Abstract
EFSA was asked to deliver a scientific opinion regarding the effect on public health of a possible increase of the maximum level (ML) for ‘aflatoxin total’ (AFT; sum of aflatoxin B1, aflatoxin B2, aflatoxin G1 and aflatoxin G2) from 4 to 10 μg/kg in peanuts and processed products thereof. Aflatoxins are genotoxic and cause hepatocellular carcinomas in humans. The Panel on Contaminants in the Food Chain (CONTAM Panel) evaluated 8,085 samples of peanuts and 472 samples of peanut butter, with > 60% left‐censored. The mean concentration of AFT in peanuts was 2.65/3.56 μg/kg (lower bound (LB)/upper bound (UB)) with a maximum of 1,429 μg/kg. The mean concentration in peanut butter was 1.47/1.92 μg/kg (LB/UB) with a maximum of 407 μg/kg. Peanut oil was not included since all data were left‐censored and the ML does not apply for oil. Exposure was calculated for a ‘Current ML’ and ‘Increased ML’ scenario, and mean chronic exposure estimates for consumers only, amounted to 0.04–2.74 ng/kg body weight (bw) per day and 0.07–4.28 ng/kg bw per day, respectively. The highest exposures were calculated for adolescents and other children. The CONTAM Panel used the cancer potencies estimated by the Joint FAO/WHO Expert Committee on Food Additives for the risk characterisation. Under the scenario of the current ML, the cancer risk was estimated to range between 0.001 and 0.213 aflatoxin‐induced cancers per 100,000 person years. Under the scenario of the increased ML, it ranged between 0.001 and 0.333 aflatoxin‐induced cancers per 100,000 person years. Comparing these data calculated under the current ML scenario with the yearly excess cancer risk of 0.014 shows a higher risk for consumers of peanuts and peanut butter in some surveys. The calculated cancer risks indicate that an increase of the ML would further increase the risk by a factor of 1.6–1.8.
Keywords: aflatoxin, peanuts, occurrence, exposure, maximum level (ML), public health
Summary
Following a request from the European Commission, the Panel on Contaminants in the Food Chain (CONTAM Panel) was asked to deliver a scientific opinion on the effect on public health of a possible increase of the maximum level (ML) for ‘aflatoxin total’ (AFT) from 4 to 10 μg/kg in peanuts and processed products thereof, intended for direct human consumption or used as an ingredient in foodstuffs. The assessment should include vulnerable groups of the population and take the consumption patterns within the European Union (EU) into account.
Aflatoxins are mycotoxins produced primarily by the fungi Aspergillus flavus and Aspergillus parasiticus. A. parasiticus produces aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1) and aflatoxin G2 (AFG2), whereas A. flavus mainly produces AFB1 and AFB2. However, A. flavus isolates producing AFG1 and AFG2 also have been identified. These fungi are especially found in areas with a hot and humid climate. AFB1 is the most frequently found aflatoxin in contaminated samples and the three others are generally not reported in the absence of AFB1. AFT refers to the sum of these four aflatoxins.
Aflatoxins have primarily been detected in imported foods such as peanuts, tree nuts, dried fruit, spices, crude oil, cocoa beans, maize and rice. Processes such as heating, roasting and baking can reduce the levels of aflatoxins but cannot completely eliminate the toxins. Extraction and refining of oil can reduce the levels of aflatoxins to less than 1 μg/kg even from highly contaminated samples of peanuts.
AFB1 is a genotoxic and potent liver carcinogen in experimental animals and humans. In experimental animals, AFG1 is also carcinogenic, whereas there is limited evidence for carcinogenicity of AFB2 and inadequate evidence for carcinogenicity of AFG2.
EFSA assessed the toxicology of aflatoxins in 2007 and the most recent evaluation was carried out by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 2016. Given the short deadline of this request, no new toxicological evaluation was performed by the CONTAM Panel. Therefore, the CONTAM Panel decided to use the cancer potencies estimated by JECFA. The cancer potencies for an AFB1 exposure of 1 ng/kg body weight (bw) per day (in 100,000 person years) were estimated to be 0.017 (mean estimate) for hepatitis B surface antigen negative (HBsAg–) individuals and 0.269 for HBsAg+ individuals using model averaging. The 95% UB potency estimates were 0.049 and 0.562, respectively. No reference point or cancer potency has been identified for AFT, AFB2, AFG1 or AFG2 in previous assessments. Considering that AFB1 is the major contributor to the exposure, the CONTAM Panel used a conservative approach and evaluated the aflatoxins as a group and applied the cancer potencies calculated for AFB1 to AFT.
Occurrence data on aflatoxins in peanuts, peanut butter and peanut oil were available in the EFSA Chemical Occurrence database. Only samples in which AFB1, AFB2, AFG1 and AFG2 were analysed, were used in the assessment. In total, 34,324 analytical results for AFB1, AFB2, AFG1 and AFG2 were taken into account. These correspond to 8,581 samples, of which 8,095 were peanuts, 472 samples were peanut butter and 14 were peanut oil. Most of the samples had AFT concentrations less than 10 μg/kg. About 66% of the data were provided by two countries. Due to time constraints, it was not feasible to issue a complementary call for data.
The highest concentrations of AFT were found in peanuts with a mean value of 2.65/3.56 μg/kg (lower bound (LB)/upper bound (UB)). Most of the samples (87%) were left‐censored with a maximum concentration of 1,429 μg/kg. Seven per cent of the samples had a concentration above the ML of 4 μg/kg. Concentrations of AFT in peanut butter were lower with a mean value of 1.47/1.92 μg/kg (LB/UB) and a maximum of 407 μg/kg. Five per cent of the samples had a concentration above the ML of 4 μg/kg and 64% of the samples were left‐censored. All 14 samples of peanut oil were left‐censored. Because the ML of 4 μg/kg does not apply to crude or refined vegetable oil, peanut oil was not included in the exposure assessment.
Contribution of the individual aflatoxins to the AFT concentration varied but the major contributor was AFB1 representing 61% of the MB AFT concentration for samples containing less than 1 μg/kg, and up to 78% for samples containing more than 10 μg/kg.
Consumers of peanuts and peanut butter represent on average 7% (range: 0–36%) of the total population, so the CONTAM Panel concluded that exposure estimates, for consumers only, were the most adequate.
No data were available regarding consignments that were not shipped to the EU due to levels exceeding the current ML of 4 μg/kg but would have been placed on the EU market if a higher ML was in place. Therefore, the CONTAM Panel made a simulation by bootstrapping instead. It is anticipated that changing the ML from 4 to 10 μg/kg for peanuts will increase the mean LB estimates by a factor of 1.75 and the mean UB estimates by a factor of 1.56. Such a simulation could not be carried out for peanut butter due to few samples above 4 μg/kg, but it was assumed that the factor for peanut butter would be proportional to the factor for peanuts. The bootstrapping approach relied on the assumption that the proportion of non‐compliant samples (based on the reported data) would remain the same after an increase of the ML.
Chronic dietary exposure was calculated for two scenarios namely a ‘Current ML’ and ‘Increased ML’ scenario. The current scenario represents exposure to the current situation with an ML of 4 μg/kg, using the mean occurrence values for AFT in peanuts and peanut butter as reported. The ‘Increased ML’ scenario represents exposure to a simulated market situation with an ML of 10 μg/kg. In this scenario, the mean occurrence values for AFT in both peanuts and peanut butter were multiplied with the calculated increase factors.
Under the ‘Current ML’ scenario, mean chronic exposure estimates, for consumers only, ranged from 0.04 (lowest LB estimate) to 2.74 (highest UB estimate) ng/kg bw per day. If the ML is changed to 10 μg/kg, the exposure estimates increased to 0.07–4.28 ng/kg bw per day, respectively. The highest exposures were calculated for adolescents and other children.
Due to the low number of consumers in most dietary surveys and age classes, a reliable exposure assessment for highly exposed consumers was not possible. Approximate estimations for the adult population indicated that exposure of high consumers may be two to four times higher compared to the exposure of average consumers.
Based on the cancer potencies estimated by JECFA in 2016, the CONTAM Panel estimated the cancer risk based on the mean cancer potency estimates and a prevalence of HBsAg+ of 0.01%, as well as on the UB potency estimates and a prevalence of HBsAg+ of 5.61%. Under the scenario of the current ML, the cancer risk was estimated to range between 0.001 and 0.213 aflatoxin‐induced cancers per year per 100,000 persons. Under the scenario of the increased ML, it ranged between 0.001 and 0.333 aflatoxin‐induced cancers per year per 100,000 persons.
According to the WHO Guideline for drinking‐water quality, an excess lifetime cancer risk of 10–5 or less is considered to be of low risk for public health and, assuming a lifetime expectancy of 70 years, this corresponds to a yearly excess cancer risk of 0.014 additional cancer cases per 100,000 subjects. Comparing the aflatoxin‐induced cancers calculated under the current ML scenario with this yearly excess cancer risk, a higher risk for consumers of peanut and peanut butter is identified in some surveys. The calculated cancer risks indicate that an increase of the ML would further increase the risk by a factor of 1.6–1.8.
The CONTAM Panel recommends that a full risk assessment should be carried out considering the observation of elevated aflatoxin levels in some food commodities originating from European countries and that the last full risk assessment by the CONTAM Panel was carried out in 2007. Knowledge on contamination levels of consignments that were not shipped to the EU due to exceedance of the current ML but would have been shipped if a higher ML was in place, would be needed when assessing the effect on the risk for public health of a possible increase of an ML in the future.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
In Codex Alimentarius, and more specific in the Codex Committee on Contaminants in Food (CCCF), discussions on a maximum level (ML) and associated sampling plan for ‘aflatoxin total’ (AFT) in ready‐to‐eat peanuts are ongoing.
At the 11th session of CCCF in April 2017, it was concluded to request comments on the suggested MLs of 10 or 15 μg/kg for AFT in ready‐to‐eat peanuts.
In preparation of the 11th session of CCCF, the EU had agreed to a position1 whereby the EU objected to the proposed level of 15 μg/kg for the reasons outlined in the position.
As one of the proposed MLs (i.e. 10 μg/kg for AFT in ready‐to‐eat peanuts) is higher than the current EU ML of 4 μg/kg for AFT in ready‐to‐eat peanuts, it is necessary that the European Food Safety Authority (EFSA) assesses if the increase of the current EU ML of 4 μg/kg for AFT in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs to 10 μg/kg of AFT would not result in an unacceptable increase of the risk for public health taking into account vulnerable groups of the population and the EU consumption patterns.
The final possible acceptance by the EU of the ML of 10 μg/kg of AFT in ready‐to‐eat peanuts in Codex Alimentarius Commission shall depend on the outcome of the abovementioned EFSA risk assessment. Specific reference is made to the EFSA risk assessment in the EU position in preparation to the 12th Session of the CCCF.2
1.1.2. Terms of Reference
In accordance with Art. 29 (1) of Regulation (EC) No 178/2002, the European Commission asks the European Food Safety Authority to assess the effect on public health of a possible increase of the ML for AFT from 4 to 10 μg/kg in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs taking into account vulnerable groups of the population and the EU consumption patterns.
1.2. Interpretation of the Terms of Reference
In order to fulfil the terms of reference, the EFSA Panel on Contaminants in the Food Chain (CONTAM) concluded that this opinion should comprise an:
estimation of the influence of a possible increase of the ML for AFT, from 4 to 10 μg/kg in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs, on the dietary exposure of the EU population to AFT from such peanuts, including the consumption patterns of specific groups of the population if appropriate;
assessment of the human health risks for the EU population, including specific groups of the population if appropriate, as the consequence of the estimated dietary exposure.
A re‐evaluation of the toxicological properties of aflatoxins is beyond the scope of this assessment.
1.3. Supporting information for the assessment
Aflatoxins are mycotoxins produced primarily by the fungi Aspergillus flavus and Aspergillus parasiticus. A. parasiticus produces aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1) and aflatoxin G2 (AFG2), whereas A. flavus mainly produces AFB1 and AFB2. However, A. flavus isolates producing AFG1 and AFG2 also have been identified (Moore et al., 2017). When concentrations or MLs mention ‘total’, it refers to the sum of these four aflatoxins and the structures are shown in Figure 1. AFB1 is the most frequently found aflatoxin in contaminated samples and the three others are generally not reported in the absence of AFB1 (FAO/WHO, 2017).
Figure 1.
