Abstract
Following a request from the European Commission, EFSA periodically updates the database on the host plants of Xylella spp. While previous editions of the database (2015 and 2016) dealt with the species Xylella fastidiosa only, this database version addresses the whole genus Xylella, including therefore both species X. fastidiosa and Xylella taiwanensis. The database now includes information on host plants of Xylella spp. retrieved from scientific literature up to November 2017 and from EUROPHYT notifications up to May 2018. An extensive literature search was performed to screen the scientific and technical literature published between the previous database update conducted in December 2015 and December 2017. The literature screening was supported by the DistillerSR software platform. The applied protocol for the extensive literature review and extensive information search, together with examples of data extraction, are described in detail in this report. This report also includes published information on resistance or tolerance of plant varieties to Xylella spp. The current database includes 563 plant species reported to be infected by X. fastidiosa, of which for 312 plant species the infection has been determined with at least two different detection methods. These species cover hundreds of host plant genera in 82 botanical families (61 botanical families when considering only records with at least two different detection methods). The update of this database of host plants of Xylella spp. reported world‐wide provides a key tool for risk management, risk assessment and research on this polyphagous bacterial plant pathogen.
Keywords: data extraction, host plants database, literature review, sequence type, ST, subspecies, Xylella fastidiosa, Xylella taiwanensis
1. Introduction
Xylella spp. is a well‐studied plant pathogenic bacterium (Janse and Obradovic, 2010; Purcell, 2013; Almeida and Nunney, 2015). Xylella fastidiosa is listed as one of the top 10 plant pathogenic bacteria in Molecular Plant Pathology based on a survey among the international community (Mansfield et al., 2012). It is known to cause many different diseases like Pierce's disease of grapes in California, citrus variegated chlorosis in Brazil, bacterial leaf scorch in shaded trees in North America, oleander leaf scorch in California, olive diseases in Europe. Xylella spp. are polyphagous pathogens. They can cause severe diseases, but might also remain asymptomatic, without causing any serious damage. To understand a host range and host–bacteria relationship, it is necessary to review existing studies and ongoing research with new techniques of identification of the pathogen (Baldi and La Porta, 2017).
The first list of host plant species of X. fastidiosa published by the European Food Safety Authority (EFSA) was compiled in 2013 on the basis of the online list provided by the University of Berkeley in California (EFSA, 2013) and it was focused mostly on the strains/subspecies related to Pierce's disease – disease of grapevine.
In January 2015, EFSA published a Scientific Opinion of the EFSA PLH Panel on the risk to plant health posed by X. fastidiosa in the EU territory (EFSA PLH Panel, 2015), which included a table (Appendix B in EFSA PLH Panel, 2015) listing known host plants of X. fastidiosa together with the relevant references for each of them. This table provided information on host plant species, their botanical family, the country and location of the records, the detection methods used and also the subspecies (recorded from the publication and putatively assigned on the basis of the strain, host plant and location). In 2015, EFSA published an electronic version of the database of the host plants of X. fastidiosa, together with a categorisation of plants for planting on the risk of introduction of X. fastidiosa (EFSA, 2015). EFSA updated its X. fastidiosa host plant database at the end of 2015 (EFSA, 2016). Some preliminary results from the current EFSA Xylella spp. host plant database were presented at the European Conference on Xylella fastidiosa 1 held in Palma de Mallorca in November 2017 (Figure 1).
Figure 1.
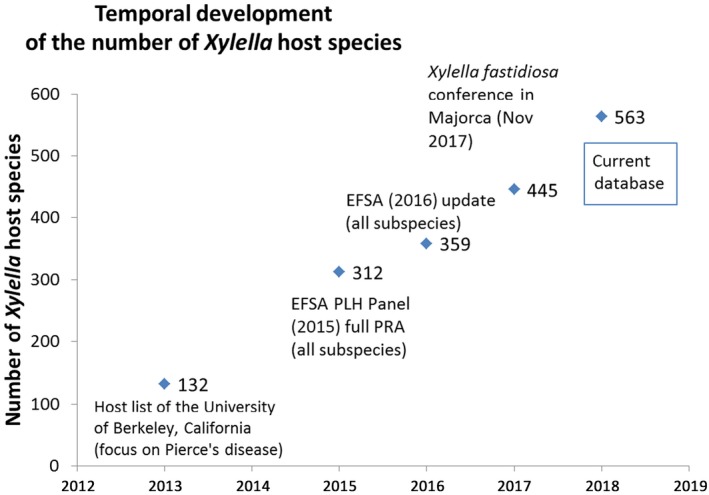
Temporal pattern of the total number of reported Xylella spp. host plants (2013–2018)
The increase in the total number of hosts of Xylella spp. reported in the database from 2013 to 2018 is particularly related to the new plant species reported being infected by X. fastidiosa in Europe since 2013. However, it also reflects the most extensive coverage of scientific literature in all languages and the inclusion of reports on the other species Xylella taiwanensis.
1.1. Background
The extensive literature search (ELS) protocol was used in the context of the EFSA mandate M‐2013‐0321 on urgent technical assistance on the regulated harmful organism Xylella spp. periodical updates of a database of host plants of Xylella spp. (EFSA‐Q‐2017‐00215). This update integrates the list of X. fastidiosa host plants, published on 20 November 2016.
1.2. Terms of Reference as provided by the requestor
EFSA was asked to deliver by the end of March 2017 a preliminary report on the hosts of the Apulian strain of Xylella fastidiosa subsp. pauca, which was delivered within the deadline (EFSA, 2017).
EFSA is asked to further specify and update the host plants database of Xylella fastidiosa currently available, taking into account the different Xylella fastidiosa subspecies and strains (with particular reference to the European isolates), with inclusion of information on non‐susceptible host plants and varieties and negative results of diagnostic tests when available. EFSA is asked to maintain and update this database periodically and to make new releases available on the EFSA website, together with a report. Such report should specify the list of plants confirmed to be infected by at least two detection methods in field conditions or via vector transmission under experimental conditions and be published at least annually.
1.3. Interpretation of the Terms of Reference
This scientific report provides a description of the methodology of the review undertaken, approach made to list the hosts and results obtained from this review (sample size, time span cover of the literature extracted, exclusion criteria for publications, etc.), as well as a detailed view on the different host plants listed (diagnostic tests used – including negative tests, isolates, subspecies and sequence types, susceptibility/resistance information retrieved from the publications on different hosts). This edition of the database covers both species Xylella fastidiosa and Xylella taiwanensis.
2. Data and methodologies
The process was divided into the following steps:
An extensive literature search to identify the relevant references.
A selection of the identified studies based on titles, abstracts and full text.
Data extraction of the relevant information from the selected references for the creation of an updated global database of Xylella host plants.
Data analysis and reporting (EFSA data warehouse).
2.1. Extensive literature search
During the search process, two main aspects were considered: the sources of information (literature databases) to be consulted (Table 1) and the development of the search strategy (Table 2).
Table 1.
Sources of information
| Database | Time coverage | Platform |
|---|---|---|
| Web of Science Core Collection | 1975–present | Web of Science |
| CABI: CAB Abstracts | 1973–present | Web of Science |
| BIOSIS Citation Index | 1926–present | Web of Science |
| Chinese Science Citation Database | 1989–present | Web of Science |
| Current Contents Connect | 1998–present | Web of Science |
| Data Citation Index | 1900–present | Web of Science |
| FSTA | 1969–present | Web of Science |
| KCI‐Korean Journal Database | 1980–present | Web of Science |
| Russian Science Citation Index | 2005–present | Web of Science |
| MEDLINE | 1950–present | Web of Science |
| SciELO Citation Index | 1997–present | Web of Science |
| Zoological Record | 1864–present | Web of Science |
Table 2.
Search string applied
| Search string | Platform: Web of Science |
|---|---|
| Results | |
| TS=(xylella OR xyllela OR xylela OR (pierce* NEAR/2 disease) OR (((Plum OR plums) AND “leaf scald*”)) OR ((Phony NEAR/2 (peach* OR disease*))) OR ((citrus AND variegat* AND chlorosis)) OR crespera OR “almond leaf scorch*” OR “bacterial leaf scorch*” OR “coffee leaf scorch*” OR “mulberry leaf scorch*” OR “oleander leaf scorch*” OR “sycamore leaf scorch*” OR “Periwinkle wilt” OR “Ragweed stunt” OR ((Olive NEAR “quick decline syndrome”)) OR “Xylem inhabiting bacteri*” OR “Xylem limited bacteri*” OR FXIB OR FXJB OR “rickettsialike bacteri*” OR “rickettsia like bacteri*”) | 3,544 |
The review question (i.e. ‘which plant species can host Xylella/Xylella‐associated diseases?’) was broken down into key stages using the P/O conceptual model listed in the EFSA systematic review guidance (EFSA, 2010):
Population of interest (P)
The population of interest is that of plant species, world‐wide.
Outcome (condition of interest) (O)
The outcome (condition of interest) is that of Xylella infection.
2.1.1. Information sources
The established search strategy was run in all the databases listed in Table 1 via the Web of Science platform (Clarivate Analytics). No language, date or document type restrictions were applied to retrieve as many relevant publications as possible.
2.1.2. Search terms
The search strategy was designed combining the different terms describing both the pathogen and the diseases caused in the different host plants. The established search string is detailed in Table 2. The search was run in all the selected information sources (Table 1) on 16 May 2017 and 3,544 potentially relevant references were retrieved.
The search string was run again on 24 November 2017 and 51 additional references were obtained.
The collected records were downloaded and imported into the EndNote X8 bibliographic management software (Clarivate Analytics). Duplicate entries were removed using EndNote and the remaining records were uploaded on DistillerSR online2 together with the full texts in portable document format (pdf). Additional redundant references were excluded by the Duplicate Detection function of DistillerSR.
Nineteen references (e.g. from grey literature, information obtained via official requests to the different research groups, national authorities, references cross‐check in publications, conference proceedings) were included at a later stage of the process. Moreover, additional information was retrieved consulting the EUROPHYT outbreak notification database on 8 May 2018. Some data were also provided through personal communications by experts.
2.2. Study selection
The collected references were screened for relevance in two steps in the DistillerSR Web‐Based Systematic Review Software (Evidence Partners):
Title/abstract screening of all the references.
Full‐text screening of those references that passed the previous step.
Specific inclusion/exclusion criteria (described in Tables 3 and 4, respectively) were applied at each step and two reviewers worked in parallel screening all the references. Whenever a discrepant outcome was identified by the software, the reviewers had to solve the conflict and reach a common agreement on that reference.
Table 3.
Inclusion criteria for the title/abstract screening
| Question text | Type of answer | Answer text | Exclusion criteria |
|---|---|---|---|
| Is Xylella/a Xylella‐associated disease/a Xylella synonym the topic of the study? | Only one of the possible alternative answers can be selected | Yes | Included |
| No | Excluded | ||
| Is it a primary research study? | Only one of the possible alternative answers can be selected | Yes | Included |
| No | Excluded |
Table 4.
Inclusion criteria for the full‐text screening
| Question text | Type of answer | Answer text | Exclusion criteria |
|---|---|---|---|
| Is an English abstract present? | Only one of the possible alternative answers can be selected | Yes | Neutral |
| No | Neutral | ||
| Which is the type of the publication? | Only one of the possible alternative answers can be selected | Peer‐reviewed article | Neutral |
| Article | Neutral | ||
| Book | Neutral | ||
| Conference proceedings | Neutral | ||
| Abstract | Neutral | ||
| Technical publication/Report | Neutral | ||
| Other | Neutral | ||
| Is the Xylella host plant the main scope of the study? | Only one of the possible alternative answers can be selected | Yes | Neutral |
| No | Neutral | ||
| Is Xylella/a Xylella‐associated disease/a Xylella synonym studied in association to a host plant? | Only one of the possible alternative answers can be selected | Yes | Included |
| No | Excluded |
The first step required the reviewers to reply to two questions (Table 3) considering only the title and abstract (if available) of the reference. The aim of this step was to include only the publications presenting original research data (i.e. primary research studies) on Xylella or Xylella‐associated disease. So, in both questions, a positive answer was needed to select the reference.
A negative reply to one of the two questions was enough to exclude the reference. Whenever the information provided in the title and abstract was insufficiently clear, the reference was accepted and passed to the following step for further consideration.
All publications that passed the title/abstract screening were subjected to the full‐text screening (second step), except for 10 publications for which the full text was not retrieved (despite best efforts to carry this out).
This step required the reviewers to reply to four questions (Table 4): the first three questions were descriptive (neutral), whereas the fourth question had an inclusion/exclusion role. The descriptive questions were added to collect information about the type of reference. On the fourth question, only publications describing Xylella studied in association with a host plant (i.e. in vivo) were selected for the data extraction phase.
2.3. Data extraction
The last step of the procedure was the extraction of informative data from the selected references. The data extraction covered the information listed in Table 5. For each reference, one or more forms were filled to extract all relevant data reported in the publication. Each form represents a unique combination of data.
Table 5.
Data extraction structure
| Extracted data | Description |
|---|---|
| General information | In this section, the general information about the study is reported |
| RecordID | Unique number allocated to each row |
| RefID | Unique number allocated to each reference within the DistillerSR software |
| Reference | Full reference |
| Publication year | Year of the publication |
| Starting year | Starting year of the study, as reported in the publication |
| Ending year | Ending year of the study, as reported in the publication |
| Botanical identification | The botanical identification of the plant, both as reported in the publication and according to the updated taxonomy of the EPPO Global Database, is reported in this section |
| Plant EPPO code | EPPO code of the plant species, from the EPPO global databasea |
| Plant family | Plant family, from the EPPO global databasea |
| Plant genus | Plant genus, from the EPPO global databasea |
| Plant species | Plant species, from the EPPO global databasea |
| Reported plant species | Name of the plant species as reported in the publication |
| Common name | Common name of the plant species, as reported in the publication |
| Cultivar | Cultivar or plant variety, as reported in the publication |
| Infection information | Detailed information about the infection and location of the plant is reported in this section |
| Infection method (Level 1) | The infection of the plant can be natural, artificial or not specified |
| Infection method (Level 2) | Subcategories of natural infection: during survey activity, during research activity. ‘Research activity’ is used when plants are planted under natural inoculum pressure and infection development was monitored without interfering. Subcategories of artificial infection: mechanical inoculation (detailed at level 3a), vector transmission (detailed at level 3b) |
| Mechanical inoculation (Level 3a) | Subcategories of mechanical inoculation: budding, grafting, needle, root uptake, stem absorption, syringe |
| Infection vector species (Level 3b) | Insect species used in the artificial vector transmission |
| Location type | The place where the plant was placed or found: natural habitat, greenhouse, screenhouse, interception, not specified |
| Geographical information | In this section, the geographical location of the plant is reported, as detailed as possible. In case of intercepted plant, the reported location is the geographical origin of the plant and not the country and location where it was intercepted |
| Country code | From the EFSA catalogue |
| Country | From the EFSA catalogue |
| Location | From the EFSA catalogue, with additional detailed information as reported in the publication |
| Coordinates explanation | The reported coordinates (latitude and longitude) can represent the centroid of the area (region or country), or the exact location XY coordinates of the point of sample, or the near location XY coordinates based on village, town or identifiable geographical features (national park, lake, river etc.), or XY of study site coordinates indicates the centroid of the area sampled |
| Latitude | Latitude as reported in the publication or derived from Google maps (use WGS84, decimal format) |
| Longitude | Longitude as reported in the publication or derived from Google maps (use WGS84, decimal format) |
| Pest description | Information about the pest is reported in this section, together with genetic data |
| Pest EPPO code | EPPO code of the pest, from the EPPO global databasea |
| Pest species | Name of Xylella spp. as reported in the publication (from 1930 up to now): Alfalfa dwarf virus, Morus suffodiens virus, Phony peach bacterium, Pierce's disease bacterium, Pierce's disease virus, Rickettsia‐like bacteria, Rod‐shaped bacteria, Xylella fastidiosa, Xylella taiwanensis, Xylem‐inhabiting bacteria |
| Pest subspecies | Xylella fastidiosa subspecies reported in the publication: fastidiosa, morus, multiplex, pauca, sandyi, tashke |
| Reported pest | Name of Xylella spp. as reported in the publication (from 1930 up to now). |
| Disease | Name of the disease caused by Xylella spp., as reported in the publication: Alfalfa dwarf, Almond leaf scorch, Bacterial leaf scorch, Blueberry bacterial leaf scorch, Citrus variegated chlorosis, Coffee leaf scorch, Crespera, Elm leaf scorch, Leaf scorch disease, Mulberry leaf scorch, Oleander leaf scorch, Olive quick decline syndrome, Pear leaf scorch, Pecan bacterial leaf scorch, Periwinkle wilt, Phony peach disease, Pierce disease, Plum leaf scald, Potato purple top disease, Ragweed stunt, Sweetgum dieback, Sycamore leaf scorch |
| Strain | Name of the strain of Xylella spp., as reported in the publication |
| MLST (multilocus sequence type) | Sequence type (ST) of Xylella fastidiosa, as reported in the publication. If the ST is inferred from another publication, a note is added in the genotyping comment |
| Genotyping comment | Additional information retrieved in the publication about the Xylella spp. strain or sequence type |
| Methods of identification | In this section, the identification methods applied to detect a Xylella spp. infection are listed. Eight detection methods were considered and for each of them the outcome of the analysis (positive or negative), together with the number of infected plants and the total number of analysed plants, were reported. Moreover, additional information could be added in the comment column beside each detection method |
| Symptoms | Observation of symptoms in the plant, as reported in the publication |
| Symptoms expression in test plants | Observation of symptom development in test plants after an attempt to transmit the pathogen through vectors |
| Culture | Isolation of cultivable bacteria from tissue samples on solid culture media |
| Microscopy | Observation of Xylella bacteria through microscopic analysis techniques |
| ELISA | Enzyme‐linked immunosorbent assay |
| Other immunological techniques | Immunological techniques other than ELISA |
| PCR‐based methods | Polymerase chain‐reaction‐based methods (PCR, nested PCR, qPCR, etc.) |
| Sequencing | Sequencing technique (Sanger, next generation sequencing, etc.) and sequence analysis (MLST, phylogenetic tree, etc.) |
| Host status | Information about the tolerance and resistance response of the plant |
| Tolerance/Resistance reported | Tolerant/Resistant status of the plant, as reported in the publication |
| Tolerance/Resistance category | Categories describing the response of the tolerant/resistant plant: lack of infection or negative reading, Lack of systemic movement, Lack or reduction of symptoms, Lack or reduction of symptoms – Lower bacterial population, Lack or reduction of symptoms – Lower bacterial population – Lower disease incidence, Lack or reduction of symptoms – Lower disease incidence, Lower bacterial population, Lower bacterial population – Lower disease incidence, Lower disease incidence, Infection not persistent, Reported as tolerant/resistant (no details) |
| Tolerance/Resistance comment | Comment reporting detailed information about the tolerant/resistant response of the plant, as reported in the publication |
| General comment | General comment on the study |
| Comment | Additional relevant information |
EPPO (2018) EPPO Global Database (available online). https://gd.eppo.int
Two reviewers worked in sequence: the first reviewer performed the data extraction whereas the second reviewer conducted the quality control of the extracted data.
There was no language limit in the search and also publications written in different languages than English, such Chinese, French, German, Italian, Portuguese, Slovenian, Spanish and Russian, were retrieved. Those publications were sent for an official translation and some of these were included in the data extraction step.
2.4. Data warehouse
The Xylella spp. host plant database has been considered a good candidate for the prototyping of a pest in the plant data repository within the EFSA Scientific Data Warehouse (S‐DWH).
A harmonised data model has been established, also taking into account the feedback of a previous pilot focusing on the creation of a database about pests in apple. The aim was to establish a harmonised data flow for the collection and the collation of an extensive literature review generated data in the plant health domain.
2.4.1. Data management
Data have been collected through DistillerSR and then submitted to the EFSA Data Collection Framework (DCF). DCF is the upfront system in the EFSA pipeline of data collection tools and allows a first step of harmonisation against the EFSA controlled reference terminology (as known as EFSA catalogues). Data have been then included in the S‐DWH by means of a standardised Extract Transform Load (ETL) procedure and they have been further analysed and managed to generate needed statistics.
Raw data and related metadata are published in Zenodo in the EFSA Knowledge Junction community (https://doi.org/10.5281/zenodo.1339344).
Data will be available soon as interactive reports at the following link (expected to be active by end September 2018): https://www.efsa.europa.eu/en/microstrategy/xylella
2.4.2. Data reporting
Data reporting was designed to distinguish the Xylella spp. host plant species, based on the number and type of detection methods applied for each finding. Different combinations of detection methods were considered:
Plant species positive with at least two detection methods (among: symptoms observation on the test plant in experimental vector transmission, enzyme‐linked immunosorbent assay (ELISA), other immunological techniques, polymerase chain reaction (PCR)‐based methods, sequencing and culture) or positive with one method (between: sequencing, culture).
The same as point A, but also including microscopy: plant species positive with at least two detection methods (among: microscopy, symptoms observation on the test plant in experimental vector transmission, ELISA, other immunological techniques, PCR‐based methods, sequencing and culture) or positive with one method (between: sequencing, culture).
Plant species positive with at least one detection method (among: symptoms observation on the test plant in experimental vector transmission, ELISA, other immunological techniques, PCR‐based methods, sequencing and culture).
Plant species positive with at least one detection method including microscopy (microscopy, symptoms observation on the test plant in experimental vector transmission, ELISA, other immunological techniques, PCR‐based methods, sequencing and culture).
All positives plant species reported, regardless of the detection methods (positive records but without the detection method specified, symptom observations, microscopy, symptoms observation on the test plant in experimental vector transmission, ELISA, other immunological techniques, PCR‐based methods, sequencing, culturing).
3. Results
3.1. Results of the literature review
3.1.1. Collected literature and screening for relevance
The literature search was conducted twice, in May and in November 2017, and 3,595 references were obtained. Nineteen additional references were retrieved by other sources.
All the collected references were uploaded in DistillerSR and 3,098 references were selected after the removal of duplicates. These references were then screened (Figure 2).
Figure 2.
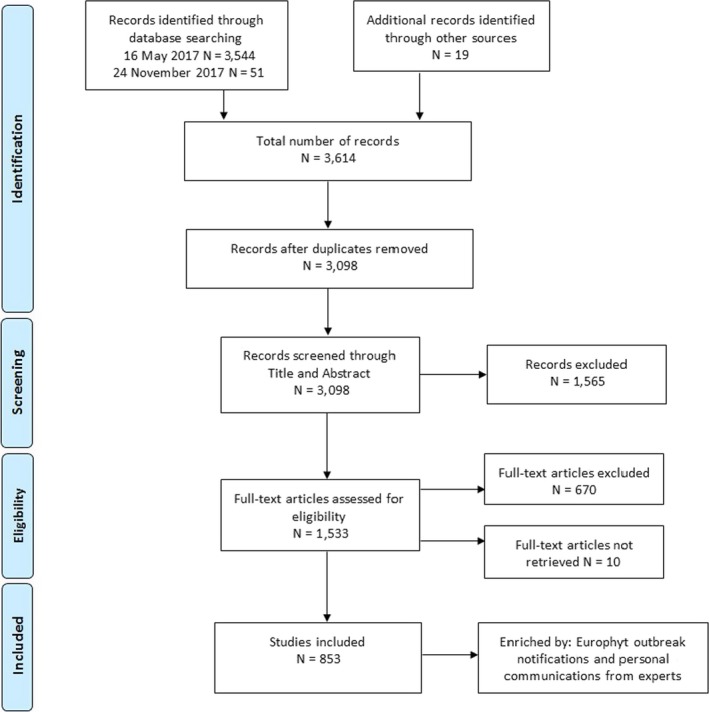
Flow diagram of the screening process in the DistillerSR tool
In the first step, the title and abstract screening, 1,565 references were excluded because they either did not focus on Xylella or Xylella‐associated diseases and/or because they were not primary research studies.
The accepted 1,533 references went through to the second step, the full‐text screening. Ten references were not evaluated as the full text was not retrieved and 670 references were excluded at this step.
The 853 references in which Xylella or Xylella‐associated diseases were studied in association with a host plant (i.e. in vivo) were selected for the data extraction phase. To catch the latest available information on the topic, the EUROPHYT outbreak database was consulted on 8 May 2018 and additional information was provided by scientific experts and national authorities. The full list of the selected references used for data extraction step is reported in Appendix E.
In total, 8,391 data extraction forms were filled in with informative data and subsequently analysed to retrieve the list of Xylella host plants.
3.1.2. Trend of publications
The oldest publications retrieved through the literature search and included in the data extraction step were published in the USA in 1930 on the phony disease of peach (Hutchins, 1930). The publication of Saponari et al. (2017) that describes the isolation and pathogenicity of X. fastidiosa‐associated with the olive quick decline syndrome in southern Italy was published in December 2017 and it is the most recent publication retrieved through the literature search.
The temporal trend of published publications about Xylella spp. and related diseases is shown in Figure 3. The number of publications used to extract data for this database stayed more or less constant between 1930 and the 1960s, increased to 10–20 per year between the 1970s and the 1990s, increased again in the period 2000–2010, with the highest number of publications in 2007 (47 publications).
Figure 3.

Number of publications published per year from 1930 to 2017 and used for data extraction
3.1.3. Unconfirmed records
A subset of unconfirmed studies (or of dubious relevance) or single records, included in the data extraction, was excluded from the data analysis. The records excluded from the data analysis are as follows:
Berisha et al. (1996, 1998) were considered unconfirmed/dubious. The EPPO Global Database states: ‘Absent, invalid record. EPPO Reporting Service (98/006): in an abstract (Berisha et al., 1996), it was claimed that X. fastidiosa had been isolated from diseased grapevine grown in Kosovo. The authors of the abstract have not, when requested, provided any details to substantiate this claim, which can only be regarded as dubious. EPPO Reporting Service (98/157): a fuller report (Berisha et al., 1998) stated that the material came from Cermjan, Kosovo (near Albanian border). Isolations and further study were undertaken in the USA. Lack of further study in the concerned area leaves considerable doubt about the nature of the original material. So the report remains dubious’.
Fliege (1974) was considered unconfirmed/dubious as the symptoms described in roots of Erica gracilis do not resemble those of X. fastidiosa.
Güldür et al. (2005) was considered unconfirmed/dubious. The EPPO Global Database states: ‘Absent, invalid record. EPPO Reporting Service (2016/192): the results of the study by Güldür et al. (2005) which suggested the occurrence of almond leaf scorch in Turkey have not been confirmed by any other studies or surveys. The NPPO of Turkey confirmed in 2014 that this record should be considered as invalid, and restated its declaration in 2016’.
Gutierrez‐Ibanez et al. (2009) was considered unconfirmed/dubious for Solanum tuberosum in Mexico as for the described disease (potato purple top disease or ‘zebra chip’) there are no further reports or publications associating it with X. fastidiosa.
The publications of Sadovskii (1985), Sadovskii and Shevchenko (1991) and Gvozdyak et al. (1990) were considered unconfirmed/dubious as the presence of X. fastidiosa has never been confirmed in Russia and Ukraine and the given publications describe only symptoms of the disease on plums.
The record by Jindal and Sharma (1987) in India was considered unconfirmed/dubious. The EPPO Global Database states: ‘Unreliable record. The identification requires confirmation by modern techniques’.
Temsah et al. (2015) was considered an unconfirmed/dubious record, as a later publication (Habib et al., 2016) stated that X. fastidiosa does not occur in Lebanon. The EPPO Global Database states: ‘Absent, invalid record. EPPO Reporting Service (2016/037): in 2015, a publication suggesting the presence of Xylella fastidiosa (EPPO A1 List) in Lebanon was published (Temsah et al., 2015). However, later studies confirmed that the ELISA‐positive samples initially obtained were false‐positive’.
The report stating the presence of Pyrus sp. infected by X. fastidiosa in Oregon (United States Department of Agriculture (USDA) National Clonal Germplasm Repository – Corvallis, Oregon/Xylella fastidiosa response plan, 2015) is not confirmed so far, as no further information has been published or released about this finding since the first reporting.
In Wendland et al. (2003), the Australian origin of the X. fastidiosa strain (9715 (755/95)) isolated from Vitis vinifera was considered unconfirmed/dubious according to a personal communication of Rui P. Leite Junior (Instituto Agronomico do Paranà, Brazil).
The record of Rosa floribunda infected by X. fastidiosa in Corsica reported in Cabassut (2015) and Denancé et al. (2017) was considered unconfirmed/dubious, according to a personal communication of Marie‐Agnès Jacques (INRA, France).
The record of Malus domestica infected by X. fastidiosa in France reported in Denancé et al. (2017) was considered unconfirmed/dubious as the same publication reported that ‘The contamination of this apple tree appeared transient, as subsequent samplings of the same tree maintained in containment conditions failed to reveal any contamination.’
In addition to the above unconfirmed records, some publications report findings of Xylella ST types which are divergent from previous analyses performed by other laboratories. Such is the case for example of the paper by Denancè et al. (2017) which reports from four samples from Corsica (France) a complete MLST profile while stating that the samples were not detected positive based on the EPPO protocol for MLSA used by the French National Laboratory of Reference. Such cases are included in the database and the stated divergences are reported in the column ‘Comment_PCR’ of the ‘observation’ spreadsheet of the Excel in Zenodo.
3.2. Host plants of Xylella spp. – data analysis
3.2.1. Identity of Xylella spp. – different aspects
Xylella spp. – the agent of many plant diseases – has been known by researchers and agronomists from the end of 19th and beginning of 20th century (Hutchins, 1930; Anonymous, 1984) when it was called by various names (Table 6). Very often the name followed the host plant name, e.g. ‘phony peach virus’ in peaches or ‘alfalfa dwarf virus’ in alfalfa plants. Sometimes the name of the agent was related to the outbreak zone or the person who first described the disease phenomenon, e.g. Anaheim disease in California (Anonymous, 1984), which was later called Pierce's disease after Newton B. Pierce (a Californian plant pathologist). The currently recognised taxonomic description and nomenclature of this organism were given by Wells et al. in 1987 with the name of Xylella fastidiosa. In 2016, X. taiwanensis – a new species within the Xylella genus – was proposed (Su et al., 2016). The X. fastidiosa species is divided into at least six genetically different subspecies but only the subspecies fastidiosa and multiplex are officially considered viable by the International Society of Plant Pathology Committee on the Taxonomy of Plant Pathogenic Bacteria (ISPP‐CTPPB) (Bull et al., 2012). The other remaining subspecies are: morus, pauca, sandyi and tashke. All the subspecies are listed in the Xylella spp. host plant database and a list of hosts for each subspecies is shown in Appendices A and B.
Table 6.
Temporal development of the nomenclature for Xylella spp
| Decades | Names of the disease/causal agent | Selected references |
|---|---|---|
| 1920s | Phony peach virus | Hutchins (1930); Hutchins and Rue (1949) |
| 1930s | Alfalfa dwarf virus, Phony peach virus, Xylem‐inhabiting bacteria | Hewitt et al. (1946); Hutchins et al. (1953); Hutchins and Rue (1949); Millikan (1955); Turner and Pollard (1958); Turner (1949) |
| 1940s | Alfalfa dwarf virus, Phony peach virus, Pierce's disease virus, Xylem‐inhabiting bacteria | Cochran (1951); Hewitt et al. (1946); Hutchins et al. (1953); Hutchins and Rue (1949); Kenknight (1951); Turner (1949) |
| 1950s | Morus suffodiens virus, Phony peach virus, Pierce's disease bacterium, Pierce's disease virus, Xylem‐inhabiting bacteria | Bruer (1951); Cochran (1951); Hewitt (1958); Loomis (1961); Millikan and Anderson (1954); Millikan (1955); Mortensen et al. (1977); Stoner (1953a,b); Stoner et al. (1951); Wester and Jylkka (1959) |
| 1960s | Pierce's disease bacterium, Pierce's disease virus, Rickettsia‐like bacteria | Goheen et al. (1973); Mortensen and Knight (1968); Mortensen (1968); Mortensen et al. (1977) |
| 1970s | Pierce's disease bacterium, Rickettsia‐like bacteria, Rod‐shaped bacteria, Xylem‐inhabiting bacteria | Auger et al. (1974); Brlansky and Timmer (1982); Davis et al. (1980); Evert et al. (1981); Feldman (1984); French (1977); Goheen et al. (1973); Hearon et al. (1980); Hopkins and Adlerz (1980); Hopkins and Mollenhauer (1975); Hopkins and Mortensen (1974); McCoy et al. (1978); Purcell (1975); Raju et al. (1983); Weaver et al. (1980); Wells and Weaver (1980) |
| 1980s | Phony peach bacterium, Pierce's disease bacterium, Rickettsia‐like bacteria, Xylem‐inhabiting bacteria, Xylella fastidiosa | Evert (1987); Hopkins (1984); Hopkins and Thompson (1984); Jimenez (1985); Kostka et al. (1984); Yonce and Chang (1987); Wells et al. (1981, 1987) |
| 1990s onwards | Xylella fastidiosa | Chang and Donaldson (1993); Laranjeira et al. (1998); Leite et al. (1997); McElrone et al. (1999); Purcell and Saunders (1995); Su and Leu (1995) |
3.2.2. Host plant species in artificial vs natural infections for different Xylella species and subspecies
The EFSA Xylella spp. host plant database contains data from different types of studies. Some of the studies reported natural infections (e.g. surveys in the fields) and some were performed under controlled artificial conditions (laboratory or controlled conditions screenhouses).
Following the classification of the species into A, B, C, D, E detection categories (see Section 2.4.2), two lists of host plant species were created:
Appendix A and Table 9 – Xylella fastidiosa subspecies in experimentally infected plants.
Appendix B and Table 10 – Xylella fastidiosa subspecies in naturally infected plants.
Table 9.
Number of host plant species, experimentally infected, susceptible to the different X. fastidiosa subspecies
| Category | fastidiosa | multiplex | pauca | sandyi | tashke | Unknown |
|---|---|---|---|---|---|---|
| A | 35 | 12 | 7 | 3 | 0 | 93 |
| B | 35 | 12 | 7 | 3 | 0 | 98 |
| C | 41 | 17 | 17 | 3 | 1 | 209 |
| D | 41 | 17 | 17 | 3 | 1 | 215 |
| E | 42 | 18 | 17 | 3 | 1 | 224 |
Table 10.
Number of host plant species, naturally infected, susceptible to the different X. fastidiosa subspecies
| Category | fastidiosa | fastidiosa/sandyi | morus | multiplex | pauca | sandyi | tashke | Unknown |
|---|---|---|---|---|---|---|---|---|
| A | 32 | 2 | 4 | 108 | 41 | 6 | 1 | 148 |
| B | 32 | 2 | 4 | 108 | 41 | 6 | 1 | 154 |
| C | 32 | 2 | 4 | 116 | 43 | 7 | 1 | 345 |
| D | 32 | 2 | 4 | 116 | 43 | 7 | 1 | 353 |
| E | 33 | 2 | 4 | 117 | 43 | 7 | 1 | 363 |
Both appendices show the results for the subspecies fastidiosa, multiplex and pauca, as they are the most studied in the database. Few records are available for the subspecies sandyi, morus and tashke, which are presented in the tables below (Tables 7 and 8).
Table 7.
Xylella fastidiosa subspecies in experimentally infected plants (subspecies: sandyi and tashke)
| Xylella subspecies | sandyi | tashke | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plant species in different classifications (A–E) | A | B | C | D | E | A | B | C | D | E |
| Nerium oleander | 6 | 6 | 6 | 6 | 9 | |||||
| Nicotiana benthamiana | 0 | 0 | 1 | 1 | 1 | |||||
| Prunus dulcis | 1 | 1 | 1 | 1 | 1 | |||||
| Vinca major | 2 | 2 | 2 | 2 | 2 | |||||
Table 8.
Xylella fastidiosa subspecies in naturally infected plants (subspecies: morus and sandyi)
| Xylella subspecies | morus | sandyi | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plant species in different classifications(A–E) | A | B | C | D | E | A | B | C | D | E |
| Nandina domestica | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
The total number of positive host plant species of X. fastidiosa was counted, regardless of the detection method (Category E: 563 species, 264 genera, 82 families). Excluding the records with only symptoms observation or when the detection method was not specified (Category D), the total number of host plant species recorded was reduced to 554. When microscopy was also excluded (Category C), 543 host plant species were recorded. When considering only records determined with at least two detection methods (excluding only symptoms and unspecified detection method) or by sequencing or culturing (Category B), 316 host plant species remained. When microscopy was also excluded (Category A), the number of host plants resulted in 312 species, 152 genera and 61 families (Figure 4). The sample size for this calculation was 853 scientific publications published between 1930 and 2017, with 6,464 records (positive findings) in the extraction table.
Figure 4.

Number of host plant species according to different classification systems described in Section 2.4.2
Artificial inoculations were positive in 122 plant species while 234 plant species were reported positive in natural infections (according to the classification A described in Section 2.4.2).
The number of the host plant species was calculated according to the reporting system, described in Section 2.4.2 and it is presented in the triangle scheme below (Tables 9 and 10).
Xylella taiwanensis was recorded only in one publication (Su et al., 2016) naturally infecting Pyrus pyrifolia in Taiwan.
Host plants of X. fastidiosa were detected using different methods and some of the results were contradictory. Those contradicting host plant species were compared with all other host plant species in the database and if there was the same host plant species in the other studies without contradiction positive, we counted it as positive. If the host plant species occurred in the extraction table only once and it was contradictory, the species was not counted in the total number of species but is listed in Table 11. In most cases, contradictions of results occurred for positive ELISA and negative PCR‐based methods, but in a few cases three detection methods were used.
Table 11.
Contradictory resultsa
| Plant species | Contradicting methods | Citation |
|---|---|---|
| Heteromeles arbutifolia (Rosaceae) | ELISA POS vs PCR‐based methods NEG | Costa et al. (2004) |
| Hibiscus syriacus (Malvaceae) | ELISA POS vs PCR‐based methods NEG | McGaha et al. (2007) |
| Juglans californica (Juglandaceae) | ELISA POS vs PCR‐based methods NEG | Costa et al. (2004) |
| Phyla nodiflora (Verbenaceae) | ELISA and PCR‐based methods POS vs Other immunological technique NEG | Buzombo et al. (2006) |
| Pistacia vera (Anacardiaceae) | ELISA POS vs PCR‐based methods NEG | Costa et al. (2004) |
| Platanus racemosa (Platanaceae) | ELISA POS vs PCR‐based methods NEG | Costa et al. (2004) |
| Rubus trivialis (Rosaceae) | ELISA and PCR‐based methods POS vs Other immunological technique NEG | Buzombo et al. (2018) |
| Schinus molle (Anacardiaceae) | ELISA POS vs PCR‐based methods NEG | Costa et al. (2004) |
| Solanum elaeagnifolium (Solanaceae) | ELISA POS vs PCR‐based methods NEG | Costa et al. (2004) |
| Tillandsia usneoides (Bromeliaceae) | ELISA POS vs PCR‐based methods NEG and Other immunological technique NEG | Buzombo et al. (2006) |
Complete list of contradicting results is available in Appendix D.
3.2.3. Geographical distribution of Xylella spp. host plant species
The geographical distribution of plant species infected by Xylella spp. is shown in Figure 5.
Figure 5.
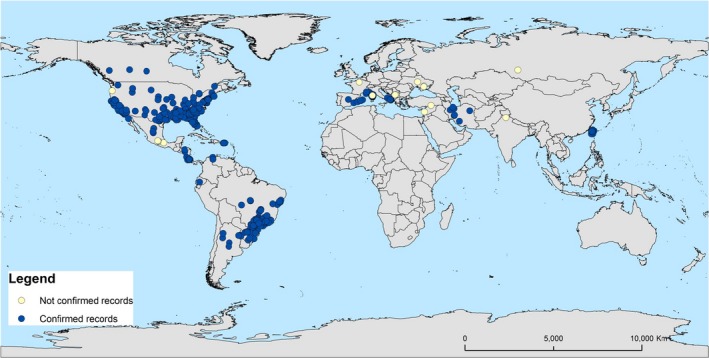
Geographical distribution of Xylella spp.
Confirmed findings of X. fastidiosa have been reported in several countries of North, Central and South America, in Asia (only in Iran) and more recently in Europe (Italy, France and Spain). The species Xylella taiwanensis has been reported so far only in Taiwan (China).
Unconfirmed findings (Section 3.1.3), such as in Kosovo, Turkey and India, are also shown on the map.
3.2.4. Host plants and Xylella fastidiosa sequence type association
Information about the genetic characterisation, such as the sequence type (ST), has also been recorded from all the selected publications. The full list of records of plant species infected by the different STs in artificial and natural conditions is shown in Appendix C. The country has been reported for plant species that have been found naturally infected.
In total, 889 records have been reported in the database, describing 176 plant species in which the X. fastidiosa ST has been characterised. Actually, 81 different STs have been described world‐wide.
The highest number of records refers to plant species in which the detected X. fastidiosa STs belong to the subspecies pauca. Those plants have been identified in Central and South America (Argentina, Brazil, Costa Rica and Ecuador) and in European countries (Italy, France and Spain).
STs of subspecies multiplex have been found in plant species distributed in USA, France, Spain and Brazil. In USA, Costa Rica, Spain and Mexico several plant species have been identified as infected by STs of subspecies fastidiosa.
The plant species with more reported records are Olea europaea, Prunus dulcis and V. vinifera, whereas the most recorded STs are ST53 (subspecies pauca) and ST1 (subspecies fastidiosa).
