Abstract
Background
Cerebral ischemia is a major player of acute ischemic stroke (AIS) and mainly caused by blood vessels obstruction-induced reduced blood flow. Furthermore, miR-218-5p level was elevated in patients with AIS compared with controls. The present study investigated the biochemical mechanisms underlying the role of miR-218-5p in AIS in vitro.
Material/Methods
PC12 cells were chosen to establish oxidative-glucose deprivation/re-oxygenation (OGD/R) injury model. The interaction between miR-218-5p and N-myc downstream regulated gene 4 (NDRG4) was evaluated by Luciferase reporter assay. The levels of NDRG4, endothelial nitric oxide synthase (eNOS) and protein related to cell apoptosis were quantitatively analyzed with real-time quantitative polymerase chain reaction (RT-qPCR) or western blotting. Inflammatory cytokines, myeloperoxidase (MPO) and oxidative stress status were measured using specific commercial assay kits. Further, the cells apoptosis was analyzed with flow cytometry assay.
Results
MiR-218-5p level was notably increased in OGD/R injured PC12 cells and directly targeted NDRG4. MiR-218-5p inhibitor significantly inhibited inflammatory cytokines release, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and monocyte chemotactic protein 1 (MCP-1). In addition, miR-218-5p downregulation ameliorated nitric oxide (NO) and eNOS levels and suppressed the inducible nitric oxide synthase (iNOS) expression and cell apoptosis. However, NDRG4 silencing abolished all corrective effects of miR-218-5p inhibitor in OGD/R injured PC12 cells.
Conclusions
Downregulation of miR-218-5p protect against OGDR-induced injuries of PC12 cells through reducing inflammatory cytokines secretion, oxidative stress status, apoptosis rate and maintenance of endovascular homeostasis via upregulating NDRG4. MiR-218-5p may serve as a novel effective biomarker to monitor AIS progression.
MeSH Keywords: Encephalitis; Hypoxia-Ischemia, Brain; MicroRNAs; PC12 Cells
Background
Stroke is emerged as one of the main causes of death and disability broadly, which can be categorized into 2 major types of stroke, namely, cerebral ischemia and hemorrhagic stroke [1]. Cerebral ischemia is the major player of acute ischemic stroke (AIS) and mainly caused by blood vessels obstruction-induced reduced blood flow [2,3]. In addition, excessive inflammation and oxidative stress may aggravate AIS symptoms and increased mortality of patients with AIS [4]. With the increasing morbidity and mortality of stroke-related deaths worldwide, AIS has aroused huge attention all over the world. However, owing to the limitations of magnetic resonance imaging (MRI) and enhanced computed tomography (CT) in the diagnosis of AIS [5], it is more and more crucial and urgent to investigate the underlying mechanism of AIS pathogenesis and identify precise diagnosis to monitor disease progression. Multiple pathological and physiological processes are involved in the occurrence and progression of AIS, including energy failure, post ischemic inflammatory response, ischemic oxidative stress and even cell apoptosis [6]. It is well-known that AIS is a neurological deficit accompanied by high intracranial vascular inflammation. Thus, we presume that inhibiting inflammatory response and oxidative stress may be potential therapeutic strategies to alleviate the neural damage induced by oxidative-glucose deprivation/re-oxygenation (OGD/R).
MicroRNAs (miRNAs) are a new class of small, non-coding RNAs consisting of 21–25 nucleotides, that repress translation or degrade messenger RNA transcripts through interaction with their target coding messenger RNAs (mRNA) at the 3′-untranslated region (3′UTR) [7]. Accumulating studies have shown that miRNAs are closely associated with cell proliferation, inflammation, and apoptosis, and it offers tremendous potential evidences in unraveling the underlying mechanisms of stroke pathogenesis [8,9]. Recent study demonstrated that the level of miR-218-5p in plasma was elevated in patients with AIS compared with controls [10]. In addition, Jin et al. disclosed that elevated miR-185 plasma levels could be served as promising and independent predicting factors for AIS [11]. As N-myc downstream regulated gene (NDRG) family members, NDRG1–NDRG4 are cytoplasmic protein, which consists of 340 to 394 amino acid residues and have amino acid sequence homology [12]. NDRG4 was reported to be specifically expressed in the brain and heart [13]. Previous studies indicated that NDRG4 plays an important role in the maintenance of intracerebral brain-derived neurotrophic factor levels, contributing to the resistance to neuronal cell injury induced by ischemic stress. Furthermore, NDRG4 knockout animals are at risk of cerebral ischemia, implying that NDRG4 may be a potential therapeutic target to alleviate ischemia-induced injury [12]. However, the underlying mechanism is still unclear. Interestingly, NDRG4 was found that it is one of the potential target genes of miR-218-5p by predicting on the Targetscan database (www.targetscan.org).