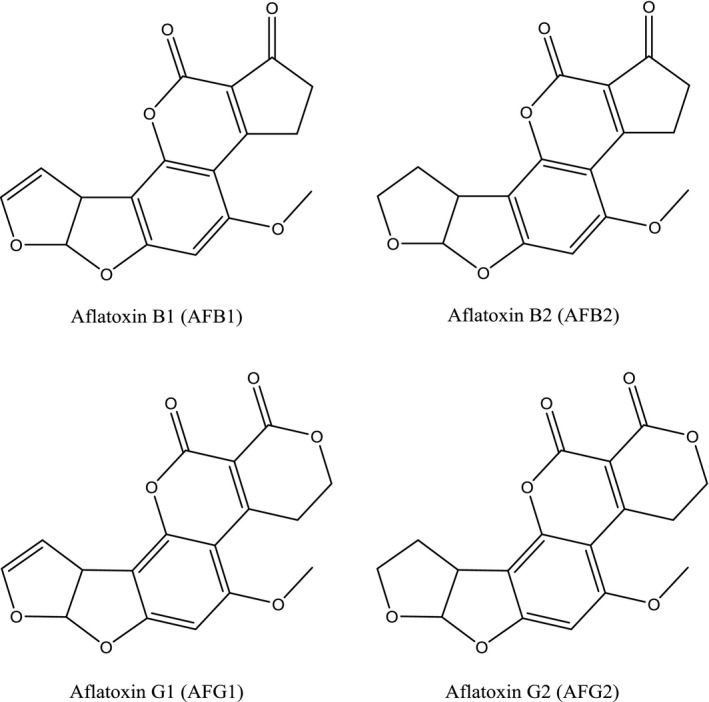
Chemical structures of aflatoxins B1, B2, G1 and G2
The aflatoxin‐producing fungi are especially found in areas with hot, humid climate and aflatoxins are found in food as a result of both pre‐ and post‐harvest fungal contamination (EFSA, 2007a). The rate and degree of contamination depend on temperature, humidity, soil and storage conditions. A. flavus is ubiquitous, favouring the aerial parts of the plants, e.g. leaves and flowers, while A. parasiticus is more adapted to a soil environment and is more limited distributed than A. flavus (EFSA, 2007a).
Findings of aflatoxins have primarily been associated to imported foods such as peanuts, tree nuts, dried fruit, spices, figs, crude vegetable oil, cocoa beans, maize and rice (EFSA, 2007a). However, aflatoxins have in recent years also been found in cereals, especially maize grown in Kosovo, Serbia and Italy (Battilani et al., 2016; Rapid Alert System for Food and Feed3).
Aflatoxins M1 (AFM1) and M2 (AFM2) are the hydroxylated metabolites of AFB1 and AFB2 and can be found in milk or milk products obtained from livestock that have ingested contaminated feed. Therefore, AFM1 and AFM2 were not considered in this opinion.
A wide spectrum of methods have been used for analysis of aflatoxins and the most widely applied methods for quantitative analysis are high‐performance liquid chromatography (HPLC) combined with mass spectrometry (MS) or fluorescence detection (FD) (EFSA, 2007a; FAO/WHO, 2017).
1.3.1. Previous assessments
Aflatoxins have previously been evaluated by EFSA's CONTAM Panel in 2007 when EFSA was asked to advice on the potential increase in risk to consumer health associated with a proposed change of the existing EU ML in almonds, hazelnuts and pistachios (EFSA, 2007a). Aflatoxins were also evaluated at several occasions by JECFA (i.e. at its 46th, 49th, 56th and 68th meetings, and last time at its 83rd meeting in 2016 (FAO/WHO, 2017). The International Agency for Research on Cancer (IARC) evaluated aflatoxins latest in 2012 (IARC, 2012).
Following absorption, aflatoxins undergo first pass metabolism in the liver where they exert their toxicity due to formation of toxic metabolites. The available toxicological knowledge on aflatoxins is mostly related to AFB1. Aflatoxins are genotoxic and cause cancer in the liver, and it is therefore not possible to identify an intake without risk. As only the carcinogenicity of aflatoxins is of concern in this opinion, no further description of other toxic effects occurring at higher doses is given.
Carcinogenicity and mode of action
IARC (2012) classified aflatoxins as a group as carcinogenic to humans (Group 1) causing hepatocellular carcinomas. In experimental animals, both AFB1 and AFG1 are carcinogenic, whereas there is limited evidence for carcinogenicity of AFB2 and inadequate evidence for carcinogenicity of AFG2. AFB1 and AFG1 are mutagenic in bacteria and AFB1 induces point mutations, mitotic recombination in mammalian cells and genetic instability. There is strong evidence that the carcinogenicity is due to a genotoxic mode of action (IARC, 2012). AFB1 is more potent than AFG1 both with respect to mutagenicity and liver carcinogenicity (Wong and Hsieh, 1976), but AFG1 induced a higher incidence of kidney tumours than AFB1 (EFSA, 2007a).
The double bond in the furan ring of AFB1 and AFG1 can be oxidised and form a reactive 8,9‐exo‐epoxide that readily reacts with DNA and other nucleophiles (FAO/WHO, 2017). In DNA, the AFB1‐N7‐guanine is formed resulting in persistent lesions, which may subsequently lead to transversion mutations (IARC, 2012). Detoxication of AFB1 8,9‐exo‐epoxide can take place by several pathways such as hydrolysis, and enzyme‐mediated conjugations with glutathione, glucuronic acid and sulfate, and excretion. In particular, glutathione conjugation of the reactive epoxide catalysed by glutathione S‐transferase (GST) isoforms in the liver appears to be critical and account for interspecies susceptibility to AFB1 carcinogenicity. While mice with high α‐class GST activity are relatively resistant, hepatic GST activity is much less in rats, trout and humans and these species are therefore more susceptible. Monkeys show intermediate activity (IARC, 2012; FAO/WHO, 2017). AFB1 is also directly detoxified by oxidation. Due to human polymorphisms in cytochrome P450 enzymes responsible for detoxicating AFB1 and inactivation of AFB1‐8,9‐exo‐epoxide by GST isoforms, there is also some interindividual variability in susceptibility to AFB1 among humans (EFSA, 2007a; IARC, 2012; FAO/WHO, 2017).
AFB1 dihydrodiol, a hydrolytic product of AFB1 8,9‐epoxide, may bind to lysine residues of proteins forming adducts, i.e. in serum albumin, which is used as a biomarker of aflatoxin exposure in many studies (Guengerich et al., 2002; EFSA, 2007a; FAO/WHO, 2017).
Co‐exposure to hepatitis viruses, in particular hepatitis B has a strong impact on the carcinogenic risk to aflatoxins. In epidemiological studies, there is an interaction with hepatitis B infection, and subjects positive for hepatitis B surface antigen (HBsAg) show at least a multiplicative risk when present together with aflatoxin exposure (FAO/WHO, 2017). The interaction between aflatoxin and hepatitis C remains to be resolved.
Dose–response considerations of carcinogenic risk
In its 49th meeting, JECFA (FAO/WHO, 1999) evaluated a large number of epidemiological studies and identified the Chinese study on mortality from liver cancer by Yeh et al. (1989) as the pivotal study. In this study, the mortality from liver cancer associated with exposure to aflatoxins both in HBsAg+ and HBsAg– individuals were examined. JECFA estimated AFB1 potencies, which corresponded to 0.3 cancers/year per 100,000 subjects per ng AFB1/kg body weight (bw) per day (uncertainty range: 0.05–0.5) in HBsAg+ individuals and 0.01 cancers/year per 100,000 subjects per ng AFB1/kg bw per day (uncertainty range: 0.002–0.03) in HBsAg− individuals.
In 2007, EFSA's CONTAM Panel also considered a large number of epidemiological studies on aflatoxin exposure and hepatocellular carcinoma and identified the liver carcinogenicity of aflatoxins to be the pivotal effect for the risk assessment (EFSA, 2007a). The Panel noted that the available database from experimental animals and epidemiological studies were sufficient for dose‐response modelling of AFB1. The CONTAM Panel considered many studies on aflatoxin and liver cancer in rats and as a result used the 2 years carcinogenicity study in male Fisher rats given AFB1 by Wogan et al. (1974) in its hazard characterisation. A benchmark dose lower confidence limit for an extra cancer risk of 10% (BMDL10) of 170 ng/kg bw per day was calculated. In its assessment of the cancer risk, the CONTAM Panel conducted benchmark dose (BMD) analyses for the Chinese study on mortality from liver cancer (Yeh et al., 1989) and for a group of studies from Africa on risk of liver cancer (Peers et al., 1976 as corrected by Carlborg, 1979; Van Rensburg et al., 1985; Peers et al., 1987). The prevalence of HBsAg+ was 23% in the Chinese cohort, between 21% and 28% for two studies from Africa, and unknown for one study. The CONTAM Panel derived a BMDL10 on a background risk of 10.5% of 870 ng/kg bw per day from the study by Yeh et al. (1989), and a BMDL01 of 78 ng/kg bw per day on a background risk of 0.17–0.50% was derived from the other studies. The CONTAM Panel used these values for the risk characterisation. In addition, cancer rates for adults with a high AFB1 intake were estimated based on cancer potency estimates made by JECFA as referenced above for HBsAg– and HBsAg+ of populations with 0.2% and 7% prevalence of HBsAg+.
At its 83rd meeting in 2016, JECFA reviewed and updated toxicological evidence on aflatoxin hepatocarcinogenicity. JECFA re‐confirmed its previous conclusion that the lifetime dietary study in male F344 rats (Wogan et al., 1974) is the most suitable study in experimental animals for modelling. Male F344 rats appear to be particularly susceptible, and in this study, AFB1 as low as 1 μg/kg diet produced liver tumours. Even lower levels of AFB1 in feed caused liver tumours in rainbow trout. Using a 4‐week exposure period with termination after 1 year showed a hepatotumourigenic response over a dose range of 0.05–110 μg/kg diet in rainbow trout (Williams et al., 2009; Williams, 2012). JECFA (FAO/WHO, 2017) noted that the dose‐related tumourigenesis did not seem to deviate from a log‐linear relationship and that a similar relationship was observed between the dose of AFB1 and AFB1 – DNA adducts in trout and rat liver (Bailey et al., 1998; Pottenger et al., 2014). These observations with doses approaching human exposures lend support to the application of a linear non‐threshold model in AFB1 cancer risk assessment.
JECFA (FAO/WHO, 2017) concluded at its 83rd meeting that the prospective Chinese study by Yeh et al. (1989), that demonstrated a close to linear relationship between aflatoxin exposure and mortality from hepatocellular carcinoma, was still the pivotal study for the risk assessment. The risk was recalculated using a Bayesian model averaging approach, as model uncertainty was a concern. Using this technique, the posterior potency4 is estimated from observed data and prior weighing based on the potential relationship between dose and the response at the low dose range, the posterior weights were then calculated using the Akaike information criterion (AIC) representing each model fit.
In line with the low‐dose linear relationship observed for the animal data, JECFA gave higher weights to the additive linear model of Wu‐Williams et al. (1992) (40%) and the multistage model of Bowers et al. (1993) (40%). The exponential multiplicative of Hoseyni (1992) and the multiplicative model of Wu‐Williams et al. (1992) received a lower weight (10% each). The posterior weights were 47%, 14%, 30% and 9%, respectively. Using model averaging, potency estimates of 0.017 (mean) and 0.049 (95% upper bound (UB)) per 105 person years per ng/kg bw were calculated for HBsAg– individuals. And potency estimates of 0.269 (mean) and 0.562 (95% UB) per 105 person years per ng/kg bw were calculated for HBsAg+ individuals (FAO/WHO, 2017).
JECFA also modelled the rat studies of Wogan et al. (1974) using model averaging of several models down to a risk of 0.001 and used this value to linearly extrapolate further down to a risk of 1 ng/kg bw per day in rats. This risk value for rats was extrapolated to a unit risk for humans using a conversion factor of body weight 0.75, which resulted in a risk of 4.6 (95% confidence interval (CI): 1.3, 74.9) per 105 person years per ng/kg bw (FAO/WHO, 2017).
Exposure assessments
In 2007, the CONTAM Panel assessed the average dietary exposure to AFT truncating the occurrence data to the current EU MLs and using GEMS/Food Consumption Cluster diets data and data from individual surveys (EFSA, 2007a). This assessment included exposure from almonds, hazelnuts, pistachios, other nuts, maize, oilseeds, dried fruits and spices. For adults, this exposure ranged from 0.35 to 1.93 ng/kg bw per day (minimum lower bound (LB)–maximum UB) and for children from 0.56 to 1.91 ng/kg bw per day (minimum LB–maximum UB). Since 2007, no updated assessment of the total dietary exposure to AFT has been carried out at the level of the European Union.
In 2016, JECFA calculated international estimates of chronic dietary exposure using the food consumption data from the GEMS/Food cluster diets and a standard body weight of 60 kg (FAO/WHO, 2017). The calculations covered the exposure from cereals and pseudocereals, tree nuts and groundnuts, spices, figs and soy (Lipp, 2018). The mean UB dietary AFT exposure ranged from 1.3 ng/kg bw per day (cluster G08, including Austria, Germany, Poland and Spain) to 34.8 ng/kg bw per day (cluster G13, including African countries and Haiti). JECFA reported that a similar pattern of exposure was observed under the LB scenario. The dietary exposure for a high consumer was considered to be twice the mean dietary exposure. Wheat was the main contributor to the UB dietary AFT exposure (range 37–76.5%) for several countries, including many European countries. However, for cluster G10 (including European countries such as Italy, Bulgaria, Estonia, Latvia and Lithuania), rice was the main contributor to the UB dietary AFT exposure (range 34.5–80.3%). No information was provided regarding the major contributors to the LB dietary AFT exposure. Based on these calculations and on national estimates, JECFA concluded that with the exception of very high estimates of dietary exposure to AFT for some African countries (105–850 ng/kg bw), all mean dietary AFT exposure was in the range < 0.01–58 ng/kg bw per day with high consumer estimates in the range < 0.01–200 ng/kg bw per day. Considering the different foods included in the exposure assessment, a direct comparison with the results generated by the CONTAM Panel in 2007 is difficult.
