Abstract
The conclusions of EFSA following the peer review of the initial risk assessment carried out by the competent authority of the rapporteur Member State, the United Kingdom, for the pesticide risk assessment for the triazole derivative metabolites are reported. The context of the peer review was that requested by the European Commission following the submission and evaluation of confirmatory data in relation to mammalian toxicology, metabolism and residue data. The conclusions were reached on the basis of the evaluation of various uses for a number of triazole fungicides. Recommendations are proposed. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
Keywords: triazole derivative metabolites, peer review, confirmatory data, risk assessment, pesticide, fungicide
Summary
During the second and third stage of the European Commission's pesticides review programme (referred to in Article 8(2) of Council Directive 91/414/EEC), data gaps and confirmatory data requirements were identified for a number of triazole fungicides. A group of manufactures of the triazole‐containing fungicides (BASF SE, Bayer CropScience AG, DOW Agrosciences LLC, Isagro S.p.A. and Syngenta Crop Protection AG) formed an industry taskforce, known as the Triazole Derivative Metabolite Group (TDMG) which made a joint submission of new toxicological, metabolism and residues data to meet these data requirements. The new data submitted by the TDMG were intended to support the derivation of revised toxicological reference values for each of the triazole derivative metabolites (TDMs) in order to prevent the need for the application of the additional assessment factors as concluded at the Pesticides experts' meeting (PRAPeR 14) and to also set toxicological reference values for the metabolite triazole lactic acid (TLA), which was not considered so far for the setting of reference values. These new reference values could then be used for the consumer risk assessments to address the confirmatory data requirements set for the various triazole fungicides.
The TDMG submitted data, which were evaluated by the designated rapporteur Member State (RMS), the United Kingdom, in the form of an addendum to the draft assessment reports on various triazole containing pesticides. In compliance with the guidance document SANCO 5634/2009‐rev.6.1, the RMS distributed the addendum to the Member States, the applicant TDMG and EFSA for comments on 1 December 2015. The RMS collated all comments in the format of a reporting table, which was submitted to the European Food Safety Authority (EFSA) on 24 May 2016. EFSA added its scientific views on the specific points raised during the commenting phase in column 4 of the reporting table. EFSA's scientific views and conclusions on the individual comments received were finalised in a Technical Report (EFSA supporting publication 2016:EN‐1080).
Following consideration of the Technical Report, EFSA received a mandate from the European Commission, in which EFSA was asked to set reference values, residue definitions and to perform a consumer risk assessment with regard to the TDMs for the complete group of triazole active substances that were assessed in the framework of the confirmatory data submission. In addition, a consumer dietary risk assessment on an individual substance level has also been undertaken as the approval of certain active substance has been legally conditioned by the submission of confirmatory data regarding the dietary exposure of consumers to the TDMs. According to that mandate, EFSA should deliver its assessment in the format of a conclusion. The assessment was based on the addendum prepared by the United Kingdom on the data submitted by the TDMG (United Kingdom, 2018). EFSA conducted a peer review on the updated addendum prepared by the RMS (United Kingdom, 2018) and organised experts' consultations in the sections on mammalian toxicology and residues.
Confirmatory data submitted in relation to mammalian toxicology were discussed at the Pesticides Peer Review Meeting 162 in September 2017. In particular, some toxicity issues were discussed for triazole alanine (TA), 1,2,4‐triazole (1,2,4‐T), triazole acetic acid (TAA) and triazole lactic acid (TLA) together with their reference values (acceptable daily intake (ADI) and acute reference dose (ARfD)). Finally, the issue of co‐exposure was discussed also in consideration of the mode of action of TDMs. No data gaps, issues that could be not finalised or a critical area of concern were identified in relation to the section on mammalian toxicology. The confirmatory data allowed setting of reference values for 1,2,4‐T, TAA and TA; for TLA reference values were derived bridging from the data available for TA.
The data submitted in relation to the section on residues were discussed in at the Pesticides Peer Review Meeting 171. The confirmatory data allowed setting agreed residue definitions for monitoring and risk assessment for the triazole pesticide active substances in products of plant and animal origin. The overall consumer exposure assessment for the TDMs could not be finalised in view of the identified data gaps for storage stability data for the TDMs in several crop commodities and also in relation to outstanding data to finalise the livestock exposure assessment (see data gap identified). A provisional consumer dietary intake calculation conducted with PRIMo rev.2 showed that the international estimated daily intake (IEDI) accounted for 60% of the ADI (FR toddler) for 1,2,4‐T, and 1% of the ADI (WHO Cluster diet B) for TAA. The acute intake was estimated to be 40% of the ARfD (milk) for 1,2,4‐T, and 1% of the ARfD (oranges) for TAA. Since the toxicological reference values for TLA were derived from the data available for TA, a combined dietary risk assessment for TA and TLA was performed. No chronic or acute intake concerns were identified with up to 6% of the ADI (WHO Cluster diet B), and 34% and 8% ARfD (watermelons), respectively, for children and adults. It is also emphasised that residue trials analysing all TDMs and being compliant with the European authorised uses should be provided in order to conduct a realistic consumer dietary risk assessment. Further consideration should also be given to the TDMs residue levels that may arise when several different triazole compounds are applied on a crop within the same growing season and from treatments with triazole compounds during the previous seasons. It is therefore recommended that a separate monitoring programme including 1,2,4‐T, TA, TAA and TLA compounds should be established for products of plant and animal origin to have the background residue levels of these compounds resulting from the current and past uses of the triazole active substances. Finally and for any future assessment of triazole pesticide active substances, livestock feeding studies or, alternatively metabolism studies should be conducted with the triazole active substances to carry out a complete livestock exposure assessment.
For the triazole pesticide active substances cyproconazole, difenoconazole, epoxiconazole, fenbuconazole, myclobutanil, paclobutrazol and prothioconazole, the approval has been conditioned to the submission of confirmatory data. The submitted data were, however, not considered as sufficient to fully address the confirmatory data requirements and to finalise the consumer risk assessment, which therefore, is inconclusive for all of these active substances.
Background
During the second and third stages of the European's pesticides review programme (referred to in Article 8(2) of Council Directive 91/414/EEC1), data gaps were identified, and confirmatory data requirements were set for a number of triazole fungicides. A group of manufactures of the triazole‐containing fungicides (BASF SE, Bayer CropScience AG, BASF, DOW Agrosciences LLC, Isagro S.p.A., and Syngenta Crop Protection AG) formed an industry taskforce, known as the Triazole Derivative Metabolite Group (TDMG) which made a joint submission of new toxicological, metabolism and residues data to meet these data requirements. The new data submitted by the TDMG were intended to support the derivation of revised toxicological reference values for each of the triazole derivative metabolites (TDMs) in order to prevent the need for the application of the additional assessment factors as it was concluded at the Pesticides Peer Review experts' meeting (PRAPeR 14, 2007), and to also set reference values for the metabolite triazole lactic acid (TLA), which was not considered so far for the setting of reference values. These new toxicological reference values could then be used for the consumer risk assessments to address the confirmatory data requirements set for the various triazole fungicides.
