetyl‐glyphoste, expressed as glyphosate (i.e. 0.9*0.05=0.04 mg/kg). When metabolite AMPA is below the LOQ, the LOQ was expressed as glyphosate (1.5*0.05 = 0.075 mg/kg).
MRLs referring to the residue definition for enforcement ‘sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate’ are tentative because confirmatory methods for analysis of N‐acetyl‐glyphosate and AMPA are still required.
A conversion factor of 1 was derived since AMPA (or both glyphosate and AMPA) is expected to remain ≤ LOQ. N‐acetyl‐AMPA and N‐acetyl‐glyphosate are not expected in conventional crops.
As metabolite N‐acetyl‐AMPA is not expected in conventional crops, a CF of 1 is applicable for all MRLs and risk assessment values derived under the optional residue definition.
Tentative MRL is derived because additional trials are required.
Tentative MRL is derived because the complete summary of the residue trials (including full assessment of the studies) is still required; moreover, storage stability of AMPA in high protein content commodities is not covered.
Considering that the MRL is derived from a lower number of trials compared to the northern data set and that for straw, the same MRL was derived for NEU and SEU datasets, the calculated MRL of 40 may be overestimated. Therefore, a lower MRL of 30 is proposed based on the available data set.
Tentative MRL is derived in view of the future MRL setting in feed items.
Tentative MRL is derived as a fully validated analytical method for enforcement in complex matrices is still required.
A conversion factor of 1 was derived since N‐acetyl‐AMPA is not expected in conventional crops.
B.1.2.2. Summary of residues data from the supervised residue trials on genetically modified EPSPS crops
| Crop | Region/indoora | Residue levels observed in the supervised residue trials relevant to the supported GAPs (mg/kg) | Recommendations/comments (OECD calculations) | MRL proposals(mg/kg) | HRMo (mg/kg)b | STMRMo (mg/kg)c | CFd |
|---|---|---|---|---|---|---|---|
| Genetically modified EPSPS cropsRD‐enforcement main = RD‐enforcement optional: sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate | |||||||
| Sweet corn | Import (US) | Mo: 0.185; 0.205; 0.33; 0.43; 0.58; 1.0; 1.3; 1.45 RA: – |
Trials on sweet corn with three applications at 4, 0.86 and 1.7 kg/ha considered acceptable since first applications done at an early growth stage is not expected to have a significant impact on the final residue level (Germany, 2017). Residues analysed only for glyphosate and AMPA acceptable since N‐acetyl‐glyphosate and N‐acetyl‐AMPA are not expected in EPSPS crops. MRLOECD = 2.68 |
3e , f (tentative) | 1.45 | 0.51 | 1.0 |
| Cotton seeds | Import (US) | Mo: 14.1; 7.7; 20; 21.6; 22.4; 17.5; 8.0; 6.2; 17.7; 23.7; 25.2; 30.9; 13.0; 18.8; 14.1; 7.6; 23.9 RA: – |
Trials on cotton seeds performed with higher dose rate at first application (3.3 kg/ha instead of 1.7) considered acceptable since the first application done at an early growth stage is not expected to have a significant impact on the final residue level (Germany, 2017). Residues analysed only for glyphosate and AMPA acceptable since N‐acetyl‐glyphosate and N‐acetyl‐AMPA are not expected in EPSPS crops. MRLOECD = 51.6 |
60e , f (tentative) | 30.9 | 17.7 | 1.0 |
| Sugar beets roots | Import (US) | – | No data available. | – | – | – | – |
| Sugar beets tops | Import (US) | – | Sugar beet tops not relevant for import tolerance GAP. | – | – | – | – |
| Soybeans Maize | – | – | According to the EU Register of authorised GMOs, the import of EPSPS maize and EPSPS soybeans is authorised in EU. Nevertheless, as no import tolerances on these GM crops were reported by MSs during the GAP collection phase, it was not possible to derive an MRL based on these uses | – | – | – | – |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level.
*Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue according to the residue definition for monitoring.
Supervised trials median residue according to the residue definition for monitoring.
Conversion factor for risk assessment; median of the individual conversion factors at the supported PHI for each residues trial.
Tentative MRL is derived as confirmatory methods for analysis of N‐acetyl‐glyphosate and AMPA are still required.
As N‐acetyl compounds were not analysed for in the trials, in case risk managers wish to exclude the N‐acetyl‐glyphosate from the residue definition for enforcement, the derived MRL will be still valid.
B.1.2.3. Summary of residues data from the supervised residue trials on genetically modified GOX crops
| Crop | Region/indoora | Residue levels observed in the supervised residue trials relevant to the supported GAPs (mg/kg) | Recommendations/comments (OECD calculations) | MRL proposals(mg/kg) | HRMo (mg/kg)b | STMRMo (mg/kg)c | CFd |
|---|---|---|---|---|---|---|---|
| Genetically modified GOX cropsRD‐enforcement main = RD‐enforcement optional: sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate | |||||||
| Rapeseeds | Import (US) | – | No residue trials available. | – | – | – | – |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue according to the residue definition for monitoring.
Supervised trials median residue according to the residue definition for monitoring.
Conversion factor for risk assessment; median of the individual conversion factors at the supported PHI for each residues trial.
B.1.2.4. Summary of residues data from the supervised residue trials on genetically modified GAT crops
| Crop | Region/indoora | Residue levels observed in the supervised residue trials relevant to the supported GAPs (mg/kg) | Recommendations/comments (OECD calculations) | MRL proposals(mg/kg) | HRMo (mg/kg)b | STMRMo (mg/kg)c | CFd |
|---|---|---|---|---|---|---|---|
| Genetically modified GAT cropsRD‐enforcement 1 = RD‐enforcement 2: sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate | |||||||
| Rapeseeds | Import (US) |
Mo: 1.83; 10.1; 5.6; 1.58; 2.8; 3.3; 5.8; 11.2; 3.5; 2.7; 3.3; 2.2; 0.88; 0.81; 14.8 RA: 1.88; 10.2; 5.6; 1.63; 2.9; 3.4; 5.8; 11.2; 3.5; 2.7; 3.3; 2.3; 0.93; 0.86; 15.2 |
GAT GM rapeseed is currently not authorised for placing on the market within the EU. Therefore, GAP and supporting residue trials were not considered further in the assessment. MRLOECD = 21.34 |
– | – | – | – |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue according to the residue definition for monitoring.
Supervised trials median residue according to the residue definition for monitoring.
Conversion factor for risk assessment; median of the individual conversion factors at the supported PHI for each residues trial.
B.1.2.5. Residues in succeeding crops
| Confined rotational crop study(quantitative aspect) | According to the results from the confined rotational crop studies performed up to 1.5N, residues of glyphosate or AMPA are not expected in rotational root and leafy crops following annual application of glyphosate, provided that the active substance is used according to the GAPs considered in this review. Residues of glyphosate and its metabolite AMPA above the LOQ of 0.05 mg/kg cannot be excluded in cereals grain (only AMPA), forage and chaff grown in rotation with crops treated with glyphosate. Therefore, MSs are recommended to implement proper mitigation measures when granting authorisation of plant protection products containing glyphosate, in order to avoid residues to occur in rotated cereals. Moreover, as the available studies do not cover the plateau concentration calculated for AMPA, proper mitigation measures should also be implemented to avoid accumulation of AMPA in soil and possible uptake of AMPA in rotational crops. |
| Field rotational crop study | Currently not available. |
B.1.2.6. Processing factors
| Processed commodity | Number of studiesa | Processing factor (PF) | CFP b | |
|---|---|---|---|---|
| Individual values | Median PF | |||
| Conventional crops (main residue definition) | ||||
| Robust processing factors (sufficiently supported by data) | ||||
| Citrus, juice | 6 | 0.45; < 0.71; < 0.83; < 0.83; 0.83; < 1 | 0.83 | 1c |
| Citrus, peel | 6 | < 0.83; 2.3; 2.8; 3.1; 3.1; 5.0 | 3 | 1c |
| Citrus, dry pomaced | 6 | 1.4; 1.8; 1.8; 3.3; 4.9; 5.3 | 2.6 | 1c |
| Citrus, press liquor | 6 | < 0.83; 1.7; 1.9; 2.1; 2.3; 2.7 | 2 | 1c |
| Olives, crude oil | 19 | 2× < 0.03; < 0.04; 4× < 0.05; 2× < 0.06; < 0.09; 2× < 0.13; < 0.17; < 0.25; < 0.35; < 0.38; < 0.42; 2× < 0.63 | 0.09 | 1c |
| Olives, refined oil | 6 | 2× < 0.05; 0.09; < 0.35; < 0.38; < 0.42 | 0.22 | 1c |
| Linseed, oil | 4 | < 0.1; < 0.18; 2× < 0.31 | 0.25 | 1c |
| Linseed, press cake | 4 | 1.1; < 1.5; 2×1.6 | 1.6 | 1c |
| Rapeseed, crude oil | 4 | < 0.1; < 0.13; < 0.15; < 0.27 | 0.14 | 1c |
| Rapeseed, refined oil | 5 | < 0.05; < 0.1; < 0.13; < 0.15; < 0.27 | 0.13 | 1c |
| Rapeseed, press cake | 5 | 1.2; < 1.3; 1.4; 1.5; 2.2 | 1.4 | 1c |
| Maize, fat free meal | 4 | 1.0; 1.0; 1.2; 1.2 | 1.1 | 1 |
| Maize, crude oil | 4 | < 0.05; < 0.08; < 0.11; < 0.14 | 0.1 | 1 |
| Maize, refined oil | 4 | < 0.05; < 0.08; < 0.11; < 0.14 | 0.1 | 1 |
| Rye, bran | 4 | 0.17; 1.3; 1.7; 4.8 | 1.5 | 1 |
| Rye, flour | 4 | 0.11, 0.33; 0.55; 1.5 | 0.44 | 1.1 |
| Rye, wholemeal flour | 4 | 0.01; 0.89; 1.1; 4.4 | 1 | 1.1 |
| Rye, wholemeal bread | 4 | 0.07; 0.48; 0.78; 2.6 | 0.63 | 1 |
| Rye, middlings | 4 | 0.07; 1.2; 1.5; 7.8 | 1.35 | 1 |
| Wheat, bran | 13 | 0.96; 1.2; 1.3; 1.3; 1.6; 1.7; 1.8; 1.8; 1.8; 1.9; 2.0; 2.3; 2.8 | 1.8 | 1 |
| Wheat, flour | 13 | 0.08; 0.12; 0.17; 0.29; 0.52; 0.55; 0.57; 0.58; 0.63; 0.72; 0.72; 0.77; 0.92 | 0.57 | 1 |
| Grass, hay | 6 | 2× 0.8; 1.0; 1.2; 2 × 1.7 | 1.1 | 1 |
| Grass, silage | 7 | 0.6; 2 × 0.7; 3 × 0.9; 1.2 | 0.9 | 1 |
| Indicative processing factors (limited dataset) | ||||
| Soya beans, fat free meal | 2 | 0.95; 1.0 | 0.98 | 1.2 |
| Soya beans, hulls | 2 | 4.4; 5.2 | 4.8 | 1.1 |
| Soya beans, crude oil | 2 | < 0.01; 0.01 | 0.01 | 1 |
| Maize, flour | 2 | 0.9; 0.9 | 0.9 | 1 |
| Wheat, wholemeal flour | 2 | 0.54; 1.7 | 1.1 | 1c |
| Wheat, wholemeal bread | 2 | 0.34; 0.39 | 0.37 | 1c |
| Wheat, middlings | 2 | 0.32; 0.89 | 0.61 | 1c |
| Wheat, semolina | 2 | 0.14; 0.16 | 0.15 | 1c |
| Wheat, semolina bran | 2 | 1.4; 2.2 | 1.8 | 1c |
| Genetically modified GAT crops | ||||
| Robust processing factors (sufficiently supported by data) | ||||
| Rapeseed, refined oil | 3 | < 0.004; 2×< 0.01 | 0.01 | 1 |
| Rapeseed, press cake | 3 | 1.6; 1.5; 0.31 | 1.5 | 1 |
| Indicative processing factors (limited dataset) | ||||
| Soya beans, fat free meal | 1 | 0.68 | 0.68 | 1.3 |
| Soya beans, hulls | 1 | 5.3 | 5.3 | 1.2 |
| Soya beans, refined oil | 1 | < 0.05 | 0.05 | 1 |
| Maize, meal | 2 | 1.1; 0.97 | 1.1 | 1.2 |
| Maize, refined oil | 2 | < 0.53; < 0.83 | 0.68 | 1 |
| Maize, flour | 2 | 0.85; 1.0 | 0.93 | 1.2 |
| Maize, starch | 2 | < 0.53; < 0.83 | 0.68 | 1 |
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
Conversion factor for risk assessment in the processed commodity; median of the individual conversion factors for each residues trial.
Since residues of AMPA were below the LOQ in both row and processed commodities, a CF of 1 is proposed for risk assessment.
Reported as citrus feed meal by the RMS.
B.2. Residues in livestock
| Relevant groups | Dietary burden expressed in | Most critical dieta | Most critical commoditya | Trigger exceeded(Y/N) | |||
|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | ||||||
| Med. | Max. | Med. | Max. | ||||
| Cattle (all diets) | 1.81 | 13.2 | 47.2 | 342 | Cattle (dairy) | Grass, forage (fresh) | Yes |
| Cattle (dairy only) | 1.81 | 13.2 | 47.2 | 342 | Cattle (dairy) | Grass, forage (fresh) | Yes |
| Sheep (all diets) | 2.1 | 17.7 | 62.7 | 530 | Sheep (ram/ewe) | Grass, forage (fresh) | Yes |
| Sheep (ewe only) | 2.1 | 17.7 | 62.7 | 530 | Sheep (ram/ewe) | Grass, forage (fresh) | Yes |
| Swine (all diets) | 0.58 | 2.85 | 25.1 | 123 | Swine (breeding) | Grass, forage (fresh) | Yes |
| Poultry (all diets) | 1.16 | 2.28 | 17.0 | 33.4 | Poultry (layer) | Wheat, straw | Yes |
| Poultry (layer only) | 1.16 | 2.28 | 17.0 | 33.4 | Poultry (layer) | Wheat, straw | Yes |
Calculated for the maximum dietary burden.
B.2.1. Nature of residues and methods of analysis in livestock
B.2.1.1. Metabolism studies, methods of analysis and residue definitions in livestock
| Livestock(available studies) | Animal | Dose(mg/kg bw per day) | Duration(days) | N rate/comment |
|---|---|---|---|---|
| Glyphosate | ||||
| Laying hen | 18.2 | 5–7 | 8N compared to maximum dietary burden poultry | |
| 0.067–7.1 | 4 | Informative only (residues not sufficiently identified) | ||
| Lactating goat | 7.1–8.0 | 5 | 0.5–0.6N compared to maximum dietary burden sheep | |
| Glyphosate and AMPA (9:1) | ||||
| Laying hen | 9.7 glyphosate + 1.03 AMPA | 7 | 5N compared to maximum dietary burden poultry | |
| 32.2 glyphosate + 3.4 AMPA | 7 | 16N compared to maximum dietary burden poultry | ||
| Lactating goat | 4.1 glyphosate + 0.45 AMPA | 5 | 0.3N compared to maximum dietary burden sheep | |
| Glyphosate‐trimesium | ||||
| Laying hen | 4.1 | 10 | 2N compared to maximum dietary burden poultry | |
| Lactating goat | 2.6 | 7 | 0.2N compared to maximum dietary burden sheep | |
| 2.0a | 4 | Informative only (residues not sufficiently identified) | ||
| N ‐acetyl‐glyphosate | ||||
| Laying hen | 4.5 | 7 | 2N compared to maximum dietary burden poultry | |
| Lactating goat | 6.8 | 5 | 0.5N compared to maximum dietary burden sheep | |
| Sources: Germany (2015, 2017) | ||||
Reported in the study as 70 mg/kg in the feed and recalculated assuming a body weight of 70 kg and maximum daily intake of 2 kg feed.
