Abstract
The Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids of the European Food Safety Authority was requested to evaluate the genotoxic potential of flavouring substances from subgroup 1.1.4 of FGE.19 in the Flavouring Group Evaluation 203 Revision 2 (FGE.203Rev2). In FGE. 203 Revision 1, the Panel concluded that the genotoxic potential could not be ruled out for the flavouring substances in this FGE. The Flavour Industry provided additional genotoxicity studies for the representative substances of FGE.19 subgroup 1.1.4, namely deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140] and hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057]. In addition, new studies on hepta‐2,4‐dienal [FL‐no: 05.084], 2,4‐octadienal [FL‐no: 05.186] and tr‐2,tr‐4‐nonadienal [FL‐no: 05.194] were provided that are evaluated in the present revision of FGE.203, i.e. FGE.203Rev2. Hepta‐2,4‐dienal [FL‐no: 05.084], 2,4‐octadienal [FL‐no: 05.186] and tr‐2,tr‐4‐nonadienal [FL‐no: 05.194] did not induce gene mutations in bacteria. Hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] did not induce gene mutations in vitro in mammalian cells. Hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] was also tested in an in vivo gene mutation assay giving negative results. Both hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] and deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140] were tested in vivo for the induction of micronuclei in rats bone marrow and peripheral reticulocytes after oral or intraperitoneal administration. None of the two substances induced increased frequencies of micronuclei. The Panel concluded that the concern for genotoxicity can be ruled out for the representative substances hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] and deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140] and therefore also for the other substances in this group [FL‐no: 02.139, 02.153, 02.162, 02.188, 05.064, 05.071, 05.081, 05.084, 05.101, 05.108, 05.125, 05.127, 05.141, 05.173, 05.186, 05.194, 05.196, 09.573]. These 20 substances can be evaluated using the Procedure for the evaluation of flavouring substances.
Keywords: α,β‐unsaturated aldehydes; straight chain; FGE.203; α,β‐unsaturated conjugated double‐bonds; FGE.19; subgroup 1.1.4
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The use of flavouring is regulated under Regulation (EC) No 1334/20081 of the European Parliament and Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation, an evaluation and approval are required for flavouring substances.
The Union List of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/20122.
The list contains flavouring substances for which the scientific evaluation should be completed taking into account Commission Regulation (EC) No 1565/20003.
The genotoxicity of the twenty substances belonging to the group FGE.203 rev.1; alpha, beta‐unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.4 of FGE.19 were considered in the EFSA opinion of 26 March 2014.4
The Authority evaluated the genotoxicity of these substances on the basis of the data on the following two substances selected as representative of the group: the hexa‐2(trans),4(trans)‐dienal (FL‐no: 05.057) and deca‐2(trans),4(trans)‐dienal (FL‐no: 05.140). Overall, the Authority concluded that the safety concern regarding genotoxicity cannot be ruled out for both representative substances of the group and that this conclusion is likewise applicable to the other substances of this FGE.203.
These substances are included in the Union List with no restrictions.
Following this opinion the applicant offered to carry out a number of additional toxicology studies to address the safety concerns raised in the opinion. This set of studies were not requested and not agreed with EFSA or the Commission.
The Commission requested information on poundage and use levels of the substances in order to calculate the exposure and quantify the risks. It also requested information regarding stereoisomerism in particular regarding the substances belonging to this group and not evaluated by JECFA and currently included in the Union List. This information is also attached in the submission.
The studies offered by industry and also the information requested by the Commission were submitted by industry on 22 September 2016.
The Commission submitted for vote at the Standing Committee on Plants, Animals, Food and Feed of the 25 November 2016 a draft Regulation amending the conditions of use of these substances establishing restrictions to the food categories actually in use and also establishing maximum levels for these uses (Ref Doc SANTE 10070/2016). This measure contains the exposure to these substances and also prevents further new uses. The measure was supported by a very substantial qualified majority of the Member States. The measure will continue its usual process of adoption.
1.1.1. Terms of Reference
The European Commission requests the European Food Safety Authority (EFSA) to evaluate the studies in the submission and any new other safety information relevant, and depending on the outcome, proceed to the full evaluation on these flavouring substances, taking into account the requirements of the Commission Regulation (EC) No 1565/2000 and of Regulation (EU) No 1334/2008. The Authority is also asked to characterise the hazards and also quantify the risks also in case its concern on genotoxicity cannot be ruled out and the EFSA CEF panel procedure cannot be applied for any of the substances of the group.
2. Data and methodologies
2.1. History of the evaluation of FGE.19 substances
Flavouring Group Evaluation 19 (FGE.19) contains 360 flavouring substances from the EU Register being α,β‐unsaturated aldehydes or ketones and precursors which could give rise to such carbonyl substances via hydrolysis and/or oxidation (EFSA, 2008a).
The α,β‐unsaturated aldehyde and ketone structures are structural alerts for genotoxicity (EFSA, 2008a). The Panel noted that there were limited genotoxicity data on these flavouring substances but that positive genotoxicity studies were identified for some substances in the group.
The α,β‐unsaturated carbonyls were subdivided into subgroups on the basis of structural similarity (EFSA, 2008a). In an attempt to decide which of the substances could go through the Procedure, a (quantitative) structure–activity relationship (Q)SAR prediction of the genotoxicity of these substances was undertaken considering a number of models (DEREKfW, TOPKAT, DTU‐NFI‐MultiCASE Models and ISS‐Local Models, (Gry et al., 2007)).
The Panel noted that for most of these models internal and external validation has been performed, but considered that the outcome of these validations was not always extensive enough to appreciate the validity of the predictions of these models for these α,β‐unsaturated carbonyls. Therefore, the Panel considered it inappropriate to totally rely on (Q)SAR predictions at this point in time and decided not to take substances through the procedure based on negative (Q)SAR predictions only.
The Panel took note of the (Q)SAR predictions by using two ISS Local Models (Benigni and Netzeva, 2007a,b) and four DTU‐NFI MultiCASE Models (Gry et al., 2007; Nikolov et al., 2007) and the fact that there are available data on genotoxicity, in vitro and in vivo, as well as data on carcinogenicity for several substances. Based on these data the Panel decided that 15 subgroups (1.1.1, 1.2.1, 1.2.2, 1.2.3, 2.1, 2.2, 2.3, 2.5, 3.2, 4.3, 4.5, 4.6, 5.1, 5.2 and 5.3) (EFSA, 2008b) could not be evaluated through the Procedure due to concern with respect to genotoxicity. Corresponding to these subgroups, 15 Flavouring Group Evaluations (FGEs) were established: FGE.200, 204, 205, 206, 207, 208, 209, 211, 215, 219, 221, 222, 223, 224 and 225.
For 11 subgroups, the Panel decided, based on the available genotoxicity data and (Q)SAR predictions, that a further scrutiny of the data should take place before requesting additional data from the Flavouring Industry on genotoxicity. These subgroups were evaluated in FGE.201, 202, 203, 210, 212, 213, 214, 216, 217, 218 and 220. For the substances in FGE.202, 214 and 218, it was concluded that a genotoxic potential could be ruled out and accordingly these substances were evaluated using the Procedure. For all or some of the substances in the remaining FGEs, FGE.201, 203, 210, 212, 213, 216, 217 and 220, the genotoxic potential could not be ruled out.
To ease the data retrieval of the large number of structurally related α,β‐unsaturated substances in the different subgroups for which additional data are requested, EFSA worked out a list of representative substances for each subgroup (EFSA, 2008c). Likewise, an EFSA genotoxicity expert group has worked out a test strategy to be followed in the data retrieval for these substances (EFSA, 2008b).
The Flavouring Industry has been requested to submit additional genotoxicity data according to the list of representative substances and test strategy for each subgroup.
The Flavouring Industry has now submitted additional data and the present FGE concerns the evaluation of these data requested on genotoxicity.
2.2. History of the evaluation of the substances in subgroup 1.1.4
In November 2008, the Panel concluded based on the in vitro and in vivo genotoxicity data and carcinogenicity data available at that time as well as on the outcome of the (Q)SAR predictions that there is a safety concern for hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] since a non‐threshold mechanism cannot be excluded. The Panel requested data which clarify whether the carcinogenic effects were based on a threshold mechanism. This conclusion also applies to the other substances of this FGE.203 (EFSA, 2009).
The Panel identified two substances in FGE.19 subgroup 1.1.4 (hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] and deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140]) as representative substances (EFSA, 2008c) to be tested in accordance with the conditions set out in the ‘Genotoxicity Test Strategy for Substances belonging to Subgroups of FGE.19’ (EFSA, 2008b), and in accordance with the conclusion in FGE.203. The representative substances for subgroup 1.1.4 are shown in Table 1.
Table 1.
Representative substances for subgroup 1.1.4 of FGE.19 (EFSA, 2008c)
| FL‐no | EU register name | Structural formula |
|---|---|---|
| 05.057 | Hexa‐2(trans),4(trans)‐dienal |
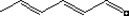
|
| 05.140 | Deca‐2(trans),4(trans)‐dienal |
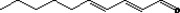
|
Since FGE.203 was published, three additional substances had been included in the subgroup 1.1.4 of FGE.19 (2,4‐decadienal [FL‐no: 05.081], 2,4‐octadienal [FL‐no: 05.186] and tr‐2,tr‐4‐nonadienal [FL‐no: 05.194]); therefore, FGE.203Rev1 concerned the genotoxicity evaluation of 20 flavouring substances.
In response to the requested genotoxicity data in FGE.203 on representative substances for subgroup 1.1.4, new data on the representative substance deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140] and literature data on the representative substance hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] were submitted by Industry (EFFA, 2013) and evaluated in FGE.203 revision 1 (EFSA CEF Panel, 2014).
In FGE.203Rev1, the Panel considered that a non‐threshold mechanism of action cannot be excluded for both representative substances based on the data available and the Panel concluded that the safety concern cannot be ruled out for hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] and for deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140]. This conclusion was likewise applicable to the other substances in subgroup 1.1.4.
The industry has submitted additional data on hepta‐2,4‐dienal [FL‐no: 05.084], 2,4‐octadienal [FL‐no: 05.186], tr‐2,tr‐4‐nonadienal [FL‐no: 05.194] and on the representative substances deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140] and hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] that are evaluated in the present revision of FGE.203 (FGE.203Rev2), (see Table 3).
| FGE | Adopted by EFSA | Link | No. of substances |
|---|---|---|---|
| FGE.203 | 27 November 2008 | http://www.efsa.europa.eu/en/efsajournal/pub/877 | 17 |
| FGE.203Rev1 | 26 March 2014 | https://www.efsa.europa.eu/en/efsajournal/pub/3626 | 20 |
| FGE.203Rev2 | 5 June 2018 | https://www.efsa.europa.eu/en/efsajournal/pub/5322 | 20 |
Table 3.
List of genotoxicity studies evaluated in FGE.203Rev2
| Test substance | FL‐no | In vitro | In vivo | |
|---|---|---|---|---|
| Gene mutation assay | Micronucleus test | Transgenic rodent mutation assay | ||
| Hexa‐2(trans),4(trans)‐dienal | 05.057 | Lloyd (2015) |
Whitwell (2016a) (gavage) Whitwell (2016b) (i.p.) |
McKeon and Ciubotaru (2016) |
| Hepta‐2,4‐dienal | 05.084 | Higton (2015a) | ||
| 2,4‐Octadienal | 05.186 | Higton (2015b) | ||
| tr‐2,tr‐4‐Nonadienal | 05.194 | Higton (2015c) | ||
| deca‐2(trans),4(trans)‐dienal | 05.140 |
Keig‐Shevlin (2016a) (gavage) Keig‐Shevlin (2016b) (i.p.) |
||
FL‐no: FLAVIS number; i.p.: intraperitoneal.
2.3. Presentation of the substances in flavouring group evaluation 203Rev2
FGE.203Rev2 concerns 20 substances, corresponding to subgroup 1.1.4 of FGE.19. Fifteen of these substances are α,β‐unsaturated aldehydes with two or more conjugated double‐bonds with and without additional non‐conjugated double‐bonds [FL‐no: 05.057, 05.064, 05.071, 05.081, 05.084, 05.101, 05.108, 05.125, 05.127, 05.140, 05.141, 05.173, 05.186, 05.194 and 05.196] and five are precursors for such aldehydes [FL‐no: 02.139, 02.153, 02.162, 02.188 and 09.573] (see Appendix A, Table A.1).
Table A.1.
Specification summary of the substances in the present group evaluation
| FL‐no JECFA‐no | EU register name | Structural formula | FEMA no CoE no CAS no | Phys. form Mol. formula Mol. weight | Solubilitya Solubility in ethanolb | Boiling point, °Cc Melting point, °C ID test Assay minimum | Refrac. indexd Spec. gravitye | Comments |
|---|---|---|---|---|---|---|---|---|
|
02.139 1189 |
Deca‐2,4‐dien‐1‐ol |

|
3911 11748 18409‐21‐7 |
Liquid C10H18O 154.25 |
Insoluble Soluble |
112 (13 hPa) IR NMR 95% (sum of isomers) |
1.485–1.495 0.861–0.871 |
Predominantly E,E |
|
02.153 1784 |
Hepta‐2,4‐dien‐1‐ol |

|
33467‐79‐7 |
Liquid C7H12O 112.17 |
Freely soluble |
80 (19 hPa) MS 95% (sum of isomers) |
1.487–1.493 | |
|
02.162 1174 |
Hexa‐2,4‐dien‐1‐ol |

|
3922 111‐28‐4 |
Solid C6H10O 98.16 |
Insoluble Soluble |
n.a. 24–33 IR NMR 95% (sum of isomers) |
n.a. n.a. |
|
|
02.188 1183 |
Nona‐2,4‐dien‐1‐ol |

|
3951 11802 62488‐56‐6 |
Liquid C9H16O 140.23 |
Insoluble Soluble |
85 (0.7 hPa) IR NMR 92% |
1.486–1.496 0.862–0.872 |
At least 92% (predominantly E,E); secondary component 3‐4% 2‐none‐1‐ol |
|
05.057 1175 |
Hexa‐2(trans),4(trans)‐dienal |

