Abstract
In compliance with Article 43 of Regulation (EC) No 396/2005, EFSA received a mandate from the European Commission to provide an update of the reasoned opinion on the review of existing maximum residue levels (MRLs) for imazalil published on 5 September 2017, taking into account the additional information provided on the toxicity of the metabolites R014821, FK‐772 and FK‐284. EFSA did not derive MRL proposals from the post‐harvest uses reported on citrus fruits, apples, pears, potatoes, bananas and melons because the assessment of the toxicological properties of the metabolite R014821 (expected to occur following post‐harvest application of imazalil) could not be finalised. Risk managers should be made aware that the genotoxic potential of the metabolite R014821 could not be ruled out. For all these commodities, a decision on the residue definition for risk assessment could not be taken, which is a perquisite to perform a reliable dietary risk assessment. For the other commodities and considering fall‐back Good Agricultural Practices (GAPs) when possible, some information required by the regulatory framework was missing. Hence, although no apparent risk to consumers was identified, the consumer risk assessment is considered indicative only and some MRL proposals derived by EFSA still require further consideration by risk managers. It is noted that MRL proposals in commodities of animal origin were not derived because, provided that GAPs with post‐harvest applications would be withdrawn, the livestock exposure is not expected to exceed the trigger value. Nevertheless, it is noted that lacking of information/data (in particular on the toxicity of metabolites FK‐772 ad FK‐284) was also identified, which prevent from proposing residue definition for enforcement and risk assessment in livestock commodities.
Keywords: imazalil, MRL review, Regulation (EC) No 396/2005, consumer risk assessment, imidazole, pesticide, fungicide
Summary
Imazalil was included in Annex I to Directive 91/414/EEC on 1 January 1999 by Commission Directive 97/73/EC. The active substance has been approved under Regulation (EC) No 1107/2009, by Commission Implementing Regulation (EU) No 705/2011, which entered into force on 1 January 2012 amending the Annex to Commission Implementing Regulation (EU) No 540/2011, as amended by Commission Implementing Regulation (EU) No 541/2011.
In 2014, the Imazalil Task Force submitted an application in accordance with Article 6(1) and 7 of the Regulation to the Netherlands, the evaluating Member State (EMS), requesting a modification of existing maximum residue levels (MRLs) for citrus fruits, apples, pears, bananas and potatoes. The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 8 May 2015. When assessing the evaluation report, EFSA identified data gaps, related to the toxicological profiles of metabolites R014821, FK‐722 and FK‐284, which needed further clarifications and suspended its evaluation in October 2015 to request additional information to the EMS. In 2017, the Imazalil Task force provided to the EMS additional clarifications and information which were still considered not sufficient to address the identified data gaps.
The assessment of the all existing MRLs in compliance with Article 12(2) of Regulation (EC) No 396/2005 was issued by EFSA on 9 August 2017 and the data gaps related to the afore mentioned metabolites were highlighted, indicating that these data were needed to confirm some tentatively proposed MRLs and existing EU MRLs.
In the meantime, in March 2018, EFSA received an updated Evaluation Report from the Netherlands where additional information was assessed. The EMS concluded that the data on metabolites R014821, FK‐772 and FK‐284 were still not sufficient to conclude on the genotoxicity of these three compounds. EFSA issued a reasoned opinion on 8 June 2018 pursuant to Article 10 of the Regulation, supporting the conclusions from the EMS. Following the latest conclusions from the reasoned opinion on imazalil and the related toxicological information on the metabolites R014821, FK‐772 and FK‐284; on 29 June 2018, EFSA received from the European Commission the mandate to deliver, in accordance with Article 43 of Regulation (EC) No 396/2005, an updated reasoned opinion on the existing MRLs for imazalil. Specifically, EFSA was requested to assess the impact of this new toxicological information on the uses assessed under the Article 12 review, reconsidering, where relevant:
the residue definitions for risk assessment for plant and animal commodities;
the MRLs and risk assessment values derived for plant and animal commodities;
the acceptability of the existing codex maximum residue limits (CXLs);
the consumer risk assessment.
In addition, on the basis of the Good Agricultural Practice (GAPs) and supporting data already submitted in the framework of the MRL review carried out under Article 12 of the Regulation, EFSA was asked to identify possible fall‐back MRLs. No further Member States consultation was considered needed in the framework of this mandate.
The metabolism of imazalil was investigated for three different modes of applications (foliar, post‐harvest and seed treatment) in three different crop groups (cereals, fruit crops and root crops), hereby covering all uses under assessment. Based on the available studies, the residue definition for enforcement was proposed as imazalil (any ratio of constituent isomers). Imazalil can be enforced with a limit of quantification (LOQ) of 0.01 mg/kg in the four main plant matrices. For risk assessment purpose, the residue definition imazalil (any ratio of constituent isomers) could be proposed for commodities subject to foliar and seed treatment. However, a decision on the residue definition for risk assessment could not be derived for commodities subject to post‐harvest treatment. The tentative residue definition for risk assessment previously proposed as the ‘sum of imazalil and R014821, expressed as imazalil’ has been suspended because toxicological data for the metabolite R014821, which is expected to occur following post‐harvest uses of imazalil, are missing.
The nature of residues is unchanged through standard hydrolysis. No conclusion could be proposed regarding the nature of residues in rotational crops as no metabolism studies are available.
The available residue trials data allowed deriving (tentative) MRL proposals as well as risk assessment values for all commodities under evaluation, except for peppers (where no data were available) and melons (for which the number of data was insufficient to derive a MRL). Tentative MRLs were also derived for cereal straw in view of the future need to set MRLs in feed items. Considering that a residue definition for commodities underdoing post‐harvest treatment was not proposed, risk assessment values could not be derived from the GAPs with post‐harvest applications. Therefore, although MRLs could be calculated from different post‐harvest uses reported on citrus fruits, apples, pears, bananas and potatoes, no risk assessment values were derived from these GAPs. For potatoes, fall‐back MRL and risk assessment values could be derived from the seed treatment GAP, which is fully supported by data. However, for citrus fruits, apples, pears, bananas and melons, no alternative GAPs were available to derive fall‐back MRL and risk assessment values.
Robust processing factors were derived for peeled fruits (citrus fruits, bananas and melons) as well as for many processed commodities of oranges (juice, dry pomace, wet pomace and marmalade), apples (juice and wet pomace) and potatoes (unpeeled/boiled, peeled/boiled and fried). For the other processed commodities assessed in this review, the processing factors are considered tentative because of the limited number of data.
Considering that no risk assessment values could be derived for the critical GAPs authorised on citrus fruits, apples and potatoes, a comprehensive dietary burden considering all GAPs reported in this review could not be calculated. Livestock dietary burden calculations were then calculated without considering the GAPs with post‐harvest applications. For potatoes, the risk assessment values derived from the seed treatment GAP were considered while for citrus fruits and apples no input values could be considered. The calculated dietary burdens hereby calculated were found to be below the trigger value of 0.1 mg/kg dry matter (DM) for all groups of livestock. Therefore, MRL and risk assessment values in livestock commodities are not needed provided that GAPs with post‐harvest treatment would be withdrawn.
Chronic and acute consumer exposure was calculated using revision 2 of the EFSA Pesticide Residues Intake Model (PRIMo). In the absence of a final conclusion on the residue definition for risk assessment for the plant commodities subject to post‐harvest applications, the consumer exposure calculations were performed without considering the GAPs with post‐harvest applications authorised on citrus fruits, apples, pears, potatoes, bananas and melons. For potatoes, fall‐back MRL and risk assessment values could be derived from the seed treatment GAP and for melons a GAP with foliar application was tentatively assessed although not supported by data. For those commodities where data were insufficient to derive a MRL, EFSA considered the existing EU MRL for an indicative calculation. For melons, an exceedance of the acute reference dose (ARfD) was identified representing 606.7% of the ARfD. Excluding this MRL from the calculation, the highest chronic exposure represented 1.7% of the acceptable daily intake (ADI) (WHO Cluster diet B) and the highest acute exposure amounted to 18.6% of the ARfD (tomatoes).
Apart from the MRLs evaluated in the framework of this review, internationally recommended CXLs have also been established for imazalil. Additional calculations of the consumer exposure, considering these CXLs, were therefore carried out, noting that the CXLs derived from post‐harvest uses have not been considered. Exceedance of the ARfD was identified for the existing CXL in persimmon (131%). Excluding this CXL from the calculation, the highest chronic exposure represented 6.0% of the ADI (German children) and the highest acute exposure amounted to 62.4% of the ARfD (strawberries).
Background and Terms of Reference as provided by the requestor
Imazalil was included in Annex I to Council Directive 91/414/EEC on 1 January 1999 by means of Commission Directive 97/73/EC,1 and has been approved under Regulation (EC) No 1107/2009,2 by Commission Implementing Regulation (EU) No 705/2011, which entered into force on 1 January 2012 amending the Annex to Commission Implementing Regulation (EU) No 540/2011,3 as amended by Commission Implementing Regulation (EU) No 541/2011.4
On 5 February 2014, the Imazalil Task Force submitted to the Netherlands, the evaluating Member State (EMS), an application in accordance with Article 6(1) and 7 of the Regulation to modify the existing maximum residue levels (MRLs) for citrus fruits, apples, pears, bananas and potatoes. The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/20055 , which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 8 May 2015. When assessing the evaluation report (Netherlands, 2015), EFSA identified data gaps, related to the toxicological profiles of metabolites R014821, FK‐722 and FK‐284, which needed further clarifications and suspended its evaluation on 23 October 2015 to request additional information to the EMS. On 17 December 2017, EFSA received the updated evaluation report from the EMS. As not all the data gaps were considered addressed, on 10 January 2018, the clock stop was maintained in order to address the outstanding issues.
In the meantime, the assessment of the all existing MRLs in compliance with Article 12(2) of Regulation (EC) No 396/2005 was issued by EFSA on 9 August 2017 and the data gaps related to the afore mentioned metabolites were highlighted, indicating that these data were needed to confirm some tentatively proposed MRLs and existing EU MRLs.
On 16 March 2018, EFSA received an updated evaluation report from the Netherlands related to the MRL application, where the additional information was assessed. The EMS concluded that the data on metabolites R014821, FK‐772 and FK‐284 were still not sufficient to conclude on the genotoxicity of these three compounds. EFSA issued a reasoned opinion on 8 June 2018 pursuant to Article 10 of the Regulation, supporting the conclusions from the Netherlands (EFSA, 2018). Following the latest conclusions from the reasoned opinion on imazalil and the related toxicological information on the metabolites R014821, FK‐772 and FK‐284, on 29 June 2018, EFSA received from the European Commission the mandate to deliver, in accordance with Article 43 of Regulation (EC) No 396/2005, an updated reasoned opinion on the existing MRLs for imazalil. Specifically, EFSA was requested to assess the impact of this new toxicological information on the uses assessed under the Article 12 review, reconsidering, where relevant:
the residue definitions for risk assessment for plant and animal commodities;
the MRLs and risk assessment values derived for plant and animal commodities;
the acceptability of the existing codex maximum residue limits (CXLs);
the consumer risk assessment.
In addition, on the basis of the Good Agricultural Practice (GAPs) and supporting data already submitted in the framework of the MRL review carried out under Article 12 of the Regulation (Belgium, 2016; EURL, 2016; France, 2016; Germany, 2016; Italy, 2016; Netherlands, 2016; Portugal, 2016; Spain, 2016; EFSA, 2017; Greece, 2018), EFSA was asked to identify possible fall‐back MRLs. No further collection of GAPs or data from Member States (MSs) was considered needed in the framework of this mandate.
The chronic and acute exposure calculations for all crops reported in the framework of the present updated review performed using the EFSA Pesticide Residues Intake Model (PRIMo) (excel file) and the PROFile are key supporting documents and made publicly available as background documents to this reasoned opinion. Furthermore, a screenshot of the Report sheet of the PRIMo is presented in Appendix C.
Regulatory information on the active substance and its use pattern
Imazalil is the ISO common name for (RS)‐1‐(β‐allyloxy‐2,4‐dichlorophenethyl)imidazole or allyl (RS)‐1‐(2,4‐dichlorophenyl)‐2‐imidazol‐1‐ylethyl ether (IUPAC). Imazalil is a racemic mixture.
Imazalil belongs to the group of imidazole compounds which are used as fungicide.
The chemical structure of the active substance and its main metabolites are reported in Appendix E.
Imazalil was evaluated in the framework of Directive 91/414/EEC with Belgium designated as rapporteur Member State (RMS). The representative uses supported for the peer review process were dipping/drenching or spraying waxing for citrus (post‐harvest), foliar spray applications for tomatoes grown on artificial substrate, and seed treatment for winter and spring barley and wheat.
Following the first peer review, in which EFSA was not yet involved, a decision on inclusion of the active substance in Annex I to Directive 91/414/EEC was published by means of Commission Directive 97/73/EC, which entered into force on 1 January 1999. EFSA carried out the peer review of the pesticide risk assessment for imazalil for its renewal in the framework of Commission Regulation (EC) No 737/20076, with the Netherlands designated as RMS and Spain as co‐rapporteur Member State (co‐RMS). Imazalil has been approved under Regulation (EC) No 1107/2009 by means of Commission Implementing Regulation (EU) No 705/20117 which entered into force on 1 January 2012. It was a specific provision of the approval that the applicant was required to submit to the European Commission further studies on confirmatory information as regards the environmental fate and behaviour of imazalil and its residues in processed commodities. EFSA finalised a technical report on the confirmatory assessment for imazalil on 8 October 2014.
The EU MRLs for imazalil are established in Annexes II and III of Regulation (EC) No 396/2005, as amended by Commission Regulation (EU) No 750/20108 . The review of the all existing MRLs in compliance with Article 12 of Regulation (EC) No 396/2005 was issued by EFSA on 9 August 2017 (EFSA, 2017). The proposed modifications have not yet been implemented in the EU MRL legislation
A reasoned opinion on the modification of existing MRLs in various commodities was issued in June 2018 (EFSA, 2018) taking into account new toxicological information concerning the metabolites R014821, FK‐772 and FK‐284.
For the purpose of this updated review, the critical GAPs and the possible fall‐back GAPs already reported in the MRL review, were considered. The details of the authorised GAPs for imazalil considered in this assessment, including the identified fall‐back GAPs, are given in Appendix A.
Data and methodologies
EFSA has based its assessment on the previous reasoned opinion on the MRL review under Article 12 (EFSA, 2017), including all the data made available by RMS and MSs in that framework (Belgium, 2016; EURL, 2016; France, 2016; Germany, 2016; Italy, 2016; Netherlands, 2016; Portugal, 2016; Spain, 2016; Greece, 2018), the assessment reports and their addenda prepared under the first peer review (Belgium, 1996) and under Regulation (EC) No 1107/2009 (Netherlands, 2009a,b, 2014), the EFSA conclusion on the peer review of the pesticide risk assessment of the active substance imazalil in the context of the renewal procedure under Commission Regulation (EC) No 737/2007 (EFSA, 2010), the Joint Meeting on Pesticide residues (JMPR) Evaluation report (FAO, 1977, 1984, 1985, 1994) as well as the recent reasoned opinion on the modification of the existing MRLs for imazalil (EFSA, 2018) and the related evaluation report prepared by the Netherlands (2015). The assessment is performed in accordance with the legal provisions of the uniform principles for evaluation and authorisation of plant protection products as set out in Commission Regulation (EU) No 546/20119 and the currently applicable guidance documents relevant for the consumer risk assessment of pesticide residues (European Commission, 1997a,b,c,d,e,f,g, 2000,b, 2017; OECD, 2011, 2013).
More detailed information on the available data and on the conclusions derived by EFSA can be retrieved from the list of end points reported in Appendix B.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of imazalil was investigated for foliar application (tomatoes), post‐harvest application (oranges, apples, potatoes) and seed treatment (wheat and potatoes) (Belgium, 1996, Netherlands, 2009a, 2015), hereby covering all uses under assessment.
