Abstract
The duration of the vaccination campaign sufficient to eliminate lumpy skin disease (LSD) mainly depends on the vaccination effectiveness and coverage achieved. By using a spread epidemiological model, assuming a vaccination effectiveness of 65%, with 50% and 90% coverage, 4 and 3 years campaigns, respectively, are needed to eliminate LSD. When vaccination effectiveness is 80% to 95%, 2 years of vaccination at coverage of 90% is sufficient to eliminate LSD virus (LSDV). For shorter campaigns, LSD is predicted to persist. When the infection is eliminated by vaccination, two pathways for disease recurrence are possible, (i) by new introduction from a neighbouring affected area, especially by introduction of infected animals, or, less likely (ii) the infection persisting either in the environment, in vectors or in wild animals. For planning surveillance, several elements should be considered: the objectives and related design prevalence, the epidemiological situation, the immunological status of the host population, the geographical area and the season, the type of surveillance (active or passive), the diagnostic methods including clinical detection (considered the most effective method for early detection of LSD), the target population, the sample size and frequency. According to the model, for early detecting new introductions of LSD, it may be needed to clinically check a large number of herds (e.g. 2–3,000 herds) monthly. Lower sample sizes can be considered, when a greater delay in detecting the virus is acceptable. Where vaccination is maintained, active surveillance for verifying the effectiveness of vaccination would be needed. Demonstrating disease absence can rely on serological surveillance, which should consider the test sensitivity, the design prevalence (estimated value: 3.5%), the onset and duration of serum antibodies. Important knowledge gaps on LSD are about within‐herd transmission, duration of protective immunity, role of vectors, diagnostic tests, farm location and type in the at‐risk countries and the epidemiological status of neighbouring countries.
Keywords: lumpy skin disease, spread, vaccine, mathematical model, surveillance, diagnostic test
Summary
The Standing Group of Experts on lumpy skin disease (LSD) for South‐East Europe under the Global Framework for the Progressive Control of Transboundary Animal Diseases (GF‐TADs) umbrella recommended that all countries in South‐East Europe, affected or at risk for LSD, should collaborate within the GF‐TADs to draft a regional roadmap on an LSD exit strategy from 2018 onwards, based on the experience gained in the region in the previous years as well as the latest available scientific information and World Organisation for Animal Health (OIE) recommendations. This recommendation has triggered a mandate to the European Food Safety Authority (EFSA) in which it is asked to: (i) assess the most suitable duration of an LSD vaccination campaign, using live homologous vaccines, to achieve disease freedom in a country or region; (ii) assess the probability of LSD recurrence in LSD‐affected areas, after ceasing LSD vaccination, considering possible persistence of LSD virus (LSDV) in these areas and the possible threat posed by outbreaks occurring in neighbouring countries or regions; and (iii) assess the effectiveness of different surveillance systems (active or passive) and consider all the components of surveillance, such as type of samples to be collected, sampling strategy and frequency, diagnostic methods, etc.
The latter point is asked to be assessed according to the different objectives for surveillance, i.e. early detection or demonstration of absence of disease, in the following contexts: (a) in areas or countries at risk of LSD, where no LSD outbreaks have occurred and no LSD vaccination was carried out; and (b) where LSD vaccination is carried out; (c) in areas where no LSD outbreaks have occurred and LSD preventive vaccination was carried out, and then stopped; and finally, (d) in areas where LSD outbreaks have been confirmed, and vaccination is stopped.
For the assessment of the suitable duration of vaccination to eliminate the disease (no infected animals are left after stopping vaccination), predictive simulations run with a spread model were performed to explore the percentage of herds infected in relation to the number of years of vaccination. This was aimed to estimate the probability to decrease disease incidence to a level form which it cannot restart again, as a function of the number of years of the vaccination campaign and at the same time to assess the probability the disease would persist. The simulations were presented for two case studies, one for Albania and one for Bulgaria and Greece, treated as a single region.
The duration of the vaccination to eliminate LSD depends on the vaccination effectiveness and the vaccination coverage achieved. Assuming a median vaccine effectiveness of 65%, it was concluded that 3 years of vaccination at coverage of 90% are most likely sufficient to eliminate LSDV from the population, and the absence of new introductions into the country. At a coverage of 50%, 4 years of vaccination are most likely sufficient to eliminate LSDV from the population, assuming the absence of new introductions into the country. Assuming a median vaccine effectiveness of 80–95%, 2 years of vaccination at coverage of 90% are most likely sufficient to eliminate LSDV from the population, assuming the absence of new introductions into the country. The duration of vaccination to eliminate LSDV from the population would increase to 3–5 years when the vaccination coverage is 70%, assuming the absence of new introductions into the country.
In the absence of control measures or when the duration of vaccination campaign is less than 3 years (2 years in the best‐case scenario with vaccination coverage of 90% and effectiveness of 80–95%), LSDV was predicted to persist and re‐emerge in all scenarios simulated by the spread model.
As the above conclusions depend on the level of vaccination effectiveness, it is important to monitor vaccination in the field and to correctly and timely report outbreaks in vaccinated regions, to be able to test the assumptions that underlie the calculations in this report.
The above conclusions are based on scenarios when vaccination is completely stopped at a precise moment in time in the whole country or area. Nevertheless, methods for discontinuing the vaccination programme can be differentiated or combined based on the risk profile of each situation or country, mostly determined by the epidemiological status of the country itself and of the neighbouring countries, and the immunological status of the cattle population. In particular, considering areas bordering endemic regions, which are most at risk for LSD introduction and new outbreaks once vaccination is lifted, special attention should be put on the surveillance in these regions and the option of stopping vaccination at a regional level and, accordingly, different timelines in the same country may be considered.
For the assessment of the probability of disease recurrence when the infection is eliminated by vaccination, two pathways were assessed: (i) the spill‐over infection from a neighbouring affected country or area; or (ii) the infection source within the area but different from the cattle population, either the virus persists outside the domestic host, e.g. in the environment, or in vectors or in wild animals, the former being a more effective route for LSD recurrence.
The recurrence of LSD by introduction of infected animals from neighbouring endemic countries into neighbouring naïve countries is likely, especially if uncontrolled movement of animals across borders can occur.
The recurrence due to active movement of vectors from an infected area into a naïve area is only likely at a short distance, although indirect evidence based on wind trajectories indicate that long‐distance dissemination of infected vectors by winds is a potential route. These events are also linked to the time period LSDVs remain viable in vectors, which it seems to be long enough only in ticks. In general, no studies about vector species of LSDV have been performed in Europe and particularly in the LSD‐affected countries, so specific evidence on vector competence is still missing.
The probability of recurrence of LSD linked to the probability of wildlife being carriers of LSDV or the occurrence of a sylvatic cycle of the virus cannot be assessed because of lack of information. The probability of recurrence of LSD due to the role of subclinically infected animals is low, as the most likely source of virus transmission is linked to the high levels of virus present in skin lesions, so in animals with evident clinical symptoms that are usually removed from the population.
The probability of recurrence of LSD linked to the virus remaining viable in the external environment (e.g. in shaded pens or beddings) is not known, as well as the probability of an animal acquiring the infection by contact with contaminated bedding is unknown, although it is known that transmission by direct contact or indirect contract with fomites is less effective than by vector transmission.
For the assessment of surveillance strategies (ToR 2), the surveillance options for each of the given four scenarios were assessed, according to the main surveillance objectives, the epidemiological situation, the immunological status of the host population, the geographical area and the best period of time for performing the surveillance activities, the type of surveillance (active or passive), the performance of diagnostic methods to be used including clinical detection (which is considered the most important component of early warning for LSD and for which awareness‐raising campaigns should be continuously encouraged for farmers and veterinarians) and other diagnostic methods, the target population of the surveillance activities and criteria for its selection, and the parameters for the calculation of sample size.
Three approaches were taken using the LSD spread model to inform the design of the surveillance programmes, in particular the design prevalence in the different scenarios. First, the model was used to calculate the time to detection and the prevalence of infected herds at detection, assuming no control measures. Second, the model was used to compute the prevalence of infected herds at a determined time lag after an incursion (21, 28 or 35 days). Third, the model was used to compute the time at which a determined level of threshold prevalence (0.1%, 1% and 5%) was reached. This was used to determine the possible design prevalence to be used accordingly for both early detection and demonstration of absence of disease.
The spread model was also used to estimate zone size beyond which LSD may spread with a certain probability. This model was helpful because it is an element to plan risk‐based surveillance according to the distance from an infected region (surveillance zones or buffer zones). It was estimated that LSD may spread up to 80 km with 99.9% probability, when this probability is computed over the entire infectious period of a farm. Early warning of new introductions of LSD in a country could be targeted in areas bordering infected countries, whereas early warning for re‐emergence of LSD should be targeted at previously infected areas of the whole country.
According to the spread model, the median expected prevalences at 3, 4 and 5 weeks after introduction that could be used as design prevalence for early detection, are below 0.2%. This showed that for active surveillance to be effective in early detection, given the low values of design prevalence, clinical examination performed by veterinarians of a large number of herds (2,000–3,000 herds) at high frequency (monthly) would be needed.
As this level of surveillance would not be feasible in many situations given the logistical and organisational difficulties in planning repeated visits to a such high numbers of cattle herds, the surveillance activities can be strengthened or partially replaced by adding systematic clinical examinations for LSD at live animal markets, during pre‐movement clinical checks or at ante‐mortem examinations on animals to be slaughtered. These activities could be also combined with other surveillance programme on cattle population in place in the country. Active surveillance can be feasible only in at‐risk areas and during the risk period.
The values of design prevalence used for early detection of LSD introduction were derived from the epidemiological model. However, higher design prevalence values can be considered to reduce the sample size, but this will lead to a larger delay in detecting the infection (1–2 weeks). This should be evaluated case by case, according to specific conditions such as geographical, animal density and distribution, type of farms, surveillance programmes already in place, etc. In particular, the possible consequences of greater delays in detecting the infection should be evaluated in line with the planned preventive and control measures, including the number of vaccine doses for stockpiling.
For the demonstration of absence of LSD in a previously affected area, the availability of the serological assay is important, although its performance under field conditions still has to be properly evaluated. In any case, according to OIE, at least 2 years is recommended after stopping vaccination, before disease‐free status can be demonstrated.
The design prevalence that could be used for demonstrating absence of disease, could be derived from the proportion of herds ever infected during a simulation, the lowest 25th percentile of this distribution is equal to 3.5%, which can be used as design prevalence for this surveillance purpose. For countries where mass vaccination or vaccination of susceptible animals is kept in place, rather than a surveillance system to detect infection, active surveillance for verifying the effectiveness of vaccination would be needed.
Important knowledge gaps are about within‐herd transmission parameters, duration of protective immunity from vaccination and natural infection, duration of passive immunity in calves, role of vectors, diagnostic test performance under field conditions, exact farm location and farm type in all affected and at‐risk countries and the epidemiological status of neighbouring countries. Further research studies and data collection should be encouraged on these aspects.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. LSD occurrence
Lumpy skin disease (LSD) is a viral disease of cattle, mechanically transmitted primarily by blood‐ feeding insects (vectors) and to a lesser extent through direct contact between cattle.
Mortality due to LSD is not very high (up to 10%), however occurrence of the disease is associated with a drop in production and serious trade restrictions.
Since 2013, Turkey reports regularly outbreaks of LSD practically in all parts of its territory and to date it is considered an LSD endemic country, despite vaccination, albeit with a vaccine considered by experts, including EFSA, of low effectiveness.
2014: In Cyprus LSD was reported for the first time in November 2014 in those areas of the Republic of Cyprus in which the Government of the Republic of Cyprus does not exercise effective control (no occurrence to the rest of the island).
2015: LSD was confirmed in the European part of Turkey in June 2015 and then in the border areas of Greece in August 2015. The measures undertaken by the Greek authorities (stamping out of outbreaks and mass vaccination campaign in the north eastern provinces), combined with winter brought LSD to a controlled state by December 2015.
2016: LSD returned in April 2016 in Greece (in an LSD restricted area of low vaccine coverage) and a week later in Bulgaria (first ever report on 13 April 2016). In the months that followed, the disease gained entry in 7 countries of South East Europe (apart from Turkey): Greece, Bulgaria, Serbia, Kosovo, Albania and Montenegro.
2017: Only sporadic LSD reports from the Former Yugoslav Republic of Macedonia (4 outbreaks) and Greece (2 outbreaks), no outbreaks reported in the other countries of SE Europe (excluding Turkey and Albania). Albania was the only country in South East Europe to experience a full‐scale recurrence of LSD in 2017 (more than 400 outbreaks).
1.1.2. LSD vaccination
All seven affected countries in South‐East Europe have implemented annual vaccination of all their cattle in 2016 and 2017, using live homologous vaccines and plan to repeat this programme in 2018.
In addition, Croatia (2016 and 2017) and Bosnia and Herzegovina (2017) implemented preventive vaccination against LSD in 2016 due to disease presence in neighbouring countries.
So far, uniform (all cattle in all holdings in an area) mass vaccination against LSD with high vaccination coverage (over 95%) appears to be the most effective preventive measure against the disease, as confirmed by EFSA in an LSD urgent opinion adopted in July 2016.
However, despite the high LSD vaccine coverage achieved across South‐East Europe, for 2 consecutive years now (2016–2017), there is evidence to indicate that:
The LSD virus (LSDV) is still present in the region (outbreaks in Albania, the Former Yugoslav Republic of Macedonia and Greece in 2017).
Non‐immune animals remain at risk of LSD infection, even in areas with relatively high vaccine coverage.
On occasion, the disease can still spread from vaccinated to non‐vaccinated areas, especially if vaccine coverage has been insufficient.
During the last meeting of the Standing Group of Experts on lumpy skin disease (LSD) for South‐East Europe under the GF‐TADs umbrella (Budva, Montenegro 19–20 October 2017) it was proposed, among other recommendations, that:
‘All countries of South‐East Europe, affected or at risk for LSD, should collaborate within the GF‐TADs to draft a regional roadmap on an LSD exit strategy from 2018 onwards, based on the experience gained in the region in the previous years as well as the latest available scientific information and OIE recommendations.’
In light of the above, the Commission is in need of an updated opinion on specific aspects of LSD based, at least, on:
the data collected from the countries affected by, or at risk of, LSD including the countries that implemented preventive vaccination;
the latest available scientific information;
any other relevant information available through the EU Reference Laboratory for LSD;
the LSD data collected by EFSA and their analysis/evaluations in previous EFSA LSD opinions/reports;
the epidemiological situation of LSD in South‐East Europe over the past years, including 2018.
Therefore, in the context of Article 31 of Regulation (EC) No. 178/2002, EFSA should provide scientific and technical assistance to the Commission based on the Terms of Reference that follow.
1.1.3. Terms of Reference (ToR)
ToR 1. LSD vaccination
ToR 1.1 Assess the most suitable duration of an LSD vaccination campaign, using live homologous vaccines, like the ones used so far in the region of SE Europe, intended to achieve disease freedom in a country or region, considering any relevant factors that may affect and influence disease spread and persistence.
ToR 1.2 Assess the probability of LSD recurrence in LSD‐affected areas, after ceasing of LSD vaccination, bearing in mind the possible persistence of LSDV in these areas but also the possible threat posed by outbreaks occurring in neighbouring countries or regions.
The above assessments, under points (1.1) and (1.2) should take into account all factors that may have an impact on the optimum duration of a vaccination campaign or the probability of LSD recurrence as above, including, but not limited to, control measures in case of disease confirmation, vaccine coverage, spatial and temporal distribution of disease outbreaks, LSD presence in neighbouring countries, disease seasonality, maternal immunity, time elapsed since cease of vaccination etc.
ToR 2. LSD surveillance
Assess the effectiveness of different surveillance systems (active, passive, etc.) bearing in mind the samples that may be used for LSD diagnosis (e.g. skin, blood, other) and all LSD diagnostic methods available (clinical, serological, molecular, including Differentiation of Infected from Vaccinated Animals (DIVA) methods that can differentiate LSD vaccine from field viruses) in the following contexts:
ToR 2.1. In areas or countries at risk of LSD (e.g. due to LSD outbreaks in neighbouring countries), where no LSD outbreaks have occurred and no LSD vaccination was carried out.
ToR 2.2. In areas or countries at risk of LSD (e.g. due to LSD outbreaks in neighbouring countries), where LSD vaccination is carried out.
ToR 2.3. In areas or countries at risk of LSD (e.g. due to LSD outbreaks in neighbouring countries), where no LSD outbreaks have occurred and LSD preventive vaccination was carried out, after cease of vaccination.
ToR 2.4 In areas or countries where LSD outbreaks have been confirmed, after cease of vaccination.
The above assessment should include at least, but not be limited to, features such as type of surveillance (active, passive), duration of surveillance, types of samples to be collected and their tests, selection criteria for animals to be sampled (spatial–temporal, other), etc., and address in particular the issues raised below:
For areas or countries under point (2.1) and point (2.2): assessment of the effectiveness of different surveillance systems aiming to achieve early detection of a primary disease incursion.