|
3429 640 142‐83‐6 |
Liquid C6H8O 96.13 |
Slightly soluble Soluble |
64 (20 hPa) MS 95% min |
1.538–1.543 0.896–0.902 (20°) |
Secondary components 5% hexa‐2(trans),4(cis)‐dienal, < 1% hexa‐2(cis),4(cis)‐dienal, < 1% hexa‐2(cis),4(trans)‐dienal, < 0.1% 2,4‐hexadecanoic acid |
|
05.064 1198 |
Trideca‐2(trans),4(cis),7(cis)‐trienal |
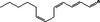
|
3638 685 13552‐96‐0 |
Liquid C13H20O 192.30 |
Insoluble Soluble |
138 (0.4 hPa) NMR 71% |
1.472–1.478 0.801–0.809 |
At least 71%; secondary components 14% 4‐(cis)‐7‐(cis)‐tridecadienol; 6% 3‐(cis)‐7‐(cis)‐tridecadienol; 5% 2‐(trans)‐7‐(cis)‐tridecadienal; 3% 2‐(trans)‐4‐(trans)‐7‐(cis)‐tridecatrienal |
|
05.071 1185 |
Nona‐2,4‐dienal |

|
3212 732 6750‐03‐4 |
Liquid C9H14O 138.21 |
Insoluble Soluble |
97 (13 hPa) IR 89% |
1.522–1.525 0.850–0.870 |
At least 89% (predominantly E,E); secondary components 5‐6% 2,4‐nonadien‐1‐ol and 1–2% 2‐nonen‐1‐ol |
| 05.081 | 2,4‐Decadienal |

|
3135 2120 2363‐88‐4 |
Liquid C10H16O 152.24 |
Insoluble Soluble |
104 MS 89% |
1.512–1.517 0.866–0.876 |
At least 89%; secondary components: mixture of the (cis, cis)‐; (cis, trans)‐ and (trans, cis)‐2,4‐decadienals (sum of all isomers 95%); acetone and isopropanol |
|
05.084 1179 |
Hepta‐2,4‐dienal |

|
3164 729 4313‐03‐5 |
Liquid C7H10O 110.16 |
Insoluble Soluble |
84 (1 hPa) IR 92% |
1.478–1.480 0.822–0.828 |
At least 92% (predominantly E,E); secondary components 2‐4% (E,Z)‐2,4‐heptadienal and 2–4% 2,4‐heptadienoic acid |
|
05.101 1173 |
Penta‐2,4‐dienal |

|
3217 11695 764‐40‐9 |
Liquid C5H6O 82.13 |
n.a. Soluble |
60 (91 hPa) NMR 95% (sum of isomers) |
1.525–1.532 0.801–0.809 |
Predominantly E,E |
|
05.108 1195 |
Undeca‐2,4‐dienal |

|
3422 10385 13162‐46‐4 |
Liquid C11H18O 166.26 |
Insoluble Soluble |
129 (17 hPa) NMR 95% |
1.500–1.505 0.896–0.906 |
Up to 95% E,E with 5–10% E,Z |
|
05.125 1196 |
Dodeca‐2,4‐dienal |

|
3670 11758 21662‐16‐8 |
C12H20O 180.28 |
85% | At least 85% (predominantly E,Z); secondary component 11‐12% 2‐(trans)‐4‐(cis) isomer | ||
|
05.127 1181 |
Octa‐2(trans),4(trans)‐dienal |
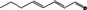
|
3721 11805 30361‐28‐5 |
Liquid C8H12O 124.18 |
Insoluble Soluble |
105–106 (10 hPa) IR NMR 95% min |
1.519–1.525 0.832–0.839 |
90–98% E,E with 0.1–8% E,Z |
|
05.140 1190 |
Deca‐2(trans),4(trans)‐dienal |

|
3135 2120 25152‐84‐5 |
Liquid C10H16O 152.24 |
Insoluble Soluble |
104 IR 90% min |
1.512–1.517 0.866–0.876 |
Secondary components 4‐5% deca‐2(trans),4(cis)‐dienal, < 1% deca‐2(cis),4(cis)‐dienal, < 0.5% deca‐2(cis),4(trans)‐dienal and < 0.1% 2,4‐decadienoic acid |
|
05.141 1786 |
Deca‐2,4,7‐trienal |

|
4089 51325‐37‐2 |
Liquid C10H14O 150.22 |
Very slightly soluble Very soluble |
233 n.a. IR NMR MS 95% (sum of isomers) |
1.538–1.544 0.898–0.905 |
81–83% (6E,4E,7Z); 5–6% (2E,4Z,7Z) and 10–11% (2E,4E,7E) |
|
05.173 1785 |
Nona‐2,4,6‐trienal |

|
4187 57018‐53‐8 |
Liquid C9H12O 136.19 |
Freely soluble |
194 MS 95% (sum of isomers) |
0.867–0.873 | |
| 05.186 | 2,4‐Octadienal |
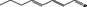
|
3721 11805 5577‐44‐6 |
Liquid C8H12O 124.18 |
Insoluble Soluble |
106 (1.1 hPa) MS 95% (sum of isomers) |
1.519–1.525 0.832–0.839 |
Up to 85% E,E With 10% E,Z |
| 05.194 | tr‐2,tr‐4‐Nonadienal |

|
3212 732 5910‐87‐2 |
Liquid C9H14O 138.21 |
Insoluble Soluble |
97 (1.3 hPa) MS 89% |
1.522–1.525 0.850–0.870 |
Name in the Union List to be changed to (2E, 4E)‐nona‐2,4‐dienal At least 89%; secondary components at least 5% 2,4‐nonadien‐1‐ol and 2‐nonen‐1‐ol and other isomers of 2,4‐nonadienal |
| 05.196 | tr‐2,tr‐4‐Undecadienal |

|
3422 10385 30361‐29‐6 |
Liquid C11H18O 166.26 |
Practically insoluble or insoluble Freely soluble |
129 (1.73 hPa) NMR 95% |
1.500–1.505 0.896‐0.906 |
Name in the Union List to be changed to (2E, 4E)‐undeca‐2,4‐dienal. 90–95% E,E with 0.1–8% E,Z |
|
09.573 1780 |
Hexa‐2,4‐dienyl acetate |

|
10675 1516‐17‐2 |
Liquid C10H20O2 140.18 |
Freely soluble |
80 (20 hPa) MS 95% |
1.470–1.476 0.908–0.914 |
Predominantly E,E |
FL‐no: FLAVIS number; JECFA‐no: The Joint FAO/WHO Expert Committee on Food Additives number; FEMA no: Flavor and Extract Manufacturers Association number; CoE no: Council of Europe number; CAS no: Chemical Abstract Service number; ID: Identity; IR: infrared spectroscopy; NMR: nuclear magnetic resonance; MS: mass spectra.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1,013.25 hPa, if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
A summary of their current evaluation status by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) is given in Appendix B, Table B.1 (JECFA, 2004). Four substances [FL‐no: 05.081, 05.186, 05.194 and 05.196] have not been previously evaluated by JECFA.
Table B.1.
Summary of safety evaluation of the JECFA substances in the present group
| FL‐no JECFA‐no | EU register name | Structural formula | EU MSDIa US MSDI (μg/capita per day) | Classb Evaluation procedure pathc | JECFA Outcome on the named compoundd or e | EFSA conclusion on the named compound |
|---|---|---|---|---|---|---|
|
02.139 1189 |
Deca‐2,4‐dien‐1‐ol |

|
ND 26 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
02.162 1174 |
Hexa‐2,4‐dien‐1‐ol |

|
ND 0.4 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
02.188 1183 |
Nona‐2,4‐dien‐1‐ol |

|
ND 26 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.057 1175 |
Hexa‐2(trans),4(trans)‐dienal |

|
0.97 0.1 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.064 1198 |
Trideca‐2(trans),4(cis),7(cis)‐trienal |
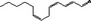
|
0.18 0.009 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.071 1185 |
Nona‐2,4‐dienal |

|
1.5 0.7 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.084 1179 |
Hepta‐2,4‐dienal |

|
3.0 23 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.101 1173 |
Penta‐2,4‐dienal |

|
0.12 0.2 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.108 1195 |
Undeca‐2,4‐dienal |

|
3.2 0.4 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.125 1196 |
Dodeca‐2,4‐dienal |

|
0.57 0.1 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.127 1181 |
Octa‐2(trans),4(trans)‐dienal |
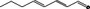
|
0.55 0.007 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.140 1190 |
Deca‐2(trans),4(trans)‐dienal |

|
22 70 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
02.153 1784 |
Hepta‐2,4‐dien‐1‐ol |

|
0.061 0.01 |
Class I B3: Intake below threshold B4: Adequate NOAEL exists |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.141 1786 |
Deca‐2,4,7‐trienal |

|
0.12 0.01 |
Class I B3: Intake below threshold B4: Adequate NOAEL exists |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.173 1785 |
Nona‐2,4,6‐trienal |

|
0.0012 ND |
Class I B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
09.573 1780 |
Hexa‐2,4‐dienyl acetate |

|
0.61 0.01 |
Class I B3: Intake below threshold B4: Adequate NOAEL exists |
d | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 05.081 | 2,4‐Decadienal |

|
27 | No evaluation | Not evaluated by the JECFA | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 05.186 | 2,4‐Octadienal |

|
0.65 | No evaluation | Not evaluated by the JECFA | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 05.194 | tr‐2,tr‐4‐Nonadienal |

|
2.9 | No evaluation | Not evaluated by the JECFA | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 05.196 | tr‐2,tr‐4‐Undecadienal |

|
3.2 | No evaluation | Not evaluated by the JECFA | Evaluated in FGE.203Rev2 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
FL‐no: FLAVIS number; JECFA‐no: The Joint FAO/WHO Expert Committee on Food Additives number; MSDI: maximised Survey‐derived Daily Intake; ND: not determined; NOAEL: no‐observed‐adverse‐effect‐level.
EU MSDI: Amount added to food as flavour in (kg/year) x 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita per day.
Thresholds of concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
No safety concern based on intake calculated by the MSDI approach of the named compound.
Data must be available on the substance or closely related substances to perform a safety evaluation.
The Panel has also taken into consideration the outcome of the predictions from five selected (Q)SAR models (Benigni and Netzeva, 2007a; Gry et al., 2007; Nikolov et al., 2007) on 13 aldehydes [FL‐no: 05.057, 05.064, 05.071, 05.081, 05.084, 05.101, 05.108, 05.125, 05.127, 05.140, 05.141, 05.173 and 05.196]. The 13 aldehydes and their (Q)SAR predictions are shown in Appendix C, Table C.1.
Table C.1.
(Q)SAR predictions on mutagenicity for 13 aldehydes from subgroup 1.1.4
| FL‐no JECFA‐no | EU register name | Structural formulaa | ISS local model Ames Test TA100b | MultiCASE Ames testc | MultiCASE mouse lymphoma testd | MultiCASE chromosomal aberration test in CHOe | MultiCASE chromosomal aberration test in CHLf |
|---|---|---|---|---|---|---|---|
|
05.101 1173 |
Penta‐2,4‐dienal |

