Abstract
In accordance with Article 6 of Regulation (EC) No 396/2005, the Agriculture and Horticulture Development Board (AHDB) submitted a request to the competent authority in the United Kingdom (evaluating Member State (EMS)) to modify the existing maximum residue level (MRL) for the active substance deltamethrin in kale. To accommodate for the intended use of deltamethrin, it was considered necessary to raise the existing MRL. Based on the evaluation report prepared by the EMS in accordance with Article 8 of Regulation (EC) No 396/2005, EFSA concludes that the applicant provided sufficient data to derive an MRL proposal of 0.15 mg/kg for the proposed uses in kale. Adequate analytical enforcement methods are available to control compliance with the proposed MRL for deltamethrin in kale. In a tentative risk assessment, no consumer concern has been identified; however, the risk assessment was affected by non‐standard uncertainties. Further risk management considerations are required to decide whether the MRL proposal is acceptable.
Keywords: deltamethrin, kale, MRL application, consumer risk assessment
Summary
In accordance with Article 6 of Regulation (EC) No 396/2005, the Agriculture and Horticulture Development Board (AHDB) submitted a request to the competent authority of the United Kingdom (evaluating member state (EMS)) to modify the existing maximum residue levels (MRLs) for the active substance deltamethrin in kale. To accommodate for the intended uses of deltamethrin in this crop, it was considered necessary to raise the existing MRL. The United Kingdom drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 15 December 2016.
EFSA based its assessment on the evaluation report submitted by the EMS, the draft assessment report (DAR) prepared under Council Directive 91/414/EEC, the Commission review report on deltamethrin as well as the conclusion from the previous EFSA reasoned opinions on deltamethrin including the review of the existing MRLs under Article 12 of Regulation (EC) No 396/2005.
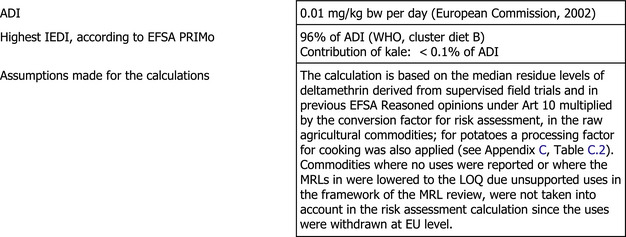
The toxicological profile of deltamethrin was evaluated in the framework of Directive 91/414/EEC and the data were sufficient to derive an acceptable daily intake (ADI) of 0.01 mg/kg body weight (bw) per day and acute reference dose (ARfD) of 0.01 mg/kg bw.
The metabolism of deltamethrin in primary crops belonging to the group of fruits and fruiting vegetables (apples and tomatoes), pulses and oilseeds (cotton seed) and cereals (maize) was investigated in the framework of Directive 91/414/EEC and the MRL review. The metabolism studies showed that the metabolic pathway is similar in all crop groups investigated. EFSA concluded that for the crops under consideration sufficient information on the metabolic behaviour in primary crops is available; the residue definitions derived in the framework of the MRL review are equally applicable for the use in kale.
Analytical methods for enforcement of the proposed residue definition are available; the available method is sufficiently validated to demonstrate that it is appropriate for MRL enforcement.
In support of the application, eight overdosed residue trials on kale have been submitted which were scaled down to reflect the envisaged good agricultural practice (GAP). The kale samples of the supervised field trials were analysed for the parent compound and are appropriate to derive an MRL proposal for kale. To estimate the residue concentration covering the full residue definition for risk assessment (sum of deltamethrin and its alpha‐R‐isomer and trans‐isomer), it is proposed to use the conversion factor derived in the framework of the MRL review for vegetables. This approach is acceptable, taking into account that the application to support the new use on kale was submitted to the EMS on 23 June 2015, thus, before the new residue definition was established. However, it would be desirable to verify the appropriateness of the conversion factor with residue trials where the samples are analysed for the full residue definition for risk assessment.
The possible transfer of deltamethrin residues in crops that can grow in a rotational crop scenario has been investigated and it was concluded that no residues of deltamethrin are expected according to the proposed GAP.
Due to the use of kale as possible feed item, the residues of deltamethrin in commodities of animal origin were assessed under this MRL application.
In order to estimate the dietary burden for livestock, EFSA performed the calculation according to old European methodology; in addition, the livestock exposure was calculated according to the current internationally agreed methodology. Considering that the European Union (EU) MRLs for animal products were derived from the existing Codex MRLs, the results of the EU dietary burden calculations were compared with the calculated dietary intake of livestock which was the basis of the Codex MRLs. Since the EU animal burden calculations are lower than the intake of livestock estimated at Codex level and since kale is not a main contributor, EFSA concludes that the intended use on kale will not trigger a revision of the current MRLs set for animal products.
The residue definitions for animal commodities have been previously derived during the MRL review only on tentative basis. Neither new metabolism data nor adequately feeding studies have been submitted under the current application.
The chronic exposure calculations performed in the framework of the MRL review under Article 12 of Regulation (EC) No 396/2005 has been updated, taking into account expected residues for the MRLs implemented in the EU Regulation and the expected residues in the crops for which MRL proposals were implemented in the EU Regulation after the MRL review. EFSA also performed an acute risk assessment for the commodities under consideration using the highest residue found in the residue trials multiplied by the conversion factor for risk assessment derived in the framework of the MRL review for vegetables. The result of the chronic exposure assessment was below the ADI with the highest international estimated daily intake (IEDI) being 96% of ADI (WHO cluster diet B). The contribution of kale to the total exposure was ca 0.1% (expressed as percentage of the ADI). The estimated short‐term intake for deltamethrin residues expected in kale accounted for 84.5% of the ARfD.
Thus, under the assumption that the isomers included in the risk assessment residue definition are equal or less toxic than the parent deltamethrin and that the amount of the metabolites is not more than 25% of the residues of deltamethrin (reflected by the conversion factor of 1.25), it is concluded that the long‐term and short‐term intake of residues of deltamethrin resulting from the envisaged uses in kale are unlikely to present a public health concern. Overall, the consumer risk assessment should be considered as tentative due to the non‐standard uncertainties identified in the current risk assessment.
The process for renewal of the approval of deltamethrin in accordance with Regulation (EC) No 1107/2009 is currently ongoing; thus, the conclusions derived in this reasoned opinion might be reconsidered in the light of the new data in the renewal process.
Based on the detailed assessment, EFSA derives the following summary table below.
| Codea | Commodity | Existing EU MRLb (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: deltamethrin | ||||
| 0243020 | Kale | 0.01* | Further risk management considerations required | EFSA derived a MRL proposal of 0.15 mg/kg based on the residue trials submitted in support of the NEU residue trials according to the intended use in kale. In a tentative risk assessment, no consumer concern has been identified; however, the risk assessment was affected by non‐standard uncertainties. Thus, further risk management considerations are required to decide whether the MRL proposal is acceptable |
MRL: maximum residue level; NEU: northern European Union.