The TDMG submitted data, which were evaluated by the designated rapporteur Member State (RMS), the United Kingdom, in the form of an addendum to the draft assessment reports on various triazole containing pesticides (United Kingdom, 2015). In compliance with the guidance document SANCO 5634/2009‐rev.6.1 (European Commission, 2013), the RMS distributed the addendum to the Member States, the applicant TDMG and EFSA for comments on 1 December 2015. The RMS collated all comments in the format of a reporting table, which was submitted to EFSA on 24 May 2016. EFSA added its scientific views on the specific points raised during the commenting phase in column 4 of the reporting table. EFSA's scientific views and conclusions on the individual comments received were finalised in a Technical Report (EFSA, 2016).
Following consideration of the Technical Report (EFSA, 2016), EFSA received a mandate from the European Commission, in which EFSA was asked to set reference values, residue definitions and to perform a consumer risk assessment with regard to the TDMs for the complete group of triazole active substances that were assessed in the framework of the confirmatory data submission. In addition, a consumer dietary risk assessment on an individual substance level has also been undertaken as the approval of certain active substance has been legally conditioned to the submission of confirmatory data regarding the dietary exposure of consumers to the TDMs. According to that mandate, EFSA should deliver its assessment in the format of a conclusion. The assessment was based on the addendum prepared by the United Kingdom on the data submitted by the TDMG (United Kingdom, 2018). EFSA conducted a peer review on the updated addendum prepared by the RMS (United Kingdom, 2018) and organised experts' consultation in the sections on mammalian toxicology and residues.
The addendum (United Kingdom, 2018) and the reporting table were discussed at the Pesticides Peer Review Meeting 162 on mammalian toxicology and Pesticides Peer Review Meeting 171 on residues. Details of the issues discussed, together with the outcome of these discussions were recorded in the meeting reports.
A final consultation on the conclusions arising from the peer review took place with Member States via a written procedure in June 2018.
The conclusions laid down in this report were reached on the basis of the peer review of the RMS's evaluation of the confirmatory data submitted in relation to mammalian toxicology, metabolism and residue data based on the various Draft Assessment reports (DAR) submitted in the framework of the Directive 91/414/EEC and that were compiled by EFSA in order to investigate the residue levels and respective proportions of the TDMs present in primary crops and in rotational crops and by the TDMG regarding the products of animal origin. A key supporting document to this conclusion is the peer review report, which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review. The peer review report (EFSA, 2018) comprises the following documents, in which all views expressed during the course of the peer review, including minority views, can be found:
the reports of the scientific consultation with Member State experts;
the comments received on the draft EFSA conclusion.
Given the importance of the revised addendum (United Kingdom, 2018) and the peer review report, these documents are considered as background documents to this conclusion.
It is recommended that this conclusion report and its background documents would not be accepted to support any registration outside the European Union (EU) for which the applicant has not demonstrated to have regulatory access to the information on which this conclusion report is based.
Conclusions of the evaluation
‘The triazole derivative metabolites triazole acetic acid (TAA), triazole alanine (TA), 1,2,4‐triazole (1,2,4‐T) and triazole lactic acid (TLA), are common metabolites of the triazole‐containing fungicides. PRAPeR Expert Meeting (no. 14, January 2007) previously evaluated the existing toxicological data for the triazole derivative metabolites (TDMs) and set reference values (ADI and Acute RfD) for 1,2,4‐T and TA. Whilst a substantial package of toxicological studies was available at that time for 1,2,4‐T and TA, only very limited data existed for triazole acetic acid (TAA). Due to the lack of toxicological information on TAA, PRAPeR took a precautionary approach and proposed the use of the lower endpoints of 1,2,4‐T for when reference values for TAA were needed (TLA was not considered at PRAPeR 14).
The extent to which these common metabolites are formed in each crop or in environmental matrices is dependent on the particular triazole‐containing active substance and the way in which it is used. The relative toxicity of the common metabolites compared to the individual substances also differs by substance. Conclusions regarding the relevance of these common metabolites to the risk assessments were therefore considered separately for each active substance. PRAPeR 14 only considered the hazards, NOAELs and potential data gaps for these metabolites'. (Quoted from: United Kingdom, 2015)
The TDMG, a group of manufactures of the triazole‐containing fungicides consisting of BASF SE, Bayer CropScience AG, BASF, DOW Agrosciences LLC, Isagro S.p.A. and Syngenta Crop Protection AG, submitted new toxicological, metabolism and residues data to meet the data requirements, that have been identified during the peer review and approval procedure for a number of triazole fungicides. The assessment of the information was presented in a confirmatory data addendum (United Kingdom, 2015), which has been updated after the commenting phase on that addendum and after the Pesticides Peer Review Experts' meeting (United Kingdom, 2018).
A ‘worst‐case’ consumer dietary intake assessment with regard to the TDMs for the complete group of triazole active substances that were assessed in the framework of these confirmatory data has been conducted and it was demonstrated that the risk for the consumers is unlikely. The overall consumer exposure assessment for the TDMs could, however, not be finalised in view of the identified data gaps for additional storage stability data for the TDMs in several crop commodities and missing data to finalise the livestock exposure assessment.
1. Mammalian toxicology
Triazole derivative metabolites were discussed at the Pesticides Peer Review Experts' Teleconference 162 (September 2017). New toxicological data submitted for 1,2,4‐triazole (1,2,4‐T), triazole alanine (TA), triazole acetic acid (TAA), and triazole lactic acid (TLA) were taken into consideration. When referring to the different studies used to derive reference values, it is expressly indicated if such studies were newly submitted; in the absence of specific indications, the studies mentioned should be considered as submitted under the previous assessment.
During the meeting, the experts discussed the setting of an acceptable daily intake (ADI) and the majority of the experts agreed to set the ADI for 1,2,4‐triazole at 0.023 mg/kg body weight (bw) per day based on the no‐observed‐adverse‐effect‐level (NOAEL) of 6.9 mg/kg bw per day, considering the decreased body weight gain in the newly submitted 12‐month rat study. At the same time an increased uncertainty factor (UF) of 300 was applied to cover the lack of a developmental neurotoxicity (DNT) study and carcinogenicity and dog studies. The RMS disagreed with such proposal, since they considered it to be more appropriate to set an ADI at 0.05 mg/kg bw per day based on the NOAEL of 15 mg/kg bw per day observed in the rat two‐generation study and by applying an UF of 300. The ARfD was agreed to be 0.1 mg/kg bw based on the NOAEL of 30 mg/kg bw per day from the rabbit developmental toxicity studies. An increased UF of 300 was applied to cover the lack of a DNT study and to ensure a sufficient margin of safety in relation to the lowest‐observed‐adverse‐effect level (LOAEL) for developmental effects. Both the ADI and the ARfD are slightly different compared to the values agreed during the PRAPeR 14 meeting, where the ADI was set at 0.02 mg/kg bw per day, based on the NOAEL of 17 mg/kg bw per day in the rat multigeneration study with an UF of 1,000; the ARfD was set at 0.06 mg/kg bw, based on the NOAEL of 30 mg/kg bw per day in the rat developmental study with an UF of 500.