JECFA also performed an impact assessment of the implementation of different MLs in ready‐to‐eat peanuts. The application of an ML of 15 μg/kg for ready‐to‐eat peanuts reduced the chronic UB dietary exposure to AFT by a maximum of 20%. A further reduction of the ML to 4 μg/kg resulted in a negligible additional reduction in dietary exposure. Based on this assessment, JECFA concluded that ‘enforcing an ML of 10, 8 or 4 μg/kg for ready‐to‐eat peanuts would have little further impact on dietary exposure to AFT for the general population, compared with setting an ML of 15 μg/kg’ (FAO/WHO, 2017).
Risk characterisation
In 2007, the CONTAM Panel calculated margins of exposure (MOEs) based on both BMDL10 and BMDL01 values derived from the epidemiological data and the BMDL10 value derived from the animal data. When evaluating AFT, the CONTAM Panel took into account that AFG1 and AFB2 were also shown to be carcinogenic in rodents and assumed that the carcinogenic potency of AFT would be similar to that of AFB1. The Panel (EFSA, 2007a) considered this to be a conservative approach. The MOEs based on the BMDL10 from the animal data were considered to indicate a potential concern for human health. The BMDLs from the epidemiological studies on populations with a high rate of HBsAg+ indicated a sensitivity similar to that of the sensitive rats. However, other subgroups were considered likely to be less sensitive. The CONTAM Panel concluded that changing the ML for AFT from 4 to 8 or 10 μg/kg in almonds, hazelnuts and pistachios would have minor effects on estimates of dietary exposures, calculated MOEs and cancer risk.
JECFA calculated at its 83rd meeting the cancer risk associated with estimated aflatoxin exposure in different regions and concluded that the lowest cancer risks were estimated for clusters G07 and G08, which include European and other developed countries. The cancer risk estimates for these clusters ranged from < 0.01 to 0.1 aflatoxin‐induced cancers per year and per 100,000 subjects. The highest cancer risk was estimated for cluster G13 (sub‐Saharan African countries and Haiti) and ranged from 0.21 to 3.94 aflatoxin‐induced cancers per year and per 100,000 subjects (FAO/WHO, 2017).
1.3.2. Legislation
In this opinion, where reference is made to Regulations, the reference should be understood as relating to the most recent amendment, unless otherwise stated.
In order to protect public health, Article 2 of Council Regulation (EEC) No 315/935 of 8 February 1993 laying down Community procedures for contaminants in food stipulates that, where necessary, maximum tolerances for specific contaminants shall be established. Subsequently, a number of MLs for aflatoxins and other mycotoxins in various foodstuffs were laid down in the Annex, Section 2 of Commission Regulation (EC) No. 1881/20066 of 19 December 2006 setting MLs for certain contaminants in foodstuffs. The MLs for aflatoxins are set following the principle of ‘as low as reasonably achievable’ (ALARA), derived from the frequency distribution of the respective food classes (usually at the 90–95th percentile), taking into account the outcome of the risk assessment and the analytical capabilities.
The currently applicable MLs for aflatoxins in peanuts are shown in Table 1. The MLs refer to the edible part of peanuts. If peanuts ‘in shell’ are analysed, it is assumed when calculating the aflatoxin content all the contamination is on the edible part.
Table 1.
EU maximum levels for aflatoxins (μg/kg) in peanuts
| Foodstuff | Aflatoxin B1 | Sum of aflatoxins B1, B2, G1 and G2 |
|---|---|---|
|
Peanuts, to be subjected to sorting, or other physical treatment, before human consumption or use as an ingredient in foodstuffs, with the exception of:
|
8.0 | 15.0 |
|
Peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs, with the exception of:
|
2.0 | 4.0 |
According to Article 1 of Commission Regulation (EC) No 1881/2006, foodstuffs shall not be placed on the market where they contain aflatoxins at a level exceeding the MLs. Article 3 of the Regulation stipulates that foodstuffs not complying with the MLs shall not be used as food ingredients and/or shall not be mixed with foodstuffs complying with the MLs.
Foodstuffs to be subjected to sorting or other physical treatment to reduce aflatoxin levels shall not be mixed with foodstuffs intended for direct human consumption or with foodstuffs intended for use as a food ingredient. Moreover, foodstuffs containing aflatoxins shall not be deliberately detoxified by chemical treatments.
The following specific provisions for peanuts are laid down in Articles 4 and 5 of Commission Regulation (EC) No 1881/2006. According to Article 4, peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs not complying with the respective MLs can be placed on the market provided that these foodstuffs:
are not intended for direct human consumption or use as an ingredient in foodstuffs;
comply with the MLs for peanuts, to be subjected to sorting, or other physical treatment, before human consumption or use as an ingredient in foodstuffs;
are subjected to a treatment involving sorting or other physical treatment and that after this treatment the MLs for peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs are not exceeded, and this treatment does not result in other harmful residues are labelled clearly showing their use, and bearing the indication ‘product shall be subjected to sorting or other physical treatment to reduce aflatoxin contamination before human consumption or use as an ingredient in foodstuffs’. The indication shall be included on the label of each individual bag, box, etc., and on the original accompanying document. The consignment/batch identification code shall be indelibly marked on each individual bag, box, etc., of the consignment and on the original accompanying document.
Article 5 of Commission Regulation (EC) No 1881/2006 states that in the absence of a clear indication that their intended use is not for human consumption, the MLs for peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs shall apply to all peanuts and derived products thereof placed on the market.
As regards the exception of peanuts for crushing and the application of the respective MLs, the exception only applies to consignments which are clearly labelled showing their use and bearing the indication ‘product to be subject to crushing for the production of refined vegetable oil’.
Specific criteria for sampling and analysis of aflatoxins in peanuts are specified in Commission Regulation (EC) No 401/20067 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. The Regulation specifies inter alia the number and weight of samples to be collected in dependence on the size of the lot and the place of sampling. A lot or sublot is only accepted if the laboratory sample is conform to the MLs, taking into account the correction for recovery and measurement uncertainty. On the other hand, a commodity is rejected if the laboratory sample exceeds the ML beyond reasonable doubt taking into account the correction for recovery and measurement uncertainty.
Further requirements are laid down in this Regulation for sample preparation, including treatment of the samples in the laboratory, calculation of proportion of shell/kernel of whole nuts and preparation of replicate samples for enforcement, trade (defence) and reference (referee) purposes. Finally, Regulation (EC) 401/2006 defines the analytical criteria, such as recovery, repeatability and reproducibility, which a method has to fulfil for the official control on the levels of aflatoxins in foodstuffs.
Commission Regulation (EC) No 669/20098 lays down rules concerning the increased level of official controls to be carried out at the point of entry on imports of specific feed and food commodities of non‐animal origin. It provides inter alia that the competent authorities at the designated point of entry shall carry out with undue delay documentary checks, identity and physical checks, including laboratory analysis at an increased frequency rate of 50% for peanuts in shell, peanuts shelled, peanut butter and peanuts otherwise prepared or preserved from Bolivia, Gambia, Madagascar, Senegal and Sudan. A release for free circulation within the EU is subject to favourable results from the required checks.
Commission Implementing Regulation (EU) No 884/20149 imposes special conditions governing the import of feed and food from various third countries due to contamination risk by aflatoxins. The Regulation applies inter alia to the import of peanuts in shell and shelled, peanut butter and peanuts otherwise prepared or preserved (food and feed) originating in or consigned from China, Egypt, Ghana, India, Brazil and Argentina. Each consignment must be accompanied by a specific health certificate, and the results of sampling and analysis performed by the competent authorities of the country of origin, or of the country where the consignment is consigned from if that country is different from the country of origin, to ascertain compliance with Union legislation on MLs of aflatoxins. The competent authorities at the designated points of import must carry out an identity check and a physical check by taking a sample for analysis of AFB1 for feed or AFB1 and AFT contamination for food on the above‐mentioned consignments from Argentina at a frequency of 5%, from India and Brazil at a frequency of 10%, from China and Egypt at a frequency of 20%, and from Ghana at a frequency of 50%.
Codex Alimentarius10 has set an ML of 15 μg/kg for aflatoxins in peanuts intended for further processing. The ML is accompanied by requirements for sampling depending on the size of the lot, and performance criteria for the analytical determination which are quite similar to the European Regulation. In contrast, the Codex Standard does not stipulate an ML for peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs.
2. Data and methodologies
2.1. Hazard identification and characterisation, and supporting information for the assessment
2.1.1. Collection, selection of evidence
Given the short deadline of this request, previous assessments relevant for the current risk assessment were used i.e. FAO/WHO (1999, 2008, 2017) and EFSA (2007a, 2009). No additional search for scientific literature was conducted.
2.1.2. Appraisal of evidence
The information retrieved was screened and evaluated by relevant domain experts from the CONTAM working group (WG) on ML of aflatoxins in peanuts and was used for the present assessment.
2.2. Occurrence data submitted to EFSA
2.2.1. Data collection and validation
For this statement, EFSA relied on occurrence data on aflatoxins in peanuts, peanut butter and peanut oil available in the EFSA Chemical Occurrence database. These data were collected through the continuous call for data which includes aflatoxins. Only samples in which AFB1, AFB2, AFG1 and AFG2 were analysed, were used in the present assessment.
The data submission to EFSA followed the requirements of the EFSA Guidance on Standard Sample Description for Food and Feed (EFSA, 2010a); occurrence data were managed following the EFSA standard operational procedures (SOPs) on ‘Data collection and validation’ and on ‘Data analysis of food consumption and occurrence data’.
All data on aflatoxins in peanut, peanuts butter and peanut oil available in the EFSA database by 29 September 2017 were extracted and further considered. Data received after this date were not included.
2.2.2. Data analysis
A detailed quality control of the occurrence data was performed in order to identify duplicates, to identify potential errors in the coding of the different samples under FoodEx classification and to ensure the overall comparability of the data. Upon identification of potential inconsistencies, data providers were contacted to provide further clarification. The outcome of the data analysis is shown in Section 3.2.1.
For analytical results of the four individual aflatoxins, the left‐censored data (analytical data below the limit of detection (LOD)/limit of quantification (LOQ)) were treated by the substitution method as recommended in the ‘Principles and Methods for the Risk Assessment of Chemicals in Food’ (WHO/IPCS, 2009). The same method is described in the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010b), as an option in the treatment of left‐censored data. The guidance suggests that the LB and UB approach should be used for chemicals likely to be present in the food (e.g. naturally occurring contaminants, nutrients and mycotoxins). At the LB, results below the LOQ or LOD were replaced by zero; at the UB, the results below the LOD were replaced by the numerical value of the LOD and those below the LOQ were replaced by the numerical value of the LOQ. Additionally, a middle bound (MB) approach was used by assigning a value of LOD/2 or LOQ/2 to the left‐censored data.
In addition, occurrence values for AFT were calculated from the analytical results of the individual aflatoxins. Considering that AFB1 is the aflatoxin that is most frequently found and at the highest concentration, and that not all aflatoxin producing moulds produce all four aflatoxins, simply adding the four LOQ values for samples in which none of the aflatoxins are quantified, would overestimate the UB AFT level. Therefore, the concentration of AFT was calculated for each sample following the principles listed below:
when quantified results were available for all four aflatoxins, the concentration of AFT was calculated as the sum of all concentrations;
when the results for all four aflatoxins were left‐censored, the UB concentration of AFT was calculated as twice the LOD/LOQ for AFB1 (the main contributor) unless the sum of the four LOD/LOQ was lower;
when there were both, quantified and left‐censored results, the UB concentration of AFT was calculated as the sum of quantified values and twice the LOD/LOQ for AFB1, unless the sum of the quantified values and the LOD/LOQ of the unquantified aflatoxins was lower.
2.3. Food consumption data
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database) provides a compilation of existing national information on food consumption at individual level. It was first built in 2010 (EFSA, 2011a; Huybrechts et al., 2011; Merten et al., 2011). Details on how the Comprehensive Database is used are published in the Guidance of EFSA (EFSA, 2011a). The latest version of the Comprehensive Database11 was used with subjects classified in different age classes as follows:
Infants: < 12 months old
Toddlers: ≥ 12 months to < 36 months old
Other children: ≥ 36 months to < 10 years old
Adolescents: ≥ 10 years to < 18 years old
Adults: ≥ 18 years to < 65 years old
Elderly: ≥ 65 years to < 75 years old
Very elderly: ≥ 75 years old.
