Abstract
In compliance with Article 43 of Regulation (EC) No 396/2005, EFSA received from the European Commission a mandate to provide its reasoned opinion on the existing maximum residue levels (MRLs) for acetamiprid which might lead to consumers intake concerns on the basis of the new toxicological reference values agreed upon by Member States (MSs) in October 2017. In order to identify the MRLs of potential concern that require a more detailed assessment, EFSA performed a preliminary risk assessment, identifying a risk for consumers for 12 commodities. Measures for reduction of the consumer exposure were assessed by EFSA and should be considered by risk managers. Furthermore, in accordance with Article 6 of Regulation (EC) No 396/2005, ADAMA Makhteshim Ltd submitted two requests to modify the existing MRL for acetamiprid in table olives, olives for oil production, barley and oats. The data submitted in support of the requests were found to be sufficient to derive MRL proposals for all crops under assessment. Based on the risk assessment results, EFSA concluded that the short‐term and long‐term intake of residues resulting from the use of acetamiprid according to the intended agricultural practices on table olives, olives for oil production, barley and oats is unlikely to present a risk to consumer health.
Keywords: Acetamiprid, MRL, Regulation (EC) No 396/2005, consumer risk assessment, neonicotinoid, insecticide
Summary
Acetamiprid was firstly included in Annex I to Directive 91/414/EEC on 1 January 2005 by Commission Directive 2004/99/EC. After the first approval, the European Food Safety Authority (EFSA) published several reasoned opinions on the modifications of the existing maximum residue levels (MRLs), including the assessment of the all existing MRLs in compliance with Article 12(2) of Regulation (EC) No 396/2005.
Acetamiprid was evaluated for renewal of approval in the framework of Commission Regulation (EC) No 1107/2009 and the toxicological reference values for the substance were lowered.
EFSA therefore received on 16 October 2017, a mandate from the European Commission in accordance with Article 43 of Regulation (EC) No 396/2005 to perform a focussed review of the existing MRLs for acetamiprid taking into consideration the new toxicological reference values as noted by the Standing Committee on Plants, Animals, Food and Feed and, in case of consumer intake concerns, to derive fall‐back MRLs that would not lead to unacceptable risk for consumers.
Furthermore, in accordance with Article 6 of Regulation (EC) No 396/2005, the evaluating Member States (EMSs), Italy and Poland, received two applications from the company ADAMA Makhteshim Ltd to modify the existing MRLs for acetamiprid in table olives, olives for oil production, barley and oats. EMSs drafted evaluation reports in accordance with Article 8 of Regulation (EC) No 396/2005, which were submitted to the European Commission and forwarded to EFSA. For reasons of efficiency, EFSA assessed also these applications in this reasoned opinion.
Subsequent to the request from the European Commission, EFSA performed a preliminary risk assessment of the existing EU MRLs for acetamiprid and for 12 plant commodities (scarole, apples, spinaches, pears, lettuce, kale, celery, peaches, beet leaves (chard), purslane, Chinese cabbage and head cabbage) an acute consumer intake concerns could not be excluded. Therefore, EFSA asked Member States (MSs) to provide fall‐back good agricultural practices (GAPs) with supporting residue data for those commodities for which the existing MRL leads to a potential acute intake concern.
For this assessment, EFSA mainly relied on its previous reasoned opinions, its conclusion on the peer review and the evaluation reports prepared by the EMSs in accordance with Article 8 of Regulation (EC) No 396/2005. The additional information provided by the MSs during the MS consultation was also considered.
The following conclusions are derived.
The residue data which were submitted by the MSs in support of the fall‐back GAPs were sufficient to derive fall‐back MRLs safe for consumers for all commodities possibly of concern, except for celery, kale and Chinese cabbages for which no fall‐back data were available. Nevertheless, the fall‐back MRL derived for escaroles is only tentative and needs to be confirmed by the following data:
full data set supporting the southern outdoor fall‐back GAP for escarole and the confirmation that trials were performed on open leaf varieties.
Furthermore, it is highlighted that some of the MRLs derived result from a GAP in one climatic zone only, whereas other GAPs reported by the RMS were not fully supported by data. EFSA therefore identified the following data gaps which are not expected to impact the validity of the fall‐back MRLs derived but which might have an impact on national authorisations:
full data set compliant with the southern outdoor fall‐back GAP for head cabbages with residue analysed in the whole plant after removal of roots and decayed leaves in line with the Annex I of Regulation 396/2005;
full data set supporting the northern outdoor fall‐back GAP for escarole;
full data set supporting the southern outdoor fall‐back GAPs for spinaches and chards and the confirmation that trials were performed on open leaf varieties.
full data sets supporting the southern outdoor and the indoor fall‐back GAPs for celeries.
If the above‐reported data gaps are not addressed in the future, MSs are recommended to withdraw or modify the relevant authorisations at national level.
MSs are in any case recommended to withdraw their national authorisations for celeries, kales and Chinese cabbages where no fall‐back MRL could be derived by EFSA. For apples, pears, peaches, head cabbages, lettuce, escaroles, spinaches, chards and purslanes, EFSA recommends that the national authorisations are being modified in order to comply with the fall‐back MRLs derived by EFSA.
In the framework of this assessment, it can be concluded that there is no need to modify the existing European Union (EU) MRLs for commodities of animal origin. Nevertheless, it is noted that additional analytical methods for enforcement in animal commodities addressing the data gap identified during the MRL review and currently reflected in the EU legislation were evaluated during the peer review for the renewal.
Furthermore, EFSA concludes that available data were sufficient to derive MRL proposals accommodating the intended uses on table olives, olives for oil production, barley and oats. According to the results of the risk assessment, these intended uses are unlikely to pose a health risk for consumers.
Background
Acetamiprid was firstly included in Annex I to Directive 91/414/EEC1 on 1 January 2005 by Commission Directive 2004/99/EC2. After the first approval, the European Food Safety Authority (EFSA) published several reasoned opinions on the modifications of the existing maximum residue levels (MRLs), including the assessment of the all existing MRLs in compliance with Article 12(2) of Regulation (EC) No 396/20053 (EFSA, 2011, 2012, 2013, 2014, 2015, 2016a).
Acetamiprid was evaluated for renewal of approval in the framework of Commission Regulation (EC) No 1107/2009. In 17 October 2016 EFSA published its conclusion on the peer review of the pesticide risk assessment of the active substance acetamiprid (EFSA, 2016b) and concluded on lower toxicological reference values (TRV) (acceptable daily intake (ADI) and acute reference dose (ARfD)). The lower TRV were agreed in the Standing Committee on Plant, Animal, Food and Feed in October 2017 (European Commission, 2017b).
On 16 October 2017, in accordance with Article 43 of Regulation (EC) No 396/2005, the European Commission requested EFSA to perform a focussed review of the existing MRLs for acetamiprid taking into consideration the new TRV and to derive fall‐back MRLs that would not lead to unacceptable risk for consumers.
To address the request from the European Commission, on 20 November 2017 EFSA asked Member States (MSs) to provide fall‐back good agricultural practice (GAP) with supporting residue data for those commodities (scarole, apples, spinaches, pears, lettuce, kale, celery, peaches, beet leaves (chard), purslane, Chinese cabbage and head cabbage) for which the existing MRL leads to potential acute intake concerns.
All fall‐back data received by 18 January 2018 were evaluated and considered by EFSA during the finalisation of the reasoned opinion.
Furthermore, in accordance with Article 6 of Regulation (EC) No 396/2005, the evaluating Member States (EMSs) Italy and Poland, received two applications from the company ADAMA Makhteshim Ltd to raise the existing MRLs for acetamiprid in table olives, olives for oil production, barley and oats. EMSs drafted two evaluation reports in accordance with Article 8 of Regulation (EC) No 396/2005, which were submitted to the European Commission and forwarded to EFSA. For reasons of efficiency, both applications were also assessed in this reasoned opinion.
The active substance and its use pattern
Acetamiprid is the ISO common name for (E)‐N1‐[(6‐chloro‐3‐pyridyl)methyl]‐N2‐cyano‐N1‐ methylacetamidine (IUPAC).
Acetamiprid belongs to the group of neonicotinoids compounds which are used as insecticides. It is used by foliar application to control a range of herbivorous insect Hemiptera, Thysanoptera, Lepidoptera and Coleoptera, both outdoor and indoor. Acetamiprid affects the nicotinic acetylcholine receptor, impacting the synapses in the insect central nervous system.
The chemical structure of the active substance and its main metabolites are reported in Appendix E.
Acetamiprid was evaluated in the framework of Directive 91/414/EEC with Greece designated as rapporteur Member State (RMS). The representative uses evaluated in the first peer review were foliar applications on various fruit crops, cotton and tobacco. Acetamiprid has been recently peer reviewed by EFSA in the framework of the renewal of the approval of the active substance under Regulation (EC) No 1107/2009 (EFSA, 2016b). The representative uses evaluated for the renewal included applications by foliar spraying to control aphids on pome fruit and on protected tomato and against aphids and Colorado beetle on potato. Following the peer review under the renewal procedure, a decision on renewal of the approval of the active substance acetamiprid in accordance with Regulation (EC) No 1107/2009 was published by Commission Implementing Regulation (EU) 2018/1134, which entered into force on 1 March 2018. This approval is restricted to uses as insecticide only (European Commission, 2017c).
The EU MRLs for acetamiprid are established in Annex II of Regulation (EC) No 396/2005. Codex maximum residue limits (CXLs) for this active substance were also established by the Codex Alimentarius Commission (CAC). Since the entry into force of this regulation EFSA has issued several reasoned opinions on the modification of MRLs for acetamiprid. The proposals from these reasoned opinions have been considered in the preparation of EU legislation. An overview of the MRL changes that occurred since the entry into force of the above mentioned Regulation is provided below (Table 1).
Table 1.
Overview of the MRL changes since the entry into force of Regulation (EC) No 396/2005
| Procedure | Legal implementation | Remarks |
|---|---|---|
| Art. 12 (EFSA, 2011) | (EU) No 87/2014 | Review of existing MRLs |
| Implementation of CXL | (EU) No 500/2013 | CAC 2012 |
| Art 10 (EFSA, 2012) | (EU) 500/2013 | Purslane, legume vegetables and pulses |
| Art 10 (EFSA, 2013) | (EU) 2015/401 | Apricots and tree nuts (import tolerance USA) |
| Art. 10 (EFSA, 2014) | (EU) 2015/846 | Bananas |
| Art. 10 (EFSA, 2015) | (EU) 2016/1003 | Leafy brassicas. No MRL was proposed by EFSA due to acute intake concerns identified when using the lower toxicological reference values (TRV) recommended by the EFSA PPR Panel. As these TRV were not yet in force at the time of the assessment, the derived MRLs were legally implemented |
| Art. 10 (EFSA, 2016a) | (EU) 2016/1902 | Table olives, tomatoes, gherkins, peas and beans with pods, pulses, olives for oil production, rapeseeds, wheat grain |
| Implementation of CXL | (EU) 2017/626 | CAC 2016 |
MRL: maximum residue level; CXL: codex maximum residue limit.
Assessment
In order to identify the potential MRLs of concern when considering the new TRV, EFSA performed a preliminary risk assessment of the existing EU MRLs established in the Regulation (EC) 2017/626, using the revision 2 of EFSA Pesticide Residue Intake Model (PRIMo).
The results of the preliminary risk assessment indicated that for 12 commodities of plant origin (scarole, apples, spinaches, pears, lettuce, kale, celery, peaches, beet leaves (chard), purslane, Chinese cabbage and head cabbage) the current MRL might pose an acute risk to European consumers (see also Section 3). For these commodities, EFSA asked MSs to report fall‐back GAPs (Appendix A.1) with supporting residue data, which were then further considered by EFSA to derive fall‐back MRLs. All fall‐back data received are detailed in the MS Consultation Report (EFSA, 2018).