In the present study, PC12 cells were chosen to establish oxidative-glucose deprivation/re-oxygenation (OGD/R) injury model. The present study aimed to investigate the underlying mechanisms of miR-218-5p effects on acute ischemic stroke. Taken together, all results implied that miR-218-5p downregulation protects against OGD/R-induced injuries via upregulating NDRG4 in vitro.
Material and Methods
Cell culture and OGD/R treatment
Rat pheochromocytoma cells (PC12 cells) were purchased from ATCC and maintained in Dulbecco’s Modified Eagle Medium (DMEM) contained with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin, and 100 units/mL penicillin with normal growth conditions (5% CO2 and 95% N2) in a 37°C humidified atmosphere. PC12 cells were treated with OGD/R or without pre-treatment as previous study described with minor revision [14]. In brief, PC12 cells were cultured in glucose-free DMEM, and then the cells were placed in an anaerobic chamber supplemented with 5% CO2 and 95% N2 at 37°C for 2 hours. The glucose (4.5 mg·mL−1) was added subsequently. Finally, PC12 cells were maintained with normoxic conditions (95% air and 5% CO2) for 12 hours.
PC12 cells transfection
PC12 cells were seeded randomly in 6-well plates. After the medium was replaced with serum-free DMEM, PC12 cell lines were transfected by miR-NC (100 nM), miR-218-5p mimic (100 nM), miR-218-5p inhibitor (100 nM), shRNA-NC (1 μM), shRNA- NDRG4-1, or shRNA- NDRG4-2 (1 μM) by using transfection reagent Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the manufacturer’s instructions. The miR-218-5p mimic, miR-NC, shRNA-NDRG4, and shRNA-NC were obtained from Biomics Biotechnologies Co., Ltd. The culture medium was replaced with fresh DMEM containing 10% FBS following a 6 to 8 hours incubation period. After culture for another 48 hours, western blotting and real-time quantitative polymerase chain reaction (RT-qPCR) were employed to detect the efficiency of transfection.
RNA Isolation and RT-qPCR
Total RNA was obtained from PC12 cells with TRIzol reagent (Ambion, USA) following the manufacturer’s instructions. Subsequently, 1 μg total RNAs were reverse transcribed with PrimeScript RT Reagent Kit with gDNA Eraser (TaKaRa, Otsu, Shiga, Japan) to synthesize cDNAs. Quantitative PCR was performed by using SYBR Premix EX Taq (Takara, Otsu, Shiga, Japan). The mRNA levels for each gene were calculated using the2−ΔΔCt method with normalization to expression level of GAPDH. The primer sequences of miR-218-5p and NDRG4 for qPCR amplification were shown below:
miR-218-5p, F: 5′-ACACTCCAGCTGGGTTGTGCT-3′,
R: 5′-TGTCGTGGAGTCGGCAATTC-3′;
NDRG4, F: 5′-TCTTCCCTGATTTGGTGGAG-3′,
R: 5′-CCAGAAGAGCTGAAGGTTGG-3′;
GAPDH, F: 5′-CTCACCGGATGCACCAATGTT-3′,
R: 5′-CGCGTTGCTCACAATGTTCAT-3′
Western blotting analysis
Total proteins were obtained from PC12 cell lines by radioimmunoprecipitation assay buffer (RIPA) lysis buffer after being washed with cold phosphate-buffered saline (PBS). Total protein extract was fractionated with 10% SDS-PAGE and transferred onto PVDF membranes. Each membrane was incubated with specific primary antibodies overnight at 4°C after being blocked with 5% skim milk. Rabbit anti- NDRG4 antibodies (1: 1000), rabbit anti-p-eNOS antibodies (1: 1000), rabbit anti-eNOS antibodies (1: 1000), rabbit anti-iNOS antibodies (1: 1000), rabbit anti-Bcl2 antibodies (1: 1000), rabbit anti-Bax antibodies (1: 1000), rabbit anti-cleaved caspase-3 antibodies (1: 1000), and rabbit anti-caspase-3 antibodies (1: 1000) were acquired from Cell Signaling Technology (Danvers, MA, USA). After being washed with 1×TBST, 3 times, the PVDF membranes were reacted with appropriate horseradish peroxidase-conjugated secondary antibodies for 2 hours at room temperature. Further, the target proteins expression was analyzed by using chemiluminescence detection kit (Millipore, Billerica, MA, USA).
