Abstract
The European Commission asked EFSA for an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of a riboflavin‐based additive (minimum 80%) produced by a genetically modified strain of Ashbya gossypii (DSM 23096). It is intended to be used in feed for all animal species and categories. The additive under assessment does not give rise to safety concerns on the genetic modification of the production strain. The additive contains 80% of riboflavin (vitamin B2) and 20% of spent growth medium. The additive is safe for target animals with a wide margin of safety. The use of riboflavin 80% produced by A. gossypii DSM 23096 in animal nutrition does not represent a safety concern for consumers. The Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) cannot draw a final conclusion on the risk posed for the user by inhalation of riboflavin produced by A. gossypii DSM 23096 and on the potential to be irritant to skin or eyes. The product under assessment is not a skin sensitiser; however, riboflavin is a known photosensitiser. The use of riboflavin produced by A. gossypii DSM 23096 in animal nutrition does not pose a risk to the environment. The additive is regarded as an effective source of riboflavin in covering the animal's requirement when administered via feed. The FEEDAP Panel made recommendations on the description of the additive.
Keywords: nutritional additive, vitamin B2 , riboflavin, safety, Ashbya gossypii, genetically modified microorganism, efficacy
Summary
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of vitamin B2 (riboflavin) produced by Ashbya gossypii DSM 23096 as a feed additive for all animal species and categories.
Riboflavin is primarily found as an integral component of the coenzymes flavin adenine dinucleotide and flavin mononucleotide. Flavocoenzymes participate in redox reactions of carbohydrates, fats and proteins. Riboflavin is therefore involved in the energy metabolism of men and animals.
The product under assessment contains 80% of riboflavin (vitamin B2) and 20% of spent growth medium. It is produced by fermentation with a genetically modified strain of the fungus Ashbya gossypii DSM 23096. None of the sequences integrated in the genome of the production strain raises a safety concern. Neither the production strain nor its recombinant DNA was detected in the final product. The final product is considered to be safe with regard to the genetic modification of the production strain.
Riboflavin produced by A. gossypii DSM 23096 is safe for the target animals with a wide margin of safety.
The use of riboflavin 80% produced by A. gossypii DSM 23096 in animal nutrition does not represent a safety concern for consumers.
FEEDAP Panel cannot draw a final conclusion on the risk posed for the user by inhalation of riboflavin produced by A. gossypii DSM 23096 and on the potential to be irritant to skin or eyes. The product under assessment is not a skin sensitiser; however, riboflavin is a known photosensitiser.
The use of riboflavin produced by A. gossypii DSM 23096 in animal nutrition does not pose a risk to the environment.
Riboflavin produced by A. gossypii DSM 23096 is regarded as effective in covering the animal's requirement when administered via feed.
The FEEDAP Panel made a recommendation on the description of the additive.
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No. 1831/20031 sets out the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 10(2) of that Regulation also specifies that for existing products within the meaning of Article 10(1), an application shall be submitted in accordance with Article 7, at the latest 1 year before the expiry date of the authorisation given pursuant to Directive 70/524/EEC for additives with a limited authorisation period, and within a maximum of seven years after the entry into force of this Regulation for additives authorised without a time limit or pursuant to Directive 82/471/EEC.
The European Commission received a request from the Company BASF SE2 for the evaluation of the product vitamin B2 (riboflavin) produced by Ashbya gossypii DSM 23096, when used as a feed additive for all animal species (category: nutritional additives; functional group: vitamins, provitamins and chemically well‐defined substances having similar effect).
According to Article 7(1) of Regulation (EC) No. 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 10(2) (re‐evaluation of an authorised feed additive). EFSA received directly from the applicant the technical dossier in support of this application. The particulars and documents in support of the application were considered valid by EFSA as of 23 May 2012.3
According to Article 8 of Regulation (EC) No. 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product vitamin B2 (riboflavin) produced by Ashbya gossypii DSM 23096, when used under the proposed conditions of use (see Section 3.1.7).
1.2. Additional information
Riboflavin is the generic name for the water‐soluble vitamin B2. Riboflavin is primarily found as an integral component of the coenzymes, flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN). Flavocoenzymes participate in redox reactions of carbohydrates, fats and proteins from which living organisms derive most of their energy.
The Scientific Committee on Food (SCF) expressed an opinion on riboflavin as a colouring matter authorised for use in foodstuffs produced by fermentation using genetically modified Bacillus subtilis (European Commission, 1998) and another opinion on the tolerable upper intake level of vitamin B2 (European Commission, 2000). The EFSA Panel on Food Additives and Nutrient Sources added to food (ANS) issued a statement on the inability to assess the safety of riboflavin‐enriched yeast added for nutritional purposes as a source of riboflavin in food supplements and the bioavailability of riboflavin from this source, based on the supporting dossier (EFSA, 2009). The EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) issued several opinions on the substantiation of several health claims related to riboflavin (EFSA NDA Panel, 2010, 2012, 2013a,b). The ANS Panel issued an opinion on the re‐evaluation for riboflavin (E101(i)) and riboflavin‐5’‐phosphate (E 101(ii)) as part of the food additives re‐evaluation programme specified under Regulation (EU) No 257/20104 (EFSA ANS Panel, 2013). The EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) issued an opinion on the safety and efficacy of vitamin B2 (80%) as riboflavin produced by Bacillus subtilis KCCM‐10445 for all animal species (EFSA FEEDAP Panel, 2014, 2018) and another opinion on the safety and efficacy of vitamin B2 as riboflavin and riboflavin‐5’‐phosphate ester monosodium salt, produced by either Bacillus subtilis DSM 17339 or Bacillus subtilis DSM 23984 (EFSA FEEDAP Panel, 2016).
Riboflavin is included in the European Pharmacopeia (PhEur), Monograph (MG) 0292 (PhEur, 2010).
