Abstract
Following a request from the European Commission, the Panel on Dietetic Products, Nutrition and Allergies (NDA) was asked to revise the tolerable upper intake level (UL) for vitamin D for infants (≤ 1 year) set in 2012. From its literature review, the Panel concluded that the available evidence on daily vitamin D intake and the risk of adverse health outcomes (hypercalciuria, hypercalcaemia, nephrocalcinosis and abnormal growth patterns) cannot be used alone for deriving the UL for infants. The Panel conducted a meta‐regression analysis of collected data, to derive a dose–response relationship between daily supplemental intake of vitamin D and mean achieved serum 25(OH)D concentrations. Considering that a serum 25(OH)D concentration of 200 nmol/L or below is unlikely to pose a risk of adverse health outcomes in infants, the Panel estimated the percentage of infants reaching a concentration above this value at different intakes of vitamin D. Based on the overall evidence, the Panel kept the UL of 25 μg/day for infants aged up to 6 months and set a UL of 35 μg/day for infants 6–12 months. The Panel was also asked to advise on the safety of the consumption of infant formulae with an increased maximum vitamin D content of 3 μg/100 kcal (Commission Delegated Regulation (EU) 2016/127 repealing Directive 2006/141/EC in 2020). For infants aged up to 4 months, the intake assessment showed that the use of infant formulae containing vitamin D at 3 μg/100 kcal may lead some infants to receive an intake above the UL of 25 μg/day from formulae alone without considering vitamin D supplemental intake. For infants aged 4–12 months, the 95th percentile of vitamin D intake (high consumers) estimated from formulae and foods fortified or not with vitamin D does not exceed the ULs, without considering vitamin D supplemental intake.
Keywords: vitamin D, infants, adverse health outcomes, 25(OH)D, UL, intake
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2018.EN-1456/full
Summary
Following a request from the European Commission, the Panel on Dietetic Products, Nutrition and Allergies (NDA) revised its Opinion of 2012 on the tolerable upper intake level (UL) for vitamin D, regarding the UL set for infants, i.e. aged less than 1 year.1 The UL is the maximum level of total chronic daily intake of a nutrient from all sources (i.e. foods, including supplements2) judged to be unlikely to pose a risk of adverse health effects in humans.
This request arises in the context of Commission Delegated Regulation (EU) 2016/1273, which will repeal Directive 2006/141/EC4 from 2020, and increases the maximum allowed vitamin D content from 2.5 to 3 μg/100 kcal for infant formulae (IF), i.e. to reach the same content as for follow‐on formulae (FoF). In order to ensure the highest level of protection of infants consuming formulae, the European Commission seeks European Food Safety Authority (EFSA)'s advice on the safety of the consumption of IF and FoF containing 3 μg/100 kcal of vitamin D by infants.
The previous EFSA Opinion of 2012 on the revision of the ULs for vitamin D set by the Scientific Committee on Food (SCF) in 2003 concluded that there was a paucity of data on infants on which to base a no‐ or a lowest observed adverse effect level (NOAEL or LOAEL). Having considered in particular available evidence on ‘high’ vitamin D intake and the absence of retarded growth or hypercalcaemia in infants, and as newer data from intervention studies in healthy infants had not become available since the previous risk assessment (SCF, 2003), the Panel decided in 2012 to retain the UL of 25 μg vitamin D/day previously derived for infants.5
Vitamin D is the generic term for ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3), which are present in foods. Vitamin D3 is also synthesised endogenously in the skin following exposure to ultraviolet (UV)‐B irradiation. While for other age groups beyond infancy, the synthesis of vitamin D3 in the skin during the summer months may be the main source of vitamin D and may fulfil the requirement, the Panel considers that this may not be the case for infants, who therefore have greater reliance on dietary intake. In the body, vitamin D is hydroxylated in the liver to 25‐hydroxyvitamin D (25(OH)D), which is then hydroxylated primarily in the kidneys to the biologically active metabolite 1,25‐hydroxyvitamin D (1,25(OH)2D), whose concentration in blood is tightly regulated. Vitamin D, 25(OH)D and 1,25(OH)2D are transported in the blood mainly by the vitamin D‐binding protein (DBP). The principal function of 1,25(OH)2D is to maintain calcium and phosphorus homoeostasis.
Serum 25(OH)D concentration is used as a biomarker of vitamin D intake in populations with low exposure to UV‐B irradiation and of vitamin D status. All analytical methods available for its measurement have uncertainties, which could lead to over‐ or underestimation. Specifically in infants, some analytical methods may overestimate serum concentration of 25(OH)D, due to the fact that they also detect the C3‐epimer of 25(OH)D, which may contribute up to about 40% of total 25(OH)D during the first 3 months of life (contrary to older children and adults). However, the metabolic role of the C3‐epimer is unclear. Serum 25(OH)D concentration in infants can be influenced by vitamin D intake level, form and frequency of consumption, baseline serum 25(OH)D concentration, sun exposure, season, body mass (or age) and body fat and maternal characteristics during pregnancy and lactation (e.g. maternal supplementation with vitamin D).
Vitamin D toxicity resulting from ‘long‐term’ ingestion of ‘large’ doses of vitamin D is associated with an increase in the serum concentration of 25(OH)D and some other vitamin D metabolites but usually not of the active metabolite 1,25(OH)2D. Some hypotheses for mechanisms of toxicity have been proposed. The presence of elevated serum‐free 1,25(OH)2D concentration despite normal total 1,25(OH)2D suggests that, in states of vitamin D toxicity, 1,25(OH)2D is displaced from DBP by 25(OH)D or other metabolites, and that this could promote entry of free 1,25(OH)2D into target cells where it could stimulate gene transcription.
Based on this proposed mechanism of toxicity and in line with previous assessments by EFSA and other bodies, the Panel focussed on the following four adverse health outcomes: hypercalciuria, hypercalcaemia, ectopic calcification, e.g. nephrocalcinosis, and abnormal growth patterns.
The Panel collected data on serum 25(OH)D concentration in relation to vitamin D intake or adverse health outcomes, recognising that a ‘high’ concentration is not an adverse health outcome per se, but can be considered as a surrogate endpoint.
The Panel defined upfront the strategy and methodology to evaluate possible relationships between daily intake of vitamin D, i.e. D2 or D3 from all dietary sources including fortified foods and supplements, and adverse health outcomes or endpoints in infants. In June 2017, the Panel undertook a systematic literature search of trials and observational studies on full‐term healthy infants, either breastfed or fed with a standard IF or FoF, possibly receiving complementary foods, and appraised the risk of bias (RoB) of the studies.
The Panel discussed the available evidence from trials and observational studies on the four adverse health outcomes (hypercalciuria, hypercalcaemia, nephrocalcinosis and abnormal growth patterns). Although more data in infants are available since 2012, the Panel concludes that these data on daily vitamin D intake and adverse health outcomes cannot be used alone for deriving the UL of vitamin D for infants. This is because of methodological limitations of the studies, including the use of unclear reference values for serum or urinary calcium in infants, and of single measurements that may not provide an indication of the risk of sustained elevated concentrations. Moreover, the studies showed inconsistent results, or the absence of a dose–response relationship between vitamin D intake (or serum 25(OH)D concentration) and adverse health outcomes. The Panel notes the absence of data on the systematic administration of defined vitamin D doses above 50 μg/day to healthy infants for ethical reasons.
The level of daily vitamin D intake or serum 25(OH)D concentration that is associated with the occurrence of adverse health outcomes could not be determined on the basis of the available data and the Panel could not define a NOAEL for vitamin D intake. However, having considered previous assessments of EFSA and of other bodies that discussed ‘high’ serum 25(OH)D concentration (not specifically for infants), as well as available data collected through the dedicated literature search on infants, the Panel concludes that a serum 25(OH)D concentration of 200 nmol/L or below is unlikely to pose a risk of adverse health outcomes in healthy infants. This should not be regarded as a cut‐off for toxicity but as a conservative value from which a UL could be derived.
Case reports described adverse health outcomes in infants associated with chronic daily administration of vitamin D doses of about 4.5–20 times the adequate intake (AI) of 10 μg/day set by EFSA in 2016 for this age group; these outcomes were associated with elevated serum 25(OH)D concentrations. However, as these reports are based on incidental findings, they are not suitable to define a LOAEL for vitamin D in infants.
The Panel conducted a meta‐regression analysis of collected data to derive a dose–response relationship between daily supplemental intake of vitamin D and mean achieved serum 25(OH)D concentrations from data collected per arm of the trials selected through the literature search, adjusted for baseline serum 25(OH)D concentrations, age and duration of supplementation. This analysis was based on aggregated data from six trials with limited RoB, corresponding to 17 arms and 58 time points, which used vitamin D from supplements and generally did not assess the intake of vitamin D from the diet. From this model, an empirical distribution of mean achieved serum 25(OH)D concentrations was generated from a large number of simulations. From this, a distribution of serum 25(OH)D concentrations that may be achieved by individuals was then simulated, and the percentage of infants reaching a concentration above 200 nmol/L was estimated for different vitamin D intakes.
For infants aged up to 6 months, the Panel concludes that the available body of evidence supports keeping the previous UL of 25 μg/day. The Panel notes that predictions from the model support this conclusion. These predictions also show that, at a given intake of vitamin D, infants older than 6 months achieve lower serum 25(OH)D concentration than infants younger than 6 months, which may be explained by the increase in body mass. Thus, for infants aged 6 to less than 12 months, the Panel considers that evidence from the predictions supports a UL of 35 μg/day.
The Panel draws its conclusions on the UL for vitamin D from data mainly on vitamin D3, as the dose–response analysis to predict the percentage of individuals that would have serum 25(OH)D concentrations above 200 nmol/L included studies that used supplementation with vitamin D3. However, the updated UL of 25 and 35 μg/day applies both to vitamin D2 and D3.
In order to characterise the risk of infants having an intake above the UL for vitamin D, the Panel assessed the daily vitamin D intake in Europe, considering in particular the consumption of IF and FoF containing 3 μg/100 kcal of vitamin D. The intake assessment was conducted separately for infants aged up to 4 months, and for those aged 4 to less than 12 months, to account for the additional vitamin D intake in relation to the introduction of complementary feeding.6
The Panel notes that there are different national supplementation policies for vitamin D in infants in the European Union (EU) and compliance with these policies may vary between countries. In addition, limited information was available to the Panel from surveys on infants in Europe on the percentage of consumers of vitamin D‐containing supplements without information on their vitamin D content. Furthermore, limited information was available on possible disparities between the labelled and the actual vitamin D content of a product (fortified foods or supplements). Thus, the Panel did not make any assumption regarding such disparities or regarding vitamin D supplementation in its intake assessment.
With the objective of comparing the outcome of the intake assessment undertaken by EFSA with other available data, data were collected either from published studies or reports from national authorities or through a consultation of Member States undertaken by the European Commission.
For infants aged up to 4 months, according to calculations based on the maximal regulated vitamin D content in IF and the default ‘high’ IF consumptions set by the EFSA Scientific Committee in 2017, the Panel concludes that, depending on the reference body weight, age of the infants and energy content of IF (without considering additional intake due to supplementation or possible disparities between the labelled and the actual composition of IF):
-
–
the use of a maximum vitamin D content of 3 μg/100 kcal in IF (as per Delegated Regulation (EU) 2016/127) may lead some infants to consume amounts of vitamin D above the UL of 25 μg/day from formulae alone. The precise percentage of such infants could not be determined from the available data.
-
–
the use of a maximum vitamin D content of 2.5 μg/100 kcal in IF (as per Directive 2006/141/EC) does not result in intakes of vitamin D above the UL of 25 μg/day from formulae alone. The margin between the calculated intakes via formulae and the UL varies between 1 and 9 μg/day.
For infants aged from 4 to 12 months, the intake assessment was conducted separately for consumers and non‐consumers of IF and FoF. The intake assessment was done:
-
–
using individual data available to EFSA in 2017 of food consumption of infants from six surveys, conducted between 2001 and 2011 and most of them nationally representative of their respective EU countries;
-
–
and considering eight intake scenarios, based on the minimum or maximum amount of vitamin D in IF and FoF according to the two regulatory texts (Directive 2006/141/EC and Commission Delegated Regulation (EU) 2016/127) and based on the composition of foods fortified or not with vitamin D (without considering possible disparities between the labelled and the actual composition of foods), coming from the EFSA Nutrient Composition Database, and from the Mintel Global New Products Database.
These eight scenarios were assumed to describe situations that may occur on the EU market taking into account voluntarily fortified foods, different possible food patterns in infants but excluding the contribution from supplements and considering the two extremes in the range of the vitamin D content in mandatorily fortified foods. They were not meant to reflect the fortification practices of the six EU countries that provided food consumption data. Means and 95th percentiles (P95) of estimated vitamin D intake were used to characterise the risk of exceeding the UL (without considering additional intake due to supplementation) for infants aged from 4 to 12 months:
-
–
For infants consuming neither formulae nor fortified foods and for those not consuming formulae but consuming fortified foods, the estimated means and P95 of vitamin D intake are below or at the AI of 10 μg/day.
-
–
For infants consuming IF or FoF containing the maximum amount of vitamin D of 3 μg/100 kcal, but not consuming fortified foods, the estimated means and P95 of vitamin D intake are higher than in the above situation, but do not exceed the UL of 35 μg/day (i.e. for infants 6–12 months). The margin between the estimated P95 of vitamin D intake in formula consumers and the UL of 35 μg/day varies between about 15 and 22 μg/day. The margin calculated with the UL of 25 μg/day (i.e. for infants 4–6 months), varies between about 5 and 12 μg/day.
-
–
With the additional intake of fortified foods, the estimated means and P95 of vitamin D intake are higher than in the above situation, but do not exceed the UL of 35 μg/day (i.e. for infants 6–12 months). The margin between the estimated P95 of vitamin D intake in formula consumers and the UL of 35 μg/day varies between about 10 and 18 μg/day. The margin calculated with the UL of 25 μg/day (i.e. for infants 4–6 months), varies between about 0.4 and 8 μg/day.
Observed food patterns were not available for infants in nationally representative surveys from all EU Member States and food fortification practices vary substantially from one country to the other. The Panel notes that this should be born in mind when discussing estimated intakes of vitamin D in different scenarios. The margin between the estimated P95 of vitamin D intake in formula consumers and the UL in the various defined scenarios of food consumption could be considered as an indication of the possible additional impact of supplementation. This indication should be considered bearing in mind the regulatory compositional requirements of formulae and in the specific context of national supplementation policies and compliance with these policies.
As supporting documents, Annex A contains the full details of the statistical approach of the analysis of the response in infants of a vitamin D biomarker to daily intake of this vitamin, and Annex B (Excel® file) contains the full details (P5, P95, median and mean) of the calculations for the intake assessment for age from 4 months to < 1 year. Both results are summarised in the following sections.
The NDA Panel endorsed a draft of this scientific opinion for public consultation on 9 April 2018. The draft document has been revised and updated according to the comments received, where appropriate, and the comments received were addressed and published in a technical report.
1. Introduction
1.1. Background as provided by the European Commission
Commission Directive 2006/141/EC4 lays down requirements for infant formulae and follow‐on formulae placed on the market in the EU. Commission Delegated Regulation (EU) 2016/1273 repeals Directive 2006/141/EC from 2020 and revises the rules applicable to infant formulae and follow‐on formulae, taking account of the opinion of the European Food Safety Authority (EFSA) of 2014.7
Directive 2006/141/EC requires infant formula to contain vitamin D at amounts in the range 1–2.5 μg/100 kcal and follow‐on formula in the range 1–3 μg/100 kcal. In Delegated Regulation (EU) 2016/127, the Commission has set vitamin D levels for infant formula and follow‐on formula in the range 2–3 μg/100 kcal. The minimum amount of 2 μg/100 kcal was recommended by EFSA in its Scientific Opinion of 2014 and the maximum amount of 3 μg was extended also to infant formula based on its history of safe use in follow‐on formula and taking into account technological considerations brought forward by manufacturers.
Concerns have been raised that high consumption of formula containing 3 μg/100 kcal of vitamin D, combined with vitamin D intake via national supplementation policies (e.g. supplemental 10 μg per day recommended in Finland) could lead some infants to consume vitamin D at amounts that could pose safety risks. These concerns derive from calculations showing that the total daily intake of vitamin D resulting from the consumption of high amounts of formula containing 3 μg/100 kcal of vitamin D together with the amounts consumed according to national supplementation policies could be, for some infants, higher than the Tolerable Upper Intake Level set by EFSA for vitamin D in 2012 (25 μg/d for infants).8 Furthermore, the issue was brought up that the present Tolerable Upper Intake Level for vitamin D for infants was derived from experiments with a form of vitamin D (vitamin D2), which may be less efficient than the form of vitamin D currently preferred for fortification and supplementation (vitamin D3).
In order to ensure the highest level of protection of infants consuming formulae, the Commission would like to seek EFSA's advice on the matter. More specifically, the Commission would like EFSA to assess whether consumption of formula containing 3 μg/100 kcal of vitamin D, assuming additional vitamin D intakes through supplementation, is safe for infants and under what conditions.
In order to best address this question, EFSA should look into the latest scientific evidence and revise, if necessary, its opinion of 2012 on the Tolerable Upper Intake Level of vitamin D as regards infants. As the EFSA opinion is relatively recent, EFSA should focus only on infants and not address the matter for other population groups covered by the 2012 opinion.
1.2. Terms of reference as provided by the European Commission
In accordance with Article 29 (1) (a) of Regulation (EC) No 178/20029, the European Commission asks the EFSA to issue an opinion revising, if necessary, the tolerable upper intake level (UL) for vitamin D that is unlikely to pose a risk of adverse health effects for infants on the basis of the latest scientific evidence.
1.3. Interpretation of the Terms of Reference and identified subquestions (hazard characterisation, intake assessment and risk characterisation)
Taking into account considerations in the following sections in particular on hazard identification (Section 1.10), EFSA interprets this mandate as follows:
-
–
Hazard characterisation:
-
a
Based on a systematic literature search (Section 3), to evaluate possible relationships between intake of vitamin D and adverse health outcomes and endpoints in infants (i.e. up to 12 months of age), with the aim of answering the following questions:
Is there new available evidence on ‘high’ intake of vitamin D and adverse health outcomes/adverse change in endpoints?
Is there enough new evidence to change the current UL for vitamin D of 25 μg/day in infants?
-
a
-
b
To perform a quantitative assessment of the dose–response, where applicable.
-
–
Intake assessment: to assess the daily vitamin D intake in infants in Europe (Section 4).
-
–
Risk characterisation: to evaluate the risk that some infants have an intake above the UL for vitamin D, considering in particular the ‘high’ consumption of formulae containing up to 3 μg/100 kcal of vitamin D (Section 6).
-
–
1.4. Previous assessments on vitamin D
The UL is the maximum level of total chronic daily intake of a nutrient from all sources (i.e. foods, including supplements2) judged to be unlikely to pose a risk of adverse health effects in humans (EFSA NDA Panel, 2010).
In the EU, the Scientific Committee on Food (SCF, 2003) established a UL of 25 μg/day for infants and young children aged 0–24 months based on the absence of hypercalcaemia attributable to intervention with vitamin D2 in two studies (Ala‐Houhala, 1985; Vervel et al., 1997). In these studies, breastfed or formula‐fed infants received 25 μg vitamin D/day plus the amount ingested via fortified infant formula (IF) for up to 5 months after birth. This amount of 25 μg vitamin D/day was treated as a no observed adverse effect level (NOAEL) and an uncertainty factor (UF) of 1 was deemed appropriate because, in both studies, serum 25‐hydroxy‐vitamin D (25(OH)D) concentration (Section 1.8) was below the threshold of increased risk for hypercalcaemia that was considered to be above 200 nmol/L in adults. In the following sections of this Scientific Opinion, the term ‘serum’ 25(OH)D concentration will be used consistently, regardless of whether the measurements were indeed obtained in serum or in plasma.
In North America, a UL of 25 μg/day was set for infants 0–6 months by the US Institute of Medicine based on a NOAEL of 45 μg/day, applying an UF of 0.5 to ensure the absence of toxicity also in small infants, and rounding up (IOM, 2011). This NOAEL derives from data on normal growth in infants receiving a mean of 45 μg vitamin D/day (Fomon et al., 1966) (Section 3.3.5). The tolerance of infants aged 6–12 months with a greater body mass was assumed to increase, thus IOM added 12.5 μg/day to the UL set for young infants 0–6 months, yielding a UL of 37.5 μg/day.
Upon the request from the European Commission in 2010 to revise the ULs for vitamin D set by SCF in 2003 for all population groups, EFSA noted that there was still a paucity of data on infants on which to base a NOAEL or a lowest observed adverse effect level (LOAEL) and discussed seven references (EFSA NDA Panel, 2012a). This scientific opinion describes historical evidence of retarded growth from one small study in nine infants who received various regimens of vitamin D exceeding 45 μg/day up to 1 year of age, compared to six infants who received 8.5 μg daily or less (Jeans and Stearns, 1938). However, another small study using doses up to 54 μg vitamin D3/day (mean of 45 μg/day) until about 5 months of age did not show such an effect (Fomon et al., 1966) (Section 3.3.5). A large long‐term prospective cohort study (Hyppönen et al., 2011) (Section 3.3.5), comparing height in subjects who received 50 μg/day vitamin D (unspecified), < 50 μg/day or > 50 μg/day in infancy, did not confirm the findings of Jeans and Stearns (1938) on retarded growth. Early intervention studies using doses up to 25 μg vitamin D2/day (plus the amount ingested via breast milk or fortified IF) for up to 5 months after birth did not indicate that these intakes were associated with hypercalcaemia in infants (Ala‐Houhala, 1985; Vervel et al., 1997). Two other studies on infants were discussed but not used for setting the UL because one was dealing with large vitamin D dose (‘stoss’) therapies (Barrueto et al., 2005) and the other study was of short duration (Gordon et al., 2008). Considering the limited evidence available since the last risk assessment (SCF, 2003), the Panel considered that the UL of 25 μg vitamin D/day previously derived for infants from 0 to 12 months of age should be retained. In this same opinion, ULs were set at 50 μg/day for children 1–10 years.
The Scientific Advisory Committee on Nutrition (SACN, 2016) in line with the Committee on toxicity of chemicals in food, consumer products and the environment (COT, 2015) agreed that retaining the UL of 25 μg/day of vitamin D set by EFSA NDA Panel (2012a) for infants, was appropriate.
In a different context, upon request from the European Commission to update the previous dietary reference values (DRVs) set by SCF (1993), EFSA analysed the dose–response relationship between total vitamin D intake and mean achieved serum 25(OH)D concentration (biomarker for vitamin D status) in each of the 83 arms of 35 trials using vitamin D3 and carried out under conditions of assumed minimal cutaneous vitamin D synthesis (meta‐regression) (EFSA NDA Panel, 2016). This model was used to derive the vitamin D intake in adults and children required to achieve a target serum 25(OH)D concentration of 50 nmol/L, which was based on associations with musculoskeletal health outcomes. The resulting intake corresponded to the definition of an adequate intake (AI) for vitamin D.10 However, too few data on infants met the inclusion criteria for such an analysis, and the AI of 10 μg/day for infants was set based on other data (EFSA NDA Panel, 2016).
In this short overview, the Panel highlights the two reference values set for vitamin D for infants in the EU, i.e. an AI of 10 μg/day (EFSA NDA Panel, 2016) and a UL of 25 μg/day (EFSA NDA Panel, 2012a). The assessment of the dose–response relationship between vitamin D intake and a biomarker was previously used by the Panel to set AIs for adults and children, but not for infants due to lack of data. The Panel notes that previously set ULs for infants (in the EU or outside) were derived considering data on vitamin D intake and linear growth and data on vitamin D intake and serum calcium or risk of hypercalcaemia. The Panel also notes that, while IOM set different values for the UL for vitamin D during the first and second half year of life, both SCF and EFSA set one single UL for the first year of life.
1.5. Forms of vitamin D (vitamers) and their food sources
Vitamin D is a fat‐soluble and the generic term for vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol).11 Vitamin D2 is synthesised by exposure of ergosterol in plants, fungi and yeast to ultraviolet (UV)‐B irradiation, whereas vitamin D3 may be formed in humans and in animals following UV‐B irradiation of the skin (Section 1.7.1.1) and is also produced in some plants (lichens).
The major food sources for naturally occurring vitamin D have been discussed (EFSA NDA Panel, 2016). In brief:
-
–
for vitamin D3, these sources include animal foods such as fish (and especially fatty fish and fish liver), offal (particularly liver), meat and meat products and egg yolks.
-
–
25(OH)D is present in some foods of animal origin in varying amounts. ‘Due to the suggested higher biological activity of 25(OH)D in foods compared with the native vitamin D, a conversion factor of 5 has been used for 25(OH)D3 in the calculation of total vitamin D3 in some food composition tables, including those in the UK, Denmark and Switzerland’.
-
–
for vitamin D2, some higher fungi, e.g. mushrooms, are a natural source if exposed to UV‐B.
Vitamin D3 and vitamin D2 may be added to foods12 including dietary supplements.13
1.6. General considerations on food patterns in infants
Infants may be fed from birth exclusively with breast milk or exclusively with formulae or both, with the addition, at a certain age, of complementary foods (EFSA NDA Panel, 2009).
Mean vitamin D concentrations in breast milk of healthy lactating women who are unsupplemented (or supplemented with vitamin D below the UL) are low, ranging from 0.25 to 2.0 μg/L (Dawodu and Tsang, 2012; EFSA NDA Panel, 2013, 2016). Infant and follow‐on14 formulae (IF and FoF) are currently fortified with vitamin D15 as are some commercially available complementary foods.16 Other complementary foods may be naturally low (e.g. fruits or vegetables) or naturally rich (e.g. fish) or fortified with vitamin D. The Panel notes that the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition recommends a daily oral supplementation of 10 μg vitamin D for all infants during the first year of life starting from birth onwards (Braegger et al., 2013; EFSA NDA Panel, 2016).
1.7. Functions, physiology and metabolism of vitamin D
The functions of vitamin D in the body are mediated by its active metabolite 1,25‐dihydroxy‐vitamin D (1,25(OH)2D: 1,25(OH)2D2 or 1,25(OH)2D3 called calcitriols). They include the maintenance and regulation of calcium and phosphorus concentrations that are critically important for the development and maintenance of healthy bones but also significant effects in other tissues (Section 1.7.2). General aspects of vitamin D physiology, functions and metabolism have been discussed previously (EFSA NDA Panel, 2016) and are summarised in the subsequent sections where special consideration is given to infants where possible.
1.7.1. Physiology of vitamin D
1.7.1.1. Cutaneous synthesis of vitamin D
Vitamin D3 is synthesised in the skin from 7‐dehydrocholesterol upon exposure to sunlight or other sources of UV‐B irradiation via the formation of previtamin D3 that thermally isomerises immediately to vitamin D3. Vitamin D3 produced in the skin enters the circulation bound to the vitamin D‐binding protein (DBP). Prolonged dermal exposure to sunlight/UV‐B irradiation leads to photodegradation of both previtamin D3 and vitamin D3 in the skin to biologically inert isomers (Webb et al., 1989), thus preventing vitamin D toxicity due to prolonged sun exposure (Holick, 1994).
Endogenous cutaneous vitamin D synthesis depends on many factors (e.g. latitude, season, ozone layer, clouds, surface type such as snow, time spent outdoors, sunscreen use, clothing, skin type, age) (EFSA NDA Panel, 2016). With increasing latitude, both the quality and quantity of sunlight are not sufficient in parts of the year for vitamin D3 synthesis in the skin to take place (Engelsen et al., 2005; EFSA NDA Panel, 2016).
Concerns regarding UV‐related skin and eye damage have led to recommendations to reduce UV exposure in infants because it is considered a health hazard (American Academy of Pediatrics, 1999; Wagner and Greer, 2008; Balk, 2011). As a result of this advice, the endogenous vitamin D synthesis may be low in infants.
While for other age groups beyond infancy, the synthesis of vitamin D3 in the skin during the summer months may be the main source of vitamin D and may fulfil the requirement, the Panel considers that this may not be the case for infants, who therefore have greater reliance on dietary intake.
1.7.1.2. Intestinal absorption of vitamin D
Vitamin D from foods is absorbed throughout the small intestine with an efficiency varying generally between 55 and 99% and with no discrimination between vitamins D2 and D3 (Thompson et al., 1966; Lo et al., 1985; Jones, 2014; Borel et al., 2015; Reboul, 2015). The absorption process is more efficient in the presence of bile salts and when dietary fat is present in the lumen of the small intestine. Vitamin D absorbed from the intestine is incorporated into chylomicrons, which reach the systemic circulation through the lymphatic system (Jones, 2013). In a study on term infants, vitamin D2 (provided by orogastric tube) was well absorbed both in young and older infants (one or more than 10 days of age, respectively), with increasing absorption efficiency with age due to increased bile salt secretion (Hollis et al., 1996). Vitamin D3‐palmitate was equivalent with free vitamin D2 in raising circulating vitamin D concentrations only at an age of about 3 months, due to the dependence on gastrointestinal tract maturation and secretion of esterases (Hollis et al., 1996).
1.7.1.3. Transport in blood and metabolism of vitamin D
Vitamins D2 and D3, released either from chylomicrons or DBP, undergo 25‐hydroxylation in the liver to 25(OH)D2 and 25(OH)D3, respectively (IOM, 2011; Jones, 2014). 25(OH)D is then released into the circulation bound to DBP. In adults, 85–90% of 25(OH)D is transported in the blood bound to DBP, 10–15% to albumin and < 1% is free (Bikle et al., 1985; Powe et al., 2013; Chun et al., 2014; Yousefzadeh et al., 2014).
Serum 25(OH)D concentrations reflect the amount of vitamin D from both cutaneous synthesis and ingested foods. Serum 25(OH)D2 is of dietary origin only, while serum 25(OH)D3 may be of dietary or dermal origin. The kidneys are the main site where 25(OH)D undergoes 1α‐hydroxylation (Jones, 2014) to form 1,25(OH)2D. The activity of the 1α‐hydroxylase is regulated by calcium, phosphate and their regulating hormones. In the blood, 1,25(OH)2D is primarily transported bound to DBP and albumin (Bikle et al., 1986; Jones et al., 1998; Powe et al., 2013). The serum concentration of 25(OH)D is approximately 1,000 times higher than that of 1,25(OH)2D. In adults, serum 25(OH)D has a mean half‐life of approximately 13–15 days (Jones et al., 2014), while serum 1,25(OH)2D has a half‐life of hours (Jones et al., 1998; IOM, 2011). The Panel is not aware of data suggesting that the half‐life of serum 25(OH)D would be different in infants on usual intakes.
The C3‐epimer of 25(OH)D (3‐epi‐25(OH)D) has been detected in the serum in 20–98% of infants (e.g. (Singh et al., 2006; Gallo et al., 2013a)). The 3‐epi‐25(OH)D may represent up to about 40% of total serum 25(OH)D concentration measured in young infants (Stepman et al., 2011; Ooms et al., 2016). In a cohort of 1,050 infants (Kiely et al., 2017), 3‐epi‐25(OH)D3 was also detected in cord sera of infants who had 25(OH)D3 > 15 nmol/L (in 99.4% of all infants), representing on average (range) 11.2 (6–35)% of total 25(OH)D concentration. In this cohort, the cord 3‐epi‐25(OH)D3 concentrations were significantly positively associated with cord 25(OH)D3, and negatively with gestational age and maternal age.
Ooms et al. (2016) reported that 3‐epi‐25(OH)D3 concentrations were relatively low in the first weeks of life (mean (range) of 3 (1–7) nmol/L), with a low‐relative contribution to 25(OH)D3 concentration (< 10%). 3‐epi‐25(OH)D3 concentrations increased from the second week of life with highest levels observed in the second and third months, reaching as high as 200 nmol/L in some infants. Relative 3‐epi‐25(OH)D3 contribution to 25(OH)D3 was up to 36% in full‐term infants. From age 3 months, a sharp decline in absolute or relative 3‐epi‐25(OH)D3 level was seen, thereafter to remain at a relatively constant low concentration (mean (range) 8 (1–62) nmol/L, < 10% of 25(OH)D3).
High 3‐epi‐25(OH)D concentrations in infancy are probably due to postnatal formation rather than foetal stores (Bailey et al., 2014). The metabolic role of 3‐epi‐25(OH)D is unclear. It is converted to 3‐epi‐1,25(OH)2D, which binds to the vitamin D receptor (VDR) with a lower affinity than 1,25(OH)2D. It has also been detected in the serum of older children and adults, but at much smaller concentrations (e.g. (Bailey et al., 2013)). Specificities of the analysis of serum concentration of 25(OH)D in infants, in particular with regard to the concentration of 3‐epi‐25(OH)D, are discussed in Section 1.8.6.3.
1.7.1.4. Distribution to tissues and storage
Within hours of ingestion or synthesis in the skin, vitamin D is distributed to the liver or delivered as either vitamin D or its metabolites 25(OH)D and 1,25(OH)2D to various tissues of which adipose tissue is considered a major repository in the body for vitamin D (Blum et al., 2008).
25(OH)D is transported via the placenta to the foetus and also converted there to 1,25(OH)2D or 24,25(OH)2D (Paulson and DeLuca, 1986; Salle et al., 2002; Kovacs, 2008; Dror and Allen, 2010; Shin et al., 2010; Young et al., 2014).
1.7.1.5. Degradation and elimination
Free 1,25(OH)2D taken up by target cells is either rapidly metabolised or bound to VDR (Lehmann and Meurer, 2010). Vitamin D metabolites in the body are degraded in an oxidative pathway by the actions of CYP24A1 (24‐hydroxylase) (EFSA NDA Panel, 2016). 1,25(OH)2D is a strong controller of its own degradation by stimulating the 24‐hydroxylase (IOM, 2011). The majority of the metabolites of the vitamin D degradative pathway are excreted in the bile (Jones, 2013) and thus in the faeces. The Panel is not aware of any specific data on vitamin D degradation and elimination in infants.
1.7.2. Functions of the biologically active metabolite 1,25(OH)2D
The principal function of 1,25(OH)2D (i.e. 1,25(OH)2D3 and 1,25(OH)2D2) is to maintain calcium and phosphorus homoeostasis in the circulation, together with parathyroid hormone (PTH) and fibroblast growth factor (FGF‐23) (EFSA NDA Panel, 2012a, 2016; Jones, 2013). The main target tissues of 1,25(OH)2D are the intestine, the kidneys and the bone. In the intestine, 1,25(OH)2D binds to the VDR to facilitate calcium and phosphorus absorption by active transport. In the kidney, 1,25(OH)2D stimulates the tubular reabsorption of calcium dependent on PTH that increases the production of 1,25(OH)2D from 25(OH)D in the proximal tubule (Holt and Wysolmerski, 2011). In the bone, PTH and 1,25(OH)2D interact to activate the osteoclasts responsible for bone resorption to release calcium and phosphorus into the circulation (Holick, 2006b, 2007). Maintenance of calcium and phosphate homoeostasis is especially important during infancy as this is a period of rapid skeletal growth and mineral accumulation.
1,25(OH)2D is also important in other tissues (Bouillon et al., 2008; EFSA NDA Panel, 2012a; Jones, 2014) that have VDRs as well as the 1α‐hydroxylase to convert 25(OH)D into 1,25(OH)2D (Holick, 2007). Functions of 1,25(OH)2D include cell differentiation and antiproliferative actions in various cell types, such as bone marrow, cells belonging to the immune system, skin, breast and prostate epithelial cells, muscle and intestine (Norman, 2008; Jones, 2014).
Although presenting structural differences, vitamin D2 and D3 trigger an identical set of biological responses in the body, primarily by the regulation of gene expression mediated by the same VDR. None of the steps in the specific vitamin D signal transduction cascade appears to discriminate between vitamin D2 and vitamin D3 at the molecular level (Jones, 2013). Vitamin D2 and D3 are considered biologically equivalent in terms of their ability to cure rickets (Jones, 2013).
1.7.3. Effects of genotypes on metabolism of vitamin D
Some polymorphisms of genes may influence vitamin D metabolism/endogenous synthesis (Berry and Hyppönen, 2011). Several mutations of 24‐hydroxylase (CYP24A1) (Schlingmann et al., 2011; Dinour et al., 2013) and of the renal sodium‐phosphate cotransporter 2A (SLC34A1) (Schlingmann et al., 2016) have been described in patients with idiopathic infantile hypercalcaemia (IIH). Such mutations, in both homozygous or compound heterozygous patients, are associated with hypersensitivity to vitamin D in prophylactic doses (10–12.5 μg/day) or in extremely high‐bolus doses (15 mg repeatedly) and have also been associated with nephrolithiasis and nephrocalcinosis (Jones G et al., 2012; Jones et al., 2017). The frequency of IIH due to CYP24A1 mutations in Poland has been calculated to be 1:32,465 births and the frequency of mutations of CYP24A1 in the Polish population to be 1.11% (Pronicka et al., 2017).
However, as the distribution of all genotypes is not well known and because the impact of the carrier state of one mutation on sensitivity to vitamin D is unclear, the Panel considers that the available data are insufficient to be currently considered in the derivation of a UL of vitamin D for healthy infants.
1.8. Serum 25(OH)D concentration as a biomarker for vitamin D
This section is focused on serum concentration of 25(OH)D. Other biomarkers of either vitamin D intake or status have been discussed (EFSA NDA Panel, 2016): these include free serum 25(OH)D concentration (Powe et al., 2013; Chun et al., 2014; Johnsen et al., 2014), markers of bone formation or turnover (Bonjour et al., 2014), serum concentrations of 1,25(OH)2D and PTH. For the reasons previously outlined (EFSA NDA Panel, 2016), the Panel considers that these other biomarkers cannot be used in the derivation of a UL of vitamin D for infants.
1.8.1. Serum 25(OH)D: relationship with vitamin D intake and baseline 25(OH)D concentration
Serum concentration of 25(OH)D represents total vitamin D from exposure to both UV‐B irradiation (cutaneous synthesis) and dietary sources and is used as a biomarker of dietary vitamin D intake in populations with low exposure to UV‐B irradiation from sunlight (EFSA NDA Panel, 2012a). Oral intake of vitamin D increases serum 25(OH)D concentrations without an effective regulatory mechanism (Holick, 2006a; EFSA NDA Panel, 2012a).
In adults, increasing oral vitamin D intake increases serum 25(OH)D concentration until a plateau is reached after about 6 weeks, which may indicate an equilibrium between the production and degradation of 25(OH)D (Vieth, 1999; Viljakainen et al., 2006; Seamans and Cashman, 2009). A linear relationship was reported between vitamin D intake and serum 25(OH)D concentrations up to a total vitamin D intake of 35 μg/day (Cashman et al., 2011) and 50 μg/day (Cranney et al., 2007). The IOM (2011) found a steeper rise in the serum 25(OH)D concentrations with vitamin D intakes up to 25 μg/day and a slower, more flattened response when the intake was further increased.
In adults and children, a stratified analysis of a meta‐regression model of the serum 25(OH)D response to total vitamin D intake (Section 1.4) was undertaken previously (EFSA NDA Panel, 2016). In this analysis, predicted mean serum 25(OH)D concentrations ranged between 37 and 106 nmol/L in adults (74 arms, 31 studies) and between 43 and 124 nmol/L in children aged 2–17 years (9 arms, 4 studies), at intakes of 5 up to 100 μg/day. No data from infants matching the selected inclusion criteria were available for this meta‐regression analysis (EFSA NDA Panel, 2016).
In infants, serum 25(OH)D concentration increases with vitamin D2 or D3 intake. In 113 full‐term infants, who were randomised to receive 10, 30, or 40 μg/day vitamin D3 from age 2 weeks to 3 months, median baseline serum 25(OH)D concentration of 53 nmol/L increased to mean values of 88, 124 and 153 nmol/L, respectively, at 3 months, with the maximum observed concentrations of 125, 198 and 230 nmol/L (Holmlund‐Suila et al., 2012) (Section 3.3.2.3). In another trial, 132 healthy, term, singleton, breastfed infants were randomly assigned to receive either 10, 20, 30 or 40 μg/day vitamin D3 from 1 to 12 months of age (Gallo et al., 2013a) (Section 3.3.2.2). By 3 months, 55% (95% confidence interval (CI), 38%–72%) of infants in the 10 μg/day group achieved a serum 25(OH)D concentration of 75 nmol/L or greater vs. 81% (95% CI, 65%–91%) in the 20 μg/day group, 92% (95% CI, 77%–98%) in the 30 μg/day group and 100% in the 40 μg/day group. Concentrations of 25(OH)D in serum declined from 3 to 12 months of age in all groups although dietary vitamin D (sources) increased. All dosages maintained serum 25(OH)D concentrations of 50 nmol/L or greater in 97% of infants at 3 months and sustained this in 98% to 12 months.
In healthy, breastfed, infants (n = 52) aged 1 month and who received 10 μg of either D2 or D3 daily, mean (± SD) baseline serum 25(OH)D3 concentrations were 44.2 ± 23.8 (D2) and 54.6 ± 23.7 nmol/L (D3). Mean total serum 25(OH)D concentrations were 64.8 ± 26.2 (D2) and 76.8 ± 17.4 nmol/L (D3) at the 4‐month follow‐up (Gallo et al., 2013b). In the combined groups, the baseline serum 25(OH)D concentration was inversely related to the change in total 25(OH)D (r = ‐0.52; p < 0. 001).
The Panel notes that vitamin D intake in infants increases serum 25(OH)D concentration in a dose‐dependent manner, that the extent of the increase as a result of vitamin D supplementation may depend on baseline serum concentrations of 25(OH)D and that a given intake of vitamin D results in higher serum 25(OH)D concentrations in infants < 6 months of age than in older infants.
1.8.2. Serum 25(OH)D: effect of vitamin D form (D2 or D3)
Several studies have shown that vitamin D2 supplements are less effective in raising or maintaining serum 25(OH)D concentrations compared to vitamin D3 (Jones, 2013; Lehmann et al., 2013; Itkonen et al., 2016). Although vitamins D2 and D3 are not discriminated by the specific vitamin D signal transduction cascade and are considered biologically equivalent in their ability to cure rickets (Section 1.7.2), data suggest that vitamin D3 may be the preferred substrate for hepatic conversion to 25(OH)D (Holmberg et al., 1986; Tripkovic et al., 2012). Data also suggest that vitamin D3 and its metabolites have higher binding affinity to DBP than vitamin D2 (Houghton and Vieth, 2006). Data from toxicity and repletion studies suggest some preferential non‐specific catabolism of vitamin D2 (compared to vitamin D3), accelerating its degradation, especially at ‘high’ doses (Jones, 2013).
Most data comparing the effect of vitamin D2 or D3 on serum 25(OH)D concentration in humans are from studies on adults or children.
Thacher et al. (2010) administered a single oral dose of vitamin D2 or D3 of 1.25 mg to 49 Nigerian children aged 15–120 months, of whom 28 had calcium‐deficiency rickets and 21 were healthy controls. Total serum 25(OH)D concentration peaked on day 3 and the incremental change was similar with vitamins D2 and D3 in children with rickets (72.5 ± 42.5 and 62.5 ± 27.5 nmol/L, respectively) and in control children (82.5 ± 32.5 and 77.5 ± 40 nmol/L, respectively). The disappearance rate of total serum 25(OH)D after day 3 was similar with vitamin D2 and D3 in control children. In a linear regression analysis with the serum 25(OH)D concentration on day 3 as a covariate, the decline in 25(OH)D concentrations on days 7 and 14 was significantly greater (p < 0.001) in the vitamin D2 group than the vitamin D3 group among children with rickets, consistent with a more rapid clearance of 25(OH)D2 than 25(OH)D3.
In a systematic review and meta‐analysis comparing supplementation studies in adults with vitamin D2 and D3 (Tripkovic et al., 2012), even though bolus doses of vitamin D3 (> 125 μg/day) were more effective for raising total serum 25(OH)D concentration compared with vitamin D2 doses (three studies out of four), the differences between the two forms of vitamin D supplements disappeared when given as lower daily doses (six studies). In a 12‐week randomised controlled trial (RCT) in healthy South Asian and white European women, both vitamin D3 and D2 were effective in raising serum 25(OH)D concentrations at doses of 15 μg/day during wintertime, albeit vitamin D3 was more effective than vitamin D2 (Tripkovic et al., 2017).
Data comparing the effect of vitamin D2 and vitamin D3 on serum 25(OH)D concentration in infants are scarce. In breastfed infants receiving a daily supplement of 10 μg vitamin D2 or vitamin D3 for 3 months, the change in serum 25(OH)D did not differ significantly (Gallo et al., 2013b) (Section 1.8.1). Another study on infants and young children with hypovitaminosis D (serum 25(OH)D concentration < 50 nmol/L) who were treated orally with either 50 μg/day of vitamin D2 (n = 15) or 50 μg/day of vitamin D3 for 6 weeks (n = 15), showed no statistically significant difference between the effects of vitamin D2 and vitamin D3 on the change in serum 25(OH)D concentration (from 39.3 to 109.8 nmol/L with vitamin D2 vs. from 34.3 to 103 nmol/L with vitamin D3; 7% difference in effect) (Gordon et al., 2008) (Section 3.3.3.5). The Panel notes the small sample size of these studies.
The Panel notes the limited data available on infants, which do not allow a conclusion to be drawn on whether there is a difference between vitamin D2 and D3 as regards their impact on serum 25(OH)D concentrations in infants. However, in view of the results comparing the effect of vitamin D2 and vitamin D3 on serum 25(OH)D concentration in adults, the Panel considers that, if enough data are available, the form of vitamin D (D2 or D3 or ‘unspecified’) should be considered in the dose–response analysis between vitamin D intake and serum 25(OH)D concentration of infants for the assessment of an UL for vitamin D (Section 3.5 and Annex A).
1.8.3. Serum 25(OH)D: effect of frequency of vitamin D administration
Owing to its slow turnover in the body (half‐life of about 2 months) (Jones, 2008), vitamin D is often administered as single or repeated single high doses at varying intervals or weekly in equivalent doses instead of daily. Depending on the vitamin D dose and the duration of supplementation in adults, resulting serum 25(OH)D concentrations ‘may be comparable or somewhat lower with weekly compared to daily supplementation’, respectively (EFSA NDA Panel, 2012a).
Several studies describe the treatment of infants and young children with or without rickets with single (Cesur et al., 2003; Billoo et al., 2009; Mittal et al., 2014; Harnot et al., 2017) or repeated single high‐oral doses (Pietrek et al., 1976; Markestad et al., 1987; Zeghoud et al., 1994; Shajari et al., 2009; Shakiba et al., 2010, 2014; Manaseki‐Holland et al., 2012; Huynh et al., 2017) of vitamin D up to 15 mg. In some of these studies, single high‐oral doses were compared to daily oral supplementation of vitamin D at doses between 5 and 10 μg (Shajari et al., 2009; Shakiba et al., 2010, 2014; Huynh et al., 2017). The Panel notes that high doses (typically single or repeated high‐dose oral bolus) were sometimes associated with ‘high’ serum 25(OH)D concentrations, up to > 300 nmol/L (Markestad et al., 1987). The Panel considers that these studies also demonstrate the different kinetics of an oral high‐bolus dose with a more rapid peak in serum 25(OH)D concentration after 2–3 weeks, which makes it difficult to use these data to draw conclusions about regular daily intakes required to obtain desirable serum 25(OH)D concentrations or 25(OH)D concentrations that may be associated with adverse effects. Studies testing weekly vs. daily administration in infants are scarce (Section 3.4.1 and Appendix L).
The Panel considers that, for setting the UL for vitamin D for infants, data from studies using infrequent high doses cannot be used and decided to use only studies considering daily doses.
1.8.4. Serum 25(OH)D: other factors influencing its concentration
Besides vitamin D intake, form and frequency of consumption, baseline serum 25(OH)D concentration and sun exposure, concentrations of 25(OH)D in serum vary according to season, with the lowest concentrations occurring at the end of winter and the highest concentrations in summer (Hintzpeter et al., 2008). This generally reflects the magnitude of endogenous synthesis of vitamin D following UV‐B irradiation (Section 1.7.1.1).
In infants, serum 25(OH)D concentrations follow a cyclical pattern during the first year of life, corresponding to the pattern of sun exposure (Specker et al., 1985; Specker and Tsang, 1987; Mimouni and Shamir, 2009; Kiely et al., 2017). Seasonal variation in serum 25(OH)D concentrations in breastfed infants depends on the time spent outdoors and on the area of skin exposed to sunlight. According to Specker et al. (1985), in Cincinnati, a weekly exposure of 2 hours or 20 minutes of a fully dressed (without a hat) or partially dressed infant (diaper), respectively, is sufficient for maintaining serum 25(OH)D concentration above 27.5 nmol/L. In 120 breastfeeding mother–infant pairs of different ethnicity in the US (Dawodu et al., 2014), lowest quartile of sun exposure time,17 low percentage of sun exposed body surface area (%BSA) exposure (p = 0.04) and low sun index18 (p < 0.001) were significantly associated with higher prevalence of serum 25(OH)D concentration19 < 50 nmol/L in the infants. In another prospective study on breastfeeding mother–infant pairs in the US (n = 119), China (n = 112) and Mexico (n = 113) (Dawodu et al., 2015), data on behaviour related to sunlight exposure of mothers and infants (% BSA and sun index) were collected at 4, 13, 26, 52 and 108 weeks post‐partum through questionnaires. Serum 25(OH)D concentration was measured in a subset of infants at 26 weeks of age during fall and winter. Across the three study sites, serum 25(OH)D concentration in infants was significantly associated with sun index. Cumulative sun index correlated positively with serum 25(OH)D concentration at 6 months of age in an infant population in Delhi, and a requirement of at least 30 min weekly exposure to afternoon sunlight over 40% of body area (infant clothed in diaper) for at least 16 weeks, was estimated to achieve serum 25(OH)D concentrations > 50 nmol/L (Meena et al., 2017).
Body fat impacts serum 25(OH)D concentration in adults (Saneei et al., 2013; Vanlint, 2013) and in infants (Hazell et al., 2014). Whether the age‐dependent decline in serum 25(OH)D concentrations observed from 3 to 12 months of age in the study by Gallo et al. (2013a) (Sections 1.8.1 and 3.3.2.2) was related to the increasing body mass of the infants remained unclear. In a prospective observational cohort study, infants were investigated with regard to vitamin D intake (diet and supplements), anthropometry and 25(OH)D concentrations in serum at the age of 6 months (n = 134) and 12 months (n = 98, 27% attrition) (Pludowski et al., 2011) (Section 3.3.6). Mean (± SD) daily vitamin D intakes at 6 and 12 months of age did not differ (26.6 ± 17.4 and 23.5 ± 15.5 μg). However, vitamin D intake expressed per kg body weight significantly decreased from 3.5 μg at 6 months to 2.3 μg at 12 months (p < 0.0001), which was associated with a decline in mean serum 25(OH)D concentration from 107.5 to 72.5 nmol/L. The Panel notes that, at a given vitamin D intake, body mass or age may be determinants of 25(OH)D concentrations in serum.