It is therefore highlighted that the current assessment is targeted only to the MRLs for which a risk for consumers could not be excluded according to the preliminary risk assessment.
Furthermore, in this reasoned opinion, EFSA also assessed the risks to the consumer associated with two new MRL applications, submitted to EFSA in accordance with Article 8 of Regulation (EC) No 396/2005. The detailed description of the intended use of acetamiprid in Europe on barley, oats, table olives and olives for oil production, is reported in Appendix A.2.
EFSA has based its assessment on the conclusion on the peer review of the pesticide risk assessment of the active substance acetamiprid (EFSA, 2016b), the previous reasoned opinions on acetamiprid, including the review of the existing MRLs (EFSA, 2011, 2012, 2013, 2014, 2015, 2016a), the JMPR Evaluation reports (FAO, 2011, 2015), the evaluation reports submitted during the Consultation of MSs (Belgium, 2017; Germany, 2017; Czech Republic, 2018; Finland, 2018; France, 2018; Greece, 2018; Italy, 2018; Lithuania, 2018; Portugal, 2018; Spain, 2018; Sweden, 2018 and United Kingdom, 2018) as well as the evaluation reports submitted by the EMSs according to Article 8 of Regulation (EC) No 396/2005 (Italy, 2016; Poland, 2017). These evaluation reports are considered as supporting documents to this reasoned opinion and, thus, are made publicly available.
In addition, key supporting documents to this reasoned opinion are the MSs consultation report (EFSA, 2018) and the chronic and acute exposure calculations performed using the EFSA PRIMo, revision 2 (Appendix C). Therefore, also these documents are made publicly available.
The assessment is performed in accordance with the legal provisions of the uniform principles for evaluation and authorisation of plant protection products as set out in Commission Regulation (EU) No 546/20115 and the currently applicable guidance documents relevant for the consumer risk assessment of pesticide residues (European Commission, 1997a–1997b, 1997c, 1997d, 1997e, 1997f, 1997g 2000, 2010a, b, 2017a and OECD, 2011, 2013).
More detailed information on the available data and on the conclusions derived by EFSA can be retrieved in the list of end points reported in Appendix B.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of acetamiprid in primary crops (fruit, root and leafy crop groups) was evaluated during the MRL review (EFSA, 2011) and further considered in the framework of the peer review for the renewal (EFSA, 2016b). In all plant parts, acetamiprid was identified as the major component of the radioactive residues (total radioactive residue (TRR)) accounting for ca 30–90% TRR 14–90 days after the last application, except in head cabbage where the 6‐chloronicotinic acid metabolite (IC‐0) was the sole component identified, representing 46% TRR (0.023 mg eq/kg) and in cotton seeds (24% TRR at harvest, 0.27 mg/kg). IC‐0 was also detected in carrot roots (26% TRR, 0.02 mg/kg). Other identified metabolites were observed at low levels, accounting mostly for less than 5% TRR, except metabolites IM‐1‐4 in immature carrot leaves (43% TRR).
1.1.2. Nature of residues in rotational crops
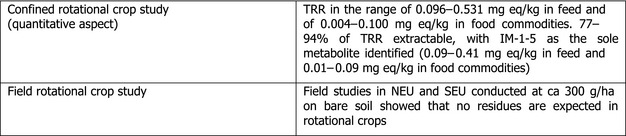
Oats and barley (relevant for the new intended EU use of acetamiprid) can be grown in rotation with other plants. Acetamiprid is of low persistence in soil (highest field DT90 43 days and 20°C lab DT90 54 days) and will therefore not be of relevance for rotational crops. However, the soil metabolite IM‐1‐5 showed to be more persistent in soil (DT50 319–663 days). Therefore, in the framework of the peer review for the renewal, the metabolism in rotational crops was investigated using the more persistent soil metabolite IM‐1‐5. In the different rotational crops investigated (wheat, turnip, spinach), metabolite IM‐1‐5 was shown to remain the main component of the radioactive residues accounting in mature plant at harvest for 77–94% TRR. Nevertheless, field rotational crop studies conducted in northern and southern EU with acetamiprid applied onto the bare soil at ca 300 g/ha, demonstrated that acetamiprid and metabolite IM‐1‐5 are not expected to be present in rotational crops (EFSA, 2016b). Considering that the conditions of application of the representative uses assessed during the renewal cover the new intended use, this conclusion is still considered relevant in the framework of the present assessment.
1.1.3. Nature of residues in processed commodities
The effect of processing on the nature of acetamiprid residues was investigated and the results indicated that acetamiprid is hydrolytically stable under standard hydrolysis conditions (EFSA, 2011, 2016b).
1.1.4. Methods of analysis in plants
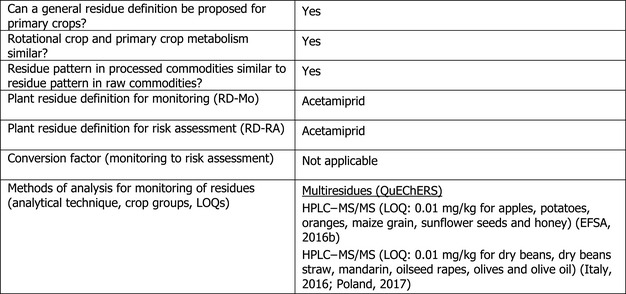
Analytical methods for the determination of acetamiprid residues in plant commodities were assessed during the MRL review and it was concluded that adequate analytical methods based on gas chromatography with electron capture detector (GC‐ECD) and high‐performance liquid chromatography with tandem mass spectrometry (HPLC–MS/MS) are available to enforce acetamiprid residues in high water, high acid, high oil content commodities and in dry commodities, at a validated limit of quantification (LOQ) of 0.01 mg/kg (EFSA, 2011).
Furthermore, during the peer review for the renewal, it was concluded that acetamiprid residues can be monitored in food and feed of plant origin with the multi‐residue method QuEChERS by HPLC–MS/MS with a LOQ of 0.01 mg/kg in all plant commodity groups (EFSA, 2016b).
Additional validation data were submitted by Italy and Poland in the framework of the new MRL application and confirmed that the multiresidue method Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) is fully validated for the enforcement of acetamiprid in the four main plant matrices and in straw at the LOQ of 0.01 mg/kg (Italy, 2016; Poland, 2017).
Therefore, EFSA concludes that sufficiently validated analytical methods are available to control residues of acetamiprid in the plant commodities under consideration in the new MRL applications.
1.1.5. Stability of residues in plants
The stability of acetamiprid residues in plant matrices under storage conditions prior to analysis was assessed during the MRL review and in the framework of the peer review for the renewal of the approval. Residues of acetamiprid were found to be stable at ≤ 18°C for up to 13 months in high water content matrices and for up to 12 months in high acid‐ and high oil content matrices as well as in dry matrices (EFSA, 2011, 2016b). An additional storage stability study on dry beans (seed and straw), apples, olives and oranges has been submitted by Italy and Poland in the framework of the MRL application for the modification of the existing MRLs for olives, barley and oats. Results of these studies confirmed that acetamiprid is stable for 12 months in the main four matrices and in straw stored at −18°C.
1.1.6. Proposed residue definitions
Since acetamiprid was identified as the major component of the residues in almost all plant matrices and since the toxicity of the IC‐0 metabolite was concluded to be covered by the toxicity of the parent acetamiprid, during the peer review for the renewal the plant residue definitions for monitoring and risk assessment were limited to acetamiprid (EFSA, 2016b). The same residue definitions were also proposed during the Article 12 MRL review (EFSA, 2011) and are applicable to primary, rotational crops and processed commodities.
The current residue definition set in Regulation (EC) No 396/2005 is identical to the residue definition for enforcement derived by EFSA.
For the new uses on olives, barley and oats, EFSA concludes that the metabolism of acetamiprid is sufficiently addressed and the residue definitions for enforcement and risk assessment derived under Article 12 MRL review and confirmed during the peer review for renewal are applicable.
Fully validated analytical methods are available to enforce the proposed residue definition in all plant commodities at the LOQ of 0.01 mg/kg.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
In order to derive fall‐back MRLs for the commodities for which a risk to consumers was identified in the preliminary risk assessment (scarole, apples, spinaches, pears, lettuce, kale, celery, peaches, beet leaves (chard), purslane, Chinese cabbage and head cabbage), EFSA considered all residue trials evaluated in the framework of the MRL review (EFSA, 2011) and additional data submitted during the consultation of MSs (Belgium, 2017; Germany, 2017; Czech Republic, 2018; Finland, 2018; France, 2018; Greece, 2018; Italy, 2018; Lithuania, 2018; Portugal, 2018; Spain, 2018; Sweden, 2018 and United Kingdom, 2018).
The authorised GAPs for acetamiprid for which a risk for consumers have been identified and the less critical GAPs that allowed EFSA to derive fall‐back MRLs are given in Appendix A.1.
Detailed results of the residue trials and the derived MRLs and risk assessment values are reported in Appendix B.1.2.1.
For celeries, no fall‐back MRLs could be derived as residue data supporting the fall‐back GAPs were not available. For kale and Chinese cabbages, according to the information provided by MSs, no uses that could be considered as fall‐back are currently authorised in EU.
For all other commodities, data were sufficient to derive at least a tentative MRL, taking note of the following considerations:
Head cabbages: a fall‐back MRL could be derived from the trials supporting the northern outdoor GAP. Nevertheless, it is noted that southern trials with all residue below the LOQ, could not be considered further as significant residues found in outer leaves, suggest that the reported residue levels refer to the head without outer leaves. Therefore, full data set compliant with southern GAPs with residue analysed in the whole plant after removal of roots and decayed leaves in line with the Annex I of Regulation 396/2005, are still required.
Escaroles: northern and southern outdoor trials were performed according to more critical GAPs. Moreover, it was not clearly reported whether southern trials were performed on open leaf varieties. Therefore, the derived fall‐back MRL should be considered tentative only and full data sets supporting the northern and the southern outdoor GAPs, including the confirmation that southern trials were performed on open leaf varieties are still required.
Spinaches and chards: although a fall‐back MRL could be derived from the northern data set, it is noted that southern trials were all performed with 2–3 applications instead of 1. Moreover, it was not clearly reported whether southern trials were performed on open leaf varieties. Therefore, a full data set supporting the southern outdoor GAP and the confirmation that trials were performed on open leaf varieties are still required.
In order to derive MRLs accommodating the new intended uses on olives, barley and oats in Europe, EFSA considered all residue trials reported by the EMSs (Italy, 2016; Poland, 2017).
The details of the new intended GAPs for acetamiprid are given in Appendix A.2.
In support of the intended SEU GAP on olives, the applicant submitted eight GAP‐compliant residue trials on olives, conducted in Italy, Spain, Greece and southern France during growing season 2013. Residues of acetamiprid were within a range of 0.46–1.30 mg/kg resulting in an MRL proposal of 3 mg/kg. This MRL proposal is extrapolated to table olives and olives for oil production (European Commission, 2017a).
In support of the intended NEU GAP on barley and oats, the applicant submitted eight GAP‐compliant residue trials on barley conducted in Germany, Hungary, Poland, Austria and northern France during growing season 2014. Residues of acetamiprid in grain were within a range of < 0.01 to 0.03 mg/kg resulting in an MRL proposal of 0.05 mg/kg. This MRL proposal is extrapolated to oats (European Commission, 2017a).