The ADI for triazole alanine was discussed during the same experts' meeting and set at 0.3 mg/kg bw per day based on the NOAEL of 30 mg/kg bw per day, taking into account the increased incidence of hyoid angulated alae in fetuses observed in the newly submitted rabbit developmental study. An UF of 100 was applied. This value is also applicable to the ARfD. The RMS disagreed with the NOAEL derived from the developmental toxicity study in rabbits. Both ADI and ARfD are different to the values agreed during the PRAPeR 14 meeting, where the ADI was set at 0.1 mg/kg bw per day, based on the NOAEL of 100 mg/kg bw per day in the rat developmental study, applying an UF of 1,000 to account for developmental effects seen at doses showing no overt signs of maternal toxicity (also in the absence of chronic study and rabbit developmental toxicity study); the same rationale was considered applicable for the derivation of the ARfD which resulted to be the same as the ADI (0.1 mg/kg bw).
The ADI of triazole acetic acid was discussed and agreed to be set at 1 mg/kg bw per day, based on the NOAEL of 100 mg/kg bw per day in the newly submitted rat two‐generation (parental toxicity: decreased body weight gain and food consumption) and rabbit developmental studies (decreased body weight gain and food consumption for maternal and developmental toxicity, plus stomach mucosal erosions or ulceration for developmental toxicity), applying an UF of 100. The same endpoint and the same UF was considered applicable for the derivation of the ARfD. In the PRAPeR 14 meeting, the reference values set for 1,2,4‐triazole were used for TAA (ADI = 0.02 mg/kg bw per day; ARfD = 0.06 mg/kg bw) in the absence of reproductive data and on the basis of several uncertainties.
No reference values were previously derived for TLA. Four studies were previously available for this metabolite: an acute oral toxicity test and three in vitro genotoxicity tests (Ames, mammalian cell gene mutation and chromosome aberration tests). During the Pesticides Peer Review Experts' Teleconference 162, it was discussed and agreed to derive reference values for TLA from TAA and TA, since no repeat dose and reproductive toxicity studies were available for TLA and because of toxicological similarities between the three metabolites. The ADI of triazole lactic acid was therefore set at 0.3 mg/kg bw per day by bridging from the reference values for triazole alanine as a worst case approach for triazole lactic acid. The same approach was considered applicable for the derivation of the ARfD for this metabolite. A summary of the derived toxicological reference values for the TDMs is presented in Table 1.
Table 1.
Overview of the derived toxicological reference values for the TDMsa
| Metabolite | Ref. values (derived at the Pesticides Peer Review TC 162 (Sept 2017) | Study | Effect observed at the LOAEL | UF | Previously set Ref. values (derived at the PRAPeR 14, Jan 2007) | Previously set UF |
|---|---|---|---|---|---|---|
|
1,2,4‐Triazole: ADI ARfD |
0.023 mg/kg bw per day 0.1 mg/kg bw |
Newly submitted rat 12‐month study Rabbit developmental study |
Decreased body weight gain Decreased body weight gain |
300 300 |
0.02 mg/kg bw per day (rat multigeneration study) 0.06 mg/kg bw (rat developmental study) |
1,000 500 |
|
Triazole alanine: ADI ARfD |
0.3 mg/kg bw per day 0.3 mg/kg bw |
Newly submitted rabbit developmental study Newly submitted rabbit developmental study |
Increased incidence of hyoid angulated alae Increased incidence of hyoid angulated alae |
100 100 |
0.1 mg/kg bw per day (rat developmental study) 0.1 mg/kg bw (rat developmental study) |
1,000 1,000 |
|
Triazole acetic acid: ADI ARfD |
1 mg/kg bw per day 1 mg/kg bw |
Newly submitted rat 2‐generation and rabbit developmental studies Newly submitted rat 2‐generation and rabbit developmental studies |
Maternal and developmental toxicity Maternal and developmental toxicity |
100 100 |
0.02 mg/kg bw per day (derived from 1,2,4‐T) 0.06 mg/kg bw (derived from 1,2,4‐T) |
1,000 1,000 |
|
Triazole lactic acid: ADI ARfD |
0.3 mg/kg bw per day 0.3 mg/kg bw |
Bridging from TA |
Not set Not set |
LOAEL: lowest‐observed‐adverse‐effect level; UF: uncertainty factor; ADI: acceptable daily intake; ARfD: acute reference dose; bw: body weight; 1,2,4‐T: 1,2,4‐triazole; TA: triazole alanine.
In the absence of specific indications, the studies mentioned should be considered as previously submitted.
2. Residues
2.1. Triazole derivative metabolites
The TDMs were discussed at the Pesticides Peer Review Experts' Meeting 171 (December 2017).
The investigation of the residue levels and respective proportions of the TDMs in plants and livestock for the derivation of the residue definitions was based on the compilation of the metabolism data in primary crops, rotational crops and in ruminant and poultry matrices conducted with the triazole active substances using the 14C‐labelling on the triazole moiety.
These data were collected by EFSA from the DARs submitted in the framework of Directive 91/414/EEC in compliance with the representative uses (Belgium, 2004, 2009a,b; Denmark, 2007; Finland, 1998; Germany, 2007, 2008; Ireland, 2000, 2005, 2010; Spain, 2007; Sweden, 2007; United Kingdom, 2005a,b, 2006a,b, 2008).
Primary crops metabolism data are reported for a total of 16 approved triazole compounds,2 and 2 triazole active substances that are not approved at EU level (bitertanol, flusilazole), on fruit crops, cereals (straw and grain), pulses and oilseeds and root crops.
For the rotational crops, metabolism data are available on leafy crops, root crops and cereal grain and straw for a total of 123 approved triazole active substances and one non approved triazole active substance (flusilazole).
Compiled metabolism data respectively for ruminants (114) and for poultry (65) from approved triazole active substances were also available.
In all plant and animal metabolism studies, the residue levels initially expressed as ‘mg parent equivalent/kg’ have been corrected to be expressed on the individual TDM molecular weight basis and the residue levels were corrected at a 1N dose rate compared to the maximum dose rate of application in the representative uses for plants or considering the maximum dietary burden calculation for livestock.
The magnitude of the TDMs residues has been determined in supervised residue trials for different crop groups and different triazole active substances both in primary and rotational crops.
As requested in the mandate, a ‘worst‐case’ consumer exposure assessment to the TDMs has been carried out in this conclusion taking into consideration the highest residue input values for risk assessment from all the individual residue data sets for plant commodities and the highest residue levels of each TDM arising in products of animal origin from the triazole active substances and from each of the TDMs.
In addition to this ‘worst‐case’ consumer exposure assessment and for the following triazole active substances (cyproconazole, difenoconazole, epoxiconazole, fenbuconazole, myclobutanil, paclobutrazol and prothioconazole), the consumer exposure assessment to the TDMs has been conducted individually as the approval of these active substances has been legally conditioned by the submission of confirmatory data regarding the dietary exposure of consumers to these metabolites.
Based on the metabolism data in primary and rotational crops that were compiled from the assessment of the 18 triazole active substances the triazole active substances were shown to degrade into the common metabolites 1,2,4‐T, TA, TLA and TAA, known as TDMs.
Besides the parent compound that was identified at significant residue levels in all crop groups, TA was predominantly found in the organs of storage (79% total radioactive residue (TRR) in potato tuber, 31–88% TRR in oil seeds, 8–69% TRR in cereal grains) but also in cereal straw (1–16% TRR) and in fruit crops (up to 80% TRR). TAA was only detected at significant proportions in cereal grain and straw (5–35% and 7–41% TRR, respectively) and TLA in fruit crops (up to 67% TRR) and in cereal straw (up to 43% TRR). 1,2,4‐T was detected at lower levels in all crop parts (up to 12% TRR). Similar metabolic patterns were depicted both in primary and in rotational crops.
