Abstract
The EFSA Panel on Plant Health performed a pest categorisation for Xanthomonas oryzae pathovars oryzae (Xoo) and oryzicola (Xoc), the causal agents of the bacterial blight and the bacterial leaf streak of rice, respectively. These pathovars are widely distributed in Asia, Africa and Australia. Xoo is also reported in some states of the USA and in some other countries of America. The identity of both pathovars is well established and efficient identification methods are available. The major host is cultivated rice (Oryza sativa), but different Oryza spp. as well as Poaceae weeds are reported as alternative hosts, with some uncertainty concerning the actual host range. Both pathovars are seed associated, despite the fact that seed transmission is still controversial for Xoo. Both pathovars are already regulated in Directives 2000/29/EC, on harmful organisms for plants, and 66/402/EEC, on the marketing of cereal seeds. The main pathway for entry is seed. Should these pathovars enter into EU, they may establish and spread, and they may have an impact on the rice crops, with uncertainties. The knowledge gaps identified are (1) the quantity of EU importation of rice seeds, (2) the risk of introduction through unprocessed rice for consumption, (3) the suitability of the EU growing climate conditions for the bacteria to establish and spread, (4) role of seed transmission (Xoo), (5) the role of weeds in the epidemiology and especially in seed transmission and dispersal, (6) host range of weeds. As none of the pathovars is known to occur in the EU, they do not meet one of the criteria for being considered as Union regulated non‐quarantine pests. Nevertheless, both pathovars meet the criteria assessed by EFSA for consideration as Union quarantine pest.
Keywords: Xanthomonas oryzae pathovars oryzae and oryzicola , Xoo, Xoc, Oryza sativa, rice, pest risk, plant health, quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorizations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/20023, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pests categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration.
for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L.,Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Xanthomonas campestris pv. oryzae (Ishiyama) Dye and Xanthomonas campestris pv. oryzicola (Fang. et al.) Dye are two of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject together to pest categorisation to determine whether they fulfil the criteria of being quarantine pests or those of being regulated non‐quarantine pests (RNQPs) for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States (MSs) referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
Both organisms belong to the same species (see Section 3.1.1) now named Xanthomonas oryzae and are therefore considered in the same pest categorisation. In this pest categorisation, they will be named under their current names, Xanthomonas oryzae pv. oryzae (Xoo) and Xanthomonas oryzae pv. oryzicola (Xoc).
2. Data and methodologies
2.1. Data
2.1.1. Literature search
Due to recent taxonomic changes, a literature search on Xoo and Xoc was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific names of the pests as search terms. Relevant papers were reviewed, and further references and information were obtained from experts, from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the EPPO Global Database (EPPO, 2017).
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the European Union (EU) and about the area of hosts grown in the EU were obtained from EUROSTAT.
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network launched by the Directorate General for Health and Consumers (DG SANCO) and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation as well as notifications of plant pests detected in the territory of the MSs and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for Xoo and Xoc, following guiding principles and steps presented in the EFSA guidance on the harmonised framework for pest risk assessment (EFSA PLH Panel, 2010) and as defined in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
In accordance with the guidance on a harmonised framework for pest risk assessment in the EU (EFSA PLH Panel, 2010), this work was initiated following an evaluation of the EU's plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union RNQP in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants and includes additional information required as per the specific ToR received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a RNQP. If one of the criteria is not met, the pest will not qualify. A pest that does not qualify as a quarantine pest may still qualify as a RNQP which needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone; thus, the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism. | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pest must be present in the risk assessment area). |
| Regulatory status (Section 3.3) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future. |
The protected zone system aligns with the pest‐free area system under the International Plant Protection Convention (IPPC). The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone). |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met. | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met. |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, while addressing social impacts is outside the remit of the Panel, in agreement with EFSA guidance on a harmonised framework for pest risk assessment (EFSA PLH Panel, 2010).
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but, following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
YES
Xanthomonas oryzae pathovars oryzae and oryzicola are two pathovars of the Xanthomonadaceae family. Their identity is clearly defined. Both pathovars were reclassified from X. campestris into a single species called X. oryzae (Swings et al., 1990), as Xoo (Ishiyama, 1922) Swings et al., 1990 and Xoc (Fang et al., 1957) Swings et al., 1990.
Previous names given to the bacteria are, for Xoo, Pseudomonas oryzae, Bacterium oryzae, Phytomonas oryzae, Xanthomonas kresek, Xanthomonas itoana, Xanthomonas translucens pv. oryzae, Xanthomonas campestris pv. oryzae and, for Xoc, Xanthomonas translucens pv. oryzicola and Xanthomonas campestris pv. oryzicola (EPPO, 2007; Bradbury, 1986).
3.1.2. Biology of the pest
Xoo and Xoc represent a very fine example of the pathovar concept, in th sense that, even if both pathovars belong to the same species and share many common features at the genotype level (Bogdanove et al., 2011), they differ strikingly in the way they enter into the rice host plant and in symptoms produced. Despite their close relatedness (Ochiai and Kaku, 1999; Vauterin et al., 1995), each pathovar clearly colonises different tissues and leads to different diseases characterised by specific symptoms (Nino‐Liu et al., 2006). While both Xoo and Xoc can be associated with the rice seed coat (Sakthivel et al., 2001; Nino‐Liu et al., 2006), only Xoc has been confirmed to be both seed‐borne and seed transmitted. The existing evidence that Xoo is seed transmitted is controversial, based on questionable methods. Therefore, the epidemiological significance of Xoo for seed‐borne transmission has not yet been determined (Cottyn Bart, personal communication, 2017). Although rice seeds are generally considered as a pathway, at least for Xoc, most rice for consumption (brown and white rice) is not able to germinate due to post‐harvest treatments. Hot water treatment is effective to cure seed (Nino‐Liu et al., 2006; Cottyn Bart, personal communication, 2017).
3.1.2.1. Xoo life cycle
Xoo infects rice by wounds made during transplantation or by wind‐driven rains (Mew, 1993). Xoo enters also through hydathodes (pores that exude drops of water), localised at the leaf tip or leaf margin (Ou, 1985) and takes advantage of the movement of guttation fluid. Rice plants may become infected by many sources, such as diseased seeds, paddy water and diseased straws and stubble (Vera Cruz et al., 2017). The organism may survive in soil for 1–3 months (Ou, 1985). High‐bacterial concentrations have been recorded in irrigation water, canals and in rice fields, although the natural presence of bacteriophages may limit such presence (Ou, 1985). Once entered into plants, the bacteria multiply in the epitheme, the cavity beneath the hydathode water pores, and then move to the xylem vessels (Mew, 1993; Noda et al., 1999) resulting in bacterial blight symptoms on the rice leaves. Xoo accumulates to very high concentrations in infected rice plants to produce a disease called bacterial leaf blight (BB). In the field, Xoo usually appears at the tillering stage. Younger plants are generally highly susceptible and, once infected, may develop a Kresek disease (Reitsma and Schure, 1950) that occurs on seedling following the transplanting from the nursery, enhanced by the common practice of cutting leaves. The Kresek disease is characterised by a seedling wilt and pale yellow new leaves (Mew, 1993). The bacteria spread within fields with the help of wind‐driven rain, rain splashes and contact between plants and potentially animals such as water rats. Asymptomatic presence has also been reported (EPPO, 2007). During very severe infections, bacterial exudates may be seen in the oozing guttation fluid. Xoo overwinters mainly in alternate hosts (Mew et al., 1984; Mew, 1987) and is able to survive on infected leaves and plant debris in soils for 1–3 months depending on soil properties (Ou, 1985). Infected straw may also serve as a source of inoculum (Mew et al., 1984).
