Abstract
The additive, ‘Zinc‐l‐selenomethionine’ (Zn‐l‐SeMet) is intended to be used as a source of selenium for all animal species. The applicant intends to market the active compound blended with inert carriers (Availa®Se). Zn‐l‐SeMet is a safe source of selenium for chickens for fattening; the conclusion is extended to all animal species. Selenium from Zn‐l‐SeMet does not elicit any adverse effects not expected for a selenium compound. The use of Zn‐l‐SeMet in animal nutrition is expected to result in a similar increase in selenium deposition in animal tissues/products as that resulting from other sources of SeMet. The use of the additive up to the maximum selenium supplementation level established for other sources of organic selenium (0.2 mg/kg complete feed) and complying with the maximum authorised total selenium content is safe for consumers. The additive is hazardous upon inhalation; owing to the high dusting potential, persons handling Availa®Se are at risk by inhalation. Availa®Se is not an irritant to the skin. In the absence of data, no conclusion on the eye irritation and skin sensitisation can be drawn. The use of Zn‐l‐SeMet in feed does not pose an additional risk to the environment, compared with other sources of selenium for which it will substitute, as long as the maximum authorised content in complete feed is not exceeded. Zn‐l‐SeMet is an effective source of selenium in chickens for fattening and laying hens; this conclusion is extended to all animal species. The maximum contribution of zinc in total feed deriving from the use of the additive (< 0.2 mg Zn/kg feed) is considered low and does not need any safety assessment except for users.
Keywords: nutritional additives, compounds of trace elements, selenium, zinc‐l‐selenomethionine, safety, efficacy
Summary
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on safety and efficacy of zinc‐l‐selenomethionine (Zn‐l‐SeMet) for all animal species.
The additive, ‘Zinc‐l‐selenomethionine’ (Zn‐l‐SeMet) is intended to be used as a source of selenium for all animal species. The preparation to be marketed is a blend of the active compound with inert carriers (in this assessment called as ‘formulated additive’; trade name: Availa®Se).
Conclusions on the safety of an additive for target species are generally based on the absence of adverse effects in a tolerance study. No such effects were seen (i) in the zootechnical endpoints for the additive under assessment and the already authorised inorganic sodium selenite, also by comparison with the unsupplemented control diet and (ii) in most haematology and serum biochemistry parameters (the few exceptions were of statistical nature as well as unclear biological relevance, if any). The response of selenium serum levels and glutathione peroxidase (GPx) activity to dietary Zn‐l‐SeMet indicates selenium bioavailability. It is therefore concluded that the Zn‐l‐SeMet is a safe source of selenium for chickens for fattening. The conclusion is extended to all animal species.
Selenium from Zn‐l‐SeMet does not elicit any adverse effects not expected for a selenium compound. The use of Zn‐l‐SeMet in animal nutrition is expected to result in a similar increase in selenium deposition in animal tissues/products as that resulting from other sources of SeMet. The use of the additive is safe for consumers provided that the maximum selenium supplementation level established for other sources of organic selenium (0.2 mg/kg complete feed) and that the maximum authorised total selenium content (0.5 mg/kg complete feed) are respected.
The additive is hazardous upon inhalation; owing to the high dusting potential, persons handling Availa®Se are at risk by inhalation. Availa®Se is not an irritant to the skin. In the absence of data, no conclusion on the eye irritation and skin sensitisation can be drawn.
The use of Zn‐l‐SeMet in feed does not pose an additional risk to the environment, compared with other sources of selenium for which it will substitute, as long as the maximum authorised content in complete feed is not exceeded.
Zn‐l‐SeMet is an effective source of selenium in chickens for fattening and laying hens; this conclusion is extended to all animal species.
The maximum contribution of zinc in total feed deriving from the use of the additive (< 0.2 mg Zn/kg feed) is considered low and does not need any safety assessment except for users.
The FEEDAP Panel recommends to use the additive in compound feed via premixtures.
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from Zinpro Animal Nutrition (Europe), Inc.2 for authorisation of the product zinc l‐selenomethionine, when used as a feed additive for all animal species (category: nutritional additives; functional group: compounds of trace elements).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). EFSA received directly from the applicant the technical dossier in support of this application. The particulars and documents in support of the application were considered valid by EFSA as of 24 October 2016.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product zinc l‐selenomethionine, when used under the proposed conditions of use (see Section 3.1.6).
1.2. Additional information
Zinc‐l‐selenomethionine (Zn‐l‐SeMet) is intended to be used as a source of selenium for all animal species. The additive has not been previously authorised as a feed additive in the European Union (EU).
The applicant holds a patent on the Zn‐l‐SeMet, with the title ‘Derivatives of seleno‐amino acids with improved bioavailability and method for assuring adequate dietary requirements of selenium for livestock’.3 The primary objective of the invention was the development of new compounds intended to enhance the bioavailability and/or increase the stability of seleno‐amino acids.
In 2014, the Food and Drug Administration (FDA) announced that Zinpro Corp. filed a petition proposing that the food additive regulations be amended to provide for the safe use of Zn‐l‐SeMet as a source of selenium in complete feed for broiler chickens.4
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier5 in support of the authorisation request for the use of Zn‐l‐SeMet as a feed additive for all animal species. The technical dossier was prepared following the provisions of Article 7 of Regulation (EC) No 1831/2003, Regulation (EC) No 429/20086 and the applicable EFSA guidance documents.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers and other scientific reports, to deliver the present output.
EFSA has verified the EURL report as it relates to the methods used for the control of Zn‐l‐SeMet in animal feed. The Executive Summary of the EURL report can be found in Annex A.7
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of Zn‐l‐Se‐Met is in line with the principles laid down in Regulation (EC) No 429/20086 and the relevant guidance documents: Guidance on nutritional additives (EFSA FEEDAP Panel, 2012a), Technical guidance: Tolerance and efficacy studies in target animals (EFSA FEEDAP Panel, 2011a), Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012b) and Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012c).
3. Assessment
The active compound under assessment is the Zn‐l‐SeMet. The preparation to be marketed is a blend of the active compound with inert carriers (in this assessment called as ‘formulated additive’; trade name: Availa®Se 1000). It is intended to supply selenium as nutritional element to all animal species.
3.1. Characterisation
3.1.1. Characterisation of the compound
The additive is ‘Zinc‐l‐Selenomethionine’. Another generic international name is also (S)‐(+)‐2‐amino‐4‐(methylseleno)butanoic acid. It has neither a Chemical Abstracts Service (CAS) number nor an International Union of Pure and Applied Chemistry (IUPAC) name. Its chemical formula is C5H10ClNO2SeZn, the structural formula is shown in Figure 1 and it has a molecular weight of 295.94 Da. The theoretical content of selenium is 26.7% and of zinc of 22.1%.
Figure 1.