The foetus cannot synthesise 25(OH)D and therefore relies on placental transfer (Section 1.7.1.4). Several observational studies have reported positive correlations between maternal serum and cord blood 25(OH)D concentrations, with coefficients between 0.42 and 0.95 (Nicolaidou et al., 2006; Bodnar et al., 2007; Josefson et al., 2013; Godang et al., 2014; Saraf et al., 2016; Wegienka et al., 2016). Thus, factors which influence maternal serum 25(OH)D concentration, including maternal intake, adiposity, skin pigmentation, covering and season, have an impact on the serum 25(OH)D concentration of the newborn. Most of the studies mentioned above also report that serum 25(OH)D concentrations are lower in the infant than in the mother. However, these studies were performed in populations where maternal vitamin D status (serum 25(OH)D concentration) was in the low or normal range. The impact of ‘high’ maternal vitamin D intake on neonatal vitamin D status was investigated in an RCT of vitamin D supplementation during pregnancy conducted in the USA (Hollis et al., 2011). Women with a singleton pregnancy (n = 502) received 10, 50 or 100 μg of vitamin D3 per day from 12 to 16 weeks’ gestation until delivery. Neonatal 25(OH)D concentration significantly correlated with maternal serum 25(OH)D concentration overall, 1 month prior to delivery, and at delivery (r2 = 0.6). Mean (± SD) neonatal concentration significantly differed by intervention group: 45.5 ± 25.3 nmol/L, 57.0 +/−24.5 nmol/L and 66.3 +/−25.8 nmol/L in the 10, 50 and 100 μg groups, respectively (p < 0.0001). The highest 25(OH)D concentration reported in neonates from the 100 μg group was 130 nmol/L. A systematic review (with meta‐analysis) of RCTs (Bi et al., 2018)20 showed that maternal vitamin D supplementation during pregnancy (compared with control group) led to significantly higher 25(OH)D concentrations (14 RCTs with 2,361 participants) and higher calcium concentrations in the neonates (nine RCTs with 1,007 participants).
Studies have investigated the potential of vitamin D supplementation of breastfeeding mothers to raise serum 25(OH)D concentrations of their infants. Results show that maternal doses of 10–25 μg/day have little or no effect on serum 25(OH)D concentrations of the breastfed infant (Greer et al., 1982; Rothberg et al., 1982; Ala‐Houhala, 1985; Ala‐Houhala et al., 1988; Hollis and Wagner, 2004). Results also show that maternal supplementation of at least 50 μg/day is required to provide a significant amount of vitamin D in the breast milk for breastfeeding infants (Ala‐Houhala et al., 1988; Saadi et al., 2009). In other studies, a maternal dose of 100 μg/day increased the mean infant 25(OH)D concentration to 75 nmol/L, which was exceeded in all infants with a maternal dose of 160 μg/day (Hollis and Wagner, 2004; Basile et al., 2006; Wagner et al., 2006). In an RCT (Hollis et al., 2015), exclusively lactating women in Charleston, South Carolina, or Rochester, New York, at 4–6 weeks postpartum, were allocated to either 10, 60 or 160 μg vitamin D3/day for 6 months. Only the infants of the women in the 10 μg group received oral 10 μg vitamin D3/day while the infants in the 60 and 160 μg groups received a placebo. Supplementation of mothers only with 60 μg/day was insufficient to prevent serum 25(OH)D concentrations < 50 nmol/L in the infants. After 4 months, the mean (± SD) serum 25(OH)D concentration of infants supplemented with 10 μg/day (109 ± 48 nmol/L, n = 74) did not differ from those infants whose mothers received 160 μg/day (106.9 ± 35.1 nmol/L, n = 74). Adverse events among mothers or infants did not differ by group and were deemed not to be related to vitamin D dose. The Panel notes that maternal supplementation with vitamin D3 of 160 μg/day during lactation raised serum 25(OH)D of infants no more than supplementation of the infants with 10 μg/day.
Besides vitamin D intake, form and frequency of consumption, baseline serum 25(OH)D concentration and sun exposure, the Panel notes that other factors influencing serum 25(OH)D concentration in infants are season, body mass (or age as a proxy) and body fat, maternal characteristics during pregnancy and lactation, in particular maternal supplementation with vitamin D.
1.8.5. Serum 25(OH)D target concentration
The Panel considered a serum 25(OH)D concentration of 50 nmol/L as a suitable target value for all age and sex groups including infants and set an AI for infants at 10 μg/day vitamin D (EFSA NDA Panel, 2016).
1.8.6. Analytical methods for measuring serum 25(OH)D
1.8.6.1. Analytical methods for measuring serum 25(OH)D: general considerations
General considerations on analytical methods for the measurement of 25(OH)D concentration in serum have been discussed previously (EFSA NDA Panel, 2016). Among the numerous methods for this measurement, liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) and high‐performance liquid chromatography (HPLC) methods are considered the gold standard (Wallace et al., 2010; Carter, 2011). These methods have the advantage that they can measure 25(OH)D3 and 25(OH)D2 separately (Tai et al., 2010; Carter, 2011). Formerly, all methods suffered from the lack of an international common standard, which contributed to the variability of results of serum 25(OH)D measurements. The Vitamin D External Quality Assessment Scheme (DEQAS) has revealed considerable differences between methods (both within and between laboratories), raising concerns about the comparability and accuracy of different assays and laboratories (Snellman et al., 2010; Carter, 2011; Farrell et al., 2012; Heijboer et al., 2012). The introduction of a standard reference material for vitamin D in human serum by the US National Institute of Standards and Technology (NIST) has been a step forward in providing a reference measurement procedure against which assays could be standardised (Carter, 2012). The Vitamin D Standardization Program (VDSP) has developed protocols for standardising procedures of 25(OH)D measurement in national health/nutrition surveys to promote 25(OH)D measurements that are accurate and comparable over time, location and laboratory, to improve the data basis for public health measures (Cashman et al., 2013).
1.8.6.2. Analytical methods for measuring serum 25(OH)D: specificities in infants
When comparing two commercially available immunoassays for measuring serum 25(OH)D concentration in infants against LC‐MS/MS, results (mean ± SD) obtained with both immunoassays (46.9 ± 17.1 nmol/L with radioimmunoassay (RIA) and 75.5 ± 29.3 nmol/L with an enzyme immune assay (EIA)) were significantly different from those analysed with LC‐MS/MS (58.9 ± 21.6 nmol/L) (Gallo et al., 2014a).
The C3‐epimer of 25(OH)D (Section 1.7.1.3) may interfere with the determination of 25(OH)D, especially in HPLC and LC‐MS/MS assays. LC‐MS/MS is dependent on monitoring mass transitions and LC‐MS/MS methods are unable to differentiate coeluting isomeric compounds with identical elemental composition but different structure. This has led to the overestimation of serum 25(OH)D concentrations. Thus, LC columns capable of providing sufficient chromatographic selectivity prior to MS identification must be employed (Bailey et al., 2013). As this problem has been recognised, many LC‐MS/MS laboratories now apply this latter approach (e.g. (Singh et al., 2006; Gallo et al., 2014a; Kiely et al., 2017)).
The C3‐epimer may not interfere with the determination of serum 25(OH)D concentration in most, but not all, immunoassays (Bailey et al., 2013), but there may be other metabolites that do so, as reported by Gallo et al. (2014a).
1.8.6.3. Analytical methods for measuring serum 25(OH)D: conclusions
The Panel considers that all analytical methods available to measure serum 25(OH)D concentration in infants have uncertainties. The Panel considers that, if enough data are available, the analytical method used to measure serum 25(OH)D concentration should be considered in the dose–response analysis between vitamin D intake and serum 25(OH)D concentration for the assessment of a UL for vitamin D in infants (Section 3.5 and Annex A).
The Panel notes that the concentration of 3‐epi‐25(OH)D should be considered when interpreting serum 25(OH)D concentrations of young infants in the first 3 months of life: otherwise, the vitamin D status may be erroneously classified as sufficient due to a high proportion of 3‐epi‐25(OH)D in total 25(OH)D determination (Singh et al., 2006). Older LC‐MS/MS methods may have overestimated serum 25(OH)D concentration in the first 3 months of life because they were also measuring the C3‐epimer, whereas newer LC‐MS/MS methods have accounted for this. Some immunoassay methods do not detect the C3‐epimer while others do.
1.8.7. Conclusion on serum 25(OH)D concentration as a biomarker for vitamin D
The Panel considers that serum 25(OH)D concentration is an accepted biomarker of vitamin D intake in populations with low exposure to UV‐B irradiation and of vitamin D status at population level (EFSA NDA Panel, 2016). Serum 25(OH)D concentration in infants can be influenced by vitamin D intake, form and frequency of consumption, baseline serum 25(OH)D concentration, sun exposure, season, body mass (or age) and body fat, maternal characteristics during pregnancy and lactation, in particular maternal supplementation with vitamin D. Factors such as the form of vitamin D (D2 or D3) or the analytical method should be considered in the dose–response analysis between vitamin D intake and serum 25(OH)D concentration for the assessment of an UL for vitamin D in infants if enough data are available (Sections 3.5 and 7 and Annex A).
1.9. Proposed mechanisms of toxicity of vitamin D
Mechanisms of toxicity of vitamin D were summarised previously (EFSA NDA Panel, 2012a; SACN, 2016).
Various studies in adults and children indicate that ingestion of large doses of vitamin D (e.g. 125–1,000 μg/day over a period of at least 1 month results in an increase in the serum concentration of 25(OH)D, while that of the active metabolite 1,25(OH)2D is unchanged (Jones, 2008) or even reduced (IOM, 2011).
Some hypotheses for mechanisms of toxicity have been proposed. The presence of elevated serum‐free 1,25(OH)2D concentration despite normal total 1,25(OH)2D suggests that, in states of vitamin D toxicity, 1,25(OH)2D is displaced from DBP by 25(OH)D or other metabolites, and that this could promote entry of free 1,25(OH)2D into target cells where it could stimulate gene transcription (Pettifor et al., 1995; SACN, 2016). It has also been suggested that serum 25(OH)D concentrations exceed DBP binding capacity; thus, free 25(OH)D enters the cell and has direct effects on gene expression (SACN, 2016).
As mentioned before (Section 1.7.2), the principal function of 1,25(OH)2D is to maintain calcium and phosphorus homoeostasis. Under normal conditions, serum calcium is tightly regulated. Serum calcium concentrations are detected by surface calcium‐sensing receptors found in parathyroid and the clear cells of the thyroid glands, kidney, intestine, bone marrow and other tissues. ‘Long‐term’ ingestion of ‘large’ doses of vitamin D results in disturbance of calcium homoeostasis.
Excessive vitamin D intake leading to ‘very high’ serum 25(OH)D concentrations may lead to hypercalcaemia (Vieth, 1990; Pettifor et al., 1995; Holick, 2006a). An epidemic of infantile hypercalcaemia was observed in the UK in the 1950s when foods including those for infants provided up to 100 μg/day. The reported incidence of hypercalcaemia in infants declined when vitamin D content in these foods was reduced (Fraser, 1967; SCF, 2003). An increase in serum calcium concentration inhibits PTH release and stimulates calcitonin release, which, in infants and children, is a principal defence against hypercalcaemia (Weaver and Heaney, 2014). The clinical manifestations associated with hypercalcaemia can include fatigue, muscular weakness, anorexia, nausea, vomiting, constipation, tachyarrhythmia; soft tissue calcification; failure to thrive; polyuria, osteolysis, dehydration and weight loss (Debre, 1948). A study in the 1930s suggested retarded linear growth in infants who received 45–113 μg vitamin D daily (Jeans and Stearns, 1938) (Section 1.4). Soft tissue calcification due to excessive intake of vitamin D and associated hypercalcaemia is found especially in kidneys, the heart and arterial tissues with subsequent effects on function (Peng and Taylor, 1980).
Kidney function is affected because high concentrations of calcium (in the serum then filtered in the urine) alter the antidiuretic action of vasopressin on the renal tubules. The net result is reduced urinary concentration ability which usually presents as polyuria. Hypercalciuria is one of the earliest signs of vitamin D toxicity and precedes the occurrence of hypercalcaemia. The initial hypercalciuria may be ameliorated as renal failure progresses because of reduced calcium clearance. When reduced renal blood flow occurs, less calcium is presented to the renal glomerulus and hypercalcaemia can rapidly progress (Cusano et al., 2011).
Consequences of sustained hypercalcaemia and hypercalciuria are nephrolithiasis (kidney stones), nephrocalcinosis and a decrease in kidney function (Ronnefarth and Misselwitz, 2000; EFSA NDA Panel, 2012a,b). Mortality from hypercalcaemia itself is rare.
The Panel notes that vitamin D toxicity resulting from ‘long‐term’ ingestion of ‘large’ doses of vitamin D is associated with an increase in the serum concentration of 25(OH)D and some other vitamin D metabolites but usually not of the active metabolite 1,25(OH)2D. Some hypotheses for mechanisms of toxicity have been proposed. The presence of elevated serum‐free 1,25(OH)2D concentration despite normal total 1,25(OH)2D suggests that, in states of vitamin D toxicity, 1,25(OH)2D is displaced from DBP by 25(OH)D or other metabolites, and that this could promote entry of free 1,25(OH)2D into target cells where it could stimulate gene transcription. Disturbances of calcium homoeostasis are also observed.
1.10. Hazard identification: adverse health outcomes and endpoints associated with excessive vitamin D intake in infants
The most suitable adverse health outcomes and endpoints regarding hypervitaminosis D in infants were identified and selected by the Panel, considering those listed in previous assessments on ULs (SCF, 2003; IOM, 2011; EFSA NDA Panel, 2012a; COT, 2015) (Section 1.4) and based on Section 1.9 and expert knowledge.
The Panel focussed on the following four outcomes to inform the setting of a UL for vitamin D in infants: hypercalciuria, hypercalcaemia, ectopic calcification, e.g. nephrocalcinosis, and abnormal growth patterns.
Taking into account the previous assessment for the setting of AIs for vitamin D (EFSA NDA Panel, 2016) (Section 1.4), which was derived in particular from the dose–response relationship between total vitamin D intake and serum 25(OH)D concentration, the Panel also focussed on serum 25(OH)D concentration to inform the setting of a UL. Excessive vitamin D intakes are reflected by increasing serum 25(OH)D concentrations that are associated with an increased risk for the above‐mentioned adverse health outcomes. Increased serum 25(OH)D concentrations may therefore indicate a hazardous vitamin D intake before this results in clinically manifest adverse health outcomes.
Other possible signs/symptoms of hypervitaminosis D may be, e.g. gastrointestinal symptoms (anorexia, vomiting, abdominal pain, colic, constipation), polyuria and neurologic symptoms (loss of consciousness), but they are not specific to hypervitaminosis D. The Panel did not consider them among the primary outcomes and endpoints for this assessment.
The Panel selected the following adverse health outcomes and endpoints for this scientific opinion on infants: hypercalciuria/urinary calcium, hypercalcaemia/serum calcium, ectopic calcification, e.g. nephrocalcinosis, abnormal growth/anthropometric parameters, serum 25(OH)D concentration.
2. Data and methodologies
The following guidance documents of EFSA and SCF were taken into account by the Panel:
-
–
Guidelines of the Scientific Committee on Food for the development of ULs for vitamins and minerals (SCF, 2000);
-
–
Guidance of EFSA Scientific Committee on the risk assessment of substances present in food intended for infants below 16 weeks of age (EFSA Scientific Committee et al., 2017b);
-
–
Guidance of EFSA Scientific Committee on the use of the benchmark dose approach in risk assessment (EFSA Scientific Committee et al., 2009);
-
–
Updated guidance on the use of the benchmark dose approach in risk assessment (EFSA Scientific Committee et al., 2017a);
-
–
Guidance of EFSA on the application of systematic review methodology to food and feed safety assessments to support decision making (EFSA, 2010).
As mentioned in Section 1.3, the Panel undertook a systematic literature review. The approach for this review is described in Sections 3.1 and 3.2 and the results of the identified papers are described in Sections 3.3 and 3.5 (quantitative dose–response analysis).
The Panel undertook an assessment of the intake of vitamin D by infants, based on data available in EFSA (Section 4).
With the objective of comparing the outcome of the intake assessment undertaken by EFSA (Section 4) with other available data, data were collected either from published studies or reports from national authorities, or through a consultation of Member States (MS) via e‐mail undertaken by the European Commission.21 From this search, data or contributions discussing aspects within the remit of this scientific opinion (e.g. ‘high’ vitamin D intake in infants, composition data of infant foods) are presented in Sections 4.1.1 and 4.2.1.
3. Assessment of adverse effects of excess intake of vitamin D in infants (hazard characterisation)
The strategy and methodology for the assessment of possible relationships between vitamin D intake and adverse health outcomes in infants, including dose–response relationship(s) between vitamin D intake and biomarker(s) where applicable, was defined upfront by the Panel. The steps described below were applied in line with this definition.
3.1. Daily vitamin D intake and adverse health outcomes in infants: EFSA's extensive literature search
3.1.1. Literature search: sources and forms of vitamin D
In line with the remit of this assessment, the systematic literature search dealt with daily vitamin D intake (i.e. vitamin D2 or D3) from all dietary sources, including fortified foods and dietary supplements. The Panel excluded data on administration modes other than the oral route.
3.1.2. Literature search: target population
In line with the remit of this assessment, the literature search dealt with full‐term22 healthy infants, up to 12 months of age, either breastfed or fed orally with a standard IF or FoF, and possibly receiving complementary foods.
For this assessment, the Panel decided not to set specific ULs for subgroups of the infant population on the basis of, e.g. ethnicity or environmental conditions (e.g. higher or lower exposure to the sun).
3.1.3. Literature search: eligibility criteria
Eligibility criteria for this assessment include criteria related to the characteristics of the literature (report characteristics) and criteria related to study characteristics and study population.
English, German and French were the selected languages and no limit on publication date was applied to the search. The criterion related to language derives from a mapping of the 34 different languages of the literature search in Embase using the proposed search strings (Appendix A). English was by far the most frequent language (4,450 hits), followed by a similar number of hits in French and German (162 and 161 hits, respectively). The choice of these three languages is a proposed trade‐off between the aim for completeness and time constraints.
Primary research papers published in scientific journals were selected. Thus, other publication types were excluded: expert opinions, editorials, position statements, guidelines, articles from the popular media, abstracts, conference proceedings, protocols, PhD theses, letters to the editor, narrative or systematic reviews.
Experimental and observational studies (retrospective or prospective cohort or case–control) in humans were selected by the Panel. Detailed information on the initial eligibility criteria on study characteristics and study populations is available in Appendix B. In particular, preclinical studies (animals, in vitro, in silico data) can produce valuable knowledge, e.g. on mechanisms, but extrapolation of these data to infants is subject to uncertainties; thus, they were excluded. In addition, the Panel focussed on oral daily vitamin D consumption (alone or not), and, for trials, doses above 10 μg/day, which corresponds to the AI for vitamin D for infants (EFSA NDA Panel, 2016). Both vitamin D forms (vitamin D2 and vitamin D3) were considered as well as studies on ‘vitamin D’ (unspecified), but studies/arms on vitamin D metabolites were excluded. Studies/arms using bolus/weekly/monthly doses, and case reports, were excluded from the further steps of the literature search, but identified separately for possible qualitative consideration in the scientific opinion (Section 3.4). No specific eligibility criterion on assessment methods, e.g. method to measure vitamin D intake or serum 25(OH)D concentration, was applied. Even though carefully collected 24 h urine would be the preferred method for the measurement of calcium excretion (Section 3.3.2), this is difficult in infants; therefore, other types of urine collection (e.g. spot urine) were considered. Based on previous work (Brouwer‐Brolsma et al., 2016; EFSA NDA Panel, 2016), no specific eligibility criterion on latitude or country was applied.
Study populations composed only of ill infants were excluded from this assessment because the relationship between vitamin D intake and health outcomes in these infants may be affected by the disease and/or medication used. An exception to this is infants with vitamin D deficiency rickets undergoing treatment, possibly receiving high vitamin D doses: it was initially considered that these studies might provide useful information for setting a UL for healthy infants. However, after a preliminary statistical analysis of the selected studies as described in Section 3.5, an amendment to the predefined methodology was agreed and the two papers (Seino et al., 1981; Emel et al., 2012) that comprised subjects with vitamin D deficiency rickets were excluded by the Panel.
3.1.4. Literature search: bibliographic databases and search strategies
Three databases were searched: Embase, Pubmed and Cochrane.23 Embase and PubMed were considered as the leading databases for biomedical sciences, Cochrane Library is a source for systematic reviews and RCTs of human health care interventions. These databases were identified in line with the defined scope of the review, based also on the EFSA inventory of information sources,24 and in line with the approach of the comprehensive literature search provided by an external contractor to EFSA as supporting evidence for a previous assessment on AIs for vitamin D (Brouwer‐Brolsma et al., 2016).
Specific search strategies were created by the information specialist of EFSA. Date of search per database, search strategies and related number of hits are indicated in Appendix C.
3.1.5. Literature search: study selection
Inclusion of papers in the assessment was undertaken through a title and abstract then full text screening, based on the identified eligibility criteria (Section 3.1.3 and Appendix B).
At title and abstract screening, all papers proposed for inclusion by a first reviewer went to full text screening directly. However, all those proposed for exclusion by the first reviewer required the agreement of a second reviewer for exclusion. In case of doubt, papers were moved to full‐text screening.
Full‐text screening was done in duplicate: if two reviewers agreed to exclude a paper, it was excluded from the further steps; if two reviewers agreed to include a paper, it was included for the further steps; possible divergences between the reviewers or doubts were discussed with the experts.
During the screening phase, reviewers took note of the availability of data related to information on the mothers of the infants investigated (e.g. supplementation in pregnancy, level of education, parity), sun exposure, season of birth or of study or skin type. This was done for possible further qualitative discussion, as the Panel expected the number of studies mentioning these aspects to be limited (thus, for statistical reasons, these parameters could not be considered as covariates at the step of the quantitative analysis).
3.1.6. Literature search: included studies after the study selection process
Among the 25 papers finally included, some contained two or three studies, yielding 31 studies to go through data extraction and appraisal. The flow chart in Appendix E reports the results of the different phases of the study selection process.
3.1.7. Data extraction and check of the dataset
The list of parameters to be extracted was predefined (Appendix D). Data expressed only in graphs/curves/figures were identified as such. Numerical outcomes were extracted as expressed in the papers, e.g. absolute values or changes, percentage changes between time points, and with the unit used in the papers. The data set was created for all time points investigated in the studies selected after screening and was checked by a different reviewer to correct mistakes.
3.1.8. Transformation of numerical values after data extraction
EFSA applied a series of transformations to the data in order to:
-
–
Harmonise the measurement unit used in the various studies,
-
○
age of infants and duration of supplementation were converted into weeks,25
-
○
values for the intake of vitamin D were converted into μg (1 IU = 0.025 μg),
-
○
length/height measurements were converted to cm,
-
○
for calcium, the molecular mass of 40.1 g/mol was considered,
-
○
serum concentrations of 25(OH)D were converted to nmol/L (1 ng/mL = 2.5 nmol/L).
-
○
-
–
Transform changes from baseline to absolute values.
-
–
Convert values of body length, weight, head circumference expressed in z‐scores according to the growth standards of WHO Multicentre Growth Reference Study Group (2006) back to the original values. As these WHO standards are age‐ and sex‐specific, the gender‐specific values after transformation were averaged assuming an equal proportion of both sexes.
-
–
Transform mean and standard deviation into a ln‐transformed scale where appropriate. A moment‐based approach as described by Higgins et al. (2008) was used.
-
–
Average data when given separately by gender (Ziegler et al., 2014).
When cut‐off values for hypercalciuria are discussed (Section 3.3.2), for ease of reading, ratios of urinary calcium/creatinine were converted to mmol/mmol by using the respective molecular masses of 40 and 113 g/mol.
3.2. Daily vitamin D intake and adverse health outcomes in infants: appraisal of the included studies
3.2.1. Appraisal: risk of bias tool and methodology to assess the internal and external validity
The methodology for the assessment of the internal26 and external27 validity of the selected papers was defined upfront. For the appraisal of internal validity, the risk of bias (RoB) of each individual included study was appraised using the Office of Health Assessment and Translation/National Toxicology Program (OHAT/NTP) RoB tool.28 The use of the tool was limited to the aspects relevant to clinical trials (randomised and not, controlled and not) and prospective observational studies (cohort and case–control) in humans. The tool was structured in six bias domains (selection, confounding, performance, attrition/exclusion, detection and selective reporting), plus ‘other sources of bias’ (e.g. inappropriate statistical methods) (Appendix F).
As a trade‐off between methodological requirements and time constraints, judgements were made by applying a two‐level rating scale:
-
–
low RoB: there was direct or indirect evidence of low RoB practices OR it was deemed that deviations from low RoB practices for these criteria during the study would not appreciably bias results
-
–
high RoB: there was direct or indirect evidence of high RoB practices OR there was insufficient information provided on the study conduct in order to exclude high RoB practices.
Although the indication of the expected direction of the bias is useful (i.e. the true effect is underestimated/overestimated/biased in both directions), this was not deemed mandatory for this assessment.
For each paper selected, the appraisal of internal validity was done at outcome level for the RoB that are outcome‐dependent, because, for the same study, the design and conduct may affect the risk of bias differently depending on the outcomes measured. If the available information was unclear (e.g. simple mention of randomisation without further details), the more conservative judgement was selected (i.e. high RoB). Selected papers in English and German were appraised in duplicate,29 and possible discrepancies were discussed collegially. The results of the appraisal of the internal validity per bias domain, selected paper and health outcome/endpoint are presented in Appendices Appendix G – Synthesis of the risk of bias by individual studies investigating hypercalciuria/urinary calcium, Appendix H – Synthesis of the risk of bias by individual studies investigating hypercalcaemia/serum calcium, Appendix I – Synthesis of the risk of bias by individual studies investigating nephrocalcinosis, Appendix J – Synthesis of the risk of bias by individual studies investigating anthropometric parameters–K.
After pilot testing of the tool, the Panel decided to appraise the RoB related to external validity on the overall body of evidence (and not per paper) (Section 3.3.1).
3.2.2. Appraisal: definition of the risk of bias tiers per outcome
An overall judgement on the RoB was reached, resulting in each paper being allocated to a tier of RoB by health outcome/endpoint. This information can be read vertically, i.e. for each paper, in the tables in Appendices Appendix G – Synthesis of the risk of bias by individual studies investigating hypercalciuria/urinary calcium, Appendix H – Synthesis of the risk of bias by individual studies investigating hypercalcaemia/serum calcium, Appendix I – Synthesis of the risk of bias by individual studies investigating nephrocalcinosis, Appendix J – Synthesis of the risk of bias by individual studies investigating anthropometric parameters–K. This was based first on the definition of three key domains: randomisation (for trials) or confounding factors (for observational studies), exposure characterisation (assessment of vitamin D intake), outcome assessment. Then, for each outcome, each study was assigned to a tier of RoB defined as follows:
-
–
Tier 1 (low overall RoB): no high RoB in key domains and at least 50% domains with low RoB.
-
–
Tier 2 (medium overall RoB): one or more high RoB in key domains and at least 50% domains with Low RoB.
-
–
Tier 3 (high overall RoB): one or more high RoB in key domains and less 50% domains with Low RoB.
This means that the same study could be in different tiers of RoB according to the health outcome/endpoint considered: for example, the paper by Gallo et al. (2013a) was included in tier 1 of RoB for hypercalciuria/urinary calcium, or hypercalcaemia/serum calcium, and tier 2 for serum 25(OH)D concentration.
3.2.3. Appraisal: potential confounders and modifiers
The Panel identified a priori a preliminary list of potential confounders30 and/or modifiers31 that could affect the relationship between vitamin D intake and health outcomes (Figure 1). Such a list was useful for the appraisal as well as for discussion of potential covariates for the statistical analysis (Section 3.5 and Annex A).
Figure 1.
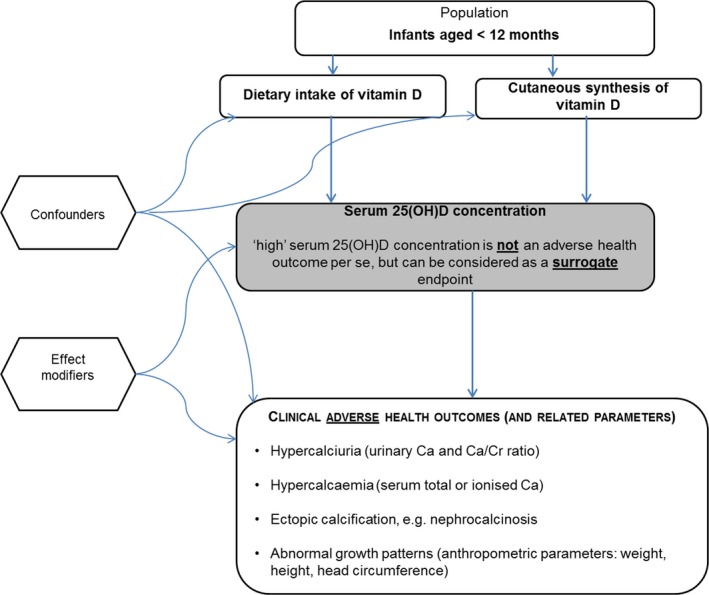
Confounders and modifiers
For this assessment:
Maternal characteristics could be considered as confounders for all outcomes of interest, e.g. level of education, income, age, adolescent vs. adult mothers.
Type of feeding of the infants, frequency of vitamin D supplementation and health status of the infants (e.g. healthy or infants with vitamin D deficiency rickets under treatment) could be considered as confounders or modifiers (on a case‐by‐case basis), for all outcomes of interest.
Genotype was considered as a possible modifier for all outcomes of interest (not reported in any of the selected papers).
Calcium intake/supplementation of the infants was considered as a possible modifier in particular for serum calcium/urinary calcium of the infants.
For serum 25(OH)D concentration of the infants, the list of possible modifiers included vitamin D exposure of the infants before the start of the study; season of study; season of birth; sunlight exposure of the infant (or time spent outdoors); latitude (as a proxy for sun exposure); skin type (e.g. ethnicity, skin sensitivity, Fitzpatrick categorisation); baseline serum 25(OH)D concentration at the start of the study (for trials); characteristics of the mother, e.g. supplementation of vitamin D during pregnancy, health or vitamin D status or dietary intakes during pregnancy, sun exposure of the mothers, sunlight‐related behaviour (e.g. use of sunscreen, time spent outdoors etc.). Age of the infants was initially not considered as a confounder/modifier for the blood/urinary parameters; however, after discussing the available evidence (Sections 1.8.4 and 3.5 and Annex A), the influence of body mass, which was highly correlated to age, was investigated for serum 25(OH)D concentration (and finally, age was considered in the dose–response analysis).
For abnormal growth and anthropometric parameters, the list included length and head circumference at birth (in case of preterm infants) (modifier), height of the parents (in relation to height of the child) (modifier), for retrospective cohort studies (if any) infections/health status (e.g. healthy or with vitamin D deficiency rickets) (modifier), maternal smoking (modifier), the sex of the infants (confounder or modifier on a case‐by‐case basis), birth weight of the infants (confounder or modifier on a case‐by‐case basis). For studies on mixed populations of term and preterm infants (without results reported separately for each of these subpopulations): length of gestation may have an influence on the growth pattern. However, for studies on exclusively full‐term infants, length of gestation was not expected to have an impact on the outcomes of interest for this assessment.
If randomisation was appraised as acceptable for a selected trial, the potential confounders were not considered as being an issue. In its appraisal of the RoB of the 25 selected publications, the Panel considered that 16 were at high RoB with regard to randomisation (for randomised trials) or adequate comparison groups (non‐randomised trials, prospective cohort studies) (Appendices Appendix G – Synthesis of the risk of bias by individual studies investigating hypercalciuria/urinary calcium, Appendix H – Synthesis of the risk of bias by individual studies investigating hypercalcaemia/serum calcium, Appendix I – Synthesis of the risk of bias by individual studies investigating nephrocalcinosis, Appendix J – Synthesis of the risk of bias by individual studies investigating anthropometric parameters–K).
3.2.4. Appraisal: sources of uncertainty in the intake of vitamin D in the included studies
3.2.4.1. Appraisal: criterion used to appraise the risk of bias for the included studies
For the appraisal of the exposure characterisation (intake of vitamin D), the Panel decided to focus on whether or not the included studies mention an analytical check of the actual vitamin D content of the supplement or fortified food consumed. Where such a check was absent, there is a risk of under‐ or overestimation of the actual vitamin D content. The exposure characterisation was generally considered by the Panel to be of high RoB, i.e. 19 publications out of 25 selected (Appendices Appendix G – Synthesis of the risk of bias by individual studies investigating hypercalciuria/urinary calcium, Appendix H – Synthesis of the risk of bias by individual studies investigating hypercalcaemia/serum calcium, Appendix I – Synthesis of the risk of bias by individual studies investigating nephrocalcinosis, Appendix J – Synthesis of the risk of bias by individual studies investigating anthropometric parameters–K).
3.2.4.2. Other sources of uncertainty in the assessment of the intake in the included studies
Compliance in trials (risk of overestimation of the actual vitamin D intake in case of limited compliance). This information was extracted from the selected papers (Appendix D).
Type of infant feeding at inclusion: included studies may be undertaken on infants that are all exclusively breastfed, or all exclusively formula‐fed, or who receive, e.g. breast milk and formula and possibly complementary feeding (Section 1.6). This information was extracted from the selected papers (Appendix D). In general, mean vitamin D concentrations in breast milk of healthy lactating women, unsupplemented or supplemented with vitamin D below the UL, are low (Section 1.6). Hence, the amount of vitamin D provided by breast milk in exclusively breastfed infants in addition to the supplements provided in a given study might be considered to be negligible.
Background vitamin D intake in trials: if this intake is not reported, there is a risk of underestimation of the actual total intake of vitamin D. This information was extracted from the selected papers (Appendix D); however, it was absent in most of them. In particular, among the papers included in the statistical analysis (Holst‐Gemeiner et al., 1978; Gordon et al., 2008; Holmlund‐Suila et al., 2012; Gallo et al., 2013a; Grant et al., 2014; Ziegler et al., 2014) (Section 3.5 and Annex A), only one (Gallo et al., 2013a) assessed background vitamin D intake.
Forms of supplemental vitamin D provided: supplements as tablets/pills, supplements as drops (as they may be more difficult to count), supplements (unspecified form), formula or any other fortified food/meal. This information was extracted from the selected papers.
3.2.5. Appraisal: sources of uncertainty in the assessment of the outcomes/endpoints in the included studies
3.2.5.1. Appraisal: outcome assessments with generally low risk of bias (RoB)
For hypercalciuria/urinary calcium:
Measurement of urinary calcium (as concentration or ratio over creatinine) is standard hospital practice, so the RoB for this assessment was considered to be low for the publications that investigated this outcome. This can be seen when reading horizontally across the table on the appraisal of the internal validity presented in Appendix G. However, definition of hypercalciuria may differ according to authors/laboratories. Different methods of urine collection (24 h vs. one or more spot samples, number of collection days, complete or incomplete urine collection, etc.) may also prevent direct comparisons between studies (Section 3.3.2.1).
For hypercalcaemia/serum calcium:
Measurement of serum calcium concentration is standard hospital practice, so the RoB for this assessment was considered to be low for the selected publications that investigated this outcome. This can be seen when reading horizontally across the table on the appraisal of the internal validity in Appendix H. However, definition of hypercalcaemia may differ according to authors/laboratories (Section 3.3.3.1).
For nephrocalcinosis/ectopic calcification:
There is no standardised diagnostic criterion for nephrocalcinosis from the ultrasound image, i.e. interpreting whether the observed spots are calcifications or just noise on the image depends on the skills of the radiologist/paediatrician involved in the studies. The RoB for this assessment was considered to be low (Appendix I).
3.2.5.2. Appraisal: outcome assessment with low or high risk of bias (growth/anthropometric parameters)
For results reported as z‐scores, growth references or standards may be different according to studies, preventing direct comparison of the results. However, the included papers for which z‐scores were extracted all used the WHO growth standards (Gallo et al., 2013a, 2016).
In addition, length/height or weight may be measured by the investigators or self‐reported by the parents, and the method of measurement may or may not be described in the papers with or without an indication of repeated measurements (e.g. for head circumference of an infant, repeated measurements with a non‐elastic band are indicative of a better measurement quality).
This information was extracted from the studies (Appendix D), and taken into account in the appraisal process, four publications were considered of high RoB for this outcome (Appendix J).
3.2.5.3. Appraisal: outcome assessment with generally high risk of bias (serum 25(OH)D)
All analytical methods available to measure serum 25(OH)D concentration in infants have uncertainties (Section 1.8.6) and ‘high’ serum 25(OH)D concentration may have been defined differently by the authors of some of the included studies (e.g. as a stopping rule for the assessment of supplementation safety). Information on analytical method(s) for 25(OH)D measurements, possible definition of ‘high’ serum 25(OH)D concentration by the authors and the presence or absence of an external quality control/standardisation were extracted (Appendix D). The Panel considered that the issues related to the possible lack of external quality control and standardisation (Section 1.8.6.1) are important for measurement of serum 25(OH)D concentration. Thus, the RoB for this assessment was appraised as high for 19 publications that investigated this outcome (Appendix K).
3.3. Results of the included studies on daily intake of vitamin D in infants
This assessment is based on the body of evidence obtained from the extensive literature search performed by the EFSA (Section 3.1), i.e. studies in infants considering a daily intake of vitamin D (at least in one arm). For the reasons described in the following sections for each outcome/endpoint, the Panel decided to:
-
–
meta‐analyse collected data on the dose–response relationship between daily vitamin D supplemental intake and serum 25(OH)D concentration using quantitative models (Section 3.5 and Annex A), in order to predict the expected population mean responses and individual responses.
-
–
whenever possible, to show graphically in forest plots or numerically in tables, the collected data on daily vitamin D supplemental intake and the other measurements (i.e. total and ionised serum calcium, urinary calcium/creatinine ratio, weight, length/height) and summarise narratively (by tiers of RoB, study type and publication date) the study results (Sections 3.3.2 to 3.3.6).
In addition, arms/studies with results not provided in relation to vitamin D intake were excluded from the dose–response analysis or graphical representations (e.g. in the study by Pittard et al. (1991), serum total and ionised calcium and body weight were reported by time point only and not by dose).
The Panel was aware of a large systematic review on clinical trials in children (0–18 years), either healthy or not, that were selected on the basis of a ‘high’ supplement dose of vitamin D used, defined as a value (daily or not) above the DRV set by the IOM for infants and children (no dose limit for low birth weight and premature neonates) (Nama et al., 2016). The inclusion and exclusion criteria applied for this systematic review (undertaken for a large age range of children) are close, although not identical, to those applied by the Panel for this assessment on infants. Considering trials identified by Nama et al. (2016) in healthy neonates or infants receiving daily doses of vitamin D above the DRV set by the IOM, the Panel notes that 14 papers are in common with the literature selected by the Panel (four additional trials being discussed by the Panel).
3.3.1. Characteristics of the included studies used for the assessment (body of evidence)
Of 31 studies from 25 papers identified after the literature screening, 24 were trials (of different designs) and 7 were prospective studies (Table 1). As mentioned in Section 3.1.3, two papers (three studies) that comprised subjects with vitamin D deficiency rickets were excluded after a preliminary statistical analysis.
Table 1.
Frequency distribution by study design among the included studies
| Study frequency distribution by study design | ||
|---|---|---|
| Design | Absolute frequency | Relative frequency (%) |
| RCT | 14 | 45 |
| RNCT | 3 | 10 |
| NRCT | 3 | 10 |
| NRNCT | 4 | 13 |
| PROSP | 7 | 22 |
| TOTAL | 31 | 100 |
| ARMS | 74 | |
| TIME POINTS | 223 | |
RCT: randomised controlled trial; RNCT: randomised non‐controlled trial; NRCT: non‐randomised controlled trial; NRNCT: non‐randomised non‐controlled trial; PROSP: prospective study.
For serum 25(OH)D concentration, among these 31 studies, 74 arms from trials were available, related to different supplementation doses and corresponding to 223 measurements,32 some of which were repeated measurements on the same study arm at different time points (Table 1).
Table 2 describes, in the 31 selected studies, the frequency of the assessment of the outcomes/endpoints considered relevant for this scientific opinion. Data for hypercalcaemia, hypercalciuria, abnormal growth patterns/anthropometric parameters and nephrocalcinosis are reported in italics. Concentrations of serum 25(OH)D and total calcium were the endpoints most frequently measured (87% and 74%, respectively), followed by anthropometric measurements (particularly body weight and length/height).
Table 2.
Frequency of assessed outcomes/endpoints (possible multiple outcomes/endpoints per study) in the included studies
| Outcome | Number of studies | % of studies | Definition used by authors |
|---|---|---|---|
| Urinary calcium | 2 | 6 | |
| Urinary calcium/creatinine ratio | 8 | 26 | |
| Hypercalciuria | 4 | 13 |
0.8 calcium/creatinine ratio(mg/mg) 2.2 calcium/creatinine ratio (mmol/mmol) |
| Serum 25(OH)D | 27 | 87 |
For ‘high’ serum 25(OH)D: 150, 250 nmol/L; 60, 100 ng/mL |
| Serum total calcium | 23 | 74 | |
| Serum ionised calcium | 4 | 13 | |
| Hypercalcaemia | 7 | 23 | 2.6 mmol/L; 11.2 mg/dL |
| Nephrocalcinosis | 2 | 6 | Number of infants |
| Body weight | 12 | 39 | |
| Length/height | 12 | 39 | |
| Head circumference | 4 | 13 | |
| Abnormal growth patterns/anthropometric parameters | 3 | 10 | Number of infants |
For hypercalcaemia, hypercalciuria and ‘high’ serum 25(OH)D concentration, cut‐off values used for the diagnosis may change depending on the laboratory and the analytical methods applied. In the following sections, in line with a previous assessment (EFSA NDA Panel, 2012a), the occurrence of adverse health outcomes will be defined according to the cut‐off selected by the authors in each individual study (Table 2). More information on the use of these cut‐offs in the context of the studies (e.g. age of the infant population to which they were applied) are reported in the following sections.
Studies on exclusively breast‐ and formula‐fed infants were equally frequent (Table 3), and the most frequent studies were those including infants receiving mixed feeding (e.g. partial breastfeeding or partial formula‐feeding with complementary feeding).
Table 3.
Frequency distribution by feeding type of the included infants at the start of the studies
| Type of feeding at inclusion in the studies | Number of studies | % of studies |
|---|---|---|
| Exclusively breastfed | 6 | 19 |
| Exclusively formula‐fed | 6 | 19 |
| Mixed feeding | 13 | 42 |
| Not reported | 2 | 7 |
| Unclear | 4 | 13 |
Of the 31 studies, 19 clearly indicated that the included subjects were all full term (while the remaining 12 did not give a clear indication on this aspect).
Regarding the source of vitamin D, this was most often supplements in an unspecified form (11 of 25 papers), then supplements as drops (10 papers), tablet/pills (two papers), a fortified formula (one paper) and one paper reporting on a study that used both a supplement (unspecified) and a fortified formula. Most often, the form of vitamin D (vitamer) used was vitamin D3 (13 studies of 31) or was not mentioned (10 studies of 31), and a limited number of studies used vitamin D2 (7 of 31) or both vitamers (1 study). The highest dose considered among the selected studies was 50 μg/day. Only 8 studies of 31 provided information on the background vitamin D intake. Supplementation duration and age of the infants were variable, ranging from some days up to age of 1 year.
A large range of countries, thus of latitudes, was also considered (Austria, Canada, Germany, Finland, France, Gabon, Japan, New Zealand, Poland, Switzerland, Turkey, USA).
Information on the mothers (e.g. supplementation in pregnancy, level of education) was available for 18 of 25 papers), on sun exposure for 6 of 25 papers, on season of birth (10 of 25 papers), on season of study (15 of 25 papers), or on skin type or pigmentation (10 of 25 papers). As anticipated (Section 3.1.5), this information was not provided in all selected papers and, where provided, the level of detail was variable.
The Panel notes the variability in the design of the studies selected after the literature search. The Panel also considers that the diversity of the infant population supports the external validity of the selected studies.
3.3.2. Assessment of data on hypercalciuria
Hypercalciuria was discussed as an outcome in four studies reported in three publications (Czech‐Kowalska et al., 2012; Holmlund‐Suila et al., 2012; Gallo et al., 2013a). Details of study designs are given in Appendix L. Among the studies in which urinary calcium was measured, irrespective of whether hypercalciuria was observed or not, five studies mentioned urinary calcium/creatinine ratio, which were rated tier 1 (Gallo et al., 2013a), 2 (Czech‐Kowalska et al., 2012; Holmlund‐Suila et al., 2012) or 3 for this outcome (Hoppe et al., 1997; Siafarikas et al., 2011).
3.3.2.1. Hypercalciuria: definitions
Hypercalciuria has been defined based on urinary calcium concentration (mmol or mg per L and/or per kg body weight per day). Whilst most authors agree that the upper limit of calcium excretion in urine of healthy subjects is 4 mg/kg body weight per day, both in adults (Heaney et al., 1999; Curhan et al., 2001; Chandhoke, 2007; Jones et al., 2010; Jones AN et al., 2012)) and in children (Ghazali and Barratt, 1974; Ring and Borkenstein, 1987; Dumas, 1997; Tekin et al., 1997; Audran and Legrand, 2000; Leslie and Taneja, 2017), the applicability of definitions for adult hypercalciuria particularly to infants is less clear. From the value of 4 mg/kg body weight per day, the other widely accepted definitions for men (> 275 mg/day) and women (> 250 mg/day) are derived (Chandhoke, 2007) (Leslie and Taneja, 2017), or alternatively a calcium concentration > 200 mg/L (or 5 mmol/L) in a 24 h urine collection (Leslie and Taneja, 2017).
Although ideally the diagnosis of hypercalciuria should be based on the measurement of urinary calcium excretion in 24 h urine, this is difficult to apply in infants for practical reasons. Thus, the ratio of calcium over creatinine (Ca/Cr) in a random or timed urine sample has been considered to define thresholds for hypercalciuria, and random urinary Ca/Cr ratio is used as a standard hospital practice. Most authors agree that a urinary Ca/Cr ratio (mg/mg) of 0.2–0.25 (or 0.56–0.70 mmol/mmol) is the upper limit of a normal range in adults and children above 2 years of age (Ring and Borkenstein, 1987; Sargent et al., 1993; Dumas, 1997; Matos et al., 1997; Tekin et al., 1997; Koyun et al., 2007; Jones et al., 2010; Arrabal‐Polo et al., 2016; Leslie and Taneja, 2017). Urinary Ca/Cr ratios are higher in infancy and decrease with age (EFSA NDA Panel, 2012b; Gallo et al., 2014b). In randomly collected urine samples, urinary Ca/Cr ratios of 0.9, 0.6, 0.42 and 0.22 (mg/mg) (or 2.5, 1.7, 1.2, 0.62 mmol/mmol) have been reported for ages < 7 months, 7–18 months, 19 months to 6 years and adults, respectively (Sargent et al., 1993).
The Panel notes that the gold standard for diagnosing hypercalciuria is the analysis of calcium in a 24h urine, from which the calcium excretion per kg body weight per day can be calculated. An increased urinary Ca/Cr ratio in a single urine sample can raise suspicion of hypercalciuria, but needs confirmation by repeated measurements and preferably by the measurement of the urinary calcium excretion over 24 hours.
3.3.2.2. Hypercalciuria: studies from risk of bias tier 1
Gallo et al. (2013a) (Section 1.8.1) investigated the impact of vitamin D3 supplementation on serum 25(OH)D concentrations (dose–response), different health outcomes and side effects in 132 healthy initially breastfed infants. From 1 to 12 months of age, the infants were randomly assigned to receive one of the following vitamin D intervention (VIDI) groups: 10 μg/day (n = 39), 20 μg/day (n = 39), 30 μg/day (n = 38) or 40 μg/day (n = 16). Dietary intake of vitamin D was assessed by the volume of breast milk consumed and by 3‐day dietary records, whereas the endogenous synthesis of the vitamin was estimated by %BSA exposed to sunlight on a weekly basis. Infants were monitored for safety outcomes related to calcium homoeostasis including the urinary Ca/Cr ratio (in 4‐h urine collection) at baseline, at 2, 3, 6, 9 and 12 months of age. Urinary Ca/Cr ratios progressively declined with infant growth. ‘Suspected hypercalciuria’ (urinary Ca/Cr ratio > 3.3 (mmol/mmol), above the hospital reference range) was found in one child from each of the groups treated with 20, 30 and 40 μg/day; however, all the subsequent safety checks (electrocardiogram and renal ultrasound) were normal.
3.3.2.3. Hypercalciuria: studies from risk of bias tier 2
Holmlund‐Suila et al. (2012) (Section 1.8.1) conducted a double‐blind dose–response RCT aiming at determining the daily dose of vitamin D3 required to achieve serum 25(OH)D concentrations of 80 nmol/L or above in breastfed infants without any signs of side effects. Term infants with adequate for gestational age birth weight (n = 113) were recruited at age 2 weeks and were randomised to receive for 10 weeks vitamin D3 at 10, 30 or 40 μg/day. Urinary Ca/Cr ratio in spot urine samples was analysed at the end of the intervention (when the infants were 3 months old) and did not show differences between intervention groups. Hypercalciuria (defined as urinary Ca/Cr ratio > 2.2 mmol/mmol) was found in 39% of all participants.33 Individual urinary Ca/Cr ratios were reported for infants who had serum 25(OH)D concentrations > 150 nmol/L (range 150–230 nmol/L) and ranged from 0.31 to 6.34 mmol/mmol. The Panel notes that the prevalence of hypercalciuria was similar between groups. In addition, the potential impact of differences in the feeding of infants was not taken into consideration in the analysis and the duration of the study was comparatively short (10 weeks), which limits the ability of this investigation to provide reliable evidence on the safety aspects of the tested levels of vitamin D intake.
Czech‐Kowalska et al. (2012) followed prospectively 30 term infants with hypovitaminosis D (baseline serum 25(OH)D concentration < 50 nmol/L) for 10 weeks from age 15 days to 94 days. Infants were supplemented with vitamin D3 at a median intake of about 14 μg/day (median total intake of about 18 μg/day from food and supplements).34 Infants were separated into deficient (baseline serum 25(OH)D concentration < 28 nmol/L) and insufficient (baseline serum 25(OH)D concentration 28–50 nmol/L), but data were reported for the combined group. Vitamin D and calcium status parameters were analysed at baseline and at the end of the study. Compared with baseline, serum 25(OH)D concentrations were six times higher at the end of the intervention (from 21.1 nmol/L to 136.5 nmol/L). Urinary Ca/Cr ratios, measured in spot urine samples, were similar at the two time points; however, hypercalciuria, defined as urinary Ca/Cr ratio > 0.8 mg/mg (2.27 mmol/mmol35), was found in seven infants (three of these infants had serum 25(OH)D > 150 nmol/L and one had serum 25(OH)D > 200 nmol/L) at the end of the investigation. The Panel notes that the lack of a control group limits the value of this investigation.