In temperate regions, Xoo survives the winter in the rhizosphere of weeds of the genera Leersia and Zizania (see Section 3.4.1) as well as in the base of the stem and the roots of rice stubble (Mizukami and Wakimoto, 1969). There is some uncertainty about the exact host range of Xoo. Some authors mention ’Poaceae’ as hosts. Although Xoo may be associated with rice seeds, seed‐borne transmission remains a matter of controversy (Fang et al., 1956; Mew and Misra, 1994; Sakthivel et al., 2001; Vera Cruz et al., 2017). This question should be further investigated. Reported transmission by insects or birds is considered as anecdotic (EPPO, 2007).
3.1.2.2. Xoc life cycle
Xoc enters mostly through stomata or leaf lesions, multiplies in the parenchyma to produce linear leaf streaks (bacterial leaf streak (BLS)), which are sometimes translucent (Mew, 1993). Bacteria multiply beneath the leaf epidermis and expand up and down from the entry point between the vascular bundles, but do not spread through the xylem vessels like Xoo. Xoc may produce typical yellow orange exudates extruding from stomata on the leaf surface. The bacteria spread within the field through leaf contact, rain splashes, wind‐driven rains and animals. Asymptomatic presence has also been reported. During very severe infections, bacterial exudates may be seen oozing out of the streaks. Xoc is seed transmitted (Mew, 1993; Xie and Mew, 1998). The bacteria may survive on alternate hosts like Leersia hexandra and Zizania aquatica (see Section 3.4.1, Bradbury, 1984; Leyns et al., 1984; Reddy and Nayak, 1974), although some authors report that none have been yet clearly identified (Ou, 1985; Nino‐Liu et al., 2006). The bacterium was also found at high concentrations in water and also survives in plant debris (Ou, 1985).
3.1.2.3. Molecular interplay between Xoo/Xoc and rice
The pathogenicity of both Xoo and Xoc relies on a type‐3 secretion system to deliver a whole repertoire of type‐3 effectors within rice cells (Nino‐Liu et al., 2006). This includes members of the transcription activator‐like effector family some of which are major virulence factors in both pathovars as they greatly promote bacterial virulence by activating the expression of host S susceptibility genes, preventing the activation of S genes results in attenuated disease (Hutin et al., 2015).
3.1.2.4. Bacterial leaf blight and bacterial leaf streak symptoms
Xoo and Xoc can be distinguished from each other at early stages by the disease symptoms they produce on their rice host plant, BB and BLS, respectively, while later confusion may occur on symptoms. BB and BLS may occur simultaneously in the same field, and sometimes even in the same leaf when disease pressure is high (Goto, 2004; Mew, 1993).
3.1.3. Intraspecific diversity
Xoo, contrary to Xoc, is characterised by a very high degree of race–cultivar specificity (Zhang et al., 2017). Races are classified by the use of a standard differential set of rice near‐isogenic lines (NILs, also known as IRBB lines, Ogawa et al., 1991) that differ from the susceptible parent by single resistance genes that were introgressed from resistant cultivars. So far, up to 41 BB resistance genes (also called Xa) have been identified and registered in the OryzaBase database (http://www.shigen.nig.ac.jp/rice/oryzabase/gene/list) (Zhang et al., 2017). On the pathogen side, at least 30 races have been evidenced, including 3 in West Africa, 11 in the Philippines, 14 in Sri Lanka, and more in Japan, Nepal, India and Korea (Triplett et al., 2014).
The probably high but still underestimated number of Xoo races underlies a long history of co‐evolution between that pathogen and its host. Studies on diversity of pathogen populations first based on DNA fingerprinting methods (such as AFLP, RFLP, rep‐PCR, AFLP) (Leach et al., 1992; Nelson et al., 1994; Choi et al., 1998; Gonzalez et al., 2007; Hajri et al., 2012; Zhao, 2012; Mishra et al., 2013; Wonni, 2016) and more recently sequencing‐based methodologies (i.e. MLST, MLVA) highlight the occurrence of genetic lineages discriminating the pathovar by continent so that African Xoo and Xoc form two distinct lineages, and Asian Xoo and Xoc are two others. The use of a 16‐MLVA minisatellites schemes (Poulin et al., 2015) enabled to show that allelic richness is generally lower in African pathovars as compared to Asian ones, suggesting that diversity of Asian Xoo and Xoc populations is higher, which is in line with a longer history of intensive rice cultivation in this part of the world. Yet, because of the changes that African rice agriculture is facing since two or three decades, it is expected that African Xoo and Xoc pathovars are actually undergoing important diversification events (Verdier et al., 2012).
3.1.4. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, detection and identification methods are available for both pests, Xoo and Xoc
Symptoms‐based detection is recommended by EPPO before bacterial isolation and further identification processes, which may involve pathogenicity tests on susceptible rice varieties like Azucena or Kitaake, and the use of ELISA with monoclonal antibodies (Benedict et al., 1989). Today, PCR‐based approaches (Cui et al., 2016; Lang et al., 2010; Cho et al., 2011; Song et al., 2012; EPPO, 2007) provide tools to accurately detect and identify both Xoo and Xoc. A loop‐mediated isothermal amplification protocol is also available (Lang et al., 2014).
Seed testing methodologies have been proposed, based on seedlings grow out tests (Mew and Misra, 1994) or seed washing procedures followed by plating on semiselective media (Gnanamanickam et al., 1994) or serological detection methods (Benedict et al., 1989), although such approaches are not considered sensitive enough to detect low concentrations of the pathogens.
A comprehensive description of the currently available and validated detection and identification methods has been recently proposed by Vera Cruz et al. (2017).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
Both Xoo and Xoc are present in the main rice‐producing areas of the world (see Figures 1 and 2 plus Tables 2 and 3).
Figure 1.
