Abstract
The Panel on Food Additives and Nutrient Sources added to Food (ANS) provides a scientific opinion re‐evaluating the safety of gellan gum (E 418) as a food additive. Following the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010, the Panel considered that adequate exposure and toxicity data were available. Based on the reported use levels, a refined exposure of up to 72.4 mg/kg body weight (bw) per day in toddlers at the 95th percentile was estimated. Gellan gum is unlikely to be absorbed intact and would not be fermented by human intestinal microbiota. There is no concern with respect to carcinogenicity and genotoxicity. No adverse effects were reported in chronic studies at the highest doses tested in mice and rats (3,627 and 1,460 mg gellan gum/kg bw per day, respectively). Repeated oral intake up to 200 mg/kg bw per day for 3 weeks had no adverse effects in humans. The Panel concluded that there is no need for a numerical acceptable daily intake (ADI) for gellan gum (E 418), and that there is no safety concern at the refined exposure assessment for the reported uses and use levels of gellan gum (E 418) as a food additive. The Panel recommended to better define the specifications of gellan gum including the absence of viable cells of the microbial source and the presence of polyhydroxybutyrate (PHB), protein and residual bacterial enzymatic activities.
Keywords: gellan gum (E 418), food additive
Summary
The present opinion deals with the re‐evaluation of the safety of gellan gum (E 418) used as a food additive.
Gellan gum (E 418) is authorised as a food additive in the European Union (EU) in accordance with Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/20121.
In the EU, gellan gum (E 418) has been evaluated by the Scientific Committee for Food (SCF) in 1990 (SCF, 1992). The committee allocated an acceptable daily intake (ADI) ‘not specified’ based on the toxicological data and the use levels, typically ranging from 0.1% to 1%, as a gelling, stabilising or thickening agent. Specific dietary or medical uses were not covered by the SCF evaluation. Furthermore in the SCF evaluation was pointed out that the specifications should exclude the presence of viable Pseudomonas elodea. The Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated gellan gum in 1991 (JECFA, 1991b) and revised the specifications twice thereafter (JECFA, 2006, 2014). JECFA ‘Committee allocated an ADI ‘not specified’ to gellan gum, and pointed out that its potential laxative effect at high intakes should be taken into account when it is used as a food additive’.
According to Commission Regulation (EU) No 231/2012, gellan gum (E 418) is a high molecular weight polysaccharide gum produced by a pure culture fermentation of a carbohydrate by strains of P. elodea. The Panel noted that according to available information gellan gum (E 418) is produced by only one bacterial strain ATCC 31461.
The repeating unit of the polysaccharide is a tetrasaccharide composed of two d‐glucose units, one d‐glucuronic acid residue and one of l‐rhamnose residue and is substituted with acyl groups (glycerate and acetate groups as O‐glycosidically linked esters).
According to the industry, in addition to the gellan gum polysaccharide, typical samples contain water (2–14%), proteinaceous material measured by nitrogen content (%N = 0–3.0%) (Documentation provided to EFSA No 3) and may contain polyhydroxybutyrate (PHB) up to 25 wt% (Baird and Cleary, 1994).
There are two basic forms: ‘high acyl’ and ‘low acyl’ form of the food additive gellan gum (E 418), which are distinguished by the degree of substitution by O‐acyl groups. For ‘low acyl’ (including fully deacylated) type, there are both clarified and non‐clarified products available on the market.
The Panel noted that, according to the available information from the industry, the content of PHB in dried gellan gum was estimated to be from less than 1% up to 25%, depending on the degree of deacylation and clarification. The Panel noted that PHB may be a major component of the food additive E 418 resulting from the manufacturing process. In the absence of any justification about its technological need, the Panel considered that its presence should be limited, and/or at least indicated in the specifications of the food additive E 418. In this regard, the Panel noted that clarification is a feasible mean to reduce the amount of PHB in E 418.
In the period from the date of US Patent of Kang and Veeder (1982) to which the production of gellan gum originally refers until the present time, no reports have been identified that the strain ATCC 31461 was the cause of any human infection.
The biological and toxicological testing has been performed with a deacylated gellan gum or with a gellan gum of unknown degree of acylation. However, the Panel considered that the structural similarities of the different types of gellan gum allowed for read across. No information was available on the purity of the test material in these studies, including the concentration of PHB.
The in vivo metabolic and physiological studies of gellan gum indicated that this compound would not be absorbed intact. In rats, there is indication of limited increased production of short‐chain fatty acid (SCFA) and the faecal release of acetate and butyrate could be the consequence of both the hydrolysis of the acetyl side chain of gellan gum and the possible presence of PHB. In humans, gellan gum would not be absorbed intact and there is no indication of significant fermentation by the intestinal microbiota.
Gellan gum is of low acute toxicity.
Subchronic toxicity studies with gellan gum conducted in rats and dogs did not reveal adverse effects at the highest doses tested (equal to 2,950 mg/kg body weight (bw) per day for males and 3,760 mg/kg bw per day for females in rats and 1,870 mg/kg bw day for males and 2,070 mg/kg bw per day for females in dogs). In a short‐term study in rhesus monkeys, no adverse effects were seen at 3,000 mg gellan gum/kg bw per day, the highest dose tested.
Based on the available data, the Panel considered that gellan gum did not raise concern with respect to genotoxicity.
Gellan gum is not of concern with respect to carcinogenicity. Chronic toxicity studies with gellan gum did not reveal adverse effects at the highest doses tested equal to 2,867 mg gellan gum/kg bw per day for male mice and 3,627 mg gellan gum/kg bw per day for female mice, or equivalent to 1,460 mg gellan gum/kg bw per day in rats.
A dietary two‐generation reproductive toxicity study, an one‐generation study (in utero phase of a chronic/carcinogenicity study) and a prenatal developmental toxicity study in rats with gellan gum up to 5% in the diet (1,460 mg/kg bw per day), the highest dose tested, did not show adverse effects.
The consumption of up to 200 mg/kg bw per day gellan gum over 3 weeks had no adverse health effects in humans. There was no indication for allergenic reaction to gellan gum both in animals and humans.
To assess the dietary exposure to gellan gum (E 418) from its use as a food additive, the exposure was calculated based on (1) maximum reported use levels for food categories in which gellan gum (E 418) is authorised at quantum statis (QS) and maximum permitted levels (MPLs) for the two food categories with numerical maximum levels (defined as the maximum level exposure assessment scenario) and (2) the reported use levels (defined as the refined exposure assessment scenario).
Based on the available data set, the Panel calculated three refined exposure estimates based on different assumptions: a brand‐loyal consumer scenario, a non‐brand‐loyal scenario and the food supplement consumers’ only scenario. The Panel considered that the refined exposure assessment approach resulted in more realistic long‐term exposure estimates compared to the maximum level exposure assessment scenario.
The Panel noted that the estimated long‐term exposures based on the maximum level exposure assessment scenario are very likely conservative, as this scenario assumes that all foods and beverages listed under the annex II to Regulation No 1333/2008 contain gellan gum (E 418) at the MPL or at the maximum reported use levels that were in this case mainly provided from a food additive producer.
For gellan gum (E 418), few reported uses were available on eight food categories. However, not all available data could be included in the assessment owing to specific restrictions/exceptions regarding products not referenced in the FoodEx classification. This may have resulted in an underestimation of exposure to gellan gum (E 418). On the other hand, several food categories for which use data were available were included without considering specific restrictions/exceptions, which may have overestimated the exposure to gellan gum (E 418). In total, 7 out of 71 authorised food categories were taken into account in the refined exposure assessment scenarios. Added to that, approximately 67% of the food products labelled with gellan gum (E 418) in the Mintel's Global New Products Database (GNPD) belonged to food subcategories that were considered in the refined exposure assessment scenarios (Appendix B).
Several uncertainties were identified in the exposure assessment (Table 5). Overall, for the maximum level exposure scenario, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure to gellan gum (E 418) as a food additive according to Annex II to Regulation (EC) No 1333/2008 in European countries included in the EFSA Comprehensive database. Based on the assumption that the food additive is not used in those food categories in which it is permitted but for which no usage data were provided, also the refined scenario would in general result in an overestimation of exposure.
Table 5.
Summary of dietary exposure to gellan gum (E 418) from its use as a food additive in the maximum level exposure assessment scenario and in the refined exposure scenarios, in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Maximum level exposure assessment scenario | ||||||
| Mean | 16–173 | 71–231 | 50–199 | 32–92 | 21–67 | 16–62 |
| 95th percentile | 72–690 | 213–446 | 106–377 | 66–188 | 45–126 | 35–109 |
| Refined estimated exposure assessment scenario | ||||||
| Brand‐loyal scenario | ||||||
| Mean | < 0.1–4.3 | 0.2–18.5 | 0.7–21.0 | 0.57–13.3 | 0.2–4.7 | 0.1–1.8 |
| 95th percentile | 0.4–25.2 | 0.7–72.4 | 3.5–54.3 | 3.4–33.9 | 1.4–17.1 | 0.5–7.5 |
| Non‐brand‐loyal scenario | ||||||
| Mean | < 0.1–0.9 | < 0.1–4.1 | 0.2–4.6 | 0.1–3.0 | < 0.1–1.1 | < 0.1–0.4 |
| 95th percentile | 0.1–5.2 | 0.4–14.7 | 0.7–11.3 | 0.7–7.2 | 0.3–3.6 | 0.1–1.7 |
The Panel noted that the exposure to gellan gum (E 418) from its use according the Annex III (Parts 2, 3, 4 and 5A) was not considered in the exposure assessment.
Since gellan gum (E 418) is authorised and used in a certain type of flavoured drinks, to which consumers may be brand loyal, the Panel selected the refined brand‐loyal scenario as the most relevant exposure scenario for the safety evaluation of this food additive.
Due to the discrepancies observed between the data reported from food industry and Mintel database, where gellan gum (E 418) is labelled in more products than in food categories for which data were reported from industry, the Panel noted that the collection of data on use and use levels of gellan gum (E 418) would allow for a more realistic exposure assessment. The Panel also noted that according to the Mintel's GNPD, gellan gum was listed as an ingredient in seven products of subcategory ‘growing‐up milk’ (soya‐drink products recommended for young children 1–3 years old) and in five products of subcategory ‘baby juices & drinks’. Gellan gum (E 418) is not authorised as a food additive in those subcategories.
The Panel also noted that the refined exposure estimates are based on reported use levels of gellan gum (E 418). If current practice changes, this refined estimates may no longer be representative and should be updated.
Following the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA, 2014), and given that:
from all the data received, data were adequate for a refined exposure assessment for 7 out of 71 food categories;
based on the reported use levels, a refined exposure (brand‐loyal scenario) of up to 72.4 mg/kg bw per day in toddlers at the 95th percentile was estimated;
gellan gum is unlikely to be absorbed intact and would not be fermented by human intestinal microbiota;
adequate toxicity data were available;
there was no concern with respect to genotoxicity and carcinogenicity;
no adverse effects were reported in chronic studies at the highest doses tested in mice and rats (3,627 and 1,460 mg gellan gum/kg bw per day, respectively).
repeated oral intake up to 200 mg/kg bw per day for 3 weeks had no adverse effects in humans,
the Panel concluded that there is no need for a numerical ADI for gellan gum (E 418), and that there is no safety concern at the refined exposure assessment for the reported uses and use levels of gellan gum (E 418) as a food additive.
The Panel recommended the European Commission to consider:
changing in the definition of the European Commission specifications the chemical names of the acyl groups ‘glyceryl’ and ‘acetyl’ to ‘glycerate’ and ‘acetate’;
indicating in the definition of the European Commission specifications that only the non‐genetically modified strain ATCC 31461 should be used for the production of gellan gum
establishing specifications for the individual types of gellan gum with respect to acylation and clarification;
including specifications for the absence of viable cells of the microbial source;
defining purity in the specifications including the presence of PHB and residual bacterial enzymatic activities;
revising the current limits for the toxic elements lead, mercury, cadmium and arsenic in the European Commission specification for gellan gum (E 418) in order to ensure that gellan gum (E 418) as a food additive will not be a significant source of exposure to these toxic elements in food.
1. Introduction
The present opinion deals with the re‐evaluation of gellan gum (E 418) when used as a food additive.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
Regulation (EC) No 1333/20082 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union. In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the European Union before 20 January 2009 has been set up under the Regulation (EU) No 257/20103. This Regulation also foresees that food additives are re‐evaluated whenever necessary in light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU4 of 2001. The report ‘Food additives in Europe 2000,5 submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010 the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference
The Commission asks the European Food Safety Authority to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.1.3. Interpretation of terms of reference
This re‐evaluation refers exclusively to the uses of gellan gum (E 418) as a food additive in food, including food supplements, and does not include a safety assessment of other uses of gellan gum (as described in Section 3.4.2).
1.2. Information on existing authorisations and evaluations
Gellan gum (E 418) is authorised as a food additive in the European Union (EU) in accordance with Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/20126.
In the EU, gellan gum (E 418) has been evaluated by the SCF in 1990 (SCF, 1992). The committee allocated an acceptable daily intake (ADI) ‘not specified’ based on the toxicological data and the use levels, typically ranging from 0.1% to 1%, as a gelling, stabilising or thickening agent. Specific dietary or medical uses were not covered by the SCF evaluation. Furthermore in the SCF evaluation, it was pointed out that the specifications should exclude the presence of viable Pseudomonas elodea.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated gellan gum in 1991 (JECFA, 1991b) and revised the specifications twice thereafter (JECFA, 2006, 2014). JECFA ‘Committee allocated an ADI ‘not specified’ to gellan gum, and pointed out that its potential laxative effect at high intakes should be taken into account when it is used as a food additive’.
Gellan gum (E 418) is one of the food additives that composed jelly mini‐cups which were suspended in 2004 by the European Commission to be placed on the market and import (Commission Decision 2004/37/EC), following the measures taken and information provided by different Member States. Jelly mini‐cups are defined as ‘jelly confectionery of a firm consistence, contained in semi rigid mini‐cups or mini‐capsules, intended to be ingested in a single bite by exerting pressure on the mini‐cups or minicapsule to project the confectionery into the mouth’.
In 2004, the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC Panel) prepared a scientific opinion on a request from the European Commission related to the use of certain food additives derived from seaweed or non‐seaweed origin, including gellan gum (E 418) in jelly mini cups (EFSA AFC Panel, 2004). The AFC Panel concluded that any of these gel‐forming additives or of any other type that gave rise to a confectionery product of a similar size, with similar physical and/or physicochemical properties and that could be ingested in the same way as the jelly mini‐cups, would give rise to a risk for choking (EFSA AFC Panel, 2004). The use of these additives in jelly mini‐cups is not authorised in EU.7
Gellan gum (E 418) is included in the European Union Register8 of feed additives (Regulation (EC) No 1831/20039).