For areas or countries under point (2.3) and point (2.4): assessment of the effectiveness of different surveillance systems aiming to demonstrate the absence of disease with a relatively high level of confidence.
1.2. Introduction and interpretation of the Terms of Reference
After the successful implementation of regional mass vaccination against LSD in the Balkan region, LSD epidemics have been contained and no new outbreaks have been reported in all those countries homogeneously and sufficiently covered by vaccination. At this stage, the question about the kind of exit strategy to be followed is raised among the risk managers and the veterinary authorities of the affected countries. This is mostly linked to whether, when and how to lift the vaccination measures (ToR 1) and the kind of surveillance strategy to implement in the different situations (ToR 2), basically according to the epidemiological and immunisation status of the different countries and/or regions.
The aspects for optimising the vaccination are mainly the proportion of animals vaccinated in a certain country or area (vaccination coverage), how quickly this level is reached (when the vaccination starts compared with the disease insurgence; see EFSA AHAW Panel, 2016), and how long the vaccination is in place, i.e. how vaccination campaigns are performed. According to ToR 1, if the goal of the risk manager is to stop vaccination only when the likelihood of recurrence of an epidemic is very low, this implies that the probability of at least one farm being infected, and the probability of reintroduction of LSDV from outside, should be very low at the time herd immunity becomes insufficient. For this objective what is relevant is the probability that the infection is absent in the country at the time vaccination is stopped or, similarly, the probability that after stopping vaccination the epidemic will not start again. To assess this scenario, an epidemiological model of LSD spread is used as a tool to estimate the numbers of vaccination campaigns needed to bring the infection to zero. Different scenarios can be explored according to vaccination coverage (% of vaccinated animals), vaccination effectiveness (the probability that a vaccinated animal is really protected from the infection), replacement rate, etc. ToR 1 is addressed in Sections 3.1 and 3.2 of the present document.
As baseline information about those aspects, in the chapter about LSD infection of the OIE's Terrestrial Animal Health Code (TAHC) revised in July 2017 (OIE, 2017)1 a country is free from LSD infection if:
Historically free, i.e. (i) there has never been occurrence of disease; or (ii) the disease has been eradicated or ceased to occur for at least 25 years, under the condition that, for at least during the past 10 years, LSD has been notifiable and an early detection system is in place, no vaccination is in place and the infection is not known to be established in wildlife.
Vaccination has been prohibited for at least 3 years in the country and a clinical surveillance programme in accordance with Article 11.9.15 has demonstrated no occurrence of infection with LSDV.
Vaccination has been prohibited in the country for at least 2 years and a clinical, virological and serological surveillance programme in accordance with Article 11.9.15 has demonstrated no occurrence of infection with LSDV.
In the light of the above, before adopting a new strategy not based on vaccination, according to OIE, evidence of the absence of virus circulation in the last 2 or 3 years should be provided after stopping vaccination. Depending on the type of diagnostic tests available to prove the absence of virus circulation, if this would be based on polymerase chain reaction (PCR) tests, these should be carried out in a large number of animals and the cost of this type of surveillance may be not sustainable. The length of the period without any detected virus circulation depends on the strength of the evidence: at least 3 years if the lack of occurrence of infection is based on a clinical surveillance programme only or at least 2 years if a clinical, virological and serological surveillance programme has been put in place (OIE, 2017).
1.2.1. ToR 2
The general concepts of surveillance for LSD are described in Article 11.9.5 of the OIE code Chapter 11.9 on infection with LSDV, as well as some principles of clinical, virological and serological surveillance for LSD. The choice of the surveillance strategy to detect the presence of infection with LSDV, even in the absence of clinical signs, is up to the veterinary authority of each country, considering the epidemiological situation in the country.
In this report, the European Commission requested an assessment on how the surveillance should be implemented according to different situations. The two main aspects to consider for the surveillance strategy planning in the different countries/regions are the infection status (free country at risk of introduction, active or past infection) and the control measures applied, basically constituting vaccination.
For countries never affected by the disease, under the objective of early detection and early warning (the situation under the scenarios as in points 2.1 and 2.2 of ToR 2, but could be also for point 2.3), it is most important to detect infection at the time that a corrective process is still possible and effective. This implies identifying a threshold at which action can still be taken (with regard to vaccination, how long will it take to vaccinate the area and how long before herd immunity is sufficient to confer protection) and the complexity here is that the prevalence in the population changes with time. Therefore, because of the time factor, in addition to sample size, the frequency of sampling is important, and both aspects should be estimated and discussed in each of the scenarios. Because of this relevance of sampling frequency, for diseases such as LSD that are easily recognised, passive surveillance usually outperforms active surveillance.
For countries previously affected, and where surveillance aims at demonstrating the absence of disease (the situation under the scenarios as in ToR as in point 2.4), the concept of design prevalence is commonly used. The biological reasoning is that, should LSDV be present in a region, where herd immunity is below the threshold allowing major spread, it will spread and should easily reach the design prevalence. Therefore, the absence of the disease is demonstrated when LSDV is not detected based on design prevalence. The vaccination status of the population may create two possible situations: first in a non‐vaccinated population, the disease if present, will spread widely and reach that level of prevalence. Second, when vaccination is effective, properly and widely applied, the virus would not spread and the utility of design prevalence is limited.
These aspects related to ToR 2 are addressed in Sections 2.3 and 3.2 of the present document.
2. Data and methodologies
2.1. Mathematical model for studying the dynamics of LSDV in a vaccinated population to achieve disease freedom and set design of surveillance
The intention with the concept of disease freedom intended here is that no infected animals are left after stopping vaccination, i.e. true freedom from the disease. The assessment methodology used is based on predictive simulations run with the spread model previously presented (EFSA, 2018) to explore the percentage of herds infected in relation to the number of years the vaccination campaign runs for and to estimate the probability to decrease disease incidence to a level form which it cannot restart again, as a function of the number of years of the vaccination campaign. Model simulations are presented for two case studies, one for Albania and one for Bulgaria and Greece, treated as a single region.
2.1.1. Data
Demographic data for Albania, Bulgaria and Greece were obtained from the national veterinary services in each country. For Albania, the data provided consisted of the number of cattle (as of February 2017) in each herd and the location of the herd at the level of village as administrative unit (latitude and longitude), which was used as a proxy for herd location (because herds do not have unique locations). For Bulgaria and Greece (treated as a single region in the simulated outbreaks), this provided the number of cattle (as of January 2017 for Bulgaria and June 2018 for Greece) in each herd and the location of the herd (latitude and longitude). In some cases, this was the herd's location, while for others it was the village in which the herd was located (again, used as a proxy for herd location). Furthermore, an appreciable number of herds in Greece had zero cattle recorded in the dataset (9,452 out of 24,889). The Greek veterinary services advised that these herds are most likely to be small herds which had no cattle on the date for which data were extracted, but which would usually have a small number of cattle. Accordingly, these herds were assumed to have one, two or three cattle (with the number sampled uniformly).
Rate of annual replacement in the region was reported to range between 6% and 30% (GF‐TADs2), in Bulgaria, for example, is reported to be 8–10% in dairy cattle herds and 3% in beef cattle herds, while life expectancy for dairy cattle is around 10 years, for beef cattle 30 months, so mean life expectancy of the cattle population could be considered around 5–6 years (data reported by the Bulgarian Food Safety Agency, 2018).
Temperature data for Albania, Bulgaria and Greece were obtained from the European Commission Joint Research Centre MARS Meteorological Database3, which provides daily meteorological data spatially interpolated on a 25 km by 25 km grid cell. Specifically, we extracted the daily minimum and daily maximum temperatures for 2013–2017 and computed the midpoint of these for each of the 575 grid cells covering Albania, Bulgaria and Greece. For each farm, the temperature for the grid cell in which it was located was assumed. The default temperature dataset was that for 2016, but the sensitivity of the model predictions to the data used in the simulations was assessed.
2.1.2. Methodology: model of LSD spread for estimating the duration of a vaccination campaign and probability of recurrence
The model used to describe the spread and control of LSD was adapted from that used for previous opinions (EFSA AHAW Panel, 2015; EFSA, 2018), principally to allow for immunity in the cattle population (both natural and vaccine derived) and for population turnover. In the model the animals are assigned to four classes: Susceptible (i.e. uninfected) (S), Infected (I), Recovered (R) and Vaccinated (i.e. uninfected and temporarily protected) (V), with the number of cattle in each class recorded. The model structure and its components are presented below.
Host demography
Natural (i.e. non‐LSD‐associated) mortality was assumed to occur at a constant rate in a herd (equal to the reciprocal of the mean life expectancy). Host reproduction was assumed to be continuous, with the number of replacements born each day chosen to restore the herd size to its initial level.
Vaccination
Vaccination was assumed to be implemented for all herds on 1 April in the year following the initial incursion and on 1 April for a number of years subsequently (for a further 1–4 years). In the first year of the vaccination campaign, each herd was vaccinated, with the probability given by the vaccination coverage at farm level, i.e. the proportion of herds that were vaccinated. If the farm was vaccinated all animals on the farm were assumed to be vaccinated. Susceptible (i.e. uninfected) cattle were assumed to be protected, with the probability given by the vaccination effectiveness (VE), i.e. the proportion of vaccinated animals that were protected from infection under field conditions (EFSA, 2018), while infected and recovered cattle were unaffected by vaccination (reflecting lifelong immunity assumed to occur following natural infection). For herds in which all animals had been vaccinated previously, it was assumed that all animals were revaccinated in subsequent years, with the probability that an animal is protected given by 1− (1−VE)y (where y is the number of years for which the vaccine was used). This increase in vaccination effectiveness over time was used to allow a simulation of the increase in effectiveness following repeated vaccinations, i.e. the booster effect in which animals that were previously vaccinated, but did not develop protective immunity, do so following a second or following vaccination. Consequently, in the first year of vaccination, the proportion protected is equal to VE of the total vaccinated population. In the second year of vaccination, the additional proportion protected is equal to VE of the population not protected in the first year, and so on. For herds that did not vaccinate previously, they did so in the next year with probability given by the farm‐level coverage, so that the proportion of herds vaccinated at least once increases during a campaign (Figure 1). One year after a vaccination campaign stops, all vaccinated animals were assumed to become susceptible again (i.e. the duration of immunity is assumed to be 1 year).
Figure 1.
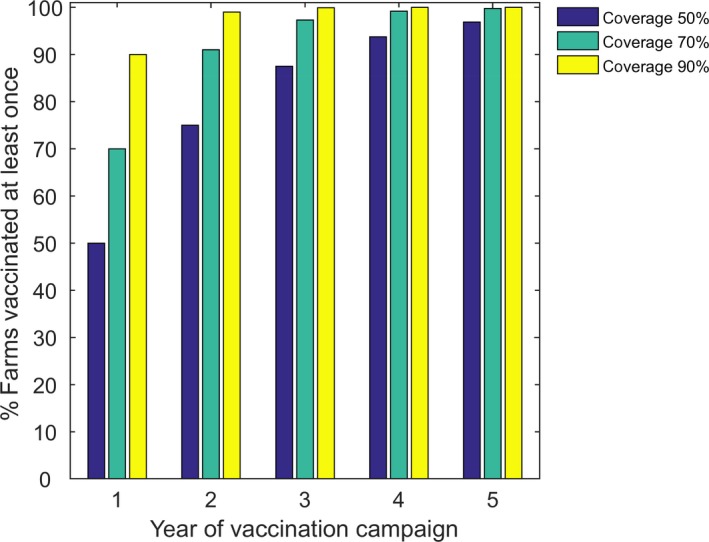
Percentage of herds vaccinated at least once in the model for each year of a vaccination campaign, assuming a farm‐level coverage of 50% (blue), 70% (green) or 90% (yellow)
Farm‐level coverage for Albania was assumed to be 50% or 90%, while for Bulgaria and Greece it was assumed to be 70% or 90%, based on the data provided by the affected countries (EFSA, 2018). Vaccination effectiveness for Albania was sampled from the posterior distribution obtained by fitting the model to outbreak data for four districts in Albania (Bulqizë, Dibër, Kukës and Mat) from 2016 (EFSA, 2018; Gubbins et al., 2018) (see Table 1, the median vaccination effectiveness is 65%, with 57.3–71.6 as 95% interval). For Bulgaria and Greece, vaccination effectiveness was assumed to be 80 or 95%, as estimated in previous works by a survival and a kernel‐based model (EFSA, 2018). In particular, with the survival model, vaccination effectiveness was calculated as 1 − hazard ratio, with the hazard ratio of an outbreak occurring in vaccinated versus non‐vaccinated herds. In fact, the performance of LSD vaccination under field conditions is likely to differ among countries and situations and it may be related to several factors such as how the vaccine is administered, if the cold chain is well maintained, the animal may escape the vaccination or not react properly to the vaccine, etc.
Table 1.
Summary of the parameters in the model for the transmission of LSDV
| Parameter | Symbol | Posterior median | 95% credible interval |
|---|---|---|---|
| Outbreak duration | |||
| Shape | sD | 1.93 | (1.83, 2.04) |
| Mean (intercept) | γ0 | 43.9 | (41.3, 46.8) |
| Mean (log10 herd size) | γ1 | 11.0 | (5.4, 16.6) |
| Transmission between herds | |||
| Transmission parameter | h0 | −11.19 | (−11.34, −11.05) |
| Transmission rescalinga | δh | −2.50 | (−3.28, −1.72) |
| Seasonality parameter | h1 | 1.78 | (1.63, 1.94) |
| Kernel power | α | 1.76 | (1.61, 1.92) |
| Distance scaling (km) | d0 | 0.73 | (0.56, 0.92) |
| Vaccine effectiveness (%) | VE | ||
| Albania | 65.0 | (57.3, 71.6) | |
| Bulgaria and Greece (low) | 70 | – | |
| Bulgaria and Greece (high) | 90 | – | |
Change in the transmission parameter (i.e. h0 → h0 + δh) when applying the model to Bulgaria and Greece.
LSD spread within a herd
When a herd becomes infected, the total cumulated number of animals infected at the end of the outbreak can be obtained from a binomial distribution with population S (i.e. the number of susceptible cattle) and probability f, the final size fraction of the outbreak. Within the Susceptible‐Infected‐Removed (SIR) framework, this f is given by the non‐zero root of the equation,
| (1) |
where R0 is the within‐herd basic reproduction ratio (Keeling and Rohani, 2011). A value of R0 = 2 was used in the simulations (Magori‐Cohen et al., 2012; Molla et al., 2017). However, the sensitivity of the model predictions to changes in R0 was considered by simulating outbreaks assuming R0 = 1.5 or R0 = 5.0.
The duration of a within‐herd outbreak was sampled from a gamma distribution with shape parameter s D and mean μ D given by,
| (2) |
where γ 0 and γ 1 are parameters and N(= S + I + R + V) is the herd size. The parameters were estimated by fitting the model to data from reported outbreaks from Albania using Bayesian methods (Table 1).
At the end of the outbreak (i.e. after the outbreak duration has lapsed), the infected animals move into the recovered class, where they remain until they are removed from the herd (as part of routine herd management). Mortality due to LSD was assumed to be negligible.
LSD spread between herds
Transmission of LSDV between herds was modelled using a kernel‐based approach. In this case, the force of infection, λi(t), experienced by herd i on day t is
| (3) |
where h(t) is the seasonally varying transmission parameter, Si(t) is the number of susceptible cattle in herd i on day t, K(dij) is the distance kernel (see below), dij is the great circle distance between herds i and j and Ij(t) is the number of infected cattle in herd j on day t. A fat‐tailed kernel was used, so that,
| (4) |
where α controls how rapidly the force of infection decays with distance and d 0 is the distance at which the force of infection is reduced by half.
The impact of seasonality was incorporated by assuming that the transmission parameter depends on the relative abundance of Stomoxys calcitrans, one of the putative vectors of LSDV and known to be abundant in the areas where LSDV is circulating in the Balkans, typically from April until October (EFSA, 2018). In this case the transmission parameter is
| (5) |
where h0 and h1 are the baseline transmission and seasonality parameters, respectively, and A(t) is the relative vector abundance at time t (normalised so the maximum is equal to one). The relative vector abundance is given by
| (6) |
where F, E, L and P are temperature‐dependent functions describing fecundity, egg survival, larval survival and pupal survival, respectively, c is the normalising constant and Tm–1 is the monthly mean temperature for the preceding month. Appropriate functional forms for F, E, L and P were obtained from experiments using laboratory colonies of S. calcitrans (Lysyk, 1998; Kahana‐Sutin et al., 2017).
Transmission parameters for scenarios in Albania were sampled from the posterior distribution obtained by fitting the model to outbreak data from 2016 for four districts in Albania (Bulqizë, Dibër, Kukës and Mat) (EFSA, 2018; Gubbins et al., 2018). For scenarios in Bulgaria and Greece, considered as a single region, the same posterior distribution (i.e. based on outbreaks in Albania) was used initially. However, this resulted in unrealistically large outbreaks. Because the force of infection between herds is assumed to be proportional to herd size, the larger herd sizes in Bulgaria and Greece (mean herd size: 16 cattle) resulted in a considerably larger force of infection compared with Albania (mean herd size: 2 cattle). Accordingly, the seasonal transmission rate, h(t), was rescaled (Table 1), so that the mean transmission rate over a year was the same as that estimated previously for outbreaks in Israel (EFSA AHAW Panel, 2015). This was chosen because previous simulated outbreaks in Bulgaria and Greece had used the Israeli estimates (EFSA AHAW Panel, 2015, 2016). Transmission parameters are summarised in Table 1.