| Time needed to reach a plateau concentration in milk and eggs (days) | Milk: < 7 daysEggs: 14 days (based on 28‐day feeding study, no plateau reached within 8 days in metabolism studies) |
| Metabolism in rat and ruminant similar (Yes/No) | Yes |
| Animal residue definition for monitoring (RD‐Mo) | Sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate |
| Animal residue definition for risk assessment (RD‐RA) | Sum of glyphosate, AMPA, N‐acetyl‐glyphosate and N‐acetyl‐AMPA, expressed as glyphosate |
| Conversion factor (monitoring to risk assessment) | See Appendix B.2.2.1 |
| Fat soluble residues (Yes/No) | No |
| Methods of analysis for monitoring of residues(analytical technique, crop groups, LOQs) | HPLC‐MS/MS; ILV available; LOQ for glyphosate, AMPA and N‐acetyl‐glyphosate: 0.025 mg/kg each in meat, milk and egg and 0.05 mg/kg each in liver, kidney and fat. A confirmatory GC‐MS method is only available for glyphosate in milk, eggs and meat. A confirmatory method for glyphosate in fat and in liver/kidney as well as a confirmatory method for AMPA and N‐acetyl‐glyphosate in all matrices is missing. |
B.2.1.2. Stability of residues in livestock
| Animal products (available studies) | Animal | Commodity | T (°C) | Stability (Months/years) |
|---|---|---|---|---|
| Glyphosate | ||||
| swine | Fat | −20 | 26 months | |
| swine | Muscle | −20 | 26 months | |
| swine | Liver | −20 | 26 months | |
| swine | Kidney | −20 | 26 months | |
| cow | Milk | −20 | 16 months | |
| chicken | Egg | −20 | ≤ 14 months | |
| AMPA | ||||
| swine | Fat | −20 | 26 months | |
| swine | Muscle | −20 | 26 months | |
| swine | Liver | −20 | 26 months | |
| swine | Kidney | −20 | 26 months | |
| cow | Milk | −20 | 16 months | |
| chicken | Egg | −20 | ≤ 14 months | |
|
Source: Germany, 2015 Storage stability of N‐acetyl‐glyphosate and N‐acetyl‐AMPA not investigated. | ||||
B.2.2. Magnitude of residues in livestock
B.2.2.1. Summary of the residue data from livestock feeding studies
| Animal commodity | Residues at the closest feeding level (mg/kg) | Estimated value at 1N | MRL proposal (mg/kg) | CFc | ||
|---|---|---|---|---|---|---|
| Mean | Highest | STMRa (mg/kg) | HRb (mg/kg) | |||
| Cattle (all diets) – Closest feeding level (19.4 mg/kg bw per day; 1.5N dietary burden)d | ||||||
| Muscle | 0.20 | 0.20 | 0.17 | 0.18 | 0.2e(tentative) | 1 |
| Fat | 0.20 | 0.22 | < 0.2 | < 0.2 | 0.2*, e , f (tentative) | 1 |
| Liver | 0.71 | 0.85 | 0.54 | 0.69 | 0.7e , f (tentative) | 1 |
| Kidney | 8.39 | 10.2 | 0.69 | 6.82 | 7e , f (tentative) | 1 |
| Cattle (dairy only) – Closest feeding level (19.4 mg/kg bw per day; 1.5N dietary burden)d | ||||||
| Milkg | 0.10 | n.a. | < 0.1 | < 0.1 | 0.1*, e (tentative) | 1 |
| Sheep (all diets)h – Closest feeding level (19.4 mg/kg bw; 1.1N dietary burden)d | ||||||
| Muscle | 0.20 | 0.20 | 0.17 | 0.19 | 0.2e (tentative) | 1 |
| Fat | 0.20 | 0.22 | 0.17 | 0.21 | 0.3e , f (tentative) | 1 |
| Liver | 0.71 | 0.85 | 0.54 | 0.81 | 0.9e , f (tentative) | 1 |
| Kidney | 8.39 | 10.2 | 0.81 | 9.28 | 10e , f (tentative) | 1 |
| Sheep (dairy only)h – Closest feeding level (19.4 mg/kg bw; 1.1N dietary burden)d | ||||||
| Milkg | 0.10 | n.a. | < 0.1 | < 0.1 | 0.1*, e (tentative) | 1 |
| Swine – Closest feeding level (3.91 mg/kg bw per day; 1.4N rate)i | ||||||
| Muscle | < 0.17 | < 0.17 | < 0.17 | < 0.17 | 0.2e (tentative) | 1 |
| Fat | < 0.17 | < 0.17 | < 0.2 | < 0.2 | 0.2*, e , f (tentative) | 1 |
| Liver | 0.42 | 0.46 | < 0.17 | 0.35 | 0.4e , f (tentative) | 1 |
| kidney | 3.07 | 3.58 | 0.22 | 2.46 | 3e , f (tentative) | 1 |
| Poultry (all diets) – Closest feeding level (2.96 mg/kg bw per day; 1.3N rate)j | ||||||
| Muscle | < 0.17 | < 0.17 | < 0.17 | < 0.17 | 0.2e (tentative) | 1 |
| Fat | < 0.17 | < 0.17 | < 0.2 | < 0.2 | 0.2*, e , f (tentative) | 1 |
| Liver | 0.19 | 0.20 | < 0.2 | < 0.2 | 0.2*, e , f (tentative) | 1 |
| Poultry (layer only) – Closest feeding level (2.96 mg/kg bw per day; 1.3N rate)j | ||||||
| Eggs | < 0.10 | < 0.10 | < 0.10 | < 0.10 | 0.1*, e (tentative) | 1 |
n.a.: not applicable.
*Indicates that the MRL is proposed at the limit of quantification.
The mean residue level for milk and the mean residue levels for eggs and tissues were recalculated at the 1N rate for the median dietary burden.
The mean residue level in milk and the highest residue levels in eggs and tissues were recalculated at the 1N rate for the maximum dietary burden.
Conversion factor from enforcement to risk assessment. CF of 1 is proposed because N‐acetyl‐AMPA is not expected at significant levels.
Closest feeding level and N dose rate related to the maximum dietary burden. Study performed with glyphosate‐trimesium with dose rate expressed as glyphosate equivalents.
MRL proposal is tentative because a confirmatory method for AMPA and N‐acetyl‐glyphosate is still required for all animal matrices.
MRL proposal is tentative because a confirmatory method for glyphosate is still required for fat, liver and kidney.
Highest residue level from day 1 to day 28 (daily mean of 2 cows).
Since extrapolation from cattle to other ruminants and swine is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in sheep.
Closest feeding level and N dose rate related to the maximum dietary burden. Study performed on pigs dosed with glyphosate and AMPA at 9:1. Dose rate reported refer to the sum of glyphosate and AMPA, expressed as glyphosate.
Closest feeding level and N dose rate related to the maximum dietary burden. Study performed on hens dosed with glyphosate and AMPA at 9:1. Dose rate reported refer to the sum of glyphosate and AMPA, expressed as glyphoate.
B.3. Consumer risk assessment
B.3.1. Consumer risk assessment without consideration of the existing CXLs
| ADI | 0.5 mg/kg bw per day (EFSA, 2015) |
| Highest IEDI, according to EFSA PRIMo |
Scenario 1 (considering the main RD‐monitoring): 9.1% ADI (WHO, cluster diet B) Scenario 2 (considering the optional RD‐monitoring): 9.9% ADI (WHO, cluster diet B) |
| Assumptions made for the calculations |
Scenario 1 (considering the main RD‐monitoring): The calculation is based on the median residue levels and conversion factors in the raw agricultural commodities derived from the reported uses on conventional and genetically modified crops. For those commodities where data were insufficient to derive a MRL, EFSA considered the existing EU MRL multiplied by a conversion factor for an indicative calculation. For sunflower, soyabeans and mustard seed, the conversion factor of 1.1 (as derived from trials performed on other oildseeds) was considered. For buckwheat and rice grain, the conversion factor of 2.3 (as derived from trials performed on other cereals) was considered. For cultivated fungi, the conversion factor of 2.3 (worst‐case CF derived in this review) was considered. The contributions of commodities where no GAP was reported in the framework of this review were not included in the calculation. Scenario 2 (considering the optional RD‐monitoring): The calculation is based on the median residue levels in the raw agricultural commodities derived from the reported uses on conventional and genetically modified crops and expressed under the optional residue definition for monitoring (i.e. including glyphosate, AMPA and N‐acetyl‐glyphosate for all commodities). No CF was considered because residues of N‐acetyl‐AMPA above the LOQ are not expected. For MRLs proposed at the LOQ, risk assessment was performed considering a combinded LOQ (summing up individual LOQs of glyphosate, AMPA and N‐acetyl‐glyphosate). For those commodities where data were insufficient to derive a MRL, EFSA considered the existing EU MRL. |
| ARfD | 0.5 mg/kg bw (EFSA, 2015) |
| Highest IESTI, according to EFSA PRIMo |
Scenario 1 (considering the main RD‐monitoring): 55.7% ARfD (dry beans) Scenario 2 (considering the optional RD‐monitoring): 55.7% ARfD (dry beans) |
| Assumptions made for the calculations |
Scenario 1 (considering the main RD‐monitoring): The calculation is based on the highest residue levels and conversion factors in the raw agricultural commodities derived from the reported uses on conventional and genetically modified crops. For those commodities where data were insufficient to derive a MRL, EFSA considered the existing EU MRL multiplied by a conversion factor for an indicative calculation, as follows. For sunflower, soyabeans and mustard seed, the conversion factor of 1.1 (as derived from trials performed on other oilseeds) was considered. For buckwheat and rice grain, the conversion factor of 2.3 (as derived from trials performed on other cereals) was considered. For cultivated fungi, the conversion factor of 2.3 (worst‐case CF derived in this review) was considered. The contributions of commodities where no GAP was reported in the framework of this review were not included in the calculation.Scenario 2 (considering the optional RD‐monitoring): The calculation is based on the highest residue levels in the raw agricultural commodities derived from the reported uses on conventional and genetically modified crops and expressed under the optional residue definition for monitoring (i.e. including glyphosate, AMPA and N‐acetyl‐glyphosate for all commodities). No CF was considered because residues of N‐acetyl‐AMPA above the LOQ are not expected. For MRLs proposed at the LOQ, risk assessment was performed considering a combinded LOQ (summing up individual LOQs of glyphosate, AMPA and N‐acetyl‐glyphosate). For those commodities where data were insufficient to derive a MRL, EFSA considered the existing EU MRL. |
ADI: acceptable daily intake; bw: body weight; IEDI: international estimated daily intake; PRIMo: (EFSA) Pesticide Residues Intake Model; WHO: World Health Organization; ARfD: acute reference dose; IESTI: international estimated short‐term intake.
B.3.2. Consumer risk assessment with consideration of the existing CXLs
| ADI | 0.5 mg/kg bw per day (EFSA, 2015) |
| Highest IEDI, according to EFSA PRIMo |
Scenario 1 (considering the main RD‐monitoring): 18.7% ADI (UK todder)Scenario 2 (considering the optional RD‐monitoring): 19.0% ADI (UK todder) |
| Assumptions made for the calculations |
Scenario 1 (considering the main RD‐monitoring): For those commodities having a CXL higher than the EU MRL proposal, the median residue levels from the EU scenario were replaced by the median residue levels derived by JMPR. CXLs for sweet corn, cotton seeds, soybean, maize and all livestock commodities having a different residue definition (not comparable with the definition derived by EFSA), could not be included in the calculation. Scenario 2 (considering the optional RD‐monitoring): For those commodities having a CXL higher than the EU MRL proposal, the median residue levels from the EU scenario were replaced by the median residue levels derived by JMPR. CXLs for sweet corn, cotton seeds, soybean, maize, dry beans, dry lentils, dry peas, sunflower seeds, sugar canes and all livestock commodities having a different residue definition (not comparable with the optional definition), could not be included in the calculation. |
| ARfD | 0.5 mg/kg bw per day (EFSA, 2015) |
| Highest IESTI, according to EFSA PRIMo |
Scenario 1 (considering the main RD‐monitoring): 91% ARfD (sugar beet roots) Scenario 2 (considering the optional RD‐monitoring): 91% ARfD (sugar beet roots) |
| Assumptions made for the calculations |
Scenario 1 (considering the main RD‐monitoring): For those commodities having a CXL higher than the EU MRL proposal, the highest residue levels from the EU scenario were replaced by the highest residue levels derived by JMPR. CXLs for sweet corn, cotton seeds, soybean, maize and all livestock commodities having a different residue definition (not comparable with the definition derived by EFSA), could not be included in the calculation. Scenario 2 (considering the optional RD‐monitoring): For those commodities having a CXL higher than the EU MRL proposal, the median residue levels from the EU scenario were replaced by the median residue levels derived by JMPR. CXLs for sweet corn, cotton seeds, soybean, maize, dry beans, dry lentils, dry peas, sunflower seeds, sugar canes and all livestock commodities having a different residue definition (not comparable with the optional definition), could not be included in the calculation. |
B.4. Proposed MRLs
B.4.1. Main residue definition for enforcement
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
| Enforcement residue definition: glyphosate | |||||
| 110010 | Grapefruits | 0.1* | – | 0.05* | Recommendeda |
| 110020 | Oranges | 0.5 | – | 0.05* | Recommendeda |
| 110030 | Lemons | 0.1* | – | 0.05* | Recommendeda |
| 110040 | Limes | 0.1* | – | 0.05* | Recommendeda |
| 110050 | Mandarins | 0.5 | – | 0.05* | Recommendeda |
| 120010 | Almonds | 0.1* | – | 0.05* | Recommendeda |
| 120020 | Brazil nuts | 0.1* | – | 0.05* | Recommendeda |
| 120030 | Cashew nuts | 0.1* | – | 0.05* | Recommendeda |
| 120040 | Chestnuts | 0.1* | – | 0.05* | Recommendeda |
| 120050 | Coconuts | 0.1* | – | 0.05* | Recommendeda |
| 120060 | Hazelnuts/cobnuts | 0.1* | – | 0.05* | Recommendeda |
| 120070 | Macadamias | 0.1* | – | 0.05* | Recommendeda |
| 120080 | Pecans | 0.1* | – | 0.05* | Recommendeda |
| 120090 | Pine nut kernels | 0.1* | – | 0.05* | Recommendeda |
| 120100 | Pistachios | 0.1* | – | 0.05* | Recommendeda |
| 120110 | Walnuts | 0.1* | – | 0.05* | Recommendeda |
| 130010 | Apples | 0.1* | – | 0.05* | Recommendeda |
| 130020 | Pears | 0.1* | – | 0.05* | Recommendeda |
| 130030 | Quinces | 0.1* | – | 0.05* | Recommendeda |
| 130040 | Medlars | 0.1* | – | 0.05* | Recommendeda |
| 130050 | Loquats/Japanese medlars | 0.1* | – | 0.05* | Recommendeda |
| 140010 | Apricots | 0.1* | – | 0.05* | Recommendeda |
| 140020 | Cherries (sweet) | 0.1* | – | 0.05* | Recommendeda |
| 140030 | Peaches | 0.1* | – | 0.05* | Recommendeda |
| 140040 | Plums | 0.1* | – | 0.05* | Recommendeda |
| 151010 | Table grapes | 0.5 | – | 0.05* | Recommendeda |
| 151020 | Wine grapes | 0.5 | – | 0.05* | Recommendeda |
| 152000 | Strawberries | 0.1* | – | 0.05* | Further consideration neededb |
| 153010 | Blackberries | 0.1* | – | 0.05* | Further consideration neededb |
| 153020 | Dewberries | 0.1* | – | 0.05* | Further consideration neededb |
| 153030 | Raspberries (red and yellow) | 0.1* | – | 0.05* | Further consideration neededb |
| 154010 | Blueberries | 0.1* | – | 0.05* | Further consideration neededb |
| 154020 | Cranberries | 0.1* | – | 0.05* | Further consideration neededb |
| 154030 | Currants (black, red and white) | 0.1* | – | 0.05* | Further consideration neededb |
| 154040 | Gooseberries (green, red and yellow) | 0.1* | – | 0.05* | Further consideration neededb |
| 154050 | Rose hips | 0.1* | – | 0.05* | Further consideration neededb |
| 154060 | Mulberries (black and white) | 0.1* | – | 0.05* | Further consideration neededb |
| 154070 | Azaroles/Mediterranean medlars | 0.1* | – | 0.05* | Further consideration neededb |
| 154080 | Elderberries | 0.1* | – | 0.05* | Further consideration neededb |
| 161020 | Figs | 0.1* | – | 0.05* | Recommendeda |
| 161030 | Table olives | 1 | – | 0.05* | Recommendeda |
| 161040 | Kumquats | 0.1* | – | 0.05* | Recommendeda |
| 161060 | Kaki/Japanese persimmons | 0.1* | – | 0.05* | Recommendeda |
| 162010 | Kiwi fruits (green, red, yellow) | 0.1* | – | 0.05* | Recommendeda |
| 162020 | Litchis/lychees | 0.1* | – | 0.05* | Recommendeda |
| 162030 | Passionfruits/maracujas | 0.1* | – | 0.05* | Recommendeda |
| 163010 | Avocados | 0.1* | – | 0.05* | Recommendeda |
| 163020 | Bananas | 0.1* | 0.05* | 0.05* | Recommendedf |
| 163030 | Mangoes | 0.1* | – | 0.05* | Recommendeda |
| 163040 | Papayas | 0.1* | – | 0.05* | Recommendeda |
| 163050 | Granate apples/pomegranates | 0.1* | – | 0.05* | Recommendeda |
| 163060 | Cherimoyas | 0.1* | – | 0.05* | Recommendeda |
| 211000 | Potatoes | 0.5 | – | 1 | Further consideration neededb |
