Abstract
Moniliformin (MON) is a mycotoxin with low molecular weight primarily produced by Fusarium fungi and occurring predominantly in cereal grains. Following a request of the European Commission, the CONTAM Panel assessed the risk of MON to human and animal health related to its presence in food and feed. The limited information available on toxicity and on toxicokinetics in experimental and farm animals indicated haematotoxicity and cardiotoxicity as major adverse health effects of MON. MON causes chromosome aberrations in vitro but no in vivo genotoxicity data and no carcinogenicity data were identified. Due to the limitations in the available toxicity data, human acute or chronic health‐based guidance values (HBGV) could not be established. The margin of exposure (MOE) between the no‐observed‐adverse‐effect level (NOAEL) of 6.0 mg/kg body weight (bw) for cardiotoxicity from a subacute study in rats and the acute upper bound (UB) dietary exposure estimates ranged between 4,000 and 73,000. The MOE between the lowest benchmark dose lower confidence limit (for a 5% response ‐ BMDL05) of 0.20 mg MON/kg bw per day for haematological hazards from a 28‐day study in pigs and the chronic dietary human exposure estimates ranged between 370 and 5,000,000 for chronic dietary exposures. These MOEs indicate a low risk for human health but were associated with high uncertainty. The toxicity data available for poultry, pigs, and mink indicated a low or even negligible risk for these animals from exposure to MON in feed at the estimated exposure levels under current feeding practices. Assuming similar or lower sensitivity as for pigs, the CONTAM Panel considered a low or even negligible risk for the other animal species for which no toxicity data suitable for hazard characterisation were identified. Additional toxicity studies are needed and depending on their outcome, the collection of more occurrence data on MON in food and feed is recommended to enable a comprehensive human risk assessment.
Keywords: moniliformin, MON, exposure, toxicity, occurrence, human and animal risk assessment
Summary
In a request from the European Commission, the Panel on Contaminants in the Food Chain (CONTAM Panel) was asked to assess on the basis of the available information the risk for public health and farm and companion animals related to the presence of moniliformin (MON) in food and feed. The potential risks for the different animal species and specific (vulnerable) groups of the human population should be considered.
MON is a mycotoxin with low molecular weight typically, but not exclusively, produced by several plant pathogenic Fusarium species. It has mainly been detected in cereal grains and cereal‐based food and feed. Naturally occurring modified forms of MON have not been reported. Analytical methods for MON in food, feed and biological samples have been mostly based on liquid chromatography coupled to ultraviolet detection (LC‐UV) and LC with tandem mass spectrometry (LC–MS/MS). Currently, LC–MS/MS is the most widely used and preferred analytical technique. However, none of the applied analytical methods for MON have been formally validated in inter‐laboratory studies. Certified reference materials were not available for MON but calibrants were commercially available.
Occurrence of MON in various cereal grains, such as maize, wheat, barley and oats, and in products produced from them has been reported in the literature, and co‐occurrence with other mycotoxins, in particular with trichothecenes, enniatins, beauvericin and zearalenone, was found. Within the available occurrence data sampled between 2001 and 2016 was a total of 3,205, 806, and 504 analytical results of MON for food, feed and unprocessed grains of undefined end‐use, respectively, that fulfilled the required quality criteria of EFSA. However, the proportion of left‐censored data (results below the limit of detection (LOD) or limit of quantification (LOQ)) was high and reached 90% for MON in food, 60% for MON in feed and 70% for MON in unprocessed grains of undefined end‐use. The LODs ranged between 1 and 20 μg/kg food, 5 and 52 μg/kg feed and were equal to 8 μg/kg grains. The LOQs ranged between 4 and 66 μg/kg food, 7 and 171 μg/kg feed and were equal to 39 μg/kg grains. The highest mean concentrations of MON were recorded for food in the categories ‘Grains for human consumption’, ‘Snack food’ and ‘Breakfast cereals’, for feed in the category ‘Cereal grains’ (i.e. maize and barley) and for unprocessed grains of undefined end‐use in the category ‘Grains as crops’ (i.e. wheat and oat grain).
Cleaning and sorting of grains resulted in a reduction of MON in subsequently produced products. Milling of grains led to a redistribution of MON into different fractions. Semolina, flour and feed flour contained the highest concentrations of MON. Cooking and baking generally led to reductions of MON concentrations in contaminated samples. MON was unstable under high temperatures in combination with alkaline conditions. In the absence of studies with feed materials, the CONTAM Panel considered the effects of the processing of animal feeds were similar to those reported for food. Although the effects of ensiling on MON appeared not to have been studied so far, in view of the relatively high levels of MON reported in maize grains, plants intended for silage were considered as also potential sources of exposure to MON for ruminant livestock.
The estimates of mean acute human exposure to MON across dietary surveys and age groups ranged from 82 to 530 ng/kg body weight (bw) per day based on the mean upper bound (UB) concentrations The estimates based on the UB of the 95th percentile acute exposure ranged from 202 to 1,489 ng/kg bw per day. The highest acute dietary exposures were for infants, toddlers and other children. The estimates of mean chronic human exposure to MON across dietary surveys and age groups ranged from 0.04 to 226 ng/kg bw per day (minimum lower bound (LB) to the maximum UB). The estimates at the 95th percentile ranged from 0.06 to 528 ng/kg bw per day (minimum LB–maximum UB). The highest chronic dietary exposures were for infants, toddlers, other children and adolescents. The most important contributors to the chronic dietary exposure to MON were ‘Grains and grain‐based products’, especially ‘cereal flakes’. The limited available consumption data on vegetarians did not indicate a major difference in the dietary exposure to MON between them and the general population.
Exposure of farm and companion animals to MON was primarily from consuming cereal grains and cereal by‐products. Levels reported for grass‐based forages were generally low. For ruminants, the estimated lowest LB and highest UB mean dietary exposures were 0.04 and 1.6 μg/kg bw per day, and the 95th percentile exposures were 0.27 and 2.4 μg/kg bw per day, respectively. For pigs, the estimated lowest LB and highest UB mean dietary exposures were 0.65 and 2.2 μg/kg bw per day, and the 95th percentile exposures were 2.8 and 5.6 μg/kg bw per day, respectively. For poultry, the estimated lowest LB and highest UB mean dietary exposures were 0.71 and 3.2 μg/kg bw per day, and the 95th percentile exposures were 3.6 and 10 μg/kg bw per day, respectively. For horses, the estimated LB and UB mean dietary exposures to MON were 0.06 and 0.27 μg/kg bw per day, and the 95th percentile exposures were 0.31 and 0.68 μg/kg bw per day, respectively. For farmed fish (salmonids and carp), the estimated lowest LB and highest UB mean dietary exposures were 0.10 and 0.51 μg/kg bw per day, and the 95th percentile exposures were 0.38 and 1.5 μg/kg bw per day, respectively. For farmed rabbits, the estimated LB and UB mean dietary exposures were 0.39 and 1.0 μg/kg bw per day, and the 95th percentile exposures were 2.5 μg/kg bw per day (both, LB and UB). For farmed mink, the estimated LB and UB mean dietary exposures were 0.24 and 0.33 μg/kg bw per day, and the 95th percentile exposures were 0.99 and 1.05 μg/kg bw per day, respectively. For dogs and cats, the estimated lowest LB and highest UB mean dietary exposures were 0.22 and 0.33 μg/kg bw per day, respectively, and the 95th percentile exposures were 0.80 μg/kg bw per day for dogs and 0.75 μg/kg bw per day for cats (both, LB and UB).
The data on the toxicokinetics of MON in experimental animals were limited. In rats, a large portion of MON was absorbed and excreted rapidly after administration with no apparent accumulation in any tissue. However, the fate of at least half of the amount ingested remained unknown. In this opinion, no data on toxicokinetics were identified for farm and companion animals. The only available study on the transfer of MON from feed to food products of animal origin was identified for broiler chickens, where no transfer was found.
Acute toxicity of MON was identified in rats, with oral LD50 values ranging from 19 to 25 mg MON/kg bw. The oral acute toxicity in mice was lower with LD50 values of about 50 mg MON/kg bw. Acute toxicity was accompanied by muscular weakness, respiratory and cardiovascular changes, the latter including faint heart beats and cardiac arrhythmia. The CONTAM Panel identified the presence of ultrastructural lesions in the myocardium, reduction of contractility in aorta, pulmonary artery and terminal ileus, decreased myocardial contractile force and ventricular arrhythmia and congestive heart failure as prominent acute adverse health effects in experimental animals. The CONTAM Panel identified only one subacute toxicity study in rats, which allowed the identification of a no‐observed‐adverse‐effect level (NOAEL) of 6 mg/kg bw per day. Cardiotoxicity was observed at 15 mg/kg bw per day and indications of cardiotoxicity were seen at doses as low as 9 mg MON/kg bw per day. Only one subchronic toxicity study based on a limited number of rats was identified. MON induced cardiotoxicity and mortality at 32.5 mg MON/kg bw per day and higher, while no adverse effects were observed at the lowest dose tested (16.6 mg MON/kg bw per day), which was identified as NOAEL for mortality. Data on haematotoxicity or myelotoxicity and on immunotoxicity were too scarce to conclude on the hazard of MON in experimental animals. No chronic toxicity studies or any carcinogenicity study on MON were identified in experimental animals. For developmental and reproductive toxicity of MON, only one study in mink was identified from which the lowest dose of 0.92 mg MON/kg bw per day was identified as NOAEL. Exposure to 1.94 mg MON/kg bw per day, the other dose tested, resulted in significant neonatal mortality and reduced offspring body weights. There was no evidence that MON induces bacterial reverse mutation. MON has been shown to be clastogenic in vitro inducing chromosomal damage. No data were identified to conclude on whether in vitro genotoxicity is caused by a direct or indirect mechanism. No data were available on genotoxicity of MON in vivo.
No relevant human epidemiological data on MON were identified. Although it has been hypothesised in published literature that dietary exposure to MON was involved in past incidence and prevalence of Keshan disease (KD) in some regions in China, the CONTAM Panel noted that the evidence for a causal relation between dietary exposure to MON and the incidence of KD was too weak and insufficient for human hazard characterisation.
Only a limited amount of data was available on the mode of action of MON and the mode was unclear. The inhibition of enzymes involved in glucose metabolism could lead to cellular energy deprivation and may partially explain the respiratory stress, including myocardial effects. The available database of possible effects of combined exposure to MON and other mycotoxins was weak and insufficient to establish the nature of combined effects.
Data on adverse health effects in farm and companion animals were lacking for most of the animal species. Information was available on poultry and, however limited, for pigs, farmed fish and farmed mink. Mortality and reduced body weight gain were identified as chronic adverse effects both in pigs and poultry.
From the few available studies on the toxicity of MON in pigs, reduced weight gain, adverse haematological effects, cardiotoxicity and mortality accompanied with lesions in heart were identified as critical adverse health effects. The NOAEL for reduced body weight gain ranged between 50 and 100 mg MON/kg feed, corresponding to 1.2 and 2.2 mg MON/kg bw per day. For haematological adverse effects, a NOAEL of 25 mg MON/kg feed corresponding to 1.0 mg MON/kg bw per day was identified. A lowest BMDL05 of 0.20 mg MON/kg bw per day was calculated from the dose–response data on the decrease of haematocrit and haemoglobin levels and this was the most sensitive endpoint for pigs exposed to MON. Mortality was observed at a dose as low as 4.17 mg MON/kg bw per day. A study in miniature pigs showed cardiotoxicity at 3 mg MON/kg bw per day.
In poultry, the heart was the main target organ causing heart failure at acute doses. Repeated dietary exposure to MON not only generated cardiomegaly but also changed haematological parameters and affected body weight gain and egg production. For 1‐day‐old chickens, oral LD50 values of 4.0 and 5.4 mg MON/kg bw were reported. Ascites with oedema of the mesenteries and small haemorrhages in the proventriculus, gizzard, small and large intestine, and skin were observed in surviving chickens. For 7‐day‐old ducks, an oral LD50 value of 3.7 mg MON/kg bw with increasing heart rates followed by arrhythmia and ultimately cessation of contraction were reported. In broiler chickens, the dose of 2.8 mg MON/kg bw per day resulted in reduced body weight gain, cardiomyopathy, changes in the major haematological parameters and increased mortality rates, while at 1.4 mg MON/kg bw per day no adverse effects were observed. In the only available study on laying hens, the dose of 8.5 mg MON/kg bw per day reduced egg production and body weight gain, while 3.8 mg MON/kg bw per day did not generate any adverse effects. In turkeys, no adverse effects were observed at 1.6 mg MON/kg bw per day, while a dose of 3.2 mg MON/kg bw per day induced cardiomegaly. Based on two studies on ducks, the dose of 2.8 mg MON/kg bw per day generated cardiomegaly, while no adverse effects were observed at 2.3 mg MON/kg bw per day.
Only two studies on farmed fish were identified. Reduced weight gain was reported for channel catfish at the lowest dose of 0.8 mg MON/kg bw per day. Nile tilapia appeared to be more resistant and no effects were observed at 1.8 mg/kg bw per day.
In farmed mink, a dose of 1.94 mg MON/kg bw per day resulted in significant neonatal mortality and it reduced body weight of the offspring. This dose was identified as the lowest‐observed‐adverse‐effect level (LOAEL), whereas 0.92 mg MON/kg bw per day was the NOAEL.
Given that no toxicity data suitable for hazard characterisation of MON were identified for ruminants, farmed rabbits, horses, farmed fish, dogs and cats and no NOAELs/LOAELs could be determined for these farm and companion animals, the CONTAM Panel considered the benchmark dose lower confidence limit for a benchmark response of 5% (BMDL05) of 0.20 mg MON/kg bw identified for pigs as an indicative reference point for those. The CONTAM Panel noted that the conclusion on animals other than poultry, pigs and farmed mink would be affected by a higher degree of uncertainty than that on the animal species for which sufficient toxicity data were available.
The CONTAM Panel could not establish an acute reference dose (ARfD) for MON due to the limitations of the available acute and subacute toxicity data. The CONTAM Panel identified cardiotoxicity as a critical adverse health effect of acute and subacute exposure to MON and identified a NOAEL of 6.0 mg/kg bw from a subacute study in rats as reference point for the acute exposure of humans to MON. The CONTAM Panel calculated the margin of exposure (MOE) between the NOAEL of 6.0 mg/kg bw from a subacute study in rats and the acute UB dietary exposure estimates. The MOEs ranged across age groups and consumption studies from 11,000 to 73,000 at the mean and from 4,000 to 29,000 at the 95th percentile dietary exposures, respectively, indicating a low risk for human health.
Due to limitations in the available toxicity data on chronic effects, the CONTAM Panel could not establish a tolerable daily intake (TDI) for MON. However, haematotoxicity was the critical chronic adverse effects of MON in pigs and the CONTAM Panel identified the lowest BMDL05 of 0.20 mg MON/kg bw per for the decrease of the haematocrit and haemoglobin levels as reference point for chronic exposure of humans. In order to get an indication of the possible chronic risk from MON exposure, the CONTAM Panel calculated the MOE between the lowest BMDL05 of 0.20 mg MON/kg bw per day calculated for haematological adverse effects from a 28‐day study in pigs and the chronic dietary human exposure estimates. The MOEs ranged across age groups and consumption studies from 3,900 to 5,000,000 (LB) and from 880 to 25,000 (UB) at the mean exposure, and from 1,400 to 3,300,000 (LB) and from 370 to 4,500 (UB) at the 95th percentile exposure estimates. The CONTAM Panel concluded that these MOE values were sufficiently large to indicate a low risk for human health from current chronic dietary exposure to MON. However, the CONTAM Panel stressed that in the absence of quantitative dose–response data on cardiotoxicity this risk characterisation was based on haematological effects from very limited toxicity database. The limited data on exposure among vegetarians did not indicate notable differences in acute or chronic dietary exposure between the vegetarians and the general population. Therefore, the conclusions on the general population remained valid also for vegetarians.
The margins (MOEs) between the UB estimates of the dietary exposure and the reference point for adverse health effects, ranged for pigs between 90 and 160 for the mean and 35 and 60 for the 95th percentile exposure, for poultry between 430 and 1,400 for the mean and 140 and 460 for the 95th percentile and it was 2,700 for the mean and 830 for the 95th percentile exposure in the farmed mink. The CONTAM Panel concluded that the MOE calculated for pigs, poultry and farmed mink indicated overall a low or even negligible risk for these animal species at the estimated exposure levels of MON under current feeding practices. The MOEs or the other farm and companion animals for which no toxicity data suitable for hazard characterisation of MON were identified, ranged between 120 and 1,400 for the mean and 80 and 290 for the 95th percentile exposure. The CONTAM Panel noted that these MOEs were similar to those observed for animals for which data on adverse effects were observed and concluded that the risk for the other farm and companion animals was low or even negligible at the estimated exposure levels of MON under current feeding practices. The CONTAM Panel also noted that the conclusion on animals other than poultry, pigs and farmed mink would be affected by a higher degree of uncertainty than that on the animal species for which sufficient toxicity data were available.
The CONTAM Panel concluded that in the human risk assessment of MON overall the uncertainty was large. The impact of the uncertainties in the risk assessment of farm and companion animals was also large. Therefore, the CONTAM Panel recommends that a well‐designed 90‐day toxicity study in rats using purified MON and according to relevant Organisation for Economic Co‐operation and Development (OECD) guidelines with special focus on the assessment of haematotoxicity, myelotoxicity and cardiotoxicity should be performed. Furthermore, in vivo studies on the genotoxicity of MON and more data on the mode of action of MON are needed. The CONTAM Panel also recommends well‐designed studies of the toxicokinetics and adverse effects of MON in experimental and farm and companion animals, particularly, in animal species other than poultry. The CONTAM Panel further recommends, depending on the outcome of the above suggested toxicity studies, the collection of more occurrence data on MON in foods and feeds with state‐of‐the‐art validated analytical methods, such as LC–MS/MS, to enable a comprehensive risk assessment for humans, and farm and companion animals.
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
Moniliformin (MON) is formed in cereals by a number of Fusarium species that include F. avenaceum, F. subglutinans and F. proliferatum and occurs as the sodium or potassium salt of 3‐hydroxy‐3‐cyclobutene‐1,2‐dione. Samples of oats, wheat, maize, rye and triticale have been shown to be contaminated with MON.
Available information (not exhaustive)
In accordance with Article 36 of Regulation (EC) No 178/2002, a report ‘Scientific information on mycotoxins and natural plant toxicants’ has been produced following a grant agreement between the European Food Safety Authority (EFSA) and the author(s) of the report (CFP/EFSA/CONTAM/2008/01). The report presents information, inter alia, regarding MON in feed and food and is available on the EFSA website (http://www.efsa.europa.eu/en/scdocs/doc/24e.pdf).
Issue
In the above mentioned report produced on the request of EFSA, it is concluded that MON is a toxin of possible concern in animal feed (especially maize‐based) but the lack of data on occurrence and its transfer into animal products make it impossible to evaluate its significance to animal and human health.
Following this conclusion indicating that MON is a toxin of possible concern, the European Commission asks EFSA to assess on the basis of the available information the risk for farm animals and public health related to the presence of MON in feed and food in order to enable the European Commission and the competent authorities in the Member States to consider the need for a possible follow up including to fill the knowledge gaps.
1.1.2. Terms of Reference
In accordance with Art. 29 (1) of Regulation (EC) No 178/2002, the European Commission asks the European Food Safety Authority to provide a scientific opinion on the risks for public and animal health related to the presence of MON in feed and food.
The assessment should, based upon the available information, assess if the presence of MON in food and feed is a potential risk for public and animal health taking into account the toxicity of MON and the occurrence in feed and food. For the assessment of the risks, the situation for the different animal species and the specific (vulnerable) groups of the human population (e.g. high consumers, children, people following specific diets, etc.) should be considered.
1.2. Interpretation of the Terms of Reference
The CONTAM Panel concluded that the terms of reference provided by the Commission were clear.
1.3. Supporting information for the assessment
1.3.1. Chemistry
Moniliformin (MON) (Figure 1) is a mycotoxin with low molecular weight (free acid: 98.00 g/mol; molecular formula: C4H2O3; Chemical Abstract Service (CAS) number 31876‐38‐7) produced by several Fusarium species (Sydenham et al., 1996) and by Penicillium melanoconidium (Hallas‐Møller et al., 2016). It was discovered in the USA while screening for toxigenic products of Fusarium moniliforme from the damaged maize seeds which were naturally infected with southern leaf blight by Cole et al. (1973) who assigned its trivial name moniliformin. Another Fusarium species, F. fujikuroi, which is a complex of several Fusarium species, producing several different mycotoxins (e.g. beauvericin, fusaproliferin, and fumonisins B1, B2 and B3) is known to produce substantial amounts of MON (Fotso et al., 2002). Therefore, these species of Fusarium have often been used as a source of MON in various studies (see Section 3.1). The CONTAM Panel noted that, since MON was found in 1973, there has been taxonomic reclassification of the MON producing Fusarium species and new evidence has also shown that MON is not only produced by Fusarium species (Battilani et al., 2009; Hallas‐Møller et al., 2016). Franck and Breipohl (1984) studied the biosynthesis of MON and concluded from experimental work that MON is formed through the condensation of two acetate moieties via malonyl coenzyme A to form 1,3 the intermediate cyclobutanedione, followed by oxidation, tautomerisation and dehydration.
Figure 1.
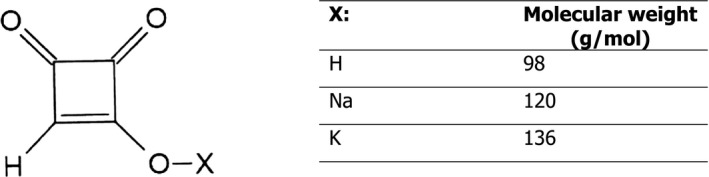
Chemical structure of moniliformin (MON)
MON generally occurs in nature as the sodium (CAS number 71376‐34‐6) or potassium salt (CAS number 52591‐22‐7) of 3‐hydroxy‐3‐cyclobutene‐1,2‐dione, also known as semisquaric acid (Appell et al., 2007; Battilani et al., 2009; Diaz Toro et al., 2015). Springer et al. (1974) elucidated the MON chemical structure by X‐ray crystallography of the potassium salt. MON is a water soluble, polar, strong acid with a pKa value of 0.88 (Scharf et al., 1978; Franck and Breipohl, 1984; Verniest et al., 2005). The ultraviolet (UV) absorbance of MON in distilled water has a maximum at 227 nm and a shoulder at 258 nm with molar absorption coefficients (ε) of 1,990 m2/mol and 540 m2/mol, respectively, (Sydenham et al., 1996). The melting point for the crystalline acid is at 158°C (Cole and Cox, 1981) and for the sodium and potassium salts above 320°C (Cole and Cox, 1981; Sydenham et al., 1996). In aqueous buffer solutions, MON was most stable at pH 4. After 60 min at pH 4 and 150°C, MON was reduced by only 5%. Heating at pH 10 caused major reduction of the concentration of MON. After 60 min at pH 10 and 100, 125 and 150°C, MON was reduced by 56, 72 and 83%, respectively (Pineda‐Valdes and Bullerman, 2000). The free acid of MON is instable in both methanol and water (Scott and Lawrence, 1987).
The CONTAM Panel noted that at the time of development of this opinion, no naturally occurring modified forms of MON have been identified. Chemically synthesised methyl‐ and phenyl forms of MON have been reported by Mrozek (1988).
1.3.2. Methods of analysis
The analytical methodology described in this section mainly relates to the determination of MON in food and feed. Methods of analysis used for biological samples were applied in studies described in Sections 3.1.1 and 3.1.3.
1.3.2.1. Sampling and storage
To date, no specific recommendations concerning sampling and storage of samples intended for the determination of MON have been established. However, to ensure the reliability of the generated analytical data, a representative sample must be provided. Due to the possible inhomogeneous distribution of MON in lots (of grains), sampling may contribute to a significant extent to the variability in analytical results. Samples should be stored under appropriate conditions (dry, preferably frozen) prior to analysis in order to prevent the growth of fungi and associated production of toxins.
1.3.2.2. Determination of MON
Analytical methods typically consist of MON extraction from the samples with an extraction solvent, usually followed by a clean‐up step to eliminate interferences from the sample matrix, and a final detection/quantification step of MON by suitable techniques. Analytical methods for MON have been reviewed by Zoellner and Mayer‐Helm (2006), Krska et al. (2007), Jestoi (2008), Battilani et al. (2009), Cigić and Prosen (2009). Examples on the methods can be found in Table 1. Besides methods especially developed for MON analysis (single analyte methods), MON has also been part of multi‐mycotoxin/multianalyte liquid chromatography–mass spectrometry (LC–MS) methods although it is a highly polar acid raising a particular demand for chromatographic separation (Apfelthaler et al., 2008). Examples are multianalyte methods including 79 fungal metabolites (Apfelthaler et al., 2008), several mycotoxins (Sulyok et al., 2006; Herebian et al., 2009; Delgado et al., 2014), or 295 microbial metabolites (Malachova et al., 2014) where extracts are directly injected into the LC–MS equipment (Kokkonen and Jestoi, 2009) or after solid‐phase clean‐up (Jin et al., 2010). Sometimes, high limits of quantification (LOQs) for MON are achieved in multi‐mycotoxin LC–MS methods (Kokkonen and Jestoi, 2009).
Table 1.
Typical examples of the method characteristics and limits of quantification (LOQ) of analytical methods used for the determination of MON in food and feed
| Analytical technique | Method characteristics | LOQ (μg/kg) | References |
|---|---|---|---|
| TLC | Screening (qualitative–semiquantitative) | nr (LOD = 50–1,000) |
Kamimura et al. (1981) Kostecki et al. (1997) Romer et al. (1997) |
| HPLC‐UV/DAD |
Confirmation (semiquantitative–quantitative) Possible multianalyte detection |
25–136 |
Shepherd and Gilbert (1986) Scott and Lawrence (1987) Lauren and Agnew (1991) Filek and Lindner (1996) Kostecki et al. (1997) Scudamore et al. (1998) Munimbazi and Bullerman (1998) Parich et al. (2003) Maragos (2004) Sorensen et al. (2007) |
| HPLC‐FLD |
Confirmation (semiquantitative–quantitative) Possible multianalyte detection |
20 | Filek and Lindner (1996) |
| HPLC–MS(/MS) |
Confirmation (semiquantitative–quantitative) Possible multianalyte detection |
0.25–1,250 |
Sewram et al. (1999) Jestoi et al. (2003) Sulyok et al. (2006) Sorensen et al. (2007) Herebian et al. (2009) Kokkonen and Jestoi (2009) Jin et al. (2010) Scarpino et al. (2013) Delgado et al. (2014) Nazari et al. (2015) |
| HPLC–HRMS |
Confirmation (semiquantitative–quantitative) Possible multianalyte detection Identification of unknown compounds |
2.5 | von Bargen et al. (2012) |
LOQ: limit of quantification; LOD: limit of detection; nr: not reported; TLC: thin‐layer chromatography; HPLC: high‐performance liquid chromatography; UV: ultra violet; DAD: diode array detection; FLD: fluorescence detection; MS: mass spectrometry; MS/MS: tandem mass spectrometry; HRMS: high‐resolution mass spectrometry.
Analyte isolation
MON is soluble in water, but because of possible extraction of undesired impurities from the sample matrix and because of instant swelling of cooked matrices after application, water is not suitable as an extraction solvent (Chung et al., 2005). Therefore, extraction is generally carried out with acetonitrile/water (84–95% acetonitrile) (Scott and Lawrence, 1987; Bosch et al., 1989; Jestoi et al., 2003; Parich et al., 2003; Jin et al., 2010). In addition, an ion pair reagent tetra‐n‐butyl ammonium hydroxide (TBAH) or tetra‐n‐butyl ammonium hydrogen sulfate (TBAHS) was used to facilitate extraction (Shepherd and Gilbert, 1986; Munimbazi and Bullerman, 1998). A method combining TBAHS and α‐amylase has also been reported resulting in a higher recovery of MON and lesser interferences from matrix (Chung et al., 2005). Clean‐up steps may involve the use of strong anion exchange columns (Munimbazi and Bullerman, 1998; Parich et al., 2003) or other solid‐phase extraction columns including MycoSep™ columns (Scarpino et al., 2013). Appell et al., 2007, synthesised molecularly imprinted polymers to bind MON which were further used as sorbents for molecularly imprinted solid‐phase extraction to pre‐concentrate and clean‐up maize extracts.
Chromatographic methods
Although MON can be analysed by thin‐layer chromatography (TLC), gas chromatography (Gilbert et al., 1986) and capillary zone electrophoresis (Böhs et al., 1995; Maragos, 2004), high‐performance liquid chromatography coupled to ultraviolet detection (HPLC‐UV) or LC–MS is predominantly used. Enzyme‐linked immunosorbent assays are non‐existing as no antibodies were developed against MON (Appell et al., 2007).
For TLC analysis, MON is visualised by spraying 3‐methyl‐2‐benzothiazolinone hydrazone (MBTH) (Kostecki et al., 1997) or 2,4‐dinitrophenylhydrazine (Kamimura et al., 1981; Jansen and Dose, 1984) and quantified by densitometric analysis (Kamimura et al., 1981). Romer et al. (1997) used a one‐step clean‐up column MycoSep™ followed by TLC as a rapid detection technique. Determination of MON in Fusarium cultures/isolates mostly relied on TLC (Bosch et al., 1989; Desjardins et al., 1997; Kostecki et al., 1997; Schütt et al., 1998).
HPLC‐UV and HPLC with diode array detection (HPLC‐DAD) (Parich et al., 2003; Sorensen et al., 2007) are mainly used with ion‐pair reversed‐phase chromatography (Shepherd and Gilbert, 1986; Scott and Lawrence, 1987; Thiel, 1990; Munimbazi and Bullerman, 1998) or hydrophilic interaction (HILIC) chromatography (Sorensen et al., 2007) to achieve good chromatographic separation. Derivatisation of MON also allows the samples to be analysed by fluorescence detection (HPLC‐FLD) (Filek and Lindner, 1996).
LC–MS(/MS) has been used since more recent years (Sewram et al., 1999; Jestoi et al., 2003; Nazari et al., 2015). MON is efficiently ionised in the negative mode of electrospray ionisation and atmospheric pressure chemical ionisation (Jestoi et al., 2003; Herebian et al., 2009; Jin et al., 2010), but may have to be derivatised in order to achieve retention on reversed phase LC columns (Zoellner and Mayer‐Helm, 2006). Alternatively, ion‐pairing (Sewram et al., 1999) or HILIC chromatography (Sorensen et al., 2007) can be used for this purpose. Also, because of its low molecular weight, only one fragment ion can be produced out of the precursor ion (Jestoi et al., 2003; Herebian et al., 2009). High‐resolution mass spectrometry for MON analysis in cereal samples was reported by von Bargen et al. (2012) who used the isotopically labelled 13C2‐MON internal standard, as well as by Lim et al. (2015).
1.3.2.3. Analytical quality assurance: performance criteria, reference materials and proficiency testing for analysis of food
While criteria for methods of analysis for the official control of the levels of various other mycotoxins are laid down in the Regulation (EU) No 401/2006 of 23 February 20061, as amended by the Regulation (EU) No 519/2014 of 16 May 20142, performance criteria for methods of analysis of MON have not been established to date. Currently, certified reference materials were not available for MON, but non‐certified calibrant solutions of the sodium salt of MON were commercially available (Battilani et al., 2009). The free acid of MON must not be used as an analytical standard because of its instability in both methanol and water (Scott and Lawrence, 1987). Proficiency tests for the determination of MON have not been organised.
1.3.3. Previous risk and exposure assessments on MON
No previous scientific risk assessments on MON in food and/or feed by national agencies, national and international independent expert advisory committees were identified by the CONTAM Panel. However, one scientific paper of Peltonen et al. (2010) proposed a tolerable daily intake (TDI) of 0.1 mg/kg body weight (bw) per day based on a preliminary no‐observed‐adverse‐effect levels (NOAEL) of 10 mg/kg bw per day from the pathological and histopathological data on a single 28‐day rat toxicity study (toxicity data were unpublished at the time). These authors estimated that the exposures of Finnish children and adults to MON were 5‐ and 45‐fold lower, respectively, than this proposed TDI. Using a worst‐case scenario exposure for Norway, these authors concluded that exposure to adults was 15‐fold lower than this proposed TDI. However, the CONTAM Panel noted that when this 28‐day toxicity study was completed and published later by Jonsson et al. (2015) these authors could not confirm the suggested NOAEL. The Norwegian Scientific Committee for Food Safety (VKM, 2013) conducted a risk assessment for Fusarium mycotoxins but due to lack of sufficient toxicological evidence no TDI could be derived for MON.
1.3.4. Legislation
In the European Union (EU) and worldwide, no legal maximum levels or guidance levels have been set for MON in foods and feeds (FAO, 2004; Leatherhead Food Research Association, 2010; Council Regulation (EEC) No 315/933; Commission Regulation (EC) No 1881/20064; EU Directive 2002/32/EC5; Recommendation 2006/576/EC6).
1.3.5. Other supporting information
The CONTAM Panel also noted that several reviews on trichothecenes and Fusarium toxins identified the possible adverse effects of MON to humans and to several farm animal species, in particular, poultry (Ueno, 1973; Jestoi et al., 2004; Peltonen et al., 2010; Marin et al., 2013; Escriva et al., 2015). Two recent papers of Jonsson et al., 2013 and Jonsson et al., 2015; and the recent review of Freayman et al. (2017) confirmed this.
2. Data and methodologies
2.1. Methodology of data collection for supporting information for the assessment
2.1.1. Collection and selection of evidence (search strategy, eligibility criteria) for supporting information
No systematic literature search was carried out for scientific evidence for the Sections ‘1.3 Supporting information for the assessment’, ‘3.3.1 Occurrence data on food and feed reported in the available literature’ and ‘3.4 Food and feed processing’. The collected scientific evidence in these sections, used as background information for the assessment, was limited to the most relevant information identified by the experts of the CONTAM Panel working group on Fusarium toxins.
2.1.2. Appraisal of evidence for supporting information
The inclusion of studies for the Sections ‘1.3 Supporting information for the assessment’, and ‘3.4 Food and feed processing’ was based on consideration by the expert judgement of the CONTAM working group (WG) on Fusarium toxins of the extent to which the study was informative and relevant for the assessment accounting for study quality considerations. With regard to the Section ‘3.3.1 Occurrence data on food and feed reported in the available literature’, the appraisal and reporting of selected data used for the exposure assessment were in compliance with the quality requirements of EFSA for the occurrence data (see Section 2.3).
2.2. Methodology of data collection for hazard identification and characterisation
2.2.1. Collection and selection of evidence (search strategy, eligibility criteria) for hazard identification and characterisation
A first systematic literature search in scientific databases aimed at identifying studies that have been published in the open scientific literature and in scientific peer‐reviewed journals until 17 November 2015. The collection of scientific studies available in the public domain was done through searching scientific literature databases (Web of Science and PubMed) using the word ‘moniliformin’ as key term word. The search aimed to retrieve as many studies as possible that might be relevant for hazard identification and hazard characterisation of MON. The search was not limited to the evidence published in English language. The references resulting from the literature search were imported and managed using a software package (EndNoteX8). Deletion of the duplicate references (automatically and manually) resulted in 671 references.
The titles and abstracts were screened for the relevant evidence for hazard identification and characterisation of MON. Publications which were not in the field of laboratory animals and human health, and the health of farm and companion animal were excluded in the screening. These papers reported in particular data on mycology, plant physiology and invasion of plant diseases linked to MON producing fungi. Conference proceedings and abstracts which were part of the outcome of the literature search were also reviewed and included when they provided relevant supporting scientific information as it was, in particular, the case for the hazard characterisation of poultry.
The identified publications included in the assessment of MON in this opinion were:
Papers that had been published in a scientific journal and were subject to an independent scientific peer‐review process (i.e. the process that scientific journals generally use to ensure that the articles to be published represent the best information available in terms of solid scientific soundness and quality control).
Study reports written in English or in other languages which included an abstract in English were considered. The only exceptions to this were the studies on human epidemiology written fully in Chinese (without an English abstract): these were also included when their relevance of effects of MON in humans observed in toxicosis outbreaks in Asia was clear. When identified as relevant by the working group, these and also papers written in other languages than English were submitted for the translation to the Translation Centre of the Bodies of the European Union in Luxemburg. The received translation was then included for hazard identification and hazard characterisation of MON and this translation of the paper was indicated as footnote in this opinion.
Reviews and book chapters were considered as source of background information and as an additional source of scientific evidence unless otherwise stated in this scientific opinion.
The selection process above resulted in total of 220 publications for human and animal hazard identification and characterisation; among them were 102 publications that contained information on experimental animals and humans and, partially overlapping, 179 that contained information for farm and companion animals, mainly poultry (54 publications). Ten papers on toxicosis outbreaks in humans in Asia and only published in China were obtained from a member of WG on Fusarium toxins during the development of the scientific opinion and considered for Section 3.1.7.
To update the published literature for the hazard identification and characterisation of MON first collected up to 17 November 2015, the alerts of the table of contents of the journals within mycotoxin area namely World Mycotoxin Journal, Food Additives and Contaminants, Food and Chemical Toxicology, Toxicology and Applied Pharmacology, Toxicology, Toxicology Letters, Poultry Science and Avian Diseases were monitored for relevant publications on MON up to 31 May 2017, and relevant publications were identified. This outcome was confirmed by a second systematic literature search for the time span from 1 January 2016 to 29 May 2017.
2.2.2. Appraisal of evidence for hazard identification and characterisation
The retrieved evidence was reviewed by the CONTAM WG on Fusarium toxins and has been used for this assessment as considered relevant by expert judgement. Any limitations noted by the WG in the evidence used for the risk assessment of MON in food and feed are described in this scientific opinion. Selection of the scientific papers considered study quality and the extent to which the study was relevant (e.g. sufficient details on the methodology, performance and outcome of the study, information on dosing and route of administration and details of reporting).
The amount of available data on MON for different sections of the assessment varied greatly. In a first step, only those data from which it could clearly be concluded that the adverse effects in experimental and farm and companion animals were associated with an oral exposure to MON alone were included in the sections on hazard characterisation of humans and farm and companion animals. Second, papers reporting oral co‐exposure to MON and other mycotoxins were included when it was clear from the study description and content that the co‐exposure did not have a substantial impact on toxicity of MON: e.g. (1) when the other identified mycotoxins had concentrations that were not considered to induce or notably contribute to the observed adverse effects, or (2) when the other identified mycotoxins were known to have specific adverse effects which could not be attributed to MON, or (3) when the other identified mycotoxins were not expected to interact with the effects of MON.
Papers were excluded when the reported data were from experiments: (1) designed for using naturally contaminated feed in which not only MON but also other Fusarium toxins such as fumonisins, beauvericin or other mycotoxins not produced by Fusarium species were (or might have been) present in the diet, or (2) the diets were prepared from fermented grains contaminated with Fusarium strains producing MON and other mycotoxins. Papers on studies in which feed was artificially contaminated with pure MON or with added Fusarium culture material reported to contain MON alone were considered as of providing useful information on the adverse effects induced by MON.
With regard to the evidence on toxicokinetics in experimental animals, the CONTAM Panel decided to report the available ex vivo data under the header of in vitro data emphasising clearly the nature of each experiment and which information was considered.
For the study of combined effects of MON with other mycotoxins (Section 3.1.6), only those studies were included in the assessment in which the experiment design was clearly set up to study combined effects and in which methodologically sound conclusions were substantiated and reported on the combined effects.
In this opinion, if not indicated in the text explicitly, the term ‘significant’ always indicates the presence of statistical significance at the level of 0.05.
2.3. Occurrence data on MON used for the assessment
2.3.1. Data collection and validation
Following an European Commission mandate to EFSA, a call for annual collection of chemical contaminant occurrence data in food and feed, including MON, was issued by the former EFSA Dietary and Chemical Monitoring Unit (now DATA Unit)7 in December 2010 with a closing date of 1 October of each year.8 European national authorities and similar bodies, research institutions, academia, food business operators and other stakeholders were invited to submit analytical data on MON in food and feed. The data for the present assessment were provided by national authorities from Finland, Italy, the Netherlands, Norway, Sweden and the United Kingdom (UK).
The data submission to EFSA followed the requirements of the EFSA Guidance on Standard Sample Description for Food and Feed (EFSA, 2010a); occurrence data were managed following the EFSA standard operational procedures on ‘Data collection and validation’ and on ‘Data analysis of food consumption and occurrence data’.
In the data validation phase, data reported as suspect samples9 were excluded from the present analysis. Suspect samples are usually samples taken from the same site as a consequence of evidence or suspicion of contamination, and are often taken as a follow‐up of demonstrated non‐compliance with legislation. Some of the remaining samples may also have been collected in a more targeted way (i.e. selective sampling, convenient sampling)10 (see also Section 2.3.2).
By the end of October 2016, 2,800 analytical results of food (including one result from suspect sampling to be excluded) and 528 results of unprocessed grains of undefined end‐use (including 24 from suspect sampling to be excluded) with analytical data on MON were available in the EFSA database. Data received after the 31 of October 2016 were not included in the data set used for further evaluation for this opinion.
In addition to the occurrence data collected from the Member States within the call for data, the CONTAM Panel also searched the published literature for occurrence data of MON in food and feed for possible inclusion as additional data in the occurrence data sets submitted to EFSA within the call for data and to be used for the exposure assessment. The literature data were included when they conformed to the most important EFSA requirements on data collection and validation, and the details on country of origin, product, sampling, analytical method, LODs/LOQs and occurrence levels (e.g. mean, median) were adequately reported. As an outcome of this exercise, additional occurrence data on MON in food (i.e. 406 analytical results) were obtained from the scientific literature (Jestoi et al., 2004; Van der Fels‐Klerx et al., 2012; Lindblad et al., 2013; Scarpino et al., 2013; Uhlig et al., 2013). Additional occurrence data on MON in feed (i.e. 191 analytical results) were obtained from the scientific literature (Goertz et al., 2010; Van der Fels‐Klerx et al., 2012). Furthermore, in the beginning of the development of this opinion, it was brought to the attention of the CONTAM Panel that two research groups at research institutes in Austria and Norway wished to provide recent occurrence data on MON in feed to EFSA. These feed data on MON of 380 analytical results from Austria and 235 analytical results from Norway were also included in the occurrence data set.