3.3.2.4. Hypercalciuria: studies from risk of bias tier 3
Siafarikas et al. (2011) conducted a trial in 40 newborns who were randomised into equal groups to receive vitamin D3 at 6.25 μg/day or 12.5 μg/day from birth until the age of 6 weeks. All infants were breastfed and, in order to take into account the differences in sun exposure, half of the infants completed the study during autumn/winter whereas the other half did the study during spring/summer. There were no significant differences in urinary calcium concentrations in spot urine samples and urinary Ca/Cr ratios between the two groups both at baseline and at the end of the intervention. It was unknown whether there were any cases of hypercalciuria within the two groups of infants. The Panel notes that this is a short study and the levels of vitamin D supplementation used for intervention were close to the AI of 10 μg/day for infants (EFSA NDA Panel, 2016); thus, side effects cannot be expected.
Hoppe et al. (1997) followed prospectively 37 term infants from days 1 to 60 of age. Infants were fed with unfortified breast milk (n = 16) or formula (n = 21) and received vitamin D3 supplements of 12.5 μg/day, and urinary Ca/Cr ratios were measured in spot urine samples. The Panel notes that it was unknown whether there were any cases of hypercalciuria among the infants; however, the level of vitamin D supplementation used was close to the AI of 10 μg/day for infants (EFSA NDA Panel, 2016); thus, side effects cannot be expected. In addition, the infants were fed with different formulae with unknown content of vitamin D, which does not allow calculation of the total daily intake of the vitamin for this group.
3.3.2.5. Daily intake of vitamin D and mean urinary calcium/creatinine ratio in infants
Appendix M, showing data from the studies described in the previous sections, provides no evidence of a dose–response relationship between vitamin D supplemental intake over the range of doses tested and mean urinary Ca/Cr ratios.
3.3.2.6. Hypercalciuria: conclusion
The Panel notes that most of these studies were short‐term interventions of a small scale and none of them measured urinary calcium excretion over 24 hours. Although one study reported several cases of hypercalciuria in a group of infants with vitamin D intake of about 20 μg/day (Czech‐Kowalska et al., 2012), the two RCTs which tested multiple vitamin D doses between 10 and 40 μg/day (Holmlund‐Suila et al., 2012; Gallo et al., 2013a) did not find significant differences in the prevalence of hypercalciuria as defined by the authors between vitamin D doses. The Panel also notes that these RCTs which involved a higher number of participants, provided inconsistent results with considerable discrepancies in the rate of hypercalciuria (from 3–6% to 39%), and the urinary Ca/Cr ratios were not related to the administered dose of vitamin D in any of these investigations.
The Panel considers that these studies are not informative for determining the association between vitamin D supplemental doses and the risk of hypercalciuria.
3.3.3. Assessment of data on hypercalcaemia
Hypercalcaemia or ‘high’ serum calcium was reported as an outcome in eight studies reported in six publications (Vervel et al., 1997; Czech‐Kowalska et al., 2012; Holmlund‐Suila et al., 2012; Gallo et al., 2013a; Grant et al., 2014; Ziegler et al., 2014). Details of study design are given in Appendix L.
3.3.3.1. Hypercalcaemia: definitions
Hypercalcaemia or ‘high’ serum calcium is defined based on serum total calcium concentration and/or ionised calcium concentration. Measurement of serum calcium concentration is standard hospital practice. However, the cut‐off used to define hypercalcaemia or ‘high’ serum calcium may differ according to the laboratory or author. Hypercalcaemia has been defined as serum calcium concentration above 2.75 mmol/L (EFSA NDA Panel, 2012a,b) or ionised calcium above 1.35 mmol/L (SCF, 2003). However, reported serum calcium values differ by age, and the reported 97.5th centile for infants < 12 months is higher than in adults, varying between 2.75 and 3.00 mmol/L (Gallo et al., 2014b).
Gallo et al. (2014b) measured a number of plasma and urinary analytes in 132 healthy, term, breastfed infants participating in a vitamin D supplementation trial (Gallo et al., 2013a) (Sections 1.8.1 and 3.3.2.2). Results were not reported per group but per age in months; thus, this paper was not among the studies included after the extensive systematic review (Section 3.1). However, it provides additional information, as its purpose was to define reference values for analytes in blood and urine. Ionised and total calcium in serum declined significantly between 1 and 12 months (median values from 1.40 to 1.33 mmol/L and from 2.53 to 2.47 mmol/L for ionised calcium and total calcium, respectively), while serum creatinine increased significantly. Ionised calcium was elevated at 1 month, with a range (1.29–1.52 mmol/L) exceeding the upper limits for adults (1.15–1.29 mmol/L, Radiometer America®). Despite the decrease over time, 25% of infants still exceeded the 97.5th centile of reference ranges at 12 months. Similarly, total calcium concentrations were elevated compared to reference data for older children, with about 25% of infants over the upper adult/child limits (2.23–2.58 mmol/L, Beckman Coulter®). The decline in total calcium occurred later than that in ionised calcium. However, clinical safety evaluations (e.g. for signs of hypercalcaemia) were normal.
The reference range for ionised calcium also differs according to the method used and there are no agreed reference ranges for infants. Valkama et al. (2017) reported ionised calcium concentrations in 762 infants of the VIDI study, using reference ranges from the Finnish HUSLAb laboratory36 (< 4 days 1.05–1.5 mmol/L pH 7.4; 1–30 days 1.1–1.4 mmol/L pH 7.4; 1–12 months 1.16–1.39 mmol/L pH 7.4). They observed a marked decrease in the prevalence of ‘mild hypercalcaemia’, defined as up to 10% above the upper limit for age, from 6 to 12 months. Higher ionised calcium concentrations were associated with female sex and younger age. They concluded that it was uncertain whether these mildly elevated values represented ‘true’ hypercalcaemia or rather ‘false’ reference values. Only 4 of the 762 infants (0.5%) had both mild hypercalcaemia and subnormal PTH concurrently at 12 months, and most of the infants had normal PTH, suggesting that these mildly elevated ionised calcium concentrations were physiological and not clinically relevant.
The Panel notes that the available data suggest that both total and ionised calcium concentrations may be higher in infants compared to older children and adults, and that it is not possible to define a clear cut‐off for hypercalcaemia. In the discussion of the following publications, the definition of hypercalcaemia used by the authors, generally based on a ‘high’ serum calcium concentration, are reported. However, the Panel notes that as serum calcium is tightly regulated (Section 1.9), single measurements of serum calcium, which are generally reported in the following publications, may not provide an indication of the risk of sustained elevated serum calcium (indicative of hypercalcaemia per se).
3.3.3.2. Hypercalcaemia: studies from risk of bias tier 1
Gallo et al. (2013a) (Sections 1.8.1 and 3.3.2.2) randomised healthy breastfed infants to receive daily doses of vitamin D3 between 10 and 40 μg from 1 to 12 months. ‘Suspected hypercalcaemia’ was based on serum‐ionised calcium concentration above the hospital reference range (1.15–1.38 nmol/L) and was reported in 0, 2, 2 and 2 infants in each of the study groups, respectively. However, electrocardiograms and renal ultrasound in these suspected cases revealed no abnormalities.
Ziegler et al. (2014) conducted an RCT on healthy breastfed infants randomised to receive 5 (n = 56), 10 (n = 60), 15 (n = 56) or 20 (n = 41) μg vitamin D/day from 28 days to 9 months of age. Calcium concentrations > 12.0 mg/100 mL (3 mmol/L) were observed on one occasion with 5 μg/day, on four occasions with 10 μg/day, on three occasions with 15 μg/day and on three occasions with 20 μg/day. The rationale for this cut‐off was not provided and it was not referred to as ‘high serum calcium’ or as ‘hypercalcaemia’ by the authors, although in the discussion, they comment that there was no risk of hypercalcaemia even with 20 μg/day. No relationship could be derived between serum 25(OH)D concentration and risk of hypercalcaemia.
3.3.3.3. Hypercalcaemia: studies from risk of bias tier 2
Holmlund‐Suila et al. (2012) randomised healthy breastfed infants to receive daily doses of vitamin D3 between 10 and 40 μg/day from 2 weeks to 3 months of age (Sections 1.8.1 and 3.3.2.3). Mean serum calcium concentration did not differ between groups and there was no correlation between serum calcium and 25(OH)D concentrations. Individual serum calcium concentrations were reported for infants who had serum 25(OH)D > 150 nmol/L (range: 150–230 nmol/L) and ranged from 2.44 to 2.78 mmol/L (3 being above 2.75 mmol/L). No infant developed hypercalcaemia, which in this study was assessed based on symptoms (nausea, vomiting, poor feeding or prolonged constipation) rather than defined by serum calcium concentration.
Czech‐Kowalska et al. (2012) studied prospectively 30 term infants with baseline serum 25(OH)D concentration < 50 nmol/L receiving vitamin D3 supplements (median total intake of about 18 μg/day) (Section 3.3.2.3). Although no definition was provided, the authors reported that none of the infants had ‘hypercalcaemia’ after 10 weeks.
Grant et al. (2014) conducted an RCT investigating daily supplementation of pregnant women (from 27 weeks of gestation to birth) and their infants (from birth to 6 months; 95% breastfed at birth) with placebo (n = 87) or vitamin D3 at 25 μg to the mother/10 μg to the infant (n = 87) or 50 μg to the mother/20 μg to the infant (n = 86). Hypercalcaemia, defined as a serum total calcium concentration above 11.2 mg/dL (2.8 mmol/L), was not reported in any infant, including five infants with serum 25(OH)D concentration > 250 nmol/L at 2 months.
3.3.3.4. Hypercalcaemia: studies from risk of bias tier 3
Vervel et al. (1997) (Section 1.4) conducted an RCT to measure serum 25(OH)D concentration at 1 (n = 70) and 3 months (n = 52) in infants born to mothers with or without vitamin D supplementation during pregnancy (Section 1.4). Infants were fed vitamin D fortified milk and randomised to receive either 12.5 μg or 25 μg D2 per day. Mean serum total calcium concentrations did not differ between groups at 1 and 3 months of age. Serum calcium concentrations at 3 months ranged from 2.42 to 2.80 mmol/L in infants who received 12.5 μg/day in addition to fortified IF and from 2.46 to 2.79 mmol/L in those supplemented with 25 μg/day. Among infants from unsupplemented mothers,37 the percentage of infants with serum calcium concentration > 2.60 mmol/L was 15% and 50% in the 25 μg D2 group at 1 and 3 months, compared to 36% and 60% of the 12.5 μg D2 group. However, the choice of this cut‐off of 2.60 mmol/L was not justified by the authors and it was not defined by them as ‘hypercalcaemia’.
3.3.3.5. Hypercalcaemia: other data
One additional publication mentioned hypercalcaemia in the discussion section, but it was not described or defined in the methods or results. Gordon et al. (2008) (rated as tier 1 for serum calcium, Sections 1.4 and 1.8.2) randomised 40 infants and young children aged 8–24 months with hypovitaminosis D (serum 25(OH)D concentration < 50 nmol/L) to 50 μg/day vitamin D2 or D3 for 6 weeks. A higher overall incidence of ‘mild hypercalcaemia’ (not defined) was reported at baseline in contrast to at the end of the study, possibly related to vitamin D insufficiency (hypovitaminosis). All subjects were reported to be asymptomatic, and there was no statistically significant correlation between the presence of ‘mild hypercalcaemia’ at baseline and after each intervention.
One study presented data on serum ionised calcium but did not define or discuss hypercalcaemia. In the study by Pittard et al. (1991) in term infants supplemented with vitamin D at 10 (n = 11) or 20 μg/day (n = 5), serum total and ionised calcium at eight time points from 0 to 16 weeks of age were reported as 2.2–2.4 mmol/L and 1.2–1.4 mmol/L, respectively. However, the values were not reported in relation to the dose of vitamin D (some infants received 10 or 20 μg of vitamin D daily, others received 0.85 or 1.5 μg 25(OH)D3).
The Panel notes that two papers published after the end of the period of the systematic literature search (Section 3.1) reported first the results of a preliminary analysis (Valkama et al., 2017) (Section 3.3.3.1), then the final analysis from the VIDI study (published after the end of the public consultation on this opinion) (Rosendahl et al., 2018). The incidence of hypercalcaemia was evaluated in 987 eligible healthy infants who randomly received 10 or 30 μg of vitamin D3 supplements daily from age 2 weeks to 24 months (762 included in Valkama et al. (2017); 12 infants were excluded after randomisation, leaving 489 and 486 in the 10 or 30 μg group, respectively, in Rosendahl et al. (2018)). At birth, about 95% of the infants had serum 25(OH)D concentration above 50 nmol/L. Hypercalcaemia was defined by the authors as ionised calcium above 1.39 mmol/L at 6 and 12 months (Section 3.3.3.1) and above 1.35 mmol/L at 24 months. ‘Severe hypercalcaemia’ was defined as ionised calcium above 1.53 mmol/L (10% higher than the upper limit of the reference range) at 6 and 12 months and above 1.48 mmol/L at 24 months. In cases of ‘severe hypercalcaemia’, serum 25(OH)D and calcium concentrations would be measured again, and symptoms indicative of hypercalcaemia would be investigated. Mean ionised calcium concentrations did not differ between groups. Numbers (%) of subjects with calcium concentrations above the value defining hypercalcaemia were 121 (27%) and 124 (28%) at age 6 months in the 10 μg and 30 μg group, respectively, 6 (1%) and 10 (2%) at age 12 months and 27 (7%) and 32 (9%) at age 24 months. No ‘severe hypercalcaemia’ occurred and no clinical signs suggestive of hypercalcaemia were reported. The Panel notes that this RCT had the longest duration of daily vitamin D supplementation in infants and young children.
3.3.3.6. Daily intake of vitamin D and mean serum total calcium concentration in infants
Mean serum total calcium concentration was reported as an outcome in five RCTs (Gordon et al., 2008; Holmlund‐Suila et al., 2012; Gallo et al., 2013a; Grant et al., 2014; Ziegler et al., 2014) and one observational study (Czech‐Kowalska et al., 2012) (tiers 1 or 2) previously described. It was also measured in eight studies assessed as tier 3 (Pincus et al., 1954; Fomon et al., 1966; Ala‐Houhala, 1985; Pittard et al., 1991; Vervel et al., 1997; Zeghoud et al., 1997; Nguema‐Asseko et al., 2005; Siafarikas et al., 2011). Details of study design are given in Appendix L; a brief description of the studies is provided below for those not already discussed. Data on serum calcium were also reported by Hazell et al. (2017) in a 3‐year follow‐up of subjects from the study by Gallo et al. (2013a). However, the Panel considered that the 3‐year time interval was too long for these measurements to indicate an effect of vitamin D intake during infancy on serum calcium.
Ala‐Houhala (1985) (Section 1.4) studied 100 healthy mother‐term infant pairs during summer and winter and measured serum calcium and 25(OH)D concentrations. All infants were exclusively breastfed and subjects were randomly allocated to three supplementation strategies: a maternal supplement of 25 μg/day or an infant supplement of 10 μg or 25 μg/day. Fomon et al. (1966) (Section 1.4) measured serum calcium at monthly intervals in healthy term male infants. The infants received formula feeding providing vitamin D at around (i) 45 μg/day (n = 13) or (ii) 11 μg/day (n = 11) or (iii) were breastfed and additionally obtained a single formula feeding together with a supplement which accounted to a total daily vitamin D intake of approximately 7.5 μg (n = 26). Pincus et al. (1954) measured serum calcium on days 1 and 5 of life in healthy term infants divided into five groups: breastfed with 15 μg (n = 13) vitamin D/day vs. no supplement (n = 15); fed formula without vitamin D plus either 15 μg (n = 30) vitamin D supplement per day or no supplement (n = 46); fed formula containing 10 μg vitamin D/day (n = 52). Nguema‐Asseko et al. (2005) measured serum calcium at birth, 3 and 6 months in healthy term infants from Gabon. All infants were exclusively breastfed until 3 months and were assigned to receive vitamin D at 25 μg/day (n = 41) vs. no supplement (n = 38). Siafarikas et al. (2011) measured serum calcium at delivery and 6 weeks of age in 40 healthy term breastfed infants, randomised to either 6.25 μg or 12.5 μg vitamin D3/day (Section 3.3.2.4). Zeghoud et al. (1997) measured serum calcium in 80 healthy term infants 3–6 days after delivery; repeated samples were obtained from 52 formula‐fed infants who received either 12.5 or 25 μg vitamin D2/day at 1 and 3 months of age.
Data on mean serum total calcium from the studies appraised as tier 1 or 2 are shown in a forest plot (Figure 2). The Panel concludes that this forest plot does not suggest any clear trend between vitamin D intake ranging from 5 to 50 μg/day or infant age and mean serum total calcium concentration. Data on mean serum total calcium according to different doses of vitamin D from the studies appraised as tier 3 are shown in Appendix N. The Panel concludes that these studies do not provide useful information with respect to the definition of a UL for vitamin D.
Figure 2.
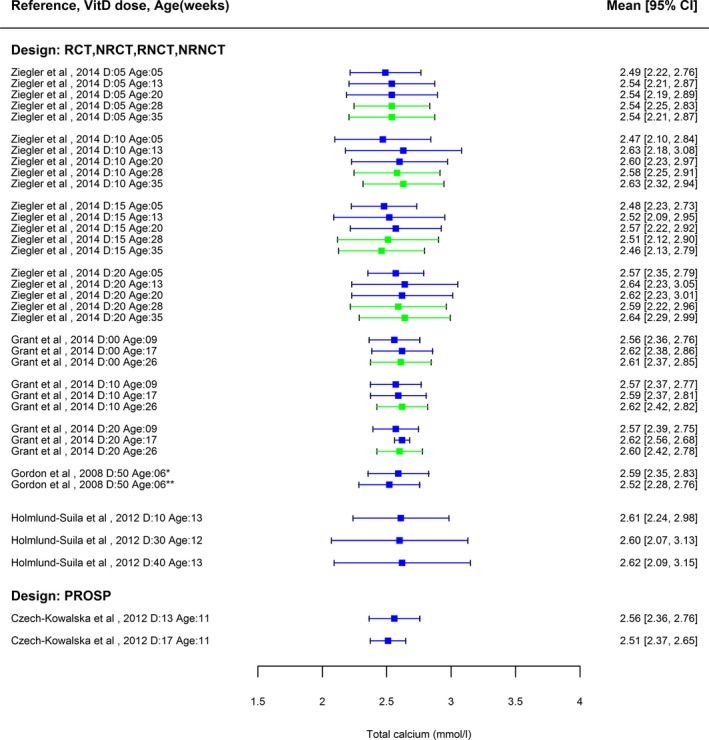
Forest plot of total calcium in serum for studies from tiers 1 and 2 for the RoB
-
*Vitamin D3 **Vitamin D2CI: confidence interval; D: vitamin D dose (μg/day).Values for trials (NRCT: non‐randomised controlled trial; NRNCT: non‐randomised non‐controlled trial; RCT: randomised controlled trial; RNCT: randomised non‐controlled trial) and prospective studies (PROSP) are indicated separately.Age: age of the infants in weeks (rounded). Calcium concentrations at baseline are not shown. Measurements before 6 months of age (blue) or after 6 months of age (green).
3.3.3.7. Daily intake of vitamin D and mean serum ionised calcium concentration in infants
The RCT by Gallo et al. (2013a) described above (tier 1) provides data on daily vitamin D intake and mean serum concentrations of ionised calcium (Appendix O), in infants who were randomised to receive vitamin D supplements of 10, 20, 30 or 40 μg/day. The data show no dose–response relationship between vitamin D supplemental intake and mean ionised calcium concentrations, although concentrations were lower in older infants, as reported by Gallo et al. (2014b) in line with Valkama et al. (2017).
3.3.3.8. Hypercalcaemia: conclusion
The Panel concludes that the available data on the relationship between vitamin D intake and ‘high serum calcium’ or ‘hypercalcaemia’ have a number of limitations which make interpretation problematic, including variable quality as indicated by the risk of bias assessments. The Panel notes that as serum calcium is tightly regulated (Section 1.9), single measurements of serum calcium, often reported in the discussed publications, may not provide an indication of the risk of sustained elevated serum calcium (indicative of hypercalcaemia per se).
Among the publications providing data on these outcomes, one did not observe hypercalcaemia but did not provide any definition (Czech‐Kowalska et al., 2012), three (Vervel et al., 1997; Grant et al., 2014; Ziegler et al., 2014) used a cut‐off for total calcium in serum (2.8, 2.6 or 3.0 mmol/L), but only the one (Grant et al., 2014) used it to define hypercalcaemia, one diagnosed hypercalcaemia based on symptoms (Holmlund‐Suila et al., 2012) and two (in three publications) diagnosed hypercalcaemia based on a serum‐ionised calcium above the upper limit for a hospital range (Gallo et al., 2013a; Valkama et al., 2017; Rosendahl et al., 2018). One study referred to ‘mild’ hypercalcaemia in the discussion only without providing definition (Gordon et al., 2008), and a further study presented data on ionised calcium (Pittard et al., 1991), but did not discuss or define hypercalcaemia. No clinical signs suggestive of hypercalcaemia were reported in healthy infants receiving up to 40 μg vitamin D/day (for 2.5 months or 11 months), including infants with serum 25(OH)D concentrations above 150 or 250 nmol/L. One study in infants with baseline serum 25(OH)D < 50 nmol/L who received 50 μg/day reported no cases with symptoms of ‘hypercalcaemia’ (Gordon et al., 2008). The evaluated studies do not provide an indication of a clear relationship between vitamin D intake and risk of hypercalcaemia. The forest plot (Figure 2) and table (Appendix N) also provided no evidence of a dose–response relationship between vitamin D supplemental intake over the range of doses tested and mean serum total calcium. The single study included from the systematic literature search (Gallo et al., 2013a) reporting mean serum concentration of ionised calcium in infants receiving different vitamin D supplements also showed no indication of a dose–response relationship (Appendix O).
The Panel considers that these studies are not informative for determining the association between vitamin D supplemental doses and the risk of hypercalcaemia.
3.3.4. Assessment of data on ectopic calcification (e.g. nephrocalcinosis)
Nephrocalcinosis was discussed as an outcome only in one study, which was rated tier 3 of RoB for this outcome (Hoppe et al., 1997). It was also checked in Gallo et al. (2013a) (tier 1 for urinary and serum calcium, and anthropometric parameters, tier 2 for serum 25(OH)D concentration). Details of the study design are given in Appendix L.
3.3.4.1. Ectopic calcification, e.g. nephrocalcinosis: definition
Calcinosis is a chronic disease condition, whilst calcification can be acute and may even be reversible. Nephrocalcinosis is one manifestation of ectopic calcification; besides nephrocalcinosis, other calcium depositions can be observed in skin, adipose tissue and blood vessels of very severely ill infants.
3.3.4.2. Nephrocalcinosis: available studies
Hoppe et al. (1997) studied prospectively 37‐term infants from days 1 to 60 of age, fed with unfortified breast milk or formulae and supplemented daily with 12.5 μg vitamin D3 (Section 3.3.2.4). Renal ultrasonography conducted in 26 infants did not find nephrocalcinosis. The Panel also notes that the dose was close to the AI of 10 μg/day (EFSA NDA Panel, 2016) and the period of intervention was short; therefore, nephrocalcinosis could not be expected.
In the RCT by Gallo et al. (2013a) (Sections 1, 3.3.2 and 3.3.3), infants were supplemented with vitamin D3 doses between 10 and 40 μg/day from 1 to 12 months. Infants with ‘suspected hypercalciuria’ or ‘suspected hypercalcaemia’ underwent renal ultrasound and electrocardiogram in a secondary analysis, but no abnormalities were detected.
3.3.4.3. Nephrocalcinosis: conclusion
The Panel notes that only two studies monitored the risk of developing nephrocalcinosis as a result of vitamin D supplementation with a dose up to 40 μg/day and did not find any cases of nephrocalcinosis. The Panel notes that, in one of these studies, the supplemental vitamin D intake was close to the AI (total daily intake unknown) and the duration of the study was short.
The Panel considers that these studies are not informative for determining the association between vitamin D supplemental doses and the risk of nephrocalcinosis.
3.3.5. Assessment of data on abnormal growth patterns
Eleven papers discussed abnormal growth patterns and/or anthropometric parameters (Fomon et al., 1966; Pittard et al., 1991; Nguema‐Asseko et al., 2005; Hyppönen et al., 2011; Siafarikas et al., 2011; Czech‐Kowalska et al., 2012; Holmlund‐Suila et al., 2012; Gallo et al., 2013a, 2016; Ziegler et al., 2014; Hazell et al., 2017) (all tiers). Details of study design are given in Appendix L and tiers of RoB for all studies are indicated below.
3.3.5.1. Abnormal growth patterns: definition
Various anthropometric measurements such as weight, length/height, head circumference and their respective z‐scores have been used to assess growth in studies on vitamin D intake. Abnormal growth is typically defined as growth rate outside the normal range (3rd–97th centile) of the standard WHO growth curves for boys and girls or z‐scores of below ‐2 or above +2.
Some of the available studies presented changes of anthropometric measurements or z‐scores between different time points, which can be considered as growth. However, others have expressed the results in absolute values of attained weight/length/head circumference, which cannot be considered characteristics of growth, but these measurements might be still informative for discrimination of the effects of different doses of vitamin D.
3.3.5.2. Abnormal growth patterns: studies measuring growth
In the RCT by Gallo et al. (2013a) (tier 1) (Sections 1, 3.3.2, 3.3.3 and 3.3.4), infants were supplemented with vitamin D3 doses between 10 and 40 μg/day from 1 to 12 months. Weight, length and head circumference were measured and the respective z‐scores were evaluated at baseline (1 month of age) and at 2, 3, 6, 9 and 12 months of age. The growth of all the infants participating in the trial was within the normal rate and there were no significant differences between groups in the growth parameters at any of the investigated time points. There was, however, a significant attrition in the highest dose group. The participants in this study were followed further at the age of 3 years and the z‐scores of height for age or weight for age did not show any significant differences among the vitamin D groups (Gallo et al., 2016; Hazell et al., 2017) (tier 1).
Ziegler et al. (2014) (tier 1) (Section 3.3.3.2) randomised healthy breastfed infants to four groups supplemented with vitamin D between 5 and 20 μg/day from the age of 28 days to 9 months. Weight and length gain from 1 to 4 months and from 4 to 9 months were not significantly different between groups in each sex.
Holmlund‐Suila et al. (2012) (tier 2) (Sections 1.8.1, 3.3.2 and 3.3.3) randomised breastfed infants to receive vitamin D3 doses between 10 and 40 μg/day from 2 weeks to 3 months of age. Weight, length, head circumference, length of left tibia, circumference of the left calf and their changes before and after intervention (both sexes assessed together) did not show significant difference among groups.
Fomon et al. (1966) (tier 3) (Sections 1.4 and 3.3.3.6) followed 50 healthy full‐term male infants receiving either formulae with different vitamin D content or breast milk plus formulae and supplement from 9 days until 168 days of age. The rates of length and weight gain of the different feeding groups were similar at all time points of the investigation. The Panel notes that this is a non‐randomised investigation based on a small number of infants only of male sex, which limits the generalisability of its results.
3.3.5.3. Studies measuring attained weight/height/head circumference
Hyppönen et al. (2011) (tier 3) analysed the link between vitamin D supplementation in infancy and the attained height in childhood and early adulthood in 10,060 participants of the Northern Finland Birth Cohort study. Data on vitamin D dose in infancy (< 50 μg/day, 50 μg/day or > 50 μg/day) and frequency of supplement usage (none, irregular or regular) were obtained when the children were 1‐year old whereas information for body height as well as various relevant confounders was collected at ages 1 year, 14 years and 31 years. The study did not find a significant association between the dose or frequency of vitamin D supplement use and the height of participants at the three different time points after adjustment for multiple confounders. The Panel notes the high RoB due to the fact that a large proportion of the data on height, vitamin D supplement use and various confounders was based on self‐reporting.
Nguema‐Asseko et al. (2005) (tier 3) (Section 3.3.3.6) measured similar weight, length and head circumference in infants in Gabon in the supplemented (25 μg/day of vitamin D) and non‐supplemented groups, at the end of the 3‐month intervention.
Pittard et al. (1991) (tier 3) (Section 3.3.3.5) measured the weight of term infants supplemented with 10 or 20 μg/day from birth to the age of 16 weeks. The postnatal weight gain of all infants during the study period was consistent with the accepted normal values and both groups grew normally; however, comparison between groups was not presented.
Siafarikas et al. (2011) (tier 3) (Sections 3.3.2.4 and 3.3.3.6) randomised newborns to receive either 6.25 or 12.5 μg/day vitamin D3 from birth until the age of 6 weeks. At the end of the intervention period, weight and length were significantly lower in the infants supplemented with vitamin D at 12.5 μg/day compared with those who received vitamin D at 6.25 μg/day (4,195 vs. 4,768 g, p = 0.02; 56 vs. 58 cm, p = 0.02). The Panel notes that this is a short study and the intervention dose of vitamin D was close to the AI.
Czech‐Kowalska et al. (2012) (tier 3) (Sections 3.3.2.3 and 3.3.3.3) provided vitamin D3 supplements (median total intake of about 18 μg/day) to 30 term infants. Weight and length of the infants were within the normal range at the age of 15 and 94 days.
Forest plots representing the results on mean attained length and weight in arms receiving different vitamin D doses in two RCTs (Holmlund‐Suila et al., 2012; Gallo et al., 2013a) and two prospective observational studies (Gallo et al., 2016; Hazell et al., 2017) (tiers 1 and 2) are presented in Appendix P. The forest plots provide no evidence of a dose–response relationship between vitamin D supplemental intake over the range of doses tested and mean attained length and weight.
3.3.5.4. Abnormal growth patterns: conclusion
The Panel notes that the results of the studies investigating the association between levels of vitamin D intake ranging from 6.25 μg/day to over 50 μg/day and growth patterns and/or anthropometric parameters are consistent between themselves. They do not show any relationship between vitamin D intake and abnormal growth patterns, and therefore contradict the conclusions of the early study by Jeans and Stearns (1938) (Section 1.4). Most of the evidence is based on small‐scale observational studies of short duration and/or not primarily designed to investigate this question.
The Panel considers that these studies are not informative for determining the association between vitamin D supplemental doses and the risk of abnormal growth patterns.
3.3.6. Assessment of data on ‘high’ serum 25(OH)D concentration
‘High’ serum 25(OH)D was reported as an outcome in seven studies from five publications (Vervel et al., 1997; Czech‐Kowalska et al., 2012; Holmlund‐Suila et al., 2012; Gallo et al., 2013a; Grant et al., 2014), rated as tier 2 except Vervel et al. (1997) rated as tier 3. There were no studies rated tier 1 for the RoB.
Thirteen studies were not used in the dose–response analysis (Section 3.5 and Annex A), because they were either trials rated tier 3 for RoB or prospective observational studies. Of these 13 studies, six trials (Ala‐Houhala, 1985; Pittard et al., 1991; Vervel et al., 1997; Zeghoud et al., 1997; Nguema‐Asseko et al., 2005; Siafarikas et al., 2011) and three prospective observational studies (Czech‐Kowalska et al., 2012; Gallo et al., 2016; Hazell et al., 2017) were already discussed in earlier sections. Three other trials (Ribot, 1981; Kunz et al., 1982; Pehlivan et al., 2003), and one other prospective observational study (Pludowski et al., 2011) were also excluded from the dose–response analysis. Details of study design are summarised in Appendix L.
3.3.6.1. ‘High’ serum 25(OH)D concentration: definitions from official bodies and medical societies
Different values have been considered by different authors and expert groups as representing a ‘high’ serum 25(OH)D concentration, generally considering this concentration in relation to symptoms of vitamin D toxicity, hypercalcaemia or chronic disease outcomes and mortality. The rationale for the different values selected is summarised below.
The SCF (2003) reported that the ‘upper reference level of 25(OH)D for infants is 130–150 nmol/L’ (Markestad, 1984; Holick, 1999). It considered that 130–150 nmol/L was well below the threshold of increased risk for hypercalcaemia, which was said to be above 200 nmol/L in adults.
The previous EFSA opinion on the UL for vitamin D highlighted the lack of additional data available at that time and noted that serum 25(OH)D concentrations associated with hypercalcaemia vary over a wide range, such that the 25(OH)D concentration in serum cannot be considered a suitable predictor of hypercalcaemia (EFSA NDA Panel, 2012a). It also indicates that the IOM (2011) considered, among other data, ‘evidence from observational studies on chronic disease outcomes suggesting an increase in risk associated with 25(OH)D concentrations above approximately 125–150 nmol/L’, and discusses a ‘U’ or ‘J’‐shaped relationship between serum 25(OH)D concentrations and mortality or other chronic disease outcomes in adults (EFSA NDA Panel, 2012a).
Several epidemiological studies have explored the relationship between serum 25(OH)D concentration and mortality in adult and elderly populations (Autier and Gandini, 2007; Melamed et al., 2008; Pilz et al., 2009; Hutchinson et al., 2010; Michaelsson et al., 2010;). The studies generally had measurements of serum 25(OH)D concentration at a single time point and examined associations between this measurement and the outcome (e.g. mortality) reported a few years later. Although the analyses adjusted for potential confounders, they cannot exclude the possibility of residual confounding or demonstrate causality. Furthermore, the concentrations associated with increased risk in these studies did not exceed 125 nmol/L and were well below what would be considered to be ‘high’ serum 25(OH)D concentration based on risk of hypercalcaemia or toxicity. The Panel considers that these data on chronic exposures and outcomes in adults cannot be used to determine what constitutes a ‘high’ serum 25(OH)D concentration or to inform the setting of an UL for infants, especially taking into account the relatively short period of exposure to a given amount of vitamin D during infancy and the generally decreasing intake of vitamin D per kg body weight with age.
The Scientific Opinion which described how AIs for vitamin D were set (EFSA NDA Panel, 2016), noted that ‘high serum 25(OH)D concentrations (> 220 nmol/L) may lead to hypercalcaemia, which may eventually lead to soft tissue calcification and resultant renal and cardiovascular damage’. One of the supporting references for this statement itself refers to a study by Heaney et al. (2003), in which vitamin D3 was administered daily in doses of 0, 25, 125 and 250 μg for approximately 20 weeks during the winter to 67 healthy men living in Omaha. No change in serum calcium was observed with a serum 25(OH)D concentration of 220 nmol/L at an oral intake of 250 μg vitamin D3/day.
The Endocrine Society (Holick et al., 2011) stated ‘although it is not known what the safe upper value for 25(OH)D is for avoiding hypercalcaemia, most studies in children and adults have suggested that the blood levels need to be above 150 ng/mL [375 nmol/L] before there is any concern. Therefore, a UL of 100 ng/mL [250 nmol/L] provides a safety margin in reducing risk of hypercalcaemia’.
A global consensus recommendation from 11 international scientific organisations, 10 of them paediatric (Munns et al., 2016), also stated that ‘toxicity is defined as hypercalcemia and serum 25(OH)D > 250 nmol/L with hypercalciuria and suppressed PTH. […] To allow a large safety margin, the consensus group felt it prudent to use the concentration of 250 nmol/L as the recommended upper limit of serum 25(OH)D – even if symptomatic toxicity from RCTs has only been reported at levels > 500 nmol/L’.
The Panel notes the heterogeneity of the values proposed by the scientific bodies and medical societies to define ‘high’ serum 25(OH)D concentration, and that they are not specifically derived for infants.
3.3.6.2. ‘High’ serum 25(OH)D concentration: studies from risk of bias tier 2
Holmlund‐Suila et al. (2012) randomised breastfed infants to receive vitamin D3 doses between 10–40 μg/day from 2 weeks to 3 months of age (Sections 1, 3.3.2, 3.3.3 and 3.3.5). The authors defined ‘high’ serum 25(OH)D concentration in this study as > 150 nmol/L measured by automated chemiluminescence immunoassay (CLIA, which does not recognise the C3‐epimer according to Bailey et al. (2013)). Individual ‘high’ serum 25(OH)D concentration > 150 nmol/L was reported, in 7/35 and 20/37 infants from the 30 and 40 μg/day groups, respectively. No infant developed hypercalcaemia and there was no difference in the rate of hypercalciuria between groups. Although the concentration was not used in this study as ‘high’ serum 25(OH)D concentration, the Panel also notes that some of the infants (n = 4 out 37) receiving 40 μg/day of vitamin D had 25(OH)D concentrations beyond 200 nmol/L.
Gallo et al. (2013a) (Sections 1, 3.3.2, 3.3.3, 3.3.4 and 3.3.5) supplemented infants with vitamin D3 doses between 10 and 40 μg/day from 1 to 12 months. The authors reported that 15/16 infants in the 40 μg per day group reached the ‘high’ serum 25(OH)D concentration specified in the stopping rules for the trial (250 nmol/L at the age of 3 months measured using enzyme‐linked immunosorbent assay (ELISA)) and were subsequently given 10 μg/day. However, no infant was symptomatic and no abnormalities of growth were observed during the study. Although this analytical method was not used in this study for the stopping rules concentration, the Panel also notes that, reading on the figure reporting the individual curves of serum 25(OH)D concentration measured by LC‐MS/MS according to age in each group, at age 3 months, some of the infants receiving 30 μg/day (n = 3 out 27) and more infants receiving 40 μg/day (n = 5 out of 13) had serum 25(OH)D concentrations beyond 200 nmol/L.
Grant et al. (2014) (Section 3.3.3.3) randomly supplemented pregnant women daily during pregnancy and their infants from birth to 6 months (vitamin D3 at 25 μg to the mother/10 μg to the infant or 50 μg to the mother/20 μg to the infant, compared with placebo). At 2 months, ‘high’ serum 25(OH)D concentration, defined in this study as > 250 nmol/L measured by LC‐MS, was reported in one infant from the 25/10 μg/day group (325 nmol/L) and in four infants from the 50/20 μg/day group (260, 320, 325, 335 nmol/L). None of these infants had ‘hypercalcaemia’ defined by ‘elevated serum calcium concentration’ (> 11.2 mg/dL, i.e. 2.8 mmol/L).
Czech‐Kowalska et al. (2012) (Sections 3.3.2.3 and 3.3.3.3) studied prospectively 30 term infants with baseline serum 25(OH)D concentration < 50 nmol/L receiving vitamin D3 supplements (median total intake of about 18 μg/day). The authors reported data on ‘high’ serum 25(OH)D concentration for 30 term infants, measured by CLIA. Serum 25(OH)D concentration > 150 nmol/L was reported in 12 infants (40%), of whom five had 25(OH)D > 200 nmol/L. None of the infants had hypercalcaemia, and the incidence of hypercalciuria did not differ between infants with ‘normal’ or ‘high’ serum 25(OH)D concentration. Seven infants from the study had hypercalciuria (defined as urinary Ca/Cr ratio > 0.8 mg/mg, i.e. 2.27 mmol/mmol), of whom three had serum 25(OH)D concentrations > 150 and 1 > 250 nmol/L.
3.3.6.3. ‘High’ serum 25(OH)D concentration: studies from risk of bias tier 3
Vervel et al. (1997) fed infants (born to mothers with or without vitamin D supplementation during pregnancy) vitamin D fortified milk and randomised them to receive daily either 12.5 μg or 25 μg D2 (Sections 1.4 and 3.3.3.4). None of the infants had serum 25(OH)D concentration, measured by competitive protein‐binding assay (CPBA), above the ‘upper limit of observed values in healthy adults’ (100 nmol/L)’, the highest reaching 87.5 nmol/L at 1 month or 92.5 nmol/L at 3 months.
3.3.6.4. ‘High’ serum 25(OH)D concentration: other data
In a preliminary analysis (n = 762 included out of 987 eligible) (Valkama et al., 2017)38 and the final analysis (n = 975 randomised out of 987 eligible) (Rosendahl et al., 2018)39 from the VIDI study (Sections 3.3.3.1 and 3.3.3.5), serum 25(OH)D concentration was measured using CLIA in infants randomised to receive either 10 or 30 μg/day vitamin D3 from 2 weeks to 2 years (489 and 486 subjects, respectively). At birth, about 95% of the infants had serum 25(OH)D concentration above 50 nmol/L. Results for the whole study population showed that 18% had serum 25(OH)D concentration > 125 nmol/L at 12 months, but this was not associated with clinical symptoms suggestive of hypercalcaemia (Valkama et al., 2017). The numbers (%) of individuals with serum 25(OH)D concentration above 125 nmol/L were 23 (4.8%) and 19 (4.0%) in cord blood in the 10 and 30 μg group, respectively, and 6 (1.5%) and 130 (32.2%) at 12 months and 13 (3.2%) and 159 (38.8%) at 24 months (Rosendahl et al., 2018). No infant had a concentration greater than 250 nmol/L. Sharma et al. (2017) reported an increase of subclinical and overt hypervitaminosis D over a 6‐year period (2011–2016) in a hospital setting in India based on 5,527 reports from patients of all ages. Hypervitaminosis D, defined in this study as serum 25(OH)D concentrations > 250 nmol/L, was found in 4.1% of the patients of whom 2.7% had serum 25(OH)D > 375 nmol/L. Patients aged < 20 years had significantly higher hypervitaminosis D than the older age groups. Serum 25(OH)D concentration had a significant positive correlation with serum calcium concentration (σ = 0.212, p < 0.001). Among the patients with serum 25(OH)D concentration in the categories 250–375 and > 375 nmol/L, 8.11% and 30.5%, respectively, had elevated serum calcium concentration (> 2.55 mmol/L). However, among patients with serum 25(OH)D concentrations in the categories 75–250 and 50–75 nmol/L, only 0.58% and 0.1%, respectively, had elevated serum calcium concentration, and no cases of elevated serum calcium were found in patients with serum 25(OH)D concentrations < 50 nmol/L.
From these papers and those discussed in the previous sections (i.e. Sections 3.3.6.2, 3.3.6.3–3.3.6.4), the Panel notes that one study (Grant et al., 2014) reported ‘high’ serum 25(OH)D concentration measured using LC‐MS when infants were 2 months of age, which could have included the C3‐epimer (thus leading to a possible overestimation of serum 25(OH)D concentration); the remaining studies used assays which do not measure the C3‐epimer (Bailey et al., 2013).
3.3.6.5. ‘High’ serum 25(OH)D concentration: conclusion
From the previous Sections (Section 3.3.6.2, 3.3.6.3–3.3.6.4) on available data from collected studies on achieved serum 25(OH)D concentration after (varying levels of) daily vitamin D supplementation in infants, no clinical symptoms suggestive of hypercalcaemia or abnormal growth were observed in infants who had serum 25(OH)D concentration above 125, or 150, or 200 or 250 nmol/L (depending on the study considered).
In the previous sections, the Panel also considered previous assessments of EFSA and other bodies that discussed ‘high’ serum 25(OH)D concentration (not specifically for infants). These values range between 125 and 250 nmol/L. The Panel considers that epidemiological data on chronic exposures to vitamin D and adverse outcomes in adults cannot be used to determine what constitutes a ‘high’ serum 25(OH)D concentration or to inform the setting of a UL for infants, especially taking into account the short period of exposure to a given amount of vitamin D during infancy and the decreasing intake of vitamin D per kg body weight with age. The Panel considers that the evidence used to define previously described cut‐offs for ‘high’ serum 25(OH)D concentrations is not sufficient to define an ‘evidence‐based’ value for ‘high’ serum 25(OH)D concentration as a safety cut‐off in infants.
However, based on previous opinions/assessments in particular that of the EFSA NDA Panel (2016) which considered that a concentration above 220 nmol/L may lead to hypercalcaemia (Section 3.3.6.1), together with available data from infants assessed in this Opinion, the Panel considers that a serum 25(OH)D concentration of 200 nmol/L or below is unlikely to pose a risk of adverse health outcomes (hypercalciuria, hypercalcaemia, nephrocalcinosis, abnormal growth) in healthy infants. This should not be regarded as a cut‐off for toxicity but as a conservative value from which a UL could be derived using a meta‐regression analysis on the relationship between daily supplemental vitamin D intake and serum 25(OH)D concentration (Section 3.5).
3.4. Case reports of vitamin D intoxication and studies using non‐daily doses of vitamin D in infants
3.4.1. Identification of papers
During the screening of the literature by EFSA, case reports on at least one of the adverse health outcomes/endpoints identified, following vitamin D intake in infants, were not considered for further data extraction, appraisal and dose–response analysis due to the high RoB. However, reports on individual cases with a clear identification of the ingested dose of vitamin D and of at least one of the adverse health outcomes/endpoints observed in infants were flagged during the screening, for possible qualitative consideration in the scientific opinion, separately from the other included study designs.
Although no requirement on frequency of vitamin D consumption was applied to the search (Appendix B), the Panel considered that changes in serum or urinary calcium concentrations or serum 25(OH)D concentrations after non‐daily or single large doses of vitamin D cannot be directly compared with those occurring after daily consumption. Thus, while trials on daily doses were selected during the literature search, for further data extraction, appraisal and quantitative analysis, trials on weekly or monthly doses, bolus doses or ‘stoss’ prophylaxis were flagged at full‐text screening, as for case reports, for possible qualitative consideration in the scientific opinion, separately from the other included study designs (Section 1.8.3). For these study types, the aim of this non‐exhaustive data collection was to possibly identify supportive evidence.
A limited data extraction from case reports and studies using non‐daily vitamin D doses was undertaken, for an easier comparison between papers (Appendix D). Through the literature search, 116 case reports were identified, from which several were excluded for diverse reasons, e.g. not on adverse outcomes in infants, not on vitamin D2 or D3, vitamin D administered to the mother and not the infant, not in the included languages, not on oral route. The remaining 89, of which the majority (2/3) were in English, and the rest in German or French, were screened by reviewers, and most of them were further excluded for diverse reasons (e.g. not in infants, premature infants, not a case report, vitamin D dose unknown, etc.). Additional case reports were identified through hand‐search.
Through the literature search by EFSA, three studies comparing one arm receiving daily dose with at least one receiving a non‐daily dose were identified (Holst‐Gemeiner et al., 1978; Gordon et al., 2008; Emel et al., 2012), as well as 18 studies only on non‐daily doses (Pietrek et al., 1976; Markestad et al., 1987; Zeghoud et al., 1994; Cesur et al., 2003; Billoo et al., 2009; Shajari et al., 2009; Shakiba et al., 2010, 2014; Manaseki‐Holland et al., 2012; Mittal et al., 2014; Harnot et al., 2017; Huynh et al., 2017) were identified and briefly discussed (Section 1.8.3).
3.4.2. Results of the case reports on vitamin D intoxication in infants
Case reports of hypervitaminosis D – when carefully documented with regard to total daily vitamin D dose and length of administration/intake as well as appearance of adverse effects – might provide an indication of the approximate magnitude of vitamin D dose that overwhelms the homoeostatic control mechanisms in infants. Cases of prolonged daily consumption of vitamin D can be stratified according to dose, e.g. a group 1 with up to 20 times the AI vs. a group 2 with much higher multiples, up to >1,000 times the AI set for infants (EFSA NDA Panel, 2016); and they should be differentiated from reports on repeated administration of single milligram doses (5–15 mg) of vitamin D which was the custom for rickets prophylaxis in some countries (Dietzsch, 1957; Braun and Kauderer, 1966; West et al., 1971; Ulbrych‐Jablonska, 1972; Misselwitz and Hesse, 1986; Mete et al., 1997; Doneray et al., 2008; Sharawat, 2016) up to the 1980s. The Panel considers that case reports on repeated vitamin D administration of single milligram doses are not informative for the identification of a LOAEL or NOAEL of chronic daily vitamin D consumption.
From the eligible case reports listed, only five belong to group 1 (Table 4) and may be considered informative because the vitamin D intake consisted of multiples (4.5–20 times) of recommended dose for the prevention of rickets (AI of 10 μg/day) for durations of 1–4 months in infants aged 2 weeks to 2 months (Marie et al., 1969; Chambellan‐Tison et al., 2007; Ahmad and Al‐Agha, 2013; Pavlovic and Berenji, 2014; Radlovic et al., 2014). All infants showed hypercalcaemia and hypercalciuria with decreased concentrations of PTH and serum concentrations of 25(OH)D at or above 175 nmol/L. Two infants had nephrocalcinosis (Ahmad and Al‐Agha, 2013; Chambellan‐Tison et al., 2007) and one of them also developed nephrocalcinosis with nephrogenic diabetes insipidus after 3 months (Ahmad and Al‐Agha, 2013). One infant who had a vitamin D intake 16 times the AI for 1 month did not develop clinical symptoms but needed more than 2 months of treatment for normalisation of abnormal laboratory values (Radlovic et al., 2014). In summary, these case reports indicate that daily vitamin D intakes between 44 and 200 μg/day by young infants can lead to signs of hypervitaminosis D within 1 month, with clinical symptoms taking somewhat longer to develop. Four of the five cases were breastfed infants, which mean that the total vitamin D intake would not be much higher than the intake from supplements. The intake in all of these cases was higher than the present UL for vitamin D.
Table 4.
Case reports of infants with hypervitaminosis D due to daily ingestion of vitamin D
| Author | Patient | Vit D dose per day | Duration of vit D intake | Symptoms | Laboratory |
|---|---|---|---|---|---|
| Daily administration of vitamin D at doses up to 20times the AI | |||||
| Marie et al. (1969) | 8‐month‐old boy born at term, 3 months full formula‐fed, then start complementary feeding; anorexia | Birth to 4 months ‘normal’ vitamin D prophylaxis;At age 4 months, start 100 μg/dayAt age 6 months, 200 μg/day |
4 months 2 months 2 months |
Anorexia starting 1 month after increase of vitamin D dose, abnormal ECG (shortening QT interval), decreased glomerular filtration, X‐ray dense end metaphyseal bands |
Ca in serum 4.5 mmol/L Ca in urine 8 mg/kg per day |
| Chambellan‐Tison et al. (2007) | 4‐month‐old boy, breastfed, prescribed 30 μg vitamin D/day, received about 70 μg/day | 70 μg/day | 4 months | Since 2 weeks anorexia, hypotonia, weakness, constipation, polyuria, dehydration, neurological symptoms, nephrocalcinosis, abnormal ECG (prolonged QT interval) |
Ca in serum 4.28 mmol/L Ionised Ca 2.52 mmol/L (normal < 1.32) Ca in urine 3.6 mmol/kg per day Ca/Cr in urine 5.3 (unit not reported) (normal 0.5) 25(OH)D 800 nmol/LPTH ↓ |
| Ahmad and Al‐Agha (2013) | Premature (30 weeks, birth weight 950 g, hyaline membrane disease treated with TPN (4 weeks) | 2–5 months of age: 35 μg/day plus vit D from formula: 40–50 μg/day) (calculated) | 3 months |
5 months of age: Weight 2.2 kg < 5th centile, hypotonia, polyuria, hypernatraemia, nephrogenic diabetes insipidus, medullary nephrocalcinosis |
5 months of age: Ca in serum 2.95 mmol/L, Ca in urine 6.52 mmol/L, 25(OH)D 175 nmol/L, PTH ↓ Na 156 mmol/L, Cl 112 mmol/L, osmolality in serum 320, in urine 20 mosmol/kg |
| Radlovic et al. (2014) | 1.5‐month‐old girl. Full‐term healthy, birth weight 3,600 g, exclusively breastfed; received by misunderstanding 16 times prophylactic dose. At age 1.5 mo, length and weight 50th centile | From age 15 days to 45 days 167 μg vitamin D3/day | 1 month | No clinical symptoms |
1.5 month: Ca in serum 2.72 mmol/L 25(OH)D > 400 nmol/L PTH ↓ Ca/Cr in urine 1.6 mg/mg (normal < 0.8 mg/mg) |
| Pavlovic and Berenji (2014) | 4.5 months with weight loss (4.25 kg i.e < 3rd centile), Breastfed for 2.5 months, thereafter formula |
33 μg/day 44 μg/day |
From 2 weeks to 2.5 months of age (D2) = 8 weeks from 2.5 months of age plus 11 μg (D3) from formula = 8 weeks |
Vomiting, constipation |
Ca in serum 4.91 mmol/L, Ca/Cr in urine 2.81 (unit not reported) (normal < 0.2), 25(OH)D >375 nmol/L PTH ↓ |
25(OH)D: 25‐hydroxy‐vitamin D, Ca/Cr: calcium/creatinine ratio, ECG: electrocardiogram, PTH: parathyroid hormone.