| 212010 | Cassava roots/manioc | 0.1* | – | 0.05* | Further consideration neededb |
| 212020 | Sweet potatoes | 0.1* | – | 0.05* | Further consideration neededb |
| 212030 | Yams | 0.1* | – | 0.05* | Further consideration neededb |
| 212040 | Arrowroots | 0.1* | – | 0.05* | Further consideration neededb |
| 213010 | Beetroots | 0.1* | – | 0.05* | Further consideration neededb |
| 213020 | Carrots | 0.1* | – | 0.05* | Recommendeda |
| 213030 | Celeriacs/turnip rooted celeries | 0.1* | – | 0.05* | Further consideration neededb |
| 213040 | Horseradishes | 0.1* | – | 0.05* | Further consideration neededb |
| 213050 | Jerusalem artichokes | 0.1* | – | 0.05* | Further consideration neededb |
| 213060 | Parsnips | 0.1* | – | 0.05* | Further consideration neededb |
| 213070 | Parsley roots/Hamburg roots parsley | 0.1* | – | 0.05* | Further consideration neededb |
| 213080 | Radishes | 0.1* | – | 0.05* | Further consideration neededb |
| 213090 | Salsifies | 0.1* | – | 0.05* | Further consideration neededb |
| 213100 | Swedes/rutabagas | 0.1* | – | 0.05* | Further consideration neededb |
| 213110 | Turnips | 0.1* | – | 0.05* | Further consideration neededb |
| 220010 | Garlic | 0.1* | – | 0.05* | Further consideration neededb |
| 220020 | Onions | 0.1* | – | 0.05* | Further consideration neededb |
| 220030 | Shallots | 0.1* | – | 0.05* | Further consideration neededb |
| 220040 | Spring onions/green onions and Welsh onions | 0.1* | – | 0.05* | Further consideration neededb |
| 231010 | Tomatoes | 0.1* | – | 0.05* | Recommendeda |
| 231020 | Sweet peppers/bell peppers | 0.1* | – | 0.05* | Further consideration neededb |
| 231030 | Aubergines/eggplants | 0.1* | – | 0.05* | Recommendeda |
| 231040 | Okra/lady's fingers | 0.1* | – | 0.05* | Further consideration neededb |
| 232010 | Cucumbers | 0.1* | – | 0.05* | Further consideration neededb |
| 232020 | Gherkins | 0.1* | – | 0.05* | Further consideration neededb |
| 232030 | Courgettes | 0.1* | – | 0.05* | Further consideration neededb |
| 233010 | Melons | 0.1* | – | 0.05* | Further consideration neededb |
| 233020 | Pumpkins | 0.1* | – | 0.05* | Further consideration neededb |
| 233030 | Watermelons | 0.1* | – | 0.05* | Further consideration neededb |
| 241010 | Broccoli | 0.1* | – | 0.05* | Further consideration neededb |
| 241020 | Cauliflowers | 0.1* | – | 0.05* | Further consideration neededb |
| 242010 | Brussels sprouts | 0.1* | – | 0.05* | Further consideration neededb |
| 242020 | Head cabbages | 0.1* | – | 0.05* | Further consideration neededb |
| 243010 | Chinese cabbages/pe‐tsai | 0.1* | – | 0.05* | Further consideration neededb |
| 243020 | Kales | 0.1* | – | 0.05* | Further consideration neededb |
| 244000 | Kohlrabies | 0.1* | – | 0.05* | Further consideration neededb |
| 251010 | Lamb's lettuces/corn salads | 0.1* | – | 0.05* | Further consideration neededb |
| 251020 | Lettuces | 0.1* | – | 0.05* | Further consideration neededb |
| 251030 | Escaroles/broad‐leaved endives | 0.1* | – | 0.05* | Further consideration neededb |
| 251040 | Cresses and other sprouts and shoots | 0.1* | – | 0.05* | Further consideration neededb |
| 251050 | Land cresses | 0.1* | – | 0.05* | Further consideration neededb |
| 251060 | Roman rocket/rucola | 0.1* | – | 0.05* | Further consideration neededb |
| 251070 | Red mustards | 0.1* | – | 0.05* | Further consideration neededb |
| 251080 | Baby leaf crops (including brassica species) | 0.1* | – | 0.05* | Further consideration neededb |
| 252010 | Spinaches | 0.1* | – | 0.05* | Further consideration neededb |
| 252020 | Purslanes | 0.1* | – | 0.05* | Further consideration neededb |
| 252030 | Chards/beet leaves | 0.1* | – | 0.05* | Further consideration neededb |
| 253000 | Grape leaves and similar species | 0.1* | – | 0.05* | Further consideration neededb |
| 254000 | Watercresses | 0.1* | – | 0.05* | Further consideration neededb |
| 255000 | Witloofs/Belgian endives | 0.1* | – | 0.05* | Further consideration neededb |
| 256010 | Chervil | 0.1* | – | 0.05* | Further consideration neededb |
| 256020 | Chives | 0.1* | – | 0.05* | Further consideration neededb |
| 256030 | Celery leaves | 0.1* | – | 0.05* | Further consideration neededb |
| 256040 | Parsley | 0.1* | – | 0.05* | Further consideration neededb |
| 256050 | Sage | 0.1* | – | 0.05* | Further consideration neededb |
| 256060 | Rosemary | 0.1* | – | 0.05* | Further consideration neededb |
| 256070 | Thyme | 0.1* | – | 0.05* | Further consideration neededb |
| 256080 | Basil and edible flowers | 0.1* | – | 0.05* | Further consideration neededb |
| 256090 | Laurel/bay leave | 0.1* | – | 0.05* | Further consideration neededb |
| 256100 | Tarragon | 0.1* | – | 0.05* | Further consideration neededb |
| 260010 | Beans (with pods) | 0.1* | – | 0.05* | Recommendeda |
| 260020 | Beans (without pods) | 0.1* | – | 0.05* | Recommendeda |
| 260030 | Peas (with pods) | 0.1* | – | 0.05* | Recommendeda |
| 260040 | Peas (without pods) | 0.1* | – | 0.05* | Recommendeda |
| 260050 | Lentils (fresh) | 0.1* | – | 0.05* | Recommendeda |
| 270010 | Asparagus | 0.1* | – | 0.05* | Further consideration neededb |
| 270020 | Cardoons | 0.1* | – | 0.05* | Further consideration neededb |
| 270030 | Celeries | 0.1* | – | 0.05* | Further consideration neededb |
| 270040 | Florence fennels | 0.1* | – | 0.05* | Further consideration neededb |
| 270050 | Globe artichokes | 0.1* | – | 0.05* | Recommendeda |
| 270060 | Leeks | 0.1* | – | 0.05* | Further consideration neededb |
| 270070 | Rhubarbs | 0.1* | – | 0.05* | Further consideration neededb |
| 270080 | Bamboo shoots | 0.1* | – | 0.05* | Further consideration neededb |
| 270090 | Palm hearts | 0.1* | – | 0.05* | Further consideration neededb |
| 280010 | Cultivated fungi | 0.1* | – | 0.1 | Further consideration neededc |
| 280020 | Wild fungi | 50 | – | 0.05* | Recommendeda |
| 300010 | Beans (dry) | 2 | 2 | 15 | Further consideration neededd |
| 300020 | Lentils (dry) | 10 | 5 | 15 | Further consideration neededd |
| 300030 | Peas (dry) | 10 | 5 | 15 | Further consideration neededd |
| 300040 | Lupins/lupine beans (dry) | 10 | – | 15 | Further consideration neededb |
| 401010 | Linseeds | 10 | – | 15 | Recommendeda |
| 401020 | Peanuts/groundnuts | 0.1* | – | 0.05* | Further consideration neededb |
| 401030 | Poppy seeds | 0.1* | – | 0.05* | Further consideration neededb |
| 401040 | Sesame seeds | 0.1* | – | 0.05* | Further consideration neededb |
| 401050 | Sunflower seeds | 20 | 7 | 20 | Further consideration needede |
| 401080 | Mustard seeds | 10 | – | 10 | Further consideration neededc |
| 401100 | Pumpkin seeds | 0.1* | – | 0.05* | Further consideration neededb |
| 401110 | Safflower seeds | 0.1* | – | 0.05* | Further consideration neededb |
| 401120 | Borage seeds | 0.1 | – | 10 | Recommendeda |
| 401130 | Gold of pleasure seeds | 0.1 | – | 0.05* | Further consideration neededb |
| 401140 | Hemp seeds | 0.1* | – | 0.05* | Further consideration neededb |
| 401150 | Castor beans | 0.1 | – | 0.05* | Further consideration neededb |
| 402010 | Olives for oil production | 1 | – | 30 | Recommendeda |
| 402020 | Oil palms kernels | 0.1 | – | 0.05* | Recommendeda |
| 402030 | Oil palms fruits | 0.1 | – | 0.05* | Further consideration neededb |
| 402040 | Kapok | 0.1 | – | 0.05* | Recommendeda |
| 500010 | Barley grains | 20 | 30 | 30 | Recommendedf |
| 500020 | Buckwheat and other pseudo‐cereal grains | 0.1* | 30 | 30 | Recommendedg |
| 500040 | Common millet/proso millet grains | 0.1* | 30 | 30 | Recommendedh |
| 500050 | Oat grains | 20 | 30 | 30 | Recommendedf |
| 500060 | Rice grains | 0.1* | – | 0.1 | Further consideration neededc |
| 500070 | Rye grains | 10 | 30 | 30 | Recommendedh |
| 500080 | Sorghum grains | 20 | 30 | 30 | Recommendedh |
| 500090 | Wheat grains | 10 | 30 | 30 | Recommendedh |
| 610000 | Teas | 2 | – | 0.05* | Further consideration neededb |
| 620000 | Coffee beans | 0.1 | – | 0.05* | Further consideration neededb |
| 631000 | Herbal infusions from flowers | 2* | – | 0.05* | Further consideration neededb |
| 632000 | Herbal infusions from leaves and herbs | 2* | – | 0.05* | Further consideration neededb |
| 633000 | Herbal infusions from roots | 2* | – | 0.05* | Further consideration neededb |
| 650000 | Carobs/Saint John's breads | 0.1* | – | 0.05* | Further consideration neededb |
| 700000 | Hops | 0.1* | – | 0.05* | Further consideration neededb |
| 810000 | Seed spices | 0.1* | – | 0.05* | Further consideration neededb |
| 820000 | Fruit spices | 0.1* | – | 0.05* | Further consideration neededb |
| 830000 | Bark spices | 0.1* | – | 0.05* | Further consideration neededb |
| 840000 | Root and rhizome spices | 0.1* | – | 0.05* | Further consideration neededb |
| 850000 | Bud spices | 0.1* | – | 0.05* | Further consideration neededb |
| 860000 | Flower pistil spices | 0.1* | – | 0.05* | Further consideration neededb |
| 870000 | Aril spices | 0.1* | – | 0.05* | Further consideration neededb |
| 900020 | Sugar canes | 0.1* | 2 | 2 | Recommendedm |
| 900030 | Chicory roots | 0.1* | – | 0.05* | Further consideration neededb |
| – | Other commodities of plant origin | – | – | Further consideration neededi | |
|
Enforcement residue definition (existing): glyphosate Enforcement residue definition (proposed): sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate | |||||
| 234000 | Sweet corn | 3 | 3 | 3 | Further consideration neededj |
| 401060 | Rapeseeds/canola seeds | 10 | 30 | 30 | Further consideration neededk |
| 401070 | Soyabeans | 20 | 20 | 20 | Further consideration neededl |
| 401090 | Cotton seeds | 10 | 40 | 60 | Further consideration neededj |
| 500030 | Maize/corn grains | 1 | 5 | 3 | Further consideration neededj |
| 900010 | Sugar beet roots | 15 | 15 | 15 | Further consideration neededk |
| 1011010 | Swine muscle | 0.05* | 0.05* | 0.2 | Further consideration neededj |
| 1011020 | Swine fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededj |
| 1011030 | Swine liver | 0.05* | 0.5 | 0.4 | Further consideration neededj |
| 1011040 | Swine kidney | 0.5 | 0.5 | 3 | Further consideration neededj |
| 1012010 | Bovine muscle | 0.05* | 0.05* | 0.2 | Further consideration neededj |
| 1012020 | Bovine fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededj |
| 1012030 | Bovine liver | 0.2 | 5 | 0.7 | Further consideration neededj |
| 1012040 | Bovine kidney | 2 | 5 | 7 | Further consideration neededj |
| 1013010 | Sheep muscle | 0.05* | 0.05* | 0.2 | Further consideration neededj |
| 1013020 | Sheep fat tissue | 0.05* | 0.05* | 0.3 | Further consideration neededj |
| 1013030 | Sheep liver | 0.05* | 5 | 0.9 | Further consideration neededj |
| 1013040 | Sheep kidney | 0.05* | 5 | 10 | Further consideration neededj |
| 1014010 | Goat muscle | 0.05* | 0.05* | 0.2 | Further consideration neededj |
| 1014020 | Goat fat tissue | 0.05* | 0.05* | 0.3 | Further consideration neededj |
| 1014030 | Goat liver | 0.05* | 5 | 0.9 | Further consideration neededj |
| 1014040 | Goat kidney | 0.05* | 5 | 10 | Further consideration neededj |
| 1015010 | Equine muscle | 0.05* | 0.05* | 0.2 | Further consideration neededj |
| 1015020 | Equine fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededj |
| 1015030 | Equine liver | 0.05* | 5 | 0.7 | Further consideration neededj |
| 1015040 | Equine kidney | 0.05* | 5 | 7 | Further consideration neededj |
| 1016010 | Poultry muscle | 0.05* | 0.05* | 0.2 | Further consideration neededj |
| 1016020 | Poultry fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededj |
| 1016030 | Poultry liver | 0.05* | 0.5 | 0.2* | Further consideration neededj |
| 1020010 | Cattle milk | 0.05* | 0.05 | 0.1* | Further consideration neededj |
| 1020020 | Sheep milk | 0.05* | 0.05 | 0.1* | Further consideration neededj |
| 1020030 | Goat milk | 0.05* | 0.05 | 0.1* | Further consideration neededj |
| 1020040 | Horse milk | 0.05* | 0.05 | 0.1* | Further consideration neededj |
| 1030000 | Birds eggs | 0.05* | 0.05* | 0.1* | Further consideration neededj |
| – | Other commodities of animal origin | – | – | Further consideration neededi | |
MRL: maximum residue level; CXL: codex maximum residue limit.
*Indicates that the MRL is set at the limit of quantification.
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available (combination G‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; no CXL is available (combination E‐I in Appendix E).
GAP evaluated at EU level is not supported by data, but no risk to consumers was identified for the existing EU MRL; no CXL is available (combination C‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; existing CXL is covered by the tentative MRL (combination E‐III in Appendix E).
GAP evaluated at EU level is not supported by data, but no risk to consumers was identified for the existing EU MRL; existing CXL is covered by the existing EU MRL (combination C‐III in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; existing CXL is covered by the recommended MRL (combination G‐III in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level is not supported by data, but the existing EU MRL is lower than the existing CXL (combination C‐VII in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level, which is also fully supported by data, leads to a lower MRL (combination G‐VII in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; CXL is not compatible with EU residue definitions (combination E‐II in Appendix E).
MRL is derived from the existing CXL, which is not sufficiently supported by data but for which no risk to consumers is identified; GAP evaluated at EU level, which is also not fully supported by data, would lead to a lower tentative MRL (combination E‐V in Appendix E).
GAP evaluated at EU level is not supported by data, but no risk to consumers was identified for the existing EU MRL; CXL is not compatible with EU residue definitions (combination C‐II in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level, which is not fully supported by data, leads to a lower tentative MRL (combination E‐VII in Appendix E).
B.4.2. Optional residue definition for enforcement
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
| Enforcement residue definition (existing): glyphosateEnforcement residue definition (proposed ‐ optional): sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate | |||||
| 110010 | Grapefruits | 0.1* | – | 0.2* | Further consideration neededa |
| 110020 | Oranges | 0.5 | – | 0.2* | Further consideration neededa |
| 110030 | Lemons | 0.1* | – | 0.2* | Further consideration neededa |
| 110040 | Limes | 0.1* | – | 0.2* | Further consideration neededa |
| 110050 | Mandarins | 0.5 | – | 0.2* | Further consideration neededa |
| 120010 | Almonds | 0.1* | – | 0.2* | Further consideration neededa |
| 120020 | Brazil nuts | 0.1* | – | 0.2* | Further consideration neededa |
| 120030 | Cashew nuts | 0.1* | – | 0.2* | Further consideration neededa |
| 120040 | Chestnuts | 0.1* | – | 0.2* | Further consideration neededa |
| 120050 | Coconuts | 0.1* | – | 0.2* | Further consideration neededa |
| 120060 | Hazelnuts/cobnuts | 0.1* | – | 0.2* | Further consideration neededa |
| 120070 | Macadamias | 0.1* | – | 0.2* | Further consideration neededa |
| 120080 | Pecans | 0.1* | – | 0.2* | Further consideration neededa |
| 120090 | Pine nut kernels | 0.1* | – | 0.2* | Further consideration neededa |
| 120100 | Pistachios | 0.1* | – | 0.2* | Further consideration neededa |
| 120110 | Walnuts | 0.1* | – | 0.2* | Further consideration neededa |
| 130010 | Apples | 0.1* | – | 0.2* | Further consideration neededa |
| 130020 | Pears | 0.1* | – | 0.2* | Further consideration neededa |
| 130030 | Quinces | 0.1* | – | 0.2* | Further consideration neededa |
| 130040 | Medlars | 0.1* | – | 0.2* | Further consideration neededa |
| 130050 | Loquats/Japanese medlars | 0.1* | – | 0.2* | Further consideration neededa |
| 140010 | Apricots | 0.1* | – | 0.2* | Further consideration neededa |
