Abstract
The EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) provides a scientific opinion on the refined exposure assessment of extracts of rosemary (E 392) when used as a food additive. Extracts of rosemary (E 392) was evaluated by the AFC Panel in 2008. Following this EFSA evaluation, extracts of rosemary (E 392) was authorised for use as a food additive in the EU in several food categories with maximum levels. In 2015, the ANS Panel provided a scientific opinion on the safety of the proposed extensions of use for extracts of rosemary (E 392) in fat‐based spreads. In 2016, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) has evaluated this food additive and established a temporary acceptable daily intake (ADI) of 0–0.3 mg/kg body weight (bw) for rosemary extract, expressed as carnosic acid plus carnosol. Based on the data provided by food industry, the Panel was able to refine the exposure estimates of extracts of rosemary (E 392). The highest mean refined exposure estimate (non‐brand loyal scenario) was 0.09 mg/kg bw per day in children (3–9 years) and the highest 95th percentile of exposure was 0.20 mg/kg bw per day in children. Taking uncertainties into account, the Panel concluded that these exposure estimates very likely overestimate the real exposure to extracts of rosemary (E 392) from its use as a food additive according to Annex II. Margins of safety were estimated for children and adults using the refined exposure estimate; these are higher than the ones calculated in 2015. Intake of carnosic acid and carnosol from natural diet (herbs) was estimated. It was maximally 1.66 mg/kg bw per day (p95).
Keywords: extracts of rosemary, E 392, refined exposure assessment
Summary
Following a request from the European Commission, the EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) performed a refined exposure assessment of extracts of rosemary (E 392) when used as a food additive. The Panel was not provided with a newly submitted dossier and based this assessment on concentration data available following a public call for data.
Extracts of rosemary (E 392) was evaluated in 2008 for its safety, by the EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (EFSA AFC Panel, 2008), for its use as a food additive. Following this EFSA evaluation, extracts of rosemary (E 392) was authorised for use as a food additive in the European Union (EU) according to Annexes II and III to Regulation (EC) No 1333/2008. The AFC Panel estimated that the toxicological data on the rosemary extracts are insufficient to establish an acceptable daily intake (ADI) but that the existing data, including the absence of effects in the 90‐day studies on reproductive organs and negative genotoxicity data, did not give reason for concern. In 2015, the EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) provided a scientific opinion on the safety of the proposed extensions of use for extracts of rosemary (E 392) in fat‐based spreads at 30 mg/kg and 100 mg/kg. In 2016, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) has evaluated this food additive and concluded that there are sufficient data to establish an ADI for rosemary extract prepared according to the specifications established at this meeting. Thus, JECFA established a temporary ADI of 0–0.3 mg/kg body weight (bw) for rosemary extract, expressed as carnosic acid plus carnosol.
In 2017, the European Food Safety Authority (EFSA) launched a public call for data aiming at collecting reported use levels from industry or analytical data on several food additives, including extracts of rosemary (E 392). Use levels were reported by industry. Added to these new data, information on the presence of food additives on the label of foods was retrieved from the Mintel's Global New Products Database (GNPD), an online database monitoring new introductions of packaged goods in the market worldwide. Consumption data were available through the EFSA Comprehensive Database.
Dietary exposure to extracts of rosemary (E 392) from its use as a food additive according to Annex II was calculated for different exposure scenarios based on the provided use levels. If actual practice changes, this refined estimates may no longer be representative and should be updated. The Panel also noted that the exposure to extracts of rosemary (E 392) from its use according the Annex III (Part 2, 4, 5A) was not considered in the exposure assessment.
Extracts of rosemary (E 392) is authorised in 33 food categories of which none was identified as a food category to which consumers may be brand loyal. Therefore, the Panel selected the refined non‐brand‐loyal scenario as the most relevant exposure scenario for the safety evaluation of this food additive.
Food subcategories from the Mintel's GNPD included in the exposure assessment represented approximately 83% of the food products labelled with extracts of rosemary (E 392) in the database. In all exposure scenarios, it was assumed that 100% of food products contained extracts of rosemary (E 392), whereas information from the Mintel's GNPD showed that the additive was used in only a small percentage of food products.
Based on the data provided by food industry, the Panel was able to refine the exposure estimates of extracts of rosemary (E 392). The highest mean refined exposure estimate (non‐brand loyal scenario) was 0.09 mg/kg bw per day in children (3–9 years) and the highest 95th percentile of exposure was 0.20 mg/kg bw per day in children. Taking uncertainties into account, the Panel concluded that these exposure estimates very likely overestimate the real exposure to extracts of rosemary (E 392) from its use as a food additive according to Annex II.
A range of margins of safety (MOS) values was calculated by the Panel by dividing the lowest value of the range of NOAELs of 20–60 mg carnosol plus carnosic acid/kg bw per day identified by the AFC Panel (EFSA AFC Panel, 2008) by the highest p95 exposure level in each population and the highest value of the range of NOAELs by the lowest p95 exposure level. Using this approach the range of MOS was 100–2,000 and 200–3,000 for children and adults, respectively. These new MOS estimates are higher than the ones calculated in 2015 (25–240 for children and 60–600 for adults) using an maximum permitted limit (MPL) scenario.
Intake of carnosic acid and carnosol from natural diet was estimated at the maximum up to 1.66 mg/kg bw per day (p95) in toddlers.
1. Introduction
The present opinion deals with the refined exposure estimation of extracts of rosemary (E 392) when used as a food additive.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
Regulation (EC) No 1333/20081 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union. In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the European Union before 20 January 2009 has been set up under the Regulation (EU) No 257/2010.2 This Regulation also foresees that food additives are re‐evaluated whenever necessary in the light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU3 of 2001. The report “Food additives in Europe 20004” submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010, the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference
The Commission asks the European Food Safety Authority to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.1.3. Interpretation of terms of Reference
In 2013, EFSA received a communication from European Commission suggesting to limit this evaluation to a refined exposure assessment of extracts of rosemary (E 392) by 2018 instead of a full re‐evaluation.5
Therefore, this opinion provides only a refined exposure assessment.
1.2. Information on existing authorisations and evaluations
Extracts of rosemary (E 392) is derived from Rosmarinus officinalis L. and contains several compounds which have been proven to exert antioxidative functions. These compounds belong mainly to the classes of phenolic acids, flavonoids, diterpenoids (carnosol and carnosic acid) and triterpenes.
Extracts of rosemary (E 392) was evaluated in 2008 for its safety, by the EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) Panel (EFSA AFC Panel, 2008), for its use as a food additive. Following this EFSA evaluation, extracts of rosemary (E 392) was authorised for use as a food additive in the European Union (EU) in several food categories with maximum levels, in accordance with Annexes II and III to Regulation (EC) No 1333/2008 on food additives. The AFC Panel estimated that the toxicological data on the rosemary extracts are insufficient to establish an acceptable daily intake (ADI), because the toxicity data set did not provide reproductive and developmental toxicity studies or a long‐term study. On the other hand, the existing data, including the absence of effects in the 90‐day studies on reproductive organs and negative genotoxicity data, did not give reason for concern.