Luciferase reporter assay
The potential target genes of miR-218-5p were searched on the Targetscan database. Among these candidates, NDRG4 was selected because of its protective effect on neuronal cell injury. To investigate the miR-218-5p on the 3′-UTR of NDRG4, luciferase reporter plasmid, pMIR- NDRG4-3′-UTR wild type (Wt), and pMIR-NDRG4-3′-UTR mutant (Mut) were synthesized and co-transfected into PC12 cells along with miR-218-5p mimics or miR-NC in 96-well plates by Lipofectamine 2000. The luciferase activities were detected at 48 h after transfection and normalized to Renilla activity using the Dual-Luciferase reporter assay system (Promega).
Biochemical analysis
According to the manufacturer’s protocols, the levels of reactive oxygen species (ROS), malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), myeloperoxidase (MPO) and inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and monocyte chemotactic protein 1 (MCP-1) in PC12 cells supernatants of respective group were analyzed with commercial test kits and enzyme-linked immunosorbent assay (ELISA) Kits (Duo-Set; R&D Systems), respectively. The optical density (OD) value at 450–650 nm was measured using a microplate reader.
Cell apoptosis analysis
PC12 cells (1×106 cells/mL) were incubated and transfected with miR-218-5p mimic or miR-NC followed by washing and trypsinization. Cells were centrifuged and resuspended with binding buffer (100 μL). Subsequently, PC12 cells were stained with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining solution. Finally, the solubilized PC12 cells were maintained on ice for 0.5 hours in the darkness following the manufacturer’s instructions. The Annexin FITC-positive and PI-negative cells were identified as apoptotic cells, and the percentage of apoptotic cells in each sample was conducted on a flow cytometer (Becton Dickinson, USA) and analyzed with BD LSRFortessa software. Moreover, the expression levels of proteins related to cell apoptotic are detected by western blotting.
Statistical analysis
All data are expressed as the means±standard deviation (SD) and analyzed with SPSS 17.0 software. Two-tailed Student’s t-tests were used to compare the differences between groups and one-way analysis of variance (ANOVA) test followed by Tukey post-test was performed to compare the parameters of multi-groups. P-value <0.05 was defined as statistically significant difference.
Results
MiR-218-5p level was increased and directly targeted NDRG4 in OGD/R injured PC12 cells
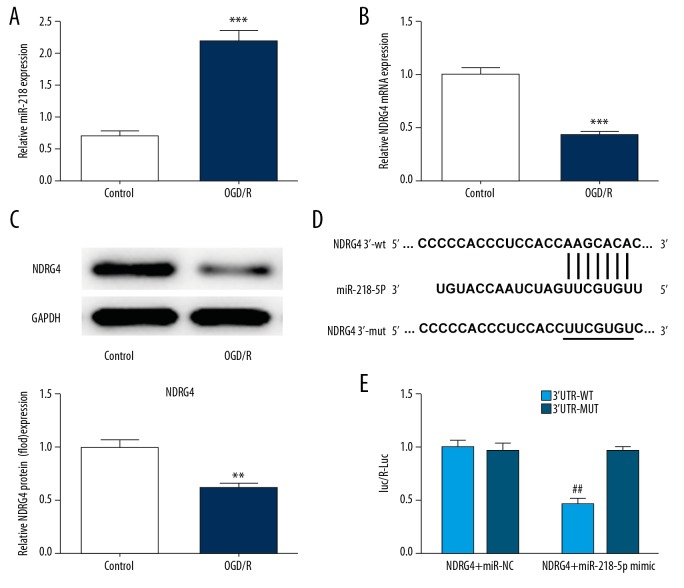
To investigate the role of miR-218-5p in AIS, its expression level was first detected by RT-qPCR. As presented in Figure 1A, miR-218-5p expression was notably increased in OGD/R injured PC12 cells compared to controls. The RT-qPCR and western blot were employed to detect the expression of NDRG4. In contrast, the expression level of NDRG4 was decreased in OGD/R injured PC12 cells compared to controls (Figure 1B, 1C). Interestingly, luciferase reporter assay results suggested that miR-218-5p directly targets NDRG4 gene to inhibit its expression (Figure 1D, 1E). Therefore, these results indicated that the miR-218-5p level is increased and directly targets NDRG4 in OGD/R injured PC12 cells.
Figure 1.
MiR-218-5p level is increased and directly targets NDRG4 in OGD/R injured PC12 cells. (A) MiR-218-5p level was measured via RT-qPCR. (B, C) The expression level of NDRG4 was measured with RT-qPCR and western blotting. (D) The putative binding sites of miR-218-5p. (E) Luciferase reporter assay was employed to investigate the interaction between miR-218-5p and NDRG4. The data were expressed as means±SD; ** P<0.01, *** P<0.001 versus Control. ## P<0.01 versus NDRG4+miR-NC. NDRG4 – N-myc downstream regulated gene 4; OGD/R – oxidative-glucose deprivation/re-oxygenation; RT-qPCR – real-time quantitative polymerase chain reaction; SD – standard deviation.
