Abstract
The Panel on Plant Health performed a pest categorisation of Popillia japonica (Coleoptera: Scarabaeidae) for the EU. P. japonica is a distinguishable species listed in Annex IAII of Council Directive 2000/29/EC. It is native to Japan but established in the USA in the early 20th century. It spreads from New Jersey to most US states east of the Mississippi, some to the west and north into Canada. P. japonica feeds on over 700 plant species. Adults attack foliage and fruit surfaces. They can cause serious injury to tree fruits and soft fruit, vegetable crops, ornamental herbaceous plants, shrubs, vines and trees. Larvae are root feeders regarded as serious pests of lawns and turf, vegetables and nursery stock. Adults emerge during the summer and can fly short distances on warm sunny days. The life cycle is usually completed in one year. In cooler regions, development takes two years. P. japonica occurs in the EU in the Azores (Portugal), Lombardy and Piedmont (Italy) where it is under official control. Adults are suspected of being able to spread on aircraft as hitchhikers, i.e. without host plants. Soil accompanying plants for planting provides a pathway for further introductions. Hosts are widely available within the EU. Climatic conditions across central and parts of southern EU are suitable for development in one year. Across parts of northern Europe development over two years is likely. Without control, impacts could be expected on a range of plants. Phytosanitary measures are available to reduce the likelihood of introduction of P. japonica. All criteria assessed by EFSA for consideration as a potential Union quarantine pest are met. Plants for planting are not necessarily the main means of spread so P. japonica does not satisfy all criteria necessary for it to be regarded as a Union regulated non‐quarantine pest (RNQP).
Keywords: hitch hiker, Japanese beetle, pest risk, plant health, plant pest, quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorisations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/20023, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L.. and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pests categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocantus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Popillia japonica is one of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest (RNQP) for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States (MS) referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores. It is noted that within the original plant health directive 2000/29 EC the genus of the organism is misspelt as Popilia.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on P. japonica was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as a search term. Relevant papers were reviewed and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plan Protection Organization (EPPO) Global Database (EPPO, 2018) and relevant publications.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission, and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the MS and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for P. japonica, following guiding principles and steps in the International Standard for Phytosanitary Measures No 11 (FAO, 2013), No 21 (FAO, 2004) and EFSA PLH Panel (2018).
This work was initiated following an evaluation of the EU plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union RNQP in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required in accordance with the specific terms of reference received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a RNQP. If one of the criteria is not met, the pest will not qualify. A pest that does not qualify as a quarantine pest may still qualify as a RNQP that needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone; thus, the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism. | Is the pest present in the EU territory? If not, it cannot be a regulated non‐quarantine pest. (A regulated non‐quarantine pest must be present in the risk assessment area) |
| Regulatory status (Section 3.3 ) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future |
The protected zone system aligns with the pest free area system under the International Plant Protection Convention (IPPC) The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone) |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? |
| Available measures (Section 3.6 ) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential regulated non‐quarantine pest were met, and (2) if not, which one(s) were not met |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, whereas addressing social impacts is outside the remit of the Panel.
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
Yes, Popillia japonica is a clearly defined insect species in the order Coleoptera (beetles), family Scarabaeidae (scarab beetles).
P. japonica Newman, 1841 (Coleoptera: Scarabaeidae) is a well‐established species with stable taxonomy although it is very similar in appearance and habits to Popillia quadriguttata which occurs in Korea and China (Lee et al., 2014). It has the common name Japanese beetle (Bosik, 1997).
3.1.2. Biology of the pest
In most areas of its native range in Japan, the life cycle is completed in one year. On Honshu, around Tokyo (central Japan), development occurs within a year, but in northern, cooler, areas of Honshu, perhaps 25% of individuals take 2 years to complete development (King, 1931; Fleming, 1972). In Hokkaido (northern Japan), most individuals have a 2‐year life cycle (Clausen et al., 1927).
In the USA, King (1931) noted that most individuals developed within 12 months in Pennsylvania and New Jersey but rarely some took two years to complete development. In Massachusetts, around 90% of individuals completed development within 1 year but about 10% took 2 years (Vittum, 1986). In Canada, the life cycle can take 1 or 2 years depending on summer temperatures (Campbell et al., 1989). In Italy, the life cycle is completed in one year (EPPO, 2016b).
Adult emergence, subsequent mating, oviposition and larval development vary with latitude and from year to year according to temperature (Fleming, 1972). Nevertheless, in general, adults emerge in the summer (June–July) and fly or climb to feed on foliage at the top of low growing hosts before later moving to feed on trees. Shortly after emergence and maturation feeding adults mate. Adults live for 30–45 days during which time there can mate more than once. Adults tend to aggregate to feed and mate on individual host plants such that some will be heavily infested whilst the nearby hosts of the same species are not attacked (Campbell et al., 1989). Adults feed on the foliage and fruit of hosts (Metcalf and Metcalf, 1993). Adults are most active, feeding and flying, on warm sunny days. In Italy, whilst adults peak in July, some adults can be active until September and rarely in October. In the Azores, adults can be found between May and November (EPPO, 2016b).
After mating, females burrow up to 10 cm into the soil to oviposit up to six eggs at a time (Metcalf and Metcalf, 1993). After laying a single egg or a small group of eggs, females exit the soil to feed, and then return to oviposit in the soil again. A female will usually lay between 40 and 60 eggs in total (Campbell et al., 1989). Eggs are not cold hardy and viability decreases at temperatures below 10°C; seven days at 0°C led to 100% egg mortality (Fleming, 1972). Depending on temperature, eggs usually hatch after about 2 weeks. Larvae feed on decaying matter and then the roots of a variety of grasses, garden and field crops, and ornamental plants in the upper 7.5 cm of soil (Metcalf and Metcalf, 1993). Larvae are most abundant in lawns, pastures and golf courses, i.e. areas of abundant grass. There are three larval instars. The first instar develops in 2–3 weeks; the second in 3–4 weeks. The third larval instar burrows deeper and overwinters at depths of 10–20 cm, presumably to avoid cooler or freezing temperatures. In the spring, as the soil warms, larvae rise to shallower depths in the soil where they form a chamber in which they pupate and emerge in mid‐summer to repeat the cycle. In cases where development takes 2 years, second and third instars overwinter during the first and second winters, respectively (Vittum, 1986).
3.1.3. Intraspecific diversity
No intraspecific diversity has been described for this species.
3.1.4. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, there is an EPPO phytosanitary standard diagnostic protocol for P. japonica. It refers to detection and trapping techniques as well as addressing the identification of the pest to species level (EPPO, 2016a).
Symptoms of adult P. japonica include the feeding holes they cause in host leaves. When there are high numbers of adults, leaves can be skeletonised (EPPO, 2016a). Adults feed gregariously, usually beginning to feed at the top of a host and working down (Fleming, 1972). Discoloured grass patches, expanding over time or the death of turf grass can indicate the presence or larvae in the soil.
Commercially available lures are available. Lures combine the female produced sex attractant ((R,Z)‐5‐(1‐decenyl)dihydro‐2(3H)‐furanone) with a mixture of 2‐phenethyl propionate, eugenol and geraniol (3:7:3). The lure is very attractive to both sexes (Ladd et al., 1981). Traps baited with a lure are useful for detecting new infestations and mass trapping can be used to suppress pest populations (Porter and Held, 2002).
Detection of larvae requires soil sampling. Larval populations are aggregated and often occur in the vicinity of plants that had had adults aggregating on them to feed and mate during the summer; well drained moderately textured soils in sunlight also favour higher densities of larvae. Soil with high levels of organic matter tends to have lower larval densities (Dalthorp et al., 2000; Porter and Held, 2002).