Experiments of artificial infection have been especially performed using X. fastidiosa STs belonging to subspecies fastidiosa, with 89 records reported in the table.
3.2.5. Botanical characterisation of the hosts
The most abundant families in different plant species are: Fabaceae, Asteraceae, Vitaceae, Poaceae, Rosaceae, Rutaceae, Fagaceae, Rubiaceae, Lamiaceae and Oleaceae (Figure 6). Many host plant species are very well studied (such as citrus and grapevine) by many research groups in different parts of the world. Among the listed host plants, there are many important crops, different tree species, shrubs, weeds and ornamentals. Xylella spp. are polyphagous, but many different host plant species can play different roles in the pathogen epidemiology, for example asymptomatic reservoir plants. Xylella spp. can be hosted by plants classified in the different botanical higher clades, such as Monocotyledons, Dicotyledons and also Gymnosperms.
Figure 6.
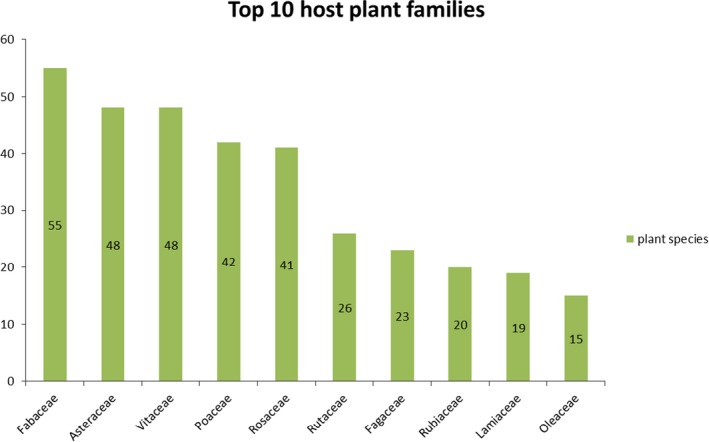
Xylella spp. host plant families – the most abundant in species
3.2.6. Tolerant or resistant host plants
Eighty‐seven out of 853 publications contained information about the tolerance and resistance status of 64 plant species. As expected, the most studied genera belonged to economically important crops: Vitis and Citrus followed by Prunus. The fourth more studied genus, and with the most recent publications, was Olea. The full list of plant species together with the number of records of tolerant/resistant response for each plant species is listed in Table 12.
Table 12.
Number of records in Xylella host plant database of tolerant/resistant response for each plant species
| Plant species | Number of records |
|---|---|
| Arabidopsis thaliana | 4 |
| Citrus celebica | 1 |
| Citrus clementina | 2 |
| Citrus jambhiri | 2 |
| Citrus junos | 1 |
| Citrus latifolia | 1 |
| Citrus limettioides | 1 |
| Citrus limon | 14 |
| Citrus medica | 1 |
| Citrus natsudaidai | 1 |
| Citrus paradisi | 4 |
| Citrus reticulata | 9 |
| Citrus reticulata × C. sinensis × C. paradisi | 1 |
| Citrus sinensis | 7 |
| Citrus spp. | 82 |
| Citrus tangerina | 32 |
| Citrus × nobilis | 11 |
| Citrus × tangelo | 13 |
| Coffea arabica | 4 |
| Coffea spp. | 1 |
| Fortunella margarita | 1 |
| Olea europaea | 13 |
| Platanus spp. | 2 |
| Poncirus trifoliata | 3 |
| Prunus angustifolia | 1 |
| Prunus armeniaca | 3 |
| Prunus avium | 4 |
| Prunus cerasus | 2 |
| Prunus domestica | 4 |
| Prunus dulcis | 8 |
| Prunus persica | 8 |
| Prunus salicina | 10 |
| Prunus spp. | 13 |
| Prunus × amygdalo‐persica | 8 |
| Quercus ilex | 4 |
| Vaccinium corymbosum | 5 |
| Vitis aestivalis | 2 |
| Vitis arizonica | 5 |
| Vitis arizonica hybrid | 6 |
| Vitis arizonica × V. rupestris | 6 |
| Vitis arizonica × V. vinifera | 1 |
| Vitis arizonica/candicans | 3 |
| Vitis arizonica/candicans × V. rupestris | 2 |
| Vitis arizonica/girdiana | 1 |
| Vitis berlandieri × riparia hybrids | 6 |
| Vitis berlandieri × V. rupestris | 4 |
| Vitis candicans | 2 |
| Vitis cinerea × V. berlandieri | 2 |
| Vitis girdiana | 2 |
| Vitis munsoniana | 3 |
| Vitis popenoei | 1 |
| Vitis rotundifolia | 58 |
| Vitis rotundifolia × V. rupestris | 1 |
| Vitis simpsonii | 1 |
| Vitis spp. | 76 |
| Vitis tiliaefolia | 1 |
| Vitis vinifera | 25 |
| Vitis × champinii | 1 |
| Vitis aestivalis var. smalliana | 5 |
| Vitis aestivalis var. smalliana × V. simpsonii | 4 |
| Vitis aestivalis var. smalliana × V. vinifera | 1 |
| Vitis nesbittiana | 1 |
| Vitis rufotomentosa | 1 |
| Vitis shuttleworthii | 5 |
| Total | 507 |
For each record, the host status as reported in the publication has been inserted in the database. Moreover, categories have been created to group and analyse the outcome of the tolerant/resistance response. Those categories reflect the response (one or more than one) for which the authors of the studies considered the plant species as tolerant or resistant. The most described outcomes of the tolerant/resistant behaviour are the lack or reduction of symptoms expression, the lower amount of bacterial population, the lack of infection and the lack of systemic movement of bacteria (Table 13).
Table 13.
Number of records and publications for each tolerant/resistance response category
| Category | Number of records | Number of publications | ||
|---|---|---|---|---|
| Artificial infection | Natural infection | Not specified | ||
| Lack or reduction of symptoms | 74 | 75 | 10 | |
| Lower bacterial population | 47 | 6 | 19 | |
| Lack of systemic movement | 71 | 7 | ||
| Lack of infection or Negative reading | 41 | 77 | 12 | |
| Lack or reduction of symptoms – Lower bacterial population | 11 | 2 | 5 | |
| Lack or reduction of symptoms – Lower disease incidence | 2 | 1 | ||
| Lower bacterial population – Lower disease incidence | 2 | 2 | ||
| Lack or reduction of symptoms – Lower bacterial population – Lower disease incidence | 2 | 2 | ||
| Lower disease incidence | 3 | 1 | ||
| Not persistent infection | 5 | 2 | ||
| Reported as tolerant/resistant – no details | 16 | 22 | 51 | 42 |
The lack or reduction of symptoms is the most considered outcome of the tolerant/resistant status, with 166 records equally distributed between natural (81) and artificial (85) way of infection. The lack of infection, both under natural inoculum pressure and artificial conditions, demonstrated the tolerant/resistant behaviour of the plant in 12 publications and 118 single records.
The lack of systemic movement was retrieved in 71 records through artificial inoculation, so in these plant species the infection occurred but the bacteria remain localised in proximity to the point of inoculum.
The occurrence of the bacterial population was tested 70 times in 23 different publications and the lower rate compared with other plant species or varieties let the authors of the studies consider these plants as tolerant/resistant.
In 89 records, the plant was considered tolerant/resistant but no details were described. In 51 of those records, the kind of infection was also not specified.
Table 14 proposes a list of plant species with negative result(s) in artificial infection, and never detected positive under natural conditions. Nevertheless, it should be stated that such a list was drafted by compiling the results of different studies performed under different conditions of inoculum, strains, incubation period, etc. Therefore, one should keep in mind that there is so many unknown (bacterial diversity, insect vectors, environmental conditions) that such experiments are fraught with difficulty and uncertainty, and should be considered with caution (EFSA, 2015).
Table 14.
List of plant species with negative results in artificial infection and never detected positive under natural conditions
| Plant family | Plant species | Number of records |
|---|---|---|
| Aizoaceae | Tetragonia tetragonioides | 1 |
| Amaranthaceae | Beta vulgaris | 1 |
| Anacardiaceae | Pistacia lentiscus | 1 |
| Anacardiaceae | Rhus laurina | 1 |
| Anacardiaceae | Rhus ovata | 1 |
| Asteraceae | Acmella ciliata | 2 |
| Asteraceae | Artemisia californica | 1 |
| Asteraceae | Eclipta prostrata | 1 |
| Asteraceae | Matricaria discoidea | 1 |
| Asteraceae | Solidago microglossa | 1 |
| Brassicaceae | Brassica rapa | 1 |
| Calycanthaceae | Calycanthus occidentalis | 2 |
| Commelinaceae | Commelina virginica | 1 |
| Convolvulaceae | Jacquemontia grandifolia | 1 |
| Cyperaceae | Cyperus acuminatus | 1 |
| Ericaceae | Vaccinium ashei | 3 |
| Fabaceae | Acacia cowleana | 2 |
| Hydrangeaceae | Philadelphus californicus | 1 |
| Juglandaceae | Juglans californica | 1 |
| Lamiaceae | Prostanthera ovalifolia | 2 |
| Myrtaceae | Callistemon viminalis | 2 |
| Myrtaceae | Eucalyptus erythrocorys | 2 |
| Myrtaceae | Melaleuca lateritia | 2 |
| Nyctaginaceae | Bougainvillea sp. | 1 |
| Phrymaceae | Mimulus aurantiacus | 1 |
| Pinaceae | Pseudotsuga menziesii | 1 |
| Poaceae | Distichlis spicata | 1 |
| Poaceae | Poa pratensis | 1 |
| Poaceae | Polypogon monspeliensis | 1 |
| Polygonaceae | Eriogonum fasciculatum | 1 |
| Proteaceae | Banksia serrata | 2 |
| Rhamnaceae | Frangula californica | 1 |
| Rosaceae | Cotoneaster franchetii | 1 |
| Rosaceae | Heteromeles arbutifolia | 2 |
| Rosaceae | Malus sylvestris | 1 |
| Rosaceae | Prunus davidiana | 3 |
| Rosaceae | Prunus tomentosa | 2 |
| Rosaceae | Prunus virginiana var. demissa | 1 |
| Rosaceae | Pyracantha angustifolia | 1 |
| Rutaceae | Citrus limettioides | 1 |
| Rutaceae | Citrus maxima | 1 |
| Rutaceae | Correa glabra | 2 |
| Salicaceae | Populus sp. | 1 |
| Salicaceae | Salix sessilifolia | 1 |
| Verbenaceae | Aloysia virgata | 2 |
| Vitaceae | Vitis popenoei | 1 |
4. Conclusions
Following the request of the European Commission, EFSA was asked to update and regularly maintain a Xylella spp. host plant database. In July 2018, the ‘Updated Xylella fastidiosa pest categorisation’ was published, which was an update of part of the EFSA PLH Panel, 2015 scientific opinion. Some of the information from the Xylella spp. host plant database was used in this categorisation.
The Xylella host plant database was enriched with recent world‐wide scientific literature, grey literature, EUROPHYT notifications, internet sources and communications from different scientific groups.
An ELS was performed in 2017 starting from 3,614 publications with no time and language limits. In total, 853 publications were selected for data extraction and information on botanical identification of the plant, kind of infection, geographical data, detection methods, host status (resistance/tolerance) were retrieved from the publications. A new detailed distribution map has been drawn and findings of unconfirmed records distinguished. The nomenclature of the host plants was linked to the automatic EPPO codes, to facilitate further updates or changes. All data have been stored in Data Warehouse, which allows the storage and harmonisation of data.
The data of natural and artificial studies were distinguished and all numeric data recalled. A total of 122 species with evidence of artificial infection and 234 from natural infections were recorded.
In this scientific report, a comprehensive list of host plant species of X. fastidiosa and X. taiwanensis was created taking into account different detection methods and new genetic characterisations (multilocus sequence typing). The total number of plants reported infected by X. fastidiosa regardless of the detection method was 563 species, 264 genera and 82 families. When considering only records determined with at least two detection methods (excluding only symptoms, microscopy and unspecified detection method) or by sequencing or culturing, 312 host plant species remained from 152 genera and 61 botanical families.
Host status has been considered in the current database. Special categories with extensive comments have been added. In total, 87 publications were identified having information on resistance, tolerance response of the plants.
This update of the database of host plants of Xylella spp. reported world‐wide provides a key tool for risk management, risk assessment and research on this polyphagous bacterial plant pathogen.
Abbreviations
- DCF
Data Collection Framework
- EFSA PLH Panel
EFSA Panel on Plant Health
- ELISA
enzyme‐linked immunosorbent assay
- ELS
extensive literature search
- EPPO
European and Mediterranean Plant Protection Organization
- ETL
Extract Transform Load
- ISPP‐CTPPB
International Society of Plant Pathology Committee on the Taxonomy of Plant Pathogenic Bacteria
- MLST
multilocus sequence type
- PCR
polymerase chain reaction
- S‐DWH
EFSA Scientific Data Warehouse
- ST
sequence type
- USDA
United States Department of Agriculture
Appendix A – Xylella fastidiosa subspecies in experimentally infected plants
1.
| Xylella subspecies | fastidiosa | multiplex | pauca | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant species in different classifications(A‐E) | A | B | C | D | E | A | B | C | D | E | A | B | C | D | E |
| Acer rubrum | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Amaranthus blitoides | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Ambrosia acanthicarpa | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Ambrosia artemisiifolia | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Carya illinoinensis | 4 | 4 | 12 | 12 | 12 | ||||||||||
| Catharanthus roseus | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 6 | 6 | 6 | |||||
| Chenopodium quinoa | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Citrus sinensis | 2 | 2 | 3 | 3 | 3 | ||||||||||
| Citrus sp. | 0 | 0 | 20 | 20 | 20 | ||||||||||
| Conium maculatum | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Convolvulus arvensis | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Cyperus esculentus | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Datura wrightii | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Dendranthema × grandiflorum | 0 | 0 | 1 | 1 | 1 | ||||||||||
| Echinochloa crus‐galli | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Erigeron canadensis | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Eriochloa gracilis | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Erodium moschatum | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Eucalyptus camaldulensis | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Eucalyptus globulus | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Helianthus annuus | 3 | 3 | 3 | 3 | 3 | ||||||||||
| Ipomoea purpurea | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Lactuca serriola | 3 | 3 | 3 | 3 | 3 | ||||||||||
| Liquidambar styraciflua | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Malva parviflora | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Medicago sativa | 18 | 18 | 18 | 18 | 18 | 15 | 15 | 15 | 15 | 15 | |||||
| Nerium oleander | 4 | 4 | 8 | 8 | 8 | ||||||||||
| Nicotiana clevelandii | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Nicotiana glauca | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Nicotiana tabacum | 0 | 0 | 10 | 10 | 12 | 0 | 0 | 3 | 3 | 4 | 1 | 1 | 1 | 1 | 1 |
| Olea europaea | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 4 | 4 | 4 | 11 | 11 | 29 | 29 | 30 |
| Platanus occidentalis | 3 | 3 | 4 | 4 | 4 | ||||||||||
| Polygala myrtifolia | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 5 | 5 | 5 | |||||
| Portulaca oleracea | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Prunus avium | 0 | 0 | 4 | 4 | 4 | ||||||||||
| Prunus cerasifera | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Prunus domestica | 0 | 0 | 1 | 1 | 1 | ||||||||||
| Prunus dulcis | 23 | 23 | 23 | 23 | 26 | 12 | 12 | 12 | 12 | 16 | 0 | 0 | 5 | 5 | 5 |
| Prunus persica | 0 | 0 | 1 | 1 | 1 | ||||||||||
| Prunus persica × P. webbii | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | |||||
| Prunus salicina | 0 | 0 | 1 | 1 | 1 | ||||||||||
| Prunus sp. | 3 | 3 | 4 | 4 | 4 | 3 | 3 | 4 | 4 | 5 | |||||
| Prunus webbii | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | |||||
| Prunus × amygdalo‐persica | 0 | 0 | 6 | 6 | 6 | ||||||||||
| Quercus ilex | 0 | 0 | 1 | 1 | 1 | ||||||||||
| Quercus pubescens | 0 | 0 | 1 | 1 | 1 | ||||||||||
| Rubus ursinus | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | |||||
| Rumex crispus | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Simmondsia chinensis | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Solanum lycopersicum | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Solanum melongena | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Sonchus oleraceus | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Sorghum halepense | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Vaccinium corymbosum | 0 | 0 | 4 | 4 | 6 | 0 | 0 | 3 | 3 | 4 | |||||
| Vaccinium sp. | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 4 | |||||
| Vicia faba | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Vicia sativa | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Vitis vinifera | 28 | 28 | 32 | 33 | 35 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 2 | 2 |
| Xanthium strumarium | 3 | 3 | 3 | 3 | 3 | ||||||||||
| Grand Total (Sum of records) | 120 | 120 | 143 | 144 | 157 | 46 | 46 | 69 | 69 | 80 | 25 | 25 | 95 | 95 | 96 |
| Plant species | 35 | 35 | 41 | 41 | 42 | 12 | 12 | 17 | 17 | 18 | 7 | 7 | 17 | 17 | 17 |
Appendix B – Xylella fastidiosa subspecies in naturally infected plants
1.
| Xylella subspecies | fastidiosa | multiplex | pauca | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant species in different classifications (A–E) | A | B | C | D | E | A | B | C | D | E | A | B | C | D | E |
| Acacia dealbata | 1 | 1 | 2 | 2 | 2 | ||||||||||
| Acacia saligna | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | |||||
| Acacia sp. | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Acer griseum | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Acer platanoides | 0 | 0 | 2 | 2 | 2 | ||||||||||
| Acer pseudoplatanus | 2 | 2 | 3 | 3 | 3 | ||||||||||
| Acer rubrum | 1 | 1 | 2 | 2 | 2 | ||||||||||
| Acer sp. | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Alnus rhombifolia | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Ambrosia psilostachya | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Ambrosia trifida | 9 | 9 | 9 | 9 | 9 | ||||||||||
| Ambrosia trifida var. texana | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Ampelopsis cordata | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Anthyllis hermanniae | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Artemisia arborescens | 2 | 2 | 3 | 3 | 3 | ||||||||||
| Asparagus acutifolius | 2 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | |||||
| Baccharis halimifolia | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Calicotome spinosa | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Calicotome villosa | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Carya illinoinensis | 11 | 11 | 12 | 12 | 12 | ||||||||||
| Carya sp. | 4 | 4 | 4 | 4 | 4 | ||||||||||
| Catharanthus roseus | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Celtis occidentalis | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Cercis canadensis | 3 | 3 | 3 | 3 | 3 | ||||||||||
| Cercis occidentalis | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||||
| Cercis siliquastrum | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Chenopodium album | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Chionanthus sp. | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Cistus creticus | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Cistus monspeliensis | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 4 | 4 | 4 | |||||
| Cistus salviifolius | 2 | 2 | 3 | 3 | 3 | ||||||||||
| Cistus sp. | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Citrus sinensis | 2 | 2 | 2 | 2 | 2 | 54 | 54 | 60 | 60 | 60 | |||||
| Citrus sp. | 36 | 36 | 36 | 36 | 36 | ||||||||||
| Coffea arabica | 17 | 17 | 17 | 17 | 17 | 74 | 74 | 74 | 74 | 74 | |||||
| Coffea sp. | 25 | 25 | 25 | 25 | 25 | ||||||||||
| Coronilla valentina | 2 | 2 | 3 | 3 | 3 | ||||||||||
| Coronilla valentina subsp. glauca | 0 | 0 | 1 | 1 | 1 | ||||||||||
| Cytisus scoparius | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Cytisus sp. | 1 | 1 | 2 | 2 | 2 | ||||||||||
| Cytisus villosus | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Dodonaea viscosa | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Encelia farinosa | 4 | 4 | 5 | 5 | 5 | ||||||||||
| Eremophila maculata | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Erigeron bonariensis | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Erigeron sumatrensis | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Erysimum hybrids | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Euphorbia terracina | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Euryops chrysanthemoides | 1 | 1 | 2 | 2 | 2 | ||||||||||
| Fallopia japonica | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Ficus carica | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Fraxinus americana | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Fraxinus angustifolia | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Fraxinus sp. | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Genista corsica | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Genista ephedroides | 2 | 2 | 3 | 3 | 3 | ||||||||||
| Genista lucida | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Genista sp. | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Genista × spachiana | 1 | 1 | 2 | 2 | 2 | ||||||||||
| Ginkgo biloba | 3 | 3 | 3 | 3 | 3 | ||||||||||
| Gleditsia triacanthos | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Grevillea juniperina | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Hebe sp. | 2 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | |||||
| Helianthus annuus | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Helianthus sp. | 3 | 3 | 3 | 3 | 3 | ||||||||||
| Helichrysum italicum | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Heliotropium europaeum | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Hibiscus rosa‐sinensis | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Iva annua | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Juglans regia | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Koelreuteria bipinnata | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Lagerstroemia indica | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Lagerstroemia sp. | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Laurus nobilis | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Lavandula angustifolia | 2 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | |||||
| Lavandula dentata | 3 | 3 | 4 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | |||||
| Lavandula sp. | 3 | 3 | 4 | 4 | 4 | ||||||||||
| Lavandula stoechas | 2 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | |||||
| Lavandula × heterophylla | 1 | 1 | 2 | 2 | 2 | ||||||||||
| Lavandula × intermedia | 2 | 2 | 3 | 3 | 3 | ||||||||||
| Liquidambar styraciflua | 12 | 12 | 12 | 12 | 12 | ||||||||||
| Liriodendron tulipifera | 0 | 0 | 1 | 1 | 1 | ||||||||||
| Lupinus aridorum | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Lupinus villosus | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Magnolia grandiflora | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Medicago sativa | 4 | 4 | 4 | 4 | 4 | 1 | 1 | 2 | 2 | 2 | |||||
| Metrosideros excelsa | 1 | 1 | 2 | 2 | 2 | ||||||||||
| Metrosideros sp. | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Myoporum insulare | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Myrtus communis | 2 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | |||||
| Nerium oleander | 1 | 1 | 1 | 1 | 1 | 9 | 9 | 12 | 12 | 13 | |||||
| Olea europaea | 9 | 9 | 10 | 10 | 10 | 93 | 93 | 151 | 151 | 160 | |||||
| Olea europaea subsp. sylvestris | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | |||||
| Olea sp. | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Pelargonium fragrans | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Pelargonium graveolens | 3 | 3 | 4 | 4 | 4 | ||||||||||
| Pelargonium sp. | 5 | 5 | 6 | 6 | 6 | ||||||||||
| Periwinkle (common name) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Phagnalon saxatile | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Phillyrea latifolia | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Platanus occidentalis | 9 | 9 | 11 | 11 | 11 | ||||||||||
| Pluchea odorata | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Polygala myrtifolia | 2 | 2 | 2 | 2 | 2 | 55 | 55 | 60 | 60 | 60 | 5 | 5 | 9 | 9 | 10 |
| Polygala sp. | 0 | 0 | 1 | 1 | 1 | ||||||||||
| Polygala × dalmaisiana | 0 | 0 | 1 | 1 | 1 | ||||||||||
| Polygala × grandiflora nana | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Prunus armeniaca | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Prunus avium | 6 | 6 | 6 | 6 | 6 | 0 | 0 | 1 | 1 | 1 | 4 | 4 | 8 | 8 | 9 |
| Prunus cerasifera | 19 | 19 | 21 | 21 | 21 | ||||||||||
| Prunus cerasus | 0 | 0 | 1 | 1 | 1 | ||||||||||
| Prunus domestica | 11 | 11 | 11 | 11 | 11 | 1 | 1 | 1 | 1 | 1 | |||||
| Prunus dulcis | 20 | 20 | 20 | 20 | 20 | 21 | 21 | 21 | 21 | 22 | 6 | 6 | 6 | 6 | 7 |
| Prunus persica | 1 | 1 | 1 | 1 | 1 | 4 | 4 | 4 | 4 | 4 | 0 | 0 | 1 | 1 | 1 |
| Prunus sp. | 23 | 23 | 23 | 23 | 23 | ||||||||||
| Quercus coccinea | 3 | 3 | 4 | 4 | 4 | ||||||||||
| Quercus falcata | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Quercus ilex | 0 | 0 | 1 | 1 | 1 | ||||||||||
| Quercus laevis | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Quercus macrocarpa | 1 | 1 | 2 | 2 | 2 | ||||||||||
| Quercus nigra | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Quercus palustris | 14 | 14 | 16 | 16 | 16 | ||||||||||
| Quercus phellos | 2 | 2 | 3 | 3 | 3 | ||||||||||
| Quercus robur | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Quercus rubra | 8 | 8 | 10 | 10 | 10 | ||||||||||
| Quercus shumardii | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Quercus sp. | 6 | 6 | 6 | 6 | 6 | ||||||||||
| Quercus suber | 2 | 2 | 3 | 3 | 3 | ||||||||||
| Ratibida columnifera | 3 | 3 | 3 | 3 | 3 | ||||||||||
| Rhamnus alaternus | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Rosa canina | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Rosa sp. | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Rosmarinus officinalis | 1 | 1 | 1 | 1 | 1 | 4 | 4 | 5 | 5 | 5 | 2 | 2 | 2 | 2 | 2 |
| Rubus sp. | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Salvia mellifera | 3 | 3 | 4 | 4 | 4 | ||||||||||
| Sambucus canadensis | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Sambucus sp. | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Sapindus saponaria | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Solidago virgaurea | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Spartium junceum | 2 | 2 | 2 | 2 | 2 | 5 | 5 | 7 | 7 | 7 | 1 | 1 | 1 | 1 | 2 |
| Spartium sp. | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Streptocarpus hybrids | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Ulmus americana | 4 | 4 | 6 | 6 | 6 | ||||||||||
| Ulmus crassifolia | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Vaccinium corymbosum | 0 | 0 | 0 | 0 | 3 | ||||||||||
| Vaccinium sp. | 8 | 8 | 8 | 8 | 8 | ||||||||||
| Vinca minor | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Vinca sp. | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Vitis aestivalis | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Vitis aestivalis hybrid | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Vitis candicans | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Vitis cinerea var. helleri × V. vulpina | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Vitis girdiana | 1 | 1 | 2 | 2 | 2 | ||||||||||
| Vitis rotundifolia | 3 | 3 | 3 | 3 | 3 | ||||||||||
| Vitis sp. | 44 | 44 | 44 | 44 | 45 | ||||||||||
| Vitis vinifera | 31 | 31 | 31 | 31 | 39 | ||||||||||
| Westringia fruticosa | 1 | 1 | 1 | 1 | 1 | 4 | 4 | 4 | 4 | 5 | |||||
| Westringia glabra | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Xanthium strumarium | 1 | 1 | 2 | 2 | 2 | ||||||||||
| Grand Total(Sum of records) | 157 | 157 | 158 | 158 | 168 | 379 | 379 | 440 | 440 | 444 | 346 | 346 | 423 | 423 | 439 |
| Plant species | 32 | 32 | 32 | 32 | 33 | 108 | 108 | 116 | 116 | 117 | 41 | 41 | 43 | 43 | 43 |
Appendix C – Xylella sequence types (STs)
1.
List of records of plant species infected by different Xylella sequence types (STs) in artificial, natural or not specified kind of infection. The records of plant species naturally found infected are divided per country.
|
X. fastidiosa subspecies ST Plant species |
Natural infection | Artificial infection | Not specified | Grand Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Argentina | Brazil | Costa Rica | Ecuador | France | Italy | Mexico | Spain | USA | unknown | Total | Total | Total | ||
| fastidiosa | 22 | 2 | 11 | 93 | 128 | 89 | 217 | |||||||
| ST1 | 2 | 11 | 81 | 94 | 89 | 183 | ||||||||
| Acer sp. | 1 | 1 | 1 | |||||||||||
| Amaranthus blitoides | 1 | 1 | ||||||||||||
| Ambrosia acanthicarpa | 2 | 2 | ||||||||||||
| Calicotome spinosa | 1 | 1 | 1 | |||||||||||
| Catharanthus roseus | 2 | 2 | ||||||||||||
| Cercis occidentalis | 1 | 1 | 1 | |||||||||||
| Chenopodium quinoa | 2 | 2 | ||||||||||||
| Cistus monspeliensis | 1 | 1 | 1 | |||||||||||
| Citrus sinensis | 1 | 1 | 1 | |||||||||||
| Conium maculatum | 2 | 2 | ||||||||||||
| Convolvulus arvensis | 1 | 1 | ||||||||||||
| Cyperus esculentus | 1 | 1 | ||||||||||||
| Datura wrightii | 1 | 1 | ||||||||||||
| Echinochloa crus‐galli | 1 | 1 | ||||||||||||
| Erigeron canadensis | 1 | 1 | ||||||||||||
| Eriochloa gracilis | 1 | 1 | ||||||||||||
| Erodium moschatum | 2 | 2 | ||||||||||||
| Eucalyptus camaldulensis | 2 | 2 | ||||||||||||
| Eucalyptus globulus | 1 | 1 | ||||||||||||
| Genista lucida | 1 | 1 | 1 | |||||||||||
| Helianthus annuus | 3 | 3 | ||||||||||||
| Ipomoea purpurea | 2 | 2 | ||||||||||||
| Juglans regia | 1 | 1 | 1 | |||||||||||
| Lactuca serriola | 3 | 3 | ||||||||||||
| Malva parviflora | 2 | 2 | ||||||||||||
| Medicago sativa | 3 | 3 | 6 | 9 | ||||||||||
| Metrosideros sp. | 1 | 1 | 1 | |||||||||||
| Nicotiana glauca | 2 | 2 | ||||||||||||
| Olea europaea | 1 | 1 | ||||||||||||
| Pluchea odorata | 1 | 1 | 1 | |||||||||||
| Polygala myrtifolia | 2 | 2 | 2 | |||||||||||
| Portulaca oleracea | 1 | 1 | ||||||||||||
| Prunus avium | 2 | 2 | 4 | 4 | ||||||||||
| Prunus dulcis | 1 | 17 | 18 | 18 | 36 | |||||||||
| Rhamnus alaternus | 1 | 1 | 1 | |||||||||||
| Rubus ursinus | 2 | 2 | ||||||||||||
| Rumex crispus | 1 | 1 | ||||||||||||
| Sambucus canadensis | 2 | 2 | 2 | |||||||||||
| Simmondsia chinensis | 2 | 2 | ||||||||||||
| Solanum lycopersicum | 1 | 1 | ||||||||||||
| Solanum melongena | 1 | 1 | ||||||||||||
| Sonchus oleraceus | 1 | 1 | ||||||||||||
| Sorghum halepense | 1 | 1 | ||||||||||||
| Spartium junceum | 1 | 1 | 1 | |||||||||||
| Vicia faba | 1 | 1 | ||||||||||||
| Vicia sativa | 1 | 1 | ||||||||||||
| Vitis aestivalis | 2 | 2 | 2 | |||||||||||
| Vitis girdiana | 1 | 1 | 1 | |||||||||||
| Vitis sp. | 1 | 29 | 30 | 30 | ||||||||||
| Vitis vinifera | 1 | 1 | 19 | 21 | 17 | 38 | ||||||||
| Xanthium strumarium | 3 | 3 | ||||||||||||
| ST17 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST18 | 1 | 1 | 1 | |||||||||||
| Vitis sp. | 1 | 1 | 1 | |||||||||||
| ST19 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST2 | 8 | 8 | 8 | |||||||||||
| Vitis rotundifolia | 3 | 3 | 3 | |||||||||||
| Vitis sp. | 5 | 5 | 5 | |||||||||||
| ST20 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST21 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST3 | 1 | 1 | 1 | |||||||||||
| Lupinus aridorum | 1 | 1 | 1 | |||||||||||
| ST33 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST4 | 3 | 3 | 3 | |||||||||||
| Vitis sp. | 3 | 3 | 3 | |||||||||||
| ST47 | 2 | 2 | 2 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| Vitis sp. | 1 | 1 | 1 | |||||||||||
| ST52 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST54 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST55 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST56 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST57 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST59 | 1 | 1 | 1 | |||||||||||
| Vitis vinifera | 1 | 1 | 1 | |||||||||||
| ST60 | 1 | 1 | 1 | |||||||||||
| Vitis vinifera | 1 | 1 | 1 | |||||||||||
| ST61 | 3 | 3 | 3 | |||||||||||
| Citrus sinensis | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 2 | 2 | 2 | |||||||||||
| ST72 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST76 | 2 | 2 | 2 | |||||||||||
| Coffea arabica | 2 | 2 | 2 | |||||||||||
| ST77 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| fastidiosa/sandyi | 3 | 1 | 4 | 4 | ||||||||||
| ST72 | 2 | 2 | 2 | |||||||||||
| Coffea arabica | 2 | 2 | 2 | |||||||||||
| ST75 | 1 | 1 | 1 | |||||||||||
| Coffea canephora | 1 | 1 | 1 | |||||||||||
| ST76 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| morus | 22 | 22 | 22 | |||||||||||
| ST29 | 7 | 7 | 7 | |||||||||||
| Morus alba | 3 | 3 | 3 | |||||||||||
| Morus rubra | 4 | 4 | 4 | |||||||||||
| ST30 | 5 | 5 | 5 | |||||||||||
| Morus alba | 4 | 4 | 4 | |||||||||||
| Nandina domestica | 1 | 1 | 1 | |||||||||||
| ST31 | 6 | 6 | 6 | |||||||||||
| Morus sp. | 6 | 6 | 6 | |||||||||||
| ST62 | 4 | 4 | 4 | |||||||||||
| Morus alba | 4 | 4 | 4 | |||||||||||
| multiplex | 3 | 77 | 21 | 159 | 260 | 19 | 12 | 291 | ||||||
| ST10 | 7 | 7 | 7 | |||||||||||
| Prunus domestica | 1 | 1 | 1 | |||||||||||
| Prunus persica | 3 | 3 | 3 | |||||||||||
| Prunus sp. | 3 | 3 | 3 | |||||||||||
| ST15 | 3 | 3 | 3 | |||||||||||
| Prunus cerasifera | 3 | 3 | 3 | |||||||||||
| ST22 | 3 | 3 | 1 | 4 | ||||||||||
| Ambrosia psilostachya | 1 | 1 | 1 | |||||||||||
| Ambrosia trifida | 2 | 2 | 1 | 3 | ||||||||||
| ST23 | 10 | 10 | 10 | |||||||||||
| Acer rubrum | 1 | 1 | 1 | |||||||||||
| Ambrosia trifida | 2 | 2 | 2 | |||||||||||
| Helianthus sp. | 2 | 2 | 2 | |||||||||||
| Iva annua | 1 | 1 | 1 | |||||||||||
| Quercus rubra | 1 | 1 | 1 | |||||||||||
| Ratibida columnifera | 2 | 2 | 2 | |||||||||||
| Solidago virgaurea | 1 | 1 | 1 | |||||||||||
| ST24 | 5 | 5 | 5 | |||||||||||
| Cercis occidentalis | 1 | 1 | 1 | |||||||||||
| Liquidambar styraciflua | 3 | 3 | 3 | |||||||||||
| Ulmus crassifolia | 1 | 1 | 1 | |||||||||||
| ST25 | 4 | 4 | 4 | |||||||||||
| Encelia farinosa | 4 | 4 | 4 | |||||||||||
| ST26 | 1 | 12 | 13 | 13 | ||||||||||
| Alnus rhombifolia | 1 | 1 | 1 | |||||||||||
| Prunus cerasifera | 2 | 2 | 2 | |||||||||||
| Prunus domestica | 1 | 1 | 2 | 2 | ||||||||||
| Prunus sp. | 8 | 8 | 8 | |||||||||||
| ST27 | 6 | 6 | 2 | 8 | ||||||||||
| Ginkgo biloba | 1 | 1 | 1 | |||||||||||
| Lagerstroemia sp. | 1 | 1 | 1 | |||||||||||
| Prunus cerasifera | 1 | 1 | ||||||||||||
| Prunus dulcis | 2 | 2 | 1 | 3 | ||||||||||
| Prunus sp. | 2 | 2 | 2 | |||||||||||
| ST28 | 4 | 4 | 1 | 5 | ||||||||||
| Ambrosia trifida | 2 | 2 | 1 | 3 | ||||||||||
| Helianthus sp. | 1 | 1 | 1 | |||||||||||
| Iva annua | 1 | 1 | 1 | |||||||||||
| ST32 | 2 | 2 | 1 | 3 | ||||||||||
| Rubus fruticosus | 1 | 1 | ||||||||||||
| Rubus sp. | 2 | 2 | 2 | |||||||||||
| ST34 | 1 | 1 | 1 | |||||||||||
| Prunus cerasifera | 1 | 1 | 1 | |||||||||||
| ST35 | 1 | 1 | 1 | |||||||||||
| Xanthium strumarium | 1 | 1 | 1 | |||||||||||
| ST36 | 1 | 1 | 1 | |||||||||||
| Prunus sp. | 1 | 1 | 1 | |||||||||||
| ST37 | 1 | 1 | 1 | |||||||||||
| Lupinus villosus | 1 | 1 | 1 | |||||||||||
| ST38 | 1 | 1 | 1 | |||||||||||
| Platanus occidentalis | 1 | 1 | 1 | |||||||||||
| ST39 | 6 | 6 | 6 | |||||||||||
| Koelreuteria bipinnata | 1 | 1 | 1 | |||||||||||
| Liquidambar styraciflua | 4 | 4 | 4 | |||||||||||
| Prunus sp. | 1 | 1 | 1 | |||||||||||
| ST40 | 4 | 4 | 1 | 5 | ||||||||||
| Prunus cerasifera | 3 | 3 | 1 | 4 | ||||||||||
| Sambucus sp. | 1 | 1 | 1 | |||||||||||
| ST41 | 3 | 3 | 3 | |||||||||||
| Prunus sp. | 1 | 1 | 1 | |||||||||||
| Ulmus americana | 2 | 2 | 2 | |||||||||||
| ST42 | 6 | 6 | 3 | 9 | ||||||||||
| Ambrosia trifida | 2 | 2 | 1 | 3 | ||||||||||
| Sapindus saponaria | 1 | 1 | 1 | |||||||||||
| Vaccinium corymbosum | 1 | 1 | ||||||||||||
| Vaccinium corymbosum × V. angustifolium hybrid | 1 | 1 | ||||||||||||
| Vaccinium sp. | 3 | 3 | 3 | |||||||||||
| ST43 | 4 | 4 | 2 | 6 | ||||||||||
| Vaccinium corymbosum | 1 | 1 | ||||||||||||
| Vaccinium corymbosum × V. angustifolium hybrid | 1 | 1 | ||||||||||||
| Vaccinium sp. | 4 | 4 | 4 | |||||||||||
| ST44 | 2 | 2 | 2 | |||||||||||
| Quercus palustris | 1 | 1 | 1 | |||||||||||
| Quercus rubra | 1 | 1 | 1 | |||||||||||
| ST45 | 6 | 6 | 6 | |||||||||||
| Acer griseum | 1 | 1 | 1 | |||||||||||
| Ampelopsis cordata | 1 | 1 | 1 | |||||||||||
| Cercis canadensis | 3 | 3 | 3 | |||||||||||
| Gleditsia triacanthos | 1 | 1 | 1 | |||||||||||
| ST46 | 3 | 3 | 3 | |||||||||||
| Celtis occidentalis | 1 | 1 | 1 | |||||||||||
| Chionanthus sp. | 1 | 1 | 1 | |||||||||||
| Prunus armeniaca | 1 | 1 | 1 | |||||||||||
| ST48 | 1 | 1 | 1 | |||||||||||
| Sapindus saponaria | 1 | 1 | 1 | |||||||||||
| ST49 | 1 | 1 | 1 | |||||||||||
| Prunus sp. | 1 | 1 | 1 | |||||||||||
| ST50 | 2 | 2 | 2 | |||||||||||
| Fraxinus americana | 1 | 1 | 1 | |||||||||||
| Fraxinus sp. | 1 | 1 | 1 | |||||||||||
| ST51 | 2 | 2 | 2 | |||||||||||
| Periwinkle (common name) | 1 | 1 | 1 | |||||||||||
| Vinca sp. | 1 | 1 | 1 | |||||||||||
| ST58 | 1 | 1 | 1 | 2 | ||||||||||
| Ambrosia trifida | 1 | 1 | 1 | 2 | ||||||||||
| ST6 | 2 | 2 | 10 | 14 | 11 | 25 | ||||||||
| Medicago sativa | 3 | 3 | ||||||||||||
| Olea europaea | 1 | 1 | 1 | 2 | ||||||||||
| Polygala myrtifolia | 1 | 1 | ||||||||||||
| Prunus dulcis | 1 | 10 | 11 | 4 | 15 | |||||||||
| Rubus ursinus | 1 | 1 | ||||||||||||
| Spartium junceum | 2 | 2 | 2 | |||||||||||
| Vitis vinifera | 1 | 1 | ||||||||||||
| ST6 and ST7 | 1 | 1 | 1 | |||||||||||
| Cistus monspeliensis | 1 | 1 | 1 | |||||||||||
| ST6 and/or ST7 | 72 | 72 | 72 | |||||||||||
| Acacia dealbata | 1 | 1 | 1 | |||||||||||
| Acer pseudoplatanus | 2 | 2 | 2 | |||||||||||
| Anthyllis hermanniae | 1 | 1 | 1 | |||||||||||
| Artemisia arborescens | 2 | 2 | 2 | |||||||||||
| Asparagus acutifolius | 2 | 2 | 2 | |||||||||||
| Calicotome villosa | 1 | 1 | 1 | |||||||||||
| Cercis siliquastrum | 1 | 1 | 1 | |||||||||||
| Cistus creticus | 1 | 1 | 1 | |||||||||||
| Cistus monspeliensis | 2 | 2 | 2 | |||||||||||
| Cistus salviifolius | 2 | 2 | 2 | |||||||||||
| Coronilla valentina | 2 | 2 | 2 | |||||||||||
| Cytisus scoparius | 1 | 1 | 1 | |||||||||||
| Cytisus sp. | 2 | 2 | 2 | |||||||||||
| Cytisus villosus | 1 | 1 | 1 | |||||||||||
| Euryops chrysanthemoides | 1 | 1 | 1 | |||||||||||
| Genista corsica | 1 | 1 | 1 | |||||||||||
| Genista ephedroides | 2 | 2 | 2 | |||||||||||
| Genista × spachiana | 2 | 2 | 2 | |||||||||||
| Hebe sp. | 2 | 2 | 2 | |||||||||||
| Helichrysum italicum | 2 | 2 | 2 | |||||||||||
| Lavandula angustifolia | 2 | 2 | 2 | |||||||||||
| Lavandula dentata | 2 | 2 | 2 | |||||||||||
| Lavandula sp. | 3 | 3 | 3 | |||||||||||
| Lavandula stoechas | 2 | 2 | 2 | |||||||||||
| Lavandula × heterophylla | 2 | 2 | 2 | |||||||||||
| Lavandula × intermedia | 3 | 3 | 3 | |||||||||||
| Medicago sativa | 1 | 1 | 1 | |||||||||||
| Metrosideros excelsa | 2 | 2 | 2 | |||||||||||
| Myrtus communis | 2 | 2 | 2 | |||||||||||
| Pelargonium graveolens | 2 | 2 | 2 | |||||||||||
| Pelargonium sp. | 2 | 2 | 2 | |||||||||||
| Phagnalon saxatile | 1 | 1 | 1 | |||||||||||
| Polygala myrtifolia | 4 | 4 | 4 | |||||||||||
| Polygala sp. | 1 | 1 | 1 | |||||||||||
| Prunus cerasifera | 2 | 2 | 2 | |||||||||||
| Prunus dulcis | 1 | 1 | 1 | |||||||||||
| Quercus suber | 2 | 2 | 2 | |||||||||||
| Rosa canina | 1 | 1 | 1 | |||||||||||
| Rosmarinus officinalis | 2 | 2 | 2 | |||||||||||
| Spartium junceum | 3 | 3 | 3 | |||||||||||
| Westringia fruticosa | 1 | 1 | 1 | |||||||||||
| ST63 | 1 | 1 | 1 | |||||||||||
| Prunus domestica | 1 | 1 | 1 | |||||||||||
| ST67 | 1 | 1 | 1 | |||||||||||
| Prunus domestica | 1 | 1 | 1 | |||||||||||
| ST7 | 1 | 2 | 10 | 13 | 8 | 21 | ||||||||
| Medicago sativa | 1 | 1 | ||||||||||||
| Olea europaea | 1 | 1 | 3 | 4 | ||||||||||
| Olea sp. | 1 | 1 | 1 | |||||||||||
| Polygala myrtifolia | 1 | 1 | 2 | 1 | 3 | |||||||||
| Prunus dulcis | 1 | 4 | 5 | 3 | 8 | |||||||||
| Prunus sp. | 1 | 1 | 1 | |||||||||||
| Salvia mellifera | 3 | 3 | 3 | |||||||||||
| ST79 | 1 | 1 | 1 | |||||||||||
| Polygala myrtifolia | 1 | 1 | 1 | |||||||||||
| ST8 | 9 | 9 | 9 | |||||||||||
| Alnus rhombifolia | 1 | 1 | 1 | |||||||||||
| Carya illinoinensis | 1 | 1 | 1 | |||||||||||
| Platanus occidentalis | 5 | 5 | 5 | |||||||||||
| Quercus palustris | 1 | 1 | 1 | |||||||||||
| Ulmus americana | 1 | 1 | 1 | |||||||||||
| ST81 | 17 | 17 | 17 | |||||||||||
| Acacia sp. | 1 | 1 | 1 | |||||||||||
| Ficus carica | 2 | 2 | 2 | |||||||||||
| Fraxinus angustifolia | 1 | 1 | 1 | |||||||||||
| Lavandula dentata | 1 | 1 | 1 | |||||||||||
| Olea europaea | 2 | 2 | 2 | |||||||||||
| Olea europaea subsp. sylvestris | 2 | 2 | 2 | |||||||||||
| Polygala myrtifolia | 2 | 2 | 2 | |||||||||||
| Prunus domestica | 1 | 1 | 1 | |||||||||||
| Prunus dulcis | 2 | 2 | 2 | |||||||||||
| Rhamnus alaternus | 1 | 1 | 1 | |||||||||||
| Rosmarinus officinalis | 2 | 2 | 2 | |||||||||||
| ST9 | 28 | 28 | 28 | |||||||||||
| Quercus coccinea | 2 | 2 | 2 | |||||||||||
| Quercus falcata | 1 | 1 | 1 | |||||||||||
| Quercus laevis | 2 | 2 | 2 | |||||||||||
| Quercus nigra | 1 | 1 | 1 | |||||||||||
| Quercus palustris | 11 | 11 | 11 | |||||||||||
| Quercus phellos | 1 | 1 | 1 | |||||||||||
| Quercus robur | 1 | 1 | 1 | |||||||||||
| Quercus rubra | 5 | 5 | 5 | |||||||||||
| Quercus shumardii | 1 | 1 | 1 | |||||||||||
| Quercus sp. | 3 | 3 | 3 | |||||||||||
| pauca | 3 | 94 | 8 | 2 | 3 | 167 | 7 | 1 | 285 | 40 | 325 | |||
| ST11 | 48 | 48 | 48 | |||||||||||
| Citrus sinensis | 18 | 18 | 18 | |||||||||||
| Citrus sp. | 29 | 29 | 29 | |||||||||||
| Coffea sp. | 1 | 1 | 1 | |||||||||||
| ST12 | 3 | 3 | 3 | |||||||||||
| Citrus sinensis | 2 | 2 | 2 | |||||||||||
| Citrus sp. | 1 | 1 | 1 | |||||||||||
| ST13 | 7 | 7 | 7 | |||||||||||
| Citrus sinensis | 1 | 1 | 1 | |||||||||||
| Citrus sp. | 6 | 6 | 6 | |||||||||||
| ST14 | 7 | 7 | 7 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| Coffea sp. | 6 | 6 | 6 | |||||||||||
| ST16 | 22 | 22 | 1 | 23 | ||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| Coffea sp. | 17 | 17 | 17 | |||||||||||
| Olea europaea | 4 | 4 | 1 | 5 | ||||||||||
| ST53 | 1 | 7 | 3 | 167 | 178 | 39 | 217 | |||||||
| Acacia saligna | 1 | 1 | 1 | |||||||||||
| Asparagus acutifolius | 1 | 1 | 1 | |||||||||||
| Catharanthus roseus | 2 | 2 | 4 | 6 | ||||||||||
| Chenopodium album | 2 | 2 | 2 | |||||||||||
| Cistus creticus | 1 | 1 | 1 | |||||||||||
| Citrus sinensis | 1 | 1 | ||||||||||||
| Coffea arabica | 2 | 2 | 2 | |||||||||||
| Coffea sp. | 1 | 1 | 1 | |||||||||||
| Dodonaea viscosa | 1 | 1 | 1 | |||||||||||
| Eremophila maculata | 1 | 1 | 1 | |||||||||||
| Erigeron bonariensis | 2 | 2 | 2 | |||||||||||
| Erigeron sumatrensis | 1 | 1 | 1 | |||||||||||
| Euphorbia terracina | 1 | 1 | 1 | |||||||||||
| Grevillea juniperina | 1 | 1 | 1 | |||||||||||
| Hebe sp. | 1 | 1 | 1 | |||||||||||
| Heliotropium europaeum | 2 | 2 | 2 | |||||||||||
| Laurus nobilis | 1 | 1 | 1 | |||||||||||
| Lavandula angustifolia | 1 | 1 | 1 | |||||||||||
| Lavandula stoechas | 1 | 1 | 1 | |||||||||||
| Myoporum insulare | 1 | 1 | 1 | |||||||||||
| Myrtus communis | 1 | 1 | 1 | |||||||||||
| Nerium oleander | 5 | 6 | 11 | 5 | 16 | |||||||||
| Olea europaea | 113 | 113 | 18 | 131 | ||||||||||
| Pelargonium fragrans | 1 | 1 | 1 | |||||||||||
| Periwinkle (common name) | 1 | 1 | 1 | |||||||||||
| Phillyrea latifolia | 1 | 1 | 1 | |||||||||||
| Polygala myrtifolia | 1 | 5 | 6 | 3 | 9 | |||||||||
| Prunus avium | 6 | 6 | 2 | 8 | ||||||||||
| Prunus dulcis | 4 | 4 | 4 | 8 | ||||||||||
| Prunus persica | 1 | 1 | 1 | |||||||||||
| Prunus × amygdalo‐persica | 1 | 1 | ||||||||||||
| Quercus ilex | 1 | 1 | 1 | |||||||||||
| Quercus pubescens | 1 | 1 | ||||||||||||
| Rhamnus alaternus | 1 | 1 | 1 | |||||||||||
| Rosmarinus officinalis | 1 | 1 | 1 | |||||||||||
| Spartium junceum | 1 | 1 | 1 | |||||||||||
| Vinca minor | 1 | 1 | 1 | |||||||||||
| Westringia fruticosa | 3 | 3 | 3 | |||||||||||
| Westringia glabra | 1 | 1 | 1 | |||||||||||
| ST64 | 1 | 1 | 1 | |||||||||||
| Citrus sinensis | 1 | 1 | 1 | |||||||||||
| ST65 | 1 | 1 | 1 | |||||||||||
| Citrus sinensis | 1 | 1 | 1 | |||||||||||
| ST66 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST68 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST69 | 2 | 2 | 2 | |||||||||||
| Citrus sinensis | 2 | 2 | 2 | |||||||||||
| ST70 | 1 | 1 | 1 | |||||||||||
| Hibiscus rosa‐sinensis | 1 | 1 | 1 | |||||||||||
| ST71 | 1 | 1 | 1 | |||||||||||
| Prunus domestica | 1 | 1 | 1 | |||||||||||
| ST73 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST73 and ST53 | 1 | 1 | 1 | |||||||||||
| Coffea arabica | 1 | 1 | 1 | |||||||||||
| ST74 | 2 | 2 | 2 | |||||||||||
| Coffea arabica | 2 | 2 | 2 | |||||||||||
| ST78 | 1 | 1 | 1 | |||||||||||
| Prunus dulcis | 1 | 1 | 1 | |||||||||||
| ST80 | 7 | 7 | 7 | |||||||||||
| Acacia sp. | 1 | 1 | 1 | |||||||||||
| Lavandula dentata | 1 | 1 | 1 | |||||||||||
| Olea europaea | 1 | 1 | 1 | |||||||||||
| Olea europaea subsp. sylvestris | 1 | 1 | 1 | |||||||||||
| Polygala myrtifolia | 1 | 1 | 1 | |||||||||||
| Prunus dulcis | 1 | 1 | 1 | |||||||||||
| Rosmarinus officinalis | 1 | 1 | 1 | |||||||||||
| sandyi | 2 | 1 | 23 | 26 | 4 | 30 | ||||||||
| ST5 | 23 | 23 | 4 | 27 | ||||||||||
| Hemerocallis sp. | 1 | 1 | 1 | |||||||||||
| Jacaranda mimosifolia | 1 | 1 | 1 | |||||||||||
| Magnolia grandiflora | 1 | 1 | 1 | |||||||||||
| Nerium oleander | 20 | 20 | 1 | 21 | ||||||||||
| Prunus dulcis | 1 | 1 | ||||||||||||
| Vinca major | 2 | 2 | ||||||||||||
| ST72 | 1 | 1 | 1 | |||||||||||
| Coffea sp. | 1 | 1 | 1 | |||||||||||
| ST76 | 1 | 1 | 2 | 2 | ||||||||||
| Coffea sp. | 1 | 1 | 1 | |||||||||||
| Polygala myrtifolia | 1 | 1 | 1 | |||||||||||
| Grand Total | 3 | 99 | 33 | 2 | 81 | 167 | 3 | 39 | 297 | 1 | 725 | 152 | 12 | 889 |
Appendix D – List of contradictive findings
1.