| 140020 | Cherries (sweet) | 0.1* | – | 0.2* | Further consideration neededa |
| 140030 | Peaches | 0.1* | – | 0.2* | Further consideration neededa |
| 140040 | Plums | 0.1* | – | 0.2* | Further consideration neededa |
| 151010 | Table grapes | 0.5 | – | 0.2* | Further consideration neededa |
| 151020 | Wine grapes | 0.5 | – | 0.2* | Further consideration neededa |
| 152000 | Strawberries | 0.1* | – | 0.2* | Further consideration neededa |
| 153010 | Blackberries | 0.1* | – | 0.2* | Further consideration neededa |
| 153020 | Dewberries | 0.1* | – | 0.2* | Further consideration neededa |
| 153030 | Raspberries (red and yellow) | 0.1* | – | 0.2* | Further consideration neededa |
| 154010 | Blueberries | 0.1* | – | 0.2* | Further consideration neededa |
| 154020 | Cranberries | 0.1* | – | 0.2* | Further consideration neededa |
| 154030 | Currants (black, red and white) | 0.1* | – | 0.2* | Further consideration neededa |
| 154040 | Gooseberries (green, red and yellow) | 0.1* | – | 0.2* | Further consideration neededa |
| 154050 | Rose hips | 0.1* | – | 0.2* | Further consideration neededa |
| 154060 | Mulberries (black and white) | 0.1* | – | 0.2* | Further consideration neededa |
| 154070 | Azaroles/Mediterranean medlars | 0.1* | – | 0.2* | Further consideration neededa |
| 154080 | Elderberries | 0.1* | – | 0.2* | Further consideration neededa |
| 161020 | Figs | 0.1* | – | 0.2* | Further consideration neededa |
| 161030 | Table olives | 1 | – | 0.2* | Further consideration neededa |
| 161040 | Kumquats | 0.1* | – | 0.2* | Further consideration neededa |
| 161060 | Kaki/Japanese persimmons | 0.1* | – | 0.2* | Further consideration neededa |
| 162010 | Kiwi fruits (green, red, yellow) | 0.1* | – | 0.2* | Further consideration neededa |
| 162020 | Litchis/lychees | 0.1* | – | 0.2* | Further consideration neededa |
| 162030 | Passionfruits/maracujas | 0.1* | – | 0.2* | Further consideration neededa |
| 163010 | Avocados | 0.1* | – | 0.2* | Further consideration neededa |
| 163020 | Bananas | 0.1* | 0.05* | 0.2* | Further consideration neededb |
| 163030 | Mangoes | 0.1* | – | 0.2* | Further consideration neededa |
| 163040 | Papayas | 0.1* | – | 0.2* | Further consideration neededa |
| 163050 | Granate apples/pomegranates | 0.1* | – | 0.2* | Further consideration neededa |
| 163060 | Cherimoyas | 0.1* | – | 0.2* | Further consideration neededa |
| 211000 | Potatoes | 0.5 | – | 1 | Further consideration neededa |
| 212010 | Cassava roots/manioc | 0.1* | – | 0.2* | Further consideration neededa |
| 212020 | Sweet potatoes | 0.1* | – | 0.2* | Further consideration neededa |
| 212030 | Yams | 0.1* | – | 0.2* | Further consideration neededa |
| 212040 | Arrowroots | 0.1* | – | 0.2* | Further consideration neededa |
| 213010 | Beetroots | 0.1* | – | 0.2* | Further consideration neededa |
| 213020 | Carrots | 0.1* | – | 0.2* | Further consideration neededa |
| 213030 | Celeriacs/turnip rooted celeries | 0.1* | – | 0.2* | Further consideration neededa |
| 213040 | Horseradishes | 0.1* | – | 0.2* | Further consideration neededa |
| 213050 | Jerusalem artichokes | 0.1* | – | 0.2* | Further consideration neededa |
| 213060 | Parsnips | 0.1* | – | 0.2* | Further consideration neededa |
| 213070 | Parsley roots/Hamburg roots parsley | 0.1* | – | 0.2* | Further consideration neededa |
| 213080 | Radishes | 0.1* | – | 0.2* | Further consideration neededa |
| 213090 | Salsifies | 0.1* | – | 0.2* | Further consideration neededa |
| 213100 | Swedes/rutabagas | 0.1* | – | 0.2* | Further consideration neededa |
| 213110 | Turnips | 0.1* | – | 0.2* | Further consideration neededa |
| 220010 | Garlic | 0.1* | – | 0.2* | Further consideration neededa |
| 220020 | Onions | 0.1* | – | 0.2* | Further consideration neededa |
| 220030 | Shallots | 0.1* | – | 0.2* | Further consideration neededa |
| 220040 | Spring onions/green onions and Welsh onions | 0.1* | – | 0.2* | Further consideration neededa |
| 231010 | Tomatoes | 0.1* | – | 0.2* | Further consideration neededa |
| 231020 | Sweet peppers/bell peppers | 0.1* | – | 0.2* | Further consideration neededa |
| 231030 | Aubergines/eggplants | 0.1* | – | 0.2* | Further consideration neededa |
| 231040 | Okra/lady's fingers | 0.1* | – | 0.2* | Further consideration neededa |
| 232010 | Cucumbers | 0.1* | – | 0.2* | Further consideration neededa |
| 232020 | Gherkins | 0.1* | – | 0.2* | Further consideration neededa |
| 232030 | Courgettes | 0.1* | – | 0.2* | Further consideration neededa |
| 233010 | Melons | 0.1* | – | 0.2* | Further consideration neededa |
| 233020 | Pumpkins | 0.1* | – | 0.2* | Further consideration neededa |
| 233030 | Watermelons | 0.1* | – | 0.2* | Further consideration neededa |
| 234000 | Sweet corn | 3 | 3 | 3 | Further consideration neededc |
| 241010 | Broccoli | 0.1* | – | 0.2* | Further consideration neededa |
| 241020 | Cauliflowers | 0.1* | – | 0.2* | Further consideration neededa |
| 242010 | Brussels sprouts | 0.1* | – | 0.2* | Further consideration neededa |
| 242020 | Head cabbages | 0.1* | – | 0.2* | Further consideration neededa |
| 243010 | Chinese cabbages/pe‐tsai | 0.1* | – | 0.2* | Further consideration neededa |
| 243020 | Kales | 0.1* | – | 0.2* | Further consideration neededa |
| 244000 | Kohlrabies | 0.1* | – | 0.2* | Further consideration neededa |
| 251010 | Lamb's lettuces/corn salads | 0.1* | – | 0.2* | Further consideration neededa |
| 251020 | Lettuces | 0.1* | – | 0.2* | Further consideration neededa |
| 251030 | Escaroles/broadleaved endives | 0.1* | – | 0.2* | Further consideration neededa |
| 251040 | Cresses and other sprouts and shoots | 0.1* | – | 0.2* | Further consideration neededa |
| 251050 | Land cresses | 0.1* | – | 0.2* | Further consideration neededa |
| 251060 | Roman rocket/rucola | 0.1* | – | 0.2* | Further consideration neededa |
| 251070 | Red mustards | 0.1* | – | 0.2* | Further consideration neededa |
| 251080 | Baby leaf crops (including brassica species) | 0.1* | – | 0.2* | Further consideration neededa |
| 252010 | Spinaches | 0.1* | – | 0.2* | Further consideration neededa |
| 252020 | Purslanes | 0.1* | – | 0.2* | Further consideration neededa |
| 252030 | Chards/beet leaves | 0.1* | – | 0.2* | Further consideration neededa |
| 253000 | Grape leaves and similar species | 0.1* | – | 0.2* | Further consideration neededa |
| 254000 | Watercresses | 0.1* | – | 0.2* | Further consideration neededa |
| 255000 | Witloofs/Belgian endives | 0.1* | – | 0.2* | Further consideration neededa |
| 256010 | Chervil | 0.1* | – | 0.2* | Further consideration neededa |
| 256020 | Chives | 0.1* | – | 0.2* | Further consideration neededa |
| 256030 | Celery leaves | 0.1* | – | 0.2* | Further consideration neededa |
| 256040 | Parsley | 0.1* | – | 0.2* | Further consideration neededa |
| 256050 | Sage | 0.1* | – | 0.2* | Further consideration neededa |
| 256060 | Rosemary | 0.1* | – | 0.2* | Further consideration neededa |
| 256070 | Thyme | 0.1* | – | 0.2* | Further consideration neededa |
| 256080 | Basil and edible flowers | 0.1* | – | 0.2* | Further consideration neededa |
| 256090 | Laurel/bay leave | 0.1* | – | 0.2* | Further consideration neededa |
| 256100 | Tarragon | 0.1* | – | 0.2* | Further consideration neededa |
| 260010 | Beans (with pods) | 0.1* | – | 0.2* | Further consideration neededa |
| 260020 | Beans (without pods) | 0.1* | – | 0.2* | Further consideration neededa |
| 260030 | Peas (with pods) | 0.1* | – | 0.2* | Further consideration neededa |
| 260040 | Peas (without pods) | 0.1* | – | 0.2* | Further consideration neededa |
| 260050 | Lentils (fresh) | 0.1* | – | 0.2* | Further consideration neededa |
| 270010 | Asparagus | 0.1* | – | 0.2* | Further consideration neededa |
| 270020 | Cardoons | 0.1* | – | 0.2* | Further consideration neededa |
| 270030 | Celeries | 0.1* | – | 0.2* | Further consideration neededa |
| 270040 | Florence fennels | 0.1* | – | 0.2* | Further consideration neededa |
| 270050 | Globe artichokes | 0.1* | – | 0.2* | Further consideration neededa |
| 270060 | Leeks | 0.1* | – | 0.2* | Further consideration neededa |
| 270070 | Rhubarbs | 0.1* | – | 0.2* | Further consideration neededa |
| 270080 | Bamboo shoots | 0.1* | – | 0.2* | Further consideration neededa |
| 270090 | Palm hearts | 0.1* | – | 0.2* | Further consideration neededa |
| 280010 | Cultivated fungi | 0.1* | – | 0.2* | Further consideration neededf |
| 280020 | Wild fungi | 50 | – | 0.2* | Further consideration neededa |
| 300010 | Beans (dry) | 2 | 2 | 30 | Further consideration neededc |
| 300020 | Lentils (dry) | 10 | 5 | 30 | Further consideration neededc |
| 300030 | Peas (dry) | 10 | 5 | 30 | Further consideration neededc |
| 300040 | Lupins/lupini beans (dry) | 10 | – | 30 | Further consideration neededa |
| 401010 | Linseeds | 10 | – | 15 | Further consideration neededa |
| 401020 | Peanuts/groundnuts | 0.1* | – | 0.2* | Further consideration neededa |
| 401030 | Poppy seeds | 0.1* | – | 0.2* | Further consideration neededa |
| 401040 | Sesame seeds | 0.1* | – | 0.2* | Further consideration neededa |
| 401050 | Sunflower seeds | 20 | 7 | 20 | Further consideration neededd |
| 401060 | Rapeseeds/canola seeds | 10 | 30 | 30 | Further consideration needede |
| 401070 | Soyabeans | 20 | 20 | 20 | Further consideration neededd |
| 401080 | Mustard seeds | 10 | – | 10 | Further consideration neededf |
| 401090 | Cotton seeds | 10 | 40 | 60 | Further consideration neededc |
| 401100 | Pumpkin seeds | 0.1* | – | 0.2* | Further consideration neededa |
| 401110 | Safflower seeds | 0.1* | – | 0.2* | Further consideration neededa |
| 401120 | Borage seeds | 0.1 | – | 10 | Further consideration neededa |
| 401130 | Gold of pleasure seeds | 0.1 | – | 0.2* | Further consideration neededa |
| 401140 | Hemp seeds | 0.1* | – | 0.2* | Further consideration neededa |
| 401150 | Castor beans | 0.1 | – | 0.2* | Further consideration neededa |
| 402010 | Olives for oil production | 1 | – | 30 | Further consideration neededa |
| 402020 | Oil palms kernels | 0.1 | – | 0.2* | Further consideration neededa |
| 402030 | Oil palms fruits | 0.1 | – | 0.2* | Further consideration neededa |
| 402040 | Kapok | 0.1 | – | 0.2* | Further consideration neededa |
| 500010 | Barley grains | 20 | 30 | 30 | Further consideration neededb |
| 500020 | Buckwheat and other pseudo‐cereal grains | 0.1* | 30 | 30 | Further consideration neededg |
| 500030 | Maize/corn grains | 1 | 5 | 3 | Further consideration neededc |
| 500040 | Common millet/proso millet grains | 0.1* | 30 | 30 | Further consideration needede |
| 500050 | Oat grains | 20 | 30 | 30 | Further consideration neededb |
| 500060 | Rice grains | 0.1* | – | 0.2* | Further consideration neededf |
| 500070 | Rye grains | 10 | 30 | 30 | Further consideration needede |
| 500080 | Sorghum grains | 20 | 30 | 30 | Further consideration needede |
| 500090 | Wheat grains | 10 | 30 | 30 | Further consideration needede |
| 610000 | Teas | 2 | – | 0.2* | Further consideration neededa |
| 620000 | Coffee beans | 0.1 | – | 0.2* | Further consideration neededa |
| 631000 | Herbal infusions from flowers | 2* | – | 0.2* | Further consideration neededa |
| 632000 | Herbal infusions from leaves and herbs | 2* | – | 0.2* | Further consideration neededa |
| 633000 | Herbal infusions from roots | 2* | – | 0.2* | Further consideration neededa |
| 650000 | Carobs/Saint John's breads | 0.1* | – | 0.2* | Further consideration neededa |
| 700000 | Hops | 0.1* | – | 0.2* | Further consideration neededa |
| 810000 | Seed spices | 0.1* | – | 0.2* | Further consideration neededa |
| 820000 | Fruit spices | 0.1* | – | 0.2* | Further consideration neededa |
| 830000 | Bark spices | 0.1* | – | 0.2* | Further consideration neededa |
| 840000 | Root and rhizome spices | 0.1* | – | 0.2* | Further consideration neededa |
| 850000 | Bud spices | 0.1* | – | 0.2* | Further consideration neededa |
| 860000 | Flower pistil spices | 0.1* | – | 0.2* | Further consideration neededa |
| 870000 | Aril spices | 0.1* | – | 0.2* | Further consideration neededa |
| 900010 | Sugar beet roots | 15 | 15 | 15 | Further consideration needede |
| 900020 | Sugar canes | 0.1* | 2 | 0.2* | Further consideration neededc |
| 900030 | Chicory roots | 0.1* | – | 0.2* | Further consideration neededa |
| 1011010 | Swine muscle | 0.05* | 0.05* | 0.2 | Further consideration neededc |
| 1011020 | Swine fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededc |
| 1011030 | Swine liver | 0.05* | 0.5 | 0.4 | Further consideration neededc |
| 1011040 | Swine kidney | 0.5 | 0.5 | 3 | Further consideration neededc |
| 1012010 | Bovine muscle | 0.05* | 0.05* | 0.2 | Further consideration neededc |
| 1012020 | Bovine fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededc |
| 1012030 | Bovine liver | 0.2 | 5 | 0.7 | Further consideration neededc |
| 1012040 | Bovine kidney | 2 | 5 | 7 | Further consideration neededc |
| 1013010 | Sheep muscle | 0.05* | 0.05* | 0.2 | Further consideration neededc |
| 1013020 | Sheep fat tissue | 0.05* | 0.05* | 0.3 | Further consideration neededc |
| 1013030 | Sheep liver | 0.05* | 5 | 0.9 | Further consideration neededc |
| 1013040 | Sheep kidney | 0.05* | 5 | 10 | Further consideration neededc |
| 1014010 | Goat muscle | 0.05* | 0.05* | 0.2 | Further consideration neededc |
| 1014020 | Goat fat tissue | 0.05* | 0.05* | 0.3 | Further consideration neededc |
| 1014030 | Goat liver | 0.05* | 5 | 0.9 | Further consideration neededc |
| 1014040 | Goat kidney | 0.05* | 5 | 10 | Further consideration neededc |
| 1015010 | Equine muscle | 0.05* | 0.05* | 0.2 | Further consideration neededc |
| 1015020 | Equine fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededc |
| 1015030 | Equine liver | 0.05* | 5 | 0.7 | Further consideration neededc |
| 1015040 | Equine kidney | 0.05* | 5 | 7 | Further consideration neededc |
| 1016010 | Poultry muscle | 0.05* | 0.05* | 0.2 | Further consideration neededc |
| 1016020 | Poultry fat tissue | 0.05* | 0.05* | 0.2* | Further consideration neededc |
| 1016030 | Poultry liver | 0.05* | 0.5 | 0.2* | Further consideration neededc |
| 1020010 | Cattle milk | 0.05* | 0.05 | 0.1* | Further consideration neededc |
| 1020020 | Sheep milk | 0.05* | 0.05 | 0.1* | Further consideration neededc |
| 1020030 | Goat milk | 0.05* | 0.05 | 0.1* | Further consideration neededc |
| 1020040 | Horse milk | 0.05* | 0.05 | 0.1* | Further consideration neededc |
| 1030000 | Birds eggs | 0.05* | 0.05* | 0.1* | Further consideration neededc |
| – | Other commodities of animal origin | – | – | Further consideration neededh | |
MRL: maximum residue level; CXL: codex maximum residue limit.
*Indicates that the MRL is set at the limit of quantification.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; no CXL is available (combination E‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; existing CXL is covered by the tentative MRL (combination E‐III in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; CXL is not compatible with EU residue definitions (combination E‐II in Appendix E).
GAP evaluated at EU level is not supported by data, but no risk to consumers was identified for the existing EU MRL; CXL is not compatible with EU residue definitions (combination C‐II in Appendix E).
MRL is derived from the existing CXL, which is not sufficiently supported by data but for which no risk to consumers is identified; GAP evaluated at EU level, which is also not fully supported by data, would lead to a lower tentative MRL (combination E‐V in Appendix E).
GAP evaluated at EU level is not supported by data, but no risk to consumers was identified for the existing EU MRL; no CXL is available (combination C‐I in Appendix E).
MRL is derived from the existing CXL, which is not sufficiently supported by data but for which no risk to consumers is identified; GAP evaluated at EU level is not supported by data, but the existing EU MRL is lower than the CXL (combination C‐V in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.