After excluding 25 suspect samples, a total of 4,515 analytical results (i.e. 3,205 on food, 806 on feed and 504 on unprocessed grains of undefined end‐use) were available for the exposure assessment analysis.
2.3.2. Data analysis
Following the EFSA SOP on ‘Data analysis of food consumption and occurrence data’ to guarantee an appropriate quality of the data used in the exposure assessment, the initial data set was carefully evaluated applying several data cleaning and validation steps. Special attention was paid to different parameters such as ‘Sampling strategy’, ‘Sampling method’, ‘Sampling year’, ‘Sampling country’, ‘Analytical methods’, ‘Reporting unit’, ‘LOD/LOQ’ and the codification of the different samples under FoodEx classification. Non‐targeted sampling (i.e. objective sampling11) had been applied for the samples from Austria and Norway, and it was interpreted that the non‐targeted sampling was also used for the data collected from the literature.
In the analysis of MON occurrence data, the left‐censored data (results below LOD or below LOQ) were treated by the substitution method as recommended in the ‘Principles and Methods for the Risk Assessment of Chemicals in Food’ (WHO, 2009). The same method is indicated in the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010b) as an option in the treatment of left‐censored data. The guidance suggests that the lower bound (LB) and upper bound (UB) approach should be used for chemicals likely to be present in the food (e.g. naturally occurring contaminants, nutrients and mycotoxins). The LB is obtained by assigning a value of zero (minimum possible value) to all samples reported as lower than the LOD (< LOD) or LOQ (< LOQ). The UB is obtained by assigning the numerical value of LOD to values reported as < LOD and LOQ to values reported as < LOQ (maximum possible value), depending on whether LOD or LOQ is reported by the laboratory.
2.4. Food consumption data
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database) provides a compilation of existing national information on food consumption at individual level. It was first built in 2010 (EFSA, 2011a; Huybrechts et al., 2011; Merten et al., 2011). Details on how the Comprehensive Database is used are published in the Guidance of EFSA (EFSA, 2011b). The latest version of the Comprehensive Database updated in 2015 contains results from a total of 51 different dietary surveys carried out in 23 different Member States covering 94,532 individuals.
Within the dietary studies, subjects are classified in different age classes as follows:
Infants: < 12 months old
Toddlers: ≥ 12 months to < 36 months old
Other children: ≥ 36 months to < 10 years old
Adolescents: ≥ 10 years to < 18 years old
Adults: ≥ 18 years to < 65 years old
Elderly: ≥ 65 years to < 75 years old
Very elderly: ≥ 75 years old
Two additional surveys provided information on specific population groups: ‘Pregnant women’ (≥ 15 years to ≤ 45 years old; Latvia) and ‘Lactating women’ (≥ 28 years to ≤ 39 years old; Greece).
For chronic exposure assessment, food consumption data were available from 44 different dietary surveys carried out in 19 different European countries. When for one particular country and age class two different dietary surveys were available, only the most recent one was used. This resulted in a total of 35 dietary surveys selected to estimate chronic dietary exposure. In Appendix B, Table B.1, these dietary surveys and the number of subjects available for the acute and chronic exposure assessment are described.
Table B.1.
Live weights, growth rate/productivity, dry matter intake for cattle, sheep, goats and horses, and the proportions of the diet as non‐forage
| Animal species (diet) | Live weight (kg) | Growth rate or productivity | Dry matter intake (kg/day) | % of diet as non‐forage feed | Reference |
|---|---|---|---|---|---|
| Dairy cows: grass‐based diet | 650 | 40 kg milk/day | 20.7 | 40 | OECD (2009) |
| Dairy cows: maize silage‐based diet | 650 | 40 kg milk/day | 20.7 | 45 | AFSSA (2009) |
| Beef cattle: grass silage‐based dieta | 400 | 1 kg/day | 9.6 | 15 | AFRC (1993) |
| Beef cattle: cereal‐based diet | 400 | 1.4 kg/day | 10 | 85 | |
| Fattening cattle: maize silage‐based ration | 300 | 1.4 kg/day | 6.6 | 25 | Browne et al. (2004) |
| Fattening cattle: cereal straw‐based diet | 300 | 0.9 kg/day | 8.0 | 68 | EBLEX, 2008 |
| Sheep: lactating | 80 | Feeding twin lambs | 2.8 | 50 | OECD (2009) |
| Goats: lactatingb | 60 | 6 kg milk/day | 3.4 | 65 | NRC (2007a) |
| Horses | 450 | Moderate activity | 9.0 | 50 | NRC (2007b) |
Housed castrate cattle, medium maturing breed.
Months 2–3 of lactation.
The food consumption data gathered by EFSA in the Comprehensive Database are the most complete and detailed data currently available in the EU. Consumption data were collected using single or repeated 24‐ or 48‐hour dietary recalls or dietary records covering from three to seven days per subject. Because of the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading.
2.5. Food classification
Consumption data were classified according to the FoodEx classification system (EFSA, 2011c). FoodEx is a food classification system developed by EFSA in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing the exposure to hazardous substances. It contains 20 main food categories (first level), which are further divided into subgroups having 140 items at the second level, 1,261 items at the third level and reaching about 1,800 end‐points (food names or generic food names) at the fourth level.
In 2011, a new version of FoodEx, named FoodEx2 was developed and is described in the scientific document ‘Report on the development of a Food Classification and Description System for exposure assessment and guidance on its implementation and use’ (EFSA, 2011c). The last release of FoodEx2 complements the previous hierarchical classification system of basic codes with more detailed food levels and gives the possibility of reporting additional information through the use of facets and facet descriptors (EFSA, 2015).
2.6. Feed consumption data
MON is predominantly found in cereal crops, cereal grains and in by‐products of cereal processing, all of which are widely used as feed for farm animals in Europe. They may be included as ingredients of manufactured complete feedingstuffs, or fed directly as individual feeds to livestock. In 2015, more than 90 million tonnes of cereals and cereal by‐products were used in the manufacture of compound feeds, accounting for 60% of all feed materials used, almost all of which (> 95%) are grown or produced in the EU.12 In addition, a further 51 million tonnes of cereal grains and by‐products were fed in on‐farm mixes or as single ingredients. However, there are no industry data on the partition of these cereal grains between livestock species (cattle, pigs, poultry, etc.).
There is considerable variation in both the feeds used and the feeding systems adopted for farm livestock, companion animals and fish throughout Europe. This variation is largely due to the availability of feeds and market demands for specific animal products, the quality of the feeds available and nutritional needs of the animals concerned.
Details of feed consumption of farm and companion animals and the rations used in this opinion are given in Appendix B.
2.7. Methodology for exposure assessment for MON
2.7.1. Methodology for exposure assessment for MON in humans
The CONTAM Panel considered it appropriate to estimate acute and chronic exposure to MON for all age groups (see Section 3.5). The food categories represented by either very low number of samples (≤ 5 samples) or by all data left‐censored were considered not being suitable and were not used in exposure calculation.
As reported in Section 3.3.2, quantified results were reported for ‘Grains and grain‐based products’ and for ‘Snack, desserts and other foods’. The proportion of left‐censored data was 80% for ‘Grains and grain‐based products’, 25% for ‘Snack, desserts and other foods’ and 100% for the other food categories. The chronic dietary exposure cannot be performed accurately if a large proportion of left‐censored data is included (WHO, 2009; EFSA, 2011b). Therefore, the large proportion of left‐censored data and the limited available data add uncertainty to the chronic dietary exposure assessment. Since this was the case for most of the food categories, the results of the present assessment should be interpreted with caution. It should be noted that with a high proportion of left‐censored data, the exposure is likely to be underestimated with the LB approach, whereas it may be highly overestimated with the UB approach (see also Section 4).
2.7.1.1. Acute dietary exposure
Acute dietary exposure to MON was estimated using a probabilistic approach. For calculating acute dietary exposure to MON, food consumption and body weight data at the individual level were accessed in the Comprehensive Database. The acute dietary exposure to MON was calculated for each reporting day, since individual meals are recorded for only a few countries in the consumption database. The preferred option is, therefore, to use individual days of consumption. Days of consumption offer a conservative estimate of the exposure, since it will sum the contribution of all meals during the same day. Acute exposure was assessed for each reporting day by multiplying the total consumption amount for each food category by an occurrence level randomly drawn among individual results available for that food category. Respective intakes of the foods consumed that day were summed and finally divided by the individual's body weight. This process was iterated 100 times for each day of consumption reported by each participant. For the calculations, occurrence data estimated using the UB approach was used. The UB approach is a conservative approach which better reflects the purpose of an acute exposure compared to the LB approach. For each of these endpoints, the 95% confidence interval was defined as the 2.5th and 97.5th percentiles obtained from the 1,000 iterations. All analyses were run using the SAS Statistical Software (SAS enterprise guide 5.1), including the modelling of the probabilistic acute exposure.
2.7.1.2. Chronic dietary exposure
As suggested by the EFSA WG on Food Consumption and Exposure (EFSA, 2011b), dietary surveys with only 1 day per subject were not considered for chronic exposure as they are not adequate to assess repeated exposure. Similarly, subjects who participated only 1 day in the dietary studies, when the protocol prescribed more reporting days per individual, were also excluded for the chronic exposure assessment. Not all countries provided consumption information for all age groups, and in some cases the same country provided more than one consumption survey. For calculating chronic dietary exposure to MON, food consumption and body weight data at the individual level were accessed in the Comprehensive Database. Occurrence data and consumption data were linked at the lowest (most detailed) FoodEx level possible. In addition, the different food commodities were grouped within each food category to better explain their contribution to the total dietary exposure to MON.
The mean and the high (95th percentile) chronic dietary exposures were calculated by combining MON mean occurrence values for food samples collected in different countries (pooled European occurrence data) with the average daily consumption for each food at individual level in each dietary survey and age class. Consequently, individual average exposures per day and body weight were obtained for all individuals. On the basis of distributions of individual exposures, the mean and 95th percentile exposure were calculated per survey and per age class. Dietary exposure was assessed using overall European LB and UB mean occurrence of MON.
The contribution (%) of each food category to overall mean chronic exposure of MON was calculated for each age group and dietary survey. Estimations of chronic exposure using the LB approach, which is considered to be less influenced by results below LOD/LOQ, were used to explain the contribution of the different food categories.
All analyses were run using the SAS Statistical Software (SAS enterprise guide 5.1).
2.7.2. Methodology for exposure assessment for MON in farm and companion animals
MON generally occurs in cereals crops, cereal grains and by‐products of cereal processing, both for human food and biofuel production, and as reported in Section 3.3, these may account for 60% or more of the diet of farm and companion animals. Diets do include a wide range of other feed materials, particularly vegetable proteins, but since no data are available on levels of MON in these feeds, it has been assumed that they make no contribution to exposure to MON.
No data on levels of MON in compound feeds have been reported. For forages, data on concentrations of MON in 42 samples of maize silage were provided for the EFSA database (see Section 3.3.2.3). Limited data were available for cereal straws, but levels of MON are generally low while the number of samples reported is insufficient to include the data in estimates of exposure.
A large variety of feed materials are used to formulate diets for livestock and companion animals in the EU, and information on levels of MON in these feeds is necessary if reliable estimates of exposure are to be derived. However, as reported in Section 3.3, only data on the major cereal grains (wheat, barley, oats, rice and maize), and limited data for maize silage and cereal straw, have been available to assess exposure. As a result, the estimates reported below are likely to be underestimates of exposure. For certain categories of feeds, e.g. oilseed meals and cakes, the effect of this omission is likely to be small, since the crops from which these are derived, and the feeds themselves, have not been reported to be sources of MON. However, by‐products derived from cereal grains are also widely used as animal feeds. In 2015, it was estimated that over 17 million tonnes of cereal by‐products were used in the manufacture of compound feeds,13 representing 11% of all ingredients used, and therefore, the absence of data on levels of MON in these feed materials on the underestimation is likely to be greater.
Estimates of exposure to MON by farm and companion animals are based on levels of MON in feed and the amount of feed consumed. For many livestock in Europe, part or all of the daily ration is provided in the form of manufactured compound feeds, but for this opinion data on levels of MON in species‐specific compound feeds were not available. Therefore, intakes of individual feed materials by farm and companion animals, using example diets (Appendix B), have been used to estimate exposure. It should be stressed that these do not represent either ‘average’ or ‘extreme’ diets, nor are the feeding systems ‘typical’ for all of Europe. Instead, the diets are used to estimate levels of exposure to MON that might be indicative. They are based on published guidelines on nutrition and feeding (AFRC, 1993; Carabano and Piquer, 1998; NRC, 2007a,b, Leeson and Summers, 2008; McDonald et al., 2011; EFSA FEEDAP Panel, 2012; OECD, 2013), and expert knowledge of production systems in Europe. Details of the rations used feed intakes and live weights assumed are given in Appendix B.
For all species, the mean and 95th percentile (high) exposures have been estimated based on the mean and the 95th percentile LB and UB concentrations, respectively. According to EFSA, 2011c, caution is needed when calculating acute exposure (95th percentile) where data on less than 60 samples are available, since the results may not be statistically robust. Therefore, in this Opinion estimates of the 95th percentile have not been made where data on < 60 samples are available. It should be noted that the estimates at the 95th percentile concentrations were calculated in order to characterise the farm and companion animals health risk associated with chronic dietary exposure to MON when high concentrations of MON are found in the feed, e.g. due to favourable growing season for MON production.
2.8. Methodology for risk characterisation
The CONTAM Panel applied the general principles of the risk assessment process for chemicals in food as described by WHO (2009), i.e. hazard identification and characterisation, exposure assessment and risk characterisation. Several EFSA guidance documents were applied in the assessment of MON in food and feed listed in Appendix A.
3. Assessment
3.1. Hazard identification and characterisation
3.1.1. Toxicokinetics in experimental animals and humans
In vitro studies
Behrens et al., 2015, studied the transfer of MON through a monolayer of primary porcine brain capillary endothelial cells as a model for a blood–brain barrier. The transfer of MON across the monolayer was 1.07 × 10−6 cm/s in 48 h, which was about four times the transport of the negative control sucrose (0.23 × 10−6 cm/s). In addition, the authors incubated the cell system with equimolar concentrations of MON (200 nM) on both the apical and basolateral side of the monolayer for 48 h. At the two sides, no significant differences in concentrations were observed during the 48 h and the authors concluded that MON is not a substrate for the efflux proteins since the toxin was not enriched in any compartment.
In vivo studies
Only two in vivo studies on the toxicokinetics of MON were identified.
Urine and faeces were collected 24 h pre‐exposure and 6, 12, 24, 48, 168 and 336 h after the single oral dosing of Sprague–Dawley rats (n = 3 per group) with 5 mg MON/kg bw (Jonsson et al., 2013). The study was designed according to OECD guideline 42314 and is described in Section 3.1.4 below. During the first 6 h, a mean of 38% of the total administered MON was recovered from the urine, which increased up to 42% at 24 h after exposure. MON was detectable in the urine from 24–48 h post‐dosing but not at the remaining collection times of 168 h (7 days) and 336 h (14 days) with LOD/LOQ of 0.4/0.9 μg/mL. Less than 1% of the administered MON was recovered in faeces (same LOD/LOQ as in urine). Animals at higher doses survived much less than 14 days and excretion data were not reported. However, the authors noted that the fate of more than 50% of the administered MON remained unknown and might have accumulated in body compartments or, more likely been biotransformed or degraded to metabolic products.
Five groups of Sprague–Dawley rats were exposed by gavage once per day for 28 days at the dose of 3, 6, 9, 12 and 15 mg MON/kg bw using the OECD guideline 40715 (Jonsson et al., 2015) (see details of the study in Section 3.1.4). Three animals per dose group were kept in metabolic cages, and faeces and urine samples were collected. All urine and faeces samples were analysed daily for the first week. Samples were collected on days 1, 3 and 6 in the following weeks. In the first week, the administered dose of MON recovered in urine during the following 24 h ranged from 21% to 37%. The daily excretion remained between 20% and 32% of the daily dose during the following weeks with no significant difference between dose groups. MON was not detected in the two satellite groups16 during 14 days post‐dosing. Less than 2% of the administered dose of MON was recovered in faeces during the overall period of the study and only minor traces (not quantified by the authors) of MON conjugates were found in the urine samples. The fate of more than 60% of the administered MON remained unknown in the study. The tissue concentrations were not measured. The authors of the paper speculated that in the animals MON might be biotransformed and then excreted in urine to some unknown form, e.g. by an opening of the so called ‘squaric’ ring structure of MON and carboxylation by a carboxylase into CO2 and possibly to acetate.
Conclusions
Limited data from two studies indicate that a large portion of MON was rapidly absorbed and excreted after administration with no apparent accumulation in any tissue. The urinary recovery was 20–37%, while less than 2% of the administered MON was recovered from faeces. However, the fate of more than half of the amount of MON ingested remained unknown.
3.1.2. Toxicokinetics in farm and companion animals
No data on toxicokinetics were identified for ruminants, pigs, poultry, farmed rabbits, farmed fish, horses, farmed mink, dogs and cats.
3.1.3. Transfer
Data on transfer were identified in poultry only. A trial was carried out by Zollitsch et al. (2003) using a total of 180 one‐day‐old broiler chickens. Maize grain naturally contaminated with deoxynivalenol was inoculated by F. subglutinans producing beauvericin and MON to generate contaminated maize feed material. This material was mixed with uncontaminated maize grain at different percentages to prepare the diets at levels of 0, 0.9, 1.8 and 2.7 mg MON/kg feed. No residues of MON were detected in carcass (muscles from breast, legs and wings) and selected internal organs (heart, liver, bursa of Fabricius and spleen).
The only available study on MON indicated no transfer from feed to food products of animal origin in broiler chickens. For other farm animals, no information on transfer from feed to food products of animal origin was identified.
3.1.4. Toxicity in experimental animals
3.1.4.1. Acute toxicity
The CONTAM Panel identified four studies to characterise the acute oral toxicity of MON in rodents with LD50 values which are summarised in Table 2.
Table 2.
Acute toxicity studies associated to oral exposure of MON in rodents
| Species (gender) | Origin and purity of MON | Doses tested (mg/kg bw) | LD50 (mg/kg bw) | Reference |
|---|---|---|---|---|
| White mice (breed not specified) (female) | Purified from Fusarium extract, purity not reported | 0, 10, 20, 40 and 80 |
47.6 95% CI: 34.6–67.2 |
Burmeister et al. (1980) |
| Rats, inbred BD IX black (male and female) | Purified from Fusarium extract, stated as ‘pure’ by the authors | 0, 25, 40, 63 and 100 |
Male: 50.0 95% CI: 38.5–64.9 Female: 41.6 95% CI: 33.1–52.1 |
Kriek et al. (1977) |
| Rats, Sprague–Dawley and Wistar (female) | Purified from Fusarium extract, 99% purity | 0, 2.5, 5, 10, 20, 40, 60, 80 and 100 |
18.5a Lower bound: 13.1 |
Abbas et al. (1990) |
| Rats, Sprague–Dawley (male) | Synthetic potassium salt of MON, 99% purity | 0, 5, 10, 25, 40 and 50 |
25 LD50 cut‐off valueb |
Jonsson et al. (2013) |
bw: body weight; LD50: oral median lethal dose.
No effects observed until 20 mg/kg bw where 4/5 rats died. At all higher doses 100% mortality was reported. The LD50 was calculated by the CONTAM Panel applying the BMD approach with a BMR of 50% (see Appendix C.1) with a lower 95% confidence bound of 13.1 mg MON/kg bw.
LD50 cut‐off value determined according to the Globally Harmonized System (GHS) for the classification of chemicals that cause acute toxicity.
Mice
White mice were exposed to MON by intragastric intubation (0.5 mL of MON diluted in water at doses equal to 0, 10, 20, 40 or 80 mg/kg bw as reported by the authors (number of mice per group not reported). Deaths occurred within 12 h and survivors appeared to be healthy for 14 days after dosing. An oral LD50 of 47.6 mg/kg bw for MON in white mice was calculated by Burmeister et al. (1980), with a 95% confidence interval of 95% (CI: 34.6–67.2 mg/kg bw).
Rats
The oral LD50 of chemically pure MON in diet was 50.0 and 41.6 mg/kg bw, in male and female inbred BD IX black rats, respectively (Kriek et al., 1977). The authors stated that MON was chemically pure but did not report the percentage of purity. The administered single doses were 25, 40, 63 and 100 mg MON/kg bw, including a control group. Deaths occurred within 3 h and the authors reported for those rats, in particular rapidly progressive muscular weakness, respiratory distress and terminal coma, also sternal recumbence and abdominal respiration. At autopsy, generalised congestion and cyanosis and effects on the liver, pericardium and thorax were also observed. Survivors recovered, apart from mild myocardial lesions, to clinical normality within 12 h. The authors noted that severity of histological lesions varied between the four dose groups. They provided comprehensive qualitative histopathological data illustrating dose‐dependent toxicity, but no quantitative dose–response data on the total of 50 animals used to calculate the two LD50 values.
In 20‐day virgin female rats, mainly of the Sprague–Dawley rats mixed with some Wistar rats (weight of the animals not reported), gastric intubation of MON induced haemorrhage of the small intestine and lead to death within 16 h in 5 of 5 rats exposed to 40, 60, 80 or 100 mg MON/kg bw and in 4 of 5 rats exposed to 20 mg/kg bw (Abbas et al., 1990), but no adverse effects were observed in the groups of 5 rats exposed to 0, 2.5, 5 or 10 mg MON/kg bw. Applying the benchmark dose (BMD) approach with a BMR of 50%, the CONTAM Panel identified an LD50 of 18.5 mg/kg bw for rats from this study with a lower 95% confidence bound of 13.1 mg/kg bw based on the fit of the log‐logistic model (see Table 2). These results were supported by a BMD50 interval of 13.5–17.6 mg/kg bw obtained from model averaging which is, however, not an established method for acute toxicity data. For details, see Appendix C.
The acute oral toxicity of MON was assessed in Sprague–Dawley male rats administered by gavage according to OECD Guideline 423 by Jonsson et al. (2013), applying the Globally Harmonized System (GHS) for the classification of chemicals which cause acute toxicity. The stepwise procedure started with the low dose of 5 mg MON/kg bw and the high dose of 50 mg MON/kg bw each administered to rats (n = 3), the latter expected to produce mortality. Three additional rats (only one per group used to reduce the number of experimental animals) were exposed to 10, 25 or 40 mg MON/kg bw to assist to assess the appropriate dose levels for future studies on the subacute toxicity of MON. Health condition of the animals was monitored at least twice a day for the next 13 days and complete necropsy with evaluation of macroscopic changes was performed for each rat in major organs, most detailed in the high‐dose group. At predefined time points (24 h pre‐exposure, 6 h, 12 h, and 1, 2, 7 and 14 days post‐exposure), urine and faeces samples were collected (see Section 3.1.1 above). The observed outcomes were:
The three animals in the high‐dose group of 50 mg MON/kg bw died at 48, 60 and 83 min post‐administration, respectively. These rats showed signs of toxicity such as decreased activity (at 5–10 min after exposure), altered body position, respiratory and cardiovascular changes (including faint heart beats in two and cardiac arrhythmia in one animal) and muscular weakness. The heart was unevenly contracted in two rats and there was marked congestion in the liver. Microscopically, there was mild multifocal oedema and lymphocytic infiltration in the heart muscle in all three animals.
The one rat, at the dose of 40 mg MON/kg bw, showed muscular weakness, respiratory distress, cardiovascular changes and sudden death after 75 min, all observations attributable to cardiac arrest and general toxic signs such as decreased activity and altered body position.
The one rat at the dose of 25 mg MON/kg bw showed symptoms resembling those observed at the high dose and died after 60 min.
The surviving rat at the dose of 10 mg MON/kg bw showed also decreased activity, respiratory changes, trembling and piloerection up to 3 h post‐dosing.
No clinical signs were seen at the low dose of 5 mg MON/kg bw. The activity of animals was slightly decreased but it was similar in the control group. The authors related this to the 12‐h fasting period before the start of treatment.
Based on the GHS for the classification of chemicals (OECD Guideline 423), the authors concluded that MON was acutely toxic to rats and identified 25 mg MON/kg bw as cut‐off value of for the LD50.
Conclusions
MON showed high acute toxicity in rats with oral LD50 values ranging between 18.5 and 25 mg MON/kg bw per day in experiments with 99% pure MON. The oral acute toxicity in one study in mice was lower with an LD50 of about 50 mg MON/kg bw per day.
3.1.4.2. Subacute toxicity
Only studies on cardiotoxicity were identified in the available literature.
Mice
In addition to the experiment on the acute toxicity (see Section 3.1.4.1), Burmeister et al. (1980) exposed young white mice in groups of n = 7 for 3 weeks to MON dissolved in distilled water at concentrations of 0, 0.1 and 0.5 mg/mL (initial weights ranging from 13.5 to 19.9 g) consumed on average 0, 0.7 and 2.9 mg MON daily in drinking water, respectively. All of the animals survived with an average daily water consumption and average weight gain to be calculated as of 7.0, 7.2 and 5.8 mL and 7.8, 7.2 and 5.8 g for the three groups, respectively (statistically significant reduced only in the high‐dose group based on an analysis of variance). The authors reported a slight but insignificant reduction in weight gain at the low concentration but a significant one at the high when compared to controls. The consumption of MON at the high concentration of 0.5 mg/mL was estimated to be 2.9 mg MON per day which was, however, three times higher than estimated consumption of 1.0 mg MON /per day at the LD50 of 47.6 mg MON/kg bw derived from the author's a subtrial on the acute toxicity, see Section 3.1.4.1 (assuming a bw of 20 g). The authors argued that the mice of 21‐day study either might have excreted MON readily or an efficient deactivating mechanism might occur since no deaths were observed. Because of the discrepancies between the two subtrials the CONTAM Panel did not consider these data for the risk assessment of MON.
Rats
A subacute oral toxicity study was conducted in Sprague–Dawley rats (n = 5 per group, 9–10 weeks old, 217–307 g), adapting the OECD guideline 407 (Jonsson et al., 2015). Groups of five male rats were daily exposed by gavage to 0, 3, 6, 9, 12 or 15 mg MON/kg bw per day for 28 days. The synthetic potassium salt of MON (purity > 99.8%) was used. Each animal was subject to a comprehensive check of its health conditions twice a day including movement, behaviour, respiratory, circulatory functions and excretion. Two satellite groups were treated identically with the two experimental groups at the two highest doses (12 and 15 mg MON/kg bw per day) and were kept alive for additional 14 days without treatment to study the potential reversibility of the adverse effects observed in the animals of the main part of the study. Complete necropsy was performed for each rat with macroscopic examination of all organs. Tissue samples were collected from the liver, spleen, kidneys, lungs, adrenal glands, thymus, heart, stomach, intestine, testicles and brain. There were no treatment‐related differences between control animals, treated animals or animals of the satellite groups for body weight, feed and water consumption, organ weights or measured blood parameters (such as red blood cell counts (RBC), white blood cell counts, haemoglobin (Hb), haematocrit (HCT) and platelet counts). Within the first 3 weeks after start of treatment, the mean excretion of MON in urine varied between the five dose groups ranging from 20% to 36% however without showing a clear dose dependence. There was also considerable interindividual variability per dose group with standard deviation (SD) values ranging between 5% and 13%). The authors noted that about 15% of the rats showed decreased excretion to less than 5% over a few days before they resumed excretion to normal levels possibly due to intersubject variation of metabolic enzyme activity. Overall, total mean excretion remained around 30% during for the first 3 weeks but decrease after 4 weeks to about 20%. Decreased phagocytic activity of neutrophils (luminol‐amplified chemiluminescence assay) was observed in all treated groups, including the two satellite groups and this activity did not recover. Compared with the control group, the decrease was 59% at the lowest dose and on average 48%. However, the number of neutrophils and the total number of leucocytes stayed within normal ranges and weights of lymphoid organs (thymus and spleen) remained unaffected such that the authors excluded dystrophic and dysplastic effects for MON. The CONTAM Panel noted that the neutrophil scoring method was not described and that the assay was performed on whole blood dilution. There was also a large variability of neutrophil counts in the control group (15 ± 12.5%; expressed by dividing the whole blood activity by neutrophil number) and the potential macrophage phagocytic activity was not taken into consideration in this calculation. Therefore, the CONTAM Panel decided not to consider these data for human hazard characterisation of MON.
In contrast, the CONTAM Panel summarised the clinical observations in the five experimental dose groups of 3, 6, 9, 12 or 15 mg MON/kg bw per day over the 28 days in comparison with the controls as follows:
Two rats died at the highest dose and at 12 MON/kg bw per day one rat died and one was euthanised due to respiratory distress immediately after gavage, among the 36 rats in the experimental groups, before the end of the study.
The two rats in the experimental group of 15 MON/kg bw per day died with acute heart failure on the 4th and 21st day after presenting strongly decreased activity or somnolence. They had a normal nutritional condition, however macroscopically, some blood‐stained contents were observed in the small intestine in one of them, although the gastrointestinal contents were otherwise normal. Microscopically, there was an acute congestion and leucostasis in the lungs. The other rat had no specific macroscopic or microscopic organ changes.
The two animals with premature deaths at the next lower dose of 12 MON/kg bw per day exhibited acute pulmonary congestion. The rat that died had notably little digesta in the stomach. The CONTAM Panel noted that the sacrifice of the one rat because of signs of respiratory distress just after gavage could be due to a technical error in administration by gavage when MON suspension reaches the airways. This suspicion of the CONTAM Panel is supported by dyspnea, wheezing and body secretion from nostrils or mouth in 7 of the 35 treated rats noted by the authors.
One rat in the 9 mg MON/kg bw dose group showed repeatedly symptoms (decreased activity, withdrawal and somnolence), which were similar to those seen at the two highest doses, but recovered until the end of the study.
At the three highest doses of 9–15 mg/kg bw (no dose‐specific information reported) 23% of the animals had a weak grip at the front legs compared to controls and to rats exposed to 3 MON mg/kg bw per day which showed normal grip. Grip strength at 6 MON mg/kg bw per day was not specified.
Otherwise, no other adverse health effects were reported for the doses up to 9 mg MON/kg bw. These animals were reported as clinically healthy without impairment of weight gain. A transient decrease in activity and occasionally respiratory changes and bloody nostril secretion was reported for a total of eight rats (20%) of all dose groups but also for controls; without detailed dose information given.
Accounting for the effects seen in one rat at 9 mg MON/kg bw dose the CONTAM Panel considered that at doses of 9 mg/kg bw per day and higher acute heart failure cannot be excluded for rats exposed to MON and identified 6 mg/kg bw per day as a NOAEL for adverse health effects of MON from this subacute study.
Conclusions
Very few subacute studies on MON were available in the literature. MON was well tolerated in a 28‐day study on rats at doses lower than 9 mg/kg bw per day, and a NOAEL of 6 mg/kg bw per day was identified. Cardiotoxicity was observed at 15 mg/kg bw per day and could not be excluded at doses as low as at 9 mg/kg bw per day.
3.1.4.3. Subchronic toxicity
Only two in vivo subchronic toxicity studies on MON in rats were identified.
Rats
Groups of four male and four female inbred BDIX black rats were fed ad libitum for 12 weeks with feed containing 0%, 2%, 4%, 8%, 16% and 32% of mouldy feed contaminated with Fusarium culture material producing MON (Kriek et al., 1977). The diet was not analysed for other mycotoxins, but the CONTAM Panel noted that the presence of MON was characterised by appropriate analytical techniques available at the time. All 16 animals fed with the two highest %‐proportions of mouldy feed in their diets died early (at concentration of 16% within 15–18 days and at 32% concentration within 11 days). Feed consumption of the controls and the groups given 2%, 4% or 8% of mouldy feed was determined over a 5‐day period (from day 15 to day 20) and intake decreased with increasing concentration. At 0%, 2%, 4% and 8% of mouldy feed mean feed intake was 19.1, 17.6, 18.2 and 6.9 g/day and 14.7, 12.9, 11.3 and 5.3 g/day for male and female rats, respectively. From these data and the available mean weights per %‐proportions of mouldy feed in the diet and sex (also declining with increasing %‐proportions of mouldy feed in the diet), the authors calculated doses of 0, 16.6, 34.6 and 32.5 mg MON/bw per day and 0, 16.5, 32.9 and 34.5 mg MON/bw per day for males and females, respectively. Early deaths prevented the calculation of doses at the two highest concentrations. The CONTAM Panel noted that the feed intake at the level of 8% of mouldy feed in the diet was drastically reduced such that practically only two different dose regimes were applied in this experiment, one around 17 mg MON/kg bw and another between 32 and 35 mg MON/kg bw. Mortality was high in male and females at the 8% mouldy feed (4 males and 3 females died with mean time‐to‐death of 49 and 42 days, respectively) and at 4% mouldy feed in males (3 males died mean time‐to‐death of 62 days) but no deaths were among the females at that concentration, nor among females and males at the 2% mouldy feed concentration. The most conspicuous and prominent effect in all exposed groups were lesions in the myocardium and depending on survival time myocardial degeneration, necrosis and fibrosis were evident.
Rats exposed to 32% mouldy feed in the diet showed acute degenerative lesions with pronounced granularity of the sarcoplasm and focal Zenker's degeneration and necrosis in 3 of the 8 animals, in particular, in papillary muscles and left ventricular (LV) subendocardium.
Similar lesions were seen at the exposure to 16% mouldy feed in a diet with more prominent Zenker's necrosis.
Rats exposed to 8% mouldy feed in the diet showed widely distributed Zenker's necrosis manifesting fragmentation and macrophage infiltration alternating with focal areas of myolysis and fibrosis in the papillary muscles, LV subendocardium, right ventricular wall and intraventricular septum.
Abnormalities in rats which died when being exposed to 4% mouldy feed were similar to those at 8% mouldy feed however less extended in the surviving rats (1 male and 4 females). There were also focal Zenker's necrosis, macrophage aggregates, and focal areas of early myocardial fibrosis in 6 out of the total 8 rats.
Beyond the myocardial lesions mentioned above, no specific other lesions were described for exposure to 2% mouldy feed in the diet.
No comparable lesions were observed in the controls; also not in the survivors and in two of the three males exposed to 4% mouldy feed that died before 12 weeks.
Lesions in other organs than the heart were rated by the authors as fairly mild and commonly non‐specific and not differentiated regarding the doses. However, it was noted that in one male rat exposed to 4% mouldy feed/kg diet, focal haemorrhage occurred on the rugae in the gastric fundus and that severe generalised atrophy was observed in males and females exposed to 8% or 16% mouldy feed/kg diet. Generalised venous congestion and cyanosis were evident in all the animals that died. In two animals, the myocardium was characterised according to the authors by being ‘parboiled’. Furthermore, focal or linear erosions and mild haemorrhage in the rugae of the gastric fundus were observed. The two groups of males and females exposed to 32% mouldy feed/kg diet also showed mildly emphysematous lungs, focal pulmonary haemorrhage and generalised venous congestion. The CONTAM Panel concluded that the results of Kriek et al. (1977) were relevant for the hazard characterisation of MON since they showed a qualitative dose–response relationship of the severity of adverse cardiac effects of MON in rats over the range of exposures from 2 to 32% mouldy feed/kg diet. There was also a quantitative dose–response relationship of the mortality of the rats exposed up to 8% mouldy feed/kg diet. No quantitative dose–response data were identified for any other and/or possibly milder toxicity endpoint of this study. Since myocardial lesions were observed at the lowest exposure level tested, no NOAEL for adverse cardiac health effects was identified and the dose of 16.6 mg MON/kg bw in this study was identified as a LOAEL for the cardiotoxicity of MON. Furthermore, based on the association of the severity of cardiac toxicity with the mortality, the CONTAM Panel considered the mortality of the rats as directly related to the cardiac toxicity of MON and identified 16.6 and 32.5 mg MON/kg bw per day for male rats as NOAEL and LOAEL, respectively. Since the dose at which no female rat died (32.9 mg MON/kg bw per day) was very close to that at which three of the four females rats died (34.5 mg MON/kg bw per day), no NOAEL or LOAEL was determined for female rats. Overall, the toxicity of MON appeared to be lower in female rats compared to the males.
Conclusions
Only one 12‐week subchronic study in rats was available for hazard characterisation, in which treated rats were fed diets contaminated with MON at levels of 2–32% mouldy feed/kg diet. The CONTAM Panel noted a clear qualitative dose–response relationship of the severity of adverse cardiac effects of MON in rats over that range diet and identified 17 mg MON/kg bw as a LOAEL for the cardiotoxicity of MON. Considering mortality, the CONTAM Panel identified for male rats a NOAEL of 17 and a LOAEL of 33 mg MON/kg bw per day. Toxicity of MON in rats appeared to be lower in female than males.
3.1.4.4. Chronic toxicity
No data were identified.
3.1.4.5. Developmental and reproductive toxicity
Reproductive effects in mink exposed to MON were investigated by Morgan et al. (1998). In the reproductive study, groups of pastel, adult female mink (n = 12) were fed diets containing 0, 8.1 and 17.0 mg MON/kg feed, equivalent to 0, 0.92 and 1.94 mg MON/kg bw per day as calculated by the CONTAM Panel using the body weights and dry weight feed consumption data for female mink from NRC (1982).17 These data from NRC were considered by the CONTAM Panel as the most appropriate to use, although it should be noted that there is high uncertainty in the CONTAM Panel's calculated results. First, the NRC feed intake was given as dry matter and a conversion was applied (×3) for the feed in the Morgan et al. study. Second, minks in the study of Morgan et al. were pregnant which may affect feed intake. Treatment with MON was from 2 weeks prior to the breeding season, and continued until the offspring (no data on their food consumption reported) were 8 weeks old. There were no significant differences of feed consumption, body weights, breeding performance, gestation, litter size and offspring sex ratios among the groups of dams. The number of offspring that were stillborn or died within 24 h postpartum was significantly higher in the high‐dose group (41%) compared to the low‐dose group (7.3%) and the controls (6.1%). Significant effects on the body weights of offspring were observed. At birth, the offspring in the high‐dose group weighed significantly less than those from the control group. At 3 weeks, they weighed less than offspring from the low‐dose group. No offspring weight differences were seen at 6 weeks but at 8 weeks offspring from the high‐dose group weighed less than both the low‐dose and the control group. In the high‐dose group, the mortality of offspring increased markedly between 6 and 8 weeks, reaching 72.4% at 8 weeks. An increase in offspring mortality was not seen in the low‐dose or control groups. The CONTAM Panel considered that toxicity observed in the offspring at 6 weeks and above might not be reproductive effects as the offspring were offered the same experimental diet as their dams at 3 weeks of age. No gross or histological lesions or alterations in liver, lung or heart tissues were found in 8‐week‐old offspring from the high‐dose and control groups.
Conclusions
The dose of 1.94 mg MON/kg bw per day resulted in significant neonatal mortality and reduced offspring body weights in mink. The lowest dose of 0.92 mg MON/kg bw per day was identified as a NOAEL.
3.1.4.6. Genotoxicity
Wehner et al. (1978) showed that MON was inactive in the Salmonella Typhimurium bacterial mutation assay (Ames test) using strains TA98, TA100, TA1535 and TA1537 (0.25–250 μg MON/plate) with and without metabolic activation with an induced rat liver S9 fraction. Takahashi et al. (1992) also showed that MON (10 and 100 μg/plate) was inactive in the S. Typhimurium assay with TA100. MON was also subsequently tested by Knasmüller et al. (1997) using S. Typhimurium strains TA98 and TA100 (0.7–500 μg MON/plate) with and without S9, and it did not induce mutations. The CONTAM Panel was not able to use these data from Knasmüller et al. (1997) as there were inconsistencies in the reported results. For example, the positive control caused fewer mutations than the negative control, whereas MON caused a very high level of mutations at one dose.
MON was studied in differential DNA repair assays with Escherichia coli K‐12 strains (343/753, uvrB/recA and 343/765, uvr+/rec+) at concentrations ranging from 0.7 to 500 μg/mL. In the absence of metabolic activation, a significant effect of MON on induction of reparable DNA damage was found at concentrations of 55 μg/mL and above. Addition of S‐9 mix reduced this effect (Knasmüller et al., 1997). MON showed no activity in the rec assay with Bacillus subtilis (Ueno and Kubota, 1976).
Among the responses induced in E. coli by DNA‐damaging agents is a set of functions known as the SOS‐responses (Quillardet et al., 1982). The genotoxicity of MON in E. coli was investigated by Auffray and Boutibonnes (1986) using the SOS spot test, which is a plate diffusion assay to detect the SOS response. No activity was detected. Also, an SOS chromotest of MON was conducted in E. coli strain PQ 37 by Auffray and Boutibonnes (1987) and the compound showed no SOS inducing activity, either with or without metabolic activation. The maximal concentration of MON that was tested was 5.0 μg/mL. Knasmüller et al. (1997) also showed that MON did not show activity in the SOS chromotest with E. coli strain PQ 37 (5–500 μg MON per assay) with and without S9.
Isolated primary rat hepatocytes were exposed to MON (5.0–500 μM) and unscheduled DNA synthesis (UDS) was determined by autoradiography after incubation of the cells with [3H]‐thymidine (Norred et al., 1992). MON caused no effects on UDS at concentrations of 5, 10, 50 and 500 μM, and a marginal effect at 100 μM. MON was not cytotoxic at any of the concentrations studied. The CONTAM Panel considered MON not to be active in this assay.
Knasmüller et al., 1997; observed that MON (0.01–100 μg/mL for 3‐h) induced chromosome aberrations (CAs) in primary cultures of rat hepatocytes. The largest effects on CAs were seen at MON concentrations of 1 μg/mL and 10 μg/mL, where the CA rate was approximately nine‐fold over the background level. CAs declined at the higher concentration evaluated (100 μg/mL), possibly due to inhibition of cell division. The mitotic index decreased after treatment of the cells with MON, the difference becoming significant at the concentration of 100 μg/mL. Micronuclei (MN) were also studied in the same cells using the same concentration range of MON, and no statistically significant effects were observed (Knasmüller et al., 1997).