NDRG4 silencing blocked the suppressive effect of miR-218-5p downregulation on inflammatory cytokine release and oxidative stress status
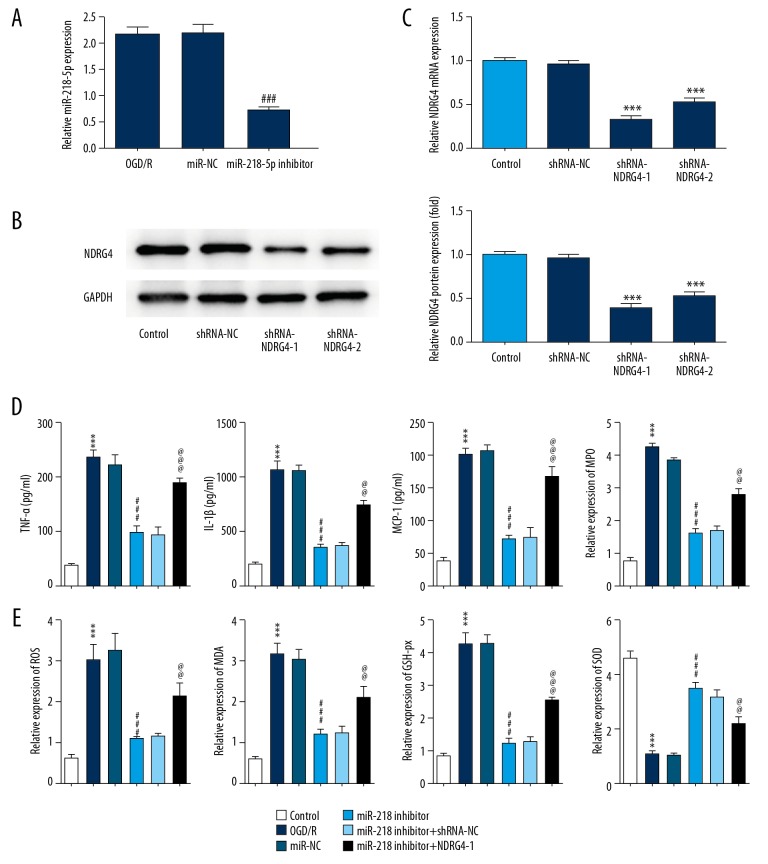
To investigate the mechanism underlying miR-218-5p in promoting cerebral ischemia, miR-218-5p inhibitor plasmid and NDRG4 interference plasmids were established to downregulate its expression level respectively. MiR-218-5p inhibitor, shRNA-NDRG4-1 and shRNA-NDRG4-2 significantly suppressed its expression level respectively. ShRNA-NDRG4-1 was chosen for the next study because of its better suppressive effect (Figure 2A–2C).
Figure 2.
NDRG4 silencing blocked the suppressive action of miR-218-5p downregulation in inflammatory cytokine release and oxidative stress status. (A) MiR-218-5p level was measured by RT-qPCR. (B, C) NDRG4 expression was analyzed by RT-qPCR and western blot. (D) The secretion levels of inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and monocyte chemotactic protein 1 (MCP-1) were detected by ELISA kits, myeloperoxidase (MPO) activities was analyzed with MPO assay kit. (E) The levels of oxygen species (ROS) production and malondialdehyde (MDA), glutathione peroxidase (GSH-Px) and activities of superoxide dismutase (SOD) were measured with specific assay kits. The data were expressed as means±SD; *** P<0.001 vs. Control. ### P<0.001 versus. OGD/R. @@ P < 0.01, @@@ P<0.001 versus miR-218-5p inhibitor. NDRG4 – N-myc downstream regulated gene 4; RT-qPCR – real-time quantitative polymerase chain reaction; ELISA – enzyme-linked immunosorbent assay; SD – standard deviation; OGD/R – oxidative-glucose deprivation/re-oxygenation.