2. Data and methodologies
2.1. Data
The Panel on Food Additives and Nutrient Sources added to Food (ANS) was not provided with a newly submitted dossier. EFSA launched public calls for data10 , 11 , 12 and, if relevant, contacted other risk assessment bodies to collect relevant information from interested parties.
The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up to 26 April 2018. Attempts were made at retrieving relevant original study reports on which previous evaluations or reviews were based, however, not always these were available to the Panel.
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database13) was used to estimate the dietary exposure.
The Mintel's Global New Products Database (GNPD) is an online resource listing food products and compulsory ingredient information that is included in labelling. This database was used to verify the use of gellan gum (E 418) in food products.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing Guidances from the EFSA Scientific Committee.
The ANS Panel assessed the safety of gellan gum (E 418) as a food additive in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the Scientific Committee on Food (SCF, 2001) and taking into consideration the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
When the test substance was administered in the feed or in the drinking water, but doses were not explicitly reported by the authors as mg/kg body weight (bw) per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012a,b,c) for studies in rodents or, in the case of other animal species, by JECFA (2000). In these cases, the daily intake is expressed as equivalent. When in human studies in adults (aged above 18 years), the dose of the test substance administered was reported in mg/person per day, the dose in mg/kg bw per day was calculated by the Panel using a body weight of 70 kg as default for the adult population as described in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012a,b,c).
Dietary exposure to gellan gum (E 418) from its use as a food additive was estimated combining food consumption data available within the EFSA Comprehensive European Food Consumption Database with the maximum permitted levels (MPLs) according to Annex II and III to Regulation (EC) No 1333/2008 and/or reported use levels and analytical data submitted to EFSA following a call for data. Different scenarios were used to calculate exposure (see Section 3.4.1). Uncertainties on the exposure assessment were identified and discussed with regard to their impact on the final exposure calculation.
In the context of this re‐evaluation, the Panel followed the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EC) No 257/2010 (EFSA ANS Panel, 2014).
3. Assessment
3.1. Technical data
3.1.1. Identity of the substance
According to Commission Regulation (EU) No 231/2012, gellan gum (E 418) is a high molecular weight polysaccharide gum produced by a pure culture fermentation of a carbohydrate by strains of Pseudomonas elodea. This strain is also named now Sphingomonas elodea.14 However, these taxonomic names have not been validly published under the rules of the International Code of Nomenclature of Bacteria (Bacteriological Code). According to information from two interested parties, the microbial source of gellan gum (E 418) production is defined by the American Type Culture Collection (ATCC) as strain ATCC 31461.15 This is the only strain used in the production of gellan gum (E 418) and is a natural strain not genetically modified by any genetic engineering techniques (Documentation provided to EFSA No 10). The two interested parties referenced a patent for the use of ATCC 31461 (assigned as Pseudomonas elodea) as a microbial source of gellan gum production (Documentation provided to EFSA No 9; No 10; Kang and Veeder, 1982). The Panel noted that in this opinion the microbial source of gellan gum will be designated as strain ATCC 31461.
Gellan gum is purified by recovery with ethanol or 2‐propanol, dried and milled. (Commission Regulation (EU) No 231/2012; Giavasis et al., 2000). The repeating unit of the polysaccharide is a tetrasaccharide composed of two d‐glucose units, one d‐glucuronic acid residue and one of l‐rhamnose residue and is substituted with acyl groups (glycerate and acetate groups as O‐glycosidically linked esters). The glucuronic acid is neutralised to a mixed potassium, sodium, calcium and magnesium salt (Commission Regulation (EU) No 231/2012; JECFA, 2014; Fialho et al., 2008).
According to the industry, in addition to the gellan gum polysaccharide, typical samples contain water (2–14%), proteinaceous material measured by nitrogen content (%N = 0–3.0%) (Documentation provided to EFSA No 3) and may contain polyhydroxybutyrate (PHB) up to 25 wt% (Baird and Cleary, 1994).
There are two basic forms: ‘high acyl’ and ‘low acyl’ form of the food additive gellan gum (E 418), which are distinguished by the percent substitution of O‐acyl groups, (Commission Regulation (EU) No 231/2012, Sworn, 2009; USDA, 2006; Documentation provided to EFSA No 7; No 8). According to the information provided by one interested party (Documentation provided to EFSA No 8), in native fermentation broth the total acyl content of the gellan molecules is around 14.7 wt%, including 2.9% acetate and 11.8% glycerate groups. In ‘high acyl’ gellan gum, two acyl substituents acetate at C6 and glycerate at C2 on the first glucose unit of the tetrasaccharide repeating unit are present, and on average, there is one glycerate per repeat and one acetate per every two repeats. The degree of acylation for commercial ‘high acyl’ gellan gum products is > 50% (> 7.35 wt%) or more typically > 80% (> 11.76 wt%). In ‘low acyl’ (partly deacylated and fully deacylated) gellan gum, the acyl groups are removed by alkaline treatment during manufacture. The degree of acylation for ‘low acyl’ gellan gum is generally ≤ 50% (≤ 7.35 wt%) or more typically often < 1% (< 0.15 wt%). Among the ‘low acyl’ gellan gum products commercially available, a very common form is the fully deacylated one, with no detectable acyl groups. According to a gellan gum manufacturer, the molecular weight of ‘high acyl’ gellan gum is 1–2 × 106 Daltons and of ‘low acyl’ gellan gum 2–3 × 105 Daltons (CP Kelco, 2007).
In the literature, deacylated gellan gum is frequently named deacetylated gellan gum. In the present report, the term deacylated is used exclusively.
For ‘low acyl’ (including fully deacylated) type, there are both clarified and non‐clarified products available on the market. In the clarified forms of gellan gum, protein residues are partly removed (Bajaj et al., 2007; Documentation provided to EFSA No 7; No 8). According to the interested party (Documentation provided to EFSA No 8), there is no commercially available clarified ‘high acyl’ gellan gum.
The structural formula of gellan gum is presented in Figure 1.
Figure 1.
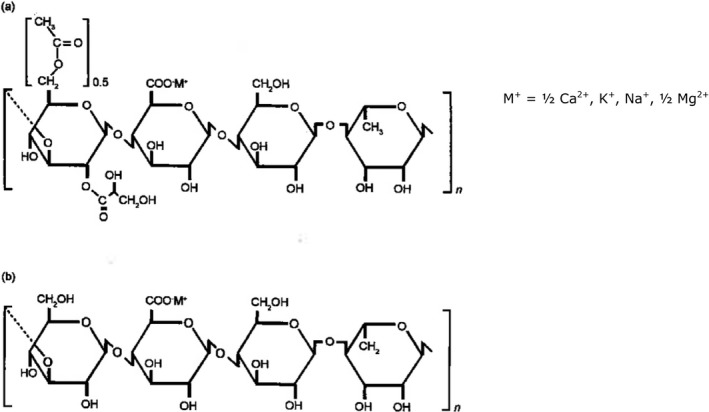
Structural formula of (a) native gellan gum (b) deacylated gellan gum (Documentation provided to EFSA No 8)
Gellan gum has the CAS Registry number 71010‐52‐1 and the EINECS No. 275‐117‐5. According to Commission Regulation (EU) No 231/2012, gellan gum is an off‐white powder which is soluble in water and insoluble in ethanol. In aqueous media, the substance produces thermoreversible gels when heated and cooled. The gelling behaviour is dependent on the acyl content, temperature and the presence of cations in the solution. While the native, non‐deacylated gellan gum forms soft and elastic gels, the deacylated gum forms firm and brittle gels. The addition of calcium, potassium, sodium and magnesium causes an increase of gel strength and brittleness. The gels are stable at temperatures up to 90°C and in a pH range between 3.5 and 8 (Paul et al., 1986; Bajaj et al., 2007).
At low temperatures, gellan forms an ordered double‐chain helix conformation, while at high temperatures a single‐chain polysaccharide structure occurs, which significantly reduces the viscosity of its solution. The transition temperature is reported to be approximately 35°C. When diluted in water, double‐chain gellan structures tend to branch and/or cyclise by aggregation and binding to one another at the ‘junction zones’ via salt bridges. Secondary interactions, including hydrogen bonds link the chains to the junction zones increasing the volume of the molecule helices (Giavasis et al., 2000).
Mei et al. (2012) investigated synergistic interaction on gel formation between konjac glucomannan and gellan gum. At lower temperatures, konjac glucomannan molecules attach by weak junctions to the helical surface of gellan polymers forming together a three‐dimensional network. Texture profile analysis (TPA) showed that properties of the mixed gels are greatly influenced by the ratio of konjac glucomannan/gellan gum and the concentration of different cations. The hardness profiles demonstrate that the addition of gellan gum increases the mixed gels strength (Mei et al., 2012).
According to the data provided by the industry (Documentation provided to EFSA No 4), based on laser diffraction analyses of six samples of typical gellan gum products expressed on a volume basis, the mean particle size goes from 84 μm (± 3 μm) to 108 μm (± 15 μm) with no particles with diameter less than 100 nm present. The interested party noted that, in order for gellan gum to perform its additive function in food and beverage systems, it must be dissolved in an aqueous medium and therefore human consumption of gellan gum in particulate form is highly unlikely from the technological standpoint.
No synonyms for gellan gum were identified in the literature nor are listed in the EU Regulation.
3.1.2. Specifications
The specifications for gellan gum (E 418) as defined in the Commission Regulation (EU) No 231/2012 and by JECFA (2014) are listed in Table 1.
Table 1.
Specifications for gellan gum (E 418) according to Commission Regulation (EU) No 231/2012 and JECFA (2014)
| Commission Regulation (EU) No 231/2012 | JECFA (2014) | |
|---|---|---|
| Definition | Gellan gum is a high molecular weight polysaccharide gum produced by a pure culture fermentation of a carbohydrate by strains of Pseudomonas elodea, purified by recovery with propan‐2‐ol or ethanol, dried and milled. The high molecular weight polysaccharide is principally composed of a tetrasaccharide repeating unit of one rhamnose, one glucuronic acid and two glucoses, and substituted with acyl (glyceryl and acetyl) groups as the O‐glycosidically linked esters. The glucuronic acid is neutralised to a mixed potassium, sodium, calcium and magnesium salt | Gellan gum is a high molecular weight polysaccharide gum produced by a pure culture fermentation of a carbohydrate by Pseudomonas elodea, purified by recovery with ethanol or 2‐propanol, dried and milled. The high molecular weight polysaccharide is principally composed of a tetrasaccharide repeating unit of one rhamnose, one glucuronic acid and two glucose units, and is substituted with acyl (glyceryl and acetyl) groups as the O‐glycosidically linked esters. The glucuronic acid is neutralised to a mixed potassium, sodium, calcium and magnesium salt. It usually contains a small amount of nitrogen containing compounds resulting from the fermentation procedures |
| Assay | Yields, on the dried basis, not less than 3.3% and not more than 6.8% of CO2 | Yields, on the dried basis, not less than 3.3% and not more than 6.8% of CO2 |
| Description | An off‐white powder | Off‐white powder |
| Identification | ||
| Solubility | Soluble in water, forming a viscous solution. Insoluble in ethanol | Soluble in water, forming a viscous solution; insoluble in ethanol |
| Gel test with calcium ion | – | Add 1.0 g of the sample to 99 mL of water and stir for about 2 h, using a motorised stirrer having a propeller‐type stirring blade. Draw a small amount of this solution into a wide bore pipet and transfer into a 10% solution of calcium chloride. A tough worm‐like gel will be formed immediately |
| Gel test with sodium ion | – | Add 1.0 g of the sample to 99 mL of water and stir for about 2 h, using a motorised stirrer having a propeller‐type stirring blade. Add 0.50 g of sodium chloride, heat to 80° with stirring and hold at 80° for 1 min. Allow the solution to cool to room temperature. A firm gel is formed |
| Purity | ||
| Loss on drying | Not more than 15% after drying (105°C, 2.5 h) | Not more than 15% (105°, 2½ h) |
| Nitrogen | Not more than 3% | Not more than 3% |
| Ethanol | – | Not more than 50 mg/kg |
| Propane‐2‐ol | Not more than 750 mg/kg | Not more than 750 mg/kg |
| Arsenic | Not more than 3 mg/kg | – |
| Lead | Not more than 2 mg/kg |
Not more than 2 mg/kg Determine using an atomic absorption technique appropriate to the specified level. The selection of sample size and method of sample preparation may be based on the principles of the method described in Volume 4, ‘Instrumental Methods’ |
| Mercury | Not more than 1 mg/kg | – |
| Cadmium | Not more than 1 mg/kg | – |
| Microbiological criteria | ||
| Total plate count | Not more than 10;000 colonies per gram | Not more than 10,000 colonies per gram |
| Yeast and mould | Not more than 400 colonies per gram | Not more than 400 colonies per gram |
| Escherichia coli | Negative in 5 g | Negative by test |
| Salmonella spp. | Negative in 10 g | Negative by test |
The Panel noted that in the EU specifications the sequence of monosaccharides (glucose‐glucuronic acid‐glucose‐rhamnose) in the repeating tetrasaccharide unit, as well as the position of substitution with acyl groups should be indicated and that the terms ‘glyceryl’ and ‘acetyl’ should be replaced with ‘glycerate’ and ‘acetate’, respectively.
The Panel also noted that in the EU specifications neither deacylated nor clarified gellan gum products are mentioned. However, it is reported by the interested parties that the low acyl (including deacylated) types are available on the market either in the clarified or non‐clarified forms.
The Panel noted that, according to the information from interested parties, the main impurity in gellan gum is PHB, the content of which in dried gellan gum was estimated to be from less than 1% up to 25%, depending on the degree of deacylation and clarification. According to US patent #5300429A by Baird and Cleary (1994), the content of PHB in dried gellan gum was estimated to be around 15–25%. One interested party (Documentation provided to EFSA No 10) informed that PHB levels in their products, measured using in‐house non‐validated (currently under validation) analytical method (no additional information on the method) are below 10% for high acylated gellan gum and below 1% for deacetylated gellan gum. Based on literature (Baird and Cleary, 1994; Bower et al., 2016), there are a few mutant strains called Sphingomonas elodea LPG‐2 (produced using classical mutagenesis) and Sphingomonas elodea PFG‐1 (produced by gene deletion technique) that can produce gellan gum with very little or no PHB. It is not clear whether these mutant strains are currently being used commercially for gellan gum production. The Panel further noted that a limit for PHB should be included in the EU specifications. The Panel noted that no proposals for the limits of PHB were provided by the interested parties.
In order to distinguish different types of gellan gum, one interested party (Documentation provided to EFSA No 8) provided the following specification proposal (Table 2).
Table 2.