Two additional surveys provided information on specific population groups: ‘Pregnant women’ (≥ 15 years to ≤ 45 years old; Latvia) and ‘Lactating women’ (≥ 28 years to ≤ 39 years old; Greece).
Overall, the food consumption data gathered by EFSA in the Comprehensive Database are the most complete and detailed data currently available in the EU. Consumption data were collected using single or repeated 24‐ or 48‐h dietary recalls or dietary records covering from 3 to 7 days per subject. As a result of the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading.
2.4. Food classification
Consumption data were classified according to the FoodEx classification system (EFSA, 2011b). FoodEx is a food classification system developed by EFSA in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing the exposure to hazardous substances. It contains 20 main food groups (first level), which are further divided into subgroups having 140 items at the second level, 1,261 items at the third level and reaching about 1,800 items (food names or generic food names) at the fourth level.
2.5. Methodology to assess the influence of increasing the maximum level on the mean concentration of ‘aflatoxin total’
No data were available regarding consignments that were not shipped to the EU due to levels exceeding the current ML of 4 μg/kg but would have been placed on the EU market if a higher ML was in place. Therefore, the impact of increasing the ML from 4 to 10 μg/kg on the mean concentration of AFT in peanuts was assessed using a simulation approach by bootstrapping. New data sets, of equal size to the observed data set, were generated by selecting (with replacement) samples from the observed data set under the constraint that the proportion of non‐compliant samples would remain the same after the increase of the ML. It should be noted that in the context of this Scientific Opinion, the measurement uncertainty was not taken into account to decide whether a sample is non‐compliant. This approach was implemented in the SAS software (version 9.4) as follows:
-
n concentration values were randomly drawn with replacement, where n is the total number of concentration values available in the initial data set, as follows
-
1
– n p values were drawn from the observed values between 10 μg/kg and the maximum AFT concentration, where p is the proportion of non‐compliance in the initial data set,
-
2
– n p p’/(1 − p’) values were drawn from the observed values between 4 and 10 μg/kg, where p’ is the proportion of samples between 4 and 10 μg/kg among the total number samples above 4 μg/kg estimated from the initial data set,
-
3
– n–n p–n p p’/(1 − p’) values were drawn from the observed values below 4 μg/kg.
-
1
The LB/MB/UB mean AFT concentration was estimated from the simulated data set and divided by the LB/MB/UB mean AFT concentration estimated from the initial data set to calculate the increase factor.
This procedure was repeated 1,000 times and the mean increase factor was calculated with its 95% CI. In a sensitivity analysis, the number of iterations was increased by a factor of 10–10,000, demonstrating that 1,000 iterations were sufficient to fully reflect the variability of the reported data set.
2.6. Exposure assessment
The CONTAM Panel considered that only chronic dietary exposure had to be assessed. As suggested by the EFSA WG on Food Consumption and Exposure (EFSA, 2011a), dietary surveys with only 1 day per subject were excluded from the current assessment because they are not adequate to assess repeated exposure. Similarly, subjects who participated only 1 day in the dietary studies, when the protocol prescribed more reporting days per individual, were also excluded from the chronic exposure assessment. When, for one particular country and age class, two different dietary surveys were available only the most recent one was used.
For calculating the chronic dietary exposure, food consumption and body weight data at the individual level were accessed in the Comprehensive Database. Occurrence data and consumption data for peanuts and relevant processed products were matched to one another. For each individual of the selected surveys, the average daily consumption of the different food items were combined with the mean occurrence values of the corresponding food samples collected (pooled European occurrence data), and the resulting exposures per food were summed in order to obtain the total chronic exposure at individual level (standardised by using the individual body weight). The mean of the individual exposures were subsequently calculated for each dietary survey and age class having at least five consumers. The 95th percentile exposure was calculated for survey and age class combinations with 61 or more consumers.
Before matching the consumption data to the corresponding occurrence data, foods that may contain peanuts were identified. Based on the available data for corresponding recipes and/or commercial products, those foods were assigned a given content of peanuts, together with a probability for that food to actually contain peanuts. A number of consumption events was selected at random for each of these foods (proportional to the above mentioned probability) and converted to the corresponding amount of peanut or its primary derivative.
2.7. Risk characterisation
The CONTAM Panel applied the general principles of the risk characterisation process for chemicals in food as described by WHO/IPCS (2009) and the relevant EFSA guidance documents (see Appendix A).
3. Assessment
3.1. Hazard identification and characterisation
Given the short deadline of the current request, no toxicological evaluation was performed by the CONTAM Panel. Instead, the Panel decided to base the assessment on the recent JECFA evaluation and used the study by Yeh et al. (1989) for the risk characterisation (FAO/WHO, 2017).
The CONTAM Panel considered the possibility to apply an MOE approach based on a BMDL value calculated from the models used by JECFA to calculate the cancer potency from the study by Yeh et al. (1989)Yeh et al. (FAO/WHO, 2017; see Section 1.3.1 for further details). In contrast to the evaluation in 2007 (see Section 1.3.1), the CONTAM Panel took the HBsAg status into account in the BMD analyses and used model averaging (EFSA Scientific Committee, 2017). However, the BMD analysis showed that based on the dose ranges studied, a very small trend in the dose–response curve was observed for HBsAg‐ subjects (see Appendix B) which results in wide BMD confidence intervals. Therefore, BMD confidence interval calculation was not appropriate and the CONTAM Panel decided to use the cancer potency estimates reported by JECFA (FAO/WHO, 2017) in the present assessment.
3.2. Occurrence data
3.2.1. Occurrence data on food submitted to EFSA
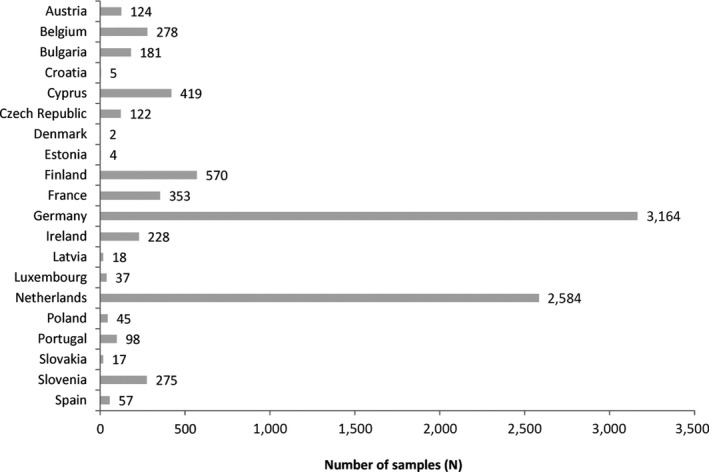
The data for the present assessment were provided by national authorities from Austria, Belgium, Bulgaria, Croatia, Cyprus, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Ireland, Latvia, Luxembourg, the Netherlands, Poland, Portugal, Slovakia, Slovenia and Spain (Figure 2).
Figure 2.
Number of samples reported by Member States
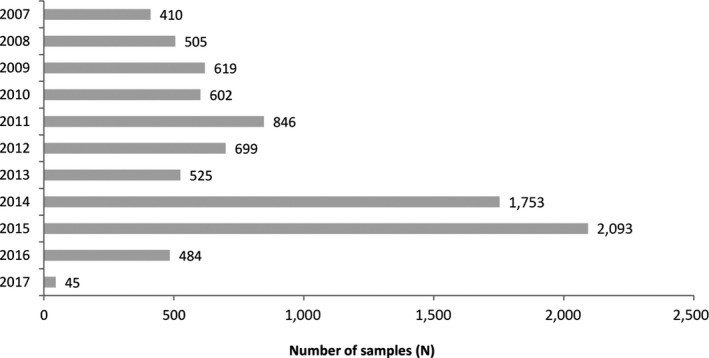
An initial number of 36,667 analytical results on aflatoxins in food were available in the EFSA database for AFB1, AFB2, AFG1 or AFG2 in peanuts, peanut butter and peanut oil collected throughout the years 2007–2017 (Figure 3).
Figure 3.
Number of samples submitted by year
These data were subject to the validation processes described in Section 2.2.1 and data providers were invited to clarify possible inconsistencies identified by EFSA's DATA Unit.
Furthermore, the overall data set was carefully analysed before being used to estimate dietary exposure and a total of 2,393 records were excluded from the final data set. Criteria for exclusion of these analytical results are also detailed in Annex A, Table A.1.
The data set was also analysed for possible correlations between mean concentration, sampling year and sampling strategy. As no specific correlations could be identified, the CONTAM Panel decided not to exclude any samples on the basis of the sampling strategy or sampling year.
In total, 34,324 analytical results for AFB1, AFB2, AFG1 and AFG2 were taken into account, which corresponds to 8,581 samples. Samples were taken in 20 Member States (Figure 2) throughout the years 2007–2017 (Figure 3).
Detailed summary statistics on the occurrence data are presented in Annex A, Table A.2. Most of the data as collected had AFT concentrations less than 10 μg/kg. The highest value in peanuts was 1,429 μg/kg and the highest value for peanut butter was 406 μg/kg. All results in peanut oil (n = 14) were left‐censored. The occurrence data for AFB1 and AFT in peanuts and peanut butter are summarised in Table 2.
Table 2.
Summary statistics of aflatoxin B1 and ‘aflatoxin total’ concentrations (μg/kg) in peanut and peanut butter
| Food group | N | Substance | LC (%) | Percentile | Concentration (μg/kg) | ||
|---|---|---|---|---|---|---|---|
| LB | MB | UB | |||||
| Peanut | 8,095 | AFB1 | 87.2 | Mean | 2.03 | 2.23 | 2.43 |
| P50 | 0 | 0.10 | 0.20 | ||||
| P95 | 4.40 | 4.40 | 4.40 | ||||
| AFT | 86.6 | Mean | 2.65 | 3.11 | 3.56 | ||
| P50 | 0 | 0.30 | 0.50 | ||||
| P95 | 5.20 | 5.80 | 6.40 | ||||
| Peanut butter | 472 | AFB1 | 64.2 | Mean | 1.07 | 1.16 | 1.25 |
| P50 | 0 | 0.10 | 0.20 | ||||
| P95 | 2.70 | 2.70 | 2.70 | ||||
| AFT | 63.8 | Mean | 1.47 | 1.69 | 1.92 | ||
| P50 | 0 | 0.20 | 0.40 | ||||
| P95 | 3.50 | 3.70 | 3.90 | ||||
AFB1: aflatoxin B1; AFT: ‘aflatoxin total’; LB: lower bound; LC: left‐censored; MB: middle bound; P50: 50th percentile; P95: 95th percentile; UB: upper bound.
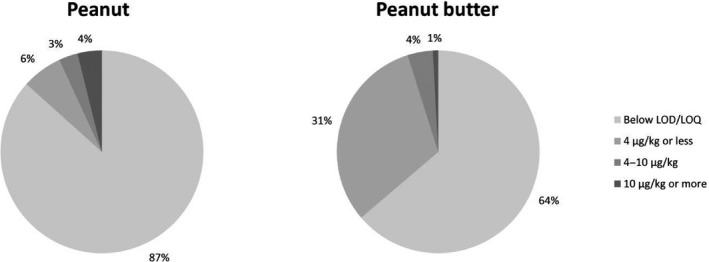
Figure 4 shows the percentage of samples for peanuts and peanut butter for different concentration ranges, which were defined by the current and suggested ML. From the 8,095 peanut samples for which the concentration of AFT was calculated, 87% of the samples had a AFT concentration below the LOD/LOQ, 6% of the samples had a concentration of 4 μg/kg or less, 3% had a concentration between 4 and 10 μg/kg and 4% of the samples had a concentration exceeding the suggested ML of 10 μg/kg. For peanut butter, the percentage of quantified results was higher, but the fractions exceeding either 4 or 10 μg/kg were lower.
Figure 4.
Comparison of ‘aflatoxin total’ concentrations with current and proposed maximum level for peanuts and peanut butter
- LOD: limit of detection; LOQ: limit of quantification.
The relative contribution of AFB1 to the AFT concentration was calculated for all samples of peanut and peanut butter where measurable amounts of AFB1 were reported (see Table 3). On average, AFB1 contributed for more than 60% to the MB concentration of AFT. It was noted that the contribution of AFB1 to AFT increased with increasing concentration of AFB1. Hence, if the ML of AFT would increase from 4 to 10 μg/kg, this would also have an impact on the number of samples exceeding the current ML of AFB1.
Table 3.
Contribution of aflatoxin B1 to the ‘aflatoxin total’ middle bound concentration in all samples of peanut and peanut butter where measurable amounts of aflatoxin B1 were reported
| Food group | Concentration AFB1 | N | Mean | Percentile | ||||
|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | ||||
| Peanut | 1 μg/kg or less | 252 | 61.2 | 23.2 | 50.0 | 66.7 | 76.9 | 85.2 |
| 1–10 μg/kg | 521 | 71.7 | 34.6 | 63.0 | 75.9 | 84.2 | 91.3 | |
| 10 μg/kg or more | 260 | 78.4 | 34.0 | 77.9 | 83.6 | 87.8 | 92.9 | |
| Peanut butter | 1 μg/kg or less | 114 | 66.5 | 45.7 | 60.0 | 66.7 | 74.7 | 85.2 |
| 1–10 μg/kg | 53 | 66.0 | n/aa | 53.8 | 67.3 | 75.5 | n/a | |
| 10 μg/kg or more | 2 | 75.3 | n/a | n/a | 75.3 | n/a | n/a | |
AFB1: aflatoxin B1; N: Number of Samples; n/a: not available.