After foliar or post‐harvest applications, imazalil is a major constituent of the residues in fruit crops and potatoes (80% of the total radioactive residues (TRR) in tomato, 89–99% TRR in orange/apples and 69–94% TRR in potatoes). No other compounds above 10% of the TRR were found in tomatoes (foliar treatment only). However, when considering a long withholding period (WHP, 6–7 months) after post‐harvest applications, the metabolite R01482110 was observed in significant proportions (11% TRR in apples and 9% TRR in potatoes) and significant absolute levels (> 0.11 mg/kg in apples and 1.27–1.54 mg/kg in potatoes peel and surface wash). Another degradation product (metabolite R04417711 ) was found in potatoes at a level remaining below 4% TRR. These studies indicate that imazalil is likely to degrade into metabolite R014821 during the storage of post‐harvest treated commodities (fruit and root crops). This degradation was not observed in fruit crops sampled early after foliar application (with a short preharvest interval (PHI)).
Studies performed with seed treatment showed much lower residue levels in cereal grains and potato tubers where the TRR always remain below 0.01 mg eq/kg. Therefore, no further attempt was carried out to characterise the residues in these matrices. However, the residue levels measured in wheat forage (1.36 mg eq/kg) and straw (0.15 mg eq/kg) indicate that, following the exaggerated application rate of 49 g a.s./100 kg seeds (> 6N compared to GAP), transfer of residues from seed to other parts of the crop may occur. The parent compound was extensively degraded in wheat forage and straw, only representing 17–24% of the TRR. The degradation products were not identified as the highest peak observed in straw does only represent 11% TRR (< 0.02 mg eq/kg). Also considering that this study is highly overdosed compared to GAP, further identification of the residue in these matrices is not deemed necessary.
Chiral analyses were performed in the study performed on potato tubers. These analyses indicate that the S/R ratios of imazalil enantiomers remain unchanged during the storage period after post‐harvest application (Netherlands, 2015).
1.1.2. Nature of residue in rotational crops
Imazalil is authorised on crops that may be grown in rotation such as cereals and potatoes (where seed treatments are authorised) as well as tomatoes, sweet peppers and cucurbits with edible peel (where foliar treatments are authorised). It is noted that although foliar treatments are only authorised for ‘indoor’ uses, there is no evidence that these GAPs are restricted to artificial substrates. Therefore, these GAPs are also relevant for rotational crops. According to the soil degradation studies evaluated in the framework of the peer review under the renewal process, the geometric mean of the DT50 value is 93.2 days (EFSA, 2010). Therefore, the DT90 value of imazalil is expected to be much higher than the trigger value of 100 days. According to the European guidelines on rotational crops (European Commission, 1997b), further investigation of residues in rotational crops is relevant.
Studies investigating the nature of residues in rotational crops are not available. The RMS made an attempt to theoretically estimate the residue levels that would occur if succeeding crops would be sown after a crop failure on potatoes previously subject to seed treatment (Netherlands, 2015). The RMS considered the case of a normal rotation (i.e. rotational crop harvested 15 months/450 days after planting of the treated seed potatoes) and a more critical scenario where the rotational crop is sown immediately after a crop failure. For this latter scenario, the RMS considered a period of 150 days between planting of the treated seed potatoes and harvest of the following crop. The theoretical estimation of the RMS took into account an ‘application rate’ of 31.5 g a.s./ha12 and a breakdown kinetic in soil corresponding to a DT50 of 93.2 days13 . With these assumptions, residue levels in rotational crops would always remain below 0.004 mg/kg assuming that the residue concentration in the crop would be equal to the residue concentration in soil (transition factor of 1). However, there is still an uncertainty regarding this potential transition factor and considering higher transition factors of 5 or 10, residues above 0.01 mg/kg may occur under the scenario of a crop failure. Furthermore, the RMS and EFSA have still reservations regarding the highest residue (HR) value of 4.6 mg/kg observed on potatoes (see also Section 1.2.1), which is an important assumption in this theoretical calculation.
In addition, the above calculation does not address the rotational crops sown after a crop failure on cereals (for which the application rate is five times higher than for potatoes14 ) or on the fruiting vegetables crops where foliar applications are authorised and for which a period lower that 150 days between planting of the treated seed potatoes and harvest of the following crop may need to be considered. Therefore, EFSA is of the opinion that the theoretical calculation reported by the RMS does not sufficiently address the data gap already identified during the peer review under the renewal procedure (EFSA, 2010). Therefore, studies investigating the nature of residues in rotational crops are still required. This data gap is linked to all uses with foliar and seed treatment. Consequently, if this data gap is not addressed in the future, MSs are recommended to withdraw or modify their relevant authorisations for foliar treatment and seed treatment (e.g. to restrict the foliar uses to artificial substrate only, etc.).
1.1.3. Nature of residues in processed commodities
The effect of processing on the nature of residues was investigated after the peer review, in the framework of the confirmatory data process (Netherlands, 2014). Studies were conducted with imazalil, simulating representative hydrolytic conditions for pasteurisation (20 minutes at 90°C, pH 4), boiling/brewing/baking (60 minutes at 100°C, pH 5) and sterilisation (20 minutes at 120°C, pH 6). Although this study was not conducted with radiolabelled material, the test compound was found at an amount of 94–99% after any kind of hydrolysis. Therefore, it was concluded that processing by pasteurisation, baking/brewing/boiling and sterilisation is not expected to have a significant impact on the composition of residues in matrices of plant origin.
1.1.4. Methods of analysis in plants
During the peer review under the renewal procedure, an analytical method using high–performance liquid chromatography with tandem mass spectrometry (HPLC–MS/MS) was validated for the determination of imazalil in high water content, high oil content and dry commodities with a limit of quantification (LOQ) of 0.01 mg/kg (EFSA, 2010). In the framework of the MRL review, the RMS provided validation data on high acid content commodities for a new analytical method also using HPLC–MS/MS (Netherlands, 2015). Independent laboratory validation (ILV) data for these methods are available for high water content commodities (EFSA, 2010), high acid content commodities (Netherlands, 2015) and dry commodities (Germany, 2016).
Hence, it is concluded that imazalil can be enforced with a LOQ of 0.01 mg/kg in high water content, high acid content, high oil content and dry commodities. This conclusion was also confirmed by the EURLs during the completeness check (EURL, 2016).
1.1.5. Stability of residues in plants
In the framework of the peer review under the renewal procedure, storage stability of imazalil was demonstrated for a period of 6 months at −18°C in commodities with high water content, dry commodities and in cereal straw (EFSA, 2010). The storage stability of the metabolite R014821 was not investigated in these studies.
New studies assessed by the RMS in the framework of the MRL review and in the recent MRL application demonstrated the storage stability for imazalil and its metabolite R014821 in matrices with water and high acid content. When stored deep frozen (at −20°C), both compounds are stable for a period of 12 months in matrices with high water content (Netherlands, 2015) and for a period of 8 months in matrices with high acid content (EFSA, 2018).
1.1.6. Proposed residue definitions
Residue definition for enforcement:
Based on the available metabolism studies performed on three different crop categories and with different modes of application, the parent compound is identified as a sufficient marker for enforcement purpose in all crop categories and for any kind of treatment. Therefore, the residue definition for enforcement can be defined as imazalil only. As imazalil is a mixture of two enantiomers, the following wording is proposed: imazalil (any ratio of constituent isomers). This residue definition also applies to processed commodities as the nature of residues is unchanged through standard hydrolysis. However, no final conclusion could be derived regarding the nature of residues in rotational crops since studies are still missing.
Residue definition for risk assessment:
Imazalil is also relevant for risk assessment purpose but is not the only toxicologically relevant compound observed in plant commodities. In particular, the metabolite R014821, which is formed by O‐dealkylation of the parent compound, was found to be formed in significant proportions in plant commodities subject to post‐harvest treatment; the amount of R014821 increased with time. This was confirmed by many residue trials including simultaneous analysis of imazalil and R014821 after a WHP of 3 months (see Section 1.2.1).
The MRL review was based on the assumption that metabolite R014821 is of similar toxicity as the parent compound; thus, the risk assessment was performed for the tentative residue definition for risk assessment defined in the peer review as the ‘sum of imazalil and R014821, expressed as imazalil’. This proposal was tentative pending the full assessment of the toxicological properties for metabolite R014821 (EFSA, 2010, 2017).
In the framework of a MRL application, the applicant provided additional toxicological studies to address the data gaps for metabolite R014821 (Netherlands, 2015). The additional data submitted were found to be insufficient to clearly rule out a genotoxic potential of this compound and to conclude whether the toxicological reference values derived for parent imazalil would be appropriate for this metabolite (Netherlands, 2015; EFSA, 2018).
Considering that metabolite R014821 is, according to the current knowledge, relevant only for commodities subject to post‐harvest treatment, the tentative residue definition for risk assessment previously proposed as the ‘sum of imazalil and R014821, expressed as imazalil’ should be suspended for crops with post‐harvest uses until the hazard characterisation for metabolite R014821 is finalised.
In metabolism studies performed with foliar treatment, metabolite R014821 is a very minor metabolite (< 0.3% TRR; < 0.01 mg/kg in tomatoes) even after an exaggerated application rate (3 × 1500 g a.s./ha) compared with the authorised foliar uses assessed in the framework of the MRL review (between 3 × 75 g a.s./ha up to 3 × 300 g a.s./ha). It is noted that these metabolism studies only provide information on samplings performed at very short PHI (1 day after the last application; 21 days after the first application), not allowing identification of degradation products that may be formed after a longer period. The findings of the metabolism studies as regards the absence of metabolite R014821 are confirmed by four residue trials performed on cucumbers with foliar applications investigating residues of parent and metabolite R014821 from day zero to day 7. Furthermore, it is noted that the overall residue levels measured after foliar treatment are much lower compared what can be found after post‐harvest applications. Based on these data, the metabolite R014821 is not expected to be found in significant concentrations in plant commodities subject to foliar applications performed according to the authorised GAPs. Consequently, the residue definition for risk assessment for commodities subject to foliar application is proposed to be restricted as parent compound only. However, considering that the formation of metabolite R014821 in commodities subject to post‐harvest treatment was found to increase with time, it is recommended that for future residue trials the absence of metabolite R014821 should be investigated, analysing samples of treated crops for parent compound and metabolite R014821, including also samples which were taken at a longer PHI than the minimum PHI defined in the GAP. If necessary, the residue definition has to be reconsidered in the future.
For seed treatment, the metabolism studies clearly show that metabolite R014821 is not expected. Therefore, the residue definition for risk assessment as parent compound also applies to this type of treatment.
As the nature of residues is not expected to be affected by processing, the conclusions on the residue definitions for risk assessment also apply to processed commodities. Therefore, for processed commodities derived from raw commodities following post‐harvest treatment, no residue definition for risk assessment can be proposed. For the processed commodities derived from raw commodities which are treated with foliar or seed treatments, the residue definition for risk assessment as parent compound applies.
In addition, EFSA emphasises that, except the metabolism study performed with ware potatoes, the available metabolism studies do not investigate the possible impact of plant metabolism on the isomer ratio of imazalil and further investigation on this matter would in principle be required. Since guidance on the consideration of isomer ratios in the consumer risk assessment is not yet available, EFSA recommends that this issue is reconsidered when such guidance becomes available.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
To assess the magnitude of imazalil residues resulting from the authorised GAPs reported in this review, EFSA considered all residue trials made available by the RMS and MSs (Netherlands, 2015, 2016; France, 2016; Greece, 2016; Spain, 2016), including residue trials evaluated in the framework of the peer reviews (Belgium, 1996; Netherlands, 2009a,b, EFSA, 2010). All residue trial samples considered in this framework were stored in compliance with the storage conditions for which storage stability of the parent compound and its metabolite R014821 were demonstrated (EFSA, 2017, 2018). Decline of residue levels during storage of the trial samples is therefore not expected.
The number of residue trials and extrapolations were evaluated in accordance with the European guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs (European Commission, 2016).
GAPs with seed treatment and foliar treatment:
Seed treatments are authorised on potatoes and cereals and foliar treatments are authorised on tomatoes, peppers, cucumbers, courgettes, gherkins and melons. For all these GAPs, the metabolite R014821 is not expected to occur (see also Section 1.1.6). Therefore, residue trials analysing only for the parent compound can be used to derive MRL and risk assessment values. However, the following data gaps were identified for sweet peppers/bell peppers, cucumbers, courgettes, gherkins and melons:
Sweet peppers/bell peppers: residue trials are not available to support the reported GAP and MRL or risk assessment values cannot be derived for this crop. Therefore, eight trials on peppers compliant with the indoor GAP are required.
Cucurbits with edible peel (cucumbers, courgettes and gherkins): only four GAP‐compliant trials performed on cucumber are available to support the indoor GAP on these crops. Tentative MRL and risk assessment values can be derived from these data but four additional trials performed on cucumber and/or courgettes and compliant with the indoor GAP are still required.
Melons: residue trials are not available to support the reported GAP and MRL or risk assessment values cannot be derived for this crop. Therefore, eight trials on melons compliant with the indoor GAP with foliar application are required.
As recommended in Section 1.1.6, the above required residue trials should provide residue decline curves for parent and metabolite R014821.
GAPs with post‐harvest treatment:
Post‐harvest treatment are authorised on citrus fruits, apples, pears, bananas, potatoes and melons, for which several residue trials were reported. Some of the available trials were performed with simultaneous analysis of the parent compound and its metabolite R014821 at different WHPs. These trials demonstrated that metabolite R014821 can reach significant levels in plant commodities subject to post‐harvest treatment, especially when stored for more than 2 or 3 months. In citrus fruits, metabolite R014821 ranged from < 0.01 to 0.03 mg/kg in whole fruit samples taken at WHP of 0 day but increase significantly at WHP of 2–3 months, where it can reach levels up to 0.13 mg/kg. Similar results were observed for this compound in apples and pears (up to 0.18 mg/kg at WHP 4 months) as well as in potatoes (up to 0.26 mg/kg at WHP 5 months).
Based on the residue levels of parent compound, (tentative) MRLs could be calculated for all these commodities except melons. However, as the residue definition for risk assessment for post‐harvest uses has been suspended until the hazard characterisation for metabolite R014821 has been completed (see Section 1.1.6), no risk assessment values were derived for these commodities.
In addition, further data gaps and/or concerns were identified for the following GAPs:
Oranges/grapefruits (waxing): a sufficient number of trials is in principle available to support this GAP. However, these trials do not include analysis of the metabolite R014821. As this metabolite was identified as a potential contributor to the toxicological burden, further trials including simultaneous analysis of imazalil and metabolite R014821 (with decline curves at different WHPs from day zero to 6 months) should be generated.
Apples/pears (smoke can): only four trials are available to support this GAP. Therefore, only tentative MRL could be derived from this GAP15
Bananas (dipping): Four trials including analysis of parent and metabolite R014821 are available (Greece, 2018). It was noted that although these trials were performed on the same site and on the same day, they can be considered independent as they were performed on different varieties. However, as four trials are not sufficient to fully support this GAP15, only tentative MRL could be derived for this commodity. Four additional trials (including simultaneous analysis of imazalil and metabolite R014821 with decline curves until WHP 6 months) should be generated.
Potatoes (post‐harvest GAP): in four of the nine available residue trials, it was indicated by the RMS that samples were washed before analysis (Netherlands, 2015). However, these four trials do also show the highest residue levels (from 3.4 to 4.6 mg/kg) of the data set. Therefore, these results are deemed questionable and should be considered on a tentative basis only. The RMS is invited to provide further clarifications with regard to these data. In the meantime, MRL derived from the post‐harvest treatment on ware potatoes is deemed tentative.