The other case reports from group 2 concern infants who were given extremely high daily vitamin D doses (up to > 1,000 times the recommended prophylactic dose) either because of misunderstanding and mislabelling of the preparation or without a clear explanation, for time periods of 10–60 days. All developed severe symptoms of vitamin D intoxication (Lukaszkiewicz et al., 1987; Besbas et al., 1989; Elarqam et al., 2007; Rajakumar et al., 2013; Kara et al., 2014; Smollin and Srisansanee, 2014; Ketha et al., 2015; Sagsak et al., 2015). In addition to these case reports, fatalities have been anecdotally reported due to overdoses of vitamin D2, including two children who died at the age of 20 and 16 months after having received a total dosage of 280 and 455 mg, respectively (Debre, 1948).
Recent cases of hypervitaminosis D were also reported in Denmark, due to the administration of a supplement which inadvertently provided about 750 μg vitamin D/day (Bøgevig et al., 2017; Stafford, 2016). Infants and young children who had taken this supplement were < 2 years of age and were found to have serum 25(OH)D > 150 nmol/L and serum ionised calcium of > 1.35 mmol/L up to > 1.49 mmol/L. Clinical symptoms included reduced appetite, vomiting, irritability and failure to thrive.
The Panel considers that these very high doses of vitamin D administered for 1–2 months are clearly toxic and need not be considered when searching for a LOAEL for vitamin D in infants. The Panel notes that there are no reports of signs of vitamin D overdose with an intake corresponding to the present UL of 25 μg/day (EFSA NDA Panel, 2012a).
3.5. Daily intake of vitamin D vs. mean achieved serum 25(OH)D: assessment of the dose–response relationship
The Panel performed a statistical analysis to characterise the dose–response relationship between ‘high’ daily supplemental intake of vitamin D and mean achieved serum 25(OH)D concentration in study arms in healthy infants. The aim of this analysis was to estimate the percentage of infants that might exceed a serum 25(OH)D concentration of 200 nmol/L (Section 3.3.6), which the Panel considered unlikely to pose a risk of adverse health outcomes (hypercalciuria, hypercalcaemia, nephrocalcinosis, abnormal growth patterns) in healthy infants. A detailed description of the statistical analysis performed to set up the intake–response model and to derive the percentage exceedance is provided in Annex A.
3.5.1. Identification of the final body of evidence for the analysis
Out of the 31 studies included after the extensive literature review undertaken by EFSA (Sections 3.1 and 3.2), 22 measured serum 25(OH)D concentration, four of which were prospective observational studies and the others were trials of various type of design.
In order to reduce methodological and biological heterogeneity:
-
–
observational studies were discussed narratively in earlier sections and excluded from the statistical analysis.
-
–
after preliminary analysis, studies/arms on infants with vitamin D deficiency rickets and/or pertaining to RoB tier 3 were excluded from the dose–response analysis.
The final body of evidence kept for the statistical analysis was composed of six studies (Holst‐Gemeiner et al., 1978; Gordon et al., 2008; Holmlund‐Suila et al., 2012; Gallo et al., 2013a; Grant et al., 2014; Ziegler et al., 2014), comprising 17 arms and 58 observations at different time points. Of these six studies, all administering vitamin D3 doses, five were RCTs (Appendix L). Transformation was applied to data in order to recategorise some variables (Table 5).
Table 5.
Categorising variables
| Variable | Categories |
|---|---|
| Latitude (degrees) |
1: between −40° and +40°a 2: (between −50° and −40°) or (between +40° and +50°) 3: (−50° and below) or (+50° and above) |
| Supplementation duration in months (weeks) |
0: baseline 1: (0,4] 3: (4,12] 6: (12,24] 12: > 24 |
| Analytical method for 25(OH)D |
CPBA: CPBAa LC‐MS/MS: LC‐MS/MS RIA: RIA or CLIAs NA: not specified |
| 25(OH)D baseline concentration (nmol/L) |
1: [10,30) 2: [30,60) 3: [60,100) |
CLIA: automated chemiluminiscence immunoassay; CPBA: competitive protein‐binding assay; LC‐MS/MS: liquid chromatography‐tandem mass spectrometry; RIA: radioimmunoassay.
NB: ranges expressed as [a–b) mean including a but excluding b.
Not present in the final body of evidence
3.5.2. Dose–response modelling of study arm mean serum 25(OH)D concentration and daily vitamin D supplemental intake
Mean serum 25(OH)D concentration, as the continuous outcome, was analysed by EFSA using the summary data extracted for each study arm and time point: the sample size, the mean and standard deviation of the baseline and achieved serum concentration of 25(OH)D.
Background intake was reported in only one of the included trials (Gallo et al., 2013a). The Panel decided not to impute the background intake to generate total vitamin D intake estimates, as the purpose of this assessment was to set a UL (and not an average requirement or an AI) and considering the time constraints for this assessment. Therefore, the intake–response relationship was established only on the basis of the additional dose of vitamin D provided in the six trials. The Panel considers that not including the background intake would lead to an underestimation of the vitamin D dose corresponding to the UL and assessed the approach as conservative.
This additional dose of vitamin D was always provided through a supplement and not a fortified food. The following analysis was thus done under the assumption that the differences in bioavailability of vitamin D when supplemented, naturally present or added to food could be considered limited, as only scarce data on this aspect are available (EFSA NDA Panel, 2016) (Section 5.6.3).
3.5.2.1. Moderator variables
A number of factors potentially influencing the dose–response relationship listed a priori (Section 3.2.3) were visually investigated in order to select those to be included in the model to characterise the heterogeneity of results across arms and repeated measurements. These factors included: baseline mean serum concentration, analytical methods, latitude categories, categories of supplementation duration, (mean) age (in weeks) vs. (mean) body weight (in g), feeding type at study start, categories of duration of gestation. Possible effects of genotypes (e.g. ethnicity) and some lifestyle habits were rarely reported in the included trials and therefore not further analysed.
Graphical investigation shows that higher values of the biomarker are achieved when the concentration at baseline is higher. The effect of the initial concentration is more evident at more extreme vitamin D intake levels (below 10 and above 30 μg/day). The variable was included by default in the model for biological reasons (Section 1.8.1).
Only two analytical methods used in the studies were finally included in the body of evidence (LC‐MS/MS and ‘RIA’, see Table 5). The measurements provided by the RIA method were higher for doses up to around 35 μg/day. The reverse occurs for higher doses.
For latitude, analysis indicated higher concentration of serum 25(OH)D for infants living in northern countries. This conflicts with the expectation of a lower vitamin D cutaneous synthesis at higher latitudes (Section 1.7.1.1). A possible explanation for this result was that the latitude in these studies was masking other country‐specific factors (e.g. country‐specific practices for maternal or infantile vitamin D supplementation) and/or infant sun exposure was too limited to expect an effect on the biomarker. The Panel thus decided to discard this factor from the model.
At different supplementation durations, differences in the shape of the dose–response relationship and in the achieved serum concentration of 25(OH)D were identified, ‘6‐months’ (i.e. more than 3–6 months, Table 5) was the category leading to the highest study arm mean concentrations at the highest vitamin D doses.
The Panel discussed whether body weight or age was more relevant to explain the achieved mean serum 25(OH)D concentration (Section 3.2.3). The two variables were highly correlated (r = 0.99) in the available body of evidence (multicollinearity), therefore, one variable was included in the model. Age was selected, because it was always reported for the study participants whereas body weight was sometimes missing.
As for type of feeding at the start of the study, the Panel considered that feeding can change quickly at this stage of the life and probably observations taken only at baseline are not very indicative of the feeding type in the following weeks. This variable was therefore discarded from further analysis.
The intake–response relationship did not result in a clear pattern for different categories of duration of gestation (i.e. arms composed of full‐term infants or mixed populations).
Eventually all the moderator variables from the original list were selected for the next steps of the analysis except latitude, mean body weight and type of feeding at start of the study for the reasons explained above.
3.5.2.2. Model assumptions: linearity, constant variance and normality
Normality, uniformity of the variance at the various doses (i.e. homoscedasticity) and linearity are standard assumptions in regression and meta‐regression analysis. Graphical representations can help to identify important deviations from these assumptions.
The assumption of linearity was confirmed after exploring the possible shape of the dose–response relationship, looking at the graphical display of mean serum 25(OH)D concentration with respect to vitamin D intake levels, without considering any adjustment for moderator variables (Annex A). It was concluded that the assumption of linearity seems to fit the data relatively well, except at high vitamin D intake (i.e. 40 μg/day and above), where most of the points systematically lie above or below the regression line.
From a biological viewpoint, it would be realistic to expect that the achieved serum 25(OH)D concentration levels off at high doses of vitamin D (instead of continuously increasing at the same rate). However, in the six selected trials, only infants with hypovitaminosis, i.e. serum 25(OH)D < 50 nmol/L (Gordon et al., 2008) received 50 μg of vitamin D/day. Thus, scarcity of data at high doses and characteristics of the population do not allow resolution of uncertainty around the true shape of the dose–response relationship at high vitamin D intakes in infants.
Potential deviation from the assumption of normality was also checked. Looking at the distribution of mean serum 25(OH)D concentration in the different arms and time points, it was concluded that approximation to normality is not fully achieved, since the right (upper) tail of the distribution is longer than in a normal distribution (right skewness) (Annex A). A distribution stratified by vitamin D intake doses and moderator variables would have allowed a better investigation of normality assumption. This was precluded by the limited number of observations.
As a consequence, besides the analysis using the original scale, a natural logarithmic transformation (ln‐transformation) of the response variable (mean achieved serum 25(OH)D concentration), explanatory variable (vitamin D intake) and one covariate (baseline serum 25(OH)D concentration) was applied. This ln‐transformation aimed to improve approximation to normality, reduce skewness and improve the uniformity of variance at a cost of slightly worsening the linear fit.
3.5.2.3. Best predictive model
A mixed effect meta‐regressive dose–response model was used, to explain the effect of moderator variables on the intake–response relationship and to account for the hierarchical structure in the data. Eventually, based on the overall goodness of fit, the biological relevance of the moderator variables and their statistical significance, the best predictive model identified (see Annex A) included, in addition to daily supplemental intake of vitamin D, a subset of the moderator variables, i.e. baseline mean serum 25(OH)D concentration, supplementation duration category and age. The formal structure of the best predictive model of study arm mean serum 25(OH)D concentration on vitamin D supplemental intake is described below:
-
–
achieved S25(OH)D = β0(baseline S25(OH)D) + β1(vitamin D intake) + β2(age) + β3 (supplementation duration category) + random effect of the study arm + random effect of the repeated measures within a study arm (model 1).
-
–
ln(achieved S25(OH)D) = β0ln(baseline S25(OH)D) + β1ln(vitamin D intake) + β2(age) + β3 (supplementation duration category) + random effect of the study arm + random effect of the repeated measures within a study arm (model 2).
The parameters of the variables were estimated for the two above models using respectively:
-
–
All variables in the original scale (model 1);
-
–
Ln‐transformation of the mean achieved serum 25(OH)D concentration), of vitamin D supplemental intake and of baseline serum 25(OH)D concentration (model 2), keeping age and duration in the original scale.
No major patterns were identified that could raise concerns for any of the two models regarding linearity of the relationship or the presence of outliers (see Section 1.1.6 of Annex A).
The Panel notes that the ln‐transformed model is better meeting the assumptions of normality and homoscedasticity, whereas the model in the original scale better fits linearity. The Panel also notes that both models (both scales) considered together give a sense of the uncertainty associated with the several choices made at a methodological level and their influence on the results.
3.5.2.4. Predicted distribution of study arm mean and individually achieved serum 25(OH)D concentration
From the six selected trials corresponding to 17 arms and 58 time points of measurements of mean 25(OH)D concentration, the distribution of the mean achieved level of serum 25(OH)D was simulated using the two predictive models, i.e. original and ln‐transformed described in the previous section. The predicted values were further analysed in order to simulate distributions of serum 25(OH)D concentration that may be achieved by individuals.
Predicted distribution of study arm mean serum 25(OH)D concentration
The hypothesis was that study arm means in the body of evidence represent a random sample from a theoretical population of study arm mean values whose distribution can be described conditionally to the value of the explanatory variables vitamin D intake, baseline serum 25(OH)D concentration and age. Since few observations were available in the body of evidence, an empirical distribution was generated with a large number of simulations (random draws) to approximate better the true distribution.
To make the model predictions more realistic, a probability distribution was used for the baseline value of serum 25(OH)D concentration, to reflect the variability that is expected for this factor in a theoretical population of studies.40 The distribution was elicited based on expert's knowledge. A truncated normal distribution of baseline serum 25(OH)D concentration, with a mean of 50 nmol/L, a standard deviation of 20 nmol/L and range between 10 and 100 nmol/L, was considered realistic by the Panel (Annex A). A truncated normal distribution was preferred to avoid values biologically unrealistic (i.e. baseline study mean concentrations below 10 and above 100 nmol/L).
In addition, a range of 5–50 μg/day for daily supplemental vitamin D intake (range covered in the body of evidence) and a range of 1–52 weeks for the age of the infants (corresponding to the target population age) were considered appropriate (Annex A).
The supplementation duration category was always set at ‘6 months’ (i.e. more than 3 months up to 6 months, Table 5). This duration was chosen since it was the one corresponding to the highest achieved serum 25(OH)D concentration at higher doses among the selected studies (Section 3.5.2.1).
Monte Carlo techniques (Burmaster and Anderson, 1994) were used to combine the empirical distribution of the baseline serum 25(OH)D concentration and values of daily vitamin D intake and age, to predicted study arm mean of the achieved serum 25(OH)D concentration. A total of 1,196,000 predictions were generated. The empirical distributions and the related quartiles of the predicted mean achieved serum 25(OH)D obtained using the model in the original and ln‐transformed scale are provided in Annex A.
Predicted distribution of individually achieved serum 25(OH)D concentration
Each predicted value of the study arm mean 25(OH)D concentration was used to simulate a distribution of individually achieved concentration of the biomarker (serum 25(OH)D).
An average coefficient of variation was estimated from the selected studies and applied to the study arm means to derive the interindividual variability of the populations of individually achieved serum 25(OH)D concentration.
Two sets of distributions of individually achieved serum 25(OH)D concentrations were generated. Each set was stratified by baseline serum 25(OH)D concentration (categories: 10 to 30 nmol/L; 30 to 60 nmol/L; 60 to 100 nmol/L) and supplemental vitamin D intake (classes from 5 to 50 μg/day, of size 5 μg/day). The percentage of infants with an individually achieved estimated serum concentration of 25(OH)D above 200 nmol/L was computed, separately for the infants below the age of 6 months and between 6 and 12 months.
The predictions of percentage of individuals above a certain serum 25(OH)D concentration obtained on the basis of the two models – the one in the original scale and the one in ln‐transformed scale for response, intake and baseline value of the biomarker – have been interpreted jointly.
3.5.2.5. Results – Percentage of infants exceeding a serum concentration of 25(OH)D
The predicted percentage of infants expected to exceed 200 nmol/L for serum 25(OH)D concentration is reported in Tables 6, 7 for the models in the original and ln‐transformed scale, (i) for infants up to the age of 6 months and (ii) and between 6 months and 12 months.
Table 6.
Percentage of infants up to 6 months of age (26 weeks included) exceeding the serum 25(OH)D concentration of 200 nmol/L. Model in the original and ln‐transformed scale
| Table 6a: original scale | Table 6b: ln‐transformed scale | |||||||
|---|---|---|---|---|---|---|---|---|
| Vitamin Dintake (μg/day) | %infants with achieved serum 25(OH)D concentration exceeding 200 nmol/L | %infants with achieved serum 25(OH)D concentration exceeding 200 nmol/L | ||||||
| For baseline serum 25(OH)D (nmol/L) | For baseline serum 25(OH)D (nmol/L) | |||||||
| [10, 30) | [30,60) | [60,100] | Any | [10, 30) | [30,60) | [60,100] | Any | |
| [5–10) | 0 | 0 | 0 | 0 | 0 | 0.1 | 1.4 | 0.5 |
| [10–15) | 0 | 0 | 1 | 0 | 0 | 0.2 | 2.2 | 0.8 |
| [15–20) | 0 | 1 | 2 | 1 | 0 | 0.3 | 2.9 | 1.0 |
| [20–25) | 0 | 2 | 4 | 2 | 0 | 0.4 | 3.5 | 1.3 |
| [25–30) | 1 | 3 | 7 | 4 | 0 | 0.5 | 4.1 | 1.5 |
| [30–35) | 3 | 6 | 11 | 7 | 0 | 0.6 | 4.6 | 1.7 |
| [35–40) | 6 | 10 | 16 | 11 | 0 | 0.7 | 5.2 | 1.9 |
| [40–45) | 10 | 15 | 21 | 17 | 0 | 0.8 | 5.6 | 2.1 |
| [45–50) | 15 | 20 | 27 | 22 | 0 | 0.9 | 6.0 | 2.3 |
Table 7.
Percentage of infants between 6 and 12 months of age (26 weeks excluded) exceeding the serum 25(OH)D concentration of 200 nmol/L. Model in the original and ln‐transformed scale
| Table 7a: original scale | Table 7b: ln‐transformed scale | |||||||
|---|---|---|---|---|---|---|---|---|
| Vitamin D intake (μg/day) | %infants with achieved serum 25(OH)D concentration exceeding 200 nmol/L | %infants with achieved serum 25(OH)D concentration exceeding 200 nmol/L | ||||||
| For baseline serum 25(OH)D (nmol/L) | For baseline serum 25(OH)D (nmol/L) | |||||||
| [10, 30) | [30,60) | [60,100] | Any | [10, 30) | [30,60) | [60,100] | Any | |
| [5–10) | 0 | 0 | 0 | 0 | 0 | 0 | 0. 6 | 0.2 |
| [10–15) | 0 | 0 | 0 | 0 | 0 | 0.1 | 1 | 0.3 |
| [15–20) | 0 | 0 | 0 | 0 | 0 | 0.1 | 1.4 | 0.5 |
| [20–25) | 0 | 0 | 1 | 0 | 0 | 0.1 | 1.7 | 0.6 |
| [25–30) | 0 | 1 | 2 | 1 | 0 | 0.2 | 2.1 | 0.7 |
| [30–35) | 1 | 2 | 4 | 2 | 0 | 0.2 | 2.4 | 0.8 |
| [35–40) | 2 | 4 | 8 | 5 | 0 | 0.3 | 2.7 | 1.0 |
| [40–45) | 4 | 7 | 12 | 8 | 0 | 0.3 | 3.0 | 1.1 |
| [45–50) | 7 | 11 | 17 | 12 | 0 | 0.3 | 3.3 | 1.2 |
NB: ranges expressed as [a–b) mean including a but excluding b.
For infants younger than 6 months, based on the results of the prediction model in the original scale (Table 6a), at a vitamin D intake of up to 25 μg/day, which is the UL previously set by EFSA NDA Panel (2012a), depending on the baseline serum 25(OH)D concentration, 0–4% of infants would achieve serum 25(OH)D concentrations above 200 nmol/L. The results of the ln‐transformed scale, up to a vitamin D supplemental dose of 25 μg/day, are consistent with the results in the original scale (Table 6b).
For infants between 6 and 12 months of age, the predicted percentage of individuals exceeding serum 25(OH)D concentrations of 200 nmol/L would be 0–1% at supplemental vitamin D intake of up to 25 μg/day, 0–2% at intakes of up to 30 μg/day and 1–4% at intakes at of up to 35 μg/day (Table 7a, original scale). For the ln‐transformed scale, these percentages range from 0 to around 2% for doses up to 35 μg/day (Table 7b).
As indicated in Section 3.5.2.3, both models (both scales) considered together give a sense of the uncertainty associated with the several choices made at a methodological level and their influence on the results. The above results represent predictions obtained from modelling, simulations and related assumptions (previously specified). The exceedance percentages should not be interpreted as precise estimates, rather as informed quantitative judgements about the expected percentage of infants that might exceed the serum 25(OH)D concentration at the various vitamin D intake, given baseline values of the biomarker and age groups (Annex A).
3.5.2.6. Unaddressed sources of uncertainty
Some uncertainty sources could not be addressed in this statistical analysis while developing the intake–response model and deriving percentages of exceedance. They are listed below:
-
–
The intake–response relationship is estimated using aggregated data, i.e. study arm mean value. The relationship observed averaging across trials might not be the same as the one observed within a trial. Some potential confounding or moderators have not been measured in the studies and could not be included in the model
-
–
The interindividual variability necessary to estimate distribution of individually achieved serum 25(OH)D concentration was unknown and it was estimated on the basis of the mean coefficient of variation across study arms assumed to be a proxy of the interindividual variability.
-
–
Sampling uncertainty, i.e. the uncertainty in the serum 25(OH)D measurement arising from the sample of studies used, was not accounted for. The mean prediction has been adopted (to calculate the percentages of individuals exceeding a certain serum 25(OH)D concentration) instead of the upper bound of the prediction interval of the prediction.
4. Assessment of the daily vitamin D intake in infants (≤ 1 year) in Europe
4.1. Food composition data
4.1.1. Food composition data either published or collected through a consultation of MS by the European Commission
4.1.1.1. Composition data on fortified foods in Finland
The highest number of fortified foods notified in the Finnish marked between 2012 and 2016 is represented by ‘milk and milk products’ (n = 197), of which 75% contained at least 1 μg/100 g of vitamin D (overall, median content of 1 μg/100 g, which is also the value of 116 milk products, i.e. 60% of the total) (Evira, 2017). The second most frequent category are ‘special diet products’, including, e.g. IF and FoF and processed cereals, with a median content of 2.5 μg/100 g, followed by other fortified foods containing between 1 and 20 μg/100 g.
4.1.1.2. Composition data of IF and FoF in France
In 2012, data declared on the labels were studied for IF (n = 36) and FoF (n = 43) and young child formulae (n = 50), covering 89% of the volume of the French market for these products (in supermarkets and pharmacies, excluding products for special medical uses) (Oqali, 2014). Median‐labelled energy contents was 67 kcal/100 mL for IF and FoF. Median‐labelled vitamin D contents were 1.0 μg/100 mL for IF and 1.10 μg/100 mL for FoF. Consumption volumes recommended on labels were about 0.7–1 L/day for the first 6 months and about 0.6 L/day for the second 6 months of age.
4.1.1.3. Composition data of fortified foods in the Netherlands
To assess vitamin D intake in Dutch infants, Verkaik‐Kloosterman et al. (2017a) used composition data from the Dutch database (NEVO 201141), complemented with labelled composition data from an inventory of vitamin D fortified foods on the Dutch market and intended for children aged 0–4 years, carried out in 2011.42 Food groups identified as voluntarily fortified were porridge and breakfast cereals, infant cookies, dairy products43 and drinks,44 with a vitamin D content that ranged from 0.74 to 16.5 μg/100 g.
4.1.1.4. Composition data from an analytically measured data set on vitamin D
Data on vitamin D composition of foods were compiled based on EuroFIR standards (Milesevic et al., 2018) with the aim of creating a data set on vitamin D content in foods based on analytical data. Analytical data were mainly collected from (i) nine national databases from or outside the EU with documented values of total vitamin D, D2, D3 and 25(OH)D (i.e. excluding estimations or imputations), or (ii) from the literature or (iii) specifically for fortified foods, from data of manufacturers (collected by the Nordic countries or the Netherlands). The most common analytical method was HPLC. Most of the data were extracted from meat, fortified foods/formulations and fish groups. Most of the data were on total vitamin D and were obtained for fortified foods, novel foods, ‘formulations’ and milk and dairy products. Data from vitamin D3 mostly come from fish, meat and dairy groups. Data on vitamin D2 were lacking in the European data set, contrary to the US Department of Agriculture (USDA) database (mushrooms and milk substitutes). Data on 25(OH)D3 came from meat and fish groups, eggs and dairy products. IF were not a stand‐alone category but were grouped by the authors in the category ‘fortified formulations’, including also meal replacements and ‘functional snacks and powders’, with varying vitamin D content per 100 g.
4.1.1.5. Data on overages45 from the Netherlands and the UK
Verkaik‐Kloosterman et al. (2017b) measured with HPLC the content of vitamin D in 29 fortified foods (mainly FoF and porridge) and 15 dietary supplements available on the Dutch market and intended for infants aged 6–12 months. For fortified foods, the results showed that the analysed content was 50–153% of the values declared on the labels. In particular, the analysed vitamin D content was between 50 and 127% of declared values for ‘instant baby porridge’ and between 74 and 81% for ‘ready‐to‐eat baby porridge’. Analysed content was 102–153% of the labelled value for FoF and between 8 and 177% for supplements.
Allen et al. (2014) described the methodology and results of the update of the National Diet and Nutrition Survey (NDNS) Nutrient Databank46 in 2011 for 257 of the 289 foods or supplements identified as fortified with vitamin D (both vitamin D2 and D3). The authors mention that information received from industry indicated that ‘overages’47 ranged between 20% and 30% for vitamin D fortified foods and 20% and 40% for vitamin D supplements. The authors considered it appropriate to apply a standard overage of 25% to all products. They also estimated a 50% reduction in ‘overage’ at consumption, after possible losses of vitamin D during processing and through degradation. Thus, for this study, a ‘blanket overage’ of 12.5% was applied to the vitamin D content of all fortified foods and supplements to cover the overages reported by the manufacturers and possible losses due to degradation and processing. When calculating the updated vitamin D intake, by using the consumption data from the NDNS 2008–2010 and the updated vitamin D composition data, the results showed an increase of 3% (6% considering the overage of 12.5%) in the daily vitamin D intake (from 3.5 to 3.6 μg/day).
4.1.1.6. Conclusion on food composition data published or collected through a consultation of MS
The Panel notes the limited published data on food fortified with vitamin D that may be consumed by infants in different EU countries. The Panel notes in particular the content of vitamin D in fortified milk and milk products in Finland. The Panel also notes a limited number of papers reporting on possible disparities between labelled values and actual (analysed) contents (overages) of fortified foods and supplements.
4.1.2. Food composition data from the EFSA nutrient composition database
For a specific intake assessment, composition data for ‘vitamin D’ and for cholecalciferol (vitamin D3) in foods and beverages were derived from the EFSA Nutrient Composition Database (Roe et al., 2013). In case, no country‐specific data were available for certain food codes, national data compilers who provided the database to EFSA borrowed compatible data from other countries and/or from similar foods. Regarding harmonisation of provided data, all food entries in this database are classified according to the food classification and description system FoodEx2 (EFSA, 2011a).
Data on vitamin D, originating from 12 countries48 and covering 3,027 records related to 394 different FoodEx2 codes, were available after data cleaning (Section 4.4.1.2). While fortification was among the facet descriptors of this database, information on whether a given food code was fortified with vitamin D was generally not available. The method used by national data compilers to obtain the composition data on vitamin D3 or ‘vitamin D’ provided to EFSA for all these food records were mostly (about 2/3) not known. For the remaining food records, depending on the country, vitamin D was analytically measured (in 6 of 12 countries) mostly via HPLC, or obtained via different calculation procedures (at recipe or ingredient level) or by imputations. Three countries stated that total vitamin D content was calculated for some food records as the sum of vitamin D3 and 25(OH)D3 49 (applying a factor of 5–25(OH)D3), and possibly vitamin D2.
4.1.3. Food composition data from Mintel Global New Products Database (GNPD)
For the intake assessment, EFSA gathered data on the levels of vitamin D in fortified foods from the Mintel Global New Products Database (GNPD). This is an online database, which monitors product introductions in markets of consumer packaged goods worldwide. Mintel GNPD started covering the food markets of the European Union in 1996, currently presenting data from 20 of its 28 member states and Norway. At the time of the search, it contained information on over 2 million food and beverage products, of which more than 900,000 are or have been available on the European food market. In particular, Mintel GNPD shows the ingredient list, the labelled table of nutrient information and pictures of the packages of the products.
For the purpose of this scientific opinion, EFSA searched Mintel GNPD, in particular:
-
–
to check the frequency of the presence of ‘vitamin D’/D2/D3 in the ingredient list, thus identifying products fortified with vitamin D within the EU's food products,
-
–
and to check the labelled content of this vitamin D.
4.1.4. Composition data for foods for infants with regulated vitamin D content
Commission Directive 2006/141/EC 4 (Section 1.1) set the range (minimum and maximum values) for vitamin D content in IF and FoF to 1–2.5 and 1–3 μg vitamin D/100 kcal, respectively. For both types of formulae, the energy content should range between 60 and 70 kcal/100 mL. Commission Delegated Regulation (EU) 2016/127 3, which will repeal Directive 2006/141/EC from 2020 (Section 1.1), revises these values and set the same vitamin D levels for IF and FoF, in the range 2–3 μg/100 kcal. According to these regulatory texts, IF are the only processed foodstuff which wholly satisfies the nutritional requirements of infants during the first months of life until the introduction of appropriate complementary feeding (while FoF are intended for infants when appropriate complementary feeding is introduced).
Commission Directive 2006/125/EC 50 prescribes that ‘baby foods’ cannot be fortified with vitamin D. ‘Cereals with an added high‐protein food which are or have to be reconstituted with water or other protein‐free liquid’ have to be mandatorily fortified at levels ranging between 1 and 3 μg/100 kcal (content in food ready for use). ‘Simple cereals which are or have to be reconstituted with milk or other appropriate nutritious liquids’, ‘pastas which are to be used after cooking in boiling water or other appropriate liquids’, ‘rusks and biscuits which are to be used either directly or after pulverisation, with the addition of water, milk or other suitable liquids’, can be fortified to a maximum of 3 μg/100 kcal (content in food ready for use). For these last three food categories, no minimum content for vitamin D is specified.
4.2. Food consumption and vitamin D intake or modelling data
4.2.1. Vitamin D intake or modelling data, either published or collected through a consultation of Member States by the European Commission
4.2.1.1. Intake of vitamin D in infants: previous assessments by EFSA
In its opinion on nutrient requirements and dietary intakes of infants and young children in the EU, EFSA indicated that ‘in 18 of the 23 countries from which information was available to EFSA, vitamin D supplementation, mainly in the form of drops or tablets, but also cod liver oil in Nordic countries, is recommended on a national level. The duration of supplementation, however, varies and ranges from the first year of life to 18 years’ (EFSA NDA Panel, 2013).
The opinion on the essential composition of IF and FoF (EFSA NDA Panel, 2014a) indicated that ‘mean/median vitamin D intakes of formula‐fed infants below six months of age are reported to be around 9–10 μg/day in infants (Noble and Emmett, 2006; Fantino and Gourmet, 2008; Lennox et al., 2013) and 3.5 μg/day in breastfed infants (Lennox et al., 2013). For infants aged 6 to < 12 months, mean/median vitamin D intakes were observed to be in the range 3.6–10.4 μg/day (Noble and Emmett, 2001; de Boer et al., 2006; DGE, 2008; Fantino and Gourmet, 2008; Marriott et al., 2008; Thorsdottir et al., 2008; Lennox et al., 2013)’.
4.2.1.2. Intake modelling and intake data from the Netherlands
Verkaik‐Kloosterman et al. (2017a) aimed to evaluate the risk of excessive vitamin D intake in children aged 0–19 months, considering food fortification and supplementation in the Netherlands, and assuming a 100% compliance with vitamin D supplementation of 10 μg/day.
For infants aged 0–6 months, as no Dutch consumption data were available, the habitual vitamin D intake (assuming no complementary food) was modelled considering daily energy requirement, an energy content of 65 kcal/100 mL of formula, and the vitamin D content of IF reported in 2011 (1.8 μg/100 kcal).
-
–
From formulae only, median‐estimated vitamin D intake in infants 0–6 months ranged from 6.4 (girls aged 0–1 month) to 11.7 μg/day (boys aged 5–6 months) and the P97.5 ranged from 7.9 to 14.7 μg/day for these age/sex groups.
-
–
Assuming a 100% compliance with vitamin D supplementation of 10 μg/day, the median‐estimated total vitamin D intake (formulae and supplements) ranged from 16.4 to 21.7 μg/day and the P97.5 ranged from 17.9 to 24.7 μg/day. Thus, none of these estimated values were exceeding the UL of 25 μg/day (EFSA NDA Panel, 2012a).
For infants 7–12 months, Verkaik‐Kloosterman et al. (2017a) used consumption data from the VoedingsstoffenInnameOnderzoek (VIO) cross‐sectional study conducted in a nationally representative population of healthy term, non‐breastfed, infants with a birth weight of at least 2.5 kg.
-
–
Median‐estimated vitamin D intake from food was 10.9 μg/day at 7 months and 5.7 μg/day at 12 months, respectively, and 95th percentile (P95) ranged from 13.5 to 16.7 μg/day.
-
–
Assuming a 100% compliance with vitamin D supplementation of 10 μg/day, median total vitamin D intake (from food including supplements) ranged between 15.4 and 21.1 μg/day at 12 and 7 months, respectively, and P95 ranged from 23 to 26.5 μg/day (P99: 27–29 μg/day). The authors concluded that 4–11% of infants in the age range 7–11 months are at risk of exceeding the UL of 25 μg/day (EFSA NDA Panel, 2012a). The authors report of some overages51 and the probable lower compliance with vitamin D supplementation.
At the age 10–11 months, median and P95 estimated vitamin D intake from foods from another (non‐nationally representative) study (Eat Complete study) (De Jong‐Rubingh and Bausch‐Goldbohm, 2015) presented by the Member State do not exceed the UL of 25 μg/day (5.3 and 13.1 μg/day).
4.2.1.3. Intake modelling from Ireland
The Food Safety Authority of Ireland52 modelled the intake of vitamin D from formulae and supplements in infants, growing at 0.4th, 50th and 99.6th centiles on the WHO growth curve. This choice of high percentile (P99.6) was to account for the fact that, at birth, infants in Ireland are heavier than in other countries and tend to be heavier than WHO growth standards,53 and thus to ensure that 95% of infants in Ireland would be covered. Most infants in Ireland are formula‐fed.
The model considered a daily intake of formula of 150 mL/kg body weight for ages 1–6 months, progressively decreasing until 12 months of age to account for introduction of complementary feeding, and the maximum vitamin D content of 3 μg/100 kcal and an energy content of 65 kcal/100 mL in formula. Supplementation with 5 μg/day vitamin D recommended for this age54 was also considered.
Modelled vitamin D intake from formula only and for boys aged 3–6 months at P99.6 was at or above the UL of 25 μg/day (EFSA NDA Panel, 2012a). Modelled total vitamin D intake (from formula and supplements) at P99.6 was higher than the UL for ages 2, 3, 4, 5, 6 and 7 months, and at P50 for ages 5 and 6 months. Data for girls were reported as being similar. The lower and higher bound (−20%, +50%) of the tolerance55 around a labelled vitamin D content of supplements of 5 μg was also considered in the modelling. Thus, the highest modelled P99.6 of total vitamin D intake was obtained at 6 months: 38 μg/day for a P99.6 of body weight of 10.5 kg.
4.2.1.4. Intake data from Belgium
A cross‐sectional study in Belgium (VITADEK study) (Moyersoen et al., 2017, 2018) aimed at assessing the intake of fat soluble vitamins from the consumption of foods (mandatorily or voluntarily fortified56 or not fortified) and dietary supplements, in particular in infants (0–11 months, n = 455). The national vitamin D supplementation is 10 μg/day for the age 0–10 years, although varying in the Flemish (10–15 μg/day) and French‐speaking (15–30 μg/day) parts of Belgium.
The P95 of estimated vitamin D intake were higher for formula‐fed infants than for breastfed infants. The P95 of estimated intake from food excluding vitamin D provided by supplements remained below 14 μg/day at all ages in formula‐fed infants thus below the UL of 25 μg/day (EFSA NDA Panel, 2012a). When considering vitamin D supplementation, the P95 of estimated total vitamin D intake were at or above 25 μg/day for infants 0–12 months and all subpopulations considered. The proportion of infants with total vitamin D intake above the UL was 6% (0–6 months) and 17% (7–12 months) in breastfed infants and 17% (0–6 months) and 26% (7–12 months) in formula‐fed infants. Supplements represented 92% and 87% of total vitamin intake in breastfed infants aged 0–6 months and 7–11 months, respectively, and 53% and 56% in formula‐fed infants aged 0–6 months and 7–11 months, respectively.
4.2.1.5. Intake data from Denmark
In the Danish National survey conducted in 2006–2007 (Trolle et al., 2013), for infants, estimated vitamin D intake from foods was reported (mean and different percentiles) for males and females, for the following age ranges: 6–7 months, 8–9 months, 10–11 months.57 The highest P95 estimated intake of vitamin D (from foods) was found between 6 and 9 months (11–12 μg/day). According to the authors, this highest intake in this age range was a result of the intake of IF and FoF and ‘synthetic porridge’. When considering the additional contribution from vitamin D supplementation, which at the time of the survey (2006) was 10 μg/day for all infants between 14 days and 1 year as recommended by national policy, the authors indicate that no infant exceeded the UL of 25 μg/day (EFSA NDA Panel, 2012a). The authors report that supplements contribute to the total vitamin D intake for around 60–70% (all age groups), and that most of the infants received (not always daily) supplementation as drops.
4.2.1.6. Intake data from the UK
Within a UK nationally representative sample of children aged 4–18 months (n = 2,246) (Lennox et al., 2013), estimated the P95 of vitamin D intake from food only was 8.2–9.6 μg/day in breastfed infants and 14.8–15.6 μg/day in non‐breastfed infants. Estimated P95 of total vitamin D intake (from foods including supplements) was always below the UL of 25 μg/day (EFSA NDA Panel, 2012a), and in the range of 9.6–15.7 μg/day in breastfed infants and 15.7–17.1 μg/day in non‐breastfed infants.
Percentage contribution of food sources (without supplements) to vitamin D intake is reported only for non‐breastfed infants. IF and FoF were the highest contributors to vitamin D intake in non‐breastfed infants (72–85% depending on age considered). They were followed by ‘commercial infant foods’ (9%–12%), particularly ‘cereal‐based foods and dishes’ (6%–7%).
4.2.1.7. Intake data from Finland
Healthy infants in Helsinki were recruited for a RCT (ongoing at the time of the publication) of vitamin D supplementation (10 or 30 μg/day, VIDI study) from age 2 weeks to 2 years, and vitamin D intake from a cross‐sectional analysis of these subjects at age 1 year are presented (Hauta‐Alus et al., 2017). The authors indicate that, since 2010, vitamin D3 fortification in Finland is 1 μg/100 mL of fluid dairy product and 20 μg/100 g of dietary fats.
Mean (SD, range) estimated vitamin D intake in partially breastfed and non‐breastfed infants was 3.8 (3.0, 0–30.7) and 7.5 (3.2, 0.6–28.3) μg/day, respectively.
Among all infants, 95% consumed ‘mass‐produced baby foods’. Porridges (milk‐based or ‘mass‐produced baby food’) and ‘infant formulae’ were the top contributors to vitamin D intake in both breastfed (25 and 11%, respectively) and non‐breastfed (19 and 31%, respectively) infants. Other major contributors to vitamin D intake were fish‐based dishes particularly in breastfed infants (24%) and milk particularly in non‐breastfed infants (17% for skimmed milk, 12% for low‐fat milk). Contribution of meat dishes and dietary fats is also described.
4.2.1.8. Conclusion on vitamin D intake or modelling data, either published or collected through a consultation of Member States by the European Commission
From these data, the Panel notes that ‘high’ percentiles (e.g. P95) of estimated vitamin D intake from foods without supplements in infants were generally below the UL of 25 μg/day (EFSA NDA Panel, 2012a). However, ‘high’ percentiles (e.g. P95) of estimated vitamin D intake from foods including supplements in infants may be beyond the UL.
4.2.2. Individual food consumption of infants in the EU available to EFSA
To estimate dietary intake of vitamin D, EFSA used food consumption data from the EFSA Comprehensive Food Consumption Database (EFSA, 2011b).58 This database provides a detailed compilation of existing information on food consumption in the EU, nationally representative (or regionally representative as for the Finnish survey), and classified according to the food classification and description system FoodEx2. Consumption data were collected at individual level using single or repeated 24‐ or 48‐hour dietary recalls or dietary records covering from 3–7 days.
For infants aged below 4 months, at the time this assessment was undertaken, food consumption data were only available from one survey carried out in Bulgaria, and thus were not used for the intake assessment. The methodology applied by EFSA for the intake assessment in this age group is described in Section 4.3.1.
For infants aged 4 to less than 12 months, the intake assessment included food consumption data from dietary surveys on subjects with at least two reporting days from six countries: Bulgaria, Denmark, Finland, Germany, the UK and Italy, covering in total 3,375 individuals (Table 1). Infants of different ages participated in these surveys. In particular, the Finnish survey included relatively young infants compared to the other surveys, since the age of all subjects was rounded to 0.5 or 1 years by the authors of the survey and all subjects at 1 year were considered as ‘toddlers’ (children aged 1–< 3 years) in the EFSA Comprehensive Food Consumption database. Considering the very few infants from the Italian survey, limited conclusions can be drawn from it.
The identification of these two age categories (below and above 4 months of age) in the population targeted in this opinion also accounts for the additional vitamin D intake in relation to the introduction of complementary feeding (EFSA NDA Panel, 2009).59 (Table 8)
Table 8.
Surveys in infants for which individual data were available to EFSA
| Country | Survey | Number of subjects | Number of formula consumers | Dietary assessment | Age (years) | |
|---|---|---|---|---|---|---|
| min | max | |||||
| Bulgaria | NUTRICHILD 2007 | 510 | 220 | 2‐d 24‐h recall | 0.42 | 0.92 |
| Denmark | IAT 2006_07 | 826 | 598 | 7‐d weighted DR | 0.44 | 0.99 |
| Finland | DIPP_2001_2009 | 500 | 303 | 3‐d weighted DR | 0.5 | 0.5 |
| Germany | VELS 2001 | 159 | 110 | 6‐d DR | 0.4 | 0.99 |
| Italy | INRAN_SCAI_2005_2006 | 11 | 6 | 3‐d DR | 0.4 | 0.9 |
| UK | DNSIYC_2011 | 1,369 | 1,188 | 4‐d weighted DR | 0.39 | 0.99 |
DIPP: Type 1 Diabetes Prediction and Prevention survey; DR: dietary record; IAT: survey on Infants And Toddlers; INRAN–SCAI: Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione – Studio sui Consumi Alimentari in Italia; min: minimum; max: maximum; VELS: Verzehrsstudie zur Ermittlung der Lebensmittelaufnahme von Säuglingen und Kleinkindern für die Abschätzung eines akuten Toxizitätsrisikos durch Rückstände von Pflanzenschutzmitteln; UK: United Kingdom.
Table 8 shows the number of formula consumers in each survey, defined as individuals who have consumed infant or FoF at least once over the reporting days. Thus, the term ‘formula consumers’ in this scientific opinion and related Annex B should not be considered as synonymous to ‘non‐breast‐fed infants’ (e.g. some infants may have consumed formula and breast milk). Previous intake assessments for scientific opinions on DRVs showed that most infants in these surveys were partially breastfed, with a proportion of breastfed infants of 58% in the Finnish survey, 40% in the German survey, 44% in the Italian survey, 21% in the UK survey. Overall surveys on infants included in this assessment, this proportion is 33%. In the Finnish survey, breast milk intake was not taken into consideration in the intake estimates as no information on the breastfeeding events was reported. The Panel notes the limitations in the methods used for assessing breast milk consumption in infants60 and related uncertainties in the vitamin D intake estimates for infants. However, considering the low vitamin D content of breast milk, this was not considered by the Panel to be a major source of uncertainty.
From these surveys on infants undertaken between 2001 and 2011, limited information was available on consumption of dietary supplements containing vitamin D:
-
–
Consumer percentages per country: vitamin supplements (any vitamin) reported to be consumed in Finland, UK and Italy; the percentage of consumers was 90% in the Finnish survey but only 6% in the UK survey and 19% in the Italian survey, which, however, reported only on three consumers;
-
–
names of the vitamin supplements consumed, without composition data.
As noted by EFSA NDA Panel (2016), the ESPGHAN Committee on Nutrition recommends a daily oral supplementation of 10 μg vitamin D for all infants during the first year of life starting from birth onwards (Braegger et al., 2013) (Section 1.6). However, there are different national supplementation policies for vitamin D in infants in the EU (Section 1.1), and compliance with these policies may also vary between countries. In addition, no information was available in these six surveys on vitamin D intake of infants through consumption of vitamin D‐containing medications. In this context, and considering the background of the mandate for this scientific opinion (Section 1.1) related to the change in regulatory content of vitamin D in IF, the Panel did not make any assumption, in its intake assessment, regarding vitamin D supplementation.
However, as an indication, EFSA undertook a search in the Mintel GNPD Database over the last 5 years (thus on products available after the latest survey considered) and for all EU countries included in the Database. Overall countries with data on vitamin D supplements (19 countries, 151 products), data on vitamin D supplements61 for infants aged < 1 year were available from 12 countries and 46 products in five different formats (capsules, drops, tablets, chewing pastilles or powder). Out of these products (44 of them containing vitamin D3, the rest unspecified), 33 provided an intake of 10 μg/day, with different conditions of use and formats (mostly drops or twist‐off capsules). The remaining products, according to the different conditions of use, provided for a vitamin D amount between 1.75 and 15 μg/day (Appendix Q). The Panel notes that, although some information on composition of the products was available in the Mintel GNPD, it showed that there was variability in the additional amount of vitamin D from supplements (1.75–15 μg/day). This database does not provide information on food consumption, in particular on supplement consumption.
The Panel decided to perform the intake assessment separately for infants below and above 4 months of age, to account for the additional vitamin D amount in relation to the introduction of complementary feeding (Section 1.6). For the intake assessment for infants aged from 4 to less than 12 months, food consumption data from nationally or regionally representative dietary surveys on infants were available from six countries. For the reasons described above, the Panel did not make any assumption in its intake assessment regarding vitamin D supplementation.
4.3. Intake calculations for infants aged 0–< 4 months (formula consumers) undertaken by EFSA
4.3.1. Approach for the calculations (formula consumers aged 0–< 4 months)
For the assessment of the intake of vitamin D for infants aged less than 4 months, the Panel used the default value for high formula consumption proposed by the EFSA Scientific Committee et al. (2017b) (Section 2). In this Guidance Document, assuming that in non‐breastfed infants, formula is the only source of nutrition for the first 16 weeks of life, the default value of 260 mL/kg body weight per day among infants aged about 1 month is defined as the highest relative consumption on a body weight basis for the whole period of the first 16 weeks. This value is also in line with data on formula consumption among formula consumers (i.e. not the full study population) in the Bulgarian survey (Section 4.2.2).
The Panel also used for these calculations the P95 of formula consumption in infants aged about 4 months, based on the same Guidance Document and derived from the same study, i.e. 195 mL/kg body weight per day at 4 months.
As the Panel sets an UL based on an intake on a daily basis (and not relative to body weight), the Panel multiplied the default value by reference body weights. For these calculations, median body weights of boys and girls were used. The choice of the median body weights was based on the need to make calculations for a reference/standard situation. The Panel considered that the choice of a higher percentile (e.g. P95) would have been reflecting an extreme scenario and therefore overconservative. The Panel assumes that the high consumption of formula expressed in mL per kg body weight per day may be observed among the heaviest infants but also the lightest who may be experiencing catch‐up growth.
Median body weights used for the calculations came from two sources:
-
–
median weights for ages 1 and 4 months of male and female infants according to the WHO Growth Standards (WHO Multicentre Growth Reference Study Group, 2006). These were created from longitudinal primary growth data and related information from 8,440 healthy breastfed infants and young children from diverse ethnic backgrounds and cultural settings (Brazil, Ghana, India, Norway, Oman and USA). The Panel notes that only one European country (Norway) was considered in this data set; however, the WHO Growth Standards are widely used in EU countries.
-
–
median measured body weights of EU male and female infants according to van Buuren et al. (2012). This external scientific report was provided to EFSA and compiles anthropometry data for infants and children (0–18 years) from the 27 EU Member States at the time of the report. For the calculations, median body weights at age 4–5 weeks (as a proxy for age 1 month) and age 18 weeks (as a proxy for age 4 months) were considered.
Calculations were performed with the maximal content of vitamin D allowed both in the Directive 2006/141/EC and Commission Delegated Regulation (EU) 2016/127 (2.5 and 3 μg/100 kcal, respectively), and an energy content from IF between 60 and 70 kcal/100 mL, as provided by both legislative texts. Although possible ‘overage’ has been reported, e.g. in formulae (Section 4.1.1.5), the Panel did not make assumptions regarding possible ‘overage’ in these calculations. The Panel also did not make assumptions on intake provided by vitamin D supplementation (Section 4.2.2).
4.3.2. Results of the calculations (formula consumers aged 0–< 4 months)
Results are provided in Table 9. Estimated intakes of vitamin D above the present UL of 25 μg/day for infants (EFSA NDA Panel, 2012a) are indicated in bold and italics.
Table 9.
Calculation for intake assessment of infants aged less than 4 months
| Age | Reference body weight (kg) | Absolute volume of formulae consumed (mL/day) | Calculated vitamin D intake according to Directive 2006/141/EC | Calculated vitamin D intake according to Delegated Regulation (EU) 2016/127 | ||||
|---|---|---|---|---|---|---|---|---|
| min–max (μg/day)c | min–max (μg/day)d | |||||||
| Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | |
| Default value at 1 mo=260 mL/kg body weight per day e | ||||||||
| 1 mo | 4.5a | 4.2a | 1,170 | 1,092 | 18–21 | 16–19 | 21–25 | 20–23 |
| 4 wk | 4.2b | 4b | 1,092 | 1,040 | 16–19 | 16–18 | 20–23 | 19–22 |
| 5 wk | 4.4b | 4.2b | 1,144 | 1,092 | 17–20 | 16–19 | 21–24 | 20–23 |
| Default value at 4 mo=195 mL/kg body weight per day e | ||||||||
| 4 mo | 7a | 6.4a | 1,365 | 1,248 | 21–24 | 19–22 | 25–29 | 23–26 |
| 18 wk | 6.6b | 6.2b | 1,287 | 1,209 | 19–23 | 18–21 | 23–27 | 22–25 |
Median reference weight according to WHO Growth Standards (WHO Multicentre Growth Reference Study Group, 2006).
Median weight according to the report from (van Buuren et al., 2012).
Range of maximal values of vitamin D content in infant formulae calculated as 2.5 μg/100 mL × 60–70 kcal = 1.5–1.75 μg/100 mL
Range of maximal values of vitamin D content in infant formulae calculated as 3 μg/100 mL × 60–70 kcal = 1.8–2.1 μg/100 mL
Default values according to EFSA Scientific Committee et al. (2017b).