Specific ELISA kits were employed to analyze the supernatant of OGD/R injured PC12 cells, the results showed that the release of pro-inflammatory cytokines, such as TNF-α, IL-1β, and MCP-1 was elevated markedly in injured PC12 cells group. MiR-218-5p inhibitor significantly inhibited the levels of TNF-α, IL-1β, and MCP-1, which was reversed by NDRG4 poor expression. Oxidative stress status was further measured using respective commercial test kits. Additionally, OGD/R treatment increased ROS production and MDA level, enhanced the activities of GSH-Px and MPO, and attenuated the activities of SOD in injured PC12 cells, which were reversed by miR-218-5p downregulation. However, the low expression of NDRG4 abolished the suppressive effect of miR-219-5p on oxidative stress status (Figure 2D, 2E). Thus, it was found that NDRG4 silencing blocked the suppressive effect of miR-218-5p downregulation on inflammatory cytokine release and oxidative stress status.
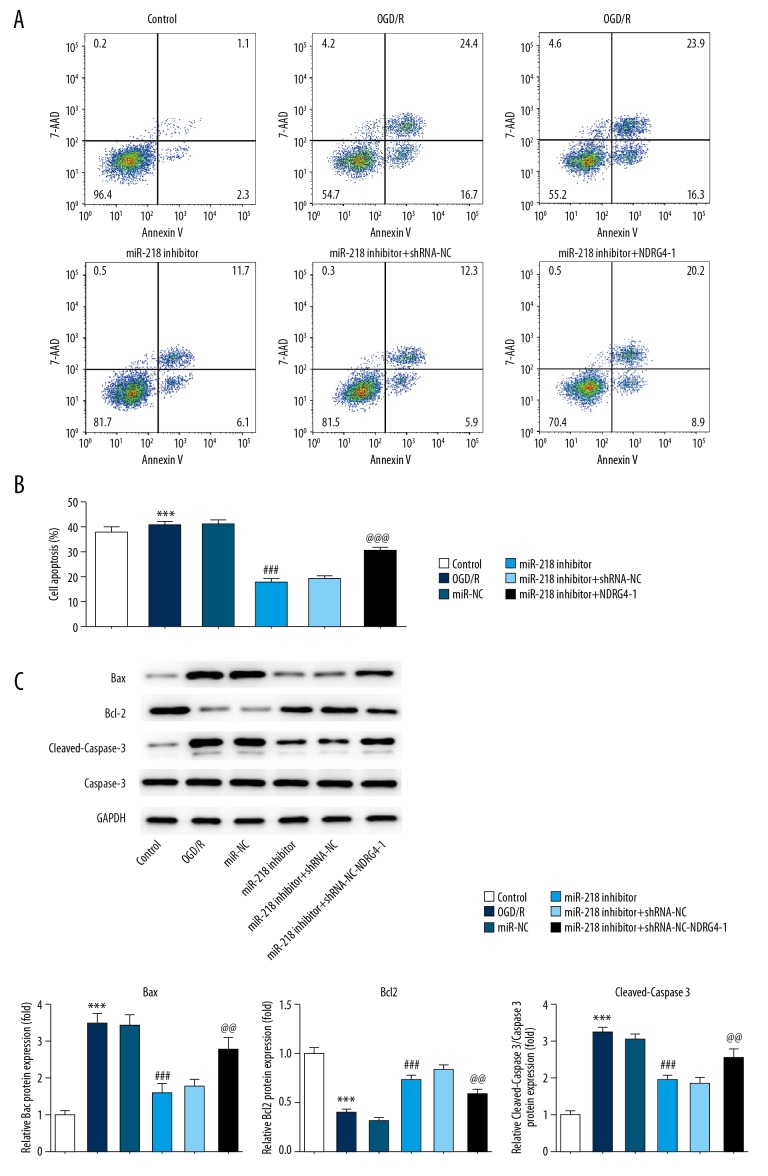
NDRG4 silencing abrogated miR-218-5p downregulation induced inhibitory effects on cell apoptosis
To further explore the mechanism underlying protective effect of miR-218-5p in cerebral ischemia stroke, flow cytometry was used to analyze the effect of miR-218-5p on cell apoptosis. As shown in Figure 3A, 3B, flow cytometry analysis results revealed that miR-218-5p inhibitor significantly decreased cell apoptosis induced by OGD/R, compared with miR-NC. However, low expression of NDRG4 notably enhanced cell apoptosis. Furthermore, to confirm the role of miR-218-5p on cell apoptosis, western blotting was employed to analyze the expression of protein related to cell apoptosis. The results showed that OGD/R condition induced increased expression levels of Bax, cleaved caspase-3 and decreased expression levels of Bcl2, which were corrected by miR-218-5p downregulation. As expected, low expression of NDRG4 abrogated the correction action of miR-218-5p downregulation in abnormal expression of above proteins (Figure 3C).
Figure 3.