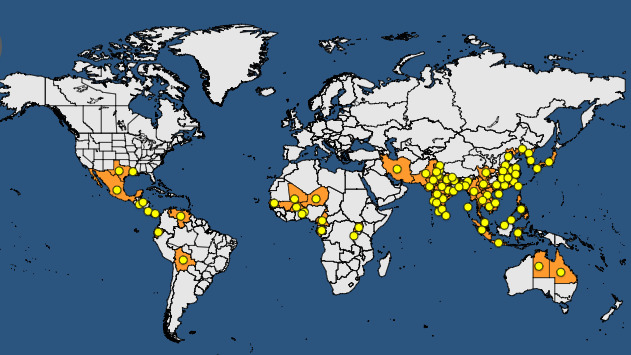
Global distribution map for Xanthomonas oryzae pathovar oryzae (extracted from the EPPO Global Database accessed on 31 October 2017)
Figure 2.
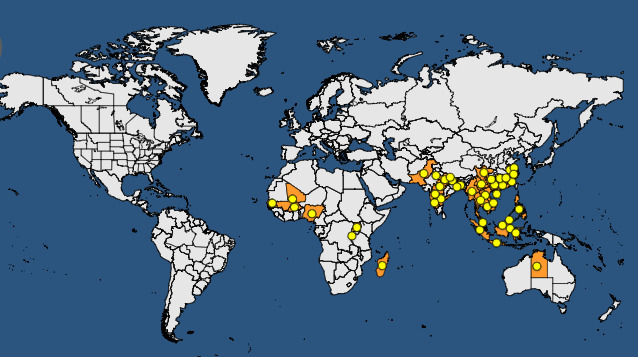
Global distribution map for Xanthomonas oryzae pathovar oryzicola (extracted from the EPPO Global Database accessed on 31 October 2017)
Table 2.
Global distribution of Xanthomonas oryzae pathovar oryzae (extracted from the EPPO Global Database accessed on 31 October 2017)
| Continent | Country | Status – EPPO GD |
|---|---|---|
| Africa | Benin | Present, restricted distribution |
| Africa | Burkina Faso | Present, no details |
| Africa | Burundi | Present, restricted distribution |
| Africa | Cameroon | Present, no details |
| Africa | Gabon | Present, few occurrences |
| Africa | Madagascar | Absent, unreliable record |
| Africa | Mali | Present, no details |
| Africa | Niger | Present, no details |
| Africa | Senegal | Present, few occurrences |
| Africa | Togo | Present, no details |
| Africa | Uganda | Present, restricted distribution |
| America | Bolivia | Present, no details |
| America | Colombia | Absent, invalid record |
| America | Costa Rica | Present, no details |
| America | Ecuador | Present, few occurrences |
| America | El Salvador | Present, no details |
| America | Honduras | Present, no details |
| America | Mexico | Present, no details |
| America | Nicaragua | Absent, unreliable record |
| America | Panama | Present, few occurrences |
| America | United States of America
|
Present, restricted distributionPresent, no details |
| America | Venezuela | Present, no details |
| Asia | Bangladesh | Present, widespread |
| Asia | Cambodia | Present, restricted distribution |
| Asia |
China Anhui, Fujian, Guangdong, Guangxi, Hainan, Henan, Hebei, Hubei, Hunan, Jiangsu, Jiangxi, Liaoning, Sichuan, Yunnan, Zhejiang |
Present, widespread Present, no details |
| Asia |
India Andaman and Nicobar Islands, Andhra Pradesh, Assam, Bihar, Chhattisgarh, Delhi, Goa, Gujarat, Haryana, Himachal Pradesh, Jammu & Kashmir, Karnataka, Kerala, Madhya Pradesh, Maharashtra, Orissa, Punjab, Rajasthan, Tamil Nadu, Tripura, Uttar Pradesh, West Bengal |
Present, widespread Present, no details |
| Asia |
Indonesia Java, Sulawesi, Sumatra |
Present, restricted distribution Present, no details |
| Asia | Iran | Present, restricted distribution |
| Asia |
Japan Honshu, Kyushu |
Present, restricted distribution Present, no details |
| Asia | Korea Dem. People's Republic | Present, restricted distribution |
| Asia | Korea, Republic | Present, restricted distribution |
| Asia | Lao | Present, widespread |
| Asia |
Malaysia Sabah, Sarawak, West |
Present, widespread Present, no details |
| Asia | Myanmar | Present, restricted distribution |
| Asia | Nepal | Present, restricted distribution |
| Asia | Pakistan | Present, restricted distribution |
| Asia | Philippines | Present, restricted distribution |
| Asia | Sri Lanka | Present, restricted distribution |
| Asia | Taiwan | Present, widespread |
| Asia | Thailand | Present, restricted distribution |
| Asia | Vietnam | Present, restricted distribution |
| Europe | Russia | Absent, pest no longer present |
| Europe | Ukraine | Absent, unreliable record |
| Oceania | Australia | Present, no details |
Table 3.
Global distribution of Xanthomonas oryzae pathovar oryzicola (extracted from the EPPO Global Database accessed on 31 October 2017)
| Continent | Country | Status – EPPO GD |
|---|---|---|
| Africa | Burkina Faso | Present, restricted distribution |
| Africa | Burundi | Present, restricted distribution |
| Africa | Madagascar | Present, no details |
| Africa | Mali | Present, restricted distribution |
| Africa | Nigeria | Present, no details |
| Africa | Senegal | Present, no details |
| Africa | Uganda | Present, restricted distribution |
| Asia | Bangladesh | Present, restricted distribution |
| Asia | Cambodia | Present, no details |
| Asia |
China Anhui, Fujian, Guangdong, Guangxi, Guizhou, Hainan, Hunan, Jiangsu, Jiangxi, Sichuan, Yunnan, Zhejiang |
Present, restricted distribution Present, no details |
| Asia |
India Andhra Pradesh, Assam, Bihar, Haryana, Karnataka, Madhya Pradesh, Maharashtra, Uttar Pradesh, West Bengal |
Present, widespread Present, no details |
| Asia | Indonesia | Present, no details |
| Asia | Malaysia | Present, no details |
| Asia | Lao | Present, restricted distribution |
| Asia | Myanmar | Present, no details |
| Asia | Nepal | Present, no details |
| Asia | Pakistan | Present, no details |
| Asia | Philippines | Present, no details |
| Asia | Taiwan | Absent, unreliable record |
| Asia | Thailand | Present, no details |
| Asia | Vietnam | Present, no details |
| Europe | Russia | Absent, pest no longer present |
| Oceania | Australia | Present, restricted distribution/no details |
Last updated: 2017‐09‐12
Last updated: 2017‐09‐12
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
NO, the pest is not known to be present in the EU territory.
Xoo and Xoc are not known to occur in the EU. As a consequence, they do not meet the criterion of the presence to qualify as a Union RNQP.
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
Xoo and Xoc are listed in Council Directive 2000/29/EC under the species campestris. Details are presented in Tables 3 and 4.
Table 4.