* Indicates that the MRL is set at the limit of quantification.
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
MRL according to the Commission Regulation (EU) 2016/1822 amending the maximum residue levels for deltamethrin and other active substances in or on certain products.
The following data gaps identified in the framework of the MRL review that affect the current MRL application in kale and that were satisfactorily addressed with the information submitted under the current application are:
confirmatory method for monitoring of residues in high water content, high oil content, acidic and dry commodities;
an independent laboratory validation (ILV) for monitoring residues in dry commodities;
confirmatory method for monitoring residues in animal commodities.
The remaining data gaps identified in the MRL review that affect this MRL application for kale and for which data have not been provided are as follows:
metabolism of trans‐deltamethrin and alpha‐R‐deltamethrin in livestock;
adequate livestock feeding studies in cows and hens, investigating all relevant tissues and animal matrices according to the residue definitions for monitoring and risk assessment simultaneously.
Background
Commission Regulation (EC) No 396/20051 (hereafter referred to as ‘MRL regulation’) establishes the rules governing the setting of pesticide maximum residue levels (MRLs) at European Union (EU) level. Article 6 of this Regulation lays down that any party having a legitimate interest or requesting an authorisation for the use of a plant protection product in accordance with Council Directive 91/414/EEC2, repealed by Regulation (EC) No 1107/20093, shall submit to a Member State an application to modify a MRL in accordance with the provisions of Article 7 of the Regulation.
On 23 June 2015, the competent authority in the United Kingdom, hereafter referred to as the evaluating Member State (EMS), received an application from the Agriculture and Horticulture Development Board (AHDB) to modify the existing MRL for the active substance deltamethrin in kale. This application was notified to the European Commission and the European Food Safety Authority (EFSA) and was subsequently evaluated by the EMS in accordance with Article 8 of the Regulation. After completion, the evaluation report was submitted to the European Commission and to EFSA on 15 December 2016.
The application was included in the EFSA Register of Questions with the reference number EFSA‐Q‐2016‐00848 and the following subject:
Deltamethrin: MRL in kale
The EMS proposed to raise the MRL of deltamethrin in kale from the limit of quantification (LOQ) (0.01 mg/kg) to the level of 0.15 mg/kg.
EFSA assessed the application and the evaluation report as required by Article 10 of the MRL regulation. EFSA identified points for which further clarifications were requested from the EMS. On 7 August 2017, the EMS replied and submitted an updated evaluation report (United Kingdom, 2017) which replaced the previously submitted evaluation report. After additional clarifications were submitted on 13 November 2017, EFSA resumed the detailed assessment of the application.
Terms of Reference
In accordance with Article 10 of Regulation (EC) No 396/2005, EFSA shall assess the application and the evaluation report and give a reasoned opinion on the risks to the consumer and where relevant to animals associated with the setting of the requested MRLs. The opinion shall include:
An assessment of whether the analytical method for routine monitoring proposed in the application is appropriate for the intended control purposes;
The anticipated LOQ for the pesticide/product combination;
An assessment of the risks of the acceptable daily intake (ADI) and acute reference dose (ARfD) being exceeded as a result of the modification of the MRL;
The contribution to the intake due to the residues in the products for which the MRLs were requested;
Any other element relevant to the risk assessment.
In accordance with Article 11 of the Regulation, the reasoned opinion shall be provided as soon as possible, at the latest within 3 months from the date of receipt of the application.
The updated evaluation report (United Kingdom, 2017) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available.
The active substance and its use pattern
The intended use of a plant protection product containing the active substance deltamethrin on kale, which is the basis for the current MRL application, is reported in Appendix A.
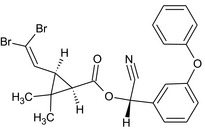
Deltamethrin is the ISO common name for (S)‐α‐cyano‐3‐phenoxybenzyl (1R, 3R)‐3‐(2,2‐dibromovinyl)‐2,2‐dimethylcyclopropanecarboxylate (IUPAC).
Deltamethrin is a non‐systemic insecticide belonging to the chemical class of pyrethroids. It prevents the transmission of nervous impulses thereby disrupting their nervous system. It is used to control many species of insects, in particular Lepidoptera, Coleoptera and Homoptera in a wide range of crops. Deltamethrin is also used topically for the control of ectoparasites in cattle and sheep.
Deltamethrin is considered as fat‐soluble (log Pow = 4.6). The chemical structure of the active substance and its main metabolites are reported in Appendix D.
Deltamethrin was evaluated in the framework of Directive 91/414/EEC with Sweden designated as rapporteur Member State (RMS). The representative uses supported for the peer review process were foliar applications as an insecticide on a large number of crops (including roots and tuber vegetables, fruits and fruiting vegetables, leafy vegetables and oilseeds) and post‐harvest uses on pulses, potatoes and cereals. Deltamethrin was included in Annex I of Directive 91/414/EEC by means of Commission Directive 2003/5/EC4, which entered into force on 1 November 2003. According to Regulation (EU) No 540/20115, deltamethrin is approved under Regulation (EC) No 1107/2009. This approval is restricted to uses as an insecticide only. As EFSA was not involved in the peer review of deltamethrin, an EFSA Conclusion on this active substance is not available. The process for renewing the approval for deltamethrin is currently ongoing.
The review of existing MRLs in the framework of Article 12 of Commission Regulation (EC) No 396/2005 has been finalised (EFSA, 2015); based on the MRLs proposed by EFSA, the legal limits were amended by Regulation 2016/18226. Following the MRL review, the MRL for deltamethrin in kale has been lowered from 0.5 mg/kg to the LOQ of 0.01 mg/kg since the authorised GAP (northern EU, 3 × 7.5 g/ha, preharvest interval (PHI) 7 days) was not fully supported by residue data and an acute consumer health risk could not be excluded. For most of the plant and animal commodities for which MRLs were proposed by EFSA certain information was missing on analytical methods for plant and animal origin commodities, insufficient residue trials, metabolism of deltamethrin in animals and unavailable robust feeding studies. A footnote was included in the EU Regulation to take into account the information previously missing if submitted by 18 October 2018.
EFSA recently published a reasoned opinion on the modification of existing MRLs for deltamethrin in celery, Florence fennel and rhubarb (EFSA, 2017); based on the MRLs proposed by EFSA, the legal limits were amended by Regulation 2017/1822.7
Deltamethrin is registered for use as a veterinary medicinal product in the EU. Within this scope, a residue definition (marker residue defined as deltamethrin only) and MRLs for animal products are already established in the framework of Regulation (EU) No 37/20108. These MRLs are lower than the residues arising from the use of deltamethrin as plant protection product.