Following a request by the European Commission in 2014, EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) provided a scientific opinion on the safety of the proposed extensions of use for extracts of rosemary (E 392) in fat‐based spreads at 30 mg/kg and 100 mg/kg in 2015 (EFSA ANS Panel, 2015). In this opinion, only the scenarios based on the maximum permitted levels (MPLs) at that time and on the MPLs and proposed new use levels at that time were performed; no refined exposure scenario was done. The Panel concluded that these two additional extensions of use for extracts of rosemary (E 392) would not change the estimated exposure to the food additive, compared with the exposure based on the already approved permitted uses, in any part of the population. The Panel also considered that the conclusions of the EFSA AFC Panel in 2008 on the safety of rosemary extracts (E 392) would remain valid and that there was no need to reconsider the available toxicological assessment to address the Terms of Reference. Thus, the Panel considered at that time that it was unlikely that there was a safety concern with the already permitted uses together with the additional proposed extension of uses compared with the already permitted uses alone. Overall, the Panel noted that the use of wider food consumption surveys led to a lower Margin of Safety (MOS) with the upper end of the range at a level similar to the MOS previously identified and used in the EFSA 2008 opinion for the safety assessment of the uses of rosemary extracts as food additives. The Panel acknowledged that there are also limitations to the available toxicity database; however, the current no observed adverse effect levels (NOAELs) were the highest doses tested in sub‐chronic studies.
The EFSA ANS Panel noted that since the publication of this scientific opinion on the extension of use for extracts of rosemary (E 392) in 2015, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) has evaluated this food additive (JECFA, 2016). This Committee concluded that there are sufficient data to establish an ADI for rosemary extract prepared according to the specifications established at this meeting. JECFA established a temporary ADI of 0–0.3 mg/kg bw for rosemary extract, expressed as carnosic acid plus carnosol, on the basis of a NOAEL of 64 mg/kg bw per day, expressed as carnosic acid plus carnosol, the highest dose tested in a short‐term toxicity study in rats, with the application of a 200‐fold uncertainty factor. The ADI was made temporary pending the submission of studies to elucidate the potential developmental and reproductive toxicity. The ANS Panel consider that the toxicological data should also be reviewed by EFSA when available.
Rosemary extracts have been registered under the REACH Regulation No 1907/20066 (ECHA, online), including consumer uses.
Rosemary extracts are permitted as an antimicrobial, refreshing and tonic in cosmetic products (European Commission database‐CosIng7). Extracts of rosemary is included in the European Union Register8 of feed additives (Regulation (EC) No 1831/20039).
Specific purity criteria for extracts of rosemary (E 392) have been defined in the Commission Regulation (EU) No 231/2012.10
2. Data and methodologies
Data
The ANS Panel was not provided with a newly submitted dossier. EFSA launched public call for data.11
The Panel based its dietary refined exposure assessment of extracts of rosemary (E 392) on information submitted to EFSA following the public call for data.
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database12) was used to estimate the dietary exposure.
The Mintel's Global New Products Database (GNPD) is an online resource listing food products and compulsory ingredient information that should be included in labelling. This database was used to verify the use of extracts of rosemary (E 392) in food products.
Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
The ANS Panel assessed the dietary refined exposure to extracts of rosemary (E 392) as a food additive in line with the principles laid down in Regulation (EU) 257/2010 and in the EFSA Statement on the approach followed for the refined exposure assessment as part of the safety assessment of food additives under re‐evaluation (EFSA ANS Panel, 2017).
Dietary exposure to extracts of rosemary (E 392) from its use as a food additive was estimated combining food consumption data available within the EFSA Comprehensive European Food Consumption Database with the maximum levels according to Annex II to Regulation (EC) No 1333/200813 and reported use levels by industry submitted to EFSA following a call for data. Different scenarios were used to calculate exposure (see Section 3.4.1). Uncertainties in the exposure assessment were identified and discussed.
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
According to Commission Regulation (EU) No 231/201214, extracts of rosemary (E 392) is a food additive identified as:
Chemical name: Rosemary extracts (Rosmarinus officinalis)
Synonyms: Extract of rosemary leaf (antioxidant)
In JECFA (2016) tentative specifications, the chemical name is:
Carnosic acid: 4a(2H)‐Phenanthrenecarboxylic acid, 1,3,4,9,10,10a‐hexahydro‐5,6‐dihydroxy‐1,1‐dimethyl‐7‐(1‐methylethyl)‐, (4aR‐trans)‐
Carnosol: 2H‐9,4a‐(Epoxymethano)phenanthren‐12‐one, 1,3,4,9,10,10a‐hexahydro‐5,6‐dihydroxy‐1,1‐dimethyl‐7(1‐ methylethyl), (4aR(4aα,9α,10aβ))‐
Chemical Formula: Carnosic acid: C20H28O4; Carnosol: C20H26O4
Molecular weight: Carnosic acid: 332.43/Carnosol: 330.42
Structural Formula: as described in Figure 1
Figure 1.
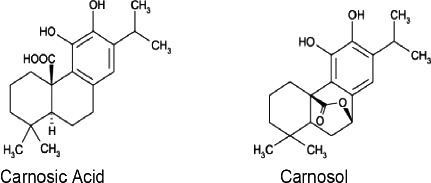
Structural formula of carnosic acid and carnosol present in extracts of rosemary (JECFA, 2016‐tentative)
3.1.2. Specifications
The specifications for extracts of rosemary (E 392) as defined in the Commission Regulation (EU) No 231/2012 and by JECFA (2016‐tentative) are listed in Table 1.
Table 1.
Specifications for extracts of rosemary (E 392) according to Commission Regulation (EU) No 231/2012 and JECFA (2016‐tentative)
| Commission Regulation (EU) No 231/2012 | JECFA (2016‐tentative) | |
|---|---|---|
| Definition | Extracts of rosemary contain several components, which have been proven to exert antioxidative functions. These components belong mainly to the classes of phenolic acids, flavonoids, diterpenoids. Besides the antioxidant compounds, the extracts can also contain triterpenes and organic solvent extractable material specifically defined in the following specification |
Rosemary extract is obtained from ground dried leaves of Rosmarinus officinalis L using food‐grade solvents, namely, acetone or ethanol. Solvent extraction is followed by filtration, solvent evaporation, drying and sieving to obtain a fine powder. Additional concentration and/or precipitation steps followed by deodorisation, decolourisation and standardisation using diluents and carriers of food grade quality maybe included to produce the final product. Rosemary extract is characterised by its content of phenolic diterpenes, carnosic acid and carnosol, the principal antioxidative agents. Other antioxidant components present include triterpenes and triterpenic acids. Rosemary extract is identified by the total content of carnosol and carnosic acid as a ratio of reference volatile compounds which are responsible for flavour. The product of commerce can be standardised to a total carnosic acid and carnosol content up to 33% |
| Description | Rosemary leaf extract antioxidant is prepared by extraction of the leaves of Rosmarinus officinalis using a food approved solvent system. Extracts may then be deodorised and decolourised. Extracts may be standardised | Beige to light brown powder |
| Assay | Not less than 5% of the total carnosic acid and carnosol | |
| Identification | ||
| Reference antioxidative compounds: phenolic diterpenes | Carnosic acid (C20H28O4) and Carnosol (C20H26O4) (which comprise not less than 90% of the total phenolic diterpenes) | |
| Reference key volatiles: | Borneol, Bornyl Acetate, Camphor, 1,8‐Cineol, Verbenone | |
| Density: | > 0.25 g/mL | |
| Solubility: | Insoluble in water | Insoluble in water; soluble in oil. |
| Purity | ||