Xanthomonas oryzae pathovars oryzae and oryzicola in Council Directive 2000/29/EC
| Annex II, Part A | Harmful organisms whose introduction into, and spread within, all member states shall be banned if they are present on certain plants or plant products | |
| Section I | Harmful organisms not known to occur in the community and relevant for the entire community | |
| (b) | Bacteria | |
| Species | Subject of contamination | |
| 5. | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) | Seeds of Oryza spp. |
3.3.2. Legislation addressing the hosts of Xanthomonas oryzae pathovars oryzae and oryzicola (Table 5)
Table 5.
Regulated hosts and commodities that may involve Xanthomonas oryzae pathovars oryzae and oryzicola in Annex V of Council Directive 2000/29/EC
| Annex V | Plants, plant products and other objects which must be subject to a plant health inspection (at the place of production if originating in the Community, before being moved within the Community—in the country of origin or the consignor country, if originating outside the Community) before being permitted to enter the Community |
| Part B | Plants, plant products and other objects originating in territories, other than those territories referred to in part A |
| 1. |
Plants, plant products and other objects which are potential carriers of harmful organisms of relevance for the entire Community Plants, intended for planting, other than seeds but including seeds of……… Oryza spp…. . |
3.3.3. Other legislation addressing the rice seed production marketed within the Community (Directive 66/402/EEC)
Council directive 66/402/EEC on the marketing of cereal seeds implies that rice seed production marketed within the Community shall be officially certified. Such a certification implies in particular that only registered varieties can be sold and that field inspections, seed sampling and testing prove the absence of regulated harmful organisms.
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
The principal host of both Xoo and Xoc is rice, Oryza sativa. O. sativa subsp. japonica is generally more resistant to Xoc than the subsp. indica. Other Oryza species are reported as hosts of Xoo, like O. glaberrima, O. barthii, O. longistamina, O. rufipogon and O. australiensis (Aldrick et al., 1973). Xoc is also able to colonise most of these other Oryza species which probably play a role as reservoir plants for both pathovars (Ou, 1985). No information is available about genetic resistance in EU grown rice cultivars.
Besides Oryza species, Xoo is able to survive during winter on hosts like Cyperus rotundus, Leptochloa chinensis and L. panacea, Leersia oryzoides, Zizania latifolia. Xoo has also been reported on different additional hosts like Cenchrus ciliaris, Cynodon dactylon, Cyperus difformis, Cyperus rotundus, Echinochloa crus‐galli, Leersia hexandra, Leersia oryzoides, Megathyrsus maximus, Oryza. longistamina, Paspalum scrobiculatum, Urochloa mutica, Zizania aquatica, Z. palustris and Zoysia japonica (Li et al., 1985; Mew, 1993; Ou, 1985; Reddy and Nayak, 1974; Valluvapridasan and Mariappan, 1989).
Xoc is also found in association with weeds even if their role in the disease cycle is less clear than for Xoo. Hosts like Leersia hexandra and Zizania aquatica have been reported (Bradbury, 1984; Leyns et al., 1984; Reddy and Nayak, 1974).
The host range of both Xoo and Xoc is probably wider, including unreported Poaceae and Cyperaceae host plants (Ou, 1985; CABI, 2017). Therefore, there is some uncertainty with regard to the exact host range of both Xoo and Xoc and the role of weed alternate hosts for their overwintering and epidemiology.
3.4.2. Entry
Is the pest able to enter into the EU territory?
Yes, with uncertainty. Both Xoo and Xoc can be found on seeds. Non‐EU rice germplasm is frequently used in national breeding programs, but the current legislation implies that seeds must be free from both bacteria when imported within the EU.
Seed transmission is not regarded as a major route for the carry‐over of Xoo. Despite the controversy on seed transmission, its association with seed is, however, frequent enough to consider a possible risk of entry (EPPO, 2007). The progressive dissemination of Xoo in the world is also to be stressed: initially reported in Asia, Xoo is now detected on a worldwide scale and reported in Africa, America and Australia. The presence of « low virulence » strains of Xo in the USA (Jones et al., 1989) illustrates a possible widespread dispersal of a bacterium like Xoo.
Seed transmission is on the contrary considered as a major pathway for Xoc dissemination (Mew, 1993; Xie and Mew, 1998). Diversity analysis of Xoc tends to point to an East (Asia) to the West (Africa) spreading pathway, as diversification continuum among Xoc populations has been observed according to an East‐West gradient (Poulin L, unpublished, personal communication 2017). This suggests that Xoc had been introduced in Madagascar and in East‐African coastal countries from Asian Xoc populations.
It is somewhat difficult to estimate precisely the volume of rice seeds imported into the EU, although it is well known that rice seeds are frequently imported for breeding purposes (Cai et al., 2013). Rice for consumption (brown or white rice) is usually processed, and hot water treatment is considered as an effective way to limit the seed transmission risk (Mew and Misra, 1994).
It shall be noticed that most seeds that are imported into the EU are for breeding reasons, so generally in small quantities (Cai et al., 2013; Kraehmer et al., 2017). Such seeds are then used as parents for crosses and submitted to many observations that may facilitate the early discovery of symptoms and then may result in effective eradication. On the other hand, as such imports lead in the end to the release of large quantities of seeds for seedling, infections that would remain undiscovered during the breeding process could then be quickly and largely disseminated within the EU.
Between 1995 and 22 August 2017, there are no records of interception of Xoo and Xoc in the Europhyt database.
There are uncertainties about the volume and geographic origin of rice seeds imports. Also to be stressed is the controversy about Xoo seed transmission. Xoo can be detected and isolated from rice seeds or rice grown up from seeds (Fang et al., 1956; Sakthivel et al., 2001).
There is a very high uncertainty about a possible entry through importation of unprocessed rice for consumption.
3.4.3. Establishment
Is the pest able to become established in the EU territory?
YES, with some uncertainties
3.4.3.1. EU distribution of main host plants
Rice cultivation in the EU is restricted to Italy, Spain, Greece, Portugal, France, Romania, Bulgaria and Hungary, by acreage importance (Table 6). Mostly ’Japonica’ rice cultivars are cultivated in Europe (Cai et al., 2013). In the EU, all rice fields are irrigated and most rice seeds are drilled (Ferrero, 2007). Rice is planted in spring and harvested in autumn. The most prevalent monocotyledonous weed species are Cyperus, Echinochloa and Heteranthera (Kraehmer et al., 2017). Non‐EU rice varieties are introduced mainly for breeding purposes (Cai et al., 2013).
Table 6.
