Abstract
The European Commission asked EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) to deliver a scientific opinion on the safety and efficacy of ADRY+®. ADRY+® consists of hydroxy analogue of methionine (HMTBa) and its calcium salt (HMTBa‐Ca), both precursors of l‐methionine. HMTBa and HMTBa‐Ca are currently authorised for use as a nutritional additives, under the functional group ‘amino acids, their salts and analogues’. ADRY+® is produced by chemical synthesis and it is intended to be used in feed for all animal species and categories. The FEEDAP Panel concluded that ADRY+® is safe for the target animals. The use of the additive in animal nutrition is not expected to result in an accumulation of HMTBa or its metabolites in edible tissues and animal products. Therefore, its use does not raise safety concerns for the consumer. ADRY+® is irritant to eyes and not irritant to the skin. The FEEDAP Panel cannot conclude on the skin sensitisation potential of this additive. The exposure of the users to the additive by inhalation is expected to be low. The use of this product as a feed additive does not represent a risk to the environment. ADRY+® is an effective source of methionine for protein synthesis in non‐ruminant animals and fish, although HMTBa may show a lower bioefficacy than dl‐methionine. In ruminants, HMTBa is more slowly degraded in the rumen than dl‐methionine.
Keywords: feed additive, hydroxy analogue of methionine, HMTBa, hydroxy analogue of methionine calcium salt, HMTBa‐Ca, safety, efficacy
Summary
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of an additive (ADRY+®) made of hydroxy analogue of methionine (HMTBa) and calcium salt of hydroxy analogue of methionine (HMTBa‐Ca) for all animal species.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts’ knowledge, to deliver the present output.
The objective of feed supplementation with essential amino acids is to complete the amino acid profile of the diet in order to closely meet individual amino acid requirements of animals or to compensate for potential imbalances. Both components of ADRY+® are precursors of l‐methionine and are currently authorised for use as a nutritional additives, under the functional group ‘amino acids, their salts and analogues’. ADRY+® is produced by chemical synthesis and it is intended to be used in feed for all animal species and categories. The applicant recommends a supplementation level from 0.02 to 0.4%.
The additive is safe for target animals under the proposed conditions of use.
The use of the additive in animal nutrition is not expected to result in an accumulation of HMTBa or its metabolites in edible tissues and animal products. Therefore, its use does not raise safety concerns for the consumer.
The additive comprising HMTBa and the calcium salt of HMTBa is irritant to eyes and not irritant to the skin. The FEEDAP Panel cannot conclude on the skin sensitisation potential of this additive. The exposure of the users to the additive by inhalation is expected to be low.
The use of this product as a feed additive does not represent a risk to the environment.
The product under application is an effective source of methionine for protein synthesis in non‐ruminant animals and fish, although HMTBa may show a lower bioefficacy than dl‐methionine. HMTBa is more slowly degraded than dl‐methionine in ruminants.
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from Adisseo France SAS2 for authorisation of the additive consisting of hydroxy analogue of methionine and calcium salt of hydroxy analogue of methionine (ADRY+®), when used as a feed additive for all animal species (category: nutritional additives; functional group: amino acids, their salts and analogues).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive. The particulars and documents in support of the application were considered valid by EFSA as of 23 August 2016.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product made of hydroxy analogue of methionine and calcium salt of hydroxy analogue of methionine (ADRY+®), when used under the proposed conditions of use (see Section 3.1.6).
1.2. Additional information
The additive commercially named as ADRY+®, consisting of hydroxy analogue of methionine (HMTBa) and calcium salt of hydroxy analogue of methionine (HMTBa‐Ca), is the subject of the present assessment. It has not been previously assessed as a feed additive as such in the European Union but the two components separately (HMTBa and HMTBa‐Ca) have been assessed previously.
The EFSA FEEDAP Panel (2012a) published a scientific opinion on dl‐methionine, dl‐methionine sodium salt, hydroxy analogue of methionine (HMTBa), calcium salt of methionine hydroxy analogue (HMTBa‐Ca) for all animal species; isopropyl ester of methionine hydroxy analogue and dl‐methionine technically pure protected with copolymer vinylpyridine/styrene for dairy cows; dl‐methionine technically pure protected with ethylcellulose for ruminants. The EFSA FEEDAP Panel (2013b) published a scientific opinion on the safety and efficacy of l‐methionine produced by Escherichia coli (KCCM 11252P and KCCM 11340P) for all animal species. The EFSA FEEDAP Panel (2015) published a scientific opinion on the safety and efficacy of dl‐methionyl‐dl‐methionine for all aquatic animal species. The EFSA FEEDAP Panel (2013a) published an opinion on the safety and efficacy of hydroxy analogue of selenomethionine for all animal species. The FEEDAP Panel published opinions on zinc/copper/manganese chelate of hydroxy analogue of methionine (EFSA FEEDAP Panel, 2008a,b,c, respectively).
The additives dl‐methionine, dl‐methionine sodium salt, hydroxy analogue of methionine (HMTBa) and calcium salt of methionine hydroxy analogue (HMTBa‐Ca), when used as a feed additive for all animal species, isopropyl ester of methionine hydroxy analogue, dl‐methionine technically pure protected with copolymer vinylpyridine/styrene and dl‐methionine protected with ethylcellulose when used as a feed additive for ruminants, are currently authorised by Regulation (EU) No 469/2013.3 HMTBa is authorised with a minimum 88% HMTBa and maximum 12% water, whereas HMTBa‐Ca is authorised with a minimum of 84% HMTBa, a minimum of 11.7% calcium and a maximum of 1% water. l‐Methionine is currently authorised as a flavouring substance in feed.
According to Commission Directive 2006/141/EC amino acids such as l‐methionine may be used in the manufacture of infant formulae and follow‐on formulae in order to satisfy the requirements on amino acids and other nitrogen compounds. It is included in the Community list of flavouring substances as FL. No. 17.027. Methionine is registered as an ingredient for use in cosmetics as antistatic and for skin conditioning (Commission Decision 2006/257/EC)4. The Scientific Committee on Animal Nutrition (European Commission, 2003) assessed the safety and efficacy of isopropyl ester of the hydroxylated analogue of methionine for dairy cows.
dl‐Methionine is described in the European Pharmacopoeia, monograph 01/2008:0624. Methionine does not require maximum residue levels in all food producing species when used as pharmacologically active substance.5
The Panel on nutrition, dietetic products, novel food and allergy of the Norwegian Scientific Committee for Food Safety (VKM, 2013) performed a risk assessment of histidine, methionine, S‐adenosylmethionine and tryptophan.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier6 in support of the authorisation request for the use of an additive made of hydroxy analogue of methionine and calcium salt of hydroxy analogue of methionine (ADRY+®) as a feed additive for all animal species. The technical dossier was prepared following the provisions of Article 7 of Regulation (EC) No 1831/2003, Regulation (EC) No 429/20087 and the applicable EFSA guidance documents.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts’ knowledge, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the additive made of hydroxy analogue of methionine and calcium salt of hydroxy analogue of methionine (ADRY+®) in animal feed. The Executive Summary of the EURL report can be found in Annex A.8
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of an additive made of hydroxy analogue of methionine and calcium salt of hydroxy analogue of methionine (ADRY+®) for all animal species is in line with the principles laid down in Regulation (EC) No 429/2008 and the relevant guidance documents: Guidance on nutritional additives (EFSA FEEDAP Panel, 2012b), Technical guidance: Tolerance and efficacy studies in target animals (EFSA FEEDAP Panel, 2011), Technical Guidance for assessing the safety of feed additives for the environment (EFSA FEEDAP Panel, 2008d), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012c) and Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012d).
