Abstract
The conclusions of EFSA following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State Italy and co‐rapporteur Member State Ireland for the pesticide active substance ethoprophos are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012. The conclusions were reached on the basis of the evaluation of the representative uses of ethoprophos as a nematicide and an insecticide on potatoes. The reliable end points, appropriate for use in regulatory risk assessment, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
Keywords: ethoprophos, peer review, risk assessment, pesticide, nematicide, insecticide
Summary
Commission Implementing Regulation (EU) No 844/2012 (hereinafter referred to as ‘the Regulation’) lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012. Ethoprophos is one of the active substances listed in Regulation (EU) No 686/2012.
In accordance with Article 1 of the Regulation, the rapporteur Member State (RMS), Italy, and co‐rapporteur Member State (co‐RMS), Ireland, received an application from AMVAC Netherlands B.V. for the renewal of approval of the active substance ethoprophos. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (Ireland), the European Commission and the European Food Safety Authority (EFSA) about the admissibility.
The RMS provided its initial evaluation of the dossier on ethoprophos in the renewal assessment report (RAR), which was received by EFSA on 15 February 2017. In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, AMVAC Netherlands B.V., for comments on 19 July 2017. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 19 September 2017.
Following consideration of the comments received on the RAR, it was concluded that additional information should be requested from the applicant and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues and ecotoxicology.
In accordance with Article 13(1) of the Regulation, EFSA should adopt a conclusion on whether ethoprophos can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 of the European Parliament and of the Council.
The conclusions laid down in this report were reached on the basis of the evaluation of the representative uses of ethoprophos as a nematicide and an insecticide on potatoes, as proposed by the applicant. Full details of the representative uses can be found in Appendix A of this report.
The use of ethoprophos according to the representative uses proposed at the European Union (EU) level results in a sufficient nematicidal and soil insecticidal efficacy against the target organisms.
A data gap was identified for a search of the scientific peer‐reviewed open literature on the active substance and its relevant metabolites (mammalian toxicology and residues area).
In the section identity, physical/chemical properties, analytical methods, data gaps were identified for determination of the oxidising properties of the active substance as manufactured; for description and validation data for the analytical methods used in the older toxicological studies in particular the repeated‐dose dietary studies; for verification of the efficiency of the extraction procedure used in the analytical method for the determination of residues in food and feed of plant origin; for additional validation data for the submitted monitoring method in air or a new method with a limit of quantification (LOQ) in compliance with the requirements for the operators, workers, residents and bystanders risk assessment and for an analytical method for analysis of the metabolite EPPA in body fluids and tissues.
In the mammalian toxicology area, data gaps were identified for the impurity profile of the batches used in the most recent toxicology studies, for the identification of the analytical methods used in the repeated‐dose dietary toxicity studies, for an in vitro interspecies comparative metabolism study, a comparative acetylcholinesterase assay after repeated dose, a reliable developmental toxicity study in rabbit, a clarification of the potential endocrine‐mediated effects for non‐EATS (oestrogen, androgen, thyroid, steroid) modalities for the thyroid C‐cell tumours and pheochromocytomas, a submission of the occupational reports on monitoring of manufacturing plant personnel conducted after the last review up to now and further analysis of the available published data relevant to the human health risk assessment; some of these data gaps led to non‐finalised issues. With regard to genotoxicity, data gaps were identified for an Ames test including the investigation of strains sensitive to cross‐linking and oxidising mutagens (TA102 or E. coli WP2 strain), clarification of the gene mutation potential in mammalian cells and robust in vivo follow up to the positive clastogenic effects observed in vitro (including the aneugenicity potential) with ethoprophos. A critical area of concern was identified considering that equivocal gene mutation and positive clastogenic effects seen in vitro were followed up with in vivo studies of limited reliability but showing also equivocal and positive results. Since a genotoxic and clastogenic potential could not be excluded, no threshold for these effects is assumed and therefore toxicological reference values could not be established and non‐dietary risk assessment could not be conducted, resulting in another critical area of concern.
In the area of residues, data gaps have been identified for sufficient residue trials compliant with the representative use on potatoes and rotational crops field trials analysing for all compounds included in the proposed risk assessment residue definitions and supported by acceptable storage stability data for all compounds. A hydrolysis study addressing the nature of residues of all compounds included in the proposed risk assessment residue definition for plants and simulating pasteurisation, baking/boiling and sterilisation is also required. With regard to the identified data gaps, a full assessment of the livestock exposure cannot be concluded on. Finally, a data gap is identified for the determination of the residues in pollen and bee products for human consumption resulting from residues taken up by honeybees from crops at blossom. A detailed assessment of the cited literature data to conclude on the non‐relevance of ethoprophos residues in pollen and honey is also required. Most notably, the consumer dietary risk assessment could not be concluded in the absence of agreed health‐based reference values for ethoprophos and is identified as a critical area of concern.
With respect to fate and behaviour in the environment, data gaps have been identified for route and rate of degradation studies in soil, updated Predicted environmental concentration ground water (PEC GW) calculations and information to address the effect of water treatment processes on the nature of the residues that might be present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water. Both lysimeter studies available and monitoring data suggest that the parametric drinking water limit of 0.1 μg/L can be occasionally exceeded in vulnerable situations. The groundwater exposure assessment cannot be finalised in view of the data gaps.
A number of data gaps were identified in the field of ecotoxicology in relation to the risk assessment to birds, aquatic vertebrates and invertebrates, bees and other non‐target arthropods and the risk assessment to earthworms and other soil macro‐organisms. The endocrine disrupting properties of ethoprophos could not be excluded. High acute risk to birds, high risk to bees and non‐target arthropods and high risk to soil macro‐organisms other than earthworms have been concluded. Critical areas of concern have been set for several non‐target organisms.
Background
Commission Implementing Regulation (EU) No 844/20121 (hereinafter referred to as ‘the Regulation’) lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/2009.2 This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of an additional 3 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3).
In accordance with Article 1 of the Regulation, the RMS Italy and co‐RMS Ireland received an application from AMVAC Netherlands B.V. for the renewal of approval of the active substance ethoprophos. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (Ireland), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on ethoprophos in the RAR, which was received by EFSA on 15 February 2017 (Italy, 2017).
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, AMVAC Netherlands B.V., for consultation and comments on 19 July 2017. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 19 September 2017. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of a reporting table. The applicant was invited to respond to the comments in column 3 of the reporting table. The comments and the applicant's response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA and the RMS on 31 October 2017. On the basis of the comments received, the applicant's response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicant and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues and ecotoxicology.
The outcome of the telephone conference, together with EFSA's further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
A final consultation on the conclusions arising from the peer review of the risk assessment took place with Member States via a written procedure in April‐May 2018.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the representative formulation, evaluated on the basis of the representative uses of ethoprophos as a nematicide and an insecticide on potatoes, as proposed by the applicant. A list of the relevant end points for the active substance and the formulation is provided in Appendix A.
In addition, a key supporting document to this conclusion is the peer review report (EFSA, 2018), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting table (31 October 2017);
the evaluation table (17 May 2018);
the report(s) of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (Italy, 2018), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion report and its background documents would not be accepted to support any registration outside the European Union (EU) for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Ethoprophos is the ISO common name for O‐ethyl S,S‐dipropyl phosphorodithioate (IUPAC).
The representative formulated product for the evaluation was ‘MOCAP 15G’, a granular formulation (GR) containing 150 g/kg ethoprophos.
The representative uses evaluated were by soil application against a spectrum of nematodes and soil dwelling insects in potatoes. Full details of the representative uses can be found in the list of end points in Appendix A.
Data were submitted to conclude that the representative uses of ethoprophos proposed at EU level result in a sufficient nematicidal and soil insecticidal efficacy against the target organisms following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014).
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion: SANCO/3029/99‐rev. 4 (European Commission, 2000a), SANCO/3030/99‐rev. 4 (European Commission, 2000b) and SANCO/825/00‐rev. 8.1 (European Commission, 2010).
The proposed specification for ethoprophos is based on batch data from industrial scale production. The proposed minimum purity of the technical material is 950 g/kg. It is proposed that the reference specification should be updated based on the data for renewal since higher minimum purity of the active substance could be set and a new significant impurity should be included. There is no FAO specification available for ethoprophos. The proposed specification is supported by (eco)toxicological assessment (see Sections 2 and 5).