Rice production area in the EU (cultivation/harvested/production) (1,000 ha) extracted from Eurostat 22 August 2017Last update 21.08.17
| GEO/TIME | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|
| European Union (28 countries) | 454.15 | 432.94 | 432.27 | 443.33 | 441.80 |
| Italy | 235.05 | 216.02 | 219.53 | 227.33 | 227.33 |
| Spain | 112.82 | 112.15 | 110.42 | 109.29 | 109.33 |
| Greece | 30.21 | 29.10 | 30.72 | 35.08 | 35.18 |
| Portugal | 31.17 | 30.18 | 28.75 | 29.14 | 29.14 |
| France | 20.73 | 20.71 | 16.68 | 16.17 | 16.78 |
| Romania | 11.30 | 11.93 | 12.72 | 11.11 | 9.11 |
| Bulgaria | 9.90 | 10.21 | 11.04 | 12.41 | 11.99 |
| Hungary | 2.96 | 2.64 | 2.40 | 2.80 | 2.95 |
3.4.3.2. Climatic conditions affecting establishment
Favourable conditions for Xoo and Xoc occur in the EU rice‐growing areas. It is generally accepted that there are no ecoclimatic limitations for bacterial diseases establishment, besides those that apply for the host. Nevertheless, a NAPFAST prediction model for Xoo predicted 0 days favourable for infection in the EU (Magarey et al., 2011), but they used a simple model based on temperature over 30°C and high relative humidity. Their map does not fit perfectly the Xoo distribution, since for instance, Ecuador or Japan has only a few days suitable for infection according to their model while the disease is present.
Despite being also present under temperate climate like in Japan, Xoo infections lead to impacts mostly in tropical and subtropical areas in Asia. BLS is favoured by the monsoon season. BB is associated with the rainy season and is known as a ‘post‐typhoon’ disease as severe epidemics happen after typhoons, because of wind and rain driven dissemination. Such weather conditions do not occur in EU although extreme weather events may happen (strong and violent wind accompanied by intense rains during hot days) (EFSA PLH Panel, 2014).
Xoc occurs mostly in tropical and subtropical climates and causes damage only under very wet conditions. Without continuous rain, secondary infections stop spreading (Mew, 1993).
Since rice straw, stubbles and debris play a role in the bacterial survival, it was hypothesised that the bacteria may survive less efficiently in temperate climates because in such climates, stubble and straws wither more rapidly, whereas in the tropics, the stubbles survive or may generate volunteer rice as a source of inoculum for the next season (Mew, 1993).
Should entry into the EU happen, both Xoo and Xoc could establish, provided host plants (rice or other susceptible hosts) are present in the vicinity and climatic conditions are conducive for the disease. Nevertheless, how far the European climate is suitable for Xoo or Xoc establishment remains uncertain.
3.4.4. Spread
Is the pest able to spread within the EU territory following establishment?
YES How: (1) via seeds (2) via wind, rain dispersal and water floods like in Asia
There is no vector clearly identified for both Xoo and Xoc. Transmission by insects or birds is considered as anecdotic (Mew, 1993). Once established, the spread may occur by seed transmission. Spread of Xoo may follow a similar pathway. In Asia, Xoo occurs mostly during the rainy season. Water contaminated by the bacteria is a way of spreading the disease from one field to the other (Ou, 1985; Mew, 1993).
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact on the EU territory?
YES, with uncertainties.
BB (caused by Xoo) is considered to cause a disease with high impact in countries where it occurs. BLS (Xoc) may also lead major damage to the crop, under favourable climatic conditions.
Xoo was listed as one of the top 10 plant pathogenic bacteria, as voted for by scientists contributing to the journal Molecular Plant Pathology (Mansfield et al., 2012). Infection of BB usually causes a reduction in grain yield and an increase in the number of sterile grains (Reddy et al., 1979; Mew, 1993). Xoo has been reported in Asia, but also in Africa, North and South America and Australia. Nevertheless, the damage it causes is more important in the tropics than in temperate areas. In the USA for instance, Xoo has not established so far, although rice has been cultivated over 200 years, suggesting that climates of rice‐producing areas in the USA and USA rice cultivation practices are not conducive to the long‐term survival or spread of Xoo (Mansfield et al., 2012). Usually, the earlier the infection takes place, the worst the symptoms will be. Yield losses also depend on cultivars susceptibility (Ou, 1985). High levels of nitrogen fertilisation also increase the disease impact. Yield loss estimates range in tropical Asia from 2% to 74% (Mizukami and Wakimoto, 1969; Reddy et al., 1979; Ou, 1985; Mew, 1993).
BLS caused by Xoc is not considered as economically important as BB, although yield losses up to 30% have been reported (Opina and Exconde, 1971; Ou, 1985). Impact of BLS is also correlated with high nitrogen fertilisation. BLS has been more recently spotted in Africa and associated with relatively high losses (Gonzalez et al., 2007) and is somehow considered as a major rice disease in the humid tropics (Xie and Wang, 1991).
There is uncertainty on how far the European climate may be suitable for Xoo or Xoc.
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
Yes
No chemical (pesticides) treatments against Xoo and Xoc are currently available or scientifically supported for rice plants or seeds in many countries. Mercuric compounds and antibiotic solutions have been used for seed disinfection, but their use is forbidden in the EU. Currently, hot water treatment is used in several countries in Asia (Mew and Misra, 1994; Nino‐Liu et al., 2006). Therefore, no field or post‐harvest treatment can ensure that seeds from plants grown in areas where Xoo or Xoc exist is free from these bacteria, except seed hot water treatment.
Measures to prevent entry of Xoo and Xoc are therefore restricted to those leading to production of healthy seeds (e.g. ban of import of seeds, seed production in pest‐free areas, pest‐free places of production or pest‐free sites of production). Analysis of seed lots for detection of Xoo and Xoc may also contribute to mitigate the risks of introduction. Except for an import ban, those measures can nevertheless not entirely eliminate the risk of introduction of contaminated seeds (Mew and Misra, 1994).
Should entry into the EU happen, both Xoo and Xoc could establish, provided host plants (rice or other susceptible hosts) are present in the vicinity.
Some measures may be used to limit spread (e.g. field tool cleaning, no reuse of flooding waters) and existing EU certification scheme may be helpful to early detect contaminated EU produced seed lots.
3.6.1. Biological or technical factors limiting the feasibility and effectiveness of measures to prevent the entry, establishment and spread of the pest
The fact that those bacteria are commonly asymptomatically associated with rice plants limits their effectiveness.
3.6.2. Control methods
In countries where the disease is known to occur, the following cultural practices and control methods (Mew, 1993) are currently used to limit its impact and spread such as:
seed treatment (e.g. hot water treatment) and certification;
use of resistant rice cultivars;
crop managing practices like weed removal, destruction of rice stubbles and straws, avoiding wounds on seedlings, machine disinfestation, avoiding reuse of flooding water.
For example, in Japan, BB has been successfully controlled through the tight implementation of such control measures.
Xoc is mainly controlled through the use of healthy seeds.