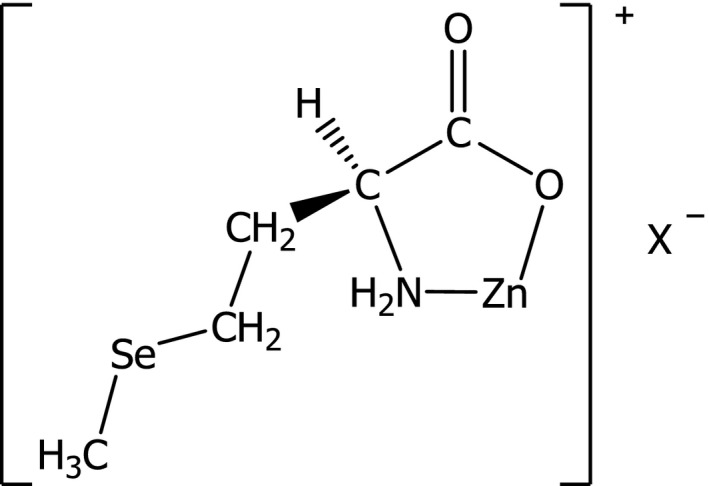
Structural Formula of zinc‐l‐selenomethionine (taken from Zinpro Patent Number EP 1594365 A1)
The molecular structure of the active compound was characterised in three lots by infrared and proton nuclear magnetic resonance spectroscopies.8
The active compound is not available from the synthesis as such (purified), but together with hydrochloric acid (see Section 3.1.3). Five batches of the reaction product were analysed for selenomethionine (SeMet), total selenium, zinc and chloride.9 The average content of SeMet was about 63.6%, selenium 25.6%, zinc 20% and chloride 21.3%
The same five batches were analysed for undesirable substances.10 Levels of heavy metals (Cd < 1 mg/kg compound11; Pb < 1 mg/kg compound;8 Hg < 0.1 mg/kg compound), F (< 25 mg/kg compound) and As (< 1 mg/kg compound) were reported. The levels of dioxins and the sum of dioxins and dioxin‐like PCBs were 0.13–0.18 ng WHO‐PCDD/F‐TEQ/kg and 0.18–0.23 ng WHO‐PCDD/F‐PCB‐TEQ/kg, respectively. The concentrations of the undesirable substances analysed comply with those set in Directive 2002/32/EC12 for compounds of trace elements or, if not mentioned in the Directive, do not represent a concern.
3.1.2. Characterisation of the formulated additive
The formulated additive is a solid preparation with a light grey to tan colour and no odour. The relative density is 2.9. It is very slightly soluble in water.13 The bulk density, measured in three samples, ranged between 1.39 and 1.41 g/cm3.
As formulated, Zn‐l‐SeMet provides a minimum of selenium and SeMet of 1,000 and 2,500 mg/kg additive, respectively.
The composition of the formulated additive is: 97.0% of calcium carbonate, 1.62% of silicon dioxide, 1.02% of vegetable oil and 0.36% of Zn‐l‐SeMet.
Five batches of the formulated additive were analysed for zinc, selenium and SeMet.14 Zinc content ranged from 875 to 910 mg/kg; total selenium content from 1,041 to 1,157 and SeMet from 2,530 to 2,751 mg/kg, thus meeting the specifications. The ratio between zinc and selenium is about 0.82.
Six batches were analysed for undesirable substances, except for fluorine in which results for only four batches were reported.15 Levels of heavy metals (Cd: 0.16 to < 1 mg/kg additive16; Pb: 1.18–2.17 mg/kg additive; Hg: < 0.05–< 0.1 mg/kg additive),13 F (16.6–66.5 mg/kg additive13) and As (< 0.1–1.16 mg/kg additive) were reported. The levels of dioxins and the sum of dioxins and dioxin‐like PCBs were 0.044–0.089 ng WHO‐PCDD/F‐TEQ per kg and 0.10–0.14 ng WHO‐PCDD/F‐PCB‐TEQ per kg, respectively. The concentrations of the undesirable substances analysed comply with those set in Directive 2002/32/EC for compounds of trace elements or, if not mentioned in the Directive, do not represent a concern.
Analysis of five batches (aged from 2 weeks to 10 months) confirmed the absence of microbial contamination. Counts of Enterobacteriaceae, moulds and yeasts were below the limit of quantification (LOQ) (< 10 cfu/g); Salmonella was not detected in 25 g.17 Levels of aflatoxin B1 and ochratoxin A analysed in three batches were below the LOQ (4 μg/kg).18 , 19
Analysis of the particle size distribution (v/v) by laser diffraction (three batches) showed that 1.1–4.1% of the particles had a diameter < 10 μm, 3.2–12.4% < 50 μm and 13.1–27.2% < 100 μm.20
Dusting potential was analysed in three batches by the Stauber–Heubach method (four measurements of each batch).21 The values reported were in the range of 6.1–11.0 g/m3. The same batches were submitted for analysis of the particle size and the zinc and selenium content (two measurements per batch) of the dust.22 The results showed that on average 93% of the particles were below 10 μm; zinc ranged from 3,458 to 7,901 mg/kg dust23 and selenium from 3,307 to 5,833 mg/kg dust. These data show that there is an enrichment of the zinc and selenium in the dust compared with the additive, likely due to the finer particles in the active compound compared to the other components of the additive.
3.1.3. Manufacturing process24
The additive is produced by complexing l‐SeMet (from chemical synthesis)19 with zinc to form Zn‐l‐SeMet complex. The manufacturing process of the active compound and of the formulated additive are described in the technical dossier.
3.1.4. Stability and homogeneity
For compounds of trace elements, stability studies are generally not required. The applicant proposed for the formulated additive a shelf‐life of 24 months. Two stability studies with three batches each under ambient (25°C/60% relative humidity (RH)) and accelerated (40°C/75% RH) conditions were on‐going at the time of the assessment, monitoring SeMet content.25 Only values up to 3 months were made available. The SeMet content decreased after 3 months storage under ambient conditions from 3,370 to 3,073 mg (91.2%) and under accelerated conditions to 2,763 mg (82.0%) per kg formulated additive.
A stability study of the additive in premixtures and mash feed monitoring selenium was provided.26 This study is part of the efficacy study submitted (see also Section 3.3.1). After about 9 months of storage, a recovery of 98.5% and 93.3% was reported for premixture and mash feed, respectively. The FEEDAP Panel notes that in this study the identified main active substance, i.e. SeMet, was not quantified.
The capacity of the additive to homogeneously distribute in premixtures27 and complete feed (mash and pelleted)28 was investigated, analysing the selenium content in 10 subsamples each. The coefficient of variation (CV) of the selenium concentration in the premixture (mean 289 mg/kg) was 7.4%. The CV of the mash feed (mean selenium content: 0.195 mg/kg) was 3.0%, that of the same feed after pelleting 3.3%.
3.1.5. Physicochemical incompatibilities or interactions
No incompatibilities resulting from the use of Zn‐L‐SeMet in compound feed are expected with the feed materials, carriers, other approved additives or medicinal products.