|
POS | OD | OD | NEG | NEG |
|
05.057 1175 |
Hexa‐2(trans),4(trans)‐dienal |

|
POS | POS | OD | NEG | NEG |
|
05.084 1179 |
Hepta‐2,4‐dienal |

|
POS | EQU | OD | NEG | NEG |
|
05.127 1181 |
Octa‐2(trans),4(trans)‐dienal |

|
POS | EQU | OD | NEG | NEG |
|
05.071 1185 |
Nona‐2,4‐dienal |

|
POS | EQU | OD | NEG | NEG |
| 05.173 | Nona‐2,4,6‐trienal |

|
NEG | EQU | OD | NEG | NEG |
| 05.081 | 2,4‐Decadienal |

|
POS | NEG | OD | NEG | NEG |
|
05.140 1190 |
Deca‐2(trans),4(trans)‐dienal |

|
POS | NEG | OD | NEG | NEG |
| 05.141 | Deca‐2,4,7‐trienal |

|
NEG | EQU | OD | NEG | NEG |
|
05.108 1195 |
Undeca‐2,4‐dienal |

|
POS | EQU | OD | NEG | NEG |
| 05.196 | tr‐2,tr‐4‐Undecadienal |

|
POS | EQU | OD | NEG | NEG |
|
05.125 1196 |
Dodeca‐2,4‐dienal |

|
POS | EQU | OD | NEG | NEG |
|
05.064 1198 |
Trideca‐2(trans),4(cis),7(cis)‐trienal |
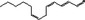
|
NEG | EQU | OD | NEG | NEG |
CHO: Chinese hamster ovary; CHL: Chinese hamster lung.
Structure group 1.1.4: α,β‐unsaturated aliphatic aldehydes with one or more conjugated double‐bonds.
Local model on aldehydes and ketones, Ames TA100. (NEG: Negative; POS: Positive; OD: out of domain).
MultiCase Ames test (OD: Out of domain; POS: Positive; NEG: Negative; EQU: Equivocal).
MultiCase Mouse lymphoma test (OD: Out of domain; POS: Positive; NEG: Negative; EQU: Equivocal).
MultiCase Chromosomal aberration in CHO (OD: Out of domain; POS: Positive; NEG: Negative; EQU: Equivocal).
MultiCase Chromosomal aberration in CHL (OD: Out of domain; POS: Positive; NEG: Negative; EQU: Equivocal).
Sections 2.4 and 2.5 of this opinion report the same information that was presented in FGE.203 and FGE.203Rev1, respectively. Section 3 reports the evaluation of the new data submitted by the Industry.
2.4. Data evaluated by the Panel in FGE.2035
2.4.1. (Q)SAR Predictions
In Table C.1, the outcomes of the (Q)SAR predictions for possible genotoxic activity in five in vitro (Q)SAR models (ISS‐Local Model‐Ames test, DTU‐NFI MultiCASE‐Ames test, chromosomal aberration test (Chinese hamster ovary (CHO) cells), chromosomal aberration test (Chinese hamster lung (CHL) cells) and mouse lymphoma test) are presented.
Out of 13 substances, 10 were predicted as positive by the ISS Local Model for the Ames test (TA100). By using the MultiCASE for the Ames test, one positive prediction (hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057]), nine equivocal predictions, two negative predictions and one out of domain were obtained. All substances were predicted as ‘out of domain’ by the MultiCASE model for the mouse lymphoma test. All substances were predicted as negative by the MultiCASE model for the chromosomal aberration test both in CHO and CHL cells.
2.4.2. Carcinogenicity studies
Groups of 50 male and 50 female F344/N rats were administered 2,4‐hexadienal (89% trans,trans‐isomer, 11% cis,trans‐isomer) in corn oil by gavage at dose levels of 0 (controls), 22.5, 45 or 90 mg/kg body weight (bw) per day, five times per week for up to 105 weeks. The survival of the dosed animals was not affected by the treatment. The mean body weights of the high‐dose males were generally lower than that of the controls. The incidences of squamous cell papillomas of the forestomach occurred with a statistically significant positive trend in male and female rats (males: 0/50; 3/50; 10/50; 29/50; females: 0/50; 1/50; 5/50; 17/50). Squamous cell carcinomas were found in one male at 45 mg/kg bw per day and in two males at the highest dose group (males papillomas and carcinomas: 0/50; 3/50; 11/50; 29/50). The incidence of epithelial hyperplasia were statistically significantly increased in rats at all dose levels (males: 3/50; 19/50; 42/50; 50/50; females: 2/50; 16/50; 37/50; 41/50) (NTP, 2003).
Groups of 50 male and 50 female B6C3F1 mice were administered 2,4‐hexadienal in corn oil by gavage at dose levels of 0 (controls), 30, 60, or 120 mg/kg bw per day, five times per week for 105 weeks. The survival and the mean body weights of the dosed animals were not affected by the treatment. The incidences of squamous cell papillomas of the forestomach occurred with a statistically significant positive trend in male and female mice (males: 2/50; 4/50; 5/50; 8/50; females: 2/50; 2/50; 11/50; 13/50). Squamous cell carcinomas were found in males and females at the highest dose group (males carcinomas: 0/50; 1/50; 0/50; 2/50; males papillomas and carcinomas: 2/50; 4/50; 5/50; 10/50; females carcinomas: 0/50; 0/49; 0/50; 7/50; females papillomas and carcinomas: 2/50; 2/49; 11/50; 18/50). Epithelial hyperplasia occurred in mice of either sex at the highest dose level (males: 14/50; 7/50; 9/50; 26/50; females: 4/50; 8/49; 12/50; 31/50). Two males from the highest dose group had squamous cell carcinoma of the tongue (NTP, 2003). Although not statistically significantly increased relative to the controls, this increase exceeded historical incidences in controls.
Additional studies were performed by NTP (2003) in order ‘to evaluate whether oral administration of 2,4‐hexadienal to F344/N rats induces the formation of the lipid peroxidation product malondialdehyde in the forestomach and/or affects the defensive antioxidant glutathione system. Forestomach samples were collected from groups of 10 male and 10 female F344/N rats administered 0, 90, or 120 mg/kg 2,4‐hexadienal in corn oil by gavage for 28 days to measure the concentrations of reduced glutathione (GSH), oxidized glutathione (GSSG), and malondialdehyde (MDA). The concentration of GSH increased significantly in males at 1 and 4 h postdosing and in females at 4 and 24 h postdosing. The concentration of GSSG increased significantly in males at all three timepoints and in females at 4 and 24 h postdosing. The concentration of GSH + GSSG increased significantly in males at 4 h postdosing and in females at 4 and 24 h postdosing. There was a significant reduction of the GSH/GSSG ratio in males at 4 h postdosing and no significant trend at other times. No statistically significant changes in the concentration of MDA were observed in the forestomach of male or female rats’.
The hypothesis that treatment with this dienal can result in an increase in the endogenous formation of acrolein and crotonaldehyde‐derived cyclic DNA adducts in the target tissues was also investigated by NTP (2003): ‘DNA adduct analysis was performed on samples of liver and forestomach tissue from male F344/N rats and forestomach tissue from B6C3F1 mice administered 0, 90 (rats only), or 120 (mice only) mg 2,4‐hexadienal/kg body weight by gavage. Vehicle control male rats were treated for 118 days; all other rats and mice were treated for 90 days.
Following 90 days of administration, there was no significant difference in the concentration of DNA adducts detected in liver samples of vehicle control and 90 mg/kg male rats. In rat forestomach samples, Acr‐dG 3 concentrations appeared to be greater in the treated group than in the vehicle control group, although the difference was not significant (p = 0.079). While neither Cro‐dG 1 nor Cro‐dG 2 were detected in forestomach tissue from vehicle control rats, Cro‐dG 2 was present in tissue from rats dosed with 90 mg/kg. These results suggest that treatment with 2,4‐hexadienal may increase cyclic adduct formation in rat forestomach DNA via a lipid peroxidation pathway. In mouse forestomach tissue, no significant change in concentration of the Acr‐dG 3 adduct was detected following 90 days of exposure to 120 mg/kg 2,4‐hexadienal. Cro‐dG adduct concentrations appeared to be greater in samples from the vehicle control group than in those from the 120 mg/kg group (p = 0.0010 for Cro‐dG 1; p = 0.0011 for Cro‐dG 2)’.
Overall, the authors of the NTP report concluded (NTP, 2003):
‘Under the conditions of these 2‐year gavage studies, there was clear evidence of carcinogenic activity of 2,4‐hexadienal in male and female F344/N rats and male and female B6C3F1 mice based on increased incidences of squamous cell neoplasms of the forestomach. The occurrence of squamous cell carcinoma of the oral cavity (tongue) in male B6C3F1 mice may have been related to the administration of 2,4‐hexadienal. Hyperplasia of the forestomach in male and female rats and mice was associated with administration of 2,4‐hexadienal’.
At its 61st meeting, JECFA has discussed the occurrence of forestomach effects in rodents:
‘The occurrence of forestomach hyperplasia and squamous cell tumours in rodents is common in bioassay studies by the National Toxicology Program in which a high concentration of an irritating material in corn oil is delivered daily by gavage into the forestomach for 2 years. High concentrations of aldehydes (e.g. malonaldehyde, furfural, benzaldehyde and trans,trans‐2,4‐hexadienal (National Toxicology Program, 1988, 1990, 1993, 2001, respectively) and other irritating substances (e.g. dihydrocoumarin, coumarin (National Toxicology Program, 1990, 1992, respectively)) delivered in corn oil by gavage are consistently associated with these phenomena in the forestomach of rodents.
Trans,trans‐2,4‐Hexadienal produced some positive results in short‐term tests for genotoxicity in vitro, but was inactive in tests in vivo. Thus, although it may be genotoxic under some conditions, this is not believed to be the basis for its effects in the rodent forestomach. There was evidence of treatment‐related injury to the forestomach epithelium and this is believed to be the primary cause of the neoplastic development. In the bioassays, development of hyperplasia in mice and rats receiving test substance by gavage in corn oil, and a low incidence of adenoma observed in mice reflect the sensitivity of the forestomach to irritation. The forestomach was the only site at which an increased incidence of neoplasia was observed in treated animals.
The relevance of the development of forestomach tumours in rodents to potential carcinogenic targets in humans has been the subject of much investigation (Grice, 1988; Wester and Kroes, 1988; Clayson et al., 1990). An International Agency for Research on Cancer Working Group (IARC, 2003) concluded that in order to evaluate the relevance of the induction of forestomach tumours in rodents to cancer in humans, the exposure conditions used in these experiments have to be considered. The exposure conditions during oral administration are unusual (particularly if dosing is effected by gavage) in that physical effects may result in high local concentrations of test substances in the forestomach and prolonged exposure of the epithelial tissue. Agents that only produce tumours in the forestomach in rodents after prolonged treatment and via mechanisms that do not involve reaction with DNA may be of less relevance to humans, since human exposure to such agents would need to surpass time‐integrated dose thresholds in order to elicit the carcinogenic response.
Therefore, the appearance of these lesions in the 2‐year bioassay in rodents given trans,trans‐2,4‐hexadienal at a high concentration by gavage has no relevance to humans, given that the results are due to the irritating effect of high bolus doses of trans,trans‐2,4‐hexadienal delivered to the contact site (the forestomach) by gavage and not the effects of systemic concentrations in the whole animal’. (JECFA, 2004).
Study validation and results are presented in Appendix D, Table D.1.
Table D.1.
Carcinogenicity studies considered by the Panel in FGE.203
| Register name [FL‐no] | Species; sex no./group | Route | Dose levels | Duration | Results | Reference | Comments |
|---|---|---|---|---|---|---|---|
| Hexa‐2(trans),4(trans)‐dienal [05.057] | Rats; Male, Female 50/sex per group | Gavage in corn oil | 0 (controls), 22.5, 45 or 90 mg/kg bw per day, five times per week | 105 weeks |
Males: Positive trend in increased squamous cell papillomas of the forestomach. One squamous cell carcinoma of the forestomach was seen in the mid‐dose group and two in the high‐dose group Females: Positive trend in increased squamous cell papillomas of the forestomach. No carcinomas were seen |
NTP (2003) |
Valid study Males: The carcinomas of the forestomach were preceded by epithelial hyperplasia and papillomas Females: Squamous cell papillomas and epithelial hyperplasia were increased at the two highest doses |
| Mice; Male, Female 50/sex per group | Gavage in corn oil | 0 (controls), 30, 60, or 120 mg/kg bw per day, five times per week | 105 weeks | Males and females: Increased incidences of squamous cell papillomas and carcinomas of the forestomach in the high‐dose groups | NTP (2003) |
Valid study The carcinomas of the forestomach were preceded by epithelial hyperplasia and squamous cell papillomas |
2.4.3. Genotoxicity studies
In subgroup 1.1.4, there are five in vitro studies and two in vivo studies on hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] and two in vitro studies on nona‐2,4‐dienal [FL‐no: 05.071] available.
Hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] was found positive in three valid studies with Salmonella Typhimurium TA100 strain (Eder et al., 1992; NTP, 2003) and TA104 strain (Marnett et al., 1985). Two valid in vivo bone marrow micronucleus assays in mice and rats which have been considered as inconclusive by NTP (2003) were considered weakly positive by the Panel. Negative results were reported in a 14‐week mouse peripheral blood micronucleus assay (NTP, 2003), considered of limited relevance due to limitations in the experimental protocol. Of limited relevance, due to several shortcomings of the studies, are considered the positive results of a SOS chromotest in Escherichia coli PQ37, the induction of DNA‐strand breaks in mouse leukaemia cells and the in vitro (nucleosides) induction of DNA adducts (Eder et al., 1993).
Nona‐2,4‐dienal [FL‐no: 05.071] was found negative in a valid study with S. Typhimurium TA104 strain (Marnett et al., 1985). The negative results of a SOS chromotest in E. coli PQ37, as well as the positive results in a test for DNA‐strand breaks in mouse leukaemia cells (Eder et al., 1993) were considered of limited relevance due to several shortcomings of these studies.
Study validation and results are presented in Appendix D, Tables D.2 and D.3.
Table D.2.
Genotoxicity data (in vitro) considered by the Panel in FGE.203
| Register name [FL‐no] | Test system | Test object | Concentration | Reported result | Reference | Commentsf |
|---|---|---|---|---|---|---|
| Hexa‐2(trans),4(trans)‐dienal [05.057] | Reverse mutation | Salmonella Typhimurium TA98, TA100, TA1535, and TA1537 | 3 mmol/plate (288 μg/plate) | Negativea , b | Florin et al. (1980) | Insufficient validity (spot test, not according to OECD guideline, methods and results insufficiently reported) |
| S. Typhimurium TA104 | < 1 μmol/plate (96 μg/plate) | Positive | Marnett et al. (1985) | Valid. Published non‐GLP study carried out only in the absence of S9; for the purpose of the study the result is considered valid | ||
| S. Typhimurium TA102 | Not reported | Negativec | Marnett et al. (1985) | Limited validity. The result is reported without details | ||
| S. Typhimurium TA100 | 0.01–0.75 μL/plate (8.95–671.3 μg/plate) | Positivec | Eder et al. (1992) | Valid | ||
| S. Typhimurium TA1535, TA98 | 0–1,500 μg/plate | Negatived | NTP (2003) | Valid. With metabolic activation in two testing centres | ||