Vitamin B2 is listed as a pharmacologically active substance in veterinary medicinal products and it is not subject to maximum residue levels when used in food‐producing animals.5 The use of riboflavin/lactoflavin as colorant is authorised in cosmetic products.6 Vitamin B2 is authorised for use in food7 and food supplements,8 for addition for specific nutritional purposes to foods for particular nutritional uses9 and for addition to processed cereal‐based foods and baby foods for infants and young children10 and to infant formulas and follow‐on formulas when reconstituted as instructed by the manufacturer.11 Riboflavin is also authorised as a colouring under number E 101 (i) for use in foodstuffs.12
The additive Vitamin B2 (riboflavin) has been authorised in the EU for all animal species without a time limit (Commission list of the authorised additives in feedingstuffs published in application of Article 9t (b) of Council Directive 70/524/EEC).13 Following the provisions of Article 10(1) of Regulation (EC) No 1831/2003, the compound was included in the EU Register of Feed Additives under the category – Nutritional additives and the functional group – Vitamins, provitamins and chemically well‐defined substances having similar effect.14
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier15 in support of the authorisation request for the use of vitamin B2 (riboflavin) as a feed additive. The technical dossier was prepared following the provisions of Article 7 of Regulation (EC) No. 1831/2003, Regulation (EC) No. 429/200816 and the applicable EFSA guidance documents.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts’ knowledge, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of riboflavin in animal feed. The Executive Summary of the EURL report can be found in Annex A.17
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of vitamin B2 produced by Ashbya gossypii DSM 23096 is in line with the principles laid down in Regulation (EC) No. 429/2008 and the relevant guidance documents: Guidance for the preparation of dossiers for the re‐evaluation of certain additives already authorised under Directive 70/524/EEC (EFSA, 2008a), Guidance on nutritional additives (EFSA FEEDAP Panel, 2012a), Technical guidance: Tolerance and efficacy studies in target animals (EFSA FEEDAP Panel, 2011), Guidance for establishing the safety of additives for the consumer (EFSA FEEDAP Panel, 2012b), Guidance on studies on the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012c), Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008b), Technical Guidance: Microbial Studies (EFSA, 2008c), Guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use (EFSA GMO Panel, 2011).
3. Assessment
The active substance/additive under assessment is riboflavin obtained by a genetically modified strain of Ashbya gossypii (DSM 23096). It is classified as a nutritional additive, functional group: vitamins, provitamins and chemically well‐defined substances having similar effect. It is intended for use in feed for all animal species and categories. The substance placed on the feed market is an additive containing 80% riboflavin and 20% of spent growth medium.
3.1. Characterisation
3.1.1. Characterisation of the active substance
Riboflavin (International Union of Pure and Applied Chemistry (IUPAC) name: 7,8‐dimethyl‐10‐[(2S,3S,4R)‐2,3,4,5,‐tetrahydroxypentyl]benzo[g] pteridine‐2,4(3H,10H)‐dione, synonyms: vitamin B2, 7,8,‐dimethyl‐10‐(1’‐D‐ribityl)isoalloxazine; lactoflavin, 1‐deoxy‐1‐(7,8,dimethyl‐2,4‐dioxo‐3,4‐dihydrobenzo[g]pteridin‐10(2H)‐yl)‐D‐ribitol), is identified by the CAS (Chemical Abstracts Service) number 83–88–5 and the EINECS (European Inventory of Existing Chemical Substances) number 201–507–1. The molecular formula of riboflavin is C17H20N4O6 and its molecular weight is 376.37. The structural formula of riboflavin is shown in Figure 1.
Figure 1.
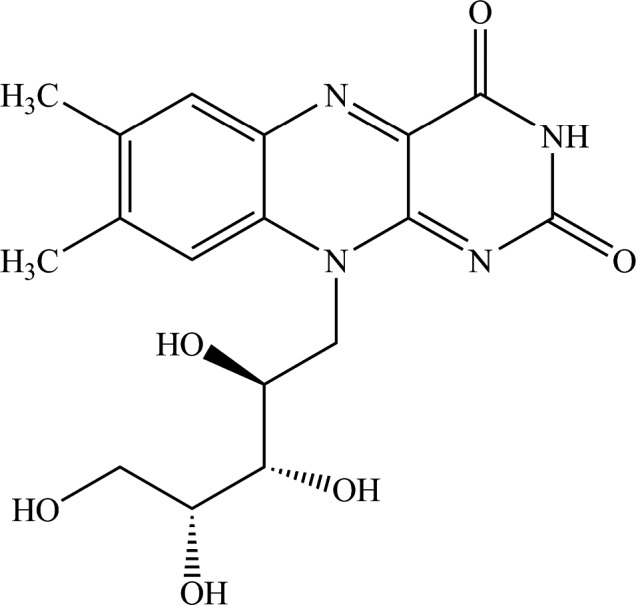
Structural formula of riboflavin
It is moderately soluble in water (10–100 mg/L at 20°C),18 and practically insoluble in ethanol, ether, alcohol, acetone and chloroform. Solutions are sensible to light, especially in the presence of alkali.
Riboflavin produced by fermentation is described in the European Pharmacopoeia monograph 0292 (PhEur, 2010) with a purity of 97–103.0% in the dried substance, less than 0.5% total related substances, < 0.025% impurity A (7,8,10‐trimethylbenzo[g]pteridine‐2,4(1H,3H)‐dione or lumiflavin), < 0.2% each for impurity B (7,8‐dimethylbenzo[g]pteridine‐2,4(3H,10H)‐dione or 8‐hydroxymethylriboflavin, 8‐HMR), impurity C (6,7‐dimethyl‐8‐[(2S,3S,4R)‐2,3,4,5‐tetrahydroxypentyl]‐pteridine‐2,4(3H,8H)‐dione or 6,7‐dimethyl‐8‐ribityllumazine, DMRL) and impurity D (hydroxymethyl)‐7‐methyl‐10‐[(2S,3S,4R)‐2,3,4,5‐tetrahydroxypentyl]benzo[g]pteridine‐2,4(3H,10H)‐dione or 8‐hydroxymethylriboflavin), loss on drying < 1.5% and sulfated ash < 0.1%.
3.1.2. Characterisation of the production organism
The production strain is a genetically modified strain of Ashbya gossypii and has been deposited in the German Collection of Microorganisms and Cell Cultures (DSMZ) under accession number DSM 23096.19 A. gossypii is a filamentous fungus evolutionarily related to the yeast Saccharomyces cerevisiae. The identity of the intermediate strain LU9868 as A. gossypii was confirmed by whole‐genome sequencing and gene sequence comparisons.20 Because LU9868 is a direct ancestor of the production strain during the genetic modification process, the identity of the production strain is confirmed as A. gossypii.
Regarding antibiotic or toxin production and presence of virulence factors, the applicant provided a comprehensive genome analysis ■■■■■21■■■■■ No clusters of concern were identified.
Antimicrobial activity of the additive (three batches) was tested ■■■■■22■■■■■ According to the National Research Council (NRC), requirements for vitamin B2 are in the range of 1.7–4.0 mg/kg feed for poultry (NRC, 1994), 2–4 mg/kg for pigs (NRC, 2012), 2.7–25 mg/kg for fish (NRC, 2011) and 2.0–4.2 mg/kg for companion animals (NRC, 2006, 2007a). Therefore, the product has no antimicrobial properties at use level.