In the study of Celik et al. (2009), human peripheral blood lymphocytes were treated for 48 h with MON (sodium salt of MON) (2.5–25 μM equals to 0.3–3.0 μg/mL as calculated by the CONTAM Panel). CAs, MN and sister chromatid exchange (SCE) increased in a concentration‐dependent manner. Statistically significant effects on CAs were seen at MON concentrations of 1.2 μg/mL and above. The structural aberrations observed were chromatid breaks, which were most common, chromosome breaks and chromatid exchanges. Numerical aberrations (polyploidy) were also observed. SCEs and MN increased significantly at MON concentrations of 1.8 μg/mL and above. The cytokinesis‐block proliferation index was not affected by MON treatment.
The CONTAM Panel noted that mode of action of MON in the in vitro studies were not investigated. No in vivo investigations of genotoxicity were identified.
Conclusions
There is no evidence that MON induces bacterial reverse mutation. MON has been shown to be clastogenic in vitro inducing chromosomal damage (CAs, MN). No data were identified to conclude on whether in vitro genotoxicity is caused by a direct or indirect mechanism. No data are available on genotoxicity in vivo.
3.1.4.7. Carcinogenicity
No data were identified.
3.1.5. Adverse effects in farm and companion animals
3.1.5.1. Ruminants
The toxicities of several isolated Fusarium species with different toxin production capabilities were analysed by Lamprecht et al. (1986). Sheep (n = 1 per group, 6–10 months of age, 29–36 kg) were dosed by gavage with either 5 g culture material/kg bw per day or a single dose of 10 mg MON/kg bw in crystalline form (98% pure). The animals who received single doses of 21.0 or 24 mg MON/kg bw18 in culture material died within 2 and 18 h, respectively. The dose of 5.2 mg/kg bw per day in culture material resulted in death within in 48 h, and the administration of 10 mg crystalline MON/kg bw within 18 h. Culture material with small concentrations of MON (< 0.1 mg/kg bw per day) showed no effects on sheep. The culture material contained also small concentrations of diacetoxyscirpenol, but no zearalenone, deoxynivalenol and T‐2 toxin. The main pathological change observed in the perished sheep was the degeneration of the proximal tubules of the kidneys. The CONTAM Panel considered that these data were too scarce to characterise the hazard of MON in sheep. No other data on adverse effects of MON in ruminants were identified.
Conclusions
Among the ruminants, only one study on sheep was identified. A dose of 5.2 mg MON/kg bw per day resulted in death within in 48 h and a dose of < 0.1 mg MON/kg bw did not show any adverse health effects in sheep. However, the CONTAM Panel considered that the data were too scarce to characterise the hazard of MON in sheep.
3.1.5.2. Pigs
Only few studies on adverse effects of MON in pigs were available.
The effects of MON on the cardiac function in miniature pigs were studied by Qiao et al. (1993, 19) for up to 16 days in one experimental group of seven weaned piglets, each receiving a dose of 3 mg MON/kg bw per day mixed into a small portion of feed given in the morning and a control group of four weaned piglets. Total feed intake per pig and day was reported as of 0.5 kg without giving details on body weights and type of housing. Four animals died after 4 and 5 h and after 5 and 6 days, respectively, in the experimental group. The carotid blood pressure in the three surviving exposed pigs (mean: 198.7 mmHg; SD: 10.8) was statistically significantly higher than in controls (mean: 118.3 mmHg; SD: 7.3). A statistically significant decrease of glutathione peroxidase was found in five pigs (surviving day 1) by an intra‐individual comparison before and after exposure, but no information was provided for the time span between the measurements in those exposed animals and for the controls. The histopathological findings in the pigs of the experimental group indicated various changes in all major organs examined, i.e. the gastrointestinal tract, liver, kidney, lung and the adrenal gland. Varying degrees of degeneration were reported for heart papillary muscles, the atrial and ventricular muscles and the conducting cells of the Purkinje type. The cardiac effects in the experimental group included tachycardia and arrhythmia, myocardial ischemia accompanied by hyperkalaemia, multiple ventricular premature beats and myocardial injury. Minor myocardial effects were mentioned for the control group from staining myocardial fibres with acidic fuchsin. The authors concluded that the pathological changes and characteristics seen at 3 mg MON/kg bw per day in the miniature pigs were ‘very similar to those of Human Keshan disease’. Considering the missing information on feed intake and body weight, the unbalanced sample size, the missing details on the methods applied to describe the of effects in experimental and control pigs, the CONTAM Panel considered this study insufficient to assess the effects against a control group. The Panel also noted that cardiomyopathy, myopathy and osteoarthropathy have been also associated of with selenium deficiency (Loscalzo, 2014; Oropeza‐Moe et al., 2015)—see also Section 3.1.7.
The impact of MON on the humoral immune response of piglets was studied by Wei et al., 2010. The animals were immunised with swine fever vaccine at the age of 25 and 60 days. At 47 days of age, the animals (average weight 12 ± 0.5 kg, n = 6 per group) were fed for 42 days either the control diet or a MON contaminated diet where MON extract from Fusarium culture material (30 mg MON/kg) was added to the feed. The swine fever antibody levels in the MON group decreased significantly from the 61 days of age compared to the control group. Dose calculation was impossible since the amount of extract added was not reported and information on the occurrence of other mycotoxins was missing. Therefore, the CONTAM Panel decided not to further consider the study for the hazard characterisation of MON.
Two feeding studies (denoted by the authors as experiment 1 and 2) based on the same design (apart from the change of the dose range) were performed by Harvey et al. (2001) on growing barrows for 28 days consecutively with graded amounts of MON from Fusarium culture material produced as reported by Ledoux et al. (1995). The concentrations of aflatoxin, zearalenone, ochratoxin A and cyclopiazonic acid were < LOD of 10 μg/kg in a control diet and fumonisins < LOD of 5 mg/kg in control diet. The pigs were fed ad libitum and feed consumption was recorded weekly per pen but not reported. Although six barrows (mean body weight 17.8–17.9 kg) per group were fed diets containing 0, 25, 50 and 100 mg MON/kg feed in the first experiment, the same number of pigs (mean body weight 15.1–15.3 kg) were fed diets containing 0, 50, 100 and 200 mg MON/kg feed in the second experiment. Compared with controls, the pigs at concentration of 100 mg MON/kg feed showed in both experiments reduced final body weight and reduced body weight gain according to the authors’ analyses. Reductions in the number of RBC, and reductions of HCT and Hb levels were statistically significantly lower at concentrations of 50 mg MON/kg feed and higher. They showed a similar concentration–effect relationship in both experiments. With increasing concentrations the levels of serum cholesterol decreased and those of creatinine increased however without reaching statistical significance even at the high concentrations (100 mg MON/kg feed in the first and at 100 and 200 mg MON/kg feed in the second experiment). The levels of cholesterol and creatinine were higher in the first experiment compared with the second. In contrast, for the levels of phosphorous, ƴ‐glutamyltransferase (GGT) and mean corpuscular haemoglobin (MCH) were lower in the first experiment compared to the second. In both experiments, one of the six pigs assigned to 100 mg MON/kg feed group died, while five of six pigs died in the group of 200 mg MON/kg of the second experiment. As the most striking gross abnormality, a straw‐coloured fluid in the pericardial sacs was reported for the pigs dying acutely within 1 week. Cardiomegaly was the most consistent post‐mortem finding. No gross lesions were seen in other pigs necropsied at day 29. Furthermore, the relative heart weight, only reported for the second trial, was higher in pigs fed the 200 mg MON/kg diet (mean (standard error of the mean SEM) of 0.75 (0.06) g/100 g bw) compared with controls and pigs exposed up to 100 mg MON/kg diet (mean (SEM) of 0.48–0.55 (0.02–0.03) g/100 g bw). The CONTAM Panel identified from this study of Harvey et al. (2001) 50 mg MON/kg feed and 100 mg MON/kg feed as a NOAEL and LOAEL, respectively, for reduced body weight gain in pigs. Regarding haematological changes, a NOAEL of 25 mg MON/kg feed and a LOAEL of 50 mg MON/kg feed were identified.
Using the reported mean body weights of each concentration group available at start of exposure and after 90 days for each of the two experiments and assuming a mean feed intake of 1,200 g/day for pig, the CONTAM Panel calculated from the concentrations 25, 50 and 100 mg MON/kg feed of the first experiment and from the concentrations 50, 100 and 200 mg MON/kg feed of the second experiment, the doses of 1.00, 2.22 and 4.17, and 1.20, 5.07 and 11.48, respectively (see Table C.3 in Appendix C.2). Therefore, the NOAEL for reduced body weight ranged between 1.20 and 2.22 and the LOAEL between 4.17 and 5.07 mg MON/kg bw per day, respectively. For haematological adverse effects, the NOAEL was calculated as 1.00 mg MON/kg bw per day (the lowest dose of the first experiment), whereas the LOAEL ranged between 1.20 and 2.22 mg MON/kg bw per day based on the two experiments. Regarding mortality and relative heart weights, only the pigs at the highest dose level of 200 mg MON/kg diet showed a statistically significant difference compared with controls. Therefore, the CONTAM Panel identified a NOAEL of 4.6 mg MON/kg bw per day and a LOAEL of 9.2 mg MON/kg bw per day for cardiotoxicity of MON in pigs from this study.
Table C.3.
Concentrations of MON, samples sizes (N) body weights, calculated doses and haematological effects of MON in pigs (means with s.e.m.) of the two experiments of Harvey et al. (2001)
| Experiment | Concentration mg MON/kg feed | N | bw at start | bw at 90 days | Mean bw dose (kg) | mg MON/kg bw per day |
|---|---|---|---|---|---|---|
| 1 | 0 | 6 | 17.9 | 42.0 | 29.95 | 0 |
| 1 | 25 | 6 | 17.8 | 41.6 | 29.70 | 1.00 |
| 1 | 50 | 6 | 17.8 | 39.8 | 27.05 | 2.22 |
| 1 | 100 | 6 | 17.8 | 36.3 | 28.80 | 4.17 |
| 2 | 0 | 6 | 15.3 | 37.2 | 26.25 | 0 |
| 2 | 50 | 6 | 15.1 | 34.9 | 25.00 | 1.2 |
| 2 | 100 | 6 | 15.3 | 32.0 | 23.65 | 5.07 |
| 2 | 200 | 6 | 15.3 | 26.5 | 20.90 | 11.48 |
| RBC | HCT | Hb | MCH |
|---|---|---|---|
| 6.22 (0.06) | 40.7 (1.1) | 13.6 (0.5) | 21.9 (0.6) |
| 5.98 (0.09) | 39.8 (0.7) | 12.9 (0.3) | 21.6 (0.7) |
| 5.01 (0.27) | 33.8 (1.2) | 11.3 (0.5) | 22.5 (0.6) |
| 5.48 (0.45) | 28.2 (1.9) | 9.3 (0.5) | 17.3 (2.5) |
| 5.02 (0.36) | 41.0 (0.5) | 14.1 (0.2) | 28.4 (2.5) |
| 3.29 (0.33) | 31.8 (1.9) | 10.6 (0.2) | 33.1 (3.0) |
| 3.05 (0.32) | 29.2 (2.3) | 9.4 (0.6) | 31.3 (3.1) |
| 2.96 (0.0) | 25.0 (0.0) | 8.4 (0.0) | 28.4 (0.0) |
MON: moniliformin; bw: body weight; Hb: haemoglobin; HCT: haematocrit; MCH: mean concentration haemoglobin; RBC: red blood cell count.
The dose–response data on RBC, HCT and Hb were considered suitable for an evaluation with the BMD approach using the data of these two consecutive experiments with overlapping dose ranges. The factor ‘experiment’ was included as covariate to account for differences in location and shape of the dose–response curves in the BMD analysis of the combined data of the two experiments. While the two blood parameters HCT and Hb could be fitted well, the fit of the RBC was inconclusive and the outcome could not be used to derive a reference point for haematological changes, see Table 3 and Appendix C. The CONTAM Panel identified from these results the lowest BMDL05 of 0.20 mg MON/kg bw per day as reference point for haematological effects in pigs.
Table 3.
Dose–response analysis of critical haematological endpoints HCT and Hb in pigs based on the combined data from two experiments of Harvey et al. (2001) using the PROAST software for the BMD approach
| Best fitting model | HCT | ||
|---|---|---|---|
| BMD 05 | BMDL 05 –BMDU 05 | ||
| mg/kg bw per day | |||
| Experiment 1 | |||
| Exponential model family | E5 | 1.14 | 0.55–2.05 |
| Hill model family | H5 | 1.30 | 0.59–2.01 |
| Experiment 2 | |||
| Exponential model family | E5 | 0.50 | 0.23–1.04 |
| Hill model family | H5 | 0.59 | 0.25–1.00 |
| Best fitting model | Hb | ||
|---|---|---|---|
| BMD05 | BMDL05–BMDU05 | ||
| mg/kg bw per day | |||
| Experiment 1 | |||
| Exponential model family | E5 | 0.92 | 0.45–1.56 |
| Hill model family | H5 | 0.97 | 0.45–1.79 |
| Experiment 2 | |||
| Exponential model family | E5 | 0.42 | 0.20–0.77 |
| Hill model family | H5 | 0.46 | 0.21–0.89 |
BMD05: benchmark dose response of 5%; BMDL05/BMDU05: 95% lower/upper confidence limit for the benchmark dose response of 5%; bw: body weight; HCT: haematocrit; Hb: haemoglobin.
Note: The two‐sided 90% confidence intervals (BMDL05–BMDU05) of the BMD05 of the best fitting models defined by the Akaike Information Criterion (AIC) are reported for the exponential and the Hill family following the EFSA guidance (EFSA Scientific Committee, 2017).
In another study the same group of authors (Harvey et al., 2002) studied under similar experimental conditions effects of MON in six 7‐week‐old crossbred barrows (mean initial body weight 11.1 kg) per group. Animals were provided 0 or 100 mg MON/kg feed ad libitum for 28 days. The MON group showed an acute mortality of 33% (2 animals died 5 and 6 days after start of exposure), one of them with clear straw‐coloured fluid in the pericardial sac and/or moderate interstitial oedema and necrosis of the myocardium. The weight gain in the group of 100 mg MON/kg feed (mean (SEM) of 12.3 (2.1) kg) was reduced compared with controls (mean (SEM) 18.9 (4.2) kg) but no apparent effects on serum biochemical and haematological values were reported. Mortality was 33% at 100 mg MON/kg feed and therefore higher than the 17% observed in Harvey et al., 2001; at the same concentration, however not statistically significant different given the small group sizes. Therefore the CONTAM Panel concluded that the data of Harvey et al. (2002) support the findings above.
Conclusions
Only few studies on the toxicity of MON in pigs were available and reduced weight gain, adverse haematological effects and mortality accompanied with lesions in heart were identified as critical on adverse health effects for pigs. The NOAEL for reduced body weight gain ranged between 1.20 and 2.22 and the LOAEL between 4.17 and 5.07 mg MON/kg bw per day, corresponding to the MON concentrations of 50 mg MON/kg and 100 mg MON/kg, respectively. The NOAEL for haematological adverse effects of 1.00 mg MON/kg bw per day was based on the lowest dose of the first experiment and the LOAEL ranged between 1.20 and 2.22 MON/kg bw per day, corresponding to MON concentrations in feed of 25 mg MON/kg and 50 mg MON/kg, respectively, based on both experiments. The lowest dose at which mortality was observed was 4.17 mg MON/kg bw per day. The CONTAM Panel identified haematological adverse effects as most sensitive adverse effects and identified the lowest BMDL05 of 0.20 mg MON/kg bw per day as reference point for pigs. The cardiotoxicity observed in a study in miniature pigs exposed to 3 mg MON/kg bw per day supported these conclusions.
3.1.5.3. Poultry
Previously, Jestoi et al. (2003), Conkova et al. (2003) and Peltonen et al. (2010) reviewed the adverse effects caused by MON in poultry. Based to the selection criteria (see Section 2.2.1), this section describes the data on experiments on adverse effects associated with the oral exposure of MON alone. The toxicity data from the exposure to MON in combination with other mycotoxins is described in Section 3.1.6.
Data on acute toxicity were only identified for 1‐day‐old broiler chickens and 7‐day‐old ducks. Cole et al. (1973) reported an LD50 of 4.0 mg/kg bw in cockerels exposed orally to purified MON (isolated from Fusarium culture material) in sterile water solution (purity of MON, number of animals and range of oral doses were not reported). An oral dose of 6.25 mg MON/kg bw was lethal to all the animals and some cockerels receiving a dose of 3.12 mg MON/kg bw died within 24 h (number of animals not reported). Those birds which survived the dose of 3.12 mg MON/kg bw recovered after 24 h and did not show apparent adverse effects. Above 3.12 mg MON/kg bw, increasing the dose decreased the time to death until a dose of 40 mg MON/kg bw which resulted in death within 45 min. The birds which died within 2 h given doses of 12.5 and 25.0 mg MON/kg bw showed no lesions in all major organs. However, ascites with oedema of the mesenteries and small haemorrhages in the proventriculus, gizzard, small and large intestine, and skin were observed in cockerels surviving for 2 h at doses of 6.25 and 12.5 mg MON/kg bw. Cockerels in the control group and the birds in the two lowest dose groups of 3.12 and 1.56 mg MON/kg bw showed no lesions.
The study of Burmeister et al. (1979) applied MON extracted and purified (estimated purity 93%) from Fusarium culture material to chickens. In the first experiment, 1‐day‐old chickens (seven birds per dose) were dosed once by gavage at 0, 1, 2, 4, 8 and 16 mg MON/kg bw. A LD50 value of 5.4 mg MON/kg bw was reported. Surviving chickens showed no adverse effects at any time during the 4‐day observation period. In a second experiment, 4‐day‐old chicken embryos (25 per treatment levels) were injected once in the air sac with MON and an LD50 of 2.8 μg/egg was identified. No overt gross teratogenic effects were reported for the survivors.
Another acute toxicity study was performed by Kriek et al. (1977) in ducks using chemically semipurified MON (purity and information on the occurrence of other mycotoxins was not reported) which was isolated from Fusarium culture material. Thirty 7‐day‐old Pekin ducklings were randomly divided into five groups of six each. They were dosed by crop intubation once with pure MON dissolved in distilled sterile water in appropriate concentrations to provide the doses of 0.0 (as control), 0.5, 1.4, 3.7 and 10.0 mg MON/kg bw. The authors identified LD50 of 3.7 mg MON/kg bw.
In the study by Zhang and Li (1989) MON was isolated from mouldy corn contaminated with Fusarium fungus (information on the occurrence of other mycotoxins was not reported) and collected from the Keshan disease (KD) region in China (see also Section 3.1.7). Three Pekin ducklings of 3‐ to 4 days old (weight 50–60 g) were reported treated by gavage with 12 mg pure MON per animal. However, purity (%) was not reported and missing information on the occurrence of other mycotoxins and mode of treatment raised questions on the actually applied dose. Normal electrocardiogram (ECG) prior to MON administration was compared to ECG after MON administration in the same animal. At approximately, 4 min after MON administration, the heart rate increased from the previous range of 240–300 bpm to 360–480 bpm, and then gradually slowed down, followed by arrhythmia and ultimately cessation of contraction. An increase in blood potassium at the time of MON introduction caused hyperkalaemia, with enlargement of the atrium due to myocardial ischemia. Myocardial injury was followed by a development of left and right ventricular dilatation in addition to atrial enlargement. Meanwhile, blood potassium levels remained excessively high, quickly leading to ventricular fibrillation and cardiac arrest causing death. The three tested ducklings all died within 14–54 min.
In conclusion, for 1‐day‐old chickens, oral LD50 values of 4.0 and 5.4 mg MON/kg bw were identified and ascites with oedema of the mesenteries and small haemorrhages in the proventriculus, gizzard, small and large intestine, and skin were observed in surviving chickens. For 7‐day‐old ducks, an oral LD50 value of 3.7 mg MON/kg bw was reported. Three‐day‐old ducklings receiving probably a high dose of 12 mg of pure MON (240 mg/kg bw) by gavage showed increased heart rates followed by arrhythmia and ultimately cessation of contraction generating the death.
Data on subchronic and chronic toxicity on MON were available for broiler chickens, laying hens, turkeys, ducks and quails, and are described below. No data were identified for other poultry species.
Broiler chickens
Allen et al. (1981) performed two trials using groups of 10 chickens (a mixture of growing Ross male and Arbor Acre female broiler chickens) for 3 weeks. In the first trial, MON was extracted and purified (98% pure) from Fusarium culture material and added to the feed to provide 0, 8 or 16 mg MON/kg of diet. In the second trial, Fusarium culture material containing MON was incorporated to the diet to provide 8, 16 and 64 mg/kg feed (information on the occurrence of other mycotoxins was not reported). The birds were examined for feed consumption, body weight and symptoms (e.g. muscular weakness, respiratory distress, cyanosis) during the conduct of the experiment. At the end of the study, they were necropsied and examined for the presence of lesions. Exposures to concentrations of 8 and 16 mg MON/kg feed (from both feed preparations) generated no adverse effects. Three of the 10 chickens fed 64 mg MON/kg diet died and surviving birds showed reduced body weight gain and reduced feed consumption. No lesions were found upon necropsy at this concentration. The authors reported that the oral dose of 64 mg MON/kg feed corresponded to 9.7 mg MON/kg bw.
In an experiment of Engelhardt et al. (1989) four groups of 10 one‐day‐old broiler chickens were fed MON‐contaminated feed for 14 days. The feed was prepared by mixing the Fusarium culture material producing MON at a concentration of 1.15 g MON/kg with maize applied in dietary percentages of 0, 12.5, 25 and 50% of feed equivalent to 0, 144, 288 and 575 mg MON/kg feed (information on the occurrence of other mycotoxins was not reported). No adverse effects were observed in controls but in the other three groups all birds died except two out of the ten birds fed 144 mg MON/kg feed which were still alive after 10 days. Adverse effects observed before death included dyspnoea, cyanosis and reluctance to move. All birds that died had cyanosis and variable amounts of ascetic and pericardial fluid. Microscopic alterations were limited to the heart and liver. Myocardial degeneration and necrosis were present indicating cardiovascular toxicosis. In the liver, alterations of multifocal vacuolation and swelling of hepatocytes were accompanied by scattered necrotic individual hepatocytes. The body weight of the surviving birds was lower than the controls.
Javed et al. (1993) fed a control and two dose groups of 10 one‐day‐old broiler chickens (Columbia x New Hampshire) from 1 to 14 days with MON, which was extracted and purified (91% minimum purity) from Fusarium culture material. Compared with the controls, the birds fed diets at 27 and 154 mg MON/kg feed were affected at day 7 and day 5 of the experiment, respectively. In both experimental groups, adverse effects started with ataxia, decreased feed intake and body weight gain, and were followed by a transient increase in water intake and seeking the heat source (within 24 h). Mortality was 40% and 70% in groups fed diets at 27 and 154 mg MON/kg feed, respectively. A further analysis of the serohaematological and immunological data by Javed et al. (1995) showed statistically significant changes of major haematological parameters in both experimental groups compared with controls: elevated levels of cholesterol, sodium, alkaline phosphatase, aspartate aminotransferase (AST), alanine aminotransferase, lactate dehydrogenase (LDH), GGT were interpreted by the authors to indicate both hepatic and renal damage, and significant decrease of glucose concentrations was interpreted by the authors to indicate pancreatic damage. Immunological changes included impaired anti‐Newcastle disease antibody haemagglutination inhibition titres associated with relative decreases in total serum for globulins and increases in albumin/globulin ratios. Hall et al. (1995) analysed vitamin A concentrations in the serum samples of the four birds fed 154 mg MON/kg feed and three control animals from the experiment of Javed et al. (1993, 1995) and noted that the vitamin A concentrations in serum were decreased in birds fed 154 mg MON/kg feed.
Based on the same experimental design used by Javed et al. (1993, 1995) (10 one‐day‐old broiler chickens fed diets at 27 and 154 mg of purified MON/kg feed during 14 days), Javed et al. (2005) observed prominent gross lesions in affected birds including ascites, hydropericardium, hepatopathy, nephropathy, cardiomyopathy, pneumonitis, gizzard ulceration and enlarged bursa of Fabricius filled with caseous material. The two concentrations of MON (27 and 154 mg MON/kg feed) produced lesions in the two groups. Histopathological changes included haemorrhage, leucocytic infiltration, fatty change or infiltration, individual cell necrosis and fibrosis in liver, kidneys, lungs, heart, intestines, gizzard, bursa of Fabricius and pancreas. Oedema and haemorrhage were prominent in brains of treated birds. Ultrastructural changes included cytoplasmic and nuclear enlargement of cells in affected liver, lungs, kidneys, heart and pancreas. There were thickened membranes of the smooth endoplasmic reticulum, dilation of the rough endoplasmic reticulum with loss of ribosomes and vacuolated or deformed mitochondria.
The CONTAM Panel identified from the studies of Javed et al. (1993, 1995, 2005) and Hall et al. (1995) a LOAEL of 27 mg MON/kg feed, the lowest concentration tested. At this concentration, adverse effects such as mortality, reduced body weight gain, cardiomyopathy and changes in the major haematological parameters were observed. No NOAEL was possible to identify from these studies.
Six groups of five 1‐day‐old broiler chickens were fed diets containing 0, 25, 50, 75, 100, 150, 200, 250 and 300 mg MON/kg feed for 21 days (Ledoux et al., 1995). MON was obtained by incorporating different percentages of a Fusarium culture material in the feed (information on the occurrence of other mycotoxins was not reported). Compared with the controls a statistically significant increased mortality of 8/30, 17/30 and 25/30 occurred at the three highest concentrations of 200, 250 and 300 mg MON/kg feed, respectively. Birds fed diets at concentrations of 100 mg MON/kg feed or higher had lower body weight gain than controls. Mortality occurred in chickens fed 200 (8 of 30), 250 (17 of 30) and 300 (25 of 30) mg MON/kg feed. Increased heart weight was observed in birds fed diet concentrations equal and above 50 mg MON/kg feed and increased liver weight was observed in birds fed diet concentrations equal and above 100 mg MON/kg feed. Cardiomegaly was observed in chickens fed 50–300 mg MON/kg feed. Histopathology revealed a generalised and high incidence of large shaped cardiomyocyte nuclei, and a loss of cardiomyocyte cross‐striations in chickens fed diet levels equal and above 75 and 200 mg MON/kg feed, respectively. From these data, the CONTAM Panel identified the dietary concentration of 50 mg/kg feed generating cardiomegaly as LOAEL and 25 mg MON/kg feed where no adverse effects were observed as NOAEL.
Zhao et al. (1996) fed 10‐day old Roman chickens a control (30 birds) and a contaminated diet (30 birds) at a level of 77 mg MON/kg feed for 30 days (provided by adding Fusarium culture material to control feed. Information on the occurrence of other mycotoxins was not reported). Seventy per cent of treated birds died with deaths starting at day 5 and peaking at 8–10 days. Surviving birds grew slowly, pathologic changes such as pulmonary congestion, severely damaged myocardium (coagulation, necrosis and interstitial oedema) were observed. Histology of myocardium showed mitochondrial hyperplasia, nucleus shape changes and decrease of myofilaments.
In addition to above data in broiler chickens, the CONTAM Panel noted results from scientific abstracts of Wu and Vesonder (1997), Nagaraj et al. (1997) and Bailey et al. (1997) that supported a LOAEL for cardiomegaly in broiler chickens at a dietary concentration of 50 mg MON/kg feed.
In the study of Kubena et al. (1997), six groups of male broiler chickens (six per group) from day‐of‐hatch to 3 weeks were fed diets containing 0 or 100 mg MON/kg feed. MON was obtained as described by Ledoux et al. (1995). When compared with controls, body weight gain was reduced by 29% in the birds of the experimental group. The efficiency of feed utilisation was adversely affected, and decreased relative weights of the bursa of Fabricius, increased relative weights of the heart, concentrations of creatinine and calcium in serum, activities of alkaline phosphatase and alanine aminotransferase, and changes in haematological values were reported.
MON from Fusarium culture material was used to prepare two dietary treatments containing 0 and 100 mg MON/kg feed (information on the occurrence of other mycotoxins was not reported) (Harvey et al., 1997). Two pens of six 1‐day old broiler chickens per dietary treatment were grown to 21 days of age. Body weight gain and feed consumption were reduced by feeding 100 mg MON/kg feed, while relative heart weight was increased. Increased alanine transferase and aspartate transaminase activities and creatinine concentration and decreased mean corpuscular volume, mean corpuscular haemoglobin and mean corpuscular haemoglobin concentration were observed. The MON diet decreased glucose, haemoglobin and mean corpuscular haemoglobin concentration. Histopathological lesions from the MON diet were limited to the kidney and consisted of extensive renal tubular epithelial degeneration plus luminal mineralisation.
Susceptibility to infection was studied in one day‐old broiler chickens fed 0, 50, 75 and 100 mg MON/kg diet prepared by mixing Fusarium culture material (10,000 mg MON/kg) with the feed (information on the occurrence of other mycotoxins was not reported) Li et al. (2000a). In the first experiment, 6 groups of two chickens were treated for 3 weeks and injected intravenously (i.v) with E. coli on day 21. Chickens fed 100 mg MON/kg feed had lower feed intakes and body weight gains than controls by that time and chickens fed 75 and 100 mg MON/kg diet had higher numbers of E. coli colonies in the circulation, liver and spleen 180 min after the injection. In the second experiment, chickens were treated for 4 weeks and were injected with 0.5 mL Newcastle disease virus vaccine intramuscularly on weeks 2 and 3. Chickens fed 75 and 100 mg MON/kg diet had reduced feed intakes and body weight gains during the 4‐week test period. Chickens fed 100 mg MON/kg diet had lower secondary antibody titres than controls 7 days after each injection. The observed decreased feed intake and body weight gain and lower secondary antibody titres against Newcastle disease virus suggest a reduced immune response in chickens fed 75 mg MON/kg diet.
One‐day‐old Cornish cross broiler chickens were treated at concentrations of 0, 25 and 50 mg MON/kg diet (each group with 30 birds) for 7 weeks (Broomhead et al., 2002). The dietary MON was obtained as described by Ledoux et al. (1995) (information on the occurrence of other mycotoxins was not reported). Compared with controls, and birds fed 25 mg MON/kg feed, reduced feed intake, reduced body weight gain, increased relative heart and increased proventriculus weights were observed after 2 weeks for birds fed 50 mg MON/kg feed. Broiler chickens fed 25 and 50 mg MON/kg also had increased serum GGT activities. In another experiment performed by the same group, each three groups of five broiler chickens were given 0, 100 and 200 mg MON/kg diet (Ledoux et al., 2003). The dietary MON was obtained as described by Ledoux et al. (1995) (information on the occurrence of other mycotoxins was not reported). Compared with controls, both feed intake and body weight gain were decreased at 100 mg MON/kg and 50–65% mortality occurred in chickens fed diets of 200 mg MON/kg feed. Surviving birds had increased kidney and liver weights. Heavier heart weights, larger pleomorphic cardiomyocyte nuclei, loss of cardiomyocytes and mild focal renal tubular mineralisation were observed at both concentrations. From the study of Broomhead et al. (2002), the CONTAM Panel identified the dietary concentrations of 50 mg MON/kg feed and 25 mg MON/kg feed to use to calculate LOAEL and NOAEL for reduced body weight gain, respectively.
In conclusion, except for one experimental design in which adverse effects were observed in broiler chickens fed a diet at 27 mg MON/kg feed (Javed et al., 1993, 1995, 2005), all the studies above on broiler chickens, and particularly Ledoux et al. (1995) and Broomhead et al. (2002) supported by the abstracts of Wu and Vesonder (1997), Nagaraj et al. (1997) and Bailey et al. (1997) reported that the level of 50 mg MON/kg feed generated adverse effects such as mortality, reduced body weight gain, cardiomyopathy and changes in the major haematological parameters in broiler chickens. Among the above studies, in one study (Allen et al., 1981) and in two others studies (Ledoux et al., 1995; Broomhead et al., 2002) levels of 16 mg and 25 MON/kg feed did not generate any adverse effects, respectively. Considering that Allen et al. (1981) used 10 birds per group for 3 weeks and Broomhead et al. (2002) used 30 birds per group for 7 weeks, the level of 25 mg MON/kg feed was identified from the Broomhead et al. study supported by the Ledoux et al. study as the level not generating adverse effects for broiler chickens. Using the reported mean body weight and mean feed consumption by the authors (Ledoux et al., 1995; Broomhead et al., 2002), the CONTAM Panel converted the dietary concentrations of 50 and 25 mg MON/kg feed to an overall LOAEL of 2.8 mg MON/kg bw per day and an overall NOAEL of 1.4 mg MON/kg bw per day for broiler chickens.
Laying hens
Beginning at 24 weeks of age, white Leghorn laying hens (six birds per group) were fed 0, 50 and 100 mg MON/kg feed for 14 months (Kubena et al., 1999). Then, the hens were fed again the control diet for an additional 2 months. The dietary MON was obtained as described by Ledoux et al. (1995) (information on the occurrence of other mycotoxins was not reported). Twenty per cent of the hens fed the 100 mg MON/kg diet died. This group of hens was the only group which had significantly lower body weights than controls and which egg production was reduced by 50% by the end of the second 28‐day laying period and remained at the reduced level for the 420 days. After the hens were fed the control diet for 60 days, the egg production returned to the level of controls or above. Egg weights were reduced during the first three 28‐day laying periods before they returned to the weights comparable with controls. The hens were artificially inseminated with semen from males fed control diets, but the fertility was not affected by MON exposure. From these data, the CONTAM Panel identified the dietary concentrations of 50 and 100 mg MON/kg feed to calculate a NOAEL and LOAEL, respectively, for decreased egg production, egg weight and body weight in laying hens. Using the body weights and feed consumptions reported by the authors, a NOAEL of 3.8 mg MON/kg bw per day for laying hens were calculated.
Turkeys
In the same experiment on broiler chickens (above), Engelhardt et al. (1989) treated 4 groups of 10 one‐day‐old turkey poults. The feed was prepared by mixing the Fusarium culture material producing MON at a concentration of 1.15 g MON/kg maize to obtain dietary percentages of 0%, 12.5%, 25% and 50% of feed equivalent to 0, 144, 288 and 575 mg MON/kg feed (information on the occurrence of other mycotoxins was not reported). While no adverse effects were observed in control birds, among the three other groups only two birds fed 288 mg MON/kg feed were still alive after 10 days. The body weights of the surviving birds were lower than the controls and the observed adverse effects were similar to the broiler chickens (see above).
Nine groups of 20 one‐day‐old female turkey poults were fed diets of 0, 25, 50, 75, 100, 150, 200, 250 and 300 mg MON/kg feed for 3 weeks (Bermudez et al., 1997). MON was from Fusarium culture material and information on the occurrence of other mycotoxins was not reported. Statistically significant mortality occurred from 200 mg MON/kg feed onwards in 6/20 to 9/20 birds. From 100 mg MON/kg feed and above, the feed intake and body weight gain were reduced compared to the controls and from 50 mg MON/kg feed and above heart weight was increased with gross lesions of cardiomegaly and focally extensive to generalised loss of cardiomyocyte cross striations (granular change). Numerous large cardiomyocyte nuclei were observed at 100 mg MON/kg feed. The diets containing MON at levels greater than or equal to 50 mg MON/kg feed induced cardiomegaly in turkey poults. From this study, the CONTAM Panel identified the dietary concentration of 25 and 50 mg MON/kg feed inducing cardiomegaly in turkeys to calculate the NOAEL and LOAEL, respectively.
Using a similar study design, Morris et al. (1999) confirmed the findings of Bermudez et al. (1997) by feeding 24 growing turkey poults two diets containing either 0 or 100 mg MON/kg for 3 weeks.
Five groups of turkeys (6 pens of 10 birds) were fed 0, 12.5, 25, 37.5 and 50 mg MON/kg feed for 14 weeks (MON from Fusarium culture material was added to a maize–soybean diet. Information on the occurrence of other mycotoxins was not reported) (Broomhead et al., 2002). Feed intake, body weight gain and feed conversion of turkeys were slightly affected at week 6 but not affected at week 14 in the end of the experiment for animals fed 50 mg MON/kg diet. Lesions in the heart were observed only in turkeys fed 50 mg MON/kg feed, where a loss of cardiomyocyte cross‐striations, increased cardiomyocyte nuclear size and an increased number of cardiomyocyte mitotic figures were also observed.
From the above studies of Bermudez et al. (1997), Morris et al. (1999) and Broomhead et al. (2002) performed by the same group of researchers, the CONTAM Panel noted that the dietary concentration of 50 mg MON/kg was cardiotoxic to turkeys, and identified the dietary concentration of 25 mg MON/kg feed to calculate a NOAEL and 50 mg MON/kg feed to calculate a LOAEL for cardiomegaly in turkeys. By using the body weights and feed consumptions reported by the authors, a NOAEL of 1.6 mg MON/kg bw per day and a LOAEL of 3.2 mg MON/kg bw per day were calculated for cardiotoxic effects in turkeys by the CONTAM Panel.
Susceptibility to infection was studied in four replicates of four 1‐day‐old turkey poults fed a control diet and 100 mg MON/kg diet (Li et al., 2000b). MON contaminated diet was prepared by mixing Fusarium culture material with the feed (information on the occurrence of other mycotoxins was not reported). In a Trial‐1, poults were fed for 4 weeks and were injected with 0.25 mL Newcastle disease vaccine (NDV) on weeks 2 and 3. Anti‐NDV antibody titres were measured 7 days after each injection. Turkey poults exposed to MON had statically lower primary and secondary antibody response, and decreased relative bursa and spleen weights were observed. In a Trial‐2, poults were fed for 3 weeks and injected with E. coli on day 21. Significantly higher numbers of E. coli colonies were observed in the blood and tissue homogenates of poults fed MON. In both experiments, feed intake and body weight gains were statically lower in turkeys poults fed MON containing diets. The level of 100 mg MON/kg feed suppressed immune response and decreased performance.
Ducks
In the above trial for LD50 determination Kriek et al. (1977), also conducted another trial (see above), in which four Pekin ducklings either 1‐day‐old or 7‐day‐old were fed 6.1, 9.7 and 11.3 g MON/kg feed (no control in the study). MON‐contaminated diets were prepared by mixing Fusarium culture material with commercial chicken mash. Both 1‐day‐old and 7‐day‐old ducklings died within 2–48 h depending of the diet levels and ages of the birds.
In the same experiment on chickens and turkeys described above, Engelhardt et al. (1989) also studied four groups of 10 one‐day‐old ducklings. The feed was prepared by mixing the Fusarium culture material producing MON at a concentration of 1.15 g MON/kg with maize to obtain dietary percentages of 0%, 12.5%, 25% and 50% of feed equivalent to 0, 144, 288 and 575 mg MON/kg feed (information on the occurrence of other mycotoxins was not reported). The control birds did not show adverse effects but all the other birds died before 10 days. Adverse effects observed before death were similar to the chickens and turkeys (see above).
In the first trial of the study of Vesonder and Wu (1998), groups of 4 one‐day‐old ducklings were provided diets of 0, 195, 308 and 1,169 mg MON/kg feed for 7 days. The control diet of corn–soy bean chick mash did not contain detectable amounts of aflatoxin B1, T‐2 toxin, deoxynivalenol, zearalenone and fumonisin B1 (LODs: 0.02 mg/kg for aflatoxin, 0.5 mg/kg for other toxins). MON was obtained from Fusarium culture material (information on the occurrence of other mycotoxins was not reported) which was mixed with control diet to provide MON concentrations in the diets. Mortalities of 50%, 67% and 100% were observed in less than 6 h at 195, 308 and 1,169 mg MON/kg feed, respectively. After 3 days, 100% mortality was observed at 195 mg MON/kg diet, whereas mortality remained unchanged for the 308 mg MON/kg diet. The CONTAM Panel noted that this observation could be due to uncertainties in the feed intake by one duck and small number of birds per group. In the second trial, groups of six 1‐day‐old ducklings were provided with either a test or control diet for 2 days. The control diet was identical to the first trial but the test diets were prepared by adding purified MON (98% purity) to the control diet to provide diets of 15, 44, 133, 400 and 1,200 mg MON/kg feed. Mortalities were observed for only birds in the groups fed 133, 400 and 1,200 mg MON/kg feed in which 0%, 25% and 75% birds died in less than 12 h. On the day 2, deaths of 25 and 50% of birds fed diet 133 and 400 mg MON/kg feed were observed, whereas the 75% death for the birds fed 1,200 mg MON/kg feed remained unchanged. No mortality within the 2‐day experiment was observed for both groups fed 15 and 44 mg MON/kg feed. However, because of the short period of time of the experiment (2 days), the CONTAM Panel did not consider these two lowest levels as NOAEL for risk assessment.
In absence of any other suitable data on adverse effects in ducks, the CONTAM Panel also noted that results from two experiments reported in two scientific abstracts of Broomhead et al. (1996) and Morris et al. (1997). Broomhead et al. (1996) (abstract), randomly allotted 140 one‐day‐old male Pekin ducklings to the groups of 0, 25, 50, 75, 100, 125 and 150 mg MON/kg feed. The dietary MON was from Fusarium culture material (information on the occurrence of other mycotoxins was not reported). Each dietary treatment was fed to 4 pen replicates of 5 ducklings per pen for 17 days. Ducklings fed a diet with 100 mg MON/kg had lower relative heart and higher relative kidney weights compared with controls. No significant differences in serum glucose, albumin, cholesterol, total protein, globulin, AST and GGT levels were observed. Cardiomegaly was observed in ducklings fed from 75 mg MON/kg diet and above. From these data, the CONTAM Panel identified the dietary concentration of 75 mg MON/kg feed generating cardiomegaly in ducklings to use for LOAEL calculation. At the dietary concentration of 50 mg MON/kg feed, no adverse effects were observed.
The group of Broomhead et al., 1996 (abstract), assigned five replicate pens of 5‐day‐old male Pekin ducklings to diet groups of 0, 50, 100 and 200 mg MON/kg feed (Morris et al., 1997, abstract). Dietary MON was obtained from Fusarium culture material (information on the occurrence of other mycotoxins was not reported). Compared to controls, feed intake was reduced in all groups, body weight gain was reduced in the group fed 100 mg/kg feed and statistical mortality occurred in the group fed 200 mg MON/kg feed.