2.1.3. Methodology: modelling LSD spread for the design of surveillance programmes
Time to detection
Three approaches were taken when using the LSD spread model (see Section 2.1.2) to inform the design of the surveillance programmes. First, it was assumed that LSD would be detected in a country when there was a substantial increase in the number of infected herds (defined below) after an incursion at different time of the year and the model was used to calculate the time to detection and the prevalence of infected herds at detection, assuming no control measures (Table 3). Second, it was assumed that LSD would be detected 21, 28 or 35 days after an incursion (or re‐emergence) and the model was used to compute the prevalence of infected herds at that time (Table 4). Third, it was assumed that LSD would be detected when the prevalence of infected herds reached 0.1%, 1% or 5% and the model was used to compute the time at which the threshold prevalence was reached (Table 5).
Table 3.
Impact of season on predicted time to detection (in days) and related number of infected herds at detection of lumpy skin disease outbreaks
| Time of incursion | Median time to detection (95% prediction interval) | Median percentage of infected herds at detection (95% prediction interval) |
|---|---|---|
| Albania | ||
| 1 January | 75 (14–134) | 0.0025 (0.0005–0.011) |
| 1 April | 30 (0–77) | 0.0015 (0.0005–0.006) |
| 1 July | 15 (0–110) | 0.0015 (0.0005–0.0205) |
| 1 October | 48 (4–75) | 0.0065 (0.0005–0.0255) |
| Bulgaria and Greece | ||
| 1 January | 62 (36–160) | 0.015 (0.001–0.072) |
| 1 April | 21 (0–107) | 0.004 (0.001–0.041) |
| 1 July | 22 (0–141) | 0.004 (0.001–0.042) |
| 1 October | 45 (6–77) | 0.027 (0.001–0.096) |
In 2017, there were 198,000 cattle herds in Albania and 88,000 in Bulgaria and Greece, considered together.
Table 4.
Median (95% prediction interval) percentage of herds infected at detection when detection occurs 21, 28 or 35 days after an incursion or re‐emergence of infection upon arrest of vaccination
| Scenario | Time to detectiona | ||
|---|---|---|---|
| 21 days | 28 days | 35 days | |
| Albania, incursion in June | 0.003 (0.0005–6.5) | 0.009 (0.0005–20.1) | 0.21 (0.0005–35.1) |
| Albania, re‐emergence (2 years of vaccination, 50% coverage) | 0.032 (0.0007–0.15) | 0.048 (0.0007–0.23) | 0.12 (0.0027–0.55) |
| Albania, re‐emergence (3 years of vaccination, 50% coverage) | 0.023 (0.0005–0.20) | 0.055 (0.0005–0.43) | 0.20 (0.0005–1.4) |
| Albania, re‐emergence (2 years of vaccination, 90% coverage) | 0.026 (0.0005–0.066) | 0.049 (0.005–0.11) | 0.12 (0.0005–0.31) |
| Bulgaria and Greece, incursion in June | 0.0045 (0.0011–2.9) | 0.015 (0.0011–9.6) | 0.074 (0.011–6.7) |
| Bulgaria and Greece, re‐emergence (2 years of vaccination, 70% coverage, 80% effectiveness) | 0.016 (0.0045–0.28) | 0.025 (0.0045–0.54) | 0.032 (0.0057–0.97) |
| Bulgaria and Greece, re‐emergence (2 years of vaccination, 70% coverage, 95% effectiveness) | 0.011 (0.0023–0.69) | 0.016 (0.0023–1.6) | 0.041 (0.0023–2.9) |
| Bulgaria and Greece, re‐emergence (2 years of vaccination, 80% coverage, 95% effectiveness) | 0.020 (0.0011–0.065) | 0.029 (0.0023–0.17) | 0.042 (0.0011–0.34) |
For re‐emergence scenarios, this is the time from 1 year after the last animal was vaccinated.
In 2017, there were 198,000 cattle herds in Albania and 88,000 in Bulgaria and Greece, considered together.
Table 5.
Median (95% prediction interval) time (days)a to reach design prevalence after an incursion or a vaccination campaign has stopped
| Scenario | Design prevalence | ||
|---|---|---|---|
| 0.1% | 1% | 5% | |
| Albania, incursion in June | 35 (12–97) | 38 (16–104) | 43 (20–110) |
| Albania, re‐emergence (2 years, 50% coverage) | 34 (14–81) | 54 (40–102) | 77 (60–125) |
| Albania, re‐emergence (3 years, 50% coverage) | 31 (15–97) | 45 (34–111) | 60 (46–125) |
| Albania, re‐emergence (2 years, 90% coverage) | 35 (28–220) | 54 (44–338) | 74 (62–112) |
| Bulgaria and Greece, incursion in June | 36 (10–118) | 47 (18–93) | 56 (24–111) |
| Bulgaria and Greece, re‐emergence (2 years, 70% coverage, 80% effectiveness) | 51 (14–75) | 88 (36–143) | 116 (64–139) |
| Bulgaria and Greece, re‐emergence (2 years, 70% coverage, 95% effectiveness) | 47 (8–147) | 75 (25–358) | 89 (43–151) |
| Bulgaria and Greece, re‐emergence (2 years, 80% coverage, 95% effectiveness) | 45 (25–95) | 70 (45–124) | 90 (66–140) |
For re‐emergence scenarios, this is the time from 1 year after the last animal was vaccinated.
In 2017, there were 198,000 cattle herds in Albania and 88,000 in Bulgaria and Greece, considered together.
In the first approach, the model was used to simulate incursions into a randomly selected farm in Albania or Bulgaria and Greece (treated as a single region) on 1 January, 1 April, 1 July and 1 October assuming no vaccination were implemented. In each scenario, a ‘substantial increase in the number of infected herds’ was determined by comparing the simulated time courses for the number of newly infected herds with the number of herds reporting cases during the first 2 weeks of the epidemic in Montenegro in 2016 (similar results were obtained if the data from the epidemic in Serbia in 2016 were used instead). For each replicate epidemic, the absolute deviance between observed number of newly reported outbreaks and the simulated number of newly infected herds was calculated at each time since the incursion (but only for those simulations in which an epidemic occurred and only for the increasing portion of the epidemic curve), with the time to detection being that for which the absolute deviance was minimised (Figure 2).
Figure 2.
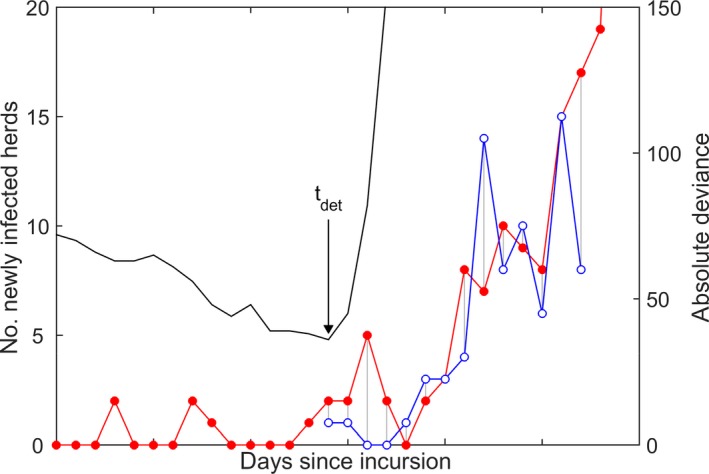
Time to detection (tdet) of an incursion of LSD
- The observed number of newly reported outbreaks in Montenegro (blue line and open circles) was compared with the simulated epidemic in Albania or Bulgaria and Greece (red line and filled circles) at each day since the incursion (left‐hand axis) by computing the absolute deviance between the two curves (black line) (i.e. the sum of the lengths of the grey lines) (right‐hand axis). Time to detection (tdet) is the days since the incursion for which the absolute deviance was minimised.
Size of surveillance areas
To estimate the zone size required for surveillance we applied the methods developed in Schley et al. (2009), which allows calculation of the probability of LSDV escaping a zone of a given radius (i.e. a farm outside the zone becoming infected). The size of surveillance zone can be defined as the minimum size of zone for which the probability of escape is below a threshold value.
The escape probability, pE(r), is given by,
| (7) |
where
| (8) |
is the probability of transmission at distance r, K(r) is the transmission kernel, Rh is the between‐farm basic reproduction number and I is the number of infected herds in the zone (assumed to be one). The probability of escape is computed over entire infectious period of a farm.
Assuming an exponential transmission kernel, K(r) = exp(−αr), the escape probability, pE(r), is given by:
| (9) |
Such a kernel means that very long‐range transmission events (usually attributed to animal movements) are highly unlikely. This was deemed appropriate because we are principally concerned here with incursions from an affected country into a disease‐free country, and cross‐border animal movements, especially from infected countries, are assumed to be controlled. Three values were considered for the kernel parameter (α), namely 0.16, 0.37 and 0.74, which are the minimum, median and maximum values estimated for 15 districts in Albania (Gubbins et al., 2018).
The between‐farm basic reproduction number has been inferred from outbreaks in the Middle East in 2012–2015 for which the estimate for Rh was 2.2 (95% confidence interval (CI): 1.2–3.5) (Alkhamis and VanderWaal, 2016). However, for outbreaks in Israel in 2013, the same authors estimated Rh to be 22.2 (95% CI: 15.2–31.5) (Alkhamis and VanderWaal, 2016). From outbreaks in Albania in 2016, lower bounds for R h were estimated to range from 1.1 to 18.3 (Gubbins et al., 2018). Accordingly, the size of surveillance zone was computed for values for Rh ranging from 1 to 20.
2.2. Probability of LSD recurrence in LSD‐affected areas due to external infection sources
The probability of recurrence of LSD in affected areas after stopping the vaccination, if the infection in animals is not eliminated by vaccination, is derived from the spread model, as explained in Section 2.1.2.
If the infection is eliminated by vaccination, the recurrence may depend either: (i) on a spill‐over infection from a neighbouring affected country or area; or (ii) on an infection source within the area but different from the cattle population, either the virus persists outside the domestic host, e.g. in the environment, or in vectors, or in wild animals. This is assessed based on the knowledge derived from literature review and expert knowledge. A case study of estimation of the probability of the possible incursion from Turkey into EU is presented based on data on LSD outbreaks reported by Turkey, Eurostat data on cattle trade movements, with the methodology as described in EFSA AHAW Panel (2015).
2.3. Surveillance strategy
To answer the ToR 2, the components of the surveillance strategy in the four different scenarios given by the ToR are analysed based on the spread model, on experimental evidence from European Reference Laboratory (EURL) on capripoxvirus, on knowledge deriving from previous EFSA outputs, on literature and expert knowledge. In particular, the surveillance scenarios are assessed based on:
The possible objective of the surveillance, i.e. early detection of primary infection or demonstration of absence of disease.
The type of surveillance, i.e. active or passive.
The possible source of infection, i.e. introduction from neighbouring countries or re‐emergence within the country.
The susceptible target population, based on data on cattle population provided by affected or at‐risk countries, and their related immunological status as result of vaccination or natural infection.
The risk areas (derived from the model kernel, as explained in Section 2.1.3), the risk periods (derived from disease seasonality) and other risk factors, e.g. outdoor vs indoor farming (EFSA, 2018).
The possible diagnostic tests to be used for LSD for the different surveillance purposes – early detection or demonstration of disease absence. The diagnostic tests and their performance (Se and Sp) are discussed with their pros and cons based on literature and experimental results by the EURL on capripoxvirus (Section 3.3.1).
The design prevalence or threshold of detection depending if the objective is early detection or for demonstration of the absence of disease.
Based on the above considerations, the sample size is estimated by RIBESS tool (EFSA, 2012).
The sampling frequency is estimated based on time to detection windows, estimated as explained in Section 2.1.3.
If a combination of different types of surveillance (e.g. passive + active) is envisaged in the different scenarios, the overall performance of the surveillance can be estimated assuming that: (1) streams are independents; and therefore, (2) the probability (performance) is equal to one minus the product of the probability of failing in each individual stream.
3. Assessment
In this section, the results of the assessment are presented. In Sections 3.1 and 3.2, the results to respond to ToR 1 (duration of vaccination and probability of recurrence) are presented and discussed, while in Section 3.3 the results related to ToR 2 (surveillance scenarios) of the mandate are shown.
3.1. Dynamics of LSD in a vaccinated population to achieve disease freedom and probability of persistence in infected areas
In this section, the probability of disease fade out to a level, from which it cannot re‐emerge, as a function of the number of years of the vaccination campaign, and the probability of persistence (i.e. the proportion of simulated outbreaks (infected herds) for which LSDV dies out) is estimated in two different regions in the Balkans, i.e. in Albania and in Greece and Bulgaria (the last two‐ones considered as one region), by using the spread model of LSD as described in Section 2.1.2.
3.1.1. Albania
As a baseline scenario, in the absence of control measures (including vaccination), LSDV was predicted to persist in Albania (without reintroduction) provided the turnover in the cattle population (mean life expectancy of cattle) is sufficiently high (Figure 3).
Figure 3.
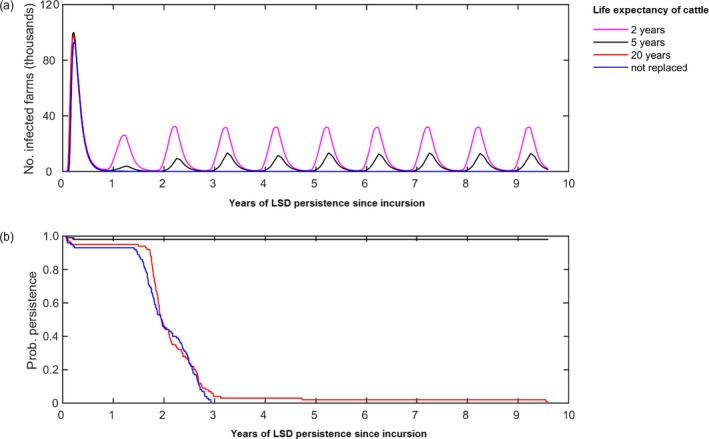
Long‐term dynamics of LSDV in Albania in the absence of control measures
- (a) Median number of infected herds over time according to different mean lifespan of cattle. (b) Probability of persistence over time. Line colours indicate the mean life expectancy assumed for cattle. In graph (b), the magenta line is below the black line. The initial incursion was to a randomly selected farm in the district of Mat on 1 June. Results are based on 100 replicates of the model.
If cattle are not replaced or if the mean lifespan of cattle was 20 years, LSD dies out typically within 3–4 years of the initial incursion, due to the onset of herd immunity. If, however, the mean lifespan of cattle is 2 or 5 years (the latter reflecting the mean lifespan of cattle), the protection derived by the herd immunity is less marked due to influx of susceptible animals and LSDV is predicted to persist for at least 10 years after the initial incursion, with recurrent epidemics of disease.
Vaccination was predicted to eliminate LSDV from Albania provided farm‐level coverage was sufficiently high and vaccination was carried out for a sufficiently long time period (Figure 4). For example, at least 4 years with 50% coverage, or at least 3 years with 90% coverage.
Figure 4.
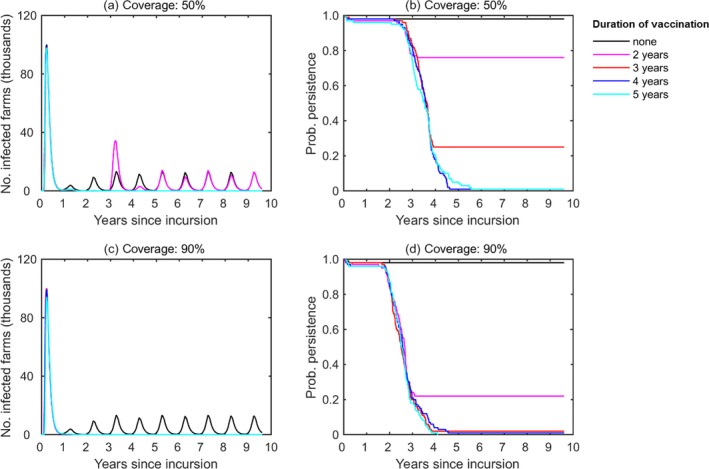
Impact of farm‐level vaccination coverage and campaign duration on the dynamics of LSD in Albania
- (a, c) Median number of infected herds over time and (b, d) Probability of persistence over time assuming a farm‐level coverage of (a, b) 50% or (c, d) 90%. Line colours indicate the campaign duration: no vaccination (black); 2 years (magenta), 3 years (red), 4 years (blue) or 5 years (cyan). The cattle mean life expectancy is 5 years. Results are based on 100 replicates of the model.