Melons (drenching): only three trials are available, which is not sufficient to derive MRL for this GAP.15 Furthermore, these trials do not include analysis of the metabolite R014821. MRL and risk assessment values cannot be derived for this commodity and 8 trials (including simultaneous analysis of imazalil and metabolite R014821 with decline curves until WHP 6 months) should be generated.
1.2.2. Magnitude of residues in rotational crops
Studies investigating the nature and/or magnitude of residues in rotational crops are not available and are still required (see Section 1.1.2).
1.2.3. Magnitude of residues in processed commodities
Studies investigating the magnitude of residues in several processed commodities of citrus fruits, apples, potatoes and melons were evaluated in the framework of the peer reviews (Belgium, 1996; Netherlands, 2009a,b). In the framework of the MRL review, the RMS has evaluated and reported additional processing studies performed on these crops (Netherlands, 2015) and peeling factors for bananas were made available by Greece (2016, 2018).
An overview of all available processing studies is available in Appendix B.1.2.3.
Residue distribution in peel/pulp:
The transfer of residues from peel to pulp has been investigated in citrus fruits, bananas and melons. Overall, it is demonstrated that residue levels observed in pulp are generally lower compared to residue levels observed in whole fruits. Based on the available data, processing factors can be derived for citrus fruits (0.07), bananas (0.13) and melons (0.12), taking into account the following considerations:
Citrus fruits: more than 50 residue trials performed with different GAPs (all post‐harvest uses) on several citrus fruits are available. In order to derive a peeling factor for all citrus fruits, EFSA considered only the sampling performed after a WHP of 0 day (compliant with the critical GAP assessed in this review) and disregarded the data where residues were below LOQ in pulp. Based on these criteria, 36 peeling factors are available, as reported in Appendix B.1.2.3. No significant difference was observed between the peeling factors derived from oranges, mandarins, lemons and grapefruits, thus a general peeling factor of 0.07 was derived from the median value of the overall data set. However, in each crop, the available data show a wide distribution of the peeling factors, ranging from 0.01 to 0.28.
Bananas: residue data in pulp following post‐harvest treatment were not available at WHP 35 days (i.e. compliant with GAP) but only at WHP 28 days (Greece, 2018). Considering the slight difference between WHP 28 and 35 days, this is considered acceptable to derive a peeling factor. However, it is noted that the available data show a wide distribution of the peeling factors, ranging from 0.05 to 0.30.
Melons: residue data in pulp were available in the three trials compliant with GAP (post‐harvest use). In addition, as the other available residue trials performed with a higher application rate provide similar peel/pulp ratios, they were all considered to derive a more robust peeling factor (Belgium, 1996).
Other processing factors assessed in this review:
Robust processing factors were derived for processed commodities of oranges (juice, dry pomace, wet pomace and marmalade), apples (juice and wet pomace) and potatoes (unpeeled/boiled, peeled/boiled and fried). For other processed commodities where the data set was limited, the processing factors are considered tentative: apples (dry pomace and sauce), potatoes (unpeeled/microwaved, crisps and granules/flakes).
The above processing factors were derived on the basis of the residue definition for enforcement in raw and processed commodities, being parent compound only.
It is noted that for all processed commodities investigated in this opinion (processed commodities of citrus fruits, apples, bananas, potatoes and melons), GAPs with post‐harvest applications are authorised on the raw agricultural commodities (RAC) while no conclusion on the risk assessment residue definition was derived for this type of use (see Section 1.1.6). Consequently, no residue definition for risk assessment is available for the investigated processed commodities and thus no conversion factor (CF) from enforcement to risk assessment can be proposed. Such conversion factors may only be proposed when a final conclusion on the residue definition for risk assessment will be derived.
Further processing studies are not required. However, if more robust processing factors were to be required by risk managers, in particular for enforcement purposes, additional processing studies would be needed.
1.2.4. Proposed MRLs
Based on the available data, EFSA was able to calculate (tentative) MRL proposals for all commodities under evaluation, except for peppers (where no data were available) and melons (for which the number of data was insufficient to derive a MRL proposal). Tentative MRLs were also derived for cereal straw in view of the future need to set MRLs in feed items.
Considering the data gaps identified on the toxicological profile of metabolite R014821, a metabolite that is expected in crops where a post‐harvest use is authorised (see Sections 1.1.6 and 1.2.1) which did not allow to confirm the previously suggested residue definition for risk assessment, risk assessment values could not be derived from the GAPs with post‐harvest applications. Therefore, although MRLs could be calculated from different post‐harvest uses reported on citrus fruits, apples, pears, bananas and potatoes, these MRL proposals are not recommended by EFSA as long as no risk assessment can be performed.
For potatoes, a fall‐back MRL and risk assessment values can be derived from the seed treatment GAP, which is fully supported by data. However, for citrus fruits, apples, pears, bananas and melons, no alternative GAPs were available to derive fall‐back MRL and risk assessment values. Based on the information available under the present assessment, imazalil is only applied post‐harvest on citrus fruits, apples, pears and bananas. It was noted that a GAP with foliar application authorised is authorised on melons but, as this GAP is not supported by data, it could also not be used to derive MRL and risk assessment values.
2. Residues in livestock
2.1. Dietary burden and need for MRL in livestock commodities
Imazalil is authorised for use on citrus fruits, apples, potatoes and small grain cereals that might be fed to livestock. Therefore, the possible transfer of residues in commodities of animal origin should be assessed.
It is highlighted that a final conclusion on the residue definition for risk assessment could not be derived for the plant commodities subject to post‐harvest applications (see Section 1). Consequently, no risk assessment values could be derived for the critical GAPs authorised on citrus fruits, apples and potatoes, which are all potential significant contributors to the dietary burden. Therefore, a comprehensive dietary burden considering all critical GAPs currently authorised cannot be calculated.
Consequently, livestock dietary burden calculations were performed excluding the GAPs with post‐harvest applications. For potatoes, the risk assessment values derived from the seed treatment GAP were considered, while for citrus fruits and apples no input values could be considered in the absence of fall‐back GAPs (see also Section 1.2.4). This scenario is referred to as scenario EU1. These calculations were performed for different groups of livestock according to OECD guidance (OECD, 2013). The input values for all relevant commodities are summarised in Appendix D.1. It is highlighted that no conclusion was achieved regarding the potential residue uptakes in rotational crops. The animal intake of imazalil residues via rotational crops has therefore not been assessed.
The dietary burdens calculated for all groups of livestock are reported in Appendix B.2. The calculated dietary burdens were found to be below the trigger value of 0.1 mg/kg dry matter (DM) for all groups of livestock. Therefore, MRL and risk assessment values in livestock commodities are not needed.
It is highlighted that the above result does not take into account the potential intake due to post‐harvest treatment on citrus fruits, apples and potatoes. It is therefore recommended to MSs to reconsider or withdraw these uses.
2.2. Nature and magnitude of residues in livestock
Considering the scenario assessed in Section 2.1, MRL and risk assessment values for commodities of livestock origin are not needed. Nevertheless, studies investigating the nature and magnitude of residues in livestock are available. These studies were considered and discussed in the previous assessments of EFSA (EFSA, 2010, 2017, 2018). An updated assessment of these data is reported here in order to provide a comprehensive review in the case where further investigation would be needed in the future.
The metabolism of imazalil was investigated in lactating goats and laying hens (Belgium, 1996). The summary of the study with laying hens initially provided during the two peer reviews was not sufficient to conclude on a metabolic pathway in poultry as the identification of the metabolites was limited. Although further detailed results were provided by the RMS in its evaluation report (Netherlands, 2015), EFSA is of the opinion that an additional study would be needed to fully depict the metabolic pathway in poultry.
The available metabolism studies showed imazalil to be extensively metabolised in livestock.
In goat tissues, the parent compound represents less than 6% of the TRR and is not detected at all in milk. Two metabolites, FK‐772 (goat kidney and muscle) and FK‐284 (goat muscle), were found in higher proportions than the parent compound. These metabolites are the only degradation products representing more than 10% of the TRR in goat tissues (15–21% TRR) and they were also present in low proportion in milk (3–6% TRR).
In poultry, imazalil was only detected in eggs and fat, representing 8% and 11% of TRR, respectively. The metabolite FK‐772 was only retrieved in liver, where it accounted for less than 9% of the TRR. One metabolite (FK‐858) was found in proportion higher than 10% of the TRR in eggs and hen muscle (11–15% TRR) but corresponding to quite low levels in these matrices (0.02–0.09 mg eq/kg). In both ruminants and poultry, the remaining radioactivity consists of several minor metabolites, all remaining in very low proportions.
Based on the above results, imazalil may not be a sufficient marker in livestock commodities. The peer review under the renewal procedure concluded that metabolites FK‐772 and/or FK‐284 should be taken into account for enforcement purpose in ruminant matrices (EFSA, 2010). Assuming that these compounds share a similar toxicity as the parent compound and considering that metabolite FK‐772 was a better marker for enforcement compared to FK‐284, a residue definition for enforcement was previously and provisionally defined as the ‘sum of imazalil and its metabolite FK‐772 (any ratio of constituent isomers), expressed as imazalil’. For risk assessment, the proposal was extended to the sum of imazalil and all identified/characterised metabolites (EFSA, 2017). These proposals were tentative pending the full assessment of the toxicological properties for metabolites FK‐772 and/or FK‐284. In the framework of a MRL application, the applicant provided additional toxicological studies to address these data gaps (Netherlands, 2015). The additional data submitted were found to be insufficient to conclude whether the toxicological reference values derived for parent imazalil would be appropriate for these metabolites (EFSA, 2018). Consequently, a decision on the residue definitions for enforcement and risk assessment cannot be derived for livestock commodities. Thus, the tentative residue definitions for enforcement and risk assessment previously proposed are suspended. In addition, it was also noted that the available metabolism studies do not investigate the possible impact of the livestock metabolism on the isomer ratio of imazalil and its metabolites.
For information purpose, it is reported that a multi‐residue analytical method using HPLC–MS/MS was validated for the determination of imazalil, FK‐772 and FK‐284 in livestock tissues, with a LOQ of 0.01 mg/kg for each compound (Netherlands, 2015, assessed in EFSA, 2017). However, EURLs informed EFSA that no validation data were available for the metabolite FK‐772.
The magnitude of imazalil residues in livestock was investigated in one study performed with lactating cows and one study performed with laying hens, both assessed during the European peer review (Netherlands, 2009a). Depending on the dietary burden that might be calculated in the future and pending a final conclusion regarding the residue definitions for livestock commodities, these studies may be used to derive MRL and risk assessment values. It is however noted that some deficiencies related to these studies were highlighted in the previous EFSA opinion (EFSA, 2017).
3. Consumer risk assessment
In the framework of this review, only the uses of imazalil reported by the RMS in Appendix A were considered; however, the use of imazalil was previously also assessed by the JMPR (FAO, 1977, 1984, 1985, 1994). The CXLs, resulting from these assessments by JMPR and adopted by the CAC, are now international recommendations that need to be considered by European risk managers when establishing MRLs. To facilitate consideration of these CXLs by risk managers, the consumer exposure was calculated both with and without consideration of the existing CXLs.
3.1. Consumer risk assessment without consideration of the existing CXLs
Chronic and acute exposure calculations were performed using revision 2 of the EFSA PRIMo (EFSA, 2007). For all crops for which MRL and risk assessment values could be derived, input values were derived according to the internationally agreed methodologies (FAO, 2009).
However, it is highlighted that in the absence of a final conclusion on the residue definition for risk assessment for the plant commodities subject to post‐harvest applications (see Section 1), no risk assessment values could be derived for the critical GAPs authorised on citrus fruits, apples, pears, potatoes, bananas and melons. Therefore, a consumer risk assessment could not be performed for the GAPs with post‐harvest applications.
For potatoes, the risk assessment values derived from the seed treatment GAP were considered as a fall‐back option. However, for citrus fruits, apples, pears and bananas, no input values could be considered in the absence of fall‐back GAPs (see also Section 1.2.4). For melons, a GAP with foliar application was reported to EFSA. However, as this GAP is not supported by residue data, no refined risk assessment values could be derived from this GAP. An indicative calculation was performed using the existing EU MRL for melons. The same approach was followed for sweet peppers/bell peppers for which the critical GAP is a foliar treatment, also not supported by residue trials. Although a peeling factor (PF) was available for melons, this peeling factor was not considered in the risk assessment as it was derived from trials performed with post‐harvest application while this GAP has to be disregarded from the assessment. For animal commodities, no input values were considered as MRLs are not needed for these commodities (see Section 2).
All input values included in the exposure calculations are summarised in Appendix D.2.
The exposure values calculated were compared with the toxicological reference values for imazalil, derived by EFSA (2010). The highest chronic exposure was calculated for Irish adults, representing 6.9% of the acceptable daily intake (ADI). With regard to the acute exposure however, an exceedance of the acute reference dose (ARfD) was identified for melons, representing 606.7% of the ARfD. A second exposure calculation was therefore performed excluding the MRL for melons. According to the results of the second calculation, the highest chronic exposure declined to 1.7% of the ADI (WHO Cluster diet B) but the highest acute exposure is then calculated for tomatoes, representing 18.6% of the ARfD.
Based on these calculations, a potential risk to consumers was identified with the MRL of melons. For the other commodities which could be assessed in this review, although uncertainties remain due to the data gaps identified in Section 1, the indicative exposure calculation did not indicate a risk to consumers.
EFSA emphasises that the above assessment does not consider the possible impact of plant and livestock metabolism on the isomer ratio of imazalil and further investigation on this matter would in principle be required. Since guidance on the consideration of isomer ratios in the consumer risk assessment is not yet available, EFSA recommends that this issue is reconsidered when such guidance becomes available.
Furthermore, it is noted that the conclusions presented under this section may need to be reconsidered in future depending on the final outcome of the assessment of residues in rotational crops.
3.2. Consumer risk assessment with consideration of the existing CXLs
CXLs are defined for imazalil. To include these CXLs in the calculations of the consumer exposure, CXLs were compared with the EU MRL proposals and all data relevant to the consumer exposure assessment have been collected from JMPR evaluations.
It is noted that no data on metabolite R014821 are available in the JMPR evaluations as this compound was not considered for risk assessment at JMPR level. However, as this compound is of relevance only for plant commodities that are subject to post‐harvest treatment and considering the data gaps on the toxicological profile of this metabolite, EFSA is of the opinion that the risk assessment for CXLs derived from post‐harvest treatment cannot be finalised, pending the submission of the data identified as missing. Therefore, as the CXLs defined on citrus fruits, pome fruits, bananas, potatoes and melons are linked to post‐harvest treatments, they were not considered in the calculation. Consequently, only the CXLs defined on strawberries, blackberries, raspberries, persimmon, cucumbers, gherkins and wheat (not derived from post‐harvest GAPs) could be considered in the present assessment.
Furthermore, it is noted that since for strawberries, cucumbers and gherkins no risk assessment values could be retrieved from JMPR evaluations, an indicative risk assessment calculation was performed considering directly the CXL values instead of the HR or STMR values. It is noted that no CXLs are currently in place for livestock commodities.
An overview of the input values used for this exposure calculation is provided in Appendix D.3.
Chronic and acute exposure calculations were performed using revision 2 of the EFSA PRIMo and the exposure values calculated were compared with the toxicological reference values derived for imazalil. The highest chronic exposure was calculated for German children, representing 6.1% of the ADI. With regard to the acute exposure, an exceedance of the ARfD was identified for persimmon representing 131% of the ARfD. A second exposure calculation was therefore performed, excluding the CXL for this commodity. According to the results of this second calculation, the highest chronic exposure was 6.0% of the ADI (German children); the highest acute exposure is then calculated for strawberries, representing 62.4% of the ARfD.
Based on these calculations, a potential risk to consumers was identified for the CXLs of imazalil on persimmon and no further refinements of the risk assessment were possible. The CXLs on citrus fruits, pome fruits, bananas, potatoes and melons could not be assessed (see above). For the remaining CXLs, the exposure calculation did not indicate a risk to consumers.