The CONTAM Panel also noted a 14‐day study of Leslie et al. (1996) on 1‐day‐old Pekin ducklings for testing the toxicity of 20 cultured fungal strains. Their ability for mycotoxin production was analysed only for fumonisins and MON (ranged 85–10 345 mg/kg culture material). Mouldy feed was mixed with commercial chicken mash (50%/50%) and fed ducklings (4 per group). A toxicity index was calculated by multiplying the amount of feed intake (g) by the mean day of death to obtain an inverse measure of toxicity for cultures. The authors concluded that MON levels were not strongly correlated with this toxicity index on ducklings due to the possible production of other non‐identified toxins interfering with the toxicity. For this reason and because no details were reported on time delay of death, body weights and feed intakes for animals, the CONTAM Panel did not consider this study further for risk assessment.
In conclusion, in the absence of any other suitable data, the CONTAM Panel identified from the abstract of Broomhead et al. (1996), the dietary concentrations of 75 mg MON/kg feed to use to calculate a LOAEL for generating cardiomegaly in ducklings, and from the abstracts of Broomhead et al. (1996) and Morris et al. (1997), the dietary concentration of 50 mg MON/kg feed to calculate a NOAEL. Because body weight and feed intake were not reported by the authors, the body weight and feed intake from Appendix C, Table C.2 were used to calculate the doses of 2.8 and 2.3 mg MON/kg bw per day as LOAEL and NOAEL, respectively, for cardiomegaly in ducks.
Table C.2.
Summary of dose–response analysis of acute toxicity data in female rats of Abbas et al. (1990)
| Models | Number of parameters | Minus Log‐likelihood | p‐value | AIC | LD50 | LDL50 | Comment |
|---|---|---|---|---|---|---|---|
| (mg/kg bw per day) | |||||||
| Full model | 9 | 2.50 | na | 23.00 | na | na | |
| Null (reduced) model | 1 | 31.09 | 64.18 | na | na | ||
| Probit | 2 | 2.50 | 1 | 9.00 | 18.71 | 14.09 | Accepted |
| Logistic | 2 | 2.50 | 1 | 9.00 | 19,32 | 14.09 | Accepted |
| LogProbit | 2 | 2.50 | 1 | 9.00 | 18.32 | 13.14 | Accepted |
| LogLogistic | 2 | 2.50 | 1 | 7.00 | 18.52 | 13.08 | Accepted |
| Multistage | No fit | ||||||
| Multistage Cancer | 1 | 4.57 | 0.85 | 11.14 | 16.54 | 11.69 | Accepted |
| Quantal‐Linear | 1 | 8.32 | 0.17 | 18.63 | 14.04 | 8.80 | Not accepted |
| Weibull | No fit | ||||||
| Gamma | No fit | ||||||
BMD50: benchmark dose response of 50%; BMDL50: 95% lower confidence limit for the benchmark dose response (BMR) of 50%; bw: body weight; na: not available, including cases where the BMD/Ls were not calculated, the fit was incomplete, or serious or conflicting comments were noted (e.g. parameters reaching boundaries).
Note: The models with the lowest AIC among the accepted models and the model with the lowest LDL50 (corresponding to a BMDL50) are in bold.
Quail
A control group of 75 one‐day‐old quail chicks (Coturnix coturnix japonica) were fed quail mash and another group of 105 birds were fed a diet of 100 mg MON/kg feed (corresponding to 5.9 mg MON/kg bw per day) for 35 days (Sharma et al., 2008, 2012). MON was from Fusarium culture material (information on the occurrence of other mycotoxins was not reported). Birds fed MON‐diet exhibited signs of poor feathering and decreased feed and water consumption and reduced body weight gain, anorexia and diarrhoea and increased AST, LDH and creatine kinase (CK) values. The changes in AST and LDH values were attributed to liver damage, while changes in CK were attributed to kidney damage. Since only one concentration was tested no reference point could be identified. Gross and microscopic observations of the heart were also reported. Cardiomegaly was observed in the group fed MON. Microscopically, hypertrophy of cardiomyocytes was observed in the group fed MON as early as after 7 days of the experiment.
Conclusions
For 1‐day‐old chickens, oral LD50 values of 4.0 and 5.4 mg MON/kg bw were reported and ascites with oedema of the mesenteries and small haemorrhages in the proventriculus, gizzard, small and large intestine, and skin were observed in surviving chickens. For 7‐day‐old ducks, an oral LD50 value of 3.7 mg MON/kg bw was reported and increasing heart rates followed by arrhythmia and ultimately cessation of contraction generating the death.
From the available database on broiler chickens, the CONTAM Panel identified a LOAEL of 50 mg MON/kg feed corresponding to the dose of 2.8 mg MON/kg bw per day for generating adverse effects such as some mortalities, reduced body weight gain, cardiomyopathy, changes in the major haematological parameters in broiler chickens and a NOAEL of 25 mg MON/kg feed corresponding to the dose of 1.4 mg MON/kg bw per day. From data from only one available study on laying hens, the CONTAM Panel identified the NOAEL of 50 mg MON/kg feed corresponding to the dose of 3.8 mg MON/kg bw per day and the LOAEL of 100 mg MON/feed corresponding to the dose of 8.5 mg MON/kg bw per day for reduced egg production and reduced body weight gain. For turkeys, the CONTAM Panel identified the NOAEL of 25 mg MON/kg feed corresponding to the dose of 1.6 mg MON/kg bw per day and the LOAEL of 50 mg MON/kg feed corresponding to the dose of 3.2 mg MON/kg bw per day for inducing cardiomegaly. From the two studies (available as abstracts) on ducks, the CONTAM Panel considered the LOAEL of 75 mg MON/kg feed corresponding to the dose of 2.8 mg MON/kg bw per day generating cardiomegaly and the NOAEL of 50 mg MON/kg feed corresponding to the dose of 2.3 mg MON/kg bw per day in ducks. The only identified study on quails supported the findings in other poultry for dietary MON generating cardiomegaly.
Overall, it can be concluded that the heart is the main target organ for poultry causing heart failure at acute doses. Chronic dietary exposure to MON generated cardiomegaly in poultry but also changed haematological parameters and affected zootechnical parameters such as reduction of body weight gain and egg production.
3.1.5.4. Solipeds
In one donkey, daily i.v. administration of 1 mg MON/kg bw per day (purity of MON not reported) resulted in acute death after 26 days accompanied by microhaemorrhages, satellitosis and neuronophagia (Buck et al., 1980). No studies on oral administration were identified for donkeys. No data on horses were identified. The CONTAM Panel noted a few studies (Marasas et al., 1976; Buck et al., 1980) on the exposure of F. moniliforme associated with horses suffering from equine leucoencephalomalacia. However, no MON was quantified in these studies. It should be noted that at the time these studies were published, fumonisins were unknown (discovered in the end of 1980s) and currently it is known that equine leucoencephalamalacia is typically associated with the exposure to fumonisins.
3.1.5.5. Farmed rabbits
No data were identified.
3.1.5.6. Farmed fish
Channel catfish with an average initial weight of 1.5 g (35 fish/aquarium, 3 aquariums/dose) were given feed containing 0, 20, 40, 60 and 120 mg MON/kg feed (estimated to correspond to 0.8, 1.7, 3.2 and 8.7 mg MON/kg bw per day by the CONTAM Panel based on data on weight and feed consumption reported in the paper) for 10 weeks (Yildirim et al., 2000). The feed was prepared by mixing MON‐containing Fusarium culture material into the feed (information on the occurrence of other mycotoxins was not reported). MON did not affect the mortality. A dose‐dependent reduction in weight gain was observed and the weight gain was significantly reduced compared to control even in the lowest dose group (20 mg MON/kg feed). The feed conversion ratio (determined as grams of feed consumed per gram of fish wet weight gain) was significantly increased in the highest dose group. The only effect found in histopathological examinations of liver and heart was smaller nuclei in liver cells from fish given 60 or 120 mg MON/kg feed. Haematocrit was significantly lowered and serum pyruvate significantly increased by 60 mg MON/kg feed.
Tuan et al. (2003) fed Nile tilapia diets containing 0, 10, 40, 70 and 150 mg MON/kg feed (3 aquariums/dose; 40 fish/ aquarium) for 8 weeks from a mean weight of 2.7 g. The doses were estimated to correspond to 0.4, 1.8, 3.9 and 9.4 mg MON/kg bw per day by the CONTAM Panel based on data on weight gain and feed conversion reported in the paper. The MON‐contaminated diets were produced by mixing Fusarium culture material (10,000 mg MON/kg) with the feed (information on the occurrence of other mycotoxins was not reported). MON did not increase the mortality rate or cause any histopathological lesions. Fish fed 70 or 150 mg MON/kg feed had a significantly (p < 0.05) lower weigh gain than controls. MON significantly increased the serum pyruvate levels in all dose groups (0.1217, 0.1254, 0.1270 or 0.1419 mmol pyruvate/L, respectively) compared to control (0.1089 mmol pyruvate/L), but there were no differences between the groups fed 10, 40 and 70 mg MON/kg feed. Haematocrit was reduced in fish fed 70 or 150 mg MON/kg feed.
Conclusions
The available data on effects of MON on fish were limited and only two feeding experiments one with channel catfish and one with Nile tilapia were identified. A reduction in weight gain was reported for fish given feed with 20 mg MON/kg, corresponding to 0.8 mg MON/kg bw per day, which was the lowest concentration used in the channel catfish feeding study. Nile tilapia may be more resistant as no effects on feed intake and weight gain were observed at 40 mg MON/kg feed, corresponding to 1.8 mg MON/kg bw per day in this species. However, serum pyruvate levels were increased in Nile tilapia at 10 mg MON/kg feed, the lowest dose tested. Due to the limited database, no critical adverse effect levels of MON for fish could be identified.
3.1.5.7. Farmed mink
Three groups of pastel, adult female mink (n = 12) were fed diets (from 2 weeks prior to the breeding season, and continued until the offspring were 8 weeks old) containing 0, 8.1 and 17.0 mg MON/kg (equivalent to 0, 0.92 and 1.94 mg MON/kg bw per day as calculated by the CONTAM Panel using the body weights and feed consumption data from NRC, 1982, see Section 3.1.4.5) (Morgan et al., 1998). MON was from Fusarium culture material (approximately 10,000 mg MON/kg) which was mixed with the diet. The culture material contained no detectable fumonisins (LOD 0.5 mg/kg) and the cereals in the diet contained no detectable concentrations of aflatoxins, zearalenone and deoxynivalenol (LODs were 0.004 mg/kg for aflatoxins B1, B2, G1 and G2, 0.2 mg/kg for zearalenone, and 0.4 mg/kg for deoxynivalenol). Throughout the trial, there were no marked differences in feed consumption or body weights of the 0.92 and 1.94 mg MON/kg bw groups compared to the controls when recorded daily and weekly, respectively. No gross lesions or alterations were observed at necropsy and there were no significant differences in brain, liver, kidney, heart, lung and adrenal gland weights (expressed as absolute weight or as percent of body weight) among the groups. The only significant difference was observed for the leucocyte differential cell counts. The percentage of segmented neutrophils in the high‐dose group was lower and the percentage of lymphocytes was larger in the high‐dose group when compared to the control and low‐dose groups. The reproductive effects reported in this paper are described in Section 3.1.4.5, and from this study, the CONTAM Panel identified the reproductive effects, neonatal mortality and reduced offspring body weights, as adverse effects in farmed mink. The LOAEL and NOAEL were calculated as 1.94 and 0.92 mg MON/kg bw per day, respectively, by the CONTAM Panel.
At birth and 3, 6, and 8 weeks afterwards, the mean body weight of the surviving offspring was in the high‐dose group 8.6, 94, 259, and 462 g (SEM: 0.4, 3.1, 12.6 and 17.8 g). This was lower than in controls with means 9.8, 99, 261, and 521 g (SEM 0.4, 2.9, 11.9 and 15.7 g) and in the low‐dose group with means 9.5, 106, 270, and 525 g (SEM: 0.3, 2.6, 10.8 and 14.2 g). The difference was statistically significant at birth and 8 weeks later. The mortality up to 8 weeks was over the first time points statistically significant, higher (74%) in the high‐dose group compared to controls and the low‐dose group (11% and 16%, respectively). The authors interpreted these findings as indications of a higher sensitivity of young mink compared with adults. However, the CONTAM Panel noted that the design of this study was limited since only two dose groups were used, with clear effects only at the highest dose. Furthermore, the study covered at the same time reproductive and developmental toxicity which would not allow a robust investigation of the sensitivity to exposure to MON of young animals in comparison to older ones, in particular, when the three groups differed substantially at birth and litter effects could not be excluded.
Conclusions
The dose of 1.94 mg MON/kg bw per day resulted in significant neonatal mortality and reduced offspring body weights in farmed mink and was identified as LOAEL. The NOAEL was 0.92 mg MON/kg bw per day for farmed mink.
3.1.5.8. Dogs and cats
No data were identified.
3.1.6. Combined effects of MON with other mycotoxins
Co‐exposure to more than one mycotoxin in animals and humans through food and feed has been reported (reviewed in Grenier and Oswald, 2011; Streit et al., 2012). Several in vivo experiments were performed to analyse the effect of MON when present with other mycotoxins. No in vitro data were identified on the combined effect of MON and other mycotoxins (Alassane‐Kpembi et al., 2017). This chapter reports only studies in which the experimental design was set to study combined effects and in which the conclusions were drawn by the authors on the combined effects (Table 4). All of these experiments were performed on farm animals. The CONTAM Panel noted that because of the lack of dose–response data, it is difficult to perform a refined statistical analysis and to draw definitive conclusion.
Table 4.
Possible combined effects between MON and other mycotoxins in vivo
| MON/Tested mycotoxin Species (exposure period) | Concentrations (MON/tested mycotoxin) (mg/kg feed) | Additive or synergistic combined effectsa | Antagonistic combined effecta | Reference |
|---|---|---|---|---|
|
MON/Aflatoxin Chicken (21 days) |
100/3.5 |
– inorganic phosphorus – body weight gain – relative weight (heart) – number of red blood cell |
– albumin – creatinine – alanine aminotransferase – relative weight (gizzard, kidney, bursa of Fabricius) – total protein – cholesterol – calcium – alkaline phosphatase |
Kubena et al. (1997) |
|
MON/FB Turkey (21 days) |
100/200 |
– feed intake (P) – relative weight (liver) – aspartate aminotransferase |
– body weight gain – mortality – glucose – lactate dehydrogenase |
Bermudez et al. (1997) |
|
MON/FB Pig (28 days) |
100/100 |
– feed intake – creatinine (P) – number of red blood cell |
– body weight gain – mortality – glucose – inorganic phosphorus – aspartate aminotransferase, alkaline phosphatase, lactate dehydrogenase, gamma‐glutamyltransferase – total iron |
Harvey et al. (2002) |
|
MON/FB Laying hens (420 days) |
50/100 |
– relative weight (pancreas) – albumin – aspartate aminotransferase – mortality |
– cytokine – relative weight (liver, kidney) – uric acid – egg production – egg weight |
Kubena et al. (1999) |
|
MON/FB Chicken (21 days) |
100/100 | – relative weight (heart) |
– feed intake – mortality – aspartate aminotransferase – body weight gain |
Ledoux et al. (2003) |
| 200/100 | – no parameter tested |
– relative weight (heart, kidney, liver) – albumin, total protein – mortality – aspartate aminotransferase |
||
| 100/200 |
– relative weight (liver, kidney) – albumin, total protein |
– relative weight (heart) – feed intake, body weight gain – mortality – aspartate aminotransferase |
||
| 200/200 | – relative weight (kidney, liver) |
– relative weight (heart) – albumin – mortality – aspartate aminotransferase – total protein |
||
|
MON/FB Turkey (21–28 days) |
100/200 | – antibody to New Castle diseases virus |
– feed intake, body weight gain – relative weight (thymus, bursa of Fabricius, spleen) – lymphocytes stimulation – bacteria in tissue and blood, mortality |
Li et al. (2000a,b) |
|
MON/FB Quail (35 days) |
100/200 |
– body weight – mortality |
– total protein – cholesterol – alanine aminotransferase, aspartate aminotransferase – albumin – lactate dehydrogenase, cytokine – creatinine – delayed type hyposensitivity reaction |
Sharma et al. (2008) |
|
MON/FB Fish (70 days) |
40/20 | – size of hepatocyte nuclei |
– body weight gain, feed intake – Sa/So liver |
Yildirim et al. (2000) |
| 40/40 |
– body weight gain – serum pyruvate – size of hepatocyte nuclei – feed intake |
– Sa/So liver | ||
|
MON/DON Turkey (21 days) |
100/20 |
– feed intake – globulin – relative weight (kidney) |
– body weight gain – relative weight (heart) – calcium |
Morris et al. (1999) |
|
MON/FB1 Chicken (14 days) |
27–154/ 27–154 |
– alkaline phosphatase – gamma glutamyltransferase – aspartate aminotransferase, – lactate dehydrogenase – cholesterol – sodium – red Blood Cell – haemoglobin – packed cell volume |
Javed et al. (1995) |
No parameter tested: In a given study, there was no parameter tested that demonstrated synergistic, additive or antagonistic combined effect.
As concluded by the authors.
Conclusions
The available database describing possible effects of combined exposure to MON and other mycotoxins was weak and insufficient for establishing the nature of combined effects.
3.1.7. Human data
No study in humans was identified that associated adverse effects and exposure to MON. However, in several reports an association was suggested between the occurrence of MON in grains and the prevalence of an endemic disease in China, called KD (International Classification of Diseases, ICD‐10 E5920) Zhang and Li (1988 and) Zhang and Li (1989); Liu (1996). KD is characterised by cardiomyopathy, with acute or chronic heart disorder. The first reported outbreak occurred Keshan county of Helongjiang province in China in 1935. The fatal KD reached a peak in 1960–1970 with reported death rates from 7.6% to 34%, and 49,408 cases and 9,614 deaths were attributed to KD between 1950 and 1982 in Helongjiang province alone. KD gradually disappeared in these regions by the 1980s.
Studies in the endemic regions confirmed that selenium deficiency was a necessary but not sufficient causative factor for KD. All KD endemic regions had low selenium levels in the soil, while no KD was found in areas with high selenium in the soil. In addition to selenium deficiency, MON has been postulated as a contributing factor in KD aetiology. This was based on several findings of Liu (1996):
Pigs fed with MON exhibited pathological changes of myocardial lesion similar to those observed in humans with KD (Qiao et al., 1993; described in Section 3.1.5.2),
MON concentrations in staple food from KD endemic regions were found to be higher than those in the non‐KD region,
MON concentrations in rice samples were lower than in other cereals which corresponded to less acute KD in areas where rice was the staple food,
the occurrence of KD was associated with both high prevalence of MON and low selenium content in cereals.
However, it has been suggested more recently that KD was due to a combination of viral infections and nutrient deficiency (Beck et al., 2003).
The CONTAM Panel noted that a study of Xiong et al. (1998)21 investigated effects of MON and selenium on the articular cartilage of Chinese mini‐pigs.
Conclusions
No human epidemiological data on MON were identified. It has been hypothesised that dietary exposure to MON was involved in past KD prevalence in some regions in China. However, the CONTAM Panel noted that the evidence for a relation between dietary exposure to MON and KD is limited.
3.1.8. Biochemical mode of action
The mode of action of MON is unclear and only a limited amount of data was available. MON and pyruvate show structural similarity, and the primary mode of action of MON seems to be the inhibition of thiamine pyrophosphate‐dependent enzymes, which compromises the tricarboxylic acid cycle. MON has been shown to impair oxidation of pyruvate and α‐ketoglutarate, and to inhibit pyruvate dehydrogenase in vitro (Thiel, 1978; Burka et al., 1982; Gathercole et al., 1986). Later, Pirrung and Nauhaus (1996) demonstrated that several enzymes sharing thiamine as common cofactor (pyruvate dehydrogenase, α‐ketoglutarate dehydrogenase, pyruvate decarboxylase and acetohydroxy acid synthase) were inhibited by MON. This could lead to cellular energy deprivation and may partially explain the respiratory stress, including myocardial effects. It could even cause mortality of test animals exposed to MON (Jonsson et al., 2015). MON may also interfere with carbohydrate metabolism through the inhibition of gluconeogenesis and aldose reductase (Deruiter et al., 1993). In rat myoblasts, MON induces an inhibition of glutathione peroxidase and glutathione reductase suggesting an implication of free radicals in the toxicity (Chen et al., 1990).
Haematotoxicity of MON was tested on human lymphocytes and on the human leukaemia cell line K‐562. The cultures were incubated for 48 h at 37°C with ~ 5% of CO2, before being examined by the MTT colorimetric assay. IC50s were equal to 22 μM and > 100 μM, respectively (Visconti et al., 1991).
Myelotoxicity of MON was tested on human haematopoietic progenitor cells in culture at concentrations equal to 0.1, 1, 5, 10, 20, 60 and 100 μM MON (Ficheux et al., 2012). Cytotoxicity, proliferation and differentiation capacities were measured. MON was cytotoxic at 10 μM for red blood cells progenitors, but not cytotoxic at tested concentrations for white blood cells progenitors and platelet progenitors. MON had no effect on proliferation from 5, 0.1 and 0.1 μM for white blood cells progenitors, platelet progenitors and red blood cells progenitors, respectively. The IC50 was equal to 31, 39 and 4.1 μM for white blood cells progenitors, platelet progenitors and red blood cells progenitors, respectively. MON disturbed also red blood cell progenitors’ differentiation from 2 μM MON, when viability was equal to 83%. Furthermore, MON was cytostatic for megakaryocytic progenitors. A general decrease in large platelet progenitors’ colony number compensated by an increase in small colony number was observed from 60 μM MON, when viability was equal to 52%. Based on these in vitro results, the authors suggested a putative in vivo production of immature red blood cells induced by MON and subsequent anaemia, and an in vivo decrease in platelet production, and consequently coagulation troubles. From this study, the CONTAM Panel concluded that MON caused cytostatic effects on platelet progenitors in vitro and platelet production was decreased. The CONTAM Panel noted that a low number of platelets could induce coagulation troubles in vivo.
Only one identified in vitro study investigated immunotoxicity of MON. Ficheux et al. (2013) observed that up to 80 μM MON induced 20% of mortality on human immature and on mature dendritic cells while no mortality was observed on human macrophages exposed to 80 μM MON. In dendritic cells, 45% of cells presented endocytosis ability in the presence of 80 μM MON. After 5 days of exposure to 80 μM MON, the expression of CD1a, a phenotypic marker of immature dendritic cells was also decreased by 59% compared to non‐treated cells. Monocytes‐derived macrophages exposed to MON during the differentiation process also presented a decrease of endocytosis ability, and a decrease of transferin receptor (CD71) and HLA‐DR expression. Expression of CD11a, CD80 and CD54 was not modified in the presence of 80 μM MON. These results showed that MON disturbs human monocytes differentiation process into macrophages and inhibits human monocytes differentiation process into immature dendritic cells, and suggested that MON could induce a decrease of immune response in case of infection. The immunotoxicity of MON was further confirmed in vivo on poultry (see Section 3.1.5.3).
Conclusions
Only a limited amount of data was identified on the mode of action of MON. The inhibition of enzymes involved in glucose metabolism could lead to cellular energy deprivation and may partially explain the respiratory stress, including myocardial effects. In vitro, scarce observations described cytotoxic effects on platelet progenitors and disturbances in differentiation process of monocytes in dendritic cells.
3.2. Consideration of critical effects, dose–response analysis and derivation of reference points for human and farm and companion animal risk assessments
3.2.1. Critical effects and derivation of reference points for human risk assessment
The toxicological database of MON for chronic studies in experimental animals was scarce. Only very few subacute and subchronic studies of sufficient quality were available for hazard characterisation, and no data on adverse health effects in humans were found. To augment the database for human hazard characterisation the CONTAM Panel examined a subchronic study on pigs (Harvey et al., 2001) and one study on developmental and reproductive toxicity in mink (Morgan et al., 1998).
3.2.1.1. Acute effects
Acute and subacute studies in rats indicate that the heart and the cardiovascular system is a central toxicity target of MON. Acute cardiac effects were observed in rats at doses as low as 10–25 mg/kg bw (Jonsson et al., 2013). Severity increased at 40 mg/kg bw and already at 50 mg/kg bw death of rats was attributed to muscular weakness, and respiratory and cardiovascular changes. This observation was in agreement with results in Kriek et al. (1977), who observed congestive heart failure at 50 mg MON/kg bw in rats. Furthermore, Zhao et al. (1993) reported ultrastructural lesions in the myocardium of rats and mice at 6 and 25 mg/kg bw. On the other hand, no adverse effects were seen in a study of acute toxicity of MON in rats at doses ranging from 2.5 to 10 mg/kg bw (Abbas et al., 1990) and the LD50 values for rats ranged from 18.5 to 50 mg MON/kg bw. In pigs, the dose of 17 mg MON/kg bw (1/6 deaths in the first experiment) was the lowest one where mortality was observed (Harvey et al., 2001), see Section 3.1.5.2. The CONTAM Panel considered the available mortality data and LD50 values from experimental animals unsuitable for derivation of an acute reference dose (ARfD) for MON, due the large uncertainties of these data and the severity of the outcome.
The CONTAM Panel identified the subacute (28 days) study in rats, performed in accordance with OECD guidance with five dose groups (3, 6, 9, 12 or 15 mg/kg bw per day) and a control group, as pivotal study for the study of cardiotoxicity in experimental animals (Jonsson et al., 2015). Acute heart failure occurred at 15 mg MON/kg bw on the 4th and 21st day after presenting strongly decreased activity or somnolence. Signs of toxicity such as decreased activity, withdrawal and somnolence were observed at 9 mg MON/kg bw and were similar to those seen at the two highest doses. Also, a weak grip at the front legs was reported at a dose of 9 mg MON/kg bw per day. No other adverse health effects were reported for the doses up to 9 mg MON/kg bw, and the study NOAEL identified as of 6 mg MON/kg bw was considered as reference point of acute adverse health effects of MON.
Accounting for the limited database with only one suitable subacute study in only one species of experimental animals, its limited number of animals and its limited information on cardiac effects in the lower dose range, the CONTAM Panel considered the available information on acute adverse health effects too limited to establish an ARfD. Therefore, the CONTAM Panel decided to assess the acute human health risk of exposure to MON by comparing the NOAEL of 6 mg MON/kg bw from the subacute study in rats with the estimated acute exposure of humans by calculating a margin of exposure (MOE) (see Section 3.7). Accounting for the severity of cardiotoxicity with the potential of acute death at only slightly higher doses, the CONTAM Panel considered this MOE as indicative to inform on the potential health concern for humans acutely exposed to MON.
3.2.1.2. Chronic effects
Only one subchronic study of MON was available in experimental animals (Kriek et al., 1977) to characterise the chronic hazard of MON. Groups of male and female rats were exposed for 12 weeks to 5 concentrations of mouldy feed containing MON and were examined for general toxicity and pathological changes in major organs, including necropsy at death or final sacrifice. Lesions in the myocardium and myocardial degeneration, necrosis and fibrosis were observed at all concentrations and an increase in severity with dose was demonstrated (see Section 3.1.4.3).
Because of high mortality and absence of dose information for the two highest concentration groups, the CONTAM Panel considered only the controls and the three concentrations 2%, 4% and 8% MON/kg feed (corresponding to 16.6, 34.6 and 32.5 mg MON/kg bw in males and to 16.5, 32.9 and 34.5 mg MON/kg bw in females) for dose–response evaluation. The CONTAM Panel concluded that the clearly increasing severity of adverse health effects over the three doses can be considered as causative for the observed mortality, for which a steep quantitative dose–response relationship could be identified. However, several limitations of the mortality data in male rats were noted in the study of Kriek et al. (1977):
The data set was small, since only a total of 16 animals (n = 4 animals per group) were available for the evaluation of the mortality.
The calculated doses at the concentration groups of 4% and 8% MON/kg diet were almost identical, probably due to lower feed consumption at 8% MON/diet. In effect, only two dose groups were available for dose–response, one in the range of 17 mg MON/kg bw per day and another in the range of 32–34 mg MON/kg bw per day.
In the absence of suitable dose–response data of experimental animals for hazard characterisation, the CONTAM Panel decided to augment the database for human hazard characterisation by available toxicity data on pigs. Therefore, the Panel identified the subchronic study on pigs (Harvey et al., 2001) as being of relevance for human hazard characterisation and identified cardiac toxicity together with haematotoxicity of MON as sensitive chronic adverse effects in the growing barrows exposed to 0, 25, 50, 100 and 200 mg MON/kg feed in two feeding experiments of this study. The Panel noted that co‐exposure with other mycotoxins was quantified as below the LOQ/LOD such that general adverse effects (reduced feed intake and body weight gain), changes in biochemical and haematological parameters and the increase of relative heart weight and mortality related to cardiotoxicity, in particular cardiomegaly, were considered as being clearly dose related and reported as statistically significant at the highest dose of 200 mg MON/kg feed. Based on the reported body weights (dose‐specific means) given in the publication, and assuming a daily feed intake of 1.2 kg feed, the CONTAM Panel identified NOAELs of 1.20–2.22, 1.00 and 4.6 mg MON/kg bw per day for reduced body weight gain, haematological adverse effects (as decreased of haematocrit and haemoglobin levels) and cardiotoxicity, respectively (see Section 3.1.5.2). Furthermore, the Panel evaluated the dose‐dependent decrease of haematocrit and haemoglobin using the BMD approach and identified the lowest BMDL05 of 0.20 mg MON/kg bw per day for haematotoxicity in pigs as a reference point for chronic effects of MON for humans.
The only available information on developmental and reproductive toxicity of MON was from a study in farmed mink exposed to two doses (calculated by the CONTAM Panel as 0.92 and 1.94 mg MON/kg bw per day). Statistically significant mortality was observed during the first 24 h after birth in offspring from dams exposed to the highest dose. The CONTAM Panel identified the significant increase of the number of still births and mortality within the first 24 h postpartum at the high dose as a possible reproductive effect of MON. Effects observed in offspring postpartum, such as reduced weight gain and early mortality after weaning, could possibly be related to postnatal MON exposure, since the kits were exposed during that period to the same dose of MON as the dams, see also Section 3.1.5.7. Therefore, these data were not considered as relevant for developmental effects but would support a reference point in the low mg MON/kg bw per day range.
Accounting for the limited database, with only one limited subchronic study in rats and a 28‐day study in pigs, and in the absence for long‐term toxicity studies, the CONTAM Panel considered the available information on chronic adverse health effects too limited to establish a TDI. Therefore, the CONTAM Panel decided to assess the chronic human health risk of exposure to MON by comparing the reference point of 0.20 mg MON/kg bw per day for haematotoxicity in pigs with the estimated chronic exposure of humans and by calculating a MOE. Accounting for the severity of cardiotoxicity with the potential of acute death at higher doses, and the limitations of the study in pigs, the CONTAM Panel considered this MOE as an indicative on the potential health concern for humans chronically exposed to MON.
3.2.2. Consideration of critical effects and derivation of reference points for farm and companion animal risk assessment
Because of the limited data on MON for some farm and companion animals, NOAELs and/or LOAELs for adverse effects of MON were only identified for poultry, pigs and farmed mink (see Section 3.1.5). While several studies on adverse effects were available for poultry that allowed the identification of NOAELs, the NOAELs identified for pigs and farmed mink were based on data on adverse health effects reported from one study only for each of the two species. The dose–reponse data available for pigs allowed the calculation of a BMDL as reference point based on the haematotoxicity of MON in that farm animal species.
3.2.2.1. Pigs
From the study on pigs (28 days of MON exposure), the CONTAM Panel identified reduced body weight gain, adverse haematological effects and mortality accompanied with lesions in heart as main adverse health effects. NOAELs were between 1.20 and 2.22 mg MON/kg bw per day for reduced body weight gain, 1.00 mg MON/kg bw per day for haematological adverse effects and 4.17 mg MON/kg bw per day for mortality.
The CONTAM Panel identified haematological adverse effects as critical effects and used the lowest BMDL05 of 0.20 mg MON/kg bw per day as a reference point for pigs.
3.2.2.2. Poultry
For broiler chickens, the CONTAM Pane noted that oral LD50 values of 4.0 and 5.4 mg MON/kg bw were reported. Ascites with oedema of the mesenteries and small haemorrhages in the proventriculus, gizzard, small and large intestine, and skin were observed in surviving chickens. The CONTAM Panel identified an overall NOAEL of 1.4 mg MON/kg bw per day and an overall LOAEL of 2.8 mg MON/kg bw per day for generating adverse effects such as some mortalities, reduced body weight gain, cardiomyopathy and changes in the major haematological parameters in broiler chickens.
From the only available study on laying hens, the CONTAM Panel identified an overall NOAEL of 3.8 mg MON/kg bw per day and an overall LOAEL of 8.5 mg MON/kg bw per day for reduced eggs production and reduced body weight gain.
The CONTAM Panel noted that the dose of 3.2 mg MON/kg bw per day did not affect body weight gain and feed intake in turkeys but cardiotoxicity was observed. Based on the data from two studies, the CONTAM Panel identified an overall NOAEL of 1.6 mg MON/kg bw per day and an overall LOAEL of 3.2 mg MON/kg bw per day for cardiotoxic effects in turkeys.
For ducks, the CONTAM Panel noted that the oral LD50 of 3.7 mg MON/kg bw was reported. High doses of MON generated heart arrhythmia and ultimately cessation of contraction causing the death. From the two studies on ducks (available as abstracts), the CONTAM Panel identified an overall NOAEL of 2.3 mg MON/kg bw per day and an overall LOAEL of 2.8 mg MON/kg bw per day for generating cardiomegaly.
3.2.2.3. Farmed mink
From the one subacute study reporting developmental effects on mink, the CONTAM Panel identified a NOAEL of 0.9 mg MON/kg bw per day and a LOAEL of 1.9 mg MON/kg bw per day for neonatal mortality.
3.2.2.4. Ruminants, farmed rabbits, horses, farmed fish, dogs and cats
Since no toxicity data suitable for hazard characterisation of MON were identified for ruminants, farmed rabbits, horses, farmed fish, dogs and cats, no NOAELs/LOAELs could be determined for these farm and companion animals. In order to obtain an indication on the risk of MON for these species, the CONTAM Panel considered the range of NOAELs of 1–4 mg MON/kg bw per day identified for pigs, poultry and farmed mink, and considered, in particular, the reference point of 0.20 mg MON/kg bw per day for haematotoxicity of MON in pigs as indicative for potential adverse health effects of MON. The CONTAM Panel noted that the range of NOAELs of 1–4 mg MON/kg bw identified for pigs, poultry and farmed mink was at least one order of magnitude lower than then LD50 values identified for experimental animals, but only slightly lower than the LD50 values identified for the poultry species, for which the LD50 values were available. Therefore, the CONTAM Panel identified the BMDL05 for pigs of 0.20 mg MON/kg bw per day as an indicative reference point for adverse health effects of MON in rabbits, horses, farmed fish, dogs and cats for which data on adverse effects were not available or insufficient. The Panel noted that this value may largely overestimate the possible hazard of MON for some, if not all, species in this group and that toxicity data are needed for the hazard identification of MON in ruminants, farmed rabbits, horses, farmed fish, dogs and cats.
3.3. Occurrence of moniliformin in food and feed
3.3.1. Occurrence data on food and feed reported in the available literature
Data on the occurrence of MON in food and feed have been published in the literature, in particular in the last decade. MON has been reported to co‐occur with many other mycotoxins, in particular Fusarium toxins including type‐A trichothecenes, type‐B trichothecenes, enniatins, beauvericin and zearalenone and related compounds (Uhlig et al., 2013) (see also Section 3.1.6). This section reports only the literature data obtained by LC–UV and LC–MS/MS techniques and published from 2002 onwards in line with the occurrence data reported to EFSA (see Section 3.3.2), and only data relating to the European market. Data from previous years were not considered, due to limitations in the performance of the analytical methods used before 2002 and to ascertain a representative picture, because changes in environmental conditions and agricultural practices might have influenced MON formation by Fusarium species. The occurrence data from the literature are reported in Table 5 for food and Table 6 for feed.
Table 5.
Published occurrence data on MON in grains and foods
| Country | Sampling | Type of sampling | Product | N | LOD μg/ kg | LOQ μg/ kg | Samples greater than LOQ | Mean of positive samples μg/kg | Mean of all samples a μg/kg | Mean (not specified) b μg/kg | Median μg/kg | Min μg/kg | Max μg/kg | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Finland | 2001 | Random | Barley | 14 | 10 | 20 | 4 | (106) | n.r. | n.r. | n.r. | (< LOQ) | 290 | Jestoi et al. (2004) |
| Wheat | 7 | 3 | (373) | n.r. | n.r. | n.r. | (< LOQ) | 810 | ||||||
| Rye | 1 | 0 | n.r. | (< LOQ) | n.r. | (< LOQ) | (< LOQ) | < LOQ | ||||||
| 2002 | Barley | 8 | 6 | (391) | n.r. | n.r. | n.r. | (< LOQ) | 750 | |||||
| Oats | 1 | 1 | (84) | (84) | n.r. | (84) | (84) | 84 | ||||||
| Spring wheat | 7 | 6 | (72) | n.r. | n.r. | n.r. | (< LOQ) | 96 | ||||||
| Italy | 2008 | Random | Maize (not reported whether for food or feed use) | 16 | 1 | 4 | 16 | n.r. | n.r. | 1127 | n.r. | 33 | 2606 | Scarpino et al. (2013) |
| 2009 | 16 | 13 | n.r. | n.r. | 106 | n.r. | < LOD | 527 | ||||||
| 2010 | 40 | 39 | n.r. | n.r. | 262 | n.r. | < LOD | 920 | ||||||
| 2011 | 36 | 33 | n.r. | n.r. | 89 | n.r. | < LOD | 409 | ||||||
| Nether‐lands | 2009 | At harvest | Winter wheat | 86 | n.r. | 6.6 | 23 | n.r | 5.7c | n.r. | n.r. | (< LOQ) | 326 | Van der Fels‐Klerx et al. (2012) |
| Preharvest | 21 | 5 | n.r | 6.7c | n.r. | n.r. | (< LOQ) | 97 | ||||||
| Norway | 2000 | Random | Oats | 20 | 40 | 130 | 1 | n.r. | n.r. | n.r. | < LOD | (< LOQ) | 70 | Uhlig et al. (2004) |
| Barley | 19 | 1 | n.r. | n.r. | n.r. | < LOD | (< LOQ) | 43 | ||||||
| Wheat | 13 | 6 | n.r. | n.r. | n.r. | < LOD | (< LOQ) | 87 | ||||||
| 2001 | Oats | 26 | 3 | n.r. | n.r. | n.r. | < LOD | (< LOQ) | 88 | |||||
| Barley | 23 | 4 | n.r. | n.r. | n.r. | < LOD | (< LOQ) | 380 | ||||||
| Wheat | 35 | 27 | n.r. | n.r. | n.r. | 69 | (< LOQ) | 420 | ||||||
| 2002 | Oats | 26 | 19 | n.r. | n.r. | n.r. | 59 | (< LOQ) | 210 | |||||
| Barley | 33 | 14 | n.r. | n.r. | n.r. | < LOD | (< LOQ) | 230 | ||||||
| Wheat | 35 | 30 | n.r. | n.r. | n.r. | 120 | (< LOQ) | 950 | ||||||
| Norway | 2002 |
Random/ targeted d |
Organic wheat | 35 | 40 | n.r. | n.r. | n.r. | n.r. | 98 | 60 | n.r. | n.r. | Bernhoft et al. (2010) |
| Conventional wheat | 35 | n.r. | n.r. | n.r. | 211 | 124 | n.r. | n.r. | ||||||
| 2003 | Organic wheat | 30 | n.r. | n.r. | n.r. | 71 | < LOD | n.r. | n.r. | |||||
| Conventional wheat | 30 | n.r. | n.r. | n.r. | 59 | < LOD | n.r. | n.r. | ||||||
| 2004 | Organic wheat | 27 | n.r. | n.r. | n.r. | 52 | < LOD | n.r. | n.r. | |||||
| Conventional wheat | 27 | n.r. | n.r. | n.r. | 47 | < LOD | n.r. | n.r. | ||||||
| Norway | 2011e | Random | Barley | 20 | n.r. | n.r. | 20 | n.r. | n.r. | n.r. | 86 | n.r. | 522 | Uhlig et al. (2013) |
| Oats | 28 | 28 | n.r. | n.r. | n.r. | 57 | n.r. | 220 | ||||||
| Wheat | 28 | 28 | n.r. | n.r. | n.r. | 88 | n.r. | 400 | ||||||
| Norway | 2014 | Random | Oats | 7 | 20 | 66 | 2 | n.r. | n.r. | 116 | (< LOQ) | n.r. | 165 | Ivanova et al. (2017) |
| Poland | 2007 | Targeted | Asparagus spears | 40f | n.r. | n.r. | n.r. | n.r. | n.r. | 121 | n.r. | (< LOQ) | 585 | Waskiewicz et al. (2010) |
| Sweden | 2009 | Random | Winter wheat | 31 | n.r. | 15 | 23 | n.r. | n.r. | n.r. | 53 | (< LOQ) | 497 | Lindblad et al. (2013) |
| 2011 | 33 | 12 | n.r. | n.r. | n.r. | < LOQ | (< LOQ) | 255 | ||||||
| 2010 | Spring wheat | 28 | 20 | n.r. | n.r. | n.r. | 61 | (< LOQ) | 1066 | |||||
| 2011 | 33 | 21 | n.r. | n.r. | n.r. | 27 | (< LOQ) | 2078 | ||||||
| Sweden | 2010 | Random | Oats | 50 | n.r. | 15 | 21 | n.r. | n.r. | n.r. | < LOQ | (< LOQ) | 156 | Fredlund et al. (2013) |
| 2011 | 43 | 18 | n.r. | n.r. | n.r. | < LOQ | (< LOQ) | 215 | ||||||
| UK | 2002–2003 | Random | Barley | 239 | n.r. | 10 | 5 | n.r. | < LOQg | n.r. | < LOQ | n.r. | 45 | Edwards (2007) |
| Oats | 196 | 0 | n.r. | < LOQg | n.r. | < LOQ | n.r. | < LOQ | ||||||
| UK | 2012 | Random | ethnic foodh | 240 | 5 | 10 | 2i | 23 | n.r. | n.r. | n.r. | (< LOQ) | 25.5 | FSA (2013) |
n.r.: not reported; N: number of samples; LOD: limit of detection; LOQ: limit of quantification.