All residue trial samples considered in this framework were stored in compliance with the demonstrated storage conditions. Decline of residues during storage of the trial samples is therefore not expected. According to the EMSs, the analytical methods used to analyse the residue trial samples have been sufficiently validated and were proven to be fit for the purpose (Italy, 2016; Poland, 2017). Results of the residue trials and the derived MRLs and risk assessment values are reported in Appendix B.1.2.2.
1.2.2. Magnitude of residues in rotational crops
Field rotational crop studies conducted in northern and southern EU with acetamiprid applied onto the bare soil at ca 300 g/ha were evaluated during the peer review for the renewal. On the basis of these studies, it was concluded that acetamiprid and metabolite IM‐1‐5 are not expected to be present in rotational crops following treatment according to the representative uses (EFSA, 2016b).
Considering that the conditions of application of the representative uses assessed during the renewal cover the new intended use, this conclusion is still relevant in the framework of the present assessment.
1.2.3. Magnitude of residues in processed commodities
For the new intended use on olives, the applicant submitted two residue trials performed at exaggerated dose rates (3x) to generate samples for processing studies (Italy, 2016). Olives were processed into oil and residues were analysed in both the raw and the processed commodity. In oil, a reduction of residues was observed. In addition, processing studies with olives have been also investigated in a previous MRL application (EFSA, 2016a). The processing factors derived for olive oil from all studies were combined and are summarised in Appendix B.1.2.4. Processing studies on barley and oats grain were not submitted. Nevertheless, since residues in raw barley and oats grain were below 0.1 mg/kg, such studies are not required.
1.2.4. Proposed MRLs
Consequently, the available residue data submitted by the MSs in support of the fall‐back GAPs are considered sufficient to derive (tentative) fall‐back MRLs as well as risk assessment values for all commodities possibly of concern, except for celery, kale and Chinese cabbages for which fall‐back data were not available. Tentative MRLs were also derived for cereal straw in view of the future need to set MRLs in feed items.
Furthermore, EFSA concludes that the data are sufficient to derive MRL proposals as well as risk assessment values accommodating the intended uses on table olives, olives for oil production, barley and oats.
2. Residues in livestock
Since barley, oats and their by‐products might be fed to livestock, the impact of the new intended uses on the livestock exposure needs to be assessed. Moreover, as a risk for consumers has been identified for the existing more critical uses on crops that can be fed to livestock (apples and kale), the impact of the withdrawal of these uses, needs also to be evaluated.
Therefore, livestock dietary burdens were calculated for different groups of livestock according to OECD guidance (OECD, 2013) considering livestock intake of all feed products containing acetamiprid residues resulting from all authorised uses, including the new intended uses on barley and oats. The input values for all relevant commodities are summarised in Appendix D. The calculated dietary burden was then compared to the intakes considered to derive the current MRLs for animal commodities (see Appendix B.2).
The calculated dietary burdens exceed the trigger value of 0.1 mg/kg dry matter (DM) for all livestock species and the main contributors are kale leaves (cattle and swine diet) and wheat straw (sheep and poultry diet). Nevertheless, the existing EU MRLs for cattle, sheep and swine tissues and milk, reflect the existing CXLs which are based on a livestock dietary exposure significantly higher than the intake calculated in this framework. Moreover, livestock intakes calculated by the JMPR are mainly driven by residues in corn forage and stover (FAO, 2015).
For poultry, the new intended uses had no impact on the dietary burdens calculated in the framework of the Article 12 MRL review, (EFSA, 2011) when the MRLs for poultry tissues and eggs were derived.
Therefore, it is concluded that the withdrawal of the most critical uses on kale and apples and the new intended use on barley and oats is not expected to have an impact on the dietary burden calculated for livestock, and thus, there is no need to modify the existing EU MRLs for commodities of animal origin.
It is noted that during the peer review for the renewal, (EFSA, 2016b) it was proposed to limit the residue definition for enforcement in animal commodities to metabolite N‐desmethyl‐acetamiprid only, while in the framework of this assessment the residue definition currently implemented in the EU legislation and by the JMPR (sum of acetamiprid and N‐desmethyl‐acetamiprid, expressed as acetamiprid) was considered.
Moreover, the Article 12 review concluded that acetamiprid and N‐desmethyl‐acetamiprid (IM‐2‐1) could be enforced in food of animal origin with a LOQ of 0.01 mg/kg in milk, muscle, fat and eggs, and a LOQ of 0.05 mg/kg in liver and kidney but that a confirmatory method was still required (EFSA, 2011). In the framework of the renewal for the approval, the QuEChERS multiresidue method with HPLC–MS/MS was considered sufficiently validated to enforce both acetamiprid and N‐desmethyl‐acetamiprid at the LOQ of 0.01 mg/kg for each compound (EFSA, 2016b). Therefore, it is concluded that the data gap identified during the MRL review is covered by the additional method evaluated during the renewal.
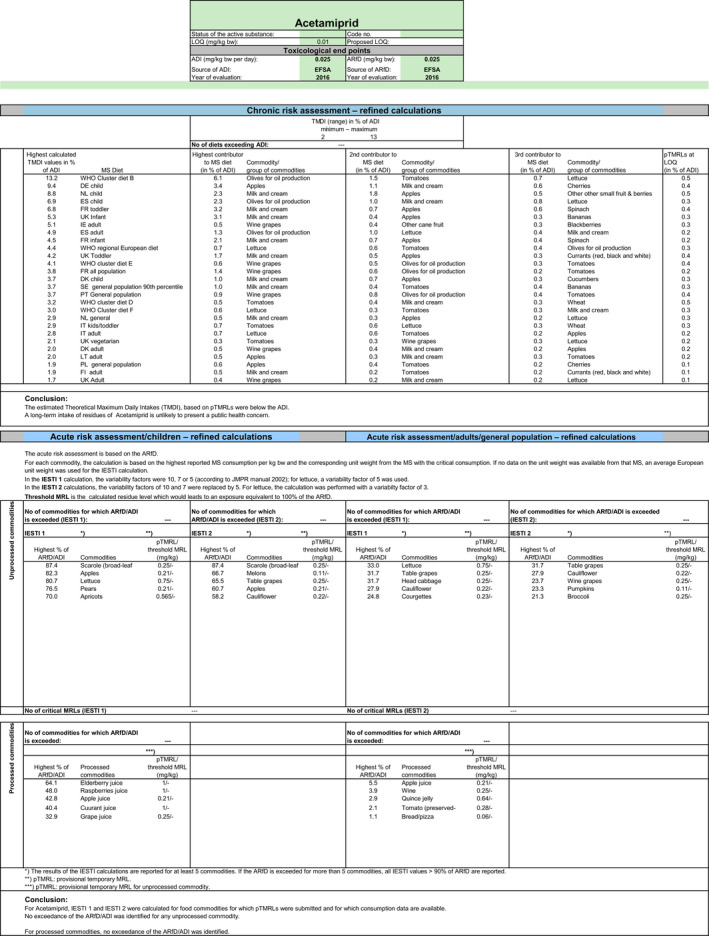
3. Consumer risk assessment
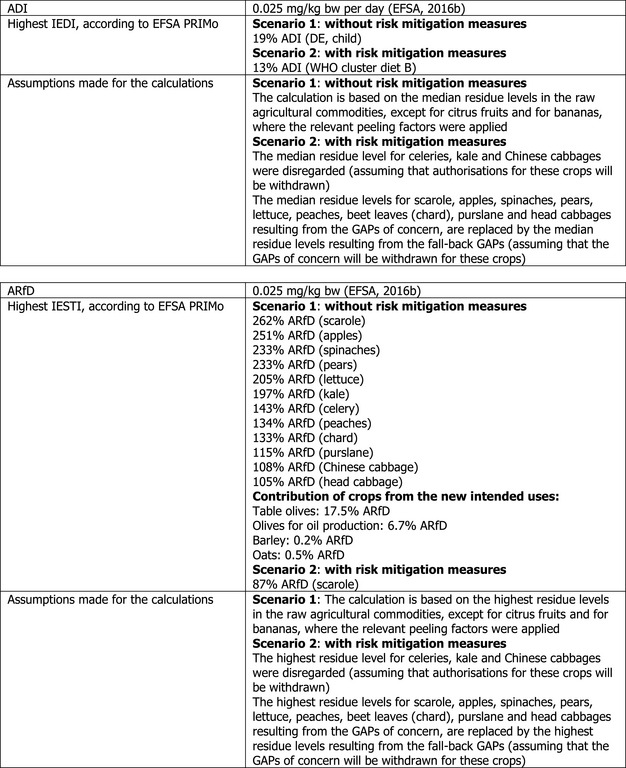
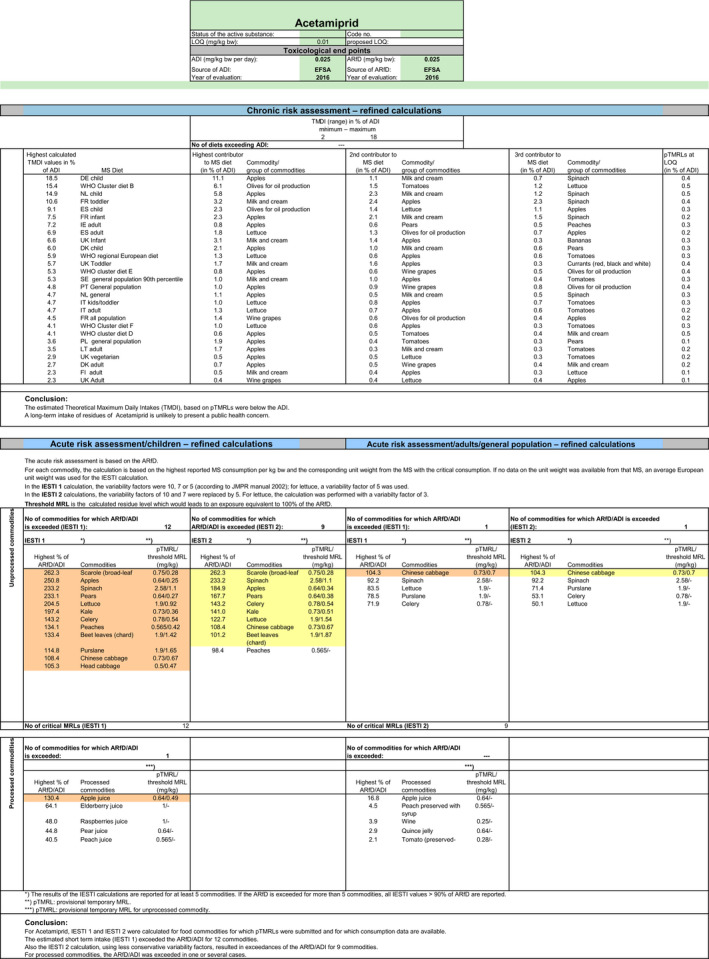
Chronic and acute exposure calculations were performed using revision 2 of the EFSA PRIMo (EFSA, 2007). The exposures calculated were compared with the TRV for acetamiprid, derived by EFSA in the framework of the renewal for the approval of the active substance (2016b).
In order to identify the potential MRLs of concern, EFSA first performed a preliminary risk assessment, using the risk assessment values derived from the existing EU uses and import tolerances assessed in the Article 12 MRL review and in the Article 10 reasoned opinions issued after the Article 12 review were considered. CXLs implemented in the EU Legislation were also covered by this risk assessment. Moreover, in order to assess the risk to consumer associated with the intended uses on olives, barley and oats, the input values derived for these commodities in Section 1.2 were included in the calculation (scenario 1). All input values considered in the exposure calculations are summarised in Appendix D.