Too limited number of samples to calculate percentile.
Available occurrence data were mostly generated with HPLC combined with FD (53%), MS (30%) or tandem mass spectrometry (MS/MS, 3%), which are generally capable of quantifying AFB1, AFB2, AFG1 and AFG2 at the concentrations of interest. For 14% of the samples, a detailed analytical method was not reported by the data provider but, based on the analytical methods reported by the same data providers over the different years, these samples were most likely analysed using HPLC‐FD. The reported LOQs for peanut and peanut butter are plotted in Figure 5.
Figure 5.
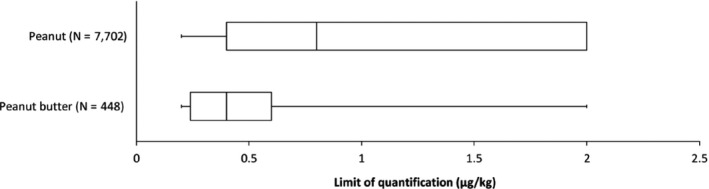
Distribution of the reported limits of quantification for ‘aflatoxin total’ in μg/kg
- Box‐plot: whiskers at 5th percentile and 95th percentile, box at 25th percentile and 75th percentile with line at 50th percentile; N: number of samples.
3.2.2. Food processing
Processes applied on foods such as heating, roasting and baking can reduce the levels of aflatoxins but do not completely eliminate the toxins, and the degree of elimination is variable and depends on the process as well as the conditions under which the process is applied (FAO/WHO, 2017). Roasting of peanuts reduces the aflatoxin level by 50–80% (FAO/WHO, 1999). During the processing of oil, levels of aflatoxins also decrease. Studies have shown that crude peanut oil contains less aflatoxins than the raw peanuts, while refined oil contains only small amounts of aflatoxins (Parker and Melnick, 1966; Abalaka and Elegbede, 1982; Abalaka, 1984; Bordin et al., 2014). For example, Parker and Melnick (1966) found that after processing a sample of peanuts to refined oil, the amount of AFB1 decreased from 5,500 μg/kg in the peanuts to a concentration of less than 1 μg/kg in the oil.
3.3. Influence of increasing the maximum level
3.3.1. Influence on the mean aflatoxin level in peanuts and products derived thereof
No data were available regarding consignments that were not shipped to the EU due to levels exceeding the current ML of 4 μg/kg but would have been placed on the EU market if a higher ML was in place. Therefore, the impact of increasing the ML from 4 to 10 μg/kg was assessed according to the methodology described in Section 2.5. As all samples for peanut oil were left‐censored and the number of samples in peanut butter with concentrations above 10 μg/kg was too small for bootstrap analysis (only four samples), this simulation was carried out for peanuts only. It should be noted that in the context of this Scientific Opinion, the measurement uncertainty was not taken into account to decide whether a sample is non‐compliant.
The number of samples in the original and simulated data sets are depicted in Figure 6. The highest AFT concentration observed in peanuts was 1,429 μg/kg.
Figure 6.
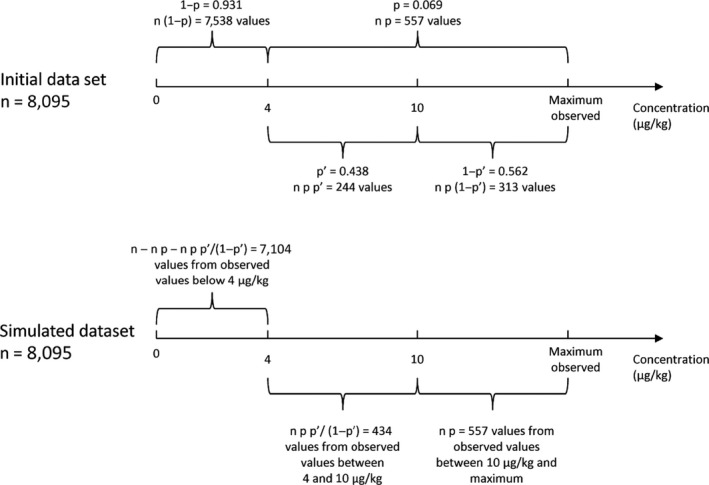
Illustration of the methodology to simulate the data set on the occurrence of aflatoxins after the increase of the maximum level
- n = number of samples; p = probability of non‐compliance with the maximum level; p’ = probability of a concentration between 4 and 10 μg/kg when non‐compliant with the current maximum level.
As a result of this analysis, it was estimated that changing the ML for AFT from 4 to 10 μg/kg in peanuts, would increase the LB, MB and UB mean AFT concentrations by factors of 1.75 (95% CI = 1.51–2.03), 1.64 (95% CI = 1.44–1.88) and 1.56 (95% CI = 1.38–1.77), respectively.
3.3.2. Influence of increasing the maximum level on chronic dietary exposure from peanuts and peanut butter
Due to time constraints, an assessment of the total dietary exposure to AFT (considering all food sources) was not considered feasible. Furthermore, considering that extraction and refining of oil may reduce the concentrations of aflatoxins considerably, that the ML under assessment does not apply to peanut oil and that available occurrence data for peanut oil were all left‐censored, peanut oil was not included in the exposure assessment.
Chronic dietary exposure to AFT in peanuts and peanut butter was hence estimated across Europe using the methodology described in Section 2.6. A total of 35 dietary surveys, carried out in 19 different Member States, were selected for this assessment. These dietary surveys, the number of subjects and the number of consumers available per age class are described in Annex A, Table A.3. Prior to the identification of the peanut and peanut butter consumers, eating occasions referring to food items that may contain peanut or peanut butter were converted assuming the contents and probabilities reported in Annex A, Table A.4. Consumers of peanuts and peanut butter represent only on average 7% (range: 0–36%) of the total population (see Annex A, Table A.3); therefore, the CONTAM Panel concluded that exposure estimates for consumers only were the most adequate.
The impact of increasing the ML on chronic dietary exposure was assessed by presenting two different exposure scenarios:
the ‘Current ML’ scenario represents exposure to the current market situation with an ML of 4 μg/kg, using the mean occurrence values for AFT in peanuts and peanut butter (see also Table 2).
the ‘Increased ML’ scenario represents exposure to a simulated market situation with an ML of 10 μg/kg. In this scenario, the mean occurrence values for AFT in both peanuts and peanut butter were multiplied with the increase factors reported for peanuts in Section 3.3.1. In the absence of a specific increase factor for peanut butter, it was assumed that the increase of aflatoxin levels in peanut butter would be proportional to the increase anticipated for peanuts.
Table 4 summarises the mean chronic dietary exposure estimates across surveys for both exposure scenarios. Detailed summary statistics on the exposure estimates calculated for each dietary survey are presented in Annex A, Table A.5. While 95th percentile estimates require a minimum of 61 observations to be considered as statistically robust, mean estimates would normally require a minimum of five observations (EFSA, 2011a). Survey and age class combinations having less than five consumers of peanuts or peanut products are therefore not presented.
Table 4.
Mean chronic dietary exposure to ‘aflatoxin total’ from peanuts, peanut butter and processed products thereof (consumers only) expressed in ng/kg bw per day across European dietary surveys, assuming two different scenarios
| Age classa | Scenario | N | Mean dietary exposure (ng/kg bw per day)b | |||||
|---|---|---|---|---|---|---|---|---|
| Minimumc | Medianc | Maximumc | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Infants | Current ML | 2 | 0.04 | 0.05 | 0.16 | 0.21 | 0.27 | 0.36 |
| Increased ML | 2 | 0.07 | 0.08 | 0.28 | 0.32 | 0.48 | 0.56 | |
| Toddlers | Current ML | 5 | 0.39 | 0.51 | 0.70 | 0.94 | 1.27 | 1.66 |
| Increased ML | 5 | 0.68 | 0.80 | 1.22 | 1.46 | 2.22 | 2.59 | |
| Other children | Current ML | 16 | 0.30 | 0.40 | 0.81 | 1.07 | 1.69 | 2.27 |
| Increased ML | 16 | 0.53 | 0.63 | 1.42 | 1.67 | 2.95 | 3.53 | |
| Adolescents | Current ML | 14 | 0.13 | 0.18 | 0.52 | 0.69 | 2.06 | 2.74 |
| Increased ML | 14 | 0.23 | 0.28 | 0.91 | 1.07 | 3.61 | 4.28 | |
| Adults | Current ML | 17 | 0.16 | 0.21 | 0.50 | 0.67 | 1.25 | 1.68 |
| Increased ML | 17 | 0.28 | 0.33 | 0.88 | 1.05 | 2.19 | 2.63 | |
| Elderly | Current ML | 12 | 0.07 | 0.10 | 0.34 | 0.46 | 0.74 | 0.99 |
| Increased ML | 12 | 0.13 | 0.15 | 0.60 | 0.71 | 1.29 | 1.54 | |
| Very elderly | Current ML | 4 | 0.21 | 0.28 | 0.36 | 0.47 | 0.73 | 0.97 |
| Increased ML | 4 | 0.37 | 0.44 | 0.62 | 0.74 | 1.27 | 1.52 | |
bw: body weight; LB: lower bound; ML: maximum level; N: number of surveys; UB: upper bound.
Section 2.4 describes the age range within each age class.
The mean estimates obtained on dietary surveys/age classes with less than five observations (consumers) may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
Estimates were rounded to two decimal places.
The highest mean chronic exposures (for consumers only) were calculated for adolescents and other children. Among the highest UB exposures for adolescents, the ‘Current ML’ scenario amounted to 2.74 ng/kg bw per day, whereas the ‘Increased ML’ scenario amounted to 4.28 ng/kg bw per day. Due to the smaller contribution of left‐censored data, the difference between LB and UB estimates was found to be smaller for the ‘Increased ML’ scenario (15–20%) compared to the ‘Current ML’ scenario (30–35%).
Regarding the highly exposed consumers within a given dietary survey and age class, only few surveys contained sufficient consumers to derive a reliable 95th percentile exposure. For adults, the only age class where a sufficient number of 95th percentiles could be derived, exposure of high consumers was found to be two to four times higher compared to the exposure of average consumers, but it is not clear whether this ratio is also applicable to the most exposed age classes such as adolescents and other children. The CONTAM Panel therefore concluded that a reliable exposure assessment for high consumers cannot be carried out, taking note of the uncertainty.
3.4. Risk characterisation
Considering that AFB1 is the major contributor to the exposure to AFT and that AFB1 is the most potent aflatoxin, the CONTAM Panel used a conservative approach and used the cancer potency estimates calculated for AFB1 for the risk characterisation of AFT. It should be noted that the exposure estimates calculated in this Scientific Opinion refer to consumers of peanuts and peanut butter only. Consumers of peanuts and peanut butter represent on average 7% (range: 0–36%) of the total population (see Section 3.3.2).
Using model averaging, JECFA calculated potency estimates of 0.017 (mean) and 0.049 (95% UB) per 105 person years per ng/kg bw for HBsAg– individuals and 0.269 (mean) and 0.562 (95% UB) per 105 person years per ng/kg bw for HBsAg+ individuals12 (FAO/WHO, 2017; see Section 1.3.1 for further details).
According to Schweitzer et al. (2015), the HBsAg seroprevalence ranges between 0.01% and 5.61% in EU Member States. The lowest seroprevalence of 0.01% was reported for the UK and the highest for Romania. No data were presented for Estonia, Latvia, Luxembourg, Malta and Finland. The overall prevalence is 2.06% for the European region.
Based on the mean potency estimates and a prevalence of HBsAg of 0.01%, the CONTAM Panel estimated the cancer risk due to peanut and peanut butter consumption, under the scenario of the current ML, to be between 0.001 and 0.047 aflatoxin‐induced cancers per year per 100,000 persons, across dietary surveys and age groups (Table 5). Under the scenario of the increased ML, it ranged between 0.001 and 0.073 aflatoxin‐induced cancers per year per 100,000 persons, across dietary surveys and age groups. In adults, the estimated cancer risk ranged between 0.003 and 0.029 aflatoxin‐induced cancers per year per 100,000 adults under the scenario of the current ML. For the scenario of the increased ML, the cancer risk is estimated to be between 0.005 and 0.045 aflatoxin‐induced cancers per year per 100,000 adults. The highest exposure and consequent cancer risk were calculated for adolescents. For this age class, the cancer risk due to peanut and peanut butter consumption was estimated to be between 0.002 and 0.047 aflatoxin‐induced cancers per year per 100,000 adolescents under the scenario of the current ML. For the scenario of the increased ML, the cancer risk is estimated to be between 0.004 and 0.073 aflatoxin‐induced cancers per year per 100,000 adolescents.