3.1.2.1. Information on the genetically modified microorganism23
Characteristics of the recipient or parental microorganism
■■■■■
Characteristics of the donor organism
■■■■■
Description of the genetic modification process
The production strain Ashbya gossypii (DSM 23096) was developed ■■■■■
■■■■■20
Compared to the recipient strain, the production strain A. gossypii DSM 23096 ■■■■■ as a result of the genetic modification and shows enhanced vitamin B2 production. ■■■■■
3.1.3. Manufacturing process
The additive under assessment is produced by ■■■■■ fermentation of the production strain.24 After fermentation, ■■■■■
■■■■■25
■■■■■26
■■■■■27
3.1.4. Characterisation of the additive
The product under assessment is specified to contain a minimum of 80% riboflavin. The product comprises dried riboflavin crystals and up to 20% residual fermentation broth.28 Analysis (photometric determination based on PhEur M 2008:0292)29 of five production batches resulted in an average of 81.9% riboflavin ‘as is’ (range 81.8–82.1%), loss on drying 2.0% (1.9–2.2%) and residue on ignition 2.8% (2.6–3.3%).30 Lumiflavine (impurity A or 7,8,10‐trimethylbenzo[g]pteridine‐2,4(3H,10H)‐dione) and other substance‐related impurities as impurity B (7,8‐dimethylbenzo[g]pteridine‐2,4(1H,3H)‐dione), impurity C (6,7‐dimethyl‐8‐[(2S,3S,4R)‐2,3,4,5‐tetrahydroxypentyl]‐pteridine‐2,4(3H,8H)‐dione) and impurity D (8‐(hydroxymethyl)‐7‐methyl‐10‐[(2S,3S,4R)‐2,3,4,5‐tetrahydroxypentyl]benzo[g]pteridine‐2,4,(3H,10H)‐dione) were not detected in three batches of the product, except impurity C which was detected in one batch (0.6 g/kg) and was well below the threshold of the PhEur.31
The applicant declared that the spent growth medium contributes to the final product with up to 20% (1.5% crude ash, 4.3% crude fat, 1.0% crude protein, 2.6% crude fibre, 9.5% nitrogen‐free extract and 1.1% water (one batch analysed)).32
The optical rotation of the active substance ■■■■■33■■■■■
3.1.4.1. Impurities
Lead determined in five batches of the additive was < 5 mg/kg and arsenic < 1 mg/kg. In the same batches, total viable aerobic counts ranged from 7 × 102 to 9 × 104 colony‐forming units (CFU)/g.34 Three batches of the additive were also monitored for aflatoxin B1, deoxynivalenol, zearalenone, HT2‐toxin, T2‐toxin or ochratoxin A. All values were below the respective limits of detection (LOD).35 Other three batches of the additive were analysed for ■■■■■■■■■■36
3.1.4.2. Physico‐chemical characteristics
The additive is a yellow to orange free‐flowing powder.34 It has a bulk density of approximately 490 kg/m3.37 It has a melting point of approximately 280°C. Its solubility in water at 20°C is about 0.07 g/L.38
The particle size distribution of six batches of the additive was determined by laser diffraction. The fractions of particles with a diameter < 10 μm, < 50 μm and < 100 μm were in the range 2–10%, 8–33% and 28–58% (v/v), respectively.39 The dusting potential measured in three batches of the product (Stauber–Heubach method) ranged between 0.43 and 0.53 g/m3.40 The particle size of the dust was analysed in the same batches (same method) and the fractions of particles < 10 μm and < 50 μm were 37–39% and 99.9%, respectively.41
3.1.5. Stability and homogeneity
No loss in stability of the additive (three batches) when stored at 25°C in polyethylene bags with light protection indicated a shelf‐life of at least 36 months.42 It is noted that the active substance is sensitive to light and the stability data provided originate from samples kept in light proof containers.
The stability of the additive (three batches) was studied in a mineral premixture for chickens for fattening supplemented with 19.5 g riboflavin/kg when kept in aluminium bottles at 25°C or 30°C for up to 7 months. The premixture contained trace elements but did not contain choline chloride. No reduction of the riboflavin content (100% of the initial value) was found.43 The stability of the additive in premixtures containing choline chloride was not demonstrated.
The stability of three batches of the additive was also examined in a compound feed for chickens for fattening (mash and pelleted) based on wheat, maize and soybean meal. The feed supplementation level was 10 mg riboflavin/kg feed. The pelleting process (conditioning at 64–66°C and pelleting at 72–76°C) resulted in a loss of riboflavin ranging between 2 and 6%. Mash and pelleted feed samples were stored at 25°C or 30°C in aluminium tightly closed bottles for 16 weeks. Riboflavin recovery (% of the initial value) ranged between 74–80% in mash feed (both at 25 and 30°C), between 81–86% in pelleted feed at 25°C and 78–84% in pelleted feed at 30°C.44
The applicant tested the capacity of the additive to distribute homogeneously ■■■■■45■■■■■As the supplementation was much higher than the normal use level (2–25 mg riboflavin/kg feed), the FEEDAP Panel considers that the capacity of the additive to distribute homogeneously in feed at normal use levels is not proven.
3.1.6. Physico‐chemical incompatibilities in feed
No physico‐chemical incompatibilities or interactions have been reported between riboflavin and feed materials, carriers, other approved additives or medicinal products when the additive was added to premixtures and feed. No such incompatibilities or interactions are expected.
3.1.7. Conditions of use
Riboflavin is intended for use in feed for all animal species and categories without a maximum limit or a withdrawal period. It can be administered via premixtures or incorporated directly into complete/complementary feed.
3.2. Safety
3.2.1. Safety aspects of the genetic modification
The genome of ■■■■■, an intermediate strain in the development of the production strain DSM 23096, was sequenced and found to be free from gene clusters known to be involved in the production of toxic secondary metabolites. Therefore, the recipient strain ■■■■■ can be reasonably considered to be safe. A. gossypii DSM 23096 ■■■■■ and shows enhanced vitamin B2 production. None of these introduced sequences raise a safety concern. As demonstrated by Southern analysis, no antibiotic resistance genes from the genetic modification process remain in the production strain.
The applicant provided sufficient information that neither the production strain nor its recombinant DNA is present in the final product. The final product, manufactured by fermentation with A. gossypii DSM 23096, does not give rise to any safety concern with regard to the genetic modification of the production strain.