The assessment of the data package revealed no issues that need to be included as critical areas of concern with respect to the identity, physical, chemical and technical properties of ethoprophos or the representative formulation. However, a data gap for determination of the oxidising properties of the active substance as manufactured was identified. The main data regarding the identity of ethoprophos and its physical and chemical properties are given in Appendix A.
Adequate methods are available for the generation of pre‐approval data required for the risk assessment. However, a data gap was identified for description and validation data for the analytical methods used in the older toxicological studies, in particular the repeated‐dose dietary studies (see Section 2). Methods of analysis are available for the determination of the active substance in the technical material and in the representative formulation and for the determination of the respective impurities in the technical material.
Ethoprophos residues can be monitored in food and feed of plant origin by liquid chromatography with tandem mass spectrometry (LC–MS/MS) with a limit of quantification (LOQ) of 0.01 mg/kg in each commodity group. However, it should be noted that the extraction procedure used is not verified (data gap). It should be noted that the residue definition in animal products is not finalised and a monitoring method might be required.
Ethoprophos residues in soil and water can be monitored by LC–MS/MS with a LOQ of 0.01 mg/kg and 0.10 μg/L, respectively. However, it should be noted that the residue definitions in soil and surface and ground water are considered open and additional monitoring methods might be required should new components be included in the residue definitions. The LC‐MS/MS method exists for monitoring ethoprophos residues in air with a LOQ of 300 μg/m3. However, a data gap for additional validation data for the submitted method or a new method with a LOQ in compliance with the requirements for the operators, workers, residents and bystanders risk assessment was identified (see Section 2).
The LC‐MS/MS method can be used for monitoring ethoprophos residues in body fluids and tissues with a LOQ of 0.01 mg/L and 0.01 mg/kg, respectively. It was concluded that the residues of metabolite EPPA should also be monitored in body fluids and tissues (see Section 2). As a consequence, a data gap for an analytical method for analysis of EPPA in body fluids and tissues was identified.
2. Mammalian toxicity
The following guidance documents were followed in the production of this conclusion: SANCO/221/2000‐rev. 10‐final (European Commission, 2003), SANCO/10597/2003‐rev. 10.1 (European Commission, 2012), Guidance on dermal absorption (EFSA PPR Panel, 2012) and Guidance on the Application of the CLP Criteria (ECHA, 2017).
Ethoprophos was discussed during the Pesticides Peer Review Meeting 172 in February 2018.
The impurity profile of the batches used in the toxicity studies is not available and a data gap is set to characterise the impurity profile of the batches used in the toxicity studies, at least for the most recent ones. A critical area of concern has not been highlighted considering that the impurities present in the technical specification (either the current or the newly proposed specification) do not present additional alerts compared to the chemical structure of the parent; some are expected to share its toxicity profile, at least with regard to its genotoxicity potential; but the relative potency to acetylcholinesterase (AChE) inhibition of the organophosphate impurities is unknown. It is noted that the newly proposed technical specification includes a new impurity of lower toxicity profile than the parent, and reduces the levels of organophosphate impurities of unknown relative toxicity (compared with the parent) and AChE inhibition potential compared to the current Annex I reference specification. The analytical methods used in the older toxicological studies have not been reported or validated, which questions the validity of the toxicological studies, in particular the repeated‐dose dietary studies (data gap and issue not finalised).
Oral absorption of ethoprophos is rapid (Tmax – time until peak blood levels achieved – below 1 hour) and extensive (> 90% of the administered dose) based on comparison of the excretion profile of the active substance after oral and intravenous exposures. It is widely distributed and completely metabolised. The excretion occurs primarily through the urine in a biphasic manner, being rapid during the first 6 h after administration. Monitoring studies in agricultural operators indicated that the metabolite EPPA is more predominant in humans than in rats (and is considered as a reasonable biomarker of ethoprophos exposure in humans), however, since no further information on human metabolites was retrieved from the studies in humans, an in vitro interspecies comparative metabolism study is needed (data gap and issue not finalised).
Ethoprophos is toxic to fatal when administered via the oral, dermal or inhalation routes (harmonised classification according to Regulation (EC) no 1272/20083 (CLP Regulation): Acute Tox. 3 – H301 ‘toxic if swallowed’, Acute Tox. 1 – H310 ‘fatal in contact with skin’ and Acute Tox. 2 – H330 ‘fatal if inhaled’. Irritation to skin and eyes could not be determined due to the high toxicity of the substance. Since all animals died after dermal administration (0.5 ml) or ocular instillation (0.1 mL, as reported in the US EPA report on ethoprophos (US EPA, 2006)), the additional labelling provision EUH070 ‘toxic by eye contact’ was proposed by the peer review to be added to the harmonised classification4. Ethoprophos may also cause allergic skin reactions (harmonised classification as Skin Sens. 1 – H317). No phototoxic potential is attributed to the substance.
AChE inhibition in erythrocytes and brain was found to be the most sensitive endpoint of ethoprophos toxicity upon short‐term exposure either via the oral, dermal or inhalation routes and in all species tested (rat, mouse, rabbit and dog). The relevant short term no‐observed adverse effect level (NOAEL) is 0.025 mg/kg body weight (bw) per day observed in the dog, 90‐day to 1‐year oral studies.
The genotoxic potential of ethoprophos could not be concluded based on incomplete investigations in bacterial cells (strains sensitive to cross‐linking and oxidising mutagens, TA102 and Escherichia coli WP2 strain were not investigated). Equivocal in vitro results in gene mutation in mammalian cells and positive clastogenic effects in vitro could not be ruled out with robust in vivo assays; one of the in vivo studies (presenting shortcomings such as low number of cells investigated) presented an increased chromosomal exchange which reflects the results observed in vitro. In addition, one of the two tests in germ cells (of limited reliability) produced positive results. On top of the clastogenic potential identified, the aneugenic potential has not been investigated (data gap). Therefore, a potential for genotoxicity or clastogenicity of ethoprophos could not be excluded, and a critical area of concern is identified considering that the equivocal gene mutation and positive clastogenic effects seen in vitro were followed up with in vivo studies of limited reliability but showing also equivocal and positive results.
Upon long‐term exposure, in addition to AChE inhibition and haematological effects observed in rats, different tumour types were observed in two different strains of rat and in independent studies: uterus endometrial adenoma and carcinoma, malignant pheochromocytoma and thyroid C‐cells carcinoma. On this basis, the peer review experts proposed to classify ethoprophos as carcinogen category 2 (Carc. 2, H351 ‘suspected of causing cancer’)4 according to current CLP criteria, while the current harmonised classification does not include classification regarding carcinogenicity but the basis of this no‐classification is unknown. The carcinogenic mode of action (MoA) has not been investigated, however a genotoxic MoA cannot be excluded. The relevant long‐term and carcinogenicity low‐observed adverse effect level (LOAEL) is 0.04 mg/kg bw per day, based on an increased incidence of malignant pheochromocytoma observed in a 2‐year study in rats. It is noted that the genotoxicity and carcinogenicity outcome are changed with regard to the previous assessment primarily due to an update of the genotoxicity assessment according to the EFSA Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment (EFSA SC, 2011) and respective update on how EFSA evaluates genotoxicity (EFSA SC, 2017).
Regarding the reproductive toxicity, ethoprophos produced reduced litter size and increased postnatal mortality in the presence of parental toxicity in rats. Abortions occurred in a rat developmental toxicity study in the presence of maternal toxicity. In rabbits, maternal toxicity was evident based on reduced body weight gain at all dose levels (LOAEL of 0.125 mg/kg bw per day); however, developmental toxicity could not be reliably concluded due to inadequate reporting of the fetal effects and low number of dams investigated (data gap and issue not finalised). In a developmental neurotoxicity study, increase in motor activity (due to a lack of habituation) and locomotor activity was observed in pups at maternal toxic doses (the RMS did not agree with this conclusion, considering the variability in the results and that the increases were not statistically significant). It was noted that a comparative AChE assay after repeated dose was available to the US EPA and according to the US EPA assessment, a higher sensitivity was observed in pups with regard to the adults (US EPA, 2007). Since this information is needed to conclude on the developmental neurotoxicity of ethoprophos, a data gap was identified and this endpoint could not be concluded and finalised. Based on the available data, the majority of the experts suggested that classification as STOT RE 2 (specific target organ toxicity – repeated exposure)4 for the nervous system may be appropriate.