The EPPO phytosanitary standard diagnostic protocol for P. japonica provides a key to the European families within the superfamily Scarabaeoidea and enables the identification of the Popillia genus. Detailed morphological descriptions of each life stage of the species are provided to allow its identification to species level (EPPO, 2016a). Fleming (1972) also provides descriptions for each life stage. However, no key to species is available and because the genus consists of more than 300 species, many from Africa and Asia, there is a chance of misidentifying some specimens (EPPO, 2016a).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
Popillia japonica is native to Japan. Reports in early literature stating that P. japonica occurs in northern China (e.g. Fleming, 1972) are regarded as invalid due to misidentification. Reports from China are interpreted as referring to the closely related species P. quadriguttata (Chen et al., 2014; EPPO Global database, 2018).
In the early 20th century, P. japonica established in North America (EPPO, 2016a). It was first reported in New Jersey in 1916 but larvae may have arrived a few years earlier in soil associated with iris plants for planting (Dickerson and Weiss, 1918) or other nursery stock from Japan (Metcalf and Metcalf, 1993; CABI, 2018). It is now well established in US states that border the Atlantic and has spread west towards the Mississippi and north into Canada. Findings in California (1961–1964; 1973–1975 and 1983–1985) were eradicated (Porter and Held, 2002).
The map in Figure 1 suggests that P. japonica occurs in Far East Russia. Careful interpretation of Figure 1 is required. P. japonica is not known to occur in continental Russia but only on the Russian island of Kunashir which sits less than 30 km to the east of Hokkaido (northern Japan) (Figure 2). Kunashir is part of the disputed Kuril Islands, an archipelago spreading from Hokkaido (Japan) to the Kamchatka Peninsula (Russia). EPPO Global database (2018) records P. japonica from Kunashir in 1977 and notes that it was sporadically observed on Kunashir Island during an expedition in 2011.
Figure 1.
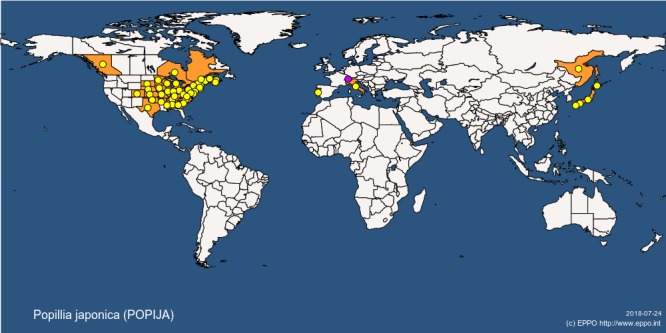
Global distribution map for Popillia japonica (extracted from the EPPO Global Database accessed on 24 July 2018). Refer to the text for notes on interpretation of the map
Figure 2.
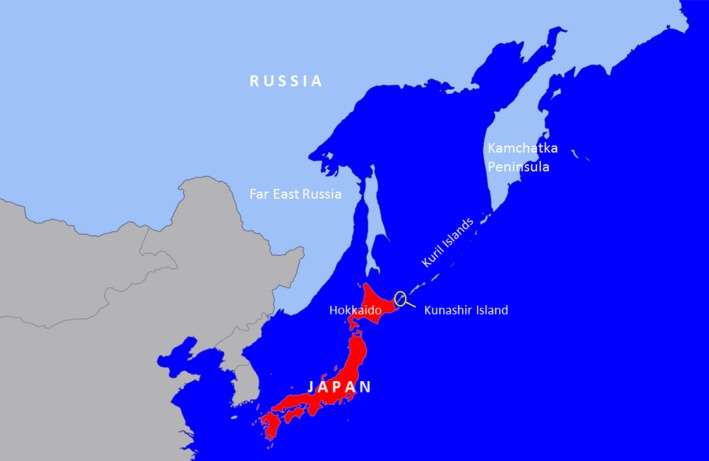
Location of Kunashir Island in relation to Far East Russia and Hokkaido
In June 2017, Switzerland reported finding P. japonica adults in a pheromone trap near the border with Italy, a few km from an outbreak site in Italy. Other than findings in the trap, no other P. japonica have been found in Switzerland (EPPO Reporting Service, 2017).
Details of the geographical distribution of P. japonica outside of the EU are provided in Table 2.
Table 2.
Global distribution of Popillia japonica, excluding EU (Source: EPPO Global Database, accessed on 24 July 2018)
| Continent | Country | Sub‐national area e.g. State | Status |
|---|---|---|---|
| America | Canada | British Columbia | Present, few occurrences |
| New Brunswick, Nova Scotia, Ontario, Prince Edward Island, Québec | Present, restricted distribution | ||
| USA | Alabama, Arkansas, Colorado, Kansas, Mississippi, Nebraska, Oklahoma, South Dakota, Texas | Present, few occurrences | |
| Connecticut, Delaware, District of Columbia, Georgia, Illinois, Indiana, Iowa, Kentucky, Maine, Maryland, Massachusetts, Michigan, Minnesota, Missouri, New Hampshire, New Jersey, New York, North Carolina, Ohio, Pennsylvania, Rhode Island, South Carolina, Tennessee, Vermont, Virginia, West Virginia, Wisconsin | Present, restricted distribution | ||
| Asia | China | (Heilongjiang, Jilin, Xianggang) | Absent, records no longer valid (misidentification) |
| Japan | General | Present, widespread | |
| Hokkaido, Honshu, Kyushu, Shikoku | Present, no details | ||
| Russia | Far East Russia (Kuril Islands) | Present, restricted distribution | |
| Europe | Switzerland | Ticino | Transient, under eradication |
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
Yes, P. japonica is present in the EU (some islands of the Azores (Portugal); Lombardy: Varese and Milano, and Piedmont: Novara (northern Italy)). It is not widely distributed and is under official control within the EU.
P. japonica was accidentally introduced into the Azores (Terceira Island) in the early 1970s (Martins and Simões, 1988; Jackson, 1992) perhaps entering via a US military airbase (Porter and Held, 2002; EPPO Global database, 2018). It has subsequently been recorded from the islands of Faial, Flores, Pico, São Jorge, Corvo and São Miguel (EPPO, 2016b). It is not known to occur in mainland Portugal.
P. japonica was reported in Italy, near Milan in 2014 (EPPO Reporting Service, 2014; Pavesi, 2014). How P. japonica arrived is unknown but two airports are close to the site where adults were initially detected (EPPO, 2016b). Although control measures were taken immediately, the European Commission considered eradication was not feasible given the extent of the infestation and the well‐established population. P. japonica remains under official control in Italy; control measures seek to contain the pest and prevent spread (European Commission, 2016).
Elsewhere in the EU, Belgium declares that P. japonica is absent from its territory on the basis that there are no records of it in the country (EPPO Global database, 2018). Lithuania and Slovenia declare that P. japonica is absent from their territories on the basis of no finds following pest surveys (EPPO Global database, 2018).
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
P. japonica is listed in Council Directive 2000/29/EC. Details are presented in Table 3.
Table 3.
Popillia japonica in Council Directive 2000/29/EC
| Annex I, Part A | Harmful organisms whose introduction into, and spread within, all member states shall be banned |
| Section II | Harmful organisms known to occur in the community and relevant for the entire community |
| (a) | Insects, mites and nematodes, at all stages of their development |
| Species | |
| 8. | Popilia japonica Newman |
3.3.2. Legislation addressing the hosts of Popillia japonica
Popillia japonica hosts prohibited from entering the EU are shown in Table 4.
Table 4.