3.2.2. Metabolic and residue studies
Riboflavin absorption, its metabolic fate and its potential accumulation in edible tissues and eggs have been described in detail in previous opinions (EFSA FEEDAP Panel, 2014, 2016).
3.2.3. Toxicological studies
A bacterial reverse mutation test46 and an in vitro mammalian cell micronucleus test47 were performed with■■■■■A subchronic oral toxicity study performed with■■■■■was also submitted.48 As the strains share a common origin, the genetic modification does not introduce safety concerns and the concentration of riboflavin in the additive is ■■■■■ the FEEDAP Panel considers that results of the subchronic oral toxicity study ■■■■■ can be applied to the product of A. gossypii DSM 23096. Other toxicological studies with riboflavin of different origin have been assessed by the ANS Panel (EFSA ANS Panel, 2013) and the conclusion was that riboflavin ‘per se’ has a low toxicity.
3.2.3.1. Genotoxicity and mutagenicity
A bacterial reverse mutation test in accordance with OECD Guideline 471 was performed ■■■■■on Salmonella Typhimurium strains TA 1535, TA 100, TA 1537, TA 98 and E. coli WP2 uvrA.■■■■■46
■■■■■ was tested in an in vitro mammalian cell micronucleus test in human lymphocytes, in accordance with OECD Guideline 487.47 ■■■■■
The additive is considered devoid of mutagenic/genotoxic potential.
3.2.3.2. Subchronic oral toxicity
A 90‐day study was conducted according to OECD Guideline 408 ■■■■■49■■■■■
3.2.3.3. Conclusions on the toxicological studies
The available toxicological studies indicate no mutagenic/genotoxic effects and low toxicity, with a NOAEL of 410 mg/kg bw day based on the effects on urinary specific gravity and volume observed in female rats.
3.2.4. Safety for the target species
The nutrient requirements (NRC, 1994, 1995, 1996, 2001, 2006, 2007a,b, 2011, 2012, GfE, 1995, 1999, 2001, 2003, 2004, 2008, 2014) and recommendations (AWT, 2002) for the target species, their tolerance limits to riboflavin excess and the toxic effect of riboflavin depending on the administration route (NRC, 1987) were discussed in previous opinions (EFSA FEEDAP Panel, 2014, 2016). According to the National Research Council (NRC), requirements for vitamin B2 are in the range of 1.7–4.0 mg/kg feed for poultry (NRC, 1994), 2–4 mg/kg for pigs (NRC, 2012), 2.7–25 mg/kg for fish (NRC, 2011) and 2.0–4.2 mg/kg for companion animals (NRC, 2006, 2007a). The FEEDAP Panel concluded that riboflavin and riboflavin 5’‐phosphate sodium are safe for the target animals with a wide margin of safety, of about 20–60 compared to the supplementation levels.
Background values of vitamin B2 in feedingstuffs may range from 1–5 mg/kg in cereals, 1–60 mg/kg in some by‐products (e.g. brewery yeast; torula yeast) and 2–47 mg/kg in feed material of animal origin (e.g. liver meal; Kirchgessner et al., 2011).
The additive under assessment is obtained by fermentation. Since it contains 80% riboflavin and unidentified material of more than 1%, tolerance data in one target species or in a laboratory species would be required (EFSA FEEDAP Panel, 2012a). No tolerance studies in the target species were provided; however, the results of a subchronic oral toxicity study in rats performed ■■■■■ supports the safety of the product under assessment for the target animal species.
3.2.4.1. Conclusions on safety for the target species
The use of riboflavin produced by Ashbya gossypii DSM 23096 as nutritional additive in feed is safe for the target animals with a wide margin of safety.
3.2.5. Safety for the consumer
The results of available toxicological information indicate that the additive under application (riboflavin, 80%) has no mutagenic/genotoxic effects and low toxicity and is as safe as riboflavin from other sources.
The EFSA ANS Panel (2013) concluded that riboflavin and riboflavin‐5′‐phosphate sodium are unlikely to be of safety concern at the currently authorised uses and use levels as food additives.
The EFSA FEEDAP Panel, based on the facts that:
supplementation of animal feed with riboflavin has been a common practice for decades and it can be assumed that the exposure estimates made by ANS Panel from non‐supplemented foods already include the potential influence of riboflavin supplementation of feed at the practical use levels;
differences in use levels of feed supplementation do not significantly alter tissue/product deposition (EFSA FEEDAP Panel, 2014, 2016)
concludes that the supplementation of feed with riboflavin would not modify the current consumer exposure to riboflavin.
3.2.5.1. Conclusions on safety for the consumer
The available toxicological studies indicate no mutagenic/genotoxic effects and low toxicity. The use of the additive under assessment in animal nutrition will not significantly alter the riboflavin content of food of animal origin. The FEEDAP Panel considers that the use of riboflavin produced by Ashbya gossypii DSM 23096 in animal nutrition is not of safety concern for consumers.
3.2.6. Safety for the user
Several studies were made available by the applicant. Of those, the acute inhalation toxicity study and the local lymph node assay were performed with ■■■■■, but the product used in the local lymph node assay had a higher concentration of riboflavin than the product under assessment (92%). The eye irritation and skin irritation studies were performed with riboflavin produced ■■■■■ and the concentration of riboflavin in the final product was unknown.48 Therefore, the results of the skin and eye irritation studies are considered only as supportive evidence that riboflavin is not skin or eye irritant.
3.2.6.1. Effects in the respiratory system
The fractions of particles with a diameter < 10 μm, < 50 μm and < 100 μm were in the range 2–10%, 8–33% and 28–58% (v/v), respectively. The dusting potential ranged between 0.43 and 0.53 g/m3.50
■■■■■ was tested in an acute inhalation toxicity study ■■■■■51■■■■■
The study does not allow to set a no observed effect concentration (NOEC) for the observed treatment‐related effects; therefore, the FEEDAP Panel cannot draw a final conclusion on the risk posed for the user by inhalation. It is noted that since there is not a final formulation subject to the authorisation, different forms of the additive can be produced having different physical properties.
3.2.6.2. Effects on eyes and skin
A 50% aqueous preparation of ■■■■■ riboflavin (purity unknown) in water was applied in a Draize (1959), skin irritation test on a patch with a layer thickness of 0.5 mm under occlusive wrapping to the intact skin on the backs of three male and three female rabbits.52 The exposed skin area was 2.5 cm × 2.5 cm. After removal of the patch, substance remnants were wiped off. Effects were scored after 15–30 min, at 24, 48, 72 h and 8 days after start of exposure. No irritation effects were noted. Based on this result, riboflavin was rated as not irritating to skin.