Assessment
EFSA has based its assessment on the evaluation report submitted by the EMS (United Kingdom, 2017), the DAR and its addendum prepared under Directive 91/414/EEC (Sweden, 1998, 2002), the review report on deltamethrin (European Commission, 2002), JMPR evaluation reports (FAO, 2002a,b, 2016) as well as the conclusion from the EFSA previous reasoned opinions, including the review of the existing MRLs for deltamethrin according to Article 12 of Regulation (EC) No 396/2005 (EFSA, 2015, 2017).
For this application, the data requirements established in Regulation (EU) No 544/20119 and the guidance documents applicable at the time of the submission of the application to the EMS are applicable (European Commission, 1996, 1997a,b,c,d,e,f,g, 2000, 2010a, b, 2015; OECD, 2011, 2013). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/201110.
A selected list of end points of the studies assessed by EFSA in the framework of the MRL review11 relevant for the current application and including relevant information provided with the current application is presented in Appendix B.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of deltamethrin in primary crops belonging to the group of fruits and fruiting vegetables (apples and tomatoes), pulses and oilseeds (cotton seed) and cereals (maize) was investigated in the framework of the MRL review. The metabolism studies showed that the metabolic pathway is similar in all crop groups investigated (EFSA, 2015).
1.1.2. Nature of residues in rotational crops
Kale may be grown in crop rotation. The metabolism of deltamethrin in rotational crops (carrots, lettuce, and barley) has been previously evaluated. In the framework of the MRL review, EFSA concluded that the metabolism in rotational crops appears to be comparable to that in primary crops (EFSA, 2015).
1.1.3. Nature of residues in processed commodities
The effect of processing on the nature of deltamethrin has been investigated in the framework of Directive 91/414/EEC (Sweden, 2002) and in the framework of the MRL review (EFSA, 2015). No additional information is needed for the current MRL application.
1.1.4. Methods of analysis in plants
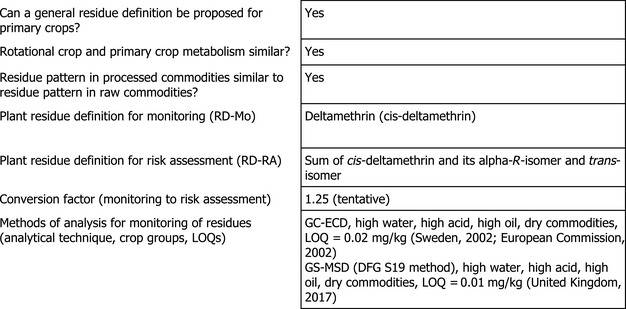
In previous assessments, EFSA assessed analytical methods to be used for enforcement purpose (EFSA, 2015). Methods for quantifying deltamethrin in plant matrices with high water content, high fat content, acidic and dry commodities using gas chromatography with electron capture detector (GC‐ECD) were provided; the LOQ for these matrices was 0.02 mg/kg. However, confirmatory data was requested in the framework of the MRL review for the following additional information related to the analytical methods for plant matrices:
confirmatory methods for monitoring of residues in high water content, high oil content, acidic and dry commodities;
an independent laboratory validation (ILV) for dry commodities;
a fully validated method of analysis for monitoring residues in complex matrices (EFSA, 2015).
Under the current application, information on the full validation of the DFG S19 method for the analysis of cis‐deltamethrin residues by gas chromatography with mass selective detection (GS‐MSD) was provided for high water content, high acid content, high fat content and dry matrices at the LOQ of 0.01 mg/kg. The method allows separating the isomers of deltamethrin (United Kingdom, 2017).
Therefore, the confirmatory data requested in the framework of the MRL review are partially addressed (first and second bullet point listed above fulfilled). A fully validated method of analysis for monitoring residues in complex matrices is still missing.
As kale belongs to the high water content commodities, EFSA concludes that analytical methods are available for monitoring of residues in the crops under this group.
1.1.5. Stability of residues in plants
Storage stability of deltamethrin was demonstrated at −20°C for a period of 24 months in high water content commodities (cabbage and tomatoes) (FAO, 2002a,b) and at −12°C for 30 months in high oil content commodities (cotton seed) and for 9 months in dry commodities (cereals grain) (Sweden, 1998). The available data were considered sufficient to conclude on the storage stability of deltamethrin in acidic matrices as well (EFSA, 2015).
1.1.6. Proposed residue definitions
EFSA concludes that for kale, sufficient information on the metabolic behaviour in primary crops is available; therefore, the residue definitions derived in the framework of the MRL review are applicable as deltamethrin for monitoring and sum of deltamethrin and its alpha‐R‐isomer and trans‐isomer for risk assessment. The risk assessment residue definition should be considered on tentative basis, pending the assessment of further toxicological data investigating the toxicological properties of the alpha‐R‐isomer and trans‐isomer of deltamethrin (EFSA, 2015). This data should be assessed in the framework of the renewal of the approval for deltamethrin.
Analytical methods for enforcement of the proposed residue definition are available; these methods demonstrate that they are appropriate for MRL enforcement in kale.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
In support of the application, eight overdosed residue trials in kale performed in northern Europe have been submitted. The trials were performed with three applications at different dose rates ranging from 7.25 to 8.81 g a.s./ha with a mean interval of 14 days between applications and a PHI of 21/22 days. The samples of these residue trials were analysed for parent deltamethrin only. Since residue data were not available according to the residue definition for risk assessment, the EMS suggested to use the tentative conversion factor of 1.25 derived in the framework of the MRL review for vegetables. In the given case, this approach might be acceptable, taking into account that the application to support the new use on kale was submitted to the EMS before the new residue definition including the deltamethrin isomers was established. It would be desirable to receive results on the concentration of the two metabolites included in the risk assessment residue definition to verify that the tentative conversion factor is appropriate for the use in kale.
It is acceptable to scale down the residues detected in the submitted overdosed residue trials by using scaling factors calculated for the individual trials which ranged from 0.73 to 0.77. More detailed information on the residue trials is provided in Appendix B.1.2.1.
The storage period of the samples was within the period for which integrity of the samples has been demonstrated. These trials are considered valid and sufficient to derive an MRL proposal for kale.
1.2.2. Magnitude of residues in rotational crops
The possible transfer of deltamethrin residues to crops that are grown in crop rotation has been assessed in previous assessments (EFSA, 2010, 2015, 2017). The available studies demonstrated that no significant residues (residues below 0.01 mg/kg) are expected in succeeding crops (spinach, carrots and radishes) planted in soil treated at 0.12 kg a.s./ha. Since the maximum annual application rate for kale (3 × 6 g a.s./ha) is lower than the use pattern tested in the rotational crop study, a non‐residue situation is expected in rotational crops provided that the active substance is applied according to the proposed GAP.