Conclusions
The metabolism of imazalil was investigated for three different modes of applications (foliar, post‐harvest and seed treatment) in three different crop groups (cereals, fruit crops and root crops), hereby covering all uses under assessment. Based on the available studies, the residue definition for enforcement was proposed as imazalil (any ratio of constituent isomers). Imazalil can be enforced with a LOQ of 0.01 mg/kg in the four main plant matrices. For risk assessment purpose, the residue definition imazalil (any ratio of constituent isomers) could be proposed for commodities subject to foliar and seed treatment. However, a decision on the residue definition for risk assessment could not be derived for commodities subject to post‐harvest treatment. The tentative residue definition for risk assessment previously proposed as the ‘sum of imazalil and R014821, expressed as imazalil’ has been suspended because toxicological data for the metabolite R014821, which is expected to occur following post‐harvest uses of imazalil, are missing.
The nature of residues is unchanged through standard hydrolysis. No conclusion could be proposed regarding the nature of residues in rotational crops as no metabolism studies are available.
The available residue trials data allowed deriving (tentative) MRL proposals as well as risk assessment values for all commodities under evaluation, except for peppers (where no data were available) and melons (for which the number of data was insufficient to derive a MRL). Tentative MRLs were also derived for cereal straw in view of the future need to set MRLs in feed items. Considering that a residue definition for commodities underdoing post‐harvest treatment was not proposed, risk assessment values could not be derived from the GAPs with post‐harvest applications. Therefore, although MRLs could be calculated from different post‐harvest uses reported on citrus fruits, apples, pears, bananas and potatoes, no risk assessment values were derived from these GAPs. For potatoes, fall‐back MRL and risk assessment values could be derived from the seed treatment GAP, which is fully supported by data. However, for citrus fruits, apples, pears, bananas and melons, no alternative GAPs were available to derive fall‐back MRL and risk assessment values.
Robust processing factors were derived for peeled fruits (citrus fruits, bananas and melons) as well as for many processed commodities of oranges (juice, dry pomace, wet pomace and marmalade), apples (juice and wet pomace) and potatoes (unpeeled/boiled, peeled/boiled and fried). For the other processed commodities assessed in this review, the processing factors are considered tentative because of the limited number of data.
Considering that no risk assessment values could be derived for the critical GAPs authorised on citrus fruits, apples and potatoes, a comprehensive dietary burden considering all GAPs reported in this review could not be calculated. Livestock dietary burden calculations were then calculated without considering the GAPs with post‐harvest applications. For potatoes, the risk assessment values derived from the seed treatment GAP were considered while for citrus fruits and apples no input values could be considered. The calculated dietary burdens hereby calculated were found to be below the trigger value of 0.1 mg/kg DM for all groups of livestock. Therefore, MRL and risk assessment values in livestock commodities are not needed provided that GAPs with post‐harvest treatment would be withdrawn.
Chronic and acute consumer exposure was calculated using revision 2 of the EFSA PRIMo. In the absence of a final conclusion on the residue definition for risk assessment for the plant commodities subject to post‐harvest applications, the consumer exposure calculations were performed without considering the GAPs with post‐harvest applications authorised on citrus fruits, apples, pears, potatoes, bananas and melons. For potatoes, fall‐back MRL and risk assessment values could be derived from the seed treatment GAP and for melons a GAP with foliar application was tentatively assessed although not supported by data. For those commodities where data were insufficient to derive a MRL, EFSA considered the existing EU MRL for an indicative calculation. For melons, an exceedance of the ARfD was identified representing 606.7% of the ARfD. Excluding this MRL from the calculation, the highest chronic exposure represented 1.7% of the ADI (WHO Cluster diet B) and the highest acute exposure amounted to 18.6% of the ARfD (tomatoes).
Apart from the MRLs evaluated in the framework of this review, internationally recommended CXLs have also been established for imazalil. Additional calculations of the consumer exposure, considering these CXLs, were therefore carried out, noting that the CXLs derived from post‐harvest uses have not been considered. Exceedance of the ARfD was identified for the existing CXL in persimmon (131%). Excluding this CXL from the calculation, the highest chronic exposure represented 6.0% of the ADI (German children) and the highest acute exposure amounted to 62.4% of the ARfD (strawberries).
Recommendations
The MRL recommendations derive by EFSA are summarised in Table 1. All MRL values listed as ‘Recommended’ in the table are sufficiently supported by data and are therefore proposed for inclusion in Annex II to the Regulation. The remaining MRL values listed in the table are not recommended for inclusion in Annex II because they require further consideration by risk managers (see Table 1 footnotes for details). In particular, some tentative MRLs and existing EU MRLs need to be confirmed by the following data:
Table 1.
Summary table
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
|
Enforcement residue definition (existing): imazalil Enforcement residue definition 1 (proposed): imazalil (any ratio of constituent isomers) | |||||
| 110010 | Grapefruits | 5 | 5 | – | Further consideration neededa |
| 110020 | Oranges | 5 | 5 | – | Further consideration neededa |
| 110030 | Lemons | 5 | 5 | – | Further consideration neededa |
| 110040 | Limes | 5 | 5 | – | Further consideration neededa |
| 110050 | Mandarins | 5 | 5 | – | Further consideration neededa |
| 130010 | Apples | 2 | 5 | – | Further consideration neededa |
| 130020 | Pears | 2 | 5 | – | Further consideration neededa |
| 130030 | Quinces | 2 | 5 | – | Further consideration neededb |
| 130040 | Medlar | 5 | 5 | – | Further consideration neededb |
| 130050 | Loquat | 5 | 5 | – | Further consideration neededb |
| 152000 | Strawberries | 0.05* | 2 | 2 | Recommendedc |
| 153010 | Blackberries | 0.05* | 2 | 2 | Recommendedc |
| 153030 | Raspberries | 0.05* | 2 | 2 | Recommendedc |
| 161060 | Persimmon | 0.05* | 2 | – | Further consideration neededd |
| 163020 | Bananas | 2 | 2 | – | Further consideration neededa |
| 211000 | Potatoes | 3 | 5 | 0.01* | Recommendede |
| 231010 | Tomatoes | 0.5 | – | 0.3 | Recommendedf |
| 231020 | Sweet peppers/bell peppers | 0.05* | – | 0.05 | Further consideration neededg |
| 232010 | Cucumbers | 0.2 | 0.5 | 0.5 | Recommendedh |
| 232020 | Gherkins | 0.2 | 0.5 | 0.5 | Recommendedh |
| 232030 | Courgettes | 0.2 | – | 0.1 | Further consideration neededi |
| 233010 | Melons | 2 | 2 | – | Further consideration neededj |
| 500010 | Barley grains | 0.05* | – | 0.01* | Recommendedf |
| 500050 | Oat grains | 0.05* | – | 0.01* | Recommendedf |
| 500070 | Rye grains | 0.05* | – | 0.01* | Recommendedf |
| 500090 | Wheat grains | 0.05* | 0.01* | 0.01* | Recommendedk |
| – | Other commodities of plant or animal origin | See Reg. 750/2010 | – | – | Further consideration neededl |
MRL: maximum residue level.
* Indicates that the MRL is set at the limit of quantification.
The previously derived tentative residue definition for risk assessment has been suspended because the toxicological data were insufficient to clearly rule out a genotoxic potential for metabolite R014821. Pending the submission of data required to finalise the hazard characterisation for this metabolite that is expected to occur following post‐harvest treatment of the crop, the risk assessment cannot be finalised and consequently no MRL recommendation was derived. A similar case applies to CXL that were derived from post‐harvest uses. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered.
There are no relevant authorisations or import tolerances reported at EU level; CXL is reflecting a post‐harvest use. Thus, pending the finalisation of the hazard characterisation for metabolite R014821, the CXL is not recommended to be taken over in EU legislation. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered.
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; there are no relevant authorisations or import tolerances reported at EU level.
There are no relevant authorisations or import tolerances reported at EU level; CXL is supported by data but a risk to consumers cannot be excluded. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered.
MRL is derived from a GAP evaluated at EU level (fall‐back GAP: seed treatment), which is fully supported by data and for which no risk to consumers is identified; CXL is higher but pending the finalisation of the hazard characterisation for metabolite R014821, the CXL is not recommended to be taken over in EU legislation.
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available.
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL; no CXL is available.
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level, which is not fully supported by data, leads to a lower tentative MRL.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; no CXL is available.
The previously derived tentative residue definition for risk assessment has been suspended because the toxicological data were insufficient to clearly rule out a genotoxic potential for metabolite R014821. Pending the submission of data required to finalise the hazard characterisation for this metabolite that is expected to occur following post‐harvest treatment of the crop, the risk assessment cannot be finalised and consequently no MRL recommendation was derived from the post‐harvest use. A similar case applies to CXL that were derived from post‐harvest uses. In addition, the foliar GAP evaluated at EU level is not supported by data and a risk to consumers cannot be excluded for the existing EU MRL. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered.
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; existing CXL is covered by the recommended MRL.
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered.
Additional residue trials supporting the GAPs on peppers and courgettes.
It is highlighted that the MRLs derived for cucumbers and gherkins result from a CXL, whereas the GAPs reported by MS were not fully supported by data for this crops. EFSA therefore identified the following data gap which is not expected to impact on the validity of the MRLs derived but which might have an impact on national authorisations:
Additional residue trials supporting the GAPs on cucumbers and gherkins (it is noted that this data gap can be covered by the major data gap identified for courgettes).
If the above‐reported data gaps are not addressed in the future, MSs are recommended to withdraw or modify the relevant authorisations at national level.
Furthermore, a representative study investigating metabolism in rotational crops was identified to be missing and should also be required. This data gap refers to all uses with foliar treatment and seed treatment. If this data gap is not addressed in the future, MSs are recommended to withdraw or modify their relevant authorisations for foliar treatment and seed treatment (e.g. to restrict the foliar uses to artificial substrate only, etc.).
It should be highlighted that EFSA did not derive MRL proposals from the post‐harvest uses reported on citrus fruits, apples, pears, potatoes, bananas and melons. The available studies on nature and magnitude of residues in plant commodities indicate that metabolite R014821 is expected following post‐harvest application of imazalil. Risk managers should be made aware that additional data on the toxicology of this compound were made available to EFSA after the MRL review. These data were insufficient to clearly rule out a genotoxic potential of the metabolite R014821 and to conclude whether the toxicological reference values derived for parent imazalil would be appropriate for the metabolite. Therefore, the tentative residue definition for risk assessment previously proposed as the ‘sum of imazalil and R014821, expressed as imazalil’ has been suspended and the consumer risk assessment linked to the GAPs with post‐harvest application has to be finalised, once the information identified as missing has been submitted (see EFSA, 2018). Therefore, although MRLs could be calculated from different post‐harvest uses reported on citrus fruits, apples, pears, bananas and potatoes, these MRLs should not be proposed pending the conclusion on the toxicological properties of metabolite R014821. For the same reasons, the CXLs derived from post‐harvest treatment (citrus fruits, pome fruits, bananas, potatoes and melons) could also not be assessed properly and thus are not recommended for implementation in the MRL Regulation.
For potatoes, a fall‐back MRL (fully supported by data) could be derived from a seed treatment GAP. However, for citrus fruits, apples, pears, bananas and melons, no further options were available to EFSA. It was noted that a GAP with foliar application authorised is authorised on melons but, as this GAP is not supported by data and since a risk for consumer was identified with the existing MRL, it could also not be used to derive a MRL for this commodity.
Consequently, a fall‐back MRL of 0.01* mg/kg is recommended for potatoes while for citrus fruits, apples, pears, bananas and melons, risk managers may consider either a specific LOQ or the default MRL of 0.01* mg/kg (see Table 1 footnotes for details). Therefore, MSs are recommended to reconsider or withdraw their national authorisations consequently. In particular, MSs may consider withdrawing their post‐harvest uses on citrus fruits, apples, pears, potatoes, bananas and melons as well as the foliar GAPs on melons.
It is noted that MRL proposals in commodities of animal origin were not derived because the livestock exposure is not expected to exceed the trigger values, excluding the GAPs with post‐harvest applications. However, it is highlighted that the hazard characterisation for the two metabolites identified in livestock metabolism studies (i.e. FK‐772 and FK‐284) could not be finalised as data were identified as missing (EFSA, 2018).
Abbreviations
- a.i.
active ingredient
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CAC
Codex Alimentarius Commission
- CF
conversion factor for enforcement residue definition to risk assessment residue definition
- CXL
codex maximum residue limit
- DALA
days after last application
- DAR
draft assessment report
- DAT
days after treatment
- DB
dietary burden
- DM
dry matter
- DS
powder for dry seed treatment
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- EMS
evaluating Member State
- eq
residue expressed as a.s. equivalent
- EC
emulsifiable concentrate
- EURLs
European Union Reference Laboratories for Pesticide Residues (former CRLs)
- EW
Emulsion, oil in water
- FAO
Food and Agriculture Organization of the United Nations
- FID
flame ionisation detector
- FU
Smoke generator
- GAP
Good Agricultural Practice
- HPLC–MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- LOQ
limit of quantification
- LS
Solution for seed treatment
- Mo
monitoring
- MRL
maximum residue level
- MS
Member States
- MS
mass spectrometry detector
- MS/MS
tandem mass spectrometry detector
- NEU
northern European Union
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- PROFile
(EFSA) Pesticide Residues Overview File
- Rber
statistical calculation of the MRL by using a non‐parametric method
- Rmax
statistical calculation of the MRL by using a parametric method
- RA
risk assessment
- RD
residue definition
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SEU
southern European Union
- SG
Water soluble granules
- SL
soluble concentrate
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- TBE
to be established
- TRR
total radioactive residue
- UV
ultraviolet (detector)
- VF
variability factor
- WHO
World Health Organization
- WHP
withholding period
Appendix A – Summary of authorised uses considered in the updated review of MRLs
A.1. Authorised uses considered to derive MRLs (foliar and seed treatment)
| Critical outdoor GAPs for Northern Europe | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop | Region | Outdoor/ Indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Potatoes | Solanum tuberosum subsp. tuberosum | NEU | Outdoor | DE, FR | Silver scurf (Helminthosporium solani), skin spot of potato (Polyscytalum pustulans), Fusarium species, dry rot (Phoma exigua) | SL | 100.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 1 | 15 | g a.i./tonnes | 0 | Fall‐back GAP. Spraying immediately after harvest. Use restricted to seed potatoes only | |||
| Barley | Hordeum vulgare | NEU | Outdoor | AT, BE, DK, FI, IE, LU, SE, UK, CZ | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | Other conc. For LS formulation are also authorised: 6 g/L and 50 g/L | |||
| Oat | Avena sativa | NEU | Outdoor | AT, BE, DK, FI, IE, LU, SE, UK | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | See comment on barley | |||
| Rye | Secale cereale | NEU | Outdoor | AT, BE, DK, FI, IE, LU, SE, UK | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | See comment on barley | |||
| Wheat | Triticum aestivum | NEU | Outdoor | AT, BE, DK, FI, IE, LU, SE, UK | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | See comment on barley | |||
| Critical outdoor GAPs for Southern Europe | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop | Region | Outdoor/ Indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) |
Comments (max. 250 characters) |
||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Potatoes | Solanum tuberosum subsp. tuberosum | SEU | Outdoor | FR | Silver scurf (Helminthosporium solani) Skin spot (Polyscytalum pustulans) | SL | 100.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 1 | 15 | g a.i./tonnes | 0 | Fall‐back GAP. Spraying immediately after harvest. Use restricted to seed potatoes only | |||
| Barley | Hordeum vulgare | SEU | Outdoor | EL, ES, IT | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | Other conc. For LS formulation are also authorised: 6 g/L and 50 g/L | |||
| Oat | Avena sativa | SEU | Outdoor | EL, ES, IT | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | See comment on barley | |||
| Rye | Secale cereale | SEU | Outdoor | EL, ES, IT | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | See comment on barley | |||
| Wheat | Triticum aestivum | SEU | Outdoor | EL, ES, IT | Helminthosporium teres, Helminthosporium gramineum | LS | 58.0 | g/L | Seed treatment – spraying | n.a. | n.a. | 1 | 3 | 7.5 | g a.i./100 kg seeds | n.a. | See comment on barley | |||
| Critical indoor GAPs for Northern and Southern Europe (including post‐harvest treatments) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop | Region | Outdoor/ Indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) |
Comments (max. 250 characters) |
||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Tomatoes | Lycopersicon esculentum | NEU/SEU | Indoor | BE, NL | Powdery mildew: Oidiopsis (Leveillula) taurica, Botrytis cinerea, Oidium lycopersici | EC | 100.0 | g/L | Foliar treatment – spraying | 51 | 89 | 1 | 3 | 7 | 14 | 300 | g a.i./ha | 1 | Based on a GAP authorised for 20 g a.i./hL and assuming 1,500 L water/ha is applied | |
| Sweet peppers | Capsicum annuum | NEU/SEU | Indoor | BE, NL | Powdery mildew: Oidiopsis (Leveillula) taurica, Botrytis cinerea, Oidium lycopersici | EC | 100.0 | g/L | Foliar treatment – spraying | 51 | 89 | 1 | 3 | 7 | 14 | 300 | g a.i./ha | 3 | Based on a GAP authorised for 20 g a.i./hL and assuming 1,500 L water/ha is applied | |
| Cucumbers | Cucumis sativus | NEU/SEU | Indoor | NL, BE, EL, IE, NL, ES, UK | Powdery mildew: Sphaerotheca fuliginea, Mycosphaerella citrulina | EC | 100.0 | g/L | Foliar treatment – spraying | 51 | 89 | 1 | 4 | 7 | 10 | 75 | g a.i./ha | 1 |
Based on a rate of 5 g a.i./hL and assuming 1,500 L water/ha). Four applications authorised in NL; only three applications authorised in the other countries |
|
| Gherkins | Cucumis sativus | NEU/SEU | Indoor | NL, BE, EL, IE, NL, ES, UK | Powdery mildew: Sphaerotheca fuliginea, Mycosphaerella citrulina | EC | 100.0 | g/L | Foliar treatment – spraying | 51 | 89 | 1 | 4 | 7 | 10 | 75 | g a.i./ha | 1 | See comment on cucumbers | |
| Courgettes | Cucurbita pepo Zucchini Group | NEU/SEU | Indoor | NL, BE, EL, IE, NL, ES, UK | Powdery mildew: Sphaerotheca fuliginea, Mycosphaerella citrulina | EC | 100.0 | g/L | Foliar treatment – spraying | 51 | 89 | 1 | 4 | 7 | 10 | 75 | g a.i./ha | 1 | See comment on cucumbers | |
| Melons | Cucumis melo | NEU/SEU | Indoor | BE | – | – | – | – | Foliar treatment – spraying | n.a. | n.a. | 1 | 3 | 7 | 70 | g a.i./ha | 3 | Not supported trials available | ||
MRL: maximum residue level; GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient.; SL: soluble concentrate; LS: Solution for seed treatment; EC: emulsifiable concentrate; EW: Emulsion, oil in water; FU: Smoke generator; SG: Water soluble granules.