PRIMo (EU_main)
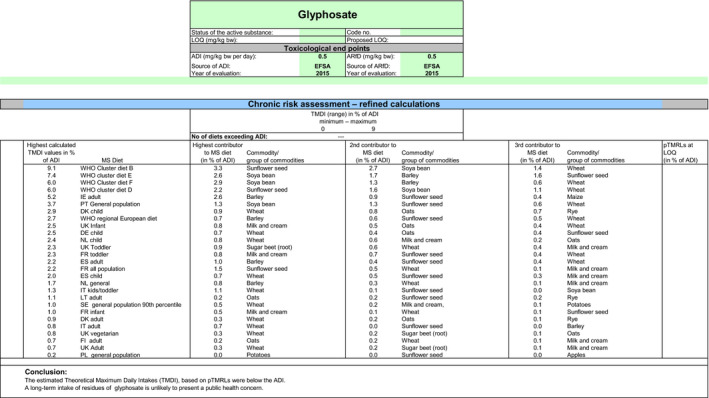
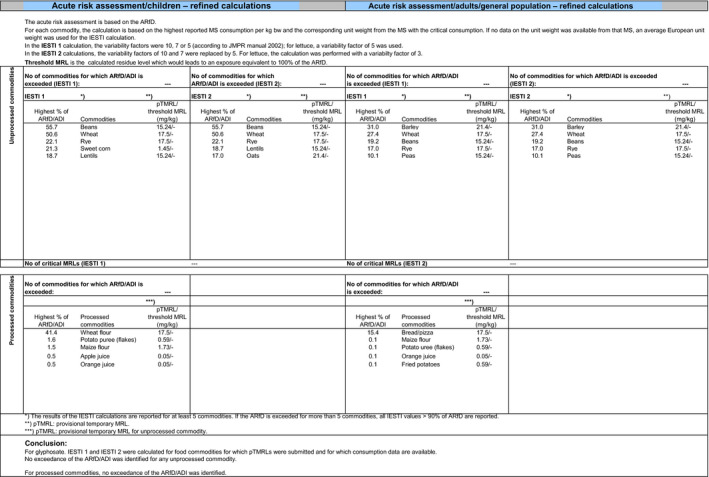
PRIMo (EU_optional)
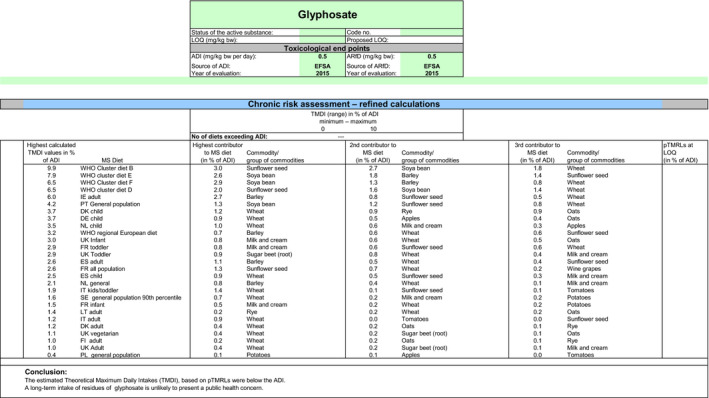
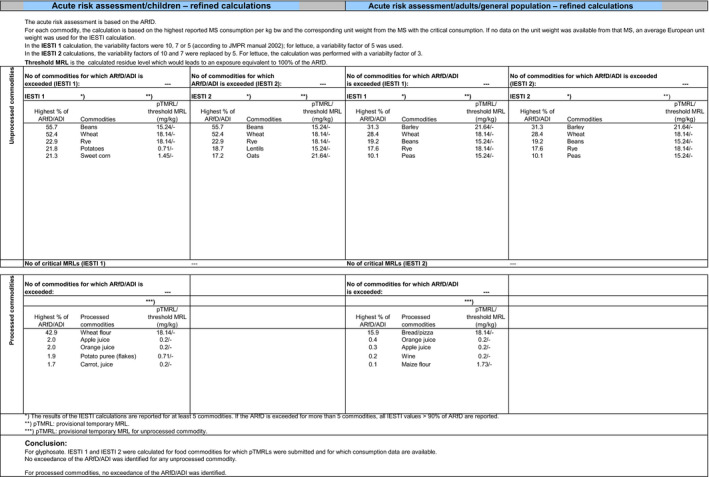
PRIMo (CXL_main)
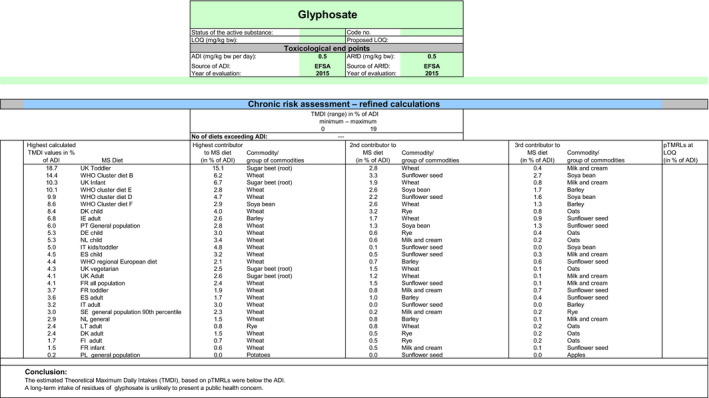

PRIMo (CXL_optional)
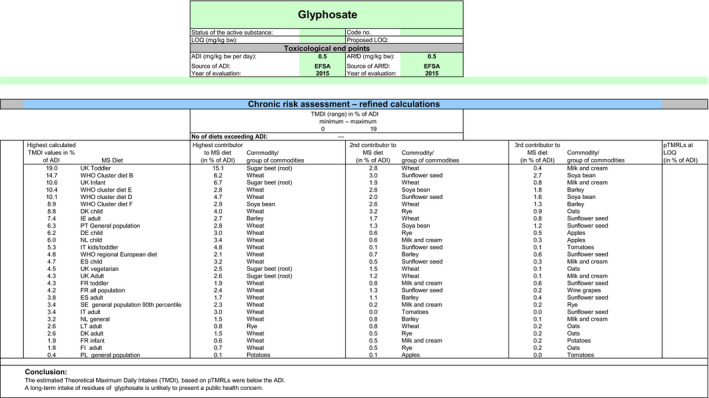
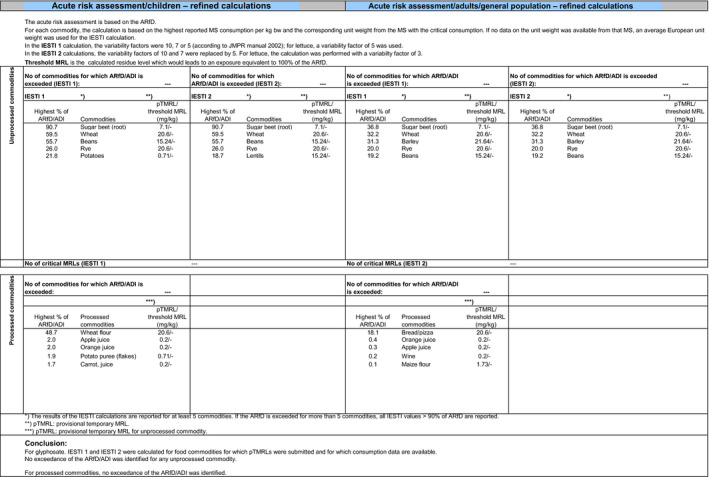
Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: sum of glyphosate, AMPA, N‐acetyl‐glyphosate and N‐acetyl‐AMPA, expressed as glyphosate | ||||
| Alfalfa, forage (green) | 0.05* | STMR × CF (tentative) | 0.05* | HR × CF (tentative) |
| Alfalfa, hay (fodder) | 0.05* | STMR × CFa (tentative) | 0.05* | HR × CFa (tentative) |
| Alfalfa, meal | 0.05* | STMR × CFa (tentative) | 0.05* | HR × CFa (tentative) |
| Alfalfa, silage | 0.05* | STMR × CFa (tentative) | 0.05* | HR × CFa (tentative) |
| Barley, straw | 57.8 | STMR × CF (tentative) | 140 | HR × CF (tentative) |
| Beet, mangel, fodder | 0.05* | STMR × CF (tentative) | 0.05* | HR × CF (tentative) |
| Beet, sugar, tops | 0.2* | STMR × CF (tentative) | 0.2* | HR × CF (tentative) |
| Cabbage, heads, leaves | 0.05* | STMR × CF (tentative) | 0.05* | HR × CF (tentative) |
| Clover, forage | 0.05* | STMR × CF (tentative) | 0.05* | HR × CF (tentative) |
| Clover, hay | 0.05* | STMR × CFa (tentative) | 0.05* | HR × CFa (tentative) |
| Clover, silage | 0.05* | STMR × CFa (tentative) | 0.05* | HR × CFa (tentative) |
| Grass, forage (fresh) | 16 | STMR × CF (tentative) | 139 | HR × CF (tentative) |
| Grass, hay | 20.5 | STMR × CF × PF (1.1) (tentative) | 153 | HR × CF × PF (1.1) (tentative) |
| Grass, silage | 16.7 | STMR × CF × PF (0.9)(tentative) | 125 | HR × CF × PF (0.9) (tentative) |
| Kale, leaves (forage) | 0.05* | STMR × CF (tentative) | 0.05* | HR × CF (tentative) |
| Oat, straw | 57.8 | STMR × CF (tentative) | 140 | HR × CF (tentative) |
| Rye, straw | 30.5 | STMR × CF (tentative) | 175 | HR × CF (tentative) |
| Triticale, straw | 30.5 | STMR × CF (tentative) | 175 | HR × CF (tentative) |
| Turnip, tops (leaves) | 0.05* | STMR × CF (tentative) | 0.05* | HR × CF (tentative) |
| Wheat, straw | 30.5 | STMR × CF (tentative) | 175 | HR × CF (tentative) |
| Carrot, culls | 0.05* | STMR × CF | 0.05* | HR × CF |
| Cassava/tapioca | 0.05* | STMR × CF (tentative) | 0.05* | HR × CF (tentative) |
| Potato, culls | 0.07 | STMR × CF (tentative) | 0.59 | HR × CF (tentative) |
| Swede, roots | 0.05* | STMR × CF (tentative) | 0.05* | HR × CF (tentative) |
| Turnip, roots | 0.05* | STMR × CF (tentative) | 0.05* | HR × CF (tentative) |
| Barley, grain | 10.7 | STMR × CF | 10.7 | HR × CF |
| Bean, seed (dry) | 0.14 | STMR × CF (tentative) | 0.14 | STMR × CF (tentative) |
| Corn, field (Maize), grain | 0.93 | STMR × CF (tentative) | 0.93 | STMR × CF (tentative) |
| Corn, pop, grain | 0.93 | STMR × CF (tentative) | 0.93 | STMR × CF (tentative) |
| Cotton, undelinted seed | 17.7 | STMR × CF (EPSPS, tentative) | 17.7 | STMR × CF (EPSPS, tentative) |
| Cowpea, seed | 0.14 | STMR × CF (tentative) | 0.14 | STMR × CF (tentative) |
| Lupin, seed | 0.14 | STMR × CF (tentative) | 0.14 | STMR × CF (tentative) |
| Millet, grain | 0.89 | STMR × CF | 0.89 | STMR × CF |
| Oat, grain | 10.7 | STMR × CF | 10.7 | STMR × CF (tentative) |
| Pea (Field pea), seed (dry) | 0.14 | STMR × CF (tentative) | 0.14 | STMR × CF (tentative) |
| Rye, grain | 0.81 | STMR × CF | 0.81 | STMR × CF |
| Sorghum, grain | 0.89 | STMR × CF | 0.89 | STMR × CF |
| Triticale, grain | 0.81 | STMR × CF | 0.81 | STMR × CF |
| Wheat, grain | 0.81 | STMR × CF | 0.81 | STMR × CF |
| Apple pomace, wet | 0.05* | STMR × CFa | 0.05* | STMR × CFa |
| Beet, sugar, dried pulp | 0.2* | STMR × CFa (tentative) | 0.2* | STMR × CFa (tentative) |
| Beet, sugar, ensiled pulp | 0.2* | STMR × CFa (tentative) | 0.2* | STMR × CFa (tentative) |
| Beet, sugar, molasses | 0.2* | STMR × CFa (tentative) | 0.2* | STMR × CFa (tentative) |
| Barley, brewer's grain (dried) | 35.2 | STMR × CF × 3.3b | 35.2 | STMR × CF × 3.3b |
| Canola (Rapeseed), meal | 1.74 | STMR × CF × PF (1.4) (tentative) | 1.74 | STMR × CF × PF (1.4) (tentative) |
| Citrus fruits, dried pulp | 0.13 | STMR × CF × PF (2.6) | 0.13 | STMR × CF × PF (2.6) |
| Coconut, meal | 0.05* | STMR × CFa | 0.05* | STMR × CFa |
| Corn, field, milled by‐products | 0.84 | STMR × CF × PF (0.9) (tentative) | 0.84 | STMR × CF × PF (0.9) (tentative) |
| Corn, field, hominy meal | 5.58 | STMR × CF × 6b (tentative) | 5.58 | STMR × CF × 6b (tentative) |
| Corn, field, gluten feed | 2.33 | STMR × CF × 2.5b (tentative) | 2.33 | STMR × CF × 2.5b (tentative) |
| Corn, field, gluten, meal | 0.93 | STMR × CF × 1b (tentative) | 0.93 | STMR × CF × 1b (tentative) |
| Cotton, meal | 22.1 | STMR × CF × 1.3b (tentative) | 22.1 | STMR × CF × 1.3b (tentative) |
| Wheat/Corn, distiller's grain (dried) | 3.07 | STMR (maize) × CF × 3.3b | 3.07 | STMR (maize) × CF × 3.3b |
| Flaxseed/Linseed, meal | 1.86 | STMR × CF × PF (1.6) | 1.86 | STMR × CF × PF (1.6) |
| Lupin seed, meal | 0.15 | STMR × CF × 1.1b (tentative) | 0.15 | STMR × CF × 1.1b (tentative) |
| Palm, kernel meal | 0.05* | STMR × CFa | 0.05* | STMR × CFa |
| Peanut, meal | 0.05* | STMR × CFa (tentative) | 0.05* | STMR × CFa (tentative) |
| Potato, process waste | 1.4 | STMR × CF × 20b (tentative) | 1.4 | STMR × CF × 20b (tentative) |
| Potato, dried pulp | 2.66 | STMR × CF × 38b (tentative) | 2.66 | STMR × CF × 38b (tentative) |
| Rapeseed, meal | 1.74 | STMR × CF × PF (1.4) (tentative) | 1.74 | STMR × CF × PF (1.4) (tentative) |
| Safflower, meal | 0.05* | STMR × CFa (tentative) | 0.05* | STMR × CFa (tentative) |
| Sugarcane, molasses | 0.05* | STMR × CFa (tentative) | 0.05* | STMR × CFa (tentative) |
| Wheat gluten, meal | 1.46 | STMR × CF × 1.8b | 1.46 | STMR × CF × 1.8b |
| Wheat, milled by‐products | 1.46 | STMR × CF × PF (1.8) | 1.46 | STMR × CF × PF (1.8) |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor.
*Indicates that the input value is proposed at the limit of quantification.
For alfalfa and clover hay, meal and silage, apples pomace, sugar beet dried pulp, ensiled pulp and molasses, coconuts meal, palm hearts kernel meal, peanut meal, safflower meal and sugarcane molasses, no default processing factor was applied because residues in the raw commodities are proposed at the LOQ. Concentration of residues in these commodities is therefore not expected.
For barley brewer's grain, corn hominy meal, corn gluten feed, corn gluten meal, cotton meal, wheat/corn distiller's grain, lupin seed meal, potatoes process waste, potato dried pulp and wheat gluten meal, in the absence of processing factors supported by data, the default processing factors were included in the calculation to consider the potential concentration of residues in these commodities.
D.2. Consumer risk assessment without consideration of the existing CXLs
| Commodity | Chronic risk assessment | Acute risk assessment | ||||
|---|---|---|---|---|---|---|
| Input value (mg/kg) (main RD‐Mo) | Comment | Input value (mg/kg) (opt. RD‐Mo) | Input value (mg/kg) (main RD‐Mo) | Comment | Input value (mg/kg) (opt. RD‐Mo) | |
| Risk assessment residue definition: sum of glyphosate, AMPA, N‐acetyl‐glyphosate and N‐acetyl‐AMPA, expressed as glyphosate | ||||||
| Grapefruits | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Oranges | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Lemons | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Limes | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Mandarins | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Almonds | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Brazil nuts | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Cashew nuts | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Chestnuts | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Coconuts | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Hazelnuts/cobnuts | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Macadamias | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Pecans | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Pine nut kernels | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Pistachios | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Walnuts | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Apples | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Pears | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Quinces | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Medlars | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Loquats/Japanese medlars | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Apricots | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Cherries (sweet) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Peaches | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Plums | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Table grapes | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Wine grapes | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Strawberries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Blackberries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Dewberries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Raspberries (red and yellow) | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Blueberries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Cranberries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Currants (black, red and white) | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Gooseberries (green, red and yellow) | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Rose hips | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Mulberries (black and white) | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Azaroles/Mediterranean medlars | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Elderberries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Figs | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Table olives | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Kumquats | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Kaki/Japanese persimmons | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Kiwi fruits (green, red, yellow) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Litchis/lychees | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Passionfruits/maracujas | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Avocados | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Bananas | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Mangoes | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Papayas | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Granate apples/pomegranates | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Cherimoyas | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Potatoes | 0.07 | STMRMo × CF (1) (tentative) | (0.2*) | 0.59 | HRMo × CF (1) (tentative) | (0.71) |
| Cassava roots/manioc | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Sweet potatoes | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Yams | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Arrowroots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Beetroots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Carrots | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Celeriacs/turnip rooted celeries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Horseradishes | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Jerusalem artichokes | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Parsnips | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Parsley roots/Hamburg roots parsley | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Radishes | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Salsifies | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Swedes/rutabagas | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Turnips | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Garlic | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Onions | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Shallots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Spring onions/green onions and Welsh onions | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Tomatoes | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Sweet peppers/bell peppers | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Aubergines/eggplants | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Okra/lady's fingers | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Cucumbers | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Gherkins | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Courgettes | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Melons | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Pumpkins | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Watermelons | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Sweet corn | 0.51 | STMRMo × CF (1) (GM EPSPS, tentative) | (0.51) | 1.45 | HRMo × CF (1) (GM EPSPS, tentative) | (1.45) |
| Broccoli | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Cauliflowers | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Brussels sprouts | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Head cabbages | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Chinese cabbages/pe‐tsai | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Kales | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Kohlrabies | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Lamb's lettuces/corn salads | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Lettuces | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Escaroles/broadleaved endives | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Cresses and other sprouts and shoots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Land cresses | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Roman rocket/rucola | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Red mustards | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Baby leaf crops (including brassica species) | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Spinaches | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Purslanes | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Chards/beet leaves | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Grape leaves and similar species | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Watercresses | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Witloofs/Belgian endives | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Chervil | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Chives | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Celery leaves | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Parsley | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Sage | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Rosemary | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Thyme | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Basil and edible flowers | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Laurel/bay leave | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Tarragon | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Beans (with pods) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Beans (without pods) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Peas (with pods) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Peas (without pods) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Lentils (fresh) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Asparagus | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Cardoons | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Celeries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Florence fennels | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Globe artichokes | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Leeks | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Rhubarbs | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Bamboo shoots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Palm hearts | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Cultivated fungi | 0.23 | EU MRL × CF (2.3)b | (0.2*) | 0.23 | EU MRL × CF (2.3)b | (0.2*) |
| Wild fungi | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Beans (dry) | 0.14 | STMRMo × CF (2) (tentative) | (0.2*) | 15.2 | HRMo × CF (2) (tentative) | (15.2) |
| Lentils (dry) | 1.45 | STMRMo × CF (2) (tentative) | (1.46) | 15.2 | HRMo × CF (2) (tentative) | (15.2) |
| Peas (dry) | 0.14 | STMRMo × CF (2) (tentative) | (0.2*) | 15.2 | HRMo × CF (2) (tentative) | (15.2) |
| Lupins/lupini beans (dry) | 0.14 | STMRMo × CF (2) (tentative) | (0.2*) | 15.2 | HRMo × CF (2) (tentative) | (15.2) |
| Linseeds | 1.28 | STMRMo × CF (1.1) | (1.28) | 12.8 | HRMo × CF (1.1) | (11.9) |
| Peanuts/groundnuts | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Poppy seeds | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Sesame seeds | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Sunflower seeds | 22 | EU MRL × CF (1.1)a | (20) | 22 | EU MRL × CF (1.1)a | (20) |
| Rapeseeds/canola seeds | 1.24 | STMRMo × CF (1) (tentative) | (1.24) | 11.9 | HRMo × CF (1) (tentative) | (11.9) |
| Soyabeans | 22 | EU MRL × CF (1.1)a | (22) | 22 | EU MRL × CF (1.1)a | (22) |
| Mustard seeds | 11 | EU MRL × CF (1.1)a | (10) | 11 | EU MRL × CF (1.1)a | (10) |
| Cotton seeds | 17.7 | STMRMo × CF (1) (GM EPSPS, tentative) | (17.7) | 30.9 | HRMo × CF (1) (GM EPSPS, tentative) | (30.9) |
| Pumpkin seeds | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Safflower seeds | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Borage seeds | 0.65 | STMRMo × CF (1) (tentative) | (0.70) | 6.80 | HRMo × CF (1) (tentative) | (6.85) |
| Gold of pleasure seeds | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Hemp seeds | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Castor beans | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Olives for oil production | 0.42 | STMRMo × CF (1) | (0.53) | 16 | HRMo × CF (1) | (16.1) |
| Oil palms kernels | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Oil palms fruits | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Kapok | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Barley grains | 10.7 | STMRMo × CF (1) | (10.8) | 21.4 | HRMo × CF (1) | (21.6) |
| Buckwheat and other pseudo‐cereal grains | 0.23 | EU MRL × CF (2.3)a | (0.2*) | 0.23 | EU MRL × CF (2.3)a | (0.2*) |
| Maize/corn grains | 0.93 | STMRMo × CF (1) (tentative) | (0.93) | 1.73 | HRMo × CF (1) (tentative) | (1.73) |
| Common millet/proso millet grains | 0.89 | STMRMo × CF (2.3) | (0.94) | 1.73 | HRMo × CF (2.3) | (1.77) |
| Oat grains | 10.7 | STMRMo × CF (1) | (10.8) | 21.4 | HRMo × CF (1) | (21.6) |
| Rice grains | 0.23 | EU MRL × CF (2.3)a | (0.2*) | 0.23 | EU MRL × CF (2.3)a | (0.2*) |
| Rye grains | 0.81 | STMRMo × CF (1) | (1.06) | 17.5 | HRMo × CF (1) | (18.1) |
| Sorghum grains | 0.89 | STMRMo × CF (2.3) | (0.94) | 1.73 | HRMo × CF (2.3) | (1.77) |
| Wheat grains | 0.81 | STMRMo × CF (1) | (1.06) | 17.5 | HRMo × CF (1) | (18.1) |
| Teas | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Coffee beans | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Herbal infusions from flowers | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Herbal infusions from leaves and herbs | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Herbal infusions from roots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Carobs/Saint John's breads | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Hops | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Seed spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Fruit spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Bark spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Root and rhizome spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Bud spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Flower pistil spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Aril spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Sugar beet roots | 0.2* | STMRMo × CF (1) (tentative) | (0.2*) | 0.2* | HRMo × CF (1) (tentative) | (0.2*) |
| Sugarcanes | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Chicory roots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Swine meat | 0.17 | STMRMo muscle × CF (1) (tentative) | (0.17) | 0.17 | HRMo muscle × CF (1) (tentative) | (0.17) |
| Swine fat tissue | 0.2* | STMRMo × CF (1) (tentative) | (0.2*) | 0.2* | HRMo × CF (1) (tentative) | (0.2*) |
| Swine liver | 0.17 | STMRMo × CF (1) (tentative) | (0.2*) | 0.35 | HRMo × CF (1) (tentative) | (0.35) |
| Swine kidney | 0.22 | STMRMo × CF (1) (tentative) | (0.22) | 2.46 | HRMo × CF (1) (tentative) | (2.46) |
| Bovine meat | 0.17 | STMRMo muscle × CF (1) (tentative) | (0.2*) | 0.18 | HRMo muscle × CF (1) (tentative) | (0.18) |
| Bovine fat tissue | 0.2* | STMRMo × CF (1) (tentative) | (0.2*) | 0.2* | HRMo × CF (1) (tentative) | (0.2*) |
| Bovine liver | 0.54 | STMRMo × CF (1) (tentative) | (0.54) | 0.69 | HRMo × CF (1) (tentative) | (0.69) |
| Bovine kidney | 0.69 | STMRMo × CF (1) (tentative) | (0.69) | 6.82 | HRMo × CF (1) (tentative) | (6.82) |
| Sheep meat | 0.17 | STMRMo muscle × CF (1) (tentative) | (0.2*) | 0.19 | HRMo muscle × CF (1) (tentative) | (0.19) |
| Sheep fat tissue | 0.17 | STMRMo × CF (1) (tentative) | (0.2*) | 0.21 | HRMo × CF (1) (tentative) | (0.21) |
| Sheep liver | 0.54 | STMRMo × CF (1) (tentative) | (0.54) | 0.81 | HRMo × CF (1) (tentative) | (0.81) |
| Sheep kidney | 0.81 | STMRMo × CF (1) (tentative) | (0.81) | 9.28 | HRMo × CF (1) (tentative) | (9.28) |
| Goat meat | 0.17 | STMRMo muscle × CF (1) (tentative) | (0.2*) | 0.19 | HRMo muscle × CF (1) (tentative) | (0.19) |
| Goat fat tissue | 0.17 | STMRMo × CF (1) (tentative) | (0.2*) | 0.21 | HRMo × CF (1) (tentative) | (0.21) |
| Goat liver | 0.54 | STMRMo × CF (1) (tentative) | (0.54) | 0.81 | HRMo × CF (1) (tentative) | (0.81) |
| Goat kidney | 0.81 | STMRMo × CF (1) (tentative) | (0.81) | 9.28 | HRMo × CF (1) (tentative) | (9.28) |
| Equine meat | 0.17 | STMRMo muscle × CF (1) (tentative) | (0.2*) | 0.18 | HRMo muscle × CF (1) (tentative) | (0.18) |
| Equine fat tissue | 0.2* | STMRMo × CF (1) (tentative) | (0.2*) | 0.2* | HRMo × CF (1) (tentative) | (0.2*) |
| Equine liver | 0.54 | STMRMo × CF (1) (tentative) | (0.54) | 0.69 | HRMo × CF (1) (tentative) | (0.69) |
| Equine kidney | 0.69 | STMRMo × CF (1) (tentative) | (0.69) | 6.82 | HRMo × CF (1) (tentative) | (6.82) |
| Poultry meat | 0.17 | STMRMo muscle × CF (1) (tentative) | (0.2*) | 0.17 | HRMo muscle × CF (1) (tentative) | (0.2*) |
| Poultry fat tissue | 0.2* | STMRMo × CF (1) (tentative) | (0.2*) | 0.2* | HRMo × CF (1) (tentative) | (0.2*) |
| Poultry liver | 0.2* | STMRMo × CF (1) (tentative) | (0.2*) | 0.2* | HRMo × CF (1) (tentative) | (0.2*) |
| Cattle milk | 0.1* | STMRMo × CF (1) (tentative) | (0.1*) | 0.1* | HRMo × CF (1) (tentative) | (0.1*) |
| Sheep milk | 0.1* | STMRMo × CF (1) (tentative) | (0.1*) | 0.1* | HRMo × CF (1) (tentative) | (0.1*) |
| Goat milk | 0.1* | STMRMo × CF (1) (tentative) | (0.1*) | 0.1* | HRMo × CF (1) (tentative) | (0.1*) |
| Horse milk | 0.1* | STMRMo × CF (1) (tentative) | (0.1*) | 0.1* | HRMo × CF (1) (tentative) | (0.1*) |
| Birds eggs | 0.1* | STMRMo × CF (1) (tentative) | (0.1*) | 0.1* | HRMo × CF (1) (tentative) | (0.1*) |
*Indicates that the input value is proposed at the limit of quantification.