| Loss of drying: | < 5% | Not more than 5% (80° under vacuum, 4 h) |
| Residual solvents: |
Acetone: Not more than 50 mg/kg Ethanol: Not more than 500 mg/kg |
|
| Arsenic: | Not more than 3 mg/kg | Not more than 3 mg/kg |
| Lead | Not more than 2 mg/kg | Not more than 2 mg/kg |
| 1 – Extracts of rosemary produced from dried rosemary leaves by acetone extraction (generic specifications applicable) | ||
| Description | Extracts of rosemary are produced from dried rosemary leaves by acetone extraction, filtration, purification and solvent evaporation, followed by drying and sieving to obtain a fine powder or a liquid. | |
| Identification | ||
| Content of reference antioxidative compounds | ≥ 10% w/w, expressed as the total of carnosic acid and carnosol | |
| Antioxidant/Volatiles — Ratio |
(Total % w/w of carnosic acid and carnosol) ≥ 15 (% w/w of reference key volatiles)* (* as a percentage of total volatiles in the extract, measured by gas chromatography–mass spectrometry detection, ‘GC‐MSD’) |
Total % of carnosic acid and carnosol/Total % of reference volatiles: (‐)‐borneol, (‐)‐bornyl acetate, (‐)‐camphor, 1,8‐Cineole (eucalyptol) and verbenone: not less than 15 |
| Purity | ||
| Residual solvents | Acetone: Not more than 500 mg/kg | |
| 2 – Extracts of rosemary prepared by extraction of dried rosemary leaves by means of supercritical carbon dioxide (not in JECFA specifications) | ||
| Description | Extracts of rosemary produced from dried rosemary leaves extracted by means of supercritical carbon dioxide with a small amount of ethanol as entrainer | |
| Identification | ||
| Content of reference antioxidative compounds | ≥ 13% w/w, expressed as the total of carnosic acid and carnosol | |
| Antioxidant/Volatiles — Ratio |
(Total % w/w of carnosic acid and carnosol) ≥ 15 (% w/w of reference key volatiles)* (* as a percentage of total volatiles in the extract, measured by gas chromatography–mass spectrometry detection, ‘GC‐MSD’) |
|
| Purity | ||
| Residual solvents | Ethanol: Not more than 2% | |
| 3 – Extracts of rosemary prepared from a deodorised ethanolic extract of rosemary (generic specifications applicable). | ||
| Description | Extracts of rosemary which are prepared from a deodorised ethanolic extract of rosemary. The extracts may be further purified, for example by treatment with active carbon and/or molecular distillation. The extracts may be suspended in suitable and approved carriers or spray‐dried | |
| Identification | ||
| Content of reference antioxidative compounds | ≥ 5% w/w, expressed as the total of carnosic acid and carnosol | |
| Antioxidant/Volatiles — Ratio |
(Total % w/w of carnosic acid and carnosol) ≥ 15 (% w/w of reference key volatiles)* (* as a percentage of total volatiles in the extract, measured by gas chromatography–mass spectrometry detection, ‘GC‐MSD’) |
Total % of carnosic acid and carnosol/Total % of reference volatiles: (‐)‐borneol, (‐)‐bornyl acetate, (‐)‐camphor, 1,8‐Cineole (eucalyptol) and verbenone: not less than 15 |
| Purity | ||
| Residual solvents | Ethanol: Not more than 500 mg/kg | |
| 4 – Extracts of rosemary decolourised and deodorised, obtained by a two‐step extraction using hexane and ethanol (not in JECFA specifications) | ||
| Description | Extracts of rosemary which are prepared from a deodorised ethanolic extract of rosemary, undergone a hexane extraction. The extract may be further purified, for example by treatment with active carbon and/or molecular distillation. They may be suspended in suitable and approved carriers or spray‐dried | |
| Identification | ||
| Content of reference antioxidative compounds | ≥ 5% w/w, expressed as the total of carnosic acid and carnosol | |
| Antioxidant/Volatiles – Ratio |
(Total % w/w of carnosic acid and carnosol) ≥ 15 (% w/w of reference key volatiles)* (* as a percentage of total volatiles in the extract, measured by gas chromatography– mass spectrometry detection, ‘GC‐MSD’) |
|
| Purity | ||
| Residual solvents | Hexane: not more than 25 mg/kg Ethanol: Not more than 500 mg/kg | |
3.2. Authorised uses and use levels
Maximum levels of extracts of rosemary (E 392) have been defined in Annex II to Regulation (EC) No 1333/2008 on food additives, as amended. In this document, these levels are named MPLs.
Currently, extracts of rosemary (E 392) is an authorised food additive in the EU with MPLs ranging from 15 to 400 mg/kg in 33 food categories listed in Table 2. All MPLs are expressed as the sum of carnosol and carnosic acid, and some are expressed on the fat basis of the food.
Table 2.
MPLs of extracts of rosemary (E 392), expressed as the sum of carnosol and carnosic acid, in foods according to the Annex II to Regulation (EC) No 1333/2008
| Food category number | Food category name | Restrictions/exception | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|
| 01.5 | Dehydrated milk as defined by Directive 2001/114/EC | Only milk powder for vending machines | 200a |
| 01.5 | Dehydrated milk as defined by Directive 2001/114/EC | Only dried milk for manufacturing of ice cream | 30 |
| 02.1 | Fats and oils essentially free from water (excluding anhydrous milkfat) | Only vegetable oils (excluding virgin oils and olive oils) and fat where content of polyunsaturated fatty acids is higher than 15% w/w of the total fatty acid, for the use in non‐heat‐treated food products | 30a |
| 02.1 | Fats and oils essentially free from water (excluding anhydrous milkfat | Only fish oil and algal oil; lard, beef, poultry sheep and porcine fat; fat and oils for the professional manufacture of heat‐treated foods; frying oils and frying fat, excluding olive oil and pomace oil | 50a |
| 02.2.2 | Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions | Only spreadable fats with a fat content less than 80% | 100a |
| 02.3 | Vegetable oil pan spray | Only fats and oils for the professional manufacture of heat‐treated foods | 50a |
| 04.2.4.1 | Fruit and vegetable preparations excluding compote | Only seaweed based fish roe analogues | 200 |
| 04.2.5.4 | Nut butters and nut spreads | 200a | |
| 04.2.6 | Processed potato products | Only dehydrated potatoes products | 200 |
| 05.3 | Chewing gum | 200 | |
| 05.4 | Decorations, coatings and fillings, except fruit based fillings covered by category 4.2.4 | Only sauces | 100a |
| 06.4.5 | Fillings of stuffed pasta (ravioli and similar) | Only in fillings of stuffed dry pasta | 250a |
| 07.2 | Fine bakery wares | 200a | |
| 08.3.1 | Non‐heat‐treated meat products | Only dried sausages | 100 |
| 08.3.1 | Non‐heat‐treated meat products | Only meat with a fat content not higher than 10%, excluding dried sausages | 15 |
| 08.3.1 | Non‐heat‐treated meat products | Only meat with a fat content higher than 10%, excluding dried sausages | 150a |
| 08.3.1 | Non‐heat‐treated meat products | Only dehydrated meat | 150 |
| 08.3.2 | Heat‐treated meat products | Only meat with a fat content not higher than 10%, excluding dried sausages | 15 |
| 08.3.2 | Heat‐treated meat products | Only meat with a fat content higher than 10%, excluding dried sausages | 150a |
| 08.3.2 | Heat‐treated meat products | Only dried sausages | 100 |
| 08.3.2 | Heat‐treated meat products | Only dehydrated meat | 150 |
| 09.2 | Processed fish and fishery products including molluscs and crustaceans | Only fish and fishery products including molluscs and crustaceans with a fat content not higher than 10% | 15 |
| 09.2 | Processed fish and fishery products including molluscs and crustaceans | Only fish and fishery products including molluscs and crustaceans with a fat content higher than 10% | 150a |
| 10.2 | Processed eggs and egg products | 200 | |
| 12.2.2 | Seasoning and condiments | 200a | |
| 12.4 | Mustard | 100a | |
| 12.5 | Soups and broths | 50 | |
| 12.6 | Sauces | 100a | |
| 15.1 | Potato‐, cereal‐, flour‐ or starch‐based snacks | 50a | |
| 15.2 | Processed nuts | 200a | |
| 17.1b | Food supplements supplied in a solid form including capsules and tablets and similar forms, excluding chewable forms | 400 | |
| 17.2b | Food supplements supplied in a liquid form | 400 | |
| 17.3b | Food supplements supplied in a syrup‐type or chewable form | 400 |
MPL: maximum permitted level.
expressed on fat basis.