1.2.3. Magnitude of residues in processed commodities
For kale, the main processing procedure will be boiling. No specific processing studies for kale are available. Processing studies in pulses and potatoes investigated the impact of boiling on the terminal deltamethrin residues. These studies showed that cooking leads to a reduction of residues (EFSA, 2015). A certain reduction of deltamethrin may be also expected in boiled kale. However, considering that not only the morphology of kale differs in terms of texture and surface/weight ratio compared to pulses and potatoes, but also the boiling duration that would be significantly shorter than in pulses and potatoes, the results of these studies cannot be extrapolated to kale (EFSA, 2017; United Kingdom, 2017).
Considering that the contribution of residues in kale to the dietary intake is low, studies investigating the magnitude of residues in processing commodities would be desirable but are currently not indispensable for a refined dietary risk assessment.
1.2.4. Proposed MRLs
Based on the available data, a MRL of 0.15 mg/kg has been derived for the use of deltamethrin in kale using the OECD calculator. In Section 3, EFSA assessed whether deltamethrin residues in kale resulting from the intended uses are likely to pose a consumer health risk.
2. Residues in livestock
As kale can be used to feed livestock, the nature and magnitude of deltamethrin residues in food commodities from animal origin need to be assessed in the framework of this application (European Commission, 1996).
2.1. Dietary burden of livestock
In the framework of the MRL review, the dietary burden for livestock was calculated using the European methodology applicable at the time of the evaluation (European Commission, 1996), taking into account the uses for which GAPs were notified. The maximum dietary burden for dairy and beef cattle was 2.8 and 2.6 mg/kg dry matter (DM), for poultry 1.8 mg/kg DM and for pig 2.2 mg/kg DM (EFSA, 2015).
JMPR assessed animal products in 2002 (FAO, 2002a) where the dietary burden of deltamethrin residues for farm animals was calculated in accordance with the approach described in the FAO Manual (FAO, 2002b). The maximum dietary burden for beef and dairy cattle were 7 mg/kg DM and 6.3 mg/kg DM. For poultry, the maximum dietary burden accounted for 2.65 mg/kg. The EU MRLs for animal products were derived from the existing Codex MRLs (CXL); although they were not fully supported by data, they have been taken over in the EU MRL legislation since they did not pose a risk to consumers (EFSA, 2015).
In the framework of the current application, two calculations were performed considering the residue values derived by EFSA in the framework of the MRL review for the crops corresponding to the MRLs implemented in the EU regulation (EFSA, 2015) and the STMR/HR value from the supervised residue trials for kale according to the risk assessment residue definition.
The first dietary burden calculation was performed considering the old European methodology (European Commission, 1996). The maximum dietary burden for dairy and beef cattle was 2.5 mg/kg and 1.9 mg/kg DM, respectively, and 1.7 mg/kg DM for poultry and 1.9 mg/kg DM for pigs (Appendix B.2). Under this methodology, new MRLs for animal origin commodities are not necessary since the existing MRLs for animal products cover the current situation.
An additional exposure calculation was performed according to the new methodology currently in use (OECD, 2013). Using this methodology, the maximum dietary burden for ruminants is 7 mg/kg DM (ram/ewe), 2.69 mg/kg DM for poultry (layer) and 4.49 mg/kg DM for swine (breeding). The result of this dietary burden calculation is in the same range as the dietary burden calculated by JMPR. Kale was not a main contributor to the animal burden calculations. Comparing the estimates for the livestock exposure derived with the methodology currently used in the EU and the JMPR calculations for the dietary burden, and considering that the CXLs were taken over in the EU MRL legislation, EFSA concludes that the intended use on kale will not trigger a revision of the current MRLs set for animal products.
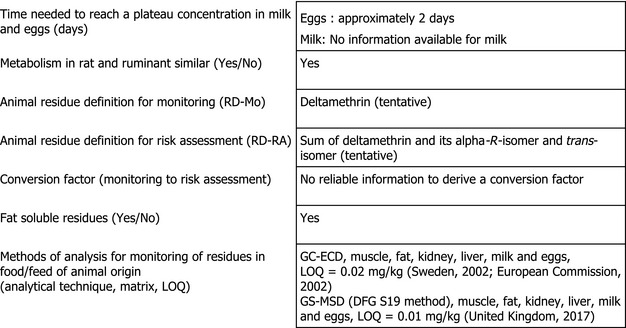
2.2. Proposed residue definitions in livestock
In the framework of the MRL review under Article 12 of Regulation (EC) No 396/2005, EFSA proposed on a tentative basis, to define the residue for enforcement as deltamethrin only; for risk assessment, the residue definition was proposed as the sum of deltamethrin and its trans‐isomer and alpha‐R‐isomer. As metabolic pathways are expected to be similar in ruminants and rodents, the results of the cow metabolism study could be extrapolated to pigs. The residue definitions were considered tentative due to some deficiencies of the animal metabolism data submitted. The following confirmatory data were identified to complete the data package (EFSA, 2015):
further information on the metabolism of trans‐deltamethrin and alpha‐R‐deltamethrin in livestock;
adequate livestock feeding studies in cows and hens, investigating all relevant tissues and matrices according to the residue definitions for monitoring and risk assessment simultaneously.
New metabolism data in livestock and feeding studies in cows and hens were not submitted in the framework of this application.
Thus, the open issues should be further addressed in the process for renewal of the approval of deltamethrin in accordance with Regulation (EC) No 1107/2009 that it is currently ongoing; thus, new information might trigger the need of a comprehensive assessment.
2.2.1. Methods of analysis in animals
In the framework of the MRL review, an analytical method using GC‐ECD for enforcing the MRLs for deltamethrin in food from animal origin was provided. The validation demonstrated that an LOQ of 0.02 mg/kg is achievable. Since the method was found to be not highly specific, EFSA identified the need to submit a confirmatory method (EFSA, 2015).
Under the current application, a full validation of the DFG S19 method for the analysis of cis‐deltamethrin residues by GS‐MSD was provided for muscle, fat, kidney, liver, milk and eggs, at the LOQ of 0.01 mg/kg. Furthermore, the method allows the separation of the three isomers of deltamethrin (United Kingdom, 2017). Therefore, the data requirement to provide a confirmatory method for animal matrices has been addressed.
3. Consumer risk assessment
The chronic exposure calculations performed in the framework of the MRL review under Article 12 of Regulation (EC) No 396/2005 (EFSA, 2015) was updated, taking into account the expected residues for kale and for all crops for which the MRL recommendations of EFSA were implemented in the EU Regulation. Since no information on the residues of the two additional metabolites included in the risk assessment residue definition was available, the risk assessment values (i.e. the supervised trials median residue and the highest residue), were multiplied by the tentative conversion factor of 1.25 derived in the framework of the MRL review for vegetables.
EFSA also performed an acute risk assessment for kale using the highest residue found in the residue trials multiplied by the tentative conversion factor.