In this simulation, the vaccination effectiveness as described in Section 2.1.2 was considered, with median value 65% (57–71%). The mean life expectancy for cattle was assumed to be 5 years and the initial incursion was to a randomly selected farm in Mat on 1 June. Where vaccination coverage was low (50%, see Figure 1) or only carried out for 2–3 years, the probability of LSDV persisting was > 20% and, when elimination did not occur, the dynamics of infection subsequently reached the same level in the absence of control. At higher coverage (90%) or for longer duration vaccination campaigns (4–5 years), LSDV was highly likely to be eliminated, but even here there were one or two simulated epidemics in which this did not occur.
Conclusion
In the absence of control measures, LSD was predicted to persist in Albania.
Three years of vaccination at a coverage of 90% is most likely to be sufficient to eliminate LSD from the population, assuming median vaccine effectiveness of 65% (vaccination effectiveness of 95.7% at the end of third year) and the absence of new introductions into the country.
Four years of vaccination at a coverage of 50% is most likely to be sufficient to eliminate LSDV from the population, assuming median vaccine effectiveness of 65% (vaccination effectiveness of 98.5% at the end of fourth year) and the absence of new introductions into the country.
In the simulated spread in Albania, LSD was predicted to persist with a probability of 80% and 25% if the vaccination coverage is 50% and vaccination lasts for 2 years and 3 years respectively, and would persist with a probability of 20% if the vaccination coverage is 90% and vaccination lasts for 2 years.
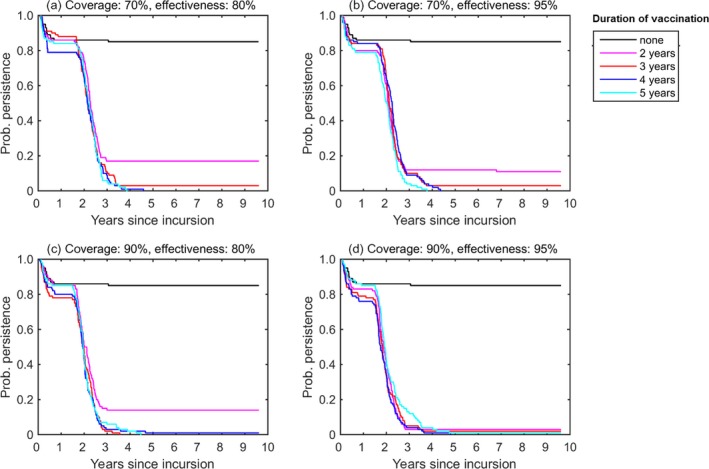
3.1.2. Bulgaria and Greece
Figures 5 and 6 show the probability of persistence of LSDV and the number of infected herds respectively, in Greece and Bulgaria (treated as a single region) for different vaccination campaigns is displayed. LSDV was predicted to be able to persist in Bulgaria and Greece in the absence of control, with recurrent annual epidemics (Figures 5 and 6, the black line).
Figure 5.
Impact of farm‐level vaccination coverage and campaign duration on the probability of LSD persisting in the region of Bulgaria and Greece (treated as a single region)
- Scenarios assume a farm‐level coverage of (a, b) 70% or (c, d) 90% and a vaccine effectiveness of (a, c) 80% or (b, d) 95%. Line colours indicate the campaign duration: no vaccination (black); 2 years (magenta), 3 years (red), 4 years (blue) or 5 years (cyan). The mean life expectancy for cattle was assumed to be 5 years and the initial incursion was to a randomly selected farm in Evros on 1 June. Results are based on 100 replicates of the model.
Figure 6.
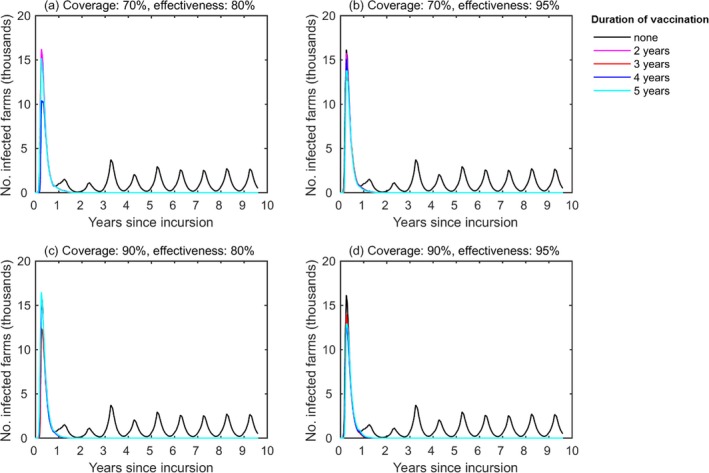
Impact of farm‐level coverage and campaign duration on the dynamics of lumpy skin disease virus in the region of Bulgaria and Greece (treated as a single region)
- Median number of infected herds assuming a farm‐level coverage of (a, b) 70% or (c, d) 90% and a vaccine effectiveness of (a, c) 80% or (b, d) 95%. Line colours indicate the campaign duration: no vaccination (black); 2 years (magenta), 3 years (red), 4 years (blue) or 5 years (cyan). The magenta, red and blue lines are not visible since below the cyan or the black lines. The mean life expectancy for cattle was assumed to be 5 years and the initial incursion was to a randomly selected farm in Evros on 1 June. Results are based on 100 replicates of the model.
For the scenario considering incursion to Bulgaria and Greece, the successful elimination of LSDV was less sensitive to assumptions about the farm‐level coverage (70% or 90%) or vaccine effectiveness (80% or 95%), but did depend on the duration of the vaccination campaign (Figure 5). In particular, the probability of LSDV re‐emerging after vaccination stopped was > 10% for campaigns that lasted only 2 years, except at very high coverage (90%) and effectiveness (95%). For longer duration campaigns (i.e. 3–5 years), the probability of re‐emergence was < 3% in all scenarios.
The above results assume no new LSDV introduction into Bulgaria or Greece. However, the recent incursion of Peste des Petits Ruminants (PPR) into Bulgaria indicates that this is a condition difficult to be maintained, due to the proximity to endemic areas.
For the scenario considering incursion to Bulgaria and Greece at vaccination of 80% effectiveness and 70% coverage, predictions did not differ for different assumed values of within‐herd basic reproduction number (1.5, 2.0 or 5.0) or of temperature data used in the simulations (year 2013–2017).
Conclusion
In the absence of control measures, LSDV was predicted to persist in Greece and Bulgaria.
Two years of vaccination at coverage of 90%, assuming a vaccination effectiveness of 95% (VE of 99.7% at the end of second year), is most likely to be sufficient to eliminate LSDV from the population, assuming the absence of new introductions into the country.
Three to five years of vaccination at coverage of 70% assuming a vaccination effectiveness of at least 80%(VE of 99.2% at the end of third year), is most likely to be sufficient to eliminate LSDV from the population, assuming the absence of new introductions into the country.
In the simulated spread in Bulgaria and Greece, LSD was predicted to persist with a probability of 20% and 5% if the vaccination coverage is 70% and vaccination lasts for only 2 years and 3 years, respectively, and would persist with a probability of 20% if the vaccination coverage is 90% and vaccination lasts for 3 years. If the vaccination coverage is 90%, LSD was predicted to persist with a probability of 20% if vaccination lasts for only 2 years.
3.1.3. Other approaches to stop vaccination
The previous simulations are based on stopping vaccination completely at a precise moment in time in the whole country or area. There may be other methods for discontinuing the vaccination programme other than: (a) stopping the vaccination and forbid it at country level. These may include (b) stopping the compulsory vaccination and leave it on a voluntary basis; or (c) stopping the vaccination in phases following a risk‐based approach.
For cases in which farmers are allowed to vaccinate their cattle on a voluntary basis, herd immunity will decrease at a slower rate, depending on the willingness to vaccinate, than stopping the vaccination at country level.
This exit strategy has been applied in Israel and over a 2‐year period, vaccination coverage dropped below 50% (Dr Galon, former Israel Chief Veterinary Officer, May 2017). Extrapolating this to Europe would mean that starting from about 2 years after the cessation of compulsory vaccination, if virus reintroduction occurs, new outbreaks can be expected. As the decision to vaccinate would be up to the farmers, the results of this strategy would be based on their commitment and may therefore vary accordingly. A drawback of the strategy is that the official authorities will not have the opportunity to oversee their vaccination campaigns and may lose the choice to have high herd immunity in areas with higher risk of virus reintroduction (e.g. at borders). In addition, the situation in Israel is different from most European countries, as Israel does not implement the same measures and does not export live cattle to Europe, so without sensitive trade impact, and the occurrence of an outbreak that is controlled in a timely and efficient manner does not imply great economic losses.
Alternatively, phasing out vaccination can be achieved in various ways: (1) relaxing the vaccination scheme within herds (e.g. single vaccination per year of only newborn animals when the maternal immunity has waned); (2) relaxing the vaccination programme in a region (not vaccinating certain types of herds); or (3) country‐wise, stopping vaccination with the exemption of those regions considered at higher risk of virus reintroduction.
Combinations of the above are possible and, consequently, a country could decide to use these based on its risk profile. For example, in areas where no outbreaks have been recorded and no outbreaks have been notified in the border regions of the neighbouring countries, strategies (a) and (b) could be considered. Alternatively, countries that border infected areas could decide for a combined strategy, including voluntary vaccination in the regions far from its borders and a compulsory (buffer) vaccination in regions and islands close to borders. If the risk of introduction from bordering infected countries becomes low(er), relaxation of the vaccination programme in the buffer regions could be considered. An example could be vaccinating dairy and breeding herds and not vaccinating fattening herds in a first phase (b). In fact, the fattening herds, with a low average age, would serve as sentinel herds and indicate whether further relaxation of vaccination approaches could be considered.
In any case, vaccination should be always monitored to correctly estimate the vaccination effectiveness: this would help to decide when the vaccination could be stopped.
3.2. Probability of recurrence of infection if the disease is eliminated by vaccination
If the disease is eliminated in an area by a specific strategy, e.g. vaccination, the probability of recurrence after a certain period may only depend on: (A) the risk of spill‐over infection from neighbouring infected countries or zones; or (B) an infection source still within the area considered but different from the live bovine hosts.
3.2.1. Risk of introduction from an affected area
The first recurrence pathway, i.e. the risk of introduction from an affected area, is the most likely, and the possible pathways were previously described in EFSA (2015), ranked in order of likelihood:
Pathway A.1 The introduction of infected animals (including germinal products) is the most efficient pathway to introduce LSDV into a country, in particular for long‐distance spread.
Pathway A.2 The active movement of flying vectors can be a pathway for LSD introduction into a naïve country over a short distance, e.g. from close infected areas.
Pathway A.3 Circumstantial evidence indicates that windborne transmission of vectors carrying the virus (after a blood meal on an infected animal) is a potential route of LSD introduction into a country.
Pathway A.1 – Risk of introduction by movement of infected live animals from bordering regions
The risk of LSD introduction from bordering regions depends primarily on:
infection status of neighbouring countries: active infection, past infection without outbreaks, freedom from infection;
distance of infection from borders;
control measures in place in neighbouring countries: none, surveillance, vaccination, etc.;
likelihood of transboundary crossing: numbers of animals moved.
The former status of the neighbouring region could be related to the disease being either in its epidemic phase, or have endemic or sporadic outbreaks, or absent (region is free of the disease). The latter status of the area regarding vaccination could be either as not vaccinated or partially or totally vaccinated with homologous or heterologous vaccines. In particular, the proximity to infected regions directly influences the probability of LSDV introduction. The combination of the infection and vaccination status leads to different levels of risk for introduction of the disease, so a case‐by‐case approach in the strategy definition should be considered for the different situations.
As the introduction of cattle from infected regions represents a risk, the possible measures to mitigate this risk are:
-
a)
to ban any introduction of animals from these regions. This can increase the risk of illegal movements if borders cannot be controlled adequately.
-
b)
allow introduction of animals from infected countries if vaccinated with a homologous vaccine at least 3–4 weeks before the movement and no later than the period for which the immunity is maintained after vaccination. The level of risk in this case is related to the effectiveness of vaccine and of vaccination activities, and the resulting capacity of each single animal to develop a solid immunity after vaccination.
-
c)
allow movement of unvaccinated animals after a quarantine period during which favourable results of diagnostic examinations are obtained. In this case, the residual level of risk is linked to the sensitivity of the overall diagnostic procedure applied and the lack of protection of animals against vectors (with consequent risk of infection) during the quarantine period.
-
d)
combining the measures from points (b) and (c).
It is important to note that the OIE code recommends that the animals before importation from a country not free from LSD:
showed no clinical sign of LSD on the day of shipment;
were kept since birth, or for the past 60 days before shipment, in an epidemiological unit where no case of LSD occurred during that period;
were vaccinated against LSD according to the manufacturer's instructions between 60 days and 1 year before shipment;
were demonstrated to have antibodies at least 30 days after vaccination;
were kept in a quarantine station for the 28 days before shipment during which time they were subjected to an agent identification test with negative results.
Case study: Turkey as possible source of virus for EU
The chance of a possible incursion of infected animals from Turkey, or similarly the time needed for the introduction of at least one infected animal from Turkey, could be estimated using a case study based on methodology applied here as described in EFSA (2015).
Based on the prevalence in the infected country of origin (P), a mean infectious period (IP) of 60 days and considering a mean duration of seropositivity by enzyme‐linked immunosorbent assay (ELISA) of 6 months (D), the probability of including at least one infected animal among N imported animals, in the absence of risk mitigating measures, would be as follows:
1 − (1 − I × P/D)N
In Table 2, the numbers of animals that should be moved in order for at least one infected case to be introduced at a certain probability (1%, 5% or 95%) are indicated for different levels of prevalence in the country of origin.
Table 2.
Number of animals to be moved from an infected country according to different probabilities such that at least one infected cases is introduced for different level of prevalence in the infected country of origin in 1 year
| Probability of introduction 1% | Probability of introduction 5% | Probability of introduction 95% | |
|---|---|---|---|
| Prevalence 0.1% | 84 | 426 | 24,850 |
| Prevalence 0.3% | 28 | 142 | 8,283 |
| Prevalence 0.5% | 17 | 86 | 4,969 |
| Prevalence 1% | 9 | 43 | 2,484 |
| Prevalence 3% | 3 | 15 | 827 |
| Prevalence 5% | 2 | 9 | 496 |
| Prevalence 10% | 1 | 5 | 248 |
If the prevalence of infection was 0.1% in the country of origin in 1 year, 426 animals would be needed to be moved to have 5% probability of introducing LSD into Europe (in the absence of any border inspection or quarantine).
Although the import of live cattle from non‐member countries such as North African and Middle Eastern countries to Member States (MSs) is forbidden according to EC Regulation No. 206/2010, it was reported by Eurostat that 51 tonnes of live cattle (approximately 100–150 heads) were traded from Turkey to EU in 2017. This discrepancy has already been pointed out in a previous EFSA output on LSD (EFSA AHAW Panel, 2015). Despite the fact that these data may be inaccurate, this situation indicates that the risk of unlawful movements of live animals from endemic countries towards the EU should always be considered. Use of these data as a proxy suggests that, considering a prevalence of LSD in Turkey of 0.65% as observed in 2013–2014 (Ince et al., 2016), the introduction of at least one infected case could be possible (5% probability). If uncontrolled animal movements were also considered, a higher probability of disease introduction should be considered.
Conclusion
Introduction of animals from endemic countries into countries with susceptible cattle population will probably result in LSD infection.
Pathway A.2 – Risk of introduction from active movement of vectors
The active movement of LSD flying vectors can potentially be a pathway for LSD introduction into a naïve country from a short distance, e.g. from infected areas close to the borders, although this appears to be the case only at very short distances, as some studies suggest (see below). Nevertheless, to date, no studies on the potential vector species of LSDV have been performed and completed in Europe and particularly in the LSD‐affected countries in recent years. Hopefully, more evidence will be produced within a short time, for example by the recently started DEFEND project,4 an international partnership of academic, industrial and governmental organisations working together to tackle the emergence of African swine fever and LSD in European livestock that will explore, among other aspects, the role of different species of vectors (in in vitro and in vivo studies).
In a recent study, Lempereur et al. (2018) showed in a mark‐release‐recapture trial in horses that vectors of equine infectious anaemia (EIA) in Belgium such as S. calcitrans flew maximum distances of 150 m and 300 m when partially fed and unfed, respectively. Another EIA vector such as Haematopota spp. (Tabanidae) travelled maximum distances of 100 m and 200 m when partially fed and unfed, respectively. The authors suggested that a distance of 150 m appeared to be the minimum required for segregation to avoid the risk for mechanical transmission of EIA, but in areas of higher vector density, this should probably be increased (Lempereur et al., 2018).
Baldacchino et al. (2013) reviewed that stable flies have a great flying capacity up to 29 km in 24 h according to laboratory flight mill studies. However, field dispersal studies showed that the flies would travel at least 3 km after 6 days in search of a blood meal. Also, a 3 years study on adult stable flies spread from larval development sites in a mixed agriculture environment in eastern Nebraska (USA) indicated that 50% of stable fly adults had dispersed beyond 1.6 km of the breeding site, and only 5% of the flies were marked flies were captured beyond 5.1 km (Taylor et al., 2010). Short‐range dispersal of stable fly is known also to depend on the host density in the area, where in places of low density of host, flight range increases and vice versa (Gersabeck and Merritt, 1985) and on extreme climate episodes such as strong winds.