A.2. Authorised uses which could not be considered to derive MRL (post‐harvest treatment)
| Crop | Region | Outdoor/ Indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) |
Comments (max. 250 characters) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Citrus fruits (grapefruits, oranges, mandarins, lemons, limes) | Citrus (paradisi, sinensis, reticulata, limon, aurantiifolia) | NEU/SEU | Indoor | ES | Penicillium digitatum, Penicillium italicum, Penicillium expansum, Diaporthe citri, Diplodia natalensis, Alternaria citri, Botrytis spp., Alternaria spp., Phomopsis spp. | EC | 500.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 50 | 50 | g a.i./hL | 0 | Drenching/dipping. Critical waiting period: 0 day. Other less critical post‐harvest treatments are also authorised as waxing and low volumes spraying (PT, EL, ES, IT) | |||
| Grapefruits, oranges | Citrus (paradisi, sinensis) | NEU/SEU | Indoor | ES, EL | Penicillium digitatum, Penicillium italicum, Penicillium expansum, Diaporthe citri, Diplodia natalensis, Alternaria citri, Botrytis spp., Alternaria spp., Phomopsis spp. | EW | 3.0 | g/L | Post‐harvest – spraying | n.a. | n.a. | 1 | 300 | g a.i./hL | 0 | Pulverisation with wax (waxing). Volume of 1 L/tonnes fruits (equivalent to 3 g a.i./tonnes fruits). | ||||
| Apples | Malus domestica | NEU/SEU | Indoor | EL | Penicillium expansum, Gloeosporium sp. | SL | 75.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 25 | 30 | g a.i./hL | 0 | Drenching/dipping. Critical waiting period: 0 day | |||
| Apples | Malus domestica | NEU/SEU | Indoor | ES, PT | Penicillium spp. | SL | 75.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 37.5 | g a.i./hL | 60 | Drenching/dipping. Critical waiting period: 60 day | ||||
| Apples | Malus domestica | NEU/SEU | Indoor | EL, ES | Post‐harvest fungi | FU | 250.0 | g/L | Post‐harvest treatment – smoke can | n.a. | n.a. | 1 | 6.0 | g a.i./tonnes | 30 | – | ||||
| Pears | Pyrus communis | NEU/SEU | Indoor | BE, NL | Penicillium expansum, Gloeosporium sp. | EC | 500.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 25 | 25 | g a.i./hL | 0 | Drenching/dipping. Critical waiting period: 0 day (1 day in NL). | |||
| Pears | Pyrus communis | NEU/SEU | Indoor | ES, PT | Penicillium spp. | SL | 75.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 37.5 | g a.i./hL | 60 | Drenching/dipping. Critical waiting period: 60 day. | ||||
| Pears | Pyrus communis | NEU/SEU | Indoor | EL, ES | Post‐harvest fungi | FU | 250.0 | g/L | Post‐harvest treatment – smoke can | n.a. | n.a. | 1 | 6.0 | g a.i./tonnes | 30 | – | ||||
| Potatoes | Solanum tuberosum subsp. tuberosum | NEU/SEU | Indoor | DE | Polyscytalum pustulans, Phoma exigua var. foveata, Helminthosporium solani, Fusarium sulphureum, Fusarium culmorum, Fusarium roseum var. sambucinum | EC | 500.0 | g/L | Post‐harvest – spraying | n.a. | n.a. | 1 | 1 | 15 | g a.i./tonnes | 0 | Spraying immediately after harvest during gathering the harvest in the storage room. No restriction of the WHP (i.e. 0 day possible). | |||
| Bananas | Musa acuminata; Musa balbisiana; Musa acuminata × Musa balbisiana | NEU/SEU | Indoor | EL | Crown rot pathogens: Colletotrichum musae, Fusarium moniliforme, Fusarium pusilum, Fusarium semitectum, Verticillium theobromae, Verticillium sp. | SG | 750.0 | g/kg | Post‐harvest treatment – dipping | n.a. | n.a. | 1 | 60 | g a.i./hL | 35 | Spray overhead on transport belt. (assuming a rate equivalent to 1.2 g a.s./tonnes). | ||||
| Melons | Cucumis melo | NEU/SEU | Indoor | ES | Penicillium sp., Fusarium sp., Alternaria sp. | EC | 500.0 | g/L | Post‐harvest treatment – drenching | n.a. | n.a. | 1 | 45 | g a.i./hL | 0 | – | ||||
GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient; WHP: withholding period; SL: soluble concentrate; LS: Solution for seed treatment; EC: emulsifiable concentrate; EW: Emulsion, oil in water; FU: Smoke generator; SG: Water soluble granules.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling |
|---|---|---|---|---|
| Fruit crops | Tomatoes | Foliar, 3 × 300 g a.s./ha (interval between applications 10 days) | 1 DALA (21 days after first application) | |
| Foliar, 3 × 1,500 g a.s./ha (interval between applications 10 days) | 1 DALA (21 days after first application) | |||
| Oranges, apples | Post‐harvest dipping, 0.05 kg/hl | From 2 hours to 7 months | ||
| Root crops | Potatoes |
Post‐harvest (ware potatoes): 15 g a.s./tonnes |
0, 14, 29, 91, 188 DAT | |
| Potatoes |
Seed treatment (seed potatoes): 15 g a.s./tonnes and 75 g a.s./tonnes |
After growing under normal conditions | ||
| Cereals/grass crops | Spring wheat | Seed‐treatment, 0.49 kg a.s./tonnes |
After growing under normal conditions. Forage: 42 DAT Grain: 150 DAT |
|
| Sources: For tomatoes (Netherlands, 2009a); for oranges, apples and spring wheat (Belgium, 1996); for potatoes (Netherlands, 2015) | ||||
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) |
| Root/tuber crops | – | – | – | |
| Leafy crops | – | – | – | |
| Cereal (small grain) | – | – | – | |
| Studies not available but still required. A theoretical calculation was presented by the RMS (Netherlands, 2015) but does not allow concluding on the residues in rotational crops | ||||
| Processed commodities (hydrolysis study) | Conditions | Investigated? | ||
| Pasteurisation (20 min, 90°C, pH 4) | Yes | |||
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | |||
| Sterilisation (20 min, 120°C, pH 6) | Yes | |||
| Source: Netherlands (2014) | ||||
| Can a general residue definition be proposed for primary crops? | Yes (only for enforcement; it is noted that significant levels of metabolite R014821 can be expected after post‐harvest treatment which may need to be considered in the risk assessment) |
| Rotational crop and primary crop metabolism similar? | Inconclusive |
| Residue pattern in processed commodities similar to residue pattern in raw commodities? | Yes |
| Plant residue definition for monitoring (RD‐Mo) | Imazalil (any ratio of constituent isomers) |
| Plant residue definition for risk assessment (RD‐RA) |
(For post‐harvest treatment and processed commodities): opena (For foliar treatment and seed treatment and processed commodities): imazalil (any ratio of constituent isomers)b |
| Conversion factor (monitoring to risk assessment) | Not relevantc |
| Methods of analysis for monitoring of residues (analytical technique, crop groups, LOQs) |
Matrices with high water content, high acid content, high oil content and dry content: HPLC–MS/MS, LOQ: 0.01 mg/kg ILV available for high water content commodities, high acid content commodities and dry commodities. |
a.s.: active substance; DALA: days after last application; DAT: days after treatment; PBI: plant‐back interval; RMS: rapporteur Member State; HPLC–MS/MS: high‐performance liquid chromatography with tandem mass spectrometry; LOQ: limit of quantification; ILV: independent laboratory validation.
In plant commodities subject to post‐harvest uses, the metabolite R014821 can be found in significant levels after long storage length. Due to the equivocal results for genotoxicity and the lack of a repeated dose study for this metabolite, the residue definition for risk assessment previously derived was suspended, pending the submission of toxicological data identified as missing (see also EFSA, 2018). The same apply for processed commodities derived from raw commodities following post‐harvest treatment.
While metabolite R014821 is not expected to occur in significant levels with foliar and seed treatments, it is recommended to still analyse it in any new studies investigating the magnitude of residues in plant commodities. This residue definition applies to processed commodities derived from raw commodities following foliar or seed treatment.
No conversion factor can be derived since no residue definition for risk assessment can be proposed.