Overall, the risk assessment is tentative because of the following elements:
use of conversion factor for risk assessment instead of information on the actual occurrence of residues of trans‐deltamethrin and alpha‐R‐deltamethrin;
lack of information on the toxicological profile of trans‐deltamethrin and alpha‐R‐deltamethrin;
lack of information on the metabolism of trans‐deltamethrin and alpha‐R‐deltamethrin in livestock;
adequate livestock feeding studies in cows and hens, investigating all relevant tissues and matrices according to the residue definitions for monitoring and risk assessment simultaneously.
The detailed input values for the chronic and acute risk assessment are listed in Appendix C.
The exposure calculations were performed using revision 2 of the EFSA PRIMo (EFSA, 2007). The Excel spreadsheet providing the risk assessment calculations are published together with this reasoned opinion.
The result of the chronic exposure assessment did not exceed the ADI; the highest international estimated daily intake (IEDI) being 96% of ADI (WHO cluster diet B). The contribution of kale to the total exposure was ca 0.1% (expressed as percentage of the ADI). The estimated short‐term intake for deltamethrin residues expected in kale accounted for 84.5% of the ARfD.
Under the assumptions that (1) the deltamethrin isomers included in the risk assessment residue definition are equal or less toxic than the parent deltamethrin and (2) that the amount of the metabolites is not more than 25% of the residues of deltamethrin (reflected by the conversion factor of 1.25), it is concluded that the long‐term and short‐term intake of residues of deltamethrin resulting from the envisaged uses in kale are unlikely to present a public health concern. Although, EFSA concluded that the new use on kale does not require a modification of the existing MRLs for animal products, the previously identified uncertainties as regards the lack of information on the metabolism of trans‐deltamethrin and alpha‐R‐deltamethrin and the deficiencies of the livestock feeding studies should be also born in mind.
Overall, the consumer risk assessment should be considered as tentative due to the non‐standard uncertainties identified in the current risk assessment.
Conclusions and recommendations
Based on the detailed assessment, EFSA derives the following summary table below.
| Codea | Commodity | Existing EU MRLb (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Deltamethrin | ||||
| 0243020 | Kale | 0.01* | Further risk management considerations required | EFSA derived a MRL proposal of 0.15 mg/kg based on the residue trials submitted in support of the NEU residue trials according to the intended use in kale. In a tentative risk assessment, no consumer concern has been identified; however, the risk assessment was affected by non‐standard uncertainties. Thus, further risk management considerations are required to decide whether the MRL proposal is acceptable |
MRL: maximum residue level; NEU: northern European Union.
* Indicates that the MRL is set at the limit of quantification.
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
MRL according to the Commission Regulation (EU) 2016/1822 amending the maximum residue levels for deltamethrin and other active substances in or on certain products.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- AHDB
Agriculture and Horticulture Development Board
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CF
conversion factor for enforcement to risk assessment residue definition
- CXL
Codex maximum residue limit
- DAR
draft assessment report
- DAT
days after treatment
- DM
dry matter
- EC
emulsion concentrate
- EMS
evaluating Member State
- GAP
Good Agricultural Practice
- GC‐ECD
gas chromatography with electron capture detector
- GC‐MSD
gas chromatography with mass selective detection
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- LOQ
limit of quantification
- Mo
monitoring
- MRL
maximum residue level
- MS
Member States
- NEU
northern European Union
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant‐back interval
- PF
processing factor
- PHI
preharvest interval
- Pow
n‐octanol/water partition coefficient
- PRIMo
(EFSA) Pesticide Residues Intake Model
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- WHO
World Health Organization
Appendix A – Summary of GAP triggering the amendment of existing EU MRLs
1.
| Crop | Region/MS | Outdoor/indoor | Member state or country | Pest controlled | Formulation | Application | PHIc or waiting period (days) | Comments (max. 250 characters) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common name | Typea | Content | Method | Growth stageb | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | |||||||||
| Kale | NEU | Outdoor | UK |
Caterpillars Flea beetle Aphids cutworms |
EC | 25.0 | g/L | Foliar treatment – spraying | 47 | 49 | – | 3 | – | 14 | – | 6 | g a.s./ha | 21 | – |
NEU: northern European Union; MS: Member State; EC: emulsion concentrate; GAP: good agricultural practice; MRL: maximum residue level; a.s.: active substance.
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI: minimum preharvest interval.
Appendix B – Selected list of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) |
|---|---|---|---|---|
| Fruit crops | Apples | Foliar, 1 × 60 g a.s./ha | 28 | |
| Tomatoes | Foliar, 2 × 50 g a.s./ha | 4, 14, 28 | ||
| Local, 14 μg/tomato | ||||
| Cereals | Maize | Foliar, 2 × 110 g a.s./ha | 0, 14, 42 | |
| Pulses/Oilseed | Cotton (I) | Local, 3–15 mg/kg leaf | 14. 42 | |
| Cotton (II) | Foliar, 0.009 mg/plant | 1, 3, 7 | ||
| Soil, 0.18 mg/plant | ||||
| Hydroponic, 6.7 mg/plant | ||||
| Cotton (III) | Foliar, 2 × 224 g a.s./ha | 4, 10, 28 | ||
|
Studies I and II on cotton cover the metabolism in leafy vegetables. Study on cotton (I) performed in open field and in glasshouse. Study on cotton (II) investigated translocation. Study on tomatoes performed in glasshouse. | ||||
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (days) |
|---|---|---|---|---|
| Root/tuber crops | Carrots (I) | Bare soil, 10 × 45 g a.s./ha | 30, 120 | |
| Carrots (II) | Bare soil, 1 × 118 g a.s./ha | 0 | ||
| Radishes | Bare soil, 1 × 118 g a.s./ha | 0 | ||
| Leafy crops | Lettuce | Bare soil, 10 × 45 g a.s./ha | 30, 120 | |
| Spinach | Bare soil, 1 × 118 g a.s./ha | 0 | ||
| Cereal (small grain) | Barley | Bare soil, 10 × 45 g a.s./ha | 30, 120 | |
|
In the study on carrots (II), radishes and spinach, the crops were cultivated immediately after soil treatment. Source: Sweden (1998). | ||||
| Processed commodities (hydrolysis study) | Conditions | Investigated? |
|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Yes | |
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | |
| Sterilisation (20 min, 120°C, pH 6) | Yes | |
|
Deltamethrin was found to be stable under standard hydrolytic conditions. | ||
PBI: plant‐back interval; DAT: days after treatment; a.s.: active substance.