Note: Values in brackets were calculated by the CONTAM Panel based on the data provided in the papers.
Mean values of all samples are calculated by setting samples in which the compounds were not detected to 0 and trace value below LOQ to LOD.
It was not reported in the paper whether the mean was calculated for all samples or for positive samples only.
Contamination levels < LOQ were set to zero.
Farms producing organic cereals were randomised, but conventional cereal farms were selected relatively randomly on the basis of neighbouring the producer of the organic cereal sampled
Exceptionally wet summer indicated.
20 Asparagus spears with brown spots (fusariosis) and 20 asparagus spears with no disease symptoms. Mycotoxins were determined in basal parts, results were not differentiated between the two types of asparagus samples.
Means based on imputation of 1.667 (LOQ/6) for all samples below LOQ (10 μg/kg).
Herbs, spices and seeds, legumes, cereals and cereal products, dairy products and root vegetables classified by the authors as ethnic foods.
Positive samples were from the category ‘cereals and cereal products’.
Table 6.
Summary of MON concentrations in animal feed reported in the literature
| Country | Sampling year(s) | Cereal | Samples (n) | LOD μg/kg | n > LOD | LOQ μg/kg | n > LOQ | Minimum μg/kg | Maximum μg/kg | Mean μg/kg | Sampling | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cereal grains | ||||||||||||
| Norway | 2000–2002 | Barley | 75 | nr | nr | 130 | 53 | < LOQ | 380 | nr | Uhlig et al. (2004) | |
| Austria | ns | Maize | ns | nr | nr | 39 | ns | 160 | 1,030 | nr | Grain from fields | Parich et al. (2003) |
| Denmark | ns | Maize | 28 | nr | nr | 12 | 0 | < LOQ | < 12 | nr | Whole maize plant | Sorensen et al. (2007) |
| Germany | 2006 | Maize | 44 | nr | nr | 10 | 19 | nr | 3,330 | 280 | Goertz et al. (2010) | |
| Germany | 2007 | Maize | 40 | nr | nr | 10 | 18 | nr | 1,850 | 110 | Goertz et al. (2010) | |
| Italy | 2008 | Maize | 16 | 1 | nr | 4 | 16 | 33 | 2,606 | 1,127 | Maize grain (not reported whether for food or feed use) | Scarpino et al. (2013) |
| Italy | 2009 | Maize | 16 | 1 | nr | 4 | 13 | < LOQ | 527 | 106 | ||
| Italy | 2010 | Maize | 40 | 1 | nr | 4 | 39 | < LOQ | 920 | 282 | ||
| Italy | 2011 | Maize | 36 | 1 | nr | 4 | 33 | < LOQ | 409 | 89 | ||
| Netherlands | 2010 | Maize cobs | 42 | 50 | 1 | 25 | nr | < LOD | 332 | nr | Cobs from standing plants intended for silage | Van Asselt et al. (2012) |
| Finland | 2001–2002 | Oats | 1 | nr | nr | 20 | 1 | nr | 84 | nr | Jestoi et al. (2004) | |
| Norway | 2000–2002 | Oats | 73 | nr | nr | 130 | 38 | < LOQ | 210 | nr | Uhlig et al. (2004) | |
| UK | 2002–2003 | Oats | 196 | nr | nr | 10 | 0 | nr | < 10 | nr | Grain from commercial feed manufacturers | Edwards (2009) |
| Norway | 2000–2002 | Wheat | 83 | nr | nr | 130 | 76 | < LOQ | 950 | nr | Grain from fields | Uhlig et al. (2004) |
| Netherlands | 2009 | Wheat | 86 | nr | nr | 6.6 | 23 | 6.8 | 119 | 5.5 | Grain from fields | Van der Fels‐Klerx et al. (2012) |
| Poland | ns | Wheat | 10 | ns | 4 | ns | nr | nr | 200 | nr | Grain from fields | Krysinska‐Traczyk et al. (2001) |
| Poland | 1993 | Wheat | 25 | 25 | 7 | nr | nr | nr | 198 | 63 | Grain from fields | Grabarkiewicz‐Szczesna et al. (2001) |
| Complete feeds | ||||||||||||
| Slovakia | 2003–2004 | Poultry feed | 50 | nr | nr | 39 | 26 | nr | 1214 | nr | Commercial compound feed | Labuda et al. (2005) |
N: number of samples; LOD: limit of detection; LOQ: limit of quantification; nr: not reported.
3.3.1.1. Occurrence data on food reported in the available literature
MON has been reported in the literature to occur in particular in grains (maize, wheat, barley, oats and triticale). Incidentally MON was found in asparagus (Knaflewski et al., 2008; Karolewski et al., 2011) and in pineapple juice (Stępień et al., 2013). The occurrence data of MON in food and food products, sampled from 2000 to 2014 (reported in the literature from 2004 to 2017) were summarised in Table 5.
Ten studies reported the occurrence of MON in various grains, one study reported the occurrence of MON in asparagus and one study reported the occurrence of MON in ethnic foods (as classified by the authors). Most data were from grains and mostly from northern Europe. Altogether, data were reported from 1,683 samples, of which 108 samples of maize, 541 samples of wheat, 356 samples of barley, 397 samples of oats, 1 sample of rye, 40 samples of asparagus and 240 samples of ethnic foods. It was noted by the CONTAM Panel that some articles provided only limited information which sometimes made their interpretation of the analytical data difficult. Concerning grains, the highest values reported for maize, wheat, barley and oats were 2,606, 2,078, 522 and 220 μg/kg, respectively. For asparagus, the highest reported value was 585 μg/kg, while for ethnic food the highest reported value was 25.5 μg/kg. Mean values ranged from 89 to 1127 μg/kg for maize, from 5.7 to 373 μg/kg for wheat, from < LOQ to 391 μg/kg for barley and from < LOQ to 166 μg/kg for oats.
3.3.1.2. Occurrence data on feed reported in the available literature
The studies identified by the CONTAM Panel on the occurrence of MON in feeds for farm animals are summarised in Table 5. The studies specifically relating to levels of MON in feeds reveal considerable variation between cereal types in the occurrence and maximum concentrations. Generally, concentrations tend to be higher in maize (corn) grains than in other cereals (Uhlig et al., 2004) and are lowest in oats (Edwards, 2009).
As reported below (Section 3.4), processing can result in changes in concentrations of MON in the different fractions produced. Krysinska‐Traczyk et al. (2001) analysed 10 samples of stored wheat grain and 10 samples of settled grain dust released during machine threshing of wheat grain were collected on 10 farms located in Lublin province (eastern Poland). MON was detected in 4/10 samples of grain (highest 200 μg/kg) but in 8/10 samples of grain dust (maximum 780 μg/kg).
Only one report of levels of MON in complete feedingstuffs has been identified. Labuda et al. (2005) analysed 50 samples of poultry feed from farms in Slovakia. MON was detected in 26 of the samples, with concentrations ranging from 42 to 1,214 μg/kg (mean 217 μg/kg) (LOD = 39 μg/kg).
The European corn borer (ECB) is a major pest of maize (corn), and strategies to control it have included the development of genetically modified maize (Bt maize). A study by Magg et al. (2003) examined concentrations of MON in maize grains from genetically modified maize (Bt maize) and non‐genetically modified maize across five locations. The mean concentration in grains from the insecticide‐protected plants was 66.2 μg MON/kg, while that for the non‐GM (Fusarium‐infested) maize grains was 296.0 μg MON/kg. In a 4‐year study of maize plants in north‐west Italy, Scarpino et al. (2013) also reported an increase in levels of MON in grains associated with an increasing incidence of the ECB.
Forages are important – and frequently the sole – feeds for ruminant livestock, and may be fed fresh or preserved (e.g. as hay or silage). MON has been reported particularly in maize plants and maize silage (Sorensen et al., 2007; Storm et al., 2010). In view of the relatively high levels of MON reported in maize grains, it might be expected that whole maize plants intended for silage are also potential sources of exposure for ruminant livestock. However, Sorensen et al. (2007) did not detect MON in any of the 28 samples analysed from whole plants intended for ensiling. Van Asselt et al. (2012) also analysed maize cobs intended for ensiling; of the 42 samples only one had a level of MON (332 μg/kg) greater than the LOD. In the current occurrence data on feed provided to EFSA, 31% per cent of the 42 samples of maize silage reported were blow LOD, and there was a maximum of 44 μg/kg, suggesting that maize silage is not likely to be a major contributor to exposure to MON by farm animals (see Section 3.3.2.3).
3.3.2. Occurrence data in food, feed and unprocessed grains of undefined end‐use used for the assessment
3.3.2.1. Analytical methods
Considering all the available analytical results (i.e. the results submitted to EFSA by Member States and the results extracted from the published literature), where classification of the analytical method used for determination of MON in food/feed/crops was reported by the Member States, results were obtained by LC–MS based methods (70%) and by HPLC (10%). For the remaining 20% of the data, no information on analytical methods was reported.
3.3.2.2. Food occurrence data used for the assessment
The 2,799 analytical results submitted to EFSA were available from two European countries (Section 2.3.1) namely the Netherlands reported 84% of the MON data and UK the remaining 16%. Data were reported on samples collected between the years 2002 and 2015, with the majority of the data collected in 2014 and 2015. In addition to the data submitted to EFSA by the Netherlands and UK the data on the 406 samples extracted from the scientific literature (Section 2.3.1) were from Italy (n = 108), the Netherlands (n = 86), Sweden (n = 91), Norway (n = 83) and Finland (n = 38), and referred to samples collected between 2001 and 2015 (the majority collected in 2009–2011).
Overall, 3,205 analytical results on MON in food were included in the exposure assessment analysis. These include the data reported by the Netherlands and the UK (i.e. 2,799 analytical results, 87%) and literature data reported for Europe (i.e. 406 analytical results, 13%). The origin of the samples was not always the European country who reported the data, i.e. the data set also contained samples originating from North and South America, Africa, Asia and Australia.
The LODs/LOQs of the MON data from the EFSA database and the scientific literature data varied between laboratories and food matrices (i.e. LOD minimum–maximum 1–20 μg/kg, mean 5 μg/kg; LOQ minimum–maximum 4–66 μg/kg, mean 41 μg/kg). The lowest mean LOQs were reported for the FoodEx level 2 categories of ‘Snack, desserts and other foods’ (LOQ=10), ‘Fine bakery wares’ (LOQ=10), and ‘Grains for human consumption’ (LOQ = 18 μg/kg). A high percentage of results below LOD/LOQ in combination with high LODs/LOQs with substantial differences between LB and UB scenarios were observed, increasing the uncertainty associated with the dietary exposure estimations. In order to reduce this impact, but also not to exclude data on foods mainly contributing to the exposure to MON, an evaluation of LOQs was performed for those MON data for which the results were considered to be suitable for the dietary exposure assessment. This evaluation was based on the EFSA internal guidance on the application of LOD/LOQ cut‐offs. A special attention was paid to food categories which are considered to be potentially important contributors to the dietary exposure to MON. Such a main food category identified was FoodEx level 1 ‘Grains and grain‐based products’. The analytical results were submitted to EFSA as corrected for recovery in all cases. Fifty‐three per cent of analytical results retrieved from the scientific literature were reported as being corrected for recovery.
Approximately, 99% of the data were obtained for samples collected within official monitoring programs and 1% for samples from specific studies on occurrence on MON in food. Regarding the sampling method, a minor part of analytical results (1%) were obtained from pooled samples meaning that the result represented an average of a number of samples taken in equal parts from different consignments/batches and pooled together for the laboratory analysis. Since the level of aggregation for pooled samples matched the level of classification of the individual samples (only similar food matrices were pooled together) results from pooled samples were retained for further evaluation. To ensure a proportionate representation of the individual samples, and thus an accurate use of occurrence data in assessing the dietary exposure, the mean concentrations per food category were calculated by weighting the reported analytical results for the number of samples pooled.
All analytical results were expressed on whole weight basis, thus no conversion had to be applied.
The MON occurrence data were available for 14 FoodEx level 1 food categories. An overview of the number of data points available for exposure assessment, the percentage of results below LOD/LOQ, the mean and 95th percentile concentrations of MON, are presented in Appendix B, Table B.2–B.4.
Table B.2.
Assumed live weights and feed intake for pigs, poultry and fish
| Live weight (kg) | Feed intake (kg dry matter/day) | Reference | |
|---|---|---|---|
| Pigs: starter | 20 | 1.0 | EFSA (2012) |
| Pigs: finishing | 100 | 3.0 | EFSA (2012) |
| Pigs: gilts | 50 | 2.0 | |
| Pigs: lactating sows | 200 | 6.0 | EFSA (2012) |
| Poultry: broilersa | 2 | 0.12 | EFSA (2012) |
| Poultry: laying hens | 2 | 0.12 | EFSA (2012) |
| Turkeys: fattening turkeys | 12 | 0.40 | EFSA (2012) |
| Ducks: fattening ducks | 3 | 0.14 | Leeson and Summers (2008) |
| Salmonids | 2 | 0.04 | EFSA 2012 |
| Carp | 1 | 0.02 | Schultz et al. (2012) |
Fattening chickens.
Table B.4.
Feed intakes of high yielding lactating dairy cows (40 litres/day) and beef cattle fed diets based on different forages with non‐forage feeds adjusted for milk yield, and beef cattle
| Ruminants | Quantities of feed consumed (kg dry matter/day) | Reference | ||
|---|---|---|---|---|
| Forage | Maize grain | Barley grain | ||
| Lactating dairy cows: maize silage‐based diet | 15.0 | 9.5 | ni | AFSSA (2009) |
| Fattening beef cattle: maize silage‐based diet | 4.9 | ni | ni | EBLEX (2012) |
| Beef cattle: cereal straw‐based diet | 2.5 | ni | 4.1 | EBLEX (2008) |
| Beef cattle: ‘Cereal beef’ | 1.5 | ni | 5.5 | EBLEX (2005) |
ni: not included in the diet formulations.
MON was quantified in the following FoodEx level 1 categories: ‘Grains and grain‐based products’ (n = 1,457, LB/UB means of 32.2/56.7 μg/kg) and ‘Snacks, desserts, and other foods’ (n = 4, LB/UB mean = 73/75.5 μg/kg).
Within FoodEx level 1 category ‘Grains and grain‐based products’, quantified values were obtained in a number of food categories at FoodEx level 2: ‘Grains for human consumption’ (LB/UB mean = 47.1/60.4 μg/kg), ‘Grain milling products’ (LB/UB mean = 0.6/48.8 μg/kg), ‘Pasta (raw)’ (LB/UB mean = 0.2/49.3 μg/kg), ‘Breakfast cereals’ (LB/UB mean = 1.5/49.4 μg/kg) and ‘Fine bakery wares’ (LB/UB mean = 8.4/13.4 μg/kg). Overall, 99% of the quantified results were on ‘Grains and grain‐based products’ and in particular on ‘Grains for human consumption’. Therefore, the mean concentration for the food category was strongly influenced by the results obtained on the samples of ‘Wheat grain’, ‘Barley grain’, and ‘Corn grain’ and ‘Oats grain’. These data were reported by 6 different countries.
3.3.2.3. Feed occurrence data used for the assessment
The 806 results for the feed samples analysed on MON were from four European countries, including Austria (n = 380, 47%), Norway (n = 235, 29%), the Netherlands (n = 107, 13%) and Germany (n = 84, 10%). The results from Austria and Norway were provided to EFSA and the data from the Netherlands and Germany were collected from the published literature (see Section 2.3.1). The origin of the data was not always the EU, i.e. the data set also contained samples of feed imported from Turkey, Ukraine and Russia. The analytical results were from samples collected from 2006 to 2016, with the majority of the data collected between 2015 and 2016.
The LODs/LOQs of the MON data reported to EFSA varied between countries (i.e. Austria LOD = 4.8 and LOQ = 16.1 μg/kg; Norway LOD = 51.3 and LOQ = 171 μg/kg; Germany LOQ = 10 μg/kg; the Netherlands LOQ = 6.6 μg/kg).
In the final data set, MON occurrence data were available for three feed categories at the FoodEx level 1. An overview of the number of data points available for exposure assessment, the percentage of results below LOD/LOQ, the mean and 95th percentile concentrations of MON, are presented in Annex A, Tables A5–A7.
The reported feed categories were ‘Cereal grains, their products and by‐products’ (n = 739), ‘Forages and roughage, and products derived thereof’ (n = 65) and ‘Oil seeds, oil fruits, and products derived thereof’ (n = 2). MON was quantified in all three feed categories.
In the category ‘Cereal grains, their products and by‐products’ quantified values were reported for a number of feed categories at FoodEx level 2: ‘Barley’ (LB/UB mean = 40.5/84.8 μg/kg), ‘Wheat’ (LB/UB mean = 9.7/46.9 μg/kg), ‘Maize’ (LB/UB mean = 69.6/73.7 μg/kg), ‘Oats’ (LB/UB mean = 18.1/136.9 μg/kg), ‘Rye’ (LB/UB mean = 11.4/147.9 μg/kg), ‘Spelt’ (LB/UB mean = 0.0/171.0 μg/kg) and ‘Cereal grains, their products and by‐products, unspecified’ (LB/UB mean = 2.3/16.2 μg/kg). In the category ‘Oil seeds, oil fruits, and products derived thereof’, the reported levels were LB/UB mean = 3.9–11.9 μg/kg. In the category ‘Forages and roughage, and products derived thereof’, quantified values were reported for ‘Maize silage’ (LB/UB = 15.9/20.9 μg/kg), ‘Cereals straw’ (LB/UB = 6.2/17.8 μg/kg) and ‘Grass, field dried, [Hay]’ (LB/UB = 5.7/15.4 μg/kg).
3.3.2.4. Unprocessed grains of undefined end‐use occurrence data used for the assessment
The category ‘Unprocessed grains’ comprised grains of undefined end‐use, defined also as ‘Grains as crops’. As the end‐use of the grains at harvest is not established and because normally grains for human and animal consumption undergo several processing steps before being used, it was considered appropriate to report their concentrations separately.
An initial number of 504 results of ‘Unprocessed grains of undefined end‐use’ samples analysed for MON reported to EFSA by Sweden were available for the assessment (Section 2.3.1). The origin of the samples was not always the European country who reported the data, i.e. the data set also contained samples originating from Asia, North America and Africa. Data were reported on samples collected from 2010 to 2015. The LODs/LOQs of the MON data were LOD = 8 μg/kg and LOQ = 39 μg/kg.
In the final data set, MON occurrence data were available for three categories at the FoodEx level 3. An overview of the number of data points available for exposure assessment, the percentage of results below LOD/LOQ, the mean and 95th percentile concentrations of MON, are presented in Annex A, Tables A8–A10.
The reported categories were ‘Wheat grain’ (n = 105), ‘Barley grain’ (n = 46), ‘Rye grain’ (n = 17), ‘Buckwheat grain’ (n = 4), ‘Millet grain’ (n = 2), ‘Oats grain’ (n = 139) and rice (n = 191). MON was quantified in ‘Wheat grain’ (LB/UB mean = 102.4/109.6 μg/kg) and ‘Oats grain’ (LB/UB mean = 15.3/39.0 μg/kg).
3.3.3. Conclusions on the occurrence data
Twelve studies on the occurrence of MON in food (sampling years 2001–2014) and 12 studies on the occurrence of MON in feed (sampling years 2006–2016) were published. For food, most data were obtained for food grains, with the highest values reported for maize, wheat, barley and oats at 2,606, 2,078, 522 and 220 μg/kg, respectively. For asparagus, the highest reported value was 585 μg/kg, while for ethnic food the highest reported value was 25.5 μg/kg. Mean values ranged from 89 to 1,127 μg/kg for maize, from 5.7 to 373 μg/kg for wheat, from < LOQ to 391 μg/kg for barley and from < LOQ to 166 μg/kg for oats.
Twelve studies on the occurrence of MON in feeding stuffs sampled between 2000 and 2011 were identified in the literature. For most of the studies, the data were obtained for whole feed grains, with maximum levels for maize (corn), wheat, barley and oats of 3,330, 950, 380 and 210 μg/kg, respectively. In two studies, MON was detected in maize cobs (maximum concentration 84 μg/kg) but not in whole maize plants. In one study involving commercially manufactured compound feed for poultry, a maximum of 1,214 μg/kg was reported.
For food, current occurrence data included data submitted to EFSA (n = 2,799 from two countries) and data extracted from the scientific literature (n = 406 from five countries). Quantified values of data submitted to EFSA and the ones extracted from the scientific literature were in the same range. MON was quantified in ‘Grains and grain‐based products’ (LB/UB mean = 32.2/56.7 μg/kg, 80% left‐censored) and in ‘Snacks, desserts, and other foods’ (LB/UB mean = 73.0/75.5 μg/kg, 25% left‐censored). For feed, 806 analytical results from four countries were available for three feed categories (i.e. cereal grains, oil seeds, and forages and roughage). The levels ranged from LB/UB mean = 9.7/46.9 μg/kg for ‘Wheat’ to LB/UB mean = 11.4/147.9 μg/kg for ‘Spelt’. The levels were lower for ‘Oil seeds, oil fruits, and products derived thereof’ and ‘Forages and roughage, and products derived thereof’. The 504 analytical results available from Sweden for unprocessed grains of undefined end‐use were in a similar range.
Overall, although there was considerable variation in levels of MON reported in foods and feeds, the data used to estimate exposure are broadly similar to those reported in the literature for foods and feeds.
Furthermore, MON has been reported to co‐occur with many other mycotoxins, in particular with Fusarium toxins.
3.4. Food and feed processing
The extent to which cereals are processed depends on the cereal type and the final feed/food product. In general, it is known that processing reduces Fusarium toxin concentrations in products for human consumption but may increase levels in food or feed by‐products. This is because mechanical cleaning of cereals (de‐hulling) may lead to by‐products (for the food and feed industry) in which Fusarium toxins concentrate significantly. While this may generally result in (much) higher concentrations of Fusarium toxins in these materials than in the cereals before cleaning, scarce studies on the effect of milling on MON show this may not be the case for this mycotoxin. The effects of processing of cereals and cereal products, in ways common to the food and feed industry, on MON have been investigated in a limited amount of studies, while the focus was sometimes in combination with the effects of processing on other Fusarium toxins.
3.4.1. Food processing
3.4.1.1. Cleaning and sorting
Studies on the specific effect of cleaning and sorting on MON could not be identified. In general, sorting of cereals by removing extensively damaged or infected kernels has the effect of lowering the concentrations of mycotoxins in subsequently produced products (Abbas and Mirocha, 1985; Scudamore and Patel, 2009).
3.4.1.2. Rolling and milling
Tittlemier et al. (2014) investigated the fate of MON during milling of durum wheat, as well as during the production and cooking of spaghetti. Samples of clean durum wheat were fortified with kernels contaminated by F. avenaceum, resulting in durum wheat samples containing MON ranging from 0.16 to 0.90 mg/kg. After milling in a laboratory mill, semolina, bran, shorts, flour and feed flour were analysed for MON, showing concentrations of MON at 57–135, < 4–8, < 4–11, 56–106 and 24–31%, respectively, of the initial concentrations. Semolina, flour and feed flour not only contained the highest concentrations of MON, but they also represented the largest compartment of MON in the milling mass balance. On average semolina, flour and feed flour contained 85%, 6.8% and 1.4% of the MON in the whole wheat prior to milling, respectively. The authors indicated that this association between MON and endosperm was different from what has been observed with other Fusarium mycotoxins, such as DON, which has been associated with outer bran layers of wheat. They explained the differences in distribution between MON and DON in milling products to be due to the translocation of MON from mycelium to endosperm, because physical characteristics of MON suggest it could be more mobile than DON within a kernel.
3.4.1.3. Cooking and baking
Scott and Lawrence (1987) investigated the stability of MON in cereal grains during heating. Ground maize and ground wheat both spiked at a level of 1 mg MON/kg were held at 50, 100 and 150°C for 0.5–2 h in a constant‐temperature cabinet. Heating at all temperatures led to losses of MON and the reductions were correlated with increases in temperature. At 50°C, approximately 80% and approximately 60% of MON remained in the maize and in the wheat, respectively. At 100°C, 38% of MON remained in the maize and 22% in the wheat, while at 150°C these percentages were 38% and 15%, respectively.
Castelo (1999) studied the fate of MON during extrusion cooking. Food grade yellow maize grits were spiked with MON at 5 mg/kg grits (dry basis). The moisture content of the grits was raised to 26% by adding water. Samples were extruded at different temperatures (140, 160, 180 and 200°C) and screw speeds (40, 80, 120 and 160 rpm). Extrusion cooking resulted in statistically significant losses of MON. The percentage loss of MON ranged from 24% to 34%. Statistical analysis showed no significant differences between the different screw speeds and no significant differences between the different temperatures. The study showed that MON is a relatively heat‐stable compound that can largely survive most conditions used in thermally processed food.
A complete reduction (100%) of MON was observed by Pineda‐Valdes et al. (2002), when a naturally contaminated maize sample containing 1.4 mg MON/kg was used in a pilot‐scale alkaline tortilla manufacturing process. In the first step with alkaline cooking of maize, the concentration of MON was already reduced by 97%, while MON was not detected in any steps after alkaline cooking, including the rinsing water (study did not report the results for the remaining 3% of MON). A parallel experiment performed at laboratory scale, using a maize sample containing 17.6 mg MON/kg, resulted in a reduction of 71% from the initial concentration in the maize to the final product. The greatest reduction of MON (54%) resulted from the alkaline cooking step. Based on these findings, the authors concluded that MON was reduced significantly by the nixtamalisation process, probably due to both heat and the alkaline pH. This confirms the earlier finding (see also Section 1.3.1) that heating at pH 10 caused a major reduction of the concentration of MON (Pineda‐Valdes and Bullerman, 2000). Pineda‐Valdes et al. (2003) investigated the effects of common commercial food processing methods, including baking, autoclaving, extrusion, frying and roasting, on reduction of MON in maize‐based food products. Maize muffin baking from maize meal, spiked with 5 mg MON/kg (dry basis) at 204°C and 218°C led to MON concentration decreases by 38% and 42%, respectively. Autoclaving at 121°C for 65 min of creamed maize for infants and cream style maize, both spiked with 5 mg MON/kg (wet basis), showed MON reductions of 10% and 23%, respectively. Extrusion cooking of maize grits mixing screw (with 18% moisture content and spiked with 5 mg MON/kg (dry basis)) at 180°C decreased MON concentration by 27%. Frying of maize masa spiked with 5 mg MON/kg (dry basis) at 190°C for 10 min for maize chips production reduced MON concentration by 30%. Roasting of maize meal spiked with 5 mg MON/kg (dry basis) at 218°C for 15 min had the most significant effect of the food processing methods on reduction of MON, inducing a 45% decrease in its concentration.
Tittlemier et al. (2014) studied the fate of MON during production and cooking of spaghetti. Spaghetti was processed from 3 samples of semolina with MON concentrations 0.14, 0.52 and 1.02 mg/kg using a customised microextruder, and dried in a pilot‐scale pasta dryer. Dried uncooked spaghetti was placed into boiling water and cooled down for 10 min followed by water removal. The cooked spaghetti was air dried for approximately 60 h at room temperature prior to MON analysis. There was a large decrease in the amount of MON measured, when raw spaghetti was prepared. MON could only be measured in raw spaghetti obtained from the two semolina samples with the highest levels of MON and 27% of MON measured in semolina appeared to be retained in the raw spaghetti from these two samples. During the cooking process, a further reduction of the MON concentration in the cooked spaghetti of approximately 50% was observed, with a part of the water‐soluble MON leaching into the cooking water. A balance study revealed an overall increase in the amount of MON in the cooked spaghetti plus cooking water as compared to the uncooked spaghetti. The authors hypothesised that the loss of MON from semolina to raw pasta is due to the binding or conjugation of MON during dough formation, which is then released into the cooking water during the cooking of spaghetti.
3.4.1.4. Malting process
Studies were not identified.
3.4.2. Feed processing
Except for one study in which it was reported that the occurrence of MON in the dust released during wheat threshing was greater than in the original grains (Krysinska‐Traczyk et al., 2001), no information on impact of feed processing on MON concentrations in feed materials has been identified. However, cereal grains intended for use as an animal feed are usually subject to some of the processes used for processing grains for human consumption (cleaning, sorting, drying, rolling/grinding and/or extrusion) before being fed to livestock, and therefore many of the effects reported above for food (Section 3.3.1) apply equally to cereal grains for animal feed. In addition, by‐products of processing grains for human consumption are widely used as feeds for livestock. Ensiling is extensively used as a way of preserving forages which allows them to be stored for long periods without deteriorating. Forage maize is widely grown and ensiled as feed for livestock in Europe, and in view of the relatively high levels of MON reported in maize grains it might be expected that whole maize plants intended for silage are also potential sources of exposure for ruminant livestock. Moreover, Fusarium mycotoxins, produced before ensiling, are highly stable substances and usually are not affected by ensiling (Scudamore and Livesey, 1998; Dorn et al., 2009). Few data are available on the presence of MON in maize silage. Sorensen et al., 2007, did not detect MON in whole plants intended for ensiling and in the current occurrence data on feed (see Section 3.3.1.2) the maximum concentration was 44 μg MON/kg in maize silage, while in 31% of the 42 samples of maize silage levels were below LOD.
3.4.3. Conclusions
Published studies on the effect of food and feed processing on MON were limited, which requires caution in drawing firm conclusions. During cleaning of grains the majority of infected kernels are removed, which is expected to result in lower MON concentrations in subsequently produced products. Milling of grains leads to a redistribution of MON into different fractions. Semolina, flour and feed flour contain the highest concentrations of MON and they represent the largest compartment of MON in the milling balance. Due to its physical characteristics, MON appears to be associated with endosperm, while other Fusarium toxins are merely associated with outer bran layers of grains. Cooking and baking generally lead to reductions of MON concentrations, where the observed reductions are higher at increased temperatures. MON is unstable under high temperatures in combination with alkaline conditions, such as during the nixtamilisation process. There is limited information on the effects of processing of animal feeds on levels of MON, particularly in cereals and cereal by‐products although it may be reasonable to assume that the effects are similar to those for food. There is some evidence that MON may be present in maize silage, but little appears to be known on the effect of ensiling on MON.
3.5. Human exposure assessment
3.5.1. Exposure assessment for humans
3.5.1.1. Mean and 95th percentile acute dietary exposure
The mean and 95th percentile of acute exposure estimates at the UB to MON obtained for different age groups are shown in Table 7. The range represents the minimum (Min) to the maximum (Max) from the different countries and the number in the brackets are the 95% confidence intervals (for more detail see Section 2.7.1.1). The mean and 95th percentile of acute exposure to MON obtained for different age groups is shown in Annex A, Table A11.
Table 7.
Summary statistics of probabilistic acute dietary exposure assessment to moniliformin (at the upper bound) across European dietary surveys (ng/kg bw per day) by age group
| Age groupa | n | Mean dietary exposure (ng/kg bw per day) | 95th percentile dietary exposure (ng/kg bw per day) | ||
|---|---|---|---|---|---|
| Min | Max | Min | Max | ||
| Infantsa | 6 | 121 (81–287) | 300 (294–304) | 506 (487–530) | 1,489 (1,313–1,596) |
| Toddlersa | 11 | 293 (265–360) | 506 (409–848) | 757 (635–853) | 1,480 (1,096–2,201) |
| Other children | 20 | 228 (220–239) | 530 (460–625) | 566 (530–615) | 1,455 (1,138–1,572) |
| Adolescents | 20 | 145 (138–158) | 337 (321–359) | 384 (363–406) | 821 (785–873) |
| Adults | 22 | 94 (89–100) | 219 (212–231) | 278 (273–284) | 656 (639–673) |
| Elderly | 16 | 82 (76–96) | 253 (246–263) | 202 (179–229) | 675 (645–708) |
| Very elderlya | 14 | 88 (83–95) | 196 (189–207) | 212 (187–240) | 552 (503–627) |
bw: body weight; n: number of surveys; Min: minimum; Max: maximum.
Note: The corresponding 95% confidence intervals are presented in the brackets.
One dietary survey for infants, and three dietary surveys for toddlers and very elderly had less than 60 survey participants and therefore could not be included in calculation of the 95th percentile exposure.
Infants (< 12 months)
The mean acute dietary exposure ranged from 121 to 300 ng/kg bw per day, and the 95th percentile from 506 to 1,489 ng/kg bw per day.
Toddlers (≥ 12 months to < 36 months old)
The mean acute dietary exposure ranged from 293 to 506 ng/kg bw per day, and the 95th percentile from 757 to 1,480 ng/kg bw per day.
Other children (≥ 36 months to < 10 years old)
The mean acute dietary exposure ranged from 228 to 530 ng/kg bw per day, and the 95th percentile from 566 to 1,455 ng/kg bw per day.
Adolescents (≥ 10 years to < 18 years old)
The mean acute dietary exposure ranged from 145 to 337 ng/kg bw per day, and the 95th percentile ranged from 384 to 821 ng/kg bw per day.
Adults (≥ 18 years to < 65 years)
The mean acute dietary exposure ranged from 94 to 219 ng/kg bw per day, and the 95th percentile ranged from 278 to 656 ng/kg bw per day. Acute dietary exposure in ‘Pregnant women’ and ‘Lactating women’ were within the range of exposure estimates in the adult population.
Elderly and very elderly (≥ 65 years old)
The mean dietary exposure ranged from 82 to 253 ng/kg bw per day for the elderly and very elderly, and the 95th percentiles ranged from 202 to 675 ng/kg bw per day.
3.5.1.2. Mean and 95th percentile chronic dietary exposure
Table 8 shows summary statistics of the chronic dietary exposure assessment to MON using the available occurrence data. Detailed mean and 95th percentile dietary exposure estimates calculated for each dietary survey are presented in Annex A, Table A12.
Table 8.
Summary statistics of the chronic dietary exposure to moniliformin (ng/kg bw per day) across European countries
| Age groupa | n | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | ||
| Mean dietary exposure in total population (ng/kg bw per day)b | |||||||
| Infants | 6 | 0.06 | 11 | 9.2 | 73 | 38 | 106 |
| Toddlers | 10 | 0.10 | 20 | 6.7 | 73 | 51 | 196 |
| Other children | 18 | 0.04 | 15 | 6.4 | 74 | 23 | 226 |
| Adolescents | 17 | 0.04 | 18 | 3.4 | 43 | 11 | 116 |
| Adults | 17 | 0.20 | 12 | 1.3 | 31 | 8.0 | 78 |
| Elderly | 14 | 0.20 | 7.7 | 0.69 | 24 | 5.8 | 93 |
| Very elderly | 12 | 0.28 | 7.9 | 0.72 | 24 | 22 | 78 |
| 95th percentile dietary exposure in total population (ng/kg bw per day)b | |||||||
| Infantsa | 6 | 0.54 | 144 | 54 | 278 | 123 | 489 |
| Toddlersa | 10 | 0.34 | 213 | 61 | 383 | 135 | 528 |
| Other children | 18 | 0.39 | 79 | 35 | 238 | 63 | 406 |
| Adolescents | 17 | 0.08 | 72 | 14 | 150 | 35 | 280 |
| Adults | 17 | 0.22 | 57 | 7.5 | 99 | 33 | 265 |
| Elderly | 14 | 0.10 | 44 | 3.8 | 61 | 23 | 267 |
| Very elderlya | 12 | 0.06 | 44 | 2.6 | 68 | 16 | 201 |
bw: body weight; LB: lower bound; UB: upper bound.
One dietary survey for infants, and three dietary surveys for toddlers and very elderly had less than 60 survey participants and therefore could not be included in calculation of the 95th percentile exposure.
Rounded to the first or second decimal place or to a whole number.
Infants (< 12 months)
The mean chronic dietary exposure ranged from 0.06 to 106 ng/kg bw per day (minimum LB and maximum UB) and the 95th percentile dietary exposure from 0.54 to 489 ng/kg bw per day (minimum LB to maximum UB).
Toddlers (≥ 12 months to < 36 months old)
The mean chronic dietary exposure ranged from 0.10 to 196 ng/kg bw per day (minimum LB to maximum UB) and the 95th percentile dietary exposure from 0.34 to 528 ng/kg bw per day (minimum LB to maximum UB).
Other children (≥ 36 months to < 10 years old)
The mean chronic dietary exposure ranged from 0.04 to 226 ng/kg bw per day (minimum LB to maximum UB) and the 95th percentile dietary exposure from 0.39 to 406 ng/kg bw per day (minimum LB to maximum UB).
Adolescents (≥ 10 years to < 18 years old)
The mean chronic dietary exposure ranged from 0.04 to 116 ng/kg bw per day (minimum LB to maximum UB) and the 95th percentile dietary exposure from 0.08 to 280 ng/kg bw per day (minimum LB to maximum UB).
Adults (≥ 18 years to < 65 years)
The mean chronic dietary exposure to MON ranged from 0.20 to 78 ng/kg bw per day (minimum LB to maximum UB) and the 95th percentile dietary exposure estimate from 0.22 to 265 ng/kg bw per day (minimum LB to maximum UB). Chronic dietary exposure in ‘Pregnant women’ and ‘Lactating women’ were within the range of exposure estimates in the adult population.
Elderly and very elderly (≥ 65 years old)
The mean dietary exposure to MON ranged from 0.20 to 93 ng/kg bw per day (minimum LB to maximum UB), the 95th percentiles dietary exposure estimate from ranged from 0.10 to 267 ng/kg bw per day (minimum LB to maximum UB).
3.5.1.3. Contribution of different food groups to the exposure
The contribution of individual food groups to chronic dietary exposure to MON (see Annex A, Table A13) varied between the dietary surveys. This is explained by the specific food consumption patterns in the individual European countries and even in the different regions of one country. The contribution to chronic dietary exposure to MON for the individual food groups was assessed separately for each survey and age group. The results are reported as a number of surveys for the following contribution ranges: < 1, 1–5, 5–10, 10–25, 25–50, 50–75, > 75%. ‘Cereal flakes’ made the largest contribution to the dietary exposure to MON in all age groups.
3.5.1.4. Dietary exposure to MON for specific groups
Dietary exposure to MON for vegetarian diets includes more cereal and cereal‐based products and therefore it was considered that the exposure in this consumer group could be higher. The Comprehensive Database contains only limited data on food consumption of vegetarians. Dietary exposure was calculated and compared to the exposure of all subjects included in the respective dietary study. Generally, for acute and chronic exposure, higher or marginally higher means were observed compared to the general population for infants (based on one study), toddlers, other children and the elderly (Tables 9 and 10). Thus, the limited data on vegetarians do not indicate a substantial difference in the dietary exposure to MON between the vegetarians and the general population.
Table 9.
Summary statistics of probabilistic acute dietary exposure assessment to MON (at mean, the lower and upper bound) (ng/kg bw per day) by age group in vegetarians. The corresponding 95% confidence intervals are presented in the brackets
| Age groupa | Mean dietary exposure (ng/kg bw per day) | 95th percentile dietary exposure (ng/kg bw per day)a | |||
|---|---|---|---|---|---|
| n | Min | Max | Min | Max | |
| Infants | 1 | 240 (182–461) | – | – | |
| Toddlers | 3 | 402 (305–701) | 1,010 (688–1,126) | – | – |
| Other children | 2 | 173 (108–286) | 673 (504–1,083) | – | – |
| Adolescents | 4 | 182 (150–240) | 256 (224–389) | – | – |
| Adults | 10 | 132 (95–249) | 258 (217–337) | 371 (318–443) | 594 (411–714) |
| Elderly | 7 | 42 (14–71) | 219 (172–266) | – | – |
| Very elderly | 4 | 50 (39–65) | 178 (147–207) | – | – |
bw: body weight; n: number of surveys; Min: minimum, Max: maximum.
Not calculated for the other age groups because estimates were only available from one dietary survey.
Table 10.
Summary statistics of the mean chronic dietary exposure to MON (ng/kg bw per day) by age group in vegetarians
| Age groupa | n | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | ||
| Mean dietary exposure in total population (ng/kg bw per day)b | |||||||
| Infants | 1 | 15 | 162 | 15 | 162 | 15 | 162 |
| Toddlers | 3 | 0.02 | 4.5 | 13 | 131 | 14 | 379 |
| Other children | 2 | 12 | 41 | 16 | 150 | 19 | 260 |
| Adolescents | 4 | 2.2 | 47 | 2.3 | 72 | 7.9 | 102 |
| Adults | 8 | 0.01 | 23 | 1.6 | 54 | 4.4 | 86 |
| Elderly | 7 | < 0.01 | < 0.01 | 1.3 | 17 | 11 | 42 |
| Very elderly | 3 | < 0.01 | < 0.01 | < 0.01 | 48 | 15 | 77 |
| 95th percentile dietary exposure in total population (ng/kg bw per day)b | |||||||
| Adults | 8 | 10 | 10 | 10 | 165 | 165 | 165 |
n: number of surveys; bw: body weight; Min: minimum, Max: maximum.
Not calculated for the other age groups because estimates were only available from one dietary survey.
Rounded to the first or second decimal place or to a whole number.
Conclusions
Mean acute dietary exposure to MON across 23 European countries in different age groups, using UB concentrations, ranged from 82 ng/kg bw per day in the elderly to 530 ng/kg bw per day in other children. The 95th percentile ranged from 202 ng/kg bw per day in the elderly to 1,489 ng/kg bw per day in infants. Infants, toddlers and children had the highest estimates of chronic dietary exposure to MON (mean exposure 0.04–226 ng/kg bw per day, 95th percentile 0.34–528 ng/kg bw per day). This can be explained by the higher intake of food per kg bw in younger age groups. Acute and chronic dietary exposure in ‘Pregnant women’ and ‘Lactating women’ were within the range of exposure estimates in the adult population.
The highest contributions to the exposure of MON were from grain‐based products across all age groups. Other relevant contributions were found in food for infants and small children, fruit and fruit products in infants and toddlers, and composite foods in adults.