Pesticide residues monitoring data (DE survey, 2014–2015) on unprocessed food commodities (mainly fruits and vegetables) showed residue levels above the limit of quantification (LOQ) for each of the TDMs, i.e. 0.035–0.064 mg/kg for 1,2,4‐T, 1.2–1.4 mg/kg for TA, 0.39–0.45 mg/kg for TAA and 0.78–2.4 mg/kg for TLA. These results confirmed the occurrence of each of the TDMs in primary and rotational crops from the compiled metabolism data in plants.
The TDMs remained stable under the standard hydrolysis conditions simulating processing of pasteurisation, baking, brewing and boiling and sterilisation.
The experts of the meeting agreed that general residue definitions for enforcement and risk assessment for primary, rotational crops and processed commodities can be derived based on the available data:
The TDMs were not considered to be suitable markers to be included in the residue definition for enforcement purposes since all the triazole pesticide active substances need to be assessed together and complete residue data sets are not available for all the uses of all the triazole active substances. Moreover, the TDMs may also result from the use of fertilisers, an assessment which is not in the remit of EFSA. For risk assessment purposes, although 1,2,4‐T generally occurred in lower proportions in plant matrices compared to the other TDMs, the experts agreed to include this compound in the risk assessment residue definition in view of its higher toxicity compared to the other TDMs and the quantifiable residue levels of this compound observed in primary and rotational crop field trials. The agreed residue definitions for the TDMs in plants are reported in Table 2.
Table 2.
Agreed residue definitions for triazole pesticide active substances in plants
| Plant commodities | |
| RD for enforcement: | Triazole parent compound only |
| RDs for risk assessment: |
|
RD: residue definition; TA: triazole alanine; TLA: triazole lactic acid; TAA: triazole acetic acid; 1,2,4‐T: 1,2,4‐triazole.
The magnitude of the TDMs have been determined in numerous residue trials conducted on crops covering most of the crop categories and for different triazole active substances both in primary and rotational crops. These trials were submitted in the framework of the confirmatory data (United Kingdom, 2015). The submitted residue trials were performed according to specific good agricultural practices (GAPs) authorised for the triazole active substances and residue trials conducted outside Europe were also available. In some cases, these residue trials were compliant with the representative uses of triazole active substances that were approved at EU level. All the residue trials that were used to perform the consumer dietary intake assessment involve only the use of a single triazole active substance, these residue trials do not reflect the situation where several different triazole active substances may be applied on a crop during the same growing season or from treatments with triazole active substances during the previous seasons. However, it is noted that significant residue levels were often found in untreated control samples of residue trials on primary and rotational crops suggesting the use of triazole pesticide active substances in previous seasons. Despite these uncertainties, the experts were of the opinion that these trials should be considered with the purpose of performing a ‘worst case’ consumer dietary intake calculation. It was, however, emphasised that residue trials analysing all TDMs and compliant with the European authorised uses should be provided in order to conduct a realistic consumer dietary risk assessment and also the need for monitoring data on the occurrence and background levels of all TDMs in plants. For each commodity the input residue values for risk assessment (supervised trials median residues (STMR) and the supervised trials highest residues (HR)) were calculated based on all the residue trials conducted with the same active substance on this commodity and for a commodity group, the highest STMR and HR values derived from all the individual data sets have been applied to each crop within the commodity group in order to conduct the ‘worst‐case’ consumer dietary intake calculation.
All the submitted trials were supported by validated analytical methods.
Regarding the storage stability data that are part of the addendum prepared by the RMS (United Kingdom, 2018), the experts agreed on the maximum storage time periods for which acceptable storage stability is observed for all TDMs in several commodity categories. These are reported in Table 3.
Table 3.
Stability of residues
| Plant products (category) | Commodity | Storage stability (Months) | |||
|---|---|---|---|---|---|
| 1,2,4‐Triazole | TA | TAA | TLA | ||
| High water content | Apples, tomatoes, mustard leaves, wheat forage, radishes tops/roots, turnips roots, sugar beet roots, cabbages, lettuces | 6 | 53 | 53 | 48 (lettuce only) |
| High starch content | Barley, wheat | 12 | 26 | 26 | 48 |
| High oil content | Rapeseeds, soya beans | 12 (soya bean only; not stable in rape seed) | 26 (soya bean only; not stable in rape seed) | 53 | 48 |
| High protein content | Peas, dry; Navy beans | No data | 15 | 25 | 48 |
| High acid content | Oranges | No data | No data | No data | 48 |
| Cereal straw | Barley, wheat | 12 | 53 | 40 | No data |
| Animal products | |||||
| Milk | 18 | No data | No data | No data | |
| Eggs | 12 | No data | No data | No data | |
| Liver | 12 | No data | No data | No data | |
| Muscle | 12 | No data | No data | No data | |
| Fat | 12 | No data | No data | No data | |
TA: triazole alanine; TAA: triazole acetic acid; TLA: triazole lactic acid.
From the submitted storage stability data, it can be concluded that the residue trials analysing TA, TAA and TLA residues in high water‐, high oil‐, high protein‐ and high starch content commodities were supported by acceptable storage stability data on these compounds, except for TA (raspberries, peas, rapeseeds) and TAA (raspberries). The residue trials analysed 1,2,4‐T residues in most of the crops within a time interval for which acceptable storage stability of this compound could not be demonstrated, except for stone fruit, stem vegetables, soya beans and oats grain. Storage stability data were not provided and are required for 1,2,4‐T, TA and TAA in high acid‐content commodities, for 1,2,4‐triazole in high protein‐content commodities and for TLA in cereal straw to cover the maximum storage time interval of all residue trials in primary and rotational crops (data gap). For products of animal origin, the available storage stability data demonstrated acceptable freezer storage stability of 1,2,4‐T in milk for 18 months and in eggs, liver, muscle and fat for 12 months. Additional storage stability data analysing for the residues of TA and TAA in milk and eggs were also provided but were not considered as acceptable since the homogenised samples of milk and eggs were fortified with a mixture of TA and TAA and not with the individual compound, respectively.
The compilation of the poultry and ruminant metabolism studies conducted with the triazole pesticide active substances with the 14C labelling on the triazole moiety showed that besides the parent compound that was detected in significant proportions in all animal matrices ranging between 27% and 81% TRR in milk, eggs and tissues, 1,2,4‐T was also found to be a predominant compound of the total residues with levels ranging from 31% to 86% TRR in those matrices. TA was identified at very low levels in poultry muscle only (< 10% TRR) and at levels between 22% and 39% TRR in ruminant matrices.
Since TA is a major component in feed items, the potential transfer of this compound in poultry and ruminant matrices was further investigated in a metabolism study conducted with 14C‐TA. TA remains the major compound of the total residues in all poultry matrices (84–97.2% TRR) and in ruminant tissues (56–76% TRR) while TA and 1,2,4‐T accounted for 8% and 86% TRR, respectively, in milk. TLA and TAA were detected in very low levels in all matrices (< 1% TRR). The potential transfer of TAA, TLA and 1,2,4‐T present in feed items to the animal matrices was not further investigated. Although there are indications from the ruminant metabolism study conducted with the 14C‐TA, that there is no accumulation of TAA and TLA (4.2% and < 1% of the total administered dose in urine, respectively), these metabolites were however detected in the ruminant matrices from the feeding study conducted with TA. Based on the metabolism studies conducted, respectively, with triazole pesticide active substances and TA and considering the results of the livestock feeding studies carried out with TA and TAA, respectively, the experts agreed on the following residue definitions (Table 4).