The highest chronic exposure was calculated for German child, representing 19% of the ADI. With regard to the acute exposure; however, an exceedance of the ARfD was identified for scarole, apples, spinaches, pears, lettuce, kale, celery, peaches, beet leaves (chard), purslane, Chinese cabbage and head cabbage, representing 262%, 251%, 233%, 233%, 205%, 197%, 143%, 134%, 133%, 115%, 108% and 105% of the ARfD, respectively. No risk for consumer was identified for table olives, olives for oil production, barley and oats.
A second exposure calculation (scenario 2) was therefore performed, considering the fall‐back residue data for all crops, except for celery, Chinese cabbages and kales which were excluded from the calculation as no fall‐back risk assessment values could be derived. According to the results of this second calculation, the highest chronic exposure declined to 13% of the ADI for WHO cluster diet B; the highest acute exposure is then calculated for escaroles, representing 87% of the ARfD.
Conclusions and recommendations
The residue data which were submitted by the MSs in support of the fall‐back GAPs were sufficient to derive fall‐back MRLs safe for consumers for all commodities possibly of concern, except for celery, kale and Chinese cabbages for which no fall‐back data were available. Nevertheless, the fall‐back MRL derived for escaroles is only tentative and needs to be confirmed by the following data:
Full data set supporting the southern outdoor fall‐back GAP for escarole and the confirmation that trials were performed on open leaf varieties.
Furthermore, it is highlighted that some of the MRLs derived result from a GAP in one climatic zone only, whereas other GAPs reported by the RMS were not fully supported by data. EFSA therefore identified the following data gaps which are not expected to impact the validity of the fall‐back MRLs derived but which might have an impact on national authorisations:
full data set compliant with the southern outdoor fall‐back GAP for head cabbages with residue analysed in the whole plant after removal of roots and decayed leaves in line with the Annex I of Regulation 396/2005;
full data set supporting the northern outdoor fall‐back GAP for escarole;
full data set supporting the southern outdoor fall‐back GAPs for spinaches and chards and the confirmation that trials were performed on open leaf varieties.
full data sets supporting the southern outdoor and the indoor fall‐back GAPs for celeries.
If the above‐reported data gaps are not addressed in the future, MSs are recommended to withdraw or modify the relevant authorisations at national level.
MSs are in any case recommended to withdraw their national authorisations for celeries, kales and Chinese cabbages where no fall‐back MRL could be derived by EFSA. For apples, pears, peaches, head cabbages, lettuce, escaroles, spinaches, chards and purslanes, EFSA recommends that the national authorisations are being modified in order to comply with the fall‐back MRLs derived by EFSA.
In the framework of this assessment, it can be concluded that there is no need to modify the existing EU MRLs for commodities of animal origin. Nevertheless it is noted that additional analytical methods for enforcement in animal commodities addressing the data gap identified during the MRL review and currently reflected in the EU legislation were evaluated during the peer review for the renewal (EFSA, 2016b).
Furthermore, EFSA concludes that available data were sufficient to derive MRL proposals accommodating the intended uses on table olives, olives for oil production, barley and oats. According to the results of the risk assessment, these intended uses are unlikely to pose a health risk for consumers (see Table 2).
Table 2.
Summary table
| Code number b | Commodity | Existing EU MRL (mg/kg) | Outcome of the assessment | |
|---|---|---|---|---|
| MRL (mg/kg) | Comment | |||
| Enforcement residue definition: acetamiprid | ||||
| 130010 | Apples | 0.8 | 0.4 | Fall‐back MRL is proposedc |
| 130020 | Pears | 0.8 | 0.4 | Fall‐back MRL is proposedc |
| 140030 | Peaches | 0.8 | 0.2 | Fall‐back MRL is proposedc |
| 242020 | Head cabbages | 0.7 | 0.4 | Fall‐back MRL is proposedc |
| 243010 | Chinese cabbages | 1.5 | – | A fall‐back MRL could not be proposedd |
| 243020 | Kales | 1.5 | – | A fall‐back MRL could not be proposedd |
| 251020 | Lettuces | 3 | 1.5 | Fall‐back MRL is proposedc |
| 251030 | Escaroles/broad‐leaved endives | 1.5 | 0.4 | Tentative fall‐back MRL is proposede |
| 252010 | Spinaches | 5 | 0.6 | Fall‐back MRL is proposedc |
| 252020 | Purslanes | 3 | 0.6 | Fall‐back MRL is proposedc |
| 252030 | Chards/beet leaves | 3 | 0.6 | Fall‐back MRL is proposedc |
| 270030 | Celeries | 1.5 | – | A fall‐back MRL could not be proposedf |
| 161030 | Table olives | 0.9 | 3 | New intended EU uses are sufficiently supported by data and no risk for consumers has been identified |
| 402010 | Olives for oil production | 0.9 | 3 | |
| 500010 | Barley grains | 0.01a | 0.05 | |
| 500050 | Oat grains | 0.01a | 0.05 | |
| – | Other products of plant origin | See Regulation 2017/626 | See Regulation 2017/626 | Existing MRLs can be maintainedg |
| Enforcement residue definition: sum of acetamiprid and N‐desmethyl acetamiprid, expressed as acetamiprid | ||||
| – | Other products of animal origin | See Regulation 2017/626 | See Regulation 2017/626 | Existing MRLs can be maintainedh |
MRL: maximum residue level.
Indicates that the MRL is set at the limit of quantification.
Commodity code number, as listed in Annex I of Regulation (EC) No 396/2005.
The existing EU MRL was identified as a potential MRL of concern. Data supporting a fall‐back MRL were submitted by MSs and no risk to consumers is identified for this fall‐back MRL.
The existing EU MRL was identified as a potential MRL of concern. No uses are currently authorised in EU that could be considered to derive a fall‐back MRL. EFSA proposes to lower the MRL to the appropriate LOQ and to withdraw the relevant authorisations within the EU.
The existing EU MRL was identified as a potential MRL of concern. Data supporting a fall‐back MRL were submitted by MSs and no risk to consumers is identified for this fall‐back MRL. Nevertheless the derived fall‐back MRL should be confirmed by the submission of additional data.
The existing EU MRL was identified as a potential MRL of concern. Residue data supporting the fall‐back GAPs were not available and a fall‐back MRL cannot be derived. EFSA proposes to lower the MRL to the appropriate LOQ and to withdraw the relevant authorisations within the EU.
The existing EU MRL was not identified as a potential MRL of concern.
The existing EU MRL was not identified as a potential MRL of concern. Moreover the withdrawal of the most critical existing uses on kale and apples and the intended uses on barley and oats are not expected to have an impact on the MRLs calculated for livestock.
Abbreviations
- a.i.
active ingredient
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CAC
Codex Alimentarius Commission
- CS
capsule suspension
- CV
coefficient of variation (relative standard deviation)
- CXL
codex maximum residue limit
- DAT
days after treatment
- DM
dry matter
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- ECD
electron capture detector
- EMS
evaluating Member State
- eq
residue expressed as a.s. equivalent
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- GC‐ECD
gas chromatography with electron capture detector
- HPLC‐MS/MS
high performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ISO
International Organisation for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- LOQ
limit of quantification
- Mo
monitoring
- MRL
maximum residue level
- MS
Member States
- NEU
northern European Union
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant‐back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged, and Safe (analytical method)
- RA
risk assessment
- RD
residue definition
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SEU
southern European Union
- SMILES
simplified molecular‐input line‐entry system
- SG
water‐soluble granule
- SL
soluble concentrate
- SP
water‐soluble powder
- STMR
supervised trials median residue
- TRR
total radioactive residue
- TRV
toxicological reference values
- WG
water‐dispersible granule
- WHO
World Health Organization
- WP
wettable powder
Appendix A – Summary of authorised or intended uses considered in the assessment
A.1. Authorised uses for which a risk for consumers was identified in the preliminary risk assessment (in bold) and identified fall‐back GAPs
| Crop | Region | Outdoor/Indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Apples | Malus domestica | Non‐EU | Outdoor | USA | Aphids | WP | 70.0 | g/kg | Foliar treatment – spraying | 4 | 10 | 17 | 0.17 | kg a.i./ha | 7 | EFSA ( 2011 ) | ||||
| Apples | Malus domestica | NEU | Outdoor | FR | Laspeyresia pomonella, Phyllonorycter spp., Leucoptera malifoliella | WG | 200.0 | g/kg | Foliar treatment – spraying | 69 | 81 | 2 | 14 | 0.10 | kg a.i./ha | 14 |
Fall‐back GAP Covers also UK, CZ, DE, FI and LT GAPs |
|||
| Apples | Malus domestica | SEU | Outdoor | FR, PT, ES, IT | Laspeyresia pomonella, Phyllonorycter spp., Leucoptera malifoliella | WG | 200.0 | g/kg | Foliar treatment – spraying | 69 | 81 | 2 | 14 | 30 | 0.10 | kg a.i./ha | 14 |
Fall‐back GAP. Covers also EL GAP |
||