Considering the maximal regulated vitamin D in IF in Commission Delegated Regulation (EU) 2016/127 (3 μg vitamin D/100 kcal), the Panel notes that, depending on the reference body weight, ‘high’ formula consumption, age of the infants (about 1 or 4 months) and energy content of formulae (without considering additional intake due to supplementation and without considering possible overages62):
-
–
some of these calculated ‘high’ vitamin D intake are below the UL of 25 μg/day set in 2012,
-
–
others are above this UL of 25 μg/day and remain below 30 μg/day.
Considering the maximal regulated vitamin D content in IF in Directive 2006/141/EC (2.5 μg vitamin D/100 kcal), the Panel notes that all estimated vitamin D intakes indicated in Table 9 were below the UL of 25 μg/day set in 2012 (without considering additional intake due to supplementation and without considering possible overages).
4.4. Intake assessment for infants aged 4–< 12 months undertaken by EFSA
4.4.1. Approach for the intake assessment (age 4–< 12 months)
4.4.1.1. Selection of data on natural vitamin D content in foods
Among foods that naturally contain vitamin D (i.e. that are not fortified with this vitamin), sources of vitamin D3 include animal foods (e.g. fish, offal, meat, egg yolks) (Section 1.5), for which vitamin D content may differ according to factors including the vitamin D content of the animal feed (EFSA NDA Panel, 2016). For potential natural sources of vitamin D2, i.e. higher fungi, the content may depend on whether or not mushrooms have been cultivated in the dark or whether yeast has been exposed to UV (Kristensen et al., 2012; EFSA NDA Panel, 2014b).
In this intake assessment, the effect of cooking practices (baking, steaming, etc.) was not taken into account. For milk or fermented milk products, skimmed, semiskimmed or whole milk were not considered separately (vitamin D content was averaged for unfortified milk, see Section 4.4.1.3). Similarly, for meat and meat products, the different meat cuts for a given animal (e.g. collar, shoulder, leg, fillet) were not considered separately.
4.4.1.2. Selection of data on fortified foods: outlier analysis and data cleaning
As information on the level of fortification of foods was generally not available from the EFSA Nutrient Composition Database (Section 4.1.2), EFSA performed an outlier analysis of its data set to identify any value which deviated from the others for a given food code. The objective was to discriminate values that were due to variability in their natural vitamin D content from values due to fortification. In addition, possible misclassifications of food codes in a given food group were corrected and/or the records deleted.
EFSA performed the outlier analysis on food categories for which a more than 10‐fold difference between the minimum and the maximum values of vitamin D content was identified in the EFSA Nutrient Composition Database, with more than 10 eating occasions in the EFSA Comprehensive Food Consumption Database.
-
–
When no outliers were identified for a given food code, the mean content of vitamin D was taken as a unique value.
-
–
Whenever outliers were identified for a given food code, highest and lowest values were compared with published data, national food composition databases available online as well as with data from the Mintel Database and recipes available on the Internet (for composite foods, e.g. biscuits). If not part of the natural variability according to this search, the highest vitamin D contents were assumed to be due to vitamin D fortification. A few foods (e.g. avocado, meat burger) showing implausible vitamin D content (as provided to EFSA by some national databases) were excluded.
As additional check specifically to discriminate foods with natural content of vitamin D from foods fortified with vitamin D, EFSA performed searches in Mintel GNPD to identify foods containing vitamin D as an ingredient, marketed in the EU in the last 5 years (2012–2017). This search showed a high variability in fortification practices within the EU, with regard to the number of foods identified with vitamin D as an ingredient per country63 or the frequency of such foods within each Mintel food category per country.64
As a result of the outlier analysis and the reality check in Mintel, 58 food categories for which food consumption data were available for infants in the EFSA Comprehensive Food Consumption Database, and which could contain vitamin D according to the EFSA Nutrient Composition Database, were considered for this assessment of vitamin D intake. For 14 of them, the possibility of fortification with vitamin D was taken into account. For each food category, for the fortified and non‐fortified versions when applicable, an average content of vitamin D was estimated (Section 4.4.1.3).
Within the 58 food categories which could contain vitamin D, in case of ‘zero’ values for some foods, only values considered plausible and part of the natural variability were included in the calculations (e.g. non‐fortified breakfast cereals). The ‘zero’ values considered as not plausible were excluded from the calculations (e.g. zero vitamin D in whole cow milk) or reclassified accordingly.
For some food codes of animal origin, identified discrepancies in vitamin D content between countries that provided data to EFSA may have been due to the use by some national food composition databases of a factor of 5 to convert 25(OH)D present in animal foods into total vitamin D (EFSA NDA Panel, 2016) (Section 4.1.2). In such cases, corrections were applied by EFSA to convert all values to vitamin D3.
Dilution/conversion factors were used based on a previous report65 that defined them on the basis of information from the Mintel GNPD database, which confirmed the usual factors used in other risk assessment areas.
-
–
For IF and FoF reported in the Consumption Database as powder, a conversion factor of 8 was used to convert all forms into liquid.
-
–
For processed cereal‐based foods for infants, as the regulated vitamin D contents are expressed for the reconstituted forms (i.e. forms ready for use, see Section 4.1.4), different factors were used to convert all consumption data into values for these forms. A conversion factor of 4 was used for ‘simple cereals which have to be reconstituted with milk or other appropriate nutritious liquids’.66 A factor of 7 was instead used for ‘cereals with an added high protein food which have to be reconstituted with water or other protein‐free liquid’.67
4.4.1.3. Selection of values for vitamin D content in non‐fortified and fortified foods
For its intake assessment, the Panel considered the vitamin D content of the following fortified foods:
-
–
The regulated contents of IF and FoF and processed cereals‐based foods for infants and young children (Section 4.1.4). As the regulated vitamin D contents were expressed per 100 kcal of foods ready for use, EFSA collected data on the specific energy content of each specific food category among processed cereals‐based foods for infants (Directive 2006/125/EC50) in the Mintel GNPD Database. EFSA used the median energy content to convert the regulated vitamin D contents into values per 100 g or mL (Table 10). For IF and FoF, the minimal and maximal regulated contents were considered (thus, no distribution of vitamin D content in formulae between these two extremes was considered) (Table 10).
-
–
For fortified milk, the Panel chose the vitamin D content of 1 μg/100 mL, as the maximum content present in the EU market, in Finland (Table 11).
-
–
For the remaining foods consumed by infants, the mean vitamin D content as indicated in the EFSA Nutrient Composition Database was considered (Section 4.1.2). For the remaining 11 food categories possibly fortified, EFSA used the mean vitamin D content (Table 11) as indicated in this EFSA Database for the fortified and non‐fortified versions after data cleaning (Section 4.4.1.4).
Table 10.
Vitamin D content considered for three mandatorily fortified food categories for infants
| Food category | Mean content (μg/100 g or 100 mL) | |||||
|---|---|---|---|---|---|---|
| ‘Old’ regulation (Directive 2006/141/EC) | ‘New’ regulation (Del. Reg.(EU) 2016/127) | Directive 2006/125/EC | ||||
| Min | Max | Min | Max | Min | Max | |
| Infant formulae | 0.60 | 1.75 | 1.20 | 2.10 | ||
| Follow‐on formulae | 0.60 | 2.10 | 1.20 | 2.10 | ||
| Cereals with an added high‐protein food reconstituted | 0.81 | 2.43 | ||||
Table 11.
Vitamin D content considered for 11 food categories considering or not fortification
| Food category | Mean content (μg/100 g or 100 mL) | |
|---|---|---|
| Non‐fortified | Fortified | |
| Biscuits, rusks and cookies for children | 2.80 | 10.00 |
| Biscuits | 0.40 | 2.20 |
| Cheese | 0.29 | 2.10 |
| Margarines and similar | 0.28 | 5.97 |
| Milk and dairy powders | 0.20 | 2.10 |
| Dairy dessert and similar | 0.15 | 0.44 |
| Milk | 0.14 | 1.00 |
| Fermented milk products | 0.07 | 0.27 |
| Breakfast cereals | 0.00 | 2.08 |
| Dairy imitates | 0.00 | 1.04 |
| Simple cereals for infants or children, reconstituted | 0.00 | 2.43 |
Although ‘overage’ in fortified foods (or dietary supplements) has been reported (Section 4.1.1.5), EFSA did not make any assumptions regarding possible overage for this intake assessment.
An additional reality check specifically on the vitamin D content of the fortified versions of the food categories in Table 11 was conducted by EFSA in the Mintel GNPD Database. This search was done over the last 5 years and for five of the countries that provided infant food consumption data from dietary surveys (Italy, Denmark, Finland, UK, Germany, Table 8).68 This reality check (Table 12) confirmed that the mean values of vitamin D content taken into account in the intake assessment for the foods considered to be fortified were equal or below the most frequent values reported for these fortified foods available on the market according to Mintel (no overestimation). As the available data on intake of vitamin D in infants collected from the literature and Members States showed that the main contributors to vitamin D intake were food supplements followed by mandatorily fortified foods (e.g. formulae) (Section 4.2.1), further refinements on the composition data for voluntarily fortified foods were not deemed necessary for this assessment.
Table 12.
Comparison of the vitamin D content of fortified food categories in EFSA Nutrient Composition Database and in Mintel GNPD database
| Food category fortified with vitamin D | Vitamin D content (from EFSA's data)a | Vitamin D content (fromMintel GNPD) | Number of products in Mintel GNPD |
|---|---|---|---|
| mean (μg/100 mL or /100 g) | mode (μg/100 mL or /100 g) | ||
| Dairy imitates | 1 | 0.75 | 241 |
| Cheese | 2.10 | 6.25 | 29 |
| Margarines and similar | 5.97 | 7.5 | 191 |
| Milk and dairy powders | 2.10 | 2 (as prepared/reconstituted) | 7 |
| Dairy dessert and similar | 0.44 | 0.75 | 138 |
| Milk | 1.00 | 1 | 26 |
| Fermented milk products | 0.27 | 0.75 | 272 |
| Breakfast cereals | 2.08 | 4.2 | 281 |
| Biscuits | 2.2 | 1.5 | 34 |
| Biscuits and rusks for infants | 10 | 10 | 5 |
GNPD: Global New Products Database.
EFSA Nutrient Composition Database (Roe et al., 2013). Date of searches: December 2017–February 2018.
4.4.1.4. Proposed intake scenarios for infants aged 4–< 12 months
The intake of vitamin D from food (without dietary supplements) was calculated at the individual level by multiplying the average daily consumption for each food or food groups with the corresponding vitamin D content, summing up the respective intakes throughout the diet. In principle, inaccuracies in this matching of food consumption and food composition data may lead to uncertainties (either under or overestimation); however, these were assumed to be limited. No assumptions were made about the consumption of dietary supplements or medications containing vitamin D by infants (Section 4.2.2).
In the context of the present mandate, EFSA estimated distributions of vitamin D intake from food (P5, P95, median and mean) in two subgroups: formula consumers and non‐formula consumers. Formula consumers were defined as those consuming formula at least once during the day (Section 4.2.2). EFSA considered different scenarios to estimate vitamin D intake from food (without considering dietary supplements) in infants aged 4–< 12 months. Summary results for formula consumers are discussed in the core text of this opinion, while detailed results for formula and non‐formula consumers are available in Annex B (Excel® file).
Two main scenarios were defined: ‘fortified’ and ‘non‐fortified’ with vitamin D. Further detailed scenarios were defined based on the minimal and maximal vitamin D content of infant and FoF, which are the main source of vitamin D in infants, according to the two regulatory texts for these food categories (Table 10).
Scenarios 1–4 (‘non‐fortified’ scenarios) consider all foods at their natural vitamin D content (including zero) and the three food categories mandatorily fortified with vitamin D (Table 10): infant and FoF and cereals with an added high protein food reconstituted with water or other protein‐free liquid (a subcategory of processed cereal‐based foods for infants and young children).
Scenarios 5–8 (‘fortified’ scenarios) consider the 11 food categories (Table 11) with a vitamin D content considered to be due to fortification and the three food categories mandatorily fortified with vitamin D.
The different scenarios take into account IF and FoF fortified at the minimum (scenarios 1 and 3, 5 and 7) or the maximum (scenarios 2 and 4, 6 and 8) regulated vitamin D content, from both regulatory texts (Directive 2006/141/EC and Commission Delegated Regulation (EU) 2016/127) (Sections 1.1 and 4.1.4). They also take into account processed cereals‐based foods for infants and young children at their minimum (scenarios 1 and 3) and/or maximum (scenarios 6 and 8) regulated values (Directive 2006/125/EC) (Section 4.1.4).
Depending on the scenario, for the 11 food categories listed in Table 4, the vitamin D content which is considered natural, or the content after fortification, was used for the calculations for the ‘non‐fortified’ or ‘fortified’ scenarios, respectively. For example:
-
–
in scenarios 1–4, whenever biscuit consumption was reported at individual level, it was assumed that all foods from the category ‘biscuit’ were non‐fortified. Thus, the average vitamin D content of the ‘non‐fortified version’ observed in the EFSA Nutrient Composition Database for this food category (i.e. 0.4 μg/100 g) was used.
-
–
On the contrary, in scenarios 5–8, whenever biscuit consumption was reported at individual level, it was assumed that all foods from the category ‘biscuit’ were fortified at the average level of 2.2 μg/100 g.
Detailed results on estimated vitamin D intake (P5, P95, median and mean) for each scenario are available in Annex B (Excel® file), and summary results are discussed in Section 4.4.2.
From this approach, major points need to be highlighted:
-
–
In the ‘fortified scenarios’ (scenarios 5–8), no assumption was made on the proportion of foods within each of the 11 food categories that may be voluntarily fortified.
-
–
Consumption of none, one or up to all of the 11 food categories that can be voluntarily fortified may have been reported at individual level. This means that, for example, if for a given individual, eating occasions were reported for all of these 11 food categories, all were considered as non‐fortified (scenarios 1–4) or as fortified (scenarios 5–8) at the level indicated in Table 11.
-
–
This means that, while the food consumption data are representative of the country in which the survey was made (e.g. Bulgaria, UK, Denmark), the composition data used in the fortified scenarios were not meant to reflect the fortification practices of this specific country. The scenarios were rather defined as describing ‘extreme’ situations that may occur on the EU market regarding voluntarily fortified foods, taking into account different possible food patterns in infants and the two ‘extremes’ in the range of the vitamin D content in mandatorily fortified foods.
4.4.2. Results of the intake assessment for infants aged 4–< 12 months
Among formula consumers, across the eight scenarios and the six surveys considered by EFSA for its intake assessment, the mean contribution of IF or FoF to vitamin D intake from food (without dietary supplements) was variable and detailed results are reported in Annex B (Excel® file). The mean contribution of IF and FoF (combined) across scenarios and countries ranged from 31% to 97%. This is in line with available data published or collected from Member States (Section 4.2.1) indicating that the main contributors to vitamin D intake from food (without dietary supplements) were the mandatorily fortified foods (including processed cereal‐based foods).
As previously mentioned (Section 4.4.1.4), the eight scenarios were defined as describing situations that may occur on the EU market regarding voluntarily fortified foods, taking into account different possible food patterns in infants and the two extremes in the range of the vitamin D content in mandatorily fortified foods (they were not meant to reflect the fortification practices of specific countries). Observed food patterns were not available for infants in nationally representative surveys from all EU Member States and food fortification practices vary substantially from one country to the other. The Panel notes that this should be born in mind when discussing estimated intakes of vitamin D in different scenarios.
Tables 13 and 14 report estimated intake of vitamin D (mean, and P95, i.e. ‘high consumers’ for vitamin D) in formula consumers, for the scenarios based on Commission Delegated Regulation (EU) 2016/127. Detailed results for these scenarios and this regulatory text as well as results for scenarios based on Directive 2006/141/EC and results for non‐formula consumers are available in Annex B (Excel® file). The Panel notes that estimated vitamin D intakes from food (without dietary supplements) are much lower in non‐formula consumers than in formula consumers (discussed below): estimated mean intakes in non‐formula consumers across surveys and scenarios were all below the AI for infants of 10 μg/day and even estimated intakes at P95 of non‐formula consumers were at or below the AI (EFSA NDA Panel, 2016).
Table 13.
Mean vitamin D intake from food (without dietary supplements) of infants 4–12 months, formula consumers only, under Commission Delegated Regulation (EU) 2016/127, in four scenarios (S)
| Country | Survey (food consumption data) | N of subjects | Mean vitamin D intake (μg/day) | |||
|---|---|---|---|---|---|---|
| ‘non‐fortified’ scenariosa | ‘fortified’ scenariosb | |||||
| Min | Max | Min | Max | |||
| S3 | S4 | S7 | S8 | |||
| Bulgaria | NUTRICHILD | 220 | 7.1 | 11.3 | 9.6 | 13.9 |
| Germany | VELS | 110 | 4.5 | 7.1 | 7.2 | 9.8 |
| Denmark | IAT 2006_07 | 598 | 5.3 | 8.5 | 6.9 | 10.1 |
| Finland | DIPP_2001_2009 | 303 | 5.6 | 9.5 | 6.2 | 10.2 |
| UK | DNSIYC_2011 | 1,188 | 7.2 | 11.8 | 9.1 | 13.8 |
| Italy | INRAN_SCAI_2005_06 | 6 | 5.1 | 8.7 | 6.5 | 10 |
DIPP: Type 1 Diabetes Prediction and Prevention survey; DR: dietary record; IAT: survey on Infants And Toddlers; INRAN‐SCAI: Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione – Studio sui Consumi Alimentari in Italia; min: minimum; max: maximum; VELS: Verzehrsstudie zur Ermittlung der Lebensmittelaufnahme von Säuglingen und Kleinkindern für die Abschätzung eines akuten Toxizitätsrisikos durch Rückstände von Pfl anzenschutzmitteln; UK: United Kingdom.
Minimum (S3) and maximum (S4) vitamin D regulated content in formulae, no voluntarily fortified foods considered.
Minimum (S7) and maximum (S8) vitamin D regulated content in formulae, mandatory and voluntarily fortified foods considered.
Table 14.
P95 vitamin D intake from food (without dietary supplements) of infants 4–12 months, formula consumers only, under Commission Delegated Regulation (EU) 2016/127, in four scenarios (S)
| Country | Survey (food consumption data) | N of subjects | P95 vitamin D intake (μg/day) | |||
|---|---|---|---|---|---|---|
| “non‐fortified” scenariosa | “fortified” scenariosb | |||||
| Min | Max | Min | Max | |||
| S3 | S4 | S7 | S8 | |||
| Bulgaria | NUTRICHILD | 220 | 12.1 | 19.9 | 16.7 | 24.6 |
| Germany | VELS | 110 | 8.2 | 13.2 | 12.9 | 16.9 |
| Denmark | IAT 2006_07 | 598 | 9.7 | 16.4 | 11.2 | 17.7 |
| Finland | DIPP_2001_2009 | 303 | 10.3 | 17.9 | 10.4 | 18.1 |
| UK | DNSIYC_2011 | 1,188 | 11.2 | 18.8 | 13.5 | 20.5 |
| Italy | INRAN_SCAI_2005_06 | 6 | * | * | * | * |
DIPP: Type 1 Diabetes Prediction and Prevention survey; DR: dietary record; IAT: survey on Infants And Toddlers; INRAN‐SCAI: Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione – Studio sui Consumi Alimentari in Italia; min: minimum; max: maximum; VELS: Verzehrsstudie zur Ermittlung der Lebensmittelaufnahme von Säuglingen und Kleinkindern für die Abschätzung eines akuten Toxizitätsrisikos durch Rückstände von Pflanzenschutzmitteln; UK: United Kingdom.
* 95th percentile intakes calculated from fewer than 60 subjects require cautious interpretation as the results may not be statistically robust (EFSA, 2011b) and, therefore, for these dietary surveys, the 95th percentile estimates are not presented in the intake results.
Minimum (S3) and maximum (S4) vitamin D regulated content in formulae, no voluntarily fortified foods considered.
Minimum (S7) and maximum (S8) vitamin D regulated content in formulae, mandatory and voluntarily fortified foods considered.
Annex B and Tables 13, 14 indicate (in red in Annex B, and in italics in the Tables) estimated intakes below the AI for infants of 10 μg/day. Intakes close to the UL of 25 μg/day for infants set in 2012 (EFSA NDA Panel, 2012a) were also identified (in bold and italics in the Tables). This information is provided for the reader in order to help interpret the magnitude and distribution of the estimated intakes in the different scenarios. The reader of Annex B is also kindly invited to take into account national food consumption patterns, national food fortifications practices and national supplementation policies to estimate vitamin D intakes in his/her own country.
Scenarios 3 and 4 represent, respectively, the estimates for the scenarios with the minimum (2 μg/100 kcal) and the maximum (3 μg/100 kcal) regulated content of vitamin D in infant and FoF according to the Commission Delegated Regulation (EU) 2016/127 that will come into force in 2020 (further called ‘new’ regulation).
For comparison purposes, scenarios 7 and 8 represent the ‘worst‐case’ scenarios with, respectively, the minimum and the maximum vitamin D regulated content set according to the proposed values in Commission Delegated Regulation (EU) 2016/127 that will come into force in 2020.
The Panel notes that, for formula consumers, the mean intakes are mostly below the AI for the ‘non‐fortified’ scenarios (3 and 4) and around the AI for the fortified scenarios (7 and 8). The P95 intake for the ‘non‐fortified’ scenarios is around the AI considering the minimum vitamin D regulated content, whilst P95 intakes for scenarios 7 and 8 are above the AI but below 25 μg/day.
Considering the range of vitamin D intake coming from formula consumption with composition according to Commission Delegated Regulation (EU) 2016/127, depending on the food patterns of formula consumers observed in a specific country, the Panel notes that:
-
–
High consumers (P95 of vitamin D intake) may consume approximately between about 10 and 25 μg/day vitamin D from foods in countries with high frequency of food fortification (scenarios 7–8)
-
–
High consumers (P95 of vitamin D intake) may consume approximately between about 8 and 20 μg/day vitamin D from foods in countries with low frequency of food fortification (scenarios 3–4)
Assuming all formulae contain vitamin D at the maximum regulated content, the impact of voluntary food fortification at current vitamin D content is shown by comparing scenarios 4 and 8. Depending on the food patterns and the age of the infants, the Panel notes that the impact of food fortification may contribute up to around 5 μg/day extra at P95.
In comparison, the difference in estimated intakes at P95 between the ‘old’ (Directive 2006/141/EC) (scenario 6) and ‘new’ regulation (Commission Delegated Regulation (EU) 2016/127) (scenario 8) is smaller (e.g. around 3 μg at P95) (Annex B).
The Panel also notes that although the consumption of IF or FoF has a large impact on vitamin D intake, the additional impact of changing from the ‘old’ to the ‘new’ maximum vitamin D content is relatively small. There is little difference between the highest estimated intake at the mean or P95 for the ‘old’ and ‘new’ scenarios (Annex B), i.e. comparing:
-
–
Scenarios 2 and 4 among the ‘non‐fortified scenarios’ (highest mean of about 11 vs. about 12 μg/day, and highest P95 about 17 vs. about 20 μg/day, respectively)
-
–
Scenarios 6 and 8 among the ‘fortified scenarios’ (highest mean of about 13 vs. about 14 μg/day, and highest P95 about 22 vs. about 25 μg/day, respectively).
5. Derivation of a Tolerable Upper Intake Level for vitamin D for infants
Based on the proposed mechanism of toxicity (Section 1.9) and previous assessments by EFSA and other bodies (Sections 1.4 and 1.10), the Panel focussed on the following four adverse health outcomes: hypercalciuria, hypercalcaemia, ectopic calcification, e.g. nephrocalcinosis, and abnormal growth patterns. The adverse outcomes associated with ‘high’ daily vitamin D intake in infants were examined by the Panel in trials and prospective observational studies obtained through a systematic literature review (Section 3). Hypercalciuria is considered as the earliest sign of excessive vitamin D intake (Section 1.9).
5.1. Hypercalciuria
Two prospective observational studies and three RCTs were available, which measured urinary Ca/Cr ratios in healthy infants supplemented with vitamin D up to 40 μg/day for a period of 6 weeks up to 11 months (Section 3.3.2). These studies showed inconsistent results with considerable discrepancies in the rate of hypercalciuria (from 3–6% to 39%). There was no dose–response relationship between urinary Ca/Cr ratios and the administered dose of vitamin D in any of these studies (Appendix M).
The Panel notes the methodological limitations of these studies in relation to design that the definition of hypercalciuria based on the value of urinary Ca/Cr ratio varied between studies and that urinary Ca/Cr ratios were assessed in single spot urine samples which limit their ability to provide reliable information on the total amount of calcium excreted with the urine.
The Panel considers that available data on the relationship between vitamin D intake and the risk of hypercalciuria cannot be used for deriving the UL of vitamin D for infants.
5.2. Hypercalcaemia
Sixteen papers (each covering one or several studies) measured serum calcium and/or reported on hypercalcaemia in healthy infants supplemented with vitamin D up to 50 μg/day for up to 11 months (Section 3.3.3). This number includes the few papers used in the former EFSA Opinion (EFSA NDA Panel, 2012a), and two additional papers published after the end of the literature search (Section 3.1). These studies analysed either serum total calcium or ionised calcium. Three of the studies (four papers) reported cases of hypercalcaemia defined as ‘high’ serum calcium concentration. Among them, one RCT reported suspected hypercalcaemia based on ionised calcium in a few infants in each of the study groups supplemented with vitamin D doses from 10 to 40 μg/day. Clinical symptoms suggestive of hypercalcaemia were not observed in any of these studies, and there was no indication of an association between the dose of vitamin D and risk of hypercalcaemia. This absence of a dose–response relationship was also obvious from forest plots of mean serum total calcium and supplemental intake of vitamin D (up to 50 μg/day) in study arms at different time points, in infants below and above 6 months of age (Figure 2).
The Panel notes that the definition of hypercalcaemia varied between studies, and often only single measurements of serum calcium were reported which may not provide an indication of the risk of sustained elevated serum calcium. The Panel also notes that in case reports, clinical symptoms suggestive of hypercalcaemia have been associated with vitamin D intakes of 44 μg/day and above (Section 3.4).
The Panel considers that available data on the relationship between vitamin D intake and the risk of hypercalcaemia do not allow conclusions to be drawn for deriving the UL of vitamin D for infants.
5.3. Ectopic calcification (e.g. nephrocalcinosis)
The risk of developing nephrocalcinosis as a result of vitamin D3 supplementation with 12.5 μg/day for 2 months was considered in one study in healthy infants (Section 3.3.4). This study did not find any cases of nephrocalcinosis, but the Panel notes that the dose of vitamin D was well below the current UL (close to the AI of 10 μg/day (EFSA NDA Panel, 2016)). In another study on healthy infants supplemented with doses up to 40 μg/day of vitamin D3 for 11 months, additional safety procedures (electrocardiogram or renal ultrasound) in those with ‘suspected’ hypercalcaemia or ‘suspected’ hypercalciuria did not reveal any abnormalities in secondary analysis (Sections 3.3.2 and 3.3.3).
The Panel notes the scarcity of data on ectopic calcification in relation to vitamin D intake and considers that the available data cannot be used for deriving the UL of vitamin D for infants.
5.4. Abnormal growth patterns
Eleven papers (some of them on studies of small scale) investigated growth and/or anthropometric parameters at specific ages (rather than increments over time which would better represent growth) in healthy infants supplemented with vitamin D up to 40 μg/day for up to 11 months (Section 3.3.5). Since the previous Opinion (EFSA NDA Panel, 2012a), which described the results from earlier studies that did not provide consistent results, three additional RCTs reported on growth of infants supplemented with vitamin D (up to 40 μg/day) as secondary outcomes. The forest plots (Appendix P) provided no evidence of a dose–response relationship between daily vitamin D supplemental intake over the range of doses tested (i.e. up to 40 μg/day) and absolute mean values of attained length and weight at different time points. The Panel notes that growth was not a primary endpoint of the investigation in most of the studies.
The Panel considers that the available data on the relationship between vitamin D intake and the risk of abnormal growth patterns cannot be used for deriving the UL of vitamin D for infants.
5.5. Conclusion on daily vitamin D intake and adverse health outcomes
The Panel notes the absence, for ethical reasons, of data on the systematic administration of defined vitamin D doses above 50 μg/day to healthy infants.
The Panel concludes that the available data on adverse health outcomes (hypercalciuria, hypercalcaemia, nephrocalcinosis, abnormal growth patterns) cannot be used alone for deriving the UL of vitamin D for infants.
5.6. Serum 25(OH)D concentration
The level of daily vitamin D intake or serum 25(OH)D concentration that is associated with the occurrence of adverse health outcomes could not be determined by the Panel on the basis of the available data. In consideration of the uncertainties associated with the available evidence from studies on daily vitamin D intake and various adverse health outcomes in infants (Section 5.5), the Panel could not define a NOAEL for vitamin D intake. However, the Panel notes the proposed mechanisms by which sustained elevated serum 25(OH)D concentrations could lead to adverse health outcomes (Section 3.3.6). For reasons described in Section 3.3.6, the Panel considers that a serum 25(OH)D concentration of 200 nmol/L or below is unlikely to pose a risk of adverse health outcomes (hypercalciuria, hypercalcaemia, nephrocalcinosis, abnormal growth) in infants. This should not be regarded as a cut‐off for toxicity but as a conservative value from which a UL could be derived.
Thus, the Panel derives the UL of vitamin D for infants based on the dose–response relationship of daily supplemental intake of vitamin D and serum 25(OH)D concentrations that are unlikely to pose a risk of adverse health outcomes. The Panel recognises, however, that serum 25(OH)D concentration is not an adverse health outcome per se but can be considered as a surrogate endpoint.
Hence, the Panel used an approach for the derivation of the UL for vitamin D in infants similar to that used for setting AIs for vitamin D (EFSA NDA Panel, 2016), i.e. the assessment of the dose–response relationship between daily vitamin D intake and achieved serum 25(OH)D concentrations, based on aggregated data (meta‐regression). In this opinion, only supplemental vitamin D doses could be considered (instead of the total vitamin D intake, for setting AIs for vitamin D), the correlated structure in the data (several arms within one study and repeated measurements at different time points within one arm) was accounted for and, in order to be as protective as possible, varying values for the covariates were considered (and not e.g. their mean values).
This choice of approach by the Panel is in line with the guidance of EFSA's Scientific Committee on Benchmark Dose Modelling (BMD), that recommends using a dose–response modelling approach to establish the reference point (point of departure), in order to set health‐based guidance values (EFSA Scientific Committee et al., 2017a). The Panel chose to select serum 25(OH)D concentrations as a reference point. For selection of the model, the Akaike information criterion was applied (Section 3.5 and Annex A).
From the systematic literature review undertaken by EFSA, six trials in healthy infants (corresponding to 17 arms, and 58 time points) with limited risk of bias were included in a quantitative meta‐analytical synthesis, in order to characterise the dose–response relationship between daily supplemental intake of vitamin D (between 5 and 50 μg/day) and mean achieved serum 25(OH)D concentrations in each study arm (Sections 3.1 and 3.5 and Annex A). This model was stratified by baseline serum 25(OH)D concentrations (10–30, 30–60, 60–100 nmol/L) and age groups (< 6 months, ≥ 6 months) and the duration of vitamin D supplementation was set at 3–6 months. From this model, an empirical distribution of mean achieved serum 25(OH)D concentrations was generated from a large number of simulations. From this, a distribution of serum 25(OH)D concentrations that may be achieved by individuals was then simulated, and the Panel estimated, for different vitamin D intakes, the percentage of infants possibly reaching a concentration above 200 nmol/L as a numerical threshold.
In this approach, models based on original or ln‐transformed scales were derived: the ln‐transformed model is the one better meeting the assumptions of normality and homoscedasticity, whereas the model in the original scale better fits linearity, and both models (both scales) considered together give a sense of the uncertainty associated with the several choices performed at the methodological level and their influence on the results.
As a result of the analysis, the percentages of infants exceeding the serum 25(OH)D concentration of 200 nmol/L increase with increasing supplemental vitamin D intakes. This percentage is higher with increasing baseline serum 25(OH)D concentrations and decreases with age.
5.6.1. Infants aged up to 6 months
Based on the results of the prediction model in original scale (Table 6), 25 μg/day can be identified as the daily intake of vitamin D at which, depending on the baseline serum 25(OH)D concentration, 0–4% of individuals aged < 6 months would exceed serum 25(OH)D concentrations of 200 nmol/L. The results of the ln‐transformed scale, up to a vitamin D supplemental dose of 25 μg/day, are consistent with the results in the original scale. The Panel notes that predictions from the model for the age 0–6 months support the UL of 25 μg/day set by SCF (2003) and retained by EFSA NDA Panel (2012a).
For infants aged up to 6 months, the Panel concludes that the available body of evidence supports keeping the previous UL of 25 μg/day.
5.6.2. Infants aged 6 to less than 12 months
The Panel notes that, in the prediction model, at a given intake of vitamin D, infants older than 6 months achieve lower serum 25(OH)D concentrations than infants younger than 6 months, which may be explained by the increase in body mass (Tables 6 and 7). The Panel also notes that the UL set for children aged 1–10 years is 50 μg/day (EFSA NDA Panel, 2012a).
Based on the results of the prediction model (original scale) (Table 7), for infants aged between 6 and 12 months of age, the predicted percentage of individuals exceeding serum 25(OH)D concentrations of 200 nmol/L would be 0–1% at supplemental vitamin D intake of up to 25 μg/day, 0–2% at intakes of up to 30 μg/day and 0–4% at intakes of up to 35 μg/day. For the ln‐transformed scale, these percentages of individuals exceeding serum 25(OH)D concentrations of 200 nmol/L range from 0 to around 2% for doses up to 35 μg/day. Thus, for infants aged 6 to less than 12 months, the Panel considers that evidence from the predictions supports a UL for vitamin D of 35 μg/day.
5.6.3. General comments on the model
Acknowledging the heterogeneity in the body of evidence and the uncertainties in the modelling, this dose–response analysis offers the opportunity to identify the level of vitamin D intake from supplements associated with the minimum percentage of infants with ‘high’ 25(OH)D concentrations in serum (Section 3.5 and Annex A).
In this model, only supplemental vitamin D intake is considered because data on the vitamin D intake from the other foods of the diet were not available in most of the studies. Therefore, the infants exceeding the serum 25(OH)D concentration of 200 nmol/L are likely to have total vitamin D intakes somewhat beyond the intakes provided by supplements.
The derived UL for vitamin D in infants is based on studies using supplemental vitamin D rather than formula fortified with specific amounts of vitamin D. It is unknown whether vitamin D from formula will have different absorption and bioavailability from vitamin D provided as a supplement as there are only limited data on the effect of the food matrix on vitamin D absorption (EFSA NDA Panel, 2016).
5.6.4. Case reports
Case reports describe adverse health outcomes (mentioned above) associated with the administration to one or a few infants of repeated mg doses of vitamin D as well as with chronic daily administration of vitamin doses of about 4.5–20 times the AI of 10 μg/day for this age (EFSA NDA Panel, 2016) (Section 3.4). The Panel notes that adverse health outcomes reported in these case reports were associated with elevated serum 25(OH)D and reduced PTH concentrations. As these reports either cannot be converted into daily doses or are based on incidental findings, the Panel concludes that they are not suitable to define a LOAEL for vitamin D in infants.
6. Characterisation of the risk
The characterisation of the risk of an intake above the UL for vitamin D is presented separately for infants aged up to 6 months and infants aged 6 to less than 12 months.
The characterisation of the risk is derived from the intake assessment, which was conducted separately for infants aged up to 4 months, and for those aged 4 to less than 12 months, to account for the additional vitamin D intake in relation to the introduction of complementary feeding (Section 4.2.2). For reasons previously described (Sections 4.1.1.5 and 4.2.2), the Panel did not make assumption in its intake assessment regarding vitamin D supplementation or possible overages from fortified foods or supplements.
6.1. Characterisation of the risk for infants < 6 months of age
For exclusively breastfed infants, no risk of exceeding the UL is expected because of the low vitamin D content in breast milk (ranging from 0.25 to 2.0 μg/L, (EFSA NDA Panel, 2016)).
For infants exclusively fed with formula, the calculations are based on default ‘high’ IF consumptions of 260 and 195 mL/kg body weight per day at about 1 and 4 months, respectively (EFSA Scientific Committee et al., 2017b), on the 50th percentile of reference body weights (WHO Multicentre Growth Reference Study Group, 2006; van Buuren et al., 2012), and the maximum regulated vitamin D content in IF of 2.5 μg/100 kcal (Directive 2006/141/EC) or 3 μg/100 kcal (Commission Delegated Regulation (EU) 2016/127).
-
–
The daily vitamin D intake from IF alone, containing the maximal regulated vitamin D content of 3 μg/100 kcal, may exceed the UL of 25 μg/day at age about 4 months in some infants, depending on the energy content of formulae which may vary between 60 and 70 kcal/100 mL (Section 4.3.2, Table 9). The precise percentage of such infants could not be determined from the available data. Estimated vitamin D intakes from IF alone would remain below 30 μg/day.
-
–
The daily vitamin D intake from IF alone, containing the maximal regulated vitamin D content of 2.5 μg/100 kcal, will not exceed the UL of 25 μg/day (Section 4.3.2, Table 9). The margin between the calculated intakes via formulae and the UL varies between 1 and 9 μg/day, depending on the reference body weight, ‘high’ formula consumption, age (about 1 or 4 months) and energy content considered.
6.2. Characterisation of the risk for infants ≥ 6 months of age
The intake assessment for infants aged 4 to less than 12 months was conducted separately for consumers and non‐consumers of IF and FoF (Section 4.4). The intake assessment was done:
-
–
using food consumption data of infants from six surveys, most of them nationally representative of EU countries, conducted between 2001 and 2011 and for which the individual data were available to EFSA in 2017;
-
–
and considering eight intake scenarios. These were defined based on the minimum or maximum amount of vitamin D in IF and FoF according to the two regulatory texts (Directive 2006/141/EC and Commission Delegated Regulation (EU) 2016/127) and based on the composition of foods fortified or not with vitamin D, coming from the EFSA Nutrient Composition Database and from the Mintel GNPD.
The scenarios were assumed to describe situations that may occur on the EU market taking into account voluntarily fortified foods, different possible food patterns in infants, but excluding the contribution from supplements (for the reasons explained in Section 4.2.2), and considering the two extremes in the range of the vitamin D content in mandatorily fortified foods. They were not meant to reflect the fortification practices of the six EU countries that provided food consumption data.
For the characterisation of the risk of an intake above the UL for vitamin D, means and P95 of estimated vitamin D intake from European populations were used (Tables 13 and 14).
-
–
For infants consuming neither formulae nor fortified foods and for those not consuming formulae but consuming fortified foods, the estimated means and P95 of vitamin D intake are below or at the AI of 10 μg/day.
-
–
For infants consuming IF or FoF containing the maximum regulated amount of vitamin D (i.e. 3 μg/100 kcal) but not consuming fortified foods, the estimated means and P95 of vitamin D intake range from about 7 to 12 μg/day and from about 13 to 20 μg/day, respectively. The margin between the estimated P95 of vitamin D intake in formula consumers and the UL of 35 μg/day varies between about 15 and 22 μg/day.
-
–
With the additional intake of fortified foods, the estimated means and P95 vitamin D intake range from about 10 to 14 μg/day and from about 17 to 24.6 μg/day, respectively. The margin between the estimated P95 of vitamin D intake and the UL of 35 μg/day varies between about 10 and 18 μg/day.
The Panel concludes that data from various European infant populations indicate that for infants aged 6 to less than 12 months, vitamin D intake in high consumers of formulae and fortified foods is below the UL of 35 μg/day.
7. Conclusions
7.1. Conclusion regarding the UL for vitamin D in infants
The Panel concludes that the UL for infants < 6 months of age is set at 25 μg/day and the UL for infants ≥ 6 months of age is set at 35 μg/day (Section 5).
The Panel concludes on the UL for vitamin D from data mainly on vitamin D3 (Annex A), as the dose–response analysis to predict the percentage of individuals that would have serum 25(OH)D concentrations above 200 nmol/L included studies that used supplementation with vitamin D3. However, the updated UL of 25 and 35 μg/day applies both to vitamin D2 and D3.
7.2. Conclusion regarding the maximal regulated vitamin D content in infant formulae (infants below 4 months)
The use of a maximum vitamin D content of 3 μg/100 kcal in IF as per Commission Delegated Regulation (EU) 2016/127 may lead some infants aged at about 4 months to consume amounts of vitamin D above the UL of 25 μg/day from formulae alone (without considering additional intake due to supplementation and without considering possible overages) (Sections 4.1.1.5, 4.3.1, 4.3.2 and 6.1). The precise percentage of such infants could not be determined from the available data.
The use of a maximum vitamin D content of 2.5 μg/100 kcal in IF in Directive 2006/141/EC does not result in intakes of vitamin D above the UL of 25 μg/day from formulae alone (without considering additional intake due to supplementation and without considering possible overages). The margin between the calculated intakes via formulae and the UL varies between 1 and 9 μg/day (Sections 4.1.1.5, 4.3.1 and 4.3.2).
7.3. Conclusion regarding infants aged 4–12 months
For infants aged 4 to less than 12 months and consuming IF or FoF containing the maximum amount of vitamin D (i.e. 3 μg/100 kcal as per Commission Delegated Regulation (EU) 2016/127) but not consuming fortified foods or supplements (Sections 4.2.2, 4.4.2 and Annex B):
-
–
the margin between the estimated P95 of vitamin D intake in formula consumers and the UL of 35 μg/day (i.e. for infants 6–12 months) varies between about 15 and 22 μg/day (scenario 4).
-
–
the margin calculated with the UL of 25 μg/day (i.e. for infants 4–6 months) varies between about 5 and 12 μg/day (scenario 4).
With the additional intake of fortified complementary foods (scenarios in Section 4.4.2 and Annex B):
-
–
the margin between the estimated P95 of vitamin D intake in formula consumers and the UL of 35 μg/day (i.e. for infants 6–12 months) varies between about 10 and 18 μg/day (scenario 8).
-
–
the margin calculated with the UL of 25 μg/day (i.e. for infants 4–6 months) varies between about 0.4 and 8 μg/day (scenario 8).
For infants aged 4 to less than 12 months and consuming IF or FoF containing the maximum amount of vitamin D (i.e. 2.5 or 3 μg/100 kcal, respectively, as per Directive 2006/141/EC) but not consuming fortified foods or supplements (Sections 4.2.2, 4.4.2 and Annex B):
-
–
the margin between the estimated P95 of vitamin D intake in formula consumers and the UL of 35 μg/day (i.e. for infants 6–12 months) varies between about 18 and 22 μg/day (scenario 2).
-
–
the margin calculated with the UL of 25 μg/day (i.e. for infants 4–6 months), varies between about 8 and 12 μg/day (scenario 2).
With the additional intake of fortified complementary foods (scenarios in Section 4.4.2 and Annex B):
-
–
the margin between the estimated P95 of vitamin D intake in formula consumers and the UL of 35 μg/day (i.e. for infants 6–12 months) varies between about 13 and 20 μg/day (scenario 6).
-
–
the margin calculated with the UL of 25 μg/day (i.e. for infants 4–6 months), varies between about 3 and 10 μg/day (scenario 6).
Observed food patterns were not available for infants in nationally representative surveys from all EU Member States and food fortification practices vary substantially from one country to the other. The Panel notes that this should be born in mind when discussing estimated intakes of vitamin D in different scenarios. The margin between the estimated P95 of vitamin D intake in formula consumers and the UL in the various defined scenarios of food consumption could be considered as an indication of the possible additional impact of supplementation. This indication should be considered bearing in mind the regulatory compositional requirements of formulae and in the specific context of national supplementation policies and compliance with these policies.
8. Recommendations for research
Consumption surveys providing information on dietary (including fortified foods) and supplemental vitamin D intake of infants and compliance with vitamin D supplementation policies in different countries. Intake data modelling of contribution of foods, including fortified foods and supplements, to vitamin D intake of infants, and their impact on the intake of the low and high consumers (to minimise both the risk of vitamin D deficiency and the risk of exceeding the UL);
Studies on infants that compare the effect of vitamin D2 and D3 on serum 25(OH)D concentrations;
Studies on infants providing information on the normal range of physiological values of serum and urinary calcium;
Longitudinal studies with regular, reliable and standardised measurements of calcium in serum and urine, serum 25(OH)D and PTH concentrations and ectopic calcification in relation to total vitamin D intake (foods including supplements); information may also be obtained by revisiting previously generated datasets;
Studies on the functional role of the C3‐epimer of 25(OH)D;
Studies providing information on the dose–response relationship between vitamin D intake and serum concentration of 25(OH)D and vitamin D metabolites;
Studies to better characterise the influence of various factors on serum 25(OH)D concentration (in particular in infants), e.g.: vitamin D frequency of consumption, baseline serum 25(OH)D concentration, sun exposure, season, body mass (or age) and body fat, maternal characteristics during pregnancy and lactation, in particular maternal supplementation with vitamin D.
Abbreviations
- 1,25(OH)2D
1,25‐dihydroxy‐vitamin D
- 25(OH)D
25‐hydroxy‐vitamin D
- AI
adequate intake
- BMD
Benchmark Dose Modelling
- BMI
body mass index
- BSA
body surface area
- CI
confidence interval
- CLIA
automated chemiluminiscence immunoassay
- COT
UK Committee on toxicity of chemical in food, consumer products and the environment
- CPBA
competitive protein‐binding assay
- CYP24A1
cytochrome P450 family 24 subfamily A member 1
- DBP
vitamin D‐binding protein
- DEQAS
Vitamin D External Quality Assessment Scheme
- DGE
Deutsche Gesellschaft für Ernährung
- DRV
dietary reference value
- ECG
electrocardiogram
- EIA
enzyme immune assay
- ELISA
enzyme‐linked immunosorbent assay
- ESPGHAN
European Society for Paediatric Gastroenterology, Hepatology and Nutrition
- FFQ
food frequency questionnaire
- FGF
fibroblast growth factor
- FoF
follow‐on formula
- FoodEx2
EFSA food classification and description system (revision 2)
- GNPD
Global New Products Database
- HPLC
high‐performance liquid chromatography
- IF
infant formula
- IIH
idiopathic infantile hypercalcaemia
- IOM
US Institute of Medicine
- IU
international unit
- LC–MS/MS
liquid chromatography‐tandem mass spectrometry
- LOAEL
lowest observed adverse effect level
- MS
member state
- NDA
Panel on Dietetic Products, Nutrition and Allergies
- NDNS
National Diet and Nutrition Survey
- NEVO
Nederlands Voedingsstoffenbestand
- NIST
US National Institute of Standards and Technology
- NOAEL
no observed adverse effect level
- NRCT
non‐randomised controlled trial
- NRNCT
non‐randomised non‐controlled trial
- NTP
National Toxicology Program
- OHAT
Office of Health Assessment and Translation
- OR
odds ratio
- P95
95th percentile
- PROSP
prospective cohort study
- PTH
parathyroid hormone
- RCT
randomised controlled trial
- RIA
radioimmunoassay
- RNCT
randomised non‐controlled trial
- RoB
risk of bias
- RIA
radioimmunoassay
- SACN
Scientific Advisory Committee on Nutrition
- SCF
Scientific Committee on Food
- SD
standard deviation
- SLC
solute carrier
- UF
uncertainty factor
- UL
tolerable upper intake level
- USDA
US Department of Agriculture
- UV
ultraviolet
- VDSP
Vitamin D Standardization Program
- VDR
vitamin D receptor
- VIDI
vitamin D intervention
- VIO
VoedingsstoffenInnameOnderzoek (Nutrient Intake Study)
- VITADEK
study on the intake of vitamin A, D, E and K
- Vitamin D2
ergocalciferol
- Vitamin D3
cholecalciferol
- WHO
World Health Organization
Appendix A – Systematic literature search for hazard characterisation: mapping of results of the literature search by languages (e.g. in Embase)
1.
Date: June 2017
| Language | Embase |
|---|---|
| English | 4450 |
| French | 162 |
| German | 161 |
| Spanish | 81 |
| Polish | 59 |
| Italian | 42 |
| Russian | 27 |
| Turkish | 22 |
| Czech | 21 |
| Japanese | 20 |
| Chinese | 19 |
| Dutch | 19 |
| Hungarian | 11 |
| Portuguese | 9 |
| Swedish | 8 |
| Danish | 7 |
| Croatian | 6 |
| Norwegian | 6 |
| Bulgarian | 5 |
| ‘Unknown’ | 5 |
| Persian | 4 |
| Ukrainian | 4 |
| Romanian | 3 |
| Serbian | 3 |
| Slovak | 3 |
| Arabic | 2 |
| Finnish | 2 |
| Icelandic | 2 |
| Afrikaans | 1 |
| Catalan | 1 |
| Estonian | 1 |
| Greek | 1 |
| Korean | 1 |
Appendix B – Systematic literature search for hazard characterisation: initial eligibility criteria related to study characteristics/populations
1.