Proposal for modification of the specifications (Documentation provided to EFSA No 8)
| Specifiations | Gellan variants | |||
|---|---|---|---|---|
| HA gellan | LA gellan (non‐clarified) | LA gellan (clarified) | Deacylated gellan (clarified) | |
| Degree of acylation | > 50% | ≤ 50% | ≤ 50% | < 1% |
| Total acyl content (wt%) | > 7.35% | ≤ 7.35% | ≤ 7.35% | < 0.15% |
| Residual solvent 2‐propanol* | ≤ 750 mg/kg | ≤ 750 mg/kg | ≤ 750 mg/kg | ≤ 750 mg/kg |
| Residual solvent ethanol* | < 0.9% | < 0.9% | < 0.9% | < 0.9% |
| Nitrogen | < 3% | < 3% | < 1% | < 1% |
| Loss of drying | ≤ 15% | ≤ 15% | ≤ 15% | ≤ 15% |
| Transmittance (0.5% solution) | NA | NA | ≥ 80% | ≥ 80% |
* ‘Separate limits should be set for 2‐propanol and ethanol. The interested party does ‘not support the setting of combined limits, typically referred to as “singly or in combination” as found frequently in European specifications (Regulation 231/2012 laying down specifications for food additives)’. The interested party ‘supports that separate limits be set for each solvent used. This does not even require more analytical efforts as these determinations can be done with the same gas chromatography system’.
The interested party provided a narrow range of analytical data for recently produced batches (the number of tested batches not indicated) demonstrating that purity of tested products complied with the EU specifications. Range levels of nitrogen content in gellan gum averages 0.065% N (Documentation provided to EFSA No 3). One interested party (Documentation provided to EFSA No 7) provided analytical results for three batches of ‘high acyl’ unclarified gellan gum demonstrating that the identity and purity of tested products complied with the EU specifications. Range levels for nitrogen content were 1.67–1.96%.
According to the industry, during the fermentation process, the bacteria produce enzymes (i.e. amylases, cellulases and protease). However, producers reduce enzyme activity as much as possible. The enzymes are deactivated throughout the manufacturing process, as residual enzyme activities would impact the performance of the product. According to internal non‐validated tests, there are no enzymatic activities in the final products (Documentation provided to EFSA No 3). No analytical data confirming the absence of enzymatic activities in the final products were provided to EFSA. The Panel noted that specifications for residual enzyme activity using validated methods should be included in the EU specifications.
The Panel noted that, according to the EU specifications for gellan gum (E 418) impurities of the toxic elements arsenic, cadmium, lead and mercury are accepted up to concentrations of 3, 1, 2 and 1 mg/kg, respectively. Contamination at such levels could have a significant impact on the exposure to these elements, for which the exposures already are close to the health‐based guidance values or benchmark doses (lower confidence limits) established by EFSA (EFSA CONTAM Panel, 2009a,b, 2010, 2012a,b,c, 2014).
In the period from the date of US Patent of Kang and Veeder (1982) to which the production of gellan gum originally refers until the present time no, reports have been identified that the strain ATCC 31461 was the cause of any human infection. The Panel recommended that the strain ATCC 31461 should be identified in the definition of the European Commission specifications as the only production microorganism for gellan gum (E 418) as a food additive.
Regarding the additional specifications for the fermentation organism, according to one interested party (Documentation provided to EFSA No 8; No 10), the manufacturing process always includes a pasteurisation step at high temperatures, plus treatment with alcohol and, in the deacylation process in addition exposure to a high pH values, that generally assures that microorganisms per se and the fermentation organism in particular, do not survive the process. They also reported that viable colonies of cells in the finished gellan powder could be detected visibly as characteristic yellow pigmented colonies on a standard plate count agar, but that they have never detected any viable cells in their products. Another interested party (Documentation provided to EFSA, No 7; No 9) provided analytical method for detection of viable cells in gellan gum products, but without any supporting analytical results. It also reported that they do not have a test method for non‐viable cells, but however, they measure total nitrogen according to the current EU and JECFA specifications.
The Panel noted that specifications for absence of viable cells of the gellan gum producing organism ATCC 31461 should be included in the EU specifications.
3.1.3. Manufacturing process of gellan gum as a food additive
According to the literature data (Giavasis et al., 2000; Bajaj et al., 2007; Sworn, 2009), as well as from the information provided by the industry (Documentation provided to EFSA No 8), the gellan gum manufacturing process can be divided into two main parts: the fermentation process and the downstream recovery processes of the gellan gum.
The fermentation process is carried out by the pure culture of the ATCC 31461 bacterial strain in the fermentation medium containing a carbon source (usually sugars), nitrogen sources and mineral salts under sterile conditions with strict control of aeration, agitation, temperature and pH.
After fermentation, the polysaccharide can be recovered by several ways. Precipitation by addition of alcohol yields the high acylated form, while treatment of the broth with alkali prior to alcohol precipitation leads to the formation of the low acylated form. Clarified gellan gum is produced by filtration of low acylated gellan gum to remove cell residues and other insoluble components.
Sworn et al. (2003) described a patented manufacturing process employing the treatment of the gum with a weak base for production of modified gellan gum in which the ratio of acetate to glycerate substituent groups is higher than 1 (Patent No.: US 6,602,996 B1), but it is not clear if this product is used as a food additive E 418.
The Panel noted that the clarification step is not performed for the production of the high acylated gellan gum.
3.1.4. Methods of analysis in food
No analytical methods for the quantification of gellan gum in foods were identified in the literature.
Instead, a method for the estimation in gels is described in EPA (2003). The method is based on heating and cooling of the gel in the presence of a dilute sequestrant solution (e.g. 0.1% w/v sodium hexametaphosphate). The gellan gum assay is based upon the presence of rhamnose which can be determined using the cysteine‐sulfuric acid procedure.
Different polysaccharides (xanthan gum, locust bean gum, guar gum, gum arabic, tragacanth, arabinogalactan, carrageenan, furcellaran, agar) were analysed quantitatively in dairy products (Glueck and Thier, 1980). The polysaccharides are extracted from foodstuff, and then fat, starch, milk proteins and carbohydrates are removed by extraction or degradation. The resulting polysaccharide fraction is analysed by gas chromatography after hydrolysis with trifluoroacetic acid, derivatisation of the resulting monosaccharides with hydroxylamine hydrochloride and acetic acid anhydride to form the aldonitrilacetate derivatives. The polysaccharides can be qualitatively identified by their characteristic monosaccharide pattern, and quantified via the single monosaccharide peaks. In the case of gellan gum glucose, rhamnose and glucuronic acid should be identified as hydrolysis products. For the polysaccharides, recoveries of 80–90% were obtained when adding 0.05% of the thickeners to skim milk or 1–2% to mixtures of ice cream or pudding constituents (Glueck and Thier, 1980). In a later investigation, the analytical procedure was improved by Preuss and Thier (1983). Changes in the separation of interfering substances (fats, proteins and starch) allowed the quantitative determination of polysaccharide gums in a variety of foods like blancmange powder, glaze, fruit ice, and cream cheese. Recoveries for most of the thickeners and gums are about 60–85% with a coefficient of variation of 2–8%.
For the qualitative test of gums in mayonnaise and French dressing, the AOAC Official Method 937.12 is reported by the Association of Official Agricultural Chemists (now AOAC International) (AOAC, 2002). The gums are precipitated from the food sample, hydrolyzed to monosaccharides which are qualitatively identified. This method is not applicable in presence of starch. A similar method (AOAC Official Method 935.61) for qualitative determination of gums in salad dressing based on a precipitation reaction is applicable in the presence of starch (AOAC, 2002). Both methods are applicable for the determination of any kinds of different gums used in foodstuff (i.e. it is not selective for gellan gum).
3.1.5. Stability of the substance, and reaction and fate in food
Information on reactions in food was not identified on the literature searches in Toxline, Medline and SciFinder. During the production process, a gellan gum solution is heated to 90–95°C for 10–15 min (Bajaj et al., 2007) which indicates its stability at elevated temperatures. The Panel noted that polysaccharides are known to hydrolyse under acid conditions and elevated temperature, but it is unlikely that this reaction occurs in food.
According to one interested party (Documentation provided to EFSA No 10), the stability of gellan gum in final food products cannot be determined by means of classic chemical analyses and they suggest using of physical/rheological tests such as determining ‘suspension power’ and ‘gel strength’ as approximations for stability tests. Provided results for 0.03% highly acylated gellan gum in chocolate milk (elastic modulus, 1 month at 37°C), 0.02% low acylated gellan gum in basil seed drinks (visual observation, 6 months at room temperature) and 0.20% water gels (gel strength, 3 months at room temperature) indicated excellent stability in tested food applications, as they almost fully retain their physical properties during storage. No data on partially deacetylated gellan gum were available from the industry.
3.2. Authorised uses and use levels
Maximum levels of gellan gum (E 418) have been defined in Annex II to Regulation (EC) No 1333/200816 on food additives, as amended. In this document, these levels are named MPLs.
Currently, gellan gum (E 418) is authorised as a Group I food additive in 67 food categories and has a specific authorised use in other 4 food categories (for a total of 71 food categories). Gellan gum (E 418) is an authorised food additive in the EU at quantum satis (QS) in almost all foods apart from FC 04.2.5.2 (jam, jellies and marmalades and sweetened chestnut purée as defined by Directive 2001/113/EC) and FC 04.2.5.3 (Other similar fruit or vegetable spreads) in which MPLs of 10,000 mg/kg are reported.
Table 3 summarises foods that are permitted to contain gellan gum (E 418) and the corresponding MPLs as set by Annex II to Regulation (EC) No 1333/2008.
Table 3.
MPLs of gellan gum (E 418) in foods according to the Annex II to Regulation (EC) No 1333/2008
| Food category number | Food categories name | E‐number | Restrictions/exception | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|---|
| 01.3 | Unflavoured fermented milk products, heat‐treated after fermentation | Group I | Quantum satis | |
| 01.4 | Flavoured fermented milk products including heat‐treated products | Group I | Quantum satis | |
| 01.6.3 | Other creams | Group I | Quantum satis | |
| 01.7.1 | Unripened cheese excluding products falling in category 16 | Group I | Except mozzarella | Quantum satis |
| 01.7.5 | Processed cheese | Group I | Quantum satis | |
| 01.7.6 | Cheese products (excluding products falling in category 16) | Group I | Quantum satis | |
| 01.8 | Dairy analogues, including beverage whiteners | Group I | Quantum satis | |
| 02.2.2 | Other fat and oil emulsions including spreads as defined by Council Regulation (EC) No 1234/2007 and liquid emulsions | Group I | Quantum satis | |
| 02.3 | Vegetable oil pan spray | Group I | Quantum satis | |
| 03 | Edible ices | Group I | Quantum satis | |
| 04.2.1 | Dried fruit and vegetables | Group I | Quantum satis | |
| 04.2.2 | Fruit and vegetables in vinegar, oil, or brine | Group I | Quantum satis | |
| 04.2.4.1 | Fruit and vegetables preparations excluding compote | Group I | Quantum satis | |
| 04.2.5.2a | Jam, jellies and marmalades and sweetened chestnut purée as defined by Directive 2001/113/EC | E 418 | 10,000 | |
| 04.2.5.3a | Other similar fruit or vegetable spreads | E 418 | 10,000 | |
| 04.2.5.4 | Nut butters and nut spreads | Group I | Quantum satis | |
| 04.2.6 | Processed potato products | Group I | Quantum satis | |
| 05.1 | Cocoa and Chocolate products as covered by Directive 2000/36/EC | Group I | Only energy‐reduced or with no added sugar | Quantum satis |
| 05.2b | Other confectionery including breath freshening microsweets | Group I | Quantum satis | |
| 05.3 | Chewing gum | Group I | Quantum satis | |
| 05.4 | Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4 | Group I | Quantum satis | |
| 06.2.2 | Starches | Group I | Quantum satis | |
| 06.3 | Breakfast cereals | Group I | Quantum satis | |
| 06.4.2 | Dry pasta | Group I | Only gluten free and/or pasta intended for hypoproteic diets in accordance with Directive 2009/39/EC | Quantum satis |
| 06.4.4 | Potato gnocchi | Group I | Except fresh refrigerated potato gnocchi | Quantum satis |
| 06.4.5 | Fillings of stuffed pasta (ravioli and similar) | Group I | Quantum satis | |
| 06.5 | Noodles | Group I | Quantum satis | |
| 06.6 | Batters | Group I | Quantum satis | |
| 06.7 | Pre‐cooked or processed cereals | Group I | Quantum satis | |
| 07.1 | Bread and rolls | Group I | Except products in 7.1.1 and 7.1.2 | Quantum satis |
| 07.2 | Fine bakery wares | Group I | Quantum satis | |
| 08.3.1 | Non‐heat‐treated meat products | Group I | Quantum satis | |
| 08.3.2 | Heat‐ treated meat products | Group I | Except foie gras, foie gras entier, blocs de foie gras, Libamáj, libamáj egészben, libamáj tömbben | Quantum satis |
| 08.3.3 | Casings and coatings and decorations for meat | Group I | Quantum satis | |
| 09.2 | Processed fish and fishery products including molluscs and crustaceans | Group I | Quantum satis | |
| 09.3 | Fish roe | Group I | Only processed fish roe | Quantum satis |
| 10.2 | Processed eggs and egg products | Group I | Quantum satis | |
| 11.2 | Other sugars and syrups | Group I | Quantum satis | |
| 11.4.1 | Table‐top sweeteners in liquid form | E 418 | Quantum satis | |
| 11.4.2 | Table‐top sweeteners in powder form | E 418 | Quantum satis | |
| 12.1.2 | Salt substitutes | Group I | Quantum satis | |
| 12.2.2 | Seasonings and condiments | Group I | Quantum satis | |
| 12.3 | Vinegars | Group I | Quantum satis | |
| 12.4 | Mustard | Group I | Quantum satis | |
| 12.5 | Soups and broths | Group I | Quantum satis | |
| 12.6 | Sauces | Group I | Quantum satis | |
| 12.7 | Salads and savoury‐based sandwich spreads | Group I | Quantum satis | |
| 12.8 | Yeast and yeast products | Group I | Quantum satis | |
| 12.9 | Protein products, excluding products covered in category 1.8 | Group I | Quantum satis | |
| 13.2 | Dietary foods for special medical purposes defined in Directive 1999/21/EC (excluding products from food category 13.1.5) | Group I | Quantum satis | |
| 13.3 | Dietary foods for weight control diets intended to replace total daily food intake or an individual meal (the whole or part of the total daily diet) | Group I | Quantum satis | |
| 13.4 | Foods suitable for people intolerant to gluten as defined by Regulation (EC) No 41/2009 | Group I | Including dry pasta | Quantum satis |
| 14.1.2 | Fruit juices as defined by Directive 2001/112/EC and vegetable juices | Group I | Only vegetable juices | Quantum satis |
| 14.1.3 | Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products | Group I | Only vegetable nectars | Quantum satis |
| 14.1.4 | Flavoured drinks | Group I | Quantum satis | |
| 14.1.5.2 | Other | Group I | Excluding unflavoured leaf tea; including flavoured instant coffee | Quantum satis |
| 14.2.3 | Cider and perry | Group I | Quantum satis | |
| 14.2.4 | Fruit wine and made wine | Group I | Quantum satis | |
| 14.2.5 | Mead | Group I | Quantum satis | |
| 14.2.6 | Spirit drinks as defined in Regulation (EC) No 110/2008 | Group I | Except whisky or whiskey | Quantum satis |
| 14.2.7.1 | Aromatised wines | Group I | Quantum satis | |
| 14.2.7.2 | Aromatised wine‐based drinks | Group I | Quantum satis | |
| 14.2.7.3 | Aromatised wine‐product cocktails | Group I | Quantum satis | |
| 14.2.8 | Other alcoholic drinks including mixtures of alcoholic drinks with non‐alcoholic drinks and spirits with less than 15% of alcohol | Group I | Quantum satis | |
| 15.1 | Potato‐, cereal‐, flour‐ or starch‐based snacks | Group I | Quantum satis | |
| 15.2 | Processed nuts | Group I | Quantum satis | |
| 16 | Desserts excluding products covered in category 1, 3 and 4 | Group I | Quantum satis | |
| 17.1c | Food supplements supplied in a solid form including capsules and tablets and similar forms, excluding chewable forms | Group I | Quantum satis | |
| 17.2c | Food supplements supplied in a liquid form | Group I | Quantum satis | |
| 17.3c | Food supplements supplied in a syrup‐type or chewable form | Group I | Quantum satis | |
| 18 | Processed foods not covered by categories 1 to 17, excluding foods for infants and young children | Group I | Quantum satis |
MPL: maximum permitted level.