| Pears | Pyrus communis | Non‐EU | Outdoor | USA | Aphids | WP | 70.0 | g/kg | Foliar treatment – spraying | 4 | 10 | 17 | 0.17 | kg a.i./ha | 7 | EFSA ( 2011 ) | ||||
| Pears | Pyrus communis | NEU | Outdoor | FR | Laspeyresia pomonella, Phyllonorycter spp., Leucoptera malifoliella | WG | 200.0 | g/kg | Foliar treatment – spraying | 69 | 81 | 2 | 14 | 0.10 | kg a.i./ha | 14 |
Fall‐back GAP Covers also UK, CZ, DE, FI and LT GAPs |
|||
| Pears | Pyrus communis | SEU | Outdoor | FR, PT, ES, IT | Laspeyresia pomonella, Phyllonorycter spp., Leucoptera malifoliella | WG | 200.0 | g/kg | Foliar treatment – spraying | 69 | 81 | 2 | 14 | 30 | 0.10 | kg a.i./ha | 14 |
Fall‐back GAP Covers also EL GAP |
||
| Peaches | Persica vulgaris, syn: Prunus persica | non‐EU | Outdoor | USA | WP | 70.0 | g/kg | Foliar treatment – spraying | n.a. | n.a. | 4 | 10 | 12 | 0.17 | kg a.i./ha | 7 | EFSA ( 2013 ) | |||
| Peaches | Persica vulgaris, syn: Prunus persica | SEU | Outdoor | PT, ES, EL, IT | Foliar treatment – spraying | 2 | 14 | 30 | 0.10 | kg a.i./ha | 14 | Fall‐back | ||||||||
| Head cabbages | Brassica oleracea var. capitata | non‐EU | Outdoor | USA | Aphids | WP | 70.0 | g/kg | Foliar treatment ‐ spraying | 5 | 6 | 8 | 0.08 | kg a.i./ha | 7 | EFSA ( 2011 ) | ||||
| Head cabbages | Brassica oleracea var. capitata | NEU | Outdoor | SE | Biting and sucking insects | SG | 200.0 | g/kg | Foliar treatment – spraying | 2 | 7 | 14 | 0.05 | kg a.i./ha | 7 | Covers also FR, DE and LT GAPs | ||||
| Head cabbages | Brassica oleracea var. capitata | SEU | Outdoor | IT | Aphids, Altica | SP | 50.0 | g/kg | Foliar treatment – spraying | 2 | 0.07 | 0.08 | kg a.i./ha | 7 | Covers also ES, PT and EL GAPs | |||||
| Chinese cabbages | Brassica rapa subsp. pekinensis | NEU | Outdoor | DE | Brevicoryne brassicae , Aleyroidae | Foliar treatment – spraying | 12 | 2 | 14 | 0.06 | kg a.i./ha | 7 | EFSA ( 2015 ) | |||||||
| Kales | Brassica oleracea var. sabellica; Brassica oleracea var. viridis | NEU | Outdoor | DE | Brevicoryne brassicae , Aleyroidae | Foliar treatment – spraying | 12 | 2 | 14 | 0.07 | kg a.i./ha | 7 | EFSA ( 2015 ) | |||||||
| Lettuces | Lactuca sativa | NEU/SEU | Indoor | UK, IT | Aphids, Altica , leafhopper, Liriomyza , thrips | SP | 200.0 | g/kg | Foliar treatment – spraying | 1 | 2 | 0.05 | kg a.i./ha | 3 | EFSA ( 2011 ) | |||||
| Lettuces | Lactuca sativa | NEU | Outdoor | DE | Aphids | Foliar treatment – spraying | 1 | 2 | 7 | 14 | 0.05 | kg a.i./ha | 3 |
Fall‐back (EFSA, 2011) Covers also FI and LT GAPs |
||||||
| Lettuces | Lactuca sativa | SEU | Outdoor | ES | Foliar treatment – spraying | 2 | 0.06 | kg a.i./ha | 3 |
Fall‐back (EFSA, 2011) Covers also PT, EL and IT GAPs. |
||||||||||
| Escaroles | Cichorium endivia var. latifolia | NEU | Outdoor | DE | Aphids | Foliar treatment – spraying | 1 | 2 | 7 | 14 | 0.05 | kg a.i./ha | 3 | EFSA ( 2011 ) | ||||||
| Escaroles | Cichorium endivia var. latifolia | SEU | Outdoor | FR, IT | Aphids, Altica , leafhopper, Liriomyza , thrips | SP | 50.0 | g/kg | Foliar treatment – spraying | 1 | 2 | 0.05 | kg a.i./ha | 7 | EFSA ( 2011 ) | |||||
| Escaroles | Cichorium endivia var. latifolia | NEU | Outdoor | FR | Aphids | 0.1 | g/L | Foliar treatment – spraying | 2 | 5 | 10 | 0.03 | kg a.i./ha | 14 | Fall‐back GAP | |||||
| Escaroles | Cichorium endivia var. latifolia | SEU | Outdoor | EL, IT | Aphids, Altica, leafhopper, Liriomyza, thrips | SL | 50.0 | g/kg | Foliar treatment – spraying | 19 | 49 | 1 | 0.10 | kg a.i./ha | 10 |
Fall‐back GAP. Covers also FR GAP |
||||
| Spinaches | Spinacia oleracea | NEU/SEU | Indoor | UK | Foliar treatment – spraying | 2 | 0.05 | kg a.i./ha | 3 | EFSA ( 2011 ) | ||||||||||
| Spinaches | Spinacia oleracea | non‐EU | Outdoor | USA | Aphids | WP | 70.0 | g/kg | Foliar treatment – spraying | 5 | 6 | 8 | 0.08 | kg a.i./ha | 7 | EFSA ( 2011 ) | ||||
| Spinaches | Spinacia oleracea | NEU | Outdoor | UK | Foliar treatment – spraying | 2 | 0.05 | kg a.i./ha | 7 |
Fall‐back (EFSA, 2011) Covers also FI and LT GAPs |
||||||||||
| Spinaches | Spinacia oleracea | SEU | Outdoor | EL, IT | Aphids, Altica, leafhopper, Liriomyza, thrips | SL | 50.0 | g/kg | Foliar treatment – spraying | 19 | 49 | 1 | 0.10 | kg a.i./ha | 10 | Fall‐back | ||||
| Purslanes | Portulaca oleracea | NEU/SEU | Indoor | Aphids | SP | 200.0 | g/kg | Foliar treatment – spraying | 1 | 2 | 14 | 0.05 | kg a.i./ha | 3 | EFSA ( 2012 ) | |||||
| Purslanes | Portulaca oleracea | NEU | Outdoor | FR | SG | Foliar treatment – spraying | 2 | 0.05 | kg a.i./ha | 7 | Fall‐back (EFSA, 2011) | |||||||||
| Purslanes | Portulaca oleracea | SEU | Outdoor | FR | Foliar treatment – spraying | 20 | 49 | 2 | 0.05 | kg a.i./ha | 7 | Fall‐back (EFSA, 2011) | ||||||||
| Beet leaves (Chards) | Beta vulgaris var. flavescens | NEU/SEU | Indoor | BE | Aphids | SP | 200.0 | g/kg | Foliar treatment – spraying | 1 | 2 | 0.05 | kg a.i./ha | 7 | EFSA ( 2011 ) | |||||
| Beet leaves (Chards) | Beta vulgaris var. flavescens | NEU | Outdoor | BE | Aphids | SP | 200.0 | g/kg | Foliar treatment – spraying | 1 | 2 | 0.05 | kg a.i./ha | 7 | Fall‐back (EFSA, 2011) | |||||
| Beet leaves (Chards) | Beta vulgaris var. flavescens | SEU | Outdoor | EL, IT | Aphids, Altica, leafhopper, Liriomyza, thrips | SL | 50.0 | g/kg | Foliar treatment – spraying | 19 | 49 | 1 | 0.10 | kg a.i./ha | 10 | Fall‐back | ||||
| Celeries | Apium graveolens var. dulce | non‐EU | Outdoor | USA | Aphids | WP | 70.0 | g/kg | Foliar treatment – spraying | 5 | 6 | 8 | 0.08 | kg a.i./ha | 7 | EFSA ( 2011 ) | ||||
| Celeries | Apium graveolens var. dulce | SEU | Outdoor | IT | Aphids | SL | 50 | g/L | Foliar treatment – spraying | 19 | 49 | 1 | 0.1 | kg a.i./ha | 10 | Fall‐back | ||||
| Celeries | Apium graveolens var. dulce | NEU/SEU | Indoor | IT | Aphids | SL | 50 | g/L | Foliar treatment – spraying | 19 | 49 | 1 | 0.1 | kg a.i./ha | 5 | Fall‐back | ||||
GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient; WP: wettable powder; WG: water‐dispersible granule; SG: water‐soluble granule; SP: water‐soluble powder; SL: soluble concentrate.
A.2. New intended uses
| Outdoor GAPs for Northern Europe | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop | Region | Outdoor/Indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) | Comments | ||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Barley | Hordeum vulgare | NEU | Outdoor | DE, CZ, UK | Aphids | Foliar treatment – spraying | 12 | 69 | 2 | 10 | 0.04 | kg a.i./ha | n.a. | New intended use evaluated by PL (Poland, 2017) | ||||||
| Oat | Avena sativa | NEU | Outdoor | DE, CZ, UK | Aphids | Foliar treatment – spraying | 12 | 69 | 2 | 10 | 0.04 | kg a.i./ha | n.a. | New intended use evaluated by PL (Poland, 2017) | ||||||
| Outdoor GAPs for Southern Europe | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop | Region | Outdoor/Indoor | Member state or country | Pest controlled | Formulation | Application | PHI or waiting period (days) |
Comments (max. 250 characters) |
||||||||||||
| Common name | Scientific name | Type | Content | Method | Growth stage | Number | Interval (days) | Rate | ||||||||||||
| Conc. | Unit | From BBCH | Until BBCH | Min. | Max. | Min. | Max. | Min. | Max. | Unit | ||||||||||
| Table olives | Olea europaea | SEU | Outdoor | EL, ES, IT, PT | Prays oleae | Foliar treatment – spraying | 65 | 89 | 2 | 14 | 0.10 | kg a.i./ha | 7 | New intended use evaluated by IT. Covers the use assessed in EFSA (2016a) | ||||||
| Olives for oil production | Olea europaea var. europaea | SEU | Outdoor | EL, ES, IT, PT | Prays oleae | Foliar treatment – spraying | 65 | 89 | 2 | 14 | 0.10 | kg a.i./ha | 7 | New intended use evaluated by IT. Covers the use assessed in EFSA (2016a) | ||||||
GAP: Good Agricultural Practice; BBCH: growth stages of mono‐ and dicotyledonous plants; PHI: preharvest interval; NEU: northern European Union; SEU: southern European Union; a.i.: active ingredient.
Appendix B – Residues in plants
B.1. List of end points
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crops | Applications | Sampling (DAT) |
|---|---|---|---|---|
| Fruit crops | Eggplants | Dotting on leave and fruit surface, 1 × 9.5 g a.s./hL | 7, 14 | |
| Apples | Foliar, 1 × 208 g/ha | 0, 7, 14, 28, 62, 90 | ||
| Fruit dotting, 1 × 104 g/ha | 0, 14, 28, 62 | |||
| Root crops | Carrots | Foliar, 2 × 100 g/ha | 14 | |
| Leafy crops | Cabbages | Foliar, 1 × 302 g/ha | 0, 7, 14, 21, 28, 63 | |
| Soil treatment, 1 × 5,940 g/ha | 7, 14, 28 | |||
| Foliar, 1 × 299 g/ha | 0, 7, 14, 28, 63 | |||
| Pulses/oilseeds | Cotton |
Foliar, 4 × 123 Foliar, 4 × 1,230 g/ha |
14, 28 DAT | |
| Sources: EFSA (2011, 2016b) | ||||
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) |
| Root/tuber crops | Turnips | Bare soil, 266 g a.s./ha | 0 | |
| Leafy crops | Spinaches | Bare soil, 266 g a.s./ha | 0 | |
| Cereal (small grain) | Wheat | Bare soil, 266 g a.s./ha | 0 | |
|
Source: EFSA (2016b) The study was conducted with the most persistent soil metabolite IM‐1‐5 (DT50 319–663 days) |
||||
| Processed commodities (hydrolysis study) | Conditions | Investigated? | ||
| Pasteurisation (20 min, 90°C, pH 4) | Yes | |||
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | |||
| Sterilisation (20 min, 120°C, pH 6) | Yes | |||
| Sources: EFSA (2011, 2016b) | ||||
a.s.: active substance; DAT: days after treatment; PBI: plant‐back interval; HPLC–MS/MS: high‐performance liquid chromatography with tandem mass spectrometry; LC–MS/MS: liquid chromatography with tandem mass spectrometry; LOQ: limit of quantification.