| Plant family | Plant species | Detection methods | POS/NEG | Reference |
|---|---|---|---|---|
| Asteraceae | Baccharis pilularis | ELISA | POS | Costa et al. (2004) |
| PCR‐based methods | NEG | |||
| Rutaceae | Citrus limon | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Rutaceae | Citrus sp. | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Asteraceae | Encelia farinosa | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Araliaceae | Hedera helix | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Rosaceae | Heteromeles arbutifolia | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Juglandaceae | Juglans californica | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Apocynaceae | Nerium oleander | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Oleaceae | Olea europaea | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Anacardiaceae | Pistacia vera | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Platanaceae | Platanus racemosa | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Rosaceae | Prunus americana | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Rosaceae | Prunus sp. | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Fagaceae | Quercus agrifolia | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Salicaceae | Salix sp. | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Adoxaceae | Sambucus sp. | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Anacardiaceae | Schinus molle | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Solanaceae | Solanum elaeagnifolium | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Rutaceae | Citrus sinensis | Immunological detection method | POS | Damsteegt et al. (2006) |
| PCR‐based methods | NEG | |||
| Rutaceae | Citrus clementina | ELISA | NEG | GonzaLez et al. (2002) |
| PCR‐based methods | POS | |||
| Rutaceae | Citrus clementina × C. sinensis | ELISA | NEG | |
| PCR‐based methods | POS | |||
| Rutaceae | Citrus clementina × C. sinensis | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Rutaceae | Citrus reticulata | ELISA | NEG | |
| PCR‐based methods | POS | |||
| Rutaceae | Citrus × tangelo | ELISA | NEG | |
| PCR‐based methods | POS | |||
| Fagaceae | Quercus palustris | ELISA | NEG | Harris et al. (2013) |
| PCR‐based methods | POS | |||
| Fagaceae | Quercus rubra | ELISA | NEG | |
| PCR‐based methods | POS | |||
| Ginkgoaceae | Ginkgo biloba | ELISA | POS | Harris et al. (2014) |
| PCR‐based methods | NEG | |||
| Magnoliaceae | Liriodendron tulipifera | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Apocynaceae | Nerium oleander | ELISA | POS | Hernandez‐Martinez et al. (2006) |
| PCR‐based methods | NEG | |||
| Vitaceae | Vitis vinifera | ELISA | POS | |
| PCR‐based methods | NEG | |||
| Rosaceae | Prunus persica | ELISA | NEG | Hopkins and Adlerz (1988) |
| Immunological detection method | POS | |||
| Anacardiaceae | Rhus sp. | ELISA | POS | |
| Immunological detection method | NEG | |||
| Asteraceae | Solidago fistulosa | ELISA | POS | |
| Immunological detection method | NEG | |||
| Rutaceae | Citrus × tangelo | Immunological detection method | NEG | Laranjeira et al. (1998) |
| PCR‐based methods | POS | |||
| Malvaceae | Hibiscus syriacus | ELISA | POS | McGaha et al. (2007) |
| PCR‐based methods | NEG | |||
| Vitaceae | Vitis vinifera | ELISA | POS | Amanifar et al. (2014) |
| PCR‐based methods | NEG | |||
| Vitaceae | Vitis sp. | ELISA | NEG | Qi (2007) |
| PCR‐based methods | POS | |||
| Rutaceae | Citrus sinensis | ELISA | POS | Souza et al. (2000) |
| PCR‐based methods | NEG | |||
| Rutaceae | Citrus sinensis | ELISA | NEG | Souza et al. (2000) |
| PCR‐based methods | POS | |||
| Oleaceae | Olea sp. | ELISA | NEG | Yaseen et al. (2015) |
| PCR‐based methods | POS | |||
| Verbenaceae | Phyla nodiflora | ELISA | POS | Buzombo et al. (2006) |
| Immunological detection method | NEG | |||
| PCR‐based methods | POS | |||
| Rosaceae | Rubus trivialis | ELISA | POS | |
| Immunological detection method | NEG | |||
| PCR‐based methods | POS | |||
| Bromeliaceae | Tillandsia usneoides | ELISA | POS | |
| Immunological detection method | NEG | |||
| PCR‐based methods | NEG | |||
| Apocynaceae | Vinca minor | ELISA | POS | |
| Immunological detection method | NEG | |||
| PCR‐based methods | NEG | |||
| Vitaceae | Vitis candicans | ELISA | POS | |
| Immunological detection method | NEG | |||
| PCR‐based methods | POS |
Appendix E – References used for data extraction
1.
Abrahams BR and Norton JD, 1994. Transmission of plum leaf scald or phony peach disease, Xylella fastidiosa Wells, by two budding methods in peach and plum. Hortscience, 29(7), 736–736.
Adams JP, Rousseau RJ and Leininger TD, 2012. Genetic control of growth traits and inheritance of resistance to bacterial leaf scorch in American sycamore. Silvae Genetica, 61(4–5), 198–206. https://doi.org/10.1515/sg-2012-0025
Adlerz WC and Hopkins DL, 1981. Detection of Pierce's disease bacterium in wild plants in Florida. Phytopathology, 71(8), 856–856.
Agostini JP and Haberle TJ, 2000. The effect of Citrus tree age on citrus variegated chlorosis. Proceedings of the 14th Conference of the International Organization of Citrus Virologists, Campinas, Sao Paulo State, Brazil, 13–18 September 1998:232–237.
Agricultural Research Administration, 1945. Report of the Chief of the Bureau of Entomology and Plant Quarantine, Agricultural Research Administration, 1945, 63 pp.
Aguilar E, Moreira L and Rivera C, 2008. Confirmation of Xylella fastidiosa infecting grapes Vitis vinifera in Costa Rica. Tropical Plant Pathology, 33(6), 444–448.
Aguilar E, Villalobos W, Garita L and Rivera C, 2006. Confirmation of the presence of Xylella fastidiosa in plants of grapevine in Costa Rica. Phytopathology, 96(6):S162–S162.
Aguilar E, Villalobos W, Moreira L, Rodriguez CM, Kitajima EW and Rivera C, 2005. First report of Xylella fastidiosa infecting Citrus in Costa Rica. Plant Disease, 89(6), 687–687. https://doi.org/10.1094/Pd-89-0687b
Ahern SJ, Das M, Bhowmick TS, Young R and Gonzalez CF, 2014. Characterization of novel virulent broad‐host‐range phages of Xylella fastidiosa and Xanthomonas. Journal of Bacteriology, 196(2), 459–471. https://doi.org/10.1128/JB.01080-13
Albibi R, Chen J and Lamikanra O, 1997. Evaluation of RAPD in grape Pierce's disease bacterium study. Phytopathology, 87(6 Suppl.), S3–S3.
Albibi R, Chen J, Lamikanra O, Banks D, Jarret RL and Smith BJ, 1998. RAPD fingerprinting Xylella fastidiosa Pierce's disease strains isolated from a vineyard in north Florida. FEMS Microbiology Letters, 165(2), 347–352. https://doi.org/10.1016/s0378-1097(98)00300-0
Aldrich JH, Gould AB and Martin FG, 1991. A study of the distribution of Xylella fastidiosa within the roots of peach. Phytopathology, 81(10), 1232–1232.
Aldrich JH, Gould AB and Martin FG, 1992. Distribution of Xylella fastidiosa within Roots of Peach. Plant Disease, 76(9), 885–888. https://doi.org/10.1094/pd-76-0885
Aldrich TJ, Rolshausen PE, Roper MC, Reader JM, Steinhaus MJ, Rapicavoli J, Vosburg DA and Maloney KN, 2015. Radicinin from Cochliobolus sp. inhibits Xylella fastidiosa, the causal agent of Pierce's disease of grapevine. Phytochemistry, 116, 130–137. https://doi.org/10.1016/j.phytochem.2015.03.015
Almeida RP and Purcell AH, 2003. Biological traits of Xylella fastidiosa strains from grapes and almonds. Appl Environ Microbiol, 69(12), 7447–7452.
Almeida RP and Purcell AH, 2003. Transmission of Xylella fastidiosa to grapevines by Homalodisca coagulata (Hemiptera: Cicadellidae). Journal of Economic Entomology, 96(2), 264–271.
Almeida RP, Chau JH, Nascimento FE and Lopes JS, 2007. Genetic structure of Citrus and coffee isolates of Xylella fastidiosa from Brazil. Phytopathology, 97(7), S3–S3.
Almeida RP, Mann R and Purcell AH, 2004. Xylella fastidiosa cultivation on a minimal solid defined medium. Current Microbiology, 48(5), 368–372. https://doi.org/10.1007/s00284-003-4219-x
Almeida RP, Nascimento FE, Chau J, Prado SS, Tsai CW, Lopes SA and Lopes JR, 2008. Genetic structure and biology of Xylella fastidiosa strains causing disease in Citrus and coffee in Brazil. Applied and Environmental Microbiology, 74(12), 3690–3701. https://doi.org/10.1128/AEM.02388-07
Almeida RPP and Purcell AH, 2002. Homalodisca coagulata (Hemiptera, Cicadellidae) transmission of Xylella fastidiosa to almonds. Phytopathology, 92(6 Supplement), S3–S3.
Almeida RPP and Purcell AH, 2003. Homalodisca coagulata (Hemiptera, Cicadellidae) transmission of Xylella fastidiosa to almond. Plant Disease, 87(10), 1255–1259. https://doi.org/10.1094/pdis.2003.87.10.1255
Almeida RPP and Purcell AH, 2006. Patterns of Xylella fastidiosa colonization on the precibarium of sharpshooter vectors relative to transmission to plants. Annals of the Entomological Society of America, 99(5), 884–890. https://doi.org/10.1603/0013-8746(2006)99[884:poxfco]2.0.co;2
Almeida RPP, Pereira EF, Purcell AH and Lopes JRS, 2001. Multiplication and movement of a Citrus strain of Xylella fastidiosa within sweet orange. Plant Disease, 85(4), 382–386. https://doi.org/10.1094/pdis.2001.85.4.382
Almeida RPP, Wistrom C, Hill BL, Hashim J and Purcell AH, 2005. Vector transmission of Xylella fastidiosa to dormant grape. Plant Disease, 89(4), 419–424. https://doi.org/10.1094/Pd-89-0419
Alves E, Kitajima EW and Leite B, 2003. Interaction of Xylella fastidiosa with different cultivars of Nicotiana tabacum: a comparison of colonization patterns. Journal of Phytopathology‐Phytopathologische Zeitschrift, 151(9), 500–506. https://doi.org/10.1046/j.1439-0434.2003.00759.x
Alves E, Leite B, Marucci RC, Pascholati SF, Lopes JR and Andersen PC, 2008. Retention sites for Xylella fastidiosa in four sharpshooter vectors (Hemiptera: Cicadellidae) analyzed by scanning electron microscopy. Current Microbiology, 56(5), 531–538. https://doi.org/10.1007/s00284-008-9119-7
Alves E, Leite B, Pascholati SF, Ishida ML and Andersen PC, 2009. Citrus sinensis leaf petiole and blade colonization by Xylella fastidiosa: Details of xylem vessel occlusion. Scientia Agricola, 66(2), 218–224. https://doi.org/10.1590/s0103-90162009000200011
Alves E, Marucci CR, Lopes JRS and Leite B, 2004. Leaf symptoms on plum, coffee and Citrus and the relationship with the extent of xylem vessels colonized by Xylella fastidiosa. Journal of Phytopathology, 152(5), 291–297. https://doi.org/10.1111/j.1439-0434.2004.00843.x
Alves E, Marucci RC, Pascholati SF, Lopes JRS and Leite B, 2003. Relationship between leaf symptoms and the proportions of xylem‐colonized vessels of plum, coffee and Citrus colonized by Xylella fastidiosa. Phytopathology, 93(6 Supplement), S4–S4.
Alves E, Pascholati SF and Leite B, 2002. Varieties of Nicotiana tabacum as alternative experimental hosts for the study of plant – Xylella fastidiosa interactions. XXXIV Brasilian Phytopathological Congress and XI Latinamerican Phytopathological Congress, Sao Pedro, SP, Brazil, August 5–10, 2001. Fitopatologia, 37(1), 10–66.
Alves E, Wulff NA, Pascholati SF and Leite B, 2002. Xylella fastidiosa colonizes preferentially pitted xylem vessels. XXXIV Brasilian Phytopathological Congress and XI Latin American Phytopathological Congress, Sao Pedro, SP, Brazil, August 5–10, 2001. Fitopatologia, 37(1), 10–66.
Amanifar N, Taghavi M and Salehi M, 2016. Xylella fastidiosa from almond in Iran: overwinter recovery and effects of antibiotics. Phytopathologia Mediterranea, 55(3), 337–345. https://doi.org/10.14601/Phytopathol_Mediterr-17682
Amanifar N, Taghavi M, Izadpanah K and Babaei G, 2014. Isolation and pathogenicity of Xylella fastidiosa from grapevine and almond in Iran. Phytopathologia Mediterranea, 53(2), 318–327.
Amaral AMD, Paiva LV and Souza MD, 1994. Effect of pruning in Valencia and Pera Rio orange trees (Citrus sinensis (L.) Osbeck) with symptoms of citrus variegated chlorosis (CVC). Ciencia e Pratica, 18(3), 306–307.
Amorim L, Bergamin Filho A, Palazzo DA, Bassanezi RB, Godoy CV and Torres GAM, 1993. Citrus variegation chlorosis: A diagrammatic scale for the evaluation of the severity of the disease. Fitopatologia Brasileira, 18(2), 174–180.
Amsden BF, Vincelli P and Hartman JR, 2010. Detection of Xylella fastidiosa in petioles is independent of sample storage time and temperature. Phytopathology, 100(6), S6‐S6.
Anas O, Harrison UJ, Brannen PM and Sutton TB, 2008. The effect of warming winter temperatures on the severity of Pierce's disease in the Appalachian Mountains and Piedmont of the southeastern United States. Plant Health Progress(July):0718–0701.
Appel DN and Torres CP, 2008. Comparative epidemiology of Pierce's disease in grape varieties in Texas. Phytopathology, 98(6), S210–S210.
Araujo WL, Marcon J, Maccheroni W, van Elsas JD, van Vuurde JWL and Azevedo JL, 2002. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in Citrus plants. Applied and Environmental Microbiology, 68(10), 4906–4914. https://doi.org/10.1128/Aem.68.10.4906-4914.2002
Auger J, Mircetich SM and Nyland G, 1974. Interrelation between bacteria causing Pierce's disease of grapevines and almond leaf scorch. Proceedings of the American Phytopathological Society, 1, (1975)‐(1975).
Auger JG, Shalla TA and Kado CI, 1974. Pierce's disease of grapevines evidence for a bacterial etiology. Science (Washington D.C.), 184(4144), 1375–1377.
Ayres AJ, Gimenes‐Fernandes N and Barbosa JC, 2001. Intensity of the citrus variegated chlorosis in the state of Sao Paulo and South of Triangulo Mineiro. Summa Phytopathologica, 27(2), 189–197.
Ayres AJ, Gimenes‐Fernandes N and Barbosa JC, 2002. Citrus variegated chlorosis (CVC): current status in commercial orange groves in the states of Sao Paulo and Minas Gerais (southern Triangulo Mineiro). Proceedings of the Fifteenth Conference of the International Organization of Citrus Virologists, Paphos, Cyprus, 11–16 November 2001:288–292.
Baccari C and Lindow SE, 2011. Assessment of the Process of Movement of Xylella fastidiosa Within Susceptible and Resistant Grape Cultivars. Phytopathology, 101(1), 77–84. https://doi.org/10.1094/Phyto-04-10-0104
Baccari C, Killiny N, Ionescu M, Almeida RP and Lindow SE, 2014. Diffusible signal factor‐repressed extracellular traits enable attachment of Xylella fastidiosa to insect vectors and transmission. Phytopathology, 104(1), 27–33. https://doi.org/10.1094/PHYTO-06-13-0151-R
Backus EA and Morgan DJ, 2011. Spatiotemporal colonization of Xylella fastidiosa in its vector supports the role of egestion in the inoculation mechanism of foregut‐borne plant pathogens. Phytopathology, 101(8), 912–922. https://doi.org/10.1094/PHYTO-09-10-0231
Banks D, Albibi R, Chen J, Lamikanra O, Jarret RL and Smith BJ, 1999. Specific detection of Xylella fastidiosa Pierce's disease strains. Curr Microbiol, 39(2), 85–88.
Barbosa D, Alencar VC, Santos DS, de Freitas Oliveira AC, de Souza AA, Coletta‐Filho HD, de Oliveira RS and Nunes LR, 2015. Comparative genomic analysis of coffee‐infecting Xylella fastidiosa strains isolated from Brazil. Microbiology, 161(Pt 5), 1018–1033. https://doi.org/10.1099/mic.0.000068
Barbosa FFL, Muller GW, Vaz Filho D and Pompeu Junior J, 2001. Rootstocks do not affect expression of symptoms of citrus variegated chlorosis (CVC) in sweet oranges. Revista Brasileira de Fruticultura, 23(1), 212–214.
Barnard EL, Ash EC, Hopkins DL and McGovern RJ, 1998. Distribution of Xylella fastidiosa in oaks in Florida and its association with growth decline in Quercus laevis. Plant Disease, 82(5), 569–572. https://doi.org/10.1094/pdis.1998.82.5.569
Basha SM, Mazhar H and Vasanthaiah HK, 2010. Proteomics approach to identify unique xylem sap proteins in Pierce's disease‐tolerant Vitis species. Appl Biochem Biotechnol, 160(3), 932–944. https://doi.org/10.1007/s12010-009-8620-1
Baumgartel J and Walker MA, 2007. Optimizing greenhouse evaluations of Pierce's disease resistance. American Journal of Enology and Viticulture, 58(3), 413a‐414a.
Baumgartner K and Warren J, 2005. Role of Xylella fastidiosa populations in systemic riparian hosts and the spread of Pierce's disease to grapevines in Northern California. Phytopathology, 95(6), S8–S8.
Baumgartner K and Warren JG, 2005. Persistence of Xylella fastidiosa in riparian hosts near northern California vineyards. Plant Disease, 89(10), 1097–1102. https://doi.org/10.1094/Pd-89-1097
Bazzi C, Stefani E, Padovan F and Mazzucchi U, 1990. Xylella fastidiosa Wells. et al. is not associated with ‘mal dell'esca’ of grapevine in the Emilia‐Romagna region. Phytopathologia Mediterranea, 24(1), 56–58.
Behringer G and Kobayashi D, 2013. The genetic characterization and radiation of bacterial leaf scorch of oak in New Jersey. Phytopathology, 103(6), 14–14.
Behringer G, Gould AB and Kobayashi D, 2012. Characterizing Xylella fastidiosa subsp multiplex in symptomatic northeastern and mid‐Atlantic oak trees. Phytopathology, 102(7), 11–11.
Beretta MJG, Barthe GA, Ceccardi TL, Lee RF and Derrick KS, 1997. Survey for strains of Xylella fastidiosa in Citrus affected by Citrus variegated chlorosis and Citrus blight in Brazil. Plant Disease, 81(10), 1196–1198. https://doi.org/10.1094/pdis.1997.81.10.1196
Beretta MJG, Derrick KS, Lee RF and Laranjeira FF, 1994. Observations on the spread of citrus variegated chlorosis and declinio/blight in Sao Paulo State, Brazil. Phytopathology, 84(8), 866–866.
Beretta MJG, Harakava R and Chagas CM, 1996. First report of Xylella fastidiosa in coffee. Plant Disease, 80(7), 821–821.
Bergsma‐Vlami M, van de Bilt JLJ, Tjou‐Tam‐Sin NNA, Helderman CM, Gorkink‐Smits PPMA, Landman NM, van Nieuwburg JGW, van Veen EJ and Westenberg M, 2017. Assessment of the genetic diversity of Xylella fastidiosa in imported ornamental Coffea arabica plants. Plant Pathology, 66(7), 1065–1074. https://doi.org/10.1111/ppa.12696
Bergsma‐Vlami M, van de Bilt JLJ, Tjou‐Tam‐Sin NNA, van de Vossenberg BTLH and Westenberg M, 2015. Xylella fastidiosa in Coffea arabica Ornamental Plants Imported from Costa Rica and Honduras in the Netherlands. Journal of Plant Pathology, 97(2), 395–395.
Berisha B, Chen YD, Xu BY and Chen TA, 1996. Isolation of Pierce's disease bacteria from grapevine in Europe. Phytopathology, 86(11 SUPPL.), S119–S119.
Berisha B, Chen YD, Zhang GY, Xu BY and Chen TA, 1998. Isolation of Peirce's disease bacteria from grapevines in Europe. European Journal of Plant Pathology, 104(5), 427–433. https://doi.org/10.1023/a:1008655621235
Bextine B and Child B, 2007. Xylella fastidiosa genotype differentiation by SYBR Green‐based QRT‐PCR. FEMS Microbiol Lett, 276(1), 48–54. https://doi.org/10.1111/j.1574-6968.2007.00910.x
Bextine BR and Miller TA, 2003. Improved detection of Xylella fastidiosa in asymptomatic grapevine using xylem fluid collection technique. Phytopathology, 93(6 Supplement), S8–S8.
Bextine BR and Miller TA, 2004. Comparison of whole‐tissue and xylem fluid collection techniques to detect Xylella fastidiosa in grapevine and oleander. Plant Disease, 88(6), 600–604. https://doi.org/10.1094/pdis.2004.88.6.600
Bextine BR, Harshman D, Johnson MC and Miller TA, 2004. Impact of pymetrozine on glassy‐winged sharpshooter feeding behavior and rate of Xylella fastidiosa transmission. Journal of Insect Science, 4(34), 34.
Bianchi GL, 2016. The Xylella fastidiosa monitoring in Friuli Venezia Giulia: recent developments and future developments. Notiziario ERSA(2), 24–29.
Black M, Sanchez A, Davis J, Kamas J and Adams P, 2008. More Texas Xylella fastidiosa isolates colonized Helianthus annuus and Iva annua than Ambrosia trifida var. texana and Vitis vinifera ‘Chardonnay’. Phytopathology, 98(6), S23–S23.
Black M, Sanchez A, Davis J, Kamas J and Ortiz S, 2005. Supplemental Xylella fastidiosa hosts found near four central Texas vineyards with or without Pierce's disease histories. Phytopathology, 95(6), S10–S10.
Blake JH, 1993. Distribution of Xylella fastidiosa in Oak, Maple, and Sycamore in South Carolina. Plant Disease, 77(12), 1262–1262.
Bleve G, Marchi G, Ranaldi F, Gallo A, Cimaglia F, Logrieco AF, Mita G, Ristori J and Surico G, 2016. Molecular characteristics of a strain (Salento‐1) of Xylella fastidiosa isolated in Apulia (Italy) from an olive plant with the quick decline syndrome. Phytopathologia Mediterranea, 55(1), 139–146. https://doi.org/10.14601/Phytopathol_Mediterr-17867
Bolanos C, Zapata M, Brodbeck B, Andersen P, Wessel‐Beaver L and Estevez de Jensen C, 2015. Spatial distribution of coffee trees (Coffea arabica L.) potentially diseased with coffee leaf scorch caused by Xylella fastidiosa in Puerto Rico. Journal of Agriculture of the University of Puerto Rico, 99(2), 157–165.
Boscia D, Altamura G, Ciniero A, Carolo Md, Dongiovanni C, Fumarola G, Giampetruzzi A, Greco P, Notte Pl, Loconsole G, Manni F, Melcarne G, Montilon V, Morelli M, Murrone N, Palmisano F, Pollastro P, Potere O, Roseti V, Saldarelli P, Saponari A, Saponari M, Savino V, Silletti MR, Specchia F and Susca L, 2017. Resistance to Xylella fastidiosa in different olive cultivars. Informatore Agrario, 73(11), 59–63.
Boscia D, Occurrence of Xylella fastidiosa in Apulia. International symposium on the European outbreak of Xylella fastidiosa in olive. Gallipoli, Locorotondo, Italy, 21–24 October 2014:30.
Boyhan GE, Abrahams BR, Norton JD and Huang HW, 1996. Budding method affects transmission of Xylella fastidiosa in plum. Hortscience, 31(1), 89–90.
Boyhan GE, Tangsukkasemsan B, Norton JD and Himelrick DG, 1997. Incidence of Xylella fastidiosa Wells et al. on plum and peach in Alabama. Fruit Varieties Journal, 51(1), 31–35.
Brady J, Faske J, Faske T and McGahan D, 2010. Evaluating the impact of nutritional treatments on Xylella fastidiosa in grapevine. Phytopathology, 100(6), S16‐S16.
Brannen P and Chang CJ, 2009. Expansion of Xylella fastidiosa into blueberries in Georgia and Florida. Phytopathology, 99(6), S170–S170.
Brannen PM and Chang CJ, 2002. Survey of North Georgia wine grapes for Pierce's disease as related to elevation. Phytopathology, 92(6 Supplement), S9–S9.
Brannen PM, Nissen L, Denny T, Chang C and Tertuliano M, 2010. Bacterial leaf scorch of blueberries: A new threat to the southeastern industry. Phytopathology, 100(6), S199–S199.
Britton KO, Leininger T and Chang CJ, 1999. Sycamore dieback in the southeastern United States. Phytopathology, 89(6 SUPPL.), S9–S9.
Brlansky RH and Howd DS, 1993. Light and transmission electron microscopy of Citrus leaves affected by citrus variegated chlorosis and pecosita. Phytopathology, 83(12), 1399–1399.
Brlansky RH and Raju BC, 1981. Scanning Electron‐Microscopy of the Xylem of Plants Affected by Pierce's Disease of Grapes, Almond Leaf Scorch, Periwinkle Wilt, and Citrus Blight. Phytopathology, 71(2), 205–205.
Brlansky RH and Timmer LW, 1982. Detection and Transmission of a Gram‐Negative, Xylem‐Limited Bacterium in Sharpshooters from a Citrus Grove in Florida. Plant Disease, 66(7), 590–592. https://doi.org/10.1094/pd-66-590
Brlansky RH, Damsteegt VD and Hartung JS, 2002. Transmission of the citrus variegated chlorosis bacterium Xylella fastidiosa with the sharpshooter Oncometopia nigricans. Plant Disease, 86(11), 1237–1239. https://doi.org/10.1094/pdis.2002.86.11.1237
Brlansky RH, Damsteegt VD, Howd DS and Hartung JS, 1996. Transmission of the causal agent of citrus variegated chlorosis, Xylella fastidiosa, with a sharpshooter leafhopper vector from Florida. Phytopathology, 86(11 SUPPL.), S74–S74.
Brlansky RH, Dvis CL, Timmer LW, Howd DS and Contreras J, 1991. Xylem‐limited bacteria in citrus from Argentina with symptoms of citrus variegated chlorosis. Phytopathology, 81(10), 1210–1210.
Brlansky RH, Lee RF and Timmer LW, 1981. Detection of Plant Rickettsia‐Like Bacteria In‐situ Using Immunofluorescence. Phytopathology, 71(8), 863–863.
Bruening G, Civerolo EL, Jernstedt J, Re EB and Buzayan JM, 2001. Reaction of Chenopodium quinoa leaves to infiltrated Xylella fastidiosa (Xf). Phytopathology, 91(6 Supplement), S11–S11.
Bruer HL, 1951. Survey of phony peach incidence in wild Prunus. The Plant Disease Reporter, 35(4), 186–188.
Burbank LP and Stenger DC, 2017. The DinJ/RelE Toxin‐Antitoxin System Suppresses Bacterial Proliferation and Virulence of Xylella fastidiosa in Grapevine. Phytopathology, 107(4), 388–394. https://doi.org/10.1094/PHYTO-10-16-0374-R
Buzkan N and Walker MA, 2004. Effect of tissue on the inoculation and detection of Xylella fastidiosa in the grapevine. Turkish Journal of Agriculture and Forestry, 28(5), 341–347.
Buzkan N, Kocsis L and Walker MA, 2005. Detection of Xylella fastidiosa from resistant and susceptible grapevine by tissue sectioning and membrane entrapment immunofluorescence. Microbiology Research, 160(3), 225–231. https://doi.org/10.1016/j.micres.2004.05.006
Buzkan N, Kocsis L, Krivanek AF and Walker MA, 2003. Developing rapid evaluations for resistance to Xylella fastidiosa, the causal agent of Pierce's disease. Proceedings of the 8th International Conference on Grape Genetics and Breeding, Vols 1 and 2(603), 433–440. https://doi.org/10.17660/actahortic.2003.603.55
Buzkan N, Krivanek AF, Eskalen A and Walker MA, 2003. Improvements in sample preparation and polymerase chain‐reaction techniques for detection of Xylella fastidiosa in grapevine tissue. American Journal of Enology and Viticulture, 54(4), 307–312.
Buzombo P and Morano L, 2005. Strain differences in Xylella fastidiosa observed using indirect immunofluorescence. American Journal of Enology and Viticulture, 56(3), 316A‐316A.
Buzombo P, Jaimes J, Lam V, Cantrell K, Harkness M, McCullough D and Morano L, 2006. An American hybrid vineyard in the Texas Gulf Coast: Analysis within a Pierce's disease hot zone. American Journal of Enology and Viticulture, 57(3), 347–355.
Cabassut G, 2015. Mise à jour n° 8 en date du 27/11/2015 notification de la presence d'organismes nuisibles et des mesures de lutte.