In an eye irritation test according to Draize (1959), 22 mg of ■■■■■ riboflavin (purity unknown) was applied to the eyes of two female and four male white rabbits as a single application into the conjunctival sac.53 Readings of irritation were performed 1, 24, 48 and 72 h after application. The test substance was not washed out. Slight irritation of the mucous membrane was noted in one animal, which was reversible within 2 days. Based on this result, riboflavin was rated as not irritating to the eyes.
The FEEDAP Panel considers that since these assays were not performed with the product under assessment, the results cannot be directly extrapolated to riboflavin (80%) produced using Ashbya gossypii DSM 23096. Nevertheless, they provide reassurance that the active substance riboflavin does not possess any significant potential for skin and eye irritancy.
Riboflavin ■■■■■ was tested for skin sensitisation in the local lymph node assay according to the OECD Guideline 429 in mice.54 In this study, stimulation indexes of 1.1, 1.4 and 1.5 were determined with the test item at concentrations of 5, 10 and 25% (w/v) in N,N‐dimethylformamide (DMF), respectively. Riboflavin fine powder was therefore found to be a non‐sensitiser for the skin when tested up to the highest applicable concentration of 25% in DMF.
Photoallergenic skin reaction has been shown with riboflavin on guinea pigs (Joshi and Pathak, 1987). In addition, riboflavin is a recognised photosensitiser inducing oxidative damage to light‐exposed tissues; therefore, it may elicit skin and eye photoallergic reactions (Cardoso et al., 2012).
3.2.6.3. Conclusions on safety for the user
FEEDAP Panel cannot draw a final conclusion on the risk posed for the user by inhalation of riboflavin produced by A. gossypii DSM 23096. The product under assessment is not a skin sensitiser; however, riboflavin is a known photosensitiser.
Data provided demonstrating that the active substance riboflavin is not a skin or eye irritant do not allow to conclude on the irritancy potential of the product under assessment.
3.2.7. Safety for the environment
The active substance riboflavin occurs in nature. Its use in animal nutrition is not expected to substantially increase the concentration in the environment. Therefore, a risk for the environment resulting from the use of riboflavin in animal nutrition is not foreseen.
Neither the production strain nor its recombinant DNA was detected in the final product. The riboflavin produced with A. gossypii DSM 23096 does not give raise to environmental safety concerns with respect to the genetic modification of the production strain.
3.3. Efficacy
Vitamin B2 has been used worldwide in animal nutrition for decades. Dietary requirements are set for domestic animals except for ruminants, owing to microbial synthesis of riboflavin in the rumen (GfE, 1995, 2001, 2003; NRC, 1996, 2001, 2007b). Owing to the long history of use and its established nutritional role in domestic animals, riboflavin is regarded as effective in covering the animal's requirement. Data on requirement, allowances and recommendations for feed supplementation are easily accessible in the standard literature on animal nutrition.
The FEEDAP Panel considers that riboflavin from Ashbya gossypii DSM 23096 is effective in covering the animal's requirement when administered via feed.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those set out in the Feed Hygiene Regulation55 and Good Manufacturing Practice.
4. Conclusions
Neither the production strain A. gossypii DSM 23096 nor its recombinant DNA was detected in the final product. Vitamin B2 (riboflavin 80%), manufactured by fermentation with A. gossypii DSM 23096, does not give rise to any safety concern with regard to the genetic modification of the production strain.
Riboflavin produced by A. gossypii DSM 23096 is safe for the target animals with a wide margin of safety.
The use of riboflavin 80% produced by A. gossypii DSM 23096 in animal nutrition does not represent a safety concern for consumers.
FEEDAP Panel cannot draw a final conclusion on the risk posed for the user by inhalation of riboflavin produced by A. gossypii DSM 23096 and on the potential to be irritant to skin or eyes. The product under assessment is not a skin sensitiser; however, riboflavin is a known photosensitiser.
The use of riboflavin produced by A. gossypii DSM 23096 in animal nutrition does not pose a risk to the environment.
Riboflavin produced by A. gossypii DSM 23096 is regarded as effective in covering the animal's requirement when administered via feed.
5. Recommendations
The description of the additive (riboflavin 80%) should contain the statement ― ‘produced by fermentation with Ashbya gossypii DSM 23096.’
Documentation provided to EFSA
Vitamin B2 in the form of riboflavin as a feed additive for all animal species. November 2010. Submitted by BASF SE.
Vitamin B2 in the form of riboflavin as a feed additive for all animal species. Supplementary information. March 2015. Submitted by BASF SE.
Vitamin B2 in the form of riboflavin as a feed additive for all animal species. Supplementary information. August 2015. Submitted by BASF SE.
Vitamin B2 in the form of riboflavin as a feed additive for all animal species. Supplementary information. June 2016. Submitted by BASF SE.
Vitamin B2 in the form of riboflavin as a feed additive for all animal species. Supplementary information. August 2017. Submitted by BASF SE.
Evaluation report of the European Union Reference Laboratory for Feed Additives on the methods(s) of analysis for vitamin B2.
Comments from Member States.
Chronology
| Date | Event |
|---|---|
| 14/11/2012 | Dossier received by EFSA |
| 12/11/2012 | Reception mandate from the European Commission |
| 23/05/2013 | Application validated by EFSA – Start of the scientific assessment |
| 29/05/2013 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation, manufacturing process and safety for the user |
| 08/07/2013 | Complementary request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended on 29/05/2013. Issues: characterisation of the production strain |
| 23/08/2013 | Comments received from Member States |
| 03/09/2013 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 02/03/2015 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 21/05/2015 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended Issues: Characterisation of the production strain, characterisation of the additive (impurities) |
| 24/08/2015 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 14/01/2016 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended Issues: Characterisation of the additive, characterisation of the strain, safety for the consumer and for the user |
| 14/06/2016 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 19/07/2016 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended Issues: Safety for the consumer |
| 18/08/2017 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 13/06/2018 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- ANS
EFSA Panel on Food Additives and Nutrient Sources added to food
- bw
body weight
- CAS
Chemical Abstracts Service
- DMF
N,N‐dimethylformamide
- DMRL
6,7‐dimethyl‐8-ribityl‐lumazine
- DSMZ
German Collection of Microorganisms and Cell Cultures
- EINECS
European Inventory of Existing Chemical Substances
- EURL
European Union Reference Laboratory
- FAD
Flavin adenine dinucleotide
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- FMN
Flavin mononucleotide
- GfE
Gesellschaft für Ernährungsphysiologie
- GMO
EFSA Panel on Genetically Modified Organisms
- 8‐HMR
8‐hydroxymethyl‐riboflavin
- IUPAC
International Union of Pure and Applied Chemistry
- KCCM
Korean Culture Center of Microorganisms
- LC50
median lethal concentration
- LOD
Limit of detection
- MIC
minimum inhibitory concentration
- NOAEL
no observed adverse effect level
- NOEC
no observed effect concentration
- NRC
National Research Council
- OECD
Organisation for Economic Co‐operation and Development
- PCR
Polymerase chain reaction
- PhEur
European Pharmacopoeia
- SCF
Scientific Committee on Food
- TEF
Thyrotroph embryonic factor
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for Vitamin B2
1.