FCS 17 refers to food supplements as defined in Directive 2002/46/EC of the European Parliament and of the Council excluding food supplements for infants and young children.
Table 2 summarises foods that are permitted to contain extracts of rosemary (E 392) and the corresponding MPLs as set by Annex II to Regulation (EC) No 1333/2008.
According to Annex III, Part 2 of Regulation (EC) No 1333/2008, extracts of rosemary (E 392) is authorised as a food additive other than carrier in food colour preparations with a maximum level of 1,000 mg/kg in the preparation, and 5 mg/kg in the final product expressed as the sum of carnosic acid and carnosol.
According to Annex III, Part 4, extracts of rosemary (E 392) is also authorised as a food additive in all food flavourings at the maximum level of 1,000 mg/kg (expressed as the sum of carnosol and carnosic acid).
In addition, according to Annex III, Part 5, Section A of Regulation (EC) No 1333/2008, extracts of rosemary (E 392) is also authorised at the level of 1,000 mg/kg in the preparation of β‐carotene and lycopene and 5 mg/kg in the final product expressed as the sum of carnosol and carnosic acid.
3.3. Exposure data
3.3.1. Reported use levels or data on analytical levels of extracts of rosemary (E 392)
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued a public call15 for occurrence data (usage level and/or concentration data) on extracts of rosemary (E 392). In response to this public call, updated information on the actual use levels of extracts of rosemary (E 392) in foods was made available to EFSA by industry. No analytical data on the concentration of extracts of rosemary (E 392) in foods were made available by the Member States.
Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with data on use levels (n = 44) of extracts of rosemary (E 392) in foods for 12 out of the 33 food categories in which extracts of rosemary (E 392) is authorised.
The Panel noted that data were submitted for one food category which is not authorised to contain the food additive as such: noodles (FC 06.5). For this food category, levels for their seasoning were provided. These levels were not taken into account as seasoning as an ingredient is already covered by the FC 12.2.2.
Updated information on the actual use levels of extracts of rosemary (E 392) in foods was made available to EFSA by FoodDrinkEurope (FDE), the International Chewing Gum Association (ICGA), the Association of the European Self‐Medication Industry (AESGP), l'Alliance 7, IMACE ‐ European Margarine Association, European Potato Processors’ Association (EUPPA), EU Fish Processors and Traders Association ‐ European Federation of National Organizations of Importers and Exporters of Fish (AIPCE‐CEP) and Intersnack.
The Panel noted that two use levels for a niche product were provided: one on other fat (FC 02.2.2) and one for sauce (FC 12.6). Since other use levels were available for sauces, the Panel did not consider the niche level for sauce in the analysis. The level provided for the FC 02.2.2 was used in the refined exposure assessment scenario as no other data were available.
MPLs for extracts of rosemary (E 392) are expressed as the sum of carnosol and carnosic acid. Exposure estimates should then be expressed also in mg of carnosol and carnosic acid/kg bw per day. However, some data providers buy a preparation from suppliers, e.g. seasonings in which rosemary extract is a component. Therefore, only the specifications for the seasoning are known by food industry, but not for the rosemary extract itself, because it is only a subcomponent. Thus, some levels reported to EFSA are expressed as extracts of rosemary in total and not as the sum of carnosol and carnosic acid. However, by doing so food industry is on the safe side, since as long as the levels of rosemary extract are lower than the MPLs, the amount of carnosol and carnosoic acid as well is below these maximum limits. This introduce an uncertainty on the exposure assessment as it should overestimate the intake of carnosol and carnosic acid.
Appendix A provides data on the use levels of extracts of rosemary (E 392) in foods as reported by industry.
3.3.2. Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information of over 2.5 million food and beverage products of which more than 1,000,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 20 out of its 28 member countries and Norway presented in the Mintel GNPD.16
For the purpose of this Scientific Opinion, the Mintel's GNPD17 was used for checking the labelling of food and beverages products and food supplements for extracts of rosemary (E 392) within the EU's food market as the database contains the compulsory ingredient information on the label.
According to the Mintel's GNPD, extracts of rosemary (E 392) was labelled on more than 4,700 products between January 2013 and February 2018. The main Mintel's GNPD food subcategories containing the food additive were ‘dry soup’, ‘pizzas’ and ‘stocks’. Most food subcategories are covered in the current assessment and food subcategories from the Mintel GNPD included in the exposure assessment represented approximately 83% of the food products labelled with extracts of rosemary (E 392) in the database. The Mintel categories which could not be included in the present assessment are meat substitutes, spreads (nut spread, spreadable cheese, chocolate spread, etc.), confectionary, meat pastes and pâtés, rice.
Appendix B lists the percentage of the food products labelled with extracts of rosemary (E 392) out of the total number of food products per food subcategories according to the Mintel's GNPD food classification. The percentages ranged from less than 0.1% in many food subcategories to 17.8% in Mintel's GNPD food subcategory ‘dry soup’. The average percentage of foods labelled to contain extracts of rosemary (E 392) was 1%.
3.3.3. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). Consumption surveys added in the Comprehensive database in 2015 were also taken into account in this assessment.18
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database includes the currently best available food consumption data across Europe.
Food consumption data from the following population groups were used for the exposure assessment: infants, toddlers, children, adolescents, adults and the elderly. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 3).
Table 3.
Population groups considered for the exposure estimates of extracts of rosemary (E 392)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlersa | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrenb | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlyb | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Netherlands, Sweden, UK |
The term ‘toddlers’ in the EFSA Comprehensive Database corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013.
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure estimates. In practice, the FoodEx food codes were matched to the FCS food categories.
Food categories considered for the exposure assessment of extracts of rosemary (E 392)
The food categories in which the use of extracts of rosemary (E 392) is authorised were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
Some food categories (or their restrictions/exceptions) for which MPLs were available and/or use levels were submitted are not referenced in the EFSA Comprehensive Database and could therefore not be taken into account in the present estimate. This was the case for four food categories (Appendix C) and may have resulted in an underestimation of the exposure. The food categories which were not taken into account are described below (in ascending order of the FCS codes):
01.5 Dehydrated milk as defined by Council Directive 2001/114/EC19, only milk powder for vending machines. The FoodEx codes do not allow restricting only to foods sold in vending machine. The whole food category was not taken into account because the restriction represents only a very small part of the food category;
01.5 Dehydrated milk as defined by Council Directive 2001/114/EC,19 only dried milk for manufacturing of ice‐cream. The restriction indicates that the dried milk referred to in this food category is not sold directly to the consumer. In order to take into account this food category, the edible ices (FC 03) made of dried milk should be taken into account. However, no information on the consumption of this type of ice‐cream is available in the EFSA Comprehensive database. To avoid overestimation of the exposure, the whole FC 03 was not considered in the exposure assessment;
02.3 Vegetable oil pan spray;
04.2.4.1 Fruit and vegetable preparations excluding compote, only seaweed based fish roe analogues
No foods correspond to the two above food categories in the EFSA Comprehensive database, therefore they cannot be taken into account.
For the following food categories, the restrictions/exceptions which apply to the use of extracts of rosemary (E 392) could not be taken into account, and therefore the whole food category was considered in the exposure assessment. This applies also to four food categories (Appendix D) and may have resulted in an overestimation of the exposure:
02.1 Fats and oils essentially free from water (excluding anhydrous milkfat), only vegetable oils (excluding virgin oils and olive oils) and fat where content of polyunsaturated fatty acids is higher than 15% w/w of the total fatty acid, for the use in non‐heat‐treated food products: the polyunsaturated fatty acids content could not be checked as well as their use in non‐heat‐treated food products. Thus, vegetable oils and fat with the exception of olive oil, were taken into account.