| S. Typhimurium TA98 | 0–150 μg/plate | Negativec | NTP (2003) | Valid. Without metabolic activation in two testing centres | ||
| S. Typhimurium TA1535 | 0–166 μg/plate | Negativec | NTP (2003) | Valid. Without metabolic activation in two testing centres | ||
| S. Typhimurium TA100 | 0–333 μg/plate | Positivec | NTP (2003) | Valid. Without metabolic activation, Positive in 1 of 2 testing centres | ||
| S. Typhimurium TA100 | 0–1,500 μg/plate | Positived | NTP (2003) | Valid. With metabolic activation in 2 testing centres | ||
| SOS chromotest | Escherichia coli PQ37 and PQ243 | < 590 nmol | Negative | Eder et al. (1992) | Limited validity (only without S9‐mix) | |
| E. coli PQ37 | Not reported | Positive | Eder et al. (1993) | Limited validity (results poorly reported, concentrations and bacteriotoxicity not reported) | ||
| DNA strand breaks | L1210 mouse leukaemia cells | 20 μmol/mL (1,923 μg/mL) 300 and 500 μmol/mL (28,839 and 48,065 μg/mL) |
Negative Positive |
Eder et al. (1993) | Limited validity (results poorly reported) | |
| DNA adducts | Nucleosides | 100 mmol/L | Positive | Eder et al. (1993) | Validity cannot be evaluated (result poorly reported) | |
| Nona‐2,4‐dienal [05.071] | Reverse mutation | S. Typhimurium TA104 | < 0.4 μmol/plate (< 55 μg/plate) | Negativec | Marnett et al. (1985) | Valid. Published non‐GLP study, considered valid |
| S. Typhimurium TA102 | Not reported | Negativec | Marnett et al. (1985) | Limited validity | ||
| SOS chromotest | E. coli PQ37 | Not reported | Negative | Eder et al. (1993) | Limited validity | |
| DNA strand breaks | L1210 mouse leukaemia cells | 400 μmol/mL (55,284 μg/mL) | Negativee | Eder et al. (1993) | Limited validity | |
| 500 μmol/mL (69,105 μg/mL) | Positive |
OECD: Organisation for Economic Co‐operation and Development; GLP: Good Laboratory Practice.
Spot test method.
With and without metabolic activation.
Without metabolic activation.
With metabolic activation.
Results demonstrated in the presence of cytotoxicity.
Validity of genotoxicity studies:
Valid.
Limited validity (e.g. if certain aspects are not in accordance with OECD guidelines or current standards and/or limited documentation).
Insufficient validity (e.g. if main aspects are not in accordance with any recognised guidelines (e.g. OECD) or current standards and/or inappropriate test system).
Validity cannot be evaluated (e.g. insufficient documentation, short abstract only, too little experimental details provided).
Table D.3.
Genotoxicity data (in vivo) considered by the Panel in FGE.203
| Register name [FL‐no] | Test system | Test object | Route | Dose | Result | Reference | Commentsa |
|---|---|---|---|---|---|---|---|
| Hexa‐2(trans),4(trans)‐dienal [05.057] | Micronucleus formation | B6C3F1 mice bone marrow | Administered three times by intraperitoneal injection at 24‐h intervals | 40, 80, 120 or 160 mg/kg | Inconclusive | NTP (2003) |
Valid Administered three times at 24‐h intervals. Bone marrow studied at 24 h after the last dosing. A very weak positive response was observed at the highest dose level in conjunction with a slight decrease in PCE/NCE ratio. Technically the study is not flawed. The test was not repeated. Despite the presence of a significant positive trend, NTP decided that the study was inconclusive |
| B6C3F1 mice peripheral blood | Administered by gavage for 14 weeks | 7.5, 15, 30, 60 or 120 mg/kg | Negative |
Limited validity Administered by gavage for 14 weeks. No increase in MN‐NCE was observed. PCE/NCE ratios were not affected either. The study is of limited validity, due to shortcomings in the experimental protocol (no‐standard assay) |
|||
| Male F344/N rats bone marrow | Administered as a single i.p. injection | 50, 100, 150 or 200 mg/kg | Inconclusive |
Valid Administered once. Bone marrow studied at 24 h post‐dosing. A very weak non‐significant positive response was observed at the highest dose level but no decrease in PCE/NCE ratio. Technically the study is not flawed. The test was not repeated. Despite the presence of a significant positive trend, NTP decided that the study was inconclusive |
i.p: intraperitoneal; PCE: polychromatic Erythrocytes; NCE: normochromatic erythrocytes; MN: micronuclei.
Validity of genotoxicity studies:
Valid.
Limited validity (e.g. if certain aspects are not in accordance with OECD guidelines or current standards and/or limited documentation).
Insufficient validity (e.g. if main aspects are not in accordance with any recognised guidelines (e.g. OECD) or current standards and/or inappropriate test system).
Validity cannot be evaluated (e.g. insufficient documentation, short abstract only, too little experimental details provided).
2.4.4. Conclusion on genotoxicity and carcinogenicity
The Panel concluded that 2,4‐hexadienal [FL‐no: 05.057] increased the incidence of neoplasms in the forestomach of male and female rats and mice. In addition, squamous cell carcinoma of the tongue was observed in two mice of the high‐dose group. Based on the data available, a non‐threshold genotoxic mechanism cannot be excluded. This conclusion also applies to the other substances in this FGE likewise.
2.4.5. Conclusions for FGE.203
Based on the available data on carcinogenicity and genotoxicity, there is a safety concern for hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] since a non‐threshold mechanism cannot be excluded. Therefore, the substances of this FGE cannot be evaluated through the Procedure. The Panel requests data which clarify whether the carcinogenic effects were based on a threshold mechanism.
2.5. Additional genotoxicity data evaluated by the Panel in FGE.203Rev16
In response to the EFSA request in FGE.203 for additional genotoxicity data for subgroup 1.1.4, the Flavour Industry (EFFA, 2013; IOFI, 2013) has submitted genotoxicity data on deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140] (Table 2).
Table 2.
Overview of New Data Submitted for Subgroup 1.1.4
| Test substance | Additional data submitted | Reference |
|---|---|---|
| Deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140] |
Ames test. S. Typhimurium TA97, TA98, TA100, TA102, TA104 and TA1535 Dosed from 0.1 to 1,000 μg/plate ± S9‐mix |
NTP (2011) |
| Micronucleus induction. Male rat bone marrow polychromatic erythrocytes. Dosed from 100 to 600 mg/kg bw | ||
| Micronucleus induction. Male and female mice bone marrow and peripheral blood polychromatic erythrocytes. Dosed from 25 to 600 mg/kg bw | ||
| Hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] | Data review. Cytotoxicity, genotoxicity, carcinogenicity | IARC (2012) |
bw: body weight.
2.5.1. In vitro data
Bacterial reverse mutation assay
2,4‐Decadienal was tested independently in two laboratories in S. Typhimurium TA97, TA98, TA100, TA102, TA104 and TA1535 in the absence and presence of rat or hamster S9‐mix, using the pre‐incubation method. Concentrations from 0.3 to 666 μg/plate in strains TA97 and TA1535 and from 0.3 to 1,000 μg/plate in TA 98 and TA 100 were tested in the first study and from 0.1 to 100 μg/plate in strains TA97, TA98, TA100, TA102, TA104, TA1535 were evaluated in the second study. The test (NTP, 2011) was performed according the OECD Guideline 471 (OECD, 1997a), following the Good Laboratory Practice (GLP) principles. In the absence of S9‐mix, evidence of toxicity above 10 μg/plate and cell killing at 33 μg/plate or above was observed in all tester strains. In the presence of S9‐mix, signs of toxicity were observed starting from 1,000 in strain TA98 (with 30% hamster S9‐mix) and from 333 or 666 μg/plate in the other tester strains. The vehicle and positive control substances produced appropriate responses. No evidence of mutagenicity was observed in any of the tester strains.
Study validation and results are presented in Appendix E, Table E.1.
Table E.1.
Additional genotoxicity data (in vitro) considered by the Panel in FGE.203Rev1
| Register name [FL‐no] | Test system | Test object | Concentration | Result | Reference | Comments |
|---|---|---|---|---|---|---|
| Deca‐2(trans),4(trans)‐dienal [05.140] | Reverse mutation | Salmonella Typhimurium TA1535, TA97 | 0.3, 1.0, 3.0, 10.0, 16.0, 33.0, 100.0, 166.0, 333.0 and 666.0 μg/platea , b | Negative | NTP (2011) |
Valid. The test was performed in two testing centres. Study design complies with OECD Guideline 471 and GLP principles. The highest concentration tested is limited by the toxicity |
| S. Typhimurium TA98, TA100 | 0.3, 1.0, 3.0, 10.0, 16.0, 33.0, 100.0, 333.0 and 1,000.0 μg/platea , b | Negative | ||||
| S. Typhimurium TA100, TA102, TA104, TA1535, TA97, TA98 | 0.1, 0.3, 1.0, 3.0, 10.0, 33.0 and 100.0 μg/platea , b | Negative |
OECD: Organisation for Economic Co‐operation and Development; GLP: Good Laboratory Practice.
With and without S‐9 metabolic activation.
Pre‐incubation method.
2.5.2. In vivo data
Micronucleus assay
2,4‐Decadienal was evaluated in a micronucleus assay in bone marrow polychromatic erythrocytes (PCE) for its ability to induce chromosomal damage in rats. 2,4‐Decadienal dissolved in corn oil as a carrier was administered by a single intraperitoneal (i.p.) injection to F344/N rats (5 males/dose) at doses of 100, 200, 400 and 600 mg/kg bw. Cyclophosphamide (CPA, 25 mg/kg bw) was given as the positive control. Rats from all dose groups were sampled 24 h after dosing. At least 1,000 PCE were scored for each animal for micronuclei (MN). No cytotoxic effects were observed at any dose, as determined by a reduction in the number of PCE vs vehicle controls. Statistically significant increase in MN frequency was observed in the groups dosed with 100–400 mg 2,4‐decadienal/kg bw (up to 6‐fold compared to control) but not for the highest dose 600 mg/kg bw, which produced marked clinical toxicity (NTP, 2011). The p‐value for the trend test was not significant for this study due to the downturn in micronuclei induction at the highest dose.
In a parallel study, 2,4‐decadienal dissolved in corn oil as a carrier was administered to mice (5 males/dose) by three i.p. injections at 24 h intervals, at doses of 25, 50, 100 and 200 mg/kg bw. CPA (25 mg/kg bw) was given as the positive control. Mice from all the groups were sampled 24 h after the final dosing. Only 1,000 PCE were scored for each animal for MN instead of 2,000 as recommended in OECD Guidelines 474 (OECD, 1997b). A trend of increase in micronuclei frequency is evident in the range of doses 25–200 mg/kg bw, but no statistically significant difference with respect to the control was observed at any dose level of 2,4‐decadienal. It should be noted that the mean micronuclei frequency in the control group (1.2 per 1,000 cells) is twofold compared with the value at the lowest dose tested (NTP, 2011).
In a second experiment of the above study, mice (5 males/dose) were administered a single i.p. injection of 400 or 600 mg/kg bw of 2,4‐decadienal dissolved in corn oil. Bone marrow and peripheral blood were sampled 48 h post‐dosing. A statistically significant increase in micronucleated PCE was observed for the 600 mg/kg bw group (3.5‐fold compared to control). Analysis of peripheral blood PCE in these same mice did not show a statistically significant increase in the frequency of micronucleated cells.
The evaluation of the peripheral blood sampled from male and female mice at the end of a 90‐day gavage toxicity study at doses of 0, 50, 100, 200, 400 or 800 mg/kg, 5 days per week for 14 weeks, by the same laboratory, showed no increase in the frequency of micronucleated reticulocytes in treated groups compared with controls. No relevant treatment‐related haematological effects were described with the exception of a minimal treatment‐related, but not dose‐related, decreases in haematocrit values, haemoglobin concentrations and erythrocyte counts occurred in the higher dosed male and/or female mice. No data on clinical signs, bone marrow toxicity and blood analysis are available to demonstrate the systemic exposure (NTP, 2011).
Overall, the Panel noted that a statistically significant increase of micronucleated PCE was observed in both rats and mice up to 6‐fold and 3.5‐fold compared to control, respectively. Therefore, the Panel considered that 2,4‐decadienal cannot be considered non‐genotoxic in vivo after i.p. injection.
Study validation and results are presented in Appendix E, Table E.2.
Table E.2.
Additional genotoxicity data (in vivo) considered by the Panel in FGE.203Rev1
| Register name [FL‐no] | Test system in vivo | Test object | Route | Dose | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|
| Deca‐2(trans),4(trans)‐dienal [05.140] | Micronucleus induction | Male rat bone marrow polychromatic erythrocytes | i.p. | 100, 200, 400 and 600 mg/kg bw | Positivea | NTP (2011) | Study design complies with OECD Guideline 474 |
| Male mouse bone marrow polychromatic erythrocytes | i.p. | 25, 50, 100 and 200 mg/kg bw | Equivocalb | A trend of increase but not statistically significant. Study design complies with OECD Guideline 474 | |||
| Male mouse bone marrow polychromatic erythrocytes | i.p. | 400 and 600 mg/kg bw | Positivea | Significant increase only at the highest dose. Study design complies with OECD Guideline 474 | |||
| Male mouse peripheral blood polychromatic erythrocytes | i.p. | 400 and 600 mg/kg bw | Negativea | No statistically significant increase of micronucleated cells was observed. Study design complies with OECD Guideline 474 | |||
| Mouse peripheral blood reticulocytes | gavage | 50, 100, 200, 400 and 800 mg/kg bw per day | Negativec | No statistically significant increase of micronucleated cells was observed. Study design complies with OECD Guideline 474 |
bw: body weight; OECD: Organisation for Economic Co‐operation and Development.
Administered as a single intraperitoneal injection.
Administered 3x by intraperitoneal injection at 24‐h intervals.
Administered by gavage for a period of 14 weeks.
2.5.3. Literature data on hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] and 2,4‐decadienal [FL‐no: 05.140]
For hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057], no new experimental data have been submitted by Industry, but additional data from literature including a IARC monograph (IARC, 2012).
Hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] tested in V79 and in Caco‐2 cells through a comet assay, induced a concentration‐dependent induction of DNA damage, in association with a depletion of GSH levels (Glaab et al., 2001). The production of oxidative DNA damage (FPG‐sensitive sites detected by comet assay) by 2,4‐hexadienal was demonstrated to be the consequence of the GSH depletion in V79 cells (Janzowski et al., 2003). 2,4‐Hexadienal produced 1,N2‐cyclic‐deoxyguanosine and 7,8‐cyclic‐guanosine adducts in a cell‐free system (Eder et al., 1993). Crotonaldehyde‐deoxyguanosine‐2 adduct levels determined by a 32P‐post‐labelling technique were increased in forestomach but not in liver of rats exposed to 2,4‐hexadienal at a dose of 90 mg/kg bw by gavage for 90 days (NTP, 2003). These results suggest that treatment with 2,4‐hexadienal may increase cyclic adduct formation in rat forestomach DNA via a lipid peroxidation pathway (NTP, 2003). Reactive oxygen species (ROS) can cause DNA damage in forestomach in the form of 8‐hydroxydeoxy‐guanosine. According to IARC (2012), the increase in chronic inflammation of the forestomach and the presence of forestomach ulcers observed in the high‐dose group of male rodents in the 2‐year study (NTP, 2003) does not support the hypothesis that the dose‐related increases in forestomach neoplasms in male and female rodents is due only to 2,4‐hexadienal cytotoxicity. IARC classified 2,4‐hexadienal as possible carcinogen to humans and concluded that ‘mechanistic data provide additional support for the relevance of the animal carcinogenicity data to humans’ and that ‘there is a moderate evidence that tumour induction occurs via a genotoxic mechanism’.