The limited consumption data on vegetarians do not indicate a major difference in the acute and chronic dietary exposure to MON between vegetarians and the general population.
3.6. Exposure assessment for farm and companion animals
Exposure estimates at the 95th percentile and mean concentrations for farm and companion animals are reported below (see also Appendix B). The farm and companion animal exposure estimates22 were calculated by using the occurrence data set which comprised the data on feed (Section 3.3.2.3) and the grains reported as ‘Unprocessed grains of undefined end‐use’ (Section 3.3.2.4). The feed consumption is reported in Section 2.6 and in Appendix B. For the methodology of exposure calculations for farm and companion animals, see Section 2.7.
No data on levels of MON in species‐specific compound feeds have been reported, and therefore exposures to MON have been based on concentrations in individual feed materials and their levels of inclusion in the diets. For non‐forage feeds, information on levels of MON was only available for cereal grains. For forages, data on concentrations of MON in 42 samples of maize silage were provided for the EFSA database (see Section 3.3.2.3). Limited data were provided for cereal straws (n = 18), and these have been used to estimate mean LB and UB exposure for certain ruminant livestock.
It should be noted that in contrast to human exposure, in which estimates of exposure are presented as ng/kg bw per day, the exposure estimates in this section for farm and companion animals are presented as μg/day or μg/kg bw per day.
3.6.1. Ruminants and horses
Two scenarios have been considered in estimating exposure by dairy cows to MON. The first reflects diets in which grass – fresh or conserved – is supplemented with compound feeds containing cereals as described in Appendix B, Section B.1. In this scenario, it is assumed that the forages make no contribution to exposure. In the second scenario, maize silage is assumed to be the sole forage, supplemented with maize grain. In view of the relatively small number of samples of maize silage in the database (n = 42), 95th percentile estimates of exposure have not been made.
For fattening beef cattle, four different feeding systems have been considered, namely (a) grass (fresh or conserved) supplemented with cereals, (b) fattening beef cattle on a maize silage‐based diet, (c) beef cattle on a cereal straw‐based diet and (d) intensively reared beef cattle on a ‘cereal beef’ diet. Details of the levels of maize silage, cereal straw and cereals in these different rations are given in Appendix B, Section B.1.
For lactating sheep and goats, and for horses, diets have been assumed to be predominantly grass‐based (fresh or conserved) supplemented with cereals, the quantities of which are given in Appendix B.
Estimates of exposures for ruminants and horses are given in Table 11.
Table 11.
Mean and 95th percentile (P95) moniliformin concentration in the diet of ruminants and horses derived from concentrations in individual feed materials and their relative proportions in their diets and the corresponding estimated exposure (μg/day and ng/kg bw per day)
| Animal species (diet) | LB/UB | Diet concentration μg/kg dry matter | Exposure μg/day | Exposure ng/kg bw per day | |||
|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | ||
| Dairy cows (grass‐based diet) | LB | 23 | 4.5 | 483 | 94 | 740 | 140 |
| UB | 25 | 9.9 | 518 | 205 | 800 | 320 | |
| Dairy cows (maize silage‐based diet)a | LB | – | 36 | – | 979 | – | 1,500 |
| UB | – | 37 | – | 1013 | – | 1,600 | |
| Beef cattle (grass silage‐based diet) | LB | 11 | 1.8 | 106 | 17 | 270 | 40 |
| UB | 11 | 4.5 | 106 | 43 | 270 | 110 | |
| Beef cattle (cereal‐based diet) | LB | 94 | 15 | 941 | 148 | 2,400 | 370 |
| UB | 94 | 38 | 941 | 383 | 2,400 | 960 | |
| Beef cattle (maize silage‐based diet)a | LB | – | 12 | – | 78 | – | 260 |
| UB | – | 16 | – | 102 | – | 340 | |
| Beef cattle (straw‐based diet)a | LB | – | 19 | – | 152 | – | 510 |
| UB | – | 50 | – | 396 | – | 1,300 | |
| Lactating sheep | LB | 27 | 5.2 | 75 | 15 | 1,200 | 180 |
| UB | 29 | 11 | 80 | 32 | 1,300 | 400 | |
| Lactating goats | LB | 15 | 9.7 | 52 | 33 | 860 | 550 |
| UB | 15 | 32 | 52 | 108 | 860 | 1,800 | |
| Horses | LB | 16 | 3.2 | 141 | 29 | 310 | 60 |
| UB | 34 | 14 | 307 | 122 | 680 | 270 | |
LB: lower bound; UB: upper bound.
Insufficient samples were available to undertake 95th percentile exposure estimates
Rounded to the first or second decimal place or to a whole number.
3.6.2. Pigs and poultry
Estimates of 95th percentile and mean exposure by pigs and poultry to MON were derived from data for assumed inclusion rates of cereal grains in their diets, and are given in Table 12. Details of assumed inclusion rates of cereals are given in Appendix B.
Table 12.
Mean and 95th percentile (P95) moniliformin concentration in the diet of pigs and poultry derived from concentrations in individual feed materials and their relative proportion in diets and the corresponding estimated exposure (μg/day and ng/kg bw per day)a
| Animal species | LB/UB | Diet concentration μg/kg dry feed matter | Exposure μg/day | Exposure ng/kg bw per day | |||
|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | ||
| Pig starter | LB | 98 | 22 | 98 | 22 | 4,900 | 1,100 |
| UB | 111 | 43 | 111 | 43 | 5,600 | 2,200 | |
| Pig finisher | LB | 105 | 24 | 317 | 70 | 3,200 | 700 |
| UB | 119 | 46 | 357 | 138 | 3,600 | 1,400 | |
| Lactating sow | LB | 92 | 22 | 552 | 129 | 2,800 | 650 |
| UB | 106 | 41 | 634 | 245 | 3,200 | 1,200 | |
| Fattening chickens | LB | 160 | 40 | 19 | 4.8 | 9,700 | 2,400 |
| UB | 171 | 53 | 21 | 6.3 | 10,000 | 3,200 | |
| Laying hens | LB | 107 | 35 | 13 | 4.2 | 6,500 | 2,100 |
| UB | 149 | 45 | 18 | 5.4 | 9,000 | 2,700 | |
| Fattening turkeys | LB | 107 | 21 | 43 | 8.5 | 3,600 | 710 |
| UB | 115 | 46 | 46 | 18 | 3,900 | 1,500 | |
| Fattening ducks | LB | 92 | 21 | 13 | 2.9 | 4,300 | 980 |
| UB | 104 | 41 | 15 | 5.7 | 4,900 | 1,900 | |
LB: lower bound; UB: upper bound.
Rounded to the first or second decimal place or to a whole number.
3.6.3. Farmed fish (salmonids and carp), farmed rabbits and farmed mink
In the absence of any data on concentrations of MON in species‐specific compound feeds, estimates of exposure were made by using example rations and concentrations in individual feed materials (see Appendix B for details of rations used) and are reported in Table 13.
Table 13.
Mean and 95th percentile (P95) MON concentration in the diet of farmed rabbits, farmed fish and farmed mink derived from concentrations in individual feed materials and their relative proportions in diets and the corresponding estimated exposure (μg/day and ng/kg bw per day)a
| Animal species | LB/UB | Diet concentration μg/kg dry feed matter | Exposure μg/day | Exposure ng/kg bw per day | |||
|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | ||
| Salmonids | LB | 19 | 4.9 | 0.76 | 0.19 | 380 | 100 |
| UB | 23 | 8.6 | 0.90 | 0.34 | 450 | 170 | |
| Carp | LB | 62 | 16 | 1.4 | 0.34 | 1,400 | 340 |
| UB | 69 | 23 | 1.5 | 0.51 | 1,500 | 510 | |
| Farmed rabbits | LB | 33 | 5.3 | 5.0 | 0.79 | 2,500 | 390 |
| UB | 33 | 14 | 5.0 | 2.0 | 2,500 | 1,000 | |
| Farmed mink | LB | 27 | 6.6 | 2.0 | 0.50 | 1,000 | 240 |
| UB | 29 | 9.1 | 2.2 | 0.68 | 1,100 | 330 | |
LB: lower bound; UB: upper bound.
Rounded to the first or second decimal place or to a whole number.
3.6.4. Dogs and cats
No data on levels of MON in proprietary feeds for dogs and cats were available, and therefore exposure was estimated using example rations (see Appendix B for details) and concentrations MON in cereal grains used. The estimated exposures are reported in Table 14.
Table 14.
Mean and 95th percentile (P95) MON concentration in the diet of cats and dogs derived from concentrations in individual feed materials and their relative proportions in diets and the corresponding estimated exposure (μg/day and ng/kg bw per daya
| Animal species | LB/ UB | Diet concentration μg/kg dry feed matter | Exposure μg/day | Exposure ng/kg bw per day | |||
|---|---|---|---|---|---|---|---|
| P95 | Mean | P95 | Mean | P95 | Mean | ||
| Cats | LB | 50 | 15 | 3.0 | 0.9 | 750 | 220 |
| UB | 50 | 18 | 3.0 | 1.1 | 750 | 260 | |
| Dogs | LB | 55 | 19 | 20 | 7.0 | 800 | 280 |
| UB | 56 | 23 | 20 | 8.1 | 800 | 330 | |
LB: lower bound; UB: upper bound.
Rounded to the first or second decimal place or to a whole number.
3.7. Risk characterisation
3.7.1. Human health risk characterisation
3.7.1.1. Acute human health risk from the dietary exposure to MON
Since the available data were not sufficient to derive an ARfD for MON, the CONTAM Panel characterised the acute human health risk associated with acute dietary exposure to MON by comparing the mean and 95th percentile probabilistic acute dietary exposure estimates across European dietary surveys and the age groups as summarised in Table 7 (see Section 3.5.1.1) with the NOAEL of 6 mg MON/kg bw (i.e. 6,000,000 ng MON/kg bw) for cardiotoxicity identified based on a subacute study in rats (see Section 3.2.1.2).
Estimated exposure levels to MON across the surveys and age groups varied at the mean UB from 82 to 530 ng/kg bw, with smallest lower and largest upper confidence bounds of 76 and 848 ng/kg bw. At the 95th percentile, UB exposure varied from 202 to 1,489 ng/kg bw, with smallest lower and largest upper confidence bounds of 179 and 2,201 ng/kg bw. The resulting MOE values (rounded down) ranged from 11,000 to 73,000 at the mean and from 4,000 to 29,000 at the 95th percentile dietary exposures, respectively, see Table 15. The CONTAM Panel concluded that these MOE values were sufficiently large to indicate a low health concern for humans from current acute dietary exposure to MON.
Table 15.
Margins of exposure (MOE) values based on the NOAEL of 6 mg MON/kg bw identified in a subacute study in rats and the acute dietary exposure across age groups
| Age group | MOE calculated from mean dietary exposure | MOE calculated from 95th percentile dietary exposure | ||
|---|---|---|---|---|
| Minimum | Maximum | Minimum | Maximum | |
| Infants | 49,000 | 20,000 | 11,000 | 4,000 |
| Toddlers | 20,000 | 11,000 | 7,000 | 4,000 |
| Other children | 26,000 | 11,000 | 10,000 | 4,000 |
| Adolescents | 41,000 | 17,000 | 15,000 | 7,000 |
| Adults | 63,000 | 27,000 | 21,000 | 9,000 |
| Elderly | 73,000 | 23,000 | 29,000 | 8,000 |
| Very elderly | 68,000 | 30,000 | 28,000 | 10,000 |
NOAEL: no‐observed‐adverse‐effect level.
Note: Minimum and maximum refer to the exposure scenarios.
The data on dietary habits of vegetarians were available from only five European countries and four of them had only very few subjects. These limited data did not indicate notable differences in acute dietary exposure between the vegetarians and the general population. Therefore, the conclusions on the general population remained valid also for the subpopulation of vegetarians.
3.7.1.2. Chronic human health risk from the dietary exposure to MON
Since the available toxicity data were not sufficient to establish a chronic HBGV for MON, the CONTAM Panel assessed the risk from chronic dietary exposure to MON by comparing the mean and 95th percentile acute dietary LB and UB exposure estimates across European dietary surveys and the age groups as summarised in Table 8 (see Section 3.5.1.1) with the lowest BMDL05 of 0.20 mg MON/kg bw per day calculated for haematological adverse effects (i.e. 200,000 ng MON/kg bw per day) (see Section 3.2.1.2).
The mean chronic exposure to MON across the surveys and age groups varied at LB from 0.04 to 51 and at UB from 7.7 to 226 ng/kg bw per day. High exposure (95th percentile) varied at LB from 0.06 to 135 and at UB from 44 to 528 ng/kg bw per day. The MOE values (rounded down) ranged from to 3,900 to 5,000,000 (LB) and from 880 to 25,000 (UB) at the mean exposure, and from 1,400 to 3,300,000 (LB) and from 370 to 4,500 to (UB) at the 95th percentile exposure estimates, see Table 16.
Table 16.
Margins of exposure (MOE) values based on the BMDL05 of 0.20 mg MON/kg bw per day for haematological effects identified in a 28‐day study in pigs and the chronic dietary exposure across age groups
| Age group | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| MOE calculated from mean dietary exposure | ||||||
| Infants | 3,300,000 | 18,000 | 21,000 | 2,700 | 5,200 | 1,800 |
| Toddlers | 2,000,000 | 10,000 | 29,000 | 2,700 | 3,900 | 1,000 |
| Other children | 5,000,000 | 13,000 | 31,000 | 2,700 | 8,600 | 880 |
| Adolescents | 5,000,000 | 11,000 | 58,000 | 4,600 | 18,000 | 1,700 |
| Adults | 1,000,000 | 16,000 | 153,000 | 6,400 | 25,000 | 2,500 |
| Elderly | 1,000,000 | 25,000 | 289,000 | 8,300 | 34,000 | 2,100 |
| Very elderly | 700,000 | 25,000 | 277,000 | 8,300 | 9,000 | 2,500 |
| MOE calculated from 95th percentile dietary exposure | ||||||
| Infants | 370,000 | 1,300 | 3,700 | 700 | 1,600 | 400 |
| Toddlers | 580,000 | 930 | 3,200 | 520 | 1,400 | 370 |
| Other children | 510,000 | 2,500 | 5,700 | 840 | 3,100 | 490 |
| Adolescents | 2,500,000 | 2,700 | 14,000 | 1,300 | 5,700 | 710 |
| Adults | 900,000 | 3,500 | 26,000 | 2,000 | 6,000 | 750 |
| Elderly | 2,000,000 | 4,500 | 52,000 | 3,200 | 8,600 | 750 |
| Very elderly | 3,300,000 | 4,500 | 76,000 | 2,900 | 12,000 | 990 |
BMDL: Benchmark dose lower confidence limit; LB: lower bound; UB: upper bound.
The CONTAM Panel concluded that these MOE values were sufficiently large to indicate a low health concern for humans from current chronic dietary exposure to MON.
However, the CONTAM Panel stresses that in the absence of quantitative dose–response data on cardiotoxicity this risk characterisation was based on haematological effects from very limited toxicity database.
The data on dietary habits of vegetarians were available from only five European countries and four of them had only very few subjects. These limited data did not indicate notable differences in chronic dietary exposure between the vegetarians and the general population. Therefore, the conclusions on the general population remained valid also for the subpopulation of vegetarians.
3.7.2. Animal health risk characterisation
Because of the limited knowledge on the effects of MON on farm and companion animals and on the absence of a comprehensive database on feed consumption by livestock in the EU, it has not been possible to properly assess the risk of MON for animal health. However, the exposure estimates at the LB and UB concentrations for MON in diets have been estimated for most relevant farm livestock and companion animal categories, based on expected feed intakes and example diets and these have been compared with the species‐specific BMDL for pigs or NOAELs for poultry and farmed mink only, see Table 17. In particular, the CONTAM Panel took into account the dietary exposure assessment of MON using recent analytical results on the occurrence of MON in feed as described in Sections 2 and 3.3.2 and the diet composition and feed consumption of farm and companion animals as described in Sections 2.6 and Appendix B. The estimates of exposure to MON are presented in Section 3.6.
Table 17.
Margins of exposure (MOE) values based on NOAEL or BMDL05 identified in relevant studies and the estimated UB in mean and 95th percentile dietary exposure across animal species
| Species | MOE calculated from mean dietary exposure | MOE calculated from 95th percentile dietary exposure |
|---|---|---|
| Highest UB | Highest UB | |
| Ruminants | 120 | 80 |
| Pig | ||
| – Fattening pig | 90 | 35 |
| – Lactating sow | 160 | 60 |
| Poultry | ||
| – Broiler chicken | 430 | 140 |
| – Laying hens | 1,400 | 420 |
| – Fattening turkey | 1,000 | 410 |
| – Fattening duck | 1,200 | 460 |
| Horse | 740 | 290 |
| Farmed rabbit | 200 | 80 |
| Farmed mink | 2,700 | 830 |
| Dog | 600 | 250 |
| Cat | 760 | 260 |
UB: upper bound.
Note: values were rounded down (to hundreds above 1,000 or to sets of ten).
For pigs, the CONTAM Panel characterised the health risk associated with dietary exposure to MON by comparing the estimated exposures at the UB mean and UB 95th percentile dietary concentrations for MON (see Section 3.6 and Appendix B), with the identified reference point BMDL05 for haematological hazard) of 0.20 mg MON/kg bw per day (see Table 17).
For poultry and farmed mink, the CONTAM Panel characterised the health risk associated with dietary exposure to MON by comparing the estimated exposures at the UB mean and UB 95th percentile dietary concentrations for MON (see Section 3.6 and Appendix B), with the identified NOAELs and calculating MOE values rounded down to the next lower unit of 10 or 100, see details in Table 17 and Appendix D.
For all other farm and companion animal species where a specific reference point could not be identified, the CONTAM Panel applied a conservative approach and characterised the health risk associated with dietary exposure to MON by comparing the estimated exposures at the UB mean and UB 95th percentile dietary concentrations for MON (see Section 3.6 and Appendix B), with the lowest indicative reference point of 0.20 mg MON/kg bw per day, identified for pigs.
3.7.2.1. Pigs
The dietary exposure for fattening pigs at the UB mean and 95th percentile ranged between 0.1% and 0.2% of the NOAEL of 1.2 mg/kg bw per day and was slightly higher than for lactating sows. The MOE values comparing the UB mean and 95th percentile dietary exposure with the reference point of 0.20 mg MON/kg bw per day ranged from 90 to 160 (mean) and from 35 to 60 (95th percentile) for fattening pigs and lactating sows, respectively, see Table 17 and Appendix B. Therefore, the risk for adverse health effects from feed containing MON was considered low for pigs at the estimated exposure levels under current feeding practices.
3.7.2.2. Poultry
Amongst the poultry species, the highest dietary exposure was for broiler chickens, for which the exposure at the at the UB mean and 95th percentile ranged between 0.2% and 0.7% of the NOAEL of 1.4 mg/kg bw per day (see Table 17). The MOE values comparing the UB mean and 95th percentile dietary exposure with the NOAELs identified as 1.4, 3.8, 1.6 and 2.3 μg/kg bw for broiler chickens, laying hens, fattening turkeys and fattening ducks, respectively, ranged overall between 430 and 1400 for the mean and between 140 and 460 at the 95th percentile exposure – see Table 17 and Appendix D. Therefore, the risk for adverse health effects from feed containing MON was considered as negligible for poultry species at the estimated exposure levels under current feeding practices.
3.7.2.3. Farmed mink
For farmed mink, the dietary exposure estimates at the UB mean and 95th percentile ranged between 0.03 and 0.1% of the NOAEL of 0.92 mg/kg bw per day. The MOE values comparing the UB mean and 95th percentile dietary exposure with the NOAEL were 2,700 and 830 – see Table 17 and Appendix D. Therefore, the risk for adverse health effects from feed containing MON was considered as negligible for farmed mink at the estimated exposure levels under current feeding practices.
3.7.2.4. Other farm and companion animals
The CONTAM Panel noted that the margin between the estimated mean and 95th percentile exposures and the assumed indicative reference point of 0.20 mg MON/kg bw adopted for other animals (see Section 3.2.2) was not smaller than those observed for animals for which data on adverse effects were available. Specifically the MOEs between the UB dietary exposure estimates and the NOAELs for the other farm and companions animals for which data on adverse effects were not available or insufficient ranged between 90 and 2,700 for the mean and 35 and 800 for the 95th percentile exposure, respectively – see Table 17 and Appendix D. Therefore, it seems reasonable to conclude that the incidence of adverse health effects from feed containing MON is unlikely for them at the levels of exposure estimated for current feeding practices. The CONTAM Panel noted that the conclusion on animals other than poultry, pigs and farmed mink would be affected by a higher degree of uncertainty than that on the animal species for which toxicity data were available.
4. Uncertainty analysis
Evaluation of the inherent uncertainties in the assessment of exposure to MON has been performed following the guidance given in the Opinion of the Scientific Committee related to Uncertainties in Dietary Exposure Assessment (EFSA Scientific Committee, 2006). In addition, the report on ‘Characterizing and Communicating Uncertainty in Exposure Assessment’ has been considered (WHO/IPCS, 2008). According to the guidance provided by EFSA, 2006, the following sources of uncertainties have been considered: assessment objectives, exposure scenario, exposure model and model input (parameters). In addition to the EFSA opinion, 2006, the CONTAM Panel also considered other uncertainties.
4.1. Assessment objectives
The objectives of the assessment were defined in the terms of reference and no uncertainty was associated in the objectives.
4.2. Exposure scenario and model
The results of the samples collected in 2001–2016 on the occurrence of MON in food, feed and grains of undefined end‐use reported to EFSA, identified suitable for evaluation, were limited. These data were augmented by the results extracted from the published literature collected between 2001 and 2015 for food, and between 2006 and 2016 for feed. For the final data set, analytical results for food were available from six European countries and for feed, including the grains of undefined‐end use, from five European countries. However, these samples of food/feed did not always originate from the European country who reported the data. The data showed a high percentage of left censored results and about 90% and 60% of the results available to assess human exposure and farm and companion animal exposure, respectively, were left censored. Non‐targeted sampling was reported only for the samples submitted to EFSA and this was assumed also for the other contributing data in the absence of further information. Therefore, the evaluation data set may not be fully representative at the European level, which adds to the uncertainty for the exposure assessments for humans and animals. The use of LB values tends to underestimate, while the use of UB values tends to overestimate dietary exposure.
The lack of analytical methods formally validated through interlaboratory studies hampered a reliable conclusion on the uncertainty of the currently available results on MON. The lack of certified reference materials for MON (as calibrants and in particular matrix materials) is an additional limitation that hampered an uncertainty estimate for individual laboratories. Both issues contribute to the overall uncertainty of occurrence data and are equally valid for the chemical analysis used in toxicokinetic and toxicity studies. Available information on correction/non‐correction for recovery was not complete and contributes to that uncertainty.
There were considerable differences in the number of analytical results across the food categories with high prevalence of grains and grain‐based foods and only few or no samples for other food categories, except for asparagus, adding to overall uncertainty of the human exposure estimates.
For several food categories, the estimation of the highest reliable percentile was not possible due to the small numbers of samples. There was a lack of dietary surveys on the consumption data for infants and for vegetarians adding to the uncertainty of exposure estimates for these subgroups in human population.
The CONTAM Panel noted a wide range between the LB and UB values of the chronic dietary exposure of humans across European countries and the age groups which clearly indicate the considerable uncertainty of these estimates.
Regarding the exposure of animals through feed consumption, only data on the major cereal grains (wheat, barley, oats, rice and maize), and limited data for maize silage and cereal straw, were available to assess exposure. As cereal by‐products are typically used for animal feeding, the absence of data on MON concentrations in these products led to a likely underestimation of exposure. A large variety of feed materials are used to formulate diets for farm and companion animals in Europe, and the lack of information on levels of MON in these feeds needed for reliable estimation of exposure added to the overall uncertainty of the animal exposure.
4.3. Other uncertainties
Data on toxicokinetics were missing for humans and were very limited for experimental animals, while the fate of more than 50% of ingested MON remains unknown. For farm and companion animals, no data on toxicokinetrics were identified. Distribution and metabolism are unknown and more information is needed on the potential crossing of the blood‐brain barrier for MON. Therefore, the uncertainty of the toxicokinetics of MON is large.
There is a lack of long‐term toxicity studies and reproduction toxicity studies for MON in experimental animals. There is also a lack of information on general toxicity, haematotoxicity and cardiotoxicity of MON in experimental animals and most farm and companion animals. In particular, studies on quantitative dose–response data for key cardiac endpoints, such as cardiomegaly, muscular distress of the heart and heart failure in both well‐designed short‐term and long‐term studies, are lacking. In addition, available in vitro and in vivo data on the mode of action of MON are currently too limited and the relationship between cardiotoxicity and mortality of MON in key animal species needs to be based on a sufficiently large toxicity database, such that a convincing dose–response relationship would be evident. The CONTAM Panel also noted that the dose–response data on the acute adverse effects of MON in rats of Jonsson et al. (2015) and on chronic effects in pigs of Harvey et al. (2001) may not fully inform on the critical endpoints chosen. The mechanism for the in vitro genotoxic effects is unknown, and whether the observed positive in vitro results are relevant for the situation in vivo, where data are so far lacking. For these reasons, both acute and chronic risk characterisation remains incomplete and the hazard characterisation of MON remains highly uncertain.
Overall, the CONTAM Panel considered that the uncertainty, particularly resulting from the lack of toxicity data on MON, prevented reliable risk assessment for humans and most farm and companion animals.
4.4. Summary of uncertainties
In Table 18, a summary of the uncertainty evaluation is presented, highlighting the main sources of uncertainty and indicating an estimate of whether the respective source of uncertainty might have led to an over‐ or underestimation of the exposure or the resulting risk.
Table 18.
Summary of qualitative evaluation of the impact of uncertainties on the risk assessment of the human and animal dietary exposure to MON
| Sources of uncertainty | Directiona |
|---|---|
| Assuming non‐targeted sampling for literature data used for exposure | + |
| Uncertainty of the analytical measurements | +/− |
| Effects of food and feed processing | +/− |
| No data for most grain‐based products and other foods and no data on other feed than grains | +/− |
| High variability of the composition of feedstuffs used and feeding systems for farm animals in Europe | +/− |
| Use of UB occurrence data in the exposure estimations | + |
| Use of LB occurrence data in the exposure estimations | − |
| Limited data on exposure of infants | +/− |
| Use of Fusarium culture materials in the toxicity studies | +/− |
| Lack of toxicity data on general toxicity in experimental and farm and companion animals | +/− |
| Limited data on toxicokinetics | +/− |
| Lack of data on in vivo genotoxicity and carcinogenicity | − |
| Limited data on the mode of action | +/− |
+ = uncertainty with potential to cause over‐estimation of exposure/risk; − = uncertainty with potential to cause under‐estimation of exposure/risk.
The CONTAM Panel considered the impact of the uncertainties on the risk assessment of animal and human exposure to MON and concluded that overall uncertainty is large.
5. Conclusions
Moniliformin (MON) is a mycotoxin with low molecular weight typically, but not exclusively, produced by several plant pathogenic Fusarium species. It has mainly been detected in cereal grains and cereal‐based food and feed. Naturally occurring modified forms of MON have not been reported.
Methods of analysis
Analytical methodology for MON in foods, feeds and biological samples has been mostly based on LC‐UV and LC–MS/MS. Currently, LC–MS/MS is the most widely used and preferred technique.
None of the applied analytical methods for MON have been formally validated in interlaboratory studies.
Rapid immunochemical test kits, able to detect MON, have not been developed.
Certified reference materials (both reference matrices and reference calibrants) are not available for MON but calibrants are commercially available.
Occurrence
MON has mainly been reported to occur in various cereal grains, such as maize, wheat, barley and oats, and in cereal products.
MON has been found to co‐occur with other mycotoxins, in particular with trichothecenes, enniatins, beauvericin and zearalenone.
A total of 3,205, 806, and 504 analytical results of MON for food, feed and unprocessed grains of undefined end‐use, respectively, sampled between 2001 and 2016 fulfilled the quality criteria applied by EFSA.
The proportion of left‐censored data in the data set (results below the LOD or LOQ) were 90% for MON in food, 60% for MON in feed and 70% for MON in unprocessed grains of undefined end‐use.
The highest mean concentrations of MON were recorded for food in the categories ‘Grains for human consumption’, ‘Snack food’ and ‘Breakfast cereals’, for feed in the categories ‘Cereal grains’ (i.e. maize and barley) and for unprocessed grains of undefined end‐use in the category ‘Grains as crops’ (i.e. wheat and oat grain).
Effects of processing
Cleaning and sorting of grains resulted in a reduction of MON in subsequently produced products.
Milling of grains led to a redistribution of MON into different fractions. Semolina, flour and feed flour contained the highest concentrations of MON.
Cooking and baking generally led to reductions of MON concentrations in contaminated samples.
MON was unstable under high temperatures in combination with alkaline conditions.
In the absence of studies with feed materials, the CONTAM Panel considered the effects of the processing of animal feeds were similar to those reported for food.
Although the effects of ensiling on MON appeared not to have been studied so far, in view of the relatively high levels of MON reported in maize grains, plants intended for silage were considered as also potential sources of exposure for ruminant livestock.
Human exposure
The estimates of mean acute exposure to MON across 39 different dietary surveys and all age groups using the UB concentrations ranged from 82 to 530 ng/kg bw per day. The estimates of 95th percentile acute exposure ranged from 202 to 1,489 ng/kg bw per day. The highest acute dietary exposures were for infants, toddlers, and other children.
The estimates of mean chronic exposure to MON across 33 different dietary surveys and all age groups using the minimum LB and the maximum UB concentrations ranged from 0.04 to 226 ng/kg bw per day. The estimates of 95th percentile chronic exposure ranged from 0.06 to 528 ng/kg bw per day. The highest chronic dietary exposures were for infants, toddlers, other children and adolescents.
The most important contributors to the chronic dietary exposure to MON were ‘Grains and grain‐based products’, especially ‘cereal flakes’.
The limited available consumption data on vegetarians do not indicate a major difference in the dietary exposure to MON between them and the general population.
Farm and companion animal exposure
Animal exposure to MON was primarily from consuming cereal grains and cereal by‐products. Levels in forages were generally low.
For ruminants, the estimated lowest LB and highest UB mean dietary exposures were 0.04 and 1.6 μg/kg bw per day, and the 95th percentile exposures were 0.27 and 2.4 μg/kg bw per day, respectively.
For pigs, the estimated lowest LB and highest UB mean dietary exposures were 0.65 and 2.2 μg/kg bw per day, and the 95th percentile exposures were 2.8 and 5.6 μg/kg bw per day, respectively.
For poultry, the estimated lowest LB and highest UB mean dietary exposures were 0.71 and 3.2 μg/kg bw per day, and the 95th percentile exposures were 3.6 and 10 μg/kg bw per day, respectively.
For horses, the estimated LB and UB mean dietary exposures were 0.06 and 0.27 μg/kg bw per day, and the 95th percentile exposures were 0.31 and 0.68 μg/kg bw per day, respectively.
For farmed fish (salmonids and carp), the estimated lowest LB and highest UB mean dietary exposures were 0.10 and 0.51 μg/kg bw per day, and the 95th percentile exposures were 0.38 and 1.5 μg/kg bw per day, respectively.
For farmed rabbits, the estimated LB and UB mean dietary exposures were 0.39 and 1.0 μg/kg bw per day, and the 95th percentile exposures were 2.5 μg/kg bw per day (LB and UB).
For farmed mink, the estimated LB and UB mean dietary exposures were 0.24 and 0.33 μg/kg bw per day, and the 95th percentile exposures were 0.99 and 1.05 μg/kg bw per day, respectively.
For dogs and cats, the estimated lowest LB and highest UB mean dietary exposures were 0.22 and 0.33 μg/kg bw per day, respectively. The 95th percentile exposures ranged between 0.80 and 0.75 μg/kg bw per day.
Toxicokinetics
In experimental animals, a large portion of MON was absorbed and excreted rapidly after administration with no apparent accumulation in any tissue. However, the fate of at least half of the amount ingested remains unknown.
No data on toxicokinetics were identified for farm and companion animals considered in this opinion.
The only available study on the transfer of MON from feed to food products of animal origin was identified for broiler chickens, and no transfer was found.
Toxicity of MON in experimental animals
Acute toxicity of MON was identified in rats, with oral LD50 values ranging from 19 to 25 mg MON/kg bw in experiments using 99% pure MON. The oral acute toxicity in mice was lower with LD50 values of about 50 mg MON/kg bw.
Acute toxicity was accompanied by muscular weakness, respiratory and cardiovascular changes, the latter including faint heart beats and cardiac arrhythmia.
Prominent acute adverse health effects in experimental animals were presence of ultrastructural lesions in the myocardium, reduction of contractility in aorta, pulmonary artery and terminal ileus, decreased myocardial contractile force and ventricular arrhythmia and congestive heart failure.
The CONTAM Panel identified only one subacute study of MON in rats that allowed identification of a NOAEL of 6 mg/kg bw per day. Cardiotoxicity was observed at 15 mg/kg bw per day and indications of cardiotoxicity were seen at 9 mg MON/kg bw per day.
Only one subchronic study with a limited number of rats was identified. MON induced cardiotoxicity and mortality at 32.5 mg MON/kg bw per day and higher, while no adverse effects were observed at the lowest dose tested (16.6 mg MON/kg bw per day) which was identified as a NOAEL for mortality for male rats. Data on haematotoxicity or myelotoxicity and on immunotoxicity were too scarce to conclude on the hazard of MON in experimental animals.
No chronic studies or any carcinogenicity study on MON were identified in animals.
For developmental and reproductive toxicity of MON, only one study in mink was identified from which the lowest dose of 0.92 mg MON/kg bw per day was identified as a NOAEL. Exposure to 1.94 mg MON/kg bw per day, the other dose tested, resulted in significant neonatal mortality and reduced offspring body weights.
There was no evidence that MON induces bacterial reverse mutation. MON has been shown to be clastogenic in vitro inducing chromosomal damage.
No data were identified to conclude on whether in vitro genotoxicity is caused by a direct or indirect mechanism.
No data were available on genotoxicity of MON in vivo.
No relevant human epidemiological data on MON were identified.
Although it has been hypothesised in published literature that dietary exposure to MON was involved in past prevalence of KD in some regions in China, the CONTAM Panel noted that the evidence for a causal relation between dietary exposure to MON and the incidence of KD was too weak and insufficient to be considered for human hazard characterisation.
The mode of action of MON is unclear and only a limited amount of data was available. The inhibition of enzymes involved in glucose metabolism could lead to cellular energy deprivation and may partially explain the respiratory stress, including myocardial effects.
The available database of possible effects of combined exposure to MON and other mycotoxins was weak and insufficient for establishing the nature of combined effects.
Considerations on derivation of human health‐based guidance values (HBGV)
Since the toxicological database of MON for experimental animals was scarce, and only one subacute study and one subchronic study were available to characterise the hazard of MON, the CONTAM Panel also examined the toxicological data for farm animals to augment the database for human hazard characterisation.
Acute adverse health effects
The CONTAM Panel could not establish an ARfD for MON due to the limitations of the available acute and subacute toxicity data.
The CONTAM Panel identified cardiotoxicity as a critical adverse health effect of acute and subacute exposure to MON and identified a NOAEL of 6.0 mg/kg bw from a subacute study in rats as reference point for the acute exposure of humans to MON.
Chronic adverse health effects
The CONTAM Panel could not establish a TDI for MON due to limitations in the available toxicity data on chronic effects.
The CONTAM Panel identified haematotoxicity as the critical chronic adverse effects of MON in pigs and identified the lowest BMDL05 of 0.20 mg MON/kg bw per for the decrease of the haematocrit and haemoglobin levels as reference point for chronic exposure of humans.
Adverse effects and reference points in farm and companion animals
Data on adverse health effects in farm and companion animals were lacking for most of the animal species. Information was available on poultry and, however limited, for pigs, farmed fish and farmed mink.
Mortality and reduced body weight gain were identified as chronic adverse effects both in pigs and poultry.
-
From the few available studies on the toxicity of MON in pigs, reduced weight gain, adverse haematological effects, cardiotoxicity and mortality accompanied with lesions in heart were identified as sensitive adverse health effects.
-
o
The NOAEL for reduced body weight gain ranged between 50 and 100 mg MON/kg feed, corresponding to 1.2 and 2.2 mg MON/kg bw per day.
-
o
For haematological adverse effects, a NOAEL of 25 mg MON/kg feed, corresponding to 1.0 mg MON/kg bw per day, was identified. A lowest BMDL05 of 0.20 mg MON/kg bw per day was calculated on the decrease of haematocrit and haemoglobin levels and this was the most sensitive endpoint for pigs exposed to MON.
-
o
Mortality was observed at a dose as low as 4.2 mg MON/kg bw per day.
-
o
A study in miniature pigs showed cardiotoxicity at 3 mg MON/kg bw per day.
-
o
-
In poultry, the heart was the main target organ and MON caused heart failure after acute dosing. Repeated dietary exposure to MON generated cardiomegaly but also changed haematological parameters and affected body weight gain and egg production.
-
o
For day‐old chickens, oral LD50 values of 4.0 and 5.4 mg MON/kg bw were reported. Ascites with oedema of the mesenteries and small haemorrhages in the proventriculus, gizzard, small and large intestine, and skin were observed in surviving chickens.
-
o
For 7‐day‐old ducks, an oral LD50 value of 3.7 mg MON/kg bw, and increasing heart rates followed by arrhythmia and ultimately cessation of contraction were reported.
-
o
In broiler chickens, the dose of 2.8 mg MON/kg bw per day resulted in reduced body weight gain, cardiomyopathy, changes in the major haematological parameters and increased mortality rates, while at the dose of 1.4 mg MON/kg bw per day no adverse effects were observed.
-
o
In the only available study on laying hens, the dose of 8.5 mg MON/kg bw per day reduced egg production and body weight gain, while the dose of 3.8 mg MON/kg bw per day did not generate any adverse effects.
-
o
In turkeys, no adverse effects were observed at 1.6 mg MON/kg bw per day, while a dose of 3.2 mg MON/kg bw per day induced cardiomegaly.
-
o
Based on two studies on ducks, the dose of 2.8 mg MON/kg bw per day generated cardiomegaly, while no adverse effects were observed at 2.3 mg MON/kg bw per day.
-
o
Only two studies on farmed fish were identified. Reduced weight gain was reported for channel catfish at the lowest dose of 0.8 mg MON/kg bw per day. Nile tilapia appeared to be more resistant and no effects were observed at 1.8 mg/kg bw per day in this species.
In farmed mink, the dose of 1.94 mg MON/kg bw per day resulted in significant neonatal mortality and reduced offspring body weights and was identified as the LOAEL. The NOAEL was 0.92 mg MON/kg bw per day.
No toxicity data suitable for hazard characterisation of MON were identified for ruminants, farmed rabbits, horses, farmed fish, dogs and cats. Therefore, the CONTAM Panel considered the BMDL05 of 0.20 mg MON/kg bw identified for pigs as an indicative reference point.
The CONTAM Panel noted that the conclusion on animals other than poultry, pigs and farmed mink would be affected by a higher degree of uncertainty than that on the animal species for which sufficient toxicity data were available.
Human health risk characterisation
Acute risk
Since an ARfD could not be established, and in order to get an indication of the risk from MON exposure, the CONTAM Panel calculated the MOE between the NOAEL of 6.0 mg/kg bw from a subacute study in rats and the acute UB dietary exposure estimates.
The MOEs ranged across age groups and consumption studies from 11,000 to 73,000 at the mean and from 4,000 to 29,000 at the 95th percentile dietary exposures, respectively, indicating a low risk for human health.
Chronic risk
Since a TDI could not be established, and in order to get an indication of the possible chronic risk from MON exposure, the CONTAM Panel calculated the MOE between the lowest BMDL05 of 0.20 mg MON/kg bw per day calculated for haematological hazards from a 28‐day study in pigs and the chronic dietary human exposure estimates.
The MOEs ranged across age groups and consumption studies from 3,900 to 5,000,000 (LB) and from 880 to 25,000 (UB) at the mean exposure, and from 1,400 to 3,300,000 (LB) and from 370 to 4,500 (UB) at the 95th percentile exposure estimates.
The CONTAM Panel concluded that these MOE values were sufficiently large to indicate a low risk for human health from current chronic dietary exposure to MON.
However, the CONTAM Panel stressed that in the absence of quantitative dose–response data on cardiotoxicity this risk characterisation was based on haematological effects from very limited toxicity database.
The limited data on exposure among vegetarians did not indicate notable differences in acute or chronic dietary exposure between the vegetarians and the general population. Therefore, the conclusions on the general population remained valid also for vegetarians.
Farm and companion animal health risk characterisation
The margins between the UB estimates of the dietary exposure and the reference point for adverse health effects ranged for pigs between 90 and 160 for the mean and 35 and 60 for the 95th percentile exposure, for poultry between 430 and 1400 for the mean and 140 and 460 for the 95th percentile and it was 2,700 for the mean and 800 for the 95th percentile exposure, respectively, in the farmed mink.
The CONTAM Panel concluded that the MOE calculated for pigs, poultry and farmed mink indicated overall a low or even negligible risk for these animal species at the estimated exposure levels of MON under current feeding practices.
The MOEs for the other farm and companion animals for which no toxicity data suitable for hazard characterisation of MON were identified, ranged between 120 and 760 for the mean and 80 and 290 for the 95th percentile exposure, respectively.
The CONTAM Panel noted that these MOEs were similar to those observed for animals for which data on adverse effects were observed and conclude that the risk for the other farm and companion animals were therefore also low or even negligible at the estimated exposure levels of MON under current feeding practices.
The CONTAM Panel noted that the conclusion on animals other than poultry, pigs and farmed mink would be affected by a higher degree of uncertainty than that on the animal species for which sufficient toxicity data were available.
6. Recommendations
A well‐designed 90‐day toxicity study in rats using purified MON performed according to relevant OECD guidelines with special focus on the assessment of haematotoxicity, myelotoxicity and cardiotoxicity, is needed.
Furthermore, in vivo studies on the genotoxicity of MON and more data on its mode of action are needed.
Well‐designed studies of the toxicokinetics of MON in experimental, farm and companion animals are required.