Although ethoprophos is proposed to be classified as carcinogenic category 2, it is not classified or proposed to be classified as toxic for reproduction or development category 2 (even though the latter could not be finalised), in accordance with the provisions of Regulation (EC) No 1272/2008, and therefore, the conditions of the interim provisions of Annex II, Point 3.6.5 of Regulation (EC) No 1107/2009 concerning human health for the consideration of endocrine disrupting properties are not met. The mechanistic data do not indicate an oestrogen, androgen or thyroid‐mediated MoA. Ethoprophos was considered positive in the steroidogenesis assay since it increased estradiol production but it demonstrated an equivocal effect on testosterone production and there were no oestrogen related effects observed in the in vivo mammalian studies. The apical effects observed in the levels 4 and 5 studies according to the OECD conceptual framework (OECD, 2012) such as thyroid C cell tumours and pheochromocytoma could be endocrine‐mediated through non‐EATS (oestrogen, androgen, thyroid, steroid) modalities (regarding calcitonin or catecholamine). This is a data gap and a non‐finalised issue. However, there are no validated test guidelines or guidance to interpret these effects. Ethoprophos did not suppress the immune function, as assessed by the measurement of antigen‐specific, T‐cell dependent antibody formation in the spleen.
Regular monitoring of manufacturing plant personnel for cholinesterase inhibition has been reported in the previous assessment (United Kingdom, 2004), an update of the monitoring performed until now and submission of the respective occupational report is needed (data gap).
Four ethoprophos metabolites were tested for their acute oral toxicity and AChE inhibition. EPPA, SME and OME presented inhibition of the AChE and toxicity characteristic of organophosphates; M5 presented lower acute toxicity and reduced or absence of AChE inhibition compared to the parent. Regarding the toxicity profile of the metabolites M5, EPPA and M18, all experts agreed that it is covered by toxicity and concerns established for the parent ethoprophos.
Taking into consideration that a genotoxic and clastogenic potential could not be ruled out for ethoprophos, no threshold for these effects is assumed and therefore no toxicological reference values (dietary, such as the acceptable daily intake (ADI) and the acute reference dose (ARfD) or non‐dietary, such as the acceptable operator exposure level (AOEL) and the acute acceptable operator exposure level (AAOEL)) can be derived. This represents a critical area of concern for the approval of the active substance.
It is noted that the experts discussed the point of departure (PoD) for hypothetical toxicological reference values assuming that the genotoxic potential could be excluded. For short‐ to long‐term exposure reference values (ADI and AOEL), a new PoD should take into consideration the newly established LOAEL of 0.04 mg/kg bw per day for carcinogenic effects, with the application of an additional uncertainty factor (UF) – possibly of 10 (overall 1,000). This would lower the currently set ADI of 0.0004 mg/kg bw per day and the AOEL of 0.001 mg/kg bw per day (European Commission 2013) to 0.00004 mg/kg bw per day. The PoD for acute exposure (ARfD and AAOEL) already established could be retained (ARfD is previously set at 0.01 mg/kg bw) but an additional UF should be applied to take into consideration the uncertainties and possible data gaps related to developmental neurotoxicity – possibly of 2 (overall 200). This indicates that, even if the genotoxic potential could be excluded, the toxicological reference values should be lowered significantly with respect to the values set during the previous assessment and currently in place.
Since non‐dietary toxicological reference values were not established, the exposure risk assessment for operators, workers, bystanders and residents cannot be calculated. This leads to a critical area of concern.
Regarding the search of the scientific peer‐reviewed open literature on the active substance and its relevant metabolites, even though new published reviews have been included regarding epidemiological/biomonitoring studies on organophosphates, it is unclear whether ethoprophos was included in these analyses. Additionally, it is noted that data such as ToxCast, studies on a molecular level and non‐EU monitoring studies would be relevant to the human health risk assessment and should have been assessed and summarised in the RAR and a data gap is identified for further analysis of these studies.
3. Residues
The assessment in the residue section is based on the OECD guidance document on overview of residue chemistry studies (OECD, 2009), the OECD publication on maximum residue level (MRL) calculations (OECD, 2011), the European Commission guideline document on MRL setting (European Commission, 2011) and the Joint Meeting on Pesticide Residues (JMPR) recommendations on livestock burden calculations (JMPR, 2004, 2007).
Ethoprophos was discussed during the Pesticides Peer Review Meeting 173 in February 2018.
Metabolism was investigated upon soil incorporation of [1‐ethyl‐14C]‐ethoprophos in potatoes (root crops) (2N rate), head/leafy cabbages (leafy crops) and sweet corn (fruit crops). M5 (ethyl phosphate) compound was the predominant compound of the total radioactive residues (TRR) in potato tuber and vines (38% and 12.5% TRR, respectively), in leafy and head cabbages (up to 24% TRR) and in sweet corn grain (35% TRR). In all crop groups, the parent compound and the identified metabolites EPPA, SME and OME were either never detected in potato tuber and in sweet corn grain, or found only in very low proportions, never exceeding 10% TRR in all other crop parts and the major part of the radioactive residues was found to be incorporated into natural plant constituents. The main degradation pathway of ethoprophos in primary crops was the loss of the S‐propyl alkyl groups with the formation of M5 compound. As for its very simple structure, M5 might not be specific to ethoprophos and cannot therefore be considered as a suitable residue marker compound despite its significant occurrence in all crop parts. For all crop categories, the residue definition for monitoring is set as ethoprophos only. The agreed residue definition for risk assessment is set as ethoprophos and M5, expressed as ethoprophos. The toxicity of M5 was considered covered by the toxicity of the parent compound (refer to Section 2).
Old and new rotational crops metabolism studies were conducted following a bare soil application of [1‐ethyl‐14C]‐ethoprophos (2N rate). From the old data, metabolite M5 was shown to be the most pertinent residue across the tested rotational crops and at the different plant‐back intervals (PBIs) (17–51% TRR). Ethoprophos and EPPA were significant residues in specific crops (e.g. ethoprophos in immature radish plants, 18% TRR; EPPA in immature wheat and mature radish foliage, 10% and 21% TRR, respectively) but very minor in the other rotational crop commodities.
As for in the most recent study (2N rate), M5 compound was recovered at insignificant proportions in all crop parts and at all PBIs (residue levels < 10% TRR), while the parent compound was identified in wheat forage and radish root (12.5% TRR and 25% TRR, respectively) at the 21‐day PBI only, and degraded rapidly along with the longer PBIs. EPPA compound was the major residue across the different rotational crops at 21‐day PBI (13–23% TRR), except in wheat straw and grain where it was hardly detected and accounted for up to 25% TRR in immature spinaches at 152‐day PBI. The conjugates of M50 compound (M38, M39, M41 and M42) were the predominant compounds of the total residues in radish foliage (up to 38.5% TRR) and in mature spinaches (23% TRR) at 152‐day PBI. Finally, M18 compound accounted for up to 30% TRR in wheat straw at 152‐day PBI only.
For rotational crops and for monitoring, it was agreed that the same definition as for primary crops, should be proposed. The residue definition for risk assessment in rotational crops is proposed as ethoprophos, EPPA and M5, expressed as ethoprophos. The toxicity of M5 and EPPA was considered covered by the toxicity of the parent compound (see Section 2).
Ethoprophos was shown to be stable under frozen conditions for up to 19 months in potatoes and in tomatoes whilst acceptable storage stability of EPPA was demonstrated for only 3 months in potato tuber. Storage stability data on M5 were not provided. All the submitted residue trials analysing for ethoprophos were considered as compliant with the northern Europe (NEU) and southern Europe (SEU) Good Agricultural Practices (GAPs) on potatoes provided that the proportionality principle could be applied and are supported by acceptable storage stability data and validated analytical methods. Since the magnitude of M5 residues was not determined in these trials, a data gap was identified for a sufficient number of residue trials on potatoes compliant respectively with the NEU and SEU GAPs for the determination of M5 and supported by acceptable storage stability data on this compound.