-
02.1 Fats and oils essentially free from water (excluding anhydrous milkfat),
-
–
only fish oil and algal oil; lard, beef, poultry, sheep and porcine fat: no algal oil is available in the EFSA Comprehensive database, all fish oil and lard, beef, poultry, sheep and porcine fat available in the EFSA Comprehensive database were taken into account.
-
–
fat and oils for the professional manufacture of heat‐treated foods: this information is not available in the EFSA Comprehensive database and this restriction was not taken into account
-
–
frying oils and frying fat, excluding olive oil and pomace oil: oils and fat that can be used for frying were all taken into account
-
–
02.2.2 Other fat and oil emulsions, including spreads as defined by Council Regulation (EC) No 1234/200720 and liquid emulsions, only spreadable fats with a fat content less than 80%: low‐fat butter and margarine were taken into account.
06.4.5 Fillings of stuffed pasta (ravioli and similar), only in fillings of stuffed dry pasta: all filled pasta were taken into account
Furthermore, for the FCs 08.3.1 Non‐heat‐treated meat products and 08.3.2 Heat‐treated meat products, it is not possible to distinguish heat‐treated from non‐heat‐treated meat products. Meat products were separated between dried sausages, dehydrated meat and other meat. For each of these subcategories, for the regulatory scenario, MPLs as in Table 1 were applied depending on fat content of the foods. It has to be mentioned that use levels were only reported for cooked smoked sausage only; thus, only these kinds of meat products were taken into account in the refined exposure assessment. Thus, dried sausages, dehydrated meat and other meat products were included in the regulatory scenario but not in the refined exposure scenarios.
For the FCs 17.1/17.2/17.3 Food supplements, in solid, liquid, syrup‐type or chewable form, the form consumed cannot be differentiated in the EFSA Comprehensive database and therefore the same use level was applied to the whole FC 17.
For the refined scenario, 13 additional food categories were not taken into account because no use levels were provided for these food categories to EFSA (Appendix A). For the remaining food categories, the refinements considering the restrictions/exceptions as set in Annex II to Regulation No 1333/2008 were applied.
Overall, 26 food categories were included in the regulatory maximum level exposure scenario, and 12, in the refined scenarios of the exposure assessment to extracts of rosemary (E 392) (Appendix C).
3.4. Exposure estimates
3.4.1. Exposure to extracts of rosemary (E 392), expressed as the sum of carnosol and carnosic acid, from its use as a food additive
The Panel estimated the chronic dietary exposure to extracts of rosemary (E 392) for the following population groups: infants, toddlers, children, adolescents, adults and the elderly. Dietary exposure to extracts of rosemary (E 392) was calculated by multiplying concentrations of extracts of rosemary (E 392) per food category (Appendix C) with their respective consumption amount per kilogram body weight for each individual in the Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only one day per subject were excluded as they are considered as not adequate to assess repeated exposure.
This was carried out for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 3). On the basis of these distributions, the mean and 95th percentile of exposure were calculated per survey and per population group. The 95th percentile of exposure was only calculated for those population groups with a sufficiently large sample size (EFSA, 2011a). Therefore, in the present assessment, the 95th percentile of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain were not estimated.
Exposure assessment to extracts of rosemary (E 392) was carried out by the ANS Panel based on two different sets of concentration data: (1) MPLs as set down in the EU legislation (defined as the regulatory maximum level exposure assessment scenario); and (2) reported use levels (defined as the refined exposure assessment scenario). These two scenarios are discussed in detail below.
These scenarios do not consider the consumption of food supplements, which are covered in an additional scenario detailed below (food supplements consumers only scenario).
A possible additional exposure from the use of extracts of rosemary (E 392) as a food additive in food additives (Part 2), flavourings (Part 4) and nutrients (Part E, Section A) in accordance with Annex III to Regulation (EC) No 1333/2008 was not considered in any of the exposure assessment scenarios, as no concentration data were available reflecting this use of the food additive.
Regulatory maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario is based on the MPLs as set in Annex II to Regulation (EC) No 1333/2008. For extracts of rosemary (E 392), the MPLs as listed in Table 2 were used to assess the exposure according to this scenario.
MPLs expressed for extracts of rosemary (E 392) on fat basis were converted to whole weight based on fat content information obtained from the EFSA Comprehensive Database.
The Panel considers the exposure estimates derived following this scenario as the most conservative since it is assumed that that the population will be exposed to the food additive present in food at the MPLs over a longer period of time.
Refined exposure assessment scenario
The refined exposure assessment scenario is based on use levels reported by food industry and analytical results reported by Member States. For extracts of rosemary (E 392), the refined exposure assessment scenario was only based on use levels reported by food industry. This exposure scenario can consider only food categories for which these data were available to the Panel.
Reported use levels expressed for extracts of rosemary (E 392) on fat basis were converted to whole weight based on fat content information per food obtained from the EFSA Comprehensive Database.
Based on the available data set, the Panel calculated two refined exposure estimates based on two model populations:
-
The brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to extracts of rosemary (E 392) present at the maximum reported use level for one food category. This exposure estimate is calculated as follows:
-
–
Combining food consumption with the maximum of the reported use levels for the main contributing food category at the individual level.
-
–
Using the mean of the typical reported use levels for the remaining food categories.
-
–
The non‐brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to extracts of rosemary (E 392) present at the mean reported use levels in food. This exposure estimate is calculated using the mean of the typical reported use levels for all food categories.
Appendix C summarised the concentration levels of extracts of rosemary (E 392) used in the refined exposure scenarios.
‘Food supplement consumers only’ scenario
Extracts of rosemary (E 392) is authorised in the FC 17 Food supplements as defined in Directive 2002/46/EC excluding food supplements for infants and young children. As exposure via food supplements may deviate largely that via food, and the number of food supplement consumers may be low depending on populations and surveys, an extra scenario was calculated in order to reflect additional exposure to food additives from the intake of food supplements. This additional exposure was estimated assuming that consumers only of food supplements were exposed to extracts of rosemary (E 392) present at the maximum reported use levels in these supplements on a daily basis. For the remaining food categories (12/33 categories), the mean of the typical reported use levels of extracts of rosemary (E 392) were used.
As FC 17 does not consider food supplements for infants and toddlers as defined in the legislation, exposure to extracts of rosemary (E 392) via food supplements was not estimated for these two population groups.
Dietary exposure to extracts of rosemary (E 392), expressed as the sum of carnosol and carnosic acid
Table 4 summarises the estimated exposure to extracts of rosemary (E 392) from its use as a food additive in six population groups (Table 3) according to the different exposure scenarios. Detailed results per population group and survey are presented in Appendix E.
Table 4.