Cabrera J, Groves R, Chen J, Lin H, Francis M and Civerolo E, 2005. Seasonal population biology of Xylella fastidiosa genotypes in almond and movement by insect vectors. Phytopathology, 95(6), S16‐S16.
Cabrera‐La Rosa JC, Johnson MW, Civerolo EL, Chen J and Groves RL, 2008. Seasonal population dynamics of Draeculacephala minerva (Hemiptera: Cicadellidae) and transmission of Xylella fastidiosa. Journal of Economic Entomology, 101(4), 1105–1113.
Calsa T and Figueira A, 2007. Citrus plastid‐related gene profiling based on expressed sequence tag analyses. Genetics and Molecular Biology, 30(3), 848–856. https://doi.org/10.1590/s1415-47572007000500013
Camargo LEA, 2001. Genetic diversity of Xylella fastidiosa in three Citrus producing regions of the State of Sao Paulo, Brazil. Summa Phytopathologica, 27(1), 148–148.
Campus L, Pucci N, Modesti V, Lucchesi S, D'Amaro P and Loreti S, 2016. Monitoring of Xylella fastidiosa in areas free from the pathogen. Informatore Agrario, 72(26), 48–51.
Cao T, Connell JH and Kirkpatrick BC, 2007. Almond leaf scorch disease: Cultivar and seasonal susceptibility. Phytopathology, 97(7), S17–S17.
Cao T, DeJong TM and Kirkpatrick BC, 2013. Almond Leaf Scorch Disease Development on Almond Branches High‐Grafted on Peach Rootstock. Plant Disease, 97(2), 277–281. https://doi.org/10.1094/Pdis-06-12-0580-Re
Cao TS, Connell JH, Wilhelm M and Kirkpatrick BC, 2011. Influence of Inoculation Date on the Colonization of Xylella fastidiosa and the Persistence of Almond Leaf Scorch Disease Among Almond Cultivars. Plant Disease, 95(2), 158–165. https://doi.org/10.1094/Pdis-05-10-0327
Carazzolle MF, Rabello FR, Martins NF, de Souza AA, do Amaral AM, Freitas‐Astua J, Pereira GAG, Machado MA and Mehta A, 2011. Identification of defence‐related genes expressed in coffee and Citrus during infection by Xylella fastidiosa. European Journal of Plant Pathology, 130(4), 529–540. https://doi.org/10.1007/s10658-011-9775-5
Carbajal D, Morano KA and Morano LD, 2004. Indirect immunofluorescence microscopy for direct detection of Xylella fastidiosa in xylem sap. Curr Microbiol, 49(5), 372–375. https://doi.org/10.1007/s00284-004-4369-5
Cariddi C, Saponari M, Boscia D, De Stradis A, Loconsole G, Nigro F, Porcelli F, Potere O and Martelli GP, 2014. Isolation of a Xylella fastidiosa Strain Infecting Olive and Oleander in Apulia, Italy. Journal of Plant Pathology, 96(2), 425–429.
Carrer GMM, Silva MdSS, Munari CRR, Takita MAA and Souza AAA, 2009. Expression of nbs‐LRR gene in Citrus plant infected with Xylella fastidiosa. Plant Biology (Rockville), 2009(Suppl. S), 335–335.
Cartagena LV, Sanchez E, Vargas M, Solorzano A, Hernandez F, Iwasawa H and Freer E, 2002. Presence of bacterial in the xylem of coffee (Rubiaceae: Coffea arabica) affected by the disease known as “Crespera”. Revista De Biologia Tropical, 50(1), 45–48.
Casais VO, do Patrocinio E, de Oliveira SAS, Schnadelbach AS, Barbosa CDJ and Barbosa LV, 2014. Genetic diversity of Xylella fastidiosa in Citrus producing regions in the state of Bahia, Brazil. Pesquisa Agropecuaria Brasileira, 49(1), 26–33. https://doi.org/10.1590/S0100-204x2014000100004
Caserta R, Souza‐Neto RR, Takita MA, Lindow SE and De Souza AA, 2017. Ectopic Expression of Xylella fastidiosa rpfF Conferring Production of Diffusible Signal Factor in Transgenic Tobacco and Citrus Alters Pathogen Behavior and Reduces Disease Severity. Molecular Plant‐Microbe Interactions, 30(11), 866–875. https://doi.org/10.1094/MPMI-07-17-0167-R
Caserta R, Takita MA, Targon ML, Rosselli‐Murai LK, de Souza AP, Peroni L, Stach‐Machado DR, Andrade A, Labate CA, Kitajima EW, Machado MA and de Souza AA, 2010. Expression of Xylella fastidiosa fimbrial and afimbrial proteins during biofilm formation. Appl Environ Microbiol, 76(13), 4250–4259. https://doi.org/10.1128/AEM.02114-09
Castro PRC, Kluge RA, Medina CL and Corrente JE, 2004. Management of citrus variegated chlorosis (CVC) with bioregulators. Proceedings of the Interamerican Society for Tropical Horticulture, 47, 161–163.
Castro PRC, Medina CL and Almeida M, 2001. Response of citrus variegated chlorosos (CVC)‐infected ‘Pera’ sweet orange to growth regulators. Proceedings of the Interamerican Society for Tropical Horticulture, 43, 104–107.
Cervantes K, Ray D, Stamler R, French J, Soneji J, Heerema R, Grauke L and Randall J, 2016. Evidence for seed transmission of Xylella fastidiosa in pecan (Carya illinoinensis). Phytopathology, 106(12), 109–110.
Chagas CM, Rossetti V and Beretta MJG, 1992. Electron‐Microscopy Studies of a Xylem‐Limited Bacterium in Sweet Orange Affected with citrus variegated chlorosis Disease in Brazil. Journal of Phytopathology‐Phytopathologische Zeitschrift, 134(4), 306–312. https://doi.org/10.1111/j.1439-0434.1992.tb01238.x
Chakraborty S, Nascimento R, Zaini PA, Gouran H, Rao BJ, Goulart LR and Dandekar AM, 2016. Sequence/structural analysis of xylem proteome emphasizes pathogenesis related proteins, chitinases and beta‐1, 3‐glucanases as key players in grapevine defense against Xylella fastidiosa. Peerj, 4 ARTN e2007
Chang C, Amerson M and Donaldson R, 2005. Movement of Xylella fastidiosa grape strain 17AV97 in muscadines and French hybrid grapes. Phytopathology, 95(6), S18–S18.
Chang C, Brannen P, Krewer G, Boland R and Donaldson R, 2007. Bacterial leaf scorch of blueberry: A new disease caused by Xylella fastidiosa. Phytopathology, 97(7), S20–S20.
Chang CJ and Donaldson R, 1997. Periwinkles as artificial host plants of various Xylella fastidiosa strains. Phytopathology, 87(6 SUPPL.), S17–S17.
Chang CJ and Donaldson RC, 1993. Xylella fastidiosa – Cultivation in Chemically Defined Medium. Phytopathology, 83(2), 192–194. https://doi.org/10.1094/phyto-83-192
Chang CJ and Scott RE, 2004. Pierce's disease severity in relation to various rootstocks. Phytopathology, 94(6), S15–S15.
Chang CJ and Walker JT, 1988. Bacterial Leaf Scorch of Northern Red Oak – Isolation, Cultivation, and Pathogenicity of a Xylem‐Limited Bacterium. Plant Disease, 72(8), 730–733. https://doi.org/10.1094/pd-72-0730
Chang CJ and Yonce C, 1984. Plum Leaf Scald Bacteria – Survival through Winter. Phytopathology, 74(7), 879–879.
Chang CJ and Yonce CE, 1987. Overwintering of plum leaf scald bacteria in infected trees. Annals of the Phytopathological Society of Japan, 53(3), 345–353.
Chang CJ, Donaldson R, Brannen P, Krewer G and Boland R, 2009. Bacterial Leaf Scorch, a New Blueberry Disease Caused by Xylella fastidiosa. Hortscience, 44(2), 413–417.
Chang CJ, Garnier M, Zreik L, Rossetti V and Bove JM, 1993. Culture and Serological Detection of the Xylem‐Limited Bacterium Causing citrus variegated chlorosis and Its Identification as a Strain of Xylella fastidiosa. Current Microbiology, 27(3), 137–142. https://doi.org/10.1007/bf01576010
Chang CJ, Leininger TD and Britton KO, 2002. Screening for sycamores that may be tolerant to leaf scorch disease caused by Xylella fastidiosa. Phytopathology, 92(6 Supplement), S13–S13.
Chang CJ, Robacker CD and Lane RP, 1990. Further Evidence for the Isolation of Xylella fastidiosa on Nutrient Agar from Grapevines Showing Pierce's Disease Symptoms. Canadian Journal of Plant Pathology‐Revue Canadienne De Phytopathologie, 12(4), 405–408. https://doi.org/10.1080/07060669009500981
Chatelet DS, Matthews MA and Rost TL, 2006. Xylem structure and connectivity in grapevine (Vitis vinifera) shoots provides a passive mechanism for the spread of bacteria in grape plants. Annals of Botany, 98(3), 483–494. https://doi.org/10.1093/aob/mcl124
Chatterjee S, Newman KL and Lindow SE, 2008. Cell‐to‐cell signaling in Xylella fastidiosa suppresses movement and xylem vessel colonization in grape. Molecular Plant‐Microbe Interactions, 21(10), 1309–1315. https://doi.org/10.1094/MPMI-21-10-1309
Chatterjee S, Wistrom C and Lindow SE, 2008. A cell‐cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proceedings of the National Academy of Sciences of the United States of America, 105(7), 2670–2675. https://doi.org/10.1073/pnas.0712236105
Chen HY, Kandel PP, Cruz LF, Cobine PA and De la Fuente L, 2017. The Major Outer Membrane Protein MopB Is Required for Twitching Movement and Affects Biofilm Formation and Virulence in Two Xylella fastidiosa strains. Molecular Plant‐Microbe Interactions, 30(11), 896–905. https://doi.org/10.1094/Mpmi-07-17-0161-R
Chen J, Groves R, Civerolo EL, Viveros M, Freeman M and Zheng Y, 2005. Two Xylella fastidiosa Genotypes Associated with Almond Leaf Scorch Disease on the Same Location in California. Phytopathology, 95(6), 708–714. https://doi.org/10.1094/PHYTO-95-0708
Chen J, Han S, Civerolo E, Stenger DC and Van Sluys M, 2007. Two whole genome sequences of Xylella fastidiosa almond leaf scorch strains. Phytopathology, 97(7), S21–S22.
Chen J, Ledbetter C and Groves R, 2007. Susceptibility of Prunus rootstock seedlings to Xylella fastidiosa strains isolated from almond in California. Phytopathology, 97(7), S22–S22.
Chen J, Livingston S, Groves R and Civerolo EL, 2008. High throughput PCR detection of Xylella fastidiosa directly from almond tissues. J Microbiol Methods, 73(1), 57–61. https://doi.org/10.1016/j.mimet.2008.01.011
Chen JC, Civerolo EL, Jarret RL, Van Sluys MA and de Oliveira MC, 2005. Genetic discovery in Xylella fastidiosa through sequence analysis of selected randomly amplified polymorphic DNAs. Current Microbiology, 50(2), 78–83. https://doi.org/10.1007/s00284-004-4412-6
Chen JC, Groves R, Zheng YW, Civerolo EL, Viveros M and Freeman M, 2007. Colony morphology of Xylella fastidiosa almond leaf scorch strains. Canadian Journal of Plant Pathology, 29(3), 225–231.
Cheng DW, Lin H, Takahashi Y, Walker MA, Civerolo EL and Stenger DC, 2010. Transcriptional regulation of the grape cytochrome P450 monooxygenase gene CYP736B expression in response to Xylella fastidiosa infection. BMC Plant Biol, 10, 135. https://doi.org/10.1186/1471-2229-10-135
Choat B, Gambetta GA, Wada H, Shackel KA and Matthews MA, 2009. The effects of Pierce's disease on leaf and petiole hydraulic conductance in Vitis vinifera cv. Chardonnay. Physiol Plant, 136(4), 384–394. https://doi.org/10.1111/j.1399-3054.2009.01231.x
Choi HK, da Silva FG, Lim HJ, Iandolino A, Seo YS, Lee SW and Cook DR, 2010. Diagnosis of Pierce's Disease Using Biomarkers Specific to Xylella fastidiosa rRNA and Vitis vinifera Gene Expression. Phytopathology, 100(10), 1089–1099. https://doi.org/10.1094/Phyto-01-10-0014
Choi HK, Iandolino A, da Silva FG and Cook DR, 2013. Water deficit modulates the response of Vitis vinifera to the Pierce's disease pathogen Xylella fastidiosa. Molecular Plant‐Microbe Interactions, 26(6), 643–657. https://doi.org/10.1094/MPMI-09-12-0217-R
Ciapina LP, Carareto Alves LM and Lemos EG, 2004. A nested‐PCR assay for detection of Xylella fastidiosa in Citrus plants and sharpshooter leafhoppers. Journal of Applied Microbiology, 96(3), 546–551.
Clifford JC, Rapicavoli JN and Roper MC, 2013. A rhamnose‐rich O‐antigen mediates adhesion, virulence, and host colonization for the xylem‐limited phytopathogen Xylella fastidiosa. Molecular Plant‐Microbe Interactions, 26(6), 676–685. https://doi.org/10.1094/MPMI-12-12-0283-R
Cochran LC, 1951. Natural occurrence of the phony virus in wild Chickasaw plums near peach orchards in Georgia. The Plant Disease Reporter, 35(4), 181–182.
Coletta‐Filho HD, Borges KM and Machado MA, 2000. Occurrence of Xylella fastidiosa in sweet orange candidate mother trees and percentage of transmission to nursery tree using infected budwood. Laranja, 21(2), 335–343.
Coletta‐Filho HD, Carlos EF, Targon MLPN, Cristofani M, Souza AA and Machado MA, 2000. Distribution of Xylella fastidiosa within sweet orange trees: influence of age and level of symptom expression of citrus variegated chlorosis. Proceedings of the 14th Conference of the International Organization of Citrus Virologists, Campinas, Sao Paulo State, Brazil, 13–18 September 1998:243–248.
Coletta HD, Goncalves FP, Amorim L, de Souza AA and Machado MA, 2013. Survey of Xylella fastidiosa and citrus variegated chlorosis in Sao Paulo State, Brazil. Journal of Plant Pathology, 95(3), 493–498.
Coletta HD, Pereira EO, Souza AA, Takita MA, Cristofani‐Yale M and Machado MA, 2007. Analysis of resistance to Xylella fastidiosa within a hybrid population of Pera sweet orange x Murcott tangor. Plant Pathology, 56(4), 661–668. https://doi.org/10.1111/j.1365-3059.2007.01605.x
Coletta‐Filho HD, Bittleston LS and Almeida RP, 2011. Spatial genetic structure of a vector‐borne generalist pathogen. Applied and Environmental Microbiology, 77(8), 2596–2601. https://doi.org/10.1128/AEM.02172-10
Coletta‐Filho HD, Francisco CS and Almeida RP, 2014. Temporal and spatial scaling of the genetic structure of a vector‐borne plant pathogen. Phytopathology, 104(2), 120–125. https://doi.org/10.1094/PHYTO-06-13-0154-R
Coletta‐Filho HD, Francisco CS, Lopes JR, Muller C and Almeida RP, 2017. Homologous Recombination and Xylella fastidiosa Host‐Pathogen Associations in South America. Phytopathology, 107(3), 305–312. https://doi.org/10.1094/PHYTO-09-16-0321-R
Coll ORd, Lenicov AMMR, Agostini JP and Paradell S, 2000. Detection of Xylella fastidiosa in weeds and sharpshooters in orange groves affected with citrus variegated chlorosis in Misiones, Argentina. Proceedings of the 14th Conference of the International Organization of Citrus Virologists, Campinas, Sao Paulo State, Brazil, 13–18 September 1998:216–222.
Coll ORd, Lenicov AMMR, Agostini JP and Paradell S, 2000. Some factors in a pest management program for Valencia sweet orange groves with citrus variegated chlorosis (CVC). Proceedings of the 14th Conference of the International Organization of Citrus Virologists, Campinas, Sao Paulo State, Brazil, 13–18 September 1998:238–242.
Consejería de Medio Ambiente, Agricultura y Pesca del Gobierno de las Islas Baleares Dirección General de Agricultura y Ganadería. Servicio de Agricultura, 2017. Update 27 June 2018.
Cordeiro AB, Sugahara VH, Stein B and Leite RP, 2014. Evaluation by PCR of Xylella fastidiosa subsp pauca transmission through Citrus seeds with special emphasis on lemons (Citrus limon (L.) Burm. f). Crop Protection, 62, 86–92. https://doi.org/10.1016/j.cropro.2014.03.017
Cornara D, Cavalieri V, Dongiovanni C, Altamura G, Palmisano F, Bosco D, Porcelli F, Almeida RPP and Saponari M, 2017. Transmission of Xylella fastidiosa by naturally infected Philaenus spumarius (Hemiptera, Aphrophoridae) to different host plants. Journal of Applied Entomology, 141(1–2), 80–87. https://doi.org/10.1111/jen.12365
Cornara D, Saponari M, Zeilinger AR, de Stradis A, Boscia D, Loconsole G, Bosco D, Martelli GP, Almeida RPP and Porcelli F, 2017. Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy. Journal of Pest Science, 90(2), 521–530. https://doi.org/10.1007/s10340-016-0793-0
Cornara D, Sicard A, Zeilinger AR, Porcelli F, Purcell AH and Almeida RP, 2016. Transmission of Xylella fastidiosa to Grapevine by the Meadow Spittlebug. Phytopathology, 106(11), 1285–1290. https://doi.org/10.1094/PHYTO-05-16-0202-R
Costa HS, Blua MS, Bethke JA and Redak RA, 2000. Transmission of Xylella fastidiosa to oleander by the glassy‐winged sharpshooter, Homalodisca coagulata. Hortscience, 35(7), 1265–1267.
Costa HS, Guzmán A, Hernandez‐Martinez R, Gispert C and Cooksey DA, 2006. Detection and differentiation of Xylella fastidiosa strains acquired and retained by glassy‐winged sharpshooters (Hemiptera : Cicadellidae) using a mixture of strain‐specific primer sets. Journal of Economic Entomology, 99(4), 1058–1064. https://doi.org/10.1603/0022-0493-99.4.1058
Costa HS, Raetz E, Pinckard TR, Gispert C, Hernandez‐Martinez R, Dumenyo CK and Cooksey DA, 2004. Plant hosts of Xylella fastidiosa in and near southern California vineyards. Plant Disease, 88(11), 1255–1261.
Costello MJ, Steinmaus SJ and Boisseranc CJ, 2017. Environmental variables influencing the incidence of Pierce's disease. Australian Journal of Grape and Wine Research, 23(2), 287–295. https://doi.org/10.1111/ajgw.12262
Cruz AC, Luvisi A, De Bellis L and Ampatzidis Y, 2017. X‐FIDO: An Effective Application for Detecting Olive Quick Decline Syndrome with Deep Learning and Data Fusion. Frontiers in Plant Science, 8, 1741. https://doi.org/10.3389/fpls.2017.01741
Cursino L, Athinuwat D, Patel KR, Galvani CD, Zaini PA, Li Y, De La Fuente L, Hoch HC, Burr TJ and Mowery P, 2015. Characterization of the Xylella fastidiosa PD1671 gene encoding degenerate c‐di‐GMP GGDEF/EAL domains, and its role in the development of Pierce's disease. PloS One, 10(3), e0121851. https://doi.org/10.1371/journal.pone.0121851
Cursino L, Galvani CD, Athinuwat D, Zaini PA, Li YX, De La Fuente L, Hoch HC, Burr TJ and Mowery P, 2011. Identification of an Operon, Pil‐Chp, That Controls Twitching Motility and Virulence in Xylella fastidiosa. Molecular Plant‐Microbe Interactions, 24(10), 1198–1206. https://doi.org/10.1094/Mpmi-10-10-0252
Cursino L, Li Y, Zaini PA, De La Fuente L, Hoch HC and Burr TJ, 2009. Twitching motility and biofilm formation are associated with tonB1 in Xylella fastidiosa. FEMS Microbiology Letters, 299(2), 193–199. https://doi.org/10.1111/j.1574-6968.2009.01747.x
da Costa PI, Franco CF, Miranda VS, Teixeira DC and Hartung JS, 2000. Strains of Xylella fastidiosa rapidly distinguished by arbitrarily primed‐PCR. Current Microbiology, 40(4), 279–282.
da Silva MM, Andrade MdS, Bauermeister A, Merfa MV, Forim MR, Fernandes JB, Vieira PC, Silva MFdGFd, Lopes NP, Machado MA and Souza AAd, 2017. A simple defined medium for the production of true diketopiperazines in Xylella fastidiosa and their identification by ultra‐fast liquid chromatography‐electrospray ionization ion trap mass spectrometry. Molecules, 22(6), 985–985.
da Silva VS, Shida CS, Rodrigues FB, Ribeiro DC, de Souza AA, Coletta‐Filho HD, Machado MA, Nunes LR and de Oliveira RC, 2007. Comparative genomic characterization of Citrus‐associated Xylella fastidiosa strains. BMC Genomics, 8, 474. https://doi.org/10.1186/1471-2164-8-474
Daane KM, Wistrom CM, Shapland EB and Sisterson MS, 2011. Seasonal abundance of Draeculacephala minerva and other Xylella fastidiosa vectors in California almond orchards and vineyards. Journal of Economic Entomology, 104(2), 367–374.
Dalbo MA, Bruna ED, Nodari RO and Saifert L, 2016. Plum selections with total resistance to leaf scald (Xylella fastidiosa). XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (Ihc2014): International Symposium on Plant Breeding in Horticulture, 1127(1127), 61–64. https://doi.org/10.17660/ActaHortic.2016.1127.11
Dalbo MA, Hoffman RL, Melo D and Moraes LKAd, 2005. Production of plum (Prunus salicina) vegetative material free of leaf scald (Xylella fastidiosa). Agropecuaria Catarinense, 18(3), 98–102.
Dalbo MA, Klabunde GHF, Nodari RO, Fernandes D and Basso MF, 2010. Evolution of the response of segregating populations of plums and the association with microsatellite markers of leaf scald. Crop Breeding and Applied Biotechnology, 10(4), 337–344. https://doi.org/10.1590/s1984-70332010000400008
Damsteegt VD, Brlansky RH, Phillips PA and Roy A, 2003. Glassy‐winged sharpshooter transmission of Xylella fastidiosa, causal agent of citrus variegated chlorosis. Phytopathology, 93(6 Supplement), S19–S19.
Damsteegt VD, Brlansky RH, Phillips PA and Roy A, 2006. Transmission of Xylella fastidiosa, causal agent of citrus variegated chlorosis, by the glassy‐winged sharpshooter, Homalodisca coagulata. Plant Disease, 90(5), 567–570. https://doi.org/10.1094/Pd-90-0567
Daniell JW and Krewer GW, 1984. Effect of number of bacteria on cold injury of rooted cuttings from phony‐infected and uninfected peach‐trees. Hortscience, 19(3), 423–424.
Das M, Bhowmick TS, Ahern SJ, Young R and Gonzalez CF, 2015. Control of Pierce's Disease by Phage. PloS One, 10(6), e0128902. https://doi.org/10.1371/journal.pone.0128902
Daugherty MP and Almeida RPP, 2009. Estimating Xylella fastidiosa transmission parameters: decoupling sharpshooter number and feeding period. Entomologia Experimentalis et Applicata, 132(1), 84–92. https://doi.org/10.1111/j.1570-7458.2009.00868.x
Daugherty MP, Bosco D and Almeida RPP, 2009. Temperature mediates vector transmission efficiency: inoculum supply and plant infection dynamics. Annals of Applied Biology, 155(3), 361–369. https://doi.org/10.1111/j.1744-7348.2009.00346.x
Daugherty MP, Lopes J and Almeida RPP, 2010. Vector within‐host feeding preference mediates transmission of a heterogeneously distributed pathogen. Ecological Entomology, 35(3), 360–366. https://doi.org/10.1111/j.1570-7458.2009.00868.x
Daugherty MP, Lopes JRS and Almeida RPP, 2010. Strain‐specific alfalfa water stress induced by Xylella fastidiosa. European Journal of Plant Pathology, 127(3), 333–340. https://doi.org/10.1007/s10658-010-9598-9
Daugherty MP, Rashed A, Almeida RPP and Perring TM, 2011. Vector preference for hosts differing in infection status: sharpshooter movement and Xylella fastidiosa transmission. Ecological Entomology, 36(5), 654–662. https://doi.org/10.1111/j.1365-2311.2011.01309.x
Daugherty MP, Zeilinger AR and Almeida RPP, 2017. Conflicting effects of climate and vector behavior on the spread of a plant pathogen. Phytobiomes, 1(1), 46–53.
Davis MJ and Thomson SV, 1977. Pierce's disease isolation of the causal agent from grapevines. Proceedings of the American Phytopathological Society, (4), 138–138.
Davis MJ, French WJ and Schaad NW, 1981. Axenic culture of the bacteria associated with phony disease of peach and plum leaf scald. Current Microbiology, 6(5), 309–314. https://doi.org/10.1007/bf01566883
Davis MJ, French WJ and Schaad NW, 1981. Isolation and Culture of the Bacteria Associated with Phony Peach Disease and Plum Leaf Scald. Phytopathology, 71(8), 869–870.
Davis MJ, Purcell AH and Thomson SV, 1977. Pierce's disease consistent isolation of a bacterium from diseased grapevines. Proceedings of the American Phytopathological Society, (4), 204–204.
Davis MJ, Purcell AH and Thomson SV, 1978. Pierce's Disease of Grapevines – Isolation of Causal Bacterium. Science, 199(4324), 75–77. https://doi.org/10.1126/science.199.4324.75
Davis MJ, Purcell AH and Thomson SV, 1980. Isolation Media for the Pierce's Disease Bacterium. Phytopathology, 70(5), 425–429. https://doi.org/10.1094/phyto-70-425
Davis MJ, Raju BC, Brlansky RH, Lee RF, Mccoy RE and Norris RC, 1982. Relationship of the Xylem‐Limited Bacteria Causing Periwinkle Wilt and Pierce's Diseases. Phytopathology, 72(7), 936–936.
Davis MJ, Raju BC, Brlansky RH, Lee RF, Timmer LW, Norris RC and McCoy RE, 1983. Periwinkle wilt bacterium – axenic culture, pathogenicity, and relationships to other Gram‐negative, xylem‐inhabiting bacteria. Phytopathology, 73(11), 1510–1515. https://doi.org/10.1094/phyto-73-1510
Davis MJ, Thomson SV and Purcell AH, 1980. Etiological Role of the Xylem‐Limited Bacterium Causing Pierce Disease in Almond Leaf Scorch. Phytopathology, 70(6), 472–475. https://doi.org/10.1094/phyto-70-472
De Benedictis M, De Caroli M, Baccelli I, Marchi G, Bleve G, Gallo A, Ranaldi F, Falco V, Pasquali V, Piro G, Mita G and Di Sansebastiano GP, 2017. Vessel occlusion in three cultivars of Olea europaea naturally exposed to Xylella fastidiosa in open field. Journal of Phytopathology, 165(9), 589–594.
de Carvalho Nunes WM, Machado MA, Corazza‐Nunes MJ and Furtado EL, 2001. Spatial dynamics of citrus variegated chlorosis (CVC) foci by symptoms and serology. Acta Scientiarum Universidade Estadual de Maringa, 23(5), 1215–1219.
de Carvalho Nunes WM, Zanutto CA, Corazza‐Nunes MJ and e Oliveira Molina R, 2006. Spatio‐temporal analysis of the citrus variegated chlorosis (CVC) in the Northwest of Parana, using PCR for detection of Xylella fastidiosa. Acta Scientiarum: Agronomy, 28(3), 421–425.
De La Fuente L, Parker JK, Oliver JE, Granger S, Brannen PM, van Santen E and Cobine PA, 2013. The bacterial pathogen Xylella fastidiosa affects the leaf ionome of plant hosts during infection. PloS one, 8(5), e62945. https://doi.org/10.1371/journal.pone.0062945
de Lima JEO, Miranda VS, Hartung JS, Brlansky RH, Coutinho A, Roberto SR and Carlos EF, 1998. Coffee leaf scorch bacterium: Axenic culture, pathogenicity, and comparison with Xylella fastidiosa of Citrus. Plant Disease, 82(1), 94–97.
De Miranda MP, Villada ES, Lopes SA, Fereres A and Lopes JRS, 2013. Influence of Citrus Plants Infected With Xylella fastidiosa on Stylet Penetration Activities of Bucephalogonia xanthophis (Hemiptera: Cicadellidae). Annals of the Entomological Society of America, 106(5), 610–618.
De Nadai Fernandes EA, Tagliaferro FS, Turra C, De Franca EJ and Bacchi MA, 2008. Elemental composition changes in Citrus affected by the CVC disease. Journal of Radioanalytical and Nuclear Chemistry, 278(2), 371–374.
de Oliveira Lins SR, e Abreu MS, Alves E, Barbosa JF and e Souza RM, 2008. Report of Xylella fastidiosa in petioles and hypocotyls of coffee plants with symptoms of Buttery spot. Ciencia E Agrotecnologia, 32(1), 42–47.
de Souza AA, Ionescu M, Baccari C, Silva AM and Lindow SE, 2013. Phenotype Overlap in Xylella fastidiosa Is Controlled by the Cyclic Di‐GMP Phosphodiesterase Eal in Response to Antibiotic Exposure and Diffusible Signal Factor‐Mediated Cell‐Cell Signaling. Applied and Environmental Microbiology, 79(11), 3444–3454.
de Souza AA, Takita MA, Coletta HD, Caldana C, Goldman GH, Yanai GM, Muto NH, e Oliveira RC, Nunes LR and Machado MA, 2003. Analysis of gene expression in two growth states of Xylella fastidiosa and its relationship with pathogenicity. Molecular Plant‐Microbe Interactions, 16(10), 867–875.
de Souza AA, Takita MA, Coletta HD, Caldana C, Yanai GM, Muto NH, e Oliveira RC, Nunes LR and Machado MA, 2004. Gene expression profile of the plant pathogen Xylella fastidiosa during biofilm formation in vitro. Fems Microbiology Letters, 237(2), 341–353.
de Souza AA, Takita MA, Coletta‐Filho HD, Campos MA, Teixeira JEC, Targon MLPN, Carlos EF, Ravasi JF, Fischer CN and Machado MA, 2007. Comparative analysis of differentially expressed sequence tags of sweet orange and mandarin infected with Xylella fastidiosa. Genetics and Molecular Biology, 30(3), 965–971.
de Souza AA, Takita MA, Pereira EO, Coletta HD and Machado MA, 2005. Expression of pathogenicity‐related genes of Xylella fastidiosa in vitro and in planta. Current Microbiology, 50(4):223–228.
Decarvalho SA and Desouza M, 1991. Occurrence of Plum Leaf Scald Symptoms in Delfim‐Moreira, Mg‐Orchards (Brazil). Pesquisa Agropecuaria Brasileira, 26(11–12), 2015–2020.
Della Coletta‐Filho H and Machado MA, 2002. Evaluation of the genetic structure of Xylella fastidiosa populations from different Citrus sinensis varieties. Applied and Environmental Microbiology, 68(8), 3731–3736.
Della Coletta‐Filho H and Machado MA, 2003. Geographical genetic structure of Xylella fastidiosa from Citrus in Sao Paulo State, Brazil. Phytopathology, 93(1), 28–34.
Della Coletta‐Filho H, Carvalho SA, Carvalho Silva LF and Machado MA, 2014. Seven years of negative detection results confirm that Xylella fastidiosa, the causal agent of CVC, is not transmitted from seeds to seedlings. European Journal of Plant Pathology, 139(3), 593–596.
Della Coletta‐Filho H, Francisco CS, Spotti Lopes JR, De Oliveira AF and e Oliveira Da Silva LF, 2016. First report of olive leaf scorch in Brazil, associated with Xylella fastidiosa subsp pauca. Phytopathologia Mediterranea, 55(1), 130–135.
Dellape G, Paradell S, Semorile L and Delfederico L, 2016. Potential vectors of Xylella fastidiosa: a study of leafhoppers and treehoppers in Citrus agroecosystems affected by citrus variegated chlorosis. Entomologia Experimentalis Et Applicata, 161(2), 92–103. https://doi.org/10.1111/eea.12491
Denancé N, Legendre B, Briand M, Olivier V, de Boisseson C, Poliakoff F and Jacques MA, 2017. Several subspecies and sequence types are associated with the emergence of Xylella fastidiosa in natural settings in France. Plant Pathology, 66(7), 1054–1064. https://doi.org/10.1111/ppa.12695
Deng W, Hsu S, Tzeng Y, Huang T, Su C, Jan F and Chang C, 2011. Nutritional requirements and possible alternate hosts of Xylella fastidiosa that causes pear leaf scorch in Taiwan. Phytopathology, 101(6), S41–S41.
DeStefano DA, Grybauskas AP, Sherald JL, Momen B, Huang Q and Sullivan JH, 2007. Effect of the growth regulator paclobutrazol on growth of the bacterial pathogen Xylella fastidiosa. Arboriculture & Urban Forestry, 33(4), 246–252.
Di Bello PL, Balci Y, Martin D, Huang Q and Lear M, 2012. Occurrence of Xylella fastidiosa subsp multiplex on Washington DC street trees. Phytopathology, 102(6), 2–2.
Djelouah K, Frasheri D, Valentini F, D'Onghia AM and Digiaro M, 2014. Direct tissue blot immunoassay for detection of Xylella fastidiosa in olive trees. Phytopathologia Mediterranea, 53(3), 559–564.
Doddapaneni H, Lin H, Yao J and Walker AM, 2006. Gene expression profiling of the grape I Xylella fastidiosa interaction. Plant Biology (Rockville), 2006, 174–174.
Dominiak JD and Olson BR, 2006. Detection of Xylella fastidiosa in Oklahoma. Phytopathology, 96(6), S30–S30.
Ducroquet JPHJ and Dalbo MA, 2007. SCS 409 Camila e SCS 410 Piuna – new plum cultivars with resistance to leaf scald. Agropecuaria Catarinense, 20(1), 67–70.
Elbeaino T, Valentini F, Abou Kubaa R, Moubarak P, Yaseen T and Digiaro M, 2014. Multilocus sequence typing of Xylella fastidiosa isolated from olive affected by “olive quick decline syndrome” in Italy. Phytopathologia Mediterranea, 53(3), 533–542.
Ellis EA, McEachern GR, Clark S and Cobb BG, 2010. Ultrastructure of pit membrane dissolution and movement of Xylella fastidiosa through pit membranes in petioles of Vitis vinifera. Botany‐Botanique, 88(6), 596–600. https://doi.org/10.1139/B10-025
EPPO (European and Mediterranean Plant Protection Organization), online. EPPO Global Database. Available online: https://gd.eppo.int [Accessed: May 2018]
Esau K, 1948. Anatomic effects of the viruses of Pierce's disease and phony peach. Hilgardia, 18(12), 423–481.
EUROPHYT notification n. 246
EUROPHYT notification n. 305–383–470
EUROPHYT notification n. 325
EUROPHYT notification n. 325–384
EUROPHYT notification n. 384
EUROPHYT notification n. 442
EUROPHYT notification n. 442–501
EUROPHYT notification n. 470
EUROPHYT notification n. 501
EUROPHYT notification n. 521
EUROPHYT notification n. 524
EUROPHYT notification n. 819
EUROPHYT notification n. 946
EUROPHYT notification n. 325–384–442–501
Evert DR and Smittle DA, 1989. Phony disease influences peach leaf characteristics. Hortscience, 24(6), 1000–1002.
Evert DR, 1987. Influence of Phony disease of peach on stem hydraulic conductivity and leaf xylem pressure potential. Journal of the American Society for Horticultural Science, 112(6), 1032–1036.
Evert DR, Gaines TP and French WJ, 1981. Rickettsia‐like bacteria in peach roots preceded development of visual symptoms of phony peach disease and changes in leaf elemental concentrations. Journal of the American Society for Horticultural Science, 106(6), 780–782.
Fadel AL, Stuchi ES, de Carvalho SA, Federici MT and Della Coletta H, 2014. Navelina ISA 315: A cultivar resistant to citrus variegated chlorosis. Crop Protection, 64, 115–121. https://doi.org/10.1016/j.cropro.2014.06.014
Fatmi M, Damsteegt VD and Schaad NW, 2003. A combined agar absorbent and BIO‐PCR assay for rapid, sensitive detection of Xylella fastidiosa (Xf) in grape and Citrus. Phytopathology, 93(6 Supplement), S25–S25.
Fatmi M, Damsteegt VD and Schaad NW, 2005. A combined agar‐absorption and BIO‐PCR assay for rapid, sensitive detection of Xylella fastidiosa in grape and Citrus. Plant Pathology, 54(1), 1–7. https://doi.org/10.1111/j.1365-3059.2004.01114.x
Fedatto LM, Silva‐Stenico ME, Etchegaray A, Pacheco FTH, Rodrigues JLM and Tsai SM, 2006. Detection and characterization of protease secreted by the plant pathogen Xylella fastidiosa. Microbiological Research, 161(3), 263–272. https://doi.org/10.1016/j.micres.2005.10.001
Federal I and Disease, 1992. Vanquever forest region by Rod Turnquist and Dennis Clarke. Fids report, 40 pp.
Feil H and Purcell AH, 2001. Temperature‐dependent growth and survival of Xylella fastidiosa in vitro and in potted grapevines. Plant Disease, 85(12), 1230–1234. https://doi.org/10.1094/pdis.2001.85.12.1230
Feil H, Feil WS and Lindow SE, 2007. Contribution of fimbrial and afimbrial adhesins of Xylella fastidiosa to attachment to surfaces and virulence to grape. Phytopathology, 97(3), 318–324. https://doi.org/10.1094/PHYTO-97-3-0318
Feil H, Feil WS and Purcell AH, 2003. Effects of date of inoculation on the within‐plant movement of Xylella fastidiosa and persistence of Pierce's disease within field grapevines. Phytopathology, 93(2), 244–251. https://doi.org/10.1094/phyto.2003.93.2.244
Feil H, Feil WS, Detter JC, Purcell AH and Lindow SE, 2003. Site‐Directed Disruption of the fimA and fimF Fimbrial Genes of Xylella fastidiosa. Phytopathology, 93(6), 675–682. https://doi.org/10.1094/PHYTO.2003.93.6.675
Feldman AW, 1984. Young tree decline (blight) not reproduced in citrus inoculated with Pierce's disease bacterium. Soil and Crop Science Society of Florida Proceedings, 43, 81–85.
Ferguson MH, Clark C and Smith B, 2016. Xylella fastidiosa in rabbiteye blueberry in Louisiana is genetically similar to a strain found in southern highbush blueberry in Georgia. Phytopathology, 106(2), 8–8.
Ferguson MH, Clark CA and Smith BJ, 2014. Xylella fastidiosa infection is correlated with lower yield in a rabbiteye blueberry orchard in Louisiana. Phytopathology, 104(5), 4–4.
Ferguson MH, Clark CA and Smith BJ, 2017. Association of Xylella fastidiosa with Yield Loss and Altered Fruit Quality in a Naturally Infected Rabbiteye Blueberry Orchard. Hortscience, 52(8), 1073–1079. https://doi.org/10.21273/Hortsci12044-17
Ferreira Filho AS, Quecine MC, Bogas AC, Rossetto Pde B, Lima AO, Lacava PT, Azevedo JL and Araujo WL, 2012. Endophytic Methylobacterium extorquens expresses a heterologous beta‐1,4–endoglucanase A (EglA) in Catharanthus roseus seedlings, a model host plant for Xylella fastidiosa. World Journal of Microbiology & Biotechnology, 28(4), 1475–1481. https://doi.org/10.1007/s11274-011-0949-2
Ferreira GM, Mascaro FD, Dalla Pria M, Ribeiro PJ and De Mio LLM, 2016. Spatial Analysis of Plum Leaf Scald in Sao Paulo State, Brazil. Journal of Plant Pathology, 98(3), 511–518. https://doi.org/10.4454/Jpp.V98i3.035
Fishleder A and Walker MA, 1999. Evaluating grape rootstocks and species for resistance to Pierce's disease bacteria. American Journal of Enology and Viticulture, 50(3), 375–375.
Fliege HF, 1974. Electron microscopic investigations on the occurrence of rickettsia‐like bacteria in the roots of Erica gracilis. Zeitschrift fuer Pflanzenkrankheiten und Pflanzenschutz, 81(12), 765–767.
Floyd LE and Sutton TB, 2008. Reservoir hosts of Xylella fastidiosa, causal agent of Pierce's disease of grapevines, in North Carolina. Phytopathology, 98(6), S54–S54.
Fogaca AC, Zaini PA, Wulff NA, da Silva PI, Fazio MA, Miranda A, Daffre S and da Silva AM, 2010. Effects of the antimicrobial peptide gomesin on the global gene expression profile, virulence and biofilm formation of Xylella fastidiosa. FEMS Microbiology Letters, 306(2):152–159. https://doi.org/10.1111/j.1574-6968.2010.01950.x
Fonseca HS, Furtado EL, Kuramae EE, Machado SR, Minhoni MAT and Nozaki DN, 2002. Rubber tree a new host of Xylella fastidiosa in Brazil. XXXIV Brasilian Phytopathological Congress and XI Latinamerican Phytopathological Congress, Sao Pedro, SP, Brazil, August 5–10, 2001. Fitopatologia, 37(1):10–66.