3. Assessment
An additive made of hydroxy analogue of methionine (HMTBa) and calcium salt of hydroxy analogue of methionine (HMTBa‐Ca), for all animal species, commercially named ADRY+®, is the subject of the present assessment. It is proposed to be used in animal nutrition in the category nutritional feed additive, amino acids, their salts and analogues as a source of methionine.
3.1. Characterisation
3.1.1. Characterisation of the active substances
HMTBa has the IUPAC name 2‐hydroxy‐4‐(methylthio)butanoic acid and the CAS number 583‐91‐5. Its molecular weight is 150.2 g/mol, its molecular formula is C5H10O3S and its molecular structure is given in Figure 1.
Figure 1.
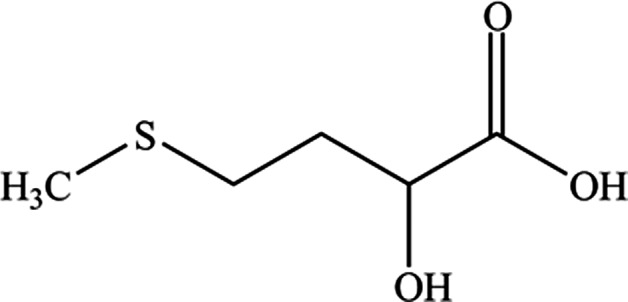
Molecular structure of hydroxy analogue of methionine
HMTBa‐Ca has the IUPAC name 2‐hydroxy‐4‐(methylthio)butanoic acid, calcium salt; and the CAS number 4857‐44‐7. The molecular weight of the active substance is 338.45 g/mol, its molecular formula is (C5H9O3S)2Ca and its molecular structure is shown in Figure 2.
Figure 2.
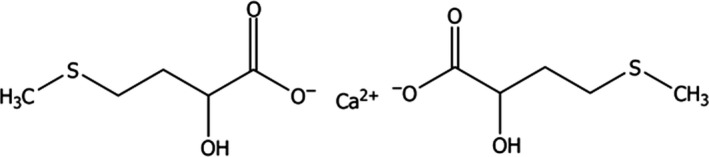
Molecular structure of the calcium salt of hydroxy analogue of methionine
3.1.2. Manufacturing process9
The dossier contains information on the manufacturing process of the additive which is based on chemical synthesis. The substances involved have been described in the dossier and the respective material safety data sheets were made available.10 , 11 , 12 A part of monomeric HMTBa, the additive contains a fraction of oligomers of HMTBa (condensation products in the form of dimers and trimers).13
3.1.3. Characterisation of the additive14
The product is specified to contain ≥ 88% HMTBa (originating from HMTBa and HMTBa‐Ca) and > 8% calcium. Analysis of five batches by a titrimetric method (total organic sulfur; TOS) indicated an average content of HMTBa of 89.7% (range 89.4–90.0%).15 Calcium and water content were not provided.
Further analytical data, specific for HMTBa, of five batches showed that the HMTBa content was on average 88.5% (range 88.4–88.7%, TOS method), the calcium content is on average 8.8% (range 8.8–8.9%) and the loss on drying 1.3% (range 1.2–1.4%). The amount of identified material on dry matter basis is 98.6%.16 The distribution of different oligomers of HMTBa in the product was further investigated by liquid chromatography and the results are described in the technical dossier.17 From the information provided (percentage distribution), it is not possible however to assess the absolute concentration of HMTBa and its oligomers in the final product. Thus, it is possible that the amount of unidentified substances is larger than indicated by the applicant.
The isomeric composition of the product is described in the dossier. The d‐ and l‐forms of HMTBa are both precursors of l‐methionine.
3.1.3.1. Impurities
Three batches were analysed for heavy metals and arsenic content. Lead ranged from 0.09 to 0.14 mg/kg, cadmium from 0.039 to 0.058 mg/kg, mercury from 0.013 to 0.018 mg/kg and arsenic from 0.11 to 0.16 mg/kg. Fluorine was < 20 mg/kg. Dioxins ranged from 0.062 to 0.064 ng WHO‐PCDD/F‐TEQ/kg; the sum of dioxins and dioxin‐like polychlorinated biphenyls (PCBs) from 0.101 to 0.104 ng/kg, and non‐dioxin‐like PCBs ranged from 0.362 to 0.374 ng WHO‐PCDD/F‐TEQ/kg.18 Aflatoxins B1, B2, G1, G2 (only one batch analysed) were below the limit of quantification.19
Regarding the microbiological contamination of the final product (three batches analysed), total count, Escherichia coli, Enterobacteriaceae and anaerobic sulfite reducers were < 10 CFU/g (except one batch that had total counts of 13 CFU/g). Salmonella spp. were absent (25 g sample) and yeast and moulds were < 100 CFU/g.20
Hydrocyanic acid is an undesirable substance that may originate from the production process. The analysis of three batches of the final product showed values ranging from 7.7 to 8.6 mg/kg.
3.1.3.2. Physicochemical properties
The additive is a light beige powder with a sulfurous odour.21 Solubility in water is 5% at 25°C.22 The bulk density of five batches ranged from 0.39 to 0.41 g/cm3.23
Particle size distribution was analysed by laser diffraction (v/v) in five batches of the additive; the percentage of particles < 100 μm diameter ranged from 2.4 to 8.4%, that of < 50 μm diameter from 0.1 to 2.1% and that of < 10 μm diameter from 0.2 to 1.1%.24 The average particle size ranged from 240 to 365 μm diameter. The dusting potential (Stauber–Heubach method) of these five batches ranged from 0.15 to 0.19 g/m3.
3.1.4. Stability and homogeneity
3.1.4.1. Shelf life
HMTBa was determined by potentiometric titration followed by an oxidation‐reduction reaction, where only organic sulfur is measured. Thus, the survival of HMTBa itself is uncertain.