3.1.6. Conditions of use
The additive is intended to be used in feed for all animal species. It should be used up to a maximum supplementation rate of 0.2 mg Se/kg feed; a total selenium concentration of 0.5 mg/kg feed should not be exceeded. The additive should be incorporated to feed via a premixture.
3.2. Safety
3.2.1. Absorption, distribution, metabolism and excretion (ADME)
No specific studies on the toxicokinetics of Zn‐l‐SeMet were provided. It can reasonably be assumed that the zinc moiety is dissociated in the intestine (see also Section 3.3). Taking into account the conditions of use of the additive, the content of zinc is negligible and will not be further considered (see also Section 3.1.6).29 Consequently, SeMet is the only relevant fraction. The ADME of l‐SeMet, including its hydroxy‐analogue and the dl‐form, has been described in previous EFSA FEEDAP opinions (EFSA, 2006, 2009; EFSA FEEDAP Panel, 2011b,c; EFSA FEEDAP Panel, 2013a,b, 2014a). Ingested SeMet is actively absorbed in the gut like methionine, the sulfur analogue of SeMet (Schrauzer, 2000); a common Na+ dependent transport mechanism of SeMet and Met in the jejunal brush border membrane of pigs was shown as early as in 1989 (Wolffram et al., 1989); rapid distribution of the absorbed SeMet occurs in most organs (Alexander, 2015). SeMet is non‐specifically incorporated into body proteins as a substitute for the common amino acid Met (Schrauzer, 2000); it can act as a selenium reservoir for the organism. Another portion of SeMet is metabolised resulting into dihydrogen selenide to be utilised in selenium pathways. Urine is the major excretory pathway of selenium (Alexander, 2015).
3.2.2. Toxicological studies
No specific toxicological studies for the product under assessment were provided by the applicant.
Considering the contribution of zinc by the highest supplementation of the additive, any exposure from zinc from the additive is considered toxicologically negligible. The Met from the additive will be incorporated in body proteins or completely metabolised (EFSA FEEDAP Panel, 2012d). Both components are therefore not considered a toxicological concern.
The toxicology of selenium, including organic forms, namely SeMet, has been extensively reviewed by the Scientific Committee on Food (SCF) (European Commission, 2000), the Agency for Toxic Substances and Disease Registry (ATSDR, 2003), the WHO (2011), the European Medicines Agency (EMA, 2015) and the FEEDAP Panel (EFSA, 2006, 2009; EFSA FEEDAP Panel, 2011b,c, 2013a,b, 2014a,b, 2015, 2016a,b).
Briefly, selenium excess (selenosis) is a well‐known disease in humans and animals. Although selenium may induce oxidative DNA damage in vitro, genotoxic effects could be shown in vivo only when toxic doses (> 3 mg Se/kg body weight (bw)) were applied (European Commission, 2000). Teratogenic effects occur at high doses in birds (> 3 mg Se (as SeMet)/kg diet; Spallholz and Hoffman, 2002) and fishes (> 10 mg Se/kg diet; Lemly, 2002); however, developmental toxicity in mammals is controversial (EMA, 2015). Endocrine‐disrupting effects targeting thyroid, growth hormone and insulin‐like growth factor, have been described in humans (Maggio et al., 2010; Valdiglesias et al., 2010) and in mice (Ren et al., 2016); however, such effects are reversible in adult individuals upon discontinuation of exposure.
The FEEDAP Panel considers that selenium supplementation using the additive Zn‐l‐SeMet does not introduce any different toxicological concerns compared to other organic sources of selenium.
3.2.3. Safety for the target species
3.2.3.1. Safety for target species
Where a feed additive application is made as a nutritional additive for all animal species, tolerance data may be limited to one species. The applicant provided a tolerance study with Zn‐l‐SeMet (Availa®Se) in chickens for fattening with duration of 36 days.30 , 31
A total of 1,200 one‐day‐old male broiler chickens (Ross 308) were allocated to five treatment groups in a randomised complete block design. Each treatment had 12 replicates (pens) containing 20 birds each. The treatments consisted of a negative control (starter and grower feeds containing 0.04 and 0.07 mg Se/kg, respectively), two groups with added sodium selenite (0.2 and 2.0 mg Se/kg) and two treatments with added Zn‐l‐SeMet (0.2 and 2.0 mg Se/kg). The birds did not receive any medical treatment during the trial.
All chickens were fed ad libitum a pelleted feed (starter/grower). The diets were mainly composed of wheat, maize and soybean meal and contained as a starter (day 1–21) 21.0% crude protein (CP) and 12.1 MJ AME/kg and as grower (from day 22 onwards) 19.6% CP and 12.5 MJ AME/kg. Final feeds were analysed for nutritional composition, energy and selenium concentration.
The birds were inspected at least once a day for general health. Mortality was daily monitored. Body weight was measured at the end of the starter period (day 21) and at the end of the study (day 36), feed intake was recorded for both periods. Body weight gain and feed to gain ratio were calculated for the corresponding intervals. Blood samples were taken from two birds per pen on day 36 (haematology,32 clinical biochemistry33) and on day 37 (selenium, glutathione peroxidase (GPx) for the treatments without selenium supplementation and with 0.2 mg Se from both sources/kg feed). GPx was analysed on the individual samples, selenium on pooled pen samples. The birds used for blood collection on day 36 were subsequently killed; samples of liver, kidney, muscle tissue (left breast) and skin plus fat of the control group and of the groups with feed supplemented with 0.2 mg Se/kg were taken for tissue analysis (pooled samples per pen). Neither macroscopic nor microscopic examination of organs and tissues was made at necropsy.
The data were subjected to analysis of variance (ANOVA) considering blocks and treatments as variables. Treatments were compared by the least significant differences (LSD) test after significant effects had been confirmed by ANOVA. Differences with a p value ≤ 0.05 were considered significant, based on a two‐sided test. The pen was the statistical unit for the zootechnical parameters and the pooled selenium levels in serum and tissues, and the individual bird for other blood parameters.
No adverse health effects were seen. At the end of the study, there were no significant differences between the groups for body weight gain, feed to gain ratio and mortality (Table 1). The haematological endpoints were not significantly different (except the monocytes, which were lower at the 0.2 mg Se from Zn‐l‐Se‐Met than for all other groups; Table 2). Concerning blood biochemistry, the only significant differences were seen for alkaline phosphatase (ALP) and calcium (see Table 2). ALP increased in the Zn‐l‐SeMet groups, the value for the high dose being significantly different to the control; both selenite groups did not differ from the control. Calcium was significantly higher in the high selenium Zn‐L‐SeMet group compared to the two 0.2 mg Se/kg groups. The GPx levels in serum were significantly higher in the two selenium supplemented groups (only the 0.2 mg Se supplementation groups analysed) without differences between the selenium sources. The supplemented birds had a 30 fold increase in GPx activity, whereas the increase in serum selenium was only five‐ to sixfold (see Section 3.3). Selenium in serum was highest in the Zn‐l‐SeMet group, followed by the sodium selenite group with lowest levels in the control group. GPx activity is considered as a biomarker of selenium bioavailability, and the observed increase, together with elevated selenium serum levels, indicates selenium bioavailability from Zn‐l‐SeMet. The few other differences seen in haematology and serum biochemistry parameters were, if any, of unclear biological relevance.