3.7. Uncertainty
Quantity of seed importation from outside EU;
Possible entry with unprocessed rice imported for consumption;
Suitability of the EU climate conditions for pathogen establishment and spread;
Role of seed transmission (Xoo);
Role of weed in the epidemiology, e.g. in seed transmission and dispersal;
Host range on weeds, e.g. imports of Cyperus esculentus tubers from Africa.
4. Conclusions
Xoo and Xoc meet the criteria assessed by EFSA for consideration as potential Union quarantine pest (Table 7).
Table 7.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1) | The identity of both pests, Xoo and Xoc, is clearly established. They can be identified with reliable and sensitive techniques. | The identity of both pests, Xoo and Xoc, is clearly established. They can be identified with reliable and sensitive techniques. | No uncertainty |
| Absence/presence of the pest in the EU territory (Section 3.2) | None of the two pests (Xoo and Xoc) is known to occur within the EU. | None of the two pests (Xoo and Xoc) is known to occur within the EU; therefore, they do not qualify as a RNQP | No uncertainty |
| Regulatory status (Section 3.3) | Xoo and Xoc are currently regulated on seeds of Oryza spp. by Directive 2000/29/EC. | Xoo and Xoc are currently regulated on seeds of Oryza spp. by Directive 2000/29/EC. | No uncertainty |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | Xoo and Xoc can enter, establish and spread within the EU through the seed pathway. | Xoo and Xoc can enter establish and spread within the EU through the seed pathway. |
Quantity of rice seed importation within the EU; Risk of introduction through unprocessed rice for consumption considered as limited, but with a major uncertainty; Suitability of the EU growing climate conditions for the bacteria establishment and spread; Role of seed transmission (Xoo); Role of weed in the epidemiology, e.g. in seed transmission and dispersal Host range of weeds |
| Potential for consequences in the EU territory (Section 3.5) | If introduced in the EU, the potential impact of Xoo and Xoc is very difficult to assess. | If introduced in the EU, the potential impact of Xoo and Xoc is very difficult to assess. | Suitability of the EU growing climate conditions for the bacteria |
| Available measures (Section 3.6) | Prohibition or restriction of the importation of rice seed. | Seed certification. | No uncertainty |
| Conclusion on pest categorisation (Section 4) | Xoo and Xoc meet the criteria assessed by EFSA for consideration as potential Union quarantine pests. | Xoo and Xoc do not meet the presence on the territory criterion and therefore do not qualify as a Union RNQPs. | |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | The knowledge gaps identified are (1) the quantity of EU importation of rice seeds and (2) risk of introduction through unprocessed rice for consumption (3), the suitability of the EU growing climate conditions for the bacteria establishment and spread, (4) role of seed transmission (Xoo), (5) role of weeds in the epidemiology, especially in seed transmission and dispersal, (6) host range of weeds | ||
Abbreviations
- AFLP
Amplified fragment length polymorphism
- BB
Bacterial leaf blight
- BLS
Bacterial leaf streak
- DG SANCO
Directorate General for Health and Consumers
- ELISA
enzyme‐linked immunosorbent assay
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- MLST
Multilocus sequence typing
- MLVA
Multiple‐Locus Variable number tandem repeat Analysis
- MS
Member State
- NIL
near‐isogenic line
- PCR
polymerase chain reaction
- PLH
EFSA Panel on Plant Health
- RFLP
Restriction Fragment Length Polymorphism
- rep‐PCR
Repetitive extragenic palindromic PCR
- RNQP
regulated non‐quarantine pest
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
- Xoc
Xanthomonas oryzae pv. oryzicola
- Xoo
Xanthomonas oryzae pv. oryzae
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Grégoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Bragard C, Szurek B, Hollo G and Caffier D, 2018. Scientific Opinion on the pest categorisation of Xanthomonas oryzae pathovars oryzae and oryzicola . EFSA Journal 2018;16(1):5109, 25 pp. 10.2903/j.efsa.2018.5109
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00426
Panel members: Claude Bragard, David Caffier, Thierry Candresse, Elisavet Chatzivassiliou, Katharina Dehnen‐Schmutz, Gianni Gilioli, Jean‐Claude Grégoire, Josep Anton Jaques Miret, Michael Jeger, Alan MacLeod, Maria Navajas Navarro, Björn Niere, Stephen Parnell, Roel Potting, Trond Rafoss, Vittorio Rossi, Gregor Urek, Ariena Van Bruggen, Wopke Van der Werf, Jonathan West and Stephan Winter.
Adopted: 22 November 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1 and 2: © EPPO
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
References
- Aldrick SJ, Buddenhagen IW and Reddy APK, 1973. The occurrence of bacterial leaf blight in wild and cultivated rice in Northern Australia. Australian Journal of Agricultural Research, 24, 219–227. [Google Scholar]
- Benedict AA, Alvarez AM, Berestecky J, Imanaka W, Mizumoto CY, Pollard LW, Mew TW and Gonzalez CF, 1989. Pathovar‐specific monoclonal antibodies for Xanthomonas campestris pv. oryzae and for Xanthomonas campestris pv. oryzicola . Phytopathology, 79, 322–328. [Google Scholar]
- Bogdanove AJ, Koebnik R, Lu H, Furutani A, Angiuoli SV, Patil PB, Van Sluys MA, Ryan RP, Meyer DF, Han SW, Aparna G, 2011. Two new complete genome sequences offer insight into host and tissue specificity of plant pathogenic Xanthomonas spp. Journal of Bacteriology, 193, 5450–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury J, 1984. Xanthomonas. Bergey's manual of systematic bacteriology, Vol. 1.