From the residue trials, ethoprophos residues can occur in potato tubers at a level higher than 0.01 mg/kg; therefore, a hydrolysis study addressing the nature of residues of all compounds included in the proposed risk assessment residue definition for plants and simulating pasteurisation, baking/boiling and sterilisation is required (data gap and issue that could not be finalised). Pending upon the outcome of the requested study addressing the nature of the residues at processing, processing trials in potatoes involving a heating step (cooked potatoes, purée, fried potatoes) and analysing for ethoprophos and all relevant degradation products might be needed.
Rotational crops field residue trials were conducted in the US and in the Northern and Southern zones of Europe following a soil incorporation of ethoprophos at 10 kg a.s./ha (1.6 N rate). In the US trials conducted on leafy crops, root crops, legume vegetables and cereal small grains, quantifiable residues of ethoprophos and EPPA were recovered in radish foliage (< 0.01 and 0.126 mg/kg, respectively) and in radish root (up to 0.028 and 0.048 mg/kg, respectively) only at the 1 and 4 months PBIs. In the European trials on leafy crops, root crops and cereals, residues of ethoprophos, EPPA, OME and SME were below the LOQ of the method (0.001 mg/kg) in all crop parts, except in carrot leaf (0.002 mg/kg for ethoprophos). Since these trials were not supported by storage stability data on EPPA, and M5 residues were not analysed for, a data gap is set for the submission of sufficient rotational crops residue trials analysing for ethoprophos, EPPA and M5 residues and covered by acceptable storage stability data (data gap and issue that could not be finalised).
Currently, in the absence of residue trials analysing all the compounds included in the risk assessment residue definitions in primary and rotational crops, a full assessment of the livestock exposure cannot be concluded yet as it is not possible to estimate the total livestock dietary burden and to assess the potential for the occurrence of significant residues in food of animal origin. This is an issue that could not be finalised. It is noted that metabolism studies in lactating goats and in laying hens with ethoprophos are available but these were not conducted in accordance with the current guidance recommendations in terms of metabolites’ identification in all matrices. EPPA, M5, OME and SME metabolites were tentatively characterised in poultry liver and kidney whilst EPPA and M5 were identified in ruminant liver and kidney only but without any further quantification. Whether or not a metabolism study in fish is necessary is also pending finalisation of the fish dietary intake calculation. Meanwhile, and based on the available data, residue definitions for monitoring and risk assessment in livestock cannot be proposed.
The data requirement for the determination of the residues in pollen and bee products for human consumption resulting from residues taken up by honeybees from crops at blossom could not be addressed considering the outstanding residue field trials on potatoes and on rotational crops analysing all compounds included in the risk assessment residue definition and as uptake and translocation of ethoprophos residues throughout the plants was demonstrated to occur from the available plant metabolism studies (data gap). Furthermore, the detailed assessment of the cited literature data to conclude on the non‐relevance of ethoprophos residues in pollen and honey was not provided and is required (data gap).
Considering that a genotoxic potential cannot be ruled out for ethoprophos, reference values cannot be established for this substance and a dietary risk assessment for the consumer cannot be conducted. This is a critical area of concern (see also Section 2).
4. Environmental fate and behaviour
The rates of dissipation and degradation in the environmental matrices investigated were estimated using FOCUS (2006) kinetics guidance. Degradation of ethoprophos was investigated in laboratory studies under dark aerobic conditions at 20–22°C with the active substance either 14C‐labelled in the propyl or in the ethyl moiety in three different soils and in three additional soils with non‐labelled material. The RMS considered the experiment performed with the ethyl labelled ethoprophos not acceptable. In these studies, ethoprophos exhibited low to moderate persistence. No metabolites were identified. Mineralisation of the 14C radiolabelled propyl moiety to carbon dioxide accounted for 50–60% applied radioactivity (AR) after 90 days. The formation of unextractable residues for this radiolabel accounted for 11–14% AR after 90 days. Only the range of pH 5.5–7.0 is covered by available studies.
A data gap was identified for route and rate of degradation studies in soil performed under Good laboratory practice (GLP) with identification of metabolites (especially to confirm whether or not metabolite EPPA needs to be assessed further) and covering the range of pH established in the Regulation (EC) No 283/20135. Especially the soil pH range of 7.5–8 is not covered by current rate of degradation data set. Depending on the results other studies on the metabolites identified may be eventually needed.
The available field dissipation studies were not considered fully reliable to derive exposure or modelling end points. No further field dissipation studies are triggered or required.
The available anaerobic soil incubations of ethoprophos have been considered not reliable. The available soil photolysis study demonstrated that ethoprophos was not degraded during 30 days of irradiation and can be considered stable to photolytic breakdown.
Reliable batch adsorption/desorption studies in seven soils are available for ethoprophos. Ethoprophos exhibited very high to medium mobility in soil. It was concluded that the adsorption of ethoprophos was not pH dependent (pH 5.7–7.4).
In a lysimeter study of 2 years duration (UK, Ongar, Essex) with application of 9.44 kg a.s./ha the first year, ethoprophos reached levels of 0.143–4.02 μg/L as annual average concentrations in the leachate. In confined field leaching studies in the Netherlands with application rates of up to 10 kg a.s./ha, ethoprophos was not found in the leachate.
Ethoprophos was stable to hydrolysis under sterile conditions at pH in the range of 3–7 (20–25°C). At pH 9 and the same temperature range, hydrolysis was observed with formation of ethyl alcohol and S,S‐dipropyl phosphorodithioic acid. In an aqueous photolysis study, the compound was found to be photolytically stable (25°C, pH 7). Ethoprophos should be classified as a non‐readily biodegradable substance according to the available study.
In laboratory incubations in dark aerobic natural sediment water systems at 20°C, ethoprophos exhibited medium to high persistence. The unextractable sediment fraction accounted for up to 10.2–11.6% AR at study end (100 days). Mineralisation accounted for up to 22% AR at the end of the study. The necessary surface water and sediment exposure assessments (predicted environmental concentrations (PEC) calculations) were carried out for ethoprophos up to step 3 and step 4 calculations (FOCUS, 2001). The step 4 calculations followed the FOCUS (2007) guidance, with no‐spray drift buffer zones of up to 10 m being implemented for the drainage scenarios, and combined no‐spray buffer zones with vegetative buffer strips of up to 20 m being implemented for the run‐off scenarios. The RMS disagreed with the applicant on the way dust drift was implemented in the calculations and provided new estimations based on EFSA opinion (2005). However, risk managers and others may wish to note that whilst run‐off mitigation is included in the step 4 calculations available, the FOCUS report (FOCUS, 2007) acknowledges that for substances with KFoc < 2,000 mL/g (i.e. ethoprophos), the general applicability and effectiveness of run‐off mitigation measures had been less clearly demonstrated in the available scientific literature, than for more strongly adsorbed compounds. In addition, the PEC SW calculations may need to be revisited or completed once the data gap for route and rate of degradation investigations in soil is completed.
The groundwater exposure assessments were carried out using FOCUS (2009) scenarios and the models PEARL 4.4.4 and PELMO 5.5.3 for the active substance ethoprophos applied to potatoes (6 kg/ha). According to the available calculations, the potential for groundwater exposure from the representative use in potatoes by ethoprophos above the parametric drinking water limit of 0.1 μg/L was concluded to be in principle low in geoclimatic situations that are represented by all nine FOCUS groundwater scenarios. However, adsorption input parameters used (organic carbon adsorption coefficient (Koc) = 101 mL/g, 1/n = 0.89) included results of a study for which stability of the test substance during the experiment cannot be guaranteed. When these results are not considered, a more critical geometric mean is obtained (Koc = 79.9 mL/g, 1/n = 0.92). Taking this into account, the potential contamination of groundwater by ethoprophos for at least one or more scenarios cannot be completely ruled out. Both lysimeter studies available and monitoring data suggest that the limit of 0.1 μg/L can be occasionally exceeded in vulnerable situations. In addition, the calculations may need to be revisited or completed once the data gap for route and rate of degradation investigations in soil is completed. Therefore, a data gap for updated PEC GW calculations is identified and the groundwater exposure assessment cannot be considered finalised at this stage.
The applicant did not provide appropriate information to address the effect of water treatment processes on the nature of the residues that might be present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water. This has led, at the time of writing this conclusion, to the identification of a data gap (see Section 7) and results in the consumer risk assessment not being finalised (see Section 9).