Table 1.
Effects of various dosages of sodium selenite and zinc‐l‐selenomethionine (Availa®Se) on performance of chickens for fattening (day 36) (240 birds per treatment)
| Selenium supplemented (mg/kg) | 0 | Na‐selenite | Availa®Se | ||
|---|---|---|---|---|---|
| 0.2 | 2.0 | 0.2 | 2.0 | ||
| Selenium content 1 | |||||
| Starter diet | 0.04 | 0.23 | 2.04 | 0.29 | 2.26 |
| Grower diet | 0.07 | 0.27 | 2.13 | 0.33 | 2.51 |
| Mortality (%) | 4.6 | 4.6 | 5.7 | 3.4 | 8.4 |
| Feed intake (g/bird) | 3,847 a , b | 3,834 a , b | 3,753 a | 3,914 b | 3,800 a |
| Body weight gain (g/bird) | 2,683 | 2,734 | 2,661 | 2,782 | 2,730 |
| Feed to gain ratio (kg/kg) | 1.434 | 1.403 | 1.412 | 1.407 | 1.392 |
1 Analysed (mg/kg).
a,b Different superscripts within a given row indicate statistical differences (p < 0.05).
Table 2.
Effects of various dosages of sodium selenite and zinc‐l‐selenomethionine (Availa®Se) on haematology and biochemistry parameters of chickens for fattening (day 36) (24 birds per treatment)
| Selenium supplemented (mg/kg) | 0 | Na‐selenite | Availa®Se | ||
|---|---|---|---|---|---|
| 0.2 | 2.0 | 0.2 | 2.0 | ||
| Monocytes (109/L) | 0.09 b | 0.07 a , b | 0.09 b | 0.02 a | 0.10 b |
| Alkaline phosphatase (IU/L) | 4,951 a , b | 4,920 a , b | 4,416 a | 6,142 b , c | 7,252 c |
| Calcium (mmol/L) | 2.59 b | 2.45 a | 2.49 a , b | 2.45 a | 2.60 b |
| Glutathione peroxidase in serum (U/mL) | 48 a | 1,448 b | ND 1 | 1,414 b | ND |
1 ND: Not Determined.
a,b,c Different superscripts within a given row indicate statistical differences (p < 0.05).
3.2.3.2. Conclusions on safety for the target species
Conclusions on the safety of an additive for target species are generally based on the absence of adverse effects in a tolerance study. No such effects were seen (i) in the zootechnical endpoints for the additive under assessment and the already authorised inorganic sodium selenite, also by comparison with the unsupplemented control diet and (ii) in most haematology and serum biochemistry parameters (the few exceptions were of statistical nature as well as unclear biological relevance, if any). The response of selenium serum levels and GPx activity to dietary Zn‐l‐SeMet indicates selenium bioavailability. It is therefore concluded that the Zn‐l‐SeMet is a safe source of selenium for chickens for fattening. The conclusion is extended to all animal species.
3.2.4. Safety for the consumer
3.2.4.1. Deposition studies
Deposition data in tissues and eggs were taken from the tolerance study in chickens for fattening (described in Section 3.2.3.1) and from an efficacy study in laying hens (described in Section 3.3.2). Results of the negative control, the group with sodium selenite at 0.2 mg/kg and the group with Zn‐l‐SeMet at 0.2 mg/kg are reported in Table 3.
Table 3.
Data on tissue and egg deposition of sodium selenite and zinc‐l‐selenomethionine (Availa®Se) from a study in chickens for fattening (day 36) (24 birds per treatment) and a study in laying hens (day 59)
| Selenium supplemented (mg/kg) | 0 | Na‐selenite | Availa®Se | Ratio Se from Availa®Se to Se from Na‐selenite |
|---|---|---|---|---|
| 0.2 | 0.2 | |||
| Selenium content in: | ||||
| Breast muscle (ng/g) | 100 a | 103 a | 265 b | 2.57 |
| Liver (ng/g) | 250 a | 513 b | 655 c | 1.27 |
| Kidney (ng/g) | 253 a | 695 b | 809 c | 1.16 |
| Skin/fat (ng/g) | 79 a | 159 b | 232 c | 1.46 |
| Egg (ng/g liquid egg) 1 | < 50 2 | 129 a | 231 b | 1.79 |
1 From the Efficacy study (see also Section 3.3.2).
2 Corresponds to LOQ.
a,b,c Different superscripts within a given row indicate statistical differences (p < 0.05).
There were significant effects of selenium level and source on the selenium content of the four tissues examined and eggs. In chickens for fattening, the negative control had significantly lower selenium levels in liver, kidney and skin than the standard sodium selenite treatment, which in turn had significantly lower selenium levels than the standard Zn‐l‐SeMet treatment. The selenium levels in breast muscle were not different between the negative control and the standard sodium selenite treatment, but both were significantly lower than the Zn‐l‐SeMet treatment.
In both deposition studies performed on chickens for fattening or on laying hens, the level of selenium is higher in the tissues and products in the Zn‐l‐SeMet treatment compared to control treatment or to sodium selenite treatment.
3.2.4.2. Assessment of the safety for the consumer
The upper tolerable level (UL) for selenium has been set by the Scientific Committee on Food (European Commission, 2000) (adults: 300 μg/day; toddlers: 60 μg/day), and used by the FEEDAP Panel in previous assessments of the consumer safety for different selenium compounds.
Data provided by the applicant showed increased tissue deposition of selenium from dietary Zn‐l‐SeMet compared with sodium selenite (see Table 3). The FEEDAP Panel has several times expressed its view that SeMet yeast sources would result in similar selenium deposition (EFSA FEEDAP Panel, 2011b,c, 2012e); this view was later extended to the hydroxy‐analogue of SeMet (HMSeBA) (EFSA FEEDAP Panel, 2013a), to l‐SeMet (EFSA FEEDAP Panel, 2013b) and to dl‐SeMet (EFSA FEEDAP Panel, 2014a). There is no reason to assume that SeMet from different sources would result in an essentially different deposition pattern in edible tissues/products.
The FEEDAP Panel has previously concluded that the selenium supplementation of feed by selenised yeast, by HMSeBA, by l‐SeMet or by dl‐SeMet should be limited to a maximum of 0.2 mg Se/kg feed. Therefore, with respect to consumer safety, the Panel concludes that supplemental selenium from Zn‐ l‐SeMet should equally be limited to a maximum of 0.2 mg/kg feed.
3.2.4.3. Conclusions on safety for the consumer
The use of Zn‐l‐SeMet in animal nutrition is expected to result in a similar increase in selenium deposition in animal tissues/products as that resulting from other sources of SeMet. To ensure consumer safety from consumption of food originating from animals fed Zn‐l‐SeMet, the FEEDAP Panel concludes that dietary selenium supplementation from the additive should not exceed a maximum of 0.2 mg/kg complete feed, within the maximum total selenium content of 0.5 mg/kg authorised in the EU for complete feedingstuffs.