Table 4.
Agreed residue definitions for triazole pesticide active substances in animal matrices
| Animal commodities | |
| RD for enforcement: | Triazole parent compound only |
| RDs for risk assessment: |
|
RD: residue definition; TA: triazole alanine; TAA: triazole acetic acid; TLA: triazole lactic acid.
The livestock dietary burden calculation has been performed respectively for each TDM compound and triggered livestock feeding studies for 1,2,4‐T, TA, TAA and TLA, see chapter B.7.4 of the addendum (United Kingdom, 2015, 2018). Poultry and ruminants feeding studies were conducted respectively with TA and TAA and analysed for the magnitude of TA, TAA, 1,2,4‐T and TLA residues. The poultry feeding study conducted with TA showed that TA remained predominant in all matrices and a slight metabolisation to 1,2,4‐T in whole eggs, liver and muscle at the highest dosing level was noted. When the animals were fed with TAA, this compound was detected in eggs, fat and liver with residues of TA in liver only at all dosing levels. From the ruminant feeding study conducted with TA, TA remained predominant in all tissues but with a significant metabolisation of TA into 1,2,4‐T in milk and to a minor extent into 1,2,4‐T and TAA in tissues. TLA was identified in fat only but its detection was rather attributed to a contamination as the respective levels were independent from the dosing levels. When ruminants were fed with TAA, this metabolite was only detected at the highest dose level in whole milk and in all tissues whilst TA was identified in liver, muscle and kidney at all the dosing levels. 1,2,4‐T and TLA compounds were never detected (< 0.01 mg/kg). Animal tissues, milk and eggs samples were analysed within 30 days of sampling.
Since livestock feeding studies were not conducted to address the potential transfer of 1,2,4‐T and TLA in products of animal origin, the experts agreed that transfer factors for TA derived from the feeding studies conducted with TA should be applied to 1,2,4‐T, assuming that the absorption and excretion behaviour of TA and 1,2,4‐T are similar. Similarly transfer factors for TAA derived from the feeding studies conducted with TAA should be applied to TLA assuming that the absorption and excretion behaviour of TAA and TLA are comparable and because of the similarity of the functional groups. From the available toxicological studies, the absorption and excretion of TA, 1,2,4‐T and TAA were shown to be similar and the experts agreed to estimate the 1,2,4‐T residue levels in animal matrices by applying transfer factors for TA derived from the feeding study conducted with TA. A feeding study conducted with 1,2,4‐T is therefore not required as no further metabolism of this compound in animal matrices is expected. In contrast and since a similar absorption and excretion behaviour of TLA compared to the other TDMs could not be demonstrated, livestock feeding studies conducted with TLA or metabolism studies performed in accordance with the current recommendations as a surrogate to these feeding studies should be provided (data gap). Meanwhile and provisionally, transfer factors for TAA derived from the feeding study conducted with TAA were applied to estimate the residue levels of TLA in animal commodities. The magnitude of residues of each TDM in animal matrices were therefore estimated by using the approach of a separate dietary burden calculation for each TDM and the application of transfer factors respectively to 1,2,4‐T and to TLA for which feeding studies are not available.
Furthermore, the residues of the TDMs (mainly 1,2,4‐T and to a minor extent, TA) arising from the metabolism of triazole pesticide active substances in livestock should also be considered to derive the total residue levels of the individual TDMs in animal matrices. In the framework of these confirmatory data assessments and since feeding studies conducted with the triazole compounds were not available, the residue levels of 1,2,4‐T and TA were estimated from the metabolism studies conducted with the triazole compounds when these were available. For any future assessment of triazole pesticide active substances, livestock feeding studies or, alternatively metabolism studies should be conducted with the triazole compounds to carry out a complete livestock exposure assessment.
The ‘worst‐case’ consumer dietary intake assessment with regard to the TDMs for the complete group of triazole active substances that were assessed in the framework of these confirmatory data has been conducted by the RMS using the EFSA PRIMo rev.3 and by EFSA using the EFSA PRIMo rev.2A since PRIMo rev.3 is not applicable in the framework of confirmatory data assessed here.
The chronic and acute dietary intakes have been carried out using the highest input residue values for risk assessment (STMR values and the HR values), derived for each TDM for each crop groups and each product of animal origin. Since in most of the residue trials in primary and rotational crops, higher residue levels of the TDMs in the control samples were observed, these levels were also considered in the dietary intake calculation. Using the EFSA PRIMo rev.3, the IEDI accounted for 93% of the ADI (NL toddler) for 1,2,4‐T, 6% of the ADI (NL toddler) for TA, 1% of the ADI (NL toddler) for TAA and 1% of the ADI (NL toddler) for TLA. No acute intake concern was identified as the calculated international estimated short‐term intake (IESTI) accounted for up to 40% of the ARfD (cattle milk) for 1,2,4‐T, 28% of the ARfD (oranges) for TA, 1% of the ARfD (oranges) for TAA and 7% of the ARfD (potatoes) for TLA. Using the EFSA PRIMo rev.2A, the IEDI accounted for 60% of the ADI (FR toddler) for 1,2,4‐T, 5% of the ADI (WHO Cluster diet B) for TA, 1% of the ADI (WHO Cluster diet B) for TAA and < 1% of the ADI (FR toddler) for TLA. The acute intake was estimated to be 40% of the ARfD (milk) for 1,2,4‐T, 28% of the ARfD (oranges) for TA, 1% of the ARfD (oranges) for TAA and 6.7% of the ARfD (potatoes) for TLA. Since the toxicological reference values for TLA were derived by bridging with the reference values of TA, a combined dietary risk assessment for TA and TLA was performed. No chronic or acute intake concerns were identified with up to 6% ADI (WHO Cluster diet B), and 34% and 8% ARfD (watermelons) respectively for children and adults.
Summary for the TDMs
The overall consumer exposure assessment to the triazole derivative metabolites is affected by the following uncertainties: absence of storage stability data for some of the TDMs in several crop categories (see data gaps identified) and lack of residue trials analysing 1,2,4‐T residues in most of the crops and supported by acceptable storage stability data, outstanding data to finalise the livestock exposure assessment (see data gap identified), the need for residue trials conducted in compliance with the European authorised uses and analysing all the TDMs in order to conduct a realistic consumer dietary risk assessment. Further consideration should also be given to the TDMs residue levels that may arise when several different triazole compounds are applied on a crop within the same growing season and from treatments with triazole compounds that occurred during the previous seasons. It is therefore recommended that a separate monitoring programme including 1,2,4‐T, TA, TAA and TLA compounds should be established for products of plant and animal origin to have the background residue levels of these compounds resulting from the current and past uses of the triazole active substances.
2.2. Individual triazole active substances
For the triazole pesticide active substances cyproconazole, difenoconazole, epoxiconazole, fenbuconazole, myclobutanil, paclobutrazol and prothioconazole, the approval has been conditioned by the submission of confirmatory data regarding the consumer dietary exposure to the TDMs. Under this section, the confirmatory data requirements set as a condition for the approval of each of these active substances have been assessed in the light of the confirmatory data on the TDMs evaluated in this conclusion. The consumer exposure assessment with regard to each of these active substances has been undertaken individually as requested in the mandate.