Summary of dietary exposure to extracts of rosemary (E 392), expressed as the sum of carnosol and carnosic acid, from their use as food additives in the maximum level exposure assessment scenario and in the refined exposure scenarios, in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Regulatory maximum level exposure assessment scenario | ||||||
|
0.03–0.12 0.08–0.32 |
0.08–0.44 0.24–0.70 |
0.14–0.34 0.28–0.85 |
0.06–0.24 0.15–0.58 |
0.05–0.16 0.11–0.40 |
0.04–0.17 0.09–0.38 |
| Refined estimated exposure assessment scenario | ||||||
| Brand‐loyal scenario | ||||||
|
< 0.01–0.04 < 0.01–0.20 |
0.01–0.12 0.03–0.31 |
0.02–0.16 0.06–0.35 |
0.01–0.08 0.03–0.22 |
0.01–0.05 0.02–0.15 |
0.01–0.04 0.02–0.12 |
| Non‐brand‐loyal scenario | ||||||
|
< 0.01–0.02 < 0.01–0.11 |
< 0.01–0.08 0.02–0.19 |
0.01–0.09 0.03–0.20 |
0.01–0.05 0.02–0.13 |
0.01–0.03 0.02–0.10 |
< 0.01–0.03 0.01–0.08 |
In the regulatory maximum level exposure assessment scenario, the mean exposure to extracts of rosemary (E 392) from its use as a food additive ranged from 0.03 mg/kg bw per day in infants to 0.44 mg/kg bw per day in toddlers. The 95th percentile of exposure to extracts of rosemary (E 392) ranged from 0.08 mg/kg bw per day in infants to 0.85 mg/kg bw per day in children.
In the brand‐loyal refined exposure scenario, the mean exposure to extracts of rosemary (E 392) from its use as a food additive ranged from below 0.01 mg/kg bw per day in infants to 0.16 mg/kg bw per day in children. The high exposure (p95) to extracts of rosemary (E 392) ranged from below 0.01 mg/kg bw per day in infants to 0.35 mg/kg bw per day in children. In the non‐brand‐loyal refined exposure scenario, the mean exposure to extracts of rosemary (E 392) from its use as a food additive ranged from below 0.01 mg/kg bw per day in almost all population groups to 0.09 mg/kg bw per day in children. The 95th percentile of exposure ranged from below 0.01 mg/kg bw per day in infants to 0.20 mg/kg bw per day in children.
In the food supplements consumers only scenario, the mean exposure to extracts of rosemary (E 392) from its use as a food additive ranged from 0.01 mg/kg bw per day for adolescents and adults to 0.09 mg/kg bw per day for children. The 95th percentile of exposure to extracts of rosemary (E 392) ranged from 0.02 mg/kg bw per day for adolescents to 0.13 mg/kg bw per day for children. These exposure levels did not exceed the exposure levels calculated for the refined exposure scenario.
Main food categories contributing to exposure to extracts of rosemary (E 392) using the regulatory maximum level exposure assessment scenario
In the regulatory maximum level exposure assessment scenario, the main contributing food categories to the total mean exposure estimates for infants were fats and oils essentially free from water (FC 02.1), and soups and broths (FC 12.5), meat products (FC 08.3) and fine bakery wares (FC 07.2). For all other population groups (toddlers, children, adolescents, adults and the elderly), the main contributing food categories were meat products (FC 08.3), fine bakery wares (FC 07.2) and soups and broths (FC 12.5).
Main food categories contributing to exposure to extracts of rosemary (E 392) using the refined exposure assessment scenario
The main contributing food category from the refined estimated exposure scenario, both brand‐loyal and non‐brand‐loyal scenarios were fine bakery wares (FC 07.2) and soups and broths (FC 12.5) for infants; and fine bakery wares (FC 07.2) only for the others population groups.
Appendix E summarises the contributing food categories for the regulatory maximum level and the refined exposure assessment scenario.
Uncertainty analysis
Uncertainties in the exposure assessment of extracts of rosemary (E 392) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 5.
Table 5.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/– |
| Use of data from food consumption surveys covering only a few days to estimate high percentiles (95th) long‐term (chronic) exposure | + |
| Correspondence of reported use levels to the food items in the EFSA Comprehensive Food Consumption Database: uncertainties to which types of food the levels refer | +/– |
| Uncertainty in possible national differences in use levels of food categories | +/– |
|
Concentration data: ‐ use levels considered applicable to all foods within the entire food category, whereas on average 1% of the foods, belonging to food categories with foods labelled with extracts of rosemary (E 392), was labelled with the additive |
+ |
| Food categories selected for the exposure assessment: exclusion of food categories due to missing FoodEx linkage (n = 4/33 food categories) | – |
| Food categories selected for the exposure assessment: inclusion of food categories without considering the restriction/exception (n = 4 for the MPL scenario/n = 1 for the refined scenarios out of 33 food categories) | + |
| Food categories selected in the exposure assessment: no concentration data for certain food categories (n=14/33 food categories for the refined scenarios) | – |
| Foods which may contain the food additive according to Annex III to Regulation (EC) No 1333/2008 not taken into account | – |
|
Regulatory maximum level exposure assessment scenario: ‐ exposure calculations based on the MPL according to Annex II to Regulation (EC) No 1333/2008 |
+ |
|
Refined exposure assessment scenarios: ‐ exposure calculations based on the maximum or mean levels (reported use from industries) |
+/– |
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
Extracts of rosemary (E 392) is authorised in 33 food categories. Use levels of the additive were made available by industry for 12 food categories.
The Panel calculated that out of the foods authorised to contain extracts of rosemary (E 392) according to Annex II to Regulation (EC) No 1333/2008, 3% (for infants) to 86% (for children) of the amount of food consumed (by weight) per population group was taken into account in the current assessment.
The Panel also noted that information from the Mintel's GNPD (Appendix B) indicated that many food sub‐categories, as categorised according to the Mintel's GNPD nomenclature, were labelled with the food additive. The main ones were included in the current exposure assessment: soups, fine bakery wares, sauces and snacks. Food subcategories from the Mintel GNPD included in the exposure assessment represented approximately 83% of the food products labelled with extracts of rosemary (E 392) in the database.
Furthermore, the percentage of foods per subcategory labelled to contain extracts of rosemary (E 392) was maximally up to 18% (Appendix B); in the assessment, it was assumed that the additive was present in 100% of the foods belonging to the food categories included in the different exposure scenarios.
As mentioned above, the refined exposure assessment scenario is based on use levels reported by food industry. This exposure scenario can consider only food categories for which these data were available to the Panel. Regarding extracts of rosemary (E 392), the main contributing food categories in the regulatory maximum exposure assessment scenario were fine bakery wares, meat products and soups and broth. These three food categories were also included in the refined exposure scenario as reported use levels were made available to EFSA.
Given these observations, the Panel considered overall that the uncertainties identified would, in general, result in an overestimation of the exposure to extracts of rosemary (E 392) from its use as a food additive according to Annex II, in European countries considered in the EFSA European database for all exposure scenarios.
The Panel noted that food categories which may contain extracts of rosemary (E 392) due to carry‐over (Annex III, Part 2, 4, 5A) were not considered in the current exposure assessment.
3.4.2. Exposure via the regular diet
Carnosic acid and carnosol are substances naturally present in foods. Their main sources are rosemary (Rosmarinus officinalis) and sage (Salvia officinalis and other Salvia species).
Natural content of carnosic acid and carnosol was retrieved from publications and databases to estimate their intake from natural sources. Levels of carnosic acid and carnosol were available for dried rosemary leaves (112 mg/kg (Loussouarn et al., 2017)), fresh rosemary leaves (12.18 mg/kg (Luis and Johnson, 2005)) and dried sage (5.3 mg/kg (FooDB version 1.0 database, 21)). In other herbs and spices and tea, carnosic acid and carnosol were detected but not quantified.
The foods taken into account to estimate natural intake of both compounds are rosemary (dried and fresh), sage (dried, infusion leaves), as well as aromatic herbs undefined (dried and fresh). The level of carnosic acid and carnosol applied to the latter was the one of dried sage (5.3 mg/kg).
Dietary intake of carnosic acid and carnosol
End April 2018, EFSA published a new release of the Comprehensive European Food Consumption Database.22 This database includes now new surveys. This updated database was used to estimate the intake of carnosic acid and carnosol from the natural diet.