The stability of three batches of the additive was studied at three different temperatures (5, 25 and 30°C). The additive was packed in sealed low‐density polyethylene bags (semi‐permeable) and placed inside a cardboard envelope (similar to market packaging) and stored for one year.25 Losses were ≤ 1.2% in all cases.
3.1.4.2. Stability in premixtures
HMTBa was measured using reverse phase high‐performance liquid chromatography (RP‐HPLC) with UV detection after alkaline hydrolysis of the polymers contained in extracts obtained after solid ‘dilution’ of the premixes with maize meal (to avoid interactions due to the high concentrations of minerals).26 All the HMTBa components of the additive are transformed to HMTBa monomers.
The stability of the additive (six batches) was studied in two different vitamin/mineral premixtures, one for poultry and one for pigs (three batches each), both supplemented at 8% and stored at 25°C for 6 months.27 Packaging was made as described above (see Section 3.1.4.1). The pig premixture contained choline chloride (167 g/kg). Losses observed in the poultry and pig premixtures ranged from 16% to 22% and from 6% to 17% HTMBa on a dry matter basis, respectively.
3.1.4.3. Stability in feedingstuffs
HMTBa was measured using RP‐HPLC with UV detection after alkaline hydrolysis, which causes all the HMTBa oligomers to be transformed to HMTBa monomers.
The stability of the additive (three batches) was studied in two different feedingstuffs, one for poultry and one for pigs, in mash and in pelleted form. Poultry feed was supplemented at 0.35% and pig feed at 0.15%. Poultry feed was based on wheat, soybean meal, extruded soybeans and palm oil; pig feed was based on wheat, soybean meal and barley and contained choline chloride. Feeds were stored at 25 and 30°C for 3 months.28 Packaging was done as described above (see Section 3.1.4.1). In poultry feed, losses observed in meal at 25°C ranged from 7% to 15% HMTBa on dry matter basis and those observed at 30°C ranged from 1% to 10%; as regards pelleted feed, losses observed at 25°C were up to 0.2% and losses at 30°C ranged from 3% to 4%. In pig feed, losses observed in meal at 25°C ranged from 1% to 17% HMTBa on dry matter basis and those observed at 30°C ranged from 12% to 18%; as regards pelleted feed, losses observed at 25°C ranged from 5% to 6% and losses at 30°C ranged from 1 to 13%. Pelleting process represented a loss of 0.5% to 5.6% in chicken feed and of 0% to 6.6% in pig feed.
3.1.4.4. Homogeneity
The capacity of the additive to distribute homogeneously in premixtures was studied in the premixtures described above (see Section 3.1.4.2). Ten subsamples per premixture were analysed. The coefficient of variation (CV) of the poultry premixture was 2.7% and the one of the pig premixture 1.2%.26
The capacity of the additive to distribute homogeneously in feedingstuffs was studied in the compound feeds described above (see Section 3.1.4.3). Ten subsamples of mash and ten of pelleted feed were analysed from both poultry and pigs feed, each mash or pelleted. The CV of poultry meal was 2.8% and that of poultry pelleted feed 1.7%. The CV of pig meal was 1.8% and that of pig pelleted feed 2.9%.29
3.1.5. Physicochemical incompatibilities or interactions
No physicochemical incompatibilities in feed are expected when used with other additives, medicinal products or other feed materials.
3.1.6. Conditions of use
The additive is intended to be used in feed for all animal species and categories with no minimum or maximum content specified. It is proposed to be supplemented in feedingstuffs via premixtures or directly into complete feed and complementary feed. The level of supplementation will depend on the dietary composition and animal requirements of methionine. The applicant recommends a supplementation level from 0.02% to 0.4%.30
3.2. Safety
HMTBa is absorbed and metabolised to methionine in the small intestine, in liver and kidney of non‐ruminants (including poultry and fish); and mainly in non‐hepatic tissues in ruminants (Dibner and Knight, 1984; Zhang et al., 2015). The two‐step metabolic conversion involves the oxidation of the alpha‐C of HMTBa, resulting in the formation of 2‐oxo‐4‐(methylthio)butanoic acid, followed by the transamination of the alpha‐C to form methionine (HMTBa is more slowly metabolised than dl‐methionine in the rumen (Belasco, 1980)). Like methionine, HMTBa forms dimethyl sulfide (Clark and Salsbury, 1980) and volatile fatty acids (Belasco, 1980) in the rumen, but both are formed more slowly from HMTBa.
Hydrocyanic acid is an undesirable substance that may originate from the production process. The analysis of three batches of the final product showed values ranging from 7.7 to 8.6 mg/kg. Annex I of directive 2002/32/EC sets maximum levels of 50 mg hydrocyanic acid/kg complete feed for all food producing species except for young chickens (< 6 weeks) that have a maximum level of 10 mg hydrocyanic acid/kg complete feed. Thus the residual concentrations of hydrocyanic acid in the product raise no safety concerns for the target species, consumer, user or the environment.
3.2.1. Safety for the target species
The additive under application is a mixture of two already authorised products for which EFSA has already assessed safety for the target animals (EFSA FEEDAP Panel, 2012a, Section 3.1.1). HMTBa originates from the same producer and the manufacturing process has not changed; and the production process of HMTBa‐Ca is essentially the same described in the EFSA opinion and not expected to introduce undesirable substances of concern. The combination of HMTBa and HMTBA‐Ca in a feed additive will not result in additional risks for the target animals. The additive contains on average 98.6% of identified material. Based on the review of the literature and on the reasons explained above, the FEEDAP Panel considers that the additive under assessment is safe for the target species and that no further tolerance studies are required. Nonetheless, a tolerance study in chickens for fattening was provided as supportive evidence.31 The study, however, could not be considered in the assessment because the negative control group was fed a methionine deficient diet without supplemented methionine.
3.2.2. Safety for the consumer
Absorption, distribution, metabolism and excretion of methionine and HMTBa were described in a previous opinion (EFSA FEEDAP Panel, 2012a). The d‐ and l‐ isomers of HMTBa will be oxidised to keto analogue of methionine and then transaminated to l‐methionine by different HMTBa‐converting enzymes present in tissues such as intestine, liver or kidney (Gordon and Sizer, 1965; Dibner and Knight, 1984; Dupuis et al., 1989, 1990; Ferjancic‐Biagini et al., 1995; Rangel‐Lugo and Austic, 1998; Martín‐Venegas et al., 2006). Neither HMTBa nor its metabolites are expected to accumulate in edible tissues and animal products. Using the combination of HMTBa and HMTBa‐Ca is not expected to result in an additional exposure for the consumer. Therefore, the use of this feed additive does not raise safety concerns for the consumer.
3.2.3. Safety for the user
3.2.3.1. Effects on the respiratory system
The physicochemical properties of the additive are described in Section 3.1.3. Data on particle size distribution and on dusting potential of the additive indicate a low potential for inhalation exposure.