NDRG4 silencing abrogated miR-218-5p downregulation induced inhibitory effects on cell apoptosis. (A, B) The cells apoptosis was assayed with flow cytometry. (C) Expressions levels of Bax, Bcl-2, cleaved caspase-3, and caspase-3 proteins were evaluated by western blotting. The data were expressed as means±SD; *** P<0.001 versus Control. ### P<0.001 versus OGD/R. @@ P<0.01, @@@ P<0.001 versus miR-218-5p inhibitor. NDRG4 – N-myc downstream regulated gene 4; SD – standard deviation; OGD/R – oxidative-glucose deprivation/re-oxygenation.
NDRG4 silencing abolished the protective effect of miR-218-5p downregulation in vascular endothelial functions
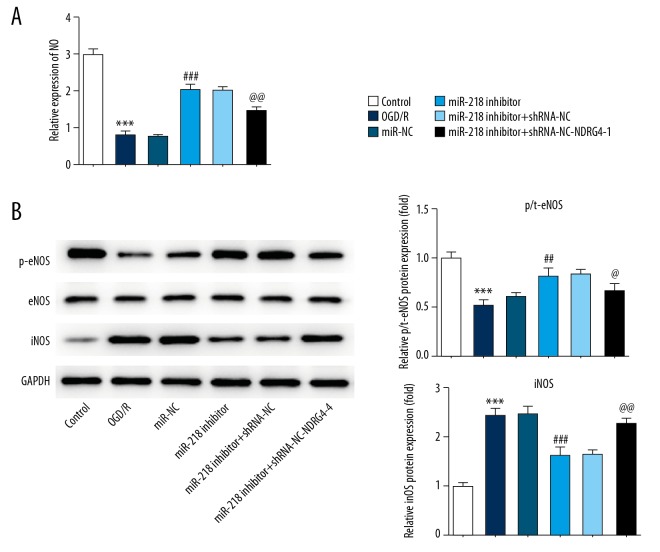
To evaluate the role of miR-218-5p in vascular endothelial functions, the level of NO and expression levels of eNOS and iNOS were analyzed by commercial assay kits and western blotting. The content of NO and p-eNOS expression were markedly inhibited in OGD/R injured PC12 cells. On the contrary, the iNOS expression was significantly increased by OGD/R treatment compared with miR-NC. MiR-218-5p inhibitor significantly ameliorated NO, p-eNOS levels and suppressed the iNOS expression level, which were abrogated by NDRG4 silencing (Figure 4).
Figure 4.
NDRG4 silencing blocked the protective effect of miR-218-5p downregulation on vascular endothelial functions. (A) The level of NO was analyzed with NO assay kit. (B) The expression levels of p-eNOS/eNOS and iNOS were measured by western blot. The data were expressed as means±SD; *** P<0.001 vs. Control. ## P<0.001, ### P<0.001 versus OGD/R. @ P<0.05, @@ P<0.01 versus miR-218-5p inhibitor. NDRG4 – N-myc downstream regulated gene 4; NO – nitric oxide; eNOS – endothelial nitric oxide synthase; iNOS – inducible nitric oxide synthase; SD – standard deviation; OGD/R – oxidative-glucose deprivation/re-oxygenation.
Discussion
Acute ischemic stroke (AIS) is primarily caused by vessel occlusion and induces a loss of brain function. AIS possesses potentially adverse outcomes and imposes a heavy burden on society [15]. Although in recent decades, great advances have achieved in prevention, diagnose and therapy of AIS [16,17], the molecular biology of AIS remains unclear. Identifying novel biomarkers with high feasibility is crucial for precise diagnosis and monitoring of disease progression. Recent studies have reported that miR-218-5p level in plasma is increased in patients with AIS [10,11]. In order to prove it, PC12 cells were chosen to establish OGD/R injury model. Present study revealed that miR-218-5p expression was elevated in OGD/R injured PC12 cells, in accordance with the previous clinical trials [10,11]. Moreover, miR-218-5p was also exhibited to target NDRG4 gene directly and inhibited its expression. In present study, miR-218-5p and NDRG4 expression was regulated to investigate molecular mechanism of miR-218-5p in potential pathogenesis of AIS.