Regulated hosts and commodities that might provide a pathway for Popillia japonica and which are listed in Annex III of Council Directive 2000/29/EC
| Annex III, Part A | Plants, plant products and other objects the introduction of which shall be prohibited in all Member States | |
| Description | Country of origin | |
| 1. | Plants of […] Larix | Non‐European countries |
| 2. | Plants of Castanea Mill., and Quercus L. with leaves, other than fruit and seeds | Non‐European countries |
| 3. | Plants of Populus L., with leaves, other than fruit and seeds | North American countries |
| 9. | Plants of Chaenomeles Ldl., Cydonia Mill., Crateagus L., Malus Mill., Prunus L., Pyrus L., and Rosa L., intended for planting, other than dormant plants free from leaves, flowers and fruit | Non‐European countries |
| 14. | Soil and growing medium as such, which consists in whole or in part of soil or solid organic substances such as parts of plants, humus including peat or bark, other than that composed entirely of peat | […] Russia […] and third countries not belonging to continental Europe […] |
| 15. | Plants of Vitis L., other than fruits | Third countries other than Switzerland |
| 16. | Plants of Citrus L., […] | Third countries |
| 18. | Plants of Cydonia Mill., Malus Mill., Prunus L. and Pyrus L. and their hybrids, and Fragaria L intended for planting, other than seeds | Without prejudice to the prohibitions applicable to the plants listed in Annex III A (9), where appropriate, non‐European countries, other than […], Canada, the continental states of the USA |
| 19. | Plants of the family Graminacae, other than plants of ornamental perennial grasses of the subfamilies Bambusoideae and Panicoideae and of the genera Buchloe, Bouteloua Lag., Calamagrostis, Cortaderia Stapf., Glyceria R. Br., Hakonechloa Mak. ex Honda, Hystrix, Molinia, Phalaris L., Shibataea, Spartina Schreb., Stipa L. and Uniola L., intended for planting, other than seeds | Third countries, […] |
In this section of previous pest categorisations, special requirements described in Annexes IV and V of 2000/29/EC, necessary for the import of hosts of the pest being categorised, have been highlighted e.g. EFSA PLH Panel (2017). However, P. japonica is a highly polyphagous pest and given the large number of hosts on which it feeds, the large amount of relevant legislation that can be extracted from Annexes IV and V is not repeated here.
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
P. japonica is one of the most polyphagous plant pests (Potter and Held, 2002). In the USA, adult P. japonica can be found feeding on over 300 species in 79 families. Hosts include trees such as Acer, Betula, Fagus, Juglans, Larix, Malus, Populus, Prunus, Quercus, Tilia and Ulmus; shrubs such as Althaea, Hibiscus, Rhododendron, Rosa, Vaccinium and Viburnum; soft fruit crops such as Fragaria, Rubus and Vitis; and field crops such as Asparagus officinalis, Glycine max and Zea mays. Larvae are known to feed on the roots of grasses (e.g. Festuca, Poa, Lolium) and pasture plants, such as Trifolium, and are particularly pests of lawns, golf courses and pastures. They also feed on the roots of vegetables and nursery stock (Metcalf and Metcalf, 1993; EPPO 2016b). Adults are known as defoliators, skeletonising leaves but they can also feed on fruit.
Table 5 lists some of the principle P. japonica hosts. More extensive lists of hosts can be found in Fleming (1972), Ladd (1987, 1989) and CABI (2018).
Table 5.
Major and main plant hosts of Popillia japonica according to EPPO Global database (2018) and CABI (2018) (accessed on 24 July 2018)
| EPPO (major)/CABI (main) justification | Species | Common name | Family |
|---|---|---|---|
| Main | Asparagus officinalis | Asparagus | Liliaceae |
| Major | Fragaria x ananassa | Garden strawberry | Rosaceae |
| Major | Malus domestica | Apple | Rosaceae |
| Major | Prunus domestica | European plum | Rosaceae |
| Major | Prunus persica | Peach | Rosaceae |
| Main | Rheum hybridum | Rhubarb | Polygonaceae |
| Major | Rosa large‐flowered bush hybrids | Hybrid tea roses | Rosaceae |
| Main | Rubus | Blackberry, raspberry | Rosaceae |
| Main | Tilia | Limes | Tiliaceae |
| Major/Main | Zea mays | Maize | Poaceae |
Contradictory classification of hosts: The following plants are regarded by CABI as ‘main hosts’ although the EPPO Global database (2018) classify them as ‘minor hosts’: Acer, Glycine max (soya bean), Malus (ornamental species), Prunus (stone fruit), Rosa (roses), Ulmus (elms), Vitis (grape). Of these, Fleming (1972) reports ‘extensive feeding’ on Acer, Malus, Prunus, Rosa, Ulmus and Vitis suggesting these are favoured or main hosts.
The existing plant health directive does not explicitly list all P. japonica hosts or link P. japonica to specific hosts. However, as a pest listed in Annex I/AII of 2000/29 EC, P. japonica is a pest whose introduction and spread in the EU is banned irrespective of what it is found on. As a pest that spends the majority of its life in the soil, the prohibition of soil from third countries not belonging to continental Europe (See Annex III, point 14) will assist in inhibiting the entry of P. japonica into the EU with host plants for planting not specifically listed in the plant health directive, 2000/20 EC.
3.4.2. Entry
Is the pest able to enter into the EU territory?
Yes, P. japonica has already entered the EU (Azores, Portugal; the regions of Lombardy and Piedmont in the vicinity of Milan, Italy).
Pathways include infested soil and growing media accompanying host plants for planning (i.e. eggs, larvae and pupae); leaves and flowers on plants for planting, cut flowers and cut branches (i.e. adults) and hitch‐hiking adults on aircraft, independent of host plants.
Pathways include:
soil and growing media accompanying host plants for planting containing eggs, larvae and/or pupae,
soil from tools and machinery,
soil containing eggs, larvae and/or pupae,
plants for planting with foliage leaf and flower feeding adults,
cut flowers with flower feeding adults,
adults hitchhiking on aircraft, independent of host plants.
Existing legislation closes the soil pathway.
Appendix A details EU imports of some fruit hosts and plants for planting in general from USA, Canada and Japan.
There are no reports of interceptions of P. japonica in the EUROPHYT interceptions database. In the more recently developed EUROPHYT outbreaks database, there are two records of P. japonica outbreaks. One refers to the outbreak in Italy (EUROPHYT Outbreaks 2016) and the other to the finding in Switzerland (EUROPHYT Outbreaks 2017).
In the UK, adult P. japonica were found in the 1950s at Prestwick in Scotland, in association with military aircraft (Cameron, 1954). Fleming (1972) cites Bourke (1961) as reporting that occasionally large numbers of P. japonica beetles were removed from civil and military aircraft arriving in Europe from the United States (the adults were alive). There was no mention of any plants or plant products in the aircraft.
3.4.3. Establishment
Is the pest able to become established in the EU territory?
Yes, P. japonica has established in the EU in the Azores and in continental Europe in northern Italy (see Section 3.2.2).
3.4.3.1. EU distribution of main host plants
As a pest of grassland and a wide range of other plants (trees, shrubs, vegetable and field crops and wild plants), many hosts are widely available to P. japonica across the EU. More than one‐third of the European agricultural area is grassland (Smit et al., 2008). Figure 3 shows agricultural grassland as a percentage of land cover across the EU at NUTS 2 resolution. Appendix B details EU MS crop area of some hosts (maize, asparagus, berries, strawberries, plum, peaches, apples and grapes).
Figure 3.