The PEC in soil, surface water, sediment and groundwater covering the representative uses assessed can be found in Appendix A of this conclusion.
5. Ecotoxicology
The risk assessment was based on the following documents: European Commission (2002a,b), SETAC (2001), EFSA (2009), EFSA PPR Panel (2013) and EFSA (2013). According to Regulation (EU) No. 283/2013, data should be provided regarding the acute and chronic toxicity to honeybees and data to address the development of honeybee brood and larvae. As the European Commission (2002a) does not provide a risk assessment scheme which is 1) suitable for soil application (applicable for the current assessment) and 2) which is able to use the chronic toxicity data for adult honeybees and the honeybee brood, when performing the risk assessment according to European Commission (2002a), the risk to adult honeybees from chronic toxicity and the risk to bee brood could not be finalised due to the lack of a risk assessment scheme. Additionally, under the current assessment, the chronic endpoint for honey bees is missing; thus the chronic risk assessment could not be assessed. The EFSA (2013) was used for risk assessment in order to reach a conclusion for the representative uses.
Ethoprophos was discussed at the Pesticides Peer Review Meeting 174 in February 2018.
The impurity profile of the batches used in the (eco)toxicity studies is not available and a data gap is set to characterise the impurity profile of the batches used in the toxicity studies, at least for the most recent ones (data gap – see Section 2).
High acute risk from dietary exposure has been identified for birds ingesting granules when eating soil‐contaminated food, birds consuming seedlings with residues from granular applications, and birds consuming earthworms with residues from granular applications. This is a critical area of concern. Low risk to birds could be concluded for birds ingesting granules when eating soil‐contaminated food after the refinement considering the granules incorporation depth. The additionally available refinements (body burden model and refinement of the PT (proportion of an animal's daily diet obtained in habitat treated with pesticide) were discussed at the Pesticides Peer Review Meeting 174 and were not considered acceptable. A data gap has been set to provide information to identify the most suitable focal species for southern Europe for further refinement. In addition, a low acute risk was concluded for birds ingesting granules with/as grit and for birds ingesting granules when seeking seeds as food. The birds’ exposure to granules that can be ingested as source of food was considered not relevant since the granules of the plant protection product are considered as of low caloric value. The acute risk to birds from consumption of contaminated water was assessed as low.
Considering the available data, the experts at the Pesticides Peer Review Meeting 174 agreed that bobwhite quail was the most sensitive species and key driver for long‐term risk assessment to birds. In the absence of the above‐mentioned endpoint for birds (data gap), the long‐term risk assessment, the risk from consumption of contaminated food, contaminated water and the risk from secondary poisoning in birds could not be finalised (data gap). It is noted that even by considering the non‐conservative endpoint from the modified study on bobwhite quail, a low risk for the representative uses cannot be concluded.
The acute and long‐term dietary risk and the risk via consumption of contaminated water to mammals have been considered low for all the relevant granular‐applications scenarios. Additionally, low risk was concluded for fish‐eating mammals.
Considering the lack of information on the nature and characterisation of ethoprophos metabolites in soils (data gap, Section 4), the secondary poisoning from soil metabolites could not be finalised (data gap). The risk assessment to birds and mammals from metabolites needs to be performed (data gap).
For aquatic organisms, toxicity data were available for fish, aquatic invertebrates, sediment‐dwelling organisms and algae. One mesocosm study was available and discussed at the Pesticides Peer Review Meeting 174. No minimal detectable difference (MDD) was provided (data gap) and due to the uncertainties linked to the lack of some representative taxa in the test system, the experts agreed to apply an assessment factor of 3 to the relevant endpoint (NOEC = 1.78 μg/L) and to use in the refined risk assessment an ecological threshold option–regulatory acceptable concentration (ETO‐RAC) of 0.59 μg/L.
For the in‐furrow application of ethoprophos, high risk (acute and chronic) to fish were identified in 1/6 FOCUS scenarios provided that mitigation measures are applied (data gap). High acute risk to aquatic invertebrates, including sediment‐dwelling organisms, has been identified in 2/6 FOCUS scenarios even including mitigation measures (data gap). High chronic risk to aquatic invertebrates was concluded for 5/6 FOCUS scenarios considering mitigation measures (data gap). By using the available ETO‐RAC, when mitigation measures are considered, a high risk was still identified for 2/6 FOCUS scenarios (D6 Ditch and R3 Stream) (data gap).
For the use pattern that includes the application of ethoprophos following a broadcast application, high acute risk to fish was identified in 2/6 FOCUS scenarios even if mitigation measures are applied (data gap). High chronic risk to fish was identified in 4/6 FOCUS scenarios considering vegetated buffer strips up to 20 m (data gap). High acute risk to aquatic invertebrates, including sediment‐dwelling organisms, has been identified in 4/6 FOCUS scenarios even including mitigation measures (data gap). High chronic risk to aquatic invertebrates was concluded for 5/6 FOCUS scenarios also considering mitigation measures. By using the available ETO‐RAC, and when mitigation measures are considered, low risk could not be concluded for 4/6 FOCUS scenarios (only in D3 and D4 FOCUS scenario low risk is confirmed) (data gap).
Low risk has been concluded to algae for both representative use patterns of ethoprophos in potatoes.
Pending on the data gap on degradation in soil (see Section 4), the risk to non‐target aquatic organisms might need to be revised.
It is noted that in the available AMA test (amphibians metamorphosis assay), an indication of thyroid disruption was observed; therefore, it is unclear whether the amphibians are covered by the current aquatic vertebrates risk assessment. This might need to be revised while addressing the data gap for aquatic vertebrates risk assessment.
Only acute ecotoxicity tests for honey bees were available. A data gap has, therefore, been identified for further studies addressing the chronic effects and the chronic risk to honeybees (adult and larvae) and this has been indicated as an issue that could not be finalised. No risk assessment was provided by the applicant, according to the guidance document European Commission (2002a), because this guidance is not suitable for the representative uses of ethoprophos. The EFSA bee guidance (2013) has been used for the risk assessment. High risk to bees could not be excluded for exposure via guttation water. A high risk via exposure from contaminated water (surface water) was concluded (data gap). Low risk to bees was concluded for the puddle water scenarios and in the relevant field scenarios (treated crop, weeds). A high acute risk to bees in the succeeding crop scenario was identified (data gap). Insufficient information was available to perform a risk assessment for sublethal effects on bees (i.e. hypopharyngeal glands (HPG), data gap), accumulative effects, and effects from occurring metabolites in pollen and nectar was not investigated (data gap). Information to perform a risk assessment for bumble bees and solitary bees were not available.
As regards other non‐target arthropods (NTAs), laboratory studies were not available with the standard tier 1 indicator species (i.e. Aphidius rhopalosiphi and Typhlodromus pyri). However, toxicity studies with other NTAs were available (Poecilus cupresus and Aleochara bilineata). High risk could not be refined by considering existing higher tier data and further information for addressing the risk to NTAs other than bees has to be provided (data gap and critical area of concern).
For earthworms, available data were not considered enough to conclude on a low risk (data gap, issue that could not be finalised). For soil macro‐organisms other than earthworms, high chronic risk has been identified for Hypoaspis aculeifer and Folsomia candida in the first tier for both representative uses. Data to refine this risk were unavailable (data gap and critical area of concern). Low risk has been identified to soil microorganisms. In the absence of the identification and characterisation of ethoprophos metabolites in soils (data gap, see Section 4), the risk from soil metabolites to soil organisms was not performed (data gap and issue that cannot be finalised).
Low risk is expected to non‐target terrestrial plants due to the nearly dust‐free condition of the granular formulated product. Besides, low risk was identified for organisms involved in biological methods for sewage treatment.
Regarding the potential for endocrine disruption of ethoprophos, all the available information was discussed by the experts at the Pesticides Peer Review Meeting 172 (mammalian toxicology) and at the Pesticides Peer Review Meeting 174 (ecotoxicology). It is noted that in Section 2 a data gap was identified for further addressing the endocrine disruption in mammals via non‐EATS modalities. As reported in Section 2, regarding the EATS modalities, the mechanistic data did not indicate an oestrogen, androgen or thyroid‐mediated endocrine activity whilst a positive steroidogenesis assay was available. A fish short‐term reproduction assay was available; due to the high variability in the results in this study, specifically for what concerns the VTG (vitellogenin) levels and cumulative eggs production, a firm conclusion could not be reached. An amphibian metamorphosis assay (AMA) test was available and possible effects due to thyroid modality cannot be excluded on the basis of this study. Pending on the data gap in Section 2, further information might be needed to address the endocrine disrupting properties in non‐target organisms, particularly relevant for the amphibians.
6. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1–4)
Table 1.
Soil
| Compound (name and/or code) | Persistence | Ecotoxicology |
|---|---|---|
| Ethoprophosa | Low to moderate persistence (DT50 = 7.9–17.3 days) |
High risk to soil macro‐organisms other than earthworms. Data gap for earthworms. |
DT50: period required for 50% dissipation.
Open for potential metabolites.
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| Ethoprophos | Rat LC50 inhalation = 0.123 mg/L air (4 h, nose only) – Acute Tox. 2 – H330 ‘fatal if inhaled’ |
LC50: lethal concentration, 50%.
Table 2.
Groundwater
| Compound (name and/or code) | Mobility in soil | > 0.1 μg/L at 1 m depth for the representative usesa | Pesticidal activity | Toxicological relevance |
|---|---|---|---|---|
| Ethoprophosb | Very high to medium (KFoc = 38–169 mL/g) |
FOCUS: No in available calculations, update needed for input parameters (data gap). Lysimeter: Yes, up to 0.143 μg/L and 4.02 μg/L in two lysimeters after application 9.44 kg a.s./ha in UK Confined field leaching studies: No (Netherlands) |
Yes | Yes |
KFoc: Freundlich organic carbon adsorption coefficient; FOCUS: Forum for the Co‐ordination of Pesticide Fate Models and their Use; a.s.: active substance.
FOCUS scenarios or a relevant lysimeter.
Open for potential metabolites.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Ethoprophosa | High risk to aquatic invertebrates and fish |
Open for potential metabolites.
7. Data gaps
This is a list of data gaps identified during the peer review process, including those areas in which a study may have been made available during the peer review process but not considered for procedural reasons (without prejudice to the provisions of Article 56 of Regulation (EC) No 1107/2009 concerning information on potentially harmful effects).
The oxidising properties of the active substance as manufactured (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 1).
Efficiency of the extraction procedure used in the analytical method for the determination of residues in food and feed of plant origin (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 1).
Additional validation data for the submitted method or a new method with a LOQ in compliance with the requirements for the operators, workers, residents and bystanders risk assessment (relevant for representative uses evaluated; submission date proposed by the applicant: unknown; see Section 1).
An analytical method for analysis of metabolite EPPA in body fluids and tissues (relevant for representative uses evaluated; submission date proposed by the applicant: unknown; see Section 1)
Characterisation of the impurity profile of the batches used in the (eco)toxicological studies (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Sections 2 and 5).
Analytical methods used in the older toxicity studies, in particular repeated‐dose dietary studies (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Sections 1 and 2).
In vitro interspecies comparative metabolism study (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 2).
Ames test including the investigation of strains sensitive to cross‐linking and oxidising mutagens (TA102 or E. coli WP2 strain), clarification of the gene mutation potential in mammalian cells and robust in vivo follow up to the positive clastogenic effects observed in vitro (including the aneugenicity potential) with ethoprophos (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 2).
Comparative AChE assay after repeated dose (available to the US EPA) and reliable developmental toxicity study in rabbit (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 2).
Clarification of the potential endocrine‐mediated apical effects observed in levels 4 and 5 studies according to the OECD conceptual framework (OECD, 2012) such as thyroid C cell tumours and pheochromocytoma that may be endocrine‐mediated through non‐EATS modalities (regarding calcitonin or catecholamine), although it is acknowledged that there are no validated OECD test guidelines to address this gap. Pending on the outcome of this data gap, further information might be needed to address the endocrine disrupting properties in non‐target organisms, particularly relevant for the amphibians (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Sections 2 and 5).
Submission of the occupational reports on monitoring of manufacturing plant personnel conducted after the last review (2004) up to now (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 2).
Further analysis of the available epidemiological/biomonitoring studies to clarify whether ethoprophos has been investigated, and other published data such as ToxCast, studies on a molecular level and non‐EU monitoring studies that are considered relevant to the human health risk assessment (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 2).
Sufficient residue trials on potatoes compliant respectively with the NEU and SEU GAPs for the determination of M5 and supported by acceptable storage stability data on this compound (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 3).
Sufficient rotational crops residue trials analysing for ethoprophos, EPPA and M5 residues and covered by acceptable storage stability data for these compounds (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 3).
A hydrolysis study addressing the nature of residues of all compounds included in the proposed risk assessment residue definition for plants and simulating pasteurisation, baking/boiling and sterilisation (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 3).
Determination of the residues in pollen and bee products for human consumption resulting from residues taken up by honeybees from crops at blossom (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 3).
Detailed assessment of the cited literature data to conclude on the non‐relevance of ethoprophos residues in pollen and honey was not provided and is required (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 3).
Route and rate of degradation studies in soil performed under GLP with identification of metabolites (especially to confirm whether or not metabolite EPPA needs to be assessed further) and covering the range of pH established in the regulation (EU) No 283/2013. Especially the soil pH range of 7.5–8 is not covered by the current rate of degradation data set. Depending on the results, other studies on the metabolites identified may be eventually needed and the risk assessment might need to be performed (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Sections 4 and 5).
Updated PEC GW calculations with geometric mean KFoc = 79.9 mL/g (1/n = 0.92) and with consideration of results of new route and rate of degradation studies in soil required (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 4).
Information to address the effect of water treatment processes on the nature of the residues that might be present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water (relevant for all representative uses evaluated; submission date proposed by the applicant: unknown; see Section 4).
Further information to address the risk to birds for ethoprophos and its metabolites might need to be considered. The long‐term risk to birds for ethoprophos should be addressed including a long term study on bobwhite quail (relevant for all the representative uses; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the risk to mammals for ethoprophos metabolites might need to be further considered (relevant for all the representative uses; submission date proposed by the applicant: unknown; see Section 5).
Further information for the identification of proper birds focal species for southern Europe needs to be provided (relevant for all the representative uses; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the risk to fish and amphibians for ethoprophos (relevant for all the representative uses; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the risk to aquatic invertebrates (relevant for all representative uses; submission date proposed by the applicant: unknown; see Section 5).
The MDD data and evaluation of the available mesocosm study need to be provided (relevant for all representative uses; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the acute risk throughout the succeeding crops scenario and the chronic risk to bees (adults and larvae) (relevant for all representative uses; submission date proposed by the applicant: unknown; see Section 5).
Further information on the risk to bees due to consumption of contaminated water and guttation water needs to be addressed (relevant for all representative uses; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the risk from sublethal effects on bees (i.e. HPG) and effects from occurring metabolites in pollen and nectar (relevant for all representative uses; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the risk to NTAs (relevant for all representative uses; submission date proposed by the applicant: unknown; see Section 5).
Further information to address the risk for ethoprophos and its possible metabolites to earthworms and other soil meso‐ and macrofauna (relevant for all representative uses; submission date proposed by the applicant: unknown; see Sections 4 and 5).
8. Particular conditions proposed to be taken into account to manage the risk(s) identified
Vegetated buffer strips (VBS) up to 10 m are necessary to achieve a low acute and chronic risk to fish in R3/stream scenarios for in‐furrow application of ethoprophos.
VBS up to 10 m are necessary to achieve a low acute risk to aquatic invertebrates in R1–R2/stream for in‐furrow application of ethoprophos.
VBS up to 20 m are necessary to achieve a low acute risk to fish in R1–R2/stream scenarios for the broadcast application of ethoprophos.
Nearly dust‐free condition of the granular formulated product is necessary to achieve low risk to non‐target terrestrial plants (off‐field risk assessment).
9. Concerns
9.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/20116 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
-
1
The analytical methods used in the older toxicological studies were not reported and therefore not validated, which questions the validity of the studies, in particular the repeated‐dose dietary studies (see Section 2).
-
2
The need for further tests and risk assessment to unique human metabolites could not be finalised whilst an in vitro interspecies comparative metabolism study is not submitted (see Section 2).