B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability (Months/years) |
|---|---|---|---|---|
| High water content | Apple (raw and processed) | −20 | 12 months | |
| High acid content | Oranges | −18 | 8 months | |
| Dry/ high starch | Cereal grain | −18 | 6 months | |
| Specific matrices | Cereal straw | −18 | 6 months | |
|
For high water content commodities: storage stability was separately demonstrated for imazalil and R014821, in raw and processed commodities (Netherlands, 2015) For high acid content commodities: storage stability was separately demonstrated for imazalil and R014821, in raw and processed commodities (EFSA, 2018) For other matrices: storage stability only demonstrated for imazalil; no data available for the metabolite R014821 (EFSA, 2010) | ||||
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Crop | Region/indoora | Residue levels observed in the supervised residue trials relevant to the supported GAPs (mg/kg) | Recommendations/comments (OECD calculations) | MRL proposals (mg/kg) | HRMo (mg/kg)b | STMRMo (mg/kg)c | CFd |
|---|---|---|---|---|---|---|---|
| Residue definition for enforcement: imazalil; Residue definition for risk assessment: Open (the residue definition previously derived was suspended, pending the submission of toxicological data identified as missing) | |||||||
| Citrus fruits | EU (drenching: 50 g a.i./hl; WHP: 0 day) |
Mo [whole fruit] – Oranges: 0.57; 0.59; 0.6; 0.66; 0.66; 0.84; 1.36; 1.4; 2.06; 2.09; 2.2; 2.2; 2.46; 2.58; 2.59; 2.81; 2.89; 3.12; 4.95 Mo [whole fruit] – Mandarins: 0.49; 0.6; 1.3; 1.51; 1.6; 1.8; 1.8; 2.19; 2.26; 2.4; 2.66; 2.72; 3.34; 4.84 RA: no data (the residue definition for RA was suspended) |
Combined data set on oranges and mandarins, compliant with GAP (EFSA, 2010; Netherlands, 2015; Spain, 2016). Residue levels of R014821 increase with the withholding period (0.02–0.13 mg/kg at WHP 2–3 months). Rber = 5.25 Rmax = 4.46 MRLOECD: not relevante |
6 (tentative)f |
4.95 | 2.09 | TBE |
|
Mo [Pulp] – Oranges: 5 × < 0.01; 0.02; 0.03; 0.04; 0.09; 0.12; 0.21; 0.69 Mo [Pulp] – Mandarins: 0.02; 0.02; 0.05; 0.08; 0.12; 0.18; 0.43; 0.48 |
Residue levels directly measured in pulp were reported for information purpose | – | 0.69 | 0.05 | TBE | ||
| Oranges Grapefruits | EU (waxing: 300 g a.i./hl equivalent to 3 g a.i./tonnes; WHP: 0 day) |
Mo [whole fruit] – Oranges: 0.43; 0.47; 1.1; 1.3; 1.4; 1.5; 1.7; 2.1 Mo [whole fruit] – Mandarins: 1.2; 1.5; 1.6; 1.7; 2.1; 2.3 RA: no data (the residue definition for RA was suspended) |
Combined data set on oranges and mandarins, compliant with GAP (Spain, 2016). No results available for R014821. Rber = 3.60 Rmax = 2.89 MRLOECD: not relevante |
4 (tentative)f |
2.3 | 1.5 | TBE |
|
Mo [Pulp] – Oranges: 4 × < 0.01 Mo [Pulp] – Mandarins : < 0.01; 0.02 |
Residue levels directly measured in pulp were reported for information purpose. | – | 0.02 | 0.01 | TBE | ||
|
Apples Pears |
EU (drenching: 25–30 g a.i./hl; WHP: 0 day) |
Mo Apples: 0.3; 0.39; 0.42; 0.55; 0.57; 0.59; 0.63; 0.65; 0.76; 0.84; 0.89; 0.9; 1; 1.33 Mo Pears: 0.34; 0.35; 0.38; 0.45; 0.50; 0.57; 0.66; 0.7; 0.82; 1.02; 1.15; 1.23; 1.24; 1.45; 2.0; 2.1; 2.28; 3.5 RA: no data (the residue definition for RA was suspended) |
Combined data set on apples and pears, compliant with GAP (Belgium, 1996; Netherlands, 2016, Spain, 2016). Residue levels of R014821 increase with the withholding period (up to 0.18 mg/kg at WHP 4 months). Rber = 2.42 Rmax = 2.47 MRLOECD: not relevante |
4 (tentative)f |
3.50 | 0.73 | TBE |
| EU (drenching: 38 g a.i./hl; WHP: 60 days) |
Mo Apples: 0.4; 0.53; 0.66; 0.75; 1.55; 1.71; 1.81; 2.11; 2.38; 2.77 Mo Pears: 1.2; 1.4 RA: no data (the residue definition for RA was suspended) |
Combined data set on apples and pears, compliant with GAP (Netherlands, 2015; Spain, 2016). Residue levels of R014821 increase with the withholding period (up to 0.14 mg/kg at WHP 3 months). Rber = 3.57 Rmax = 4.07 MRLOECD: not relevante |
4 (tentative)f |
2.77 | 1.48 | TBE | |
| EU (smoke can: 6 g a.i./tonnes; WHP: 30 days) |
Mo Apples: 0.36, 0.38, Mo Pears: 0.74, 0.79 RA: no data (the residue definition for RA was suspended) |
Combined data set on apples and pears, compliant with GAP (Spain, 2016). No results available for R014821. Rber = 1.56 Rmax = 1.75 MRLOECD: not relevante |
2 |
0.79 | 0.56 | TBE | |
| Bananas | EU (post‐harvest dipping) |
Mo [whole fruit] : 2.12; 2.61; 2.71; 2.80 RA [whole fruit]: no data (the residue definition for RA was suspended) |
Trials compliant with GAP (Greece, 2018). Residue levels of R014821 range between (0.03–0.05 mg/kg) at WHP 1 month (no data available at longer WHP). Rber = 5.56 Rmax = 4.12 MRLOECD: not relevante |
5 |
2.80 | 2.66 | TBE |
|
Mo [pulp]: 0.15; 0.23; 0.42; 0.93 RA [pulp]: no data (the residue definition for RA was suspended) |
Residue levels directly measured in pulp were reported for information purpose. Data available on pulp were obtained at PHI 28 days, which within the 25% tolerance compared to GAP (PHI 35 days) | – | 0.93 | 0.33 | TBE | ||
| Potatoes | EU (ware potatoes, post‐harvest spraying) |
Mo: 0.46; 0.88; 1.51; 1.83; 2.65; 3.4i; 4.1i; 4.5i; 4.6i RA: no data (the residue definition for RA was suspended) |
Trials compliant with GAP (Netherlands, 2015). Residue levels of R014821 increase with the withholding period (up to 0.26 mg/kg at WHP 5 months). Rber = 8.6 Rmax = 7.43 MRLOECD: not relevante |
9 |
4.60 | 2.65 | TBE |
| Melons | EU (post‐harvest drenching |
Mo: 0.47; 0.47; 0.47 RA: no data (the residue definition for RA was suspended) |
Trials performed with drench application (25% deviation on the application rate) (Belgium, 1996). No results available for R014821 | – | – | – | TBE |
| Residue definition for enforcement and for risk assessment: imazalil (any ratio of constituent isomers) | |||||||
| Potatoes | NEU (fall‐back GAP: seed treatment) | 10 × < 0.01 | Trials compliant with GAP (France, 2016) | 0.01* | < 0.01 | < 0.01 | 1 |
| SEU (fall‐back GAP: seed treatment) | 10 × < 0.01 | Trials compliant with GAP (France, 2016) | 0.01* | < 0.01 | < 0.01 | 1 | |
| Tomatoes | EU | 0.03; 0.03; 0.08; 0.08; 0.09; 0.16; 0.15; 0.14 |
Trials on tomatoes compliant with GAP. MRLOECD = 0.3 |
0.3 | 0.16 | 0.09 | 1 |
| Sweet peppers/bell peppers | EU | – | No data available | – | – | – | 1 |
|
Cucumbers Gherkins Courgettes |
EU | 0.02; 0.05; 0.01; 0.02 |
Trials performed on cucumbers. Two first trials compliant with GAP. Two additional trials performed with three applications instead of 4 (Netherlands, 2016). Metabolite R014821 was analysed <LOQ in all trials samples (decline studies up to PHI 28 days) MRLOECD = 0.09 |
0.1 (tentative)g | 0.05 | 0.02 | 1 |
| Melons | Indoor (foliar treatment) | – | No trials available (Belgium, 2016) | – | – | – | 1 |
|
Barley grains Oats grains Rye grains Wheat grains |
NEU | 4 × < 0.05; 4 × < 0.01 | Trials performed on barley and compliant with GAP (Belgium, 1996; EFSA, 2010). Based on NEU and SEU trials, an MRL of 0.01* mg/kg can be proposed | 0.01* | < 0.01 | < 0.01 | 1 |
| SEU | 4 × < 0.01 | ||||||
|
Barley straw Oat straw Rye straw Wheat straw |
NEU | 4 × < 0.01 | Trials on barley compliant with GAP (EFSA, 2010). Based on NEU and SEU trials, an MRL of 0.01* mg/kg can be proposed | 0.01* (tentative)i | < 0.01 | < 0.01 | 1 |
| SEU | 4 × < 0.01 | ||||||
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level; WHP: withholding period; PHI: preharvest interval. Mo: monitoring; RA: risk assessment; a.i.: active ingredient; Rber: statistical calculation of the MRL by using a non‐parametric method; Rmax: statistical calculation of the MRL by using a parametric method; TBE: to be established.
* Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue according to the residue definition for monitoring.
Supervised trials median residue according to the residue definition for monitoring.
Conversion factor for risk assessment. To be established (TBE) upon a decision on the residue definition for risk assessment after post‐harvest uses.
MRLOECD calculator is not relevant for data obtained by post‐harvest treatment as it may artificially overestimate the MRL calculation derived from homogeneous data set.
MRL is tentative because a residue definition for risk assessment could not be derived for the commodities subject to post‐harvest treatment (risk assessment cannot be performed for this commodity).
MRL is tentative because additional residue data trials are still required.
Highest residue levels were observed after a DAT longer than 0 day in samples that were washed before analysis; therefore the whole results are deemed questionable and are used on a tentative basis to derive MRL and risk assessment values.
Tentative MRL is derived for feed items.
B.1.2.2. Residues in succeeding crops
| Confined rotational crop study (quantitative aspect) | Not available but still required. A theoretical calculation was presented by the RMS (Netherlands, 2015) but does not allow concluding on the potential residues uptake in rotational crops |
| Field rotational crop study | Not available |
RMS: rapporteur Member State.
B.1.2.3. Processing factors
| Processed commodity | Number of studiesa | Processing factor (PF) | ||
|---|---|---|---|---|
| Individual values | Median PF | CFf | ||
| Robust processing factors (sufficiently supported by data) | ||||
| Citrus, peeled | 36b | 0.01; 0.01; 0.01; 0.02; 0.03; 0.04; 0.04; 0.04; 0.04; 0.04; 0.04; 0.04; 0.05; 0.05; 0.05; 0.05; 0.06; 0.07; 0.07; 0.07; 0.08; 0.08; 0.08; 0.08; 0.10; 0.11; 0.12; 0.13; 0.14; 0.15; 0.15; 0.16; 0.20; 0.21; 0.25; 0.28 | 0.07 | TBE |
|
Oranges, juice (→ extrapolated to other citrus) |
10 | 0.01; 0.02; 0.03; 0.05; 0.05; 0.10; 0.11; 0.14; 0.33; 0.35 | 0.08 | TBE |
|
Oranges, dry pomace (→ extrapolated to other citrus) |
10 | 1.0; 1.1; 2.3; 4.03; 4.05; 4.39; 4.48; 4.86; 6.7; 9.6 | 4.2 | TBE |
|
Oranges, wet pomace (→ extrapolated to other citrus) |
7 | 1.74; 1.98; 2.03; 2.04; 2.2; 2.29; 2.7 | 2 | TBE |
|
Oranges, marmalade (→ extrapolated to other citrus) |
7 | 0.15; 0.25; 0.25; 0.27; 0.28; 0.56; 0.68 | 0.27 | TBE |
|
Apples, juice (→ extrapolated to pears) |
3 | < 0.01; 0.03; 0.2 | 0.03 | TBE |
|
Apples, wet pomace (→ extrapolated to pears) |
3 | 1.36; 1.52; 1.9 | 1.5 | TBE |
| Bananas, peeled | 4c | 0.05; 0.09; 0.16; 0.30 | 0.13 | TBE |
| Potatoes, unpeeled and boiled | 4 | 0.12; 0.15; 0.28; 0.50 | 0.22 | TBE |
| Potatoes, peeled and boiled | 2d | < 0.01; < 0.01 | 0.01 | TBE |
| Potatoes, fried | 4 | < 0.01; < 0.01; < 0.01; 0.02 | 0.01 | TBE |
| Melons, peeled | 6 | 0.06e; 0.07e; 0.11; 0.13; 0.18e; 0.20 | 0.12 | TBE |
| Indicative processing factors (limited data set) | ||||
|
Apples, dry pomace (→ extrapolated to pears) |
2 | 3.39; 3.94 | 3.7 | TBE |
| Apples, sauce | 2 | 0.07; 0.31 | 0.19 | TBE |
| Potatoes, unpeeled and microwaved | 2 | 1.09; 1.58 | 1.3 | TBE |
| Potatoes, crisps | 2 | 0.02; 0.02 | 0.02 | TBE |
| Potatoes, granules or flakes | 2 | 0.01; 0.01 | 0.01 | TBE |
TBE: to be established.
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
Based on residue trials compliant with the critical GAP (drenching 50 g/hl; WHP 0 d, including replicates) performed on oranges (n = 15 ranging from 0.01 to 0.28) and mandarins (n = 16 ranging from 0.01 to 0.25) (Netherlands, 2015; Spain, 2016) and considering the processing factors derived during the peer review on oranges (0.08), lemons (0.04; 0.04; 0.05) and grapefruits (0.13) (EFSA, 2010).
Based on sampling performed at PHI 28 days (Greece, 2018).
Although only 2 studies are available, this PF is considered robust because the two available data show that significant residues are not expected in boiled potatoes (unpeeled and peeled).
Based on residue trials performed with a higher application rate compared to GAP (Belgium, 1996).
Conversion factor for risk assessment. Considering that all processing factors derived in this table are linked to raw commodities subject to post‐harvest uses, no CF can be derived: To be established (TBE) upon a decision on the residue definition for risk assessment after post‐harvest uses.
B.2. Residues in livestock
| Relevant groups | Dietary burden expressed in | Most critical dieta | Most critical commoditya |
Trigger exceeded (Y/N) |
|||
|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | ||||||
| Med. | Max. | Med. | Max. | ||||
| Scenario EU1: excluding GAPs with post‐harvest treatment (citrus fruits, apples and potatoes). For potatoes, a fall‐back GAP (seed treatment) was considered. | |||||||
|
Cattle (all diets) |
0.002 | 0.002 | 0.05 | 0.05 | Cattle (dairy) | Potato, process waste | No |
|
Cattle (dairy only) |
0.002 | 0.002 | 0.04 | 0.04 | Cattle (dairy) | Potato, process waste | No |
|
Sheep (all diets) |
0.002 | 0.002 | 0.05 | 0.05 | Sheep (raw/ewe) | Potato, process waste | No |
|
Sheep (ewe only) |
0.002 | 0.002 | 0.05 | 0.05 | Sheep (raw/ewe) | Potato, process waste | No |
|
Swine (all diets) |
0.001 | 0.001 | 0.05 | 0.05 | Swine (breeding) | Potato, process waste | No |
|
Poultry (all diets) |
0.001 | 0.001 | 0.02 | 0.02 | Poultry (turkey) | Potato, culls | No |
|
Poultry (layer only) |
0.001 | 0.001 | 0.02 | 0.02 | Poultry (layer) | Potato, culls | No |
bw: body weight; DM: dry matter; GAP: Good Agricultural Practice.
Calculated for the maximum dietary burden.
B.2.1. Nature of residues and methods of analysis in livestock
B.2.1.1. Metabolism studies, methods of analysis and residue definitions in livestock
| Livestock (available studies) | Animal | Dose (mg/kg bw per day) | Duration (days) | Comment |
|---|---|---|---|---|
| Laying hen | 4.6 | 10 | Belgium (1996); Netherlands (2015) | |
| Lactating goat | 10 | 3 | Belgium (1996) | |
| For laying hens, the study reported in Belgium, 1996 was not deemed sufficient; additional analyses were provided by the RMS in Netherlands (2015). Even though further detailed results were provided by the RMS in its evaluation report (Netherlands, 2015), EFSA is of the opinion that an additional study would still be needed to fully depict the metabolic pathway in poultry | ||||
| Time needed to reach a plateau concentration in milk and eggs (days) | Not reported |
| Metabolism in rat and ruminant similar (Yes/No) | Yes |
| Animal residue definition for monitoring (RD‐Mo) | No proposala |
| Animal residue definition for risk assessment (RD‐RA) | No proposala |
| Conversion factor (monitoring to risk assessment) | Not relevantb |
| Fat soluble residues (Yes/No) | No |
| Methods of analysis for monitoring of residues (analytical technique, crop groups, LOQs) |
Muscle, fat, liver, kidney and milk: HPLC–MS/MS Method validated for the parent compound and FK‐722 with LOQ of 0.01 mg/kg for each compound. Combined LOQ: 0.02 mg/kg ILV available for each compound Source: Netherlands (2015) Eggs: HPLC–MS/MS Method validated for the parent compound only LOQ: 0.01 mg/kg ILV available Source: Netherlands (2015) Missing method for metabolite FK‐772 |
bw: body weight; RMS: rapporteur Member State; HPLC–MS/MS: high‐performance liquid chromatography with tandem mass spectrometry; LOQ: limit of quantification; ILV: independent laboratory validation.
No residue definition is needed according to the dietary burden calculated under scenario EU1. It is noted that metabolites FK‐772 and FK‐284 can be found in significant levels in livestock tissues and products. Due to the equivocal results for genotoxicity for metabolite FK‐772, the lack of a repeated dose study for metabolite FK‐772 and FK‐284 and the lack of information on the impact livestock of metabolism on the isomer ratio of imazalil, the previously derived residue definitions have been suspended, pending submission of further data as specified in EFSAs reasoned opinion published in June 2018 (EFSA, 2018).
No conversion factor can be derived since no residue definition for risk assessment can be proposed.
B.2.1.2. Stability of residues in livestock
|
Animal products (available studies) |
Animal | Commodity | T (°C) | Stability (Months/years) |
|---|---|---|---|---|
| – | Tissues | – | – | |
| – | Milk | – | – | |
| – | Egg | – | – | |
| No studies available | ||||
B.2.2. Magnitude of residues in livestock
B.2.2.1. Summary of the residue data from livestock feeding studies
MRLs and risk assessment values for livestock commodities considering a comprehensive dietary burden with all authorised critical GAPs could not be calculated.
Considering a scenario (EU1) excluding GAPs with post‐harvest treatment (citrus fruits, apples and potatoes), MRLs are not needed for livestock commodities.