B.1.1.2. Stability of residues in plants
|
Plant products (available studies) |
Category | Commodity | T (°C) | Stability (Months/years) |
|---|---|---|---|---|
| High water content | Apple, tomato | −18 | ≤ 13 | |
| High oil content | Cotton seed, cotton oil, orange oil, olives | −18 | 12 | |
| Dry/high protein | Fodder peas | −18 | 12 | |
| Dry/high starch | Potato tuber | −18 | 8 | |
| High acid content | Orange | −18 | 12 | |
| Specific matrices | Dry bean straw | −18 | 12 | |
| Processed commodities |
Apple juice/wet pomace Cotton gin trash/hulls/meal Orange dried pulp, orange juice |
−18 | 12 | |
|
Additional studies on lettuce (15 months), cabbages/cucumbers and apples (12 months) were also evaluated during the renewal and in the framework of the MRL application | ||||
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials – fall‐back GAPs
| Crop |
Region/ indoora |
Residue levels observed in the supervised residue trials relevant to the supported GAPs (mg/kg) |
Recommendations/comments (OECD calculations) |
MRL proposals (mg/kg) |
HR (mg/kg)b |
STMR (mg/kg)c |
|---|---|---|---|---|---|---|
|
Apples Pears |
NEU | 0.071; 0.034; 0.034; 0.068; 0.058; 0.032; 0.21; 0.08; 0.07; 0.03; 0.07; 0.05; 0.13; 0.05; 0.05; 0.07; 0.14; 0.21; 0.09; 0.12; 0.21; 0.03; 0.04 |
Combined data set of trials on apples (21) and pears (2) compliant with GAP or with dose rate within the 25% deviation (Germany, 2017; France, 2018; Spain, 2018) MRLOECD: 0.32 |
0.4 | 0.21 | 0.07 |
| SEU | 0.08; 0.02; 0.06; 0.07; 0.09; 0.05; 0.02; 0.20; 0.028; 0.017; 0.029; 0.034; 0.02; 0.178; 0.04; 0.09; 0.08; 0.14; 0.18; 0.05; 0.20; 0.12 |
Trials on apples compliant with GAP or with dose rate within the 25% deviation (France, 2018; Greece, 2018; Italy, 2018; Portugal, 2018; Spain, 2018) MRLOECD: 0.33 |
0.4 | 0.20 | 0.07 | |
| Peaches | SEU | 0.095; 0.07; 0.028; 0.084; 0.10; 0.04; 0.02; 0.04 |
Trials on peaches compliant with GAP (Greece, 2018; Italy, 2018; Portugal, 2018; Spain, 2018) MRLOECD: 0.19 |
0.2 | 0.10 | 0.06 |
| Head cabbages | NEU | < 0.01; < 0.01; < 0.01; < 0.01; 0.02; 0.02; 0.04; 0.25 |
Trials on head cabbage with dose rate within the 25% deviation (Germany, 2017; France, 2018; Lithuania, 2018; Sweden, 2018) MRLOECD: 0.38 |
0.4 | 0.25 | 0.02 |
| SEU | < 0.01; < 0.01; < 0.01; < 0.01; < 0.01 | Trials on head cabbage with dose rate within the 25% deviation (Portugal, 2018). In two trials, highest residues were found in outer leaves (0.62; 0.25) giving indication that results were reported for the head without outer leaves. Therefore, data were considered not appropriated to derive an MRL reflecting the GAP | – | – | – | |
| Lettuces | NEU | 0.15; 0.19; 0.32; 0.39; 0.58; 0.63; 0.66; 0.75 |
Trials on lettuce compliant with GAP (EFSA, 2011) MRLOECD= 1.38 |
1.5 | 0.75 | 0.49 |
| SEU | 0.07; 0.11; 0.14; 0.14; 0.16; 0.19; 0.4; 0.68 |
Trials on lettuce compliant with GAP (EFSA, 2011) MRLOECD= 1.06 |
1.5 | 0.68 | 0.15 | |
| Escaroles | NEU | 0.04; 0.06; 0.075; 0.08; 0.12; 0.125; 0.13; 0.16 | Trials on lettuce overdosed with residues measured at shorter PHI of 7 days instead of 14 days. Although residues were recalculated according to the proportionality principle, as residues were analysed at shorter PHI, data can be only used to derive a tentative MRL for scarole (France, 2018)MRLOECD= 0.30 |
0.3 (tentative) |
0.16 | 0.10 |
| SEU | 0.02; 0.03; 0.03; 0.06; 0.06; 0.07; 0.25 |
Trials on lettuce performed with 2–3 applications instead of 1 used to derive a tentative MRL for scarole (Greece, 2018; Italy, 2018). Confirmation that trials were performed on open‐leaf varieties is still required MRLOECD= 0.39 |
0.4 (tentative) |
0.25 | 0.06 | |
|
Spinaches Chards |
NEU | 0.08; 0.14; 0.16; 0.16; 0.24; 0.25; 0.28; 0.31 |
Trials on lettuce compliant with GAP extrapolated to spinaches and chards (EFSA, 2011) MRLOECD= 0.61 |
0.6 | 0.31 | 0.20 |
| SEU | 0.02; 0.03; 0.03; 0.06; 0.06; 0.07; 0.25 |
Trials on lettuce performed with 2–3 applications instead of 1 used to derive a tentative MRL for spinaches and chards (Greece, 2018; Italy, 2018). Confirmation that trials were performed on open‐leaf varieties is still required MRLOECD= 0.39 |
0.4 (tentative) |
0.25 | 0.06 | |
| Purslanes | NEU | 0.08; 0.14; 0.16; 0.16; 0.24; 0.25; 0.28; 0.31 |
Trials on lettuce compliant with GAP extrapolated to purslanes (EFSA, 2011) MRLOECD= 0.61 |
0.6 | 0.31 | 0.20 |
| SEU | 0.17; 0.06; 0.3; 0.06; 0.11; 0.14; 0.10; 0.04 |
Trials on lettuce compliant with GAP extrapolated to purslanes (EFSA, 2011) MRLOECD= 0.46 |
0.5 | 0.30 | 0.11 | |
| Celeries | SEU | – | No residue trials available | – | – | – |
| Indoor | – | No residue trials available | – | – | – |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level.
When more than one possible fall‐back GAP is available, MRL and risk assessment values considered as fall‐back are reported in bold.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue.
Supervised trials median residue.
B.1.2.2. Summary of residues data from the supervised residue trials – new intended uses
| Crop |
Region/ indoora |
Residue levels observed in the supervised residue trials relevant to the supported GAPs (mg/kg) |
Recommendations/comments (OECD calculations) |
MRL proposals (mg/kg) |
HR (mg/kg)b |
STMR (mg/kg)c |
|---|---|---|---|---|---|---|
|
Table olives Olives for oil production |
SEU | 0.7; 0.8; 0.9; 0.56; 0.46; 1.3; 0.9; 0.8 |
Trials on olives compliant with GAP for table olives and olives for oil production (Italy, 2016) MRLOECD: 2.41 |
3 | 1.3 | 0.80 |
|
Barley grain Oats grain |
NEU | 6 × < 0.01; 0.03; 0.022 |
Trials on barley compliant with GAP (Poland, 2017). Extrapolation to oats possible MRLOECD: 0.04 |
0.05 | 0.03 | 0.01 |
|
Barley straw Oats straw |
NEU | 0.044; 0.066; 0.077; 0.14; 0.21; 0.23; 0.30; 0.32 |
Residue trials on barley compliant with GAP (Poland, 2017). Extrapolation to oats possible MRLOECD: 0.60 |
0.6 (tentative)d |
0.32 | 0.18 |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue.
Supervised trials median residue.
Tentative MRLs derived in view of the future need to set MRLs in feed items.
B.1.2.3. Residues in succeeding crops
TRR: total radioactive residue; eq: residue expressed as a.s. equivalent; NEU: northern Europe; SEU: southern Europe.
B.1.2.4. Processing factors
B.2. Residues in livestock
| Relevant groups | Dietary burden expressed in | Most critical dieta | Most critical commoditya |
Trigger exceeded (Y/N) |
Previous assessment (Max) (mg/kg DM) |
|||
|---|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | |||||||
| Med. | Max. | Med. | Max. | |||||
| Cattle (all diets) | 0.0219 | 0.0545 | 0.57 | 1.42 | Cattle (dairy) | Kale, leaves | Y |
18b (FAO, 2015) |
| Cattle (dairy only) | 0.0219 | 0.0545 | 0.57 | 1.42 | Cattle (dairy) | Kale, leaves | Y |
9.5c (FAO, 2015) |
| Sheep (all diets) | 0.0090 | 0.0347 | 0.22 | 0.82 | Sheep (lamb) | Wheat, straw | Y |
18b (FAO, 2015) |
| Sheep (ewe only) | 0.0072 | 0.0273 | 0.22 | 0.82 | Sheep (ram/ewe) | Wheat, straw | Y |
18b (FAO, 2015) |
| Swine (all diets) | 0.0093 | 0.0191 | 0.40 | 0.83 | Swine (breeding) | Kale, leaves | Y |
18b (FAO, 2015) |
| Poultry (all diets) | 0.0041 | 0.0143 | 0.06 | 0.21 | Poultry (layer) | Wheat, straw | Y |
0.22 (EFSA, 2011) |
| Poultry (layer only) | 0.0041 | 0.0143 | 0.06 | 0.21 | Poultry (layer) | Wheat, straw | Y |
0.22 (EFSA, 2011) |
bw: body weight; DM: dry matter.
Calculated for the maximum dietary burden.
Highest maximum beef or dairy cattle dietary burden suitable for maximum residue level estimates for mammalian meat and edible offal. Dietary burden based on Australian diet and mainly driven by corn stover and forage.
Highest maximum dairy cattle dietary burden suitable for maximum residue level estimates for milk. Dietary burden based on US/Canada diet and mainly driven by corn stover and forage.
B.3. Consumer risk assessment
ADI: acceptable daily intake; bw: body weight; IEDI: international estimated daily intake; PRIMo: (EFSA) Pesticide Residues Intake Model; WHO: World Health Organization; GAP: good agricultural practice; ARfD: acute reference dose; IESTI: international estimated short‐term intake.
B.4. Proposed MRLs
| Code number b | Commodity | Existing EU MRL (mg/kg) | Outcome of the assessment | |
|---|---|---|---|---|
| MRL (mg/kg) | Comment | |||
| Enforcement residue definition: acetamiprid | ||||
| 130010 | Apples | 0.8 | 0.4 | Fall‐back MRL is proposedc |
| 130020 | Pears | 0.8 | 0.4 | Fall‐back MRL is proposedc |
| 140030 | Peaches | 0.8 | 0.2 | Fall‐back MRL is proposedc |
| 242020 | Head cabbages | 0.7 | 0.4 | Fall‐back MRL is proposedc |
| 243010 | Chinese cabbages | 1.5 | – | A fall‐back MRL could not be proposedd |
| 243020 | Kales | 1.5 | – | A fall‐back MRL could not be proposedd |
| 251020 | Lettuces | 3 | 1.5 | Fall‐back MRL is proposedc |
| 251030 | Escaroles/broad‐leaved endives | 1.5 | 0.4 | Tentative fall‐back MRL is proposede |
| 252010 | Spinaches | 5 | 0.6 | Fall‐back MRL is proposedc |
| 252020 | Purslanes | 3 | 0.6 | Fall‐back MRL is proposedc |
| 252030 | Chards/beet leaves | 3 | 0.6 | Fall‐back MRL is proposedc |
| 270030 | Celeries | 1.5 | – | A fall‐back MRL could not be proposedf |
| 161030 | Table olives | 0.9 | 3 | New intended EU uses are sufficiently supported by data and no risk for consumers has been identified |
| 402010 | Olives for oil production | 0.9 | 3 | |
| 500010 | Barley grains | 0.01a | 0.05 | |
| 500050 | Oat grains | 0.01a | 0.05 | |
| – | Other products of plant origin | See Regulation 2017/626 | See Regulation 2017/626 | Existing MRLs can be maintainedg |
| Enforcement residue definition: sum of acetamiprid and N‐desmethyl acetamiprid, expressed as acetamiprid | ||||
| – | Other products of animal origin | See Regulation 2017/626 | See Regulation 2017/626 | Existing MRLs can be maintainedh |
MRL: maximum residue level.
Indicates that the MRL is set at the limit of quantification.
Commodity code number, as listed in Annex I of Regulation (EC) No 396/2005.
The existing EU MRL was identified as a potential MRL of concern. Data supporting a fall‐back MRL were submitted by MSs and no risk to consumers is identified for this fall‐back MRL.
The existing EU MRL was identified as a potential MRL of concern. No uses are currently authorised in EU that could be considered to derive a fall‐back MRL. EFSA proposes to lower the MRL to the appropriate LOQ and to withdraw the relevant authorisations within the EU.
The existing EU MRL was identified as a potential MRL of concern. Data supporting a fall‐back MRL were submitted by MSs and no risk to consumers is identified for this fall‐back MRL. Nevertheless the derived fall‐back MRL should be confirmed by the submission of additional data.