Central nervous system inflammation is closely related to the development of brain damage induced by acute ischemia [18]. Furthermore, Hu et al. reported that Gualou Guizhi decoction have protective effect on ischemia-reperfusion brain damage through inactivation of the nuclear factor κ-B inflammation pathway [19]. In present study, marked pro-inflammatory factors, including TNF-α, IL-1β, and MCP-1, were observed after OGD/R treatment. MPO enzymatic activity was assessed to determine the extent of inflammation in injured PC12 cells. These data suggested that miR-218-5p downregulation remarkably inhibited inflammatory cytokines levels in OGD/R conditions, which were abolished by low expression of NDRG4. Thus, inflammation inhibition by miR-218-5p may be related to the neuroprotective effects. Furthermore, recent studies have centered on ameliorating patient outcomes and neurological dysfunction by inhibiting cell apoptosis and inflammatory responses after ischemic injury. Wu et al. demonstrated that Germacrone, identified as an anti-inflammatory compound extracted from Rhizoma curcuma, possesses neuroprotective effects in rat models of cerebral ischemia/reperfusion injury via antioxidative and antiapoptotic mechanisms [20]. Our data suggested that miR-215-5p downregulation decreased ROS production and MDA level, suppressed activities of GSH-Px and MPO, and elevated activities of SOD in OGD/R injured PC12 cells. However, low expression of NDRG4 elevated the oxidative stress status significantly. Echinocystic acid suppressed cell apoptosis and excessive inflammation and possessed neuroprotective effects through the inactivation of JNK pathway in cerebral I/R injury [21]. Xie et al. demonstrated that notoginseng leaf triterpenes inhibited the apoptosis of neuronal cells induced by cerebral ischemia and exert neuroprotective effects [22], which is consistent with our results. Our data implied that miR-218-5p downregulation induced inhibitory effects on cell apoptosis induced by OGD/R treatment, which were also abrogated by NDRG4 silencing. Therefore, all results demonstrated that miR-218-5p downregulation protect against OGD/R-induced injury through attenuating secretion levels of inflammatory cytokines, oxidative stress status and cell apoptosis rate by upregulating NDRG4.
Endothelial nitric oxide synthase (eNOS) is found to express predominantly in the vasculature and catalyzes the conversion of amino acid L-arginine to NO [23]. Impairment of eNOS activity is involved in the pathogenesis of endothelial dysfunction in numerous diseases including ischemic stroke [24]. Thus, eNOS plays crucial roles in the maintenance of endovascular homeostasis. The data confirmed that miR-218-5p downregulation significantly ameliorated NO and p-eNOS levels and suppressed the iNOS expression level. In accordance with our study, Zhang et al. demonstrated that the improvement of eNOS uncoupling and activation eNOS/NO ameliorated ischemia/reperfusion-induced brain endothelial permeability [25]. Maedeh et al. also reported that chronic morphine protected against IR injury via downregulating I/R-induced iNOS expression and modifying oxidative stress and NO production in the hippocampal tissue [26]. However, the protective action of miR-218-5p downregulation in vascular endothelial functions was significantly abolished by NDRG4 silencing. These data further confirm the protective action of miR-218-5p deletion on cerebral OGD/R-induced brain damage.
Conclusions
MiR-218-5p was related to pathogenesis of AIS through inflammation response, oxidative stress, cell apoptosis and vascular endothelial functions. Downregulation of miR-218-5p protects against oxygen-glucose deprivation/reperfusion-induced injuries of PC12 cells through reducing the secretion levels of inflammatory cytokines, oxidative stress status, cell apoptosis rate and maintenance of endovascular homeostasis by upregulating NDRG4. All results suggested that miR-218-5p may serve as a novel effective marker to monitor disease progression. Furthermore, these findings provide novel insight for defining alternative neuroprotective strategies in AIS.
Footnotes
Source of support: This work was supported by Wuhan Municipal Health Bureau Project (No. WX14C02)
References
- 1.Hankey GJ. Stroke. Lancet. 2017;389:641–54. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–24. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 3.Liberale L, Carbone F, Montecucco F, et al. Ischemic stroke across sexes: What is the status quo? Front Neuroendocrinol. 2018;50:3–17. doi: 10.1016/j.yfrne.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Reiche EMV, Gelinksi JR, Alfieri DF, et al. Immune-inflammatory, oxidative stress and biochemical biomarkers predict short-term acute ischemic stroke death. Metab Brain Dis. 2019;34:789–804. doi: 10.1007/s11011-019-00403-6. [DOI] [PubMed] [Google Scholar]