Francis M, Civerolo E and Bruening G, 2005. Nicotiana tabacum cv. SR‐1 is highly susceptible to Xylella fastidiosa associated with Pierce's disease in California. Phytopathology, 95(6), S31–S31.
Francis M, Civerolo EL and Bruening G, 2008. Improved Bioassay of Xylella fastidiosa using Nicotiana tabacum cultivar SR1. Plant Disease, 92(1), 14–20. https://doi.org/10.1094/Pdis-92-1-0014
Francis M, Lin H, Cabrera‐La Rosa J, Doddapaneni H and Civerolo EL, 2006. Genome‐based PCR primers for specific and sensitive detection and quantification of Xylella fastidiosa. European Journal of Plant Pathology, 115(2), 203–213. https://doi.org/10.1007/s10658-006-9009-4
Francisco CS, Ceresini PC, Almeida RPP and Coletta‐Filho HD, 2017. Spatial Genetic Structure of Coffee‐Associated Xylella fastidiosa Populations Indicates that Cross Infection Does Not Occur with Sympatric Citrus Orchards. Phytopathology, 107(4), 395–402.
Frazier NW and Freitag JH, 1946. Ten additional leafhopper vectors of the virus causing Pierce's disease of grapes. Phytopath, 36(8), 634–637.
Freitag JH, 1951. Host range of Pierce's disease virus of grapes as determined by insect transmission. Phytopath, 41(10), 920–934.
French JM, Randall JJ, Heerema RJ, Hanson SF and Goldberg NP, 2007. Improved ELISA detection of Xylella fastidiosa in woody plant tissue using sap extracted by a pressure chamber. Phytopathology, 97(7):S37–S37.
French WJ and Kitajima EW, 1978. Occurrence of Plum Leaf Scald in Brazil and Paraguay. Plant Disease Reporter, 62(12), 1035–1038.
French WJ, 1974. A method for observing rickettsia‐like bacteria associated with phony peach diseaSE. Phytopathology, 64(2), 260–261.
French WJ, 1974. Vacuum extraction of rickettsia‐like bacteria from peach trees affected with phony disease. Proceedings of the American Phytopathological Society, 1, (1975)‐(1975).
French WJ, 1976. Field diagnosis of peach phony disease based on the presence of a Rickettsia‐like bacterium. Proceedings of the American Phytopathological Society, 3, (1977)‐(1977).
French WJ, 1977. The incidence of phony disease in wild plum trees as determined by histochemical and microscopic methods. Proceedings of the Florida State Horticultural Society, 89, 241–243.
French WJ, 1982. Distribution and severity of plum leaf scald disease in Brazil. Phytopathology, 72(4), 452–452.
French WJ, 1982. Reciprocal Transmission of Plum Leaf Scald and Phony Disease of Peach. Phytopathology, 72(4), 452–453.
French WJ, Christis RG and Stassi DL, 1977. Recovery of Rickettsia‐Like Bacteria by Vacuum Infiltration of Peach Tissues Affected with Phony Disease. Phytopathology, 67(7), 945–948.
French WJ, Latham AJ and Stassi DL, 1977. Phony peach bacterium associated with leaf scald of plum trees. Proceedings of the American Phytopathological Society, (4), 223–223.
French WJ, Stassi DL and Schaad NW, 1978. Use of Immunofluorescence for the Identification of Phony Peach Bacterium. Phytopathology, 68(7), 1106–1108. https://doi.org/10.1094/phyto-68-1106
Fritschi FB, Lin H and Walker A, 2006. Responses to Xylella fastidiosa infection differ among Vitis genotypes. Plant Biology (Rockville), 2006, 180–180.
Fritschi FB, Lin H and Walker MA, 2007. Xylella fastidiosa population dynamics in grapevine genotypes differing in susceptibility to Pierce's disease. American Journal of Enology and Viticulture, 58(3), 326–332.
Fritschi FB, Lin H and Walker MA, 2008. Scanning electron microscopy reveals different response pattern of four Vitis genotypes to Xylella fastidiosa infection. Plant Disease, 92(2), 276–286. https://doi.org/10.1094/Pdis-92-2-0276
Fry SM and Milholland RD, 1988. Multiplication and translocation of the Pierce's disease bacterium in grapevines. Phytopathology, 78(12 PART 1), 1541–1541.
Fry SM and Milholland RD, 1990. Multiplication and translocation of Xylella fastidiosa in petioles and stems of grapevine resistant, tolerant, and susceptible to Pierce's disease. Phytopathology, 80(1), 61–65. https://doi.org/10.1094/phyto-80-61
Fry SM and Milholland RD, 1990. Response of resistant, tolerant, and susceptible grapevine tissues to invasion by the Pierce's disease bacterium, Xylella fastidiosa. Phytopathology, 80(1), 66–69. https://doi.org/10.1094/phyto-80-66
Fry SM, Huang JS and Milholland RD, 1994. Isolation and Preliminary Characterization of Extracellular Proteases Produced by Strains of Xylella fastidiosa from Grapevines. Phytopathology, 84(4), 357–363. https://doi.org/10.1094/phyto-84-357
Fry SM, Milholland RD and Huang PY, 1988. Isolation and growth of the Pierce's disease bacterium on simple bacteriological media. Phytopathology, 78(12 PART 1), 1602–1602.
Fry SM, Milholland RD and Huang PY, 1990. Isolation and Growth of Strains of Xylella fastidiosa from Infected Grapevines on Nutrient Agar Media. Plant Disease, 74(7), 522–524. https://doi.org/10.1094/pd-74-0522
Galvani CD, Li YX, Burr TJ and Hoch HC, 2007. Twitching motility among pathogenic Xylella fastidiosa isolates and the influence of bovine serum albumin on twitching‐dependent colony fringe morphology. FEMS Microbiology Letters, 268(2), 202–208. https://doi.org/10.1111/j.1574-6968.2006.00601.x
Gambetta GA, Fei J, Rost TL and Matthews MA, 2007. Leaf scorch symptoms are not correlated with bacterial populations during Pierce's disease. Journal of Experimental Botany, 58(15–16), 4037–4046.
Gambetta GA, Rost TL and Matthews MA, 2009. Passive Pathogen Movement via Open Xylem Conduits in Grapevine Graft Unions. American Journal of Enology and Viticulture, 60(2), 241–245.
Garcia AL, Torres SCZ, Heredia M and Lopes SA, 2012. Citrus Responses to Xylella fastidiosa Infection. Plant Disease, 96(9), 1245–1249. https://doi.org/10.1094/Pdis-10-11-0868-Re
Giampetruzzi A, Morelli M, Saponari M, Loconsole G, Chiumenti M, Boscia D, Savino VN, Martelli GP and Saldarelli P, 2016. Transcriptome profiling of two olive cultivars in response to infection by the CoDiRO strain of Xylella fastidiosa subsp. pauca. BMC Genomics, 17(475), 475. https://doi.org/10.1186/s12864-016-2833-9
Goheen AC and Lowe SK, 1973. Use of electron microscopy for indexing grapevines for Pierce's disease. Rivista di Patologia Vegetale, 4, 9(3), 279–280.
Goheen AC, Nyland G and Lowe SK, 1973. Association of a Rickettsia‐like organism with Pierce's disease of grapevines and alfalfa dwarf and heat therapy of the disease in grapevines. Phytopathology, 63(3), 341–345.
Goheen AC, Raju BC and Frazier NW, 1980. Alternative hosts of Pierce's disease in Napa Valley, California. Pages 171–176 in: Proc. VIIth Int. Conf. Viruses Grapevines (ICVG), Niagara Falls, Canada, 159–180.
Goheen AC, Raju BC, Lowe SK and Nyland G, 1979. Pierce's Disease of Grapevines in Central America. Plant Disease Reporter, 63(9), 788–792.
Gomes MdMdA, Lagôa AMMA, Machado EC, Medina CL and Machado MA, 2003. Gas exchanges and carbohydrate metabolism in orange trees with citrus variegated chlorosis. Brazilian Journal of Plant Physiology, 15(1), 25–31.
Gomes MMA, Lagôa AMMA, Machado EC and Medina CL, 2003. Abscisic acid and indole‐3‐acetic acid contents in orange trees infected by Xylella fastidiosa and submitted to cycles of water stress. Plant Growth Regulation, 39(3), 263–270. https://doi.org/10.1023/a:1022854405898
Goncalves FP, Lourenco SA, Stuchi ES, Hau B and Amorim L, 2011. Comparative analysis for quantification of citrus variegated chlorosis in the field. Scientia Agricola, 68(5), 562–565. https://doi.org/10.1590/s0103-90162011000500008
Goncalves FP, Stuchi ES, da Silva SR, Reiff ET and Amorim L, 2011. Role of healthy nursery plants in orange yield during eight years of citrus variegated chlorosis epidemics. Scientia Horticulturae, 129(2), 343–345. https://doi.org/10.1016/j.scienta.2011.03.038
Goncalves FP, Stuchi ES, Lourenco SA, Hau B and Amorim L, 2012. Relationship between sweet orange yield and intensity of citrus variegated chlorosis. Plant Pathology, 61(4), 641–647. https://doi.org/10.1111/j.1365-3059.2011.02557.x
Goncalves FP, Stuchi ES, Lourenco SA, Kriss AB, Gottwald TR and Amorim L, 2014. The effect of irrigation on development of citrus variegated chlorosis symptoms. Crop Protection, 57, 8–14. https://doi.org/10.1016/j.cropro.2013.11.016
González‐Jaimes EP, Souza PSD, Wickert E and Donadio LC, 2002. Avaliação da resistência à Xylella fastidiosa em germoplasma de tangerina e híbridos introduzidos da Itália e Córsega. Revista Brasileira de Fruticultura, 24(2), 579–582.
Goodwin PH and Zhang S, 1997. Distribution of Xylella fastidiosa in southern Ontario as determined by the polymerase chain reaction. Canadian Journal of Plant Pathology, 19(1), 13–18. https://doi.org/10.1080/07060669709500564
Goodwin PH, Devay JE and Meredith CP, 1988. Physiological‐Responses of Vitis vinifera Cv – Chardonnay to Infection by the Pierce's Disease Bacterium. Physiological and Molecular Plant Pathology, 32(1), 17–32. https://doi.org/10.1016/s0885-5765(88)80003-1
Goodwin PH, Devay JE and Meredith CP, 1988. Roles of Water‐Stress and Phytotoxins in the Development of Pierce's Disease of the Grapevine. Physiological and Molecular Plant Pathology, 32(1), 1–15. https://doi.org/10.1016/s0885-5765(88)80002-x
Gould A, Hamilton G, Vodak M, Grabosky J and Lashomb J, 2013. Street‐tree incidence and severity of bacterial leaf scorch of oak in the New Jersey urban forest. Phytopathology, 103(6), 51–51.
Gould A, Zhang J, Staniszewska‐Goraczniak H, Hamilton G, Hillman B, Goraczniak R and Lashomb J, 2005. Preliminary characterization of Xylella fastidiosa strains isolated from oak in New Jersey. Phytopathology, 95(6), S36‐S36.
Gould AB, French WJ, Aldrich JH, Brodbeck BV, Mizell RF and Andersen PC, 1991. Rootstock influence on occurrence of Homalodisca coagulata, peach xylem fluid amino‐acids, and concentrations of Xylella fastidiosa. Plant Disease, 75(8), 767–770. https://doi.org/10.1094/pd-75-0767
Gould AB, Hamilton G, Vodak M, Grabosky J and Lashomb J, 2004. Bacterial leaf scorch of oak in New Jersey: Incidence and economic impact. Phytopathology, 94(6), S36‐S36.
Gould AB, Hamilton G, Vodak M, Grabosky J, Staniszewska H and Lashomb J, 2007. Incidence and severity of bacterial leaf scorch of oak in the New Jersey urban forest. Phytopathology, 97(7), S178–S178.
Gould AB, Wells JM and Clarke BB, 1992. Distribution of oak leaf scorch in the Delaware Valley, New Jersey. Phytopathology, 82(10), 1160–1160.
Gouran H, Gillespie H, Nascimento R, Chakraborty S, Zaini PA, Jacobson A, Phinney BS, Dolan D, Durbin‐Johnson BP, Antonova ES, Lindow SE, Mellema MS, Goulart LR and Dandekar AM, 2016. The Secreted Protease PrtA Controls Cell Growth, Biofilm Formation and Pathogenicity in Xylella fastidiosa. Scientific Reports, 6, 31098. https://doi.org/10.1038/srep31098
Grebus ME, Henry JM, Hartin JE and Wilen CA, 1996. Bacterial leaf scorch of oleander: A new disease in southern California. Phytopathology, 86(11 SUPPL.), S110–S110.
Groenteman R, Forgie SA, Hoddle MS, Ward DF, Goeke DF and Anand N, 2015. Assessing invasion threats: novel insect‐pathogen‐natural enemy associations with native New Zealand plants in southern California. Biological Invasions, 17(5), 1299–1305. https://doi.org/10.1007/s10530-014-0804-0
Groves R, Chen J, Civerolo E, Viveros M and Freeman M, 2004. Almond leaf scorch disease in the San Joaquin Valley of California: Factors affecting pathogen distribution. Phytopathology, 94(6), S36‐S36.
Groves R, Chen J, Viveros M, Freeman M, Lynn K, Cabrera J and Civerolo E, 2005. Temporal patterns of almond leaf scorch disease progress and associated Xylella fastidiosa genotypes. Phytopathology, 95(6), S37–S37.
Groves RL, Chen J, Civerolo EL, Freeman MW and Viveros MA, 2005. Spatial analysis of almond leaf scorch disease in the San Joaquin Valley of California: Factors affecting pathogen distribution and spread. Plant Disease, 89(6), 581–589. https://doi.org/10.1094/Pd-89-0581
Guan W, Shao J, Elbeaino T, Davis RE, Zhao T and Huang Q, 2015. Specific Detection and Identification of American Mulberry‐Infecting and Italian Olive‐Associated Strains of Xylella fastidiosa by Polymerase Chain Reaction. PloS one, 10(6), e0129330. https://doi.org/10.1371/journal.pone.0129330
Guan W, Shao J, Singh R, Davis RE, Zhao T and Huang Q, 2013. A TaqMan‐based real‐time PCR assay for specific detection and quantification of Xylella fastidiosa strains causing bacterial leaf scorch in oleander. J Microbiol Methods, 92(2), 108–112. https://doi.org/10.1016/j.mimet.2012.11.008
Guevara J, 1997. Occurrence of Pierce's Disease (Xyllela fastidiosa) in grape of the Guadalupe Valley, Baja California, Mexico. Phytopathology, 87(6 SUPPL.), S36‐S36.
Guilhabert MR and Kirkpatrick BC, 2005. Identification of Xylella fastidiosa antivirulence genes: Hemagglutinin adhesins contribute to X. fastidiosa biofilm maturation and colonization and attenuate virulence. Molecular Plant‐Microbe Interactions, 18(8), 856–868. https://doi.org/10.1094/Mpmi-18-0856
Güldür ME, Caglar BK, Castellano MA, Unlu L, Guran S, Yilmaz MA and Martelli GP, 2005. First report of almond leaf scorch in Turkey. Journal of Plant Pathology, 87(3), 246–246.
Guo X, Zhang S and Jiang L, 2003. Direct detection of Xylella fastidiosa of Pierce's disease in xylem fluids of grapevines. Journal of Agricultural Biotechnology, 11(4), 435–436.
Guo XR and Lu J, 2004. Use of a pressure chamber to isolate and detect Xylella fastidiosa in xylem exudates of grapevines. American Journal of Enology and Viticulture, 55(2), 202–205.
Gupta AK and Sharma RC, 1997. Leaf scorch disease of Behmi‐a promising root stock. Plant Disease Research, 12(2), 166–167.
Gutierrez‐Ibanez AT, Laguna‐Cerda A, Rojas‐Martinez RI, Gonzalez‐Garza R, Salgado‐Siclan ML, Aguilar‐Ortigoza C and Gonzalez‐Esquivel C, 2009. Molecular association of Xylella fastidiosa in potato plants (Solanum tuberosum L.) with purple top symptoms. Revista Chapingo Serie Horticultura, 15(3), 275–279.
Gvozdyak RI, Shevchenko SI and Sadovskii YP, 1990. Study of the plum leaf scald pathogen. Mikrobiologicheskii Zhurnal (Kiev), 52(2), 70–77.
Habermann G and Rodrigues JD, 2009. Leaf gas exchange and fruit yield in sweet orange trees as affected by citrus variegated chlorosis and environmental conditions. Scientia Horticulturae, 122(1), 69–76. https://doi.org/10.1016/j.scienta.2009.04.003
Habermann G, Alvarez RDF, Modesto JC, Fortes AMT, Rodrigues JD and Ono EO, 2006. Rooting of healthy and CVC‐affected ‘Valencia’ sweet orange stem cuttings, through the use of plant regulators. Brazilian Archives of Biology and Technology, 49(1), 29–36. https://doi.org/10.1590/s1516-89132006000100004
Habermann G, Machado EC, Rodrigues JD and Medina CL, 2000. Evaluation of Xylella fastidiosa effects on leaf gas exchange of Pera sweet orange grafted on Rangpur lime rootstock. Proceedings of the 14th Conference of the International Organization of Citrus Virologists, Campinas, Sao Paulo State, Brazil, 13–18 September 1998, 249–253.
Habermann G, Machado EC, Rodrigues JD and Medina CL, 2003. CO2 assimilation, photosynthetic light response curves, and water relations of ‘Pera’ sweet orange plants infected with Xylella fastidiosa. Brazilian Journal of Plant Physiology, 15(2), 79–87.
Habermann G, Machado EC, Rodrigues JD and Medina CL, 2003. Gas exchange rates at different vapor pressure deficits and water relations of ‘Pera’ sweet orange plants with citrus variegated chlorosis (CVC). Scientia Horticulturae, 98(3), 233–245. https://doi.org/10.1016/S0304-4238(02)00228-5
Habib W, Nigro F, Gerges E, Jreijiri F, Al Masri Y, El Riachy M and Choueiri E, 2016. Xylella fastidiosa does not occur in Lebanon. Journal of Phytopathology, 164(6), 395–403.
Haelterman RM, Tolocka PA, Roca ME, Guzmán FA, Fernandez FD and Otero ML, 2015. First Presumptive Diagnosis of Xylella fastidiosa Causing Olive Scorch in Argentina. Journal of Plant Pathology, 97(2), 393–393.
Hao L, Johnson K, Cursino L, Mowery P and Burr TJ, 2017. Characterization of the Xylella fastidiosa PD1311 gene mutant and its suppression of Pierce's disease on grapevines. Molecular Plant Pathology, 18(5), 684–694. https://doi.org/10.1111/mpp.12428
Harkness M and Morano L, 2004. Initial analysis of hybrid varieties Blanc du Bois, Cynthiana, and Black Spanish within a Pierce's disease hot zone. American Journal of Enology and Viticulture, 55(3), 318A‐318A.
Harmon PF and Hopkins DL, 2009. First report of bacterial leaf scorch caused by Xylella fastidiosa on southern highbush blueberry in Florida. Plant Disease, 93(11), 1220–1220. https://doi.org/10.1094/Pdis-93-11-1220a
Harper SJ, Ward LI and Clover GR, 2010. Development of LAMP and real‐time PCR methods for the rapid detection of Xylella fastidiosa for quarantine and field applications. Phytopathology, 100(12), 1282–1288. https://doi.org/10.1094/PHYTO-06-10-0168
Harris JL and Balci Y, 2015. Population Structure of the Bacterial Pathogen Xylella fastidiosa among Street Trees in Washington DC. PloS one, 10(3), ARTN e0121297
Harris JL, Di Bello P, Lear M, Hoang Q and Balci Y, 2013. Bacterial leaf scorch of amenity trees caused by Xylella fastidiosa in Washington, DC: Distribution, host range and presence of the pathogen within street trees. Phytopathology, 103(10)
Harris JL, Di Bello PL, Lear M and Balci Y, 2014. Bacterial leaf scorch in the District of Columbia: distribution, host range, and presence of Xylella fastidiosa among urban trees. Plant Disease, 98(12), 1611–1618. https://doi.org/10.1094/Pdis-02-14-0158-Sr
Harrison UJ, Anas O and Sutton TB, 2002. Geographical distribution of Pierce's disease in North Carolina's winegrowing region. Phytopathology, 92(6 Supplement), S35–S35.
Hartman JR, Eshenaur BC and Jarlfors UE, 1995. Bacterial leaf scorch caused by Xylella fastidiosa: A Kentucky survey: A unique pathogen; and bur oak, a new host. Journal of Arboriculture, 21(2), 77–82.
Hartman JR, Jarlfors UE, Fountain WM and Thomas R, 1996. First report of bacterial leaf scorch caused by Xylella fastidiosa on sugar maple and sweetgum. Plant Disease, 80(11), 1302–1302. https://doi.org/10.1094/pd-80-1302b
Hartman JR, Kaiser CA, Jarlfors UE, Eshenaur BC, Bachi PR and Dunwell WC, 1991. Occurrence of oak bacterial leaf scorch caused by Xylella fastidiosa in Kentucky. Plant Disease, 75(8), 862–862. https://doi.org/10.1094/pd-75-0862d
Hartung JS, Beretta J, Brlansky RH, Spisso J and Lee RF, 1994. Citrus Variegated chlorosis – bacterium – axenic culture, pathogenicity, and serological relationships with other strains of Xylella fastidiosa. Phytopathology, 84(6), 591–597. https://doi.org/10.1094/phyto-84-591
Hartung JS, Nian S, Lopes S, Ayres AJ and Brlansky R, 2014. Lack of evidence for transmission of Xylella fastidiosa from infected sweet orange seed. Journal of Plant Pathology, 96(3), 497–506.
Haygood RA, Witcher W and Jones RK, 1988. Outbreak of sycamore leaf scorch in the Carolinas. Plant Disease, 72(7), 644–644. https://doi.org/10.1094/pd-72-0644c
He CX, Li WB, Ayres AJ, Hartung JS, Miranda VS and Teixeira DC, 2000. Distribution of Xylella fastidiosa in Citrus rootstocks and transmission of citrus variegated chlorosis between sweet orange plants through natural root grafts. Plant Disease, 84(6), 622–626. https://doi.org/10.1094/pdis.2000.84.6.622
Hearon SS, Sherald JL and Kostka SJ, 1980. Association of xylem‐limited bacteria with elm, sycamore, and oak leaf scorch. Canadian Journal of Botany‐Revue Canadienne De Botanique, 58(18),1986–1993. https://doi.org/10.1139/b80-228
Hendson M, Purcell AH, Chen D, Smart C, Guilhabert M and Kirkpatrick B, 2001. Genetic diversity of Pierce's disease strains and other pathotypes of Xylella fastidiosa. Appl Environ Microbiol, 67(2),895–903. https://doi.org/10.1128/AEM.67.2.895-903.2001
Henneberger T, Stevenson KL and Britton KO, 2003. Effect of low temperature on populations of Xylella fastidiosa in naturally infected sycamore. Phytopathology, 93(6 Supplement), S34‐S35.
Henneberger TSM, Stevenson KL, Britton KO and Chang CJ, 2004. Distribution of Xylella fastidiosa in sycamore associated with low temperature and host resistance. Plant Disease, 88(9), 951–958. https://doi.org/10.1094/pdis.2004.88.9.951
Hernandez Garboza L and Ochoa Corona F, 1994. Diagnosis of Xylella fastidiosa in grape and weeds associated with this crop. Manejo Integrado de Plagas, (33), 7–10.
Hernandez L and Ochoa Corona FM, 1997. ELISA‐DAS detection of Xylella fastidiosa Wells et al. in grapevine (Vitis vinifera L.) and weeds in vineyards of Mara county, Zulia state, Venezuela. Revista de la Facultad de Agronomia, Universidad del Zulia, 14(3), 297–306.
Hernandez‐Martinez R, Cooksey DA and Wong FP, 2009. Leaf Scorch of Purple‐Leafed Plum and Sweetgum Dieback: Two New Diseases in Southern California Caused by Xylella fastidiosa Strains with Different Host Ranges. Plant Disease, 93(11), 1131–1138. https://doi.org/10.1094/Pdis-93-11-1131
Hernandez‐Martinez R, Costa HS, Cooksey DA and Wong FP, 2006. Leaf scorch in ornamental purple‐leaf plum (Prunus cerasifera) in southern California caused by Xylella fastidiosa. Phytopathology, 96(6), S47–S47.
Hernandez‐Martinez R, Costa HS, Cooksey DA and Wong FP, 2006. Sweet gum dieback in southern California caused by Xylella fastidiosa. Phytopathology, 96(6), S47–S47.
Hernandez‐Martinez R, Costa HS, Dumenyo CK and Cooksey DA, 2006. Differentiation of strains of Xylella fastidiosa infecting grape, almonds, and oleander using a multiprimer PCR assay. Plant Disease, 90(11), 1382–1388. https://doi.org/10.1094/Pd-90-1382
Hernandez‐Martinez R, de la Cerda KA, Costa HS, Cooksey DA and Wong FP, 2007. Phylogenetic Relationships of Xylella fastidiosa Strains Isolated from Landscape Ornamentals in Southern California. Phytopathology, 97(7), 857–864. https://doi.org/10.1094/PHYTO-97-7-0857
Hernandez‐Martinez R, Dumenyo CK, Azad H, Costa HS, Wong FP and Cooksey DA, 2004. Phylogenetic analyses of Xylella fastidiosa strains isolated from ornamental hosts. Phytopathology, 94(6), S152–S152.
Hernandez‐Martinez R, Pinckard T, Costa H, Cooksey D and Wong F, 2005. Isolation and characterization of Xylella fastidiosa strains from mulberry in southern California and inability to infect oleander and grape. Phytopathology, 95(6), S41–S41.
Hernandez‐Martinez R, Pinckard TR, Costa HS, Cooksey DA and Wong FP, 2006. Discovery and characterization of Xylella fastidiosa strains in southern California causing mulberry leaf scorch. Plant Disease, 90(9), 1143–1149. https://doi.org/10.1094/Pd-90-1143
Hewitt WB, 1958. Pierce's disease virus in Mississippi and other southern states. The Plant Disease Reporter, 42(2), 207–210.
Hewitt WB, Frazier NW and Houston BR, 1942. Transmission or Pierce's Disease of Grapevines with a Leaf Hopper. Phytopathology, 32(1), p‐8 p.
Hewitt WB, Houston BR and et al., 1946. Leafhopper transmission of the virus causing Pierce's disease of grape and dwarf of alfalfa. Phytopathology, 36(2), 117–128.
Hill BL and Purcell AH, 1995. Acquisition and Retention of Xylella fastidiosa by an Efficient Vector, Graphocephala atropunctata. Phytopathology, 85(2), 209–212. https://doi.org/10.1094/phyto-85-209
Hill BL and Purcell AH, 1995. Multiplication and Movement of Xylella fastidiosa within Grapevine and 4 Other Plants. Phytopathology, 85(11), 1368–1372. https://doi.org/10.1094/phyto-85-1368
Hill BL and Purcell AH, 1997. Populations of Xylella fastidiosa in Plants Required for Transmission by an Efficient Vector. Phytopathology, 87(12), 1197–1201. https://doi.org/10.1094/PHYTO.1997.87.12.1197
Hilton A, Wang XW, Jo YK and Grauke LJ, 2017. Improved diagnostic methods for Xylella fastidiosa infecting pecan and related Carya species. Phytopathology, 107(3), 6–6.
Hilton AE, Jo YK, Cervantes K, Stamler RA, Randall JJ, French JM, Heerema RJ, Goldberg NP, Sherman J, Wang X and Grauke LJ, 2017. First Report of Pecan Bacterial Leaf Scorch Caused by Xylella fastidiosa in Pecan (Carya illinoinensis) in Arizona, New Mexico, California, and Texas. Plant Disease, 101(11), 1949–1949. https://doi.org/10.1094/Pdis-02-17-0298-Pdn
Holland RM, Christiano RSC, Gamliel‐Atinsky E and Scherm H, 2014. Distribution of Xylella fastidiosa in Blueberry Stem and Root Sections in Relation to Disease Severity in the Field. Plant Disease, 98(4), 443–447. https://doi.org/10.1094/Pdis-06-13-0680-Re
Holley JD, 1993. Diseases diagnosed on herbaceous and woody ornamentals. Canadian Plant Disease Survey, 73(45–50)
Hopkins D, 2005. Biological control of Pierce's disease in the vineyard with a benign strain of Xylella fastidiosa. Phytopathology, 95(6), S44–S44.
Hopkins D, Harmon P and Brannen P, 2012. Host range of Xylella fastidiosa strains that cause blueberry leaf scorch. Phytopathology, 102(7), 55–55.
Hopkins DL and Adlerz WC, 1977. Transmission of the Pierces disease bacterium from citrus trees with blight to indicator. Proceedings of the American Phytopathological Society, (4), 224–224.
Hopkins DL and Adlerz WC, 1980. Pierce Disease Bacterium Causes a Disease of Rough Lemon Citrus. Phytopathology, 70(6), 568–568.
Hopkins DL and Adlerz WC, 1988. Natural Hosts of Xylella fastidiosa in Florida. Plant Disease, 72(5), 429–431. https://doi.org/10.1094/pd-72-0429
Hopkins DL and Mollenhauer HH, 1973. Rickettsia‐like bacterium associated with Pierce's disease of grapes. Science (Washington D C), 179(4070), 298–300.
Hopkins DL and Mollenhauer HH, 1975. Tylose and gum formation in the xylem of Pierce's disease infected grapevines. Proceedings of the American Phytopathological Society, 2, 65–65.
Hopkins DL and Mortensen JA, 1971. Suppression of Pierce's disease symptoms by tetracycline antibiotics. Plant Disease Reporter, 55(7), 610–612.
Hopkins DL and Mortensen JA, 1974. Pierce's disease in muscadine grapes. Proceedings of the American Phytopathological Society, 1, (1975)‐(1975).
Hopkins DL and Thompson CM, 1982. Multiplication of virulent and avirulent Pierce's disease bacterial isolates in grapevine tissue. Proceedings of the Fifth International Conference on Plant Pathogenic Bacteria, 225–234.
Hopkins DL and Thompson CM, 1984. Seasonal Concentration of the Pierce's Disease Bacterium in Carlos and Welder Muscadine Grapes Compared with Schuyler Bunch Grape. Hortscience, 19(3), 419–420.
Hopkins DL and Wichman RL, 2001. Pathogenic and molecular relationships among strains of Xylella fastidiosa from grapevine and American elder. Plant Pathogenic Bacteria, 161–164.
Hopkins DL, 1979. Seasonal concentrations of bacterial plugs in grapevines severely infected with the Pierce's disease bacterium. Phytopathology, 69(5), 528–528.
Hopkins DL, 1980. Use of pin‐prick inoculation technique demonstrates variability in virulence of Pierce's disease bacterium. Pages 177–180 in: Proc. VIIth Int. Conf. Viruses Grapevines (ICVG), Niagara Falls, Canada, 159–180.
Hopkins DL, 1981. Seasonal Concentration of the Pierce's Disease Bacterium in Grapevine Stems, Petioles, and Leaf Veins. Phytopathology, 71(4), 415–418. https://doi.org/10.1094/phyto-71-415
Hopkins DL, 1982. Relation of Pierce's Disease Bacterium to a Wilt‐Type Disease in Citrus in the Greenhouse. Phytopathology, 72(8), 1090–1092. https://doi.org/10.1094/phyto-72-1090
Hopkins DL, 1982. Variability in Virulence of the Bacterium Causing Pierce's Disease of Grapevine. Phytopathology, 72(7), 1001–1001.
Hopkins DL, 1984. Variability of Virulence in Grapevine among Isolates of the Pierce's Disease Bacterium. Phytopathology, 74(11), 1395–1398. https://doi.org/10.1094/phyto-74-1395
Hopkins DL, 1985. Effects of plant‐growth regulators on development of Pierces disease symptoms in grapevine. Plant Disease, 69(11), 944–946.
Hopkins DL, 1985. Physiological and pathological characteristics of virulent and avirulent strains of the bacterium that causes Pierces disease of grapevine. Phytopathology, 75(6), 713–717. https://doi.org/10.1094/phyto-75-713
Hopkins DL, 1985. Water‐stress in grapevines with Pierce's disease. Phytopathology, 75(4), 500–500.
Hopkins DL, 1987. Xylem‐Limited Bacteria Cause Blight Symptoms in Citrus. Phytopathology, 77(4), 641–641.
Hopkins DL, 1988. Production of Diagnostic Symptoms of Blight in Citrus Inoculated with Xylella fastidiosa. Plant Disease, 72(5), 432–435. https://doi.org/10.1094/pd-72-0432
Hopkins DL, 1990. Colonization of Pierce's Disease Resistant and Susceptible Grapevines by Xylella fastidiosa. Plant Pathogenic Bacteria, Pts a and B, 951–956.
Hopkins DL, 1991. Colonization of grapevine by various strains of Xylella fastidiosa. Phytopathology, 81(7), 812–812.
Hopkins DL, 1994. Induced resistance to Pierce's disease of grapevine by weakly virulent strains of Xylella fastidiosa. [INRA Colloquia; Plant pathogenic bacteria], 66, 951–956.
Hopkins DL, 2005. Biological control of Pierce's disease in the vineyard with strains of Xylella fastidiosa benign to grapevine. Plant Disease, 89(12), 1348–1352. https://doi.org/10.1094/Pd-89-1348
Hopkins DL, Adlerz WC and Bistline FW, 1978. Pierce's disease bacterium occurs in Citrus trees affected with blight (young tree decline). Plant Disease Reporter, 62(5), 442–445.
Hopkins DL, Bistline FW, Russo LW and Thompson CM, 1988. Seasonal detection of Xylella fastidiosa in Citrus with blight. Phytopathology, 78(12 PART 1), 1602–1602.
Hopkins DL, Bistline FW, Russo LW and Thompson CM, 1991. Seasonal fluctuation in the occurrence of Xylella fastidiosa in root and stem extracts from Citrus with blight. Plant Disease, 75(2), 145–147. https://doi.org/10.1094/pd-75-0145
Hopkins DL, French WJ and Mollenhauer HH, 1973. Association of a Rickettsia‐like bacterium with phony peach disease. Phytopathology, 63(4), 443–443.
Hopkins DL, Mollenhauer HH and French WJ, 1973. Occurrence of Rickettsia‐like bacterium in the xylem of peach trees with phony disease. Phytopathology, 63(11), 1422–1423.
Hopkins DL, Mollenhauer HH and Mortensen JA, 1974. Tolerance to Pierce's disease and the associated Rickettsia‐like bacterium in muscadine grape. Journal of the American Society for Horticultural Science, 99(5), 436–439.
Hopkins DL, Thompson CM, Bistline FW and Russo LW, 1990. Relationship between Xylem‐Limited Bacteria and Citrus Blight. Proceedings of the 102nd Annual Meeting of the Florida State Horticultural Society, 102, 21–23.
Hopkins DL, Thompson CM, Wichman RL, Bistline FW and Russo LW, 1995. Effect of inoculation of mature Citrus trees in the grove with Xylella fastidiosa on Citrus blight incidence. Proceedings of the Florida State Horticultural Society, (108), 103–106.
Hopkins DL, Thompson CM, Wichman RL, Russo LW and Bistline FW, 1996. Inoculation of mature trees with Xylella fastidiosa produces Citrus blight. Phytopathology, 86(11 SUPPL.), S106‐S106.
Houston BR, Esau K and Hewitt WB, 1947. The mode of vector feeding and the tissues involved in the transmission of Pierce's disease virus in grape and alfalfa. Phytopath, 37(4), 247–253.
Hu YLN, Coneva E, Vinson E, Kessler JR, Spiers J and Ducar J, 2012. Assessment of the Feasibility of Growing Pierce's Disease Tolerant American and French‐American Hybrid Bunch Grape Cultivars in Alabama. Journal of the American Pomological Society, 66(4), 220–222.
Huang PY, Milholland RD and Daykin ME, 1986. Structural and Morphological‐Changes Associated with the Pierce's Disease Bacterium in Bunch and Muscadine Grape Tissues. Phytopathology, 76(11), 1232–1238. https://doi.org/10.1094/phyto-76-1232
Huang Q and Sherald JL, 2004. Isolation and phylogenetic analysis of Xylella fastidiosa from its invasive alternative host, porcelain berry. Current Microbiology, 48(1), 73–76.
Huang Q, 2004. First report of Xylella fastidiosa associated with leaf scorch in black oak in Washington, DC. Plant Disease, 88(2), 224–224. https://doi.org/10.1094/pdis.2004.88.2.224c
Huang Q, 2004. Natural occurrence of Xylella fastidiosa in a commercial nursery in Maryland. Phytopathology, 94(6), S43–S43.
Huang Q, 2009. Specific detection and identification of Xylella fastidiosa Strains Causing Oleander Leaf Scorch Using Polymerase Chain Reaction. Current Microbiology, 58(4), 393–398. https://doi.org/10.1007/s00284-008-9324-4
Huang Q, Bentz J and Sherald JL, 2006. Fast, easy and efficient DNA extraction and one‐step polymerase chain reaction for the detection of Xylella fastidiosa in potential insect vectors. Journal of Plant Pathology, 88(1), 77–81.
Huang Q, Brlansky RH, Barnes L, Li W and Hartung JS, 2004. First report of oleander leaf scorch caused by Xylella fastidiosa in Texas. Plant Disease, 88(9), 1049–1049. https://doi.org/10.1094/pdis.2004.88.9.1049a
Huang Q, Li WB and Hartung JS, 2003. Association of Xylella fastidiosa with leaf scorch in Japanese beech bonsai. Canadian Journal of Plant Pathology‐Revue Canadienne De Phytopathologie, 25(4), 401–405.
Hutchins LM, 1930. The phony disease of the peach. Journal of Economic Entomology, 23(3), 555–562.
Hutchins LM, Cochran LC, Turner WF and Weinberger JH, 1953. Transmission of phony disease virus from tops of certain affected Peach and Plum trees. Phytopathology, 43(12), 691–696.
Irvin NA, Pinckard TR, Perring TM and Hoddle MS, 2014. Evaluating the potential of buckwheat and cahaba vetch as nectar producing cover crops for enhancing biological control of Homalodisca vitripennis in California vineyards. Biological Control, 76, 10–18. https://doi.org/10.1016/j.biocontrol.2014.04.006
Istituto Agronomico Mediterraneo di Bari CIHEAM/IAM.B, 2016. Comunicazione di nuova specie vegetale ospite di Xylella fastidiosa. Prot. Selge in entrata N.4 del 08/07/2016.
Istituto per la Protezione sostenibile delle Piante – CNR, 2016. Comunicazione rinvenimento infezioni di Xylella fastidiosa su nuove specie. Prot. Selge 93/2016.
Istituto per la Protezione sostenibile delle Piante – CNR, 2018. Aggiornamento attività di caratterizzazione genetica isolati di Xylella fastidiosa e segnalazione nuova specie ospite. Prot. Selge 62/2018.
Jackson BC, Blua MJ and Bextine B, 2008. Impact of duration versus frequency of probing by Homalodisca vitripennis (Hemiptera: Cicadellidae) on inoculation of Xylella fastidiosa. J Econ Entomol, 101(4), 1122–1126.
Jacques MA, Denancé N, Legendre B, Morel E, Briand M, Mississipi S, Durand K, Olivier V, Portier P, Poliakoff F and Crouzillat D, 2016. New Coffee Plant‐Infecting Xylella fastidiosa Variants Derived via Homologous Recombination. Applied and Environmental Microbiology, 82(5), 1556–1568. https://doi.org/10.1128/Aem.0329-15
Jimenez LG, 1985a. Immunological Evidence of Pierce Disease in Grapevines in Venezuela. Turrialba, 35(3), 243–247.
Jimenez LG, 1985b. Pierce's Disease in the Grapevines in Venezuela – Immunological Evidence. Phytopathology, 75(10), 1175–1175.
Jimenez LG and Morales‐Bance F, 1985. Distribution of Pierce's disease of grapevine in Costa Rica determined by the ELISA technique. Agronomia Costarricense, 9(1), 79–83.
Jimenez A LG and Ingalls A, 1990. Vitis caribaea as a source of resistance to Pierce's disease in breeding grapes for the tropics. Vitis, 262–270.
Jindal KK and Sharma RC, 1987. Outbreaks and new records india almond leaf scorch a new disease from India. FAO (Food and Agriculture Organization of the United Nations) Plant Protection Bulletin, 35(2), 64–65.
Junior RPL, Leite RMVBD and Ceresini PC, 1998. Non pathogenicity to peach cultivar Flordasun of Xylella fastidiosa. Pesquisa Agropecuaria Brasileira, 33(10), 1653–1660.
Kandel PP, Almeida RPP, Cobine PA and De La Fuente L, 2017. Natural Competence Rates Are Variable Among Xylella fastidiosa Strains and Homologous Recombination Occurs In Vitro Between Subspecies fastidiosa and multiplex. Molecular Plant‐Microbe Interactions, 30(7), 589–600. https://doi.org/10.1094/MPMI-02-17-0053-R
Kenknight G, 1951. Occurrence of phony disease in wild plum thickets distant from peach orchards in Spartanburg County, South Carolina. The Plant Disease Reporter, 35(4), 183–185.