Cyproconazole
Confirmatory data were required to address the residues of TDMs in primary crops, rotational crops and products of animal origin in order to perform the dietary exposure of consumers to the residues of TDMs. The requirements for a rotational crop metabolism study labelled on the triazole moiety and the nature of TDMs under standard hydrolysis conditions at processing have been fulfilled in the framework of the confirmatory data assessment.
Residue trials analysing all the TDMs in compliance with the representative use on wheat were provided but are not supported by acceptable storage stability data for 1,2,4‐T in grain/straw and for TLA in straw. Sufficient residue trials on wheat analysing all the TDMs and supported by acceptable storage stability data are therefore required (data gap).
A ruminant metabolism study labelled on the triazole ring has not been submitted. However, since residue definitions for monitoring and risk assessment in livestock have been derived based on the compiled metabolism data, this data gap is considered only as formally not addressed for cyproconazole. A feeding study conducted with cyproconazole to determine the magnitude of TDMs (mainly 1,2,4‐T and to a minor extent, TA) in animal commodities was however not submitted and is required (data gap).
The livestock exposure assessment and the overall consumer exposure assessment cannot be finalised pending the outstanding data to address the residue levels of the TDMs arising in animal matrices from the parent cyproconazole and from each of the TDMs and residue trials compliant with the representative use on wheat and supported by acceptable storage stability data for all TDMs. The confirmatory data requirements are not fully addressed.
Paclobutrazol
Confirmatory data were required to address the residues of TDMs in primary crops, rotational crops and products of animal origin In the framework of the confirmatory data, the metabolism of the triazole moiety of paclobutrazol was investigated in pulses and oilseeds and in rotational crops. Livestock metabolism studies labelled on the triazole ring have not been submitted. However, since residue definitions for monitoring and risk assessment in livestock have been derived based on the compiled metabolism data, this data gap is considered only as formally not addressed for paclobutrazol. Sufficient residue trials analysing for all the TDMs and compliant with the representative use on oilseed rape were submitted but are not supported by acceptable storage stability data for 1,2,4‐T and for TA, respectively. Additional residue trials on rapeseeds and supported by acceptable storage stability data for 1,2,4‐T and for TA are required (data gap). Field trials to address the residue levels of the TDMs in rotational crops are also not available (data gap). It will be reconsidered upon the submission of the requested primary crops and rotational crops field trials whether a livestock exposure assessment to the TDM residues is triggered. In the meantime, the consumer exposure assessment cannot be finalised. The confirmatory data requirements are not fully addressed.
Fenbuconazole
Confirmatory data were required to address the residues of TDMs in primary crops, rotational crops and products of animal origin in order to perform the dietary exposure of consumers to the residues of TDMs. In the framework of the confirmatory data, the metabolism of the triazole moiety of fenbuconazole was investigated in primary crops (cereals, peaches and peanuts), rotational crops and in poultry and ruminants matrices. However, and since residue trials were not provided to address the magnitude of the different TDMs in compliance with the representative uses on apples, grapes and wheat (data gap), the livestock dietary burden calculation and the consumer exposure assessment could not be performed. The confirmatory data requirements are not fully addressed.
Myclobutanil
Confirmatory data were required on the residues of myclobutanil and its metabolites in following growing seasons and information confirming that the available residue data cover all compounds of the residue definition as it cannot be excluded that 1,2,4‐T will be formed long term from continuous use of myclobutanil. There may be uptake of this compound and of other TDMs. These data were not provided in the framework of the confirmatory data as the available residue trials for myclobutanil on grapes were not compliant with the representative use on grapes and reflect only the single use of myclobutanil within the growing season. The consumer exposure assessment cannot be finalised. The confirmatory data requirement is not fully addressed.
Difenoconazole
Confirmatory data were required to address the residues of TDMs in primary crops, rotational crops, processed commodities and products of animal origin. In the framework of the assessment of the confirmatory data, the metabolism of the triazole moiety of difenoconazole was investigated in primary crops (fruit, cereals, pulses and oilseeds and root crops), in rotational crops and in poultry and ruminants matrices. The nature of the TDMs under the standard hydrolysis conditions at processing was also investigated. Sufficient residue trials analysing all TDMs on apples compliant with the northern Europe (NEU) and southern Europe (SEU) GAPs on pome fruit are available. Sufficient residue trials on wheat compliant with the SEU GAP (seed treatment) on cereals with extrapolation to barley, triticale, rye and oats are available. Residue trials compliant respectively with the NEU GAP on cereals (seed treatment) and with the NEU/SEU GAPs on carrots (foliar treatment), however, were not submitted and are required (data gap). Rotational crop field trials on cereals small grain, carrots and lettuces were submitted for the determination of all the TDMs at different plant back intervals. The maximum storage time interval of the residue samples of the trials in primary and rotational crops, however, was not provided and is required to conclude on the validity of these trials (data gap). The livestock dietary burden calculation and the overall consumer exposure assessment cannot be finalised. The confirmatory data requirements are not fully addressed.
Epoxiconazole
Confirmatory data were required to address the residues of TDMs in primary crops, rotational crops and products of animal origin in order to perform the dietary exposure of consumers to the residues of TDMs. In the framework of the assessment of the confirmatory data, the metabolism of the triazole moiety of epoxiconazole was investigated in primary crops (cereals), rotational crops and in ruminants. Residue trials analysing all TDMs and compliant respectively with the representative uses on cereals (wheat, rye, barley, oats, triticale, spelt) and on sugar beet are available. Rotational crops residue field trials were also submitted. These residue trials are however not supported by acceptable storage stability data for 1,2,4‐T in wheat grain/straw and sugar beet root and for TLA in straw. Sufficient residue trials in primary and rotational crops and supported by acceptable storage stability data on TDMs are therefore required (data gap). Although a poultry metabolism study labelled on the triazole ring of the parent triazole was not provided, residue definitions for monitoring and risk assessment in livestock have been derived based on the compiled metabolism data and the data gap for a poultry metabolism study is therefore formally not addressed for epoxiconazole. The livestock exposure assessment cannot be finalised with regard to the outstanding data on the residue levels of the TDMs in primary and rotational crops and for a poultry feeding study to determine the residue levels of the TDMs (mainly 1,2,4‐T and to a minor extent, TA) arising in animal matrices from the parent epoxiconazole (data gap). The overall consumer exposure assessment could therefore not be finalised. The confirmatory data requirements are not fully addressed.
Prothioconazole
Confirmatory data were required to address the residues of TDMs in primary crops, rotational crops and products of animal origin in order to perform the dietary exposure of consumers to the residues of TDMs. The metabolism of the triazole moiety of prothioconazole was not investigated in primary crops, rotational crops and in processed commodities. However, since residue definitions for monitoring and risk assessment in plants and animals have been derived based on the compiled metabolism data, these data gaps are considered only as formally not addressed for prothioconazole. Ruminant and poultry metabolism studies labelled on the triazole ring are available. Residue trials analysing for all TDMs and compliant with the representative uses on cereals (wheat, rye, barley, oats, triticale) and on rapeseeds together with rotational crops residue field trials were submitted in the framework of this confirmatory data assessment but were not supported by acceptable storage stability data for 1,2,4‐T in cereal grain, straw and rapeseeds and for TLA in straw. Sufficient residue trials in primary and rotational crops and supported by acceptable storage stability data are therefore required (data gap). The livestock exposure assessment therefore cannot be finalised with regard to the outstanding data for acceptable residue trials in primary and rotational crops. The overall consumer exposure assessment could not be performed. The confirmatory data requirements are not fully addressed.