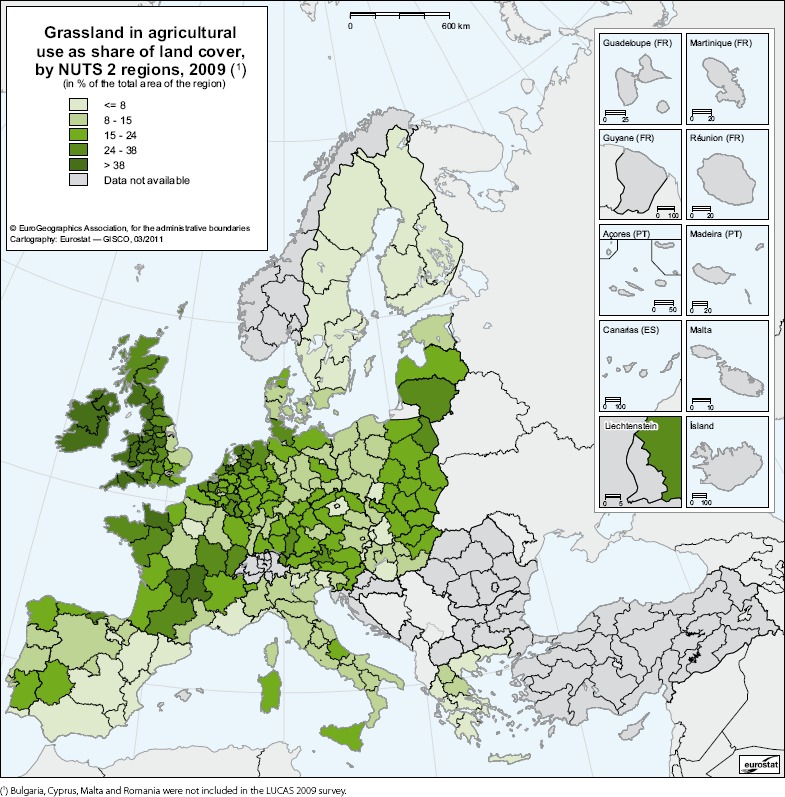
Grassland in agricultural use as share of land cover, by NUTS 2 regions (2009). Source: https://ec.europa.eu/eurostat/statisticsexplained/index.php/Archive:Land_cover_and_land_use_statistics_at_regional_level#Grasslands_maintain_Europe.E2.80.99s_livestock_farming
P. japonica is generally not regarded as a pest of plants grown in protected cultivation. However, Metzger (1933) reported adult P. japonica in glasshouses where roses were growing. It is therefore possible for P. japonica to enter glasshouses and feed on hosts although whether the pest could establish within a glasshouse regime is unknown.
3.4.3.2. Climatic conditions affecting establishment
Soil temperature and soil moisture are key abiotic factors influencing the establishment of P. japonica. Describing its distribution in North America, Fleming (1972) notes P. japonica occurs in regions where mean soil temperature is between 17.5°C and 27.5°C during the summer, and above −9.4°C in the winter. Fleming (1972) also notes that precipitation (which influences soil moisture) should be fairly uniform during the year but at least 250 mm during the summer. Fleming (1972) does not define the summer period. Meteorologically, in the northern hemisphere summer is the period 1 June to 31 August. However, culturally summer could be considered as the beginning during May and ending in September.
In the EU, P. japonica occurs in the area around Milan. Mean precipitation during June, July and August in Milan is 234 mm ( https://www.holiday-weather.com/milan/averages/), a little less than the 250 mm suggested by Fleming (1972). However, as noted above, Fleming (1972) refers only to the summer, and the precise period in which 250 mm precipitation is necessary is not clear. In addition, the effect of the water table and the irrigation on soil moisture in the Po valley could affect establishment in that area.
Describing where P. japonica could establish elsewhere around the world, Bourke (1961; in Fleming, 1972) concluded that the Mediterranean region was unsuitable for the establishment of P. japonica because of the lack of summer rainfall. Establishment in northern Europe was judged less likely because of lower summer temperatures. The most suitable climatic conditions for establishment in Europe were identified to be in central France, southern Germany, and parts of Switzerland, Austria, the Czech Republic, Hungary, Poland, Romania and Slovakia where summer rainfall is abundant and temperature is favourable. However, extensive irrigation in southern Europe could also make some areas there more suitable for the establishment of P. japonica.
In assessing the establishment potential of P. japonica in the UK, Korycinska et al. (2015) reviewed its thermal biology and used data from Régnière et al. (1981) (1,422 degree days (DD) above a threshold of 10°C) to identify where P. japonica could complete its life cycle within 12 months. It was assumed P. japonica could also complete its life cycle in two years where there were 711 DD above 10°C each year. Such information was used by EFSA to create a map (reproduced as Figure 4) showing where in the EU P. japonica could complete its life cycle in one or two years based on temperature accumulation. Figure 4 should be interpreted with care because precipitation and soil moisture is not represented but should be taken into account when considering establishment. Overlaying Figure 4 with precipitation data would more clearly identify where temperature and summer precipitation favour establishment of P. japonica. However, such analysis is beyond the scope of a pest categorisation.
Figure 4.
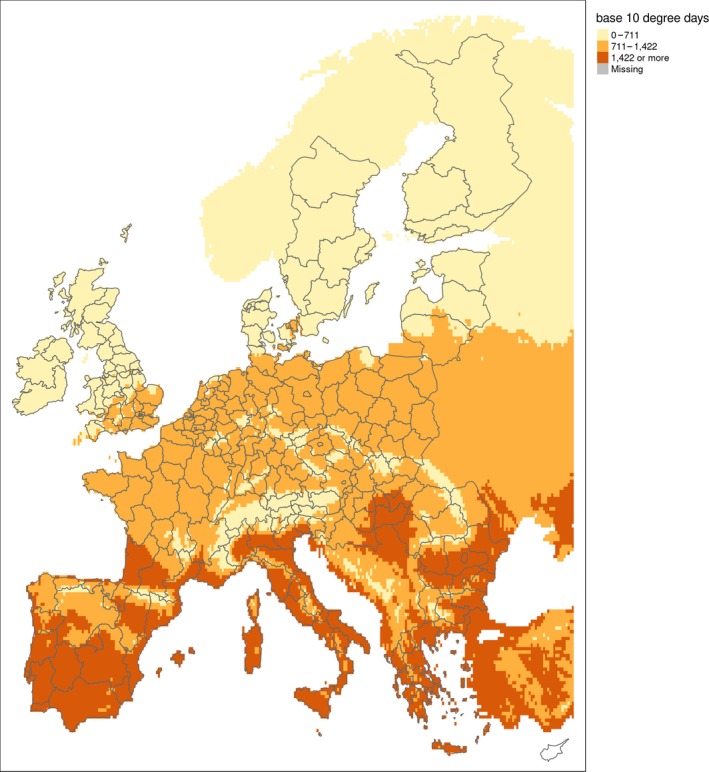
Area where accumulated temperature is suitable for development of P. japonica; darker brown indicates the life cycle can be completed in one year; tan indicates the life cycle will require two years; sandy colour indicates temperature does not favour successful development
3.4.4. Spread
Is the pest able to spread within the EU territory following establishment? How?
Yes, P. japonica can spread following establishment, as seen in the Azores and Italy.
RNQPs: Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects?
Local spread is mainly via natural dispersal of adults. Long distance spread would be facilitated by the movement of eggs, larvae and pupae in soil, with or without plants for planting. However, hitchhiking, for example, where adults are carried in aircraft and are not associated with any plants could also be responsible for long‐distance spread. Hence, plants for planting are not the main means of spread.
Adults can fly and do so on warm, sunny days when temperatures are between 29°C and 35°C (Kreuger and Potter, 2001). However, if disturbed adults will fly at 21°C (Fleming, 1972).
Most adult flights cover short distances (Fleming, 1972). In a mark–release–recapture study in the Azores, Lacey et al. (1994, 1995) found that 70% of recaptured beetles were caught within 50 m of the release point. Less than 1% were recaptured at 1 km. Sara et al. (2013) found adult density decreased significantly with increasing distance from a field edge.