Killiny N and Almeida RP, 2009. Xylella fastidiosa afimbrial adhesins mediate cell transmission to plants by leafhopper vectors. Applied and Environmental Microbiology, 75(2), 521–528. https://doi.org/10.1128/AEM.01921-08
Killiny N and Almeida RP, 2011. Gene regulation mediates host specificity of a bacterial pathogen. Environmental Microbiology Reports, 3(6), 791–797. https://doi.org/10.1111/j.1758-2229.2011.00288.x
Killiny N and Almeida RP, 2014. Factors affecting the initial adhesion and retention of the plant pathogen Xylella fastidiosa in the foregut of an insect vector. Applied and Environmental Microbiology, 80(1), 420–426. https://doi.org/10.1128/AEM.03156-13
Killiny N, Martinez RH, Dumenyo CK, Cooksey DA and Almeida RPP, 2013. The Exopolysaccharide of Xylella fastidiosa Is Essential for Biofilm Formation, Plant Virulence, and Vector Transmission. Molecular Plant‐Microbe Interactions, 26(9), 1044–1053. https://doi.org/10.1094/Mpmi-09-12-0211-R
Killiny N, Rashed A and Almeida RP, 2012. Disrupting the transmission of a vector‐borne plant pathogen. Applied and Environmental Microbiology, 78(3), 638–643. https://doi.org/10.1128/AEM.06996-11
Kitajima EW and Barros TSL, 2000. Natural co‐infection of periwinkle by a phytoplasma and a Xylella‐like bacterium. Summa Phytopathologica, 26(1), 69–70.
Kitajima EW, Bakarcic M and Fernandezvaliela MV, 1975. Association of Rickettsia‐Like Bacteria with Plum Leaf Scald Disease. Phytopathology, 65(4), 476–479. https://doi.org/10.1094/phyto-65-476
Kitajima EW, Filho C, Machado and Novaes, 2000. Escaldadura das folhas em Hibiscus schizopetalus associada à infecção por Xylella fastidiosa em Brasília, DF. Fitopatologia Brasileira, 25(Suplemento), 323.
Kitajima EW, Mohan SK, Tsuneta M, Bleicher J, French W and Leite RP, Jr., 1981. Occurrence of plum Prunus salicina leaf scald in the states of Parana and Santa Catarina Brazil. Fitopatologia Brasileira, 6(2), 285–292.
Koide T, Zaini PA, Moreira LM, Vencio RZ, Matsukuma AY, Durham AM, Teixeira DC, El‐Dorry H, Monteiro PB, da Silva AC, Verjovski‐Almeida S, da Silva AM and Gomes SL, 2004. DNA microarray‐based genome comparison of a pathogenic and a nonpathogenic strain of Xylella fastidiosa delineates genes important for bacterial virulence. Journal of Bacteriology, 186(16), 5442–5449. https://doi.org/10.1128/JB.186.16.5442-5449.2004
Kostka SJ, Sherald JL and Tattar TA, 1982. Isolation of Bacteria from 3 Elm Species and Mulberry Exhibiting Leaf Scorch. Phytopathology, 72(7), 936–936.
Kostka SJ, Sherald JL and Tattar TA, 1984. Culture of Fastidious, Xylem‐Limited Bacteria from Declining Oaks in the Northeastern States. Phytopathology, 74(7), 803–803.
Kostka SJ, Sherald JL, Hearon SS and Rissler JF, 1980. Serological Relatedness of Elm and Oak Scorch‐Associated Bacteria to the Pierce's Disease (Pd) Bacterium. Phytopathology, 70(7), 689–690.
Kostka SJ, Sherald JL, Hearon SS and Rissler JF, 1981. Cultivation of the Elm Leaf Scorch‐Associated Bacterium (Esb). Phytopathology, 71(7), 768–768.
Kostka SJ, Tattar TA and Sherald JL, 1983. Mulberry Leaf Scorch – Pathogenicity of the Associated Bacterium. Phytopathology, 73(9), 1344–1344.
Kostka SJ, Tattar TA and Sherald JL, 1985. Suppression of bacterial leaf scorch symptoms in American elm through oxytetracycline microinjection. Journal of Arboriculture, 11(2), 54–58.
Kostka SJ, Tattar TA and Sherald JL, 1986. Elm Leaf Scorch – Abnormal Physiology in American Elms Infected with Fastidious, Xylem‐Inhabiting Bacteria. Canadian Journal of Forest Research‐Revue Canadienne De Recherche Forestiere, 16(5), 1088–1091. https://doi.org/10.1139/x86-188
Kostka SJ, Tattar TA, Sherald JL and Hurtt SS, 1986. Mulberry Leaf Scorch, New Disease Caused by a Fastidious, Xylem‐Inhabiting Bacterium. Plant Disease, 70(7), 690–693. https://doi.org/10.1094/pd-70-690
Krell RK, Boyd EA, Nay JE, Park YL and Perring TM, 2007. Mechanical and insect transmission of Xylella fastidiosa to Vitis vinifera. American Journal of Enology and Viticulture, 58(2), 211–216.
Krell RK, Perring TM, Farrar CA, Park YL and Gispert C, 2006. Intraplant sampling of grapevines for Pierce's disease diagnosis. Plant Disease, 90(3), 351–357. https://doi.org/10.1094/Pd-90-0351
Krell RK, Perring TM, Hashim‐Buckey JM and Pinckard TR, 2008. Susceptibility of Vitis vinifera L. cv. Redglobe and Thompson Seedless to Pierce's disease. American Journal of Enology and Viticulture, 59(1), 61–66.
Krewer GW, Daniell JW and Couvillon GA, 1981. The Effect of Phony Peach on the Rooting of Cuttings from Prunus Persica (L) Batsch. Hortscience, 16(3), 282–282.
Krewer GW, Daniell JW and Couvillon GA, 1982. The Effect of Phony Peach on the Rooting of Peach Cuttings. Hortscience, 17(2), 225–226.
Krivanek AF and Walker MA, 2005. Vitis resistance to Pierce's disease is characterized by differential Xylella fastidiosa populations in stems and leaves. Phytopathology, 95(1), 44–52. https://doi.org/10.1094/Phyto-95-0044
Krivanek AF, Famula TR, Tenscher A and Walker MA, 2005. Inheritance of resistance to Xylella fastidiosa within a Vitis rupestris x Vitis arizonica hybrid population. Theoretical and Applied Genetics, 111(1), 110–119. https://doi.org/10.1007/s00122-005-1999-3
Krivanek AF, Riaz S and Walker MA, 2006. Identification and molecular mapping of PdR1, a primary resistance gene to Pierce's disease in Vitis. Theoretical and Applied Genetics, 112(6), 1125–1131. https://doi.org/10.1007/s00122-006-0214-5
Krivanek AF, Stevenson JF and Walker MA, 2005. Development and Comparison of Symptom Indices for Quantifying Grapevine Resistance to Pierce's Disease. Phytopathology, 95(1), 36–43. https://doi.org/10.1094/PHYTO-95-0036
Krugner R and Ledbetter CA, 2016. Rootstock Effects on Almond Leaf Scorch Disease Incidence and Severity. Plant Disease, 100(8), 1617–1621. https://doi.org/10.1094/Pdis-01-16-0125-Re
Krugner R, Ledbetter CA, Chen J and Shrestha A, 2012. Phenology of Xylella fastidiosa and Its Vector Around California Almond Nurseries: An Assessment of Plant Vulnerability to Almond Leaf Scorch Disease. Plant Disease, 96(10), 1488–1494. https://doi.org/10.1094/Pdis-01-16-0125-Re
Krugner R, Sisterson MS and Lin H, 2012. Effects of Gender, Origin, and Age on Transmission of Xylella fastidiosa to Grapevines by Homalodisca vitripennis (Hemiptera: Cicadellidae). Annals of the Entomological Society of America, 105(2), 280–286. https://doi.org/10.1603/An11117
Krugner R, Sisterson MS, Chen JC, Stenger DC and Johnson MW, 2014. Evaluation of Olive as a Host of Xylella fastidiosa and Associated Sharpshooter Vectors. Plant Disease, 98(9), 1186–1193. https://doi.org/10.1094/Pdis-01-16-0125-Re
Kuriger WE, Schaad NW and French WJ, 1981. Purification of rickettsia‐like bacteria (Rlb) from phony peach‐trees by renografin density gradient centrifugation. Phytopathology, 71(2), 233–233.
Kuzina LV, Miller TA and Cooksey DA, 2006. In vitro activities of antibiotics and antimicrobial peptides against the plant pathogenic bacterium Xylella fastidiosa. Letters in Applied Microbiology, 42(5), 514–520. https://doi.org/10.1111/j.1472-765X.2006.01898.x
Labroussaa F, Zeilinger AR and Almeida RP, 2016. Blocking the transmission of a noncirculative vector‐borne plant pathogenic bacterium. Molecular Plant‐Microbe Interactions, 29(7), 535–544. https://doi.org/10.1094/MPMI-02-16-0032-R
Lacava PT, Andreote FD, Araujo WL and Azevedo JL, 2006. Characterization of the endophytic bacterial community from Citrus by isolation, specific PCR and DGGE. Pesquisa Agropecuaria Brasileira, 41(4), 637–642. https://doi.org/10.1590/s0100-204x2006000400013
Lacava PT, Araujo WL, Maccheroni W and Azevedo JL, 2001. RAPD profile and antibiotic susceptibility of Xylella fastidiosa, causal agent of citrus variegated chlorosis. Letters in Applied Microbiology, 33(4), 302–306.
Lacava PT, Araujo WL, Marcon J, Maccheroni W and Azevedo JL, 2004. Interaction between endophytic bacteria from Citrus plants and the phytopathogenic bacteria Xylella fastidiosa, causal agent of Citrus‐variegated chlorosis. Letters in Applied Microbiology, 39(1), 55–59. https://doi.org/10.1111/j.1472-765X.2004.01543.x
Laranjeira FF and Palazzo DA, 1999. Qualitative damage to ‘Natal’ sweet orange fruits caused by citrus variegated chlorosis. Laranja, 20(1), 77–91.
Laranjeira FF and Pompeu Junior J, 2002. Performance of 15 cultivars of sweet oranges affected by citrus variegated chlorosis. Laranja, 23(2), 401–411.
Laranjeira FF, Bergamin Filho A and Amorim L, 1998. Dynamics and structure of citrus variegated chlorosis (CVC) foci. Fitopatologia Brasileira, 23(1), 36–41.
Laranjeira FF, Bergamin Filho A, Amorim L and Gottwald TR, 2004. Spatial dynamics of citrus variegated chlorosis in three regions of Sao Paulo, Brazil. Fitopatologia Brasileira, 29(1), 56–65.
Laranjeira FF, Bergamin Filho A, Amorim L and Lopes JRS, 2003. Seasonal behaviour of citrus variegated chlorosis in three regions of Sao Paulo State, Brazil. Fitopatologia Brasileira, 28(6), 633–641.
Laranjeira FF, Bergamin Filho A, Amorim L, Berger R and Gottwald TR, 2003. Temporal dynamics of citrus variegated chlorosis in three regions of Sao Paulo, Brazil. Fitopatologia Brasileira, 28(5), 481–488.
Laranjeira FF, Filho AB, Amorim L and Berger RD, 1998. Practical aspects of citrus variegated chlorosis epidemiology. Laranja, 19(1), 79–90.
Laranjeira FF, Gottwald TR, Amorim L, Berger RD and Bergamin Filho A, 2000. Spatio‐temporal dynamics of citrus variegated chlorosis: a preliminary analysis. Proceedings of the 14th Conference of the International Organization of Citrus Virologists, Campinas, Sao Paulo State, Brazil, 13–18 September 1998, 223–231.
Laranjeira FF, Mueller GW, Trindade J and Silva LMS, 1996. Observation of citrus variegated chlorosis (CVC) in Sergipe, Brazil. Fitopatologia Brasileira, 21(4), 521–521.
Laranjeira FF, Pompeu Junior J and Palazzo D, 2000. Seeds from sweet orange fruits ‘Natal’ affected by citrus variegated chlorosis: germination, seedling growth and non‐transmission of Xylella fastidiosa. Laranja, 21(1), 161–173.
Laranjeira FF, Pompeu Junior J, Garcia Junior A, Vieira M, Harakava R and Beretta MJG, 1998. Screening for tolerance of Citrus to Xylella fastidiosa, the causal agent of citrus variegated chlorosis CVC. Fruits (Paris), 53(5), 345–349.
Laranjeira FF, Pompeu Junior J, Harakava R, Figueredo JO, Carvalho SA and Coletta‐Filho HD, 1998. Citrus varieties and species host of Xylella fastidiosa under field conditions. Fitopatologia Brasileira, 23(2), 147–154.
Laranjeira FF, Silva LG, Fonseca EL, Silva SXB, Rocha JB, Santos HP, Ledo CAS and Hau B, 2008. Prevalence, incidence and distribution of citrus variegated chlorosis in Bahia, Brazil. Tropical Plant Pathology, 33(5), 339–347. https://doi.org/10.1590/s1982-56762008000500001
Latham AJ and Norton JD, 1980. Incidence of Plum Leaf Scald in Alabama. Alabama Agricultural Experiment Station Bulletin, (525), 3–15.
Latham AJ, Norton JD and Folsom MW, 1980. Leaf scald on plum shoots growing from disease‐free buds. Plant Disease, 64(11), 995–996.
Ledbetter CA and Rogers EE, 2009. Differential Susceptibility of Prunus Germplasm (Subgenus Amygdalus) to a California Isolate of Xylella fastidiosa. Hortscience, 44(7), 1928–1931.
Ledbetter CA, Chen JC, Livingston S and Groves RL, 2009. Winter curing of Prunus dulcis cv ‘Butte,’ P. webbii and their interspecific hybrid in response to Xylella fastidiosa infections. Euphytica, 169(1), 113–122. https://doi.org/10.1007/s10681-009-9954-z
Lee RF, Beretta MJG, Derrick KS and Hooker ME, 1992. Development of a Serological Assay for citrus variegated chlorosis – a New Disease of Citrus in Brazil. Proceedings of the 105th Annual Meeting of the Florida State Horticultural Society, 105, 32–35.
Lee RF, Beretta MJG, Hartung JH, Hooker ME and Derrick KS, 1993. Citrus variegated chlorosis: Confirmation of a Xylella fastidiosa as the causal agent. Summa Phytopathologica, 19(2), 123–125.
Leininger TD, Britton KO and Chang CJ, 2001. Determining the role of bacterial leaf scorch, canker stain, and Botryosphaeria canker in the dieback of plantation sycamores in the southeastern United States. Shade Tree: Wilt Diseases, 209–216.
Leininger TD, Britton KO, Chang CJ and Schiff NM, 2001. Sycamore dieback research in Mississippi and Alabama. Phytopathology, 91(6 Supplement), S54‐S54.
Leininger TD, Schiff NM and Corbin KC, 2004. The glassy‐winged sharpshooter transmits Xylella fastidiosa between sycamore trees. Phytopathology, 94(6), S59–S59.
Leite B and Andersen PC, 2009. Localized accumulation of silicon (Si) in grape leaves affected by Pierce's disease. Microscopy and Microanalysis, 15, 918–919. https://doi.org/10.1017/S1431927609097463
Leite B, Ishida ML, Alves E, Carrer H, Kitajima EW and Pascholati SF, 2002. Evidences on the involvement of sulfur and calcium in the adhesion of Xylella fastidiosa from Citrus. XXXIV Brasilian Phytopathological Congress and XI Latinamerican Phytopathological Congress, Sao Pedro, SP, Brazil, August 5–10, 2001. Fitopatologia, 37(1), 10–66.
Leite Junior RP, Campos Leite RMVBd and Ceresini PC, 1998. Non pathogenicity to peach cultivar Flordasun of Xylella fastidiosa. Pesquisa Agropecuaria Brasileira, 33(10), 1653–1660.
Leite RMVBC, Leite Junior RP and Ceresini PC, 1997. Alternative hosts of Xylella fastidiosa in plum orchards with leaf scald disease. Fitopatologia Brasileira, 22(1), 54–57.
Leite RMVBC, Leite Junior RP and Ceresini PC, 1997. Fluctuation of Xylella fastidiosa population in susceptible and resistant plums to the leaf scald disease. Fitopatologia Brasileira, 22(1), 58–63.
Leite RP, Jr H and Giovanina F U, 1997. Report of citrus variegated chlorosis in the state of Santa Catarina, Brazil. Fitopatologia Brasileira, 22(2), 214–219.
Leonard Nunney, personal communication (2018).
Leu HH, Leu LS and Lin CP, 1998. Development and application of monoclonal antibodies against Xylella fastidiosa, the causal bacterium of pear leaf scorch. Journal of Phytopathology‐Phytopathologische Zeitschrift, 146(1), 31–37. https://doi.org/10.1111/j.1439-0434.1998.tb04747.x
Leu LS and Su CC, 1993. Isolation, Cultivation, and Pathogenicity of Xylella fastidiosa, the Causal Bacterium of Pear Leaf Scorch Disease in Taiwan. Plant Disease, 77(6), 642–646. https://doi.org/10.1094/pd-77-0642
Li W, Teixeira DC, Hartung JS, Huang Q, Duan Y, Zhou L, Chen J, Lin H, Lopes S, Ayres AJ and Levy L, 2013. Development and systematic validation of qPCR assays for rapid and reliable differentiation of Xylella fastidiosa strains causing citrus variegated chlorosis. J Microbiol Methods, 92(1), 79–89. https://doi.org/10.1016/j.mimet.2012.10.008
Li WB, Ayres AJ, He CX and Donadio LC, 2000. Susceptibility of tangerines to citrus variegated chlorosis. Proceedings of the First International Symposium on Citrus Biotechnology, (535), 253–257. https://doi.org/10.17660/actahortic.2000.535.31
Li WB, Pria WD, Lacava PM, Qin X and Hartung JS, 2003. Presence of Xylella fastidiosa in Sweet Orange Fruit and Seeds and Its Transmission to Seedlings. Phytopathology, 93(8), 953–958. https://doi.org/10.1094/PHYTO.2003.93.8.953
Li WB, Pria WD, Teixeira C, Miranda VS, Ayres AJ, Franco CF, Costa MG, He CX, Costa PI and Hartung JS, 2001. Coffee leaf scorch caused by a strain of Xylella fastidiosa from Citrus. Plant Disease, 85(5), 501–505. https://doi.org/10.1094/pdis.2001.85.5.501
Li WB, Zhou CH, Pria WD, Teixeira DC, Miranda VS, Pereira EO, Ayres AJ and Hartung JS, 2002. Citrus and coffee strains of Xylella fastidiosa induce Pierce's disease in grapevine. Plant Disease, 86(11), 1206–1210. https://doi.org/10.1094/pdis.2002.86.11.1206
Li WB, Zreik L, Fernandes NG, Miranda VS, Teixeira DC, Ayres AJ, Garnier M and Bov JM, 1999. A triply cloned strain of Xylella fastidiosa multiplies and induces symptoms of citrus variegated chlorosis in sweet orange. Current Microbiology, 39(2), 106–108.
Li ZT, Hopkins DL and Gray DJ, 2015. Overexpression of antimicrobial lytic peptides protects grapevine from Pierce's disease under greenhouse but not field conditions. Transgenic Research, 24(5), 821–836.
Lieth JH, Meyer MM, Yeo KH and Kirkpatrick BC, 2011. Modeling Cold Curing of Pierce's Disease in Vitis vinifera ‘Pinot Noir’ and ‘Cabernet Sauvignon’ Grapevines in California. Phytopathology, 101(12), 1492–1500. https://doi.org/10.1094/Phyto-08-10-0207
Lima JEO, Miranda VS, Coutinho A, Roberto SR and Carlos EF, 1996. Distribution of Xylella fastidiosa in coffee plants, coffee growing areas, and its in vitro culture. Fitopatologia Brasileira, 21(3), 392–393.
Lima JEOD, Miranda VS, Roberto SR, Coutinho A, Palma RR and Pizzolitto AC, 1997. Diagnosis of citrus variegated chlorosis through light microscopy. Fitopatologia Brasileira, 22(3), 370–374.
Lin H, Doddapaneni H, Takahashi Y and Walker MA, 2007. Comparative analysis of ESTs involved in grape responses to Xylella fastidiosa infection. BMC Plant Biol, 7:8. https://doi.org/10.1186/1471-2229-7-8
Lin H, Islam MS, Cabrera‐La Rosa JC, Civerolo EL and Groves RL, 2015. Population Structure of Xylella fastidiosa Associated with Almond Leaf Scorch Disease in the San Joaquin Valley of California. Phytopathology, 105(6), 825–832. https://doi.org/10.1094/PHYTO-09-14-0254-R
Lin H, Islam MS, Morano L, Groves R, Bextine B, Civerolo E and Walker MA, 2013. Genetic Variation of Xylella fastidiosa Associated with Grapevines in Two Major Viticultural Regions in the United States: California and Texas. Journal of Plant Pathology, 95(2), 329–337.
Lin Y‐S and Chang Y‐L, 2012. The Insect Vectors of Pierce's Disease on Grapevines in Taiwan. Formosan Entomologist, 32(2), 155–167.
Lindow S, Newman K, Chatterjee S, Baccari C, Lavarone AT and Ionescu M, 2014. Production of Xylella fastidiosa diffusible signal factor in transgenic grape causes pathogen confusion and reduction in severity of Pierce's disease. Molecular Plant‐Microbe Interactions, 27(3), 244–254. https://doi.org/10.1094/MPMI-07-13-0197-FI
Livingston S, Chen JC and Civerolo EL, 2010. Seasonal Behavior of Xylella fastidiosa Causing Almond Leafscorch Disease under Field Conditions and Improved Detection of the Bacteria by Means of Array‐PCR. Journal of Phytopathology, 158(1), 40–45. https://doi.org/10.1111/j.1439-0434.2009.01577.x
Loconsole G, Potere O, Boscia D, Altamura G, Djelouah K, Elbeaino T, Frasheri D, Lorusso D, Palmisano F, Pollastro P, Silletti MR, Trisciuzzi N, Valentini F, Savino V and Saponari M, 2014. Detection of Xylella fastidiosa in Olive Trees by Molecular and Serological Methods. Journal of Plant Pathology, 96(1), 7–14.
Loconsole G, Saponari M, Boscia D, D'Attoma G, Morelli M, Martelli GP and Almeida RPP, 2016. Intercepted isolates of Xylella fastidiosa in Europe reveal novel genetic diversity. European Journal of Plant Pathology, 146(1), 85–94. https://doi.org/10.1007/s10658-016-0894-x
Loomis NH, 1961. Symptom expression and occurrence of Pierce's disease virus at Meridian, Miss. Proc Amer Soc Hort Sci, 77, 331–336.
Lopes JRS, Daugherty MP and Almeida RPP, 2009. Context‐dependent transmission of a generalist plant pathogen: host species and pathogen strain mediate insect vector competence. Entomologia Experimentalis Et Applicata, 131(2), 216–224. https://doi.org/10.1111/j.1570-7458.2009.00847.x
Lopes JRS, Daugherty MP and Almeida RPP, 2010. Strain origin drives virulence and persistence of Xylella fastidiosa in alfalfa. Plant Pathology, 59(5), 963–971. https://doi.org/10.1111/j.1365-3059.2010.02325.x
Lopes SA and Torres SC, 2006. An effective and low‐cost culture medium for isolation and growth of Xylella fastidiosa from Citrus and coffee plants. Current Microbiology, 53(6), 467–469. https://doi.org/10.1007/s00284-005-0477-0
Lopes SA, Marcussi S, Torres SCZ, Souza V, Fagan C, Franca SC, Fernandes NG and Lopes JRS, 2003. Weeds as alternative hosts of the Citrus, coffee, and plum strains of Xylella fastidiosa in Brazil. Plant Disease, 87(5), 544–549. https://doi.org/10.1094/pdis.2003.87.5.544
Lopes SA, Ribeiro DM, Roberto PG, Franca SC and Santos JM, 2000. Nicotiana tabacum as an experimental host for the study of plant‐Xylella fastidiosa interactions. Plant Disease, 84(8), 827–830. https://doi.org/10.1094/pdis.2000.84.8.827
Lopes SA, Teixeira DC, Fernandes NG, Ayres AJ, Torres SCZ and Barbosa JC, 2005. An experimental inoculation system to study Citrus‐Xylella fastidiosa interactions. Plant Disease, 89(3), 250–254. https://doi.org/10.1094/Pd-89-0250
Lowe SK, Nyland G and Mircetich SM, 1976. Ultrastructure of Almond Leaf Scorch Bacterium with Special Reference to Topography of Cell‐Wall. Phytopathology, 66(2), 147–151. https://doi.org/10.1094/phyto-66-147
Lowe SK, Nyland G and Mircetich SM, 1977. A simple and rapid staining procedure for the in‐situ detection of almond leaf scorch bacterium. Proceedings of the American Phytopathological Society, (4), 208–208.
Lu J, 2000. The Pierce's disease resistant grapes in the southeast United States. American Journal of Enology and Viticulture, 51(3), 285–286.
Lu J, Ren ZB and Cousins P, 2008. Evaluation of Grape Rootstocks for Resistance to Pierce's Disease and Adaptation to North Florida Environment. Proceedings of the International Symposium on Enhancing Economic and Environmental Sustainability of Fruit Production in a Global Economy, (772), 257‐+.
Lu J, Xu X, Ren Z, Yun H and Liu X, 2003. Interaction between the pathogen and host plants during the Pierce's disease development of grapevines. Hortscience, 38(5), 687–688.
Luvisi A, Aprile A, Sabella E, Vergine M, Nicoli F, Nutricati E, Miceli A, Negro C and De Bellis L, 2017. Xylella fastidiosa subsp pauca (CoDiRO strain) infection in four olive (Olea europaea L.) cultivars: profile of phenolic compounds in leaves and progression of leaf scorch symptoms. Phytopathologia Mediterranea, 56(2), 259–273. https://doi.org/10.14601/Phytopathol_Mediterr-20578
Machado EC, De Oliveira RF, Ribeiro RV, Medina CL, Stuchi ES and Pavani LC, 2007. Water deficiency intensifies physiological symptoms of Citrus variegated clorosis in ‘Natal’ sweet orange plants. Bragantia, 66(3), 373–379.
Machado EC, de Oliveira RF, Ribeiro RV, Medina CL, Stuchi ES, Marin FR, da Silva AB and da Silva SR, 2006. Sap flow and photosynthesis of ‘Natal’ sweet orange plants with citrus variegated chlorosis. Pesquisa Agropecuaria Brasileira, 41(6), 911–918. https://doi.org/10.1590/s0100-204x2006000600003
Machado EC, Quaggio JA, Lagôa AMMA, Ticelli M and Furlani PR, 1994. Gas exchange and water relations of orange trees with citrus variegated chlorosis. Revista Brasileira de Fisiologia Vegetal, 6(1), 53–57.
Machado MA, Targon MLPN, Beretta MJG, Laranjeira FF and Carvalho SAD, 1997. Detection of Xylella fastidiosa in species and varieties of Citrus top‐grafted to ‘Pera’ sweet orange infected with citrus variegated chlorosis. Fitopatologia Brasileira, 22(1), 30–33.
Mang SM, Frisullo S, Elshafie HS and Camele I, 2016. Diversity Evaluation of Xylella fastidiosa from Infected Olive Trees in Apulia (Southern Italy). Plant Pathology Journal, 32(2), 102–111. https://doi.org/10.5423/PPJ.OA.08.2015.0153
Martinati JC, Lacava PT, Miyasawa SKS, Guzzo SD, Azevedo JL and Tsai SM, 2007. Reduction of the symptoms caused by Xylella fastidiosa subsp pauca through application of benzothiadiazole and silicon. Pesquisa Agropecuaria Brasileira, 42(8), 1083–1089. https://doi.org/10.1590/s0100-204x2007000800004
Marucci RC, Giustolin TA, Miranda MP, Ferraz PC and Lopes JRS, 2002. Sharpshooter transmission of a coffee strain of Xylella fastidiosa to coffee seedlings. XXXIV Brasilian Phytopathological Congress and XI Latinamerican Phytopathological Congress, Sao Pedro, SP, Brazil, August 5–10, 2001. Fitopatologia, 37(1), 10–66.
Marucci RC, Giustolin TA, Miranda MPd, Miquelote H, Almeida RPPd and Lopes JRS, 2003. Identification of a non‐host plant of Xylella fastidiosa to rear healthy sharpshooter vectors. Scientia Agricola, 60(4), 669–675.
Marucci RC, Lopes JR and Cavichioli RR, 2008. Transmission efficiency of Xylella fastidiosa by sharpshooters (Hemiptera: Cicadellidae) in coffee and Citrus. J Econ Entomol, 101(4), 1114–1121. https://doi.org/10.1603/0022-0493(2008)101[1114:TEOXFB]2.0.CO;2
Marucci RC, Lopes JRS, Vendramim JD and Corrente JE, 2005. Influence of Xylella fastidiosa infection of Citrus on host selection by leafhopper vectors. Entomologia Experimentalis Et Applicata, 117(2), 95–103. https://doi.org/10.1111/j.1570-7458.2005.00336.x
Matsumoto A, Huston SL, Killiny N and Igo MM, 2012. XatA, an AT‐1 autotransporter important for the virulence of Xylella fastidiosa Temecula1. Microbiologyopen, 1(1), 33–45. https://doi.org/10.1002/mbo3.6
Matsumoto A, Young GM and Igo MM, 2009. Chromosome‐based genetic complementation system for Xylella fastidiosa. Appl Environ Microbiol, 75(6), 1679–1687. https://doi.org/10.1128/AEM.00024-09
McCoy RE, Thomas DL and Williams DS, 1977. Noncultivable xylem inhabiting bacteria associated with periwinkle wilt. Proceedings of the American Phytopathological Society, (4), 179–179.
McCoy RE, Thomas DL, Tsai JH and French WJ, 1978. Periwinkle wilt, a new disease associated with xylem delimited rickettsial‐like bacteria transmitted by a sharpshooter. Plant Disease Reporter, 62(12), 1022–1026.
McElrone AJ and Forseth IN, 2004. Photosynthetic responses of a temperate liana to Xylella fastidiosa infection and water stress. Journal of Phytopathology, 152(1), 9–20. https://doi.org/10.1046/j.1439-0434.2003.00794.x
McElrone AJ, Jackson S and Habdas P, 2008. Hydraulic disruption and passive migration by a bacterial pathogen in oak tree xylem. J Exp Bot, 59(10), 2649–2657. https://doi.org/10.1093/jxb/ern124
McElrone AJ, Sherald JL and Forseth IN, 2001. Effects of water stress on symptomatology and growth of Parthenocissus quinquefolia infected by Xylella fastidiosa. Plant Disease, 85(11), 1160–1164. https://doi.org/10.1094/pdis.2001.85.11.1160
McElrone AJ, Sherald JL and Forseth IN, 2003. Interactive effects of water stress and xylem‐limited bacterial infection on the water relations of a host vine. J Exp Bot, 54(381), 419–430.
McElrone AJ, Sherald JL and Pooler MR, 1999. Identification of alternative hosts of Xylella fastidiosa in the Washington, DC, area using nested polymerase chain reaction (PCR). Journal of Arboriculture, 25(5), 258–263.
McGaha LA, Jackson B, Bextine B, McCullough D and Morano L, 2007. Potential plant reservoirs for Xylella fastidiosa in south Texas. American Journal of Enology and Viticulture, 58(3), 398–401.
McGovern RJ and Hopkins DL, 1994. Association of Xylella fastidiosa with Leaf Scorch and Decline of Live Oak in Florida. Plant Disease, 78(9), 924–924.
Mehta A, Leite RP, Jr. and Rosato YB, 2001. Assessment of the genetic diversity of Xylella fastidiosa isolated from Citrus in Brazil by PCR‐RFLP of the 16S rDNA and 16S‐23S intergenic spacer and rep‐PCR fingerprinting. Antonie Van Leeuwenhoek, 79(1), 53–59.
Melanson RA, Sanderlin RS and Ham J, 2011. Classification of strains of Xylella fastidiosa isolated from pecan in Louisiana as Xylella fastidiosa subspecies multiplex. Phytopathology, 101(6), S267–S267.
Melanson RA, Sanderlin RS, McTaggart AR and Ham JH, 2012. A Systematic Study Reveals that Xylella fastidiosa Strains from Pecan Are Part of X. fastidiosa subsp multiplex. Plant Disease, 96(8), 1123–1134. https://doi.org/10.1094/Pdis-09-11-0730-Re
Meng Y, Li Y, Galvani CD, Hao G, Turner JN, Burr TJ and Hoch HC, 2005. Upstream migration of Xylella fastidiosa via pilus‐driven twitching motility. Journal of Bacteriology, 187(16), 5560–5567. https://doi.org/10.1128/JB.187.16.5560-5567.2005
Meyer M and Kirkpatrick BC, 2009. Application of abscisic acid increases curing of Pierce's disease‐affected potted grapevines. Phytopathology, 99(6), S85–S85.
Meyer M, Kocsis L and Walker A, 2002. Transmission of Pierce's disease by chip‐budding and bench‐grafting. American Journal of Enology and Viticulture, 53(3), 248A‐248A.
Meyer MM and Kirkpatrick BC, 2011. Exogenous Applications of Abscisic Acid Increase Curing of Pierce's Disease‐Affected Grapevines Growing in Pots. Plant Disease, 95(2), 173–177. https://doi.org/10.1094/Pdis-06-10-0446
Milholland RD, Huang PY, Clayton CN and Jones RK, 1981. Pierce's disease on muscadine grapes in North Carolina. Plant Disease, 65(1), 73–74. https://doi.org/10.1094/pd-65-73
Millikan DF and Anderson JR, 1954. The phony peach virus in Missouri. The Plant Disease Reporter, 38(12), 834–835.
Millikan DF, 1955. The phony peach virus. University of Missouri, Bulletin 661.
Minsavage GV, Thompson CM, Hopkins DL, Leite RMVBC and Stall RE, 1994. Development of a Polymerase Chain‐Reaction Protocol for detection of Xylella fastidiosa in Plant Tissue. Phytopathology, 84(5), 456–461. https://doi.org/10.1094/phyto-84-456
Miranda VS, Farias PRS, Roberto SR and Lacava PM, 2007. Genetic characterization of Xylella fastidiosa isolated from Citrus and coffee plants. Scientia Agricola, 64(5), 482–485. https://doi.org/10.1590/s0103-90162007000500005
Mircetich SM, 1976. Almond leaf scorch a newly recognized disease in California. Poljoprivredna Znanstvena Smotra, 39(49), 245–252.
Mircetich SM, Lowe SK, Moller WJ and Nyland G, 1976. Etiology of Almond Leaf Scorch Disease and Transmission of Causal Agent. Phytopathology, 66(1), 17–24. https://doi.org/10.1094/phyto-66-17
Mizubuti ESG, Matsuoka K and Parizzi P, 1994. Association of Xylella‐like bacteria with sweet orange with variegated chlorosis symptoms in the Zona da Mata region of Minas Gerais State, Brazil. Fitopatologia Brasileira, 19(2), 241–244.
Modesti V, Pucci N, Lucchesi S, Campus L and Loreti S, 2016. Experience of the Latium region (Central Italy) as a pest‐free area for monitoring of Xylella fastidiosa: distinctive features of molecular diagnostic methods. European Journal of Plant Pathology, 1–10.
Mollenhauer HH and Hopkins DL, 1974. Ultrastructural study of Pierce's disease bacterium in grape xylem tissue. Journal of Bacteriology, 119(2), 612–618.
Mollenhauer HH and Hopkins DL, 1976. Xylem Morphology of Pierce's Disease‐Infected Grapevines with Different Levels of Tolerance. Physiological Plant Pathology, 9(1), 95–100. https://doi.org/10.1016/0048-4059(76)90079-5
Montague T, Hellman EW, Appel D and Krawitzky M, 2016. Asexual Propagation of Grapevine Transmits Pierce's Disease Pathogen (Xylella fastidiosa) to Rooted Cuttings. International Journal of Fruit Science, 16(2), 135–149. https://doi.org/10.1080/15538362.2015.1061961
Monteiro PB, Renaudin J, Jagoueix‐Eveillard S, Ayres AJ, Garnier M and Bove JM, 2001. Catharanthus roseus, an experimental host plant for the Citrus strain of Xylella fastidiosa. Plant Disease, 85(3), 246–251. https://doi.org/10.1094/pdis.2001.85.3.246
Monteiro PB, Teixeira DC, Palma RR, Garnier M, Bove JM and Renaudin J, 2001. Stable transformation of the Xylella fastidiosa citrus variegated chlorosis strain with oriC plasmids. Applied and Environmental Microbiology, 67(5), 2263–2269. https://doi.org/10.1128/AEM.67.5.2263-2269.2001
Montero‐Astua M, Aguilar E, Chacon C, Garita‐Cambronero J, Garita L, Villalobos W, Moreira L, Li W, Godoy C, Hartung JS and Rivera C, 2006. Characterization of Xylella fastidiosa and epidemiology of the plant diseases caused by the bacterium in Costa Rica. Phytopathology, 96(6), S81–S81.
Montero‐Astua M, Chacon‐Diaz C, Aguilar E, Rodriguez CM, Garita L, Villalobos W, Moreira L, Hartung JS and Rivera C, 2008. Isolation and molecular characterization of Xylella fastidiosa from coffee plants in Costa Rica. J Microbiol, 46(5), 482–490. https://doi.org/10.1007/s12275-008-0072-8
Montero‐Astua M, Hartung JS, Aguilar E, Chacon C and Rivera C, 2006. Molecular comparison of Xylella fastidiosa isolates from Costa Rica, North and South America. Phytopathology, 96(6), S164–S164.
Montero‐Astua M, Hartung JS, Aguilar E, Chacon C, Li W, Albertazzi FJ and Rivera C, 2007. Genetic Diversity of Xylella fastidiosa Strains from Costa Rica, Sao Paulo, Brazil, and United States. Phytopathology, 97(10), 1338–1347. https://doi.org/10.1094/PHYTO-97-10-1338
Montero‐Astua M, Saborio G, Chacon‐Diaz C, Villalobos W, Rodriguez CM, Moreira L and Rivera C, 2008. First report of Xylella fastidiosa in Nerium oleander in Costa Rica. Plant Disease, 92(8), 1249–1249. https://doi.org/10.1094/Pdis-92-8-1249a
Montero‐Astua M, Saborio‐R G, Chacon‐Diaz C, Garita L, Villalobos W, Hartung JS and Rivera C, 2008. First report of Xylella fastidiosa in avocado in Costa Rica. Plant Disease, 92(1), 175–175. https://doi.org/10.1094/Pdis-92-1-0175c
Montes‐Borrego M, Lopes JRS, Jimenez‐Diaz RM and Landa BB, 2015. Combined use of a new SNP‐based assay and multilocus SSR markers to assess genetic diversity of Xylella fastidiosa subsp pauca infecting Citrus and coffee plants. International Microbiology, 18(1), 13–24. https://doi.org/10.2436/20.1501.01.230
Morano LD, Bextine BR, Garcia DA, Maddox SV, Gunawan S, Vitovsky NJ and Black MC, 2008. Initial genetic analysis of Xylella fastidiosa in Texas. Curr Microbiol, 56(4), 346–351. https://doi.org/10.1007/s00284-007-9088-2
Mortensen JA and Gray DJ, 1986. Methods of breeding new seedless grapes with resistance to Pierce's disease. Hortscience, 21(3 SECT. 2), 821–821.
Mortensen JA and Gray DJ, 1986. Orlando Seedless, a bunch grape for Florida. Circular, Agricultural Experiment Stations, University of Florida (S‐335), 4 pp.
Mortensen JA and Knight RJ, Jr., 1968. Susceptibility to Pierce's disease of a plant introduction of Vitis vinifera. Proceedings of the Florida State Horticultural Society, 1967, 80, 348–350.
Mortensen JA and Stover LH, 1982. Tampa – a new bunch grape rootstock. Circular, Agricultural Experiment Stations, University of Florida(S‐295), 4 pp.
Mortensen JA, 1968. The inheritance of resistance to Pierces caused by virus disease in Vitis. Proceedings of the American Society for Horticultural Science, 92, 331–337.
Mortensen JA, 1976. Liberty – a red bunch grape for Florida. Circular, Agricultural Experiment Stations, University of Florida(5–243), 5 pp.
Mortensen JA, 1983. Conquistador. A purple bunch grape for Florida. Circular, Agricultural Experiment Stations, University of Florida(S‐300), 4 pp.
Mortensen JA, 1983. Suwannee. An early bunch grape. Circular, Agricultural Experiment Stations, University of Florida(S‐301), 3 pp.
Mortensen JA, Stover LH and Balerdi CF, 1977. Sources of Resistance to Pierce's Disease in Vitis. Journal of the American Society for Horticultural Science, 102(6), 695–697.
Mortensen. J A, 1968. Stover. An early bunch grape for Central Florida. Circ. Fla. agric. Exp. Stn.(S‐195), 6 pp.
Muranaka LS, Giorgiano TE, Takita MA, Forim MR, Silva LFC, Coletta HD, Machado MA and de Souza AA, 2013. N‐Acetylcysteine in Agriculture, a Novel Use for an Old Molecule: Focus on Controlling the Plant‐Pathogen Xylella fastidiosa. PloS one, 8(8), ARTN e72937
Myers A, Sutton T and Kennedy G, 2005. Pierce's disease of grapevines: Identifying the primary vectors in the Southeastern United States. American Journal of Enology and Viticulture, 56(4), 419–419.