GAP is not supported by data; the existing EU MRL is used for indicative exposure calculations; indicative conversion factors of 1.1 (for oilseeds) and 2.3 (for cereals) were considered for risk assessment.
GAP is not supported by data; the existing EU MRL multiplied by the worst case conversion factor of 2.3 for risk assessment is used for indicative exposure calculations.
D.3. Consumer risk assessment with consideration of the existing CXLs
| Commodity | Chronic risk assessment | Acute risk assessment | ||||
|---|---|---|---|---|---|---|
| Input value (mg/kg) (main RD‐Mo) | Comment | Input value (mg/kg) (opt. RD‐Mo) | Input value (mg/kg) (main RD‐Mo) | Comment | Input value (mg/kg) (opt. RD‐Mo) | |
| Risk assessment residue definition: sum of glyphosate, AMPA, N‐acetyl‐glyphosate and N‐acetyl‐AMPA, expressed as glyphosate | ||||||
| Grapefruits | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Oranges | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Lemons | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Limes | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Mandarins | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Almonds | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Brazil nuts | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Cashew nuts | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Chestnuts | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Coconuts | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Hazelnuts/cobnuts | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Macadamias | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Pecans | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Pine nut kernels | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Pistachios | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Walnuts | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Apples | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Pears | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Quinces | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Medlars | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Loquats/Japanese medlars | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Apricots | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Cherries (sweet) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Peaches | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Plums | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Table grapes | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Wine grapes | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Strawberries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Blackberries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Dewberries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Raspberries (red and yellow) | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Blueberries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Cranberries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Currants (black, red and white) | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Gooseberries (green, red and yellow) | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Rose hips | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Mulberries (black and white) | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Azaroles/Mediterranean medlars | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Elderberries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Figs | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Table olives | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Kumquats | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Kaki/Japanese persimmons | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Kiwi fruits (green, red, yellow) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Litchis/lychees | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Passionfruits/maracujas | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Avocados | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Bananas | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Mangoes | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Papayas | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Granate apples/pomegranates | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Cherimoyas | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Potatoes | 0.07 | STMRMo × CF (1) (tentative) | (0.2*) | 0.59 | HRMo × CF (1) (tentative) | (0.71) |
| Cassava roots/manioc | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Sweet potatoes | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Yams | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Arrowroots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Beetroots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Carrots | 0.05* | STMRMo × × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Celeriacs/turnip rooted celeries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Horseradishes | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Jerusalem artichokes | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Parsnips | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Parsley roots/Hamburg roots parsley | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Radishes | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Salsifies | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Swedes/rutabagas | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Turnips | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Garlic | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Onions | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Shallots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Spring onions/green onions and Welsh onions | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Tomatoes | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Sweet peppers/bell peppers | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Aubergines/eggplants | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Okra/lady's fingers | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Cucumbers | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Gherkins | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Courgettes | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Melons | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Pumpkins | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Watermelons | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Sweet corn | 0.51 | STMRMo × CF (1) (tentative) | (0.51) | 1.45 | HRMo × CF (1) (tentative) | (1.45) |
| Broccoli | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Cauliflowers | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Brussels sprouts | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Head cabbages | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Chinese cabbages/pe‐tsai | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Kales | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Kohlrabies | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Lamb's lettuces/corn salads | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Lettuces | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Escaroles/broad‐leaved endives | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Cresses and other sprouts and shoots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Land cresses | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Roman rocket/rucola | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Red mustards | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Baby leaf crops (including brassica species) | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Spinaches | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Purslanes | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Chards/beet leaves | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Grape leaves and similar species | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Watercresses | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Witloofs/Belgian endives | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Chervil | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Chives | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Celery leaves | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Parsley | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Sage | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Rosemary | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Thyme | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Basil and edible flowers | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Laurel/bay leave | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Tarragon | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Beans (with pods) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Beans (without pods) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Peas (with pods) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Peas (without pods) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Lentils (fresh) | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Asparagus | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Cardoons | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Celeries | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Florence fennels | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Globe artichokes | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Leeks | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Rhubarbs | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Bamboo shoots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Palm hearts | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Cultivated fungi | 0.23 | EU MRL × CF (2.3)b | (0.2*) | 0.23 | EU MRL × CF (2.3)b | (0.2*) |
| Wild fungi | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Beans (dry) | 0.14 | STMRMo × CF (2) (tentative) | (0.2*) | 15.2 | HRMo × CF (2) (tentative) | (15.24) |
| Lentils (dry) | 1.45 | STMRMo × CF (2) (tentative) | (1.46) | 15.2 | HRMo × CF (2) (tentative) | (15.24) |
| Peas (dry) | 0.14 | STMRMo × CF (2) (tentative) | (0.2*) | 15.2 | HRMo × CF (2) (tentative) | (15.24) |
| Lupins/lupini beans (dry) | 0.14 | STMRMo × CF (2) (tentative) | (0.2*) | 15.2 | HRMo × CF (2) (tentative) | (15.24) |
| Linseeds | 1.28 | STMRMo × CF (1.1) | (1.28) | 12.8 | HRMo × CF (1.1) | (11.94) |
| Peanuts/groundnuts | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Poppy seeds | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Sesame seeds | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Sunflower seeds | 22 | EU MRL × CF (1.1)a | (20) | 22 | EU MRL × CF (1.1)a | (20) |
| Rapeseeds/canola seeds | 2.98 | CXL [STMR × CF (1.01)c] (tentative) | (2.98) | 15.2 | CXL [HR × CF (1.01)c] (tentative) | (15.2) |
| Soyabeans | 22 | EU MRL × CF (1.1)a | (22) | 22 | EU MRL × CF (1.1)a | (22) |
| Mustard seeds | 11 | EU MRL × CF (1.1)a | (10) | 11 | EU MRL × CF (1.1)a | (10) |
| Cotton seeds | 17.7 | STMRMo × CF (1) (tentative) | (17.7) | 30.9 | HRMo × CF (1) (tentative) | (30.9) |
| Pumpkin seeds | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Safflower seeds | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Borage seeds | 0.65 | STMRMo × CF (1) (tentative) | (0.70) | 6.80 | HRMo × CF (1) (tentative) | (6.85) |
| Gold of pleasure seeds | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Hemp seeds | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Castor beans | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Olives for oil production | 0.415 | STMRMo × CF (1) | (0.53) | 16 | HRMo × CF (1) | (16.1) |
| Oil palms kernels | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Oil palms fruits | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Kapok | 0.05* | STMRMo × CF (1) | (0.2*) | 0.05* | HRMo × CF (1) | (0.2*) |
| Barley grains | 10.7 | STMRMo × CF (1) | (10.8) | 21.4 | HRMo × CF (1) | (21.6) |
| Buckwheat and other pseudo‐cereal grains | 3.61 | CXL [STMR × CF (1.03)c] | (3.61) | 20.6 | CXL [HR × CF (1.03)c] | (20.6) |
| Maize/corn grains | 0.93 | STMRMo × CF (1) (tentative) | (0.93) | 1.73 | HRMo × CF (1) (tentative) | (1.73) |
| Common millet/proso millet grains | 3.61 | CXL [STMR × CF (1.03)c] | (3.61) | 20.6 | CXL [HR × CF (1.03)c] | (20.6) |
| Oat grains | 10.65 | STMRMo × CF (1) | (10.8) | 21.4 | HRMo × CF (1) | (21.6) |
| Rice grains | 0.23 | EU MRL × CF (2.3)a | (0.2*) | 0.23 | EU MRL × CF (2.3)a | (0.2*) |
| Rye grain | 3.61 | CXL [STMR × CF (1.03)c] | (3.61) | 20.6 | CXL [HR × CF (1.03)c] | (20.6) |
| Sorghum grains | 3.61 | CXL [STMR × CF (1.03)c] | (3.61) | 20.6 | CXL [HR × CF (1.03)c] | (20.6) |
| Wheat grains | 3.61 | CXL [STMR × CF (1.03)c] | (3.61) | 20.6 | CXL [HR × CF (1.03)c] | (20.6) |
| Teas | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Coffee beans | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Herbal infusions from flowers | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Herbal infusions from leaves and herbs | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Herbal infusions from roots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Carobs/Saint John's breads | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Hops | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Seed spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Fruit spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Bark spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Root and rhizome spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Bud spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Flower pistil spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Aril spices | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Sugar beet roots | 3.3 | CXL [STMR × CF (1)c] (tentative) | (3.3) | 7.1 | CXL [HR × CF (1)c] (tentative) | (7.1) |
| Sugar canes | 0.32 | CXL [STMR × CF (1.19)c ] | (0.2*)d | 1.15 | CXL [HR × CF (1.19)c] | (0.2*)d |
| Chicory roots | 0.05* | STMRMo × CF (1) (tentative) | (0.2*) | 0.05* | HRMo × CF (1) (tentative) | (0.2*) |
| Swine meat | 0.17 | STMRMo muscle × CF (1) (tentative) | (0.17) | 0.17 | HRMo muscle × CF (1) (tentative) | (0.17) |
| Swine fat tissue | 0.2* | STMRMo × CF (1) (tentative) | (0.2*) | 0.2* | HRMo × CF (1) (tentative) | (0.2*) |
| Swine liver | 0.17 | STMRMo × CF (1) (tentative) | (0.2*) | 0.35 | HRMo × CF (1) (tentative) | (0.35) |
| Swine kidney | 0.22 | STMRMo × CF (1) (tentative) | (0.22) | 2.46 | HRMo × CF (1) (tentative) | (2.46) |
| Bovine meat | 0.17 | STMRMo muscle × CF (1) (tentative) | (0.2*) | 0.18 | HRMo muscle × CF (1) (tentative) | (0.18) |
| Bovine fat tissue | 0.2* | STMRMo × CF (1) (tentative) | (0.2*) | 0.2* | HRMo × CF (1) (tentative) | (0.2*) |
| Bovine liver | 0.54 | STMRMo × CF (1) (tentative) | (0.54) | 0.69 | HRMo × CF (1) (tentative) | (0.69) |
| Bovine kidney | 0.69 | STMRMo × CF (1) (tentative) | (0.69) | 6.82 | HRMo × CF (1) (tentative) | (6.82) |
| Sheep meat | 0.17 | STMRMo muscle × CF (1) (tentative) | (0.2*) | 0.19 | HRMo muscle × CF (1) (tentative) | (0.19) |
| Sheep fat tissue | 0.17 | STMRMo × CF (1) (tentative) | (0.2*) | 0.21 | HRMo × CF (1) (tentative) | (0.21) |
| Sheep liver | 0.54 | STMRMo × CF (1) (tentative) | (0.54) | 0.81 | HRMo × CF (1) (tentative) | (0.81) |
| Sheep kidney | 0.81 | STMRMo × CF (1) (tentative) | (0.81) | 9.28 | HRMo × CF (1) (tentative) | (9.28) |
| Goat meat | 0.17 | STMRMo muscle × CF (1) (tentative) | (0.2*) | 0.19 | HRMo muscle × CF (1) (tentative) | (0.19) |
| Goat fat tissue | 0.17 | STMRMo × CF (1) (tentative) | (0.2*) | 0.21 | HRMo × CF (1) (tentative) | (0.21) |
| Goat liver | 0.54 | STMRMo × CF (1) (tentative) | (0.54) | 0.81 | HRMo × CF (1) (tentative) | (0.81) |
| Goat kidney | 0.81 | STMRMo × CF (1) (tentative) | (0.81) | 9.28 | HRMo × CF (1) (tentative) | (9.28) |
| Equine meat | 0.17 | STMRMo muscle × CF (1) (tentative) | (0.2*) | 0.18 | HRMo muscle × CF (1) (tentative) | (0.18) |
| Equine fat tissue | 0.2* | STMRMo × CF (1) (tentative) | (0.2*) | 0.2* | HRMo × CF (1) (tentative) | (0.2*) |
| Equine liver | 0.54 | STMRMo × CF (1) (tentative) | (0.54) | 0.69 | HRMo × CF (1) (tentative) | (0.69) |
| Equine kidney | 0.69 | STMRMo × CF (1) (tentative) | (0.69) | 6.82 | HRMo × CF (1) (tentative) | (6.82) |
| Poultry meat | 0.17 | STMRMo muscle × CF (1) (tentative) | (0.2*) | 0.17 | HRMo muscle × CF (1) (tentative) | (0.2*) |
| Poultry fat tissue | 0.2* | STMRMo × CF (1) (tentative) | (0.2*) | 0.2* | HRMo × CF (1) (tentative) | (0.2*) |
| Poultry liver | 0.2* | STMRMo × CF (1) (tentative) | (0.2*) | 0.2* | HRMo × CF (1) (tentative) | (0.2*) |
| Cattle milk | 0.1* | STMRMo × CF (1) (tentative) | (0.1*) | 0.1* | HRMo × CF (1) (tentative) | (0.1*) |
| Sheep milk | 0.1* | STMRMo × CF (1) (tentative) | (0.1*) | 0.1* | HRMo × CF (1) (tentative) | (0.1*) |
| Goat milk | 0.1* | STMRMo × CF (1) (tentative) | (0.1*) | 0.1* | HRMo × CF (1) (tentative) | (0.1*) |
| Horse milk | 0.1* | STMRMo × CF (1) (tentative) | (0.1*) | 0.1* | HRMo × CF (1) (tentative) | (0.1*) |
| Birds eggs | 0.1* | STMRMo × CF (1) (tentative) | (0.1*) | 0.1* | HRMo × CF (1) (tentative) | (0.1*) |
*Indicates that the input value is proposed at the limit of quantification.