Baroos and Foil mentioned that the Tabanidae activity radius may extend only up to 25 m from infected cattle (Barros and Foil, 2007), so making long‐distance transmission unlikely.
The recurrence of LSD by introduction of active movement of infected LSD flying vectors is only likely in short distances (e.g. up to 5 km) between infected and susceptible herds when host are available in the area and no extreme climate events are present. It should be reminded that the primary incursion of LSD to Greece (Evros region) occurred after the confirmation of outbreaks in Turkey at short distance from the border, one outbreak was reported in the same region in Turkey in 2018.
Pathway A.3 – Risk of introduction from windborne transmission
In a previous EFSA opinion on LSD (EFSA AHAW Panel, 2015), the possibility of windborne transmission of vectors carrying the virus (after a blood meal on an infected animal) was extensively reviewed, and it was concluded that there is circumstantial evidence indicating that this is a potential route of LSDV introduction into a naïve area. This seemed likely, especially for mosquitoes and biting midges that, because of their small size, can be considered as particulate matter and have been studied in models of atmospheric tracing (Kalderon‐Asael et al., 2009; Morag et al., 2013).
Suspicion of importing LSDV into Israel from Egypt by transport of stable flies was also suggested during the epidemics in Israel in 1989–2006 (Yeruham et al., 1995; Brenner et al., 2006), when large‐scale outbreaks of LSD were taking place in adjacent countries.
Other authors provide further evidence by the analysis of backwards Lagrangian trajectories (BLTs) (i.e. Egypt to Israel), then suggesting that transport of Stomoxys by wind currents over long distances and infection by the virus at their point of arrival may occur in 24 h (Klausner et al., 2015).
Altogether, these studies suggest that long‐distance dissemination of infected vectors such as Culicoides biting midges by winds could be a potential route of transboundary transmission of vector‐borne viruses into other geographic regions, but it is less likely than for short distances. However, the risk of this type of introduction under the current situation in Europe is difficult to assess due to the limited information on the potential vector species in the area, as well as the lack of analysis of wind trajectories.
3.2.2. Risk of recurrence due to infection source within the area considered but different from live cattle
This is linked to the probability of the LSDV remaining viable in:
Pathway B.1 Vectors (flying vectors and ticks);
Pathway B.2 Wildlife;
Pathway B.3 Environment.
Pathway B.1 – Role of vectors
Some flies and mosquitoes species are thought to be the mechanical vectors of LSD and few studies carried out in the past showed that virus may not persist for more than 24 h in the body of Stomoxys (Chihota et al., 2003) and Aedes aegypti was shown to be able to transmit LSDV up to 6 days post‐feeding (Chihota et al., 2001). Nevertheless, survival of LSDV in vectors has not been fully elucidated and, to date, no studies about the potential vector species of LSDV have been performed in Europe and particularly in the LSD‐affected countries in the recent years.
Knowledge about the role of ticks as LSD vectors originates from studies conducted on African tick species Rhipicephalus appendiculatus, Rhipicephalus decoloratus and Amblyomma hebraeum (EFSA, 2015). Interstadial and transstadial transmission have been demonstrated and long persistence of the virus is possible. The virus survived the process of moulting to adults, following feeding of nymphs on LSDV‐infected cattle, and the virus could be detected by PCR test in saliva for at least 1 month (EFSA, 2015).
These findings indicate a possible role of ticks in the transmission and maintenance of LSDV in the environment as well as an overwintering pathway. Since the species and role of ticks in the recent epidemics in Europe are not known, further studies are required to investigate the importance of tick vectors for the spread and especially for the maintenance of LSDV from year to year in the affected regions of Europe.
Pathway B.2 – Role of wild animals
Various species of African wild ruminants are considered to be susceptible species. However, they do not seem to play a significant role in the epidemiology of the disease (EFSA, 2015; ANSES, 2017, 5). There are currently very few or no data on the susceptibility of wild ruminant species present in Europe, such as Cervidae. Some field trials conducted in Bulgaria showed that saliva samples taken from wild ruminants from infected areas were negative using PCR test for LSDV (Alexandrov T, personal communication, March 2018) according to a method recently published by the same author (Dietze et al., 2018).
The risk related to wildlife as carriers of LSDV or related to a possible sylvatic cycle of the virus cannot be assessed, because the epidemiological role of wild animals has not been elucidated. There may be also a possibility that, from the ecological point of view, the virus may cycle between ticks and wildlife in Europe due to the similar ecology of other diseases such as tick borne encephalitis or Crimean‐Congo haemorrhagic fever.
The role of subclinical animals in the persistence of infection is discussed later in Section 3.3.1, while the possible overwintering of the disease due to persistently infected animals remaining infectious after winter months is unknown.
Pathway B.3 – Environment
Based on a literature review on persistence of LSDV in different matrices, the longest period the virus may remain viable is up to 6 months in shaded pens or beddings (EFSA, 2015).
Transmission by direct contact or indirect contract with fomites is less effective than by vector transmission (EFSA AHAW Panel, 2015) and the event of an animal acquiring the infection by contact with contaminated bedding has never been described. Moreover, the longest period for virus viability of 6 months is shorter than immunity generated after last vaccination, which is at least 1 year but most likely to be longer in animals vaccinated more than once. This makes the probability of re‐infection with virus remaining in the environment after an outbreak, followed by vaccination, very low or negligible.
As mentioned above, further evidence will be hopefully provided by the recently started DEFEND project,4 in which a work package dedicated to LSD transmission will explore both the role of different species of vectors (in vitro and in vivo studies) and the possible indirect transmission via fomites such as feed troughs and via contaminated livestock housing.
3.3. LSD surveillance strategies
Surveillance is crucial for a country considering stopping mass vaccination. In fact, any strategy associated with a reduction of the (herd) immunity, leads to a progressive increase of the risk of virus transmission and spread if (re)introduction occurs. In addition, differently from the situation in which all animals are susceptible, for coexistence of vaccinated and unvaccinated animals, the susceptible ones can be sparse and scattered within the vaccinated population, so resulting in a greater difficulty in identifying clusters of infection based on clinical signs alone. For this reason, it could be more appropriate in such a situation to also introduce some active, laboratory‐based surveillance activities, to increase the overall sensitivity of the passive surveillance system. To date, vigilant passive surveillance and structured (risk‐based) active surveillance as main components of a surveillance system seem to be the most plausible solution for coexistence of vaccinated and unvaccinated animal populations, but the newly available serological tests for Capripoxvirus (see Section 3.3.1) may open up further possibilities. Vigilant passive surveillance is mostly served by awareness campaigns among farmers. Some examples of risk‐based active surveillance can be derived from point 3 above. In fattening herds with a high turnover rate, herd immunity will drop quickly, making these herds the first ones to become susceptible to the disease after vaccination cessation. In addition, when extensive outdoor farming is practised, this increases the probability of infection for these herds (EFSA, 2018). If active clinical surveillance is focused on such herd types, the probability of detecting the disease, following (re)introduction, can be significantly higher, as indoor herds are more intensively reared and, usually, passive surveillance is conducted anyway.
When available, the results of entomological monitoring, climatic and environmental data (e.g. altitude, land use) should also be taken into account in the identification of those animal subpopulations more at risk of being infected if virus introduction occurs. In addition, if the newly developed ELISA tests enter into routine use, other approaches, based on serological monitoring of unvaccinated animals (serological negative sentinel herds, newborn animals tested after the period of persistence of antibodies possibly acquired through colostrum or seronegative animals brought from countries where vaccination is not implemented) can be considered.
If surveillance identifies an outbreak of LSD, prompt action in setting measures is pivotal and, given that vaccination is by far the most effective measure, availability of vaccines and a vaccination plan for quick implementation should be in place. For all these reasons, to properly evaluate the best option for surveillance, it would be important to estimate the expected delay of disease recognition under different introduction and surveillance scenarios, given the direct relationship between such delay and the extent of population to be submitted to emergency vaccination for limiting the spread of the infection.
3.3.1. Items for planning surveillance in the LSD‐affected or at‐risk countries
Passive surveillance
Passive surveillance is defined by Doherr and Audigé (2001) as reporting of clinical suspect cases to the health authorities by healthcare professionals or farmers at their discretion. The advantages of passive surveillance are its low price and high coverage of the population and its continuous nature. It is especially useful for diseases that are characterised by typical clinical signs and for which subclinical infection is rare. LSD is a disease with highly apparent clinical signs. However, it has been reported that clinical appearances are heterogeneous and may range from asymptomatic to severely affected, and about 50% of infected animals may not show clinical signs (Carn and Kitching, 1995a,b; Tuppurainen et al., 2005, 2013). In a recent experiment, out of 12 infected animals, nine animals developed a generalised form of LSD with a clinical score of at least seven according to the scale proposed by Carn and Kitching (1995a) corresponding to ‘generalisation with few secondary nodules, severe lymphadenopathy, no systemic disturbance’, at 7 days post infection (Dietze et al., 2018). Altogether this evidence is only from experimental trials in which there may be limitations linked to the small number of experimental animals. Therefore, it should be verified whether this evidence is also valid under field conditions, so the importance of subclinical infection as a potential source of disease transmission is under debate.
As clinical signs of LSD are highly apparent, the probability of missing a clinical infection is low, primarily if awareness of the occurrence of disease is high among the farmers and veterinary practitioners, as is recommended by the OIE code: ‘The veterinary services should implement programmes to raise awareness among farmers and workers who have day‐to‐day contact with livestock, as well as veterinary paraprofessionals, veterinarians and diagnosticians, who should report promptly any suspicion of LSD’.
Reporting should be enhanced by explaining its importance and building trust between farmers and the authorities.
Active surveillance
Active surveillance is the detection of the disease through surveillance or monitoring activities actively performed by the official veterinarians. These activities can be randomly or not randomly carried out and they can be based on clinical, serological or virological detection of disease or infection. In active surveillance, the collection of data is specifically designed at determining levels of disease, or determining its presence or absence.
Clinical surveillance is essential for detecting cases of infection with LSDV and requires the physical examination of cattle. Surveillance based on clinical inspection provides a high level of confidence of detection of disease if a sufficient number of cattle is examined regularly at an appropriate frequency and investigations are recorded and quantified. Clinical examination and laboratory testing should be pre‐planned and applied using appropriate types of samples to clarify the status of suspected cases’.
Diagnostic tests to be used for active surveillance purposes
For surveillance purposes, we are interested in the period when the animals are infectious, i.e. viraemic with skin lesions.
Clinical detection
The clinical signs of LSD are highly apparent and so highly predictive for LSD detection. It should be noted, however, that a disease could be left unnoticed if only sight inspection is used. Performance of a clinical examination, which includes gentle stroking of the animal skin, can increase the sensitivity of this detection method. Clinical signs usually occur about 10 days after infection (Magori‐Cohen et al., 2012) and so a lag period is expected between infection and detection of the disease. However, infection during this stage is likely to be of less importance as the highest titres of virus occur in skin lesions, which at this stage do not exist. Of crucial importance for this method is the role of subclinically infected animals in disease transmission. This will be discussed below. Naturally, if subclinical infection is important in disease transmission, the sensitivity of clinical detection of infectious animals is reduced.
From the experimental trials conducted by Sciensano (Belgium) on Capripoxvirus, the sensitivity of detecting clinical signs of LSD in experimentally infected animals is 75% in the first 3 weeks after infection. Tuppurainen et al. (2005) estimated a 67% sensitivity of detecting clinical signs in experimentally infected animals within 30 days post‐infection (d.p.i.).
PCR test of blood or skin
Previous studies have shown that successful virus isolations can generally not be obtained before 6 days post‐infection (Magori‐Cohen et al., 2012). Additionally, titres of virus in skin lesions are higher by five orders of magnitude compared with their titres in blood (Babiuk et al., 2008). Taking into account the fact that not all animals show high titres in blood at such early stages, and that viremia is intermittent (Babiuk et al., 2008), the sensitivity of the PCR test for blood samples is not expected to be high. Considering the additional time needed for processing the tests, PCR testing of blood samples may not have an added value for increasing sensitivity over that achieved by clinical detection. However, PCR test is the laboratory test of choice to confirm or rule out LSD in suspected animals. In cattle population where mass vaccination against LSD is conducted, a DIVA test (Agianniotaki et al., 2017; Menasherow et al., 2016) should be used for distinction of field from vaccine virus.
The diagnostic sensitivity of different PCR assays has been reported to be between 90.5% and 100% in blood and 95.5–100% in tissues, while the specificity ranges between 96.7% and 100% in blood and 100% in tissue (Balinsky et al., 2008; Bowden et al., 2008; Stubbs et al., 2012; Haegeman et al., 2013).
ELISA
Due to the late occurrence of specific LSD antibodies, ELISA is probably not a sensitive tool for early detection of infection. However, the advantage of ELISA is that it can detect the ‘infection memory’, i.e. it can detect antibodies that indicate infection that occurred in the previous 6 months. Such a method can therefore be used every few months to monitor the sensitivity of other surveillance methods.
The immunoperoxidase monolayer assay may be more efficient for detection of an early antibody response, whereas ELISA is appropriate for detection of a prolonged antibody response (at least up to 5 months).
From the results of the experiments conducted by Sciensano (Belgium)5, for the detection of antibodies against LSDV within 1 month, both in experimentally vaccinated animals (N = 6) and in animals experimentally inoculated with a field strain of LSDV (N = 6), under experimental conditions, the following values for Se and Sp were reported:
commercial ELISA: Se = 83%; Sp = 99.7%;
immunoperoxidase monolayer assay : Se = 100%; Sp = 100%.
When testing seroconversion within 1 month in animals vaccinated under field conditions (N = 75), the detection of antibodies against LSDV is generally lower:
commercial ELISA: Se = 59%; Sp = 99.7%;
immunoperoxidase monolayer assay: Se = 53%; Sp = 100%.
As the value of sensitivity of the serological tests under field conditions appears much lower compared with those estimated under experimental conditions, the performance of this recently marketed test needs further evaluation to verify the values of Se and Sp in the field.
In Figure 7, the different time points (days PI) when LSD can be diagnosed are displayed, based on data from Sciensano (Belgium)6 and from other three experimental studies (Tuppurainen et al., 2005; Babiuk et al., 2008; Bowden et al., 2008).
Figure 7.
Diagnostic time window for Capripoxvirus infection
- Range (minimum and maximum values, black lines) and mode (red dots) of the distribution of diagnostic time windows for Capripoxvirus in different sample matrices or symptoms (fever).
According to the experimental infection trials conducting at Sciensano6 (Belgium) on 20 animals, the incubation period of LSD in experimentally infected animals generally varies between 4 and 7 days, with fever appearing usually around 5–6 d.p.i. and lasting 3–14 days. Approximately 1 day after the onset of fever, viraemia occurs and can be intermittently detected for up to 2 weeks with the most frequent detection occurring between 8 and 14 d.p.i.
The severity of clinical signs does not correlate with the length of viraemia. Skin lesions usually start to develop 1–3 days after the onset of fever. Skin nodules, scabs and crusts contain high concentrations of virus, and virus isolation up to 39 d.p.i. from skin lesions has been reported (Tuppurainen et al., 2005). The duration of infectiousness was also estimated by the spread model as 44 days, which is in line with this experimental evidence. In the study by Babiuk et al. (2008) the detection of the LSDV genome in blood was at 6–15 d.p.i., while LSDV genome detection in skin lesions was possible up to 42 d.p.i.
Together with the clinical signs and viraemia, Capripoxvirus are detectable in several secretions and excretions and internal organs, such as in oral and nasal secretions (6–64 d.p.i.), in ocular discharge (8–64 d.p.i.), in urine (10–15 d.p.i.), in faeces (4–61 d.p.i.), in semen (6–159 d.p.i.) in tissues (4–21 d.p.i.) and milk (see table in EFSA AHAW Panel, 2015). Antibodies are detectable from 6 to 8 d.p.i., peak around 3–4 weeks post infection (p.i.) and remain detectable for up to 5 months.
Importance of subclinical infection
Several studies have demonstrated the occurrence of subclinical infection in cattle (Carn and Kitching, 1995a,b; Tuppurainen et al., 2005, 2013). Transmission of LSDV by ticks that were fed on normal healthy looking skin (Tuppurainen et al., 2013), suggests a potential role of subclinically infected animals in LSDV transmission. Transmission via subclinically infected animals could potentially occur either by ticks or by contact with intact skin. However, the level of viable virus in intact skin was undetectable in cattle with mild to moderate clinical disease (Babiuk et al., 2008). Virus isolation from blood is intermittent, short term and around five orders of magnitude lower compared with skin lesions (Tuppurainen et al., 2005; Babiuk et al., 2008). Additionally, outbreaks in Israel were controlled using modified stamping out (i.e. only stamping out cases with generalised skin lesions), which was performed under the assumption that the high levels of virus present in the skin lesions are the most likely source of virus transmission (EFSA AHAW Panel, 2015), concurrently with the use of an RM‐65 sheep‐pox attenuated vaccine which was later shown to have very low effectiveness for preventing LSD (Ben‐Gera et al., 2015). Taken together, the evidence gathered to date suggests that, although subclinically infected animals may transmit the virus through bites of mechanical arthropod vectors, this route of transmission plays only a minor role in transmission of LSDV, unless other routes such as transmission by direct contact with secretions like saliva, ocular and nasal discharge were to be demonstrated.