Maximum individually or in combination with E 400–404, E 406, E 407, E 410, E 412, E 415 and E 418.
The substances listed under numbers E 400, E 401, E 402, E 403, E 404, E 406, E 407, 407a, E 410, E 412, E 413, E 414, E 415, E 417, E 418, E 425 and E 440 may not be used in jelly mini‐cups, defined, for the purpose of this Regulation, as jelly confectionery of a firm consistence, contained in semi rigid mini‐cups or mini‐capsules, intended to be ingested in a single bite by exerting pressure on the mini‐cups or mini‐capsule to project the confectionery into the mouth.
FCS 17 refers to food supplements as defined in Directive 2002/46/EC of the European Parliament and of the Council excluding food supplements for infants and young children.
According to Annex III, Part 3 of Regulation (EC) No 1333/2008, gellan gum (E 418) is also authorised as a carrier in food enzymes with a maximum level in the products (beverages or not) at QS.
According to Annex III, Parts 2 and 4, gellan gum (E 418) is authorised as a food additive having a function other than a carrier in food additives (Annex III, Part 2) and in flavourings (Annex III, Part 4) at QS in both cases.
Moreover, according to Annex III, Part 5, Section A of Regulation (EC) No 1333/2008, gellan gum (E 418) is also authorised as a food additive at QS in all nutrients. Gellan gum (E 418) is not authorised to be used in nutrients intended for food for infants and young children.
3.3. Exposure data
3.3.1. Reported use levels or data on analytical levels of gellan gum (E 418)
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment, especially for those food additives for which no MPL is set and which are authorised according to QS.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued a public call17 for occurrence data (usage level and/or concentration data) on gellan gum (E 418). In response to this public call, updated information on the actual use levels of gellan gum (E 418) in foods was made available to EFSA by industry. No analytical data on the concentration of gellan gum (E 418) in foods were made available by the Member States.
Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with data on use levels (n = 205) of gellan gum (E 418) in foods for 67 out of the 71 food categories in which gellan gum (E 418) is authorised. Out of those 67 food categories, for three food categories (02.3 Vegetable oil pan spray; 04.2.5.4 Nut butters and nut spreads; 12.1.2 Salt substitutes), data providers reported usage levels of zero and this information seemed to be supported by the Mintel's GNPD.
Updated information on the actual use levels of gellan gum (E 418) in foods was made available to EFSA by Biopolymer International (BIOPOLYMER), EuroGum A/S (DK_EUROGUM), Food Drink Europe (FDE), the International Chewing Gum Association (ICGA) and Specialised Nutrition Europe (SNE).
The Panel noted that 31 use levels in FC 13.2 and one in FC 14.1.4 referred to niche products. The use levels available for FCs 13.2 were not considered for the exposure assessment (see Section 3.3.3); the use level reported for one niche product in FC 14.1.4 was excluded for the refined scenario analysis since other use levels were available for this food category.
The Panel noted that some data providers (namely BIOPOLYMER and Eurogums A/S) are not food industries using gums in food products but food additive producers. Use levels reported by food additive producers are not considered at the same level as those provided by food industry. Food additive producers might recommend use levels to the food industry but the final levels might, ultimately, be different. Therefore, unless food additive producers confirm that the recommended levels are used by food industry, they are not considered in the refined exposure scenario. Data from food additive producers will only be used in the maximum level exposure assessment scenario in case of QS authorisation when no data are available from food industry. In this way, the most complete exposure estimates are calculated.
Appendix A provides data on the use levels of gellan gum (E 418) in foods as reported by industry and food additive producers.
3.3.2. Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information of over 2.5 million food and beverage products of which more than 1,000,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 20 out of its 28 member countries and Norway presented in the Mintel GNPD.18
For the purpose of this Scientific Opinion, the Mintel's GNPD19 was used for checking the labelling of food and beverages products and food supplements for gellan gum (E 418) within the EU's food market as the database contains the compulsory ingredient information on the label.
Between January 2013 and January 2018, a total of 1,194 products containing gellan gum (E 418) were found to be published on Mintel's GNPD.
Appendix B lists the percentage of the food products labelled with gellan gum (E 418) out of the total number of food products per food subcategories according to the Mintel's GNPD food classification. Gellan gum (E 418) was listed in a total of 51 subcategories; the top subcategories for number of products were: Plant Based Drinks (Dairy Alternatives) (n = 457); Fruit/Flavoured Still Drinks (n = 103); Nectars (n = 83); Pastilles, Gums, Jellies & Chews (n = 79); Meal Replacements & Other Drinks (n = 72); Chilled Desserts (n = 66); Shelf‐Stable Desserts (n = 64); Flavoured Milk (n = 37).
The percentages of products labelled with gellan gum (E 418) per single Mintel's GNPD food subcategory, ranged from less than 0.1% (in a range of categories) to 21.9% (in subcategory ‘Plant Based Drinks (Dairy Alternatives)’). The average percentage of foods labelled to contain gellan gum (E 418) was 0.6%.
According to Mintel's GNPD, gellan gum (E 418) was listed as ingredient in Mintel's GNPD subcategory ‘growing‐up milk’ in 7 products (soya drinks products recommended for infants 1–3 years old) and in 5 products under subcategory ‘Baby Juices & Drinks’. For those products Mintel's GNPD reported the claim ‘Babies & Toddlers (0‐4)’, this making the products to fall under FC 13.1 (Foods for infants and young children) for which gellan gum is not authorised. Moreover, 3 fruit juices products under subcategory ‘Juices’ were labelled with gellan gum on Mintel's GNPD, and they would then not be authorised under FC 14.1.2 Fruit juices as defined by Directive 2001/112/EC and vegetable juices according to the restriction ‘only vegetable juices’.
3.3.3. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). Consumption surveys added in the Comprehensive database in 2015 were also taken into account in this assessment.13
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons should be interpreted with caution. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database includes the currently best available food consumption data across Europe.
Food consumption data from the following population groups were used for the exposure assessment: infants, toddlers, children, adolescents, adults and the elderly. For the present assessment, food consumption data were available from 33 different dietary surveys carried out in 19 European countries (Table 4).
Table 4.
Population groups considered for the exposure estimates of gellan gum (E 418)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Denmark, Finland, Germany, Italy, UK |
| Toddlersa | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Denmark, Finland, Germany, Italy, Netherlands, Spain, UK |
| Childrenb | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Netherlands, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Romania, Spain, Sweden, UK |
| The elderlyb | From 65 years of age and older | Austria, Belgium, Denmark, Finland, France, Germany, Hungary, Ireland, Italy, Romania, Netherlands, Sweden, UK |
‘Toddlers’ in the EFSA Comprehensive Database corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013.
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure estimates. In practice, the FoodEx food codes were matched to the FCS food categories.
Food categories considered for the exposure assessment of gellan gum (E 418)
The food categories for which use levels of gellan gum (E 418) were provided, were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
Some food categories for which use levels were submitted are not referenced in the EFSA Comprehensive Database and could therefore not be taken into account in the present estimate. This was the case for seven food categories (Appendix C) and it may have resulted in an underestimation of the exposure. The food categories which were not taken into account are listed below:
01.7.6 Cheese products (excluding products falling in category 16).
06.6 Batters.
08.3.3 Casings and coatings and decorations for meat.
14.2.4 Fruit wine and made wine.
14.2.7.2 Aromatised wine‐based drinks.
14.2.7.3 Aromatised wine‐product cocktails.
No foods are referenced in the EFSA Comprehensive Database for the six food categories above which were therefore not taken into account.
FC 14.1.3 Fruit nectars as defined by Directive 2001/112/EC and vegetable nectars and similar products, restricted to only vegetable nectars. No vegetable nectars are available in the FoodEx nomenclature. In order not to overestimate the exposure coming from only vegetable nectars by taking into account all nectars, and considering that vegetables juices were taken into account within food category of juices (FC 14.1.2), in which vegetable nectars may have been reported within the consumption surveys, the entire food category was not taken into account.
On the other hand, for the following food categories, the restrictions/exceptions which apply to the use of gellan gum (E 418) could not be taken into account and therefore the whole food category was considered in the exposure assessment. This applies to five food categories (Appendix C) and may have resulted in an overestimation of the exposure:
5.1 Cocoa and Chocolate products as covered by Directive 2000/36/EC, restriction to ‘only energy‐reduced or with no added sugar’. The restriction could not be taken into account and the whole food category was included in the exposure assessment in order not to underestimate exposure from this food category.
06.4.4 Potato gnocchi except ‘fresh refrigerated potato gnocchi’. The full food category was taken into account because the exception refers to a small part of the whole food category.
07.1 Bread and rolls ‘except products in 7.1.1 and 7.1.2’. The exception should refer to a small part of the whole food category and thus the full food category was taken into account.
08.3.2 Heat‐treated meat products ‘except foie gras, foie gras entier, blocs de foie gras, Libamáj, libamáj egészben, libamáj tömbben’. The full food category was taken into account because the exception refers to a small part of the whole food category.
09.3 Fish roe ‘only processed fish roe’. The restriction could not be taken into account because not referenced in the Food Consumption Database. As the exception should refer to a small part of the food category, the whole food category was included in the exposure assessment.
The FCs 17.1/17.2/17.3 Food supplements, in solid, liquid, syrup‐type or chewable form, the form cannot be differentiated and the same use level was applied to the whole FC 17.
According to information from the Mintel's GNPD database, gellan gum (E 418) is not used in carbonated soft drinks. As the consumption of carbonated soft drinks can be very high, soft drinks codified as ‘cola’ were excluded from FC 14.1.4 Flavoured drinks to avoid unduly overestimation of the exposure. Despite this, taking into account flavoured drinks other than colas might result in an overestimation of the contribution to the exposure of this category because the subcategory labelled as Fruit/Flavoured Still Drinks does not necessarily correspond to all flavoured drinks other than colas.
Gellan gum (E 418) is also allowed in FCs 13.2, 13.3 and 13.4. Food items under FCs 13.2, 13.3 and 13.4 consumed by population groups‐children, adolescents, adults and the elderly may be very diverse and, in addition, there is very limited information on their consumption. Therefore, eating occasions belonging to the FCs 13.2, 13.3 and 13.4 were reclassified under food categories in accordance to their main component and included as such in the exposure assessment. The use levels available for FCs 13.2, 13.3 and 13.4 were not considered for the exposure assessment.
Considering that FC 18 (Processed foods not covered by categories 1 to 17, excluding foods for infants and young children) is extremely unspecific, the foods belonging to this food category in the EFSA Comprehensive Database (e.g. processed foods, prepared or composite dishes) were reclassified under food categories in accordance to their main component and included as such in the exposure assessment. The use levels available for FC 18 were not considered for the exposure assessment.
For the remaining food categories, the refinements considering the restrictions/exceptions as set in Annex II to Regulation No 1333/2008 were applied.
Overall, for the maximum level exposure scenario, 53 food categories were included, while for the refined scenarios (brand loyal and non‐brand loyal) only 7 food categories were included in the present exposure assessment to gellan gum (E 418) (Appendix C). Compared to the refined scenario, three additional food categories were considered (FC 17.1, 17.2 and 17.3) in the ‘food supplement consumers‐only’ scenario (Appendix C).
3.4. Exposure estimate
3.4.1. Exposure to gellan gum (E 418) from its use as a food additive
The Panel estimated the chronic dietary exposure to gellan gum (E 418) for the following population groups: infants, toddlers, children, adolescents, adults and the elderly. Dietary exposure to gellan gum (E 418) was calculated by multiplying concentrations of gellan gum (E 418) per food category (Appendix C) with their respective consumption amount per kilogram body weight for each individual in the Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only one day per subject were excluded as they are considered as not adequate to assess repeated exposure.
This was carried out for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 4). On the basis of these distributions, the mean and 95th percentile of exposure were calculated per survey and per population group. The 95th percentile of exposure was only calculated for those population groups with a sufficiently large sample size (EFSA, 2011a). Therefore, in the present assessment, the 95th percentile of exposure for infants from Italy and for toddlers from Belgium, Italy and Spain were not estimated.
Exposure assessment to gellan gum (E 418) was carried out by the ANS Panel based on two different sets of concentration data: (1) maximum levels of data provided to EFSA for food categories in which gellan gum (E 418) is authorised at QS and MPLs for the 2 food categories with numerical maximum levels (defined as the maximum level exposure assessment scenario); (2) reported use levels (defined as the refined exposure assessment scenario). These two scenarios are discussed in detail below.
These scenarios do not consider the consumption of food supplements. This exposure source was covered in one additional scenario detailed below (food supplements consumers only scenario).
A possible additional exposure from the use of gellan gum (E 418) as a food additive in food enzymes, additives, flavourings and nutrients in accordance with Annex III to Regulation (EC) No 1333/2008 (Part 2, 3, 4 and 5A) was not considered in any of the exposure assessment scenarios.
Maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario is based on the MPLs as set in Annex II to Regulation (EC) No 1333/2008 and listed in Table 3. As gellan gum (E 418) is authorised according to QS in almost all food categories, a ‘maximum level exposure assessment’ scenario was estimated based on the maximum reported use levels provided by industry (food industry and food additive producers), excluding exposure via food supplements, as described in the EFSA Conceptual framework (EFSA ANS Panel, 2014). The levels used in this exposure scenario are listed in Appendix C.
The Panel considers the exposure estimates derived following this scenario as the most conservative since it is assumed that that the population will be exposed to the food additive present in food at the MPL/maximum reported use levels over a longer period of time.
Refined exposure assessment scenario
The refined exposure assessment scenario is based on use levels reported by food industry. This exposure scenario can consider only food categories for which these data were available to the Panel.
Appendix C summarises the concentration levels of gellan gum (E 418) used in the refined exposure assessment scenario. Based on the available data set, the Panel calculated two refined exposure estimates based on two model populations:
-
The brand‐loyal consumer scenario: it was assumed that a consumer is exposed long‐term to gellan gum (E 418) present at the maximum reported use for one food category. This exposure estimate is calculated as follows:
-
–
Combining food consumption with the maximum of the reported use levels for the main contributing food category at the individual level.
-
–
Using the mean of the typical reported use levels for the remaining food categories.
-
–
The non‐brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to gellan gum (E 418) present at the mean reported use levels in food. This exposure estimate is calculated using the mean of the typical reported use levels for all food categories.
Appendix C summarised the concentration levels of gellan gum (E 418) used in the refined exposure scenarios.
‘Food supplement consumers only’ exposure assessment scenario
Gellan gum (E 418) is authorised in FC 17 (Food supplements as defined in Directive 2002/46/EC excluding food supplements for infants and young children). As exposure via food supplements may deviate largely from the one via food, and that the number of food supplement consumers may be low depending on populations and surveys, an additional scenario was calculated in order to reflect additional exposure to gellan gum (E 418) from food supplements compared to exposure to gellan gum (E 418) excluding these sources. This scenario was estimated as follow:
Consumers only of food supplements will be assumed to be exposed to gellan gum (E 418) present at the maximum reported use level on a daily basis via consumption of food supplements.
For the remaining food categories (7/71 categories), the mean of the typical reported use levels is used.
As FC 17 does not consider food supplements for infants and toddlers as defined in the legislation, exposure to gellan gum (E 418) from food supplements are not estimated for these two population groups.
This scenario included 10 food categories (Appendix C).
Dietary exposure to gellan gum (E 418)
Table 5 summarises the estimated exposure to gellan gum (E 418) from its use as a food additive in six population groups (Table 4) according to the different exposure scenarios. Detailed results per population group and survey are presented in Appendix D.
From the maximum level exposure assessment scenario, mean exposure to gellan gum (E 418) from its use as a food additive ranged from 16 mg/kg bw per day in infants and the elderly to 231 mg/kg bw per day in toddlers. The 95th percentile of exposure ranged from 35 mg/kg bw per day in the elderly to 690 mg/kg bw per day in infants.
From the refined estimated exposure scenario, brand‐loyal scenario, mean exposure to gellan gum (E 418) from its use as a food additive ranged from less than 0.1 mg/kg bw per day in infants to 21 mg/kg bw per day in children. The high exposure to gellan gum (E 418) ranged from 0.4 mg/kg bw per day infants to 72.4 mg/kg bw per day in toddlers. In the non‐brand‐loyal scenario, mean exposure to gellan gum (E 418) from its use as a food additive ranged from < 0.1 mg/kg bw per day in infants, toddlers, adults and the elderly up to 4.6 mg/kg bw per day in children. The 95th percentile of exposure to gellan gum (E 418) ranged from 0.1 mg/kg bw per day in infants and the elderly up to 14.7 mg/kg bw per day in toddlers.
For the food supplements consumers only, mean exposure to gellan gum (E 418) from its use as a food additive ranged between 0.4 mg/kg bw per day to 8.3 mg/kg bw per day in children. The 95th percentile of exposure to gellan gum (E 418) ranged between 1.9 mg/kg bw per day in the elderly and 11.7 mg/kg bw per day in children.
Main food categories contributing to exposure to gellan gum using the maximum level exposure assessment scenario
From the maximum level exposure assessment scenario, the main contributing food categories to the total mean exposure estimates for infants, toddlers and children were unflavoured fermented milk products, meat products and flavoured fermented milk products including heat‐treated products. For adolescent and adults the main contributor was meat products followed by flavoured fermented milk products including heat‐treated products and unflavoured fermented milk products, including natural unflavoured buttermilk (excluding sterilised buttermilk) non‐heat‐treated after fermentation; for the elderly, the main contributing food categories were meat products, unflavoured fermented milk products, including natural unflavoured buttermilk (excluding sterilised buttermilk) non‐heat‐treated after fermentation and bread and rolls (more details in Appendix E).
Main food categories contributing to exposure to gellan gum using the refined exposure assessment scenario
Flavoured drinks and Sauces were the most contributing food categories for all population groups under the brand‐loyal and the non‐brand‐loyal scenarios (more details in Appendix E).
Uncertainty analysis
Uncertainties in the exposure assessment of gellan gum (E 418) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 6.
Table 6.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/– |
| Use of data from food consumption surveys covering only a few days to estimate high percentiles (95th) long‐term (chronic) exposure | + |
| Correspondence of reported use levels to the food items in the EFSA Comprehensive Food Consumption Database: uncertainties to which types of food the levels refer to | +/– |
| Uncertainty in possible national differences in use levels of food categories | +/– |
|
Concentration data:
|
+ +/– |
| Food categories selected for the exposure assessment: exclusion of food categories due to missing FoodEx linkage (n = 11/67) | – |
| Food categories selected for the exposure assessment: inclusion of food categories without considering the restriction/exception (n = 5 out of 53 in maximum scenario; none out of 7 food categories in the refined scenarios) | + |
| Food categories included in the exposure assessment: no data for certain food categories which were therefore not considered in the exposure estimates (n=18/71 for maximum scenario or 64/71 for refined scenarios) | – |
|
Maximum level exposure assessment scenario:
|
+ – |
|
Refined exposure assessment scenarios:
|
+/– – |
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
Gellan gum (E 418) is authorised as a Group I food additive in 67 food categories and has a specific authorised use in 4 other food categories for a total of 71 food categories (Table 3). Since, the majority of food categories correspond to the general Group I food additives authorisation, the food additive may not necessarily be used in some of these food categories. This may explain why reported use levels were provided by food industry only for 8 food categories of which one category could not be included in the assessment as no consumption data are available in the EFSA Comprehensive Database. The Panel calculated that out of the foods authorised to contain gellan gum (E 418) according to Annex II to Regulation (EC) No 1333/2008, 4.4–55.1% of the amount of food consumed (by weight) per population group was reported to potentially contain gellan gum (E 418) as a food additive.
Based on this, the Panel noted that the information from the Mintel GNPD supported the observation that due to its Group I authorisation, the food additive may not be used in all food categories in which it is authorised among which FCs 01.7.6 Cheese products (excluding products falling in category 16), 01.8 Dairy analogues, including beverage whiteners, 04.2.4.1 Fruit and vegetable preparations excluding compote, 04.2.5.2 Jam, jellies and marmalades and sweetened chestnut puree as defined by Directive 2001/113/EC, 04.2.5.3 Other similar fruit or vegetable spreads, 04.2.6 Processed potato products, 06.3 Breakfast cereals, 06.4.2 Dry pasta, 06.4.5 Fillings of stuffed pasta (ravioli and similar), 06.5 Noodles, 06.7 Pre‐cooked or processed cereals (full list available in Appendix B).
Suitable data available for the refined exposure assessment scenario were provided by food industry for the following food categories: 04.2.4.1 Fruit and vegetable preparations excluding compote; 05.2 Other confectionery including breath freshening microsweets; 05.3 Chewing gum; 05.4 Decorations, coatings and fillings, except fruit‐based fillings covered by category 4.2.4; 12.6 Sauces; 12.9 Protein products, excluding products covered in category 1.8; 14.1.4 Flavoured drinks.
On the other side, for some categories for which the Mintel GNPD supported the presence of gellan gum (E 418), data were missing (including for instance FC 01.3 Unflavoured fermented milk products, heat‐treated after fermentation, FC 01.4 Flavoured fermented milk products including heat‐treated products and 7.2. Fine bakery wares) and this might have resulted in an underestimation of the exposure; the full list is available in Appendix B.
Furthermore, the Panel noted that information from the Mintel GNPD (Appendix B) indicated that 41 out of 51 food subcategories, categorised according to the Mintel GNPD nomenclature, in which gellan gum (E 418) was labelled were included in the maximum level exposure scenario; these 41 food subcategories represented approximately 89% of the food products labelled with gellan gum (E 418) in the database. For the refined scenarios, only 16 Mintel GNPD food subcategories were included, these 16 food subcategories representing approximately 67% of the food products labelled with gellan gum (E 418) in the database.
A total of 15 food products for which the use of gellan gum (E 418) is not authorised according to the EU legislation were found to be labelled in the Mintel's GNPD to contain the additive (in subcategories ‘growing‐up milk’, ‘Baby Juices & Drinks’ and ‘Juices’).
According to the Mintel's GNPD database, gellan gum (E 418) is not used in carbonated soft drinks but it is used in Fruit/Flavoured Still Drinks. Consumption of soft drinks codified as ‘cola’ were excluded from consumption within FC 14.1.4 Flavoured drinks in order to avoid unduly overestimation of the exposure. Despite exclusion of colas, still a likely overestimation of the contribution of FC 14.1.4 Flavoured to the exposure is present.
Furthermore, the percentage of foods per subcategory labelled to contain gellan gum (E 418) was maximally about 21% (Appendix B), while in the assessment it was assumed that 100% of the foods belonging to an authorised food category contained the additive.
Given these observations, the Panel considered overall that the uncertainties identified would, in general, result in an overestimation of the exposure to gellan gum (E 418) as a food additive in European countries considered in the EFSA Comprehensive database for the maximum level exposure scenario. Based on the assumption that the food additive is not used in those food categories in which it is permitted but for which no usage data were provided, also the refined scenario would in general result in an overestimation of exposure.
The Panel noted that food categories which may contain gellan gum (E 418) due to carry‐over (Annex III, Part 2, 3, 4, 5) were not considered in the current exposure assessment.
3.4.2. Exposure via other sources
Exposure to gellan gum due to the following uses was not considered in this opinion.
Pharmaceutical use
From data provided by the European Medicines Agency (EMA) information about the current medicinal usage of gellan gum and the usage as excipient in medicinal products was retrieved (Letter from EMA to EFSA, personal communication, May 2015).
Gellan gum is used as an excipient in oral drug delivery systems, e.g. as a matrix‐forming agent for sustained release tablets, as a disintegrating agent in immediate release tablets, to form beads and capsules and for ophthalmic and nasal formulations (Osmałek et al., 2014; Zia et al., 2017). Gellan gum is used as an excipient in medicinal products authorised in centralised procedures as seen from the answer of EMA.
For gellan gum as an active ingredient, no authorised medicinal products exist within the EU.
3.5. Biological and Toxicological data
The biological and toxicological testing has been performed with gellan gum preparations of different purities, with a deacylated gellan gum or with a gellan gum of unknown degree of acylation. However, the Panel considered that the structural similarities of the different types of gellan gum allowed for read across. In the studies summarised below, no information on the content of the impurity PHB was available.
3.5.1. Absorption, distribution, metabolism and excretion
In vitro studies
Adiotomre et al. (1990) investigated the effects of dietary fibres, including gellan gum (Kelco mc, San Diego, MW 0.5‐1 × 106), on caeacal fermentations by using fresh human microbiota. In the gellan gum used, one of the glucose residues in the tetrasaccharide repeat unit carries acetic ester groups at C6 and a glyceric ester group at C2. Evolution of short‐chain fatty acids (SCFAs) and water‐holding capacity after fermentation were also measured. Among other gums, gellan gum yielded a twice amount of total SCFAs (37.2 vs 15.5 mmol/L for controls). The major SCFAs produced were acetic and propionic acids, with smaller amounts of butyric, isobutyric, valeric and isovaleric acids. By contrast, the amount of water held by 1 g of the fermented residue was high in case of gellan gum (3.08 vs 0.91 g/g for controls).
In vivo studies
The absorption, distribution and excretion of gellan gum (MW, level of deacylation and impurities not specified) is described in a study provided to EFSA (Documentation provided to EFSA No 30) (report only available in part). In this study, nine male and female Sprague–Dawley rats received a single dose of 848–980 mg/kg bw of 3H‐ and 14C‐labelled gellan gum (not further specified) by gavage in a corn oil suspension. The respiratory 14C levels over 24 h were measured in one male and one female rat treated once with 950 and 980 mg/kg bw radiolabelled gellan gum. Only 0.5% of the applied 14C was expired as 14CO2. The study of faecal and urinary excretions as well as on blood levels were conducted in cages without air filtering. Urinary and faecal excretions as well as tissue distribution were assessed in four female and four male animals over 7 days. The administered 3H was completely excreted via faeces (98–101% of the administered dose) and urine (4–5%), organ and tissue samples as well as the remaining carcass had 3H levels close to background signal. The excretion and distribution of 14C gave a similar pattern as most of the radioactivity was found in faeces (85–87 % of the administered dose) followed by urine (2–3%), whereas blood, analysed single tissues and the remaining carcass contained only minor amounts (3–4%). In an additional experiment, blood was sampled over 7 days in four males and four females receiving radiolabelled gellan gum. The peak concentration of 14C of about 0.4 % of the applied dose appeared about 5 h after administration. According to the authors, the majority of orally ingested gellan gum was excreted via faeces.
Edwards and Eastwood (1995) investigated the caecal and faecal short‐chain fatty acids and stool output in rats fed on diets containing non‐starch polysaccharides, including gellan gum (Kelco Inc., MW 0.5–1 × 106, one of the glucose residues in the tetrasaccharide repeat unit carries acetic ester groups at C6 and a glyceric ester group at C2, impurities not specified). The basal diet of male Wistar rats (n = 7) was supplemented or not with 50 g/kg of gellan gum for 28 days. Faeces were then collected over 2 days and caecal contents obtained post‐mortem. Caecal and faecal wet and dry weights and SCFA were measured. Gellan gum increased caecal pH, faecal water and did not influence total caecal and faecal SCFAs. However, in faeces, gellan gum increased molar proportions of acetic and butyric acids and decrease that of propionic acid. The faecal release of acetate and butyrate could also be the consequence of the hydrolysis of the acetyl side chain of gellan gum and the possible presence of PHB, respectively.