-
3
The developmental neurotoxicity could not be concluded in the absence of a comparative AChE assay after repeated dose to clarify whether the young animals may be more sensitive to ethoprophos exposure than the adults; in addition, the results of the rabbit developmental toxicity study cannot be relied upon with regard to the developing fetuses (see Section 2).
-
4
Interim criteria are not met, however, the potential endocrine‐mediated apical effects observed in levels 4 and 5 studies according to the OECD conceptual framework (OECD, 2012) such as thyroid C‐cell tumours and pheochromocytoma that may be endocrine‐mediated through non‐EATS modalities (regarding calcitonin or catecholamine) cannot be concluded; although it is acknowledged that there are no validated OECD test guidelines to address this concern (see Section 2).
-
5
The consumer dietary risk assessment could not be finalised with respect to residues in primary and rotational crops, the nature of the residues in processed commodities and the livestock exposure assessment (see Section 3).
-
6
Groundwater exposure assessment cannot be finalised (see Section 4).
-
7
Consumers risk assessment cannot be finalised with respect to residues that might be present in surface water and groundwater, when surface water or groundwater is abstracted for drinking water (see Section 4).
-
8
The long‐term risk assessment for ethoprophos to birds could not be finalised in the absence of a valid Tier I reproductive endpoint (see Section 5).
-
9
Secondary poisoning from soil metabolites for birds and mammals (see Section 5).
-
10
Risk from soil metabolism to soil organisms cannot be finalised in the absence of the identification and characterisation of ethoprophos metabolites in soils (see Section 5).
-
11
The chronic risk to bees could not be finalised in absence of a Tier I endpoint (see Section 5).
-
12
The risk to earthworms could not be carried out in absence of a valid Tier I endpoint (see Section 5).
9.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
-
13
Equivocal gene mutation and positive clastogenic effects seen in vitro were followed up with in vivo studies of limited reliability but showing also equivocal and positive results (see Section 2).
-
14
Since a genotoxic and clastogenic potential could not be excluded, no threshold for these effects is assumed and therefore toxicological reference values could not be established. Dietary and non‐dietary exposure risk assessment could not be conducted (see Sections 2 and 3).
-
15
High acute risk to birds has been identified (see Section 5).
-
16
High risk to soil dwelling NTAs has been identified which could not be refined by existing higher tier data (see Section 5).
-
17
High risk to soil organisms other than microorganisms and earthworms for all representative uses has been identified (see Section 5).
9.3. Overview of the concerns identified for each representative use considered
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 5.)
Table 5.
Overview of concerns
| Representative use | Potato (in‐furrow application) | Potato (broadcast application) | |
|---|---|---|---|
| Operator risk | Risk identified | X13,14 | X13,14 |
| Assessment not finalised | |||
| Worker risk | Risk identified | X13,14 | X13,14 |
| Assessment not finalised | |||
| Resident/bystander risk | Risk identified | X13,14 | X13,14 |
| Assessment not finalised | |||
| Consumer risk | Risk identified | X13,14 | X13,14 |
| Assessment not finalised | X5,7 | X5,7 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | X15 | X15 |
| Assessment not finalised | X8,9 | x8,9 | |
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | X16,17 | X16,17 |
| Assessment not finalised | X10,11,12 | X10,11,12 | |
| Risk to aquatic organisms | Risk identified | X | X |
| Assessment not finalised | |||
| Groundwater exposure to active substance | Legal parametric value breached | X | X |
| Assessment not finalised | X6 | X6 | |
| Groundwater exposure to metabolites | Legal parametric value breached | ||
| Parametric value of 10 µg/La breached | |||
| Assessment not finalised | X6 | X6 | |
Columns are grey if no safe use can be identified. The superscript numbers relate to the numbered points indicated in Sections 9.1 and 9.2. Where there is no superscript number, see Sections 2–6 for further information.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission, 2003.
Abbreviations
- 1/n
slope of Freundlich isotherm
- a.s.
active substance
- AAOEL
acute acceptable operator exposure level
- AChE
acetylcholinesterase
- ADI
acceptable daily intake
- AMA
amphibian metamorphosis assay
- AOEL
acceptable operator exposure level
- AR
applied radioactivity
- ARfD
acute reference dose
- bw
body weight
- CLP
classification, labelling and packaging
- DAR
draft assessment report
- DT50
period required for 50% dissipation (define method of estimation)
- EATS
oestrogen, androgen, thyroid, steroid
- ECHA
European Chemicals Agency
- EEC
European Economic Community
- ETO
ecological threshold option
- FAO
Food and Agriculture Organization of the United Nations
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- GLP
Good laboratory practice
- GR
granule
- HPG
hypopharyngeal glands
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- KFoc
Freundlich organic carbon adsorption coefficient
- Koc
organic carbon adsorption coefficient
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOAEL
lowest observable adverse effect level
- LOQ
limit of quantification
- MDD
minimal detectable difference
- MRL
maximum residue level
- NEU
northern Europe
- NOAEL
no observed adverse effect level
- NOEC
no observed effect concentration
- NOEL
no observed effect level
- NTA
non‐target arthropod
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant‐back interval
- PD
proportion of different food types
- PEC
predicted environmental concentration
- PECair
predicted environmental concentration in air
- PECgw
predicted environmental concentration in groundwater
- PECsed
predicted environmental concentration in sediment
- PECsoil
predicted environmental concentration in soil
- PECsw
predicted environmental concentration in surface water
- PoD
point of departure
- PT
proportion of diet obtained in the treated area
- RAC
regulatory acceptable concentration
- RAR
Renewal Assessment Report
- RMS
rapporteur Member State
- SEU
southern Europe
- SMILES
simplified molecular‐input line‐entry system
- Tmax
time until peak blood levels achieved
- TRR
total radioactive residue
- UF
uncertainty factor
- VBS
vegetated buffer strips
- VTG
vitellogenin
- WHO
World Health Organization
Appendix A – List of end points for the active substance and the representative formulation
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2018.5290
Appendix B – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|
|
M5 Ethyl‐phosphate, O‐ethyl phosphoric acid |
ethyl dihydrogen phosphate O=P(O)(O)OCC ZJXZSIYSNXKHEA‐UHFFFAOYSA‐N |
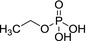
|
|
EPPA (M31), M1M, M1, O‐ethyl S‐propyl phosphorothioate, |
O‐ethyl S‐propyl hydrogen phosphorothioate O=P(O)(SCCC)OCC JHUCCRNKXWBDTO‐UHFFFAOYSA‐N |
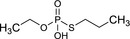
|
| SME (M49) |
O‐ethyl S‐methyl S‐propyl phosphorodithioate O=P(SCCC)(SC)OCC YAYATFRQCMZXSD‐UHFFFAOYSA‐N |
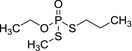
|
| OME (M48) |
O‐ethyl O‐methyl S‐propyl phosphorothioate O=P(OCC)(SCCC)OC BLMDNYFPFQLYIY‐UHFFFAOYSA‐N |
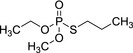
|
| M50 |
O‐ethyl S‐(3‐hydroxypropyl) S‐propyl phosphorodithioate O=P(SCCCO)(SCCC)OCC ZXOJSNLUTAJCAO‐UHFFFAOYSA‐N |
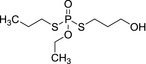
|
|
M38 Glycoside conjugate of O‐ethyl‐S‐propyl‐S‐hydroxy‐propylphosphorodithioate |
O‐ethyl S‐[4‐(d‐glucopyranosyloxy)butyl] S‐propyl phosphorodithioate O[C@H]([C@H]([C@@H]([C@@H](CO)O1)O)O)C1OCCCCSP(SCCC)(OCC)=O ZLMSGJFSHJSNFI‐MRVUMPFXSA‐N |
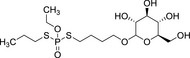
|
|
M39 Glycosyl‐sulfate of O‐ethyl‐S‐propyl‐S‐hydroxy‐propylphosphorodithioate |
O‐ethyl S‐propyl S‐{4‐[(6‐O‐sulfo‐d‐glucopyranosyl)oxy]butyl} phosphorodithioate O[C@H]([C@H]([C@@H]([C@@H](COS(=O)(O)=O)O1)O)O)C1OCCCCSP(SCCC)(OCC)=O JOXFUGSSDWXSRD‐FQGYJZIPSA‐N |
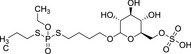
|
|
M41 glycosyl‐malonate of O‐ethyl‐S‐propyl‐S‐hydroxy‐propylphosphorodithioate |
4‐{[ethoxy(propylsulfanyl)phosphoryl]sulfanyl}butyl 6‐O‐(carboxyacetyl)‐d‐glucopyranoside O=C(O)CC(=O)OC[C@H]1OC(OCCCCSP(=O)(OCC)SCCC)[C@H](O)[C@@H](O)[C@@H]1O GEQKSYDZTVEGOW‐HNSOARQOSA‐N |
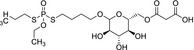
|
|
M42 glycosyl‐malonyl‐sulfate of O‐ethyl‐S‐propyl‐S‐hydroxy‐propylphosphorodithioate |
O‐ethyl S‐[4‐({6‐O‐[3‐oxo‐3‐(sulfooxy)propanoyl]‐d‐glucopyranosyl}oxy)butyl] S‐propyl phosphorodithioate O=S(=O)(O)OC(=O)CC(=O)OC[C@H]1OC(OCCCCSP(=O)(OCC)SCCC)[C@H](O)[C@@H](O)[C@@H]1O DQTLNGPTYYQLKX‐ZEYCPOLWSA‐N |
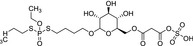
|
|
M18 conjugate of O‐ethylphosphorothioate: R’’‐glycoside |
1‐S‐[ethoxy(hydroxy)phosphoryl]‐1‐thio‐d‐glucopyranose OP(OCC)(SC1[C@@H]([C@@H](O)[C@H](O)[C@H](O1)CO)O)=O PISMICJMECDZFX‐KEWYIRBNSA‐N |
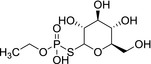
|
| S , S ‐dipropyl phosphorodithioic acid |
S,S‐dipropyl hydrogen phosphorodithioate O=P(SCCC)(SCCC)O WRKUPFMPTHCAHW‐UHFFFAOYSA‐N |
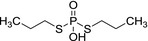
|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2015 ACD/Labs 2015 Release (File version N20E41, Build 75170, 19 December 2014).