Myers AL, Sutton TB, Abad JA and Kennedy GG, 2007. Pierce's Disease of Grapevines: Identification of the Primary Vectors in North Carolina. Phytopathology, 97(11), 1440–1450. https://doi.org/10.1094/PHYTO-97-11-1440
Nascimento R, Gouran H, Chakraborty S, Gillespie HW, Almeida‐Souza HO, Tu A, Rao BJ, Feldstein PA, Bruening G, Goulart LR and Dandekar AM, 2016. The Type II Secreted Lipase/Esterase LesA is a Key Virulence Factor Required for Xylella fastidiosa Pathogenesis in Grapevines. Scientific Reports, 6.
Navarrete F and De La Fuente L, 2015. Zinc Detoxification Is Required for Full Virulence and Modification of the Host Leaf lonome by Xylella fastidiosa. Molecular Plant‐Microbe Interactions, 28(4), 497–507. https://doi.org/10.1094/Mpmi-07-14-0221-R
Nesbitt WD and Byrd T, 1976. New grape variety released. Research and Farming, 35(1/2), 5–5.
Newman KL, Almeida RPP, Purcell AH and Lindow SE, 2003. Use of a green fluorescent strain for analysis of Xylella fastidiosa colonization of Vitis vinifera. Applied and Environmental Microbiology, 69(12), 7319–7327. https://doi.org/10.1128/Aem.69.12.7319-7327.2003
Newman KL, Almeida RPP, Purcell AH and Lindow SE, 2004. Cell‐cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proceedings of the National Academy of Sciences of the United States of America, 101(6), 1737–1742. https://doi.org/10.1073/pnas.0308399100
Newman KL, Chatterjee S, Ho KA and Lindow SE, 2008. Virulence of plant pathogenic bacteria attenuated by degradation of fatty acid cell‐to‐cell signaling factors. Molecular Plant‐Microbe Interactions, 21(3), 326–334. https://doi.org/10.1094/MPMI-21-3-0326
Nome SF, Docampo D, Goheen AC, Raju BC and Nyland G, 1981. An enzyme‐linked immunosorbent‐assay (ELISA) for detection of Pierces disease bacteria in plant‐tissues. Phytopathology, 71(1), 107–107.
Nome SF, Haelterman RM, Docampo DM, Prataviera AG and Di Feo LDV, 1992. Almond leaf scorch in Argentina. Fitopatologia Brasileira, 17(1), 57–60.
Nome SF, Raju BC, Goheen AC, Nyland G and Docampo D, 1980. Enzyme‐linked immunosorbent‐assay for Pierce's disease bacteria in plant‐tissues. Phytopathology, 70(8), 746–749. https://doi.org/10.1094/phyto-70-746
Northover PR and Dokken‐Bouchard F, 2012. Diseases diagnosed on crop samples submitted in 2011 to the Saskatchewan Ministry of Agriculture Crop Protection Laboratory. Canada Plant Disease Survey, 92(26–30)
Norton JD and Latham AJ, 1979. Tolerance of Plum to Leaf Scald. Hortscience, 14(2), 129–129.
Norton JD and Latham AJ, 1980. Tolerance of Plum to Leaf Scald. Hortscience, 15(3), 395–395.
Norton JD, 1986. Resistance to Leaf Scald in Plum. Hortscience, 21(4), 938–938.
Norton JD, Boyhan GE and Abrahams BR, 1992. Resistance to leaf scald in plum. Hortscience, 27(6), 609–610.
Norton JD, Boyhan GE, Smith DA and Abrahams BR, 1990. New early season plum developed. AU‐Rubrum cultivar combines high yielding ability with disease resistance and excellent fruit quality. Circular – Alabama Agricultural Experiment Station, Auburn University(301), 7 pp.
Nunes WMC, Medina CL, Machado MA, Machado EC, Corazza‐Nunes MJ and Muller GW, 2004. Transmission of Xylella fastidiosa by approach grafting method. Laranja, 25(2), 348–349.
Nunney L, 2011. Homologous recombination and the invasion of a new plant host by the pathogenic bacterium, Xylella fastidiosa. Phytopathology, 101(6), S130–S130.
Nunney L, Hopkins DL, Morano LD, Russell SE and Stouthamer R, 2014. Intersubspecific recombination in Xylella fastidiosa Strains native to the United States: infection of novel hosts associated with an unsuccessful invasion. Applied and Environmental Microbiology, 80(3), 1159–1169. https://doi.org/10.1128/AEM.02920-13
Nunney L, Ortiz B, Russell SA, Ruiz Sanchez R and Stouthamer R, 2014. The complex biogeography of the plant pathogen Xylella fastidiosa: genetic evidence of introductions and Subspecific introgression in Central America. PloS one, 9(11), e112463. https://doi.org/10.1371/journal.pone.0112463
Nunney L, Schuenzel EL, Scally M, Bromley RE and Stouthamer R, 2014. Large‐Scale Intersubspecific Recombination in the Plant‐Pathogenic Bacterium Xylella fastidiosa Is Associated with the Host Shift to Mulberry. Applied and Environmental Microbiology, 80(10), 3025–3033. https://doi.org/10.1128/Aem.04112-13
Nunney L, Vickerman DB, Bromley RE, Russell SA, Hartman JR, Morano LD and Stouthamer R, 2013. Recent evolutionary radiation and host plant specialization in the Xylella fastidiosa subspecies native to the United States. Applied and Environmental Microbiology, 79(7), 2189–2200. https://doi.org/10.1128/AEM.03208-12
Nunney L, Yuan XL, Bromley R, Hartung J, Montero‐Astua M, Moreira L, Ortiz B and Stouthamer R, 2010. Population Genomic Analysis of a Bacterial Plant Pathogen: Novel Insight into the Origin of Pierce's Disease of Grapevine in the US. PloS One, 5(11), ARTN e15488
Nunney L, Yuan XL, Bromley RE and Stouthamer R, 2012. Detecting Genetic Introgression: High Levels of Intersubspecific Recombination Found in Xylella fastidiosa in Brazil. Applied and Environmental Microbiology, 78(13), 4702–4714. https://doi.org/10.1128/Aem.01126-12
Nyland G, Goheen AC, Lowe SK and Kirkpatrick HC, 1973. The ultrastructure of a rickettsia‐like organism from a peach tree affected with phony disease. Phytopathology, 63(10), 1275–1278.
Official document of the French Ministry of Agriculture. Espèces trouvées positive en Corse au 31/12/2017.
Official document of the French Ministry of Agriculture. Xylella fastidiosa en région Provence‐Alpes‐Cote‐d'Azur, situation au 05/06/2018.
O'Keefe K, Del Cid C, Pinedo CA, Puetz W and Springer CJ, 2013. Elevated CO2 Does Not Ameliorate the Negative Consequences of Infection with the Xylem‐Limited Bacteria Xylella fastidiosa in Quercus rubra Seedlings. Castanea, 78(3), 216–226.
Oliveira AC, Vallim MA, Semighini CP, Araujo WL, Goldman GH and Machado MA, 2002. Quantification of Xylella fastidiosa from Citrus Trees by Real‐Time Polymerase Chain Reaction Assay. Phytopathology, 92(10), 1048–1054. https://doi.org/10.1094/PHYTO.2002.92.10.1048
Oliver JE, Arnold TT, Cobine PA and De La Fuente L, 2012. The effects of diverse Xylella fastidiosa isolates on the model host Nicotiana tabacum. Phytopathology, 102(7), 88–88.
Oliver JE, Brannon JM, Cobine PA and De La Fuente L, 2013. Comparing the effects of southeastern US strains of Xylella fastidiosa subsp. fastidiosa and multiplex on blueberry and tobacco. Phytopathology, 103(6), 107–107.
Oliver JE, Cobine PA and De La Fuente L, 2015. Xylella fastidiosa Isolates from both subsp multiplex and fastidiosa Cause Disease on Southern Highbush Blueberry (Vaccinium sp.) Under Greenhouse Conditions. Phytopathology, 105(7), 855–862. https://doi.org/10.1094/Phyto-11-14-0322-Fi
Oliver JE, Sefick SA, Parker JK, Arnold T, Cobine PA and De la Fuente L, 2014. Ionome Changes in Xylella fastidiosa‐Infected Nicotiana tabacum Correlate With Virulence and Discriminate Between Subspecies of Bacterial Isolates. Molecular Plant‐Microbe Interactions, 27(10), 1048–1058. https://doi.org/10.1094/Mpmi-05-14-0151-R
Olmo D, Nieto A, Adrover F, Urbano A, Beidas O, Juan A, Marco‐Noales E, Lopez MM, Navarro I, Monterde A, Montes‐Borrego M, Navas‐Cortes JA and Landa BB, 2017. First Detection of Xylella fastidiosa Infecting Cherry (Prunus avium) and Polygala myrtzfolia Plants, in Mallorca Island, Spain. Plant Disease, 101(10), 1820–1820. https://doi.org/10.1094/Pdi5-04-17-0590-Pdn
Olson BR, Dominiak J, von Broembsen S, Bergs M and Bextine BR, 2006. First report of Xylella fastidiosa in Oklahoma. Plant Disease, 90(1), 108–108. https://doi.org/10.1094/Pd-90-0108b
Osiro D, Colnago LA, Otoboni AM, Lemos EG, de Souza AA, Coletta‐Filho HD and Machado MA, 2004. A kinetic model for Xylella fastidiosa adhesion, biofilm formation, and virulence. FEMS Microbiol Lett, 236(2), 313–318. https://doi.org/10.1016/j.femsle.2004.06.003
Ouyang P, Arif M, Fletcher J, Melcher U and Ochoa Corona FM, 2013. Enhanced reliability and accuracy for field deployable bioforensic detection and discrimination of Xylella fastidiosa subsp. pauca, causal agent of citrus variegated chlorosis using razor ex technology and TaqMan quantitative PCR. PloS one, 8(11), e81647. https://doi.org/10.1371/journal.pone.0081647
Overall LM and Rebek EJ, 2015. Seasonal Abundance and Natural Inoculativity of Insect Vectors of Xylella fastidiosa in Oklahoma Tree Nurseries and Vineyards. J Econ Entomol, 108(6), 2536–2545. https://doi.org/10.1093/jee/tov261
Paradela Filho Osvald MHSVJAR, Garcia Junior A, Beretta MJG, Harakawa R, Machado MA, Laranjeira FF, Rodrigues Neto J and Beriam LOS, 1997. Occurrence of Xylella fastidiosa in coffee plants in Brazil. Summa Phytopathologica, 23(1), 46–49.
Parent JG, Desjardins S and Brisson JD, 1986. Detection of Xylem‐Associated Rickettsia‐Like Bacteria in Goldenrod in Quebec. Canadian Plant Disease Survey, 66(2), 55–57.
Park YL, Perring TM, Krell RK, Farrar CA and Gispert C, 2006. Spatial distribution of Pierce's disease in the Coachella Valley: Implications for sampling. American Journal of Enology and Viticulture, 57(2), 220–225.
Park YL, Perring TM, Krell RK, Hashim‐Buckey JM and Hill BL, 2011. Spatial Distribution of Pierce's Disease Related to Incidence, Vineyard Characteristics, and Surrounding Land Uses. American Journal of Enology and Viticulture, 62(2), 229–238. https://doi.org/10.5344/ajev.2011.10064
Parker LD, Bordallo PN and Colova VM, 2009. Phylogenetics Analysis of North American Native ‘Cynthiana’/’Norton’ Grape Cultivar Using DNA Microsatellite Markers. Ix International Conference on Grape Genetics and Breeding, 827, 225–228.
Pavan A, Calixto MC, CArdoso SC, Mendes BMJ, Bergamin A, Lopes JRS, de Carvalho CR and Mourao FDA, 2007. Evaluation of ‘Hamlin’ sweet orange plus ‘Montenegrina’ mandarin somatic hybrid for tolerance to Xanthomonas axonopodis pv. citri and Xylella fastidiosa. Scientia Horticulturae, 113(3), 278–285. https://doi.org/10.1016/j.scienta.2007.03.022
Perez‐Donoso AG, Greve LC, Walton JH, Shackel KA and Labavitch JM, 2007. Xylella fastidiosa infection and ethylene exposure result in xylem and water movement disruption in grapevine shoots. Plant Physiol, 143(2), 1024–1036. https://doi.org/10.1104/pp.106.087023
Perez‐Donoso AG, Lenhof JJ, Pinney K and Labavitch JM, 2016. Vessel embolism and tyloses in early stages of Pierce's disease. Australian Journal of Grape and Wine Research, 22(1), 81–86. https://doi.org/10.1111/ajgw.12178
Peroni LA, Reis JR, Coletta‐Filho HD, de Souza AA, Machado MA and Stach‐Machado DR, 2008. Assessment of the diagnostic potential of Immmunocapture‐PCR and Immuno‐PCR for citrus variegated chlorosis. J Microbiol Methods, 75(2), 302–307. https://doi.org/10.1016/j.mimet.2008.06.024
Perring TM, Farrar CA and Blua MJ, 2001. Proximity to Citrus influences Pierce's disease in Temecula Valley vineyards. California Agriculture, 55(4), 13–18.
Perry RL, Mollenhauer HH and Bowen HH, 1974. Electron photo microscopy verification of Pierces disease on grape plants from Texas. Plant Disease Reporter, 58(9), 780–782.
Pierce BK and Kirkpatrick BC, 2015. The PhoP/Q two‐component regulatory system is essential for Xylella fastidiosa survival in Vitis vinifera grapevines. Physiological and Molecular Plant Pathology, 89, 55–61. https://doi.org/10.1016/j.pmpp.2014.12.003
Pierce BK, Voegel T and Kirkpatrick BC, 2014. The Xylella fastidiosa PD1063 Protein Is Secreted in Association with Outer Membrane Vesicles. PloS one, 9(11), ARTN e113504
Plant Disease Survey, 1930. Phony disease of peach. US Dept Agric Bur Plant Indust Plant Dis Reptr, 14(15), 148–149.
Poltronieri LS, Cunha Junior JO, Trindade DR, Cardoso SS and Brioso PST, 2005. Molecular Detection of Xylella fastidiosa in Citrus in the state of Para, Brazil. Fitopatologia Brasileira, 30(2), 199–199.
Pompeu J, Jr L, F. F H, R F, J. O. de C and S. A F, 1998. Citrus varieties and species hosts of Xylella fastidiosa at field level. Laranja, 19(2), 321–330.
Pooler MR and Hartung JS, 1995. Genetic‐Relationships among Strains of Xylella fastidiosa from Rapd‐PCR Data. Current Microbiology, 31(2), 134–137. https://doi.org/10.1007/bf00294290
Pooler MR and Hartung JS, 1995. Specific PCR detection and identification of Xylella fastidiosa strains causing citrus variegated chlorosis. Current Microbiology, 31(6), 377–381.
Potere O, Susca L, Loconsole G, Saponari M, Boscia D, Savino V and Martelli GP, 2015. Survey for the presence of Xylella fastidiosa subsp. pauca (strain codiro) in some forestry and ornamental species in the Salento peninsula. Journal of Plant Pathology, 97(2), 373–376.
Prado SD, Lopes JRS, Demetrio CGB, Borgatto AF and Almeida RPP, 2008. Host colonization differences between Citrus and coffee isolates of Xylella fastidiosa in reciprocal inoculation. Scientia Agricola, 65(3), 251–258. https://doi.org/10.1590/s0103-90162008000300005
Pria Junior WD, Lacava PM, Li W, Costa PId and Hartung JS, 2003. Detection of Xylella fastidiosa in seeds and its translocation to sweet orange seedlings. Laranja, 24(2), 397–412.
Purcell AH and Saunders S, 1995. Harvested Grape Clusters as Inoculum for Pierce's Disease. Plant Disease, 79(2), 190–192. https://doi.org/10.1094/pd-79-0190
Purcell AH and Saunders SR, 1999. Fate of Pierce's disease strains of Xylella fastidiosa in common riparian plants in California. Plant Disease, 83(9), 825–830. https://doi.org/10.1094/pdis.1999.83.9.825
Purcell AH and Saunders SR, 1999. Glassy‐winged sharpshooters expected to increase plant disease. California Agriculture, 53(2), 26–27.
Purcell AH, 1974. Spatial patterns of Pierces disease in the Napa Valley. American Journal of Enology and Viticulture, 25(3), 162–167.
Purcell AH, 1975. Role of Blue‐Green Sharpshooter, Hordnia circellata in Epidemiology of Pierce's Disease of Grapevines. Environmental Entomology, 4(5), 745–752. https://doi.org/10.1093/ee/4.5.745
Purcell AH, 1979. Control of the Blue‐Green Sharpshooter (Homoptera, Cicadellidae) and Effects on the Spread of Pierce's Disease of Grapevines. Journal of Economic Entomology, 72(6), 887–892. https://doi.org/10.1093/jee/72.6.887
Purcell AH, 1981. Vector Preference and Inoculation Efficiency as Components of Resistance to Pierce's Disease in European Grape Cultivars. Phytopathology, 71(4), 429–435. https://doi.org/10.1094/phyto-71-429
Purcell AH, Saunders SR, Hendson M, Grebus ME and Henry MJ, 1999. Causal Role of Xylella fastidiosa in Oleander Leaf Scorch Disease. Phytopathology, 89(1), 53–58. https://doi.org/10.1094/PHYTO.1999.89.1.53
Purcino RP, Medina CL, Martins de Souza D, Winck FV, Machado EC, Novello JC, Machado MA and Mazzafera P, 2007. Xylella fastidiosa disturbs nitrogen metabolism and causes a stress response in sweet orange Citrus sinensis cv. Pera. Journal of Experimental Botany, 58(11), 2733–2744. https://doi.org/10.1093/jxb/erm138
Qi HA, 2007. Natural occurrence of Xylella fastidiosa in a commercial nursery in Maryland. Canadian Journal of Plant Pathology, 29(3), 299–303.
Qin X, Miranda VS, Machado MA, Lemos EG and Hartung JS, 2001. An Evaluation of the Genetic Diversity of Xylella fastidiosa Isolated from Diseased Citrus and Coffee in Sao Paulo, Brazil. Phytopathology, 91(6), 599–605. https://doi.org/10.1094/PHYTO.2001.91.6.599
Queiroz‐Voltan RB and Paradela Filho O, 1999. Characterization of anatomical structure in Citrus plants infected with Xylella fastidiosa. Laranja, 20(1), 55–76.
Queiroz‐Voltan RB, Cabral LP and Paradela Filho O, 2004. Seasonal comparactions of Xylella fastidiosa effect in coffee cultivars. Bragantia, 63(3), 381–393.
Queiroz‐Voltan RB, Cabral LP and Paradela Filho O, 2004. Severity symptoms of Xylella fastidiosa on coffee cultivars. Bragantia, 63(3), 395–404.
Queiroz‐Voltan RB, Cabral LP, Fazuoli LC and Paradela Filho O, 2005. Susceptibility valuation to Xylella fastidiosa in different coffee species. Bragantia, 64(4), 615–624.
Queiroz‐Voltan RB, Cabral LP, Paradela Filho O, Carvalho Carelli ML, Fahl JI and Fazuoli LC, 2005. Effect of Xylella fastidiosa in coffee plants at different edaphoclimatic regions. Bragantia, 64(1), 89–100.
Queiroz‐Voltan RB, Cabral LP, Paradela O and Fazuoli LC, 2007. “Decote” type pruning effect upon Xylella fastidiosa control in coffee cultivars. Bragantia, 66(1), 69–80.
Queiroz‐Voltan RB, Paradela Filho O, Carelli MLC and Fahl JI, 1998. Structural aspects in infected coffee plant with Xylella fastidiosa. Bragantia, 57(1), 23–33.
Raju BC and Goheen AC, 1981. Relative Sensitivity of Selected Grapevine Cultivars to Pierce's Disease Bacterial Inoculations. American Journal of Enology and Viticulture, 32(2), 155–158.
Raju BC, Goheen AC and Frazier NW, 1983. Occurrence of Pierce's Disease Bacteria in Plants and Vectors in California. Phytopathology, 73(9), 1309–1313. https://doi.org/10.1094/phyto-73-1309
Raju BC, Goheen AC, Teliz D and Nyland G, 1979. Occurrence of Pierce's Disease of Grapevines in Mexico. Phytopathology, 69(8), 919–919.
Raju BC, Goheen AC, Teliz D and Nyland G, 1980. Pierce's disease of grapevines in Mexico. Plant Disease, 64(3), 280–282.
Raju BC, Nome SF, Docampo DM, Goheen AC, Nyland G and Lowe SK, 1980. Alternative Hosts of Pierce's Disease of Grapevines That Occur Adjacent to Grape Growing Areas in California. American Journal of Enology and Viticulture, 31(2), 144–148.
Raju BC, Nyland G, Goheen AC, Nome SF, Wells JW, Weaver DJ and Lee RF, 1981. Serological Relationships of Rickettsia‐Like Bacteria in Diseased Plants. Phytopathology, 71(1), 108–108.
Raju BC, Wells JM, Mircetich SM and Nyland G, 1984. Pathogenic Relationships between Pierce's Disease and Phony Peach Bacteria. Phytopathology, 74(7), 857–857.
Raju BC, Wells JM, Nyland G, Brlansky RH and Lowe SK, 1982. Plum Leaf Scald – Isolation, Culture, and Pathogenicity of the Causal Agent. Phytopathology, 72(11), 1460–1466. https://doi.org/10.1094/phyto-77-1460
Ramming DW, Walker MA, Tenscher A and Krivanek AF, 2009. Breeding Table and Raisin Grapes with Increased Fruit Quality while Retaining Pierce's Disease Resistance. Ix International Conference on Grape Genetics and Breeding, 827, 445–450.
Randall JJ, French J, Yao S, Hanson SF and Goldberg NP, 2011. First Report of Xylella fastidiosa in Peach in New Mexico. Plant Disease, 95(7), 871–872. https://doi.org/10.1094/Pdis-10-10-0719
Randall JJ, Goldberg NP, Kemp JD, Radionenko M, French JM, Olsen MW and Hanson SF, 2009. Genetic analysis of a novel Xylella fastidiosa subspecies found in the southwestern United States. Applied and Environmental Microbiology, 75(17), 5631–5638.
Randall JJ, Radionenko M, French JM, Goldberg NP and Hanson SF, 2007. First report of Pierce's disease in New Mexico. Plant Health Progress (October), 1002–1001.
Randall JJ, Radionenko M, French JM, Goldberg NP and Hanson SF, 2007. Pierce's disease detected in New Mexico grapevines. Phytopathology, 97(7), S96‐S96.
Randall JJ, Radionenko M, French JM, Olsen MW, Goldberg NP and Hanson SF, 2007. Distribution and genetic analysis of Xylella fastidiosa strains found in chitalpa in the southwestern United States. Phytopathology, 97(7), S96‐S96.
Randall JJ, Radionenko M, French JM, Olsen MW, Goldberg NP and Hanson SF, 2007. Xylella fastidiosa detected in New Mexico in chitalpa, a common landscape ornamental plant. Plant Disease, 91(3), 329–329. https://doi.org/10.1094/Pdis-91-3-0329b
Rashed A, Daugherty MP and Almeida RPP, 2011. Grapevine Genotype Susceptibility to Xylella fastidiosa does not Predict Vector Transmission Success. Environmental Entomology, 40(5), 1192–1199.
Rashed A, Kwan J, Baraff B, Ling D, Daugherty MP, Killiny N and Almeida RPP, 2013. Relative Susceptibility of Vitis vinifera Cultivars to Vector‐Borne Xylella fastidiosa through Time. PloS One, 8(2), ARTN e55326
Rathe AA, Pilkington LJ, Gurr GM and Daugherty MP, 2012. Potential for persistence and within‐plant movement of Xylella fastidiosa in Australian native plants. Australasian Plant Pathology, 41(4), 405–412. https://doi.org/10.1007/s13313-011-0116-0
Reddy JD, Reddy SL, Hopkins DL and Gabriel DW, 2007. ToIC is required for pathogenicity of Xylella fastidiosa in Vitis vinifera grapevines. Molecular Plant‐Microbe Interactions, 20(4), 403–410. https://doi.org/10.1094/Mpmi-20-4-0403
Reis S, Tenscher A, Krivanek A, Ramming D and Walker A, 2002. Use of bulked segregant analysis to identify an AFLP marker linked to Xylella fastidiosa resistance. American Journal of Enology and Viticulture, 53(3), 255A‐255A.
Riaz S, Tenscher AC, Rubin J, Graziani R, Pao SS and Walker MA, 2008. Fine‐scale genetic mapping of two Pierce's disease resistance loci and a major segregation distortion region on chromosome 14 of grape. Theoretical and Applied Genetics, 117(5), 671–681. https://doi.org/10.1007/s00122-008-0802-7
Ribeiro RV, Machado EC and Oliveira RF, 2003. Early photosynthetic responses of sweet orange plants infected with Xylella fastidiosa. Physiological and Molecular Plant Pathology, 62(3), 167–173. https://doi.org/10.1016/S0885-5765(03)00038-9
Ribeiro RV, Machado EC and Oliveira RF, 2004. Growth‐ and leaf‐temperature effects on photosynthesis of sweet orange seedlings infected with Xylella fastidiosa. Plant Pathology, 53(3), 334–340. https://doi.org/10.1046/j.1365-3059.2004.01012.x
Ricci AP, Mourao Filho FdAA, Araujo PSRd, Beretta MJG and Derrick K, 2001. Nutrient content of ‘Pera’ sweet orange trees affected by citrus variegated chlorosis. Laranja, 22(2), 517–531.
Robacker CD and Chang CJ, 1992. Shoot‐Tip Culture of Muscadine Grape to Eliminate Pierce's Disease Bacteria. Hortscience, 27(5), 449–450.
Roberto SR, Coutinho A, de Lima JEO, Miranda VS and Carlos EF, 1996. Transmission of Xylella fastidiosa by the sharpshooters Dilobopterus costalimai, Acrogonia terminalis and Oncometopia facialis in Citrus. Fitopatologia Brasileira, 21(4), 517–518.
Roberto SR, Farias PRS and Bergamin Filho A, 2002. Geostatiscal analysis of spatial dynamics of citrus variegated chlorosis. Fitopatologia Brasileira, 27(6), 599–604.
Roberto SR, Sanches AL and Caetano AC, 2000. Evaluation of seeds of CVC‐affected ‘Valencia’ sweet orange (Citrus sinensis L. Osb.) fruits. Revista Brasileira de Fruticultura, 22(3), 478–480.
Rocha JG, Zambolim L, Maciel‐Zambolim E and Ribeiro do Vale FX, 2010. Temporal and spatial dynamics of coffee leaf scorch caused by Xylella fastidiosa. Australasian Plant Pathology, 39(3), 234–240.
Rocha JG, Zambolim L, Zambolim EM, do Vale FXR, Junior WCJ and Bergamin A, 2010. Quantification of yield loss due to coffee leaf scorch. Crop Protection, 29(10), 1100–1104. https://doi.org/10.1016/j.cropro.2010.04.011
Rodrigues CM, de Souza AA, Takita MA, Kishi LT and Machado MA, 2013. RNA‐Seq analysis of Citrus reticulata in the early stages of Xylella fastidiosa infection reveals auxin‐related genes as a defense response. BMC Genomics, 14, Artn 676
Rodrigues JLM, Silva‐Stenico ME, Gomes JE, Lopes JRS and Tsai SM, 2003. Detection and diversity assessment of Xylella fastidiosa in field‐collected plant and insect samples by using 16S rRNA and gyrB sequences. Applied and Environmental Microbiology, 69(7), 4249–4255. https://doi.org/10.1128/Aem.69.7.4249.4255.2003
Rodriguez CM, Obando JJ, Villalobos W, Moreira L and Rivera C, 2001. First report of Xylella fastidiosa infecting coffee in Costa Rica. Plant Disease, 85(9), 1027–1027.
Rodriguez Solis CM, Sanchez Saborio F and Godoy C, 2003. Isolation of Xylella fastidiosa from coffee plants with symptoms of curling. Boletin PROMECAFE (97), 11–13.
Rogers E, 2011. Evaluation of Arabidopsis thaliana as a model host for Xylella fastidiosa. Phytopathology, 101(6), S155–S155.
Rogers EE and Ledbetter CA, 2015. Susceptibility to Xylella fastidiosa in a First‐generation Hybrid from a non‐traditional Peach‐Almond Cross. Hortscience, 50(3), 337–340.
Rogers EE, 2010. Arabidopsis thaliana ecotypes with differential susceptibility to the bacterial pathogen Xylella fastidiosa. Phytopathology, 100(6), S110–S110.
Rogers EE, 2012. Evaluation of Arabidopsis thaliana as a Model Host for Xylella fastidiosa. Molecular Plant‐Microbe Interactions, 25(6), 747–754. https://doi.org/10.1094/Mpmi-11-10-0270
Roper MC, Greve LC, Labavitch JM and Kirkpatrick BC, 2007. Detection and visualization of an exopolysaccharide produced by Xylella fastidiosa in vitro and in planta. Applied and Environmental Microbiology, 73(22), 7252–7258. https://doi.org/10.1128/AEM.00895-07
Roper MC, Greve LC, Warren JG, Labavitch JM and Kirkpatrick BC, 2007. Xylella fastidiosa requires polygalacturonase for colonization and pathogenicity in Vitis vinifera grapevines. Molecular Plant‐Microbe Interactions, 20(4), 411–419. https://doi.org/10.1094/MPMI-20-4-0411
Rosato YB, Neto JR, Miranda VS, Carlos EF and Manfio GP, 1998. Diversity of a Xylella fastidiosa population isolated from Citrus sinensis affected by citrus variegated chlorosis in Brazil. Systematic and Applied Microbiology, 21(4), 593–598. https://doi.org/10.1016/s0723-2020(98)80072-6
Rossetti V, 1991. Citrus Variegated Chlorosis – a New Severe Disease in Brazil. Proceedings of the International Citrus Symposium, 628–632.
Rossetti V, Garnier M, Bove JM, Beretta MJG, Teixeira ARR, Quaggio JA and Denegri JD, 1990. Occurrence of Xylem‐Restricted Bacteria in Sweet Orange Trees Affected by Chlorotic Variegation, a New Citrus Disease in Brazil. Comptes Rendus De L Academie Des Sciences Serie Iii‐Sciences De La Vie‐Life Sciences, 310(8), 345–349.
Rossetto EA and Duarte V, 2001. Epidemiological aspects of citrus variegated chlorosis at Rio Grande do Sul State, Brazil. Phytopathology, 91(6 Supplement), S77–S77.
Ruel J and Walker A, 2004. Resistance to pierce's disease in Muscadinia rotundifolia and other native grape species. American Journal of Enology and Viticulture, 55(3), 301A‐301A.
Ruel JJ and Walker MA, 2006. Resistance to Pierce's disease in Muscadinia rotundifolia and other native grape species. American Journal of Enology and Viticulture, 57(2), 158–165.
Sadovskii YP, 1985. Rickettsia‐like bacteria transformation of cytoplasmic cell membrane of peach trees affected by grafted node necrosis. Mikrobiologicheskii Zhurnal (Kiev), 47(4), 44–53.
Sadovskii YP and Shevchenko SI, 1991. Electron microscopic studies of inclusions in plant tissues infected with rickettsia‐like bacteria. Tsitologiya i Genetika, 25(3), 7–12.
Sanchez A, Black M and Kamas J, 2013. Pierce's disease in three susceptible grape cultivars grafted on hybrid rootstocks or own‐rooted. Phytopathology, 103(6), 126–126.
Sanderlin RS and Heyderich‐Alger KI, 2000. Evidence that Xylella fastidiosa can cause leaf scorch disease of pecan. Plant Disease, 84(12), 1282–1286. https://doi.org/10.1094/pdis.2000.84.12.1282
Sanderlin RS and Heyderich‐Alger KI, 2003. Effects of pecan bacterial leaf scorch on growth and yield components of cultivar Cape Fear. Plant Disease, 87(3), 259–262. https://doi.org/10.1094/pdis.2003.87.3.259
Sanderlin RS and Melanson RA, 2006. Transmission of Xylella fastidiosa through pecan rootstock. Hortscience, 41(6), 1455–1456.
Sanderlin RS and Melanson RA, 2008. Reduction of Xylella fastidiosa transmission through pecan scion wood by hot‐water treatment. Plant Disease, 92(7), 1124–1126. https://doi.org/10.1094/Pdis-92-7-1124
Sanderlin RS, 1998. Evidence that Xylella fastidiosa is associated with pecan fungal leaf scorch. Plant Disease, 82(2), 264–264. https://doi.org/10.1094/pdis.1998.82.2.264a
Sanderlin RS, 2005. Cultivar and seedling susceptibility to pecan bacterial leaf scorch caused by Xylella fastidiosa and graft transmission of the pathogen. Plant Disease, 89(5), 446–449. https://doi.org/10.1094/Pd-89-0446
Sanderlin RS, 2015. Susceptibility of Some Common Pecan Rootstocks to Infection by Xylella fastidiosa. Hortscience, 50(8), 1183–1186.
Sanderlin RS, 2017. Host Specificity of Pecan Strains of Xylella fastidiosa subsp multiplex. Plant Disease, 101(5), 744–750. https://doi.org/10.1094/Pdis-07-16-1005-Re
Sanderlin RS, Li B, Melanson RA and Gil S, 2009. Spread of Xylella fastidiosa in a pecan orchard and presence of potential vectors in orchards. Phytopathology, 99(6), S114–S114.
Santos Filho HP, Barbosa CJ, Matrangolo WJR, Ribeiro JS, Meissner Filho PE and Miranda MP, 1999. Identification of Citrus variegation chlorosis in the state of Bahia, Brazil. Fitopatologia Brasileira, 24(2), 190–190.
Saponari M, 2015. Host of Xylella fastidiosa strain CoDiRO_Apulia_Dec_2015.
Saponari M, Boscia D, Altamura G, D'Attoma G, Cavalieri V, Loconsole G, Zicca S, Dongiovanni C, Palmisano F, Susca L, Morelli M, Potere O, Saponari A, Fumarola G, Di Carolo M, Tavano D, Savino V and Martelli GP, 2016. Pilot project on Xylella fastidiosa to reduce risk assessment uncertainties. EFSA Supporting Publication 2016:13(3):1013, 60 pp https://doi.org/10.2903/sp.efsa.2016.EN-1013
Saponari M, Boscia D, Nigro F and Martelli GP, 2013. Identification of DNA Sequences Related to Xylella fastidiosa in Oleander, Almond and Olive Trees Exhibiting Leaf Scorch Symptoms in Apulia (Southern Italy). Journal of Plant Pathology, 95(3), 668–668.
Saponari M, Boscia D, Loconsole G, Palmisano F, Savino V, Potere O and Martelli GP, 2014. New hosts of Xylella fastidiosa strain CoDiRO in Apulia. Journal of Plant Pathology, 96(3), 611–611.
Saponari M, Boscia D, Altamura G, Loconsole G, Zicca S, D'Attoma G, et al. Isolation and pathogenicity of Xylella fastidiosa associated to the olive quick decline syndrome in southern Italy. Scientific Reports. 2017;7:17723.
Saponari M, Loconsole G, Cornara D, Yokomi RK, De Stradis A, Boscia D, Bosco D, Martelli GP, Krugner R and Porcelli F, 2014. Infectivity and Transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. Journal of Economic Entomology, 107(4), 1316–1319. https://doi.org/10.1603/Ec14142
Saunders MS and French WJ, 1983. Enzyme‐Linked Immunosorbent Assays for Detection of Xylem‐Limited Bacteria – Use of Trinder Reagent. Applied and Environmental Microbiology, 46(2), 344–347.
Schaad NW, Opgenorth D and Gaush P, 2002. Real‐Time Polymerase Chain Reaction for One‐Hour On‐Site Diagnosis of Pierce's Disease of Grape in Early Season Asymptomatic Vines. Phytopathology, 92(7), 721–728. https://doi.org/10.1094/PHYTO.2002.92.7.721
Schreiber HL, Koirala M, Lara A, Ojeda M, Dowd SE, Bextine B and Morano L, 2010. Unraveling the First Xylella fastidiosa subsp fastidiosa genome from Texas. Southwestern Entomologist, 35(3), 479–483. https://doi.org/10.3958/059.035.0336
Schuenzel EL, Scally M, Stouthamer R and Nunney L, 2005. A multigene phylogenetic study of clonal diversity and divergence in North American strains of the plant pathogen Xylella fastidiosa. Appl Environ Microbiol, 71(7), 3832–3839. https://doi.org/10.1128/AEM.71.7.3832-3839.2005
Severin HHP, 1949. Transmission of the virus of Pierce's disease of grapevines by leafhoppers. Hilgardia, 19(6), 190–190.
Severin HHP, 1950. Spittle‐insect vectors of Pierce's disease virus. II. Life history and virus transmission. Hilgardia, 19(11), 357–382.
Shapland EB, Daane KM, Yokota GY, Wistrom C, Connell JH, Duncan RA and Viveros MA, 2006. Ground vegetation survey for Xylella fastidiosa in California almond orchards. Plant Disease, 90(7), 905–909. https://doi.org/10.1094/Pd-90-0905
Sherald J, Hearon S, Kostka S and Morgan D, 1982. Pathogenicity of a Pierce's Disease (Pd)‐Like Bacterium Cultured from Leaf Scorch Affected Sycamores. Phytopathology, 72(6), 710–710.
Sherald JL and Lei J, 1989. Survey of the national mall Washington DC USA for elm leaf scorch associated with Xylella fastidiosa. Phytopathology, 79(10), 1165–1165.
Sherald JL and Lei JD, 1991. Evaluation of a Rapid ELISA Test Kit for Detection of Xylella fastidiosa in Landscape Trees. Plant Disease, 75(2), 200–203. https://doi.org/10.1094/pd-75-0200
Sherald JL, 1990. Pathogenicity of Xylella fastidiosa to American elm. Phytopathology, 80(10), 1066–1066.
Sherald JL, 1993. Pathogenicity of Xylella Fastidiosa in American Elm and Failure of Reciprocal Transmission between Strains from Elm and Sycamore. Plant Disease, 77(2), 190–193. https://doi.org/10.1094/pd-77-0190
Sherald JL, Hearon SS, Kostka SJ and Morgan DL, 1983. Sycamore Leaf Scorch – Culture and Pathogenicity of Fastidious Xylem‐Limited Bacteria from Scorch‐Affected Trees. Plant Disease, 67(8), 849–852. https://doi.org/10.1094/pd-67-849
Sherald JL, Kostka SJ and Hurtt SS, 1985. Pathogenicity of Fastidious, Xylem‐Inhabiting Bacteria (Fxib) on American Sycamore. Phytopathology, 75(11), 1294–1294.
Sherald JL, Patton EN, Stidham TM and Favre CL, 1994. Incidence and development of bacterial leaf scorch of elm on the National Mall. Journal of Arboriculture, 20(1), 18–23.
Sherald JL, Wells JM and Hurtt SS, 1987. Association of Fastidious, Xylem‐Inhabiting Bacteria with Leaf Scorch in Red Maple. Plant Disease, 71(10), 930–933. https://doi.org/10.1094/pd-71-0930
Sherman WB and Rouse RE, 2001. Characteristics of plums from the University of Florida breeding program. Proceedings of the Florida State Horticultural Society, 114, 30–32.
Shi X and Lin H, 2015. Characterization of Xylella fastidiosa popP gene required for pathogenicity. Journal of Plant Pathology and Microbiology, 6(8), 295–295.
Shi X, Liang Z, Bi J, Morse JG and Cooksey DA, 2010. The virulence mechanisms of Xylella fastidiosa in xylem fluid from resistant and susceptible grapevines. Phytopathology, 100(6), S118–S118.
Shi X, Tian L and Lin H, 2013. Characterization of Xylella fastidiosa popP gene required for pathogenicity. Phytopathology, 103(6), 132–132.
Shi XY, Dumenyo CK, Hernandez‐Martinez R, Azad H and Cooksey DA, 2007. Characterization of regulatory pathways in Xylella fastidiosa: genes and phenotypes controlled by algU. Appl Environ Microbiol, 73(21), 6748–6756. https://doi.org/10.1128/AEM.01232-07
Shi XY, Dumenyo CK, Hernandez‐Martinez R, Azad H and Cooksey DA, 2009. Characterization of regulatory pathways in Xylella fastidiosa: genes and phenotypes controlled by gacA. Appl Environ Microbiol, 75(8), 2275–2283. https://doi.org/10.1128/AEM.01964-08
Silva GFD, Lim H, Choi H, Iandolino A, Baek J, Leslie A, Xu J, Cook D and Iandolino A, 2004. Characterizing the grape transcriptome: identification of transcripts correlated with berry development and host responses to Pierce's Disease of grapes. Plant Biology (Rockville), 2004, 136–136.
Silva Monteiro de B, da JC, Peres M and L A, 1997. A dry rot of Citrus fruit. Comunicado Tecnico – Empresa de Pesquisa Agropecuaria do Estado do Rio de Janeiro(232), 2 pp.
Silva SRd, Oliveira JCd, Stuchi ES, Donadio LC, Souza PSD and González‐Jaimes EP, 2004. Avaliação de tangerinas, tangores e tangelos em relação à clorose variegada dos citros. Revista Brasileira de Fruticultura, 26(1), 57–60.
Silva‐Stenico ME, Pacheco FT, Pereira‐Filho ER, Rodrigues JL, Souza AN, Etchegaray A, Gomes JE and Tsai SM, 2009. Nutritional deficiency in Citrus with symptoms of citrus variegated chlorosis disease. Brazilian Journal of Biology, 69(3), 859–864.
Simonetti LM, Cristofani‐Yaly M, Schinor EH and Machado MA, 2011. Response of Citrus hybrids to citrus variegated chlorosis. Citrus Research and Technology, 32(2), 77–83.