- 5.Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: A systematic review. JAMA. 2015;313:1451–62. doi: 10.1001/jama.2015.3058. [DOI] [PubMed] [Google Scholar]
- 6.Zheng T, Shi Y, Zhang J, et al. MiR-130a exerts neuroprotective effects against ischemic stroke through PTEN/PI3K/AKT pathway. Biomed Pharmacother. 2019;117:109117. doi: 10.1016/j.biopha.2019.109117. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Lei J, Ding L, et al. MicroRNA: function, detection, and bioanalysis. Chem Rev. 2013;113:6207–33. doi: 10.1021/cr300362f. [DOI] [PubMed] [Google Scholar]
- 8.Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics. 2011;43:521–28. doi: 10.1152/physiolgenomics.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan JR, Koo YX, Kaur P, et al. MicroRNAs in stroke pathogenesis. Curr Mol Med. 2011;11:76–92. doi: 10.2174/156652411794859232. [DOI] [PubMed] [Google Scholar]
- 10.Jin F, Xing J. Circulating miR-126 and miR-130a levels correlate with lower disease risk, disease severity, and reduced inflammatory cytokine levels in acute ischemic stroke patients. Neurol Sci. 2018;39:1757–65. doi: 10.1007/s10072-018-3499-7. [DOI] [PubMed] [Google Scholar]
- 11.Jin F, Xing J. Circulating pro-angiogenic and anti-angiogenic microRNA expressions in patients with acute ischemic stroke and their association with disease severity. Neurol Sci. 2017;38:2015–23. doi: 10.1007/s10072-017-3071-x. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto H, Kokame K, Okuda T, et al. NDRG4 protein-deficient mice exhibit spatial learning deficits and vulnerabilities to cerebral ischemia. J Biol Chem. 2011;286:26158–65. doi: 10.1074/jbc.M111.256446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou RH, Kokame K, Tsukamoto Y, et al. Characterization of the human NDRG gene family: A newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics. 2001;73:86–97. doi: 10.1006/geno.2000.6496. [DOI] [PubMed] [Google Scholar]
- 14.Zuo Y, Wang Y, Hu H, Cui W. Atorvastatin protects myocardium against ischemia-reperfusion injury through inhibiting miR-199a-5p. Cell Physiol Biochem. 2016;39:1021–30. doi: 10.1159/000447809. [DOI] [PubMed] [Google Scholar]
- 15.Lau AY, Wong KS, Lev M, et al. Burden of intracranial steno-occlusive lesions on initial computed tomography angiography predicts poor outcome in patients with acute stroke. Stroke. 2013;44:1310–16. doi: 10.1161/STROKEAHA.111.672741. [DOI] [PubMed] [Google Scholar]
- 16.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 17.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 18.Silva B, Sousa L, Miranda A, et al. Memory deficit associated with increased brain proinflammatory cytokine levels and neurodegeneration in acute ischemic stroke. Arq Neuropsiquiat. 2015;73:655–59. doi: 10.1590/0004-282X20150083. [DOI] [PubMed] [Google Scholar]
- 19.Hu HX, Lin RH, Zhu XQ, et al. Anti-inflammatory effects of Gualou Guizhi decoction in transient focal cerebral ischemic brains. [Corrected] Mol Med Rep. 2015;12:1321–27. doi: 10.3892/mmr.2015.3511. [DOI] [PubMed] [Google Scholar]
- 20.Wu T, Yin F, Kong H, Peng J. Germacrone attenuates cerebral ischemia/reperfusion injury in rats via antioxidative and antiapoptotic mechanisms. J Cell Biochem. 2019;120(11):18901–9. doi: 10.1002/jcb.29210. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Li W, Cao X, et al. Echinocystic acid, a natural plant extract, alleviates cerebral ischemia/reperfusion injury via inhibiting the JNK signaling pathway. Eur J Pharmacol. 2019;861:172610. doi: 10.1016/j.ejphar.2019.172610. [DOI] [PubMed] [Google Scholar]
- 22.Xie W, Zhu T, Dong X, et al. HMGB1-triggered inflammation inhibition of notoginseng leaf triterpenes against cerebral ischemia and reperfusion injury via MAPK and NF-kappaB signaling pathways. Biomolecules. 2019;9(10) doi: 10.3390/biom9100512. pii: E512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang T, Zhou S, Li X, et al. MicroRNA-155 induces protection against cerebral ischemia/reperfusion injury through regulation of the Notch pathway in vivo. Exp Ther Med. 2019;18:605–13. doi: 10.3892/etm.2019.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava K, Bath PM, Bayraktutan U. Current therapeutic strategies to mitigate the eNOS dysfunction in ischaemic stroke. Cell Mol Neurobiol. 2012;32:319–36. doi: 10.1007/s10571-011-9777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Hu X, Guo S, et al. Myricetin ameliorated ischemia/reperfusion-induced brain endothelial permeability by improvement of eNOS uncoupling and activation eNOS/NO. J Pharmacol Sci. 2019;140:62–72. doi: 10.1016/j.jphs.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Arabian M, Aboutaleb N, Ajami M, Habibey R. Interaction of mTOR and iNOS pathways in protection against Ischemia/Reperfusion injury. Iran J Pharm Res. 2019;18:785–92. doi: 10.22037/ijpr.2019.1100680. [DOI] [PMC free article] [PubMed] [Google Scholar]