A rate of spread of 16–24 km per year was reported in the decade after P. japonica first established in the USA (EPPO, 2016b). Later, Fox (1932) reported spread varied between 3 and 24 km per year in the USA. Allsopp (1996) estimated P. japonica spread at 7.7 km/year between 1927 and 1938 then at 11.9 km/year from 1939 to 1951. Such spread could have been a mix of natural dispersal and movement assisted by man, such as with plants for planting.
In Italy, the initial demarcated area was significantly increased indicating that P. japonica had spread from the area where first detected (European Commission, 2016).
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes. When adults are abundant they can cause serious injury to tree fruits, soft fruit, vegetable crops, ornamental herbaceous garden plants, shrubs, vines and trees (Campbell, 1989). Larvae are serious pests of grasses, lawns and turf and of vegetables and nursery stock (Metcalf and Metcalf, 1993).
RNQPs: Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? 4
Yes. Larvae can feed on the roots of nursery stock, girdling the roots, severely stunting or killing plants.
Larval feeding on the roots of hosts reduces their vitality and can cause plant mortality (Fleming, 1972). Impacts on pasture and other grassed areas such as golf courses and lawns are reported by many authors, such as Dalthorp et al. (2000) and Hamilton et al. (2007). USDA/APHIS (2015) reported P. japonica was the most widespread turf‐grass pest in the USA. Costs due to larvae were estimated to be US$234 million per year. This consisted of US$78 million for control costs and US$156 million for the replacement of damaged turf and ornamental plants. Potter and Held (2002) note that there is substantial insecticide usage, especially on home lawns, golf courses, and in urban landscapes to manage P. japonica.
Adults can skeletonise the foliage of trees and shrubs, vegetables and weeds and feed on many field crops. Adults can also feed on the surface of deciduous fruits (Metcalf and Metcalf, 1993). Adults can aggregate and feed in large numbers on the fruit of early ripening varieties of apple, peach, nectarine, plum, raspberries and quince making the fruit unmarketable (CABI, 2018). Maize is the field crop most seriously damaged in North America. Adults cut off the maturing silk, preventing pollination resulting in reduced yield (Smith et al., 1997). USDA/APHIS (2015) estimated adult P. japonica causes losses of US$226 million per year.
In Japan, surveys indicate that P. japonica is relatively uncommon and it has never been a major turf pest (Lee et al., 2014), presumably due to it being kept under control by natural enemies and resistant plants. However, as the area used of grassland has grown it has become a more serious pest with damage reported on various crops including peach and cherry (Ando, 1986).
In the Lombardy region of Italy, populations were reported as very low in July 2016 and only in rare cases were larvae found above a density of 50 m−2. No damage was reported (EUROPHYT Outbreaks, 2016). In the Piedmont region, EUROPHYT Outbreaks (2016) reported small damage on plants, except in a mixed grove for the production of nectarines where 95% damage occurred. The nature of the damage is not reported in the Europhyt and so it is difficult to interpret what 95% means.
P. japonica has not caused extensive damage in the Azores (CABI, 2018).
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
Yes, existing measures prohibit the entry of soil and some host plants into the EU as plants for planting (see Section 3.3). Additional measures are also available (see below).
RNQPs: Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated?
Yes, plants for planting could be sourced from pest free areas, or imported dormant and bare rooted and inspected on arrival (see below).
3.6.1. Identification of additional measures
Phytosanitary measures are currently applied to many P. japonica hosts but not in relation specifically to P. japonica. Several key hosts are prohibited from entering the EU as plants for planting (see Section 3.3). As a pest listed in Annex I/AII of 2000/29 EC, P. japonica is a pest whose introduction and spread in the EU is banned irrespective of what it is found on. As a pest that spends the majority of its life in the soil, the prohibition of soil from third countries not belonging to continental Europe assists in inhibiting the entry of P. japonica into the EU both in soil and with all host plants for planting.
3.6.1.1. Additional control measures
Control measures are measures that have a direct effect on pest abundance.
Potential control measures relevant to P. japonica are listed in Table 6. Many of the measures are designed to reduce pest abundance at source and hence would be applied in third countries to reduce the likelihood of entry into the EU. Specific requirements could be for pest free areas for nursery stock or protected cultivation or soil free dormant plants. Specific requirements for pest freedom of consignments of host fruit could also be considered.
Table 6.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) inhibiting pest entry, establishment or spread in relation to those hosts without specific regulation
| Information sheet (with hyperlink to information sheet if available) | Control measure summary | Risk component (entry/establishment/spread/impact) |
|---|---|---|
| http://doi.org/10.5281/zenodo.1175887 | As a pest that is so polyphagous it will be difficult to grow plants outdoors that are isolated from other potential hosts. However, if plants can be grown in physical protection e.g. within a glasshouse then some protection can be provided | Entry (limits infestation at source) |
| Chemical treatments on crops including reproductive material (Work in progress, not yet available) | In the US, insecticides have been applied to the foliage and flowers of susceptible plants to target and manage adult P. japonica (Potter and Held, 2002) |
Entry (reduces population at source) Spread (causes morality within established populations, reducing pressure to spread) |
| http://doi.org/10.5281/zenodo.1175929 | The physical and chemical cleaning and disinfection of facilities, tools, machinery, transport means, facilities and other accessories. Infested soil could carry eggs, larvae and pupae so should be cleaned from tools and machinery. Adults are known to hitchhike and so could be transported, e.g. in packing boxes. Cleaning the packaging (boxes) may help |
Entry (reduces infestation on vectors at source) Spread (reduces infestation on vectors in outbreak area) |
| http://doi.org/10.5281/zenodo.1175956 |
Eggs, larvae and pupae develop in the soil and efforts targeting the soil could be considered In the USA, large amounts of pesticides are applied to grassland to manage P. japonica. (USDA/APHIS, 2015) |
Entry (reduces population at source) Spread (causes morality within established populations, reducing pressure to spread) |
| http://doi.org/10.5281/zenodo.1181442 |
Treatment of the waste (deep burial, composting, incineration, chipping, production of bio‐energy, etc.) in authorised facilities and official restriction on the movement of waste Consignments intercepted with P. japonica should be disposed of appropriately |
Establishment (reduces population of pests that enter) |
| Use of resistant and tolerant plant species/varieties (Work in progress, not yet available) | Field trials and laboratory assays have revealed significant variation in susceptibility to P. japonica amongst Betula spp., Glycine max, Tilia spp. and Ulmus spp. (Potter and Held, 2002) | Entry (limits infestation at source) |
| http://doi.org/10.5281/zenodo.1181717 |
Crop rotation, associations and density, weed/volunteer control are used to prevent problems related to pests and are usually applied in various combinations to make the habitat less favourable for pests Good sanitation is an effective way to reduce populations in nursery fields. Smitley (1996) reported ten times more larvae in weedy fields than in clean fields |
Entry (reduces infestation at source) Spread (reduces population build up, reducing pressure to spread) |
| Biological control and behavioural manipulation (Work in progress, not yet available) |
Other pest control techniques not covered by 1.03 and 1.13 a) biological control b) sterile insect technique c) mating disruption d) mass trapping Entomopathogenic nematodes have potential to control many soil‐dwelling insect pests but have been limited in their usage due to unpredictable performance (Helmberger et al., 2017). Nevertheless, the entomopathogenic nematodes Steinerenema glaseri and Heterorhabditis bacteriophora can be effective in controlling larvae in turf and potted nursery stock but are expensive and have limited shelf life (Potter and Held, 2002). The entomopathogens Metarhizium anisopliae and Beauveria bassiana have provided inconsistent control over P. japonica (Potter and Held, 2002) Mass trapping using lures can be used to reduce numbers in isolated populations (Wawrzynski and Ascerno, 1998) |
Establishment and Spread (use of mass trapping in isolated populations reduces population build up, reducing pressure to spread) There are several known predators and pathogens of P. japonica, a few of which are commercially available. However, none are consistently effective (Potter and Held, 2002). |
| Post‐entry quarantine and other restrictions of movement in the importing country (Work in progress, not yet available) |
This information sheet covers post‐entry quarantine of relevant commodities; temporal, spatial and end‐use restrictions in the importing country for import of relevant commodities; Prohibition of import of relevant commodities into the domestic country. Relevant commodities are plants, plant parts and other materials that may carry pests, either as infection, infestation, or contamination |
This measure is appropriate for pests infesting plants for planting that are difficult to detect. Given that P. japonica larvae and pupae develop in the soil and adults are detectable upon emergence, this measure could be considered |
3.6.1.2. Additional supporting measures
Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance.