Time to detection and possible design prevalence
To assess the effectiveness of different surveillance systems aiming to achieve early detection, as described in Section 2.1.3, the model as described in Section 2.1.2 was used to estimate the time to detection of an epidemic. LSD incursions into Albania or Bulgaria and Greece (the two latter treated as a single region) were simulated assuming no control measures were implemented (Table 3).
The time to detection of an incursion was similar for both Albania and Bulgaria and Greece (Table 3). The time of year at which the incursion can occur did have a large impact on the time to detection (Table 3). In the season for favourable LSD transmission, typically from April until October (EFSA, 2018), the time to detection is shorter than in winter months because it is easier to detect cases. In particular, it took 60–75 days before an incursion was detected in January, compared with 15–30 days when an incursion occurred in April or July. Incursions in October had an intermediate detection times of around 45 days. The number of infected herds at detection is typically small (Table 3) and corresponds to a prevalence of infected herds < 0.1% in all instances.
If the aim of surveillance is to detect an incursion or to detect re‐emergence after a vaccination campaign stops within a certain time, the necessary median design prevalence is very low and is typically < 0.2% (Table 4; cf Table 3).
Table 4 shows that the median estimates of the percentage of herds infected increases with time until detection, but are all less than 0.2% of the herds. However, the 95% prediction ranges following a new introduction have a much higher upper range (even as large as 35% in Albania when the interval is 35 days), than those for re‐emergence of the virus. The reason for this difference is the presence of remaining vaccination immunity in the latter, which slows down between‐herd spread. Upper ranges of the percentage of infected herds are also higher when the effectiveness of vaccination increases, the reason being that immunity is higher in the population, resulting in fewer animals with clear clinical signs.
If the aim of surveillance is to detect an incursion or re‐emergence once a threshold (i.e. design) prevalence is reached, the time to detection increases with design prevalence (Table 5). The time to detection is typically shorter when detecting an incursion in a naïve population than when detecting re‐emergence after a vaccination campaign stops.
For the surveillance purpose of demonstrating absence of disease, the design prevalence that could be used for a serological survey, could be derived from the proportion of herds ever infected during a simulation (Figure 8). The lowest 25th percentile in the graph is equal to 3.5%, which can be used as design prevalence of the surveillance scenario aiming at demonstrating absence of disease (Section 3.3.2).
Figure 8.
Box plot (lower, median and upper quartile) of the proportion of herds ever infected during the spread simulation (scenarios for Albania in pink, for Bulgaria/Greece in blue, according to different levels of vaccination coverage (C) and effectiveness (E))
The above two tables and figure could provide risk managers and veterinary services with helpful aspects under different scenarios to set surveillance strategies, according to the level of preparedness and level of acceptable risk.
Probability of LSD spreading in relation to distance from infected areas: size of surveillance areas
The probability of LSDV ‘escaping’ from a zone declines with distance, with the probability of escape for a zone of > 50 km being < 6% even for the widest kernel estimated for Albania (Figure 9a). This distance increases as the between‐farm basic reproduction number (Rh) increases (Figure 9b).
Figure 9.
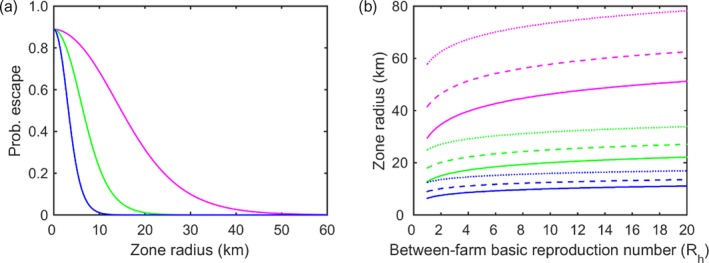
Spreading distance for lumpy skin disease virus
- Probability of LSDV escaping a zone of a given size (km) assuming the between‐herd basic reproduction number, Rh = 2.2. (b) Size of surveillance zone (km) and its dependence on the between‐farm basic reproduction number (R h) when the threshold probability of escape used to calculate the zone size is 5% (solid line), 1% (dashed line) or 0.1% (dotted line). Results in each panel are shown for an exponential kernel with kernel parameter α = 0.16 (magenta), α = 0.37 (green) or α = 0.74 (blue) (i.e. the minimum, median and maximum values estimated for 15 districts in Albania)
Distance sizes range from 6.4 km to 78.2 km, depending on assumptions made about Rh (values derived from Alkhamis and VanderWaal, 2016; Gubbins et al., 2018) and the threshold probability used (Table 6). This information may be useful in setting buffer zones for surveillance around infected area or from borders of endemic countries.
Table 6.
Size of surveillance zone (km) for lumpy skin disease virus
| Kernel parameter (α) | Between‐farm basic reproduction number (rh) | Threshold probability of escape | ||
|---|---|---|---|---|
| 5% | 1% | 0.1% | ||
| 0.16 | 1 | 29.5 | 41.5 | 57.8 |
| 20 | 51.2 | 62.5 | 78.2 | |
| 0.37 | 1 | 12.8 | 18.0 | 25.0 |
| 20 | 22.2 | 27.1 | 33.9 | |
| 0.74 | 1 | 6.4 | 9.0 | 12.5 |
| 20 | 11.1 | 13.6 | 17.0 | |
For comparison, the OIE recommends in Chapter 11.9. of the Code for infection with LSDV that surveillance should be carried out over a distance of at least 20 km from the border with that country or zone, but a lesser distance could be acceptable if there are relevant ecological or geographical features likely to interrupt the transmission of LSDV. This would correspond to an intermediate value in the range presented in Table 6.
According to the range of the above‐mentioned sizes of surveillance zones and considering the abundance and location of cattle populations in Greece and Bulgaria (when data on location are available, Figure 10), the number of herds and animals in different surveillance zones in these two countries can be estimated (Table 7).
Figure 10.
Cattle herds in Greece (GR) and Bulgaria (BG) and different sizes of surveillance zones from the border with Turkey
Table 7.
Number of herds and animals in different surveillance belt zones from the Turkish border in Greece and Bulgaria
| Total | 10 km | 40 km | 80 km | |||||
|---|---|---|---|---|---|---|---|---|
| herds | Animals | herds | Animals | herds | Animals | herds | Animals | |
| Greece | 32,455 | 729,687 | 633 | 10,630 | 1,159 | 19,023 | 2,236 | 43,595 |
| Bulgaria | 60,480 | 669,787 | 127 | 3453 | 967 | 22,378 | 10,287 | 115,618 |
This example may provide an idea of how many herds should be targeted with surveillance in different buffer zones to have a certain level of probability that the disease would not ‘jump’ from the risk border to somewhere else within the country.
3.3.2. Surveillance in different scenarios
A set of items must be taken into account to evaluate which surveillance options are the best for the given situation:
The epidemiological situation such as the absence of infection (never occurred), current or past infection, the time from the last LSD occurrence, possible source of introduction or re‐emergence of the infection.
The immunological status of the host population (as a result of vaccination or natural infection).
The main surveillance objectives.
The geographical area and the best period of time for performing the surveillance activities, according to the selected objectives.
The type of surveillance (active or passive). In this context, passive surveillance is meant as the detection of the disease based on clinical suspicion, detected and reported by farmers or veterinarians, whereas active surveillance is meant as the detection of the disease through surveillance or monitoring activities actively performed by the official veterinarians. These activities can be carried out at random or not and they can be based on clinical, serological or virological detection of disease or infection. Considering that LSD is a notifiable disease with relevant economic impact and capacity for international spread, the passive surveillance should be in place in the whole territory of all countries. Informative campaigns and other initiative could be considered to increase the awareness of farmers and veterinarians for this disease, especially in the area most at risk for LSD introduction. For this reason, the scenarios considered below will take into account only the feasibility of active surveillance actions.
The target population of the surveillance activities and criteria for its selection (census, random, targeted, based on the risk).
The parameters for the calculation of sample size (i.e. design prevalence).
These aspects are discussed below for each of the scenarios given by the ToR 2. A synoptic table summarising these elements is available at this link: https://doi.org/10.5281/zenodo.1451440.
Scenario 1: Areas or countries at risk of LSD (e.g. due to LSD outbreaks in neighbouring countries), where no LSD outbreaks have occurred and no LSD vaccination was carried out
Objective of the surveillance: early detection of primary infection following LSDV introduction.
Possible source of infection: introduction from neighbouring infected countries.
Susceptible population: the whole cattle population is fully susceptible.
Risk areas: assuming a good level of control of animal transboundary movements and imports of live cattle, the risk areas are only those closer to the infected neighbouring countries, where the infection can propagate by contiguity from the infected territories or introduced through limited local uncontrolled animal movements. According to the model, an area up to 80 km from the borders can be considered (see Chapter 3.3.1), then geographical factors and distribution of cattle population should be considered. Article 11.9.15. of OIE's TAHC considers a distance of at least 20 km from the border with an infected country as the area at risk where surveillance activities should be put in place. Considering the wide range of possible vectors, although as mechanical vectors only, special considerations should be made for the possibility of LSDV introduction through windborne dissemination of infected vectors (see Chapter 3.2.2), especially for those countries sharing close sea borders with infected territories (as, for example, Italy).
Period at major risk: from late spring to early autumn. Considering the results of model estimates for best time to detection (see Chapter 3.3.1), and the season of concern, a window between 3 (21 days) and 5 (35 days) weeks should be considered for the early detection of LSDV introduction.
Type of surveillance: active surveillance can be feasible only in areas at risk and during the period at major risk.
-
Diagnostic tests of choice:
-
–
Considering that the whole cattle population is fully susceptible, clinical examinations of animals may be considered as the best diagnostic tool for early detection of the introduction of infection.
-
–
The suspected cases detected through clinical examinations must be confirmed by laboratory tests, e.g. by PCR, to detect the presence of LSDV.
-
–
Threshold for detection (design prevalence for early detection): the value that can be assumed for the calculation as in the example below is 0.0045%, the median of the expected herd prevalence with an incursion in Bulgaria and Greece detected at 21 days after incursion in June (see Table 4).
-
–
Sampling frequency: considering the time to detection window (3–5 weeks), the clinical examination rounds in cattle herds should be repeated at least every 5 weeks during the whole period at risk. Nevertheless, it should be considered that, according to Table 5, the expected prevalence may increase rapidly after introduction i.e. up to 70 times in a 2 week period.
Duration of surveillance: until the risk of introduction exists, i.e. when bordering with an area or country with an active infection or not free from LSD.
-
Target areas/population:
-
☐
All cattle herds located in the areas at risk. Larger herds (with higher herd size) could be preferred for the higher probability of detecting animals showing clinical signs, but only if this choice does not cause any bias (for example, due to a non‐homogeneous spatial distribution of these herds or different outdoor access compared with smaller herds).
-
☐
Given the logistic and organisational difficulties in planning repeated visits to cattle herds, the surveillance activities can be strengthened by adding systematic clinical examinations for LSD at live animal markets, before cattle leaving their herds for any reasons (pre‐movement clinical checks) and during ante‐mortem examinations on animals to be slaughtered. These activities could be also combined with other surveillance programme on cattle populations in place in the country.
-
☐
Example 7 of a scheme of active surveillance under Scenario 1 for the early detection of LSD incursion under Scenario 1, e.g. in a country like Romania where no disease was reported and no vaccination has been in place, but bordering with infected countries, considering a buffer zone of 80 km (a zone with 99.9% probability of finding an LSD outbreak, if introduced – see Table 6) along the border with Bulgaria, based on clinical detection with a sensitivity of 75% to detect clinical signs at a prevalence of 0.0045% (the median of the expected prevalence with an incursion in Greece and Bulgaria detected at 21 days after incursion in June) with a total cattle population of 78,000 of which 4,290 are located in the buffer area (location randomly allocated based on cattle population size at NUTS2 level derived from data from Eurostat, Figure 11) with a confidence level of 95%, 3,619 herds (within‐herd correlation is unknown, thus it is not considered, for practical purposes all animals in the herds could be clinically examined) must be checked every 5 weeks from April until October.
Figure 11.
Cattle herds in Romania that would fall within the buffer zone of 80 km along the border Bulgaria–Romania
Scenario 2. Areas or countries at risk of LSD (e.g. due to LSD outbreaks in neighbouring countries), where LSD vaccination is carried out
Objective of the surveillance: under the conditions of Scenario 2, considering a mass‐ vaccination campaign in place, with the vaccination of all susceptible bovine animals in the country or in the areas at risk, active surveillance activities, aiming at early detection of the possible introduction of virus, would not be feasible due to the small proportion of susceptible populations and possibly not appropriate as the resources used to properly conduct the vaccination campaign could be diverted away. A more appropriate surveillance objective could be represented by quantification of the immunity level achieved in the population through the vaccination.
Possible source of infection: it is not relevant as the objective is not to detect virus introduction early but the level of immunity achieved in the population.
Susceptible population: non‐immunised fraction of cattle population. This includes animals not already vaccinated due to not complete vaccination coverage, animals recently introduced from LSD‐free countries or areas and calves under vaccination age, i.e. younger than 6 months, if not born from immunised dams.
Risk areas: as for the Scenario 1, the areas at major risk are those bordering with infected neighbouring countries, for a width up to 80 km from the borders (see Table 4 above). Therefore, this is the area where the level of immunity should be primarily assessed.
Period at major risk: from late spring to early autumn. It is important, therefore, that the maximum level of immunity is reached before the start of this period.
Type of surveillance: active surveillance for virus detection cannot be feasible. If clinical signs are detected by passive surveillance, those animals should be tested by DIVA PCR to differentiate an infection by field or vaccine virus. A serological cross‐sectional survey can be performed to assess the level of immunity of population.
Test of choice: a serological test can be used to assess the level of immunity of population.
Threshold for detection: in this case, the main parameter to be considered is the required level of precision for the estimation of the proportion of immune (i.e. serological positive) animals in the population. A value of expected prevalence of 70%, considering, e.g. the average level of vaccination coverage achieved in Greece after 1 year of vaccination (EFSA, 2018), or more could be chosen for this type of survey.
Sampling frequency: considering the period at risk (April–October), the level of immunity should be assessed just before this period.
Target areas/population: all cattle living in the country or in the areas at risk. The animals should be randomly selected.
Scenario 3. Areas or countries at risk of LSD (e.g. due to LSD outbreaks in neighbouring countries), where no LSD outbreaks have occurred and LSD preventive vaccination was carried out, after cease of vaccination
Objective of the surveillance: early detection of primary infection and demonstration of absence of LSD without vaccination according to Article 11.9.4 point 2 of OIE's TAHC.
Possible source of infection: introduction from neighbouring infected countries.
Susceptible population: the fraction of cattle population not vaccinated. This includes animals born after vaccination cessation and those not already vaccinated due to incomplete vaccination coverage and animals recently introduced from LSD‐free countries/areas and calves under vaccination age, if not born from immunised dams. It can be also considered the fraction of those vaccinated animals losing immunity after 1–2 years. However, this part of the population cannot be currently estimated due to the lack of knowledge about the duration of immunity after vaccination, under different situation of animals vaccinated once or more times. For the sake of simplicity, this possible fraction of susceptible animals has been not considered here.
Risk areas: as for Scenario 1, the risk areas for LSDV introduction are only those closer to the infected neighbouring countries, for a width up to 80 km from the borders.
Period at major risk: from spring to autumn. As for Scenario 1, a window between 3 (21 days) and 5 (35 days) weeks should be considered for the early detection of LSDV introduction.
Type of surveillance: while active surveillance for early detecting the virus introduction should be concentrated in the at‐risk areas, surveillance aimed at demonstrating absence of disease should be performed over the whole country. In fact, according to the Article 11.9.4., point 2 of OIE's TAHC:
‘When preventive vaccination is conducted in a country or zone free from LSD, in response to a threat but without the occurrence of a case of LSD, free status may be regained eight months after the last vaccination when clinical, virological and serological surveillance conducted in accordance with Article 11.9.15. has demonstrated no occurrence of infection with LSDV.’
-
Diagnostic tests of choice:
-
–
For early detection of virus introduction, as for Scenario 1, considering that noticeable clinical signs can be observed in the susceptible population, clinical surveillance can be considered the best and feasible option. The suspected cases detected through clinical examinations must be confirmed by laboratory tests (e.g. PCR).
-
–
For demonstrating the absence of infection, a random survey on non‐vaccinated animals could be considered, based on antibody detection (through ELISA, for which a sensitivity value of 83% can be considered for detecting antibodies against natural infection; see Section 3.3.1).
-
–
Threshold for detection for early detection: as for the Scenario 1, 0.0045%, which is the median of the expected herd prevalence with an incursion in Bulgaria and Greece detected at 21 days after incursion in June, can be considered.