B.3. Consumer risk assessment
B.3.1. Consumer risk assessment without consideration of the existing CXLs
| ADI | 0.025 mg/kg bw per day (EFSA, 2010) |
| Highest IEDI, according to EFSA PRIMo |
Scenario EU1: 6.9% ADI (IE adult) Scenario EU2: 1.7% (WHO Cluster diet B) |
| Assumptions made for the calculations |
Scenario EU1: This scenario reflects the authorised uses for seed treatment and foliar uses, excluding the post‐harvest uses. The calculation is based on the median residue levels in the raw agricultural commodities. For potatoes, the risk assessment values derived from the seed treatment GAP were considered as a fall‐back option. However, for citrus fruits, apples, pears, bananas and melons, no fall‐back GAPs could be identified. For melons and sweet peppers/bell peppers where data were insufficient to derive a MRL, EFSA considered the existing EU MRL for an indicative calculation. The contributions of commodities where no GAP was reported in the framework of the MRL review were not included in the calculation Scenario EU2: The EU MRL for melons was excluded from this calculation. All other input values remain unchanged, compared to Scenario EU1 |
| ARfD | 0.05 mg/kg bw (EFSA, 2010) |
| Highest IESTI, according to EFSA PRIMo |
Scenario EU1: 606.7% ARfD (melons) Scenario EU2: 18.6 (tomatoes) |
| Assumptions made for the calculations |
Scenario EU1: This scenario reflects the authorised uses for seed treatment and foliar uses, excluding the post‐harvest uses. The calculation is based on the highest residue levels in the raw agricultural commodities. For potatoes, the risk assessment values derived from the seed treatment GAP were considered as a fall‐back option. However, for citrus fruits, apples, pears, bananas and melons, no fall‐back GAPs could be identified. For melons and sweet peppers/bell peppers where data were insufficient to derive a MRL, EFSA considered the existing EU MRL for an indicative calculation. The contributions of commodities where no GAP was reported in the framework of the MRL review were not included in the calculation Scenario EU2: The EU MRL for melons was excluded from this calculation. All other input values remain unchanged |
ADI: acceptable daily intake; IEDI: international estimated daily intake; bw: body weight; PRIMo: (EFSA) Pesticide Residues Intake Model; WHO: World Health Organization; GAP: Good Agricultural Practice; MRL: maximum residue level; ARfD: acute reference dose; IESTI: international estimated short‐term intake; CXL: codex maximum residue limit.
B.3.2. Consumer risk assessment with consideration of the existing CXLs
| ADI | 0.025 mg/kg bw per day (EFSA, 2010) |
| Highest IEDI, according to EFSA PRIMo |
Scenario CX1: 6.1% ADI (DE, child) Scenario CX2: 6.0% ADI (DE, child) |
| Assumptions made for the calculations |
Scenario CX1: This scenario is based on scenario EU2, including CXLs which are not derived from post‐harvest uses (strawberries, blackberries, raspberries, persimmon, cucumbers, gherkins and wheat). For citrus fruits, pome fruits, bananas, potatoes and melons, the CXLs are based on post‐harvest treatments and were therefore not considered in the assessment For those commodities having a CXL higher than the EU MRL proposal, median residue levels applied in the EU2 scenario were replaced by the median residue levels derived by JMPR. When the median residue level derived by JMPR was not available, the HR or the CXL was considered for an indicative calculation Scenario CX2: The CXL for persimmon was excluded from the calculation. All other input values remain unchanged |
| ARfD | 0.05 mg/kg bw (EFSA, 2010) |
| Highest IESTI, according to EFSA PRIMo |
Scenario CX1: 131% ARfD (persimmon) Scenario CX2: 62.4% ARfD (strawberries) |
| Assumptions made for the calculations |
Scenario CX1: This scenario is based on scenario EU2, including CXLs which are not derived from post‐harvest uses (strawberries, blackberries, raspberries, persimmon, cucumbers, gherkins and wheat). For citrus fruits, pome fruits, bananas, potatoes and melons, the CXLs are based on post‐harvest treatments and were therefore not considered in the assessment For those commodities having a CXL higher than the EU MRL proposal, highest residue levels applied in the EU2 scenario were replaced by the highest residue levels derived by JMPR. When the highest residue level derived by JMPR was not available, the CXL was considered for an indicative calculation Scenario CX2: The CXL for persimmon was excluded from the calculation. All other input values remain unchanged |
ADI: acceptable daily intake; IEDI: international estimated daily intake; bw: body weight; PRIMo: (EFSA) Pesticide Residues Intake Model; WHO: World Health Organization; GAP: Good Agricultural Practice; MRL: maximum residue level; ARfD: acute reference dose; IESTI: international estimated short‐term intake; CXL: codex maximum residue limit.
B.4. Proposed MRLs
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
|
Enforcement residue definition (existing): imazalil Enforcement residue definition 1 (proposed): imazalil (any ratio of constituent isomers) | |||||
| 110010 | Grapefruits | 5 | 5 | – | Further consideration neededa |
| 110020 | Oranges | 5 | 5 | – | Further consideration neededa |
| 110030 | Lemons | 5 | 5 | – | Further consideration neededa |
| 110040 | Limes | 5 | 5 | – | Further consideration neededa |
| 110050 | Mandarins | 5 | 5 | – | Further consideration neededa |
| 130010 | Apples | 2 | 5 | – | Further consideration neededa |
| 130020 | Pears | 2 | 5 | – | Further consideration neededa |
| 130030 | Quinces | 2 | 5 | – | Further consideration neededb |
| 130040 | Medlar | 5 | 5 | – | Further consideration neededb |
| 130050 | Loquat | 5 | 5 | – | Further consideration neededb |
| 152000 | Strawberries | 0.05* | 2 | 2 | Recommendedc |
| 153010 | Blackberries | 0.05* | 2 | 2 | Recommendedc |
| 153030 | Raspberries | 0.05* | 2 | 2 | Recommendedc |
| 161060 | Persimmon | 0.05* | 2 | – | Further consideration neededd |
| 163020 | Bananas | 2 | 2 | – | Further consideration neededa |
| 211000 | Potatoes | 3 | 5 | 0.01* | Recommendede |
| 231010 | Tomatoes | 0.5 | – | 0.3 | Recommendedf |
| 231020 | Sweet peppers/bell peppers | 0.05* | – | 0.05 | Further consideration neededg |
| 232010 | Cucumbers | 0.2 | 0.5 | 0.5 | Recommendedh |
| 232020 | Gherkins | 0.2 | 0.5 | 0.5 | Recommendedh |
| 232030 | Courgettes | 0.2 | – | 0.1 | Further consideration neededi |
| 233010 | Melons | 2 | 2 | – | Further consideration neededj |
| 500010 | Barley grains | 0.05* | – | 0.01* | Recommendedf |
| 500050 | Oat grains | 0.05* | – | 0.01* | Recommendedf |
| 500070 | Rye grains | 0.05* | – | 0.01* | Recommendedf |
| 500090 | Wheat grains | 0.05* | 0.01* | 0.01* | Recommendedk |
| – | Other commodities of plant or animal origin | See Reg. 750/2010 | – | – | Further consideration neededl |
MRL: maximum residue level; CXL: codex maximum residue limit.
* Indicates that the MRL is set at the limit of quantification.
The previously derived tentative residue definition for risk assessment has been suspended because the toxicological data were insufficient to clearly rule out a genotoxic potential for metabolite R014821. Pending the submission of data required to finalise the hazard characterisation for this metabolite that is expected to occur following post‐harvest treatment of the crop, the risk assessment cannot be finalised and consequently no MRL recommendation was derived. A similar case applies to CXL that were derived from post‐harvest uses. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered.
There are no relevant authorisations or import tolerances reported at EU level; CXL is reflecting a post‐harvest use. Thus, pending the finalisation of the hazard characterisation for metabolite R014821, the CXL is not recommended to be taken over in EU legislation. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered.
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; there are no relevant authorisations or import tolerances reported at EU level.
There are no relevant authorisations or import tolerances reported at EU level; CXL is supported by data but a risk to consumers cannot be excluded. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered.
MRL is derived from a GAP evaluated at EU level (fall‐back GAP: seed treatment), which is fully supported by data and for which no risk to consumers is identified; CXL is higher but pending the finalisation of the hazard characterisation for metabolite R014821, the CXL is not recommended to be taken over in EU legislation.
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available.
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL; no CXL is available.
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level, which is not fully supported by data, leads to a lower tentative MRL.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified; no CXL is available.
The previously derived tentative residue definition for risk assessment has been suspended because the toxicological data were insufficient to clearly rule out a genotoxic potential for metabolite R014821. Pending the submission of data required to finalise the hazard characterisation for this metabolite that is expected to occur following post‐harvest treatment of the crop, the risk assessment cannot be finalised and consequently no MRL recommendation was derived from the post‐harvest use. A similar case applies to CXL that were derived from post‐harvest uses. In addition, the foliar GAP evaluated at EU level is not supported by data and a risk to consumers cannot be excluded for the existing EU MRL. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered.
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; existing CXL is covered by the recommended MRL.
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered.
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.
PRIMO EU1
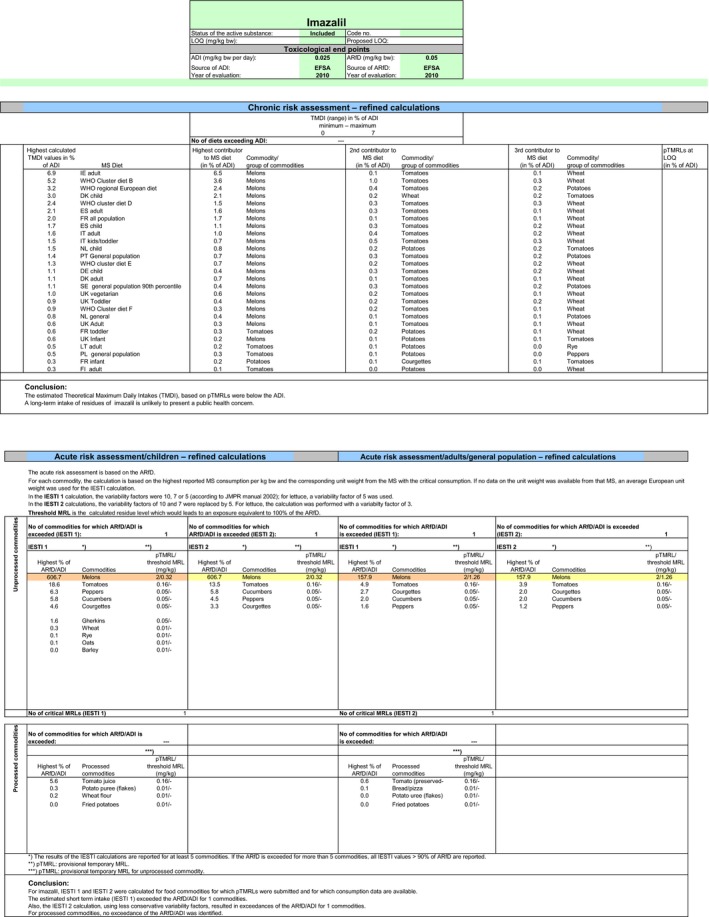
PRIMO EU2
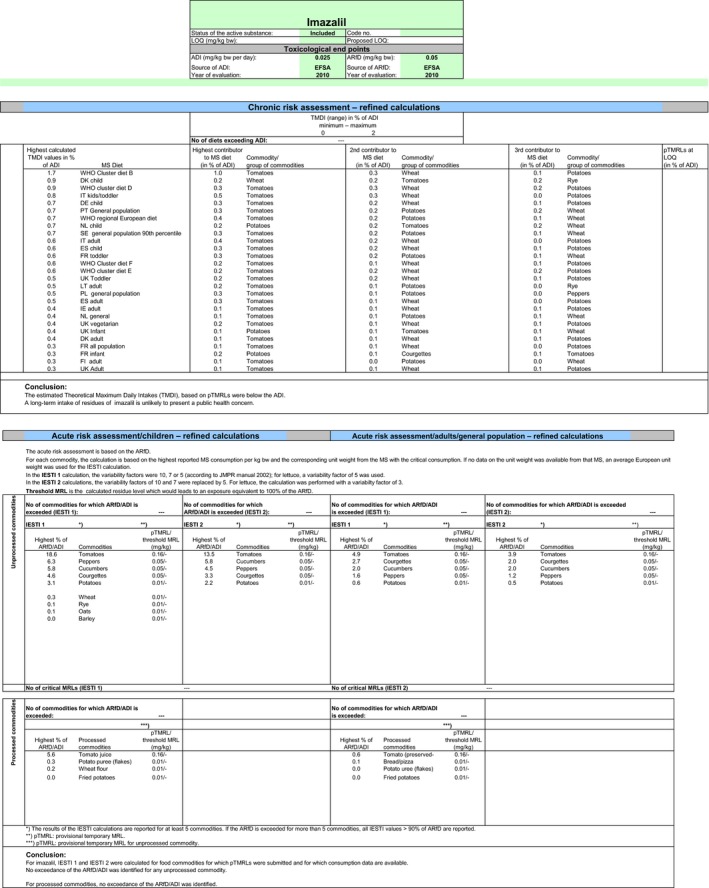
PRIMO CX1
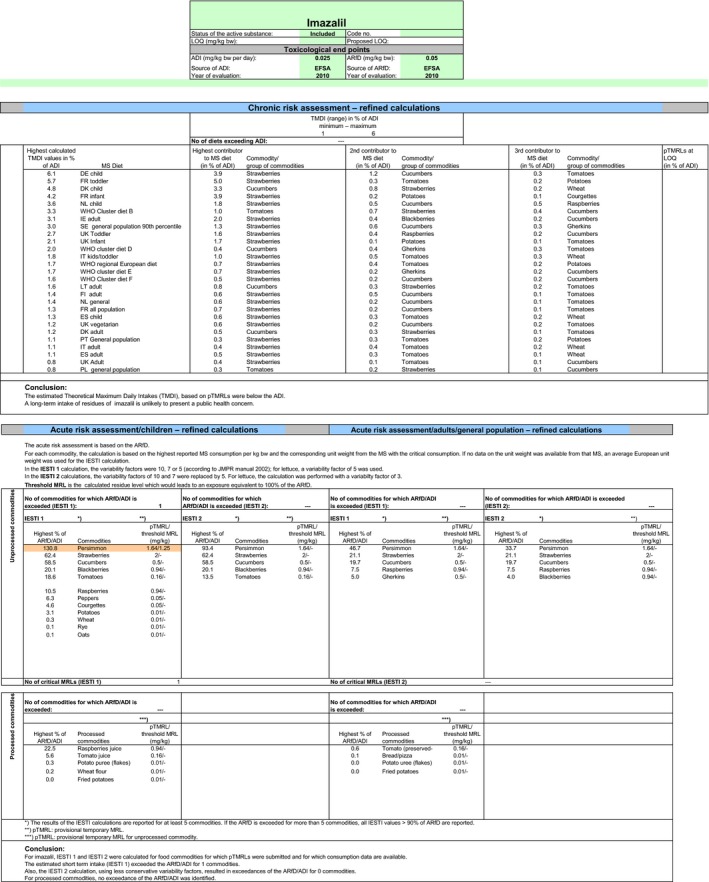
PRIMO CX2
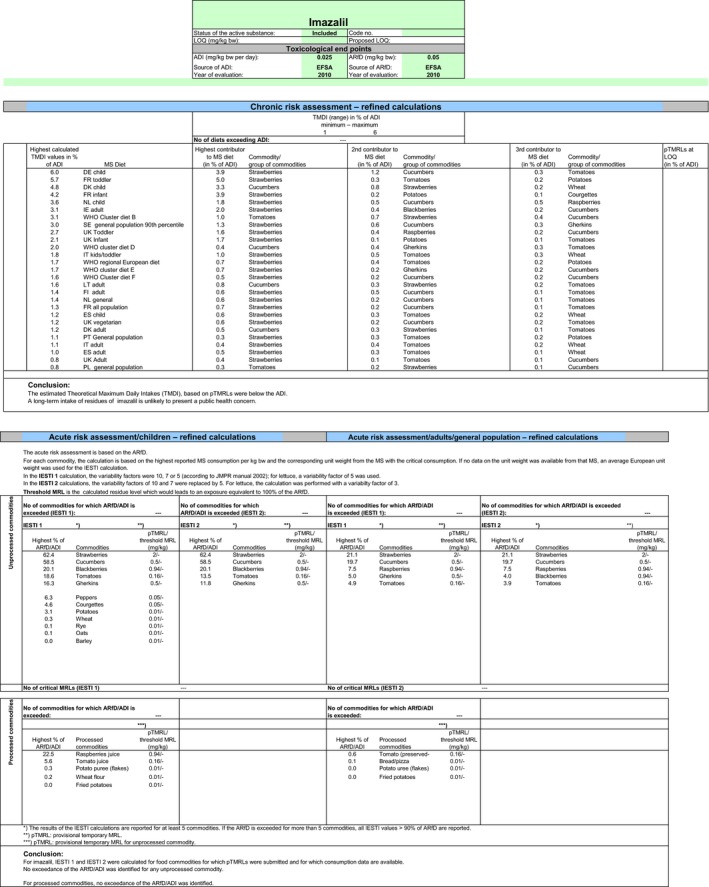
Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Scenario EU1: excluding GAPs with post‐harvest treatment (citrus fruits, apples and potatoes)a. For potatoes, a fall‐back GAP (seed treatment) was considered. | ||||
| Potato, culls | 0.01* | STMRa | 0.01* | HRa |
| Potato, process waste | 0.01* | STMRa,b | 0.01* | STMRa,b |
| Potato, dried pulp | 0.01* | STMRa,b | 0.01* | STMRa,b |
| Brewer's grain, dried | 0.01* | STMRb | 0.01* | STMRb |
| Wheat, distiller's grain (dry) | 0.01* | STMRb | 0.01* | STMRb |
| Wheat gluten, meal | 0.01* | STMRb | 0.01* | STMRb |
| Wheat, milled by‐products | 0.01* | STMRb | 0.01* | STMRb |
| Small grain cereals, grain | 0.01* | STMR | 0.01* | STMR |
| Small grain cereals, straw | 0.01* | STMR | 0.01* | HR |
STMR: supervised trials median residue; HR: highest residue.