A number of papers are also available in the scientific literature related to the mechanism of action of the genotoxic damage induced by 2,4‐decadienal.
The reaction of 2,4‐decadienal with 2‐deoxyguanosine results in the production of a number of base derivatives. Six different stable DNA adducts (hydroxyl‐etheno‐dGua derivatives) were isolated by reverse‐phase high‐performance liquid chromatography (HPLC) and fully characterised with spectroscopic measurements, following in vitro treatment of calf thymus DNA with 2,4‐decadienal (Loureiro et al., 2000, 2004).
A number of studies report the induction of DNA damage in human cells in culture.
Treatment of human erythroleukemia cell line (HEL cells) with 2,4‐decadienal leads to a marked variation of the cellular GSH level and induces DNA fragmentation, as revealed by the presence of low molecular weight DNA fragments upon electrophoresis (Nappez et al., 1996).
It has been shown that 2,4‐decadienal induces intracellular ROS (determined by dichlorofluorescein assay) and causes significant oxidative damage of the 8‐hydroxy‐2′‐deoxyguanosine in lung adenocarcinoma cell line A549 at concentrations from 50 to 200 μM (Wu and Yen, 2004).
Significant induction of DNA strand breaks, detected by comet assay, was observed in vitro in human bronchiolar epithelial cells (BEAS‐2B) after 4 h of exposure to 1 μM of 2,4‐decadienal. The extent of DNA fragmentation was significantly reduced by the co‐treatment with antioxidants, such as N‐acetylcysteine (NAC), superoxide dismutase (SOD) and catalase, indicating that an oxidative stress is involved in the process of DNA breakage.
A significant enhancement of the DNA damage induced by the treatment with 2,4‐decadienal was observed through an in vitro challenge with Endo III/Fpg (a group of repair enzymes that specifically recognise and repair oxidised purines and pyrimidines) after 1 h of treatment, and with nucleotide excision repair (NER) enzymes after 4 h of treatment (Young et al., 2010). These results reveal that 2,4‐decadienal induces two different types of DNA damage: oxidised DNA bases and formation of bulky adducts. The results indicate that, in addition to early oxidative DNA damage, non‐oxidative DNA damage, such as bulky adduct formation, was also induced by 2,4‐decadienal (Young et al., 2010).
2.5.4. Discussion of available data
In FGE.203, the Panel noted that 2,4‐hexadienal [FL‐no: 05.057] increased the incidence of neoplasms in the forestomach of male and female rats and mice in a 2‐year carcinogenicity study. In addition, squamous cell carcinoma of the tongue has been observed in two mice of the high‐dose group (NTP, 2003). The Panel noted that tongue cancer is generally rare in laboratory animals and that it could be relevant for humans.
On the basis of the evidence from the additional papers reporting the induction of DNA adducts in different systems in vitro and in vivo and of the IARC classification of 2,4‐hexadienal as ‘possible carcinogen to humans’ and considering the conclusion drawn by IARC that ‘mechanistic data provide additional support for the relevance of the animal carcinogenicity data to humans’ and that ‘there is a moderate evidence that tumour induction occurs via a genotoxic mechanism’ the Panel confirms the safety concern for 2,4‐hexadienal.
2,4‐Decadienal was tested for genotoxicity in a NTP study (NTP, 2011). No increase in revertants was observed in any of the several strains of S. Typhimurium tested with and without liver S9 activation enzymes. According to the authors of the NTP report, the in vivo micronucleus tests in rats and mice produced mixed results. The conclusion of the NTP study report is that 2,4‐decadienal was not mutagenic in vitro or in vivo. The Panel, however, noted that statistically significant increases in the frequency of micronuclei in PCE were observed with 2,4‐decadienal up to 6‐fold in rats without a dose–response relationship and in mice at a single dose level (3.5‐fold compared to controls), after i.p. injection in the NTP study. The Panel also noted that the negative result of the micronucleus assay performed in the 90‐day study by gavage, without any evidence of a systemic exposure, cannot overrule the effects observed in rats and mice after an acute exposure. Based on these considerations, the Panel did not agree with the authors of the NTP report and concluded that 2,4‐decadienal cannot be considered non‐genotoxic in vivo in rats and mice after i.p. injection.
On the basis of the overall evaluation of the genotoxicity data of 2,4‐decadienal showing some indication for genotoxicity in vivo and considering the evidence from in vitro studies for the induction of different types of DNA damage (oxidised DNA bases and bulky adducts), a non‐threshold mechanism of genotoxicity cannot be excluded for 2,4‐decadienal.
2.5.5. Conclusion for FGE.203Rev1
The Panel considered that a non‐threshold mechanism of action cannot be excluded for both representative substances based on the data available. The Panel concluded that the safety concern cannot be ruled out for the representative substances hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] and for 2,4‐decadienal [FL‐no: 05.140]. Therefore, the substances of this FGE cannot be evaluated through the Procedure.
3. Assessment
3.1. Additional data evaluated by the Panel in FGE.203Rev2
The applicant has submitted in vitro genotoxicity studies for hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057], hepta‐2,4‐dienal [FL‐no: 05.084], 2,4‐octadienal [FL‐no: 05.186], tr‐2,tr‐4‐nonadienal [FL‐no: 05.194], and in vivo genotoxicity data for hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] and deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140], that are listed in Table 3. These studies are evaluated in the present revision of FGE.203 (FGE.203Rev2). A summary of results is reported in Appendix F, Tables F.1 and F.2. All these studies were performed in accordance with the respective OECD test guidelines and in compliance with GLP.
Table F.1.
Summary of Additional Genotoxicity Data submitted for FGE.203Rev2 in vitro
| FL‐no | Chemical name | Test system in vitro | Test object | Concentrations of substance | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|
| 05.057 | Hexa‐2(trans),4(trans)‐dienal | Gene mutation assay in mammalian cells | Mouse lymphoma L5178Y cells |
2, 4, 6, 8, 10, 12, 14 μg/mLb 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 3.5 μg/mLc 2, 4, 6, 8, 10, 12, 13, 14, 15 μg/mLb 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4 μg/mLc 0.125, 0.25, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 1.2d μg/mL |
Positive Negative Negative Negative Negative |
Lloyd (2015) | Reliable with restrictions (the range of historical controls was not provided). Study performed in accordance with OECD TG 476. Positive results were observed at toxic concentrations |
| 05.084 | Hepta‐2,4‐dienal | Bacterial reverse mutation test |
Salmonella Typhimurium TA98, TA100, TA102, TA1535 and TA1537 S. Typhimurium TA98, TA100, TA1535 and TA1537 S. Typhimurium TA102 S. Typhimurium TA98, TA100, TA1535 and TA1537 |
5, 16, 50, 160, 500, 1,600, 5,000 μg/platea 80, 160, 300, 625, 1,250, 2,500, 5,000 μg/platea 19.53, 39.06, 78.13, 156.3, 312.5, 625, 1,250, 2,500 μg/platef 9.766, 19.53, 39.06, 78.13, 156.3, 312.5, 625, 1,250 μg/platee |
Negative Negative Negative Negative |
Higton (2015a) | Reliable without restrictions. Study performed in accordance with OECD TG 471 |
| 05.186 | 2,4‐Octadienal | Bacterial reverse mutation test | S. Typhimurium TA98, TA100, TA102, TA1535 and TA1537 |
5, 16, 50, 160, 500, 1,600, 5,000 μg/platea 8.192, 20.48, 51.2, 128, 320, 800 and 2,000 μg/platea |
Negative | Higton (2015b) | Reliable without restrictions. Study performed in accordance with OECD TG 471 |
| 05.194 | tr‐2,tr‐4‐Nonadienal | Bacterial reverse mutation test | S. Typhimurium TA98, TA100, TA102, TA1535 and TA1537 |
5, 16, 50, 160, 500, 1,600, 5,000 μg/platea 8.192, 20.48, 51.2, 128, 320, 800, 2,000 μg/platea |
Negative | Higton (2015c) | Reliable without restrictions. Study performed in accordance with OECD TG 471 |
OECD: Organisation for Economic Co‐operation and Development; TG: Test Guideline.
With and without S9 metabolic activation.
3 h treatment with metabolic activation.
3 h treatment without metabolic activation.
24 h treatment without metabolic activation.
With S9 metabolic activation.
Without S9 metabolic activation.
Table F.2.
Summary of Additional Genotoxicity Data submitted for FGE.203Rev2 in vivo
| FL‐no | Chemical name | Test system in vivo | Test object route | Dose mg/kg bw per day | Result | Reference | Comments |
|---|---|---|---|---|---|---|---|
| 05.057 | Hexa‐2(trans),4(trans)‐dienal | Micronucleus assay in bone marrow and peripheral blood | Han Wistar rats (males) Oral gavage | 0 (corn oil), 88, 175 and 350 | Negative | Whitwell (2016a) | Reliable without restrictions. Study performed in accordance with OECD TG 474 |
| Micronucleus assay in bone marrow and peripheral blood | Han Wistar rats (males) Intraperitoneal | 0 (corn oil), 19, 38 and 75 | Negative | Whitwell (2016b) | Reliable without restrictions. Study performed in accordance with OECD TG 474 | ||
| Transgenic rodent gene mutation assay (cII gene), liver and forestomach |
Big Blue® B6C3F1 male mice Oral gavage |
0 (corn oil), 10, 30, 90 and 120 | Negative | McKeon and Ciubotaru (2016) | Reliable without restrictions. Study performed in accordance with OECD TG 488 | ||
| 05.140 | Deca‐2(trans),4(trans)‐dienal | Micronucleus assay in bone marrow and peripheral blood | Han Wistar rats (males) Oral gavage | 0 (corn oil), 350, 700 and 1,400 | Negative | Keig‐Shevlin (2016a) | Reliable without restrictions. Study performed in accordance with OECD TG 474 |
| Micronucleus assay in bone marrow and peripheral blood | Han Wistar rats (males) Intraperitoneal | 0 (corn oil), 25, 50 and 100 | Negative | Keig‐Shevlin (2016b) | Reliable without restrictions. Study performed in accordance with OECD TG 474 |
bw: body weight; OECD: Organisation for Economic Co‐operation and Development; TG: Test Guideline.
The applicant provided information on specifications that are considered in the present opinion. During the evaluation process, the Panel requested data on stability and decomposition products of the representative substances hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] and deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140]. In reply to the Panel's request, the applicant provided information that is evaluated in the present opinion.
Slug mucosa irritation assay studies were submitted for 2,4‐hexadienal, 2,4‐heptadienal, 2,4‐decadienal (Adriaens, 2014a), 2,4‐octadienal, 2,4‐nonadienal (Adriaens, 2014b) and deca‐2(trans),4(trans)‐dienal (Adriaens, 2013). This assay was developed to predict the mucosal irritation potency of pharmaceutical formulations and ingredients. Since these studies are not relevant for genotoxicity, they are not described in this opinion.
3.2. Specifications
Specifications, including purity criteria of the flavouring substances [FL‐no: 02.139, 02.153, 02.162, 02.188, 05.057, 05.064, 05.071, 05.081, 05.084, 05.101, 05.108, 05.125, 05.127, 05.140, 05.141, 05.173, 05.186, 05.194, 05.196 and 09.573], are summarised in Appendix A, Table A.1.
3.2.1. Stability and decomposition products
The Panel noted that in the recently provided in vivo genotoxicity studies for hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] and deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140], both substances were stored under nitrogen; this however, does not correspond to the conditions of storage of the flavouring substances expected under normal conditions of use (i.e. storage for 12 months at temperatures < 18°C and out of direct light and air) (EFFA, 2018).
To decide whether the substances subjected to genotoxicity testing can be considered representative of the materials of commerce, the Panel requested information on the stability of hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] and deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140] under their intended conditions of use. The applicant provided data from capillary gas chromatographic analyses of freshly prepared flavouring substances and of flavouring substances stored close to the end of their shelf‐life. For both substances, the only changes observed after 12 months of storage under normal conditions in air, were related to cis/trans‐isomerisation of the substances (i.e. 5–8% of the [FL‐no: 05.057] was isomerised to its 2(trans),4(cis) isomer and ~ 5% of [FL‐no: 05.140] was isomerised to its 2(trans),4(cis) isomer) and to oxidation of the aldehydes to their corresponding acids (< 0.5%) (EFFA, 2018).
The Panel concluded that the materials tested in the genotoxicity studies are representative of the material of commerce.
3.3. In vitro gene mutation assays
3.3.1. Gene mutation assay in mammalian cells
Hexa‐2(trans),4(trans)‐dienal (stored at 2–8°C, under inert gas and protected from light; purity 82.4% as trans,trans‐isomer, 97.5% as sum of two isomers) was tested in an in vitro gene mutation assay at the hypoxanthine‐guanine phosphoribosyl transferase (hprt) locus in mouse lymphoma L5178Y cell line (Lloyd, 2015). Cells were treated for 24‐h in the absence of metabolic activation (S9‐mix from rats induced with Aroclor 1254) or for 3‐h in the presence or in the absence of S9‐mix. The test is GLP and OECD test guideline 476 (OECD, 1997c) compliant; data are summarised in Appendix F, Table F.1. Dimethyl sulfoxide (DMSO) was used as a solvent and negative control. The positive controls were 4‐nitroquinoline‐1‐oxide (NQO) and benzo[a]pyrene (B[a]P).
Based on a range‐finding cytoxicity test, the following concentrations range were chosen for the first experiment: 0.25–7.5 μg/mL and 2–25 μg/mL for the 3‐h treatment in the absence and in the presence of S9‐mix, respectively. Seven days after treatment the highest concentrations were too toxic; therefore, the highest concentrations considered for viability and 6‐thioguanine (6TG) resistance analysis were 3.5 μg/mL in the absence of S9‐mix and 14 μg/mL in the presence of S9‐mix, resulting in a percent relative survival (RS) of 12% and 6% RS, respectively. No increase in mutant frequency was observed for the 3‐h treatment without metabolic activation. For the 3‐h treatment in the presence of S9‐mix, a statistically significant increase of mutant frequency of 7.14 (%RS 33), 6.02 (%RS 21) and 5.94 (%RS 6) was observed at the concentrations of 10, 12 and 14 μg/mL, respectively. The mutant frequency in the vehicle control was 2.14 (%RS 100).