Table 7.
Selected additional supporting measures (a full list is available in EFSA PLH Panel, 2018) inhibiting pest entry, establishment or spread in relation to those hosts without specific regulation
| Information sheet (with hyperlink to information sheet if available) | Supporting measure summary | Risk component (entry/establishment/spread/impact) |
|---|---|---|
| http://doi.org/10.5281/zenodo.1181430 |
Imported host plants for planting and fruit could be inspected for compliance from freedom of P. japonica Traps with lures are used in USA (e.g. California) to aid with early detection of incursions (Potter and Held, 2002) |
Entry |
| Sampling (Work in progress, not yet available) | According to ISPM 31, it is usually not feasible to inspect entire consignments, so phytosanitary inspection is performed mainly on samples obtained from a consignment | Entry |
| Phytosanitary certificate and plant passport (Work in progress, not yet available) | An official paper document or its official electronic equivalent, consistent with the model certificates of the IPPC, attesting that a consignment meets phytosanitary import requirements (ISPM 5) | Entry |
| http://doi.org/10.5281/zenodo.1180845 | Mandatory/voluntary certification/approval of premises is a process including a set of procedures and of actions implemented by producers, conditioners and traders contributing to ensure the phytosanitary compliance of consignments. It can be a part of a larger system maintained by a National Plant Protection Organization in order to guarantee the fulfilment of plant health requirements of plants and plant products intended for trade | Entry |
| Certification of reproductive material (voluntary/official) (Work in progress, not yet available) | Reproductive material could be examined and certified free from P. japonica. However, certification of reproductive material is usually applied with respect to plant pathogens and nematodes | Entry |
| http://doi.org/10.5281/zenodo.1180597 |
In third countries: Sourcing plants from a pest free place of production, site or area, surrounded by a buffer zone, would minimise the probability of spread into the pest free zone In the EU: delimiting a buffer zone around an infested area |
Entry Spread |
| Surveillance (Work in progress, not yet available) | ISPM 5 defines surveillance as an official process which collects and records data on pest occurrence or absence by survey, monitoring or other procedures | Spread (from infested areas of the EU) |
3.6.1.3. Biological or technical factors limiting the effectiveness of measures to prevent the entry, establishment and spread of the pest
Eggs, larvae and pupae develop underground/in soil and are difficult to detect.
Adults can disperse by flight.
The pest feeds on many plants.
Hosts are widely available throughout the EU.
3.6.1.4. Biological or technical factors limiting the ability to prevent the presence of the pest on plants for planting
Adults can disperse by flight.
The pest feeds on many plants.
Adults are attracted to herbivore induced plant volatiles (HIPVs), resulting in aggregation on damaged plants.
Hosts are widely available throughout the EU.
3.7. Uncertainty
The pathway for introduction into the EU is unknown; hitch hiking is suspected but is uncertain.
Noting the impact of the pest in the USA, there is uncertainty as to why relatively little impact has been reported from the areas where P. japonica occurs in the EU. For example, there are no reports of extensive damage from the islands of the Azores although the pest has been present on them for many years (CABI, 2018). The European Commission update of the outbreak in Lombardy noted that no damage was reported (EUROPHYT Outbreaks, 2016). Lack of damage may be because of the rather dry summer conditions, and the subsequent impact of drier soils on larvae.
The threshold of precipitation required for establishment is unclear (at least 250 mm in the ‘summer’).
Whether plants for planting are the principle means of spread is uncertain.
4. Conclusions
Popillia japonica meets the criteria assessed by EFSA for consideration as a Union quarantine pest (Table 8).
Table 8.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | Popillia japonica Newman, 1841 is an established insect species in the order Coleoptera (beetles), family Scarabaeidae (scarab beetles) | Popillia japonica Newman, 1841 is an established insect species in the order Coleoptera (beetles), family Scarabaeidae (scarab beetles) | No uncertainty |
| Absence/presence of the pest in the EU territory (Section 3.2 ) | P. japonica is present in the EU (some islands of the Azores, Portugal; Lombardy and Piedmont, northern Italy). It is not widely distributed within the EU | P. japonica is present in the EU (some islands of the Azores, Portugal; Lombardy and Piedmont, northern Italy). It is not widely distributed within the EU | No uncertainty |
| Regulatory status (Section 3.3 ) | Popillia japonica is listed in Annex I A II of Council Directive 2000/29/EC as a harmful organism known to occur in the community and whose introduction into, and spread within, all member states is banned. P. japonica is under official control in Portugal and Italy | Popillia japonica is listed in Annex I A II of Council Directive 2000/29/EC as a harmful organism known to occur in the community and whose introduction into, and spread within, all member states is banned. P. japonica is under official control in Portugal and Italy. As a quarantine pest, it cannot also be a regulated non‐quarantine pest | No uncertainty |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | P. japonica has entered the EU already and has established in an area of northern Italy and in the Azores (Portugal). Pathways for further introductions include infested soil accompanying host plants for planning and as hitch hikers on aircraft | Local spread is mainly via natural dispersal of adults. Long distance spread would be facilitated by the movement of plants for planting. However, adult P. japonica are suspected to hitchhike without host plants hence plants for planting have not been proven to be the main means of spread |
The pathway for introduction into the EU is unknown, hitch hiking is suspected but is uncertain Regarding establishment, the level of necessary precipitation and soil moisture is unknown |
| Potential for consequences in the EU territory (Section 3.5 ) | In North America, when adults are abundant they can cause serious injury to tree fruits, soft fruit, vegetable crops, ornamental herbaceous garden plants, shrubs, vines and trees. Larvae are serious pests of grasses, lawns and turf and of vegetables and nursery stock. However, in the EU no damage has been reported in Lombardy; some damage has been reported from Piedmont particularly to nectarines; extensive damage has not been reported from the Azores | Adults feeding on foliage and larvae damaging roots would cause impacts on plants for planting | Why no more damage has been reported in Italy and the Azores is uncertain (perhaps due to dry summer?) |
| Available measures (Section 3.6 ) | Phytosanitary measures are available to inhibit the likelihood of entry into the EU e.g. source plants for planting from pest free areas. Measures are also available to inhibit spread from areas of the EU where the pest already occurs (e.g. control of movement of soil with plants for planting) | Phytosanitary measures are available to prevent the presence of the pest on plants for planting | Whether plants for planting are the principle means of spread is uncertain |
| Conclusion on pest categorisation (Section 4 ) | Popillia japonica satisfies all of the criteria assessed by EFSA to satisfy the definition of a Union quarantine pest | Popillia japonica does not meet the criteria of (a) plants for planting being the principal means of spread. Hence it does not satisfy all of the criteria that are within the remit of EFSA to assess for it to be regarded as a Union RNQP | No uncertainty |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | Future assessments could investigate why there is not greater impacts reported in the Azores and Lombardy | ||
Abbreviations
- DD
degree days
- DG SANTÉ
Directorate General for Health and Food Safety
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- HIPV
herbivore induced plant volatile
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- PZ
protected zone
- RNQP
regulated non‐quarantine pest
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Glossary
(terms are as defined in ISPM 5 unless indicated by +)
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 1995, 2017)
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 1995, 2017)
- Control measures+
Measures that have a direct effect on pest abundance.