Table 6 summarises the estimated intake of carnosic acid and carnosol in six population groups. Detailed results per population group and survey are presented in Appendix F.
Table 6.
Summary of dietary intake to the sum of carnosol and carnosic acid from the natural diet in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Dietary intake of rosemary, sage and undefined aromatic herbs (g/person per day) | ||||||
|
0.0–0.33 0.0–1.95 |
0.0–0.74 0.0–3.50 |
0.0–0.89 0.0–4.05 |
0.0–0.79 0.0–4.85 |
0.0–1.19 0.0–7.5 |
0.0–1.72 0.0–7.90 |
| Dietary intake of carnosic acid and carnosol from natural diet (mg/kg bw per day) | ||||||
|
0.0–0.26 0.0–1.59 |
0.0–0.34 0.0–1.66 |
0.0–0.22 0.0–0.99 |
0.0–0.09 0.0–0.48 |
0.0–0.09 0.0–0.52 |
0.0–0.13 0.0–0.51 |
The mean intake of carnosic acid and carnosol from natural diet was up to 0.34 mg/kg bw per day in toddlers, while the high intake (p95) reached 1.66 mg/kg bw per day also in toddlers.
Exposure from both dietary sources (as a food additive and from natural diet)
The range of exposure from both food additive and natural diet is indicated in Table 7. The percentage of carnosol and carnosic acid coming from the food additive is at the maximum of 35% for the population of adolescents.
Table 7.
Summary of dietary exposure to extracts of rosemary (E 392), expressed as the sum of carnosol and carnosic acid, from their use as a food additive in the non‐brand loyal exposure scenario, summary of dietary intake of carnosol and carnosic acid from natural diet, and sum of both sources (as a range and percentage) in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Dietary exposure to extracts of rosemary (E 392), non‐brand‐loyal scenario (mg/kg bw per day) | ||||||
|
< 0.01–0.02 < 0.01–0.11 |
< 0.01–0.08 0.02–0.19 |
0.01–0.09 0.03–0.20 |
0.01–0.05 0.02–0.13 |
0.01–0.03 0.02–0.10 |
< 0.01–0.03 0.01–0.08 |
| Dietary intake of carnosic acid and carnosol from natural diet (mg/kg bw per day) | ||||||
|
0.0–0.26 0.0–1.59 |
0.0–0.34 0.0–1.66 |
0.0–0.22 0.0–0.99 |
0.0–0.09 0.0–0.48 |
0.0–0.09 0.0–0.52 |
0.0–0.13 0.0–0.51 |
| Sum of both sources (Food additives and natural diet) (mg/kg bw per day) | ||||||
|
0.0–0.28 7% |
0.0–0.42 19% |
0.0–0.31 29% |
0.0–0.14 35% |
0.0–0.12 25% |
0.0–0.16 19% |
3.4.3. Exposure via other sources
Extracts of rosemary (E 392) is also permitted as an antimicrobial, refreshing and tonic in cosmetic products. According to the Regulation (EC) No 1223/200923 on cosmetic products, there is no limit.
Rosemary extracts have been registered under the REACH Regulation and is used in the following products: washing & cleaning products, air care products, biocides (e.g. disinfectants, pest control products), polishes and waxes, perfumes and fragrances and cosmetics and personal care products. Other release to the environment of this substance is likely to occur from: indoor use (e.g. machine wash liquids/detergents, automotive care products, paints and coating or adhesives, fragrances and air fresheners) and outdoor use as processing aid.
Data to calculate the exposure via all these sources were not available to the Panel and therefore the exposure resulting from these other sources could not be taken into account in this opinion
3.5. Discussion
To assess the dietary exposure to extracts of rosemary (E 392) from its use as a food additive, the exposure was calculated based on (1) MPLs set out in the EU legislation (defined as the regulatory maximum level exposure assessment scenario) and (2) the reported use levels (defined as the refined exposure assessment scenario).
Extracts of rosemary (E 392) is authorised in 33 food categories of which none was identified as a food category to which consumers may be brand loyal. Therefore, the Panel selected the refined non‐brand‐loyal scenario as the most relevant exposure scenario for the safety evaluation of this food additive.
In total, 12 out of 33 food categories were taken into account in the refined exposure assessment scenarios.
The exposure estimates in the non‐brand‐loyal exposure assessment scenario was maximally 0.20 mg/kg bw per day (95th percentile for children) (Table 4).
The Panel noted that the main food category contributing to exposure in the refined scenarios was fine bakery wares which is a highly consumed food category and for which the second highest use level (after the one for food supplements) was reported. More specific data on the foods belonging to this food category that contain the additive will result in a more refined exposure estimate.
The assessments were hampered by several uncertainties and overall it was estimated that the exposure was overestimated due to the reported use levels used and assumptions made in the exposure assessment. For an elaborate discussion of the uncertainties, see Section 3.4.1.
The Panel also noted that the refined exposure estimates are based on information provided on the reported level of use of extracts of rosemary (E 392). If actual practice changes, this refined estimates may no longer be representative and should be updated.
The Panel noted that more food categories were labelled with extract of rosemary (E 392) than for which use levels were reported by industry. However, the main ones were included in the current exposure assessment: soups, fine bakery wares, sauces and snacks. Food subcategories from the Mintel GNPD included in the exposure assessment represented approximately 83% of the food products labelled with extracts of rosemary (E 392) in the database.
Intake of carnosic acid and carnosol from natural diet was at the maximum up to 1.66 mg/kg bw per day (p95) in toddlers. This would represent approximately nine times the intake from the food additive for this population.
The Panel noted that the exposure to extracts of rosemary (E 392) from its use according the Annex III (Parts 2, 4 and 5A) was not considered in the exposure assessment, neither the use of rosemary as an ingredient in composite foods. This may have led to an underestimation of overall exposure. Exposure to the food additive via other sources was also not considered.
The Panel noted that the collection of concentration data of extracts of rosemary (E 392) could support a more refined assessment on dietary exposure.
The Panel calculated a range of MOS of 100–2,000 for children. This was calculated by dividing the lowest value of the range of NOAELs of 20–60 mg carnosol plus carnosic acid/kg bw per day identified by the AFC Panel (EFSA AFC Panel, 2008) by the highest p95 exposure level (0.2 mg/kg bw per day) in this population and the highest value of the range of NOAELs by the lowest p95 exposure level (0.03 mg/kg bw per day). Using the same approach for adults, the range of MOS was 200–3,000.
The Panel noted that the current refined exposure estimates were based on the EFSA Comprehensive consumption database as in the 2015 exposure estimates (EFSA ANS Panel 2015). The margins of safety for children and adults (100–2,000 and 200–3,000 respectively) using the refined exposure estimate are higher than the ones calculated in 2015 (25–240 for children and 60–600 for adults) using an MPL scenario.
4. Conclusions
Based on the data provided by food industry, the Panel was able to refine the exposure estimates of extracts of rosemary (E 392). The highest mean refined exposure estimate (non‐brand loyal scenario) was 0.09 mg/kg bw per day in children (3–9 years) and the highest 95th percentile of exposure was 0.20 mg/kg bw per day in children. Taking uncertainties into account, the Panel concluded that these exposure estimates very likely overestimate the real exposure to extracts of rosemary (E 392) from its use as a food additive according to Annex II.
Documentation provided to EFSA
FDE (FoodDrinkEurope), 2017. Data on usage levels of extracts of rosemary (E 392) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 29 November 2017.
ICGA (International Chewing Gum Association), 2017. Data on usage levels of extracts of rosemary (E 392) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 30 November 2017.
Association of the European Self‐Medication Industry (AESGP), 2017. Data on usage levels of extracts of rosemary (E 392) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 1 February 2018.