3.2.3.2. Effects on skin and eyes
The following tests were performed with the product under assessment (ADRY+® containing 88.3% HMTBa and 8.9% calcium).
Skin irritation potential was investigated in an in vitro study (Episkin™ test) in accordance with OECD Guideline 439.32 In an artificial three‐dimensional model of human skin, a dose of 10 mg ADRY+® was applied as a solid test item to the model skin surface. The exposure lasted 15 min and was followed by 42 h incubation in fresh medium. A negative control was exposed to 10 μL Dulbecco's phosphate‐buffered saline, and a positive control to 10 μL of 5% aqueous sodium dodecyl sulfate. Three tissues per treatment group were used. The mean viability of ADRY+®‐exposed cells was 92% of the negative control. As the mean viability of exposed cells was above the cut‐off for a 15‐min exposure of 50%, the interleukin‐1 (IL‐1) alpha concentrations in culture media retained samples from the three negative controls and the three test item‐treated tissues were analysed by enzyme‐linked immunosorbent assay (ELISA). The mean IL‐1 alpha concentration for treated tissues was 34.4 pg/mL. As it was below the threshold of 60 pg/mL, the additive is classified as non‐irritant to skin.
Skin irritation potential was also investigated in an in vivo dermal irritation/corrosion test in accordance with OECD Guideline 404.33 No effects on body weight and no mortality were observed. A slight erythema (score 1 on a scale of 4) was observed in all three rabbits at 1 and 24 h and in only one at 48 h, having disappeared at 72 h. The primary irritation index was 0.44. Hence, according to the UN Globally Harmonised System of Classification and Labelling of Chemicals (GHS, 2011), the product is not considered a skin irritant.
The eye irritation potential was investigated in an in vitro study (bovine corneal opacity and permeability test) in accordance with OECD Guideline 437.34 The opacity value was 106 and the permeability value (quantity of sodium fluorescein dye that passes across the full thickness of the cornea) was 0.4 compared with the negative control, resulting in an in vitro irritancy score of 112 (cut off for a test item inducing serious eye damage is > 55). Hence, ADRY+ was considered as a chemical inducing serious eye damage.
As the in vitro test was clearly positive, no in vivo ocular irritation/corrosion test was performed.
Skin sensitisation was not investigated for this product.
3.2.3.3. Conclusions on safety for the user
The additive is irritant to eyes and not irritant to the skin. The FEEDAP Panel cannot conclude on the skin sensitisation potential of this additive. The exposure of the users to the additive by inhalation is expected to be low.
3.2.4. Safety for the environment
Methionine is a physiological and natural component in animals and plants. Like its salts and the hydroxy analogue, it is not excreted as such (but as urea/uric acid, sulfate and CO2). The use of methionine, its analogue and their salts in animal nutrition would not lead to any localised increase in the concentration in the environment. It is concluded that the use of ADRY+® as a feed additive does not represent a risk to the environment.
3.3. Efficacy
3.3.1. Efficacy in non‐ruminants
The applicant submitted a long‐term efficacy study in chicken for fattening where a dose (0.4%) of ADRY+® was tested in comparison with an unsupplemented control group deficient in sulfur amino acids (0.3%).35 Over the 35‐day treatment period with the additive supplementation in feed, an increased body weight gain and feed intake and improved feed to gain ratio was observed. However, there is no indication from this experiment of the efficacy of the additive relative to a treatment group with a balanced diet in sulfur amino acids.
The efficacy of dl‐methionine and of HMTBa in non‐ruminant animals including fish is described in numerous publications, and has been assessed previously by the EFSA FEEDAP Panel (2012a). In particular, a meta‐analysis of 46 dose–response studies from 27 peer‐reviewed papers (Sauer et al., 2008) concluded that HMTBa and its salts have lower bioavailability than dl‐methionine in chickens for fattening. The meta‐analysis showed that biological efficiencies of HMTBa were 81 and 79% of the values for dl‐methionine, on an equimolar basis, for average daily gain (ADG) and feed/gain, respectively. Although some more recent papers (Zou et al., 2015; Agostini et al., 2016; Kluge et al., 2016; Vazquez‐Añon et al., 2017) appeared to demonstrate bioequivalence between HMTBa and dl‐methionine in poultry, the FEEDAP Panel concludes on the weight of evidence that HMTBa is likely to be less efficacious than dl‐methionine in all non‐ruminant species. The main reasons for this lower bioefficacy are (i) the fact that the gut microbiota of the small intestine compete with the host more for HMTBa than for dl‐methionine (Drew et al., 2003; Malik et al., 2009), (ii) commercial HMTBa and its salts may contain besides the free acid/salt, significant amounts of dimers, trimers and oligomers, known to have a lower bioefficacy depending on the manufacturing process (Boebel and Baker, 1982; Saunderson, 1991; Van Weerden et al., 1992; Liu et al., 2004; Hoehler et al., 2005; Zimmerman et al., 2005; Dilger and Baker, 2008; Elwert et al., 2008; Shoveller et al., 2010; Opapeju et al., 2012; Zelenka et al., 2013; Sangali et al., 2014) (see Appendix A).
3.3.2. Efficacy of HMTBa in ruminants
As in non‐ruminants, HMTBa is a precursor of dl‐methionine once absorbed (EFSA FEEDAP Panel, 2012a). Although the degradation of HMTBa in the rumen lowers its efficacy as a source of dl‐methionine for the host animal, the ruminal degradation rate of HMTBa is about 40% that of dl‐methionine (Patterson and Kung, 1988), and 20–60% of dietary HMTBa may escape ruminal degradation depending on the liquid passage rate (Koenig et al., 1999, 2002). Dietary HMTBa leads to increased plasma methionine concentrations (Koenig et al., 1999), demonstrating its nutritive value in ruminants. The HMTBa can also be utilised by ruminal microorganisms, and has been shown to increase microbial protein synthesis by up to 22% at a 0.1% supplementation rate (Vazquez‐Anon, 2005). Other effects observed with HMTBa include increased protozoal numbers (Patton et al., 1970a), decreased ruminal stearate concentration (Patton et al., 1970b), increased total tract ADF digestibility (+ 17%; Bull and Vandersall, 1973) and ruminal neutral detergent fibre (NDF) (+ 9%; Noftsger et al., 2005) degradability.
3.3.3. Conclusions on efficacy for the target species
Based on the available literature, the additive under application is considered an effective source of methionine for protein synthesis in non‐ruminant animals and fish. HMTBa shows a somewhat lower bioefficacy than dl‐methionine in non‐ruminants. In contrast, HMTBa is more slowly degraded than dl‐methionine in ruminants and therefore may partially escape ruminal degradation.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation36 and Good Manufacturing Practice.
4. Conclusions
The additive is safe for target animals under the proposed conditions of use.