Threshold for detection for demonstration of absence of LSD: the lowest 25th percentile as from the graph at Figure 8, 3.5%.
Sampling frequency: for early detection of LSDV introduction, considering the time to detection window (3–5 weeks), the clinical examinations should be repeated at least every 5 weeks during the whole period at risk. For the objective of demonstrating absence of disease, the sampling programme can be performed at any period of the year, but preferably after the period at major risk, when the expected prevalence of serological positive animals is higher.
Duration of surveillance: for early detection, surveillance should last whenever the risk of introduction exists, i.e. when bordering with an area or country with an active infection or not yet free from LSD. For recovering the free status, according to the Article 11.9.4. point 2 of OIE's TAHC, the serological survey should be performed at least 8 months after the last vaccination.
Target areas/population: all non‐vaccinated cattle in areas at major risk (for early detection) or on the whole country (for demonstrating absence of disease). Calves born from vaccinated dams can be included in the target population after 6 months of age, when maternal immunity can be considered waned and does not interfere with serological tests. The herds should be randomly selected. The same considerations as Scenario 1 about the logistic and organisational difficulties of performing clinical surveillance can be made.
Example 7 of active surveillance for the objective of early detecting the virus introduction under the conditions of Scenario 3: e.g. in Croatia, a country at risk where preventive vaccination was carried out, under an active surveillance plan conducted in a buffer zone of 80 km (a zone with 99.9% probability of finding a LSD outbreak, if introduced – see Table 6) along the border with Serbia and Montenegro (the two countries bordering with Croatia that were affected in 2016, in the hypothesis they were still actively infected), where 3,500 cattle herds are present out of 25,500 and based on clinical detection of the disease, with a sensitivity of 75% to detect clinical signs at a prevalence of 0.0045%, the sample size should be 3,450 herds (within‐herd correlation is unknown thus it is not considered, for practical purposes all animals in the herds could be clinically examined) (Figure 12), to be checked every 5 weeks from April until October.
Figure 12.
Cattle herds in Croatia that would fall within the buffer zone of 80 km along the border with Serbia and Montenegro
Example 7 of active surveillance for the objective of demonstration of absence of disease under the conditions of Scenario 3: Using the same example of Croatia with a total cattle population of 25,500 cattle herds, for demonstrating absence of disease, after 8 months from the cessation of vaccination, the use of ELISA test (Se = 83%) and a design prevalence of 3.5%, 103 herds should be randomly selected and tested. As stated before, the sampling programme can be performed at any period of the year, but preferably after the period at major risk (April–October), when the expected prevalence of serological positive animals is higher. The sample of herds to be selected can be stratified according to the population of herds in the different administrative subdivisions in the country, if there are important differences in the geographical distribution of herds.
Scenario 4. Areas or countries where LSD outbreaks have been confirmed, after cease of vaccination
Objective of the surveillance: early detection of primary infection following reintroduction from neighbouring infected countries and demonstration of absence of LSD.
Possible source of infection: introduction from neighbouring infected countries and possible re‐emergence of infection due to the persistence of LSDV in reservoirs.
Susceptible population: the fraction of cattle population not immune, which includes animals born after vaccination and LSDV circulation cessation and animals recently introduced from LSD‐free countries/areas. The same considerations as Scenario 3 about vaccinated animals no longer immune can be made.
Risk areas: as for the Scenario 1, the risk areas for LSDV introduction are only those closer to the infected neighbouring countries, for a width up to 80 km from the borders. For the possible re‐emergence of the virus from residual sources of infection, all the territories of the country where the virus circulated must be considered as areas at risk.
Period at major risk: from spring to autumn. As for Scenario 1, a window between 3 (21 days) and 5 (35 days) weeks should be considered for an early detection of LSDV introduction.
Type of surveillance: the active surveillance for early detecting the virus introduction could be focused on at‐risk areas only, but that aiming at detecting the possible re‐emergence of virus must be applied at least in all those territories where the virus circulated. The active surveillance aiming at demonstrating absence of disease should be performed over the whole country. According to the Article 11.9.4., point 1 of OIE's TAHC, when a case of LSD occurs in a country or zone previously free from LSD, the waiting periods to be considered to regain free status vary depending on whether a stamping‐out policy has been applied (14 months or 26 months after the slaughter/killing of the last case, or after the last vaccination, when respectively clinical, virological and serological surveillance or clinical surveillance alone are conducted) or not (2 years or 3 years after the last vaccination, when respectively clinical, virological and serological surveillance or only clinical surveillance alone are conducted).
-
Diagnostic tests of choice:
-
–
For early detection of virus introduction or re‐emergence: as for Scenario 1, considering that noticeable clinical signs can be observed in susceptible populations, clinical surveillance can be considered the best and feasible option.
-
–
For demonstrating the absence of disease: a random serological survey on susceptible animals, as for Scenario 3.
-
–
-
Design prevalence:
-
–
Threshold for early detection: 0.02%, which is the estimated median prevalence value in Bulgaria and Greece, with the re‐emergence of the infection after 2 years of vaccination, 80% coverage, 95% effectiveness (see Table 4). In fact, this is the scenario in which outbreaks were reported, vaccination was performed and then stopped.
-
–
Threshold for demonstrating the absence of disease: 3.5%, as the lowest 25th percentile as from the graph at Figure 8.
-
–
-
Sampling frequency:
For early detection of LSDV reintroduction or re‐emergence, considering the time to detection window (3–5 weeks), the sampling rounds on animals should be repeated at least every 5 weeks during the whole period at risk.
For the objective of demonstrating absence of disease, the sampling programme can be performed at any period of the year, but preferably after the period of major risk, when the expected prevalence of serological positive animals is higher.
Duration of surveillance: for early detection, surveillance should last until the risk of introduction exists, i.e. bordering with a territory or country with an active infection or not yet free from LSD. For recovering the free status, according to OIE's TAHC, surveillance may vary based on whether a stamping‐out policy has been applied or not. In the first case, the serological surveillance should be performed at least during the 14 months after the slaughter/killing of the last case, or after the last vaccination (Article 11.9.4 point 1.a.i.). If a stamping‐out policy has not been applied, serological surveillance should be carried out at least during the 2 years after the last vaccination (Article 11.9.4 point 1.b. and Article 11.9.3).
Target areas/population: all cattle herds with susceptible animals in areas at major risk and those experiencing virus circulation (for early detection of reintroduction and re‐emergence respectively) or on the whole country (for demonstrating absence of disease). Calves born from vaccinated dams can be included in the target population after 6 months of age, when the maternal immunity can be considered waned and do not interfere with serological tests. The same considerations as for Scenario 1 about the logistic and organisational difficulties of performing clinical surveillance can be made. For detecting the possible re‐emergence of the virus in previously infected territories, surveillance should be focused on those possible virus reservoirs with the highest probability of being already infected (the last infected areas and herds, with more susceptible animals, with the best favourable vectors conditions).
Example 7 of active surveillance for early detecting the virus reintroduction under the conditions of Scenario 4: e.g. in Greece and Bulgaria with a buffer zone of 80 km (a zone with 99.9% probability of finding a LSD outbreak, if introduced – see Table 6) along the border with Turkey where there is an active infection, based on clinical detection with a sensitivity of 75% to detect clinical signs at a prevalence of 0.02% (based on median of re‐emergence in Bulgaria and Greece, after 2 years vaccination, 80% coverage, 95% effectiveness) in a cattle population of 12,200 herds in the buffer area out of 90,000 total cattle herds (Figure 13) the sample size would be 2,630 cattle herds (within‐herd correlation is unknown thus it is not considered, for practical purposes all animals in the herds could be clinically examined) with a confidence of 95%, to be checked every 5 weeks from April until October.
Figure 13.
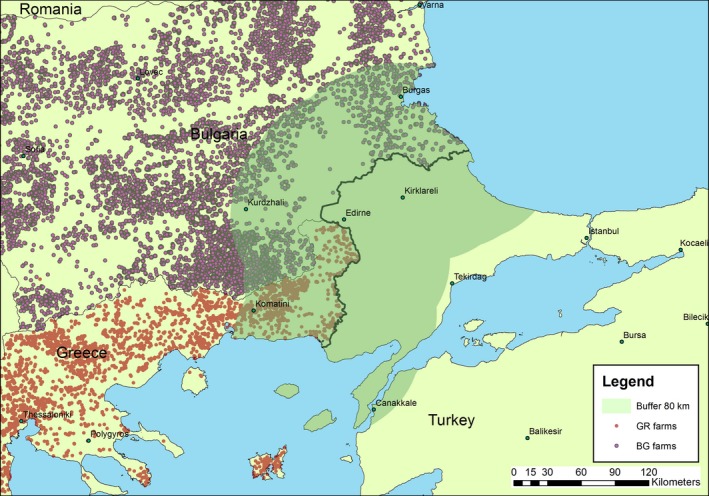
Cattle herds in Greece and Bulgaria that would fall within the buffer zone of 80 km along the border with Turkey
Example 7 of active surveillance for the objective of demonstration of absence of disease under the conditions of Scenario 4: using the same example of Greece and Bulgaria, for demonstrating absence of disease, after 2 years from the cessation of vaccination, the use of ELISAs (Se = 83%) and a design prevalence of 3.5%, 103 herds should be randomly selected and tested in each country. As stated before, the sampling programme can be performed at any period of the year, but preferably after the period of major risk (April–October), when the expected prevalence of serological positive animals is higher. The sample of herds to be selected can be stratified according to the population of herds in the different administrative subdivisions in the country, if there are important differences in the geographical distribution of herds.
Sentinel animals
Although possibly feasible under some local conditions, in the above‐described scenarios, the use of sentinel animals as possible alternatives for active surveillance was not considered. Sentinel‐based surveillance implies repeatedly testing (clinically, serologically or virologically) the same susceptible animals with a given frequency. This surveillance approach could be the unique option during mass‐vaccination campaigns when susceptible animals are no longer available. In this case, some animals are deliberately left unvaccinated and recruited as sentinel animals. Apart from the logistic difficulties in finding a sufficient number of farmers willing to provide their animals as sentinels and the costs for the veterinary services in performing repeated sampling rounds (sometimes at very short intervals) in some herds the approach based on sentinels can have variable success for the early detection of infection according to the disease and the epidemiological conditions. Firstly, for vector‐borne diseases, the geographical distribution of the risk of infection is frequently not homogeneous, as it is influenced by climate and environmental factors. When these conditions are not fully known (as for LSD), the geographical distribution and localisation of sentinel animals may not be optimal for early detection of virus circulation. For these reasons, for diseases transmitted by flying vectors, sentinel systems may work better, whereas for diseases that tend to cluster within herds, the sentinel system may fail to detect early infection, unless a very high number of sentinel herds are used. For LSD, finally, the presence of characteristic skin lesions in a good percentage of infected animals offers a valid (and less expensive) alternative to the sentinel system, when a sufficient number of susceptible animals are available.
4. Conclusions
4.1. ToR 1: LSD vaccination
ToR 1.1: Assess the most suitable duration of an LSD vaccination campaign, using live homologous vaccines, like the ones used so far in the region of South‐East Europe, intended to achieve disease freedom in a country or region, considering any relevant factors that may affect and influence disease spread and persistence:
Assuming a median vaccine effectiveness of 65%, 3 years of vaccination at coverage of 90% are most likely sufficient to eliminate LSDV from the population, and result in the absence of new introductions into the country. At a coverage of 50%, 4 years of vaccination are most likely sufficient to eliminate LSDV from the population, assuming the absence of new introductions into the country.
Assuming a median vaccine effectiveness of 80–95%, 2 years of vaccination at coverage of 90% are most likely sufficient to eliminate LSDV from the population, assuming the absence of new introductions into the country. The duration of vaccination to eliminate LSDV from the population would increase to 3–5 years when the vaccination coverage is 70%, assuming the absence of new introductions into the country.
As the above conclusions depend on the level of vaccination effectiveness, it is important to monitor vaccination in the field and to correctly and timely report outbreaks in vaccinated regions, to be able to test the assumptions underlying the calculations in this report.
Methods for discontinuing the vaccination programme (e.g. stop it and forbid at national level, stopping compulsory vaccination and allowing it on a voluntary basis or stopping the vaccination in phases) can be differentiated or combined based on the risk profile of each situation or country, mostly determined by the epidemiological status of the country itself and of the neighbouring countries, and the immunological status of the cattle population.
ToR 1.2: Assess the probability of LSD recurrence in LSD‐affected areas, after ceasing LSD vaccination, bearing in mind the possible persistence of LSDV in these areas but also the possible threat posed by outbreaks occurring in neighbouring countries or regions:
In the absence of control measures or when the duration of vaccination campaign is less than 3 years (2 years in the best case scenario with vaccination coverage of 90% and effectiveness of 80–95%), LSDV was predicted to persist and re‐emerge in all scenarios simulated by the spread model (Albania, Greece and Bulgaria).
-
If the disease is eliminated in an area by a specific strategy, e.g. vaccination, the probability of recurrence after a certain period may only depend on: (a) the risk of spill‐over infection from neighbouring infected countries or zones; or (b) on an infection source from within the area considered but different from the live bovine host, the latter being a less effective route for LSD recurrence:
-
–
The recurrence of LSD by introduction of infected animals from neighbouring endemic countries into neighbouring naïve countries is likely, especially if uncontrolled movement of animals across borders can occur.
-
–
Up to date, no studies about vector species of LSDV have been performed in the affected countries, thus specific evidence on vector competence is still missing.
-
–
Based on the available knowledge, active movement of potential vector species of LSDV (i.e. S. calcitrans) in agricultural landscape is in general known to be limited up to 5 km; therefore, the introduction of LSDV carrying vectors by their active movement from an infected area into a naive area is only likely at a short distance.
-
–
Indirect evidence based on wind trajectories analysis indicate that long‐distance dissemination of infected vectors by winds is a potential route of transboundary transmission of LSDV.
-
–
The recurrence of LSD linked to the LSDV remaining viable for longer time in vectors seems to be likely only in ticks, although specific studies are needed to identify the causal European tick species and to elucidate its role under European conditions.
-
–
The probability of recurrence of LSD linked to the probability of wildlife being carriers of LSDV or the occurrence of a sylvatic cycle of the virus cannot be assessed, because of lack of information.
-
–
The probability of recurrence of LSD due to the role of subclinically infected animals is low since the most likely source of virus transmission is linked to the high levels of virus present in the skin lesions, so in animals with evident clinical symptoms, which are usually removed from the population.
-
–
The probability of recurrence of LSD linked to the virus remaining viable in the external environment (e.g. in shaded pens or beddings) is not known, as well as the probability of an animal acquiring the infection by contact with contaminated bedding is unknown, although it is known that transmission by direct contact or indirect contract with fomites is less effective than by vector transmission. Nevertheless, the available evidence for the longest period of virus viability in the external environment is 6 months, which is shorter than immunity generated after last vaccination, which is at least 1 year, so making the probability of re‐infection by this route very low.
-
–
4.2. ToR 2 Assess the effectiveness of different surveillance systems (active, passive, etc.) bearing in mind the samples that may be used for LSD diagnosis (e.g. skin, blood, other) and all LSD diagnostic methods available (clinical, serological, molecular, including DIVA methods that can differentiate LSD vaccine from field viruses) in four different scenarios
Passive surveillance is the most important component of early warning for LSD and in general for diseases with clear clinical manifestation. It should always be in place, as LSD is a notifiable disease.
Each of the four given scenarios were assessed based on the following items for LSD surveillance: objective of the surveillance, possible source of infection, the target areas and population, the period at major risk, the type of surveillance, the diagnostic tests of choice, the design prevalence, the sampling size and sampling frequency.
According to the spread model, following introduction, the virus would not spread beyond a radius of up to 80 km from the infected area with a probability of 99.9%.
According to the spread model, the median expected prevalences at 3, 4 or 5 weeks after introduction that could be used as design prevalence for early detection are below 0.2%. There could be up to a 70‐fold increase in prevalence at 2 weeks after disease introduction in a free country.
For active surveillance to be effective in early detection, given the low values of design prevalence, clinical examination performed by veterinarians of a large number of herds (2,000–3,000 herds) at high frequency (monthly) would be needed. The suspected clinical cases need to be confirmed by laboratory test (e.g. PCR) and in particular by DIVA test in vaccinated populations.
As this level of surveillance would not be feasible in many situations, given the logistic and organisational difficulties in planning repeated visits to a such high number of cattle herds, the surveillance activities can be strengthened or partially replaced by adding systematic clinical examinations for LSD at live animal markets, before cattle leaving their herds for any reasons (pre‐movement clinical checks) and during ante mortem examinations on animals to be slaughtered. These activities could be also combined with other surveillance programmes on cattle population in place in the country. Active surveillance can be feasible only in at‐risk areas and during the risk period.
The values of design prevalence used for early detection of LSD introduction in the examples of Chapter 3.3.2 were derived from the epidemiological model. Higher design prevalence values can be considered to reduce the sample size, but this will lead to a larger delay in detecting the infection (1–2 weeks). This should be evaluated case by case, according to specific conditions such as geographic, animal density and distribution, type of herds surveillance programmes already in place, etc. In particular, the possible consequences of greater delays in detecting the infection should be evaluated in line with the actions to be put in place in response to LSD introduction and the preparatory activities to be implemented, including the number of vaccine doses for stockpiling.