Studies on adverse effects of MON in farm animals and companion animals other than poultry are needed.
Depending on the outcome of the above recommended toxicity studies, more occurrence data on MON in foods and feed with state‐of‐the‐art validated analytical methods, such as LC–MS/MS, might be required to enable a comprehensive risk assessment for humans and farm and companion animals to be undertaken.
Documentation provided to EFSA
Jonsson_M. 2017. Additional data_submitted to EFSA_24.4.2017 on repeated dose 28‐day oral toxicity study of moniliformin in rats published by Jonsson M, Atosuo J, Jestoi M, Nathanail A, Kokkonen U‐M, Anttila M, Koivisto P, Lilius E‐M and Peltonen K in 2015.
Abbreviations
- AFRC
Agricultural and Food Research Council
- ALT
alanine aminotransferase
- ARfD
acute reference dose
- AST
aspartate aminotransferase
- BMD
Benchmark dose
- BMDL
Benchmark dose lower confidence limit
- BMDU
Benchmark dose upper confidence limit
- bw
body weight
- CA
chromosome aberration
- CAS
Chemical Abstract Service
- CI
confidence interval
- CK
creatine kinase
- CONTAM Panel
EFSA Panel on Contaminants in the Food Chain
- DAD
diode array detection
- DDT
dichlorodiphenyltrichloroethane
- DL‐PCBs
dioxin‐like polychlorinated biphenyls
- ECB
European corn borer
- ECG
electrocardiogram
- FAO
Food and Agriculture Organization of the United Nations
- FEEDAP Panel
EFSA Panel on Additives and Products or Substances used in Animal Feed
- FEFAC
European Feed Manufactures Federation
- FLD
fluorescence detection
- GGT
gamma glutamyltransferase
- GHS
Globally Harmonized System
- Hb
haemoglobin
- HBGV
health‐based guidance value
- HCT
haematocrit
- HILIC
hydrophilic interaction chromatography
- HPLC
high‐performance liquid chromatography
- HRMS
high‐resolution mass spectrometry
- IC50
half maximal inhibitory concentration
- ICD
International Classification of Diseases
- IPCS
International Programme on Chemical Safety (WHO)
- i.v.
intravenous
- LB
lower bound
- LC–MS
liquid chromatography‐mass spectrometry
- LC‐UV
liquid chromatography‐ultraviolet
- LD50
lethal dose (median)
- LDH
lactate dehydrogenase
- LOAEL
lowest‐observed‐adverse‐effect level
- LOD
limit of detection
- LOQ
limit of quantification
- KD
Keshan disease
- MCH
Mean concentration haemoglobin
- Max
Maximum
- Min
minimum
- MOE
Margin of exposure
- MON
moniliformin
- MN
micronucleus
- MS
mass spectrometry
- NDV
Newcastle disease vaccine
- NOAEL
no‐observed‐adverse‐effect levels
- NRC
National Research Council
- OECD
Organisation for Economic Co‐operation and Development
- PCBs
polychlorinated biphenyls
- PCDDs
polychlorinated dibenzo‐p‐dioxins
- PCDD/Fs
polychlorinated dibenzo‐p‐dioxins and polychlorinated dibenzofurans
- PCDFs
polychlorinated dibenzofurans
- RBC
red blood cell count
- SCE
sister chromatid exchange
- SD
Standard deviation
- SEM
Standard error of the mean
- TBAH
tetra‐n‐butyl ammonium hydroxide
- TBAHS
tetra‐n‐butyl ammonium hydrogen sulfate
- TCDD
tetrachlorodibenzo‐p‐dioxin
- TDI
tolerable daily intake
- TEQ
TCDD Toxic equivalents
- TLC
thin‐layer chromatography
- UB
upper bound
- UDS
unscheduled DNA synthesis
- UV
ultraviolet
- VKM
Norwegian Scientific Committee for Food Safety
- WG
Working group
- WHO
World Health Organization
Appendix A – EFSA guidance documents applied in the assessment of MON in food and feed
The CONTAM Panel applied the general principles of the risk assessment process for chemicals in food as described by WHO (2009), i.e. hazard identification and characterisation, exposure assessment and risk characterisation. The following EFSA guidance documents were applied in the assessment of MON in food and feed:
EFSA (European Food Safety Authority), 2006. Guidance of the Scientific Committee on a request from EFSA related to uncertainties in Dietary Exposure Assessment. EFSA Journal 2007;4(12):438, 54 pp. https://doi.org/10.2903/j.efsa.2007.438
EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: General principles. EFSA Journal 2009;6(5):1051, 22 pp. https://doi.org/doi org/10.2903/j.efsa.2009.1051
EFSA (European Food Safety Authority), 2010. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. https://doi.org/10.2903/j.efsa.2010.1557
EFSA (European Food Safety Authority), 2011. Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. https://doi.org/10.2903/j.efsa.2011.2379
EFSA (European Food Safety Authority), 2011. Overview of the procedures currently used at EFSA for the assessment of dietary exposure to different chemical substances. EFSA Journal 2011;9(12):2490, 33 pp. https://doi.org/10.2903/j.efsa.2011.2490
EFSA Scientific Committee, 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. https://doi.org/10.2903/j.efsa.2012.2579
EFSA Scientific Committee, 2012. Scientific Opinion on Risk Assessment Terminology. EFSA Journal 2012;10(5):2664, 43 pp. https://doi.org/10.2903/j.efsa.2012.2664
EFSA Scientific Committee, Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017. Update: Guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. https://doi.org/10.2903/j.efsa.2017.4658
EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534, 26 pp. https://doi.org/10.2903/j.efsa.2012.2534
Appendix B – Feed intakes and diet composition (farm and companion animals)
To estimate exposure to moniliformin (MON), information on both the amount of feed consumed and the concentration of MON in the feed is required. This Appendix gives details of the feed intakes, live weights and diet compositions for different livestock, fish and companion animals used as the basis to estimate exposures. These are based on published guidelines on nutrition and feeding (e.g. Carabano and Piquer, 1998; NRC 2000, 2007a,b, Leeson and Summers, 2008, OECD, 2009; McDonald et al., 2011; EFSA FEEDAP Panel, 2012) and information provided by European feed manufacturers. They are therefore estimates of the Panel on Contaminants in the Food Chain (CONTAM Panel), but agree with common practice. In Annex A, Table A5, the concentrations of MON in feeds used to estimate exposure are presented. It should be noted that the farm and companion animal exposure estimates were calculated by using the occurrence data set which comprised the data on feed (Section 3.3.2.3 and Annex A, Table A5) and the grains reported as ‘Unprocessed grains of undefined end‐use’ (Section 3.3.2.4 and Annex A, Table A8 (crops)).
B.1. Feed intakes
B.1.1. Cattle, sheep, goats and horses
Dairy cows
The amounts of feed given to lactating dairy cows varies according to the amount and quality of forages and other feeds available, the milk yield and the size of the cow. Two scenarios have been considered in estimating exposure by dairy cows to MON. The first reflects diets in which grass – fresh or conserved – is supplemented with cereal grains. In this scenario, it is assumed that the forages make no contribution to exposure, and that non‐forage feeds are fed at the rate of 0.3 kg/kg of milk produced (Nix, 2010). Exposures to MON have been estimated for a 650‐kg dairy cow, with a milk yield of 40 kg per day, reflecting a relatively high milk yield. Assumptions on the amounts of forages and non‐forage feed are given in Table B.1. In the second scenario, maize silage is assumed to be the sole forage, supplemented with maize grain (amounts given in Table B.4). In view of the relatively small number of samples of maize silage in the database (n = 42), the 95th percentile estimates of exposure have not been made.
Beef cattle
There are a wide variety of beef production and husbandry systems in Europe. They may be categorised broadly as forage‐based or cereal‐based systems, although combinations of these systems are commonly found. In this opinion, four feeding systems are considered, in which the forages are (1) grass hay or silage (in which case the forage is assumed to make no contribution to MON exposure), (2) maize silage and (3) cereal straw with, in each case, appropriate supplementation with non‐forage feed materials. A fourth system, namely ‘cereal beef’, is also considered. For exposure estimates, live weights of 300 or 400 kg, and feed intakes of between 6.6 and 10 kg dry matter/day have been assumed, depending on the feeding regime, based on guidelines published by EBLEX (2008, 2012) and details are given in Table B.1.
Sheep and goats
Many breeds and systems of management have been developed for sheep and goats to suit the land, climate and husbandry conditions in the EU. As for other ruminants, forages may be the only feeds used after weaning (NRC, 2007a). Common exceptions to this are pregnant and lactating animals, whose feed is usually supplemented with non‐forage feeds or commercial compound (complementary) feeds (AFRC, 1993; NRC, 2007a). In this opinion, exposure estimates have been made for lactating sheep and goats. The CONTAM Panel has used a daily dry matter intake of 2.8 kg for an 80‐kg lactating sheep feeding twin lambs to estimate the exposures. For lactating goats, the CONTAM Panel has used daily dry matter intakes of 3.3 kg for a 60‐kg goat for milking (4 kg milk/day). No estimates of exposure have been made for fattening sheep and goats. For these livestock, fresh or conserved forages are frequently the sole feed, and levels of MON in these feeds are generally low or not reported.
Horses
Horses are non‐ruminant herbivores. They generally consume 2–3.5% of their body weight in feed (dry matter) each day, of which a minimum of 50% should be as forage (pasture or hay) (NRC, 2007b). Mature horses with minimal activity can be fed forage alone, but for growing and active horses supplementary feeding with cereal grains, cereal by‐products (e.g. oats, barley and wheat bran) and vegetable proteins is necessary. The CONTAM Panel has estimated the exposure for a 450‐kg horse, with a daily intake of 9 kg dry matter/day; as for ruminant livestock it is assumed that fresh grass is the forage, and therefore makes no contribution to MON exposure (Table B.1).
B.1.2. Non‐ruminant animals
Pigs
Although there is a considerable range of pig production systems in Europe, exposure estimates have been made for piglets (pig starter), finishing pigs and lactating sows (using feed intakes proposed by EFSA (2012)) (Table B.2)
Poultry
The CONTAM Panel applied the live weights and feed intakes reported for fattening chickens (broilers), laying hens and turkeys proposed by EFSA (2012) and for ducks by Leeson and Summers (2008) (Table B.2)
Farmed fish (salmonids and carp)
Commercially reared species include Atlantic salmon, rainbow trout, sea bass, sea bream, cod, halibut, tuna, eel and turbot. In this Scientific Opinion, exposures to MON have been made for farmed salmon and carp. Details of the body weights and feed intakes used are given in Table B.2.
B.1.3. Farmed rabbits
Feed intakes of 65–80 g/kg bw per day have been reported (Carabano and Piquer, 1998). For the exposure estimates, the CONTAM Panel have assumed a live weight of 2 kg, and a daily feed intake of 75 g/kg bw (derived from Carabano and Piquer, 1998).
B.1.4. Farmed mink
For estimating exposure, the CONTAM Panel have assumed a live weight of 2.07 kg for a male mink at pelting, and with a feed intake of 227 g/day (75 g dry matter) (NRC, 1982).
B.1.5. Dogs and cats
The amount of food consumed is largely a function of the mature weight of the animal, level of activity, physiological status (e.g. pregnancy or lactation) and the energy content of the diet. In this opinion, the CONTAM Panel assumed body weights (kg) and feed intakes (g dry matter/day) for dogs and cats were 25/360 and 4/60, respectively (derived from NRC, 2006).
B.2. Diet compositions and inclusion of diet ingredients to the exposure calculations
As reported in Section 3.3.2, most of the data on levels of MON provided to EFSA have been for whole cereal grains, and therefore, estimates of exposure have been made using assumed levels of cereal grains in the diets of farmed animals (including fish) and companion animals. In addition, levels of MON in maize silages (n = 42) and cereal straw (n = 18) have been reported and these, together with levels in cereal grains, have been used to estimate mean LB and UB exposures to MON for dairy and beef cattle.
B.2.1. Cattle, sheep, goats and horses
For most ruminants and horses, forages (either fresh or conserved) are important ingredients in their diet, but they are normally supplemented with non‐forage feeds such as cereals, cereal by‐products, oilseed meals and by‐products of human food production. These may be fed either as individual feeds, mixtures of feed materials or as species‐specific complementary feeds in the form of compound feeds. In some situations, however, forages may represent the total diet.
Fresh (grazed) or conserved grass (as silage or hay) are the principal forages for ruminants and horses in the EU, but in the absence of any data on levels of MON in these feeds, it has been assumed that they make no contribution to exposure. For other maize silage and cereal straw, however, the presence of MON has been reported. Therefore, two estimates of exposure have been reported for ruminants and horses, the first of which assumes no exposure from forages (i.e. the main forages are fresh grass and/or grass silage). Exposures have also been estimated for diets in which maize silage or cereal straw are the main forage. AFSSA (2009) have provided example intakes of dairy cows fed maize silage supplemented with maize grain and soybean meal, while example diets of beef cattle on maize silage or cereal straw‐based diets are taken from EBLEX (2008, 2012), and these are given in Table B.4.
Horses are non‐ruminant herbivores, and their diet should contain a minimum of 50% forages (NRC, 2007b). While mature horses with minimal activity can be fed forage alone, for growing and active horses supplementary feeding with cereal grains, cereal by‐products (e.g. oats, barley, and wheat bran) and vegetable proteins is necessary. Although oats are the preferred cereal for many horse owners, other cereal grains and cereal by‐products are also routinely used. In this Opinion, the CONTAM Panel has estimated the exposure for a 450‐kg horse, with a daily intake of 9 kg dry matter/day, of which half is in the form of non‐forage feeds and where oat grains represent 40% of the non‐forage component of the daily ration.
B.2.2. Pigs and poultry
In the absence of data for species‐specific compound feeds or pigs and poultry, estimates of exposure to MON have been made using levels of MON in whole cereal grains and their inclusion in diets for each category of livestock as given in Table B.3.
Table B.3.
Estimated example diet composition (%) for farmed species and companion animals (cats and dogs)
| Feeds | Wheat | Barley | Oats | Maize |
|---|---|---|---|---|
| Ruminants and horses | ||||
| Dairy: high yielding | 15 | 20 | ni | ni |
| Beef: intensive cereal | ni | 60 | ni | ni |
| Beef: fattening | ni | 40 | ni | ni |
| Sheep: lactating | 14 | 18 | ni | ni |
| Goats: lactating | ni | 25 | 35 | ni |
| Goats: fattening | ni | 20 | 40 | ni |
| Horses | ni | ni | 40 | ni |
| Pigs and poultry | ||||
| Pig starter | 48 | 16 | ni | ni |
| Pig finisher | 48 | 20 | ni | ni |
| Lactating sow | 50 | 11 | ni | ni |
| Broilers: starter | 32 | ni | ni | 35 |
| Broilers: grower | 38 | ni | ni | 38 |
| Laying hens | 30 | ni | ni | 35 |
| Turkeys: grower | 30 | 35 | ni | ni |
| Ducks: grower | 45 | 15 | ni | ni |
| Farmed fish | ||||
| Salmonids | 13 | ni | ni | ni |
| Carp | 24 | ni | ni | 10 |
| Farmed rabbits and mink | ||||
| Rabbits | ni | 18 | ni | ni |
| Mink | 6 | 1 | ni | 6 |
| Cats and dogs | ||||
| Cats | 10 | ni | ni | 5 |
| Dogs | 10 | ni | 0.5 | 6 |
ni: not included in the diet formulations.
B.2.3. Farmed rabbits
Rabbits are usually fed a pelleted diet (in the form of complete feedingstuffs) consisting of dried forages, cereals and vegetable proteins supplemented with minerals, vitamins and trace elements. Lebas and Renouf (2009) reviewed diet formulations used in experimental studies: in 58 diets, cereals and cereal by‐products (mostly wheat brans represented 18–20%. Dried lucerne is a particularly important ingredient in some diets, and has been included at levels of up to 65% (Lebas and Renouf, 2009). In this opinion, the cereal grain inclusion rates used in a typical French commercial rabbit compound, as provided by T. Gidenne, (Personal communication, 2011) have been used, details of which are given in Table B.3.
B.2.4. Farmed mink
Mink are carnivorous animals and are fed high protein diets, and therefore commercially manufactured mink feed consists largely of fish and land animal by‐products, with lesser amounts of cereals and cereal by‐products. The proportions of cereal grains used in estimating the exposure are given in Table B.3, and are based on information translated from Finnish to English provided by the Finnish Fur Breeders Association in 2015.23
B.2.5. Farmed fish (salmonids and carp)
Traditionally, the principal raw materials used for the manufacture of fish feeds in Europe have been fishmeal and fish oils, and although alternative sources of oil and protein (e.g. soybean meals and vegetable oils) are increasingly being used fish‐derived feeds remain the major ingredients.
For many fish species, digestion of complex carbohydrates and the metabolic utilisation of the absorbed glucose is low, reflecting the scarcity of carbohydrates in the aquatic environment (Guillaume et al., 2001). Instead, fish obtain much of their energy from protein in the diet. Where carbohydrates are used, they generally require some form of pre‐treatment (e.g. cooking, flaking or toasting).
Berntssen et al. (2010) provided details of the composition of a diet for growing salmonids, and the CONTAM Panel used this feed formulation to estimate the exposures (Table B.3).
In contrast, studies with the common carp (Cyprinus carpio) have demonstrated greater intestinal amylase activity than in carnivorous fish, which accounts for the better utilisation of carbohydrates by these fish. The optimum level of carbohydrates appears to be 30–40% (FAO, Aquaculture Feed and Fertiliser Resources Information System24), which allows for higher levels of cereals than in diets for salmonids. The CONTAM Panel have adopted the ingredients of commercial compound feeds for carp reported by Schultz et al. (2012) to estimate exposure to MON.
B.2.6. Cats and dogs
Most small companion animals derive their nutritional needs from processed food, and in 2010 EU annual sales of pet food products was approximately 8.3 million tonnes.25 Although a wide range of ingredients is used in commercial diets, most dog and cat diets contain cereals (predominantly wheat, rice or maize), cereal by‐products, vegetable proteins and by‐products of human food production.
The European Pet Food Industry Federation (FEDIAF) has provided information on typical ingredient compositions of dry cat and dog food,26 and in the absence of data on species‐specific manufactured complete feedingstuffs, this has been used to estimate exposure to MON (details given in Table B.3). However, these should be regarded as indicative only, since actual ingredients will vary depending both on the availability of feed materials and the nutrient requirements of the animals.
Appendix C – Benchmark dose modelling
This Appendix provides details on the calculation of the LD50 for female rats of the study by Abbas et al. (1990) and BMDL values from the dose–response evaluation of pigs reported by Harvey et al. (2001) using the BMD approach (EFSA Scientific Committee, 2017). Further details on the application of BMDS and Proast software are provided as supplementary information in its Appendix A.
For the quantal response data from Abbas et al., all models available in the BMDS software were selected using the default benchmark response (BMR) of 10% extra risk. For the quantitative response data of Harvey et al. (2001), the BMR was defined as a percent change of the magnitude of the response when compared to that predicted at background, i.e. a relative deviation from background. The default value of 5% (BMR = 0.05) was used as recommended by EFSA Scientific Committee (2017) in the absence of statistical or toxicological considerations supporting a deviation from that default value. The BMD analyses of quantitative data were based on means and standard errors of the mean available from the reports of the selected studies. For interpreting the graphs and tables obtained by PROAST, it should be noted that the data of each dose group are assumed to be log‐normally distributed such that the means are geometric means and the whiskers are based on geometric standard deviations.
C.1. Calculation of the LD50 for female rats reported by Abbas et al. (1990)
The dose–response data on female rats reported by Abbas et al. (1990) were analysed using the BMD approach to calculate a LD50 and its lower 95% confidence limit. Doses of MON, number of animals per dose group and mortality are shown in Table C.1.
Table C.1.
Doses of MON, number of animals per dose group and incidences for the acute toxicity of rats by Abbas et al. (1990)
| Dose (mg MON/kg bw) | Number of animals | Incidence |
|---|---|---|
| 0 | 5 | 0 |
| 2.5 | 5 | 0 |
| 5 | 5 | 0 |
| 10 | 5 | 0 |
| 20 | 5 | 4 |
| 40 | 5 | 5 |
| 60 | 5 | 5 |
| 80 | 5 | 5 |
| 100 | 5 | 5 |
MON: moniliformin; bw: body weight.
Minimum BMDL approach for BMD = 50% – female rats
The CONTAM Panel modelled the acute toxicity data of female rats of Abbas et al. (1990) (Table C.1) to calculate an LD50 using the BMD approach with a BMR of 50% using the BMDS software accounting the EFSA guidance (EFSA Scientific Committee, 2017). Thereby, the CONTAM Panel estimated the LD50 of 18.52 mg/kg bw with a lower 90% confidence bound LDL50 of 13.08 mg/kg bw. The results of the BMD analysis are presented in Table C.2. The multistage model in its default form in BMDS and the Weibull and Gamma models could not be fitted. The probit and logistic as well as the log‐probit and log‐logistic models were saturated models. The AIC of the full model was AICFull = 23.00 with AICFull +2 = 25.00. All models selected for the BMD analysis showed an AIC < AICFull +2 = 25.00. The AIC of the null model was AICNull = 64.18 with AICNull −2 = 62.18. No selected model showed an AIC > AICNull −2 = 62.18. The AICMIN = 7.00 was obtained by the log‐logistic model and all other models, except the multistage cancer and the quantal linear model, showed AIC values not larger than AICMIN +2 = 9.00 and were accepted. The lowest BMDL50, denoted here as LDL50, of the accepted models was 13.08 mg MON/kg bw per day obtained with the log‐logistic model. The fit is shown in Figure C.2 and more details of the analysis are given in Supplementary Information Abbas et al. (1990)
Figure C.2.
Dose–response curves for the modelling averaging of the fitted models of the data of Abbas et al. (1990)
Figure C.1.
Dose–response curve of the fitted model of lowest BMDL 50 on the acute toxicity data reported by Abbas et al. (1990) (see Table C.1). The (red) curve is the fitted dose–response curve and the dotted line curve indicates the 95% lower confidence limit of the calculated BMDL
Model averaging – female rats
Model averaging (MA) implemented in the EFSA tool based on the two‐stage, probit, logistic, log‐probit, log‐logistic, two‐stage, Weibull, Gamma models and the exponential and hill model modified for quantal data provided the BMC confidence interval (MA‐BMC‐CI) of 13.5–17.6 mg/kg bw per day (for details see in Supplementary Information Abbas et al., 1990). The corresponding Bootstrap plot of MA is shown in Figure C.2.
C.2. Calculation of BMDL values for haematological effects of MON in pigs reported by Harvey et al. (2001)
Concentration–response data on the haematotoxicity of MON in barrows abotaine in two consecutively conducted 28‐day experiments, each designed for n = 6 animals per group, were reported by Harvey et al. (2001). Concentrations of MON in feed, mean body weights and number of animals per dose group and haematological effects of MON are shown in Table C.3. The CONTAM Panel calculated for each dose group of the two experiments a mean body weight based on the reported mean body weights of the pigs at start and after 28 weeks. Based on that and assuming a 1.2 kg feed intake per day, doses were calculated as shown in Table C.3.
Figure C.3.
Result of the dose–response evaluation the two experiments of Harvey et al. (2001) combined for haemoglobin (Hb)
Figure C.4.
Result of the dose–response evaluation the two experiments of Harvey et al. (2001) combined for haematocrit (HCT)
Figure C.5.
Result of the dose–response evaluation the two experiments of Harvey et al. (2001) combined for the red blood cell count (RBC)
Appendix D – Margin of exposure (MOE) values based on the NOAEL or BMDL identified in relevant studies and the estimated UB or LB 95th percentile and mean dietary exposure across animal species
Table D.1.
Margin of exposure (MOE) values based on upper bound (UB) 95th percentile and mean dietary exposure across animal species
| Animal species | NOAEL/ BMDL μg/kg bw | 95th percentile dietary exposure (UB) | Mean percentile dietary exposure (UB) | ||
|---|---|---|---|---|---|
| Exposure μg/kg bw per day | MOE value | Exposure μg/kg bw per day | MOE value | ||
| Ruminants£ | 200* | 2.4 | 83 | 1.6 | 125 |
| • Dairy cows | 200* | 0.8 | 250 | 0.32 | 625 |
| • Beef cattle | 200* | 2.4 | 83 | 1.3 | 154 |
| Lactating sheep | 200* | 1.3 | 154 | 0.4 | 500 |
| Lactating goat | 200* | 1.8 | 111 | 0.86 | 233 |
| Fattening pig (starter) | 200* | 5.6 | 36 | 2.2 | 90 |
| Lactating sow | 200* | 3.2 | 62 | 1.2 | 166 |
| Poultry | |||||
| • Broiler chicken | 1,400 | 10 | 140 | 3.2 | 438 |
| • Laying hens | 3,800 | 9.0 | 422 | 2.7 | 1407 |
| • Fattening turkey | 1,600 | 3.9 | 410 | 1.5 | 1067 |
| • Fattening duck | 2,300 | 4.9 | 469 | 1.9 | 1211 |
| Horse | 200* | 0.68 | 294 | 0.27 | 741 |
| Farmed fish | 800# | 1.5 | 533 | 0.51 | 1569 |
| Farmed rabbit | 200* | 2.5 | 80 | 1 | 200 |
| Farmed mink | 920 | 1.1 | 836 | 0.33 | 2788 |
| Dog | 200* | 0.8 | 250 | 0.33 | 606 |
| Cat | 200* | 0.75 | 267 | 0.33 | 769 |
NOAEL: no‐observed‐adverse‐effect levels; BMDL: benchmark dose lower confidence limit; bw: body weight; UB: upper bound. *: based on pig BMDL05; #: reduced body weight gain reported in catfish, £: based on various types of ruminants
For ‘ruminants’, it is reported the lowest calculated value from the various diets (as listed in Table 11 in the body of the opinion).
Table D.2.
Margin of exposure (MOE) based on lower bound (LB) 95th percentile and mean dietary exposure across animal species
| Animal species | NOAEL/ BMDL μg/kg bw | 95th percentile dietary exposure (LB) | Mean percentile dietary exposure (LB) | ||
|---|---|---|---|---|---|
| Exposure μg/kg bw per day | MOE values | Exposure μg/kg bw per day | MOE values | ||
| Ruminants | 200* | 0.27 | 741 | 0.04 | 5,000 |
| • Dairy cows | 200* | 0.74 | 270 | 0.14 | 1,429 |
| • Beef cattle | 200* | 0.27 | 741 | 0.04 | 5,000 |
| Lactating sheep | 200* | 1.2 | 167 | 0.18 | 1,111 |
| Lactating goat | 200* | 0.86 | 233 | 0.55 | 364 |
| Fattening pig (starter) | 200* | 4.9 | 40.8 | 1.1 | 181.8 |
| Lactating sow | 200* | 2.8 | 71 | 0.65 | 308 |
| Poultry | |||||
| • Broiler chicken | 1,400 | 9.7 | 144 | 2.4 | 583 |
| • Laying hens | 3,800 | 6.5 | 585 | 2.1 | 1,810 |
| • Fattening turkey | 1,600 | 3.6 | 444 | 0.71 | 2,254 |
| • Fattening duck | 2,300 | 4.3 | 535 | 0.98 | 2,347 |
| Horse | 200* | 0.31 | 645 | 0.06 | 3,333 |
| Farmed fish | 800# | 0.38 | 2,105 | 0.1 | 8,000 |
| Farmed rabbit | 200* | 2.5 | 80 | 0.39 | 513 |
| Farmed mink | 920 | 0.99 | 929 | 0.24 | 3833 |
| Dog | 200* | 0.8 | 250 | 0.28 | 714 |
| Cat | 200* | 0.75 | 267 | 0.22 | 909 |
NOAEL: no‐observed‐adverse‐effect levels; BMDL: benchmark dose lower confidence limit; bw: body weight; LB: lower bound. *: based on pig BMDL05; #: reduced body weight gain reported in catfish.
Annex A – Supporting tables on food and feed occurrence and human exposure
Annex A can be found in the online version of this output (‘Supporting information’ section): http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2018.5082/abstract
Description: Supporting tables on food and feed occurrence and human exposure
Supporting information
Supporting tables on food and feed occurrence and human exposure
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Nebbia CS, Oswald IP, Petersen A, Rose M, Roudot A‐C, Schwerdtle T, Vleminckx C, Vollmer G, Wallace H, De Saeger S, Eriksen GS, Farmer P, Fremy J‐M, Gong YY, Meyer K, Naegeli H, Parent‐Massin D, van Egmond H, Altieri A, Colombo P, Eskola M, van Manen M and Edler L, 2018. Scientific Opinion on the risks to human and animal health related to the presence of moniliformin in food and feed. EFSA Journal 2018;16(3):5082, 95 pp. 10.2903/j.efsa.2018.5082
Requestor: European Commission (EC)
Question number: EFSA‐Q‐2010‐01004
Panel members: Jan Alexander, Lars Barregård, Margherita Bignami, Beat Brüschweiler, Sandra Ceccatelli, Bruce Cottrill, Michael Dinovi, Lutz Edler, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Helle Katrine Knutsen, Carlo Stefano Nebbia, Isabelle P Oswald, Annette Petersen, Martin Rose, Alain‐Claude Roudot, Tanja Schwerdtle, Christiane Vleminckx, Günter Vollmer and Heather Wallace.
Acknowledgements: The CONTAM Panel wishes to acknowledge all European competent authorities and other stakeholders that provided occurrence data on moniliformin in food and feed, and supported the consumption data collection for the Comprehensive European Food Consumption Database.
Adopted: 21 November 2017
Notes
Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. OJ L 70, 9.3.2006, p. 12–34.
Commission Regulation (EU) No 519/2014 of 16 May 2014 amending Regulation (EC) No 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T‐2, HT‐2 toxin and citrinin and screening methods of analysis. OJ L 147, 17.5.2014, p. 29–43.
Council Regulation (EEC) No 315/93 of February 1993 laying down Community procedures for contaminants in food. OJ L 37, 13.2.1993, p. 1–3.
Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. OJ L 364, 20.12.2006, p. 5–24.
Directive 2002/32/EC of the European Parliament and the Council of 7 May 2002 on undesirable substances in animal feed. OJ L 140, 30.5.2002, p. 10–21.
Commission Recommendation 2006/576/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T‐2 and HT‐2 and fumonisins in products intended for animal feeding. OJ L 229, 23.8.2006, p. 7–9.
From 1 January 2014 onwards, Evidence Management Unit (DATA).
Suspect sampling means ‘selection of an individual product or establishment in order to confirm or reject a suspicion of non‐conformity. It's not a random sampling, therefore there is no sample extracted from the population’. EFSA Journal 2013;11(10):3424, 114 pp. https://doi.org/10.2903/j.efsa.2013.3424
For the definition of selective and convenient sampling, see EFSA Journal 2013;11(10):3424, 114 pp. https://doi.org/10.2903/j.efsa.2013.3424
Objective sampling means ‘strategy based on the selection of a random sample from a population on which the data are reported. Random sample is a sample which is taken under statistical consideration to provide representative data.’
Source: FEFAC Feed and Food Statistical Yearbook 2015. www.fefac.org
Food and Feed Statistical Yearbook 2015. www.FEFAC.org
OECD Guidelines for the Testing of Chemicals, Test No. 423 (2002): Acute Oral toxicity ‐ Acute Toxic Class Method. Available online: http://www.oecd-ilibrary.org/environment/test-no-423-acute-oral-toxicity-acute-toxic-class-method_9789264071001-en
OECD Guidelines for the Testing of Chemicals, Test No. 407 (2008): Repeated Dose 28‐day Oral Toxicity Study in Rodents. Available online: http://www.oecd-ilibrary.org/environment/test-no-407-repeated-dose-28-day-oraltoxicity-study-in-rodents_9789264070684-en
The term satellite group is used by OECD guideline 407 http://www.oecd.org/chemicalsafety/testing/37477972.pdf
Assuming a female mink eats 38 g dry weight feed/kg bw per day (equivalent to 38 g × 3 = 114 g wet weight feed/kg bw per day) and the consumption of feed containing 8.1 mg MON/kg feed (17.0 mg MON/kg feed) this is equivalent to an intake of 0.114 kg × 8.1 mg MON/kg feed = 0.92 mg MON/kg bw per day (0.114 kg × 17.0 mg MON/kg feed = 1.94 mg MON/kg bw per day).
Doses calculated by the CONTAM Panel from the data reported by the authors.
Original paper in Chinese, text based on the translation to English.
Original paper in Chinese, text based on the translation to English.
Dietary exposures assume a 12% moisture content in the feed (i.e. they are expressed on an 88% dry matter (DM) basis).
Available online: www.Fedif.org.
FEDIAF, Personal communication by email, May 2016
References
- Abbas HK and Mirocha CJ, 1985. Production of moniliformin by Fusarium moniliforme var. subglutinans isolated from wheat kernels originating from Minnesota. Microbiologie – Aliments –. Nutrition, 3, 223–227. [Google Scholar]
- Abbas HK, Mirocha CJ, Vesonder RF and Gunther R, 1990. Acute toxic effects of an isolate of moniliformin‐producing Fusarium oxysporum and purified moniliformin on rats. Archives of Environmental Contamination and Toxicology, 19, 433–436. [DOI] [PubMed] [Google Scholar]
- AFRC (Agricultural and Food Research Council), 1993. Energy and protein requirements of ruminants: An advisory manual. Alderman G and Cottrill BR (eds.). CAB International, Wallingford, UK, 159 pp. [Google Scholar]
- AFSSA (Agence Francaise de Securite Sanitaire de Aliments), 2009. Évaluation des risques liés à la présence de mycotoxines dans les chaînes alimentaires humaine et animale. Fremy JM and Thomann C (eds.). AFSSA , Rennes, France, 308 pp. [Google Scholar]
- Alassane‐Kpembi I, Schatzmayr G, Marin D, Taranu D, Puel O and Oswald IP, 2017. Mycotoxins co‐contamination: Methodological aspects and biological relevance of combined toxicity studies. Critical Reviews in Food Science and Nutrition, 16, 3489–3507. [DOI] [PubMed] [Google Scholar]
- Allen NK, Burmeister HR, Weaver GA and Mirocha CJ, 1981. Toxicity of dietary and intravenously administered moniliformin to broiler chickens. Poultry Science, 60, 1415–1417. [DOI] [PubMed] [Google Scholar]
- Apfelthaler E, Bicker W, Lämmerhofer M, Sulyok M, Krska R, Lindner W and Schuhmacher R, 2008. Retention pattern profiling of fungal metabolites on mixed‐mode reversed‐phase/weak anion exchange stationary phases in comparison to reversed‐phase and weak anion exchange separation materials by liquid chromatography–electrospray ionisation‐tandem mass spectrometry. Journal of Chromatography A, 1191, 171–181. 10.1016/j.chroma.2007.12.067 [DOI] [PubMed] [Google Scholar]
- Appell M, Kendra DF, Kim EK and Maragos CM, 2007. Synthesis and evaluation of molecularly imprinted polymers as sorbents of moniliformin. Food Additives and Contaminants, 24, 43–52. 10.1080/02652030600887586 [DOI] [PubMed] [Google Scholar]
- Auffray Y and Boutibonnes P, 1986. Evaluation of the genotoxic activity of some mycotoxins using Escherichia coli in the SOS spot test. Mutation Research, 171, 79–82. [DOI] [PubMed] [Google Scholar]
- Auffray Y and Boutibonnes P, 1987. Genotoxic activity of some mycotoxins using the SOS chromotest. Mycopathologia, 100, 49–53. [DOI] [PubMed] [Google Scholar]
- Bailey RH, Kubena LF, Harvey RB, Buckley SA, Richard JR, Bennett GW, Dombrink‐Kurtzman MA, Bermudez AJ and Rottinghaus GE, 1997. Toxicity of purified moniliformin and moniliformin naturally produced in Fusarium fujikoroi culture material in young broiler chicks. 86th Annual Meeting of the Poultry Science Association, Georgia, USA.
- Battilani P, Costa LG, Dossena A, Gullino ML, Marchelli R, Galaverna G, Pietri A, Dall'Asta C, Giorni P, Spadaro D and Gualla A, 2009. Scientific information on mycotoxins and natural plant toxicants. EFSA Supporting Publications, 6, EN‐24. 467 pp. 10.2903/sp.efsa.2009.en-24 [DOI] [Google Scholar]
- Beck MA, Levander OA and Handy J, 2003. Selenium deficiency and viral infection. The Journal of Nutrition, 133, 1463S–1467S. [DOI] [PubMed] [Google Scholar]
- Behrens M, Huewel S, Galla HJ and Humpf HU, 2015. Blood‐brain barrier effects of the Fusarium mycotoxins Deoxynivalenol, 3 Acetyldeoxynivalenol, and Moniliformin and their transfer to the brain. PLoS ONE, 10, e0143640. 10.1371/journal.pone.0143640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez AJ, Ledoux DR, Rottinghaus GE and Bennett GA, 1997. The individual and combined effects of the Fusarium mycotoxins moniliformin and fumonisin B‐1 in turkeys. Avian Diseases, 41, 304–311. [PubMed] [Google Scholar]
- Bernhoft A, Clasen PE, Kristoffersen AB and Torp M, 2010. Less Fusarium infestation and mycotoxin contamination in organic than in conventional cereals. Food Additives and Contaminants, 27, 842–852. [DOI] [PubMed] [Google Scholar]
- Berntssen MHG, Maage A, Julshamm K, Oeye BE and Lundebye AK, 2010. Carry‐over of dietary organochlorine pesticides, PCDD/Fs, PCBs, and brominated flame retardants to Atlantic salmon (Salmo salar L.) fillets. Chemosphere, 83, 95–103. [DOI] [PubMed] [Google Scholar]
- Böhs B, Seidel V and Lindner W, 1995. Analysis of selected mycotoxins by capillary electrophoresis. Chromatographia, 41, 631–637. [Google Scholar]
- Bosch U, Mirocha CJ, Abbas HK and di Menna M, 1989. Toxicity and toxin production by Fusarium isolates from New Zealand. Mycopathologia, 108, 73–79. [DOI] [PubMed] [Google Scholar]
- Broomhead JN, Ledoux DR, Bermudez AH and Rottinghaus GE, 1996. Effects of Fusarium fujikoroi culture material containing known levels of moniliformin on ducklings. SPSS, Abstract S18 – Poultry Science. [DOI] [PubMed]
- Broomhead JN, Ledoux DR, Bermudez AJ and Rottinghaus GE, 2002. Chronic effects of moniliformin in broilers and turkeys fed dietary treatments to market age. Avian Diseases, 46, 901–908. [DOI] [PubMed] [Google Scholar]
- Browne EM, Juniper DT, Bryant MJ and Fisher AV, 2004. Intake, live‐weight gain and carcass characteristics of beef cattle given diets based on forage maize silage harvested at different stages of maturity. Animal Science, 79, 405–413. 10.1017/S1357729800090275 [DOI] [Google Scholar]
- Buck WB, Haliburton JC, Thilste dJP, Lock TF and Vesonder RF, 1980. Equine leucoencephalomalacia: comparative pathology of naturally occurring and experimental cases. Amer. Ass. Veterinary Laboratory Diagnosticians 22nd Annual Proceedings, 239‐258.
- Burka LT, Doran J and Wilson BJ, 1982. Enzyme inhibition and the toxic action of moniliformin and other vinylogous a‐ketoacids. Biochemical Pharmacology, 31, 79–84. [DOI] [PubMed] [Google Scholar]
- Burmeister HR, Ciegler A and Vesonder RF, 1979. Moniliformin, a metabolite of Fusarium moniliforme NRRL 6322: Purification and toxicity. Applied and Environmental Microbiology, 37, 11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister HR, Grove MD and Kwolek WF, 1980. Moniliformin and butenolide: effect on mice of high‐level, long‐term oral intake. Applied and Environmental Microbiology, 40, 1142–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabano R and Piquer J, 1998. The digestive system of the rabbit. In: de Blas C and Wiseman J (eds.). The Nutrition of the Rabbit. CABI Publishing, Oxfordshire, UK. pp. 1–16. [Google Scholar]
- Castelo M, 1999. Stability of mycotoxins in thermally processed corn products. PhD Thesis. University of Nebraska, United States. 141 pp.