Shimizu et al. (1999) compared the physiological functions of rats fed diets containing 5% of either gellan gum (San‐eigen, MW 600–700 KDa, level of deacylation and impurities not specified) or curdlan or cellulose powder (considered as the control group) for 4 week. Therefore, their effects on the lipid concentrations of serum and liver, faecal bile acid composition and caecal fermentation products were investigated in rats fed cholesterol‐free diets. The gastrointestinal transit time in the group fed gellan gum was significantly shorter than that of the other groups. No significant differences were observed in the serum concentrations of total cholesterol and high‐density lipoprotein (HDL) cholesterol. Amounts of SCFAs (acetic, propionic and butyric acid) and lactic acid in the caecal contents were similar in gellan gum and control groups. These results indicated that gellan gum shortened the gastrointestinal transit time and would not be degraded and fermented by intestinal bacteria in the caecum.
Human study
Five female and five male volunteers were given gellan gum (MW, level of deacylation and impurities not specified) corresponding to 175 mg/kg bw for 7 days, followed by 200 mg gellan gum/kg bw for a further 16 days (Documentation provided to EFSA No 17). Measurements before and at the end of the 23‐day test period showed that the gellan gum acted as a faecal bulking agent for the male volunteers and for four of the females. In two females and two males, the dietary transit time increased but it decreased for three females and three males. Among several investigated parameters (plasma biochemistry, haematological indices, urinalysis parameters, blood glucose and plasma insulin concentrations), gellan gum ingestion had no significant effect on breath hydrogen and methane concentrations. According to the authors, the data indicated that the ingestion of gellan gum at a high level for 23 days caused no adverse dietary or physiological effects in any of the volunteers. In particular, the enzymatic and other indicators of adverse toxicological effects remained unchanged. The Panel noted that the unchanged breath hydrogen and methane concentrations would be related to the absence of significant fermentation of gellan gum in the large intestine of the volunteers.
Overall, the in vivo metabolic and physiological studies of gellan gum in rats indicated that this compound would not be absorbed intact. There is indication of limited increased production of SCFA in the rats. The faecal release of acetate and butyrate could also be the consequence of the hydrolysis of the acetyl side chain of gellan gum and the possible presence of PHB, respectively. In humans, gellan gum would not be absorbed intact and there is no indication of significant fermentation by the intestinal microbiota.
3.5.2. Acute toxicity
Acute oral toxicity of gellan gum in Sprague–Dawley rats was reported to be above 5,000 mg/kg bw (Documentation provided to EFSA No 32). The Panel noted that gellan gum was of low acute toxicity.
3.5.3. Short‐term and subchronic toxicity
Rats
In a 13‐week oral study, 20 Sprague–Dawley rats (CD‐Crl: (SD) BR) per sex and group were fed with diet containing 0%, 3%, 4.5% or 6% gellan gum (MW, level of deacylation and impurities not specified) (Documentation provided to EFSA No 21). These doses were equal to 3,480, 5,230 and 6,990 mg/kg bw per day for males and 3,660, 5,460 and 7,260 mg/kg bw per day for females in the first week of treatment. Over the course of the study doses declined to 1,440, 2,140 and 2,950 mg/kg bw per day in males and 1,850, 2,790 and 3,760 mg/kg bw per day in females in the last week of the study. Time weighted average doses were not reported and original data were not available. Clinical observations, body weight, food intake, food efficiency, haematological, clinical chemistry and urinalysis parameters, ophthalmological examinations, gross and microscopic examinations, organ weights did not demonstrate differences among the groups. The faecal moisture content was statistically significantly increased as compared to the controls only in males from the mid‐dose group in week 6 and 12 and from the high‐dose group in week 12. This transient increase in faecal moisture was considered by the authors as of no toxicological significance. A treatment‐related slight irritation of the stomach mucosa (reddening) was observed at necropsy, but this was not verified histologically and therefore it was not considered as adverse. From week 6 to week 7 of the study, the majority of animals had a sialodacryoadenitis viral infection but recovered without any persistent signs of infection. The no‐observed‐adverse‐effect‐level (NOAEL) suggested by the authors was 6% gellan gum in the diet, the highest concentration tested (equal to 2,950 mg/kg bw per day for males and 3,760 mg/kg bw per day for females). The Panel agreed with this conclusion.
Dogs
Five Beagle dogs per sex and group were fed diets containing 0%, 3%, 4.5% and 6% gellan gum (MW, level of deacylation and impurities not specified) over 52 weeks. The average doses were reported as 950, 1,460 and 1,870 mg/kg bw per day for males and 940, 1,490 and 2,070 mg/kg bw per day for females in the low‐, mid‐ and high‐dose groups, respectively. Twice daily clinical observations, weekly records on body weight, ophthalmoscopic, haematologic and biochemical analysis as well as necropsy, selected organ weights and histopathology did not reveal any treatment‐related changes (Documentation provided to EFSA No 19). Only food intake was increased in the treated groups. The Panel considered the NOAEL of this study to be 6% of gellan gum in the diet (equal to 1,870 mg/kg bw per day in males and 2,070 mg/kg bw per day in females), the highest dose tested.
Monkeys
A 28‐day oral study with rhesus monkeys (2 per sex and group) dosed with 1,000, 2,000 or 3,000 mg gellan gum (MW, level of deacylation and impurities not specified)/kg bw per day by gavage did not show any substance‐related toxicity. Animals were observed for clinical signs, haematology and biochemistry (Documentation provided to EFSA No 31). The NOAEL of this study was 3,000 mg/kg bw per day, the highest dose tested. The Panel noted the limitations of the study because no organ weights and histopathological assessments were recorded as animals were not sacrificed.
Overall, the Panel noted that subchronic toxicity studies with gellan gum conducted in rats and dogs did not reveal adverse effects at the highest doses tested (equal to 2,950 mg/kg bw per day for males and 3,760 mg/kg bw per day for females in rats and 1,870 mg/kg bw per day for males and 2,070 mg/kg bw per day for females in dogs). In a short‐term study in rhesus monkeys, no adverse effects were seen at 3, 000 gellan gum mg/kg bw per day, the highest dose tested.
3.5.4. Genotoxicity
In vitro
Gellan gum was tested for induction of gene mutation in Salmonella Typhimurium strains TA1535, TA1537, TA1538, TA98 and TA100, both in the absence and presence of S9 metabolic activation at dose‐levels of 10, 30, 100, 300 and 1,000 μg/plate, as limited by its solubility (MW, level of deacylation and impurities not specified) (Documentation provided to EFSA No 25). The results obtained did not show any increase in revertant numbers compared to the concurrent vehicle control. The Panel noted that no confirmatory assay was performed and that gellan gum was not tested in TA102 or E. coli WP2 tester strains. Since oxidising or cross‐linking activities are not expected to occur, overall, the Panel, considered the negative results observed as reliable with limitations.
Gellan gum (MW, level of deacylation and impurities not specified) was tested for gene mutation at the hypoxanthine‐guanine phosphoribosyl transferase (HPRT) locus in V79 cells both in the absence and presence of S9 metabolic activation at dose levels of 3, 5, 10 and 20 mg/mL. Cells were treated for 3 h and plated in selective medium after an expression period of 5 days (Documentation provided to EFSA No 26). The Panel noted that, despite all test concentrations used exceeded the highest test concentration of 2 mg/mL recommended by the current OECD guideline no. 476 the results obtained indicated that gellan gum did not induce significant increases in mutation frequency at any concentration assayed in any treatment condition. The Panel also noted that the time lapse of 5 h used for the expression of the mutant phenotype was shorter than that recommended in the current OECD guideline No. 476. Overall, the Panel considered the study as acceptable.
In the study by Documentation provided to EFSA (No 27), hepatocytes isolated from male Sprague–Dawley rats (Crl:CD (SD) BR) by liver perfusion with collagenase were treated with gellan gum (MW, level of deacylation and impurities not specified) at dose levels of 0.3, 1, 3, 5, 10 and 20 mg/mL in triplicate cultures for 20 h. Unscheduled DNA synthesis (UDS) was measured by autoradiography using 3H‐thymidine at dose levels of 3, 5, 10 and 20 mg/mL. Results obtained did not show any increase in the grain counts of treated cells compared to the negative control cultures, indicating that the test compound does not induce unscheduled DNA synthesis.
An alkaline elution rat hepatocyte assay with gellan gum was also performed to assess its potential DNA breaking activity. However, this study was demonstrated to be not valid by the author since the fluorescent signal indicating the induction of DNA fragments was a false positive result due to the reaction of gellan gum with diaminobenzoic acid (DABA) used in the assay to measure DNA fluorescence (Documentation provided to EFSA No 25). The Panel agreed with this conclusion.
In vivo
An in vivo mouse micronucleus test was performed to evaluate the ability of gellan gum (MW, level of deacylation and impurities not specified) to induce micronuclei in the bone marrow polychromatic erythrocytes of ICR mice (Documentation provided to EFSA No 24). Groups of five males and five females were dosed orally by gavage at dose levels of 45, 225 and 450 mg/kg bw, as limited by the solubility of test compound for two consecutive days. Vehicle and positive control group animals were also included. Test and control animals were euthanised after approximately 24 and 48 h from the last treatment. Gellan gum did not induce any increase in micronuclei in bone marrow polychromatic erythrocytes over the vehicle control group at any dose level and sampling time assayed. However, the Panel noted that data on bone marrow toxicity were not reported, and therefore, there are no indication of target tissue exposure. In addition, the Panel, based on the evidence that gellan gum is hardly, if not at all absorbed, considers the results of this study of limited relevance for risk assessment.
Overall, based on the available data the Panel concluded that the use of gellan gum did not raise concern with respect to genotoxicity.
3.5.5. Chronic toxicity and carcinogenicity
Mice
Chronic and carcinogenic effects of gellan gum (a polysaccharide, Code No. EX4967, Lot No. 86001A, 58.5% gellan, MW, level of deacylation and impurities not specified) were assessed in a feeding study (Good Laboratory Practice (GLP) compliant) with CD‐1 mice over 96 (female) and 98 weeks (male), respectively (Documentation provided to EFSA No 18). Gellan gum was administered in the diet at concentrations of 0%, 1%, 2% or 3% (equal to 0, 936, 1,872 or 2,867 mg gellan gum/kg bw per day for males and 0, 1,287, 2,457 or 3,627 mg gellan gum/kg bw per day for females). The study was terminated after 96 weeks for females and 98 weeks for males due to the low survival of the respective control group. Clinical examination for mortality and morbidity was performed twice daily, whereas from week 26 onwards a weekly detailed palpation examined for the presence of masses. Within the low‐ and mid‐dose groups, only liver, kidney, ovaries, testes, adrenals, pituitary, lungs, heart and gross abnormalities were histopathologically examined. No organ weights measurements, haematological, biochemical, urine or faecal analyses were performed. The study revealed no substance‐related non‐neoplastic or neoplastic changes. The Panel identified a NOAEL of 3% of the diet (corresponding to 2,867 mg gellan gum/kg bw per day for males and 3,627 mg gellan gum/kg bw per day for females), the highest dose tested.
Rats
In a GLP‐compliant study groups of 50 F1 generation Sprague–Dawley rats of each sex were exposed to gellan gum in utero (a polysaccharide, Code No. EX4967, Lot No. 86001A, 58.5% gellan, MW, level of deacylation and impurities not specified) and continued on gellan gum containing diets for approximately 104 weeks (Documentation provided to EFSA No 20). These animals were derived from the two‐generation reproduction toxicity study which is described in Section 3.5.6. The dietary levels of gellan gum were 0%, 2.5%, 3.8% or 5.0% (equivalent to 0, 730, 1,110, or 1,460 mg gellan gum/kg bw per day). The rats were observed daily for the first 4 weeks of treatment and weekly thereafter for clinical signs of toxicity. Individual bodyweights and food consumption were measured on a weekly basis for the first 26 weeks of treatment and every 2 weeks thereafter. Ophthalmoscopic and histopathological examinations were conducted on the control and 5% groups during weeks 1, 13, 26, 52, 78 and 103. Clinical chemistry and haematological samples were collected at weeks 13, 25, 39 and 51. After 104 weeks, ophthalmoscopic examinations, haematology, clinical chemistries and organ weight data revealed no changes which could be attributed to the feeding of gellan gum. Survival of male treated rats was poor when compared to controls, whereas female treated rats exhibited better survival than their concurrent controls. Male rats, fed gellan gum at the 2.5% and 3.8% in the diet exhibited lower body weights after 76 weeks. The initial bodyweights were 5.2% and 3.4% lower than the control values for the 2.5% and 3.8% groups, respectively. The authors concluded that the growth of the treated animals was comparable to that of the controls. There were no neoplastic or non‐neoplastic changes that could be associated with the feeding of gellan gum. The authors concluded that under the conditions of this bioassay, gellan gum was non‐carcinogenic to Sprague–Dawley rats. The Panel agreed with this conclusion. The Panel considered that the 5% of gellan gum in the diet (equivalent to 1,460 mg gellan gum/kg bw per day) was the NOAEL, the highest dose tested.
Overall, the Panel concluded that gellan gum is not of concern with respect to carcinogenicity. Chronic toxicity studies with gellan gum did not reveal adverse effects at the highest doses tested equal to 2,867 mg gellan gum/kg bw per day for male mice and 3,627 mg gellan gum/kg bw per day for female mice, or equivalent to 1,460 mg gellan gum/kg bw per day in rats.
3.5.6. Reproductive and developmental toxicity
Reproductive toxicity studies
Gellan gum (a polysaccharide, Code No. EX4967, Lot No. 86001A, 58.5% gellan. MW, level of deacylation and impurities not specified) was administered to Sprague–Dawley rats (n = 26/sex per group) via the diet at dose levels for of 0%, 2.5%, 3.8% and 5% (equivalent to 0, 730, 1,110, or 1,460 mg gellan/kg bw per day) in a two‐generation reproduction toxicity study (Documentation provided to EFSA No 28). The study was performed in compliance with OECD guideline 416 and GLP. Male rats were treated continuously from 70 days prior to mating to three weeks after mating, whereas female rats received the gellan gum containing diet from 14 days prior to mating until sacrifice after weaning. For the F1 generation, 26 male and female pups of F0 were selected and maintained until mating for F2 generation of this study. Some males of F0 suffered from a sialodacryoadenitis viral infection. The authors stated that no treatment‐related effects were observed in any generation on survival, clinical observations, body weight, food consumption, mating or fertility or gestation index, conception rate, gestation or parturition length, pup survival, post‐implantation loss, lactation index and necropsy as well as pathological examinations. The Panel agreed with this conclusion and considered the highest dose tested, 5% in the diet (1,460 mg/kg bw per day), to be the NOAEL.