3.2.5. Safety for the user
3.2.5.1. Effects on the respiratory system
No specific inhalation toxicity studies for the product under assessment were provided by the applicant.
However, due to the high dusting potential of the formulated additive (maximum determined: 11.0 g/m3), an estimation of inhalation exposure was performed.
Taking in consideration the selenium content in dust (average concentration of 4,777 mg/kg), it can therefore be expected that 52.5 mg Se/m3 (11.0 g dust/m3 × 4,777 mg/kg) will be the selenium content in the dust. The respirable fraction could be estimated to be 93% (see Section 3.1.2). Thus, the selenium concentration in the respirable dust would then be 49 mg/m3 (93% × 52.5 mg Se/m3).
Following the above‐described approach for zinc, a value of 59 mg Zn/m3 is estimated in the respirable dust.
The estimated value for selenium and for zinc exceeds, respectively, by 3 and by 1 orders of magnitude the most recent internationally accepted proposed thresholds for selenium (0.02 mg/m3, set by the Deutsche Forschungsgemeinschaft as maximum concentration in the workplace (Maximale Arbeitsplatz Konzentration, MAK) (DFG, 2012)) and for zinc (2 mg/m3, set by the American Conference of Governmental Industrial Hygienists as threshold limit value (TLV) (ACGIH, 2003), respectively. Consequently, the FEEDAP Panel considers that the additive poses a risk upon inhalation to users.
3.2.5.2. Effects on the eyes and skin
The applicant submitted an acute skin irritation assay in the rabbit, performed with the formulated additive according to the OECD Guidance 404.34 Availa®Se was found to be non‐irritant for the skin of the rabbit.
No specific studies on eye irritation and skin sensitisation were submitted.
3.2.5.3. Conclusions on safety for the user
The additive is hazardous upon inhalation. Owing to the high dusting potential, persons handling Availa®Se are at risk by inhalation.
Availa®Se is not an irritant to the skin. In the absence of data, no conclusion on the eye irritation and skin sensitisation can be drawn.
3.2.6. Safety for the environment
It is well documented in the scientific literature that, owing to the specific metabolic fate of organic selenium compounds, which reasonably include the Zn‐l‐SeMet, significantly more selenium from organic sources than from inorganic sources is retained in the animal body (see Section 3.2.4.1, Table 3), and therefore less selenium is expected to be released to the environment.
Therefore, the FEEDAP Panel considers that the use of Zn‐l‐SeMet in feed does not pose an additional risk to the environment, compared with other sources of selenium for which it will substitute, as long as the maximum authorised content in complete feed is not exceeded. Moreover, the maximum permitted selenium supplementation from organic selenium sources is about half of that for inorganic sources.
Since the contribution of zinc from the additive will be negligible (see also Section 3.2.1), the environmental impact of zinc needs not to be addressed.
3.3. Efficacy
For demonstration of the efficacy of nutritional additives, one study in a single animal species or category, including laboratory animals, is considered sufficient. Zn‐l‐SeMet is a compound of trace elements not already authorised as a feed additive, a short‐term study is required by to support efficacy (Commission Regulation (EC) No 429/2008; EFSA FEEDAP Panel, 2012a).
The applicant made for that purpose reference to the studies provided to investigate safety of the additive in chickens for fattening and selenium deposition in chicken and eggs (see also Section 3.2.4). Also, a third study published by Chantiratikul et al. (2008a,b) could be considered for that purpose.
3.3.1. Study in chickens for fattening
The study has been described above (see Section 3.2.3.1). The results of this study clearly indicate that at the highest use level of selenium (0.2 mg/kg) from Zn‐l‐Se‐Met (i) selenium is absorbed, serum selenium increased from 29 in the unsupplemented control to 172 ng/mL, (ii) selenium is deposited in tissues and organs (see Table 3) and (iii) selenium is incorporated into the functional enzyme GPx (increase in serum from 48 to 1,414 U/mL (see Table 2)).
3.3.2. Studies in laying hens
After a preperiod of 42 days on a basal diet without selenium supplementation, a total of 180 HyLine Brown laying hens (23‐week‐old, mean body weight: 1,728 g) were distributed to five treatments with 12 replicates each.35 The replicate was a cage with three hens. The treatments were (i) no selenium supplementation (basal diet), (ii) supplementation of 0.2 and 0.3 mg Se/kg from Na‐selenite (concentrations confirmed by analysis) and (iii) supplementation of 0.2 and 0.3 mg Se/kg from Zn‐l‐SeMet. These diets (mash maize soybean meal type diet with 14.8% CP, 12.5% ash, and 11.4 MJ ME/kg) were fed for 60 days.
Performance (body weight, feed intake, feed to egg ratio, egg production) was assessed at day 28 and day 59 while eggs (all eggs pooled/replicate) and blood samples (2 hens/replicate) for analysis of Se and GPx (serum only) were collected on day 59 and day 60 for backup samples. Statistical examination was done by ANOVA (mixed models, Tukey adjustment), differences with a p < 0.05 were considered significant.
The main results are summarised in Table 4. No deaths occurred during the study. No significant differences were seen for the performance parameters, except for the ratio feed to egg mass for which an increase was found in the highest dose of Na‐selenite. The Panel suggests that the number of hens per group was not high enough and that the study duration (and that of the depletion period) was not long enough to detect effects on performance by the addition of selenium to a Se‐deficient diet.
Table 4.
Cumulative data for feed intake, laying rate, egg weight and ratio feed to egg mass, Serum GPx and selenium (24 hens per treatment), and selenium in eggs (all eggs pooled/replicate) on day 59
| Selenium supplemented (mg/kg) | 0 | Na‐selenite | Availa®Se | ||
|---|---|---|---|---|---|
| 0.2 | 0.3 | 0.2 | 0.3 | ||
| Selenium content (mg/kg) 1 | 0.03 | 0.20 | 0.31 | 0.23 | 0.35 |
| Feed intake (g/hen per day) | 113 | 113 | 113 | 114 | 111 |
| Laying rate (%) | 98.4 | 96.8 | 95.3 | 98.1 | 98.3 |
| Egg weight (g) | 60.7 | 61.2 | 59.9 | 60.0 | 59.3 |
| Ratio feed to egg mass (kg/kg) | 1.89 a | 1.91 a | 1.98 b | 1.91 a | 1.91 a |
| Serum Se (ng/mL) | 29 c | 150 b | 170 a , b | 181 a | 195 a |
| Egg Se (ng/g liquid egg) | < 50 | 129 d | 157 c | 231 b | 333 a |
| GPx (U/mL serum) | 100 b | 1,398 a | 1,736 a | 1,570 a | 1,510 a |
1 Analysed.
a,b,c Different superscripts within a given row indicate statistical differences (p < 0.05).