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2017)
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2017)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2017)
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2017)
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2017)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2017)
- Protected zones (PZ)
A protected zone is an area recognised at EU level to be free from a harmful organism, which is established in one or more other parts of the Union
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2017)
- Regulated non‐quarantine pest (RNQP)
A non‐quarantine pest whose presence in plants for planting affects the intended use of those plants with an economically unacceptable impact and which is therefore regulated within the territory of the importing contracting party (FAO, 2017)
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2017)
- Supporting measures+
Organisational measures or procedures supporting the choice of appropriate Risk Reduction Options that do not directly affect pest abundance
Appendix A – EU imports of hosts (100 of kg)
1.
| Canada | Japan | USA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Product (CN code) | 2013 | 2014 | 2015 | 2016 | 2017 | 2013 | 2014 | 2015 | 2016 | 2017 | 2013 | 2014 | 2015 | 2016 | 2017 |
| Maize or corn (01005) | 2,862,919 | 14,175,780 | 2,329,571 | 9,051,676 | 8,216,216 | 9 | 4 | 1 | 1 | 42 | 988,331 | 10,174,620 | 4,042,839 | 6,492,303 | 8,385,418 |
| Fresh apples (0808 10) | 1,250 | 1,979 | 2,450 | 2,355 | 1,377 | 2 | 2 | 2 | 8 | 123 | 120,811 | 90,049 | 62,117 | 42,907 | 24,264 |
| Fresh berries a (0809 40) | 6,336 | 7,649 | 5,262 | 3,950 | 2,420 | 0 | 2 | 5 | 2 | 84 | 62,318 | 68,722 | 39,473 | 46,137 | 24,688 |
| Fresh plums and sloes (0809 40) | 56 | 11 | 0 | 37 | 144 | 118 | 10 | 130 | |||||||
| Roses, whether or not grafted (0602 40) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 20 | 11 | 10 | 31 | 6 | 5 |
| Live plants b (0602 90) | 42 | 146 | 10 | 9 | 58 | 10,964 | 8,594 | 6,169 | 13,383 | 11,451 | 32,078 | 31,316 | 33,723 | 34,508 | 27,514 |
Fresh berries – strawberries, raspberries, blackberries, back, white or red currants, gooseberries and other edible fruits (excluding nuts, bananas, dates, figs, pineapples, avocados, guavas, mangoes, mangosteens, papaws ‘papayas’, citrus fruit, grapes, melons, apples, pears, quinces, apricots, cherries, peaches, plums and sloes).
Live plants – including their roots – and mushroom spawn (excluding bulbs, tubers, tuberous roots, corms, crowns and rhizomes, including chicory plants and roots, unrooted cuttings and slips, fruit and nut trees, rhododendrons, azaleas and roses).
Appendix B – EU member state production area (cultivation/harvested/production in 1,000 ha) of some Popillia japonica hosts (accessed 21/9/2018)
1.
| Category of the host | Eurostat code | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|---|
| Grain maize and corn‐cob mix | C1500 | 9,774.71 | 9,610.16 | 9,255.94 | 8,563.11 | 8,376.20 |
| Green maize | G3000 | 6,036.69 | 6,147.80 | 6,262.31 | 6,252.89 | 6,134.06 |
| Asparagus | V2600 | 47.81 | 52.04 | 53.90 | 58.54 | : |
| Strawberries | S0000 | 97.17 | 109.48 | 107.57 | 108.76 | : |
| Berries (excluding strawberries) | F3000 | : | : | : | 144.73 | : |
| Apples | F1110 | 536.77 | 524.50 | 538.50 | 523.70 | 523.61 |
| Plum | F1250 | 162.01 | 157.36 | 154.79 | 152.73 | : |
| Peaches | F1210 | 163.87 | : | 157.81 | 156.38 | 154.21 |
| Grapes | W1000 | : | : | 3,167.97 | 3,141.30 | : |
‘:’ data not available.
Suggested citation: EFSA Plant Health Panel (EFSA PLH Panel) , Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Czwienczek E and MacLeod A, 2018. Scientific Opinion on the pest categorisation of Popillia japonica . EFSA Journal 2018;16(11):5438, 30 pp. 10.2903/j.efsa.2018.5438
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00489
Panel members: Claude Bragard, Katharina Dehnen‐Schmutz, Francesco Di Serio, Paolo Gonthier, Marie‐Agnès Jacques, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A. Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe Lucien Reignault, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà.
Acknowledgements: The PLH Panel wishes to thank the following for the support provided to this scientific output: Carsten Behring for providing map (Figure 4).
Adopted: 27 September 2018
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © EPPO; Figure 3: Eurostat / EuroGeographics Association
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
See Section 2.1 on what falls outside EFSA's remit.
References
- Allsopp PG, 1996. Japanese beetle, Popillia japonica Newman (Coleoptera: Scarabaeidae): rate of movement and potential distribution of an immigrant species. The Coleopterists Bulletin, 50, 81–95. [Google Scholar]
- Ando Y, 1986. Seasonal prevalence and outbreaks of the Japanese beetle, Popillia japonica Newman (Coleoptera: Scarabaeidae). Japanese Journal of Applied Entomology and Zoology, 30, 111–116 (In Japanese). [Google Scholar]
- Bosik JJ, 1997. Common names of insects and related organisms. Committee on common names of insects. Entomological Society of America, 232 pp. [Google Scholar]
- Bourke PMA. 1961. Climatic aspects of the possible establishment of the Japanese beetle in Europe. Technical Note 41, World Meteorological Organization, Geneva, 9 pp.
- CABI , 2018. Popillia japonica (Japanese beetle). CABI Invasive Species Compendium. Datasheet last modified 14 July 2018. Available online: https://www.cabi.org/isc/datasheetreport?dsid=43599 [Accessed: 30 July 2018]
- Cameron WPL, 1954. Japanese beetle found in aircraft at Prestwick, Ayrshire. Plant Pathology, 3, 34–34. [Google Scholar]
- Campbell JM, Sarazin MJ and Lyons B. 1989, Canadian beetles (Coleoptera) injurious to crops, ornamentals, stored products, and buildings, 491 pp.
- Chen RZ, Klein MG, Li QY and Li Y, 2014. Mass trapping Popillia quadriguttata using Popillia japonica (Coleoptera: Scarabaeidae) pheromone and floral lures in North eastern China. Environmental Entomology, 43, 774–781. [DOI] [PubMed] [Google Scholar]
- Clausen C, King J and Teranishi C, 1927. The parasites of Popillia japonica in Japan and Chosen (Korea) and their introduction into the United States. United States Department of Agriculture Bulletin, 1429, 1–55. [Google Scholar]
- Dalthorp D, Nyrop J and Villani MG, 2000. Spatial ecology of the Japanese beetle, Popillia japonica . Entomologia Experimentalis et Applicata, 96, 129–139. [Google Scholar]
- Dickerson EL and Weiss HB, 1918. Popilia japonica Newm., a recently introduced Japanese pest. Canadian Entomologist, 50, 217–221. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2017. Scientific Opinion on the pest categorisation of Anthonomus signatus . EFSA Journal 2017;15 (7):4882, 22 pp. 10.2903/j.efsa.2017.4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gr_egoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Hart A, Schans J, Schrader G, Suffert M, Kert_esz V, Kozelska S, Mannino MR, Mosbach‐Schulz O, Pautasso M, Stancanelli G, Tramontini S, Vos S and Gilioli G, 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO , 2014. First report of Popillia japonica in Italy. EPPO Reporting Service 2014 (10):179. Available online: https://gd.eppo.int/reporting/article-3272 [Accessed: 24 August 2018]
- EPPO , 2016a. Diagnostics Popillia japonica, PM 7/74 (1). EPPO Bulletin, 36, 447–450. [Google Scholar]
- EPPO , 2016b. PM 9/21(1) Popillia japonica: procedures for official control. EPPO Bulletin, 46, 543–555. [Google Scholar]
- EPPO Global database , 2018. (European and Mediterranean Plant Protection Organization) online. Available online: https://gd.eppo.int/ [Accessed: 24 July 2018]
- EPPO Reporting Service , 2017. First report of Popillia japonica in Switzerland. EPPO Reporting Service, 2017, 160. [Google Scholar]
- European Commission , 2016. Final report of an audit carried out in Italy from 12 September 2016 to 23 September 2016 in order to evaluate the situation and control of Japanese beetle (Popillia Japonica) European Commission DG (SANTE) 2016‐8795 – MR.