GAP is not supported by data; the existing EU MRL is used for indicative exposure calculations; indicative conversion factors of 1.1 (for oilseeds) and 2.3 (for cereals) were considered for risk assessment.
GAP is not supported by data; the existing EU MRL multiplied by the worst‐case conversion factor of 2.3 for risk assessment is used for indicative exposure calculations.
CXL is higher than the MRL derived in Section 1; the corresponding risk assessment values are used for the (indicative) exposure calculations.
CXL on sugarcane could not be considered in the optional scenario since the optional residue definition (sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate) does not allow comparison with this CXL (defined for glyphosate only while residues of AMPA and/or N‐acetyl compounds above the LOQ are not excluded).
Appendix E – Decision tree for deriving MRL recommendations
1.
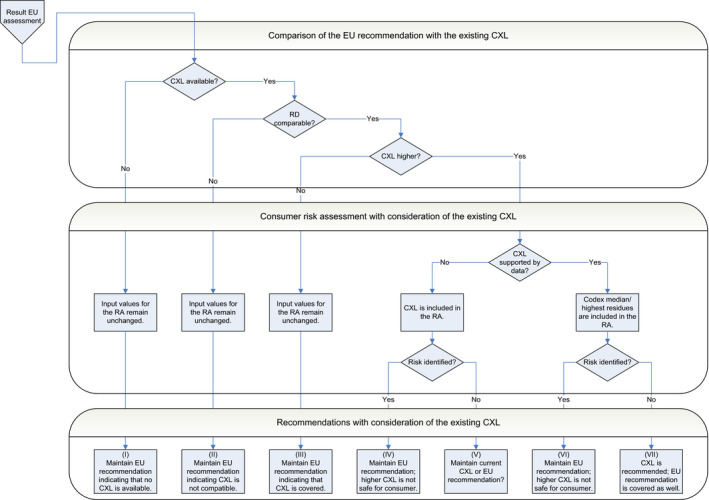
Appendix F – Used compound codes
1.
| Code/trivial name | Chemical name/SMILES notation | Structural formula |
|---|---|---|
| Glyphosate |
N‐(phosphonomethyl)glycine OC(=O)CNCP(=O)(O)O |
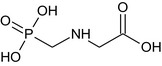
|
| Glyphosate‐trimesium |
trimethylsulfonium N‐[(hydroxyphosphinato)methyl]glycine [O‐]P(=O)(O)CNCC(O)=O.C[S+](C)C |
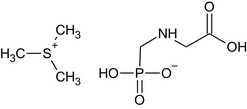
|
| Trimethyl‐sulfonium (TMS‐cation) |
trimethylsulfanium C[S+](C)C |
|
| PMG‐anion | N‐[(hydroxyphosphinato)methyl]glycine |
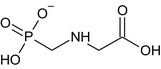
|
| N‐acetyl‐glyphosate |
N‐acetyl‐N‐(phosphonomethyl)glycine OC(=O)CN(CP(=O)(O)O)C(C)=O |
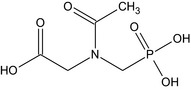
|
| AMPA |
(aminomethyl)phosphonic acid NCP(=O)(O)O |
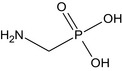
|
| N‐acetyl‐AMPA |
[(carboxyamino)methyl]phosphonic acid O=C(O)NCP(=O)(O)O |
|
| N‐methyl‐AMPA |
[(methylamino)methyl]phosphonic acid CNCP(=O)(O)O |
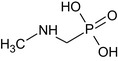
|
SMILES: simplified molecular‐input line‐entry system.
Appendix G – Assessment of the uses previously evaluated by EFSA but not yet legally implemented
1.
It is noted that uses on GAT rapeseed, GAT soybean and GAT maize were evaluated by EFSA in the framework of previous MRL applications (EFSA, 2009, 2013). As these uses are not legally implemented, they were not considered in the framework of the present MRL review. However, in order to support risk managers in the decision‐making process by providing a full overview of the available data, EFSA also reported the assessment of these uses in the present Appendix. The details on these uses are reported in Appendix G.1 (Intended Good Agricultural Practices). A summary of the assessment is presented below focusing on the data and the key calculations specific to these MRL applications (see Appendices G.2, G.3, G.4 and G.5). For what regards the core assessment (nature of residues, storage stability, methods of analysis), reference is made to the reasoned opinion on MRL review and to the list of end points, where all the available studies were already evaluated and reported.
To assess the magnitude of residues in plants resulting from the intended GAPs, EFSA considered all residue trials reported by the evaluating Member State (EMS) in the evaluation reports submitted in the framework of the previous MRL applications (Germany, 2009, 2013a). In these trials, residues were analysed for glyphosate, AMPA, N‐acetyl‐glyphosate and N‐acetyl‐AMPA. MRLs and risk assessment values were recalculated according to the residue definitions for genetically modified crops proposed in the MRL review and considering the most recent agreed methodology (OECD, 2011). Detailed results of the residue trials, derived MRLs and risk assessment values are reported in Appendix G.2. Data were sufficient to derive the MRLs which would accommodate the intended uses on GAT rapeseed, GAT soybean and GAT maize. The following considerations should be made:
Rapeseed: the MRLs derived from the intended use on GAT crop (20 mg/kg) is lower than the MRL proposed in the MRL review (30 mg/kg). Therefore, the intended use on the GAT rapeseed is expected to be covered by the MRL proposed in the MRL review. This MRL was derived from the existing CXL for which no risk to consumers was identified (see Table 2 of the MRL review).
Soybean: the MRL derived from the intended use on GAT crop (15 mg/kg) is lower than the MRL proposed in the MRL review (20 mg/kg). Therefore, the intended use on the GAT soybean is expected to be covered by the MRL proposed in the MRL review. However, it is highlighted that this MRL was proposed at the existing EU MRL, as no residue trials were available to support the existing uses on conventional and EPSPS soybeans (see footnote (l) in Table 2 of the MRL review).
Maize: the MRLs derived from the intended use on GAT crop (0.6 mg/kg) is lower than the MRL proposed in the MRL review (3 mg/kg). Therefore, the intended use on the GAT maize is expected to be covered by the MRL proposed in the MRL review.
In conclusion, the MRLs proposed in the MRL review are expected to cover also the intended uses on GAT crops. It is reminded that, since a fully validated method for enforcement of N‐acetyl‐glyphosate and AMPA in plant commodities is not available, the MRLs derived for rapeseed, soybean and maize were considered tentative.
For information purpose, EFSA also calculated MRLs and risk assessment values for N‐acetyl‐glyphosate only. This additional information may be useful in case risk managers would have interest to define a separate residue definition for this compound as well as for assessing the specific intake of this compound in livestock (see below).
EFSA assessed the possible impact of intended uses on the total livestock dietary burden. First of all, an overall dietary burden considering existing uses and intended uses was calculated according to the residue definition for risk assessment in plant commodities. For rapeseed, soybean and maize, risk assessment values derived from the existing uses and from the intended uses on GAT crops were compared and the most critical values were selected. For all other feed items, the risk assessment values derived from the authorised uses were considered. Livestock dietary burden calculations were performed for different groups of livestock according to OECD guidance (OECD, 2013). The input values used in this calculation are summarised in Appendix G.3.1. This first calculation showed that the intended uses do not modify the dietary burden already assessed based on the existing uses (see comparison in Appendix G.4). This is mainly due to the overwhelming contribution of the existing uses on grass forage and wheat (straw). Furthermore, a theoretical dietary burden which would result from the intended uses only (see input values in Appendix G.3.2) was also calculated and showed extremely lower results compared to the overall dietary burden (see Appendix G.4). Based on these results, it is concluded that the intended uses on rapeseed, soybean and maize do not alter the overall dietary burden for what regards glyphosate and AMPA.
An additional livestock dietary burden calculation was performed to assess the intake of specific metabolites (N‐acetyl compounds). According to the residue trials, N‐acetyl‐glyphosate is the major residue in GAT‐modified crops (see Appendix G.2). Therefore, the calculation was based on the risk assessment values derived for N‐acetyl‐glyphosate only (see input values in Appendix G.3.3). Results of this calculation indicated that the intake of N‐acetyl‐glyphosate in livestock exceed the trigger value (ranging between 0.02 and 0.10 mg/kg bw per day) and represented 60–70% of the total residues intake, resulting from the intended uses calculated according to the residue definition for risk assessment (see Appendix G.4). Consequently, a specific assessment on the magnitude of residues in livestock was performed with a particular focus on the metabolite N‐acetyl‐glyphosate.
Livestock feeding studies conducted on dairy cows and laying hens fed with N‐acetyl‐glyphosate were evaluated in the framework of a previous MRL application (Germany, 2009). Detailed results of these studies were reported in the corresponding EFSA reasoned opinion (EFSA, 2009). These studies indicate that transfer of N‐acetyl‐glyphosate to animal tissues and products was very limited. Based on these studies and the estimated N‐acetyl‐glyphosate intakes by livestock, N‐acetyl‐glyphosate is expected to remain below the LOQ in all animal commodities. Therefore, in case risk managers wish to define a separate residue definition for N‐acetyl‐glyphosate only, MRLs for this compound could be set at the LOQs (see Appendix G.5). Since confirmatory method for N‐acetyl‐glyphosate in all matrices is missing, those MRLs would be tentative only.
EFSA assessed the possible impact of the intended uses on the consumer exposure. Based on the results of the studies on the magnitude of residues in plant and animal commodities, the MRLs proposed in the MRL review are expected to cover the intended uses on GAT crops (see Table G.1 below). Therefore, the consumer risk assessment performed in the MRL review does not need to be reconsidered and it can be concluded that the short‐term and long‐term intake of residues resulting from the intended uses on GAT soybeans, maize and rapeseeds is unlikely to present a risk to consumer health.
Table G.1.
Conclusion and recommendations
| Code | Commodity | Existing MRL (mg/kg) | MRL proposed in MRL review (mg/kg) | MRL derived from intended uses (mg/kg) | Comment and recommendation |
|---|---|---|---|---|---|
|
Enforcement residue definition (existing): glyphosate Enforcement residue definition (proposed): sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate | |||||
| 401060 | Rapeseeds/canola seeds | 10 | 30 | 20 | MRL proposed in the MRL review is sufficient to cover the intended use |
| 401070 | Soyabeans | 20 | 20 | 15 |
MRL proposed in the MRL review is sufficient to cover the intended use It is noted that no residue data were available to support the existing uses |
| 500030 | Maize/corn grains | 1 | 3 | 0.6 | MRL proposed in the MRL review is sufficient to cover the intended use |
| – | Commodities of animal origin | See Table 2 in MRL review | – | The residue levels in GAT‐modified rapeseeds, soybeans, maize and their by‐products resulting from the intended uses do not require a modification of the MRLs for animal products derived in the MRL review | |
G.1. Intended Good Agricultural practice (GAPs)
| GAPs for import tolerances (non‐European indoor, outdoor or post‐harvest treatments) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GAT genetically modified crops | ||||||||||||||||||||
| Crop | Region | Outdoor/indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Rapeseeds | Brassica napus subsp. napus | non‐EU | Outdoor | CAN | Broadleaf weeds and grasses | SL | 500.0 | g/L | Foliar treatment ‐ broadcast spraying | 11 | 89 | 1 | 3 | 0.68 | 0.90 | kg a.i./ha | 7 | Dessicant use (EFSA, 2013) | ||
| Soyabeans | Glycine max | non‐EU | Outdoor | USA | Broadleaf weeds and grasses | SL | 500.0 | g/L | Foliar treatment ‐ spraying | 8 | 99 | 1 | 4 | 0.82 | 3.33 | kg a.i./ha | 14 |
Maximum glyphosate per season: 6.77 kg a.i./ha Dessicant use (EFSA, 2009) |
||
| Maize | Zea mays | non‐EU | Outdoor | USA | Broadleaf weeds and grasses | SL | 600.0 | g/L | Foliar treatment ‐ spraying | 7 | 99 | 1 | 4 | 0.87 | 4.10 | kg a.i./ha | 7 |
Maximum glyphosate per season: 6.77 kg a.i./ha Dessicant use (EFSA, 2009) |
||
GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient.
G.2. Overview of the available residue trials data
| Crop | Region/indoora | Residue levels observed in the supervised residue trials relevant to the supported GAPs (mg/kg) | Recommendations/comments (OECD calculations) | MRL proposals (mg/kg) | HRMo (mg/kg)b | STMRMo (mg/kg)c | CFd |
|---|---|---|---|---|---|---|---|
|
Genetically modified GAT crops RD‐enforcement 1=RD‐enforcement 2: sum of glyphosate, AMPA and N‐acetyl‐glyphosate, expressed as glyphosate Values into parentheses refer to the residues of N‐acetyl‐glyphosate only | |||||||
| Rapeseeds | Import (CAN) |
Mo: 0.8; 0.86; 14.76; 1.48; 2.48; 3.71; 1.81; 2.72; 2.73; 3.10; 3.49; 4.92; 5.61; 10.38; 9.23 RA: 0.85; 0.91; 15.1; 1.53; 2.53; 3.76; 1.86; 2.77; 2.78; 3.15; 3.54; 4.97; 5.66; 10.43; 9.28 |
Trials on rapeseeds compliant with GAP (Germany, 2013a) MRLOECD: 20.43. Glyphosate ranged from 0.41 to 8.95 mg/kg N‐acetyl‐glyphosate ranged from 0.46 to 14 mg/kg. AMPA ranged from < 0.05 to 0.082 mg/kg N‐acetyl‐AMPA always below or at 0.05 mg/kg, apart from 1 sample (0.34) mg/kg |
(20)e |
14.8 (14) |
3.1 (0.46) |
1 – |
| Soybeans | Import (USA) |
Mo: < 0.15; 0.28; 0.41; 0.48; 0.59; 0.76; 0.77; 0.93; 0.96; 1.01; 1.13; 1.16; 1.19; 1.36; 1.70; 1.80; 1.87; 1.92; 2.04; 2.07; 2.54; 2.54; 2.62; 2.92; 3.11; 3.31; 3.47; 4.42; 5.27; 5.55; 5.77; 5.94; 6.05; 6.19; 6.77; 8.07 RA: < 0.20; 0.33; 0.49; 0.64; 0.73; 0.82; 0.86; 1.04; 1.19; 1.19; 1.26; 1.30; 1.42; 1.52; 2.02; 2.04; 2.18; 2.04; 2.36; 2.21; 3.03; 3.08; 2.9; 3.01; 3.53; 3.59; 3.6; 5.52; 5.65; 5.66; 6.13; 6.82; 6.87; 6.61; 8.07; 8.64 |
Trials on soyabeans compliant with GAP (Germany, 2009.) MRLOECD: 11.36 Glyphosate ranged from < 0.05 to 1.7 mg/kg N‐acetyl‐glyphosate ranged from < 0.05 to 7.9 mg/kg. AMPA ranged from < 0.05 to 0.16 mg/kg. N‐acetyl‐AMPA ranged from < 0.05 to 1.3 mg/kg |
(15)e |
8.07 (7.9) |
1.98 (1.65) |
1.1 – |
| Maize, grain | Import (US) |
Mo: < 0.06; < 0.06; 0.06; 0.06; 0.07; 0.07; 0.07; 0.07; 0.07; 0.07; 0.07; 0.07; 0.08; 0.08; 0.08; 0.08; 0.08; 0.08; 0.08; 0.09; 0.09; 0.09; 0.09; 0.09; 0.1; 0.1; 0.1; 0.11; 0.11; 0.13; < 0.15; < 0.15; < 0.15; < 0.15; 0.15; 0.15; 0.16; 0.17; 0.17; 0.18; 0.2; 0.21; 0.25; 0.3; 0.34; 0.4; 0.56 RA: < 0.08; < 0.08; 0.08; 0.08; 0.09; 0.09; 0.09; 0.09; 0.09; 0.09; 0.09; 0.09; 0.1; 0.1; 0.1; 0.1; 0.1; 0.1; 0.1; 0.11; 0.11; 0.11; 0.11; 0.11; 0.12; 0.12; 0.12; 0.13; 0.13; 0.15; 0.17; < 0.2; < 0.2; < 0.2; < 0.2; 0.2; 0.21; 0.22; 0.22; 0.23; 0.25; 0.23; 0.3; 0.32; 0.36; 0.43; 0.6 |
Trials on maize compliant with GAP (Germany, 2009). MRLOECD: 0.56 Glyphosate ranged from < 0.02 to 0.08 mg/kg N‐acetyl‐glyphosate ranged from < 0.02 to 0.52 mg/kg. AMPA always below or at the LOQs of 0.02 and 0.05 mg/kg N‐acetyl‐AMPA always below or at the LOQs of 0.02 and 0.05 mg/kg apart from 2 samples (0.03 and 0.04 mg/kg). |
(0.6)e |
0.56 (0.52) |
0.09 (0.04) |
1.2 – |
| Maize, stover | Import (US) | – | Cereals straw not relevant for import tolerance GAP | – | – | – | – |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level.
*Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue according to the residue definition for monitoring.
Supervised trials median residue according to the residue definition for monitoring.
Conversion factor for risk assessment; median of the individual conversion factors at the supported PHI for each residues trial (unless otherwise specified).
MRLs are tentative because confirmatory methods for analysis of N‐acetyl‐glyphosate and AMPA are still required.
In case risk managers wish to restrict the RD to glyphosate and AMPA only, an MRL of 15 mg/kg would be sufficient to accommodate the new use on GAT rapeseeds.
In case risk managers wish to restrict the RD to glyphosate and AMPA only, an MRL of 2 mg/kg would be sufficient to accommodate the new use on GAT soybeans.
In case risk managers wish to restrict the RD to glyphosate and AMPA only, an MRL of 0.2 mg/kg would be sufficient to accommodate the new use on GAT maize.
G.3. Input values for the dietary burden calculations
G.3.1. Input values considering all existing uses and the intended uses on GAT crops
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: sum of glyphosate, AMPA, N‐acetyl‐glyphosate and N‐acetyl‐AMPA, expressed as glyphosate | ||||
| Soybeans, seed | 2.18 | STMR × CF (tentative)a | 2.18 | STMR × CF (tentative)a |
| Rapeseed, meal | 4.65 | STMR × CF × PF (1.5)b (tentative)a | 4.65 | STMR × CF × PF (1.5)b (tentative)a |
| Soybeans, meal | 1.75 | STMR × CF × PF (0.68)b (tentative)a | 1.75 | STMR × CF × PF (0.68)b (tentative)a |
| Soybeans, hulls | 12.6 | STMR × CF × PF (5.3)b (tentative)a | 12.6 | STMR × CF × PF (5.3)b (tentative)a |
| All other feed commodities | See Appendix D.1 | |||
STMR: supervised trials median residue; CF: conversion factor; PF: processing factor.