NB: Criteria described below are those as initially selected by the Panel. A few of these criteria have later been adapted by the Panel (Section 3)
| Study populations (at the time of the intake of vitamin D) | In |
1) Studies on healthy full‐term infants (from birth to < 12 months). In case of a study on a mixed population, e.g. a study on infants (i.e. > 12 months) and young children (1–3 years), for which the results would not be reported separately), the study may be included, in case it respects the other eligibility criteria. 2) Studies on full‐term infants with vitamin D deficiency rickets under treatment. 3) Studies on an undistinguishable mix of full and preterm infants, or studies on infants with no clear/explicit information on whether the infants were born at term or not, may be considered as well. |
| Out |
1) Studies and study arms explicitly restricted to preterm infants (defined as born before the 37th week of gestation). It was considered that extrapolating from premature/preterm infants, which is a heterogeneous group, to full‐term infants was not possible. 2) Trials or prospective studies restricted to infants with (a) disease(s) 69 (apart from full‐term infants with vitamin D deficiency rickets under treatment) are excluded. This criterion does not apply to retrospective cohort studies, case–control studies or case reports on the outcomes of interest. Retrospective studies undertaken only on people with a disease should be excluded. In case of a study on a mixed population (e.g. infants with vitamin D deficiency rickets with or without additional diseases, for which the results would not be reported separately), the study may be included, in case it respects the other eligibility criteria. 3) Studies investigating the association between dietary or supplemental vitamin D intake of pregnant or breastfeeding women on (adverse) health outcomes in their offspring. 4) Studies investigating very low birth weight infants. Studies on low birth weight infants (defined as weighing less than 2.5 kg or also called small for gestational age infants) will not be excluded, as healthy full‐term low birth weight infants could still be fed with standard infant formula or breastfed and could even receive higher amount of formulae. However, very low birth weight infants (defined as infants weighing less than 1.5 kg) are often (very) preterm or born at term but with adverse health outcomes and/or fed parenterally or enterally via a tube and/or fed with a preterm formula. Thus, studies on these infants will be excluded from this assessment, because the results that cannot be extrapolated to the general population of healthy infants born at term |
|
| Study designs | In |
1) Randomised controlled trials, which assessed the effect of different levels of intake of vitamin D on the selected adverse health outcomes and endpoints In view of the variety of adverse health outcomes considered, no minimal duration for the study will be requested 2) non‐controlled randomised trials (e.g. comparing the same dose of vitamin D2 and vitamin D3), non‐randomised controlled trials, non‐randomised non‐controlled trials 3) Observational studies (retrospective and prospective cohort studies, case–control), excluding cross‐sectional studies. For case–control and retrospective cohort studies, the case individuals would need to present at least one of the adverse health outcomes identified In view of the different adverse health outcomes considered, which could occur within different time frames, no limits on the follow‐up will be requested (e.g. a study reporting outcomes in childhood or adulthood after vitamin D consumption in infancy would be considered). Special attention will be paid to: – the consideration of main confounding factors – the quality of the study design (e.g. was the body weight measured, was the sun exposure assessed, was the time of the year reported, was the dose analytically checked?) |
| Out |
1) Cross‐sectional observational studies This study type provides only summary information on intake/dose of supplementation and its association with frequency of occurrence of adverse effects 2) Preclinical studies (animals, in vitro, in silico data)3) Case reports: Case reports will not be considered for further screening, data extraction, appraisal and dose–response analysis, as referring to acute/short‐term consumption of vitamin D and due to the high risk of bias. However, reports on case individuals presenting at least one of the adverse health outcomes identified may be considered qualitatively in the scientific opinion, but separately from the other considered study designs, and in case of clear identification of the ingested dose of vitamin D and the adverse health outcome observed in infants under clinical care. Thus, data from case reports may be used as supporting evidence. For this, they will be flagged to go to a specific category in order to allow an easy retrieval |
|
| Intervention (study designs 1 or 2) or exposure (study design 3) | In |
For trials, oral vitamin D consumption, alone or with a concomitant intake of other nutrient(s) (e.g. vitamin D + calcium). Trials should consider dose(s) of vitamin D above 10 μg/day (value set for the adequate intake for infants (EFSA NDA Panel, 2016)). In view of the low content of vitamin D in breast milk, and the often limited content of vitamin D in some foods (e.g. fruits and vegetables), studies that do not assess the vitamin D intake from sources other than the intervention will not be excluded (e.g. a study on vitamin D supplementation in breastfed infants with no information on vitamin D in breast milk would not be excluded) |
| Out |
1) Interventions with parenteral administration of vitamin D 2) Interventions using only doses of vitamin D of 10 μg/day or less. 3) Although no requirement on frequency of vitamin D oral consumption will be applied to the search or during the title/abstract screening, changes in serum or urinary calcium concentrations or serum 25(OH)D concentrations after weekly, monthly or single large doses of vitamin D cannot be directly compared with those occurring after daily or weekly consumption. Thus, while trials on daily doses may be considered for further quantitative analysis, as for case reports mentioned above, trials (or arms of trials) on weekly, monthly doses, bolus doses or ‘stoss’ therapy will be flagged to go to a specific category in order to allow an easy retrieval |
|
| Outcomes of interest | In |
Above‐mentioned health outcomes – hypercalciuria: number of cases or percentage of cases, data on urinary calcium concentrations, data or urinary ratios of calcium/creatinine – hypercalcaemia: number of cases or percentage of cases, data on serum calcium concentrations, ionised calcium concentrations – ectopic calcification in particular nephrocalcinosis: number of cases or % of cases of nephrocalcinosis – failure to thrive: weight, length/height, head circumference longitudinally reported – ‘high’ serum 25(OH)D concentration |
| Out |
Any other health outcomes or endpoints In particular, studies only on cord blood would not be relevant (unless measurements of serum/plasma 25(OH)D concentration later during infancy are also available), as 25(OH)D concentration in cord blood would rather be indicative of maternal vitamin D status and the provision of vitamin D to the foetus by the mother |
Appendix C – Systematic literature search for hazard characterisation: search strategies
1.
Final number of hits for each database (bold) are given before the removal of duplicates.
Pubmed
Date of the search 19/6/2017
Limits: items published in English, French or German
| Search | Query | Hits |
|---|---|---|
| #24 | Search #22 AND #23 | 3,304 |
| #23 | Search English[Language] OR French[Language] OR German[Language] | 24,243,150 |
| #22 | Search #20 NOT #21 | 3,551 |
| #21 | Search “Editorial” [Publication Type] OR “Letter” [Publication Type] | 1,381,109 |
| #20 | Search #18 NOT #19 | 3,585 |
| #19 | Search (rat[ti] OR rats[ti] OR mouse[ti] OR mice[ti] OR murine[ti] OR rodent[ti] OR rodents[ti] OR hamster[ti] OR hamsters[ti] OR pig[ti] OR pigs[ti] OR porcine[ti] OR rabbit[ti] OR rabbits[ti] OR animal[ti] OR animals[ti] OR dogs[ti] OR dog[ti] OR cats[ti] OR bovine[ti] OR sheep[ti] OR ovine[ti] OR monkey[ti] OR monkeys[ti]) NOT medline[sb] | 96,381 |
| #18 | Search #16 NOT #17 | 3,595 |
| #17 | Search “animals”[MeSH Terms] NOT “humans”[MeSH Terms] | 4,338,097 |
| #16 | Search #15 AND #2 | 3,750 |
| #15 | Search #13 OR #14 | 18,602 |
| #14 | Search “Vitamin D/adverse effects”[Mesh:noexp] OR “Vitamin D/complications”[Mesh:noexp] OR “Vitamin D/toxicity”[Mesh:noexp] OR “Vitamin D/poisoning”[Mesh:noexp] OR “Cholecalciferol/adverse effects”[Mesh:noexp] OR “Cholecalciferol/complications”[Mesh:noexp] OR “Cholecalciferol/toxicity”[Mesh:noexp] OR “Cholecalciferol/poisoning”[Mesh:noexp] OR “Ergocalciferols/adverse effects”[Mesh:noexp] OR “Ergocalciferols/complications”[Mesh:noexp] OR “Ergocalciferols/toxicity”[Mesh:noexp] OR “Ergocalciferols/poisoning”[Mesh:noexp] | 1,846 |
| #13 | Search #12 AND #5 | 17,413 |
| #12 | Search #11 OR #10 OR #9 OR #8 OR #7 OR #6 | 5,552,060 |
| #11 | Search (((((“Hydroxycholecalciferols”[Mesh] OR “25‐Hydroxyvitamin D 2”[Mesh] OR hydroxycholecalciferol*[tiab] OR hydroxycolecalciferol*[tiab] OR hydroxyergocalciferol*[tiab] OR “hydroxyvitamin d”[tiab] OR “hydroxyvitamins d”[tiab] OR “hydroxyvitamin d3”[tiab] OR “hydroxyvitamins d3”[tiab] OR “hydroxivitamin d”[tiab] OR “hydroxivitamins d”[tiab] OR “hydroxivitamin d3”[tiab] OR “hydroxivitamins d3”[tiab] OR calcifediol[tiab] OR 25ohd[tiab] OR “25 ohd”[tiab] OR “25 oh d”[tiab] OR “25ohd3”[tiab] OR “25 ohd3”[tiab] OR “25 oh d3”[tiab] OR “25 oh d 3”[tiab] OR calcidiol[tiab]) AND (serum[tiab] OR plasma[tiab] OR blood[tiab]))) OR (“Hydroxycholecalciferols/blood”[Mesh] OR “25‐Hydroxyvitamin D 2/blood”[Mesh]))) AND (((concentration*[tiab] OR elevat*[tiab] OR high[tiab] OR higher[tiab] OR increas*[tiab]))) | 14,786 |
| #10 | Search “Hypercalcaemia”[Mesh] OR “Calcium/blood”[Mesh] OR Hypercalcaemia[tiab] OR hypercalciema[tiab] OR hypercalcaemia[tiab] OR calcemia[tiab] OR ((blood[tiab] OR serum[tiab] OR plasma[tiab]) AND calcium[tiab]) OR “Hypercalciuria”[Mesh] OR “Calcium/urine”[Mesh] OR hypercalciuria[tiab] OR hypercalcinuria[tiab] OR calciuria[tiab] OR (urinary[tiab] AND calcium[tiab]) OR calcium ratio[tiab] OR calcium homeostasis[tiab] OR (calcium[tiab] AND homoeostasis[tiab]) | 124,450 |
| #9 | Search “Urolithiasis”[Mesh] OR ((urinary[tiab] OR kidney*[tiab] OR renal[tiab] OR bladder[tiab] OR vesical[tiab] OR ureter*[tiab]) AND (calcu*[tiab] OR stone*[tiab] OR lithias*[tiab])) OR nephrolith*[tiab] OR urolith*[tiab] OR cystolith*[tiab] | 78,590 |
| #8 | Search “Nephrocalcinosis”[Mesh] OR nephrocalcinos*[tiab] OR ((kidney*[tiab] OR renal[tiab] OR tubulus[tiab] OR tubular[tiab] OR nephric[tiab]) AND (calcinosis[tiab] OR calcinoses[tiab] OR calcification[tiab] OR calcified[tiab])) OR ectopic calcification*[tiab] OR abnormal calcification*[tiab] OR acute calcification*[tiab] OR ectopic calcinos*[tiab] | 10,013 |
| #7 | Search “Drug‐Related Side Effects and Adverse Reactions”[Mesh] OR “Toxicity Tests”[Mesh] OR “Poisoning”[Mesh:noexp] OR “Substance‐Related Disorders”[Mesh:noexp] OR “Drug Overdose”[Mesh] OR “No‐Observed‐Adverse‐Effect Level”[Mesh] OR “Dose‐Response Relationship, Drug”[Mesh] OR ((adverse[tiab] OR side[tiab] OR undesirable[tiab] OR unwanted[tiab]) AND (effect[tiab] OR effects[tiab] OR reaction*[tiab] OR event[tiab] OR events[tiab] OR outcome*[tiab])) OR complication*[tiab] OR upper intake*[tiab] OR upper limit*[tiab] OR intake limit*[tiab] OR noael[tiab] OR noel[tiab] OR no observed effect*[tiab] OR toxic*[tiab] OR intoxic*[tiab] OR poison*[tiab] OR excess*[tiab] OR risk[tiab] OR risks[tiab] OR hypervitaminos*[tiab] OR hyper vitaminos*[tiab] OR supervitaminos*[tiab] OR “super vitaminosis”[tiab] OR “super vitaminoses”[tiab] OR “dose response”[tiab] OR “dose effect”[tiab] OR “dosage effect”[tiab] OR “dosage response”[tiab] OR “dose activity”[tiab] OR “dosage activity”[tiab] | 4,048,172 |
| #6 | Search ((“Body Weight”[Mesh] OR “Growth and Development”[Mesh:NoExp] OR “Growth”[Mesh] OR “Body Height”[Mesh] OR “Body Mass Index”[Mesh] OR growth[tiab] OR weight[tiab] OR “body size”[tiab] OR “body mass”[tiab] OR BMI[tiab] OR length[tiab] OR height[tiab] OR “head circumference”[tiab] OR development*[tiab]) AND (retard*[tiab] OR disorder*[tiab] OR reduc*[tiab] OR trajector*[tiab] OR disturb*[tiab] OR curve*[tiab] OR limit*[tiab] OR negative[tiab] OR adverse[tiab] OR rate[tiab] OR factor affect*[tiab] OR factors affect*[tiab] OR factor influenc*[tiab] OR factors influenc*[tiab] OR factor alter*[tiab] OR factors alter*[tiab] OR factor chang*[tiab] OR factors chang*[tiab] OR failure[tiab] OR abnormal*[tiab])) OR “Growth Disorders”[Mesh] OR “Body Weight Changes”[Mesh] OR “Failure to Thrive”[Mesh] OR “failure to thrive”[tiab] | 1,922,815 |
| #5 | Search #3 AND #4 | 24,263 |
| #4 | Search “Dietary Supplements”[Mesh:NoExp] OR “Food, Fortified”[Mesh] OR “Infant Food”[Mesh] OR “Milk”[Mesh] OR intake*[tiab] OR consum*[tiab] OR supplement*[tiab] OR diet[tiab] OR diets[tiab] OR dieta*[tiab] OR diete*[tiab] OR food*[tiab] OR administrat*[tiab] OR intervention[tiab] OR fortif*[tiab] OR milk[tiab] OR formula*[tiab] OR high dose[tiab] OR higher dose[tiab] OR high dosage[tiab] OR higher dosage[tiab] OR overdos*[tiab] OR over dosage*[tiab] OR over dose[tiab] | 2,696,723 |
| #3 | Search “Vitamin D”[Mesh:NoExp] OR “Cholecalciferol”[Mesh:NoExp] OR “Ergocalciferols”[Mesh:NoExp] OR “vitamin d”[tiab] OR “vit d”[tiab] OR vitd[tiab] OR “vitamin d2”[tiab] OR “vit d2”[tiab] OR vitd2[tiab] OR “vitamin d3”[tiab] OR “vit d3”[tiab] OR vitd3[tiab] OR ergocalciferol*[tiab] OR calciferol*[tiab] OR cholecalciferol*[tiab] OR colecalciferol*[tiab] OR calciol[tiab] | 64,610 |
| #2 | Search “Infant”[Mesh] OR “Pediatrics”[Mesh:NoExp] OR “Neonatology”[Mesh] OR infan*[tiab] OR newborn*[tiab] OR “new born”[tiab] OR “new borns”[tiab] OR perinat*[tiab ] OR neonat*[tiab] OR baby[tiab] OR babies[tiab] OR toddler*[tiab] OR preschoolchild*[tiab] OR boy[tiab] OR boys[tiab] OR girl[tiab] OR girls[tiab] OR child[tiab] OR children[tiab] OR childhood[tiab] OR kid[tiab] OR kids[tiab] OR pediatric*[tiab] OR paediatric*[tiab] OR “month old”[tiab] OR “months old”[tiab] | 2,290,689 |
Embase
Date of the search 20/6/2017
Limits: items published in English, French or German
| Search | Query | Hits |
|---|---|---|
| #22 | #19 NOT #20 AND ([english]/lim OR [french]/lim OR [german]/lim) | 4,688 |
| #21 | #19 NOT #20 | 5,085 |
| #20 | [conference abstract]/lim OR [editorial]/lim OR [letter]/lim OR ‘conference review’/it | 4,127,436 |
| #19 | #15 NOT #18 | 6,779 |
| #18 | #16 NOT #17 | 5,248,835 |
| #17 | ‘human’/exp OR ‘human experiment’/de | 18,295,966 |
| #16 | ‘animal’/exp OR ‘animal experiment’/exp | 23,543,619 |
| #15 | #1 AND #14 | 7,079 |
| #14 | #12 OR #13 | 33,627 |
| #13 | ‘vitamin d intoxication’/exp OR ‘vitamin d’/dd_ae,dd_to OR ‘ergocalciferol’/dd_ae,dd_to OR ‘colecalciferol’/dd_ae,dd_to | 3,065 |
| #12 | #4 AND #11 | 31,975 |
| #11 | #5 OR #6 OR #7 OR #8 OR #9 OR #10 | 6,731,707 |
| #10 | ‘Hypercalcaemia’/exp OR ‘calcium blood level’/exp OR ‘calcium homeostasis’/exp OR Hypercalcaemia:ab,ti OR hypercalciema:ab,ti OR hypercalcaemia:ab,ti OR calcemia:ab,ti OR ((blood OR serum OR plasma) NEAR/3 calcium):ab,ti OR ‘hypercalciuria’/exp OR ‘calcium urine level’/exp OR hypercalciuria:ab,ti OR hypercalcinuria:ab,ti OR calciuria:ab,ti OR (urinary NEAR/3 calcium):ab,ti OR ‘calcium ratio’:ab,ti OR ‘calcium homeostasis’:ti,ab OR ‘calcium homoeostasis’:ti,ab | 94,693 |
| #9 | ‘hydroxycolecalciferol’/exp OR ‘25 hydroxyvitamin d’/exp OR ‘calcifediol’/exp OR hydroxycholecalciferol*:ab,ti OR hydroxycolecalciferol*:ab,ti OR hydroxyergocalciferol*:ab,ti OR ‘hydroxyvitamin d’:ab,ti OR ‘hydroxyvitamins d’:ab,ti OR ‘hydroxyvitamin d3’:ab,ti OR ‘hydroxyvitamins d3’:ab,ti OR ‘hydroxivitamin d’:ab,ti OR ‘hydroxivitamins d’:ab,ti OR ‘hydroxivitamin d3’:ab,ti OR ‘hydroxivitamins d3’:ab,ti OR calcifediol:ab,ti OR 25ohd:ab,ti OR ‘25 ohd’:ab,ti OR ‘25 oh d’:ab,ti OR ‘25ohd3’:ab,ti OR ‘25 ohd3’:ab,ti OR ‘25 oh d3’:ab,ti OR ‘25 oh d 3’:ab,ti OR calcidiol:ab,ti AND (serum:ti,ab OR plasma:ti,ab OR blood:ti,ab OR ‘blood level’/de OR ‘vitamin blood level’/exp) AND (concentration*:ti,ab OR elevat*:ti,ab OR high*:ti,ab OR increas*:ti,ab OR level*:ti,ab) | 21,352 |
| #8 | ‘urolithiasis’/exp OR ((urinary OR ‘urinary tract’ OR kidney* OR renal OR bladder OR vesical OR ureter*) NEAR/3 (calcul* OR stone* OR lithias*)):ti,ab OR nephrolith*:ti,ab OR urolith*:ti,ab OR cystolith*:ti,ab | 72,056 |
| #7 | ‘kidney calcification’/exp OR ((kidney* OR renal OR tubulus OR tubular OR nephric) NEAR/3 (calcinosis OR calcinoses OR calcification OR calcified)):ti,ab OR nephrocalcinos*:ti,ab OR ((ectopic OR abnormal OR acute) NEXT/3 calcification*):ti,ab OR (ectopic NEXT/3 calcinos*):ti,ab | 8,171 |
| #6 | ‘growth disorder’/de OR ‘failure to thrive’/exp OR ‘growth retardation’/exp OR ‘growth, development and aging disorders’/de OR ‘body weight disorder’/exp OR ((growth OR weight OR ‘body size’ OR height OR ‘body mass’ OR bmi OR length OR ‘head circumference’ OR development*) NEAR/7 (retard* OR disorder* OR disturb* OR trajector* OR curve* OR reduc* OR limit* OR negative OR adverse OR rate OR failure OR abnormal*)):ti,ab OR (growth:ti,ab OR weight:ti,ab OR ‘body size’:ti,ab OR height:ti,ab OR ‘body mass’:ti,ab OR bmi:ti,ab AND (factor* NEAR/3 (affect* OR influenc* OR alter* OR chang*)):ti,ab) OR ‘failure to thrive’:ti,ab OR (‘body growth’/exp OR ‘body weight’/exp OR ‘body size’/exp OR ‘body height’/exp OR ‘body mass’/exp AND (retard*:ti,ab OR disorder*:ti,ab OR disturb*:ti,ab OR trajector*:ti,ab OR curve*:ti,ab OR reduc*:ti,ab OR limit*:ti,ab OR negative:ti,ab OR adverse:ti,ab OR rate:ti,ab OR failure:ti,ab OR abnormal*:ti,ab)) | 1,251,502 |
| #5 | ‘drug overdose’/exp OR ‘drug safety’/exp OR ‘adverse drug reaction’/de OR ‘complication’/de OR ‘hypervitaminosis’/de OR ‘no‐observed‐adverse‐effect level’/exp OR ‘intoxication’/de OR ‘toxicity’/exp OR ‘risk’/de OR ‘dose response’/exp OR ((adverse OR side OR undesirable OR unwanted) NEAR/3 (effect* OR reaction* OR event OR events OR outcome*)):ti,ab OR complication*:ti,ab OR ((upper OR intake) NEAR/3 limit*):ti,ab OR ‘upper intake’:ti,ab OR noael:ti,ab OR noel:ti,ab OR ‘no observed effect level’:ti,ab OR ‘no observed effect levels’:ti,ab OR toxic*:ti,ab OR intoxic*:ti,ab OR poison*:ti,ab OR excess*:ti,ab OR safety:ti,ab OR risk:ti,ab OR risks:ti,ab OR hypervitaminos*:ti,ab OR ‘hyper vitaminosis’:ab,ti OR ‘hyper vitaminoses’:ti,ab OR supervitaminos*:ti,ab OR ‘super vitaminosis’:ti,ab OR ‘super vitaminoses’:ti,ab OR ‘dose response’:ti,ab OR ‘dose effect’:ti,ab OR ‘dosage effect’:ti,ab OR ‘dosage response’:ti,ab OR ‘dose activity’:ti,ab OR ‘dosage activity’:ti,ab | 5,839,586 |
| #4 | #2 AND #3 | 46,331 |
| #3 | ‘vitamin intake’/exp OR ‘vitamin supplementation’/exp OR ‘dietary supplement’/exp OR ‘baby food’/exp OR ‘milk’/exp OR intake*:ti,ab OR consum*:ti,ab OR supplement*:ti,ab OR diet*:ti,ab OR food*:ti,ab OR administrat*:ti,ab OR intervention:ti,ab OR fortif*:ti,ab OR milk:ti,ab OR formula*:ti,ab OR (high* NEAR/3 (dose OR dosage)):ti,ab OR overdos*:ti,ab OR ‘over dosage’:ti,ab OR ‘over dose’:ti,ab | 3,668,797 |
| #2 | ‘vitamin d’/de OR ‘ergocalciferol’/exp OR ‘colecalciferol’/exp OR ‘vitamin d’:ab,ti OR ‘vit d’:ab,ti OR vitd:ab,ti OR ‘vitamin d2’:ab,ti OR ‘vit d2’:ab,ti OR vitd2:ab,ti OR ‘vitamin d3’:ab,ti OR ‘vit d3’:ab,ti OR vitd3:ab,ti OR ergocalciferol*:ab,ti OR calciferol*:ab,ti OR cholecalciferol*:ab,ti OR colecalciferol*:ab,ti OR calciol:ab,ti | 113,962 |
| #1 | ‘infant’/exp OR ‘toddler’/exp OR ‘neonatology’/exp OR ‘pediatrics’/de OR infan*:ab,ti OR newborn*:ab,ti OR ‘new born’:ab,ti OR ‘new borns’:ab,ti OR perinat*:ab,ti OR neonat*:ab,ti OR baby:ab,ti OR babies:ab,ti OR toddler*:ab,ti OR preschoolchild*:ab,ti OR boy:ab,ti OR boys:ab,ti OR girl:ab,ti OR girls:ab,ti OR child:ab,ti OR children:ti,ab OR childhood:ti,ab OR kid:ab,ti OR kids:ab,ti OR pediatric*:ab,ti OR paediatric*:ab,ti OR ‘month old’:ti,ab OR ‘months old’:ti,ab | 2,907,454 |
Cochrane Library
Date of the search 20/6/2017
| ID | Search | Hits |
|---|---|---|
| #1 | [mh infant] or [mh ^paediatrics] or [mh neonatology] or infan*:ti,ab,kw or newborn*:ti,ab,kw or “new born”:ti,ab,kw or “new borns”:ti,ab,kw or perinat*:ti,ab,kw or neonat*:ti,ab,kw or baby:ti,ab,kw or babies:ti,ab,kw or toddler*:ti,ab,kw or preschoolchild*:ti,ab,kw or boy:ti,ab,kw or boys:ti,ab,kw or girl:ti,ab,kw or girls:ti,ab,kw or child:ti,ab,kw or children:ti,ab,kw or childhood:ti,ab,kw or kid:ti,ab,kw or kids:ti,ab,kw or pediatric*:ti,ab,kw or paediatric*:ti,ab,kw or “month old”:ti,ab,kw or “months old”:ti,ab,kw | 134,248 |
| #2 | [mh ^”Vitamin D”] or [mh ^Cholecalciferol] or [mh ^Ergocalciferols] or “vitamin d”:ti,ab,kw or “vit d”:ti,ab,kw or vitd:ti,ab,kw or “vitamin d2”:ti,ab,kw or “vit d2”:ti,ab,kw or vitd2:ti,ab,kw or “vitamin d3”:ti,ab,kw or “vit d3”:ti,ab,kw or vitd3:ti,ab,kw or ergocalciferol*:ti,ab,kw or calciferol*:ti,ab,kw or cholecalciferol*:ti,ab,kw or colecalciferol*:ti,ab,kw or calciol:ti,ab,kw (Word variations have been searched) | 6,704 |
| #3 | (([mh “Body Weight”] or [mh ^”Growth and Development”] or [mh Growth] or [mh “Body Height”] or [mh “Body Mass Index”] or growth:ti,ab,kw or weight:ti,ab,kw or “body size”:ti,ab,kw or “body mass”:ti,ab,kw or BMI:ti,ab,kw or length:ti,ab,kw or height:ti,ab,kw or “head circumference”:ti,ab,kw or development*:ti,ab,kw) and (retard*:ti,ab,kw or disorder:ti,ab,kw or reduc*:ti,ab,kw or limit*:ti,ab,kw or negative:ti,ab,kw or adverse:ti,ab,kw or rate:ti,ab,kw or failure:ti,ab,kw or abnormal*:ti,ab,kw or (factor*:ti,ab,kw near/3 (affect*:ti,ab,kw or influenc*:ti,ab,kw or alter*:ti,ab,kw or chang:ti,ab,kw)))) or [mh “Growth Disorders”] or [mh “Body Weight Changes”] or “failure to thrive”:ti,ab,kw or [mh “Failure to thrive”] | 116,445 |
| #4 | [mh “Drug‐Related Side Effects and Adverse Reactions”] or [mh “Toxicity Tests”] or [mh ^Poisoning] or [mh ^”Substance‐Related Disorders”] or [mh “Drug Overdose”] or [mh “No‐Observed‐Adverse‐Effect Level”] or [mh “Dose‐Response Relationship, Drug”] or ((adverse:ti,ab,kw or side:ti,ab,kw or undesirable:ti,ab,kw or unwanted:ti,ab,kw) near/3 (effect:ti,ab,kw or effects:ti,ab,kw or reaction*:ti,ab,kw or event:ti,ab,kw or events:ti,ab,kw or outcome*:ti,ab,kw)) or complication*:ti,ab,kw or upper intake*:ti,ab,kw or upper limit*:ti,ab,kw or intake limit*:ti,ab,kw or noael:ti,ab,kw or noel:ti,ab,kw or no observed effect*:ti,ab,kw or toxic*:ti,ab,kw or intoxic*:ti,ab,kw or poison*:ti,ab,kw or excess*:ti,ab,kw or risk:ti,ab,kw or risks:ti,ab,kw or hypervitaminos*:ti,ab,kw or hyper vitaminos*:ti,ab,kw or supervitaminos*:ti,ab,kw or “super vitaminosis”:ti,ab,kw or “super vitaminoses”:ti,ab,kw or “dose response”:ti,ab,kw or “dose effect”:ti,ab,kw or “dosage effect”:ti,ab,kw or “dosage response”:ti,ab,kw or “dose activity”:ti,ab,kw or “dosage activity”:ti,ab,kw | 403,722 |
| #5 | [mh Nephrocalcinosis] or nephrocalcinos*:ti,ab,kw or ((kidney*:ti,ab,kw or renal:ti,ab,kw or tubulus:ti,ab,kw or tubular:ti,ab,kw or nephric:ti,ab,kw) near/3 (calcinosis:ti,ab,kw or calcinoses:ti,ab,kw or calcification:ti,ab,kw or calcified:ti,ab,kw)) or ((ectopic:ti,ab,kw or abnormal:ti,ab,kw or acute:ti,ab,kw) near/2 calcification*:ti,ab,kw) or (ectopic:ti,ab,kw near/3 calcinos*:ti,ab,kw) | 97 |
| #6 | [mh Hypercalcaemia] or [mh Calcium/BL] or Hypercalcaemia:ti,ab,kw or hypercalciema:ti,ab,kw or hypercalcaemia:ti,ab,kw or calcemia:ti,ab,kw or ((blood:ti,ab,kw or serum:ti,ab,kw or plasma:ti,ab,kw) and calcium:ti,ab,kw) or [mh Hypercalciuria] or [mh Calcium/UR] or hypercalciuria:ti,ab,kw or hypercalcinuria:ti,ab,kw or calciuria:ti,ab,kw or (urinary:ti,ab,kw near/3 calcium:ti,ab,kw) or “calcium ratio”:ti,ab,kw or “calcium homeostasis”:ti,ab,kw or (calcium:ti,ab,kw near/3 homoeostasis:ti,ab,kw) | 13,420 |
| #7 | ((([mh Hydroxycholecalciferols] or [mh “25‐Hydroxyvitamin D 2”] or hydroxycholecalciferol*:ti,ab,kw or hydroxycolecalciferol*:ti,ab,kw or hydroxyergocalciferol*:ti,ab,kw or “hydroxyvitamin d”:ti,ab,kw or “hydroxyvitamins d”:ti,ab,kw or “hydroxyvitamin d3”:ti,ab,kw or “hydroxyvitamins d3”:ti,ab,kw or “hydroxivitamin d”:ti,ab,kw or “hydroxivitamins d”:ti,ab,kw or “hydroxivitamin d3”:ti,ab,kw or “hydroxivitamins d3”:ti,ab,kw or calcifediol:ti,ab,kw or 25ohd:ti,ab,kw or “25 ohd”:ti,ab,kw or “25 oh d”:ti,ab,kw or “25ohd3”:ti,ab,kw or “25 ohd3”:ti,ab,kw or “25 oh d3”:ti,ab,kw or “25 oh d 3”:ti,ab,kw or calcidiol:ti,ab,kw) and (serum:ti,ab,kw or plasma:ti,ab,kw or blood:ti,ab,kw)) or ([mh Hydroxycholecalciferols/BL] or [mh “25‐Hydroxyvitamin D 2”/BL])) and (concentration*:ti,ab,kw or elevat*:ti,ab,kw or high:ti,ab,kw or higher:ti,ab,kw or increas*:ti,ab,kw) | 2,360 |
| #8 | [mh Urolithiasis] or ((urinary:ti,ab,kw or kidney*:ti,ab,kw or renal:ti,ab,kw or bladder:ti,ab,kw or vesical:ti,ab,kw or ureter*:ti,ab,kw) near/3 (calcu*:ti,ab,kw or stone*:ti,ab,kw or lithias*:ti,ab,kw)) or nephrolith*:ti,ab,kw or urolith*:ti,ab,kw or cystolith*:ti,ab,kw | 2,649 |
| #9 | #8 or #7 or #6 or #5 or #4 or #3 | 456,639 |
| #10 | #9 and #2 | 5,156 |
| #11 | [mh ^”Vitamin D”/AE,CO,TO,PO] or [mh ^Cholecalciferol/AE,CO,TO,PO] or [mh ^Ergocalciferols/AE,CO,TO,PO] | 160 |
| #12 | #10 or #11 | 5,168 |
| #13 | #12 and #1 | 829 |
| #14 | [mh ^”Dietary Supplements”] or [mh “Food, Fortified”] or [mh milk] or [mh “Infant Food”] or intake*:ti,ab,kw or consum*:ti,ab,kw or supplement*:ti,ab,kw or diet*:ti,ab,kw or food*:ti,ab,kw or administrat*:ti,ab,kw or intervention:ti,ab,kw or fortif*:ti,ab,kw or milk:ti,ab,kw or formula*:ti,ab,kw or (high* near/3 (dose or dosage)):ti,ab,kw or overdos*:ti,ab,kw or (over near/1 dosage*):ti,ab,kw or “over dose”:ti,ab,kw | 397,557 |
| #15 | #13 and #14 | 718 |
Appendix D – Systematic literature search for hazard characterisation: predefined list of parameters for data extraction from the included studies
1.
For the included studies (that considered daily intake of vitamin D)
-
1
Data on study setting extracted for each paper:
Study number (in case of several studies in the same paper)
-
Study design:
-
–
Type of design
-
–
For trials: if compliance was assessed (yes/no)
-
–
Duration (for trials) or length of follow‐up (for observational studies)
-
–
Latitude (keeping one decimal after the comma). If not available, city/region, country code. If not reported in the paper, latitude was imputed at the phase of the data analysis, based on the information on city/country.
-
–
-
Study population:
-
–
Age category when vitamin D was consumed (infants only, or mixed populations comprising infants)
-
–
Sex: all males, all females, mixed
-
–
Health status, e.g. healthy infants, infants with vitamin D‐deficient rickets, infants with hypovitaminosis D
-
–
category of length of gestation: all full‐term (either explicitly mentioned in the paper or if inclusion criterion of the study subjects was defined as at or more than 37 weeks of gestation), mixed (i.e. clearly stated or suspected/unspecified)
-
–
Type of feeding at inclusion: all exclusively breastfed, all exclusively formula‐fed, mixed (i.e. any other cases, e.g. partially breastfed, with complementary feeding)
-
–
Information on sample size (full study population or per group) and if information on the characteristics/reasons of lost to follow‐up was provided.
-
–
-
2
Data on outcomes/endpoints at different time points (including baseline) will be extracted for each paper:
Study number
-
Study design:
-
–
Study group
-
–
Additional variables to be filled‐in in case of definition of groups not only by vitamin D intake (e.g. one group receiving vitamin D2 and another group receiving vitamin D3)
-
–
Time points (0–N) or date of each time point (if reported in the paper)
-
–
Age at each time point.
-
–
-
Vitamin D intake:
-
–
Source of supplemental vitamin D intake (e.g. supplements as tablets/pills, supplements as drops, supplements (unspecified form), formula/meal/any other fortified food)
-
–
Form of the vitamin D supplemental intake (D2, D3, D unspecified, D2+D3)
-
–
Frequency of supplementation per group (daily to be extracted for further analysis; weekly, monthly, bolus, single doses to be flagged in a separate category; injections not to be considered in this mandate)
-
–
Duration of supplementation
-
–
If the dose was analytically checked.
-
–
If compliance/adherence was assessed
-
–
Information on vitamin D intake (observational studies) or background vitamin D intake (trials, i.e. apart from the vitamin D intervention dose).
-
–
Information on calcium supplementation/intake
-
Outcomes of interest at the different time points, including at birth if available) (there could be several outcomes per study):
-
–
Weight
-
–
If using z‐scores: if the WHO growth standards were used or other standards
-
–
Percentage of small for gestational age infants (if reported)
-
–
Length/height
-
–
If using z‐scores: if the WHO growth standards were used or other standards
-
–
Head circumference
-
–
If anthropometry data were measured or reported, and if measured, if they were repeatedly measured
-
–
number of cases or % of cases of failure to thrive
-
–
number of cases or % of cases of hypercalcaemia
-
–
definition of hypercalcaemia by the authors, cut‐off value in mmol/L
-
–
serum total calcium concentrations;
-
–
serum‐ionised calcium concentrations if available
-
–
number of cases or % of cases of hypercalciuria
-
–
definition of hypercalciuria by the authors, cut‐off value used
-
–
urinary calcium concentrations;
-
–
daily urinary volume
-
–
duration of urinary collection (24h, spot urine, others)
-
–
one or more spot urine samples
-
–
number of collection days?
-
–
data on urinary ratios of calcium/creatinine; urinary creatinine concentrations
-
–
number of cases or % of cases of nephrocalcinosis/ectopic calcification
-
–
serum 25(OH)D concentration
-
–
If ‘high’ 25(OH)D was defined in the paper and how; if so: number of cases or % of cases
-
–
analytical method(s) for serum 25(OH)D measurement: HPLC, LC‐MS/MS, CPBA, enzyme‐linked immunosorbent assay (ELISA), radioimmunoassay (RIA), others, unspecified)
-
–
external quality control (yes/no): mention of the laboratory certification by the Vitamin D External Quality Assessment Scheme (DEQAS) and National Institute of Standards and Technology (NIST) standard reference material.
-
–
Additional comments whenever required.
Simplified data extraction for study types not considered for further data extraction, appraisal and dose–response analysis, but considered qualitatively in the scientific opinion (Section 3.4 ):
For case reports: information such as number of cases, age, vitamin D intake, vitamin D form (D2, D3, both, unspecified), consumption duration, were identified.
For studies using non‐daily vitamin D doses, the following information was identified:
study type (e.g. RCT),
if compliance was assessed (yes/no),
study duration/follow‐up,
latitude if available otherwise city and country,
health status of the infants (e.g. healthy, with vitamin D deficiency rickets, low birth weight infants),
sample size,
full‐term infants or not,
mixed populations of males and females or not,
type of feeding at inclusion,
age,
number of groups,
vitamin D dose,
form of vitamin D,
frequency of supplementation,
duration of supplementation,
mode of administration (e.g. drops),
calcium intake,
and whether data were assessed or reported (yes/no) on urinary calcium, hypercalciuria, serum calcium, hypercalcaemia, nephrocalcinosis, 25(OH)D concentration in serum, anthropometric parameters, abnormal growth.
Appendix E – Systematic literature search for hazard characterisation: outcome of the search and screening
1.
Figure 3.
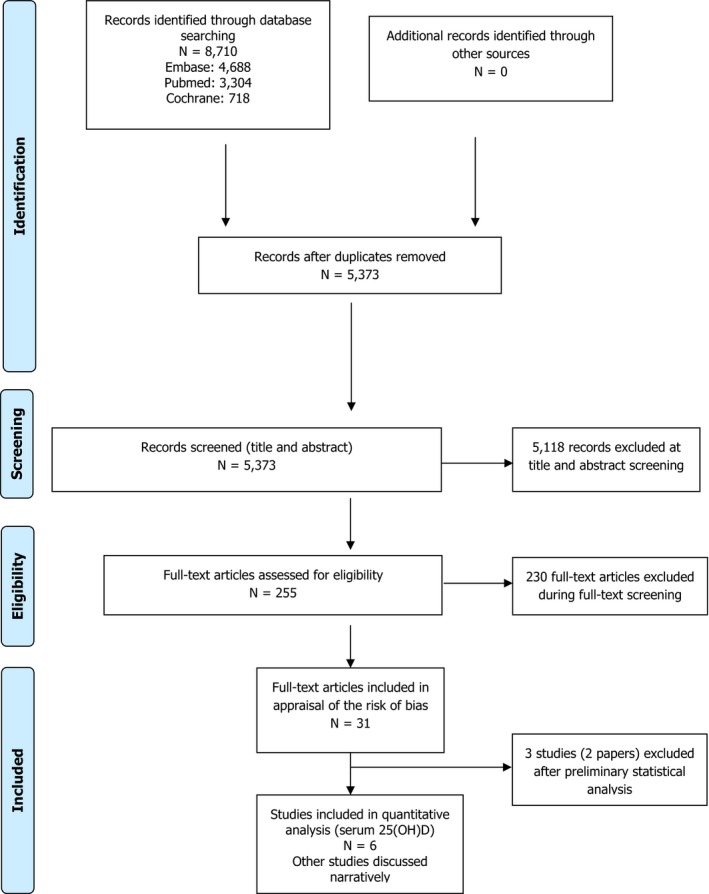
Appendix F – Appraisal of the risk of bias: tool with domain specific questions and relevant study design
1.
| Bias Domains and Questions | Study designs | |||||
|---|---|---|---|---|---|---|
| Randomised controlled trials | Non‐randomised controlled trials | Randomised non‐controlled trials | Non‐randomised non‐controlled trials | Prospective observational studies | Retrospective studies | |
|
Appraisal of internal validity The ability of a study to provide an unbiased estimate of the true value of the parameter(s) that the study is intended to estimate. | ||||||
| SELECTION BIAS | ||||||
| 1. Was administered dose or exposure level adequately randomised? | X | X | ||||
| 2. Was allocation to study groups adequately concealed? | X | X | ||||
| 3. Did selection of study participants result in appropriate comparison groups? | X | X | X | X | ||
| CONFOUNDING BIAS | ||||||
| 4. Did the study design or analysis account for important confounding and modifying variables of the outcome? Were the confounding and modifying variables measured reliably and consistently? | X | X | X | X | X | X |
| PERFORMANCE BIAS | ||||||
| 5. Were the research personnel and human subjects blinded to the study group during the study considering the health‐outcome ‘failure to thrive’? | X | X | X | X | ||
| ATTRITION / EXCLUSION BIAS | ||||||
| 6. Were outcome data complete without attrition or exclusion from analysis? | X | X | X | X | X | |
| DETECTION BIAS | ||||||
| 7. Can we be confident in the exposure characterisation? | X | X | X | X | X | X |
| 8. Can we be confident in the outcome assessment considering the outcome? | X | X | X | X | X | X |
| SELECTIVE REPORTING BIAS | ||||||
| 9. Were all measured outcomes reported when considering the outcome? | X | X | X | X | X | X |
| OTHER SOURCES OF BIAS | ||||||
| 10. Are there no other potential threats to internal validity (e.g. statistical methods were appropriate and researchers adhered to the study protocol)? | X | X | X | X | X | X |
Adapted from OHAT/NTP risk of bias tool (https://ntp.niehs.nih.gov/ntp/ohat/pubs/riskofbiastool_508.pdf).
Appendix G – Synthesis of the risk of bias by individual studies investigating hypercalciuria/urinary calcium
1.
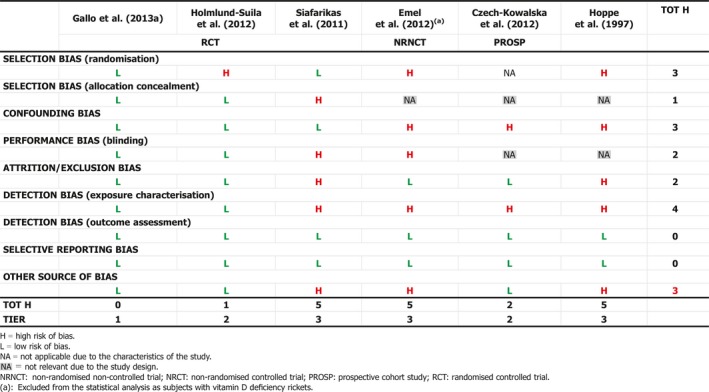
Appendix H – Synthesis of the risk of bias by individual studies investigating hypercalcaemia/serum calcium
1.
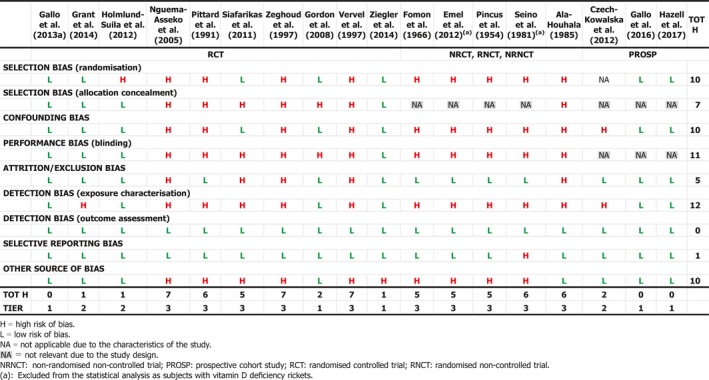
Appendix I – Synthesis of the risk of bias by individual studies investigating nephrocalcinosis
1.
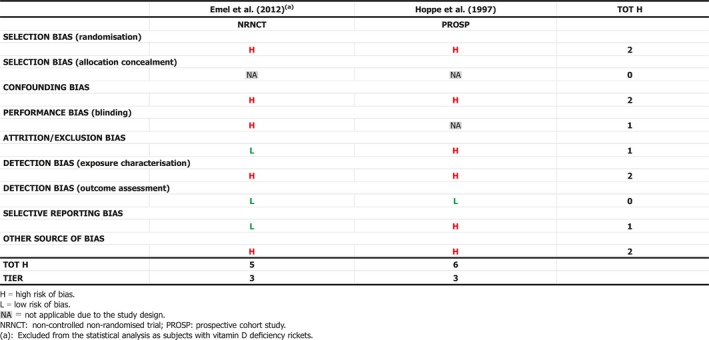
Appendix J – Synthesis of the risk of bias by individual studies investigating anthropometric parameters
1.
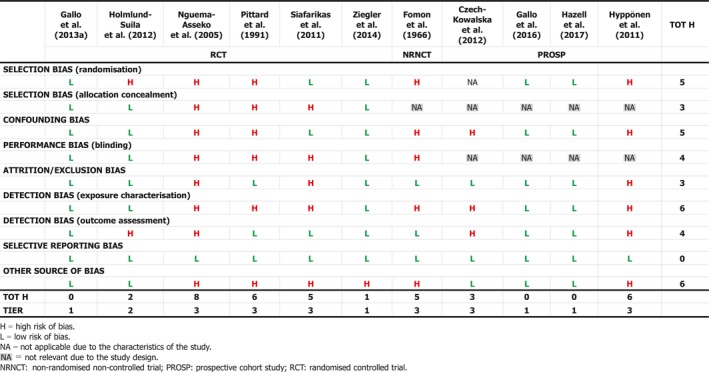
Appendix K – Synthesis of the risk of bias by individual studies investigating high serum 25(OH)D concentration
1.