The selenium levels analysed in serum and egg reflected the dietary selenium concentration. Selenium deposition in the liquid egg indicated the better availability of dietary Zn‐l‐SeMet compared to sodium selenite. The selenium content per egg was nearly double in the Zn‐l‐SeMet groups at both dietary levels when compared to the sodium selenite groups. No differences between selenium sources and levels were obtained for the GPx activity in serum, however there was a clear and significant increase compared to control level.
Chantiratikul et al. (2008a) compared the effects of sodium selenite and Zn‐l‐SeMet on plasma selenium concentration and glutathione peroxidase (GPx) activity in the red blood cells (RBC) of laying hens. The supplementation rates were 0.3, 1.0 and 3.0 mg Se/kg feed from both additives. Plasma selenium concentration statistically increased (p < 0.01) with increasing selenium levels. Plasma Se concentration of hens receiving Zn‐l‐SeMet supplemented diets was higher (p < 0.05) than that of hens receiving sodium selenite supplemented diets. The Se sources did not dramatically alter GPx activity in RBC. However, GPx activity significantly increased (p < 0.01) with increasing dietary selenium levels. In a subsequent publication of the same study, the authors (Chantiratikul et al., 2008b) described the effects of both additives on performance and egg selenium concentration in laying hens. Feed consumption/kg egg, egg production, egg weight, Haugh units and eggshell thickness were not affected by sources and levels of selenium (p > 0.05). Increasing level of dietary selenium significantly increased (p < 0.05) selenium content in eggs. Zn‐l‐SeMet markedly increased (p < 0.05) egg selenium concentrations as compared with sodium selenite. The results indicated that the use of Zn‐l‐SeMet resulted in higher (p < 0.05) egg selenium concentrations than that of sodium selenite.
3.3.3. Conclusions on efficacy for the target species
Zn‐l‐SeMet is an effective source of selenium in chickens for fattening and laying hens; this conclusion is extended to all animal species.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation36 and Good Manufacturing Practice.
4. Conclusions
Zn‐l‐SeMet is a safe source of selenium for chickens for fattening; the conclusion is extended to all animal species.
Selenium from Zn‐l‐SeMet does not elicit any adverse effects not expected for a selenium compound. The use of Zn‐l‐SeMet in animal nutrition is expected to result in a similar increase in selenium deposition in animal tissues/products as that resulting from other sources of SeMet. The use of the additive is safe for consumers provided that the maximum selenium supplementation level established for other sources of organic selenium (0.2 mg/kg complete feed) and that the maximum authorised total selenium content (0.5 mg/kg complete feed) are respected.
The additive is hazardous upon inhalation; owing to the high dusting potential, persons handling Availa®Se are at risk by inhalation. Availa®Se is not an irritant to the skin. In the absence of data, no conclusion on the eye irritation and skin sensitisation can be drawn.
The use of Zn‐l‐SeMet in feed does not pose an additional risk to the environment, compared with other sources of selenium for which it will substitute, as long as the maximum authorised content in complete feed is not exceeded.
Zn‐l‐SeMet is an effective source of selenium in chickens for fattening and laying hens; this conclusion is extended to all animal species.
The maximum contribution of zinc in total feed deriving from the use of the additive (< 0.2 mg Zn/kg feed) is considered low and does not need any safety assessment except for users.
5. Recommendation
The additive, either as Zn‐l‐SeMet or as the formulated additive containing Zn‐l‐SeMet (i.e. Availa®Se 1000), should be added to feed via premixture to ensure homogeneous distribution in complete feed.
Documentation provided to EFSA
Zinc‐l‐selenomethionine (Availa Se). August 2016. Submitted by Zinpro Animal Nutrition (Europe), Inc.
Zinc‐l‐selenomethionine (Availa Se). Supplementary information. December 2016. Submitted by Zinpro Animal Nutrition (Europe), Inc.
Zinc‐l‐selenomethionine (Availa Se). Supplementary information. April 2017. Submitted by Zinpro Animal Nutrition (Europe), Inc.
Evaluation report of the European Union Reference Laboratory for Feed Additives on the Methods(s) of Analysis for zinc‐l‐selenomethionine.
Comments from Member States.
Abbreviations
- AAS
atomic absorption spectrometry
- ADME
absorption, distribution, metabolism and excretion
- ALP
alkaline phosphatase
- AME
apparent metabolizable energy
- ANOVA
analysis of variance
- ATSDR
Agency for Toxic Substances and Disease Registry
- bw
body weight
- CAS
Chemical Abstracts Service
- CFU
colony forming unit
- CP
crude protein
- CV
coefficient of variation
- DFG
Deutsche Forschungsgemeinschaft
- EMA
European Medicines Agency
- EURL
European Union Reference Laboratory
- FDA
Food and Drug Administration
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- GPx
glutathione peroxidase
- HGAAS
hydride generation atomic absorption spectrometry
- HPLC‐FLD
high performance liquid chromatography with fluorescence detection
- ICP‐AES
inductively coupled plasma‐atomic emission spectrometry
- ICP‐MS
inductively coupled plasma‐mass spectrometry
- ICP‐OES
inductively coupled plasma‐optical emission spectrometry
- IUPAC
International Union of Pure and Applied Chemistry
- LOQ
limit of quantification
- LSD
least significant differences
- MAK
Maximale Arbeitsplatz Konzentration
- OECD
Organisation for Economic Co‐operation and Development
- OPA
ortho‐phthalaldehyde
- PCB
polychlorinated biphenyl
- PCDD/F
polychlorinated dibenzo‐p‐dioxin/dibenzofuran
- RBC
red blood cells
- RH
relative humidity
- SCF
Scientific Committee on Food
- SeMet
selenomethionine
- TEQ
toxic equivalent
- TLV
threshold limit value
- UL
upper tolerable level
- WHO
World Health Organization
- Zn‐l‐SeMet
zinc‐l‐selenomethionine
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for zinc‐l‐selenomethionine
1.
In the current application authorisation is sought under article 4(1) for Zinc‐L‐selenomethionine under the category/functional group (3b) “nutritional additives”/“compounds of trace elements”, according to the classification system of Annex I of Regulation (EC) No 1831/2003. Specifically, authorisation is sought for the use of the feed additive for all animal species and categories.
The feed additive (Zinc‐L‐selenomethionine) is a complexation product of zinc chloride and L‐selenomethionine containing about 63% of L‐selenomethionine and about 20% of zinc. It is to be marketed as a solid preparation, containing a minimum of 0.36% of Zinc‐L‐selenomethionine, which corresponds to a minimum of 0.1% of selenium and 0.05% of zinc; it also contains calcium carbonate, silicon dioxide and vegetable oil as carriers. The feed additive is intended to be incorporated into feedingstuffs through premixtures with a proposed maximum total selenium content of 0.5 mg/kg feedingstuffs to comply with legal requirements.