STMR and CF derived from the intended uses on GAT‐soybean and GAT‐rapeseed (see Appendix G.2).
Processing factors for soybean‐ and rapeseed‐processed items were assessed in Appendix B.1.2.6 (PF for genetically modified GAT crops).
G.3.2. Input values considering only the intended uses on GAT crops
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: sum of glyphosate, AMPA, N‐acetyl‐glyphosate and N‐acetyl‐AMPA, expressed as glyphosate | ||||
| Corn, field (Maize), grain | 0.11 | STMR × CF (tentative) | 0.11 | STMR × CF (tentative) |
| Corn, pop, grain | 0.11 | STMR × CF (tentative) | 0.11 | STMR × CF (tentative) |
| Soybeans, seed | 2.18 | STMR × CF (tentative) | 2.18 | STMR × CF (tentative) |
| Rapeseed, meal | 4.65 | STMR × CF × PF (1.5)a (tentative) | 4.65 | STMR × CF × PF (1.5)a (tentative) |
| Corn, field, milled by‐products | 0.10 | STMR × CF × PF (0.93)a (tentative) | 0.10 | STMR × CF × PF (0.93)a (tentative) |
| Corn, field, hominy meal | 0.68 | STMR × CF × PFb (tentative) | 0.68 | STMR × CF × PFb (tentative) |
| Corn, field, gluten feed | 0.28 | STMR × CF × PFb (tentative) | 0.28 | STMR × CF × PFb (tentative) |
| Corn, field, gluten, meal | 0.11 | STMR × CF × PFb (tentative) | 0.11 | STMR × CF × PFb (tentative) |
| Corn, field, distiller's grain (dry) | 0.37 | STMR × CF × PFb (tentative) | 0.37 | STMR × CF × PFb (tentative) |
| Soybeans, meal | 1.75 | STMR × CF × PF (0.68)a (tentative) | 1.75 | STMR × CF × PF (0.68)a (tentative) |
| Soybeans, hulls | 12.6 | STMR × CF × PF (5.3)a (tentative) | 12.6 | STMR × CF × PF (5.3)a (tentative) |
STMR: supervised trials median residue; CF: conversion factor; PF: processing factor.
Processing factors for soybean (meal and hulls), corn (milled by‐products) and rapeseed (meal) were assessed in Appendix B.1.2.6 (PF for genetically modified GAT crops).
For corn hominy meal, corn gluten feed, corn gluten meal and corn distiller's grain, in the absence of processing factors supported by data, the default processing factors were included in the calculation to consider the potential concentration of residues in these commodities.
G.3.3. Input values considering only the intended uses on GAT crops (N‐acetyl‐glyphosate only)
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: N‐acetyl‐glyphosate, expressed as glyphosate | ||||
| Corn, field (Maize), grain | 0.04 | STMR (tentative) | 0.04 | STMR (tentative) |
| Corn, pop, grain | 0.04 | STMR (tentative) | 0.04 | STMR (tentative) |
| Soybeans, seed | 1.65 | STMR (tentative) | 1.65 | STMR (tentative) |
| Rapeseed, meal | 0.69 | STMR × PF (1.5)a (tentative) | 0.69 | STMR × PF (1.5)a (tentative) |
| Corn, field, milled by‐products | 0.05 | STMR × PF (1.3)a (tentative) | 0.05 | STMR × PF (1.3)a (tentative) |
| Corn, field, hominy meal | 0.24 | STMR × PFb (tentative) | 0.24 | STMR × PFb (tentative) |
| Corn, field, gluten feed | 0.10 | STMR × PFb (tentative) | 0.10 | STMR × PFb (tentative) |
| Corn, field, gluten, meal | 0.04 | STMR × PFb (tentative) | 0.04 | STMR × PFb (tentative) |
| Corn, field, distiller's grain (dry) | 0.13 | STMR × PFb (tentative) | 0.13 | STMR × PFb (tentative) |
| Soybeans, meal | 1.15 | STMR × PF (0.70)a (tentative) | 1.15 | STMR × PF (0.70)a (tentative) |
| Soybeans, hulls | 8.58 | STMR × PF (5.2)a (tentative) | 8.58 | STMR × PF (5.2)a (tentative) |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor.
Processing factors for soybean (meal and hulls), corn (milled by‐products) and rapeseed (meal) were assessed in previous MRL applications (EFSA, 2009 for corn and soybeans; EFSA, 2013 for rapeseed).
For corn hominy meal, corn gluten feed, corn gluten meal and corn distiller's grain, in the absence of processing factors supported by data, the default processing factors were included in the calculation to consider the potential concentration of residues in these commodities.
G.4. Results of the livestock dietary burden calculations
| Relevant groups | Max. Dietary burden expressed in mg/kg bw per day | Trigger exceeded(Y/N) | ||||||
|---|---|---|---|---|---|---|---|---|
| Existing uses onlya | Existing uses and intended usesb | Most critical commodity | Intended uses only: total residues for risk assessmentc | Most critical commodity | Intended uses only: N‐acetyl‐glyphosate only (% compared to total residues for risk assessment)d | Most critical commodity | ||
| Cattle (all diets) | 13.2 | 13.2 | Grass, forage (fresh) | 0.0396 | Soybean, hulls | 0.0273 (69%) | Soybean, hulls | Yes |
| Cattle (dairy only) | 13.2 | 13.2 | Grass, forage (fresh) | 0.0300 | Canola, meal | 0.0192 (64%) | Soybean, seed | Yes |
| Sheep (all diets) | 17.7 | 17.7 | Grass, forage (fresh) | 0.1402 | Soybean, hulls | 0.0968 (69%) | Soybean, hulls | Yes |
| Sheep (ewe only) | 17.7 | 17.7 | Grass, forage (fresh) | 0.1016 | Soybean, hulls | 0.0697 (69%) | Soybean, hulls | Yes |
| Swine (all diets) | 2.85 | 2.85 | Grass, forage (fresh) | 0.0570 | Soybean, hulls | 0.0397 (70%) | Soybean, hulls | Yes |
| Poultry (all diets) | 2.28 | 2.28 | Wheat, straw | 0.1025 | Canola, meal | 0.0616 (60%) | Soybean, seed | Yes |
| Poultry (layer only) | 2.28 | 2.28 | Wheat, straw | 0.0736 | Soybean, hulls | 0.0516 (70%) | Soybean, hulls | Yes |
Dietary burden calculation considering all authorised uses reported and assessed in the MRL review (see details in the core assessment Appendix B.2).
Overall dietary burden calculation considering all authorised uses reported in the MRL review and the intended uses assessed in previous MRL applications (EFSA, 2009, 2013).
Dietary burden calculation considering only the intended uses assessed in previous MRL applications (EFSA, 2009, 2013).
Dietary burden calculation considering only the intended uses assessed in previous MRL applications (EFSA, 2009, 2013), N‐acetyl‐glyphosate only (in percentage: contribution of N‐acetyl‐glyphosate to the dietary burden intended uses only expressed according to the residue definition for risk assessment).
G.5. Summary of the residue data from livestock feeding studies performed with N‐acetyl‐glyphosate
| Animal commodity | Residues at the closest feeding level (mg/kg) | Estimated value at 1N | MRL proposal (mg/kg) | ||
|---|---|---|---|---|---|
| Mean | Highest | STMRa (mg/kg) | HRb (mg/kg) | ||
| Residue definition for enforcement and risk assessment: N‐acetyl‐glyphosate | |||||
| Cattle (all diets) – Closest feeding level (1.25 mg/kg bw per day; 46N dietary burden)c | |||||
| Muscle | < 0.025 | < 0.025 | < 0.025 | < 0.025 | 0.025*, d (tentative) |
| Fat | < 0.05 | < 0.05 | < 0.05 | < 0.05 | 0.05*, d (tentative) |
| Liver | < 0.05 | < 0.05 | < 0.05 | < 0.05 | 0.05*, d (tentative) |
| Kidney | 0.08 | 0.11 | < 0.05 | < 0.05 | 0.05*, d (tentative) |
| Cattle (dairy only – Closest feeding level (1.25 mg/kg bw per day; 66N dietary burden)c | |||||
| Milke | < 0.025 | n.a. | < 0.025 | < 0.025 | 0.025*, d (tentative) |
| Sheep (all diets)e – Closest feeding level (1.25 mg/kg bw; 13N dietary burden)c | |||||
| Muscle | < 0.025 | < 0.025 | < 0.025 | < 0.025 | 0.025*, d (tentative) |
| Fat | < 0.05 | < 0.05 | < 0.05 | < 0.05 | 0.05*, d (tentative) |
| Liver | < 0.05 | < 0.05 | < 0.05 | < 0.05 | 0.05*, d (tentative) |
| Kidney | 0.08 | 0.11 | < 0.05 | < 0.05 | 0.05*, d (tentative) |
| Sheep (dairy only)e – Closest feeding level (1.25 mg/kg bw; 18N dietary burden)c | |||||
| Milke | < 0.025 | n.a. | < 0.025 | < 0.025 | 0.025*, d (tentative) |
| Swinee – Closest feeding level (1.25 mg/kg bw per day; 31N dietary burden) c | |||||
| Muscle | < 0.025 | < 0.025 | < 0.025 | < 0.025 | 0.025*, d (tentative) |
| Fat | < 0.05 | < 0.05 | < 0.05 | < 0.05 | 0.05*, d (tentative) |
| Liver | < 0.05 | < 0.05 | < 0.05 | < 0.05 | 0.05*, d (tentative) |
| kidney | 0.08 | 0.11 | < 0.05 | < 0.05 | 0.05*, d (tentative) |
| Poultry (all diets) – Closest feeding level (1.5 mg/kg bw per day; 25N dietary burden)c | |||||
| Muscle | 0.03 | 0.04 | < 0.025 | < 0.025 | 0.025*, d (tentative) |
| Fat | 0.11 | 0.13 | < 0.05 | < 0.05 | 0.05*, d (tentative) |
| Liver | 0.19 | 0.21 | < 0.05 | < 0.05 | 0.05*, d (tentative) |
| Poultry (layer only) – Closest feeding level (1.5 mg/kg bw per day; 30N dietary burden)c | |||||
| Eggs | 0.03 | 0.05 | < 0.025 | < 0.025 | 0.025*, d (tentative) |
n.a.: not applicable.
*Indicates that the MRL is proposed at the limit of quantification.
The mean residue level for milk and the mean residue levels for eggs and tissues were recalculated at the 1N rate for the median dietary burden.
The mean residue level in milk and the highest residue levels in eggs and tissues were recalculated at the 1N rate for the maximum dietary burden.
Closest feeding level and N dose rate related to the maximum dietary burden. Study performed with N‐acetyl‐glyphosate.
MRL proposal is tentative because a confirmatory method for N‐acetyl‐glyphosate is still required for all animal matrices.
Highest residue level from day 1 to day 28 (daily mean of 3 cows).
Since extrapolation from cattle to other ruminants and swine is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in sheep and swine.
Suggested citation: EFSA (European Food Safety Authority) , 2018. Reasoned Opinion on the review of the existing maximum residue levels for glyphosate according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2018;16(5):5263, 230 pp. 10.2903/j.efsa.2018.5263
Requestor: European Commission
Question number: EFSA‐Q‐2008‐00561
Acknowledgement: EFSA wishes to thank the rapporteur Member State, Germany, for the preparatory work on this scientific output.
Approved: 17 April 2018
This publication is linked to the following EFSA Journal articles: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2018.5283/full, http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2019.5862/full
NOTE: This reasoned opinion is superseded by an updated version.
Notes
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Commission Directive 2001/99/EC of 20 November 2001 amending Annex I to Council Directive 91/414/EEC concerning the placing of plant protection products on the market to include glyphosate and thifensulfuron‐methyl as active substances. OJ L 304, 21.11.2001, p. 14–16.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Implementing Regulation (EU) No 541/2011 of 1 June 2011 amending Implementing Regulation (EU) No 540/2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 187–188.
Commission Implementing Regulation (EU) 2016/1056 of 29 June 2016 amending Implementing Regulation (EU) No 540/2011 as regards the extension of the approval period of the active substance glyphosate. OJ L 173, 30.6.2016, p. 52–54.
Commission Implementing Regulation (EU) 2016/1313 of 1 August 2016 amending Implementation Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance glyphosate. OJ L 208, 2.8.2016, p. 1–3.
Commission Regulation (EU) No 1141/2010 of 7 December 2010 laying down the procedure for the renewal of the inclusion of a second group of active substances in Annex I to Council Directive 91/414/EEC and establishing the list of those substances, OJ L 322, 8.12.2010, p. 10–19.
Commission Implementing Regulation (EU) No 380/2013 of 25 April 2013 amending Regulation (EU) No 1141/2010 as regards the submission of the supplementary complete dossier to the Authority, the other Member States and the Commission, OJ L 116, 26.4.2013, p. 4–4.
Commission Regulation (EC) No 149/2008 of 29 January 2008 amending Regulation (EC) No 396/2005 of the European Parliament and of the Council by establishing Annexes II, III and IV setting maximum residue levels for products covered by Annex I thereto, OJ L 58, 1.3.2008, p. 1–398.
Commission Regulation (EC) No 839/2008 of 31 July 2008 amending Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards Annexes II, III and IV on maximum residue levels of pesticides in or on certain products, OJ L 234, 30.8.2008, p. 1–216.
EPSPS: In conventional plants, glyphosate inhibits the 5‐enolpyruvylshikimate‐3‐phosphate synthase (EPSPS) protein, a key enzyme in the biosynthesis of aromatic amino acids (e.g. tyrosine, phenylalanine…), leading to plant death. Tolerance to glyphosate is obtained by the introduction of a gene from Rhizobium radiobacter that codes for the expression of a modified EPSPS protein, insensitive towards glyphosate inhibition.
GOX: Glyphosate oxidoreductase, protein obtained by the introduction of a gene from Ochrobactrum anthrop acting by breaking down glyphosate to AMPA and glyoxylate which have no herbicidal activity.
GAT: Glyphosate N‐acetyltransferase, protein obtained by the introduction of a gene from Bacillus licheniformis, giving rise to N‐acetyl‐glyphosate which denotes no herbicidal activity.
Available online: http://ec.europa.eu/food/dyna/gm_register/index_en.cfm
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Molecular weight of glyphosate (169.1 g/mol)/molecular weight of AMPA (111.1 g/mol).
Molecular weight of glyphosate (169.1 g/mol)/molecular weight of N‐acetyl‐glyphoate (211.1 g/mol).
At the time of finalisation of the present review, it is currently under assessment in the framework of Regulation (EC) No 1829/2003.
The combined LOQ was calculated considering the sum of LOQs and molecular factors of 1.5 (to convert AMPA to glyphosate) and 0.8 (to convert N‐acetyl‐AMPA to glyphosate).
References
- EFSA (European Food Safety Authority), 2007. Reasoned opinion on the potential chronic and acute risk to consumers’ health arising from proposed temporary EU MRLs. EFSA Journal 2007;5(3):32r, 1141 pp. 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Reasoned opinion on the modification of the residue definition of glyphosate in genetically modified maize grain and soybeans, and in products of animal origin on request from the European Commission. EFSA Journal 2009;7(9):2009, 42 pp. 10.2903/j.efsa.2009.1310 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Reasoned Opinion on modification of the existing MRL for glyphosate in lentils. EFSA Journal 2012;10(1):2550, 25 pp. 10.2903/j.efsa.2012.2550. Available online: www.efsa. europa.eu [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. Reasoned opinion on the import tolerance for glyphosate in genetically modified oilseed rape. EFSA Journal 2013;11(11):3456, 30 pp. 10.2903/j.efsa.2013.3456 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA Journal 2015;13(11):4302, 107 pp. 10.2903/j.efsa.2015.4302. Available online: www.efsa.europa.eu [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. Reasoned opinion on the modification of the existing maximum residue levels for glyphosate in borage and corn gromwell seeds. EFSA Journal 2016;14(4):4468, 20 pp. 10.2903/j.efsa.2016.4468 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Completeness check report on the review of the existing MRLs of glyphosate prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 21 September 2017. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2018a. Member States consultation report on the review of the existing MRLs of glyphosate prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 26 March 2018. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2018b. Evaluation of the impact of glyphosate and its residues in feed on animal health. EFSA Journal 2018;16(5):5283, 25 pp. 10.2903/j.efsa.2018.5283. Available online: www.efsa. europa.eu [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2016. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev. 10.2, September 2016.
- FAO (Food and Agriculture Organization of the United Nations), 2005. Glyphosate. In: Pesticide residues in food – 2005. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticides residues. 20 ‐ 29 September 2005. FAO Plant Production and Protection Paper 183.
- FAO (Food and Agriculture Organization of the United Nations), 2009. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 2nd Edition. FAO Plant Production and Protection Paper 197, 264 pp.
- FAO (Food and Agriculture Organization of the United Nations), 2011. Glyphosate. In: Pesticide residues in food – 2011. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticides residues. 20 ‐ 29 September 2011. FAO Plant Production and Protection Paper 211.
- FAO (Food and Agriculture Organization of the United Nations), 2013. Glyphosate. In: Pesticide residues in food – 2013. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticides residues. 17 ‐ 26 September 2013. FAO Plant Production and Protection Paper 219.
- Germany , 2009. Evaluation Report prepared under Article 8 of Regulation (EC) No 396/2005. MRLs for glyphosate in genetically modified soybeans and maize. 23 January 2009.
- Germany , 2013a. Evaluation Report prepared under Article 8 of Regulation (EC) No 396/2005. MRLs for glyphosate in genetically modified rapeseeds. 13 May 2013.
- Germany , 2013b. Renewal assessment report (RAR) on the active substance glyphosate prepared by the rapporteur Member State Germany in the framework of Regulation (EU) No 1141/2010, December 2013. Available online: www.efsa.europa.eu
- Germany , 2015. Final addendum to the renewal assessment report (RAR) on the active substance glyphosate prepared by the rapporteur Member State Germany in the framework of Commission Regulation (EU) No 1141/2010, compiled by EFSA in October 2015. Available online: www.efsa.europa.eu
- Germany , 2017. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Review of the existing MRLs for glyphosate, 9 June 2017. Available online: www.efsa.europa.eu
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 4 September 2013.
- United Kingdom , 2015. Evaluation Report prepared under Article 8 of Regulation (EC) No 396/2005. MRLs for glyphosate in corn gromwell seeds. 25 June 2015.