Table 5.
Cancer risk calculated from the mean chronic dietary exposure to ‘aflatoxin total’a, the mean potency estimates of the cancer risk and a prevalence of HBsAg of 0.01%
| Age classb | Scenario | N | Cancer risk (aflatoxin‐induced cancers per year and per 100,000 subjects) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Infants | Current ML | 2 | 0.001 | 0.001 | 0.003 | 0.004 | 0.005 | 0.006 |
| Increased ML | 2 | 0.001 | 0.001 | 0.005 | 0.005 | 0.008 | 0.010 | |
| Toddlers | Current ML | 5 | 0.007 | 0.009 | 0.012 | 0.016 | 0.022 | 0.028 |
| Increased ML | 5 | 0.012 | 0.014 | 0.021 | 0.025 | 0.038 | 0.044 | |
| Other children | Current ML | 16 | 0.005 | 0.007 | 0.014 | 0.018 | 0.029 | 0.039 |
| Increased ML | 16 | 0.009 | 0.011 | 0.024 | 0.028 | 0.050 | 0.060 | |
| Adolescents | Current ML | 14 | 0.002 | 0.003 | 0.009 | 0.012 | 0.035 | 0.047 |
| Increased ML | 14 | 0.004 | 0.005 | 0.015 | 0.018 | 0.061 | 0.073 | |
| Adults | Current ML | 17 | 0.003 | 0.004 | 0.009 | 0.011 | 0.021 | 0.029 |
| Increased ML | 17 | 0.005 | 0.006 | 0.015 | 0.018 | 0.037 | 0.045 | |
| Elderly | Current ML | 12 | 0.001 | 0.002 | 0.006 | 0.008 | 0.013 | 0.017 |
| Increased ML | 12 | 0.002 | 0.003 | 0.010 | 0.012 | 0.022 | 0.026 | |
| Very elderly | Current ML | 4 | 0.004 | 0.005 | 0.006 | 0.008 | 0.012 | 0.017 |
| Increased ML | 4 | 0.006 | 0.008 | 0.011 | 0.012 | 0.022 | 0.026 | |
LB: lower bound; ML: maximum level; N: number of surveys; UB: upper bound.
Mean chronic dietary exposure to ‘aflatoxin total’ from peanuts, peanut butter and processed products thereof (consumers only) calculated from mean LB and UB occurrence values for two different scenarios.
Section 2.4 describes the age range within each age class.
Based on the UB potency estimates and a prevalence of HBsAg of 5.61%, the CONTAM Panel estimated the cancer risk due to peanut and peanut butter consumption, under the scenario of the current ML, to be between 0.003 and 0.213 aflatoxin‐induced cancers per year per 100,000 persons, across dietary surveys and age groups, (Table 6). Under the scenario of the increased ML, it ranged between 0.006 and 0.333 aflatoxin‐induced cancers per year per 100,000 persons, across dietary surveys and age groups. In adults, the cancer risk ranged between 0.012 and 0.131 aflatoxin‐induced cancers per year per 100,000 adults under the scenario of the current ML. For the scenario of the increased ML, the cancer risk is estimated to be between 0.022 and 0.204 aflatoxin‐induced cancers per year per 100,000 adults. For adolescents, the cancer risk due to peanut and peanut butter consumption was estimated to be between 0.010 and 0.213 aflatoxin‐induced cancers per year per 100,000 adolescents under the scenario of the current ML. For the scenario of the increased ML, the cancer risk is estimated to be between 0.018 and 0.333 aflatoxin‐induced cancers per year per 100,000 adolescents.
Table 6.
Cancer risk calculated from the mean chronic dietary exposure to ‘aflatoxin total’a, the upper bound potency estimate of the cancer risk and a prevalence of HBsAg of 5.61%
| Age classb | Scenario | N | Cancer risk (aflatoxin‐induced cancers per year and per 100,000 subjects) | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | ||||||
| LB | UB | LB | UB | LB | UB | |||
| Infants | Current ML | 2 | 0.003 | 0.004 | 0.012 | 0.016 | 0.021 | 0.028 |
| Increased ML | 2 | 0.006 | 0.007 | 0.021 | 0.025 | 0.037 | 0.043 | |
| Toddlers | Current ML | 5 | 0.030 | 0.040 | 0.054 | 0.073 | 0.099 | 0.129 |
| Increased ML | 5 | 0.053 | 0.062 | 0.095 | 0.114 | 0.173 | 0.202 | |
| Other children | Current ML | 16 | 0.023 | 0.032 | 0.063 | 0.083 | 0.131 | 0.176 |
| Increased ML | 16 | 0.041 | 0.049 | 0.110 | 0.130 | 0.230 | 0.275 | |
| Adolescents | Current ML | 14 | 0.010 | 0.014 | 0.040 | 0.053 | 0.160 | 0.213 |
| Increased ML | 14 | 0.018 | 0.022 | 0.070 | 0.083 | 0.281 | 0.333 | |
| Adults | Current ML | 17 | 0.012 | 0.017 | 0.039 | 0.052 | 0.097 | 0.131 |
| Increased ML | 17 | 0.022 | 0.026 | 0.068 | 0.081 | 0.171 | 0.204 | |
| Elderly | Current ML | 12 | 0.006 | 0.008 | 0.027 | 0.035 | 0.057 | 0.077 |
| Increased ML | 12 | 0.010 | 0.012 | 0.046 | 0.055 | 0.100 | 0.120 | |
| Very elderly | Current ML | 4 | 0.017 | 0.022 | 0.028 | 0.037 | 0.057 | 0.076 |
| Increased ML | 4 | 0.029 | 0.034 | 0.048 | 0.057 | 0.099 | 0.118 | |
LB: lower bound; ML: maximum level; N: number of surveys; UB: upper bound.
Mean chronic dietary exposure to ‘aflatoxin total’ from peanuts, peanut butter and processed products thereof (consumers only) calculated from mean LB and UB occurrence values for two different scenarios.
Section 2.4 describes the age range within each age class.
According to the WHO Guideline for drinking‐water quality (WHO, 2011), an excess lifetime cancer risk of 10−5 or less is considered to be of low risk for health concern.13 Assuming a lifetime expectancy of 70 years, this corresponds to a yearly excess cancer risk of 0.014 additional cancer cases14 per 100,000 subjects. Comparing the estimated aflatoxin‐induced cancers calculated under the current ML scenario with this yearly excess cancer risk, a higher risk for consumers of peanut and peanut butter is identified in some surveys. The calculated cancer risks indicate that an increase of the ML would further increase the risk by a factor of 1.6–1.8.
3.5. Analysis of the divergent opinion with JECFA
As explained in Section 1.3.1, JECFA concluded in a similar assessment that ‘enforcing an ML of 10, 8 or 4 μg/kg for ready‐to‐eat peanuts would have little further impact on dietary exposure to AFT for the general population, compared with setting an ML of 15 μg/kg’.
This divergent opinion relates to methodological differences in the exposure assessment, namely the treatment of samples exceeding the considered ML, the foods included in the exposure assessment and the target population (consumers only vs general population).
The CONTAM Panel notes that the exposure to aflatoxins from peanuts and products derived thereof comes in addition to dietary aflatoxin exposure from other sources. Due to the short deadline of this mandate, an assessment of the total chronic dietary exposure to AFT (considering all food sources) was not considered feasible, and the CONTAM Panel limited the exposure assessment to peanuts ready‐to‐eat, peanut butter and processed products thereof. The exposure was calculated for consumers only, since consumers of peanuts and peanut butter represent on average 7% (range: 0–36%) of the total population. The CONTAM Panel consequently based its conclusion on the assessment of the risk for consumers only, whereas JECFA based its conclusions on the risk for the general population. The CONTAM Panel recommends performing a full risk assessment on aflatoxins that would allow the interpretation of the exposure from peanuts in the perspective of the total dietary exposure to AFT.
JECFA and the CONTAM Panel also deviate in the treatment of samples exceeding the considered ML. JECFA excluded all samples for which the concentration of AFT exceeded the considered ML (4, 8, 10 or 15 μg/kg). The CONTAM Panel and EFSA used this approach also in previous assessments (e.g. EFSA, 2007a, 2012). A limitation of this approach is the assumption that all samples comply with the ML that is in place. In practice, a certain percentage of samples on the market will exceed the ML, and therefore, an underestimation of the risk is made. To improve and refine risk assessment methods, the methodology used by EFSA for dietary exposure assessment has been revised. In the opinion on MLs of deoxynivalenol (DON) in certain cereal products (EFSA CONTAM Panel, 2013), another approach was used to create a data set representing the occurrence of DON for a hypothetical ML. In this approach, a new data set was simulated by bootstrapping, assuming that the proportion of non‐compliant samples observed in the initial data set will remain the same after increase of the ML and no samples above the considered ML were excluded. The CONTAM Panel used a similar approach in the present evaluation where, according to the occurrence data available in the EFSA database, 7% of the samples on peanut exceed the EU ML of 4 μg/kg. Hence, the CONTAM Panel simulated a new data set assuming that also 7% of the samples would be non‐compliant under the increased ML.
3.6. Uncertainty analysis
The evaluation of the inherent uncertainties in the assessment of effect on public health of a possible increase of the ML for AFT from 4 to 10 μg/kg in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs has been performed following the guidance of the Opinion of the Scientific Committee related to Uncertainties in Dietary Exposure Assessment (EFSA, 2007b). In addition, the report on ‘Characterizing and Communicating Uncertainty in Exposure Assessment’ has been considered (WHO/IPCS, 2008). According to the guidance provided by the EFSA opinion (2007b), the following sources of uncertainties have been considered: assessment objectives, exposure scenario/exposure model and model input (parameters).
3.6.1. Assessment objectives
The objectives of the assessment are clearly specified in the terms of reference. The assessment is only focused on the free parent aflatoxins and because of lack of data does not take into account potentially modified aflatoxins. A re‐evaluation of the toxicological properties of aflatoxins is beyond the scope of this assessment.
3.6.2. Exposure scenario/exposure model
The occurrence data used in the exposure assessment were mainly the results of monitoring programmes. Due to the sampling strategy (selective/targeted sampling design) and to the performance of the analytical methods which aimed to verify compliance with MLs but not to determine background levels, the data are considered to overestimate the background level of aflatoxins in peanuts and peanut butter, especially the upper bound estimates. For peanuts and peanut butter, 87% and 64%, respectively, of the AFT concentrations were left‐censored. Moreover, around 66% of the samples were reported from only two European countries. Thus, the data set may not be representative of aflatoxins on the European market.
The mean value of aflatoxins in peanuts is also influenced by some high values, and insufficient information was available in the EFSA Chemical Occurrence database, to separate peanut samples that were not subject to sorting or any other physical treatment from peanuts that are intended for direct human consumption. Therefore, all available peanut samples were used in the exposure assessment. Although it is expected that the majority of samples referred to peanuts for direct human consumption, this may lead to an overestimation of the exposure.
No data were available regarding consignments that were not shipped to the EU due to levels exceeding the current ML of 4 μg/kg but that would have been placed on the EU market if a higher ML was in place. Data were instead generated by a simulation approach. The approach relied on the assumption that the proportion of non‐compliant samples (based on the reported data) would remain the same after the increase of the ML. The calculated confidence intervals indicated that the approach may lead to either an over‐ or underestimation by approximately 10–15%. This introduces some uncertainty into the assessment.
Processing could only be partially taken into account, as limited occurrence data on roasted peanuts and peanut butter were included in the exposure assessment. On the other hand, effects of other processing methods, such as during manufacturing of peanut sauce or peanut oil could not be addressed because of lack or limited data. This introduces uncertainty into the exposure assessment.
Before matching the consumption data to the corresponding occurrence data, eating occasions for foods that may contain peanuts were identified and amounts were converted to the corresponding amount of peanuts and peanut butter. Assumptions applied for this conversion may however not be accurate and representative of all possible recipes and commercial products. Furthermore, it cannot be excluded that peanuts or peanut butter have been used as a minor or exceptional ingredient of other composite foods. This conversion is therefore to be considered as an approximation rather than an accurate calculation.
The total diet has not been taken into account in the exposure assessments, but only the exposure from peanuts ready‐to‐eat, peanut butter, and processed products thereof. Since consumers of peanuts and peanut butter represent on average 7% (range: 0–36%) of the total population, exposure estimates for AFT in peanuts, peanut butter and processed products thereof was calculated for consumers only. Considering that most consumption surveys covered only few days, the mean consumption of consumers only, is most likely overestimated. On the other hand, a reliable exposure assessment for highly exposed consumers was not possible due to the low number of consumers in most dietary survey and age classes. This introduces additional uncertainty to the assessment. Approximate estimations for the adult population indicated that for high consumers, exposures may be underestimated by a fold‐factor between 2 and 4, although it is not clear whether this ratio is also applicable to the most exposed age classes such as adolescents and other children.