The use of the additive in animal nutrition is not expected to result in an accumulation of HMTBa or its metabolites in edible tissues and animal products. Therefore, the use of this feed additive does not raise safety concerns for the consumer.
The additive comprising HMTBa and the calcium salt of HMTBa is irritant to eyes and not irritant to the skin. The FEEDAP Panel cannot conclude on the skin sensitisation potential of this additive. The exposure of the users to the additive by inhalation is expected to be low.
The use of this product as a feed additive does not represent a risk to the environment.
The product under application is an effective source of methionine for protein synthesis in non‐ruminant animals and fish, although HMTBa shows a lower bioefficacy than dl‐methionine. HMTBa is more slowly degraded than dl‐methionine in ruminants.
Documentation provided to EFSA
Preparation of HMTBa and calcium salt of HMTBa (ADRY+®). August 2016. Submitted by Adisseo France SAS.
Preparation of HMTBa and calcium salt of HMTBa (ADRY+®). Supplementary information January 2017. Submitted by Adisseo France SAS.
Preparation of HMTBa and calcium salt of HMTBa (ADRY+®). Supplementary information April 2017. Submitted by Adisseo France SAS.
Evaluation report of the European Union Reference Laboratory for Feed Additives on the Methods of Analysis for Preparation of hydroxy analogue of Methionine (HMTBa) and calcium salt of HMBTa.
Comments from Member States.
Abbreviations
- AAS
atomic absorption spectrometry
- ADF
acid detergent fibre
- ADG
average daily gain
- CAS
Chemical Abstracts Service
- CFU
colony forming unit
- CV
coefficient of variation
- ELISA
enzyme‐linked immunosorbent assay
- EURL
European Union Reference Laboratory
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- ICP‐AES
inductively coupled plasma atomic emission spectrometry
- IL‐1
interleukin‐1
- IUPAC
International Union of Pure and Applied Chemistry
- NDF
neutral detergent fibre
- OECD
Organisation for Economic Co‐operation and Development
- PCB
polychlorinated biphenyl
- PCDD/F
polychlorinated dibenzo‐p‐dioxin/dibenzofuran
- RP‐HPLC
reversed phase high performance liquid chromatography
- RSDr
relative standard deviation for repeatability
- RSDip
relative standard deviation for intermediate precision
- Rrec
recovery rate
- SCAN
Scientific Committee on Animal Nutrition
- TEQ
toxic equivalent
- TOS
total organic sulfur
- UV
ultraviolet
- WHO
World Health Organization
Appendix A – Bioefficacy of monomers compared to dimers, trimers and oligomers of HMTBa/HMTBa‐Ca
1.
The efficacy of dl‐methionine and of HMTBa in non‐ruminant animals including fish is described in numerous publications, and has been assessed previously by the EFSA FEEDAP Panel (2012a) (see also Section 3.3.1).
Regarding the bioefficacy of the monomers compared to the dimers, trimers and oligomers of HMTBA/HMTBA‐Ca, indeed only a few authors compared specifically the efficacy of HMTBa/HMTBa‐Ca of both monomers/non‐monomers vs dl‐methionine. However, there is also supporting data with physiological studies:
-
Van Weerden et al. (1992) used two products: (1) product dl‐HMTBa containing 68% monomers on a total of 87.4% free acids (based on analysis) 2) product containing 95% polymers and 5% monomers (based on analysis). Two trials were performed. Bioefficacy on equimolar (isosulfurous) basis:
-
–
Experiment I: dl‐methionine 100%; dl‐HMTBa 77%; dl‐HMTBa‐polymers 57%;
-
–
Experiment II: dl‐methionine 100%; dl‐HMTBa 76%; dl‐HMTBa‐polymers 56%.
-
–
-
Boebel and Baker (1982), used four products: dl‐methionine, HMTBa‐Ca, HMTBa (Alimet Degussa) containing 65% monomers and 17% dimers (analysed) and HMTBa‐P containing 35% dimers, 30% trimers and 12% higher oligomers (analysed). Two trials were performed. Efficacy on molar/isosulfurous basis:
-
–
Experiment I: dl‐methionine 100%, HMTBa‐Ca 87.4%, HMTBa 78.0%, HMTBa‐polymers 69.4%;
-
–
Experiment II: dl‐methionine 100%, HMTBa‐Ca 81.8%, HMTBA 80.8%, HMTBa‐polymers 53.6%.
-
–
The lower bioefficacy of dimers, trimers and oligomers normally present in commercial products of HMTBa/HMTBa‐Ca, compared to the main component being monomers, was further supported by the experiments of Koban and Koberstein (1984), showing a lower hydrolysis of oligomeric HMTBa, under physiological conditions of pH, temperature and retention time simulating gastric conditions, than dimeric and trimeric HMTBa. This is also in accordance with the outcome of the studies from Saunderson (1991). In these experiments, HMTBa from a different producer was compared with different methionine compounds: dl‐Meth, HMTBa‐Ca and MHB‐oligomers. Chickens fed oligomers isolated from HMTBA showed significantly more residual HMTBa in ileal digesta (8–10 animals, TiO2 as a marker) and excreta (24 h, 6 animals) than animals fed equimolar levels of dl‐methionine or HMTBa/HMTBa‐Ca, indicating a significant lower utilisation of this oligomer fraction.
From the data reported above:
There is convincing evidence that HMTBa/HMTBa‐Ca, on an equimolar/isosulfurous basis, have a significant lower bioefficacy than dl‐methionine (75% vs 100%).
There is substantial evidence that, on an equimolar/isosulfurous basis, the non‐monomeric components (dimers, trimers and oligomers) in commercial HMTBa/HMTBa‐Ca products have a significant lower bioefficacy than the monomer fraction (53.6% to 69.4% vs 76% to 80.8%; dl‐methionine set at 100%).
References
Boebel KP and DH Baker, 1982. Efficacy of calcium salt and free acid forms of methionine hydroxy analog for chicks. Poultry Science, 61, 1167–1175.
Koban HG and Koberstein E, 1984. Kinetics of hydrolysis of dimeric and trimeric methionine hydroxy analogue free acid under physiological conditions of pH and temperature. Journal of Agricultural and Food Chemistry, 32, 393‐396.
Saunderson CL, 1991. Metabolism of methionine and its nutritional analogs. Poultry International, 30, 34–38.
Van Weerden EJ, Schutte JB and Bertram HL, 1992. Utilization of the polymers of methionine hydroxy analogue free acid (HMTBa) in broiler chicks. Archiv für Geflügelkunde, 56,63–68.
Annex A – European Union Reference Laboratory evaluation report on the analytical methods submitted with the application Preparation of hydroxy analogue of Methionine (HMTBa) and calcium salt of HMBTa
1.