- Celik M, Yilmaz S, Aksoy H, unal F, Yuzbasoglu D and Donbak L, 2009. Evaluation of the genotoxicity of Fusarium mycotoxin moniliformin in human peripheral blood lymphocytes. Environmental and Molecular Mutagenesis, 50, 431–434. [DOI] [PubMed] [Google Scholar]
- Chen L, Tian X and Yang B, 1990. A study on the inhibition of rat myocardium glutathione peroxidase and glutathione reductase by moniliformin. Mycopathologia, 110, 119–124. [DOI] [PubMed] [Google Scholar]
- Chung SH, Ryu D, Kim EK and Bullerman LB, 2005. Enzyme‐assisted extraction of moniliformin from extruded corn grits. Journal of Agriculture and Food Chemistry, 53, 5074–5078. 10.1021/jf0580014 [DOI] [PubMed] [Google Scholar]
- Cigić IK and Prosen H, 2009. An overview of conventional and emerging analytical methods for the determination of mycotoxins. International Journal of Molecular Sciences, 10, 62–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RJ and Cox RH, 1981. Fusarium toxins. In: Cole RJ (ed.). Handbook of Toxic Fungal Metabolites. Elsevier, Amsterdam, the Netherlands. pp. 893–910. [Google Scholar]
- Cole RJ, Kirksey JW, Cutler HG, Doupnik BL and Peckham JC, 1973. Toxin from Fusarium moniliforme: effects on plants and animals. Science, 179, 1324–1326. 10.1126/science.179.4080.1324 [DOI] [PubMed] [Google Scholar]
- Conkova E, Laciakova A, Kovac G and Seidel H, 2003. Fusarial toxins and their role in animal diseases. The Veterinary Journal, 165, 214–220. 10.1016/S1090-0233(02)00127-2 [DOI] [PubMed] [Google Scholar]
- Delgado RM, Sulyok M, Jirsa O, Spitzer T, Krska R and Polišenská I, 2014. Relationship between lutein and mycotoxin content in durum wheat. Food Additives and Contaminants: Part A, 31, 1274–1283. 10.1080/19440049.2014.925589 [DOI] [PubMed] [Google Scholar]
- Deruiter J, Jarp JM, Cutler HG and Davis RA, 1993. Studies on aldose reductase inhibitors from fungi: Moniliformin and small ring analogues. Journal of Enzyme Inhibition, 7, 249–256. [Google Scholar]
- Desjardins AE, Plattner RD and Nelson PE, 1997. Production of Fumonisin B1 and Moniliformin by Gibberella fujikuroi from Rice from Various Geographic Areas. Applied and Environmental Microbiology, 63, 1838–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Toro PCD, Arévalo FJ, Zon MA and Fernández H, 2015. Studies of the electrochemical behavior of moniliformin mycotoxin and its sensitive determination at pretreated glassy carbon electrodes in a non‐aqueous medium. Journal of Electroanalytical Chemistry, 738, 40–46. 10.1016/j.jelechem.2014.11.026 [DOI] [Google Scholar]
- Dorn B, Forrer HR, Schürch S and Vogelgsang S, 2009. Fusarium species complex on maize in Switzerland: Occurrence, prevalence, impact and mycotoxins in commercial hybrids under natural infection. European Journal of Plant Pathology, 125, 51–61. [Google Scholar]
- EBLEX (Division of the Agriculture and Horticulture Development Board, AHDB), 2005. Beef Action for Profit 3: Better Returns from Dairy‐Bred Bulls. EBLEX, Huntingdon. Available online: http://beefandlamb.ahdb.org.uk/wp-content/uploads/2016/01/BRP-Improving-cattle-handling-manualE-3-190116.pdf
- EBLEX (English Beef and Lamb Executive), 2008. Feeding growing and finishing cattle for better returns. In: Brown D, Rumans K and Vickers M (eds.). EBLEX Better Return Programme, Huntington, UK, 20 pp. [Google Scholar]
- EBLEX (English Beef and Lamb Executive), 2012. Experts view: Maize in beef systems. In: Marsh S (ed.). EBLEX Better Returns Programme, Huntington, UK, 3 pp. [Google Scholar]
- Edwards SG, 2007. Investigation of Fusarium mycotoxins in UK barley and oats production. Home Grown Cereals Authority, Project Report No. 415.
- Edwards SG, 2009. Fusarium mycotoxin content of UK organic and conventional oats. Food Additives and Contaminants, 26, 1063–1069. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2006. Guidance of the Scientific Committee on a request from EFSA related to uncertainties in Dietary Exposure Assessment. EFSA Journal 2006;4(5):438, 54 pp. 10.2903/j.efsa.2006.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011c. Report on the development of a food classification and description system for exposure assessment and guidance on its implementation and use. EFSA Journal 2011;9(12):2489, 84 pp. 10.2903/j.efsa.2011.2489 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. The food classification and description system FoodEx2 (revision 2). EFSA Supporting Publication 2015:EN‐804, 90 pp. 10.2903/sp.efsa.2015.en-804 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products and Substance used in Animal Feed), 2012. Guidance for the preparation of dossiers for technological additives. EFSA Journal 2012;10(1):2528, 23 pp. 10.2903/j.efsa.2012.2528 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017. Update: use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JA, Carlton WW and Tuite JF, 1989. Toxicity of Fusarium moniliforme var. subglutinans for chicks, ducklings, and turkey poults. Avian Diseases, 33, 357–360. [PubMed] [Google Scholar]
- Escriva L, Font G and Manyes L, 2015. In vivo toxicity studies of Fusarium mycotoxins in the last decade: a review. Food and Chemical Toxicology, 78, 185–206. 10.1016/j.fct.2015.02.005 [DOI] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 2004. Worldwide regulations for mycotoxins in food and feed in 2003. FAO Food and Nutrition Paper 81. Food and Agriculture Organization of the United Nations, Rome, Italy. Available online: http://www.fao.org/3/a-y5499e.pdf
- Ficheux AS, Sibiril Y, Le Garrec R and Parent‐Massin D, 2012. In vitro myelotoxicity assessment of the emerging mycotoxins Beauvericin, Enniatin b and Moniliformin on human haematopoietic progenitors. Toxicon, 59, 182–191. 10.1016/j.toxicon.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Ficheux AS, Sibiril Y and Parent‐Massin D, 2013. Effects of beauvericin, enniatin b and moniliformin on human dendritic cells and macrophages: An in vitro study. Toxicon, 71, 1–10. 10.1016/j.toxicon.2013.04.024 [DOI] [PubMed] [Google Scholar]
- Filek G and Lindner W, 1996. Determination of the mycotoxin moniliformin in cereals by high performance liquid chromatography and fluorescence detection. Journal of Chromatography A, 732, 291–298. [Google Scholar]
- Fotso J, Leslie JF and Smith S, 2002. Production of beauvericin, moniliformin, fusaproliferin, and fumonisins b(1), b(2), and b(3) by fifteen ex‐type strains of Fusarium species. Applied and Environment Microbiology, 68, 5195–5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck B and Breipohl G, 1984. Biosynthesis of moniliformin, a fungal toxin with cyclobutanedione structure. Angewandte Chemie (International ed. in English), 23, 996–998. 10.1002/anie.198409961 [DOI] [Google Scholar]
- Freayman S, Croubels S, Devreese M and Antonissen G, 2017. Emerging Fusarium and Alternaria mycotoxins: occurrence, toxicity and toxicokinetics. Toxins, 9, 228. 10.3390/toxins9070228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredlund E, Gidlund A, Sulyok M, Börjesson T, Krska R, Olsen M and Lindblad M, 2013. Deoxynivalenol and other selected Fusarium toxins in Swedish oats – occurrence and correlation to specific Fusarium species. International Journal of Food Microbiology, 167, 276–283. [DOI] [PubMed] [Google Scholar]
- FSA (Food Standards Agency), 2013. Mycotoxins in ethnic food. Food Survey information sheet 04/13, 50 pp.
- Gathercole PS, Thiel PG and Hofmeyr JHS, 1986. Inhibition of pyruvate dehydrogenase complex by moniliformin. Biochemical Journal, 233, 719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J, Startin JR, Parker I, Shepherd MJ, Mitchell JC and Perkins MJ, 1986. Derivatization of the Fusarium mycotoxin moniliformin for gas‐chromatography mass‐spectrometry analysis. Journal of Chromatography, 369, 408–414. 10.1016/S0021-9673(00)90148-1 [DOI] [Google Scholar]
- Goertz A, Zuehlke S, Spiteller M, Steiner U, Dehne HW, Waalwijk C, de Vries I and Oerke EC, 2010. Fusarium species and mycotoxin profiles on commercial maize hybrids in Germany. European Journal of Plant Pathology, 128, 101–111. [Google Scholar]
- Grabarkiewicz‐Szczesna Kostecki M, Golinski P and Kiecana I, 2001. Fusariotoxins in kernels of winter wheat cultivars field samples collected during 1993 in Poland. Nahrung/Food, 45, 28–30. [DOI] [PubMed] [Google Scholar]
- Grenier B and Oswald IP, 2011. Mycotoxin co‐contamination of food and feed: meta‐analysis of publications describing toxicological interactions. World Mycotoxin journal, 4, 285–313. [Google Scholar]
- Guillaume J, Kaushik S, Bergot P and Metailler R, 2001. Nutrition and feeding of fish and crustaceans. Praxis Publishing, UK. 408 pp. [Google Scholar]
- Hall JO, Javed T, Bennett GA, Richard JL, Dombrink‐Kurtzman MA, Cote LM and Buck WB, 1995. Serum vitamin A (retinol) reduction in broiler chicks on feed amended with Fusarium proliferatum culture material or fumonisin B1 and moniliformin. Journal of Veterinary Diagnostic Investigation, 7, 416–418. [DOI] [PubMed] [Google Scholar]
- Hallas‐Møller M, Nielsen KF and Frisvad JC, 2016. Production of the Fusarium Mycotoxin Moniliformin by Penicillium melanoconidium . Journal of Agricultural and Food Chemistry, 64, 4505–4510. [DOI] [PubMed] [Google Scholar]
- Harvey RB, Kubena LF, Rottinghaus GE, Turk JR, Casper HH and Buckley SA, 1997. Moniliformin from Fusarium fujikouroi culture material and deoxynivalenol from naturally contaminated wheat incorporated into diets of broiler chicks. Avian Diseases, 41, 957–963. [PubMed] [Google Scholar]
- Harvey RB, Edrington TS, Kubena LF, Rottinghaus GE, Turk JR, Genovese KJ and Nisbet DJ, 2001. Toxicity of moniliformin from Fusarium fujikuroi culture material to growing barrows. Journal of food protection, 64, 1780–1784. [DOI] [PubMed] [Google Scholar]
- Harvey RB, Edrington TS, Kubena LF, Rottinghaus GE, Turk JR, Genovese KJ, Ziprin RL and Nisbet DJ, 2002. Toxicity of fumonisin from Fusarium verticillioides culture material and moniliformin from Fusarium fujikuroi culture material when fed singly and in combination to growing barrows. Journal of Food Protection, 65, 373–377. [DOI] [PubMed] [Google Scholar]
- Herebian D, Zhülke S, Lamshöft M and Spiteller M, 2009. Multi‐mycotoxin analysis in complex biological matrices using LC‐ESI/MS: Experimental study using triple stage quadrupole and LTQ‐Orbitrap. Journal of Separation Science, 32, 939–948. 10.1002/jssc.200800589 [DOI] [PubMed] [Google Scholar]
- Huybrechts I, Sioen I, Boon PE, Ruprich J, Lafay L, Turrini A, Amiano P, Hirvonen T, De Neve M, Arcella D, Moschandreas J, Westerlund A, Ribas‐Barba L, Hilbig A, Papoutsou S, Christensen T, Oltarzewski M, Virtanen S, Rehurkova I, Azpiri M, Sette S, Kersting M, Walkiewicz A, Serra‐Majem L, Volatier J‐L, Trolle E, Tornaritis M, Busk L, Kafatos A, Fabiansson S, De Henauw S and Van Klaveren JD, 2011. Dietary exposure assessments for children in Europe (the EXPOCHI project): rationale, methods and design. Archives of Public Health, 69, 1169–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova L, Sahlstrom S, Rud I, Uhlig S, Feaste CK, Eriksen GS and Divon HH, 2017. Effect of primary processing on the distribution of free and modified Fusarium mycotoxins in naturally contaminated oats. World Mycotoxin Journal, 10, 73–88. [Google Scholar]
- Jansen C and Dose K, 1984. Quantitative determination of moniliformin in vegetable foods and feeds. Fresenius’ Zeitschrift für Analytische Chemie, 319, 60–62. [Google Scholar]
- Javed T, Bennett GA, Richard JL, Dombrink‐Kurtzman MA, Cote LM and Buck WB, 1993. Mortality in broiler chicks on feed amended with Fusarium proliferatum culture material or with purified fumonisin B1 and moniliformin. Mycopathologia, 123, 171–183. [DOI] [PubMed] [Google Scholar]
- Javed T, Dombrink‐Kurtzman MA, Richard JL, Bennett GA, Cote LM and Buck WB, 1995. Serohematologic alterations in broiler chicks on feed amended with Fusarium proliferatum culture material or fumonisin B1 and moniliformin. Journal of Veterinary Diagnostic Investigation, 7, 520–526. [DOI] [PubMed] [Google Scholar]
- Javed T, Bunte RM, Dombrink‐Kurtzman MA, Richard JL, Bennett GA, Cote LM and Buck WB, 2005. Comparative pathologic changes in broiler chicks on feed amended with Fusarium proliferatum culture material or purified fumonisin B1 and moniliformin. Mycopathologia, 159, 553–564. 10.1007/s11046-005-4518-9 [DOI] [PubMed] [Google Scholar]
- Jestoi MN, 2008. Emerging Fusarium ‐Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin: A Review. Critical Reviews in Food Science and Nutrition, 48, 21–49. 10.1080/10408390601062021 [DOI] [PubMed] [Google Scholar]
- Jestoi MN, Rokka M, Rizzo A, Peltonen K, Parikka P and Yli‐Matilla T, 2003. Moniliformin in Finnish grains: analysis with LC‐MS/MS. Aspects of Applied Biology, 68, 2011–2016. [Google Scholar]
- Jestoi MN, Rokka M, Yli‐Mattila T, Parikka P, Rizzo A and Peltonen K, 2004. Presence and concentrations of Fusarium‐related mycotoxins beauvericin, enniatins and moniliformin in Finnish grain samples. Food Additives and Contaminants, 21, 794–802. 10.1080/02652030410001713906 [DOI] [PubMed] [Google Scholar]
- Jin PG, Han Z, Cai ZX, Wu YJ and Ren YP, 2010. Simultaneous determination of 10 mycotoxins in grain by ultra‐high‐performance liquid chromatography–tandem mass spectrometry using 13C15‐deoxynivalenol as internal standard. Food Additives and Contaminants: Part A, 27, 1701–1713. 10.1080/19440049.2010.517222 [DOI] [PubMed] [Google Scholar]
- Jonsson M, Jestoi MN, Nathanail AV, Kokkonen UM, Anttila M, Koivisto P, Karhunen P and Peltonen K, 2013. Application of OECD Guideline 423 in assessing the acute oral toxicity of moniliformin. Food and Chemical Toxicology, 53, 27–32. [DOI] [PubMed] [Google Scholar]
- Jonsson M, Atosuo J, Jestoi MN, Nathanail AV, Kokkonen UM, Anttila M, Koivisto P, Lilius EM and Peltonen K, 2015. Repeated dose 28‐day oral toxicity study of moniliformin in rats. Toxicology Letters, 233, 38–44. [DOI] [PubMed] [Google Scholar]
- Kamimura H, Motohiro N, Kazuo Y Kazuo S, Akihiro I, Toshihiro N, Hirofumi U and Yasuta N, 1981. Simultaneous detection of several Fusarium mycotoxins in cereals, grains, and foodstuffs. Journal of the Association of Official Analytical Chemists, 64, 1067–1073. [PubMed] [Google Scholar]
- Karolewski Z, Waskiewicz A, Irzykowska L, Bocianowsski J, Kostecki M, Golinski P, Knaflewski M and Weber Z, 2011. Fungi presence and their mycotoxin distribution in asparagus spears. Polish Journal of Environmental Studies, 20, 911–918. [Google Scholar]
- Knaflewski M, Golinski P, Kostecki M, Waskiewicz A and Weber Z, 2008. Mycotoxins and mycotoxin‐producing fungi occurring in asparagus spears. Proceedings XIth IS on Asparagus. Eds: J.H. Mulder et al. Acta Hort., 776, pp 183‐190.
- Knasmüller S, Bresgen N, Kassie F, Mersch‐Sundermann V, Gelderblom W, Zohrer E and Eckl PM, 1997. Genotoxic effects of three Fusarium mycotoxins, fumonisin B1, moniliformin and vomitoxin in bacteria and in primary cultures of rat hepatocytes. Mutation Research, 391, 39–48. [DOI] [PubMed] [Google Scholar]
- Kokkonen MK and Jestoi MN, 2009. A Multi‐compound LC‐MS/MS Method for the Screening of Mycotoxins in Grains. Food Analytical Methods, 2, 128–140. 10.1007/s12161-009-9078-z [DOI] [Google Scholar]
- Kostecki M, Grabarkiewicz‐Szczesna J and Golinski P, 1997. Simultaneous analysis of beauvericin and moniliformin in fungal cultures and in cereal grain samples. Mycotoxin Research, 13, 17–22. [DOI] [PubMed] [Google Scholar]
- Kriek NPJ, Marasas WFO, Steyn PS, vanRensburg SJ and Steyn M, 1977. Toxicity of a moniliformin‐producing strain of fusarium moniliforme var. subglutinans isolated from maize. Food and Cosmetics Toxicology, 15, 579–587. https://doi.org/0.1016/0015-6264(77)90073-6 [DOI] [PubMed] [Google Scholar]
- Krska R, Welzig E and Boudra H, 2007. Analysis of Fusarium toxins in feed. Animal Feed Science and Technology, 137, 241–264. 10.1016/j.anifeedsci.2007.06.004 [DOI] [Google Scholar]
- Krysinska‐Traczyk E, Kiecana I, Perkowski J and Dutkiewicz J, 2001. Levels of fungi and mycotoxins in samples of grain and grain dust collected on farms in eastern Poland. Annals of Agricultural and Environmental Medicine, 8, 269–274. [PubMed] [Google Scholar]
- Kubena LF, Harvey RB, Buckley SA, Edrington TS and Rottinghaus GE, 1997. Individual and combined effects of moniliformin present in Fusarium fujikuroi culture material and aflatoxin in broiler chicks. Poultry Science, 76, 265–270. [DOI] [PubMed] [Google Scholar]
- Kubena LF, Harvey RB, Buckley SA, Bailey RH and Rottinghaus GE, 1999. Effects of long‐term feeding of diets containing moniliformin, supplied by Fusarium fujikuroi culture material, and fumonisin, supplied by Fusarium moniliforme culture material, to laying hens. Poultry Science, 78, 1499–1505. [DOI] [PubMed] [Google Scholar]
- Labuda R, Parich A, Vekiru E and Tancinova D, 2005. Incidence of fumonisins, moniliformin and Fusarium species in poultry feed mixtures from Slovakia. Annals of Agricultural and Environmental Medicine, 12, 81–86. [PubMed] [Google Scholar]
- Lamprecht SC, Marasas WFO, Thiel PG, Schneider DJ and Knox‐Davies PS, 1986. Incidence and toxigeniticy of seedborne Fusarium species from annual Medicago species in South Africa. Phytopathology, 76, 1040–1042. [Google Scholar]
- Lauren DR and Agnew MP, 1991. Multitoxin Screening Method for Fusarium Mycotoxins in Grains. Journal of Agriculture and Food Chemistry, 39, 502–507. [Google Scholar]
- Leatherhead Food Research Association , 2010. Guide to Contaminants in Foodstuffs. An international overview of maximum limits. Leatherhead Food International Limited, Leatherhead, United Kingdom. ISBN 987‐907895‐09‐8. [Google Scholar]
- Lebas F and Renouf B, 2009. Raw materials utilization and feeding techniques: new contributions in the 9th World Rabbit Congress. Journée d’étude ASFC, 5 February 2009, Vérone ‐ Ombres & Lumières, 30‐36
- Ledoux DR, Bermudez AJ, Rottinghaus GE and Broomhead J, 1995. Effects of feeding Fusarium fujikuroi culture material containing known levels of moniliformin in young broiler chicks. Poultry Science, 74, 297–305. [DOI] [PubMed] [Google Scholar]
- Ledoux DR, Broomhead JN, Bermudez AJ and Rottinghaus GE, 2003. Individual and combined effects of the Fusarium Mycotoxins fumonisin B‐1 and moniliformin in broiler chicks. Avian Diseases, 47, 1368–1375. [DOI] [PubMed] [Google Scholar]
- Leeson S and Summers JD, 2008. Commercial Poultry Nutrition, 3rd Edition. Nottingham University Press, Guelph, Ontario, Canada. 412 pp. [Google Scholar]
- Leslie JF, Marasas WFO, Shephard GS, Sydenham EW, Stockenholm S and Thiel PG, 1996. Duckling toxicity and the production of fumonisin and moniliformin by isolates in the A and F mating populations of Gibberella fujikuroi (Fusarium moniliforme). Applied and Environmental Microbiology, 62, 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ledoux D, Bermudez A, Fritsche K and Rottinhaus G, 2000a. Effects of moniliformin on performance and immune function of broiler chicks. Poultry Science, 79, 26–32. [DOI] [PubMed] [Google Scholar]
- Li Y, Ledoux D, Bermudez A, Fritsche K and Rottinhaus G, 2000b. The individual and combined effects of fumonisins and moniliformin on performance and selected immune parameters in turkey poults. Poultry Science, 79, 871–878. [DOI] [PubMed] [Google Scholar]
- Lim CW, Lai KY, Yeo JF, Tai SH and Chan SH, 2015. Quantitative assessment of moniliformin in cereals via alternative precipitation pathways, aided by LC‐LIT‐MS and LC‐Q‐TOF‐MS. Food Chemistry, 174, 372–379. [DOI] [PubMed] [Google Scholar]
- Lindblad M, Gidlund A, Sulyok M, Börjesson T, Krska R, Olsen M and Fredlund E, 2013. Deoxynivalenol and other selected Fusarium toxins in Swedish wheat ‐ Occurrence and correlation to specific Fusarium species. International Journal of Food Microbiology, 167, 284–291. 10.1016/j.ijfoodmicro.2013.07.002 [DOI] [PubMed] [Google Scholar]
- Liu X, 1996. Studies on Moniliformin and the etiology of Keshan disease. Journal of Hygiene Research, 25, 151–156. [Google Scholar]
- Loscalzo J, 2014. Keshan disease, selenium deficiency, and the selenoproteome. New England Journal of Medicine, 370, 1756–1760. 10.1056/NEJMcibr1402199 [DOI] [PubMed] [Google Scholar]
- Magg T, Bohn M, Klein D, Merditaj V and Melchinger AE, 2003. Concentration of moniliformin produced by Fusarium species in grains of transgenic Bt maize hybrids compared to their isogenic counterparts and commercial varieties under European corn borer pressure. Plant Breeding, 122, 322–327. [Google Scholar]
- Malachova A, Sulyok M, Beltran E, Berthiller F and Krska R, 2014. Optimization and validation of a quantitative liquid chromatography‐tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all relevant mycotoxins in four model food matrices. Journal of Chromatography A, 1362, 145–156. 10.1016/j.chroma.2014.08.037 [DOI] [PubMed] [Google Scholar]
- Maragos CM, 2004. Detection of moniliformin in maize using capillary zone electrophoresis. Food Additives & Contaminants, 21, 803–810. 10.1080/02652030410001717812. [DOI] [PubMed] [Google Scholar]
- Marasas WFO, Kellerman TS, Pienaar JG and Naude TW, 1976. Leukoencephalomalacia: a mycotoxicosis of equidae caused by Fusarium moniliforme Sheldon. Onderstepoort Journal of Veterinary Research, 43, 113–122. [PubMed] [Google Scholar]
- Marin S, Ramos AJ, Cano‐Sancho G and Sanchis V, 2013. Mycotoxins: occurrence, toxicology and exposure assessment. Food and Chemical Toxicology, 60, 218–237. 10.1016/j.fct.2013.07.047 [DOI] [PubMed] [Google Scholar]
- McDonald P, Greenhalgh JFD, Morgan CA, Edwards R, Sinclair L and Wilkinson R, 2011. Animal Nutrition, 7th edition. Pearson, London, UK. 712 pp. [Google Scholar]
- Merten C, Ferrari P, Bakker M, Boss A, Hearty Á, Leclercq C, Lindtner O, Tlustos C, Verger P, Volatier JL and Arcella D, 2011. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food Additives and Contaminants: Part A, 28, 975–995. [DOI] [PubMed] [Google Scholar]
- Morgan MK, Bursian SJ, Rottinghaus GE, Bennett GA, Render JA and Aulerich RJ, 1998. Subacute and Reproductive Effects in Mink from Exposure to Fusarium fujikuroi Culture Material (M‐1214) Containing Known Concentrations of Moniliformin. Archives of Environmental Contamination and Toxicology, 35, 513–517. [DOI] [PubMed] [Google Scholar]
- Morris CM, Ledoux DR, Broomhead J, Bermudez A, Rottinghaus GE and Logan A, 1997. Effects of pelleting on the toxicity of moniliformin in ducklings. 86th Annual Meeting of Poultry Science Association, August 3‐6, 1997, Georgia, USA.
- Morris CM, Li YC, Ledoux DR, Bermudez AJ and Rottinghaus GE, 1999. The individual and combined effects of feeding moniliformin, supplied by Fusarium fujikuroi culture material, and deoxynivalenol in young turkey poults. Poultry Science, 78, 1110–1115. [DOI] [PubMed] [Google Scholar]
- Mrozek J, 1988. MNDO computations of the structure and properties of the moniliformin, phenylmoniliformin and methylmoniliformin. Acta Physica Polonica, A74, 247–254. [Google Scholar]
- Munimbazi C and Bullerman LB, 1998. High‐performance liquid chromatographic method for the determination of moniliformin in corn. Journal of AOAC International, 81, 999–1004. [PubMed] [Google Scholar]
- Nagaraj RY, Wu W and Vesonder RF, 1997. Electrocardiographic changes in broiler chickens with ascites syndrome induced by the feeding of Fusarium proliferatum culture producing moniliformin. 86th Annual Meeting of the Poultry Science Association, August 3‐6, 1997, Georgia, USA.
- Nazari F, Sulyok M, Kpbarfard F, Yazdanpanah H and Krska R, 2015. Evaluation of Emerging Fusarium mycotoxins beauvericin, Enniatins, Fusaproliferin and Moniliformin in Domestic Rice in Iran. Iranian Journal of Pharmaceutical Research, 14, 505–512. [PMC free article] [PubMed] [Google Scholar]
- Norred WP, Plattner RD, Vesonder RF, Bacon CW and Voss KA, 1992. Effects of slected secondary metabolites of Fusarium moniliformin on unscheduled synthesis of DNA by rat primary hepatocytes. Food and Chemical Toxicology, 30, 233–237. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council), 1982. Nutrient requirements of Mink and Foxes. 2nd Revised edition. The National Academies Press, Washington, USA. 72 pp. 10.17226/1114 [DOI] [Google Scholar]
- NRC (National Research Council), 2000. Nutrient Requirements of Beef Cattle: Seventh Revised Edition: Update 2000. The National Academies Press, Washington, USA, 248 pp. 10.17226/9791 [DOI] [Google Scholar]
- NRC (National Research Council), 2006. Nutrient Requirements of Dogs and Cats. The National Academies Press, Washington, DC, 424 pp. 10.17226/10668 [DOI] [Google Scholar]
- NRC (National Research Council), 2007a. Nutrient requirements of small ruminants: sheep, goats, cervids and new world camelids. The National Academies Press, Washington, DC, 384 pp. 10.17226/11654 [DOI] [Google Scholar]
- NRC (National Research Council), 2007b. Nutrient requirement of horses, 6th Revised Edition. The National Academies Press, Washington, DC, 360 pp. 10.17226/11653 [DOI] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2009. Guidance Document on Overview of residue chemistry study. Series on Pesticides No. 32, Paris, France, 93 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance Document on Residues in Livestock. Series on Pesticides No. 73. OECD, Paris, France, 77 pp.
- Oropeza‐Moe M, Wisloff H and Bernhoff A, 2015. Selenium deficiency associated porcine and human cardiomyopathies. Journal of Trace Elements in Medicine and Biology, 31, 148–156. 10.1016/j.jtemb.2014.09.011 [DOI] [PubMed] [Google Scholar]
- Parich A, Boeira LS, Castro SP and Krska R, 2003. Determination of moniliformin using SAX column clean‐up and HPLC/DAD‐detection. Mycotoxin Research, 19, 203–206. 10.1007/BF02942966 [DOI] [PubMed] [Google Scholar]
- Peltonen K, Jestoi MN and Eriksen GS, 2010. Health effects of moniliformin: a poorly understood Fusarium mycotoxin. World Mycotoxin Journal, 3, 403–414. [Google Scholar]
- Pineda‐Valdes G and Bullerman LB, 2000. Thermal Stability of Moniliformin at Varying Temperature, pH, and Time in an Aqueous Environment. Journal of Food Protection, 63, 1598–1601. [DOI] [PubMed] [Google Scholar]
- Pineda‐Valdes G, Ryu D, Jackson DS and Bullerman LB, 2002. Reduction of moniliformin during alkaline cooking of corn. Cereal Chemistry, 79, 779–782. [Google Scholar]
- Pineda‐Valdes G, Ryu D, Hanna MA and Bullerman LB, 2003. Reduction of moniliformin in corn by heat processing. Journal of Food Science, 68, 1031–1035. [Google Scholar]
- Pirrung MC and Nauhaus SK, 1996. Cofactor‐directed, time‐dependent inhibition of thiamine enzymes by the fungal toxin moniliformin. Journal of Organic Chemistry, 61, 2592–2593. [DOI] [PubMed] [Google Scholar]
- Qiao H, Ruiping S, Qizhi L, Xiumei L and Yixin M, 1993. Effects of moniliformin on cardiac function in miniature pigs. Chines Journal of Veterinary Medicine, 19, 3–6. [Google Scholar]
- Quillardet P, Huisman O, D'ari R and Hofnung M, 1982. SOS chromotest, a direct assay of induction of an SOS function in Escherichia coli K‐12 to measure genotoxicity. Proceedings of the National Academy of Sciences, 79, 5971–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer T, Malone B and Brinker T, 1997. A rapid thin layer chromatographic method for moniliformin. Proceedings of the Fifth European Fusarium Seminar, September 1‐5, 1997, Szeged, Hungary, pp. 385–388.
- Scarpino V, Blandino M, Negre M, Reyneri A and Vanara F, 2013. Moniliformin analysis in maize samples from North‐West Italy using multifunctional clean‐up columns and the LS‐MS/MS detection method. Food Additives and Contaminants Part A, 30, 876–884. 10.1080/19440049.2013.793825 [DOI] [PubMed] [Google Scholar]
- Scharf HD, Frauenrath H and Pinske W, 1978. Synthese und Eigenschaften der Semiquadratsaure und ihrer Alkalisalze (Moniliformin). European Journal of Inorganic Chemistry, 111(1), 168–182. 10.1002/cber.19781110115 [DOI] [Google Scholar]
- Schultz S, Vallant B and Kainz MJ, 2012. Preferential feeding on high quality diets decreases methyl mercury of farm‐raised common carp (Cyprinus carpio L.). Aquaculture, 29, 338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütt F, Nirenberg HI and Deml G, 1998. Moniliformin production in the genus Fusarium . Mycotoxin Research, 14, 35–40. 10.1007/BF02945091 [DOI] [PubMed] [Google Scholar]
- Scott PM and Lawrence J, 1987. Liquid chromatography determination and stability of the Fusarium mycotoxin moniliformin in cereal grains. Journal of the Association of Official Analytical Chemists, 70, 850–853. [PubMed] [Google Scholar]
- Scudamore KA and Livesey CT, 1998. Occurrence and significance of mycotoxins in forage crops and silage: A review. Journal of the Science of Food and Agriculture, 77, 1–17. [Google Scholar]
- Scudamore KA and Patel S, 2009. Fusarium mycotoxins in milling streams from the commercial milling of maize imported to the UK, and the relevance to current legislation. Foos Additives and Contaminants: Part A, 26, 744–753. 10.1080/02652030802688394 [DOI] [PubMed] [Google Scholar]
- Scudamore KA, Nawaz S and Hetmanski MT, 1998. Mycotoxins in ingredients of animal feeding stuffs: II. determination of mycotoxins in maize and maize products. Food Additives & Contaminants, 15, 30–55. [DOI] [PubMed] [Google Scholar]
- Sewram V, Nieuwoudt TW, Marasas WFO, Shephard GS and Ritieni A, 1999. Determination of the mycotoxin moniliformin in cultures of Fusarium subglutinans and in naturally contaminated maize by high‐performance liquid chromatography‐atmospheric pressure chemical ionization mass spectrometry. Journal of Chromatography A, 848, 185–191. [DOI] [PubMed] [Google Scholar]
- Sharma D, Asrani RK, Ledoux DR, Jindal N, Rottinghaus GE and Gupta VK, 2008. Individual and combined effects of fumonisin B1 and moniliformin on clinicopathological and cell‐mediated immune response in Japanese quail. Poultry Science, 87, 1039–1051. 10.3382/ps.2007-00204 [DOI] [PubMed] [Google Scholar]
- Sharma D, Asrani RK, Ledoux DR, Rottinghaus GE and Gupta VK, 2012. Toxic interaction between fumonisin B1 and moniliformin for cardiac lesions in Japanese quail. Avian Diseases, 56, 545–554. [DOI] [PubMed] [Google Scholar]
- Shepherd MJ and Gilbert J, 1986. Method for the analysis in maize of the Fusarium mycotoxin Moniliformin employing ion‐pairing extraction and high‐performance liquid chromatography. Journal of Chromatography, 358, 415–422. 10.1016/S0021-9673(01)90355-3 [DOI] [PubMed] [Google Scholar]
- Sorensen JL, Nielsen KF and Ulf T, 2007. Analysis of moniliformin in maize plants using hydrophilic interaction chromatography. Journal of Agriculture and Food Chemistry, 55, 9764–9768. [DOI] [PubMed] [Google Scholar]
- Springer JP, Clardy J, Cole RJ, Kirksey JW, Hill RK, Carlson RM and Isidor JL, 1974. Structure and synthesis of moniliformin, a novel cyclobutane microbial toxin. Journal of the American Chemical Society, 96, 2267–2268. [DOI] [PubMed] [Google Scholar]
- Stępień L, Koczyk G and Waskiewicz A, 2013. Diversity of Fusarium species and mycotoxins contaminating pineapple. Journal of Applied Genetics, 54, 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm IM, Kristensen NB, Raun BM, Smedsgaard J and Thrane U, 2010. Dynamics in the microbiology of maize silage during whole‐season storage. Journal of Applied Microbiology, 109, 1017–1026. 10.1111/j.1365-2672.2010.04729.x [DOI] [PubMed] [Google Scholar]
- Streit E, Naehrer K, Rodrigues I and Schatzmayr G, 2012. Current situation of mycotoxin contamination and co‐occurrence in animal feed‐focus on Europe. Journal of the Science of Food and Agriculture, 93, 2892–2899. [DOI] [PubMed] [Google Scholar]
- Sulyok M, Berthiller F, Krska R and Schuhmacher R, 2006. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Communications in Mass Spectrometry, 20, 2649–2659. 10.1002/rcm.2640 [DOI] [PubMed] [Google Scholar]
- Sydenham EW, Thiel PG and Vleggelaar R, 1996. Physicochemical Data for Some Selected Fusarium Toxins. Journal of AOAC International, 79, 1365–1379. [PubMed] [Google Scholar]
- Takahashi H, Osada K, Yazaki H and Kimura S, 1992. Detection of Mutagenic Activity of Mycotoxins by Salmonella typhimurium/microsome Assay and ultra‐weak Chemiluminescence. Journal of the Japanese Society of Nutrition and Food Science, 45, 169–173. [Google Scholar]
- Thiel PG, 1978. A molecular mechanism for the toxic action of moniliformin, a mycotoxin produced by Fusarium moniliforme . Biochemical Pharmacology, 27, 483–486. [DOI] [PubMed] [Google Scholar]
- Thiel PG, 1990. Determination of moniliformin by High‐performance Liquid Chromatography. Presented at the 4th International IUPAC Symposium on Mycotoxins and Phycotoxins. Lausanne, Switzerland. August 1979. Journal of Environmental Pathology, Toxicology and Oncology, 10, 162–165. [PubMed] [Google Scholar]
- Tittlemier SA, Roscoe M, Trelka R Patrick SK, Bamforth JM, Gräfenhan T, Schlichting L and Fu BX, 2014. Fate of moniliformin during milling of Canadian durum wheat, processing, and cooking of spaghetti. Canadian Journal of Plant Science, 94, 555–563. [Google Scholar]
- Tuan NA, Manning BB, Lovell RT and Rottinghaus GE, 2003. Responses of Nile tilapia fed (Oreochromis niloticus) fed diets containing different concentrations of moniliformin or fumonisin B1. Aquaculture, 217, 515–528. [Google Scholar]
- Ueno Y, 1973. Akakabi Toxins (Fusarium Toxins) Part 2. Food Hygiene and Safety Science, 14, 501–510. [Google Scholar]
- Ueno Y and Kubota K, 1976. DNA‐attaching ability of carcinogenic mycotoxins in recombination‐deficient mutant cells of Bacillus subtilis . Cancer Research, 36, 445–451. [PubMed] [Google Scholar]
- Uhlig S, Torp M, Jarp J, Parich A, Gutleb AC and Krska R, 2004. Moniliformin in Norwegian grain. Food Additives and Contaminants, 21, 598–606. [DOI] [PubMed] [Google Scholar]
- Uhlig S, Eriksen GS, Hofgaard IS, Krska R, Beltran E and Sulyok M, 2013. Faces of a changing climate: semi‐quantitative multimycotoxin analysis of grain grown in exceptional climatic conditions in Norway. Toxins, 5, 1682–1697. 10.3390/toxins5101682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Asselt ED, Azambuja W, Moretti A, Kastelein P, De Rijk TC, Stratakou I and Van der Fels‐Klerx HJ, 2012. A Dutch field survey on fungal infection and mycotoxin concentrations in maize. Food Additives and Contaminants Part A, 29, 1556–1565. [DOI] [PubMed] [Google Scholar]
- Van der Fels‐Klerx HJ, De Rijk TC, Booy CJH, Goedhart PW, Boers EAM, Zhao C, Waalwijk C, Mol HGT and van der Lee TAJ, 2012. Occurrence of Fusarium head blight species and Fusarium mycotoxins in winter wheat. Food Additives and Contaminants Part A, 29, 1716–1726. 10.1080/19440049.2012.685891 [DOI] [PubMed] [Google Scholar]
- Verniest G, Colpeart J, Tornroos KW and De Kimpe N, 2005. New synthesis of semisquaric acid derivatives via chlorinated N‐(Cyclobutylidene)amines. Journal of Organic Chemistry, 70, 4549–4552. 10.1021/jo0502044 [DOI] [PubMed] [Google Scholar]
- Vesonder RF and Wu W, 1998. Correlation of moniliformin, but not fumonisin B1 levels, in culture materials of Fusarium isolates to acute death to ducklings. Poultry Science, 77, 67–72. [DOI] [PubMed] [Google Scholar]
- Visconti A, Minervini F, Lucivero G and Gambatesa V, 1991. Cytotoxic and immunotoxic effects of Fusarium mycotoxins using a rapid colorimetric bioassay. Mycopathologia, 113, 181–186. [DOI] [PubMed] [Google Scholar]
- VKM (Norwegian Scientific Committee for Food Safety), 2013. Risk assessment of mycotoxins in cereal grain in Norway. Opinion of the Scientific Steering Committee of the Norwegian Scientific Committee for Food Safety. VKM Report, 21, 287 pp. Available online: http://www.vkm.no/dav/eee04d10c4.pdf
- Von Bargen KW, Lohrey L, Cramer B and Humpf HU, 2012. Analysis of the Fusarium mycotoxin moniliformin in cereal samples using 13C2‐moniliformin and high‐resolution mass spectrometry. Journal of Agriculture and Food Chemistry, 60, 3586–3591. 10.1021/jf300323d [DOI] [PubMed] [Google Scholar]
- Waskiewicz A, Irzykowska L, Bocianowski J, Karolewski Z, Kostecki M, Weber Z and Golinski P, 2010. Occurrence of Fusarium fungi and mycotoxins in marketable asparagus spears. Polish Journal of Environmental Studies, 19, 219–225. [Google Scholar]
- Wei W‐k, Huang L‐h,, Wang K, Tang Z‐x, Wei X‐h, Zhou Z‐r and Luo S‐j, 2010. Effects of moniliformin on antibody of virus of classical swine fever in weaned piglets. Guangdong Agricultural Science, 9, Article No.: 1004‐874X (2010) 09‐0032‐02 [Google Scholar]
- Wehner FC, Marasas WFO and Thiel PG, 1978. Lack of Mutagenicity to Salmonella typhimurium of Some Fusarium Mycotoxins. Applied and Environmental Microbiology, 35, 659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2008. Harmonization Project Document No. 6. Guidance document on characterizing and communicating uncertainty in exposure assessment (Part 1), WHO/IPCS. 152 pp. Available online: http://www.who.int/ipcs/methods/harmonization/areas/uncertainty%20.pdf
- WHO (World Health Organization), 2009. Principles and Methods for the Risk Assessment of Chemicals in Food. Environment Health Criteria 240. Available online: http://www.who.int/foodsafety/publications/chemical-food/en/
- Wu W and Vesonder RF, 1997. Moniliformin but not fumonisin B1 induced cardiomegaly and elevated serum lactate in broilers. Poultry Science Association, 76(Supplement 1), S96. [Google Scholar]
- Xiong Y, Dongxu M, Sichun L, Xiong Z, Shiyuan Z, Zhilun W, Huayin B and Jiru X, 1998. Experimental study of effects of moniliformin (MON) and selenium on the articular cartilage of Chinese miniature pigs. Endemic Diseases Bulletin, 13, 1–4. [Google Scholar]
- Yildirim M, Manning BB, Lovell RT, Grizzle JM and Rottinghaus GE, 2000. Toxicity of moniliformin and fumonisin B1 fed singly and in combination in diets for young channel catfish Ictalurus punctatus . Journal of the World Aquaculture Society, 31, 599–608. [Google Scholar]
- Zhang H and Li JL, 1988. Mechanism of toxicity of moniliformin. JSM Mycotoxins, 1988(Suppl. 1), 109–110. [Google Scholar]
- Zhang H and Li JL, 1989. Moniliformin and its toxicity. Acta Microbiologica Sinica, 29, 93–100. [PubMed] [Google Scholar]
- Zhao D, Feng Q, Yan Z, Li C, Pan Y and Cui Q, 1993. Ultrastructural study of moniliformin induced lesions of myocardium in rats and mice. Biomedical and Environmental Sciences, 6, 37–44. [PubMed] [Google Scholar]
- Zhao X, Sheng Q, Zhaozian W, Yufen X, Guangrong C and Shaojun L, 1996. A study on the pathology of moniliformin poisoning in chickens. Acta Veterinaria et Zootechnica Sinica, 27, 87–93. [Google Scholar]
- Zoellner P and Mayer‐Helm B, 2006. Trace mycotoxin analysis in complex biological and food matrices by liquid chromatography‐atmospheric pressure ionisation mass spectrometry. Journal of Chromatography A, 1136, 123–169. 10.1016/j.chroma.2006.09.055 [DOI] [PubMed] [Google Scholar]
- Zollitsch W, Raffaseder C, Bohm J, Wagner E and Leitgeb R, 2003. Impact of the mycotoxins moniliformin and beauvericin on growth and carcass traits of broilers. Wiener Tierärztliche Monatsschrift, 90, 238–243. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting tables on food and feed occurrence and human exposure