L'Alliance 7, 2017. 2017. Data on usage levels of extracts of rosemary (E 392) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 30 November 2017.
IMACE, 2017. 2017. Data on usage levels of extracts of rosemary (E 392) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 29 November 2017.
European Potato Processors’ Association (EUPPA), 2017. 2017. Data on usage levels of extracts of rosemary (E 392) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 30 November 2017.
EU Fish Processors and Traders Association ‐ European Federation of National Organizations of Importers and Exporters of Fish (AIPCE‐CEP), 2017. Data on usage levels of extracts of rosemary (E 392) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 30 November 2017.
Intersnack, 2017. Data on usage levels of 2017. Data on usage levels of extracts of rosemary (E 392) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 29 November 2017.
Abbreviations
- ADI
acceptable daily intake
- AESGP
Association of the European Self‐Medication Industry
- AFC
EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food
- AIPCE‐CEP
EU Fish Processors and Traders Association ‐ European Federation of National Organizations of Importers and Exporters of Fish
- ANS
EFSA Panel on Food Additives and Nutrient Sources added to Food
- bw
body weight
- CAS
Chemical Abstracts Service
- EUPPA
European Potato Processors’ Association
- FC
food category
- FCS
food categorisation system
- FDE
Food Drink Europe
- GNPD
Global New Products Database
- ICGA
International Chewing Gum Association
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- MOS
margin of safety
- MPL
maximum permitted limit
- NOAEL
no observed adverse effect level
- SCF
Scientific Committee on Food
Appendix A – Summary of the reported use levels (mg/kg or mg/L as appropriate) of extracts of rosemary (E 392) provided by industry
Appendix B – Number and percentage of food products labelled with extracts of rosemary (E 392) out of the total number of food products present in the Mintel GNPD per food sub‐category between 2013 and 2018
Appendix C – Concentration levels of extracts of rosemary (E 392) used in the refined exposure scenarios (mg/kg or mL/kg as appropriate)
Appendix D – Summary of total estimated exposure of extracts of rosemary (E 392) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Appendix E – Main food categories contributing to exposure to extracts of rosemary (E 392) using the regulatory maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Appendix F – Summary of dietary intake of carnosol and carnosic acid from natural diet per population group and survey: mean and 95th percentile (mg/kg bw per day)
1.
Appendix Appendix A – Summary of the reported use levels (mg/kg or mg/L as appropriate) of extracts of rosemary (E 392) provided by industry, Appendix B – Number and percentage of food products labelled with extracts of rosemary (E 392) out of the total number of food products present in the Mintel GNPD per food sub‐category between 2013 and 2018, Appendix C – Concentration levels of extracts of rosemary (E 392) used in the refined exposure scenarios (mg/kg or mL/kg as appropriate), Appendix D – Summary of total estimated exposure of extracts of rosemary (E 392) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day), Appendix E – Main food categories contributing to exposure to extracts of rosemary (E 392) using the regulatory maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)–F can be found in the online version of this output (‘Supporting information’ section)
Supporting information
Summary of the reported use levels (mg/kg or mg/L as appropriate) of extracts of rosemary (E 392) provided by industry
Number and percentage of food products labelled with extracts of rosemary (E 392) out of the total number of food products present in the Mintel GNPD per food sub‐category
Concentration levels of extracts of rosemary (E 392) used in the refined exposure scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of extracts of rosemary (E 392) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to extracts of rosemary (E 392) using the regulatory maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Summary of dietary intake of carnosol and carnosic acid from natural diet per population group and survey: mean and 95th percentile (mg/kg bw per day)
Suggested citation: EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Lambré C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Boon P, Lindtner O, Tlustos C, Tard A and Leblanc J‐C, 2018. Scientific Opinion on the refined exposure assessment of extracts of rosemary (E 392) from its use as food additive. EFSA Journal 2018;16(8):5373, 25 pp. 10.2903/j.efsa.2018.5373
Requestor: European Commission
Question number: EFSA‐Q‐2013‐00691
Panel members: Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipič, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Gunter Georg Kuhnle, Claude Lambré, Jean‐Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The Panel wishes to thank the WG on Exposure Assessment for the preparatory work on this scientific output The ANS Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 28 June 2018
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © FAO/WHO 2016
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
COM(2001) 542 final.
Food Additives in Europe 2000, Status of safety assessments of food additives presently permitted in the EU, Nordic Council of Ministers, TemaNord 2002, 560.
Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). OJ L 396, 30.12.2006.
Available online: http://ec.europa.eu/consumers/cosmetics/cosing/index.cfm?fuseaction=search.simple
Available online: http://ec.europa.eu/food/food/animalnutrition/feedadditives/comm_register_feed_additives_1831-03.pdf
Regulation (EC) No 1831/2003 of the European Parliament and the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, 29–43.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p 1.
Call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 6) http://www.efsa.europa.eu/sites/default/files/consultation/170223.pdf
Available online: http://www.efsa.europa.eu/en/food-consumption/comprehensive-database
Commission Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1–295.
Missing Bulgaria, Cyprus, Estonia, Latvia, Lithuania, Luxembourg, Malta and Slovenia.
http://www.gnpd.com/sinatra/home/ accessed on 26/02/2018.
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
Council Directive 2001/114/EC of 20 December 2001 relating to certain partly or wholly dehydrate preserved milk for human consumption. OJ L 15, 17.1.2002, p. 19.
Council Regulation (EC) No 1234/2007 of 22 October 2007 establishing a common organisation of agricultural markets and on specific provisions for certain agricultural products. OJ L 299, 16.11.2007, p. 1.
Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. OJ L 342, 22.12.2009, p. 59.
References
- ECHA , online. Available online: https://echa.europa.eu/brief-profile/-/briefprofile/100.075.694
- EFSA (European Food Safety Authority), 2007. Scientific opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA AFC Panel (EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food), 2008. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission on the use of rosemary extracts as a food additive. EFSA Journal 2008;6(6):721, 29 pp. 10.2903/j.efsa.2008.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2015. Scientific Opinion on extension of use of extracts of rosemary (E 392) in fat‐based spreads. EFSA Journal 2015;13(5):4090, 22 pp. 10.2903/j.efsa.2015.4090 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2017. Statement on the approach followed for the refined exposure assessment as part of the safety assessment of food additives under re‐evaluation. EFSA Journal 2017;15(10):5042, 9 pp. 10.2903/j.efsa.2017.5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: General Principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- FooDB version 1.0 database . Available online: http://foodb.ca/compounds/FDB014905
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2016. Evaluation of certain food additives. Eighty‐second report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization Technical Report Series, 1000, 45–54.
- Loussouarn M, Krieger‐Liszkay A, Svilar L, Bily A, Birtić S and Havaux M 2017. Carnosic Acid and Carnosol, Two Major Antioxidants of Rosemary, Act through Different Mechanisms. Plant Physiology, 175, 1381–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis JC and Johnson CB, 2005. Seasonal variations of rosmarinic and carnosic acids in rosemary extracts. Analysis of their in vitro antiradical activity. Spanish Journal of Agricultural Research, 3, 106–112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the reported use levels (mg/kg or mg/L as appropriate) of extracts of rosemary (E 392) provided by industry
Number and percentage of food products labelled with extracts of rosemary (E 392) out of the total number of food products present in the Mintel GNPD per food sub‐category
Concentration levels of extracts of rosemary (E 392) used in the refined exposure scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of extracts of rosemary (E 392) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to extracts of rosemary (E 392) using the regulatory maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Summary of dietary intake of carnosol and carnosic acid from natural diet per population group and survey: mean and 95th percentile (mg/kg bw per day)