For the quantification of Selenomethionine in the feed additive the Applicant submitted a single‐laboratory validated and further verified method based on high performance liquid chromatography with fluorescence detection (HPLC‐FLD) after pre‐column derivatisation with ortho‐phthalaldehyde (OPA). Based on the acceptable performance characteristics presented the EURL recommends for official control this HPLC‐FLD method to quantify Selenomethionine in the feed additive.
For the quantification of total selenium in the feed additive the Applicant submitted the official AOAC 2006.03 method designed for the analysis of elements in fertilisers, based on microwave digestion with nitric acid followed by inductively coupled plasma‐optical emission spectrometry (ICP‐OES). In the frame of other dossiers related to selenium containing feed additives the EURL evaluated several alternative single‐laboratory validated and further verified methods, and recommended for official control either inductively coupled plasma‐atomic emission spectrometry (ICP‐AES), or inductively coupled plasma‐mass spectrometry (ICP‐MS) for the quantification of total selenium in the feed additive.
For the quantification of total selenium in premixtures and feedingstuffs the Applicant did not propose any method for official control. However, the EURL previously evaluated and recommended the CEN method EN 16159:2012 based on hydride generation atomic absorption spectrometry (HGAAS) after microwave digestion with HNO3/H2O2. For the quantification of total selenium in premixtures, the EURL suggests diluting the premixtures samples with ground cereal feed and applying the HGAAS method mentioned above. Based on the performance characteristics available, the EURL recommends for official control the CEN method EN 16159:2012 for the quantification of total selenium in premixtures and feedingstuffs.
For the quantification of total zinc in the feed additive, premixtures and feedingstuffs the Applicant submitted the internationally recognised ring‐trial validated method EN 15510 based on inductively coupled plasma‐atomic emission spectrometry (ICP‐AES). Two additional ring‐trial validated methods were previously evaluated by the EURL in the frame of the Zinc group dossiers: EN 15621 based on ICP‐AES after pressure digestion and the Community method based on atomic absorption spectrometry (AAS). Based on the performance characteristics available the EURL recommends for the official control these two CEN methods for the quantification of total zinc in the feed additive.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Suggested citation: EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Pechova A, López‐Gálvez G and Mantovani A, 2018. Scientific Opinion on the safety and efficacy of Zinc‐l‐Selenomethionine as feed additive for all animal species. EFSA Journal 2018;16(3):5197, 18 pp. 10.2903/j.efsa.2018.5197
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00554
Panel members: Gabriele Aquilina, Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Georges Bories, Andrew Chesson, Pier Sandro Cocconcelli, Gerhard Flachowsky, Jürgen Gropp, Boris Kolar, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Alberto Mantovani, Baltasar Mayo, Fernando Ramos, Guido Rychen, Maria Saarela, Roberto Edoardo Villa, Robert John Wallace and Pieter Wester.
Acknowledgements: The FEEDAP Panel wishes to thank the following for the support provided to this scientific output (in alphabetical order of the last name): Rosella Brozzi, Jaume Galobart and Paola Manini.
Adopted: 20 February 2018
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Zinpro Animal Nutrition (Europe), Inc. Akkerdistel 2E. 5831 PJ. Boxmeer. The Netherlands.
Patent number EP 1594365 A1. The primary objective of the invention was the development of new compounds intended to enhance the bioavailability and/or increase the stability of seleno‐amino acids. Available online: https://www.google.com/patents/EP1594365A1?cl=en
FEED dossier reference: FAD‐2016‐0056.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep-fad-2016-0056-zinc-selenomethionine.pdf
Technical Dossier/Section II.
Technical Dossier/Section II/Annexes II‐32 to II‐36.
Technical Dossier/Section II/Annexes II‐37 to II‐41.
These values are not consistent with the limit of quantification of the analytical method (LOQ: 0.1 mg/kg). Technical Dossier/Supplementary information.
Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. OJ L 140, 30.5.2002, p. 10.
Technical Dossier/Section II/Annex II‐53.
Dossier/Section II/Annexes II‐1 to II‐5.
Technical Dossier/Section II/Annexes II‐10 to II‐15.
Assuming that the LOQs for the analysis of undesirable substances in the additive are the same as those for the active compound (see footnote 8), these values are found not to be consistent with a LOQ of 0.1 mg/kg for Cd and Hg and of 25 mg/kg for F.
Technical Dossier/Section II/Annexes II‐21 to II‐25.
Technical Dossier/Section II/Annexes II‐26 to II‐28.
Technical Dossier/Supplementary information.
Technical Dossier/Section II/Annex II‐29.
Technical Dossier/Section II/Annex II‐30.
Technical Dossier/Section II/Annex II‐31.
Zinc in one batch was not determined.
This section has been amended following the confidentiality claims made by the applicant.
Technical Dossier/Section II/Annexes II‐48 and II‐49.
Technical Dossier/Section II/Annex II‐50.
Technical Dossier/Section II/Annex II‐51.
Technical Dossier/Section II/Annex II‐52.
Considering the maximum supplementation rate of the formulated additive (i.e. 200 mg Availa®Se 1,000 per kg complete feed), the contribution of zinc from the additive to the total dietary zinc would be below 0.2 mg/kg.
Technical Dossier/Section III/Annex III_1.
Some animals were kept for 37 days.
Red blood cells, packed cell volume, haemoglobin, white blood cell, lymphocytes, neutrophil granulocytes, monocytes, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, mean corpuscular volume.
Albumin, albumin/globulin ratio, total protein, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, gamma‐glutamyl transferase, glutamate dehydrogenase, creatinine, creatine phosphokinase, bilirubin, glucose, triglycerides, cholesterol, urea, Ca, P, K, Na, Cl.
Technical Dossier/Section III/Annex_III_3.