B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability (months/years) |
|---|---|---|---|---|
| High water content |
Lettuce Cabbage Tomato |
−20 −20 −20 |
16 months 24 months 24 months |
|
| High oil content | Cotton seed | −12 | 30 months | |
| Dry/high starch | Cereals grain | −12 | 9 months | |
| High acid content | – | −20 | 24 months | |
|
Studies cover also the stability of the isomers included in the residue definition. Result from the storage stability study on tomatoes (borderline between high water and acidic commodity) are extrapolated to the acidic commodities. Source: EFSA (2015). | ||||
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Crop | Region/indoora | Residue levels observed in the supervised residue trials relevant to the supported GAPs (mg/kg) | Recommendations/comments (OECD calculations) | MRL proposals (mg/kg) | HR (mg/kg)b | STMR (mg/kg)c | CF |
|---|---|---|---|---|---|---|---|
| Kale | NEU |
RD‐Mo(overdosed trials): 4 × < 0.05, 2 × 0.06, 0.08, 0.14 RD‐Mo (scalded): 0.037, 2 × 0.039, 0.040, 0.044, 0.046, 0.060, 0.102 RD‐RA: no results reported |
MRLOECD = 0.15/0.15 Residues from overdosed residue trials were scaled down (individual scaling factors between 0.73 and 0.77) to match the intended GAP considering the proportionality principle |
0.15 | 0.13 | 0.05 | 1.25d |
GAP: good agricultural practice; MRL: maximum residue level; RD: residue definition; Mo: monitoring; RA: risk assessment; OECD: Organisation for Economic Co‐operation and Development.
NEU: Outdoor trials conducted in northern Europe; SEU: Outdoor trials conducted in southern Europe; Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue according to the residue definition for risk assessment.
Supervised trials median residue according to the residue definition for risk assessment.
Since no data were available for the residue definition for risk assessment, the tentative CF derived in the framework of the MRL review for vegetables was used (EFSA, 2015).
B.1.2.2. Residues in succeeding crops

B.1.2.3. Processing factors
| Processed commodity | Number of studiesa | Processing factor (PF) | CFP b | |
|---|---|---|---|---|
| Individual values | Median PF | |||
| Indicative processing factors relevant for the crops under consideration (limited data set and residues not analysed according to the proposed residue definitions) | ||||
| Potatoes, unpeeled and boiled | 4 | 0.22; 0.27; 0.19; 0.34 | 0.26 | 1.25 |
| Dry pulses, cooked | 1 | 0.10 | 0.10 | 1.25 |
| Source: EFSA (2015) | ||||
| No processing studies were submitted under the current application | ||||
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
Tentative conversion factor for risk assessment in the processed commodity is the same as derived from the raw commodities.
B.2. Residues in livestock
B.2.1. Result of dietary burden calculation
| Relevant groupsa | Dietary burden expressed in | Most critical commodity | Trigger exceeded (Y/N) | ||
|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | ||||
| Med. | Max. | Max. | |||
| Dairy ruminants | 0.073 | 0.089 | 2.47 | Rye Bran | Y |
| Meat ruminants | 0.081 | 0.090 | 1.89 | Oat grain | Y |
| Pigs | 0.075 | 0.081 | 1.88 | Oat grain | Y |
| Poultry | 0.089 | 0.107 | 1.71 | Oat grain | Y |
bw: body weight; DM: dry matter.
Results of the dietary burden calculation following the methodology of the EU guidance document (European Commission, 1996).
B.2.2. Nature of residues and methods of analysis in livestock
B.2.2.1. Metabolism studies, methods of analysis and residue definitions in livestock
| Livestock (available studies) | Animal | Dose (mg/kg bw per day) | Duration (days) | N rate/comment |
|---|---|---|---|---|
| Lactating cow | 10 | 3 | ca 125N | |
| Laying hens | 5 | 3 | ca 30N | |
|
Source: EFSA (2015). N rate updated according to the current MRL application. | ||||
bw: body weight.
B.2.2.2. Stability of residues in livestock
| Animal products (available studies) | Animal | Commodity | T (°C) | Stability (months/years) |
|---|---|---|---|---|
| Poultry | Muscle, liver, kidney, eggs | −12 | 11 months | |
| Cow | Milk | −20 | 7 months | |
| Source: EFSA (2015). | ||||
B.2.2.3. Magnitude of residues in livestock
No new feeding studies were submitted.
B.3. Consumer risk assessment
B.3.1. Consumer risk assessment without consideration of the existing CXLs

Appendix C – Input values for the exposure calculations
C.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input valuea (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Alfalfa (fresh and silage) | 0.11 | EFSA (2015) | 0.23 | EFSA (2015) |
| Clover (fresh and silage) | 0.11 | EFSA (2015) | 0.23 | EFSA (2015) |
| Grass (fresh and silage) | 0.05 | EFSA (2015) | 0.08 | EFSA (2015) |
| Cabbage | 0.03 | EFSA (2015) | 0.08 | EFSA (2015) |
| Kale | 0.05 |
STMR Current application |
0.13 |
HR Current application |
| Sugar beet leaves | 0.03 | EFSA (2015) | 0.03 | EFSA (2015) |
| Fodder beet leaves | 0.03 | EFSA (2015) | 0.05 | EFSA (2015) |
| Alfalfa hay | 0.43 | EFSA (2015) | 0.90 | EFSA (2015) |
| Clover hay | 0.43 | EFSA (2015) | 0.90 | EFSA (2015) |
| Grass hay | 0.20 | EFSA (2015) | 0.30 | EFSA (2015) |
| Citrus pomace | 0.03 | EFSA (2015) | 0.03 | EFSA (2015) |
| Apple pomace | 0.21 | EFSA (2015) | 0.21 | EFSA (2015) |
| Oat grain | 0.63 | EFSA (2015) | 1.79 | EFSA (2015) |
| Rye, maize, barley grain | 0.88 | EFSA (2015) | 1.38 | EFSA (2015) |
| Wheat grain | 0.56 | EFSA (2015) | 0.73 | EFSA (2015) |
| Wheat bran | 5.00 | EFSA (2015) | 5.00 | EFSA (2015) |
| Rye bran | 7.00 | EFSA (2015) | 7.00 | EFSA (2015) |
| Wheat and rye straw | 0.32 | EFSA (2015) | 0.51 | EFSA (2015) |
| Barley and oat straw | 0.23 | EFSA (2015) | 0.59 | EFSA (2015) |
| Pulses (bean, lupin, pea) | 0.25 | EFSA (2015) | 0.33 | EFSA (2015) |
| Potatoes | 0.09 | EFSA (2015) | 0.14 | EFSA (2015) |
| Turnips | 0.03 | EFSA (2015) | 0.03 | EFSA (2015) |
| Swedes | 0.03 | EFSA (2015) | 0.03 | EFSA (2015) |
| Rape seed meal | 0.13 | EFSA (2015) | 0.13 | EFSA (2015) |
| Cotton seed/cotton seed meal | 0.01 | EFSA (2015) | 0.01 | EFSA (2015) |
| Linseed meal | 0.03 | EFSA (2015) | 0.03 | EFSA (2015) |
| Sunflower seed meal | 0.13 | EFSA (2015) | 0.13 | EFSA (2015) |
All the details of the residue data and processing factors used for dietary exposure to livestock can be found in EFSA (2015). Input values have been updated considering expected residue values of the implemented MRLs.