Early warning of new introductions of LSD in a country could be targeted in areas bordering infected countries, whereas early warning for re‐emergence of LSD should be targeted at previously infected areas of the whole country.
For the demonstration of absence of LSD in a previously affected area, the availability of the serological assay is important, although its performance under field conditions still has to be properly evaluated. In any case, according to OIE, at least 2 years is recommended after stopping vaccination, before disease‐free status can be demonstrated.
The design prevalence that could be used for demonstrating absence of LSD, could be derived from the proportion of herds ever infected during a simulation, the lowest 25th percentile of this distribution is equal to 3.5%, which can be used as design prevalence for this surveillance purpose.
For countries where mass vaccination or vaccination of susceptible animals is kept in place, rather than a surveillance system to detect infection, active surveillance for verifying the effectiveness of vaccination would be needed.
Important knowledge gaps are about within‐herd transmission parameters, duration of protective immunity from vaccination and natural infection, duration of passive immunity in calves, role of vectors and the epidemiological status of neighbouring countries.
According to the spread model, stopping vaccination in areas bordering endemic countries would most probably lead to new outbreaks due to new introductions of the virus.
5. Recommendations
Vaccination campaigns should be monitored in the field to correctly estimate the vaccination effectiveness, which is useful in planning surveillance activities before the vaccination is lifted.
-
To improve LSD control and surveillance plans, more and better information is needed on:
-
–
within‐herd transmission;
-
–
duration of protective immunity from vaccination and natural infection, and duration of maternal immunity;
-
–
which vector species play a role as LSD vectors, this information should be gathered by targeting a number of farms experiencing LSD outbreaks and followed up during the entire LSD season;
-
–
diagnostic test performances, especially under field conditions;
-
–
exact farm location and farm type in all affected and at‐risk countries.
-
–
Time to detection and number of infected farms at detection should be carefully considered to allow the evaluation of possible consequences and costs of disease incursion and the feasibility to quickly implement control measures (vaccination in particular).
As areas bordering endemic regions or countries are most at risk for LSD introduction and new outbreaks once vaccination is lifted, special attention should be put on surveillance in these regions and the option of stopping vaccination at regional level and according to different timelines in the same country may be considered.
As passive surveillance is the most important component of early warning for LSD, awareness‐raising campaigns on LSD should be implemented for farmers, veterinarians and all involved stakeholders.
Glossary
- Vaccination coverage
The proportion of herds that are vaccinated in a target population.
- Vaccination effectiveness
The proportion of vaccinated animals that are protected from infection under field conditions. It should not be confused with ‘vaccine efficacy’, which is instead the proportion of vaccinated animals which are protected from infection under experimental conditions (laboratory studies), usually expressed as a percentage.
Abbreviations
- BLT
backwards Lagrangian trajectory
- DIVA
Differentiation of Infected from Vaccinated Animals
- d.p.i
days post‐infection
- EIA
equine infectious anaemia
- ELISA
enzyme‐linked immunosorbent assay
- EURL
European Reference Laboratory
- GF‐TADs
Global Framework for the Progressive Control of Transboundary Animal Diseases
- LSD
lumpy skin disease
- LSDV
lumpy skin disease virus
- MS
Member State
- OIE
World Organisation for Animal Health
- PCR
polymerase chain reaction
- PPR
Peste des Petits Ruminants
- SIR
Susceptible‐Infected‐Removed
- TAHC
Terrestrial Animal Health Code
- ToR
Terms of Reference
Suggested citation: EFSA (European Food Safety Authority) , Calistri P, DeClercq K, De Vleeschauwer A, Gubbins S, Klement E, Stegeman A, Cortiñas Abrahantes J, Antoniou S‐E, Broglia A and Gogin A, 2018. Scientific report on lumpy skin disease: scientific and technical assistance on control and surveillance activities. EFSA Journal 2018;16(10):5452, 46 pp. 10.2903/j.efsa.2018.5452
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00289
Acknowledgements: EFSA wishes to thank the members of the Working Group on lumpy skin disease: Paolo Calistri, Kris DeClercq, Annebel De Vleeschauwer, Simon Gubbins, Eyal Klement, Arjan Stegeman for the preparatory work on this scientific output; Julio Alvarez, Dominique Bicout, Bruno Garin‐Bastuji, Virginie Michel, Soeren Nielesen, Helen Roberts, Liisa Sihvonen, Karl Ståhl for reviewing the document, and EFSA staff members: Alessandro Broglia, José Cortiñas Abrahantes, Andrey Gogin, Sotiria‐Eleni Antoniou for the support provided to this scientific output. A special thanks is due to: Ledi Pite from Ministry of Agriculture, Rural Development and Water Administration, Albania; Aleksandra Miteva and Tsviatko Alexandrov from Food Safety Agency, Bulgaria; Žaklin Acinger‐Rogić from Ministry of Agriculture, Croatia; Ilektra Fragkou and Chrysoula Dile of the Animal Health Directorate of the Greek Ministry of Rural Development and Food, Greece; Bafti Murati from the Food and Veterinary Agency, Kosovo; Marko Nikolic from the Ministry of Agriculture and Rural Development, Montenegro; Vanja Kondratenko, Food and Veterinary Agency, Former Yugoslav Republic of Macedonia; Tatjana Labus and Budimir Plavšić from the Ministry of Agriculture and Environmental Protection, Republic of Serbia for the timely provision of the epidemiological data.
Amendment: An editorial correction was carried out that does not materially affect the contents or outcome of this scientific output. In the abstract, ‘By using a spread epidemiological model, assuming a vaccination effectiveness of 65%, with 50% and 90% coverage, 3 and 4 years campaigns, respectively, are needed to eliminate LSD.’ was changed to ‘By using a spread epidemiological model, assuming a vaccination effectiveness of 65%, with 50% and 90% coverage, 4 and 3 years campaigns, respectively, are needed to eliminate LSD.’ The definitions of the abbreviations ‘EURL’ and ‘SIR’ were modified. To avoid confusion, the original version has been removed from the EFSA Journal, but is available on request, as is a version showing all the changes made.
Approved: 28 September 2018
Amended: 2 November 2018
Notes
Sample sizes based on different parameters about host population, sensitivity test, design prevalence and risk factors can be calculated by using the tool available at https://efsa.openanalytics.eu/app/ribess (log in requested); for more information, see also https://zenodo.org/record/167306#.W6NteqSFOUk
References
- Agianniotaki EI, Tasioudi KE, Chaintoutis SC, Iliadou P, Mangana‐Vougiouka O, Kirtzalidou A, Alexandropoulos T, Sachpatzidis A, Plevraki E, Dovas CI and Chondrokouki E, 2017. Lumpy skin disease outbreaks in Greece during 2015–16, implementation of emergency immunization and genetic differentiation between field isolates and vaccine virus strains. Veterinary Microbiology, 201, 78–84. [DOI] [PubMed] [Google Scholar]
- Alkhamis MA and VanderWaal K, 2016. Spatial and temporal epidemiology of lumpy skin disease in the Middle East, 2012–2015. Frontiers in Veterinary Science, 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANSES (French Agency for Food, Environmental and Occupational Health & Safety), 2017. Risk of introduction of lumpy skin disease into France. Scientific opinion, Request No 2016‐SA‐0120, https://www.anses.fr/fr/system/files/SABA2016SA0120RaEN.pdf
- Babiuk S, Bowden TR, Parkyn G, Dalman B, Manning L, Neufeld J, Embury‐Hyatt C, Copps J and Boyle DB, 2008. Quantification of lumpy skin disease virus following experimental infection in cattle. Transboundary and Emerging Diseases, 55, 299–307. [DOI] [PubMed] [Google Scholar]
- Baldacchino F, Muenworn V, Desquesnes M, Desoli F, Charoenviriyaphap T and Duvallet G, 2013. Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): a review. Parasite, 20, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balinsky C, Delhon G, Smoliga G, Prarat M, French R, Geary S, Rock D and Rodriguez L, 2008. Rapid preclinical detection of sheeppox virus by a real‐time PCR assay. Journal of Clinical Microbiology, 46, 438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros ATM and Foil LD, 2007. The influence of distance on movement of tabanids (Diptera: Tabanidae) between horses. Veterinary Parasitology, 144, 380–384. [DOI] [PubMed] [Google Scholar]
- Ben‐Gera J, Klement E, Khinich E, Stram Y and Shpigel NY, 2015. Comparison of the efficacy of Neethling lumpy skin disease virus and x10RM65 sheep‐pox live attenuated vaccines for the prevention of lumpy skin disease – the results of a randomized controlled field study. Vaccine, 33, 4837–4842. [DOI] [PubMed] [Google Scholar]
- Bowden TR, Babiuk SL, Parkyn GR, Copps JS and Boyle DB, 2008. Capripoxvirus tissue tropism and shedding: a quantitative study in experimentally infected sheep and goats. Virology, 371, 380–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner J, Haimovitz M, Oron E, Stram Y, Fridgut OBV, Kuznetzova L, Oved Z, Waserman A, Garazzi S, Perl S, Lahav D, Edery N and Yadin H, 2006. Lumpy skin disease (LSD) in a large dairy herd in Israel. Israel Journal of Veterinary Medicine, 61, 3–4. [Google Scholar]
- Carn VM and Kitching RP, 1995a. An investigation of possible routes of transmission of lumpy skin disease virus (Neethling). Epidemiology and Infection, 114, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carn VM and Kitching RP, 1995b. The clinical response of cattle experimentally infected with lumpy skin disease (Neethling) virus. Archives of Virology, 140, 503–513. [DOI] [PubMed] [Google Scholar]
- Chihota CM, Rennie LF, Kitching RP and Mellor PS, 2001. Mechanical transmission of lumpy skin disease virus by Aedes aegypti (Diptera: Culicidae). Epidemiology and Infection, 126, 317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihota CM, Rennie LF, Kitching RP and Mellor PS, 2003. Attempted mechanical transmission of lumpy skin disease virus by biting insects. Medical and Veterinary Entomology, 17, 294–300. [DOI] [PubMed] [Google Scholar]
- Dietze K, Moritz T, Alexandrov T, Krstevski K, Schlottau K, Milovanovic M, Hoffmann D and Hoffmann B, 2018. Suitability of group‐level oral fluid sampling in ruminant populations for lumpy skin disease virus detection. Veterinary Microbiology, 221, 44–48. [DOI] [PubMed] [Google Scholar]
- Doherr MG and Audigé L, 2001. Monitoring and surveillance for rare health‐related events: a review from the veterinary perspective. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 356, 1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. A framework to substantiate absence of disease: the risk based estimate of system sensitivity tool (RiBESS) using data collated according to the EFSA Standard Sample Description – an example on Echinococcus multilocularis . EFSA supporting publication 2012:EN‐366, 44 pp. Available online: https://www.efsa.europa.eu/en/supporting/pub/en-366
- EFSA (European Food Safety Authority), 2018. Lumpy skin disease: II. Data collection and analysis. EFSA Journal 2018;16(2):5176, 33 pp. 10.2903/j.efsa.2018.5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2015. Scientific Opinion on lumpy skin disease. EFSA Journal 2015;13(1):3986, 73 pp. 10.2903/j.efsa.2015.3986 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2016. Urgent advice on lumpy skin disease. EFSA Journal 2016;14(8):4573, 27 pp. 10.2903/j.efsa.2016.4573 [DOI] [Google Scholar]
- Gersabeck EF and Merritt RW, 1985. Dispersal of adult Stomoxys calcitrans (L.) (Diptera: Muscidae) from known immature developmental areas. Journal of Economic Entomology, 78, 617–621. [DOI] [PubMed] [Google Scholar]
- Gubbins S, Stegeman A, Klement E, Pite L, Broglia A and Cortiñas Abrahantes J, 2018. Inferences about the transmission of lumpy skin disease virus between farms from outbreaks in Albania during 2016. Preventive Veterinary Medicine, submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman A, Zro K, Vandenbussche F, Demeestere L, Van Campe W, Ennaji MM and De Clercq K, 2013. Development and validation of three Capripoxvirus real‐time PCRs for parallel testing. Journal of Virological Methods, 193, 446–451. [DOI] [PubMed] [Google Scholar]
- Ince ÖB, Çakir S and Dereli MA, 2016. Risk analysis of lumpy skin disease in Turkey. Indian Journal of Animal Research, 50, 1013–1017. [Google Scholar]
- Kahana‐Sutin E, Klement E, Lensky I and Gottlieb Y, 2017. High relative abundance of the stable fly Stomoxys calcitrans is associated with lumpy skin disease outbreaks in Israeli dairy farms. Medical and Veterinary Entomology, 31, 150–160. [DOI] [PubMed] [Google Scholar]
- Kalderon‐Asael B, Erel Y, Sandler A and Dayan U, 2009. Mineralogical and chemical characterization of suspended atmospheric particles over the east Mediterranean based on synoptic‐scale circulation patterns. Atmospheric Environment, 43, 3963–3970. [Google Scholar]
- Keeling MJ and Rohani P, 2011. Modeling infectious diseases in humans and animals. Princeton University Press, Princeton, New Jersey, USA: p. 408. [Google Scholar]
- Klausner Z, Fattal E and Klement E, 2015. Using synoptic systems’ typical wind trajectories for the analysis of potential atmospheric long‐distance dispersal of lumpy skin disease virus. Transboundary and Emerging Diseases, 64, 398–410. [DOI] [PubMed] [Google Scholar]
- Lempereur L, Sohier C, Smeets F, Maréchal F, Berkvens D, Madder M, Francis F and Losson B, 2018. Dispersal capacity of Haematopota spp. and Stomoxys calcitrans using a mark–release–recapture approach in Belgium. Medical and Veterinary Entomology, 32, 298–303. [DOI] [PubMed] [Google Scholar]
- Lysyk T, 1998. Relationships between temperature and life‐history parameters of Stomoxys calcitrans (Diptera: Muscidae). Journal of Medical Entomology, 35, 107–119. [DOI] [PubMed] [Google Scholar]
- Magori‐Cohen R, Louzoun Y, Herziger Y, Oron E, Arazi A, Tuppurainen E, Shpigel NY and Klement E, 2012. Mathematical modelling and evaluation of the different routes of transmission of lumpy skin disease virus. Veterinary Research, 43, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasherow S, Erster O, Rubinstein‐Giuni M, Kovtunenko A, Eyngor E, Gelman B, Khinich E and Stram Y, 2016. A high‐resolution melting (HRM) assay for the differentiation between Israeli field and Neethling vaccine lumpy skin disease viruses. Journal of Virological Methods, 232, 12–15. [DOI] [PubMed] [Google Scholar]
- Molla W, Frankena K and De Jong MCM, 2017. Transmission dynamics of lumpy skin disease in Ethiopia. Epidemiology and Infection, 145, 2856–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morag N, Mullens BA and Gottlieb Y, 2013. Assessment of survival and body size variation of Culicoides imicola (Diptera: Ceratopogonidae) as functions of “Candidatus Cardinium” (Bacteroidetes) infection status. Applied and Environmental Microbiology, 79, 6260–6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE (World Organisation for Animal Health), 2017. Infection with lumpy skin disease virus. Terrestrial Animal Health Code, Chapter, 11, 9. [Google Scholar]
- Schley D, Gubbins S and Paton DJ, 2009. Quantifying the risk of localised animal movement bans for foot‐and‐mouth disease. PLoS ONE, 4, e5481 10.1371/journal.pone.0005481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs S, Oura CA, Henstock M, Bowden TR, King DP and Tuppurainen ES, 2012. Validation of a high‐throughput real‐time polymerase chain reaction assay for the detection of capripoxviral DNA. Journal of Virological Methods, 179, 419–422. [DOI] [PubMed] [Google Scholar]
- Taylor DB, Moon RD, Campbell JB, Berkebile DR, Scholl PJ, Broce AB and Hogsette JA, 2010. Dispersal of stable flies (Diptera: Muscidae) from larval development sites in a Nebraska landscape. Environmental Entomology, 39, 1101–1110. [DOI] [PubMed] [Google Scholar]
- Tuppurainen ESM, Venter EH and Coetzer JA, 2005. The detection of lumpy skin disease virus in samples of experimentally infected cattle using different diagnostic techniques. The Onderstepoort Journal of Veterinary Research, 72, 153–164. [DOI] [PubMed] [Google Scholar]
- Tuppurainen ES, Lubinga JC, Stoltsz WH, Troskie M, Carpenter ST, Coetzer JA, Venter EH and Oura CA, 2013. Mechanical transmission of lumpy skin disease virus by Rhipicephalus appendiculatus male ticks. Epidemiology and Infection, 141, 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeruham I, Nir O, Braverman Y, Davidson M, Grinstein H, Haymovitch M and Zamir O, 1995. Spread of lumpy skin disease in Israeli dairy herds. Veterinary Record, 137, 91–93. [DOI] [PubMed] [Google Scholar]