In the current application authorisation is sought under article 4(1) for the preparation of hydroxy analogue of Methionine (HMTBa) and calcium salt of HMTBa under the category/functional group 3(c) ‘nutritional additives’/‘amino acids, their salts and analogues’ according to the classification system of Regulation (EC) No 1831/2003. Specifically, authorisation is sought for the use of the feed additive for all animal species. The preparation (feed additive) is a light beige powder, consisting of a minimum of 88% of HMTBa and minimum of 8% of calcium in the feed additive. The feed additive is intended to be incorporated directly into feedingstuffs or through premixtures with no minimum or maximum dose proposed. However, the Applicant suggested typical inclusion levels ranging from 0.2 to 4 g of the feed additive/kg complete feedingstuffs.
The Applicant submitted two single‐laboratory validated and further verified methods based on: (i) a potentiometric titration after a redox reaction for the quantification of HMTBa in the feed additive; and (ii) Reversed Phase High Performance Liquid Chromatography coupled with UV detection at 214 nm (RP‐HPLC‐UV) for the quantification of HMTBa in premixtures and feedingstuffs containing the preparation.
The following performance characteristics were reported for the above mentioned titrimetric method:
a relative standard deviation for repeatability (RSDr) ranging from 0.1 to 0.2%;
a relative standard deviation for intermediate precision (RSDip) of 0.2%; and
a recovery rate (Rrec) ranging from 100 to 101%.
The performance characteristics reported for the above mentioned RP‐HPLC‐UV method for the mass fractions of HMTBa in premixtures and feedingstuffs ranging from 0.16 to 6.6 g/kg, were as follows:
a RSDr ranging from 0.4 to 2.7%;
a RSDip ranging from 1.6 to 2.7%; and
a Rrec ranging from 89 to 99%.
Furthermore, both methods were previously recommended by the EURL in the frame of report FAD‐2010‐0023.
Based on the performance characteristics presented, the EURL recommends for official control these two methods for the quantification of HMTBa in the feed additive, premixtures and/or feedingstuffs.
The Applicant did not submit any method for the quantification of calcium in the feed additive, however the EURL identified three suitable CEN methods: EN ISO 6869:2000, based on Atomic Absorption Spectrometry (AAS), EN 15510:2007 or EN 15621:2012, based on Inductively Coupled Plasma Atomic Emission Spectrometry (ICP‐AES) after dissolution of the feed additive.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa Roberto Edoardo, Wester P, Costa L, Dierick N, Leng L, Tarrés‐Call J and Wallace RJ, 2018. Scientific Opinion on the safety and efficacy of hydroxy analogue of methionine and its calcium salt (ADRY+®) for all animal species. EFSA Journal 2018;16(3):5198, 17 pp. 10.2903/j.efsa.2018.5198
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00370
Panel members: Gabriele Aquilina, Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Georges Bories, Andrew Chesson, Pier Sandro Cocconcelli, Gerhard Flachowsky, Jürgen Gropp, Boris Kolar, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Alberto Mantovani, Baltasar Mayo, Fernando Ramos, Guido Rychen, Maria Saarela, Roberto Edoardo Villa, Robert John Wallace and Pieter Wester.
Acknowledgements: The EFSA FEEDAP Panel wishes to thank the following for the support provided to this scientific output: Jaume Galobart, Matteo Innocenti and Gloria López Gálvez.
Note: This scientific opinion is published following the EC decision on the confidentiality claims submitted by the applicant as per Article 8(6) and Article 18 of Regulation (EC) No 1831/2003. The sections amended are identified in the opinion. The original version has been removed from the EFSA Journal.
Adopted: 20 February 2018
Amended: 13 Oct 2020
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Adisseo France S.A.S. lmmeuble Antony Parc II‐10 place du General de Gaulle, 92160 Antony, France.
Commission implementing Regulation (EU) No 469/2013 of 22 May 2013 concerning the authorisation of dl‐methionine, dl‐methionine sodium salt, hydroxy analogue of methionine, calcium salt of hydroxy analogue of methionine, isopropyl ester of hydroxy analogue of methionine, dl‐methionine protected with copolymer vinylpyridine/styrene and dl‐methionine protected with ethylcellulose as feed additives. Official Journal, L 136, 23.5.2013, p. 1‐8.
Commission Decision of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products. OJ L 97, 5.4.2006, p. 1.
Commission Regulation (EC) No 1931/1999, amending Annexes I, II and III of Council Regulation (EEC) No 2377/90 laying down a Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. OJ L 240, 10.09.1999, p. 3.
FEED dossier reference: FAD‐2016‐0030.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep-fad-2016-0030-hmtba.pdf
This section has been edited following the provisions of Article 8(6) and Article 18 of Regulation (EC) No 1831/2003.
Technical dossier/Supplementary information January 2017/Annex 1.3.
Technical dossier/Supplementary information January 2017/Annexes 1.2 and 1.3.
Technical dossier/Section 2.3 and supplementary information January 2017/Annex 1.3.
Technical dossier/Supplementary information January 2017/ADRY + Sin January 2017, Question 4.
This section has been edited following the provisions of Article 8(6) and Article 18 of Regulation (EC) No 1831/2003.
Technical dossier/Section II/Annex II.1.2.
Technical dossier/Supplementary information January 2017/Annex 2.2.
Technical dossier/Supplementary information April 2017/Annex 1.3.
Technical dossier/Section II/Annex II.1.4.
Technical dossier/Section II/Annex II.1.3. limit of quantification (in µg/kg) was < 0.1 except for aflatoxin G2 that was < 0.2
Technical dossier/Section II/Annex II.1.5.
Technical dossier/Section 2.1.3.1.
Technical dossier/Section II/Annex II.5.1 SDS.
Technical dossier/Section II/Annex II.1.10.
Technical dossier/Section II/Annexes II.1.10 and 11.
Technical dossier/Section 2.4.1.1.2 and Annex II.4.1; supplementary information January 2017/Annex 5, potentiometric titration followed by oxidation reduction reaction.
Technical dossier/Section II/Annex II.4.2.
Technical dossier/Section II/Annex II.4.2, reverse phase HPLC with UV detection after alkaline hydrolysis of the polymers contained in the extracts.
Technical dossier/Section II/Annex II.4.3, reverse phase HPLC with UV detection after alkaline hydrolysis of the polymers contained in the extracts.
Technical dossier/Section II/Annex II.4.3.
Technical dossier/Section 2.5.1.1.
Technical dossier/Section III/Annex III.1.2a, b and c.
Technical dossier/Section III/Annex III.3.2.
Technical dossier/Section III/Annex III.3.3.
Technical dossier/Section III/Annex III.3.1.