In the second experiment, for the 3‐h treatment, concentrations ranging from 0.25 to 5 μg/mL in the absence of S9‐mix and from 2 to 20 μg/mL in the presence of S9‐mix, were tested. Seven days after treatment, the highest concentrations were too toxic; therefore, the highest concentrations considered for viability and 6TG resistance analysis were 4 μg/mL in the absence of S9‐mix and 15 μg/mL in the presence of S9‐mix, which gave 14% and 13% RS, respectively. No increase of mutation frequency was observed at 3‐h treatment in the presence or absence of S9‐mix.
In the second experiment, for the 24‐h treatment without metabolic activation, concentrations ranging from 0.125 to 1.5 μg/mL were tested. Seven days after treatment, the highest concentration of 1.5 μg/mL was too toxic; therefore, the highest concentration analysed for cell viability and 6TG resistance was 1.2 μg/mL, which gave 5% RS. No increase of mutation frequency was observed.
Since one of the assay acceptance criteria indicates that the mutation frequency of the vehicle control should be within three times the historical mean value (3.76 for the 3‐h treatment in the presence of S9‐mix, in this laboratory), and considering that the increase of mutation frequency at 3‐h in the presence of S9‐mix, was not confirmed in the second experiment, the author of the study considered the increase of mutation frequency observed in the first experiment as not biologically relevant. The linear trend test was statistically significant in both experiments for all the treatment conditions.
The Panel noted that a weak, but statistical significant, increase in mutation frequency was observed in experiment 1 at the three highest concentrations tested for 3‐h treatment in the presence of metabolic activation and a significant test for trend (p < 0.05) was reported. The comparison of the results with the distribution of the historical negative control was not feasible, as only the historical mean values was reported. These results were not confirmed in the second experiment carried out at the same range of concentrations following the same experimental conditions. No increase of mutation frequency was reported at any other condition tested. The Panel concluded that hexa‐2(trans),4(trans)‐dienal is not mutagenic in mammalian cells.
3.3.2. Bacterial reverse mutation assay
Hepta‐2,4‐dienal [FL‐no: 05.084]
Hepta‐2,4‐dienal [FL‐no: 05.084] (stored at 15–25°C, protected from light; purity 95%) was tested in the Ames assay (Higton, 2015a) to assess its potential for induction of mutagenicity in five histidine‐requiring S. Typhimurium strains: TA98, TA100, TA1535, TA1537 and TA102. The assay was performed in the absence and presence of metabolic activation (S9‐mix from Aroclor 1254‐induced rat livers), in two separate experiments, in triplicate (quintuplicate for negative control and triplicate for positive controls). DMSO was used as a solvent and negative control. In the first experiment, hepta‐2,4‐dienal was tested at concentrations of 5, 16, 50, 160, 500, 1,600 and 5,000 μg/plate using the plate incorporation method (in the absence and presence of S9‐mix). Evidence of toxicity characterised by a slight reduction of background bacterial lawn was observed at 1,600 μg/plate and above in all strains in the absence and presence of S9‐mix. Based on these results, concentrations applied in the second experiment were: 80, 160, 300, 625, 1,250, 2,500 and 5,000 μg/plate for TA98, TA100, TA1535 and TA1537 (in the absence and presence of S9‐mix). For TA102, concentrations up to 2,500 μg/plate (in the absence of S9‐mix) and up to 1,250 μg/plate (in the presence of S9‐mix) were applied. In the second experiment, all treatments in the presence of S9‐mix were modified using the pre‐incubation method. Due to evidence of toxicity observed at 1,250 and/or 2,500 μg/plate and above in all strains in the absence of S9‐mix, at 300 and/or 625 μg/plate and above in strains TA98, TA100, TA1535 and TA1537 and at 312.5 μg/plate and above in strain TA102 in the presence of S9‐mix, a third experiment was performed at the same test conditions but at lower concentrations and narrower concentration range (9.766, 19.53, 39.06, 78.13, 156.3, 312.5, 625 and 1,250 μg/plate), using the pre‐incubation method in the presence of S9‐mix. Strain TA102 was not tested in the third experiment. No increase in revertants was observed in any strain for any test conditions.
Appropriate positive controls were included for all five strains, and the assay was performed in accordance with OECD TG 471 (OECD, 1997a); data are summarised in Appendix F, Table F.1. The Panel considered that hepta‐2,4‐dienal [FL‐no: 05.084] was not mutagenic in this assay.
2,4‐Octadienal [FL‐no: 05.186]
2,4‐Octadienal [FL‐no: 05.186] (stored at 2–8°C, protected from light; purity was stated as 91.4% for the trans,trans‐isomer and 96.4% for both isomers) was tested in the Ames assay (Higton, 2015b) to assess its potential for induction of mutagenicity in five histidine‐requiring Salmonella Typhimurium strains: TA98, TA100, TA1535, TA1537 and TA102. The assay was performed in the absence and presence of metabolic activation (S9‐mix from Aroclor 1254‐induced rat livers) in two separate experiments, in triplicate (quintuplicate for negative control and triplicate for positive controls). DMSO was used as a solvent and negative control. In the first experiment, 2,4‐octadienal was tested at concentrations up to 5,000 μg/plate using the plate incorporation method (in the absence and presence of S9‐mix). In the first experiment, 2,4‐octadienal gave continuous toxicity at and above 1,600 μg/plate and a slight reduction in the background lawn at 500 μg/plate in all five strains (in the absence and presence of S9‐mix). Based on the toxicity results in the first experiment, concentrations up to 2,000 μg/plate were applied for all five strains (in the absence and presence of S9‐mix). In the second experiment, the pre‐incubation method was applied in the presence of S9‐mix.
In experiment 2, using the plate incorporation method and in the absence of metabolic activation, toxicity occurred at 2,000 μg/plate and a slight decrease in the background lawn was observed at 800 μg/plate in all strains. In experiment 2, using the pre‐incubation method and in the presence of metabolic activation, toxicity occurred at 800 μg/plate and a slight decrease in the background lawn occurred at 320 μg/plate in strains TA98, TA100, TA1535 and TA102; for strain TA1537, toxicity occurred at 320 μg/plate and no decrease in the background lawn was observed at any concentration. No increase in revertants was observed in any strain for any test conditions.
Appropriate positive controls were included for all five strains, and the assay was performed in accordance with OECD TG 471 (OECD, 1997a); data are summarised in Appendix F, Table F.1. The Panel considered that 2,4‐octadienal [FL‐no: 05.186] was not mutagenic in this assay.
tr‐2,tr‐4‐Nonadienal [FL‐no: 05.194]
tr‐2,tr‐4‐Nonadienal [FL‐no: 05.194] (stored at 2–8°C, protected from light; purity 89.2%) was tested in the Ames assay (Higton, 2015c) to assess its potential for induction of mutagenicity in five histidine‐requiring Salmonella Typhimurium strains: TA98, TA100, TA1535, TA1537 and TA102. The assay was performed in the absence and presence of metabolic activation (S9‐mix from Aroclor 1254‐induced rat livers), in two separate experiments, in triplicate (quintuplicate for negative control and triplicate for positive controls). DMSO was used as a solvent and negative control. In the first experiment, tr‐2,tr‐4‐nonadienal was tested at concentrations up to 5,000 μg/plate using the plate incorporation method (in the absence and presence of S9‐mix). In the first experiment, tr‐2, tr‐4‐nonadienal gave continuous toxicity at and above 1,600 μg/plate and a slight reduction in the background lawn at 500 μg/plate in all five strains (in the absence and presence of S9‐mix). Based on the toxicity results in the first experiment, concentrations up to 2,000 μg/plate were applied in the confirmatory assay for all five strains (in the absence and presence of S9‐mix). In the second experiment, the pre‐incubation method was applied in all treatments in the presence of S9‐mix.
In the second experiment, using the plate incorporation method and in the absence of metabolic activation, toxicity occurred at 800 μg/plate and a slight decrease in the background lawn occurred at 320 μg/plate in all strains. In the second experiment, using the pre‐incubation method and in the presence of metabolic activation, toxicity occurred at 320 μg/plate and a slight decrease in the background lawn was seen at 128 μg/plate in all strains. No increase in revertants was observed in any strain for any test conditions.
Appropriate positive controls were included for all five strains, and the assay was performed in accordance with OECD TG 471 (OECD, 1997a); data are summarised in Appendix F, Table F.1. The Panel considered that tr‐2,tr‐4‐nonadienal [FL‐no: 05.194] was not mutagenic in this assay.
3.4. In vivo micronucleus assays in the bone marrow and peripheral blood
3.4.1. In vivo micronucleus assay with hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057], oral gavage administration
Hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] (stored at 2–8°C protected from light and under nitrogen; purity 81% (trans, trans‐isomer), 97.6% as sum of two isomers) was tested for a potential clastogenic or aneugenic effect in an in vivo micronucleus assay with scoring in bone marrow cells and peripheral blood reticulocytes of Han Wistar rats (Whitwell, 2016a). The study was performed in accordance with GLP and OECD TG 474 (OECD, 2014).
Based on an oral gavage range‐finding experiment with doses up to 500 mg/kg bw per day, where no differences in response between female and male rats were seen, a maximum tolerated dose (MTD) of 350 mg/kg bw per day was established. In this dose range‐finding experiment, clinical signs of toxicity (e.g. decreased activity, eye closure and hunched posture) were observed only at the highest dose tested. In the main experiment, 12 male rats were dosed twice – at 0 (day 1) and 24 h (day 2) – by oral gavage at dose levels of 0 (corn oil), 88, 175 and 350 mg/kg bw per day. Six male rats were given 10 mg/kg bw per day CPA, as the positive control. Corn oil was used as vehicle following the same treatment schedule.
Test animals were examined daily for signs of overt toxicity and body weights were recorded. No clinical signs of toxicity were seen at any of the test conditions applied in the main experiment. Dose‐related decreases in group mean bodyweights were seen as compared to vehicle control.
Bone marrow was sampled from six rats per dose level, 24 h after the last administration of the test substance (subgroups 1). Peripheral blood reticulocytes were sampled from another group of six rats 48 h after the last administration (subgroups 2). Only for the positive control group both bone marrow and peripheral blood were sampled at 24 h after the second administration of CPA.
A total of at least 500 PCE and normochromatic erythrocytes (NCE) was scored to calculate the degree of bone marrow toxicity by the relative decrease in PCE. A total of 4,000 PCE per animal was scored for the presence of MN by visual analysis.
In the peripheral blood reticulocyte, a total of 20,000 reticulocytes/animal were analysed for MN by high speed flow cytometry.
No decrease in PCE was observed in the bone marrow of rats treated with the test compound nor with CPA compared to the vehicle control. On the contrary, in peripheral blood, a dose‐related decrease in the percentage of reticulocytes was observed compared to the negative control. At the dose levels of 88, 175 and 350 mg/kg per day, the percentage of reticulocytes was 3.16%, 2.83% and 1.59% respectively compared to 2.74% for the concurrent vehicle control (the historical vehicle control range for reticulocytes is 1.01–4.37%). This decrease in the percentage of reticulocytes was considered an indication of bone marrow exposure.
Group mean results of MN frequencies were similar to the concurrent vehicle control and no statistically significant (p ≤ 0.05) increases in MN were seen for any of the dose groups, both in bone marrow and in peripheral blood. The positive control group showed statistically significant increases in MN frequencies.
Negative and positive control values were within the laboratory's historical control data. Data are summarised in Appendix F, Table F.2.
The Panel considered the results of this study as negative.
3.4.2. In vivo micronucleus assay with hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057], intraperitoneal administration
Administration via the i.p. route followed a similar study design as the study by Whitwell (2016a).
Hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] (stored at 2–8°C under nitrogen, protected from light; purity 81% (trans,trans‐isomer), 97.6% as sum of two isomers) was tested for a potential clastogenic or aneugenic effect in the in vivo micronucleus assay with scoring in bone marrow cells and peripheral blood reticulocytes of Han Wistar rats (Whitwell, 2016b). The study was performed in accordance with GLP and OECD TG 474 (OECD, 2014).
In a range‐finding experiment with i.p. administration of doses up to 350 mg/kg bw per day, clinical signs of toxicity were observed including, increased activity, clonic and tonic convulsions, twitching, gasping and piloerection. No differences in response between female and male rats were observed. From this dose range‐finding experiment study, a MTD of 75 mg/kg bw per day was established. In the main experiment, 12 male rats were dosed twice at 0 (day 1) and 24 h (day 2), via i.p. injection at dose levels of 0 (corn oil), 19, 38 and 75 mg/kg bw per day. Six male rats were given 10 mg/kg bw per day CPA, as the positive control, following the same treatment schedule. Corn oil was used as vehicle.
Test animals were examined daily for signs of overt toxicity and body weights were recorded. With the exception of hunched posture observed in one animal in the dose group of 75 mg/kg bw per day, no clinical signs of toxicity were observed. Dose‐related decreases in group mean body weight gains were observed.
Bone marrow was sampled from six rats per dose level 24 h after the final test substance administration (subgroups 1) and peripheral blood reticulocytes were sampled from another six rats per dose level, 48 h after the last administration (subgroups 2) except for the positive control group where both bone marrow and peripheral blood were sampled at 24 hs after the second administration of CPA.
A total of at least 500 PCE and NCE was scored to calculate the degree of bone marrow toxicity by the relative decrease in PCE. A total of 4,000 PCE per animal was scored for the presence of MN by visual analysis.
In the peripheral blood reticulocyte, 20,000 reticulocytes from each animal were analysed for MN by high speed flow cytometry.
No decrease in PCE was seen in the bone marrow of treated rats except a slight decrease (7.6%) at the high dose. There was no difference in MNPCE (micronucleated polychromatic erythrocytes) frequencies (significant level p ≤ 0.05) between treated rats and vehicle controls. The positive control group showed statistically significant increases in MN frequencies.