In the current application, authorisation is sought under articles 10(2) for Vitamin B2 (Riboflavin) under the category/functional group 3(a) ‘nutritional additives’/’vitamins, provitamins and chemically well defined substances having similar effect’ according to Annex I of Regulation (EC) No 1831/2003. Authorisation is sought for the use of the feed additive for all animal species and categories. Riboflavin is produced by fermentation using Ashbya gossypii strains. According to the Applicant, the feed additive contains at least 80% of riboflavin. The feed additive is intended to be incorporated in feedingstuffs, directly or through premixtures. The Applicant did not specify any maximum or minimum concentration of Vitamin B2 in feedingstuffs; however, typical inclusion levels range from 3 to 20 mg/kg feedingstuffs.
For the determination of riboflavin per se (with a minimum purity of 97%), the European Union Reference Laboratory (EURL) proposes the European Pharmacopoeia method (Ph. Eur. 6.0, 01/2008:0292). Identification is based on specific optical rotation, thin‐layer chromatography and ultraviolet/visible spectrophotometry while quantification is based on spectrophotometry at 444 nm. The EURL recommends this method for official control to determine riboflavin per se.
For the determination of riboflavin in premixtures and feedingstuffs, the Applicant submitted the AOAC 940.33 microbiological method based on the titration with Lactobacillus casei. However, the EURL evaluated already several analytical methods in the frame of the FAD‐ 2010‐0304 dossier and recommended for official control: the VDLUFA Bd. III, 13.9.1 method, using ion pair reversed phase high‐performance liquid chromatography coupled to UV detector (HPLC‐UV), to determine riboflavin in premixtures; and ‐ the EN 14152 method based on acidic hydrolysis and enzymatic dephosphorylation followed by HPLC with fluorescence detector, to determine riboflavin (as total Vitamin B2) in feedingstuffs.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Suggested citation: The EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wester P, Costa L, Dierick N, Glandorf B, Herman L, Kärenlampi S, Leng L, Tebbe C, Aguilera J, Manini P, Tarrés‐Call J and Wallace RJ,2018. Scientific Opinion on the safety and efficacy of vitamin B2 (riboflavin) produced by Ashbya gossypii DSM 23096 for all animal species based on a dossier submitted by BASF SE. EFSA Journal 2018;16(7):5337, 19 pp. 10.2903/j.efsa.2018.5337
Requestor: European Commission
Question number: EFSA‐Q‐2012‐00953
Panel members: Gabriele Aquilina, Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Georges Bories, Andrew Chesson, Pier Sandro Cocconcelli, Gerhard Flachowsky, Jürgen Gropp, Boris Kolar, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Alberto Mantovani, Baltasar Mayo, Fernando Ramos, Guido Rychen, Maria Saarela, Roberto Edoardo Villa, Robert John Wallace and Pieter Wester.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the European Commission decision on the confidentiality requests formulated by the applicant. A previous, provisional version of this output which had been made publicly available pending the adoption of the decision has been replaced by this version. The full output has been shared with the European Commission, EU Member States and the applicant.
Acknowledgements The EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) wishes to thank the following for the support provided to this scientific output: Jaume Galobart, Lucilla Gregoretti, Orsolya Holczknecht, Gloria López Gálvez, and Paola Manini.
Adopted: 13 June 2018
Amended: 19 Feb 21
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
BASF SE, Carl‐Bosch‐Strasse, 38, 67056 Ludwigshafen, Germany.
A new mandate was received in EFSA on 12/11/2012.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 29.
Commission Regulation (EU) 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 15, 20.1.2010, p. 1.
Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetics product (recast). OJ L 342 22.12.2009, p. 59.
Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. OJ L 404 30.12.2006, p. 26, last amended by Commission Regulation (EC) No 1170/2009 of 30 November 2009 amending Directive 2002/46/EC of the European Parliament and of Council and Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards the lists of vitamin and minerals and their forms that can be added to foods, including food supplements. OJ L 314 1.12.2009, p. 36.
Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. OJ L 183 12.7.2002, p. 51.
Commission Regulation (EC) 953/2009 of 13 October 2009 on substances that may be added for specific nutritional purposes in foods for particular nutritional uses. OJ L 269, 14.10.2009, p. 9.
Commission Directive 2006/125 EC of 5 December 2006 on processed cereal‐based foods and baby foods for infants and young children. OJ L 339 6.12.2006, p. 16.
Commission Directive 2006/141 EC of 22 December 2006 on infant formulas and follow‐on formulas and amending Directive 1999/21/EC. OJ L 401 30.12.2006, p. 1.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83 22.3.2012, 295 pp.
2004/C 50/01. OJ C 50 25.2.2004, 144 pp. Council Directive of 23 November 1970 concerning additives in feedingstuffs 70/524/EEC. OJ L 270 14.12.70.
European Union Register of Feed Additives pursuant to Regulation (EC) No 1831/2003. Available online: http://ec.europa.eu/food/food/animalnutrition/feedadditives/comm_register_feed_additives_1831-03.pdf
FEED dossier reference: FAD‐2010‐0177.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/default/files/FinRep-FAD-2010-0177-Riboflavin.pdf
Technical dossier/Section II.2.2.2.1.
Technical dossier/Section II/Annex II.6.
Technical dossier/Supplementary information August 2015.
Technical dossier/Supplementary information June 2016 and March 2015/Annex G ■■■■■
Technical dossier/Supplementary information June 2016/Annex 3■■■■■
Technical dossier/Supplementary information March 2015.
Technical dossier/Section II.2.3.1.
Technical dossier/Supplementary information June 2016/Annex 5.
Technical dossier/Supplementary information March 2015/Annex M.
Technical dossier/Supplementary information August 2015/Annex B.
Technical dossier/Section II.2.1.3.