Technical dossier/Section IV/Annexes IV.1.A to D.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- Agostini PS, Dalibard P, Mercier Y, Van der Aar P and Van der Klis JD, 2016. Comparison of methionine sources around requirement levels using a methionine efficacy method in 0 to 28 day old broilers. Poultry Science, 95, 560–569. [DOI] [PubMed] [Google Scholar]
- Belasco IJ, 1980. Fate of carbon‐14 labeled methionine hydroxy analog and methionine in the lactating dairy cow. Journal of Dairy Science, 63, 775–784. [Google Scholar]
- Boebel KP and Baker DH, 1982. Efficacy of calcium salt and free acid forms of methionine hydroxy analog for chicks. Poultry Science, 61, 1167–1175. [Google Scholar]
- Bull LS and Vandersall JH, 1973. Sulfur source for in vitro cellulose digestion and in vivo ration utilization, nitrogen metabolism, and sulfur balance. Journal of Dairy Science, 56, 106–112. [DOI] [PubMed] [Google Scholar]
- Clark WA and Salsbury RL, 1980. Dimethyl sulfide in milk of lactating dairy cows fed various sulfur compounds. Journal of Dairy Science, 63, 375–378. [DOI] [PubMed] [Google Scholar]
- Dibner JJ and Knight CD, 1984. Conversion of 2‐hydroxy‐4‐(methylthio) butanoic acid to L‐methionine in the chick: a stereospecific pathway. Journal of Nutrition, 114, 1716–1723. [DOI] [PubMed] [Google Scholar]
- Dilger R and Baker D, 2008. Cyst(e)ine imbalance and its effect on methionine precursor utilisation in chicks. Journal of Animal Science, 86, 1832–1840. [DOI] [PubMed] [Google Scholar]
- Drew MD, Van Kessel AG and Maenz DD, 2003. Absorption of methionine and 2‐Hydroxy‐4‐methylthiobutoanic acid in conventional and germ‐free chickens. Poultry Science, 82, 1149–1153. [DOI] [PubMed] [Google Scholar]
- Dupuis L, Saunderson CL, Puigserver A and Brachet P, 1989. Oxidation of methionine and 2‐hydroxy‐4‐methylthio‐butanoic acid stereoisomers in chicken tissues. British Journal of Nutrition, 62, 63–75. [DOI] [PubMed] [Google Scholar]
- Dupuis L, Brachet P and Puigserver A, 1990. Oxidation of the supplemental methionine source L‐2‐hydroxy‐4‐methylthiobutanoic acid pure L‐2‐hydroxy acid oxidase from chicken liver. Journal of Nutrition, 120, 1171–1178. [DOI] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2008a. Scientific opinion on the safety and efficacy of Mintrex®Zn (Zinc chelate of hydroxy analogue of methionine) as feed additive for all species. EFSA Journal 2008;6(5):694, 16 pp. 10.2903/j.efsa.2008.694 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2008b. Scientific Opinion on the safety and efficacy of Mintrex®Cu (Copper chelate of hydroxy analogue of methionine) as feed additive for all species. EFSA Journal 2008;5(10):693, 19 pp. 10.2903/j.efsa.2008.693 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2008c. Scientific opinion on the safety and efficacy of Mintrex®Mn (Manganese chelate of hydroxy analogue of methionine) as feed additive for all species. EFSA Journal 2008;6(5):692, 17 pp. 10.2903/j.efsa.2008.692 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA European Food Safety Authority of the Scientific Panel on Additives and Products or Substances used in Animal Feed), 2008d. Technical Guidance for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011. Technical guidance: Tolerance and efficacy studies in target animals. EFSA Journal 2011;9(5):2175, 15 pp. 10.2903/j.efsa.2011.2175 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Scientific Opinion on DL‐methionine, DL‐methionine sodium salt, the hydroxy analogue of methionine and the calcium salt of methionine hydroxy analogue in all animal species; on the isopropyl ester of methionine hydroxy analogue and DL‐methionine technically pure protected with copolymer vinylpyridine/styrene in dairy cows; and on DL‐methionine technically pure protected with ethylcellulose in ruminants. EFSA Journal 2012;10(3):2623, 42 pp. 10.2903/j.efsa.2012.2623 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance for the preparation of dossiers for nutritional additives. EFSA Journal 2012;10(1):2535, 14 pp. 10.2903/j.efsa.2012.2535 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Guidance for establishing the safety of additives for the consumer. EFSA Journal 2012;10(1):2537, 12 pp. 10.2903/j.efsa.2012.2537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012d. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2013a. Scientific Opinion on safety and efficacy of hydroxy‐analogue of selenomethionine as feed additive for all species. EFSA Journal 2013;11(1):3046. 30 pp. 10.2903/j.efsa.2013.3046 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2013b. Scientific Opinion on the safety and efficacy of L‐methionine produced by Escherichia coli (KCCM 11252P) and Escherichia coli (KCCM 1134P) for all animal species. EFSA Journal 2013;11(10):3428, 16 pp. 10.2903/j.efsa.2013.3428 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015. Scientific Opinion on the safety and efficacy of DL‐methionyl‐DL‐methionine for all aquatic animal species. EFSA Journal 2015;13(2):4012, 14 pp. 10.2903/j.efsa.2015.4012 [DOI] [Google Scholar]
- Elwert C, de Abreu Fernandes E and Lemme A, 2008. Biological effectiveness of methionine hydroxy‐analogue calcium salt in relation to DL‐methionine in broiler chickens. Asian‐Australasian Journal of Animal Science, 21, 1506–1515. [Google Scholar]
- European Commission , 2003. Report of the Scientific Committee on Animal Nutrition on the use of a HMBi (isopropyl ester of the hydroxylate analogue of methionine). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_out129.pdf
- Ferjancic‐Biagini A, Dupuis L, De Caro J and Puigserver A, 1995. In vitro oxidative decarboxylation of L‐2‐hydroxy‐4‐methylthiobutanoic acid by L‐2‐hydroxy acid oxidase A from chicken liver. Biochimie, 77, 249–55. [DOI] [PubMed] [Google Scholar]
- GHS (Globally Harmonised system of classification and labelling of chemicals), 2011. Fourth edition. United Nations, New York, Geneva. Available online: http://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev04/English/ST-SG-AC10-30-Rev4e.pdf [Google Scholar]
- Gordon RS and Sizer IW, 1965. Conversion of methionine hydroxy analogue to methionine in chick. Poultry Science, 44, 673–678. [DOI] [PubMed] [Google Scholar]
- Hoehler D, Lemme A, Roberson K and Turner K, 2005. Impact of methionine sources on performance in turkeys. Journal of Applied Poultry Research, 14, 296–305. [Google Scholar]
- Kleemann A, Leuchtenberger W, Hoppe B and Tanner H, 1985. Amino acids. In: Ulmann's Encyclopedia of Industrial Chemistry, Vol. A2, VCH, Verlagsgesellschaft mbH, Weinheim.