Technical Dossier/Section IV/Annex_IV_4_2.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- ACGIH (American Conference of Governmental Industrial Hygienists), 2003. Zinc oxide fume. US Department of Labor. Available online: https://www.osha.gov/dts/chemicalsampling/data/CH_277000.html
- Alexander J, 2015. Selenium. Chapter 52. In: Nordberg G, Fowler B and Nordberg M (eds). Handbook on the toxicology of metals. 4th Edition. Academic Press Publications. UK, pp. 1175–1208. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry), 2003. Toxicological profile for Selenium. US Department of Health and Human Services. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp92.pdf [PubMed]
- Chantiratikul A, Aengwanich W, Chinrasri O and Chantiratikul P, 2008a. Plasma selenium concentration and glutathione peroxidase activity in red blood cells of laying hens fed sodium selenite or zinc‐l‐selenomethionine. International Journal of Poultry Science, 7, 692–695. [Google Scholar]
- Chantiratikul A, Chinrasri O and Chantiratikul P, 2008b. Effect of Sodium Selenite and Zinc‐L‐selenomethionine on Performance and Selenium Concentrations in Eggs of Laying Hens. Asian‐Australasian Journal of Animal Sciences, 21, 1048–1052. [Google Scholar]
- DFG (Deutsche Forschungsgemeinschaft), 2012. List of MAK and BAT Values Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area Report No. 4. Available online: http://onlinelibrary.wiley.com/doi/10.1002/9783527666034.oth01/pdf
- EFSA (European Food Safety Authority), 2006. Opinion of the Scientific Panel on Additives Products or Substances used in Animal Feed on the safety and efficacy of the product Sel‐Plex® 2000 as a feed additive according to Regulation (EC) No 1831/2003. EFSA Journal 2006;4(5):348, 40 pp. 10.2903/j.efsa.2006.348 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Safety and efficacy of SELSAF (Selenium enriched yeast from Saccharomyces cerevisiae CNCM I‐3399) as feed additive for all species. Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed. EFSA Journal 2009;7(4):992, 24 pp. 10.2903/j.efsa.2009.992 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (Panel on Additives and Products or Substances used in Animal Feed), 2011a. Technical guidance: tolerance and efficacy studies in target animals. EFSA Journal 2011;9(5):2175, 15 pp. 10.2903/j.efsa.2011.2175 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (Panel on Additives and Products or Substances used in Animal Feed), 2011b. Scientific Opinion on Safety and efficacy of Sel‐Plex® (organic form of selenium produced by Saccharomyces cerevisiae CNCM I‐3060) for all species. EFSA Journal 2011;9(4):2110, 52 pp. 10.2903/j.efsa.2011.2110 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (Panel on Additives and Products or Substances used in Animal Feed), 2011c. Scientific Opinion on the safety and efficacy of selenium in the form of organic compounds produced by the selenium‐enriched yeast Saccharomyces cerevisiae NCYC R645 (SelenoSource AF 2000) for all species. EFSA Journal 2011;9(6):2279, 15 pp. 10.2903/j.efsa.2011.2279 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for the preparation of dossiers for nutritional additives. EFSA Journal 2012;10(1):2535, 14 pp. 10.2903/j.efsa.2012.2535 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance for establishing the safety of additives for the consumer. EFSA Journal 2012;10(1):2537, 12 pp. 10.2903/j.efsa.2012.2537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012d. Scientific Opinion on dl‐methionine, dl‐methionine sodium salt, the hydroxy analogue of methionine and the calcium salt of methionine hydroxy analogue in all animal species; on the isopropyl ester of methionine hydroxy analogue and dl‐methionine technically pure protected with copolymer vinylpyridine/styrene in dairy cows; and on dl‐methionine technically pure protected with ethylcellulose in ruminants. EFSA Journal 2012;10(3):2623, 42 pp. 10.2903/j.efsa.2012.2623 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (Panel on Additives and Products or Substances used in Animal Feed), 2012e. Scientific Opinion on safety and efficacy of selenium in the form of organic compounds produced by the selenium‐enriched yeast Saccharomyces cerevisiae NCYC R646 (Selemax 1000/2000) as feed additive for all species. EFSA Journal 2012;10(7):2778, 17 pp. 10.2903/j.efsa.2012.2778 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (Panel on Additives and Products or Substances used in Animal Feed), 2013a. Scientific Opinion on safety and efficacy of hydroxy‐analogue of selenomethionine as feed additive for all species. EFSA Journal 2013;11(1):3046, 30 pp. 10.2903/j.efsa.2013.3046 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (Panel on Additives and Products or Substances used in Animal Feed), 2013b. Scientific Opinion on the safety and efficacy of l‐selenomethionine as feed additive for all animal species. EFSA Journal 2013;11(5):3219, 18 pp. 10.2903/j.efsa.2013.3219 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014a. Scientific Opinion on the safety and efficacy of dl‐selenomethionine as a feed additive for all animal species. EFSA Journal 2014;12(2):3567, 20 pp. 10.2903/j.efsa.2014.3567 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014b. Scientific Opinion on the safety for the target animals and for the users of selenium in the form of organic compounds produced by the selenium‐enriched yeast Saccharomyces cerevisiae NCYC R645 (SelenoSource AF 2000) for all animal species. EFSA Journal 2014;12(7):3797, 8 pp. 10.2903/j.efsa.2014.3797 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015. Scientific Opinion on the safety and efficacy of selenium compounds (E8) as feed additives for all animal species: sodium selenite (coated granulated preparation), based on a dossier submitted by Doxal Italia S.p.A. EFSA Journal 2015;13(11):4271, 27 pp. 10.2903/j.efsa.2015.4271 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016a. Scientific Opinion on the safety and efficacy of selenium compounds (E8) as feed additives for all animal species: sodium selenite, based on a dossier submitted by Retorte GmbH Selenium Chemicals and Metals. EFSA Journal 2016;14(2):4398, 26 pp. 10.2903/j.efsa.2016.4398 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016b. Scientific opinion on the safety and efficacy of selenium compounds (E8) as feed additives for all animal species: sodium selenite, based on a dossier submitted by Todini and Co SpA. EFSA Journal 2016;14(3):4442, 24 pp. 10.2903/j.efsa.2016.4442 [DOI] [Google Scholar]
- EMA (European Medicines Agency), 2015, online. European public MRL assessment report (EPMAR) – potassium selenate (all food producing species), sodium selenate (all food producing species), sodium selenite (all food producing species). Committee for Veterinary Medicinal Products. Available online: http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500185181
- European Commission , 2000. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Selenium. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out80g_en.pdf
- Lemly AD, 2002. Selenium assessment in aquatic ecosystems: a guide for hazard evaluation and water quality criteria. Springer Verlag, New York, NY, USA. pp. 89–100. [Google Scholar]
- Maggio M, Ceda GP, Lauretani F, Bandinelli S, Dall'Aglio E, Guralnik JM, Paolisso G, Semba RD, Nouvenne A, Borghi L, Ceresini G, Ablondi F, Benatti M and Ferrucci L, 2010. Association of plasma selenium concentrations with total IGF‐1 among older community‐dwelling adults: the InCHIANTI study. Clinical Nutrition, 29, 674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Ali T, Chen W, Han D, Zhang L, Gu X, Zhang S, Ding L, Fanning S and Han B, 2016. The role of selenium in insulin‐like growth factor I receptor (IGF‐IR) expression and regulation of apoptosis in mouse osteoblasts. Chemosphere, 144, 2158–2164. [DOI] [PubMed] [Google Scholar]
- Schrauzer GN, 2000. Selenomethionine: a review of its nutritional significance, metabolism and toxicity. Journal of Nutrition, 130, 1653–1656. [DOI] [PubMed] [Google Scholar]
- Spallholz JE and Hoffman DJ, 2002. Selenium toxicity: cause and effects in aquatic birds. Aquatic Toxicology, 57, 27–37. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2011. Selenium in drinking‐water – Background document for development of WHO Guidelines for Drinking‐water Quality. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/selenium.pdf
- Wolffram S, Berger B, Grenacher B and Scharrer E, 1989. Transport of selenoamino acids and their sulfur analogues across the intestinal brush border membrane of pigs. Journal of Nutrition, 119, 706–712. [DOI] [PubMed] [Google Scholar]