* Indicates that the input value is proposed at the limit of quantification.
For citrus fruits, apples and potatoes, the critical GAPs are post‐harvest treatments. However, as risk assessment could not be derived from GAPs with post‐harvest treatments, no input values are available for the feed items derived from these GAPs. For potatoes, risk assessment values could be derived from a fall‐back GAP (seed treatment).
For processed commodities of potatoes (process waste and dried pulp) and cereals (brewer's and distiller's grain, gluten and milled by‐products), no default processing factor was applied because imazalil is applied as a seed treatment and residues are expected to be below the LOQ. Concentration of residues in these commodities is therefore not expected.
D.2. Consumer risk assessment without consideration of the existing CXLs
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition (applicable only to foliar uses and seed treatments): imazalil (any ratio of constituent isomers) | ||||
|
Grapefruits Oranges Lemons Limes Mandarins |
– | Not assesseda | – | Not assesseda |
|
Apples Pears |
– | Not assesseda | – | Not assesseda |
| Bananas | – | Not assesseda | – | Not assesseda |
| Potatoes | 0.01* | STMRb | 0.01* | HRb |
| Tomatoes | 0.09 | STMR | 0.16 | HR |
| Sweet peppers/bell peppers | 0.05 | EU MRLc | 0.05 | EU MRLc |
|
Cucumbers Gherkins Courgettes |
0.02 | STMR (tentative) | 0.05 | HR (tentative) |
| Melons | 2 | EU MRLd | 2 | EU MRLd |
| – | Not assessede | – | Not assessede | |
| Small grain cereals | 0.01* | STMR | 0.01* | STMR |
STMR: supervised trials median residue; HR: highest residue; MRL: maximum residue level.
* Indicates that the input value is proposed at the limit of quantification.
For citrus fruits, apples, pears and bananas, the critical GAPs are post‐harvest treatments. However, as risk assessment could not be derived from GAPs with post‐harvest treatments, these commodities could not be assessed. No fall‐back GAPs were identified for these crops.
For potatoes, the critical GAP is a post‐harvest treatment. However, as risk assessment could not be derived from GAPs with post‐harvest treatments, this GAP could not be assessed. A fall‐back GAP (seed potatoes) was therefore considered.
In the absence of supporting data, the existing EU MRL is used for indicative exposure calculations.
For melons, the critical GAP is a post‐harvest treatment. However, as risk assessment could not be derived from GAPs with post‐harvest treatments, this GAP could not be assessed. A GAP with foliar treatment might be used as a fall‐back GAP but in the absence of supporting data, the existing EU MRL was used for indicative exposure calculations.
A second exposure was calculated excluding the EU MRL on melons as an acute exposure concern was identified with the EU MRL (Scenario EU2).
D.3. Consumer risk assessment with consideration of the existing CXLs
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition (applicable only to foliar uses and seed treatments): imazalil (any ratio of constituent isomers) | ||||
|
Grapefruits Oranges Lemons Limes Mandarins |
– | Not assesseda | – | Not assesseda |
|
Apples Pears Quinces Medlar Loquat |
– | Not assesseda | – | Not assesseda |
| Strawberries | 2 | CXLb | 2 | CXLb |
| Blackberries | 0.94 | HR (CXL)c | 0.94 | HR (CXL) |
| Raspberries | 0.94 | HR (CXL)c | 0.94 | HR (CXL) |
| Persimmon | 1.64 | HR (CXL)c | 1.64 | HR (CXL) |
| – | Not assessedd | – | Not assessedd | |
| Bananas | – | Not assesseda | – | Not assesseda |
| Potatoes | 0.01* | STMRe | 0.01* | HRe |
| Tomatoes | 0.09 | STMR | 0.16 | HR |
| Sweet peppers/ bell peppers | 0.05 | EU MRLf | 0.05 | EU MRLf |
| Cucumbers | 0.05 | CXLb | 0.05 | CXLb |
| Gherkins | 0.05 | CXLb | 0.05 | CXLb |
| Courgettes | 0.02 | STMR (tentative) | 0.05 | HR (tentative) |
| Melons | – | Not assesseda | – | Not assesseda |
| Small grain cereals | 0.01* | STMR | 0.01* | STMR |
STMR: supervised trials median residue; HR: highest residue; MRL: maximum residue level; CXL: codex maximum residue limit.
* Indicates that the input value is proposed at the limit of quantification.
For citrus fruits, pome fruits, bananas and melons, the CXL are linked to post‐harvest uses. As risk assessment could not be derived from GAPs with post‐harvest treatments, these CXLs could not be assessed. For the same reason, no EU GAPs could be assessed for these crops.
In the absence of risk assessment values available for this CXL, the CXL value is directly used for an indicative calculation.
As the median value is not available, the highest value (instead of the median) is used for an indicative chronic calculation.
A second exposure was calculated excluding the CXL (and associated risk assessment values) on persimmon as an acute exposure concern was identified for this commodity.
For potatoes, the CXL is linked to a post‐harvest use. As risk assessment could not be derived from GAPs with post‐harvest treatments, this CXL could not be assessed. STMR and HR values assesse for the EU GAP (seed potatoes) were considered.
In the absence of supporting data, the existing EU MRL is used for indicative exposure calculations.
Appendix E – Used compound codes
1.
| Code/trivial name | Chemical name/SMILES notationa | Structural formulab |
|---|---|---|
| imazalil |
(RS)‐1‐(β‐allyloxy‐2,4‐dichlorophenethyl)imidazole Clc2ccc(C(OCC=C)Cn1 ccnc1)c(Cl)c2 |
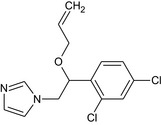
|
| R014821 |
(1RS)‐1‐(2,4‐dichlorophenyl)‐2‐(1H‐imidazol‐1‐yl)ethanol OC(Cn1ccnc1)c2ccc(Cl)cc2Cl |
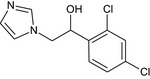
|
| R044177 |
(2RS)‐2‐(allyloxy)‐2‐(2,4‐dichlorophenyl)ethanamine hydrochloride (1:1) Cl.Clc1cc(Cl)ccc1C(OCC=C)CN |
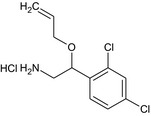
|
| FK‐772 |
3‐{(2RS)‐2‐(2,4‐dichlorophenyl)‐2‐[(2RS)‐2,3‐dihydroxypropoxy]ethyl}‐2,4‐imidazolidinedione Clc2ccc(C(OCC(O)CO)CN1C(=O)CNC1=O)c(Cl)c2 |
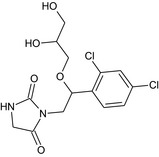
|
| FK‐284 |
3‐[(2RS)‐2‐(2,4‐dichlorophenyl)‐2‐hydroxyethyl]‐2,4‐imidazolidinedione O=C2NCC(=O)N2CC(O)c1ccc(Cl)cc1Cl |
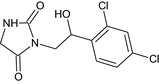
|
| FK‐858 |
(2RS)‐3‐[(1RS)‐1‐(2,4‐dichlorophenyl)‐2‐(1H‐imidazol‐1‐yl)ethoxy]‐1,2‐propanediol Clc2ccc(C(OCC(O)CO)Cn1ccnc1)c(Cl)c2 |
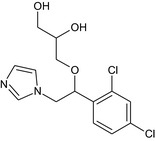
|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; SMILES: simplified molecular‐input line‐entry system.
ACD/Name 2015 ACD/Labs 2015 Release (File version N20E41, Build 75170, 19 December 2014).
ACD/ChemSketch 2015 ACD/Labs 2015 Release (File version C10H41, Build 75059, 17 December 2014).
Suggested citation: EFSA (European Food Safety Authority) , Brancato A, Brocca D, Carrasco Cabrera L, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Jarrah S, Kardassi D, Leuschner R, Lostia A, Lythgo C, Medina P, Miron I, Molnar T, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B and Villamar‐Bouza L, 2018. Reasoned Opinion on the updated review of the existing maximum residue levels for imazalil according to Article 12 of Regulation (EC) No 396/2005 following new toxicological information. EFSA Journal 2018;16(10):5453, 52 pp. 10.2903/j.efsa.2018.5453
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00608
Approved: 28 September 2018
Notes
Commission Directive 97/73/EC of 15 December 1997 including an active substance (imazalil) in Annex I to Council Directive 91/414/EEC concerning the placing of plant protection products on the market, OJ L 353, 24.12.1997, p. 26–28.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Implementing Regulation (EU) No 541/2011 of 1 June 2011 amending Implementing Regulation (EU) No 540/2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 187–188.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Commission Regulation (EC) No 737/2007 of 27 June 2007 on laying down the procedure for the renewal of the inclusion of a first group of active substances in Annex I to Council Directive 91/414/EEC and establishing the list of those substances. OJ L 169, 29.6.2007, p. 10–18.
Commission Implementing Regulation (EU) No 705/2011 of 20 July 2011 approving the active substance imazalil, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJ L 190, 21.7.2011, p. 43–49.
Commission Regulation (EU) No 750/2010 of 7 July 2010 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for certain pesticides in or on certain products. OJ L 220, 21.8.2010, p. 1–56.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Metabolite R014821 ((1RS)‐1‐(2,4‐dichlorophenyl)‐2‐(1H‐imidazol‐1‐yl)ethanol): formed from the dealkylation of the parent compound (see Appendix E).
Metabolite R044177 ((2RS)‐2‐(allyloxy)‐2‐(2,4‐dichlorophenyl)ethanamine hydrochloride (1:1)): formed from the loss of the imidazole ring and carboxylation (see Appendix E).
Considering the highest residues observed in potatoes after post‐harvest treatment of 4.6 mg/kg (see Appendix B.1.2) and assuming a planting density of 7 tonnes potatoes per ha.
Geometric mean derived from different DT50 values calculated in laboratory (EFSA, 2010).
Application rate for cereals is 75 g a.s./tonnes tubers while application rate for potatoes is 15 g a.s./tonnes seeds.
Considering that apples, pears, bananas and melons are worldwide major crops (European Commission, 2016), eight trials are required to derive MRLs for these commodities. Furthermore, these trials do not include analysis of the metabolite R014821. A minimum of four additional residue trials including simultaneous analysis of imazalil and metabolite R014821 (with decline curves at different WHPs from day zero to 6 months) should be generated. As a clear difference was observed between apples (0.36–0.38 mg/kg) and pears (0.74‐0.79 mg/kg), these trials should equitably be performed on apples and pears.
References
- Belgium , 1996. Draft assessment report on the active substance imazalil prepared by the rapporteur Member State Belgium in the framework of Council Directive 91/414/EEC, July 1996.
- Belgium , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2007. Reasoned opinion on the potential chronic and acute risk to consumers’ health arising from proposed temporary EU MRLs. EFSA Journal 2007;5(3):32r, 1141 pp. 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Conclusion on the peer review of the pesticide risk assessment of the active substance imazalil. EFSA Journal 2010;8(2):1526, 69 pp. 10.2903/j.efsa.2010.1526 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Brancato A, Brocca D, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Janossy J, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Medina P, Miron I, Molnar T, Nougadere A, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017. Reasoned opinion on the review of the existing maximum residue levels for imazalil according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2017;15(9):4977, 66 pp. 10.2903/j.efsa.2017.4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Brancato A, Brocca D, Carrasco Cabrera L, Chiusolo A, Civitella C, Court Marques D, Crivellente F, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Istace F, Jarrah S, Kardassi D, Leuschner R, Lostia A, Lythgo C, Medina P, Mineo D, Miron I, Molnar T, Parra Morte JM, Pedersen R, Reich H, Riemenschneider C, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Terron A, Theobald A, Vagenende B and Villamar‐Bouza L, 2018. Reasoned opinion on the modification of the existing maximum residue levels for imazalil in various commodities. EFSA Journal 2018;16(6):5329, 29 pp. 10.2903/j.efsa.2018.5329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EURL (European Union Reference Laboratories for Pesticide Residues), 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Analytical methods validated by the EURLs and overall capability of official laboratories to be considered for the review of the existing MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2016. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev. 10.2, September 2016.
- European Commission , 2017. Appendix D. Guidelines on comparability, extrapolation, group tolerances and datarequirements for setting MRLs. 7525/VI/95‐rev.10.3, June 2017.
- FAO (Food and Agriculture Organization of the United Nations), 1977. Imazalil. 1977 Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues, December 1977. FAO Plant Production and Protection Paper 10
- FAO (Food and Agriculture Organization of the United Nations), 1984. Imazalil. 1984 Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues, September‐October 1984. FAO Plant Production and Protection Paper 62
- FAO (Food and Agriculture Organization of the United Nations), 1985. Imazalil. 1985 Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues, September‐October 1985. FAO Plant Production and Protection Paper 72/2, 226 pp
- FAO (Food and Agriculture Organization of the United Nations), 1994. Imazalil. 1994 Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues, September 1994. Available online: http://www.fao.org
- FAO (Food and Agriculture Organization of the United Nations), 2009. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 2nd Edition. FAO Plant Production and Protection Paper 197, 264 pp.
- France , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- Germany , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- Greece , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- Greece , 2018. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil (Corrigendum), March 2018. Available online: http://www.efsa.europa.eu
- Italy , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- Netherlands , 2009a. Assessment Report on the active substance imazalil prepared by the rapporteur Member State Netherlands in consultation with Spain in the framework of Commission Regulation (EC) No 737/2007, May 2009. Available online: http://www.efsa.europa.eu
- Netherlands , 2009b. Final Addendum to Assessment Report on imazalil, prepared by the rapporteur Member State Netherlands in consultation with Spain in the framework of Commission Regulation (EC) No 737/2007, compiled by EFSA, November 2009. Available online: http://www.efsa.europa.eu
- Netherlands , 2014. Post inclusion addendum to the draft assessment report on imazalil on confirmatory data. Vol 3 B.7 Residues, May 2014. Available online: http://www.efsa.europa.eu
- Netherlands , 2015. Evaluation report prepared under Article 8 of Regulation (EC) No 396/2005. Modification of MRLs for imazalil in various crops and products of animal origin, March 2015, revised in March 2018. Available online: http://www.efsa.europa.eu
- Netherlands , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 04 September 2013.
- Portugal , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu
- Spain , 2016. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Authorised uses to be considered for the review of the existing EU MRLs for imazalil, May 2016. Available online: http://www.efsa.europa.eu