On the contrary, in peripheral blood, a dose‐related decrease in the percentage of reticulocytes was observed compared to the negative control. At the dose levels of 19, 38 and 75 mg/kg bw per day, the percentage of reticulocytes was 2.35%, 1.82% and 1.31%, respectively, compared to 3.11% for the concurrent vehicle control (the historical vehicle control range for reticulocytes is 1.01–4.37%). This decrease in the percentage of reticulocytes was considered an indication of bone marrow exposure.
Group mean results of MN frequencies were similar to the concurrent vehicle control and no statistically significant (p ≤ 0.05) increases in MN were seen for any of the dose groups. The positive control group showed statistically significant increases in MN frequencies.
Negative and positive control values were within the laboratory's historical control data. Data are summarised in Appendix F, Table F.2.
The Panel considered the results of this study as negative.
3.4.3. In vivo micronucleus assay with deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140], oral gavage administration
Deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140] (stored at 2–8°C protected from light under nitrogen; purity 97.8%, sum of isomers) was tested for a potential clastogenic or aneugenic effect in the in vivo micronucleus assay with scoring in bone marrow cells and peripheral blood reticulocytes of Han Wistar rats (Keig‐Shevlin 2016a). The study was performed in accordance with GLP and OECD TG 474 (OECD, 2014).
In a range‐finding experiment with oral gavage, deca‐2(trans),4(trans)‐dienal was tested at 2,000 and 1,400 mg/kg bw per day. Following the second dose, clinical signs of toxicity were observed, including anogenital soiling, piloerection, arched gait, no differences in response between female and male rats were observed. In all animals, a body weight lost up to 10% was observed. Based on this dose range‐finding experiment a MTD of 1,400 mg/kg bw per day was established. In the main experiment, 12 male rats were dosed twice, at 0 (day 1) and 24 h (day 2), by oral gavage at dose levels of 0 (corn oil), 350, 700 and 1,400 mg/kg bw per day. Six male rats were given 10 mg/kg bw per day CPA, as the positive control, following the same treatment schedule. Corn oil was used as a vehicle.
Test animals were examined daily for signs of overt toxicity and body weights were recorded. No clinical signs of toxicity were observed at any of the test conditions after the first dosing. After the second dosing skin and fur staining were noted around the anus and soft faeces in animals of the 700 and 1,400 mg/kg bw per day dose groups. Dose‐related decreases in group mean bodyweights were observed at the highest doses up to 9.3% compared to vehicle control.
Bone marrow was sampled from six rats per dose level 24 h after the final test substance administration (subgroups 1) and peripheral blood reticulocytes were sampled from another six rats per dose level, 48 h after the last test substance administration (subgroups 2) except for the positive control group where both bone marrow and peripheral blood were sampled at 24 h after the second administration of CPA.
A total of at least 500 PCE and NCE was scored to calculate the degree of bone marrow toxicity by the relative decrease in PCE. For MN analysis, 4,000 PCE per animal were scored for the presence of MN by visual analysis.
A total of 20,000 reticulocytes from each animal were analysed for MN by high speed flow cytometry.
No decrease in PCE was seen in the bone marrow of treated rats. There was no difference in MNPCE frequencies between treated rats and vehicle controls. The positive control group showed statistically significant increases in MN frequencies.
In peripheral blood, a dose‐related decrease in the percentage of reticulocytes was observed compared to the negative control. At the dose levels of 350, 700 and 1,400 mg/kg bw per day, the percentage of reticulocytes was 1.92%, 1.65% and 0.83%, respectively, compared to 2.09% for the concurrent vehicle control. This decrease in the percentage of reticulocytes was considered an indication of bone marrow exposure. Group mean results of MN frequencies were similar to the concurrent vehicle control and no statistically significant increases in MN were seen for any of the dose groups. The positive control group showed statistically significant increases in MN frequencies.
Negative and positive control values were within the laboratory's historical control data. Data are summarised in Appendix F, Table F.2.
The Panel considered the results of this study as negative.
3.4.4. In vivo micronucleus assay deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140], intraperitoneal administration
Administration via the i.p. route followed a similar study design as the in vivo micronucleus study by Keig‐Shevlin (2016a).
Deca‐2(trans),4(trans)‐dienal (stored at 2–8°C protected from light under nitrogen; purity 97.8%, sum of isomers) was tested for a potential clastogenic or aneugenic effect in the in vivo micronucleus assay with scoring in bone marrow cells and peripheral blood reticulocytes of Han Wistar rats (Keig‐Shevlin, 2016b). The study was performed in accordance with GLP and OECD TG 474 (OECD, 2014).
In a range‐finding experiment with i.p. administration, deca‐2(trans),4(trans)‐dienal was tested at 100, 200 and 400 mg/kg bw per day. Clinical signs of toxicity were observed, including arched gait, ataxia, decreased activity, piloerection and ptosis, no differences in response between female and male rats were observed. At 100 mg/kg bw per day, body weight lost up to 14% was observed. Based on this dose range‐finding experiment, a MTD of 100 mg/kg bw per day was established.
In the main experiment, 12 male rats were dosed twice, at 0 (day 1) and 24 h (day 2), by i.p. at dose levels of 0 (corn oil), 25, 50 and 100 mg/kg bw per day. Six male rats were given 10 mg/kg bw per day CPA as the positive control, following the same treatment schedule. Corn oil was used as vehicle.
Test animals were examined daily for signs of overt toxicity and body weights were recorded. No clinical signs of toxicity were observed except for the death of one animal in the vehicle group after the second dosing. Dose‐related decreases in group mean bodyweights were observed at the highest doses (50 and 100 mg/kg bw per day) up to 11.1% compared to vehicle control.
Bone marrow was sampled from six rats per dose level 24 h after the final test substance administration (subgroups 1) and peripheral blood reticulocytes were sampled from another six rats per dose level, 48 h after the last administration (subgroups 2) except for the positive control group where both bone marrow and peripheral blood were sampled at 24 h after the second administration of CPA.
A total of at least 500 PCE and NCE was scored to calculate the degree of bone marrow toxicity by the relative decrease in PCE. A total of 4,000 PCE per animal was scored for the presence of MN by visual analysis.
A total of 20,000 reticulocytes from each animal were analysed for MN by high speed flow cytometry.
No decrease in PCE was observed in the bone marrow of treated rats. There was no difference in MNPCE frequencies between treated rats and vehicle controls. The positive control group showed statistically significant increases in MN frequencies.
In peripheral blood, a dose‐related decrease in the percentage of reticulocytes was observed compared to the negative control. At the dose levels of 25, 50 and 100 mg/kg bw per day, the percentage of reticulocytes was 1.95%, 1.71% and 1.23%, respectively, compared to 2.29% for the concurrent vehicle control. This decrease in the percentage of reticulocytes was considered an indication of bone marrow exposure. Group mean results of MN frequencies were similar to the concurrent vehicle control and no statistically significant increases in MN were seen for any of the dose groups. The positive control group showed statistically significant increases in MN frequencies.
Negative and positive control values were within the laboratory's historical control data. Data are summarised in Appendix F, Table F.2.
The Panel considered the results of this study as negative.
3.5. Transgenic rodent mutation assay with hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057]
Hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] (purity 95.9% trans,trans‐isomer) was tested in a transgenic rodent gene mutation assay for its potential to induce gene mutations at the cII locus in Big Blue® transgenic B6C3F1 mice; forestomach and liver were analysed (McKeon and Ciubotaru, 2016). The study was performed in accordance with GLP and OECD TG 488 (OECD, 2013). Data are summarised in Appendix F, Table F.2.
In a dose range‐finding study, five groups of six non‐transgenic B6C3F1 mice/sex per group were administered via gavage doses of 10, 30, 90 and 120 mg hexa‐2(trans),4(trans)‐dienal/kg bw per day for five consecutive days. The determination of hexa‐2(trans),4(trans)‐dienal in corn oil (vehicle) dosing formulation showed that the low dose level was likely 5.2–6 mg/kg bw per day instead of 10 mg/kg per day, and the high dose level was likely 101.9 mg/kg bw per day. No statistically significant changes in body weight were observed. The microscopic analysis showed sub mucosal inflammation, ulceration and/or erosion of forestomach. In general, male mice were more severely affected than female mice. Only in females, dilation of kidney tubules was observed.
The same range of doses (10, 30, 90 and 120 mg/kg bw per day) were tested in the 28‐day gavage study in Big Blue® B6C3F1 male mice, six animals per group. Animals were sacrificed after 3 days of recovery following the last administration. Corn oil was the vehicle control. Six male Big Blue® B6C3F1 mice served as the positive control and received N‐ethyl‐N‐nitrosourea (ENU) 40 mg/kg bw per day at days 1, 2 and 3 by oral gavage.
No mortality or clinical signs, no statistically significant changes in body weights or body weight gains were noted during the dosing period of this study.
Hexa‐2(trans),4(trans)‐dienal did not show any significant increase in cII mutant frequency compared to control. One animal (in dose group 10 mg/kg bw per day) showed a mutant frequency in the forestomach more than four times higher than all of the other dosed groups and control and was considered by the Panel as a clonal expansion of a pre‐existing mutation unrelated to test article treatment. With respect to the laboratory's historical control data, the number of studies performed in the period 2013–2015 for which data are provided has not been given. From the data provided, it looks as only one study has been performed with scoring in the forestomach in the period. Nevertheless, the Panel considered the results as negative.
3.6. Conclusions
Hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] was found negative in the basic battery of S. Typhimurium strains (TA100, TA98, TA1535, TA1537, TA102) tested in the Ames test both with and without metabolic activation. An increase in the frequency of revertants was observed in S. Typhimurium TA104 strain and in three studies with S. Typhimurium TA100 strain. No induction of gene mutations was observed in mammalian cells. Inconclusive results were reported in in vivo bone marrow micronucleus assays in mice and rats described in the NTP report. Two in vivo micronucleus studies in rats performed by gavage and by i.p. treatment did not report any statistically significant increase of MN frequency at any dose tested in peripheral blood reticulocytes and in PCE of the bone marrow. A dose‐related decrease of percent reticulocytes for both administration routes and a slight decrease in PCE at the high dose after the i.p. administration were detected in treated animals compared with the controls. This was considered as an indication of bone marrow exposure to the compound. A transgenic rodent mutagenicity study (Big Blue® assay) carried out in B6C3F1 mice did not show any significant increase in mutant frequency in liver and forestomach of treated animals compared with the controls.
Deca‐2(trans),4(trans)‐dienal did not induce any increase in revertants in the basic battery of S. Typhimurium strains with and without metabolic activation. Statistically significant increases in the frequency of micronuclei in PCE were observed with 2,4‐decadienal up to 6‐fold in rats without a dose–response relationship and in mice at a single dose level (3.5‐fold compared to controls), after i.p. injection in the NTP study. The Panel, however, noted that neither the purity nor the composition of isomers was reported in the genotoxicity section of the NTP report.
Two new in vivo micronucleus studies in rats performed by gavage and by i.p. treatment did not show any statistically significant increase of MN frequency at any dose tested in peripheral reticulocytes and in PCE of the bone marrow. A dose‐related decrease in the percentage of reticulocytes in treated animals compared to control was observed for both treatments, which could be considered as an indication of bone marrow exposure to the compound. Since information on purity and composition of isomers is available for the new studies, the Panel considers the new studies more relevant than the study performed by NTP.
Hepta‐2,4‐dienal, 2,4‐octadienal and tr‐2,tr‐4‐nonadienal tested for potential induction of gene mutation up to 5,000 μg/plate in five strains of S. Typhimurium TA98, TA100, TA1535, TA1537 and TA102 in the absence and presence of metabolic activation by S9‐mix did not induce any increase of revertants at any concentration tested.
Overall, the Panel concluded based on the new available results obtained in a comprehensive battery of in vitro and in vivo tests that the concern for genotoxicity can be ruled out for the representative substances hexa‐2(trans),4(trans)‐dienal [FL‐no: 05.057] and deca‐2(trans),4(trans)‐dienal [FL‐no: 05.140] and therefore also for the other substances in this group [FL‐no: 02.139, 02.153, 02.162, 02.188, 05.064, 05.071, 05.081, 05.084, 05.101, 05.108, 05.125, 05.127, 05.141, 05.173, 05.186, 05.194, 05.196, 09.573]. These 20 substances can be evaluated using the Procedure for the evaluation of flavouring substances.
Documentation provided to EFSA
Adriaens E, 2013. Slug mucosal irritation assay, 1 day stinging itching burning. InvertTox study number: 13D26. Unpublished report submitted by EFFA to EFSA.
Adriaens E, 2014a. Slug mucosal irritation assay, 1 day stinging itching burning. InvertTox study number: 14F13. Unpublished report submitted by EFFA to EFSA.
Adriaens E, 2014b. Slug mucosal irritation assay, 1 day stinging itching burning. InvertTox study number: 14I26. Unpublished report submitted by EFFA to EFSA.
Benigni R and Netzeva T, 2007a. Report on a QSAR model for prediction of genotoxicity of α,β‐unsaturated aldehydes in S. typhimurium TA100 and its application for predictions on α,β‐unsaturated aldehydes in Flavouring Group Evaluation 19 (FGE.19). Unpublished report submitted by FLAVIS Secretariat to EFSA.