Based on these considerations, the CONTAM Panel concluded that there is substantial uncertainty in the exposure assessment.
3.6.3. Model input (parameters)
There are no prescribed fixed official methods for the analysis of aflatoxins in peanuts and laboratories can use any appropriate method of analysis, provided it can be demonstrated in a traceable manner that they fulfil the requirements according to Commission Regulation (EC) No 401/2006. This may have added to the uncertainty in the analytical results but only to a minor extent.
3.6.4. Other uncertainties
The CONTAM Panel used the cancer potencies calculated by JECFA at its 83rd meeting. The liver cancer potencies were calculated using an epidemiological study where the lowest exposure group had an estimated exposure of 12 ng AFB1/kg bw (FAO/WHO, 1999). The potencies were expressed as the liver cancer cases per 100,000 person years per ng aflatoxin. Applying these potency estimates for AFB1 exposure of around 1 ng/kg bw per day and below implies an extrapolation outside the dose range. However, considering that AFB1 is a carcinogen showing a linear dose–response in the range of low doses tested in experimental studies (FAO/WHO, 2017), the CONTAM Panel concluded that this extrapolation is appropriate, but is uncertain at very low doses and might overestimate the risk.
The cancer potencies were calculated by JECFA for both HBsAg+ and HBsAg– individuals. The cancer potency for HBsAg– individuals is based on relatively few cases and is therefore more uncertain than the estimated potency for HBsAg+ subjects.
The interaction between aflatoxin and hepatitis C is unknown and has not been taken into account, which might underestimate the risk.
The CONTAM Panel assumed that the cancer potency of AFT is equal to that of AFB1. Given that AFB1 is the most potent aflatoxin, this might overestimate the risk.
Use of UB cancer potencies may cause an overestimation of the cancer incidence.
3.6.5. Summary of uncertainties
In Table 7, a summary of the uncertainty evaluation is presented, highlighting the main sources of uncertainty and indicating an estimate of whether the respective source of uncertainty might have led to an over‐ or underestimation of the exposure or the resulting risk.
Table 7.
Summary of qualitative evaluation of the impact of uncertainties on the risk assessment on effect on public health of a possible increase of the maximum level for ‘aflatoxin total’ from 4 to 10 μg/kg in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs
| Sources of uncertainty | Direction |
|---|---|
| Same proportion of samples exceeding the current and the suggested increased maximum level | +a |
| Resampling of occurrence data by bootstrapping | +/− |
| Limited occurrence data in peanut butter and peanut oil | +/− |
| Use of lower bound/upper bound data for ‘aflatoxin total’ | +/− |
| Mean value of ‘aflatoxin total’ in peanuts influenced by some high values, potentially being removed from or not placed on the market | + |
| Conversion of foods that may contain peanuts into the corresponding amount of peanuts and peanut butter | +/− |
| Assessment for consumers only, while most consumption surveys cover few days | + |
| Reliable assessment for highly exposed consumers was not possible | − |
| Extrapolating cancer risk outside dose range | + |
| Estimated cancer potency for hepatitis B surface antigen‐subjects is more uncertain because based on relatively few cases | +/− |
| Cancer potency for aflatoxin B1 applied to ‘aflatoxin total’ | + |
| Use of upper bound cancer potencies | + |
| Possible interaction between aflatoxin and hepatitis C | − |
+ = uncertainty with potential to cause overestimation of exposure/risk; − = uncertainty with potential to cause underestimation of exposure/risk.
The CONTAM Panel considered that the impact of the uncertainties on the risk assessment on effect on public health of a possible increase of the ML for AFT from 4 to 10 μg/kg in ‘peanuts and processed products thereof’, intended for direct human consumption or use as an ingredient in foodstuffs, is large. The assessment is more likely to overestimate than to underestimate the additional risk.
4. Conclusions
4.1. General
Aflatoxins are mycotoxins produced primarily by the fungi A. flavus and A. parasiticus. A. parasiticus produces AFB1, AFB2, AFG1 and AFG2, whereas A. flavus mainly produces AFB1 and AFB2. However, A. flavus isolates producing AFG1 and AFG2 also have been identified. These fungi are especially found in areas with a hot and humid climate. AFB1 is the most frequently found aflatoxin in contaminated samples and the three others are generally not reported in the absence of AFB1. AFT refers to the sum of these four aflatoxins.
Aflatoxins have primarily been detected in imported foods such as peanuts, tree nuts, dried fruit, spices, crude oil, cocoa beans, maize and rice. Processes such as heating, roasting and baking can reduce the levels of aflatoxins but cannot completely eliminate the toxins. Extraction and refining of oil can reduce the levels of aflatoxins to less than 1 μg/kg even from highly contaminated samples of peanuts.
4.2. Occurrence/Exposure
To estimate the occurrence of aflatoxins in peanuts and processed products thereof, EFSA used a data set containing 8,095 samples for peanuts, 472 samples of peanut butter and 14 samples of peanut oil. Samples for peanut oil were all left‐censored.
The highest concentrations of AFT were found in peanuts with a mean value of 2.65/3.56 μg/kg (LB/UB estimates) and a maximum concentration of 1,429 μg/kg. A total of 87% of the samples were left‐censored and 7% of the samples had a concentration higher than the current ML of 4 μg/kg.
Compared to the findings in peanuts, concentrations of AFT in peanut butter were lower with a mean value of 1.47/1.92 μg/kg (LB/UB estimates) and a maximum value of 407 μg/kg. Also, the number of left‐censored samples was lower (64%) and 5% of the samples had a concentration higher than the current ML of 4 μg/kg.
Contribution of the individual aflatoxins to the AFT concentrations varied, but the major contributor was identified as AFB1. On average, AFB1 represented 61% of the AFT MB concentration for samples containing less than 1 μg/kg, and up to 78% for samples containing more than 10 μg/kg.
Based on a simulation by bootstrapping, it is anticipated that changing the ML from 4 to 10 μg/kg for peanuts will increase the mean LB estimates by a factor of 1.75 and the mean UB estimates by a factor of 1.56. Although such a simulation could not be carried out for peanut butter, it was assumed that the increase of aflatoxin levels in peanut butter would be proportional to the increase anticipated for peanuts.
Due to time constraints, an assessment of the total chronic dietary exposure to AFT (considering all food sources) was not considered feasible. Furthermore, considering that extraction and refining of oil may reduce the concentrations of aflatoxins considerably, that the ML under this assessment does not apply to peanut oil and that available occurrence data for peanut oil were all left‐censored, peanut oil was not included in the exposure assessment.
Since consumers of peanuts and peanut butter represent on average 7% (range: 0–36%) of the total population, exposure estimates for AFT in peanuts, peanut butter and processed products thereof was calculated for consumers only.
Assuming the current market situation with the ML of 4 μg/kg, mean chronic exposure estimates for consumers only, ranged from 0.04 (lowest LB estimate) to 2.74 (highest UB estimate) ng/kg bw per day. If the ML is changed to 10 μg/kg, it is expected that these exposure estimates may increase up to 0.07 and 4.28 ng/kg bw per day, respectively. The highest exposures were calculated for adolescents and other children.
Due to the low number of consumers in most dietary surveys and age classes, a reliable exposure assessment for highly exposed consumers was not possible. Approximate estimations for the adult population indicated that exposure of high consumers may be two to four times higher compared to the exposure of average consumers. However, it is not clear whether this ratio is also applicable to the most exposed age classes such as adolescents and other children.
4.3. Risk characterisation
AFB1 is a genotoxic compound and potent liver carcinogen in animals and humans. In experimental animals, AFG1 is also carcinogenic, whereas there is limited evidence for carcinogenicity of AFB2 and inadequate evidence for carcinogenicity of AFG2.
The CONTAM Panel used the cancer potencies estimated by JECFA in 2016. The cancer potencies were estimated to be 0.017 (mean) and 0.049 (95% UB) per 105 person years per ng/kg bw for HBsAg– individuals and 0.269 (mean) and 0.562 (95% UB) per 105 person years per ng/kg bw for HBsAg+ individuals.
The cancer risk was estimated based on the mean cancer potency estimates and a prevalence of HBsAg+ of 0.01%, as well as on the UB potency estimates and a prevalence of HBsAg+ of 5.61%.
Under the scenario of the current ML, the cancer risk was estimated to range between 0.001 and 0.213 aflatoxin‐induced cancers per year per 100,000 persons. Under the scenario of the increased ML, it ranged between 0.001 and 0.333 aflatoxin‐induced cancers per year per 100,000 persons.
According to the WHO Guideline for drinking‐water quality, an excess lifetime cancer risk of 10−5 or less is considered to be of low risk for public health and, assuming a lifetime expectancy of 70 years, this corresponds to a yearly excess cancer risk of 0.014 additional cancer cases per 100,000 subjects. Comparing the estimated aflatoxin‐induced cancers calculated under the current ML scenario with this yearly excess cancer risk, a higher risk for consumers of peanuts and peanut butter is identified in some surveys. The calculated cancer risks indicate that an increase of the ML would further increase the risk by a factor of 1.6–1.8.
5. Recommendations
Considering that the last full risk assessment by the CONTAM Panel was carried out in 2007 and that elevated aflatoxin levels were observed in some food commodities originating from European countries, a full risk assessment would be appropriate.
Knowledge on contamination levels of consignments that were not shipped to the EU due to exceedance of the current ML but would have been shipped if a higher ML was in place, would be needed when assessing the effect on the risk for public health of a possible increase of an ML in the future.
Abbreviations
- AFB1
aflatoxin B1
- AFB2
aflatoxin B2
- AFG1
aflatoxin G1
- AFG2
aflatoxin G2
- AFM1
aflatoxin M1
- AFM2
aflatoxin M2
- AFT
aflatoxin total
- AIC
Akaike information criterion
- ALARA
as low as reasonably achievable
- BMD
benchmark dose
- BMDL
benchmark dose lower confidence limit
- bw
body weight
- CCCF
Codex Committee on Contaminants in Food
- CI
confidence interval
- CONTAM
EFSA Panel on Contaminants in the Food Chain
- DON
deoxynivalenol
- FD
fluorescence detection
- GST
glutathione S‐transferase
- HBsAg
hepatitis B surface antigen
- HPLC
high‐performance liquid chromatography
- IARC
International Agency for Research on Cancer
- IPCS
International Programme on Chemical Safety
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LB
lower bound
- LC
left‐censored
- LOD
limit of detection
- LOQ
limit of quantification
- MB
middle bound
- ML
maximum level
- MOE
margin of exposure
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- N
Number of samples/surveys
- p
probability of non‐compliance with the maximum level
- p’
probability of a concentration between 4 and 10 μg/kg when non‐compliant with the current maximum level
- P50
50th percentile
- P95
95th percentile
- SOP
standard operational procedure
- UB
upper bound
- WG
Working Group
- WHO
World Health Organization
Appendix A – EFSA guidance documents applied for the risk assessment
1.
The following EFSA guidance documents pertaining to risk assessment were followed for the development of the risk assessment:
EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA Journal 2005;3(10):282, 33 pp. https://doi.org/10.2903/j.efsa.2005.282
EFSA (European Food Safety Authority), 2007. Guidance of the Scientific Committee on a request from EFSA related to Uncertainties in Dietary Exposure Assessment. EFSA Journal 2007;5(1):438, 54 pp. https://doi.org/10.2903/j.efsa.2007.438
EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009:7(5);1051, 22 pp. https://doi.org/10.2903/j.efsa.2009.1051
EFSA (European Food Safety Authority), 2010. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. https://doi.org/10.2903/j.efsa.2010.1457
EFSA (European Food Safety Authority), 2010. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. https://doi.org/10.2903/j.efsa.2010.1557
EFSA (European Food Safety Authority), 2011. Use of the EFSA Comprehensive European Food Consumption Database in Intakes Assessment. EFSA Journal 2011;9(3):2097, 34 pp. https://doi.org/10.2903/j.efsa.2011.2097
EFSA Scientific Committee 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. https://doi.org/10.2903/j.efsa.2011.2379
EFSA Scientific Committee 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. https://doi.org/10.2903/j.efsa.2012.2579
EFSA Scientific Committee, 2012. Scientific Opinion on Risk Assessment Terminology. EFSA Journal 2012;10(5):2664, 43 pp. https://doi.org/10.2903/j.efsa.2012.2664
EFSA Scientific Committee, Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017. Update: Guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. https://doi.org/10.2903/j.efsa.2017.4658
Appendix B – Dose–response models fitted by JECFA to the data of Yeh et al. (1989)
1.
Figure B.1.
Dose–response models fitted by JECFA to the data of Yeh et al. (1989)
- Curves show the number of primary hepatocellular carcinoma cases per person years in function of the dose (ng/kg bw per day) for HBsAg positive (red) and HBsAg negative (blue) cases (see Section 1.3.1 and FAO/WHO, 2017 for further details).
Annex A – Occurrence data submitted to EFSA and influence of increasing the maximum level on dietary exposure
1.