C.2. Consumer risk assessment
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Citrus fruits | 0.01 | EFSA (2015) | ||
| Tree nuts | 0.03 | EFSA (2015) | ||
| Pome fruits | 0.04 | EFSA (2015) | ||
| Apricots | 0.04 | EFSA (2015) | ||
| Cherries | 0.05 | EFSA (2015) | ||
| Peaches | 0.04 | EFSA (2015) | ||
| Plums | 0.01 | EFSA (2015) | ||
| Table and wine grapes | 0.08 | EFSA (2015) | ||
| Strawberries | 0.03 | EFSA (2015) | ||
| Cane fruit | 0.03 | EFSA (2015) | ||
| Other small fruits and berries | 0.10 | EFSA (2015) | ||
| Table olives | 0.26 | EFSA (2015) | ||
| Kiwi | 0.03 | EFSA (2015) | ||
| Potatoes | 0.09 | EFSA (2015) | ||
| Other root and tuber vegetables | 0.03 | EFSA (2015) | ||
| Garlic | 0.03 | EFSA (2015) | ||
| Onions | 0.03 | EFSA (2015) | ||
| Shallots | 0.03 | EFSA (2015) | ||
| Spring onions | 0.07 | EFSA (2015) | ||
| Tomatoes | 0.03 | EFSA (2015) | ||
| Peppers | 0.04 | EFSA (2015) | ||
| Aubergines (egg plants) | 0.07 | EFSA (2015) | ||
| Cucurbits edible peel | 0.03 | EFSA (2015) | ||
| Cucurbits inedible peel | 0.03 | EFSA (2015) | ||
| Sweet corn | 0.03 | EFSA (2015) | ||
| Flowering brassica | 0.03 | EFSA (2015) | ||
| Head cabbage | 0.03 | EFSA (2015) | ||
| Chinese cabbage | 0.03 | EFSA (2015) | ||
| Kale | 0.05 | Current application | 0.13 | Current application |
| Lamb's lettuce | 0.43 | EFSA (2015) | ||
| Lettuce | 0.19 | EFSA (2015) | ||
| Scarole (broad‐leaf endive) | 0.04 | EFSA (2015) | ||
| Cress | 0.43 | EFSA (2015) | ||
| Land cress | 0.43 | EFSA (2015) | ||
| Rocket, Rucola | 0.43 | EFSA (2015) | ||
| Red mustard | 0.43 | EFSA (2015) | ||
| Leaves and sprouts of Brassica spp. | 0.16 | EFSA (2015) | ||
| Vine leaves | 0.16 | EFSA (2015) | ||
| Water cress | 0.16 | EFSA (2015) | ||
| Witloof | 0.03 | EFSA (2015) | ||
| Herbs | 0.43 | EFSA (2015) | ||
| Beans (fresh, with pods) | 0.01 | EFSA (2015) | ||
| Beans (fresh, without pods) | 0.01 | EFSA (2015) | ||
| Peas (fresh, with pods) | 0.01 | EFSA (2015) | ||
| Peas (fresh, without pods) | 0.01 | EFSA (2015) | ||
| Lentils (fresh) | 0.01 | EFSA (2015) | ||
| Celery | 0.08 | EFSA (2017) | ||
| Florence fennel | 0.08 | EFSA (2017) | ||
| Rhubarb | 0.08 | EFSA (2017) | ||
| Globe artichokes | 0.07 | EFSA (2015) | ||
| Leek | 0.07 | EFSA (2015) | ||
| Cultivated fungi | 0.03 | EFSA (2015) | ||
| Dry beans | 0.25 | EFSA (2015) | ||
| Pulses | 0.66 | EFSA (2015) | ||
| Linseed | 0.03 | EFSA (2015) | ||
| Poppy seed | 0.06 | EFSA (2015) | ||
| Sesame seed | 0.01 | EFSA (2015) | ||
| Sunflower seed | 0.06 | EFSA (2015) | ||
| Rape seed | 0.06 | EFSA (2015) | ||
| Mustard seed | 0.06 | EFSA (2015) | ||
| Cotton seed | 0.01 | EFSA (2015) | ||
| Pumpkin seeds | 0.01 | EFSA (2015) | ||
| Safflower | 0.01 | EFSA (2015) | ||
| Borage | 0.06 | EFSA (2015) | ||
| Gold of pleasure | 0.06 | EFSA (2015) | ||
| Hempseed | 0.06 | EFSA (2015) | ||
| Castor bean | 0.06 | EFSA (2015) | ||
| Olives for oil production | 0.26 | EFSA (2015) | ||
| Barley grain | 0.88 | EFSA (2015) | ||
| Buckwheat grain | 0.63 | EFSA (2015) | ||
| Maize grain | 0.88 | EFSA (2015) | ||
| Millet grain | 0.63 | EFSA (2015) | ||
| Oats grain | 0.63 | EFSA (2015) | ||
| Rice grain | 0.56 | EFSA (2015) | ||
| Rye grain | 0.88 | EFSA (2015) | ||
| Sorghum grain | 0.63 | EFSA (2015) | ||
| Wheat grain | 0.56 | EFSA (2015) | ||
| Tea | 2.75 | EFSA (2015) | ||
| Herbal infusions (dried, flowers) | 1.31 | EFSA (2015) | ||
| Herbal infusions (dried, leaves) | 1.31 | EFSA (2015) | ||
| Herbal infusions (dried, roots) | 0.09 | EFSA (2015) | ||
| Spices (seeds) | 0.13 | EU MRL × CF | ||
| Spices (fruits and berries) | 1.31 | EFSA (2015) | ||
| Spices (roots and rhizome) | 0.09 | EFSA (2015) | ||
| Spices (buds) | 1.31 | EFSA (2015) | ||
| Spices (flower stigma) | 1.31 | EFSA (2015) | ||
| Sugar beet (root) | 0.03 | EFSA (2015) | ||
| Chicory roots | 0.01 | EFSA (2015) | ||
| Swine meat | 0.06 | EFSA (2015) | ||
| Swine fat (free of lean meat) | 0.16 | EFSA (2015) | ||
| Swine liver | 0.03 | EFSA (2015) | ||
| Swine kidney | 0.03 | EFSA (2015) | ||
| Ruminant meat | 0.06 | EFSA (2015) | ||
| Ruminant fat | 0.16 | EFSA (2015) | ||
| Ruminant liver | 0.03 | EFSA (2015) | ||
| Ruminant kidney | 0.03 | EFSA (2015) | ||
| Poultry meat | 0.02 | EFSA (2015) | ||
| Poultry fat | 0.04 | EFSA (2015) | ||
| Poultry liver | 0.02 | EFSA (2015) | ||
| Ruminant milk | 0.02 | EFSA (2015) | ||
| Birds' eggs | 0.02 | EFSA (2015) | ||
STMR: supervised trials median residue; Mo: monitoring; CF: conversion factor.