Gellan gum (a polysaccharide, Code No. EX4967, Lot No. 86001A, 58.5% gellan, MW, level of deacylation and impurities not specified) was also administered to Sprague–Dawley rats within an in utero/chronic/carcinogenicity study (Documentation provided to EFSA No 20). This study in utero phase is comparable with a one‐generation reproduction study. Groups of Sprague–Dawley rats (75/sex) were administered via the diet at dose levels for of 0%, 2.5%, 3.8% and 5% (equivalent to 0, 730, 1,110, or 1,460 mg gellan gum mg/kg bw per day) for 63 days prior to mating and throughout mating. The females were treated during gestation and lactation. Apart from some not dose‐related effects, significant lower body weights were observed in males of the F0 generation of the 5% group. The authors considered this effect not dose related but related to increased levels of non‐nutritive gellan gum in the diet. The reproductive and developmental parameters were not affected in this study. The Panel considered the highest dose tested, 5% in the diet (corresponding to 1,460 mg gellan/kg bw per day), to be the NOAEL, the highest dose tested.
Developmental studies
A dietary prenatal developmental toxicity study was reported in Sprague–Dawley rats (n = 25/group) with dose levels of 0%, 2.5%, 3.8% or 5% gellan gum (a polysaccharide Code No. EX4967, Lot No. 86001A, MW, level of deacylation and impurities not specified) (equivalent to, 0, 730, 1,110, or 1,460 mg gellan gum/kg bw per day (Documentation provided to EFSA No 29). The test diets were fed from gestational day (GD) 6–15. Maternal survival, food consumption, body weight and gross pathology at caesarean section as well as pregnancy rate, corpora lutea, number of implantation sites, pre‐ and post‐implantation losses, number of resorptions, number of live and dead fetuses, malformations were unaffected by treatment with gellan gum. Reduced ossification only occurred in the 2.5% and 3.8% groups, variations of the sternebrae only in the 3.8% group and abnormalities (visceral, skeletal and external) were either comparable to the control group, within normal variation, not dose dependent and/or not changed due to treatment with gellan gum. The authors stated that gellan gum did not induce treatment‐related effects up to dose levels of 5% in the diet (corresponding 1,460 mg gellan gum/kg bw per day), the highest dose tested. The Panel agreed with this conclusion.
Overall, a dietary two‐generation reproductive toxicity study, an one‐generation study (in utero phase of a chronic/carcinogenicity study) and a prenatal developmental toxicity study in rats with gellan gum up to 5% in the diet (1,460 mg/kg bw per day), the highest dose tested did not show adverse effects.
3.5.7. Hypersensitivity, allergenicity and food intolerance
Animal studies
Gellan gum was applied in a guinea pig sensitisation test following the protocol of Magnusson and Kligman (Documentation provided to EFSA No 22). This study did not reveal any dermal contact dermatitis up to 2.5% dermally applied gellan gum.
Humans
In order to exclude a possible allergic reaction towards gellan gum), a study (Documentation provided to EFSA No 23) was performed, analysing the blood samples of 10 volunteers including two volunteers who had elevated eosinophils as shown in another study provided to EFSA (Documentation provided to EFSA No 17; see Section 3.5.1). The blood samples were analysed by paper radioimmunosorbent test (PRIST) and radioallergosorbent test (RAST), for total immunoglobulin E (IgE) and allergen‐specific IgE, respectively. Although overall IgE levels were occasionally elevated in some samples, no IgE specific for gellan gum was detected in the serum of the participants.
The Panel did not identify in the literature any case report of allergic reaction in human after oral exposure to gellan gum.
Overall, the Panel noted that both in animals and humans there were no indications for hypersensitivity reaction to gellan gum.
3.6. Discussion
According to Commission Regulation (EU) No 231/2012, gellan gum (E 418) is a high molecular weight polysaccharide gum produced by a pure culture fermentation of a carbohydrate by strains of Pseudomonas elodea. The Panel noted that according to available information gellan gum (E 418) is produced by only one bacterial strain ATCC 31461.
The repeating unit of the polysaccharide is a tetrasaccharide composed of two d‐glucose units, one d‐glucuronic acid residue and one of l‐rhamnose residue and is substituted with acyl groups (glycerate and acetate groups as O‐glycosidically linked esters).
According to the industry, in addition to the gellan gum polysaccharide, typical samples contain water (2–14%), proteinaceous material measured by nitrogen content (%N = 0–3.0%) (Documentation provided to EFSA No 3) and may contain PHB up to 25 wt% (Baird and Cleary, 1994).
There are two basic forms: ‘high acyl’ and ‘low acyl’ form of the food additive gellan gum (E 418), which are distinguished by the degree of substitution by O‐acyl groups. For ‘low acyl’ (including fully deacylated) type, there are both clarified and non‐clarified products available on the market.
The Panel noted that, according to the available information from the industry, the content of PHB in dried gellan gum was estimated to be from less than 1% up to 25%, depending on degree of deacylation and clarification. The Panel noted that PHB may be a major component of the food additive E 418 resulting from the manufacturing process. In the absence of any justification about its technological need, the Panel considered that its presence should be limited, and/or at least indicated in the specifications of the food additive E 418. In this regard, the Panel noted that clarification is a feasible mean to reduce the amount of PHB in E 418.
In the period from the date of US Patent of Kang and Veeder (1982) to which the production of gellan gum originally refers until the present time no reports have been identified that the strain ATCC 31461 was the cause of any human infection.
The biological and toxicological testing has been performed with a deacylated gellan gum or with a gellan gum of unknown degree of acylation. However, the Panel considered that the structural similarities of the different types of gellan gum allowed for read across. No information was available on the purity of the test material in these studies, including the concentration of PHB.
The in vivo metabolic and physiological studies of gellan gum indicated that this compound would not be absorbed intact. In rats, there is indication of limited increased production of SCFA and the faecal release of acetate and butyrate could be the consequence of both the hydrolysis of the acetyl side chain of gellan gum and the possible presence of PHB. In humans, gellan gum would not be absorbed intact and there is no indication of significant fermentation by the intestinal microbiota.
Gellan gum is of low acute toxicity.
Subchronic toxicity studies with gellan gum conducted in rats and dogs did not reveal adverse effects at the highest doses tested (equal to 2,950 mg/kg bw per day for males and 3,760 mg/kg bw per day for females in rats and 1,870 mg/kg bw per day for males and 2,070 mg/kg bw per day for females in dogs). In a short‐term study in rhesus monkeys, no adverse effects were seen at 3,000 mg gellan gum/kg bw per day, the highest dose tested.
Based on the available data, the Panel considered that gellan gum did not raise concern with respect to genotoxicity.
Gellan gum is not of concern with respect to carcinogenicity. Chronic toxicity studies with gellan gum did not reveal adverse effects at the highest doses tested equal to 2,867 mg gellan gum/kg bw per day for male mice and 3,627 mg gellan gum/kg bw per day for female mice, or equivalent to 1,460 mg gellan gum/kg bw per day in rats.
A dietary two‐generation reproductive toxicity study, an one‐generation study (in utero phase of a chronic/carcinogenicity study) and a prenatal developmental toxicity study in rats with gellan gum up to 5% in the diet (1,460 mg/kg bw per day), the highest dose tested, did not show adverse effects.
The consumption of up to 200 mg/kg bw per day gellan gum over 3 weeks had no adverse health effects in humans. There was no indication for allergenic reaction to gellan gum both in animals and humans.
According to the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA, 2014), the Panel considered that sufficient toxicity data were available in animals showing no adverse effects at highest doses tested up to 3,627 mg gellan gum/kg bw per day. Therefore, the Panel considered that there is no need to allocate a numerical ADI for gellan gum (E 418).
To assess the dietary exposure to gellan gum (E 418) from its use as a food additive, the exposure was calculated based on (1) maximum reported use levels for FCs in which gellan gum (E 418) is authorised at QS and MPLs for the 2 FCs with numerical maximum levels (defined as the maximum level exposure assessment scenario) and (2) the reported use levels (defined as the refined exposure assessment scenario).
Based on the available data set, the Panel calculated three refined exposure estimates based on different assumptions as described in Section 3.4: a brand‐loyal consumer scenario, a non‐brand‐loyal scenario and the food supplement consumers’ only scenario. The Panel considered that the refined exposure assessment approach resulted in more realistic long‐term exposure estimates compared to the maximum level exposure assessment scenario.
The Panel noted that the estimated long‐term exposures based on the maximum level exposure assessment scenario are very likely conservative, as this scenario assumes that all foods and beverages listed under the annex II to Regulation No 1333/2008 contain gellan gum (E 418) at the MPL or at the maximum reported use levels that were in this case mainly provided from a food additive producer.
For gellan gum (E 418), few reported uses were available on eight food categories. However, not all available data could be included in the assessment owing to specific restrictions/exceptions regarding products not referenced in the FoodEx classification. This may have resulted in an underestimation of exposure to gellan gum (E 418). On the other hand, several food categories for which use data were available were included without considering specific restrictions/exceptions, which may have overestimated the exposure to gellan gum (E 418). In total, 7 out of 71 authorised food categories were taken into account in the refined exposure assessment scenarios. Added to that, approximately 67% of the food products labelled with gellan gum (E 418) in the Mintel's GNPD belonged to food subcategories that were considered in the refined exposure assessment scenarios (Appendix B).
Several uncertainties were identified in the exposure assessment (Table 6). Overall, for the maximum level exposure scenario, the Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure to gellan gum (E 418) as a food additive according to Annex II to Regulation (EC) No 1333/2008 in European countries considered in the EFSA Comprehensive database. Based on the assumption that the food additive is not used in those food categories in which it is permitted but for which no usage data were provided, also the refined scenario would in general result in an overestimation of exposure.
The Panel noted that the exposure to gellan gum (E 418) from its use according the Annex III (Parts 2, 3, 4 and 5A) was not considered in the exposure assessment.
Since gellan gum (E 418) is authorised and used in a certain type of flavoured drinks, to which consumers may be brand loyal, the Panel selected the refined brand‐loyal scenario as the most relevant exposure scenario for the safety evaluation of this food additive.
Due to the discrepancies observed between the data reported from food industry and Mintel database, where gellan gum (E 418) is labelled in more products than in food categories for which data were reported from industry, the Panel noted that the collection of data on use and use levels of gellan gum (E 418) would allow for a more realistic exposure assessment. The Panel also noted that according to the Mintel's GNPD, gellan gum was listed as an ingredient in seven products of subcategory ‘growing‐up milk’ (soya‐drink products recommended for young children 1–3 years old) and in five products of subcategory ‘baby juices & drinks’. Gellan gum (E 418) is not authorised as a food additive in those subcategories.
The Panel also noted that the refined exposure estimates are based on reported use levels of gellan gum (E 418). If current practice changes, this refined estimates may no longer be representative and should be updated.
4. Conclusions
Following the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA, 2014), and given that:
from all the data received, data were adequate for a refined exposure assessment for 7 out of 71 food categories;
based on the reported use levels, a refined exposure (brand‐loyal scenario) of up to 72.4 mg/kg bw per day in toddlers at the 95th percentile was estimated;
gellan gum is unlikely to be absorbed intact and would not be fermented by human intestinal microbiota;
adequate toxicity data were available;
there was no concern with respect to genotoxicity and carcinogenicity;
no adverse effects were reported in chronic studies at the highest doses tested in mice and rats (3,627 and 1,460 mg gellan gum/kg bw per day respectively).
repeated oral intake up to 200 mg/kg bw per day for 3 weeks had no adverse effects in humans.
the Panel concluded that there is no need for a numerical ADI for gellan gum (E 418), and that there is no safety concern at the refined exposure assessment for the reported uses and use levels of gellan gum (E 418) as a food additive.
5. Recommendations
The Panel recommended the European Commission to consider:
changing in the definition of the European Commission specifications the chemical names of the acyl groups ‘glyceryl’ and ‘acetyl’ to ‘glycerate’ and ‘acetate’;
indicating in the definition of the European Commission specifications that only the non‐genetically modified strain ATCC 31461 should be used for the production of gellan gum
establishing specifications for the individual types of gellan gum with respect to acylation and clarification;
including specifications for the absence of viable cells of the microbial source;
defining purity in the specifications including the presence of PHB and residual bacterial enzymatic activities;
revising the current limits for the toxic elements lead, mercury, cadmium and arsenic in the European Commission specification for gellan gum (E 418) in order to ensure that gellan gum (E 418) as a food additive will not be a significant source of exposure to these toxic elements in food.
Documentation provided to EFSA
Pre‐evaluation documents on carrageenan (E 407). Frauenhofer ITEM. December 2012.
Biopolymer International, 2010. Response to EFSA. EFSA call for data on emulsifiers, stabilisers and gelling agents. Submitted to EFSA on 22 October 2010.
Biopolymer International, 2015. Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Submitted to EFSA on 15 December 2015.
Biopolymer International, 2016. Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 06 April 2016.
Biopolymer International, 2016. Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
CP Kelco, 2017. Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 5 September 2017.
Biopolymer International, 2018. Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information from CP Kelco on gellan gum. Submitted to EFSA on 11 January 2018.
Biopolymer International, 2018. Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information from DSM China on gellan gum. Submitted to EFSA on 11 January 2018.
Biopolymer International, 2018. Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information from CP Kelco on gellan gum. Submitted to EFSA on 14 March 2018.
Biopolymer International, 2018. Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information from DSM China on gellan gum. Submitted to EFSA on 14 March 2018.
EMA (European Medicines Agency): communication to EFSA request for information on a certain group of substances used as food additives, December 2015.
Biopolymer International (BIOPOLYMER), Data on usage levels of gellan gum (E 418) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 3) Submitted to EFSA on 30th Sept 2014.
EuroGum A/S (DK_EUROGUM), 2014. Data on usage levels of gellan gum (E 418) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 3) Submitted to EFSA on 30th Sept 2014.
Food Drink Europe (FDE), 2013. Data on usage levels of gellan gum (E 418) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 3) Submitted to EFSA on 29th Nov 2013.
International Chewing Gum Association (ICGA), 2014. Data on usage levels of gellan gum (E 418) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 3) Submitted to EFSA on 30th Sept 2014.
Specialised Nutrition Europe (SNE), 2014. Data on usage levels of gellan gum (E 418) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 3) Submitted to EFSA on 30th Sept 2014.
CP Kelco, 2017 The dietary effects of gellan gum in humans. Food Additives and Contaminants, 5, 237–249. Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 05 September 2017.