- Kluge H, Gessner DK, Herzog E and Eder K, 2016. Efficacy of DL‐methionine hydroxy analogue‐free acid in comparison to DL‐methionine in growing male white Pekin ducks. Poultry Science, 95, 590–594. [DOI] [PubMed] [Google Scholar]
- Koenig KM, Rode LM, Knight CD and McCullough PR, 1999. Ruminal escape, gastrointestinal absorption, and response of serum methionine to supplementation of liquid methionine hydroxy analog in dairy cows. Journal of Dairy Science, 82, 355–361. [DOI] [PubMed] [Google Scholar]
- Koenig KM, Rode LM, Knight CD and Vazquez‐Anon M, 2002. Rumen degradation and availability of various amounts of liquid methionine hydroxy analog in lactating dairy cows. Journal of Dairy Science, 85, 930–938. [DOI] [PubMed] [Google Scholar]
- Liu Z, Bateman A, Bryant M and Roland D, 2004. Estimation of bioavailability of DL‐methionine hydroxy analogue relative to DL methionine in layers with exponential and slope ratio models. Poultry Science, 83, 1580–1586. [DOI] [PubMed] [Google Scholar]
- Malik G, Hoehler D, Rademacher M, Drew MD and Van Kessel AG, 2009. Apparent absorption of methionine and 2‐hydroxy‐4‐methylthiobutanoic acid from gastrointestinal tract of conventional and gnotobiotic pigs. Animal, 3, 1378–1386. [DOI] [PubMed] [Google Scholar]
- Martín‐Venegas R, Geraert PA and Ferrer R, 2006. Conversion of the methionine hydroxy analogue DL‐2‐hydroxy‐(4‐methylthio) butanoic acid to sulfur‐containing amino acids in the chicken small intestine. Poultry Science, 85, 1932–1938. [DOI] [PubMed] [Google Scholar]
- Noftsger S, St‐Pierre NR and Sylvester JT, 2005. Determination of rumen degradability and ruminal effects of three sources of methionine in lactating cows. Journal of Dairy Science, 88, 223–237. [DOI] [PubMed] [Google Scholar]
- Opapeju F, Htoo J, Dapoza C and Nyachoti C, 2012. Bioavailability of methionine hydroxy analog‐calcium salt relative to DL‐methionine to support nitrogen retention and growth in starter pigs. Animal, 6, 1750–1756. [DOI] [PubMed] [Google Scholar]
- Patterson JA and Kung L Jr, 1988. Metabolism of DL‐methionine and methionine analogs by rumen microorganisms. Journal of Dairy Science, 71, 3292–3301. [DOI] [PubMed] [Google Scholar]
- Patton RA, McCarthy RD, Keske LG, Griel LC Jr and Baumgardt BR, 1970a. Effect of feeding methionine hydroxy analog on the concentration of protozoa in the rumen of sheep. Journal of Dairy Science, 53, 933–935. [DOI] [PubMed] [Google Scholar]
- Patton RA, McCarthy RD and Griel LC Jr, 1970b. Observations on rumen fluid, blood serum, and milk lipids of cows fed methionine hydroxy analog. Journal of Dairy Science, 53, 776–780. [DOI] [PubMed] [Google Scholar]
- Rangel‐Lugo M and Austic RE, 1998. Transamination of 2‐oxo‐4‐[methylthio]butanoic acid in chicken tissues. Poultry Science, 77, 98–104. [DOI] [PubMed] [Google Scholar]
- Sangali CP, Giusti Bruno LD, Vianna Nunes R, de Oliveira Rodriguez, Neto A, Pozza PC, Moraes de Oliveira TM, Frank R and Schone RA, 2014. Bioavailability of different methionine sources for growing broilers. Revista Brasileira de Zootecnia, 43, 140–145. [Google Scholar]
- Sauer N, Emrich K, Piepho HP, Lemme A, Redshaw MS and Mosenthin R, 2008. Meta‐analysis of the relative efficiency of methionine‐hydroxy‐analogue‐free‐acid compared with DL‐methionine in broilers using nonlinear mixed models, 2008. Poultry Science, 87, 2023–2031. 10.3382/ps.2007-00514 [DOI] [PubMed] [Google Scholar]
- Saunderson CL, 1991. Metabolism of methionine and its nutritional analogs. Poultry International, 30, 34–38. [Google Scholar]
- Shoveller A, Moehn S, Rademacher M, Htoo J and Ball R, 2010. Methionine‐hydroxy analogue was found to be significantly less available compared to DL‐methionine for protein deposition in growing pigs. Animal, 4, 61–66. [DOI] [PubMed] [Google Scholar]
- Van Weerden EJ, Schutte JB and Bertram HL, 1992. Utilization of the polymers of methionine hydroxy analogue free acid (MHA‐FA) in broiler chicks. Archiv für Geflügelkunde, 56, 63–68. [Google Scholar]
- Vazquez‐Anon M, 2005. The effects of Alimet feed supplement and Sequent feed supplement on rumen digestibility, protein synthesis and ruminal disappearance. Journal of Animal Science, 83(Suppl. 1), 319. [Google Scholar]
- Vazquez‐Añon M, Bertin G, Mercier Y, Reznik G and Roberton JL, 2017. Review of the chemistry, metabolism and dose response of two supplemental methionine sources and the implications in their relative bioefficacy. World's Poultry Science Journal, 73, 1–12. 10.1017/S0043933917000551 [DOI] [Google Scholar]
- VKM (Norvegian scientific committee for food safety) , 2013. Opinion of the Panel on nutrition, dietetic products, novel food and allergy: Risk assessment of histidine, methionine, S‐adenosylmethionine and tryptophan, 34 pp. Available online: https://vkm.no/download/18.175083d415c86c573b59c3a7/1501675375589/ba7a85274a.pdf
- Zelenka J, Heger J, Machander V, Wiltafsky M and Lešták M, 2013. Bioavailability of liquid methionine hydroxy analogue‐free acid relative to DL methionine in broilers. Acta Universitatis Agriculturae et Sivilculturae Mendelianae Brunensis, LXI, 5, 1513–1520. [Google Scholar]
- Zhang S, Wong EA and Gilbert ER, 2015. Bioavailability of different dietary supplemental methionine sources in animals. Frontiers in Bioscience, Elite, 7, 478–490. [DOI] [PubMed] [Google Scholar]
- Zimmerman B, Mosenthin R, Rademacher M and Esteve‐Garcia E, 2005. Comparative studies on the relative efficacy of DL‐methionine and liquid methionine hydroxy analogue in growing pigs. Asian‐Australasian Journal of Animal Science, 18, 1003–1010. [Google Scholar]
- Zou L, Wang D, Liu J, Bai Y, Liang Z and Zhang T, 2015. Effects of DL‐2‐hydroxy‐4‐(methylthio) butanoic acid on broilers at different dietary inclusion rates. British Poultry Science, 56, 337–344. 10.1080/00071668.2015.1021296 [DOI] [PubMed] [Google Scholar]