- Bradbury JF, 1986. Guide to Plant Pathogenic Bacteria. CAB International, Egham, UK. [Google Scholar]
- CABI (Centre for Agriculture and Bioscience International), 2017. Xanthomonas oryzae pv. oryzicola (bacterial leaf streak of rice) datasheet. https://www.cabi.org/isc/datasheet/56977, [Accessed: 31 October 2017]
- Cai X, Fan J, Jiang Z, Basso B, Sala F, Spada A, Grassi F and Lu B‐R, 2013. The puzzle of Italian rice origin and evolution: determining genetic divergence and affinity of rice germplasm from Italy and Asia. PLoS ONE, 8, e80351. 10.1371/journal.pone.0080351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MS, Kang MJ, Kim CK, Seol YJ, Hahn JH, Park SC, Hwang DJ, Ahn T‐Y, Park DH, Lim CK and Park DS, 2011. Sensitive and specific detection of Xanthomonas oryzae pv. oryzae by real‐time bio‐PCR using pathovar‐specific primers based on an rhs family gene. Plant disease, 95, 589–594. [DOI] [PubMed] [Google Scholar]
- Choi SH, Vera Cruz CM and Leach J, 1998. Distribution of Xanthomonas oryzae pv. oryzae DNA modification systems in Asia. Applied and Environment Microbiology, 64, 1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottyn B, 2017. personal communication. E‐mail message to Claude Bragard on the 29th of October, 2017
- Cui Z, Ojaghian MR, Tao Z, Kakar KU, Zeng J, Zhao W, Duan Y, Vera Cruz CM, Li B, Zhu B and Xie G, 2016. Multiplex PCR assay for simultaneous detection of six major bacterial pathogens of rice. Journal of Applied Microbiology, 120, 1357–1367. 10.1111/jam.13094 [DOI] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2010. PLH Guidance on a harmonised framework for pest risk assessment and the identification and evaluation of pest risk management options by EFSA. EFSA Journal 2010;8(2):1495, 66 pp. 10.2903/j.efsa.2010.1495 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2014. Scientific Opinion on the risk to plant health of Xanthomonas citri pv. citri and Xanthomonas citri pv. aurantifolii for the EU territory. EFSA Journal 2014;12(2):3556, 178 pp. 10.2903/j.efsa.2014.3556 [DOI] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 2007. Diagnostic of Xanthomonas oryzae . Bulletin OEPP/EPPO Bulletin, 37, 543–553. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 2017. EPPO global database. Available online: https://gd.eppo.int [Accessed: 31 October 2017]
- Fang CT, Lin CF and Chu CL, 1956. A preliminary study on the disease cycle of the bacterial leaf blight of rice. Acta Phytopathologica Sinica, 2, 173–185. [Google Scholar]
- Fang CT, Ken HC, Chen TY, Chu YK, Faan HC and Wu SC, 1957. A comparison of the Bice bacterial leaf blight organism with the bacterial leaf streak organisms of Rice and Leersia hexandra Swartz. Acta Phytopathologica Sinica, 3. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- Ferrero A, 2007. Rice in the European Union. Research Book: Agro‐economical Traits of Rice Cultivation in Europe and India, 1995(5.1), 17.
- Gnanamanickam SS, Shigaki T, Medalla ES, Mew TW and Alvarez AM, 1994. Problems in detection of Xanthomonas oryzae pv. oryzae in rice seed and potential for improvement using monoclonalantibodies. Plant Disease, 78, 173–178. [Google Scholar]
- Gonzalez C, Szurek B, Manceau C, Mathieu T, Séré Y and Verdier V, 2007. Molecular and pathotypic characterization of new Xanthomonas oryzae strains from West Africa. Molecular Plant‐Microbe Interactions, 20, 534–546. [DOI] [PubMed] [Google Scholar]
- Goto M, 1992. Fundamentals of Bacterial Plant Pathology. Academic Press, San Diego. [Google Scholar]
- Hajri A, Brin C, Zhao S, David P, Feng J, Koebnik R, Szurek B, Verdier V, Boureau T and Poussier S, 2012. Multilocus sequence analysis and type III effector repertoire mining provide new insights into the evolutionary history and virulence of Xanthomonas oryzae . Molecular Plant Pathology, 13, 288–302. 10.1111/j.1364-3703.2011.00745.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutin M, Pérez‐Quintero AL, Lopez C and Szurek B, 2015. MorTAL Kombat: the story of defense against TAL effectors through loss‐of‐susceptibility. Frontiers in Plant Science, 6, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama S, 1922. Studies of bacterial leaf blight of rice. Report of the Imperial Agricultural Station. Nishigahara (Konosu), 45, 233–261. [Google Scholar]
- Jones RK, Barnes LW, Gonzalez CF, Leach JE, Alvarez AM and Benedict AA, 1989. Identification of low‐virulence strains of Xanthomonas campestris pv. oryzae from rice in the United States. Phytopathology, 79, 984–990. [Google Scholar]
- Kraehmer H, Thomas C and Vidotto F, 2017. Rice Production in Europe. In Rice Production Worldwide (pp. 93–116). Springer International Publishing.
- Lang JM, Hamilton JP, Diaz MG, Van Sluys MA, Burgos MR, Vera Cruz CM, Buell CR, Tisserat NA and Leach JE, 2010. Genomics‐based diagnostic marker development for Xanthomonas oryzae pv. oryzae and X. oryzae pv. oryzicola . Plant Disease 94:311–319. https://doi.org/10.1094/PDIS‐94‐3‐0311 [DOI] [PubMed] [Google Scholar]
- Lang JM, Langlois P, Nguyen MHR, Triplett LR, Purdie L, Holton TA, Djikeng A, Vera Cruz CM, Verdier V and Leach JE, 2014. Sensitive detection of Xanthomonas oryzae pathovars oryzae and oryzicola by loop‐mediated isothermal amplification. Applied and Environment Microbiology 80, 4519–4530. 10.1128/aem.00274-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JE, Rhoads ML, Vera Cruz CM, White FF, Mew TW and Leung H, 1992. Assessment of genetic diversity and population structure of Xanthomonas oryzae pv. oryzae with a repetitive DNA element. Applied Environmental Microbiology, 58, 2188–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns F, De Cleene M, Swings JG and De Ley J, 1984. The host range of the genus Xanthomonas . The Botanical Review, 50, 308–356. [Google Scholar]
- Li ZZ, Zhao H and Ying XD, 1985. The weed carriers of bacterial leaf blight of rice. Acta Phtopathologica Sinica, 15, 246–248. English Abstract. [Google Scholar]
- Magarey RD, Borchert DM, Engle JS, Colunga‐Garcia M, Koch FH and Yemshanov D, 2011. Risk maps for targeting exotic plant pest detection programs in the United States. EPPO Bulletin, 41, 46–56. [Google Scholar]
- Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA, Toth I, Salmond G and Foster GD, 2012. Top 10 plant pathogenic bacteria in molecular plant pathology. Molecular Plant Pathology, 13, 614–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mew TW, Mew IP and Huang JS, 1984. Scanning electron microscopy of virulent and avirulent strains of Xanthomonas campestris pv. oryzae on rice leaves. Phytopathology, 74, 635–641. [Google Scholar]
- Mew TW, 1987. Current status and future prospects of research on bacterial blight of rice. Annual review of phytopathology, 25, 359–382. [Google Scholar]
- Mew TW, 1993. Xanthomonas oryzae pathovars on rice: cause of bacterial blight and bacterial leaf streak. Chapman and Hall, Xanthomonas. New York. pp. 30–40. [Google Scholar]
- Mew TW and Misra JK, 1994. A manual of rice seed health testing. International Rice Research Institute, Manila, Philippines. [Google Scholar]
- Mishra D, Vishnupriya MR, Anil MG, Konda K, Raj Y and Sonti RV, 2013. Pathotype and genetic diversity amongst Indian isolates of Xanthomonas oryzae pv. oryzae . PLoS ONE, 8, e81996. 10.1371/journal.pone.0081996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami T and Wakimoto S, 1969. Epidemiology and control of bacterial leaf blight of rice. Annual Review of Phytopathology, 7, 51–72. [Google Scholar]
- Nelson RJ, Baraoidan MR, Vera Cruz CM, Yap IV, Leach JE, Mew TW and Leung H, 1994. Relationship between phylogeny and pathotype for the bacterial blight pathogen of rice. Applied Environmental Microbiology, 60, 3275–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nino‐Liu DO, Ronald PC and Bogdanove AJ, 2006. Xanthomonas oryzae pathovars: model pathogens of a model crop. Molecular Plant Pathology, 7, 303–324. [DOI] [PubMed] [Google Scholar]
- Noda T, Du PV, Dinh V, Dinh ELH and Kaku H, 1999. Pathogenicity of Xanthomonas oryzae pv. oryzae strains in Vietnam. Annual Phytopathology Society Japan, 65, 293–296. 10.3186/jjphytopath.65.293 [DOI] [Google Scholar]
- Ochiai H and Kaku H, 1999. Genetic relationships among Xanthomonas species and pathovars based on RFLP analyses of PCR‐amplified 16S, 23S rDNA and rDNA internal transcribed spacer. Japanese Journal of Phytopathology, 65, 437–446. [Google Scholar]
- Ogawa T, Yamamoto K, Khush G and Mew T, 1991. Breeding of near‐isogenic lines of rice with single genes for resistance to bacterial blight pathogen (Xanthomonas campestris pv. oryzae). Japanese Journal of Breeding, 41, 523–529. [Google Scholar]
- Opina OS and Exconde OR, 1971. Assessment of yield loss due to bacterial leaf streak of rice. Philippine Phytopathology, 7, 35–39. [Google Scholar]
- Ou SH, 1985. Rice diseases. IRRI, Philippines. [Google Scholar]
- Poulin L, 2017. unpublished. Personal communication. E‐mail message to Boris Szurek on the 1st of December 2017.