Kelco Division of Merck and Co., Inc., 1987. A dietary carcinogenicity study of Gellan gum in the albino mouse. Project No. 81833. Confidential Research Report from the Bio‐Research Laboratories Ltd. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
Kelco Division of Merck and Co., Inc., 1986. A 52‐week oral toxicity study of the Gellan gum in the Beagle Dog. Project No. 81779. Confidential Research Report from the Bio‐Research Laboratories Ltd. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
Kelco Division of Merck and Co., Inc., 1985. An in utero/chronic toxicity/carcinogenicity study of Gellan gum administered in the diet to the rat (in utero phase) Vol.1. Project No. 81835. Confidential Research Report from the Bio‐Research Laboratories Ltd. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
Kelco Division of Merck and Co., Inc., 1983. A 13‐week toxicity study of a Polysaccharide Gum (K9850) during dietary administration to the albino rat. Project No. 81274. Confidential Research Report from the Bio‐Research Laboratories Ltd. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
Kelco Division of Merck and Co., Inc., 1986. Guinea pig sensitisation test. Confidential Report from the Laboratories Merck Sharp & Dohme‐Chibret. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
Kelco Division of Merck and Co., Inc., 1989. Clinical laboratory study of Prist and Rast and Gellan gum. Confidential Report from the Advanced Allergy Management. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
Kelco Division of Merck and Co., Inc., 1989. Mutagenicity test on Gellan gum EX‐4967 in vivo mouse micronucleus assay. HLA Study No. 10708‐0‐455. Confidential Final Report from the Hazleton Laboratories America, Inc. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
Kelco Division of Merck and Co., Inc., 1985. Gellan gum, genotoxicity evaluation. Confidential Report from the Merck Institute for Therapeutic Research. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
MSDRL, 1984a. Gellan gum, V‐79 Mammalian Cell Mutagenesis. Confidential Report from the Department of Safety Assessment, MSDRL. Biopolymer International. 2017 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 5 September 2017.
MSDRL, 1984b. Gellan gum, unscheduled DNA synthesis in rat hepatocytes. Confidential Report from the Department of Safety Assessment, MSDRL. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 5 September 2017.
Kelco Division of Merck and Co., Inc., 1985a. A two generation reproduction study of Gellam gum administration in the diet to the rat. Project No. 81834. Confidential Research Report from the Bio‐Research Laboratories Ltd. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
Kelco Division of Merck and Co., Inc., 1985b. The teratology study of Gellan gum administration in the diet to the rat. Project No. 81890. Confidential Research Report from the Bio‐Research Laboratories Ltd. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
Kelco Division of Merck and Co., Inc., 1984a. Rat balance study, tissue distribution and blood level of 14C and 3H labeled Gellan gum. Study Number KE‐162r. Confidential Final Report from the Primate Research Institute. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
Kelco Division of Merck and Co., Inc., 1984b. A 28‐day subchronic toxicity stud in rhesus monkeys. PRI Study No. KE‐170m. Confidential Final Report from the Promate Research Institute. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
Kelco Division of Merck and Co., Inc., 1980. Acute oral Toxicity study in rats. Project No. 2123‐103. Confidential Final Report from the Hazelton Laboratories America, Inc. Biopolymer International. 2016 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information. Submitted to EFSA on 12 September 2016.
Abbreviations
- ADI
acceptable daily intake
- AFC Panel EFSA
Former Panel on Additives, Flavourings, Processing Aids and Materials in Contact
- ANS Panel
EFSA Panel on Food Additives and Nutrient Sources added to Food
- ATCC
American Type Culture Collection
- BIOPOLYMER
Biopolymer International
- CAS
Chemical Abstracts Service
- DABA
diaminobenzoic acid
- DK_EUROGUM
EuroGum A/S
- EINECS
European List of Notified Chemical Substances
- EMA
European Medicines Agency
- FAO
Food and Agriculture Organization
- FC
food category
- FCS
Food Classification System
- FDE
Food and Drink Europe
- GD
gestational day
- GLP
Good Laboratory Practice
- GNPD
Global New Products Database
- GRAS
Generally Recognised As Safe
- HDL
high‐density lipoprotein
- HPRT
hypoxanthine‐guanine phosphoribosyl transferase
- ICGA
International Chewing Gum Association
- IgE
immunoglobulin E
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- MPL
maximum permitted level
- NOAEL
no‐observed‐adverse‐effect‐level
- OECD
Organisation for Economic Co‐operation and Development
- PHB
polyhydroxybutyrate
- PRIST
paper radioimmunosorbent test
- RAST
radioallergosorbent test
- QS
quantum statis
- SCF
Scientific Committee for Food)
- SCFA
short‐chain fatty acid
- SNE
Specialised Nutrition Europe
- TPA
texture profile analysis
- UDS
Unscheduled DNA synthesis
Appendix A – Summary of reported use levels (mg/kg or mg/L as appropriate) of gellan gum (E 418) provided by industry
Appendix B – Number and percentage of food products labelled with gellan gum (E 418) out of the total number of food products present in the Mintel GNPD per food subcategory between 2013 and 2018
Appendix C – Concentration levels of gellan gum (E 418) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Appendix D – Summary of total estimated exposure of gellan gum (E 418) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Appendix E – Main food categories contributing to exposure to gellan gum (E 418) using the maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
1.
Appendixes A–E can be found in the online version of this output (‘Supporting information’ section) https://doi.org/10.2903/j.efsa.2018.5296
Supporting information
Summary of reported use levels (mg/kg or mg/L as appropriate) of gellan gum (E 418) provided by industry
Number and percentage of food products labelled with gellan gum (E 418) out of the total number of food products present in the Mintel GNPD per food subcategory between 2013 and 2018
Concentration levels of gellan gum (E 418) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of gellan gum (E 418) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to gellan gum (E 418) using the maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Suggested citation: EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) , Younes M, Aggett P, Aguilar F, Crebelli R, Filipic M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Lambré C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Brimer L, Mosesso P, Christodoulidou A, Cascio C, Tard A, Lodi F and Dusemund B, 2018. Scientific Opinion on the re‐evaluation of gellan gum (E 418) as food additive. EFSA Journal 2018;16(6):5296, 39 pp. 10.2903/j.efsa.2018.5296
Requestor: European Commission
Question number: EFSA‐Q‐2011‐00517
Panel members: Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipic, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Gunter Georg Kuhnle, Claude Lambré, Jean‐Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Jacqueline Wiesner and Pasquale Mosesso. The ANS Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 17 May 2018
Notes
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1–295
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
COM(2001) 542 final.
Food Additives in Europe 2000, Status of safety assessments of food additives presently permitted in the EU, Nordic Council of Ministers, TemaNord 2002, 560.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1–295
Annex II of Regulation (EC) No 1333/2008 on food additives for use in foodstuffs.
Available online: http://ec.europa.eu/food/food/animalnutrition/feedadditives/comm_register_feed_additives_1831-03.pdf
Regulation (EC) No 1831/2003 of the European Parliament and the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, 29–43.
Call for scientific data on food additives permitted in the EU and belonging to the functional classes of emulsifiers, stabilisers and gelling agents. Published: 22 November 2009. Available from: http://www.efsa.europa.eu/en/dataclosed/call/ans091123
Call for technical data on certain thickening agents permitted as food additives in the EU – Extended Deadline: 31 December 2015. Available online: http://www.efsa.europa.eu/it/data/call/141219
Call for food additives usage level and/or concentration data in food and beverages intended for human consumption ‐ Extended deadline: 30 September 2014. https://www.efsa.europa.eu/it/dataclosed/call/datex140310
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
Sphingomonas sp. (ATCC® 31461™)
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16.
Missing Bulgaria, Cyprus, Estonia, Latvia, Lithuania, Luxembourg, Malta and Slovenia.
http://www.gnpd.com/sinatra/home/ accessed on 11/01/2018.
References
- Adiotomre J, Eastwood MA, Edwards C and Brydon WG, 1990. Dietary fiber: in vitro methods that anticipate nutrition and metabolic activity in humans. The American Journal of Clinical Nutrition, 52, 128–134. [DOI] [PubMed] [Google Scholar]
- AOAC (Association of Official Agricultural Chemists), 2002. AOAC Official Methods 937.12 and 935.61. Chapter 43 – Spices and Other Contaminants. Food compensition; Additives; Natural Contaminants. Official Methods of Analysis of AOC International, 17th Edition, Vol. II., 14 p.
- Baird JK and Cleary JM, 1994. U.S. Patent No. 5,300,429. U.S. Patent and Trademark Office, Washington, DC. [Google Scholar]
- Bajaj IB, Survase SA, Saudagar PS and Singhal RS, 2007. Gellan gum: fermentative production, downstream processing and applications. Food Technology and Biotechnology, 45, 341. [Google Scholar]
- Bower S, Burke E, Harding NE, Patel YN, Schneider JC, Meissner D, Morrison NA and Bezanson R, 2016. U.S. Patent No. 9,290,783. Washington, DC: U.S. Patent and Trademark Office.
- Edwards CA and Eastwood MA, 1995. Caecal and faecal short‐chain fatty acids and stool output in rats fed on diets containing non‐starch polysaccharides. British Journal of Nutrition, 73, 773–781. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Scientific opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA AFC Panel (EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food), 2004. Opinion on a request from the Commission related to the use of certain food additives in jelly mini cups. EFSA Journal 2004;2(8):82, 11 pp. 10.2903/j.efsa.2004.82 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources), 2014. Statement on a conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010. EFSA Journal 2014;12(6):3697, 11 pp. 10.2903/j.efsa.2014.3697 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009a. Scientific opinion on cadmium in food. EFSA Journal 2009;7(10):980, 139 pp. 10.2903/j.efsa.2009.98 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009b. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351, 199 pp. 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012a. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 241 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012b. Scientific Opinion on lead dietary exposure in the European population. EFSA Journal 2012;10(7):2831, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012c. Scientific Opinion on cadmium dietary exposure in the European population. EFSA Journal 2012;10(1):2551, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on dietary exposure to inorganic arsenic in the European population. EFSA Journal 2014;12(3):3597, 68 pp. 10.2903/j.efsa.2014.3597 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012a. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012b. Scientific Opinion on Exploring options for providing advice about possible human health risks based on the concept of Threshold of Toxicological Concern (TTC). EFSA Journal 2012;10(7):2750, 103 pp. 10.2903/j.efsa.2012.2750 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012c. Statement on the applicability of the Margin of Exposure approach for the safety assessment of impurities which are both genotoxic and carcinogenic in substances added to food/feed. EFSA Journal 2012;10(3):2578, 5 pp. 10.2903/j.efsa.2012.2578 [DOI] [Google Scholar]
- EPA , 2003. Gellan gum; Notice of filling a pesticide petition to establish a tolerance for a certain pesticide chemical in or on food. Federal Register, 68, 42026–42030. [Google Scholar]
- Fialho AM, Moreira LM, Granja AT, Popescu AO, Hoffmann K and Sa‐Correia I, 2008. Occurrence, production, and applications of gellan: current state and perspectives. Applied Microbiology and Biotechnology, 79, 889–900. [DOI] [PubMed] [Google Scholar]
- Giavasis I, Harvey LM and McNeil B, 2000. Gellan gum. Critical Reviews in Biotechnology, 20, 177–211. [DOI] [PubMed] [Google Scholar]
- Glueck U and Thier HP, 1980. Quantitative determination of some thickeners in dairy products. Zeitschrift für Lebensmittel‐Untersuchung und ‐Forschung, 170, 272–279. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1991b. Evaluation of certain food additives and contaminants. Twenty‐seventh report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series No.806.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Gellan gel. Combined Compendium of Food Additive Specifications ‐ all Specifications. Monographs from the 1st to the 65th Meeting (1956–2005) Food Additives Vol.1‐3.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2014. Monograph 16. Combined compendium of food additive specifications. Available online: http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/en/
- Kang KS and Veeder GT, 1982. U.S. Patent No. 4,326,053. Washington, DC: U.S. Patent and Trademark Office. Biopolymer International. 2018 Response to EFSA. Call for technical data on certain thickening agents permitted as food additives in the EU. Additional information from CP Kelco on gellan gum. Submitted to EFSA on 14 March 2018
- Kelco CP, 2007. Kelcogel gellan gum, 5th Edition. World's Leading Hydrocolloid Solutions Provider, 30 pp. [Google Scholar]
- Mei T, Xu X, Li B, Li J, Cui B, Zhou B and Ablaye W, 2012. Synergistic interaction of konjac glucomannan and gellan gum investigated by rheology and texture analysis. Journal of Applied Polymer Science, 125, 1363–1370. [Google Scholar]
- Osmałek T, Froelich A and Tasarek S, 2014. Application of gellan gum in pharmacy and medicine. International Journal of Pharmaceutics, 466, 328–340. [DOI] [PubMed] [Google Scholar]
- Paul F, Morin A and Monsan P, 1986. Microbial polysaccharides with actual potential industrial applications. Biotechnology Advances, 4, 245–259. [DOI] [PubMed] [Google Scholar]
- Preuss A and Thier HP, 1983. Isolation of natural thickeners from food by capillary gas chromatographic determination. Zeitschrift für Lebensmittel‐Untersuchung und ‐Forschung, 176, 5–11. [Google Scholar]
- SCF (Scientific Committee for Food), 1992. Opinion expressed. Reports of the Scientific Committee for Food, 26th series. Food science and techniques, 1992.
- SCF (Scientific Committee on Food), 2001. Guidance on submissions for food additive evaluations by the scientific committee on food. Opinion expressed on 11 July 2001. Available online: http://ec.europa.eu/food/fs/sc/scf/out98_en.pdf
- Shimizu J, Wada M, Takita T and Innami S, 1999. Curdlan and gellan gum, bacterial gel‐forming polysaccharides, exhibit different effects on lipid metabolism, cecal fermentation and fecal bile acid excretion in rats. Journal of Nutritional Science and Vitaminology, 45, 251–262. [DOI] [PubMed] [Google Scholar]
- Sworn G, 2009. Gellan gum. In: Phillips GO and Williams PA (eds.). Handbook of hydrocolloids, 2nd Edition. CRC Press, Boca Raton, FL ‐, USA. pp. 204–227. [Google Scholar]
- Sworn G, Chen YL, Morrison NA, Talashek T and Clark R, 2003. U.S. Patent No. 6,602,996. U.S. Patent and Trademark Office, Washington, DC. [Google Scholar]
- USDA (National Organic Program), 2006. Gellan gum. Handling/Processing, Technical Evaluation Report from the ICF Consulting. 6 p. [Google Scholar]
- Zia KM, Tabasum S, Khan MF, Akram N, Akhter N and Noreemand Zuber M, 2017. Recent trends on gellan gum blends with natural and synthetic polymers: a review. International Journal of Biological Macromolecules Pii: S0141‐8130 (17)32675‐2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of reported use levels (mg/kg or mg/L as appropriate) of gellan gum (E 418) provided by industry
Number and percentage of food products labelled with gellan gum (E 418) out of the total number of food products present in the Mintel GNPD per food subcategory between 2013 and 2018
Concentration levels of gellan gum (E 418) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of gellan gum (E 418) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to gellan gum (E 418) using the maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)