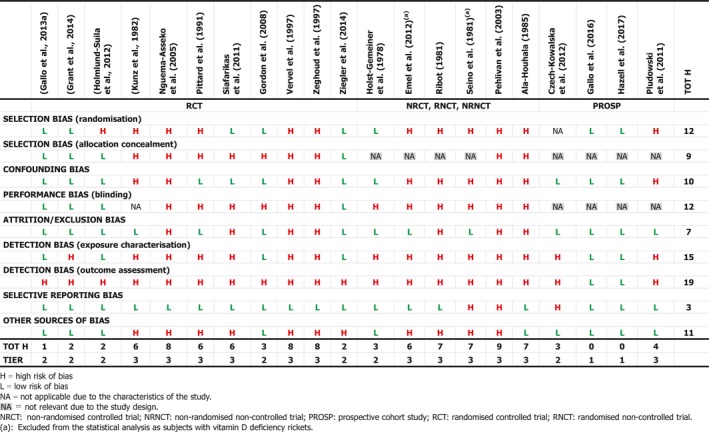
Appendix L – Assessment: summary characteristics of the studies included after the extensive literature search
1.
| Study population | Diet characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Biblio graphy |
Nb | Health status | Full term | Vit D source ‐trials | Vit D form | D_ wks |
Method for serum 25(OH)D |
VitD dose analytically checked | Compliance check | Ca‐ supp | Comments on study characteristics |
|
(Country) Hemisphere: Latitude |
TofFeed | Vit D dose μg/day | L‐ wks | > | |||||||
|
DESIGN: RCT AGE CLASS: ALL INFANTS | |||||||||||
| Gallo et al. (2013a) | 132 | Healthy | Full term | Drop | D3 | 12 | LC‐MS,MS | Yes | Yes | No | Authors mentioned that infants grew in an age‐appropriate way over time, with no differences over groups. Vitamin D from other foods than breast milk assessed using 3‐d dietary records. |
|
(CA) N:>= 40 & <= 50 |
Mixed |
G1:10 G2:20 G3:30 G4:40 |
11 | ||||||||
| Grant et al. (2014) | 200 | Healthy | Mixed | Drop | D3 | 7 | LC‐MS,MS | No | Yes | No | All mean weight at birth above 3.4 kg. Preterm received vitamin D through parenteral and oral nutrition. Mothers of infants in groups 2 and 3 supplemented during pregnancy. |
|
(NZ) S:< 40 |
Mixed |
G1:0 G2:10 G3:20 |
6 | ||||||||
| Holmlund‐Suila et al. (2012) | 113 | Healthy | Full term | Drop | D3 | 3 | Other | Yes | Yes | No | Analytical method for 25(OH)D: automated chemiluminiscence immunoassay (CLIA). |
|
(FI) N:>= 50 |
Mixed |
G1:10 G2:30 G3:40 |
3 | ||||||||
| Kunz et al. (1982) | 29 | Healthy | Mixed | Pill | NA | 2 | CPBA | No | No | No | Infants received formula containing 10 μg/L vitamin D. |
|
(DE) N:>= 50 |
Formula |
G1:25 G2:12.5 |
1.4 | ||||||||
| Nguema‐Asseko et al. (2005) | 79 | Healthy | Mixed | S‐NA | NA | 7 | NA | No | No | No |
Vitamin D provided via Uvestero®. Unclear if exclusively breastfed (but assumed to). All infants born in August. |
|
(GA) N:< 40 |
Breast |
G1:25 G2:0 |
6 | ||||||||
| Pittard et al. (1991) | 25 | Healthy | Full term | S‐NA | NA | 4 | NA | No | No | No | Throughout the study, each neonate was given an 84J/30 mL formula, that contained 12.8 mmol/L of calcium, and no added vitamin D. |
|
(US) N:< 40 |
Unclear |
G1:1 0G2:20 |
3.7 | ||||||||
| Siafarikas et al. (2011) | 40 | Healthy | Full term | Pill | D3 | 2 | RIA | No | No | No | In each group, an even number of infants born in summer and in winter |
|
(DE) N:>= 50 |
Breast |
G1:6.25 G2:12.5 |
1.4 | ||||||||
| Vervel et al. (1997)(Study 1 and Study 2) | 80 | Healthy | Full term | Drop | D2 | 4 | CPBA | No | Yes | No |
Results reported i) according to whether the mothers were supplemented (n = 22) or not (n = 48) and ii) according to the dose given to infants. Content of vitamin D in formula reported. Intake of formula measured but not reported Study 1: infants from unsupplemented mothers Study 2: infants from supplemented mothers |
|
(FR) N:>= 40 & <= 50 |
Formula |
G1:12.5 G2:25 |
3 | ||||||||
| Zeghoud et al. (1997) |
14 (Study 1); 36 (Study 2); 29 (Study 3) |
Healthy | Full term | S‐NA | D2 | 4 | CPBA | No | No | No |
Vitamin D provided via Uvesterol®. Vitamin D content in formula 11 +/− 1 μg/L D3. Study nr 1: neonates with vitamin D deficiency (25(OH)D < 30 nmol/L) and with high PTH (> 60 ng/L). Study nr 2: neonates with 25(OH)D < 30 nmol/L. Study nr 3: neonates with 25(OH)D strictly > 30 nmol/L |
|
(FR) N:>= 40 & = 50 |
Formula |
G1:25 G2:12.5 |
3 | ||||||||
| Ziegler et al. (2014) | 213 | Healthy | Full term | Drop | D3 | 9 | RIA | Yes | Yes | No |
Complementary feeding possible but not formula until 9 mo. Mean vitamin D supplement really consumed, according to time points: Group 1: 5.5–6 μg/day Group 2: 10–11 μg/day Group 3: 14–15 μg/day Group 4: 19–20.5 μg/day |
|
(US) N:< 40 |
Breast |
G1:5 G2:10 G3:15 G4:20 |
11 | ||||||||
|
DESIGN: RCT AGE CLASS: MIXED POPULATION | |||||||||||
| Gordon et al. (2008) | 26 | HypovitD | Mixed | Drop | D2,D3 | 2 | Other | Yes | Yes | Yes |
25(OH)D measured by chemiluminescence. Data on arm on weekly dose (n = 14) not extracted |
| (US) N:>= 40 & <= 50 | Mixed |
G1:50 G2:50 |
1.4 | ||||||||
|
DESIGN: RNCT, NRCT,NRNCT AGE CLASS: ALL INFANTS | |||||||||||
| Ala‐Houhala (1985) |
47 (Study 1) 45 (Study 2) |
Healthy | Full term | S‐NA | NA | 5 | CPBA | No | No | No |
From the main study (n = 100, including 8 in a pilot study, results not extracted), 9 infants excluded as mothers interrupted exclusive breastfeeding, but unclear if they belong to the pilot or the main study. Half of the mothers were supplemented during pregnancy. Mothers of group 1 are supplemented after delivery, while those of group 2 and 3 are not. Study 1: infants followed in winter Study 2: infants followed in summer |
|
(FI) N:>= 50 |
Breast |
G1:0 G2:10 G3:25 |
4.6 | ||||||||
| Fomon et al. (1966) | NA | Healthy | Full term | FORT | NA | 6 | NA | No | No | No | Commercial complementary feeding free of vitamin D was permitted. Mean birth weight of 3.4–3.6 kg in each group. Group 1: received formula with 10 μg/405 mL, median vitamin D intake is 11.25 μg/day (range 9–14 μg/day). 13 subjects at inclusion (11 completed 112 days and 2 were lost to follow up before). Group 2: received 40 μg/405 mL. Median intake of vitamin D 45 μg/day (range 34.5–54 μg/day). Group 3: Control group of 31 breastfed infants (not exclusively breastfed, as they receive 1 μg/100 mL of formula). Vitamin D content in breast milk not measured. Average vitamin D intake of 2 μg/day from formula and supplements consumed (5 μg/day). Authors say total vitamin D intake in this group was about 7.5 μg/day |
|
(US) N:>= 40 & <= 50 |
Mixed |
G1:11.25 G2:45 G3:7.5 |
5.6 | ||||||||
| Holst‐Gemeiner et al. (1978) | 10 | Healthy | Mixed | Drop | D3 | 2 | RIA | No | No | No | Infants received breastmilk or formula but no other vitamin D supplementation. Data for the group receiving a bolus dose not extracted |
| (AT) N:>= 40 & <= 50 | Mixed | G1:30 | 1.2 | ||||||||
| Pehlivan et al. (2003) | 40 | Healthy | Mixed | S‐NA | NA | 4 | Other | No | No | No | The paper mentions 65 infants and 40 breastfed infants. Not clear if the 40 are included in the 65 or not. Only data for 40 breastfed infants extracted. Supplementation started at the start of the second week in both groups. Baseline values not reported |
|
(TR) N:>= 40 & <= 50 |
Breast |
G1:10 G2:20 |
4 | ||||||||
| Pincus et al. (1954) | NA | Healthy | Mixed | FORT/S‐NA | NA | 1 | NA | No | No | No |
All infants were breastfed but unclear if exclusively. Group 1: no supplement. Group 2: supplement was an aqueous preparation. Group 3: fed with a formula not containing vitamin D. Group 4: fed with formula containing 10 μg/L vitamin D. Unclear how much formula was consumed, so real vitamin D intake is unknown. Group 5: fed with a formula not containing vitamin D. Infants received vitamin D supplement in an aqueous preparation |
|
(US) N:>= 40 & <= 50 |
Mixed |
G1:0 G2:15 G3:0 G4:10 G5:15 |
1 | ||||||||
| Ribot (1981) | 35 | Healthy | Mixed | S‐NA | NA | 1 | CPBA | No | No | No |
Vitamin D provided via Uvesterol®. Group1: newborns, group 2: aged 2–6 mo. Data for the group of premature infants not extracted |
|
(FR) N:>= 40 & <= 50 |
NA |
G1:25 G2:25 |
1 | ||||||||
|
DESIGN: PROSP AGE CLASS: ALL INFANTS | |||||||||||
|
Czech‐Kowalska et al. (2012) (PL) N:>= 50 |
19 (Study 1), 11 (Study 2) |
Hypovit D | Full term | Drop | D3 | 3 | Other | No | Yes | No |
No signs of rickets. Median birth weight of 3570 g (3405;3890). Seventeen infants (56.7%) born in summer and 13 infants (43.3%) in winter. Twenty‐five infants (83.3%) were regularly supplemented (1 drop daily), while in 5 cases (4 deficient and 1 insufficient at T1) the supplementation scheme was different, i.e. 2 drops of vitamin D ingested occasionally in a time period of 2–10 weeks. Most of the infants were exclusively breastfed. |
| Mixed | 2.3 |
Median intake at time point 1 for the full study 14 μg/day (P25–P75 12.5–17). Analytical method for 25OHD: immunochemiluminescence. Study 1 : newborn infants with 25OHD less than 27.5 nmol/L (‘deficient’ group). All infants were supplemented, but they did not receive vitamin D supplementation at baseline. Time point 0, median (IQR): vitamin D supplements (μg/day) 0 (0;0); total vitamin D intake (μg/day) 0.5 (0.5;0.5) Time point 1, median (IQR): vitamin D supplements (μg/day) 17 (12.5;19); total vitamin D intake (μg/day) 18 (13.5;20). Study 2: newborn infants with 25OHD between 27.5 and 50 nmol/L (‘insufficient’ group). Time point 0, median (IQR): vitamin D supplements (μg/day) 0 (0;0); total vitamin D intake (μg/day) 0.5 (0.5;0.5) Time point 1, median (IQR): vitamin D supplements (μg/day) 12.5 (12.5;15); total vitamin D intake (μg/day) 19 (13.5;20). Both groups did not differ by maternal vitamin D supplementation, infants’ age, gender, birth weight, season of birth, type of feeding and vitamin D intake. 86% of mothers reported vitamin D supplementation during pregnancy. |
|||||||||
| Hoppe et al. (1997) | 37 | Healthy | Full term | S‐NA | D3 | 3 | NA | No | No | No |
Birth weight in term infants, mean (SD): formula‐fed 3,316 (562), breastfed 3,047 (710) g. Calcium content of infant feedings (mg/100 mL)‐Human milk 30‐ formula A 57 – Formula B 45. Authors indicate that serum ca and creatinine were in the normal age‐related range (range not given). Group 1: only formula‐fed term infants. Group 2: only breastfed term infants. |
| (CH, DE) N:>= 40 & <= 50 | Mixed |
G1:12.5 G2:12.5 |
2 | ||||||||
| Pludowski et al. (2011) | 75 | Healthy | Full term | Drop | D3 | 7 | Other | No | Yes | No |
Analytical method for 25OHD: radioreceptor method. A subgroup from the full study group (n = 132) of 82 infants with a complete data set was selected, divided into 3 subgroups according to vitamin D supplementation scheme reported by caregivers: 1. Regular supplementation at both 6 and 12 months (n = 43) (Group 1). 2. Supplementation scheme changed between the 6th and the 12th month (n = 32) i.e. regular supplementation, then occasional, or the other way round, or discontinued supplementation. Group 2) 3. Occasional supplementation at both 6 and 12 months (n = 7). Questionnaire used to collect dietary data and data on vitamin D supplements. Vitamin D intake from breast milk not taken into account. Group 1: Infants regularly supplemented with vitamin D). T0: Total intake (food + supplements): 29 +/− 18 μg/day. T1: Total intake (food+supplements): 29 +/− 14 μg/day. Group 2: Infants with reported changes in the supplementation scheme. T0: Total intake (food+supplements): 28.5 +/− 15.5 μg/day. T1: Total intake (food+supplements): 19 +/− 14 μg/day. |
|
(PL) N:>= 50 |
Mixed |
G1:NA G2:NA |
6 | ||||||||
|
DESIGN: PROSP AGE CLASS: MIXED POPULATION | |||||||||||
| Gallo et al. (2016) | 132 | Healthy | Full term | Drop | D3 | 12 | LC‐MS, MS | Yes | Yes | No |
Same cohort as Hazell et al., 2017. Dietary intake from other foods assessed using three 24h recalls and validated FFQ for preschool children used to assess calcium and vitamin D (from food and supplements) intake over the past month. Background intake (food only) at 36 months not significantly different among groups, in particular for calcium and vitamin D: calcium: 931–970 mg/day; vitamin D: 6–7.5 μg/day Vitamin D, total diet and supplements (group 1,2,3,4) Tertile 1: % ≤ 9 μg/day 40.0, 25.0, 19.0, 36.0Tertile 2: % 9–13 μg/day 16.0, 42.0, 46.0, 9.0Tertile 3: % ≥ 13 μg/day 44.0, 33.0, 35.0, 55.0. No information given on intake between the end of the trial and one month before the follow‐up. |
|
(CA) N:>= 40 & <= 50 |
Mixed |
G1:10 G2:20 G3:30 G4:40 |
0.1 | ||||||||
| Hazell et al. (2017) | 132 | Healthy | Full term | Drop | D3 | 12 | LC‐MS,MS | Yes | Yes | No | Same cohort as Gallo et al., 2016; follow‐up of the trial Gallo et al., 2013a; From the original 132 participants in the trial, 66% (49 boys and 38 girls) returned for the 3‐year follow‐up. |
| (CA) N:>= 40 & <= 50 | Mixed |
G1:10 G2:20 G3:30 G4:40 |
0.1 | ||||||||
| Hyppönen et al. (2011) | 12058 | Mixed | Mixed | Drop | NA | 13 | NA | No | Yes | No |
200 infants identified as having rickets. Daily dose of vitamin D calculated on the basis of the concentration of vitamin D in the product used and the reported dosage (as number of droplets) of the product. 84 children who had received cod liver oil were classified as having received the recommended dose (50 μg/day). No information on vitamin D supplementation after the first year. According to contemporary recommendations, vitamin D supplementation of 50 μg/day was recommended to all children up to 2 y, which may suggest that systematic supplementation in many cases was restricted to the first 2 y of life. Subjects grouped according to frequency of supplementation: none, irregularly, regularly. Analysis according to daily doses done only in those that received supplementation regularly |
| NA |
G1:0 G2:< 50 G3:50 G4:> 50 |
0.6 | |||||||||
AT: Austria; Breast: exclusively breastfed at inclusion; CA: Canada; Ca‐supp: calcium supplementation (yes/no); CBPA: competitive protein‐binding assay; CH: Switzerland; CLIA: automated chemiluminiscence immunoassay; D: Duration of vitamin D additional intake (converted into weeks and rounded); DE: Germany; FI: Finland; Formula : exclusively formula‐fed at inclusion; FORT: fortified food (formula); FR: France; GA: Gabon; L: Length of follow‐up (converted into weeks and rounded); LCMS‐MS: liquid chromatography‐tandem mass spectrometry; N: North; Nb: number of subjects at randomisation/inclusion; NA: type of feeding at inclusion not specified; NRCT: non‐randomised controlled trial; NRNCT: non‐randomised non‐controlled trial; NZ: New Zealand; PL: Poland; PROSP: prospective cohort study; RCT: randomised controlled trial; RNCT: randomised non‐controlled trial; RIA: radioimmunoassay; S: South; S‐NA: supplements in an unspecified form; TofFeed: type of feeding at inclusion; TR: Turkey; US: United States; wks: weeks.
Appendix M – Assessment: studies reporting on urinary calcium/creatinine ratio
1.
| Design | Citation | Tier | Vitamin D dose | Age | Urinary Ca/Cr ratio | ||
|---|---|---|---|---|---|---|---|
| (μg/day) | (weeks) | unit | (mean) | (SD) | |||
| RCT | Holmlund‐Suila et al. (2012) | 2 | 10 | 13 | mmol/mmol | 2 | n.a. |
| 30 | 12 | mmol/mmol | 1.95 | n.a. | |||
| 40 | 13 | mmol/mmol | 2.1 | n.a. | |||
| Gallo et al. (2013a) | 1 | 10 | 0 | othera | 1.9 | n.a. | |
| 10 | 4 | othera | 1.6 | n.a. | |||
| 10 | 8 | othera | 1.7 | n.a. | |||
| 10 | 21 | othera | 1.7 | n.a. | |||
| 10 | 34 | othera | 0.9 | n.a. | |||
| 10 | 48 | othera | 0.85 | n.a. | |||
| 20 | 0 | othera | 2.2 | n.a. | |||
| 20 | 4 | othera | 2.1 | n.a. | |||
| 20 | 8 | othera | 1.85 | n.a. | |||
| 20 | 21 | othera | 1.7 | n.a. | |||
| 20 | 34 | othera | 0.8 | n.a. | |||
| 20 | 48 | othera | 0.5 | n.a. | |||
| 30 | 0 | othera | 1.9 | n.a. | |||
| 30 | 4 | othera | 2.1 | n.a. | |||
| 30 | 8 | othera | 2 | n.a. | |||
| 30 | 21 | othera | 1.6 | n.a. | |||
| 30 | 34 | othera | 0.6 | n.a. | |||
| 30 | 48 | othera | 0.85 | n.a. | |||
| 40 | 0 | othera | 1.7 | n.a. | |||
| 40 | 4 | othera | 1.9 | n.a. | |||
| 40 | 8 | othera | 2.1 | n.a. | |||
| 40 | 21 | othera | 1.6 | n.a. | |||
| PROSP | Hoppe et al. (1997) | 3 | 12.5 | 0 | mol/mol | 0.1 | 0.41 |
| 12.5 | 0 | mol/mol | 0.22 | 0.48 | |||
| 12.5 | 9 | mol/mol | 0.27 | 0.87 | |||
| 12.5 | 9 | mol/mol | 0.36 | 0.6 | |||
| Czech‐Kowalska et al. (2012) | 2 | 16.75b | 0 | mg/mg | 0.46 | 0.53 | |
| 16.75b | 11 | mg/mg | 0.57 | 0.28 | |||
| 12.5c | 0 | mg/mg | 0.39 | 0.27 | |||
| 12.5c | 11 | mg/mg | 0.61 | 0.7 | |||
PROSP: prospective cohort study; RCT: randomised controlled trial; SD: standard deviation.
The values of urinary Ca/Cr ratio from the study by Siafarikas et al. (2011) not included as the unit was not reported in the paper.
outside of the normal range of the hospital (Section 3.3.2.2).
vitamin D deficient subjects (Section 3.3.2.3).
vitamin D insufficient subjects (Section 3.3.2.3).
Appendix N – Assessment: studies reporting on serum total calcium (tier 3)
1.
| Design | Citation | Vitamin D dose | Age | Total blood calcium | ||
|---|---|---|---|---|---|---|
| (μg/day) | (weeks) |
N (subjects included in the measurement) |
(mean) | (SD) | ||
| RCT | Nguema‐Asseko et al. (2005) | 0 | 13 | 27 | 2.23 | 0.6 |
| 0 | 26 | 28 | 2.32 | 0.2 | ||
| 25 | 13 | 37 | 2.39 | 0.2 | ||
| 25 | 26 | 24 | 2.45 | 0.2 | ||
| Siafarikas et al. (2011) | 6.25 | 0 | 14 | 2.38 | 2.29 | |
| 6.25 | 6 | 14 | 2.66 | 2.58 | ||
| 12.5 | 0 | 14 | 2.38 | 2.29 | ||
| 12.5 | 6 | 14 | 2.55 | 2.47 | ||
| Vervel et al. (1997) a | 12.5 | 0 | 28 | 2.44 | 0.15 | |
| 12.5 | 4 | 28 | 2.57 | 0.08 | ||
| 12.5 | 13 | 20 | 2.62 | 0.09 | ||
| 25 | 0 | 20 | 2.46 | 0.16 | ||
| 25 | 4 | 20 | 2.53 | 0.06 | ||
| 25 | 13 | 14 | 2.62 | 0.07 | ||
| Vervel et al. (1997) b | 12.5 | 0 | 6 | 2.58 | 0.09 | |
| 12.5 | 4 | 6 | 2.56 | 0.11 | ||
| 12.5 | 13 | 4 | 2.58 | 0.14 | ||
| 25 | 0 | 16 | 2.51 | 0.07 | ||
| 25 | 4 | 16 | 2.56 | 0.08 | ||
| 25 | 13 | 14 | 2.58 | 0.07 | ||
| Zeghoud et al. (1997) | 12.5 | 0 | n.a. | 2.41 | 0.21 | |
| 12.5 | 4 | n.a. | 2.58 | 0.49 | ||
| 12.5 | 13 | n.a. | 2.65 | 0.51 | ||
| 25 | 0 | n.a. | 2.41 | 0.21 | ||
| 25 | 4 | n.a. | 2.53 | 0.07 | ||
| 25 | 13 | n.a. | 2.6 | 0.12 | ||
| 12.5 | 0 | n.a. | 2.48 | 0.12 | ||
| 12.5 | 4 | n.a. | 2.58 | 0.49 | ||
| 12.5 | 13 | n.a. | 2.58 | 0.49 | ||
| 25 | 0 | n.a. | 2.48 | 0.12 | ||
| 25 | 4 | n.a. | 2.52 | 0.09 | ||
| 25 | 13 | n.a. | 2.6 | 0.12 | ||
| 12.5 | 0 | n.a. | 2.52 | 0.1 | ||
| 12.5 | 4 | n.a. | 2.58 | 0.49 | ||
| 12.5 | 13 | n.a. | 2.6 | 0.5 | ||
| 25 | 0 | n.a. | 2.62 | 0.1 | ||
| 25 | 4 | n.a. | 2.57 | 0.06 | ||
| 25 | 13 | n.a. | 2.6 | 0.12 | ||
| RNCT, NRCT,NRNCT | Ala‐Houhala (1985) c | 0 | 0 | n.a. | 2.78 | 0.23 |
| 0 | 8 | n.a. | 2.7 | 0.22 | ||
| 0 | 20 | n.a. | 2.7 | 0.22 | ||
| 10 | 0 | n.a. | 2.8 | 0.2 | ||
| 10 | 8 | n.a. | 2.75 | 0.2 | ||
| 10 | 20 | n.a. | 2.7 | 0.2 | ||
| 25 | 0 | n.a. | 2.8 | 0.13 | ||
| 25 | 8 | n.a. | 2.8 | 0.13 | ||
| 25 | 20 | n.a. | 2.75 | 0.13 | ||
| Ala‐Houhala (1985) d | 0 | 0 | n.a. | 2.78 | 0.23 | |
| 0 | 8 | n.a. | 2.7 | 0.22 | ||
| 0 | 20 | n.a. | 2.7 | 0.22 | ||
| 10 | 0 | n.a. | 2.6 | 0.19 | ||
| 10 | 8 | n.a. | 2.7 | 0.2 | ||
| 10 | 20 | n.a. | 2.7 | 0.2 | ||
| 25 | 0 | n.a. | 2.7 | 0.12 | ||
| 25 | 8 | n.a. | 2.7 | 0.12 | ||
| 25 | 20 | n.a. | 2.65 | 0.12 | ||
| Fomon et al. (1966) | 7.5 | 4 | 24 | 2.52 | 0.16 | |
| 7.5 | 8 | 23 | 2.45 | 0.08 | ||
| 7.5 | 12 | 23 | 2.53 | 0.14 | ||
| 7.5 | 16 | 24 | 2.51 | 0.12 | ||
| 7.5 | 20 | 20 | 2.48 | 0.11 | ||
| 7.5 | 24 | 18 | 2.42 | 0.13 | ||
| 11.25 | 4 | 10 | 2.48 | 0.16 | ||
| 11.25 | 8 | 8 | 2.53 | 0.11 | ||
| 11.25 | 12 | 10 | 2.48 | 0.1 | ||
| 11.25 | 16 | 7 | 2.52 | 0.12 | ||
| 11.25 | 20 | 9 | 2.49 | 0.11 | ||
| 11.25 | 24 | 9 | 2.49 | 0.13 | ||
| 45 | 4 | 9 | 2.47 | 0.12 | ||
| 45 | 8 | 9 | 2.52 | 0.21 | ||
| 45 | 12 | 11 | 2.57 | 0.09 | ||
| 45 | 16 | 7 | 2.46 | 0.12 | ||
| 45 | 20 | 11 | 2.44 | 0.18 | ||
| 45 | 24 | 11 | 2.42 | 0.2 | ||
| Pincus et al. (1954) | 0 | 0 | 15 | 2.23 | 0.16 | |
| 0 | 0 | 46 | 2.36 | 0.17 | ||
| 0 | 1 | 15 | 2.26 | 0.14 | ||
| 0 | 1 | 46 | 2.28 | 0.23 | ||
| 10 | 0 | 52 | 2.27 | 0.21 | ||
| 10 | 1 | 52 | 2.15 | 0.37 | ||
| 15 | 0 | 13 | 2.29 | 0.08 | ||
| 15 | 0 | 30 | 2.27 | 0.11 | ||
| 15 | 1 | n.a. | 2.22 | 0.09 | ||
| 15 | 1 | 30 | 2.04 | 0.29 | ||
n.a.: not available; NRCT: non‐randomised controlled trial; NRNCT: non‐randomised non‐controlled trial;; RCT: randomised controlled trial; RNCT: randomised non‐controlled trial; SD: standard deviation.
Study 1, infants from unsupplemented mothers
Study 2, infants from supplemented mothers
Study 1, infants followed in winter
Study 2, infants followed in summer
Appendix O – Assessment: studies reporting ionised calcium values
1.
| Design | Citation | Dose | Age | Ionised blood calcium | ||
|---|---|---|---|---|---|---|
| (weeks) | Units | (mean) | (SD) | |||
| RCT | Gallo et al. (2013a) [Link] | 10 | 0 | mmol/L | 1.38 | n.a. |
| 10 | 4 | mmol/L | 1.37 | n.a. | ||
| 10 | 8 | mmol/L | 1.37 | n.a. | ||
| 10 | 21 | mmol/L | 1.35 | n.a. | ||
| 10 | 34 | mmol/L | 1.33 | n.a. | ||
| 10 | 48 | mmol/L | 1.32 | n.a. | ||
| 20 | 0 | mmol/L | 1.4 | n.a. | ||
| 20 | 4 | mmol/L | 1.4 | n.a. | ||
| 20 | 8 | mmol/L | 1.37 | n.a. | ||
| 20 | 21 | mmol/L | 1.35 | n.a. | ||
| 20 | 34 | mmol/L | 1.33 | n.a. | ||
| 20 | 48 | mmol/L | 1.32 | n.a. | ||
| 30 | 0 | mmol/L | 1.39 | n.a. | ||
| 30 | 4 | mmol/L | 1.37 | n.a. | ||
| 30 | 8 | mmol/L | 1.36 | n.a. | ||
| 30 | 21 | mmol/L | 1.35 | n.a. | ||
| 30 | 34 | mmol/L | 1.33 | n.a. | ||
| 30 | 48 | mmol/L | 1.32 | n.a. | ||
| 40 | 0 | mmol/L | 1.4 | n.a. | ||
| 40 | 4 | mmol/L | 1.38 | n.a. | ||
| 40 | 8 | mmol/L | 1.37 | n.a. | ||
| 40 | 21 | mmol/L | 1.35 | n.a. | ||
| PROSP | Hazell et al. (2017) | 10 | 48 | mmol/L | 1.29 | 0.03 |
| 20 | 48 | mmol/L | 1.3 | 0.05 | ||
| 30 | 48 | mmol/L | 1.28 | 0.06 | ||
| 40 | 48 | mmol/L | 1.27 | 0.05 | ||
n.a.: not available; PROSP: prospective cohort study; RCT: randomised controlled trial; SD: standard deviation.
Units as reported in the paper are nmol/L, but here assumed to be mmol/L based on Gallo et al. (2014b).
Appendix P – Forest plot on studies reporting on mean body length (cm) and mean body weight (g) (tiers 1–2)
1.
Figure 4.
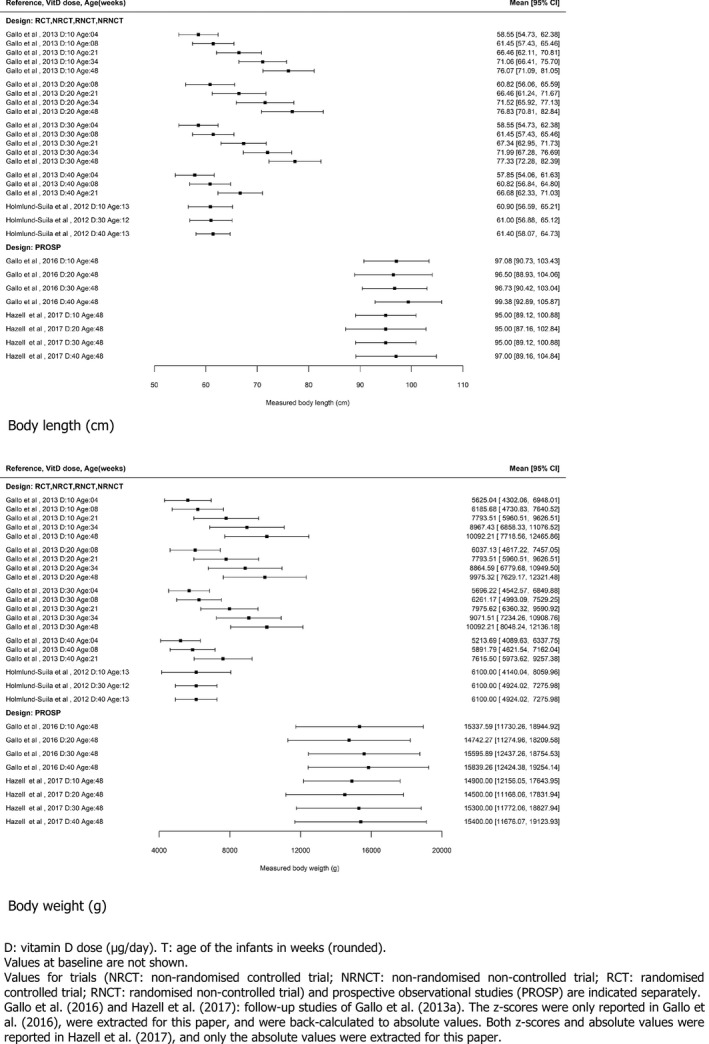
Appendix Q – Assessment: vitamin D content in 46 food supplements targeted for infants < 1 year
1.
| Country | Format type | Serving size/conditions of use |
Vitamin D (daily dose in μg)a |
Vitamin D form |
|---|---|---|---|---|
| Belgium | Liquid | 2 drops/day | 10 | D3 |
| Belgium | Tablet | 1 tablet/day | 10 | D3 |
| Croatia | Liquid | 6 drops/day | 10 | D3 |
| Denmark | Liquid | 5 drops/day | 10 | D3 |
| Denmark | Liquid | 5 drops/day | 10 | D3 |
| Denmark | Liquid | 5 drops/day | 10 | D3 |
| Denmark | Tablet | 1 tablet/day | 10 | D3 |
| Denmark | Tablet | 1 tablet/day | 10 | vit D unspecified |
| Denmark | Tablet | 1 tablet/day | 10 | D3 |
| Denmark | Tablet | 1 tablet/day | 10 | D3 |
| Germany | Tablet | 1 tablet/day | 12.5 | D3 |
| Hungary | Liquid | 2 drops/day | 10 | D3 |
| Ireland | Capsules | 1 capsule/day | 5 | D3 |
| Italy | Liquid | 30 drops/day | 10 | D3 |
| Italy | Liquid | 4 drops/day | 10 | D3 |
| Italy | Liquid | 0.5 mL/day | 7.5 | D3 |
| Netherlands | Liquid | 2 drops/day | 10 | D3 |
| Netherlands | Liquid | 10 drops/day | 10 | D3 |
| Norway | Liquid | 10 drops/day | 10 | D3 |
| Norway | Liquid | 1 capsule/day | 10 | D3 |
| Norway | Liquid | 5 drops/day | 10 | D3 |
| Poland | Liquid | 1 capsule/day | 10 | D3 |
| Poland | Liquid | 1 capsule/day | 10 | D3 |
| Poland | Capsules | 1 capsule/day | 10 | D3 |
| Poland | Capsules | 1 capsule/day | 10 | D3 |
| Poland | Capsules | 1 capsule/day | 10 | D3 |
| Poland | Capsules | 1 capsule/day | 10 | D3 |
| Poland | Liquid | 1 capsule/day | 10 | D3 |
| Poland | Capsules | 1 capsule/day | 15 | D3 |
| Poland | Capsules | 1 capsule/day | 10 | D3 |
| Poland | Capsules | 1 capsule/day | 10 | D3 |
| Sweden | Liquid | 5 drops/day | 10 | D3 |
| Sweden | Liquid | 0.14 mL/day | 7.5 | D3 |
| UK | Liquid | 1 spray/day | 10 | D3 |
| UK | Liquid | 1 drop/day | 10 | D3 |
| UK | Liquid | 2.5 mL/day | 1.75 | vit D unspecified |
| UK | Powder | 0.5–1 sachet/day | 1.13–2.5 | D3 |
| UK | Liquid | 0.3 mL/day | 10 | D3 |
| UK | Chew | 1 pastille/day | 5 | D3 |
| UK | Liquid | half teaspoon/day | 7 | D3 |
| UK | Liquid | 4 drops/day | 7.5 | D3 |
| UK | Liquid | 0.5 mL/day | 8.5 | D3 |
| UK | Liquid | 0.5 mL/day | 10 | D3 |
| UK | Liquid | 1 spray/day | 7.5 | D3 |
| UK | Liquid | 8 drops/day | 8 | D3 |
| UK | Liquid | 5 drops/day | 10 | D3 |
n.a.: information not available from which to calculate the vitamin D content by g/mL; UK: United Kingdom.
Date of search in Mintel GNPD database: February 2018.
Based on serving size and conditions of use as indicated on the label of the products.
Annex A – Statistical methods used to estimate the intake–response of serum 25(OH)D concentration on daily supplemental intake of vitamin D and to derive the percentage of infants exceeding a serum 25(OH)D concentration
1.
Annex A can be found in the online version of this output, under the section ‘Supporting information’, at: https://doi.org/10.2903/j.efsa.2018.5365
Description: The annex describes in details the statistical methods used to estimate the intake–response of serum 25(OH)D concentration on daily supplemental intake of vitamin D and to derive the percentage of infants exceeding a serum 25(OH)D concentration. These methods and the results obtained are described in brief in Section 3.5 of this output.
Annex B – Results of the assessment of the daily intake of vitamin D for infants aged 4–< 12 months and 8 intake scenarios
1.
Annex B can be found in the online version of this output, under the section ‘Supporting information’, at: https://doi.org/10.2903/j.efsa.2018.5365
Description: The annex is an Excel® file which presents in Tables A1–A6 the detailed data of the characteristics of the populations, food composition and food consumption used in the eight intake scenarios applied for the intake assessment for infants aged 4–< 12 months. The results are described in brief in Section 4.4.2 of this output.
Supporting information
Statistical methods used to estimate the intake–response of serum 25 (OH)D concentration on daily supplemental intake of vitamin D and to derive the percentage of infants exceeding a serum 25(OH)D concentration
Results of the assessment of the daily intake of vitamin D for infants aged 4–< 12 months and 8 intake scenarios
Suggested citation: EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , Turck D, Bresson J‐L, Burlingame B, Dean T, Fairweather‐Tait S, Heinonen M, Hirsch‐Ernst KI, Mangelsdorf I, McArdle HJ, Naska A, Nowicka G, Pentieva K, Sanz Y, Siani A, Sjödin A, Stern M, Tomé D, Van Loveren H, Vinceti M, Willatts P, Fewtrell M, Lamberg‐Allardt C, Przyrembel H, Arcella D, Dumas C, Fabiani L, Martino L, Tomcikova D and Neuhäuser‐Berthold M, 2018. Scientific opinion on the update of the tolerable upper intake level for vitamin D for infants. EFSA Journal 2018;16(8):5365, 118 pp. 10.2903/j.efsa.2018.5365
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00208
Panel members: Jean‐Louis Bresson, Barbara Burlingame, Tara Dean, Susan Fairweather‐Tait, Marina Heinonen, Karen Ildico Hirsch‐Ernst, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Monika Neuhäuser‐Berthold, Grażyna Nowicka, Kristina Pentieva, Yolanda Sanz, Alfonso Siani, Anders Sjödin, Martin Stern, Daniel Tomé, Dominique Turck, Henk Van Loveren, Marco Vinceti and Peter Willatts.
Acknowledgements: The Panel wishes to thank EFSA staff: Mathias Amundsen, Krizia Ferrini, Irene Muñoz Guajardo, Rita Sousa, Qingqing Sun and Ermolaos Ververis, for the support provided to this scientific opinion.
Adopted: 28 June 2018
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2018.EN-1456/full
Notes
The definition of vitamin D supplementation policies for infants or of public health actions in favour of breastfeeding is outside the scope of this opinion.
Prophylactic administration of vitamin D in some countries may be considered as medication.
Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow‐on formula and as regards requirements on information relating to infant and young child feeding, OJ L 25, 2.2.2016, p. 1.
Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow‐on formulae and amending Directive 1999/21/EC, OJ L 401, 30.12.2006, p. 1.
ULs set in the previous EFSA Opinion of 2012 for other population groups were outside the scope of this opinion, thus remain unchanged, i.e. 50 µg/day for children aged 1–10 years, and 100 µg/day for children aged 11–17 years and all adults.
In its scientific opinion of 2009 on the appropriate age for introduction of complementary feeding of infants, ‘the Panel concludes that the introduction of complementary food into the diet of healthy term infants in the EU between the age of 4 and 6 months is safe and does not pose a risk for adverse health effects’. At the time the present scientific opinion was drafted, this opinion on appropriate age for introduction of complementary feeding was under revision (EFSA‐Q‐2016‐00482).
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2014. Scientific Opinion on the essential composition of infant and follow‐on formulae. EFSA Journal 2014;12(7):3760.
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Scientific Opinion on the Tolerable Upper Intake Level of vitamin D. EFSA Journal 2012;10(7):2813.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety, OJ L 31, 1.2.2002, p. 1.
Adequate Intake (AI): the value estimated when a Population Reference Intake cannot be established because an average requirement cannot be determined. An Adequate Intake is the average observed daily level of intake by a population group (or groups) of apparently healthy people that is assumed to be adequate.
For conversion between µg and International Units (IU) of vitamin D intake: 1 µg = 40 IU and 0.025 µg = 1 IU. For conversion between nmol/L and ng/mL for serum 25(OH)D concentration: 2.5 nmol/L = 1 ng/mL.
Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. OJ L 404, 30.12.2006, p. 26.
Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. OJ L 183, 12.7.2002, p. 51.
IF is the only processed foodstuff which wholly satisfies the nutritional requirements of infants during the first months of life until the introduction of appropriate complementary feeding (while FoF are intended for infants when appropriate complementary feeding is introduced).
Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow‐on formula and as regards requirements on information relating to infant and young child feeding. OJ L 25, 2.2.2016, p. 1.
Commission Directive 2006/125/EC of 5 December 2006 on processed cereal‐based foods and baby foods for infants and young children. OJ L 339, 6.12.2006, p. 16.
Part of the body exposed, type of covering, assessed via questionnaire.
% BSA x weekly hours of sun exposure, lower in winter than in other seasons.
Concentration analysed at 4, 26 and 52 weeks.
Published after the end of the period of the systematic literature search (Section 3.1).
EFSA wishes to acknowledge all Member State bodies that provided data on vitamin D through this consultation.
Full‐term infants are defined as infants born at or after the 37th week of gestation.
Dates covered by each database: 1946 to present (Pubmed), 1947 to present (Embase), inception to present (Cochrane).
1 day = 1/7 week and 1 month = 4.33 weeks.
The ability of a study to provide an unbiased estimate of the true value of the parameter(s) that the study is intended to estimate.
The extent to which external bias is minimised in the studies, and the results are generalisable to the assessment question.
Taking into account the mother tongue of the reviewers, papers in French were not assessed in duplicate. The question related to statistical aspects was not assessed in duplicate either.
Confounding occurs when a factor is associated with both the exposure and the outcome but does not lie on the causative pathway. Confounding factors need to be eliminated to prevent distortion of association.
A modifier occurs when exposure has a different effect among different subgroups. Effect modification is associated with the outcome but not the exposure.
The count of the (intermediate and final) time‐points above does not include observations taken at the start of the studies (later used as baseline serum 25(OH)D concentrations in the dose‐response analysis, Section 3.5 and Annex A).
No obvious difference in the prevalence of hypercalciuria between the three groups can be seen in Figure 3 of this paper.
P25–P75 intake: about 12.5–17 μg/day from supplements, about 13.5–20 µg/day from food including supplements.
Considering that 1 mg Ca/ L = 0.025 mmol Ca/L and that 1 mg creatinine/L = 0.0088 mmol creatinine/L.
% Infants from supplemented women above 2.60 mmol/L: in the 25 µg group, 14 % at 3 months (19% at 1 month); in the 12.5 µg group, 25 % at 3 months (17 % at 1 month).
A paper published after the end of the literature search undertaken by the Panel.
A paper published after the end of the public consultation for this opinion.
Using e.g. average values for these variables would not have been sufficiently conservative for establishing a UL
Combining data from two databases (http://www.levensmiddelendatabank.nl, and http://www.innovadatabase.com), a supermarket inventory, and searches on websites of manufacturers.
Fruit fromage frais, ready‐to‐drink milk porridge, yogurt drink.
Instant cacao powder and soya drink.
As indicated in this publication: ‘Some studies indicate that manufacturers of such products [fortified foods and dietary supplements] may add higher amounts of micronutrients, in general, to compensate for losses during processing and shelf life (so‐called overages)’.
Database of nutrient composition data used to estimate nutrient intake for all foods, supplements and recipes in the NDNS.
As indicated in this publication: ‘the additional amount or ‘overage’ added during fortification and supplementation above the label value to allow for any processing losses and degradation.’
Germany, Denmark, Finland, France, UK, Iceland, Italy, the Netherlands, Portugal, Serbia, Sweden, Slovenia.
Denmark, Finland and the UK.
Commission Directive 2006/125/EC of 5 December 2006 on processed cereal‐based foods and baby foods for infants and young children. OJ L 339, 6.12.2006, p. 16.
See Section 4.1.1.5.
Approaches for modelling range of micronutrient intakes for infants consuming infant formula in Ireland (2017). M. A. T. Flynn and O. C. Lyons, Public Health Nutrition, Food Safety Authority of Ireland. (unpublished)
Food Safety Authority of Ireland (2007). Recommendations for a National Policy on Vitamin D Supplementation for Infants in Ireland, 46 pp.
European Commission. Guidance document for competent authorities for the control of compliance with EU legislation on: Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 and Council Directive 90/496/EEC of 24 September 1990 on nutrition labelling of foodstuffs and Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements with regard to the setting of tolerances for nutrient values declared on a label. 2012.
For this age group, voluntarily fortified foods were milk‐based cereals and cooking fats.
157 males (6–7 months), 159 females (6–7 months), 153 males (8–9 months), 136 females (8–9 months), 114 males (10–11 months), 107 females (10–11 months).
In this scientific opinion of 2009 on the appropriate age for introduction of complementary feeding of infants, ’the Panel concludes that the introduction of complementary food into the diet of healthy term infants in the EU between the age of 4 and 6 months is safe and does not pose a risk for adverse health effects’. At the time the present scientific opinion was drafted, this opinion on appropriate age for introduction of complementary feeding was under revision (EFSA‐Q‐2016‐00482).
For example, for the German study, the total amount of breast milk was calculated based on the observations by Paul et al. (1988) on breast milk consumption during one eating occasion at different age groups: the amount of breast milk consumed on one eating occasion was set to 135 g/eating occasion for infants between 6 and 7 months of age and to 100 g/eating occasion for infants between 8 and 12 months of age (Kersting and Clausen, 2003). For the UK survey, the amount of breast milk consumed was either directly quantified by the mother (expressed breast milk) or extrapolated from the duration of each breastfeeding event.
This covers only products available in supermarkets (e.g. not drops or tablets with vitamin D for infants available in pharmacy under a medical prescription).
For example, over all food categories followed in the Mintel GNPD database, over the past 5 years, nine products were identified in Slovakia with vitamin D in the ingredient list, and about 430 in the UK (search in September 2017).
For example, the percentage of butter fortified with vitamin D was calculated by EFSA to be 1.2% overall Europe from the data available in Mintel, but the analysis by country showed that e.g. 23.7% of butter in Norway was fortified with vitamin D (search in September 2017).
EFSA internal report on the harmonization of dilution factors to be used in the assessment of dietary exposure. Available at: https://zenodo.org/record/1256085#.WxTweC5uaUm
As ‘the most frequent reconstitution indication presented by manufacturers is 1 part of cereals to 4 parts of water’.
As, in the range of dilution factors (5–10) provided by the producers, more than half of the products presenting indications of lower dilutions ratios.
At the time of this assessment, the Bulgarian market was not followed in Mintel GNPD.
In particular, studies on ‘idiopathic infantile hypercalcaemia’ should be excluded.
References
- Ahmad IA and Al‐Agha AE, 2013. Hypervitaminosis D causing nephrogenic diabetes insipidus in a 5‐month‐old infant. Saudi Medical Journal, 34, 187–189. [PubMed] [Google Scholar]
- Ala‐Houhala M, 1985. 25‐Hydroxyvitamin D levels during breast‐feeding with or without maternal or infantile supplementation of vitamin D. Journal of Pediatric Gastroenterology and Nutrition, 4, 220–226. [DOI] [PubMed] [Google Scholar]
- Ala‐Houhala M, Koskinen T, Parviainen MT and Visakorpi JK, 1988. 25‐Hydroxyvitamin D and vitamin D in human milk: effects of supplementation and season. American Journal of Clinical Nutrition, 48, 1057–1060. [DOI] [PubMed] [Google Scholar]
- Allen RE, Dangour AD and Tedstone AE, 2014. Update of the vitamin D content of fortified foods and supplements in the UK National Diet and Nutrition Survey Nutrient Databank. Nutrition Bulletin, 39, 247–245. [Google Scholar]
- American Academy of Pediatrics , 1999. Ultraviolet light: a hazard to children. American Academy of Pediatrics. Committee on Environmental Health. Pediatrics, 104, 328–333. [PubMed] [Google Scholar]
- Arrabal‐Polo MA, del Carmen Cano‐Garcia M and Arrabal‐Martin M, 2016. The fasting calcium/creatinine ratio in patients with calcium stones and the relation with hypercalciuria and phosphocalcium metabolism. Archivos Espanoles de Urologia, 69, 117–120. [PubMed] [Google Scholar]
- Audran M and Legrand E, 2000. Hypercalciuria. Joint, Bone, Spine, 67, 509–515. [DOI] [PubMed] [Google Scholar]
- Autier P and Gandini S, 2007. Vitamin D supplementation and total mortality: a meta‐analysis of randomized controlled trials. Archives of Internal Medicine, 167, 1730–1737. [DOI] [PubMed] [Google Scholar]
- Bailey D, Veljkovic K, Yazdanpanah M and Adeli K, 2013. Analytical measurement and clinical relevance of vitamin D(3) C3‐epimer. Clinical Biochemistry, 46, 190–196. [DOI] [PubMed] [Google Scholar]
- Bailey D, Perumal N, Yazdanpanah M, Al Mahmud A, Baqui AH, Adeli K and Roth DE, 2014. Maternal‐fetal‐infant dynamics of the C3‐epimer of 25‐hydroxyvitamin D. Clinical Biochemistry, 47, 816–822. [DOI] [PubMed] [Google Scholar]
- Balk SJ, 2011. Ultraviolet radiation: a hazard to children and adolescents. Pediatrics, 127, e791–e817. [DOI] [PubMed] [Google Scholar]
- Barrueto F Jr, Wang‐Flores HH, Howland MA, Hoffman RS and Nelson LS, 2005. Acute vitamin D intoxication in a child. Pediatrics, 116, e453–e456. [DOI] [PubMed] [Google Scholar]
- Basile LA, Taylor SN, Wagner CL, Horst RL and Hollis BW, 2006. The effect of high‐dose vitamin D supplementation on serum vitamin D levels and milk calcium concentration in lactating women and their infants. Breastfeeding Medicine, 1, 27–35. [DOI] [PubMed] [Google Scholar]
- Berry D and Hyppönen E, 2011. Determinants of vitamin D status: focus on genetic variations. Current Opinion in Nephrology and Hypertension, 20, 331–336. [DOI] [PubMed] [Google Scholar]
- Besbas N, Oner A, Akhan O, Saatci U, Bakkaloglu A and Topaloglu R, 1989. Nephrocalcinosis due to vitamin D intoxication. Turkish Journal of Pediatrics, 31, 239–244. [PubMed] [Google Scholar]
- Bi WG, Nuyt AM, Weiler H, Leduc L, Santamaria C and Wei SQ, 2018. Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta‐analysis. JAMA Pediatrics, 172, 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Siiteri PK, Ryzen E and Haddad JG, 1985. Serum protein binding of 1,25‐dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. Journal of Clinical Endocrinology and Metabolism, 61, 969–975. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E and Haddad JG, 1986. Assessment of the free fraction of 25‐hydroxyvitamin D in serum and its regulation by albumin and the vitamin D‐binding protein. Journal of Clinical Endocrinology and Metabolism, 63, 954–959. [DOI] [PubMed] [Google Scholar]
- Billoo AG, Murtaza G, Memon MA, Khaskheli SA, Iqbal K and Rao MH, 2009. Comparison of oral versus injectable vitamin‐D for the treatment of nutritional vitamin‐D deficiency rickets. Journal of the College of Physicians and Surgeons Pakistan, 19, 428–431. [PubMed] [Google Scholar]
- Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, Saltzman E and Dawson‐Hughes B, 2008. Vitamin D(3) in fat tissue. Endocrine, 33, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Catov JM, Roberts JM and Simhan HN, 2007. Prepregnancy obesity predicts poor vitamin D status in mothers and their neonates. Journal of Nutrition, 137, 2437–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer E, Hulshof K and Doest D, 2006. Voedselconsumptie van jonge peuters [Food consumption of young children]. TNO Report, 6269, 37. [Google Scholar]
- Bøgevig S, Hoegberg LCG, Schou AJ, Schmidt IM, Vojdeman FJ, Kamperis K, Mølgaard C, Brot C and Christesen H, 2017. National vitamin D intoxication outbreak among infants due to a manufacturing error of vitamin D droplets: challenges for the healthcare system. Clinical Toxicology, 55, 385–386. [Google Scholar]
- Bonjour JP, Kohrt W, Levasseur R, Warren M, Whiting S and Kraenzlin M, 2014. Biochemical markers for assessment of calcium economy and bone metabolism: application in clinical trials from pharmaceutical agents to nutritional products. Nutrition Research Reviews, 27, 252–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel P, Caillaud D and Cano NJ, 2015. Vitamin D bioavailability: state of the art. Critical Reviews in Food Science and Nutrition, 55, 1193–1205. [DOI] [PubMed] [Google Scholar]
- Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C and Demay M, 2008. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocrine Reviews, 29, 726–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, Hojsak I, Mihatsch W, Molgaard C, Shamir R, Turck D and Van Goudoever J on Behalf of the ESPGHAN Committee on Nutrition , 2013. Vitamin D in the healthy European paediatric population. Journal of Pediatric, Gastroenterology and Nutrition, 56, 692–701. [DOI] [PubMed] [Google Scholar]
- Braun OH and Kauderer B, 1966. Nil nocere. Prevention of rickets in the neonatal period. Münchener Medizinische Wochenschrift, 108, 104–108. [PubMed] [Google Scholar]
- Brouwer‐Brolsma EM, Berendsen AAM, Vaes AMM, Dullemeijer C, de Groot LCPGM and Feskens EJM, 2016. Collection and analysis of published scientific information as preparatory work for the setting of Dietary Reference Values for Vitamin D. EFSA supporting publication 2016:EN‐766, 171 pp.
- Burmaster DE and Anderson PD, 1994. Principles of good practice for the use of Monte Carlo techniques in human health and ecological risk assessments. Risk Analysis, 14, 477–481. [DOI] [PubMed] [Google Scholar]
- van Buuren S, Schönbeck Y and van Dommelen P, 2012. Collection, collation and analysis of data in relation to reference heights and reference weights for female and male children and adolescents (0‐18 years) in the EU, as well as in relation to the age of onset of puberty and the age at which different stages of puberty are reached in adolescents in the EU. Project developed on the procurement project CT/EFSA/NDA/2010/01. EFSA Supporting publication 2012:EN‐255, 59 pp. 10.2903/sp.efsa.2012.en-2255 [DOI]
- Carter GD, 2011. Accuracy of 25‐hydroxyvitamin D assays: confronting the issues. Current Drug Targets, 12, 19–28. [DOI] [PubMed] [Google Scholar]
- Carter GD, 2012. 25‐hydroxyvitamin D: a difficult analyte. Clinical Chemistry, 58, 486–488. [DOI] [PubMed] [Google Scholar]
- Cashman KD, FitzGerald AP, Viljakainen HT, Jakobsen J, Michaelsen KF, Lamberg‐Allardt C and Molgaard C, 2011. Estimation of the dietary requirement for vitamin D in healthy adolescent white girls. American Journal of Clinical Nutrition, 93, 549–555. [DOI] [PubMed] [Google Scholar]
- Cashman KD, Kiely M, Kinsella M, Durazo‐Arvizu RA, Tian L, Zhang Y, Lucey A, Flynn A, Gibney MJ, Vesper HW, Phinney KW, Coates PM, Picciano MF and Sempos CT, 2013. Evaluation of Vitamin D Standardization Program protocols for standardizing serum 25‐hydroxyvitamin D data: a case study of the program's potential for national nutrition and health surveys. American Journal of Clinical Nutrition, 97, 1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesur Y, Caksen H, Gundem A, Kirimi E and Odabas D, 2003. Comparison of low and high dose of vitamin D treatment in nutritional vitamin D deficiency rickets. Journal of Pediatric Endocrinology and Metabolism, 16, 1105–1109. [DOI] [PubMed] [Google Scholar]
- Chambellan‐Tison C, Horen B, Plat‐Wilson G, Moulin P and Claudet I, 2007. Severe hypercalcemia due to vitamin D intoxication. Archives de Pédiatrie, 14, 1328–1332. [DOI] [PubMed] [Google Scholar]
- Chandhoke PS, 2007. Evaluation of the recurrent stone former. Urologic Clinics of North America, 34, 315–322. [DOI] [PubMed] [Google Scholar]
- Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS and Hewison M, 2014. Vitamin D and DBP: the free hormone hypothesis revisited. Journal of Steroid Biochemistry and Molecular Biology, 144 Pt A, 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COT (Committee on toxicity of chemicals in foods, consumer products and the environment), 2015. Statement on adverse effects of high levels of vitamin D. 78 pp.
- Cranney A, Horsley T, O'Donnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, Fang M, Yazdi F, Garritty C, Sampson M, Barrowman N, Tsertsvadze A and Mamaladze V, 2007. Effectiveness and safety of vitamin D in relation to bone health. Evidence Report/Technology Assessment (Full Report), 158, 1–235. [PMC free article] [PubMed] [Google Scholar]
- Curhan GC, Willett WC, Speizer FE and Stampfer MJ, 2001. Twenty‐four‐hour urine chemistries and the risk of kidney stones among women and men. Kidney International, 59, 2290–2298. [DOI] [PubMed] [Google Scholar]
- Cusano NE, Thys‐Jacobs S and Bilezikian JP, 2011. Hypercalcemia due to vitamin D toxicity In: Feldman D, Pike JW. and Adams JS. (eds). Vitamin D. Academic Press, Elsevier, London: pp. 1381–1402. [Google Scholar]
- Czech‐Kowalska J, Pludowski P, Dobrzanska A, Kryskiewicz E, Karczmarewicz E, Gruszfeld D, Pleskaczynska A and Golkowska M, 2012. Impact of vitamin D supplementation on markers of bone mineral metabolism in term infants. Bone, 51, 781–786. [DOI] [PubMed] [Google Scholar]
- Dawodu A and Tsang RC, 2012. Maternal vitamin D status: effect on milk vitamin D content and vitamin D status of breastfeeding infants. Advances in Nutrition, 3, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawodu A, Zalla L, Woo JG, Herbers PM, Davidson BS, Heubi JE and Morrow AL, 2014. Heightened attention to supplementation is needed to improve the vitamin D status of breastfeeding mothers and infants when sunshine exposure is restricted. Maternal and Child Nutrition, 10, 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawodu A, Davidson B, Woo JG, Peng YM, Ruiz‐Palacios GM, de Lourdes Guerrero M and Morrow AL, 2015. Sun exposure and vitamin D supplementation in relation to vitamin D status of breastfeeding mothers and infants in the global exploration of human milk study. Nutrients, 7, 1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong‐Rubingh C and Bausch‐Goldbohm RA, 2015. De Eet Compleet Test: 2‐daags voedselconsumptie onderzoek onder kinderen van 1‐4 jaar die een kinderdagverblijf bezoeken. TNO 2014 R11714 98 pp.