The existing EU MRL was identified as a potential MRL of concern. Residue data supporting the fall‐back GAPs were not available and a fall‐back MRL cannot be derived. EFSA proposes to lower the MRL to the appropriate LOQ and to withdraw the relevant authorisations within the EU.
The existing EU MRL was not identified as a potential MRL of concern.
The existing EU MRL was not identified as a potential MRL of concern. Moreover the withdrawal of the most critical existing uses on kale and apples and the intended uses on barley and oats are not expected to have an impact on the MRLs calculated for livestock.
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.
PRIMo (scenario 1)
PRIMo (scenario 2)
Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: acetamiprid | ||||
| Alfalfa, forage (green) | 0.09 |
STMR (EFSA, 2011) |
0.41 |
HR (EFSA, 2011) |
| Alfalfa, hay (fodder) | 0.23 |
STMR × 2.5b (EFSA, 2011) |
1.03 |
HR × 2.5b (EFSA, 2011) |
| Alfalfa, meal | 0.23 |
STMR × 2.5b (EFSA, 2011) |
1.03 |
HR × 2.5b (EFSA, 2011) |
| Alfalfa, silage | 0.10 |
STMR × 1.1b (EFSA, 2011) |
0.45 |
STMR × 1.1b (EFSA, 2011) |
|
Barley, straw Oat, straw |
0.18 |
STMR (intended use) |
0.32 |
HR (intended use) |
| Cabbage, heads leaves | 0.10 |
STMR (EFSA, 2011) |
0.50 |
HR (EFSA, 2011) |
| Kale, leaves (forage) | 0.10 |
STMR (EFSA, 2015) |
0.73 |
HR (EFSA, 2015) |
| Triticale, strawWheat, straw | 0.27 |
STMR (EFSA, 2011) |
1.6 |
HR (EFSA, 2011) |
| Potato, culls | 0.01a |
STMR (EFSA, 2011) |
0.01a |
STMR (EFSA, 2011) |
|
Barley, grain Oat, grain |
0.01 |
STMR (intended use) |
0.01 |
STMR (intended use) |
|
Bean, seed (dry) Cowpea, seed Lupin, seed Pea (Field pea), seed (dry) |
0.02 |
STMR (EFSA, 2016a) |
0.02 |
STMR (EFSA, 2016a) |
| Cotton, undelinted seed | 0.09 |
STMR (EFSA, 2011) |
0.09 |
STMR (EFSA, 2011) |
|
Triticale, grain Wheat, grain |
0.01 |
STMR (EFSA, 2016a) |
0.01 |
STMR (EFSA, 2016a) |
| Apple, pomace, wet | 0.30 |
STMR × PF (1.3) (EFSA, 2011) |
0.30 |
STMR × PF (1.3) (EFSA, 2011) |
|
Brewer's grain, dried Wheat, distiller's grain (dry) |
0.03 |
STMR × 3.3b (EFSA, 2016a) |
0.03 |
STMR × 3.3b (EFSA, 2016a) |
| Canola (Rape seed), meal | 0.06 |
STMR × 2 b (EFSA, 2016a) |
0.06 |
STMR × 2 b (EFSA, 2016a) |
| Citrus fruits, dried pulp | 1.90 |
STMR × 10 b (EFSA, 2011) |
1.90 |
STMR × 10 b (EFSA, 2011) |
| Coconut, meal | 0.02 |
STMR × 1.5 b (EFSA, 2011) |
0.02 |
STMR × 1.5 b (EFSA, 2011) |
| Cotton, meal | 0.04 |
STMR × PF (0.4) (EFSA, 2011) |
0.04 |
STMR × PF (0.4) (EFSA, 2011) |
| Lupin seed, meal | 0.02 |
STMR × 1.1b (EFSA, 2016a) |
0.02 |
STMR × 1.1b (EFSA, 2016a) |
| Potato, process waste | 0.01a |
STMRc (EFSA, 2011) |
0.01a |
STMRc (EFSA, 2011) |
| Potato, dried pulp | 0.01a |
STMRc (EFSA, 2011) |
0.01a |
STMRc (EFSA, 2011) |
| Rape, meal | 0.06 |
STMR × 2b (EFSA, 2016a) |
0.06 |
STMR × 2b (EFSA, 2016a) |
| Wheat gluten, meal | 0.02 |
STMR × 1.8b (EFSA, 2016a) |
0.02 |
STMR × 1.8b (EFSA, 2016a) |
| Wheat, milled by‐pdts | 0.07 |
STMR × 7b (EFSA, 2016a) |
0.07 |
STMR × 7b (EFSA, 2016a) |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor; CXL: codex maximum residue limit.
Crops in bold indicate the commodities of relevance in the assessment.
Indicates that the input value is proposed at the limit of quantification.
For alfalfa hay forage and silage, for distiller's grains, for meals of oilseeds, coconuts, wheat gluten and lupin seeds and for wheat milled by‐products, in the absence of processing factors supported by data, default processing factors were included in the calculation to consider the potential concentration of residues in these commodities.
For potatoes process waste and dried pulp, no default processing factor was applied because residues in the raw commodities were below the LOQ. Concentration of residues in these commodities is therefore not expected.
D.2. Consumer risk assessment
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: acetamiprid | ||||
| Citrus fruits | 0.01 |
STMR × PF (0.03) (EFSA, 2011) |
0.02 |
HR × PF (0.03) (EFSA, 2011) |
| Tree nuts | 0.01 |
STMR (EFSA, 2013) |
0.05 |
HR (EFSA, 2013) |
|
Apples Pears |
0.23 |
STMR |
0.64 |
HR |
| 0.07 |
STMR (Fall‐back) |
0.21 |
HR (Fall‐back) |
|
| Quinces | 0.23 |
STMR |
0.64 |
HR |
| Medlars | 0.23 |
STMR |
0.64 |
HR |
| Loquats/Japanese medlars | 0.23 |
STMR |
0.64 |
HR |
| Apricots | 0.22 |
STMR (EFSA, 2013) |
0.57 |
HR (EFSA, 2013) |
| Cherries (sweet) | 0.45 |
STMR CXL (FAO, 2011) |
0.88 |
HR CXL (FAO, 2011) |
| Peaches | 0.22 |
STMR |
0.57 |
HR |
| 0.06 |
STMR (Fall‐back) |
0.10 |
HR (Fall‐back) |
|
| Plums | 0.04 |
STMR CXL (FAO, 2011) |
0.11 |
HR CXL (FAO, 2011) |
| Table and wine grapes | 0.09 |
STMR CXL (FAO, 2011) |
0.25 |
HR CXL (FAO, 2011) |
| Strawberries | 0.10 |
STMR |
0.25 |
HR |
|
Cane fruits Other small fruits and berries |
0.64 |
STMR CXL (FAO, 2011) |
1.00 |
HR CXL (FAO, 2011) |
| Figs | 0.01 |
STMR (EFSA, 2011) |
0.01 |
HR (EFSA, 2011) |
|
Table olives Olives for oil production |
0.80 |
STMR (intended use) |
1.30 |
HR (intended use) |
| Bananas | 0.05 |
STMR x PF (0.49) (EFSA, 2014) |
0.11 |
HR x PF (0.49) (EFSA, 2014) |
| Potatoes | 0.01a |
STMR (EFSA, 2011) |
0.01a |
HR (EFSA, 2011) |
| Onions | 0.01 |
STMR (EFSA, 2011) |
0.02 |
HR (EFSA, 2011) |
| Garlic | 0.01 |
STMR CXL (FAO, 2011) |
0.01 |
HR CXL (FAO, 2011) |
| Spring onions | 0.38 |
STMR CXL (FAO, 2011) |
2.00 |
HR CXL (FAO, 2011) |
| Tomatoes | 0.13 |
STMR (EFSA, 2016a) |
0.28 |
HR (EFSA, 2016a) |
| Sweet peppers/bell peppers | 0.10 |
STMR (EFSA, 2011) |
0.19 |
HR (EFSA, 2011) |
| Aubergines/eggplants | 0.04 |
STMR |
0.11 |
STMR |
| Okra, lady's fingers | 0.04 |
STMR CXL (FAO, 2011) |
0.14 |
HR CXL (FAO, 2011) |
| Cucumbers | 0.05 |
STMR (EFSA, 2011) |
0.23 |
HR (EFSA, 2011) |
| Gherkins | 0.14 |
STMR (EFSA, 2016a) |
0.37 |
HR (EFSA, 2016a) |
| Courgettes | 0.05 |
STMR (EFSA, 2011) |
0.23 |
HR (EFSA, 2011) |
| Cucurbits with inedible peel | 0.05 |
STMR CXL (FAO, 2011) |
0.11 |
HR CXL (FAO, 2011) |
| Sweet corn | 0.01a |
STMR CXL (FAO, 2015) |
0.01a |
HR CXL (FAO, 2015) |
| Broccoli | 0.03 |
STMR |
0.25 |
HR |
| Cauliflowers | 0.02 |
STMR CXL (FAO, 2011) |
0.22 |
HR CXL (FAO, 2011) |
| Brussels sprouts | 0.02 |
STMR (EFSA, 2011) |
0.03 |
HR (EFSA, 2011) |
| Head cabbages | 0.10 |
STMR |
0.50 |
HR |
| 0.02 |
STMR (Fall‐back) |
0.25 |
HR (Fall‐back) |
|
| Chinese cabbages | 0.10 |
STMR (EFSA, 2015) |
0.73 |
HR (EFSA, 2015) |
| – | No fall‐back available | – | No fall‐back available | |
| Kales | 0.10 |
STMR (EFSA, 2015) |
0.73 |
HR (EFSA, 2015) |
| – | No fall‐back available | – | No fall‐back available | |
| Lamb's lettuces/corn salads | 0.83 |
STMR (EFSA, 2011) |
1.90 |
HR (EFSA, 2011) |
| Lettuces | 0.83 |
STMR (EFSA, 2011) |
1.90 |
HR (EFSA, 2011) |
| 0.49 |
STMR (Fall‐back) |
0.75 |
HR (Fall‐back) |
|
| Escaroles/broad‐leaved endives | 0.49 |
STMR (EFSA, 2011) |
0.75 |
HR (EFSA, 2011) |
| 0.10 |
STMR (Fall‐back, tentative) |
0.25 |
HR (Fall‐back, tentative) |
|
|
Cresses and other sprouts and shoots Roman rocket/rucola Baby leaf crops (including brassica species) |
0.83 |
STMR (EFSA, 2011) |
1.90 |
HR (EFSA, 2011) |
|
Land cresses Red mustards |
0.81 |
STMR (EFSA, 2011) |
1.90 |
HR (EFSA, 2011) |
| Spinaches | 0.83 |
STMR (EFSA, 2011) |
2.58 |
HR (EFSA, 2011) |
| 0.20 |
STMR (Fall‐back) |
0.31 |
HR (Fall‐back) |
|
| Purslanes | 0.83 |
STMR (EFSA, 2012) |
1.90 |
HR (EFSA, 2012) |
| 0.20 |
STMR (Fall‐back) |
0.31 |
HR (Fall‐back) |
|
| Chards/beet leaves | 0.81 |
STMR (EFSA, 2011) |
1.90 |
HR (EFSA, 2011) |
| 0.20 |
STMR (Fall‐back) |
0.31 |
HR (Fall‐back) |
|
| Fresh herbs | 0.83 |
STMR (EFSA, 2011) |
1.90 |
HR (EFSA, 2011) |
|
Beans (with pods) Peas (with pods) |
0.06 |
STMR (EFSA, 2016a) |
0.32 |
HR (EFSA, 2016a) |
|
Beans (without pods) Peas (without pods) |
0.03 |
STMR CXL (FAO, 2011) |
0.18 |
HR CXL (FAO, 2011) |
| Asparagus | 0.26 |
STMR CXL (FAO, 2015) |
0.43 |
HR CXL (FAO, 2015) |
| Celeries | 0.32 |
STMR |
0.78 |
HR |
| – | No fall‐back available | – | No fall‐back available | |
| Globe artichokes | 0.11 |
STMR (EFSA, 2011) |
0.25 |
HR (EFSA, 2011) |
| Pulses | 0.02 |
STMR (EFSA, 2016a) |
0.08 |
HR (EFSA, 2016a) |
| Rapeseeds/canola seeds | 0.03 |
STMR (EFSA, 2016a) |
0.20 |
HR (EFSA, 2016a) |
| Cotton seeds | 0.09 |
STMR |
0.50 |
STMR |
| Barley and oat grains | 0.01 |
HR (intended use) |
0.03 |
STMR (intended use) |
| Wheat grains | 0.01 |
STMR (EFSA, 2016a) |
0.06 |
HR (EFSA, 2016a) |
|
Cardamom Peppercorn (black, green and white) |
0.10 |
STMR CXL (FAO, 2015) |
0.10 |
HR CXL (FAO, 2015) |
| Risk assessment residue definition: sum of acetamiprid and N‐desmethyl‐acetamiprid, expressed as acetamiprid | ||||
| Swine meat | 0.02 | STMR CXL (FAO, 2015) | 0.27 | HR CXL (FAO, 2015) |
| Swine fat tissue | 0.02 | STMR CXL (FAO, 2015) | 0.16 | HR CXL (FAO, 2015) |
| Swine liver | 0.11 | STMR CXL (FAO, 2015) | 0.89 | HR CXL (FAO, 2015) |
| Swine kidney | 0.11 | STMR CXL (FAO, 2015) | 0.89 | HR CXL (FAO, 2015) |
| Bovine, sheep, goat and equine meat | 0.02 | STMR CXL (FAO, 2015) | 0.27 | HR CXL (FAO, 2015) |
| Bovine, sheep, goat and equine fat tissue | 0.02 | STMR CXL (FAO, 2015) | 0.16 | HR CXL (FAO, 2015) |
| Bovine, sheep, goat and equine liver | 0.11 | STMR CXL (FAO, 2015) | 0.89 | HR CXL (FAO, 2015) |
| Bovine, sheep, goat and equine kidney | 0.11 | STMR CXL (FAO, 2015) | 0.89 | HR CXL (FAO, 2015) |
| Poultry meat | 0.02a | STMR (EFSA, 2011) | 0.02a | HR (EFSA, 2011) |
| Poultry fat tissue | 0.02a | STMR (EFSA, 2011) | 0.02a | HR (EFSA, 2011) |
| Poultry liver | 0.1a | STMR (EFSA, 2011) | 0.1a | HR (EFSA, 2011) |
| Cattle, sheep, goat and horse milk | 0.02 |
STMR CXL (FAO, 2015) |
0.11 |
HR CXL (FAO, 2015) |
| Birds eggs | 0.02a | STMR (EFSA, 2011) | 0.02a | HR (EFSA, 2011) |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor; CXL: codex maximum residue limit.