All the details of the residue data used for consumer exposure calculations can be found in EFSA (2015).
Appendix D – Used compound/metabolite codes
1.
| Code/trivial name | Chemical name/SMILES notationa | Structural formulaa |
|---|---|---|
| Deltamethrin |
(S)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐(2,2‐dibromovinyl)‐2,2‐dimethylcyclopropanecarboxylate or (S)‐α‐cyano‐3‐phenoxybenzyl (1R)‐cis‐3‐(2,2‐dibromovinyl)‐2,2‐dimethylcyclopropanecarboxylate Br\C(Br)=C/[C@H]3[C@@H](C(=O)O[C@H](C#N)c2cccc(Oc1ccccc1)c2)C3(C)C |
|
| trans‐Isomer |
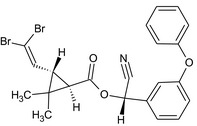
(S)‐cyano(3‐phenoxyphenyl)methyl (1R,3S)‐3‐(2,2‐dibromovinyl)‐2,2‐dimethylcyclopropanecarboxylate Br\C(Br)=C/[C@@H]3[C@@H](C(=O)O[C@H](C#N)c2cccc(Oc1ccccc1)c2)C3(C)C |
|
| alpha‐R‐Isomer |
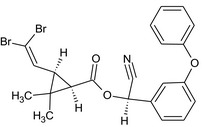
(R)‐cyano(3‐phenoxyphenyl)methyl (1R,3R)‐3‐(2,2‐dibromovinyl)‐2,2‐dimethylcyclopropanecarboxylate Br\C(Br)=C/[C@H]3[C@@H](C(=O)O[C@@H](C#N)c2cccc(Oc1ccccc1)c2)C3(C)C |
|
SMILES: simplified molecular‐input line‐entry system.
ACD/ChemSketch, Advanced Chemistry Development, Inc., ACD/Labs Release: 12.00 Product version: 12.00 (Build 29305, 25 Nov 2008).
Suggested citation: EFSA (European Food Safety Authority) , Brancato A, Brocca D, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Medina P, Miron I, Molnar T, Nougadere A, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Jose T, Anne T, Vagenende B, Verani A and Villamar‐Bouza L, 2018. Reasoned Opinion on the modification of the existing maximum residue level for deltamethrin in kale. EFSA Journal 2018;16(1):5153, 26 pp. 10.2903/j.efsa.2018.5153
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00848
Approved: 21 December 2017
Notes
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Directive 2003/5/EC of 10 January 2003 amending Council Directive 91/414/EEC to include deltamethrin as active substance. OJ L 8, 14.1.2003, p. 7–9.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Regulation (EU) 2016/1822 of 13 October 2016 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for aclonifen, deltamethrin, fluazinam, methomyl, sulcotrione and thiodicarb in or on certain products. OJ L 281, 18.10.2016, p. 1–44.
Commission Regulation (EU) 2017/1016 of 14 June 2017 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for benzovindiflupyr, chlorantraniliprole, deltamethrin, ethofumesate, haloxyfop, Mild Pepino Mosaic Virus isolate VC1, Mild Pepino Mosaic Virus isolate VX1, oxathiapiprolin, penthiopyrad, pyraclostrobin, spirotetramat, sunflower oil, tolclofos‐methyl and trinexapac in or on certain products. OJ L 159, 21.6.2017, p. 1–47.
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 15, 20.1.2010, p. 1–72.
Commission Regulation (EU) No 544/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the data requirements for active substances. OJ L 155, 11.6.2011, p. 1–66.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
The updated list of endpoints reflects the decisions on implementing MRL recommendations taken in Commission Regulation (EU) 2016/1822.
References
- EFSA (European Food Safety Authority), 2007. Reasoned opinion on the potential chronic and acute risk to consumers' health arising from proposed temporary EU MRLs according to Regulation (EC) No 396/2005 on Maximum Residue Levels of Pesticides in Food and Feed of Plant and Animal Origin. EFSA Journal 2007;5(3):32r, 104 pp. 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Reasoned opinion on the modification of the existing MRL(s) for deltamethrin in potatoes. EFSA Journal 2010;8(11):1900, 28 pp. 10.2903/j.efsa.2010.1900 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for deltamethrin according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2015;13(11):4309, 104 pp. 10.2903/j.efsa.2015.4309 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Reasoned opinion on the modification of the existing MRL(s) for deltamethrin in celery, Florence fennel and rhubarb. EFSA Journal 2017;15(1):4683, 24 pp. 10.2903/j.efsa.2017.4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 1996. Appendix G. Livestock Feeding Studies. 7031/VI/95 rev.4, 22 July 1996.
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev.3.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev.6.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev.2.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev.5.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev.3.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev.5.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals. 7039/VI/95. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev.4.
- European Commission , 2002. Review report for the active substance deltamethrin. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 18 October 2002 in view of the inclusion of active substance in Annex I of Council Directive 91/414/EEC. 6504/VI/99‐final, 17 October 2002.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010 Rev. 0, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev.8‐1.
- European Commission , 2015. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev.10.1.
- FAO (Food and Agriculture Organisation of the United Nations), 2002a. Deltamethrin. In: Pesticide residues in food – 2002‐ Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues. FAO Plant Production and Protection Paper 172, 2002.
- FAO (Food and Agriculture Organisation of the United Nations), 2002b. Submission and evaluation of pesticide residues data for the estimation of maximum residue levels in food and feed. First edition. FAO Plant Production and Protection Paper 173, 2002.
- FAO (Food and Agriculture Organisation of the United Nations), 2016. Deltamethrin. In: Pesticide residues in food – 2016‐ Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues. FAO Plant Production and Protection Paper 229, 2016.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL Calculator: User Guide. In: Series on Pesticides No 56. ENV/JM/MONO(2011)2, 01 March 2011.
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 04 September 2013.
- Sweden , 1998. Draft assessment report on the active substance deltamethrin prepared by the rapporteur Member State Sweden in the framework of Council Directive 91/414/EEC, October 1998.
- Sweden , 2002. Addendum to the draft assessment report on the active substance deltamethrin prepared by the rapporteur Member State Sweden in the framework of Council Directive 91/414/EEC, June 2002.
- United Kingdom , 2017. Evaluation report prepared under Article 8 of Regulation (EC) No 396/2005. MRL application on the setting of MRL(s) in deltamethrin, as revised in July 2017.