Benigni R and Netzeva T, 2007b. Report on a QSAR model for prediction of genotoxicity of α,β‐unsaturated ketones in S. typhimurium TA100 and its application for predictions on α,β‐unsaturated aldehydes in Flavouring Group Evaluation 19 (FGE.19). Unpublished report submitted by FLAVIS Secretariat to EFSA.
EFFA (European Flavour Association), 2013. Submission toxicity data on FGE.19 materials: Subgroup 1.1.4 – FGE.203.
EFFA (European Flavour Association), 2018. Submission of additional information on substances within FGE.203 (FGE.19 Subgroup 1.1.4). Supplementary data submitted by EFFA to EFSA.
Higton T, 2015a. 2,4‐Heptadienal: Bacterial Reverse Mutation Assay. Covance Laboratories Ltd. England. Study no. 8322118. December 2015. Unpublished report submitted by EFFA to EFSA.
Higton T, 2015b. 2,4‐Nonadienal: Bacterial Reverse Mutation Assay. Covance Laboratories Ltd. England. Study no. 8322123. December 2015. Unpublished report submitted by EFFA to EFSA.
Higton T, 2015c. 2,4‐Octadienal: Bacterial Reverse Mutation Assay. Covance Laboratories Ltd. England. Study no. 8322119. December 2015. Unpublished report submitted by EFFA to EFSA.
IOFI (International Organization of the Flavor Industry), 2013. Flavouring Group Evaluation 19 Subgroup 1.1.4: 18 Flavouring Substances (Flavouring Substances) of the Chemical Group 3 (Annex I of 1565/2000/EC) structurally related to straight‐chain aliphatic acyclic α,β‐unsaturated aldehydes, with two or more conjugated double‐bonds with or without additional non‐conjugated double‐bonds, used as flavouring substances. Revised submission. FGE.203. 21/02/2013. FLAVIS.8.189.
Keig‐Shevlin Z, 2016a. 2,4‐Decadienal: Oral Gavage in vivo Micronucleus Study in the Bone Marrow and Peripheral Blood of Treated Rats. Covance Laboratories Ltd. Study no. 8321403. August 2016. Unpublished final report submitted by EFFA to EFSA.
Keig‐Shevlin Z, 2016b. 2,4‐Decadienal: Intraperitoneal in vivo Micronucleus Study in the Bone Marrow and Peripheral Blood of Treated Rats. Covance Laboratories Ltd. Study no. 8321404. July 2016. Unpublished final report submitted by EFFA to EFSA.
Lloyd M, 2015. 2,4‐Hexadienal: in vitro L5178Y Gene Mutation Assay at the hprt locus. Covance Laboratories Ltd. Study no. 8304487. February 2015. Unpublished final report submitted by EFFA to EFSA.
McKeon ME and Ciubotaru C, 2016. In vivo mutation assay at the cII Locus in Big Blue® transgenic B6C3F1 mice with a 5‐day dose range finder. BioReliance Study Number AE28GY.170.BTL. August 2016. Unpublished final report submitted by EFFA to EFSA.
Nikolov N, Jensen GE, Wedebye EB and Niemelä J, 2007. Report on QSAR predictions of 222 α,β‐unsaturated aldehydes and ketones from Flavouring Group Evaluation 19 (FGE.19) on 360 α,β‐unsaturated aldehydes and ketones and precursors for these. Unpublished report submitted by FLAVIS Secretariat to EFSA.
Whitwell J, 2016a. 2,4‐Hexadienal: Oral gavage in vivo micronucleus study in the bone marrow and peripheral blood of treated rats. Covance Laboratories Ltd, England. Study no. 8321401. January 2016. Unpublished report submitted by EFFA to EFSA.
Whitwell J, 2016b. 2,4‐Hexadienal: Intraperitoneal in vivo micronucleus study in the bone marrow and peripheral blood of treated rats. Covance Laboratories Ltd, England. Study no. 8321402. January 2016. Unpublished report submitted by EFFA to EFSA.
Abbreviations
- 6TG
6‐thioguanine
- B[a]P
benzo[a]pyrene
- bw
body weight
- CAS
Chemical Abstract Service
- CEF
Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CHL
Chinese hamster lung (cells)
- CHO
Chinese hamster ovary (cells)
- CoE
Council of Europe
- CPA
cyclophosphamide
- dGuo
2‐deoxyguanosine
- DMSO
dimethyl sulfoxide
- EFFA
European Flavour Association
- ENDOIII
endonuclease III
- ENU
N‐ethyl‐N‐nitrosourea
- FAO
Food and Agriculture Organization
- FEMA
Flavor and Extract Manufacturers Association
- FGE
Flavouring Group Evaluation
- FLAVIS
(FL) Flavour Information System (database)
- FPG
formamidopyrimidine DNA glycosylase
- GLP
Good Laboratory Practice
- HPRT
hypoxanthine‐guanine phosphoribosyl transferase
- GSH
glutathione
- GSSG
oxidised glutathione
- HPLC
high‐performance liquid chromatography
- IARC
International Agency for Research on Cancer
- ID
Identity
- IOFI
International Organization of the Flavor Industry
- i.p.
intraperitoneal
- IR
infrared spectroscopy
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- MDA
malondialdehyde
- MN
micronuclei
- MNBN
micronucleated binucleate cells
- MNPCE
micronucleated polychromatic erythrocytes
- MS
mass spectra
- MSDI
maximised Survey‐derived Daily Intake
- MTD
maximum tolerated dose
- NAC
N‐acetylcysteine
- NCE
normochromatic erythrocytes
- NER
nucleotide excision repair
- NOAEL
no‐observed‐adverse‐effect‐level
- NMR
nuclear magnetic resonance
- No
Number
- NQO
4‐nitroquinoline‐1‐oxide
- NTP
National Toxicology Program
- OECD
Organisation for Economic Co‐operation and Development
- PCE
polychromatic erythrocytes
- (Q)SAR
(Quantitative)Structure–Activity Relationship
- ROS
reactive oxygen species
- RS
relative survival
- SCF
Scientific Committee on Food
- SOD
superoxide dismutase
- TG
Test Guideline
- WHO
World Health Organization
Appendix A – Specification summary of the substances in the Flavouring Group Evaluation 203Rev2
1.
Appendix B – Summary of safety evaluation applying the procedure
1.
Appendix C – (Q)SAR predictions on mutagenicity
1.
Appendix D – Genotoxicity and carcinogenicity studies evaluated in FGE.203
1.
Appendix E – Genotoxicity studies evaluated in FGE.203Rev1
1.
Appendix F – Genotoxicity studies evaluated in FGE.203Rev2
1.
Suggested citation: EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , Silano V, Bolognesi C, Castle L, Chipman K, Cravedi J‐P, Engel K‐H, Fowler P, Franz R, Grob K, Husøy T, Kärenlampi S, Mennes W, Milana MR, Pfaff K, Riviere G, Srinivasan J, Tavares Poças MF, Tlustos C, Wölfle D, Zorn H, Binderup M‐L, Marcon F, Marzin D, Mosesso P, Anastassiadou M, Carfì M and Gürtler R, 2018. Scientific Opinion on Flavouring Group Evaluation 203, Revision 2 (FGE.203Rev2): α,β‐unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.4 of FGE.19 with two or more conjugated double‐bonds and with or without additional non‐conjugated double‐bonds. EFSA Journal 2018;16(7):5322, 39 pp. 10.2903/j.efsa.2018.5322
Requestor: European Commission
Question numbers: EFSA‐Q‐2017‐00003, EFSA‐Q‐2017‐00004, EFSA‐Q‐2017‐00005, EFSA‐Q‐2017‐00006, EFSA‐Q‐2017‐00007, EFSA‐Q‐2017‐00008, EFSA‐Q‐2017‐00009, EFSA‐Q‐2017‐00010, EFSA‐Q‐2017‐00011, EFSA‐Q‐2017‐00012, EFSA‐Q‐2017‐00013, EFSA‐Q‐2017‐00014, EFSA‐Q‐2017‐00015, EFSA‐Q‐2017‐00016, EFSA‐Q‐2017‐00017, EFSA‐Q‐2017‐00018, EFSA‐Q‐2017‐00019, EFSA‐Q‐2017‐00020, EFSA‐Q‐2017‐00021, EFSA‐Q‐2017‐00022
Panel members: Claudia Bolognesi, Laurence Castle, Kevin Chipman, Jean‐Pierre Cravedi, Karl‐Heinz Engel, Paul Fowler, Roland Franz, Konrad Grob, Rainer Gürtler, Trine Husøy, Sirpa Kärenlampi, Wim Mennes, Maria Rosaria Milana, Karla Pfaff, Gilles Riviere, Jannavi Srinivasan, Vittorio Silano, Maria de Fátima Tavares Poças, Christina Tlustos, Detlef Wölfle and Holger Zorn.
Acknowledgements: The Panel wishes to thank the hearing experts: Vibe Beltoft and Karin Nørby and EFSA staff: Annamaria Rossi for the support provided to this scientific output.
Adopted: 5 June 2018
Notes
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
Scientific Opinion on Flavouring Group Evaluation 203 Rev 1 (FGE.203 Rev1): alpha, beta‐unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.4 of FGE.19 with two or more conjugated double‐bonds and with or without additional non‐conjugated double‐bonds. EFSA Journal 2014;12(4):3626, 31 pp. https://doi.org/10.2903/j.efsa.2014.32626. Available online: www.efsa.europa.eu/efsajournal
The data presented in Section 2.4 are cited from the first Scientific Opinion on FGE.203. These data are the basis for the conclusions in FGE.203 requesting additional genotoxicity data.
The data presented in Section 2.5 are cited from the Scientific Opinion FGE.203Rev1.
References
- Clayson DB, Iverson F, Nera EA and Lok E, 1990. The significance of induced forestomach tumors. Annual Review of Pharmacology and Toxicology, 30, 441–463. [DOI] [PubMed] [Google Scholar]
- Eder E, Deininger C, Neudecker T and Deininger D, 1992. Mutagenicity of beta‐alkyl substituted acrolein congeners in the Salmonella typhimurium strain TA100 and genotoxicity testing in the SOS chromotest. Environmental and Molecular Mutagenesis, 19, 338–345. [DOI] [PubMed] [Google Scholar]
- Eder E, Scheckenbach S, Deininger C and Hoffman C, 1993. The possible role of α,β‐unsaturated carbonyl compounds in mutagenesis and carcinogenesis. Toxicology Letters, 67, 87–103. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Minutes of the 26th Plenary meeting of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food, Held in Parma on 27 ‐ 29 November 2007. Parma, 7 January 2008. Available online: http://www.efsa.europa.eu/EFSA/Event_Meeting/afc_minutes_26thplen_en.pdf
- EFSA (European Food Safety Authority), 2008b. Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) on Genotoxicity Test Strategy for Substances belonging to Subgroups of FGE.19. EFSA Journal 2008;6(12):854, 5 pp. 10.2903/j.efsa.2008.854 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008c. Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) on List of alpha, beta‐unsaturated aldehydes and ketones representative of FGE.19 substances for genotoxicity testing. EFSA Journal 2008;6(12):910, 5 pp. 10.2903/j.efsa.2008.910 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Scientific Opinion of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) on Flavouring Group Evaluation 203: α,ß‐Unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.4 of FGE.19 with two or more conjugated double bonds and with or without additional non‐conjugated double bonds. EFSA Journal 2009;7(4):877, 23 pp. 10.2903/j.efsa.2009.877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2014. Scientific Opinion on Flavouring Group Evaluation 203 Revision 1 (FGE.203Rev1): α,β‐Unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.4 of FGE.19 with two or more conjugated double‐bonds and with or without additional non‐conjugated double‐bonds. EFSA Journal 2014;12(4):3626, 31 pp. 10.2903/j.efsa.2014.3626 [DOI] [Google Scholar]
- Florin I, Rutberg L, Curvall M and Enzell CR, 1980. Screening of tobacco smoke constituents for mutagenicity using the Ames’ test. Toxicology, 18, 219–232. [DOI] [PubMed] [Google Scholar]
- Glaab V, Collins AR, Eisenbrand G and Janzowski C, 2001. DNA‐damaging potential and glutathione depletion of 2‐cyclohexene‐1‐one in mammalian cells, compared to food relevant 2‐alkenals. Mutation Research, 497, 185–197. [DOI] [PubMed] [Google Scholar]
- Grice HC, 1988. Safety evaluation of butylated hydroxyanisole from the perspective of effects on forestomach and oesophageal squamous epithelium. Food and Chemical Toxicology, 26, 717–723. [DOI] [PubMed] [Google Scholar]
- Gry J, Beltoft V, Benigni R, Binderup M‐L, Carere A, Engel K‐H, Gürtler R, Jensen GE, Hulzebos E, Larsen JC, Mennes W, Netzeva T, Niemelä J, Nikolov N, Nørby KK and Wedebye EB, 2007. Description and validation of QSAR genotoxicity models for use in evaluation of flavouring substances in Flavouring Group Evaluation 19 (FGE.19) on 360 α,(‐unsaturated aldehydes and ketones and precursors for these. Unpublished report submitted by FLAVIS Secretariat to EFSA.
- IARC (International Agency for Research on Cancer), 2003. Predictive value of rodent forestomach and gastric neuroendocrine tumours in evaluating carcinogenic risks to humans. IARC Technical Publication No. 39.
- IARC (International Agency for Research on Cancer), 2012. 2,4‐Hexadienal. IARC monographs on the evaluation of carcinogenic risks to humans., 101, 391–402. [PMC free article] [PubMed] [Google Scholar]
- Janzowski C, Glaab V, Mueller C, Straesser U, Kamp HG and Eisenbrand G, 2003. α, β‐Unsaturated carbonyl compounds: induction of oxidative DNA damage in mammalian cells. Mutagenesis, 18, 465–470. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2004. Safety evaluation of certain food additives and contaminants. Sixty‐first Meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 52. IPCS, WHO, Geneva.
- Loureiro AP, Di Mascio P, Gomes OF and Medeiros MH, 2000. trans, trans‐2,4‐decadienal‐induced 1, N(2)‐etheno‐2′‐deoxyguanosine adduct formation. Chemical Research in Toxicology, 13, 601–609. [DOI] [PubMed] [Google Scholar]
- Loureiro AP, de Arruda Campos IP, Gomes OF, di Mascio P and Medeiros MH, 2004. Structural characterization of diastereoisomeric ethano adducts derived from the reaction of 2′‐deoxyguanosine with trans, trans‐2,4‐decadienal. Chemical Research Toxicology, 17, 641–649. [DOI] [PubMed] [Google Scholar]
- Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H and Ames BN, 1985. Naturally‐occurring carbonyl compounds are mutagens in Salmonella tester strain TA104. Mutation Research, 148, 25–34. [DOI] [PubMed] [Google Scholar]
- Nappez C, Battu S and Beneytout JL, 1996. trans, trans‐2,4‐decadienal: cytotoxicity and effect on glutathione level in human erythroleukemia (HEL) cells. Cancer Letters, 99, 115–119. [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program), 2003. Toxicology and carcinogenesis studies of 2,4‐hexadienal in F344/N rats and B6C3F1 mice (gavage studies). NTP TRS 509, NIH Publication No. 04–4443. Available online: http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr509.pdf
- NTP (National Toxicology Program), 2011. NTP technical report on the toxicity studies of 2,4‐decadienal (CAS No. 25152‐84‐5) administered by gavage to F344/N Rats and B6C3F1 Mice F344. Toxicity Report Series Number 76. NIH Publication No. 11–5969.
- OECD (Organisation for Economic Co‐operation and Development), 1997a. Test No. 471: Bacterial reverse mutation test. OECD Guideline for testing of chemicals. Section 4. Available online: http://www.oecd.org/dataoecd/18/31/1948418.pdf
- OECD (Organisation for Economic Co‐operation and Development), 1997b. Test No. 474: Mammalian erythrocyte micronucleus test. OECD Guideline for testing of chemicals. Section 4. Available online: http://www.oecd.org/dataoecd/18/34/1948442.pdf
- OECD (Organisation for Economic Co‐operation and Development), 1997c. Test No. 476: In vitro Mammalian Cell Gene Mutation Test. OECD Guidelines for the Testing of Chemicals, Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 2013. Test No. 488: Transgenic Rodent Somatic and Germ Cell Gene Mutation Assays. OECD Guidelines for the Testing of Chemicals, Section 4.
- OECD (Organisation for Economic Co‐operation and Development), 2014. Test No. 474: Mammalian erythrocyte micronucleus test. OECD Guideline for testing of chemicals. Section 4.
- Wester PW and Kroes R, 1988. Forestomach carcinogens: pathology and relevance to man. Toxicologic Pathology, 16, 165–171. [DOI] [PubMed] [Google Scholar]
- Wu SC and Yen GC, 2004. Effects of cooking oil fumes on the genotoxicity and oxidative stress in human lung carcinoma (A‐549) cells. Toxicology In Vitro, 18, 571–580. [DOI] [PubMed] [Google Scholar]
- Young SC, Chang LW, Lee HL, Tsai LH, Liu YC and Lin P, 2010. DNA damages induced by trans, trans‐2,4‐decadienal (tt‐DDE), a component of cooking oil fume, in human bronchial epithelial cells. Environmental and Molecular Mutagenesis, 51, 315–321. [DOI] [PubMed] [Google Scholar]