Annex A can be found in the online version of this output (‘Supporting information’ section): http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2018.5175/abstract
Description: The annex is an excel file which represents tables (from Table A.1 to A.6) on aflatoxin occurrence and the influence of increasing the maximum level on dietary exposure.
Supporting information
Occurrence data submitted to EFSA and influence of increasing the maximum level on dietary exposure
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Nebbia CS, Oswald IP, Rose M, Roudot A‐C, Schwerdtle T, Vleminckx C, Vollmer G, Wallace H, Fürst P, Baert K, Cortiñas Abrahantes J, Dujardin B, Ferrini K and Petersen A, 2018. Statement on the effect on public health of a possible increase of the maximum level for ‘aflatoxin total’ from 4 to 10 μg/kg in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs. EFSA Journal 2018;16(2):5175, 32 pp. 10.2903/j.efsa.2018.5175
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00698
Panel members: Jan Alexander, Lars Barregård, Margherita Bignami, Beat Brüschweiler, Sandra Ceccatelli, Bruce Cottrill, Michael Dinovi, Lutz Edler, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Helle Katrine Knutsen, Carlo Stefano Nebbia, Isabelle Oswald, Annette Petersen, Martin Rose, Alain‐Claude Roudot, Tanja Schwerdtle, Christiane Vleminckx, Günter Vollmer and Heather Wallace.
Acknowledgements: The Panel wishes to thank the EFSA staff members: Davide Arcella and Valentina Bocca for the support provided to this scientific output. The CONTAM Panel acknowledges all European competent institutions and other stakeholders that provided occurrence data on aflatoxins in peanut, peanut butter and peanut oil, and supported the data collection for the Comprehensive European Food Consumption Database.
Adopted: 23 January 2018
Notes
https://ec.europa.eu/food/safety/rasff_en; searched on 14 December 2017
Posterior estimates are obtained from the posterior distribution of the parameter of interest (potency), which is defined as the probability distribution from an unknown parameter considered as a random variable, and it is determined by the prior knowledge about the parameter and the evidence gathered in the collected data about the parameter of interest (Congdon, 2006).
OJ L 37, 13.2.1993, p. 1–3.
OJ L 364, 20.12.2006, p. 5–24.
OJ L 70, 9.3.2006, p. 12–34.
Commission Regulation (EC) No 669/2009 of 24 July 2009 implementing Regulation (EC) No 882/2004 of the European Parliament and of the Council as regards the increased level of official controls on imports of certain feed and food of non‐animal origin and amending Decision 2006/504/EC. OJ L 194, 25.7.2009, p. 11–21.
Commission Implementing Regulation (EU) No 884/2014 of 13 August 2014 imposing special conditions governing the import of certain feed and food from certain third countries due to contamination risk by aflatoxins and repealing Regulation (EC) No 1152/2009. OJ L 242, 14.8.2014, p. 4–19.
Codex Alimentarius: General Standard for Contaminants and Toxins in Food and Feed (CXS 193‐1995, last amended in 2017).
These values can be interpreted as follows: when 100,000 HBsAg+ subjects are exposed for 1 year to 1 ng/kg bw per day, it is estimated that this relates to 0.269 aflatoxin‐induced cancer cases with an 95% UB of 0.562.
An excess lifetime cancer risk of 10−5 is equivalent to one additional case of cancer per 100,000 of the population ingesting drinking water containing the substance at the guideline value for 70 years (WHO, 2011). This risk level is used by the WHO to set guidance values for chemicals in drinking water.
The yearly extra risk was calculated by dividing the excess lifetime cancer risk of 10−5 by the lifetime expectancy of 70 years and expressing it per 100,000 subjects.
References
- Abalaka JA, 1984. Aflatoxin distribution in edible oil‐extracting plants and in poultry feedmills. Food and Chemical Toxicology, 22, 461–463. [DOI] [PubMed] [Google Scholar]
- Abalaka JA and Elegbede JA, 1982. Aflatoxin distribution and total microbial counts in an edible oil extracting plant. I Preliminary observations. Food and Chemical Toxicology, 20, 43–46. [DOI] [PubMed] [Google Scholar]
- Bailey GS, Dashwood R, Loveland PM, Pereira C and Hendricks JD, 1998. Molecular dosimetry in fish: quantitative target organ DNA adduction and hepatocarcinogenicity for four aflatoxins by two exposure routes in rainbow trout. Mutation Research, 399, 233–244. 10.1016/s0027-5107(97)00258-3 [DOI] [PubMed] [Google Scholar]
- Battilani P, Toscano P, Van der Fels‐Klerx HJ, Moretti A, Camardo Leggieri M, Brera C, Rortais A, Goumperis T and Robinson T, 2016. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Scientific Reports, 6, 24328. 10.1038/srep24328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordin K, Sawada MM, Rodrigues CED, da Fonseca CR and Oliveira CAF, 2014. Incidence of aflatoxins in oil seeds and possible transfer to oil: a review. Food Engineering Reviews, 6, 20–28. 10.1007/s12393-014-9076-9 [DOI] [Google Scholar]
- Bowers J, Brown B, Springer J, Tollefson L, Lorentzen R and Henry S, 1993. Risk assessment for aflatoxin: an evaluation based on the multistage model. Risk Analysis, 13, 637–642. [DOI] [PubMed] [Google Scholar]
- Carlborg FW, 1979. Cancer, mathematical models and aflatoxin. Food and Cosmetics Toxicology, 17, 159–166. 10.1016/0015-6264(79)90216-5 [DOI] [PubMed] [Google Scholar]
- Congdon P, 2006. Bayesian statistical modeling. 2nd Edition. Chapter 1 Introduction: The Bayesian Method, its Benefits and Implementation. John Wiley, West Sussex, England, 1–23.
- EFSA (European Food Safety Authority), 2007a. Opinion of the scientific panel on contaminants in the food chain (CONTAM) related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived products. EFSA Journal 2007;5(3):446, 127 pp. 10.2903/j.efsa.2007.446 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007b. Guidance of the Scientific Committee on a request from EFSA related to Uncertainties in Dietary Exposure Assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Statement of the Scientific Panel on Contaminants in the Food Chain on a request from the European Commission on the effects on public health of an increase of the levels for aflatoxin total from 4 μg/kg to 10 μg/kg for tree nuts other than almonds, hazelnuts and pistachios. EFSA Journal 2009;7(6):1168, 11 pp. 10.2903/j.efsa.2009.1168 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Effect on dietary exposure of an increase of the levels for aflatoxin total from 4 μg/kg to 10 μg/kg for dried figs. EFSA supporting publication 2012:EN‐311, 6 pp. 10.2903/sp.efsa.2012.en-311 [DOI] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017. Update: use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization), 1999. Aflatoxins. Safety evaluation of certain food additives and contaminants. Prepared by the sixty‐eighth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additive Series 40, Available online: http://www.inchem.org/documents/jecfa/jecmono/v040je16.htm
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization), 2008. Aflatoxins. Safety evaluation of certain food additives and contaminants. Prepared by the sixty‐eighth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). 305–356.
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization), 2017. Aflatoxins. Evaluation of certain contaminants in food (Eighty‐third report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). 11‐40.
- Guengerich FP, Arneson KO, Williams KM, Deng Z and Harris TM, 2002. Reaction of aflatoxin B1 oxidation products with lysine. Chemical Research in Toxicology, 15, 780–792. 10.1021/tx010156s [DOI] [PubMed] [Google Scholar]
- Hoseyni MS, 1992. Risk assessment for aflatoxin: III. Modeling the relative risk of hepatocellular carcinoma. Risk Analysis, 12, 123–128. [DOI] [PubMed] [Google Scholar]
- Huybrechts I, Sioen I, Boon PE, Ruprich J, Lafay L, Turrini A, Amiano P, Hirvonen T, De Neve M, Arcella D, Moschandreas J, Westerlund A, Ribas‐Barba L, Hilbig A, Papoutsou S, Christensen T, Oltarzewski M, Virtanen S, Rehurkova I, Azpiri M, Sette S, Kersting M, Walkiewicz A, Serra‐Majem L, Volatier JL, Trolle E, Tornaritis M, Busk L, Kafatos A, Fabiansson S, De Henauw S and Van Klaveren JD, 2011. Dietary exposure assessments for children in Europe (the EXPOCHI project): rationale, methods and design. Archives of Public Health, 69, 4. 10.1186/0778-7367-69-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 2012. Aflatoxins. Chemical Agents and Related Occupations. A review of Human Carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans, 100F, 225–248. [PMC free article] [PubMed] [Google Scholar]
- Lipp M, 2018. Re: JECFA evaluation of aflatoxins_dietary exposure. Message to Baert K. 8.1.2018. e‐mail.
- Merten C, Ferrari P, Bakker M, Boss A, Hearty A, Leclercq C, Lindtner O, Tlustos C, Verger P, Volatier JL and Arcella D, 2011. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food Additives and Contaminants. Part A, 28, 975–995. 10.1080/19440049.2011.576440 [DOI] [PubMed] [Google Scholar]
- Moore GG, Olarte RA, Horn BW, Elliott JL, Singh R, O'Neal CJ and Carbone I, 2017. Global population structure and adaptive evolution of aflatoxin‐producing fungi. Ecology Evolution, 7, 9179–9191. 10.1002/ece3.3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WA and Melnick D, 1966. Absence of aflatoxin from refined vegetable oils. Journal of the American Oil Chemists’ Society, 43, 635–638. [DOI] [PubMed] [Google Scholar]
- Peers FG, Gilman GA and Linsell CA, 1976. Dietary aflatoxins and human liver cancer. A study in Swaziland. International Journal of Cancer, 17, 167–176. [DOI] [PubMed] [Google Scholar]
- Peers F, Bosch X, Kaldor J, Linsell A and Pluijmen M, 1987. Aflatoxin exposure, hepatitis B virus infection and liver cancer in Swaziland. International Journal of Cancer, 39, 545–553. [DOI] [PubMed] [Google Scholar]
- Pottenger LH, Andrews LS, Bachman AN, Boogaard PJ, Cadet J, Embry MR, Farmer PB, Himmelstein MW, Jarabek AM, Martin EA, Mauthe RJ, Persaud R, Preston RJ, Schoeny R, Skare J, Swenberg JA, Williams GM, Zeiger E, Zhang F and Kim JH, 2014. An organizational approach for the assessment of DNA adduct data in risk assessment: case studies for aflatoxin B1, tamoxifen and vinyl chloride. Critical Reviews in Toxicology, 44, 348–391. 10.3109/10408444.2013.873768 [DOI] [PubMed] [Google Scholar]
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G and Ott JJ, 2015. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet, 386, 1546–1555. 10.1016/s0140-6736(15)61412-x [DOI] [PubMed] [Google Scholar]
- Van Rensburg SJ, Cook‐Mozaffari P, Van Schalkwyk DJ, Van der Watt JJ, Vincent TJ and Purchase IF, 1985. Hepatocellular carcinoma and dietary aflatoxin in Mozambique and Transkei. British Journal of Cancer, 51, 713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2011. Guideline for Drinking‐water Quality. Available online: http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2008. Uncertainty and data quality in exposure assessment. Harmonisation project document No. 6. ISBN 978 92 4 156376 5.U. Available online: http://www.inchem.org/documents/harmproj/harmproj/harmproj6.pdf.
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2009. Environmental Health Criteria 240. Principles and methods for the assessment of chemicals in food. Available online: http://www.who.int/foodsafety/publications/chemical-food/en/
- Williams DE, 2012. The rainbow trout liver cancer model: response to environmental chemicals and studies on promotion and chemoprevention. Comparative Biochemistry and Physiology Part C. Toxicology and Pharmacology, 155, 121–127. 10.1016/j.cbpc.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DE, Orner G, Willard KD, Tilton S, Hendricks JD, Pereira C, Benninghoff AD and Bailey GS, 2009. Rainbow trout (Oncorhynchus mykiss) and ultra‐low dose cancer studies. Comparative Biochemistry and Physiology Part C. Toxicology and Pharmacology, 149, 175–181. 10.1016/j.cbpc.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogan GN, Paglialunga S and Newberne PM, 1974. Carcinogenic effects of low dietary levels of aflatoxin B1 in rats. Food and Cosmetics Toxicology, 12, 681–685. [DOI] [PubMed] [Google Scholar]
- Wong JJ and Hsieh DP, 1976. Mutagenicity of aflatoxins related to their metabolism and carcinogenic potential. Proceedings of the National Academy of Sciences of the United States of America, 73, 2241–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu‐Williams AH, Zeise L and Thomas D, 1992. Risk assessment for aflatoxin B1: a modeling approach. Risk Analysis, 12, 559–567. [DOI] [PubMed] [Google Scholar]
- Yeh FS, Yu MC, Mo CC, Luo S, Tong MJ and Henderson BE, 1989. Hepatitis B virus, aflatoxins, and hepatocellular carcinoma in southern Guangxi, China. Cancer Research, 49, 2506–2509. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Occurrence data submitted to EFSA and influence of increasing the maximum level on dietary exposure