- EUROPHYT Outbreaks , 2016. European Commission Notification of the presence of a harmful organism to the Commission and to other member states. Update No. 2. Outbreak No. 574.
- EUROPHYT Outbreaks , 2017. European Commission Notification of the presence of a harmful organism to the Commission and to other member states. Update No. 1. Outbreak No. 243.
- FAO (Food and Agriculture Organization of the United Nations), 1995. ISPM (International standards for phytosanitary measures) No 4. Requirements for the establishment of pest free areas. Available online: https://www.ippc.int/en/publications/614/
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents/1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2017. ISPM (International Standards for Phytosanitary Measures) 5 —Glossary of phytosanitary terms. FAO, Rome, 34 pp. Available online: http://www.fao.org/fileadmin/user_upload/faoterm/PDF/ISPM_05_2016_En_2017-05-25_PostCPM12_InkAm.pdf
- Fleming WE, 1972. Biology of the Japanese beetle. USDA Technical Bulletin 1449, Washington, DC. Available online: https://naldc.nal.usda.gov/download/CAT87201410/PDF [Accessed: 30 July 2018]
- Fox H, 1932. The known distribution of the Japanese beetle in 1930 and 1931, with special reference to the area of continuous infestation. Journal of Economic Entomology, 25, 396–407. [Google Scholar]
- Hamilton RM, Foster RE, Gibb TJ, Sadof CS, Holland JD and Engel BA, 2007. Distribution and dynamics of Japanese beetles along the Indianapolis airport perimeter and the influence of land use on trap catch. Environmental Entomology, 36, 287–296. [DOI] [PubMed] [Google Scholar]
- Helmberger MS, Shields EJ and Wickings KG, 2017. Ecology of belowground biological control: entomopathogenic nematode interactions with soil biota. Applied Soil Ecology, 121, 201–213. [Google Scholar]
- Jackson TA, 1992. Scarabs–pests of the past or the future? In: Jackson TA. and Glare TR. (eds.). Use of Pathogensin Scarab Pest Management. Intercept Ltd., Andover, Hampshire, UK: pp.1–10. [Google Scholar]
- King JL, 1931. The present status of the established parasites of Popillia japonica Newman. Journal of Economic Entomology, 24, 453–462. [Google Scholar]
- Korycinska A, Baker RHA and Eyre D, 2015. Rapid Pest Risk Analysis (PRA) for: Popillia japonica. Defra, 34 pp. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/downloadExternalPra.cfm?id=4106 [Accessed: 22 July 2018]
- Kreuger B and Potter DA, 2001. Diel feeding activity and thermoregulation by Japanese beetles (Coleoptera: Scarabaeidae) within host plant canopies. Environmental Entomology, 30, 172–180. [Google Scholar]
- Lacey LA, Amaral JJ, Coupland J and Klein MG, 1994. The influence of climatic factors on the flight activity of the Japanese beetle (Coleoptera: Scarabaeidae): implications for use of a microbial control agent. Biological Control, 4, 298–303. [Google Scholar]
- Lacey LA, Amaral JJ, Coupland J, Klein MG and Simoes AM, 1995. Flight activity of Popillia japonica (Coleoptera: Scarabaeidae) after treatment with Metarhizium anisopliae . Biological Control, 5, 167–172. [Google Scholar]
- Ladd TL Jr, 1987. Japanese beetle (Coleoptera: Scarabaeidae): influence of favored food plants on feeding response. Journal of Economic Entomology, 80, 1014–1017. [Google Scholar]
- Ladd TL Jr, 1989. Japanese beetle (Coleoptera: Scarabaeidae): feeding by adults on minor host and nonhost plants. Journal of Economic Entomology, 82, 1616–1619. [Google Scholar]
- Ladd TL, Klein MG and Tumlinson JH, 1981. Phenethyl propionate: eugenol: geraniol (3:7:3) and Japonilure: a highly effective joint lure for Japanese beetles. Journal of Economic Entomology, 74, 665–667. [Google Scholar]
- Lee DW, Smitley DR, Lee SM, Kaya HK, Park CC and Choo HY, 2014. Seasonal phenology and diurnal activity of Promachus yesonicus (Diptera: Asilidae), a predator of scarabs, on Korean golf courses. Journal of Asia‐Pacific Entomology, 17, 169–174. [Google Scholar]
- Martins A and Simões N, 1988. Suppression of the Japanese beetle in the Azores: an ecological approach. Ecological Bulletins, 39, 99–100. [Google Scholar]
- Metcalf RL and Metcalf RA, 1993. Destructive and useful insects: their habits and control. 5th Edition, McGraw‐Hill, New York. [Google Scholar]
- Metzger FW, 1933. Preliminary report on controlling the winter emergence of the Japanese beetle in rose greenhouses by application of chemicals to the soil. Journal of Economic Entomology, 26, 205–210. [Google Scholar]
- Pavesi M, 2014. Popillia japonica specie aliena invasiva segnalata in Lombardia. L'Informatore Agrario no. 32, 53–55.
- Potter DA and Held DW, 2002. Biology and management of the Japanese beetle. Annual Review of Entomology, 47, 175–205. [DOI] [PubMed] [Google Scholar]
- Régnière J, Rabb RL and Stinner RE, 1981. Popillia japonica: simulation of temperature‐dependent development of the immatures, and prediction of adult emergence. Environmental Entomology, 10, 290–296. [Google Scholar]
- Sara SA, McCallen EB and Switzer PV, 2013. The spatial distribution of the Japanese Beetle, Popillia japonica, in soybean fields. Journal of Insect Science, 13, 36 10.1673/031.013.3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit HJ, Metzger MJ and Ewert F, 2008. Spatial distribution of grassland productivity and land use in Europe. Agricultural Systems, 98, 208–219. [Google Scholar]
- Smith IM, McNamara DG, Scott PR and Holderness M (Eds), 1997. Popillia japonica In: Quarantine Pests for Europe. 2nd Edition, CABI/EPPO, Wallingford, 1425 pp. [Google Scholar]
- Smitley DR, 1996. Incidence of Popillia japonica (Coleoptera: Scarabaeidae) and other scarab larvae in nursery fields. Journal of Economic Entomology, 89, 1262–1266. [Google Scholar]
- USDA/APHIS (United States Department of Agriculture, Animal and Plant Health Inspection Service) , 2015. Managing the Japanese Beetle: A Homeowner's Handbook. Program Aid 1599. 16 pp.
- Vittum PJ, 1986. Biology of the Japanese beetle (Coleoptera: Scarabaeidae) in eastern Massachusetts. Journal of Economic Entomology, 79, 387–391. [Google Scholar]
- Wawrzynski R and Ascerno ME, 1998. Mass trapping for Japanese beetle (Coleoptera: Scarabaeidae) suppression in isolated areas. Journal of Arboriculture, 24, 303–307. [Google Scholar]