- Poulin L, Grygiel P, Magne M, Gagnevin L, Rodriguez‐R LM, Forero Serna F, Zhao S, El Rafii M, Dao S, Tekete C, Wonni I, Koita O, Pruvost O, Verdier V, Vernière C and Koebnik R, 2015. New multilocus variable‐number tandem‐repeat analysis tool for surveillance and local epidemiology of bacterial leaf blight and bacterial leaf streak of rice caused by Xanthomonas oryzae. Applied and Environmental Microbiology, 81, 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PR and Nayak P, 1974. New host for bacterial leaf blight pathogen of rice. Current science. [Google Scholar]
- Reddy APK, Mackenzie DR, Rouse DI and Rao AV, 1979. Relationship of bacterial leaf blight severity to grain yield of rice. Phytopathology, 69, 967–969. [Google Scholar]
- Reitsma J and Schure PSJ, 1950. ’Kresek’, a bacterial disease of Rice. Contributions from the General Agricultural Research Station, Bogor, 117.
- Sakthivel N, Mortensen C and Mathur S, 2001. Detection of Xanthomonas oryzae pv. oryzae in artificially inoculated and naturally infected rice seeds and plants by molecular techniques. Applied Microbiology and Biotechnology, 56, 435–441. [DOI] [PubMed] [Google Scholar]
- Song ES, Lee BM, Lee CS and Park YJ, 2012. PCR‐based Rapid Assay for Discriminative Detection of Latent Infections of Rice Bacterial Blight. Journal of Phytopathology, 160, 195–200. [Google Scholar]
- Swings J, Van den Mooter M, Vauterin L, Hoste B, Gillis M, Mew TW and Kersters K, 1990. Reclassification of the Causal Agents of Bacterial Blight (Xanthomonas campestris pv. oryzae) and Bacterial Leaf Streak (Xanthomonas campestris pv. oryzicola) of Rice as Pathovars of Xanthomonas oryzae (ex Ishiyama 1922) sp. nov., nom. rev. International Journal of Systematic and Evolutionary Microbiology, 40, 309. [Google Scholar]
- Triplett L, Koebnik R, Verdier V and Leach JE, 2014. The Genomics of Xanthomonas oryzae . In: Gross DC, Lichens‐Park A and Chittaranjan K (eds.). Genomics of Plant‐Associated Bacteria. Springer‐Verlag, Berlin Heidelberg. pp. 127–150. [Google Scholar]
- Valluvapridasan V and Mariappan V, 1989. Alternate hosts of rice bacterial blight (BB) pathogen Xanthomonas campestris pv. oryzae . International Rice Research Newsletter, 14, 27–28. [Google Scholar]
- Vauterin L, Hoste B, Kersters K and Swings J, 1995. Reclassification of xanthomonas. International Journal of Systematic and Evolutionary Microbiology, 45, 472–489. [DOI] [PubMed] [Google Scholar]
- Vera Cruz CM, Cottyn B, Nguyen MH, Lang J, Verdier V, Mew TW and Leach JE, 2017. Detection of Xanthomonas oryzae pv. oryzae, and X. oryzae pv. oryzicola in rice seeds (Chapter 8). In APS Manual on Detection of Plant Pathogenic Bacteria in Seed and Other Planting Material. APS Press, Minneapolis, MN. ISBN 978‐0‐89054‐539‐3.
- Verdier V, Cruz CV and Leach JE, 2012. Controlling rice bacterial blight in Africa: needs and prospects. Journal of Biotechnology, 159, 320–328. [DOI] [PubMed] [Google Scholar]
- Wonni I, Ouedraogo SL, Ouedraogo I and Sanogo L, 2016. Antibacterial activity of extracts of three aromatic plants from Burkina Faso against rice pathogen, Xanthomanas oryzae . African Journal of Microbiology Research, 10, 681–686. [Google Scholar]
- Xie GL and Mew TW, 1998. A leaf inoculation method for detection of Xanthomonas oryzae pv. oryzicola from rice reed. Plant Disease, 82, 1007–1011. 10.1094/PDIS.1998.82.9.1007 [DOI] [PubMed] [Google Scholar]
- Xie GL and Wang H, 1991. Comparison of several bactericides against bacterial leaf streak of rice. Journal of Zhejiang Agricultural Science, 5, 233–235. [Google Scholar]
- Zhang F, Wu ZC, Wang MM, Dingkuhn M, Xu JL, Zhou YL and Li ZK, 2017. Genome‐wide association analysis identifies resistance loci for bacterial blight in a diverse collection of indica rice germplasm. PLoS ONE, 12, e0174598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Poulin L, Rodriguez‐R LM, Serna NF, Liu SY, Wonni I, Szurek B, Verdier V, Leach JE, He QY, Feng JX and Koebnik R, 2012. Development of a variable number of tandem repeats typing scheme for the bacterial rice pathogen Xanthomonas oryzae pv. oryzicola . Phytopathology, 102, 948–956. [DOI] [PubMed] [Google Scholar]