Technical dossier/Supplementary information June 2016/Annex 1.
Technical dossier/Section II/Annex 2.1.
Technical dossier/Supplementary information March 2015/Annex A. High‐performance liquid chromatography analyses with limit of detection (in g/100 g) of 0.025 for impurity A –lumiflavin‐, and 0.05 for impurities B, C and D.
Technical dossier/Section II/Annex 2.4.
Technical dossier/Supplementary information June 2016/Annex 2.
Technical dossier/Section II/Annex II.1.
Technical dossier/Section II/Annex 2.3. LOD (in µg/kg) was 0.3 for aflatoxin B1, 10 for deoxynivalenol, 5 for HT2‐toxin and ochratoxin‐A, and 1 for zearalenone and T2‐toxin.
Technical dossier/Supplementary information March 2015/Annex L. ■■■■■
Technical dossier/Section II.2.1.5 and Annex II.1.
Technical dossier/Section II/Annex II.13.
Technical dossier/Section II/Annex 2.5.
Technical dossier/Supplementary information March 2015/Annex D.
Technical dossier/Supplementary information March 2015/Annex E.
Technical dossier/Section II.2.4.1 and Annex 2.9.
Technical dossier/Section II/Annex 2.10.
Technical dossier/Section II/Annex 2.11 and Annex 2.12.
Technical dossier/Supplementary information June 2016/Annex 6.
Technical dossier/Supplementary information August 2017/Appendix 1.
Technical dossier/Supplementary information August 2017/Appendix 2.
Technical dossier/Supplementary information June 2016/Response to Q8.
Technical dossier/Section III/Annex III.7.
Technical dossier/Section II/Annex II.5 and Supplementary information March 2015/Annexes E and D.
Technical dossier/Section III/Annex III.6 and Supplementary information June 2016/Response to Q8.
Technical dossier/Section III/References annex III‐1/83‐88‐5 Skin irrit rabbit.
Technical dossier/Section III/References annex III‐1/83‐88‐5 Eye irrit rabbit.
Technical dossier/Section III/Annexes Section III/References annex III‐1/83‐88‐5 Local lymph node and Supplementary information June 2016/Response to Q8.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- AWT (Arbeitsgemeinschaft für Wirkstoffe in der Tierernährung e.V.), 2002. Vitamins in animal nutrition, Agrimedia GmbH, ISBN 3‐86037‐167-3.
- Cardoso DR, Libardi SH and Skibsted LH, 2012. Riboflavin as a photosensitizer. Effects on human health and food quality. Food & Function, 3, 487–502. [DOI] [PubMed] [Google Scholar]
- Dietrich FS, Voegeli S, Brachat S, Lerch A, Gates K, Steiner S, Mohr C, Pöhlmann R, Luedi P, Choi S, Wing RA, Flavier A, Gaffney TD and Philippsen P, 2004. The Ashbya gossypii genome as a tool for mapping the ancient Saccharomyces cerevisiae genome. Science, 304, 304–307. [DOI] [PubMed] [Google Scholar]
- Draize JH, 1959. Appraisal of the safety of chemicals in foods, drugs and cosmetics. In: Dermal Toxicity, pp. 46–59. Association of Food and Drug Officials of the United States, Texas, State Department of Health. Austin, Texas, USA.
- EFSA (European Food Safety Authority), 2008a. Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for the preparation of dossiers for the re‐evaluation of certain additives already authorised under Directive 70/524/EEC. EFSA Journal 2008;6(9):779, 9 pp. 10.2903/j.efsa.2008.779 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008b. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008c. Technical Guidance: Microbial Studies. EFSA Journal 2008;6(10):836, 3 pp. 10.2903/j.efsa.2008.836 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Opinion of the Scientific Panel on Food Additive and Nutrient Sources added to food on the inability to assess the safety of riboflavin‐enriched yeast added for nutritional purposes as a source of riboflavin in food supplements and the bioavailability of riboflavin from this source, based on the supporting dossier. EFSA Journal 2009;7(6):1135, 6 pp. 10.2903/j.efsa.2009.1135 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additive and Nutrient Sources added to food), 2013. Scientific Opinion on the re‐evaluation for Riboflavin (E101(i)) and Riboflavin‐5′‐phosphate (E 101(ii)). EFSA Journal 2013;11(10):3357, 49 pp. 10.2903/j.efsa.2013.3357 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2011. Technical guidance: Tolerance and efficacy studies in target animals. EFSA Journal 2011;9(5):2175, 15 pp. 10.2903/j.efsa.2011.2175 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for the preparation of dossiers for nutritional additives. EFSA Journal 2012;10(1):2535, 14 pp. 10.2903/j.efsa.2012.2535 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance for establishing the safety of additives for the consumer. EFSA Journal 2012;10(1):2537, 12 pp. 10.2903/j.efsa.2012.2537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014. Scientific Opinion on the safety and efficacy of vitamin B2 (80%) as riboflavin produced by Bacillus subtilis for all animal species, based on a dossier submitted by VITAC EEIG. EFSA Journal 2014;12(1):3531, 2 pp. 10.2903/j.efsa.2014.3531 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016. Scientific Opinion on Safety and efficacy of vitamin B2 (riboflavin and riboflavin 5’‐phosphate ester monosodium salt) produced by Bacillus subtilis (DSM 17339 and DSM 23984) for all animal species based on a dossier submitted by DSM. EFSA Journal 2016;14(1):4349, 26 pp. 10.2903/j.efsa.2016.4349 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances usedin Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Herman L, Glandorf B, Karenlampi S and Aguilera Jand Cocconcelli PS, 2018. Scientific Opinion on the safety of vitamin B2 (80%) as riboflavin produced by Bacillus subtilis KCCM‐10445 for all animal species. EFSA Journal 2018;16(3):5223, 8 pp. 10.2903/j.efsa.2018.5223 [DOI] [Google Scholar]