ACD/ChemSketch 2015 ACD/Labs 2015 Release (File version C10H41, Build 75059, 17 December 2014).
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Arena M, Auteri D, Barmaz S, Brancato A, Brocca D, Bura L, Carrasco Cabrera L, Chiusolo A, Civitella C, Court Marques D, Crivellente F, Ctverackova L, De Lentdecker C, Egsmose M, Erdos Z, Fait G, Ferreira L, Goumenou M, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Magrans JO, Medina P, Mineo D, Miron I, Molnar T, Padovani L, Parra Morte JM, Pedersen R, Reich H, Riemenschneider C, Sacchi A, Santos M, Serafimova R, Sharp R, Stanek A, Streissl F, Sturma J, Szentes C, Tarazona J, Terron A, Theobald A, Vagenende B, Van Dijk J and Villamar‐Bouza L, 2018. Conclusion on the peer review of the pesticide risk assessment of the active substance ethoprophos. EFSA Journal 2018;16(10):5290, 26 pp. 10.2903/j.efsa.2018.5290
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00105
Update: This scientific output, approved on 17 May 2018, supersedes the previous output published on 28 April 2006 (EFSA, 2006).
Approved: 17 May 2018
Notes
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26–32.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355.
It should be noted that harmonised classification and labelling is formally proposed and decided in accordance with Regulation (EC) No 1272/2008.
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 1–84.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- ECHA (European Chemicals Agency), 2017. Guidance on the Application of the CLP Criteria; Guidance to Regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures. Version 5.0, July 2017. Reference: ECHA‐17‐G‐21‐EN; ISBN: 978‐92‐9020‐050‐5; available online: https://echa.europa.eu/guidance-documents/guidance-on-clp
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Panel on Plant Health, Plant Protection Products and their Residues on a request of EFSA related to FOCUS sw scenarios. EFSA Journal, 2005;3(1):145, 31 pp. 10.2903/j.efsa.2005.145 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006. Conclusion on the peer review of the pesticide risk assessment of the active substance ethoprophos. EFSA Journal 2006;4(4):66r, 72 pp. 10.2903/j.efsa.2006.66r [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance on risk assessment for birds and mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp., 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance ethoprophos. Available online: http://www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2012. Guidance on dermal absorption. EFSA Journal 2012;10(4):2665, 30 pp. 10.2903/j.efsa.2012.2665 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 186 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- EFSA SC (European Food Safety Authority Scientific Committee), 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 68 pp, 10.2903/j.efsa.2011.2379. Available online: http://www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA SC (European Food Safety Authority Scientific Committee), 2017. Hardy A, Benford D, Halldorsson T, Jeger M, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Younes M, Aquilina G, Crebelli R, Gurtler R, Hirsch‐Ernst KI, Mosesso P, Nielsen E, vanBenthem J , Carfi M, Georgiadis N, Maurici D, Parra Morte J and Schlatter J, 2017. Scientific Opinion on the clarification of some aspects related to genotoxicity assessment. EFSA Journal 2017;15(12):5113, 25 pp. 10.2903/j.efsa.2017.5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000.
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission , 2002a. Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002‐rev. 2 final, 17 October 2002
- European Commission , 2002b. Guidance Document on Aquatic Ecotoxicology Under Council Directive 91/414/EEC. SANCO/3268/2001‐rev. 4 final, 17 October 2002.
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2011. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. SANCO 7525/VI/95‐rev. 9. March 2011. p.1–46
- European Commission , 2012. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003‐rev. 10.1, 13 July 2012.
- European Commission , 2013. Review report for the active substance ethoprophos. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 16 March 2007 in view of the inclusion of ethoprophos in Annex I of Council Directive 91/414/EEC. SANCO/03307/2006‐Rev.6, 3 October 2013, 9 pp.
- European Commission , 2014. Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.4, May 2015.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2006. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005‐v. 2.0, 434 pp.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2007. Landscape and mitigation factors in aquatic risk assessment. Volume 1. Extended summary and recommendations. Report of the FOCUS Working Group on Landscape and Mitigation Factors in Ecological Risk Assessment. EC Document Reference SANCO/10422/2005 v. 2.0, 169 pp.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2009. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 1, 604 pp., as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.0, January 2011
- Italy , 2017. Renewal Assessment Report (RAR) on the active substance ethoprophos prepared by the rapporteur Member State Italy, in the framework of Commission Implementing Regulation (EU) No 844/2012, January 2017. Available online: http://www.efsa.europa.eu
- Italy , 2018. Revised Renewal Assessment Report (RAR) on ethoprophos prepared by the rapporteur Member State Italy in the framework of Regulation (EC) No 844/2012, March 2018. Available online: http://www.efsa.europa.eu
- JMPR (Joint Meeting on Pesticide Residues), 2004. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Rome, Italy, 20–29 September 2004, 383 pp.
- JMPR (Joint Meeting on Pesticide Residues), 2007. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Switzerland, 18–27 September 2007, 164 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2009. Guidance document on overview of residue chemistry studies. ENV/JM/MONO(2009)31, 28 July 2009.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2012. Series on Testing and Assessment: No 150: Guidance document on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption. ENV/JM/MONO(2012)22, 524 pp.
- SETAC (Society of Environmental Toxicology and Chemistry), 2001. Guidance document on regulatory testing and risk assessment procedures for plant protection products with non‐target arthropods. ESCORT 2.
- United Kingdom , 2004. Draft Assessment Report (DAR) on the active substance ethoprophos prepared by the rapporteur Member State United Kingdom in the framework of Directive 91/414/EEC, April 2004. Available online: http://www.efsa.europa.eu
- US EPA (Environmental Protection Agency), 2006. US Environmental Protection Agency, Office of Pesticide Programs, Reregistration Eligibility Decision for Ethoprophos, July 31, 2006
- US EPA (United States Environmental Protection Agency), 2007. OPP (Office of Prevention, Pesticides and Toxic Substances) Official Record, Health Effects Division, Scientific Data Reviews, EPA Series 361. Ethoprop (Ethoprophos). Review of Developmental Neurotoxicity Study, April 17, 2007.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