Singh R, Ferrin DM and Huang Q, 2010. First Report of Xylella fastidiosa Associated with Oleander Leaf Scorch in Louisiana. Plant Disease, 94(2), 274–274. https://doi.org/10.1094/Pdis-94-2-0274b
Sisterson M, Chen J, Daane K, Groves R, Higbee B and Ledbetter C, 2010. Epidemiology of almond leaf scorch disease in the San Joaquin Valley of California. Phytopathology, 100(6), S119–S119.
Sisterson M, Daane K, Thammiraju S and Groves R, 2008. Assessment of the role of alfalfa in the spread of Xylella fastidiosa in California. Phytopathology, 98(6), S147–S147.
Sisterson MS, Chen JC, Viveros MA, Civerolo EL, Ledbetter C and Groves RL, 2008. Effects of almond leaf scorch disease on almond yield: Implications for management. Plant Disease, 92(3), 409–414. https://doi.org/10.1094/Pdis-92-3-0409
Sisterson MS, Thammiraju SR, Daane K and Groves RL, 2007. Alfalfa as an important inoculum source of Xylella fastidiosa. Phytopathology, 97(7), S108–S109.
Sisterson MS, Thammiraju SR, Lynn‐Patterson K, Groves RL and Daane KM, 2010. Epidemiology of Diseases Caused by Xylella fastidiosa in California: Evaluation of Alfalfa as a Source of Vectors and Inocula. Plant Disease, 94(7), 827–834. https://doi.org/10.1094/Pdis-94-7-0827
Smart CD, Hendson M, Guilhabert MR, Saunders S, Friebertshauser G, Purcell AH and Kirkpatrick BC, 1998. Seasonal detection of Xylella fastidiosa in grapevines with culture, ELISA and PCR. Phytopathology, 88(9 SUPPL.), S83–S83.
Smith DL, Dominiak‐Olson J and Sharber CD, 2009. First Report of Pierce's Disease of Grape Caused by Xylella fastidiosa in Oklahoma. Plant Disease, 93(7), 762–762. https://doi.org/10.1094/Pdis-93-7-0762b
Smith DL, Dominiak‐Olson J, Mulder P and von Broembsen S, 2008. Presence of Xylella fastidiosa in Oklahoma. Phytopathology, 98(6), S212‐S212.
Soares MS, da Silva DF, Forim MR, da Silva MF, Fernandes JB, Vieira PC, Silva DB, Lopes NP, de Carvalho SA, de Souza AA and Machado MA, 2015. Quantification and localization of hesperidin and rutin in Citrus sinensis grafted on C. limonia after Xylella fastidiosa infection by HPLC‐UV and MALDI imaging mass spectrometry. Phytochemistry, 115, 161–170. https://doi.org/10.1016/j.phytochem.2015.02.011
Souza PSD, Donadio LC and Jaimez EPG, 2000. Evaluation of some Citrus genotypes in relation to citrus variegated chlorosis (CVC). Revista Brasileira de Fruticultura, 22(2), 148–152.
Souza PSD, Goes Ad, Stuchi ES, Jaimes EPG, Wickert E, Silva SRd and Donadio LC, 2006. Reaction of oranges varieties and clones to Xylella fastidiosa. Revista Brasileira de Fruticultura, 28(1), 145–147.
Stenger DC, Lee MW, Rogers EE and Chen JC, 2010. Plasmids of Xylella fastidiosa mulberry‐infecting strains share extensive sequence identity and gene complement with pVEIS01 from the earthworm symbiont Verminephrobacter eiseniae. Physiological and Molecular Plant Pathology, 74(3–4), 238–245. https://doi.org/10.1016/j.pmpp.2010.03.003
Stevenson JF, Matthews MA and Rost TL, 2005. The developmental anatomy of Pierce's disease symptoms in grapevines: Green islands and matchsticks. Plant Disease, 89(6), 543–548.
Stevenson JF, Matthews MA, Greve LC, Labavitch JM and Rost TL, 2004. Grapevine susceptibility to Pierce's disease II: Progression of anatomical symptoms. American Journal of Enology and Viticulture, 55(3), 238–245.
Stoner WN, 1953. Leafhopper transmission of a degeneration of grape in Florida and its relation to Pierce's disease. Phytopath, 43(11), 611–615.
Stoner WN, 1953. Pierce's disease virus infection, a cause of Grape degeneration in Florida. Phytopathology, 43(5), 293 pp.
Stoner WN, 1958. Field symptoms indicate occurrence of “alfalfa dwarf” or “Pierce's disease” virus in Rhode Island. The Plant Disease Reporter, 42(5), 573–580.
Stoner WN, Stover LH and Parris GK, 1951. Field and laboratory investigations indicate grape degeneration in Florida is due to Pierce s disease virus infection. The Plant Disease Reporter, 35(8), 341–344.
Stout GL, 1943. A report on surveys for Pierce's disease of grape in California. Bull California Dept Agric, 32(2), 134–144.
Stover E, Riaz S and Walker MA, 2008. PCR Screening for Xylella fastidiosa in Grape Genebank Accessions Collected in the Southeastern United States. American Journal of Enology and Viticulture, 59(4), 437–439.
Stover LH and Dennison RH, 1963. The potential of Blue Lake grapes for fresh juice processing. Sunshine State Agric Res Rept, 8(3), 16–17.
Stover LH, 1960. Blue Lake, a new bunch grape for Florida home gardens. Circ. Fla. agric. Exp. Stn., S‐120, 10 pp.
Stover LH, 1961. New varieties may make Florida a grape producer. Sunshine State Agricultural Research Report, 6, 17–19; 17.
Su C and Leu L, 1995. Distribution of pear leaf scorch and monthly isolation of its causal organism, Xylella fastidiosa from infected trees. Plant Pathology Bulletin, 4(1), 30–33.
Su C, Chang C, Chang C, Su W, Chu J, Deng W and Shih H, 2013. Occurrence of Pierce's disease and its control strategies in Taiwan. Plant Pathology Bulletin, 22(3), 245–258.
Su CC, Chang CJ, Chang C‐M, Shih H‐T, Tzeng K‐C, Jan F‐J, Kao C‐W and Deng W‐L, 2013. Pierce's Disease of Grapevines in Taiwan: Isolation, Cultivation and Pathogenicity of Xylella fastidiosa. Journal of Phytopathology, 161(6), 389–396.
Su CC, Chang CJ, Yang WJ, Hsu ST, Tzeng KC, Jan FJ and Deng WL, 2012. Specific characters of 16S rRNA gene and 16S‐23S rRNA internal transcribed spacer sequences of Xylella fastidiosa pear leaf scorch strains. European Journal of Plant Pathology, 132(2), 203–216. https://doi.org/10.1007/s10658-011-9863-6
Su CC, Deng WL, Jan FJ, Chang CJ, Huang H and Chen J, 2014. Characterization of Xylella fastidiosa pear leaf scorch strain in Taiwan through whole genome sequence analyses. Phytopathology, 104(11), 115–115.
Su CC, Deng WL, Jan FJ, Chang CJ, Huang H, Shih HT and Chen J, 2016. Xylella taiwanensis sp. nov., causing pear leaf scorch disease. Int J Syst Evol Microbiol, 66(11), 4766–4771. https://doi.org/10.1099/ijsem.0.001426
Su CC, Yang WJ, Feng CY, Hsu ST and Tzeng KC, 2008. The application of DNA fingerprintings amplified by arbitrary primers in differentiating pear leaf scorch bacterium from other Xylella fastidiosa strains. Plant Pathology Bulletin, 17(4), 261–269.
Su CC, Yang WJ, Hsu ST and Tzeng KC, 2008. Specific detection of Xylella fastidiosa strains causing pear leaf scorch by polymerase chain reaction. Plant Pathology Bulletin, 17(3), 183–194.
Sun Q, Greve LC and Labavitch JM, 2011. Polysaccharide compositions of intervessel pit membranes contribute to Pierce's disease resistance of grapevines. Plant Physiol, 155(4), 1976–1987. https://doi.org/10.1104/pp.110.168807
Sun Q, Sun YL, Walker MA and Labavitch JM, 2013. Vascular Occlusions in Grapevines with Pierce's Disease Make Disease Symptom Development Worse. Plant Physiology, 161(3), 1529–1541. https://doi.org/10.1104/pp.112.208157
Svyantek AW, Coneva ED, Kessler JR, Spiers JD, Vinson EL and Pitts JA, 2016. Exploring the Growth and Cropping Potential of Pierce's Disease Resistant Vitis vinifera L. Selections for Enhanced Viticultural Sustainability in Alabama and the Southeast. Journal of the American Pomological Society, 70(4), 224–227.
Tangsukkasemsan B, Norton JD and Boyhan GE, 1995. The occurrence of plum leaf scald on plum cultivars in Alabama. Hortscience, 30(3), 437–437.
Technical report by POnTE and XF‐Actors, 2017. Institute for sustainable Plant Protection, CNR, Bari (Italy) with the contributions of members of the consortium POnTE and XF‐Actors. Studies on the host plants of Xylella fastidiosa in Europe. Provided to EFSA following official request on the 14 March 2017.
Teixeira DD, Rocha SRP, Santos MD, Mariano AG, Bin Li W and Monteiro PB, 2004. A suitable Xylella fastidiosa CVC strain for post‐genome studies. Current Microbiology, 49(6), 396–399. https://doi.org/10.1007/s00284-004-4363-y
Temsah M, Hanna L, Saad A. First Report of Xylella fastidiosa associated with Oleander Leaf Scorch in Lebanon. Journal of Crop Protection. 2015;4(1), 131–7.
Teresa Federici M, Marcondes JA, Picchi SC, Stuchi ES, Fadel AL, Laia ML, Lemos MVF and Macedo Lemos EG, 2012. Xylella fastidiosa: An in vivo system to study possible survival strategies within Citrus xylem vessels based on global gene expression analysis. Electronic Journal of Biotechnology, 15(3)
Theodoro GdF, Nesi CN, Verona LAF and Andrade TPRd, 2005. Intensity of citrus variegated chlorosis in sweet orange orchards in the West of Santa Catarina State, Brazil. Agropecuaria Catarinense, 18(1), 91–94.
Thorne ET, Stevenson JF, Rost TL, Labavitch JM and Matthews MA, 2006. Pierce's disease symptoms: Comparison with symptoms of water deficit and the impact of water deficits. American Journal of Enology and Viticulture, 57(1), 1–11.
Timmer LW, Brlansky RH, Lee RF and Raju BC, 1983. A Fastidious, Xylem‐Limited Bacterium Infecting Ragweed. Phytopathology, 73(7), 975–979. https://doi.org/10.1094/phyto-73-975
Timmer LW, Brlansky RH, Raju BC and Lee RF, 1981. A Xylem‐Limited, Rickettsia‐Like Bacterium Infecting Ragweed. Phytopathology, 71(8), 909–909.
Tolocka PA, Mattio MF, Otero ML, Paccioretti MD, Roca M, Guzmán FA and y Haelterman RM, Nueva secuencia tipo de Xylella fastidiosa subsp. pauca ST78, obtenida de un aislamiento de almendro de Argentina. Libro de resúmenes, 4° Congreso Argentino de Fitopatologia, 19, 20 y 21 de Abril de 2017 Mendoza, Argentina:177.
Torres CP and Appel DN, 2008. Isolation of Xylella fastidiosa from seven grape varieties in a Texas vineyard. Phytopathology, 98(6), S212–S213.
Torres CP, Appel DN and Morano L, 2008. Differentiation of Xylella fastidiosa subspecies piercei isolates from a Texas vineyard into strain groups utilizing simple sequence repeat markers. Phytopathology, 98(6), S157–S157.
Tuan SJ, Hu FT, Chang HY, Chang PW, Chen YH and Huang TP, 2016. Xylella fastidiosa Transmission and Life History of Two Cicadellinae Sharpshooters, Kolla paulula and Bothrogonia ferruginea (Hemiptera: Cicadellidae), in Taiwan. Journal of Economic Entomology, 109(3), 1034–1040. https://doi.org/10.1093/jee/tow016
Tubajika KM, Civerolo EL, Bartels D and Hashim JM, 2002. Spatial patterns of grapevines with Pierce's disease in the lower San Joaquin Valley. Phytopathology, 92(6 Supplement), S81–S82.
Tubajika KM, Civerolo EL, Ciomperlik MA, Luvisi DA and Hashim JM, 2004. Analysis of the Spatial Patterns of Pierce's Disease Incidence in the Lower San Joaquin Valley in California. Phytopathology, 94(10), 1136–1144. https://doi.org/10.1094/PHYTO.2004.94.10.1136
Tubajika KM, Civerolo EL, Puterka GJ, Hashim JM and Luvisi DA, 2007. The effects of kaolin, harpin, and imidacloprid on development of Pierce's disease in grape. Crop Protection, 26(2), 92–99. https://doi.org/10.1016/j.cropro.2006.04.006
Turner WF and Pollard HN, 1955. Additional leafhopper vectors of phony Peach. Journal of Economic Entomology, 48(6), 771–772.
Turner WF and Pollard HN, 1958. Insect transmission of phony peach disease. U S Dept Agric Tech Bull, 1192, 1–30.
Turner WF, 1949. Insect Vectors of Phony Peach Disease. Science, 109(2822), 87–88. https://doi.org/10.1126/science.109.2822.87
Tyson GE, Stojanovic BJ, Kuklinski RF, Divittorio TJ and Sullivan ML, 1985. Scanning Electron‐Microscopy of Pierce's Disease Bacterium in Petiolar Xylem of Grape Leaves. Phytopathology, 75(3), 264–269. https://doi.org/10.1094/phyto-75-264
Ueno B and Uesugi CH, 2002. Survey of Xylella fastidiosa occurrence on coffee plants in the Distrito Federal and Vicinity. Fitopatologia Brasileira, 27(2), 223–223.
Ueno B, Funada CK, Yorinori MA and Leite RP, 1998. First report of Xylella fastidiosa on Catharantus roseus in Brazil. Plant Disease, 82(6), 712–712.
Upchurch W, 1979. Two new muscadines released. Research and Farming, 37(3/4), 6–6.
USDA National Clonal Germplasm Repository‐Corvallis, Oregon/Xylella fastidiosa response plan
Van Horn C, Chang CJ and Chen J, 2017. De Novo Whole‐Genome Sequence of Xylella fastidiosa subsp. multiplex Strain BB01 Isolated from a blueberry in Georgia, USA. Genome Announc, 5(6). https://doi.org/10.1128/genomeA.01598-16
Vargas Cartagena L, Sanchez E, Vargas M, Solorzano A, Hernandez F, Iwasawa H and Freer E, 2002. [Bacterial present in the xylem of coffee (Rubiaceae: Coffea arabica) with “Crespera” disease]. Rev Biol Trop, 50(1), 45–48.
Vasanthaiah HKN, Katam R and Basha SM, 2007. Application of functional genomics approach to analyze Pierce's disease in grapes. Plant Biology (Rockville), 2007, 73–73.
Villalobos W, Rodriguez CM and Rivera C, 2006. Geographical distribution and incidence of Xylella fastidiosa in coffee plantations in Costa Rica. Phytopathology, 96(6), S165–S165.
Voegel TM, Doddapaneni H, Cheng DW, Lin H, Stenger DC, Kirkpatrick BC and Roper MC, 2013. Identification of a response regulator involved in surface attachment, cell‐cell aggregation, exopolysaccharide production and virulence in the plant pathogen Xylella fastidiosa. Molecular Plant Pathology, 14(3), 256–264. https://doi.org/10.1111/mpp.12004
Von Broembsen SL and Olson B, 2005. Surveys of Oklahoma grapevines for Xylella fastidiosa. Phytopathologia Mediterranea, 44(1), 97–98.
Walker A and Tenscher A, 2005. Progress toward breeding Pierce's disease resistant winegrapes. American Journal of Enology and Viticulture, 56(3), 292A‐292A.
Walker MA, Tenscher A and Riaz S, 2011. Pierce's Disease Resistant Winegrapes Are Approaching Wine Quality and Field Testing. American Journal of Enology and Viticulture, 62(3), 393a‐393a.
Wallingford AK, Tolin SA, Myers AL, Wolf TK and Pfeiffer DG, 2007. Expansion of the range of Pierce's disease in Virginia. Plant Health Progress(October):1004–1001.
Wallingford AK, Wallis CM and Chen J, 2013. Effects of rootstock on Xylella fastidiosa infection and grapevine sap phenolics. Phytopathology, 103(6), 154–154.
Wallis CM and Chen JC, 2012. Grapevine Phenolic Compounds in Xylem Sap and Tissues Are Significantly Altered During Infection by Xylella fastidiosa. Phytopathology, 102(9), 816–826. https://doi.org/10.1094/Phyto-04-12-0074-R
Wallis CM, Wallingford AK and Chen J, 2013. Grapevine rootstock effects on scion sap phenolic levels, resistance to Xylella fastidiosa infection, and progression of Pierce's disease. Front Plant Sci, 4, 502. https://doi.org/10.3389/fpls.2013.00502
Wallis CM, Wallingford AK and Chen JC, 2013. Effects of cultivar, phenology, and Xylella fastidiosa infection on grapevine xylem sap and tissue phenolic content. Physiological and Molecular Plant Pathology, 84, 28–35. https://doi.org/10.1016/j.pmpp.2013.06.005
Wang P, Lee Y, Igo MM and Roper MC, 2017. Tolerance to oxidative stress is required for maximal xylem colonization by the xylem‐limited bacterial phytopathogen, Xylella fastidiosa. Molecular Plant Pathology, 18(7), 990–1000. https://doi.org/10.1111/mpp.12456
Weaver DJ, Raju BC, Wells JM and Lowe SK, 1980. Occurrence in Johnson grass Sorghum halepense of Rickettsia‐like bacteria related to the phony peach disease organism. Plant Disease, 64(5), 485–487.
Wells JM and Raju BC, 1983. Isolation and culture of the bacterium causing phony disease of peach. Phytopathology, 73(2), 377–377.
Wells JM and Weaver DJ, 1980. Distribution of rickettsia‐like bacteria in peach, and occurrence in plum, cherry and some perennial weeds. Phytopathology, 70(6), 572–572.
Wells JM, Horton BD and Raju BC, 1983. A Highly Infectious Strain of the Plum Leaf Scald Bacterium. Phytopathology, 73(2), 377–377.
Wells JM, Raju BC and Nyland G, 1983. Isolation, Culture, and Pathogenicity of the Bacterium Causing Phony Disease of Peach. Phytopathology, 73(6), 859–862. https://doi.org/10.1094/phyto-73-859
Wells JM, Raju BC, Nyland G and Lowe SK, 1981. Medium for isolation and growth of bacteria associated with plum leaf scald and phony peach diseases. Appl Environ Microbiol, 42(2), 357–363.
Wells JM, Raju BC, Thompson JM and Lowe SK, 1981. Common Etiology of Phony Peach and Plum Leaf Scorch Diseases. Phytopathology, 71(8), 912–912.
Wells JM, Raju BC, Thompson JM and Lowe SK, 1981. Etiology of Phony Peach and Plum Leaf Scald Diseases. Phytopathology, 71(11), 1156–1161. https://doi.org/10.1094/phyto-71-1156
Wendland A, Truffi D, Leite Junior RP and Camargo LEA, 2003. Sequencing and variability of the Xylella fastidosa – specific genomic fragment amplified by the primer pair RST 31/33. Fitopatologia Brasileira, 28(3), 298–301.
Wester HV and Jylkka EW, 1959. Elm scorch, graft transmissible virus of American elm. The Plant Disease Reporter, 43(5), 519–519.
Wichman RL and Hopkins DL, 2002. Differentiation of pathogenic groups of Xylella fastidiosa strains with whole‐cell protein profiles. Plant Disease, 86(8), 875–879. Unsp D‐2002–0528–05r
Wichman RL, Hopkins DL and Wichman TA, 2000. First report of oleander leaf scorch caused by Xylella fastidiosa in Florida. Plant Disease, 84(2), 198–198.
Wistrom C and Purcell AH, 2005. The fate of Xylella fastidiosa in vineyard weeds and other alternate hosts in California. Plant Disease, 89(9), 994–999. https://doi.org/10.1094/pd-89-0994
Wistrom C, Sisterson MS, Pryor MP, Hashim‐Buckey JM and Daane KM, 2010. Distribution of glassy‐winged sharpshooter and threecornered alfalfa hopper on plant hosts in the San Joaquin Valley, California. J Econ Entomol, 103(4), 1051–1059.
Wong F, 2005. Update on Xylella fastidiosa in landscape plant hosts. CoHort (Outreach and Cooperation, University of California), 7.2(Winter 2005), 1–3.
Wong F, Cooksey DA and Costa HS, 2004. Documentation and characterization of Xylella fastidiosa strains in landscape hosts. Pierce's Disease Research Symposium Proceedings: 238–241.
Xu X, Ren ZB and Lu J, 2004. Appearance of Xylella fastidiosa in Pierce's Disease resistant and Susceptible Grapevines. Hortscience, 39(4), 826–826.
Yamamoto PT, Felippe MR, Caetano AC, Sanches AL and Lopes JRS, 2007. First report of Fingeriana dubia cavichioli transmitting Xylella fastidiosa to Citrus. Fitopatologia Brasileira, 32(3), 266–266.
Yamamoto PT, Roberto SR, Dalla Pria Jr W, Felippe MR, Caetano A, Miranda VS, Teixeira DC, Sanches AL, Costa MG, Lopes JRS and Purcell AH, 2002. Time thresholds for inoculation of Xylella fastidiosa by sharpshooters in Citrus. XXXIV Brasilian Phytopathological Congress and XI Latinamerican Phytopathological Congress, Sao Pedro, SP, Brazil, August 5–10, 2001. Fitopatologia, 37(1), 10–66.
Yamamoto PT, Roberto SR, Pria WD, Felippe MR, Miranda VS, Teixeira DdC and Lopes JRS, 2002. Transmission of Xylella fastidiosa by Acrogonia virescens and Homalodisca ignorata (Hemiptera: Cicadellidae) to Citrus plants. Summa Phytopathologica, 28(2), 178–181.
Yang LT, Lin H, Takahashi Y, Chen F, Walker MA and Civerolo EL, 2011. Proteomic analysis of grapevine stem in response to Xylella fastidiosa inoculation. Physiological and Molecular Plant Pathology, 75(3), 90–99. https://doi.org/10.1016/j.pmpp.2010.11.002
Yaseen T, Drago S, Valentini F, Elbeaino T, Stampone G, Digiaro M and D'Onghia AM, 2015. On‐site detection of Xylella fastidiosa in host plants and in “spy insects” using the real‐time loop‐mediated isothermal amplification method. Phytopathologia Mediterranea, 54(3), 488–496.
Yonce CE and Chang CJ, 1987. Detection of Xylem‐Limited Bacteria from Sharpshooter Leafhoppers and Their Feeding Hosts in Peach Environs Monitored by Culture Isolations and ELISA Techniques. Environmental Entomology, 16(1), 68–71. https://doi.org/10.1093/ee/16.1.68
Yorinori MA, Ribas AF, Ueno B, Massola Junior NS and Leite Junior RP, 2003. Detection of Xylella fastidiosa in coffee germplasm. Fitopatologia Brasileira, 28(4), 427–430.
Yuan X, Morano L, Bromley R, Spring‐Pearson S, Stouthamer R and Nunney L, 2010. Multilocus sequence typing of Xylella fastidiosa causing Pierce's disease and oleander leaf scorch in the United States. Phytopathology, 100(6), 601–611. https://doi.org/10.1094/PHYTO-100-6-0601
Zapata M, Hartung J, Brodbeck B and Andersen P, 2011. Endophytic bacteria from the vascular tissue of coffee (Coffea arabica L.) and Citrus (Citrus sinensis L.) leaves found during the attempt to isolate the pathogen, Xylella fastidiosa in Puerto Rico. Phytopathology, 101(6), S279–S279.
Zhang J, Lashomb J, Gould A and Hamilton G, 2011. Cicadomorpha insects associated with bacterial leaf scorch infected oak in central New Jersey. Environ Entomol, 40(5), 1131–1143. https://doi.org/10.1603/EN10083
Zhang S, Chakrabarty PK, Fleites LA, Rayside PA, Hopkins DL and Gabriel DW, 2015. Three New Pierce's Disease Pathogenicity Effectors Identified Using Xylella fastidiosa Biocontrol Strain EB92–1. PloS one, 10(7)
Zhang YP, Uyemoto JK and Kirkpatrick BC, 1998. A small‐scale procedure for extracting nucleic acids from woody plants infected with various phytopathogens for PCR assay. Journal of Virological Methods, 71(1), 45–50.
Suggested citation: EFSA (European Food Safety Authority) , 2018. Scientific report on the update of the Xylella spp. host plant database. EFSA Journal 2018;16(9):5408, 87 pp. 10.2903/j.efsa.2018.5408
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00215
Acknowledgements: EFSA wishes to thank EFSA staff members ALPHA UNIT: Ewelina Czwienczek, Alice Delbianco, Tomasz Kaluski, Svetla Kozelska, Marco Pautasso, Giuseppe Stancanelli, Sara Tramontini, AMU UNIT: Andrea Baù, Olaf Mosbach‐Schulz, Irene Muñoz Guajardo, DATA UNIT: Mario Monguidi and the external contractor Minh Ngoc Quan for the preparatory work on this report, and to the experts of the WG: Rodrigo Almeida, Marie‐Agnès Jacques, Joao Lopes and Leonard Nunney, Claude Bragard and Thierry Candresse for reviewing and providing suggestions for the draft.
Approved: 30 July 2018
Notes
References
- Almeida RPP and Nunney L, 2015. How Do Plant Diseases Caused by Xylella fastidiosa Emerge? Plant Disease, 99, 1457–1467. [DOI] [PubMed] [Google Scholar]
- Anonymous , 1984. The Anaheim disease. San Francisco Call, 10 December 1894, p. 9
- Auger J, Mircetich SM and Nyland G, 1974. Interrelation between bacteria causing Pierce's disease of grapevines and almond leaf scorch. Proceedings of the American Phytopathological Society, 1, 1975. [Google Scholar]
- Baldi P and La Porta N, 2017. Xylella fastidiosa: Host Range and Advance in Molecular Identification Techniques. Frontiers in Plant Science, 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berisha B, Chen YD, Xu BY and Chen TA, 1996. Isolation of Pierce's disease bacteria from grapevine in Europe. Phytopathology, 86, S119. [Google Scholar]
- Berisha B, Chen YD, Zhang GY, Xu BY and Chen TA, 1998. Isolation of Peirce's disease bacteria from grapevines in Europe. European Journal of Plant Pathology, 104, 427–433. 10.1023/A:1008655621235 [DOI] [Google Scholar]
- Brlansky RH and Timmer LW, 1982. Detection and transmission of a Gram‐negative, xylem‐limited bacterium in sharpshooters from a citrus grove in Florida. Plant Disease, 66, 590–592. 10.1094/Pd-66-590 [DOI] [Google Scholar]
- Bruer HL, 1951. Survey of phony peach incidence in wild Prunus . The Plant Disease Reporter, 35, 186–188. [Google Scholar]
- Bull CT, De Boer SH, Denny TP, Firrao G, Saux MF‐L, Saddler GS, Scortichini M, Stead DE and Takikawa Y, 2012. List of new names of plant pathogenic bacteria (2008–2010). Journal of Plant Pathology, 94, 21–27. [Google Scholar]
- Buzombo P, Jaimes J, Lam V, Cantrell K, Harkness M, McCullough D and Morano L, 2006. An American hybrid vineyard in the Texas Gulf Coast: analysis within a Pierce's disease hot zone. American Journal of Enology and Viticulture, 57, 347–355. [Google Scholar]
- Cabassut G, 2015. Mise à jour no. 8 en date du 27/11/2015 notification de la presence d'organismes nuisibles et des mesures de lutte.
- Chang CJ and Donaldson RC, 1993. Xylella fastidiosa – cultivation in chemically defined medium. Phytopathology, 83, 192–194. 10.1094/Phyto-83-192 [DOI] [Google Scholar]
- Cochran LC, 1951. Natural occurrence of the phony virus in wild Chickasaw plums near peach orchards in Georgia. The Plant Disease Reporter, 35, 181–182. [Google Scholar]
- Costa HS, Raetz E, Pinckard TR, Gispert C, Hernandez‐Martinez R, Dumenyo CK and Cooksey DA, 2004. Plant hosts of Xylella fastidiosa in and near southern California vineyards. Plant Disease, 88, 1255–1261. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Purcell AH and Thomson SV, 1980. Isolation media for the Pierce's disease bacterium. Phytopathology, 70, 425–429. 10.1094/Phyto-70-425 [DOI] [Google Scholar]
- Denancé N, Legendre B, Briand M, Olivier V, de Boisseson C, Poliakoff F and Jacques MA, 2017. Several subspecies and sequence types are associated with the emergence of Xylella fastidiosa in natural settings in France. Plant Pathology, 66, 1054–1064. 10.1111/ppa.12695 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Journal 2010;8(6):1637, 90 pp. 10.2903/j.efsa.2010.1637 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. Statement of EFSA on host plants, entry and spread pathways and risk reduction options for Xylella fastidiosa Wells et al. EFSA Journal 2013;11(11):3468, 50 pp. 10.2903/j.efsa.2013.3468 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Categorisation of plants for planting, excluding seeds, according to the risk of introduction of Xylella fastidiosa . EFSA Journal 2015;13(3):4061, 31 pp. 10.2903/j.efsa.2015.4061 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. Scientific report on the update of a database of host plants of Xylella fastidiosa: 20 November 2015. EFSA Journal 2016;14(2):4378, 40 pp. 10.2903/j.efsa.2016.4378 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2015. Scientific Opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory, with the identification and evaluation of risk reduction options. EFSA Journal 2015;13(1):3989, 262 pp. 10.2903/j.efsa.2015.3989 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Baù A, Delbianco A, Stancanelli G and Tramontini S, 2017. Statement on susceptibility of Olea europaea L. varieties to Xylella fastidiosa subsp. pauca ST53: systematic literature search up to 24 March 2017. EFSA Journal 2017;15(4):4772, 18 pp. 10.2903/j.efsa.2017.4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evert DR, 1987. Influence of phony disease of peach on stem hydraulic conductivity and leaf xylem pressure potential. Journal of the American Society for Horticultural Science, 112, 1032–1036. [Google Scholar]
- Evert DR, Gaines TP and French WJ, 1981. Rickettsia‐like bacteria in peach roots preceded development of visual symptoms of phony peach disease and changes in leaf elemental concentrations. Journal of the American Society for Horticultural Science, 106, 780–782. [Google Scholar]
- French WJ, 1977. The incidence of phony disease in wild plum trees as determined by histochemical and microscopic methods. Proceedings of the Florida State Horticultural Society, 89, 241–243. [Google Scholar]
- Goheen AC, Nyland G and Lowe SK, 1973. Association of a rickettsia‐like organism with Pierces disease of grapevines and alfalfa dwarf and heat therapy of the disease in grapevines. Phytopathology, 63, 341–345. [Google Scholar]
- Hearon SS, Sherald JL and Kostka SJ, 1980. Association of xylem‐limited bacteria with elm, sycamore, and oak leaf scorch. Canadian Journal of Botany – Revue Canadienne De Botanique, 58, 1986–1993. 10.1139/b80-228 [DOI] [Google Scholar]
- Hewitt WB, 1958. Pierce's disease virus in Mississippi and other southern states. The Plant Disease Reporter, 42, 207–210. [Google Scholar]
- Hewitt WB and Houston BR, 1946. Leafhopper transmission of the virus causing Pierce's disease of grape and dwarf of alfalfa. Phytopathology, 36, 117–128. [PubMed] [Google Scholar]
- Hopkins DL, 1984. Variability of virulence in grapevine among isolates of the Pierce's disease bacterium. Phytopathology, 74, 1395–1398. 10.1094/Phyto-74-1395 [DOI] [Google Scholar]
- Hopkins DL and Adlerz WC, 1980. Pierce disease bacterium causes a disease of rough lemon citrus. Phytopathology, 70, 568. [Google Scholar]
- Hopkins DL and Mollenhauer HH, 1975. Tylose and gum formation in the xylem of Pierce's disease infected grapevines. Proceedings of the American Phytopathological Society, 2, 65. [Google Scholar]
- Hopkins DL and Mortensen JA, 1974. Pierce's disease in muscadine grapes. Proceedings of the American Phytopathological Society, 1, 1975. [Google Scholar]
- Hopkins DL and Thompson CM, 1984. Seasonal concentration of the Pierce's disease bacterium in Carlos and welder muscadine grapes compared with Schuyler bunch grape. HortScience, 19, 419–420. [Google Scholar]
- Hutchins LM, 1930. The phony disease of the peach. Journal of Economic Entomology, 23, 555–562. [Google Scholar]
- Hutchins LM and Rue JL, 1949. Natural spread of phony disease to apricot and plum. Phytopathology, 39, 503. [Google Scholar]
- Hutchins LM, Cochran LC, Turner WF and Weinberger JH, 1953. Transmission of phony disease virus from tops of certain affected peach and plum trees. Phytopathology, 43, 691–696. [Google Scholar]
- Janse JD and Obradovic A, 2010. Xylella fastidiosa: its biology, diagnosis, control and risks. Journal of Plant Pathology, 92, S35–S48. [Google Scholar]
- Jimenez LG, 1985. Immunological evidence of Pierce's disease in grapevines in Venezuela. Turrialba, 35, 243–247. [Google Scholar]
- Jindal KK and Sharma RC, 1987. Outbreaks and new records india almond leaf scorch a new disease from India. FAO (Food and Agriculture Organization of the United Nations) Plant Protection Bulletin, 35, 64–65. [Google Scholar]
- Kenknight G, 1951. Occurrence of phony disease in wild plum thickets distant from peach orchards in Spartanburg County, South Carolina. The Plant Disease Reporter, 35, 183–185. [Google Scholar]
- Kostka SJ, Sherald JL and Tattar TA, 1984. Culture of fastidious, xylem‐limited bacteria from declining oaks in the northeastern States. Phytopathology, 74, 803. [Google Scholar]
- Laranjeira FF, Bergamin Filho A and Amorim L, 1998. Dynamics and structure of citrus variegated chlorosis (CVC) foci. Fitopatologia Brasileira, 23, 36–41. [Google Scholar]
- Leite RMVBC, Leite Junior RP and Ceresini PC, 1997. Alternative hosts of Xylella fastidiosa in plum orchards with leaf scald disease. Fitopatologia Brasileira, 22, 54–57. [Google Scholar]
- Loomis NH, 1961. Symptom expression and occurrence of Pierce's disease virus at Meridian, Miss. Proceedings of the Society for Horticultural Science, 77, 331–336. [Google Scholar]
- Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA, Toth I, Salmond G and Foster GD, 2012. Top 10 plant pathogenic bacteria in molecular plant pathology. Molecular Plant Pathology, 13, 614–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy RE, Thomas DL, Tsai JH and French WJ, 1978. Periwinkle wilt, a new disease associated with xylem delimited rickettsia‐like bacteria transmitted by a sharpshooter. The Plant Disease Reporter, 62, 1022–1026. [Google Scholar]
- McElrone AJ, Sherald JL and Pooler MR, 1999. Identification of alternative hosts of Xylella fastidiosa in the Washington, DC, area using nested polymerase chain reaction (PCR). Journal of Arboriculture, 25, 258–263. [Google Scholar]
- McGaha LA, Jackson B, Bextine B, McCullough D and Morano L, 2007. Potential plant reservoirs for Xylella fastidiosa in South Texas. American Journal of Enology and Viticulture, 58, 398–401. [Google Scholar]
- Millikan DF, 1955. The phony peach virus. University of Missouri, Bulletin 661.
- Millikan DF and Anderson JR, 1954. The phony peach virus in Missouri. The Plant Disease Reporter, 38(12), 834–835. [Google Scholar]
- Mortensen JA, 1968. The inheritance of resistance to Pierce's caused by virus disease in Vitis . Proceedings of the American Society for Horticultural Science, 92, 331–337. [Google Scholar]
- Mortensen JA and Knight RJ Jr, 1968. Susceptibility to Pierce's disease of a plant introduction of Vitis vinifera . Proceedings of the Florida State Horticultural Society, 1967, 348–350. [Google Scholar]
- Mortensen JA, Stover LH and Balerdi CF, 1977. Sources of resistance to Pierce's disease in Vitis . Journal of the American Society for Horticultural Science, 102, 695–697. [Google Scholar]
- Purcell AH, 1975. Role of blue‐green sharpshooter, Hordnia circellata in epidemiology of Pierce's disease of grapevines. Environmental Entomology, 4, 745–752. 10.1093/ee/4.5.745 [DOI] [Google Scholar]
- Purcell AH and Saunders S, 1995. Harvested grape clusters as inoculum for Pierce's disease. Plant Disease, 79, 190–192. 10.1094/Pd-79-0190 [DOI] [Google Scholar]
- Purcell A, 2013. Paradigms: Examples from the Bacterium Xylella fastidiosa . Annual Review of Phytopathology, 51, 339–356. [DOI] [PubMed] [Google Scholar]
- Raju BC, Goheen AC and Frazier NW, 1983. Occurrence of Pierce's disease bacteria in plants and vectors in California. Phytopathology, 73, 1309–1313. 10.1094/Phyto-73-1309 [DOI] [Google Scholar]
- Sadovskii YP, 1985. Rickettsia‐like bacteria transformation of cytoplasmic cell membrane of peach trees affected by grafted node necrosis. Mikrobiologicheskii Zhurnal (Kiev), 47, 44–53. [Google Scholar]
- Sadovskii YP and Shevchenko SI, 1991. Electron microscopic studies of inclusions in plant tissues infected with Rickettsia‐like bacteria. Tsitologiya i Genetika, 25, 7–12. [Google Scholar]
- Saponari M, Boscia D, Altamura G, Loconsole G, Zicca S, D'Attoma G, Morelli M, Palmisano F, Saponari A, Tavano D, Savino VN, Dongiovanni C and Martelli GP, 2017. Isolation and pathogenicity of Xylella fastidiosa associated to the olive quick decline syndrome in southern Italy. Scientific Reports, 7, 17723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner WN, 1953a. Leafhopper transmission of a degeneration of grape in Florida and its relation to Pierce's disease. Phytopathology, 43, 611–615. [Google Scholar]
- Stoner WN, 1953b. Pierce's disease virus infection, a cause of grape degeneration in Florida. Phytopathology, 43, 293. [Google Scholar]
- Stoner WN, Stover LH and Parris GK, 1951. Field and laboratory investigations indicate grape degeneration in Florida is due to Pierce's disease virus infection. The Plant Disease Reporter, 35, 341–344. [Google Scholar]
- Su C and Leu L, 1995. Distribution of pear leaf scorch and monthly isolation of its causal organism, Xylella fastidiosa from infected trees. Plant Pathology Bulletin, 4, 30–33. [Google Scholar]
- Su CC, Deng WL, Jan FJ, Chang CJ, Huang H, Shih HT and Chen J, 2016. Xylella taiwanensis sp. nov., causing pear leaf scorch disease. International Journal of Systematic and Evolutionary Microbiology, 66, 4766–4771. 10.1099/ijsem.0.001426 [DOI] [PubMed] [Google Scholar]
- Temsah M, Hanna L and Saad A, 2015. First Report of Xylella fastidiosa associated with oleander leaf scorch in Lebanon. Journal of Crop Protection, 4, 131–137. [Google Scholar]
- Turner WF, 1949. Insect vectors of phony peach disease. Science, 109, 87–88. 10.1126/science.109.2822.87 [DOI] [PubMed] [Google Scholar]
- Turner WF and Pollard HN, 1958. Insect transmission of phony peach disease. US Department of Agriculture Technical Bulletin, 1192, 1–30. [Google Scholar]
- Weaver DJ, Raju BC, Wells JM and Lowe SK, 1980. Occurrence in Johnson grass Sorghum halepense of Rickettsia‐like bacteria related to the phony peach disease organism. Plant Disease, 64, 485–487. [Google Scholar]
- Wells JM and Weaver DJ, 1980. Distribution of Rickettsia‐like bacteria in peach, and occurrence in plum, cherry and some perennial weeds. Phytopathology, 70, 572. [Google Scholar]
- Wells JM, Raju BC, Nyland G and Lowe SK, 1981. Medium for isolation and growth of bacteria associated with plum leaf scald and phony peach diseases. Applied and Environmental Microbiology, 42, 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JM, Raju BC, Hung HY, Weisburg WG, Mandelco‐Paul L and Brenner DJ, 1987. Xylella fastidiosa gen. nov., sp. nov.: Gram‐negative, xylem‐limited fastidious plant bacteria related to Xanthomonas spp. International Journal of Systematic Bacteriology, 37, 136–143. [Google Scholar]
- Wendland A, Truffi D, Leite Junior RP and Camargo LEA, 2003. Sequencing and variability of the Xylella fastidiosa – specific genomic fragment amplified by the primer pair RST 31/33. Fitopatologia Brasileira, 28, 298–301. [Google Scholar]
- Wester HV and Jylkka EW, 1959. Elm scorch, graft transmissible virus of American elm. The Plant Disease Reporter, 43, 519. [Google Scholar]
- Yonce CE and Chang CJ, 1987. Detection of xylem‐limited bacteria from sharpshooter leafhoppers and their feeding hosts in peach environs monitored by culture isolations and ELISA techniques. Environmental Entomology, 16, 68–71. 10.1093/ee/16.1.6 [DOI] [Google Scholar]