- Debre R, 1948. Toxic effects of overdosage of vitamin D2 in children. American Journal of Diseases of Children, 75, 787–791. [DOI] [PubMed] [Google Scholar]
- DGE (Deutsche Gesellschaft für Ernährung), 2008. Ernährungsbericht 2008 [Nutrition Report 2008]. 442 pp.
- Dietzsch HJ, 1957. Avoidable disorders due to overdosage with vitamin D in infant welfare clinics. Deutsche Gesundheitswesen, 12, 266–272. [PubMed] [Google Scholar]
- Dinour D, Beckerman P, Ganon L, Tordjman K, Eisenstein Z and Holtzman EJ, 2013. Loss‐of‐function mutations of CYP24A1, the vitamin D 24‐hydroxylase gene, cause long‐standing hypercalciuric nephrolithiasis and nephrocalcinosis. Journal of Urology, 190, 552–557. [DOI] [PubMed] [Google Scholar]
- Doneray H, Ozkan B, Caner I, Ozkan A and Karakelleoglu C, 2008. Intragastric alendronate therapy in two infants with vitamin D intoxication: a new method. Clinical Toxicology, 46, 300–302. [DOI] [PubMed] [Google Scholar]
- Dror DK and Allen LH, 2010. Vitamin D inadequacy in pregnancy: biology, outcomes, and interventions. Nutrition Reviews, 68, 465–477. [DOI] [PubMed] [Google Scholar]
- Dumas R, 1997. Hypercalciuria: etiologies and treatment. Archives de Pédiatrie, 4, 351–358. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Guidance for those carrying out systematic reviews. EFSA Journal 2010; 8(6):1637, 90 pp. 10.2903/j.efsa.2010.1637 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Report on the development of a food classification and description system for exposure assessment and guidance on its implementation and use. EFSA Journal 2011;9(12):2489, 84 pp. 10.2903/j.efsa.2011.2489 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2009. Scientific Opinion on the appropriate age for introduction of complementary feeding of infants. EFSA Journal 2009;7(12):1423, 38 pp. 10.2903/j.efsa.2009.1423 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2010. Scientific Opinion on principles for deriving and applying Dietary Reference Values. EFSA Journal 2010;8(3):1458, 30 pp. 10.2903/j.efsa.2010.1458 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products Nutrition and Allergies), 2012a. Scientific Opinion on the Tolerable Upper Intake Level of vitamin D. EFSA Journal 2012;10(7):2813, 45 pp. 10.2903/j.efsa.2012.2813 10.2903/j.efsa.2012.2813 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products Nutrition and Allergies), 2012b. Scientific Opinion on the Tolerable Upper Intake Level of calcium. EFSA Journal 2012;10(7):2814, 44 pp. 10.2903/j.efsa.2012.2814 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013. Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA Journal 2013;11(10):3408, 103 pp. 10.2903/j.efsa.2013.3408 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2014a. Scientific Opinion on the essential composition of infant and follow‐on formulae. EFSA Journal 2014;12(7):3760, 106 pp. 10.2903/j.efsa.2014.3760 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products Nutrition and Allergies), 2014b. Scientific Opinion on the safety of vitamin D‐enriched UV‐treated baker's yeast. EFSA Journal 2014;12(1):3520, 19 pp. 10.2903/j.efsa.2014.3520, 19 pp. 10.2903/j.efsa.2014.3520 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2016. Scientific Opinion on Dietary Reference Values for vitamin D. EFSA Journal 2016;14(10):4547, 179 pp. 10.2903/j.efsa.2016.4547 [DOI] [Google Scholar]
- EFSA Scientific Committee , Barlow S, Chesson A, Collins JD, Flynn A, Hardy A, Jany K‐D, Knaap A, Kuiper H, Larsen J‐C, Lovell D, Le Neindre P, Schans J, Schlatter S, Silano V, Skerfving S and Vannier P, 2009. Guidance of the Scientific Committee on a request from EFSA on the use of the benchmark dose approach in risk assessment. EFSA Journal 2009;7(6):1150, 72 pp. 10.2903/j.efsa.2009.1150 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017a. Update: Guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Bresson J‐L, Dusemund B, Gundert‐Remy U, Kersting M, Lambre C, Penninks A, Tritscher A, Waalkens‐Berendsen I, Woutersen R, Arcella D, Court Marques D, Dorne J‐L, Kass GEN and Alicja M, 2017b. Guidance on the risk assessment of substances present in food intended forinfants below 16 weeks of age. EFSA Journal 2017;15(5):4849, 58 pp. 10.2903/j.efsa.2017.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elarqam L, Babakhouya A, Chaouki S, Atmani A, Bouharrou A and Hida M, 2007. L'intoxication par la vitamine D chez le nourrisson. Journal de Pédiatrie et de Puériculture, 20, 203–205. [Google Scholar]
- Emel T, Dogan DA, Erdem G and Faruk O, 2012. Therapy strategies in vitamin D deficiency with or without rickets: efficiency of low‐dose stoss therapy. Journal of Pediatric Endocrinology and Metabolism, 25, 107–110. [DOI] [PubMed] [Google Scholar]
- Engelsen O, Brustad M, Aksnes L and Lund E, 2005. Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochemistry and Photobiology, 81, 1287–1290. [DOI] [PubMed] [Google Scholar]
- Evira , 2017. D‐vitamiinilla täydennetyt elintarvikkeet Suomessa, tuotteiden markkinoilletulo ja koostumus vuosina 2012‐2016. Eviran raportti. DNo 7187/0071/2016, 35 pp.
- Fantino M and Gourmet E, 2008. Nutrient intakes in 2005 by non‐breast fed French children of less than 36 months. Archives de Pédiatrie, 15, 446–455. [DOI] [PubMed] [Google Scholar]
- Farrell CJ, Martin S, McWhinney B, Straub I, Williams P and Herrmann M, 2012. State‐of‐the‐art vitamin D assays: a comparison of automated immunoassays with liquid chromatography‐tandem mass spectrometry methods. Clinical Chemistry, 58, 531–542. [DOI] [PubMed] [Google Scholar]
- Fomon SJ, Younoszai MK and Thomas LN, 1966. Influence of vitamin D on linear growth of normal full‐term infants. Journal of Nutrition, 88, 345–350. [DOI] [PubMed] [Google Scholar]
- Fraser D, 1967. The relation between infantile hypercalcemia and vitamin D–public health implications in North America. Pediatrics, 40, 1050–1061. [PubMed] [Google Scholar]
- Gallo S, Comeau K, Vanstone C, Agellon S, Sharma A, Jones G, L'Abbe M, Khamessan A, Rodd C and Weiler H, 2013a. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. Journal of the American Medical Association, 309, 1785–1792. [DOI] [PubMed] [Google Scholar]
- Gallo S, Phan A, Vanstone CA, Rodd C and Weiler HA, 2013b. The change in plasma 25‐hydroxyvitamin D did not differ between breast‐fed infants that received a daily supplement of ergocalciferol or cholecalciferol for 3 months. Journal of Nutrition, 143, 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo S, Comeau K, Agellon S, Vanstone C, Sharma A, Jones G, L'Abbe M, Khamessan A, Weiler H and Rodd C, 2014a. Methodological issues in assessing plasma 25‐hydroxyvitamin D concentration in newborn infants. Bone, 61, 186–190. [DOI] [PubMed] [Google Scholar]
- Gallo S, Comeau K, Sharma A, Vanstone CA, Agellon S, Mitchell J, Weiler HA and Rodd C, 2014b. Redefining normal bone and mineral clinical biochemistry reference intervals for healthy infants in Canada. Clinical Biochemistry, 47, 27–32. [DOI] [PubMed] [Google Scholar]
- Gallo S, Hazell T, Vanstone CA, Agellon S, Jones G, L'Abbe M, Rodd C and Weiler HA, 2016. Vitamin D supplementation in breastfed infants from Montreal, Canada: 25‐hydroxyvitamin D and bone health effects from a follow‐up study at 3 years of age. Osteoporosis International, 27, 2459–2466. [DOI] [PubMed] [Google Scholar]
- Ghazali S and Barratt TM, 1974. Urinary excretion of calcium and magnesium in children. Archives of Disease in Childhood, 49, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godang K, Froslie KF, Henriksen T, Qvigstad E and Bollerslev J, 2014. Seasonal variation in maternal and umbilical cord 25(OH) vitamin D and their associations with neonatal adiposity. European Journal of Endocrinology, 170, 609–617. [DOI] [PubMed] [Google Scholar]
- Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A and Cox JE, 2008. Treatment of hypovitaminosis D in infants and toddlers. Journal of Clinical Endocrinology and Metabolism, 93, 2716–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CC, Stewart AW, Scragg R, Milne T, Rowden J, Ekeroma A, Wall C, Mitchell EA, Crengle S, Trenholme A, Crane J and Camargo CA Jr, 2014. Vitamin D during pregnancy and infancy and infant serum 25‐hydroxyvitamin D concentration. Pediatrics, 133, e143–e153. [DOI] [PubMed] [Google Scholar]
- Greer FR, Searcy JE, Levin RS, Steichen JJ, Steichen‐Asche PS and Tsang RC, 1982. Bone mineral content and serum 25‐hydroxyvitamin D concentrations in breast‐fed infants with and without supplemental vitamin D: one‐year follow‐up. Journal of Pediatrics, 100, 919–922. [DOI] [PubMed] [Google Scholar]
- Harnot J, Verma S, Singhi S, Sankhyan N, Sachdeva N and Bharti B, 2017. Comparison of 300,000 and 600,000 IU Oral Vitamin‐D Bolus for Vitamin‐D Deficiency in Young Children. Indian Journal of Pediatrics, 84, 111–116. [DOI] [PubMed] [Google Scholar]
- Hauta‐Alus HH, Korkalo L, Holmlund‐Suila EM, Rosendahl J, Valkama SM, Enlund‐Cerullo M, Helve OM, Hytinantti TK, Makitie OM, Andersson S and Viljakainen HT, 2017. Food and nutrient intake and nutrient sources in 1‐year‐old infants in Finland: A cross‐sectional analysis. Nutrients, 9, pii: E1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell TJ, Gallo S, Berzina I, Vanstone CA, Rodd C and Weiler HA, 2014. Plasma 25‐hydroxyvitamin D, more so than its epimer, has a linear relationship to leaner body composition across infancy in healthy term infants. Applied Physiology, Nutrition, and Metabolism, 39, 1137–1143. [DOI] [PubMed] [Google Scholar]
- Hazell TJ, Gallo S, Vanstone CA, Agellon S, Rodd C and Weiler HA, 2017. Vitamin D supplementation trial in infancy: body composition effects at 3 years of age in a prospective follow‐up study from Montreal. Pediatric Obesity, 12, 38–47. [DOI] [PubMed] [Google Scholar]
- Heaney RP, Recker RR and Ryan RA, 1999. Urinary calcium in perimenopausal women: normative values. Osteoporosis International, 9, 13–18. [DOI] [PubMed] [Google Scholar]
- Heaney RP, Davies KM, Chen TC, Holick MF and Barger‐Lux MJ, 2003. Human serum 25‐hydroxycholecalciferol response to extended oral dosing with cholecalciferol. American Journal of Clinical Nutrition, 77, 204–210. [DOI] [PubMed] [Google Scholar]
- Heijboer AC, Blankenstein MA, Kema IP and Buijs MM, 2012. Accuracy of 6 routine 25‐hydroxyvitamin D assays: influence of vitamin D binding protein concentration. Clinical Chemistry, 58, 543–548. [DOI] [PubMed] [Google Scholar]
- Higgins JP, White IR and Anzures‐Cabrera J, 2008. Meta‐analysis of skewed data: combining results reported on log‐transformed or raw scales. Statistics in Medicine, 27, 6072–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ and Scheidt‐Nave C, 2008. Vitamin D status and health correlates among German adults. European Journal of Clinical Nutrition, 62, 1079–1089. [DOI] [PubMed] [Google Scholar]
- Holick MF, 1994. McCollum Award Lecture, 1994: vitamin D–new horizons for the 21st century. American Journal of Clinical Nutrition, 60, 619–630. [DOI] [PubMed] [Google Scholar]
- Holick MF, 1999. Vitamin D In: Shils ME, Olson JA, Shike M, Ross AC.(ed.). Modern nutrition in health and disease. Lippincott Willams & Wilkins, Philadelphia, USA, pp 329‐345. [Google Scholar]
- Holick MF, 2006a. Vitamin D In: Shils ME, Shike M, Ross AC, Caballero B. and Cousins RJ. (eds.). Modern Nutrition in Health and Disease. Lippincott Williams & Wilkins, Philadelphia, PA, USA: pp. 376–395. [Google Scholar]
- Holick MF, 2006b. Resurrection of vitamin D deficiency and rickets. Journal of Clinical Investigation, 116, 2062–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF, 2007. Vitamin D deficiency. New England Journal of Medicine, 357, 266–281. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff‐Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM and Endocrine Society , 2011. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism, 96, 1911–1930. [DOI] [PubMed] [Google Scholar]
- Hollis BW and Wagner CL, 2004. Vitamin D requirements during lactation: high‐dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. American Journal of Clinical Nutrition, 80, 1752S–1758S. [DOI] [PubMed] [Google Scholar]
- Hollis BW, Lowery JW, Pittard WB 3rd, Guy DG and Hansen JW, 1996. Effect of age on the intestinal absorption of vitamin D3‐palmitate and nonesterified vitamin D2 in the term human infant. Journal of Clinical Endocrinology and Metabolism, 81, 1385–1388. [DOI] [PubMed] [Google Scholar]
- Hollis BW, Johnson D, Hulsey TC, Ebeling M and Wagner CL, 2011. Vitamin D supplementation during pregnancy: double‐blind, randomized clinical trial of safety and effectiveness. Journal of Bone and Mineral Research, 26, 2341–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis BW, Wagner CL, Howard CR, Ebeling M, Shary JR, Smith PG, Taylor SN, Morella K, Lawrence RA and Hulsey TC, 2015. Maternal Versus Infant Vitamin D Supplementation During Lactation: A Randomized Controlled Trial. Pediatrics, 136, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg I, Berlin T, Ewerth S and Bjorkhem I, 1986. 25‐Hydroxylase activity in subcellular fractions from human liver. Evidence for different rates of mitochondrial hydroxylation of vitamin D2 and D3. Scandinavian Journal of Clinical and Laboratory Investigation, 46, 785–790. [DOI] [PubMed] [Google Scholar]
- Holmlund‐Suila E, Viljakainen H, Hytinantti T, Lamberg‐Allardt C, Andersson S and Makitie O, 2012. High‐dose vitamin d intervention in infants–effects on vitamin d status, calcium homeostasis, and bone strength. Journal of Clinical Endocrinology and Metabolism, 97, 4139–4147. [DOI] [PubMed] [Google Scholar]
- Holst‐Gemeiner D, Gemeiner M, Pilz I and Swoboda W, 1978. Plasma 25‐hydroxycholecalciferol after daily vitamin D administration in comparison with massive single‐dose prophylaxis (author's transl). Wiener Klinische Wochenschrift, 90, 509–512. [PubMed] [Google Scholar]
- Holt E and Wysolmerski JJ, 2011. Parathyroid hormone, parathyroid hormone‐related protein, and calcitonin In: Vitamin D. (ed.). Feldman D, Pike JW and Adams JS. Academic Press, San Diego, CA, USA: pp. 725–745. [Google Scholar]
- Hoppe B, Hesse A, Neuhaus T, Fanconi S, Blau N, Roth B and Leumann E, 1997. Influence of nutrition on urinary oxalate and calcium in preterm and term infants. Pediatric Nephrology, 11, 687–690. [DOI] [PubMed] [Google Scholar]
- Houghton LA and Vieth R, 2006. The case against ergocalciferol (vitamin D2) as a vitamin supplement. American Journal of Clinical Nutrition, 84, 694–697. [DOI] [PubMed] [Google Scholar]
- Hutchinson MS, Grimnes G, Joakimsen RM, Figenschau Y and Jorde R, 2010. Low serum 25‐hydroxyvitamin D levels are associated with increased all‐cause mortality risk in a general population: the Tromso study. European Journal of Endocrinology, 162, 935–942. [DOI] [PubMed] [Google Scholar]
- Huynh J, Lu T, Liew D, Doery JC, Tudball R, Jona M, Bhamjee R and Rodda CP, 2017. Vitamin D in newborns. A randomised controlled trial comparing daily and single oral bolus vitamin D in infants. Journal of Paediatrics and Child Health, 53, 163–169. [DOI] [PubMed] [Google Scholar]
- Hyppönen E, Fararouei M, Sovio U, Hartikainen AL, Pouta A, Robertson C, Whittaker JC and Jarvelin MR, 2011. High‐dose vitamin D supplements are not associated with linear growth in a large Finnish cohort. Journal of Nutrition, 141, 843–848. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine), 2011. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press, Washington, DC, USA, 1115 pp. [PubMed] [Google Scholar]
- Itkonen ST, Skaffari E, Saaristo P, Saarnio EM, Erkkola M, Jakobsen J, Cashman KD and Lamberg‐Allardt C, 2016. Effects of vitamin D2‐fortified bread v. supplementation with vitamin D2 or D3 on serum 25‐hydroxyvitamin D metabolites: an 8‐week randomised‐controlled trial in young adult Finnish women. British Journal of Nutrition, 115, 1232–1239. [DOI] [PubMed] [Google Scholar]
- Jeans PC and Stearns G, 1938. The effect of vitamin D on linear growth in infancy: II. The effect of intakes above 1,800 U.S.P. units daily. Journal of Pediatrics, 13, 730–740. [Google Scholar]
- Johnsen MS, Grimnes G, Figenschau Y, Torjesen PA, Almas B and Jorde R, 2014. Serum free and bio‐available 25‐hydroxyvitamin D correlate better with bone density than serum total 25‐hydroxyvitamin D. Scandinavian Journal of Clinical and Laboratory Investigation, 74, 177–183. [DOI] [PubMed] [Google Scholar]
- Jones G, 2008. Pharmacokinetics of vitamin D toxicity. American Journal of Clinical Nutrition, 88, 582S–586S. [DOI] [PubMed] [Google Scholar]
- Jones G, 2013. Extrarenal vitamin D activation and interactions between vitamin D(2), vitamin D(3), and vitamin D analogs. Annual Review of Nutrition, 33, 23–44. [DOI] [PubMed] [Google Scholar]
- Jones G, 2014. Vitamin D In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR. (ed.). Modern nutrition in health and disease. Wolters Kluwer Health/Lippincott Williams & Wilkins, Baltimore, Philadelphia, PA, USA. pp. 278–292. [Google Scholar]
- Jones G, Strugnell SA and DeLuca HF, 1998. Current understanding of the molecular actions of vitamin D. Physiological Reviews, 78, 1193–1231. [DOI] [PubMed] [Google Scholar]
- Jones AN, Blank RD, Lindstrom MJ, Penniston KL and Hansen KE, 2010. Adjustment for body mass index and calcitrophic hormone levels improves the diagnostic accuracy of the spot urine calcium‐to‐creatinine ratio. Osteoporosis International, 21, 1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AN, Shafer MM, Keuler NS, Crone EM and Hansen KE, 2012. Fasting and postprandial spot urine calcium‐to‐creatinine ratios do not detect hypercalciuria. Osteoporosis International, 23, 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Prosser DE and Kaufmann M, 2012. 25‐hydroxyvitamin D‐24‐hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Archives of Biochemistry and Biophysics, 523, 9–18. [DOI] [PubMed] [Google Scholar]
- Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A and Schoenmakers I, 2014. 25(OH)D2 half‐life is shorter than 25(OH)D3 half‐life and is influenced by DBP concentration and genotype. Journal of Clinical Endocrinology and Metabolism, 99, 3373–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Kottler ML and Schlingmann KP, 2017. Genetic diseases of vitamin D metabolizing enzymes. Endocrinology and Metabolism Clinics of North America, 46, 1095–1117. [DOI] [PubMed] [Google Scholar]
- Josefson JL, Feinglass J, Rademaker AW, Metzger BE, Zeiss DM, Price HE and Langman CB, 2013. Maternal obesity and vitamin D sufficiency are associated with cord blood vitamin D insufficiency. Journal of Clinical Endocrinology and Metabolism, 98, 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara C, Gunindi F, Ustyol A and Aydin M, 2014. Vitamin D intoxication due to an erroneously manufactured dietary supplement in seven children. Pediatrics, 133, e240–e244. [DOI] [PubMed] [Google Scholar]
- Kersting M and Clause K, 2003. Ernährungsphysiologische Auswertung einer repräsentativen Verzehrsstudie bei Säuglingen und Kleinkindern VELS mit dem Instrumentarium der DONALD Studie, 103 pp.
- Ketha H, Wadams H, Lteif A and Singh RJ, 2015. Iatrogenic vitamin D toxicity in an infant–a case report and review of literature. Journal of Steroid Biochemistry and Molecular Biology, 148, 14–18. [DOI] [PubMed] [Google Scholar]
- Kiely M, O'Donovan SM, Kenny LC, Hourihane JO, Irvine AD and Murray DM, 2017. Vitamin D metabolite concentrations in umbilical cord blood serum and associations with clinical characteristics in a large prospective mother‐infant cohort in Ireland. Journal of Steroid Biochemistry and Molecular Biology, 167, 162–168. [DOI] [PubMed] [Google Scholar]
- Kovacs CS, 2008. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. American Journal of Clinical Nutrition, 88, 520S–528S. [DOI] [PubMed] [Google Scholar]
- Koyun M, Guven AG, Filiz S, Akman S, Akbas H, Baysal YE and Dedeoglu N, 2007. Screening for hypercalciuria in schoolchildren: what should be the criteria for diagnosis? Pediatric Nephrology, 22, 1297–1301. [DOI] [PubMed] [Google Scholar]
- Kristensen HL, Rosenqvist E and Jakobsen J, 2012. Increase of vitamin D(2) by UV‐B exposure during the growth phase of white button mushroom (Agaricus bisporus). Food & Nutrition Research, 56, 7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz C, von Lilienfeld‐Toal H, Niesen M and Burmeister W, 1982. 25‐hydroxy‐vitamin‐D in serum of newborns and infants during continuous oral vitamin D treatment (author's transl). Padiatrie und Padologie, 17, 181–185. [PubMed] [Google Scholar]
- Lehmann B and Meurer M, 2010. Vitamin D metabolism. Dermatologic Therapy, 23, 2–12. [DOI] [PubMed] [Google Scholar]
- Lehmann U, Hirche F, Stangl GI, Hinz K, Westphal S and Dierkes J, 2013. Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo‐controlled trial. Journal of Clinical Endocrinology and Metabolism, 98, 4339–4345. [DOI] [PubMed] [Google Scholar]
- Lennox A, Sommerville J, Ong K, Henderson H and Allen R (Department of Health, Food Standards Agency), 2013. Diet and Nutrition Survey of Infants and Young Children, 2011. 108 pp.
- Leslie SW and Taneja A, 2017. Hypercalciuria. In: StatPearls. Treasure Island (FL).
- Lo CW, Paris PW, Clemens TL, Nolan J and Holick MF, 1985. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. American Journal of Clinical Nutrition, 42, 644–649. [DOI] [PubMed] [Google Scholar]
- Lukaszkiewicz J, Proszynska K, Lorenc RS and Ludwiczak H, 1987. Hepatic microsomal enzyme induction: treatment of vitamin D poisoning in a 7 month old baby. British Medical Journal (Clinical Research Edition), 295, 1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaseki‐Holland S, Maroof Z, Bruce J, Mughal MZ, Masher MI, Bhutta ZA, Walraven G and Chandramohan D, 2012. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet, 379, 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie J, Hennequet A, Marandian H and Momenzadeh A, 1969. Hypervitaminosis D2 due to daily ingestion of hydro‐alcoholic solution of 400 U.I. per drop. Recovery following calcium‐free diet and corticotherapy. Annales de Pédiatrie, 16, 24–26. [PubMed] [Google Scholar]
- Markestad T (Doctoral Thesis. University of Bergen, Bergen, Norway), 1984. Studies on vitamin D requirements and vitamin D metabolism in infancy and early childhood.
- Markestad T, Hesse V, Siebenhuner M, Jahreis G, Aksnes L, Plenert W and Aarskog D, 1987. Intermittent high‐dose vitamin D prophylaxis during infancy: effect on vitamin D metabolites, calcium, and phosphorus. American Journal of Clinical Nutrition, 46, 652–658. [DOI] [PubMed] [Google Scholar]
- Marriott LD, Robinson SM, Poole J, Borland SE, Godfrey KM, Law CM, Inskip HM and Southampton Women's Survey Study G , 2008. What do babies eat? Evaluation of a food frequency questionnaire to assess the diets of infants aged 6 months. Public Health Nutrition, 11, 751–756. [DOI] [PubMed] [Google Scholar]
- Matos V, van Melle G, Boulat O, Markert M, Bachmann C and Guignard JP, 1997. Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. Journal of Pediatrics, 131, 252–257. [DOI] [PubMed] [Google Scholar]
- Meena P, Dabas A, Shah D, Malhotra RK, Madhu SV and Gupta P, 2017. Sunlight Exposure and Vitamin D Status in Breastfed Infants. Indian Pediatrics, 54, 105–111. [DOI] [PubMed] [Google Scholar]
- Melamed ML, Michos ED, Post W and Astor B, 2008. 25‐hydroxyvitamin D levels and the risk of mortality in the general population. Archives of Internal Medicine, 168, 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mete E, Dilmen U, Energin M, Ozkan B and Guler I, 1997. Calcitonin therapy in vitamin D intoxication. Journal of Tropical Pediatrics, 43, 241–242. [DOI] [PubMed] [Google Scholar]
- Michaelsson K, Baron JA, Snellman G, Gedeborg R, Byberg L, Sundstrom J, Berglund L, Arnlov J, Hellman P, Blomhoff R, Wolk A, Garmo H, Holmberg L and Melhus H, 2010. Plasma vitamin D and mortality in older men: a community‐based prospective cohort study. American Journal of Clinical Nutrition, 92, 841–848. [DOI] [PubMed] [Google Scholar]
- Milesevic J, Samaniego L, Kiely M, Glibetic M, Roe M and Finglas P, 2018. Specialized food composition dataset for vitamin D content in foods based on European standards: Application to dietary intake assessment. Food Chemistry, 240, 544–549. [DOI] [PubMed] [Google Scholar]
- Mimouni FB and Shamir R, 2009. Vitamin D requirements in the first year of life. Current Opinion in Clinical Nutrition and Metabolic Care, 12, 287–292. [DOI] [PubMed] [Google Scholar]
- Misselwitz J and Hesse V, 1986. Hypercalcemia following prophylactic vitamin D administration. Kinderarztliche Praxis, 54, 431–438. [PubMed] [Google Scholar]
- Mittal H, Rai S, Shah D, Madhu SV, Mehrotra G, Malhotra RK and Gupta P, 2014. 300,000 IU or 600,000 IU of oral vitamin D3 for treatment of nutritional rickets: a randomized controlled trial. Indian Pediatrics, 51, 265–272. [DOI] [PubMed] [Google Scholar]
- Moyersoen I, Demarest S, De Ridder K, Tafforeau J, Lachat C and Van Camp J, 2017. Fat‐soluble vitamin intake from the consumption of food, fortified food and supplements: design and methods of the Belgian VITADEK study. Archives of Public Health, 75, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyersoen I, Lachat C, Cuypers K, Ridder K, Devleesschauwer B, Tafforeau J, Vandevijvere S, Vansteenland M, De Meulenaer B, Van Camp J and Van Oyen H, 2018. Do current fortification and supplementation programs assure adequate intake of fat‐soluble vitamins in Belgian infants, toddlers, pregnant women, and lactating women? Nutrients, 10, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Makitie O, Ramos‐Abad L, Ward L, DiMeglio LA, Atapattu N, Cassinelli H, Braegger C, Pettifor JM, Seth A, Idris HW, Bhatia V, Fu J, Goldberg G, Savendahl L, Khadgawat R, Pludowski P, Maddock J, Hypponen E, Oduwole A, Frew E, Aguiar M, Tulchinsky T, Butler G and Hogler W, 2016. Global consensus recommendations on prevention and management of nutritional rickets. Journal of Clinical Endocrinology and Metabolism, 101, 394–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nama N, Menon K, Iliriani K, Pojsupap S, Sampson M, O'Hearn K, Zhou LL, McIntyre L, Fergusson D and McNally JD, 2016. A systematic review of pediatric clinical trials of high dose vitamin D. PeerJ, 4, e1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguema‐Asseko B, Ganga‐Zandzou PS, Ovono F, Lendoye E, Lemamy GJ, Akendengue B and Milama EN, 2005. Vitamin D status in Gabonese children. Archives de Pédiatrie, 12, 1587–1590. [DOI] [PubMed] [Google Scholar]
- Nicolaidou P, Hatzistamatiou Z, Papadopoulou A, Kaleyias J, Floropoulou E, Lagona E, Tsagris V, Costalos C and Antsaklis A, 2006. Low vitamin D status in mother‐newborn pairs in Greece. Calcified Tissue International, 78, 337–342. [DOI] [PubMed] [Google Scholar]
- Noble S and Emmett P, 2001. Food and nutrient intake in a cohort of 8‐month‐old infants in the south‐west of England in 1993. European Journal of Clinical Nutrition, 55, 698–707. [DOI] [PubMed] [Google Scholar]
- Noble S and Emmett P, 2006. Differences in weaning practice, food and nutrient intake between breast‐ and formula‐fed 4‐month‐old infants in England. Journal of Human Nutrition and Dietetics, 19, 303–313. [DOI] [PubMed] [Google Scholar]
- Norman AW, 2008. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. American Journal of Clinical Nutrition, 88, 491S–499S. [DOI] [PubMed] [Google Scholar]
- Ooms N, van Daal H, Beijers AM, Gerrits GP, Semmekrot BA and van den Ouweland JM, 2016. Time‐course analysis of 3‐epi‐25‐hydroxyvitamin D3 shows markedly elevated levels in early life, particularly from vitamin D supplementation in preterm infants. Pediatric Research, 79, 647–653. [DOI] [PubMed] [Google Scholar]
- Oqali (Observatoire de la qualité de l'alimentation), 2014. Laits infantiles. Première caractérisation du secteur (données 2012). 93 pp.
- Paul AA, Black AE, Evans J, Cole TJ and Whitehead RG, 1988. Breastmilk intake and growth from two to ten months. Journal of Human Nutrition and Dietetics, 1, 437–450. [Google Scholar]
- Paulson SK and DeLuca HF, 1986. Vitamin D metabolism during pregnancy. Bone, 7, 331–336. [DOI] [PubMed] [Google Scholar]
- Pavlovic M and Berenji K, 2014. From Rickets Prevention to Vitamin D Intoxication. European Journal of General Medicine, 11, 200–202. [Google Scholar]
- Pehlivan I, Hatun S, Aydogan M, Babaoglu K and Gokalp AS, 2003. Maternal vitamin D deficiency and vitamin D supplementation in healthy infants. Turkish Journal of Pediatrics, 45, 315–320. [PubMed] [Google Scholar]
- Peng SK and Taylor CB, 1980. Probably role of excesses of vitamin D in genesis of arteriosclerosis. Paroi Arterielle, 6, 63–67. [PubMed] [Google Scholar]
- Pettifor JM, Bikle DD, Cavaleros M, Zachen D, Kamdar MC and Ross FP, 1995. Serum levels of free 1, 25‐dihydroxyvitamin D in vitamin D toxicity. Annals of Internal Medicine, 122, 511–513. [DOI] [PubMed] [Google Scholar]
- Pietrek J, Preece MA, Windo J, O'Riordan JL, Dunnigan MG, McIntosh WB and Ford JA, 1976. Prevention of vitamin‐D deficiency in Asians. Lancet, 1, 1145–1148. [DOI] [PubMed] [Google Scholar]
- Pilz S, Dobnig H, Nijpels G, Heine RJ, Stehouwer CD, Snijder MB, van Dam RM and Dekker JM, 2009. Vitamin D and mortality in older men and women. Clinical Endocrinology, 71, 666–672. [DOI] [PubMed] [Google Scholar]
- Pincus JB, Gittleman JF, Sobel AE and Schmerzler E, 1954. Effects of vitamin D on the serum calcium and phosphorus levels in infants during the first week of life. Pediatrics, 13, 178–185. [PubMed] [Google Scholar]
- Pittard WB 3rd, Geddes KM, Hulsey TC and Hollis BW, 1991. How much vitamin D for neonates? American Journal of Diseases of Children, 145, 1147–1149. [DOI] [PubMed] [Google Scholar]
- Pludowski P, Socha P, Karczmarewicz E, Zagorecka E, Lukaszkiewicz J, Stolarczyk A, Piotrowska‐Jastrzebska J, Kryskiewicz E, Lorenc RS and Socha J, 2011. Vitamin D supplementation and status in infants: a prospective cohort observational study. Journal of Pediatric Gastroenterology and Nutrition, 53, 93–99. [DOI] [PubMed] [Google Scholar]
- Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR and Thadhani R, 2013. Vitamin D‐binding protein and vitamin D status of black Americans and white Americans. New England Journal of Medicine, 369, 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronicka E, Ciara E, Halat P, Janiec A, Wojcik M, Rowinska E, Rokicki D, Pludowski P, Wojciechowska E, Wierzbicka A, Ksiazyk JB, Jacoszek A, Konrad M, Schlingmann KP and Litwin M, 2017. Biallelic mutations in CYP24A1 or SLC34A1 as a cause of infantile idiopathic hypercalcemia (IIH) with vitamin D hypersensitivity: molecular study of 11 historical IIH cases. Journal of Applied Genetics, 58, 349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radlovic N, Lekovic Z, Ristic D, Radlovic V, Djuricic G, Dimitrijevic A and Vuletic B, 2014. Case report of acute vitamin D intoxication in an infant. Srpski Arhiv za Celokupno Lekarstvo, 142, 736–739. [DOI] [PubMed] [Google Scholar]
- Rajakumar K, Reis EC and Holick MF, 2013. Dosing error with over‐the‐counter vitamin D supplement: a risk for vitamin D toxicity in infants. Clinical Pediatrics, 52, 82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul E, 2015. Intestinal absorption of vitamin D: from the meal to the enterocyte. Food & Function, 6, 356–362. [DOI] [PubMed] [Google Scholar]
- Ribot C, 1981. Etude des taux plasmatiques de 25‐OH‐D, chez le nouveau‐né à terme et le prématuré, après administration de 1000 UI de vitamine D par voie orale. Le Journal des Agreges, 14, 5. [Google Scholar]
- Ring E and Borkenstein M, 1987. Use of the calcium‐creatinine ratio in diagnosis and therapy. Padiatrie und Padologie, 22, 245–250. [PubMed] [Google Scholar]
- Roe MA, Bell S, Oseredczuk M, Christensen T, Westenbrink S, Pakkala H, Presser K and Finglas PM, 2013. Updated food composition database for nutrient intake. Project developed on the procurement project CFT/EFSA/DCM/2011/03. EFSA Supporting publication 2013:EN‐355, 21 pp. 10.2903/sp.efsa.2013.en-2355 [DOI]
- Ronnefarth G and Misselwitz J, 2000. Nephrocalcinosis in children: a retrospective survey. Members of the Arbeitsgemeinschaft fur padiatrische Nephrologie. Pediatric Nephrology, 14, 1016–1021. [DOI] [PubMed] [Google Scholar]
- Rosendahl J, Valkama S, Holmlund‐Suila E, Enlund‐Cerullo M, Hauta‐Alus H, Helve O, Hytinantti T, Levalahti E, Kajantie E, Viljakainen H, Makitie O and Andersson S, 2018. Effect of higher vs standard dosage of vitamin D3 supplementation on bone strength and infection in healthy infants: a randomized clinical trial. JAMA Pediatrics, 172, 646–654. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg AD, Pettifor JM, Cohen DF, Sonnendecker EW and Ross FP, 1982. Maternal‐infant vitamin D relationships during breast‐feeding. Journal of Pediatrics, 101, 500–503. [DOI] [PubMed] [Google Scholar]
- Saadi HF, Dawodu A, Afandi B, Zayed R, Benedict S, Nagelkerke N and Hollis BW, 2009. Effect of combined maternal and infant vitamin D supplementation on vitamin D status of exclusively breastfed infants. Maternal and Child Nutrition, 5, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACN (Scientific Advisory Committee on Nutrition), 2016. Vitamin D and Health report. 304 pp.
- Sagsak E, Savas‐Erdeve S, Keskin M, Cetinkaya S and Aycan Z, 2015. The use of pamidronate for acute vitamin D intoxication, clinical experience with three cases. Journal of Pediatric Endocrinology and Metabolism, 28, 709–712. [DOI] [PubMed] [Google Scholar]
- Salle BL, Delvin E and Glorieux F, 2002. [Vitamin D and pregnancy]. Bulletin de L'Academie Nationale de Medecine, 186, 369–376; discussion 376‐367. [PubMed] [Google Scholar]
- Saneei P, Salehi‐Abargouei A and Esmaillzadeh A, 2013. Serum 25‐hydroxy vitamin D levels in relation to body mass index: a systematic review and meta‐analysis. Obesity Reviews, 14, 393–404. [DOI] [PubMed] [Google Scholar]
- Saraf R, Morton SM, Camargo CA Jr and Grant CC, 2016. Global summary of maternal and newborn vitamin D status ‐ a systematic review. Maternal and Child Nutrition, 12, 647–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent JD, Stukel TA, Kresel J and Klein RZ, 1993. Normal values for random urinary calcium to creatinine ratios in infancy. Journal of Pediatrics, 123, 393–397. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food), 1993. Nutrient and energy intakes for the European Community. Reports of the Scientific Committee for Food, 31st Series. Food ‐ Science and Technique, European Commission, Luxembourg, 248 pp.
- SCF (Scientific Committee on Food), 2000. Guidelines of the Scientific Committee on Food for the development of tolerable upper intake levels for vitamins and minerals. SCF/CS/NUT/UPPLEV/11 Final, 11 pp.
- SCF (Scientific Committee on Food), 2003. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of vitamin D. 35 pp.
- Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz‐Broking E, Fehrenbach H, Wingen AM, Guran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G and Konrad M, 2011. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. New England Journal of Medicine, 365, 410–421. [DOI] [PubMed] [Google Scholar]
- Schlingmann KP, Ruminska J, Kaufmann M, Dursun I, Patti M, Kranz B, Pronicka E, Ciara E, Akcay T, Bulus D, Cornelissen EA, Gawlik A, Sikora P, Patzer L, Galiano M, Boyadzhiev V, Dumic M, Vivante A, Kleta R, Dekel B, Levtchenko E, Bindels RJ, Rust S, Forster IC, Hernando N, Jones G, Wagner CA and Konrad M, 2016. Autosomal‐recessive mutations in SLC34A1 encoding sodium‐phosphate cotransporter 2A cause idiopathic infantile hypercalcemia. Journal of the American Society of Nephrology, 27, 604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans KM and Cashman KD, 2009. Existing and potentially novel functional markers of vitamin D status: a systematic review. American Journal of Clinical Nutrition, 89, 1997S–2008S. [DOI] [PubMed] [Google Scholar]
- Seino Y, Ishii T, Shimotsuji T, Ishida M and Yabuuchi H, 1981. Plasma active vitamin D concentration in low birthweight infants with rickets and its response to vitamin D treatment. Archives of Disease in Childhood, 56, 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shajari A, Shakib M, Nourani F, Zaki M and Kheirandish M, 2009. Urinary calcium/creatinin ratio with different dosages of vitamin D3 prophylaxis in infants. Iranian Journal of Pediatrics, 19, 159–163. [Google Scholar]
- Shakiba M, Sadr S, Nefei Z, Mozaffari‐Khosravi H, Lotfi MH and Bemanian MH, 2010. Combination of bolus dose vitamin D with routine vaccination in infants: a randomised trial. Singapore Medical Journal, 51, 440–445. [PubMed] [Google Scholar]
- Shakiba M, Pahloosye A, Mirouliaei M and Islami Z, 2014. Comparison of two regimens of vitamin D supplementation for vitamin D‐deficient neonates. Singapore Medical Journal, 55, 266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharawat IK, 2016. Hypervitaminosis D with dyslipidemia: an unusual scenario. Indian Pediatrics, 53, 174–175. [PubMed] [Google Scholar]
- Sharma LK, Dutta D, Sharma N and Gadpayle AK, 2017. The increasing problem of subclinical and overt hypervitaminosis D in India: An institutional experience and review. Nutrition, 34, 76–81. [DOI] [PubMed] [Google Scholar]
- Shin JS, Choi MY, Longtine MS and Nelson DM, 2010. Vitamin D effects on pregnancy and the placenta. Placenta, 31, 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siafarikas A, Piazena H, Feister U, Bulsara MK, Meffert H and Hesse V, 2011. Randomised controlled trial analysing supplementation with 250 versus 500 units of vitamin D3, sun exposure and surrounding factors in breastfed infants. Archives of Disease in Childhood, 96, 91–95. [DOI] [PubMed] [Google Scholar]
- Singh RJ, Taylor RL, Reddy GS and Grebe SK, 2006. C‐3 epimers can account for a significant proportion of total circulating 25‐hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. Journal of Clinical Endocrinology and Metabolism, 91, 3055–3061. [DOI] [PubMed] [Google Scholar]
- Smollin C and Srisansanee W, 2014. Vitamin D toxicity in an infant: case files of the University of California, San Francisco medical toxicology fellowship. Journal of Medical Toxicology, 10, 190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellman G, Melhus H, Gedeborg R, Byberg L, Berglund L, Wernroth L and Michaelsson K, 2010. Determining vitamin D status: a comparison between commercially available assays. PLoS ONE, 5, e11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specker BL and Tsang RC, 1987. Cyclical serum 25‐hydroxyvitamin D concentrations paralleling sunshine exposure in exclusively breast‐fed infants. Journal of Pediatrics, 110, 744–747. [DOI] [PubMed] [Google Scholar]
- Specker BL, Valanis B, Hertzberg V, Edwards N and Tsang RC, 1985. Sunshine exposure and serum 25‐hydroxyvitamin D concentrations in exclusively breast‐fed infants. Journal of Pediatrics, 107, 372–376. [DOI] [PubMed] [Google Scholar]
- Stafford N, 2016. News. Vitamin D supplements poison dozens of Danish children. BMJ. British Medical Journal, 354, i4534. [DOI] [PubMed] [Google Scholar]
- Stepman HC, Vanderroost A, Stockl D and Thienpont LM, 2011. Full‐scan mass spectral evidence for 3‐epi‐25‐hydroxyvitamin D(3) in serum of infants and adults. Clinical Chemistry and Laboratory Medicine, 49, 253–256. [DOI] [PubMed] [Google Scholar]
- Tai SS, Bedner M and Phinney KW, 2010. Development of a candidate reference measurement procedure for the determination of 25‐hydroxyvitamin D3 and 25‐hydroxyvitamin D2 in human serum using isotope‐dilution liquid chromatography‐tandem mass spectrometry. Analytical Chemistry, 82, 1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin N, Kural N and Torun M, 1997. Renal function in children with hypercalciuria. Turkish Journal of Pediatrics, 39, 335–339. [PubMed] [Google Scholar]
- Thacher TD, Fischer PR, Obadofin MO, Levine MA, Singh RJ and Pettifor JM, 2010. Comparison of metabolism of vitamins D2 and D3 in children with nutritional rickets. Journal of Bone and Mineral Research, 25, 1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GR, Lewis B and Booth CC, 1966. Absorption of vitamin D3‐3H in control subjects and patients with intestinal malabsorption. Journal of Clinical Investigation, 45, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsdottir I, Thorisdottir AV and Pálsson GI, 2008. Mataræði íslenskra ungbarna 2005–2007. University Press, Reykjavík, Iceland: p. 59. [Google Scholar]
- Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, Chope G, Hyppönen E, Berry J, Vieth R and Lanham‐New S, 2012. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25‐hydroxyvitamin D status: a systematic review and meta‐analysis. American Journal of Clinical Nutrition, 95, 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripkovic L, Wilson LR, Hart K, Johnsen S, de Lusignan S, Smith CP, Bucca G, Penson S, Chope G and Elliott R, 2017. Daily supplementation with 15 μg vitamin D2 compared with vitamin D3 to increase wintertime 25‐hydroxyvitamin D status in healthy South Asian and white European women: a 12‐wk randomized, placebo‐controlled food‐fortification trial. The American Journal of Clinical Nutrition, 106, 481–490. [DOI] [PubMed] [Google Scholar]
- Trolle E, Holmboe Gondolf U, Ege M, Kørup K, Hess Ygill K and Christensen T, 2013. Danskernes kostvaner Spaed‐ og småbørn 2006‐2007. DTU Fødevareinstituttet, pp. 82.
- Ulbrych‐Jablonska A, 1972. The hypocalcemic effect of glucagon in cases of hypercalcemia. Helvetica Paediatrica Acta, 27, 613–615. [PubMed] [Google Scholar]
- Valkama S, Holmlund‐Suila E, Enlund‐Cerullo M, Rosendahl J, Hauta‐alus H, Helve O, Hytinantti T, Viljakainen H, Andersson S and Mäkitie O, 2017. No Severe Hypercalcemia with Daily Vitamin D3 Supplementation of up to 30 μg during the First Year of Life. Hormone Research in Paediatrics, 88, 147–154. [DOI] [PubMed] [Google Scholar]
- Vanlint S, 2013. Vitamin D and Obesity. Nutrients, 5, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkaik‐Kloosterman J, Beukers MH, Jansen‐van der Vliet M and Ocke MC, 2017a. Vitamin D intake of Dutch infants from the combination of (fortified) foods, infant formula, and dietary supplements. European Journal of Nutrition, 56, 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkaik‐Kloosterman J, Seves SM and Ocke MC, 2017b. Vitamin D concentrations in fortified foods and dietary supplements intended for infants: Implications for vitamin D intake. Food Chemistry, 221, 629–635. [DOI] [PubMed] [Google Scholar]
- Vervel C, Zeghoud F, Boutignon H, Tjani JC, Walrant‐Debray O and Garabedian M, 1997. Fortified milk and supplements of oral vitamin D. Comparison of the effect of two doses of vitamin D (500 and 1,000 UI/d) during the first trimester of life. Archives de Pédiatrie, 4, 126–132. [DOI] [PubMed] [Google Scholar]
- Vieth R, 1990. The mechanisms of vitamin D toxicity. Bone and Mineral, 11, 267–272. [DOI] [PubMed] [Google Scholar]
- Vieth R, 1999. Vitamin D supplementation, 25‐hydroxyvitamin D concentrations, and safety. American Journal of Clinical Nutrition, 69, 842–856. [DOI] [PubMed] [Google Scholar]
- Viljakainen HT, Palssa A, Karkkainen M, Jakobsen J and Lamberg‐Allardt C, 2006. How much vitamin D3 do the elderly need? Journal of the American College of Nutrition, 25, 429–435. [DOI] [PubMed] [Google Scholar]
- Wagner CL and Greer FR, 2008. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics, 122, 1142–1152. [DOI] [PubMed] [Google Scholar]
- Wagner CL, Hulsey TC, Fanning D, Ebeling M and Hollis BW, 2006. High‐dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6‐month follow‐up pilot study. Breastfeeding Medicine, 1, 59–70. [DOI] [PubMed] [Google Scholar]
- Wallace AM, Gibson S, de la Hunty A, Lamberg‐Allardt C and Ashwell M, 2010. Measurement of 25‐hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids, 75, 477–488. [DOI] [PubMed] [Google Scholar]
- Weaver C and Heaney R, 2014. Calcium In: Ross A, Caballero B, Cousins RJ, Tucker KL, Ziegler TR. (ed.). Modern Nutrition in Health and Disease. Lippincott Williams & Wilkins / Wolters Kluwer, Baltimore. pp. 133–149. [Google Scholar]
- Webb AR, DeCosta BR and Holick MF, 1989. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. Journal of Clinical Endocrinology and Metabolism, 68, 882–887. [DOI] [PubMed] [Google Scholar]
- Wegienka G, Kaur H, Sangha R and Cassidy‐Bushrow AE, 2016. Maternal‐Cord Blood Vitamin D Correlations Vary by Maternal Levels. Journal of Pregnancy, 2016, 7474192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West TE, Sinclair L, Joffe M and O'Riordan JL, 1971. Treatment of hypercalcaemia with calcitonin. Lancet, 1, 675–678. [DOI] [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group (World Health Organization), 2006. WHO Child Growth Standards: Length/height‐for‐age, weight‐for‐age, weight‐for‐length, weight‐for‐height and body mass index‐for‐age: Methods and development. 312 pp.
- Young BE, Cooper EM, McIntyre AW, Kent T, Witter F, Harris ZL and O'Brien KO, 2014. Placental vitamin D receptor (VDR) expression is related to neonatal vitamin D status, placental calcium transfer, and fetal bone length in pregnant adolescents. FASEB Journal, 28, 2029–2037. [DOI] [PubMed] [Google Scholar]
- Yousefzadeh P, Shapses SA and Wang X, 2014. Vitamin D binding protein impact on 25‐Hydroxyvitamin D levels under different physiologic and pathologic conditions. International Journal of Endocrinology, 2014, 981581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeghoud F, Ben‐Mekhbi H, Djeghri N and Garabedian M, 1994. Vitamin D prophylaxis during infancy: comparison of the long‐term effects of three intermittent doses (15, 5, or 2.5 mg) on 25‐hydroxyvitamin D concentrations. American Journal of Clinical Nutrition, 60, 393–396. [DOI] [PubMed] [Google Scholar]
- Zeghoud F, Vervel C, Guillozo H, Walrant‐Debray O, Boutignon H and Garabedian M, 1997. Subclinical vitamin D deficiency in neonates: definition and response to vitamin D supplements. American Journal of Clinical Nutrition, 65, 771–778. [DOI] [PubMed] [Google Scholar]
- Ziegler EE, Nelson SE and Jeter JM, 2014. Vitamin D supplementation of breastfed infants: a randomized dose‐response trial. Pediatric Research, 76, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical methods used to estimate the intake–response of serum 25 (OH)D concentration on daily supplemental intake of vitamin D and to derive the percentage of infants exceeding a serum 25(OH)D concentration
Results of the assessment of the daily intake of vitamin D for infants aged 4–< 12 months and 8 intake scenarios