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms), 2011. Scientific Opinion on Guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use. EFSA Journal 2011;9(6):2193, 54 pp. 10.2903/j.efsa.2011.2193 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2010. Scientific Opinion on the substantiation of health claims related to riboflavin (vitamin B2) and contribution to normal energy‐yielding metabolism (ID 29, 35, 36, 42), contribution to normal metabolism of iron (ID 30, 37), maintenance of normal skin and mucous membranes (ID 31, 33), contribution to normal psychological functions (ID 32), maintenance of normal bone (ID 33), maintenance of normal teeth (ID 33), maintenance of normal hair (ID 33), maintenance of normal nails (ID 33), maintenance of normal vision (ID 39), maintenance of normal red blood cells (ID 40), reduction of tiredness and fatigue (ID 41), protection of DNA, proteins and lipids from oxidative damage (ID 207), and maintenance of the normal function of the nervous system (ID 213) pursuant to Article 13(1) of Regulation (EC) No. 1924/2006. EFSA Journal 2010;8(10):1814, 28 pp. 10.2903/j.efsa.2010.1814 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2012. Scientific Opinion on the substantiation of a health claim related to a combination of thiamin, riboflavin, niacin, pantothenic acid, pyridoxine, D‐biotin and pumpkin seed oil and maintenance of normal hair pursuant to Article 13(5) of Regulation (EC) No. 1924/2006. EFSA Journal 2012;10(7):2807, 8 pp. 10.2903/j.efsa.2012.2807 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013a. Scientific Opinion on the substantiation of a health claim related to the combination of artichoke leaf dry extract standardised in caffeoylquinic acids, monacolin K in red yeast rice, sugar‐cane derived policosanols, OPC from French maritime pine bark, garlic dry extract standardised in allicin, d‐α‐tocopheryl hydrogen succinate, riboflavin and inositol hexanicotinate in Limicol® and reduction of blood LDL‐cholesterol concentrations pursuant to Article 14 of Regulation (EC) No. 1924/2006. EFSA Journal 2013;11(7):3327, 16 pp. 10.2903/j.efsa.2013.3327 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013b. Scientific Opinion on the substantiation of a health claim related to riboflavin (vitamin B2) and contribution to normal energy‐yielding metabolism pursuant to Article 14 of Regulation (EC) No. 1924/2006. EFSA Journal 2013;11(10):3410, 10 pp. 10.2903/j.efsa.2013.3410 [DOI] [Google Scholar]
- European Commission , 1998. Opinion on Riboflavin as a colouring matter authorized for use in foodstuffs produced by fermentation using genetically modified Bacillus subtilis. Available online: http://ec.europa.eu/food/fs/sc/scf/out18_en.html
- European Commission , 2000. Opinion of the Scientific Committee on Food on the Upper Tolerable Intake of Vitamin B2 . Available online: http://ec.europa.eu/food/fs/sc/scf/out80i_en.pdf
- GfE (Gesellschaft für Ernährungsphysiologie), 1995. Recommendations for the supply of energy and nutrients to beef cattle, DLG‐Verlag, Frankfurt am Main, 85 pp. [in German]
- GfE (Gesellschaft für Ernährungsphysiologie), 1999. Recommendations for the supply of energy and nutrients to laying hens and chicken for fattening (broilers). DLG‐Verlag, Frankfurt am Main, 185 pp. [in German]
- GfE (Gesellschaft für Ernährungsphysiologie), 2001. Recommendations for the supply of energy and nutrients to dairy cows and heifers. DLG‐Verlag, Frankfurt am Main, 136 pp. [in German]
- GfE (Gesellschaft für Ernährungsphysiologie), 2003. Recommendations for the supply of energy and nutrients to goats. DLG‐Verlag, Frankfurt am Main, 121 pp.
- GfE (Gesellschaft für Ernährungsphysiologie), 2004. Recommendations for the supply of energy and nutrients to fattening turkeys (in German). Proceedings of the Society of. Nutrition Physiology, 13, 199–233. [Google Scholar]
- GfE (Gesellschaft für Ernährungsphysiologie), 2008. Recommendations for the supply of energy and nutrients to pigs. DLG‐Verlag, Frankfurt am Main, 245 pp.
- GfE (Gesellschaft für Ernährungsphysiologie), 2014. Recommendations to the supply of energy and nutrients to horses, DLG‐Verlag, Frankfurt am Main, 190 pp. [in German]
- Joshi PC and Pathak MA, 1987. Skin photosensitizing effects of riboflavin and its photodegradation products. Photochemistry and Photobiology, 45, 99S. [Only an abstract of this study was available]. [Google Scholar]
- Kirchgessner M, Roth FX, Schwarz FJ and Stangl GL, 2011. Animal Nutrition: Guide for study, service and practice. 13th Edition, DLG‐Verlag Frankfurt, 648 pp. [in German]
- NRC (National Research Council), 1987. Vitamin tolerance of animals. The National Academies Press, Washington, DC, USA. pp. 53–57. [Google Scholar]
- NRC (National Research Council), 1994. Nutrient requirements of poultry: 9th, Revised edition. The National Academies Press, Washington DC, USA. p. 176. [Google Scholar]
- NRC (National Research Council), 1995. Nutrient requirements of laboratory animals: 4th, Revised edition. The National Academies Press, Washington DC, USA. p. 173. [Google Scholar]
- NRC (National Research Council), 1996. Nutrient requirements of beef cattle: 7th, Revised edition. The National Academies Press, Washington DC, USA. p. 232. [Google Scholar]
- NRC (National Research Council), 2001. Nutrient requirements of dairy cattle: 7th, Revised edition. The National Academies Press, Washington DC, USA. p. 381. [Google Scholar]
- NRC (National Research Council), 2006. Nutrient requirements of dogs and cats. The National Academies Press, Washington DC, USA. p. 398. [Google Scholar]
- NRC (National Research Council), 2007a. Nutrient requirements of horses. The National Academies Press, Washington DC, USA. p. 341. [Google Scholar]
- NRC (National Research Council), 2007b. Nutrient requirements of small ruminants. Sheep, goats, cervids, and new world camelids. The National Academies Press, Washington DC, USA. p. 362. [Google Scholar]
- NRC (National Research Council), 2011. Nutrient requirements of fish and shrimp. The National Academies Press, Washington DC, USA. p. 376. [Google Scholar]
- NRC (National Research Council), 2012. Nutrient requirements of swine, 11th, Revised edition. The National Academies Press, Washington DC, USA. p. 400. [Google Scholar]
- PhEur (European Pharmacopeia), 2010. Riboflavin, Monograph (MG) 0292. 7th Edition. Strasbourg, France. Council of Europe (COE)—European Directorate for the Quality of Medicines.
- Stahmann KP, Revuelta JL and Seulberger H, 2000. Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical riboflavin production. Applied Microbiology and Biotechnology, 53, 509–516. [DOI] [PubMed] [Google Scholar]
- VDLUFA III 28.4.1 , 2007. Mikrobiologisches Verfahren zum Nachweis von antimikrobiell virksamen Substanzen; Grundmodul (Screening). VDLUFA Methodenbuch III (7. Erg.)