Although confirmatory data have been requested for the active substances cyproconazole, paclobutrazol, fenbuconazole, myclobutanil, difenoconazole, epoxiconazole and prothiaconazole, the submitted data were not considered sufficient to finalise the consumer risk assessment, which therefore, is inconclusive for all of these active substances.
3. Data gaps
Data gaps relevant for the risk assessment to cover the complete group of triazole derivate metabolites
-
1
Storage stability data on 1,2,4‐T, TA and TAA in high acid content commodities, on 1,2,4‐T in high protein content commodities and on TLA in cereal straw and covering the maximum storage time interval of the residue samples of the residue trials in primary and rotational crops.
-
2
Poultry and ruminant feeding studies conducted with TLA or, alternatively, metabolism studies performed in accordance with the current recommendations as a surrogate to these feeding studies to determine the magnitude of TLA residues in products of animal origin.
For the triazole pesticide active substances cyproconazole, difenoconazole, epoxiconazole, fenbuconazole, myclobutanil, paclobutrazol and prothioconazole, data gaps have been identified in the framework of their approval procedure and confirmatory data have been requested. These data requirements have not been addressed entirely and are confirmed here below for each of the substances:
-
3
A feeding study conducted with cyproconazole to determine the magnitude of TDMs (mainly 1,2,4‐T and to a minor extent, TA) in animal commodities (cyproconazole).
-
4
Sufficient residue trials analysing for all the TDMs in compliance with the representative use on wheat and supported by acceptable storage stability on 1,2,4‐T in cereal grain/straw and on TLA in straw (cyproconazole).
-
5
Sufficient residue trials analysing for all the TDMs compliant with the representative use on oilseed rapeseeds and supported by acceptable storage stability data for 1,2,4‐T and for TA (paclobutrazol).
-
6
Rotational crops field residue trials to address the residue levels of the TDMs (paclobutrazol).
-
7
Residue trials to address the magnitude of the TDMs in compliance with the representative uses on apples, grapes and wheat (fenbuconazole).
-
8
The consumer exposure data to triazole metabolites from grapes in following growing seasons as it cannot be excluded that 1,2,4‐T will be formed long term from continuous use of myclobutanil (myclobutanil).
-
9
Sufficient residue trials analysing for all TDMs and compliant respectively with the NEU GAP on cereals (seed treatment) and the NEU/SEU GAPs on carrots (foliar treatment) (difenoconazole).
-
10
The maximum storage time interval of the residue samples of the residue trials analysing for all TDMs in primary and rotational crops (difenoconazole).
-
11
Sufficient residue trials analysing for all TDMs and compliant respectively with the representative use on cereals (wheat, rye, barley, oats, triticale, spelt) and on sugar beet and supported by acceptable storage stability data on TDMs (epoxiconazole).
-
12
Rotational crops residue field trials supported by acceptable storage stability data on TDMs (epoxiconazole).
-
13
A poultry feeding study to determine the residue levels of the TDMs (mainly 1,2,4‐T and to a minor extent, TA) arising in animal matrices from the parent epoxiconazole (epoxiconazole).
-
14
Residue trials analysing for all TDMs and compliant with the representative use on cereals (wheat, rye, barley, oats, triticale) and on oilseed rapeseeds and supported by acceptable storage stability data on TDMs (prothioconazole).
-
15
Rotational crops field residue trials supported by acceptable storage stability data on TDMs (prothioconazole).
4. Recommendations
Residue trials analysing all TDMs in compliance with the European authorised uses should be provided in order to conduct a realistic consumer dietary risk assessment.
A separate monitoring programme including all the TDMs should be established to have information on the background levels of these TDMs in products of plant and animal origin and resulting from the current and previous uses of the triazole active substances.
For any future assessment of triazole pesticide active substances, livestock feeding studies or, alternatively metabolism studies should be conducted with the triazole active substances to carry out a complete livestock exposure assessment.
5. Concerns
5.1. Issues that could not be finalised
An issue is listed as an issue that could not be finalised where there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the Uniform Principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/20116, and where the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
The overall consumer exposure assessment to the triazole derivative metabolites could not be finalised as the validity of the residue trials in primary and rotational crops could not be concluded on in relation to the storage stability data on TDMs. Due to the outstanding poultry and ruminant feeding studies with TLA, or alternatively metabolism studies that can be used as waivers for feeding studies, the livestock exposure assessment could not be finalised.
Although confirmatory data have been requested for the active substances cyproconazole, paclobutrazol, fenbuconazole, myclobutanil, difenoconazole, epoxiconazole and prothiaconazole, the submitted data were not considered sufficient to fully address the confirmatory data requirements and to finalise the consumer risk assessment, which therefore, is inconclusive for all of these active substances.
5.2. Critical areas of concern
An issue is listed as a critical area of concern where there is enough information available to perform an assessment for the representative uses in line with the Uniform Principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and where this assessment does not permit to conclude that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern where the assessment at the higher tier level could not be finalised due to lack of information, and where the assessment performed at the lower tier level does not permit to conclude that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater or any unacceptable influence on the environment.
No critical areas of concern have been identified.
Abbreviations
- ADI
acceptable daily intake
- ARfD
acute reference dose
- DAR
draft assessment report
- DNT
developmental neurotoxicity
- GAP
Good Agricultural Practice
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- IUPAC
International Union of Pure and Applied Chemistry
- LOAEL
lowest‐observed‐adverse‐effect level
- LOQ
limit of quantification (determination)
- NEU
northern Europe
- NOAEL
no‐observed‐adverse‐effect‐level
- PRIMo
Pesticide Residue Intake Model
- RMS
rapporteur Member State
- SEU
southern Europe
- STMR
supervised trials median residue
- 1,2,4‐T
1,2,4‐triazole
- TA
triazole alanine
- TAA
triazole acetic acid
- TDM
triazole derivative metabolite
- TDMG
Triazole Derivative Metabolite Group
- TLA
triazole lactic acid
- TRR
total radioactive residue
- UF
uncertainty factor
- WHO
World Health Organization
Appendix A – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|
|
1,2,4‐triazole 1,2,4‐T |
1H‐1,2,4‐triazole c1ncnn1 NSPMIYGKQJPBQR‐UHFFFAOYSA‐N |
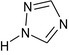
|
|
Triazole alanine TA |
3‐(1H‐1,2,4‐triazol‐1‐yl)‐D,L‐alanine NC(Cn1cncn1)C(=O)O XVWFTOJHOHJIMQ‐UHFFFAOYSA‐N |
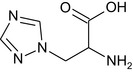
|
|
Triazole acetic acid TAA |
1H‐1,2,4‐triazol‐1‐ylacetic acid O=C(O)Cn1cncn1 RXDBSQXFIWBJSR‐UHFFFAOYSA‐N |
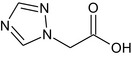
|
|
Triazole lactic acid or Triazole hydroxy propionic acid TLA |
(2RS)‐2‐hydroxy‐3‐(1H‐1,2,4‐triazol‐1‐yl)propanoic acid OC(Cn1cncn1)C(=O)O KJRGHGWETVMENC‐UHFFFAOYSA‐N |
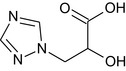
|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2015 ACD/Labs 2015 Release (File version N20E41, Build 75170, 19 December 2014).