Crops in bold indicate the commodities of relevance in the assessment.
Indicates that the input value is proposed at the limit of quantification.
Appendix E – Used compound codes
1.
| Code/trivial namea | Chemical name/SMILES notation | Structural formula |
|---|---|---|
|
N‐desmethyl‐acetamiprid (IM‐2‐1) |
(E)‐N‐[(6‐chloro‐3‐pyridyl)methyl]‐N’‐cyanoacetamidine Clc1ccc(CNC(\C)=N\C#N)cn1 |
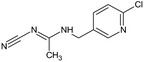
|
| IM‐1‐4 |
1‐(6‐chloro‐3‐pyridyl)‐N‐ methylmethanamine Clc1ccc(CNC)cn1 |
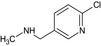
|
| IM‐1‐5 |
N‐[(6‐chloro‐3‐pyridyl)methyl]‐N‐methylacetamidine Clc1ccc(CN(C)C(C)=N)cn1 |
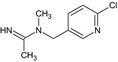
|
| 6‐chloronicotinic acid (IC‐0) |
6‐chloronicotinic acid OC(=O)c1cnc(Cl)cc1 |
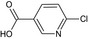
|
SMILES: simplified molecular‐input line‐entry system.
The metabolite name in bold is the name used in the conclusion.
Suggested citation: EFSA (European Food Safety Authority) , Brancato A, Brocca D, Carrasco Cabrera L, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Medina P, Miron I, Molnar T, Pedersen R, Reich H, Riemenschneider C, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B and Villamar‐Bouza L, 2018. Reasonded Opinion on the focussed assessment of certain existing MRLs of concern for acetamiprid and modification of the existing MRLs for table olives, olives for oil production, barley and oats. EFSA Journal 2018;16(5):5262, 39 pp. 10.2903/j.efsa.2018.5262
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00723, Q‐2016‐00628 and Q‐2017‐00063
Approved: 13 April 2018
Notes
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 9.8.1991, p. 1–32, as last amended.
Commission Directive 2004/99/EC of 1 October 2004 amending Council Directive 91/414/EEC to include acetamiprid and thiacloprid as active substances. OJ L 309, 6.10.2004, p. 6–8.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Commission Implementing Regulation (EU) 2018/113 of 24 January 2018 renewing the approval of the active substance acetamiprid in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJ L 20, 25.1.2018, p. 7–10.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- Belgium , 2017. Evaluation report on authorised uses to be considered for the review of the existing MRLs for acetamiprid prepared by the evaluating Member State Belgium under Article 43 of Regulation (EC) No 396/2005, 20 December 2017, 5 pp.
- Czech Republic , 2018.Evaluation report on authorised uses to be considered for the review of the existing MRLs for acetamiprid prepared by the evaluating Member State Czech Republic under Article 43 of Regulation (EC) No 396/2005, 11 January 2018, 6 pp.
- EFSA (European Food Safety Authority), 2007. Reasoned opinion on the potential chronic and acute risk to consumers’ health arising from proposed temporary EU MRLs. EFSA Journal 2007;5(3):32r, 1141 pp. 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Review of the existing maximum residue levels (MRLs) for acetamiprid according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2011;9(7):2328, 59 pp. 10.2903/j.efsa.2011.2328 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Reasoned opinion on the modification of the existing MRLs for acetamiprid in purslane, legume vegetables and pulses (beans and peas). EFSA Journal 2012; 10(12):3051, 28 pp. 10.2903/j.efsa.2012.3051 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. Reasoned opinion on the modification of the existing maximum residue level (MRL) for acetamiprid in apricots and tree nuts. EFSA Journal 2013;11(12):3506, 30 pp. 10.2903/j.efsa.2013.3506 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Reasoned opinion on the modification of the existing MRL for acetamiprid in bananas. EFSA Journal 2014;12(9):3824, 20 pp. 10.2903/j.efsa.2014.3824 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Reasoned opinion on the modification of the existing maximum residue levels for acetamiprid in leafy brassicas. EFSA Journal 2015;13 (9):4229, 20 pp. 10.2903/j.efsa.2015.4229 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016a. Reasoned opinion on the modification of the existing maximum residue levels for acetamiprid in various crops. EFSA Journal 2016;14(2):4385, 25 pp. 10.2903/j.efsa.2016.4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2016b. Conclusion on the peer review of the pesticide risk assessment of the active substance acetamiprid. EFSA Journal 2016;14(11):4610, 26 pp. 10.2903/j.efsa.2016.4610 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2018. Member States consultation report on the focused assessment of certain existing MRLs of concern for acetamiprid prepared by EFSA in the framework of Article 43 of Regulation (EC) No 396/2005, 13 April 2018. Available online: www.efsa.europa.eu
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2017a. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev.10.3, June 2017.
- European Commission , 2017b. Summary report of the Standing Committee on Plants, Animals, Food and Feed held in Brussels on 5–6 October 2017 (Section Phytopharmaceuticals – Plant Protection Products – Legislation), sante.ddg2.g.5(2018)475819.
- European Commission , 2017c. Final renewal report for the active substance acetamiprid. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 13 December 2017 in view of the inclusion of active substance in Annex I of Council Directive 91/414/EEC. SANTE/10502/2017 Rev 4, 13 December 2017.
- FAO (Food and Agriculture Organization of the United Nations), 2011. Acetamiprid. In: Pesticide residues in food – 2011. Evaluations. Part I. Residues. FAO Plant Production and Protection Paper 212, p. 246.
- FAO (Food and Agriculture Organization of the United Nations), 2015. Acetamiprid. In: Pesticide residues in food – 2015. Evaluations. Part I. Residues. FAO Plant Production and Protection Paper 226, p. 129.
- Finland , 2018. Evaluation report on authorised uses to be considered for the review of the existing MRLs for acetamiprid prepared by the evaluating Member State Finland under Article 43 of Regulation (EC) No 396/2005, 15 January 2018, 3 pp.
- France , 2018. Evaluation report on authorised uses to be considered for the review of the existing MRLs for acetamiprid prepared by the evaluating Member State France under Article 43 of Regulation (EC) No 396/2005, 15 January 2018, 54 pp.
- Germany , 2017. Evaluation report on authorised uses to be considered for the review of the existing MRLs for acetamiprid prepared by the evaluating Member State Germany under Article 43 of Regulation (EC) No 396/2005, 22 December 2017, 14 pp.
- Greece , 2018. Evaluation report on authorised uses to be considered for the review of the existing MRLs for acetamiprid prepared by the evaluating Member State Greece under Article 43 of Regulation (EC) No 396/2005, 15 January 2018, 26 pp.
- Italy , 2016. Evaluation report on the modification of MRLs for acetamiprid in olives prepared by the evaluating Member State Italy under Article 8 of Regulation (EC) No 396/2005, September 2016, 65 pp.
- Italy , 2018. Evaluation report on authorised uses to be considered for the review of the existing MRLs for acetamiprid prepared by the evaluating Member State Italy under Article 43 of Regulation (EC) No 396/2005, January 2018, 42 pp.
- Lithuania , 2018. Evaluation report on authorised uses to be considered for the review of the existing MRLs for acetamiprid prepared by the evaluating Member State Lithuania under Article 43 of Regulation (EC) No 396/2005, 15 January 2018, 11 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 04 September 2013.
- Poland , 2017. Evaluation report on the modification of MRLs for acetamiprid in barley and oats prepared by the evaluating Member State Poland under Article 8 of Regulation (EC) No 396/2005, 20 December 2016, 86 pp.
- Portugal , 2018. Evaluation report on authorised uses to be considered for the review of the existing MRLs for acetamiprid prepared by the evaluating Member State Portugal under Article 43 of Regulation (EC) No 396/2005, 15 January 2018, 31 pp.
- Spain , 2018. Evaluation report on authorised uses to be considered for the review of the existing MRLs for acetamiprid prepared by the evaluating Member State Finland under Article 43 of Regulation (EC) No 396/2005, 22 January 2018, 29 pp.
- Sweden , 2018. Evaluation report on authorised uses to be considered for the review of the existing MRLs for acetamiprid prepared by the evaluating Member State Sweden under Article 43 of Regulation (EC) No 396/2005, 12 January 2018, 9 pp.
- United Kingdom , 2018. Evaluation report on authorised uses to be considered for the review of the existing MRLs for acetamiprid prepared by the evaluating Member State United Kingdom under Article 43 of Regulation (EC) No 396/2005, 12 January 2018, 5 pp.