ACD/ChemSketch 2015 ACD/Labs 2015 Release (File version C10H41, Build 75059, 17 December 2014).
Suggested citation: EFSA (European Food Safety Authority) , Brancato A, Brocca D, Carrasco Cabrera L, Chiusolo A, Civitella C, Court Marques D, Crivellente F, De Lentdecker C, Erdös Z, Ferreira L, Goumenou M, Greco L, Istace F, Jarrah S, Kardassi D, Leuschner R, Medina P, Mineo D, Miron I, Molnar T, Nave S, Parra Morte JM, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Terron A, Theobald A, Vagenende B and Villamar‐Bouza L, 2018. Conclusion on the peer review of the pesticide risk assessment for the triazole derivative metabolites in light of confirmatory data submitted. EFSA Journal 2018;16(7):5376, 20 pp. 10.2903/j.efsa.2018.5376
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00529
Erratum: A correction of the following conclusion was needed due to the absence of data on the storage stability of 1,2,4‐triazole in eggs. Indeed, the Table 3 in part 2.1 indicated “no data” when a value was available. The descriptive paragraph following the table was amended to match the updated table and some of the text was adapted accordingly.
Approved: 29 June 2018
Amended: 17 April 2019
Notes
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32. Repealed by Regulation (EC) No 1107/2009.
Myclobutanil, cyproconazole, difenoconazole, fenbuconazole, penconazole, metconazole, propiconazole, bromuconazole, epoxiconazole, fluquinconazole, flutriafol, tebuconazole, tetraconazole, ipconazole, triticonazole, paclobutrazole.
Epoxiconazole, penconazole, tebuconazole, fenbuconazole, flutriafol, paclobutrazole, metconazole, fluquiconazole, difenoconazole, tetraconazole, propiconazole, ipconazole.
Epoxiconazole, metconazole, triticonazole, prothioconazole, prothioconazole‐desthio, tebuconazole, fenbuconazole, tetraconazole, propiconazole, difenoconazole, fluquinconazole.
Metconazole, prothioconazole, tebuconazole, fenbuconazole, propiconazole, difenoconazole.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- Belgium , 2004. Draft Assessment Report (DAR) on the active substance metconazole prepared by the rapporteur Member State Belgium in the framework of Council Directive 91/414/EEC, January 2004. Available online: www.efsa.europa.eu
- Belgium , 2009a. Draft Assessment Report (DAR) on the active substance bromuconazole prepared by the rapporteur Member State Belgium in the framework of Council Directive 91/414/EEC, October 2009. Available online: www.efsa.europa.eu
- Belgium , 2009b. Revised Draft Assessment Report (DAR) on the active substance myclobutanil prepared by the rapporteur Member State Belgium in the framework of Council Directive 91/414/EEC, October 2009. Available online: www.efsa.europa.eu
- Denmark , 2007. Draft Assessment Report (DAR) on the active substance tebuconazole prepared by the rapporteur Member State Denmark in the framework of Council Directive 91/414/EEC, February 2007. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2016. Technical report on the outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for triazole derivative metabolites in light of confirmatory data. EFSA supporting publication 2016:EN‐1080, 90 pp. 10.2903/sp.efsa.2016.EN-1080 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2018. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the triazole derivative metabolites. Available online: www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- European Commission , 2013. Guidance document on the procedures for submission and assessment of confirmatory information following approval of an active substance in accordance with Regulation (EC) No 1107/2009. SANCO 5634/2009‐rev. 6.1.
- Finland , 1998. Draft Assessment Report (DAR) on the active substance propiconazole prepared by the rapporteur Member State Finland in the framework of Council Directive 91/414/EEC, October 1998. Available online: www.efsa.europa.eu
- Germany , 2007. Draft Assessment Report (DAR) on the active substance penconazole prepared by the rapporteur Member State Germany in the framework of Council Directive 91/414/EEC, October 2007. Available online: www.efsa.europa.eu
- Germany , 2008. Draft Assessment Report (DAR) on the active substance ipconazole prepared by the rapporteur Member State Germany in the framework of Council Directive 91/414/EEC, May 2008. Available online: www.efsa.europa.eu
- Ireland , 2000. Draft Assessment Report (DAR) on the active substance flusilazole prepared by the rapporteur Member State Ireland in the framework of Council Directive 91/414/EEC, October 2000. Available online: www.efsa.europa.eu
- Ireland , 2005. Draft Assessment Report (DAR) on the active substance fluquinconazole prepared by the rapporteur Member State Ireland in the framework of Council Directive 91/414/EEC, February 2005. Available online: www.efsa.europa.eu
- Ireland , 2010. Draft Assessment Report (DAR) on the active substance cyproconazole prepared by the rapporteur Member State Ireland in the framework of Council Directive 91/414/EEC, February 2010. Available online: www.efsa.europa.eu
- Spain , 2007. Draft Assessment Report (DAR) on the active substance tetraconazole prepared by the rapporteur Member State Spain in the framework of Council Directive 91/414/EEC, February 2007. Available online: www.efsa.europa.eu
- Sweden , 2007. Draft Assessment Report (DAR) on the active substance difenoconazole prepared by the rapporteur Member State Sweden in the framework of Council Directive 91/414/EEC, May 2006. Available online: www.efsa.europa.eu
- United Kingdom , 2005a. Draft Assessment Report (DAR) on the active substance bitertanol prepared by the rapporteur Member State the United Kingdom in the framework of Council Directive 91/414/EEC, March 2005. Available online: www.efsa.europa.eu
- United Kingdom , 2005b. Draft Assessment Report (DAR) on the active substance fenbuconazole prepared by the rapporteur Member State the United Kingdom in the framework of Council Directive 91/414/EEC, December 2005. Available online: www.efsa.europa.eu
- United Kingdom , 2006a. Draft Assessment Report (DAR) on the active substance flutriafol prepared by the rapporteur Member State the United Kingdom in the framework of Council Directive 91/414/EEC, May 2006. Available online: www.efsa.europa.eu
- United Kingdom , 2006b. Draft Assessment Report (DAR) on the active substance paclobutrazol prepared by the rapporteur Member State the United Kingdom in the framework of Council Directive 91/414/EEC, September 2006. Available online: www.efsa.europa.eu
- United Kingdom , 2008. Draft Assessment Report (DAR) on the active substance triticonazole prepared by the rapporteur Member State the United Kingdom in the framework of Council Directive 91/414/EEC, September 2008. Available online: www.efsa.europa.eu
- United Kingdom , 2015. Triazole Derivate Metabolites, addendum – confirmatory data prepared by the rapporteur Member State, the United Kingdom in the framework of Regulation (EC) No 1107/2009, revised version of 2015. Available online: www.efsa.europa.eu
- United Kingdom , 2018. Triazole Derivate Metabolites, addendum – confirmatory data prepared by the rapporteur Member State, the United Kingdom in the framework of Regulation (EC) No 1107/2009, revised version of February 2018. Available online: www.efsa.europa.eu


